Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/44/01. The contractual start date was in March 2012. The draft report began editorial review in December 2013 and was accepted for publication in June 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dr Price reports grants from UK Medical Research Council, during the conduct of the study; Dr Turner reports grants from University of Birmingham/National Institute of Health Research (NIHR), during the conduct of the study; Dr Jordan, Professor Riley, Dr Moore, Professor Singh, Professor Adab, Professor Fitzmaurice, Dr Jowett and Professor Jolly report grants from the NIHR during the duration of the study; Professor Singh reports that the University Hospitals of Leicester NHS Trust holds the intellectual property for a self-management manual for chronic obstructive pulmonary disease.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Jordan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Chronic obstructive pulmonary disease: definition, prognosis and burden
Chronic obstructive pulmonary disease (COPD) is a long-term condition characterised by ‘persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles and gases’. 1 The most important cause of COPD is cigarette smoking, although other risk factors are thought to be indoor and outdoor air pollution, occupational exposures and diet. 2 Over time, patients experience increasing breathlessness and more frequent exacerbations of respiratory symptoms, leading to increasing disability, reduced quality of life (QoL) and often repeated hospitalisations. 1
Chronic obstructive pulmonary disease affects 5–10% of people worldwide,3 is rising in prevalence,4 and is a leading cause of death. 5 In the UK it is the second most common cause of emergency admissions,6 costing the NHS over £800M per year. 7 Increasing recognition of the importance of this disease8,9 culminated in a new National Clinical Outcomes Strategy in 2011. 6
Diagnosis and severity of chronic obstructive pulmonary disease
A diagnosis of COPD is suspected among people with breathlessness or cough and is supported by post-bronchodilator spirometry to confirm irreversible airflow obstruction. 10 Although definitions of airflow obstruction are inconsistent and controversial,11 National Institute for Health and Care Excellence (NICE) guidance for COPD currently defines airflow obstruction when the ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) is < 0.7 (i.e. FEV1/FVC < 0.7). 10 Despite the requirement for confirmation with spirometry, there are many people with a clinical diagnosis of COPD who do not meet these spirometric criteria. 12 Late-onset asthma, other comorbidities and difficulty obtaining spirometry data may contribute to misdiagnoses.
Severity of airflow obstruction in the UK is graded using categories of FEV1 as a percentage of predicted normal values of a healthy reference population (Table 1),10 although these definitions may vary across countries, and have changed over time.
| Category | FEV1% pred |
|---|---|
| Mild | > 80 |
| Moderate | 50–79 |
| Severe | 30–49 |
| Very severe | < 30 |
Severity of airflow obstruction does not necessarily reflect either the level of disability experienced or the frequency of exacerbation and composite measures to capture the global impact of the disease have been proposed. 1 However, they are not yet widely used as the basis for treatment decisions. Most research studies evaluating treatments use FEV1% pred (forced expiratory volume in 1 second percentage predicted) to select and describe patients. FEV1 is also often used as an outcome measure to describe prognosis of patients, as are clinical measures (such as dyspnoea and exacerbations), global measures such as health-related quality of life (HRQoL) and health service utilisation (e.g. hospital admissions).
Exacerbations of chronic obstructive pulmonary disease
Exacerbations or ‘flare-ups’ of COPD occur in approximately 50–60% of moderate/severe patients with COPD, per year, in published cohorts and trials13,14 and similar rates are also observed in primary care (unpublished data from the Birmingham COPD cohort study). They are a characteristic component of disease progression, often requiring hospitalisation1 and are associated with long-term poor outcome. Exacerbations are caused primarily by viral respiratory infections, particularly the common cold (associated with about two-thirds of exacerbations). 15 They result in worsening of a patient’s symptoms for several days, this being more frequent during winter months. 16
Approximately 15% of patients with COPD per year have exacerbations that are severe enough to lead to hospital admission,7 which contributes to over half of the total direct costs of COPD to the NHS. 7 Readmission for an exacerbation within 3 months is high at > 30%,17 as is 30-day mortality. Exacerbations are often not independent events, and there are a group of people who are frequent exacerbators. 18 Exacerbations are usually treated with an increase in usual medication, a course of antibiotics and/or steroids. 10
Management of chronic obstructive pulmonary disease
In early-stage disease patients may not display or recognise their symptoms but, as the disease progresses, varying degrees of cough, sputum, wheeze and dyspnoea1 may develop until eventually patients may require long-term oxygen therapy. 10 Other than the acute treatment of exacerbations, therapy is aimed at reducing progression and managing symptoms and is primarily based around smoking cessation, inhaled medications, pulmonary rehabilitation (PR) and, increasingly, more preventative disease management approaches [including self-management (SM)]. 10
Management of long-term conditions in the UK
More than 15 million people in England are living with long-term conditions such as COPD, diabetes, heart disease and asthma. 19 Long-term conditions represent > 70% of hospital bed-days and more than half of general practitioner (GP) consultations, and account for at least 70% of the total health and social care budget. 19 For patients, long-term conditions reduce QoL and ability to carry out daily tasks, as well as contributing to premature mortality. In the past, treatment of people with long-term conditions would have been more reactive. However, in 2004, the NHS Improvement Plan set out the plans for the future care of these patients by focusing on avoiding admissions and caring for patients at the primary care level, and encouraging patients to manage their own condition (SM). 20
Patients access health-care professionals relatively infrequently and, therefore, in order to optimise their health patients must be able to manage their own condition successfully on a daily basis. Support should be available to help patients (and their families/carers) manage their own condition and make healthier choices about their diet, physical activity and lifestyle. 20 Since the NHS Improvement Plan was published, this approach has been embedded in subsequent policy documents,21 which clearly emphasise the important role of SM. However, it is clear that clinicians are often reluctant to take this approach and, therefore, the support for patient SM is likely to be suboptimal. 19
Surveys indicate that > 90% of patients with long-term conditions would like to become more active self-managers, although in many conditions report insufficient knowledge or support to do so. 22
Self-management: definition and models
‘Self-management’ has been defined as the ability of a patient to deal with all that a chronic disease entails, including symptoms, treatment, physical and social consequences and lifestyle changes. 23 The exact nature of SM will vary from condition to condition and person to person. Indeed, there is debate about the interpretation of the goals of SM, which may differ between health-care professionals and patients, and between countries and health-care systems.
There are many factors that may affect a patient’s ability to self-manage (e.g. severity, presence of comorbidities, depression, education, psychological factors, ethnicity). 24–27 One behavioural model that describes SM is Patient Activation,28 which emphasises that patients should have the knowledge, skills and confidence to manage their own health and health care. Interventions to promote SM should aim to address each of these components.
Interventions to support self-management
Self-management support involves collaboration between the health-care professional and the patient so that the patient acquires and demonstrates the knowledge and skills required to manage his/her medical regimens, change their health behaviour, improve control of their disease and improve their well-being. 29 Patient education alone is not sufficient; monitoring and assessment of progress is also essential. SM interventions should teach skills that promote health behaviour modification with the aim of increasing self-efficacy (the belief that one can successfully execute particular behaviours), thus improving clinical outcomes, including adherence. 30 Strategies to promote self-efficacy include personal experience and practice, feedback and reinforcement, analysis of causes of failure and shared experience with successful peers. 30 Indeed, the established NHS Expert Patient Programme for managing chronic diseases is based on Bandura’s theory of self-efficacy. 31 Evaluations of SM programmes should therefore first assess patients’ self-efficacy, change in behaviour and then patient outcomes and health-care utilisation.
Self-management programmes can be delivered in a number of ways (e.g. series of workshops, written material, by telephone, internet or a mixture) by various professionals or lay personnel, and can have a range of components. Systematic reviews of SM programmes for long-term conditions have concluded that such programmes tend to lead to small improvements in some outcomes for some chronic diseases (but not all) and that further research is needed. 32,33 More recently there have been some unsuccessful high-profile trials in primary care settings,34–36 some of which suggest that only a subgroup of patients may be able to self-manage.
Self-management of chronic obstructive pulmonary disease: principles and current practice
Self-management for patients with COPD is complex and challenging. 10,25 It requires patients to be able to manage various facets of their condition on a daily basis, including understanding and taking their medications appropriately with good inhaler technique, early recognition of exacerbations of symptoms and early instigation of treatment during an exacerbation, receiving annual influenza vaccinations, managing their breathlessness (including stress management/relaxation) to allow them to undertake activities of daily living, bronchial clearance techniques, taking regular exercise to maintain their lung function and exercise capacity, quitting smoking and maintaining a healthy diet. 29,30,37
In reality, the true extent to which patients manage these aspects is not well described but it is likely to be suboptimal. A survey published in 2009 in Canada38 revealed that although patients felt that their knowledge about the disease was good, in reality their knowledge of the causes of COPD, the consequences of not adhering to their medication and how to manage exacerbations was inadequate. A small study in one GP practice in the UK in 200439 indicated that only 48% of patients with COPD had discussed levels of exercise with their GP/nurse and only 50% had spare antibiotics/steroids at home in case of exacerbations, although > 80% reported understanding their inhalers, knowing what to do if they had an exacerbation and having given up smoking.
Current self-management support for chronic obstructive pulmonary disease in the UK
Self-management support for COPD is less well developed than in other long-term conditions both in the UK and worldwide. NICE quality standards state that patients with COPD should have a comprehensive, up-to-date personalised management plan, including information/educational material about the condition and its management. 40 NICE guidance also emphasises that patients at risk of having an exacerbation of COPD should be given SM advice/treatment that encourages them to respond promptly to the symptoms of an exacerbation. 10 Other aspects of SM advice include promoting proactive behaviour change, such as smoking cessation and increased exercise. However, the evidence about the exact nature and the effectiveness and cost-effectiveness of potential components of a SM package is acknowledged to be inadequate. 10
A variety of tools are available, such as the ‘Living Well with COPD’ programme developed by the Montreal Chest Institute and mentioned in the American Thoracic Society statement,30 materials provided by the British Lung Foundation,41 and materials developed by individual hospitals/universities or private health-care companies, but there is no one consistent recommended approach. 6,10 Limited evidence suggests that programmes are patchily provided and unlikely to be individualised. 42 Qualitative studies in the UK and elsewhere suggest that patients report a lack of SM support and a lack of understanding of their condition. 43,44
This heterogeneity is reflected in the literature describing trials of a wide variety of interventions. It is accepted, however, that the optimum package of care is not known,10 and this fact is one of the premises upon which this report is based.
There is considerable overlap between programmes that are defined as SM and other more complex supervised programmes, such pulmonary rehabilitation (PR). 37,45 PR is defined as ‘an evidence-based, multidisciplinary, and comprehensive intervention for patients with chronic respiratory diseases who are symptomatic and often have decreased daily life activities . . . programs involve patient assessment, exercise training, education, nutritional intervention, and psychosocial support’. 30 A continuum of support is now recognised, which should, ideally, be personalised to reflect an individual patient’s needs, including disease severity and other comorbidities. 37,45
For this reason, in the second study within our evidence report, we have included trials of a wide range of care packages including PR in order to identify which features of SM are most important, as long as they involve one or more of the specified components of SM.
Evidence for the effectiveness and cost-effectiveness of self-management support for chronic obstructive pulmonary disease: existing literature
Current literature on SM for COPD largely addresses the effectiveness of SM support when delivered to patients in a stable state. There are now many trials and overlapping systematic reviews of interventions (such as PR, integrated care), which include a SM component, although to varying degrees. 46–50 A Cochrane systematic review of SM education interventions48 (excluding studies on PR, updated in 2009) identified 14 randomised controlled trials (RCTs) that showed that SM interventions delivered to patients with COPD in the stable state could significantly reduce hospital admissions compared with usual care (UC) [odds ratio (OR) = 0.64, 95% confidence interval (CI) 0.47 to 0.89], significantly improve some domains of QoL and effect a small improvement in dyspnoea. However, many of the other results were inconclusive, possibly because of the great heterogeneity in the populations studied, nature of the interventions, outcomes measured and length of follow-up. The authors concluded that ‘data were still insufficient to formulate clear recommendations regarding the form and contents of SM education programmes in COPD . . . with a need for more large RCTs with long-term follow-up’. 48
A systematic review of five trials on the effectiveness of action plans only (with only limited education) found that although patients were significantly more likely to recognise exacerbations and initiate treatment, there was no reduction in health-care utilisation, and they concluded that a more significant SM approach might be needed. 50 A further systematic review of COPD disease management programmes,49 including 10 trials and three before-and-after studies, indicated that such programmes (which often include SM components) may decrease hospital admission and improve QoL, although further exploration of the elements that bring the greatest benefit are needed.
A more recent systematic review of integrated disease management demonstrated a significant improvement in QoL and respiratory admissions,47 and there are other recent systematic reviews of breathing exercises,51 outreach nurses52 and exercise training. 53 These reviews are significantly overlapping in their inclusion but none of them comprehensively reviews all of the latest trials relating to SM interventions/components or attempts to delineate the relative effectiveness of the different components.
One important factor that varies among the trials already reviewed48 is the nature of the populations involved. It has been suggested that SM programmes should target those patients with more severe COPD and frequent exacerbations in order to be beneficial. 29,30 Patients who are admitted to hospital have a high risk of readmission within 90 days. 17 Thus a focus on patients who are currently hospitalised for COPD (or recently discharged) could have the most potential for health gain and reduction in resource use. Data on such interventions following hospitalisation (other than PR programmes) are limited. 54
Rationale for evidence review
Although there is a plethora of RCTs published and increasing numbers of systematic reviews on different aspects of SM support for COPD, results are conflicting about which of the many types of interventions, and particularly which components, are the most effective. 10 Furthermore, there remain significant unanswered questions about the timing and delivery of SM support, particularly whether SM support provided soon after discharge from hospital is effective or cost-effective.
In 2010, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme published a commissioning brief: supported SM for patients with moderate to severe COPD. It asked for a wide systematic review of the literature, particularly focusing on patients, around or soon after discharge, to answer: ‘What are the elements of supported SM that prevent readmission to hospital and adverse outcomes?’ We report a series of systematic reviews and an economic model to address this question.
Chapter 2 Aims and objectives
There were two main aims of this research project. The first was to undertake a systematic review of the effectiveness and cost-effectiveness of supported self-management among people with moderate to severe chronic obstructive pulmonary disease who had recently been discharged from hospital following an acute exacerbation of their condition, and to use this evidence to undertake a model-based cost-effectiveness analysis from the UK NHS perspective. With a wider systematic review, we also planned to identify the features and elements of self-management interventions that are most effective.
Each aim had specific objectives.
Aim 1
Among patients with chronic obstructive pulmonary disease at discharge, or recently discharged from hospital within the last 6 weeks, to undertake:
-
a systematic review of:
-
the evidence for the effectiveness of self-management support evaluating health behaviour change, self-efficacy, health service utilisation and patient-reported outcomes such as QoL (review 1)
-
the qualitative evidence about patient satisfaction, acceptance and barriers to self-management support (review 2)
-
the cost-effectiveness of self-management support (review 3)
-
-
a cost-effectiveness analysis and economic model of self-management support compared with usual care (economic model).
Aim 2 (review 4)
Among patients with chronic obstructive pulmonary disease, at any time point, to:
-
undertake a wider systematic review of the evidence of the effectiveness of self-management support [including interventions (such as pulmonary rehabilitation) for which there are significant components of self-management] in reducing exacerbations, hospital admissions/readmissions and improving QoL
-
describe the features and elements of self-management interventions in relation to their effectiveness by simple categorisation and tabulation
-
perform subgroup analysis and meta-regression to explore features such as the effect of study quality, population, setting and nature of intervention on the effectiveness of self-management interventions compared with usual care
-
use mixed-treatment comparison meta-analysis methods to explore which components or combinations of components are most effective.
Structure of the report
The following chapters report separately on:
-
Chapter 3: Aim 1 – clinical effectiveness review (review 1)
-
Chapter 4: Aim 1 – qualitative evidence review (review 2)
-
Chapter 5: Aim 1 – cost-effectiveness review (review 3)
-
Chapter 6: Aim 1 – economic model
-
Chapter 7: Aim 2 – review of effectiveness of components of self-management (review 4).
Each of the above chapters incorporates methods, results and discussion, and then, finally, Chapter 8 provides an overall summary.
Chapter 3 A systematic review of the clinical effectiveness of supported self-management interventions delivered shortly after hospital discharge: review 1
The aim of this chapter is to present the findings of a systematic review of the evidence for the effectiveness of SM support evaluating health behaviour change, self-efficacy, health service utilisation and patient-reported outcomes, such as quality of life (QoL).
Methods
A systematic review of published evidence of the effectiveness of interventions to support self-management (SM) among patients with chronic obstructive pulmonary disease (COPD) who had recently been discharged from hospital.
Definition of self-management used for this review
‘Self-management’ has been defined as the ability of a patient to deal with all that a chronic disease entails, including symptoms, treatment, physical and social consequences and lifestyle changes. 23 SM interventions involve collaboration between the health-care professional and the patient so that the patient acquires and demonstrates the knowledge and skills required to manage their medical regimens, change their health behaviour, improve control of their disease and improve their well-being. 29 This definition of SM was used as a basis to devise a list of SM interventions/components that were considered for this review (Table 2). Because SM interventions are so heterogeneous, we specifically chose to include all possible aspects of SM to ensure completeness. However, we excluded interventions of smoking cessation alone, as there is already good evidence of the benefits of smoking cessation in general, and a large number of systematic reviews addressing the effectiveness of smoking cessation interventions (currently 60 Cochrane systematic reviews alone). Most evidence of the effectiveness of smoking cessation relates to general populations, rather than people with a particular condition. Any study that included smoking cessation as one component of a multicomponent package in people with COPD was included. Similarly, there is already a systematic review of pulmonary rehabilitation (PR) at this time point;55 therefore, it was not considered necessary to repeat it but rather use it for comparison.
| Intervention/component | Included/excluded | Comments |
|---|---|---|
| Adherence to medication | Include | Education about taking treatment correctly, promoting adherence |
| Ambulatory oxygen | Exclude | Unless it concerns education or support to take prescribed treatments such as ambulatory oxygen |
| Breathing techniques | Include | For example, pursed lip breathing |
| Bronchial hygiene techniques | Include | Mucus/airways clearance |
| Case management | Exclude | Unless elements of SM |
| Community matrons | Exclude | Unless elements of SM |
| Complementary therapies | Exclude | Exclude anything on acupuncture and massage, etc. |
| Early recognition of symptoms/action plans | Include | Must be self-monitoring, not external monitoring by external agency, unless there is a teaching/training element (e.g. patient being taught how to recognise the symptoms and act accordingly) |
| Education | Include | Any topics |
| Exercise | Include | Any type of exercise |
| Hospital at home | Exclude | Unless elements of SM |
| Inhaler technique | Include | Including assessment of inhaler technique |
| Integrated care | Exclude | Unless elements of SM |
| Nutritional programmes | Include | Include anything which encourages/helps people to maintain good nutrition or modify their diet; exclude anything to do with (proprietary) supplements, dietary programmes or trials of effectiveness |
| Patient empowerment | Include | As recommended by patient advisory group |
| Relaxation | Include | Any types |
| Respiratory muscle training | Include | Including both inspiratory and EMT |
| Smoking cessation | Exclude | Unless as a component of a larger package (not as a single active intervention) |
| Stress management | Include | Any types including counselling |
| Support groups | Include | As recommended by patient advisory group |
| Telecare | Include | Exclude if purely telemonitoring – not just about contact; include if there is an encouragement/support component, e.g. help to promote adherence to medication |
Search strategy for effectiveness studies
A comprehensive search strategy was designed and conducted by an experienced information specialist. The searches were kept broad to capture evidence to suit both aims.
Searches for relevant studies were conducted across the following bibliographic databases: MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations and EMBASE (via Ovid), Cochrane Central Register of Controlled Trials (CENTRAL – Wiley) and Science Citation Index (Institute of Scientific Information). Subject-specific databases were also searched: PEDro physiotherapy evidence database, PsycINFO (via Ovid) and the Cochrane Airways Group Register of Trials. Ongoing studies were sourced through the metaRegister of Current Controlled Trials, International Standard Randomised Controlled Trial Number database, World Health Organization International Clinical Trials Registry Platform Portal and ClinicalTrials.gov. Specialist abstract and conference proceedings were sourced through the Institute of Science Information’s Conference Proceedings Citation Index and British Library’s Electronic Table of Contents (Zetoc). Hand-searching through European Respiratory Society, the American Thoracic Society and British Thoracic Society (BTS) conference proceedings from 2010 to 2012 was also undertaken, and selected websites were also examined. No language restrictions or methodological filters were applied to the searches. Electronic database searching was carried out from inception to May 2012, and no updated searches were undertaken beyond this time point. The search strategies used for electronic databases can be found in Appendix 1; terms for COPD were combined with those for SM and, where possible, utilised appropriate medical subject headings.
The citation lists of all included studies and any citations within relevant reviews were scanned for additional relevant studies. Consultations with experts in the field through the investigators identified additional relevant literature.
Reference Manager version 11 (Thomson ResearchSoft, San Francisco, CA, USA) was used to store and manage all search results.
Study selection process
After removal of duplicates, titles and abstracts of the remaining search results were independently reviewed by two reviewers. Full texts were obtained for papers meeting the inclusion criteria or when the abstract was unclear. Full texts were then independently reviewed by two reviewers using detailed and piloted selection criteria concerning study design, populations, interventions, comparators and outcomes for each review. Any discrepancies were resolved by a third reviewer. Any non-English language papers were assessed, based on titles and abstract, but when information was lacking or unclear, translators were used to decide final inclusion. A reviewer worked alongside translators to avoid misinterpretation of the selection criteria. During full-text screening, papers were categorised into their appropriate objectives or were excluded with reasons.
Selection criteria
The selection criteria for this review are summarised in Table 3.
| Study designs | RCTs |
|---|---|
| Population | Patients with moderate to severe COPD (defined clinically, with or without spirometry) recruited specifically at discharge or up to 6 weeks post discharge for an acute exacerbation of their condition (patients with mild or very severe COPD were included if they were a minority of the population group) Approximately 90% of patients in studies should have COPD The setting could be either hospital or community |
| Intervention | SM packages or important components of SM Excluding trials of smoking cessation and PR |
| Comparator | No intervention, UC, control/sham, other SM intervention |
| Primary outcomes | Any of: Health service outcomes and mortality Primary care consultations Hospital admissions Readmissions Duration of admissions Mortality Emergency department visits |
| Secondary outcomes | Any considered but to include: Behaviour change Self-efficacy Specific behaviours, e.g. increase in exercise/activity Patient-reported outcomes Exacerbations HRQoL Anxiety/depression Patient satisfaction Dyspnoea Other Lung function (FEV1 and FEV1/FVC) |
Only primary studies were included. Studies concerning patients with moderate to severe COPD were included, and those with patients with mild or very severe COPD were included only if the majority of the study population was moderate/severe. A COPD study population of approximately 90% was required for inclusion unless data on the subset of patients with COPD were provided separately. Studies were included if the intervention was set within either a hospital or a community. Studies of any SM intervention/package or components of SM interventions were included. For example, medication management, action plans, exercise, inhaler technique and stress management (see Table 2). Comparators consisting of usual care (UC), control/sham or other SM interventions were accepted.
Risk of bias assessment
All RCTs were assessed using the recommended and validated Cochrane Risk of Bias tool. 56 The following six domains were assessed: sequence generation, allocation concealment, blinding of personnel and participants (by outcome), incomplete outcome data (by outcome), selective outcome reporting and other potential threats to validity. Domains were judged as high risk of bias, low risk of bias and unclear risk of bias. For trials with multiple papers, information from all of the studies was used to judge risk of bias. After a piloting process, all studies were assessed by two independent reviewers with a third reviewer overseeing the process. The GRADE57 framework was used to denote overall quality of evidence across studies for each of the primary outcomes and also HRQoL, using a scoring system of 4 (high) to 1 (very low) quality. The findings were summarised in a table, incorporating the results but also aspects that led to the final judgement.
Data extraction and manipulation
Approach
Data were extracted into piloted tables by the first reviewer with a second reviewer checking the extraction and a third reviewer overseeing the process. The results of all studies were tabulated and described and considered for combination in meta-analyses. Authors of included studies were contacted to clarify details and provide additional data required for analyses.
Types of data extracted
The following types of data were extracted from all papers:
-
Study characteristics Including sample size, mean age, severity according to mean FEV1% pred, place of recruitment, descriptions of intervention and control groups, outcomes, length of intervention and length of follow-up. When multiple papers were derived from the same trial, study characteristics were obtained from the original paper.
-
Study results Summary results from baseline and all follow-up times were extracted, including treatment effects, p-values, confidence intervals (CIs), mean scores at follow-up and/or mean changes in each group, numbers of events, hazard ratios (HRs), rates, loss to follow-up, etc. If multiple interventions were considered in a study then data were extracted for each pair of interventions compared.
Data manipulation
In order to maximise and prepare the data for statistical analyses, a number of steps were taken:
-
Lengths of intervention and follow-up were converted to weeks as a proportion of a 52-week year and rounded to the nearest week.
-
For continuous outcomes, for example QoL, reported mean difference (MD) estimates and 95% CIs calculated from an analysis of covariance (ANCOVA) were preferred, as this method adjusts for baseline imbalances. If not reported, the following methods were used in order of priority:
-
MDs reported from an analysis of change scores
-
MDs reported from an analysis of final scores
-
MDs calculated indirectly by ourselves from other information (e.g. mean change score for each group or the mean final score for each group).
-
If standard errors (SEs) were not reported directly, they were calculated from other information where available (such as p-values, 95% CIs, number in each group) at the end of follow-up, and the standard deviation (SD) of values in each group at the end of follow-up.
-
For effect estimates for numbers of events over time, for example number of admissions or exacerbations over follow-up, we preferentially used HRs (e.g. from a Cox regression analysis) because they compare the rate of events over the whole follow-up period and account for individuals lost to follow-up (censored). We used only first admissions, as it is not possible to combine different types of measures (e.g. with mean number of admissions per patient) without making very strong assumptions, and this was the most common measure. Where not reported, the following methods were used to estimate the HR and its 95% CI indirectly, used in this priority order:
-
Methods of Parmar et al. ,58 which allowed indirect estimation of the HR and its CI from the p-value, and the number of patients and outcomes in each group.
-
If numbers of events and sample size were available, the method of Perneger59 was used. Where there were zero cells then a continuity correction (1/sample size of the opposite group) was added to each cell to allow HRs to be calculable. 60
-
-
Where necessary, MD results and loge HRs presented on the same plots were multiplied by –1 to ensure that all estimates and intervals obtained related to the same direction of effect (e.g. that a MD in HRQoL of < 0 meant the same thing in each study).
-
To utilise more results on emergency department (ED) visits, reported mean numbers of visits during follow-up were converted to rate of ED visit by assuming that all patients not lost to follow-up were observed for the full duration of the trial.
Forest plots
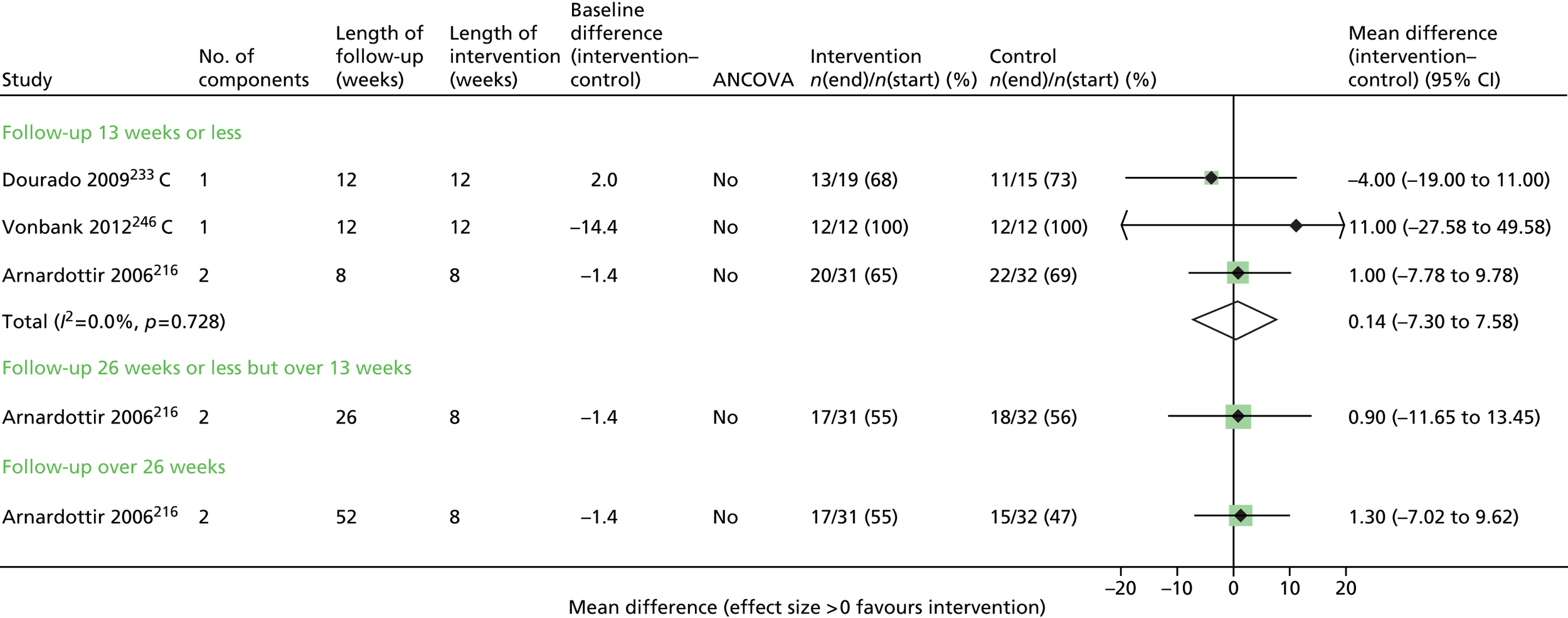
Results for each outcome were presented, where relevant, on a forest plot. Interventions were heterogeneous across the studies so results were placed in subgroups most consistent with the intensity and duration of support provided:
-
more-supported SM package – six or more contacts or unspecified contacts but ≥ 6 weeks’ duration
-
less-supported SM package – fewer than six contacts or unspecified contacts and < 6 weeks’ duration
-
exercise-based intervention.
Within each of these subgroups, studies were displayed in order of length of follow-up except for QoL outcomes, which were also grouped by questionnaire [St George’s Respiratory Questionnaire (SGRQ), Chronic Respiratory (Disease) Questionnaire (CRQ), EuroQoL-5 Dimensions (EQ-5D)]. As there were multiple follow-up points, it was decided that for each outcome, only data from the final follow-up period would be displayed in the forest plot and used in any subsequent meta-analysis. The subgroups were specified prior to inspection of the results to allow sensible exploration of the different types of interventions. Meta-regression was not possible owing to the limited number of studies.
Meta-analyses
General approach
For each outcome the core group met to discuss whether or not meta-analysis was appropriate. Meta-analysis was considered only when at least three studies were available.
All analyses were undertaken using Stata statistical software, version 12 (StataCorp LP, College Station, TX, USA). When it was not appropriate to pool data, studies were displayed graphically in a forest plot but without pooling.
Meta-analysis methods
A random-effects meta-analysis model was used to synthesise effect estimates across trials61 to account for between-trial heterogeneity in intervention effects across the trials. MDs were pooled on the original scale, but HRs were pooled on the loge scale.
Heterogeneity across studies was summarised using the I2-statistic (which gives the percentage of the total variability in the data due to between-trial heterogeneity)62 and the tau-squared statistic (the between-trial variance). 61
When two or more interventions from the same study contributed to the same meta-analysis with the same control group, an adjustment was required:
-
For continuous outcomes, the SE of each estimate was inflated by first obtaining the pooled SD (assuming equal variances) using the estimates of SE and sample size in each group. An inflated SE was then calculated using the full sample size in the intervention group, and the sample size in the control group divided by the number of comparisons it contributed to within the meta-analysis.
-
For one study the same control group appeared twice or more in the analysis when using a HR outcome. As the HRs for this study had been calculated using two-by-two tables,59 adjustment was made by modifying the number of control events and the total sample size in the control group by dividing by the number of comparisons in which that control group was incorporated. The modified two-by-two tables were then used to calculate new HRs to be used in the meta-analyses where appropriate. 59
Assessing publication bias
This was not possible as there were fewer than 10 studies for each of the outcomes.
Patient advisory group
A patient advisory group was established from local patients with COPD, chaired by Mr Michael Darby. Meetings were held at the University of Birmingham, and the group provided advice on how COPD affected their lives, their understanding of the importance of SM and different components, and their experiences of SM programmes. This assisted in the development of the definition of SM for the inclusion criteria of this review. For example, they suggested the need for including peer support groups as an essential component. They also commented on the plain English summary.
Results
Search results
Study identification and flow chart
Initial database searches identified 13,355 records, of which 836 remained after scanning titles and abstracts using the inclusion/exclusion criteria (Figure 1). After the same criteria were applied to the full papers, 12 papers reporting 10 trials were finally included in the review. 63–74 Appendix 2 details the reasons for exclusion at each stage. These were largely because patients were not recruited at the appropriate time point during/after discharge. Overall, 5% of all full texts required arbitration by a third reviewer.
FIGURE 1.
The selection process for clinical effectiveness studies.
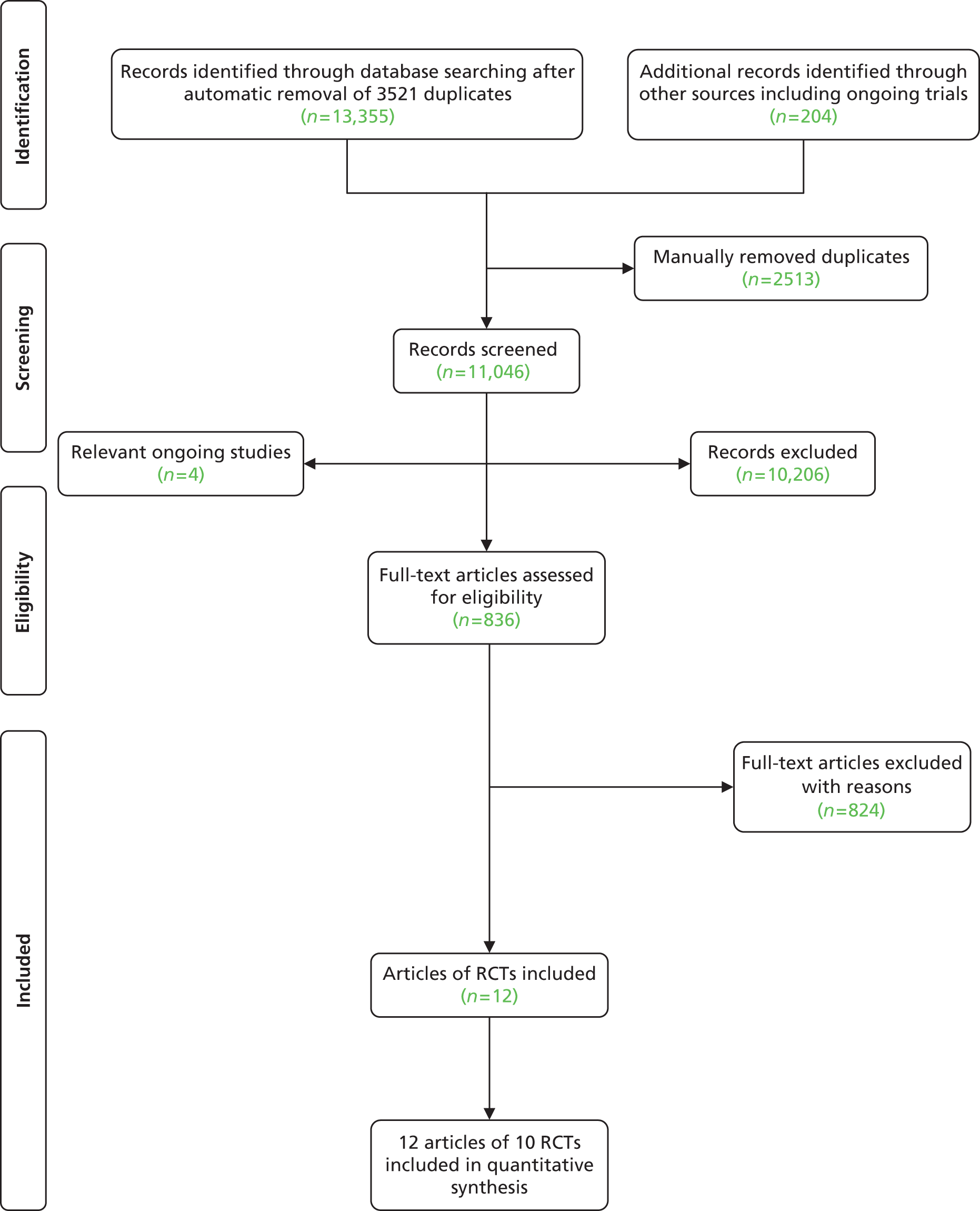
The inclusion of two trials was particularly difficult to assess. 68,75 Both were comparing ‘hospital at home’ with UC and had substantial SM components. One trial75 was excluded because all patients were seen in the ED then randomised to home compared with hospital (and therefore patients were not admitted at all unless in the control group). In the second study,68 although patients were assessed in the ED, a substantial proportion of patients in both arms were initially admitted and then discharged from hospital. The difference between the two arms was (a) the proportion of patients requiring admissions and (b) the intervention arm had ongoing SM support at home, whereas, once discharged, the control group had usual primary care support. Thus, this trial was included. 68
Conference abstracts meeting the inclusion criteria for this review are listed in Appendix 3. There were a further four trials that were ongoing at the time of the search end date (see Appendix 4).
Characteristics of included studies
There were 10 RCTs (from 12 papers). 63–74 One study69 had a limited qualitative element referring to patient satisfaction, which will be discussed in the following chapter (see Chapter 4), and one study68 included a cost analysis, which is presented in Chapter 5. Table 4 details the characteristics of the RCTs.
| Author, year, country, study design | Population inclusion criteria | Participants | Intervention (n) | Comparator (n) | Outcomes |
|---|---|---|---|---|---|
| Behnke, 2000,64 Germany, RCT | Inclusion: Severe COPD; patients admitted owing to acute exacerbation Exclusion: Unstable cardiac disease, cor pulmonale or other comorbidities preventing exercise participation, e.g. orthopaedic inabilities or peripheral vascular disease |
N = 46 Recruited in hospital 4–7 days post hospital admission Of 30 completers: Mean age (years) (SD): Int: 64.0 (1.9) Cont: 68.0 (2.2) Sex (male) n (%): Int: 12 (80.0) Cont: 11 (73.3) Mean FEV1% pred (SD): Int: 34.1 (7.4) Cont: 37.5 (6.6) |
TRAINING (n = 23) Usual medication and 30 minutes daily breathing exercises Ten-day hospital-based training including daily 6-minute treadmill and five self-controlled walking sessions Followed by 6 months individually tailored home-based walking programme, three times a day Diaries of exercise Two-weekly visits for 3 months then monthly telephone calls for 3 months |
CONTROL (n = 23) Usual medication and 30 minutes’ daily breathing exercises Ten-day hospital-based training, including daily 6-minute treadmill and five self-controlled walking sessions Advised to perform exercise at home without specific instruction |
Mortality (6 months) QoL – CRQ (3 and 6 months) Exercise capacity: 6-MWT treadmill (1, 2, 3 and 6 months) Dyspnoea: Baseline/Transitional Dyspnoea Index (every visit post discharge) Lung function: FEV1, FVC, TLC, ITGV, DLCO, RV (days 0 and 11, 3 and 6 months) Blood gas analysis, BP, heart rate (days 1 and 11, and 6 months) |
| Behnke, 2003,65 Germany, RCT | Inclusion: Severe COPD; patients admitted due to acute exacerbation Exclusion: Unstable cardiac disease, cor pulmonale or other comorbidities preventing exercise participation, e.g. orthopaedic inabilities or peripheral vascular disease |
N = 46 Follow-up of 26 of 30 patients who had participated in the Behnke et al.64 6-month trial Of 26 completers: Mean age (years) (SD): Int: 64.0 (7.5) Cont: 69.0 (6.9) Sex (male) n (%): Int: 11 (76) Cont: 9 (75) FEV1% pred (SD): Int: 34.9 (7.1) Cont: 37.5 (6.9) |
TRAINING (n = 23) Usual medication and 30 minutes’ daily breathing exercises Ten-day hospital-based training, including daily 6-minute treadmill and five self-controlled walking sessions Eighteen-month home-based training programme, three times a day for 15 minutes based on 125% of 6-MWT for 3 months and then advised to continue regular exercise Diaries of exercise Two-weekly visits for 3 months then monthly telephone calls for 3 months |
CONTROL (n = 23) Usual medication and 30 minutes’ daily breathing exercises No exercise training instructions in hospital or home No visits, but did receive monthly telephone calls |
QoL: CRQ (6, 12, 18 months) Exercise capacity: 6-MWT treadmill (6, 12, 18 months) Dyspnoea: Borg Scale at rest; Baseline/Transitional Dyspnoea Index (6, 12, 18 months) Lung function: FEV1, VC, TLC, ITGV, DLCO, RV (6, 12, 18 months) Hospital admissions (6-month periods for 18 months) Activity data (training group only) (each month) Inhaler and medications use |
| Lee, 2002,66 Hong Kong, cluster RCT | Inclusion: COPD; aged 65+ years; present residents of participating nursing home; at least one admission in previous 6 months Exclusion: Terminal illness (not expected to survive > 6 months) Communication problems |
N = 45 nursing homes N = 112 patients Patients recruited from the geriatric units of two hospitals with main diagnosis of COPD and soon to be discharged Of 89 completers: Mean (SD) age (years): Int: 81.08 ± 6.03 Cont: 79.68 ± 6.53 Sex (male) n (%): Int: 27 (56.3) Cont: 20 (48.8) Mean FEV1% pred (SD): Int: 30.64 (10.12) Cont: 31.08 (13.25) Severity n (%):
|
CARE SUPPORT TO NURSING HOME (n = 48 completers) Support to nursing home staff provided by community nurses Visit 1: Within 1 week of discharge:
Monthly visits by same nurse to provide ongoing support and education to the staff Between visits and as necessary community nurse would additionally provide advice via:
This may include advice on need for ED visit or admission If readmitted, protocol and visits recommenced on discharge back to the home |
CONTROL (n = 41 completers) Usual community nursing, e.g. wound/catheter management |
Hospitalisation (6 months)
Respiratory status (6 months): FEV1% pred Psychological status (6 months): GHQ: total and subscales Patient satisfaction (6 months): Thirteen-item Likert scale; not administered to control arm Nursing health staff satisfaction (1 month): Eleven-item Likert scale; not administered to control arm |
| Egan 2002,69 Australia, RCT plus qualitative element (n = 18) | Inclusion: COPD; ≥ 18 years; history of chronic bronchitis (with infection), emphysema, chronic obstruction, chronic asthma, or combination; admission to respiratory unit bed within 72 hours of hospital admission Exclusion: Cognitive function insufficient to complete questionnaire |
N = 66 Patients admitted with COPD to a major private hospital; recruited during admission Mean age (years): Int: 67.8 Cont: 67.2 Sex (male) n (%): Int: 12 (36) Cont: 20 (60) FEV1% pred: NR Severe (FEV1 < 35% pred) Int: 19 (57.6%) Cont: 19 (57.6%) Mild/moderate (FEV1 35–50% pred) Int: 14 (42.4) Cont: 14 (42.4) |
CASE MANAGEMENT (n = 33) Nursing assessment and review: comprehensive – to identify physical, psychological, social, spiritual, resource needs; standardised clinical pathway of care during hospital admission Coordination between medical, nursing and allied health personnel by case manager Coordinated case management with patient and carer education on managing the disease, medication, rehabilitation, available community services and arranged discharge planning Regular telephone calls to patient and carer at 1 week and 6 weeks |
UC (n = 33) Nursing assessment (not clear); standardised clinical pathway of care during hospital admission No contact with case manager, no case conferences and no post-discharge follow-up |
Hospital readmission: 3 months QoL: SGRQ and Subjective Well-Being Scale: 1 and 3 months Social support survey: 1 and 3 months Anxiety and depression: HADS (1 and 3 months) Patient satisfaction: qualitative semistructured interview with 18 participants, 3 months |
| Hermiz, 2002,67 Australia, RCT | Inclusion: COPD; 30–80 years; patients attending hospital ED or admitted with COPD Exclusion: Resided outside region; insufficient English skills; resident in nursing home; confused or demented |
N = 177 Patients attending hospital ED or admitted with COPD; not clear exactly when recruited but visit 1 occurred 1 week after discharge Mean age (years): Int: 67.1 Cont: 66.7 Sex (male), n (%): Int: 41 (48.8) Cont: 43 (46.2) FEV1% pred: NR |
HOME VISITS (n = 84) Two home visits (community nurse) Visit 1: Within 1 week of discharge
Progress review Patient encouraged to refer to education booklet for guidance |
UC (n = 93) UC (GP) |
Mortality (3 months) Readmissions or ED visits (3 months) GP consultations or nurse home visits (3 months) GP prescribed drugs GP arranged follow-up GP provided patient with education GP provided carer with education QoL: SGRQ (3 months) Behaviour change (3 months)
|
| Dheda, 2004,73 UK, RCT | Inclusion: Diagnosis of COPD; first admission of COPD Exclusion: Another dominant medical condition; mandatory reason for hospital follow-up, e.g. suspected cancer; already under outpatient follow-up; refused consent |
N = 33 First admission of COPD Not clear when recruited but implies at discharge (data may be completers only – not clear): Mean age (years) (SD): Int: 68.4 (5.8) Cont: 71.3 (8.4) Sex (male) n (%): NR Mean FEV1% pred (SD): Int: 44.7 (21.8) Cont: 39 (11.9) Disease severity (BTS guidelines) Int: 20% mild, 20% moderate, 60% severe Cont: 20% mild, 27% moderate, 53% severe |
HOSPITAL OUTPATIENT FOLLOW-UP (n = 15) Visit to respiratory nurse and/or chest physician: (n = 4+) over 6-month period (3, 6, 8, 12 or 16 weeks)
|
PRIMARY CARE FOLLOW-UP (n = 18) Visit primary care teams as required |
Hospital admissions (6 months) Exacerbations (two or more) (6 months) QoL: SGRQ, SF-36 (6 months) Lung function: FEV1 Oxygen saturation Pharmacological prescriptions: oxygen, nebuliser, theophylline, bronchodilators |
| Hernandez, 2003,68 Spain, RCT | Inclusion: COPD exacerbation; absence of any criteria for imperative hospitalisation as stated by the BTS guidelines Exclusion: Not living in the area or admitted from a nursing home, lung cancer and other advanced neoplasm, extremely poor social conditions, severe neurological or cardiac comorbidities, illiteracy, no telephone |
N = 222 Patients with COPD exacerbation. Recruited at emergency room of two tertiary hospitals Mean age (years) (SD): Int: 71 (9.9) Cont: 70.5 (9.4) Sex (male)%: Int: 96.7 Cont: 97 Mean (SD) FEV1 l (% pred): NR at baseline |
HOME-BASED HOSPITALISATION (n = 121) Assessed by specialised team in emergency room At discharge Standard pharmacological treatment was used in accordance with national guidelines Non-pharmacological treatment, 2 hour, including:
Duration of home hospitalisation determined by nurse; up to five visits permitted during 8-week period, but no limit of telephone contact; action plan revisited and education reinforced Failure was based on referral to emergency room or more than five nurse visits required |
UC (n = 101) Standard assessment by physician in emergency room Standard pharmacological treatment No post-discharge follow-up |
Mortality (2 months) Readmissions or ED visits (2 months) Hospitalisation: hospital days (2 months) QoL: SGRQ and SF-12 scale (2 months) Lung function: FEV1, FVC (2 months) Patient satisfaction (2 months) Disease knowledge (2 months) Inhaler technique (2 months) Medication prescriptions and home rehabilitation (2 months) Costs (2 months) |
| Kwok, 2004,70 Hong Kong, RCT | Inclusion: CLD (89% had COPD); 60+ years; having at least one hospital admission for CLD in the 6 months before index admission Exclusion: Resided outside region; communication difficulties; no family caregiver; resident in institutional care; terminal disease with life expectancy < 6 months |
N = 157 Hospitalised patients with principal diagnosis of CLD recruited from medical wards of two hospitals within 3 days of admission Of 149 completers: Mean age (years) (SD): Int: 75.3 ± 7 Cont: 74.2 ± 5.7 Sex (male) n (%): Int: 56 (73) Cont: 55 (69) Mean FEV1: NR |
INTERVENTION (n = 77) A community nurse Visit 1: Before discharge:
|
UC (n = 80) Routine follow-up by same medical teams Some patients received home visit if referred |
Hospital readmissions (4 weeks, 6 months) Period of hospitalisation (bed-days) ED visits (6 months) Psychosocial scores: London Handicap Scale, GHQ score, Multidimensional Health Locus of Control Scales (6 months) Exercise capacity: 6-MWT (6 months) Mortality (6 months) Care burden (6 months): Cost of Care Index |
| Wong, 2005,74 Hong Kong, RCT | Inclusion: Diagnosis of COPD; alert and orientated; contactable by telephone Exclusion: Discharged to an old-age home; serious alcohol or drug abuse or psychiatric disease; diagnosed with IHD, musculoskeletal disorders or other disabling diseases that may limit rehabilitation; dying and/or unable to provide informed consent |
N = 60 At discharge from medical department of acute care hospital Mean age (years) (SD): 73.6 (7.8) Sex (male) n (%): 47 (78.3) FEV1% pred: NR |
TELEPHONE FOLLOW-UP (n = 30) Structured, individualised educational and supportive telephone follow-up programme delivered by a respiratory nurse Based on Bandura’s theory of self-efficacy31
|
ROUTINE CARE (n = 30) UC |
Health service utilisation: ED, outpatient, admissions (1, 3 months) Self-efficacy: Modified Chinese COPD Self-Efficacy Scale for dyspnoea (day 35) |
| Casas, 2006,71 Spain, RCT | Inclusion: COPD; hospital admission > 48 hours due to exacerbation Exclusion: Not living in health-care area; severe comorbid conditions; logistical limitations due to poor social conditions, e.g. no telephone access; admitted to nursing home |
N = 155 (n = 113, Barcelona; n = 42, Leuven) Recruited immediately after hospital discharge from two tertiary hospitals (Barcelona, Leuven) Mean age (years) (SD): Int: 70 (9) Cont: 72 (9) Sex (male) n (%): Int: 50 (77) Cont: 79 (78) Mean FEV1% pred (SD): Int: 43 (20) Cont: 41 (15) |
INTEGRATED CARE (n = 65) Four-part integrated care:
|
UC (n = 90) UC: Hospital physician decided on outpatient control regime. Standard protocol for pharmacological prescription and in-hospital treatment Physician visit every 6 months |
Mortality (6, 12 months) Hospital admissions (12 months) Health-care resource utilisation (12 months): includes GP consultations |
| Garcia-Aymerich, 2007,72 Spain (subset of Casas et al.71), RCT | Inclusion: COPD; admitted because of an episode of exacerbation requiring hospitalisation for > 48 hours Exclusion: Not living in the health-care area or living in a nursing home; lung cancer or other advanced malignancies; logistic limitations due to poor social conditions, illiteracy or no telephone access; extremely severe neurological or cardiovascular comorbidities |
N = 113 Recruited immediately after discharge from one tertiary hospital Of 62 completers: Mean age (years) (SD): Int: 72 (10) Cont: 73 (9) Sex (male) n (%): Int: 16 (80.0) Cont: 37 (90) FEV1% pred: NR Described as ‘severe’ |
INTEGRATED CARE (n = 44) Four-part integrated care:
|
UC (n = 69) UC |
Mortality (6 and 12 months) QoL: SGRQ, EQ-5D (6 and 12 months) Dyspnoea: MRC (6 and 12 months) Treatment adherence and inhaler technique: Medication Adherence Scale, Inhaler Adherence Scale and observation; medication use (6 and 12 months) Medications and oxygen therapy (6 and 12 months) Lung function – FEV1, FVC, PaO2, PaCO2 (6 and 12 months) Vaccination uptake (influenza, pneumococcal): 6 and 12 months Patient satisfaction (6 and 12 months) Smoking (6 and 12 months) Exercise (6 and 12 months) Knowledge: about disease and identification/treatment of exacerbations BMI |
| Bucknall, 2012,63 UK, RCT | Inclusion: Patients with COPD admitted to hospital with acute exacerbation; FEV1 < 70% pred and FEV1/FVC < 0.7 Exclusion: History of asthma or left ventricular failure; active malignant disease; evidence of confusion or poor memory |
N = 464 During or shortly after hospital admission; six acute hospitals and contributing hospitals with eligible patients; augmented by review of patients attending PR and checking for evidence of hospital admission Mean age (years) (SD): 69.1 (9.3) Sex (male) n (%): 170 (37%) Mean FEV1% pred (SD): 40.5 (13.6) |
SUPPORTED SM (n = 232) Long-term treatment optimised, inhaler techniques checked, offered appropriate smoking cessation advice and PR Symptom daily diaries Supported SM by nurses trained in ‘self-regulation theory’; this aims to empower patients to manage COPD by improved knowledge and understanding of the disease and skills to monitor symptoms and carry out appropriate actions, such as altering treatment early in early stages of an exacerbation SM material based on ‘Living Well with COPD programme’ Content included:
|
UC (n = 232) Long-term treatment optimised, inhaler techniques checked, offered appropriate smoking cessation advice and PR Symptom daily diaries UC: continuing management by GP, hospital clinicians or both |
Mortality (12 months) Hospital admission with exacerbation of COPD (12 months) Successful SM (initiating treatment during exacerbation) (12 months) QoL: SGRQ, EQ-5D (6 and 12 months) Anxiety/depression – HADS (6 and 12 months) Self-efficacy – COPD Self Efficacy Scale (6 and 12 months) |
Characteristics of included randomised controlled trials
Size, setting, recruitment
Randomised controlled trials ranged in size from 3373 to 46463 total participants. One66 was a cluster RCT, based in 45 nursing homes. One paper was the 18-month follow-up of the original study,64,65 and one paper72 referred to the Spanish centre of a European study. 71
Participants were largely recruited in hospital during an exacerbation of COPD or at (or immediately after) discharge. Two papers67,68 also included patients recruited at the ED who may not have been admitted to hospital.
The definition of COPD for inclusion was generally based on a clinical diagnosis (except for Bucknall et al. ,63 which also required patients to meet the spirometric criteria for airflow obstruction). One study70 included a mixed population of patients with chronic lung disease, although 89% had COPD.
Patient exclusion from trials was usually based on inability to provide consent; terminal illness or extreme comorbidities preventing inclusion in rehabilitation/exercise; or social conditions/lack of access to a telephone. All of the studies were set among patients living at home except for the cluster RCT, which was specifically based in nursing homes. 66
Description of included patients
Mean age of participants was similar across the included RCTs (66–74 years), except in the cluster RCT based in nursing homes,66 where the mean age was approximately 80 years. Sex distribution, however, was variable across studies (ranging from 37% to 97% males). Where reported, severity of disease was similar with mean FEV1 ranging from approximately 31% to 42% of predicted values. Most patients were described as having moderate or severe COPD.
Description of self-management interventions and comparators
Interventions were varied and have been described in full in Table 4. Figure 2 provides a summary diagram of the included RCTs, with interventions grouped into three categories:
FIGURE 2.
Study characteristics of the included RCTs.
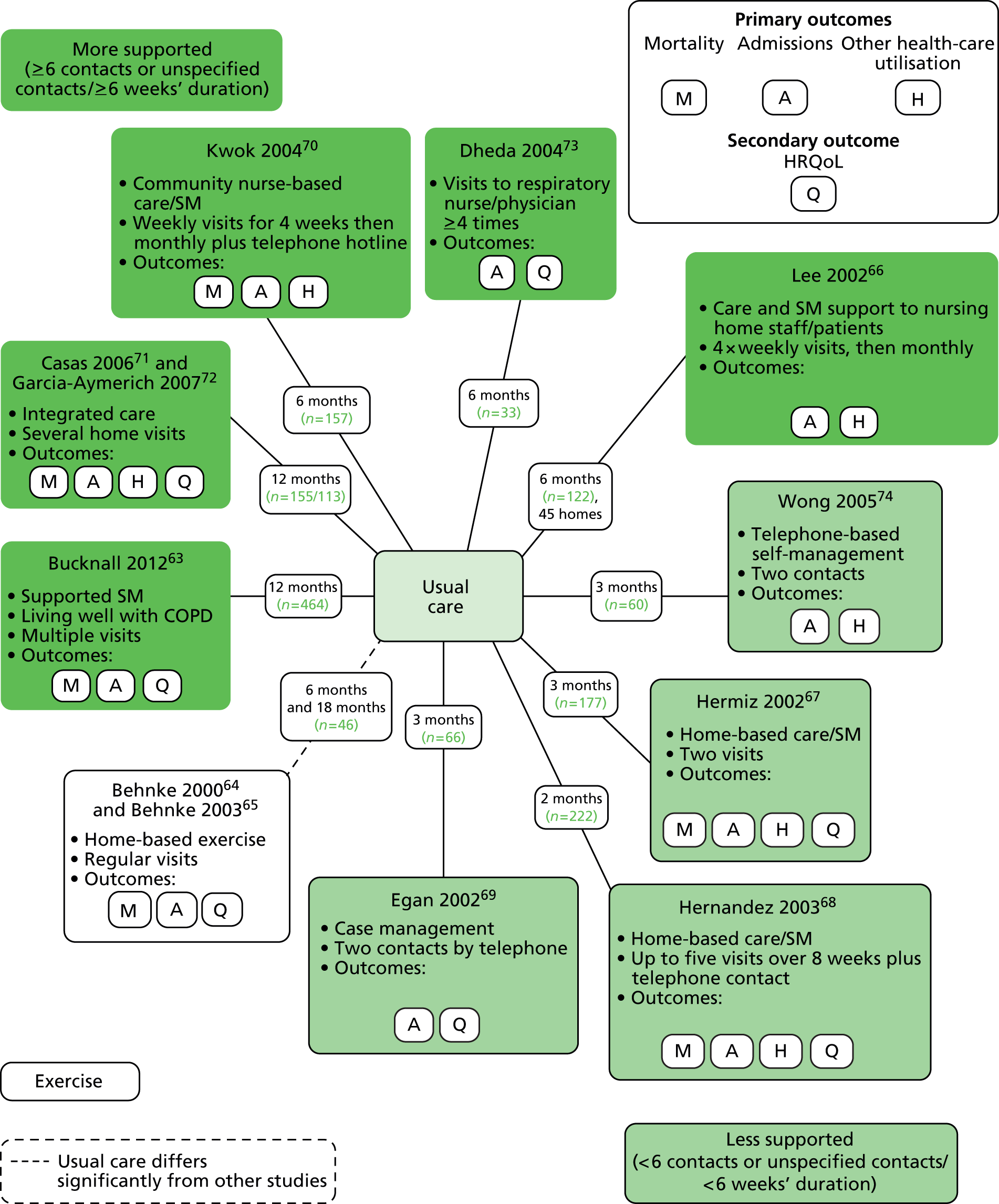
-
‘More supported’ Six or more contacts or ≥ 6 weeks’ duration if contacts not specified. This category included:
-
– large RCT in the UK of supported SM (based on the Living Well with COPD materials) for 12 months, compared with UC63
-
– RCT in Spain/The Netherlands of integrated care including supported SM for 12 months,71,72 compared with UC
-
– RCT in Hong Kong70 of a community nurse-supported discharge programme, including SM support, with weekly visits for 4 weeks and then monthly, with additional telephone hotline and a total follow-up of 6 months, compared with UC
-
– small RCT in the UK73 of hospital outpatient visit-based SM support over 16 weeks with total 6 months’ follow-up, compared with UC
-
– cluster RCT in Hong Kong66 of support by community nurses to nursing home staff and patients with a supported SM component, weekly visits for 1 month and thereafter monthly visits for a total of 6 months, compared with UC.
-
-
‘Less supported’ Fewer than six contacts or < 6 weeks’ duration if contacts not specified. Including:
-
– RCT in China of telephone-based SM (based on Bandura’s theories of self-efficacy31), with two telephone calls before week three and total follow-up for 3 months, compared with UC74
-
– RCT in Australia of SM support provided by two visits after 1 week and 1 month, with total 3 months’ follow-up, compared with UC67
-
– RCT in Spain of home-based hospitalisation, including SM education and action plans, reinforced during up to five home-visits and telephone contacts over an 8-week period, compared with UC68
-
– RCT in Australia of case management with SM support with review and two telephone calls and follow-up for 3 months in total, compared with UC. 69
-
-
‘Exercise-only intervention’ Home-based exercise-only interventions:
Description of comparators
Comparators were ‘UC’ [often with little description but focused on usual GP management] except for the exercise trials,64,65 for which the control group had some initial exercise training in hospital and were then advised to perform exercise at home.
Range of outcomes reported
All included trials measured at least one of the primary outcomes. Mortality was reported in six trials;63,64,67,68,70–72 hospital admissions (measured in multiple ways) in all 10 trials;63–74 and other health-care utilisation in six trials. 66–68,70–72,74
Of the secondary outcomes, HRQoL was assessed in seven trials63–68,71–73 and was provided as an overall score as well as subdomains. The most common score was the SGRQ.
Exacerbations were reported in only one trial. 73 Self-efficacy was measured in two trials;63,74 behaviour change in four trials;63,67,68,72 anxiety/depression in five trials;63,66,69,70 exercise capacity in two trials;64,65,70 dyspnoea in two trials;64,65,72 and lung function in five trials. 64–66,68,72,73
Patient satisfaction with the intervention was described in five trials,66–68,72 one of which is described in full in the next chapter, as it involved qualitative interviews. 69 Costs were described in one trial,68 but no trials described days lost from work.
Quality of included randomised controlled trials
Risk of bias evaluations are presented in Table 5 and are described as high, low or unclear risk for each aspect of potential bias.
| Sources of bias | aBehnke 200064 | aBehnke 200365 | Egan 200269 | Hermiz 200267 | Lee 200266 | Hernandez 200368 | Dheda 200473 | Kwok 200470 | Wong 200574 | bCasas 200671 | bGarcia-Aymerich 200772 | Bucknall 201263 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sequence generation | Unclear: Randomly allocated but method not described |
Unclear: Randomly allocated but method not described |
Low: Stratified and then random number tables |
Unclear: ‘Simple randomisation’ at one site and permutated block at another |
Unclear: Intervention and control nursing homes matched by readmission rates and stratified into high, medium, low risk. Randomised in pairs but details not given |
Low: Computer-generated random numbers in 1 : 1 or 2 : 1 ratio |
Unclear: Randomly allocated but method not described |
Low: ‘Random number table’ |
Low: ‘Research randomiser’ |
Low: Computer-generated random numbers |
Low: Computer-generated random numbers |
Low: Computer-generated sequence using permuted blocks and minimisation |
| 2. Allocation concealment | Unclear: Insufficient information |
Unclear: Insufficient information |
Unclear: Insufficient information |
Unclear: Insufficient information |
Unclear: Insufficient information |
Unclear Although described as ‘blindly assigned to groups’ |
Unclear: Insufficient information |
Unclear: Insufficient information |
Unclear: Insufficient information |
Unclear: Although described as ‘blindly assigned to groups’ |
Unclear: Although described as ‘blindly assigned to groups’ |
Low: Treatment group allocation were obtained by telephone after baseline assessment had been made |
| 3. Blinding of outcomes | ||||||||||||
| a. Hospital admissions | n/a | Low | Low | Low | Low | Low | Low | Low | Low | Low | n/a | Low |
| b. ED visits | n/a | n/a | n/a | n/a | Low | Low | n/a | Low | Low | n/a | n/a | n/a |
| c. Primary care consultations | n/a | n/a | n/a | Low Available from GP |
n/a | n/a | n/a | n/a | n/a | Low | n/a | n/a |
| d. Mortality | Low | n/a | n/a | Low | n/a | Low | n/a | Low | n/a | Low | Low | Low |
| e. Patient-reported outcomes | HRQoL: high Dyspnoea: high Patient not blinded |
HRQoL: high Patient not blinded |
HRQoL: high Anxiety and depression: high Patient not blinded |
HRQoL: high Behaviour change: high Patient satisfaction: high Patient not blinded |
Psychological status: high Patient satisfaction: high Patients not blinded |
HRQoL: high Patient satisfaction: high Investigator administrating questionnaire blinded but patient not blinded |
HRQoL: high Patients not blinded |
GHQ score: high Patients not blinded |
Self-efficacy: high Patients not blinded although assessors blinded |
n/a | High HRQoL; dyspnoea; treatment adherence/inhaler technique; vaccine uptake; patient satisfaction; smoking; exercise; knowledge |
High HRQoL; anxiety and depression; self-efficacy Patients not blinded |
| f. Other outcomes of interest | Lung function: unclear No information provided but interviews conducted by the physicians managing the care so unlikely to be blind Exercise capacity: low risk because assessor gave no encouragement |
Lung function: unclear No information provided, but interviews conducted by the physicians managing the care so unlikely to be blind Exercise capacity: low risk because assessor gave no encouragement |
n/a | n/a | Lung function: unclear Not known whether or not assessors were blinded |
Lung function: unclear Not known whether or not assessors were blinded |
Lung function: unclear Not known whether or not assessors were blinded Exacerbations: unclear No information |
Exercise capacity: low Assessors were blinded |
n/a | Unclear Lung function No information provided on blinding |
n/a | |
| 4. Incomplete outcome data | Outcomes only provided on completers 14/23 (61%) in intervention arm 12/23 (52%) in control arm |
Ignoring deaths, 11% loss to follow-up, although reasons not provided for withdrawals | Outcomes only provided on completers (79.5% overall) Insufficient reporting of attrition/exclusions |
Implies that the only attrition during 8-week period was due to death | Most outcomes only provided on completers (89%) | Low: all participants accounted for, 3.3% dropout; missing values replaced by group mean | ||||||
| a. Hospital admissions | n/a | High | Unclear | Low 89% followed up |
High | Unclear | Unclear: Unclear follow-up rate |
Low: 89% followed up |
Low: 97% followed up |
Low | n/a | Low |
| b. ED visits | n/a | n/a | n/a | n/a | High | Unclear | Low 89% followed up |
Low 97% followed up |
n/a | n/a | n/a | |
| c.Primary care consultations | n/a | n/a | n/a | Low: 89% followed up |
n/a | n/a | n/a | n/a | Low: 97% followed up |
Low: Withdrawals reported; other than deaths < 10% lost |
n/a | n/a |
| d. Mortality | Low | n/a | n/a | Low: 100% followed up |
Low | n/a | Low | n/a | Low | Low | Low | |
| e. Other | High: Withdrawals reported; outcomes provided only on completers (65% in each arm) |
High |
High: Other than deaths, 12% loss to-follow-up, although reasons/characteristics not provided for withdrawals Data not provided for all participants |
Low: 89% followed up |
HRQoL: unclear | HRQoL: high Lung function: high 66.7% followed up in intervention arm and 83.3% followed up in control arm Withdrawals reported but no information on characteristics reported and, not accounted for in analysis |
Exercise capacity: high 77% took part due to loss to follow-up and mobility problems |
Low: 97% followed up |
n/a | High: High loss to follow-up (other than deaths, 14.2% lost) Reasons for withdrawals reported but analyses undertaken only on completers Lost to follow-up appeared more severely affected than completers |
High: HRQoL; self-efficacy; anxiety and depression high loss to follow-up; only 61% completed questionnaires at 6 months Non-completers had greater morbidity and worse baseline self-efficacy, and more likely to be in the control arm |
|
| 5.Selective outcome reporting | Unclear: Protocol not identified |
Unclear Protocol not identified Mention of collection of GP consultations and exacerbations, but not reported |
Unclear: Protocol not identified |
Unclear: Protocol not identified |
Unclear: Protocol not identified |
Unclear: Protocol not identified |
Unclear: Protocol not identified |
Unclear: Protocol not identified |
Unclear: Protocol not identified |
High: Data not available for HRQoL |
Unclear: Protocol not identified |
Unclear: Protocol not identified |
| Other comments | Methodology of lung function measurement not given Table of characteristics provided only on completers |
Baseline differences for age, CRQ, lung function and 6-MWT Table of characteristics only provided on completers |
Clear imbalance of gender at baseline, and possibly other characteristics Outcome data very difficult to interpret as change provided between interim time points only |
Study design problematic Although a cluster design analysis does not take this into account Unknown validity of satisfaction questionnaire Methodology of FEV1 measurement not given |
Baseline differences with respect to smokers, oxygen therapy, although comparable to disease severity (FEV1% pred) Short follow-up period Outcome assessment not clear; percentage not always correct Lung function analyses not adjusted for baseline |
Very small study Methods of outcome assessment not described Numerical data not available for lung function Rather limited information provided throughout One patient excluded from analysis owing to visiting GP (not ITT) No table of characteristics Confusion over SEM or SD |
Three subjects in control were undergoing PR | Change in sample size calculation External validity of Chinese Self-Efficacy Scale Gender may not be very well balanced across arms |
Differences in text and Table 2 for differences in rate of admissions Not well balanced on previous hospitalisations, and receipt of influenza vaccination |
No description of lung function test methods Intervention arm seemed to have higher number of admissions in the previous year and possibly worse SGRQ score |
In general, the quality of reporting and conduct of the included studies was low, with some very small, poorly conducted studies. 64,65,73 Out of the 10 RCTs,63–74 appropriate methods of randomisation (e.g. computer random number generator) were used in six trials63,68–72,74 suggesting a low risk of bias, although methods were unclear in the remaining four. 64–67,73 Allocation concealment was insufficiently described in all except the largest most recent trial,63 which used a central telephone method of allocation to reduce the risk of bias.
Blinding of patients and health-care personnel would not have been appropriate for this type of SM and similar such interventions; therefore, the results of any patient-reported outcomes or non-blinded, investigator-assessed outcomes would be potentially subject to bias. In this review, the important patient-reported outcomes were consistently judged to be subject to high risk of bias across all of the trials, including HRQoL, dyspnoea, anxiety and depression, self-efficacy, patient satisfaction and behaviour change.
Measurements of lung function and exercise capacity both rely on assessors’ encouragement and could be subject to bias if not blinded. It was usually unclear whether or not investigators were blind to treatment when assessing lung function, although when measured, exercise capacity was judged to have low risk of bias because either the assessors were blind70 or it was explicitly stated that they did not provide encouragement. 64,65 In general, conduct of outcome measurement were frequently poorly described, with standards and conduct of lung function testing particularly unclear. 64,65,68,72
However, assessment of hospital admissions, other health-care utilisation and mortality would be likely to have a low risk of bias (either self-reported or obtained from records), as concluded for most of the included trials.
The most obvious flaw in the conduct of some of the included studies was the lack of completeness in follow-up, which was considerably < 70% in some trial arms and would be likely to bias most clinical measures and HRQoL (and other questionnaire) outcomes in particular,63–65,73 and any other questionnaire/clinical measures. This was even discussed by the authors of the largest, most recent trial with only 61% of randomised patients with HRQoL reported at follow-up,63 who concluded that the data were therefore unreliable. Several of the studies64,65 reported characteristics of only the completing participants rather than all randomised participants, or gave no table of characteristics at all. 73 This weakens any attempt to assess baseline imbalance and any bias introduced at this stage.
As is usual, it was difficult to assess selective outcome reporting without availability of protocols. In addition, outcome data were unclearly analysed in several studies,64–66,69,73 and the older studies (pre 2005) were often limited in their description of methods in general. There were signs of baseline imbalance between arms in some studies, which was not addressed in the analyses. 65 The best conducted and reported trials tended to be the most recent. 63,70–72,74
Primary outcomes
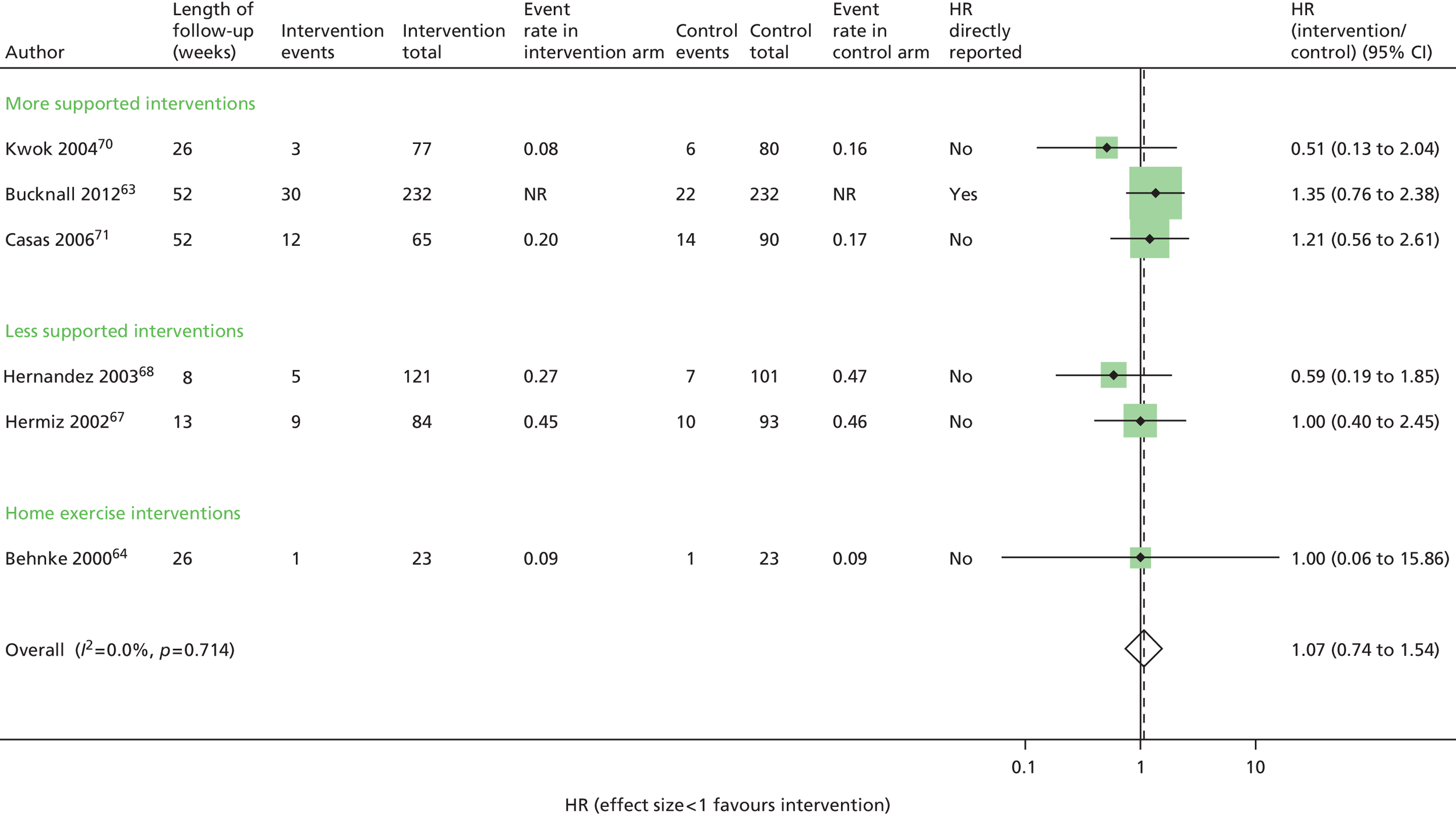
All-cause mortality: no evidence of effect
Six trials63,64,67,68,70–72 contributed mortality results (Figure 3 and Appendix 5). There was a wide range of event rates across the trials. Despite the general heterogeneity of interventions, there was no statistical heterogeneity and the overall results indicated that there was no evidence of effect on mortality (HR 1.07, 95% CI 0.74 to 1.54). Using the GRADE system, we would judge that this is moderate-quality evidence (Table 6).
FIGURE 3.
All-cause mortality. NR, not reported.
Hospital admissions: no evidence of effect
Seven trials63,65,67,68,70,71,73 contributed data to the admissions results (Figure 4 and Appendix 6). The results of three trials66,69,74 could not be included in the combined results because they reported mean number of admissions rather than first admission and one69 also did not provide sufficient information to calculate a SE.
FIGURE 4.
First hospital admission. NR, not reported.
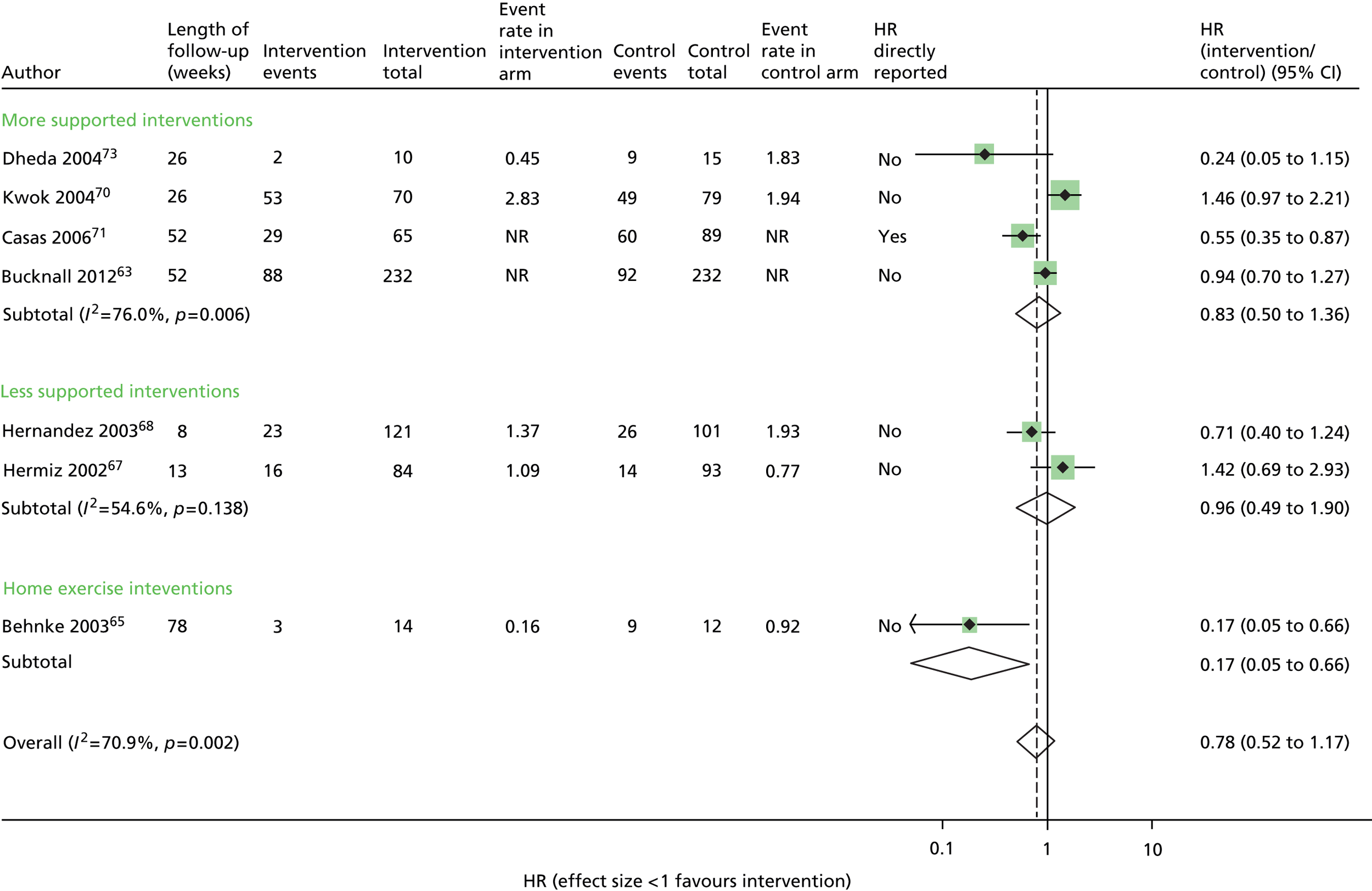
Overall, statistical heterogeneity was high (I2 = 70.9%), and subdividing by level of support explained only a fraction of this. Estimates are provided by level of SM support, although there was no evidence of any effect for the non-exercise-based interventions and substantial remaining heterogeneity.
One of the studies that may have contributed to the remaining heterogeneity in the non-exercise-based studies is the small study of 33 participants by Dheda et al. ,73 which was poorly reported, had signs of inadequate randomisation and very high loss to follow-up, especially in the intervention arm. This study73 had the most extreme results in its category.
The trial of home-based exercise65 demonstrated a large relative reduction on the rate of first admission (HR 0.17, 95% CI 0.05 to 0.66), although this trial was very small and these results were based only on participants who completed the study (< 60% of those randomised), and thus would also be subject to high risk of bias. Furthermore, although subgrouping by intervention category was an a priori identified analysis, care must be taken in the interpretation of results due to multiple comparisons being performed. The evidence above was judged to be of low quality according to GRADE (see Table 6).
General practitioner consultations: no evidence of effect
Two trials reported GP-related health-care activity (see Appendix 7). 67,71 Neither trial reported any evidence of differences between physician contacts, drug prescriptions or amount of education provided between the intervention arm and the control arm. Note that one trial67 reported mean number of visits, although it is likely that median values would be more appropriate. The evidence was of very low quality (see Table 6).
Emergency department visits: no evidence of effect
The effect on ED visits is displayed in Figure 5 (see Appendix 8), for which five trials66–68,70,72 contributed data. Four trials66,68,70,74 reported mean visits per patient and two67,68 reported first visit. The two trials with a longer follow-up of 6 months66,70 failed to demonstrate any evidence of an effect on ED visits. This evidence was also of low quality (see Table 6).
FIGURE 5.
Emergency department visits. NR, not reported.
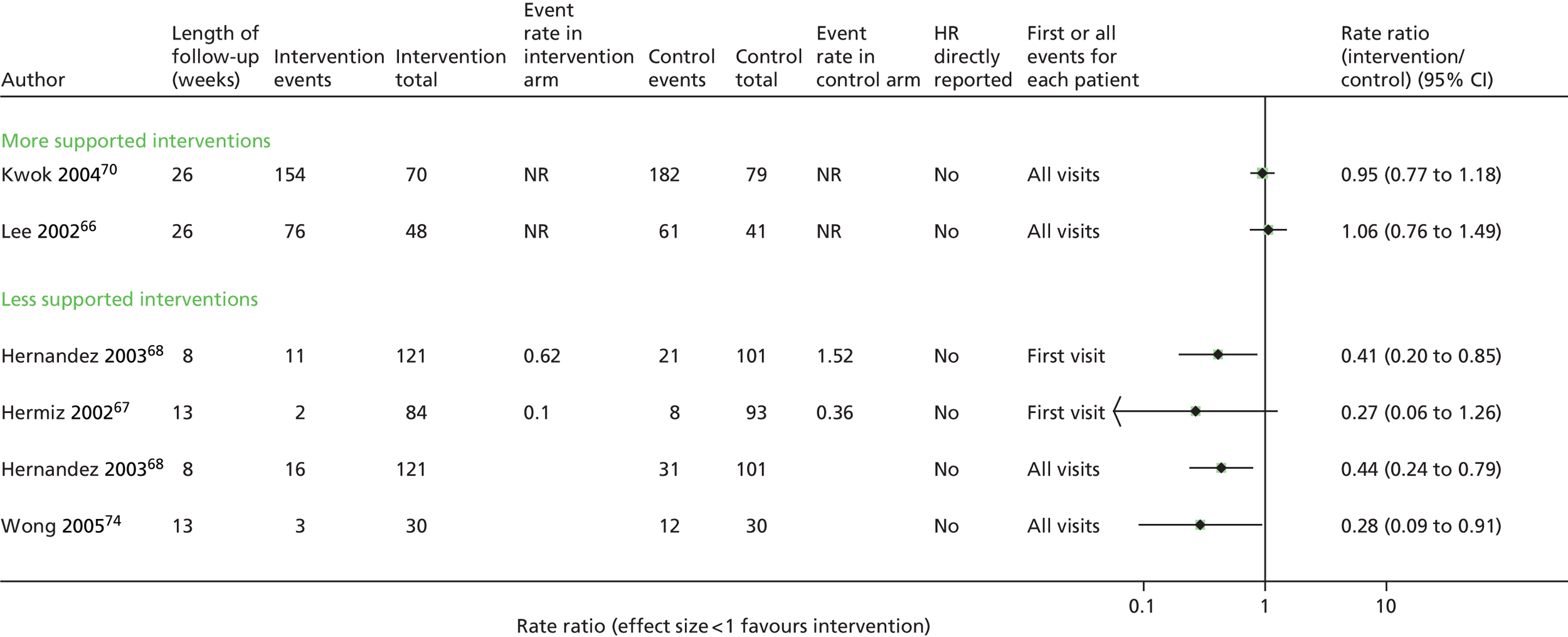
| Outcome | Control risk | Intervention risk | Results | No. of participants (trials) | Quality rating | Comments |
|---|---|---|---|---|---|---|
| Mortality | 56/598 | 59/581 | 1.15 (95% CI 0.79 to 1.67) | 1179 (6) | +++; moderate | Outcome measurement likely to be unbiased I2 = 0% Generally, low risk of bias except allocation concealment rarely mentioned |
| Hospital admissions | 259/621 | 211/596 | 0.78 (95% CI 0.52 to 1.17) | 1217 (7) | ++; low | Outcome measurement problematic in some trials due to loss to follow-up I2 = 70.9% Wide CIs crossing line of no effect Some study results based on completers only |
| ED visits | n/a | n/a | Not combined RR ranged from 0.27 to 1.06 |
932 (5) | ++; low | Unclear methods of randomisation and allocation in two trials Follow-up was generally short and results inconsistent |
| GP consultations | n/a | n/a | Not combined No significant effects |
332 (2) | +; very low | Very little information |
| HRQoL | n/a | n/a | SGRQ 3.84-point improvement (95% CI 1.29 to 6.40 points) | 845 (6) | +; very low | Biased follow-up – enormous loss to follow-up Outcome measurement likely to be biased I2 = low |
Secondary outcomes
Health-related quality of life: consistent with improvement although potential bias
Six trials63–66,67,68,72,73 contributed data on HRQoL using the SGRQ, the CRQ or the EQ-5D (Figure 6 and Appendix 9). The data from one study69 could not be used as they reported median change only. Five trials63,67,68,72,73 used the SGRQ, two trials63,72 reported the EQ-5D and one trial64 the CRQ.
Overall, SM interventions resulted in an improvement of 3.84 (95% CI 1.29 to 6.40) points on the SGRQ scale compared with control (close to the minimal clinically important difference of four points), although follow-up (where reported) ranged from about 25% to 83% across studies, and, therefore, this result should be treated with caution and contributed to the overall judgement that this evidence was of very low quality (see Table 6). In particular, the study by Dheda et al. ,73 which produced the most extreme results, had many methodological flaws.
FIGURE 6.
Health-related quality of life as measured by SGRQ, CRQ and EQ-5D. NR, not reported.

Exercise capacity: possible effect with exercise-based intervention only
Two trials64,65,70 reported on exercise capacity (see Appendix 10). For the study by Behnke et al. ,64 we analysed results from the longest follow-up time point (18 months). At this point, the home exercise programme showed strong statistical evidence of a large benefit compared with exercise advice only (MD in treadmill distance in 6 minutes = 355.0 m, 95% CI 269.2 m to 440.9 m). Note that this trial was likely to be biased, as only completing participants were described, and the trial had a loss to follow-up of > 40%. In neither trial was the substantial baseline imbalance taken into account, which would have exaggerated the effect size. In contrast, the trial of nurse-supported discharge in Hong Kong retained nearly 90% of its patients, and there was no statistical evidence of a difference after 6 months in mean distance walked compared with the UC arm (MD 24 m, 95% CI –7.1 m to 55.1 m).
Lung function: no evidence of effect
Data from four trials64–66,68,72 were plotted (see Appendix 11). Three trials provided results on raw FEV1 values (Figure 7),64,65,68,72 and two trials the effect on percentage predicted FEV1 (Figure 8). 65,66 There was no evidence of any effect from any of the individual trial results, and it was not deemed appropriate to pool the individual trials due to the small number of studies and heterogeneity of outcomes, follow-up time and methods of analysis. The findings are in agreement with the fifth trial73 which reported no evidence of effect on FEV1 but did not provide data. Again, the proportion of patients followed up across the trials was very variable.
FIGURE 7.
Lung function (FEV1, l). NR, not reported.

FIGURE 8.
Lung function (FEV1% pred). NR, not reported.

Anxiety and depression: possible improvement in scores
Four trials (see Appendix 12)63,66,69,70 reported on psychological health outcomes; however, only two trials63,66 contributed to the analysis on anxiety (Figure 9) because one trial69 reported only median changes and the other70 did not provide separate results for anxiety. Although there were data on less than half of the sample in the larger study,63 the intervention group had a mean reduction of 1.06 points (95% CI 0.04 to 2.08 points) in the ‘anxiety’ component of the Hospital Anxiety and Depression Scale (HADS) score compared with the control group, and the other trial66 demonstrated a mean reduction of 1.5 points (95% CI 0.62 to 2.38 points) in the ‘anxiety and insomnia’ component of the General Health Questionnaire (GHQ) relative to the control group. 66
FIGURE 9.
Anxiety. NR, not reported.

One of the above trials66 also showed some evidence of a reduction in depression score (MD –1.0, 95% CI –1.97 to –0.03), although follow-up rates were not reported, whereas there was no evidence of effect in the larger trial63 (Figure 10).
FIGURE 10.
Depression. NR, not reported.
Exacerbations: no evidence of effect
Only one small, poor-quality trial73 of hospital outpatient follow-up (n = 33) reported on exacerbations. This study reported that there were ‘fewer patients with two or more exacerbations within a six-month period (2 v. 3) in the intervention group but the small numbers precluded meaningful statistical analysis’.
Dyspnoea: possible effects in exercise trial
Two RCTs64,65,70 reported effects on dyspnoea (see Appendix 13) reporting a variety of different measures. The Behnke trial64,65 of home-based exercise reported effects at 1, 2, 3, 6, 12 and 18 months using the Baseline/Transitional Dyspnoea Index and the ‘dyspnoea’ domain of the CRQ questionnaire. Significant improvements in dyspnoea score in the intervention arm were observed throughout the trial, with all measures. However, > 45% of the 46 original patients had dropped out by the end of the follow-up period.
A trial of integrated care72 also reported dyspnoea among 62 of 113 completers using the Medical Research Council (MRC) dyspnoea scale, finding no evidence of effect after 12 months (MD between two arms –0.38, 95% CI –1.1 to 0.34).
Behaviour change: improvement in knowledge but inconsistent evidence of effects on behaviour
Three trials67,68,72 reported effects of the intervention on a range of health behaviours (see Appendix 14). None of the studies used validated questionnaires.
All three trials67,68,72 reported significantly better knowledge about the disease and how to recognise and treat exacerbations among patients receiving the SM intervention, and two trials68,72 reported significantly better adherence to inhaler treatment and inhaler technique. This was not matched by improvements in smoking behaviours or uptake of vaccines. Effects on physical activity were inconsistent.
Self-efficacy: inconsistent effects
Two trials63,74 reported effects on self-efficacy (see Appendix 15). Significant improvements in self-efficacy were observed in the trial74 of a community nurse-supported discharge programme in Hong Kong after 3 months (p = 0.009), which was most marked in the physical exertion and weather/environment domains. Despite a high loss to follow-up, a much larger and more intensive trial63 of supported SM reported no evidence of improvement in self-efficacy after 12 months.
Patient satisfaction: inconsistent results
Four trials66–68,72 reported effects on patient satisfaction with the intervention using different questionnaires (often with little detail provided; see Appendix 16). Two66,68 of the trials66–68,72 indicated increased satisfaction with their care compared with the control arm. However, loss to follow-up was generally high.
Discussion
Key results
Despite a rigorous search we identified only 10 RCTs63–74 that evaluated the effectiveness of interventions providing SM support to patients shortly after being discharged from hospital with an acute exacerbation of their COPD.
Few of the trials had consistently low risk of bias. Many studies were small and suffered from inadequate reporting and high loss to follow-up, particularly affecting patient-reported outcomes such as HRQoL.
Although the participants seemed relatively homogeneous, interventions were very heterogeneous, with some trials67–69,74 providing low-intensity, short-term support of 2–3 months and others63 a very intensive package lasting for 12 months.
Overall, there was limited evidence of effect on health-related behaviours and outcomes. There was some evidence of improvement in patient knowledge, treatment of exacerbations and inhaler technique,68,69,73 but there was inconsistent evidence of effect on other health-promoting behaviours68,73 or on self-efficacy. 64,75
In terms of health outcomes, the most consistent effects were observed on patients’ QoL, with an overall improvement with data from five trials63,67,68,72,73 of 3.8 points on the SGRQ score (close to the minimally clinically important difference of four points). Notably, though, this estimate should be treated with caution because, although reaching statistical significance, there was substantial and differential loss to follow-up in both arms, which could bias the results in favour of a positive effect. The authors of the largest trial63 indicated, themselves, that the results from their trial could be unreliable. The reduction in anxiety exhibited in two trials,63,66 however, supports some potential effect on patients’ psychological health (although it is not clear whether or not this would be clinically important).
An important outcome for these patients is whether the SM package had any effect on subsequent hospital admissions. We were able to use data that reported time to first all-cause admission. Despite subdividing by intensity of intervention, we were unable to explain the substantial heterogeneity observed, but, overall, there was no clear evidence of effect on hospitalisation (HR 0.78, 95% CI 0.52 to 1.17; I2 = 71%). Post hoc inspection of the data suggested a possibility of a greater effect with the exercise-based intervention but would require more data to be explored in depth. It is possible, however, that the effects on admissions would be diluted because we extracted admissions due to any cause (although in our analysis the majority were for respiratory causes).
There was no apparent evidence of effect on mortality and no clear patterns with duration of intervention.
In general, the most positive results across the outcomes were observed in the small trial of home-based exercise64,65 but, given the multiple methodological limitations of the trial in terms of reporting and analysis, the results have to be interpreted with caution.
How this fits with other literature
This is the first systematic review that addresses the effectiveness of SM support provided to patients with COPD soon after hospital discharge. The only other review related to this time point is a Cochrane review of PR,76 which identified nine trials and showed significant reduction in hospital admissions [pooled odds ratio (OR) 0.22, 95% CI 0.08 to 0.58], over 25 weeks and mortality (OR 0.28, 95% CI 0.10 to 0.84) over 107 weeks. Effects of PR on HRQoL were well above the minimal important difference when measured by the CRQ and the SGRQ total score (MD –9.88, 95% CI –14.40 to –5.37). However, in common with our review, trials were small and methodologically inadequate, and, although loss to follow-up was not discussed or assessed in the risk of bias section, a large proportion did not complete the rehabilitation. There was also significant heterogeneity across many of the outcomes. The authors discussed the possibility of publication bias and possible overestimate of effect with small trials but suggested this would not account for the whole effect. The results would fit with our tentative observation that trials with an exercise component might be more effective.
The majority of the studies and reviews of SM support are set among patients who have COPD in a stable state. Our results, although showing few significant effects, are consistent with some of the other systematic reviews. For example, a systematic review of SM education48 showed evidence of a significant reduction in respiratory admissions (OR 0.64, 95% CI 0.47 to 0.89; n = 8 RCTs) and a significant mean improvement of 2.6 points (95% CI 0.2 to 5.0 points) on the SGRQ score (n = 7 trials). A review of PR46 reported an overall mean improvement in SGRQ score of 6 points (95% CI 3 to 9 points; n = 6 trials) and a review of integrated disease management47 found a similar improvement in HRQoL: SGRQ 3.71 points (95% CI 1.6 to 5.8 points; n = 13); CRQ 1.02 points (95% CI 0.67 to 1.36 points; n = 4) and respiratory admissions (OR 0.68, 95% CI 0.47 to 0.99; n = 7) and a similar lack of effect on mortality. Conversely, a review of action plans found little evidence of benefit on HRQoL or health-care utilisation. 50
In the last couple of years, and particularly since the completion of our searches, there have also been a number of individual trials and commentaries that question whether patients are actually able to self-manage. 34,36,77,78 Two of these among patients with COPD35,62 identify a group of successful self-managers in post hoc exploratory analyses. The first of these63 is included in our review but no other studies have explored these subgroups so we were unable to examine this point further.
Strengths and weaknesses
A strength of this review was the comprehensive search and selection process, which made it unlikely that we would have missed relevant studies. In addition, we followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance with respect to study selection, data extraction, risk of bias assessment, reporting and analysis.
The main limitation relates to the paucity of evidence and methodological weaknesses of many of the available studies, which limits our conclusions, and the heterogeneous nature of the interventions that makes comparisons hard and conclusions difficult to draw. The particular problems with these studies, especially the older ones, include generally inadequate reporting of important items, particularly methods of randomisation and limited data on baseline characteristics. Many studies were small, with data reported only for participants completing the trial and had substantial loss to follow-up of > 30% in some arms, which is likely to bias all self-reported items and HRQoL in particular. There was a lack of information about the assessment of some outcomes, especially lung function measurements and analyses were often unclear or inappropriate. In addition, the admission results were reported in several different ways, for example first admission, mean admissions, etc. Although ideally we would like to be able to capture all of this information – especially as some patients may have multiple admissions – current methodologies are inadequate to do so. We chose rate of first admission because there were more data available; however, it is unclear how the effect of the interventions would vary if all admissions could be considered.
With the limited number of trials it was not possible to assess publication bias, but it is possible that because of the small size of the studies showing positive effects this would be a potential problem.
Another issue is that of generalisability, as only two RCTs63,73 were set in the UK health-care setting. Studies in China and other areas of Europe may not necessarily be relevant; the feasibility and effectiveness of different types of support may be dependent on both financial and practical issues in individual settings. With the limited data available it was not possible to explore the effect of different settings.
Implications for research and practice
It is difficult to recommend any type of SM support to be provided immediately after discharge with the evidence available as there is no clear evidence of effect across most of the outcomes. This conclusion is in contradiction to the current recommendations in the COPD discharge care bundle. 79 Notwithstanding, the point estimate is consistent with ≈20% reduction in admissions which has been observed in other systematic reviews.
However, to move forward with this area of research, there should be:
-
more in-depth work to explore the needs/views of patients with regard to SM support after a recent discharge from hospital before designing novel interventions aimed at behaviour change
-
an adequate standard of reporting ensured in future trials, and they should be conducted to modern standards with an adequate number of participants
-
a clear framework for describing and classifying SM interventions and their comparators
-
an exercise component included in future studies
-
clear reporting of outcomes to include self-efficacy, behaviour change and clinical outcomes, including separate reporting of COPD-related and all-cause admissions
-
consideration that patients may be too ill at this point (both physically and psychologically) to take up the more rigorous parts of SM interventions until they are in a more stable state; the difficulty in recruitment and retention in the included studies bears this out.
Conclusions
-
Self-management support delivered shortly after an acute exacerbation may have some benefit in terms of HRQoL and possibly admissions but the evidence is thin and unconvincing.
-
Exercise may play an important role but there are not enough data to explore this.
-
Any future trials should address issues of bias, particularly loss to follow-up, but this may be inherent in the nature of the intervention and the fact that patients are still trying to recover from an exacerbation.
-
The evidence is not in support of SM interventions to be put into practice for patients with COPD at, or recently after, hospital discharge.
Chapter 4 A systematic review of the qualitative evidence about patient satisfaction, acceptance and barriers to supported self-management interventions delivered shortly after hospital discharge: review 2
The aim of this chapter is to present the findings of a systematic review of the qualitative evidence about patient satisfaction, acceptance and barriers to self-management (SM) support.
Methods
A systematic review of published evidence of the qualitative evidence about patient satisfaction, acceptance and barriers to SM support programmes among patients with chronic obstructive pulmonary disease (COPD) recently discharged from hospital.
Definition of self-management
As described for review 1 and tabulated in Table 2.
Search strategy
A comprehensive search strategy was undertaken as described as for review 1. The search was broad and covered many databases and no study design filters were applied. Search terms related to qualitative evidence included ‘patient-centred’ and ‘patient focus’.
Study selection process
As described for review 1.
Selection criteria
Selection criteria were as described for review 1, with the difference of two elements: study design and outcomes. Only qualitative study designs (interviews and focus groups) were sought and outcomes relating to patient satisfaction, acceptance and barriers to SM were assessed.
Study quality, data extraction and synthesis
Study quality was assessed using the Critical Appraisal Skills Programme (CASP) checklist for qualitative research. 80 As well as extracting data related to study and patient characteristics, any quotes, key themes and concepts identified were extracted. As outlined in the protocol, an interpretive analysis (meta-ethnography)81 was planned if findings allowed. This involved looking for similarities (reciprocal translation), differences (refutational translation) or creating a line of argument using concepts proposed in included primary studies. However, as only one study described a small element of qualitative interviewing, this was not undertaken.
Results
Search results
Figure 11 outlines the flow of included studies. One of the included randomised controlled trials (RCTs)69 from review 1 had a limited qualitative element referring to patient satisfaction and was therefore included in this review. There was also one ongoing study (see Appendix 4).
FIGURE 11.
Selection process for qualitative studies.

Characteristics of included study
The included trial was a trial of nursing-based case management among 66 patients in Australia admitted to hospital with COPD. The intervention comprised SM support with review and two telephone calls and follow-up for 3 months in total, compared with usual care. 69 A subgroup of 18 patients and their carers from both arms of the trial were interviewed in more depth using semistructured interviews focusing on issues associated with patients and caregiver satisfaction with care. The interviews were recorded, and then transcribed and coded to identify themes. These interviews revealed that patients were very satisfied with their care in hospital and, for those in the intervention arm, ongoing contact with the community nurse was very helpful in improving their access to resources and communication with the health professionals. For those in the control arm, those with family support or medical contact were satisfied but those without were not. Table 7 describes the patient and caregiver quotes in relation to case managers.
| Context | Quotation |
|---|---|
| The caregiver of a patient in the control group without support – such as extensive family, medical support from health-care professionals | It is absolutely hopeless |
| A patient from the intervention group who received a CM talking about benefits of having a CM | I became more involved with (the CM) and it was good to know that she cared . . . kept on your hammer all the time . . . So I think that . . . it will give some peace of mind to the patients, you know. The big thing is to know what is happening |
| A patient from the intervention group commenting on benefits that a CM provides | (The CM) made me aware of things that were available that I didn’t bother to want to know about before |
| A caregiver of a patient of the intervention group commenting on benefits that a CM provides | (The CM) helped me organize (a nebulizer); she pointed out a lot of things to me, different things that should be done (for the patient) |
Quality of included studies
Table 8 presents an assessment of the quality of this study. The aims of the study were not very clear from the outset, which means that it was difficult to assess which methodology would be appropriate. It was possible to infer that patients’/carers’ views and satisfaction with the intervention would be sought, which would mean that these semistructured qualitative interviews would be appropriate. However, although the authors mentioned that patients were selected to maximise variability, the only factor that they mention is about representing both intervention and control groups.
There is mention of the home setting, although not the justification for it, but they do not mention a topic guide, any modification of the methods, details of any interviewer biases, how the themes were identified or whether or not saturation was reached. Thus, the findings are really not very valuable.
| CASP checklist questions | Judgement and comments/quotes |
|---|---|
| Was there a clear statement of the aims of the research? | Unclear Aims of the RCT made clear Authors state a lot of focus is around economic outcomes rather than patient-focused outcomes; no clear aim relating to qualitative research |
| Is a qualitative methodology appropriate? | UnclearThis is an RCT which has both quantitative and qualitative analysesUnclear as the research goal is not clearly stated although they mention focusing on issues to do with patient and caregiver satisfaction |
| Was the research design appropriate to address the aims of the research? | Unclear Very little detail of qualitative methodology reported, although semistructured interviews seem appropriate |
| Was the recruitment strategy appropriate to the aims of the research? | UnclearParticipants were selected to maximise variability and to represent both intervention and control groupsVariability not detailed; subgroup of 18 patients from RCT selected for qualitative |
| Were the data collected in a way that addressed the research issue? | Unclear Audiotaped interviews Patient interviews – semistructured Patients and caregivers interviewed at home No topic guide, no mention of any modifications during study, no justifications for setting for data collection |
| Has the relationship between researcher and participants been adequately considered? | Unclear No details of biases; not clear who led interviews |
| Have ethical issues been taken into consideration? | Unclear ‘The study received ethical clearance from the participating hospital’ – This is in reference to the RCT; no other details regarding ethics |
| Was the data analysis sufficiently rigorous? | Unclear Not enough detail to permit judgementAll interviews, including those with the respiratory physicians were audiotaped then transcribed and coded to identify recurring themes and patterns |
| Is there a clear statement of findings? | Unclear Not particularly adequateBased on the qualitative interviews, all patients were very satisfied with their care in hospital |
| How valuable is the research? | Not very valuable as it stands with limited details |
Discussion
Key results
A comprehensive search of the literature revealed only one RCT69 with a limited qualitative aspect and limited conclusions.
Strengths and weaknesses
The search strategy was broad, conducted across several databases and with no study design filters applied. Additionally, reviewers adhered to a systematic methodology with two independent reviewers assessing studies for inclusion and exclusion against a prespecified selection criteria; therefore, it was unlikely that any relevant qualitative evidence will have been overlooked. However, after a thorough search and identification process, only one study69 was included.
How this fits with the findings of the effectiveness review (review 1)
Unfortunately, the included study69 only gave scant information regarding the qualitative aspects, and it is difficult to be sure of the true purpose of the interviews and how they were actually conducted. The study,69 however, reported that patients who received a case manager were very satisfied with their care, and were made aware of resources available to them and how to use them. Those in the control arm without family support were more concerned about their situation, although those in the control arm with family support seemed reasonably satisfied. This reflects the evidence from the quantitative studies for which the evidence was inconsistent (two studies66–68,72 showed better satisfaction with the intervention and two did not) but does not really gain much further insight.
Conclusions
There is almost no qualitative evidence about patients’ views on SM support programmes that are delivered post discharge, and, given that the quantitative evidence reveals uncertainty about the effectiveness of such interventions in their current form, it is therefore a potential area of research need.
Chapter 5 A systematic review of the cost-effectiveness of supported self-management interventions delivered shortly after hospital discharge: review 3
The aim of this chapter is to present the findings of a systematic review of the cost-effectiveness of self-management (SM) interventions delivered post discharge in patients with chronic obstructive pulmonary disease (COPD) compared with usual care (UC).
Methods
A systematic review of the literature was conducted to identify all published studies assessing the cost-effectiveness of SM interventions delivered to patients with COPD within 6 weeks of hospital discharge following an acute exacerbation.
Search strategy
A comprehensive search strategy was undertaken by an experienced information specialist from inception to May 2012. Three electronic databases were searched: MEDLINE and EMBASE via Ovid and Cochrane NHS Economic Evaluation Database (Wiley). Searches were not limited by date nor were any language restrictions applied. The search strategies used for each database can be found in Appendix 17. Relevant literature from the clinical effectiveness searches were also identified and included for review, if they had not already been captured in the searches for cost-effectiveness.
Reference Manager version 11 was used to store and manage all references.
Study selection process
The inclusion and exclusion criteria outlined below were used to select studies. A two-stage review process was applied by two independent reviewers: first, screening titles and abstracts, and then reviewing full papers. Discrepancies were resolved by a third reviewer with expertise/knowledge in the field of health economics.
Selection criteria
Study design
Full and partial economic studies, costing studies and costing models were included.
Population
Studies including patients admitted to hospital with an acute exacerbation of COPD, who were recruited at the point of discharge or within 6 weeks after discharge were included (see Chapter 3, Methods).
Intervention
Any SM programme, package or intervention including adherence to medication, inhaler technique, breathing techniques, exercise, education and support groups among others. Pulmonary rehabilitation was not included for this review.
Comparator
Comparators considered were UC, other SM interventions or no intervention.
Outcomes
Cost-related outcomes included health service utilisation, hospital admissions and readmissions, duration of admissions, ED visits, days lost from work, drug utilisation and cost-effectiveness. Effectiveness outcomes were as reported for the clinical effectiveness review (see Chapter 3).
Risk of bias assessment
Risk of bias of included studies was assessed using the Drummond checklist, as suggested in the Cochrane Handbook. 56 Risk of bias assessment was undertaken by two reviewers independently, one of whom had expertise in the field of health economics.
Data extraction
Study characteristics and results were extracted independently by two reviewers. Meta-analysis was not considered appropriate for this review because of the paucity of evidence.
Results
Search results
Figure 12 outlines the study identification process. A total of 1611 references were imported into Reference Manager, and 240 duplicates were removed automatically and manually. Overall, 1131 titles and abstracts were screened, with 129 articles being identified for full-text review. Only one study68 met the inclusion criteria for this review, which also formed part of the clinical effectiveness review. A further four studies met initial inclusion criteria but were ongoing and so have been listed in Appendix 4.
FIGURE 12.
Selection process for cost-effectiveness studies.

