Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/36/02. The contractual start date was in December 2011. The draft report began editorial review in June 2013 and was accepted for publication in March 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Marie Lordkipanidzé has received speaker honoraria from Eli Lilly, which manufactures the antiplatelet agent prasugrel. David Fitzmaurice has received honoraria from Boehringer Ingelheim, Sanofi-aventis and AstraZeneca, but not in relation to antiplatelet therapy. All other authors declare no competing interests.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Dretzke et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Cardiovascular disease is the leading cause of death in the developed world, with coronary artery disease (CAD) and stroke accounting for one-quarter of all deaths in the UK. 1 Important progress has been made in the management of heart disease over the last century, driving the incidence of disease down in both men and women. Among the many beneficial medical therapies which have been shown to decrease the risk of recurrent vascular events, antiplatelet agents have become the cornerstone of therapy in patients suffering from atherosclerotic vascular disease. It is thus not surprising that over 40,000 tons of aspirin are produced every year worldwide, and 35,000 kg of aspirin are consumed every day in the USA alone (the figure for the UK is 6000 kg per day). 2 In the UK, aspirin was the second most prescribed drug in 2011, with 32.4 million prescriptions dispensed in the community, 95% of which were for cardioprotection. 3
Indications for antiplatelet therapy
The use of antiplatelet agents covers a large spectrum of vascular diseases. 4 In primary prevention, antiplatelet agents can be given to patients at high risk of thrombotic events, such as patients with multiple risk factors for CAD or diabetes. 5 In secondary prevention, antiplatelet agents can be given either acutely in patients with acute coronary syndromes (ACSs), following percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG), or chronically in patients with stable CAD, in patients with a history of transient ischaemic attacks (TIAs) or strokes and patients with peripheral arterial disease (PAD). 5 The benefit of aspirin therapy in each of these pathologies is related to the underlying thrombotic risk, and is usually greatest in high-risk individuals and lowest in individuals with no overt atherosclerotic disease (Figure 1).
FIGURE 1.
The benefit of aspirin in terms of risk prevention in different patient groups. From Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl.):e89–119. 5 Reproduced with permission from the American College of Chest Physicians. MI, myocardial infarction.