A number of other studies (n = 27) were identified as potentially useful to inform the independent economic analysis (see Chapter 6). These studies included relevant primary or secondary data on the cost or utilisation of health care associated with SM in patients with COPD; however, the intervention was not delivered to patients in hospital at the point of discharge or within 6 weeks of hospital discharge. Data from some of these studies were used to estimate the costs of SM in the model (see Chapter 6).
Characteristics of included studies
The single included trial by Hernandez et al. 68 was conducted in two tertiary hospitals in Barcelona, Spain, with a total sample size of 222 patients: 121 patients were randomised to the home hospitalisation group and 101 were randomised to conventional care. The hospital-at-home intervention included four phases: assessment by a specialist team during admission to the emergency room; treatment at discharge; home hospitalisation with follow-up; and assessment after 8-week follow-up. Specific SM components implemented as part of the hospital-at-home service included 2 hours of the following delivered at the point of discharge and later reinforced during home visits (along with action plan reinforcement):
-
education on disease, adherence to medication and recognition/prevention of triggers of exacerbations
-
selecting appropriate equipment at home and training on the correct administration of pharmacological therapy
-
smoking cessation
-
patient empowerment for daily life activities, including hygiene, dressing, household tasks, leisure activities, breathing exercises and skeletal muscle activity
-
nutrition recommendations
-
socialisation and changes in lifestyle.
The following outcomes were reported: mortality, readmissions or ED visits, hospitalisation, HRQoL, lung function, patient satisfaction, disease knowledge, inhaler technique, medication prescriptions, and home rehabilitation and costs.
Quality of included studies
The details of a cost analysis economic evaluation68 were described and are summarised in Table 9. The research question was stated with reasons for its importance as well as for the rationale for the intervention and control under comparison. A public insurer perspective was taken but not justified; however, the limitations of taking this viewpoint were highlighted. The source of effectiveness estimates was stated and details of the design and results of the effectiveness study were provided. Outcome measures were clearly outlined and quantities of resource use (per patient) were given separately from unit costs. Details of direct and indirect costs were provided with nurse home visits, prescriptions, telephone calls and transport being calculated directly and inpatient hospital stay, emergency room visits, outpatient visits, primary care consultations and social support visits being calculated indirectly. Costs incurred by patients or carers were not considered. Currency and price data were reported using 2000 price data (euros); however, no details were provided regarding any price adjustments for inflation or currency conversions. The time horizon for the study was 1 year; thus, discount rates were not applied. Tariff prices were applied to resource-use data that were collected to calculate an average annual health-care cost per patient in both arms. Although differences in both costs and outcomes were reported, they did not conduct a full economic evaluation by presenting the relative cost-effectiveness. As the costs for the intervention were lower and outcomes better, this study68 suggests the intervention dominated UC. Sensitivity analyses, and the details thereof, were discussed only briefly.
| Author | Hernandez et al.68 |
| Date | 2003 |
| Type of economic evaluation | Cost analysis |
| Currency used | Euros (€) |
| Year to which costs apply | 2000 |
| Perspective used | Public insurer |
| Comparators | Home hospitalisation compared with conventional care |
| Source(s) of effectiveness data | Clinical effectiveness data from RCT |
| Source(s) of resource-use data | Based on data from RCT |
| Sources of unit cost data | Directly calculated from data from trial, as well as indirectly calculated from tariffs for patients with COPD in a public insurance company |
| Modelling approach used | Not applicable |
| Summary of effectiveness results | Mortality: HR = 0.59 (95% CI 0.19 to 1.85) First ED visit: HR = 0.41 (95% CI 0.20 to 0.85) All ED visits: HR = 0.44 (95% CI 0.24 to 0.79) Hospital admission: HR = 0.71 (95% CI 0.40 to 1.24) QoL: MD = 4.50 (95% CI 0.66 to 8.34) Lung function: MD = 0.20 (95% CI –0.04 to 0.44) |
| Summary of cost-effectiveness results | Intervention dominates UC |
| Sensitivity analysis | Intervention dominates UC when resources released by the intervention were 75% or 50% of the average cost |
Cost-effectiveness
As only one study met inclusion criteria for this review, no meta-analysis was undertaken; instead, the cost analysis results from the included study68 are reported.
Costs were reported for the following outcomes (categories) following an 8-week follow-up period: length of hospital stay, emergency room visits (excluding visits that required further hospital admission), outpatient visits, primary care consultations, social support visits, home visits by nurse, prescriptions, telephone calls (both to the nurse from the patient and from the nurse to the patient) and transport costs. Details of the costs reported for each outcome are provided in Table 10.
| Resource-use item | Cost per patient (€) | p-valuea | |
|---|---|---|---|
| Home hospitalisation | Conventional care | ||
| Inpatient hospital stay | 941.40 | 1795.47 | < 0.001 |
| Emergency room visits | 10.31 | 24.59 | 0.01 |
| Outpatient visits | 5.49 | 22.04 | – |
| Primary care physician visits | 8.19 | 7.57 | – |
| Prescriptions | 217.21 | 172.06 | 0.001 |
| Nurse home visits | 41.94 | – | – |
| Social support visits | 1.62 | 2.19 | – |
| Telephone calls | 20.99 | – | – |
| Transport | 7.97 | 9.59 | – |
| Average cost (€) per patient (95% CI) | 1255.12 | 2033.51 | 0.003 |
The cost analysis found the home hospitalisation intervention to be significantly less costly than conventional care (average cost per patient: €1255.12 vs. €2033.51; p = 0.003). Hospital stay, emergency room visits, outpatient and social support visits were at a greater cost per patient for the conventional care group than for the home hospitalisation group, with the difference for hospital stay and emergency room visits reaching significance (p < 0.001 and p = 0.01, respectively). Prescription costs were significantly higher in the intervention group than in the control group (cost per patient: €217.21 vs. €172.06; p = 0.001). Primary care visits were also greater in the intervention group, although the difference was not reported to be statistically significant.
A sensitivity analysis based on resources for home hospitalisation at 50% and 75% of the average cost per patient (to capture intervention costs in the longer term) was undertaken. Cost savings in favour of home hospitalisation were conserved across each assumption.
Discussion
Summary of results
A comprehensive search strategy identified one study68 that met the inclusion criteria for this review. The overall quality of the study was high, with some issues related to reporting. Meta-analysis was not possible. The study68 revealed that home hospitalisation is less costly than conventional care [£1041.75 vs. £1687.81 (conversion rate €1 = £0.83)].
How this fits with other literature
One relevant study82 published after the search strategy had been completed was subsequently identified. Xin Lie et al. 82 developed a Markov model to evaluate the impact of a hypothetical exacerbation management programme that could detect the risk of exacerbation and divert the risk of hospitalisation. In patients without prior history of exacerbation, they estimated that this would result in savings of US$2900 per patient over 12 years, and in higher-risk patients – with a history of one or two exacerbations per year – this estimate increased to US$16,000 per patient.
Strengths and limitations
This is the first systematic review of the cost-effectiveness literature of SM interventions for patients who have recently been discharged from hospital after an acute exacerbation of COPD. The methods used throughout this cost-effectiveness review were systematic. A comprehensive search strategy was undertaken and the results were reviewed independently by two reviewers, including a health economist. The recommended quality assessment checklist was used.
It should be noted that the patients in this study were recruited from the emergency room rather than after discharge post hospital admission, which may or may not cause variations in the applicability of the results. A scarcity of evidence of the cost-effectiveness of SM interventions was evident. The identified study included the cost of implementing a hospital-at-home programme with components of SM, thus the SM components reflect only a proportion of the costs and cost savings incurred.
Implications for research
There is a need for more economic evaluations, alongside randomised controlled trials, specifically addressing patients who have recently been discharged from an inpatient hospital admission stay after an acute exacerbation of COPD, and who have been treated with SM interventions or components of SM, such as education, action plans, breathing techniques, relaxation and stress management amongst others.
Chapter 6 Economic evaluation
Methods
This section provides a detailed description of the economic model that was developed and used to evaluate the cost-effectiveness (cost–utility) of self-management (SM) support delivered within 6 weeks of hospital discharge compared with usual care (UC) in a patient population with chronic obstructive pulmonary disease (COPD) who have been admitted for an exacerbation. The evidence for the effectiveness of SM programmes (see Chapter 3) demonstrated that there was considerable uncertainty for the outcome measures of mortality, quality of life (QoL) and admissions. The model presented here considers the potential impact of reduced admissions due to a SM programme, in terms of costs, mortality and QoL. However, owing to the uncertainty around the point estimate of reduction in admission and the considerable heterogeneity between studies, this effect must be considered with some caution. Therefore, the economic model should be viewed as speculative, with the aim of estimating the potential cost–utility of SM if it is truly effective at reducing hospital admissions for exacerbations.
Model description
A Markov decision model, built in TreeAgePro 2014 (TreeAge Software, Inc., Williamstown, MA, USA) was structured to consider short-term increased risks of readmission and mortality, and the long-term natural history of the disease, taking into account exacerbations, increasing COPD severity and mortality (Figure 13). This structure was an adapted version of other COPD Markov models83 with health states linked to GOLD (Global Initiative for Chronic Obstructive Lung Disease) severity. It incorporated additional health states to capture the higher risks reported in patients immediately after discharge in audits of patients admitted to hospital. 84 The model had a time cycle of 1 month and a lifetime time horizon (30 years) was used. All costs and outcomes were considered from a NHS perspective for a price year of 2012.
FIGURE 13.
Markov model structure.
Severity of COPD was defined according to the GOLD classification. GOLD stage 2 was defined as having a forced expiratory volume in 1 second (FEV1) of ≥ 50%, < 80% predicted; GOLD stage 3, a FEV1 of ≥ 30%, < 50% predicted; and GOLD stage 4, a FEV1 of < 30% predicted. GOLD stage 1 (mild COPD) was excluded, as < 16% of patients admitted to UK hospitals with COPD had a FEV1 of ≥ 80% predicted.
A patient started in the model in one of three health states representing their first month post admission, taking into account their current GOLD severity stage. Those who continued to recover without further exacerbations moved to health states to represent the second and third month of recovery, again related to their GOLD stage. Within this 3-month recovery period, a patient could die from COPD or other causes or have a further exacerbation requiring readmission, which could be fatal. If the patient survived, they were discharged to restart the 3-month recovery period in a ‘first month post-admission’ health state. The patient pathway within each post-admission health state (Figure 14) was similar for each month and severity stage. The post-admission health states allowed the model to consider the immediate increased risk of readmission and COPD-related mortality for 3 months after discharge. Once a patient survived 3 months of recovery with no readmissions, they moved into a stable health state for their GOLD stage.
FIGURE 14.
Pathways within post-admission states.
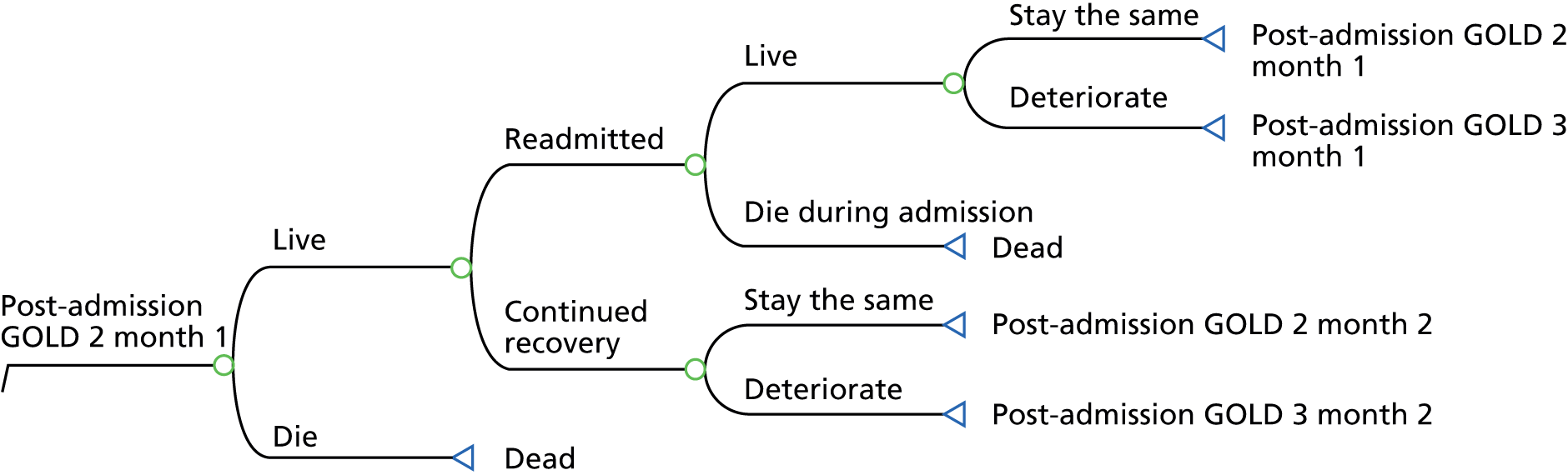
Once in the stable GOLD stage 2, 3 or 4, a patient could remain in that health state, deteriorate to the next more severe health state, have an exacerbation or die. An exacerbation could be moderate (managed in primary care) or severe (admitted to hospital). Patients who recovered from moderate exacerbations either remained in the same health state or deteriorated. Severe exacerbations could result in death, and surviving patients moved to the relevant ‘first month post-admission’ health state. It was assumed that no patients could improve into a better GOLD stage health state. Figure 15 illustrates the patient pathway in a stable health state.
FIGURE 15.
Pathways within stable health states.
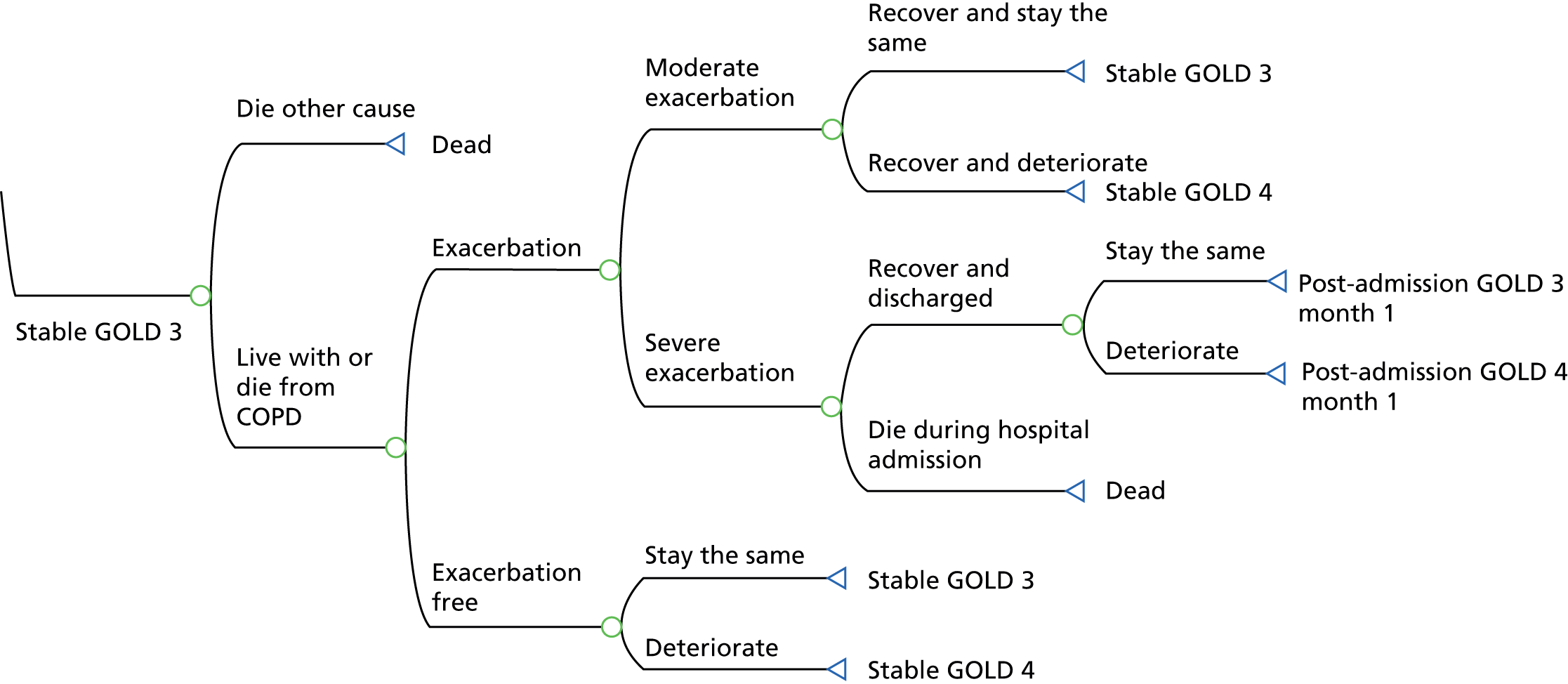
Base-case cohort
For the base-case analysis, the characteristics of the cohort were taken from the 2011 report by the European Audit84 of UK patients with COPD admitted to hospital (Table 11). The proportions of men and current smokers were assumed to remain constant. The baseline distribution of patients entering the model was 35% in GOLD stage 2, 35% in GOLD stage 3 and 30% in GOLD stage 4.
| Characteristic | Median or % |
|---|---|
| Age (median) | 72 |
| Sex (% male) | 47.4 |
| Smoking status (% current smokers) | 39.4 |
| GOLD stage (%): 2; 3; 4 | 35; 35; 30 |
Estimation of model parameters
This section outlines the assumptions applied, and sources used, to populate the base-case, usual-care arm.
Transition probabilities within post-admission health states
Published data on exacerbation rates in patient cohorts who had been admitted to hospital demonstrated an elevated risk of readmission and mortality immediately after discharge. 84,85 In addition, exacerbation rates and severity of exacerbations increased with disease severity. The risk of a mild or moderate exacerbation was not considered in the post-admission health states.
The majority of the transition probabilities for post-admission health states were obtained from the European Audit84 and are reported in Table 12. Risks of readmission and mortality were the same for each of the three post-admission months and did not differ by GOLD stage. Post-admission COPD-related mortality and readmission risks were assumed to be evenly distributed over the 3-month period.
| Definition | Probability | Beta distributiona |
|---|---|---|
| COPD-related death during admissionb | ||
| Men | 0.050 | α = 118, β = 2243 |
| Women | 0.051 | α = 133, β = 2490 |
| 90-day COPD-related death post admissionb | ||
| Men | 0.047 | α = 104, β = 2097 |
| Women | 0.049 | α = 120, β = 2324 |
| 90-day COPD-related readmissionb | ||
| Men | 0.323 | α = 705, β = 1496 |
| Women | 0.295 | α = 721, β = 1723 |
Age- and sex-specific all-cause mortality rates were obtained from Office for National Statistics life tables and adjusted to avoid double counting of COPD-related mortality. Appendix 18 lists the COPD adjusted all-cause mortality rates applied in the economic model. Age- and smoking-related disease progression rates were obtained from a published model86 (see Appendix 19). All annual rates were converted to monthly probabilities.
Transition probabilities within stable health states
The patient pathways for each GOLD stage health state were assumed to be the same (see Figure 15); however, the probabilities, costs and utilities differed by COPD severity. The probability of progressing to a more severe GOLD stage was not assumed to vary by exacerbation history; thus, the transition probabilities for movement between stable health states were the same as those described above.
The probabilities for exacerbations and hospitalisations were obtained from the TORCH (TOwards a Revolution in COPD Health)85 and ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints)13 studies, respectively. Patients who survived, exacerbation free, for the 3-month post-admission period were assumed to have exacerbation and hospitalisation rates similar to those reported in stable cohorts for each severity stage. As the exacerbation rates from the TORCH trial85 were reported by type of treatment, assumptions were required in the model regarding the proportion of patients on each type of treatment in each GOLD stage health state. These proportions were obtained from unpublished data collected from a cohort of UK patients with COPD who were recruited as part of BLISS (Birmingham Lung Improvement Studies) in the West Midlands. Exacerbation rates were then weighted by the proportion of patients on each treatment in each GOLD stage severity group. As the TORCH study85 did not report the proportion actually admitted to hospital for an exacerbation, this was obtained from the ECLIPSE study. 13
The base-case proportions on each type of treatment, annual exacerbation rates and the proportion resulting in a hospital admission are reported in Tables 13 and 14. Probabilities for inpatient mortality, all-cause mortality and disease progression are as described previously for the post-admission states.
| Severity stage | Treatment type | |||
|---|---|---|---|---|
| Othera | ICS | LABA | ICS/LABA | |
| GOLD 2 | ||||
| Proportion on treatment (%) (BLISS) | 42.92 | 0.02 | 6.31 | 50.75 |
| Annual exacerbation rate (TORCH)85 | 0.82 | 0.68 | 0.71 | 0.57 |
| GOLD 3 | ||||
| Proportion on treatment (%) (BLISS) | 26.27 | 0.85 | 5.08 | 67.80 |
| Annual exacerbation rate (TORCH)85 | 1.24 | 0.99 | 1.08 | 0.91 |
| GOLD 4 | ||||
| Proportion on treatment (%) (BLISS) | 16.28 | 4.65 | 2.33 | 76.74 |
| Annual exacerbation rate (TORCH)85 | 1.79 | 1.53 | 1.40 | 1.54 |
Estimate for effectiveness of self-management support
Data and assumptions regarding the effectiveness of SM were based on the results of the review presented in Chapter 3. As previously highlighted, although evidence suggested a potential reduction in readmissions, there was considerable uncertainty around the point estimate of effect as the 95% CI crossed 1 (see Chapter 3, Hospital admissions: no evidence of effect). The HR used for the base-case model was the weighted ratio of the more intense SM interventions. Two alternative HRs reported were subsequently applied in a one-way sensitivity analysis. The estimate of effect was applied to all monthly probabilities for readmission for severe exacerbation in the SM strategy in the model (Table 15).
Although the effectiveness estimate was estimated over a short period of time in the trials, the model assumed that the effect of the SM intervention would last for 2 years.
The results of the review indicated no evidence of higher all-cause mortality associated with SM, as the HR reported was very close to 1. As the model takes into account a reduced risk of readmission, which leads to improved survival, no further adjustment to mortality was undertaken.
| Analysis | HR | 95% CI | Meta-analysis inclusion criteria | |
|---|---|---|---|---|
| Base case | 0.83 | 0.50 to 1.36 | Review 1: More supported SM interventions | |
| One-way sensitivity analysis | Low | 0.96 | 0.49 to 1.90 | Review 1: Less supported SM interventions |
| High | 0.78 | 0.52 to 1.17 | Review 1: All studies, including those with an exercise component | |
Estimation of quality-adjusted life-years
Utility values were required for all health states and exacerbation events, and were combined with information on survival in order to calculate quality-adjusted life-years (QALYs). The model health states were based on COPD severity defined by GOLD stages 2–4. Utility values for these health states were calculated from unpublished data collected from the BLISS cohort. Utility scores for GOLD stages 2–3 were derived from the EQ-5D-5L (a revised version of the EQ-5D questionnaire). The five dimensions (mobility, self-care, usual activities, pain and discomfort, and anxiety and depression) have five levels, compared with the older version, which used three levels. The addition of two more levels may have made the EQ-5D more sensitive to differences in health states and avoid ceiling effects. 87
The EQ-5D-5L was completed by 917 participants enrolled in the BLISS study, with a confirmed diagnosis of COPD, at GOLD stage 2, 3 or 4, and converted to utility scores using the interim crosswalk value set for a UK population reported by EuroQoL. 88 Data from this cohort were deemed suitable for stable health states in the model for two reasons. First, participants were not recovering from an exacerbation at the time of questionnaire completion, therefore the utility scores were expected to reflect QoL in the stable condition. Second, > 80% of patients in each GOLD stage had been admitted to hospital at least once in the past year, therefore presenting a population who suffered exacerbations. EQ-5D-5L responses were converted to utility scores and are reported in Table 16.
| Descriptor | GOLD 2 | GOLD 3 | GOLD 4 |
|---|---|---|---|
| Sample size (n) | 650 | 229 | 38 |
| Mean utility score (SE) | 0.7041 (0.0102) | 0.6765 (0.0174) | 0.6014 (0.0415) |
The utility scores were compared with values applied in other COPD models that defined health states by GOLD severity stage. The utility values obtained from the BLISS cohort study were similar to those reported in other studies. 83,89–91 Data on utility loss suffered immediately after a moderate or severe exacerbation were extracted from previously published models; however, estimates varied greatly and the evidence underpinning these was poor. 83,89,92–94 It was assumed that there was a loss of utility for 1 month for moderate exacerbations and a utility loss for 3 months for severe exacerbations due to full recovery taking a longer period of time. 95–97 However, in line with other studies, the utility loss for severe exacerbations was assumed to be greatest in the first month, with improvement in QoL in the second and third months post admission.
The utility loss estimate of 15% for moderate exacerbation and 50% for severe exacerbation was obtained from Rutten-van Mölken et al. 89 This was applied to the mean utility score across all three severity stages (as opposed to each individually) to ensure that the utility loss suffered in stage 2 or 3 was not greater than loss experienced by stage 4 patients. The mean utility score found in the BLISS cohort across stages 2–4 was 0.693; therefore, a moderate exacerbation was assumed to result in a loss of 0.104 QALYs for 1 month and a severe exacerbation was assumed to result in a 0.346 loss of QALYs in the first month, reducing to a loss of 0.173 QALYs for months 2 and 3. A summary of all the utilities applied in the base-case analysis is provided in Table 17.
| Severity of COPD | Base case | ||
|---|---|---|---|
| GOLD 2 | GOLD 3 | GOLD 4 | |
| Stable conditiona | 0.7041 | 0.6765 | 0.6014 |
| Moderate exacerbation | 0.6001 (1 month) | 0.5725 (1 month) | 0.4974 (1 month) |
| Severe exacerbation | 0.3581 (first month) | 0.3305 (first month) | 0.347 (first month) |
| 0.5311 (months 2 and 3) | 0.5035 (months 2 and 3) | 0.4284 (months 2 and 3) | |
The review presented in Chapter 3 found evidence that SM may have a positive impact on QoL; however, the results were highly uncertain. As the model takes into account reduced rates of readmissions in the SM strategy, which leads to reduced loss of QoL, no further utility gains were applied in the model.
Resource use and costs
The resource use considered within the model was broadly concerned with the SM intervention, primary and secondary health-care professional contacts, and pharmacotherapy. Health-care contacts for each GOLD severity group were estimated with reference to National Institute of Health and Care Excellence (NICE) guidelines and expert opinion. Use of pharmacotherapy was estimated from data provided by the BLISS cohort. Unit costs were primarily obtained from NHS Reference costs98 and Unit Costs of Health and Social Care. 99 When appropriate, unit costs were inflated to 2012 prices using NHS Health Index inflation rates. Annual costs were divided by 12 to derive a monthly cost. Moderate and severe exacerbations were treated as additional one-off costs and assumed to be the same, irrespective of the underlying GOLD stage.
Routine health-care visits
It is recommended by NICE10 that stable patients with COPD are followed up at least once a year and those with very severe COPD at least twice a year, with rapid access to hospital assessment as necessary. Based on these guidelines it was assumed that patients at GOLD stages 2, 3 and 4 would attend 1, 2 and 2.5 assessments per year, respectively, and that spirometry tests were conducted once per year in GOLD stage 2 and twice in GOLD stage 3 and 4 patients. As follow-up arrangements in primary or secondary care were not specified within the guidelines, an even split between both types of services was assumed for each severity group.
The cost of follow-up and spirometry in secondary care were obtained from NHS Reference Costs. 98 Costs for follow-up in primary care were based on the cost of a home visit by a community nurse, as published by the Personal Social Services Research Unit (PSSRU)99 and the cost of spirometry was extracted from a costing document published by NHS Commissioning Support for London. 100 Additional health-care costs included were the provision of annual influenza vaccinations, home oxygen therapy and the cost of prescribing. As the median age of the population was > 70 years, it was assumed that 75%101 of patients in each severity group received the vaccination at the current estimated cost of £6.21. 101 The average number of days and cost of home oxygen therapy received in each severity group was obtained from estimates reported in Hertel et al. ,92 derived from expert opinion.
Smoking cessation advice and pulmonary rehabilitation are also recommended by NICE as UC for patients with COPD. 10 However, these costs were assumed to be the same for both strategies, thus cancelling each other out, and were omitted from the model.
The total annual costs of health-care visits in GOLD stages 2, 3 and 4 were estimated to be £180, £332 and £453, respectively. A summary of the assumptions and reference costs applied to derive these estimates is provided in Table 18.
| Health care | GOLD 2 | GOLD 3 | GOLD 4 | Unit cost (£) | Source |
|---|---|---|---|---|---|
| Secondary care follow-up | 0.5 visit | 1 visit | 1.25 visits | 139 | NHS Reference Costs 2010/11,98 inflated to 2012 |
| Primary care follow-up | 0.5 visit | 1 visit | 1.25 visits | 57 | PSSRU 2012;99 hourly cost of a community nurse home visit |
| Secondary care spirometry | 0.5 test | 1 test | 1 test | 52 | NHS Reference Costs 2010–11,98 inflated to 2012 prices |
| Primary care spirometry | 0.5 test | 1 test | 1 test | 18 | North Central London costing report for a community-led COPD pathway100 |
| Influenza vaccination | 75% uptake | 75% uptake | 75% uptake | 6.21 | Department of Health 2011101 |
| Oxygen therapy | 0 days | 1.22 days | 6.08 days | 15 | Hertel et al.92 |
| Prescription costs per consultation (£) | 42.70 (assuming one per annum) | PSSRU 201299 | |||
| Annual cost (£) | 180.20 | 331.88 | 453.40 | ||
| Monthly cost (£) | 15.02 | 26.12 | 37.78 | ||
Routine pharmacotherapy
The NICE guidance is not prescriptive for each GOLD stage, and suggests that the number and type of treatments prescribed should be determined by patient symptoms and response. Therefore, the model utilised data from the previously described BLISS cohort for the proportion of patients on each line of therapy, by GOLD stage (Table 19). 10 As 100% of patients were reported to be on an inhaled short-acting β2-agonist (SABA), assumptions on the number of delivery devices in each severity stage were made by clinical experts. Drug reference costs reported by NICE 2011102 (Table 20) were compared with current unit costs listed on the NHS Drug Tariff database103 in 2014. Most of the drug prices were consistent with those listed in the NICE 2011 report; however, some were higher and some were lower. 102 As there did not appear to be a consistent drug inflation rate during this period (2011–14), it was not appropriate to inflate the 2011 prices or deflate the 2014 prices to estimate the costs in 2012 prices, thus the prices listed in the NHS Drug Tariff database103 for 2014 were applied. Annual and monthly costs were calculated by applying the same unit cost to annual costs reported by NICE. When there was more than one drug in each treatment class, an overall average cost was applied.
| Severity stage | Assumed no. of SABAs used per month | Proportion on type of pharmacotherapy | |||||
|---|---|---|---|---|---|---|---|
| SABA | ICS | LABA | Combinationa | LAMA | SAMA | ||
| GOLD 2 (n = 599) | 1 | 1.00 | 0.04 | 0.06 | 0.51 | 0.46 | 0.05 |
| GOLD 3 (n = 216) | 2 | 1.00 | 0.01 | 0.05 | 0.68 | 0.62 | 0.04 |
| GOLD 4 (n = 37) | 2.5 | 1.00 | 0.05 | 0.02 | 0.77 | 0.65 | 0.05 |
| Monthly cost (£) | GOLD stage 2 | 43.72 | |||||
| GOLD stage 3 | 60.91 | ||||||
| GOLD stage 4 | 67.57 | ||||||
| Class | Drug formulation and dose | Price (£) per pack (NICE 2011)102 | Price (£) per pack (NHS 2012)103 | Annual cost (£) estimated by NICE102 | Annual cost in 2012 prices (£) | Monthly cost (£) |
|---|---|---|---|---|---|---|
| SABA | Salbutamol 100 µg metered inhalation (generic) | 3.52 | 3.31 | 25.70 | 24.17 | 2.01 |
| Terbutaline 500 µg metered inhalation (Bricanyl®, AstraZeneca) | 6.92 | 6.92 | 101.03 | 101.03 | 8.42 | |
| SABA average cost | 5.22 | |||||
| ICS | Beclometasone 250 µg metered inhalation (generic) | 18.74 | 12.31 | 34.20 | 22.45 | 1.87 |
| SAMA | Ipratropium 20 µg metered inhalation (Atrovent®, Boehringer Ingelheim) | 5.05 | 5.05 | 27.65 | 27.65 | 2.30 |
| LABA | Salmeterol 25 µg metered inhalation (Serevent) | 29.26 | 29.26 | 356.00 | 356.00 | 29.67 |
| LAMA | Tiotropium 18 µg inhalation capsule (Spiriva) | 32.49 | 33.50 | 395.30 | 407.58 | 33.97 |
| LABA and ICS | Budesonide 200 µg + formoterol 6 µg metered inhalation (Symbicort® turbohaler, Astrazeneca) | 38.00 | 11.84 + 24.80 | 462.33 | 445.78 | 37.15 |
| Budesonide 400 µg + formoterol 12 µg metered inhalation (Symbicort turbohaler) | 38.00 | 13.86 + 30.06 | 462.33 | 534.36 | 44.53 | |
| Fluticasone propionate 500 µg + salmeterol 50 µg metered inhalation (Seretide® accuhaler, Allen & Hanburys Ltd) | 40.92 | 40.92 | 497.86 | 497.92 | 41.49 | |
| LABA + ICS average cost | 41.06 |
Cost of exacerbations
Moderate exacerbations were assumed to be predominantly managed in primary care through GP appointments, with a proportion attending accident and emergency (A&E) without admission. As no data were found on the split between GP and A&E visits, assumptions were derived from expert opinion reported in Hertel et al. ,92 which assumed that two out of three patients would see a GP, and one out of three patients would attend A&E. Prescribed additional medication for a moderate exacerbation was assumed to be a course of prednisolone (5 mg tablets, six times per day for 5 days) and antibiotics when exacerbations were associated with a history of purulent sputum (NICE10). The total cost of treating a moderate exacerbation was estimated to be £114, and a breakdown of how this cost was calculated is presented in Table 21.
| Resource-use item | % requiring resource | Unit cost (£) | Source of cost estimate |
|---|---|---|---|
| GP visit (12 minutes) | 66.7 | 44.40 | PSSRU99 |
| A&E visit without admission | 33.3 | 112.00 | PSSRU99 |
| Prednisolone (5-mg tablets, six times per day for 5 days) | 100 | 0.11 | NHS Drug Tariff database103 |
| Amoxicillin (Amoxil®, GlaxoSmithKline) (500-mg capsules, three times a day for 5 days) | 100 | 0.09 | NHS Drug Tariff database103 |
| Prescription costs per consultation | 100 | 42.70 | PSSRU99 |
| Estimated cost (£) of moderate exacerbation | 114.28 |
The majority of severe exacerbations were assumed to be managed in hospital but 20% were assumed to be managed through hospital-at-home or early discharge schemes. The 2011 NICE102 costing study estimated the average cost of a COPD hospital admission to be £1978. These costs were not inflated as the NHS tariff prices98 applied appeared similar to those listed in 2012. No data were available on the tariffs for hospital-at-home or early discharge schemes; however, a UK-based cost analysis estimated the costs incurred in a similar scheme to be £1653 in 2009 prices,104 inflated to £1769 for 2012. Following discussion with our clinical experts, it was assumed that 20% of those who suffered an exacerbation requiring admission accessed the non-inpatient type of service.
Guidance from NICE also recommends that patients should be followed up after discharge therefore this cost was included in the average cost of a severe exacerbation and was assumed to include one follow-up visit, 30% seen by a community nurse, 30% attending a GP appointment and 40% attending an outpatient appointment. The total cost of managing a severe exacerbation was estimated to be £2053 (Table 22).
| Resource-use item | Proportion requiring resource (%) | Unit cost (£) | Source |
|---|---|---|---|
| Average cost of COPD hospital stay | 80 | 1978 | NICE 2011102 |
| Average cost of hospital-at-home programme | 20 | 1769 | Bakerley et al.,104 inflated to 2012 prices |
| Community nurse follow-up | 30 | 57 | PSSRU99 |
| GP follow-up (12-minute visit) | 30 | 44 | PSSRU99 |
| Outpatient appointment follow-up | 40 | 139 | NHS tariff prices98 |
| Estimated cost (£) of severe exacerbation | 2053 |
Cost of self-management
The cost of providing a SM programme to patients with COPD post discharge was estimated with reference to the activities described in a sample of studies selected from review 1 in Chapter 3. Studies were chosen to reflect different levels of intensity of SM. The estimated cost of delivering a SM programme of low, moderate/high and high intensity is detailed in Tables 23–25. The costs estimated for Wong et al. 74 and Bucknall et al. 63 were estimates based on the resource use described.
| Description of activity | Resource required | Unit cost (£) | Total cost per patient (£) | Source |
|---|---|---|---|---|
| Two 10- to 20-minute telephone calls within 4 weeks of discharge; each telephone call was assumed to take 45 minutes of staff nurse time, taking into account missed calls and processing information before and after | Staff nurse time | 43 | 64.50 | Hour of staff nurse time (PSSRU 201299) |
| A senior nurse specialist supervised this service; this was 15 minutes of time per patient | Senior staff nurse time | 81 | 20.25 | Hour of senior nurse specialist (PSSRU 201299) |
| Total cost | 84.75 |
| Description of activity | Resource required | Unit cost (£) | Total cost per patient (£) | Source |
|---|---|---|---|---|
| Two 1-hour one-to-one education sessions by specialist respiratory nurse | Specialist nurse | 91 | 182 | Hour of clinical nurse specialist patient contact time (PSSRU 201299) |
| Care plan development, sharing plan with primary care team (30 minutes of nurse specialist and 30 minutes of community nurse time) | Specialist nurse | 58 | 50 | Hour of clinical nurse specialist and community nurse (PSSRU 201299) |
| Community nurse | 42 | |||
| One follow-up by respiratory care team including respiratory specialist, GP, nurse and social worker (30 minutes of each health-care professional’s time) | Specialist nurse home visit | 91 | 2440 | Hour of specialist nurse home visit time; hour of GP home visiting time; hour of community nurse time; hour of social worker for adult services time (PSSRU 201299) |
| GP home visit | 282 | |||
| Community nurse | 61 | |||
| Social worker | 54 | |||
| Four weekly telephone calls in the first month (10 minutes per call, plus 10 minutes follow-up administration) | Specialist nurse | 58 | 77.33 | Hour of clinical nurse specialist time (PSSRU 201299) |
| Two follow-up telephone calls (10 minutes per call, plus 10 minutes’ follow-up administration) | Specialist nurse | 58 | 38.67 | Hour of clinical nurse specialist time (PSSRU 201299) |
| 0.03 telephone calls per patient triggered through access to specialist case manager via telephone service (20 minutes each) | Specialist nurse | 58 | 0.58 | Hour of clinical nurse specialist time (PSSRU 201299) |
| 0.05 follow-up home visits per patient triggered by telephone calls | Specialist nurse | 91 | 3.05 | Hour of home visit by community nurse (PSSRU 201299) |
| Total cost | 597.13 |
| Description of activity | Resource required | Unit cost (£) | Total cost per patient (£) | Source |
|---|---|---|---|---|
| Four 40-minute training sessions at home from study nurse (each visit is 60 minutes community nurse specialist time) | Community nurse | 61.00 | 244.00 | Hour of community nurse home visiting time (PSSRU 201299) |
| Seven home visits every 6 weeks for 12 months (each visit takes an hour of community nurse specialist time) | Community nurse | 61.00 | 427.00 | Hour of community nurse home visiting time (PSSRU 201299) |
| Total cost | 671.00 |
Calculated costs were compared with estimates of other SM programmes that are targeted at patients with COPD but not delivered at discharge (see Appendix 20).The majority of SM programmes cost between £500 and £600, and are therefore similar to the estimate of the SM programme described by Casas et al. ;71 thus this was chosen for the base case for the moderate- to high-intensity programme. Sensitivity analyses were conducted to evaluate the cost-effectiveness of SM assuming low- and high-intensity programmes and are outlined in the sensitivity analysis subsection.
Assessment of cost-effectiveness
The incremental analysis was designed to generate the cost per additional QALY gained for SM delivered within 6 weeks of discharge when compared with UC in a cohort of patients with COPD. In summary, the key assumptions for the base case were as follows:
-
The starting cohort was assumed to be aged 72 years, 47.4% male with 39.4% current smokers (see Chapter 6, Base-case cohort).
-
The starting distribution of COPD severity was 35% GOLD stage 2, 35% GOLD stage 3 and 30% GOLD stage 4 (see Chapter 6, Base-case cohort).
-
Mortality and readmission risks during admission and immediately after discharge were taken from the European Audit84 and applied for 3 months’ post-admission (see Chapter 6, Transition probabilities within post-admission health states).
-
Long-term exacerbation, hospitalisation risk and disease progression were taken from large cohort studies of three years or more (see Chapter 6, Transition probabilities within stable health states).
-
The estimate for reduction in risk of admission with SM was taken from moderate- to high-intensity programmes (see Chapter 6, Estimate for effectiveness of self-management report).
-
Utility values were obtained from the BLISS cohort and an estimate was applied for the utility loss associated with exacerbation (see Chapter 6, Estimation of quality-adjusted life-years).
-
The cost of UC was estimated with reference to pharmacotherapy use amongst the BLISS cohort, best practice guidance, expert opinion and NHS reference prices (see Chapter 6, Resource use and costs).
-
The cost of SM was estimated to be £597, incurred in the first month and the effect was assumed to last for 2 years (see Chapter 6, Cost of self-management).
Where available, data were entered into the model as distributions so as to fully incorporate the uncertainty around parameter values in order that a probabilistic sensitivity analysis could be undertaken. Beta distributions were applied to the proportion on different treatments and accessing services in primary and secondary care; they were also applied to annual exacerbation rates and the proportion resulting in hospital admissions, as well as the risk reduction expected in the SM arm. Normal distributions were applied to utilities and utility losses. The probabilistic sensitivity analysis was run with 1000 simulations, and cost-effectiveness planes and acceptability curves were produced.
Sensitivity analysis
Additional model runs were undertaken to determine the impact of changing key parameters on the model results. Those parameters for which the incremental cost-effectiveness ratio (ICER) was demonstrated to be particularly sensitive to change were explored in more detail. The following analyses were undertaken:
-
The time horizon was varied, changing from the base-case assumption of 30 years to 6 months, 2 years, 10 years and 20 years.
-
The effect of SM on admissions was varied by substituting the base-case HR of 0.83 (95% CI 0.50 to 1.36) with two alternative HRs reported in Chapter 3. This included a HR derived from a meta-analysis of two low-intensity programmes of 0.96 (95% CI 0.49 to 1.90) and a meta-analysis that included exercise interventions representing a high-intensity programme of 0.78 (95% CI 0.52 to 1.17).
-
The duration of effect was tested for the base-case moderate-high-intensity SM programme, assuming the effect lasted for only (1) 6 months and (2) the lifetime of the cohort (see Chapter 6, Duration of effect).
-
The cost of SM was tested applying a low estimate of £85 and a high estimate of £671 (see Chapter 6, Cost of self-management).
-
An alternative set of utility scores obtained from Borg et al. 83 were applied (higher utility scores for GOLD stages 2 and 3, lower utility scores for GOLD stage 4 and a proportional deduction in utility for each severity stage lasting for 1 month in both moderate or severe exacerbation); see Table 26 (see also Utility values for chronic obstructive pulmonary disease).
-
Subgroup analysis was conducted to test if the decision rules changed if targeted at different subpopulations. This was tested by assuming that (1) all patients were GOLD stage 2; (2) all patients were GOLD stage 3; (3) all patients were GOLD stage 4; (4) there were different start ages; (5) all of the cohort were male; (6) all of the cohort were female; (7) all were smokers; (8) and all were non-smokers (see Chapter 6, Subgroup analysis).
-
Two scenario analyses were conducted: scenario 1 applied the highest estimate of effect 0.78 (95% CI 0.52 to 1.17) and the highest estimate of SM costs, £671; scenario 2 applied the lowest estimate of effect 0.96 (95% CI 0.49 to 1.90) and the lowest estimate of costs of £85 (see Chapter 6, Scenario analysis).
| Sensitivity analysis 1 | |||
|---|---|---|---|
| Stable condition | 0.7551 | 0.7481 | 0.5493 |
| Moderate exacerbation | 0.6418 (1 month) | 0.6359 (1 month) | 0.4669 (1 month) |
| Severe exacerbation | 0.378 (1 month) | 0.374 (1 month) | 0.2747 (1 month) |
Results
Base-case analysis
The base-case results presented in Table 27 show that, compared with UC, SM (delivered within 6 weeks of discharge) was more costly and resulted in better outcomes, with a £683 cost difference and gain of 0.0831 QALYs. The ICER was £8218 per QALY gained – well below the threshold values of £20,000–30,000 per QALY gained as recommended by NICE. 10
| Strategy | Mean cost (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY) | Probability cost-effective at a specified threshold (%)a | |
|---|---|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||||
| UC | 18,872 | 5.767 | |||||
| SM | 19,556 | 683 | 5.850 | 0.0831 | 8218 | 68 | 71 |
Results from the probabilistic sensitivity analysis are shown in the cost-effectiveness plane in Figure 16, which shows the distribution of 1000 resampled cost–effect difference pairs. The probabilistic sensitivity analysis clearly shows that SM is the more expensive strategy; however, the effectiveness is less certain, with a number of points indicating that SM may give fewer QALYs. The cost-effectiveness acceptability curve in Figure 17 shows that the intervention has a 68% probability of being cost-effective at £20,000 per QALY gained and 71% at a £30,000 threshold.
FIGURE 16.
Base-case cost-effectiveness plane.

FIGURE 17.
Base-case cost-effectiveness acceptability curve.
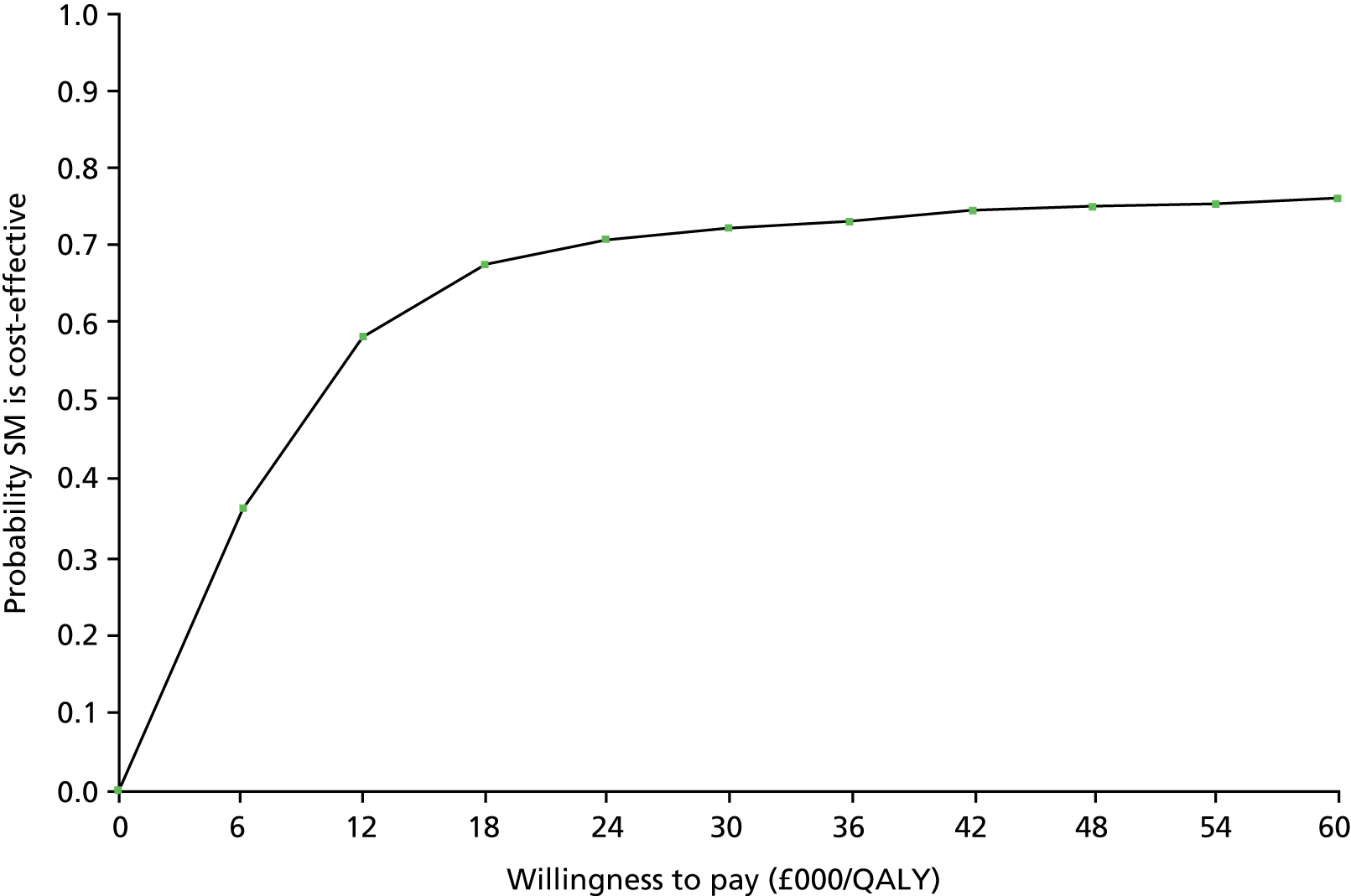
Sensitivity analysis
Alternative model time horizon
Table 28 presents the results of the model when varying the time horizon of the model. At a short time horizon of 6 months, when the intervention has been effective for 6 months, the ICER was £52,487 – above the NICE thresholds for cost-effectiveness; however, this is unlikely to represent a realistic time frame. At 2 years, the ICER reduces to £5954 as a result of most of the additional cost of implementing a SM programme being offset by savings from a reduction in hospital admissions. This changes over time, as higher costs are incurred in the surviving arm as a result of lower mortality. The probability of SM being cost-effective at £20,000/QALY remains > 62% beyond 2 years but no longer dominates UC.
| Time horizons | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|
| 6 months | 175 | 0.0033 | 52,487 | 32 | 39 |
| 2 years | 95 | 0.0160 | 5954 | 62 | 65 |
| 10 years | 489 | 0.0624 | 7838 | 65 | 72 |
| 20 years | 664 | 0.0812 | 8180 | 68 | 71 |
| Base case (30 years) | 683 | 0.0831 | 8218 | 68 | 71 |
Alternative hazard ratios for readmissions in the self-management strategy
Owing to the considerable uncertainty around the effectiveness estimate (HR) for readmissions, this model parameter was expected to be the biggest driver of the cost-effectiveness results. To test this, two alternative HRs reported in the meta-analysis for review 1 were applied in the model and the results are shown in Table 29. The first alternative HR applied was a higher estimate of the effect. This was derived from a meta-analysis that included SM interventions with an exercise component. Applying the higher estimate of effect, the ICER decreased to £6249, and the likelihood of SM being cost-effective at a threshold value of £20,000 per QALY increased to 82%. The second alternative HR applied was a lower estimate of effect derived from a meta-analysis of two low-intensity SM programmes. This increased the ICER to £38,265. This was above the threshold value of £30,000/QALY and hence the probability of SM being cost-effective at £30,000/QALY reduced to 45%.
| HR | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|
| Base case (0.83, 95% CI 0.50 to 1.36) | 683 | 0.0831 | 8218 | 68 | 71 |
| High estimate (0.78, 95% CI 0.52 to 1.17) | 676 | 0.1082 | 6249 | 82 | 84 |
| Low estimate (0.96, 95% CI 0.49 to 1.90) | 703 | 0.0184 | 38,265 | 41 | 45 |
Figure 18 illustrates the relationship between changing the point estimates of the HR and the mean ICER. At values of < 1 the ICERs are positive, and at all values of < 0.95 the ICERs are below the threshold of £30,000 per QALY. As the HR approaches 0.5, the ICER decreases and the benefits increase. At all values of > 1, SM is a less favourable option. If SM has no effect or increases the risk of hospital admission, it is dominated by UC (negative ICERs in Figure 18) hence why the ICER drops sharply. As the ratio increases to 1.5 the ICER decreases as UC becomes less cost-effective due to lower mortality in the UC arm. The 95% CIs for all three reported estimates crossed 1.
FIGURE 18.
Sensitivity analysis: relationship between HR for readmissions and ICER. a, Illustration of how the ICER changes by varying the HR between 0.5 and 1.5; and (b) magnification of how the ICER changes within the boundaries of a threshold of plus or minus £30,000 per QALY.
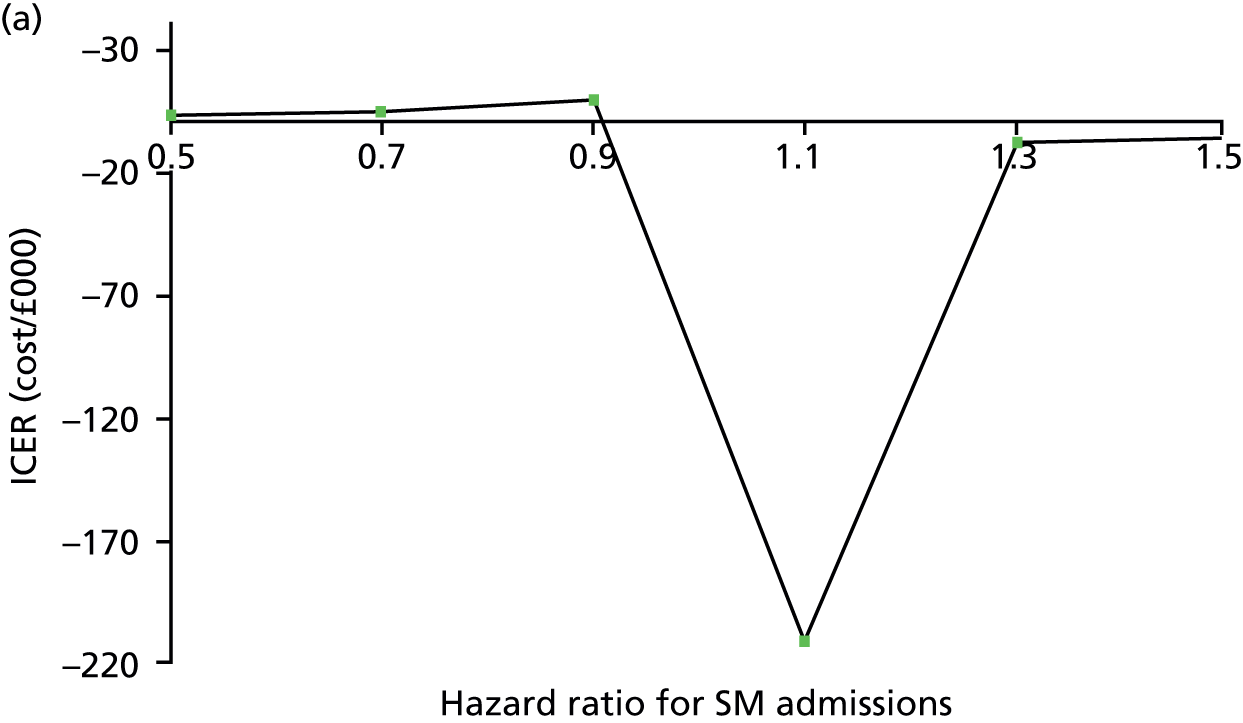
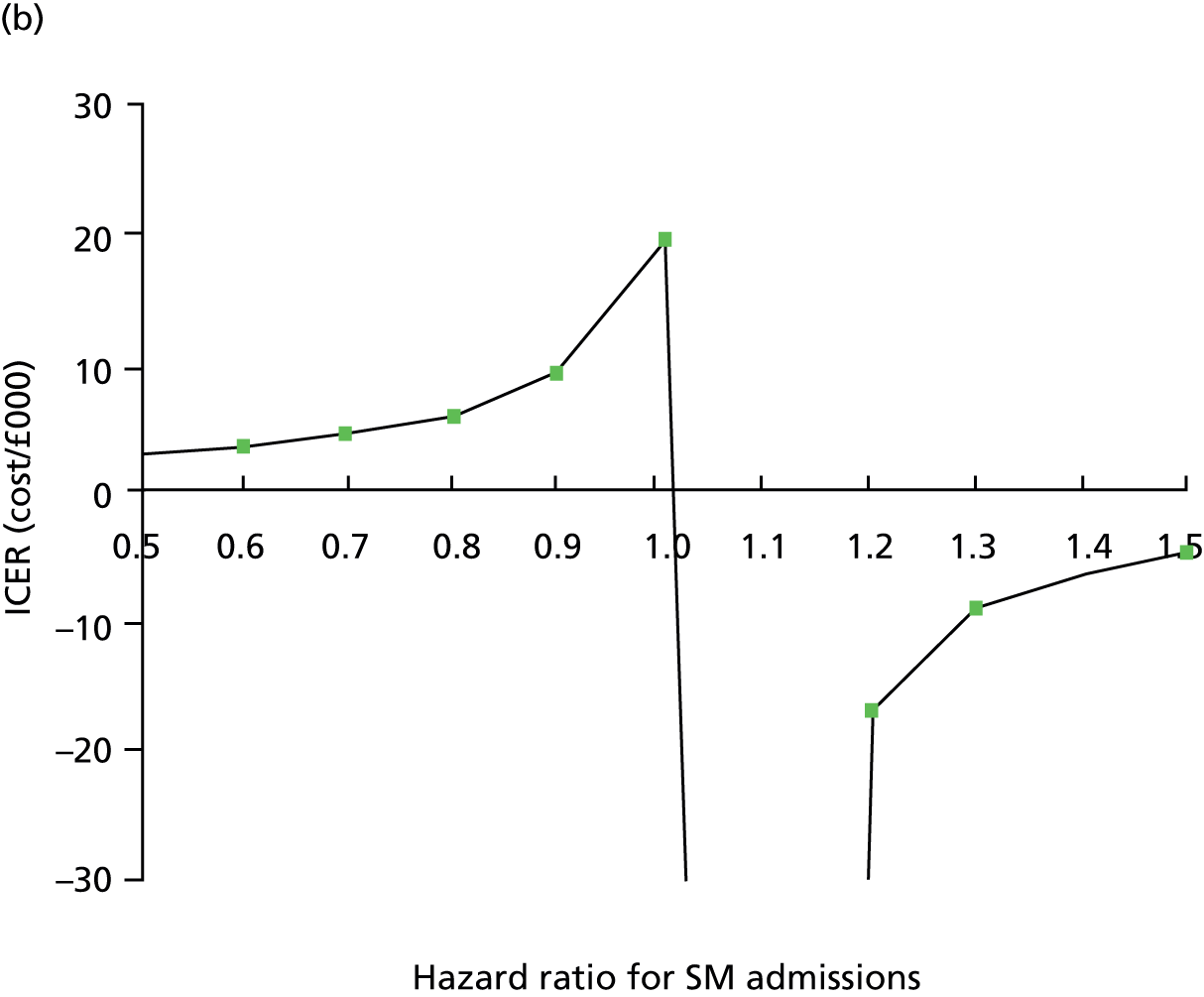
Duration of effect
Table 30 presents the results applying different assumptions regarding the duration of effect of SM support. In the base case it was assumed that the effect of SM would last for 2 years. The values were varied in the sensitivity analysis from 6 months to 30 years. When a shorter duration of effect was applied, the ICER increased and the probability of SM being cost-effective decreased. Conversely, applying a higher duration of effect decreased the ICER and increased the likelihood of SM being cost-effective.
| Duration of effect | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|
| Base case (2 years) | 683 | 0.0831 | 8218 | 68 | 71 |
| High estimate: 30 years | 383 | 0.2876 | 1333 | 77 | 77 |
| Low estimate: 6 months | 686 | 0.0414 | 16,570 | 55 | 63 |
Figure 19 illustrates the relationship between changing the duration of effect and the ICER. At between 6 and 24 months the ICERs drop sharply and the decision rule changes. Most of the studies identified in the effectiveness review were short term in nature, resulting in uncertainty on the duration of this effect.
FIGURE 19.
Sensitivity analysis: relationship between ICER and duration of effect of SM. (a) Illustration of how the ICER changes between 10 months and 30 years; and (b) magnification of how the ICER changes between 3 and 25 months.


Cost of self-management
The impact of changing the cost of SM is presented in Table 31. Applying the high estimate of £671 increases the ICER to £9257 and the probability of SM being cost-effective at a threshold of £20,000 per QALY is similar at 69%. Applying the low estimate of £85 decreases the ICER to £1033 and increases the probability that SM is cost-effective at a threshold of £20,000 per QALY to 76%.
| Cost of SM (£) | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|
| Low estimate: 85 | 86 | 0.083 | 1033 | 76 | 77 |
| High estimate: 671 | 768 | 0.0831 | 9257 | 69 | 69 |
Figure 20 shows the relationship between SM costs and the ICER. At all values for the cost of SM between £50 and £2200 the ICER is below a willingness-to-pay threshold of £30,000. At costs above £2200 the mean ICER in the base-case scenario is not cost-effective.
FIGURE 20.
Relationship between cost of SM and ICER.

Utility values for chronic obstructive pulmonary disease
The effect of applying alternative utility scores and assumptions is shown in Table 32, demonstrating that QALYs are gained in both strategy arms, irrespective of the changes in utility values for stable health states and utility loss associated with exacerbation changes. Therefore, there is little impact on QALY differences between strategies, and all estimates of the utility values for stable and exacerbating health states give similar results.
| Analysis | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY) | |
|---|---|---|---|---|---|
| SM + UC | UC | ||||
| Base case | 683 | 5.850 | 5.767 | 0.083 | 8218 |
| Values obtained in Borg et al.83 | 680 | 6.126 | 6.044 | 0.082 | 8304 |
Subgroup analysis
GOLD severity stage
Table 33 presents the ICERs when assuming that only one GOLD stage severity group entered the model. The mean difference (MD) in QALYs gained between SM and UC increased in more severe groups. The lowest ICER is £3323 per QALY gained in GOLD stage 4, with a 73% likelihood that SM is cost-effective at £20,000 per QALY. There is greater uncertainty around the cost-effectiveness of SM in patients entering the model at GOLD stage 2 or 3, with the probability of being cost-effective at £20,000 per QALY 64% or 62%, respectively.
| Cohort enter at: | Mean cost (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY) | % cost-effectiveness at: | |||
|---|---|---|---|---|---|---|---|---|---|
| SM | UC | SM | UC | £20,000/QALY | £30,000/QALY | ||||
| GOLD 2 | 15,835 | 15,245 | 591 | 7.437 | 7.367 | 0.069 | 8511 | 64 | 68 |
| GOLD 3 | 21,078 | 19,989 | 1089 | 5.783 | 5.697 | 0.086 | 12,629 | 62 | 68 |
| GOLD 4 | 22,120 | 21,803 | 317 | 4.078 | 3.983 | 0.0955 | 3323 | 73 | 75 |
Age
The starting age of the model cohort was varied and results are presented in Table 34. The probability of SM being cost-effective does not change by age but the ICERs are lower in the older cohort. Therefore, although the ICERs are different for different start ages, the decision rules are similar at all start ages and the probability of being cost-effective is similar.
| Start age (years) | Mean cost (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY) | % cost-effectiveness at: | |||
|---|---|---|---|---|---|---|---|---|---|
| SM (£) | UC (£) | SM | UC | £20,000/QALY | £30,000/QALY | ||||
| 55 | 28,747 | 27,738 | 1009 | 8.5814 | 8.464 | 0.1174 | 8591 | 66 | 71 |
| 85 | 10,409 | 10,045 | 364 | 3.1137 | 3.0681 | 0.0456 | 7980 | 67 | 71 |
Gender
The results for separate male and female cohorts are shown in Table 35. There was very little difference in the ICERs or probability of SM being cost-effective when targeted at solely men or women.
| Gender | Cost difference (£) | QALY difference | ICER (£/QALY) | % cost-effectiveness at: | |
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| Male | 642 | 0.0814 | 7895 | 70 | 73 |
| Female | 725 | 0.085 | 8534 | 68 | 71 |
Smoking status
Table 36 presents the results for current smokers and ex-/non-smokers. Again, there was very little difference in the ICERs or probability of SM being cost-effective when targeted at only smokers or ex-smokers.
| Smoking status | Cost difference (£) | QALY difference | ICER (£/QALY) | % cost-effective at: | |
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| Smoker | 679 | 0.0829 | 8188 | 67 | 71 |
| Ex-/non-smoker | 679 | 0.0829 | 8189 | 67 | 71 |
Scenario analysis
Table 37 presents the results of the scenario analysis. The first scenario applied the highest effect on reducing admissions (HR 0.78) and the highest cost estimate of SM (£671). The second scenario applied the lowest effect (HR 0.96) and the lowest estimate of the cost of SM (£85). This suggests that the likelihood of SM being cost-effective is greater in the higher cost, higher-effect scenario, relative to the lower-cost, lowest-effect scenario, but still < £20,000 per QALY.
| Scenario | Cost difference (£) | QALY difference | ICER (£/QALY) | % cost-effectiveness at: | |
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| Cost applied: £671, HR applied: (0.78) | 758 | 0.108 | 7007 | 78 | 81 |
| Cost applied: £85, HR applied: (0.96) | 107 | 0.018 | 5832 | 54 | 55 |
Discussion
Key results
This is the first economic model to consider the cost-effectiveness of SM support compared with UC in patients with COPD within 6 weeks of discharge from hospital admission for an exacerbation. Owing to the considerable uncertainty on the impact on readmissions, and the heterogeneity of the trial results, this model-based analysis should be viewed as speculative, and therefore providing only estimates of the potential impact of a SM programme.
The base-case model results suggested that SM support was a cost-effective intervention at the threshold at which NICE is willing to pay at £20,000 per QALY gained, if the assumption that the provision of SM support leads to a reduction in hospital admissions is met. The impact of reduced readmissions in the model led to lower mortality and morbidity from severe exacerbations over the long term. There were fewer costly hospital admissions, and intervention costs were relatively low compared with the cost of a readmission.
The probabilistic sensitivity analysis, which considers parameter uncertainty in the model, suggested that SM had a probability of 68% of being cost-effective at a threshold of £20,000/QALY, demonstrating the uncertainty around the impact of SM on readmissions. The remaining probability, when SM was not cost-effective, was due to the intervention potentially having worse outcomes while being more costly. Furthermore, the one-way sensitivity analyses undertaken were informative in highlighting the key drivers of the model results. As expected, cost-effectiveness was affected by the estimate of effect on readmissions, duration of effect and cost of a SM programme. The base case considered the intervention to have an impact for 2 years; however, the data from trials were collected only over the short term, for example 6 months. The results demonstrated that if the effect lasts for only 6 months then at a threshold of £20,000 per QALY gained the SM support was unlikely to be cost-effective. Currently, the model suggests that as long as the cost of the intervention is < £2200 then it is likely to be cost-effective; however, this threshold value will drop if the intervention is less effective.
Subgroup analysis, changing by considering different cohorts with regards to gender, age or smoking status, found no evidence of effect on the overall result. There was some evidence that SM might be more cost-effective in GOLD stage 4 patients. This is most likely to be due to a higher baseline risk of exacerbation and a higher proportion of exacerbations resulting in hospital admission. Therefore, a risk reduction is likely to have a greater effect.
Strengths and limitations
A key strength of this analysis is that this is the first economic model to consider the cost-effectiveness of SM in this particular patient group and illustrates the key variables that impact on the results. Although no good-quality, long-term data currently exist on the effectiveness of SM, a model structure exists for reanalysis once additional data become available.
The model structure applied was a further strength of this study. It is a modified version of previously published decision models whereby additional post-admission health states were added to incorporate emerging evidence on the higher risks in patients with COPD immediately after discharge. 13 This was thought to be particularly important for measuring costs and outcomes in this model, as the patient population were assumed to receive the intervention within 6 weeks of discharge.
Although there are concerns about the effectiveness estimates, robust data were included in the model to represent the natural history of the condition. The risks applied in the first 3 months were obtained from the UK cohort included in the European Audit84 of patients with COPD admitted to hospital. The model also captures long-term outcomes by disease severity, applying data on long-term exacerbation risks, mortality and disease progression from large longitudinal studies – TORCH85 and ECLIPSE. 13 This study also applies patient-level utility data obtained from a representative sample of UK patients (unpublished data obtained from BLISS cohort). Finally, although there was a great deal of uncertainty around effectiveness data and assumptions applied to this model, distributions were applied to reflect this uncertainty. The probabilistic sensitivity analysis was also supplemented by a one-way sensitivity analysis of all key parameters, thus demonstrating which parameters were mostly likely to influence decisions to implement SM.
There are a number of caveats when considering the results of this economic model. Most importantly, this is a speculative decision model and therefore can be considered as indicative of only the potential cost-effectiveness of SM. As highlighted in the clinical effectiveness and cost-effectiveness review, there is a dearth of high-quality evidence on the long-term costs and outcomes associated with this intervention. This model was based on this weak evidence of effect and thus incorporates considerable uncertainty when assumptions from the literature and estimates from clinical experts were applied in the absence of better-quality data.
In addition to the uncertainty around the effect of SM, there was also some uncertainty around parameters and assumptions applied in the base case for UC. Although the model was able to reflect mortality and readmission risks in the first 3 months after discharge, it was assumed that after those 3 months, those who were not readmitted would move to a stable health state. It is unclear if this really reflects natural history or if the risk of readmissions and mortality remain higher beyond 3 months among those with recent history of exacerbation. Similarly, the data extracted from the TORCH85 and ECLIPSE13 studies represent average exacerbation and hospitalisation rates in stable COPD cohorts over a 3-year period and these data were applied over a 30-year time horizon. In reality this may change over time.
The model highlights a number of areas in which further research is required. Crucially, further evidence is needed on the effectiveness of SM support to confirm if it is indeed cost-effective and with greater certainty. Longer-term evidence beyond 6 months is also required. Follow-up data on cohorts of patients admitted to hospital is needed to provide better estimates of long-term outcomes. A review of costs applied of other SM programmes in patients with COPD suggests that the cost of implementing SM support are likely to range somewhere between £8568 and £671. 63 Although more research is required to develop more accurate cost estimates for implementing SM programmes in this cohort, this is unlikely to change the outcome of this analysis. Finally, better data are required on utility values in COPD populations, particularly the utility loss associated with exacerbation.
Although outside the scope of this report – in light of the uncertainty around the effectiveness and cost-effectiveness of the intervention – it would be beneficial for a value of information analysis to be undertaken in the future. Value of information analysis allows a comparison of the potential benefits of additional research with the costs of further investigation. The value of any further research is based on how much this extra information will reduce the overall decision uncertainty.
Summary
-
Currently, there is no published evidence on the cost-effectiveness of SM compared with UC in patients with COPD who have recently been discharged from hospital.
-
This is the first economic model to attempt to estimate the cost-effectiveness of SM in this patient group.
-
This speculative model indicates that SM is cost-effective if it is assumed that the intervention has a small positive effect on reducing admissions for a minimum of 6 months.
-
The model has a number of limitations, the most important related to the large amount of uncertainty around the effectiveness estimate driving the model results.
-
The analysis highlights the importance of conducting further research on the effect and duration of effect of the SM intervention delivered post discharge to allow a more robust analysis of cost-effectiveness.
Chapter 7 A systematic review to identify the features and elements of self-management support interventions that are most effective: review 4
The aim of this chapter is to present the findings from a broad systematic review to assess the effectiveness, and identify the most effective components, of self-management (SM) interventions.
Methods
A systematic review of the evidence of effectiveness of interventions to support SM among patients with chronic obstructive pulmonary disease (COPD), at any time point, to identify the features and elements that are most effective.
Definition of self-management
As described for review 1 and tabulated in Table 2.
Search strategy
A comprehensive search strategy described as for review 1. Only citation lists of relevant reviews were examined for additional relevant studies.
Study selection process
As described for review 1.
Selection criteria
In contrast with the first review, owing to the likely high volume of relevant studies, the selection criteria included only RCTs and a more limited range of outcomes (Table 38). Although RCTs purely of smoking cessation were excluded, trials described as ‘pulmonary rehabilitation (PR)’ were included, as many PR trials include components of SM and aim to enable participants to self-manage their condition after the PR programme ends. Furthermore, many interventions describe supported SM with a supervised structured exercise programme, which is similar to PR. There is a large overlap of intervention content even with different definitions and we wanted to include as complete a range of evidence as possible.
| Study designs | RCTs |
|---|---|
| Population | Any patients with moderate to severe COPD (defined clinically with or without spirometry) including those in the stable state (patients with mild or very severe COPD were included if they were a minority of the population group) > 90% of patients in studies had COPD The setting could be either hospital or community |
| Intervention | SM packages, larger packages of care that included a significant component of SM (e.g. PR) or important components of SM Excluding trials of smoking cessation |
| Comparator (where appropriate) | No intervention, usual care, control/sham, other SM intervention |
| Primary outcomes | Exacerbations Hospital admissions/readmissions HRQoL |
| Secondary outcomes | Mortality Anxiety, depression Exercise capacity Lung function Health service utilisation ED visits Dyspnoea |
Risk of bias assessment
As for review 1, all of the RCTs were assessed using the Cochrane Risk of Bias tool. 56 Assessment was limited to primary outcomes. All studies were assessed by one independent reviewer, with a second reviewer independently checking at least 10% of studies, and a third reviewer overseeing the complete process.
Data extraction
Approach
Data extraction of study characteristics was undertaken by a single reviewer, except for key fields such as sample size, duration of intervention and duration of follow-up, which were extracted in duplicate on to a piloted table of characteristics. The components of interventions were mapped by a single reviewer after the research team had each mapped 30 studies and discussed discrepancies and component definitions/criteria. For the results, data were extracted only from papers that reported any of the three primary outcomes (QoL, hospital admissions/readmissions or exacerbations). The reporting in papers of secondary outcomes (mortality, anxiety, depression, exercise capacity, lung function, health-care utilisation, ED visits and breathlessness) was documented but the results were not extracted. Owing to high volume, one reviewer extracted all of the data on to piloted tables using Microsoft Excel version 2010 (Microsoft Corporation, Redmond, WA, USA), with at least 10% of the extracted data being checked by a statistician. If necessary any differences were resolved via discussion with a third reviewer. When relevant data were lacking or unclear, authors were contacted via e-mail.
Types of data extracted
As described for review 1.
Data manipulation
As described for review 1.
Analyses
Owing to a large volume of literature, only those papers that reported any one of the three primary outcomes were taken forward for further analyses:
-
HRQoL scores including subdomain scores
-
numbers of/time to first hospital admissions/readmissions
-
numbers of/time to first exacerbation/s.
Papers with secondary, but no primary, outcomes were tabulated only.
Analyses consisted of mapping and description of the features and elements of the interventions from the included papers; presentation of the results of various combinations and comparisons of components on forest plots; meta-analysis of the data where appropriate; meta-regression; and subgroup analysis to explore heterogeneity.
Description of the features and elements of self-management interventions by simple categorisation and tabulation
To describe the features and elements of SM interventions, a mapping process was undertaken whereby interventions were broken down into a visual representation of components (defined in Table 39). Components included disease knowledge/education, exercise, breathing techniques, smoking cessation and inhaler technique among others. Each component was subdivided into either an information element only or a support/training element. All treatment arms of the trials were mapped in this way. The numbers of components within intervention and control arms were identified.
| Component | Broad inclusion/definition | |
|---|---|---|
| Disease knowledge | Education about disease, disease management, treatments, SM, chronic illness, activities of daily life, end of life, self-care tips, travel and COPD | |
| SM unspecified | SM education/skills | |
| RMT | IMT, EMT (pressure, threshold, resistance devices) | |
| Action planning | Managing exacerbations, coping plan, management of COPD symptoms, recognising when to call a doctor | |
| Breathing management and techniques | Breathing exercises, breathing retraining, respiratory biofeedback, managing breathlessness and coping with triggers for breathlessness, t’ai chi, vocal exercises | |
| Smoking cessation | Advice, counselling, groups, interventions to help reduce/quit smoking as required | |
| Medication/adherence | Information about medication and adherence, promoting adherence (pharmacological or non-pharmacological) | |
| Bronchial hygiene techniques | Postural drainage/coughing technique | |
| Nutrition | Advice, counselling, groups, supplements as required | |
| Psychological intervention | Psychosocial support, cognitive–behavioural therapy, cognitive training, relaxation (including exercises, e.g. progressive muscle relaxation), stress management, general goal-setting, mood disturbance, handling emotions (how to cope with the disease), psychosocial problems associated with respiratory disability, self-talk and panic control, health, qigong | |
| Preventative | Avoiding exacerbations, pollution and environmental hazards, managing infections, personal hygiene | |
| Inhaler technique and use | Assessing inhaler technique, teaching correct use and handling of inhalers | |
| Energy conservation | Pacing and good posture, home modifications and ADL, work simplification | |
| Support groups/patient empowerment | Peer support self-help groups/networks, e.g. Breathe Easy, developing confidence to negotiate with clinicians | |
| Exercise | Strength | Upper limb, lower limb strength/resistance exercises |
| Aerobic | Cycling, walking, stair climbing as aerobic/endurance exercises | |
| Other | Flexibility and balance exercises, sham training, unspecified exercises | |
| Enhanced access/care | Access to health professionals, access to call centre/hotline, health professional home visits and/or telephone support | |
| Other | Any miscellaneous uncommon components, e.g. sleep or other symptom control | |
| Usual care | Usual medications and visits to GP or routine secondary care | |
Exploring significant components of self-management interventions in reducing exacerbations, hospital admissions/readmissions and improving quality of life
Planned analyses and comparisons
To explore the effectiveness of different SM components (or groups of components), a series of 18 analyses were planned (Table 40) in collaboration with the steering group to ensure clinical relevance. The analysis plan was developed prior to collation of any of the data and followed two main objectives:
To:
-
explore clinically relevant interventions
-
avoid repeating any recent high-quality systematic review, such as a Cochrane review.
| Intervention | Comparator |
|---|---|
| 1. Multicomponent interventions | UC/control |
| 2. Addition of one component | |
| 3. Exercise-only interventions | UC/control/sham intervention |
| 4. Enhanced care | |
| 5. Multicomponent interventions with supervised exercise | UC/control |
| 6. Multicomponent interventions with structured unsupervised exercise | UC/control |
| 7. Multicomponent interventions with exercise counselling only | UC/control |
| 8. Multicomponent interventions without an exercise element | UC/control |
| 9. Multicomponent interventions including an exercise component consisting of aerobic and strength training | UC/control |
| 10. Strength and aerobic exercise training | Aerobic training only |
| 11. Endurance/aerobic training | Strength/resistance training |
| 12. Upper limb and lower limb training | Lower limb training only |
| 13. Interval training | Continuous training |
| 14. IMT or EMT | UC/control/sham intervention |
| 15. More sessions/longer-duration interventions | Fewer sessions/shorter duration interventions |
| 16. Hospital-based interventions | Home-based interventions |
| 17. Pharmacist-delivered interventions | |
| 18. Maintenance programme post PR | No maintenance programme post PR |
We explored the effectiveness of any single component interventions that were delivered either alone or as part of a wider package for which the only difference between the two arms was this single component. A multicomponent SM package was included in many analyses and we defined multicomponent as including three or more relevant components. The definition of three components was used because most exercise programmes would require some discussion of managing breathlessness. From a clinical perspective it seemed likely that some interventions would describe both components and others only the exercise component.
To avoid repeating current systematic reviews, we chose not to explore the effectiveness of integrated care but instead explored the effects of ‘enhanced care’. We defined ‘enhanced care’ to be interventions that gave patients access to additional contact with health-care professionals through regular telephone contact or visits. This is distinct from integrated care, which required delivery by a multidisciplinary team.
The effectiveness of exercise-only interventions was explored by examining different combinations of exercise (e.g. strength, aerobic, and combined strength and aerobic exercises). The inclusion of these different modes of exercise is important for professionals developing and delivering SM and rehabilitation programmes for COPD. Exercise as part of multicomponent packages was categorised into groups of supervised exercise (which mirrors PR), unsupervised exercised (mirroring home-based rehabilitation programmes) and exercise education only.
As well as the components, we were also interested in delivery mechanisms. These were discussed and agreed by the steering group before any analyses were undertaken. We considered that the location of the intervention was an important delivery issue, for example hospital or centre-based compared with a home-based programme and the duration or intensity of programmes to be important delivery issues also. To explore these questions we sought trials that had direct comparisons.
Post hoc analyses were decided upon after mapping the content of the SM intervention components. Post hoc analyses included an exploration of the effectiveness of interventions delivered by pharmacists and the effectiveness of maintenance programmes post PR.
Presentation on forest plots
For each analysis, the first stage involved presenting extracted study results on forest plots alongside key study characteristics so that the wider team could determine whether it was sensible to performmeta-analysis. For each intervention, the effectiveness across each outcome was presented. Data were presented in forest plots when there were ≥ 10 studies. As there were multiple follow-up points, results were divided into three time periods: up to and including 3 months; above 3 months to 6 months; and beyond 6 months since the start of the study. If a study had more than one follow-up point within each time period, the latest follow-up within the period was used.
All forest plots were then ordered according to the number of components in the intervention arm, followed by the length of follow-up and then alphabetically by author name.
Owing to a large volume of different QoL measures used, only data from the disease-specific total SGRQ and CRQ were included in the forest plots. In this review, SGRQ is presented on a reversed scale (i.e. higher scores are better).
For QoL data, the numbers of patients followed up were displayed, as well as baseline differences between intervention and control arms, and whether or not ANCOVA was used to adjust for the baseline value. For plots of HRs, details were also displayed of whether or not the effect size was used directly from data within the trials or whether or not they were estimated using other available data.
Meta-analysis methods
As described for review 1, but in addition to the summary estimate and its 95% confidence interval (CI), each random-effects analysis was also summarised by reporting a 95% prediction interval. This predicts how the effectiveness of the intervention could vary from the average in different circumstances, for example for different contexts, populations and lengths of follow-up. 62,105 This is important to ascertain whether the intervention is likely to work in the majority of settings, or whether – due to unexplained heterogeneity – the intervention may work well in some settings but work less well (or not at all) in other settings. Prediction intervals were calculated where there were five or more studies per analysis and were tabulated separately from the forest plots.
Assessing publication bias
For each meta-analysis containing ≥ 10 studies, the likelihood of publication bias was investigated through the construction of funnel plots and Egger’s test for ‘small-study effects’, i.e. the tendency for smaller studies to provide more positive findings. It is important to note that when heterogeneity exists, publication bias may be one of a number of reasons for any small-study effects identified. The restriction of 10 studies was due to the low power of identifying small-study effects with few studies. 106
Meta-regression and subgroup or sensitivity analyses
For each meta-analysis, if there were sufficient numbers of studies (at least 10 per meta-analysis), meta-regression was considered to explore whether the following prespecified variables explained any of the heterogeneity: severity of disease in the study population, length of intervention, number of components of intervention and study quality.
Mixed-treatment comparisons
Although mixed-treatment comparison meta-analyses were planned, the assumptions to undertake the analysis were not considered to have been met. In particular, the large heterogeneity in follow-up time, the included patient population and the study design suggested that the consistency assumption required was unlikely to be sensible. 107 Therefore, no mixed-treatment comparisons were explored.
Patient advisory group
See review 1 for details.
Search results
Included studies
From 13,355 identified titles, 836 full papers were obtained and 283 papers were finally included. Of these, 174 RCTs from 194 papers reported one of the three primary outcomes: HRQoL, hospital admissions/readmissions and exacerbations (Figure 21). A total of 89 papers reported outcomes other than our three primary outcomes and are listed in Appendix 21 alongside the secondary outcomes that they reported. Overall, 553 papers were excluded (see Appendix 2 for full list with reasons for exclusion). Arbitration by a third reviewer was required for 5% of all full texts. In total, 40 ongoing studies were identified as relevant (see Appendix 4).
FIGURE 21.
Summary of the selection process for clinical effectiveness studies.

Within the 174 trials with primary outcomes several studies had multiple arms. Thus there were 229 comparisons of interventions compared with usual care (UC), control or another active intervention.
Characteristics of studies
The study and population characteristics of the 174 included RCTs with relevant primary outcomes are summarised in Appendix 22.
Country/setting/recruitment
The majority of the trials were set in high-income countries, with 33 (19%) from the USA and 21 (12.1%) from the UK. However, trials were set in 31 different countries, including eight from China, six from Hong Kong, three from India and two from the Republic of Korea. A breakdown is given in Table 41.
| Country | n | % |
|---|---|---|
| USA108–140 | 33 | 19.0 |
| UK63,73,141–159 | 21 | 12.1 |
| Australia67,69,160–170 | 13 | 7.5 |
| Spain68,171–180 | 12 | 6.9 |
| The Netherlands181–190 | 10 | 5.8 |
| Canada191–198 | 8 | 4.6 |
| China74,199–205 | 8 | 4.6 |
| Germany64,206–211 | 7 | 4.0 |
| Hong Kong66,70,212–215 | 6 | 3.4 |
| Sweden216–221 | 6 | 3.4 |
| Denmark222–226 | 5 | 2.9 |
| New Zealand227–231 | 5 | 2.9 |
| Brazil232–235 | 4 | 2.3 |
| Turkey236–239 | 4 | 2.3 |
| India240–242 | 3 | 1.7 |
| Italy75,243,244 | 3 | 1.7 |
| Austria245,246 | 2 | 1.1 |
| Belgium247,248 | 2 | 1.1 |
| France249,250 | 2 | 1.1 |
| Ireland251,252 | 2 | 1.1 |
| Israel253,254 | 2 | 1.1 |
| Norway255,256 | 2 | 1.1 |
| Switzerland257,258 | 2 | 1.1 |
| Taiwan259,260 | 2 | 1.1 |
| Japan261,262 | 2 | 1.1 |
| Argentina263 | 1 | 0.6 |
| Egypt264 | 1 | 0.6 |
| Greece265 | 1 | 0.6 |
| Jordan266 | 1 | 0.6 |
| Korea267 | 1 | 0.6 |
| Republic of Korea268 | 1 | 0.6 |
| Spain and Belgium70 | 1 | 0.6 |
| Venezuela269 | 1 | 0.6 |
Size
The sample size of the 174 included trials ranged from 10 to 743 [median 53, interquartile range (IQR) 38–100]. Trials were generally small with 81 (46.6%) trials including < 50 participants, 47 (27.0%) with 50–99 participants, 34 (19.5%) with 100–199 participants, and 12 (6.9%) with ≥ 200 participants (Table 42).
| Size of trial | n | % |
|---|---|---|
| < 25 | 13 | 7.5 |
| 25–49 | 68 | 39.1 |
| 50–74 | 29 | 16.7 |
| 75–99 | 18 | 10.3 |
| 100–149 | 23 | 13.2 |
| 150–199 | 11 | 6.3 |
| 200+ | 12 | 6.9 |
Population characteristics
Table 43 summarises the characteristics of the populations in the trials. The characteristics reported were frequently of those who completed the trial rather than those who were randomised.
The mean age of the participants was between 52 and 80 years, with the majority of trials (72%) reporting a mean age of between 60 and 69 years. The proportion of male participants ranged from 15% to 100%. In the trials that provided data on the gender of the participants, males tended to be in the majority. Thirty-four trials66,67,69,70,74,111,112,118,127,133,136,142,144,151–153,168,185,192,195,199,201–205,209,210,212,214,218,238,264,270–272 did not report the FEV1% pred but reported the proportions within severity groups. Of the trials that did provide these data, the mean FEV1% pred of the trial participants ranged from 26.3% to 69.0%. More than half of trials had a population mean in the 30–59% range, which is equivalent to GOLD stage 3 – severe COPD.
Recruitment of participants was mainly from secondary care or PR programmes.
| Characteristic | n (%) |
|---|---|
| Age (mean, years) | |
| 50–59 | 11 (6.3) |
| 60–69 | 111 (63.8) |
| 70–79 | 29 (16.7) |
| 80+ | 1 (0.5) |
| NR | 22 (12.6) |
| Males (n, %) | |
| 1–25 | 4 (2.3) |
| 26–50 | 36 (20.7) |
| 51–75 | 62 (35.6) |
| 75–100 | 51 (29.3) |
| NR | 21 (12.1) |
| FEV1% pred (mean) | |
| 50–79 | 44 (25.3) |
| 30–49 | 90 (51.7) |
| < 30 | 5 (2.9) |
| NR | 35 (20.1) |
| Recruited from: | |
| Secondary care inpatient | 15 (8.6) |
| Secondary care outpatient/unspecified | 83 (47.7) |
| ED | 1 (0.5) |
| PR programme/referred | 21 (12.1) |
| Primary care | 9 (5.2) |
| Primary and secondary care | 3 (1.7) |
| Community | 3 (1.7) |
| Primary or secondary care and advertisement | 18 (10.3) |
| NR/unclear | 21 (12.1) |
Follow-up of trial participants
Length of follow-up ranged from 4 weeks to 2 years from the start of the intervention. In 78 (44.8%), follow-up was ≤ 3 months, in 120 (69.0%) it was ≤ 6 months and in 174 (94.3%) it was ≤ 1 year. Twelve trials65,116,155,166,172,182,190,248,271,273–277 had follow-up of > 1 year (Table 44).
Time from the end of intervention (delivery of last element of SM support) to last follow-up varied considerably (see Table 44). A total of 106 (60.9%) of the trials reported follow-up data only at the end of the intervention period (details provided within appendices). Only 18 trials111,121,141,161,166,170,172,192,195,198,210,213,217,229,250,251,270,271,276–279 (10.9%) reported a follow-up of > 6 months after the end of the intervention.
| Time to last follow-up | n (studies) | % |
|---|---|---|
| Time to last follow-up (weeks) | ||
| ≤ 13 | 76 | 43.7 |
| 14–26 | 40 | 23.0 |
| 27–52 | 44 | 25.3 |
| > 52 | 12 | 6.9 |
| Unclear | 2 | 1.1 |
| Time from end of intervention to last follow-up (weeks) | ||
| 0 | 106 | 60.9 |
| ≤ 13 | 27 | 15.5 |
| 14–26 | 16 | 8.6 |
| 27–52 | 14 | 8.0 |
| > 52 | 4 | 2.3 |
| Unclear | 7 | 4.0 |
Interventions
The interventions were very heterogeneous. They included structured group-based PR programmes; more limited one-to-one educational SM interventions delivered in an outpatient setting or at a patient’s home, sometimes with telephone follow-up; integrated disease management with multidisciplinary input and often some element of monitoring by health professionals; exercise-only interventions (with some dyspnoea management) and respiratory muscle training (RMT) using threshold devices. Within these various broad categories, there was a range of individual SM components, including some that might be less traditionally part of SM, such as qigong, t’ai chi and singing. Appendix 23 provides detailed descriptions of the intervention and comparator groups with intensity and frequency of interventions delivered.
Description of self-management components in intervention and comparator arms
Within the arms of the 174 trials we categorised 15 types of components (plus other and unspecified). In the intervention groups exercise was the most commonly reported component (76.9%), followed by breathing techniques and management of dyspnoea (64.2%), and general education about COPD and its management (47.2%). Details of the numbers of individual components for the intervention and comparator groups are shown in Table 45. Appendix 24 displays which components were present within the intervention and comparator groups of each study.
| Component | Intervention (no. of studies) | Comparator (no. of studies) |
|---|---|---|
| n (%) | n (%) | |
| Exercise | 176 (76.9) | 96 (41.9) |
| Breathing techniques/dyspnoea management | 147 (64.2) | 52 (22.7) |
| Disease knowledge | 108 (47.2) | 68 (29.7) |
| Psychological including relaxation and stress management | 77 (33.6) | 34 (14.8) |
| Medication advice | 77 (33.6) | 43 (18.8) |
| Nutrition advice | 51 (22.3) | 28 (12.2) |
| Enhanced access | 50 (21.8) | 15 (6.6) |
| Action planning for self-treating exacerbations | 43 (18.8) | 9 (3.9) |
| Smoking cessation advice/support | 44 (19.2) | 18 (7.9) |
| Inhaler technique | 36 (15.7) | 19 (8.3) |
| Bronchial hygiene/secretion clearance techniques | 30 (13.1) | 16 (7.0) |
| Unspecified | 24 (10.5) | 9 (3.9) |
| RMT | 32 (14.0) | 11 (4.8) |
| Energy conservation | 22 (9.6) | 7 (3.1) |
| Other | 18 (7.9) | 5 (2.2) |
| Preventative measures to avoid infection | 18 (7.9) | 11 (4.8) |
| COPD support groups | 7 (3.1) | 3 (1.3) |
Up to 13 different SM components were included in any one of the intervention arms, and up to 11 in any one of the comparator groups (Table 46). Seventy-three (31.9%) of the intervention arm interventions had six or more components. In the intervention group, 38 (16.6%) were single components with the vast majority of these being exercise-only interventions. In contrast, the majority of the comparators had two or fewer described components [167 (72.9%)], with 34.9% not providing any detail about the SM education or support provided to the comparator group as part of UC (see Table 46).
| No. of components in the comparator groups | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total, n (%) | ||
| No. of components in the intervention groups | 1 | 10 | 22 | 2 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 38 (16.6) |
| 2 | 20 | 18 | 13 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 54 (23.6) | |
| 3 | 7 | 5 | 11 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29 (12.7) | |
| 4 | 6 | 3 | 2 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 (8.3) | |
| 5 | 8 | 2 | 1 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 16 (7.0) | |
| 6 | 7 | 3 | 0 | 0 | 3 | 1 | 6 | 1 | 0 | 0 | 0 | 0 | 21 (9.2) | |
| 7 | 8 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 18 (7.9) | |
| 8 | 6 | 0 | 0 | 1 | 2 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 14 (6.1) | |
| 9 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 5 (2.2) | |
| 10 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 7 (3.1) | |
| 11 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 5 (2.2) | |
| 12 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 (0.9) | |
| 13 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.4) | |
| Total no. of studies | 80 (34.9) | 57 (24.9) | 30 (13.1) | 17 (7.4) | 14 (6.1) | 2 (0.9) | 12 (5.2) | 8 (3.5) | 4 (1.7) | 2 (0.9) | 1 (0.4) | 2 (0.9) | 229 | |
The content of the components of the intervention are shown according to the total number of components in the intervention in Table 47. Of the single-component interventions, 25 of 38 (65.8%) were exercise only, 9 of 38 (23.7%) were RMT and three (7.9%) were breathing exercises. In the two- and three-component interventions, exercise is frequently combined with breathing/dyspnoea management and disease knowledge. Overall, the most common components were exercise [176 (76.9%) of studies], breathing techniques and dyspnoea management [147 (64.2%)], disease knowledge [108 (47.2%)] and psychological interventions [77 (33.6%)].
In those interventions with six or more components, the most common components were exercise [69 (94.5%) of studies], breathing techniques and dyspnoea management [66 (90.4%)], disease knowledge [65 (89.0%)] and medication advice [60 (82.5%)]. Notably, smoking cessation was mentioned in only 38 (52.1%) of the interventions with six or more components.
| Intervention components | No. of SM components in intervention | Total no. of studies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
| Action planning for self-treating exacerbations | 0 | 3 | 2 | 2 | 4 | 2 | 7 | 6 | 4 | 7 | 3 | 3 | 1 | 43 |
| Breathing techniques/dyspnoea management | 3 | 37 | 16 | 15 | 10 | 18 | 16 | 12 | 5 | 7 | 5 | 24 | 1 | 147 |
| Bronchial hygiene/secretion clearance techniques | 0 | 0 | 0 | 4 | 2 | 3 | 5 | 4 | 1 | 3 | 5 | 2 | 1 | 30 |
| Disease knowledge | 0 | 8 | 11 | 10 | 14 | 17 | 16 | 13 | 4 | 7 | 5 | 2 | 1 | 108 |
| Energy conservation | 0 | 0 | 0 | 2 | 0 | 3 | 1 | 8 | 1 | 2 | 4 | 1 | 0 | 22 |
| Enhanced access | 0 | 4 | 6 | 3 | 6 | 4 | 8 | 7 | 3 | 4 | 3 | 1 | 1 | 50 |
| Exercise | 25 | 37 | 21 | 14 | 10 | 18 | 17 | 14 | 5 | 7 | 5 | 2 | 1 | 176 |
| Inhaler technique | 0 | 0 | 0 | 1 | 2 | 4 | 8 | 7 | 4 | 5 | 2 | 2 | 1 | 36 |
| Medication advice | 0 | 1 | 6 | 3 | 7 | 16 | 13 | 13 | 5 | 6 | 5 | 1 | 1 | 77 |
| Nutrition advice | 0 | 2 | 3 | 1 | 4 | 8 | 10 | 6 | 4 | 5 | 5 | 2 | 1 | 51 |
| Preventative measures to avoid infection | 0 | 0 | 3 | 0 | 0 | 2 | 2 | 3 | 2 | 2 | 2 | 1 | 1 | 18 |
| Psychological including relaxation and stress management | 1 | 3 | 8 | 14 | 8 | 14 | 8 | 7 | 2 | 4 | 5 | 2 | 1 | 77 |
| RMT | 9 | 7 | 7 | 1 | 0 | 3 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 32 |
| Smoking cessation advice/support | 0 | 0 | 0 | 1 | 5 | 6 | 9 | 8 | 4 | 5 | 3 | 2 | 1 | 44 |
| COPD support groups | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 7 |
| Unspecified | 0 | 0 | 1 | 4 | 3 | 6 | 0 | 3 | 0 | 4 | 1 | 1 | 1 | 24 |
| Other | 0 | 6 | 3 | 1 | 4 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 18 |
Figure 22 displays the range of different interventions included.
FIGURE 22.
Range of interventions included across 174 trials.
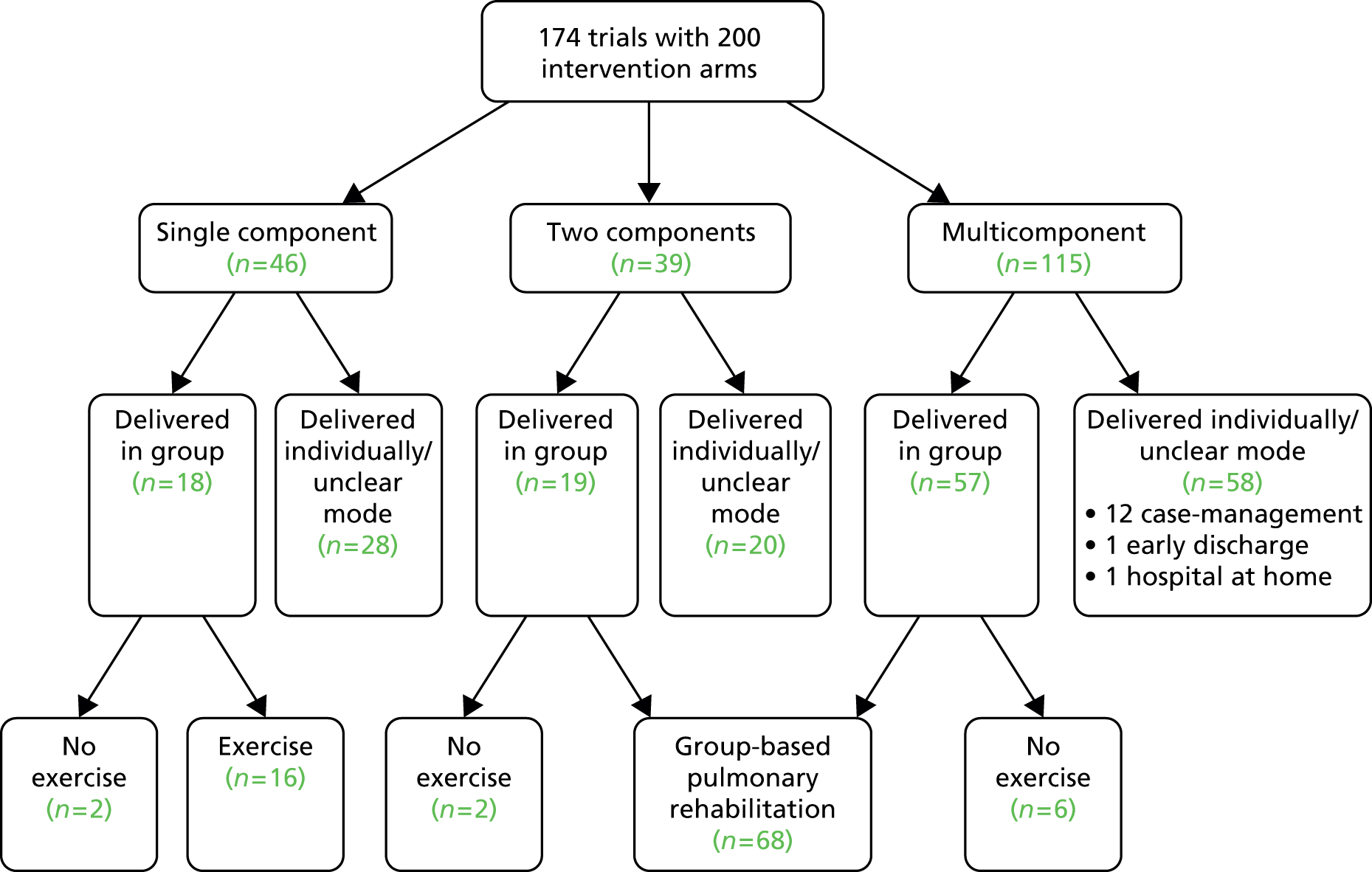
Duration of the intervention
The duration of the intervention was measured to the last behavioural or supportive contact and ranged from 1 day to 2 years (Table 48). A total of 114 trials (58.8%) reported interventions of ≤ 3 months’ duration, with nine trials (4.6%) being longer than 1 year. Five trials71,72,117,142,215,255,280,281 did not report an intervention duration or had a variable duration intervention (see Appendix 23).
| Intervention duration (weeks) | No. of studies | % |
|---|---|---|
| < 1 | 2 | 1.1 |
| 4 | 19 | 10.9 |
| 5–8 | 59 | 33.9 |
| 9–13 | 34 | 19.5 |
| 14–26 | 29 | 16.7 |
| 27–52 | 22 | 12.6 |
| 53–104 | 4 | 2.3 |
| NR/variable duration | 5 | 2.9 |
Mode of delivery of the intervention
The majority of the interventions were delivered by nurses and respiratory physiotherapists. Half of the interventions had a group-based component; 63 (36.2%) were entirely group based; and an additional 24 (13.8%) had a group component followed by individual support. In 20 studies the mode of delivery was unclear. Details of the mode of delivery are in Table 49 and in the detailed characteristics of intervention table in Appendix 23.
| Mode of delivery | n (studies) | % |
|---|---|---|
| Group based | 63 | 36.2 |
| Individual | 63 | 36.2 |
| Mixed: group and one to one | 24 | 13.8 |
| Remote (internet/telemonitoring) | 4 | 2.3 |
| Unclear | 20 | 11.5 |
| Total | 174 | 100.0 |
Comparator arms
There were 141 comparisons (from 127 trials) of an intervention compared with UC or a control group that was not an active intervention. The UC arm was frequently not described; in other cases it was the standard primary and/or secondary care for people with COPD.
A total of 107 comparisons (from 85 trials) were of two active interventions. Details are provided in Appendix 23.
Primary outcome measures
Most trials (163, 96.6%) reported HRQoL; 42 (24.1%) reported hospital admissions or readmissions and only 20 (11.5%) reported exacerbations.
Other outcome measures reported
The included studies reported a wide range of outcomes; 12 reported mortality, 103 dyspnoea, 34 anxiety and 41 depression outcomes. Exercise capacity was reported in 135 studies and lung function in 92 studies. Health service utilisation was reported in 41 trials and ED visits in 29 trials. The details of which trial reported which outcomes are displayed in Appendix 25.
Risk of bias of included studies
Table 50 summarises the risk of bias. Details of the risk of bias assessment for all of the included studies are tabulated in Appendix 26.
| Risk of bias | Low, n (%) | High, n (%) | Unclear, n (%) | Total |
|---|---|---|---|---|
| Sequence generation | 66 (37.9) | 0 (0) | 108 (62.1) | 174 |
| Allocation concealment | 27 (15.5) | 1 (0.6) | 146 (83.9) | 174 |
| Blinding of HRQoL outcome | 34 (19.5) | 117 (62.7) | 23 (13.2) | 174 |
| Blinding of admission outcome | 44 | 0 | 1 | 45 |
| Incomplete outcome data | 46 (26.4) | 83 (47.7) | 45 (25.8) | 174 |
| Selective outcome reporting | 55 (31.6) | 2 (1.1) | 117 (67.2) | 174 |
| Other biases | 44 (25.3) | 86 (49.4) | 44 (25.3) | 174 |
Reporting of the method of generating the randomisation sequence was generally poor, with only 71 (36%) of the trials providing adequate information. Where reported, the randomisation method was adequate to produce a low risk of bias. Similarly, the majority of studies [146 (84%)] did not provide sufficient information about allocation concealment to be able to determine the risk of bias. We considered the risk of bias for self-reported HRQoL to be high unless the participant was blinded to the intervention, either by randomisation to an active intervention in each study arm, or through a sham intervention. This resulted in a high rate of categorisation of high risk of bias for this outcome measure [117 (63%)].
We also assumed that reporting of hospital admission would be unlikely to be influenced by knowledge of allocation; thus, the majority of trials reporting this outcome were categorised as at low risk of bias for this outcome. Loss to follow-up was frequently high, and authors often failed to adequately account for those with missing outcome data or did not describe their characteristics. Relatively few trials reported that they had published a protocol or were registered on a clinical trials database, so only 63 (30%) were categorised as at low risk of bias of selective outcome reporting; however, in most cases all the outcome measures described in the methods were reported in the results section.
A significant other potential cause of bias was due to authors reporting in the abstract the numbers completing the trial rather than randomised and the characteristics of only those who completed the trial. Furthermore, baseline imbalances (e.g. caused by small sample sizes) were not routinely adjusted for in statistical analyses, and thus differences at follow-up (e.g. in QoL) might (partly) be due to baseline differences.
Effectiveness results
Appendix 25 gives an overall summary of direction of effects for each trial for reference purposes.
The following sections refer to the results of specific analyses described previously in Table 40.
All trials
Figures 23–26 plot the outcomes at all reported time points for all trials for HRQoL measured by the SGRQ and the CRQ, hospital admission and exacerbations. These results have not been combined by meta-analysis due to the heterogeneity of the interventions and comparators.
The trials were ordered by the number of components, and upon visual inspection there does not appear to be any relationship between the size of the effect and the number of components. For HRQoL, many trials reported a large difference between the intervention and comparator group at baseline and, when present, this was rarely adjusted for in the analysis using ANCOVA.
At the last follow-up point, 11 of 56 (19.6%) resulted in a statistically significant reduction in hospital admissions and 4 of 28 (14.3%) a statistically significant reduction in exacerbations. A total of 22 of 87 (25.3%) comparisons showed a statistically significant improvement in total SGRQ score, 16 of 41 (39.0%) in total CRQ, 10 of 24 (41.7%) on the physical components of the Short Form questionnaire-36 items (SF-36) but only 2 of 21 (9.5%) on the mental component of the SF-36. For individual components, the CRQ ‘dyspnoea’ and ‘mastery components had the highest proportions of reported significant improvements (26.4% and 25.8%, respectively).
FIGURE 23.
(a) Health-related quality of life as measured by the SGRQ at all reported follow-up points for all included studies; and (b) key for figure. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22. b, Indicates that the number in the control group has been halved, with half of the group used as control for one comparison (nurse-assisted medical management vs. UC) and half for the other comparison (nurse-assisted collaborative management vs. UC).



FIGURE 24.
Health-related quality of life as measured by the CRQ at all reported follow-up points for all included studies. A = rehabilitation (traditional and modern) + qigong + breathing training + limb training vs. UC. B = modern rehabilitation + breathing training + limb training vs. UC. C = traditional rehabilitation + qigong vs. UC. D = rehab (traditional and modern) + qigong + breathing training + limb training vs. modern rehabilitation + breathing training + limb training. E = rehab (traditional and modern) + qigong + breathing training + limb training vs. traditional rehabilitation + qigong. F = rehab (traditional and modern) + qigong + breathing training + limb training vs. traditional rehabilitation + qigong.
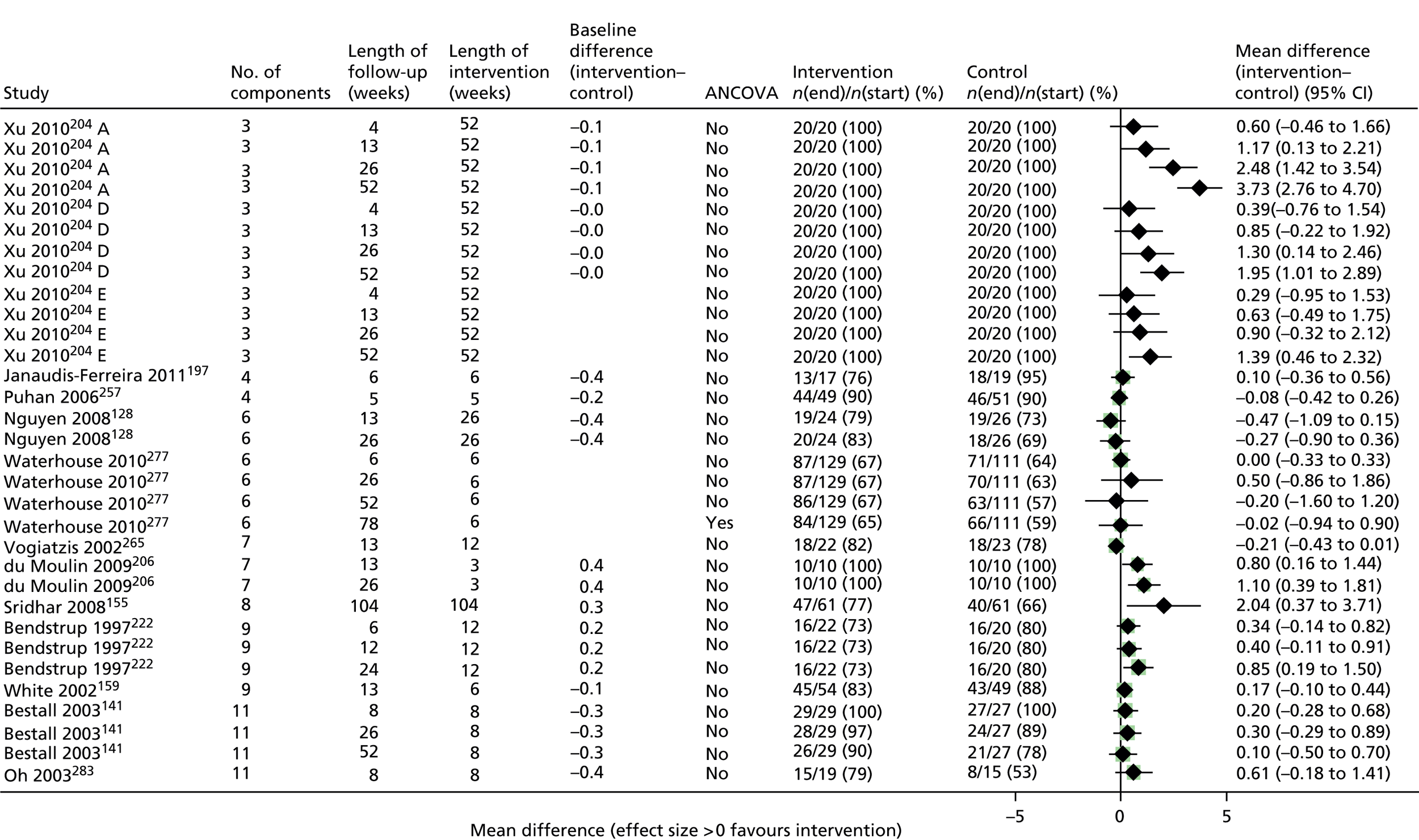
FIGURE 25.
Hospital admissions at all reported follow-up points for all included studies. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22.

FIGURE 26.
Exacerbations at all reported follow-up points for all included studies. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22.

Multicomponent interventions compared with usual care
Health-related quality of life
Multicomponent interventions were defined as those with three or more components. Forty-one trials reporting 44 interventions compared with UC reported usable total SGRQ or CRQ results; 31 trials reported hospital admissions, of which 18 could be used in meta-analysis; and 12 trials reported exacerbations of which three could be used in the meta-analysis (see Appendix 25).
The meta-analysis findings are reported for three time periods: up to and including 3 months, greater than 3 months but up to 6 months (hereafter described as 3–6 months) and greater than 6 months.
For SGRQ followed up to 3 months, most trials reported a greater improvement from baseline to follow-up in favour of the intervention group. 68,121,142,144,148,149,161,186,194,213,214,221,226,227,237–239,257 The summary meta-analysis result reveals that on average the multicomponent arm had a SGRQ score of 6.50 points (95% CI 3.62 to 9.39 points) higher than the UC arm. However, this is the average of a distribution of trial effects and this distribution is wide due to high heterogeneity (I2 = 82.4%). The prediction interval reports the range in which 95% of the distribution of the effects lies. The majority of the interval is > 0 and thus mainly in favour of multicomponent interventions; however, it does overlap zero (95% CI –4.66 to 17.67) indicating that the interventions are not always effective. At the longer follow-up point of 3–6 months, the estimate of the average effect was 4.47 points on the SGRQ (95% CI 1.93 to 7.02 points; I2 = 79.6%) and at > 6 months it was 2.40 points (95% CI 0.75 to 4.04 points; I2 = 57.9%), both favouring the intervention group. There is some suggestion that the size of the effect was smaller as follow-up was longer, but loss to follow-up was also a more significant problem at the longest follow-up point, which may have biased the effect estimate. The upper boundary of the prediction intervals also lowers with the longer follow-up time.
There were fewer trials reporting the CRQ. At all three time points the estimate of the average effect was in favour of the intervention group, suggesting that, on average, multicomponent SM interventions were more effective than UC at up to 6 months’ follow-up. However, heterogeneity was high and the prediction intervals at all three time points included zero. The results for HRQoL are summarised in Figures 27 and 28 and Table 51.
FIGURE 27.
Health-related quality-of-life (SGRQ) outcomes for multicomponent SM intervention vs. UC. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22. b, Number in the control group has been halved with half of the group used as control for one comparison (nurse-assisted medical management vs. UC) and half for the other comparison (nurse-assisted collaborative management vs. UC). Chan 2010212 B = exercise vs. control; Coultas 2005112 A = nurse-assisted collaborative management vs. UC; Coultas 2005112 B = nurse-assisted medical management vs. UC.



FIGURE 28.
Health-related quality-of-life (CRQ) outcomes for multicomponent SM intervention vs. UC. Xu 2010204 A = rehabilitation (traditional and modern) + qigong + breathing training + limb training vs. UC.

| Outcome | Time frame | No. of studies (comparisons) | Summary MD (95% CI) | I2 (%) | 95% prediction interval |
|---|---|---|---|---|---|
| SGRQ | ≤ 3 months | 18 | 6.50 (3.62 to 9.39) | 82.4 | –4.66 to 17.67 |
| > 3 to ≤ 6 months | 14 (15) | 4.47 (1.93 to 7.02) | 79.6 | –4.71 to 13.65 | |
| > 6 months | 16 | 2.40 (0.75 to 4.04) | 57.9 | –2.38 to 7.17 | |
| CRQ | ≤ 3 months | 7 | 0.40 (0.01 to 0.79) | 75.7 | –0.84 to 1.64 |
| > 3 to ≤ 6 months | 5 | 1.02 (0.05 to 1.98) | 93.2 | –2.66 to 4.70 | |
| > 6 months | 3 | 1.21 (–0.47 to 2.88) | 96.2 |
Hospital admissions
The results of 18 studies63,65,67,68,70,71,73,120,140,142,148,160,168,212,213,227,266,270 of multicomponent interventions compared with UC which reported hospital admissions have been analysed; eight with follow-up at ≤ 3 months,67,68,70,120,148,160,212,227 four with follow-up to 6 months;70,73,160,266 and eight with follow-up at ≥ 1 year. 63,65,71,140,142,168,213,270 Although the summary HRs from meta-analysis favoured the intervention groups at all three follow-up periods, there was much uncertainty leading to only weak statistical evidence of an effect and heterogeneity was high at follow-up times of > 3 months. Details are given in Figure 29 and Table 52.
FIGURE 29.
Hospital admissions for multicomponent SM interventions vs. UC. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22. Chan 2010212 B = exercise vs. control.
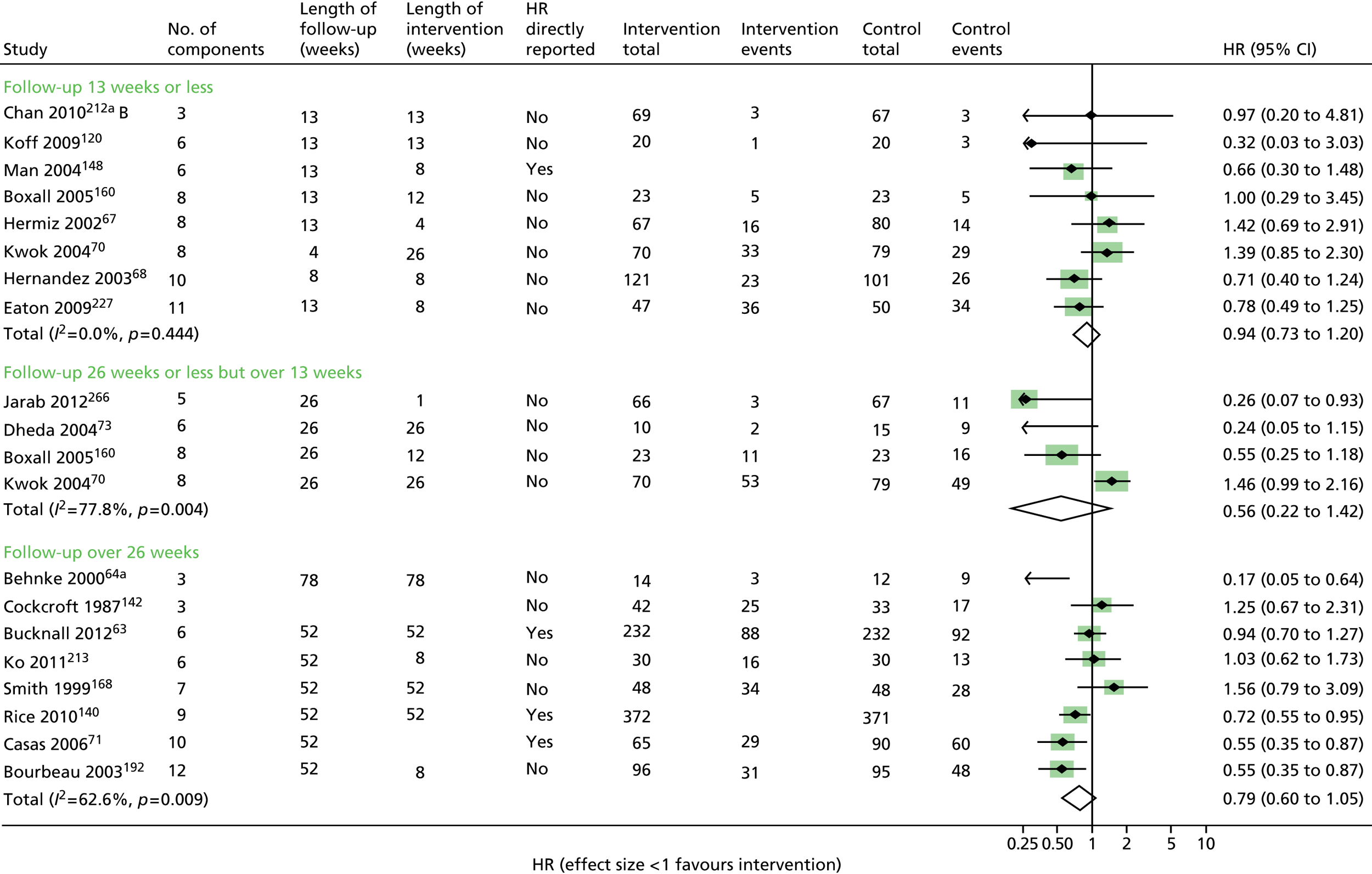
Exacerbations
Exacerbations were reported in an analysable format by only four studies. 73,182,213,284 The multicomponent interventions had no evident effect – details in Figure 30 and Table 52. At the last follow-up point, only 2 of 12 studies that reported exacerbations reported a statistically significant effect in favour of the multicomponent SM intervention. 140,172
FIGURE 30.
Exacerbations for multicomponent SM interventions vs. UC. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22.

| Outcome | Time frame | No. of studies | Summary HR (95% CI) | I2 (%) | 95% prediction interval |
|---|---|---|---|---|---|
| Admissions | ≤ 3 months | 8 | 0.94 (0.73 to 1.20) | 0.0 | 0.69 to 1.28 |
| > 3 to ≤ 6 months | 4 | 0.56 (0.22 to 1.42) | 77.8 | – | |
| > 6 months | 8 | 0.79 (0.60 to 1.05) | 62.6 | 0.36 to 1.77 | |
| Exacerbations | ≤ 3 months | 1 | 3.01 (0.31 to 28.96) | n/a | – |
| > 3 to ≤ 6 months | 2 | 1.01 (0.59 to 1.74) | 0.0 | – | |
| > 6 months | 2 | 1.09 (0.77 to 1.53) | 37.4 | – |
Evidence of effectiveness of multicomponent interventions on HRQoL, but considerable uncertainty for hospital admissions and exacerbations.
Exploring specific individual components of self-management interventions
We aimed to explore the effectiveness of specific individual components of SM interventions by examining the trials for which there was a difference in one component between intervention and control arms.
Action plans
Four trials170,188,229,231 reported the addition of an action plan to a SM package. There was no difference in the average effect on HRQoL of the arms including action plans compared with the comparator groups (SGRQ 0.43, 95% CI –1.69 to 2.54; I2 = 0%) (Figure 31).
FIGURE 31.
Health-related quality of life (SGRQ) at final follow-up for comparisons assessing the effects of one additional component of SM.
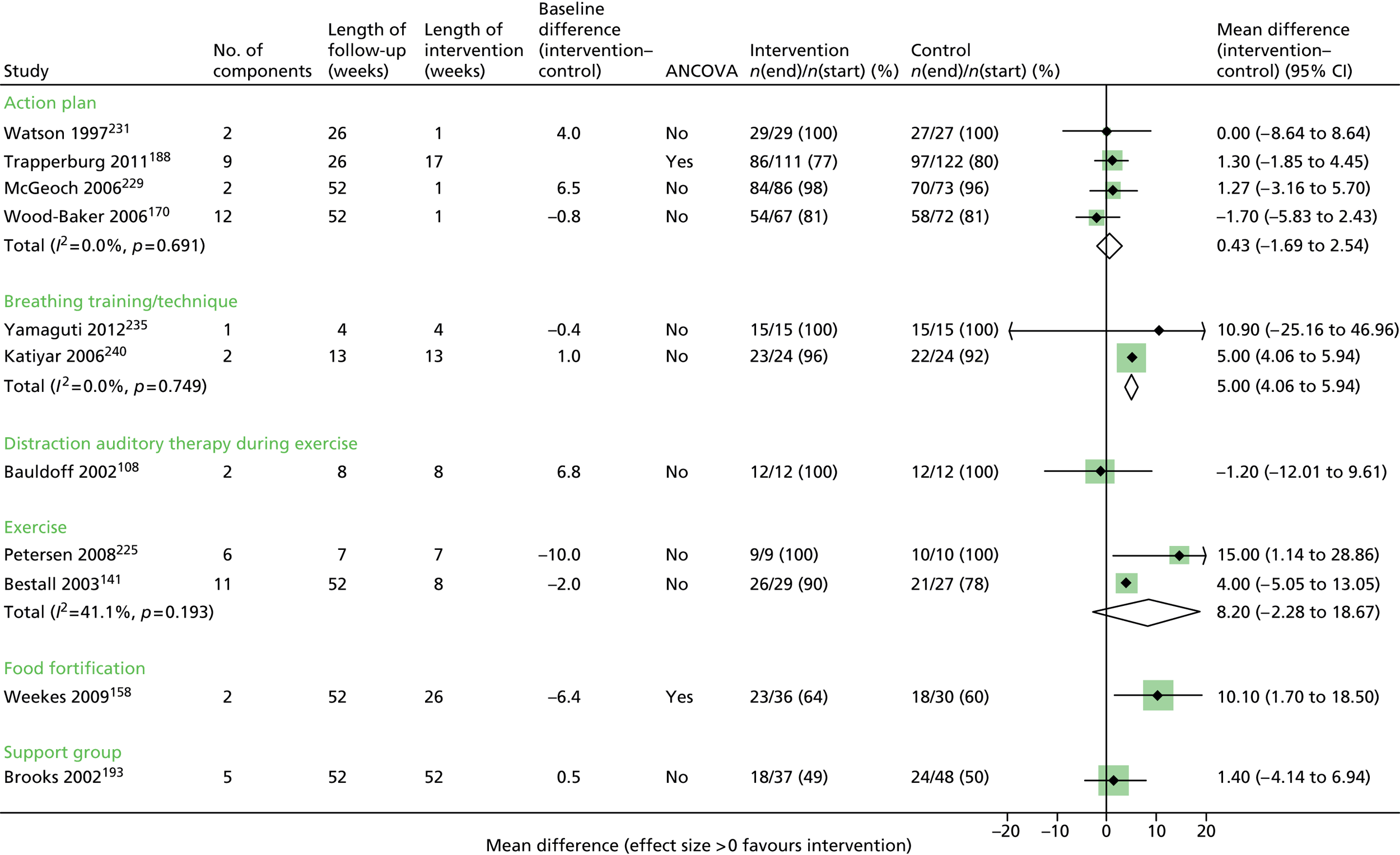
McGeoch et al. 229 further undertook 1-year follow-up of people who were given an action plan and reported no effect on hospital admissions (HR 0.97, 95% CI 0.33 to 2.89) (Figure 32). At 6 months’ follow-up, a large trial by Trappenburg et al. 188 found no additional effect of action plans on exacerbations (HR 1.12, 95% CI 0.77 to 1.62) (Figure 33).
FIGURE 32.
Admissions at final follow-up for comparisons assessing the effects of one additional component of SM.

FIGURE 33.
Exacerbations at final follow-up for comparisons assessing the effects of one additional component of SM.

Breathing techniques
Two trials235,240 reported breathing training or techniques. On average, the breathing training groups had a SGRQ score that was 5.0 points (95% CI 4.06 to 5.94 points) higher than the comparison groups. Although the trials were of a small size, the heterogeneity was low (I2 = 0%). Van Gestal et al. 208 reported no difference in the CRQ at 4 weeks’ follow-up (0.17, 95% CI –0.09 to 0.43 points) (Figure 34; see also Figure 31).
FIGURE 34.
Health-related quality of life (CRQ) at final follow-up for comparisons assessing the effects of one additional component of SM. Xu 2010204 D = rehabilitation (traditional and modern) + qigong + breathing training + limb training vs. modern rehabilitation + breathing training + limb training.

Distraction auditory therapy during exercise
Bauldoff et al. 108 reported no significant difference in SGRQ between the group with distraction auditory therapy and a group with an exercise intervention only (see Figure 31).
Exercise techniques/dyspnoea management
We did not include the exercise-only interventions in this analysis, as these have been separately analysed and are described in the next section. This analysis investigates the effect of adding exercise to other SM components.
Two trials141,225 reported HRQoL using the SGRQ, with an average effect of 8.20 points (95% CI –2.28 to 18.67 points). The trial by Petersen et al. 225 was small (n = 19), with a difference at baseline not accounted for and short follow-up. Three trials141,204,272 reported the CRQ with the exercise group achieving an average of 0.71 points (95% CI –0.30 to 1.73 points) more improvement than the comparison group (see Figures 31 and 34).
A small trial by Moore et al. ,271 which investigated exacerbations, reported no evidence of a reduction at 6 weeks’ follow-up (see Figure 33).
Patient support groups
Brooks et al. 193 investigated the effect of the addition of a patient support group to a multicomponent SM package, but although only half of the participants completed the trial, no difference in HRQoL was found at 1-year follow-up (SGRQ 1.40, 95% CI –4.14 to 6.94) (see Figure 31).
Exercise-only interventions compared with usual care or a sham intervention
We further examined the effect of individual components by reviewing trials in which exercise was a single component. The exercise could be supervised or unsupervised, but there were no other SM components.
Eight trials64,183,207,212,240,248,252,282 reported the effects of exercise-only interventions on HRQoL, with no other SM components in a way through which the data could be incorporated in the meta-analyses. The four trials183,212,240,252 with five comparator groups, which reported SGRQ at up to 3 months, reported a significant benefit from the exercise-only intervention (4.87 points, 95% CI 3.96 to 5.79). The prediction interval (3.39–6.36) provides strong evidence that exercise-only interventions are effective in improving the SGRQ score at up to 13 weeks’ follow-up. Only Gohl et al. 207 reported the SGRQ at 1-year follow-up with a large but non-significant effect in favour of exercise, although the sample size was very small with only 19 participants. Three trials64,248,282 reported the CRQ outcome, although all had low rates of follow-up, so results must be interpreted with caution. At 3 months’ follow-up there was a modest effect in favour of the exercise group but not statistically significant, and at 6 months there was a larger, non-significant effect, with a high level of heterogeneity. Details are shown in Figures 35 and 36, and Table 53.
FIGURE 35.
Health-related quality-of-life (SGRQ) outcomes for exercise-only interventions vs. UC/sham intervention. a, Refers to the fact that number in the control group has been halved with half of the group used as control for one comparison (t’ai chi qigong vs. control) and half for the other comparison (exercise vs. control). Chan 2010212 A = t’ai chi qigong vs. control. Chan 2010212 B = exercise vs. control.

FIGURE 36.
Health-related quality-of-life (CRQ) outcomes for exercise-only interventions vs. UC/sham intervention.

| Outcome | Time frame | No. of trials (comparisons) | Summary MD (95% CI) | I2 (%) |
|---|---|---|---|---|
| SGRQ | ≤ 3 months | 4 (5) | 4.87 (3.96 to 5.79) | 0 |
| > 6 months | 1 | 8.50 (–2.29 to 19.29) | n/a | |
| CRQ | ≤ 3 months | 2 | 0.70 (–0.07 to 1.47) | 68.6 |
| > 3 to ≤ 6 months | 2 | 1.17 (–0.35 to 2.69) | 92.5 | |
| Summary HR (95% CI) | ||||
| Admissions | ≤ 3 months | 1 (2) | 1.12 (0.29 to 4.36) | 0 |
| Exacerbations | ≤ 3 months | 1 | 0.02 (0.00 to 28.17) | n/a |
| > 3 to ≤ 6 months | 1 | 0.34 (0.07 to 1.77) | n/a |
Chan et al. 212 compared t’ai chi and exercise to UC in separate comparisons and reported no effects on hospital admission rates (Figure 37). Behnke et al. 64 undertook follow-up at the end of an 18-month exercise-only intervention and showed a significantly lower hospital admission rate in the intervention group (3/14) compared with 9 of 12 in the UC group (HR 0.17, 95% CI 0.05 to 0.64). In addition, Hernandez et al. 282 reported no statistical difference in admission rates between the exercise group and comparator at last follow-up. A small trial by Murphy et al. 252 reported exacerbations at 3 and 6 months, with a suggestion of lower rates, but participant numbers were very small (HR at 3 months 0.02, 95% CI 0.00 to 28.17; HR at 6 months 0.34, 95% CI 0.07 to 1.77) (Figures 37 and 38). Chan et al. 212 reported no difference on exacerbations between groups at 3 months’ follow-up.
FIGURE 37.
Admission outcomes for exercise-only interventions vs. UC/sham intervention. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22. b, Refers to the fact that number in the control group has been halved with half of the group used as control for one comparison (t’ai chi qigong vs. control) and half for the other comparison (exercise vs. control). Chan 2010212 A = t’ai chi qigong vs. control; Chan 2010212 B = exercise vs. control.

FIGURE 38.
Exacerbation outcomes for exercise-only interventions vs. UC/sham intervention.
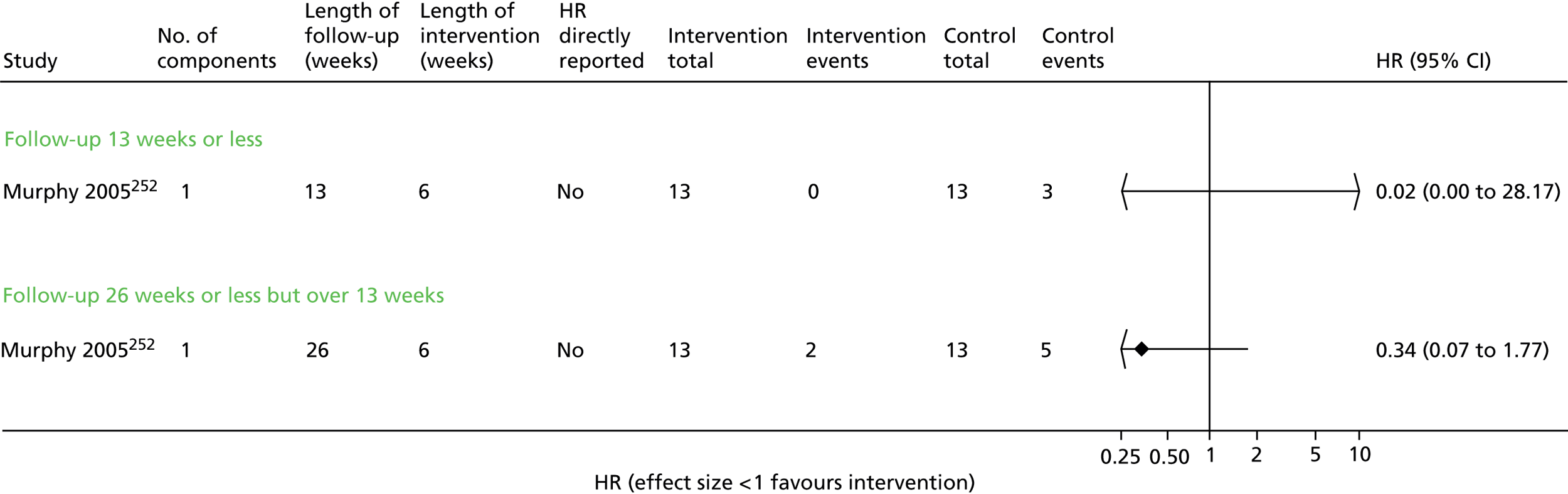
For individual components we are limited by insufficient evidence. There is no evidence of effectiveness of action plans, there was only one trial193 of support groups showing no difference when added to a multicomponent package; breathing management and techniques may have a positive effect.
There is strong evidence that exercise-only interventions increase HRQoL in the short-term, but limited evidence on hospital admissions and exacerbations.
Enhanced care
We wanted to explore the general effects of providing support to patients over just giving simple information approaches, which we termed ‘enhanced care’. It included regular telephone contact to reinforce information or behaviour change techniques, provided encouragement or included scheduled home visits for assessment with reinforcement or encouragement.
Fifteen studies63–65,67,71,72,110,120,140,155,180,188–190,192,193,206,251,270,271,283 provided information in the form of the total SGRQ or CRQ and were included in the meta-analyses. Only three studies67,120,193 reported the SGRQ at 3 months’ follow-up with very heterogeneous results; estimate of average effect was 1.27 points (95% CI –4.28 to 6.82 points, I2 = 73.8). At 3–6 months’ and 12 months’ follow-up the enhanced-care arm had a higher SGRQ score than the UC arm – details are given in Figure 39 and Table 54. The estimates of the average CRQ at the three follow-up times all favoured the enhanced-care arm but were not statistically significant and heterogeneity was very high (Figure 40).
FIGURE 39.
Health-related quality-of-life (SGRQ) outcomes for enhanced care (± SM package) vs. UC/SM package. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22.

| Outcome | Time frame | No. of studies (comparisons) | Summary MD (95% CI) | I2 (%) | 95% prediction interval |
|---|---|---|---|---|---|
| SGRQ | ≤ 3 months | 3 | 1.27 (–4.28 to 6.82) | 73.8 | – |
| > 3 to ≤ 6 months | 4 | 3.09 (1.28 to 4.90) | 0.0 | – | |
| > 6 months | 7 | 4.05 (2.23 to 5.87) | 8.4 | 1.00 to 7.10 | |
| CRQ | ≤ 3 months | 4 | 0.54 (–0.18 to 1.26) | 87.5 | –2.18 to 8.12 |
| > 3 to ≤ 6 months | 3 | 0.93 (–0.49 to 2.35) | 95.5 | –3.40 to 8.28 | |
| > 6 months | 2 | 0.85 (–1.12 to 2.82) | 82.1 | – | |
| Summary HR (95% CI) | |||||
| Admissions | ≤ 3 months | 4 | 1.05 (0.67 to 1.66) | 38.3 | – |
| > 3 to ≤ 6 months | 2 | 0.75 (0.20 to 2.86) | 93.2 | – | |
| > 6 months | 10 | 0.78 (0.62 to 0.99) | 55.1 | 0.40 to 1.54 | |
| Exacerbations | > 3 to ≤ 6 months | 2 | 1.11 (0.76 to 1.60) | 0.0 | |
| > 6 months | 1 | 1.00 (0.68 to 1.46) | n/a |
FIGURE 40.
Health-related quality-of-life (CRQ) outcomes for enhanced care (± SM package) vs. UC/SM package.

On average, enhanced care had a similar risk of hospital admission at 3 and 6 months (Figure 41 and Table 54), but a lower risk at 1 year or longer (HR 0.78, 95% CI 0.62 to 0.99). However, there was moderate heterogeneity at 1 year and the prediction interval showed that the intervention would frequently not be effective in lowering hospital admissions. Seven studies did not provide data that could be included in the meta-analyses,69,74,136,140,180,188,251 of which three also reported a statistically significant reduction in hospital admissions at 1-year follow-up. 140,180,251
FIGURE 41.
Admissions outcomes for enhanced care (± SM package) vs. UC/SM package. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22.

Only three trials155,188,206 were included in the meta-analyses for exacerbations with no evidence of any effect of enhanced care on risk of exacerbation (Figure 42). Four other trials120,140,180,251 reported exacerbation rates at last follow-up, one140 of which reported a statistically significant reduction.
FIGURE 42.
Exacerbation outcomes for enhanced care (± SM package) vs. UC/SM package.

Positive effect on HRQoL, particularly at medium-/longer-term follow-up. There may be a reduction in hospital admissions with longer-term follow-up, but there is considerable heterogeneity. There is insufficient evidence to establish the effect on exacerbations.
The contribution of exercise to multicomponent self-management packages
The following analyses explore the contribution of exercise and its mode of delivery as a contributor to the heterogeneity. Multicomponent interventions:
-
with a supervised exercise element compared with UC
-
a structured, unsupervised exercise element compared with UC
-
exercise counselling only compared with UC
-
without an exercise element compared with UC.
Multicomponent interventions with a supervised exercise element compared with usual care
Trials were included in this category if the exercise component of a larger package of care was directly supervised. The majority were group, centre-based interventions (generally referred to as PR).
Health-related quality of life
Of the 47 trials that reported HRQoL, 26 trials (27 interventions) reported disease-specific HRQoL using the total SGRQ or CRQ (see Appendices 13 and 27). Findings are similar to those for multicomponent interventions overall, with the largest estimate of the average effect in SGRQ at up to 3 months’ follow-up (7.75, 95% CI 3.49 to 12.01) points. There was no difference in average effect between the intervention and UC arms in SGRQ at follow-up of > 6 months. Heterogeneity was very high for most outcomes and follow-up times and loss to follow-up variable. Small but significant improvements in the CRQ were seen at 3 and 6 months’ follow-up favouring the SM group. Details are given in Figures 43 and 44 and Table 55.
FIGURE 43.
Health-related quality-of-life (SGRQ) outcomes for multicomponent SM interventions including supervised exercise vs. UC/control. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22. Chan 2010212 B = exercise vs. control.

FIGURE 44.
Health-related quality-of-life (CRQ) outcomes for multicomponent SM interventions including supervised exercise vs. UC/control. Xu 2010204 A = rehabilitation (traditional and modern) + qigong + breathing training + limb training vs. UC.
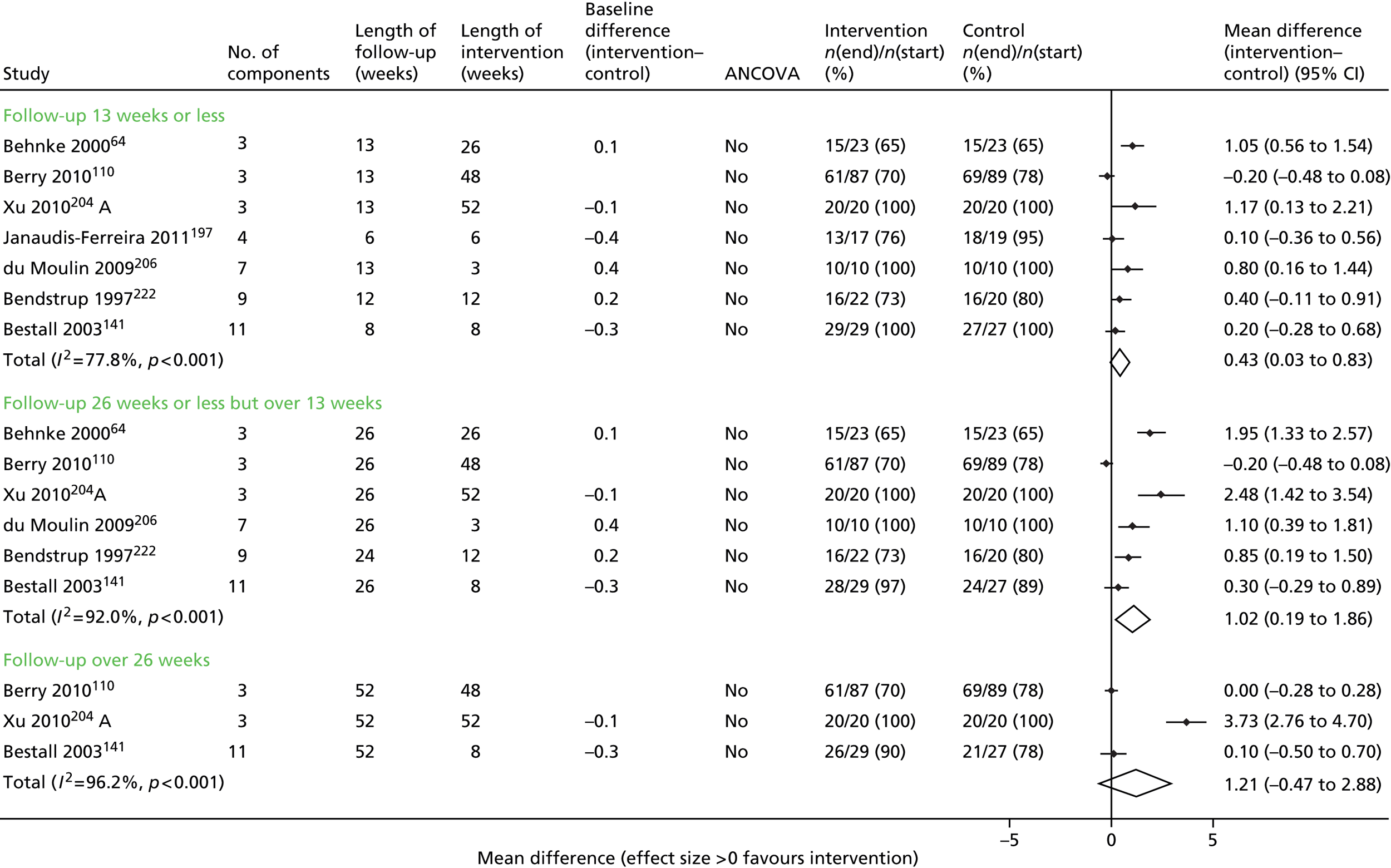
| Outcome | Time frame | No. of studies (comparisons) | Summary MD (95% CI) | I2 (%) | 95% prediction interval |
|---|---|---|---|---|---|
| SGRQ | ≤ 3 months | 14 | 7.75 (3.49 to 12.01) | 80.1 | –8.25 to 23.75 |
| > 3 to ≤ 6 months | 6 | 6.57 (3.24 to 9.90) | 77.6 | –3.66 to 16.79 | |
| > 6 months | 4 | 1.13 (–2.81 to 5.08) | 0 | – | |
| CRQ | ≤ 3 months | 7 | 0.43 (0.03 to 0.83) | 77.8 | –0.86 to 1.72 |
| > 3 to ≤ 6 months | 6 | 1.02 (0.19 to 1.86) | 92.0 | –1.95 to 3.99 | |
| > 6 months | 3 | 1.21 (–0.47 to 2.88) | 95.2 | – | |
| Summary HR (95% CI) | |||||
| Admissions | ≤ 3 months | 4 | 0.78 (0.54 to 1.14) | 0.0 | – |
| > 3 to ≤ 6 months | 1 | 0.55 (0.25 to 1.18) | n/a | – | |
| > 6 months | 2 | 0.47 (0.08 to 2.60) | 83.8 | – | |
| Exacerbations | > 3 to ≤ 6 months | 2 | 0.99 (0.56 to 1.75) | 0 | – |
| > 6 months | 2 | 1.09 (0.77 to 1.53) | 37.5 | – |
Hospital admissions
We were able to combine the reports of hospital admission rates in six trials. 65,148,160,192,212,213,227 Although the trend was for the average effect on admission rates to be lower in the intervention arms, this was not a significant effect at any of the follow-up time points (Figure 45). Four other trials172,182,212,213 reported hospital admissions at last follow-up; only one reported a statistically significant reduction in hospital admissions. 172
FIGURE 45.
Admissions outcomes for multicomponent SM interventions including supervised exercise vs. UC/control. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22. Chan 2010212 B = exercise vs. control.

Exacerbations
Three trials182,206,213 reported exacerbation rates in such a way that a HR could be computed; heterogeneity was low or moderate but no evidence of effect was observed (Figure 46 and Table 55). Two additional trials172,212 reported exacerbations at last study follow-up, with Güell et al. 172 reporting a statistically significant reduction in favour of the intervention group.
FIGURE 46.
Exacerbation outcomes for multicomponent SM interventions including supervised exercise vs. UC/control. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22.
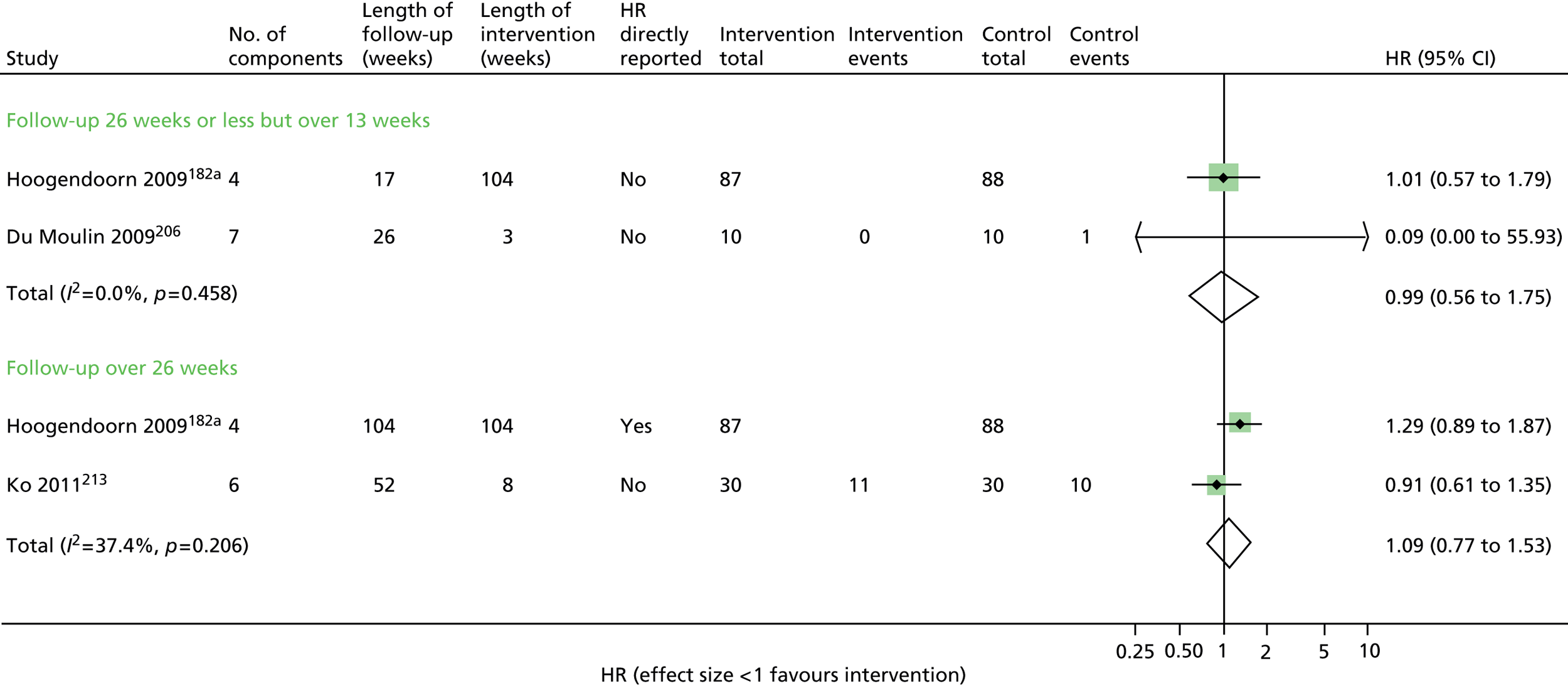
Multicomponent interventions with a structured, unsupervised exercise element compared with usual care
Trials were included in this category if they provided detail about a structured home exercise programme including duration and proposed frequency of exercise within a larger package of care. These were home-based interventions.
Of the eight trials186,251,255,262,264,270,283,284 that reported HRQoL, five used the total SGRQ186,251,255,262,270 and one the CRQ. 283 On average, the multicomponent SM package with structured unsupervised exercise had a larger improvement in SGRQ at 3–6 months’ follow-up than UC [3.59 points (95% CI 1.28 to 5.91 points; I2 = 0%)]. However, by 1-year follow-up there was no evidence of effect: SGRQ 0.80 points (95% CI –1.03 to 2.63 points; prediction interval –2.39 to 3.99). Only one trial283 reported the CRQ at 8 weeks’ follow-up (0.61, 95% CI –0.18 to 1.41 points) (Figures 47 and 48, and Table 56).
FIGURE 47.
Health-related quality-of-life (SGRQ) outcomes for multicomponent SM interventions with structured, unsupervised exercise vs. UC/control.
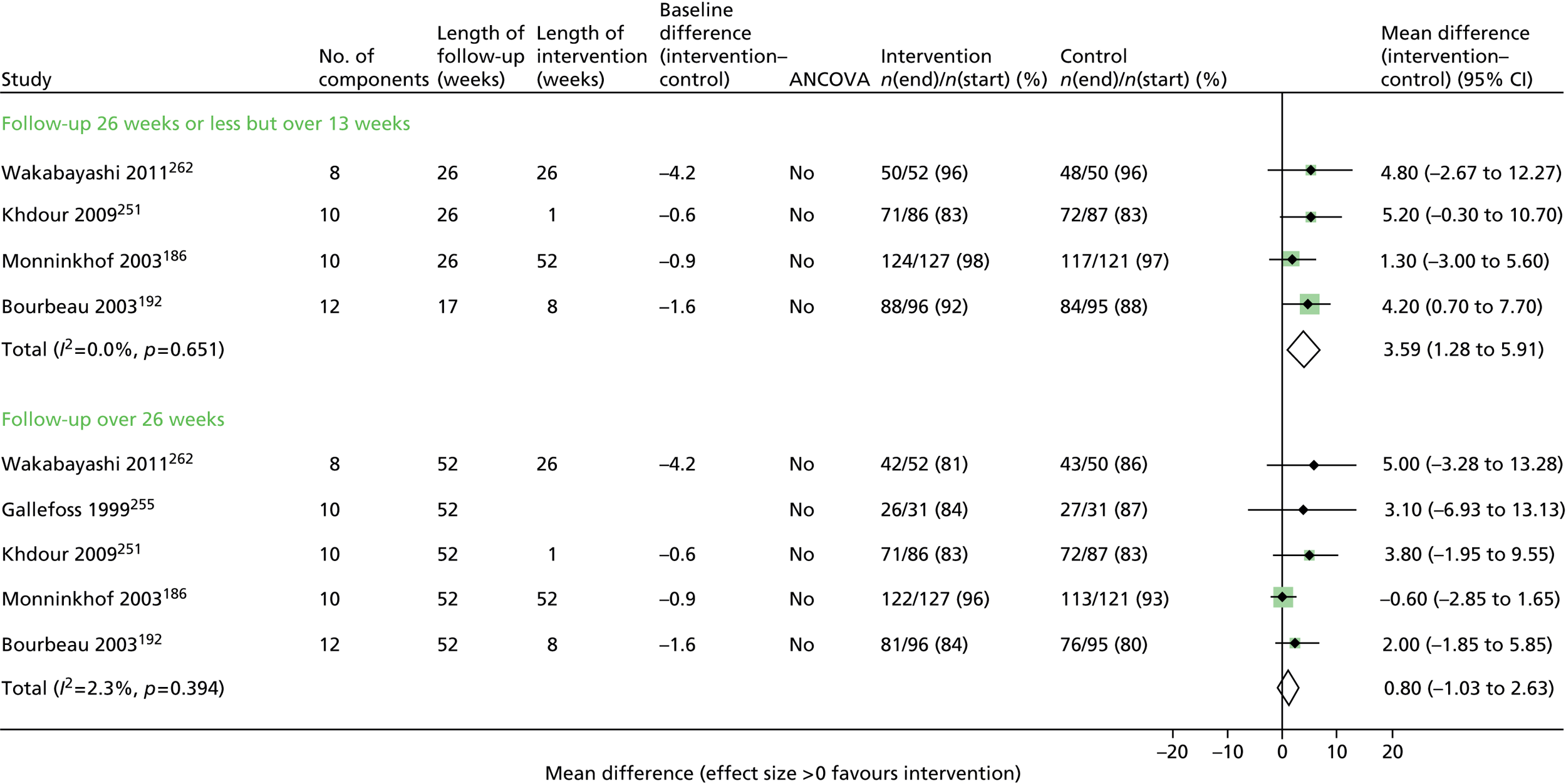
FIGURE 48.
Health-related quality-of-life (CRQ) outcomes for multicomponent SM interventions with structured, unsupervised exercise vs. UC/control.
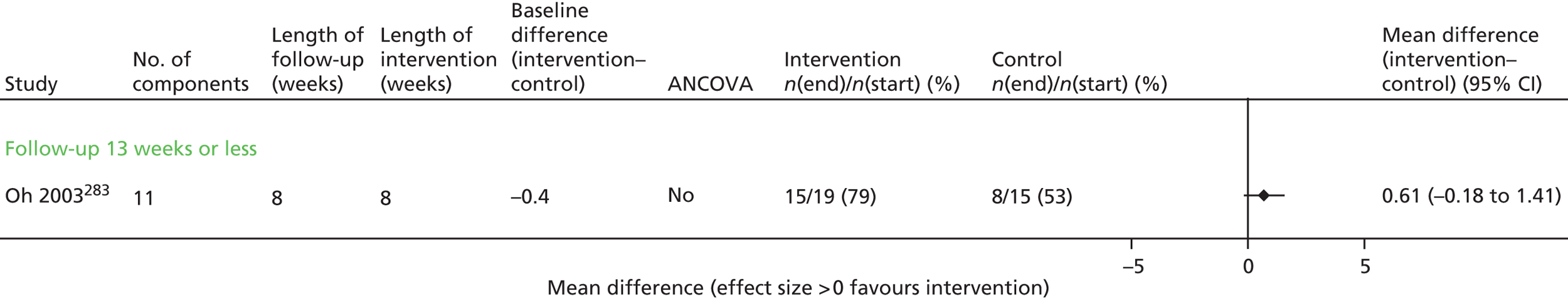
| Outcome | Time frame | No. of studies | Summary MD (95% CI) | I2 (%) |
|---|---|---|---|---|
| SGRQ | > 3 to ≤ 6 months | 4 | 3.59 (1.28 to 5.91) | 0.0 |
| > 6 months | 5 | 0.80 (–1.03 to 2.63) | 2.3 | |
| CRQ | ≤ 3 months | 1 | 0.61 (–0.18 to 1.41) | n/a |
| Summary HR (95% CI) | ||||
| Admissions | > 6 months | 1 | 0.55 (0.35 to 0.87) | n/a |
| Exacerbations | ≤ 3 months | 1 | 3.01 (0.31 to 28.96) | n/a |
Of the four trials182,255,262,270 that reported hospital admissions at last follow-up, two182,270 reported a statistically significant reduction in the intervention group. A HR could be calculated for only one trial,270 which reported a significant reduction in hospital admissions (HR 0.55, 95% CI 0.35 to 0.87) (Figure 49). 270 Moore et al. ,284 who had only 27 participants (and wide CIs), reported exacerbations (HR 3.01, 95% CI 0.31 to 28.96) (see Figure 50). An additional two trials192,251 reported no significant difference in exacerbations at last follow-up point.
FIGURE 49.
Admissions outcomes for multicomponent SM interventions with structured, unsupervised exercise vs. UC/control.
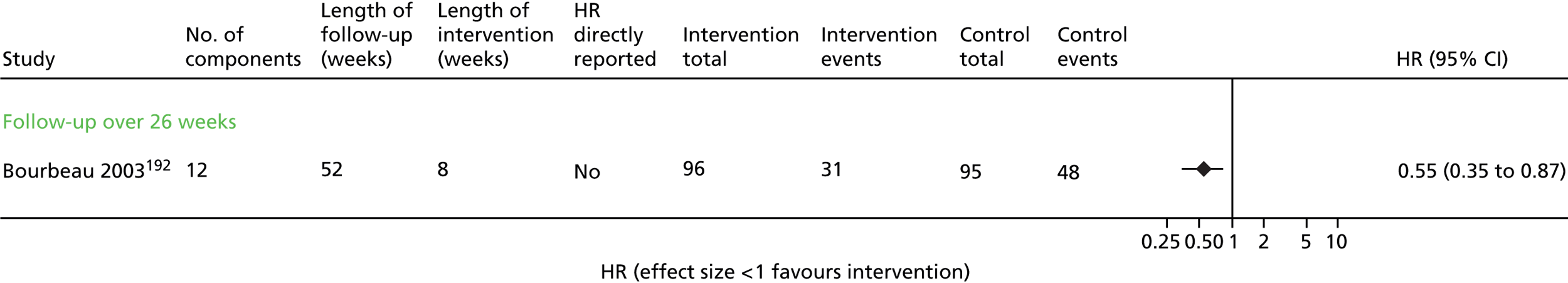
FIGURE 50.
Exacerbation outcomes for multicomponent SM interventions with structured, unsupervised exercise vs. UC/control.
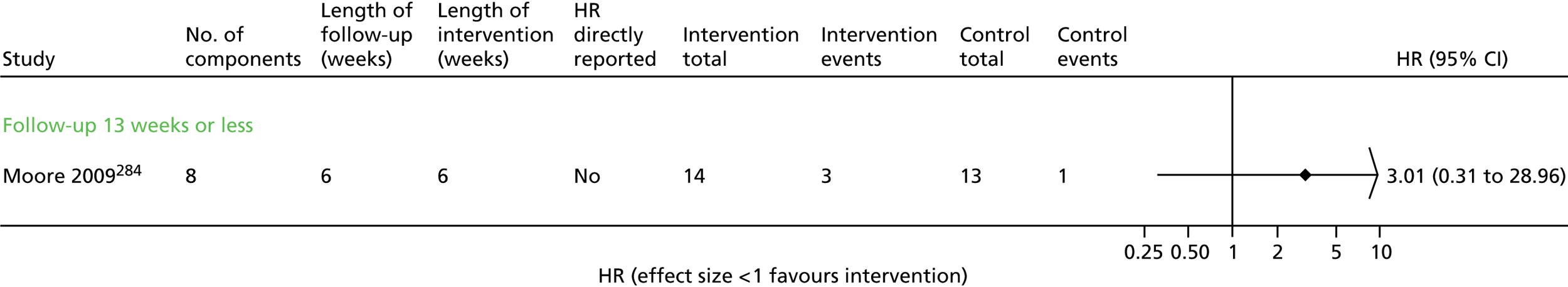
Multicomponent interventions with exercise counselling only compared with usual care
Seven trials67,72,73,140,170,180,266 reported disease-specific HRQoL using total SGRQ or CRQ following multicomponent interventions that included advice about increasing exercise. There were no significant effects on the SGRQ in the combined analyses at any of the three follow-up points and heterogeneity was high (Figure 51 and Table 57). Two trials72,180 had large differences in the SGRQ score at baseline that were not accounted for, and three trials72,73,140 had low or imbalanced follow-up rates.
FIGURE 51.
Health-related quality-of-life (SGRQ) outcomes for multicomponent SM interventions with exercise counselling only vs. UC/control. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22.

| Outcome | Time frame | No. of studies | Summary MD (95% CI) | I2 (%) |
|---|---|---|---|---|
| SGRQ | ≤ 3 months | 1 | 1.32 (–2.97 to 5.61) | n/a |
| > 3 to ≤ 6 months | 3 | 1.87 (–4.43 to 8.18) | 71.2 | |
| > 6 months | 4 | 3.88 (–1.39 to 9.14) | 74.6 | |
| Summary HR (95% CI) | ||||
| Admissions | ≤ 3 months | 2 | 1.40 (0.93 to 2.11) | 0 |
| > 3 to ≤ 6 months | 3 | 0.52 (0.13 to 2.09) | 81.0 | |
| > 6 months | 3 | 0.79 (0.50 to 1.26) | 67.9 | |
| Exacerbations | > 3 to ≤ 6 months | 1 | 1.0 (0.17 to 5.98) | n/a |
Eight trials reported hospital admissions,67,70,71,73,75,140,168,266 of which one168 was not included in the meta-analyses. There were no significant effects on admissions at any of the three follow-up points, and heterogeneity was high at the 6- and 12-month follow-up points. Details are given in Figure 52 and Table 57.
FIGURE 52.
Admission outcomes for multicomponent SM interventions with exercise counselling only vs. UC/control.

Only Dheda et al. 73 reported exacerbation rates at 6 months’ follow-up in a form enabling a HR to be calculated, with no difference between study arms (Figure 53). Three other trials140,180,266 reported exacerbations at 6 months265 or a year,140,180 with Rice et al. 140 reporting a significant reduction in exacerbations at 1 year.
FIGURE 53.
Exacerbation outcomes for multicomponent SM interventions with exercise counselling only vs. UC/control.

Multicomponent interventions without an exercise element compared with usual care
We included five trials63,112,120,185,256 of multicomponent interventions that did not include exercise or even advice about exercise as a component in the meta-analyses.
Although the estimates of average effects of QoL (SGRQ) at all three follow-up points were in the direction favouring the intervention, none was statistically significant and heterogeneity was high (Figure 54 and Table 58).
FIGURE 54.
Health-related quality-of-life (SGRQ) outcomes for multicomponent SM interventions without an exercise element vs. UC/control. a, Indicates that the control group that has been halved in size (split between two comparisons). Coultas 2005112 A = nurse-assisted collaborative management vs. UC; Coultas 2005112 B = nurse-assisted medical management vs. UC.
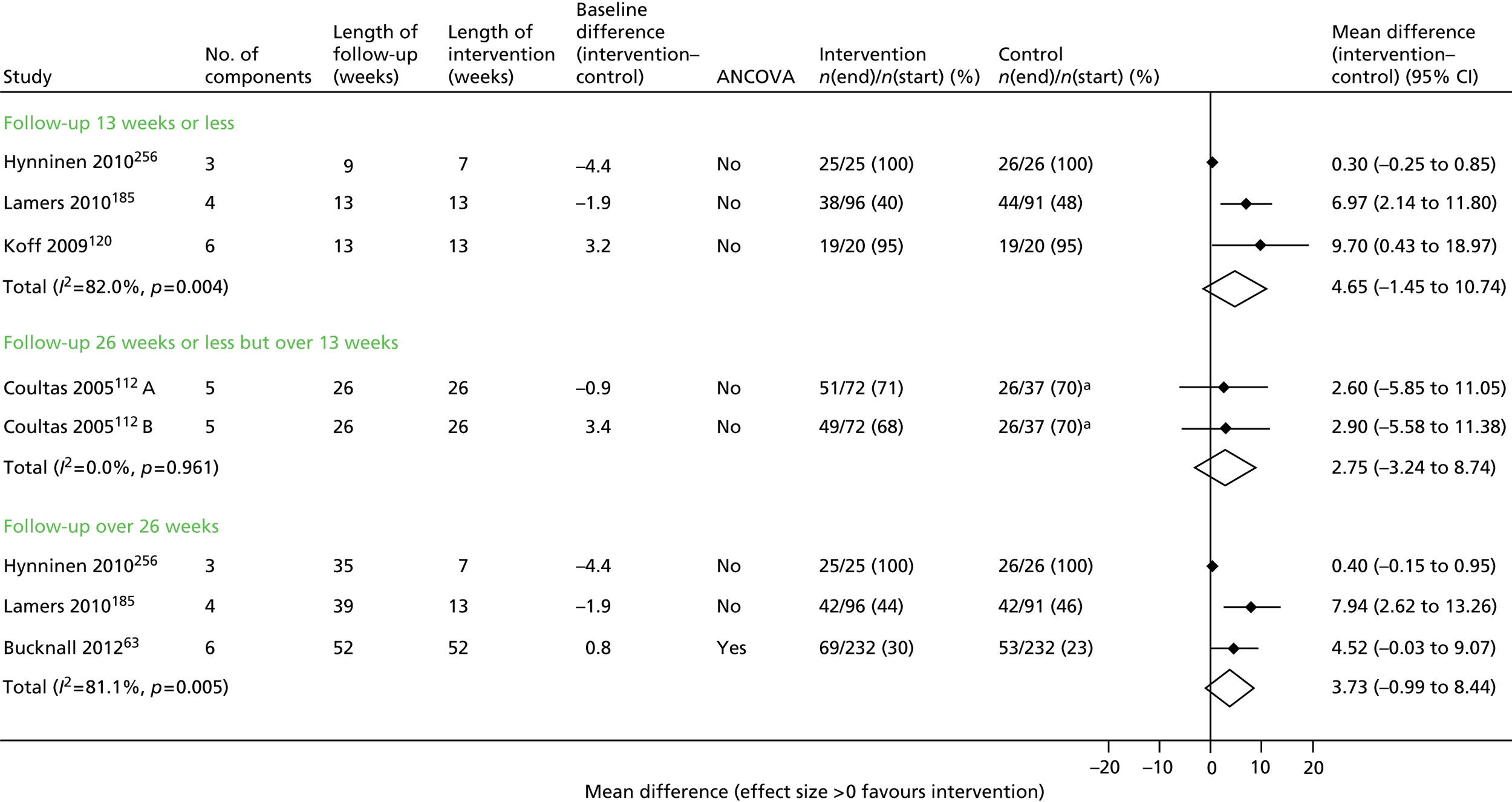
| Outcome | Time frame | No. of studies | Summary MD (95% CI) | I2 (%) |
|---|---|---|---|---|
| SGRQ | ≤ 3 months | 3 | 4.65 (–1.45 to 10.74) | 82.0 |
| > 3 to ≤ 6 months | 2 | 2.75 (–3.24 to 8.74) | 0 | |
| > 6 months | 3 | 3.73 (–0.99 to 8.44) | 81.1 | |
| Summary HR (95% CI) | ||||
| Admissions | ≤ 3 months | 1 | 0.32 (0.03 to 3.03) | n/a |
| > 6 months | 2 | 0.99 (0.76 to 1.30) | 0 |
Results were combined for two trials that reported hospital admission rates at 3–6 months. 63,142 Neither reported a statistically significant reduction in admissions (Figure 55). Three other trials112,136,142 that reported hospital admissions at last follow-up did not find any significant difference between study groups. Koff et al. 120 reported no significant difference in exacerbations at 3 months’ follow-up.
FIGURE 55.
Admissions outcomes for multicomponent SM interventions without an exercise element vs. UC/control.
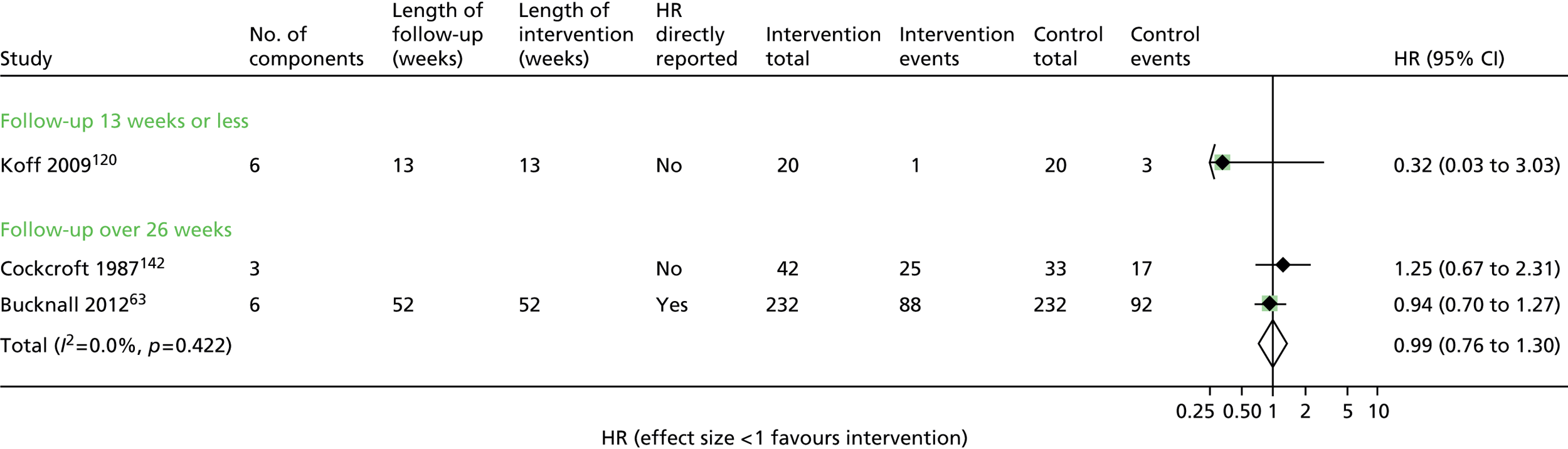
-
Multicomponent interventions with supervised exercise compared with UC have a positive effect on HRQoL and positive trend for hospital admissions but not reaching statistical significance.
-
For multicomponent interventions with structured, unsupervised exercise there is evidence of effectiveness on HRQoL in the medium term, but insufficient evidence for hospital admissions and exacerbations.
-
For interventions with advice to increase exercise in an unstructured manner there were few trials, but no evidence of overall effect on HRQoL, hospital admissions or exacerbations.
-
There were limited numbers of studies of multicomponent interventions without any exercise counselling. There is some evidence that they may lead to short-term improvements in HRQoL, but there was inconclusive evidence for hospital admissions.
Self-management interventions including an exercise component consisting of aerobic and strength training
Given the possible influence of exercise, we further explored the effect of different types of exercise interventions by first examining the effects of interventions that include both aerobic and strength training.
The summary meta-analysis result indicates that, on average, the combined aerobic/strength exercise arm has a SGRQ score of 7.80 points higher than the UC arm (95% CI 2.82 to 12.79 points). However, this is the average of the distribution of intervention effects and this distribution was wide as a result of high heterogeneity (I2 = 81.5%). The prediction interval, in which 95% of the distribution of the effects occur, is –10.60 to 26.21, which is mainly in favour of the intervention, but also indicates that the intervention is not always effective. At the mid follow-up point of 3–6 months, the estimate of the average effect was 3.76 points on the SGRQ (95% CI 2.13 to 5.39 points; I2 = 0%) favouring the intervention group. However, at > 6 months there was no evidence of effect (Figure 56 and Table 59). The CRQ results favoured the intervention group up to 6 months’ follow-up (Figure 57).
FIGURE 56.
Health-related quality-of-life (SGRQ) outcomes for combined strength and aerobic exercise interventions with SM components vs. UC. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22.

FIGURE 57.
Health-related quality-of-life (CRQ) outcomes for combined strength and aerobic exercise interventions with SM components vs. UC.

We identified six trials148,154,160,213,227,270 reporting hospital admissions but at no follow-up time point was the average effect significantly in favour of the intervention arm (Figure 58 and Table 59).
FIGURE 58.
Admission outcomes for combined strength and aerobic exercise interventions with SM components vs. UC.

| Outcome | Time frame | No. of studies (comparisons) | Summary MD (95% CI) | I2 (%) | 95% prediction interval |
|---|---|---|---|---|---|
| SGRQ | ≤ 3 months | 13 | 7.80 (2.82 to 12.79) | 81.5 | –10.60 to 26.21 |
| > 3 to ≤ 6 months | 6 | 3.76 (2.13 to 5.39) | 0.0 | 1.45 to 6.07 | |
| > 6 months | 6 | 0.27 (–1.47 to 2.01) | 0.0 | –2.20 to 2.74 | |
| CRQ | ≤ 3 months | 4 | 0.27 (0.00 to 0.53) | 0 | – |
| > 3 to ≤ 6 months | 2 | 0.55 (0.02 to 1.09) | 32.1 | – | |
| > 6 months | 1 | 0.10 (–0.50 to 0.70) | 70.1 | – | |
| Summary HR (95% CI) | |||||
| Admissions | ≤ 3 months | 4 | 0.67 (0.42 to 1.09) | 23.1 | – |
| > 3 to ≤ 6 months | 1 | 0.55 (0.25 to 1.18) | n/a | – | |
| > 6 months | 2 | 0.75 (0.41 to 1.37) | 68.2 | – | |
| Exacerbations | ≤ 3 months | 2 | 0.80 (0.11 to 5.83) | 65.4 | – |
| > 3 to ≤ 6 months | 1 | 1.01 (0.57 to 1.79) | n/a | – | |
| > 6 months | 2 | 1.09 (0.77 to 1.53) | 37.4 | – |
Similarly, the average effects of the four trials reporting exacerbations,154,182,213,284 showed no evidence of effect of aerobic and strength training (Figure 59).
FIGURE 59.
Exacerbation outcomes for combined strength and aerobic exercise interventions with SM components vs. UC. a, Indicates that several papers are represented by this lead publication. Details are given in Appendix 22.

Favourable effect on HRQoL and hospital admissions (although not statistically significant). No evidence of effect on exacerbations.
Strength and aerobic exercise training compared with aerobic training only
To investigate the effect of strength training, we evaluated the effects of studies reporting the addition of strength training over aerobic training. Two trials reported this comparison at 3 months’ follow-up. 233,246 On average combined training has a SGRQ 4.23 points (95% CI –8.75 to 17.22 points) higher than aerobic training alone, but this effect is not statistically significant (Figure 60).
FIGURE 60.
Health-related quality-of-life (SGRQ) outcomes for strength and aerobic exercise training interventions vs. aerobic exercise only. Dourado 2009233 B = strength training and low-intensity general training vs. low-intensity general training; Vonbank 2012246 B = strength and endurance training vs. endurance training.
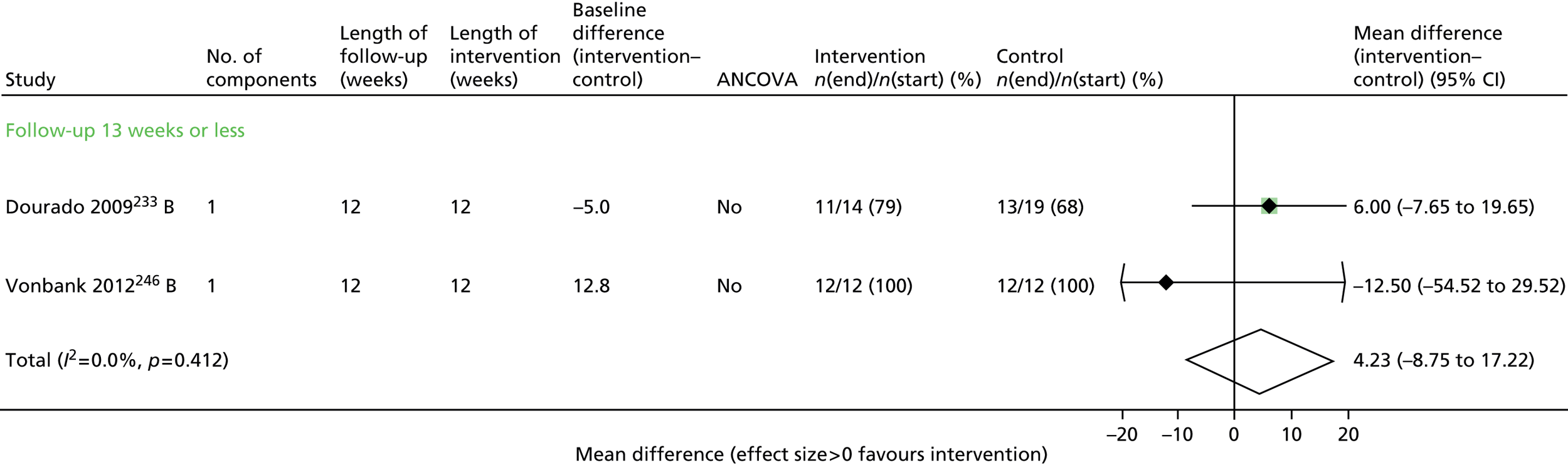
Endurance training compared with strength/resistance training
To explore which of the strength or endurance training was more effective we examined trials directly comparing both. Four trials reported HRQoL for this comparison (Figures 61 and 62). 216,233,246,247 At none of the follow-up points was there any evidence of a significant difference in average effect (Table 60). Only one trial reported hospital admission rates, which showed no evidence of effect (HR 0.68, 95% CI 0.22 to 2.13) (Figure 63). 247
FIGURE 62.
Health-related quality-of-life (CRQ) outcomes for endurance training vs. strength/resistance exercise training.

| Outcome | Time frame | No. of studies | Summary MD (95% CI) | I2 (%) |
|---|---|---|---|---|
| SGRQ | ≤ 3 months | 3 | 0.14 (–7.30 to 7.58) | 0 |
| > 3 to ≤ 6 months | 1 | 0.90 (–11.65 to 13.45) | n/a | |
| > 6 months | 1 | 1.30 (–7.02 to 9.62) | n/a | |
| CRQ | ≤ 3 months | 1 | 0.0 (–0.90 to 0.90) | n/a |
| Summary HR (95% CI) | ||||
| Admissions | ≤ 3 months | 1 | 0.68 (0.22 to 2.13) | n/a |
FIGURE 63.
Admission outcomes for endurance training vs. strength/resistance exercise training.

Limited evidence; no evidence of effect.
Upper and lower limb training compared with lower limb training only
This analysis aimed to explore the addition of upper limb training, which trains accessory respiratory muscles. Only one trial263 of 48 participants reported the CRQ immediately post intervention at 8 weeks with no evidence of effect (–0.21, 95% CI –0.85 to 0.42) (Figure 64).
FIGURE 64.
Health-related quality-of-life (CRQ) outcomes of upper and lower limb training vs. lower limb training only.

Limited evidence from one trial263 only; no evidence of effect.
Interval compared with continuous exercise
We explored whether interval or continuous exercise training was more effective. In a direct comparison, three trials reported the CRQ at ≤ 3 months (Figure 65). 124,257,265 There was no evidence of a difference in average effect of interval exercise compared with continuous exercise interventions (CRQ –0.14, 95% CI –0.32 to 0.04; I2 = 0%) (see Figure 65).
FIGURE 65.
Health-related quality-of-life (CRQ) outcomes of interval vs. continuous training.

Limited evidence; no evidence of effect.
Inspiratory or expiratory muscle training compared with usual care or a sham intervention
Four trials163,184,253,254 reported RMT compared with a UC or sham intervention. Two small trials253,254 reported the SGRQ at three follow-up points but with wide CIs around a non-significant effect. Two small trials163,184 reported the CRQ immediately post intervention at 5 and 8 weeks, with an average CRQ score of 0.44 points (95% CI –0.27 to 1.15) higher than UC, indicating a non-statistically significant improvement in HRQoL. Only one trial253 reported hospital admissions, with a non-significantly lower HR in the RMT arm (HR 0.77, 95% CI 0.34 to 1.72) (Figures 66–68 and Table 61).
FIGURE 66.
Health-related quality-of-life (SGRQ) outcomes for inspiratory and expiratory muscle training interventions vs. UC/control/sham training.
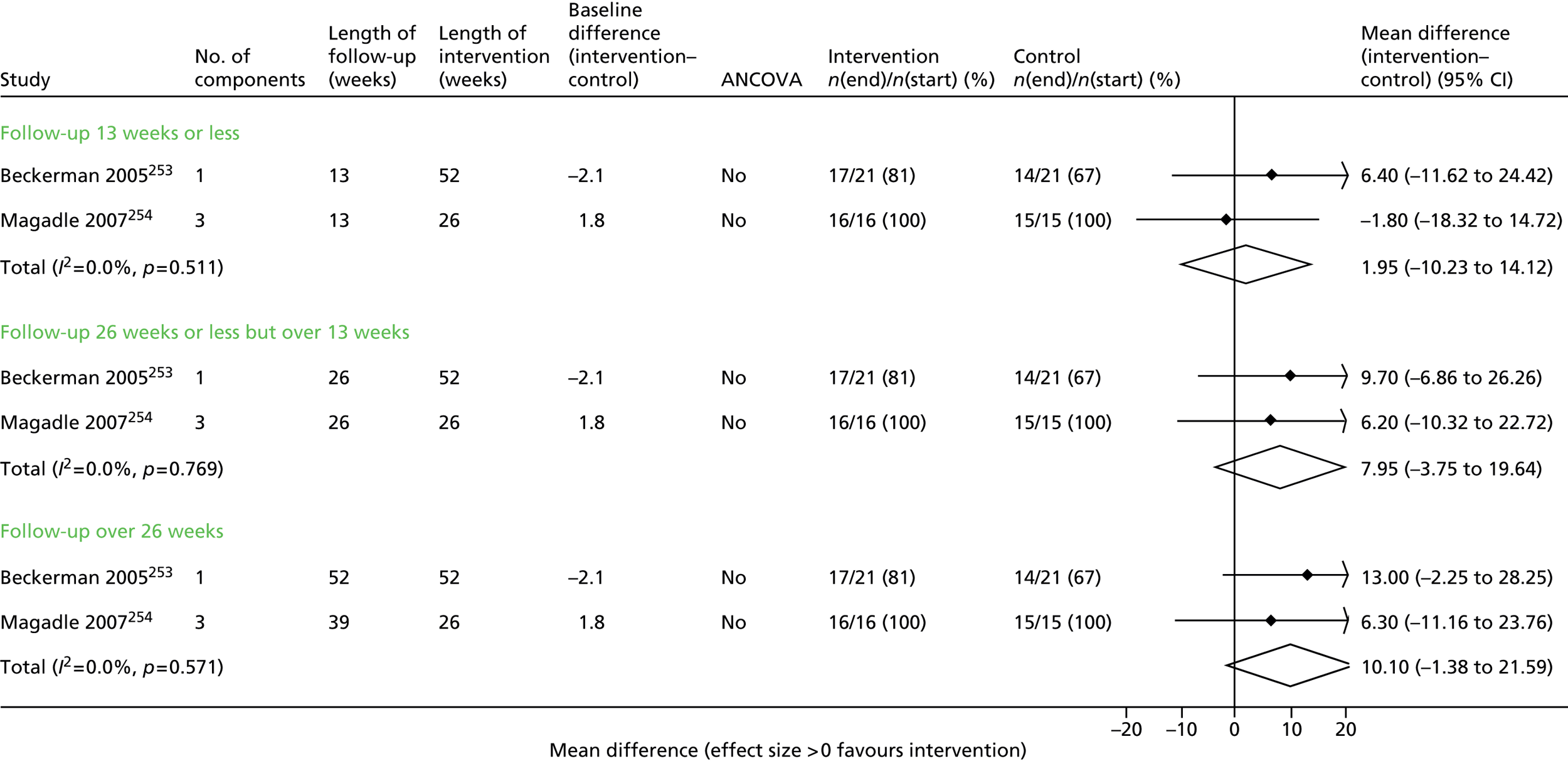
FIGURE 67.
Health-related quality-of-life (CRQ) outcomes for inspiratory and expiratory muscle training interventions vs. UC/control/sham training.

FIGURE 68.
Admission outcomes for inspiratory muscle training and expiratory muscle training interventions vs. UC/control/sham training.
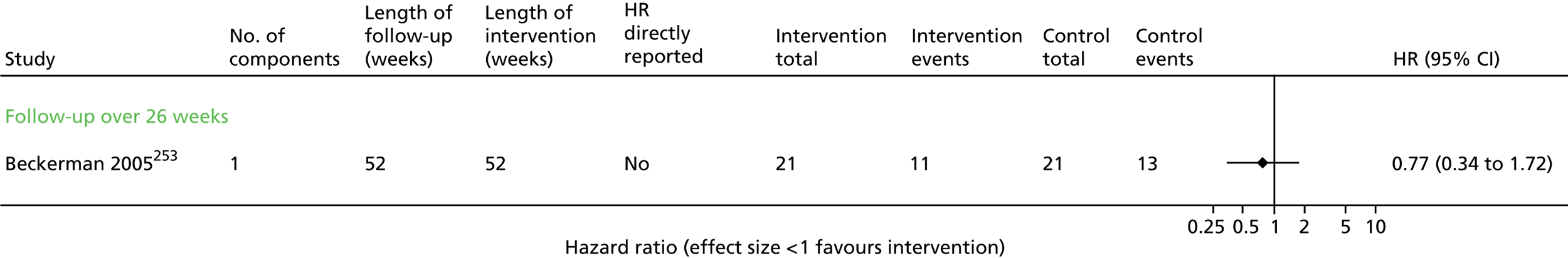
| Outcome | Time frame | No. of studies | Summary MD (95% CI) | I2 (%) |
|---|---|---|---|---|
| SGRQ | ≤ 3 months | 2 | 1.95 (–10.23 to 14.12) | 0 |
| > 3 to ≤ 6 months | 2 | 7.95 (–3.75 to 19.64) | 0 | |
| > 6 months | 2 | 10.10 (–1.38 to 21.59) | 0 | |
| CRQ | ≤ 3 months | 2 | 0.80 (–0.27 to 1.15) | 69.8 |
| Summary HR (95% CI) | ||||
| Admissions | ≤ 3 months | 1 | 0.77 (0.34 to 1.72) | n/a |
Limited evidence. Some evidence of potential effect on HRQoL in mid to longer term.
Direct comparison of more sessions/longer duration with shorter programmes
This analysis investigated the effect of longer programmes: 7 weeks’ duration compared with 4 weeks’ duration,144 additional exercise sessions following a course of PR224 or two compared with one repeat PR sessions. 244 It did not investigate the intensity of exercise undertaken within a session. There is no evidence that longer programmes or more sessions lead to improved SGRQ scores or reduced exacerbations (Figures 69–71 and Table 62).
FIGURE 69.
Health-related quality-of-life (SGRQ) outcomes for SM interventions of a longer duration vs. SM interventions of a shorter duration.
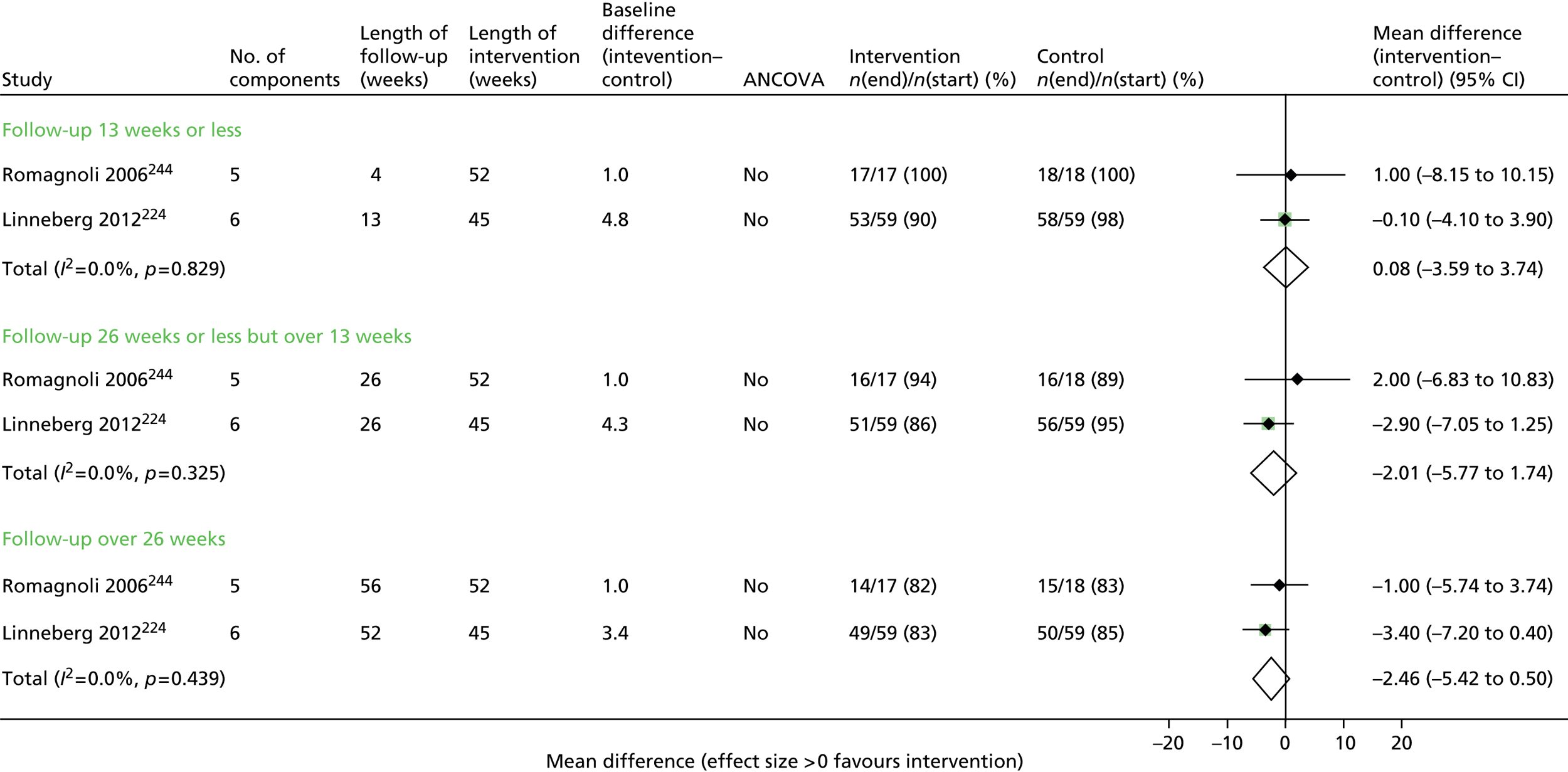
FIGURE 70.
Health-related quality-of-life (CRQ) outcomes for SM interventions of a longer duration vs. SM interventions of a shorter duration.

FIGURE 71.
Exacerbation outcomes for SM interventions of a longer duration vs. SM interventions of a shorter duration.
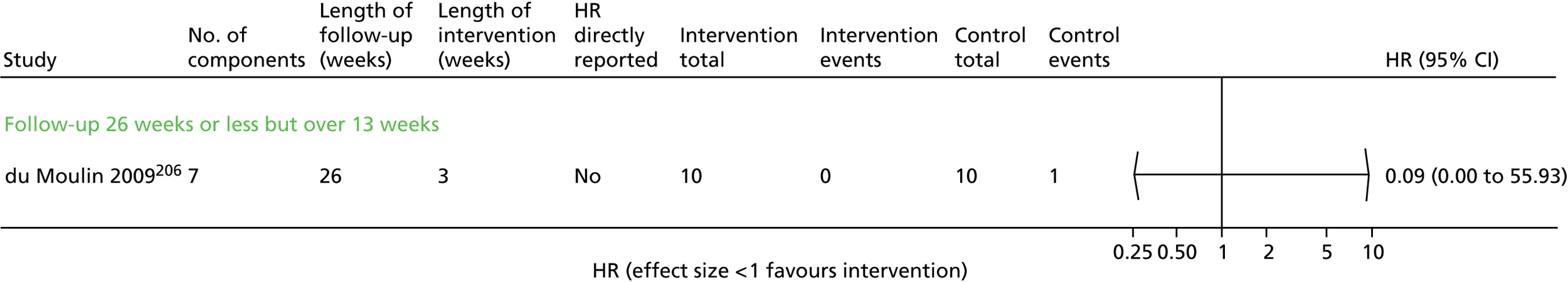
| Outcome | Time frame | No. of studies | Summary MD (95% CI) | I2 (%) |
|---|---|---|---|---|
| SGRQ | ≤ 3 months | 2 | 0.08 (–3.59 to 3.74) | 0.0 |
| > 3 to ≤ 6 months | 2 | –2.01 (–5.77 to 1.74) | 0.0 | |
| > 6 months | 2 | –2.46 (–5.42 to 0.50) | 0.0 | |
| Summary HR (95% CI) | ||||
| Exacerbations | > 3 to ≤ 6 months | 1 | 0.09 (0.00 to 55.93) | n/a |
Limited evidence; no evidence of effect.
Hospital compared with home location
Four trials reported disease-specific HRQoL outcomes in hospital with or without home locations compared with a home-based programme (Table 63). 64,169,177,198 There was no evidence of an average effect that differed between the comparison groups in HRQoL at any of the follow-up points except for one small trial at 6 months’ follow-up64 (Figures 72 and 73). In Maltais et al. 198 both groups received a 4-week outpatient supervised educational package, while the exercise component was either hospital- or home-based.
| Outcome | Time frame | No. of studies | Summary MD (95% CI) | I2 (%) |
|---|---|---|---|---|
| SGRQ | ≤ 3 months | 2 | –0.25 (–4.58 to 4.09) | 28.2 |
| > 3 to ≤ 6 months | 1 | 3.00 (–4.94 to 10.94) | n/a | |
| > 6 months | 2 | –1.94 (–5.26 to 1.38) | 15.6 | |
| CRQ | ≤ 3 months | 2 | 0.33 (–1.09 to 1.75) | 93.7 |
| > 3 to ≤ 6 months | 1 | 1.95 (1.33 to 2.57) | n/a |
FIGURE 72.
Health-related quality-of-life (SGRQ) outcomes for SM interventions delivered in hospital vs. delivered at home.
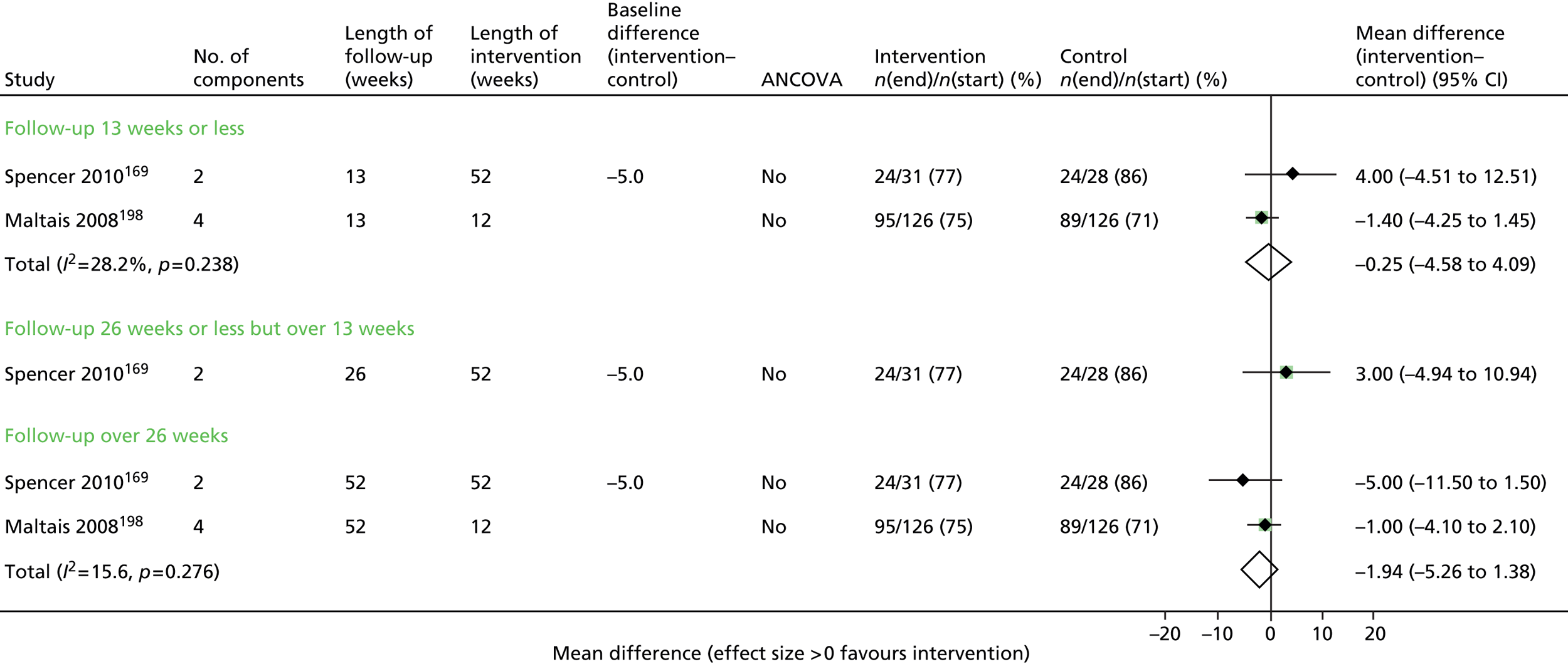
FIGURE 73.
Health-related quality-of-life (CRQ) outcomes for SM interventions delivered in hospital vs. delivered at home.

Limited evidence; no evidence of effect.
Delivery by pharmacists
We aimed to explore any effects by professional delivering care. The majority of interventions were delivered by nurses or physiotherapists. Three trials136,251,266,285 had a SM intervention delivered by a pharmacist with a UC comparator. Of these, two trials251,266 reported the SGRQ, with a higher average effect in favour of the pharmacist-led intervention at 6 months (2.74, 95% CI –1.54 to 7.03; I2 = 30.3%), but this was not statistically significant (Figure 74). At 1-year follow-up, Khdour et al. 251 reported a non-significant difference of 3.80 SGRQ points in favour of the pharmacist-led intervention (95% CI –1.95 to 9.55 points). Jarab et al. 266 reported a significant reduction in hospital admissions at 6 months (HR 0.26, 95% CI 0.07 to 0.93) (Figure 75).
FIGURE 74.
Health-related quality-of-life (SGRQ) outcomes for pharmacist-led interventions.
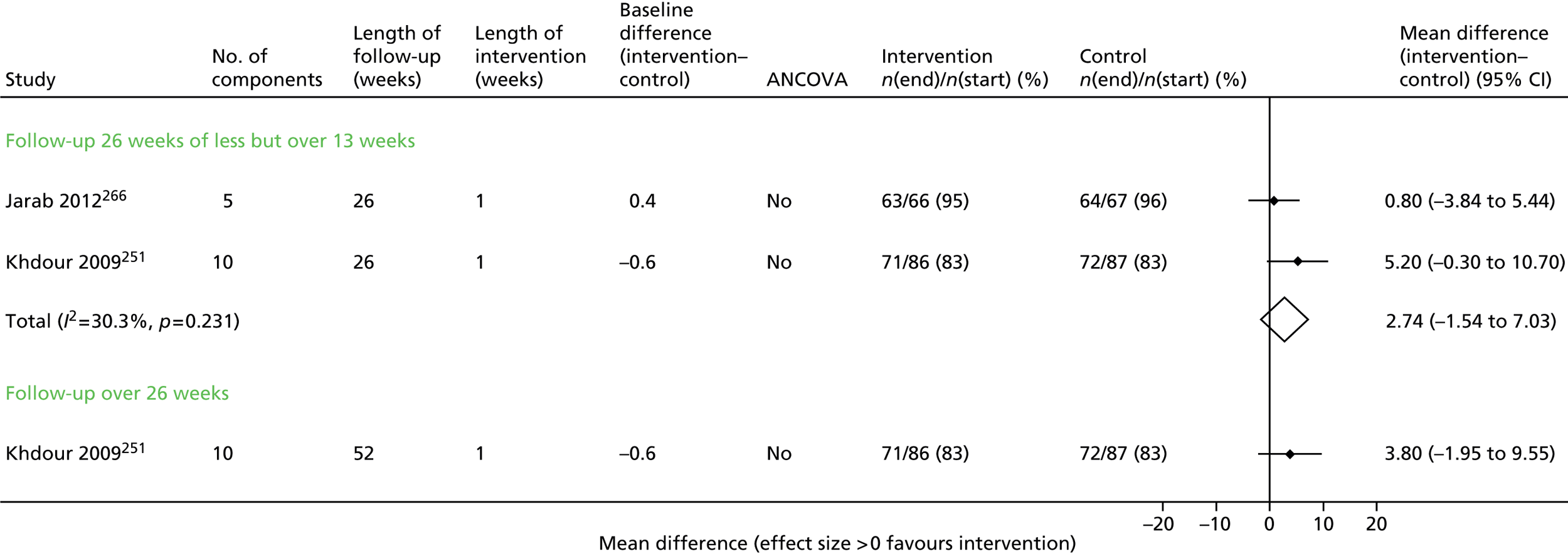
FIGURE 75.
Admission outcomes for pharmacist-led interventions.
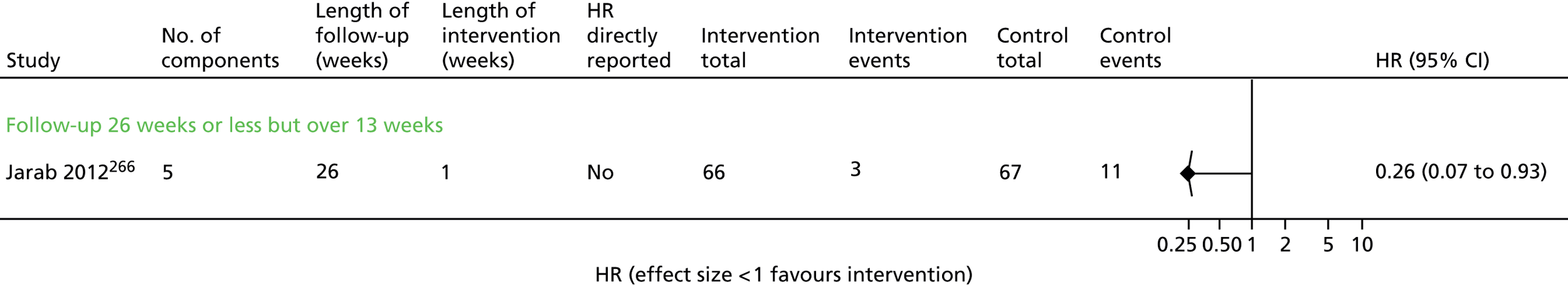
Insufficient evidence; no evidence of effect.
Maintenance programme post pulmonary rehabilitation compared with no maintenance programme
No combined analyses were possible for this analysis. Romagnoli et al. 244 found no evidence of effect on HRQoL from a maintenance programme following PR at 4, 26 or 52 weeks (Figure 76). At 2 years’ follow-up, Sridhar et al. 155 reported a significantly greater CRQ score (2.04, 95% CI 0.37 to 3.71) but no effect on hospital admissions (HR 1.11, 95% CI 0.65 to 1.91) or exacerbations (HR 1.00, 95% CI 0.68 to 1.46) (Figures 77–79).
FIGURE 76.
Health-related quality-of-life (SGRQ) outcomes for maintenance PR vs. PR with no maintenance period.
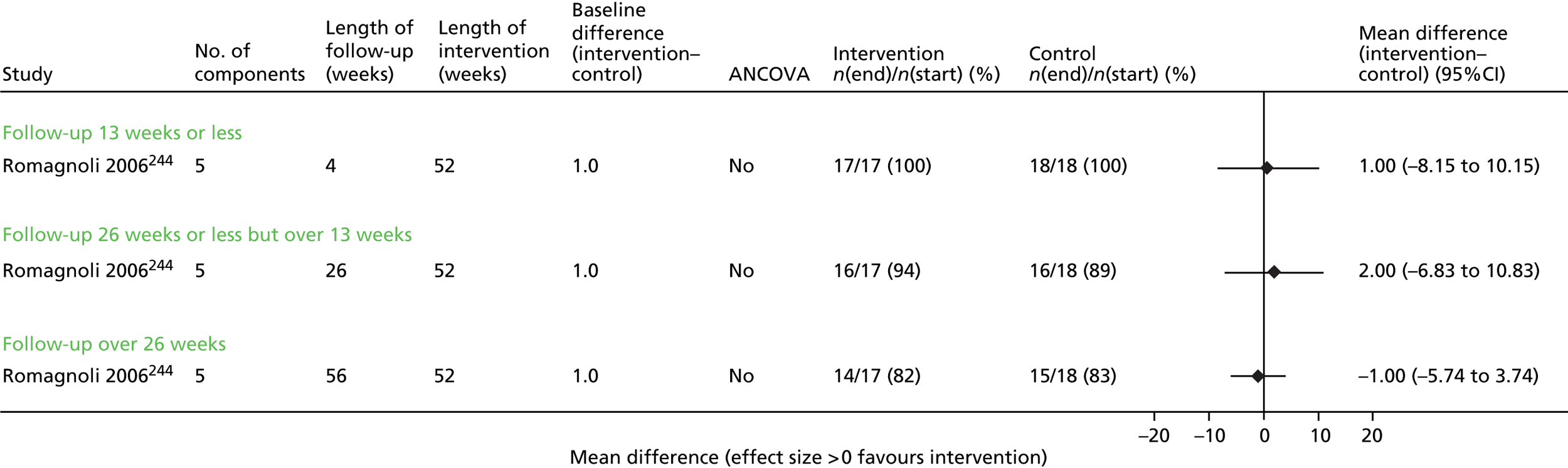
FIGURE 77.
Health-related quality-of-life (CRQ) outcomes for maintenance PR vs. PR with no maintenance period.
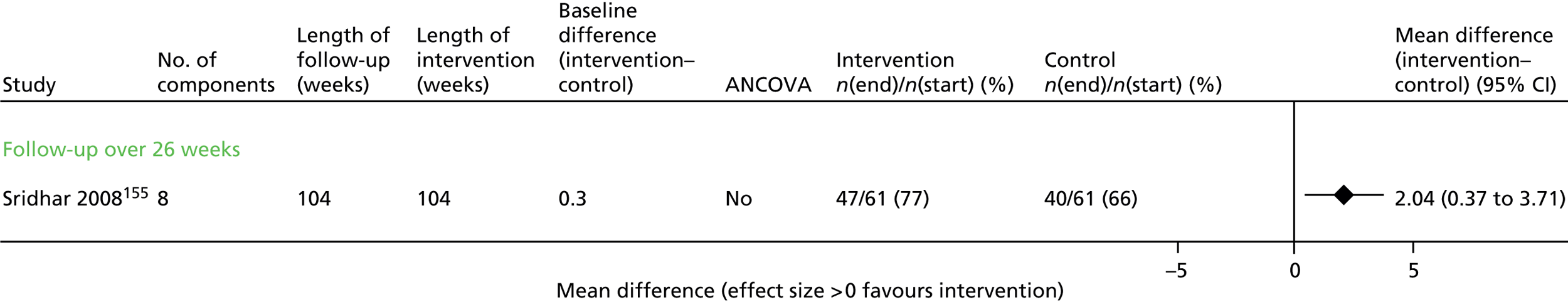
FIGURE 78.
Admission outcomes for maintenance PR vs. PR with no maintenance period.

FIGURE 79.
Exacerbation outcomes for maintenance PR vs. PR with no maintenance period.