Antiplatelet therapy in primary prevention of cardiovascular disease
There is little clinical evidence to support the use of antiplatelets for the primary prevention of cardiovascular events in patients with a cardiovascular disease risk less than 20%. 4 In patient groups carrying the highest cardiovascular disease risk, the benefit (i.e. the expected number of individuals avoiding a serious vascular event by using aspirin) exceeds the risk associated with aspirin treatment (i.e. experiencing a major bleed). 5 The latest meta-analysis by the Antithrombotic Trialists’ Collaboration found that aspirin therapy in primary prevention of cardiovascular events resulted in a 12% proportional reduction in the incidence of serious vascular events [rate ratio (RR) 0.88, 95% confidence interval (CI) 0.82 to 0.94] and an 18% proportional reduction in the incidence of major coronary events (RR 0.82, 95% CI 0.75 to 0.90). 4 On the other hand, aspirin was associated with an increase in major gastrointestinal (GI) and other extracranial bleeds (RR 1.54, 95% CI 1.30 to 1.82). In absolute numbers, however, the decrease in major coronary events from 0.34% to 0.28% per year is only slightly superior to the increase in bleeding events from 0.07% to 0.10% per year. 4 As a consequence, most guidelines advise against daily aspirin therapy in men and women without evidence of manifest vascular disease. However, daily aspirin therapy (75–160 mg) can be considered in apparently healthy individuals in whom the vascular risk is considered high and the bleeding risk low. 5,6
Within the primary prevention populations, patients suffering from diabetes mellitus have specific guidelines when it comes to antiplatelet therapy in prevention of vascular events. 7 This stems from epidemiological studies which have shown that diabetic patients have a two- to three-fold increase in risk of major ischaemic events. Despite the higher risk of cardiovascular disease, the benefit of giving aspirin in patients suffering from diabetes alone is, however, less certain. 8 Recent guidelines reflect this by moving away from a universal recommendation for aspirin in all diabetic patients, and advising daily aspirin therapy only in diabetic patients with concomitant risk factors for CAD where the most benefit can be gained. 7,8 As a consequence, daily administration of aspirin is usually initiated in primary prevention in diabetic patients at increased cardiovascular risk (10-year risk > 10%). This includes most men aged > 50 years or women aged > 60 years who have at least one additional major risk factor (family history of cardiovascular disease, hypertension, smoking, dyslipidaemia or albuminuria). 7
Antiplatelet therapy in secondary prevention of cardiovascular disease
Daily low-dose aspirin therapy (75–325 mg) is strongly recommended for all patients with established cardiovascular disease. In patients with a prior cardiovascular event, evidence that daily aspirin therapy reduces the risk of major adverse cardiovascular events is arguably strong. 4 Although the proportional reduction in risk of any serious vascular event does not differ significantly between primary and secondary prevention trials, the absolute risk reduction is much greater in secondary prevention, thus rendering the benefit-to-risk ratio unquestionably in favour of aspirin therapy. 4 It is therefore not surprising that all US, European and UK guidelines recommend life-long aspirin therapy in all patients with established cardiovascular disease. 5
Although aspirin is recommended in all patients indefinitely, in patients who have suffered an ACS, which may or may not have required revascularisation, additional antiplatelet therapy on top of daily aspirin treatment is recommended. Thus, in patients who have had a ST-elevation myocardial infarction (MI) or non-ST-elevation ACS (including unstable angina), and in patients who have undergone PCI, addition of an adenosine diphosphate (ADP) receptor blocker such as clopidogrel, prasugrel (Efient®, Eli Lilly) or ticagrelor (Brilique®, AstraZeneca) is recommended for up to 1 year. 9 Although the ADP receptor blocker is usually discontinued at the end of the year, thus covering the acute phase of thrombotic disease, aspirin is continued indefinitely, thus maintaining antiplatelet coverage into stable CAD.
Antiplatelet therapy in stroke
Stroke is a leading cause of functional impairments, with 20% of survivors requiring institutional care after 3 months and 15–30% being permanently disabled. 10 Although the role of anticoagulation is well established in stroke prevention, the role of aspirin therapy is less clear in this patient group. 11 As such, most recent guidelines do not recommend the use of aspirin in primary prevention, but warrant the use of aspirin cardiovascular prophylaxis (including but not specific to stroke) in individuals whose risk is sufficiently high for the benefits to outweigh the risks associated with treatment (a 10-year risk of cardiovascular events of 6–10%). 11
In patients suffering from atrial fibrillation, aspirin is recommended either on top of or in replacement of anticoagulation in low-risk and some moderate-risk patients. The decision is based on patient preference, estimated bleeding risk if anticoagulated and access to anticoagulation monitoring. 11 For high-risk patients with atrial fibrillation deemed unsuitable for anticoagulation, dual antiplatelet therapy with aspirin and clopidogrel might be reasonable; the combination offers more protection against stroke than aspirin alone but with increased risk of major bleeding.
Antiplatelet therapy in peripheral arterial disease
Lower-extremity artery disease (LEAD) is a relatively common pathology. The disease is often asymptomatic, with approximately one-third of all LEAD patients in the community presenting with symptoms. A recent study has reported a LEAD prevalence of 18%, with 7% of patients reporting symptoms of intermittent claudication. 12 In the latest meta-analysis by the Antithrombotic Trialists’ Collaboration, the incidence of vascular death, non-fatal MI and non-fatal stroke at follow-up was significantly decreased by 23% by antiplatelet drugs in patients with intermittent claudication. 4 It follows that antiplatelet therapy is recommended in patients with symptomatic PAD, with low-dose aspirin (75–150 mg daily) at least as effective as higher daily doses. 13 Moreover, antiplatelet therapy with aspirin is recommended in all patients with angioplasty for LEAD to reduce the risk of systemic vascular events, and dual antiplatelet therapy with aspirin and clopidogrel is recommended for a minimum of 1 month in cases of peripheral revascularisation, after which time clopidogrel may be discontinued but with aspirin prescribed indefinitely.
Defining aspirin response
The efficacy of aspirin to prevent thrombotic events in cardiovascular patients is well established, with > 100 randomised trials having been conducted in high-risk patients and demonstrating a reduction in vascular death of approximately 15% and a further reduction in non-fatal vascular events of approximately 30%. 4 Few drugs have demonstrated similar efficacy, with up to 50 major vascular events avoided per 1000 patients treated for 1 year, at a cost of one to two patients experiencing a major GI bleeding event. 5 Both the benefit and the risk associated with aspirin are attributed to its ability to prevent thrombus formation via inhibition of platelet function. 14
The best-characterised mechanism of aspirin is acetylation of a key enzyme in platelet function, the cyclo-oxygenase (COX)-1 enzyme. This enzyme transforms arachidonic acid into thromboxane A2 (TxA2), a platelet agonist and vasoconstrictor. 15–17 Aspirin is effective in inhibiting platelet activity at doses as low as 20–40 mg per day,18 and is clinically effective in preventing thrombotic events in daily doses as low as 75 mg with little benefit of higher doses. 5 This is particularly important in view of the fact that though low doses of aspirin appear effective in preventing thrombotic events in patients at risk, the effect on bleeding (especially GI bleeding) has been shown to be aspirin dose dependent. 19 In recent years, it has been shown that even acutely well-managed major bleeding events are associated with worse outcomes in cardiovascular patients, in terms of both major adverse cardiovascular events and mortality. 20,21 It follows that most treatment guidelines advocate the use of the lowest aspirin dose effective in preventing thrombotic complications so as to minimise the risk of major bleeding. 22–25 From this, a need for monitoring of aspirin therapy has emerged and prompted the development and investigation of numerous assays of platelet function.
Platelet function testing in routine clinical practice
Current clinical guidelines do not recommend routine platelet function testing for aspirin in cardiovascular patients. 26–28 Although platelet function testing may be considered in certain contexts, for example ‘in patients at high risk for poor clinical outcomes’27 or if ‘a diagnosis of non-compliance is likely to aid management’,26 the general message from both European and US guidelines, as well as from the Working Group on Aspirin Resistance of the International Society on Thrombosis and Haemostasis, is that monitoring of antiplatelet response by platelet function assays should remain restricted to clinical research, and not be introduced in daily clinical practice.
A number of reasons may explain the lack of enthusiasm for platelet function testing in recently published guidelines. These include the lack of consensus on the platelet function assay to be used; on the definition of inadequate platelet response to aspirin; and on the clinical management of patients with insufficient platelet inhibition by aspirin. 14 Although there are a number of platelet function tests (PFTs) available, it remains to be established how best to use these assays, and whether or not adjusting antiplatelet therapy based on these results will improve clinical outcome.
Platelet function assays
A vast array of platelet function assays is available to test the response of platelets to the inhibitory effect of aspirin (Table 1). Some assays are laboratory based and require extensive expertise to operate, whereas others have been specifically developed to be point of care. Although some assays study global haemostasis, most platelet function assays target a specific phase of platelet function, from platelet adhesion to platelet activation, secretion and aggregation. Important methodological disparities make the assays unique in the way that they assess platelet responses. For example, some of these assays are carried out in whole blood [including whole-blood aggregometry (WBA), platelet counting, platelet function analyser-100 (PFA-100®; Siemens, Malvern, PA, USA), VerifyNow® Aspirin (Accumetrics Inc., San Diego, CA, USA), Impact-R® (DiaMed, Cresier, Switzerland) and flow cytometry], whereas others require sample preparation [such as light transmission aggregometry (LTA), plasma or serum thromboxane B2 (TxB2) measurement], and others can be performed on urine (levels of the TxB2 metabolite 11-dehydro-TxB2). There is no official guideline recommending one assay over another, and platelet function testing is not recommended for routine clinical testing in patients requiring aspirin therapy. As a result, many of the available platelet function assays have been used in a research capacity, and part of the uncertainty surrounding the definition and clinical relevance of aspirin resistance is due to the non-interchangeable nature of these assays.
| Platelet function assay | Principle | Specificity for COX-1 | Advantages | Disadvantages |
|---|---|---|---|---|
| Pharmacological perspective | ||||
| Serum/plasma TxB2 | Assessment of the major TxA2 metabolite in blood, TxB2, following clotting of whole blood (serum) or aggregation of platelet-rich plasma (plasma) | Almost exclusively dependent on platelet COX-1 activity | Requires small volume of blood | Prone to artefact Non-linear relationship with TxA2-dependent platelet aggregation |
| Urinary 11-dehydro-TxB2 | Assessment of the major TxA2 metabolite in urine, 11-dehydro-TxB2 | Largely dependent on platelet COX-1 activity | Non-invasive Global measure of TxA2 formation |
Non-platelet sources of TxA2 will also contribute to this measure Relationship to in vivo platelet activity is unknown |
| Functional perspective | ||||
| LTA | Measurement of light transmission in a platelet-rich plasma sample following stimulation with a platelet agonist | COX-1 specific: AA-induced COX-1 non-specific: collagen-, epinephrine- or ADP-induced |
Historical gold standard | Time and labour intensive Requires large volume of blood Non-physiological milieu for platelets |
| VerifyNow® Aspirin (Accumetrics Inc., San Diego, CA, USA) | Platelet agglutination onto fibrinogen-coated beads in response to agonist stimulation in whole blood | COX-1 specific: aspirin cartridge COX-1 non-specific: P2Y12 cartridge |
Point of care | Expensive Inflexible |
| PFA-100® (Siemens, Malvern, PA, USA) | High-shear platelet plug formation on a membrane coated with platelet agonists in whole blood | COX-1 non-specific: CEPI cartridge CADP cartridge (CEPI more sensitive than CADP for detecting aspirin) |
Point of care Easy to use Includes an element of flowing blood |
Detects a high number of patients as poor aspirin responders Correlates poorly with other platelet function assays Sensitive to other factors including vWF, platelet reactivity, platelet count and haematocrit |
| WBA | Measurement of impedance between electrodes immersed in whole blood stimulated with an agonist | COX-1 specific: AA-induced COX-1 non-specific: collagen- or ADP induced |
Physiological milieu for platelets | Time and labour intensive Sensitive to artefactual activation (especially due to haemolysis) |
| Multiplate® (Roche, Munich, Germany) | Automated WBA | COX-1 specific: AA-induced COX-1 non-specific: collagen- or ADP-induced |
Point-of-care assay | Sensitive to artefactual activation (especially due to haemolysis) |
| Flow cytometry | Fluorescent measurement of platelet activation markers (e.g. P-selectin) and conformational changes in platelet glycoproteins (e.g. PAC-1 for activated GPIIb/IIIa) | COX-1 specific: AA-induced COX-1 non-specific: collagen- or ADP-induced |
Requires small volume of blood Fixation of samples allows for sending to a core laboratory for analysis |
Time and labour intensive Requires specialised equipment and operator Expensive |
| Plateletworks® (Helena Laboratories, Beaumont, TX, USA) | Single-platelet counting in a whole-blood before-and-after stimulation with a platelet agonist | COX-1 specific: AA-induced COX-1 non-specific: collagen- or ADP-induced |
Easy to use Does not require specialised equipment |
Poor correlation with other platelet function assays |
| Impact-R® (DiaMed, Cresier, Switzerland) | Monitoring of platelet adhesion to a polystyrene surface coated with plasma proteins | COX-1 specific: addition of AA COX-1 non-specific: plate coated with fibrinogen and vWF |
Requires small volume of blood Incorporates an element of shear in whole blood |
Poor correlation with other platelet function assays |
| TEG [TEG® (Haemonetics, Braintree, MA) or ROTEM® (Tem International GmbH, Munich, Germany)] | Monitoring of the rate and quality of clot formation | COX-1 specific: platelet mapping technology with AA COX-1 non-specific: coagulation-based assay |
Provides a readout of global haemostasis | Largely platelet insensitive, even with platelet mapping technology |
From a pharmacological perspective, the monitoring of aspirin efficacy requires assessment of the ability of aspirin to inhibit its pharmacological target (platelet COX-1), and thus inhibit the conversion from arachidonic acid to TxA2. 29 This is the accepted measurement of the European Agency for the Evaluation of Medicinal Products to assess the efficacy of aspirin. 30 Assays measuring TxA2 formation in clotting blood or in aggregating platelet-rich plasma thus appear ideal. However, TxA2 cannot be easily measured in biological samples as it has a very short half-life in plasma (30–60 seconds). 31 As a consequence, assays measuring stable metabolites of TxA2, most commonly TxB2 (in serum/plasma) or 11-dehydro-TxB2 (in urine), are the most widely used.
From a functional perspective, a multitude of platelet function assays are available to assess platelet responsiveness to aspirin. 32 Some assays require extensive technical expertise and are limited to specialised laboratories, whereas others are point of care and are meant as bedside tools. The assays that use arachidonic acid as the agonist require a functioning COX-1 to convert it to the active TxA2 molecules which then elicit a platelet response; these are referred to as COX-1-specific (Table 2). TxA2 is a secondary mediator of platelet activation and synergises with other platelet pathways33 to elicit full platelet responses. Therefore, aspirin therapy can also partly inhibit platelet activation induced by other agonists, such as collagen and epinephrine. 34,35 Platelet function assays based on these agonists have been used to quantify the platelet reactivity of platelets in patients taking aspirin, although these do not specifically assess the pharmacological efficacy of aspirin. 36 These are referred to as COX-1-non-specific assays (see Table 2).
| Platelet function assays (aspirin): eligible in any population | COX-1-non-specific and global assays of platelet function: eligible in patients on aspirin alone | Platelet function assays (clopidogrel): not eligible to assess platelet responses to aspirin |
|---|---|---|
COX-1-specific assays: TxA
2
|
COX-1-non-specific assays
|
Activation downstream of the P2Y12-ADP receptor
|
Arguably, COX-1-specific assays may capture more faithfully the effect of aspirin on platelets and may therefore be preferable when looking at the pharmacological efficacy of aspirin. Moreover, COX-1-specific assays are directly targeted by aspirin and are not affected by concomitant antiplatelet therapy, whereas COX-1-non-specific and global assays will be influenced by other antiplatelet therapy used (e.g. in cases of dual antiplatelet therapy with aspirin and an ADP receptor blocker, such as clopidogrel).
Prevalence and natural history of ‘aspirin resistance’
When response to aspirin is assessed by COX-1-specific assays, little variability in platelet responses is seen, with almost complete inhibition of TxA2-dependent platelet aggregation in almost all patients. 36–40 Far greater biological variability in aspirin-induced platelet inhibition has been reported36,37,39,41,42 when COX-1-non-specific assays have been used to assess platelet inhibition by aspirin. The definition of normal response to aspirin has also lacked standardisation, and insufficient platelet response to aspirin, or ‘aspirin resistance’, has been reported in various fashions, including tertiles/quartiles of response as well as dichotomisation based on arbitrary cut-off values. Strikingly, the correlation between the results obtained with the various platelet function assays is disappointingly low,36,37,39,41,42 thus making the studies using different platelet function assays difficult to compare.
Despite the uncertainties surrounding the best way to test for aspirin effects, platelet function assays have provided a number of potential mechanisms to explain some of the variability seen in platelet reactivity in patients taking aspirin. 43,44 As none of these factors fully explain the variability seen in patients, the phenomenon of aspirin resistance is likely to be multifactorial.
In order to assess the efficacy of aspirin, it must be ascertained that the person being assessed has indeed ingested aspirin. However, non-compliance with prescribed aspirin therapy is common and thus compliance needs to be verified. 45,46 Although crucial to the determination of platelet response to aspirin, assessment of compliance is often lacking in studies of aspirin resistance. In a recent report on the use of secondary prevention drugs in patients with established cardiovascular disease, Prospective Urban Rural Epidemiology (PURE) study investigators found that approximately one-quarter of patients with an indication for aspirin therapy were actually taking it,47 making assessment of compliance a necessity prior to platelet function testing. In studies where aspirin administration was actively monitored, the majority of patients who were aspirin resistant on initial testing became responsive to aspirin upon retesting following observed ingestion. 46,48 Thus, in fully compliant patients, aspirin resistance may be a rare but important biological phenomenon. 45,49,50 Another important variable to control for in studies of aspirin resistance is the presence of interacting drugs. A well-described interaction between aspirin and NSAIDs such as ibuprofen and naproxen [but not rofecoxib (Vioxx®, Merck Sharpe & Dohme), celecoxib (Celebrex®, Pfizer), meloxicam, acetaminophen or diclofenac] has been shown to have an impact on platelet aggregation responses. 51–54 These drugs prevent aspirin from binding to its target, platelet COX-1. Therefore, current guidelines recommend that concomitant use of NSAIDs with aspirin should be carefully avoided. 23
Other factors have been consistently associated with altered platelet responses to aspirin. Genetic factors are known to be associated with variability in platelet responses to aspirin. 40 In a large study of over 1800 participants treated with aspirin, heritable factors contributed to 27–77% of variability in platelet function assay results, most importantly in COX-1-non-specific assays, whereas COX-1-specific assays were influenced by less than 2% by heritable factors. 40 Among considerable environmental factors, obesity plays an important role. Indeed, increased waist circumference and higher body mass index have been associated with reduced efficacy of aspirin to inhibit platelets. 48 This is especially important when enteric-coated aspirin tablets are used, as these also further reduce aspirin bioavailability. 48,55 In diabetic patients, aspirin resistance is more common, and platelets have an enhanced sensitivity to platelet agonists, which has been associated with metabolic alterations, oxidative stress and endothelial dysfunction. 56–62
Finally, recent evidence suggests that accelerated platelet function recovery may be a potential source of variability in platelet responsiveness to aspirin. The most striking example of platelet turnover involvement in platelet responsiveness to aspirin is in patients suffering from essential thrombocythemia (ET), a natural disease model of enhanced platelet generation. In ET, recovery of platelet function occurs within 24 hours despite daily aspirin therapy and is due to the formation of a large number of new uninhibited platelets from megakaryocytes, resulting in an increased rate of platelet turnover. 63–65 The phenomenon is not, however, limited to ET; both in healthy volunteers and in patients suffering from CAD or diabetes, increased platelet turnover has been associated with insufficient platelet inhibition by aspirin. 66–70 Increasing the frequency of aspirin administration to twice daily has been shown to effectively improve the inhibition of platelet function by aspirin in these settings, although the clinical benefit of this therapy modification remains unknown. 63,64,71–73
Although the characteristics associated with poor response have been explored in detail,74 it is noteworthy that the different studies have used different platelet function methodologies to explore the determinants of platelet responses. In parallel, a number of different studies have shown platelet function assay results to lack correlation and agreement among themselves, thus identifying different patients as poor responders to aspirin and having different determinants of response. 37,75,76 Which platelet function assay, if any, is the most clinically predictive of future major adverse cardiovascular events remains to be established. 77 As a consequence, the natural history of aspirin resistance remains somewhat uncertain. There is a need to address basic questions on the prognostic and diagnostic utility and cost-effectiveness of platelet function testing in the context of aspirin therapy before testing can be recommended in clinical practice. A number of systematic reviews attempting to address this basic question have been published in recent years. In general, these have failed to sufficiently capture the volume of available evidence or consider the heterogeneous nature of the evidence reviewed. These reviews are explored in more detail as part of the results section of this report (see Chapter 5, Systematic reviews). As detailed in Chapter 3, the aims of this report were to address this question of prognostic and diagnostic utility of platelet function testing in the context of aspirin therapy.
Chapter 2 Decision problem
This project was commissioned to review the evidence currently available on the association between the result of a PFT and the occurrence of clinically relevant cardiovascular and cerebrovascular events, in those patients receiving long-term aspirin therapy for cardiovascular disease or cerebrovascular disease (CVD), and to consider the cost-effectiveness of the use of such tests. Specifically, this entailed (i) determining prognostic utility (whether or not a test is able to distinguish between groups of patients with different average outcome risks, even if it does not accurately predict individual outcome risk); (ii) determining diagnostic utility [if such tests exist, to determine whether or not they have high diagnostic/predictive utility (e.g. sensitivity, specificity and positive and negative predictive values close to 1) in order to determine, for individual patients, if treatment modification should be considered based on the test result]; and (iii) undertaking an exploratory model-based cost-effectiveness analysis.
The commissioning brief produced in 2010 by the National Institute for Health Research (NIHR), prior to this project being funded, was titled The Diagnostic Utility of Identifying Aspirin Resistance, and asked:
In patients being considered for long term aspirin therapy is there evidence to show which tests of ‘aspirin resistance’ predict which patients will benefit from a change in management? Should all such patients be assessed and if not in which groups of patients is testing cost-effective?
The questions posed in the commissioning brief are much wider than those examined by the project that was eventually commissioned and require extensive consideration of the clinical pathway of treating patients with cardiovascular disease or CVD, in whom long-term therapy with aspirin is traditionally viewed as the mainstay of antithrombotic therapy. To review the evidence for each step of the pathway is beyond the scope of the commissioned project. Thus, there are a plethora of questions that cannot be answered by the work undertaken for this project, yet answers are required in order to determine if patients correctly identified as likely to be at higher risk of adverse clinical outcomes while receiving long-term aspirin therapy should have their management changed, and if so, when, and to what alternative therapeutic regimen. These questions include but are not limited to the following:
-
If patients could be correctly identified by a PFT as being at greater risk of adverse clinical outcomes than other patients, do such patients gain some benefit, no matter how small, from the aspirin therapy?
-
Does platelet function, as measured by a given test, change over time in a given individual, and if so, to what degree, when and why?
-
When, if at all, should platelet function testing be undertaken, and should testing be repeated and when?
-
At what threshold of risk of adverse outcomes should a change in therapy be considered?
-
Which therapeutic regimen should patients considered at high risk be switched to and when?
Some of these questions are intrinsically linked, and there is potentially published evidence related to some of these that could be systematically reviewed in the future.
This project therefore only reviews the available evidence on the prognostic and diagnostic utility of PFTs, applied to patients on long-term aspirin therapy, in order to determine if patient groups or individual patients with high risk of adverse clinical outcomes can be identified correctly. The cost-effectiveness of using these tests is considered through a review of economic evidence and a speculative de novo model-based economic evaluation using, where necessary, clinician-derived assumption-based inputs relating to parts of the clinical pathway outside of the scope of this project for which definitive published evidence was not readily available.
In this context, ‘aspirin resistance’ is defined as elevated platelet reactivity measured using a PFT. This definition does not specify a threshold for defining elevated reactivity but relies on that specified by the authors of the studies concerned. As such, there is likely to be considerable variability in the characterisation of aspirin resistance employed in individual studies. Based on this definition, the term ‘aspirin resistant’ is defined as those individuals classified as having elevated platelet reactivity based on the PFT and threshold specified by the authors of the studies, and ‘aspirin sensitive’ is defined as those not having elevated platelet reactivity based on the PFT and threshold specified by the authors of the studies.
An evaluation of prognostic utility of aspirin resistance requires assessment of whether or not PFTs are able to distinguish between groups of patients with different average risks of clinically important outcomes.
Providing prognostic utility can be demonstrated, an evaluation of the diagnostic/predictive utility of aspirin resistance requires assessment of whether or not PFTs are able to determine, for individual patients, if they are at increased risk of clinically important outcomes and thus warrant consideration of treatment modification.
Chapter 3 Aim of the review
The aims of the review were as follows:
-
To review systematically the evidence relating platelet function testing to the risk of adverse clinical outcome(s) in patients on aspirin therapy with established cardiovascular disease or CVD, or diabetes. More specifically, to determine whether or not different PFTs have prognostic utility or diagnostic/predictive utility with regard to such clinical outcomes.
-
Prognostic utility To establish whether or not any of the available PFTs has prognostic ability, i.e. is able to distinguish between groups of patients with different average outcome risks. For PFTs demonstrating prognostic utility, to explore:
-
Diagnostic/predictive utility To establish whether or not any of the available PFTs to determine aspirin resistance has sufficiently high diagnostic/predictive utility (e.g. sensitivity, specificity and positive and negative predictive values close to 1) in order to determine, for individual patients, if treatment modification should be considered based on the test result.
-
-
To review systematically the evidence relating to the economic utility of platelet function testing in patients on aspirin therapy with established cardiovascular disease or CVD, or diabetes.
-
To undertake exploratory, model-based cost-effectiveness analysis of the use of platelet function testing in patients on long-term aspirin therapy with consideration of the potential for populating the model with data based on the results of the systematic review outlined in (1).
Within this report, the methods and results for the aims outlined in (1) are reported in Chapters 4 and 5 respectively, and those for the aims outlined in (2) are reported in Chapter 6. The findings for all aims are discussed in Chapter 7.
The protocol for this project was registered with PROSPERO (2012:CRD42012002151) and has been published on the NIHR Health Technology Assessment (HTA) programme website (www.hta.ac.uk/2468). A version of the protocol was also published in the journal BMC Systematic Reviews. 78
Chapter 4 Methods of prognostic and diagnostic utility review
This section describes the methods for the systematic review of the evidence relating platelet function testing to the risk of adverse clinical outcome(s) in patients on aspirin therapy with established cardiovascular disease or CVD, or diabetes.
The review will specifically target studies which relate platelet function testing to clinical outcome in patients with established cardiovascular disease or CVD or diabetes who are being treated with aspirin. Analysis will consider whether or not PFTs have prognostic ability in that they are able to distinguish between groups of patients with different average outcome risks. If demonstrable, analysis will subsequently consider diagnostic/predictive ability, i.e. whether or not given tests have sufficiently high diagnostic/predictive utility to accurately distinguish those individual patients who will have an adverse outcome from those who will not.
A standard systematic review approach was used and is described below.
Selection criteria
Two broad types of study were considered relevant for this review: those studies that provide information on the prognostic or diagnostic/predictive utility of PFTs and those that report prognostic models, in which a PFT is one of multiple prognostic factors predicting clinical outcomes in a population of interest. The selection criteria for each are outlined below.
Prognostic utility and diagnostic utility studies
Types of study
Any prospective primary studies, or systematic reviews of such studies, assessing PFT(s) in relation to clinical outcomes.
Types of participants
Patients aged ≥ 18 years on aspirin (as monotherapy or in combination with other antiplatelet agents), with established cardiovascular disease or CVD, or diabetes. Studies with mixed populations were included as long as data for relevant patients were extractable. Studies with patients on aspirin for peripheral vascular disease were noted.
Setting
Studies in any setting were included.
Technology
Either a COX-1-specific PFT (which measures aspirin response specifically) or a global PFT in patients receiving aspirin as the only antiplatelet therapy. The selection process was guided by the information in Table 2.
Outcomes
Clinical outcomes, such as vascular events [non-fatal and fatal ischaemic stroke, TIA, systemic embolism (pulmonary embolism, peripheral arterial embolism), MI, revascularisation procedures]; haemorrhagic events; all-cause mortality; mortality due to vascular events; composite outcomes containing the above [e.g. major adverse cardiac events (MACEs)].
Timing
Reported outcomes had to occur after the undertaking of a PFT and the post-test follow-up period had to be 7 days or longer. Thus, studies performing platelet function testing after clinical events, with no further follow-up after the testing, were excluded (unless the testing was undertaken on stored samples retrieved prior to the clinical event, as this retains the temporal relationship between testing and subsequent outcome occurrence).
Prognostic model studies
Studies reporting prognostic models, in which a PFT was one of multiple prognostic factors predicting clinical outcomes in a population of interest, were eligible for review, in order to examine the contribution of the PFT to the overall performance of the prognostic model, and to establish whether or not predictive accuracy of clinical outcomes was improved by combining test results with other prognostic factors. The following criteria were used to select such studies:
-
Was a statistical model outlined to predict a relevant clinical outcome outlined above?
-
Did the model include a factor for PFT result or aspirin resistance?
-
Was the model developed for use in patients aged ≥ 18 years and on aspirin (alone or in combination with another therapy) for established cardiovascular disease or CVD or diabetes?
Searches
The following bibliographic databases were searched:
-
The Cochrane Library (Wiley) (issue 4 of 12) [including the Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, HTA Database, NHS Economic Evaluation Database (NHS EED) and Cochrane Central Register of Controlled Trials] to April 2012, MEDLINE (Ovid) from 1950 to 2012, MEDLINE In-Process & Other Non-Indexed Citations (Ovid) to 25 April 2012 and EMBASE (Ovid) from 1980 to 2012.
Search strategies combined index and text words encompassing the technologies (platelet function testing) and the patient group (cardiovascular disease, CVD and diabetes), as well as focusing on aspirin resistance. The Zetoc database (The British Library), Conference Proceedings Citation Index and Science Citation Index (Web of Science) were searched for conference proceedings. ClinicalTrials.gov, the UK Clinical Research Network Study Portfolio Database, the World Health Organization International Clinical Trials Registry Platform and the metaRegister of Controlled Trials were also searched for ongoing studies.
Reference lists of relevant articles, particularly systematic reviews, were hand-searched to identify other potentially relevant articles. Furthermore, a subject expert was used to identify any studies which may not be identified using standard methods.
Restrictions on publication language and date were not applied to the searches.
Copies of the search strategies used in electronic databases can be found in Appendix 1.
In addition, abstracts from the following national and international proceedings were hand-searched from 2009 onwards:
-
platelet conferences (Platelets International Symposium)
-
cardiology conferences (British Cardiovascular Society, American College of Cardiology, European Society of Cardiology, American Heart Association, American College of Chest Physicians)
-
stroke conferences (International Stroke Conference, American Stroke Association)
-
haematology conferences (British Society for Haematology, International Society on Thrombosis and Haemostasis, International Society for Laboratory Haematology).
Abstracts that were identified were considered for relevance in a similar way to fully published studies/articles.
Search results were entered into reference management software [Reference Manager version 11 (Thomson ResearchSoft, San Francisco, CA, USA)]. Duplicate records were removed by built-in algorithms and subsequent manual checking.
The searches of electronic databases were undertaken in April 2012 and were not updated after this time. A note was made of any additional relevant studies published subsequently that came to the attention of the authors of this report. These studies were not reviewed to avoid bias. A brief comment is made about these studies in Chapter 5, Relevant studies identified after the search cut-off dates.
Study selection
Study selection was undertaken as a two-step process. Titles (and abstracts where available) in records were initially screened by two reviewers, using prespecified screening criteria. These criteria were kept necessarily broad as it was anticipated that not all relevant information would necessarily be presented in an abstract, and thus the use of stricter criteria was likely to lead to the exclusion of relevant articles at this screen stage. These criteria were based on whether or not the records indicated that articles were about, or likely to be about, platelet function testing; reported, or were likely to report, clinical outcomes measured after a PFT; and were about patients who had or were likely to have cardiovascular/cerebrovascular or diabetic disease and were receiving aspirin therapy.
An additional criterion for conference abstracts was that these needed to be published from 2009 onwards to be retained. Letters to journals were not automatically classed as irrelevant, because often new results relevant to this field are made available through this medium.
Full texts of any potentially relevant articles or those where a decision could not be made were sought. In the second part of the two-step selection process, full-text articles were assessed against the full inclusion criteria by two reviewers independently. Any discrepancies between reviewers were resolved by discussion or by referral to a third reviewer. A copy of the selection form used for this process is available on request.
Both stages of the selection process were piloted prior to full implementation.
At title and abstract screening and for full-text screening, appropriate portions of non-English-language articles were translated where necessary to aid the selection process.
A record was kept of all decisions made, the reason for exclusion from the review at the full-text screening stage, articles that were not obtainable even by The British Library and also cases where decisions could not be made owing to missing information in a paper or abstract. In the case of this last scenario, an e-mail was sent to an author requesting further information.
During the selection process, any study identified that was thought to be of relevance to the cost-effectiveness review was cross-checked against the search results for that review to ensure comprehensiveness.
Assessment of risk of bias
Risk of bias was assessed by one reviewer and independently checked by a second reviewer. Disagreements were resolved by discussion.
Prognostic and diagnostic/predictive utility
As the review involved assessment of both prognostic and diagnostic/predictive utility, the quality assessment strategy involved using criteria of relevance from both the Quality Assessment of Diagnostic Accuracy Studies (revised tool) (QUADAS-2) guidelines79 for diagnostic test studies and criteria for checking the quality of prognostic studies suggested by Hayden et al. 80
These criteria were compiled under the five domains outlined below with their corresponding assessment questions.
-
Domain 1: patient selection
-
Was a consecutive or random sample of patients enrolled?
-
Was patient selection independent of patient outcomes?
-
Were reasons for any posteligibility exclusions provided?
-
-
Domain 2: PFT
-
If a threshold was used, was it prespecified?
-
How was the threshold derived (e.g. literature cut-off, based on study data)?
-
Is the undertaking and interpretation of the index test blinded to the patient characteristics (including clinical outcomes)?
-
-
Domain 3: outcomes
-
Were the outcomes of interest clearly defined in advance?
-
Were the outcome results interpreted without knowledge of the results of the PFT?
-
-
Domain 4: study attrition
-
What was the proportion of missing data? (State reasons for loss to follow-up or differences in those who completed or were lost.)
-
-
Domain 5: confounding
-
Are confounders accounted for in the design or analysis (e.g. adjustment, stratification)?
-
If there is an adjusted outcome measure [e.g. odds ratio (OR), hazard ratio (HR)], what were the factors that were adjusted for?
-
If a HR was presented, was the proportional hazards assumption met?
-
Was compliance measured?
-
How was compliance measured?
-
Level of compliance.
-
Prognostic models
If any prognostic models were included, the quality criteria described by Altman81 were to be used in addition to those of Hayden et al. 80 Specific elements to be considered were:
-
methods of model development (selection of candidate risk variables, relative weighting, handling of continuous variables)
-
internal and external model validations
-
study design (prospective/retrospective)
-
sample size (considered a priori)
-
missing data (quantity, and how missing data were handled in the statistical analysis)
-
criteria for inclusion of prognostic factors into the model (adequately described, and whether or not well-known prognostic factors were included regardless of significance).
Any prognostic models identified were to be summarised qualitatively (summarising, for example, included variables, calculation of risk score, predictive accuracy and whether or not the model was validated internally and externally) and quantitatively by extracting performance statistics for calibration (such as observed/expected outcomes) and discrimination (such as sensitivity and specificity) of the model. Similarly, where studies reported the incremental value of including PFTs in prognostic models, these data were to be summarised.
Data extraction
Data extraction was conducted by one reviewer using a standardised, piloted data extraction form, and independently checked by a second. Disagreements were resolved through discussion or referral to a third reviewer.
The data extraction process was necessarily complex owing to the nature and variability of the included studies. Data extraction was undertaken directly into a specially created sheet in Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA). Extensive data related to the following domains were extracted: study design and characteristics; patient characteristics; antiplatelet regimens; PFT utilised; outcome measures and length of follow-up; data required for analyses; statistical methods employed and their appropriateness. Studies were grouped according to whether patients were on monotherapy (aspirin only) or dual therapy (with a second antiplatelet agent such as clopidogrel added to aspirin), in order to distinguish between patients in a stable (monotherapy) or acute phase (dual therapy) of thrombotic disease. Patients who have experienced ACS, or who have undergone PCI, will generally have a second agent added to their therapy for up to 1 year before reverting back to monotherapy. Note that for reasons outlined in Presentation of results, only results pertaining to monotherapy studies have been presented in this report.
For further details on data extracted, readers can consult a copy of the database via information presented in Appendix 4.
With regard to the data extracted for analysis, details are given in the following section.
Analysis
Data extraction for potential meta-analysis
A key analytical aim was to conduct meta-analysis for each test in relation to each clinical outcome reported by the individual studies. To do this, relevant data reported by the included studies needed to be extracted. Data extraction was conducted independently by two reviewers, and if necessary any differences were resolved via discussion with a third reviewer. If multiple cut-off levels were considered in a study (e.g. to define test ‘positive’ and test ‘negative’), then results were sought for each cut-off reported. Both unadjusted and adjusted results were extracted, as both were considered to be important. Unadjusted results help ascertain the prognostic ability of a test when it is used in isolation. Adjusted results reveal whether or not a test has prognostic utility over and above other prognostic factors; a true causal factor of poor outcome will retain strong prognostic value even after adjustment, and so this further informs the clinical value of a test.
Two groups of summary results were sought during data extraction, as follows.
Prognostic ability: unadjusted and adjusted odds ratios and hazard ratios
The prognostic ability of each test reveals its association with clinical outcome and provides the relative risk between groups defined by test values; for example, the odds of poor outcome in test-positive patients compared with test-negative patients.
For binary outcomes, the reported unadjusted OR and its 95% CI and p-value were extracted. If these were not available, data were sought to populate a 2 × 2 table, from which the values could be calculated directly. Any adjusted ORs (with CIs and p-values) reported were extracted along with the reported set of adjustment factors that were used.
When the follow-up is longer and/or there are patients lost to follow-up (censored), time-to-event analyses are more appropriate to account for different lengths of follow-up. When time-to-event analyses were reported (e.g. Cox regression analyses, log-rank tests), the unadjusted HR and its 95% CI and p-value were sought and extracted. If these were not provided directly, then the methods of Parmar et al. 82 to indirectly estimate them from other available data were used. If these were not possible, and a 2 × 2 table was available for a particular time point, the method of Perneger83 was used; this method assumes that all patients are followed up for the same length of time. Any adjusted HRs (with CIs and p-values) reported and the set of adjustment factors that were used were also extracted. For studies using Cox regression, whether or not the proportional hazards assumption had been checked and was considered valid was recorded.
If studies reported results according to the test on its continuous scale, that prognostic result was extracted directly (and so did not force a categorisation). If results were presented for the test categorised into three or more groups (e.g. according to tertiles or quartiles), results for each comparison presented were extracted, but where possible the groups were collapsed down to a binary comparison (to be most comparable with other studies, which generally used a dichotomisation). This collapsing was only possible for calculating unadjusted ORs or unadjusted HRs when 2 × 2 tables could be derived; it was not possible for adjusted results.
If studies provided a 2 × 2 table with one or both groups with a zero cell, then a continuity correction was added to these in order to calculate effect sizes, using the method of Sweeting et al. 84 The continuity correction added was 1/(sample size of the opposite group).
Diagnostic/predictive accuracy
If prognostic utility can be demonstrated, an evaluation of diagnostic/predictive utility of aspirin resistance requires assessment of whether or not PFTs are able to determine, for individual patients, if they are at increased risk of clinically important outcomes and thus warrant consideration of treatment modification.
Ordinarily, test accuracy is assessed on ability to distinguish between patients who are subject to a risk factor/carry a marker for disease, etc., and those who are not. However, in the current context of platelet function testing predicting future adverse clinical outcomes, diagnostic utility requires the test to identify the risk factor, and then the risk factor has to be intrinsically linked to the outcome. Thus, the diagnostic utility contains elements of the accuracy of the test in measuring platelet function and the strength of the association between the platelet function and the outcome. Furthermore, there is no single outcome in the current context and the risk of each possible outcome might vary over time. This means that, prior to assessment of diagnostic utility, it is important to have demonstrable association between the marker and outcome(s).
As will be seen in Chapter 5, no strong association was identified between any PTF and clinical outcome, thus determination of diagnostic utility is mute. However, where data were available to consider an assessment of diagnostic utility, the presence of these data was noted and they were extracted. Speculative analysis of sensitivities and specificities was undertaken and this is presented in Appendix 3 along with a description of the relevant analysis methods.
Meta-analysis methods
Once the summary results were extracted for each study and for each test, the clinical experts and researchers met to identify groups of similar patient groups and clinical outcomes across studies. For each patient group and outcome identified, the possibility for meta-analysis was considered; that is, whether or not suitable data were available from multiple studies for the same clinical outcome and test in relation to prognostic ability (relative risk scale: synthesis of ORs or HRs, taking unadjusted and adjusted results separately) and, speculatively, the diagnostic/predictive ability (absolute risk scale: sensitivity and specificity). Where possible, a separate meta-analysis for each cut-off level was considered. The intended methods for any meta-analyses were outlined in the protocol. As a result of the clinical and methodological heterogeneity between studies, pooling of data was determined to be inappropriate even in subgroups of studies employing the same PFT. However, data are presented in this report in forest plots (without the summary estimate) along with some relevant study characteristics highlighting heterogeneity.
Amendments to protocol
Initially the protocol did not specify that studies of patients on dual/triple antiplatelet therapy [i.e. aspirin with additional antiplatelet agent(s)] had to employ an aspirin-specific PFT, rather than any PFT. This was changed prior to study selection and the pertinent platelet function assays are reflected in Table 2.
It was originally stated that studies which met all of the inclusion criteria except for reporting clinical outcomes would be noted, as these might provide useful information for cost-effectiveness analysis (e.g. uncertainty around the prevalence of those defined as aspirin resistant from specific assays in specific populations). From very early in the study selection process, the protocol was amended to omit this owing to the very large number of studies being identified and limited benefit of identifying these across all the tests and populations.
These amendments were reported to the NIHR and a revised protocol was submitted.
Presentation of results
Throughout the following sections, our aim has been to highlight the heterogeneity between studies with regard to population, PFT, outcomes and analysis of studies.
Results have therefore been separated according to whether patients were receiving only aspirin as antiplatelet therapy (monotherapy) or aspirin and a second antiplatelet agent (dual therapy) at the time of the PFT. There are a number of reasons for this:
-
Populations receiving monotherapy are potentially likely to differ from those receiving dual therapy (e.g. they are less likely to have very recently had an acute cardiovascular event or to be undergoing non-elective PCI).
-
The influence of a second antiplatelet agent on an aspirin-specific PFT is unclear.
-
The second antiplatelet agent is likely to influence occurrence of clinical outcomes, and occurrence of outcome is fundamental to determination of prognostic utility.
-
Resistance to other antiplatelet agents is known, and may affect event rates.
The original intention was to report and analyse studies relating to both patients receiving monotherapy and those receiving dual therapy. It was decided to undertake a stepwise approach to the analysis, starting with monotherapy studies and then moving on to dual-therapy studies; based on the reasons listed above, it is possible that an association between aspirin resistance and clinical outcome may be more apparent within those populations receiving aspirin therapy alone, as it might be more difficult to demonstrate prognostic utility in patients receiving aspirin with additional antiplatelet therapy because of the potential added confounding effect of the other antiplatelet agent.
Furthermore, it is debateable whether or not analysis of studies with dual therapy is warranted in the absence of demonstrated prognostic utility of platelet function testing in patients treated with aspirin as monotherapy. As this criterion was not met (i.e. prognostic utility could not be adequately demonstrated), all results presented in the following sections relate to monotherapy only. However, in the interest of transparency the authors wish for all extracted and analytical data (including those from dual-therapy studies) to be available to readers of this report. The data have been made available through a web portal and further details can be found in Appendix 4, including how to access the data.
Monotherapy studies were further defined as those where all, or the vast majority of, patients were on monotherapy at the time of the PFT, given that treatment strategies may change over time depending on disease progression. Adding a second agent may affect the rate of clinical events, and this may not be independent of the underlying risk, as higher-risk patients are more likely to be receiving or to commence dual therapy. Where studies have clearly specified where a proportion of patients have at some point during the follow-up period switched therapy or received additional therapy, this information has been extracted. It is, however, possible that not all studies have reported this information.
Populations have been broadly classified as having (i) stable CAD, (ii) stable CVD/stroke, (iii) PAD/peripheral vascular disease (PVD) or (iv) unstable angina (UA)/ACS. Where patients are undergoing elective PCI (PCI) or primary PCI (PPCI), this has also been indicated. Where the population comprises several patient groups, this has been classified as miscellaneous. Note that some acute populations have been included where the PFT was undertaken when patients were on monotherapy.
Results have been separated for different PFTs, and where several thresholds or agonists have been used, this has been indicated. Where different PFTs have been used within the same study, results have been presented in Chapter 5, Studies with more than one test.
Outcomes have been classified as (i) death, (ii) MACE, (iii) ischaemic/thrombotic or (iv) haemorrhagic/bleeding. A consistent definition for MACE is not used in the literature;85 for example, it may or may not include stroke. For a composite outcome that includes cerebrovascular complications, the abbreviation MACCE is sometimes used (with the additional ‘C’ indicating the cerebrovascular component), but again, this is not consistent. Rather than devise a definition of what constitutes MACE or MACCE for this report, studies with a composite outcome of adverse cardiovascular events have been grouped together using the abbreviation MACE. Where stroke has been reported as a separate outcome, this has also been highlighted.
Within the categories of MACE/MACCE there are some inconsistencies between studies in how this has been defined; this has been appropriately highlighted where necessary. The category of ‘ischaemic/thrombotic’ events is broad and encompasses a number of different events such as revascularisation, angina, bypass surgery, cardiovascular readmission, graft occlusion, MI, etc.
The different outcome measures used in the studies have been summarised as a first step in deciding whether or not pooling is possible and to give an idea of the range of outcome measures used. They have been grouped according to the following: sensitivity and specificity, unadjusted or adjusted ORs, or unadjusted or adjusted HRs. Where HRs or ORs have not been presented but have been calculated for this report, this has been indicated. Additionally, where groups have been collapsed in order to provide a single threshold, this has also been indicated. Note that where outcomes have been reported for different test characteristics (e.g. different agonist, threshold, etc.), not all results will necessarily have been summarised using the same outcome measures.
Odds ratios and HRs provide information on the usefulness of a PFT as a prognostic risk factor. Adjusted ORs or HRs may take into account differences in clinical characteristics, which are linked to adverse events. At the least informative level, articles have only provided a narrative statement regarding the relationship between PFT results and clinical events.
Quality assessment of studies is also clearly presented to aid interpretation of findings.
Owing to the extensive nature of the data extracted from included studies for this project, it was deemed unfeasible to adequately present all the data in this report (even as appendices). The results section of the prognostic utility review in this report contains, where necessary, details of the studies, including the populations studied, test characteristics and quality-related features, and data for key outcomes are presented in illustrative forest plots.
Chapter 5 Results of prognostic utility review
Quantity of research available
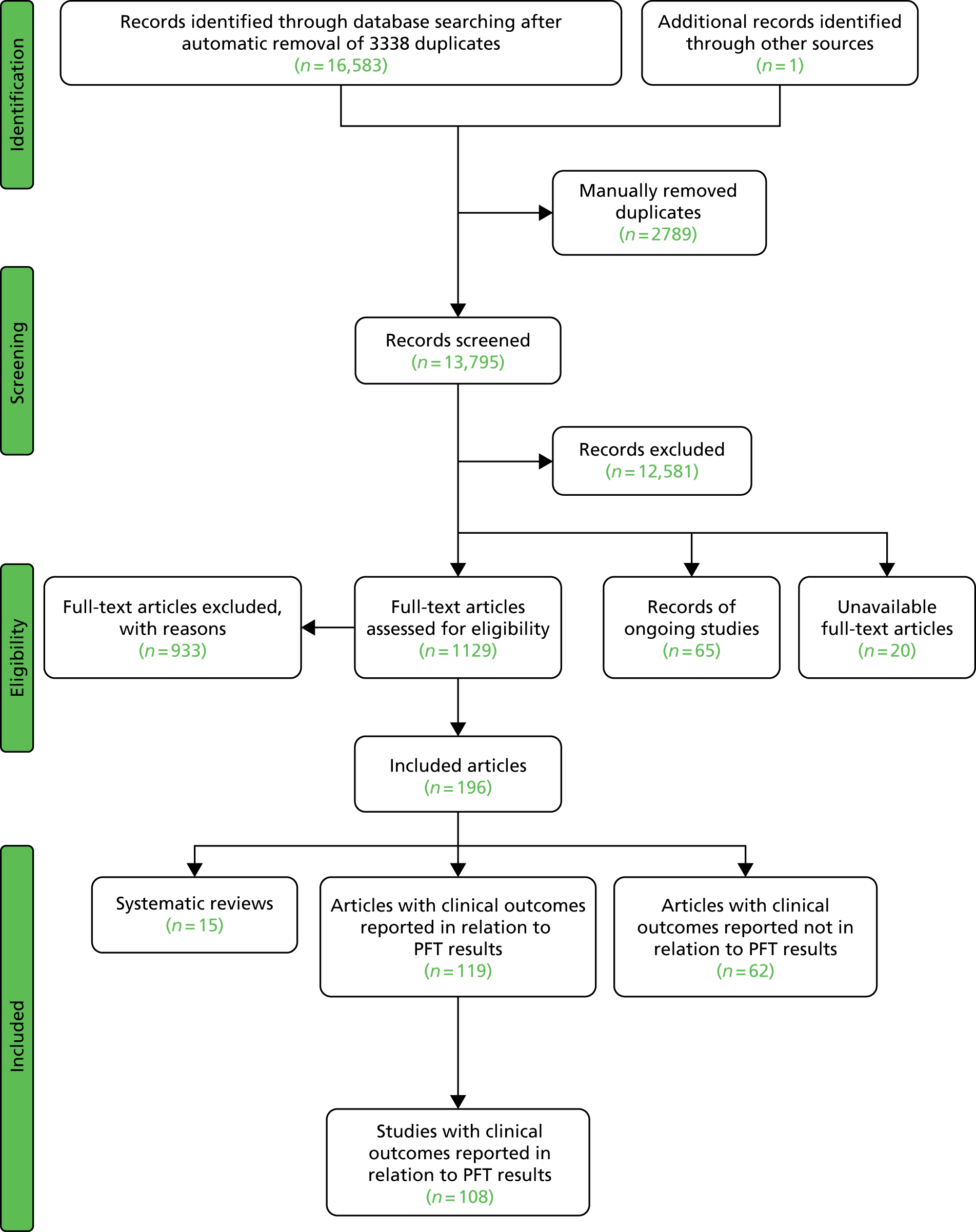
The searches resulted in the identification of 16,583 records (after automatic removal of duplicate records) and one further record from checking reference lists of relevant systematic reviews. Manual removal of duplicate records left 13,795 article records. Screening of titles and abstracts in these records indicated that 12,581 were not relevant. Full-text articles of the remaining 1214 were sought. Twenty of these articles were unobtainable and these are listed in Appendix 5; 65 were reports of ongoing studies and these are commented on later in this chapter (see Ongoing studies); and 1129 full-text articles were obtained for assessment against the inclusion criteria. Nine hundred and thirty-three articles were excluded and these are listed in Appendix 6, Table 85 with reasons for exclusion; 12 of these were excluded because there was insufficient information available to make a decision despite requests by e-mail to the authors for further details (see Appendix 6, Table 86).
One hundred and ninety-six articles met the inclusion criteria. Of these, 62 contained details of PFT results and clinical outcome data but failed to report the outcome data in relation to the test result, and thus provided no relevant information on prognostic utility of the PFT. These studies are listed in Appendix 7.
A further 119 included articles all reported clinical outcome data in relation to the result of one or more PFTs. 46,76,86–202 These articles report the findings of 108 studies that are detailed in the subsequent sections of this report. The remaining 15 articles203–217 reported systematic reviews and these are described below (see Systematic reviews).
A flow diagram presenting the process of selecting studies can be found in Figure 2.
FIGURE 2.
Flow diagram showing study selection.
Study mapping
As outlined in more detail below (see Monotherapy), included studies were separated into categories based on whether enrolled patients were receiving aspirin as their only antiplatelet agent (monotherapy) or aspirin combined with one or more other agents (dual/triple therapy) at the time of the PFT, and by the type of PFT employed in the study. Subcategorisation was undertaken to distinguish studies in which the therapy at the time of platelet function testing remained the same during follow-up from those in which this changed (e.g. patients on monotherapy at the time of testing but subsequently receiving dual therapy). Subjective decision-making was required in some cases where a proportion of patients was receiving a different therapeutic regimen at the time of testing and/or follow-up (e.g. some on monotherapy and some on dual therapy at the time of testing and/or follow-up). If the proportion was considered small (≤ 5%) then these studies were categorised under the therapy of the larger proportion. If large (≥ 11%), then these studies were put into a separate category.
The result of this mapping of studies is shown in Table 3.
| PFT | LTA | VerifyNow® Aspirin | PFA-100® | Thromboxane metabolites | WBA | TEG | Miscellaneous tests |
|---|---|---|---|---|---|---|---|
| Monotherapy | |||||||
| Studies: 19 | Studies: 7 | Studies: 21 | Studies: 11 Urinary: 9 Serum/plasma: 3 (N.B. One study is both urinary and serum) |
Studies: 8 Multiplate®: 1 Impedance: 7 |
Studies: 3 | Studies: 7 | |
| Monotherapy at time of PFT and during follow-up | Abumiyah95 Cha121 De Boni159 Feher88 Gum149 Kempfert113 Ohmori142 Payne147 Sørensen155 van der Loo90 |
Chen133 Lee171 Ozben86 |
Bevilacqua118 Boncoraglio116 Christiaens127 Morawski144 Poulsen132 Sambola145 Silver189,193 |
Bruno148 (urinary) Cotter46 (plasma) Eikelboom151 (urinary) Eskandarian202 (urinary) Thomson110 (urinary) |
Multiplate®: Orta166 Impedance: Gengo128 Mueller153 |
Sahin168 | Buchanan152 [bleeding time by Surgicutt II® (ITC Commercial Group, USA)] Grotemeyer154 (platelet reactivity test) Stejskal146,198 [Apact II® (Labitec GmbH, Ahrensburg, Germany) cationic propyl gallate platelet aggregometry] |
| Studies with more than one test | |||||||
| Addad108 | Addad108 (urinary) | ||||||
| Gluckman99 | Gluckman99 | Gluckman99 (urinary) | Gluckman99 | ||||
| Linnemann112 | Linnemann112 | ||||||
| Lordkipanidzé162 | Lordkipanidzé162 | Lordkipanidzé162 | Lordkipanidzé162 (urinary) | Lordkipanidzé162 | |||
| Majeed117 | Majeed117 | ||||||
| Miyata164 | Miyata164 (serum and urinary) | ||||||
| Schwammenthal125 | Schwammenthal125 (Impact-R®) | ||||||
| Sobol186 | Sobol186 | ||||||
| Tan174 | Tan174 | ||||||
| Monotherapy at time of PFT, proportion on monotherapy and proportion on dual therapy during follow-up | Feng201 Zanow169 |
Chu105 | Aksu109 Campo123 Hobikoglu135 Pamukcu137 Ziegler150 Fuchs138 |
Eikelboom195 (urinary) | |||
| Studies with more than one test | |||||||
| Frelinger76 | Frelinger76 (serum) | Frelinger76 (flow cytometry) | |||||
| Modica187 [PA-20/200® (Kowa Inc., Tokyo, Japan) test] | Modica187 | ||||||
| Monotherapy at time of PFT, dual therapy during follow-up | Kim92 | Foussas115 | |||||
| Studies with more than one test | |||||||
| Kaminska196 | Kaminska196 (flow cytometry) | ||||||
| Spectre93,163 | Spectre93,163 (Impact-R®) | ||||||
| Monotherapy or dual therapy | |||||||
| Monotherapy or dual therapy at time of PFT. Monotherapy, dual therapy or triple therapy during follow-up | Campo103,107 | ||||||
| Dual (triple) therapy | |||||||
| Studies: 14 | Studies: 12 | Studies: 14 | Studies: 0 | Studies: 10 Multiplate®: 8 Impedance: 2 |
Studies: 3 | Studies: 3 | |
| Dual therapy at time of PFT and during follow-up | Abumiyah95 Angiolillo96,172,188 Aradi126 Blindt130 Cuisset111 Cuisset143 De Boni159 Gurbel122,136 Marcucci160 Payne147 |
Amoah91 Ko182 Kim194 Lee171 Lee200 Lee87 (dual/triple) Marcucci161 Range190 Ripley178 Ryu170 Saw119 |
Catakoglu185 Chiu89 Fateh-Moghadam181 Foussas131 Gianetti141 Grdinic94 Jacopo129 Marcucci139 Pamukcu140 Smit,102 Bouman173 (dual/triple) Ziegler150 |
Impedance: Toth175 Ivandic114 Multiplate®: Eshtehardi106 Bobescu176,177,180 Colic184 Milicic158,191 Tokgoz183 Kaymaz165,167 |
Tang156 Gurbel197 Sambu199 |
Buch134 [Ultegra RPFA-ASA® (Accumetrics,San Diego, CA, USA)] Vaduganathan192 (Ultegra RPFA) |
|
| Studies with more than one test | |||||||
| Breet98,101,157 | Breet98,101,157 | Breet98,101,157 | Breet98,101,157 (Impact-R®) | ||||
| Gori120,124 Marcucci104 |
Gori120 | ||||||
| Ko100 | Ko100 | ||||||
| Diabetes (dual therapy) | Kuliczkowski97,179 | ||||||
Of the 108 included studies with test data linked to clinical outcome data, 57 studies reported on a patient group solely or predominantly receiving aspirin as monotherapy at the time of testing,46,76,86,88,90,92,93,95,99,105,108–110,112,113,115–118,121,123,125,127,128,132,133,135,137,138,142,144–155,159,162–164,166,168,169,171,174,186,187,189,193,195,196,198,201,202 51 studies reported on a group of patients solely or predominantly receiving dual therapy87,89,91,94–98,100–102,104,106,111,114,119,120,122,124,126,129–131,134,136,139–141,143,147,150,156–161,165,167,170–173,175–185,188,190,191,192,194,197,199,200 and one study103,107 contained a mixed population of monotherapy and dual therapy. Five studies95,147,150,159,171 were able to be mapped to both monotherapy and dual therapy categories. Turning to categories of test, LTA and the PFA-100® were the most frequently used tests in included studies, with VerifyNow® Aspirin, thromboxane metabolites and WBA also frequently encountered. Thromboelastography (TEG) was less well represented. Several tests that fell outside of these categories were placed in a miscellaneous category and this included small numbers of studies that employed, for example, tests such as flow cytometry methods and various commercial assays not included in other categories. Proportions of tests used within the monotherapy studies were: LTA (25%, 19 studies16,88,90,93,95,112,113,121,125,142,147,149,155,159,163,164,169,174,187,201), VerifyNow® Aspirin (9%, 7 studies86,92,99,105,133,162,171), PFA-100® (28%, 21 studies76,99,108,109,112,115,116,118,123,127,132,135,137,138,144,145,150,162,186,187,189,193), thromboxane metabolites measurement (14%, 11 studies46,76,99,108,110,148,151,162,164,195,202), WBA (10%, 8 studies99,117,128,153,162,166,186,196), TEG (4%, 3 studies117,168,174) and miscellaneous tests (9%, 7 studies76,93,125,146,152,154,163,196,198). The corresponding proportions for dual-therapy studies were: LTA (25%, 14 studies95,96,98,100,101,104,111,120,122,124,126,130,136,143,147,157,159,160,172,188), VerifyNow® Aspirin (21%, 12 studies87,91,98,101,119,157,161,170,171,178,182,190,194,200), PFA-100® (25%, 14 studies89,94,98,101,102,120,124,129,131,139–141,150,157,173,181,185), thromboxane metabolites measurement (0%), WBA (18%, 10 studies97,100,106,114,158,165,167,175–177,179,180,183,184,191), TEG (5%, 3 studies156,197,199) and miscellaneous tests (5%, 3 studies98,101,134,157,192). Note that several studies utilised a range of tests concurrently in the same study population. These are also identified, along with the tests used, in Table 3.
Prognostic utility of tests
Population characteristics and quality assessment of studies are presented in the following sections. As outlined in more detail in Chapter 4, Presentation of results, the structuring of results has been guided by:
-
population receiving monotherapy or dual therapy at the time of the PFT
-
therapy received after the PFT
-
PFT used
-
outcome (death, MACE, ischaemic/thrombotic event, bleeding)
-
outcome measures presented or calculable [(un)adjusted OR and HR, sensitivity and specificity]; note that sensitivities and specificities are presented in Appendix 3.
This is followed by a summary for each PFT. Studies where more than one PFT were performed concurrently are reported in Studies with more than one test.
Monotherapy
The tests identified for assessing platelet function in patients on monotherapy (aspirin only) are (i) LTA, (ii) VerifyNow® Aspirin, (iii) measurement of urinary or serum/plasma 11-dehydro-TxB2 concentrations, (iv) PFA-100®, (v) WBA, (vi) TEG and (vii) other miscellaneous tests.
Light transmission aggregometry
Population and test characteristics
Nineteen studies88,90,93,95,112,113,121,125,142,147,149,155,159,162,164,169,174,187,201 were identified in this category, four of which were reported in abstract form only,162,164,169,174 and one as a letter. 88 Populations had CAD (six studies113,142,149,162,164,201), CVD/stroke (six studies88,95,121,125,155,159) or PAD/PVD (four studies90,112,147,169). There were three studies93,174,187 in patients with UA/ACS; in one of these93 patients were all undergoing PPCI. None of the studies reported how long patients had had their primary underlying condition for.
In 12 studies88,90,95,112,121,142,147,155,159,162,164,174 it appeared that patients were exclusively on monotherapy both at the time of the PFT and during follow-up. In two studies,113,125 around 4% and 5% of patients were on dual therapy (aspirin + clopidogrel) at the time of the PFT. Given the small proportion on dual therapy, these studies have been included in the ‘monotherapy’ category.
In a further four studies,149,169,187,201 patients were on monotherapy at the time of the PFT, and around 4%,149 25%169,201 or 45%187 of patients respectively went on to receive an additional antiplatelet agent (clopidogrel) at some point during follow-up. It is possible that not all studies have reported where a proportion of patients commenced additional therapies during follow-up.
In the study where patients underwent PPCI,93 patients were on monotherapy at the time of the PFT and all were on dual therapy (aspirin + clopidogrel) during follow-up. This study has been listed separately, as the addition of clopidogrel therapy in all patients may affect the rate of events, and may also be a reflection of underlying population differences compared with the other studies.
Comedications, where reported, included statins, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, calcium channel blockers, nitrate esters, proton pump inhibitors and dalteparin (Fragmin®, Pfizer). NSAIDs were not permitted (or had to be discontinued within a certain time period) in seven studies;88,90,112,142,149,159,201 one study155 stated that drugs known to affect PFTs were discontinued, and there were no details on NSAIDs in the remaining studies.
The number of participants in the studies ranged from 32 to 583 (see Table 4). Where reported, mean ages of patients ranged from 60 to 75 years, with most means around the mid to late 60s or early 70s. There were more men than women in 14 out of 15 studies that reported on this,88,90,93,95,112,121,125,147,149,155,159,163,174,187,201 with proportions of men ranging from 53% to 81%. Only one study142 included more women (54%). The proportion of patients with diabetes ranged from 11% to 47%, and that of smokers from 5% to 66% (where reported, see Table 4). All studies were conducted in hospital settings.
The dose of aspirin ranged between 75 mg/day and 325 mg/day, with the exception of one study155 where the dose was high, at 1000 mg/day. This study included patients with TIAs or reversible ischaemic neurological deficits. There were no details on dose in one study. 95 Details were variable across studies regarding the length of time patients had been receiving aspirin therapy, with some noting a minimum period and some whether patients were chronic or first-time users, but many giving no details (see Table 4). No study stated whether aspirin was provided in enteric or plain form, though one study93 noted that aspirin was in chewable form.
The main study characteristics are listed in Table 4 below. Note that in some studies baseline characteristics have been reported only according to resistant/sensitive groups or groups with/without adverse clinical events, rather than for the total study population.
| Study/country | Number of patients | Age (years) | Therapy | Main underlying condition | Selected other population details | Due to undergo vascular intervention? | Aspirin dose/frequency | Duration of aspirin therapy (prior to PFT) | Percentage aspirin resistant | Derivation of threshold/comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Abumiya 2011,95 Japan | 144 | Mean 66.22 (SD 9.73) | Mono | CVD/stroke | Smokers: 16.7% Diabetes: 22.2% |
No | No details | No details | No details | Mean % aggregation stated by groups ± adverse event |
| Cha 2008,121 South Korea | 107 | Mean 64.3 (SD 12.0) | Mono | CVD/stroke | Smokers: 66% Diabetes: 46% |
No | 100 mg/day | PFT 5 days after being on aspirin | 24 or 75 | Depending on how tertiles were aggregated |
| De Boni 2011,159 Italy | 32 | Mean/median? 62.8 (range 40–84) for total group (includes patients on monotherapy, dual therapy and clopidogrel only) | Mono | CVD/stroke | No details | No details | 100 mg or 200 mg (frequency not stated) | No details | 0 | Aggregation (AA) ≥ 20% |
| Feher 2011,88 Hungary (letter) | 281 | Mean 64.13 (SD 10.92) sensitive, mean 66.74 (SD 10.17) resistant | Mono | CVD/stroke | Smokers: n = 78 (28%) Diabetes: n = 72 (26%) |
No | 100 mg/day | Mean 8.4 (SD 3.6) months | 18.1 | No details |
| Feng 2011,201 China | 136 | Mean 74.9 (SD 7), range 60–89 | Mono (25% dual during follow-up) |
CAD | Smokers: n = 41 (30%) Diabetes: n = 37 (27%) |
No details | 100 mg/day | No details but not previously on aspirin | No details | Quartiles: aggregation results given for patients with events |
| Gum 2003,149 USA | 326 | Mean 59 (SD 15) resistant, mean 62 (SD 11) sensitive | Mono (3.7% dual at follow-up) | CAD | Smokers: Aspirin resistant (n = 17): 0% Aspirin sensitive (n = 309): 6% Diabetes: Aspirin resistant (n = 17): 18% Aspirin sensitive (n = 309): 25% |
Yes (in ‘some’). Elective cardiac catheterisation | 325 mg/day | ≥ 7 days before enrolment | 5.2 | Aggregation ≥ 20% (AA), ≥ 70% (ADP). Unclear if resistance based on AA and/or ADP |
| Kempfert 2009,113 Germany | 59 | No details | Mono (5% dual at time of PFT) | CAD | No details | Yes: CABG and combined procedures | 500 mg aspirin intravenously postoperatively, then 100 mg/day | No details | 28.8 | Defined as aspirin resistant if platelet aggregation exceeded the threshold of 30% despite in vitro addition of 25 µM aspirin |
| Linnemann 2009,112 Germany | 57 | Median 67.7 (range 44–90) | Mono | PAD/PVD | Smokers: n = 31 (31.6%) Diabetes: n = 45 (45.9%) |
No | 100 mg/day | At least 14 days | 3.5 | Resistance defined as the maximum aggregation values within the reference range (≥ 78%) despite aspirin medication |
| Lordkipanidzé 2011,162 Canada (abstract) | 198 | No details | Mono | CAD | No details | No | 80–325 mg/day | No details | No details | No details (ORs reported) |
| Miyata 2011,164 Japan (abstract) | 583 | No details | Mono | CAD | No details | No | More or less than 100 mg/day (proportions not stated) | No details | No details | No details (narrative statement regarding event rate and platelet function) |
| Modica 2009,187 Sweden | 334 | Mean 72 | Mono (45% dual at discharge) | UA/ACS | Smokers: 21% Diabetes: 20% |
No details | 75 mg/day | No details | No details | No details (HRs reported) |
| Ohmori 2006,142 Japan | 136 | Mean 75.4 (SD 9.4) | Mono | CAD | Smokers: n = 7 (5.1%) Diabetes: n = 15 (11%) |
No | 81 mg (frequency not stated) | No details | No details | Significant event difference noted for upper quartiles compared with others |
| Payne 2004,147 UK | 54 | Mean 69 (SD 8.5) | Mono | PAD/PVD | Smokers: 32% Diabetes: 19% |
Yes: carotid endarterectomy | 150 mg (frequency not stated) | 4 weeks before surgery | 0 | Aggregation (AA) ≥ 20% |
| Schwammenthal 2008,125 Israel | 105 (79 eligible for analysis) | Mean 63 (SD 12) | Mono (4% dual at time of PFT) | CVD/stroke | Smokers: Good response (N = 40): current smokers, n = 7 (18%); past smokers, n = 13 (33%) Partial response (N = 34): current smokers, n = 5 (15%); past smokers, n = 11 (33%) Complete unresponsiveness (N = 31): current smokers, n = 8 (26%); past smokers, n = 5 (16%) Diabetes: Good response (N = 40): n = 7 (16%) Partial response (N = 34): n = 11 (32%) Complete unresponsiveness (N = 31): n = 10 (32%) |
No | 100 mg (55%) or 325 mg (45%) (frequency not stated) | 40% > 1 week before index event | 60.7 | Aggregation (AA) ≥ 20% (partial and complete unresponsiveness groups aggregated) |
| Sørensen 1983,155 Denmark | 41 | Mean 58 (range 34–73) based on total population, not stated for aspirin group | Mono | CVD/stroke | No details | No | 1000 mg/day | No details | 41.5 | Platelet hyperaggregability defined as ‘secondary aggregation obtained by ADP concentration ≤ 1 µmol/l.’ No further details |
| Tan 2010,174 China (abstract) | 250 | Mean 62 (SD 17), range 48–71 | Mono | UA/ACS | No details | No details | 150–250 mg/day | No details | 18.8 | Aggregation (AA) ≥ 20% |
| van der Loo 2011,90 Switzerland | 109 | Mean 68.1 (SD 11.4) with events, mean 72.3 (SD 9.7) without events | Mono | PAD/PVD | Smokers: Reported by pack-years (1 pack-year of smoking based on someone who had smoked one pack of cigarettes per day, i.e. 20 cigarettes daily for 1 year) With events at follow-up (n = 66): 36.4 (SD 38.8) pack-years Without events at follow-up (n = 43): 27.9 (SD 28.2) pack-years Diabetes: With events at follow-up (N = 66): n = 23 (35%) Without events at follow-up (N = 43): n = 11 (26%) |
Yes: percutaneous angioplasty | 100 mg/day | No details | No details | Mean levels of platelet aggregation shown for groups with and without events |
| Zanow 2010,169 Germany (abstract) | 75 and 54 (different aspirin regimens) | No details | Mono (25% dual during follow-up) | PAD/PVD | No details | Yes: endovascular revascularisation, peripheral bypass | 100 mg/day or one-off dose postoperatively | No details | Daily aspirin group: 31 One-off aspirin group: 32 |
Aggregation (AA) ≥ 30% |
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||||||
| Spectre 2011,93 Israel | 54 | Mean 59.7 | Mono at PFT, all patients on dual post PFT | UA/ACS (undergoing PPCI) | Smokers: 30% Diabetes: 24% |
Yes: PPCI | 100 mg/day | Previous long-term use of aspirin in 73% | 33.3 or 66.7 | Depending on whether upper two or lower two tertiles were grouped together to provide a single cut-off |
The test performed in 18 out of 19 studies was LTA. Most tests used arachidonic acid as an agonist, with some also using collagen and ADP, and sodium citrate as the anticoagulant (where reported). One study187 used a variant of LTA, an aggregometer that uses laser light scattering (the PA-200).
Most studies reported no details on the timing of the PFT after aspirin ingestion. One study125 noted that there were at least 6 hours between aspirin dose and PFT, and three other studies112,147,149 stated that there were up to 24 hours between aspirin dose and PFT. Table 5 provides details of test characteristics.
| Study | Details of kit/manufacturer | Anticoagulant (concentration) | Agonist (concentration) | Time since last aspirin dose |
|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||
| Abumiya 201195 | MC Medical, Tokyo, Japan | ‘Citrated blood’ | Collagen (2 µM) Collagen (5 µM) ADP (1 µM) ADP (10 µM) |
No details |
| Cha 2008121 | Model 560 VS (Chrono-log Corporation, Havertown, PA, USA) | Sodium citrate | AA (0.5 mg/ml = 1.6 mM) ADP (10 µM) |
No details |
| De Boni 2011159 | Chrono-log 700-4 lumi-aggregation systems (Chrono-log Corporation, Havertown, PA, USA) | Sodium citrate (3.2%) | AA (0.5 mM increasing up to 1 mM) | No details |
| Feher 201188 (letter) | LTA (no further details) | No details | No details (reported elsewhere) | No details |
| Feng 2011201 | LTA (no further details) | No details | AA | No details |
| Gum 2003149 | PAP4 platelet aggregometer (BioData, Horsham, PA, USA) | Sodium citrate (3.8%) | AA (0.5 mg/ml = 1.6 mM) ADP 10 µM |
1–24 hours before blood sampling |
| Kempfert 2009113 | PAP-4 (moeLab, Berlin, Germany) | Citrate | AA (1 mM) | No details |
| Linnemann 2009112 | Behring Coagulation Timer® (BCT®) (Dade Behring, Düdingen, Switzerland) | Sodium citrate (3.2%) | AA (0.5 mg/ml = 1.6 mM) | 1–24 hours |
| Lordkipanidzé 2011162 (abstract) | LTA (no further details) | No details | AA (1.6 mM) ADP (5 µM) ADP (10 µM) ADP (20 µM) |
No details |
| Miyata 2011164 (abstract) | LTA (no further details) | No details | AA Collagen |
No details |
| Modica 2009187 | PA-200 | Sodium citrate (0.129 M) | Epinephrine (30 µl of a solution containing 0.1 mg epinephrine) | No details |
| Ohmori 2006142 | LTA (no further details) and PA-20 platelet aggregation analyser | Sodium citrate (10%) | Collagen (1 µg/ml) | No details |
| Payne 2004147 | PAP4 platelet aggregometer | Trisodium citrate (3.8% wt/vol) | AA (2.5 mM) | < 24 hours |
| Schwammenthal 2008125 | PACKS-4 (Helena Laboratories, Beaumont, TX, USA) | ‘Citrated blood’ | AA (1.6 mM) | At least 6 hours before blood sampling |
| Sørensen 1983155 | Turbidimetric aggregation (Born method) | No details | ADP (lowest ADP concentration that could produce secondary aggregation) | No details |
| Tan 2010174 (abstract) | LTA (no further details) | No details | AA | Unclear: blood samples collected every 2 hours up to 24 hours before and after aspirin administration |
| van der Loo 201190 | APACT 4 aggregometer (Labitec GmbH, Ahrensburg, Germany) | Sodium citrate (3.8%) | Epinephrine (0.1 mM) Collagen (5 µg/ml) ADP (2 mM) |
No details |
| Zanow 2010169 (abstract) | LTA (no further details) | No details | AA ADP |
No details |
| Monotherapy at time of PFT, dual therapy during follow-up | ||||
| Spectre 201193 | PACKS-4 | Sodium citrate | AA (1.6 mM) | No details |
Study design and quality
Results of the risk-of-bias assessment can be found in Tables 6–9.
| Domain 1: patient selection | Was a consecutive or random sample of patients enrolled? | Was patient selection independent of patient outcomes? | Were reasons for any posteligibility exclusions provided? |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Abumiya 201195 | No details | Yes | No details |
| Cha 2008121 | Unclear; patients selected from a larger, consecutively enrolled group | Yes | No details |
| De Boni 2011159 | No details | Yes | Patients who changed therapy, with low/no compliance, intolerance/allergy to aspirin, contraindications to anticoagulants, who did not attend follow-up |
| Feher 201188 (letter) | Consecutive | Yes | No details |
| Feng 2011201 | Consecutive | Yes | No details |
| Gum 2003149 | Unclear; patients recruited from consecutive patients presenting to the outpatient clinic | Yes | No details |
| Kempfert 2009113 | Consecutive | Yes | No details |
| Linnemann 2009112 | Consecutive | Yes | No details |
| Lordkipanidzé 2011162 (abstract) | No details | Yes | No details |
| Miyata 2011164 (abstract) | Consecutive | Yes | No details |
| Modica 2009187 | Consecutive | Yes | No details |
| Ohmori 2006142 | No details | Yes | No details |
| Payne 2004147 | Consecutive | Yes | Unclear; 38/138 patients excluded before randomisation, but unclear if any would have met the inclusion criteria |
| Schwammenthal 2008125 | Consecutive | Yes | No details |
| Sørensen 1983155 | No details | Yes | No details |
| Tan 2010174 (abstract) | Consecutive | Yes | No details |
| van der Loo 201190 | Unclear (substudy of a trial) | Yes | No details |
| Zanow 2010169 (abstract) | No details | Yes | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | |||
| Spectre 201193 | Consecutive | Yes | No details |
| Domain 2: PFT | If a threshold was used, was it prespecified? | How was the threshold derived? (e.g. literature cut-off, based on study data) | Is the undertaking and interpretation of the index test blinded to the patient characteristics (including clinical outcomes)? |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Abumiya 201195 | No | Quartiles | No details |
| Cha 2008121 | Yes (> 20%) | Reference cited151 | No details |
| De Boni 2011159 | Yes (> 20%) | Reference cited149,218 | No details |
| Feher 201188 (letter) | No details | No details | No details |
| Feng 2011201 | No | Quartiles | No details |
| Gum 2003149 | Yes (≥ 20% for AA and ≥ 70% for ADP) | No details | No details |
| Kempfert 2009113 | Yes (aspirin resistant if platelet aggregation exceeded the threshold of 30% despite in vitro addition of 25 µM aspirin) | Unclear; states that platelet aggregation was measured according to the manufacturers’ instructions | No details |
| Linnemann 2009112 | Partially; method yes, actual value no. Based on results from group of 20 healthy volunteers. Resistance defined as the maximum aggregation values within the reference range (≥ 78%) despite aspirin medication | In accordance with recommendations given at the 53rd Annual Scientific and Standardization Committee Meeting of the ISTH in Geneva in 2007, the 5th–95th percentile of maximum aggregation measured in duplicate in a group of healthy volunteers (n = 20) was considered as the reference range (i.e. 78–96%) | No details |
| Lordkipanidzé 2011162 (abstract) | No details | No details | No details |
| Miyata 2011164 (abstract) | No details | No details | No details |
| Modica 2009187 | Yes (high residual platelet reactivity was defined as a normal CT value even when the subject was taking aspirin) | Reference cited77 | Unclear (‘test results were not accessible by the attending physicians’) |
| Ohmori 2006142 | No details | No details | Yes; laboratory staff were kept unaware of patient information |
| Payne 2004147 | Yes (> 20%) | Reference cited219 | Unclear; states that ‘all personnel involved with the trial were blinded to the nature of the patients’ current drug therapy.’ However, this may not apply to PFTs |
| Schwammenthal 2008125 | Yes (good response < 20%, partial response 20–39%, complete unresponsiveness ≥ 40%) | Reference cited149 | Unclear; treating physicians and the investigators evaluating the patients were blinded to the results of the platelet function studies |
| Sørensen 1983155 | Yes (platelet hyperaggregability defined as secondary aggregation obtained by ADP concentration ≤ 1 µM) | Reference cited220 | No details |
| Tan 2010174 (abstract) | Yes (> 20%) | No details | No details |
| van der Loo 201190 | No (mean levels of platelet aggregation shown for groups with and without events) | N/A | No details |
| Zanow 2010169 (abstract) | Yes (≥ 30%) | No details | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | |||
| Spectre 201193 | Yes: three groups (good response < 20% aggregation, intermediate 20–40%, poor response > 40%) | No details | No details |
| Domains 3 and 4: outcomes and study attrition | Were the outcomes of interest clearly defined in advance? | Were the outcome results interpreted without knowledge of the results of the PFT? | What was the proportion of missing data? (State reasons for loss to follow-up or differences in those who completed or were lost) |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Abumiya 201195 | Yes | No details | No details on whether or not there were any missing data |
| Cha 2008121 | Yes | No details | No loss to follow-up |
| De Boni 2011159 | No | No details | Unclear; patients who did not attend follow-up were excluded from study |
| Feher 201188 (letter) | Unclear | No details | No details |
| Feng 2011201 | Yes | No details | No details |
| Gum 2003149 | Yes | Those performing follow-up interviews were blinded to aspirin sensitivity status | Follow-up data were available on 97% of patients |
| Kempfert 2009113 | No | No details | 1/59 patients lost to follow-up. Reason not stated |
| Linnemann 2009112 | Yes | Unclear; reported events were only considered if they were confirmed by medical reports from GPs or admitting hospitals | Data on clinical outcome available only from patients whose platelet function was assessed twice (57/98). Of the 98, four patients died and 16 had their antithrombotic medication changed, mainly because of an acute cardiovascular event. Not clear what the remaining reasons for dropouts were. This might bias the results though authors state that there was no difference observed in aspirin resistance rates between dropouts and those remaining in the study |
| Lordkipanidzé 2011162 (abstract) | Yes | No details | No details |
| Miyata 2011164 (abstract) | Yes | No details | No details |
| Modica 2009187 | Yes | Yes (‘test results were not accessible by the attending physicians’) | No loss to follow-up |
| Ohmori 2006142 | Yes | Yes; those performing follow-up were unaware of the aspirin sensitivity status | 4/136 (three patients who developed atrial fibrillation and one who did not take aspirin were excluded from analysis) |
| Payne 2004147 | Yes | Unclear; states that ‘all personnel involved with the trial were blinded to the nature of the patients’ current drug therapy.’ However, this may not apply to PFTs and outcomes | No details |
| Schwammenthal 2008125 | Yes | Yes; treating physicians and the investigators evaluating the patients were blinded to the results of the platelet function studies | Follow-up data were available for 81/105 patients (77%) |
| Sørensen 1983155 | No | No details | 48/83 patients at last follow-up, but proportion of these in aspirin group (n = 41) unclear |
| Tan 2010174 (abstract) | Yes (though unclear if composite or individual outcomes) | No details | No details |
| van der Loo 201190 | Yes | No details | Appears to be no loss to follow-up for events (though repeat PFTs in decreasing numbers of patients over time) |
| Zanow 2010169 (abstract) | No details | No details | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | |||
| Spectre 201193 | Yes | No details | 7/63 lost to follow-up at 6 months |
| Domain 5: confounding | Are confounders accounted for in the design or analysis (e.g. adjustment, stratification)? | If there is an adjusted outcome measure (e.g. OR, HR), what were the factors that were adjusted for? | If a HR was presented, was the proportional hazards assumption met? | Was compliance measured? | How was compliance measured? | Level of compliance |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Abumiya 201195 | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Cha 2008121 | Design: N/A Analysis: yes (for OR) |
Age (> 70 years), white blood cell count > 15 points on NIHSS, statin therapy, large artery atherosclerotic infarction based on TOAST classification | N/A | No details | No details | No details |
| De Boni 2011159 | Design: N/A Analysis: no |
N/A | N/A | Yes | Exclusion criterion: those with low/no compliance | No details |
| Feher 201188 (letter) | Design: N/A Analysis: no |
N/A | N/A | Yes | ‘Study drug compliance was assessed by their general practitioners and by personal interview’ | No details |
| Feng 2011201 | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Gum 2003149 | Design: N/A Analysis: yes (for HR) |
Variables in model included age, sex, race, history of tobacco use, diabetes, hypertension, hyperlipidaemia, revascularisation, MI, haemoglobin, platelet count, creatinine, aspirin sensitivity | Yes | Yes | Patient interview both at study enrolment and follow-up | No details |
| Kempfert 2009113 | Design: N/A Analysis: no |
N/A | N/A | Yes | If platelets were not sufficiently suppressed (aggregation exceeding the 30% threshold), then the test was repeated after in vitro addition of aspirin (10 µM and 25 µM) to assess whether or not platelets exhibited resistance per se | No details on level. Stated that non-compliance or impaired intestinal uptake excluded by adding in vitro aspirin |
| Linnemann 2009112 | Design: N/A Analysis: no |
N/A | N/A | Yes | Interview at study commencement and follow-up | ‘All patients confirmed that they had taken aspirin regularly as directed over the last 14 days.’ Assume relates to start of the study |
| Lordkipanidzé 2011162 (abstract) | Design: N/A Analysis: unclear if adjusted or unadjusted OR |
No details | N/A | No details | No details | No details |
| Miyata 2011164 (abstract) | Design: N/A Analysis: appears that adjustment for possible confounders was undertaken, but no adjusted measures presented |
No details | N/A | Yes | Interview and by checking plasma concentration of salicylic acid | No details |
| Modica 2009187 | Design: N/A Analysis: yes (for HR) |
Age, sex, diabetes, smoking status, heart failure, atrial fibrillation, baseline glomerular filtration rate, troponin T, platelet aggregation, high residual platelet reactivity, intervention with CABG or PCI | Yes (the assumptions for Cox regression analysis were evaluated by Kaplan–Meier curves for all the variables included) | No details | No details | No details |
| Ohmori 2006142 | Design: N/A Analysis: yes (for HR) |
Small aggregates, medium aggregates, large aggregates | No details | Yes | By patient interview at enrolment and follow-up | Unclear; one patient who did not take aspirin was excluded from analysis |
| Payne 2004147 | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Schwammenthal 2008125 | Design: N/A Analysis: yes (for OR) |
Age, NIHSS, diabetes | N/A | No details | No details | No details |
| Sørensen 1983155 | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Tan 2010174 (abstract) | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| van der Loo 201190 | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details (N.B. appears to dismiss non-compliance as a reason for variation on PFTs; stated that ‘the variation in platelet response to agonists would imply a high degree of irregularity in taking aspirin, which makes this explanation completely speculative.’) |
| Zanow 2010169 (abstract) | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| Spectre 201193 | Design: N/A Analysis: yes (for HR) |
‘Variables chosen for inclusion into the model were those that tended to be associated with event-free survival on univariate analysis and age’ | No details | No details | No details | No details |
Patient selection was independent of study outcome in all included studies, with the PFT preceding any outcomes (as specified in the study selection criteria). Ten of 19 studies88,93,112,113,125,147,164,174,187,201 stated that consecutive patients were enrolled into the study. Only one study159 had clear details on posteligibility exclusion of patients; one criterion for exclusion was no or low compliance.
A predetermined threshold percentage (for platelet aggregation) was given in nine studies; in seven of these93,121,125,147,149,159,174 the threshold was 20% (with two studies93,125 defining a further two groups: 20–39% for partial response and ≥ 40% for complete unresponsiveness). In two studies113,169 the threshold was 30%. The remaining studies stated that quartiles were used,95,201 described the method of deriving a threshold but not an actual percentage112,155,187 or gave no details. 88,142,162,164 One study90 stated mean levels of platelet aggregation only (for groups with and without clinical events). Most studies cited a reference for their threshold or method of derivation; there were no details in seven studies. 88,90,95,142,162,164,201 Only one study142 gave clear details on blinding of laboratory staff to patient characteristics.
Outcome measures of interest were clearly predefined in all but five studies. 88,113,155,159,169 Four studies125,142,149,187 had clear details regarding blinding to the PFT results of those assessing outcomes. There appeared to be no loss to follow-up in three studies. 90,121,187 Loss to follow-up was stated in seven studies93,112,113,125,142,149,155 and ranged from 2% to 57% (see Table 8). There were no clear details in nine studies. 88,95,147,159,162,164,169,174,201 The differences in completeness of follow-up may reflect length of follow-up, study design (outcome only followed up in those that had repeat PFTs) or quality of reporting.
Compliance was measured in seven studies. 88,112,113,142,149,159,164 In three studies88,142,149 this was by a general practitioner (GP) assessment and/or patient interview, but no details on the level of compliance were stated; one patient was excluded on the basis of non-compliance in one of these studies. 142 One study112 stated that after interview all patients confirmed that they had taken aspirin as directed over the last 14 days.
A further study164 assessed compliance by interview and checking of plasma concentration of salicylic acid, but there were no details on level of compliance. One study159 did not state the method of assessing compliance, but stated that patients with low/no compliance were excluded. In the study by Kempfert et al. ,113 the PFT was repeated after in vitro addition of aspirin where platelets were not sufficiently suppressed in order to exclude non-compliance.
Thirteen studies did not appear to undertake any adjusted analyses. 88,90,95,112,113,147,155,159,162,164,169,174,201 Six studies93,121,125,142,149,187 attempted to adjust for a number of factors. There was some overlap between factors adjusted for (e.g. age), but no study used all the same factors as another. There may be selective reporting in that only variables that showed significance on univariate analysis may have been included in multivariate analyses. One study142 was unusual in that it adjusted for size of aggregates only.
Overview of outcomes
The main outcome categories reported in the LTA monotherapy studies are shown in Table 10. Note that where a study reports MACEs, individual outcomes (e.g. death, stroke) may additionally have been reported separately. There may also be more than one ischaemic/thrombotic outcome reported in the same study. Follow-up periods ranged from 30 days to 3 years.
| Study | Death | MACE | Ischaemic/thrombotic | Bleeding | Length of follow-up |
|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||||
| Abumiya 201195 | ✓ | 1 year | |||
| Cha 2008121 | ✓ | ✓ | ✓ | 90 days | |
| De Boni 2011159 | ✓ | 3 months | |||
| Feher 201188 (letter) | ✓ | 2 years | |||
| Feng 2011201 | ✓ | Up to 6 months | |||
| Gum 2003149 | ✓ | ✓ | ✓ | Mean 679 days | |
| Kempfert 2009113 | ✓ | ✓ | ✓ | 12 months | |
| Linnemann 2009112 | ✓ | ✓ | Median 17 months (range 10–37 months) | ||
| Lordkipanidzé 2011162 (abstract) | ✓ | 3 years | |||
| Miyata 2011164 (abstract) | ✓ | 2 years | |||
| Modica 2009187 | ✓ | Median 44 months (IQR 35–55 months) | |||
| Ohmori 2006142 | ✓ | Mean 172 days | |||
| Payne 2004147 | ✓ | ✓ | 30 days | ||
| Schwammenthal 2008125 | ✓ | Median 11.5 months (range 3.9–19.3 months) | |||
| Sørensen 1983155 | ✓ | Median 26 months (range 20–36 months) | |||
| Tan 2010174 (abstract) | ✓ | ✓ | ✓ | Mean 360 days (range 0–523 days) | |
| van der Loo 201190 | ✓ | Mean 80 months (range 52–94 months) | |||
| Zanow 2010169 (abstract) | ✓ | ‘Long-term’ | |||
| Monotherapy at time of PFT, dual therapy during follow-up | |||||
| Spectre 201193 | ✓ | Up to 15 months | |||
Death
Only 688,113,121,147,149,174 of the 19 studies reported this outcome, and 5113,121,147,174,188 reported solely the data needed to populate 2 × 2 tables (Table 11).
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Cha 2008121 | ✓a | ✓a | Number of events reported for three groups: low, intermediate, high ADP aggregation | ✓a | ||
| Feher 201188 (letter) | ✓a | ✓a | ✓a | |||
| Gum 2003149 | ✓ | |||||
| Kempfert 2009113 | ✓a | ✓a | ✓a | |||
| Payne 2004147 | No patients found to be aspirin resistant, therefore not represented in Figures 3 and 4 | |||||
| Tan 2010174 (abstract) | Some percentages presented, but exact numbers not clear | |||||
Figures 3 and 4 present the unadjusted ORs and unadjusted HRs reported for death. None of these were directly available from the publications (except for one HR149), but were calculated from other reported data. No study reported adjusted measures. In the study by Payne et al. (2004),147 no patients (out of a total of 54) were found to be aspirin resistant at a threshold of > 20% aggregation, therefore no summary measures could be calculated; one stroke and no deaths occurred in this patient group (follow-up 30 days). Similarly, results could not be presented in forest plots for the study by Tan et al. ;174 here the rate of death was 26% in the resistant and 11% in the sensitive group.
FIGURE 3.
Light transmission aggregometry, monotherapy: death, unadjusted ORs. Cha et al. 121 results obtained by collapsing tertiles presented into two groups defined by the threshold level shown. AA, arachidonic acid.