Limited evidence; no evidence of effect.
Exploring heterogeneity using meta-regression
Meta-regression was used to explore the effects of risk of bias within studies, the average severity of COPD in the participants, the number of intervention components and the duration of the intervention. The last analysis is also clearly related to the follow-up time point. No clear pattern emerged. Although at up to 3 months’ follow-up the trials of all multicomponent interventions with generally low risk of bias have a significantly higher SGRQ score than trials at higher risk of bias, this effect was not seen at any other time point or for subgroups of interventions. Similarly, increasing numbers of components were associated with significant improvements of SGRQ in analyses of all multicomponent interventions, but a small but opposite effect was seen for the effect of enhanced SM on hospital admissions. For multicomponent interventions, the population with severe COPD had a reduced improvement in SGRQ compared with those with moderate or less severe COPD. Inconsistent results were observed for length of follow-up (Tables 64 and 65).
| Category | Follow-up | Coefficient | 95% CI | p-value |
|---|---|---|---|---|
| All multicomponent interventions | ||||
| Low risk of biasa | ≤ 3 months | 9.86 | 6.91 to 12.80 | < 0.001 |
| Severe populationb | –3.84 | –5.85 to –1.83 | < 0.001 | |
| No. of components | 1.36 | 0.89 to 1.84 | < 0.001 | |
| Length of intervention (weeks):c | ||||
| 14–26 | – | – | – | |
| 27+ | –10.16 | –20.74 to 0.41 | 0.060 | |
| Low risk of biasa | > 3 to ≤ 6 months | –0.95 | –5.52 to 3.62 | 0.684 |
| Severe populationb | –1.97 | –7.00 to 3.06 | 0.443 | |
| No. of components | –0.51 | –1.07 to 0.05 | 0.074 | |
| Length of intervention (weeks):c | ||||
| 14–26 | 4.40 | 0.24 to 8.55 | 0.038 | |
| 27+ | –0.21 | –4.27 to 3.85 | 0.918 | |
| Low risk of biasa | > 6 months | –0.10 | –3.81 to 3.60 | 0.957 |
| Severe populationb | –2.90 | –4.69 to –1.10 | 0.002 | |
| No. of components | –0.26 | –0.81 to 0.29 | 0.358 | |
| Length of intervention (weeks):c | ||||
| 14–26 | 4.48 | –3.81 to 12.78 | 0.289 | |
| 27+ | 1.76 | 0.22 to 3.30 | 0.025 | |
| Multicomponent interventions with supervised exercise | ||||
| Low risk of biasa | ≤ 3 months | 6.27 | –1.37 to 13.91 | 0.108 |
| Severe populationb | 1.70 | –10.21 to 13.62 | 0.779 | |
| Number of components | 1.19 | –0.70 to 3.08 | 0.218 | |
| Length of intervention (weeks):c | ||||
| 14–26 | – | – | – | |
| 27+ | –11.60 | –22.11 to –1.09 | 0.031 | |
| Interventions including an exercise component consisting of aerobic and strength training | ||||
| Low risk of biasa | ≤ 3 months | 6.21 | –2.19 to 14.62 | 0.148 |
| Severe populationb | 2.08 | –8.09 to 12.24 | 0.689 | |
| Number of components | 0.60 | –1.15 to 2.36 | 0.501 | |
| Length of intervention (weeks):c | ||||
| 14–26 | – | – | – | |
| 27+ | –12.31 | –21.94 to –2.68 | 0.012 | |
| Category | Follow-up | Coefficient | 95% CI | p-value |
|---|---|---|---|---|
| Enhanced care | ||||
| Low risk of biasa | > 6 months | 0.17 | –0.34 to 0.68 | 0.513 |
| Severe populationb | –0.27 | –1.02 to 0.49 | 0.492 | |
| No. of components | –0.08 | –0.14 to –0.01 | 0.020 | |
| Length of intervention (weeks):c | ||||
| 14–26 | – | – | – | |
| 27+ | 0.39 | –0.27 to 1.04 | 0.244 | |
Publication bias
We present funnel plots of the analyses, which included at least 10 studies (see Appendices 28–33). The asymmetric distribution apparent in several of the plots is suggestive of publication bias, with an Egger’s test for asymmetry showing p < 0.1 in five of the six analyses with ≥ 10 trials. These patterns are consistent with an absence of smaller studies with negative outcomes. This would be consistent with biases observed in other literatures, particularly in the context of a comprehensive search of the literature such as the one carried out here. Although the publication bias is thus a genuine concern, the asymmetry may also be due to systematic associations between sample size and other heterogeneous characteristics that impact on outcome.
Discussion
We report the findings of a large systematic review that has explored the components and delivery of SM interventions in order to try to identify the optimal mode of delivery and make-up of such interventions.
Key results
Overall we found that:
-
There were a large number of relevant trials with our primary outcomes of interest but the majority were small, short term (≤ 3 months) and poorly reported.
-
Almost half of the trials suffered from incomplete outcome reporting, which was likely to be an important source of bias for the HRQoL results in particular.
-
Interventions were very heterogeneous and usually multicomponent.
-
Exercise was the most common component, but other common components were breathing management techniques and general education about COPD and its management.
-
Overall, the nature and results of the studies were so heterogeneous it was not appropriate to combine them all together.
-
Studies assessing the effect of individual components were few but, from the evidence available, only exercise significantly improved patient outcomes, but this was restricted to HRQoL in the short term.
-
Multicomponent interventions (with three or more components) produced combined effects which suggested that HRQoL was improved compared with UC. However, there was much statistical heterogeneity that could not be explained by length of follow-up. The results were also consistent with a potential reduction in admissions but, again, data were heterogeneous and the CIs crossing the line of no effect. Further exploration using meta-regression techniques indicated that the results could have been affected by the likely bias introduced in the study, the disease severity of the populations and the number of components in the interventions, although across time points, these findings were not stable.
-
Subgroups of the multicomponent studies revealed that interventions with more enhanced care and support were effective in improving HRQoL and reducing admission rates among the studies of ≥ 6 months’ duration.
-
Furthermore, multicomponent interventions that included supervised exercise, or an unsupervised but structured exercise element, resulted in significant and clinically important improvements in HRQoL up to 6 months, although data were sometimes heterogeneous. There was insufficient evidence to comment on other outcomes.
-
Further exploration of exercise did not reveal which type of exercise was more effective or whether duration/intensity was important.
-
Insufficient evidence was available to assess the effect of delivery of SM type of health professional.
Comparison of findings with other reviews
Contents of self-management interventions
Through mapping the SM interventions and their individual components we are able to show the huge range of interventions, with differing components, delivered either as brief information/education or in a more supported manner. Exercise was the most frequent component and the most common component of single/two-component interventions. A recent systematic review by Stoilkova et al. 286 has mapped educational programmes in COPD management only, including studies published in the English language. This reported that over half of educational interventions had ≥ 10 topics incorporated within a programme. We took a much broader approach to defining SM, searching for studies that might include any of the relevant aspects of SM. Although we also found that a high proportion of trials evaluated multicomponent interventions, about one-fifth were single component, usually exercise only.
Role of behaviour change strategies
From the descriptions of interventions it was frequently not clear to what extent techniques for behaviour change were used in the SM education and support. Most intervention descriptions had no description of underlying behavioural change theory or the individual behaviour change strategies used. Some papers described using self-regulation theory, or Bandura’s Social Learning Theory;31 others described strategies such as self-monitoring, goal-setting, action planning and the use of biofeedback. The use of Abraham and Michie’s287 taxonomy of behaviour change to underpin the descriptions of the SM interventions would enable their relative contributions to be ascertained. Education is an important element of COPD SM interventions, with almost half of the studies in this review including this component. However, education as directly imparted provision of information is generally not effective by itself. 288 Information is effective when accompanied by active, behavioural strategies, and it is not clear to what extent these have been included within the interventions included in this review. Dishman et al. 289 identified that knowledge alone did not predict behaviour change, but that self-efficacy is an important cognitive determinant of change, showing that people have at least acquired the confidence and belief that they can self-manage their COPD. Trials that used action planning for an exacerbation generally failed to measure self-efficacy to determine whether they increased self-efficacy for identification of an exacerbation and confidence with commencing treatment. Given that action planning has not been found to be effective,50 it is vital to explore the mechanisms by which it is proposed to work, to establish whether any lack of effect is due to a failure to commence treatment as a result of lack of confidence.
Behavioural change techniques that have been shown to be most effective in the promotion of physical activity and healthy eating in the general population may also be beneficial to encourage physical activity and exercise in people with COPD. 290 The technique associated with most effects was being prompted to self-monitor behaviour. Other techniques that appeared to be effective when combined with self-monitoring were prompting intention formation, goal-setting and providing feedback on performance. These would all be achievable as part of a SM intervention for people with COPD.
Results of effectiveness of self-management interventions
Overall the effect on HRQoL of multicomponent SM interventions was positive, with an average effect size of greater than four points on the SGRQ, which is considered to be the minimal clinically important difference. The effect of SM on admissions and exacerbations was less clear, possibly as a result of fewer trials in the analyses. Our plan was to explore the expected high levels of heterogeneity to try to identify formats of SM for COPD that looked particularly promising in terms of HRQoL and health service utilisation outcomes. These analyses were developed by the wider project steering group and aimed to have a clinical coherence. We decided not to repeat analyses that were the subject of recent Cochrane systematic reviews.
We have no evidence from our analyses that SM interventions with more components are better than those with fewer. The results from the meta-regression provided inconsistent results in relation to the risk of bias of the study, severity of the population’s COPD and number of components.
Role of specific components of self-management
There were few studies that evaluated either single components of SM compared with UC, or the addition of an individual component to a wider package of care. The exploration of single component interventions is important, as it may be the case that it is easier for participants to focus on a single component better than a multicomponent intervention.
Action plans
A Cochrane systematic review investigated the effect of action plans with a brief educational component compared with UC. 50 There was no effect on HRQoL, emergency room visits, general practitioner consultations or hospital admissions, but participants in the action plan group had more treatments for exacerbations. Our review did not identify any additional studies addressing this SM strategy alone. Action planning was a component in over half of the multicomponent interventions.
Smoking cessation interventions
Smoking cessation was a surprisingly low proportion of the SM components (in < 20% of interventions); however, this may be a result of people with COPD being referred out to a separate smoking cessation service, rather than including it as part of a SM programme. It is possible that the inclusion of smoking cessation within SM programmes may be a source of heterogeneity, which we have not explored. In a systematic review of smoking cessation interventions for COPD five studies were included. 291 There were no comparisons of psychological interventions compared with no interventions. Direct comparisons of two active psychosocial interventions showed no significant difference, but a combination of a psychosocial and pharmacological intervention compared with no treatment showed sustained cessation.
Exercise-only interventions
We have reported the effects of exercise-only/exercise with dyspnoea management interventions compared with UC. At follow-up of ≤ 3 months the average effect of exercise only was –4.87 (95% CI –5.79 to –3.96) SGRQ points in favour of the intervention but, because of small numbers of trials reporting HRQoL with a longer follow-up or admissions or exacerbations, we do not have evidence of an effect after this short period. All but one of these trials included supervised exercise, so we were unable to explore the role of direct supervision compared with unsupervised home-based exercise in the absence of other SM components.
Effect of multicomponent interventions
In the wide range of multicomponent SM interventions and settings evaluated, our meta-analysis indicates that overall (on average) multicomponent SM interventions have a positive effect on HRQoL. Our summary estimates were larger than the minimal clinically important difference for SGRQ at follow-up to 6 months for the multicomponent interventions and at all follow-up points for the CRQ. 292 However, we did find considerable heterogeneity, making it hard to establish which particular interventions and which particular settings work best. Our findings are similar to those of a systematic review by Effing et al. ,48 who evaluated the effectiveness of SM education compared with UC. Effing et al. 48 included 14 trials, with considerable overlap with our analysis but they excluded trials of PR. Effing et al. 48 reported a smaller improvement on the SGRQ (2.6, 95% CI 0.02 to 5.0) than we found, but did report a significant reduction in respiratory admissions, which did not agree with our findings.
Integrated disease management
Several systematic reviews have addressed the effectiveness of disease management. 47,49 This has been defined by Schrijvers293 as ‘Disease management consists of a group of coherent interventions designed to prevent or manage one or more chronic conditions using a systematic, multidisciplinary approach and potentially employing multiple treatment modalities. The goal of chronic disease management is to identify persons at risk for one or more chronic conditions, to promote SM by patients and to address the illness or conditions with maximum clinical outcome, effectiveness and efficiency regardless of treatment setting(s) or typical reimbursement patterns’. The most recent review is a Cochrane review by Kruis et al. 47 The Cochrane review47 included 26 trials and reported a difference of 3.71 points on the SGRQ (95% CI 1.6 to 5.8 points) in favour of the intervention group, a reduction in respiratory admissions (OR 0.68, 95% CI 0.47 to 0.99) and a reduction in all-cause admissions up to 12 months (OR 0.62, 95% CI 0.36 to 1.07). There was no effect on exacerbations. Given this recent review, we have not repeated this analysis in this report.
Enhanced care
Our definition of enhanced care included proactive telephone calls from a respiratory health-care professional and helplines available to patients or visits from health-care professionals, all as a means to reinforce information/techniques/strategies and encourage behaviour change. The interventions were generally delivered by a respiratory nurse or physiotherapist/physiologist. This has not been addressed in other systematic reviews. We have identified improvements in HRQoL and reduced hospital admissions after 6 months of follow-up, suggesting that these enhancements should be considered further. The analyses had high levels of heterogeneity, so further exploration of the individual components would be useful.
Role of exercise alone or within larger self-management packages
Group-based self-management with supervised exercise as part of a multicomponent self-management intervention
Our analysis of SM interventions with supervised exercise is similar to that of Lacasse et al. ’s46 Cochrane review of PR programmes. Although the Lacasse et al. review46 included six trials in their analysis of total SGRQ, we included 18, and found a similar effect size at our follow-up points up to 6 months, but an attenuated effect after one year follow-up. We had higher heterogeneity than that reported by Lacasse et al. ,46 which may reflect our wider inclusion criteria. We have been able to extend the Lacasse review by reporting hospital admissions and exacerbations; however, the number of trials in these analyses was low (five and three, respectively) and no significant effects were seen.
Multicomponent self-management without supervised exercise
We undertook subgroup analyses to explore the effect of the level of supervision and amount of exercise advice and support in multicomponent SM interventions. The unsupervised exercise was structured in terms of frequency, duration and intensity, but did not take place in a centre or group setting. Although we identified an average effect on the SGRQ at 3 months that was significant, evidence of effect in the longer term was absent. Interventions that included exercise advice only or no exercise at all (as part of a multicomponent intervention) had no evidence of effectiveness.
To further explore the role of exercise we investigated the effect of strength and aerobic exercise training compared with UC. These interventions all included at least one other SM component with the exercise. This showed significantly higher HRQoL scores, but no effect on hospital admissions and exacerbations.
In a systematic review, Zainuldin et al. 294 explored intensity of leg training and type of training (interval compared with continuous). Three trials compared higher-intensity training with lower-intensity training but the pooled effect showed no significant difference between the groups on 6-Minute Walk Distance. HRQoL and hospital admissions/exacerbations were not reported. There was no significant difference between the interval and continuous training groups in the eight included studies, for any of the outcomes, including HRQoL. 294 In our study we also found no difference between interval and continuous training.
Role of aerobic and resistance exercise
To unpick the relative contributions of resistance (strength) and aerobic exercise on HRQoL, hospital admissions and exacerbations, we undertook direct comparisons. Only two trials233,246 directly compared resistance and aerobic exercise with aerobic exercise only, with no difference in average HRQoL between the exercise arms. No trials reported hospital admissions.
Four trials compared endurance with resistance exercise showing no effect of HRQoL or hospital admissions. 216,233,246,247
Respiratory muscle training
Two main groups of interventions came under this category. Sívori et al. 263 compared upper limb exercises to UC, with no significant effect on HRQoL. Our findings are similar to those of Costi et al. ,295 who reported the HRQoL outcomes individually for three trials, all of which found no significant difference.
We identified a large number of trials that evaluated inspiratory muscle training (IMT) and expiratory muscle training (EMT) using threshold devices (20 trials: see Appendix 27). These either had UC or sham devices (set at the lowest setting for resistance) as the comparison group. Only four trials163,184,253,254 reported disease-specific HRQoL using the SGRQ or CRQ and one trial253 reported hospital admissions. We did not identify any evidence of effectiveness of RMT on these outcomes.
Our findings can be compared with those of systematic reviews of IMT compared with UC,296 and IMT or IMT plus PR compared with other rehabilitation interventions. 297 Geddes et al. 296 had only two trials that compared IMT to sham treatment and reported the total CRQ (weighted MD 0.33, 95% CI 0.19 to 1.47). The review by O’Brien et al. 297 reported only the individual subscales for the CRQ, but found a significantly greater improvement in the dyspnoea subscale for the exercise-only interventions than the interventions that included IMT (CRQ dyspnoea 1.94, 95% CI 1.01 to 2.88).
Interventions delivered by particular professional groups
Three trials136,251,266,285 reported SM interventions by pharmacists. We hypothesised that the multicomponent interventions that they delivered would have a particular focus on medication management, which was a component of all three trials. The combined effects on HRQoL were not significant, but Jarab et al. 266 reported a significant reduction in hospital admissions at 6 months’ follow-up.
Other systematic reviews have reported the effects of interventions delivered by physiotherapists298 and of outreach nursing. 52 The review of outreach nursing52 has considerable overlap with the concept of integrated disease management. Wong et al. 52 reported a significant improvement in HRQoL, but no effect on mortality or hospital admissions.
How the evidence fits with other long-term conditions
All our included trials took a patient-based approach in which the SM was delivered to patients in the form of group-based or individual education and other support. A large UK-based cluster randomised trial (WISE: Whole System informing self-management engagement),34 published after our search was completed, sought to support primary care practitioners to embed SM support into their everyday practice. The trial recruited 1634 patients with COPD in primary care but did not find statistically significant improvements in self-efficacy, generic HRQoL or shared decision-making. The authors cited difficulties with implementation in a ‘real’ primary care setting with an unselected group of patients.
Recent studies of note since our searches were undertaken
A recent trial34 was halted prematurely after interim analysis identified a higher mortality rate in the group that received the SM intervention. The intervention failed to increase knowledge or use of antibiotics as part of action planning, but the findings are otherwise unexplained. An analysis of mortality rates across all of the included studies in our review might help identify whether this was an outlying result.
Strengths and limitations
Strengths
This is the largest systematic review of SM for COPD, with 174 trials reporting our three outcomes in 229 comparisons. We had no exclusions by language or publication date and included 12 trials that were reported in a language other than English. The review was undertaken with two people independently selecting titles, abstracts and full papers for inclusion/exclusion, with a third person reviewing and deciding on papers where there was a disagreement, and group discussion about papers and interventions that were difficult to categorise. We used an extensive data extraction form to extract directly and – when not reported – indirectly calculate statistical results for the intervention effects of interest. This allowed us to incorporate a larger number of studies in the meta-analysis than previous reviews, especially with regard to HRs.
Many of our subgroup analyses cover topics of published systematic reviews and we were able to extend the included papers in a number of these. We have also explored additional groups of interventions, for example in relation to the level of supervision and specification of the exercise component. Although we would have liked to undertake indirect comparisons to explore individual or groups of components further, this was not possible due to the heterogeneity of populations, interventions and comparators.
Heterogeneity was apparent in most meta-analyses but the causes of heterogeneity were difficult to identify due to the small number of studies in most meta-analyses and the potential for trial-level confounding when exploring heterogeneity. Therefore, to help summarise the heterogeneity more clearly, when five or more studies were included in the meta-analysis we reported 95% prediction intervals. These revealed the range of possible intervention effects caused by unexplained heterogeneity. However, this interval may also reflect heterogeneity caused by small-study effects and low-quality primary studies rather than just clinical causes of heterogeneity.
Limitations of studies within the review
The main limitations of our review result from the heterogeneity of both the interventions and the comparison groups, and the general poor standard of reporting and conduct in many of the identified trials. The included trials were often small, with 46% having < 50 participants; few included a power calculation; and the reporting of method of randomisation, allocation concealment and blinding of outcome assessment was often absent or poorly described. When undertaking risk of bias assessment we did not use explicit cut-offs for a certain attrition or imbalance between study groups because so many studies had small sample sizes and these thresholds were easily crossed by one or two more participants lost to follow-up in one study arm compared with the other.
As many of the trials used a ‘UC’ comparator, it was not possible to blind participants to their allocation. This is likely to lead to an attention effect, when the participants in the active intervention arm have a more positive experience and often more social support through group-based activities. As most of the trials reported HRQoL which often includes a mental or social component and follow-up was frequently only undertaken at the end of the intervention period, attention bias is likely.
Limitations of our review methods
In defining SM interventions we took a very broad perspective but tried to exclude those interventions when the intervention was largely provided by a professional. Thus hospital-at-home interventions were included only if they expressly described a SM or educational component. Disease management programmes were excluded if they were telemonitoring without an educational or SM element. Some exercise programmes that were delivered by physiotherapists were short term and appeared to describe something ‘done to’ the patient rather than teaching them to self-manage; these were excluded. Owing to the large number of papers identified and a number of people reviewing abstracts and papers for eligibility, there will inevitably be some inconsistencies in relation to inclusion/exclusion. We tried to minimise this through regular team discussions about papers that we were unsure of including.
We planned to undertake full independent double data extraction on all papers but, owing to the large number of eligible papers, only one person extracted the characteristics and outcomes, with a 20% check of the outcome data and a 100% check for key characteristics such as number of participants, duration of intervention and duration of follow-up. To ensure consistency the same person categorised the components in all trials.
In extracting HRQoL outcome data we focused on the disease-specific measures (SGRQ and CRQ) and have not reported the generic HRQoL outcomes, as a wide variety of these were reported in a small number of trials. We decided not to combine the findings of the SGRQ and CRQ in meta-analysis, as they report different domains. In addition, the reporting of the actual point differences in meta-analysis on the original scales, rather than a standardised MD, makes interpretation easier.
The admission results were reported in several different ways, for example first admission, mean admissions, etc. Although ideally we would like to be able to capture all of this information, especially because some patients may have multiple admissions, current methodologies are inadequate to do so. We chose rate of first admission because there were more data available; however, it is not clear how the effect of the interventions would vary if all admissions could be considered.
The trials reported a large number of outcomes, and trials that met our inclusion criteria in relation to population and intervention – but did not report one of our three primary outcomes (HRQoL, hospital admissions and exacerbations) – are listed but not described. Although we acknowledge the importance of other outcomes, such as exercise capacity, the focus of this review was health service utilisation and patient QoL. Mortality was rarely reported as an outcome but can be obtained from the reasons for loss to follow-up. Papers often did not report the cause of death (respiratory or other cause) and we cannot be sure about completeness, given that few trials specified mortality as an outcome measure. Therefore, we did not include mortality in our analyses.
We have undertaken a large number of comparisons, with the associated risk of identifying significant effects due to chance. However, this review was planned to be exploratory in nature and we are cautious in the interpretation of our findings.
We had planned to undertake indirect comparisons of clusters of intervention components but did not do this owing to considerable heterogeneity of the UC arms and difficulties in identifying potential comparison groups. The heterogeneity and low-quality studies led us to conclude that the consistency assumption (which is required to undertake a mixed-treatment comparison) was unlikely to be plausible, and thus indirect comparisons were not considered. 107
Generalisability
Our trials were set in 21 different countries, suggesting that our findings can be generalised across a range of different health-care settings. We did not explore the effect of location, and thus the standard level of COPD care as a potential cause of heterogeneity but it may be an important factor. In particular, we did not focus on studies undertaken only in the UK. Most of the trial participants were recruited from secondary care settings – usually hospital outpatients – and < 6% were from primary care. In addition, the participants generally had moderate or severe COPD, as defined by GOLD criteria. Thus our findings may not be generalised well to populations with milder COPD managed in primary care. In addition, trials may recruit participants who are more affluent or have a higher educational level than the general population. Given the fundamental role of self-efficacy in many SM interventions, the representativeness of the participants is key. Comparisons of the characteristics of recruited participants and people who decline to take part in a trial are rarely reported, so we are unable to comment on the generalisability of the trial participants in these aspects.
Chapter 8 Overall discussion
Introduction
This report had two aims: to evaluate the effectiveness and cost-effectiveness of SM commencing within 6 weeks of hospital discharge for an exacerbation of COPD, and to explore which components or mechanisms of delivery appear most promising in terms of the effectiveness of SM interventions for COPD in general.
Main findings
The review of SM post discharge identified no evidence of benefit of early SM support on admissions, mortality and most other health outcomes, although a modest, but possibly biased, improvement in HRQoL. However, the direction of the effect for many of the outcomes (including admissions) favoured the SM intervention. A speculative economic model was developed to explore the cost-effectiveness of such an intervention – if it were truly effective at reducing hospital admissions. The main drivers of the model were the effect on hospital readmission, the duration of the effect and the cost of a SM programme. To be cost-effective, a SM programme post admission for an acute exacerbation would need to cost no more than £2200 if there was an 18% reduction in readmissions. The sensitivity analysis suggested that SM had a probability of 68% of being cost-effective at a threshold incremental cost-effectiveness ratio of £20,000 per quality-adjusted life-year, demonstrating the uncertainty around the impact of SM on readmissions.
The second study was an exploratory review of the broad SM and PR literature to try to determine the components and mechanisms of delivery that are associated with better outcomes. Multicomponent (at least three individual components) SM interventions are likely to be effective, but the degree of heterogeneity suggests that there are important features of these interventions that need to be established; those with supervised exercise (as in a PR programme) or structured, unsupervised exercise (as in a home rehabilitation programme) appear effective. SM programmes that provide an enhanced level of care and support may reduce hospital admissions in the medium term (6 months).
Except for exercise-only interventions, there were surprisingly few trials of individual SM components, and few for which the difference between study groups was only one component. Notably, there was no evidence that action plans were effective by themselves.
Overall conclusion
It is difficult to recommend any type of SM support to be provided immediately after discharge with the evidence available, as there is no clear evidence of effect across most of the outcomes. Notwithstanding, the point estimate is consistent with ≈20% reduction in admissions, which has been observed in other systematic reviews of COPD SM interventions.
Although some components of SM interventions are associated with positive effects of HRQoL, such as structured exercise (either within a supervised group or home based), enhanced care and multicomponent interventions, it was not possible to establish the relative roles of the individual components in reducing hospital admissions and improving HRQoL.
Recommendations for future research
-
Current interventions to support patient SM that is delivered post discharge cannot currently be recommended because interventions are heterogeneous and methodology is problematic, and, despite there being potential benefit in terms of HRQoL, there is not enough good evidence to be sure that clinical outcomes could be improved. Therefore:
-
High-quality studies should be undertaken among patients with COPD disease post discharge.
-
This should include qualitative work to explore barriers and facilitators to SM when patients have recently had an exacerbation, exploration of novel approaches to affect behaviour change, and exploration of approaches tailored to the individual and their circumstances.
-
New approaches should be evaluated by properly designed and conducted trials, with special attention to reducing loss to follow-up.
-
-
Owing to the heterogeneity and complexity of interventions, it was not possible to unpick the most important components of SM interventions in general or to confirm whether they improve clinical outcomes. It is clear that action plans alone do not seem to work in their present form, but that structured exercise and more heavily supported interventions (which may not usually be defined as SM) might work better. Therefore:
-
Further in-depth work using individual patient data (e.g. an Individual Patient Data meta-analysis) should be carried out to try to identify which are the most effective components of interventions and identify patient-specific factors that may modify this. This work is ongoing by other researchers.
-
Future studies might try to identify the characteristics of patients who are more likely to be able to self-manage, and consider a more targeted approach.
-
Further qualitative work is needed to explore patients’ barriers and facilitators to SM interventions.
-
Novel approaches to influence behaviour change and to help patients manage or prevent exacerbations should be explored, first using qualitative studies and then properly designed and conducted randomised controlled trials (RCTs). Most trials include a mixture of components; more trials teasing out the individual elements, either as lone interventions, or with the addition of one component, would be useful.
-
-
Recommendations for the design and conduct of future RCTs of interventions to support patient SM:
-
In general, new trials should adhere to modern standards of design, conduct and reporting in order to reduce risks of bias, for example blinding of outcome assessment, attempts to maximise follow-up or methods to impute this, and reporting of the characteristics of all randomised patients.
-
The behaviour change theories and strategies that underpin COPD SM interventions need to be better characterised and described.
-
A clear framework for describing and classifying SM interventions and their comparators is required.
-
Trials need to be adequately powered to detect a clinically relevant difference and long enough to assess changing effects over time. There should be clear reporting of outcomes to include self-efficacy, behaviour change and clinical outcomes such as hospital admissions and exacerbations.
-
Given the wide range of HRQoL outcomes available, it would be useful to standardise their use within COPD research and ensure that they are reported accurately within publications.
-
Statistical analysis methods should be improved: in particular (1) analysis of HRQoL outcomes should routinely adjust for baseline values to overcome baseline imbalance, account for correlation between final score and baseline score, and increase statistical power; and (2) time-to-event outcomes (such as admissions, mortality, etc.) should be analysed using suitable analyses that allow for differential patient follow-up, and summarised using HRs (rather than odds ratios).
Acknowledgements
The authors thank the following:
-
Simon Stevens for his invaluable administrative support and excellent organisational skills, and keeping us all going throughout a complex project.
-
All of the people who kindly gave their time to help translate articles: Yumiko Akiya, Dom Barkos, Susan Bayliss, Matthew Blackburn, Yumi Chen, Jennifer Choi, Karin Diaconu, Janine Dretzke, Maxwell Feltham, Ditte Hedegaard, Boris Kysela, Antje Lindenmeyer, Kinga Malottki, Cristina Peñaloza and Amanda Zhang.
Contributions of authors
Rachel E Jordan (Senior Lecturer, Public Health/Epidemiology) (co-principal investigator) wrote the protocol, co-directed and developed the review, chaired the team and investigator meetings, liaised with the Public and Patient Participation group, contributed to study selection, undertook and oversaw data extraction and risk of bias assessment for reviews 1 and 2, wrote the background, oversaw and wrote the results for reviews 1 and 2, and commented on and edited all other aspects of the report.
Saimma Majothi (Research Fellow, Systematic Reviews), lead reviewer, led and coordinated the systematic review, led study selection, led the development of risk of bias and data extraction tools, undertook classification of studies, extracted results of studies for reviews 1–4, undertook risk of bias assessment for reviews 1–4, coordinated data analysis, wrote the methods sections, wrote sections for the qualitative review (review 2), wrote the cost-effectiveness review (review 3), drafted interim reports and commented on the final report.
Nicola R Heneghan (Lecturer, Physiotherapy), second reviewer, contributed to review development, study selection, data extraction and risk of bias assessment, and commented on the final report.
Deirdre B Blissett (Research Fellow, Health Economics) led and wrote the section on the economic model, undertook risk of bias for review 3 and commented on the final report.
Richard D Riley (Professor, Biostatistics) advised on methodology of the protocol, advised on statistical and reviewing methods, developed data extraction form for statistical results, undertook initial statistical analyses, supervised statistical analyses, contributed to writing the statistical methods and commented on the final report.
Alice J Sitch (Research Fellow, Biostatistics) undertook analyses for review 4, contributed to methods of analyses and commented on the final report.
Malcolm J Price (Research Fellow, Biostatistics) extracted samples of results, advised and undertook statistical analyses for review 1, wrote analysis methods for review 1, and commented on the final report.
Elizabeth J Bates (Academic Clinical Lecturer, Primary Care) contributed to risk of bias assessment, contributed to data extraction, provided clinical input, advised on costs and commented on the final report.
Alice M Turner (Clinician Scientist and Honorary Consultant Physician) contributed to study selection, provided data and advice on components of cost for the economic modelling, provided clinical input to protocol and review, and commented on the final report.
Susan Bayliss (Information Specialist) advised on, and performed, search strategies.
David Moore (Senior Lecturer, Systematic Reviews) advised on methodology of protocol, provided ongoing advice on conduct of review and methodology, contributed to study selection and risk of bias, provided technical support and commented on the final report.
Sally Singh (Professor and Head of Cardiac and Pulmonary Rehabilitation, University Hospitals of Leicester NHS Trust) provided clinical input at investigator meetings and specific questions in the interim and commented on the final report.
Peymane Adab (Professor, Public Health) contributed to risk of bias assessment, provided epidemiological input and commented on the final report.
David A Fitzmaurice (Professor, Primary Care) commented on the protocol, contributed to the study selection, provided clinical input at the investigator meetings and commented on the final report.
Susan Jowett (Senior Lecturer, Health Economics) commented on the protocol, oversaw the economic model, provided methodological input at the investigator meetings, edited the economic model chapter and commented on the final report.
Kate Jolly (Professor, Public Health) (co-principal investigator) contributed to the protocol, co-directed and developed the review, co-chaired the team and investigator meetings, contributed to the study selection, undertook and oversaw the data extraction and risk of bias assessment for review 4, oversaw the analyses, undertook the descriptive analyses and wrote results for review 4, and commented on/edited all of the other aspects of the report.
Publications
Part of this report has been presented at the International Primary Care Respiratory Group (IPCRG) conference in Stockholm (May 2013) and the British Thoracic Society conference in London (November 2013).
Funding
Rachel Jordan was funded by the NIHR with a post-doctoral research fellowship (pdf/01/2008/023). Kate Jolly is part-funded by the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care West Midlands.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Vancouver, WA: GOLD; 2013.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765-73. http://dx.doi.org/10.1016/S0140-6736(07)61380-4.
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006;28:523-32. http://dx.doi.org/10.1183/09031936.06.00124605.
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM. International variation in the prevalence of COPD (The BOLD Study): a population-based prevalence study. Lancet 2007;370:741-50. http://dx.doi.org/10.1016/S0140-6736(07)61377-4.
- Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997;349:1498-504. http://dx.doi.org/10.1016/S0140-6736(96)07492-2.
- An Outcomes Strategy for People with Chronic Obstructive Pulmonary Disease (COPD) and Asthma in England. London: DH; 2011.
- Britton M. The burden of COPD in the UK: results from the confronting COPD survey. Respir Med 2003;97:71-9. http://dx.doi.org/10.1016/S0954-6111(03)80027-6.
- Department of Health, Social Services and Public Safety . Annual Report of the Chief Medical Officer 2004. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4115781.pdf (accessed 19 January 2015).
- Clearing the Air. A National Study of Chronic Obstructive Pulmonary Disease. London: Healthcare Commission; 2006.
- Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care (Partial Update). London: NICE; 2010.
- Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax 2008;63:1046-51. http://dx.doi.org/10.1136/thx.2008.098483.
- Jones RC, Dickson-Spillmann M, Mather MJ, Marks D, Shackell BS. Accuracy of diagnostic registers and management of chronic obstructive pulmonary disease: the Devon primary care audit. Respir Res 2008;9. http://dx.doi.org/10.1186/1465-9921-9-62.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. http://dx.doi.org/10.1056/NEJMoa0909883.
- Jenkins CR, Celli B, Anderson JA, Ferguson GT, Jones PW, Vestbo J, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J 2012;39:38-45. http://dx.doi.org/10.1183/09031936.00194610.
- Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2004;1:115-20. http://dx.doi.org/10.1513/pats.2306030.
- Donaldson GC, Seemungal T, Jeffries DJ, Wedzicha JA. Effect of Environmental temperature on symptoms, lung function and mortality in COPD patients. Eur Respir J 1999;13:844-9. http://dx.doi.org/10.1034/j.1399-3003.1999.13d25.x.
- Roberts CM, Lowe D, Bucknall CE, Ryland I, Kelly Y, Pearson MG. Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax 2002;57:137-41. http://dx.doi.org/10.1136/thorax.57.2.137.
- Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. http://dx.doi.org/10.1136/thorax.57.10.847.
- Care Planning. Improving the Lives of People with Long Term Conditions. London: RCGP; 2011.
- Department of Health (DH) . The NHS Improvement Plan. Putting People at the Heart of Public Services 2004. www.bjhc.co.uk/autumnforum/docs/NHS%20Improvement%20plan.pdf (accessed 28 November 2006).
- Improving the Health and Well-Being of People with Long-Term Conditions. London: DoH; 2010.
- MORI Survey: Public Attitudes to Self Care Baseline Survey. London: DoH; 2005.
- Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns 2010;48:177-87. http://dx.doi.org/10.1016/S0738-3991(02)00032-0.
- Cramm JM, Nieboer AP. Self-management abilities, physical health and depressive symptoms among patients with cardiovascular diseases, chronic obstructive pulmonary disease, and diabetes. Patient Educ Couns 2012;87:411-15. http://dx.doi.org/10.1016/j.pec.2011.12.006.
- Disler RT, Gallagher RD, Davidson PM. Factors influencing self-management in chronic obstructive pulmonary disease: an integrative review. Int J Nurs Stud 2012;49:230-42. http://dx.doi.org/10.1016/j.ijnurstu.2011.11.005.
- Cecere LM, Slatore CG, Uman JE, Evans LE, Udris EM, Bryson CL, et al. Adherence to long-acting inhaled therapies among patients with chronic obstructive pulmonary disease (COPD). COPD 2012;9:251-8. http://dx.doi.org/10.3109/15412555.2011.650241.
- Kaptein AA, Scharloo M, Fischer MJ, Snoei L, Cameron LD, Sont JK, et al. Illness perceptions and COPD: an emerging field for COPD patient management. J Asthma 2008;45:625-9. http://dx.doi.org/10.1080/02770900802127048.
- Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004;39:1005-26. http://dx.doi.org/10.1111/j.1475-6773.2004.00269.x.
- Bourbeau J, van der Palen J. Promoting effective self-management programmes to improve COPD. Eur Respir J 2009;33:461-3. http://dx.doi.org/10.1183/09031936.00001309.
- Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006;173:1390-413. http://dx.doi.org/10.1164/rccm.200508-1211ST.
- Bandura A. Self-efficacy: toward a unifying theory of behavioural change. Psychol Rev 1977;84:191-215. http://dx.doi.org/10.1037/0033-295X.84.2.191.
- Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med 2004;164:1641-9. http://dx.doi.org/10.1001/archinte.164.15.1641.
- Chodosh J, Morton SC, Mojica W, Maglione M, Suttorp MJ, Hilton L, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med 2005;143:427-38. http://dx.doi.org/10.7326/0003-4819-143-6-200509200-00007.
- Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2882.
- Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med 2012;156:673-83. http://dx.doi.org/10.7326/0003-4819-156-10-201205150-00003.
- Bischoff EW, Akkermans R, Bourbeau J, van Weel C, Vercoulen JH, Schermer TR. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: randomised controlled trial. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e7642.
- Effing TW, Bourbeau J, Vercoulen J, Apter AJ, Coultas D, Meek P, et al. Self-management programmes for COPD: moving forward. Chron Respir Dis 2012;9:27-35. http://dx.doi.org/10.1177/1479972311433574.
- Hernandez P, Balter M, Bourbeau J, Hodder R. Living with chronic obstructive pulmonary disease: a survey of patients’ knowledge and attitudes. Respir Med 2009;103:1004-12. http://dx.doi.org/10.1016/j.rmed.2009.01.018.
- Fernandes A, Pache S, Bird W, Bryden C. Measures to improve knowledge and self-care among patients with COPD: a UK general practice audit. Prim Care Resp J 2006;15:307-9. http://dx.doi.org/10.1016/j.pcrj.2006.07.001.
- NICE COPD Quality Standards QS10. London: NICE; 2013.
- British Lung Foundation . COPD Self Management Plan. Helping Patients to Manage Their Chronic Obstructive Pulmonary Disease 2010. www.lunguk org/supporting-you/Publications/copd_self_management_plan (accessed 24 June 2010).
- Osterlund Efraimsson E, Klang B, Larsson K, Ehrenberg A, Fossum B. Communication and self-management education at nurse-led COPD clinics in primary health care. Patient Educ Couns 2009;77:209-17. http://dx.doi.org/10.1016/j.pec.2009.03.033.
- Rodgers S, Dyas J, Molyneux AW, Ward MJ, Revill SM. Evaluation of the information needs of patients with chronic obstructive pulmonary disease following pulmonary rehabilitation: a focus group study. Chron Respir Dis 2007;4:195-203. http://dx.doi.org/10.1177/1479972307080698.
- Wortz K, Cade A, Menard JR, Lurie S, Lykens K, Bae S, et al. A qualitative study of patients’ goals and expectations for self-management of COPD. Prim Care Respir J 2012;21:384-91. http://dx.doi.org/10.4104/pcrj.2012.00070.
- Wagg K. Unravelling self-management for COPD: what next?. Chron Respir Dis 2012;9:5-7. http://dx.doi.org/10.1177/1479972311435910.
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.CD003793.pub2.
- Kruis AL, Smidt N, Assendelft WJ, Gussekloo J, Boland MR, Rutten-van Mölken M, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013;10.
- Effing T, Monninkhof EM, van der Valk PD, van der Palen J, van Herwaarden CL, Partidge MR, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007;4.
- Peytremann-Bridevaux I, Staeger P, Bridevaux PO, Ghali WA, Burnand B. Effectiveness of chronic obstructive pulmonary disease-management programs: systematic review and meta-analysis. Am J Med 2008;121:433-43. http://dx.doi.org/10.1016/j.amjmed.2008.02.009.
- Walters JA, Turnock AC, Walters EH, Wood-Baker R. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2010;5. http://dx.doi.org/10.1002/14651858.CD005074.pub3.
- Holland AE, Hill CJ, Jones AY, McDonald CF. Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;10. http://dx.doi.org/10.1002/14651858.CD008250.pub2.
- Wong CX, Carson KV, Smith BJ, Wong CX, Carson KV, Smith BJ. Home care by outreach nursing for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;4.
- Zainuldin R, Mackey MG, Alison JA. Optimal intensity and type of leg exercise training for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011;11. http://dx.doi.org/10.1002/14651858.CD008008.pub2.
- Osthoff M, Leuppi JD. Management of chronic obstructive pulmonary disease patients after hospitalization for acute exacerbation. Respiration 2010;79:255-61. http://dx.doi.org/10.1159/000235721.
- Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011;10. http://dx.doi.org/10.1002/14651858.CD005305.pub3.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.2 [updated September 2009]. Chichester: John Wiley & Sons; 2009.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. http://dx.doi.org/10.1136/bmj.39489.470347.AD.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. http://dx.doi.org/10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8.
- Perneger TV. Estimating the relative hazard by the ratio of logarithms of event-free proportions. Contemp Clin Trials 2008;29:762-6. http://dx.doi.org/10.1016/j.cct.2008.06.002.
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004;23:1351-75. http://dx.doi.org/10.1002/sim.1761.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. http://dx.doi.org/10.1016/0197-2456(86)90046-2.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. http://dx.doi.org/10.1136/bmj.327.7414.557.
- Bucknall CE, Miller G, Lloyd SM, Cleland J, McCluskey S, Cotton M, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e1060.
- Behnke M, Taube C, Kirsten D, Lehnigk B, Jorres RA, Magnussen H. Home-based exercise is capable of preserving hospital-based improvements in severe chronic obstructive pulmonary disease. Respir Med 2000;94:1184-91. http://dx.doi.org/10.1053/rmed.2000.0949.
- Behnke M, Jorres RA, Kirsten D, Magnussen H. Clinical benefits of a combined hospital and home-based exercise programme over 18 months in patients with severe COPD. Monaldi Arch Chest Dis 2003;59:44-51.
- Lee DT, Lee IF, Mackenzie AE, Ho RN. Effects of a care protocol on care outcomes in older nursing home patients with chronic obstructive pulmonary disease. J Am Geriatr Soc 2002;50:870-6. http://dx.doi.org/10.1046/j.1532-5415.2002.50213.x.
- Hermiz O, Comino E, Marks G, Daffurn K, Wilson S, Harris M. Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ 2002;325. http://dx.doi.org/10.1136/bmj.325.7370.938.
- Hernandez C, Casas A, Escarrabill J, Alonso J, Puig-Junoy J, Farrero E, et al. Home hospitalisation of exacerbated chronic obstructive pulmonary disease patients. Eur Respir J 2003;21:58-67. http://dx.doi.org/10.1183/09031936.03.00015603.
- Egan E, Clavarino A, Burridge L, Teuwen M, White E. A randomized control trial of nursing-based case management for patients with chronic obstructive pulmonary disease. Lippincotts Case Manag 2002;7:170-9. http://dx.doi.org/10.1097/00129234-200209000-00002.
- Kwok T, Lum CM, Chan HS, Ma HM, Lee D, Woo J. A randomized, controlled trial of an intensive community nurse-supported discharge program in preventing hospital readmissions of older patients with chronic lung disease. J Am Geriatr Soc 2004;52:1240-6. http://dx.doi.org/10.1111/j.1532-5415.2004.52351.x.
- Casas A, Troosters T, Garcia-Aymerich J, Roca J, Hernandez C, Alonso A, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J 2006;28:123-30. http://dx.doi.org/10.1183/09031936.06.00063205.
- Garcia-Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez-Roisin R, Anto JM, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respir Med 2007;101:1462-9. http://dx.doi.org/10.1016/j.rmed.2007.01.012.
- Dheda K, Crawford A, Hagan G, Roberts CM. Implementation of British Thoracic Society guidelines for acute exacerbation of chronic obstructive pulmonary disease: impact on quality of life. Postgrad Med J 2004;80:169-71. http://dx.doi.org/10.1136/pgmj.2003.012831.
- Wong KW, Wong FK, Chan MF. Effects of nurse-initiated telephone follow-up on self-efficacy among patients with chronic obstructive pulmonary disease. J Adv Nurs 2005;49:210-22. http://dx.doi.org/10.1111/j.1365-2648.2004.03280.x.
- Aimonino Ricauda N, Tibaldi V, Leff B, Scarafiotti C, Marinello R, Zanocchi M, et al. Substitutive ‘hospital at home’ versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: a prospective randomized, controlled trial. J Am Geriatr Soc 2008;56:493-500. http://dx.doi.org/10.1111/j.1532-5415.2007.01562.x.
- Puhan M, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2009;1.
- Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD. NETT Research Group . Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD 2008;5:105-16. http://dx.doi.org/10.1080/15412550801941190.
- Wedzicha JA, Vestbo J. Can patients with COPD self-manage?. Lancet 1918;380:624-5. http://dx.doi.org/10.1016/S0140-6736(12)61339-7.
- Hopkinson NS, Englebretsen C, Cooley N, Kennie K, Lim M, Woodcock T, et al. Designing and implementing a COPD discharge care bundle. Thorax 2011;67:90-2. http://dx.doi.org/10.1136/thoraxjnl-2011-200233.
- Critical Appraisal Skills Programme (CASP) . Qualitative Research Appraisal Tool. 10 Questions to Help You Make Sense of Qualitative Research n.d. http://media.wix.com/ugd/dded87_951541699e9edc71ce66c9bac4734c69.pdf (accessed 19 January 2015).
- Noblit GW, Hare RD. Meta-Ethnography: Synthesizing Qualitative Studies. London: Sage Publications; 1988.
- Liu SX, Lee MC, Atakhorrami M, Tatousek J, McCormack M, Yung R, et al. Economic assessment of home-based COPD management programs. COPD 2013;10:640-9. http://dx.doi.org/10.3109/15412555.2013.813447.
- Borg S, Ericsson A, Wedzicha J, Gulsvik A, Lundback B, Donaldson GC, et al. A computer simulation model of the natural history and economic impact of chronic obstructive pulmonary disease. Value Health 2004;7:153-67. http://dx.doi.org/10.1111/j.1524-4733.2004.72318.x.
- An International Comparison of COPD Care in Europe: Results of the First European COPD Audit. Lausanne: European Respiratory Society; 2012.
- Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009;10. http://dx.doi.org/10.1186/1465-9921-10-59.
- Atsou K, Chouaid C, Hejblum G. Simulation-based estimates of effectiveness and cost-effectiveness of smoking cessation in patients with chronic obstructive pulmonary disease. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0024870.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. http://dx.doi.org/10.1007/s11136-011-9903-x.
- Euroqol Group . EQ-5D-5L Value Sets n.d. www.euroqol.org/about-eq-5d/valuation-of-eq-5d/eq-5d-5l-value-sets.html#c655 (accessed 19 January 2015).
- Rutten-van Mölken MP, Oostenbrink JB, Miravitlles M, Monz BU. Modelling the 5-year cost-effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. Eur J Health Econ 2007;8:123-35. http://dx.doi.org/10.1007/s10198-007-0039-4.
- Oostenbrink JB, Rutten-van Mölken MP, Monz BU, FitzGerald JM. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health 2005;8:32-46. http://dx.doi.org/10.1111/j.1524-4733.2005.03086.x.
- Oba Y. Cost-effectiveness of long-term oxygen therapy for chronic obstructive disease. Am J Manag Care 2009;15:97-104.
- Hertel N, Kotchie RW, Samyshkin Y, Radford M, Humphreys S, Jameson K. Cost-effectiveness of available treatment options for patients suffering from severe COPD in the UK: a fully incremental analysis. Int J Chron Obstruct Pulmon Dis 2012;7:183-99. http://dx.doi.org/10.2147/COPD.S29820.
- Earnshaw SR, Wilson MR, Dalal AA, Chambers MG, Jhingran P, Stanford R, et al. Cost-effectiveness of fluticasone propionate/salmeterol (500/50 microg) in the treatment of COPD. Respir Med 2009;103:12-21. http://dx.doi.org/10.1016/j.rmed.2008.10.005.
- Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost-effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics 2005;23:619-37. http://dx.doi.org/10.2165/00019053-200523060-00008.
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:1608-13. http://dx.doi.org/10.1164/ajrccm.161.5.9908022.
- Perera WR, Hurst JR, Wilkinson TM, Sapsford RJ, Mullerova H, Donaldson GC, et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J 2007;29:527-34. http://dx.doi.org/10.1183/09031936.00092506.
- Aaron SD, Donaldson GC, Whitmore GA, Hurst JR, Ramsay T, Wedzicha JA. Time course and pattern of COPD exacerbation onset. Thorax 2012;67:238-43. http://dx.doi.org/10.1136/thoraxjnl-2011-200768.
- NHS Reference Costs 2010–11. London: DH; 2011.
- Curtis L. Unit Costs of Health Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- NCL Care Pathway Review – Proposed NCL Community COPD Contracting Model. London: NHS Commissioning for London; 2010.
- The Seasonal Influenza Immunisation Programme. Consultation: a Review of the Procurement of Seasonal Flu Vaccine. London: DH; 2013.
- Chronic Obstructive Pulmonary Disease: Costing report – Implementing NICE Guidance. London: NICE; 2011.
- National Health Service of England and Wales Electronic Drug Tariffs (2013). London: DH; 2013.
- Bakerley ND, Davies C, Dyer M, Dhillon P. Cost analysis of an integrated care model in the management of acute exacerbations of chronic obstructive pulmonary disease. Chron Respir Dis 2009;6:201-8. http://dx.doi.org/10.1177/1479972309104279.
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d549.
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d4002.
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900. http://dx.doi.org/10.1136/bmj.331.7521.897.
- Bauldoff GS, Hoffman LA, Zullo TG, Sciurba FC. Exercise maintenance following pulmonary rehabilitation: effect of distractive stimuli. Chest 2002;122:948-54. http://dx.doi.org/10.1378/chest.122.3.948.
- Bauldoff GS, Rittinger M, Nelson T, Doehrel J, Diaz PT. Feasibility of distractive auditory stimuli on upper extremity training in persons with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2005;25:50-5. http://dx.doi.org/10.1097/00008483-200501000-00011.
- Berry MJ, Rejeski WJ, Miller ME, Adair NE, Lang W, Foy CG, et al. A lifestyle activity intervention in patients with chronic obstructive pulmonary disease. Respir Med 2010;104:829-39. http://dx.doi.org/10.1016/j.rmed.2010.02.015.
- Blake RL, Vandiver TA, Braun S, Bertuso DD, Straub V. A randomized controlled evaluation of a psychosocial intervention in adults with chronic lung disease. Fam Med 1990;22:365-70.
- Coultas D, Frederick J, Barnett B, Singh G, Wludyka P. A randomized trial of two types of nurse-assisted home care for patients with COPD. Chest 2005;128:2017-24. http://dx.doi.org/10.1378/chest.128.4.2017.
- Covey MK, Larson JL, Wirtz SE, Berry JK, Pogue NJ, Alex CG, et al. High-intensity inspiratory muscle training in patients with chronic obstructive pulmonary disease and severely reduced function. J Cardiopulm Rehabil 2001;21:231-40. http://dx.doi.org/10.1097/00008483-200107000-00008.
- Donesky-Cuenco D, Nguyen HQ, Paul S, Carrieri-Kohlman V. Yoga therapy decreases dyspnea-related distress and improves functional performance in people with chronic obstructive pulmonary disease: a pilot study. J Altern Complement Med 2009;15:225-34. http://dx.doi.org/10.1089/acm.2008.0389.
- Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol 1998;17:232-40. http://dx.doi.org/10.1037/0278-6133.17.3.232.
- Foy CG, Rejeski WJ, Berry MJ, Zaccaro D, Woodard CM. Gender moderates the effects of exercise therapy on health-related quality of life among COPD patients. Chest 2001;119:70-6. http://dx.doi.org/10.1378/chest.119.1.70.
- Gilmore TW, Walter RE, Davis TC, Wissing DR. Educational strategies to improve health-related quality of life in patients with COPD. Respir Care Educ Annu 2010;19:13-31.
- Guyatt G, Keller J, Singer J, Halcrow S, Newhouse M. Controlled trial of respiratory muscle training in chronic airflow limitation. Thorax 1992;47:598-602. http://dx.doi.org/10.1136/thx.47.8.598.
- Kim MJ, Larson JL, Covey MK, Vitalo CA, Alex CG, Patel M. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Nurs Res 1993;42:356-62. http://dx.doi.org/10.1097/00006199-199311000-00008.
- Koff PB, Jones RH, Cashman JM, Voelkel NF, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J 2009;33:1031-8. http://dx.doi.org/10.1183/09031936.00063108.
- Kunik ME, Veazey C, Cully JA, Souchek J, Graham DP, Hopko D, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: a randomized controlled trial. Psychol Med 2008;38:385-96. http://dx.doi.org/10.1017/S0033291707001687.
- Larson JL, Kim MJ, Sharp JT, Larson DA. Inspiratory muscle training with a pressure threshold breathing device in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1988;138:689-96. http://dx.doi.org/10.1164/ajrccm/138.3.689.
- Larson JL, Covey MK, Wirtz SE, Berry JK, Alex CG, Langbein WE, et al. Cycle ergometer and inspiratory muscle training in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:500-7. http://dx.doi.org/10.1164/ajrccm.160.2.9804067.
- Mador MJ, Krawza M, Alhajhusian A, Khan AI, Shaffer M, Kufel TJ. Interval training versus continuous training in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 2009;29:126-32. http://dx.doi.org/10.1097/HCR.0b013e31819a024f.
- Mador MJ, Deniz O, Aggarwal A, Shaffer M, Kufel TJ, Spengler CM. Effect of respiratory muscle endurance training in patients with COPD undergoing pulmonary rehabilitation. Chest 2005;128:1216-24. http://dx.doi.org/10.1378/chest.128.3.1216.
- Mador MJ, Bozkanat E, Aggarwal A, Shaffer M, Kufel TJ. Endurance and strength training in patients with COPD. Chest 2004;125:2036-45. http://dx.doi.org/10.1378/chest.125.6.2036.
- Mularski RA, Munjas BA, Lorenz KA, Sun S, Robertson SJ, Schmelzer W, et al. Randomized controlled trial of mindfulness-based therapy for dyspnea in chronic obstructive lung disease. J Altern Complement Med 2009;15:1083-90. http://dx.doi.org/10.1089/acm.2009.0037.
- Nguyen HQ, Donesky-Cuenco D, Wolpin S, Reinke LF, Benditt JO, Paul SM, et al. Randomized controlled trial of an internet-based versus face-to-face dyspnea self-management program for patients with chronic obstructive pulmonary disease: pilot study. J Med Internet Res 2008;10. http://dx.doi.org/10.2196/jmir.990.
- Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO. Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. Int J Chron Obstruct Pulmon Dis 2009;4:301-13. http://dx.doi.org/10.2147/COPD.S6643.
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007;27:237-44. http://dx.doi.org/10.1097/01.HCR.0000281770.82652.cb.
- Normandin EA, McCusker C, Connors M, Vale F, Gerardi D, Zuwallack RL. An evaluation of two approaches to exercise conditioning in pulmonary rehabilitation. Chest 2002;121:1085-91. http://dx.doi.org/10.1378/chest.121.4.1085.
- Norweg AM, Whiteson J, Malgady R, Mola A, Rey M. The effectiveness of different combinations of pulmonary rehabilitation program components: a randomized controlled trial. Chest 2005;128:663-72. http://dx.doi.org/10.1378/chest.128.2.663.
- Petty TL, Dempsey EC, Collins T, Pluss W, Lipkus I, Cutter GR, et al. Impact of customized videotape education on quality of life in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2006;26:112-17. http://dx.doi.org/10.1097/00008483-200603000-00012.
- Sassi-Dambron DE, Eakin EG, Ries AL, Kaplan RM. Treatment of dyspnea in COPD. A controlled clinical trial of dyspnea management strategies. Chest 1995;107:724-9. http://dx.doi.org/10.1378/chest.107.3.724.
- Simpson K, Killian K, McCartney N, Stubbing DG, Jones NL. Randomised controlled trial of weightlifting exercise in patients with chronic airflow limitation. Thorax 1992;47:70-5. http://dx.doi.org/10.1136/thx.47.2.70.
- Solomon DK, Portner TS, Bass GE, Gourley DR, Gourley GA, Holt JM, et al. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc (Wash) 1998;38:574-85.
- Stulbarg MS, Carrieri-Kohlman V, mir-Deviren S, Nguyen HQ, Adams L, Tsang AH, et al. Exercise training improves outcomes of a dyspnea self-management program. J Cardiopulm Rehabil 2002;22:109-21. http://dx.doi.org/10.1097/00008483-200203000-00010.
- Toshima MT, Kaplan RM, Ries AL. Experimental evaluation of rehabilitation in chronic obstructive pulmonary disease: short-term effects on exercise endurance and health status. Health Psychol 1990;9:237-52. http://dx.doi.org/10.1037/0278-6133.9.3.237.
- Yeh GY, Roberts DH, Wayne PM, Davis RB, Quilty MT, Phillips RS. exercise for patients with chronic obstructive pulmonary disease: a pilot study. Respir Care 2010;55:1475-82.
- Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2010;182:890-6. http://dx.doi.org/10.1164/rccm.200910-1579OC.
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones RW, Wedzicha AJ. Longitudinal trends in exercise capacity and health status after pulmonary rehabilitation in patients with COPD. Respir Med 2003;97:173-80. http://dx.doi.org/10.1053/rmed.2003.1397.
- Cockcroft A, Bagnall P, Heslop A, Andersson N, Heaton R, Batstone J, et al. Controlled trial of respiratory health worker visiting patients with chronic respiratory disability. Br Med J (Clin Res Ed) 1987;294:225-8. http://dx.doi.org/10.1136/bmj.294.6566.225.
- Finnerty JP, Keeping I, Bullough I, Jones J. The effectiveness of outpatient pulmonary rehabilitation in chronic lung disease: a randomized controlled trial. Chest 2001;119:1705-10. http://dx.doi.org/10.1378/chest.119.6.1705.
- Green RH, Singh SJ, Williams J, Morgan MDL. A randomised controlled trial of four weeks vs. seven weeks pulmonary rehabilitation in chronic obstructive pulmonary disease (COPD). Thorax 2001;56:143-5. http://dx.doi.org/10.1136/thorax.56.2.143.
- Liddell F, Webber J. Pulmonary rehabilitation for chronic obstructive pulmonary disease: a pilot study evaluating a once-weekly versus twice-weekly supervised programme. Physiotherapy 2010;96:68-74. http://dx.doi.org/10.1016/j.physio.2009.04.007.
- Littlejohns P, Baveystock CM, Parnell H, Jones PW. Randomised controlled trial of the effectiveness of a respiratory health worker in reducing impairment, disability, and handicap due to chronic airflow limitation. Thorax 1991;46:559-64. http://dx.doi.org/10.1136/thx.46.8.559.
- Lord VM, Cave P, Hume VJ, Flude EJ, Evans A, Kelly JL, et al. Singing teaching as a therapy for chronic respiratory disease: a randomised controlled trial and qualitative evaluation. BMC Pulm Med 2010;10. http://dx.doi.org/10.1186/1471-2466-10-41.
- Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study [published online ahead of print October 25 2004]. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38258.662720.3A.
- Chiner E, Llombart M, Martinez-Garcia MA, Fernandez-Fabrellas E, Navarro R, Cervera A, et al. Noninvasive mechanical ventilation in Valencia, Spain: from theory to practice. Arch Bronconeumol 2009;45:118-22. http://dx.doi.org/10.1016/j.arbres.2008.04.006.
- O’Neill B, McKevitt A, Rafferty S, Bradley JM, Johnston D, Bradbury I, et al. A comparison of twice- versus once-weekly supervision during pulmonary rehabilitation in chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2007;88:167-72. http://dx.doi.org/10.1016/j.apmr.2006.11.007.
- Prince KL, Helm M. Effectiveness of a rehabilitation programme in chronic bronchitis and emphysema. Clin Rehabil 1989;3:211-14. http://dx.doi.org/10.1177/026921558900300305.
- Sewell L, Singh SJ, Williams JE, Collier R, Morgan MD. Can individualized rehabilitation improve functional independence in elderly patients with COPD?. Chest 2005;128:1194-200. http://dx.doi.org/10.1378/chest.128.3.1194.
- Sewell L, Singh SJ, Williams JE, Collier R, Morgan MD. How long should outpatient pulmonary rehabilitation be? A randomised controlled trial of 4 weeks versus 7 weeks. Thorax 2006;61:767-71. http://dx.doi.org/10.1136/thx.2005.048173.
- Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010;65:423-28. http://dx.doi.org/10.1136/thx.2009.124164.
- Sridhar M, Taylor R, Dawson S, Roberts NJ, Partridge MR. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax 2008;63:194-200. http://dx.doi.org/10.1136/thx.2007.077578.
- Bustamante V, Lopez de Santa ME, Gorostiza A, Jimenez U, Galdiz JB. Muscle training with repetitive magnetic stimulation of the quadriceps in severe COPD patients. Respir Med 2010;104:237-45. http://dx.doi.org/10.1016/j.rmed.2009.10.001.
- Wedzicha JA, Bestall JC, Garrod R, Garnham R, Paul EA, Jones PW. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale. Eur Respir J 1998;12:363-9. http://dx.doi.org/10.1183/09031936.98.12020363.
- Weekes CE, Emery PW, Elia M. Dietary counselling and food fortification in stable COPD: a randomised trial. Thorax 2009;64:326-31. http://dx.doi.org/10.1136/thx.2008.097352.
- White RJ, Rudkin ST, Harrison ST, Day KL, Harvey IM. Pulmonary rehabilitation compared with brief advice given for severe chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2002;22:338-44. http://dx.doi.org/10.1097/00008483-200209000-00006.
- Boxall AM, Barclay L, Sayers A, Caplan GA. Managing chronic obstructive pulmonary disease in the community. A randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. J Cardiopulm Rehabil 2005;25:378-85. http://dx.doi.org/10.1097/00008483-200511000-00012.
- Effing T, Kerstjens H, van der Valk P, Zielhuis G, van der Palen J. (Cost)-effectiveness of self-treatment of exacerbations on the severity of exacerbations in patients with COPD: the COPE II study. Thorax 2009;64:956-62. http://dx.doi.org/10.1136/thx.2008.112243.
- Elliott M, Watson C, Wilkinson E, Musk AW, Lake FR. Short- and long-term hospital and community exercise programmes for patients with chronic obstructive pulmonary disease. Respirology 2004;9:345-51. http://dx.doi.org/10.1111/j.1440-1843.2004.00595.x.
- Hill K, Jenkins SC, Philippe DL, Cecins N, Shepherd KL, Green DJ, et al. High-intensity inspiratory muscle training in COPD. Eur Respir J 2006;27:1119-28. http://dx.doi.org/10.1183/09031936.06.00105205.
- Holland AE, Hill CJ, Nehez E, Ntoumenopoulos G. Does unsupported upper limb exercise training improve symptoms and quality of life for patients with chronic obstructive pulmonary disease?. J Cardiopulm Rehabil 2004;24:422-27. http://dx.doi.org/10.1097/00008483-200411000-00010.
- Leung RW, Alison JA, McKeough ZJ, Peters MJ. Ground walk training improves functional exercise capacity more than cycle training in people with chronic obstructive pulmonary disease (COPD): a randomised trial. J Physiother 2010;56:105-12. http://dx.doi.org/10.1016/S1836-9553(10)70040-0.
- Livermore N, Sharpe L, McKenzie D. Prevention of panic attacks and panic disorder in COPD. Eur Respir J 2010;35:557-63. http://dx.doi.org/10.1183/09031936.00060309.
- O’Shea SD, Taylor NF, Paratz JD. A predominantly home-based progressive resistance exercise program increases knee extensor strength in the short-term in people with chronic obstructive pulmonary disease: a randomised controlled trial. Aust J Physiother 2007;53:229-37. http://dx.doi.org/10.1016/S0004-9514(07)70003-X.
- Smith BJ, Appleton SL, Bennett PW, Roberts GC, Del FP, Adams R, et al. The effect of a respiratory home nurse intervention in patients with chronic obstructive pulmonary disease (COPD). Aust NZ J Med 1999;29:718-25. http://dx.doi.org/10.1111/j.1445-5994.1999.tb01621.x.
- Spencer LM, Alison JA, McKeough ZJ. Maintaining benefits following pulmonary rehabilitation: a randomised controlled trial. Eur Respir J 2010;35:571-7. http://dx.doi.org/10.1183/09031936.00073609.
- Wood-Baker R, McGlone S, Venn A, Walters EH. Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology 2006;11:619-26. http://dx.doi.org/10.1111/j.1440-1843.2006.00902.x.
- Fernandez AM, Pascual J, Ferrando C, Arnal A, Vergara I, Sevila V. Home-based pulmonary rehabilitation in very severe COPD: is it safe and useful?. J Cardiopulm Rehabil Prev 2009;29:325-31. http://dx.doi.org/10.1097/HCR.0b013e3181ac7b9d.
- Güell R, Casan P, Belda J, Sangenis M, Morante F, Guyatt GH, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest 2000;117:976-83. http://dx.doi.org/10.1378/chest.117.4.976.
- Güell R, Resqueti V, Sangenis M, Morante F, Martorell B, Casan P, et al. Impact of pulmonary rehabilitation on psychosocial morbidity in patients with severe COPD. Chest 2006;129:899-904. http://dx.doi.org/10.1378/chest.129.4.899.
- Madariaga VB, Iturri JB, Manterola AG, Buey JC, Sebastian NT, Pena VS. Comparison of 2 methods for inspiratory muscle training in patients with chronic obstructive pulmonary disease. Arch Bronconeumol 2007;43:431-8. http://dx.doi.org/10.1016/S1579-2129(07)60099-8.
- Mota S, Güell R, Barreiro E, Solanes I, Ramirez-Sarmiento A, Orozco-Levi M, et al. Clinical outcomes of expiratory muscle training in severe COPD patients. Respir Med 2007;101:516-24. http://dx.doi.org/10.1016/j.rmed.2006.06.024.
- Ortega F, Toral J, Cejudo P, Villagomez R, Sanchez H, Castillo J, et al. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:669-74. http://dx.doi.org/10.1164/rccm.2107081.
- Puente-Maestu L, Sanz ML, Sanz P, Cubillo JM, Mayol J, Casaburi R. Comparison of effects of supervised versus self-monitored training programmes in patients with chronic obstructive pulmonary disease. Eur Respir J 2000;15:517-25. http://dx.doi.org/10.1034/j.1399-3003.2000.15.15.x.
- Regiane Resqueti V, Gorostiza A, Galdiz JB, Lopez de Santa ME, Casan CP, Güell RR. Benefits of a home-based pulmonary rehabilitation program for patients with severe chronic obstructive pulmonary disease. Arch Bronconeumol 2007;43:599-604. http://dx.doi.org/10.1016/S1579-2129(07)60136-0.
- Riera HS, Rubio TM, Ruiz FO, Ramos PC, Del Castillo OD, Hernandez TE, et al. Inspiratory muscle training in patients with COPD: Effect on dyspnea, exercise performance, and quality of life. Chest 2001;120:748-56. http://dx.doi.org/10.1378/chest.120.3.748.
- Soler JJ, Martinez-Garcia MA, Roman P, Orero R, Terrazas S, Martinez-Pechuan A. Effectiveness of a specific program for patients with chronic obstructive pulmonary disease and frequent exacerbations. Arch Bronconeumol 2006;42:501-8. http://dx.doi.org/10.1016/S1579-2129(06)60576-4.
- de Blok BM, de Greef MH, Ten Hacken NH, Sprenger SR, Postema K, Wempe JB. The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: a pilot study. Patient Educ Couns 2006;61:48-55. http://dx.doi.org/10.1016/j.pec.2005.02.005.
- Hoogendoorn M, van Wetering CR, Schols AM, Rutten-van Mölken MP. Self-report versus care provider registration of healthcare utilization: impact on cost and cost-utility. Int J Technol Assess Health Care 2009;25:588-95. http://dx.doi.org/10.1017/S0266462309990432.
- Hospes G, Bossenbroek L, Ten Hacken NH, van Hengel P, de Greef MH. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns 2009;75:274-8. http://dx.doi.org/10.1016/j.pec.2008.10.005.
- Koppers RJH, Vos PJE, Boot CRL, Folgering HT. Exercise performance improves in patients with COPD due to respiratory muscle endurance training. Chest 2006;129:886-92. http://dx.doi.org/10.1378/chest.129.4.886.
- Lamers F, Jonkers CC, Bosma H, Chavannes NH, Knottnerus JA, van Eijk JT. Improving quality of life in depressed COPD patients: effectiveness of a minimal psychological intervention. COPD 2010;7:315-22. http://dx.doi.org/10.3109/15412555.2010.510156.
- Monninkhof E, van der Walk P, van der Palen J, van Herwaarden C, Zielhuis G. Effects of a comprehensive self-management programme in patients with chronic obstructive pulmonary disease. Eur Respir J 2003;22:815-20. http://dx.doi.org/10.1183/09031936.03.00047003.
- Rooyackers JM, Berkeljon DA, Folgering HT. Eccentric exercise training in patients with chronic obstructive pulmonary disease. Int J Rehabil Res 2003;26:47-9. http://dx.doi.org/10.1097/00004356-200303000-00006.
- Trappenburg JC, Monninkhof EM, Bourbeau J, Troosters T, Schrijvers AJ, Verheij TJ, et al. Effect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: a multicentre randomised controlled trial. Thorax 2011;66:977-84. http://dx.doi.org/10.1136/thoraxjnl-2011-200071.
- Wijkstra PJ, van Altena R, Kraan J, Otten V, Postma DS, Koeter GH. Quality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at home. Eur Respir J 1994;7:269-73. http://dx.doi.org/10.1183/09031936.94.07020269.
- Wijkstra PJ, ten Vergert EM, van Altena R, Otten V, Kraan J, Postma DS, et al. Long term benefits of rehabilitation at home on quality of life and exercise tolerance in patients with chronic obstructive pulmonary disease. Thorax 1995;50:824-8. http://dx.doi.org/10.1136/thx.50.8.824.
- Bernard S, Whittom F, LeBlanc P, Jobin J, Belleau R, Berube C, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:896-901. http://dx.doi.org/10.1164/ajrccm.159.3.9807034.
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 2003;163:585-91. http://dx.doi.org/10.1001/archinte.163.5.585.
- Brooks D, Krip B, Mangovski-Alzamora S, Goldstein RS. The effect of postrehabilitation programmes among individuals with chronic obstructive pulmonary disease. Eur Respir J 2002;20:20-9. http://dx.doi.org/10.1183/09031936.02.01852001.
- Busch AJ, McClements JD. Effects of a supervised home exercise program on patients with severe chronic obstructive pulmonary disease. Phys Ther 1988;68:469-74.
- Carr SJ, Hill K, Brooks D, Goldstein RS. Pulmonary rehabilitation after acute exacerbation of chronic obstructive pulmonary disease in patients who previously completed a pulmonary rehabilitation program. J Cardiopulm Rehabil Prev 2009;29:318-24. http://dx.doi.org/10.1097/HCR.0b013e3181ac7bb8.
- Goldstein RS, Gort EH, Stubbing D, Avendano MA, Guyatt GH. Randomised controlled trial of respiratory rehabilitation. Lancet 1994;344:1394-7. http://dx.doi.org/10.1016/S0140-6736(94)90568-1.
- Janaudis-Ferreira T, Hill K, Goldstein RS, Robles-Ribeiro P, Beauchamp MK, Dolmage TE, et al. Resistance arm training in patients with COPD: a randomized controlled trial. Chest 2011;139:151-8. http://dx.doi.org/10.1378/chest.10-1292.
- Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2008;149:869-78. http://dx.doi.org/10.7326/0003-4819-149-12-200812160-00006.
- Cai S, Chen P, Chen Y, Liu ZJ. Effect of health education on the lung function and life quality in patients with stable chronic obstructive pulmonary diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2006;31:189-93.
- Li Y-L. Nutritional supplementation and respiratory gym in patients with chronic obstructive pulmonary disease. Chin J Clin Rehabil 2002;6:1260-2.
- Ren L, Li Q-Y, Du J-B, Zhou J-M, Weng Q-L, Chen X-H. Comparison of different strategies of pulmonary rehabilitation for patients with COPD of different severity. J Shanghai Jiaotong Uni (Med Sci) 2011;31:620-4.
- Shao L-Z. Effects of the behavioral intervention on the life quality of the patients with chronic obstructive pulmonary disease in remission period. Chin J Clin Rehabil 2003;7:4078-9.
- Wang Z, Zeng H, Chen H. Rehabilitative treatment in patients with chronic obstructive pulmonary disease at stable stage. Chin Nurs Res 2004;18:1608-9.
- Xu Y-H, Wang J-H, Li H-F, Zhu X-H, Wang G. Efficacy of integrative respiratory rehabilitation training in exercise ability and quality of life of patients with chronic obstructive pulmonary disease in stable phase: a randomized controlled trial. J Chin Integr Med 2010;8:432-7. http://dx.doi.org/10.3736/jcim20100506.
- Zhang ZQ, Chen RC, Yang QK, Li P, Wang CZ, Zhang ZH. A randomized controlled trial study of pulmonary rehabilitation with respiratory physiology as the guide on prognosis in patients with chronic obstructive pulmonary disease. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2008;20:607-10.
- du Moulin M, Taube K, Wegscheider K, Behnke M, van den Bussche H. Home-based exercise training as maintenance after outpatient pulmonary rehabilitation. Respiration 2009;77:139-45. http://dx.doi.org/10.1159/000150315.
- Gohl O, Linz H, Schonleben T, Otte B, Weineck J, Worth H. Benefits of a multimodular outpatient training program for patients with COPD. Pneumologie 2006;60:529-36. http://dx.doi.org/10.1055/s-2006-944235.
- Van Gestel AJ, Kohler M, Steier J, Teschler S, Russi EW, Teschler H. The effects of controlled breathing during pulmonary rehabilitation in patients with COPD. Respiration 2012;83:115-24. http://dx.doi.org/10.1159/000324449.
- Warlies F, Saladin M, Hellmann A. Evaluation of a standardized specific education program ‘Lebensrhythmus Atmen’: a prospective, randomized, controlled study for COPD patients: a pilot study. Pravention Und Rehabilitation 2006;18:68-79. http://dx.doi.org/10.5414/PRP18068.
- Wittmann M, Spohn S, Schultz K, Pfeifer M, Petro W. Patient education in COPD during inpatient rehabilitation improves quality of life and morbidity. Pneumologie 2007;61:636-42. http://dx.doi.org/10.1055/s-2007-980106.
- Wright P. Effects of a resistance training on pulmonary function and performance measurements in patients with chronic obstructive pulmonary disease. European J Sport Sci 2003;3:1-6.
- Chan AW, Lee A, Suen LK, Tam WW. Effectiveness of a Tai chi Qigong program in promoting health-related quality of life and perceived social support in chronic obstructive pulmonary disease clients. Qual Life Res 2010;19:653-64. http://dx.doi.org/10.1007/s11136-010-9632-6.
- Ko FW, Dai DL, Ngai J, Tung A, Ng S, Lai K, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology 2011;16:617-24. http://dx.doi.org/10.1111/j.1440-1843.2010.01921.x.
- Lindsay M, Lee A, Chan K, Poon P, Han LK, Wong WC, et al. Does pulmonary rehabilitation give additional benefit over tiotropium therapy in primary care management of chronic obstructive pulmonary disease? Randomized controlled clinical trial in Hong Kong Chinese. J Clin Pharm Therapeut 2005;30:567-73. http://dx.doi.org/10.1111/j.1365-2710.2005.00686.x.
- Ng BH, Tsang HW, Jones AY, So CT, Mok TY. Functional and psychosocial effects of health qigong in patients with COPD: a randomized controlled trial. J Altern Complement Med 2011;17:243-51. http://dx.doi.org/10.1089/acm.2010.0215.
- Arnardottir RH, Sorensen S, Ringqvist I, Larsson K. Two different training programmes for patients with COPD: a randomised study with 1-year follow-up. Respir Med 2006;100:130-9. http://dx.doi.org/10.1016/j.rmed.2005.03.043.
- Arnardottir RH, Boman G, Larsson K, Hedenstrom H, Emtner M. Interval training compared with continuous training in patients with COPD. Respir Med 2007;101:1196-204. http://dx.doi.org/10.1016/j.rmed.2006.11.004.
- Efraimsson EO, Hillervik C, Ehrenberg A. Effects of COPD self-care management education at a nurse-led primary health care clinic. Scand J Caring Sci 2008;22:178-85. http://dx.doi.org/10.1111/j.1471-6712.2007.00510.x.
- Engstrom CP, Persson LO, Larsson S, Sullivan M. Long-term effects of a pulmonary rehabilitation programme in outpatients with chronic obstructive pulmonary disease: a randomized controlled study. Scand J Rehabil Med 1999;31:207-13. http://dx.doi.org/10.1080/003655099444371.
- Theander K, Jakobsson P, Jorgensen N, Unosson M. Effects of pulmonary rehabilitation on fatigue, functional status and health perceptions in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Clin Rehabil 2009;23:125-36. http://dx.doi.org/10.1177/0269215508096174.
- Wadell K, Sundelin G, Henriksson-Larsen K, Lundgren R. High intensity physical group training in water: an effective training modality for patients with COPD. Respir Med 2004;98:428-38. http://dx.doi.org/10.1016/j.rmed.2003.11.010.
- Bendstrup KE, Ingemann JJ, Holm S, Bengtsson B. Out-patient rehabilitation improves activities of daily living, quality of life and exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J 1997;10:2801-6. http://dx.doi.org/10.1183/09031936.97.10122801.
- Bjornshave B, Korsgaard J. Comparison of two different levels of physical training in patients with moderate to severe COPD. Lung 2005;183:101-8. http://dx.doi.org/10.1007/s00408-004-2523-2.
- Linneberg A, Rasmussen M, Buch TF, Wester A, Malm L, Fannikke G, et al. A randomised study of the effects of supplemental exercise sessions after a 7-week chronic obstructive pulmonary disease rehabilitation program. Clin Respir J 2012;6:112-19. http://dx.doi.org/10.1111/j.1752-699X.2011.00256.x.
- Petersen AM, Mittendorfer B, Magkos F, Iversen M, Pedersen BK. Physical activity counteracts increased whole-body protein breakdown in chronic obstructive pulmonary disease patients. Scand J Med Sci Sports 2008;18:557-64. http://dx.doi.org/10.1111/j.1600-0838.2007.00727.x.
- Ringbaek TJ, Broendum E, Hemmingsen L, Lybeck K, Nielsen D, Andersen C, et al. Rehabilitation of patients with chronic obstructive pulmonary disease. Exercise twice a week is not sufficient!. Respir Med 2000;94:150-4. http://dx.doi.org/10.1053/rmed.1999.0704.
- Eaton T, Young P, Fergusson W, Moodie L, Zeng I, O’Kane F, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology 2009;14:230-8. http://dx.doi.org/10.1111/j.1440-1843.2008.01418.x.
- Martin IR, McNamara D, Sutherland FR, Tilyard MW, Taylor DR. Care plans for acutely deteriorating COPD: a randomized controlled trial. Chron Respir Dis 2004;1:191-5. http://dx.doi.org/10.1191/1479972304cd047oa.
- McGeoch GR, Willsman KJ, Dowson CA, Town GI, Frampton CM, McCartin FJ, et al. Self-management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology 2006;11:611-18. http://dx.doi.org/10.1111/j.1440-1843.2006.00892.x.
- Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J 2004;34:608-14. http://dx.doi.org/10.1111/j.1445-5994.2004.00672.x.
- Watson PB, Town GI, Holbrook N, Dwan C, Toop LJ, Drennan CJ. Evaluation of a self-management plan for chronic obstructive pulmonary disease. Eur Respir J 1997;10:1267-71. http://dx.doi.org/10.1183/09031936.97.10061267.
- Bonilha AG, Onofre F, Vieira ML, Prado MY, Martinez JA. Effects of singing classes on pulmonary function and quality of life of COPD patients. Int J Chron Obstruct Pulmon Dis 2009;4:1-8.
- Dourado VZ, Tanni SE, Antunes LC, Paiva SA, Campana AO, Renno AC, et al. Effect of three exercise programs on patients with chronic obstructive pulmonary disease. Braz J Med Biol Res 2009;42:263-71. http://dx.doi.org/10.1590/S0100-879X2009000300007.
- Probst VS, Kovelis D, Hernandes NA, Camillo CA, Cavalheri V, Pitta F. Effects of 2 exercise training programs on physical activity in daily life in patients with COPD. Respir Care 2011;56:1799-807. http://dx.doi.org/10.4187/respcare.01110.
- Yamaguti WP, Claudino RC, Neto AP, Chammas MC, Gomes AC, Salge JM, et al. Diaphragmatic breathing training program improves abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Arch Phys Med Rehabil 2012;93:571-7. http://dx.doi.org/10.1016/j.apmr.2011.11.026.
- Elci A, Borekci S, Ovayolu N, Elbek O. The efficacy and applicability of a pulmonary rehabilitation programme for patients with COPD in a secondary-care community hospital. Respirology 2008;13:703-7. http://dx.doi.org/10.1111/j.1440-1843.2008.01327.x.
- Karapolat H, Atasever A, Atamaz F, Kirazli Y, Elmas F, Erdinc E. Do the benefits gained using a short-term pulmonary rehabilitation program remain in COPD patients after participation?. Lung 2007;185:221-5. http://dx.doi.org/10.1007/s00408-007-9011-4.
- Kayahan B, Karapolat H, Atyntoprak E, Atasever A, Ozturk O. Psychological outcomes of an outpatient pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease. Respir Med 2006;100:1050-7. http://dx.doi.org/10.1016/j.rmed.2005.09.031.
- Ozdemir EP, Solak O, Fidan F, Demirdal US, Evcik D, Unlu M, et al. The effect of water-based pulmonary rehabilitation on anxiety and quality of life in chronic pulmonary obstructive disease patients. Turkiye Klinikleri J Med Sci 2010;30:880-7. http://dx.doi.org/10.5336/medsci.2008-9971.
- Katiyar SK, Bihari S. Role of pranayama in rehabilitation of COPD patients: a randomized controlled study. Indian J Allergy Asthma Immunol 2006;20:98-104.
- Singh V, Khandelwal DC, Khandelwal R, Abusaria S. Pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci 2003;45:13-7.
- Subin Rao V, Prem V, Sahoo RC. Effect of upper limb, lower limb and combined training on health-related quality of life in COPD. Lung India 2010;27:4-7. http://dx.doi.org/10.4103/0970-2113.59260.
- Pomidori L, Contoli M, Mandolesi G, Cogo A. A simple method for home exercise training in patients with chronic obstructive pulmonary disease: one-year study. J Cardiopulm Rehabil Prev 2012;32:53-7. http://dx.doi.org/10.1097/HCR.0b013e31823be0ce.
- Romagnoli M, Dell’Orso D, Lorenzi C, Crisafulli E, Costi S, Lugli D, et al. Repeated pulmonary rehabilitation in severe and disabled COPD patients. Respiration 2006;73:769-76. http://dx.doi.org/10.1159/000092953.
- Breyer MK, Breyer-Kohansal R, Funk GC, Dornhofer N, Spruit MA, Wouters EF, et al. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Respir Res 2010;11. http://dx.doi.org/10.1186/1465-9921-11-112.
- Vonbank K, Strasser B, Mondrzyk J, Marzluf BA, Richter B, Losch S, et al. Strength training increases maximum working capacity in patients with COPD: randomized clinical trial comparing three training modalities. Respir Med 2012;106:557-63. http://dx.doi.org/10.1016/j.rmed.2011.11.005.
- Spruit MA, Gosselink R, Troosters T, De PK, Decramer M. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur Respir J 2002;19:1072-8. http://dx.doi.org/10.1183/09031936.02.00287102.
- Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med 2000;109:207-12. http://dx.doi.org/10.1016/S0002-9343(00)00472-1.
- Barakat S, Michele G, George P, Nicole V, Guy A. Outpatient pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2008;3:155-62.
- Ninot G, Moullec G, Picot MC, Jaussent A, Hayot M, Desplan M, et al. Cost-saving effect of supervised exercise associated to COPD self-management education program. Respir Med 2011;105:377-85. http://dx.doi.org/10.1016/j.rmed.2010.10.002.
- Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol 2009;68:588-98. http://dx.doi.org/10.1111/j.1365-2125.2009.03493.x.
- Murphy N, Bell C, Costello RW. Extending a home from hospital care programme for COPD exacerbations to include pulmonary rehabilitation. Respir Med 2005;99:1297-302. http://dx.doi.org/10.1016/j.rmed.2005.02.033.
- Beckerman M, Magadle R, Weiner M, Weiner P. The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest 2005;128:3177-82. http://dx.doi.org/10.1378/chest.128.5.3177.
- Magadle R, McConnell AK, Beckerman M, Weiner P. Inspiratory muscle training in pulmonary rehabilitation program in COPD patients. Respir Med 2007;101:1500-5. http://dx.doi.org/10.1016/j.rmed.2007.01.010.
- Gallefoss F, Bakke PS, Rsgaard PK. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:812-17. http://dx.doi.org/10.1164/ajrccm.159.3.9804047.
- Hynninen MJ, Bjerke N, Pallesen S, Bakke PS, Nordhus IH. A randomized controlled trial of cognitive behavioral therapy for anxiety and depression in COPD. Respir Med 2010;104:986-94. http://dx.doi.org/10.1016/j.rmed.2010.02.020.
- Puhan MA, Busching G, Schunemann HJ, van Oort E, Zaugg C, Frey M. Interval versus continuous high-intensity exercise in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2006;145:816-25. http://dx.doi.org/10.7326/0003-4819-145-11-200612050-00006.
- Scherer TA, Spengler CM, Owassapian D, Imhof E, Boutellier U. Respiratory muscle endurance training in chronic obstructive pulmonary disease: impact on exercise capacity, dyspnea, and quality of life. Am J Respir Crit Care Med 2000;162:1709-14. http://dx.doi.org/10.1164/ajrccm.162.5.9912026.
- Hsiao SF, Wu YT, Wu HD, Wang TG. Comparison of effectiveness of pressure threshold and targeted resistance devices for inspiratory muscle training in patients with chronic obstructive pulmonary disease. J Formosan Med Assoc 2003;102:240-5.
- Liu WT, Wang CH, Lin HC, Lin SM, Lee KY, Lo YL, et al. Efficacy of a cell phone-based exercise programme for COPD. Eur Respir J 2008;32:651-9. http://dx.doi.org/10.1183/09031936.00104407.
- Nakamura Y, Tanaka K, Shigematsu R, Nakagaichi M, Lnoue M, Homma T. Effects of aerobic training and recreational activities in patients with chronic obstructive pulmonary disease. Int J Rehabil Res 2008;31:275-83. http://dx.doi.org/10.1097/MRR.0b013e3282fc0f81.
- Wakabayashi R, Motegi T, Yamada K, Ishii T, Jones RC, Hyland ME, et al. Efficient integrated education for older patients with chronic obstructive pulmonary disease using the Lung Information Needs Questionnaire. Geriatr Gerontol Int 2011;11:422-30. http://dx.doi.org/10.1111/j.1447-0594.2011.00696.x.
- Sívori M, Rhodius E, Kaplan P, Talarico M, Gorojod G, Carreras B, et al. Exercise training in chronic obstructive pulmonary disease. Comparative study of aerobic training of lower limbs vs. combination with upper limbs. Medicina 1998;58:717-27.
- Ghanem M, Elaal EA, Mehany M, Tolba K. Home-based pulmonary rehabilitation program: effect on exercise tolerance and quality of life in chronic obstructive pulmonary disease patients. Ann Thorac Med 2010;5:18-25. http://dx.doi.org/10.4103/1817-1737.58955.
- Vogiatzis I, Nanas S, Roussos C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur Respir J 2002;20:12-9. http://dx.doi.org/10.1183/09031936.02.01152001.
- Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharm 2012;34:53-62. http://dx.doi.org/10.1007/s11096-011-9585-z.
- Jang HJ, Jung YK. The effects of self-efficacy promoting pulmonary rehabilitation program in out-patients with chronic obstructive pulmonary disease. Tuberc Respir Dis 2006;61:533-46. http://dx.doi.org/10.4046/trd.2006.61.6.533.
- Assessing care of vulnerable elders-3 quality indicators . J Am Geriatr Soc 2007;55:464-87. http://dx.doi.org/10.1111/j.1532-5415.2007.01329.x.
- Paz-Diaz H, Montes de OM, Lopez JM, Celli BR. Pulmonary rehabilitation improves depression, anxiety, dyspnea and health status in patients with COPD. Am J Phys Med Rehabil 2007;86:30-6. http://dx.doi.org/10.1097/PHM.0b013e31802b8eca.
- Bourbeau J, Collet JP, Schwartzman K, Ducruet T, Nault D, Bradley C. Economic benefits of self-management education in COPD. Chest 2006;130:1704-11. http://dx.doi.org/10.1378/chest.130.6.1704.
- Gadoury MA, Schwartzman K, Rouleau M, Maltais F, Julien M, Beaupre A, et al. Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J 2005;26:853-7. http://dx.doi.org/10.1183/09031936.05.00093204.
- Chan AW, Lee A, Suen LK, Tam WW. Qigong improves lung functions and activity tolerance in COPD clients: a single blind, randomized controlled trial. Complement Ther Med 2011;19:3-11. http://dx.doi.org/10.1016/j.ctim.2010.12.007.
- Van Wetering CR, Hoogendoorn M, Mol SJ, Rutten-van Mölken MP, Schols AM. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax 2010;65:7-13. http://dx.doi.org/10.1136/thx.2009.118620.
- Hoogendoorn M, van Wetering CR, Schols AM, Rutten-van Mölken MPMH. Is INTERdisciplinary COMmunity-based COPD management (INTERCOM) cost-effective?. Eur Respir J 2010;35:79-87. http://dx.doi.org/10.1183/09031936.00043309.
- Puente-Maestu L, Luisa SM, Sanz P, de Ona RJ, Arnedillo A, Casaburi R. Long-term effects of a maintenance program after supervised or self-monitored training programs in patients with COPD. Lung 2003;181:67-78. http://dx.doi.org/10.1007/s00408-003-1007-0.
- Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823-32. http://dx.doi.org/10.7326/0003-4819-122-11-199506010-00003.
- Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA. A randomised 2 × 2 trial of community versus hospital pulmonary rehabilitation, followed by telephone or conventional follow-up. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14060.
- Effing T, Zielhuis G, Kerstjens H, van der Valk P, van der Palen J. Community based physiotherapeutic exercise in COPD self-management: a randomised controlled trial. Respir Med 2011;105:418-26. http://dx.doi.org/10.1016/j.rmed.2010.09.017.
- Khdour MR, Agus AM, Kidney JC, Smyth BM, Elnay JC, Crealey GE. Cost-utility analysis of a pharmacy-led self-management programme for patients with COPD. Int J Clin Pharm 2011;33:665-73. http://dx.doi.org/10.1007/s11096-011-9524-z.
- Gallefoss F, Bakke PS. Impact of patient education and self-management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease. Respir Med 2000;94:279-87. http://dx.doi.org/10.1053/rmed.1999.0749.
- Gallefoss F. The effects of patient education in COPD in a 1-year follow-up randomised, controlled trial. Patient Educ Couns 2004;52:259-66. http://dx.doi.org/10.1016/S0738-3991(03)00100-9.
- Hernandez MT, Rubio TM, Ruiz FO, Riera HS, Gil RS, Gomez JC. Results of a home-based training program for patients with COPD. Chest 2000;118:106-14. http://dx.doi.org/10.1378/chest.118.1.106.
- Oh EG. The effects of home-based pulmonary rehabilitation in patients with chronic lung disease. Int J Nurs Stud 2003;40:873-9. http://dx.doi.org/10.1016/S0020-7489(03)00071-3.
- Moore J, Fiddler H, Seymour J, Grant A, Jolley C, Johnson L, et al. Effect of a home exercise video programme in patients with chronic obstructive pulmonary disease. J Rehabil Med 2009;41:195-200. http://dx.doi.org/10.2340/16501977-0308.
- Gourley GA, Portner TS, Gourley DR, Rigolosi EL, Holt JM, Solomon DK, et al. Humanistic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc (Wash) 1998;38:586-97.
- Stoilkova A, Janssen DJ, Wouters EF. Educational programmes in COPD management interventions: a systematic review. Respir Med 2013;107:1637-50. http://dx.doi.org/10.1016/j.rmed.2013.08.006.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. http://dx.doi.org/10.1037/0278-6133.27.3.379.
- Gibson PG, Coughlan J, Wilson AJ, Hensley MJ, Abramson M, Bauman A, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev 2000;2.
- Dishman RK, Sallis JF, Orenstein DR. The determinants of physical activity and exercise. Public Health Rep 1985;100:158-71.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in health eating and physical activity interventions: a meta-regression. Health Psychol 2014;28:690-701. http://dx.doi.org/10.1037/a0016136.
- Van Der Meer RM, Wagena EJ, Ostelo RW, Jacobs JE, van Schayck CP. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2003;2.
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15. http://dx.doi.org/10.1016/0197-2456(89)90005-6.
- Schrijvers G. Disease management: a proposal for a new definition. Int J Integr Care 2009;9.
- Zainuldin R, Mackey MG, Alison JA. Intensity and type of exercise for lower limb endurance training to optimise exercise capacity for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2009;1.
- Costi S, Di BM, Pillastrini P, D’Amico R, Crisafulli E, Arletti C, et al. Short-term efficacy of upper-extremity exercise training in patients with chronic airway obstruction: a systematic review. Phys Ther 2009;89:443-5. http://dx.doi.org/10.2522/ptj.20070368.
- Geddes EL, O’Brien K, Reid WD, Brooks D, Crowe J. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respir Med 2008;102:1715-29. http://dx.doi.org/10.1016/j.rmed.2008.07.005.
- O’Brien K, Geddes EL, Reid WD, Brooks D, Crowe J. Inspiratory muscle training compared with other rehabilitation interventions in chronic obstructive pulmonary disease: a systematic review update. J Cardiopulm Rehabil Prev 2008;28:128-41. http://dx.doi.org/10.1097/01.HCR.0000314208.40170.00.
- Garrod R, Lasserson T. Role of physiotherapy in the management of chronic lung diseases: an overview of systematic reviews. Respir Med 2007;101:2429-36. http://dx.doi.org/10.1016/j.rmed.2007.06.007.
- Dewan NA, Rice KL, Caldwell M, Hilleman DE, Daniel E. Economic evaluation of a disease management program for chronic obstructive pulmonary disease. COPD 2011;8:153-9. http://dx.doi.org/10.3109/15412555.2011.560129.
- Monninkhof E, van der Valk PD, Schermer T, van der Palen J, van Herwaarden C, Zielhuis G. Economic evaluation of a comprehensive self-management programme in patients with moderate to severe chronic obstructive pulmonary disease. Chron Respir Dis 2004;1:7-16.
- Tinkelman D, Corsello P, McClure D, Yin M. One-year outcomes from a disease management program for chronic obstructive pulmonary disease. Dis Manag Health Outcomes 2003;11:49-5. http://dx.doi.org/10.2165/00115677-200311010-00007.
- Alexander JL, Phillips WT, Wagner CL. The effect of strength training on functional fitness in older patients with chronic lung disease enrolled in pulmonary rehabilitation. Rehabil Nurs J 2008;33:91-7. http://dx.doi.org/10.1002/j.2048-7940.2008.tb00211.x.
- Ambrosino N, Paggiaro PL, Macchi M, Filieri M, Toma G, Lombardi FA, et al. A study of short-term effect of rehabilitative therapy in chronic obstructive pulmonary disease. Respiration 1981;41:40-4. http://dx.doi.org/10.1159/000194357.
- Bauldoff GS, Hoffman LA, Sciurba F, Zullo TG. Home-based, upper-arm exercise training for patients with chronic obstructive pulmonary disease. Heart Lung 1996;25:288-94. http://dx.doi.org/10.1016/S0147-9563(96)80064-1.
- Belman MJ, Shadmehr R. Targeted resistive ventilatory muscle training in chronic obstructive pulmonary disease. J Appl Physiol 1988;65:2726-35.
- Berry MJ, Adair NE, Sevensky KS, Quinby A, Lever HM. Inspiratory muscle training and whole-body reconditioning in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;153:1812-16. http://dx.doi.org/10.1164/ajrccm.153.6.8665039.
- Berry MJ, Rejeski WJ, Adair NE, Ettinger J, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2003;23:60-8. http://dx.doi.org/10.1097/00008483-200301000-00011.
- Bjerre-Jepsen K, Secher NH, Kok-Jensen A. Inspiratory resistance training in severe chronic obstructive pulmonary disease. Eur J Respir Dis 1981;62:405-11.
- Borghi-Silva A, Arena R, Castello V, Simoes RP, Martins LE, Catai AM, et al. Aerobic exercise training improves autonomic nervous control in patients with COPD. Respir Med 2009;103:1503-10. http://dx.doi.org/10.1016/j.rmed.2009.04.015.
- Bosch D, Feierabend M, Becker A. COPD outpatient education programme (ATEM) and BODE index. Pneumologie 2007;61:629-35. http://dx.doi.org/10.1055/s-2007-980081.
- Carrieri-Kohlman V, Gormley JM, Douglas MK, Paul SM, Stulbarg MS. Exercise training decreases dyspnea and the distress and anxiety associated with it. Monitoring alone may be as effective as coaching. Chest 1996;110:1526-35. http://dx.doi.org/10.1378/chest.110.6.1526.
- Carrieri-Kohlman V, Gormley JM, Eiser S, mir-Deviren S, Nguyen H, Paul SM, et al. Dyspnea and the affective response during exercise training in obstructive pulmonary disease. Nurs Res 2001;50:136-46. http://dx.doi.org/10.1097/00006199-200105000-00002.
- Casaburi R, Bhasin S, Cosentino L, Porszasz J, Somfay A, Lewis MI, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:870-8. http://dx.doi.org/10.1164/rccm.200305-617OC.
- Chen H, Dukes R, Martin BJ. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1985;131:251-5.
- Clark CJ, Cochrane L, Mackay E. Low intensity peripheral muscle conditioning improves exercise tolerance and breathlessness in COPD. Eur Respir J 1996;9:2590-6. http://dx.doi.org/10.1183/09031936.96.09122590.
- Clark CJ, Cochrane LM, Mackay E, Paton B. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur Respir J 2000;15:92-7. http://dx.doi.org/10.1183/09031936.00.15109200.
- Cooper CB. Desensitization to dyspnea in COPD with specificity for exercise training mode. Int J Chron Obstruct Pulmon Dis 2009;4:33-4.
- Coppoolse R, Schols AMWJ, Baarends EM, Mostert R, Akkermans MA, Janssen PP, et al. Interval versus continuous training in patients with severe COPD: a randomized clinical trial. Eur Respir J 1999;14:258-63. http://dx.doi.org/10.1034/j.1399-3003.1999.14b04.x.
- Costi S, Crisafulli E, Antoni FD, Beneventi C, Fabbri LM, Clini EM. Effects of unsupported upper extremity exercise training in patients with COPD: a randomized clinical trial. Chest 2009;136:387-95. http://dx.doi.org/10.1378/chest.09-0165.
- De Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2003;84:1154-7. http://dx.doi.org/10.1016/S0003-9993(03)00239-9.
- Dekhuijzen PN, Folgering HT, van Herwaarden CL. Target-flow inspiratory muscle training during pulmonary rehabilitation in patients with COPD. Chest 1991;99:128-33. http://dx.doi.org/10.1378/chest.99.1.128.
- Epstein SK, Celli BR, Martinez FJ, Couser JI, Roa J, Pollock M, et al. Arm training reduces the VO2 and VE cost of unsupported arm exercise and elevation in chronic obstructive pulmonary disease. J Cardiopulm Rehabil 1997;17:171-7. http://dx.doi.org/10.1097/00008483-199705000-00004.
- Esteve F, Blanc-Gras N, Gallego J, Benchetrit G. The effects of breathing pattern training on ventilatory function in patients with COPD. Biofeedback Self Regul 1996;21:311-21. http://dx.doi.org/10.1007/BF02214431.
- Falk P, Eriksen AM, Kolliker K, Andersen JB. Relieving dyspnea with an inexpensive and simple method in patients with severe chronic airflow limitation. Eur J Respir Dis 1985;66:181-6.
- Gallefoss F, Bakke PS. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication?. Am J Respir Crit Care Med 1999;160:2000-5. http://dx.doi.org/10.1164/ajrccm.160.6.9901028.
- Cabedo GV, Garces Asemany CR, Cortes BA, Oteo Elso JT, Ballester Salvador FJ. Effectiveness of the correct use of inhalation devices in patients with COPD: randomized clinical trial. Med Clin 2010;135:586-91. http://dx.doi.org/10.1016/j.medcli.2010.03.027.
- Gift AG, Moore T, Soeken K. Relaxation to reduce dyspnea and anxiety in COPD patients. Nurs Res 1992;41:242-6. http://dx.doi.org/10.1097/00006199-199207000-00011.
- Gormley JM, Carrieri-Kohlman V, Douglas MK, Stulbarg MS. Treadmill self-efficacy and walking performance in patients with COPD. J Cardiopulm Rehabil 1993;13:424-31. http://dx.doi.org/10.1097/00008483-199311000-00009.
- Harver A, Mahler DA, Daubenspeck JA. Targeted inspiratory muscle training improves respiratory muscle function and reduces dyspnea in patients with chronic obstructive pulmonary disease. Ann Intern Med 1989;111:117-24. http://dx.doi.org/10.7326/0003-4819-111-2-117.
- Heijdra YF, Dekhuijzen PNR, van Herwaarden CLA, Folgering HT. Nocturnal saturation improves by target-flow inspiratory muscle training in patients with COPD. Am J Respir Crit Care Med 1996;153:260-5. http://dx.doi.org/10.1164/ajrccm.153.1.8542126.
- Hoff J, Tjonna AE, Steinshamn S, Hoydal M, Richardson RS, Helgerud J. Maximal strength training of the legs in COPD: a therapy for mechanical inefficiency. Med Sci Sports Exerc 2007;39:220-6. http://dx.doi.org/10.1249/01.mss.0000246989.48729.39.
- Incalzi RA, Corsonello A, Trojano L, Pedone C, Acanfora D, Spada A, et al. Cognitive training is ineffective in hypoxemic COPD: a six-month randomized controlled trial. Rejuvenation Res 2008;11:239-50. http://dx.doi.org/10.1089/rej.2007.0607.
- Izumizaki M, Satake M, Takahashi H, Sugawara K, Shioya T, Homma I. Effects of inspiratory muscle thixotropy on the 6-min walk distance in COPD. Respir Med 2008;102:970-7. http://dx.doi.org/10.1016/j.rmed.2008.02.007.
- Jin X-Q, Hao L-S, Chen W-H. The influence of pulmonary rehabilitation on dyspnea, pulmonary function test and exercise tolerance in chronic obstructive pulmonary disease patients. Chin J Clin Rehabil 2002;6:662-3.
- Jones DT, Thomson RJ, Sears MR. Physical exercise and resistive breathing training in severe chronic airways obstruction. Are they effective?. Eur J Respir Dis 1985;67:159-66.
- Kheirabadi GR, Keypour M, Attaran N, Bagherian R, Maracy MR. Effect of add-on ‘Self management and behavior modification’ education on severity of COPD. Tanaffos 2008;7:23-30.
- Kongsgaard M, Backer V, Jorgensen K, Kjaer M, Beyer N. Heavy resistance training increases muscle size, strength and physical function in elderly male COPD-patients: a pilot study. Respir Med 2004;98:1000-7. http://dx.doi.org/10.1016/j.rmed.2004.03.003.
- Kirsten DK, Taube C, Lehnigk B, Jörres RA, Magnussen H. Exercise training improves recovery in patients with COPD after an acute exacerbation. Respir Med 1998;92:1191-8. http://dx.doi.org/10.1016/S0954-6111(98)90420-6.
- Kurabayashi H, Machida I, Tamura K, Iwai F, Tamura J, Kubota K. Breathing out into water during subtotal immersion: a therapy for chronic pulmonary emphysema. Am J Phys Med Rehabil 2000;79:150-3. http://dx.doi.org/10.1097/00002060-200003000-00007.
- Ruiz de Ona Lacasta JM, Garcia de PJ, Puente ML, Llorente ID, Celdran GJ, Cubillo Marcos JM. Effects of muscle training on breathing pattern in patients with severe chronic obstructive pulmonary disease. Arch Bronconeumol 2004;40:20-3. http://dx.doi.org/10.1016/S1579-2129(06)60187-0.
- Lake FR, Henderson K, Briffa T, Openshaw J, Musk AW. Upper-limb and lower-limb exercise training in patients with chronic airflow obstruction. Chest 1990;97:1077-82. http://dx.doi.org/10.1378/chest.97.5.1077.
- Levine S, Weiser P, Gillen J. Evaluation of a ventilatory muscle endurance training program in the rehabilitation of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1986;133:400-6.
- Lisboa C, Villafranca C, Pertuze J, Leiva A, Repetto P. Clinical effects of inspiratory muscle training in patients with chronic airflow limitation. Rev Med Chile 1995;123:1108-15.
- Lisboa C, Villafranca C, Leiva A, Cruz E, Pertuze J, Borzone G. Inspiratory muscle training in chronic airflow limitation: Effect on exercise performance. Eur Respir J 1997;10:537-42.
- Louie SW. The effects of guided imagery relaxation in people with COPD. Occup Ther Int 2004;11:145-59. http://dx.doi.org/10.1002/oti.203.
- Marrara KT, Marino DM, de Held PA, de Oliveira Junior AD, Jamami M, Di Lorenzo V. Different physical therapy interventions on daily physical activities in chronic obstructive pulmonary disease. Respir Med 2008;102:505-11. http://dx.doi.org/10.1016/j.rmed.2007.12.004.
- Martinez FJ, Vogel PD, Dupont DN, Stanopoulos I, Gray A, Beamis JF. Supported arm exercise vs unsupported arm exercise in the rehabilitation of patients with severe chronic airflow obstruction. Chest 1993;103:1397-402. http://dx.doi.org/10.1378/chest.103.5.1397.
- McKeon JL, Turner J, Kelly C. The effect of inspiratory resistive training on exercise capacity in optimally treated patients with severe chronic airflow limitation. Aust NZ J Med 1986;16:648-52. http://dx.doi.org/10.1111/j.1445-5994.1986.tb00005.x.
- Mehri SN, Khoshnevis MA, Zarrehbinan F, Hafezi S, Ghasemi A, Ebadi A. Effect of treadmill exercise training on VO2 peak in chronic obstructive pulmonary disease. Tanaffos 2007;6:18-24.
- Mendes De Oliveira JC, Studart Leitao Filho FS, Malosa Sampaio LM, Negrinho De Oliveira AC, Hirata RP, Costa D, et al. Outpatient vs. home-based pulmonary rehabilitation in COPD: a randomized controlled trial. Multidiscip Respir Med 2010;5:401-8. http://dx.doi.org/10.1186/2049-6958-5-6-401.
- Nasis IG, Vogiatzis I, Stratakos G, Athanasopoulos D, Koutsoukou A, Daskalakis A, et al. Effects of interval-load versus constant-load training on the BODE index in COPD patients. Respir Med 2009;103:1392-8. http://dx.doi.org/10.1016/j.rmed.2009.03.003.
- Nava S. Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil 1998;79:849-54. http://dx.doi.org/10.1016/S0003-9993(98)90369-0.
- Noseda A, Carpiaux JP, Vandeput W, Prigogine T, Schmerber J. Resistive inspiratory muscle training and exercise performance in COPD patients. A comparative study with conventional breathing retraining. Bull Eur Physiopathol Respir 1987;23:457-63.
- Nosworthy J, Barter C, Thomas S, Flynn M. An evaluation of the three elements of pulmonary rehabilitation. Aust Physiother 1993;38:189-93. http://dx.doi.org/10.1016/S0004-9514(14)60562-6.
- Phillips WT, Benton MJ, Wagner CL, Riley C. The effect of single set resistance training on strength and functional fitness in pulmonary rehabilitation patients. J Cardiopulm Rehabil 2006;26:330-7. http://dx.doi.org/10.1097/00008483-200609000-00011.
- Preusser BA, Winningham ML, Clanton TL. High- vs low-intensity inspiratory muscle interval training in patients with COPD. Chest 1994;106:110-17. http://dx.doi.org/10.1378/chest.106.1.110.
- Puente-Maestu L, Sanz ML, Sanz P, Ruiz De Ona JM, Rodriguez-Hermosa JL, Whipp BJ. Effects of two types of training on pulmonary and cardiac responses to moderate exercise in patients with COPD. Eur Respir J 2000;15:1026-32. http://dx.doi.org/10.1034/j.1399-3003.2000.01509.x.
- Ramirez-Sarmiento A, Orozco-Levi M, Güell R, Barreiro E, Hernandez N, Mota S, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med 2002;166:1491-7. http://dx.doi.org/10.1164/rccm.200202-075OC.
- Reardon J, Awad E, Normandin E, Vale F, Clark B, Zuwallack RL. The effect of comprehensive outpatient pulmonary rehabilitation on dyspnea. Chest 1994;105:1046-52. http://dx.doi.org/10.1378/chest.105.4.1046.
- Ries AL, Moser KM. Comparison of isocapnic hyperventilation and walking exercise training at home in pulmonary rehabilitation. Chest 1986;90:285-9. http://dx.doi.org/10.1378/chest.90.2.285.
- Ries AL, Ellis B, Hawkins RW. Upper extremity exercise training in chronic obstructive pulmonary disease. Chest 1988;93:688-92. http://dx.doi.org/10.1378/chest.93.4.688.
- Saunders KB, White JE. Controlled trial of breathing exercises. Br Med J 1965;2:680-2. http://dx.doi.org/10.1136/bmj.2.5463.680.
- Savci S, Ince DI, Arikan H. A comparison of autogenic drainage and the active cycle of breathing techniques in patients with chronic obstructive pulmonary diseases. J Cardiopulm Rehabil 2000;20:37-43. http://dx.doi.org/10.1097/00008483-200001000-00006.
- Singh VP, Rao V, Prem V, Sahoo RC, Keshev Pai K. Comparison of the effectiveness of music and progressive muscle relaxation for anxiety in COPD: a randomized controlled pilot study. Chron Respir Dis 2009;6:209-16.
- Spohn S, Wittmann M, Petro W. Impact of an education program on health-related control beliefs and self-efficacy expectancies in patients with COPD. Pravention Und Rehabilitation 2002;14:163-70.
- Strijbos JH, Postma DS, van Altena R, Gimeno F, Koeter GH. Feasibility and effects of a home-care rehabilitation program in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 1996;16:386-93. http://dx.doi.org/10.1097/00008483-199611000-00008.
- Strijbos JH, Postma DS, van Altena R, Gimeno F, Koeter GH. A comparison between an outpatient hospital-based pulmonary rehabilitation program and a home-care pulmonary rehabilitation program in patients with COPD. A follow-up of 18 months. Chest 1996;109:366-72. http://dx.doi.org/10.1378/chest.109.2.366.
- Su C-L, Chiang L-L, Chiang T-Y, Yu C-T, Kuo H-P, Lin H-C. Domiciliary positive expiratory pressure improves pulmonary function and exercise capacity pulmonary with chronic obstructive pulmonary disease. J Formosan Med Assoc 2007;106:204-11. http://dx.doi.org/10.1016/S0929-6646(09)60241-2.
- Tandon MK. Adjunct treatment with yoga in chronic severe airways obstruction. Thorax 1978;33:514-17. http://dx.doi.org/10.1136/thx.33.4.514.
- Tiep BL, Burns M, Kao D, Madison R, Herrera J. Pursed lips breathing training using ear oximetry. Chest 1986;90:218-21. http://dx.doi.org/10.1378/chest.90.2.218.
- Toshima MT, Blumberg E, Ries AL, Kaplan RM. Does rehabilitation reduce depression in patients with chronic obstructive pulmonary disease?. J Cardiopulm Rehabil 1992;12:261-9. http://dx.doi.org/10.1097/00008483-199207000-00005.
- Vallet G, Varray A, Fontaine JL, Prefaut C. Value of individualized rehabilitation at the ventilatory threshold level in moderately severe chronic obstructive pulmonary disease. Rev Mal Respir 1994;11:493-501.
- Vallet G, Ahmaidi S, Serres I, Fabre C, Bourgouin D, Desplan J, et al. Comparison of two training programmes in chronic airway limitation patients: standardized versus individualized protocols. Eur Respir J 1997;10:114-22. http://dx.doi.org/10.1183/09031936.97.10010114.
- Varga J, Porszasz J, Boda K, Casaburi R, Somfay A. Supervised high intensity continuous and interval training vs. self-paced training in COPD. Respir Med 2007;101:2297-304. http://dx.doi.org/10.1016/j.rmed.2007.06.017.
- Vogiatzis I, Terzis G, Nanas S, Stratakos G, Simoes DC, Georgiadou O, et al. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest 2005;128:3838-45. http://dx.doi.org/10.1378/chest.128.6.3838.
- Wanke T, Formanek D, Lahrmann H, Brath H, Wild M, Wagner C, et al. Effects of combined inspiratory muscle and cycle ergometer training on exercise performance in patients with COPD. Eur Respir J 1994;7:2205-11. http://dx.doi.org/10.1183/09031936.94.07122205.
- Weiner P, Azgad Y, Ganam R. Inspiratory muscle training combined with general exercise reconditioning in patients with COPD. Chest 1992;102:1351-6. http://dx.doi.org/10.1378/chest.102.5.1351.
- Weiner P, Magadle R, Berar-Yanay N, Davidovich A, Weiner M. The cumulative effect of long-acting bronchodilators, exercise, and inspiratory muscle training on the perception of dyspnea in patients with advanced COPD. Chest 2000;118:672-8. http://dx.doi.org/10.1378/chest.118.3.672.
- Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N. Specific expiratory muscle training in COPD. Chest 2003;124:468-73. http://dx.doi.org/10.1378/chest.124.2.468.
- Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest 2003;124:1357-64. http://dx.doi.org/10.1378/chest.124.4.1357.
- Weiner P, Magandle R, Beckerman M, Weiner M, Berar-Yanay N. Maintenance of inspiratory muscle training in COPD patients: one year follow-up. Eur Respir J 2004;23:61-5. http://dx.doi.org/10.1183/09031936.03.00059503.
- Weiner P, Weiner M. Inspiratory muscle training may increase peak inspiratory flow in chronic obstructive pulmonary disease. Respiration 2006;73:151-6.
- Wen Y-L, Huang D-F, Huang M, Huang Y-P. Evaluation on the effect of systematic exercise rehabilitation intervention in patients with chronic obstructive pulmonary disease. Chin J Clin Rehabil 2004;8:2224-5.
- Wen H, Gao Y, An JY. Comparison of high-intensity and anaerobic threshold programs in rehabilitation for patients with moderate to severe chronic obstructive pulmonary disease. Chung-Hua Chieh Ho Ho Hu Hsi Tsa Chih 2008;31:571-6.
- Wijkstra PJ, van der Mark TW, Kraan J, van Altena R, Koeter GH, Postma DS. Effects of home rehabilitation on physical performance in patients with chronic obstructive pulmonary disease (COPD). Eur Respir J 1996;9:104-10. http://dx.doi.org/10.1183/09031936.96.09010104.
- Wijkstra PJ, van der Mark TW, Kraan J, van Altena R, Koeter GH, Postma DS. Long-term effects of home rehabilitation on physical performance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;153:1234-41. http://dx.doi.org/10.1164/ajrccm.153.4.8616547.
- Wolkove N, Baltzan MA, Jr, Kamel H, Rotaple M. A randomized trial to evaluate the sustained efficacy of a mucus clearance device in ambulatory patients with chronic obstructive pulmonary disease. Can Respir J 2004;11:567-72.
- Wurtemberger G, Bastian K. Functional effects of different training in patients with COPD. Pneumologie 2001;55:553-62. http://dx.doi.org/10.1055/s-2001-19001.
- Xie S-L, Zhu M-G, Cui H-B, Liu H-Y. Influence of home-based training program on patients with COPD. Chin J Clin Rehabil 2003;7:2554-5.
- Yan Q, Sun Y. Quantitative research for improving respiratory muscle contraction by breathing exercise. Chin Med J 1996;109:771-5.
- Gallefoss F, Bakke PS. Cost–benefit and cost-effectiveness analysis of self-management in patients with COPD: a 1-year follow-up randomized, controlled trial. Respir Med 2002;96:424-31. http://dx.doi.org/10.1053/rmed.2002.1293.
- Goldstein RS, Gort EH, Guyatt GH, Feeny D. Economic analysis of respiratory rehabilitation. Chest 1997;112:370-9. http://dx.doi.org/10.1378/chest.112.2.370.
- Guyatt GH, King DR, Feeny DH, Stubbing D, Goldstein RS. Generic and specific measurement of health-related quality of life in a clinical trial of respiratory rehabilitation. J Clin Epidemiol 1999;52:187-92. http://dx.doi.org/10.1016/S0895-4356(98)00157-7.
- Carrieri-Kohlman V, Nguyen HQ, Donesky-Cuenco D, Mir-Deviren S, Neuhaus J, Stulbarg MS. Impact of brief or extended exercise training on the benefit of a dyspnea self-management program in COPD. J Cardiopulm Rehabil 2005;25:275-84. http://dx.doi.org/10.1097/00008483-200509000-00009.
- Davis AH, Carrieri-Kohlman V, Janson SL, Gold WM, Stulbarg MS. Effects of treatment on two types of self-efficacy in people with chronic obstructive pulmonary disease. J Pain Symptom Manage 2006;32:60-7. http://dx.doi.org/10.1016/j.jpainsymman.2006.01.012.
Appendix 1 Search strategies for clinical effectiveness evidence: reviews 1, 2 and 4
MEDLINE (via Ovid)
URL: https://ovid.sp.com
Date range searched: 1946 to April Week 4 2012.
Date of search: 2 May 2012.
Search strategy
-
chronic obstructive pulmonary disease.mp. or exp Pulmonary Disease, Chronic Obstructive/
-
copd.ti,ab.
-
chronic obstructive lung disease.ti,ab.
-
chronic obstructive airway disease.ti,ab.
-
chronic respiratory disorder$.ti,ab.
-
smoking-related lung disease$.ti,ab.
-
Pulmonary Emphysema/
-
exp Bronchitis/
-
emphysema.ti,ab.
-
or/1-9
-
exp Self Care/
-
self care.ti,ab.
-
self manage$.ti,ab.
-
self caring.ti,ab.
-
(self adj2 (support$ or care or caring or manage$)).ti,ab.
-
post discharge.ti,ab.
-
early discharge.ti,ab.
-
home care.ti,ab.
-
home care services/ or home nursing/
-
patient centred care.ti,ab.
-
patient education/ or patient education.ti,ab.
-
patient participation.ti,ab.
-
post hospital care.ti,ab.
-
action planning.ti,ab.
-
discharge planning.ti,ab.
-
continuity of patient care/
-
(support$ adj2 discharge).ti,ab.
-
(support$ adj2 manag$).ti,ab.
-
patient focus$.ti,ab.
-
management plan$.ti,ab.
-
management program$.ti,ab.
-
rehabilitation.mp. or exp Rehabilitation/
-
or/11-32
-
10 and 33
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Date range searched: inception to 2 May 2012.
Date of search: 2 May 2012.
Search strategy
-
chronic obstructive pulmonary disease.ti,ab.
-
copd.ti,ab.
-
chronic obstructive lung disease.ti,ab.
-
chronic obstructive airway disease.ti,ab.
-
chronic respiratory disorder$.ti,ab.
-
smoking-related lung disease$.ti,ab.
-
emphysema.ti,ab.
-
bronchitis.ti,ab.
-
or/1-8
-
(self adj2 (support$ or care or caring or manage$)).ti,ab.
-
post discharge.ti,ab.
-
early discharge.ti,ab.
-
home care.ti,ab.
-
home nursing.ti,ab.
-
patient centred care.ti,ab.
-
patient centered care.ti,ab.
-
patient education.mp.
-
patient participation.ti,ab.
-
post hospital care.ti,ab.
-
action planning.ti,ab.
-
discharge planning.ti,ab.
-
(continuity adj2 care).ti,ab.
-
(support$ adj2 (discharge or manage$)).ti,ab.
-
patient focus$.ti,ab.
-
management plan$.ti,ab.
-
management program$.ti,ab.
-
rehabilitation.ti,ab.
-
or/10-27
-
9 and 28
EMBASE (via Ovid)
Date range searched: 1980 to 2012 Week 17.
Date of search: 2 May 2012.
Search strategy
-
chronic obstructive pulmonary disease.mp. or exp chronic obstructive lung disease/
-
copd.ti,ab.
-
chronic obstructive lung disease.ti,ab.
-
chronic obstructive airway disease.ti,ab.
-
chronic respiratory disorder$.ti,ab.
-
smoking-related lung disease$.ti,ab.
-
pulmonary emphysema.mp. or exp lung emphysema/
-
emphysema.ti,ab.
-
bronchitis.mp. or exp bronchitis/
-
or/1-9
-
self care.mp. or exp self care/
-
(self adj2 (support$ or care or caring or manage$)).ti,ab.
-
post discharge.ti,ab.
-
early discharge.ti,ab.
-
exp home care/
-
home nursing.ti,ab.
-
patient centred care.ti,ab.
-
patient centered care.ti,ab.
-
patient education/
-
patient education.ti,ab.
-
patient participation.ti,ab.
-
post hospital care.ti,ab.
-
action planning.ti,ab.
-
discharge planning.ti,ab.
-
continuity of patient care.ti,ab.
-
(support$ adj2 discharge).ti,ab.
-
(support$ adj2 manage$).ti,ab.
-
patient focus$.ti,ab.
-
management plan$.ti,ab.
-
management program$.ti,ab.
-
rehabilitation.mp. or exp rehabilitation/
-
or/11-31
-
10 and 32
PsycINFO (via Ovid)
Date ranged searched: 1806 to May week 1 2012.
Date of search: 2 May 2012.
Search strategy
-
chronic obstructive pulmonary disease.mp. or exp Chronic Obstructive Pulmonary Disease/
-
copd.ti,ab.
-
chronic obstructive lung disease.ti,ab.
-
chronic obstructive airway disease.ti,ab.
-
chronic respiratory disorder$.ti,ab.
-
smoking-related lung disease$.ti,ab.
-
exp Pulmonary Emphysema/ or emphysema.ti,ab.
-
bronchitis.ti,ab.
-
or/1-8
-
(self adj2 (support$ or care or caring or manage$)).ti,ab.
-
post discharge.ti,b.
-
early discharge.ti,ab.
-
home care.mp. or exp Home Care/
-
patient centred care.ti,ab.
-
patient centered care.ti,ab.
-
client education/
-
patient education.ti,ab.
-
patient participation.ti,ab.
-
post hospital care.ti,ab.
-
action planning.ti,ab.
-
discharge planning.ti,ab.
-
‘continuum of care’/
-
continuity of patient care.ti,ab.
-
(support$ adj2 discharge).ti,ab.
-
(support$ adj manage$).ti,ab.
-
patient focus$.ti,ab.
-
management plan$.ti,ab.
-
management program$.ti,ab.
-
exp Rehabilitation/ or rehabilitation.mp.
-
or/10-29
-
9 and 30
The Cochrane Library (Wiley) 2012; Cochrane Central Register of Controlled Trials (CENTRAL); Cochrane Database of Systematic Reviews (CDSR) Issue 4 of 12; Database of Abstracts of Reviews of Effects (DARE); NHS EED issue 4 of 12
Date range searched: inception to 8 May 2012.
Date of search: 8 May 2012.
Search strategy
#1 copd
#2 chronic next obstructive next pulmonary disease
#3 MeSH descriptor Pulmonary Disease, Chronic Obstructive explode all trees
#4 chronic next obstructive next airway next disease
#5 chronic next respiratory next disorder*
#6 smoking next related next lung next disease*
#7 emphysema
#8 MeSH descriptor Pulmonary Emphysema explode all trees
#9 MeSH descriptor Bronchitis explode all trees
#10 bronchitis
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
#12 self next care
#13 MeSH descriptor Self Care explode all trees
#14 self near/2 ( support* or care or caring or manage*)
#15 post next discharge
#16 early next discharge
#17 MeSH descriptor Home Care Services explode all trees
#18 home next nursing
#19 patient next centred next care
#20 patient next centered next care
#21 MeSH descriptor Patient Education as Topic explode all trees
#22 patient next education
#23 patient next participation
#24 post next hospital next care
#25 action next planning
#26 discharge next planning
#27 continuity near/1 patient
#28 support* near/2 discharge
#29 support* near/2 manage*
#30 patient next focus*
#31 management next plan*
#32 management next program*
#33 rehabilitation
#34 MeSH descriptor Rehabilitation explode all trees
#35 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34)
#36 (#11 AND #35)
Physiotherapy Evidence Database (PEDro)
URL: www.pedro.org.au
Date range searched: 1929 to 8 May 2012.
Date of search: 8 May 2012.
Search strategy
Terms used: self-management and copd or chronic obstructive pulmonary disease.
Science Citation Index (SCI) (Web of Science)
Date range searched: 1964 to 8 May 2012.
Date of search: 8 May 2012.
Search strategy
Topic = (copd or (chronic obstructive pulmonary disease) or bronchitis or emphysema or (smoking related lung disease) or (chronic obstructive lung disease) or (chronic obstructive airway disease)) AND Topic = ((self management) or (self support*) or (self care) or (home care) or (home nursing) or (patient cent*) or (patient education) or (patient participation) or (post hospital) or (action planning) or (discharge planning) or continuity or (support* discharge) or (support* manage*) or (patient focus*) or (management plan*) or (management program*) or (rehabilitation))
Refined by: Web of Science Categories = ( RESPIRATORY SYSTEM )
Timespan = All Years. Databases = SCI-EXPANDED.
Zetoc (Mimas)
Date range searched: 1993 to 8 May 2012.
Date of search: 8 May 2012.
Search strategy
Terms used: COPD and rehabilitation; Patient education; COPD and self-management; Pulmonary and self-management.
Conference Proceedings Citation Index (CPCI) (Web of Science)
Date range searched: 1990 to 8 May 2012.
Date of search: 8 May 2012.
Search strategy
Topic = (copd or (chronic obstructive pulmonary disease) or bronchitis or emphysema or (smoking related lung disease) or (chronic obstructive lung disease) or (chronic obstructive airway disease)) AND Topic = ((self management) or (self support*) or (self care) or (home care) or (home nursing) or (patient cent*) or (patient education) or (patient participation) or (post hospital) or (action planning) or (discharge planning) or continuity or (support* discharge) or (support* manage*) or (patient focus*) or (management plan*) or (management program*) or (rehabilitation))
Timespan = All Years. Databases = CPCI-S.
Appendix 2 List of excluded papers, with reasons for exclusion: reviews 1 and 4
| Excluded article | Reasons for exclusion | |
|---|---|---|
| Review 1 | Review 4 | |
| Abad-Corp, Carrillo-Alcaraz A, Royo-Morales T, Perez-Garcia MC, Rodriguez-Mondejar JJ, Saez-Soto A, et al. Effectiveness of planning hospital discharge and follow-up in primary care for patients with chronic obstructive pulmonary disease: research protocol. J Adv Nurs 2010;66:1365–70 | Intervention | Intervention |
| Adams SG, Melo J, Luther M, Anzueto A. Antibiotics are associated with lower relapse rates in outpatients with acute exacerbations of COPD. Chest 2000;117:1345–52 | Intervention | Intervention |
| Ahmed S, Bourbeau J, Maltais F, Mansour A. The Oort structural equation modeling approach detected a response shift after a COPD self-management program not detected by the Schmitt technique. J Clin Epidemiol 2009;62:1165–72 | Time point | Study design |
| Aiken LS, Butner J, Lockhart CA, Volk-Craft BE, Hamilton G, Williams FG. Outcome evaluation of a randomized trial of the PhoenixCare intervention: program of case management and coordinated care for the seriously chronically ill. J Palliat Med 2006;9:111–26 | Population | Population |
| Aimonino RN, Tibaldi V, Leff B, Scarafiotti C, Marinello R, Zanocchi M, et al. Substitutive ‘hospital at home’ versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: a prospective randomized, controlled trial. J Am Geriatr Soc 2008;56:493–500 | Time point | – |
| Akinci AC, Olgun N. The effectiveness of nurse-led, home-based pulmonary rehabilitation in patients with COPD in Turkey. Rehabil Nurs J 2011;36:159–65 | Time point | Study design |
| Al-Showair RA, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med 2007;101:2395–401 | Time point | Outcome |
| Alexander JL, Phillips WT, Wagner CL. The effect of strength training on functional fitness in older patients with chronic lung disease enrolled in pulmonary rehabilitation. Rehabil Nurs J 2008;33:91–7 | Time point | – |
| Ambrosino N, Paggiaro PL, Macchi M, Filieri M, Toma G, Lombardi FA, et al. A study of short-term effect of rehabilitative therapy in chronic obstructive pulmonary disease. Respiration 1981;41:40–4 | Time point | – |
| Antonana J, Sobradillo V, De MD, Chic S, Galdiz J, Iriberri M. [Early discharge and home health care program for patients with exacerbated COPD and asthma.] Arch Bronconeumol 2001;37:489–94 | Population | Population |
| Antoniu SA. Self-management programs in chronic obstructive pulmonary disease: are they worthy? Exp Rev Pharmacoecon Outcome Res 2003;3:681–3 | Publication type | Publication type |
| Antoniu SA. Self-management programs in chronic obstructive pulmonary disease: do they have a sustained effect on health resource utilization? Exp Rev Pharmacoecon Outcome Res 2006;6:155–7 | Publication type | Publication type |
| Arbane G, Douiri A, Enright L, Haggis L, Poulter T, Garrod R. Effects of physical activity top up ‘pat on the back’ programme on exercise capacity and healthcare utilisation for people with chronic obstructive pulmonary disease (COPD). British Thoracic Society Winter Meeting, 1–3 December 2010, London. Thorax 2010; conference publication:A96 | Time point | Publication type |
| Armour C, Bosnic-Anticevich S, Brillant M, Burton D, Emmerton L, Krass I, et al. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax 2007;62:496–502 | Population | Population |
| Arnardottir RH, Sorensen S, Ringqvist I, Larsson K. Two different training programmes for patients with COPD: a randomised study with 1-year follow-up. Respir Med 2006;100:130–9 | Time point | – |
| Arnardottir RH, Boman G, Larsson K, Hedenstrom H, Emtner M. Interval training compared with continuous training in patients with COPD. Respir Med 2007;101:1196–204 | Time point | – |
| Ashida C, Fukata Y, Shiota M, Hayashi Y, Yoshida Y. [Acute exacerbation of chronic obstructive lung diseases and self management: observation of the early symptoms and their management.] Kango Gijutsu 1988;34:1777–81 | Study design | Study design |
| Ashikaga T, Vacek PM, Lewis SO. Evaluation of a community-based education program for individuals with chronic obstructive pulmonary disease. J Rehabil 1980;46:23–7 | Population | Population |
| Ashikaga T, Vacek PM, Lewis SO, Seckerwalker R. Impact of a COPD patient self-management program. Am Rev Respir Dis 1983;127:152 | Time point | Study design |
| Atkins CJ, Kaplan RM, Timms RM, Reinsch S, Lofback K. Behavioral exercise programs in the management of chronic obstructive pulmonary disease. J Consult Clin Psychol 1984;52:591–603 | Population | Population |
| Baarends EM, Schols AM, Slebos DJ, Mostert R, Janssen PP, Wouters EF. Metabolic and ventilatory response pattern to arm elevation in patients with COPD and healthy age-matched subjects. Eur Respir J 1995;8:1345–51 | Population | Population |
| Bagnall P, Heslop A. Chronic respiratory disease: educating patients at home. Prof Nurse 1987;2:293–6 | Outcome | Outcome |
| Baker S, Davenport P, Sapienza C. Examination of strength training and detraining effects in expiratory muscles. J Speech Lang Hear Res 2005;48:1325–3 | Population | Population |
| Baltzan MA, Kamel H, Alter A, Rotaple M, Wolkove N. Pulmonary rehabilitation improves functional capacity in patients 80 years of age or older. Can Respir J 2004;11:407–13 | Intervention | Study design |
| Barakat S, Michele G, George P, Nicole V, Guy A. Outpatient pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2008;3:155–62 | Time point | – |
| Barbanel D, Eldridge S, Griffiths C. Can a self-management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax 2003;58:851–4 | Population | Population |
| Barber CM, Bradshaw LM, Buttery P, Fishwick D, Whyte MK, Higenbottam TW. Assisted discharge for patients with exacerbations of COPD. Thorax 2001;56:417–18 | Publication type | Publication type |
| Barnestein-Fonseca P, Leiva-Fernandez J, Vidal-Espana F, Garcia-Ruiz A, Prados-Torres D, Leiva-Fernandez F. Efficacy and safety of a multifactor intervention to improve therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): protocol for the ICEPOC study. Trials 2011;12:40 | Publication type | Publication type |
| Baron K. COPD intervention investigation: Comparing the effect of an outpatient counseling session after discharge to an educational counseling session on admission day 2 on hospitalization rates in patients with COPD. 50th Annual Assembly of the New York State Council of Health-System Pharmacists, NYSCHP 2011 Verona, NY, USA, 29 April to 1 May 2011. J Pharm Pract 2011:354 | Publication type | Publication type |
| Bass H, Whitcomb JF, Forman R. Exercise training: therapy for patients with chronic obstructive pulmonary disease. Chest 1970;57:116–21 | Time point | Study design |
| Battaglia E, Fulgenzi A, Ferrero ME. Rationale of the combined use of inspiratory and expiratory devices in improving maximal inspiratory pressure and maximal expiratory pressure of patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2009;90:913–18 | Time point | Outcome |
| Battersby MW, Harris M, Reed RL, Harvey PW, Woodman RJ, Frith P. A randomised trial of the Flinders Program to improve patient self-management competencies in a range of chronic conditions: study rationale and protocol. Australasian Med J 2010;1:198–204 | Publication type | Publication type |
| Bauldoff GS, Hoffman LA, Sciurba F, Zullo TG. Home-based, upper-arm exercise training for patients with chronic obstructive pulmonary disease. Heart Lung 1996;25:288–94 | Time point | – |
| Bauldoff GS, Hoffman LA, Zullo TG, Sciurba FC. Exercise maintenance following pulmonary rehabilitation: effect of distractive stimuli. Chest 2002;122:948–54 | Time point | – |
| Bauldoff GS, Rittinger M, Nelson T, Doehrel J, Diaz PT. Feasibility of distractive auditory stimuli on upper extremity training in persons with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2005;25:50–5 | Time point | – |
| Bausewein C, Booth S, Gysels M, Kuhnbach R, Higginson IJ. Effectiveness of a hand-held fan for breathlessness: a randomised phase II trial. BMC Palliat Care 2010;9:22 | Population | Population |
| Beaulieu-Genest L, Chretien D, Maltais F, Pelletier K, Parent JG, Lacasse Y. Self-administered prescriptions of oral steroids and antibiotics in chronic obstructive pulmonary disease: are we doing more harm than good? Chron Respir Dis 2007;4:143–7 | Time point | Study design |
| Beckerman M, Magadle R, Weiner M, Weiner P. The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest 2005;128:3177–82 | Time point | – |
| Bellone A, Lascioli R, Raschi S, Guzzi L, Adone R. Chest physical therapy in patients with acute exacerbation of chronic bronchitis: Effectiveness of three methods. Arch Phys Med Rehabil 2000;81:558–60 | Intervention | Intervention |
| Bellone A, Spagnolatti L, Massobrio M, Bellei E, Vinciguerra R, Barbieri A, et al. Short-term effects of expiration under positive pressure in patients with acute exacerbation of chronic obstructive pulmonary disease and mild acidosis requiring non-invasive positive pressure ventilation. Intensive Care Med 2002;28:581–85 | Intervention | Intervention |
| Belman MJ, Kendregan BA. Physical training fails to improve ventilatory muscle endurance in patients with chronic obstructive pulmonary disease. Chest 1982;81:440–3 | Time point | Study design |
| Belman MJ, Shadmehr R. Targeted resistive ventilatory muscle training in chronic obstructive pulmonary disease. J Appl Physiol 1988;65:2726–35 | Time point | – |
| Bendstrup KE, Ingemann JJ, Holm S, Bengtsson B. Out-patient rehabilitation improves activities of daily living, quality of life and exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J 1997;10:2801–6 | Time point | – |
| Bernard S, Whittom F, LeBlanc P, Jobin J, Belleau R, Berube C, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:896–901 | Time point | – |
| Berry MJ, Adair NE, Sevensky KS, Quinby A, Lever HM. Inspiratory muscle training and whole-body reconditioning in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;153:1812–16 | Time point | – |
| Berry MJ, Rejeski WJ, Adair NE, Ettinger J, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2003;23:60–8 | Time point | – |
| Berry MJ, Rejeski WJ, Miller ME, Adair NE, Lang W, Foy CG, et al. A lifestyle activity intervention in patients with chronic obstructive pulmonary disease. Respir Med 2010;104:829–39 | Time point | – |
| Bestall JC, Paul EA, Garrod R, Garnham R, Jones RW, Wedzicha AJ. Longitudinal trends in exercise capacity and health status after pulmonary rehabilitation in patients with COPD. Respir Med 2003;97:173–80 | Time point | – |
| [Better quality of life by early diagnosis and patient education in COPD.] MMW Fortschr Med 2003;145:4–8 | Publication type | Publication type |
| Bianchi R, Gigliotti F, Romagnoli I, Lanini B, Castellani C, Grazzini M, et al. Chest wall kinematics and breathlessness during pursed-lip breathing in patients with COPD. Chest 2004;125:459–65 | Time point | Study design |
| Bird S, Noronha M, Sinnott H. An integrated care facilitation model improves quality of life and reduces use of hospital resources by patients with chronic obstructive pulmonary disease and chronic heart failure. Aust J Prim Health 2010;16:326–33 | Population | Population |
| Bissonnette J, Logan J, Davies B, Graham ID. Methodological issues encountered in a study of hospitalized COPD patients. Clin Nurs Res 2005;14:81–97 | Study design | Study design |
| Bjerre-Jepsen K, Secher NH, Kok-Jensen A. Inspiratory resistance training in severe chronic obstructive pulmonary disease. Eur J Respir Dis 1981;62:405–11 | Time point | – |
| Bjornshave B, Korsgaard J. Comparison of two different levels of physical training in patients with moderate to severe COPD. Lung 2005;183:101–8 | Time point | – |
| Blake RL, Jr, Vandiver TA, Braun S, Bertuso DD, Straub V. A randomized controlled evaluation of a psychosocial intervention in adults with chronic lung disease. Fam Med 1990;22:365–70 | Time point | – |
| Blumenthal JA, Keefe FJ, Babyak MA, Fenwick VC, Johnson JM, Stott K, et al. Caregiver-assisted coping skills training for patients with COPD: background, design, and methodological issues for the INSPIRE-II study. Clin Trials 2009;6:172–84 | Publication type | Publication type |
| Bonilha AG, Onofre F, Vieira ML, Prado MY, Martinez JA. Effects of singing classes on pulmonary function and quality of life of COPD patients. Int J Chron Obstruct Pulmon Dis 2009;4:1–8 | Time point | – |
| Borghi-Silva A, Arena R, Castello V, Simoes RP, Martins LE, Catai AM, et al. Aerobic exercise training improves autonomic nervous control in patients with COPD. Respir Med 2009;103:1503–10 | Time point | – |
| Borycki E, Kushniruk A. Development of a virtual self-management tool for COPD patients: towards a user needs ontology. AMIA Annu Symp Proc 2007;879 | Time point | Study design |
| Bosch D, Feierabend M, Becker A. [COPD outpatient education programme (ATEM) and BODE index.] Pneumologie 2007;61:629–35 | Time point | – |
| Bosma H, Lamers F, Jonkers CC, van Eijk JT. Disparities by education level in outcomes of a self-management intervention: the DELTA trial in The Netherlands. Psychiatr Serv 2011;62:793–95 | Population | Population |
| Bosnic-Anticevich SZ, Sinha H, So S, Reddel HK. Metered-dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. J Asthma 2010;47:251–6 | Population | Population |
| Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 2003;163:585–91 | Time point | – |
| Bourbeau J, Nault D, Ng-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns 2004;52:271–7 | Publication type | Publication type |
| Bourbeau J, Collet JP, Schwartzman K, Ducruet T, Nault D, Bradley C. Economic benefits of self-management education in COPD. Chest 2006;130:1704–11 | Time point | – |
| Bourbeau J. Disease management for COPD: avoiding hospitalizations and controlling cost? COPD 2011;8:143–4 | Publication type | Publication type |
| Bourbeau J. Inhaled corticosteroids and survival in chronic obstructive pulmonary disease. Eur Respir J 2003;21:202–3 | Publication type | Publication type |
| Bourjeily-Habr G, Rochester CL, Palermo F, Snyder P, Mohsenin V. Randomised controlled trial of transcutaneous electrical muscle stimulation of the lower extremities in patients with chronic obstructive pulmonary disease. Thorax 2002;57:1045–9 | Intervention | Intervention |
| Bower P, Kennedy A, Reeves D, Rogers A, Blakeman T, Chew-Graham C, et al. A cluster randomised controlled trial of the clinical and cost-effectiveness of a ‘whole systems’ model of self-management support for the management of long-term conditions in primary care: trial protocol. Implement Sci 2012;7:7 | Population | Population |
| Bowles KH, Baugh AC. Applying research evidence to optimize telehomecare. J Cardiovasc Nurs 2007;22:5–15 | Publication type | Publication type |
| Boxall AM, Barclay L, Sayers A, Caplan GA. Managing chronic obstructive pulmonary disease in the community. A randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. J Cardiopulm Rehabil 2005;25:378–85 | Time point | – |
| Bredin M, Corner J, Krishnasamy M, Plant H, Bailey C, A’Hern R. Multicentre randomised controlled trial of nursing intervention for breathlessness in patients with lung cancer. BMJ 1999;318:901–4 | Population | Population |
| Breslin EH. Breathing retraining in chronic obstructive pulmonary disease. J Cardiopulm Rehabil 1995;15:25–33 | Publication type | Publication type |
| Breyer MK, Breyer-Kohansal R, Funk GC, Dornhofer N, Spruit MA, Wouters EF, et al. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Respir Res 2010;11:112 | Time point | – |
| Brooks D, Krip B, Mangovski-Alzamora S, Goldstein RS. The effect of postrehabilitation programmes among individuals with chronic obstructive pulmonary disease. Eur Respir J 2002;20:20–9 | Time point | – |
| Brooks D, Sidani S, Graydon J, McBride S, Hall L, Weinacht K. Evaluating the effects of music on dyspnea during exercise in individuals with chronic obstructive pulmonary disease: a pilot study. Rehabil Nurs J 2003;28:192–6 | Time point | Study design |
| Brough FK, Schmidt CD, Rasmussen T, Boyer M. Comparison of two teaching methods for self-care training for patients with chronic obstructive pulmonary disease. Patient Couns Health Educ 1982;4:111–16 | Time point | Outcome |
| Brown L, Donaghy D, Jones P, Whelan R, McCormack N, Callanan I, et al. Implementation of a bundle of care reduced median hospital length of stay for patients with Chronic Obstructive Pulmonary Disease (COPD). Irish Thoracic Society Annual Scientific Meeting, 11–12 November 2011, Co. Dublin, Ireland. Ir J Med Sci 2011; conference publication: S456 | Time point | Study design |
| Brundage DJ, Swearengen P, Woody JW. Self-care instruction for patients with COPD. Rehabil Nurs J 1993;18:321–5 | Outcome | Study design |
| Busch AJ, McClements JD. Effects of a supervised home exercise program on patients with severe chronic obstructive pulmonary disease. Phys Ther 1988;68:469–74 | Time point | – |