FIGURE 4.
Light transmission aggregometry, monotherapy: death, unadjusted HRs. Cha et al. 121 results obtained by collapsing tertiles presented into two groups defined by the threshold level shown. AA, arachidonic acid. a, Three patients were on clopidogrel preoperatively, all patients were on aspirin monotherapy post-operatively.

In the studies by Cha et al. 121 and Gum et al. ,149 more deaths occurred in those patients categorised as aspirin resistant; however, none of the unadjusted ORs or HRs were statistically significant. This is also the case for the studies by Feher et al. 88 and Kempfert et al. ,113 where the extremely wide CIs are a reflection of an adjustment factor used in cases where no events occurred in the aspirin-sensitive groups.
In terms of prognostic utility, although there was a trend towards more events in the aspirin-resistant groups, no study was able to show a statistically significant difference (in CAD or CVD/stroke patients).
Major adverse cardiac events
Eleven90,93,112,113,121,142,149,162,164,174,187 of 19 studies reported this outcome (Table 12).
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Cha 2008121 | ✓a | ✓ | ✓a | ✓a | ||
| Gum 2003149 | ✓ | ✓ | ||||
| Kempfert 2009113 | ✓a | ✓a | ✓a | |||
| Linnemann 2009112 | ✓a | ✓a | ✓a | |||
| Lordkipanidzé 2011162 (abstract) | ✓ | |||||
| Miyata 2011164 (abstract) | Narrative description | |||||
| Modica 2009187 | ✓ | ✓ | ||||
| Ohmori 2006142 | ✓ | |||||
| Tan 2010174 (abstract) | Some percentages presented, but exact numbers not clear | |||||
| van der Loo 201190 | Mean platelet aggregation values for groups with and without events | |||||
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| aSpectre 201193 | ✓a | ✓a | ✓ | ✓a | ||
Note that one study112 reporting MACEs included death from cardiovascular causes, MI, ACS and stroke, but also amputation or gangrene.
The study by Miyata et al. 164 reported that no ex vivo measurements for residual platelet function were associated with cardiovascular events (no data presented). In the study by Tan et al. 174 the results for total events (MACEs) were unclear, but based on higher numbers of events for death, recurrent MI or thrombosis, it appeared that a greater number of MACEs occurred in the aspirin-resistant group. van der Loo et al. 90 reported no significant differences in mean platelet aggregation levels in groups with and without MACEs.
The remaining studies are presented in the forest plots in Figures 5–8.
FIGURE 5.
Light transmission aggregometry, monotherapy: MACEs, unadjusted ORs. AA, arachidonic acid.
FIGURE 6.
Light transmission aggregometry, monotherapy: MACEs, adjusted ORs.

FIGURE 7.
Light transmission aggregometry, monotherapy: MACEs, unadjusted HRs. AA, arachidonic acid.
FIGURE 8.
Light transmission aggregometry, monotherapy: MACEs, adjusted HRs. AA, arachidonic acid.

There were 12 unadjusted ORs based on five studies,93,112,113,121,162 (different agonists, thresholds or time points; see Figure 5), 11 of which found no statistically significant differences in event rates; there was also no consistent trend regarding direction of effect. One study121 found a statistically significant result for one of two thresholds, with more events in the aspirin-resistant group in a CVD/stroke population, but this result was based on a non-aspirin-specific agonist (ADP) and the threshold was derived by collapsing tertiles. Most ORs were not directly available from the studies (with the exception of one162).
The one statistically significant OR121 remained so after adjustment, although it moved very close to 1 (see Figure 6). There were no further adjusted ORs.
There were 10 unadjusted HRs (based on six studies,93,112,113,121,149,187 see Figure 7), all calculated from other data presented in the articles. Three showed statistically significant results (more events in the aspirin-resistant group). 121,149,187 All three included different populations (CAD, CVD/stroke and UA/ACS).
Based on the three studies where an unadjusted HR was calculable, one HR149 remained statistically significant after adjustment (see Figure 8); two previously non-significant results93 became statistically significant and one previously significant result became non-significant. 187 Of one further study included,142 one of two results was statistically significant (more events in the aspirin-resistant group).
Based on adjusted measures, there was a consistent trend towards more MACEs in the resistant groups, with some results showing statistical significance. However, this is based on a subsample of studies only (5 of 19 studies93,121,142,149,187), and the choice of adjustment factors and inclusion of certain factors into the models may have affected results. Note that two93,187 of the five studies contributing to these results were in UA/ACS patients, which may differ from the majority of stable populations, and in one study93 all patients were on dual therapy after the PFT.
Ischaemic/thrombotic events
Thirteen studies88,90,95,112,113,121,125,147,149,155,159,169,174 reported additional ischaemic/thrombotic events (Table 13).
| Study | Outcome | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||||||
| Abumiya 201195 | Recurrent cerebral infarction | Results for quartiles represented graphically. Could not extract data from forest plots | |||||
| Cha 2008121 | Non-fatal stroke | ✓a | ✓a | ✓ | |||
| MI | ✓a | ✓a | ✓a | ||||
| De Boni 2011159 | Ischaemic events/clinical relapses | No patient classified as aspirin resistant. No events occurred | |||||
| Feher 201188 (letter) | Stroke and/or TIA | ✓a | ✓a | ✓ | |||
| UA | ✓a | ✓a | ✓a | ||||
| MI | ✓a | ✓a | ✓a | ||||
| Peripheral arterial occlusion with revascularisation | ✓a | ✓a | ✓a | ||||
| Gum 2003149 | CVA | ✓ | |||||
| MI | ✓ | ||||||
| Kempfert 2009113 | Stroke | ✓a | ✓a | ||||
| Linnemann 2009112 | Peripheral arterial revascularisation | Numbers in aspirin-resistant and aspirin-sensitive groups not clear | |||||
| PTA/stenting | |||||||
| Bypass surgery or TEA | |||||||
| Payne 2004147 | Stroke | No patients classified as aspirin resistant | |||||
| Schwammenthal 2008125 | mRS score ≥ 2 or death during follow-up | ✓ | |||||
| Recurrent ischaemic event | |||||||
| Sørensen 1983155 | Stroke or death | ✓a | ✓a | ✓a | |||
| Tan 2010174 (abstract) | Recurrent MI or thrombosis | Some percentages presented, but exact numbers not clear | |||||
| van der Loo 201190 | Restenosis or reocclusion | Intrapatient variability of platelet aggregation between groups with and without events | |||||
| Zanow 2010169 (abstract) | Patency after reconstruction | No numerical data | |||||
Seven of 13 studies90,95,112,147,159,169,174 did not provide data which would have allowed their representation in forest plots. There was also heterogeneity across outcome measures (e.g. MI, UA, restenosis, etc.). Many of these measures are also captured in the MACEs described above.
In the study by Abumiya and Houkin95 there appeared to be a trend for more events (recurrent cerebral infarction) to occur in higher quartiles of platelet aggregation, but no statistical significance could be shown. The numbers in Tan et al. 174 were unclear, but it appears that a higher percentage had a recurrent MI or thrombosis in the aspirin-resistant group. van der Loo et al. 90 looked at differences in intrapatient variability of platelet aggregation between groups with and without restenosis or occlusion; no evidence for a difference was found (at adjusted p-value level). Zanow et al. 169 found that long term there was a poorer patency rate for aspirin-resistant patients, but this was not statistically significant.
The study by De Boni et al. 159 provided no useful information, as no patients were classified as aspirin resistant and no events occurred. Similarly, in the study by Payne et al. 147 no patients were classified as aspirin resistant; there was one stroke (in a group of 54 patients). Linnemann et al. assessed a number of ischaemic thrombotic outcomes, but the exact numbers in the aspirin-resistant and aspirin-sensitive groups were unclear; only 2 out of 57 patients were classified as aspirin resistant.
Twelve unadjusted ORs were presented based on four studies88,113,121,155 (different outcomes, thresholds) (Figure 9). All were calculated for this report. CIs were generally very wide and all but one showed no statistical significance, though there was a trend towards more events occurring in the aspirin-resistant groups.
FIGURE 9.
Light transmission aggregometry, monotherapy: ischaemic/thrombotic events, unadjusted ORs. AA, arachidonic acid; PAO, peripheral arterial occlusion.

A different study125 presented an adjusted OR for two thresholds, one of which was statistically significant (Figure 10).
FIGURE 10.
Light transmission aggregometry, monotherapy: ischaemic/thrombotic events, adjusted ORs. AA, arachidonic acid; mRS, modified Rankin scale.
Fourteen unadjusted HRs based on five studies88,113,121,149,155 were presented (Figure 11). Again, all but one showed no statistical significance, though there was a trend towards more events in the aspirin-resistant groups. No adjusted HRs were presented.
FIGURE 11.
Light transmission aggregometry, monotherapy: ischaemic/thrombotic events, unadjusted HRs. AA, arachidonic acid; CVA, cerebrovascular accident; PAO, peripheral arterial occlusion.
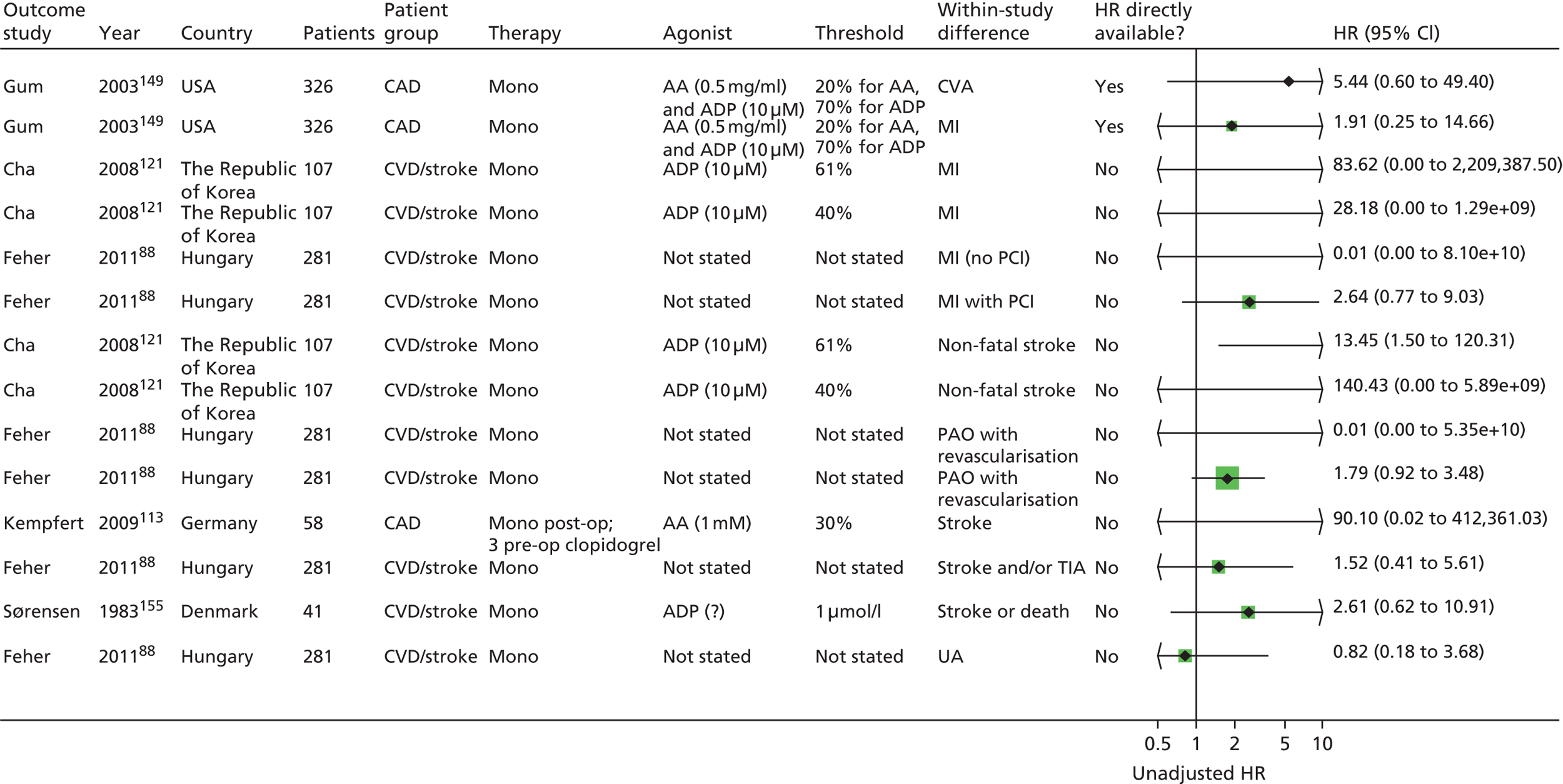
Overall, the trend towards more events in the aspirin-resistant group was consistent, but most results were not statistically significant. These results were also consistent with those of the studies not presented in the forest plots. Interestingly, there were three studies where no (or a very small proportion of) aspirin-resistant patients were identified. 112,147,159
Bleeding events
Only one study201 reported bleeding events (GI bleeds) (Table 14).
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Feng 2011201 | Aggregation values presented for individual patients (according to quartiles for some) | |||||
Of the four patients with bleeds, three were in the lowest platelet aggregation quartile (values of 2.7%, 6.75% and 9.12%). The threshold value for the lowest quartile was 9.81%. The remaining patient had a value of 11.2% (not stated which quartile). This is consistent with the assumption that GI bleeds are more likely to occur in aspirin-sensitive patients, but the small number of events precludes any firm conclusions.
Summary: light transmission aggregometry monotherapy
Nineteen studies were identified in this category. 88,90,93,95,112,113,121,125,142,147,149,155,159,162,164,169,174,187,201 There were differences in patient populations, though most appeared to have stable disease; note that although there were only three studies93,174,187 with acute (UA/ACS) populations, two of these93,187 contribute substantially to the MACE results. There was heterogeneity across studies in terms of specific patient characteristics (e.g. smoker, diabetic, comedications, etc.).
There was a lack of detail in reporting of relevant quality criteria, making an overall judgement on risk of bias difficult. Additionally, studies that do report relevant information may be more open to criticism. Lack of detail related in particular to loss-to-follow-up information, blinding and details of compliance. No study provided details on all relevant quality criteria. There were differences in threshold and method of deriving the threshold for defining aspirin resistance, but the most consistent was a threshold of 20% (seven studies93,121,125,147,149,159,174). Only one142 and four125,142,149,187 (of 19) studies respectively gave clear details on blinding to patient characteristics or PFT results. Measurement of compliance was undertaken in seven studies,88,112,113,142,149,159,164 but there was a lack of detail on the results or consequences of this; it appears that in two studies142,159 patients were excluded on the basis of low/no compliance. Some studies provided adjusted analyses; there was overlap but no consistency in factors adjusted for.
Six studies88,113,121,147,149,174 reported on differences in deaths between aspirin-resistant and aspirin-sensitive groups; there was a trend towards more events in the aspirin-resistant group (based on four studies88,113,121,149), but no significant differences were shown in any.
Eleven studies reported on MACEs. 90,93,112,113,121,142,149,162,164,174,187 There was a trend towards more events in the aspirin-resistant groups, but unadjusted measures found mostly statistically non-significant results. Five of seven results (based on five studies93,121,142,149,187) using adjusted measures were statistically significant and the trend was consistent with the unadjusted results; however, this was based on a subset of studies, different adjustment factors and two93,187 (of five93,121,142,149,187) studies in an acute population, which may not be representative of the majority of populations receiving aspirin monotherapy.
Thirteen studies reported additional ischaemic/thrombotic events. 88,90,95,112,113,121,125,147,149,155,159,169,174 Again, there was a trend towards more events in the aspirin-resistant group, but the vast majority of results (mainly unadjusted measures) were not statistically significant. Results of 7 of the 13 studies90,95,112,147,159,169,174 could not be presented in forest plots, but results were consistent (i.e. non-significant).
There was only one study reporting GI bleeds;201 this found a trend for more aspirin-sensitive patients to have more bleeds, but this was based on only four events (in 136 patients).
Note that not all studies reporting the relevant outcomes could be presented in the forest plots; the results of those studies not included in the forest plots were in the main consistent with those included or did not add much useful information. It should also be noted that some studies contributed several results to the forest plots as they presented results or could be analysed for different thresholds. Although no results have been pooled, the visual impact of these forest plots might influence how the overall results are perceived. Given the large amount of heterogeneity between the studies in terms of quality criteria, threshold, population, test characteristics (agonists), aspirin dose, etc., it was not possible to compare results across studies. Despite the heterogeneity and lack of many statistically significant results, the direction of prognostic effect appears to be largely consistent with there being more events in aspirin-resistant patients (ORs and HRs usually > 1). This suggests that LTA is a potential prognostic factor, but this is only a qualitative judgement on the evidence available; meta-analysis was not possible owing to the heterogeneity, and therefore a firm quantitative conclusion regarding whether or not LTA is prognostic is not currently possible.
Summary: light transmission aggregometry
-
Nineteen studies were identified with mainly stable populations.
-
The most frequently reported threshold was 20% platelet aggregation.
-
A lack of detail in reporting of quality criteria, particularly around loss to follow-up, blinding and details (and implications) of compliance, hampered an overall risk-of-bias assessment.
-
Heterogeneity in outcomes, patient groups and types of reported statistics meant that meta-analysis was not considered appropriate.
-
Adjusted results were rarely presented, and thus the additional prognostic value of the test over other prognostic factors is difficult to ascertain.
-
Despite clinical heterogeneity between studies, there was an overall consistent trend for more events to occur in the ‘aspirin-resistant’ group for all relevant outcomes (death, MACEs, ischaemic/thrombotic events); however, most results were not statistically significant.
-
There were more statistically significant results (more events in the resistant arm) using adjusted measures for MACEs, but these were based on only five studies.
-
One study reporting GI bleeds found a trend for more GI bleeds in ‘aspirin-sensitive’ patients, but this was based on only four events (in 136 patients).
VerifyNow® Aspirin
Population and test characteristics
Seven studies86,92,99,105,133,162,171 were identified in this category, one162 of which was reported in abstract form only. Populations were mainly classified as having CAD (three studies)99,133,162 or CVD/stroke (two studies). 86,171 One study105 was in patients with UA/ACS, and one92 was in patients with severe CAD undergoing CABG. Six studies did not report for how long patients had had their underlying condition; one study171 stated time from cerebral infarction to randomisation [≤ 90 days: 37 patients (31.1%); 91–364 days: 33 patients (27.7%); ≥ 365 days: 49 patients (41.2%)].
In six studies86,99,105,133,162,171 it appeared that patients were exclusively on monotherapy both at the time of the PFT and at follow-up. In the remaining study,92 patients were on monotherapy at the time of the PFT, and on dual therapy [+ ticlopidine (Ticlid®, Sanofi Winthrop)] during follow-up.
Comedications across the studies included, where reported, ACE inhibitors, beta-blockers, angiotensin receptor blockers, calcium channel blockers, statins, oral anticoagulants and lipid lowering agents. NSAIDs were not permitted in four studies86,92,133,171 and were taken by 28 out of 314 (9%) patients in another,105 while one study99 stated that ‘concurrent nonsteroidal anti-inflammatory drug use did not correlate with the presence of aspirin non-responsiveness defined by this method at either time point’. There were no details in one study. 162
The number of participants in the studies ranged from 106 to 468 (see Table 15). Mean ages were mainly reported by group (resistant/sensitive) and ranged from 61 years to 70 years. Overall, there were more men than women in the studies, with proportions of men ranging from 50% to 85%. All studies were conducted in hospital settings. The proportion of smokers ranged (where reported) from 11% to 39% and that of diabetics from 21% to 56%.
The dose of aspirin ranged from 75 to 325 mg/day. Four studies86,105,133,171 noted a minimum period (between 1 and 4 weeks) for which patients needed to have been taking aspirin; there were no details in the remaining studies. Two studies99,133 stated that aspirin was provided in enteric form (in 65% of patients in one study133). There were no details in the remaining studies.
The main study characteristics are listed in Table 15. Note that in some studies baseline characteristics have been reported only according to resistant/sensitive groups, rather than for the total study population. All studies used the commercially available VerifyNow® Aspirin test kit (Table 16), which uses arachidonic acid as an agonist. Four studies noted the timing of the PFT after aspirin ingestion; this was between 1 and 4 hours,86 between 2 and 30 hours,133 up to 24 hours171 or on the same day. 92 There were no details in the remaining studies.
| Study/country | Number of patients | Age (years) | Therapy | Main underlying condition | Selected other population details | Due to undergo vascular intervention? | Aspirin dose/frequency | Duration of aspirin therapy (prior to PFT) | Percentage aspirin resistant | Derivation of threshold/comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Chen 2007,133 China | 468 | Mean 66.7 (SD 10.2) resistant, mean 63.2 (SD 11.7) sensitive | Mono | CAD | Smokers: Resistant (N = 128): n = 14 (10.9%) Sensitive (N = 340): n = 44 (14.1%) Diabetes: Resistant (N = 129): n = 50 (39.1%) Sensitive (N = 340): n = 117 (34.4%) |
No | 80–325 mg/day Mean 114.4 mg (SD 42.2 mg) sensitive, mean 102.0 mg (SD 17.8 mg) resistant |
At least 4 weeks | 27.4 | Resistance defined as ARU ≥ 550. No details for derivation of threshold |
| Chu 2010,105 New Zealand | 314 | Mean 67.1 (SD 12.75) resistant, mean 70.1 (SD 11.8) sensitive | Mono (11% dual during follow-up – people who underwent PCI) | UA/ACS | Smokers: n = 7 (23.3%) resistant, n = 38 (13.4%) sensitive Diabetes: n = 10 (33.3%) resistant, n = 60 (21.1%) sensitive |
PCI in 11% CABG in 2.9% |
75–300 mg/day | At least 4 weeks | 9.6 | Resistance defined as ARU ≥ 550. No details for derivation of threshold |
| Gluckman 2011,99 USA | 229 | For patients with ≥ 1 occluded SVG (n = 70): mean 63 (range 55–72) For patients with patent SVG (n = 159): mean 63 (range 57–71) |
Mono | CAD | Smokers: n = 52 (22.7%) Diabetes: n = 84 (36.7%) |
Yes: CABG | 325 mg/day | No details | No details | Resistance defined as ARU ≥ 550. Derivation according to manufacturer’s instructions |
| Lee 2010,171 The Republic of Korea | 119 (244 enrolled; split into mono and dual therapy) All population characteristics based on 119 (mono group) |
Mean 62.8 (SD 10.0) | Mono | CVD/stroke | Smokers: n = 44 (37%) Diabetes: n = 46 (38.7%) |
No | 100 mg/day | At least 2 weeks | 10.9 | Resistance defined as ARU ≥ 550 |
| Lordkipanidzé 2011,162 UK (abstract) | 198 | No details | Mono | CAD | No details | No | 80–325 mg/day | No details | No details | No details |
| Ozben 2011,86 Turkey | 106 | Mean 64.9 (SD 14.6) | Mono | CVD/stroke | Smokers: n = 41 (38.7%) Diabetes: n = 36 (34%) |
No | 100 mg/day | At least 1 week | 33 | Resistance defined as ARU ≥ 550. Derivation according to manufacturer’s clinical studies using optical aggregometry as the comparison standard |
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||||||
| Kim 2011,92 The Republic of Korea | 220 | Mean 65.1 (SD 10.8) resistant, mean 63.9 (SD 8.8) sensitive | Mono (all patients on dual during follow-up) | MI (CABG) | Smokers: Aspirin responders (N = 181): n = 58 (32%) Aspirin non-responders (N = 39): n = 7 (17.9%) Diabetes: Aspirin responders (N = 181): n = 89 (49.2%) Aspirin non-responders (N = 39): n = 22 (56.4%) |
Yes: CABG | 100 mg/day | No details | 17.7 | Resistance defined as ARU ≥ 550. Derivation manufacturer’s reference value |
| Study | Details of kit | Anticoagulant (concentration) | Agonist (concentration) | Time since last aspirin dose |
|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||
| Chen 2007133 | VerifyNow® Aspirin | No details | AA | Between 2 and 30 hours |
| Chu 2010105 | VerifyNow® Aspirin | 3.2% citrate | AA | No details |
| Gluckman 201199 | VerifyNow® Aspirin | No details | AA | No details |
| Lee 2010171 | VerifyNow® Aspirin | No details | AA | Up to 24 hours |
| Lordkipanidzé 2011162 (abstract) | VerifyNow® Aspirin | No details | No details | No details |
| Ozben 201186 | VerifyNow® Aspirin | 3.2% citrate | AA | Between 1 and 4 hours |
| Monotherapy at time of PFT, dual therapy during follow-up | ||||
| Kim 201192 | VerifyNow® Aspirin | No details | AA | Aspirin administered on day of test |
Study design and quality
Patient selection was independent of outcome in all studies, as all patients with an available PFT were followed up. Two studies99,171 stated that consecutive patients were enrolled. Two studies92,99 provided details on posteligibility exclusions; one of these99 reported that the study population was deemed to be representative of the eligible population.
As this was a commercial test with a manufacturer-recommended threshold, it was assumed that all studies used the same threshold even where not stated. No study gave clear details on whether or not the undertaking and interpretation of the PFT was blinded to patient characteristics. Outcomes were defined in advance in all studies, and there were details in four studies86,99,105,133 regarding the blinding of outcome assessment (to the results of the PFT). Proportions of missing data were reported in four studies and were less than, or around, 1%105,133 and up to 14%171 and 32%. 99 Longer follow-up times did not correspond to greater loss to follow-up.
It was not stated whether or not the proportional hazards assumption was met in the two studies105,133 that reported HRs. Three studies86,105,133 reported adjusted measures and the factors adjusted for were listed; there was little similarity between the adjustment factors. Four studies86,99,105,171 stated that compliance was assessed (pill counts; ascertained by nurse, verified with patients). Only one study171 gave details on the actual level of compliance: six patients were excluded at 4 weeks and a further six at 6 months owing to poor drug compliance (12/119 in total).
Full details are provided in Tables 17–20.
| Domain 1: patient selection | Was a consecutive or random sample of patients enrolled? | Was patient selection independent of patient outcomes? | Were reasons for any posteligibility exclusions provided? |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Chen 2007133 | No details | Yes | No details |
| Chu 2010105 | No details | Yes | No details |
| Gluckman 201199 | No details | Yes | Patients where SVG patency not assessed or those not on aspirin monotherapy. Authors stated that the study population was representative of patients undergoing isolated CABG surgery based on comparison with the Society of Thoracic Surgeons National Database |
| Lee 2010171 | Consecutive | Yes | No details |
| Lordkipanidzé 2011162 (abstract) | No details | Yes | No details |
| Ozben 201186 | Consecutive | Yes | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | |||
| Kim 201192 | No details | Yes | 300 patients assessed for eligibility; 75 did not meet inclusion criteria, five declined to participate |
| Domain 2: PFT | If a threshold was used, was it prespecified? | How was the threshold derived (e.g. literature cut-off, based on study data)? | Is the undertaking and interpretation of the index test blinded to the patient characteristics (including clinical outcomes)? |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Chen 2007133 | Yes (ARU ≥ 550) | No details | No details |
| Chu 2010105 | Yes (ARU ≥ 550) | No details | Unclear: the clinical team managing the patients was blinded to aspirin resistance status |
| Gluckman 201199 | Yes (ARU ≥ 550) | Manufacturer’s instructions | No details |
| Lee 2010171 | Yes (ARU ≥ 550) | Manufacturer’s instructions | No details |
| Lordkipanidzé 2011162 (abstract) | No details | No details | No details |
| Ozben 201186 | Yes (ARU ≥ 550) | Manufacturer’s instructions | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | |||
| Kim 201192 | Yes (ARU ≥ 550) | Manufacturer’s instructions | No details |
| Domains 3 and 4: outcomes and study attrition | Were the outcomes of interest clearly defined in advance? | Were the outcome results interpreted without knowledge of the results of the PFT? | What was the proportion of missing data? (State reasons for loss to follow-up or differences in those who completed or were lost) |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Chen 2007133 | Yes | Yes. Personnel responsible for data collection were not aware of aspirin responsiveness results. Hospital charts were analysed to ascertain whether or not the events qualified for the definition of the end point | 4/464 (0.9%) patients lost to follow-up, one in the aspirin-resistant group, three in the aspirin-sensitive group |
| Chu 2010105 | Yes | Yes. The clinical team managing the patients was blinded to aspirin resistance status | 2/312 lost to follow-up (death during index hospitalisation) |
| Gluckman 201199 | Yes | Yes. Images were analysed by two blinded reviewers (98% concordance) with a third reviewer adjudicating as necessary | 65/229 not included at 6 months |
| Lee 2010171 | Yes | No details | 17/119 lost to follow-up [reasons: consent withdrawal (4), poor drug compliance (12), miscellaneous (1)] |
| Lordkipanidzé 2011162 (abstract) | Yes | No details | No details |
| Ozben 201186 | Yes | Yes. Personnel responsible for data collection were not aware of aspirin responsiveness results | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | |||
| Kim 201192 | Yes | No details | No details |
| Domain 5: confounding | Are confounders accounted for in the design or analysis (e.g. adjustment, stratification)? | If there is an adjusted outcome measure (e.g. OR, HR), what were the factors that were adjusted for? | If a HR was presented, was the proportional hazards assumption met? | Was compliance measured? | How was compliance measured? | Level of compliance |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Chen 2007133 | Design: N/A Analysis: yes (for HR) |
Diabetes, prior MI, haemoglobin levels | No details | No (‘Compliance to aspirin therapy was not ascertained by pill count or salicylate level monitoring’) | N/A | N/A |
| Chu 2010105 | Design: N/A Analysis: yes (for HR) |
Troponin-T (other non-significant factors were removed from model) | No details | Yes (before enrolment) | Aspirin dose and compliance were verified with patients before enrolment | No details |
| Gluckman 201199 | Design: N/A Analysis: no |
N/A | N/A | Yes | Pill counts at each postoperative encounter | No details |
| Lee 2010171 | Design: N/A Analysis: no |
N/A | N/A | Yes | Counted all returned medications after 4 weeks of treatment and calculated compliance to the trial medications. Patients with poor compliance were defined as those who missed ≥ 25% of 4-week prescribed doses | 12/119 noncompliant: six did not undergo follow-up PFT after 4 weeks of treatment owing to poor drug compliance; six excluded after having the follow-up PFT at 4 weeks owing to poor drug compliance |
| Lordkipanidzé 2011162 (abstract) | Design: N/A Analysis: unclear whether adjusted or unadjusted OR |
No details | N/A | No details | N/A | N/A |
| Ozben 201186 | Design: N/A Analysis: yes (for OR) |
Age, sex, NIHSS, prior stroke, comorbidities (hypertension, diabetes, hyperlipidaemia, coronary heart disease, renal failure) | N/A | Yes | Ascertained by nurse charts | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| Kim 201192 | Design: N/A Analysis: no |
N/A | N/A | No details | N/A | N/A |
Overview of outcomes
The most frequently reported outcome in studies using VerifyNow® Aspirin was MACEs (four studies92,105,133,162), followed by other ischaemic/thrombotic events (three studies92,99,133), death (two studies86,92) and bleeding events (two studies92,171). Outcomes and follow-up periods are shown in Table 21.
| Study | Death | MACEs | Ischaemic/thrombotic events | Bleeding | Length of follow-up |
|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||||
| Chen 2007133 | ✓ | ✓ | Mean 379 (SD 200) days | ||
| Chu 2010105 | ✓ | > 30 days and up to 6 months | |||
| Gluckman 201199 | ✓ | 6 months | |||
| Lee 2010171 | ✓ | 4 weeks | |||
| Lordkipanidzé 2011162 (abstract) | ✓ | 3 years | |||
| Ozben 201186 | ✓ | 2 years | |||
| Monotherapy at time of PFT, dual therapy during follow-up | |||||
| Kim 201192 | ✓ | ✓ | ✓ | ✓ | Responders: mean 9.8 (SD 10.5) days; non-responders: mean 10.1 (SD 10.8) days |
Death
Two studies86,92 reported this outcome (Table 22).
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Ozben 201186 | ✓a | ✓ | ✓a | ✓a | ||
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| Kim 201192 | ✓a | ✓a | ✓a | |||
Only two86,92 of seven studies reported deaths and the different outcome statistics are shown in Figures 12–14. One study (Ozben et al. 86 in patients with CVD/stroke) found a statistically significant OR and HR (greater number of deaths in the aspirin-resistant group) for both in-hospital and 2-year mortality. The OR (2-year mortality) remained statistically significant when adjusted for age, sex, National Institutes of Health Stroke Scale (NIHSS), prior stroke and comorbidities (hypertension, diabetes, hyperlipidaemia, coronary heart disease, renal failure). Only one death (in the aspirin-sensitive group) occurred in the study by Kim et al. 92 in patients undergoing CABG, and no significant difference could be shown.
FIGURE 12.
VerifyNow® Aspirin: death, unadjusted OR.
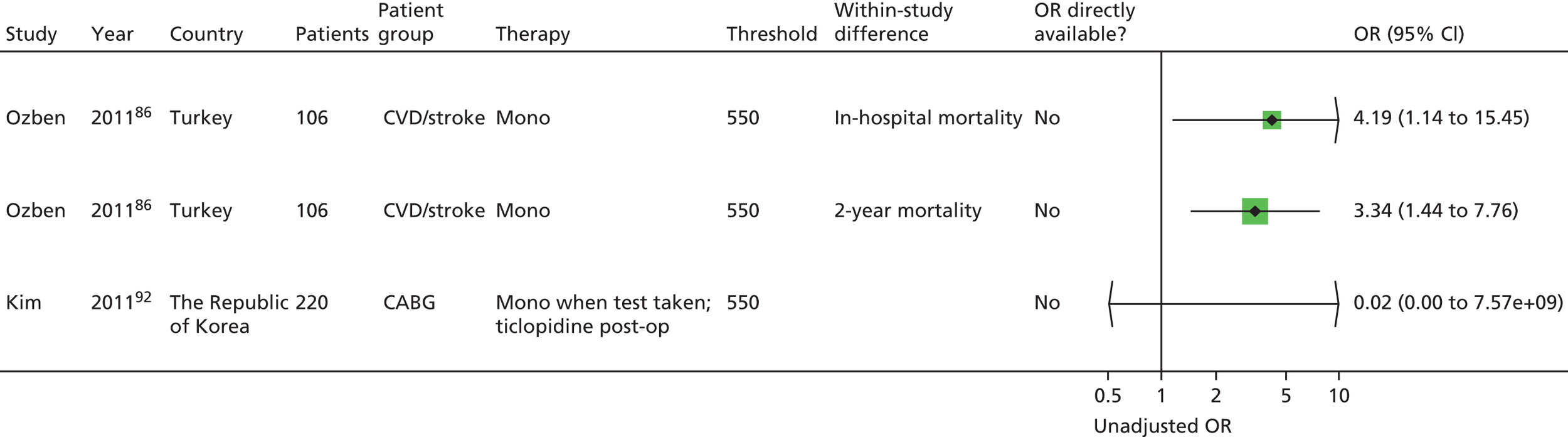
FIGURE 13.
VerifyNow® Aspirin, monotherapy: death, adjusted ORs.

FIGURE 14.
VerifyNow® Aspirin, monotherapy: death, unadjusted HRs.
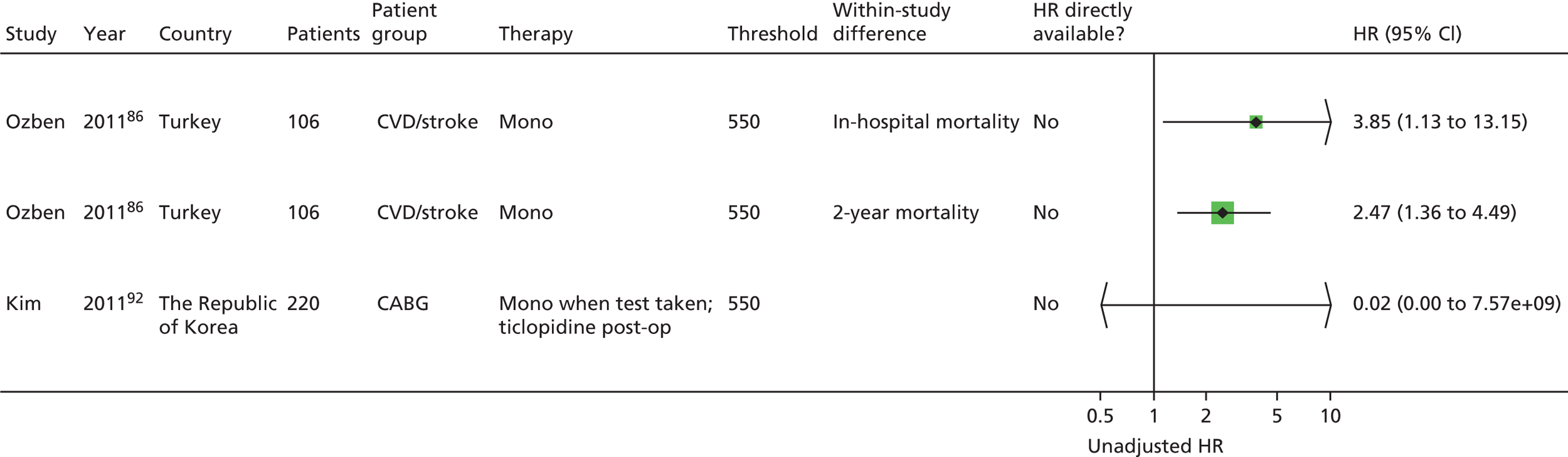
Major adverse cardiac events
Four studies92,105,133,162 reported this outcome (Table 23).
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Chen 2007133 | ✓a | ✓ | ✓ | ✓a | ||
| Chu 2010105 | ✓a | ✓/✓a | ✓ | ✓a | ||
| Lordkipanidzé 2011162 (abstract) | ✓ | |||||
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| Kim 201192 | ✓a | ✓a | ✓a | |||
Four92,105,133,162 out of seven studies reported this outcome, and the different outcome statistics are shown in Figures 15–17. The studies by Chen et al. 133 and Chu et al. (for five out of seven subgroups)105 found a statistically significant difference between groups, with more events in the resistant group (unadjusted OR). Two further studies92,162 reported more events in the sensitive group, but there were no statistically significant differences. No study reported adjusted ORs. The pattern was similar for unadjusted HRs, though with statistically significant results for five out of seven subgroups (Chu et al. 105) and a statistically significant result, with more events in the resistant group, also presented for the population undergoing CABG in the study by Chen et al. 133 Note that the unadjusted HR is not statistically significant compared with the unadjusted OR (Chen et al. 133 CAD population); given the relatively long follow-up period (mean 379 days), the HR could be considered the more useful outcome statistic. Adjusted HRs were available for three out of seven subgroups (Chu et al. 105 and Chen et al. 133 CAD population); these were all statistically significant, with more events in the resistant group. Note that the factors adjusted for in the two studies are completely different (troponin-T only in the study by Chu et al. ;105 diabetes, prior MI and haemoglobin levels in the study by Chen et al. 133).
FIGURE 15.
VerifyNow® Aspirin, monotherapy: MACEs, unadjusted ORs.
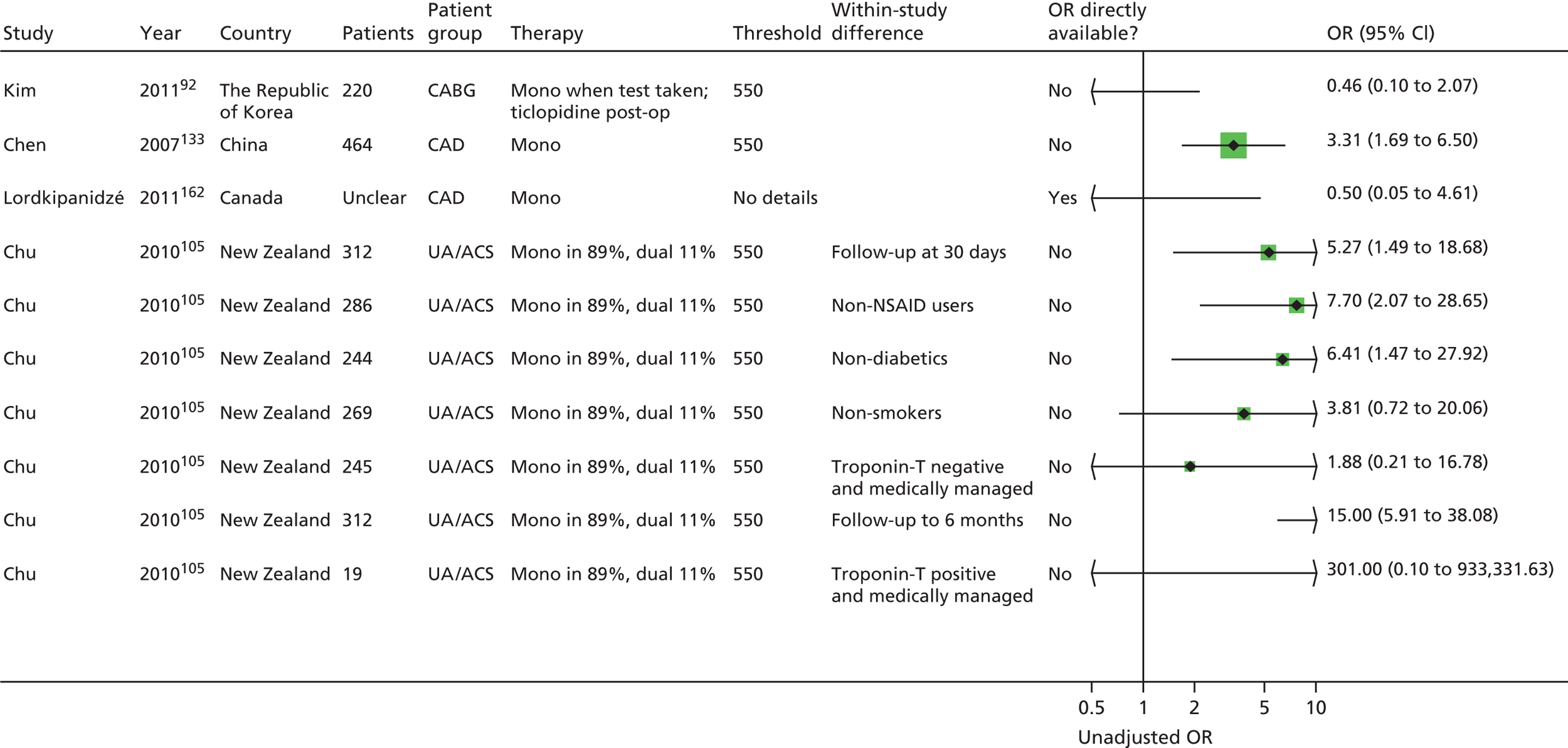
FIGURE 16.
VerifyNow® Aspirin, monotherapy: MACEs, unadjusted HRs.
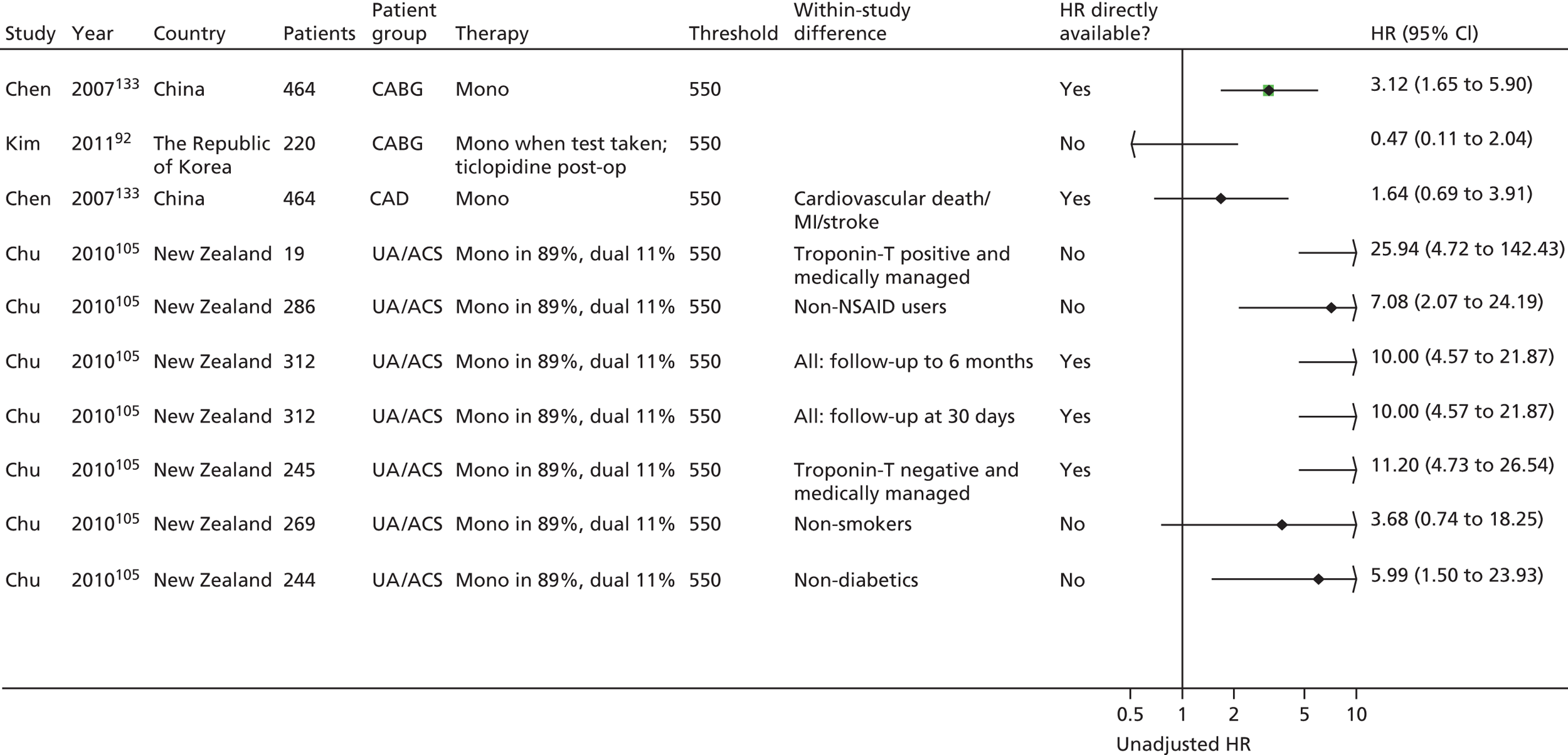
FIGURE 17.
VerifyNow® Aspirin, monotherapy: MACEs, adjusted HRs.

Thus, any statistically significant results relate to a greater number of events in the resistant group; however, not all outcome statistics (particularly adjusted HR) have been reported for all four studies/subgroups, so there is some missing information.
Ischaemic/thrombotic events
Three studies92,99,133 reported this outcome (Table 24).
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Chen 2007133 | ✓ | |||||
| Gluckman 201199 | ✓ | Mean ARU presented for groups with and without events | ||||
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| Kim 201192 | ✓a | ✓a | ✓a | |||
Three92,99,133 out of seven studies also reported additional ischaemic/thrombotic outcomes, and the different outcome statistics are reported in Figures 18 and 19. There were no statistically significant differences based on unadjusted ORs (two studies92,99). Two of seven unadjusted HRs were statistically significant (both based on one study133), with more events in the resistant group.
FIGURE 18.
VerifyNow® Aspirin, monotherapy: ischaemic/thrombotic events, unadjusted ORs. SVG, saphenous vein graft.
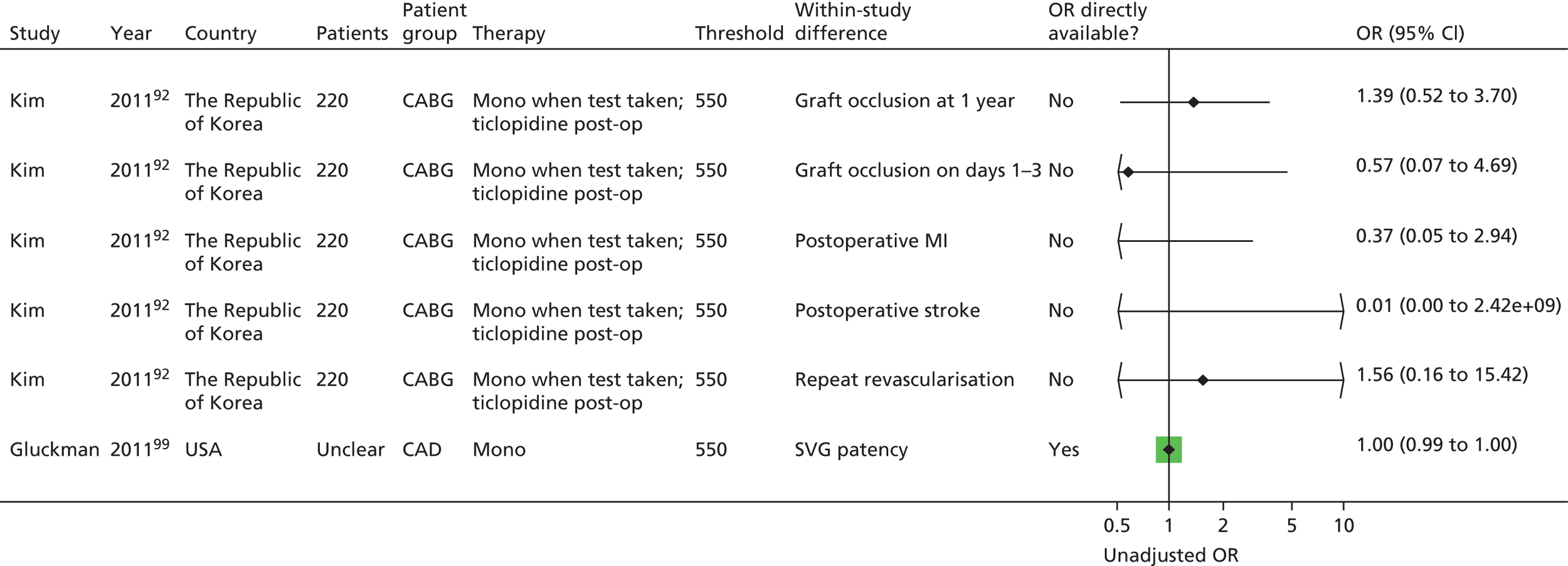
FIGURE 19.
VerifyNow® Aspirin, monotherapy: ischaemic/thrombotic events, unadjusted HRs.

The study by Gluckman et al. 99 also reported mean [standard deviation (SD)] values of aspirin reaction units (ARUs) for groups with one or more occluded saphenous vein grafts (SVGs) versus the group with no occluded SVG, and also for patients undergoing CABG. The mean values in the group with occluded SVGs were slightly higher (indicating greater platelet reactivity), but there were no significant differences and all means were below a threshold of 550 ARUs. No adjusted statistics were reported for any studies.
Thus, there is no evidence of a greater number of events in one or the other group in the two studies with CABG populations,92,99 while the only study113 with the CAD population found significant differences for two outcomes.
Bleeding events
Two studies92,171 reported this outcome (Table 25).
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Lee 2010171 | ✓a | ✓a | 0 events, therefore not calculable | |||
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| Kim 201192 | ✓a | ✓a | ✓ | |||
Only two92,171 out of seven studies reported bleeding events; these were postoperative in one study92 and over a 4-week period in ischaemic stroke patients [randomised to aspirin and placebo or aspirin and cilostazol (Pletal®, Otsuka) in the other study]. 171 The study by Kim et al. 92 also measured postoperative blood loss and transfused units of blood; there were no significant differences between the aspirin-resistant and sensitive groups. Too few events occurred to draw any overall conclusions: none in Lee et al. ,171 and two (re-exploration for bleeding) in Kim et al. 92 (Figures 20 and 21). No studies using VerifyNow® Aspirin as a PFT were identified that measured long-term adverse bleeding events.
FIGURE 20.
VerifyNow® Aspirin, monotherapy: bleeding events, unadjusted ORs. ITT, intention to treat; PP, per protocol.
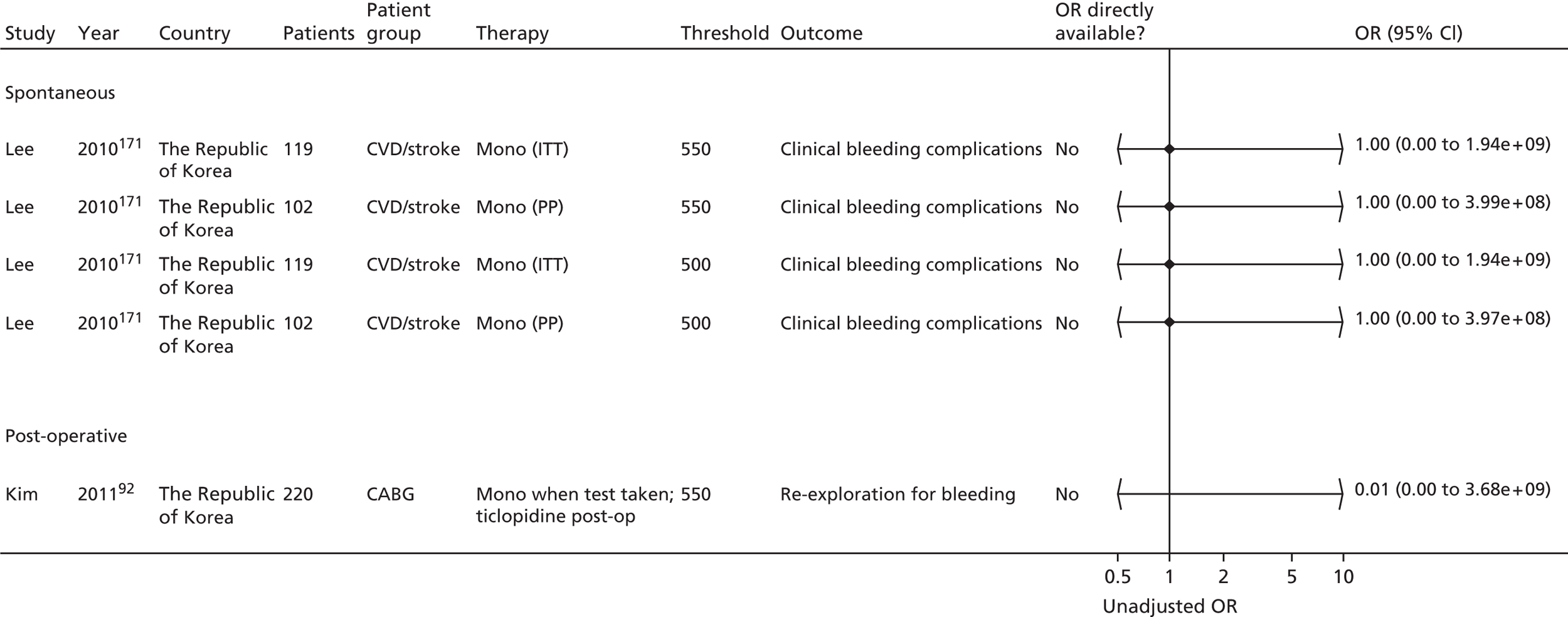
FIGURE 21.
VerifyNow® Aspirin, monotherapy bleeding events, unadjusted HRs. ITT, intention to treat.