| Cabedo Garcia VR, Garces Asemany CR, Cortes Berti A, Oteo Elso JT, Ballester Salvador FJ. [Effectiveness of the correct use of inhalation devices in patients with COPD: randomized clinical trial.] Med Clin (Barc) 2010;135:586–91 | Time point | – |
| Cai S, Chen P, Chen Y, Liu ZJ. [Effect of health education on the lung function and life quality in patients with stable chronic obstructive pulmonary diseases.] Zhong Nan Da Xue Xue Bao Yi Xue Ban 2006;31:189–93 | Time point | – |
| Callaghan S. ACTRITE: Acute Chest Triage Rapid Intervention Team. Accid Emerg Nurs 1999;7:42–6 | Study design | Study design |
| Cambach W, Chadwick-Straver RVM, Wagenaar RC, van Keimpema AR. Efficacy of a rehabilitation programme in patients with asthma and chronic obstructive pulmonary disease (COPD). Ned Tijdschr Fysiotherapie 1996;2:26–36 | Unavailable | Unavailable |
| Cambach W, Chadwick-Straver RV, Wagenaar RC, van Keimpema AR, Kemper HC. The effects of a community-based pulmonary rehabilitation programme on exercise tolerance and quality of life: a randomized controlled trial. Eur Respir J 1997;10:104–13 | Population | Population |
| Cambach W, Chadwick-Straver RVM, Wagenaar RC, van Keimpema AR. Effectiveness of a rehabilitation programme for patients with asthma and COPD carried out in the first line health care. Ned Tijdschr Fysiotherapie 1998;108:26–36 | Time point | Study design |
| Cambach W. [Rehabilitation of patients with asthma and mild to moderate chronic obstructive lung disease.] Geneesk Sport 1999;32:27–30 | Population | Population |
| Cao Z, Ong KC, Eng P, Tan WC, Ng TP. Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology 2006;11:188–95 | Study design | Study design |
| Caplan GA, Ward JA, Brennan NJ, Coconis J, Board N, Brown A. Hospital in the home: a randomised controlled trial. Med J Aust 1999;170:156–60 | Intervention | Intervention |
| Cardozo L, Steinberg J. Telemedicine for recently discharged older patients. Telemed J E Health 2010;16:49–55 | Population | Population |
| Caress A, Staples V, Towey M, Woodcock A, Niven R, Frank T, et al. Participation in treatment decisions in patients with COPD and recurrent bronchitis: a qualitative study. Thorax 2004;59:100 | Time point | Study design |
| Carone M, Bertolotti G, Cerveri I, De BF, Fogliani V, Nardini S, et al. EDU-CARE, a randomised, multicentre, parallel group study on education and quality of life in COPD. Monaldi Arch Chest Dis 2002;57:25–9 | Publication type | Publication type |
| Carr SJ, Hill K, Brooks D, Goldstein RS. Pulmonary rehabilitation after acute exacerbation of chronic obstructive pulmonary disease in patients who previously completed a pulmonary rehabilitation program. J Cardiopulm Rehabil Prev 2009;29:318–24 | Time point | – |
| Carre PC, Roche N, Neukirch F, Radeau T, Perez T, Terrioux P, et al. The effect of an information leaflet upon knowledge and awareness of COPD in potential sufferers. A randomized controlled study. Respiration 2008;76:53–60 | Population | Population |
| Carrieri-Kohlman G. Internet-based and established dyspnea self-management programs in chronic obstructive pulmonary (COPD) patients; 2005. URL: www.clinicaltrials.gov (accessed 27 January 2015) | Publication type | Publication type |
| Carrieri-Kohlman V, Gormley JM, Douglas MK, Paul SM, Stulbarg MS. Exercise training decreases dyspnea and the distress and anxiety associated with it. Monitoring alone may be as effective as coaching. Chest 1996;110:1526–35 | Time point | – |
| Carrieri-Kohlman V, Gormley JM, Eiser S, mir-Deviren S, Nguyen H, Paul SM, et al. Dyspnea and the affective response during exercise training in obstructive pulmonary disease. Nurs Res 2001;50:136–46 | Time point | – |
| Carrieri-Kohlman V, Nguyen HQ, Donesky-Cuenco D, mir-Deviren S, Neuhaus J, Stulbarg MS. Impact of brief or extended exercise training on the benefit of a dyspnea self-management program in COPD. J Cardiopulm Rehabil 2005;25:275–84 | Time point | – |
| Casaburi R, Bhasin S, Cosentino L, Porszasz J, Somfay A, Lewis MI, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:870–8 | Time point | – |
| Casciari RJ, Fairshter RD, Harrison A. Effects of breathing retraining in patients with chronic obstructive pulmonary disease. Chest 1981;79:393–8 | Time point | Study design |
| Casey D, Murphy K, Cooney A, Mee L. Developing a structured education programme for clients with COPD. Br J Community Nurs 2011;16:231–7 | Publication type | Publication type |
| Chan AW, Lee A, Suen LK, Tam WW. Effectiveness of a Tai chi Qigong program in promoting health-related quality of life and perceived social support in chronic obstructive pulmonary disease clients. Qual Life Res 2010;19:653–64 | Time point | – |
| Chan AW, Lee A, Suen LK, Tam WW. Tai chi Qigong improves lung functions and activity tolerance in COPD clients: a single blind, randomized controlled trial. Complement Ther Med 2011;19:3–11 | Time point | – |
| Chang AT, Haines T, Jackson C, Yang I, Nitz J, Low CN, et al. Rationale and design of the PRSM study: pulmonary rehabilitation or self management for chronic obstructive pulmonary disease (COPD), what is the best approach? Contemp Clin Trials 2008;29:796–800 | Publication type | Publication type |
| Chang CL, Sullivan GD, Karalus NC, Hancox RJ, McLachlan JD, Mills GD. Audit of acute admissions of chronic obstructive pulmonary disease: inpatient management and outcome. Intern Med J 2007;37:236–41 | Study design | Study design |
| Chen H, Dukes R, Martin BJ. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1985;131:251–5 | Time point | – |
| Chen KH, Chen ML, Lee S, Cho HY, Weng LC. Self-management behaviours for patients with chronic obstructive pulmonary disease: a qualitative study. J Adv Nurs 2008;64:595–604 | Time point | Study design |
| Christensen EF, Nedergaard T, Dahl R. Long-term treatment of chronic bronchitis with positive expiratory pressure mask and chest physiotherapy. Chest 1990;97:645–50 | Intervention | Intervention |
| Chuang C, Levine SH, Rich J. Enhancing cost-effective care with a patient-centric coronary obstructive pulmonary disease program. Popul Health Manag 2011;14:133–6 | Time point | Study design |
| Clark CJ, Cochrane L, Mackay E. Low intensity peripheral muscle conditioning improves exercise tolerance and breathlessness in COPD. Eur Respir J 1996;9:2590–6 | Time point | – |
| Clark CJ, Cochrane LM, Mackay E, Paton B. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur Respir J 2000;15:92–7 | Time point | – |
| Cockcroft AE, Saunders MJ, Berry G. Randomised controlled trial of rehabilitation in chronic respiratory disability. Thorax 1981;36:200–3 | Population | Population |
| Cockcroft A, Berry G, Brown EB, Exall C. Psychological changes during a controlled trial of rehabilitation in chronic respiratory disability. Thorax 1982;37:413–16 | Population | Population |
| Cockcroft A, Bagnall P, Heslop A, Andersson N, Heaton R, Batstone J, et al. Controlled trial of respiratory health worker visiting patients with chronic respiratory disability. Br Med J (Clin Res Ed) 1987;294:225–8 | Time point | – |
| Coffin SE. Bronchiolitis: in-patient focus. Pediatr Clin North Am 2005;52:1047–57 | Publication type | Publication type |
| Collins EG, Langbein WE, Fehr L, O’Connell S, Jelinek C, Hagarty E, et al. Can ventilation-feedback training augment exercise tolerance in patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2008;177:844–52 | Intervention | Intervention |
| Connolly MJ, Lowe D, Anstey K, Hosker HS, Pearson MG, Roberts CM, et al. Admissions to hospital with exacerbations of chronic obstructive pulmonary disease: Effect of age related factors and service organisation. Thorax 2006;61:843–8 | Study design | Study design |
| Cooper CB. Desensitization to dyspnea in COPD with specificity for exercise training mode. Int J Chron Obstruct Pulmon Dis 2009;4:33–43 | Time point | – |
| Coppoolse R, Schols AMWJ, Baarends EM, Mostert R, Akkermans MA, Janssen PP, et al. Interval versus continuous training in patients with severe COPD: a randomized clinical trial. Eur Respir J 1999;14:258–63 | Time point | – |
| Cortopassi F, Castro AA, Porto EF, Colucci M, Fonseca G, Torre-Bouscoulet L, et al. Comprehensive exercise training improves ventilatory muscle function and reduces dyspnea perception in patients with COPD. Monaldi Archr Chest Dis 2009;71:106–12 | Time point | Study design |
| Costes F, Roche F, Pichot V, Vergnon JM, Garet M, Barthelemy JC. Influence of exercise training on cardiac baroreflex sensitivity in patients with COPD. Eur Respir J 2004;23:396–401 | Time point | Study design |
| Costi S, Brooks D, Goldstein RS. Perspectives that influence action plans for chronic obstructive pulmonary disease. Can Respir J 2006;13:362–8 | Time point | Study design |
| Costi S, Crisafulli E, Antoni FD, Beneventi C, Fabbri LM, Clini EM. Effects of unsupported upper extremity exercise training in patients with COPD: a randomized clinical trial. Chest 2009;136:387–95 | Time point | – |
| Costi S, Di BM, Pillastrini P, D’Amico R, Crisafulli E, Arletti C, et al. Short-term efficacy of upper-extremity exercise training in patients with chronic airway obstruction: a systematic review. Phys Ther 2009;89:443–55 | Publication type | Publication type |
| Cotton MM, Bucknall CE, Dagg KD, Johnson MK, MacGregor G, Stewart C, et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax 2000;55:902–6 | Intervention | Intervention |
| Coultas D, Frederick J, Barnett B, Singh G, Wludyka P. A randomized trial of two types of nurse-assisted home care for patients with COPD. Chest 2005;128:2017–24 | Time point | – |
| Couser J, Martinez FJ, Celli BR. Pulmonary rehabilitation that includes arm exercise reduces metabolic and ventilatory requirements for simple arm elevation. Chest 1993;103:37–41 | Time point | Study design |
| Coventry PA, Hind D. Comprehensive pulmonary rehabilitation for anxiety and depression in adults with chronic obstructive pulmonary disease: systematic review and meta-analysis. J Psychosom Res 2007;63:551–65 | Publication type | Publication type |
| Covey MK, Larson JL. Exercise and COPD. Am J Nurs 2004;104:40–3 | Study design | Study design |
| Covey MK, Larson JL, Wirtz SE, Berry JK, Pogue NJ, Alex CG, et al. High-intensity inspiratory muscle training in patients with chronic obstructive pulmonary disease and severely reduced function. J Cardiopulm Rehabil 2001;21:231–40 | Time point | – |
| Cox NJ, Hendricks JC, Binkhorst RA, van Herwaarden CL. A pulmonary rehabilitation program for patients with asthma and mild chronic obstructive pulmonary diseases (COPD). Lung 1993;171:235–44 | Population | Population |
| Creutzberg EC, Wouters EF, Mostert R, Weling-Scheepers CA, Schols AM. Efficacy of nutritional supplementation therapy in depleted patients with chronic obstructive pulmonary disease. Nutrition 2003;19:120–7 | Time point | Study design |
| Crisafulli E, Costi S, de BF, Biscione G, Americi F, Penza S, et al. Effects of a walking aid in COPD patients receiving oxygen therapy. Chest 2007;131:1068–74 | Intervention | Intervention |
| Cummings E, Turner P. Patient self-management and chronic illness: evaluating outcomes and impacts of information technology. Stud Health Technol Inform 2009;143:229–34 | Study design | Study design |
| Cummings JE, Hughes SL, Weaver FM, Manheim LM, Conrad KJ, Nash K, et al. Cost-effectiveness of Veterans Administration hospital-based home care. A randomized clinical trial. Arch Intern Med 1990;150:1274–80 | Population | Population |
| Cummings E, Robinson A, Pratt HC, Cameron-Tucker H, Wood-Baker R, Walters EH, et al. Pathways Home: comparing voluntary IT and non-IT users participating in a mentored self-management project. Stud Health Technol Inform 2010;160:23–7 | Study design | Study design |
| Currie GP, Miller D. Action plans for patients with chronic obstructive pulmonary disease. BMJ 2012;344:e1164 | Publication type | Publication type |
| Cuvelier A. [Education for the early recognition of exacerbations at home. Importance of a personalized action plan.] Rev Mal Respir 2006;23:15S39–15S43 | Publication type | Publication type |
| Cydulka RK, Rowe BH, Clark S, Emerman CL, Camargo CA Jr. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: the Multicenter Airway Research Collaboration. J Am Geriatr Soc 2003;51:908–16 | Study design | Study design |
| Datta D, Zuwallack R. High versus low intensity exercise training in pulmonary rehabilitation: is more better? Chron Respir Dis 2004;1:143–9 | Publication type | Publication type |
| Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354:456–60 | Intervention | Intervention |
| Davies L, Wilkinson M, Bonner S, Calverley PM, Angus RM. ‘Hospital at home’ versus hospital care in patients with exacerbations of chronic obstructive pulmonary disease: prospective randomised controlled trial. BMJ 2000;321:1265–8 | Intervention | Intervention |
| Davis AH. Exercise adherence in patients with chronic obstructive pulmonary disease: an exploration of motivation and goals. Rehabil Nurs J 2007;32:104–10 | Time point | Study design |
| Davis AH, Carrieri-Kohlman V, Janson SL, Gold WM, Stulbarg MS. Effects of treatment on two types of self-efficacy in people with chronic obstructive pulmonary disease. J Pain Symptom Manage 2006;32:60–70 | Time point | – |
| De Blaquiere P, Christensen DB, Carter WB, Martin TR. Use and misuse of metered-dose inhalers by patients with chronic lung disease: a controlled, randomized trial of two instruction methods. Am Rev Respir Dis 1989;140:910–16 | Population | Population |
| de Blasio F. A Doubting Thomas dealing with pulmonary rehabilitation. Chest 2000;117:929–31 | Publication type | Publication type |
| De Blok BM, de Greef MH, Ten Hacken NH, Sprenger SR, Postema K, Wempe JB. The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: a pilot study. Patient Educ Couns 2006;61:48–55 | Time point | – |
| De Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2003;84:1154–7 | Time point | – |
| De Lucas RP, Rodríguez González-Moro JM, de García PJ, Santacruz SA, Tatay ME, Cubillo Marcos JM. [Training of inspiratory muscles in chronic obstructive lung disease. Its impact on functional changes and exercise tolerance.] Arch Bronconeumol 1998;34:64–70 | Time point | Study design |
| De Toledo P, Jimenez S, del PF, Roca J, Alonso A, Hernandez C. Telemedicine experience for chronic care in COPD. IEEE Trans Inf Technol Biomed 2006;10:567–73 | Study design | Study design |
| De Tullio PL, Corson ME. Effect of pharmacist counseling on ambulatory patients’ use of aerosolized bronchodilators. Am J Hosp Pharm 1987;44:1802–6 | Time point | Study design |
| DeBisschop M, Robitaille B. Can a patient information sheet reduce antibiotic use in adult outpatients with acute bronchitis? J Fam Pract 2002;51:381 | Population | Population |
| Dechman G, Wilson CR. Evidence underlying breathing retraining in people with stable chronic obstructive pulmonary disease. Phys Ther 2004;84:1189–97 | Publication type | Publication type |
| Dekhuijzen PN, Folgering HT, van Herwaarden CL. Target-flow inspiratory muscle training during pulmonary rehabilitation in patients with COPD. Chest 1991;99:128–33 | Time point | – |
| Derom E, Marchand E, Troosters T. [Rehabilitation of patients with chronic obstructive lung disease.] Ann Readapt Med Phys 2007;50:602–14 | Study design | Study design |
| Devine EC, Pearcy J. Meta-analysis of the effects of psychoeducational care in adults with chronic obstructive pulmonary disease. Patient Educ Couns 1996;29:167–78 | Publication type | Publication type |
| Dewan NA, Rafique S, Kanwar B, Satpathy H, Ryschon K, Tillotson GS, et al. Acute exacerbation of COPD: factors associated with poor treatment outcome. Chest 2000;117:662–71 | Time point | Study design |
| Dewan NA, Rice KL, Caldwell M, Hilleman DE. Economic evaluation of a disease management program for chronic obstructive pulmonary disease. COPD 2011;8:153–9 | Time point | Publication type |
| Dhein Y, Munks-Lederer C, Worth H. [Evaluation of a structured education programme for patients with COPD under outpatient conditions – a pilot study.] Pneumologie 2003;57:591–7 | Time point | Study design |
| Dickenson J. An exploratory study of patient interventions and nutritional advice for patients with chronic obstructive pulmonary disease, living in the community. Int J Disabil Hum Dev 2009;8:43–9 | Time point | Study design |
| Disler RT, Gallagher RD, Davidson PM. Factors influencing self-management in chronic obstructive pulmonary disease: an integrative review. Int J Nurs Stud 2012;49:230–42 | Publication type | Publication type |
| Dolmage TE, Maestro L, Avendano MA, Goldstein RS. The ventilatory response to arm elevation of patients with chronic obstructive pulmonary disease. Chest 1993;104:1097–100 | Time point | Study design |
| Donald KJ, McBurney H, Teichtahl H, Irving L. A pilot study of telephone based asthma management. Aust Fam Physician 2008;37:170–3 | Population | Population |
| Donesky-Cuenco D, Janson S, Neuhaus J, Neilands TB, Carrieri-Kohlman V. Adherence to a home-walking prescription in patients with chronic obstructive pulmonary disease. Heart Lung 2007;36:348–63 | Time point | Study design |
| Donesky-Cuenco D, Nguyen HQ, Paul S, Carrieri-Kohlman V. Yoga therapy decreases dyspnea-related distress and improves functional performance in people with chronic obstructive pulmonary disease: a pilot study. J Altern Complement Med 2009;15:225–34 | Time point | – |
| Dourado VZ, Tanni SE, Antunes LC, Paiva SA, Campana AO, Renno AC, et al. Effect of three exercise programs on patients with chronic obstructive pulmonary disease. Braz J Med Biol Res 2009;42:263–71 | Time point | – |
| Dowson CA, Kuijer RG, Town IG, Mulder RT. Impact of panic disorder upon self-management educational goals in chronic obstructive pulmonary disease? Chron Respir Dis 2010;7:83–90 | Study design | Study design |
| Dugan D, Walker R, Monroe DA. The effects of a 9-week program of aerobic and upper body exercise on the maximal voluntary ventilation of chronic obstructive pulmonary disease patients. J Cardiopulm Rehabil 1995;15:130–3 | Time point | Study design |
| du Moulin M, Taube K, Wegscheider K, Behnke M, van den Bussche H. Home-based exercise training as maintenance after outpatient pulmonary rehabilitation. Respiration 2009;77:139–45 | Time point | – |
| Duschek S, Schandry R, Werner B. [Changes in quality of life in patients with chronic airway diseases participating in a pilot project on home-telecare.] Pravention und Rehabilitation 2006;18:57–67 | Study design | Study design |
| Duwoos H, Naze D, Labbey JL, Deschamps D, Pingard R, Guyonnaud CD, et al. Evaluation of a method of pulmonary rehabilitation with physiotherapy and exercise training in patients with Chronic Obstructive Lung-Disease (Cold). Clin Respir Physiol Bull 1980;16:266–7 | Time point | Study design |
| Eaton T, Young P, Fergusson W, Moodie L, Zeng I, O’Kane F, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology 2009;14:230–8 | Intervention | – |
| Effing T, Kerstjens H, van der Valk P, Zielhuis G, van der Palen J. (Cost)-effectiveness of self-treatment of exacerbations on the severity of exacerbations in patients with COPD: the COPE II study. Thorax 2009;64:956–62 | Time point | – |
| Effing T, Zielhuis G, Kerstjens H, van der Valk P, van der Palen J. Community based physiotherapeutic exercise in COPD self-management: a randomised controlled trial. Respir Med 2011;105:418–26 | Time point | – |
| Effing T. Action plans and case manager support may hasten recovery of symptoms following an acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). J Physiother 2012;58:60 | Publication type | Publication type |
| Efraimsson EO, Hillervik C, Ehrenberg A. Effects of COPD self-care management education at a nurse-led primary health care clinic. Scand J Caring Sci 2008;22:178–85 | Time point | – |
| Eiser N, West C, Evans S, Jeffers A, Quirk F. Effects of psychotherapy in moderately severe COPD: a pilot study. Eur Respir J 1997;10:1581–4 | Time point | Study design |
| Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur Heart J 1998;19:1254–60 | Population | Population |
| Elci A, Borekci S, Ovayolu N, Elbek O. The efficacy and applicability of a pulmonary rehabilitation programme for patients with COPD in a secondary-care community hospital. Respirology 2008;13:703–7 | Time point | – |
| Elliott M, Watson C, Wilkinson E, Musk AW, Lake FR. Short- and long-term hospital and community exercise programmes for patients with chronic obstructive pulmonary disease. Respirology 2004;9:345–51 | Time point | – |
| Elzen H, Slaets JPJ, Snijders TAB, Steverink N. The effect of a self-management intervention on health care utilization in a sample of chronically ill older patients in the Netherlands. J Eval Clin Pract 2008;14:159–61 | Population | Population |
| Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol 1998;17:232–40 | Time point | – |
| Engstrom CP, Persson LO, Larsson S, Sullivan M. Long-term effects of a pulmonary rehabilitation programme in outpatients with chronic obstructive pulmonary disease: a randomized controlled study. Scand J Rehabil Med 1999;31:207–13 | Time point | – |
| Epstein SK, Celli BR, Martinez FJ, Couser JI, Roa J, Pollock M, et al. Arm training reduces the VO2 and VE cost of unsupported arm exercise and elevation in chronic obstructive pulmonary disease. J Cardiopulm Rehabil 1997;17:171–7 | Time point | – |
| Esteve F, Blanc-Gras N, Gallego J, Benchetrit G. The effects of breathing pattern training on ventilatory function in patients with COPD. Biofeedback Self Regul 1996;21:311–21 | Time point | – |
| Falk P, Eriksen AM, Kolliker K, Andersen JB. Relieving dyspnea with an inexpensive and simple method in patients with severe chronic airflow limitation. Eur J Respir Dis 1985;66:181–6 | Time point | – |
| Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD, NETT Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD 2008;5:105–16 | Population | Population |
| Farkas J, Kadivec S, Kosnik M, Lainscak M. Effectiveness of discharge-coordinator intervention in patients with chronic obstructive pulmonary disease: study protocol of a randomized controlled clinical trial. Respir Med 2011;105(Suppl. 1):26–30 | Publication type | Publication type |
| Farrero E, Escarrabill J, Prats E, Maderal M, Manresa F. Impact of a hospital-based home-care program on the management of COPD patients receiving long-term oxygen therapy. Chest 2001;119:364–9 | Intervention | Intervention |
| Fehrenbach C, Neville E, Holmes WF. Using a private sector partnership to provide supported early discharge for acute exacerbations of COPD in a district general hospital. Thorax 2002;57 | Publication type | Publication type |
| Fernandez AM, Pascual J, Ferrando C, Arnal A, Vergara I, Sevila V. Home-based pulmonary rehabilitation in very severe COPD: is it safe and useful? J Cardiopulm Rehabil Prev 2009;29:325–31 | Time point | – |
| Fernandez J, Martãn M, Moreno LF. Evaluation of a home-based rehabilitation program controlled with pulse-meter in COPD. Neumosur 1998;10:54–5 | Unavailable | Unavailable |
| Fernandez L, Benedicto J, Siasoco MB, Medina BJ. A randomized controlled trial on the long-term effects of multiple exposures to pulmonary rehabilitation on stable COPD patients. Philipp J Chest Dis 2000;7:40–5 | Unavailable | Unavailable |
| Finkelstein SM, Speedie SM, Potthoff S. Home telehealth improves clinical outcomes at lower cost for home healthcare. Telemed J E Health 2006;12:128–36 | Population | Population |
| Finnerty JP, Keeping I, Bullough I, Jones J. The effectiveness of outpatient pulmonary rehabilitation in chronic lung disease: a randomized controlled trial. Chest 2001;119:1705–10 | Time point | – |
| Fitzsimmons DA, Thompson J, Hawley M, Mountain GA. Preventative tele-health supported services for early stage chronic obstructive pulmonary disease: a protocol for a pragmatic randomized controlled trial pilot. Trials 2011;12:6 | Publication type | Publication type |
| Flanigan UM, Irwin A, Dagg K. An acute respiratory assessment service. Profes Nurse 1999;14:839–42 | Intervention | Intervention |
| Foglio K, Bianchi L, Ambrosino N. Is it really useful to repeat outpatient pulmonary rehabilitation programs in patients with chronic airway obstruction? A 2-year controlled study. Chest 2001;119:1696–704 | Population | Population |
| Foglio K, Bianchi L, Bruletti G, Battista L, Pagani M, Ambrosino N. Long-term effectiveness of pulmonary rehabilitation in patients with chronic airway obstruction. Eur Respir J 1999;13:125–32 | Time point | Study design |
| Foy CG, Rejeski WJ, Berry MJ, Zaccaro D, Woodard CM. Gender moderates the effects of exercise therapy on health-related quality of life among COPD patients. Chest 2001;119:70–6 | Time point | – |
| Francis PB, Jr, Petty TL, Winterbauer RH. Helping the COPD patient help himself. Patient Care 1984;18:177–80 | Publication type | Publication type |
| Franssen FM, Wouters EF, Baarends EM, Akkermans MA, Schols AM. Arm mechanical efficiency and arm exercise capacity are relatively preserved in chronic obstructive pulmonary disease. Med Sci Sports Exerc 2002;34:1570–6 | Time point | Study design |
| Gadoury MA, Schwartzman K, Rouleau M, Maltais F, Julien M, Beaupre A, et al. Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J 2005;26:853–7 | Time point | – |
| Gallefoss F. The effects of patient education in COPD in a 1-year follow-up randomised, controlled trial. Patient Educ Couns 2004;52:259–66 | Time point | – |
| Gallefoss F, Bakke PS. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication? Am J Respir Crit Care Med 1999;160:2000–5 | Time point | – |
| Gallefoss F, Bakke PS. Impact of patient education and self-management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease. Respir Med 2000;94:279–87 | Time point | – |
| Gallefoss F, Bakke PS. Patient satisfaction with healthcare in asthmatics and patients with COPD before and after patient education. Respir Med 2000;94:1057–64 | Time point | Outcome |
| Gallefoss F, Bakke PS. Cost-benefit and cost-effectiveness analysis of self-management in patients with COPD: a 1-year follow-up randomized, controlled trial. Respir Med 2002;96:424–31 | Time point | – |
| Gallefoss F, Bakke PS, Rsgaard PK. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:812–17 | Time point | – |
| Garcia-Aymerich J, Barreiro E, Farrero E, Marrades RM, Morera J, Anto JM. Patients hospitalized for COPD have a high prevalence of modifiable risk factors for exacerbation (EFRAM study). Eur Respir J 2000;16:1037–42 | Intervention | Intervention |
| Garcia-Aymerich J, Monso E, Marrades RM, Escarrabill J, Felez MA, Sunyer J, et al. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation: EFRAM Study. Am J Respir Crit Care Med 2001;164:1002–7 | Study design | Study design |
| Garcia-Aymerich J, Farrero E, Felez MA, Izquierdo J, Marrades RM, Anto JM, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003;58:100–5 | Study design | Study design |
| Garrod R, Bestall JC, Garnham R, Paul EA, Jones PW, Wedzicha JA. Randomised controlled trial of hospital out-patient pulmonary rehabilitation in moderate COPD: early effects. Physiotherapy 1997;83:367 | Publication type | Publication type |
| Garrod R, Dallimore K, Cook J, Davies V, Quade K. An evaluation of the acute impact of pursed lips breathing on walking distance in nonspontaneous pursed lips breathing chronic obstructive pulmonary disease patients. Chron Respir Dis 2005;2:67–72 | Intervention | Intervention |
| Geddes EL, Reid WD, Crowe J, O’Brien K, Brooks D. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: a systematic review. Respir Med 2005;99:1440–58 | Publication type | Publication type |
| Geddes EL, O’Brien K, Reid WD, Brooks D, Crowe J. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respir Med 2008;102:1715–29 | Publication type | Publication type |
| Ghanem M, Elaal EA, Mehany M, Tolba K. Home-based pulmonary rehabilitation program: effect on exercise tolerance and quality of life in chronic obstructive pulmonary disease patients. Ann Thorac Med 2010;5:18–25 | Intervention | – |
| Gibbons D. A nurse-led pulmonary rehabilitation programme for patients with COPD. Profes Nurse 2001;17:185–8 | Study design | Study design |
| Gift AG, Moore T, Soeken K. Relaxation to reduce dyspnea and anxiety in COPD patients. Nurs Res 1992;41:242–6 | Time point | – |
| Gilmore TW, Walter RE, Davis TC, Wissing DR. Educational strategies to improve health-related quality of life in patients with COPD. Respir Care Educ Annu 2010;19:13–31 | Time point | – |
| Gimenez M, Servera E, Vergara P, Bach JR, Polu JM. Endurance training in patients with chronic obstructive pulmonary disease: a comparison of high versus moderate intensity. Arch Phys Med Rehabil 2000;81:102–9 | Time point | Study design |
| Gimeno OF, Smith ANA, Steenhuis EJ. [Follow-up study of effect of pulmonary rehabilitation in males with COPD.] Ned Tijdschr Geneeskd 1986;130:351–3 | Time point | Study design |
| Gohl O, Linz H, Schonleben T, Otte B, Weineck J, Worth H. [Benefits of a multimodular outpatient training program for patients with COPD.] Pneumologie 2006;60:529–36 | Time point | – |
| Goldstein RS, Gort EH, Stubbing D, Avendano MA, Guyatt GH. Randomised controlled trial of respiratory rehabilitation. Lancet 1994;344:1394–7 | Time point | – |
| Goldstein RS, Gort EH, Guyatt GH, Feeny D. Economic analysis of respiratory rehabilitation. Chest 1997;112:370–9 | Time point | – |
| Golmohammadi K, Jacobs P, Sin DD. Economic evaluation of a community-based pulmonary rehabilitation program for chronic obstructive pulmonary disease. Lung 2004;182:187–96 | Time point | Study design |
| Gomez A, Roman M, Larraz C, Esteva M, Mir I, Thomas V, et al. [Efficacy of respiratory rehabilitation on patients with moderate COPD in primary care and maintenance of benefits at 2 years.] Aten Prim 2006;38:230–3. [Erratum published in Aten Prim 2006;38:369.] | Publication type | Publication type |
| Gormley JM, Carrieri-Kohlman V, Douglas MK, Stulbarg MS. Treadmill self-efficacy and walking performance in patients with COPD. J Cardiopulm Rehabil 1993;13:424–31 | Time point | – |
| Gort EH, Goldstein R, Guyatt G, Stubbing D, Avendano M. Randomized controlled trial of respiratory rehabilitation. Can J Rehabil 1993;7:13–14 | Unavailable | Unavailable |
| Gosselink R, De VJ, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J 2011;37:416–25 | Publication type | Publication type |
| Gottlieb V, Lyngso AM, Nybo B, Frolich A, Backer V. Pulmonary rehabilitation for moderate COPD (GOLD 2) – does it have an effect? COPD 2011;8:380–6 | Time point | Study design |
| Gourley GA, Portner TS, Gourley DR, Rigolosi EL, Holt JM, Solomon DK, et al. Humanistic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc (Wash) 1998;38:586–97 | Time point | – |
| Gravil JH, Al-Rawas OA, Cotton MM, Flanigan U, Irwin A, Stevenson RD. Home treatment of exacerbations of chronic obstructive pulmonary disease by an acute respiratory assessment service. Lancet 1998;351:1853–5 | Intervention | Intervention |
| Green RH, Singh SJ, Williams J, Morgan MDL. A randomised controlled trial of four weeks versus seven weeks pulmonary rehabilitation in chronic obstructive pulmonary disease (COPD). Thorax 2001;56:143–5 | Time point | – |
| Greenstone M. Self-monitored, home-based pulmonary rehab was non-inferior to outpatient, hospital-based rehab for COPD. Evid Based Med 2009;14:75 | Publication type | Publication type |
| Griffiths C, Motlib J, Azad A, Ramsay J, Eldridge S, Feder G, et al. Randomised controlled trial of a lay-led self-management programme for Bangladeshi patients with chronic disease. Br J Gen Pract 2005;55:831–7 | Population | Population |
| Griffiths TL, Burr ML, Campbell IA, Lewis-Jenkins V, Mullins J, Shiels K, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000;355:362–8. [Erratum published in Lancet 2000;355:1280.] | Population | Population |
| Griffiths TL, Phillips CJ, Davies S, Burr ML, Campbell IA. Cost-effectiveness of an outpatient multidisciplinary pulmonary rehabilitation programme. Thorax 2001;56:779–84 | Population | Population |
| Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003;124:459–67 | Study design | Study design |
| Gruffydd-Jones K, Langley-Johnson C, Dyer C, Badlan K, Ward S. What are the needs of patients following discharge from hospital after an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Prim Care Resp J 2007;16:363–8 | Time point | Study design |
| Gudmundsson G, Gislason T, Janson C, Lindberg E, Hallin R, Ulrik CS, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J 2005;26:414–19 | Study design | Study design |
| Güell R, Casan P, Sangenis M, Morante F, Belda J, Guyatt GH. Quality of life in patients with chronic respiratory disease: the Spanish version of the Chronic Respiratory Questionnaire (CRQ). Eur Respir J 1998;11:55–60 | Time point | Outcome |
| Güell R, Casan P, Belda J, Sangenis M, Morante F, Guyatt GH, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest 2000;117:976–83 | Time point | – |
| Güell R, Morante F, Sangenis M, Casan P. Effects of respiratory rehabilitation on quality of life of patients with chronic obstructive pulmonary disease. Annals de Medicina 1995;81:9 | Unavailable | Unavailable |
| Güell R, Resqueti V, Sangenis M, Morante F, Martorell B, Casan P, et al. Impact of pulmonary rehabilitation on psychosocial morbidity in patients with severe COPD. Chest 2006;129:899–904. [Erratum published in Chest 2007;132:738.] | Publication type | Publication type |
| Güell R, Resqueti V, Sangenis M, Morante F, Martorell B, Casan P, et al. Impact of pulmonary rehabilitation on psychosocial morbidity in patients with severe COPD. Chest 2006;129:899–904. [Erratum published in Chest 2007;132:738.] | Time point | – |
| Guyatt G, Keller J, Singer J, Halcrow S, Newhouse M. Controlled trial of respiratory muscle training in chronic airflow limitation. Thorax 1992;47:598–602 | Time point | – |
| Guyatt GH, King DR, Feeny DH, Stubbing D, Goldstein RS. Generic and specific measurement of health-related quality of life in a clinical trial of respiratory rehabilitation. J Clin Epidemiol 1999;52:187–92 | Time point | – |
| Haas A, Cardon H. Rehabilitation in chronic obstructive pulmonary disease: a 5-year study of 252 male patients. Med Clin North Am 1969;53:593–606 | Time point | Study design |
| Haas A, Dani A. Rehabilitation of patients with chronic obstructive pulmonary disease. AAGP 1965;31:92–8 | Time point | Study design |
| Haas A, Luczak A. The importance of rehabilitation in the treatment of chronic pulmonary emphysema. Arch Phys Med Rehabil 1961;42:733–9 | Time point | Study design |
| Haas A, Luczak AK, Kernisant R, Zotowicz V. [Studies on the use of physical therapy and rehabilitation in patients with chronic obstructive pulmonary emphysema.] Polski Tygodnik Lekarski 1963;18:1834–7 | Time point | Study design |
| Haave E. Writing About Disease: Effect on Rehabilitation; 2005. URL: www.clinicaltrials.gov (accessed 27 January 2015) | Population | Population |
| Habraken JM, Pols J, Bindels PJ, Willems DL. The silence of patients with end-stage COPD: a qualitative study. Br J Gen Pract 2008;58:844–9 | Intervention | Intervention |
| Haggerty MC, Stockdale-Woolley R, Nair S. Respi-Care. An innovative home care program for the patient with chronic obstructive pulmonary disease. Chest 1991;100:607–12 | Intervention | Intervention |
| Han SJ. [The effects of a pulmonary rehabilitation program for chronic obstructive pulmonary disease patients.] Daehan Ganho Haghoeji 2003;33:1008–17 | Time point | Study design |
| Harver A, Mahler DA, Daubenspeck JA. Targeted inspiratory muscle training improves respiratory muscle function and reduces dyspnea in patients with chronic obstructive pulmonary disease. Ann Intern Med 1989;111:117–24 | Time point | – |
| Harvey PA, Murphy MC, Dornom E, Berlowitz DJ, Lim WK, Jackson B. Implementing evidence-based guidelines: inpatient management of chronic obstructive pulmonary disease. Intern Med J 2005;35:151–5 | Study design | Study design |
| Health related quality of life changes in community based pulmonary rehabilitation for people with COPD. Data eighteen months post rehab. 07; Switzerland: Lausanne; 2007 | Study design | Study design |
| Heijdra YF, Dekhuijzen PNR, van Herwaarden CLA, Folgering HT. Nocturnal saturation improves by target-flow inspiratory muscle training in patients with COPD. Am J Respir Crit Care Med 1996;153:260–5 | Time point | – |
| Hellem E, Bruusgaard KA, Bergland A. Exercise maintenance: COPD patients’ perception and perspectives on elements of success in sustaining long-term exercise. Physiother Theory Pract 2012;28:206–20 | Time point | Study design |
| Herala M, Stalenheim G, Boman G. Effects of positive expiratory pressure (PEP), continuous positive airway pressure (CPAP) and hyperventilation in COPD patients with chronic hypercapnea. Ups J Med Sci 1995;100:223–32 | Intervention | Intervention |
| Herborg H, Soendergaard B, Froekjaer B, Fonnesbaek L, Jorgensen T, Hepler CD, et al. Improving drug therapy for patients with asthma. Part 1: Patient Outcomes. J Am Pharm Assoc (Wash) 2001;41:539–50 | Population | Population |
| Hernandez MT, Rubio TM, Ruiz FO, Riera HS, Gil RS, Gomez JC. Results of a home-based training program for patients with COPD. Chest 2000;118:106–14 | Time point | – |
| Heslop AP, Bagnall P. A study to evaluate the intervention of a nurse visiting patients with disabling chest disease in the community. J Adv Nurs 1988;13:71–7 | Time point | Study design |
| Hesselink AE, Penninx BW, van der Windt DA, van Duin BJ, de Vries P, Twisk JW, et al. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: results from a randomised controlled trial. Patient Educ Couns 2004;55:121–8 | Population | Population |
| Hill K, Jenkins SC, Philippe DL, Cecins N, Shepherd KL, Green DJ, et al. High-intensity inspiratory muscle training in COPD. Eur Respir J 2006;27:1119–28 | Time point | – |
| Hoberty RJ, Craig MW. ‘Living up to par’: a golf tournament for persons with COPD. Respir Care 1983;28:1480–3 | Population | Population |
| Hochstetter JK, Lewis J, Soares-Smith L. An investigation into the immediate impact of breathlessness management on the breathless patient: randomised controlled trial. Physiotherapy 2005;91:178–85 | Population | Population |
| Hoff J, Tjonna AE, Steinshamn S, Hoydal M, Richardson RS, Helgerud J. Maximal strength training of the legs in COPD: a therapy for mechanical inefficiency. Med Sci Sports Exerc 2007;39:220–6 | Time point | – |
| Hogan MT. Effect of pulmonary rehabilitation on quality of life in individuals with chronic obstructive pulmonary disease. Aus Health Rev 1992;52:3155 | Unavailable | Unavailable |
| Holden DA, Stelmach KD, Curtis PS, Beck GJ, Stoller JK. The impact of a rehabilitation program on functional status of patients with chronic lung disease. Respir Care 1990;35:332–41 | Time point | Study design |
| Holland AE, Hill CJ, Nehez E, Ntoumenopoulos G. Does unsupported upper limb exercise training improve symptoms and quality of life for patients with chronic obstructive pulmonary disease? J Cardiopulm Rehabil 2004;24:422–7 | Time point | – |
| Holle RHO, Williams DV, Vandree JC, Starks GL, Schoene RB. Increased muscle efficiency and sustained benefits in an outpatient community hospital-based pulmonary rehabilitation program. Chest 1988;94:1161–8 | Time point | Study design |
| Holm SM, Rodgers WM, Haennel RG, Bhutani M, MacDonald GF, Wong E. Physiological responses to treadmill and cycle ergometer exercise testing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011;183:A3965 | Publication type | Publication type |
| Honeyman P, Barr P, Stubbing DG. Effect of a walking aid on disability, oxygenation, and breathlessness in patients with chronic airflow limitation. J Cardiopulm Rehabil 1996;16:63–7 | Intervention | Intervention |
| Hoogendoorn M, van Wetering CR, Schols AM, Rutten-van Mölken MP. Self-report versus care provider registration of healthcare utilization: impact on cost and cost-utility. Int J Technol Assess Health Care 2009;25:588–95 | Time point | – |
| Hoogendoorn M, van Wetering CR, Schols AM, Rutten-van Mölken MP. Is INTERdisciplinary COMmunity-based COPD management (INTERCOM) cost-effective? Eur Respir J 2010;35:79–87 | Time point | – |
| Hospes G, Bossenbroek L, Ten Hacken NH, van Hengel P, de Greef MH. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns 2009;75:274–8 | Time point | – |
| Houchen L, Deacon S, Sandland C, Collier R, Steiner M, Morgan M, et al. Preservation of lower limb strength after a short course of pulmonary rehabilitation with no maintenance: a 6-month follow-up study. Physiotherapy 2011;97:264–6 | Time point | Study design |
| Howard JE, Davies JL, Roghmann KJ. Respiratory teaching of patients: how effective is it? J Adv Nurs 1987;12:207–14 | Time point | Study design |
| Howland J, Nelson EC, Barlow PB, McHugo G, Meier FA, Brent P, et al. Chronic obstructive airway disease. Impact of health education. Chest 1986;90:233–8 | Time point | Study design |
| Hsiao SF, Wu YT, Wu HD, Wang TG. Comparison of effectiveness of pressure threshold and targeted resistance devices for inspiratory muscle training in patients with chronic obstructive pulmonary disease. J Formosan Med Assoc 2003;102:240–5 | Time point | – |
| Hsieh MJ, Lan CC, Chen NH, Huang CC, Wu YK, Cho HY, et al. Effects of high-intensity exercise training in a pulmonary rehabilitation programme for patients with chronic obstructive pulmonary disease. Respirology 2007;12:381–8 | Time point | Study design |
| Hsu HC. [Respiratory rehabilitation in patients with chronic obstructive pulmonary disease.] Hu Li Tsa Chih 1997;44:87–92 | Publication type | Publication type |
| Huang C-H, Yang G-G, Wu Y-T, Lee C-W. Comparison of inspiratory muscle strength training effects between older subjects with and without chronic obstructive pulmonary disease. J Formosan Med Assoc 2011;110:518–26 | Time point | Study design |
| Hudson LD, Tyler ML, Petty TL. Hospitalization needs during an outpatient rehabilitation program for severe chronic airway obstruction. Chest 1976;70:606–10 | Study design | Study design |
| Hughes SL, Cummings J, Weaver F, Manheim L, Braun B, Conrad K. A randomized trial of the cost-effectiveness of VA hospital-based home care for the terminally ill. Health Serv Res 1992;26:801–17 | Population | Population |
| Hughes SL, Weaver FM, Giobbie-Hurder A, Manheim L, Henderson W, Kubal JD, et al. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA 2000;284:2877–85 | Population | Population |
| Hui KP, Hewitt AB. A simple pulmonary rehabilitation program improves health outcomes and reduces hospital utilization in patients with COPD. Chest 2003;124:94–7 | Time point | Study design |
| Hung SH, Tseng HC, Tsai WH, Lin HH, Cheng JH, Chang YM. COPD: endurance training via mobile phone. AMIA Ann Symp Proc 2007;2007:985 | Time point | Publication type |
| Hunter J, Singh SJ, Morgan MDL. Objective monitoring of adherence with home exercise training during pulmonary rehabilitation for chronic obstructive pulmonary disease. Physiotherapy 2006;92:50–4 | Time point | Study design |
| Hunter SM. Educating clients with COPD. Home Healthc Nurse 1987;5:41–3 | Publication type | Publication type |
| Hunter SM, Hall SS. The effect of an educational support program on dyspnea and the emotional status of COPD clients. Rehabil Nurs J 1989;14:200–2 | Time point | Study design |
| Hurst JR, Fitzgerald-Khan F, Quint JK, Goldring JJ, Mikelsons C, Dilworth JP, et al. Use and utility of a 24-hour Telephone Support Service for ‘high risk’ patients with COPD. Prim Care Resp J 2010;19:260–5 | Time point | Study design |
| Hynninen MJ, Bjerke N, Pallesen S, Bakke PS, Nordhus IH. A randomized controlled trial of cognitive behavioral therapy for anxiety and depression in COPD. Respir Med 2010;104:986–94 | Time point | – |
| Ige OM, Olarewaju RK, Lasebikan VO, Adeniyi YO. Outpatient pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci 2010;52:197–201 | Time point | Study design |
| Ilayaraja A, Shawesh A. A comparative study on effectiveness of autogenic drainage (AD) versus postural drainage (PD) in improving pulmonary function (FEV1, FVC) in chronic obstructive pulmonary disease (COPD). Jamahiriya Med J 2010;10:106–10 | Unavailable | Unavailable |
| Incalzi RA, Corsonello A, Trojano L, Pedone C, Acanfora D, Spada A, et al. Cognitive training is ineffective in hypoxemic COPD: a six-month randomized controlled trial. Rejuvenation Res 2008;11:239–50 | Time point | – |
| Incorvaia C, Riario-Sforza GG. Effect of patient education on adherence to drug treatment for chronic obstructive pulmonary disease. Ann Thorac Med 2011;6:242–3 | Publication type | Publication type |
| Innocenti F, Fabbri A, Guerrini M, Fonseca D, Lippi P. Results of an outpatient pulmonary rehabilitation program in patients with COPD. Eur Respir J 2000;16(Suppl. 31):46 | Unavailable | Unavailable |
| Inoue M, Ohtsu I, Tomioka S, Hagiya M, Sumi M, Aoki H, et al. [Effects of pulmonary rehabilitation on vital capacity in patients with chronic pulmonary emphysema.] Nihon Kyobu Shikkan Gakkai Zasshi 1996;34:1182–8 | Time point | Study design |
| Inoue M, Ohtsu I, Tomioka S, Sumi M, Nakayama M, Hagiya M, et al. [One year follow-up of pulmonary rehabilitation in patients with pulmonary emphysema: physiological outcome.] Nihon Kokyuki Gakkai Zasshi 1998;36:756–62 | Time point | Study design |
| Ip SP, Leung YF, Choy KL. Short-stay in-patient rehabilitation of elderly patients with chronic obstructive pulmonary disease: prospective study. Hong Kong Med J 2004;10:312–18 | Intervention | Study design |
| Ito M, Kakizaki F, Tsuzura Y, Yamada M. Immediate effect of respiratory muscle stretch gymnastics and diaphragmatic breathing on respiratory pattern. Respiratory Muscle Conditioning Group. Intern Med 1999;38:126–32 | Time point | Study design |
| Izumizaki M, Satake M, Takahashi H, Sugawara K, Shioya T, Homma I. Effects of inspiratory muscle thixotropy on the 6-minute walk distance in COPD. Respir Med 2008;102:970–7 | Time point | – |
| Janaudis-Ferreira T, Hill K, Goldstein R, Robles-Ribeiro P, Beauchamp M, Dolmage T. Resistance arm training in patients with COPD: a randomized controlled trial. European Respiratory Society Annual Congress, 18–22 September 2010, Barcelona, Spain, abstract no. 1936 | Time point | Publication type |
| Janaudis-Ferreira T, Hill K, Goldstein RS, Robles-Ribeiro P, Beauchamp MK, Dolmage T, et al. Resistance arm training in patients with COPD: a randomized controlled trial. Chest 2011;139:151–8 | Time point | – |
| Jang HJ, Jung YK. [The effects of self-efficacy promoting pulmonary rehabilitation program in out-patients with chronic obstructive pulmonary disease.] Tuberc Respir Dis 2006;61:533–46 | Time point | – |
| Janos V, Krisztina B, Attila S. [The effect of controlled and uncontrolled dynamic lower extremity training in the rehabilitation of patients with chronic obstructive pulmonary disease.] Orvosi Hetilap 2005;146:2249–55 | Time point | Study design |
| Jans MP, Schellevis FG, Le Coq EM, Bezemer PD, van Eijk JT. Health outcomes of asthma and COPD patients: the evaluation of a project to implement guidelines in general practice. Int J Qual Health Care 2001;13:17–25 | Time point | Study design |
| Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharm 2012;34:53–62 | Time point | – |
| Jeffs KJ, Lim WK, Lim M, Berlowitz DJ, Jackson B. The effect of a post acute respiratory outreach service for patients with chronic obstructive pulmonary disease on hospital readmission rates. Respirology 2005;10:239–43 | Study design | Study design |
| Jeng C, Tsao LI, Ho CH, Chang PC. Experiences of daily activities within two weeks after hospital discharge among Taiwanese elderly patients with chronic obstructive pulmonary disease. J Nurs Res 2002;10:168–76 | Intervention | Study design |
| Jensen PS. Risk, protective factors, and supportive interventions in chronic airway obstruction. Arch Gen Psychiatry 1983;40:1203–7 | Population | Population |
| Jerant A, Moore M, Lorig K, Franks P. Perceived control moderated the self-efficacy-enhancing effects of a chronic illness self-management intervention. Chron Illness 2008;4:173–82 | Population | Population |
| Jette DU, Bourgeois MC, Buchbinder R. Pulmonary rehabilitation following acute exacerbation of chronic obstructive pulmonary disease. Phys Ther 2010;90:9–12 | Publication type | Publication type |
| Jin X-Q, Hao L-S, Chen W-H. [The influence of pulmonary rehabilitation on dyspnea, pulmonary function test and exercise tolerance in chronic obstructive pulmonary disease patients.] Chin J Clin Rehabil 2002;6:662–3 | Time point | – |
| Johnston B, Wheeler L, Deuser J, Sousa KH. Outcomes of the Kaiser Permanente Tele-Home Health Research Project. Arch Fam Med 2000;9:40–5 | Population | Population |
| Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther 2003;83:424–31 | Time point | Study design |
| Jones DT, Thomson RJ, Sears MR. Physical exercise and resistive breathing training in severe chronic airways obstruction. Are they effective? Eur J Respir Dis 1985;67:159–66 | Time point | – |
| Jones RC, Hyland ME, Hanney K, Erwin J. A qualitative study of compliance with medication and lifestyle modification in Chronic Obstructive Pulmonary Disease (COPD). Prim Care Resp J 2004;13:149–54 | Time point | Study design |
| Jones RC, Wang X, Harding S, Bott J, Hyland M. Educational impact of pulmonary rehabilitation: Lung Information Needs Questionnaire. Respir Med 2008;102:1439–45 | Time point | Study design |
| Jonkers CC, Lamers F, Bosma H, Metsemakers JF, van Eijk JT. The effectiveness of a minimal psychological intervention on self-management beliefs and behaviors in depressed chronically ill elderly persons: a randomized trial. Int Psychogeriatr 2012;24:288–97 | Population | Population |
| Jonsdottir H. Life patterns of people with chronic obstructive pulmonary disease: isolation and being closed in. Nurs Sci Q 1998;11:160–6 | Intervention | Intervention |
| Jordhoy MS, Fayers P, Saltnes T, Ehlner-Elmqvist M, Jannert M, Kaasa S. A palliative-care intervention and death at home: a cluster randomised trial. Lancet 2000;356:888–93 | Population | Population |
| Joseph AM. Care coordination and telehealth technology in promoting self-management among chronically ill patients. Telemed J E Health 2006;12:156–9 | Population | Population |
| Kaelin ME, Swank AM, Barnard KL, Adams KJ, Beach P, Newman J. Physical fitness and quality of life outcomes in a pulmonary rehabilitation program utilizing symptom limited interval training and resistance training. J Exerc Physiol Online 2001;4:30–7 | Time point | Study design |
| Kagaya H, Takahashi H, Sugawara K, Kasai C, Kiyokawa N, Shioya T. Effective home-based pulmonary rehabilitation in patients with restrictive lung diseases. Tohoku J Exp Med 2009;218:215–19 | Population | Population |
| Kakizaki F, Shibuya M, Yamazaki T, Yamada M, Suzuki H, Homma I. Preliminary report on the effects of respiratory muscle stretch gymnastics on chest wall mobility in patients with chronic obstructive pulmonary disease. Respir Care 1999;44:409–14 | Time point | Study design |
| Kalter-Leibovici O. Comprehensive Disease Management Program in Chronic Obstructive Pulmonary Disease (COPD) Patients in the Community (COPD_CDM); 2009. URL: www.clinicaltrials.gov (accessed 27 January 2015) | Publication type | Publication type |
| Kamahara K, Homma T, Naito A, Matsumura T, Nakayama M, Kadono K, et al. Circuit training for elderly patients with chronic obstructive pulmonary disease: a preliminary study. Arch Gerontol Geriatr 2004;39:103–10 | Time point | Study design |
| Kanamori K, Okubo K. [Effects of pulmonary rehabilitation in patients with chronic respiratory failure.] Nihon Kyobu Shikkan Gakkai Zasshi 1996;34:397–403 | Time point | Study design |
| Kanervisto M, Kaistila T, Paavilainen E. Severe chronic obstructive pulmonary disease in a family’s everyday life in Finland: perceptions of people with chronic obstructive pulmonary disease and their spouses. Nurs Health Sci 2007;9:40–7 | Time point | Study design |
| Kapella MC, Herdegen JJ, Perlis ML, Shaver JL, Larson JL, Law JA, et al. Cognitive behavioral therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects. Int J Chron Obstruct Pulmon Dis 2011;6:625–35 | Time point | Study design |
| Kaplan RM. Randomized trial of rehabilitation in chronic obstructive pulmonary disease. J Rehabil Res Dev 1991;28:268 | Time point | Publication type |
| Kaplan RM, Atkins CJ, Reinsch S. Specific efficacy expectations mediate exercise compliance in patients with COPD. Health Psychol 1984;3:223–42 | Population | Population |
| Kara M. Using the Roper, Logan and Tierney model in care of people with COPD. J Clin Nurs 2007;16:223–33 | Intervention | Intervention |
| Kara M, Asti T. Effect of education on self-efficacy of Turkish patients with chronic obstructive pulmonary disease. Patient Educ Couns 2004;55:114–20 | Time point | Outcome |
| Karapolat H, Atasever A, Atamaz F, Kirazli Y, Elmas F, Erdinc E. Do the benefits gained using a short-term pulmonary rehabilitation program remain in COPD patients after participation? Lung 2007;185:221–5 | Time point | – |
| Kasikci MK. Using self-efficacy theory to educate a patient with chronic obstructive pulmonary disease: a case study of 1-year follow-up. Int J Nurs Pract 2011;17:1–8 | Time point | Study design |
| Katiyar SK, Bihari S. Role of pranayama in rehabilitation of COPD patients: a randomized controlled study. Indian J Allergy Asthma Immunol 2006;20:98–104 | Time point | – |
| Kayahan B, Karapolat H, Atyntoprak E, Atasever A, Ozturk O. Psychological outcomes of an outpatient pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease. Respir Med 2006;100:1050–7 | Time point | – |
| Keating A, Lee AL, Holland AE. Lack of perceived benefit and inadequate transport influence uptake and completion of pulmonary rehabilitation in people with chronic obstructive pulmonary disease: a qualitative study. J Physiother 2011;57:183–90 | Time point | Study design |
| Kelly MG, Elborn JS. Admissions with chronic obstructive pulmonary disease after publication of national guidelines. Ir J Med Sci 2002;171:16–19 | Study design | Study design |
| Kennedy S. Caring for a patient newly diagnosed with COPD: a reflective account. Nurs Stand 2011;25:43–8 | Study design | Study design |
| Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:158–64 | Study design | Study design |
| Ketelaars CA, Huyer Abu-Saad H, Halfens RJ, Schlosser MA, Mostert R, Wouters EF. Effects of specialized community nursing care in patients with chronic obstructive pulmonary disease. Heart Lung 1998;27:109–20 | Time point | Study design |
| Khdour MR, Agus AM, Kidney JC, Smyth BM, Elnay JC, Crealey GE. Cost–utility analysis of a pharmacy-led self-management programme for patients with COPD. Int J Clin Pharm 2011;33:665–73 | Time point | – |
| Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol 2009;68:588–98 | Time point | – |
| Kheirabadi GR, Keypour M, Attaran N, Bagherian R, Maracy MR. Effect of add-on ‘Self management and behavior modification’ education on severity of COPD. Tanaffos 2008;7:23–30 | Time point | – |
| Kim MJ, Larson JL, Covey MK, Vitalo CA, Alex CG, Patel M. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Nurs Res 1993;42:356–62 | Time point | – |
| Kim S, Emerman CL, Cydulka RK, Rowe BH, Clark S, Camargo CA. Prospective multicenter study of relapse following emergency department treatment of COPD exacerbation. Chest 2004;125:473–81 | Study design | Study design |
| Kinney M. Rehabilitation of patients with COLD. Am J Nurs 1967;67:2528–35 | Study design | Study design |
| Kirsten DK, Taube C, Lehnigk B, Jörres RA, Magnussen H. Exercise training improves recovery in patients with COPD after an acute exacerbation. Respir Med 1998;92:1191–8 | Time point | – |
| Kiser K, Jonas D, Warner Z, Scanlon K, Shilliday BB, DeWalt DA. A randomized controlled trial of a literacy-sensitive self-management intervention for chronic obstructive pulmonary disease patients. J Gen Intern Med 2012;27:190–5 | Time point | Outcome |
| Ko FW, Dai DL, Ngai J, Tung A, Ng S, Lai K, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology 2011;16:617–24 | Intervention | – |
| Koff PB, Jones RH, Cashman JM, Voelkel NF, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J 2009;33:1031–8 | Time point | – |
| Kokosov AN, Potashov DA. [Rehabilitation treatment of patients with chronic bronchitis with initial manifestations of obstruction at a specialized department.] Klin Med (Mosk) 1989;67:45–9 | Unavailable | Unavailable |
| Kongsgaard M, Backer V, Jorgensen K, Kjaer M, Beyer N. Heavy resistance training increases muscle size, strength and physical function in elderly male COPD-patients: a pilot study. Respir Med 2004;98:1000 | Time point | – |
| Koppers RJH, Vos PJE, Boot CRL, Folgering HT. Exercise performance improves in patients with COPD due to respiratory muscle endurance training. Chest 2006;129:886–92 | Time point | – |
| Koshioka T, Kataoka K, Ichihara S, Sawamoto A, Sawada T. [Self control of respiratory rehabilitation by patients with chronic obstructive lung diseases: group sessions on instructions in respiratory rehabilitation.] Kango gijutsu 1988;34:1773–6 | Study design | Study design |
| Kovacevic A, Schmidt KG, Nicolai T, Wisbauer M, Schuster A. Two further cases supporting nonsurgical management in congenital lobar emphysema. Klin Padiatr 2009;221:232–6 | Population | Population |
| Kuehn BM. Education key to treating airway disease: focus on inhaler users, rescue workers, athletes. JAMA 2007;298:2601–8 | Study design | Study design |
| Kunik ME, Veazey C, Cully JA, Souchek J, Graham DP, Hopko D, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: a randomized controlled trial. Psychol Med 2008;38:385–96 | Time point | – |
| Kurabayashi H, Machida I, Tamura K, Iwai F, Tamura J, Kubota K. Breathing out into water during subtotal immersion: a therapy for chronic pulmonary emphysema. Am J Phys Med Rehabil 2000;79:150–3 | Time point | – |
| Kurch TK. [Therapeutico-rehabilitation process using graded physical loads in recurrent bronchitis.] Vrach Delo 1984;6:59–61 | Population | Population |
| Kurch TK. [Effectiveness of using therapeutic and rehabilitation complexes in recurrent bronchitis.] Vrach Delo 1987;12:40–3 | Population | Population |
| Kurihara N, Shirai S. [Rehabilitation of patients with chronic obstructive lung diseases.] Kango gijutsu 1988;34:1807–11 | Study design | Study design |
| Kuver C, Beyer M, Gensichen J, Ludt S, Schmitz A, Szecsenyi J, et al. [An assessment of patient education programmes for patients with type 1 and 2 diabetes, asthma and COPD, coronary heart disease, hypertension, congestive heart failure, and breast cancer in Germany.] Z Arztl Fortbild Qualitatssich 2004;98:393–402 | Study design | Study design |
| Kyung KA, Chin PA. The effect of a pulmonary rehabilitation programme on older patients with chronic pulmonary disease. J Clin Nurs 2008;17:118–25 | Population | Population |
| Labrecque M, Rabhi K, Laurin C, Favreau H, Moullec G, Lavoie K, et al. Can a self-management education program for patients with chronic obstructive pulmonary disease improve quality of life? Can Respir J 2011;18:e77–81 | Time point | Study design |
| Lacasse Y, Wong E, Guyatt GH, King D, Cook DJ, Goldstein RS. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet 1996;348:1115–19 | Publication type | Publication type |
| Lake FR, Henderson K, Briffa T, Openshaw J, Musk AW. Upper-limb and lower-limb exercise training in patients with chronic airflow obstruction. Chest 1990;97:1077–82 | Time point | – |
| Lamers F, Jonkers CC, Bosma H, Chavannes NH, Knottnerus JA, van Eijk JT. Improving quality of life in depressed COPD patients: effectiveness of a minimal psychological intervention. COPD 2010;7:315–22 | Time point | – |
| Lamers F, Jonkers CC, Bosma H, Kempen GI, Meijer JA, Penninx BW, et al. A minimal psychological intervention in chronically ill elderly patients with depression: a randomized trial. Psychother Psychosomat 2010;79:217–26 | Population | Population |
| Lan CC, Yang MC, Lee CH, Huang YC, Huang CY, Huang KL, et al. Pulmonary rehabilitation improves exercise capacity and quality of life in underweight patients with chronic obstructive pulmonary disease. Respirology 2011;16:276–83 | Time point | Study design |
| Lange P, Brondum E, Bolton S, Martinez G. [Rehabilitation of patients with chronic obstructive pulmonary disease.] Ugeskr Laeger 2005;167:274–9 | Time point | Study design |
| Laros CD, Swierenga J. [Rehabilitation program in patients with obstructive-destructive lung disease (author’s translation).] Acta Tuberc PneumolBelg 1975;66:207–21 | Publication type | Publication type |
| Larraz C, Esteva M, Ripoll J, Mir I, Gomez A, Romãn M, et al. Efficacy of a rehabilitation program on moderate COPD conducted in primary care and the maintenance of benefits during two years. Prim Care Resp J 2010;19:A22. | Publication type | Publication type |
| Larson JL, Kim MJ, Sharp JT, Larson DA. Inspiratory muscle training with a pressure threshold breathing device in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1988;138:689–96 | Time point | – |
| Larson JL, Covey MK, Wirtz SE, Berry JK, Alex CG, Langbein WE, et al. Cycle ergometer and inspiratory muscle training in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:500–7 | Time point | – |
| Larson M, Kim MJ. Respiratory muscle training with the incentive spirometer resistive breathing device. Heart Lung 1984;13:341–5 | Time point | Study design |
| Lathlean T, Cafarella P, Rowett D, Frith P, Lawrence J. Combining chronic condition self management and pulmonary rehabilitation for COPD patients. Respirology 2008;13(Suppl. 5):A172 | Publication type | Publication type |
| Lau AC, Yam LY, Poon E. Hospital re-admission in patients with acute exacerbation of chronic obstructive pulmonary disease. Respir Med 2001;95:876–84 | Intervention | Intervention |
| Laviolette L, Bourbeau J, Bernard S, Lacasse Y, Pepin V, Breton MJ, et al. Assessing the impact of pulmonary rehabilitation on functional status in COPD. Thorax 2008;63:115–21 | Time point | Study design |
| Laviolette L, Lands LC, Dauletbaev N, Saey D, Milot J, Provencher S, et al. Combined effect of dietary supplementation with pressurized whey and exercise training in chronic obstructive pulmonary disease: a randomized, controlled, double-blind pilot study. J Med Food 2010;13:589–98 | Intervention | Intervention |
| Lawlor M, Kealy S, Agnew M, Korn B, Quinn J, Cassidy C, et al. Early discharge care with ongoing follow-up support may reduce hospital readmissions in COPD. Int J Chron Obstruct Pulmon Dis 2009;4:55–60. | Study design | Study design |
| Leal HM, Abellan AJ, Martinez CJ, Nicolas BA. [Written information on the use of aerosols in COPD patients. Can we improve their use?] Aten Prim 2004;33:6–10 | Time point | Outcome |
| Lee CY, Yeh LL, Chen CZ, Lin PY, Hsiue TR, Wang WL. [Exploring self-management in patients with chronic obstructive pulmonary disease.] Hu Li Tsa Chih 2008;55:45–55 | Time point | Study design |
| Lee DK. Pulmonary rehabilitation and readmissions in COPD: hospital readmissions did not fall. BMJ 2005;330:480 | Publication type | Publication type |
| Leff B, Burton L, Guido S, Greenough WB, Steinwachs D, Burton JR. Home Hospital program: a pilot study. J Am Geriatr Soc 1999;47:697–702 | Intervention | Intervention |
| Leidy NK, Haase JE. Functional performance in people with chronic obstructive pulmonary disease: a qualitative analysis. Adv Nurs Sci 1996;18:77–89 | Time point | Study design |
| Leidy NK, Haase JE. Functional status from the patient’s perspective: the challenge of preserving personal integrity. Res Nurs Health 1999;22:67–77 | Time point | Study design |
| Lertzman MM, Cherniack RM. Rehabilitation of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1976;114:1145–65 | Publication type | Publication type |
| Leung RW, Alison JA, McKeough ZJ, Peters MJ. Ground walk training improves functional exercise capacity more than cycle training in people with chronic obstructive pulmonary disease (COPD): a randomised trial. J Physiother 2010;56:105–12 | Time point | – |
| Leung RWM, Alison JA, McKeough ZJ, Peters MJ. A study design to investigate the effect of short-form Sun-style Tai Chi in improving functional exercise capacity, physical performance, balance and health related quality of life in people with Chronic Obstructive Pulmonary Disease (COPD). Contemp Clin Trials 2011;32:267–72 | Publication type | Publication type |
| Levine S, Weiser P, Gillen J. Evaluation of a ventilatory muscle endurance training program in the rehabilitation of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1986;133:400–6 | Time point | – |
| Lewczuk J, Piszko P. [Rehabilitation of patients with chronic obstructive pulmonary diseases.] Pneumonol Alergol Pol 1997;65:691–9 | Publication type | Publication type |
| Lewczuk J, Piszko P, Kowalska-Superlak M, Wrabec K, Knap J, Palka P, et al. [Respiratory rehabilitation of patients with chronic obstructive lung diseases in the subjective and objective evaluation.] Pneumonol Alergol Pol 1991;59:132–6 | Time point | Study design |
| Lewczuk J, Piszko P, Kowalska-Superlak M, Jagas J, Wojciak S, Wrabec K. [The impact of 2-year rehabilitation on exercise tolerance and transcutaneous oxygen saturation during exercise in patients with chronic obstructive pulmonary disease.] Pol Arch Med Wewn 1998;100:331–6 | Time point | Study design |
| Lewczuk J, Piszko P, Wojciak S, Kowalska-Superlak M, Wrabec K. [Comparison of 14-day rehabilitation and oxygen therapy on exercise tolerance and percutaneous oxygen saturation in patients with advanced COPD.] Pneumonol Alergol Pol 1998;66:464–7 | Intervention | Intervention |
| Lewis MI, Fournier M, Storer TW, Bhasin S, Porszasz J, Ren SG, et al. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol 2007;103:1299–310 | Outcome | Outcome |
| Li Y-L. [Nutritional supplementation and respiratory gym in patients with chronic obstructive pulmonary disease.] Chin J Clin Rehabil 2002;6:1260–2 | Time point | – |
| Liddell F, Webber J. Pulmonary rehabilitation for chronic obstructive pulmonary disease: a pilot study evaluating a once-weekly versus twice-weekly supervised programme. Physiotherapy 2010;96:68–74 | Time point | – |
| Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2006;144:894–903 | Study design | Study design |
| Lindsay M, Lee A, Chan K, Poon P, Han LK, Wong WC, et al. Does pulmonary rehabilitation give additional benefit over tiotropium therapy in primary care management of chronic obstructive pulmonary disease? Randomized controlled clinical trial in Hong Kong Chinese. J Clin Pharm Ther 2005;30:567–73 | Time point | – |
| Linneberg A, Rasmussen M, Buch TF, Wester A, Malm L, Fannikke G, et al. A randomised study of the effects of supplemental exercise sessions after a 7-week chronic obstructive pulmonary disease rehabilitation program. Clin Respir J 2012;6:112–19 | Time point | – |
| Lisansky DP, Clough DH. A cognitive–behavioral self-help educational program for patients with COPD. A pilot study. Psychother Psychosomat 1996;65:97–101 | Time point | Study design |
| Lisboa C, Munoz V, Beroiza T, Leiva A, Cruz E. Inspiratory muscle training in chronic airflow limitation: comparison of two different training loads with a threshold device. Eur Respir J 1994;7:1266–74 | Time point | Study design |
| Lisboa C, Villafranca C, Pertuze J, Leiva A, Repetto P. [Clinical effects of inspiratory muscle training in patients with chronic airflow limitation.] Rev Med Chil 1995;123:1108–15 | Time point | – |
| Lisboa C, Villafranca C, Leiva A, Cruz E, Pertuze J, Borzone G. Inspiratory muscle training in chronic airflow limitation: Effect on exercise performance. Eur Respir J 1997;10:537–42 | Time point | – |
| Lisboa C, Villafranca C, Caiozzi G, Berrocal C, Leiva A, Pinochet R, et al. [Quality of life in patients with chronic obstructive pulmonary disease and the impact of physical training.] Rev Med Chil 2001;129:359–66 | Time point | Study design |
| Littlejohns P, Baveystock CM, Parnell H, Jones PW. Randomised controlled trial of the effectiveness of a respiratory health worker in reducing impairment, disability, and handicap due to chronic airflow limitation. Thorax 1991;46:559–64 | Time point | – |
| Liu WT, Wang CH, Lin HC, Lin SM, Lee KY, Lo YL, et al. Efficacy of a cell phone-based exercise programme for COPD. Eur Respir J 2008;32:651–9 | Time point | – |
| Liu Y-F. [Effects of the comprehensive pulmonary rehabilitation programme on the quality of life of the patients with COPD in recovery period.] Chin J Clin Rehabil 2002;6:3170–1 | Time point | Study design |
| Livermore N, Sharpe L, McKenzie D. Prevention of panic attacks and panic disorder in COPD. Eur Respir J 2010;35:557–63 | Time point | – |
| Lolak S, Connors GL, Sheridan MJ, Wise TN. Effects of progressive muscle relaxation training on anxiety and depression in patients enrolled in an outpatient pulmonary rehabilitation program. Psychother Psychosomat 2008;77:119–25 | Population | Population |
| Lomundal BK, Steinsbekk A. Observational studies of a one year self-management program and a two year pulmonary rehabilitation program in patients with COPD. Int J Chron Obstruct Pulmon Dis 2007;2:617–24 | Time point | Study design |
| Lomundal BK, Steinsbekk A. Five-year follow-up of a one-year self-management program for patients with COPD. Int J Chron Obstruct Pulmon Dis 2012;7:87–93 | Time point | Study design |
| Conference proceedings. Long-Term Benefits of Pulmonary Rehabilitation on the Exercise Capacity and Shortness of Breath in Patient With Chronic Obstructive Pulmonary Disease (COPD). Series: Diseases of the Chest. The College; 1996. Vol. 110, no. 4, p. 159S | Unavailable | Unavailable |
| Lopez ME, Jara PM, Diaz EA, Gonzalez SM, Servian Carroquino RM, Vera VM. [Evaluation of an educational intervention for the use of inhalers in Primary Health Care.] MEDIFAM – Revista de Medicina Familiar y Comunitaria 2000;10:290–5 | Time point | Study design |
| Lord VM, Cave P, Hume VJ, Flude EJ, Evans A, Kelly JL, et al. Singing teaching as a therapy for Chron Respir Dis: a randomised controlled trial and qualitative evaluation. BMC Pulm Med 2010;10:41 | Time point | – |
| Lorenzi CM, Cilione C, Rizzardi R, Furino V, Bellantone T, Lugli D, et al. Occupational therapy and pulmonary rehabilitation of disabled COPD patients. Respiration 2004;71:246–51 | Time point | Study design |
| Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Jr, Bandura A, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care 2001;39:1217–23 | Population | Population |
| Lorig KR, Ritter PL, Gonzalez VM. Hispanic chronic disease self-management: a randomized community-based outcome trial. Nurs Res 2003;52:361–9 | Population | Population |
| Lotters F, van Tol B, Kwakkel G, Gosselink R. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J 2002;20:570–6 | Publication type | Publication type |
| Louie SW. The effects of guided imagery relaxation in people with COPD. Occup Ther Int 2004;11:145–59 | Time point | – |
| Lox CL, Freehill AJ. Impact of pulmonary rehabilitation on self-efficacy, quality of life, and exercise tolerance. Rehabil Psychol 1999;44:208–21 | Time point | Study design |
| Lucioni C, Donner CF, De BF, Lusuardi M, Mazzi S, Paggiaro PL, et al. [The costs of COPD.] Pharmacoecon 2005;7:119–34 | Intervention | Intervention |
| Macfarlane J, Holmes W, Gard P, Thornhill D, Macfarlane R, Hubbard R. Reducing antibiotic use for acute bronchitis in primary care: blinded, randomised controlled trial of patient information leaflet. BMJ 2002;324:91–4 | Population | Population |
| Mackay L. Health education and COPD rehabilitation: a study. Nurs Stand 1996;10:34–9 | Time point | Study design |
| Madariaga VB, Iturri JB, Manterola AG, Buey JC, Sebastian NT, Pena VS. [Comparison of 2 methods for inspiratory muscle training in patients with chronic obstructive pulmonary disease.] Arch Bronconeumol 2007;43:431–8 | Time point | – |
| Mador MJ, Bozkanat E, Aggarwal A, Shaffer M, Kufel TJ. Endurance and strength training in patients with COPD. Chest 2004;125:2036–45 | Time point | – |
| Mador MJ, Deniz O, Aggarwal A, Shaffer M, Kufel TJ, Spengler CM. Effect of respiratory muscle endurance training in patients with COPD undergoing pulmonary rehabilitation. Chest 2005;128:1216–24. [Erratum published in Chest 2006;129:216.] | Time point | – |
| Mador MJ, Krawza M, Alhajhusian A, Khan AI, Shaffer M, Kufel TJ. Interval training versus continuous training in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 2009;29:126–32 | Time point | – |
| Madsen H. Nurse Tele-Consultations with Discharged COPD Patients Reduce the Numbers of Readmissions; 2009. URL: www.clinicaltrials.gov (accessed 27 January 2015) | Study design | Study design |
| Magadle R, McConnell AK, Beckerman M, Weiner P. Inspiratory muscle training in pulmonary rehabilitation program in COPD patients. Respir Med 2007;101:1500–5 | Time point | – |
| Maixner J. [Effect of respiratory rehabilitation in patients with pulmonary emphysema.] Cesk Zdrav 1963;11:486–93 | Time point | Study design |
| Malicdem MG, Cruz BOD, Punzal P, De GT. Outcome of pulmonary rehabilitation among difficult to wean patients admitted at the Philippine heart center – a randomized controlled study. 15th Congress of the Asian Pacific Society of Respirology, 22–25 November 2010, Manila, The Philippines. Respirology 2010;99 | Population | Population |
| Malik SK. Aerosol therapy and rehabilitation in chronic obstructive pulmonary disease (COPD). Indian J Chest Dis 1968;10:142–8 | Time point | Study design |
| Maltais F, LeBlanc P, Simard C, Jobin J, Berube C, Bruneau J, et al. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;154:442–7 | Time point | Study design |
| Maltais F, LeBlanc P, Jobin J, Berube C, Bruneau J, Carrier L, et al. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;155:555–61 | Time point | Study design |
| Maltais F, Bourbeau J, Lacasse Y, Shapiro S, Perrault H, Penrod JR, et al. A Canadian, multicentre, randomized clinical trial of home-based pulmonary rehabilitation in chronic obstructive pulmonary disease: rationale and methods. Can Respir J 2005;12:193–8 | Publication type | Publication type |
| Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2008;149:869–78. [Summary for patients in Ann Intern Med 2008;149:I56.] | Time point | – |
| Maltais F. Pulmonary Rehabilitation at Home Versus at the Gymnasium; 2005. URL: www.clinicaltrials.gov (accessed 27 January 2015) | Publication type | Publication type |
| Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004;329:1209 | Intervention | – |
| Man WDC, Soliman MGG, Gearing J, Radford SG, Rafferty GF, Gray BJ, et al. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;168:562–7 | Time point | Study design |
| Mandigout S, Antonini M-T, Laforge Q, Lemaire F, Dalmay F, Bouteille B. Effects of training rehabilitation on the physical capacity of patients suffering from chronic obstructive pulmonary disease. Sci Sports 2007;22:300–1 | Time point | Study design |
| Mapel DW. Estimating the cost of COPD: a matter of perspective. COPD 2006;3:177–8 | Publication type | Publication type |
| Mapel DW, McMillan GP, Frost FJ, Hurley JS, Picchi MA, Lydick E, et al. Predicting the costs of managing patients with chronic obstructive pulmonary disease. Respir Med 2005;99:1325–33 | Time point | Study design |
| Marchioro JC, Belmonte G, Pradela C, Maia MN, Nascimento OA, Jardim JR. Effects of home-based pulmonary rehabilitation in COPD patients: adaptation to patient’s real life. Am J Respir Crit Care Med 2011;183:A6438 | Time point | Publication type |
| Marrara KT, Marino DM, de Held PA, de Oliveira Junior AD, Jamami M, Di L, V. Different physical therapy interventions on daily physical activities in chronic obstructive pulmonary disease. Respir Med 2008;102:505–11 | Time point | – |
| Martin IR, McNamara D, Sutherland FR, Tilyard MW, Taylor DR. Care plans for acutely deteriorating COPD: a randomized controlled trial. Chron Respir Dis 2004;1:191–5 | Time point | – |
| Martinez FJ, Vogel PD, Dupont DN, Stanopoulos I, Gray A, Beamis JF. Supported arm exercise vs unsupported arm exercise in the rehabilitation of patients with severe chronic airflow obstruction. Chest 1993;103:1397–402 | Time point | – |
| Martins JA, de Andrade AD, Britto RR, Lara R, Parreira VF. Effect of slow expiration with glottis opened in lateral posture (ELTGOL) on mucus clearance in stable patients with chronic bronchitis. Respir Care 2012;57:420–6 | Time point | Study design |
| Mattke S, Seid M, Ma S. Evidence for the effect of disease management: is $1 billion a year a good investment? Am J Manag Care 2007;13:670–6 | Publication type | Publication type |
| McBride S. Patients with chronic obstructive pulmonary disease: their beliefs about measures that increase activity tolerance. Rehabil Nurs J 1994;19:37–41 | Time point | Study design |
| McBride S, Graydon J, Sidani S, Hall L. The therapeutic use of music for dyspnea and anxiety in patients with COPD who live at home. J Holist Nurs 1999;17:229–50 | Time point | Study design |
| McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001;119:1190–209 | Publication type | Publication type |
| McGavin CR, Gupta SP, Lloyd EL, McHardy GJ. Physical rehabilitation for the chronic bronchitic: results of a controlled trial of exercises in the home. Thorax 1977;32:307–11 | Time point | Study design |
| McGeoch GR, Willsman KJ, Dowson CA, Town GI, Frampton CM, McCartin FJ, et al. Self-management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology 2006;11:611–18 | Time point | – |
| McKeon JL, Turner J, Kelly C. The effect of inspiratory resistive training on exercise capacity in optimally treated patients with severe chronic airflow limitation. Aust NZ J Med 1986;16:648–52 | Time point | – |
| McKinstry A, Tranter M, Sweeney J. Outcomes of dysphagia intervention in a pulmonary rehabilitation program. Dysphagia 2010;25:104–11 | Time point | Study design |
| McLean W, Gillis J, Waller R. The BC Community Pharmacy Asthma Study: a study of clinical, economic and holistic outcomes influenced by an asthma care protocol provided by specially trained community pharmacists in British Columbia. Can Respir J 2003;10:195–202 | Population | Population |
| Mehri SN, Khoshnevis MA, Zarrehbinan F, Hafezi S, Ghasemi A, Ebadi A. Effect of treadmill exercise training on VO2 peak in chronic obstructive pulmonary disease. Tanaffos 2007;6:18–24 | Time point | – |
| Mehuys E, Van Bortel L, De Bolle L, Van Tongelen, I, Annemans L, Remon JP, et al. Effectiveness of pharmacist intervention for asthma control improvement. Eur Respir J 2008;31:790–9 | Population | Population |
| Mendes De Oliveira JC, Studart Leitao Filho FS, Malosa Sampaio LM, Negrinho De Oliveira AC, Hirata RP, Costa D, et al. Outpatient vs. home-based pulmonary rehabilitation in COPD: a randomized controlled trial. Multidiscip Respir Med 2010;5:401–8 | Time point | – |
| Menn P, Holle R. Markov model for health-economic evaluations of COPD in Germany. 12th Annual European Congress, 24–27 October 2009, Paris, France. Value Health 2009;A303 | Publication type | Publication type |
| Menon B, Kumar S, Vishal B, V, Vijayan VK. Effect of pulmonary rehabilitation on systemic inflammation, functional parameters and muscle cross section area in COP. European Respiratory Society Annual Congress, 18–22 September 2010, Barcelona, Spain, abstract E3540 | Publication type | Publication type |
| Mercken EM, Hageman GJ, Schols AM, Akkermans MA, Bast A, Wouters EF. Rehabilitation decreases exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:994–1001 | Population | Population |
| Mertens DJ, Shephard RJ, Kavanagh T. Long-term exercise therapy for chronic obstructive lung disease. Respiration 1978;35:96–107 | Time point | Study design |
| Meulepas MA, Jacobs JE, Smeenk FWJM, Smeele I, Lucas AEM, Bottema BJAM, et al. Effect of an integrated primary care model on the management of middle-aged and old patients with obstructive lung diseases. Scand J Prim Health Care 2007;25:186–92 | Time point | Study design |
| Miller MB, Conrad WF. Pharmacist involvement in an education program for patients with chronic obstructive pulmonary disease. Am J Hosp Pharm 1975;32:909–11 | Publication type | Publication type |
| Minoguchi H, Shibuya M, Miyagawa T, Kokubu F, Yamada M, Tanaka H, et al. Cross-over comparison between respiratory muscle stretch gymnastics and inspiratory muscle training. Intern Med 2002;41:805–12 | Time point | Study design |
| Miravitlles M, Guerrero T, Mayordomo C, Sanchez-Agudo L, Nicolau F, Segu JL. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. Respiration 2000;67:495–501 | Intervention | Intervention |
| Miravitlles M, Murio C, Guerrero T. Factors associated with relapse after ambulatory treatment of acute exacerbations of chronic bronchitis. DAFNE Study Group. Eur Respir J 2001;17:928–33 | Study design | Study design |
| Miravitlles M, Calle M, varez-Gutierrez F, Gobartt E, Lopez F, Martin A. Exacerbations, hospital admissions and impaired health status in chronic obstructive pulmonary disease. Qual Life Res 2006;15:471–80 | Time point | Study design |
| Mochizuki A, Tomohisa H, Nakayama S, Kashiwazaki T, Komuro T. [Promotion of self care and organization of a social network for patients with chronic obstructive lung diseases.] Kango Gijutsu 1988;34:1786–90 | Study design | Study design |
| Molina PJ, Molina PC, de Lucas RP, Lobo Alvarez MA, Calvo CE, Lumbreras GG. [Effectiveness of a recuperative primary care intervention in patients with chronic obstructive pulmonary disease.] Aten Prim 2005;36:39–44 | Unavailable | Unavailable |
| Monninkhof E, van der Valk P, van der Palen J, van Herwaarden C, Zielhuis G. Effects of a comprehensive self-management programme in patients with chronic obstructive pulmonary disease. Eur Respir J 2003;22:815–20 | Time point | – |
| Monninkhof E, van der Aa M, van der Valk P, van der Palen J, Zielhuis G, Koning K, et al. A qualitative evaluation of a comprehensive self-management programme for COPD patients: effectiveness from the patients’ perspective. Patient Educ Couns 2004;55:177–84 | Time point | Study design |
| Monninkhof E, van der Valk P, Schermer T, van der Palen J, van Herwaarden C, Zielhuis G. Economic evaluation of a comprehensive self-management programme in patients with moderate to severe chronic obstructive pulmonary disease. Chron Respir Dis 2004;1:7–16 | Time point | – |
| Monninkhof E, van der Valk P, van der Palen J, Mulder H, Pieterse M, van Herr waarden C, et al. The effect of a minimal contact smoking cessation programme in out-patients with chronic obstructive pulmonary disease: a pre-post-test study. Patient Educ Couns 2004;52:231–6 | Time point | Study design |
| Montemayor T, Ortega F. [Strategies for muscular training in chronic obstructive pulmonary disease. Training of resistance, strength, of both?] Arch Bronconeumol 2001;37:279–85 | Publication type | Publication type |
| Montes de OM, Torres SH, Gonzalez Y, Romero E, Hernandez N, Talamo C. [Changes in exercise tolerance, health related quality of life, and peripheral muscle characteristics of chronic obstructive pulmonary disease patients after 6 weeks’ training.] Arch Bronconeumol 2005;41:413–18 | Time point | Study design |
| Moody LE, Fraser M, Yarandi H. Effects of guided imagery in patients with chronic bronchitis and emphysema. Clin Nurs Res 1993;2:478–86 | Time point | Study design |
| Moore J, Fiddler H, Seymour J, Grant A, Jolley C, Johnson L, et al. Effect of a home exercise video programme in patients with chronic obstructive pulmonary disease. J Rehabil Med 2009;41:195–200 | Time point | – |
| Mota S, Güell R, Barreiro E, Solanes I, Ramirez-Sarmiento A, Orozco-Levi M, et al. Clinical outcomes of expiratory muscle training in severe COPD patients. Respir Med 2007;101:516–24 | Time point | – |
| Moullec G, Favreau H, Lavoie KL, Labrecque M. Does a self-management education program have the same impact on emotional and functional dimensions of HRQoL? COPD 2012;9:36–45 | Time point | Study design |
| Mousing CA, Lomborg K. Self-care 3 months after attending chronic obstructive pulmonary disease patient education: a qualitative descriptive analysis. Patient Prefer Adherence 2012;6:19–25 | Time point | Study design |
| Moxham J. Early Pulmonary Rehabilitation (PR) Following Hospitalisation For Acute Exacerbations of Chronic Obstructive Pulmonary Disease (COPD) [NCT00557115.] 2007. URL: www.clinicaltrials.gov (accessed 27 January 2015) | Publication type | Publication type |
| Moxham J. The Effects of a Home Exercise Video Programme for Patients With COPD; 2007. URL: www.clinicaltrials.gov (accessed 27 January 2015) | Publication type | Publication type |
| Mularski RA, Munjas BA, Lorenz KA, Sun S, Robertson SJ, Schmelzer W, et al. Randomized controlled trial of mindfulness-based therapy for dyspnea in chronic obstructive lung disease. J Altern Complement Med 2009;15:1083–90 | Time point | – |
| Muller C, Heinl KW. [Patient training reduces the fear of cortisone in chronic obstructive respiratory tract diseases.] Prav Rehabil 1996;8:45 | Outcome | Outcome |
| Mungall IP, Hainsworth R. An objective assessment of the value of exercise training to patients with chronic obstructive airways disease. Q J Med 1980;49:77–85 | Time point | Study design |
| Murphy K, Casey D, Devane D, Cooney A, McCarthy B, Mee L, et al. A cluster randomised controlled trial evaluating the effectiveness of a structured pulmonary rehabilitation education programme for improving the health status of people with chronic obstructive pulmonary disease (COPD): The PRINCE Study protocol. BMC Pulm Med 2011;11:4 | Publication type | Publication type |
| Murphy K, Casey D, Devane D, Cooney A, McCarthy B, Mee L, et al. The effectiveness of a structured education pulmonary rehabilitation programme for improving the health status of people with Chronic Obstructive Pulmonary Disease (COPD): The PRINCE study. Irish Thoracic Society Annual Scientific Meeting, 11–12 November 2011, Co. Dublin, Ireland. Ir J Med Sci 2011:S457 | Time point | Publication type |
| Murphy M, Campbell M, Saunders J, Jackson B, Rangan N, Zimmerman F, et al. A randomised, controlled trial of pulmonary rehabilitation, weekly exercise and better health self-management in COPD. Respirology 2004;9(Suppl. 2):A48 | Publication type | Publication type |
| Murphy N, Bell C, Costello RW. Extending a home from hospital care programme for COPD exacerbations to include pulmonary rehabilitation. Respir Med 2005;99:1297–302 | Time point | – |
| Muzembo NJ, Nkakudulu BH, Frans A. [Respiratory rehabilitation in patients with bronchial asthma and chronic obstructive pulmonary disease (COPD) in Kinshasa.] Rev Pneumol Clin 2001;57:209–18 | Population | Population |
| Na JO, Kim DS, Yoon SH, Jegal YJ, Kim WS, Kim ES, et al. A simple and easy home-based pulmonary rehabilitation programme for patients with chronic lung diseases. Monaldi Arch Chest Dis 2005;63:30–6 | Time point | Study design |
| Nakamura Y, Tanaka K, Shigematsu R, Nakagaichi M, Lnoue M, Homma T. Effects of aerobic training and recreational activities in patients with chronic obstructive pulmonary disease. Int J Rehabil Res 2008;31:275–83 | Time point | – |
| Nalbant O, Nur H, Ogus C, Toraman NF. [Effects of long-term aerobic exercise program in chronic obstructive pulmonary disease.] Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2011;57:8–13 | Unavailable | Unavailable |
| Nasis IG, Vogiatzis I, Stratakos G, Athanasopoulos D, Koutsoukou A, Daskalakis A, et al. Effects of interval-load versus constant-load training on the BODE index in COPD patients. Respir Med 2009;103:1392–8 | Time point | – |
| Nava S. Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil 1998;79:849–54 | Time point | – |
| Navarre M, Patel H, Johnson CE, Durance A, McMorris M, Bria W, et al. Influence of an interactive computer-based inhaler technique tutorial on patient knowledge and inhaler technique. Ann Pharmacother 2007;41:216–21 | Population | Population |
| Navrotskii VV, Siurin SA. [Comparative efficacy of several types of physical training in restoring the physical work capacity of chronic bronchitis patients.] Vopr Kurortol Fizioter Lech Fiz Kult 1985;5:64–6 | Time point | Study design |
| Neff DF, Madigan E, Narsavage G. APN-directed transitional home care model: achieving positive outcomes for patients with COPD. Home Healthc Nurse 2003;21:543–50 | Intervention | Intervention |
| Newton DA, Bevans HG. Physiotherapy and intermittent positive-pressure ventilation of chronic bronchitis. Br Med J 1978;2:1525–8 | Intervention | Intervention |
| Ng BH, Tsang HW, Jones AY, So CT, Mok TY. Functional and psychosocial effects of health qigong in patients with COPD: a randomized controlled trial. J Altern Complement Med 2011;17:243–51 | Time point | – |
| Nguyen HQ, Carrieri-Kohlman V, Rankin SH, Slaughter R, Stulbarg MS. Is internet-based support for dyspnea self-management in patients with chronic obstructive pulmonary disease possible? Results of a pilot study. Heart Lung 2005;34:51–62 | Time point | Study design |
| Nguyen HQ, Carrieri-Kohlman V. Dyspnea self-management in patients with chronic obstructive pulmonary disease: moderating effects of depressed mood. Psychosomatics 2005;46:402–10 | Time point | Study design |
| Nguyen HQ, Donesky-Cuenco D, Wolpin S, Reinke LF, Benditt JO, Paul SM, et al. Randomized controlled trial of an internet-based versus face-to-face dyspnea self-management program for patients with chronic obstructive pulmonary disease: pilot study. J Med Internet Res 2008;10:e9 | Time point | – |
| Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO. Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. Int J Chron Obstruct Pulmon Dis 2009;4:301–13 | Time point | – |
| Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006;173:1390–413 | Study design | Study design |
| Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007;27:237–44 | Time point | – |
| Ninot G, Moullec G, Picot MC, Jaussent A, Hayot M, Desplan M, et al. Cost-saving effect of supervised exercise associated to COPD self-management education program. Respir Med 2011;105:377–85 | Time point | – |
| Nissen I, Jensen MS. [Nurse-supported discharge of patients with exacerbation of chronic obstructive pulmonary disease.] Ugeskr Laeger 2007;169:2220–3 | Intervention | Intervention |
| Nissen I, Jensen MS. [Randomised controlled trial of nurse-supported discharge of patients with exacerbation of chronic obstructive pulmonary disease.] Ugeskr Laeger 2007;169:2220–3 | Duplicate | Duplicate |
| Normandin EA, McCusker C, Connors M, Vale F, Gerardi D, Zuwallack RL. An evaluation of two approaches to exercise conditioning in pulmonary rehabilitation. Chest 2002;121:1085–91 | Time point | – |
| Norweg AM, Whiteson J, Malgady R, Mola A, Rey M. The effectiveness of different combinations of pulmonary rehabilitation program components: a randomized controlled trial. Chest 2005;128:663–72 | Time point | – |
| Noseda A, Carpiaux JP, Vandeput W, Prigogine T, Schmerber J. Resistive inspiratory muscle training and exercise performance in COPD patients. A comparative study with conventional breathing retraining. Bull Eur Physiopathol Respir 1987;23:457–63 | Time point | – |
| Nosworthy J, Barter C, Thomas S, Flynn M. An evaluation of the three elements of pulmonary rehabilitation. Aust Physiother 1993;38:189–93 | Time point | – |
| O’Bey KA, Jim LK, Gee JP, Cowen ME, Quigley AE. An education program that improves the psychomotor skills needed for metaproterenol inhaler use. Drug Intell Clin Pharm 1982;16:945–8 | Population | Population |
| O’Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med 1998;157:1489–97 | Time point | Study design |
| O’Donnell DE, McGuire M, Samis L, Webb KA. The impact of exercise reconditioning on breathlessness in severe chronic air-flow limitation. Am J Respir Crit Care Med 1995;152:2005–13 | Time point | Study design |
| Oga T, Nishimura K, Tsukino M, Sato S. Exercise responses during endurance testing at different intensities in patients with COPD. Respir Med 2004;98:515–21 | Time point | Study design |
| Oh EG. The effects of home-based pulmonary rehabilitation in patients with chronic lung disease. Int J Nurs Stud 2003;40:873–9 | Time point | – |
| O’Hara WJ, Lasachuk KE, Matheson PC. Weight training and backpacking in chronic obstructive pulmonary disease. Respir Care 1984;29:1202–10 | Time point | Study design |
| Ojoo JC, Moon T, McGlone S, Martin K, Gardiner ED, Greenstone MA, et al. Patients’ and carers’ preferences in two models of care for acute exacerbations of COPD: results of a randomised controlled trial. Thorax 2002;57:167–9 | Intervention | Intervention |
| Oka T. [Self-care and guidance of patients: nursing of patients with chronic obstructive lung diseases. Actions by self-help groups and the significance of their activities.] Kango gijutsu 1988;34:1756–60 | Study design | Study design |
| Olséni L, Midgren B, Hörnblad Y, Wollmer P. Chest physiotherapy in chronic obstructive pulmonary disease: forced expiratory technique combined with either postural drainage or positive expiratory pressure breathing. Respir Med 1994;88:435–40 | Time point | Study design |
| O’Neill B, McKevitt A, Rafferty S, Bradley JM, Johnston D, Bradbury I, et al. A comparison of twice- versus once-weekly supervision during pulmonary rehabilitation in chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2007;88:167–72 | Time point | – |
| O’Neill ES. Illness representations and coping of women with chronic obstructive pulmonary disease: a pilot study. Heart Lung 2002;31:295–302 | Time point | Study design |
| Onodera A, Yazaki K. [Effects of a short-term pulmonary rehabilitation program on patients with chronic respiratory failure due to pulmonary emphysema.] Nihon Kokyuki Gakkai Zasshi 1998;36:679–83 | Time point | Study design |
| Ortega F, Toral J, Cejudo P, Villagomez R, Sanchez H, Castillo J, et al. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:669–74 | Time point | – |
| O’Shea SD, Taylor NF, Paratz JD. A predominantly home-based progressive resistance exercise program increases knee extensor strength in the short-term in people with chronic obstructive pulmonary disease: a randomised controlled trial. Aust J Physiother 2007;53:229–37 | Time point | – |
| O’Shea SD, Taylor NF, Paratz JD. Qualitative outcomes of progressive resistance exercise for people with COPD. Chron Respir Dis 2007;4:135–42 | Time point | Study design |
| Otsuka T, Kurihara N, Fujii T, Fujimoto S, Yoshikawa J. Effect of exercise training and detraining on gas exchange kinetics in patients with chronic obstructive pulmonary disease. Clin Physiol 1997;17:287–97 | Time point | Study design |
| Ozdemir EP, Solak O, Fidan F, Demirdal US, Evcik D, Unlu M, et al. [The effect of water-based pulmonary rehabilitation on anxiety and quality of life in chronic pulmonary obstructive disease patients.] Turkiye Klinikleri J Med Sci 2010;30:880–7 | Time point | – |
| Paget T, Jones C, Davies M, Evered C, Lewis C. Using home telehealth to empower patients to monitor and manage long term conditions. Nurs Times 2010;106:17–19 | Population | Population |
| Panton LB, Golden J, Broeder CE, Browder KD, Cestaro-Seifer DJ, Seifer FD. The effects of resistance training on functional outcomes in patients with chronic obstructive pulmonary disease. Eur J Appl Physiol 2004;91:443–9 | Time point | Study design |
| Pardy RL, Rivington RN, Despas PJ, Macklem PT. Inspiratory muscle training compared with physiotherapy in patients with chronic airflow limitation. Am Rev Respir Dis 1981;123:421–5 | Time point | Study design |
| Park K, Robbins RA. ACP Journal Club. A COPD disease management program reduced a composite of hospitalizations or emergency department visits. Ann Intern Med 2011;154:JC3–5 | Publication type | Publication type |
| Pascual-Pape T, Badia JR, Marrades RM, Hernandez C, Ballester E, Fornas C, et al. [Results of a preventive program and assisted hospital discharge for COPD exacerbation. A feasibility study.] Med Clin 2003;120:408–11 | Study design | Study design |
| Paz-Diaz H, Montes de OM, Lopez JM, Celli BR. Pulmonary rehabilitation improves depression, anxiety, dyspnea and health status in patients with COPD. Am J Phys Med Rehabil 2007;86:30–6 | Time point | – |
| Pereira AM, Santa-Clara H, Pereira E, Simoes S, Remedios I, Cardoso J, et al. Impact of combined exercise on chronic obstructive pulmonary patients’ state of health. Rev Port Pneumol 2010;16:737–57 | Time point | Study design |
| Perry JA. Effectiveness of teaching in the rehabilitation of patients with chronic bronchitis and emphysema. Nurs Res 1981;30:219–22 | Time point | Study design |
| Petersen AM, Mittendorfer B, Magkos F, Iversen M, Pedersen BK. Physical activity counteracts increased whole-body protein breakdown in chronic obstructive pulmonary disease patients. Scand J Med Sci Sports 2008;18:557–64 | Time point | – |
| Petty TL, Nett LM, Finigan MM, Brink GA, Corsello PR. A comprehensive care program for chronic airway obstruction. Methods and preliminary evaluation of symptomatic and functional improvement. Ann Intern Med 1969;70:1109–20 | Time point | Study design |
| Petty TL, Nett LM. Patient education and emphysema care. Med Times 1969;97:117–30 | Publication type | Publication type |
| Petty TL, Dempsey EC, Collins T, Pluss W, Lipkus I, Cutter GR, et al. Impact of customized videotape education on quality of life in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2006;26:112–17 | Time point | – |
| Pfister T, Berrol C, Caplan C. Effects of music on exercise and perceived symptoms in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 1998;18:228–32 | Time point | Study design |
| Phillips WT, Benton MJ, Wagner CL, Riley C. The effect of single set resistance training on strength and functional fitness in pulmonary rehabilitation patients. J Cardiopulm Rehabil 2006;26:330–7 | Time point | – |
| Pilotto LS, Smith BJ, Heard AR, McElroy HJ, Weekley J, Bennett P. Trial of nurse-run asthma clinics based in general practice versus usual medical care. Respirology 2004;9:356–62 | Population | Population |
| Pinnock H, Hanley J, Lewis S, MacNee W, Pagliari C, van der Pol M, et al. The impact of a telemetric chronic obstructive pulmonary disease monitoring service: randomised controlled trial with economic evaluation and nested qualitative study. Prim Care Resp J 2009;18:233–5 | Publication type | Publication type |
| Pison C, Cano N, Cherion C, Roth H, Pichard C. [Effects of home pulmonary rehabilitation in patients with chronic respiratory failure and nutritional depletion.] Rev Mal Respir 2004;21:573–82 | Publication type | Publication type |
| Pison CM, Cano NJ, Cherion C, Caron F, Court-Fortune I, Antonini MT, et al. Multimodal nutritional rehabilitation improves clinical outcomes of malnourished patients with chronic respiratory failure: a randomised controlled trial. Thorax 2011;66:953–60 | Intervention | Intervention |
| Piszko P, Lewczuk J, Jagas J, Kowalska-Superlak K, Wrabec K. Oxygen saturation at rest, on exercise and during sleep in non oxygenated rehabilitation patients with COPD. Eur Respir J 2002;20(Suppl. 38):235 | Publication type | Publication type |
| Piszko P, Lewczuk J, Kowalska-Superlak M, Jagas J, Ludwik B, Wrabec K. [Effect of a 2-year pulmonary rehabilitation on the 7-year prognosis in patients with advanced chronic obstructive pulmonary disease.] Pol Arch Med Wewn 2004;111:57–62 | Time point | Study design |
| Pitta F, Brunetto AF, Padovani CR, Godoy I. Effects of isolated cycle ergometer training on patients with moderate-to-severe chronic obstructive pulmonary disease. Respiration 2004;71:477–83 | Time point | Study design |
| Pitta F, Takaki MY, Oliveira NH, Sant’anna TJ, Fontana AD, Kovelis D, et al. Relationship between pulmonary function and physical activity in daily life in patients with COPD. Respir Med 2008;102:1203–07 | Time point | Study design |
| Pomidori L, Contoli M, Mandolesi G, Cogo A. A simple method for home exercise training in patients with chronic obstructive pulmonary disease: one-year study. J Cardiopulm Rehabil Prev 2012;32:53–7 | Time point | – |
| Poole PJ, Chase B, Frankel A, Black PN. Case management may reduce length of hospital stay in patients with recurrent admissions for chronic obstructive pulmonary disease. Respirology 2001;6:37–42 | Time point | Study design |
| Porszasz J, Emtner M, Goto S, Somfay A, Whipp BJ, Casaburi R. Exercise training decreases ventilatory requirements and exercise-induced hyperinflation at submaximal intensities in patients with COPD. Chest 2005;128:2025–34 | Time point | Study design |
| Porta R, Vitacca M, Gile LS, Clini E, Bianchi L, Zanotti E, et al. Supported arm training in patients recently weaned from mechanical ventilation. Chest 2005;128:2511–20 | Intervention | Intervention |
| Porto EF, Castro AA, Velloso M, Nascimento O, Dal MF, Jardim JR. Exercises using the upper limbs hyperinflate COPD patients more than exercises using the lower limbs at the same metabolic demand. Monaldi Archr Chest Dis 2009;71:21–6 | Time point | Study design |
| Potashov DA, Kokosov AN. [Characteristics and results of rehabilitation of patients with chronic obstructive bronchitis at a specialized department.] Probl Tuberk 1990;3:62–6 | Time point | Study design |
| Pouw EM, Ten Velde GP, Croonen BH, Kester AD, Schols AM, Wouters EF. Early non-elective readmission for chronic obstructive pulmonary disease is associated with weight loss. Clin Nutr 2000;19:95–9 | Study design | Study design |
| Premaratne U, Sterne J, Marks G, Webb J, Azima H, Burney P. Clustered randomised trial of an intervention to improve the management of asthma: Greenwich asthma study. BMJ 1999;318:1251–5 | Population | Population |
| Preusser BA, Winningham ML, Clanton TL. High- vs low-intensity inspiratory muscle interval training in patients with COPD. Chest 1994;106:110–17 | Time point | – |
| Prigmore S. Does an Individualised Self-management Plan Help Patients with Chronic Obstructive Pulmonary Disease (COPD) Initiate Early Treatment for Infective Exacerbations? ISRCTN Register 2004. URL: www.isrctn.org (accessed 27 January 2015) | Publication type | Publication type |
| Prince KL, Helm M. Effectiveness of a rehabilitation programme in chronic bronchitis and emphysema. Clin Rehabil 1989;3:211–14 | Time point | – |
| Prior H. Randomized Controlled Trial of Home Telemonitoring for Elderly People (Dreaming); 2009. URL: www.clinicaltrials.gov (accessed 27 January 2015) | Population | Population |
| Probst VS, Troosters T, Coosemans I, Spruit MA, Pitta FO, Decramer M, et al. Mechanisms of improvement in exercise capacity using a rollator in patients with COPD. Chest 2004;126:1102–7 | Time point | Study design |
| Probst VS, Troosters T, Pitta F, Decramer M, Gosselink R. Cardiopulmonary stress during exercise training in patients with COPD. Eur Respir J 2006;27:1110–18 | Time point | Study design |
| Probst VS, Kovelis D, Hernandes NA, Camillo CA, Cavalheri V, Pitta F. Effects of 2 exercise training programs on physical activity in daily life in patients with COPD. Respir Care 2011;56:1799–807 | Time point | – |
| Puente-Maestu L, Sanz ML, Sanz P, Cubillo JM, Mayol J, Casaburi R. Comparison of effects of supervised versus self-monitored training programmes in patients with chronic obstructive pulmonary disease. Eur Respir J 2000;15:517–25 | Time point | – |
| Puente-Maestu L, Sanz ML, Sanz P, Ruiz De Ona JM, Rodriguez-Hermosa JL, Whipp BJ. Effects of two types of training on pulmonary and cardiac responses to moderate exercise in patients with COPD. Eur Respir J 2000;15:1026–32 | Time point | – |
| Puente-Maestu L, SantaCruz A, Vargas T, Martinez-Abad Y, Whipp BJ. Effects of training on the tolerance to high-intensity exercise in patients with severe COPD. Respiration 2003;70:367–70 | Time point | Study design |
| Puente-Maestu L, Luisa SM, Sanz P, de Ona RJ, Arnedillo A, Casaburi R. Long-term effects of a maintenance program after supervised or self-monitored training programs in patients with COPD. Lung 2003;181:67–78 | Time point | – |
| Puente-Maestu L, Abad YM, Pedraza F, Sanchez G, Stringer WW. A controlled trial of the effects of leg training on breathing pattern and dynamic hyperinflation in severe COPD. Lung 2006;184:159–67 | Time point | Study design |
| Puhan M, Spaar A, Frey M, Turk A, Brandli O, Ritscher D, et al. Timing of pulmonary rehabilitation – Swiss trial on pulmonary rehabilitation after COPD exacerbation (SOPRE). Joint Annual Meeting of the Swiss Respiratory Society, Swiss Society of Oto-Rhino-Laryngology, Head and Neck Surgery, Swiss Paediatric Respiratory Society and Swiss Society for Thoracic Surgery, 4–5 May 2011, Interlaken, Switzerland. Respiration 2011:92 | Intervention | Publication type |
| Puhan MA, Busching G, Schunemann HJ, van Oort E, Zaugg C, Frey M. Interval versus continuous high-intensity exercise in chronic obstructive pulmonary disease: a randomized trial. [Summary for patients in Ann Intern Med 2006;145:I49.] Ann Intern Med 2006;145:816–25 | Time point | – |
| Puhan MA, Schunemann HJ, Buesching G, van Oort E, Spaar A, Frey M. COPD patients’ ability to follow exercise influences short-term outcomes of rehabilitation. Eur Respir J 2008;31:304–10 | Time point | Study design |
| Punzal PA, Ries AL, Kaplan RM, Prewitt LM. Maximum intensity exercise training in patients with chronic obstructive pulmonary disease. Chest 1991;100:618–23 | Time point | Study design |
| Pushparajah S, McClellan R, Henry A, Kuitert LM. Use of a chronic disease management programme in COPD to reduce hospital admissions. Chron Respir Dis 2006;3:187–93 | Study design | Study design |
| Putt MT, Watson M, Seale H, Paratz JD. Muscle stretching technique increases vital capacity and range of motion in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2008;89:1103–7 | Time point | Study design |
| Ramirez-Sarmiento A, Orozco-Levi M, Güell R, Barreiro E, Hernandez N, Mota S, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med 2002;166:1491–7 | Time point | – |
| Rasekaba TM, Williams E, Hsu-Hage B. Can a chronic disease management pulmonary rehabilitation program for COPD reduce acute rural hospital utilization? Chron Respir Dis 2009;6:157–63 | Time point | Study design |
| Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J 2004;34:608–14 | Time point | – |
| Reardon J, Awad E, Normandin E, Vale F, Clark B, Zuwallack RL. The effect of comprehensive outpatient pulmonary rehabilitation on dyspnea. Chest 1994;105:1046–52 | Time point | – |
| Reema T, Adepu R, Sabin T. Impact of clinical pharmacist intervention on knowledge, attitude and practice (KAP) of patients with chronic obstructive pulmonary disease. Int J Pharm Pharm Sci 2010;2:54–7 | Time point | Study design |
| Rees PJ. A disease-specific self-management program reduced hospital utilization and improved health status in COPD. ACP J Club 2012;139:65 | Publication type | Publication type |
| Regiane R, V, Gorostiza A, Galdiz JB, Lopez de Santa ME, Casan CP, Güell RR. [Benefits of a home-based pulmonary rehabilitation program for patients with severe chronic obstructive pulmonary disease.] Arch Bronconeumol 2007;43:599–604 | Time point | – |
| Reid WD, Samrai B. Respiratory muscle training for patients with chronic obstructive pulmonary-disease. Phys Ther 1995;75:996–1005 | Publication type | Publication type |
| Rejbi IB, Trabelsi Y, Chouchene A, Ben TW, Ben SH, Zbidi A, et al. Changes in six-minute walking distance during pulmonary rehabilitation in patients with COPD and in healthy subjects. Int J Chron Obstruct Pulmon Dis 2010;5:209–15 | Population | Population |
| Ren L, Li Q-Y, Du J-B, Zhou J-M, Weng Q-L, Chen X-H. Comparison of different strategies of pulmonary rehabilitation for patients with COPD of different severity. J Shanghai Jiaotong Uni (Med Sci) 2011;31:620–4 | Time point | – |
| Renfroe KL. Effect of progressive relaxation on dyspnea and state anxiety in patients with chronic obstructive pulmonary disease. Heart Lung 1988;17:408–13 | Population | Population |
| Renzi G, Renzi P. [Role of respiratory muscle training in a rehabilitation program for patients with chronic obstructive pulmonary disease.] Union Medicale de Canada 1985;114:897–901 | Time point | Study design |
| Resnikoff PM, Ries AL. Pulmonary rehabilitation for chronic lung disease. J Heart Lung Transpl 1998;17:643–50 | Publication type | Publication type |
| Riario-Sforza GG, Incorvaia C, Paterniti F, Pessina L, Caligiuri R, Pravettoni C, et al. Effects of pulmonary rehabilitation on exercise capacity in patients with COPD: a number needed to treat study. Int J Chron Obstruct Pulmon Dis 2009;4:315–19 | Time point | Study design |
| Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2010;182:890–6 | Time point | – |
| Richards S, Coast J, Gunnell D, Peters T, Poundsford J, Darlow M-A. Randomised controlled trial comparing effectiveness and acceptability of an early discharge, hospital at home scheme with acute hospital care. BMJ 1998;316:1796–806 | Population | Population |
| Richardson J, Dunn L, Pardy R. Inspiratory resistive endurance training in patients with chronic obstructive pulmonary disease: a pilot study. Physiother Can 1989;41:85–92 | Time point | Study design |
| Riera HS, Rubio TM, Ruiz FO, Ramos PC, Del Castillo OD, Hernandez TE, et al. Inspiratory muscle training in patients with COPD: effect on dyspnea, exercise performance, and quality of life. Chest 2001;120:748–56 | Time point | – |
| Ries AL, Archibald CJ. Endurance exercise training at maximal targets in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 1987;7:594–601 | Time point | Study design |
| Ries AL, Moser KM. Comparison of isocapnic hyperventilation and walking exercise training at home in pulmonary rehabilitation. Chest 1986;90:285–9 | Time point | – |
| Ries AL, Ellis B, Hawkins RW. Upper extremity exercise training in chronic obstructive pulmonary disease. Chest 1988;93:688–92 | Time point | – |
| Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effect of pulmonary rehabilitation program on hospital days of COPD patients. Ann Intern Med 1995;122:823–32 | Unavailable | Unavailable |
| Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823–32 | Time point | – |
| Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med 2003;167:880–8 | Population | Population |
| Ries AL, Make BJ, Lee SM, Krasna MJ, Bartels M, Crouch R, et al. The effects of pulmonary rehabilitation in the national emphysema treatment trial. Chest 2005;128:3799–809 | Time point | Study design |
| Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007;131(Suppl. 5):4–42S | Publication type | Publication type |
| Ringbaek TJ, Broendum E, Hemmingsen L, Lybeck K, Nielsen D, Andersen C, et al. Rehabilitation of patients with chronic obstructive pulmonary disease. Exercise twice a week is not sufficient! Respir Med 2000;94:150–4 | Time point | – |
| Ringbaek TJ, Nielsen LL, Admasu H, Lange P. [Early supported discharge for patients with exacerbations of chronic obstructive pulmonary disease.] Ugeskr Laeger 2008;170:47–50 | Intervention | Intervention |
| Ringbaek T, Brondum E, Martinez G, Lange P, Pulmonary Rehabilitation Research Group. Rehabilitation in COPD: the long-term effect of a supervised 7-week program succeeded by a self-monitored walking program. Chron Respir Dis 2008;5:75–80 | Time point | Study design |
| Ringbaek T, Brondum E, Martinez G, Thogersen J, Lange P. Long-term effects of 1-year maintenance training on physical functioning and health status in patients with COPD: a randomized controlled study. J Cardiopulm Rehabil Prev 2010;30:47–52 | Intervention | Intervention |
| Roberts CM, Ryland I, Lowe D, Kelly Y, Bucknall CE, Pearson MG. Audit of acute admissions of COPD: standards of care and management in the hospital setting. Eur Respir J 2001;17:343–9 | Study design | Study design |
| Roberts MM, Leeder SR, Robinson TD. Nurse-led 24-h hotline for patients with chronic obstructive pulmonary disease reduces hospital use and is safe. Intern Med J 2008;38:334–40 | Time point | Study design |
| Roberts SE, Kettle G, Rogers S, Segal A, Purcell S, Fabris G, et al. Piloting and evaluating post-pulmonary rehabilitation (PR) long-term exercise (LTE) for COPD patients. British Thoracic Society Winter Meeting, 7–9 November 2011, London, UK. Thorax 2011:A127–8 | Time point | Study design |
| Roberts SE, Schreuder FM, Watson T, Stern M. A randomised control trial to investigate the effectiveness of PLB in the clinical setting. British Thoracic Society Winter Meeting, 7–9 November 2011, London, UK. Thorax 2011:A175–6 | Time point | Publication type |
| Robinson S, Fletcher C, Parrington J, Norell J, Mabbett S. COPD and me: The development and implementation of an individual patient management plan and hand-held record. British Thoracic Society Winter Meeting, 1–3 December 2010, London, UK. Thorax 2010; A174–5 | Time point | Study design |
| Robinson T. Living with severe hypoxic COPD: the patients’ experience. Nurs Times 2005;101:38–42 | Time point | Study design |
| Rodgers S, Dyas J, Molyneux AW, Ward MJ, Revill SM. Evaluation of the information needs of patients with chronic obstructive pulmonary disease following pulmonary rehabilitation: a focus group study. Chron Respir Dis 2007;4:195–203 | Time point | Study design |
| Romagnoli M, Dell’Orso D, Lorenzi C, Crisafulli E, Costi S, Lugli D, et al. Repeated pulmonary rehabilitation in severe and disabled COPD patients. Respiration 2006;73:769–76 | Time point | – |
| Roomi J, Yohannes AM, Connolly MJ. The effect of walking aids on exercise capacity and oxygenation in elderly patients with chronic obstructive pulmonary disease. Age Ageing 1998;27:703–6 | Time point | Study design |
| Rootmensen GN, van Keimpema AR, Looysen EE, van der Schaaf L, de Haan RJ, Jansen HM. The effects of additional care by a pulmonary nurse for asthma and COPD patients at a respiratory outpatient clinic: results from a double blind, randomized clinical trial. Patient Educ Couns 2008;70:179–86. | Population | Population |
| Rooyackers JM, Berkeljon DA, Folgering HT. Eccentric exercise training in patients with chronic obstructive pulmonary disease. Int J Rehabil Res 2003;26:47–4 | Time point | – |
| Rosser R, Denford J, Heslop A, Kinston W, Macklin D, Minty K, et al. Breathlessness and psychiatric morbidity in chronic bronchitis and emphysema: a study of psychotherapeutic management. Psychol Med 1983;13:93–110 | Intervention | Intervention |
| Rozman A, Butorac-Petanjek B, Plesko N, Sarajlic N, Crc M, Krstic-Buric M. [Education and training in COPD patients in Croatia.] Prav Rehabil 2001;13:125–7 | Population | Population |
| Ruiz de Ona Lacasta JM, Garcia de PJ, Puente ML, Llorente ID, Celdran GJ, Cubillo Marcos JM. [Effects of muscle training on breathing pattern in patients with severe chronic obstructive pulmonary disease.] Arch Bronconeumol 2004;40:20–3. | Time point | – |
| Ruzicka J, Zvonar J, Kolesar J, Redhammer R, Kristufek P, Karpatiova A, et al. [Effectiveness of load training in the rehabilitation of patients with chronic obstructive lung disease.] Studia Pneumologica et Phtiseologica Cechoslovaca 1989;49:544–8 | Time point | Study design |
| Ruzicka J, Zvonar J, Kolesar J, Redhammer R, Kristufek P, Karpatiova A, et al. [Resisted inspiration training and re-education of respiration in the rehabilitation programme on patients with chronic pulmonary obstructive disease.] Studia Pneumologica et Phtiseologica Cechoslovaca 1989;49:538–43 | Time point | Study design |
| Sabapathy S, Kingsley RA, Schneider DA, Adams L, Morris NR. Continuous and intermittent exercise responses in individuals with chronic obstructive pulmonary disease. Thorax 2004;59:1026–31 | Time point | Study design |
| Sala E, Alegre L, Carrera M, Ibars M, Orriols FJ, Blanco ML, et al. Supported discharge shortens hospital stay in patients hospitalized because of an exacerbation of COPD. Eur Respir J 2001;17:1138–42 | Intervention | Study design |
| Sala E, Roca J, Marrades RM, Alonso J, Gonzalez De Suso JM, Moreno A, et al. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:1726–34 | Population | Population |
| Sandland CJ, Morgan MD, Singh SJ. Detecting oxygen desaturation in patients with COPD: incremental versus endurance shuttle walking. Respir Med 2008;102:1148–52 | Intervention | Intervention |
| Santiworakul A, Jarungjitaree S, Jalayondeja W, Chantarothorn S, Supaibulpipat S. Effect of lower extremity exercise on muscle strength and physical capacity in COPD patients. J Med Assoc Thailand 2009;92:556–63 | Time point | Study design |
| Santos C, Santos J, Morais L, Rodrigues F, Rbara C. Pulmonary rehabilitation in COPD: Effects of two aerobic exercise intensity in patient-centered outcomes: a randomized study. European Respiratory Society Annual Congress, 18–22 September 2010, Barcelona, Spain, p. 2835 | Publication type | Publication type |
| Santos C, Santos J, Morais L, Rodrigues F, Barbara C. Pulmonary rehabilitation in COPD: effects of two aerobic exercise intensity in patient-centered outcomes: a randomized study. CHEST conference, 22–26 November 2011, Honolulu, HI, USA. Chest 2011:(var. pagings) | Time point | Publication type |
| Sasaki Y. [Pulmonary rehabilitation.] Hokkaido igaku zasshi 1986;61:340–3 | Study design | Study design |
| Sassi-Dambron DE, Eakin EG, Ries AL, Kaplan RM. Treatment of dyspnea in COPD. A controlled clinical trial of dyspnea management strategies. Chest 1995;107:724–9 | Time point | – |
| Satake M, Shioya T, Takahashi H, Kawatani M. Ventilatory responses to six-minute walk test, incremental shuttle walking test, and cycle ergometer test in patients with chronic obstructive pulmonary disease. Biomed Res 2003;24:309–16 | Time point | Study design |
| Saudny-Unterberger H, Martin JG, Gray-Donald K. Impact of nutritional support on functional status during an acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;156:794–9 | Intervention | Intervention |
| Saunders KB, White JE. Controlled trial of breathing exercises. Br Med J 1965;2:680–2 | Time point | – |
| Savci S, Ince DI, Arikan H. A comparison of autogenic drainage and the active cycle of breathing techniques in patients with chronic obstructive pulmonary diseases. J Cardiopulm Rehabil 2000;20:37–43 | Time point | – |
| Scherer TA, Spengler CM, Owassapian D, Imhof E, Boutellier U. Respiratory muscle endurance training in chronic obstructive pulmonary disease: impact on exercise capacity, dyspnea, and quality of life. Am J Respir Crit Care Med 2000;162:1709–14 | Time point | – |
| Scherer YK, Janelli LM, Schmieder LE. Chronic obstructive pulmonary disease: does participating in a Help Yourself to Better Breathing Program make a difference? J Cardiopulm Rehabil 1989;9:492–6 | Time point | Study design |
| Scherer YK, Janelli LM, Schmieder LE. A time-series perspective on effectiveness of a health teaching program on chronic obstructive pulmonary disease. J Healthc Educ Train 1992;6:7–13 | Time point | Study design |
| Scherer YK, Schmieder LE. The effect of a pulmonary rehabilitation program on self-efficacy, perception of dyspnea, and physical endurance. Heart Lung 1997;26:15–22 | Time point | Study design |
| Scherer YK, Schmieder LE, Shimmel S. The effects of education alone and in combination with pulmonary rehabilitation on self-efficacy in patients with COPD. Rehabil Nurs J 1998;23:71–7 | Time point | Study design |
| Schlozman DL. Rehabilitation of patients with chronic obstructive lung disease. Pneumonologia Polska 1986;54:217–21 | Publication type | Publication type |
| Schols AM, Soeters PB, Mostert R, Pluymers RJ, Wouters EF. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med 1995;152:1268–74 | Intervention | Intervention |
| Schomberg LEE, Garner JL, Porter JW, Bahadur K, Ross L, Kosky CA, et al. Does the provision of a rescue pack keep patients with chronic obstructive pulmonary disease (COPD) at home? British Thoracic Society Winter Meeting, 7–9 November 2011, London, UK. Thorax 2011:A174 | Conference abstract | Publication type |
| Schonlau M, Mangione-Smith R, Chan KS, Keesey J, Rosen M, Louis TA, et al. Evaluation of a quality improvement collaborative in asthma care: does it improve processes and outcomes of care? Ann Fam Med 2005;3:200–8 | Population | Population |
| Schucher B, Criee C-P. [Respiratory muscle training.] Atemweg Lungenkrank 2009;35:312–17 | Study design | Study design |
| Schultz K, Schwiersch M, Petro W, Muhlig S, Petermann F. [Individualized, modular structured patient behavioral training in obstructive airway diseases during inpatient rehabilitation.] Pneumologie 2000;54:296–305 | Publication type | Publication type |
| Schultz K, Stark HJ, Petro W. [New educational tasks in the rehabilitation of respiratory diseases.] Atemwegs- und Lungenkrankheiten 1996;22:38–44 | Time point | Study design |
| Schulz M, Verheyen F, Muhlig S, Muller JM, Muhlbauer K, Knop-Schneickert E, et al. Pharmaceutical care services for asthma patients: a controlled intervention study. J Clin Pharmacol 2001;41:668–76 | Population | Population |
| Sedeno MF, Nault D, Hamd DH, Bourbeau J. A written action plan for early treatment of COPD exacerbations: an important component to the reduction of hospitalizations. Proc ATS 2006;3:A603 | Publication type | Publication type |
| Sedeno MF, Nault D, Hamd DH, Bourbeau J. A self-management education program including an action plan for acute COPD exacerbations. COPD 2009;6:352–8 | Time point | Study design |
| Seron P, Riedemann P, Munoz S, Doussoulin A, Villarroel P, Cea X. [Effect of inspiratory muscle training on muscle strength and quality of life in patients with chronic airflow limitation: A randomized controlled trial.] Arch Bronconeumol 2005;41:601–6 | Population | Population |
| Serres I, Varray A, Vallet G, Micallef JP, Prefaut C. Improved skeletal muscle performance after individualized exercise training in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 1997;17:232–8 | Time point | Study design |
| Sewell L, Singh SJ, Williams JE, Collier RJ, Morgan MDL. Goal directed pulmonary rehabilitation does not significantly improve health status and domestic function. Eur Respir J 2001;18(Suppl. 33):187s | Time point | Publication type |
| Sewell L, Singh SJ, Williams JE, Collier R, Morgan MD. Can individualized rehabilitation improve functional independence in elderly patients with COPD? Chest 2005;128:1194–200 | Time point | – |
| Sewell L, Singh SJ, Williams JE, Collier R, Morgan MD. How long should outpatient pulmonary rehabilitation be? A randomised controlled trial of 4 weeks versus 7 weeks. Thorax 2006;61:767–71 | Time point | – |
| Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010;65:423–8 | Intervention | – |
| Shahin B, Germain M, Kazem A, Annat G. Benefits of short inspiratory muscle training on exercise capacity, dyspnea, and inspiratory fraction in COPD patients. Int J Chron Obstruct Pulmon Dis 2008;3:423–7 | Time point | Study design |
| Shakur H. A COPD self management programme reduced hospital use and improved health status. Evid Based Nurs 2003;6:111 | Publication type | Publication type |
| Shao L-Z. [Effects of the behavioral intervention on the life quality of the patients with chronic obstructive pulmonary disease in remission period.] Chin J Clin Rehabil 2003;7:4078–9 | Time point | – |
| Shepperd S, Harwood D, Gray A, Vessey M, Morgan P. Randomised controlled trial comparing hospital at home care with inpatient hospital care. II: cost minimisation analysis. BMJ 1998;316:1791–6 | Intervention | Intervention |
| Sheridan N, Kenealy T, Salmon E, Rea H, Raphael D, Schmidt-Busby J. Helplessness, self blame and faith may impact on self management in COPD: a qualitative study. Prim Care Resp J 2001;20:307–14 | Time point | Study design |
| Conference proceedings. Short-Term In-hospital Rehabilitation Program (SRP) in COPD Patients. Functional and Clinical Effectiveness. Series: Diseases of the chest. The College; 1996. Vol. 110, no. 4. p. 137S | Unavailable | Unavailable |
| Silverman M, Musa D, Kirsch B, Siminoff LA. Self care for chronic illness: older African Americans and whites. J Cross Cult Gerontol 1999;14:169–89 | Time point | Study design |
| Simpson K, Killian K, McCartney N, Stubbing DG, Jones NL. Randomised controlled trial of weightlifting exercise in patients with chronic airflow limitation. Thorax 1992;47:70–5 | Time point | – |
| Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 2003;290:2301–12 | Publication type | Publication type |
| Sinclair DJ, Ingram CG. Controlled trial of supervised exercise training in chronic bronchitis. BMJ 1980;280:519–21 | Study design | Study design |
| Sindhwani G, Verma A, Biswas D, Srivastava M, Rawat J. A pilot study on domiciliary pulmonary rehabilitation programme in the management of severe chronic obstructive pulmonary disease. Singapore Med J 2011;52:689–93 | Time point | Study design |
| Singh V, Khandelwal DC, Khandelwal R, Abusaria S. Pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci 2003;45:13–17 | Time point | – |
| Singh VP, Rao V, Prem V, Sahoo RC, Kenshav Pai K. Comparison of the effectiveness of music and progressive muscle relaxation for anxiety in COPD: a randomized controlled pilot study. Chron Respir Dis 2009;6:209–16 | Time point | – |
| Sirey JA, Raue PJ, Alexopoulos GS. An intervention to improve depression care in older adults with COPD. Int J Geriatr Psychiatry 2007;22:154–9 | Intervention | Intervention |
| Sirinoglu Y, Sancar M, Karagoz T, Izzettin FV. The effect of pharmacist-led education on skills of patients with chronic obstructive pulmonary disease in using inhaler device. 39th ESCP European Symposium on Clinical Pharmacy and 13th SFPC Congress: Clinical Pharmacy at the Front Line of Innovations, 21–23 October 2010, Lyon, France. Int J Clin Pharm 2011:(var. pagings):316 | Time point | Study design |
| Sívori M, Rhodius E, Kaplan P, Talarico M, Gorojod G, Carreras B, et al. [Exercise training in chronic obstructive pulmonary disease. Comparative study of aerobic training of lower limbs vs. combination with upper limbs.] Medicina 1998;58:717–27 | Time point | – |
| Skumlien S, Aure SE, Skrede RM, Bjortuft O. Endurance or resistance training in primary care after in-patient rehabilitation for COPD? Respir Med 2008;102:422–9 | Time point | Study design |
| Skwarska E, Cohen G, Skwarski KM, Lamb C, Bushell D, Parker S, et al. Randomized controlled trial of supported discharge in patients with exacerbations of chronic obstructive pulmonary disease. Thorax 2000;55:907–12 | Intervention | Intervention |
| Slinde F, Gronberg AM, Engstrom CR, Rossander-Hulthen L, Larsson S. Individual dietary intervention in patients with COPD during multidisciplinary rehabilitation. Respir Med 2002;96:330–6 | Time point | Study design |
| Smeele IJ, Grol RP, van Schayck CP, van den Bosch WJ, van den Hoogen HJ, Muris JW. Can small group education and peer review improve care for patients with asthma/chronic obstructive pulmonary disease? Qual Health Care 1999;8:92–8 | Population | Population |
| Smith BJ, Appleton SL, Bennett PW, Roberts GC, Del FP, Adams R, et al. The effect of a respiratory home nurse intervention in patients with chronic obstructive pulmonary disease (COPD). Aust NZ J Med 1999;29:718–25 | Time point | – |
| Smith J. Telehealth Research Across The Community (TRAC): an evaluation of telehealth home monitoring of home care clients with chronic obstructive pulmonary disease or chronic heart failure compared to usual care. ANZCTR 2009 | Intervention | Intervention |
| Smith JR, Mildenhall S, Noble MJ, Shepstone L, Koutantji M, Mugford M, et al. The Coping with Asthma Study: a randomised controlled trial of a home based, nurse led psychoeducational intervention for adults at risk of adverse asthma outcomes. Thorax 2005;60:1003–11 | Population | Population |
| Smith K, Cook D, Guyatt GH, Madhavan J, Oxman AD. Respiratory muscle training in chronic airflow limitation: a meta-analysis. Am Rev Respir Dis 1992;145:533–9 | Publication type | Publication type |
| Snider GL. Exacerbations of chronic obstructive pulmonary disease: should self-management be used? Am J Respir Crit Care Med 2004;170:920 | Publication type | Publication type |
| Soicher JE, Mayo NE, Gauvin L, Hanley JA, Bernard S, Maltais F, et al. Trajectories of endurance activity following pulmonary rehabilitation in COPD patients. Eur Respir J 2012;39:272–8 | Time point | Study design |
| Soler JJ, Martinez-Garcia MA, Roman P, Orero R, Terrazas S, Martinez-Pechuan A. [Effectiveness of a specific program for patients with chronic obstructive pulmonary disease and frequent exacerbations.] Arch Bronconeumol 2006;42:501–8 | Time point | – |
| Soler-Cataluna JJ, Martinez-Garcia MA, Roman SP, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925–31 | Study design | Study design |
| Solomon DK, Portner TS, Bass GE, Gourley DR, Gourley GA, Holt JM, et al. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc (Wash) 1998;38:574–85 | Time point | – |
| Spencer LM, Alison JA, McKeough ZJ. Do supervised weekly exercise programs maintain functional exercise capacity and quality of life, twelve months after pulmonary rehabilitation in COPD? BMC Pulm Med 2007;7:7 | Publication type | Publication type |
| Spencer LM, Alison JA, McKeough ZJ. Maintaining benefits following pulmonary rehabilitation: a randomised controlled trial. Eur Respir J 2010;35:571–7 | Time point | – |
| Spencer S, Jones PW. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax 2003;58:589–93 | Intervention | Intervention |
| Spiliopoulos N, Donoghue J, Clark E, Dunford M. Outcomes from a Respiratory Coordinated Care Program (RCCP) providing community-based interventions for COPD patients from 1998 to 2006. Contemp Nurse 2008;31:2–8 | Time point | Study design |
| Spohn S, Wittmann M, Petro W. [The education program for patients with COPD from Bad Reichenhall.] Pneumologie 2001;55:470–4 | Study design | Study design |
| Spohn S, Wittmann M, Petro W. [Impact of an education program on health-related control beliefs and self-efficacy expectancies in patients with COPD.] Pravention und Rehabilitation 2002;14:163–70 | Time point | – |
| Spruit MA, Gosselink R, Troosters T, De PK, Decramer M. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur Respir J 2002;19:1072–78 | Time point | – |
| Sridhar M, Taylor R, Dawson S, Roberts NJ, Partridge MR. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax 2008;63:194–200 | Time point | – |
| Steele BG, Belza B, Cain KC, Coppersmith J, Lakshminarayan S, Howard J, et al. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Arch Phys Med Rehabil 2008;89:404–12 | Population | Population |
| Steiner MC, Barton RL, Singh SJ, Morgan MD. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2003;58:745–51 | Intervention | Intervention |
| Stellefson M, Chaney BH, Chaney JD. Using exploratory focus groups to inform the development of targeted COPD self-management education DVDs for rural patients. Int J Telemed Appl 2010;2010:450418 | Time point | Study design |
| Steuten L, Vrijhoef B, Van Merode F, Wesseling GJ, Spreeuwenberg C. Evaluation of a regional disease management programme for patients with asthma or chronic obstructive pulmonary disease. Int J Qual Health Care 2006;18:429–36 | Time point | Study design |
| Steuten LM, Lemmens KM, Nieboer AP, Vrijhoef HJ. Identifying potentially cost-effective chronic care programs for people with COPD. Int J Chron Obstruct Pulmon Dis 2009;4:87–100 | Publication type | Publication type |
| Storer TW. Exercise in chronic pulmonary disease: resistance exercise prescription. Med Sci Sports Exerc 2001;33(Suppl. 7):S680–92 | Publication type | Publication type |
| Strijbos JH, Koeter GH, Meinesz AF. Home care rehabilitation and perception of dyspnea in chronic obstructive pulmonary disease (COPD) patients. Chest 1990;97(Suppl. 3):109–10S | Publication type | Publication type |
| Strijbos JH, Postma DS, van Altena R, Gimeno F, Koeter GH. A comparison between an outpatient hospital-based pulmonary rehabilitation program and a home-care pulmonary rehabilitation program in patients with COPD. A follow-up of 18 months. Chest 1996;109:366–72 | Time point | – |
| Strijbos JH, Postma DS, van Altena R, Gimeno F, Koeter GH. Feasibility and effects of a home-care rehabilitation program in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 1996;16:386–93 | Time point | – |
| Stulbarg MS, Carrieri-Kohlman V, mir-Deviren S, Nguyen HQ, Adams L, Tsang AH, et al. Exercise training improves outcomes of a dyspnea self-management program. J Cardiopulm Rehabil 2002;22:109–21 | Time point | – |
| Sturdy D, Hillman D, Green D, Jenkins S, Cecins N, Eastwood P. Feasibility of high-intensity, interval-based respiratory muscle training in COPD. Chest 2003;123:142–50 | Time point | Study design |
| Su C-L, Chiang L-L, Chiang T-Y, Yu C-T, Kuo H-P, Lin H-C. Domiciliary positive expiratory pressure improves pulmonary function and exercise capacity pulmonary with chronic obstructive pulmonary disease. J Formosan Med Assoc 2007;106:204–11 | Time point | – |
| Subin, Rao V, Prem V, Sahoo. Effect of upper limb, lower limb and combined training on health-related quality of life in COPD. Lung India 2010;27:4–7 | Time point | – |
| Sudo E, Kitade H, Kitagawa T, Kawaguchi M. [The effects of a respiratory education class on psychological status for patients with chronic respiratory disease.] Nippon Ronen Igakkai Zasshi 2006;43:630–4 | Time point | Study design |
| Sudo E, Ohga E, Matsuse T, Teramoto S, Nagase T, Katayama H, et al. [The effects of pulmonary rehabilitation combined with inspiratory muscle training on pulmonary function and inspiratory muscle strength in elderly patients with chronic obstructive pulmonary disease.] Nippon Ronen Igakkai Zasshi 1997;34:929–34 | Time point | Study design |
| Sung KY, Sung IP, So YP, Jung KP, Sung EK, Jung YK, et al. [The effect of repeated education using a computerized scoring system for the proper use of inhalation medicine.] Tuberc Respir Dis 2007;63:491–6 | Population | Population |
| Swearengen PA, Brundage DJ, Woody JW. Self-care in COPD: an assessment project by practice and education. Nurs Connect 1989;2:67–73 | Publication type | Publication type |
| Swerts PM, Kretzers LM, Terpstra-Lindeman E, Verstappen FT, Wouters EF. Exercise reconditioning in the rehabilitation of patients with chronic obstructive pulmonary disease: a short- and long-term analysis. Arch Phys Med Rehabil 1990;71:570–3 | Time point | Study design |
| Sykes K. Inspiratory muscle training in the treatment of chronic obstructive pulmonary disease: randomized controlled trial. Am J Recreat Ther 2005;4:39–48 | Publication type | Publication type |
| Tandon MK. Adjunct treatment with yoga in chronic severe airways obstruction. Thorax 1978;33:514–17 | Time point | - |
| Tang CY, Taylor NF, Blackstock FC, Clarence M. Early commencement of exercise rehabilitation for inpatients with an acute exacerbation of COPD is safe and feasible. TSANZ and ANZSRS Annual Scientific Meetings, 1–6 April 2011, Perth, WA, Australia. Respirology 2011;(var.pagings):34 | Time point | Publication type |
| Anonymous. Teaching your patient to live with C.O.P.D. Nurs Life 1985;5:31–2 | Publication type | Publication type |
| Bazian Ltd. The effects of education on patient adherence to medication. Evid Based Healthc Public Health 2005;9:398–404 | Publication type | Publication type |
| Theander K, Jakobsson P, Jorgensen N, Unosson M. Effects of pulmonary rehabilitation on fatigue, functional status and health perceptions in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Clin Rehabil 2009;23:125–36 | Time point | – |
| Thornby MA, Haas F, Axen K. Effect of distractive auditory stimuli on exercise tolerance in patients with COPD. Chest 1995;107:1213–17 | Time point | Study design |
| Tiep BL, Burns M, Kao D, Madison R, Herrera J. Pursed lips breathing training using ear oximetry. Chest 1986;90:218–21 | Time point | – |
| Tierney WM, Overhage JM, Murray MD, Harris LE, Zhou XH, Eckert GJ, et al. Can computer-generated evidence-based care suggestions enhance evidence-based management of asthma and chronic obstructive pulmonary disease? A randomized, controlled trial. Health Serv Res 2005;40:477–97 | Intervention | Intervention |
| Toevs CD, Kaplan RM, Atkins CJ. The costs and effects of behavioral programs in chronic obstructive pulmonary disease. Med Care 1984;22:1088–100 | Population | Population |
| Toshima MT, Blumberg E, Ries AL, Kaplan RM. Does rehabilitation reduce depression in patients with chronic obstructive pulmonary disease? J Cardiopulm Rehabil 1992;12:261–9 | Time point | – |
| Toshima MT, Kaplan RM, Ries AL. Experimental evaluation of rehabilitation in chronic obstructive pulmonary disease: short-term effects on exercise endurance and health status. Health Psychol 1990;9:237–52 | Time point | – |
| Tougaard L, Krone T, Sorknaes A, Ellegaard H. Economic benefits of teaching patients with chronic obstructive pulmonary disease about their illness. The PASTMA Group. Lancet 1992;339:1517–20 | Time point | Study design |
| Tougaard L, Krone T, Sorknaes A, Ellegaard H. [Health economical benefits of personalized hospital treatment of chronic bronchitis.] Ugeskr Laeger 1993;155:3657–60 | Population | Population |
| Trappenburg J, Heijneman J, Monninkhof E, Bourbeau J, Troosters T, Schrijvers G. Effectiveness of an individualized action plan on health status recovery in patients with COPD: a randomized controlled trial. European Respiratory Society Annual Congress, 18–22 September, Barcelona, Spain, p. E2168 | Publication type | Publication type |
| Trappenburg JC, Niesink A, de Weert-van Oene GH, van der Zeijden H, van Snippenburg R, Peters A, et al. Effects of telemonitoring in patients with chronic obstructive pulmonary disease. Telemed J E-Health 2008;14:138–46 | Time point | Study design |
| Trappenburg JC, Koevoets L, de Weert-van Oene GH, Monninkhof EM, Bourbeau J, Troosters T, et al. Action Plan to enhance self-management and early detection of exacerbations in COPD patients: a multicenter RCT. BMC Pulm Med 2009;9:52 | Publication type | Publication type |
| Trappenburg JC, Monninkhof EM, Bourbeau J, Troosters T, Schrijvers AJ, Verheij TJ, et al. Effect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: a multicentre randomised controlled trial. Thorax 2011;66:977–84 | Time point | – |
| Trappenburg JC, Schaap D, Monninkhof EM, Bourbeau J, de Weert-van Oene GH, Verheij TJ, et al. How do COPD patients respond to exacerbations? BMC Pulm Med 2011;11:43 | Intervention | Intervention |
| Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med 2000;109:207–12 | Time point | – |
| Utens CM, Goossens LM, Smeenk FW, van Schayck OC, van Litzenburg W, Janssen A, et al. Effectiveness and cost-effectiveness of early assisted discharge for chronic obstructive pulmonary disease exacerbations: the design of a randomised controlled trial. BMC Public Health 2010;10:618 | Publication type | Publication type |
| Vale F, Reardon JZ, Zuwallack RL. The long-term benefits of outpatient pulmonary rehabilitation on exercise endurance and quality of life. Chest 1993;103:42–5 | Population | Population |
| Vallet G, Varray A, Fontaine JL, Prefaut C. [Value of individualized rehabilitation at the ventilatory threshold level in moderately severe chronic obstructive pulmonary disease.] Rev Mal Respir 1994;11:493–501 | Time point | – |
| Vallet G, Ahmaidi S, Serres I, Fabre C, Bourgouin D, Desplan J, et al. Comparison of two training programmes in chronic airway limitation patients: standardized versus individualized protocols. Eur Respir J 1997;10:114–22. | Time point | – |
| Van der Palen J, Klein JJ, Kerkhoff AH, van Herwaarden CL, Seydel ER. Evaluation of the long-term effectiveness of three instruction modes for inhaling medicines. Patient Educ Couns 1997;32(Suppl. 1):S87–95 | Time point | Outcome |
| Van der Schans CP. Conventional chest physical therapy for obstructive lung disease. Respir Care 2007;52:1198–206 | Publication type | Publication type |
| Van Gestel AJ, Kohler M, Steier J, Teschler S, Russi EW, Teschler H. The effects of controlled breathing during pulmonary rehabilitation in patients with COPD. Respiration 2012;83:115–24 | Time point | – |
| Van Wetering CR, Hoogendoorn M, Broekhuizen R, Geraerts-Keeris GJ, De Munck DR, Rutten-van Mölken MP, et al. Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: a prespecified subgroup analysis of the INTERCOM trial. J Am Med Direct Assoc 2010;11:179–87 | Time point | Population |
| Van Wetering CR, Hoogendoorn M, Mol SJ, Rutten-van Mölken MP, Schols AM. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax 2010;65:7–13 | Time point | – |
| Vandivier W. Advanced eHealth for Chronic Obstructive Pulmonary Disease (COPD) in Colorado; 2013. URL: www.clinicaltrials.gov (accessed 27 January 2015) | Publication type | Publication type |
| Varga J, Porszasz J, Boda K, Casaburi R, Somfay A. Supervised high intensity continuous and interval training vs. self-paced training in COPD. Respir Med 2007;101:2297–304 | Time point | – |
| Vargas F, Boyer A, Bui HN, Salmi LR. Intrapulmonary percussive ventilation in acute exacerbations of COPD patients with mild respiratory acidosis: a randomized controlled trial [ISRCTN17802078.] Crit Care 2005;9:R382–9 | Intervention | Intervention |
| Verver S, Poelman M, Bogels A, Chisholm SL, Dekker FW. Effects of instruction by practice assistants on inhaler technique and respiratory symptoms of patients. A controlled randomized videotaped intervention study. Fam Pract 1996;13:35–40 | Population | Population |
| Villafranca C, Borzone G, Leiva A, Lisboa C. Effect of inspiratory muscle training with an intermediate load on inspiratory power output in COPD. Eur Respir J 1998;11:28–33 | Outcome | Outcome |
| Vitacca M, Clini E, Bianchi L, Ambrosino N. Acute effects of deep diaphragmatic breathing in COPD patients with chronic respiratory insufficiency. Eur Respir J 1998;11:408–15 | Time point | Study design |
| Vitacca M, Bianchi L, Guerra A, Fracchia C, Spanevello A, Balbi B, et al. Tele-assistance in chronic respiratory failure patients: a randomised clinical trial. Eur Respir J 2009;33:411–18 | Population | Population |
| Vivodtzev I, Pepin JL, Vottero G, Mayer V, Porsin B, Levy P, et al. Improvement in quadriceps strength and dyspnea in daily tasks after 1 month of electrical stimulation in severely deconditioned and malnourished COPD. Chest 2006;129:1540–8 | Intervention | Intervention |
| Vogiatzis I, Williamson AF, Miles J, Taylor IK. Physiological response to moderate exercise workloads in a pulmonary rehabilitation program in patients with varying degrees of airflow obstruction. Chest 1999;116:1200–7 | Time point | Study design |
| Vogiatzis I, Nanas S, Roussos C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur Respir J 2002;20:12–19 | Time point | – |
| Vogiatzis I, Terzis G, Nanas S, Stratakos G, Simoes DC, Georgiadou O, et al. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest 2005;128:3838–45 | Time point | – |
| Vonbank K, Strasser B, Mondrzyk J, Marzluf BA, Richter B, Losch S, et al. Strength training increases maximum working capacity in patients with COPD – randomized clinical trial comparing three training modalities. Respir Med 2012;106:557–63 | Time point | – |
| Votto J, Bowen J, Scalise P, Wollschlager C, Zuwallack R. Short-stay comprehensive inpatient pulmonary rehabilitation for advanced chronic obstructive pulmonary disease. Arch Phys Med Rehabil 1996;77:1115–18 | Intervention | Study design |
| Vrijhoef HJ, Van Den Bergh JH, Diederiks JP, Weemhoff I, Spreeuwenberg C. Transfer of care for outpatients with stable chronic obstructive pulmonary disease from respiratory care physician to respiratory nurse: a randomized controlled study. Chron Illn 2007;3:130–44 | Intervention | Intervention |
| Wadell K, Sundelin G, Henriksson-Larsen K, Lundgren R. High intensity physical group training in water: an effective training modality for patients with COPD. Respir Med 2004;98:428–38 | Time point | – |
| Wakabayashi R, Kida K, Yamada K, Jones RCM, Hyland ME. A randomised controlled trial of a patient education programme versus normal care for COPD using the lung information needs questionnaire (LINQ) [Abstract.] Eur Respir J 2006;28(Suppl. 50):554 | Time point | Publication type |
| Wakabayashi R, Motegi T, Yamada K, Ishii T, Jones RC, Hyland ME, et al. Efficient integrated education for older patients with chronic obstructive pulmonary disease using the Lung Information Needs Questionnaire. Geriatr Gerontol Int 2011;11:422–30 | Time point | – |
| Walker K, LeBlanc C. Neuromuscular respiratory rehabilitation. Can J Respir Ther 2001;37:36–41 | Publication type | Publication type |
| Walters J. Pathways to Lung Health: a comprehensive Self-Management Programme for Chronic Obstructive Pulmonary Disease in the community; ANZCTR 2008. URL: www.anzctr.org.au (accessed 27 January 2015) | Publication type | Publication type |
| Wang QX, Zhang XY, Li QA. Effects of a flutter mucus-clearance device on pulmonary function test results in healthy people 85 years and older in China. Respir Care 2010;55:1449–52 | Population | Population |
| Wang QY, Bourbeau J. Outcomes and health-related quality of life following hospitalization for an acute exacerbation of COPD. Respirology 2005;10:334–40 | Study design | Study design |
| Wang Z, Zeng H, Chen H. [Rehabilitative treatment in patients with chronic obstructive pulmonary disease at stable stage.] Chin Nurs Res 2004;18:1608–9 | Time point | – |
| Wanke T, Formanek D, Lahrmann H, Brath H, Wild M, Wagner C, et al. Effects of combined inspiratory muscle and cycle ergometer training on exercise performance in patients with COPD. Eur Respir J 1994;7:2205–11 | Time point | – |
| Warlies F, Saladin M, Hellmann A. [Evaluation of a standardized specific education program ‘Lebensrhythmus Atmen’: a prospective, randomized, controlled study for COPD patients: a pilot study.] Pravention und Rehabilitation 2006;18:68–79 | Time point | – |
| Warm D, Lewis K. A New Model for Continuous Care of Chronic Patients: eCare and eLearning for Patients with Chronic Obstructive Pulmonary Disease (COPD). ISRCTN Register 2008. URL: www.isrctn.org (accessed 27 January 2015) | Publication type | Publication type |
| Wasson J, Gaudette C, Whaley F, Sauvigne A, Baribeau P, Welch HG. Telephone care as a substitute for routine clinic follow-up. JAMA 1992;267:1788–93 | Population | Population |
| Waterhouse J, Walters S, Lawson R. Can Telephone Encouragement Maintain the Benefits of Pulmonary Rehabilitation for People with COPD? Data Eighteen Months Post Rehab. A Report of the CoCoRT Study of Pulmonary Rehabilitation. Lausanne: e-Learning Resources; 2007 | Study design | Study design |
| Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA. A randomised 2 x 2 trial of community versus hospital pulmonary rehabilitation, followed by telephone or conventional follow-up. Health Technol Assess 2010;14(6) | Time point | – |
| Watson PB, Town GI, Holbrook N, Dwan C, Toop LJ, Drennan CJ. Evaluation of a self-management plan for chronic obstructive pulmonary disease. Eur Respir J 1997;10:1267–71 | Time point | – |
| Wedzicha JA, Bestall JC, Garrod R, Garnham R, Paul EA, Jones PW. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale. Eur Respir J 1998;12:363–9 | Time point | – |
| Weekes CE, Emery PW, Elia M. Dietary counselling and food fortification in stable COPD: a randomised trial. Thorax 2009;64:326–31 | Time point | – |
| Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? N Engl J Med 1996;334:1441–7 | Intervention | Intervention |
| Weinberger M, Murray MD, Marrero DG, Brewer N, Lykens M, Harris LE, et al. Effectiveness of pharmacist care for patients with reactive airways disease: a randomized controlled trial. JAMA 2002;288:1594–602 | Population | Population |
| Weiner P, Azgad Y, Ganam R. Inspiratory muscle training combined with general exercise reconditioning in patients with COPD. Chest 1992;102:1351–6 | Time point | – |
| Weiner P, Azgad Y, Ganam R, Weiner M. Inspiratory muscle training in patients with bronchial asthma. Chest 1992;102:1357–61 | Population | Population |