Summary: VerifyNow® Aspirin
Seven studies86,92,99,105,133,162,171 were identified in this category, most in stable populations, but one in patients with UA/ACS105 and one92 in patients with severe CAD undergoing CABG.
There was a lack of reporting of quality criteria and no study reported all details considered to be important to assess risk of bias. No study reported on blinding to patient characteristics (when undertaking the PFT). Only one study171 gave details on the level of compliance and exclusions on the basis of this. Four99,105,133,171 of the seven studies gave details of missing data and four86,99,105,133 gave details of blinding of outcome assessors.
The risk of death in the resistant and sensitive groups was reported in only two studies. 86,92 In one of these,92 only one death occurred. The other study found statistically significant results based on unadjusted and adjusted ORs, and unadjusted HR (more events in the resistant group); this was based on 43 events in 106 patients.
Major adverse cardiac events were reported in four studies. 92,105,133,162 The direction of effect was consistent across all results (more events in the resistant group). Around half of the unadjusted ORs and unadjusted HRs were statistically significant, but it should be noted that a single study105 contributed to a large proportion of these results as several subgroup results were presented. Adjusted HRs based on two studies105,133 were also statistically significant.
Ischaemic/thrombotic events were reported in three studies;92,99,133 most unadjusted outcome measures were statistically non-significant, though there was a trend towards more events in resistant groups. There were no adjusted outcome measures.
Two studies92,171 measured (short-term) bleeding events (postoperative or re-exploration for bleeding). There were only two events in total and no conclusion can be drawn from the data.
Despite the heterogeneity and lack of many statistically significant results, the direction of prognostic effect appears to be largely consistent with more events in aspirin-resistant patients (ORs and HRs usually > 1). This suggests that VerifyNow® Aspirin is a potential prognostic factor, but this is only a qualitative judgement on the evidence available; meta-analysis was not possible owing to the heterogeneity, and therefore a firm quantitative conclusion regarding whether or not VerifyNow® Aspirin is prognostic is not currently possible.
Summary: VerifyNow® Aspirin
-
Seven studies used this commercial PFT.
-
A lack of reporting of quality criteria hampered an overall assessment of risk of bias; only one of seven studies gave details on level of compliance.
-
Heterogeneity in outcomes, patient groups and types of reported statistics meant that meta-analysis was not considered appropriate.
-
Adjusted results were rarely presented, and thus the additional prognostic value of the test over other prognostic factors is difficult to ascertain.
-
There was a consistent trend towards a greater number of events in the resistant groups within the studies; some of the results were statistically significant.
-
Some studies contributed more results by reporting on several subgroups and not all studies contributed to all outcome measures; therefore, there are potentially some missing data and/or a bias towards certain studies (though results were not pooled).
-
No studies were identified that reported on long-term bleeding events.
Thromboxane metabolite measurement
Population and test characteristics
Eleven studies46,76,99,108,110,148,151,162,164,195,202 were identified in this category, three of which were reported in abstract form only. 162,164,202 Populations had CAD (nine studies46,76,99,108,110,162,164,195,202) or CVD disease/stroke (one study148). One study151 included patients with various conditions including CAD, stroke, PVD and diabetes.
Most studies did not report for how long patients had had their primary underlying condition. One study117 reported that patients had their primary underlying condition for a mean period of 41.4 months.
In nine studies46,99,108,110,148,151,162,164,202 it appeared that patients were exclusively on monotherapy both at the time of the PFT and during follow-up. In two studies, patients were on monotherapy at the time of the PFT, and 32%76 and 54.8%195 of patients respectively went on to additionally receive clopidogrel at some point during follow-up. It is possible that not all studies have reported where a proportion of patients commenced additional therapies during follow-up.
Three studies measured thromboxane metabolite levels in serum/plasma46,76,164 and nine studies measured thromboxane metabolite levels in urine. 99,108,110,148,151,162,164,195,202 Data in these groups were analysed separately.
Comedications were reported in five studies108,148,162,164,202 and included ACE inhibitors, angiotensin II antagonists, calcium blockers, statins, beta-blockers, COX-2 antagonists, heparin, warfarin, diuretics, insulin, oral hypoglycaemics, antidepressants, anticoagulants, lipid-lowering agents and vitamin E. NSAIDs were not permitted (or had to be discontinued within a certain time period) in two studies. 108,110 One study99 stated that ‘concurrent nonsteroidal anti-inflammatory drug use did not correlate with the presence of aspirin non-responsiveness defined by this method at either time point’. In two studies, 10%76 and 24%195 of patients respectively were taking NSAIDs. There were no details on NSAIDs in the remaining studies.
The number of participants in the studies ranged from 61 to 3261 (see Table 26). Where reported, average ages of patients ranged from 53 years (mean value) to 69 years (median value), with most average ages around the early 60s. There were more men than women in the eight studies that reported this46,76,99,108,110,148,151,195 (the remaining three studies162,164,202 did not report details), with proportions of men ranging from 59% to 90%. The proportion of patients with diabetes ranged from 19% to 48%, and that of smokers from 16.6% to 71% (where reported, see Table 26). All studies were conducted in hospital settings.
The dose of aspirin ranged between 75 mg/day and 325 mg/day, with the exception of one study,148 where the dose was high at 650 mg/day. This study included patients with a non-cardioembolic, non-incapacitating cerebral infarction. There were no details on dose in one study. 151 Details were variable across studies regarding the length of time patients had been receiving aspirin therapy, with some noting a minimum period and some giving no details (see Table 26). Two studies stated aspirin was provided in enteric or plain form99,110 and no other studies provided this information.
The main study characteristics are listed in Table 26. Note that in some studies baseline characteristics have been reported only according to groups with/without adverse clinical events, or groups with occluded or patent SVG during CABG surgery, rather than for the total study population.
| Study/country | Number of patients | Age (years) | Therapy | Main underlying condition | Selected other population details | Due to undergo vascular intervention? | Aspirin dose/frequency | Duration of aspirin therapy (prior to PFT) | Percentage aspirin resistant | Derivation of threshold/comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Serum/plasma and urinary | ||||||||||
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Miyata 2011,164 Japan (abstract) | 592 (583 eligible for analyses) |
No details | Mono | CAD | No details | No | Some higher doses (> 100 mg/day), some lower doses (≤ 100 mg/day) | No details | No details | No details |
| Serum/plasma (metabolite used: TxB2) | ||||||||||
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Cotter 2004,46 Israel | 73 (61 eligible for analyses) |
Mean 53 (SD 8) | Mono | CAD | Smokers: 23% Diabetes: 19% |
No | 100 mg/day | At least 1 month | 14.8 | 5 nmol/1011 platelets (lowest TxB2 production observed in healthy volunteers) |
| Frelinger 2009,76 USA | 700 (555 eligible for analyses) |
Mean 60.7 (SEM 0.44) | Mono (32% dual during follow-up) | CAD | Smokers: 22% current smokers (72% prior smokers) Diabetes: 27% |
No | 81 or 325 mg/day | At least 3 days | 8.1 | Resistant if ≤ 3.1 ng/ml |
| Urinary (metabolite used: 11-dehydro-TxB2) | ||||||||||
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Addad 2010,108 Tunisia | 204 | Mean 59 (SD 10) | Mono | CAD | Smokers: n = 145 (71%) Diabetes: n = 79 (38.7%) |
No | 250 mg/day | At least 2 months on enrolment | No details | No details |
| Bruno 2004,148 USA | 61 (83 in total because 22 patients on ticlopidine; demographics based on 61 patients) |
Mean 61 (SD 11) | Mono | CVD/stroke | Smokers: n = 37 (45%) Diabetes: n = 40 (48%) |
No | 650 mg/day | At least 7 days | No details | No details |
| Eikelboom 2002,151 Australia | 488 (976 enrolled: 488 cases, 488 controls) |
Mean 67.3 (SD 7.2) | Mono | Miscellaneous | Smokers: n = 81 (16.6%) Diabetes: n = 159 (32.6%) |
No | No details | No details | No details | No details |
| Eikelboom 2008,195 Australia | 3261 | With an event (stroke, MI or cardiovascular death; n = 144): median 69 Without an event (n = 3117): median 64 |
Mono (54.8% dual during follow-up) | CAD | Current smokers: With an event: n = 26 (18.1%) Without an event: n = 632 (20.3%) Former smokers: With an event: n = 76 (52.8%) Without an event: n = 1547 (49.6%) Diabetes: With an event: n = 73 (50.7%) Without an event: n = 1381 (44.3%) |
No | 81 mg/day (median) Range 75–162 mg/day |
At least one month | No details | No details |
| Eskandarian 2011,202 Iran (abstract) | 124 | No details | Mono | CAD | No details | No | 80 mg/day | At least 7 days before test | 65.3 | Resistant if > 298 pg/mg Intermediate response 134–298 pg/mg Sensitive if < 134 pg/mg |
| Gluckman 2011,99 USA | 229 | For patients with ≥ 1 occluded SVG (n = 70): mean 63 (range 55–72) For patients with patent SVG (n = 159): mean 63 (range 57–71) |
Mono | CAD | Smokers: n = 52 (22.7%) Diabetes: n = 84 (36.7%) |
Yes: CABG | 325 mg/day | No details | No details | Aspirin responsive if TxB2 < 400 pg/mg creatinine |
| Lordkipanidzé 2011,162 UK (abstract) | 198 | No details | Mono | CAD | No details | No | 80–325 mg/day | No details | No details | No details |
| Thomson 2009,110 India | 63 | Mean 57 (SD 10) | Mono | CAD | Smokers: n = 43 (73%) Diabetes: n = 22 (34.9%) |
No | 75 mg/day | At least 7 days | 38.1 | Aspirin resistance defined as normalised urinary TxB2 level ≥ 67.9 ng/mmol creatinine |
Most studies reported no details on the timing of the PFT after aspirin ingestion. One study148 stated that there were up to 24 hours between aspirin dose and PFT. Table 27 shows details of test characteristics.
| Study | Details of kit/manufacturer | Anticoagulant (concentration) | Agonist (concentration) | Time since last aspirin dose |
|---|---|---|---|---|
| Serum/plasma and urinary | ||||
| Monotherapy at time of PFT and during follow-up | ||||
| Miyata 2011,164 (abstract) | Serum TxB2 Urinary 11-dehydro-TxB2 |
No details | Serum TxB2 Urinary 11-dehydro-TxB2 |
No details |
| Serum | ||||
| Monotherapy at time of PFT and during follow-up | ||||
| Cotter 200446 | TxB2 plasma (enzyme immunoassay kit obtained from Amersham, Buckinghamshire, UK) | No details | Collagen (1 µmol) | Aspirin administered on enrolment |
| Frelinger 200976 | Serum TxB2 | No details | No details | No details |
| Urinary | ||||
| Monotherapy at time of PFT and during follow-up | ||||
| Addad 2010108 | Urinary 11-dehydro-TxB2 | Enzyme-linked immunoassay | Enzyme-linked immunoassay | No details |
| Bruno 2004148 | Urinary 11-dehydro-TxB2 | No details | No details | Up to 24 hours |
| Eikelboom 2002151 | Urinary 11-dehydro-TxB2 | Enzyme immunoassay (Cayman Chemical, Ann Arbor, MI, USA) | Enzyme immunoassay (Cayman Chemical, Ann Arbor, MI, USA) | No details |
| Eikelboom 2008195 | Urinary 11-dehydro-TxB2 | No details | No details | No details |
| Eskandarian 2011202 | Urinary 11-dehydro-TxB2 | No details | No details | No details |
| Gluckman 201199 | Urinary 11-dehydro-TxB2 | No details | No details | No details |
| Lordkipanidzé 2011162 (abstract) | Urinary 11-dehydro-TxB2 | No details | No details | No details |
| Thomson 2009110 | Urinary 11-dehydro-TxB2 | No details | No details | No details |
Study design and quality
Results of the risk-of-bias assessment can be found in Tables 28–31.
| Domain 1: patient selection | Was a consecutive or random sample of patients enrolled? | Was patient selection independent of patient outcomes? | Were reasons for any posteligibility exclusions provided? |
|---|---|---|---|
| Serum/plasma and urinary | |||
| Miyata 2011164 (abstract) | Consecutive | Yes | No details |
| Serum/plasma | |||
| Cotter 200446 | Consecutive | Yes | 76/82 potentially eligible patients agreed to be interviewed; 73/76 who were on aspirin for at least 1 month were enrolled |
| Frelinger 200976 | Consecutive | Yes | Stated that less than 3% of eligible patients declined participation (reason not given) |
| Urinary | |||
| Addad 2010108 | Consecutive | Yes | No details |
| Bruno 2004148 | Unclear: consecutive patients screened for participation in the trial | Yes | 98 patients initially gave signed consent; 8/98 withdrew consent and 7/98 did not provide a urine sample |
| Eikelboom 2002151 | Control subjects randomly selected | No | 9541 patients in HOPE study; 9282 provided urine samples, samples from 5529 (Canadian centres only) sent to laboratory. Of those, only those who were taking aspirin before and at randomisation, and at each follow-up visit, were eligible for inclusion (number not stated). 488 cases and controls selected from the eligible/included |
| Eikelboom 2008195 | Unclear (patients who complied with a request to provide a sample) | Yes | No details |
| Eskandarian 2011202 (abstract) | No details | Yes | No details |
| Gluckman 201199 | No details | Yes | Patients for whom SVG patency not assessed or those not on aspirin monotherapy. Authors stated that the study population was representative of patients undergoing isolated CABG surgery based on comparison with the Society of Thoracic Surgeons National Database |
| Lordkipanidzé 2011162 (abstract) | No details | Yes | No details |
| Thomson 2012110 | Consecutive | Yes | No details |
| Domain 2: PFT | If a threshold was used, was it prespecified? | How was the threshold derived (e.g. literature cut-off, based on study data)? | Is the undertaking and interpretation of the index test blinded to the patient characteristics (including clinical outcomes)? |
|---|---|---|---|
| Serum/plasma and urinary | |||
| Miyata 2011164 (abstract) | No details | No details | No details |
| Serum/plasma | |||
| Cotter 200446 | Yes (5 nmol/1011 platelets) | Patients classified as nonresponsive: results in the range observed in volunteers not taking aspirin Patients classified as responsive: results in ranges that are observed in takers Cut-off: lowest TxB2 production value that was observed in non-takers |
No details |
| Frelinger 200976 | No (ROC analysis) | ROC analysis of serum TxB2 levels in current study with regard to MACE (resistant if ≤ 3.1 ng/ml) | No details |
| Urinary | |||
| Addad 2010108 | No (tertiles) | Tertiles | Yes; stated that all assays were performed in a blinded manner |
| Bruno 2004148 | No (median values for patients with and without events presented) | N/A | Possible; no details, but laboratory off-site |
| Eikelboom 2002151 | No (mean/median values for patients with and without events presented) | N/A | Assays were performed by laboratory staff blinded to patient status (case or control) and also assayed in random order |
| Eikelboom 2008195 | No (quartiles) | Quartiles | Yes; ‘Laboratory staff performing the assays were blinded to treatment allocation and to whether the patients had experienced a primary event’ |
| Eskandarian 2011202 (abstract) | Yes (three groups: resistant > 298 pg/mg, intermediate response 134–298 pg/mg, sensitive < 134 pg/mg) | No details | No details |
| Gluckman 201199 | Yes (aspirin responsive if < 400 pg/mg creatinine; but a threshold of 450 pg/mg creatinine used in model) | ‘According to established criteria’ (reference cited221) | No details |
| Lordkipanidzé 2011162 (abstract) | No details | No details | No details |
| Thomson 2012110 | No (population median value) | Median value of absolute urinary 11-dehydro-TxB2 level of 320 pg/ml used as cut-off in relation to clinical outcomes | No details |
| Domains 3 and 4: outcomes and study attrition | Were the outcomes of interest clearly defined in advance? | Were the outcome results interpreted without knowledge of the results of the PFT? | What was the proportion of missing data? (State reasons for loss to follow-up or differences in those who completed or were lost) |
|---|---|---|---|
| Serum/plasma and urinary | |||
| Miyata 2011164 (abstract) | Yes | No details | No details |
| Serum/plasma | |||
| Cotter 200446 | Yes | No details | Appears that there was no loss to follow-up |
| Frelinger 200976 | Yes | Yes; all clinical outcome data obtained by research personnel blinded to results of PFTs | 127/682 lost to follow-up (for MACE outcome) |
| Urinary | |||
| Addad 2010108 | Yes | Yes; follow-up clinicians were blinded to PFT results | Stated that none of the included patients was lost to follow-up |
| Bruno 2004148 | Yes | Yes; assay results not revealed to investigators until after follow-up examinations and vascular event determinations | Appears that there was no loss to follow-up |
| Eikelboom 2002151 | Yes | Yes (outcome occurred before analysis of sample) | None; retrospective [patients who had a confirmed event were defined as cases and controls were randomly selected from among those with no events (sex and age matched)] |
| Eikelboom 2008195 | Yes | No details | Appears that there was no loss to follow-up |
| Eskandarian 2011202 (abstract) | Yes | No details | No details |
| Gluckman 201199 | Yes | Yes; stated that images were analysed by two blinded reviewers (98% concordance) with a third reviewer adjudicating as necessary | 10/229 not included at 6 months |
| Lordkipanidzé 2011162 (abstract) | Yes | No details | No details |
| Thomson 2012110 | Yes | No details | 11/63 lost to follow-up, unclear if excluded from analysis |
| Domain 5: confounding | Are confounders accounted for in the design or analysis (e.g. adjustment, stratification)? | If there is an adjusted outcome measure (e.g. OR, HR), what were the factors that were adjusted for? | If a HR was presented, was the proportional hazards assumption met? | Was compliance measured? | How was compliance measured? | Level of compliance |
|---|---|---|---|---|---|---|
| Serum/plasma and urinary | ||||||
| Miyata 2011164 (abstract) | Design: N/A Analysis: appears that adjustment for possible confounders was undertaken, but no adjusted measures presented |
No details | N/A | Yes | Interview and by checking plasma concentration of salicylic acid | No details |
| Serum/plasma | ||||||
| Cotter 200446 | Design: N/A Analysis: no |
N/A | N/A | Yes | Interview and corroboration with another adult (usually spouse) | 12/21 patients (out of a total of 73) whose response was classified as non-responsive stated that they were not taking the medication, the other nine claimed they were |
| Frelinger 200976 | Design: N/A Analysis: yes (OR, HR) |
Sex, BMI, TIMI score, aspirin dose, platelet count, use of clopidogrel, statins or oral hypoglycaemic agents | Yes (the assumption of proportionality was tested and found to be valid) | Not specifically | By TxB2 levels | ‘Two patients had serum TxB2 levels in the range observed for aspirin-free healthy controls, and their platelet function was therefore consistent with aspirin noncompliance. Because “resistance” cannot be distinguished from noncompliance, these subjects were not excluded from follow-up’ |
| Urinary | ||||||
| Addad 2010108 | Design: N/A Analysis: no |
N/A | N/A | Yes | Interview at study enrolment and during follow-up period | No details |
| Bruno 2004148 | Design: N/A Analysis: yes (HR) |
Age, sex, hypertension, diabetes, smoking, ischaemic heart disease, prior stroke, hypercholesterolaemia, mean 11-dehydro-TxB2 level | No details | No details | N/A | N/A |
| Eikelboom 2002151 | Design: N/A Analysis: yes (OR) |
Included conventional vascular risk factors, co-interventions and randomised treatment allocation (full details not given) | N/A | Yes | Assessed compliance with aspirin therapy at each follow-up visit and only considered for inclusion patients who were taking aspirin before randomisation and at 6-month follow-up visits. Patients who discontinued aspirin at any time during the study were not included | No details |
| Eikelboom 2008195 | Design: N/A Analysis: yes (HR) |
Subsets of (depending on model): age, sex, BMI, current smoking, hypertension, hypercholesterolaemia, diabetes, past history of MI, stroke, TIA, peripheral artery disease, PCI, CABG, endarterectomy, peripheral angioplasty/bypass, aspirin dose, study clopidogrel, NSAIDs, statins, beta-blockers, diuretics, calcium channel blockers, ACE inhibitors, other blood pressure-lowering agents, oral hypoglycaemic agents, insulin, time of urine collection | No details | Yes | The use of medications, including aspirin dose, was recorded at each follow-up | States in the discussion that non-compliance with aspirin therapy could account for variability in thromboxane concentrations, but that ‘the present data show that factors not related to compliance with aspirin therapy, including age and sex, as well as use of several concomitant therapies, independently determine urinary 11-dehydro thromboxane B2 concentrations’ |
| Eskandarian 2011202 (abstract) | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Gluckman 201199 | Design: N/A Analysis: no |
N/A | N/A | Yes | Pill counts at each postoperative encounter | No details |
| Lordkipanidzé 2011162 (abstract) | Design: N/A Analysis: unclear whether adjusted or unadjusted OR |
No details | N/A | No details | N/A | N/A |
| Thomson 2012110 | Design: N/A Analysis: no |
N/A | N/A | No; ‘Aspirin therapy was not supervised and compliance was not verified by assaying’ | N/A | N/A |
Patient selection was independent of study outcome in 10 of the included studies,46,76,99,108,110,148,162,164,195,202 with the PFT preceding any outcomes (as specified in the study selection criteria). One study151 used a case–control design, so patient selection was not independent of outcome, but the taking of samples for the PFT still preceded the outcomes and so this study was included. Five of 11 studies stated that consecutive patients were enrolled into the study. 46,76,108,110,164 Details on posteligibility exclusion of patients were provided in five studies;46,76,99,148,151 reasons included compliance with aspirin treatment at each follow-up visit,151 patients in whom the outcome was not assessed99 or no provision of urine sample. 148
A predefined threshold was stated in only three studies46,99,202 and this was not consistent across the studies (cut-offs of 298 pg/mg creatinine,202 400 pg/mg creatinine99 and 5 nmol/1011 platelets46). The remaining studies used tertiles,108 quartiles,195 median value,110 derived the value by receiver operating characteristic (ROC) analysis,76 presented mean values for groups with and without events148,151 or gave no details. 162,164 Three studies108,151,195 gave clear details of blinding of laboratory staff to patient characteristics.
Outcome measures of interest were clearly predefined in all studies, and five studies76,99,108,148,151 had details of blinded assessment of outcomes. Five studies appeared to have no loss to follow-up46,108,148,151,195 and there were no details in three studies. 162,164,202 In the remaining three studies the loss to follow-up was 4%,99 17%110 and 19%. 76
Compliance was assessed in six studies. 46,99,108,151,164,195 Methods included interview, plasma concentration of salicylates and pill counts. In one study,46 patients who stated that they were not taking the prescribed aspirin were included as a separate subgroup in the analysis (resistant and non-compliant). Another study76 did not exclude patients as ‘resistance cannot be distinguished from non-compliance’.
Three studies76,148,151 undertook adjusted analyses, with some overlap between the adjustment factors where stated.
Overview of outcomes
Eleven studies were identified; three of these46,76,164 undertook thromboxane measurement in serum/plasma, and nine studies99,108,110,148,151,162,164,195,202 measured thromboxane in urine (one study164 in both categories) (Table 32).
| Study | Death | MACE | Ischaemic/thrombotic | Bleeding | Length of follow-up |
|---|---|---|---|---|---|
| Serum/plasma and urinary | |||||
| Miyata 2011164 (abstract) | ✓ | 2 years | |||
| Serum/plasma | |||||
| Cotter 200446 | ✓ | ✓ | 12 months | ||
| Frelinger 200976 | ✓ | ✓ | Mean 24.8 (SD 0.3) months | ||
| Urinary | |||||
| Addad 2010108 | ✓ | 1 year | |||
| Bruno 2004148 | ✓ | Mean 2 months (no SD) | |||
| Eikelboom 2002151 | ✓ | ✓ | ✓ | 5 years | |
| Eikelboom 2008195 | ✓ | ✓ | ✓ | ✓ | Median 28 months (no SD) |
| Eskandarian 2011202 (abstract) | ✓ | 1 year | |||
| Gluckman 201199 | ✓ | 6 months | |||
| Lordkipanidzé 2011162 (abstract) | ✓ | 3 years | |||
| Thomson 2012110 | ✓ | Median 36 (range 1–53) months | |||
Death
Death was reported in only 376,151,195 of 11 studies (Table 33). Outcome statistics are shown in Figures 22–25. Unadjusted ORs and HRs were calculable from Frelinger et al. 76 (measurement in serum/plasma), all of which showed a trend towards more events in the resistant arm, though none were statistically significant. The other two studies (measurement in urine) reported adjusted ORs151 and adjusted HRs. 195 Again, all reflected a greater number of events in the resistant arm; two of the adjusted ORs were statistically significant. Note that this is based on comparison of different quartiles rather than using a single cut-off. Overall, the trend was consistent (more events in the resistant arm), but based on few studies.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Serum/plasma | ||||||
| Frelinger 200976 | ✓a | ✓a | ✓a | |||
| Urinary | ||||||
| Eikelboom 2002151 | ✓ | |||||
| Eikelboom 2008195 | ✓ | |||||
FIGURE 22.
Thromboxane, monotherapy: death, unadjusted ORs.
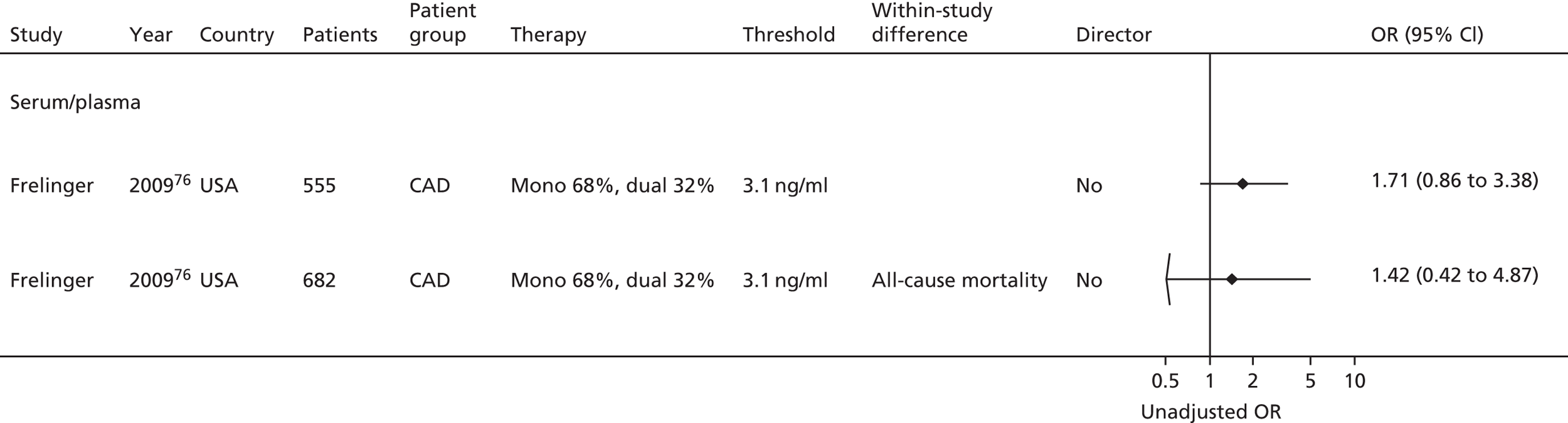
FIGURE 23.
Thromboxane metabolite measurement, monotherapy: death, adjusted ORs. Q, quartile.

FIGURE 24.
Thromboxane metabolite measurement, monotherapy: death, unadjusted HRs.

FIGURE 25.
Thromboxane metabolite measurement, monotherapy: death, adjusted HRs. Q, quartile.
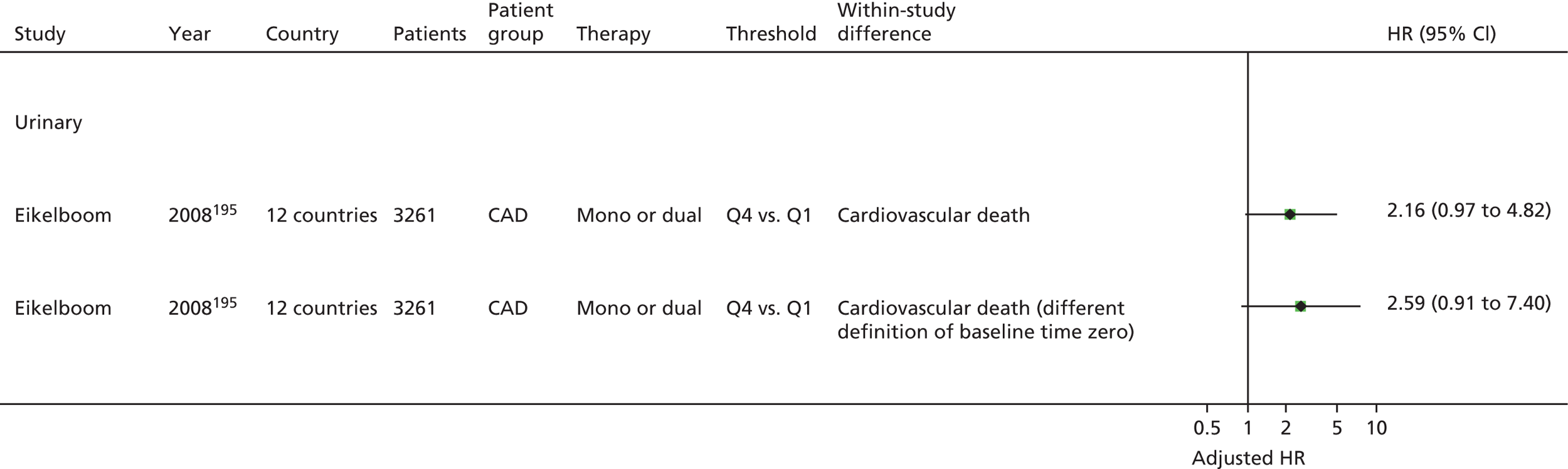
Major adverse cardiac events
Major adverse cardiac events were reported in nine studies (Table 34). 46,76,108,110,151,162,164,195,202 Outcome statistics are shown in Figures 26–29. For two studies,110,164 results could not be presented in forest plots: one164 stated that ‘no ex vivo measurements for residual platelet functions and COX activities were associated with cardiovascular events. Residual platelet functions correlated poorly with residual COX activities, and were inconsistent with assessments made 6 months later.’ The other110 found that a greater number of MACEs occurred in the upper two quartiles (higher urinary thromboxane levels) than in the lower two and that this difference was statistically significant (p = 0.04); however, the difference was not present when normalised levels of urinary thromboxane were considered.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Serum/plasma and urinary | ||||||
| Miyata 2011164 (abstract) | Narrative description of results only | |||||
| Serum/plasma | ||||||
| Cotter 200446 | ✓a | |||||
| Frelinger 200976 | ✓a | ✓ | ✓a | ✓ | ✓a | |
| Urinary | ||||||
| Addad 2010108 | ✓a | ✓a | ✓a | |||
| Eikelboom 2002151 | ✓ | |||||
| Eikelboom 2008195 | ✓ | ✓ | ||||
| Eskandarian 2011202 (abstract) | ✓a | ✓a | ✓a | |||
| Lordkipanidzé 2011162 (abstract) | ✓ | |||||
| Thomson 2012110 | Raw data not presented (only a p-value) | |||||
FIGURE 26.
Thromboxane metabolite measurement, monotherapy: MACEs, unadjusted ORs. RAVE, recurrent acute vascular event.
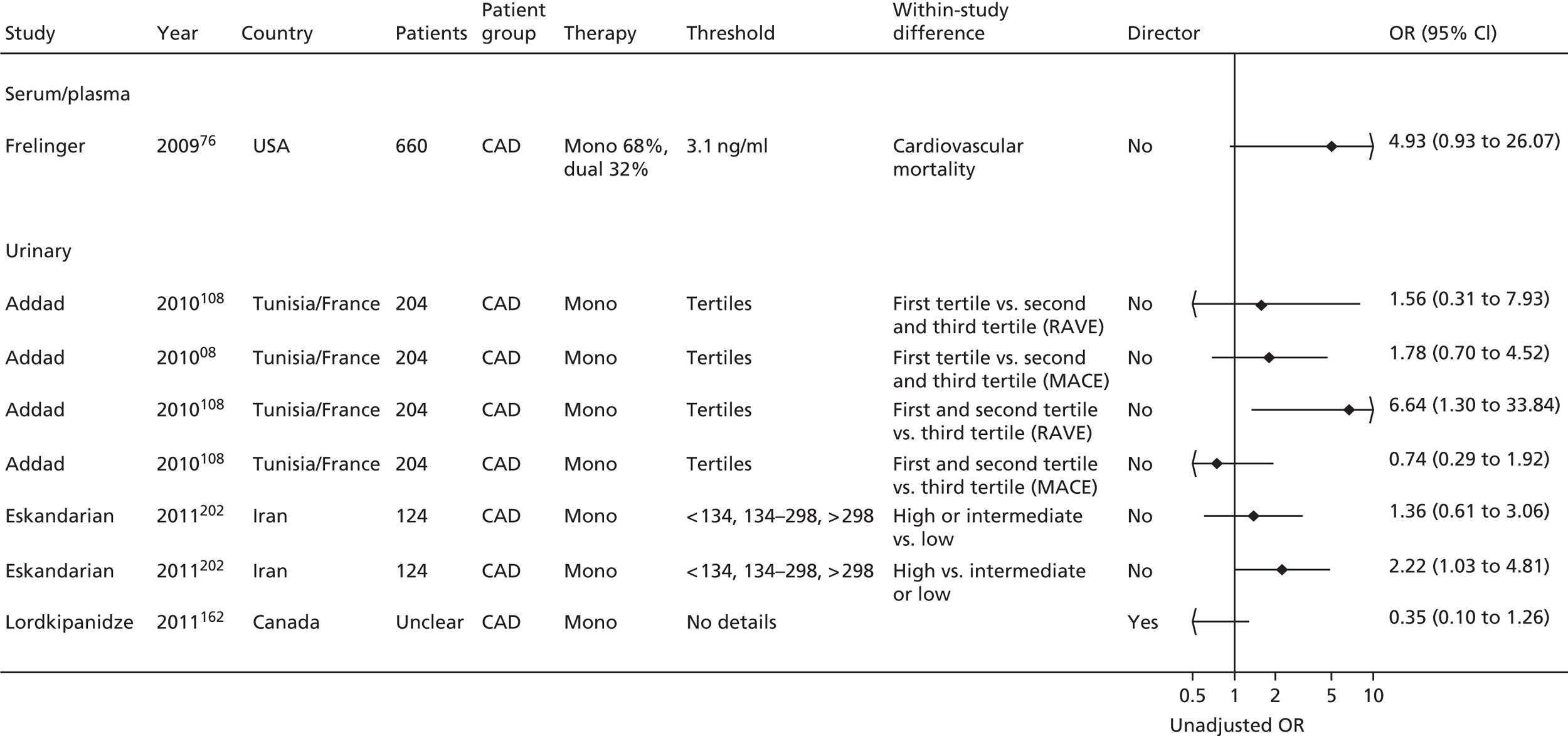
FIGURE 27.
Thromboxane metabolite measurement, monotherapy: MACEs, adjusted ORs. Q, quartile.
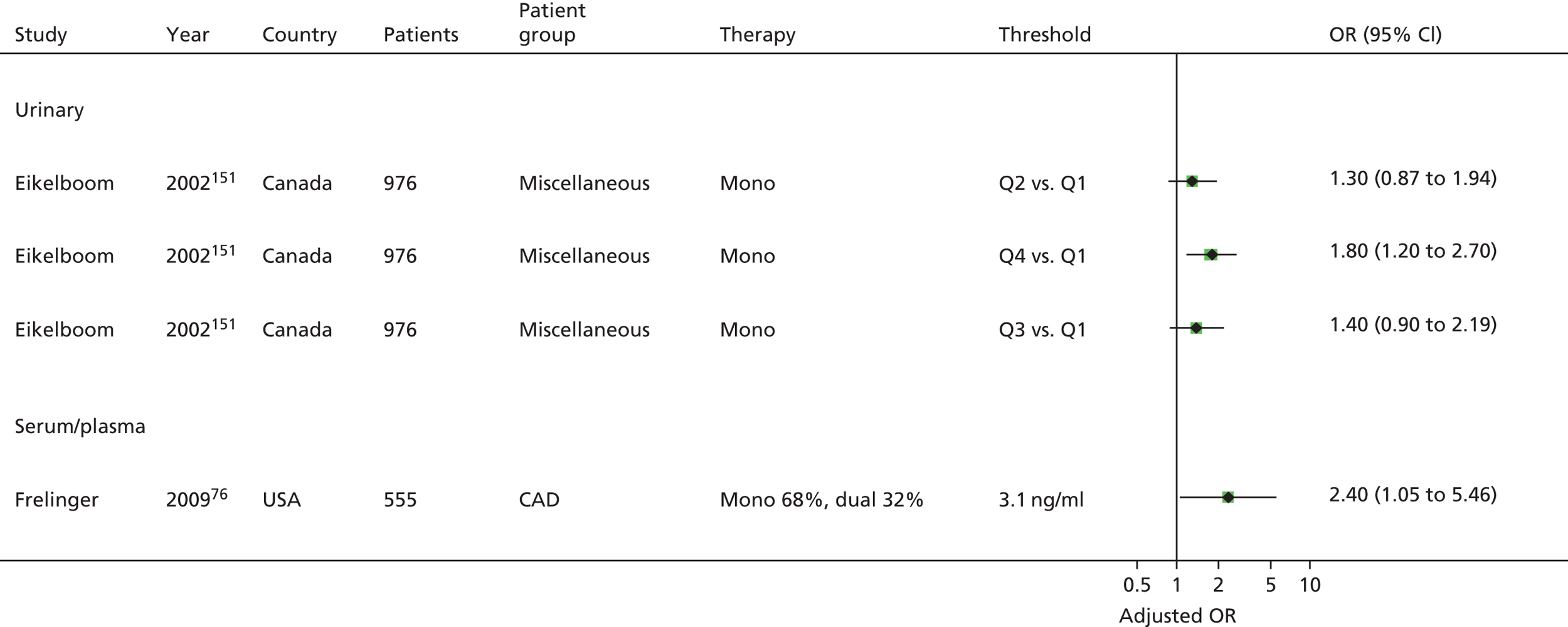
FIGURE 28.
Thromboxane metabolite measurement, monotherapy: MACEs, unadjusted HRs. RAVE, recurrent acute vascular event.
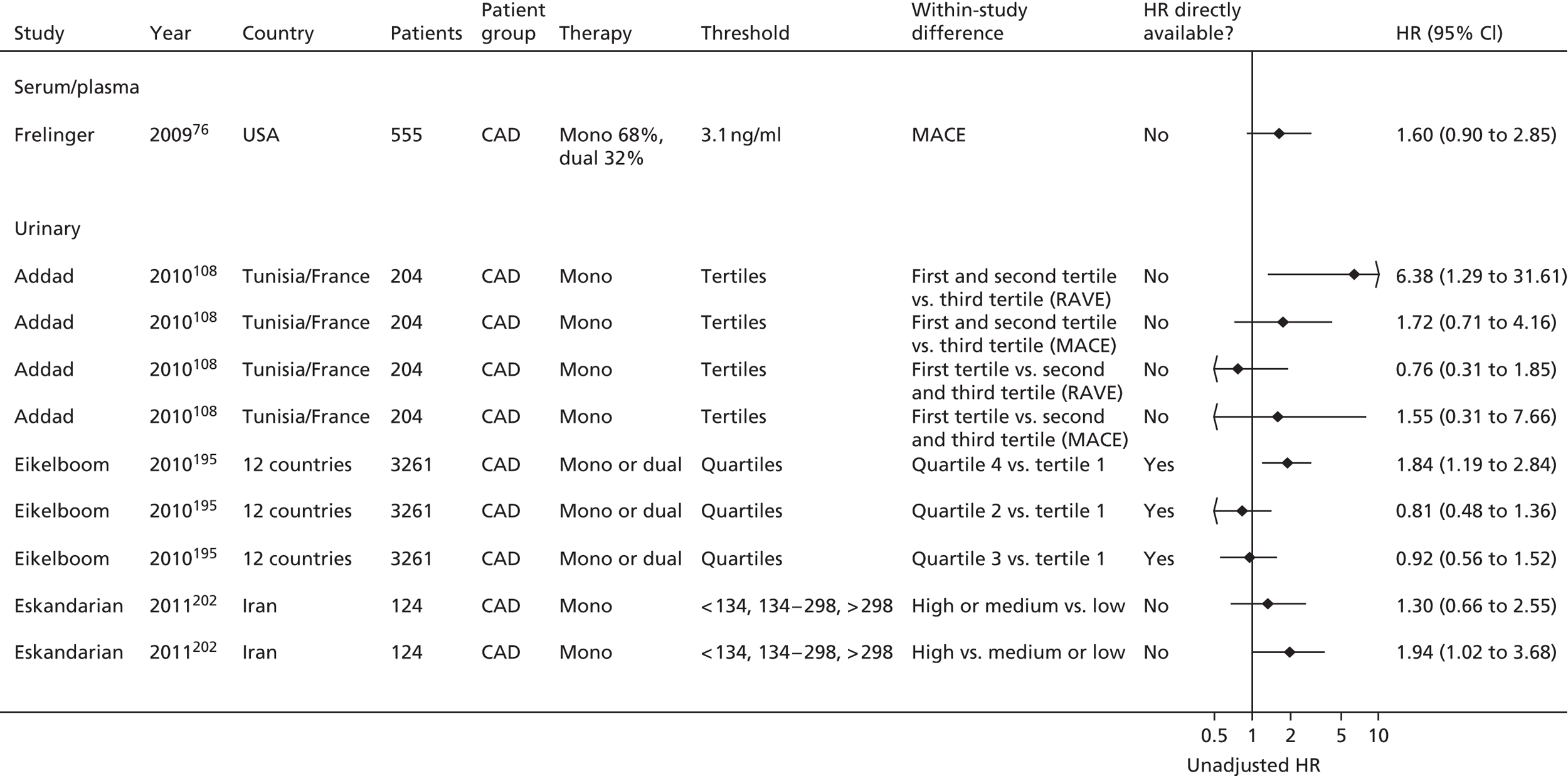
FIGURE 29.
Thromboxane metabolite measurement, monotherapy: MACEs, adjusted HRs. Q, quartile.

Eight unadjusted ORs were presented, based on four studies, three measuring thromboxane in urine108,162,202 and one in serum/plasma. 76 Six of the ORs reflected more events occurring in the resistant arm, but only two were statistically significant. Four adjusted ORs were presented, based on two studies. 76,151 The direction of effect was consistent and two were statistically significant (more events in the resistant arm).
There were 10 unadjusted HRs based on four studies. 76,108,195,202 Although overall results showed that there were more events in the resistant group, including the three statistically significant results, the direction of effect is not consistent within two studies contributing three195 and four108 unadjusted HRs each; this reflects the effect of using different cut-offs (in this case comparison of different tertiles or quartiles).
There were 12 adjusted HRs based on two studies,76,195 with 11 being based on only one study,195 reflecting the use of different models (adjustment factors) and the comparison of different quartiles rather than one cut-off. One study (serum/plasma)76 showed a statistically significant result (more events in the resistant group), whereas the direction of effect was evenly split in the study generating 11 outcome statistics.
Overall, there is a trend towards more MACEs in the resistant arm, but there is some uncertainty due to the relatively small number of studies contributing MACE results and the fact that there is some inconsistency within studies (depending on thresholds used). Only one study76 measuring thromboxane in serum/plasma was represented in the forest plots. It was therefore not possible to compare results between the two methods, though the direction of effect (more events in the resistant group) was consistent with the majority of results.
Ischaemic/thrombotic events
This outcome measure was reported in five studies (Table 35). 46,99,148,151,195 Outcome statistics are presented in Figures 30–33. Data from one study148 could not be represented in the forest plots. There was no significant difference in thromboxane levels in those with and without a vascular event.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Serum/plasma | ||||||
| Cotter 200446 | ✓a | ✓a | ✓a | |||
| Urinary | ||||||
| Bruno 2004148 | Median thromboxane levels compared in those with and without an event | |||||
| Eikelboom 2002151 | ✓ | |||||
| Eikelboom 2008195 | ✓ | |||||
| Gluckman 201199 | ✓ | ✓ | ||||
FIGURE 30.
Thromboxane metabolite measurement, monotherapy: ischaemic/thrombotic events, unadjusted ORs.

FIGURE 31.
Thromboxane metabolite measurement, monotherapy: ischaemic/thrombotic events, adjusted ORs. Q, quartile.
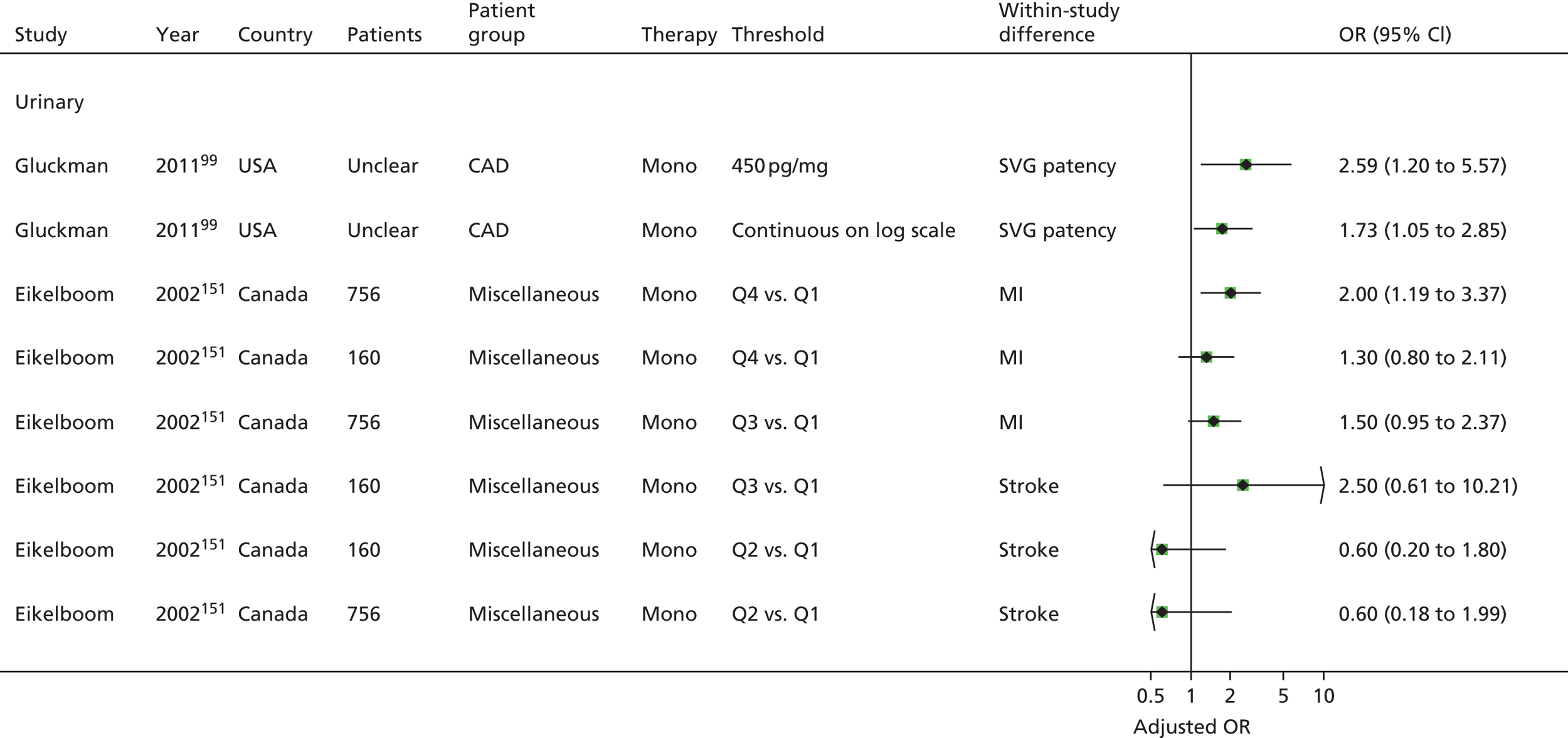
FIGURE 32.
Thromboxane metabolite measurement, monotherapy: ischaemic/thrombotic events, unadjusted HRs.
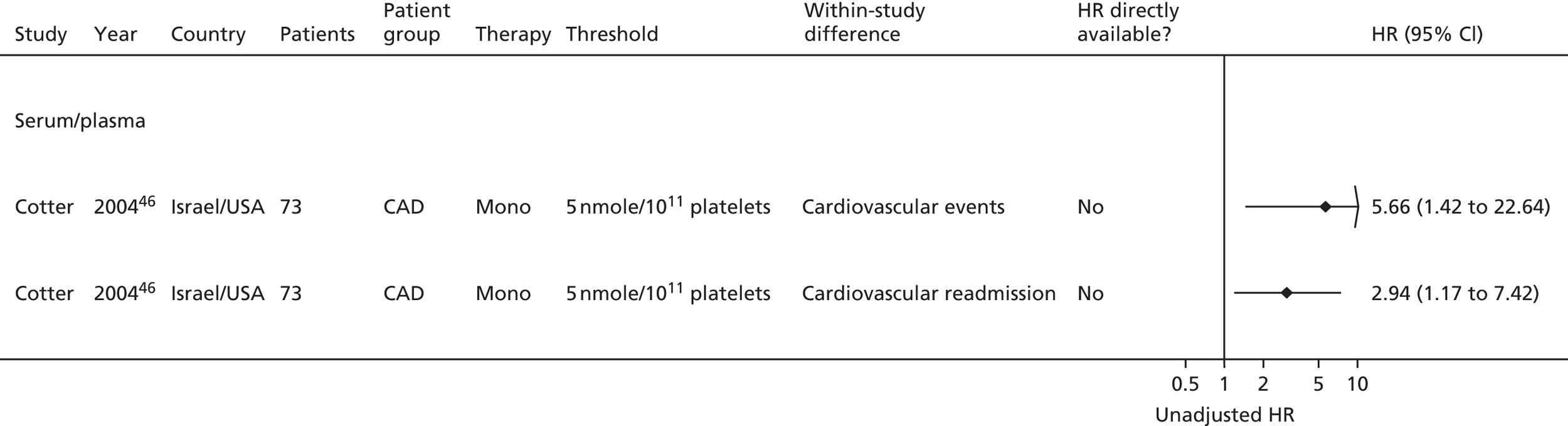
FIGURE 33.
Thromboxane metabolite measurement, monotherapy: ischaemic/thrombotic events, adjusted HRs. Q, quartile.

Unadjusted and adjusted ORs (based on three studies46,99,151) showed a consistent direction of effect (more events in the resistant group), with the exception of two of six results from one study (Eikelboom,151 adjusted ORs). All four unadjusted ORs and three of the eight adjusted ORs were statistically significant.
Two unadjusted HRs (based on one study46) were statistically significant (more events in the resistant groups), as was one of two adjusted HRs (based on a different study195).
Overall, the direction of effect is consistent (more events in the resistant group), but based on few studies. Note that one study151 contributes to six of eight adjusted ORs, and that the direction of effect is not consistent within this study (reflecting different outcomes and thresholds).
Bleeding events
One study reported this outcome (Table 36). 195
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Urinary | ||||||
| Eikelboom 2008195 | Trend across quartiles reported | |||||
Only one study195 using a thromboxane test reported bleeding events [Global Utilization of Streptokinase and tPA for Occulded Coronary Arteries (GUSTO) bleeds]. The study found no significant difference when looking at a trend for bleeding rates across quartiles.
Summary: thromboxane metabolite measurement
Eleven studies were identified in this category,46,76,99,108,110,148,151,162,164,195,202 all including stable disease populations, thus making this set of studies more homogenous in terms of population compared with studies reporting other PFTs. There was still heterogeneity, however, for example relating to specific patient characteristics and aspirin dose.
There was a lack of reporting of relevant quality criteria, making overall judgements about risk of bias difficult. No study provided details on all relevant quality criteria. Lack of details related in particular to whether or not assays were performed in a blinded manner and levels of compliance. In one study,46 patients who stated that they were not taking their prescribed aspirin were included as a separate subgroup in the analysis (both resistant and non-compliant, as opposed to resistant and compliant). In the analysis here, these groups have been merged in order to be consistent with the other studies, where it is not possible to make this distinction. There was a lack of consistency in defining thresholds, both in methods and in actual values. Only 346,99,202 of 11 studies gave a predefined threshold. One study151 used a retrospective case–control design, which is more prone to bias than prospective designs; however, as the sampling for the PFT preceded the outcomes, this study was included.
Overall, there was a consistent trend for more deaths reported in the resistant arm, with some statistically significant results, but this was based on only three studies. 76,151,195 There was also a trend for more events in the resistant groups for MACEs and ischaemic/thrombotic events, with some results showing statistical significance, but this is based on relatively few studies (no more than four studies contributed results to any one forest plot).
It is noteworthy that the direction of effect is not consistent within individual studies, reflecting different thresholds used and different outcomes (for ischaemic/thrombotic events). Some studies contributed considerably more to forest plots than others, for example where more outcomes were reported or where results could be presented for different thresholds.
Only one study195 reported bleeding events and this found no significant difference when looking at a trend for bleeding rates across quartiles.
It was not possible to assess any differences between tests measuring thromboxane in urine or serum/plasma, as only one study76 measuring thromboxane in serum/plasma was represented in the forest plots.
Summary: thromboxane
-
Eleven studies were identified, all with stable disease populations.
-
Methods for deriving thresholds and thresholds themselves were variable.
-
A lack of detail on reporting of quality criteria hampered an overall risk-of-bias assessment.
-
Heterogeneity in outcomes, test thresholds and types of reported statistics meant that meta-analysis was not considered appropriate.
-
Adjusted results were rarely presented, and thus the additional prognostic value of the test over other prognostic factors is difficult to ascertain.
-
Despite clinical heterogeneity between studies, there was a general trend for more events to occur in the ‘aspirin-resistant’ group for all relevant outcomes (death, MACEs, ischaemic/thrombotic events); however, this was often based on few studies and there was inconsistency in direction of effect within some studies (based on different thresholds or outcomes).
-
Only one study reported bleeding events and this found no significant differences when looking at a trend for bleeding rates.
-
Potential differences between measurements of thromboxane in urine or serum/plasma could not be assessed.
Platelet function analyser-100
Population and test characteristics
Twenty-one studies76,99,108,109,112,115,116,118,123,127,132,135,137,138,144,145,150,162,186,187,189 were identified in this category, of which two162,189 were reported in abstract form, and one123 in the form of a letter. Populations had CAD in 10 studies,76,99,108,112,118,127,137,144,145,162 CVD/stroke in two studies,116,186 UA/ACS in six studies109,115,132,135,138,187 and PAD/PVD in one study. 150 One large study (n = 600)189 had a mixed population (PVD, ACS and CVD/stroke) and in one further study123 all patients were undergoing PCI.
In one study, only patients with a first stroke were included. 186 In all other studies there were no details on how long patients had had their primary condition for.
In 19/21 studies, patients were on monotherapy at the time of the PFT and during follow-up. In the two other studies,115,138 patients were on dual therapy during follow-up, and in one,138 a small proportion (9%) were also on dual therapy (+ clopidogrel) at the time of the PFT.
Comedications across all studies included beta-blockers, lipid-lowering agents, anticoagulants, thrombolytic agents, ACE inhibitors, statins, heparin, COX-2 antagonists, warfarin, calcium channel blockers, diuretics, insulin, oral hypoglycaemics, antidepressants, cholesterol-lowering, antihypertensive and antidiabetic drugs, nitrate infusion and glycoprotein IIb/IIIa receptor agonists. Some studies restricted the use of some medications during a certain time period before the PFT.
Non-steroidal anti-inflammatory drugs were clearly not permitted in six studies,108,109,112,116,186,187 and in a further study137 they were not permitted during 7 days preceding the PFT.
The numbers of participants in the studies ranged from 51 to 700 (see Table 37). Where reported, mean ages ranged from 59 to 72 years, with more men than women in all studies (range 56–79%). The proportion of smokers ranged from 15% to 72% and that of diabetics from 7% to 49% (note that some proportions were presented according to resistant and sensitive groups). All studies were conducted in hospital settings.
The dose of aspirin ranged from 75 mg/day to 325 mg/day. Two studies123,189 provided no details on dose. Details were variable across studies regarding the length of time patients had been receiving aspirin therapy prior to the PFT; where reported, the time varied from a minimum of 3 days up to 2 months. One study144 stated that no patients were treated with antiplatelets for 10 days before undergoing CABG.
One study reported that aspirin was provided in enteric form. 99 There were no details in the other studies. The main study characteristics are reported in Table 37.
| Study/country | Number of patients | Age (years) | Therapy | Main underlying condition | Selected other population details | Due to undergo vascular intervention? | Aspirin dose/frequency | Duration of aspirin therapy (prior to PFT) | Percentage aspirin resistant | Derivation of threshold/comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Addad 2010,108 Tunisia | 204 | Mean 59 (SD 10) | Mono | CAD | Smokers: n = 145 (71%) Diabetes: n = 79 (38.7%) |
No | 250 mg/day | At least 2 months | No details | No details |
| Aksu 2009,109 Turkey | 240 (220 eligible for analyses; population characteristics based on 220) |
Aspirin resistant: Mean platelet volume < 8.4 fl (n = 44): mean 63.68 (SD 9.69) Mean platelet volume > 8.4 fl (n = 40): mean 62.85 (SD 12.29) Aspirin sensitive: Mean platelet volume < 8.4 fl (n = 70): mean 59.93 (SD 10.46) Mean platelet volume > 8.4 fl (n = 66): mean 57.42 (SD 11.97) |
Mono (27% dual at follow-up) | ACS | Smokers: n = 86 (39%) Diabetes: n = 107 (49%) |
No | 100 mg/day (38.2%), 300 mg/day (61.8%) | At least 7 days before hospital admission | 38.2 | Resistant if closure time ≤ 170 seconds |
| Bevilacqua 2009,118 Italy | 202 | Resistant: mean 66.7 (SD 9.6) Sensitive: mean 66.2 (SD 9.9) |
Mono | CAD | Smokers: Resistant: n = 42 (48.9%) Sensitive: n = 83 (71.6%) Diabetes: Resistant: n = 34 (39.5%) Sensitive: n = 31 (26.7%) |
Yes: CABG | 100 mg/day | At least 1 month | 42.6 | Resistant if closure time < 190 seconds |
| Boncoraglio 2009,116 Italy | 129 | Mean 59 (SD 13.9) | Mono | CVD/stroke | Smokers: n = 47 (36.4%) | No | 75–325 mg (72.1% were receiving < 160 mg/day) |
At least 4 weeks | 20.2 | Resistant if closure time < 165 seconds |
| Campo 2008,123 Italy (letter) | 135 (160 in total including 25 controls; population data based on 135 patients) |
Mean 65 (SD 12) | Mono (0.7% on dual at baseline, 15% on dual at follow-up) | PCI | Smokers: 33% Diabetes: 18% |
No | No details | Before admission 7.4% being treated with aspirin | No details | No details |
| Christiaens 2008,127 France | 97 | Mean 66 (SD 11) | Mono | CAD | Smokers: n = 15 (15%) Diabetes: n = 15 (15%) |
No | 160 mg/day | At least 1 month | 29.9 | Resistant if closure time < 187 seconds |
| Frelinger 2009,76 USA | 700 (555 eligible for analyses) |
Mean 60.7 (SEM 0.44) | Mono (32% dual during follow-up) | CAD | Smokers: 22% current (72% prior) Diabetes: 27% |
No | 81 or 325 mg/day | At least 3 days | 8.1 | Resistant if ≤ 3.1 ng/ml |
| Gluckman 2011,99 USA | 229 | For patients with ≥ 1 occluded SVG (n = 70): mean 63 (range 55–72) For patients with patent SVG (n = 159): mean 63 (range 57–71) |
Mono | CAD | Smokers: n = 52 (22.7%) Diabetes: n = 84 (36.7%) |
Yes: CABG | 325 mg/day | No details | No details | Resistant if closure time < 193 seconds |
| Hobikoglu 2007,135 Turkey | 140 | Resistant: mean 63.8 (SD 10.8) Sensitive: mean 58.3 (SD 11.2) |
Mono (12.1% on dual at follow-up) | ACS | Smokers: Resistant (N = 45): n = 22 (49%) Sensitive (N = 79): n = 31 (39%) Diabetes: Resistant (N = 45): n = 7 (15%) Sensitive (N = 79): n = 16 (20%) |
No | 100 mg/day (20% of patients); 300 mg/day (80% of patients) | At least 7 days | 32.1 | Resistant if closure time < 170 seconds |
| Linnemann 2009,112 Germany | 98 (57 eligible for analyses) |
Median 67.7 (range 44–90) | Mono | CAD | Smokers: n = 31 (31.6%) Diabetes: n = 45 (45.9%) |
No | 100 mg/day | At least 14 days | 3.5 | Resistance was the maximum aggregation values within the reference range (≥ 78%) |
| Lordkipanidzé 2011,162 UK (abstract) | 198 | No details | Mono | CAD | No details | No | 80–325 mg/day | No details | No details | No details |
| Modica 2009,187 Sweden | 334 | Mean 72 | Mono (45% dual during follow-up) | UA/ACS | Smokers: 21% Diabetes: 20% |
No | 75 mg/day | No details | No details | No details |
| Morawski 2005,144 Poland | 51 | Mean 61.3 (SD 8.4) | Mono | CAD | Diabetes: 17.6% | Yes: CABG | 150 mg/day | No patients were treated with antiplatelet agents for 10 days before the operation | No details | No details |
| Pamukcu 2007,137 Turkey | 234 | Mean 57 (SD 9) | Mono (12% on dual at follow-up) | CAD | Smokers: Resistant (N = 52): n = 32 (61.5%) Sensitive (N = 182): n = 106 (58.2%) Diabetes: Resistant (N = 52): n = 9 (17.3%) Sensitive (N = 182): n = 39 (21.4%) |
No | 100–300 mg/day (mean dose 268 mg/day, SD 70 mg/day) | At least 7 days | 22.2 | Resistant if closure time < 186 seconds |
| Poulsen 2007,132 Denmark | 298 (297 eligible for analyses) |
Based on 187 patients with no clinical outcomes: Resistant (n = 17): mean 70 (SD 10.2) Sensitive (n = 170): mean 69 (SD 12) |
Mono | UA/ACS | No details | No | 150 mg/day | At least 7 days | 70 | Resistant if closure time < 165 seconds |
| Sambola 2004,145 Spain | 100 | Mean 64 (SD 11) | Mono | CAD | No details | No | 100–125 mg/day | No details | 49 | Resistant if closure time ≤ 193 seconds |
| Silver 2009,189,193 UK (abstract) | 620 | Mean 72.5 | Mono | Miscellaneous (PVD, ACS, CVD/stroke) | No details | No | No details | No details | 25.2 | Resistant if closure time < 164 seconds |
| Sobol 2009,186 Poland | 64 (101 enrolled: 64 stroke patients, 37 controls; all population data based on 64) |
Mean 57.9 (SD 10.4) | Mono | CVD/stroke | Smokers: n = 24 (53.3%) | No | 150 mg/day | No details | 36 | Resistant if closure time ≤ 150 seconds |
| Ziegler 2002,150 Austria | 98 (64 eligible for analyses) |
Mean 66 (SD 15) | Mono (19% on dual during follow-up) | PAD/PVD | Smokers: 65.3% Diabetes: 42.9% |
Yes: PTA | 100 mg/day | At least 1 month | 5 | Resistant if closure time ≤ 170 seconds |
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||||||
| Foussas 2009,115 Greece | 496 | Resistant (n = 121): mean 67.9 (SD 9.2) Sensitive (n = 375): mean 69.8 (SD 8.8) |
Mono (dual during follow-up) | ACS | Smokers: Aspirin-resistant group (n = 121): 42.1% Aspirin-sensitive group (n = 375): 39.5% Diabetes: Aspirin-resistant group (n = 121): 24.8% Aspirin-sensitive group (n = 375): 34.7% |
No | 325 mg/day | Prior aspirin use (≥ 7 days prior): Aspirin-resistant group (n = 121): 45.5% Aspirin-sensitive group (n = 375): 40.3% |
24.4 | Resistant if closure time ≤ 193 seconds |
| Fuchs 2006,138 Austria | 208 | By clinical outcomes (recurrent ACS): Recurrent ACS (n = 58): mean 60 (SD 13) Event-free (number unclear): mean 57 (SD 12) |
Mono, with 9% on clopidogrel at time of PFT; 92% on dual during follow-up | ACS | By clinical outcomes (recurrent ACS): Recurrent ACS (n = 58): 55% smokers Event-free (number unclear): 54% smokers Recurrent ACS (n = 58): 27% diabetes Event-free (number unclear): 13% diabetes |
No | 250 mg (assume per day) | No details | No details | Resistant if closure time < 300 seconds |
All studies used the PFA-100®. The cartridge used was mainly collagen/epinephrine (CEPI), with three studies99,150,189 additionally using collagen/ADP. One study123 used collagen/ADP only. There were no details in one study. 162 Test characteristics are shown in Table 38.
| Study | Details of kit | Anticoagulant (concentration) | Agonist (concentration) | Time since last aspirin dose |
|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||
| Addad 2010108 | PFA-100® | 3.2% buffered trisodium citrate | CEPI | No details |
| Aksu 2009109 | PFA-100® | 3.8% citrate | CEPI | No details |
| Bevilacqua 2009118 | PFA-100® | 3.8% citrate | CEPI | No details |
| Boncoraglio 2009116 | PFA-100® | 3.8% citrate | CEPI | No details |
| Campo 2008123 (letter) | PFA-100® | No details | Collagen/ADP | No details |
| Christiaens 2008127 | PFA-100® | No details | CEPI | Up to 24 hours |
| Frelinger 200976 | PFA-100® | 3.8% sodium citrate | CEPI | No details |
| Gluckman 201199 | PFA-100® | 3.8% citrate | CEPI Collagen/ADP |
No details |
| Hobikoglu 2007135 | PFA-100® | No details | CEPI | Up to 24 hours |
| Linnemann 2009112 | PFA-100® | 0.129 M (3.8%) trisodium citrate | CEPI | 1–24 hours |
| Lordkipanidzé 2011162 (abstract) | PFA-100® | No details | No details | No details |
| Modica 2009187 | PFA-100® | No details | Epinephrine (30 µl of a solution containing 0.1 mg epinephrine) | Up to 24 hours |
| Morawski 2005144 | PFA-100® | No details | CEPI | Up to 12 hours |
| Pamukcu 2007137 | PFA-100® | No details | CEPI | 1–4 hours |
| Poulsen 2007132 | PFA-100® | No details | CEPI | Up to 24 hours |
| Sambola 2004145 | PFA-100® | 0.128 M buffered sodium citrate | CEPI | Approximately 3 hours |
| Silver 2009189,193 (abstract) | PFA-100® | No details | CEPI Collagen/ADP |
No details |
| Sobol 2009186 | PFA-100® | No details | CEPI | First test: before aspirin Second test: up to 24 hours |
| Ziegler 2002150 | PFA-100® | No details | CEPI Collagen/ADP |
Up to 24 hours |
| Monotherapy at time of PFT, dual therapy during follow-up | ||||
| Foussas 2009115 | PFA-100® | No details | CEPI | Up to 24 hours |
| Fuchs 2006138 | PFA-100® | 129 mM buffered sodium citrate | CEPI | No details |
Study design and quality
Results of the risk-of-bias assessment can be found in Tables 39–42.
| Domain 1: patient selection | Was a consecutive or random sample of patients enrolled? | Was patient selection independent of patient outcomes? | Were reasons for any posteligibility exclusions provided? |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Addad 2010108 | Consecutive | Yes | No details |
| Aksu 2009109 | No details | Yes | No details |
| Bevilacqua 2009118 | All patients undergoing isolated primary CABG surgery over the course of 1 year | Yes | Appears that no eligible patients were excluded |
| Boncoraglio 2009116 | Consecutive | Yes | No details |
| Campo 2008123 | Consecutive | Yes | No details |
| Christiaens 2008127 | Consecutive | Yes | No details |
| Frelinger 200976 | Consecutive | Yes | Stated that less than 3% of eligible patients declined participation (reason not given) |
| Gluckman 201199 | No details | Yes | Patients in whom SVG patency not assessed or those not on aspirin monotherapy. Authors stated that the study population was representative of patients undergoing isolated CABG surgery based on comparison with the Society of Thoracic Surgeons National Database |
| Hobikoglu 2007135 | Consecutive | Yes | No details |
| Linnemann 2009112 | Consecutive | Yes | No details |
| Lordkipanidzé 2011162 (abstract) | No details | Yes | No details |
| Modica 2009187 | Consecutive | Yes | No details |
| Morawski 2005144 | No details for whole sample, patients randomly assigned to aspirin or placebo | Yes | No details |
| Pamukcu 2007137 | Consecutive | Yes | No details |
| Poulsen 2007132 | Consecutive | Yes | No details |
| Sambola 2004145 | No details | Yes | No details |
| Silver 2009189,193 (abstract) | Consecutive | Yes | No details |
| Sobol 2009186 | Consecutive | Yes | No details |
| Ziegler 2002150 | Consecutive | Yes | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | |||
| Foussas 2009115 | Consecutive | Yes | No details |
| Fuchs 2006138 | Consecutive | Yes | No details |
| Domain 2: PFT | If a threshold was used, was it prespecified? | How was the threshold derived (e.g. literature cut-off, based on study data)? | Is the undertaking and interpretation of the index test blinded to the patient characteristics (including clinical outcomes)? |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Addad 2010108 | No (tertiles) | Tertiles | Yes; stated that all assays were performed in a blinded manner |
| Aksu 2009109 | Yes; stated (wrongly) that resistant if CEPI CT of ≥ 170 seconds, but appears that subsequent figures relate to correct definition (i.e. resistant if ≤ 170 seconds) | As described in authors’ previous studies | No details |
| Bevilacqua 2009118 | Yes (< 190 seconds) | Reference cited222 | No details |
| Boncoraglio 2009116 | Yes (< 165 seconds) | Manufacturer’s information, corroborated in authors’ laboratory | No details |
| Campo 2008123 | Yes (for CADP) | For CADP: median value as cut-off between high and low platelet reactivity; also ROC analysis for exploratory evaluation of best cut-off For CEPI: no details |
No details |
| Christiaens 2008127 | Yes (< 187 seconds) | Previously established in authors’ laboratory | No details |
| Frelinger 200976 | Yes (≤ 193 seconds) | Cut-off represents the upper limit of the 90% central interval of duplicate results measured in an aspirin-free healthy population (references given) | No details |
| Gluckman 201199 | Yes (≤ 193 seconds) | The upper limit of the normal range in the authors’ laboratory for aspirin-naive patients | No details |
| Hobikoglu 2007135 | Yes (< 170 seconds) | Mean (+ 2 SD) CT of healthy volunteers not on aspirin. Aspirin resistance defined as a normal CT (below the control group cut-off value) despite aspirin treatment | No details |
| Linnemann 2009112 | Yes (CT < 192 seconds) | Previously determined by the research group from the 95th percentile of measurements in a group of 50 healthy volunteers | No details |
| Lordkipanidzé 2011162 (abstract) | No details | No details | No details |
| Modica 2009187 | Yes | No specific value; high residual platelet reactivity defined as a normal CT value even when the subject was taking aspirin (reference cited77). In-house reference ranges were established from analyses in a control group of 278 volunteers | No details |
| Morawski 2005144 | No details | No details (appears authors were trying to measure a correlation between the CT and postoperative bleeding) | No details |
| Pamukcu 2007137 | Yes (CT < 186 seconds) | Reference cited223 for normal reference range (98–185 seconds) for the PFA-100® with CEPI cartridges | No details |
| Poulsen 2007132 | Yes (CT < 165 seconds for CEPI; no details for CADP) | Cut-off value based on the results from a previous study evaluating the performance of the PFA-100® in patients taking aspirin | No details |
| Sambola 2004145 | Yes (CT ≤ 137 seconds) | Reference cited224 | No details |
| Silver 2009189,193 (abstract) | Yes (CT < 164 seconds for CEPI; no details for CADP) | No details | No details |
| Sobol 2009186 | Yes (CT ≤ 150 seconds) | No details | No details |
| Ziegler 2002150 | Yes (CT < 170 seconds during at least one follow-up visit) | No details | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | |||
| Foussas 2009115 | Yes (CT ≤ 193 seconds) | References cited219,225 | No details |
| Fuchs 2006138 | Yes (CT < 300 seconds) | For this purpose CEPI CT was stratified according to values > or < 300 seconds, as 77% of the ACS patients reached the maximal CT value of 300 seconds after aspirin infusion. Quartiles also used in analysis | No details |
| Domains 3 and 4: outcomes and study attrition | Were the outcomes of interest clearly defined in advance? | Were the outcome results interpreted without knowledge of the results of the PFT? | What was the proportion of missing data? (State reasons for loss to follow-up or differences in those who completed or were lost) |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Addad 2010108 | Yes | Yes; follow-up clinicians were blinded to PFT results | Stated that none of the included patients was lost to follow-up |
| Aksu 2009109 | Yes | Yes; people performing follow-up interviews were blind to aspirin resistance and mean platelet volume status of the patients | 20/240 lost to follow-up; reasons not stated. Analyses based on 220 |
| Bevilacqua 2009118 | Yes | Yes; follow-up visits conducted by cardiologists not involved in the present study | Stated that follow-up 100% complete |
| Boncoraglio 2009116 | Partly (vascular cognitive impairment is mentioned in results, but not as part of composite outcome defined in methods) | Yes; personnel responsible for data collection were not aware of PFT results | 13/142 lost to follow-up (seven had changed address and telephone number, four refused to reply to the questions and two had stopped taking aspirin for reasons unconnected with vascular disease) |
| Campo 2008123 | Yes | No details | Appears there was loss to follow-up |
| Christiaens 2008127 | Yes | Yes; observers collecting follow-up data were blinded to the PFT results | All patients who enrolled completed the study |
| Frelinger 200976 | Yes | Yes; all clinical outcome data obtained by research personnel blinded to results of PFTs | 127/682 not included in follow-up (appears test not done in everyone) |
| Gluckman 201199 | Yes | Yes; stated that images were analysed by two blinded reviewers (98% concordance) with a third reviewer adjudicating as necessary | 73/229 not included at follow-up |
| Hobikoglu 2007135 | Yes | Yes; scores were determined by one of the investigators, who had no knowledge of the presence of aspirin resistance | 16/140 lost to follow-up and excluded from analysis |
| Linnemann 2009112 | Yes | Unclear; reported events were only considered if they were confirmed by medical reports from GPs or admitting hospitals | Data on clinical outcome available only from patients whose platelet function was assessed twice (57/98). Of the 41 excluded, 4 patients died and 16 had their antithrombotic medication changed. Remaining reasons for dropouts not stated. Authors state that there was no difference observed in aspirin resistance rates between dropouts and those remaining in study |
| Lordkipanidzé 2011162 (abstract) | Yes | No details | No details |
| Modica 2009187 | Yes | Yes; states that the test results were not accessible by the attending physicians | Stated that no patients were lost to follow-up |
| Morawski 2005144 | Yes | No details | Appears to be no loss to follow-up |
| Pamukcu 2007137 | Yes | No details | Appears to be no loss to follow-up |
| Poulsen 2007132 | Yes | No details | Stated that patients were excluded from follow-up if they were no longer taking aspirin, had suffered an acute MI or stroke, or had undergone mechanical revascularisation because of atherothrombotic disease within the previous 3 months 111/298 excluded from the follow-up visit for the following reasons: death (n = 39), withdrawal of aspirin treatment (n = 27), unwillingness to participate (n = 35), geographical reasons (n = 5), recurrent MI or stroke within the last 3 months (n = 4). One participant was lost to follow-up Even though these patients were excluded, clinical outcomes are linked to the full sample (297/298 patients) |
| Sambola 2004145 | No | No details | 19/100 patients lost to follow-up at 6 months. Five cardiovascular deaths and 14 patients excluded [9 declared non-compliant with aspirin treatment based on interview, 5 treated with other regimens (3 clopidogrel, 2 warfarin)] |
| Silver 2009189,193 (abstract) | Yes | Yes; treating physicians, patients and researchers were blind to the test results | No details |
| Sobol 2009186 | Yes | No details | No losses to follow-up |
| Ziegler 2002150 | Yes | No details | Appears that there was no loss to follow-up |
| Monotherapy at time of PFT, dual therapy during follow-up | |||
| Foussas 2009115 | Yes (for one outcome) No (for two outcomes) |
No details | Stated that no patients lost during follow-up |
| Fuchs 2006138 | Yes | No details | 13% of patients lost to follow-up (6% in the first year) |
| Domain 5: confounding | Are confounders accounted for in the design or analysis (e.g. adjustment, stratification)? | If there is an adjusted outcome measure (e.g. OR, HR), what were the factors that were adjusted for? | If a HR was presented, was the proportional hazards assumption met? | Was compliance measured? | How was compliance measured? | Level of compliance |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Addad 2010108 | Design: N/A Analysis: no (for groups by aspirin responder status) |
N/A | No details | Yes | Interview at study enrolment and during follow-up period | No details |
| Aksu 2009109 | Design: N/A Analysis: yes (HR) |
Troponin T, platelet number, severity score and neutrophil percentage in WBC count (but groups not defined on basis of aspirin resistance alone) | No details | No details | No details | No details |
| Bevilacqua 2009118 | Design: N/A Analysis: yes (HR) |
Age, male sex, diabetes, hypertension, obesity, hypercholesterolaemia, smoking habit, left ventricular ejection fraction, logistic EuroSCORE, incomplete revascularisation | No details | No details | No details | No details |
| Boncoraglio 2009116 | Design: N/A Analysis: no |
N/A | No details | Yes | Telephone interview using standardised questionnaire (as part of outcome assessment) | No details |
| Campo 2008123 | Design: N/A Analysis: yes (HR) |
Not specified | No details | Yes | No details | At 1 year, 116 (91%) patients still taking aspirin; at 2 years, 112 (90%) still taking aspirin |
| Christiaens 2008127 | Design: N/A Analysis: no |
N/A | No details | Yes | Personal interview at the time of inclusion | No details |
| Frelinger 200976 | Design: N/A Analysis: yes (OR) |
Sex, TIMI risk score, aspirin dose, platelet count, BMI, use of clopidogrel, statins and oral hypoglycaemic agents | N/A | Not mentioned in methods, but a TxB2 test undertaken and stated that: ‘Two patients had serum TXB2 levels in the range observed for aspirin-free healthy controls, and their platelet function was therefore consistent with aspirin noncompliance. Because “resistance” cannot be distinguished from noncompliance, these subjects were not excluded from follow-up’ | ||
| Gluckman 201199 | Design: N/A Analysis: yes (OR) |
Target vessel diameter, thromboxane levels, sex, (race in one model) | N/A | Yes | Pill counts at each postoperative encounter | No details |
| Hobikoglu 2007135 | Design: N/A Analysis: yes (HR) |
Age, platelet count, cTnT value and CAD severity score | No details | Yes | Self-report; telephone interviews at follow-up | According to the follow-up visits, all patients continued on 100 mg aspirin, and none of them discontinued treatment |
| Linnemann 2009112 | Design: N/A Analysis: no |
N/A | N/A | Yes | Interview at study commencement and follow-up | ‘All patients confirmed that they had taken aspirin regularly as directed over the last 14 days.’ Not clear which time period this relates to; probably the start of the study |
| Lordkipanidzé 2011162 (abstract) | Design: N/A Analysis: unclear whether adjusted or unadjusted OR |
No details | N/A | No details | No details | No details |
| Modica 2009187 | Design: N/A Analysis: yes (HR) |
Age, sex, diabetes, smoking status, heart failure, atrial fibrillation, baseline glomerular filtration rate, troponin T, platelet aggregation, high residual platelet reactivity, intervention with CABG or PCI | Yes (the assumptions for Cox regression analysis were evaluated by Kaplan–Meier curves for all the variables included) | No details | No details | No details |
| Morawski 2005144 | Design: N/A Analysis: no |
N/A | N/A | Yes | Aspirin was administered under controlled conditions | No details; likely to be 100% owing to RCT conditions |
| Pamukcu 2007137 | Design: N/A Analysis: no |
N/A | No details | Yes | States that: ‘Compliance with aspirin therapy was ascertained by a personal interview’ | No details |
| Poulsen 2007132 | Design: N/A Analysis: no |
N/A | No details | Yes | The patients were informed that they should take their usual aspirin 3–5 hours before blood sampling on the day of the follow-up visit. During the last week before the follow-up visit, the same investigator telephoned all patients on a daily basis to ensure that each patient remembered to take her/his daily dose of aspirin | No details |
| Sambola 2004145 | Design: N/A Analysis: no |
N/A | N/A | Yes | Personal interview at 1 and 6 months; biochemical test for salicylates in urine (not clear at which time point) | The levels of salicylate in urine for patients with good response to aspirin and resistance were 88 ± 88 mg/ml vs.78 ± 45 mg/ml, p = NS, at 1 month, and 89 ± 91 mg/ml vs. 77 ± 67 mg/ml, p = NS, at 6 months Six patients with good response to aspirin and four patients with resistance showed inadequate salicylate levels in urine (< 30 mg/ml), at both 1 and 6 months. After excluding these 10 patients, the salicylate levels were not significantly different between groups (95 ± 91mg/ml vs. 84 ± 41 mg/ml, p = NS, at 1 month; 99 ± 93 mg/ml vs. 86 ± 68 mg/ml, p = NS, at 6 months) All patients with recurrence of ischaemic events had adequate salicylate levels |
| Silver 2009189,193 (abstract) | Design: N/A Analysis: no |
N/A | No details | No details | No details | No details |
| Sobol 2009186 | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Ziegler 2002150 | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| Foussas 2009115 | Design: N/A Analysis: yes (HR) |
Age, sex, smoking, diabetes, history of MI, history of coronary bypass grafting, prior aspirin use (≥ 7 days prior), cTnI ≥ 0.4 ng/ml, hs-CRP ≥ 3 mg/l, TIMI risk score, left ventricular ejection fraction < 35% | No details | No details | No details | No details |
| Fuchs 2006138 | Design: N/A Analysis: yes (HR) |
Diabetes, beta-blockers, clopidogrel, von Willebrand factor: ristocetin cofactor | No details | No details | No details | No details |
Patient selection was independent of study outcome in all included studies, with the PFT preceding any outcomes (as specified in the study selection criteria). Fifteen of 21 studies stated that consecutive patients were enrolled. 76,108,112,115,116,123,127,132,135,137,138,150,186,187,189,193 Two studies76,99 had clear details on posteligibility exclusions.
A predetermined threshold value for defining resistance was given in 18 studies;76,99,109,112,115,116,118,123,127,132,135,137,138,145,150,186,187,189,193 thresholds varied between 150 and 193 seconds, though one study138 had a much higher threshold at 300 seconds. One study used tertiles,108 one used a median value and also conducted ROC analysis123 and one187 stated that high residual platelet reactivity was defined as a normal closure time value even when the subject was taking aspirin. There were no details on threshold in two studies. 144,162 There were a number of methods for deriving the thresholds (as reflected in the different cut-offs obtained); these included values established in previous studies or by other research groups. Only one study108 stated that assays were performed in a blinded manner.
Outcome measures of interest were (at least partly) clearly stated in the methodology of 20 out of 21 studies. Only one study145 did not clearly prespecify these. Ten studies76,99,108,109,116,118,127,135,187,193 had details on blinding of outcome assessors to the PFT results. In 10 studies108,115,118,123,127,137,144,150,186,187 it was stated or appeared that there was no loss to follow-up. There were no details in two studies. 162,193 The remaining studies reported varying proportions of loss to follow-up; this was between 8% and 58%. In the study with the largest loss to follow-up (58%),112 data on clinical outcomes were only available for those patients who had a repeat PFT.
Compliance was measured in 11 studies99,108,112,116,123,127,132,135,137,144,145 using interview, pill counts, self-reports and, in one study,145 a test for salicylates in urine. Four studies112,123,135,145 reported levels of compliance: one123 stated that 90% of patients were still taking aspirin at year 2 (though the method of how this was ascertained was not stated); one135 stated that all patients continued with their treatment (based on interviews); in one study,112 patients confirmed that they had all taken aspirin over the last 14 days (based on interview); and in one study145 patients were excluded on the basis of inadequate salicylate levels. One study76 stated that ‘Two patients had serum TXB2 levels in the range observed for aspirin-free healthy controls, and their platelet function was therefore consistent with aspirin noncompliance; as “resistance” cannot be distinguished from noncompliance, these subjects were not excluded from follow-up.’
Nine studies76,99,109,115,118,123,135,138,187 undertook adjusted analyses (based on HRs or ORs). There was some overlap in adjustment factors between the different studies, but no studies used all of the same ones.
Overview of outcomes
Twenty-one studies were found in this category,76,99,108,109,112,115,116,118,123,127,132,135,137,138,144,145,150,162,186,187,189 with MACEs being the most frequently reported outcome (Table 43). Bleeding events were reported in one study only. 144
| Study | Death | MACE | Ischaemic/thrombotic | Bleeding | Length of follow-up |
|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||||
| Addad 2010108 | ✓ | 1 year | |||
| Aksu 2009109 | ✓ | ✓ | ✓ | Mean 14.86 (SD 5.93) months | |
| Bevilacqua 2009118 | ✓ | ✓ | ✓ | Mean 32 (SD 10) months | |
| Boncoraglio 2009116 | ✓ | Mean 56.6 (range 32–91) months | |||
| Campo 2008123 | ✓ | 2 years | |||
| Christiaens 2008127 | ✓ | ✓ | ✓ | Median 2.5 years | |
| Frelinger 200976 | ✓ | ✓ | Mean 24.8 (SD 0.3) months | ||
| Gluckman 201199 | ✓ | 6 months | |||
| Hobikoglu 2007135 | ✓ | ✓ | ✓ | Mean 20 (range 18–24) months | |
| Linnemann 2009112 | ✓ | ✓ | Median 17 (range 10–37) months | ||
| Lordkipanidzé 2011162 (abstract) | ✓ | 3 years | |||
| Modica 2009187 | ✓ | Median 44 (IQR 35–55) months | |||
| Morawski 2005144 | ✓ | 7 days | |||
| Pamukcu 2007137 | ✓ | ✓ | Mean 20.6 (SD 6.9) months | ||
| Poulsen 2007132 | ✓ | ✓ | ✓ | 1 year | |
| Sambola 2004145 | ✓ | ✓ | 6 months | ||
| Silver 2009189,193 (abstract) | ✓ | Unclear; 2268 patient-years of follow-up between 2002 and 2004 | |||
| Sobol 2009186 | ✓ | 10 days | |||
| Ziegler 2002150 | ✓ | 1 year | |||
| Monotherapy at time of PFT, dual therapy during follow-up | |||||
| Foussas 2009115 | ✓ | ✓ | 1 year | ||
| Fuchs 2006138 | ✓ | Mean 859 (range 830–887) days | |||
Death
Death rates were reported in 10 studies (Table 44). 76,109,115,118,127,132,135,137,145,186 Outcome statistics are shown in Figures 34–36. Results for three of these could not be presented in forest plots. In the study by Aksu et al. ,109 results were presented according to both resistance status and a cut-off for mean platelet volume. One group (resistant and mean platelet volume > 8.4 fl) has an increased event rate compared with the other three groups, but it is unclear how much the resistance is contributing to this. In the study by Sobol et al. ,186 one death occurred in the resistant group, but it was unclear if this was in the group classified as resistant with PFA-100® or WBA. Frelinger et al. 76 does not present deaths separately by resistant and sensitive groups.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Aksu 2009109 | Results not presented according to resistant and sensitive only, but also depending on mean platelet volume | |||||
| Bevilacqua 2009118 | ✓a | ✓a | ✓a | |||
| Christiaens 2008127 | ✓a | ✓a | ✓a | |||
| Frelinger 200976 | Total number of deaths reported by resistant or sensitive groups unclear | |||||
| Hobikoglu 2007135 | ✓ | |||||
| Pamukcu 2007137 | ✓a | ✓a | ✓a | |||
| Poulsen 2007132 | ✓a | ✓a | ✓a | |||
| Sambola 2004145 | ✓a | ✓a | ✓a | |||
| Sobol 2009186 | Unclear if event in resistant group defined by PFA-100® or WBA | |||||
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| Foussas 2009115 | ✓ | |||||
FIGURE 34.
PFA-100®, monotherapy: death, unadjusted ORs. s, seconds.
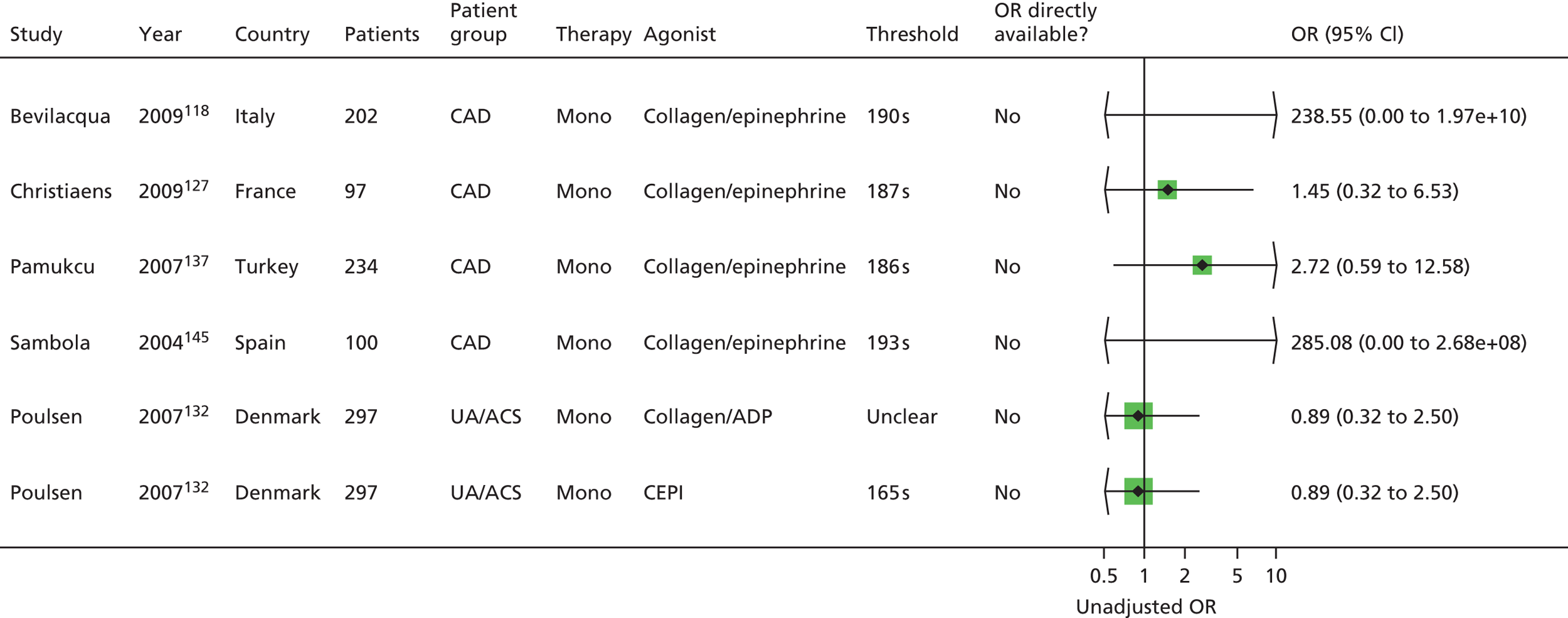
FIGURE 35.
PFA-100®, monotherapy: death, unadjusted HRs. s, seconds.