| Weiner P, Magadle R, Berar-Yanay N, Davidovich A, Weiner M. The cumulative effect of long-acting bronchodilators, exercise, and inspiratory muscle training on the perception of dyspnea in patients with advanced COPD. Chest 2000;118:672–8 | Time point | – |
| Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest 2003;124:1357–64 | Time point | – |
| Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N. Specific expiratory muscle training in COPD. Chest 2003;124:468–73 | Time point | – |
| Weiner P, Magandle R, Beckerman M, Weiner M, Berar-Yanay N. Maintenance of inspiratory muscle training in COPD patients: one year follow-up. Eur Respir J 2004;23:61–5 | Time point | – |
| Weiner P, Weiner M. Inspiratory muscle training may increase peak inspiratory flow in chronic obstructive pulmonary disease. Respiration 2006;73:151–6 | Time point | – |
| Welch HG, Johnson DJ, Edson R. Telephone care as an adjunct to routine medical follow-up. A negative randomized trial. Eff Clin Pract 2000;3:123–30 | Population | Population |
| Wen H, Gao Y, An JY. [Comparison of high-intensity and anaerobic threshold programs in rehabilitation for patients with moderate to severe chronic obstructive pulmonary disease.] Chung-Hua Chieh Ho Ho Hu Hsi Tsa Chih 2008;31:571–6 | Time point | – |
| Wen Y-L, Huang D-F, Huang M, Huang Y-P. [Evaluation on the effect of systematic exercise rehabilitation intervention in patients with chronic obstructive pulmonary disease.] Chin J Clin Rehabil 2004;8:2224–5 | Time point | – |
| White RJ, Rudkin ST, Harrison ST, Day KL, Harvey IM. Pulmonary rehabilitation compared with brief advice given for severe chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2002;22:338–44 | Time point | – |
| Whitten P, Mickus M. Home telecare for COPD/CHF patients: outcomes and perceptions. J Telemed Telecare 2007;13:69–73 | Population | Population |
| Wijkstra PJ, Strijbos JH. Home-based rehabilitation for patients with chronic obstructive pulmonary disease. Monaldi Arch Chest Dis 1998;53:450–3 | Study design | Study design |
| Wijkstra PJ, ten Vergert EM, van Altena R, Otten V, Kraan J, Postma DS, et al. Long term benefits of rehabilitation at home on quality of life and exercise tolerance in patients with chronic obstructive pulmonary disease. Thorax 1995;50:824–8 | Time point | – |
| Wijkstra PJ, van Altena R, Kraan J, Otten V, Postma DS, Koeter GH. Quality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at home. Eur Respir J 1994;7:269–73 | Time point | – |
| Wijkstra PJ, van der Mark TW, Kraan J, van Altena R, Koeter GH, Postma DS. Effects of home rehabilitation on physical performance in patients with chronic obstructive pulmonary disease (COPD). Eur Respir J 1996;9:104–10 | Time point | – |
| Wijkstra PJ, van der Mark TW, Kraan J, van Altena R, Koeter GH, Postma DS. Long-term effects of home rehabilitation on physical performance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;153:1234–41 | Time point | – |
| Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;169:1298–303 | Time point | Study design |
| Wilson A, Parker H, Wynn A, Jagger C, Spiers N, Jones J, et al. Randomised controlled trial of effectiveness of Leicester hospital at home scheme compared with hospital care. BMJ 1999;319:1542–6 | Population | Population |
| Wilson A, Wynn A, Parker H. Patient and carer satisfaction with ‘hospital at home’: quantitative and qualitative results from a randomised controlled trial. Br J Gen Pract 2002;52:9–13 | Population | Population |
| Wittmann M, Spohn S, Schultz K, Pfeifer M, Petro W. [Patient education in COPD during inpatient rehabilitation improves quality of life and morbidity.] Pneumologie 2007;61:636–42 | Time point | – |
| Wolkove N, Kamel H, Rotaple M, Baltzan M. Use of a mucus clearance device enhances the bronchodilator response in patients with stable COPD. Chest 2002;121:702–7 | Intervention | Intervention |
| Wolkove N, Baltzan MA, Jr, Kamel H, Rotaple M. A randomized trial to evaluate the sustained efficacy of a mucus clearance device in ambulatory patients with chronic obstructive pulmonary disease. Can Respir J 2004;11:567–72 | Time point | – |
| Woo J, Chan W, Yeung F, Chan WM, Hui E, Lum CM, et al. A community model of group therapy for the older patients with chronic obstructive pulmonary disease: a pilot study. J Eval Clin Pract 2006;12:523–31 | Time point | Study design |
| Wood-Baker R, McGlone S, Venn A, Walters EH. Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology 2006;11:619–26 | Time point | – |
| Worth H, Dhein Y. Does patient education modify behaviour in the management of COPD? Patient Educ Couns 2004;52:267–70 | Publication type | Publication type |
| Wright P. Effects of a resistance training on pulmonary function and performance measurements in patients with chronic obstructive pulmonary disease. Eur J Sport Sci 2003;3:1–10 | Time point | – |
| Wu X, Hou L, Bai W. [Effects of breathing training on quality of life and activities of daily living in elderly patients with stable severe chronic obstructive pulmonary disease.] Chin J Rehabil Med 2006;21:307–10 | Time point | Study design |
| Wurtemberger G, Bastian K. [Functional effects of different training in patients with COPD.] Pneumologie 2001;55:553–62 | Time point | – |
| Xie S-L, Zhu M-G, Cui H-B, Liu H-Y. Influence of home-based training program on patients with COPD. Chin J Clin Rehabil 2003;7:2554–5 | Time point | – |
| Xu Y-H, Wang J-H, Li H-F, Zhu X-H, Wang G. [Efficacy of integrative respiratory rehabilitation training in exercise ability and quality of life of patients with chronic obstructive pulmonary disease in stable phase: a randomized controlled trial.] J Chin Integr Med 2010;8:432–7 | Time point | – |
| Yamaguti WP, Claudino RC, Neto AP, Chammas MC, Gomes AC, Salge JM, et al. Diaphragmatic breathing training program improves abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Arch Phys Med Rehabil 2012;93:571–7 | Time point | – |
| Yan Q, Sun Y. Quantitative research for improving respiratory muscle contraction by breathing exercise. Chin Med J 1996;109:771–5 | Time point | – |
| Yeh GY, Roberts DH, Wayne PM, Davis RB, Quilty MT, Phillips RS. Tai chi exercise for patients with chronic obstructive pulmonary disease: a pilot study. Respir Care 2010;55:1475–82 | Time point | – |
| Zajac B. Measuring outcomes of a chronic obstructive pulmonary disease management program. Dis Manag 2002;5:9–23 | Study design | Study design |
| Zhang ZQ, Chen RC, Yang QK, Li P, Wang CZ, Zhang ZH. [A randomized controlled trial study of pulmonary rehabilitation with respiratory physiology as the guide on prognosis in patients with chronic obstructive pulmonary disease.] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2008;20:607–10 | Time point | – |
| Zimmer JG, Groth-Juncker A, McCusker J. A randomized controlled study of a home health care team. Am J Public Health 1985;75:134–41 | Population | Population |
| Zwar N, Hermiz O, Hasan I, Comino E, Middleton S, Vagholkar S, et al. A cluster randomised controlled trial of nurse and GP partnership for care of chronic obstructive pulmonary disease. BMC Pulm Med 2008;8:8 | Publication type | Publication type |
Appendix 3 Conference abstracts, relevant to review 1, between 2010 and 2012
Controlled trial of short term (3 weeks) pulmonary rehabilitation in COPD following acute exacerbation
MS Ali, D Talwar, RK Singh, D Pabreja
India
European Respiratory Society 2012Do telephone interventions of patients with COPD prevent readmission?
M Lavesen, R Overgaard, S Mazurek, A Just, D Overgaard
Denmark
Effect on prevention of readmissions of a home-based education and exercise program implemented early after a severe exacerbation of COPD
R Coll-Fernandez, N Martínez, M Arranz, H Prados, X Pomares, A Moreno, M Teixidó, F Epelde, F Caballero, E Monsó
Spain
Appendix 4 List of ongoing trials relevant to reviews 1–4
| Citation | Relevant to review | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Optimizing the effect of COPD rehabilitation | – | – | – | Y |
| A multi-center study of rehabilitation to stable chronic obstructive pulmonary disease (COPD) patients | – | – | – | Y |
| Effectiveness of incorporating tai chi in pulmonary rehabilitation program for patients with chronic obstructive pulmonary disease in primary health care | – | – | – | Y |
| Long-term respiratory rehabilitation programs in chronic obstructive pulmonary disease (COPD) patients: study of cost-effectiveness | – | – | – | Y |
| Early pulmonary rehabilitation following acute COPD exacerbation | – | – | – | Y |
| Benefits and costs of home-based pulmonary rehabilitation in chronic obstructive pulmonary disease | – | – | – | Y |
| Effects of home-based pulmonary rehabilitation in patients with severe or very severe chronic obstructive pulmonary disease (COPD) | – | – | – | Y |
| Home-based in chronic obstructive pulmonary disease | – | – | – | Y |
| Nutritional rehabilitation in chronic obstructive pulmonary disease (COPD) patients with muscle atrophy | – | – | – | Y |
| Effects of inspiratory muscle training on dyspnea in subjects with chronic obstructive pulmonary disease | – | – | – | Y |
| Eccentric exercise training as novel rehabilitation for chronic obstructive pulmonary disease (COPD) | – | – | – | Y |
| Physical activity counseling during pulmonary rehabilitation | – | – | – | Y |
| Long-term physical training in chronic obstructive pulmonary disease | – | – | – | Y |
| Multicomponent intervention to decrease chronic obstructive pulmonary disease (COPD)-related hospitalizations | – | – | – | Y |
| Impact of a hospital physical therapy program on chronic obstructive pulmonary disease (COPD) patients | Y | – | Y | Y |
| Comprehensive disease management program in chronic obstructive pulmonary disease (COPD) patients in the community | – | – | – | Y |
| Nurse managed sequential strength training and bicycle training in chronic obstructive pulmonary disease (COPD) | – | – | – | Y |
| Effects of mud bath therapy in chronic obstructive pulmonary disease | – | – | – | Y |
| Randomized trial of physical activity self-management intervention for patients with COPD | – | – | – | Y |
| Validation of an exercise DVD for maintenance after pulmonary rehabilitation | – | – | – | Y |
| Effects of respiratory muscle training and respiratory exercise in exercise tolerance, performing daily life activities and quality of life of patients with chronic obstructive pulmonary disease | – | – | – | Y |
| Balance training in patients with chronic obstructive pulmonary disease (COPD) | – | – | – | Y |
| A comprehensive care programme for patients with chronic obstructive pulmonary disease | Y | – | Y | Y |
| Problem-solving therapy for people with major depression and chronic obstructive pulmonary disease | – | – | – | Y |
| Life-long monitoring of COPD in veneto region | – | – | – | Y |
| Breathing control in patients with chronic obstructive pulmonary disease (COPD) | – | – | – | Y |
| Randomized trial of physical activity self-management intervention for patients with COPD | – | – | – | Y |
| Multicomponent intervention to decrease chronic obstructive pulmonary disease (COPD)-related hospitalizations | – | – | – | Y |
| Effectiveness of Interventions to Teach Respiratory Inhaler Technique (E-TRaIN) | – | – | – | Y |
| The COPD on Oxygen Patient Management European Trial (COMET) | – | – | – | Y |
| A randomized controlled trial to determine outcome and cost effectiveness of case management of chronic obstructive pulmonary disease (COPD) patients | – | – | – | Y |
| Home telehealth follow-up after hospital discharge for chronic obstructive pulmonary disease (COPD) patients | Y | Y | Y | Y |
| Effectiveness of incorporating tai chi in pulmonary rehabilitation program for patients with chronic obstructive pulmonary disease in primary health care | – | – | – | Y |
| Educational intervention for managing inhalers in chronic obstructive pulmonary disease (COPD) patients | – | – | – | Y |
| Disease management in asthma or chronic obstructive pulmonary disease (COPD) patients | – | – | – | Y |
| Stepping up to health – for veterans with chronic obstructive pulmonary disease (COPD) | – | – | – | Y |
| Coping skills for patients with chronic obstructive pulmonary disease (COPD) and their caregivers | – | – | – | Y |
| Prigmore S. Does an individualised self-management plan help patients with chronic obstructive pulmonary disease (COPD) initiate early treatment for infective exacerbations? ISRCTN Register 2012 | – | – | – | Y |
| Educational interventions for chronic obstructive pulmonary disease (COPD) self-management in ethno-cultural communities. Clinicaltrials.gov 2012 | – | – | – | Y |
Appendix 5 Mortality data from randomised controlled trials: review 1
| Study year | Brief intervention | Brief control | Mortality outcome, description | Follow-up (months) | Reassessment | Effect size/p-value | |
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| Behnke 200064 | Home-based walking exercise programme, N = 23 | Control: advised to exercise, no instruction, N = 23 | Deaths | 6 | 1/23 (4.3%) | 1/23 (4.3%) | NR |
| Hermiz 200267 | Home-based care focused on SM, N = 84 | UC, N = 93 | Total deaths | 3 | 9/84 (10.7%) | 10/93 (10.8%) | NR |
| Hernandez 200368 | Home hospitalisation, N = 121 | UC, N = 101 | Deaths | 2 | 5/121 (4.1) | 7/101 (6.9) | NR |
| Kwok 200470 | Community nurse-supported discharge programme, N = 77 | UC, N = 80 | Deaths | 6 | 3/77 | 6/80 | NR |
| Casas 200671 | Integrated care with SM intervention, N = 65 | UC, N = 90 | Total deaths | 6, 12 | 7/65 (10.8%), 12/65 (18.5%) | 11/90 (12.2%), 14/90 (15.6%) | NR |
| Garcia-Aymerich 200772 | Integrated care included supported SM, N = 44 | UC, N = 69 | Total deaths | 6, 12 | 6/44 (13.6%), 11/44 (25.0%) | 8/69 (11.6%), 10/69 (14.5%) | NR |
| Bucknall 201263 | Supported SM, N = 232 | UC, N = 232 | COPD deaths, all-cause deaths | 12 | 23/232 (10%), 30/232 (13%) | 16/232 (7%), 22/232 (9%) | HRs: time to death, 1.36 (95% CI 0.71 to 2.61), 1.35 (95% CI 0.77 to 2.38) |
Appendix 6 Hospital readmissions data from randomised controlled trials: review 1
| Study year | Brief | Outcome description | Follow-up (months) | End point | Effect size/p-value | ||
|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||
| Egan 200269 | Case management, N = 33 | UC, N = 33 | Unscheduled readmissions; no detail | 3 | N = 33/33 | N = 33/33 | Reported no significant difference |
| Mean (range): 2.1 (1–5) | Mean (range): 2.6 (1–6) | ||||||
| Hermiz 200267 | Home-based care focused on SM, N = 84 | UC, N = 93 | All-cause hospitalisation (also number of admissions; number of admissions due to acute respiratory disease) | 3 | N = 84/84 | N = 93/93 | |
| Hospitalisation on one or more occasion | Hospitalisation on one or more occasion | ||||||
| 16/84 (24%) | 14/93 (18%) | ||||||
| 25 readmissions | 19 readmissions | ||||||
| 12 for acute respiratory condition | 14 for acute respiratory condition | ||||||
| Lee 200266 | Care protocol (including SM) to nursing home staff and patients, N = 48 completers | UC, N = 41 completers | Mean COPD-related readmissions Mean COPD-related hospital-days Time to first hospital readmission (days) |
6 | N = 48 | N = 41 | |
| COPD admission, mean (SD) | |||||||
| 1.54 (1.75) | 1.39 (1.51) | 0.666 | |||||
| COPD hospital-days, mean (SD) | |||||||
| 14.35 (19.27) | 14.98 (20.18) | 0.882 | |||||
| Days to first readmission, mean (SD) | |||||||
| 33.58 (42.58) | 25.49 (35.67) | 0.325 | |||||
| Behnke 200365 | Home-based walking exercise programme, N = 14 | Control: no instruction N = 12 |
Total number of admissions within three consecutive 6-month periods | 18 | N = 14 | N = 12 | |
| Total admissions, months | |||||||
|
|
0.026 | |||||
| Dheda 200473 | SM hospital outpatient followed up, N = 15 | SM primary care followed up, N = 18 | Number of readmissions | 6 | N = 10, two readmissions | N = 15, nine readmissions | |
| Hernandez 200368 | Home hospitalisation, N = 121 | UC, N = 101 | Readmissions (number of admissions, duration of admission) | 2 | N = 121 | N = 101 | |
| Inpatient hospital readmissions | |||||||
|
|
0.02 | |||||
| Emergency room readmissions | |||||||
|
|
0.01 | |||||
| Kwok 200470 | Community nurse-supported discharge programme, N = 77 | UC, N = 80 | Readmissions at 28 days, 6 months, unplanned readmissions, total hospital days duration of admission) | 28 days | N = 70 | n = 79 | |
| Hospital readmissions | |||||||
| 28 days: n (%) 33 (47) | 28 days: n (%) 29 (37) | 0.244 | |||||
| 6 months: n (%) 53 (76) | 6 months: n (%) 49 (62) | 0.08 | |||||
| Unplanned readmissions, mean ± SD (median) | |||||||
| 1.5 ± 1.4 (1) | 1.5 ± 2.2 (1) | 0.319 | |||||
| Total hospital-days, mean ± SD (median) | |||||||
| 20.3 ± 25.3 (12.5) | 19.2 ± 25.6 (12) | 0.410 | |||||
| Wong 200574 | SM telephone followed up, N = 30 | Routine care, N = 30 | Health-care use (1, 3 months) (total, inpatient, outpatient) Frequency at 1 month (inpatient) Frequency at 3 months (inpatients) Days of readmission at 1 month Note that hospitalisations were attributable to respiratory problems |
1, 3 | N = 30, health-care use | N = 30, health-care use | |
| Total 1 month | |||||||
| 6/30 (21.4%) | 9/30 (31.0%) | 0.410 | |||||
| Inpatient 1 month | |||||||
| 5/30 (83.3%) | 8/30 (88.9%) | 0.410 | |||||
| Total 3 months | |||||||
| 13/30 (43.3%) | 19/30 (63.3%) | 0.195 | |||||
| Frequency 1 month | |||||||
| Inpatient, mean (SD) = 1.2 (0.4); median: 1.0 | Inpatient, mean (SD) = 1.0 (0.00); median 1.0 | 0.206 | |||||
| Outpatient: 5 | Outpatient: 6 | ||||||
| Frequency 3 months | |||||||
| Admissions: mean (SD) = 0.6 (1.0); median: 0 | Admissions: mean (SD) = 1.1 (1.3); median: 0 | 0.182 | |||||
| Duration admission (days) 1 month | |||||||
| Mean (SD) = 19.6 (2.5) | Mean (SD) = 17.3 (4.4) | 0.354 | |||||
| Casas 200671 | Integrated care with SM intervention, N = 65 | UC, N = 90 | Number of patients with readmissions (mean readmissions; rate of readmissions; difference in rate compared with previous year; survival without readmissions) Implies admissions due to exacerbations |
12 | Readmissions | ||
| 29/65 (44.6%) | 60/90 (66.7%) | ||||||
| Mean (SD) readmissions during followed-up year | |||||||
| 0.9 (1.3) | 1.3 (1.7) | 0.028 | |||||
| Rate of readmissions during followed-up year | |||||||
| Mean (SD) 1.5 (2.6) | Mean (SD): 2.1 (3.1) | 0.033; adjusted HR = 0.55 (95% CI 0.35 to 0.88) | |||||
| Difference in rate of readmissions per year | |||||||
| Mean (SD) 0.5 (2.6) | Mean (SD) 1.5 (3.1) | 0.003 | |||||
| Survival without readmissions | |||||||
| 32 (49%) | 28 (31%) | 0.03 | |||||
| Bucknall 201263 | Supported SM, N = 232 | UC, N = 232 | COPD admission or COPD death, computed COPD admissions | 12 | COPD admission or COPD death | ||
| 111/232 (48%) | 108/232 (47%) | Time to admission/death, HRs: 1.05 (95% CI 0.80 to 1.38) | |||||
| COPD admissions | |||||||
| 88/232 (37.9%) | 92/232 (39.7%) | Time to admission/death, HRs: 1.36 (95% CI 0.71 to 2.61) | |||||
Appendix 7 General practitioner consultation data from randomised controlled trials: review 1
| Study year | Brief | GP contacts – description | Follow-up | Reassessment | Effect size/p-value | ||
|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||
| Hermiz 200267 | Home-based care focused on SM, N = 84 | UC, N = 93 | GP contacts | 3 | N = 67 | N = 80 | |
| Visited GP: 60 (90%) | Visited GP: 75 (94%) | ||||||
| Mean visits to GP | |||||||
| Patient report: | |||||||
| 6.06 (n = 60) | 5.54 (n = 74) | 0.3 | |||||
| GP report: | |||||||
| 5.21 (n = 57) | 5.11 (n = 64) | 0.9 | |||||
| GP prescribed drugs | |||||||
| 42/57 (74%) | 53/64 (83%) | 0.2 | |||||
| GP arranged follow-up | |||||||
| 37/57 (65%) | 41/64 (64%) | 0.4 | |||||
| GP provided patient with education | |||||||
| 39/57 (68%) | 44/64 (69%) | 0.9 | |||||
| GP provided carer with education | |||||||
| 14/57 (25%) | 11/64 (17%) | 0.3 | |||||
| Casas 200671 | Integrated care with SM intervention, N = 65 | UC, N = 90 | Self-reported physician visits Rate per year |
12 | N = 65 | N = 90 | |
| Median (IQR) visits | |||||||
| Barcelona 2 (0–4) | Barcelona 2 (1–4) | 0.437 | |||||
| Leuven 10 (7–18) | Leuven 13 (9–27) | 0.454 | |||||
Appendix 8 Emergency department visits data from randomised controlled trials: review 1
| Study year | Brief | Outcome description | Follow-up | End point | Effect size/p-value | ||
|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||
| Lee 200266 | Care protocol (including SM) to nursing home staff and patients, N = 48 completers | UC, N = 41 completers | Mean COPD-related ED usage Time to first ED usage (days) |
6 months | N = 48 | N = 41 | |
| COPD ED usage, mean (SD) | |||||||
| 1.58 (1.75) | 1.59 (1.73) | 0.996 | |||||
| Days to first ED usage, mean (SD) | |||||||
| 33.58 (42.58) | 24.27 (35.67) | 0.251 | |||||
| Hermiz 200267 | Home-based care focused on SM, N = 84 | UC, N = 93 | All-cause hospitalisation (also number of admissions; number of admissions attributable to acute respiratory disease) | 3 months | N = 84/84 | N = 93/93 | |
| Patients visiting ED | |||||||
| 2/84 = 2.4% | 8/93 = 8.6% | ||||||
| Hernandez 200368 | Home hospitalisation, N = 121 | UC, N = 101 | Emergency room readmissions | 8 weeks | N = 121 | N = 101 | |
| Patients, n (%) | |||||||
| 11 (9.6) | 21 (22.3) | 0.02 | |||||
| Number of episodes | |||||||
| 0.13 ± 0.43 | 0.31 ± 0.62 | 0.01 | |||||
| Kwok 200470 | Community nurse-supported discharge programme, N = 77 | UC, N = 80 | Number of A&E visits | 6 months | N = 70 | N = 79 | |
| Mean ± SD (median): 2.2 ± 2.4 (2) | Mean ± SD (median): 2.3 ± 3.1 (2) | 0.997 | |||||
| Wong 200574 | SM telephone followed up, N = 30 | Routine care, N = 30 | Frequency at 3 months (emergency room) | 1, 3 months | N = 30 | N = 30 | |
| Frequency 3 months, mean (SD) | |||||||
| 0.1 (0.3), median = 0 | 0.4 (0.7), median = 0 | 0.034 | |||||
Appendix 9 Health-related quality-of-life data from randomised controlled trials: review 1
| Study year | Brief | HRQoL description | Follow-up (months) | Baseline | Reassessment | Effect size/p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| Behnke 200064 | Home-based walking exercise programme, N = 23 | Control: advised to exercise, no instruction, N = 23 | CRQ total score CRQ subdomains |
3, 6 | n/N = 15/23 | n/N = 15/23 | n/N = 15/23 | n/N = 15/23 | < 0.01 |
| CRQ total score, mean ± SD | CRQ total score estimate from graph, mean ± SEM | ||||||||
| 79.3 ± 5.3 | 79.5 ± 5.0 | 3 months: 104 ± 3.0 | 3 months: 83 ± 4.0 | ||||||
| 6 months: 116 ± 2.0 | 6 months: 77 ± 6.0 | ||||||||
| CRQ subdomains (mean ± SEM) | |||||||||
| Dyspnoea: | |||||||||
| 13.2 ± 1.1 | 13.1 ± 1.0 | 3 months 22.1 ± 1.4 | 3 months 15.7 ± 1.6 | ||||||
| 6 months 25.3 ± 1.6 | 6 months 13.9 ± 1.7 | ||||||||
| Fatigue: | |||||||||
| 17 ± 1.5 | 15.3 ± 1.4 | 3 months 20.7 ± 1.1 | 3 months 15.7 ± 1.2 | ||||||
| 6 months 23.5 ± 0.8 | 6 months 14.5 ± 1.5 | ||||||||
| Emotion: | |||||||||
| 31.5 ± 2.6 | 33.1 ± 2.4 | 3 months 38.8 ± 2.1 | 3 months 33.7 ± 2.2 | ||||||
| 6 months 42.1 ± 1.7 | 6 months 31.9 ± 2.5 | ||||||||
| Mastery: | |||||||||
| 17.7 ± 1.3 | 17.9 ± 1.5 | 3 months 23.5 ± 1.2 | 3 months 18.9 ± 1.2 | ||||||
| 6 months 25.9 ± 0.6 | 6 months 17.5 ± 1.5 | ||||||||
| Egan 200269 | Case management, N = 33 | UC, N = 33 | SGRQ – total score Subdomains also given Subjective well-being score (higher score = increased well-being) |
1, 3 | n/N = 22/33 | n/N = 24/33 | |||
| SGRQ total | |||||||||
| 1 month, median change –1.6 | 1 month, median change –1.5 | 0.621 | |||||||
| 1–3 months, median change 0.6 | 1–3 months, median change –3.2 | 0.367 | |||||||
| Symptoms | |||||||||
| 1 month, median change –17.5 | 1 month, median change –9.3 | 0.384 | |||||||
| 1–3 months, median change 2.0 | 1–3 months, median change 0.5 | 0.959 | |||||||
| Activities | |||||||||
| 1 month, median change 0 | 1 month, median change 0.4 | 0.727 | |||||||
| 1–3 months, median change 0 | 1–3 months, median change –6.4 | 0.01 | |||||||
| Impacts | |||||||||
| 1 month, median change –0.2 | 1 month, median change –0.9 | 0.849 | |||||||
| 1–3 months, median change 2.5 | 1–3 months, median change –1.5 | 0.432 | |||||||
| SWB | |||||||||
| 1 months, median change 2.81 | 1 months, median change –2.8 | 0.416 | |||||||
| 3 months, median change –2.8 | 1–3 months, median change 0 | 0.268 | |||||||
| Hermiz 200267 | Home-based care focused on SM, N = 84 | UC, N = 93 | SGRQ – total score, and subdomains | 3 | n/N = 67/84 | n/N = 80/93 | |||
| SGRQ total, mean (SD) | SGRQ total, mean change (95% CI) | SGRQ total, MD (95% CI) | |||||||
| 63.71 (18.0) | 60.69 (17.8) | 4.33 (1.05 to 7.61) | 3.00 (0.24 to 5.77) | 1.32 (–2.97 to 5.62) | |||||
| Symptoms: | |||||||||
| 64.50 | 62.97 | –1.54 (–5.64 to 2.56) | –4.72 (–7.69 to 1.74) | 3.18 (–1.83 to 8.18) | |||||
| Activities: | |||||||||
| 79.29 | 75.54 | 4.46 (0.42 to 8.50) | 1.49 (–2.42 to 5.39) | 2.97 (–2.72 to 8.66) | |||||
| Impact: | |||||||||
| 54.57 | 51.52 | 6.09 (1.91 to 10.27) | 6.30 (2.91 to 9.68) | –0.21 (–5.57 to 5.16) | |||||
| Dheda 200473 | SM advice at regular hospital outpatient followed up, N = 15 | SM primary care followed up as required, N = 18 | SGRQ – total score Subdomain scores at 6 months SF-36 |
6 | n/N = 10/15 | n/N = 15/18 | Computed MD = 20.75; p = 0.004 SF-36 ‘trend to improvement, p = 0.067 |
||
| SGRQ total, mean change (SD) | |||||||||
| –20.98 (20.36) | +0.23 (12.55) | ||||||||
| From graph: | |||||||||
| Symptoms – final score, mean (SEM): | |||||||||
| 52 (8) | 75 (3) | ||||||||
| Activity – final score, mean (SEM): | |||||||||
| 76 (4) | 83 (4) | ||||||||
| Impact – final score, mean (SEM): | |||||||||
| 42 (5) | 61 (4) | ||||||||
| SF-36: | |||||||||
| No results provided | No results provided | ||||||||
| Behnke 200365 | Home-based walking exercise programme, N = 14 | Control: no instruction, N = 12 | CRQ total score | 3, 6, 12, 18 | N = 14 | N = 12 | N = 14 | N = 12 | |
| CRQ total score, months, estimated mean ± SEM: | CRQ total score, estimated mean (SEM): | ||||||||
| 79.3 ± 5.3 | 79.5 ± 5.0 | 3 months: 104 (2.0) |
3 months: 82 (4.0) |
||||||
| 6 months: 116 ± 9.0 12 months: 116 ± 8.0 18 months: 118 ± 7.0 |
6 months: 84 ± 8.0 12 months: 80 ± 8.0 18 months: 76 ± 6.0 |
||||||||
| CRQ (mean ± SEM) 0- or 6-month time point | CRQ (mean ± SEM) | ||||||||
| Dyspnoea: | |||||||||
| 13.1 ± 1.1 | 13.9 ± 1.1 | 3 months: (22.7 ± 1.4) | 3 months: (15.8 ± 1.9) | ||||||
| 6 months: (25.9 ± 1.6) | 6 months: (14.6 ± 2.0) | < 0.001 | |||||||
| 12 months: (25.4 ± 1.4) | 12 months: (12.8 ± 1.6) | < 0.001 | |||||||
| 18 months: (25.1 ± 1.5) | 18 months: (11.2 ± 1.3) | < 0.001 | |||||||
| Fatigue: | |||||||||
| 16.7 ± 1.6 | 16.7 ± 1.5 | 3 months: (20.8 ± 1.1) | 3 months: (16.4 ± 1.3) | ||||||
| 6 months: (23.6 ± 0.9) | 6 months: (15.9 ± 1.6) | < 0.01 | |||||||
| 12 months: (23.4 ± 1.0) | 12 months: (14.7 ± 1.5) | < 0.001 | |||||||
| 18 months: (23.7 ± 1.2) | 18 months: (12.8 ± 1.3) | < 0.001 | |||||||
| Emotion: | |||||||||
| 31.9 ± 2.7 | 35.3 ± 2.3 | 3 months: (39.9 ± 2.0) | 3 months: (34.9 ± 2.3) | ||||||
| 6 months: (42.6 ± 1.7) | 6 months: (33.7 ± 2.4) | < 0.01 | |||||||
| 12 months: (41.4 ± 1.8) | 12 months: (31.3 ± 2.2) | < 0.01 | |||||||
| 18 months: (41.1 ± 1.7) | 18 months: (29.9 ± 2.2) | < 0.001 | |||||||
| Mastery: | |||||||||
| 17.7 ± 1.4 | 19.0 ± 1.6 | 3 months: (23.8 ± 1.2) | 3 months: (19.3 ± 1.5) | ||||||
| 6 months: (26.2 ± 0.6) | 6 months: (18.4 ± 1.8) | < 0.001 | |||||||
| 12 months: (26.3 ± 0.7) | 12 months: (18.2 ± 1.7) | < 0.001 | |||||||
| 18 months: (27.3 ± 0.8) | 18 months: (17.3 ± 1.6) | < 0.001 | |||||||
| Hernandez 200368 | Home hospitalisation, N = 121 | UC, N = 101 | SGRQ – total score Short Form 12-item survey |
2 | N = 121 | N = 101 | N = 116 | N = 94 | |
| SGRQ total, mean ± SD | SGRQ total, 2 months; mean change: | ||||||||
| 58 ± 17 | 59 ± 20 | –6.9 | –2.4 | 0.05 | |||||
| SF-12 physical, mean ± SD | Symptoms, 2 months; mean change: | ||||||||
| 36 ± 8 | 34 ± 8 | –8.7 | –8.4 | ||||||
| SF-12 mental, mean ± SD | Activities, 2 months; mean change: | ||||||||
| 44 ± 12 | 44 ± 13 | –4.8 | –0.09 | ||||||
| Impacts, 2 months; mean change: | |||||||||
| –7.6 | –1.9 | 0.03 | |||||||
| SF-12-Physical, 2 months; mean change: | |||||||||
| 1.7 | 1.9 | ||||||||
| SF-12-Mental, 2 months; mean change: | |||||||||
| 2.0 | –0.05 | ||||||||
| Kwok 200470 | Community nurse-supported discharge programme, N = 77 | UC, N = 80 | GHQ | 6 | N = 67 | N = 73 | N = 67 | N = 73 | |
| GHQ, mean ± SD | |||||||||
| 7.1 ± 4.1 | 7.6 ± 4.9 | 7.5 ± 5.3 | 7.9 ± 5.2 | ||||||
| Garcia-Aymerich 200772 | Integrated care included supported SM, N = 44 | UC, N = 69 | SGRQ – total score Subdomains also given Euroqol |
12 | n/N = 21/44 | n/N = 41/69 | |||
| Total SGRQ, mean change (SD) | MD (95% CI) | ||||||||
| –13.41 (13.43) | –11.02 (15.57) | –2.39 (–10.56 to 5.78) | |||||||
| Symptoms, mean change (SD) | MD (95% CI) | ||||||||
| –24.4 (19.68) | –17.11 (24.44) | –7.29 (–19.66 to 5.07) | |||||||
| Activity, mean change (SD) | MD (95% CI) | ||||||||
| –5.08 (16.61) | –8.36 (19.95) | 3.27 (–6.91 to 13.46) | |||||||
| Impact, mean change (SD) | MD (95% CI) | ||||||||
| –13.7 (15.62) | –11.29 (16.34) | –2.41 (–11.24 to 6.42) | |||||||
| Euroqol, mean change (SD) | MD (95% CI) | ||||||||
| 1.56 (1.77) | 0.93 (2.11) | 0.62 (–051 to 1.75) | |||||||
| Bucknall 201263 | Supported SM, N = 232 | UC, N = 232 | SGRQ – total score and subdomains EQ-5D |
12 | N = 232 Total SGRQ 70.5 (16.7) |
N = 232 Total SGRQ 69.7 (16.1) |
SGRQ total, mean change (SD) | ||
| –2.99 (12.56), N = 69 | 1.38 (11.33), N = 53 | –4.52 (95% CI –9.07 to 0.04) | |||||||
| Symptom | |||||||||
| –6.01 (20.85), N = 116 | –4.16 (22.52), N = 90 | –2.17 (95% CI –7.80 to 3.46) | |||||||
| Activity | |||||||||
| 1.44 (13.27), N = 91 | 0.95 (11.05), N = 69 | 0.80 (95% CI –2.58 to 4.18) | |||||||
| Impact | |||||||||
| –3.19 (17.12), N = 78 | 4.23 (15.51), N = 63 | –6.89 (95% CI –12.40 to –1.39) | |||||||
| N (%) with four-point improvement | |||||||||
| 30/69 (43%) | 18/53 (34%) | OR = 1.71 (95% CI 0.75 to 3.89) | |||||||
| EQ-5D (AUC), mean (SD) | |||||||||
| 132.8 (95.5), N = 107 | 139.8 (100.3), N = 75 | –6.9 (95% CI –36.1 to 22.4) | |||||||
Appendix 10 Exercise outcome data from randomised controlled trials: review 1
| Study year | Brief | Exercise – description | Follow-up | Baseline | Reassessment | Effect size/p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| Behnke 200365 | Home-based walking exercise programme, N = 23 | Control, N = 23 | 6-minute treadmill distance | 3, 6, 12, 18 months Results averaged over 18-month trial period |
N = 14 | N = 12 | N = 14 | N = 12 | |
| Mean metres (SEM) | Mean (SEM), estimated | ||||||||
| 273 ± 97 | 226 ± 67 | 3 months: 473 ± 40 | 3 months: 217 ± 30 | ||||||
| 6 months: 493 ± 40 | 6 months: 227 ± 30 | ||||||||
| 12 months: 513 ± 39 | 12 months: 216 ± 30 | ||||||||
| 18 months: 532 ± 39 | 18 months: 177 ± 20 | ||||||||
| Average over 18 months | |||||||||
| Mean (95% CI): 518 (438 to 597) | Mean (95% CI): 208 (148 to 270) | < 0.001 | |||||||
| Kwok 200470 | Community nurse-supported discharge programme, N = 77 | UC, N = 80 | 6-MWD | 6 months | N = 71 | N = 75 | N = 67 | N = 73 | |
| Mean ± SD: 162 ± 79 | Mean ± SD: 145 ± 71 | Mean ± SD: 174 ± 98 | Mean ± SD: 150 ± 89 | ||||||
Appendix 11 Lung function data from randomised controlled trials: review 1
| Study year | Brief | Lung function description | Follow-up | Baseline | Reassessment | Effect size/p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Brief control | Intervention | Control | Intervention | Control | ||||
| Behnke 200064 | Home-based walking exercise programme, N = 23 | Control: advised to exercise, no instruction, N = 23 | FEV1, FVC | Day 11, 3, 6 months | N = 15 | N = 15 | N = 15 | N = 15 | |
| % predicted, mean ± SEM | Mean ± SEM | ||||||||
| FEV1 | |||||||||
| 34.1 ± 7.4 | 37.5 ± 6.6 | Day 11: 2.0 ± 0.07 | Day 11: 1.15 ± 0.11 | ||||||
| 0.99 ± 0.06 | 1.02 ± 0.07 | 3 months: 1.16 ± 0.08 | 3 months: 1.05 ± 0.08 | ||||||
| 6 months: 1.22 ± 0.11 | 6 months: 1.03 ± 0.08 | ||||||||
| FVC | |||||||||
| 72.0 ± 3.8 | 73.5 ± 3.4 | Day 11: 3.2 ± 0.2 | Day 11: 2.9 ± 0.2 | ||||||
| 2.8 ± 0.2 | 2.7 ± 0.2 | 3 months: 3.1 ± 0.2 | 3 months: 2.6 ± 0.2 | ||||||
| 6 months: 3.0 ± 0.1 | 6 months: 2.5 ± 0.2 | ||||||||
| Behnke 200365 | Home-based walking exercise programme, N = 23 | Control, N = 23 | FEV1 Averaged over 18-month period Measurement of FEV1 at time points |
18 months | N = 14 | N = 12 | N = 14 | N = 12 | No significant difference between groups although difference in the time course as indicated by interaction term; p = 0.0016 |
| FEV1 | |||||||||
| % predicted, mean ± SEM | Mean + 95% CI | ||||||||
| 34.9 ± 7.1 | 37.5 ± 6.9 | 41.6 (32.9 to 50.3) | 35.7 (29.5 to 41.9) | ||||||
| FEV1 (l) | |||||||||
| Mean ± SEM, estimated from graph | |||||||||
| 0.96 ± 1.2 | 1.0 ± 0.1 | Day 11 1.18 ± 0.1 | Day 11 1.08 ± 0.1 | ||||||
| 3 months: 1.12 ± 0.1 | 3 months: 1.02 ± 0.1 | ||||||||
| 6 months: 1.14 ± 0.1 | 6 months: 1.06 ± 0.1 | ||||||||
| 12 months: 1.15 ± 0.09 | 12 months: 1.00 ± 0.1 | ||||||||
| 18 months: 1.12 ± 0.09 | 18 months: 0.94 ± 0.09 | ||||||||
| Lee 200266 | Care protocol (including SM) to nursing home staff and patients, N = 48 completers | UC, N = 41 completers | % predicted FEV1 | 6 months | N = 48 | N = 41 | N = 48 | N = 41 | 0.329 |
| % predicted, mean (SD) | |||||||||
| 30.6 (10.1) | 34.0 (15.1) | 31.1 (13.3) | 30.6 (13.1) | ||||||
| Hernandez 200368 | Home-based hospitalisation, N = 121 | UC, N = 101 | FEV1, FVC, FEV1/FVC | 8 weeks | N = 121 | N = 101 | |||
| FEV 1 (l), mean ± SD (%) | |||||||||
| 2.4. ± 0.9 (64) | 2.2. ± 0.9 (60) | ||||||||
| FVC (l), mean ± SD (%) predicted | |||||||||
| 1.2 ± 0.6 (43) | 1.1 ± 0.4 (41) | ||||||||
| FEV 1 /FVC (%) | |||||||||
| 50 ± 13.3 | 50 ± 13.1 | ||||||||
| Garcia-Aymerich 200772 | Integrated care included supported SM, N = 44 | UC, N = 69 | FEV1 (l), FEV1/FVC (%) | 12 months | N = 21 | N = 41 | |||
| FEV 1 , median (IQR) | FEV 1 , mean change (SD) | MD (95% CI) | |||||||
| 1.2 (0.8–1.4) | 1.0 (0.8–1.5) | –0.01 (0.14) | 0.06 (0.35) | –0.05 (–0.24 to 0.14) | |||||
| FEV 1 /FVC%, mean (SD) | FEV 1 /FVC | MD (95% CI) | |||||||
| 48 (17) | 51 (18) | –0.82 (8.18) | –1.66 (17.94) | 0.84 (–8.27 to 10.66) | |||||
Appendix 12 Anxiety and depression outcome data from randomised controlled trials: review 1
| Study year | Brief | Anxiety/depression – description | Follow-up | Baseline | Reassessment | Effect size/p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| Egan 200269 | Case management, N = 33 | UC, N = 33 | Hospital anxiety and depression score | 1 months, 3 months | Median change, 1 month | ||||
| Anxiety: | |||||||||
| –1.0 (N = 25) | –2.5 (N = 26) | 0.437 | |||||||
| Depression: | |||||||||
| 0.5 (N = 26) | –1 (N = 27) | 0.383 | |||||||
| 1–3 months | |||||||||
| Anxiety: | |||||||||
| 0 (N = 24) | –1.5 (N = 24) | 0.764 | |||||||
| Depression: | |||||||||
| –0.5 (n = 24) | 0.5 (n = 24) | 0.325 | |||||||
| Lee 200266 | Care protocol (including SM) to nursing home staff and patients, N = 48 completers | UC, N = 41 completers | GHQ 28-item; 4 subscales: Somatic symptoms, anxiety and insomnia, social dysfunction, depression. Likert scale 0–3 Total score = 84 obtained by summation Lower score = better psychological well-being |
6 months | N = 48 | N = 41 | N = 48 | N = 41 | |
| Total, mean (SD) | |||||||||
| 24.44 (6.70) | 22.90 (8.82) | 18.38 (4.38) | 22.61 (8.19) | ||||||
| Somatic, mean (SD) | |||||||||
| 7.19 (2.69) | 6.76 (3.59) | 4.9 (2.10) | 6.1 (2.99) | ||||||
| A&I, mean (SD) | |||||||||
| 4.31 (2.60) | 3.54 (2.83) | 2.8 (1.52) | 4.3 (2.49) | ||||||
| Social, mean (SD) | |||||||||
| 8.10 (2.20) | 7.93 (1.46) | 7.2 (0.63) | 7.7 (1.51) | ||||||
| Depression, mean (SD) | |||||||||
| 4.83 (2.40) | 4.68 (3.31) | 3.3 (1.86) | 4.3 (2.66) | ||||||
| Kwok 200470 | Community nurse-supported discharge programme, N = 77 | UC, N = 80 | GHQ | 6 months | N = 77 GHQ Mean ± SD 7.5 ± 4.5 |
N = 80 GHQ Mean ± SD 7.5 ± 4.8 |
N = 67 GHQ Mean ± SD 7.5 ± 5.3 |
N = 73 GHQ Mean ± SD 7.9 ± 5.2 |
|
| Bucknall 201263 | Supported SM, N = 232 | UC, N = 232 | HADS | 12 months | N = 232 | N = 232 | |||
| Anxiety, mean (SD) | Effect (95% CI) | ||||||||
| 10.00 (4.5) | 9.3 (4.6) | –0.37 (3.77), N = 104 | 0.93 (3.29), N = 82 | –1.06 (–2.08 to –0.03); p = 0.044 | |||||
| Depression, mean (SD) | Effect (95% CI) | ||||||||
| 8.5 (3.9) | 8.3 (4.1) | 0.54 (3.26), N = 109 | 0.75 (2.78), N = 84 | –0.27 (–1.13 to 0.59); p = 0.538 | |||||
Appendix 13 Dyspnoea outcome data from randomised controlled trials: review 1
| Study year | Brief | Dyspnoea description | Follow-up | Baseline | Reassessment | Effect size/p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| Behnke 200064 | Home-based walking exercise programme, N = 23 | Control: advised to exercise, no instruction, N = 23 | Baseline/transitional Dyspnoea Index (functional impairment, magnitude of effort, magnitude of task) Lower scores indicate greater impairment |
Day 11, 1, 2, 3 and 6 months | N = 15 Mean (SD) BDI 3.9 ± 0.6 |
N = 15 Mean (SD) BDI 3.8 ± 0.4 |
N = 15 | N = 15 | |
| Focal transitional score reported, mean change (SEM) | |||||||||
| Day 11: +6.9 ± 0.6 | Day 11: +3.1 ± 0.8 | < 0.001 | |||||||
| 1 month, estimated: +5.4 ± 0.9 | 1 month, estimated: +2.0 ± 0.7 | ||||||||
| 2 months, estimated: +4.5 ± 0.7 | 2 months, estimated: +0.3 ± 0.7 | ||||||||
| 3 months: +4.6 ± 0.7 | 3 months: +0.3 ± 0.9 | < 0.001 | |||||||
| 6 months: +4.4 ± 0.8 | 6 months: –2.8 ± 1.1 | < 0.001 | |||||||
|
Dyspnoea domain of CRQ
Lower scores indicate greater impairment |
3 and 6 months | 13.2 ± 1.1 | 13.1 ± 1.0 | 3 months: 22.1 ± 1.4 | 3 months: 15.7 ± 1.6 | < 0.001 | |||
| 6 months: 25.3 ± 1.6 | 6 months: 13.9 ± 1.7 | < 0.001 | |||||||
| Behnke 200365 | Home-based walking exercise programme, N = 23 | Control, N = 23 | N = 14, 2.4 ± 1.1 | N = 12, 2.5 ± 1.4 | N = 14 | N = 12 | |||
| Borg scale at rest | Results averaged over 18-month trial period | Mean (95% CI) | |||||||
| 0.7 (0.2 to 1.3) | 2.1 (1.4 to 2.9) | < 0.01 | |||||||
| 3, 6, 12, 18 months | Mean ± SEM, estimated from graph | ||||||||
| 3 months: 0.7 ± 0.3 | 3 months: 1.9 ± 0.3 | ||||||||
| 6 months: 0.7 ± 0.3 | 6 months: 1.9 ± 0.4 | ||||||||
| 12 months: 0.6 ± 0.3 | 12 months: 2.1 ± 0.3 | ||||||||
| 18 months: 0.9 ± 0.3: | 18 months: 2.7 ± 0.4 | ||||||||
| Baseline/transitional Dyspnoea Index (functional impairment, magnitude of effort, magnitude of task) | Results averaged over 18-month trial period | Mean (95% CI) | < 0.05 | ||||||
| 4.4 (4.4 to 2.9) | –3.9 (–5.4 to –0.7) | ||||||||
| 3, 6, 12, 18 months | Mean ± SEM, estimated from graph | ||||||||
| 3.9 ± 2.2 | 3.8 ± 1.4 | 3 months: 5.0 ± 0.9 | 3 months: 0.7 ± 0.9 | ||||||
| 6 months: 4.8 ± 0.9 | 6 months: –2.4 ± 1.5 | ||||||||
| 12 months: 4.4 ± 0.9 | 12 months: –2.8 ± 1.0 | ||||||||
| 18 months: 4.5 ± 0.9 | 18 months: –4.2 ± 1.0 | ||||||||
|
Dyspnoea domain of CRQ
Lower scores indicate greater impairment |
3, 6, 12, 18 months | 13.1 ± 1.1 | 13.9 ± 1.1 | 3 months: 22.7 ± 1.4 | 3 months: 15.8 ± 1.9 | ||||
| 6 months: 25.9 ± 1.6 | 6 months: 14.6 ± 2.0 | ||||||||
| 12 months: 25.4 ± 1.4 | 12 months: 12.8 ± 1.6 | ||||||||
| 18 months; 25.1 ± 1.5 | 18 months: 11.2 ± 1.3 | ||||||||
| Garcia-Aymerich 200772 | Integrated care included supported SM, N = 44 | UC, N = 69 | MRC Dyspnoea Scale | 12 months | Median (IQR) | N = 21 | N = 41 | Effect size estimated using linear regression: –0.38 (95% CI –1.1 to 0.34) |
|
| 3 (3–4) | 4 (3–5) | Mean change (SD) | |||||||
| –0.52 (1.12) | –0.15 (1.44) | ||||||||
Appendix 14 Behaviour change outcomes data from randomised controlled trials: review 1
| Study year | Brief | Behaviour change outcome – description | Follow-up | Baseline | Reassessment | Effect size/p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| Hermiz 200267 | Home-based care focused on SM, N = 84 | UC, N = 93 | Behaviour change as measured by
|
3 months | N = 67 | N = 80 | n/N (%) | n/N (%) | |
| Smoking | |||||||||
| 15/67 (22) | 26/80 (33) | 0.17 | |||||||
| Influenza vaccination | |||||||||
| 48/67 (72) | 60/80 (75) | 0.65 | |||||||
| Pneumococcal vaccination | |||||||||
| 42/67 (63) | 42/80 (53) | 0.28 | |||||||
| Knowledge including name of condition, role of vaccination, awareness of condition, when to seek help | Name of disease | Test of difference (p-value) | |||||||
| 36 (54) | 26 (33) | 5.9 (0.04) | |||||||
| Role of vaccination | |||||||||
| 41 (61) | 16 (20) | 26.1 (< 0.01) | |||||||
| Factors that prevent worsening of condition | |||||||||
| 26 (39) | 10 (13) | 21.9 (< 0.01) | |||||||
| When to seek help | |||||||||
| 57 (85) | 55 (69) | 7.8 (0.07) | |||||||
| Hernandez, 200268 | Home-based hospitalisation, N = 121 | UC, N = 101 | Disease knowledge | Disease knowledge | |||||
| 58% | 27% | < 0.01 | |||||||
| Compliance on inhalation technique | Compliance on inhalation technique | ||||||||
| 81% | 48% | < 0.001 | |||||||
| Rehabilitation at home | Rehabilitation at home | ||||||||
| No details about questionnaires/measures used | 51% | 21% | < 0.01 | ||||||
| Garcia-Aymerich 200772 | Integrated care included supported SM, N = 44 | UC, N = 69 | Life-style factors, SM, medical treatment | 12 months | N (%), (N = 21) | n (%), (N = 41) | |||
| Current smokers | |||||||||
| 5 (24) | 6 (15) | 0.349 | |||||||
| Physical activity | |||||||||
| 18 (86) | 34 (83) | 0.778 | |||||||
| Regular walking/exercise | |||||||||
| 18 (86) | 32 (78) | 0.470 | |||||||
| Knowledge about name of disease | |||||||||
| 17 (81) | 18 (44) | 0.005 | |||||||
| Identification of exacerbation | |||||||||
| 17 (85) | 9 (22) | < 0.001 | |||||||
| Early treatment of an exacerbation | |||||||||
| 19 (90) | 27 (66) | 0.036 | |||||||
| Adherence to oral treatment (MAS) | |||||||||
| 19 (90) | 35 (85) | 0.570 | |||||||
| Adherence to inhaled treatment (IAS scale) | |||||||||
| 15 (71) | 15 (37) | 0.009 | |||||||
| Correct inhaler manoeuvre | |||||||||
| 18 (86) | 9 (24) | < 0.001 | |||||||
| Influenza vaccination | |||||||||
| 19 (90) | 32 (78) | 0.442 | |||||||
| Pneumococcal vaccination | |||||||||
| 16 (76) | 25 (61) | 0.348 | |||||||
| Bucknall 201263 | Supported SM, N = 232 | UC, N = 232 | Initiating treatment for an exacerbation (successful SM) | 12 months | 75/180 (42%) | Not available | |||
Appendix 15 Self-efficacy outcome data from randomised controlled trials: review 1
| Study year | Brief | Self-efficacy outcome – description | Follow-up | Baseline | Reassessment | Effect size/p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| Wong 200574 | SM telephone followed up, N = 30 | Routine care, N = 30 | Modified Chinese Self Efficacy Scale: 31 items with five subscales measured using five-point Likert Scale:
|
3 months | N = 30 | N = 30 | |||
| Median (IQR): | |||||||||
| Negative affect | |||||||||
| 3.9 (0.9) | 3.8 (0.9) | 4.1 (0.7) | 3.9 (1.0) | 0.260 | |||||
| Intense emotional arousal | |||||||||
| 4.0 (0.6) | 3.9 (0.9) | 4.3 (0.6) | 4.0 (0.8) | 0.342 | |||||
| Physical exertion | |||||||||
| 3.3 (1.0) | 3.2 (1.4) | 3.9 (1.0) | 3.1 (1.0) | 0.001 | |||||
| Weather or environment | |||||||||
| 3.7 (0.7) | 3.7 (0.8) | 4.0 (0.8) | 3.7 (1.2) | 0.009 | |||||
| Behavioural risk factors | |||||||||
| 4.0 (0.8) | 4.0 (0.6) | 4.1 (1.0) | 4.0 (1.1) | 0.901 | |||||
| Total | |||||||||
| 3.8 (0.6) | 3.6 (0.8) | 4.0 (0.6) | 3.8 (1.0) | 0.009 | |||||
| Bucknall 201263 | Supported SM, N = 232 | UC N = 232 | COPD self-efficacy score Higher score = more confident |
12 months | N = 232 | N = 232 | 119/232 (48%) | 94/232 (47%) | Effect (95% CI): 2.65 (–5.85 to 11.14); p = 0.540 |
| Mean (SD) | Mean change | ||||||||
| 68.2 (27.5) | 69.8 (25.5) | –1.73 (34.04) | –5.55 (33.72) | ||||||
Appendix 16 Patient satisfaction outcome data from randomised controlled trials: review 1
| Study year | Brief | Satisfaction – description | Follow-up | Baseline | Reassessment | Effect size/p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| Hermiz 200267 | Home-based care focused on SM N = 84 |
UC, N = 93 | Patient satisfaction with GP care | 3 months | N = 67, 56/60 (93%) | N = 80, 72/75 (96%) | |||
| Lee, 200266 | Care protocol (including SM) to nursing home staff and patients, N = 48 completers | UC, N = 41 completers | Patient satisfaction 13 items with 1–5 Likert response 10 items: satisfaction about nursing home care 3 items: satisfaction about community nurse care Higher score = high satisfaction |
6 months | N = 48 | N = 41 | Significant (< 0.001) increased level of satisfaction in the intervention arm with care provided by nursing home staff | ||
| Hernandez 200368 | Home hospitalisation, N = 121 | UC, N = 101 | Patient satisfaction Questionnaire given, no details provided |
8 weeks | N = 121, mean score 8 |
N = 101, mean score 7.5 |
0.03 | ||
| Garcia-Aymerich 200772 | Integrated care included supported SM, N = 44 | UC, N = 69 | Health-care satisfaction | 12 months | N = 21 | N = 41 | 0.180 | ||
| Satisfaction with health care, n (%) | |||||||||
| 21 (100) | 34 (92) | ||||||||
Appendix 17 Search strategies for cost-effectiveness studies: review 3
MEDLINE (via Ovid)
URL: https://ovid.sp.com
Date range searched: 1946 to May week 1 2012.
Date of search: 15 May 2012.
Search strategy
-
chronic obstructive pulmonary disease.mp. or exp Pulmonary Disease, Chronic Obstructive/
-
copd.ti,ab.
-
chronic obstructive lung disease.ti,ab.
-
chronic obstructive airway disease.ti,ab
-
chronic respiratory disorder$.ti,ab.
-
smoking-related lung disease$.ti,ab.
-
Pulmonary Emphysema/
-
exp Bronchitis/
-
emphysema.ti,ab.
-
or/1-9
-
exp Self Care/
-
(self adj2 (support$ or care or caring or manage$)).ti,ab.
-
post discharge.ti,ab.
-
early discharge.ti,ab.
-
home care.ti,ab.
-
home care services/ or home nursing/
-
patient centred care.ti,ab.
-
patient centered care.ti,ab.
-
patient education/ or patient education.ti,ab.
-
patient participation.ti,ab.
-
post hospital care.ti,ab.
-
action planning.ti,ab.
-
discharge planning.ti,ab.
-
continuity of patient care/
-
(support$ adj2 discharge).ti,ab.
-
(support$ adj2 manag$).ti,ab.
-
patient focus$.ti,ab.
-
management plan$.ti,ab.
-
management program$.ti,ab.
-
rehabilitation.mp. or exp Rehabilitation/
-
or/11-30
-
10 and 31
-
economics/
-
exp ‘costs and cost analysis’/
-
cost of illness/
-
exp health care costs/
-
economic value of life/
-
exp economics medical/
-
exp economics hospital/
-
economics pharmaceutical/
-
exp ‘fees and charges’/
-
(econom$ or cost or costs or costly or costing or price or pricing or pharmacoeconomic$).tw.
-
(expenditure$ not energy).tw.
-
(value adj1 money).tw.
-
budget$.tw.
-
or/33-45
-
32 and 46
EMBASE (via Ovid)
URL: https://ovidsp.ovid.com
Date range searched: 1980 to 2012 week 19.
Date of search: 15 May 2012.
Search strategy
-
chronic obstructive pulmonary disease.mp. or exp chronic obstructive lung disease/
-
copd.ti,ab.
-
chronic obstructive lung disease.ti,ab.
-
chronic obstructive airway disease.ti,ab.
-
chronic respiratory disorder$.ti,ab.
-
smoking-related lung disease$.ti,ab.
-
pulmonary emphysema.mp. or exp lung emphysema/
-
emphysema.ti,ab.
-
bronchitis.mp. or exp bronchitis/
-
or/1-9
-
self care.mp. or exp self care/
-
(self adj2 (support$ or care or caring or manage$)).ti,ab.
-
post discharge.ti,ab.
-
early discharge.ti,ab.
-
exp home care/
-
home nursing.ti,ab.
-
patient centred care.ti,ab.
-
patient centered care.ti,ab.
-
patient education/
-
patient education.ti,ab.
-
patient participation.ti,ab.
-
post hospital care.ti,ab.
-
action planning.ti,ab.
-
discharge planning.ti,ab.
-
continuity of patient care.ti,ab.
-
(support$ adj2 discharge).ti,ab.
-
(support$ adj2 manage$).ti,ab.
-
patient focus$.ti,ab.
-
management plan$.ti,ab.
-
management program$.ti,ab.
-
rehabilitation.mp. or exp rehabilitation/
-
or/11-31
-
10 and 32
-
cost benefit analysis/
-
cost effectiveness analysis/
-
cost minimization analysis/
-
cost utility analysis/
-
economic evaluation/
-
(cost or costs or costed or costly or costing).tw.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
(technology adj assessment$).tw.
-
or/34-41
-
33 and 42
The Cochrane Library Cochrane (Wiley) NHS Economic Evaluation Database
URL: https://cochranelibrary.com
Date range searched: 1993–2012 issue 4 of 12.
Date of search: 15 May 2012.
Search strategy
#1 copd
#2 chronic next obstructive next pulmonary disease
#3 MeSH descriptor Pulmonary Disease, Chronic Obstructive explode all trees
#4 chronic next obstructive next airway next disease
#5 chronic next respiratory next disorder*
#6 smoking next related next lung next disease*
#7 emphysema
#8 MeSH descriptor Pulmonary Emphysema explode all trees
#9 MeSH descriptor Bronchitis explode all trees
#10 bronchitis
#11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
#12 self next care
#13 MeSH descriptor Self Care explode all trees
#14 self near/2 ( support* or care or caring or manage*)
#15 post next discharge
#16 early next discharge
#17 MeSH descriptor Home Care Services explode all trees
#18 home next nursing
#19 patient next centred next care
#20 patient next centered next care
#21 MeSH descriptor Patient Education as Topic explode all trees
#22 patient next education
#23 patient next participation
#24 post next hospital next care
#25 action next planning
#26 discharge next planning
#27 continuity near/1 patient
#28 support* near/2 discharge
#29 support* near/2 manage*
#30 patient next focus*
#31 management next plan*
#32 management next program*
#33 rehabilitation
#34 MeSH descriptor Rehabilitation explode all trees
#35 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34)
#36 (#11 AND #35)
MEDLINE (via Ovid)
URL: https://ovidsp.ovid.com
Date range searched: 1946 to May week 1 2012.
Date of search: 15 May 2012.
Search strategy
-
chronic obstructive pulmonary disease.mp. or exp Pulmonary Disease, Chronic Obstructive/
-
copd.ti,ab.
-
chronic obstructive lung disease.ti,ab.
-
chronic obstructive airway disease.ti,ab.
-
chronic respiratory disorder$.ti,ab.
-
smoking-related lung disease$.ti,ab.
-
Pulmonary Emphysema/
-
exp Bronchitis/
-
emphysema.mp.
-
or/1-9
-
exp Self Care/
-
(self adj2 (support$ or care or caring or manage$)).ti,ab.
-
post discharge.ti,ab.
-
early discharge.ti,ab.
-
home care.ti,ab.
-
home care services/ or home nursing/
-
patient centred care.ti,ab.
-
patient centered care.ti,ab.
-
patient education/ or patient education.ti,ab.
-
patient participation.ti,ab.
-
post hospital care.ti,ab.
-
action planning.ti,ab.
-
discharge planning.ti,ab.
-
continuity of patient care/
-
(support$ adj2 discharge).ti,ab.
-
(support$ adj2 manag$).ti,ab.
-
patient focus$.ti,ab.
-
management plan$.ti,ab.
-
management program$.ti,ab.
-
rehabilitation.mp. or exp Rehabilitation/
-
or/11-30
-
10 and 31
-
decision support techniques/
-
markov.ti,ab.
-
exp models economic/
-
decision analysis.ti,ab.
-
cost benefit analysis/
-
or/33-37
-
32 and 38
Appendix 18 Chronic obstructive pulmonary disease-adjusted all-cause mortality rates by age and sex: review 3
Appendix 18 lists the COPD-adjusted all-cause mortality rates applied in the economic model. These were derived from all-cause and COPD-related mortality rates by sex and age for a UK population, obtained from the Office for National Statistics.
| Age (years) | All-cause mortality (%) | Deaths caused by COPD (%) | COPD-adjusted mortality (%) | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| 60 | 0.8342 | 0.5361 | 4.205241 | 5.111524 | 0.7828 | 0.509483 |
| 61 | 0.8871 | 0.581 | 0.8325 | 0.552154 | ||
| 62 | 0.9507 | 0.6165 | 0.8921 | 0.585891 | ||
| 63 | 1.0509 | 0.6812 | 0.9862 | 0.647379 | ||
| 64 | 1.1558 | 0.7478 | 1.0846 | 0.710672 | ||
| 65 | 1.2725 | 0.8201 | 6.184986 | 7.25799 | 1.1941 | 0.779383 |
| 66 | 1.4205 | 0.9119 | 1.333 | 0.866625 | ||
| 67 | 1.5369 | 0.9737 | 1.4422 | 0.925356 | ||
| 68 | 1.7243 | 1.0949 | 1.6181 | 1.040539 | ||
| 69 | 1.9125 | 1.2158 | 1.7947 | 1.155436 | ||
| 70 | 2.1149 | 1.3856 | 1.9846 | 1.316806 | ||
| 71 | 2.3225 | 1.4768 | 2.1794 | 1.403478 | ||
| 72 | 2.5652 | 1.6469 | 2.4072 | 1.565133 | ||
| 73 | 2.7907 | 1.8063 | 2.6188 | 1.716619 | ||
| 74 | 3.1141 | 2.0492 | 2.9223 | 1.947459 | ||
| 75 | 3.3999 | 2.2567 | 6.654892 | 6.476441 | 3.1905 | 2.144656 |
| 76 | 3.8443 | 2.5538 | 3.6075 | 2.427006 | ||
| 77 | 4.2217 | 2.8839 | 3.9616 | 2.740716 | ||
| 78 | 4.7005 | 3.2547 | 4.4109 | 3.093106 | ||
| 79 | 5.2482 | 3.6732 | 4.9249 | 3.490828 | ||
| 80 | 5.944 | 4.1742 | 5.5778 | 3.966954 | ||
| 81 | 6.6343 | 4.662 | 6.2256 | 4.430535 | ||
| 82 | 7.4283 | 5.3215 | 6.9707 | 5.057291 | ||
| 83 | 8.1907 | 6.0585 | 7.6861 | 5.7577 | ||
| 84 | 9.2142 | 6.7739 | 8.6466 | 6.437581 | ||
| 85 | 10.2895 | 7.5849 | 5.575312 | 3.413472 | 9.6556 | 7.208315 |
| 86 | 11.2992 | 8.5749 | 10.6031 | 8.149162 | ||
| 87 | 12.7193 | 9.5838 | 11.9358 | 9.107971 | ||
| 88 | 14.0875 | 10.779 | 13.2197 | 10.24383 | ||
| 89 | 16.0713 | 12.1602 | 15.0813 | 11.55645 | ||
| 90 | 16.6367 | 13.5352 | 15.6118 | 12.86319 | ||
| 91 | 17.8196 | 14.6525 | 16.7219 | 13.92501 | ||
| 92 | 18.8878 | 16.0748 | 17.7243 | 15.2767 | ||
| 93 | 21.4681 | 18.0517 | 20.1456 | 17.15545 | ||
| 94 | 23.7662 | 20.2789 | 22.3021 | 19.27207 | ||
| 95 | 25.6292 | 22.3947 | 24.0504 | 21.28282 | ||
| 96 | 27.5704 | 24.0167 | 25.872 | 22.82429 | ||
| 97 | 29.48 | 25.97 | 27.67 | 24.68 | ||
| 98 | 31.56 | 27.82 | 29.61 | 26.44 | ||
| 99 | 32.73 | 29.69 | 30.71 | 28.22 | ||
| 100 | 34.46 | 31.82 | 32.34 | 30.24 | ||
Appendix 19 Annual disease progression risks by age and smoking status: review 3
Appendix 19 lists the annual disease progression rates applied in the economic model. These were obtained from a published COPD Markov model by Atsou et al. 86
| Age | GOLD stage 2 to 3 | GOLD stage 3 to 4 | GOLD stage 2 to 3 | GOLD stage 3 to 4 |
|---|---|---|---|---|
| Ex-smoker (%) | Ex-smoker (%) | Smoker (%) | Smoker (%) | |
| 60 | 5.803 | 5.12 | 9.338 | 7.823 |
| 61 | 5.926 | 5.229 | 9.535 | 7.989 |
| 62 | 6.049 | 5.338 | 9.733 | 8.155 |
| 63 | 6.104 | 5.386 | 9.822 | 8.229 |
| 64 | 6.159 | 5.434 | 9.912 | 8.304 |
| 65 | 6.213 | 5.482 | 10.001 | 8.379 |
| 66 | 6.268 | 5.53 | 10.091 | 8.454 |
| 67 | 6.322 | 5.579 | 10.18 | 8.529 |
| 68 | 6.367 | 5.618 | 10.252 | 8.589 |
| 69 | 6.412 | 5.658 | 10.324 | 8.65 |
| 70 | 6.457 | 5.698 | 10.396 | 8.71 |
| 71 | 6.502 | 5.737 | 10.468 | 8.77 |
| 72 | 6.547 | 5.777 | 10.54 | 8.831 |
| 73 | 6.561 | 5.789 | 10.562 | 8.849 |
| 74 | 6.575 | 5.801 | 10.584 | 8.868 |
| 75 | 6.589 | 5.814 | 10.607 | 8.887 |
| 76 | 6.603 | 5.826 | 10.629 | 8.905 |
| 77 | 6.617 | 5.838 | 10.651 | 8.924 |
| 78 | 6.638 | 5.857 | 10.686 | 8.953 |
| 79 | 6.659 | 5.876 | 10.72 | 8.982 |
| 80 | 6.681 | 5.895 | 10.755 | 9.011 |
| 81 | 6.702 | 5.914 | 10.789 | 9.04 |
| 82 | 6.724 | 5.933 | 10.824 | 9.069 |
| 83 | 6.792 | 5.993 | 10.935 | 9.161 |
| 84 | 6.861 | 6.054 | 11.045 | 9.254 |
| 85 | 6.93 | 6.114 | 11.156 | 9.347 |
| 86 | 6.998 | 6.175 | 11.266 | 9.439 |
| 87 | 7.067 | 6.236 | 11.377 | 9.532 |
| 88 | 7.136 | 6.296 | 11.487 | 9.624 |
| 89 | 7.204 | 6.357 | 11.598 | 9.717 |
| 90 | 7.273 | 6.417 | 11.708 | 9.81 |
| 91 | 7.342 | 6.478 | 11.819 | 9.902 |
| 92 | 7.41 | 6.538 | 11.929 | 9.995 |
| 93 | 7.479 | 6.599 | 12.04 | 10.088 |
| 94 | 7.547 | 6.659 | 12.15 | 10.18 |
| 95 | 7.616 | 6.72 | 12.261 | 10.273 |
| 96 | 7.685 | 6.781 | 12.372 | 10.365 |
| 97 | 7.753 | 6.841 | 12.482 | 10.458 |
| 98 | 7.822 | 6.902 | 12.593 | 10.551 |
| 99 | 3.61 | 7.891 | 12.703 | 10.643 |
| 100 | 3.61 | 7.891 | 12.703 | 10.643 |
Appendix 20 Cost of other self-management programmes in populations with chronic obstructive pulmonary disease: review 3
Appendix 20 lists cost estimates extracted from SM programmes targeted at patients with COPD, not provided within 6 weeks of discharge. Costs are listed in the year and currency they were reported and also 2012 GB pound sterling (£) prices. Costs were converted using mid-year exchange rates for the reporting year and inflated assuming an average inflation rate of 3.5%.
| Author | Type of programme | Main activities | Cost, year, currency (as reported) | Costs 2012, GB£ (estimated) |
|---|---|---|---|---|
| Khdour 2011279 | Pharmacy-led SM programme | A consultation with a pharmacist, lasting 1 hour Two follow-up telephone calls lasting 20 minutes Two follow-up consultations |
381, 2006, GB£ | 458 |
| Sridhar 2008155 | Nurse-led intermediate care programme | A 1-hour group session A home visit by a respiratory nurse A follow-up telephone call |
107, 2006, GB£ | 129 |
| Dewan 2012299 | Disease management programme | A group session lasting 1.5 hours Development of an action plan Provision of a refillable prescriptions Access to helpline Series of follow-up telephone calls |
849, 2011, US$ | 544 |
| Monninkhof 2004300 | SM programme | Five group sessions lasting 2 hours Provision of education booklet Two group training sessions lasting 1 hour |
642, 2002, euros | 508 |
| Tinkelman 2003301 | Disease management programme | A telephone education session Ongoing access to case management support via telephone service Series of follow-up telephone calls Reassessment at 6 months |
635, 2002, US$ | 573 |
Appendix 21 Outcomes as reported by studies included for review 4 but not included in analyses
| Studies | Mortality | Anxiety | Depression | Exercise capacity | Lung function | Health service utilisation | ED visits | Dyspnoea | Last follow-up (weeks) |
|---|---|---|---|---|---|---|---|---|---|
| Alexander 2008302 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 10.0 |
| Ambrosino 1981303 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 4.3 |
| Bauldoff 1996304 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 8.0 |
| Belman 1988305 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 5.0 |
| Berry 1996306 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 12.0 |
| Berry 2003307 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Bjerre-Jepsen 1981308 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 6.0 |
| Borghi-Silva 2009309 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Bosch 2007310 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | – |
| Carrieri-Kohlman 1996311 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | – |
| Carrieri-Kohlman 2001312 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 12.0 |
| Casaburi 2004313 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 10.0 |
| Chen 1985314 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 4.0 |
| Clark 1996315 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 12.0 |
| Clark 2000316 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 12.0 |
| Cooper 2009317 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 8.0 |
| Coppoolse 1999318 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 8.0 |
| Costi 2009319 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 26.0 |
| de Godoy 2003320 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 12.0 |
| Dekhuijzen 1991321 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 10.0 |
| Epstein 1997322 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 8.0 |
| Esteve 1996323 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4.0 |
| Falk 1985324 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 13.0 |
| Gallefoss 1999325 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 52.0 |
| Cabedo Garcia 2010326 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | – |
| Gift 1992327 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 4.0 |
| Gormley 1993328 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4.0 |
| Harver 1989329 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 8.0 |
| Heijdra 1996330 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 10.0 |
| Hoff 2007331 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 8.0 |
| Incalzi 2008332 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 26.0 |
| Izumizaki 2008333 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Jin 2002334 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Jones 1985335 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 10.0 |
| Kheirabadi 2008336 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13.0 |
| Kongsgaard 2004337 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 12.0 |
| Kirsten 1998338 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1.6 |
| Kurabayashi 2000339 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 8.7 |
| Ruiz de Ona Lacasta 2004340 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 52.0 |
| Lake 1990341 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 8.0 |
| Levine 1986342 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 6.0 |
| Lisboa 1995343 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | – |
| Lisboa 1997344 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 10.0 |
| Louie 2004345 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | – |
| Marrara 2008346 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 6.0 |
| Martinez 1993347 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 10.0 |
| McKeon 1986348 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 6.0 |
| Mehri 2007349 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4.0 |
| Mendes de Oliveira 2010350 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 12.0 |
| Nasis 2009351 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 10.0 |
| Nava 1998352 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Noseda 1987353 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 8.7 |
| Nosworthy 1993354 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 13.0 |
| Phillips 2006355 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 8.0 |
| Preusser 1994356 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 13.0 |
| Puente-Maestu 2000357 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 8.0 |
| Ramirez-Sarmiento 2002358 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 5.0 |
| Reardon 1994359 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 6.0 |
| Ries 1986360 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 6.0 |
| Ries 1988361 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 8.0 |
| Saunders 1965362 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 13.0 |
| Savci 2000363 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 4.0 |
| Singh 2009364 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | – |
| Spohn 2002365 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| Strijbos 1996366 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 12.0 |
| Strijbos 1996367 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Su 2007368 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 4.0 |
| Tandon 1978369 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 39.0 |
| Tiep 1986370 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | – |
| Toshima 1992371 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 52.0 |
| Vallet 1994372 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Vallet 1997373 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 4.0 |
| Varga 2007374 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 8.0 |
| Vogiatzis 2005375 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 10.0 |
| Wanke 1994376 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 8.0 |
| Weiner 1992377 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 26.0 |
| Weiner 2000378 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 22.0 |
| Weiner 2003379 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 13.0 |
| Weiner 2003380 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 13.0 |
| Weiner 2004381 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Weiner 2006382 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 8.0 |
| Wen 2004383 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Wen 2008384 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Wijkstra 1996385 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 12.0 |
| Wijkstra 1996386 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Wolkove 2004387 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 4.3 |
| Wurtemberger 2001388 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | – |
| Xie 2003389 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 12.0 |
| Yan 1996390 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 20.0 |
Appendix 22 Summary of characteristics of population and study information: review 4
| Author, year, setting | Sample size | Age, mean (SD) | Males% | FEV1% (SD) | Recruited from | Intervention details | Control details | Follow-up (weeks) | QoL | Hospital (re)admissions | Exacerbations | Mortality | Anxiety | Depression | Exercise capacity | Lung function | Health service utilisation | ED visits | Dyspnoea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aimonino Ricauda 2008 Italy75 | 104 | 79.7 (3.2) | 65 | 42.5 | ED | Hospital at home | Inpatient care | 26 | Y | Y | N | Y | N | Y | N | N | Y | N | N |
| Arnardottir 2006 Sweden216 | 63 | 66. 6 (2) | 50 | 37.5 (2.5) | Secondary care outpatient | Endurance, resistance training and calisthenics | Resistance training and calisthenics | 52 | Y | N | N | N | Y | Y | Y | Y | N | N | Y |
| Arnardottir 2007 Sweden217 | 100 | 64.5 (7.6) | 15 | 33.4 (11.5) | PR programme | Interval training | Continuous training | 16 | Y | N | N | N | Y | Y | Y | Y | N | N | Y |
| Barakat 2008 France249 | 80 | 64.8 (11.1) | 84 | 42.6 (3.1) | Secondary care outpatient | PR | Control | 14 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Bauldoff 2002 USA108 | 24 | 68.1 (8.0) | 17 | 41.3 (13.0) | PR programme | Music (distractive auditory stimulation) | Control | 8 | Y | N | N | N | Y | Y | Y | N | N | N | Y |
| Bauldoff 2005 USA109 | 30 | 63.0 (11) | 43 | 41.3 (18) | Secondary care outpatient | (1) Moderate distractive auditory stimulation during exercise; and (2) slow distractive auditory stimulation during exercise | Attention control | 4 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Beckerman 2005 Israel253 | 42 | 67.3 (15.8) | 76 | 42.5 (11.7) | Community | IMT | Sham training | 52 | Y | Y | N | N | N | N | Y | Y | Y | N | Y |
| Behnke 2000, Behnke 2003, Germany64,65 | 46 | 66.0 (2.1) | 77 | 36.0 (7.0) | Secondary care inpatient | Home-based exercise | Control | 26, 78 | Y | Y | N | N | N | N | Y | Y | Y | N | Y |
| Bendstrup 1997 Denmark222 | 47 | 64.5 (2.5) | 88 | NR | Secondary care | PR | Control | 24 | Y | N | N | N | N | N | Y | Y | N | N | N |
| Bernard 1999 Canada191 | 45 | 65.3 (7.9) | 78 | 42.5 (13.8) | NR | Aerobic and strength training | Aerobic training | 12 | Y | N | N | N | N | N | Y | N | N | N | N |
| Berry 2010 USA110 | 176 | 66.0 (10.0) | 50 | 51.8 (19.4) | Mixed | Lifestyle activity intervention | Traditional exercise therapy | 52 | Y | N | N | N | N | N | Y | Y | N | N | N |
| Bestall 2003 UK141 | 66 | 68.7 (7.5) | NR | 37.5 (11.5) | PR programme | Exercise | Control | 52 | Y | N | N | N | Y | Y | Y | Y | N | N | Y |
| Bjornshave 2005 Denmark223 | 31 | 62.6 | 35 | 34.8 | Secondary care | Middle intensity training | Low-intensity training | 4 | Y | N | N | N | N | N | Y | Y | N | N | N |
| Blake Jr 1990 USA111 | 94 | 63.4 | 81 | NR | Secondary care outpatient | Psychosocial intervention | Control | 52 | Y | N | N | Y | N | N | N | N | Y | N | N |
| Bonilha 2009 Brazil232 | 43 | 71.7 (7.5) | 75 | 51.1 (20.5) | Mixed | Singing classes | Control | 25 | Y | N | N | N | N | N | N | Y | N | N | Y |
| Bourbeau 2003, Bourbeau 2006, Gadoury 2005 Canada192,270,271 | 191 | 69.5 (7.0) | 57 | NR | Secondary care | SM programme | UC | 52, 104, 52 | Y | Y | Y | N | N | N | Y | Y | Y | Y | N |
| Boxall 2005 Australia160 | 60 | 76.7 (7.9) | 57 | 39.1 (15.5) | Secondary care outpatient | Home-based PR | Control | 26 | Y | Y | N | N | N | N | Y | N | N | N | Y |
| Breyer 2010 Austria245 | 60 | 60.3 (8.5) | 45 | 46.3 (17.6) | NR | Nordic walking | Control | 39 | Y | N | N | N | Y | Y | Y | N | N | N | N |
| Brooks 2002 Canada193 | 109 | 68.0 (7.4) | 59 | 32.0 (12.0) | PR programme | Enhanced follow-up | Conventional follow-up | 52 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Bucknall 2012 UK63 | 464 | 69.1 (9.3) | 37 | 40.5 (13.6) | Mixed | Supported SM | UC | 52 | Y | Y | N | Y | Y | Y | N | N | N | N | N |
| Busch 1988 Canada194 | 20 | 65.1 (15.5) | 79 | 26.3 (10.0) | Unclear | Home exercise | Control | 18 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Cai 2006 China199 | 82 | 61.0 (9.0) | 95 | NR | Secondary care | Education | Control | 26 | Y | N | Y | N | N | N | N | Y | N | N | Y |
| Carr 2009 Canada195 | 34 | 68.0 (8.1) | 44 | NR | Primary and secondary care | Repeat PR | UC | 52 | Y | N | N | N | N | N | Y | N | Y | N | N |
| Casas 2006, Garcia-Aymerich 2007 Spain and Belgium71,72 | 155 | 71.2 (9.0) | 83 | 41.8 (17.3) | Secondary care inpatient | Integrated care | UC | 52 | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y |
| Chan 2010, 2011 Hong Kong212,272 | 206 | 73.0 (7.7) | 91 | NR | Secondary care outpatient | (a) T’ai chi qigong; (b) exercise | Control | 13 | Y | Y | Y | N | N | N | Y | Y | Y | N | Y |
| Cockcroft 1987 UK142 | 75 | 69.8 | 68 | NR | Primary and secondary care | Respiratory health worker | Control | NR | N | Y | N | Y | N | N | N | N | Y | N | N |
| Coultas 2005 USA112 | 217 | 69.0 (8.2) | 62 | NR | Primary care | (a) Nurse-assisted collaborative management; and (b) nurse-assisted medical management | UC | 26 | Y | Y | N | N | N | Y | N | Y | Y | Y | N |
| Covey 2001 USA113 | 37 | 66.1 (8.5) | 67 | 37.8 (10.2) | NR | IMT | Education | 16 | Y | N | N | N | N | N | N | Y | N | N | Y |
| de Blok 2006 The Netherlands181 | 21 | 64.0 (11.4) | 43 | 46.8 (17.8) | PR programme | Lifestyle physical activity counselling | Control | 9 | Y | N | N | N | N | Y | Y | N | N | N | Y |
| Dheda 2004 UK73 | 33 | 70.2 (7.5) | NR | 41.3 (16.5) | Secondary care inpatient | Outpatient follow up | Primary care follow-up | 26 | Y | Y | Y | N | N | N | N | Y | Y | N | N |
| Donesky-Cuenco 2009 USA114 | 41 | 69.9 (9.5) | 28 | 47.7 (15.6) | Community | Yoga therapy | UC | 12 | Y | N | N | N | Y | Y | Y | Y | N | N | Y |
| Dourado 2009 Brazil233 | 47 | 63.1 (87.2) | 74 | 58.8 (25.0) | Secondary care inpatient | (a) Strength training with low-intensity general training; and (b) low-intensity general training | Strength training | 12 | Y | N | N | N | N | N | Y | N | N | N | Y |
| du Moulin 2009 Germany206 | 20 | 65.9 | 70 | 60.6 | PR programme | Home-based exercise | Control | 26 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Eaton 2009 New Zealand227 | 97 | 69. 9 (9.6) | 44 | 35.5 (16) | Secondary care inpatient | Early PR | UC | 13 | Y | Y | N | N | Y | Y | Y | N | N | Y | Y |
| Effing 2009, 2011 Australia161,278 | 142 | 63.4 (8.0) | 59 | 50.1 (15.8) | Secondary care outpatient | SM sessions plus COPE-active (community-based physiotherapeutic exercise) | SM | 52 | Y | Y | Y | N | Y | Y | Y | N | Y | Y | Y |
| Efraimsson 2008 Sweden218 | 52 | 67.0 (10.6) | 50 | n/a | Primary care | Self-care management education | Control | 13 | Y | N | N | N | N | N | N | N | N | N | N |
| Egan 2002 Australia69 | 66 | 52.5 | 48 | NR | Secondary care inpatient | Nursing-based case management | Control | 13 | Y | Y | N | N | Y | Y | N | N | N | N | N |
| Elci 2008 Turkey236 | 78 | 58.9 (10.1) | 81 | 47 | Secondary care | PR | Control | 13 | Y | N | N | N | Y | Y | Y | Y | N | N | Y |
| Elliott 2004 Australia162 | 43 | 66.2 (8.1) | 54 | 45.1 (18.3) | Secondary care | (a) Hospital- and home-based rehabilitation; and (b) hospital- and community-based rehabilitation | Community rehabilitation | 52 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Emery 1998 USA115 | 79 | 66.6 (6.5) | 47 | 42.0 (17.0) | Mixed | (a) Exercise, education and stress management; and(b) education and stress management | Waiting list control | 10 | Y | N | N | N | Y | Y | Y | Y | N | N | N |
| Engstrom 1999 Sweden219 | 50 | 66.4 (5.4) | 52 | 32.3 (10.8) | Secondary care outpatient | PR | UC | 52 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Fernandez 2009 Spain171 | 49 | 67 (8) | 100 | 59.76 (14.14) | Secondary care | Home-based PR | Control | 52 | Y | N | N | N | N | N | Y | Y | N | N | N |
| Finnerty 2001 UK143 | 100 | 69.5 (9.2) | 68 | 41.0 (18.5) | Secondary care outpatient | PR | Control | 26 | Y | N | N | N | N | N | Y | N | N | N | N |
| Foy 2001 USA116 | 140 | 67.7 (5.9) | 56 | 58.4 (17.8) | Mixed | Long-term exercise | Short-term exercise | 78 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Gallefoss 1999, 2000, 2002, 2004 Norway255,280,281,391 | 62 | 57.5 (9.5) | 50 | 53.5 (9.5) | Secondary care outpatient | Education | Control | 52 | Y | Y | N | N | N | N | N | Y | Y | N | N |
| Ghanem 2010 Egypt264 | 39 | 56.8 (10.8) | NR | NR | Secondary care inpatient | Home-based PR | UC | 9 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Gilmore 2010 USA117 | 37 | 59.2 (8.3) | 35 | 45.0 (15.8) | Secondary care outpatient | (a) COPD education guide and structured home visit; (b) COPD education guide; and (c) structured home visit | Control | NR | Y | N | N | N | N | N | N | N | N | N | N |
| Gohl 2006 Germany207 | 34 | 62.8 (7.7) | 68% | 53.5 (8.7) | Mixed | Training programme | UC | 52 | Y | N | N | N | N | N | Y | N | N | N | N |
| Goldstein 1994, 1997; Guyatt 1999, Canada196,392,393 | 89 | 65.5 (7.5) | 49 | 36.5 (13.2) | NR | PR | Control | 26 | Y | N | N | N | N | N | Y | Y | Y | N | Y |
| Green 2001 UK144 | 44 | 68.5 (9.0) | 64 | NR | NR | 7 weeks PR | 4 weeks PR | 7 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Güell 2000 Spain172 | 60 | 65.0 (7.0) | 100 | 35.0 (14.0) | Secondary care outpatient | PR | Control | 104 | Y | Y | Y | N | N | N | Y | Y | N | N | Y |
| Güell 2006 Spain173 | 40 | 65.0 (8.0) | 83 | 35.0 (13.0) | Secondary care | PR | Control | 17 | Y | N | N | N | Y | Y | Y | N | Y | N | N |
| Guyatt 1992 USA118 | 93 | 66.4 (7.6) | NR | NR | Secondary care | Respiratory muscle training | Sham training | 26 | Y | N | N | N | Y | Y | Y | N | N | N | Y |
| Hermiz 2002 Australia67 | 177 | 66.9 | 46 | n/a | Secondary care | Home-based care | Control | 13 | Y | Y | N | N | N | N | N | N | Y | Y | N |
| Hernandez 2000 Spain282 | 60 | 63.8 (7.7) | NR | 40.9 (16.0) | NR | Home-based training programme | Control | 12 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Hernandez 2003 Spain68 | 222 | 71.0(10.0) | 97 | 42.0 | Secondary care | Hospital at home | UC | 8 | Y | Y | N | Y | N | N | N | N | Y | Y | N |
| Hill 2006 Australia163 | 35 | 68.0 (8.6) | 67 | 36.9 (12.0) | NR | IMT | Sham IMT | 8 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Holland 2004 Australia164 | 40 | 67.8 (7.7) | 63 | 36.6 (10.3) | Secondary care outpatient | Upper limb and lower limb training | Lower limb plus sham training | 6 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Hoogendoorn 2009; Van Wetering 2010; Hoogendoorn 2010 The Netherlands182,273,274 | 199 | 66.5 (8.9) | 71 | 58.8 (16.1) | Secondary care | INTERCOM: Interdisciplinary community-based COPD management programme | UC | 104 | Y | Y | Y | N | N | N | Y | Y | Y | Y | Y |
| Hospes 2009 The Netherlands183 | 39 | 62.18 (8.7) | 60 | 64.7 (16.1) | Secondary care inpatient | Exercise counselling | UC | 12 | Y | N | N | N | N | Y | Y | N | N | N | N |
| Hsiao 2003 Taiwan259 | 42 | 69.9 (5.3) | 87 | 51.4 (13.0) | Secondary care | (a) Targeted, resistive IMT; and (b) pressure threshold IMT | Control | 8 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Hynninen 2010 Norway256 | 51 | 61.0 (8.9) | 49 | 58.8 (23.62) | Mixed | Cognitive–behavioural therapy | UC | 35 | Y | N | N | N | Y | Y | N | Y | N | N | N |
| Janaudis-Ferreira 2011 Canada197 | 36 | 66.0 (9.0) | 58 | 35.0 (15.1) | Secondary care | Unsupported upper extremity resistance training | Sham training | 6 | Y | N | N | N | N | N | Y | N | N | N | N |
| Jang 2006 Korea267 | 36 | NR | 100 | 48.7 (16.52) | Unclear | PR | Education control | 8 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Jarab 2012 Jordan266 | 133 | 62.5 (14.5) | 41 | 53.3 (16.9) | Secondary care outpatient | Pharmacist intervention | Control | 26 | Y | Y | Y | N | N | N | N | Y | Y | Y | N |
| Karapolat 2007 Turkey237 | 54 | 65.8 (9.0) | 88 | 54.9 (16.0) | NR | PR | Control | 12 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Katiyar 2006 India240 | 48 | 52.2 (2.85) | 84 | 48 (2.77) | NR | Pranayama (yogic breathing) | Control | 14 | Y | N | N | N | N | N | Y | Y | N | N | N |
| Kayahan 2006 Turkey238 | 45 | 65.8 (8.4) | 87 | NR | Secondary care outpatient | PR | Control | 9 | Y | N | N | N | Y | Y | Y | N | N | N | Y |
| Khdour 2009, 2011 Ireland251,279 | 173 | 64.5 (9.7) | 44 | 52.0 (16.9) | Secondary care outpatient | Pharmacy-led disease management programme | Control | 52 | Y | Y | Y | N | N | N | N | Y | Y | Y | N |
| Kim 1993 USA119 | 129 | 64.8 (7.4) | 76 | 40.0 (13.4) | NR | IMT | Control | 26 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Ko 2011 Hong Kong213 | 60 | 73.6 (7.10) | 98 | 54.6 (18.5) | Secondary care inpatient | Early pulmonary rehabilitation | UC | 52 | Y | Y | Y | N | N | N | Y | Y | Y | Y | N |
| Koff 2009 USA120 | 40 | 65.8 (8.7) | 48 | 32.4 (9.7) | Secondary care outpatient | Integrated care | UC | 13 | Y | Y | Y | N | N | N | N | N | Y | Y | N |
| Koppers 2006 The Netherlands184 | 39 | 55.7 (8.1) | 47 | 54.0 (14.5) | PR programme | Respiratory muscle endurance training | Sham training | 5 | Y | N | N | N | N | N | N | Y | N | N | Y |
| Kunik 2008 USA121 | 238 | 66.3 (10.3) | 96 | 46.0 (17.2) | Mixed | Cognitive–behavioural therapy | Education | 52 | Y | N | N | N | Y | Y | Y | N | Y | N | Y |
| Kwok 2004 Hong Kong70 | 157 | 74.7 (6.4) | 71 | NR | Secondary care | Community nursing programme | Control | 26 | N | Y | N | N | N | N | Y | N | N | Y | N |
| Lamers 2010 The Netherlands185 | 187 | 71 (6.7) | 60 | NR | Primary care | Minimal psychological intervention plus UC | UC | 39 | Y | N | N | N | Y | Y | N | N | N | N | N |
| Larson 1988 USA122 | 22 | 64.4 (4.6) | 91 | 31.1 (15.7) | Mixed | IMT 30% load | IMT 15% load | 8 | Y | N | N | N | N | Y | Y | Y | N | N | Y |
| Larson 1999 USA123 | 130 | 65.0 (6.0) | 66 | 50.3 (17.3) | Mixed | (a) IMT; (b) cycle ergometry training; and (c) IMT and cycle ergometry training | Health education | 17 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Lee 2002 Hong Kong66 | 112 | 80.4 (6.3) | 53 | NR (severe) | Secondary care inpatient | Care protocol nurse follow-up | Control | 26 | N | Y | N | N | Y | Y | N | Y | N | Y | N |
| Leung 2010 Australia165 | 36 | 71.5 (7.5) | 70 | 54.5 (17.5) | PR programme | Walking | Cycling | 8 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Li 2002 China200 | 74 | NR | NR | 61.8 (17.3) | Secondary care | Nutritional support | Control | 13 | Y | N | N | N | N | N | N | Y | N | N | N |
| Liddell 2010 UK145 | 30 | 69 (8.1) | 67 | 51 (21.2) | PR programme | Twice-weekly PR | Once-weekly PR | 8 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Lindsay 2005 Hong Kong214 | 50 | 69.7 (9.8) | 76 | NR | Secondary care outpatient | PR (plus tiotropium) | UC (plus tiotropium) | 13 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Linneberg 2012 Denmark224 | 118 | NR | 38 | 42.2 | PR programme | Supplemental exercise post PR programme | No supplemental exercise post-PR | 45 | Y | N | N | N | N | N | Y | N | N | N | N |
| Littlejohns 1991 UK146 | 152 | 62.7 (7.7) | 65 | 47.8 (22.7) | Secondary care outpatient | Respiratory health worker | UC | 52 | Y | N | N | N | Y | Y | Y | Y | Y | N | N |
| Liu 2008 Taiwan260 | 60 | 72.1 (7.4) | 100 | 45.6 (13.9) | NR | Cell phone-based exercise programme | Control | 52 | Y | Y | Y | Y | N | N | Y | Y | N | N | N |
| Livermore 2010 Australia166 | 41 | 73.4 (7.3) | 44 | 54.1 (20.8) | Secondary care | Cognitive–behavioural therapy | UC | 78 | Y | Y | N | N | Y | Y | N | Y | N | N | N |
| Lord 2010 UK147 | 36 | 63.7 (8.1) | NR | 37.2 (18.6) | Secondary care outpatient | Singing | Control | 7 | Y | N | N | N | Y | Y | Y | N | N | N | N |
| Madariaga 2007 Spain174 | 34 | 63.2 (10.4) | NR | 46.9 (9.5) | Secondary care outpatient | (a) RMT with a threshold device; and (b) RMT with a resistive device | Control | 6 | Y | N | N | N | N | N | N | Y | N | N | Y |
| Mador 2004 USA126 | 32 | 70.8 (6.9) | NR | 41.8 (13.9) | Secondary care outpatient | Endurance, strength training and education | Endurance training and education | 8 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Mador 2005 USA125 | 38 | 70.3 (2.0) | NR | 44.4 (4.7) | PR programme | Endurance training plus hyperpneic (combined) training) | Endurance training | 8 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Mador 2009 USA124 | 48 | 71.8 (7.4) | NR | 44.6 (13.9) | Mixed | Interval training | Continuous training | 8 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Magadle 2007 Israel254 | 34 | 65.6 (13.0) | 74 | 45.5 (10.0) | PR programme | General exercise reconditioning programme plus IMT | General exercise reconditioning programme plus sham IMT | 26 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Maltais 2008 Canada198 | 252 | 66.0 (9.0) | 56 | 44.5 (13.0) | Secondary care outpatient | Home-based PR | Hospital-based PR | 52 | Y | Y | Y | N | N | N | Y | Y | N | N | Y |
| Man 2004 UK148 | 42 | 70.2 (9.3) | 41 | 39.2 (17.0) | Secondary care inpatient | Early PR | UC | 13 | Y | Y | N | N | N | N | Y | N | N | Y | Y |
| Martin 2004 New Zealand228 | 96 | 70.1 (15.6) | 50 | 34.8 (12.0) | Primary care | Individualised care plans | UC | 52 | Y | Y | N | N | N | N | N | N | Y | Y | N |
| McGeoch 2006 New Zealand229 | 159 | 70.9 (10.9) | 65 | 53.9 (18.4) | Primary care | SM plan | UC | 52 | Y | Y | N | N | Y | Y | N | N | Y | Y | N |
| Monninkhof 2003, 2004 The Netherlands186,300 | 248 | 65.0 (7.0) | 68 | 57.0 (15.0) | Secondary care outpatient | SM programme | UC | 52 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Moore 2009 UK284 | 20 | 70.0 | 50 | 40.8 | Mixed | Home exercise video programme | Control | 6 | Y | N | N | N | Y | Y | Y | N | N | N | Y |
| Mota 2007 Spain175 | 18 | 63.5 (6.7) | NR | 28.0 (8.0) | NR | EMT | Sham training | 5 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Mularski 2009 USA127 | 86 | 67.4 (2.2) | 99 | NR | Mixed | Mindfulness-based breathing therapy | Support group control | 8 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Murphy 2005 Ireland252 | 26 | 66.0 (10.4) | 74 | 40.0 (12.0) | Secondary care inpatient | Home-based exercise | Control | 26 | Y | N | Y | N | N | N | Y | Y | N | N | Y |
| Nakamura 2008 Japan261 | 42 | 68.9 (6.8) | NR | 51.5 (19.7) | Secondary care outpatient | (a) Aerobic and strength training; and (b) aerobic training and recreational activities | Control | 12 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Ng 2011 Hong Kong215 | 80 | 72.4 (7.6) | 89 | 36.9 (13.7) | Secondary care outpatient | Health qigong | Control | 26 | Y | N | N | N | N | N | Y | N | N | N | N |
| Nguyen 2008 USA128 | 50 | 69.3 (8.8) | 50 | 50.3 (17.3) | Community | Face-to-face dyspnoea SM programme | Internet-based dyspnoea SM programme | 26 | Y | N | Y | N | N | N | Y | N | N | N | Y |
| Nguyen 2009 USA129 | 17 | 68.2 (10.5) | 94 | 40.9 (17.1) | Secondary care | Mobile coached | Mobile self-monitored | 26 | Y | N | N | N | N | N | Y | N | N | N | N |
| Nield 2007 USA130 | 40 | 65.0 (9.0) | 95 | 39.0 (13.0) | Secondary care outpatient | (a) Pursed lips breathing; and(b) EMT | Control | 12 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Ninot 2011 France250 | 45 | 63.1 | 84 | 55.1 | Secondary care | SM education programme and exercise | UC | 52 | Y | Y | N | N | N | N | Y | N | Y | Y | N |
| Normandin 2002 USA131 | 54 | 68.0 (8.1) | 53 | 49.5 (18.1) | PR programme | High intensity endurance | Low intensity calisthenics | 8 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Norweg 2005 USA132 | 43 | 75.3 (7.0) | 30 | 55.9 (17.8) | Secondary care outpatient | (a) Exercise training and activity training; and (b) exercise training and lecture series | Exercise training alone | 24 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Oh 2003 South Korea283 | 34 | 65.5 (9.6) | 61 | 43.1 (16.0) | Secondary care outpatient | Home-based PR | Control | 8 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| O’Neill 2007 UK150 | 91 | 68.5 (7.9) | 67 | 41.3 (17.8) | PR programme | Twice-weekly PR | Once-weekly PR | 26 | Y | N | N | N | Y | Y | Y | N | N | N | Y |
| Ortega 2002 Spain176 | 54 | 64.2 (7.7) | 87 | 38.3 (12.5) | NR | (a) Strength training and endurance training; and (b) endurance training | Strength training | 24 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| O’Shea 2007 Australia167 | 54 | 67.7 (8.6) | 39 | 50.5 (23.6) | Mixed | Progressive resistance exercise | Control | 24 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Ozdemir 2010 Turkey239 | 50 | 62.5 (8.9) | 100 | 54.3 (12.7) | Secondary care outpatient | Water-based PR | Control | 4 | Y | N | N | N | Y | Y | Y | Y | N | N | Y |
| Paz-Diaz 2007 Venezuela269 | 24 | 64.1 (6.3) | 75 | 31.7 (9.9) | Secondary care outpatient | PR | Control | 9 | Y | N | N | N | Y | Y | N | Y | N | N | Y |
| Petersen 2008 Denmark225 | 19 | 66.0 (2.0) | 32 | 31.0 (3.0) | NR | Lifestyle training | Control | 7 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Petty 2006 USA133 | 214 | 68.8 (9.4) | 56 | NR | Mixed | (a) Customised video; and(b) standardised video | Control | 16 | Y | N | N | N | N | N | Y | N | Y | N | N |
| Pomidori 2012 Italy243 | 36 | 72.0 (8.0) | 75 | 48.5 (12.5) | Secondary care outpatient | Paced speed walking | Walking (known distance, fixed time) | 52 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Prince 1989 Edinburgh151 | 39 | 67.5 | 64 | NR | Secondary care outpatient | Rehabilitation | Control | 6 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Probst 2011 Brazil234 | 63 | 66.0 (8.6) | 54 | 39.5 (13.5) | NR | High-intensity endurance and strength training | Low-intensity calisthenics and breathing | 12 | Y | N | N | N | N | N | Y | N | N | N | N |
| Puente-Maestu 2000, 2003 Spain177,275 | 49 | 64.4 (4.5) | NR | 40.6 (6.2) | PR programme | Supervised exercise | Self-monitored exercise | 8, 56 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Puhan 2006 Switzerland257 | 100 | 69.0 (9.2) | 66 | 34.3 (8.5) | PR programme | Interval exercise | High-intensity continuous exercise | 5 | Y | N | N | N | Y | Y | Y | N | N | N | Y |
| Rea 2004 New Zealand230 | 135 | 68.0 | 42 | 51.1 | Primary care | Disease management programme | UC | 52 | Y | Y | N | N | N | N | Y | Y | Y | Y | Y |
| Regiane Resqueti 2007 Spain178 | 38 | 67.7 (4.3) | 92 | 28.6 (8.5) | Secondary care outpatient | Home-based PR | Control | 26 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Ren 2011 China201 | 89 | NR | NR | NR | Secondary care | (a) PR strategy group 1; and (b) PR strategy group 2 | Control | 52 | N | N | Y | N | N | N | Y | Y | N | N | Y |
| Rice 2010 USA140 | 743 | 69.9 (9.6) | 98 | 37.1 (14.5) | Secondary care | Disease management programme | UC | 52 | Y | Y | N | Y | N | N | N | N | Y | Y | N |
| Riera 2001 Spain179 | 20 | 67.3 (4.5) | 90 | 39.8 (12.0) | Secondary care outpatient | IMT | Sham training | 26 | Y | N | N | N | Y | Y | Y | Y | N | N | Y |
| Ringbaek 2000 Denmark226 | 45 | 63.1 (7.2) | 16 | 47.1 (15.8) | Secondary care outpatient | PR | Control | 8 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Romagnoli 2006 Italy244 | 35 | 69.5 (8.0) | 66 | 36.5 (8.0) | PR programme | Two repeat PR sessions | One repeat PR session | 52 | Y | Y | N | N | N | N | Y | Y | N | N | Y |
| Rooyackers 2003 The Netherlands187 | 24 | 59.0 (11.6) | 83 | 41.5 (12) | NR | General exercise training and eccentric exercise training | General exercise training | 10 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Sassi-Dambron 1995 USA134 | 98 | 67.4 (8.0) | 61 | 50.0 (22.0) | Mixed | Dyspnoea management strategy | Attention control | 26 | Y | N | N | N | Y | Y | Y | Y | N | N | Y |
| Scherer 2000 Zurich258 | 34 | 69.0 (1.9) | 63 | 51.3 (4.0) | Secondary care outpatient | RMT | Control | 9 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Sewell 2005 UK152 | 180 | 68.3 (8.6) | 62 | NR | PR programme | Individually targeted exercise programme | General exercise training | 7 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Sewell 2006 UK153 | 100 | 70.1 (8.0) | 56 | NR | PR programme | 7-week PR | 4-week PR | 26 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Seymour 2010 UK154 | 60 | 66.0 (10.0) | 55 | 52.0 (17.1) | Secondary care inpatient | Post-exacerbations pulmonary rehabilitation | UC | 13 | Y | Y | Y | N | N | N | Y | Y | N | Y | N |
| Shao 2003 China202 | 38 | 63.4 (5.1) | 85 | NR | Secondary care | Rehabilitation (behavioural intervention) | Control | 52 | Y | N | N | N | Y | Y | N | N | N | N | N |
| Simpson 1992 USA135 | 34 | 71.5 (7.6) | 54 | 38.0 | Secondary care outpatient | Weight training | Control | 8 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Singh 2003 India241 | 40 | 59.4 (6.4) | 80 | 27.0 (7.3) | NR | PR | Control | 4 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Sívori 1998 Argentina263 | 28 | 64.6 (9.33) | NR | 36.1 (14.6) | Secondary care outpatient | Upper limb and lower limb training | Lower limb training | 8 | Y | Y | N | N | N | N | Y | N | Y | N | Y |
| Smith 1999 Australia168 | 96 | 69.9 (8.3) | 62 | NR | Secondary care | Home nurse | Control | 52 | Y | Y | N | Y | N | N | N | Y | Y | Y | Y |
| Soler 2006 Spain180 | 26 | 73.5 (8.1) | NR | 42.8 (15.7) | Secondary care | Education and monitoring programme | UC | 52 | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y |
| Solomon 1998, Gourley 1998 USA136,285 | 98 | 69.3 (7.9) | 100 | NR | Secondary care | Pharmaceutical care | Conventional care | 26 | Y | Y | N | N | N | N | N | N | Y | Y | Y |
| Spencer 2010 Australia169 | 59 | 66.4 (8.0) | 46 | 56.9 (19.5) | PR programme | Supervised out-patient exercise (plus PR) | Unsupervised home-based exercise (plus PR) | 52 | Y | Y | Y | N | Y | Y | Y | Y | N | Y | N |
| Spruit 2002 Belgium247 | 48 | 63.5 (7.6) | 87 | 38.0 (17.0) | Secondary care outpatient | Endurance training | Resistance training | 12 | Y | N | N | N | N | N | Y | N | N | N | N |
| Sridhar 2008 UK155 | 122 | 69.8 (10.0) | 49 | 42.0 (16.3) | Secondary care outpatient | Nurse-led intermediate care programme | Control | 104 | Y | Y | N | Y | N | N | N | Y | Y | Y | Y |
| Stulbarg 2002, Carrieri-Kohlman 2005, Davis 2006 USA137,394,395 | 115 | 66.0 (8.0) | 55 | 44.8 (14.0) | Mixed | (a) Dyspnoea SM programme with 24 training sessions; and (b) dyspnoea SM programme with four training sessions | Dyspnoea SM programme | 52 | Y | N | N | N | N | N | Y | Y | Y | N | Y |
| Subin 2010 India242 | 30 | 58.7 (8.4) | NR | 41.7 (9.5) | Secondary care | Upper and lower limb training | Upper limb training | 4 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Theander 2009 Sweden220 | 30 | 64.9 (2.0) | 50 | 33.6 (8.7) | Secondary care outpatient | PR | Control | 12 | Y | N | N | N | N | N | Y | N | N | N | N |
| Toshima 1990, Ries 1995 USA138,276 | 129 | 62.6 (7.2) | 74 | 52.0 | Secondary care | PR | Control | 26, 312 | Y | Y | N | N | N | Y | Y | Y | N | Y | Y |
| Trappenburg 2011 The Netherlands188 | 233 | 65.7 (10.8) | 69 | 55.7 (21.0) | Primary and secondary care | Individualised action plan | UC | 26 | Y | Y | Y | N | Y | Y | N | N | Y | Y | N |
| Troosters 2000 Belgium248 | 100 | 61.5 (8.1) | 87 | 42.0 (14.0) | Secondary care outpatient | Training programme | Control | 78 | Y | N | N | Y | N | N | Y | Y | N | N | N |
| Van Gestel 2012 Germany208 | 43 | 66.1 (6.4) | 43 | 45.9 (17.4) | Secondary care outpatient | Respiratory biofeedback training | Control | 4 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Vogiatzis 2002 Greece265 | 45 | 68.0 (2.0) | 83 | 45.0 (4.0) | Secondary care outpatient | Interval training | Continuous training | 13 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Vonbank 2012 Austria246 | 43 | 60.2 (6.5) | 69 | 55.8 (16.4) | Secondary care outpatient | (a) Strength and endurance training; and (b) endurance training | Strength training | 12 | Y | N | N | N | N | N | Y | Y | N | N | N |
| Wadell 2004 Sweden221 | 30 | 66.1 (8.1) | 30 | 54.6 (11.5) | Secondary care outpatient | a) Water physical aerobic training; and (b) land physical aerobic training | Control | 12 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Wakabayashi 2011 Japan262 | 102 | 71.7 (7.6) | 86 | 60.3 (21.0) | Secondary care outpatient | Integrated care | UC | 52 | Y | N | N | N | N | N | Y | Y | N | Y | N |
| Wang 2004 China203 | 100 | NR | 87 | NR | Secondary care | Resistance breathing exercises | Breathing exercises | 13 | Y | N | N | N | N | N | Y | Y | N | N | N |
| Warlies 2006 Germany209 | 60 | 63.3 | 67 | NR | Secondary care | Education | UC | 26 | Y | N | N | N | N | N | N | N | N | N | N |
| Waterhouse 2010 UK277 | 240 | 68.9 (7.9) | 52 | 46.8 (18.0) | Mixed | Hospital rehabilitation | Community rehabilitation | 78 | Y | N | N | N | N | N | Y | N | Y | N | N |
| Watson 1997 New Zealand231 | 69 | 67.5 (9.0) | 65 | 37.5 (15.0) | Primary care | SM plan | Control | 26 | Y | N | N | N | N | N | N | Y | Y | N | N |
| Wedzicha 1998 UK157 | 126 | 70.5 (7.0) | 51 | 37.3 (13.1) | Secondary care outpatient | PR | Control | 8 | Y | N | N | N | N | Y | Y | Y | N | N | N |
| Weekes 2009 UK158 | 66 | 69.1 | 51 | 31.75 (13.7) | Secondary care outpatient | Dietary counselling and food fortification | Control | 52 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| White 2002 UK159 | 103 | 67.0 (9.0) | 69 | 26.9 (7.8) | Secondary care outpatient | PR | Brief advice | 13 | Y | N | N | N | Y | Y | Y | N | N | N | Y |
| Wijkstra 1994 The Netherlands189 | 45 | 63.3 (5.0) | 91 | 44.4 (10.4) | NR | Home rehabilitation | Control | 12 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Wijkstra 1995 The Netherlands190 | 45 | 62.7 (5.0) | 83 | 43.8 (10.8) | PR programme | (a) Rehabilitation with weekly visits to a physiotherapist; and(b) rehabilitation with monthly visits to a physiotherapist | Control | 78 | Y | N | N | N | N | N | Y | Y | N | N | N |
| Wittmann 2007 Germany210 | 212 | 53.9 (6.9) | 80 | NR | Secondary care | PR plus education | PR | 52 | Y | Y | N | N | N | N | Y | Y | N | N | N |
| Wong 2005 China74 | 60 | 73.6 (7.8) | 78 | NR | Secondary care inpatient | Nurse-initiated telephone follow-up | UC | 13 | N | Y | N | N | N | N | N | N | Y | N | N |
| Wood-Baker 2006 Australia170 | 139 | 70.0 (8.1) | 84 | 45.0 (16.0) | Primary care | Action plan | UC | 52 | Y | Y | N | N | N | N | Y | Y | Y | Y | N |
| Wright 2003 Germany211 | 28 | 55.7 (6.9) | 43 | 55.9 (12.8) | NR | Resistance training | Control | 12 | Y | N | N | N | N | N | Y | Y | N | N | N |
| Xu 2010 China204 | 80 | 57.1 (7.6) | 53 | NR | Secondary care | (a) Integrative rehabilitation (traditional and modern); (b) modern rehabilitation; and(c) traditional rehabilitation | UC | 52 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Yamaguti 2012 Brazil235 | 30 | 66.5 (22.6) | 73 | 42.9 (52.9) | Secondary care | Diaphragmatic breathing | Control | 4 | Y | N | N | N | N | N | Y | N | N | N | Y |
| Yeh 2010 USA139 | 10 | 65.5 (6.0) | 60 | 50.0 (7.0) | Secondary care | T’ai chi plus UC | UC | 12 | Y | N | N | N | N | N | Y | Y | N | N | Y |
| Zhang 2008 China205 | 60 | 69.5 (3.3) | 85 | NR | Secondary care | (a) PR plus PLB; and (b) PR | Control | 8 | Y | N | N | N | N | N | Y | N | N | N | Y |
Appendix 23 Characteristics of interventions of studies included in review 4
| Author year | Intervention description | Number of components | Control description | Number of components | Intervention length (weeks) | Mode |
|---|---|---|---|---|---|---|
| Aimonino Ricauda 200875 | Hospital at home – multidimensional geriatric assessment, patient and carer education | 8 | Inpatient control – routine hospital care | 0 | 2 | Individual |
| Arnardottir 2006216 | Mixed exercise training – endurance training: 30 minutes, 2× per week with either resistance: 30 minutes, 1× per week; or calisthenics: 15 minutes 1× per week plus relaxation: 15 minutes, 1× per week | 2 | Resistance training: 30 minutes and calisthenics: 15 minutes with relaxation: 15 minutes | 2 | 8 | Group |
| Arnardottir 2007217 | Interval training: 90 minutes, 2× per week | 2 | Continuous training: 90 minutes, 2× per week | 2 | 16 | Group |
| Barakat 2008249 | PR – exercise and education: 1 hour, 3× per week | 3 | UC | 0 | 14 | Group |
| Bauldoff 2002108 | Music as a form of distractive auditory stimulation to accompany home walking programme: 20–45 minutes, 2–5× per week | 2 | Control | 1 | 8 | Individual |
| Bauldoff 2005109 | (1) Moderate tempo distractive auditory stimulation with upper extremity training: 15 minutes 2–3× per week plus warm-up; (2) slow tempo distractive auditory stimulation: 15 minutes, 2–3× per week plus warm-up | 1 | Attention control | 1 | 4 | Individual |
| Beckerman 2005253 | IMT using threshold device: 15 minutes 2× per day, 6 days per week, gradual increase in load; training in centre for 1 month then home with daily telephone call and weekly visit by respiratory therapist | 1 | Sham training, as intervention group with very low load | 1 | 52 | Unclear |
| Behnke 2000, Behnke 200364,65 | Ten-day inpatient walking training programme and breathing exercise; then walking training at home: 3× per day, with fortnightly supervision for 3 months | 3 | Control – advised to do exercise, without specific instructions | 2 | 26 | Individual |
| Bendstrup 1997222 | PR: group exercise supervised by physiotherapist 1 hour, 3× per week; 12 educational sessions and 2× occupational therapy sessions | 9 | Control – UC, waiting list until follow-up | 0 | 12 | Group |
| Bernard 1999191 | Aerobic and strength training: aerobic – 30 minutes, 3× per week; strength – 45 minutes, 3× per week | 1 | Aerobic training: 30 minutes, 3× per week plus 45 minutes’ relaxation and breathing | 4 | 12 | Group |
| Berry 2010110 | Behavioural lifestyle activity programme to promote physical activity: centre-based exercise programme 1 hour, 3× per week (reducing in frequency) plus fortnightly educational classes plus discussion about self-regulation and maintenance of exercise, individual counselling and telephone support | 3 | Exercise programme 1 hour 3× per week plus fortnightly educational classes for 3 months | 2 | 48 | Mixed |
| Bestall 2003141 | Exercise and education group programme: 2× per week | 11 | Education programme: 2× per week | 10 | 8 | Group |
| Bjornshave 2005223 | Middle-intensity home-based exercise training (steps and walking): 30 minutes, 5× per week | 1 | Low-intensity home-based exercise: 30 minutes 2× per week | 1 | 4 | Unclear |
| Blake Jr 1990111 | Psychosocial intervention (stress management strategies – relaxation, guided imagery, breathing exercises): one session of 60–90 minutes Individualised plan developed, audiotape and reading material provided At least 1 telephone contact and 2× follow-up visits, 2–4 weeks apart |
5 | Control | 0 | 8 | Individual |
| Bonilha 2009232 | Singing group class with physiotherapist and singing teacher, 1 hour per week (5 minutes’ relaxation, 10 minutes’ related respiratory exercise, 15 minutes’ vocalisation exercises and 30 minutes’ singing training) | 4 | Physiotherapy and handcraft classes, 1× per week (5 minutes’ relaxation and 50 minutes’ exercises) | 2 | 24 | Group |
| Bourbeau 2003, Bourbeau 2006, Gadoury 2005192,270,271 | Living Well with COPD programme: 8 × 1-hour home visits by health professional who was case manager | 12 | UC | 0 | 8 | Individual |
| Boxall 2005160 | Home-based PR programme: graduated exercise programme 1× per day; physiotherapist visits 1× per week for 6 weeks, then 1× per 2 weeks; education by physiotherapist, nurse and occupational therapy staff Around six education sessions and 11 home visits in total |
8 | UC | 0 | 12 | Individual |
| Breyer 2010245 | Initial 2-hour instruction then supervised outdoor Nordic walking 1 hour, 3× per week; educational session 1× per week | 7 | One educational session per week | 6 | 13 | Group |
| Brooks 2002193 | Group discussion about home programme and exercise sessions 2 hours per month led by physical therapist; telephone support from physical therapist 1× per month | 5 | Visit to physical therapist every 3 months to discuss home programme | 4 | 52 | Mixed |
| Bucknall 201263 | Supported self-management – nurse visits: 4 × 40 minutes for 2 months then every 6 months | 6 | UC | 0 | 52 | Individual |
| Busch 1988194 | Home exercise programme: endurance and resistance exercise 5× per week, fortnightly visit by physiotherapist | 2 | No home exercise, but visits by physiotherapist every 3 weeks to monitor activity level | 0 | 18 | Individual |
| Cai 2006199 | Education – disease and disease management | 6 | Control | 0 | 26 | Group |
| Carr 2009195 | 9–15 × 2-hour PR-type sessions in 3 weeks (inpatient or outpatient setting) | 6 | UC | 6 | 3 | Mixed |
| Casas 2006, Garcia-Aymerich 200771,72 | Integrated care – comprehensive assessment of patients at discharge; SM education programme by specialised respiratory nurse: 2 hours before discharge, with reinforcement sessions via telephone: 1× per week for 1 month; individually tailored care plan; access to specialised nurse through ICT platform including a web-based call centre | 10 | UC: scheduled visits from physician usually every 6 months | 0 | – | Individual |
| Chan 2010, Chan 2011212,272 | (1) T’ai chi qigong – 13 movements of breathing regulating led by a qualified t’ai chi qigong master: 60 minutes, 2× per week; patients advised to practise exercises for 1 hour daily, DVD and pictures given; | 4 | Control – usual activities (arranged to join community activities to ensure consistent attendance) | 0 | 13 | Group |
| (2) exercise – PLB and diaphragmatic breathing; advised to perform breathing and walking: 1 hour per day; leaflets with instructions/pictures given (arranged to join community activities to ensure consistent attendance) | 3 | |||||
| Cockcroft 1987142 | Respiratory health worker home visit (health education and support): approximately 1× per month | 3 | Control | 0 | – | Individual |
| Coultas 2005112 | Nurse-assisted management: 2 × 4-hour sessions | UC | 1 | 26 | Mixed | |
| (1) nurse-assisted collaborative management including self-management skills: additional 8 hours; | 5 | |||||
| (2) nurse-assisted medical management | 5 | |||||
| Covey 2001113 | IMT with loaded threshold device at home: 30 minutes per day, 5 days per week; home visit by nurse 1× per week | 1 | Education programme: nurse visit 1–1.5 hours every 2 weeks | 6 | 16 | Individual |
| de Blok 2006181 | PR plus lifestyle physical activity counselling programme with feedback (pedometer) Physical therapist-led individual exercise counselling sessions, 30 minutes pre-week 1, week 1 and week 5 of programme |
3 | PR | 3 | 9 | Mixed |
| Dheda 200473 | Regular outpatient follow-up, post discharge – review of inhaler technique/medications, smoking cessation advice, exercise and nutrition advice, introduction to a support group: ≤ 4 × over 6 months | 6 | Control – primary care follow-up, post discharge: visits made to primary care teams on need to basis | 0 | 26 | Unclear |
| Donesky-Cuenco 2009114 | Yoga training – 24 × 1-hour sessions | 4 | UC | 1 | 12 | Mixed |
| Dourado 2009233 | (1) Strength training and low intensity general exercise: 31 × hour sessions over 3 weeks; | 2 | Strength training programme: 3 × 1-hour sessions over 3 weeks | 1 | 12 | Individual |
| (2) low-intensity general exercise training: 31 × hour sessions over 3 weeks | 2 | |||||
| du Moulin 2009206 | Outpatient PR programme 6 hours per day, 5 days per week Plus maintenance walking and 4-weekly telephone call for motivation |
7 | Outpatient PR programme: 6 hours per day, 5 days per week | 6 | 3 | Remote |
| Eaton 2009227 | Inpatient PR with COPD nurse, plus exercise 30 minutes per day Outpatient visit 1 hour, 2× per week |
11 | Review by COPD nurse standardised care including advice | 1 | 8 | Mixed |
| Effing 2009, Effing 2011161,278 | Four weekly 2-hour SM sessions with training in self-management exacerbation (respiratory nurse and physiotherapist) | 11 | Four weekly 2-hour SM sessions without training in SM exacerbation (respiratory nurse and physiotherapist) | 11 | 7 | Group |
| Efraimsson 2008218 | Self-care education with motivational interviewing from primary care nurse 2 routine consultations 3–5 months apart, plus two additional nurse sessions | 13 | Two routine consultations 3–5 months apart | 0 | 13 | Individual |
| Egan 200269 | Case manager assessment, education and review: on admission, during hospitalisation and 1 and 6 weeks post discharge | 5 | UC | 0 | 6 | Individual |
| Elci 2008236 | PR 1 × 30 minutes session with nurse, then home rehabilitation: 20 minutes exercise 2× per day, 5 days per week. 24 sessions up to 90 minutes | 7 | UC plus instructions on use of respiratory medicines | 0 | 13 | Remote |
| Elliott 2004162 | (1) Hospital rehabilitation: group circuit training and aerobic exercise, 1.5 hours, 2× per week for 3 months plus home (unsupervised circuit and aerobic), 1.5 hours, 2× per week for 9 months; | 3 | Community PR: 1.5 hours, 2× per week (low-intensity group exercise) | 3 | 52 | Mixed |
| (2) hospital rehabilitation, 1.5 hours, 2× per week for 3 months plus 9 months community, 1.5 hours, 2× per week | 3 | |||||
| Emery 1998115 | (1) Exercise, education and stress management: 37 exercise sessions, 16 educational lectures, 10 stress management classes; 4 hours per day for 5 weeks, then 3× per week for 5 weeks; | 4 | Waiting list control | 0 | 10 | Group |
| (2) education and stress management: 16 educational lectures, 10 stress management classes | 3 | |||||
| Engstrom 1999219 | Exercise training sessions at hospital: 45 minutes 2× per week for 6 weeks, weekly for 6 weeks, fortnightly for 6 weeks then monthly; educational sessions 2× as group, others individualised | 7 | Usual outpatient care | 0 | 52 | Mixed |
| Fernandez 2009171 | Hospital based rehabilitation (physiotherapist): 21 × hour sessions; four home visits over 2 months for 1 hour; one home visit/month for 9 months (physiotherapist) | 7 | UC plus three sessions of respiratory education | 3 | 52 | Individual |
| Finnerty 2001143 | PR: 2 hours 2× per week for 6 weeks plus invitation to patient support group and encouragement to exercise at home a minimum of 5× per week | 7 | Control: outpatient department at 3-monthly intervals | 0 | 6 | Group |
| Foy 2001116 | Long-term exercise therapy: 1 hour 3× per week | 1 | Short-term exercise therapy: 1 hour 3× per week for 3 months | 1 | 78 | Unclear |
| Gallefoss 1999, Gallefoss 2000, Gallefoss 2002, Gallefoss 2004255,280,281,391 | Educational intervention: booklet plus multidisciplinary group education, 2 × 2 hours, individual treatment plan, 1 or 2 individual nurse and/or physiotherapist sessions | 10 | Follow-up by GP | 0 | – | Mixed |
| Ghanem 2010264 | Healthy lifestyle lectures and 4× one-to-one education sessions including exercise instruction Post discharge: Home-based exercise training (RMT, endurance and strength) alternate days with outpatient department supervision every 2 weeks |
7 | Standard medical therapy | 0 | 8 | Individual |
| Gilmore 2010117 | Factorial design (1) COPD educational SM booklet; | 7 | Control | 3 | – | Individual |
| (2) standardised home visit, including disease management, safe home environment and family support | 4 | |||||
| Gohl 2006207 | Rehabilitation/training programme – fitness studios for strength training and home training mainly on bicycle; practical and theoretical training under guidance from a trainer 90 minutes, 1× per week Mean training time between 2.4 (first phase) and 4.2 hours per week (last phase) Total training time 166 hours on average per person |
2 | Medication according to guidelines only | 0 | 52 | Group |
| Goldstein 1994, Goldstein 1997, Guyatt 1999196,392,393 | Inpatient programme for 2 months: exercise, education and relaxation; 4 month graduated discharge programme with outpatient rehabilitation, home rehabilitation, home visits from physiotherapist weekly for 1 month, fortnightly for 1 month and then monthly | 4 | Conventional community care from GP and respiratory specialist | 0 | 24 | Mixed |
| Green 2001144 | PR – 14 education sessions from a range of health-care professionals; exercise, 2× per week | 2 | PR – education from a range of health-care professionals; exercise: 4 weeks | 2 | 7 | Group |
| Güell 2000172 | Outpatient rehabilitation: Months 1–3: 30 minutes 2× per week breathing retraining Months 4–6: 30 minutes 5× per week supervised exercise for 3 months Months 7–12: Weekly supervised breathing exercises and home exercise |
5 | UC | 0 | 26 | Group |
| Güell 2006173 | PR: relaxation, breathing retraining, postural drainage strategies 2 months, 2 × 30 minutes/week. 4 × 45- to 60-minute educational sessions Exercise training, 5 × 30-minutes sessions weekly on cycle ergometer further 2 months |
6 | UC | 0 | 16 | Group |
| Guyatt 1992118 | Intervention 1: IMT with inspiratory resistance device and nose clip Intervention 2: IMT with inspiratory resistance device All groups: Used device 10 minutes 5× per day, 20 minutes training by nurse at start and 1× per week for 4 weeks, then fortnightly 4× to 3 months then monthly to 6 months |
2 | Control 1: sham training of devise with minimal resistance plus nose clip and diaphragmatic breathing Control 2: sham training of devise with minimal resistance and diaphragmatic breathing; same regime as intervention groups |
2 | 26 | Individual |
| Hermiz 200267 | Community nurse home visits for SM advice: 2 visits at 1 and 4 weeks post discharge | 7 | UC | 0 | 4 | Individual |
| Hernandez 2000282 | Home-based exercise training: 20 minutes’ distance shuttle walking externally paced with tape recording, 1 hour per day, 6 days per week, with hospital visit every 2 weeks | 2 | Hospital visits every 2 weeks for clinical supervision | 0 | 12 | Individual |
| Hernandez 200368 | Assessed by specialist team in emergency room, immediate or early discharge with specialist nurse home assessment and further visits (maximum four) or telephone support; structured assessment by nurse including knowledge of disease and compliance with treatment | 10 | UC – admission or discharge without specialist nurse support | 0 | 8 | Individual |
| Hill 2006163 | IMT with threshold loading device: supervised sessions, 21 minutes 3× per week | 1 | Sham training with 10% loading: supervised sessions, 21 minutes 3× per week | 1 | 8 | Unclear |
| Holland 2004164 | Upper limb: 15 minutes‘ weighted exercises plus lower limb training: 30 minutes‘ walking/cycling | 1 | Lower limb: 30 minutes walking/cycling plus sham upper limb training | 1 | 6 | Group |
| Hoogendoorn 2009, Van Wetering 2010, Hoogendoorn 2010182,273,274 | Supervised interdisciplinary rehabilitation (physiotherapist, nurse and dietitian), 30 minutes, 2 × per week plus 20 minutes’ active maintenance (physiotherapy) programme with one visit per month | 4 | Usual care: pharmacology according to guidelines and short smoking cessation advice | 2 | 104 | Mixed |
| Hospes 2009183 | Pedometer-based exercise counselling strategy, 5 × 30-minute individually tailored sessions Exercise counsellor |
1 | UC | 0 | 12 | Individual |
| Hsiao 2003259 | (1) Targeted resistive IMT: 15 minutes, 2 × per day, 5 × per week; | 3 | Control | 2 | 8 | Individual |
| (2) pressure threshold IMT device: 15 minutes, 2 × per day, 5 × per week | 3 | |||||
| Both groups wore nose clips, training intensity set to 50% of each patient’s maximal inspiratory pressure and adjusted as necessary every 2 weeks | ||||||
| Hynninen 2010256 | Cognitive–behavioural therapy – psychoeducation/awareness; relaxation; cognitive therapy; behavioural activation; fear-based exposure; sleep management skills: 2 hours, 1× per week | 3 | UC plus telephone contact with study personnel for assessment, monitoring and giving basic information: 5–10 minutes, 1× per 2 weeks | 1 | 7 | Group |
| Janaudis-Ferreira 2011197 | Supervised resistance arm training program 3 × per week; endurance exercise training, breathing exercises, relaxation and SM education | 4 | Sham training: Upper limb flexibility and stretching 3× per week Endurance exercise training, breathing exercises, relaxation and SM education |
1 | 6 | Individual |
| Jang 2006267 | PR – education: approximately 1 hour per week; breathing retraining and exercise training: approximately 1 hour per week, relaxation and counselling: 20 minutes per week | 6 | Control – education | 1 | 8 | Group |
| Jarab 2012266 | Pharmacist intervention at outpatient department: education about COPD and its management, motivational interviewing to increase adherence to treatment | 5 | Usual outpatient department care | 0 | 1 | Individual |
| Karapolat 2007237 | Hospital outpatient-based PR: education, aerobic and resistance exercise, 16 sessions | 8 | No rehabilitation | 0 | 8 | Group |
| Katiyar 2006240 | Pranayama – six exercises (Bhastika, Kapalabhati, Vhasya, Anulom Vilom, Bhramid, Udgeeth): at least 30 minutes, 1× per day, 6 × per week | 2 | Usual physical activity | 0 | 13 | Unclear |
| Kayahan 2006238 | Rehabilitation: education, relaxation, bronchial hygiene, breathing retraining, exercise, 2.5 hours per week, 3 days per week | 7 | UC | 0 | 8 | Group |
| Khdour 2009, Khdour 2011251,279 | Structured individually tailored education session, 1 hour from pharmacist; medication, inhaler technique, symptoms management, exercise, action plan. Reinforced at 6-month outpatient visit, and telephone at 3 and 9 months | 10 | UC | 0 | 1 | Mixed |
| Kim 1993119 | IMT | 1 | Sham training with light pressure load | 1 | 26 | Individual |
| Ko 2011213 | Early PR: 2 hours 3× per week; 20 minutes’ home exercise recommended daily | 6 | UC: one consultation with nurse specialist and recommendation to walk and stretch daily | 4 | 8 | Group |
| Koff 2009120 | Proactive integrated care (disease-specific education, teaching SM, enhanced communication, remote home monitoring) Respiratory therapist |
6 | UC | 0 | 13 | Remote |
| Koppers 2006184 | Respiratory muscle endurance training using tube: home-based, 15 minutes 2× per day, daily for 5 weeks plus weekly clinic visits to monitor training | 1 | Sham training: home-based 15 minutes 2× per day, daily for 5 weeks plus weekly visits to monitor training | 1 | 5 | Individual |
| Kunik 2008121 | Cognitive–behavioural therapy group treatment: 8 × 1 hour | 1 | COPD education | 8 | 8 | Group |
| Kwok 200470 | Trained community nurse – visit patients to provide health counselling (drug and nutrition advice, inhaler technique): before discharge; home visit to review patient, health counselling (including drug and diet regime, home modifications, encourage physical exercise), psychological support: weekly home visits for 4 weeks then monthly for 6 months | 8 | Control – followed up by same geriatricians/respiratory physicians involved in intervention group Physicians occasionally referred patients to community nurse, not more than once |
0 | 26 | Individual |
| Lamers 2010185 | Two to ten (average four) nurse contacts at home including cognitive–behavioural therapy and SM | 3 | UC according to guidelines of Dutch College of General Practitioners | 0 | 13 | Individual |
| Larson 1988122 | IMT (30% load) using nose clip: 15 minutes per day for 1 week, then gradually 30 minutes per day for 7 weeks | 1 | IMT (15% load) using nose clip: 15 minutes per day for 1 week, then gradually 30 minutes per day for 7 weeks | 1 | 8 | Unclear |
| Larson 1999123 | (1) IMT with threshold loaded device: 30 minutes per day, 5× per week with weekly nurse home visit; | 2 | Health education: nurse home visits, 1 hour every 2 weeks for 16 weeks | 1 | 17 | Individual |
| (2) cycle ergometry (interval) at home 20 minutes per day, 5× per week with weekly nurse home visit; | 2 | |||||
| (3) IMT and cycle ergometry | 3 | |||||
| Lee 200266 | Care protocol for community nurses who followed up patients for 6 months | 5 | No care protocol after discharge to nursing home | 0 | 26 | Individual |
| Leung 2010165 | Supervised indoor walking: 30–45 minutes, 3× per week | 1 | 30–45 minutes’ supervised indoor cycling 3× per week | 1 | 8 | Group |
| Li 2002200 | Nutritional support; PLB and abdominal breathing: 10–15 minutes, 2× per day | 2 | UC (normal food and exercise) | 1 | 13 | Mixed |
| Liddell 2010145 | PR (twice weekly) – supervised, individually prescribed endurance walking: 1 hour; instructions to exercise at home: ≤ 3× per week; education on managing disease delivered by multidisciplinary health professionals team: 1 hour | 6 | PR (once weekly) – supervised, individually prescribed endurance walking: 1 hour; instructions to exercise at home: ≤ 3× per week; education on managing disease delivered by multidisciplinary health professionals team: 1 hour | 7 | 8 | Mixed |
| Lindsay 2005214 | Tiotropium plus PR – psychoeducation on knowledge of SM, motivation to exercise, psychological support: 6 weekly sessions of 2 hours | 8 | Tiotropium | 0 | 13 | Group |
| Linneberg 2012224 | Post 7-week comprehensive PR programme: six supervised exercise sessions at weeks 9, 11, 13, 18, 26 and 52 (from start of PR) | 6 | No supervised exercise sessions | 6 | 45 | Group |
| Littlejohns 1991146 | Respiratory health worker – health education directed at the patient and primary care team, monitoring of treatment compliance and optimising treatment, ensuring correct inhaler technique and supervision of domiciliary oxygen, monitoring spirometry results to detect and treat exacerbations and worsening heart failure early and liaison between GP- and hospital-based services | 5 | UC | 0 | 52 | Individual |
| Liu 2008260 | Home-based endurance exercise programme with walking tempo controlled by cell phone music: daily walking at set pace until unable to keep it up Monthly hospital visits × 3 to reset walking pace with telephone support if missed daily exercise Three-monthly clinics for 9 months Home rehabilitation programme booklet and DVD |
4 | Home rehabilitation programme booklet and DVD including instructions for home walking | 3 | 52 | Individual |
| Livermore 2010166 | 4× 1-hour sessions of cognitive–behavioural therapy | 3 | UC | 0 | 4 | Individual |
| Lord 2010147 | 30 minutes’ session on breathing techniques from respiratory physiotherapists: 2× per week singing group | 5 | 30 minute session on breathing techniques from respiratory physiotherapists | 2 | 6 | Group |
| Madariaga 2007174 | Daily IMT, 15 minutes, 2× per day; increased resistance weekly to maximum tolerated load: | IMT device at minimum load, 15 minutes, 2 × per day | 0 | 6 | Individual | |
| intervention 1: IMT with threshold device; | 1 | |||||
| intervention 2: IMT with resistive device | 1 | |||||
| Mador 2004126 | Endurance training: 3× per week plus education 1 hour per week | 2 | Combined training – endurance exercise 3× per week; education 1 hour per week; strength 3× per week | 2 | 8 | Group |
| Mador 2005125 | Combined training (calisthenics, endurance training plus hyperpnea training) 15–20 minutes per day, 3 days per week plus weekly 1-hour educational class | 3 | Endurance training included calisthenics with and without weights, plus weekly 1-hour educational class | 2 | 8 | Group |
| Mador 2009124 | Continuous steady-paced exercise training: 3× per week; weekly 1-hour education | 2 | Interval exercise training: 3× per week; weekly 1-hour education | 2 | 8 | Individual |
| Magadle 2007254 | General exercise reconditioning programme plus IMT with resistive device delivered in community setting by respiratory therapist: 1 hour 3× per week | 2 | General exercise reconditioning programme plus IMT with resistive device set to level to provide ‘sham’ training: 1 hour 3× per week | 1 | 26 | Group |
| Maltais 2008198 | Group-based educational programme: 2× per week for 4 weeks followed by home exercise programme: 3× per week for 8 weeks Introductory home visit at start and weekly telephone calls from exercise trainer |
3 | Group-based educational programme: 2× per week for 4 weeks followed by outpatient exercise programme (strength and aerobic) at hospital: 2× per week for 8 weeks | 3 | 12 | Mixed |
| Man 2004148 | Community-based PR: 2 × 2 hours per week | 6 | UC | 0 | 8 | Group |
| Martin 2004228 | Action plan agreed at one consultation with respiratory nurse, reinforcement by home visits from research nurse at 3, 6 and 12 months | 2 | Visits from research nurse at start, 3, 6 and 12 months for ‘routine support’ | 0 | 52 | Individual |
| McGeoch 2006229 | Education on use of SM (action plan): early recognition of exacerbations and range of self-initiated interventions Individual 1-hour session practice nurse or respiratory educator |
1 | UC | 6 | 1 | Individual |
| Monninkhof 2003, Monninkhof 2004186,300 | SM education course and fitness programme: Education: 5 × 2 hours; exercise: 2 × 1 hour per week | 10 | UC from chest physician | 1 | 52 | Group |
| Moore 2009284 | Video multidisciplinary education (19 minutes) about benefits of exercise watched with physiotherapy Home exercise video programme: 30 minutes, 4× per week Also given educational booklet |
8 | Educational booklet only | 8 | 6 | Individual |
| Mota 2007175 | EMT using expiratory threshold device under supervision of respiratory physiotherapist: 30 minutes 3× per week | 3 | Sham training: using expiratory threshold device under supervision of respiratory physiotherapist: 30 minutes, 3× per week | 3 | 5 | Unclear |
| Mularski 2009127 | MBBT – self-administered MBBT practice: body scan meditations, sitting and walking mindfulness, mindful movements including pleasant and unpleasant events and reactions to stress: 1× per week, plus practice at home | 1 | Support group control – group sessions, semistructured conversations about COPD: 1× per week | 3 | 8 | Group |
| Murphy 2005252 | Twelve physiotherapists supervised exercise sessions – aerobic and upper limb strengthening: 30–40 minutes plus 15 minutes’ unsupervised exercise on other days, 2× per week in home | 1 | UC | 0 | 6 | Individual |
| Nakamura 2008261 | (1) Aerobic exercise 20 minutes’ walking, 3× per week plus 60 minutes’ strength training, relaxation and breathing; | 4 | Control: no exercise programme | 0 | 12 | Group |
| (2) aerobic exercise 20 minutes walking 3× per week plus 60 minutes’ recreational activities to improve balance, coordination and agility | 1 | |||||
| Ng 2011215 | Health qigong: 12 PR sessions, with four consisting of 45 minutes’ qigong | 5 | Control | 4 | – | Unclear |
| Nguyen 2008128 | Dyspnoea SM programme; 1.5- to 2-hour consultation, independent daily exercise: 30 minutes, 14 contacts weekly 1 month, then fortnightly; six group sessions, 1 hour duration | 6 | Internet-based dyspnoea SM | 6 | 26 | Mixed |
| Nguyen 2009129 | MOBILE-coached: individualised exercise plan and generic exacerbation with action plan and nurse; 150 minutes of moderate-intensity exercise with daily monitoring and weekly feedback | 4 | MOBILE-self monitored: individualised exercise plan and generic exacerbation with action plan and nurse; 150 minutes of moderate-intensity exercise | 4 | 26 | Remote |
| Nield 2007130 | Breathing training at baseline, daily practice sessions, 4× per week with clinic visits for reinforcement: | Control | 0 | 4 | Individual | |
| (1) PLB; (2) EMT | 1, 1 | |||||
| Ninot 2011250 | Two hours, 2× per week of group education and exercise | 7 | Usual primary care | 1 | 4 | Group |
| Normandin 2002131 | Comprehensive PR, including high-intensity endurance exercise: 30 minutes, 3 hours 2× per week | 2 | Comprehensive PR, including low-intensity calisthenics: 30 minutes, 3 hours, 2× per week | 2 | 8 | Group |
| Norweg 2005132 | (1) Exercise training plus activity training (dyspnoea management): 15 MBBT 1-hour exercise sessions plus home exercise for 20 minutes 2–3× per week; activity training 6 × 1 hour; | 2 | Exercise training: 15 × 1-hour exercise sessions plus home exercise for 20 minutes, 2–3× per week | 1 | 10 | Group |
| (2) exercise training plus lectures (lifestyle, stress, nutrition, relaxation): 15 × 1-hour exercise sessions plus home exercise for 20 minutes 2–3× per week | 6 | |||||
| Lectures: 6 × 45 minutes | ||||||
| Oh 2003284 | Home-based PR: IMT, aerobic, resistance exercise and stretching; relaxation: 5× per day; two nurse telephone calls per week; relaxation 2× per day | 11 | Individual education session and booklet | 7 | 8 | Individual |
| O’Neill 2007150 | PR: supervised exercise session 2× per week plus one unsupervised home session | 2 | PR: supervised exercise session 1× per week plus two unsupervised home sessions | 2 | 6 | Group |
| Ortega 2002176 | (1) Strength and endurance training: 1 hour, 3× per week; | 1 | Strength training: 1 hour 3× per week | 1 | 12 | Unclear |
| (2) endurance training: 1 hour 3× per week | 1 | |||||
| O’Shea 2007167 | Resistance exercise programme with elasticated bands: sessions 3× per week (one hospital, two at home) | 1 | UC | 0 | 12 | Mixed |
| Ozdemir 2010239 | Water-based PR: 35 minutes, 3× per week for 12 sessions | 1 | Usual medical therapy | 0 | 4 | Group |
| Paz-Diaz 2007269 | PR – breathing and exercise: 3 days per week | 2 | Optimal care according to ATS guidance with physician visit every 3 weeks | 1 | 8 | Group |
| Petersen 2008225 | Multimodal exercise training programme 2× per week for 14 sessions plus home walking | 5 | UC | 4 | 7 | Mixed |
| Petty 2006133 | (1) Tailored video – education and exercises prescribed (type, dose, repetitions, frequency) by physician or pulmonologist from a library of segments; | 2 | No video | 0 | 8 | Individual |
| (2) standard video – 2 tapes on PR exercise and education | 2 | |||||
| Pomidori 2012243 | Externally paced speed walking with metronome: 20–30 minutes per day , 4× per week; supervision by exercise therapist, 1× per week for 1 month, then fortnightly telephone support | 2 | Walking (known distance, fixed time): supervision by exercise therapist, 1× per week for 1 month, then fortnightly telephone support | 1 | 52 | Individual |
| Prince 1989151 | Rehabilitation – education, smoking cessation, diaphragmatic breathing, relaxation and exercise plan: 2 hours, 2× per week | 6 | Outpatient attendance: informal, patient-led sessions: 1 hour, 2× per week | 1 | 6 | Group |
| Probst 2011234 | High-intensity endurance and strength training: 1 hour, 3× per week | 1 | Low-intensity calisthenics and breathing: 1 hour, 3× per week | 2 | 12 | Group |
| Puente-Maestu 2000, Puente-Maestu 2003177,275 | Supervised exercise – treadmill exercise supervised by physiotherapist: 1 hour, 4× per week | 3 | Self-monitored exercise: walking 3–4 km for 1 hour 4× per week plus clinic visits 1× per week | 3 | 8 | Unclear |
| Puhan 2006257 | Inpatient PR: 12–15 sessions over 3 weeks plus high-intensity continuous exercise, target workload 70% plus followed by home exercise, 20 minutes per day Physical therapy led |
4 | Inpatient PR: 12–15 sessions over 3 weeks plus high-intensity (50%) and low-intensity (10%) interval exercise training 20 minutes per day followed by home exercise 20 minutes per day Physical therapy led |
4 | 5 | Mixed |
| Rea 2004230 | Chronic disease management programme by GP and practice nurse, care plan, assessment by respiratory physician and nurse, offer of PR, flu immunisation, 3-monthly follow-up in primary care | 7 | Assessment by GP and practice nurse who had guidelines for COPD management and access to PR | 0 | 52 | Individual |
| Regiane Resqueti 2007178 | Home-based PR: 3 × 1 hour individualised education and physical therapy; 3 × hospital exercise training; with weekly visit and telephone call by therapist for 7 weeks; home exercise for 1.5 hours, 5× per week, plus monthly telephone for 4 months | 6 | 3 × hour individualised education and physical therapy | 4 | 26 | Individual |
| Ren 2011201 | (1) PR strategy group 1: aerobic exercise training with PLB and abdominal breathing; education: 20 minutes, 5× per week; | 2 | Control: PR education, 2× per month | 0 | 20 | Group |
| (2) PR strategy group 2 – strategy 1 plus upper and lower limb training | 1 | |||||
| Rice 2010140 | 1 × 1.5 hours’ group education session, individual action plan with rescue medications; monthly telephone support from case manager; telephone number of 24-hour nursing helpline | 8 | Leaflet with summary of COPD care and telephone number of 24-hour nursing helpline | 2 | 52 | Mixed |
| Riera 2001179 | IMT with flow meter device: home training 30 minutes per day, 6× per week with monitoring every 6 weeks | 2 | Sham training with no load | 2 | 26 | Individual |
| Ringbaek 2000226 | PR – education and exercise: 2 hours 2× per week | 5 | Control: conventional community care | 0 | 8 | Group |
| Romagnoli 2006244 | Two repeat PR programmes of 18 sessions each | 4 | One repeat PR programme of 18 sessions | 4 | 52 | Group |
| Rooyackers 2003187 | Inpatient programme: general exercise training (20 minutes) plus eccentric cycle exercise training (15 minutes), 5 days per week | 1 | Inpatient programme: general exercise training (20 minutes) 5 days per week | 1 | 10 | Group |
| Sassi-Dambron 1995134 | Weekly group sessions focusing on strategies to manage dyspnoea | 4 | Six weekly educational sessions not related to COPD | 3 | 6 | Group |
| Scherer 2000258 | Respiratory muscle endurance training through portable device (using nose clip): 15 minutes, 2× per day, 5× per week, 8 weeks | 1 | Sham breathing training through incentive spirometer: 15 minutes, 2× per day, 5× per week, 8 weeks | 1 | 8 | Individual |
| Sewell 2005152 | PR: 2× per week; 1 hour exercise, 1 hour education, daily home walking plus individualised supervised strengthening exercises × 10 and unsupervised home exercise | 8 | PR 2× per week; 1 hour exercise, 1 hour education, daily home walking plus functional exercise programme based on ADL × 10 and unsupervised at home | 8 | 7 | Mixed |
| Sewell 2006153 | PR: rolling programme of 14 sessions: relaxation, disease education, chest clearance, etc., plus home exercise programme; 2 hours (1 hour supervised exercise and 1 hour education), 2× per week | 8 | PR: rolling programme of 14 sessions: relaxation, disease education, chest clearance, etc., plus home exercise programme; 2 hours (1 hour supervised exercise and 1 hour education), 2× per week | 8 | 7 | Group |
| Seymour 2010154 | PR commencing 1 week post discharge – 2 × 2-hour exercise and education sessions/week | 2 | UC | 1 | 8 | Group |
| Shao 2003202 | Rehabilitation behavioural intervention consisting of psychological, somatic and lifestyle interventions | 5 | Control | 0 | 52 | Mixed |
| Simpson 1992135 | Weight training: 3× per week | 2 | Control: no intervention | 0 | 8 | Unclear |
| Singh 2003241 | Home-based PR: breathing techniques, controlled coughing, energy conservation and walking: 1 hour per day | 4 | UC | 0 | 4 | Individual |
| Sívori 1998263 | Lower limb training: 75% max. capacity ergocycling 45 minutes, 3× per week for 8 weeks Plus upper limb training exercises: five different exercises with ball, bags of sand or wooden bars. 45 minutes’ exercise 3× per week for 8 weeks: 24 sessions total Plus respiratory exercises |
1 | Lower limb training: 75% max. capacity ergocycling 45 minutes, 3× per week for 8 weeks | 1 | 8 | Group |
| Smith 1999168 | Home-based nursing intervention: case conference between primary and secondary health-care team, home review, visits by specialist nurse every 2–4 weeks, addressed education, fitness advice and early identification of exacerbations | 7 | UC | 1 | 52 | Individual |
| Soler 2006180 | Specific programme: monthly clinical visits to specialised clinic and short educational programme; nurse-led group education; an information session for patients and families also provided | 6 | Conventional management | 4 | 52 | Group |
| Solomon 1998, Gourley 1998136,285 | Pharmacist intervention – patient assessment, therapeutic and educational interventions, collaboration with health-care team, patient follow-up through clinic visits or telephone follow-up | 3 | Control | 0 | 26 | Individual |
| Spencer 2010169 | Supervised outpatient exercise 1 hour 1× per week plus unsupervised exercise 1 hour × 4 days (walking plus strength exercises) | 1 | Unsupervised exercise 1 hour × 5 days (walking plus strength exercises), booklet and diary | 1 | 52 | Group |
| Spruit 2002247 | Supervised endurance training at outpatient department: 90 minutes 3× per week | 1 | Supervised resistance training at outpatient department: 90 minutes 3× per week | 1 | 12 | Unclear |
| Sridhar 2008155 | PR 2 hours 2× per week for 4 weeks; followed by supported SM by nurse, monthly telephone, and home visit every 3 months | 7 | PR: 2 hours 2× per week for 4 weeks | 4 | 104 | Mixed |
| Stulbarg 2002, Carrieri-Kohlman 2005, Davis 2006137,394,395 | (1) Dyspnoea SM programme (as control) and training: 24 × 30 minutes’ nurse-coached treadmill exercise sessions;(2) dyspnoea SM programme (as control) and exposure: 4 × 30 minutes’ nurse-coached treadmill exercise sessions | 66 | Dyspnoea SM programme: 3 hours’ individualised dyspnoea SM education over four sessions plus manual, walking prescription, pedometer and instructions to exercise at home for 20 minutes 4× per week | 6 | 8 | Unclear |
| Subin 2010242 | Exercise training: 5× per week | Upper limb exercise training 5× per week | 2 | 4 | Unclear | |
| (1) Upper and lower limbs; (2) lower limbs | 2, 2 | |||||
| Theander 2009220 | PR programme: physiotherapist, dietitian (3×), occupational therapist (3×), nurse (2×); 1 hour, 2 days per week | 8 | UC | 0 | 12 | Group |
| Toshima 1990, Ries 1995138,276 | Comprehensive PR programme: education, breathing techniques, psychosocial support and exercise, 12 sessions | 12 | Educational control programme – did not include exercise or behavioural elements or individualised instruction, four fortnightly sessions | 4 | 8 | Group |
| Trappenburg 2011188 | Individualised action plan: consultation with nurse case manager, telephone follow-up at 1 and 4 months | 9 | UC plus consultation with nurse case manager | 7 | 17 | Individual |
| Troosters 2000248 | Exercise training programme: 1.5 hours 3× per week for 3 months, reducing to 2× per week for the next 3 months | 1 | Usual medical care | 0 | 26 | Unclear |
| Van Gestel 2012208 | Exercise training plus respiratory feedback training (daily practice of controlled breathing) – 1.5 hours, 3× per week for 3–4 weeks | 2 | Exercise training: 1.5 hours, 3× per week for 3–4 weeks | 1 | 4 | Unclear |
| Vogiatzis 2002265 | PR including supervised interval training: cycling 40 minutes per day, 2× per week in supervised groups | 7 | PR including supervised continuous training: cycling 40 minutes per day, 2× per week in supervised groups | 7 | 12 | Group |
| Vonbank 2012246 | (1) Strength and endurance training: 2× per week; | 1 | Strength training: 2× per week | 1 | 12 | Unclear |
| (2) endurance training, building up to 60 minutes 2× per week | 1 | |||||
| Wadell 2004221 | (1) Physical aerobic training in water: 45 minutes 3× per week; | 1 | Control | 0 | 12 | Group |
| (2) physical aerobic training on land 45 minutes, 3× per week | 1 | |||||
| Wakabayashi 2011262 | Integrated care: 6 × 30 minutes’ individual tailored education | 8 | UC: standard education | 7 | 26 | Individual |
| Wang 2004203 | Resistance abdominal breathing exercises: 15–30 minutes, 2× per day | 2 | Control: simple abdominal breathing exercise | 1 | 13 | Individual |
| Warlies 2006209 | Specific education programme: Usually 2 × 3 hours week-days or 2 hours on a week-day and 4 hours on Saturday Telephone hotline available after completion of the sessions Materials included brochures, diary, inhalers, peak flow meters, anatomical models |
1 | Routine advice (approximately 15 minutes) by practice nurse on use of inhalers, description of medication plan, self-help strategies, advice on smoking, explanation of breathing apparatus | 4 | 26 | Group |
| Waterhouse 2010277 | Hospital rehabilitation: 2 hours 2× per week Half received telephone support: months 3, 4, 5, 6, 9, 12, 15 |
5 | Community rehabilitation: 2 hours 2× per week Half received telephone support: months 3, 4, 5, 6, 9, 12, 15 |
5 | 6 | Group |
| Watson 1997231 | Action plan and booklet from primary care nurse plus antibiotic and prednisolone from GP | 2 | UC | 0 | 1 | Individual |
| Wedzicha 1998157 | Exercise training and education programme: 2× per week | 10 | Education programme: 2× per week | 9 | 8 | Unclear |
| Weekes 2009158 | Leaflet of nourishing snacks, drinks and food fortification; dietary counselling provided dietitian and supply of milk powder | 1 | Control: leaflet of nourishing snacks, drinks and food fortification with no discussion | 1 | 26 | Individual |
| White 2002159 | PR programme with exercise and education: 2 hours 2× per week | 9 | Individual 1-hour educational session with booklet and exercise advice; advised 30 minutes’ exercise 4× per week | 9 | 6 | Group |
| Wijkstra 1994189 | Visit to physiotherapist 2× per week for exercise, plus twice daily practice; monthly home visit by nurse for education and SM strategies’; monthly visit to GP | 6 | Control group | 0 | 12 | Individual |
| Wijkstra 1995190 | Weeks 1–12: 30 minutes’ exercise at outpatient department physiotherapist 2× per week plus monthly coaching by GP and nurse; | No rehabilitation | 0 | 78 | Individual | |
| (1) weekly physiotherapy; | 7 | |||||
| (2) monthly physiotherapy | ||||||
| Wittmann 2007210 | As control group, with an additional component of behaviour training in group setting: 90 minutes, 4× per week; discussion with doctor to establish action plan for emergency situations: 30–60 minutes | 10 | Inpatient rehabilitation programme – including tailoring of medication, learning correct inhaler techniques, physical training, breathing and SM techniques; if necessary smoking cessation support, psychological help, nutritional advice | 7 | 3 | Group |
| Wong 200574 | Nurse-initiated telephone follow-up to increase self-efficacy: two telephone calls (weeks 1 and 3) | 2 | UC | 0 | 3 | Individual |
| Wood-Baker 2006170 | Information booklet and individual exercise session with specialist nurse, plus SM plan with action plan based on early recognition exacerbation | 12 | Information booklet and individual exercise session with specialist nurse plus UC | 11 | 1 | Individual |
| Wright 2003211 | Resistance training – muscle habituation: 2 weeks; hypertrophic training: 5 weeks each, 2× then 3× per week, 60 minutes then 120 minutes | 2 | Control | 0 | 12 | Group |
| Xu 2010204 | (1) Integrative rehabilitation (traditional and modern) – qigong, diaphragmatic breathing, PLB, upper and lower limb training; | 3 | UC | 0 | 52 | Individual |
| (2) modern rehabilitation – diaphragmatic breathing, PLB, upper and lower limb training; | 2 | |||||
| and (3) traditional rehabilitation and qigong | 2 | |||||
| Yamaguti 2012235 | Diaphragmatic breathing training programme: 45 minutes 3× per week supervised by physiotherapist | 1 | UC | 0 | 4 | Individual |
| Yeh 2010139 | T’ai chi – warm-up exercise, five simplified t’ai chi movements and meditative breathing all delivered by two certified and experienced instructors: 1 hour, 2× per week; 35 minutes’ instructional video to take home and practice at least 3× per week | 3 | UC | 1 | 12 | Group |
| Zhang 2008205 | PR 15 minutes, 3× per day: (1) PR with PLB; (2) PR | 22 | No PR | 0 | 8 | Unclear |
Appendix 24 Mapping of components of self-management interventions across intervention and comparator arms: review 4
| Author, year | Intervention | Comparator | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease knowledge | Unspecified SM | RMT | Action planning | Breathing | Smoking | Medication | Bronchial hygiene | Nutrition | Psychological | Preventative | Inhaler | Energy conservation | Support groups | Exercise | Enhanced access | Other | Total number | Disease knowledge | Unspecified SM | RMT | Action planning | Breathing | Smoking | Medication | Bronchial hygiene | Nutrition | Psychological | Preventative | Inhaler | Energy conservation | Support groups | Exercise | Enhanced access | Other | Total number | |
| Aimonino Ricauda 200875 | 1 | – | – | 1 | 1 | 1 | 1 | – | 1 | – | – | – | 1 | – | 1 | 1 | – | 9 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Arnardottir 2006216 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Arnardottir 2007217 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Barakat 2008249 | – | – | – | – | 1 | – | – | – | 1 | – | – | – | – | – | 1 | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Bauldoff 2002108 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Bauldoff 2005109 A | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Bauldoff 2005109 B | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Bauldoff 2005109 C | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Beckerman 2005253 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Behnke 200064 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 3 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Bendstrup 1997222 | 1 | – | – | – | 1 | 1 | 1 | – | 1 | 1 | – | 1 | – | – | 1 | – | 1 | 9 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Bernard 1999191 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | 1 | – | 1 | – | – | – | – | 1 | – | – | 4 |
| Berry 2010110 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | 3 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Bestall 2003141 | 1 | – | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | 1 | 1 | – | 1 | – | – | 11 | 1 | – | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | 1 | 1 | – | – | – | – | 10 |
| Bjornshave 2005223 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Blake Jr 1990111 | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | 1 | – | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Bonilha 2009232 | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | 1 | 4 | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 | 2 |
| Bourbeau 2003192 | 1 | 1 | – | 1 | 1 | 1 | – | 1 | 1 | 1 | – | 1 | 1 | – | 1 | 1 | – | 12 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Boxall 2005160 | 1 | – | – | – | 1 | – | 1 | 1 | – | 1 | – | 1 | 1 | – | 1 | – | – | 8 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Breyer 2010245 | 1 | – | – | – | 1 | 1 | 1 | 1 | 1 | – | – | – | – | – | 1 | – | – | 7 | 1 | – | – | – | 1 | 1 | 1 | 1 | 1 | – | – | – | – | – | – | – | – | 6 |
| Brooks 2002193 | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | 1 | – | 5 | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 4 |
| Bucknall 201263 | 1 | – | – | 1 | 1 | – | 1 | – | – | – | – | – | – | 1 | – | 1 | – | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Busch 1988194 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Cai 2006199 | 1 | – | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | – | – | – | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Carr 2009195 | 1 | – | – | – | 1 | – | 1 | – | – | 1 | – | – | 1 | – | 1 | – | – | 6 | 1 | – | – | – | 1 | – | 1 | – | – | 1 | – | – | 1 | – | 1 | – | – | 6 |
| Casas 200671 | 1 | 1 | – | 1 | 1 | 1 | 1 | – | 1 | – | – | 1 | – | – | 1 | 1 | – | 10 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Chan 2010212 A | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Chan 2010212 B | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Chan 2010212 C | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 4 | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 3 |
| Cockcroft 1987142 | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Coultas 2005112 A | 1 | 1 | – | 1 | – | 1 | 1 | – | – | – | – | – | – | – | – | – | – | 5 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Coultas 2005112 B | 1 | 1 | – | 1 | – | 1 | 1 | – | – | – | – | – | – | – | – | – | – | 5 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Coultas 2005112 C | 1 | 1 | – | 1 | – | 1 | 1 | – | – | – | – | – | – | – | – | – | – | 5 | 1 | 1 | – | 1 | – | 1 | 1 | – | – | – | – | – | – | – | – | – | – | 5 |
| Covey 2001113 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 | 1 | 1 | – | 1 | 1 | – | – | 1 | – | – | – | – | 6 |
| de Blok 2006181 | – | – | – | – | – | – | – | – | 1 | 1 | – | – | – | – | 1 | – | – | 3 | – | – | – | – | – | – | – | – | 1 | 1 | – | – | – | – | 1 | – | – | 3 |
| Dheda 200473 | – | – | – | – | – | 1 | 1 | – | 1 | – | – | 1 | – | 1 | 1 | – | – | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Donesky-Cuenco 2009114 | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 4 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Dourado 2009233 A | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Dourado 2009233 B | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Dourado 2009233 C | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| du Moulin 2009206 | 1 | – | – | – | 1 | 1 | – | – | 1 | 1 | – | – | – | – | 1 | 1 | – | 7 | 1 | – | – | – | 1 | 1 | – | – | 1 | 1 | – | – | – | – | 1 | – | – | 6 |
| Eaton 2009227 | 1 | – | – | 1 | 1 | – | 1 | 1 | 1 | 1 | 1 | – | 1 | – | 1 | 1 | – | 11 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Effing 2009161 | 1 | 1 | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | – | – | – | 1 | 1 | – | 11 | 1 | 1 | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | – | – | – | 1 | 1 | – | 11 |
| Efraimsson 2008218 | 1 | 1 | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | – | 1 | 1 | – | 13 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Egan 200269 | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 1 | – | 1 | 1 | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Elci 2008236 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Elliott 2004162 A | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | 3 | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | 3 |
| Elliott 2004162 B | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | 3 | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | 3 |
| Elliott 2004162 C | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | 3 | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | 3 |
| Emery 1998115 A | 1 | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Emery 1998115 B | 1 | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Emery 1998115 C | 1 | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | 4 | 1 | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | 3 |
| Engstrom 1999219 | 1 | – | – | – | 1 | 1 | 1 | – | 1 | – | – | – | 1 | – | 1 | – | – | 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Fernandez 2009171 | 1 | – | 1 | 1 | 1 | – | – | – | – | – | – | 1 | – | – | 1 | 1 | – | 7 | 1 | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | – | – | – | 3 |
| Finnerty 2001143 | 1 | – | – | 1 | 1 | – | – | – | 1 | 1 | – | – | – | 1 | 1 | – | – | 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Foy 2001116 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Gallefoss 1999255 | 1 | 1 | – | 1 | 1 | 1 | 1 | 1 | – | – | 1 | 1 | – | – | 1 | – | – | 10 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Ghanem 2010264 | 1 | – | – | – | 1 | – | 1 | – | 1 | – | 1 | 1 | – | – | 1 | – | – | 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Gilmore 2010117 A | 1 | – | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | 1 | 1 | – | 8 | 1 | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | 3 |
| Gilmore 2010117 B | 1 | – | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | 1 | – | – | 7 | 1 | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | 3 |
| Gilmore 2010117 C | 1 | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | 1 | – | 4 | 1 | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | 3 |
| Gilmore 2010117 D | 1 | – | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | 1 | 1 | – | 8 | 1 | – | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | 1 | – | – | 7 |
| Gilmore 2010117 E | 1 | – | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | 1 | 1 | – | 8 | 1 | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | 1 | – | 4 |
| Gilmore 2010117 F | 1 | – | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | 1 | – | – | 7 | 1 | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | 1 | – | 4 |
| Gohl 2006207 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Goldstein 1994196 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | 1 | – | 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Green 2001144 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Güell 2000172 | 1 | – | – | – | 1 | – | – | 1 | – | 1 | – | 1 | – | – | 1 | – | – | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Güell 2006173 | 1 | – | – | – | 1 | – | – | 1 | – | 1 | – | – | – | – | 1 | – | – | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Guyatt 1992118 | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 2 |