FIGURE 36.
PFA-100®, monotherapy: death, adjusted HRs. s, seconds.
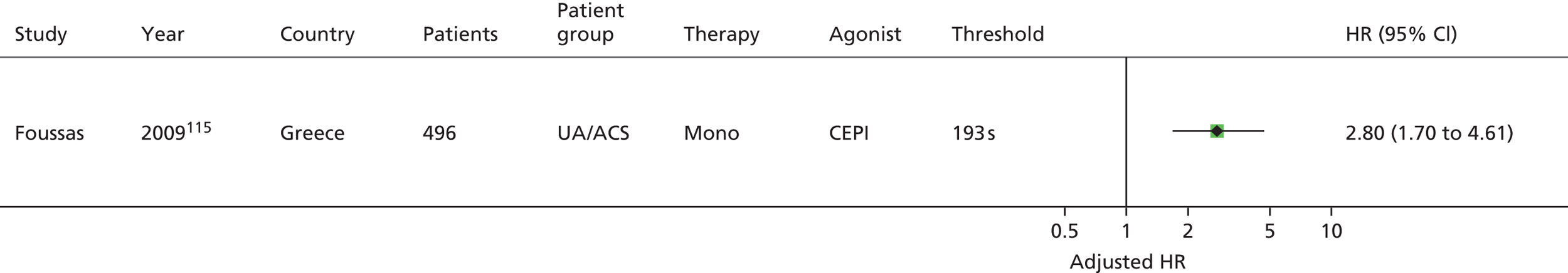
Six unadjusted ORs are presented based on five studies. 118,127,132,137,145 There were more events in the resistant arm in four of the five studies,118,127,137,145 but no differences were statistically significant. Seven of eight unadjusted HRs (based on seven studies115,118,127,132,135,137,145) are also not statistically significant. Note that the very large ORs and HRs118,145 are based on two118 and five145 events respectively in the resistant group and zero events in the sensitive group. The one statistically significant result (unadjusted HR) remained statistically significant after adjustment; this was in a UA/ACS population. 115
Overall, there was a trend towards more deaths in the resistant groups, but most results were not statistically significant.
Major adverse cardiac events
Fourteen studies reported MACEs (Table 45). 76,108,109,112,116,118,123,127,132,135,137,138,162,187 Outcome statistics are shown in Figures 37–39. One76 was not presented in a forest plot as exact numbers were not reported. The graphical representation indicates an unadjusted OR below 1 (more events in the sensitive group), but this was not statistically significant.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Addad 2010108 | ✓a | ✓a | ✓ | ✓a | ||
| Aksu 2009109 | ✓a | ✓a | ✓a | |||
| Bevilacqua 2009118 | ✓ | |||||
| Boncoraglio 2009116 | ✓a | ✓a | ✓a | |||
| Campo 2008123 | ✓ | |||||
| Christiaens 2008127 | ✓a | ✓a | ✓a | |||
| Frelinger 200976 | OR reported in graph only, but not exact numbers | |||||
| Hobikoglu 2007135 | ✓a | ✓ | ✓ | ✓a | ||
| Linnemann 2009112 | ✓a | ✓a | ✓a | |||
| Lordkipanidzé 2011162 (abstract) | ✓ | |||||
| Modica 2009187 | ✓ | ✓ | ||||
| Pamukcu 2007137 | ✓a | ✓a | ✓a | |||
| Poulsen 2007132 | ✓a | ✓a | ✓a | |||
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| Fuchs 2006138 | ✓ | ✓ | ||||
FIGURE 37.
PFA-100®, monotherapy: MACEs, unadjusted ORs. RAVE, recurrent acute vascular event; s, seconds.
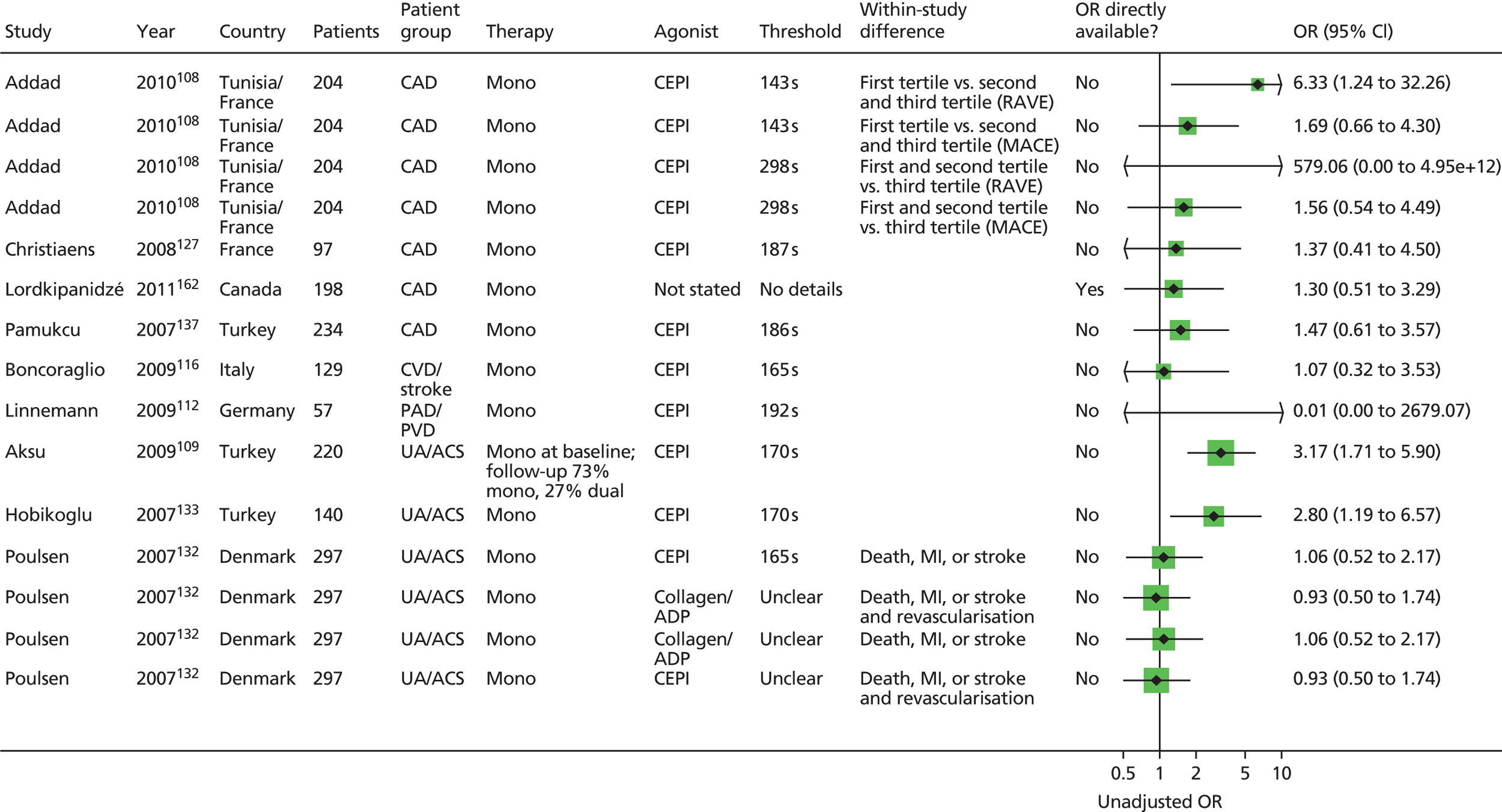
FIGURE 38.
PFA-100®, monotherapy: MACEs, unadjusted HRs. RAVE, recurrent acute vascular event; s, seconds.

FIGURE 39.
PFA-100®, monotherapy: MACEs, adjusted HRs. s, seconds.

Fifteen unadjusted ORs are presented, based on nine studies;108,109,112,116,127,132,135,137,162 note that two108,132 of these studies contribute four results each. Three ORs are statistically significant (more events in the resistant group), based on three studies. 108,109,135 Apart from one study132 contributing four results which are all close to 1, the direction of effect based on the other eight studies108,109,112,116,127,135,137,162 is consistent (more events in the resistant groups). Note that six results are based on three studies109,132,135 with an acute population.
Eighteen unadjusted HRs are presented, based on 11 studies. 108,109,112,116,118,127,132,135,137,138,187 Two108,132 of these studies contribute four results each. With the exception of two studies,132,187 the direction of effect is again consistent (more events in the resistant arm). Six unadjusted HRs (based on five studies108,109,118,135,138) were statistically significant.
Six adjusted HRs (based on five studies118,123,135,138,187) were available, four of which were statistically significant (more events in the resistant arm). One study187 clearly shows the opposite direction of effect (though this is not statistically significant); this may be a result of differences in threshold (highest vs. lowest quartile was compared rather than using a single cut-off), population differences or differences in adjustment factors.
Overall, there was a mainly consistent trend, with more MACEs in the resistant arm, and some results were statistically significant. Not all studies contributed to the results, particularly the adjusted results. In addition, five of six unadjusted HRs were based on an acute population, which may not be representative of the general population prescribed aspirin monotherapy.
Ischaemic/thrombotic events
Eleven studies reported additional ischaemic/thrombotic outcomes (Table 46). 99,109,112,115,118,127,132,135,145,150,189,193 Results from two studies could not be presented in the forest plots. In the study by Aksu et al. ,109 results were presented according to both resistance status and a cut-off for mean platelet volume. One group (resistant and mean platelet volume > 8.4 fl) had an increased event rate compared with the other three groups, but it was unclear how much the resistance was contributing to this. Sambola et al. 145 stated that no significant differences were found between resistant and sensitive groups in rates of infarction, angina or need for revascularisation.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Aksu 2009109 | Results not presented according to resistant and sensitive only, but also depending on mean platelet volume | |||||
| Bevilacqua 2009118 | ✓a | ✓ | ✓ | ✓a | ||
| Christiaens 2008127 | ✓a | ✓a | ✓a | |||
| Gluckman 201199 | ✓ | ✓ | ||||
| Hobikoglu 2007135 | ✓ | |||||
| Linnemann 2009112 | ✓a | ✓a | ✓a | |||
| Poulsen 2007132 | ✓a | ✓a | ✓a | |||
| Sambola 2004145 | Narrative description | |||||
| Silver 2009189,193 (abstract) | ✓ | |||||
| Ziegler 2002150 | ✓a | ✓a | ✓a | |||
| Monotherapy at time of PFT, dual therapy during follow-up | ||||||
| Foussas 2009115 | ✓a | ✓a | ✓a | |||
Outcome statistics are shown in Figures 40–43. Nineteen unadjusted ORs were presented based on seven studies. 99,112,115,118,127,132,150 Two ORs were statistically significant (more events in the resistant group), but for one of these (from the Gluckman et al. study99) the threshold was unclear. Note that eight ORs are derived from populations with UA/ACS. The overall direction of effect was not consistent across (or within, e.g. Poulsen et al. ,132 Linnemann et al. 112) studies. There were two adjusted ORs (based on one study99), one of which was statistically significant (more events in the resistant group).
FIGURE 40.
PFA-100®, monotherapy: ischaemic/thrombotic events, unadjusted ORs. PTA, percutaneous transluminal angioplasty; s, seconds; TEA, thoracic epidural analgesia.
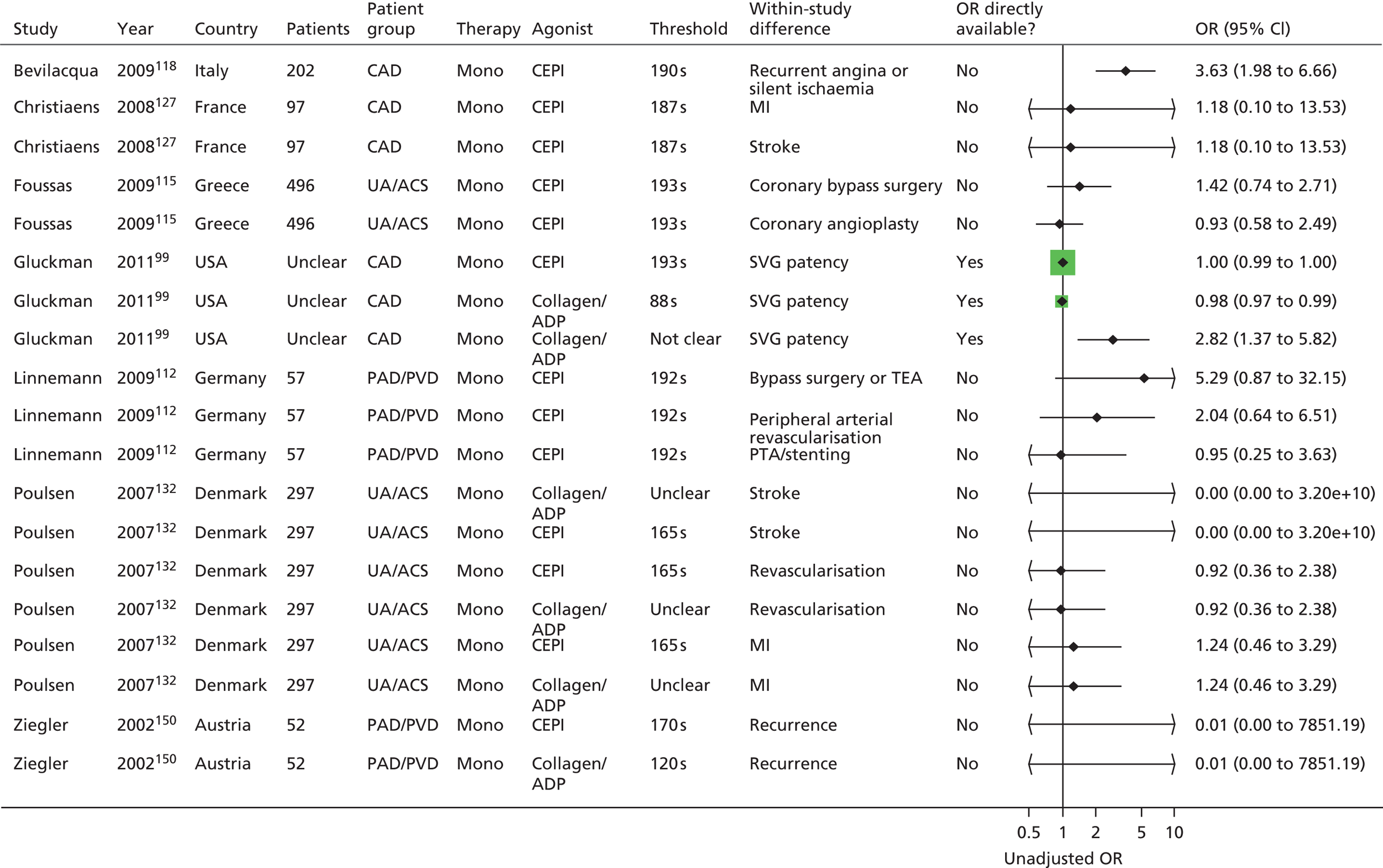
FIGURE 41.
PFA-100®, monotherapy: ischaemic/thrombotic events, adjusted ORs. s, seconds.

FIGURE 42.
PFA-100®, monotherapy: ischaemic/thrombotic events, unadjusted HRs. CVA, cerebrovascular accident; PTA, percutaneous transluminal angioplasty; s, seconds; TEA, thoracic epidural analgesia.
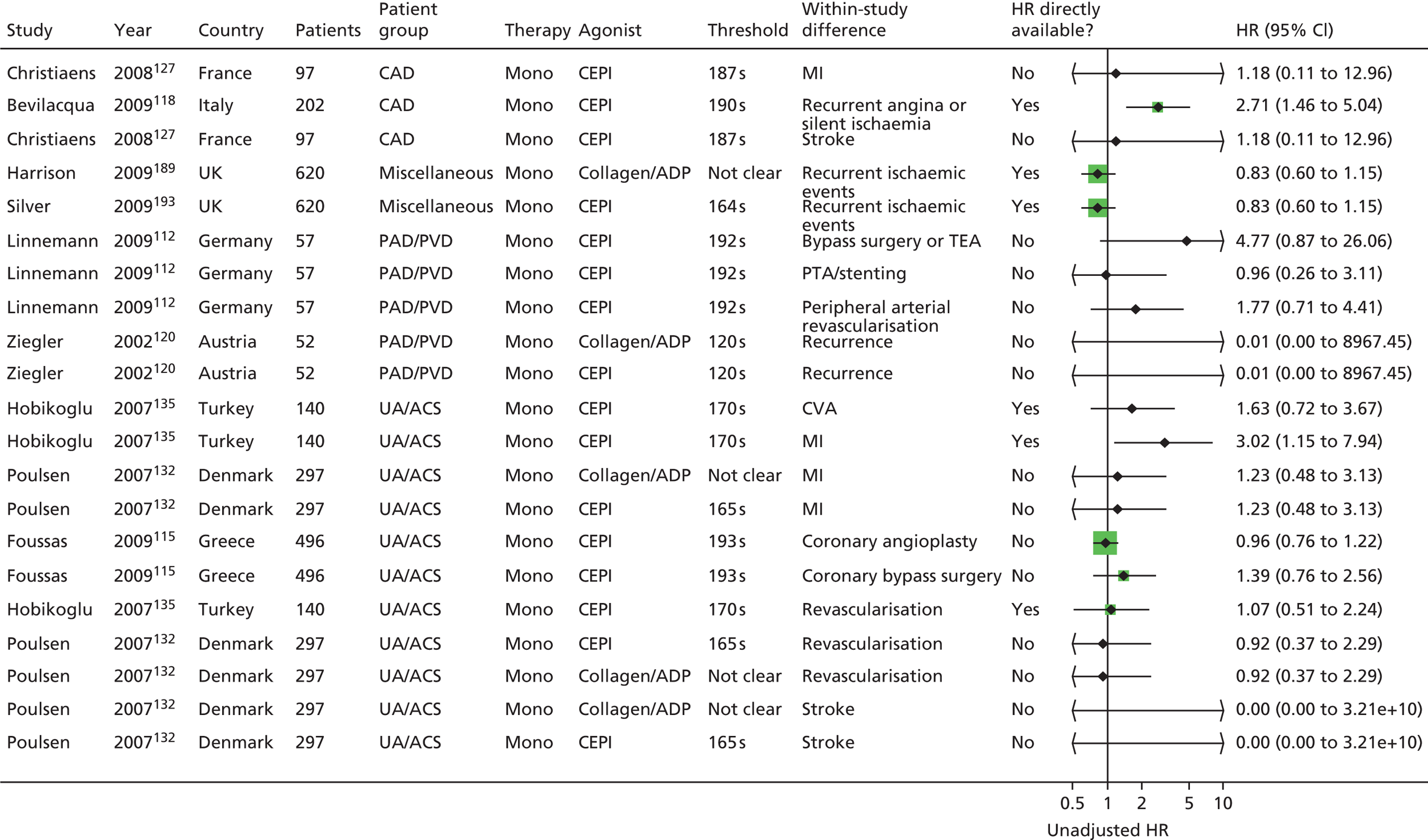
FIGURE 43.
PFA-100®, monotherapy: ischaemic/thrombotic events, adjusted HRs. s, seconds.