| Hermiz 200267 | 1 | – | – | 1 | 1 | 1 | 1 | – | – | – | – | – | 1 | – | 1 | 1 | – | 8 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Hernandez 2000282 | 1 | – | – | 1 | 1 | 1 | 1 | – | 1 | – | – | – | 1 | 1 | 1 | 1 | – | 10 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Hernandez 200368 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Hill 2006163 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Holland 2004164 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Hoogendoorn 2009182 | 1 | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | 1 | – | – | 4 | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | 2 |
| Hospes 2009183 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Hsiao 2003259 A | – | – | 1 | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 3 | – | – | 1 | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 3 |
| Hsiao 2003259 B | – | – | 1 | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 3 | – | – | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 2 |
| Hsiao 2003259 C | – | – | 1 | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 3 | – | – | – | – | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | 2 |
| Hynninen 2010256 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | 1 | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 |
| Janaudis-Ferreira 2011197 | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Jang 2006267 | 1 | – | 1 | – | 1 | – | – | – | 1 | 1 | – | – | – | – | 1 | – | – | 6 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Jarab 2012266 | 1 | – | – | – | – | 1 | 1 | 1 | – | – | – | – | – | – | 1 | – | – | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Karapolat 2007237 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | – | 1 | – | 1 | – | – | 8 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Katiyar 2006240 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Kayahan 2006238 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Khdour 2009251 | 1 | – | – | 1 | 1 | 1 | 1 | 1 | – | 1 | – | 1 | – | – | 1 | 1 | – | 10 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Kim 1993119 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Ko 2011213 | – | – | – | – | 1 | 1 | – | – | – | 1 | – | 1 | 1 | – | 1 | – | – | 6 | – | – | – | – | – | 1 | – | – | – | 1 | – | 1 | – | – | 1 | – | – | 4 |
| Koff 2009120 | 1 | 1 | – | – | 1 | – | 1 | – | 1 | – | – | – | – | – | – | 1 | – | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Koppers 2006184 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Kunik 2008121 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 2 | 1 | – | – | – | 1 | 1 | 1 | 1 | 1 | – | 1 | – | – | – | 1 | – | – | 8 |
| Kwok 200470 | – | – | – | – | – | – | 1 | – | 1 | 1 | 1 | 1 | 1 | – | 1 | 1 | – | 8 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Lamers 2010185 | – | 1 | – | 1 | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Larson 1988122 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Larson 1999123 A | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 2 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Larson 1999123 B | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 2 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Larson 1999123 C | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 3 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Larson 1999123 D | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 2 |
| Larson 1999123 E | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 3 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 2 |
| Larson 1999123 F | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 2 |
| Lee 200266 | – | – | – | – | 1 | – | 1 | – | 1 | – | – | 1 | – | – | – | 1 | – | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Leung 2010165 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Li 2002200 | – | – | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Liddell 2010145 | 1 | – | – | – | 1 | – | 1 | – | 1 | 1 | – | – | – | – | 1 | – | – | 6 | 1 | – | – | – | 1 | – | 1 | – | 1 | 1 | – | – | – | 1 | 1 | – | – | 7 |
| Lindsay 2005214 | 1 | 1 | – | – | 1 | – | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | – | 8 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Linneberg 2012224 | 1 | 1 | – | – | – | 1 | 1 | 1 | – | – | – | – | – | – | 1 | – | – | 6 | 1 | 1 | – | – | – | 1 | 1 | 1 | – | – | – | – | – | – | 1 | – | – | 6 |
| Littlejohns 1991146 | 1 | – | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | – | 1 | – | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Liu 2008260 | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | 1 | 1 | 5 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 3 |
| Livermore 2010166 | – | – | – | 1 | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Lord 2010147 | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | 1 | 5 | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 2 |
| Madariaga 2007174 A | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Madariaga 2007174 B | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Madariaga 2007174 C | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Mador 2004126 | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 3 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Mador 2005125 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | 3 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Mador 2009124 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Magadle 2007254 | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Maltais 2008198 | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 4 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 3 |
| Man 2004148 | – | 1 | – | – | 1 | 1 | 1 | – | 1 | – | – | – | – | – | 1 | – | – | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Martin 2004228 | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| McGeoch 2006229 | – | – | – | 1 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | – | – | 1 | 1 | – | – | 1 | – | 1 | 1 | – | – | 1 | – | – | 6 |
| Monninkhof 2003186 | 1 | 1 | – | 1 | 1 | – | – | – | 1 | 1 | – | – | 1 | – | 1 | 1 | 1 | 10 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 |
| Moore 2009284 | 1 | – | – | – | 1 | 1 | 1 | – | – | 1 | 1 | – | – | – | 1 | 1 | – | 8 | 1 | – | – | – | 1 | 1 | 1 | – | – | 1 | 1 | – | – | – | 1 | – | 1 | 8 |
| Mota 2007175 | – | – | 1 | – | 1 | – | – | 1 | – | 1 | – | – | – | – | – | – | – | 4 | – | – | 1 | – | – | – | – | 1 | – | 1 | – | – | – | – | – | – | – | 3 |
| Mularski 2009127 | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | – | – | 3 |
| Murphy 2005252 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Nakamura 2008261 A | – | – | – | – | 1 | – | – | 1 | – | 1 | – | – | – | – | 1 | – | – | 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Nakamura 2008261 B | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Nakamura 2008261 C | – | – | – | – | 1 | – | – | 1 | – | 1 | – | – | – | – | 1 | – | – | 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Ng 2011215 | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | 1 | 5 | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | 1 | 4 |
| Nguyen 2008128 | – | 1 | – | 1 | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 4 | – | 1 | – | 1 | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 4 |
| Nguyen 2009129 | – | – | – | – | 1 | – | 1 | – | – | 1 | 1 | – | – | – | 1 | 1 | – | 6 | – | – | – | – | 1 | – | 1 | – | – | 1 | 1 | – | – | – | 1 | 1 | – | 6 |
| Nield 2007130 A | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Nield 2007130 B | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Nield 2007130 C | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Ninot 2011250 | – | – | – | 1 | 1 | 1 | – | 1 | 1 | – | – | 1 | – | – | 1 | – | – | 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 |
| Normandin 2002131 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Norweg 2005132 A | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Norweg 2005132 B | 1 | – | – | – | – | – | 1 | – | 1 | 1 | 1 | – | – | – | 1 | – | – | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Norweg 2005132 C | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | 1 | – | – | – | – | – | 1 | – | 1 | 1 | 1 | – | – | – | 1 | – | – | 6 |
| Oh 2003283 | 1 | – | 1 | – | 1 | – | 1 | 1 | 1 | 1 | – | 1 | 1 | – | 1 | 1 | – | 11 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | – | – | 1 | 1 | – | – | – | – | 7 |
| O’Neill 2007150 | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 3 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Ortega 2002176 A | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Ortega 2002176 B | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Ortega 2002176 C | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| O’Shea 2007167 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Ozdemir 2010239 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Paz-Diaz 2007269 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 |
| Petersen 2008225 | 1 | – | – | – | 1 | – | 1 | – | 1 | 1 | – | – | – | – | 1 | – | – | 6 | 1 | – | – | – | – | – | 1 | – | 1 | 1 | – | – | – | – | – | – | – | 4 |
| Petty 2006133 A | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Petty 2006133 B | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Petty 2006133 C | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Pomidori 2012243 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Prince 1989151 | 1 | – | – | – | 1 | 1 | – | – | 1 | 1 | – | – | – | – | 1 | – | – | 6 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Probst 2011234 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Puente-Maestu 2000177 | – | – | – | – | – | – | 1 | – | 1 | – | – | – | – | – | 1 | – | – | 3 | – | – | – | – | – | – | 1 | – | 1 | – | – | – | – | – | 1 | – | – | 3 |
| Puhan 2006257 | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 4 | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 4 |
| Rea 2004230 | – | – | – | 1 | – | 1 | 1 | – | – | – | 1 | 1 | – | – | – | 1 | 1 | 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Regiane Resqueti 2007178 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Ren 2011201 A | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Ren 2011201 B | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Ren 2011201 C | 1 | – | 1 | – | 1 | – | – | 1 | – | – | – | – | – | – | 1 | 1 | – | 6 | 1 | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | 1 | – | – | 4 |
| Rice 2010140 | 1 | – | – | 1 | 1 | 1 | 1 | – | – | – | 1 | 1 | – | – | 1 | 1 | – | 9 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 2 |
| Riera 2001179 | – | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 2 | – | – | 1 | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 2 |
| Ringbaek 2000226 | 1 | – | – | – | 1 | – | – | – | 1 | 1 | – | – | – | – | 1 | – | – | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Romagnoli 2006244 | 1 | – | – | – | 1 | – | – | – | 1 | 1 | – | – | – | – | 1 | – | – | 5 | 1 | – | – | – | – | – | – | – | 1 | 1 | – | – | – | – | 1 | – | – | 4 |
| Rooyackers 2003187 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Sassi-Dambron 1995134 | 1 | – | – | – | 1 | – | – | – | – | 1 | – | – | 1 | – | – | – | – | 4 | – | – | – | – | – | – | 1 | – | 1 | – | – | – | – | – | 1 | – | – | 3 |
| Scherer 2000258 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Sewell 2005152 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | – | 1 | – | 1 | – | – | 8 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | – | 1 | – | 1 | – | – | 8 |
| Sewell 2006153 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | – | 1 | – | 1 | – | – | 8 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | – | 1 | – | 1 | – | – | 8 |
| Seymour 2010154 | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 3 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Shao 2003202 | – | – | – | – | 1 | 1 | – | – | 1 | 1 | – | – | – | – | 1 | – | – | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Simpson 1992135 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Singh 2003241 | – | – | – | – | 1 | – | – | 1 | – | – | – | – | 1 | – | 1 | – | – | 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Sívori 1998263 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Smith 1999168 | 1 | – | – | 1 | – | 1 | 1 | – | – | – | – | 1 | – | – | 1 | 1 | – | 7 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Soler 2006180 | 1 | – | – | – | 1 | 1 | – | – | 1 | – | – | 1 | – | – | 1 | 1 | – | 7 | 1 | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | 1 | – | – | 4 |
| Solomon 1998136 | 1 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Spencer 2010169 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Spruit 2002247 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Sridhar 2008155 | 1 | 1 | – | 1 | 1 | 1 | 1 | – | – | – | – | – | – | – | 1 | 1 | – | 8 | 1 | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | 4 |
| Stulbarg 2002137 A | 1 | 1 | – | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | 6 | 1 | 1 | – | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | 6 |
| Stulbarg 2002137 B | 1 | 1 | – | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | 6 | 1 | 1 | – | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | 6 |
| Stulbarg 2002137 C | 1 | 1 | – | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | 6 | 1 | 1 | – | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | 6 |
| Subin 2010242 A | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Subin 2010242 B | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Subin 2010242 C | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Theander 2009220 | 1 | 1 | – | – | 1 | 1 | 1 | – | 1 | – | – | – | 1 | – | 1 | – | – | 8 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Toshima 1990138 | 1 | – | – | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | 1 | – | 1 | – | 1 | 11 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | – | – | – | – | – | 1 | – | – | 6 |
| Trappenburg 2011188 | – | – | – | 1 | 1 | 1 | 1 | – | 1 | – | 1 | 1 | – | – | 1 | 1 | – | 9 | – | – | – | – | 1 | 1 | 1 | – | 1 | – | 1 | 1 | – | – | 1 | – | – | 7 |
| Troosters 2000248 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Van Gestel 2012208 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Vogiatzis 2002265 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | 7 | 1 | – | – | – | 1 | – | 1 | 1 | 1 | 1 | – | – | – | – | 1 | – | – | 7 |
| Vonbank 2012246 A | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Vonbank 2012246 B | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Vonbank 2012246 C | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Wadell 2004221 A | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Wadell 2004221 B | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Wadell 2004221 C | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Wakabayashi 2011262 | 1 | – | – | 1 | – | 1 | 1 | – | 1 | – | 1 | 1 | – | – | 1 | – | – | 8 | 1 | – | – | – | – | 1 | 1 | – | 1 | – | 1 | 1 | – | – | 1 | – | – | 7 |
| Wang 2004203 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
| Warlies 2006209 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | 1 | – | – | – | 1 | 1 | – | – | – | – | 1 | – | – | – | – | – | 4 |
| Waterhouse 2010277 | 1 | – | – | – | 1 | – | 1 | – | – | 1 | – | – | 1 | – | 1 | – | – | 6 | 1 | – | – | – | – | – | 1 | – | – | 1 | – | – | 1 | – | 1 | – | – | 5 |
| Watson 1997231 | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Wedzicha 1998157 | 1 | – | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | 1 | – | – | 1 | – | – | 10 | 1 | – | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | 1 | – | – | – | – | – | 9 |
| Weekes 2009158 | – | – | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | 2 | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
| White 2002159 | 1 | – | – | 1 | 1 | – | 1 | 1 | 1 | 1 | – | 1 | – | – | 1 | – | – | 9 | 1 | – | – | 1 | 1 | – | 1 | 1 | 1 | 1 | – | 1 | – | – | 1 | – | – | 9 |
| Wijkstra 1994189 | 1 | – | 1 | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | – | – | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Wijkstra 1995190 A | 1 | – | 1 | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | 1 | – | 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Wijkstra 1995190 B | 1 | – | 1 | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | 1 | – | 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Wijkstra 1995190 C | 1 | – | 1 | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | 1 | – | 7 | 1 | – | 1 | – | 1 | – | 1 | – | – | 1 | – | – | – | – | 1 | 1 | – | 7 |
| Wittmann 2007210 | 1 | 1 | – | 1 | 1 | – | 1 | – | 1 | 1 | 1 | 1 | – | – | 1 | – | – | 10 | – | 1 | – | – | 1 | – | 1 | – | 1 | 1 | – | 1 | – | – | 1 | – | – | 7 |
| Wong 200574 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Wood-Baker 2006170 | 1 | – | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | 1 | 1 | – | – | 12 | 1 | – | – | – | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | 1 | 1 | – | – | 11 |
| Wright 2003211 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Xu 2010204 A | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Xu 2010204 B | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Xu 2010204 C | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Xu 2010204 D | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 3 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 |
| Xu 2010204 E | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 3 | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 2 |
| Xu 2010204 F | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | 1 | – | – | 2 | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 2 |
| Yamaguti 2012235 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Yeh 2010139 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 |
| Zhang 2008205 A | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Zhang 2008205 B | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
| Zhang 2008205 C | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 |
| Total number | 108 | 24 | 32 | 43 | 140 | 44 | 77 | 30 | 51 | 77 | 18 | 36 | 22 | 7 | 176 | 50 | 18 | 953 | 68 | 9 | 11 | 9 | 52 | 18 | 43 | 16 | 28 | 34 | 11 | 19 | 7 | 3 | 96 | 15 | 4 | 443 |
Appendix 25 Direction of effects for hospital admissions, exacerbation and health-related quality-of-life outcomes at last follow-up: review 4
| First author | QoL | Hospital (re)admissions | Exacerbations | Follow-up (weeks) | Hospital admissions | Exacerbations | SGRQ | CRQ | SF-36 | EQ-5D | Other | Other effect | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Symptoms | Activity | Impact | Total | Dyspnoea | Fatigue | Emotional function | Mastery | PCS | MCS | Physical functioning | Role physical | Bodily pain | General health | Vitality | Social functioning | Role emotional | Mental health | Change in health | ||||||||||
| Aimonino Ricauda 200875 | 1 | 1 | 0 | 26 | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | NHP | ↑ | ||||||||||||||
| Arnardottir 2006216 | 1 | 0 | 0 | 16 | ↔ | ||||||||||||||||||||||||
| Arnardottir 2007217 | 1 | 0 | 0 | 52 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Barakat 2008249 | 1 | 0 | 0 | 14 | ↑ | • | • | • | |||||||||||||||||||||
| Bauldoff 2002108 | 1 | 0 | 0 | 8 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Bauldoff 2005109 A | 1 | 0 | 0 | 4 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Bauldoff 2005109 B | 1 | 0 | 0 | 4 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Bauldoff 2005109 C | 1 | 0 | 0 | 4 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Beckerman 2005253 | 1 | 1 | 0 | 52 | ↔ | ↔ | |||||||||||||||||||||||
| Behnke 200064 | 1 | 1 | 0 | 78 | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||||||
| Bendstrup 1997222 | 1 | 0 | 0 | 24 | ↑ | YQLQ | ↔ | ||||||||||||||||||||||
| Bernard 1999191 | 1 | 0 | 0 | 12 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Berry 2010110 | 1 | 0 | 0 | 52 | ↔ | ↔ | ↔ | ||||||||||||||||||||||
| Bestall 2003141 | 1 | 0 | 0 | 52 | ↔ | ↔ | |||||||||||||||||||||||
| Bjornshave 2005223 | 1 | 0 | 0 | 4 | • | • | |||||||||||||||||||||||
| Blake Jr 1990111 | 1 | 0 | 0 | 52 | SIP physical | ↑ | |||||||||||||||||||||||
| Bonilha 2009232 | 1 | 0 | 0 | 25 | ↔ | ||||||||||||||||||||||||
| Bourbeau 2003192 | 1 | 1 | 1 | 52 | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||
| Boxall 2005160 | 1 | 1 | 0 | 12 | ↔ | ↑ | ↔ | ↔ | ↑ | ||||||||||||||||||||
| Breyer 2010245 | 1 | 0 | 0 | 39 | ↑ | ↔ | |||||||||||||||||||||||
| Brooks 2002193 | 1 | 0 | 0 | 52 | ↔ | ↔ | ↔ | ↔ | ↔ | • | • | • | |||||||||||||||||
| Bucknall 201263 | 1 | 1 | 0 | 52 | ↔ | ↔ | ↔ | ↔ | ↑ | ↔ | |||||||||||||||||||
| Busch 1988194 | 1 | 0 | 0 | 18 | ↔ | ||||||||||||||||||||||||
| Cai 2006199 | 1 | 0 | 1 | 26 | ↑ | • | • | • | • | ||||||||||||||||||||
| Carr 2009195 | 1 | 0 | 0 | 12 | ↑ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Casas 200671 | 1 | 1 | 0 | 52 | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||
| Chan 2010212 A | 1 | 1 | 1 | 13 | ↔ | ↔ | ↔ | • | • | • | |||||||||||||||||||
| Chan 2010212 B | 1 | 1 | 1 | 13 | ↔ | ↔ | ↔ | • | • | • | |||||||||||||||||||
| Chan 2010212 C | 1 | 1 | 1 | 13 | ↔ | ↔ | ↔ | • | • | • | |||||||||||||||||||
| Cockcroft 1987142 | 0 | 1 | 0 | ↔ | |||||||||||||||||||||||||
| Coultas 2005112 A | 1 | 1 | 0 | 26 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||
| Coultas 2005112 B | 1 | 1 | 0 | 26 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||
| Coultas 2005112 C | 1 | 1 | 0 | 26 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||
| Covey 2001113 | 1 | 0 | 0 | 16 | ↔ | ||||||||||||||||||||||||
| de Blok 2006181 | 1 | 0 | 0 | 9 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||
| Dheda 200473 | 1 | 1 | 1 | 26 | ↔ | ↔ | ↑ | ↑ | ↔ | ↑ | |||||||||||||||||||
| Donesky-Cuenco 2009114 | 1 | 0 | 0 | 12 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||
| Dourado 2009233 A | 1 | 0 | 0 | 12 | ↔ | • | • | • | AQ20 | ||||||||||||||||||||
| Dourado 2009233 B | 1 | 0 | 0 | 12 | ↔ | • | • | • | AQ20 | ||||||||||||||||||||
| Dourado 2009233 C | 1 | 0 | 0 | 12 | ↔ | • | • | • | AQ20 | ||||||||||||||||||||
| du Moulin 2009206 | 1 | 0 | 0 | 26 | ↔ | ↑ | ↑ | ↑ | ↔ | ↔ | |||||||||||||||||||
| Eaton 2009227 | 1 | 1 | 0 | 13 | ↔ | ↔ | ↑ | ↔ | ↔ | ↑ | ↔ | ||||||||||||||||||
| Effing 2009161 | 1 | 1 | 1 | 52 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||
| Efraimsson 2008218 | 1 | 0 | 0 | ↑ | |||||||||||||||||||||||||
| Egan 200269 | 1 | 1 | 0 | ↔ | |||||||||||||||||||||||||
| Elci 2008236 | 1 | 0 | 0 | 13 | ↑ | SF-36 total | ↑ | ||||||||||||||||||||||
| Elliott 2004162 A | 1 | 0 | 0 | 13 | ↑ | ||||||||||||||||||||||||
| Elliott 2004162 B | 1 | 0 | 0 | 13 | • | ||||||||||||||||||||||||
| Elliott 2004162 C | 1 | 0 | 0 | 13 | • | ||||||||||||||||||||||||
| Emery 1998115 A | 1 | 0 | 0 | 10 | SIP total | ↔ | |||||||||||||||||||||||
| Emery 1998115 B | 1 | 0 | 0 | 10 | SIP total | ↑ | |||||||||||||||||||||||
| Emery 1998115 C | 1 | 0 | 0 | 10 | SIP total | ↔ | |||||||||||||||||||||||
| Engstrom 1999219 | 1 | 0 | 0 | 52 | ↔ | ↔ | ↔ | ↔ | SIP total | ↔ | |||||||||||||||||||
| Fernandez 2009171 | 1 | 0 | 0 | 52 | ↑ | • | • | • | |||||||||||||||||||||
| Finnerty 2001143 | 1 | 0 | 0 | 26 | ↑ | • | • | • | |||||||||||||||||||||
| Foy 2001116 | 1 | 0 | 0 | 78 | ↑ | ↑ | ↑ | ↑ | |||||||||||||||||||||
| Gallefoss 1999255 | 1 | 1 | 0 | 52 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Ghanem 2010264 | 1 | 0 | 0 | 9 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | • | • | • | • | • | • | • | • | • | ||||||||||
| Gilmore 2010117 A | 1 | 0 | 0 | ? | • | • | • | • | |||||||||||||||||||||
| Gilmore 2010117 B | 1 | 0 | 0 | ? | • | • | • | • | |||||||||||||||||||||
| Gilmore 2010117 C | 1 | 0 | 0 | ? | • | • | • | • | |||||||||||||||||||||
| Gilmore 2010117 D | 1 | 0 | 0 | ? | • | • | • | • | |||||||||||||||||||||
| Gilmore 2010117 E | 1 | 0 | 0 | ? | • | • | • | • | |||||||||||||||||||||
| Gilmore 2010117 F | 1 | 0 | 0 | ? | • | • | • | • | |||||||||||||||||||||
| Gohl 2006207 | 1 | 0 | 0 | 52 | ↔ | • | • | • | • | • | • | • | • | • | • | • | • | • | |||||||||||
| Goldstein 1994196 | 1 | 0 | 0 | 24 | ↑ | ↑ | ↑ | ↑ | QWB & SIP | ↔ | |||||||||||||||||||
| Green 2001144 | 1 | 0 | 0 | 7 | ↑ | ↑ | ↔ | ↑ | ↑ | ||||||||||||||||||||
| Güell 2000172 | 1 | 0 | 0 | 17 | ↑ | ↔ | ↔ | ↑ | |||||||||||||||||||||
| Güell 2006173 | 1 | 1 | 1 | 104 | ↔ | ↑ | ↑ | ↔ | ↑ | ↑ | |||||||||||||||||||
| Guyatt 1992118 | 1 | 0 | 0 | 26 | ↔ | ↔ | |||||||||||||||||||||||
| Hermiz 200267 | 1 | 1 | 0 | 13 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Hernandez 2000282 | 1 | 1 | 0 | 8 | ↑ | ↑ | ↔ | ↔ | ↑ | SF-12 | |||||||||||||||||||
| Hernandez 200368 | 1 | 0 | 0 | 12 | ↔ | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||||||||||||||
| Hill 2006163 | 1 | 0 | 0 | 8 | ↔ | ↑ | ↑ | • | • | ||||||||||||||||||||
| Holland 2004164 | 1 | 0 | 0 | 6 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Hoogendoorn 2009182 | 1 | 1 | 1 | 104 | • | ↔ | ↑ | ||||||||||||||||||||||
| Hospes 2009183 | 1 | 0 | 0 | 12 | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | CCQ | ↔ | |||||||||||
| Hsiao 2003259 A | 1 | 0 | 0 | SF-36 | ns | ||||||||||||||||||||||||
| Hsiao 2003259 B | 1 | 0 | 0 | SF-36 | ns | ||||||||||||||||||||||||
| Hsiao 2003259 C | 1 | 0 | 0 | SF-36 | • | ||||||||||||||||||||||||
| Hynninen 2010256 | 1 | 0 | 0 | 35 | ↔ | ||||||||||||||||||||||||
| Janaudis-Ferreira 2011197 | 1 | 0 | 0 | 6 | ↔ | ↔ | |||||||||||||||||||||||
| Jang 2006267 | 1 | 0 | 0 | 8 | QoL | ↑ | |||||||||||||||||||||||
| Jarab 2012266 | 1 | 1 | 1 | 26 | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||
| Karapolat 2007237 | 1 | 0 | 0 | 12 | ↑ | ↑ | ↑ | ↑ | |||||||||||||||||||||
| Katiyar 2006240 | 1 | 0 | 0 | 13 | ↑ | • | • | • | |||||||||||||||||||||
| Kayahan 2006238 | 1 | 0 | 0 | 9 | ↑ | ||||||||||||||||||||||||
| Khdour 2009251 | 1 | 1 | 1 | 52 | ↑ | • | ↔ | ↑ | ↔ | ↑ | |||||||||||||||||||
| Kim 1993119 | 1 | 0 | 0 | 26 | SIP total | • | |||||||||||||||||||||||
| Ko 2011213 | 1 | 1 | 1 | 52 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||
| Koff 2009120 | 1 | 1 | 1 | 13 | • | ↔ | ↑ | ↔ | ↔ | ↔ | |||||||||||||||||||
| Koppers 2006184 | 1 | 0 | 0 | 5 | ↔ | ||||||||||||||||||||||||
| Kunik 2008121 | 1 | 0 | 0 | 52 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||
| Kwok 200470 | 0 | 1 | 0 | ↔ | |||||||||||||||||||||||||
| Lamers 2010185 | 1 | 0 | 0 | 39 | ↑ | ↔ | ↔ | ↑ | |||||||||||||||||||||
| Larson 1988122 | 1 | 0 | 0 | SIP | ↔ | ||||||||||||||||||||||||
| Larson 1999123 A | 1 | 0 | 0 | 17 | • | • | |||||||||||||||||||||||
| Larson 1999123 B | 1 | 0 | 0 | 17 | • | • | |||||||||||||||||||||||
| Larson 1999123 C | 1 | 0 | 0 | 17 | • | • | |||||||||||||||||||||||
| Larson 1999123 D | 1 | 0 | 0 | 17 | • | • | |||||||||||||||||||||||
| Larson 1999123 E | 1 | 0 | 0 | 17 | • | • | |||||||||||||||||||||||
| Larson 1999123 F | 1 | 0 | 0 | 17 | • | • | |||||||||||||||||||||||
| Lee 200266 | 0 | 1 | 0 | 26 | ↔ | ||||||||||||||||||||||||
| Leung 2010165 | 1 | 0 | 0 | 8 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Li 2002200 | 1 | 0 | 0 | 13 | ADL, social activities | ↑ | |||||||||||||||||||||||
| Liddell 2010145 | 1 | 0 | 0 | 8 | • | ||||||||||||||||||||||||
| Lindsay 2005214 | 1 | 0 | 0 | 13 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Linneberg 2012224 | 1 | 0 | 0 | 52 | ↔ | ||||||||||||||||||||||||
| Littlejohns 1991146 | 1 | 0 | 0 | 52 | ↔ | SIP physical functioning | ↑ | ||||||||||||||||||||||
| Liu 2008260 | 1 | 1 | 1 | 52 | ↑ | • | ↑ | ||||||||||||||||||||||
| Livermore 2010166 | 1 | 1 | 0 | ↔ | ↔ | ||||||||||||||||||||||||
| Lord 2010147 | 1 | 0 | 0 | 7 | ↔ | ↑ | ↔ | ||||||||||||||||||||||
| Madariaga 2007174 A | 1 | 0 | 0 | 8 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Madariaga 2007174 B | 1 | 0 | 0 | 8 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Madariaga 2007174 C | 1 | 0 | 0 | 8 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Mador 2004126 | 1 | 0 | 0 | 8 | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||||||
| Mador 2005125 | 1 | 0 | 0 | 8 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Mador 2009124 | 1 | 0 | 0 | 8 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Magadle 2007254 | 1 | 0 | 0 | 39 | ↑ | ||||||||||||||||||||||||
| Maltais 2008198 | 1 | 1 | 1 | 52 | • | • | • | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||
| Man 2004148 | 1 | 1 | 0 | 13 | ↔ | ↑ | ↔ | ↔ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||||||||||||||
| Martin 2004228 | 1 | 1 | 0 | 52 | ↔ | ||||||||||||||||||||||||
| McGeoch 2006229 | 1 | 1 | 0 | 52 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Monninkhof 2003186 | 1 | 0 | 0 | 52 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Moore 2009284 | 1 | 0 | 0 | 6 | ↔ | QoL | |||||||||||||||||||||||
| Mota 2007175 | 1 | 0 | 0 | 5 | • | • | • | • | |||||||||||||||||||||
| Mularski 2009127 | 1 | 0 | 0 | 8 | ↔ | ↔ | ↔ | ↔ | ↑ | ↔ | |||||||||||||||||||
| Murphy 2005252 | 1 | 0 | 1 | 6 | ↔ | ↔ | • | • | • | • | |||||||||||||||||||
| Nakamura 2008261 A | 1 | 0 | 0 | 12 | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||
| Nakamura 2008261 B | 1 | 0 | 0 | 12 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↑ | |||||||||||||||||
| Nakamura 2008261 C | 1 | 0 | 0 | 12 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | • | |||||||||||||||||
| Ng 2011215 | 1 | 0 | 0 | 26 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||
| Nguyen 2008128 | 1 | 0 | 0 | 26 | ↔ | ↔ | ↔ | ||||||||||||||||||||||
| Nguyen 2009129 | 1 | 0 | 1 | 26 | • | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||
| Nield 2007130 A | 1 | 0 | 0 | 12 | • | ||||||||||||||||||||||||
| Nield 2007130 B | 1 | 0 | 0 | 12 | • | ||||||||||||||||||||||||
| Nield 2007130 C | 1 | 0 | 0 | 12 | • | ||||||||||||||||||||||||
| Ninot 2011250 | 1 | 1 | 0 | 52 | ↔ | ↔ | |||||||||||||||||||||||
| Normandin 2002131 | 1 | 0 | 0 | 8 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Norweg 2005132 A | 1 | 0 | 0 | 24 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Norweg 2005132 B | 1 | 0 | 0 | 24 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Norweg 2005132 C | 1 | 0 | 0 | 24 | ↑ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Oh 2003283 | 1 | 0 | 0 | 8 | ↑ | ↔ | ↑ | ↑ | ↑ | ||||||||||||||||||||
| O’Neill 2007150 | 1 | 0 | 0 | 26 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Ortega 2002176 A | 1 | 0 | 0 | 24 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Ortega 2002176 B | 1 | 0 | 0 | 24 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Ortega 2002176 C | 1 | 0 | 0 | 24 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| O’Shea 2007167 | 1 | 0 | 0 | 24 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Ozdemir 2010239 | 1 | 0 | 0 | 4 | • | • | • | • | • | ||||||||||||||||||||
| Paz-Diaz 2007269 | 1 | 0 | 0 | 9 | ↑ | • | • | • | |||||||||||||||||||||
| Petersen 2008225 | 1 | 0 | 0 | 7 | ↑ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Petty 2006133 A | 1 | 0 | 0 | 16 | SOLDQ | • | |||||||||||||||||||||||
| Petty 2006133 B | 1 | 0 | 0 | 16 | SOLDQ | • | |||||||||||||||||||||||
| Petty 2006133 C | 1 | 0 | 0 | 16 | SOLDQ | • | |||||||||||||||||||||||
| Pomidori 2012243 | 1 | 0 | 0 | 52 | • | VAS | • | ||||||||||||||||||||||
| Prince 1989151 | 1 | 0 | 0 | 6 | GHQ | • | |||||||||||||||||||||||
| Probst 2011234 | 1 | 0 | 0 | 12 | |||||||||||||||||||||||||
| Puente-Maestu 2000177 | 1 | 0 | 0 | 52 | • | • | • | • | |||||||||||||||||||||
| Puhan 2006257 | 1 | 0 | 0 | 5 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Rea 2004230 | 1 | 1 | 0 | 52 | ↔ | ↔ | ↑ | ↔ | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||
| Regiane Resqueti 2007178 | 0 | 0 | 1 | • | |||||||||||||||||||||||||
| Ren 2011201 A | 0 | 0 | 1 | • | |||||||||||||||||||||||||
| Ren 2011201 B | 0 | 0 | 1 | • | |||||||||||||||||||||||||
| Ren 2011201 C | 1 | 0 | 0 | 26 | • | ↑ | ↑ | ↔ | ↔ | ||||||||||||||||||||
| Rice 2010140 | 1 | 1 | 0 | 52 | ↑ | ↑ | ↑ | ||||||||||||||||||||||
| Riera 2001179 | 1 | 0 | 0 | 26 | ↑ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Ringbaek 2000226 | 1 | 0 | 0 | 8 | ↔ | PGWB index | ↔ | ||||||||||||||||||||||
| Romagnoli 2006244 | 1 | 1 | 0 | 56 | ↔ | ↔ | ↑ | ↔ | • | ||||||||||||||||||||
| Rooyackers 2003187 | 1 | 0 | 0 | 10 | ↓ | ||||||||||||||||||||||||
| Sassi-Dambron 1995134 | 1 | 0 | 0 | 26 | QWB total | ↔ | |||||||||||||||||||||||
| Scherer 2000258 | 1 | 0 | 0 | 8 | ↑ | ↔ | |||||||||||||||||||||||
| Sewell 2005152 | 1 | 0 | 0 | 7 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Sewell 2006153 | 1 | 0 | 0 | 7 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Seymour 2010154 | 1 | 1 | 1 | 13 | ↑ | ↑ | ↑ | ↔ | ↑ | ↔ | ↑ | ↔ | ↑ | ↔ | ↔ | ||||||||||||||
| Shao 2003202 | 1 | 0 | 0 | 52 | ADL, social activities | ||||||||||||||||||||||||
| Simpson 1992135 | 1 | 0 | 0 | 8 | ↑ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Singh 2003241 | 1 | 0 | 0 | 4 | ↑ | ↑ | ↑ | ↑ | |||||||||||||||||||||
| Sívori 1998263 | 1 | 1 | 0 | 8 | • | • | • | • | |||||||||||||||||||||
| Smith 1999168 | 1 | 1 | 0 | 52 | ↔ | CO-OP | • | ||||||||||||||||||||||
| Soler 2006180 | 1 | 1 | 1 | 52 | ↑ | • | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||
| Solomon 1998136 | 1 | 1 | 0 | 26 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||
| Spencer 2010169 | 1 | 1 | 1 | 52 | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ | |||||||||||||||||||
| Spruit 2002247 | 1 | 0 | 0 | 12 | ↔ | ↔ | |||||||||||||||||||||||
| Sridhar 2008155 | 1 | 1 | 0 | 104 | ↔ | ↑ | • | • | • | • | |||||||||||||||||||
| Stulbarg 2002137 A | 1 | 0 | 0 | 9 | ↑ | ↔ | ↔ | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ | ↔ | ↑ | ||||||||||
| Stulbarg 2002137 B | 1 | 0 | 0 | 9 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||
| Stulbarg 2002137 C | 1 | 0 | 0 | 9 | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ | ↔ | ↔ | ||||||||||
| Subin Rao 2010242 A | 1 | 0 | 0 | 4 | • | • | • | • | |||||||||||||||||||||
| Subin Rao 2010242 B | 1 | 0 | 0 | 4 | • | • | • | • | |||||||||||||||||||||
| Subin Rao 2010242 C | 1 | 0 | 0 | 4 | • | • | • | • | |||||||||||||||||||||
| Theander 2009220 | 1 | 0 | 0 | 12 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||
| Toshima 1990138 | 1 | 1 | 0 | 26 | ↔ | QWB total | • | ||||||||||||||||||||||
| Trappenburg 2011188 | 1 | 1 | 1 | 26 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||
| Troosters 2000248 | 1 | 0 | 0 | 78 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Van Gestel 2012208 | 1 | 0 | 0 | 4 | ↔ | ||||||||||||||||||||||||
| Vogiatzis 2002265 | 1 | 0 | 0 | 13 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Vonbank 2012246 A | 1 | 0 | 0 | 12 | ↔ | • | • | • | |||||||||||||||||||||
| Vonbank 2012246 B | 1 | 0 | 0 | 12 | ↔ | • | • | • | |||||||||||||||||||||
| Vonbank 2012246 C | 1 | 0 | 0 | 12 | ↔ | • | • | • | |||||||||||||||||||||
| Wadell 2004221 A | 1 | 0 | 0 | 12 | ↔ | ↔ | ↑ | ↔ | ↑ | ↔ | |||||||||||||||||||
| Wadell 2004221 B | 1 | 0 | 0 | 12 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||
| Wadell 2004221 C | 1 | 0 | 0 | 12 | ↔ | ↔ | ↑ | ↔ | ↑ | ↔ | |||||||||||||||||||
| Wakabayashi 2011262 | 1 | 0 | 0 | 52 | ↔ | ↔ | |||||||||||||||||||||||
| Wang 2004203 | 1 | 0 | 0 | 15 | QoL | • | |||||||||||||||||||||||
| Warlies 2006209 | 1 | 0 | 0 | 26 | ↔ | • | • | • | |||||||||||||||||||||
| Waterhouse 2010277 | 1 | 0 | 0 | 78 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||
| Watson 1997231 | 1 | 0 | 0 | 26 | ↔ | ? | ? | ? | |||||||||||||||||||||
| Wedzicha 1998157 | 1 | 0 | 0 | 8 | ↔ | ↔ | |||||||||||||||||||||||
| Weekes 2009158 | 1 | 0 | 0 | 52 | ↑ | ↔ | ↔ | ↑ | ↑ | ||||||||||||||||||||
| White 2002159 | 1 | 0 | 0 | 13 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||
| Wijkstra 1994189 | 1 | 0 | 0 | 12 | ↑ | ↔ | ↑ | ↑ | |||||||||||||||||||||
| Wijkstra 1995190 A | 1 | 0 | 0 | 78 | ↔ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Wijkstra 1995190 B | 1 | 0 | 0 | 78 | ↑ | ↔ | ↔ | ↑ | |||||||||||||||||||||
| Wijkstra 1995190 C | 1 | 0 | 0 | 78 | ↓ | ↔ | ↔ | ↔ | |||||||||||||||||||||
| Wittmann 2007210 | 1 | 1 | 0 | 52 | • | ↔ | • | • | • | ||||||||||||||||||||
| Wong 200574 | 0 | 1 | 0 | 13 | ↔ | ||||||||||||||||||||||||
| Wood-Baker 2006170 | 1 | 1 | 0 | 52 | ↔ | ↔ | ↔ | ↔ | ↔ | ||||||||||||||||||||
| Wright 2003211 | 1 | 0 | 0 | 12 | • | ||||||||||||||||||||||||
| Xu 2010204 A | 1 | 0 | 0 | 52 | ↑ | ||||||||||||||||||||||||
| Xu 2010204 B | 1 | 0 | 0 | 52 | ↑ | ||||||||||||||||||||||||
| Xu 2010204 C | 1 | 0 | 0 | 52 | ↑ | ||||||||||||||||||||||||
| Xu 2010204 D | 1 | 0 | 0 | 52 | ↑ | ||||||||||||||||||||||||
| Xu 2010204 E | 1 | 0 | 0 | 52 | ↑ | ||||||||||||||||||||||||
| Xu 2010204 F | 1 | 0 | 0 | 52 | ↔ | ||||||||||||||||||||||||
| Yamaguti 2012235 | 1 | 0 | 0 | 4 | ↑ | ↑ | ↔ | ↑ | |||||||||||||||||||||
| Yeh 2010139 | 1 | 0 | 0 | ↑ | ↔ | ↑ | ↔ | ↔ | |||||||||||||||||||||
| Zhang 2008205 A | 1 | 0 | 0 | 8 | Mood, dyspnoea, social activity, household activity, headache, appetite, anxiety | • | |||||||||||||||||||||||
| Zhang 2008205 B | 1 | 0 | 0 | 8 | |||||||||||||||||||||||||
| Zhang 2008205 C | 1 | 0 | 0 | 8 | |||||||||||||||||||||||||
Appendix 26 Risk of bias assessment for all included studies with primary outcomes: review 4
| Author year | Sequence generation | Allocation concealment | Blinding | Incomplete outcome reporting | Selective outcome reporting | Other |
|---|---|---|---|---|---|---|
| Aimonino Ricauda 200875 | LOW Computer-generated random numbers |
LOW Sealed numbered envelopes |
HRQoL: HIGH Hospital admission: UNCLEAR Method not stated |
LOW ITT analysis 23% dropout – balanced in number and similar reasons between groups No comment on the baseline characteristics of dropouts vs. completers but only 3% lost to follow-up – remainder were deaths |
UNCLEAR No protocol |
HIGH Baseline data provided for all participants Gender and percentage on home oxygen imbalanced was between groups – the home oxygen use was greater in the intervention group |
| Arnardottir 2006216 | UNCLEAR ‘Blindly randomised in block of 4’ |
LOW ‘Blindly randomised’ |
HIGH ‘Self-administered questionnaires’ |
UNCLEAR Withdrawals reported, but no ITT |
LOW All outcomes reported |
UNCLEAR Very small sample Baseline line differences for rate of perceived exertion and 12-MWD |
| Arnardottir 2007217 | UNCLEAR ‘Stratified according to disease severity . . . randomised into blocks of 4’ |
UNCLEAR ‘Closed envelope method’ |
HRQoL: HIGH | HIGH 40% loss to follow-up – higher functional/residual capacity and lung capacity = more severe disease; no other differences in dropouts; reasons given for dropouts but no numbers per arm |
LOW | HIGH No description of baseline characteristics of those lost to follow-up |
| Barakat 2008249 | LOW Randomisation was in blocks of 10, using random numbers |
UNCLEAR Not mentioned |
HRQoL: HIGH | LOW SGRQ 9/80 missing; provided reasons for dropouts, equal numbers per arm |
UNCLEAR | HIGH ‘Excluded’ participants from analysis after randomisation, including 2/40 who did not adhere to rehabilitation programme Some imbalance in FVC at baseline: 86.1 (17.8) vs. 78.7 (5.5) |
| Bauldoff 2002108 | UNCLEAR | UNCLEAR | HRQoL: HIGH | UNCLEAR Mean data given only for outcomes therefore no details on ‘n’; it could be assumed that there was complete follow-up |
UNCLEAR HRQoL mentioned in methods and reported in results |
UNCLEAR Baseline characteristics seem balanced, (age imbalance, p < 0.05 but values not stated); gender not mentioned |
| Bauldoff 2005109 | UNCLEAR | UNCLEAR Not clear when allocated to intervention |
UNCLEAR No mention |
LOW All subjects accounted for |
LOW No study protocol but report includes all outcomes |
LOW |
| Beckerman 2005253 | LOW ‘The patients were randomised using a random numbers table into two groups’ |
UNCLEAR NR |
HRQoL: LOW Sham training intervention is control; mode of data collection not reported but blinded assessor Hospital admission/length of stay: LOW Self-reported but reported on daily by blinded assessor telephone call |
HIGH Dropouts – more from control group dropped out and more deaths in control group (2 vs. 4) Characteristics of dropouts not described |
UNCLEAR No protocol |
LOW Well-matched baseline with all participants reported No discussion of limitations in discussion |
| Behnke 200064 | UNCLEAR | UNCLEAR | HRQoL: HIGH Questionnaires unblinded |
HIGH 16/46 dropped out – 8 from each group; reasons given for each and fairly similar but small numbers; possible that exacerbation may be more of a reason for dropout in the control group; no comparison of those who drop out with those who complete |
UNCLEAR HRQoL reported in methods and results |
HIGH Baseline data/demographics only given only for completers; seems balanced |
| Behnke 200365 | UNCLEAR Stated randomised, no method given |
UNCLEAR | HRQoL: LOW Self-reported intervention vs. UC control Hospital admissions: LOW Exacerbations: LOW |
UNCLEAR Only 26 of the 30 previously recruited patients took part in this follow-up study – no reasons given for the dropouts Is an ITT analysis of the 26 patients |
UNCLEAR No protocol |
HIGH Baseline reported for all 26 patients (but not the 30 previous trial completers) Intervention group average age 5 years younger |
| Bendstrup 1997222 | UNCLEAR | UNCLEAR | HRQoL: HIGH Comments indicate that patients were not blind as control group told would get intervention at end of study if it proved effective |
HIGH Only 32/47 randomised patients completed Reasons for dropouts stated but not clear for 5/15 dropouts to which group they belonged; four dropped out of intervention and five from control; small number but similar reason |
UNCLEAR Method = results section |
HIGH Demographics supplied for only those who completed the intervention in each arm Appear balanced but small numbers |
| Bernard 1999191 | UNCLEAR | UNCLEAR | HRQoL: HIGH | UNCLEAR 4/19 and 5/26 failed to complete, reasons given but not by group; no comparison with completers |
UNCLEAR HRQoL mentioned in the methods and results |
HIGH Baseline data only for completers Fairly well balanced Baseline HRQoL not reported; only comparisons of change in each group |
| Berry 2010110 | LOW ‘Randomisation was performed using a web-based randomisation application’; ‘stratified by gender and study period’. Block sizes varied randomly between 4 and 6 |
LOW ‘Only the statisticians were unblinded to the randomisation scheme’ |
HRQoL: HIGH | Lifestyle activity programme: 24/87 dropped out; traditional exercise therapy: 20/89 Details of reasons for dropout were provided by study group; higher dropouts were due to medical condition in traditional exercise therapy than lifestyle activity programme (6 vs. 2) ‘The dropouts were significantly younger and there were a relatively low percentage with comorbid illness’ |
UNCLEAR | LOW |
| Bestall 2003141 | LOW ‘Randomisation sequence was computer generated’ |
UNCLEAR Blocks of eight in sealed envelopes |
HRQoL: HIGH | LOW All patients accounted for |
LOW All outcomes reported |
LOW Baseline balanced |
| Bjornshave 2005223 | UNCLEAR ‘After inclusion, the patients were randomised’ (p. 103) |
UNCLEAR Timing of randomisation is unclear |
No information on who performed tests and whether blinded HRQoL: HIGH |
HIGH Withdrawals reported – slightly higher in intervention group However, dropouts were excluded in analysis; no imputation or baseline observation carried forward |
LOW All prespecified outcomes reported |
UNCLEAR None – no adjustments for sex or baseline FEV1, which differed at baseline – but small numbers and baseline differences not statistically significant |
| Blake Jr 1990111 | UNCLEAR Stated randomised but no method detailed |
UNCLEAR NR |
HRQoL: HIGH Self-reported intervention vs. UC control Admissions: LOW Self-reported at monthly intervals |
UNCLEAR ITT analysis < 10% dropout/incomplete data No comparison of those with incomplete data/attendance with those with complete data/attendance |
UNCLEAR No protocol |
HIGH Baseline characteristics reported for all participants; unbalanced HRQoL 11.3 vs. 14.4, intervention vs. control Intervention group more educated (40% beyond high school vs. 22%) |
| Bonilha 2009232 | UNCLEAR ‘The volunteers were then randomised to a Singing Group or to a Control Group’ No information on method of randomisation |
UNCLEAR | HRQoL: LOW Self-completed comparison of interventions (singing vs. handcrafts) No mention of single blinding of outcome assessors |
HIGH ITT analysis – NR Characteristics of patients who dropped out not discussed 30% overall dropout rate (25% from control group, 34% from intervention) Similar reasons and proportions of dropout from each group Characteristics of patients who dropped out not discussed |
UNCLEAR No protocol |
HIGH Baseline only reported for trial completers Difference in HRQoL at baseline between groups – 10 points on the SGRQ Current smokers excluded |
| Bourbeau 2003192 | LOW ‘Patients underwent randomisation with the use of a central computer-generated list of random numbers’ |
LOW Central allocation in blocks |
Independent evaluator HRQoL: HIGH Admissions: LOW Exacerbations: LOW |
LOW All patients accounted for; dropout slightly higher in control |
LOW All outcomes reported |
LOW Baseline balanced |
| Gadoury 2005271 | UNCLEAR ‘Randomly assigned’ |
UNCLEAR | LOW Patients unblinded, but outcome data from hospital and insurance database |
LOW Withdrawals reported, and ITT performed |
LOW All outcomes reported |
UNCLEAR Protocol only adhered to strictly for year 1; case manager available for some subjects |
| Boxall 2005160 | LOW Computer-generated random numbers |
UNCLEAR Coded into opaque envelopes by person blinded |
‘Neither assessors or patients were blinded’ HRQoL: HIGH Admissions: LOW |
UNCLEAR Withdrawals reported, but no ITT |
LOW All outcomes reported |
LOW |
| Breyer 2010245 | LOW ‘Randomisation . . . was done by a computer-generated algorithm . . .’ |
UNCLEAR | HRQoL: HIGH | HIGH No ITT analysis Very low dropout rate – 7.6% but no reports of characteristics of dropouts Similar dropout rate for both intervention and control groups But dropout from intervention due to exacerbation; dropout from control as a result of loss to follow-up |
LOW Protocol available |
HIGH Retired patients or on sick leave? Highly motivated patients – note low dropout Baseline well matched but only reported for trial completers |
| Brooks 2002193 | LOW Random numbers table |
UNCLEAR | HRQoL: HIGH Intervention vs. UC |
UNCLEAR Appears to be ITT analysis Similar proportions of dropouts – reasons not detailed Stated no differences in baseline characteristics between groups and details reported |
UNCLEAR No protocol |
LOW Baseline characteristics reported for all patients |
| Bucknall 201263 | LOW ‘We used a minimisation technique to stratify randomisation of participants by demographic factors . . . We constructed a computer-generated sequence by using the method of randomised permuted blocks of length four, with two allocations being made at random and two by minimisation’ |
LOW ‘The researcher did not know whether a participant was being allocated at random or by minimisation and could therefore not determine the next treatment allocation before enrolling each participant’ |
HRQoL: HIGH Hospital admissions: LOW ‘The study team were blind to information on hospital admissions when these classifications were made’ ‘Participants received monthly telephone calls from an independent researcher, blinded to the patients’ randomisation status, to collect information on health service usage and exacerbations’ |
HRQoL: HIGH Self-completed questionnaires with only 61% returned – failure to complete associated with sicker, more depressed and lower self-efficacy Time to admission or death: LOW 11% dropout rate (low) – but more withdrawals from the control group, – 21 vs. 32 Similar and small (three and four, respectively) numbers lost to follow-up in each treatment group ITT analysis of primary outcome (readmission or death) |
UNCLEAR Protocol available Additional subgroup analyses were added during the trial ‘Within the first few months, we realised that some participants accepted self-management more readily than others. Therefore, as participants in the intervention group completed their 12-month period of follow-up, we classified them as either a “successful self-manager” or not after case based review by the study team’ |
UNCLEAR Well matched at baseline across all characteristics Low recruitment rate (47%), but similar recruitment rate to other trials in this field; intervention required high level of commitment so non-participants may not have benefited from the intervention Those who declined more deprived residence area |
| Busch 1988194 | UNCLEAR ‘Assigned patients by stratified random sampling to either the control group or exercise group’ |
UNCLEAR | HRQoL: HIGH | HIGH 20 random 10 : 10; 14 follow-up 7 : 7 Reasons for dropouts documented |
UNCLEAR All outcomes in methods reported in results |
HIGH Patients excluded from follow-up in exercise group if they did not adhere to exercise regime (n = 3); control group if they exercise n = 2 Baseline data given for only 14 patients followed up |
| Cai 2006199 | Randomly | NR | HIGH | |||
| Carr 2009195 | UNCLEAR States randomised but randomisation methods not reported |
UNCLEAR | Investigator responsible for collection outcome measure data not aware of group allocation HRQoL: HIGH Interviewer delivered CRQ but intervention vs. UC |
HIGH Five dropouts due to acute exacerbation 85% completion Report on the characteristics of withdrawn patients – less dyspnoea otherwise similar to completers – no quantification of degree of difference ITT analysis – NR |
UNCLEAR No protocol |
HIGH ‘Comorbidities that might adversely affect outcome measures’ excluded – not clear what these are Acute exacerbation removed from follow-up Baseline differences 12% and 24%, respectively, on oxygen in intervention vs. control group – 53% vs. 35% male in intervention vs. control Baseline reported for all completers but only for all participants who did not have an intervening AECOPD between recruitment/baseline and randomisation |
| Casas 200671 | LOW Computer-generated randomisation |
UNCLEAR NR |
HRQoL: HIGH Self-reported comparison of intervention vs. UC control Hospital admissions: LOW from records |
LOW ITT analysis Similar drop-out rate and reasons between arms 20–26% drop-out rate with death responsible for 17% |
UNCLEAR No protocol |
UNCLEAR Baseline reported for all patients Gender imbalance (12% vs. 23% female) Difference between type of intervention at the two study sites – GP home visits in Germany; nurse, social worker and physician in Spain |
| Garcia-Aymerich 200772 | LOW ‘Assigned using computer-generated random numbers’ |
LOW ‘Blindly assigned’ |
HRQoL: HIGH Health-care resource use: LOW |
HIGH Follow-up intervention 53% Loss to follow-up 3% Follow-up control 60% Loss to follow-up 13% Imbalance in loss to follow-up, high loss |
LOW | LOW |
| Chan 2010212 | LOW Computer-generated randomisation |
UNCLEAR NR |
HRQoL: UNCLEAR | LOW ITT analysis; 23% dropout Reasons for dropout similar between groups except loss of interest higher in breathing/exercise group |
UNCLEAR No protocol |
UNCLEAR Baseline reported for a wide range of characteristics for all participants and gender differences controlled for in analysis |
| Chan 2011272 | LOW Random allocation was done using a randomiser software21 Both the total number of subjects and number of groups were entered into the computer randomiser, which then generated the random assignment of subjects; this step helped avoid yielding a highly disparate sample size in the study groups; instead, it preserved many positive attributes of simple randomisation |
UNCLEAR | Exacerbations: LOW ‘The number of COPD exacerbations and hospital admissions during the preceding 6 weeks period was recorded‘ This study utilised a single-blind, RCT. The research assistants for data collection were blinded to minimise researcher bias |
HIGH Overall dropout rate > 10% Total dropout rate = 23% With differences in dropout rates between groups:
|
UNCLEAR No protocol |
UNCLEAR Very few female patients overall Age: t’ai chi group older average age 69 years vs. 58 years for control and 61 for exercise – also fewer current smokers in t’ai chi group: 17.1% vs. 23.2% vs. 22.4% |
| Cockcroft 1987142 | UNCLEAR States randomised but no method described |
UNCLEAR NR |
HRQoL: HIGH Self-reported intervention vs. UC control Admissions: LOW from records |
LOW Low dropout (n = 2) from (n = 75) sample Not stated as ITT analysis and no comparison of dropouts vs. trial completers, but very low dropout |
UNCLEAR No protocol |
UNCLEAR Baseline data reported for all participants; limited range of variables reported; well-balanced baseline |
| Coultas 2005112 | LOW ‘Selected from electronic claims database’ ‘Computer-generated random list’ |
UNCLEAR | HRQoL: HIGH ‘Self-report health care utilisation’ ‘Assessors of health care outcomes were blinded’ |
UNCLEAR Withdrawals reported, but no ITT |
LOW All outcomes reported |
UNCLEAR Underpowered Baseline line differences for gender |
| Covey 2001113 | UNCLEAR Stated randomised But no details |
UNCLEAR NR |
HRQoL: LOW Control group is educational intervention Patients blinded to previous responses Stated single blinded |
HIGH ITT analysis not reported – appears not to be according to results Larger dropout from IMT group (7 vs. 3) ‘Sample characteristics for patients who completed the study and those who did not complete the study were not significantly different’ but no detail of this |
UNCLEAR NR |
HIGH Baseline reported for only the completers BMI higher in educational intervention group Smoking status – NR |
| de Blok 2006181 | UNCLEAR ‘Randomly assigned’ |
UNCLEAR | HIGH ‘Clinical staff blinded for group assessment‘ |
UNCLEAR Withdrawals reported but no ITT |
LOW All outcomes reported |
UNCLEAR Very small sample |
| Dheda 200473 | HIGH Stated randomised, no methods described |
UNCLEAR NR |
HRQoL: HIGH Self-reported UC vs. intervention |
UNCLEAR ITT analysis not reported; no comparison of characteristics of dropouts vs. trial completers |
UNCLEAR No protocol |
UNCLEAR Not clear if baseline reported for all participants or just trial completers; limited range of variables reported at baseline n = 25 at completion |
| Donesky-Cuenco 2009114 | UNCLEAR ‘Forty-one (41) patients were randomly assigned to the Yoga group or to the UC group‘ No details of method for assigning |
UNCLEAR | HRQoL: HIGH Self-reported Blinding not referred to except – ‘Patients were asked about their satisfaction and experiences with the programme during individual exit interviews, which were conducted by the PI, who was not involved with the study operations’ |
UNCLEAR ITT analysis – stated ITT analysis in flow diagram but numbers stated do not indicate ITT Dropout rate identical, similar reasons – 71% follow-up, all dropouts reported and characteristics compared |
No protocol | HIGH Baseline data only reported for trial completers Baseline differences between treatment group and controls – oxygen use higher in treatment group (29% vs. 6%) also mean age higher (4 years older) |
| Dourado 2009233 | UNCLEAR ‘In groups of 3, patients were randomly assigned to one of the training modalities . . .’ No comment on mode of randomisation |
UNCLEAR | HRQoL: LOW Self-completed questionnaires and comparison of intervention |
HIGH ITT analysis – NR Approximately 25% dropout rate – variable between groups Detail of reasons for dropout provided for only the whole study cohort, not by group ‘However, comparisons of these patients (dropouts) with those completing the programs did not show significant differences at baseline’ No other details |
UNCLEAR No protocol |
HIGH n = 35 but three groups, so each only small Baseline differences Baseline only reported for completing participants |
| du Moulin 2009206 | LOW For randomisation purposes, we prepared 20 envelopes, 10 with a plus, indicating intervention group, and 10 with a minus, indicating control group; patients drew these envelopes themselves; if this was not possible, a third person not involved in the study randomised the patient |
UNCLEAR Opaque envelopes |
HRQoL: HIGH Self-completion with assistance if required Intervention vs. normal care |
HIGH ITT analysis Large dropout rate – but balance between two groups and different reasons for dropout No comparison of the characteristics of dropout vs. completers |
UNCLEAR No protocol |
HIGH Very small study size n = 20 with 40% dropout Baseline reported for all patients Average age 5 years younger and 20% more smokers in intervention group |
| Eaton 2009227 | LOW ‘Randomised . . . using computer-generated randomisation’ |
UNCLEAR ‘Computer-generated randomisation with the allocation being concealed until the intervention was assigned’ No details about how |
HRQoL: HIGH Intervention vs. UC ‘The nature of the intervention precluded blinding of participants and health-care providers’ Hospital readmission: LOW ‘Data were obtained from hospital and primary health-care records and were reconciled with patient home diary records by assessors blinded to the intervention allocation’ |
HIGH ‘Both intention-to-treat and per-protocol analyses are reported’ 82 vs. 90% follow-up intervention vs. control ‘There were no significant differences in baseline characteristics between attendees and non-attendees’ But not detailed |
LOW The study was registered prospectively with the Australian Clinical Trials Registry (ACTRNO12605000372684) |
HIGH Baseline: 9% fewer current smokers in intervention group and lower total pack-years (by 5 years) – also more European patients in intervention group (83 vs. 68%) |
| Effing 2009161 | LOW Minimisation programme |
UNCLEAR | HRQoL: LOW Self-reported, comparison of two interventions Hospital admission Not entirely clear how these data were collected? From records or from diaries? Exacerbations: LOW Data collected from patient diaries but very clearly defined criteria |
UNCLEAR ITT analysis done but only after baseline measurement and substantial number of dropouts between randomisation and baseline measurement Imbalance between numbers and reasons for dropout – more dropouts for lack of motivation in self-treatment group Limited comparison between baseline characteristics of dropouts vs. completers – dropouts less dyspnoeic |
UNCLEAR No protocol |
UNCLEAR Baseline data reported for all patients but note only limited range of variables reported and substantial number of dropouts after randomisation and before baseline measurements Exacerbations clearly defined as negative change in two major symptoms with duration also clearly defined |
| Effing 2011278 | LOW ‘Patients were randomised into two study groups, using a minimisation programme, nine minimising differences between groups in gender, current smoking, FEV1 predicted (or > 50%), use of inhaled corticosteroid, and current participation in a regular physiotherapy programme’ |
UNCLEAR | HRQoL: HIGH Self-completed questionnaire |
HIGH ITT analysis done but no reporting on baseline characteristics of dropouts Low dropout rate of 8 (10.5%) Withdrawals from the control group and only three (4%) from treatment group – lost to follow-up/failure to return only in the control group, but total lost to follow-up only 7% Identical and low numbers of deaths |
UNCLEAR No protocol |
HIGH Baseline variation between groups in ISWT and endurance walking test – intervention group longer distance |
| Efraimsson 2008218 | LOW Matched pairs, independent person drew lots for allocation of either intervention or control |
UNCLEAR | HRQoL: HIGH By questionnaire in undisturbed area of clinic, nurse available to answer questions and check patients had responded to all items |
UNCLEAR 100% follow-up in tables, yet says ‘the dropout rate’ was 10 patients, may refer to eligible patients not recruited |
UNCLEAR | LOW |
| Egan 200269 | LOW Random number tables |
UNCLEAR NR |
HRQoL: HIGH Intervention vs. UC control – not entirely clear if control is UC; however, significantly different level of clinical input between intervention and control group |
HIGH Dropout rate not balanced between groups (24% from control; 15% from intervention). Reasons not listed for withdrawal and no comparison of characteristics No ITT analysis |
UNCLEAR No protocol |
HIGH Baseline data listed for all participants Marked gender imbalance between groups – 60% vs. 30% male, intervention vs. control Also variation in income levels Inclusion criteria includes chronic asthma – diagnosis not listed in baseline data |
| Elci 2008236 | LOW Randomly allocated using number tables |
UNCLEAR | HRQoL: HIGH | LOW Loss to follow-up 15.4%, equal in each arm No description of n by outcome |
LOW | LOW |
| Elliott 2004162 | UNCLEAR Randomly assigned |
UNCLEAR No mention of when measures undertaken in relation to randomisation |
HIGH No mention of blinding – unsure who undertook measures |
LOW Outcome data reported only on completed; no baseline measure carried forward or imputation; but no systematic difference in withdrawals by group (73% complete in intervention vs. 69% in control) Reason for withdrawal described (table 4) |
LOW All outcomes reported |
LOW |
| Emery 1998115 | LOW ‘Patients were randomised to one of three groups . . . Group assignments were taken from a random number schedule, printed on a piece of paper, and placed in a sealed envelope’ |
LOW ‘Participants were not given the envelope containing their group assignment until after completing the baseline assessment, and technical staff conducting the assessments were not aware of group assignments’ |
HRQoL: HIGH Using self-reported, validated questionnaire |
LOW 79 randomised with 6/79 (7.6%) dropouts, which were all reasoned Group A: 4/29 (illness) Group B: 2/25 (transport issues) Group C: no dropouts Differences between completers vs. dropouts assessed |
UNCLEAR Outcomes measures in methods section reported in results section |
LOW Baseline data reported for all patients randomised; any baseline imbalances made explicit in text |
| Engstrom 1999219 | UNCLEAR | UNCLEAR | HIGH | UNCLEAR 2/28 control plus 3/27 dropped out; reasons were given; no comparisons between dropouts and completers undertaken |
UNCLEAR HRQoL (SGRQ and generic) mentioned in methods and results |
HIGH Baseline data given for only those followed up; fairly balanced |
| Fernandez 2009171 | UNCLEAR Randomised by block allocation but no indication of how |
UNCLEAR | No direct comment on blinding for any of the outcomes HRQoL: UNCLEAR Self-completed questionnaire of intervention vs. normal care |
HIGH ITT analysis – NR 84% follow-up Similar dropout numbers Three refused from intervention, one refused in control No comment on characteristics of dropouts |
UNCLEAR No protocol |
HIGH No female patients but baseline well matched; however, only reported for those who completed the study Control group approximately half of the size of the intervention group |
| Finnerty 2001143 | LOW ‘In blocks of 10 using random numbers’ |
UNCLEAR | HRQoL: HIGH Patient completed but supervised by a blinded observer |
HIGH Many patients failed to attend assessment at baseline after randomisation. HRQoL – quality of outcome data plus whether balance between groups depends on time point for assessment |
UNCLEAR HRQoL mentioned in method and results |
HIGH Demographics not given for all randomised patients at baseline |
| Foy 2001116 | UNCLEAR | UNCLEAR | HRQoL: HIGH | 14/70 did not complete 18 months of study in short-term intervention arm; 8/70 in the long-term intervention arm; reason not reported No mention of difference between dropouts and completers |
UNCLEAR CRQ mentioned in methods and results |
LOW Baseline characteristics given for all randomised parts and seem fairly balanced |
| Gallefoss 1999255 | UNCLEAR | UNCLEAR | HRQoL: HIGH | UNCLEAR 4/31 and 5/31 in control and intervention lost to follow-up; two of those in the hospital group were due to hospitalisation; no other details given |
UNCLEAR HRQoL (HRQoL, SGRQ) mentioned in methods and results |
UNCLEAR Baseline characteristics are fairly balanced except more never smoked in the intervention group [13% (n = 4) vs. 0%] |
| Gallefoss 2000280 | LOW ‘Using random number tables’ |
UNCLEAR | HRQoL: HIGH Self-reported hospitalisations: LOW |
UNCLEAR Dropouts of patients with COPD were similar between groups: 4/31 and 5/31. Two of the control patients dropped out due to exacerbations but none in the intervention group |
UNCLEAR Hospitalisation appears presented as expected. Was HRQoL measured at baseline? (Appears not.) HRQoL data appear to be presented only as a relationship with hospitalisations/GP visits |
LOW Baseline data for patients with COPD only appears balanced for the variables tabulated Demographics are presented for all patients at baseline |
| Gallefoss 2002391 | LOW Random number table |
UNCLEAR | Hospital admission: LOW Self-reported and checked against hospital records Exacerbations: LOW Measured by use and prescribing of rescue medications from pharmacy records |
No ITT analysis Low dropout 13% Balanced number and reasons between groups |
UNCLEAR No protocol |
LOW Baseline reported for all patients – well balanced and good range of characteristic reported, not BMI |
| Gallefoss 2004281 | UNCLEAR Randomly assigned |
UNCLEAR No mention |
UNCLEAR No mention; unlikely to affect results |
LOW Withdrawals discussed; no different in withdrawal by group; no imputation |
LOW All outcomes reported |
LOW |
| Ghanem 2010264 | UNCLEAR ‘Randomly allocated’ – no details given |
UNCLEAR No details given |
HRQoL: HIGH ‘It was not possible to blind patients or assessors’ |
LOW No loss to follow-up at 2 months follow-up |
UNCLEAR | HIGH Large baseline difference in urban/rural residence; high proportion of those not allocated to rehabilitation were ‘rural’ 86% vs. 56% Considerable baseline difference in ‘respiratory failure’, more control group had respiratory failure: 72 vs. 43% |
| Gilmore 2010117 | LOW Randomly drawn cards in blocks of four |
UNCLEAR Allocation concealment – NR |
States non-blinded HRQoL: HIGH Not self-completed (read aloud to participants) Four intervention groups – three intervention variants and one UC control |
HIGH 27% dropout Reasons for dropouts not reported by group in text Home visit group appears to have higher dropouts Comparison of characteristics of completers with recruits given |
UNCLEAR No protocol |
UNCLEAR Baseline reported for all and for all completers But baseline comparison between groups reported for only the completers |
| Gohl 2006207 | Patients were randomised (‘lottery’); no further details | UNCLEAR No details |
No details | 7/17 dropouts in intervention group [two, peripheral arterial disease; one shoulder fracture; one prostate cancer, exacerbation due to infection during main training period (1) or during final assessment period (2)] 8/17 dropouts in control group (two, bereavement; two peripheral arterial disease; two exacerbation due to infection during final assessment period; two non-compliance) No details on differences between patients dropping out or remaining in study |
No obvious selective reporting; all outcomes mentioned in methods also reported in results section | Reasons for dropouts described as atypical for this patient population |
| Goldstein 1994196 | UNCLEAR Randomised, stratified by 6-MWD |
UNCLEAR No details |
HRQoL: HIGH | HIGH Follow-up: 78/89 Intervention dropouts 7/45; control 4/44 Details not provided by study arm |
UNCLEAR | HIGH Baseline imbalances in sex, number living alone, years since cessation of smoking and number receiving supplementary oxygen |
| Gourley 1998286 | UNCLEAR Stated randomised but not methods described |
UNCLEAR NR |
HRQoL: HIGH Intervention vs. control, which differs markedly in degree of contact |
HIGH Total recruitment, follow-up not reported, reported only elsewhere Not ITT analysis |
UNCLEAR No protocol |
HIGH No baseline data given in this paper; however, reported elsewhere and only for trial completers |
| Guyatt 1999393 | UNCLEAR Stated randomised, but no detail of method Full method described in Goldstein 1994196 |
UNCLEAR NR |
HRQoL: HIGH Self-reported intervention vs. UC control |
HIGH 16% vs. 10% dropout in intervention vs. control; reasons for dropout not reported by group ITT – NR No comparison of baseline characteristics of dropouts vs. trial completers |
UNCLEAR No protocol |
UNCLEAR Baseline reported in Goldstein, 1994;196 baseline reported for all participants Gender imbalance 57% vs. 43% male in intervention vs. control Baseline HRQoL well balanced |
| Green 2001144 | UNCLEAR NR |
UNCLEAR Concealed envelopes; envelopes not numbered |
HRQoL: LOW Comparison of interventions |
Not ITT analysis | UNCLEAR No protocol |
HIGH Baseline reported for all participants Mismatched baseline for pack-years (38 vs. 47 in 4-week rehabilitation vs. 7-week rehabilitation); also significant (> 0.5) differences in baseline HRQoL scores |
| Güell 2000172 | LOW | HIGH Unconcealed but consecutive patients enrolled |
Technical staff blinded No mention of other groups HRQoL: HIGH Questionnaires Admissions: LOW |
LOW 7/30 and 6/30 dropped out. Demographic data for completers and dropouts given for both groups for major variables (but not gender) |
UNCLEAR HRQoL/exacerbations/hospitalisations mentioned in methods and given in results |
LOW Baseline data given for all patients, not just completers Groups were fairly balanced |
| Güell 2006173 | UNCLEAR ‘Unconcealed randomisation’ ‘Recruitment of consecutive patients’ |
UNCLEAR ‘Not concealed’ |
HIGH ‘Neither patients or clinicians were blinded’ ‘Technicians who collected data were blinded to allocation’ |
UNCLEAR Withdrawals reported, but no detail on management of data |
LOW All outcomes reported |
HIGH Baseline differences for Millon Behavior Health Inventory (MBHI) (behaviour) and Revised Symptom Checklist (SCL-90-R) (symptoms) |
| Guyatt 1992118 | UNCLEAR Randomisation reported but method not described ‘Randomisation in sequence’ |
UNCLEAR Concealment Reported but method not described |
HRQoL: LOW Comparison of interventions and blind assessors ‘Other study personnel were blind to allocation. Patients were repeatedly instructed not to mention their impressions of the training procedure to their physician or to anyone concerned with the study’ |
HIGH No ITT analysis 15% dropout (11/74); however, dropout in run-in period due to illness ‘too great a commitment’ was 30%); different reasons for dropout given between groups and no comparison of characteristics |
UNCLEAR No protocol |
HIGH Baseline reported for only those who completed the run-in period Smoking status – NR |
| Hermiz 200267 | UNCLEAR Site 1: randomised permuted blocks with a block size of four Site 2: ‘simple randomisation’ |
UNCLEAR | HRQoL: HIGH All data collected by project officer, including administration of HRQoL measure No mention of blinding |
HIGH Loss to follow-up: intervention 8/84, control 3/93 – more withdrew from intervention group |
UNCLEAR | LOW |
| Hernandez 2000282 | UNCLEAR Stated randomised but no details |
UNCLEAR | HRQoL: UNCLEAR Self-reported: was intervention vs. control; however, both groups attended for check up and treatment supervision greater than normal care |
HIGH Not clear if ITT analysis as not stated and n not given Dropout rate 39% but balanced between groups |
UNCLEAR No protocol |
UNCLEAR Only a limited range of baseline characteristics reported – unclear if for all patients or only completers Participants were ex-smokers only; no exacerbations during course of study and no comorbidities |
| Hernandez 200368 | LOW ‘Blindly assigned using a set of computer-generated random numbers’ |
UNCLEAR | HRQoL: HIGH Self-reported intervention vs. UC trial Hospital admission: LOW Self-reported with some verified from records Exacerbations: LOW |
UNCLEAR Dropout rate not reported except deaths Unclear if ITT analysis as n not reported in data |
UNCLEAR No protocol |
Baseline given for all patients and a good range of characteristics reported 10% more current smokers in intervention group |
| Hill 2006163 | LOW Computer-generated random number sequence |
UNCLEAR NR |
HRQoL: LOW Double-blind, interviewer-administered comparison of intervention and sham intervention |
UNCLEAR Low (6%) dropout after randomisation, but dropouts (n = 2) from intervention group |
UNCLEAR No protocol |
HIGH Baseline reported for only trial completers but, as noted, only 6% dropout Baseline well matched except 6-MWT: > 10% further in intervention group |
| Holland 2004164 | UNCLEAR Not described |
UNCLEAR Not clear whether baseline measures were taken prior to randomisation |
LOW Data collector was blinded to group allocation |
LOW All withdrawals accounted for ITT analysis undertaken |
LOW All expected outcomes reported |
LOW |
| Hospes 2009183 | UNCLEAR ‘Patients were randomly assigned to an exercise counselling or a UC group’ No mention of methods of randomisation |
UNCLEAR | HRQoL: HIGH Self-reported but intervention vs. control |
HIGH Only 10% dropout and similar between groups Analysis was not ITT analysis |
UNCLEAR No protocol |
HIGH N = 40 but baseline reported for only the completing participants Baseline difference between groups in GOLD stage of COPD (22% stage 1 in intervention group vs. only 6% in control group) Smoking not reported in baseline characteristics |
| Hsiao 2003259 | UNCLEAR Method of randomisation not described |
UNCLEAR NR |
HRQoL: UNCLEAR Interventions and one UC control; no details of blinding/administration |
Dropout 12/42 No reported differences in baseline characteristics but no detail given No ITT analysis |
UNCLEAR No protocol |
HIGH Baseline given for only study completers |
| Hynninen 2010256 | UNCLEAR Stated randomly assigned in matched pairs but no details on method |
LOW Numbered sealed containers, identical in appearance |
HRQoL: HIGH | HIGH ITT analysis Overall dropout over 18 months (20%); however; imbalanced dropouts – 30% from control group, 10% from intervention No comparison of characteristics of dropouts vs. trial completers |
UNCLEAR No protocol |
UNCLEAR Baseline reported for all participants Only a limited range of characteristics reported, but well-matched baseline with exception of mental health diagnosis (40 vs. 50%, intervention vs. control) |
| Janaudis-Ferreira 2011197 | UNCLEAR ‘Patients were randomly (in blocks of four) assigned to an intervention or control group. Randomisation was stratified according to the presence or absence of the use of supplemental oxygen at rest’ No further details |
LOW ‘The sequence was kept in opaque envelopes by an investigator who was not involved in the recruitment process These envelopes were drawn by the trainer after the subjects had completed their preassessment session, allowing for concealed allocation’ |
HRQoL: LOW Control group underwent sham exercises The outcome assessor and the patients remained unaware of the group allocation Each patient exercised individually at different times and locations |
HIGH No data on characteristics of dropouts 16% lost to follow-up – 2× as many in treatment arm than control arm, but loss to follow-up unrelated to intervention ITT analysis used |
UNCLEAR No protocol available |
UNCLEAR Baseline characteristics reported for all patients recruited but smoking status not reported |
| Jang 2006267 | Tossed coins | UNCLEAR | HIGH | |||
| Jarab 2012266 | LOW ‘Participants were randomly assigned to intervention and control groups via a minimisation technique using MINIM software’ |
UNCLEAR No details given |
HRQoL: HIGH Health-care utilisation: LOW |
3/66 intervention and 3/67 control group patients withdrew by 6 months; no details given about reasons | UNCLEAR | LOW |
| Karapolat 2007237 | UNCLEAR No detail |
UNCLEAR ‘Sealed envelopes’ |
HRQoL: UNCLEAR | LOW Loss to follow-up 1/27 intervention, 3/22 control; no reasons given |
UNCLEAR | HIGH Five participants excluded from control group post randomisation ‘because they did not satisfy inclusion criteria’ |
| Katiyar 2006240 | LOW Computer-generated randomisation |
UNCLEAR NR |
HRQoL: HIGH Supervised self-administered. Intervention vs. UC |
HIGH Low dropout 3/24 but not ITT analysis |
UNCLEAR No protocol |
HIGH Baseline reported for only the completers |
| Kayahan 2006238 | UNCLEAR ‘Randomised’, no details |
UNCLEAR | HIGH | UNCLEAR Reported but no reasons given or information on management of data |
LOW All outcomes reported |
UNCLEAR Small sample |
| Khdour 2009251 | LOW Computer-generated minimisation |
UNCLEAR | HRQoL: HIGH Self-reported UC vs. intervention Admissions: LOW Patient questionnaires confirmed by hospital records |
HIGH No ITT analysis – as per-protocol analysis reported Dropouts, lost to follow-up and death balanced across groups No comparison of characteristics of dropouts vs. completers |
UNCLEAR No protocol |
LOW Baseline data reported for all participants Good range of variables reported and well balanced across all variables |
| Khdour 2011279 | LOW Minimisation |
UNCLEAR | HRQoL: HIGH Self-completed questionnaires (intervention vs. UC) Admissions: LOW Patient records verified from hospital record |
HIGH No ITT analysis 27% dropout Balanced numbers and similar reasons for dropout between groups |
UNCLEAR No protocol |
HIGH No comparison of baseline characteristics of dropouts vs. completers Baseline balanced with the exception of 10% more ‘never smokers’ in control group |
| Kim 1993119 | UNCLEAR | UNCLEAR | ‘Double-blind’ control group received sham intervention HRQoL: LOW |
HIGH 129 patients enrolled, 67 ‘provided 6 months of usable data’; detail provided on loss to follow-up but not by study arm; 17 patients dropped out before randomisation, in 4-week control period before randomisation Follow-up: 41/66 intervention; 26/46 control |
UNCLEAR All data on measures in methods were reported in results |
HIGH No detail on baseline characteristics of those lost to follow-up |
| Ko 2011213 | LOW ‘Subjects were randomised, by random number generator, to receive either PR or UC (UC). A computer programme (allocation by minimisation) was used to assist the randomisation of subjects equally into each group taking into account five factors’ |
UNCLEAR Not discussed |
HRQoL: HIGH Readmission/exacerbation: LOW From records ‘Owing to the nature of the intervention, this was an open study for patients and therapists but the technicians . . . were not involved in the delivery of the PRP and were not aware of the randomisation status’ |
HIGH ITT analysis At 6 months – loss to follow-up in both arms > 10% 20% and 17% in control and intervention arms, respectively Reasons for non-completion similar across groups No reporting of baseline characteristics of dropouts |
LOW Protocol available at www.clinicaltrials.gov NCT00287625 Verified with published protocol all reported |
HIGH Includes patients with comorbidities and there are significant differences between the control and the intervention arms (cardiac-related comorbidity 17% in intervention vs. 30% in control group), likely significant given low numbers of participants and only 10% reported improvement in HRQoL at 6 months and nil at 12 months |
| Koff 2009120 | Envelopes – not stated shuffled but presumably | UNCLEAR Envelopes – not stated sealed or numbered |
HRQoL: HIGH Self-reported but intervention vs. UC control and unblinded |
UNCLEAR No ITT analysis Very low drop outs (5% 1 participant from each branch with n = 40) |
UNCLEAR No protocol |
UNCLEAR Baseline reported for all patients and well balanced with the exception of % flu vaccinated (100% vs. 85%) and living alone 15% vs. 30%, both of which may impact on HRQoL |
| Koppers 2006184 | UNCLEAR Stated randomised but no details |
UNCLEAR NR |
HRQoL: LOW Self-completed – blinded assessor control is sham training |
HIGH Very low dropout rate – 8%; three participants, same reason (severe exacerbation) Characteristics – NR ITT analysis – NR |
UNCLEAR No protocol One of the three study arms was dropped owing to financial reasons |
UNCLEAR Baseline – reported for all participants but smoking status not reported |
| Kunik 2008121 | LOW Used ‘Statistical Analysis Systems plan procedure to create randomisation list, with blocks of size 2 to provide approximate equal number per class. The statistician provided randomised numbers and treatment codes to the study co-ordinator . . .’ |
LOW | HRQoL: HIGH | HIGH High loss to follow-up 8 weeks: 63/120 and 60/118 1 year: 56/120 and 52/118 Reasons for dropout not given by arm |
UNCLEAR | LOW |
| Kwok 200470 | LOW Random number table |
LOW Remote telephone allocation |
Readmissions: LOW Verified from electronic hospital records |
UNCLEAR Not ITT analysis 11% dropout rate, half due to death No comparison of characteristics of dropouts vs. trial completers |
UNCLEAR No protocol |
HIGH Baseline reported for all participants Appears well balanced except PEFR (misprint) However, patients with asthma and bronchiectasis included, but their distribution between groups not documented |
| Lamers 2010185 | LOW Randomisation was performed by an external agency using a computerised random number generator In order to avoid an imbalance of chronic illness and general practice (care level) over the two groups, stratification for general practice and chronic illness (diabetes mellitus or COPD) was performed. Furthermore, to obtain equal numbers in both arms, a blocked design with a block size of two was applied |
LOW Randomisation was performed by an external agency using a computerised random number generator The researchers entered patients into a computer connected to an external agency, which performed the randomisation using a computerised random number generator |
HIGH | HIGH ITT analysis Very high dropout rate from both the intervention and control groups Intervention group only 74% received the intervention Only 67% and 75% reached even the first follow-up in intervention and control group |
LOW Protocol available This paper seems to report only a subset of the outcome measure |
UNCLEAR Education level mismatched between baseline groups – up to 9% variation between percentage of highly educated in intervention and control groups |
| Larson 1988122 | UNCLEAR Randomisation stated but not described |
UNCLEAR NR |
HRQoL: LOW Comparison of interventions Double-blind trial |
HIGH n = NR Only n is number who completed study |
UNCLEAR No protocol |
HIGH Baseline characteristics table is for only the completers |
| Larson 1999123 | UNCLEAR Randomisation stated but method not reported |
UNCLEAR Concealment – NR |
HRQoL: UNCLEAR Study was comparison of interventions; however, blinding of assessors not reported; participants blinded to baseline and previous responses |
HIGH ITT analysis – NR Poor completion rate – 41% ‘There were no significant differences between those who completed the study and those who dropped out of the study in terms of demographics, pulmonary function tests, and arterial blood gases’ but no detail of this |
UNCLEAR No protocol |
HIGH Baseline data reported for only the completers of study |
| Lee 200266 | UNCLEAR Nursing home was randomised, not patient; no method given but homes were randomised based on number of admissions |
UNCLEAR | HRQoL: HIGH UC vs. intervention Hospital admission: LOW |
HIGH No reporting of the distribution of dropouts between groups No ITT analysis |
UNCLEAR No protocol |
HIGH Limited range of baseline characteristics reported: reported for only the study completers |
| Leung 2010165 | LOW Computerised telephone dial-up system – stratified randomisation |
LOW Remote allocation by telephone dial-up |
HRQoL: LOW Questionnaires were interviewer administered – and is comparison of interventions States blinded outcome assessment ‘An assessor blinded to allocation performed outcome assessment’ |
LOW ITT analysis Only 12% dropout rate Similar dropout rate between branches and has comparison of characteristics of those lost to follow-up/dropouts, different by age and FEV1/FVC ratio |
UNCLEAR No protocol |
LOW Baseline well matched data for all participants included Training intensity well standardised |
| Li 2002200 | Randomly | NR | HRQoL: UNCLEAR |
NR | ||
| Liddell 2010145 | UNCLEAR ‘Separate but identical randomisation processes were carried out at each outpatient department’ No comments on method of randomisation |
LOW ‘Equal numbers of once-weekly and twice-weekly allocations were created, and then stored in sealed envelopes. These envelopes were only opened after the initial assessment and patient consent’ Not sequentially numbered |
HRQoL: UNCLEAR Self-completed SGRQ but is an equivalence trial on intervention ‘Follow-up assessments were completed within 2 weeks of the end of the programme. At follow-up, blinding was not possible as staff and patients were aware which programme the patients had attended’ |
HIGH 33% dropout rate – all accounted for and similar rates/reasons in each group ITT analysis Baseline variables used for missing data from dropouts No comparison of completers to follow-up |
No protocol | HIGH Three participants did not have COPD on spirometry (no comment on which group they were randomised to) = 10% of participants Baseline reported for all participants Some variation in baseline characteristics > 10% in mean FEV1 Smoking not reported as a variable |
| Lindsay 2005214 | UNCLEAR ‘Randomly allocated’ |
UNCLEAR | HIGH | LOW Withdrawals reported, and ITT performed |
LOW All outcomes reported |
UNCLEAR Underpowered |
| Linneberg 2012224 | UNCLEAR ‘Patients were randomised (in blocks of 20) for the intervention group or control group’ |
UNCLEAR No information given |
HRQoL: HIGH | Reasons for dropouts were reported, but not by arm Follow-up at 1 year: 49/59 for intervention, 47/59 for control |
UNCLEAR | LOW |
| Littlejohns 1991146 | LOW Random number table |
LOW Sealed numbered envelopes kept centrally |
HRQoL: HIGH Self-reported intervention vs. UC control Admissions: LOW |
HIGH No ITT analysis 88% trial completion rate Balanced loss to follow-up Characteristics of dropouts and trial completers compared; however, characteristics reported of only those who died |
UNCLEAR No protocol |
HIGH Baseline characteristics of all patients reported for a wide range of characteristics Average HRQoL appears significantly different (9.4 vs. 7.2 – intervention vs. control) in both physical and psychosocial domains |
| Liu 2008260 | LOW ‘. . . assigned to the cell phone group according to a table of random numbers’ |
UNCLEAR | Blinding not reported in whole study HRQoL: UNCLEAR Admissions: LOW Exacerbations: HIGH Appear to be self-defined |
HIGH No ITT analysis Dropouts evenly matched between groups but no comparison of characteristics Unclear at what point data removed from analysis for dropouts |
UNCLEAR No protocol |
UNCLEAR Baseline appears very well matched but smoking history not reported and only reported for study completers |
| Livermore 2010166 | LOW ‘We used the Excel Bernoulli function to generate a random sequence of numbers, with a 50% chance of each of the two groups occurring. Randomisation was linked to subject numbers’ |
UNCLEAR ‘Randomisation . . . was concealed until after the baseline assessment was completed’ No details about how this was achieved |
HRQoL: HIGH Self-completed but intervention vs. control Admissions: LOW Record review |
UNCLEAR Graded unclear as low drop out at 6 months but higher later Dropout rate at first assessment (post intervention) = 0% Dropout rate at 6 months = 2.5% Dropout rate at 12 months = 17% Dropout rate at 18 months = 22% Similar dropout rates for each branch but no reasons given Low at 6 months, high at 12 and 18 months ITT analysis done |
UNCLEAR No protocol |
HIGH No significant baseline differences – but no report on characteristics of dropouts Blind outcome assessor for a 20% sample good correlation between assessments of non-blinded and blinded assessors |
| Lord 2010147 | UNCLEAR ‘Using block randomisation through consecutive sealed envelopes’ Not enough details to decide |
UNCLEAR Sealed but not numbered envelopes |
HRQoL: LOW Self-completed questionnaires but comparison of interventions ‘At seven weeks follow-up, study participants were again assessed by the same respiratory physiotherapists, who were blinded to treatment allocation. All subjects were instructed not to tell the physiotherapist which group they had been allocated to’ |
HIGH ITT analysis 22% drop out rate Similar dropout rate and reasons in each group Block allocation resulted in control group 20% smaller than intervention group No comparison of characteristics of dropouts |
UNCLEAR No protocol |
HIGH Baseline characteristics reported only for those completing study Although they did not reached significance (because of small numbers of participants) there were clinically significant baseline differences in recovery after ISWT and in the ISWT distance itself |
| Madariaga 2007174 | UNCLEAR No details |
UNCLEAR No details |
UNCLEAR No details |
LOW No loss to follow-up |
UNCLEAR | HIGH States 34 randomised in methods, 33 in abstract and baseline imbalances in dyspnoea score High for HRQoL |
| Mador 2004126 | UNCLEAR No description of how randomised |
LOW Used ‘opaque sealed envelope’ (p. 2037) |
LOW | LOW Dropout accounted for, and similar in two groups; no imputation for dropouts |
LOW All outcomes reported |
LOW Multiple measures and tests; no adjustment for multiple testing |
| Mador 2005125 | UNCLEAR ‘Randomly assigned’ |
UNCLEAR | HIGH | UNCLEAR Withdrawals briefly reported, but no ITT |
LOW All outcomes reported |
LOW |
| Mador 2009124 | UNCLEAR Stated randomised but no detail |
UNCLEAR Stated concealed but no detail |
HRQoL: LOW Self-reported comparison of interventions |
HIGH 12.5% dropouts balanced between groups No comparison of characteristics between dropouts and completers No ITT analysis |
UNCLEAR No protocol |
HIGH Baseline reported for only the completers |
| Magadle 2007254 | LOW ‘Using a random number table’ |
UNCLEAR Not stated |
Patients blinded by ‘sham IMT’ control; outcome assessment HRQoL: LOW |
LOW Loss to follow-up: intervention 2/10, control 2/15 |
LOW | LOW |
| Maltais 2008198 | LOW ‘We used a centrally administered, computer-generated permuted block randomisation scheme using blocks of two, stratified according to sex and participating site’ |
LOW ‘We communicated assignments by e-mail to research staff who were not otherwise involved in the trial. The case manager subsequently informed patients of their group allocation. Study personnel were unaware of the permuted block size’ |
HRQoL: LOW Comparison of interventions Home vs. hospital-based rehabilitation |
LOW ‘The primary analysis took a modified intention-to-treat approach using all patients who provided data at the specified follow-up time regardless of adherence’ ‘The withdrawal rate was similar in both treatment groups’ Although reasons for withdrawal reported, they were not reported by group ‘The baseline characteristics of the patients who withdrew were similar to those of patients who completed the trial’ |
LOW Protocol available ClinicalTrials.gov registration number: NCT00169897 |
UNCLEAR Baseline reported for all patients Baseline: home rehabilitation group 8% GOLD stage IV vs. 18% in hospital Although groups matched to dyspnoea Pack-years reported but not current smoking status |
| Man 2004148 | LOW A random number generator to assign an intervention to the first patient entering the study Minimisation method taking into account age, sex, length of hospital admission, ISWT distance and FEV1 |
LOW | It was not possible to blind patients or the assessors HRQoL: HIGH Admissions: LOW |
LOW Follow-up: 16/21 control, 18/21 intervention Details of dropouts by study arm |
UNCLEAR | LOW |
| Martin 2004228 | UNCLEAR Stated randomised but no detail given |
UNCLEAR | HRQoL: HIGH Intervention vs. control Hospital admission: LOW Method of data collection – NR |
HIGH Deaths between intervention and control were not balanced (9 vs. 4); low dropout rate (13.5%) Unclear if ITT analysis – based on missing data from baseline seems likely that it is not |
UNCLEAR No protocol |
UNCLEAR Baseline reported for all participants for some variables but not others – limited number of variables reported and gender imbalance between groups |
| McGeoch 2006229 | UNCLEAR ‘Randomly selected’ and use of ‘random numbers table’ ‘Cluster randomisation of surgeries’ |
UNCLEAR | HIGH ‘No blinding’ |
UNCLEAR Withdrawals reported, but no detail on management of data |
LOW All outcomes reported |
UNCLEAR Baseline differences for primary outcome SGRQ, ‘not clinically significant’ |
| Monninkhof 2003186 | UNCLEAR | UNCLEAR Using sealed envelopes |
HIGH No blinding |
LOW Withdrawals and dropouts reported No difference between groups |
LOW All outcomes reported |
LOW |
| Moore 2009285 | LOW Minimisation method |
UNCLEAR States concealed but no details on method |
HRQoL: UNCLEAR Intervention vs. control – control group did receive some educational material/consultation |
HIGH No ITT analysis N = 27, dropouts and lost to follow-up balanced between groups Dropout = 27% |
UNCLEAR No protocol |
HIGH Baseline reported for only the participants completing study Small sample size (n = 20) Baseline well balanced |
| Mota 2007175 | UNCLEAR No details |
UNCLEAR No details |
Participants in control given sham training Outcome assessments blinded – ‘blind regarding the assigned training or sham groups’ HRQoL: LOW |
LOW HRQoL: LOW Loss to follow-up 2/8 in control due to exacerbation Intervention 10/10 completed |
UNCLEAR | HIGH Baseline characteristics for completers only SGRQ values for baseline not given – only % change at follow-up |
| Mularski 2009127 | LOW Computer-generated random number table |
UNCLEAR Sealed, un-numbered envelopes |
HRQoL: LOW Comparison of interventions |
HIGH ITT analysis Imbalanced dropouts 52% vs. 30% (intervention vs. control) |
UNCLEAR No protocol |
HIGH Baseline reported for all participants Significant differences in average age (71 years vs. 64 years), BMI (26 kg/m2 vs. 31 kg/m2) and current smoking 17% vs. 34%) in intervention vs. control |
| Murphy 2005252 | UNCLEAR ‘Randomly assigned 1 : 1 ratio’ |
UNCLEAR ‘Blinded sealed envelopes’ |
HRQoL: HIGH Exacerbations: LOW |
UNCLEAR Withdrawals reported, but no ITT |
LOW All outcomes reported |
UNCLEAR Baseline differences for smoking history, hospitalisations |
| Nakamura 2008261 | UNCLEAR Stated as randomised but method not described |
UNCLEAR Allocation concealment – NR |
HRQoL: UNCLEAR Two interventions and one UC control However, tool was administered by a trained interviewer – blinding not reported |
HIGH Follow-up was reasonable – 22% dropout – but not balanced between groups; reasons for dropout were not reported and there was no comparison of characteristics |
UNCLEAR No protocol |
HIGH Baseline reported for only the completers No reporting of smoking status or baseline HRQoL |
| Ng 2011215 | LOW Computer-generated random number list with ‘permutated blocks’ |
LOW ‘It was then sealed in a database file with a security password’. . .’ The occupational therapist who was blind to the list opened the file and assigned the subject to treatment or control group . . .’ |
HRQoL: LOW Control group did dummy breathing exercises |
HIGH 24% lost to follow-up and discontinuation Unbalanced loss, greater in control group |
UNCLEAR No protocol available |
HIGH Baseline characteristics: more female in control (93 vs. 85%) and general health subscale (intervention vs. control 42.5% vs. 49.5%) |
| Nguyen 2008128 | LOW An investigator who was not involved in the day-to-day study operations generated the randomised sequence using random sequence generator feature [SPSS version 14 (SPSS Inc., Chicago, IL, USA)] Stratified by two clinical sites in blocks of six |
UNCLEAR ‘separated sealed opaque envelopes’ |
‘Study staff not involved in the intervention’ HRQoL: UNCLEAR Exacerbations – LOW |
HIGH n = 11 (36%) dropped out – similar in characteristics to those who remained, except more likely to be female, current smokers, reported no musculoskeletal problems, rated themselves as having advanced computer skills and less likely to have participated in face-to-face support groups or PR |
LOW NCT00102401 |
HIGH Not clear whether some baseline measures may have been completed once nurse (not patient) knew their allocation Study stopped only because of technical problems |
| Nguyen 2009129 | UNCLEAR Stated randomised but no methods detailed |
UNCLEAR | HRQoL: UNCLEAR Self-completed questionnaires, comparison of interventions, but one intervention arm had more contact with the research teams |
LOW ITT analysis Low dropout |
LOW Trial registration: ClinicalTrials.gov (NCT00373932) |
HIGH Data reported for all participants but significant differences in characteristics between groups (age and disease severity) n = 17 |
| Nield 2007130 | UNCLEAR Stated as randomised but not described |
UNCLEAR | HRQoL: UNCLEAR Administration of questionnaire is not reported; however, a comparison on two interventions against a UC/minimum intervention control |
HIGH Dropouts and reasons not reported by group ‘Loss of subjects did not impair group equivalency at either week 4 or week 12’ but no details |
UNCLEAR Not protocol |
HIGH Baseline mismatched – one intervention group had higher-than-average BMI Smoking not reported except ‘Most subjects were white men, with an average age of 65 years, with a FEV1% pred = 39; they were former smokers Were all former smokers? Also mismatch in baseline HRQoL between PLB intervention group and control (21 vs. 29) |
| Ninot 2011250 | LOW Computer-generated sequence, block randomisation |
UNCLEAR Remote allocation by fax machine |
HRQoL: HIGH Neither participants or researchers were blinded Hospital admissions: LOW From records Exacerbations: HIGH From patient interview |
HIGH No ITT analysis 16% dropout balanced in number between groups and reasons given differ between groups |
UNCLEAR No protocol |
UNCLEAR Demographics of dropouts similar to those who completed study Baseline imbalances in 6-MWD and mean age |
| Normandin 2002131 | UNCLEAR | UNCLEAR | HRQoL: HIGH Reported as an unblinded study |
HIGH 7/27 proportion lost from both groups. Reason similar between groups, except more withdrew consent in the high-intensity arm (dropout due to exacerbations, equal between groups) |
UNCLEAR HRQoL in methods and results |
HIGH Baseline data for only the completers Data available, balanced for gender and age Some potential imbalance in some biological/physical measures but not consistent in favour of one group or another |
| Norweg 2005132 | LOW Randomised using biased coin design plus probability table |
UNCLEAR No mention of allocation concealment Unclear whether baseline measures taken prior to randomisation |
HRQoL: HIGH Administered by first author who delivered intervention |
UNCLEAR Withdrawals accounted for but higher withdrawal/dropout in the exercise training-plus-activity training group at 6/52 Also, a priori, not including those who did not follow through with treatment recommendation |
LOW Outcomes stated a priori and reported; however, stated that results would be assessed according to age and depression; no results by depression are presented but probably insufficient power to do this; did adjust for depression |
HIGH No other adjustment needed Exclusion of non-compliant patients from analysis |
| Oh 2003284 | UNCLEAR ‘Patients were randomly assigned to the experimental group in order of referral . . .’ |
UNCLEAR No mention |
UNCLEAR It is not clear, but likely, that measures are not done by same person as whom delivering intervention |
LOW 11 withdrawals – accounted for High dropout – 11/34 but greater in control |
LOW All outcomes assessed |
LOW Also no difference at baseline between dropouts and those who continued |
| O’Neill 2007150 | LOW Patients were randomised in sets of 12; in each set, half were randomised to group 1 and half to group 2; random numbers were generated by an independent researcher |
LOW ‘Stored in sealed envelopes . . . opened after recruitment and assessment, before which none of the coordinator, research team and patient was aware of group allocation |
HRQoL: HIGH | HIGH | LOW | HIGH Baseline characteristics were in only the completers of the PR programmes (n = 34 and 32) |
| Ortega 2002176 | UNCLEAR | UNCLEAR | HRQoL: HIGH | HIGH Only mentioned with relation to dropouts 7/54 randomised patients dropped out and were not included in demographics or study data Numbers given by group: 1/18, 2/18, 4/18, reasons given |
UNCLEAR HRQoL mentioned in methods and results |
UNCLEAR Small numbers but some small imbalances in patient characteristics HRQoL appears somewhat imbalanced at baseline across domains but appears to be within chance variability |
| O’Shea 2007167 | LOW ‘Group allocation was generated by a member of the research team not involved in participant recruitment’ ‘Block randomisation’ |
UNCLEAR ‘Created in envelopes until after completion of baseline measurement’ |
‘All measurement sessions were conducted by an independent trained assessor, blinded to group allocation’ Exacerbations: LOW |
LOW Follow-up at 24 weeks: control 22/27, intervention 19/27; reasons given for withdrawal Those who withdrew more likely to have reduced 6-MWT distance and to have never completed PR HRQoL: LOW |
UNCLEAR | LOW |
| Ozdemir 2010239 | LOW ‘Randomised . . . according to tables of random numbers’ |
UNCLEAR No details given |
HRQoL: HIGH | LOW No loss to follow-up – last follow-up at 1 month |
UNCLEAR | LOW |
| Paz-Diaz 2007269 | UNCLEAR No detail |
UNCLEAR No detail |
No detail | HRQoL: LOW | LOW | UNCLEAR |
| Petersen 2008225 | UNCLEAR Reported as randomised but no details |
UNCLEAR Allocation concealment not considered |
HRQoL: HIGH Self-completed but intervention vs. UC |
LOW ITT analysis with 100% follow-up of all 19 participants |
UNCLEAR No protocol |
HIGH n = 19 for intervention vs. control study ‘There were no difference in any of the baseline characteristics between the “COPD training” and “COPD control” groups’, but no detail given |
| Petty 2006133 | UNCLEAR ‘Block randomisation’ |
UNCLEAR | HIGH Self-administered, ‘no blinding’ |
HIGH ‘82% retention rate’ No other information provided Disproportionate dropout in one group |
UNCLEAR ‘9-month follow-up data not provided because of poor response’ |
LOW |
| Pomidori 2012243 | UNCLEAR ‘Random assignment’ to one of two exercise regimes; no other details |
UNCLEAR No details given |
HRQoL: LOW Comparison of two exercise regimes |
36/47 (77%) follow-up at 1 year; A1 5/23 and A2 6/24 Reasons not given for dropouts, nor characteristics of dropouts |
UNCLEAR | HIGH Baseline characteristics presented for only those followed up at 1 year No data provided on SGRQ – only that from baseline to 12 months: ‘values significantly improved’ |
| Prince 1989151 | UNCLEAR Stated as randomised but method not described |
UNCLEAR NR |
HRQoL: LOW | HIGH Dropouts 2 vs. 4 on control vs. intervention No ITT analysis; no comparison of characteristics |
UNCLEAR No protocol |
HIGH Reported baseline characteristics for completers only. 6-MWD 371 vs. 412 |
| Probst 2011234 | UNCLEAR Reported randomised but no methods given |
UNCLEAR | HRQoL: LOW Comparison of interventions |
HIGH No ITT analysis 37% dropout, balanced in numbers between groups, reasons not broken down by intervention Comparison of baseline characteristics of participants who dropped out vs. trial completers |
UNCLEAR No protocol |
HIGH Baseline data reported for only the participants completing trial Good range of variables reported and groups well matched |
| Puente-Maestu 2000177 | UNCLEAR ‘Blocks of four patients established before the first patient was included |
LOW ‘The physicians who sent the patients for rehabilitation were unaware of the randomisation sequence’ |
HRQoL: UNCLEAR Not mentioned ‘HRQoL – unclear . . . measured . . . by . . . nurse’ Study almost certainly unblinded for patients and most staff |
HIGH 8/49: five patients withdrew from one group and three from another Reasons were non-medical (scheduling/no adherence/personal affairs); no mention of how these compared with completers or if reasons balanced across groups |
UNCLEAR HRQoL in results and methods |
HIGH Baseline data given for only the completers does not include gender Mostly respiratory markers Seem fairly balanced including HRQoL |
| Puente-Maestu 2003275 | UNCLEAR Patients randomly assigned but no description |
UNCLEAR No mention of allocation concealment |
No mention of blinding; outcome unlikely to be influenced on lack of blinding | LOW 10 patients dropped out, five from each group; all accounted for |
LOW All outcomes reported |
LOW |
| Puhan 2006257 | LOW ‘Online central randomisation’ |
LOW ‘Central randomisation using a computerised minimisation procedure’ |
Not reported for participants. Self-report questionnaires. Health-care staff supervising ex and those doing post intervention testing were blinded | LOW ITT performed and attrition/withdrawals reported |
LOW Protocol available but not checked All specified outcomes evident in results |
UNCLEAR Baseline differences for antibiotic use, 6-MWT |
| Rea 2004230 | LOW Practices randomised – computer-generated random number table |
UNCLEAR | HRQoL: HIGH Self-reported intervention vs. UC control Hospital admission: LOW Data collected from records |
Balanced per cent dropout (14 vs. 12%) Similar reasons for dropout between groups ITT analysis of hospital admissions LOW Not ITT analysis – for HRQoL HIGH |
UNCLEAR No protocol |
HIGH Baseline characteristics only reported for completers Intervention vs. control groups very unbalanced numbers (83 vs. 52) Randomisation based on GP practices but no data present on practice demographics/location |
| Regiane Resqueti 2007178 | UNCLEAR No details |
UNCLEAR ‘Sealed opaque envelopes’ |
HRQoL: UNCLEAR | LOW 29/38 follow-up at 6 months Intervention 14/19, control 15/19 Not reported by outcome |
UNCLEAR | LOW |
| Ren 2011201 | Randomly | NR | Questionnaires used to know the frequency of acute exacerbations | HIGH Four losses to follow-up, no details given |
LOW Exacerbations, lung function, 6-MWD, Modified Medical Research Council Scale (MMRC) |
|
| Rice 2010140 | UNCLEAR Stated as randomised but randomisation method not reported |
UNCLEAR | HRQoL: HIGH Patient completed self-reported questionnaires Admissions: LOW Records obtained |
Admissions: LOW No dropouts – 98% of records obtained HRQoL: HIGH 55% and 60% returned questionnaire in control and treatment groups, respectively |
UNCLEAR No protocol |
LOW Well matched baseline – reported for all patients |
| Riera 2001179 | UNCLEAR Stated randomised But no details |
UNCLEAR | HRQoL: LOW Questionnaires administered by blinded assessor Sham intervention as control |
LOW No dropouts 100% follow up |
UNCLEAR NR |
HIGH N = 20 Baseline; only very limited number of characteristics reported |
| Ringbaek 2000226 | UNCLEAR | UNCLEAR | HRQoL: HIGH | HIGH Dropouts all occurred in the Action arm, 7/24 (3× exacerbations, 2× myalgia, 1× lack of time, 1× no reason) ‘These patients did not differ from patients who completed the rehabilitation’ with regard to baseline characteristics |
UNCLEAR HRQoL in methods and results |
HIGH Between groups males/females unbalanced, plus ‘smoking’ unbalanced, plus ‘6-minute walk’ unbalanced; 3/15?, unbalanced, more than expected by chance Demographics given for all randomised patients |
| Romagnoli 2006244 | UNCLEAR ‘Group allocation decided according to a predetermined list’ |
UNCLEAR Allocation ‘blinded to the scientists specifically involved in the study’ |
HRQoL: HIGH ‘Self-administered SGRQ’ Hospital admissions: LOW Taken from ‘the hospitals and from the general practitioners’ registry’ |
LOW Follow-up: 14/17, group 1; 15/18 group 2 Reasons given for loss to follow-up and exclusions |
LOW | HIGH Baseline data provided only for the study completers |
| Rooyackers 2003187 | UNCLEAR Randomly allocated – no mention of how |
UNCLEAR No mention |
UNCLEAR No mention |
LOW Inpatient programme – all patients completed |
LOW All outcomes reported |
LOW Baseline balanced |
| Sassi-Dambron 1995134 | UNCLEAR ‘Randomised’ – no further details given |
UNCLEAR | HRQoL: HIGH | HIGH Dropout before treatment: intervention 1/47, control 8/51 Reasons for dropouts given by study arm – more controls lost to ‘time pressures and lack of interest!’ |
UNCLEAR | HIGH Significant difference at baseline for VAS 6-MWD between study groups |
| Scherer 2000258 | LOW Computer-generated random number table |
UNCLEAR Randomisation after consent |
HRQoL: LOW comparison of interventions | HIGH No ITT analysis No data comparing baseline characteristics of completers and dropouts |
UNCLEAR No protocol |
HIGH Baselines only presented for trial completers Imbalanced baseline – age Smoking status – NR |
| Sewell 2005152 | UNCLEAR | LOW ‘Sequentially numbered, sealed envelopes’ ‘Lead investigator blinded to subject randomisation’ |
HIGH ‘Patients not blinded’ ‘Lead investigator blinded’ ‘Self-reported CRQ’ |
UNCLEAR Withdrawals briefly reported, but no ITT |
LOW All outcomes reported |
LOW |
| Sewell 2006153 | UNCLEAR ‘Randomised’, no details |
UNCLEAR ‘Sealed envelopes’ |
LOW ‘Blinded assessor’ included ISWT and self-report questionnaires |
UNCLEAR Withdrawals reported, but no detail on management of data; similar across both groups |
LOW All outcomes reported |
LOW Underpowered at 6-month time point |
| Seymour 2010154 | UNCLEAR ‘Patients were randomised to receive either UC or peak expiratory flow rate initiated within a week of hospital discharge’ No details on how randomisation achieved |
UNCLEAR | HRQoL: HIGH Self-completed by patients but UC vs. intervention Admissions: LOW Patient diary and medical note review ‘Due to personnel required for these assessments and inevitable patient interaction, it was not possible to fully blind assessors to participant allocation’ |
HIGH ITT analysis 19% dropout rate Similar dropout between branches – only detail is ‘failed to attend’ Comparison of baseline data of dropouts – NR |
LOW Protocol available: Clinical Trials Registration Number NCT00557115 |
LOW No significant baseline differences and reported for all participants |
| Shao 2003202 | Random drawing | NR | HIGH Self-report QoL questionnaires |
HIGH Two losses to follow-up in each group; no reason/details given |
||
| Simpson 1992135 | UNCLEAR Randomisation reported but no detail ‘Stratification and random assignment yielded two groups of 17 subjects’ |
UNCLEAR NR |
HRQoL: HIGH Intervention vs. control UC ‘When the questionnaire was administered again at the end of the study subjects were informed of their previous answers . . .’ |
HIGH 3/17 dropout rate No ITT analysis |
UNCLEAR No protocol |
HIGH n = 17 Baseline reported for all participants Smoking status – NR |
| Singh 2003241 | UNCLEAR Patients divided randomly no descriptions |
UNCLEAR No mention |
UNCLEAR No mention |
LOW All patients completed |
LOW All outcomes reported |
LOW Groups balanced at baseline |
| Sívori 1998263 | LOW Randomised using random number tables |
NR | Not reported for any outcomes Patient notes were used to determine days of hospitalisation (days/patients/year calculation) |
28 patients completed Details of attrition provided. No details to suggest ITT performed |
LOW | Intervention differed with respect to training, although upper and lower limb training group had double the intervention with respect to time from what it appears to suggest from the papers; group similar at baseline; high attrition |
| Smith 1999168 | LOW Random computer-generated numbers |
UNCLEAR | HRQoL: HIGH Unblinded study, self-completed questionnaires, intervention vs. control Hospital admissions: LOW Case note review |
LOW ITT analysis Similar numbers of dropouts and deaths) 10% overall dropout Reported similar characteristics between dropouts/deaths and trial completers (not presented) |
UNCLEAR No protocol |
LOW Baseline reported for all patients – well balanced |
| Soler 2006180 | UNCLEAR | UNCLEAR | HRQoL: HIGH Self-report questionnaires Hospital admissions: LOW |
UNCLEAR Withdrawals reported but no detail on management of data |
LOW All outcomes reported |
UNCLEAR Baseline differences for ISWT, endurance shuttle walk test. Planned intervention of rehabilitation not done Small sample |
| Solomon 1998136 | LOW Random number table |
UNCLEAR NR |
Admissions: LOW | HIGH Total recruitment/follow-up – NR No ITT analysis |
UNCLEAR No protocol |
HIGH Baseline reported for only participants who completed the trial Gender imbalance 43% vs. 55% male in intervention vs. control) |
| Spencer 2010169 | LOW ‘Randomisation (performed using computerised number generation)’ |
UNCLEAR ‘Randomisation (performed using computerised number generation) was concealed in opaque envelopes and prepared by an investigator not directly involved in the study’ Numbering and sealing of envelopes not reported |
HRQoL: HIGH Self-completed questionnaire – intervention vs. control trial Hospital admission: LOW Exacerbations: HIGH Self-reported and defined as worsening symptoms ‘The assessor and subjects were not blind to group allocation’ |
ITT analysis used 18% lost to follow up Reasons listed Identical numbers dropped out due to death/illness Approximately 2× as many dropouts from intervention arm Reported characteristics of those who withdrew appear to have a lower FEV1 and be more likely to be smokers |
LOW Protocol available at Australia and New Zealand Clinical Trials registry |
LOW Baseline characteristics similar – presented for all participants |
| Spruit 2002247 | UNCLEAR | UNCLEAR Limited reporting ‘concealed envelopes’ |
HRQoL: HIGH | UNCLEAR 18/48 dropped out of the study: three in each group dropped out owing to lack of motivation (6/48), and seven in resistance training group, plus five in endurance training group (12/48) owing to hospitalisation/exacerbation (two and one of these died, respectively) Comparison in some baseline characteristics between dropouts and completers is given; no details given by outcome |
UNCLEAR HRQoL mentioned in methods and results |
HIGH Baseline data given appears balanced (only 1/8 factor statistically significant difference (diffusion capacity for CO2) but baseline data given for only the completers |
| Sridhar 2008155 | LOW ‘By the use of random numbers’ |
UNCLEAR | HRQoL: high Readmissions: LOW ‘Corroboration of self-report with hospital admissions was undertaken by respiratory research nurses against local hospital records’ |
LOW Follow-up: 55/61 Intervention: 49/61 control. All loss to follow-up due to death HRQoL: only data at 2 years follow-up reported |
UNCLEAR | HIGH Imbalance in SM activities at baseline; more control participants had reserve antibiotics or steroids |
| Stulbarg 2002137 | UNCLEAR Stratified randomisation using four strata related to oxygen saturation and anaerobic threshold |
UNCLEAR | HRQoL: HIGH Self-reported via SF-36 and CRQ, with feedback given about previous scoring by the patient |
UNCLEAR 103/115 completed the study; four dropouts from each of three groups (n = 40/37/38); reasons are given but not numbers for each reason, nor by group; those who dropped out ‘were not significantly different in baseline characteristics from those who remained in the study except for high PaCO2 and lower age’ |
UNCLEAR HRQoL tools mentioned in methods, also given in results |
HIGH Baseline characteristics given in detail for only the completers. Fairly well balanced – some small differences between the three groups but not extensive |
| Davis 2006395 | UNCLEAR ‘Randomised’ no details |
UNCLEAR | HRQoL: HIGH | UNCLEAR Overall dropout reported, but no reasons given or information on management of data |
LOW All outcomes reported |
UNCLEAR High dropout rate |
| Carrieri-Kohlman 2005394 | UNCLEAR ‘Methods and 2-month outcomes reported elsewhere’ |
UNCLEAR | HIGH ‘Blinded assessor’ |
UNCLEAR Withdrawals reported, but no ITT |
LOW All outcomes reported |
LOW |
| Subin 2010242 | UNCLEAR ‘Randomly assigned to one of three groups through block randomisation’ |
UNCLEAR No detail given |
HRQoL: LOW Three active interventions compared |
No loss to follow-up | UNCLEAR | LOW |
| Theander 2009220 | LOW The randomisation procedures were performed by an independent person from the research group, who took a random envelope from the prepared box with sealed envelopes |
UNCLEAR Opaque envelopes but not sequentially numbered |
HRQoL: HIGH Self-completed, intervention vs. UC control |
HIGH ITT analysis: NR Reasonable completion rate but three dropouts due to ill health and death in intervention group; only one dropout from control group (burden of assessment) |
UNCLEAR No protocol |
HIGH n = 26 Recruitment was terminated at 30 patients – power calculation suggested that 40 patients more appropriate – reasons for stopping recruitment not detailed Baseline reported for only the completers Gender and employment status imbalance between groups |
| Toshima 1990138 | LOW ‘Computer-generated list of the words “rehabilitation” and “education” in random order’ |
LOW ‘The recruiter reported the name to an independent person who made assignments according to the random order lists’ |
HRQoL: LOW (both groups had an intervention) | LOW Balanced losses between arms 89% follow-up at 6 months Reasons for loss to follow-up given by arm |
HIGH ‘In this article, some preliminary results are presented’ |
HIGH 10 participants dropped out between recruitment and starting the intervention. No baseline data are provided for these participants, nor allocation group |
| Ries 1995276 | LOW ‘The randomisation scheme was fixed before the trial with a block size of 8; assignment was determined by a table of random numbers . . .’ |
LOW Clinical personnel were unaware of the randomisation scheme |
HRQoL: HIGH Hospital and ED visits: HIGH Self-reported |
Nine people (six intervention; three control) dropped out prior to treatment ‘Patients who dropped out and those who remained in the study did not differ’ |
UNCLEAR | HIGH Baseline criteria for only the 119/128 randomised |
| Trappenburg 2011188 | LOW ‘To conceal the assignment sequence, a central web-based service was used. Randomisation was carried out using the minimisation technique to balance the control and intervention groups for centre and gender’ |
LOW | HRQoL: HIGH Patient-completed questionnaire Exacerbations: HIGH Self-reported, but well defined and adjudicated by three assessors Length of exacerbation time: HIGH Self-reported Health-care utilisation: LOW Telephone call verified by record |
UNCLEAR No ITT analysis Reasonably balanced reasons for loss to follow-up with 16% and 19% from control and intervention group respectively, but more comorbidity in control group (5 vs. 2) Subjects lost to follow-up had more severe airflow limitation and were more frequently recruited from an outpatient clinic (p < 0.05) |
UNCLEAR No protocol |
UNCLEAR Baseline data for all patients given Baseline: control group had a lower percentage educated to college standard (12% vs. 7%) and more with secondary education (62% vs. 68%) – potentially relevant as intervention was action plan and recording was diaries and self-reporting No details on timing of recruitment/follow-up period. i.e. more exacerbations/more severe exacerbations/longer recovery over winter months |
| Troosters 2000248 | UNCLEAR Prepared before study |
UNCLEAR Sealed envelopes |
HRQoL: HIGH | UNCLEAR 13/50 intervention 17/50 control dropped out; these appeared fairly balanced; the patients who dropped out were not dissimilar to those who were followed up (except age p < 0.004) |
UNCLEAR HRQoL mentioned in methods and results |
HIGH Baseline data given for only the completers (see above also) Groups seem balanced |
| Van Gestel 2012208 | UNCLEAR ‘RCT’ – no further details |
UNCLEAR No details given |
HRQoL: HIGH | 2/22 withdrew from intervention group; 1/21 from control group owing to exacerbation | UNCLEAR | HIGH Baseline data for only the 40 participants with follow-up data |
| Van Wetering 2010273 | LOW ‘Patients were randomised to the INTERCOM programme or to UC using a computerised procedure with concealed patient allocation’ |
UNCLEAR ‘Patients were randomised to the INTERCOM programme or to UC using a computerised procedure with concealed patient allocation’ No comment on method of concealed allocation |
HRQoL: HIGH Self-completed and comparison of intervention vs. control Exacerbations: UNCLEAR Patient defined reason for attendance at GP/ED Hospital admissions: UNCLEAR Linked paper182 reports hospital record review during economic analysis of a subset of patients – this paper on whole cohort does not |
LOW ITT analysis At 4 months, 94% completion At 12 months, 90% completion At 2 years, 85% completion Low risk of bias at 4 and 12 months Reported differences in dropouts – age in both groups (older vs. younger) |
LOW Protocol available NCT00840892 |
HIGH Baseline characteristics similar and reported on whole cohort, but 9% more current smokers in intervention group |
| Vogiatzis 2002265 | UNCLEAR NR Described as stratified randomisation; no specific details of strata mentioned |
UNCLEAR | HRQoL: HIGH | UNCLEAR HRQoL: no specific mention other than for the whole study; for the whole study, 9/45 patients did not complete the programme – four in one group, five in the other Reasons were not given by group but overall; the reasons were pulmonary infection and non-compliance ‘Characteristics were not significantly different from those of the completing patients’ |
UNCLEAR HRQoL mentioned in methods and results |
HIGH Details given for only the completers (36/45) Baseline data seem balanced Baseline HRQoL data not shown, only change |
| Vonbank 2012246 | UNCLEAR ‘Randomly assigned’ |
UNCLEAR No details given |
HRQoL: LOW All participants received active intervention |
HIGH 7/43 lost to follow-up; no details provided on dropouts |
UNCLEAR | HIGH Data provided at baseline for only the participants who completed the trial |
| Wadell 2004221 | UNCLEAR Semi-randomised Stratified by sex, FEV1 and work capacity |
UNCLEAR No mention of allocation concealment |
UNCLEAR Not mentioned |
LOW Two dropouts – discussed and from different groups |
LOW All outcomes reported |
LOW |
| Wakabayashi 2011262 | LOW ‘A case manager independent of the study randomly assigned patients to either group I or group U using a computer-generated list’ |
LOW ‘Patient allocations were sealed in numbered envelopes by an independent evaluator, not involved in the interventions . . .’ |
Hospital admissions: LOW | HIGH Does not report, as ITT analysis not done for the reported outcome of emergency attendance and admission |
UNCLEAR No mention in methods that ER attendance and admissions would be reported – only secondary outcome measures were mentioned; however, all outcome measures mentioned in methods were reported No protocol available |
HIGH Under-recruited (100 needed, 102 recruited but 17 dropped out) Baseline difference in emergency visits and hospital admissions |
| Wang 2004203 | Randomly | NR | UNCLEAR | LOW Lung function, QoL, 12-MWD reported |
||
| Warlies 2006209 | External randomisation Method not completely clear: patients were allocated a code number, chosen by randomly opening shuffled, securely closed envelopes |
Allocation number in securely closed envelopes | HRQoL: HIGH Study described as open label Patients were not given assistance with completing the SGRQ to avoid results being biased |
4/28 in intervention group and 4/32 in control group discontinued Six found participation too onerous/were not motivated, including all four in the control group; one in the intervention group withdrew without giving a reason and one developed lung cancer; including these eight, there was a total of 12 patients with missing data Variable number of patients included for different outcomes depending on completeness of assessments |
No obvious selective reporting All outcomes mentioned in methods also reported in results section |
No further comments |
| Waterhouse 2010277 | LOW ‘Random allocation sequence using the RALLOC procedure in Stata 8 . . .’ |
UNCLEAR | ‘All were blinded to the telephone intervention arm until 1 month post rehabilitation, when only the assessment team and research participants were unblinded’ HRQoL: HIGH Interviewer-led completion – unblinded participants and assessors |
HIGH No ITT analysis Only 50% and 57% follow-up for community and hospital rehabilitation, respectively, for acute outcome and 57% long-term outcomes Reported comparison of dropouts vs. completers – well matched on reported features |
LOW Trial registration: Current Controlled Trials ISRCTN86821773 |
HIGH Does not show baseline for all recruited participants, only for those who started the trial Smoking not reported as a variable |
| Watson 1997231 | UNCLEAR ‘With random assignment of subjects to either UC or enhanced care’ |
UNCLEAR No details given |
HRQoL: HIGH No details about blinding of assignments |
13/69 lost to follow-up, details not given by study group | UNCLEAR | HIGH Some large baseline imbalances, for example influenza immunisation, access to a nebuliser and marital status |
| Wedzicha 1998157 | UNCLEAR Stratified randomisation based on MRC dyspnoea score; blocks of eight No details on generation of sequence |
UNCLEAR ‘Codes held in sealed envelopes’ |
HRQoL: HIGH | UNCLEAR Eight dropouts out of 63 in exercise group; eight dropouts out of 63 in control group; reasons given More withdrawal as reason in exercise group (7 vs. 3) and more details in the control group (0 vs. 2) |
UNCLEAR SGRQ, CRQ, Extended Activities of Daily Living assessment (EADL) mentioned in methods and results given; HADS mentioned in methods and baseline data but following data appear not to be presented |
UNCLEAR Baseline data given for only the followed up patients; groups are fairly well balanced at baseline for demographics; no details of how dropouts differed from completers Possible baseline difference for shuttle walk test |
| Weekes 2009158 | UNCLEAR ‘Randomisation occurred in a standard way using sealed opaque envelopes containing randomised codes’ Not clear how the randomised codes were generated |
UNCLEAR ‘Using sealed opaque envelopes’ Not sequentially numbered |
HRQoL: HIGH Not clear if self-reported or with unblinded outcome assessor; controls given leaflet – is this different from UC? ‘A randomised controlled unblinded trial was performed’ |
LOW ‘All patients who completed at least two assessments (baseline and one other) were included in an ITT analysis to provide an unbiased assessment of the treatment effect’ Dropout reasons available online Dropout rates broadly similar between intervention and control groups 6-month 33% dropout 12-month 37% dropout and baseline characteristics of dropouts compared |
UNCLEAR No protocol |
LOW Baseline characteristics included for all randomised patients who reached baseline assessment Difference of 8% and 10% in ex/current smokers between intervention and control groups – fewer smokers in intervention group High death rate/comorbidity diagnosis rate, but similar between groups Deaths 7/59 but this probably reflects this patient group and rates that are broadly similar between groups |
| White 2002159 | UNCLEAR No description of sequence generation but groups unequal, suggesting computerised |
UNCLEAR Opaque sealed envelopes |
LOW | LOW Nine dropouts in intervention arm, six in brief advice; all accounted for |
LOW All outcomes reported |
LOW Groups balanced at baseline |
| Wijkstra 1994189 | UNCLEAR After stratification by FEV1% pred, their limiting factor in exercise capacity and maximal workload, the patients were randomly allocated |
UNCLEAR No details given |
HRQoL: HIGH | LOW Dropouts: intervention 2/30; control 0/15 Reasons for dropouts provided |
UNCLEAR | HIGH Baseline imbalance in inspiratory vital capacity |
| Wijkstra 1995190 | UNCLEAR Stratified by FEV1, maximum workload and limiting factor to exercise Randomly allocated to one of three groups |
UNCLEAR No details given |
HRQoL: HIGH | LOW Clear details given by study arm for reasons loss to follow-up
|
LOW | |
| Wittmann 2007210 | UNCLEAR External randomisation: no further details |
UNCLEAR No detail: the fact that randomisation occurred externally may contribute to concealment of allocation |
LOW | 13/107 dropouts in intervention group and 15/105 dropouts in control group during rehabilitation; reasons for both given Follow-up 1 year later was 98% (180/184) No details on differences between patients dropping out or remaining in study |
UNCLEAR No obvious selective reporting All outcomes mentioned in methods also reported in results section |
91/303 eligible patients refused to participate; 32 of these specifically requested, and received, the intervention (not as part of the study); the authors state that this means that the most motivated patients were excluded |
| Wong 200574 | LOW Computer-generated randomisation (p. 2123) |
UNCLEAR | LOW Outcomes measured by nurse blind to group assignment |
LOW Two dropouts but analysed with imputed values |
LOW All outcomes reported |
LOW |
| Wood-Baker 2006170 | LOW ‘Cluster randomisation’ ’Computer-generated randomisation package’ |
UNCLEAR | HIGH Self-administered, ‘no blinding’ |
UNCLEAR Withdrawals reported, but no detail on management of data |
LOW All outcomes reported |
HIGH Imbalance at baseline for gender, smokers, activity levels ‘Underpowered’ |
| Wright 2003211 | UNCLEAR Reported randomised but no details given |
UNCLEAR NR |
HRQoL: HIGH Intervention vs. UC control |
HIGH Large imbalance in dropouts between control and intervention groups – total recruitment not reported but completers n = 21 in intervention group and n = 5 in control |
UNCLEAR No protocol |
HIGH Not clear if baseline reported for all participants and small number of variables reported (weight not BMI) No smoking status Very heterogeneous age and symptomology within treatment group |
| Xu 2010204 | Random number | NR | UNCLEAR Use of QoL questionnaire |
6-MWD, Borg score | ||
| Yamaguti 2012235 | UNCLEAR Randomisation was stratified according to gender, using random block sizes of 2 and 4 |
UNCLEAR No information given |
HRQoL: HIGH | HIGH Follow-up: intervention 15/15, control 12/15 Information given on reasons for loss to follow-up ITT analysis done using baseline observation carried forward for missing data |
UNCLEAR Outcomes in methods all reported in results |
LOW |
| Yeh 2010139 | UNCLEAR Stated randomised but no detail |
UNCLEAR NR |
HRQoL: HIGH | LOW ITT analysis |
UNCLEAR No protocol |
HIGH Very small trial n = 10 Feasibility study Baseline reported for all participants Smoking status – NR |
| Zhang 2008205 | UNCLEAR Randomly |
UNCLEAR | HRQoL: HIGH Self-administered questionnaire |
HIGH Three losses to follow-up in group A; no reasons given |
LOW |
Appendix 27 Trials included in each analysis
| Study | Multicomponent SM interventions vs. control/UC | Single component vs. UC or multicomponent + 1 vs. multicomponent | Exercise-only interventions vs. control/UC/sham training | Enhanced care (with/without SM package) vs. UC/SM package | Multicomponent SM including supervised exercise vs. control/UC | Multicomponent SM including non-supervised exercise vs. control/UC | Multicomponent SM without exercise or exercise counselling vs. control/UC | Multicomponent SM with exercise education only vs. control/UC | Combined strength plus aerobic plus other SM vs. UC | Strength plus aerobic exercise training vs. aerobic ex only | Endurance vs. strength/resistance exercise training | Addition of upper limb training to lower limb training | Comparisons of interval and continuous exercise | Inspiratory and expiratory muscle training vs. control/sham training/UC | Exercise of a specific intensity/duration vs. exercise of different intensity/duration | Intervention in one setting vs. intervention in a different setting | Pharmacist led | Maintenance post PR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aimonino Ricauda 200875 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arnardottir 2006216 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arnardottir 2007217 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Barakat 2008249 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bauldoff 2002108 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bauldoff 2005109 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bauldoff 2005109 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bauldoff 2005109 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Beckerman 2005253 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Behnke 200064 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Bendstrup 1997222 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bernard 1999191 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Berry 2010110 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bestall 2003141 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bjornshave 2005223 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blake Jr 1990111 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bonilha 2009232 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bourbeau 2003192 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Boxall 2005160 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Breyer 2010245 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brooks 2002193 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bucknall 201263 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Busch 1988194 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cai 2006199 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Carr 2009195 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Casas 200671 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chan 2010212 A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chan 2010212 B | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chan 2010212 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cockcroft 1987142 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coultas 2005112 A | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coultas 2005112 B | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coultas 2005112 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Covey 2001113 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| de Blok 2006181 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dheda 200473 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Donesky-Cuenco 2009114 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dourado 2009233 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dourado 2009233 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dourado 2009233 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| du Moulin 2009206 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Eaton 2009227 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Effing 2009161 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Efraimsson 2008218 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Egan 200269 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elci 2008236 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elliott 2004162 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elliott 2004162 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elliott 2004162 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Emery 1998115 A | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Emery 1998115 B | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Emery 1998115 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Engstrom 1999219 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fernandez 2009171 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finnerty 2001143 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Foy 2001116 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gallefoss 1999325 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ghanem 2010264 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gilmore 2010117 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gilmore 2010117 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gilmore 2010117 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gilmore 2010117 D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gilmore 2010117 E | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gilmore 2010117 F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gohl 2006207 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Goldstein 1994196 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Green 2001144 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Güell 2000172 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Güell 2006173 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Guyatt 1992118 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hermiz 200267 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hernandez 2000282 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hernandez 200368 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hill 2006163 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Holland 2004164 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hoogendoorn 2009182 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hospes 2009183 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hsiao 2003259 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hsiao 2003259 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hsiao 2003259 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hynninen 2010256 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Janaudis-Ferreira 2011197 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Jang 2006267 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Jarab 2012266 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Karapolat 2007237 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Katiyar 2006240 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kayahan 2006238 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Khdour 2009251 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Kim 1993119 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Ko 2011213 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Koff 2009120 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Koppers 2006184 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Kunik 2008121 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kwok 200470 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lamers 2010185 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Larson 1988122 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Larson 1999123 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Larson 1999123 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Larson 1999123 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Larson 1999123 D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Larson 1999123 E | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Larson 1999123 F | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lee 200266 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leung 2010165 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Li 2002200 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liddell 2010145 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Lindsay 2005214 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Linneberg 2012224 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Littlejohns 1991146 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liu 200882 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Livermore 2010166 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lord 2010147 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Madariaga 2007174 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Madariaga 2007174 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Madariaga 2007174 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Mador 2004126 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mador 2005125 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mador 2009124 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Magadle 2007254 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Maltais 2008198 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Man 2004148 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Martin 2004228 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| McGeoch 2006229 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monninkhof 2003186 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moore 2009285 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mota 2007175 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Mularski 2009127 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Murphy 2005252 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nakamura 2008261 A | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nakamura 2008261 B | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nakamura 2008261 C | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Ng 2011215 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nguyen 2008128 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nguyen 2009129 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nield 2007130 A | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nield 2007130 B | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Nield 2007130 C | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ninot 2011250 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Normandin 2002131 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norweg 2005132 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norweg 2005132 B | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norweg 2005132 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oh 2003283 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O’Neill 2007150 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Ortega 2002176 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ortega 2002176 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ortega 2002176 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O’Shea 2007167 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ozdemir 2010239 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Paz-Diaz 2007269 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Petersen 2008225 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Petty 2006133 A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Petty 2006133 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Petty 2006133 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pomidori 2012243 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince 1989151 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Probst 2011234 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Puente-Maestu 2000357 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Puhan 200676 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Rea 2004230 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Regiane Resqueti 2007178 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ren 2011201 A | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ren 2011201 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ren 2011201 C | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rice 2010140 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Riera 2001179 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Ringbaek 2000226 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Romagnoli 2006244 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Rooyackers 2003187 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sassi-Dambron 1995134 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Scherer 2000258 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Sewell 2005152 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sewell 2006153 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Seymour 2010154 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shao 2003202 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Simpson 1992135 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Singh 2003241 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sívori 1998263 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Smith 1999168 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Soler 2006180 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Solomon 1998136 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Spencer 2010169 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Spruit 2002247 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sridhar 2008155 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Stulbarg 2002137 A | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stulbarg 2002137 B | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stulbarg 2002137 C | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subin 2010242 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subin 2010242 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subin 2010242 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Theander 2009220 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Toshima 1990138 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trappenburg 2011188 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Troosters 2000248 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Van Gestel 2012208 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vogiatzis 2002265 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Vonbank 2012246 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vonbank 2012246 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vonbank 2012246 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wadell 2004221 A | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wadell 2004221 B | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wadell 2004221 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wakabayashi 2011262 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wang 2004203 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Warlies 2006209 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Waterhouse 2010277 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Watson 1997231 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wedzicha 1998157 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Weekes 2009158 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| White 2002159 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wijkstra 1994189 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wijkstra 1995190 A | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wijkstra 1995190 B | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wijkstra 1995190 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wittmann 2007210 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wong 200574 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wood-Baker 2006170 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wright 2003211 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xu 2010204 A | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xu 2010204 B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xu 2010204 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xu 2010204 D | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xu 2010204 E | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Xu 2010204 F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yamaguti 2012235 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yeh 2010139 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Zhang 2008205 A | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Zhang 2008205 B | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Zhang 2008205 C | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Appendix 28 Funnel plot of studies for multicomponent self-management interventions vs. usual care: St George’s Respiratory Questionnaire outcomes at 13 weeks’ follow-up – review 4
FIGURE 80.
Egger’s test: coefficient of bias 1.98, p-value 0.001.
Appendix 29 Funnel plot of studies for multicomponent self-management interventions vs. usual care: St George’s Respiratory Questionnaire outcomes at between 3 and 6 months’ follow-up – review 4
FIGURE 81.
Egger’s test: coefficient of bias –1.80, p-value 0.054.

Appendix 30 Funnel plot of studies for multicomponent self-management interventions vs. usual care: St George’s Respiratory Questionnaire outcomes at ≥ 6 months’ follow-up – review 4
FIGURE 82.
Egger’s test: coefficient of bias 0.99, p-value 0.031.
Appendix 31 Funnel plot of studies for multicomponent self-management interventions including supervised exercise: St George’s Respiratory Questionnaire outcomes at ≤ 13 weeks’ follow-up – review 4
FIGURE 83.
Egger’s test: coefficient of bias 3.91, p-value 0.006.

Appendix 32 Funnel plot of studies for enhanced care interventions: hospital admissions at ≥ 6 months’ follow-up – review 4
FIGURE 84.
Egger’s test: coefficient of bias –0.43, p-value 0.749.
Appendix 33 Funnel plot of studies for combined strength and aerobic interventions: St George’s Respiratory Questionnaire outcomes at ≤ 13 weeks’ follow-up – review 4
FIGURE 85.
Egger’s test: coefficient of bias 2.79, p-value 0.061.

List of abbreviations
- A&E
- accident and emergency
- ANCOVA
- analysis of covariance
- BTS
- British Thoracic Society
- CASP
- Critical Appraisal Skills Programme
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CRQ
- Chronic Respiratory (Disease) Questionnaire
- ED
- emergency department
- EMT
- expiratory muscle training
- EQ-5D
- EuroQoL-5 Dimensions
- FEV1
- forced expiratory volume in 1 second
- FEV1% pred
- forced expiratory volume in 1 second percentage predicted
- FVC
- forced vital capacity
- GHQ
- General Health Questionnaire
- GOLD
- Global Initiative for Chronic Obstructive Lung Disease
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IMT
- inspiratory muscle training
- IQR
- interquartile range
- MD
- mean difference
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PR
- pulmonary rehabilitation
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RMT
- respiratory muscle training
- SABA
- short-acting β2-agonist
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SGRQ
- St George’s Respiratory Questionnaire
- SM
- self-management
- UC
- usual care