Two of 22 unadjusted HRs were statistically significant (more events in the resistant group); however, the direction of effect was again not consistent. Eleven of the 21 ORs were based on populations with UA/ACS. Note also that some studies contributed disproportionately to the results, for example where they measured more outcomes. There was only one adjusted HR, showing a statistically significant result (more events in the resistant group).
Bleeding events
One study reported bleeding events (Table 47). 144
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Morawski 2005144 | Narrative description | |||||
No studies were identified that looked at bleeding outcomes over the long term. One study144 evaluated postoperative bleeding and found that PFA-100® failed to correlate with postoperative bleeding, but PFA-100® measurements repeated immediately after CABG were predictive of blood loss.
Summary: platelet function analyser-100
Twenty-one studies were identified in this category. 76,99,108,109,112,115,116,118,123,127,132,135,137,138,144,145,150,162,186,187,189 There were more populations with stable disease, but those studies with acute populations contributed quite substantially to the results (roughly half of the outcome statistics). There was heterogeneity across studies in terms of specific patient characteristics.
There was a lack of reporting of relevant quality criteria, making overall judgements about risk of bias difficult. No study provided details on all relevant quality criteria. Lack of detail related in particular to whether or not assays were performed in a blinded manner, reporting of compliance levels and, to a lesser extent, whether or not outcome assessors had been blinded to PFT results. In terms of consequences of non-compliance, it appears that in one study145 patients were excluded on the basis of inadequate salicylate levels, whereas another study76 did not exclude patients with aspirin non-compliance on the basis that resistance cannot be distinguished from non-compliance. There were a number of methods for deriving the thresholds, which was reflected in the different cut-offs employed (between 150 and 193 seconds where stated, with one study138 having a much higher threshold at 300 seconds). Some studies provided adjusted results but there was no consistency between studies in terms of factors adjusted for.
Based on 10 studies76,109,115,118,127,132,135,137,145,186 reporting this outcome, there was an overall trend towards more deaths in the resistant groups, but most results were not statistically significant.
Based on 14 studies,76,108,109,112,116,118,123,127,132,135,137,138,162,187 there was a mainly consistent trend for MACEs, with more events in the resistant arm, and some results statistically significant. Note that not all studies contributed to the results, particularly the adjusted results. In addition, five of six unadjusted HRs are based on an acute population, which may not be representative of the total population.
Eleven studies reported additional ischaemic/thrombotic outcomes. 99,109,112,115,118,127,132,135,145,150,189,193 Compared with the large number of unadjusted outcome statistics reported, there were very few adjusted results. The direction of effect was not consistent for these outcomes and there were only a few statistically significant results (with more events in the resistant group). Given the large number of different outcomes and heterogeneity of other factors across studies, it was not possible to compare results for different outcomes (e.g. MI, stroke).
No studies were identified that looked at bleeding outcomes over the long term.
The mainly consistent trend for MACEs and death, with more events in the resistant arm, suggests that PFA-100® is a potential prognostic factor, but this is only a qualitative judgement on the evidence available; the trend for ischaemic/thrombotic events was, however, less consistent. Meta-analysis was not possible owing to the heterogeneity, and therefore a firm quantitative conclusion regarding whether or not PFA-100® is prognostic is not currently possible.
Summary: platelet function analyser-100
-
Twenty-one studies were identified for this test.
-
There were more stable than acute populations, though studies with acute populations contributed substantially to the results.
-
Methods for deriving thresholds and thresholds themselves were variable.
-
A lack of detail in reporting of quality criteria, for example whether or not assays were performed in a blinded manner, and in reporting of compliance levels (where measured), hampered an overall risk-of-bias assessment.
-
Adjusted results were rarely presented, and thus the additional prognostic value of the test over other prognostic factors is difficult to ascertain.
-
There was a mainly consistent trend for MACEs, with more events in the resistant arm, and some statistically significant results; this trend was reflected in studies reporting death, but most results were not statistically significant for this outcome.
-
The direction of effect was not consistent for ischaemic/thrombotic events.
-
No studies were identified that looked at bleeding outcomes over the long term.
-
Not all studies are represented in the forest plots, particularly for the adjusted outcome measures.
-
Heterogeneity in outcomes, patient groups and types of reported statistics meant that meta-analysis was not considered appropriate; there is insufficient quantitative information and methodological/clinical homogeneity across studies to enable evidence-based conclusions about the prognostic ability of PFA-100®.
Whole-blood aggregometry
Population and test characteristics
Eight studies99,117,128,153,162,166,186,196 were identified in this category, two of which were reported in abstract form only. 162,166 Populations had CAD (three studies),99,117,162 CVD/stroke (two studies),128,186 PAD/PVD (one study)153 and UA/ACS where patients were undergoing PPCI (one study). 196 There was one study166 in patients with end-stage renal disease. Two studies included only patients reporting a first event,186,196 one study consisted of patients who had suffered previous event(s)128 and one study reported patients who had their primary underlying condition for a mean period of 41.4 months. 117 Four studies did not report how long patients had been on aspirin therapy. 99,149,166,186
In seven studies99,117,128,153,162,166,186 it appeared that patients were exclusively on monotherapy both at the time of the PFT and during follow-up. It is possible that not all studies have reported where a proportion of patients commenced additional therapies during follow-up. In the remaining study,196 patients were on monotherapy at the time of the PFT and all were on dual therapy (+ clopidogrel or ticlopidine) during a portion of the follow-up period.
Two different methods of WBA were identified across studies and were analysed separately. One study166 used Multiplate® and seven studies used impedance methodology. 99,117,128,153,162,186,196 Most tests used arachidonic acid or collagen as an agonist, with some also using ADP and epinephrine, and citrate as the anticoagulant (where reported).
Most studies did not report use of other medications. Medications were reported in two studies99,117 and included statins, beta-blockers, ACE inhibitors, angiotensin receptor blockers, nitrate, lipid-lowering agents, diuretics, digoxin, spironolactone, warfarin, intravenous inotropic therapy and amiodarone. NSAIDs were not permitted (or had to be discontinued within a certain time period) in two studies. 186,196 One study99 stated that ‘concurrent nonsteroidal anti-inflammatory drug use did not correlate with the presence of aspirin non-responsiveness defined by this method at either time point’. There were no details on NSAIDs in the remaining studies.
The number of participants in the studies ranged from 26 to 653 (see Table 48). Where reported, mean ages of patients ranged from 52 to 66 years, with most means around the early 60s. There were more men than women in five out of six studies99,117,128,153,186,196 that reported this, with proportions of men ranging from 52% to 82%. Only one study128 included more women (55%). The proportion of patients with diabetes ranged from 17% to 44%, and that of smokers from 23% to 69% (where reported) (see Table 48). Where reported, studies were conducted in hospital settings.
The dose of aspirin ranged from 75 mg/day to > 325 mg/day. There were no details on dose in one study. 166 Details were variable across studies regarding the length of time patients had been receiving aspirin therapy, with some noting a minimum period and some whether or not patients were chronic users, but many giving no details (see Table 48). One study99 stated that aspirin was provided in enteric form, another reported that aspirin was provided in both enteric and plain forms117 and the other studies did not report this information.
The main study characteristics are listed in Table 48. Note that in some studies baseline characteristics have been reported only according to resistant/sensitive groups, groups with or without diabetes, or groups with occluded or patent SVG during CABG surgery, rather than for the total study population.
| Study/country | Number of patients | Age (years) | Therapy | Main underlying condition | Selected other population details | Due to undergo vascular intervention? | Aspirin dose/frequency | Duration of aspirin therapy (prior to PFT) | Percentage aspirin resistant | Derivation of threshold/comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Multiplate® (MEA) | ||||||||||
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Orta 2011,166 Turkey (abstract) | 78 | No details | Mono | Miscellaneous | No details | No details | No details | No details | 43.6 | No details |
| WBA (impedance) | ||||||||||
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Gengo 2008,128 USA | 653 | Resistant (n = 129): mean 63.7 Sensitive (n = 524): mean 66.5 |
Mono | CVD/stroke | Diabetes: Resistant (N = 129): n = 26 (20.2%) Sensitive (N = 524): n = 89 (17%) |
No | Various daily doses < 81 mg: 2% 81 mg: 68% 131 mg: 2% 162 mg: 3% 325 mg: 24% > 325 mg: 1% |
At least 2 weeks | 16.7 | Resistance if platelet response, measured in ohms of impedance, > 10 Ω |
| Gluckman 2011,99 USA | 229 | For patients with ≥ 1 occluded SVG (n = 70): mean 63 (range 55–72) For patients with patent SVG (n = 159): mean 63 (range 57–71) |
Mono | CAD | Smokers: n = 52 (22.7%) Diabetes: n = 84 (36.7%) |
Yes: CABG | 325 mg/day | No details | No details | Resistant if platelet aggregation > 1 Ω |
| Lordkipanidzé 2011,162 UK (abstract) | 198 | No details | Mono | CAD | No details | No | 80–325 mg/day | No details | No details | No details |
| Majeed 2009,117 USA | 26 | Mean 52.3 (SD 17.1) | Mono | CAD | Smokers: n = 18 (69%) Diabetes: n = 8 (31%) |
Yes | 325 mg/day | At least 7 days | 96 | Aspirin resistance defined as aggregation < 50% |
| Mueller 1997,153 Austria | 145 (100 eligible for analyses) |
Mean 62.5 (SD 11.8) | Mono | PAD/PVD | Smoking history: 57% Diabetes: 31% |
Yes: elective percutaneous balloon angioplasty | 100 mg/day | All patients stated they had not used any medication containing aspirin for at least the last 14 days prior to the baseline PFT. Aspirin was taken daily prior to follow-up PFT | Decreased platelet function: 35% No change in platelet function: 52% |
No details |
| Sobol 2009,186 Poland | 64 (101 enrolled; 64 stroke patients, 37 controls) |
Mean 57.9 (SD 10.4) | Mono | CVD/stroke | Smokers: n = 24 (53.3%) | No | 150 mg/day | No details | 36 | Lack of complete inhibition of AA-induced WBA |
| Monotherapy at time of PFT, dual during follow-up | ||||||||||
| Kaminska 2007,196 Poland | 27 (42 total sample; 27 on aspirin, 15 controls) |
Diabetes (n = 12): mean 61.5 (SD 6.0) Without diabetes (n = 15): mean 60.7 (SD 6.7) |
Mono (dual during follow-up) | UA/ACS/PPCI | Smokers: 37% Diabetes: n = 12 (44.4%) |
No details | 75–150 mg/day | All patients on aspirin previously. Duration of therapy not stated | 18.5 | Aspirin resistance was associated with the presence of platelet aggregates induced with AA |
Four studies99,128,162,166 reported no details on the timing of the PFT after aspirin ingestion. Four studies117,153,186,196 stated that there were up to 24 hours between aspirin dose and the PFT. Table 49 provides details of test characteristics.
| Study | Details of kit (manufacturer) | Anticoagulant (concentration) | Agonist (concentration) | Time since last aspirin dose |
|---|---|---|---|---|
| Multiplate® (MEA) | ||||
| Monotherapy at time of PFT and during follow-up | ||||
| Orta 2011166 (abstract) | Multiplate® analyser (Dynabyte Medical, Munich, Germany) | No details | No details | No details |
| WBA (impedance) | ||||
| Monotherapy at time of PFT and during follow-up | ||||
| Gengo 2008128 | WBA (Model 700, Chrono-Log Corporation, Havertown, PA, USA) | No details | Collagen (1 µg/ml) | No details |
| Gluckman 201199 | WBA (Model 560CA, Chrono-Log Corporation, Havertown, PA, USA) | 3.2% citrate | AA (0.5 mM) ADP (5 µM) ADP (10 µM) ADP (20 µM) Epinephrine (50 µM) Collagen (1 µg/ml) |
No details |
| Lordkipanidzé 2011162 (abstract) | WBA | No details | AA (1.6 mM) | No details |
| Majeed 2009117 | WBA (Whole-Blood Aggregometer®, Chrono-Log Corporation, Havertown, PA, USA) | No details | Collagen (1 µg/ml) Collagen (5 µg/ml) |
Up to 24 hours |
| Mueller 1997153 | WBA (CHRONO LOG® four-channel whole-blood aggregometer, Chrono-Log Corporation, Havertown, PA, USA) | No details | AA (500 µM) ADP (5 µM) ADP (10 µM) Collagen (2 µg/ml) Collagen (5 µg/ml) |
Baseline test: before starting antiplatelet therapy Follow-up tests: up to 24 hours |
| Sobol 2009186 | WBA (Chrono-Log, Havertown, PA, USA) | No details | AA (0.5 mM) | Up to 24 hours |
| Monotherapy at time of PFT, dual during follow-up | ||||
| Kaminska 2007196 | WBA (Chronolog 560, Chrono-Log Corporation, Havertown, PA, USA) | No details | AA (0.125 mM) Collagen (0.5 µg) Collagen (1 µg) Collagen (2 µg) |
Up to 24 hours |
Study design and quality
Results of the risk-of-bias assessment can be found in Tables 50–53.
| Domain 1: patient selection | Was a consecutive or random sample of patients enrolled? | Was patient selection independent of patient outcomes? | Were reasons for any posteligibility exclusions provided? |
|---|---|---|---|
| Multiplate® (MEA) | |||
| Monotherapy at time of PFT and during follow-up | |||
| Orta 2011166 (abstract) | No details | Yes | No details |
| WBA (impedance) | |||
| Monotherapy at time of PFT and during follow-up | |||
| Gengo 2008128 | Consecutive | Yes | No details |
| Gluckman 201199 | No details | Yes | Patients in whom SVG patency not assessed or those not on aspirin monotherapy. Authors stated that the study population was representative of patients undergoing isolated CABG surgery based on comparison with the Society of Thoracic Surgeons National Database |
| Lordkipanidzé 2011162 (abstract) | No details | Yes | No details |
| Majeed 2009117 | Consecutive | Yes | No details |
| Mueller 1997153 | Consecutive | Yes | 45/145 patients initially enrolled and then excluded. Reasons for exclusion: if it could be shown that patients claiming not to have used medication containing aspirin, had been using aspirin; lack of compliance in correct usage of aspirin; other exclusion criteria. All exclusion criteria seem to have been applied after enrolment and consent |
| Sobol 2009186 | Consecutive | Yes | No details |
| Monotherapy at time of PFT, dual during follow-up | |||
| Kaminska 2007196 | No details | Yes | No details |
| Domain 2: PFT | If a threshold was used, was it prespecified? | How was the threshold derived (e.g. literature cut-off, based on study data)? | Is the undertaking and interpretation of the index test blinded to the patient characteristics (including clinical outcomes)? |
|---|---|---|---|
| Multiplate® (MEA) | |||
| Monotherapy at time of PFT and during follow-up | |||
| Orta 2011166 (abstract) | No details | No details | No details |
| WBA (impedance) | |||
| Monotherapy at time of PFT and during follow-up | |||
| Gengo 2008128 | Yes (patients considered to be non-responsive to aspirin if their platelet response, measured in ohms of impedance, to 1 µg/ml of collagen was > 10 Ω, > 50% of their response to 5 µg/ml of collagen and/or > 6 Ω to 0.5 mM AA) | Criteria similar to ones cited (references given226–230) | No details (but quality control procedures described) |
| Gluckman 201199 | Yes (aspirin resistant if AA-induced platelet aggregation was > 1 Ω) | Stated that normal range in authors’ laboratory for aspirin-naive subjects was 5–7 Ω. No further details | No details |
| Lordkipanidzé 2011162 (abstract) | No details | No details | No details |
| Majeed 2009117 | Yes (aspirin resistance defined as at least 50% platelet response) | Formula and reference cited231 | No details |
| Mueller 1997153 | No threshold | Different ‘classes’ of effect of aspirin on platelet function depending on change from baseline derived from data | No details |
| Sobol 2009186 | Yes (but no numerical cut-off) | A lack of complete inhibition of AA-induced whole-blood aggregation | No details |
| Monotherapy at time of PFT, dual during follow-up | |||
| Kaminska 2007196 | Not explicitly stated; assumed that if there is any aggregation patients are classed as aspirin resistant | No details | No details |
| Domains 3 and 4: outcomes and study attrition | Were the outcomes of interest clearly defined in advance? | Were the outcome results interpreted without knowledge of the results of the PFT? | What was the proportion of missing data? (State reasons for loss to follow-up or differences in those who completed or were lost) |
|---|---|---|---|
| Multiplate® (MEA) | |||
| Monotherapy at time of PFT and during follow-up | |||
| Orta 2011166 (abstract) | Yes | No details | Appears to be no loss to follow-up |
| WBA (impedance) | |||
| Monotherapy at time of PFT and during follow-up | |||
| Gengo 2008128 | Yes | No details | Appears to be no loss to follow-up |
| Gluckman 201199 | Yes | Yes. Stated that images were analysed by two blinded reviewers (98% concordance) with a third reviewer adjudicating as necessary | 75/229 patients not included at follow-up |
| Lordkipanidzé 2011162 (abstract) | Yes | No details | No details |
| Majeed 2009117 | Yes | No details | No losses to follow-up |
| Mueller 1997153 | Yes | No details | 4/100 patients lost to follow-up by 52 weeks (for repeat PFT) but appears all patients included for outcomes |
| Sobol 2009186 | Yes | No details | No losses to follow-up |
| Monotherapy at time of PFT, dual during follow-up | |||
| Kaminska 2007196 | No; the paper did not focus on clinical outcomes (it was focused on the results of the PFTs) | Yes | The paper states that one person (out of 27) was lost to follow-up at 6 months as a result of MI; this is the same person who is recorded as having the clinical event of MI |
| Domain 5: confounding | Are confounders accounted for in the design or analysis (e.g. adjustment, stratification)? | If there is an adjusted outcome measure (e.g. OR, HR), what were the factors that were adjusted for? | If a HR was presented, was the proportional hazards assumption met? | Was compliance measured? | How was compliance measured? | Level of compliance |
|---|---|---|---|---|---|---|
| Multiplate® (MEA) | ||||||
| Monotherapy at time of PFT and during follow-up | ||||||
| Orta 2011166 (abstract) | Design: N/A Analysis: no |
N/A | No details | No details | No details | No details |
| WBA (impedance) | ||||||
| Monotherapy at time of PFT and during follow-up | ||||||
| Gengo 2008128 | Design: N/A Analysis: yes (OR) |
Age and sex; diseases including diabetes, hypertension, dyslipidaemia and CAD; nature of recurrent event (stroke vs. TIA); and the use of other drugs (clopidogrel, dipyridamole, COX-2 selective non-steroidal anti-inflammatory agents, non-selective anti-inflammatory agents) | N/A | Yes | Method described for determining presence of salicylates in urine. Patients without salicylates in their urine were excluded at baseline | No details |
| Gluckman 201199 | Design: N/A Analysis: no |
N/A | N/A | Yes | Pill counts at each postoperative encounter | No details |
| Lordkipanidzé 2011162 (abstract) | Design: N/A Analysis: unclear whether adjusted or unadjusted OR |
No details | N/A | No details | No details | No details |
| Majeed 2009117 | Design: N/A Analysis: no |
N/A | N/A | Yes | Daily aspirin administration in hospital confirmed by patients’ self-reporting, reports from nursing personnel and reviews of daily pharmacy records. After discharge, adherence to daily aspirin was assessed at each weekly visit by verbal self-reporting from patients | No details |
| Mueller 1997153 | Design: N/A Analysis: no |
N/A | N/A | Yes | A positive reaction to AA-mediated aggregometry. Non-compliance resulted in exclusion from the study | No details |
| Sobol 2009186 | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Monotherapy at time of PFT, dual during follow-up | ||||||
| Kaminska 2007196 | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
Patient selection was independent of study outcome in all included studies, with the PFT preceding any outcomes (as specified in the study selection criteria). Four studies stated that consecutive patients were enrolled into the study117,128,153,186 and the other studies did not provide details. Only two studies99,153 had clear details on posteligibility exclusion of patients; in one of these studies153 a criterion for exclusion was lack of compliance.
A predetermined threshold percentage (for platelet aggregation) was given in four studies. 99,117,128,186 Thresholds were > 1 Ω99 at least 50% platelet response;117 and > 10 Ω in response to 1 μg/ml of collagen, > 50% of the response to 5 μg/ml of collagen and/or > 6 Ω to 0.5 mM arachidonic acid. 128 In one study the numerical cut-off was not stated and patients were described as resistant if there was a ‘lack of complete inhibition of arachidonic acid induced whole blood aggregation’. 186 Another study196 stated that patients were classed as aspirin resistant if there was any aggregation at all. One study stated that quartiles were used,153 and the remaining two studies162,166 reported no details. None of the studies gave clear details on blinding of laboratory staff to patient characteristics.
Outcome measures of interest were clearly predefined in all but one study. 99,117,128,153,162,166,186 None of the studies provided clear details regarding blinding to the PFT results of those assessing outcomes.
There appeared to be no loss to follow-up in four studies. 117,128,166,186 Loss to follow-up was stated in two studies153,196 and was approximately 4% in both. There were no clear details in one study. 162 The differences in completeness of follow-up may reflect length of follow-up, study design (outcome only followed up in those that had repeat PFTs) or quality of reporting.
Compliance was measured in four studies,99,117,128,153 but there were no details on level of compliance. It was determined by presence of salicylates in urine,128 pill counts,99 patient interview, nurse assessment and pharmacy records during the period of hospitalisation and through self-report only after discharge,117 and a positive reaction to arachidonic acid-mediated aggregometry. 153 The four remaining studies reported no details on compliance. 162,166,186,196
Six studies did not appear to undertake any adjusted analyses. 99,117,153,166,186,196 One study128 attempted to adjust for a number of factors, including age, sex, presence of various comorbidities, nature of recurrent event and use of various other drugs. In one study162 it is not clear whether the ORs reported were adjusted or unadjusted. There may be selective reporting in that only variables that showed significance on univariate analysis might have been included in multivariate analyses.
Overview of outcomes
There were eight studies99,117,128,153,162,166,186,196 using WBA as a PFT and reporting on death, MACEs and ischaemic/thrombotic events (Table 54).
| Study | Death | MACEs | Ischaemic/thrombotic events | Bleeding | Length of follow-up |
|---|---|---|---|---|---|
| Multiplate® (MEA) | |||||
| Monotherapy at time of PFT and during follow-up | |||||
| Orta 2011166 (abstract) | ✓ | Mean 20.7 months (SD 6.1 months) | |||
| WBA (impedance) | |||||
| Monotherapy at time of PFT and during follow-up | |||||
| Gengo 2008128 | ✓ | 29 months | |||
| Gluckman 201199 | ✓ | 6 months | |||
| Lordkipanidzé 2011162 (abstract) | ✓ | 3 years | |||
| Majeed 2009117 | ✓ | Median 315 days (range 9–833 days) | |||
| Mueller 1997153 | ✓ | 18 months | |||
| Sobol 2009186 | ✓ | 10 days | |||
| Monotherapy at time of PFT, dual during follow-up | |||||
| Kaminska 2007196 | ✓ | ✓ | 12 months | ||
Death
Two studies186,196 reported deaths (Table 55). One of these186 could not be presented in a forest plot, as although it stated that one death occurred in the resistant group, it was unclear whether resistance was determined by WBA and/or PFA-100®. Outcome statistics are presented in Figures 44 and 45. Unadjusted ORs and HRs were calculable for the other study. 196 These were not statistically significant; the wide CIs reflect the fact that there were two events in the resistant arm and no events in the sensitive arm.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| WBA (impedance) | ||||||
| Monotherapy at time of PFT and during follow-up | ||||||
| Sobol 2009186 | Unclear which test used to classify as resistant | |||||
| Monotherapy at time of PFT, dual during follow-up | ||||||
| Kaminska 2007196 | ✓a | ✓a | ✓a | |||
FIGURE 44.
Whole-blood aggregometry, monotherapy: death, unadjusted ORs. AA, arachidonic acid.

FIGURE 45.
Whole-blood aggregometry, monotherapy: death, unadjusted HRs. AA, arachidonic acid.

Though the trend across the two studies is consistent (the only events are in the resistant group), there were too few studies and events to draw any conclusion regarding risk of death.
Major adverse cardiac events
Two studies reported MACEs162,166 (Table 56). Outcome statistics are shown in Figures 46 and 47. One study,162 in a CAD population, reported a non-statistically significant unadjusted OR. The other study166 reported an unadjusted OR and HR, which were both statistically significant. It should be noted that, although considered to be at cardiovascular risk, this was primarily a renal failure population, which might not be comparable with the other studies included here. This study also used the Multiplate® system.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Multiplate® (MEA) | ||||||
| Monotherapy at time of PFT and during follow-up | ||||||
| Orta 2011166 (abstract) | ✓a | ✓ | ✓a | |||
| WBA (impedance) | ||||||
| Monotherapy at time of PFT and during follow-up | ||||||
| Lordkipanidzé 2011162 (abstract) | ✓a | |||||
FIGURE 46.
Whole-blood aggregometry, monotherapy: MACEs, unadjusted ORs. AA, arachidonic acid.

FIGURE 47.
Whole-blood aggregometry, monotherapy: MACEs, unadjusted HRs.

Based on these two studies, no conclusions can be drawn regarding the risk of MACEs.
Ischaemic/thrombotic events
Five studies99,117,128,153,196 reported ischaemic/thrombotic outcomes (Table 57). Results from two117,153 of these could not be presented in a forest plot. In one117 it appeared that all eight thromboembolic events occurred in the resistant arm, but overall numbers of patients in the resistant and sensitive groups were unclear. In the other study,153 no patients were defined as resistant and therefore all eight events (reocclusions) occurred in sensitive patients.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| WBA (impedance) | ||||||
| Monotherapy at time of PFT and during follow-up | ||||||
| Gengo 2008128 | ✓a | ✓ | ✓a | ✓a | ||
| Gluckman 201199 | ✓ | |||||
| Majeed 2009117 | Proportions of patients in resistant and sensitive groups were unclear | |||||
| Mueller 1997153 | No patients defined as resistant | |||||
| Monotherapy at time of PFT, dual during follow-up | ||||||
| Kaminska 2007196 | ✓a | ✓a | ✓a | |||
Outcome statistics are presented in Figures 48–50. Eleven unadjusted ORs were presented based on three studies. 99,128,196 There was only one event (MI) in one study,196 therefore the four ORs are associated with very wide CIs. One study99 finds no difference in risk, whereas in one128 the ORs are statistically significant (more events in the resistant arm).
FIGURE 48.
Whole-blood aggregometry, monotherapy: ischaemic/thrombotic events, unadjusted ORs. AA, arachidonic acid.
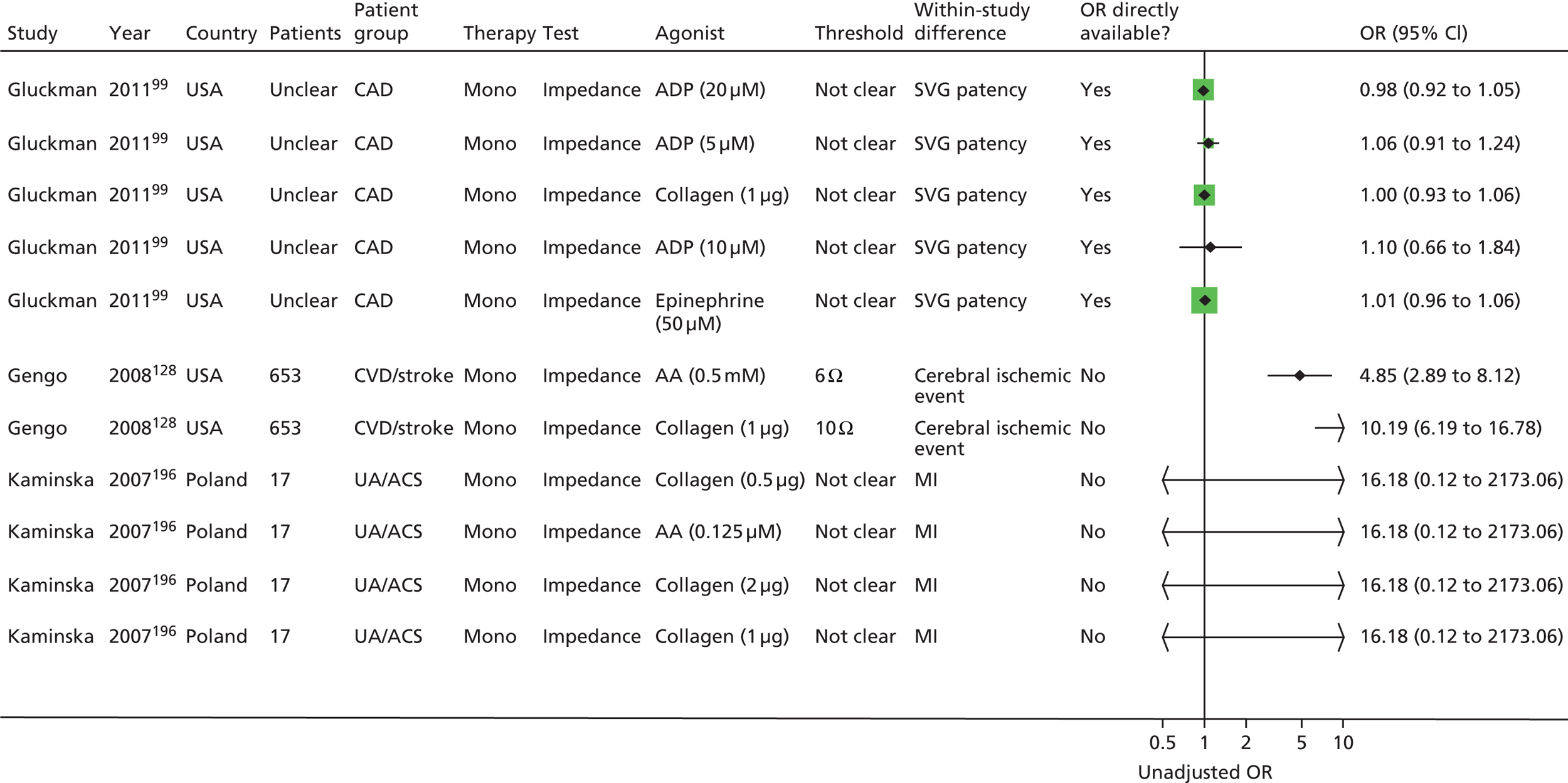
FIGURE 49.
Whole-blood aggregometry, monotherapy: ischaemic/thrombotic events, adjusted ORs. AA, arachidonic acid.

FIGURE 50.
Whole-blood aggregometry, monotherapy: ischaemic/thrombotic events, unadjusted HRs. AA, arachidonic acid.

These ORs remain statistically significant when adjusted for. Unadjusted HRs are also statistically significant for the same study,128 but not for the other study196 presenting unadjusted HRs.
Given the heterogeneity between studies (population, outcomes) and the lack of consistency in terms of direction of effect, no firm conclusions can be drawn.
Summary: whole-blood aggregometry
Eight studies were identified,99,117,128,153,162,166,186,196 with mainly stable disease populations (seven of eight studies). In one study196 with an acute disease population, dual therapy was initiated after the PFT. Only one166 of the eight studies used the Multiplate® system.
There was a lack of reporting of quality criteria, making overall judgements about risk of bias difficult. Lack of detail related to blinding in particular (to patient characteristics and results of the PFT) and the level of compliance. Only two studies99,153 gave details on posteligibility exclusions and only three studies99,117,128 gave details on the threshold used.
Only two studies reported deaths,186,196 with a total of three events across both studies. There are therefore too few data to draw any firm conclusions on the risk of death. MACEs were also only reported by two studies. 162,166 A statistically significant result (more events in the resistant arm) was shown by one of these,166 and again, firm conclusion cannot be drawn, particularly as there were differences in populations between the studies and differences in test characteristics. More data were available for ischaemic/thrombotic events, based on five studies. 99,117,128,153,196 However, there were differences in populations (e.g. CAD, UA/ACS), outcome measures (e.g. SVG patency, MI, reocclusion, cerebral ischaemic events) and treatment (e.g. dual therapy after the PFT), and there appeared to be little consistency across results, though some were statistically significant (more events in the resistant arm). There were no studies reporting bleeding events.
Given the above data it is difficult to assess the overall prognostic effect, and no conclusions can be drawn regarding the overall potential usefulness of WBA as a prognostic factor.
Summary: whole-blood aggregometry
-
Eight studies were identified, with patients with stable disease in seven of these.
-
The PFT thresholds used were not always reported or consistent across studies.
-
A lack of detail in reporting of quality criteria, particularly around blinding and details (and implications) of compliance, hampered an overall risk-of-bias assessment.
-
Heterogeneity in outcomes, patient groups and types of reported statistics meant that meta-analysis was not possible.
-
Few adjusted results were presented, and thus it is not possible to ascertain the additional prognostic value of the test over other prognostic factors.
-
Given the limited number of data, no firm conclusions could be drawn regarding risk of death or MACEs.
-
Heterogeneity around populations, outcomes and treatment (post PFT), and a lack of a clear consistent trend across the studies, meant that firm conclusions could also not be drawn for ischaemic/thrombotic events, though there were some statistically significant results (more events in the resistant arm).
-
No studies reported bleeding events.
Thromboelastography
Population and test characteristics
Three studies117,168,174 were identified in this category, two of which were reported in abstract form only. 168,174 Populations had CAD (two studies)117,168 and UA/ACS (one study). 174 One study117 reported that patients had their primary underlying condition for a mean period of 41.4 months. The two remaining studies did not report this information.
In all three studies,117,168,174 it appeared that patients were exclusively on monotherapy both at the time of the PFT and during follow-up. It is possible that not all studies have reported where a proportion of patients commenced additional therapies during follow-up.
Only one study117 reported medications used by patients. These included diuretics, ACE inhibitors, angiotensin II receptor blockers, beta-blockers, digoxin, spironolactone, nitrate, statins, warfarin, intravenous inotropic therapy and amiodarone. None of the studies reported details on NSAIDs.
The number of participants in the studies ranged from 26 to 250 (see Table 58). Mean ages of patients were 52,117 60168 and 62 years. 174 There were more men than women in two studies,117,168 with proportions of 73%117 and 68.5%. 168 The remaining study174 reported sex data for aspirin-resistant patients only (42 of the total sample of 250), 74% of whom were male.
The dose of aspirin was reported in all studies and ranged from 15 mg/day to 325 mg/day. Two studies168,174 gave no details regarding the length of time patients had been receiving aspirin therapy, and one study reported that patients had been taking aspirin for at least 7 days117 (see Table 58). One study117 reported that aspirin was provided in both enteric and plain forms, and the other studies did not report this information.
The main study characteristics are listed in Table 58.
| Study/country | Number of patients | Age (years) | Therapy | Main underlying condition | Selected other population details | Due to undergo vascular intervention? | Aspirin dose/frequency | Duration of aspirin therapy (prior to PFT) | Percentage aspirin resistant | Derivation of threshold/comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Majeed 2009,117 USA | 26 | Mean 52.3 (SD 17.1) | Mono | CAD | Smokers: n = 18 (69%) Diabetes: n = 8 (31%) |
Yes | 325 mg/day | At least 7 days | 96 | Aspirin resistance defined as aggregation ≥ 50% |
| Sahin 2011,168 Turkey (abstract) | 168 | Mean 60.1 (SD 8.4) | Mono | CAD | No details | No details | 100 mg/day | No details | 16.1 | Aspirin resistance defined as aggregation ≥ 50% |
| Tan 2010,174 China (abstract) | 250 | Mean 62 (SD 17) | Mono | UA/ACS | No details | No details | 15–250 mg/day | No details | 18.8 | Aspirin resistance defined as aggregation ≥ 50% |
The test performed in all studies was TEG. All studies used arachidonic acid as an agonist and details regarding anticoagulants were not reported in any studies.
Two studies117,174 stated that there were up to 24 hours between aspirin dose and PFT. The remaining study168 provided no details on the time between taking aspirin and the PFT. Table 59 provides details of test characteristics.
| Study | Details of kit (manufacturer) | Anticoagulant (concentration) | Agonist (concentration) | Time since last aspirin dose |
|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||
| Majeed 2009117 | TEG® 5000 (Thromboelastograph® Hemostasis Analyzer, Haemonetics Corporation, Braintree, MA) | No details | Collagen (1 µg/ml) Collagen (5 µg/ml) |
Up to 24 hours |
| Sahin 2011168 (abstract) | Modified thromboelastogram | No details | AA | No details |
| Tan 2010174 (abstract) | TEG® (Haemonetics Corporation, Braintree, MA) | No details | AA | Up to 24 hours |
Study design and quality
Results of the risk-of-bias assessment can be found in Tables 60–63.
| Domain 1: patient selection | Was a consecutive or random sample of patients enrolled? | Was patient selection independent of patient outcomes? | Were reasons for any posteligibility exclusions provided? |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Majeed 2009117 | Consecutive | Yes | No details |
| Sahin 2011168 (abstract) | Consecutive | Yes | No details |
| Tan 2010174 (abstract) | Consecutive | Yes | No details |
| Domain 2: PFT | If a threshold was used, was it prespecified? | How was the threshold derived (e.g. literature cut-off, based on study data)? | Is the undertaking and interpretation of the index test blinded to the patient characteristics (including clinical outcomes)? |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Majeed 2009117 | Yes (≥ 50%) | Formula and reference cited232 | No details |
| Sahin 2011168 (abstract) | Yes (< 50% AA-induced whole-blood thrombosit aggregation inhibition by TEG) | No details | No details |
| Tan 2010174 (abstract) | Yes (> 50%) | No details | No details |
| Domains 3 and 4: outcomes and study attrition | Were the outcomes of interest clearly defined in advance? | Were the outcome results interpreted without knowledge of the results of the PFT? | What was the proportion of missing data? (State reasons for loss to follow-up or differences in those who completed or were lost) |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Majeed 2009117 | Yes | No details | No losses to follow-up |
| Sahin 2011168 (abstract) | No details | No details | No losses to follow-up |
| Tan 2010174 (abstract) | Yes (although unclear if separate or composite) | No details | No details |
| Domain 5: confounding | Are confounders accounted for in the design or analysis (e.g. adjustment, stratification)? | If there is an adjusted outcome measure (e.g. OR, HR), what were the factors that were adjusted for? | If a HR was presented, was the proportional hazards assumption met? | Was compliance measured? | How was compliance measured? | Level of compliance |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Majeed 2009117 | Design: N/A Analysis: no |
N/A | N/A | Yes | Daily aspirin administration in hospital confirmed by patients’ self-reporting, reports from nursing personnel and reviews of daily pharmacy records. After discharge, adherence to daily aspirin was assessed at each weekly visit by verbal self-reporting from patients | No details |
| Sahin 2011168 (abstract) | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Tan 2010174 (abstract) | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
Patient selection was independent of study outcome in all three included studies,117,168,174 with the PFT preceding any outcomes (as specified in the study selection criteria). All studies stated that consecutive patients were enrolled into the study and no studies had clear details on posteligibility exclusion of patients.
A predetermined threshold percentage (for platelet aggregation) was given as > 50% or ≥ 50% for all three studies. 117,168,174 Only one study117 cited a reference232 and provided details on the method of derivation of this threshold. For the remaining two studies there were no details on threshold derivation. None of the studies gave clear details on blinding of laboratory staff to patient characteristics.
Outcome measures of interest were clearly predefined in two studies. 117,174 In one of these studies it was unclear if outcomes were separate or composite,174 and the remaining study provided no details. None of the studies had clear details regarding blinding to the PFT results of those assessing outcomes. There appeared to be no loss to follow-up in two studies117,168 and the remaining study174 provided no details on missing data or whether or not there was any loss to follow-up.
Compliance was measured in one study117 by patient interview, nurse assessment and pharmacy records during the period of hospitalisation, and through self-report only after discharge. No details on the level of compliance were stated.
All three studies did not appear to undertake any adjusted analyses.
Overview of outcomes
Three studies were identified,117,168,174 reporting on only two of the outcomes of interest (death and ischaemic/thrombotic events) (Table 64).
| Study | Death | MACEs | Ischaemic/thrombotic events | Bleeding | Length of follow-up |
|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||||
| Majeed 2009117 | ✓ | Median 315 days (range 9–833 days) | |||
| Sahin 2011168 (abstract) | ✓ | ✓ | Mean 464 days (SD 264 days) | ||
| Tan 2010174 (abstract) | ✓ | ✓ | 360 days (range 0–523 days); not stated if mean or median | ||
Death
Two studies reported on deaths (Table 65). 168,174 One study was not presented in the forest plots. 174 This found a death rate of 26% in the resistant group and 11% in the sensitive group (UA/ACS population), but there were no raw data to confirm these proportions. Outcome statistics are presented in Figures 51 and 52. In the other study, both the adjusted OR and HR were statistically non-significant. The wide CI reflects the fact that there was only one death in the resistant group and no deaths in the sensitive group.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Sahin 2011168 (abstract) | ✓a | ✓a | ✓a | |||
| Tan 2010174 (abstract) | ||||||
FIGURE 51.
Thromboelastography, monotherapy: death, unadjusted ORs.

FIGURE 52.
Thromboelastography, monotherapy: death, unadjusted HRs. AA, arachidonic acid.
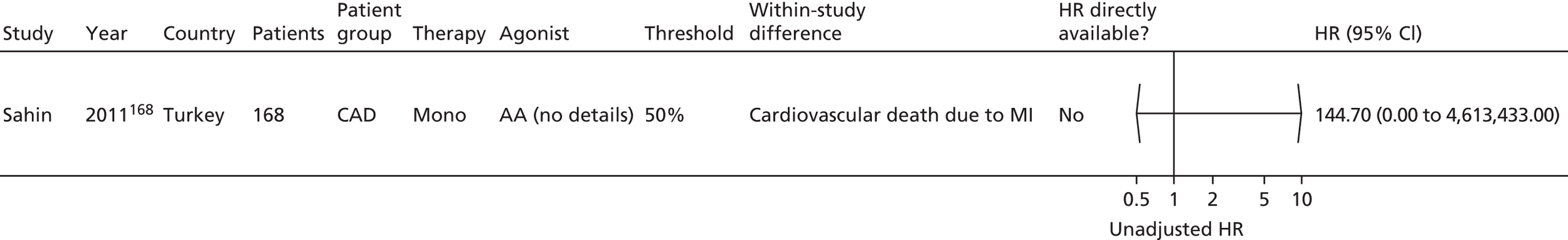
Overall, there is too little evidence on which to base any conclusions regarding risk of death.
Ischaemic/thrombotic events
Three studies reported ischaemic/thrombotic events (Table 66). 117,168,174 Two of these were not presented in the forest plots. One174 found a rate of 74% in the resistant group for recurrent MI or thrombosis and 24% in the sensitive group (UA/ACS population), but there were no raw data to confirm these proportions. In the other study,117 it appeared that all eight thromboembolic events occurred in the aspirin-resistant group.
| Study | Unadjusted OR | Adjusted OR | Unadjusted HR | Adjusted HR | Other measures related to prognosis | Sensitivity/specificity presented or calculable |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Majeed 2009117 | ||||||
| Sahin 2011168 (abstract) | ✓a | ✓a | ✓a | |||
| Tan 2010174 (abstract) | ||||||
Outcome statistics are presented in Figures 53 and 54. Unadjusted ORs and HRs of the third study168 were all statistically significant for different outcomes.
FIGURE 53.
Thromboelastography, monotherapy: ischaemic/thrombotic events, unadjusted ORs.

FIGURE 54.
Thromboelastography, monotherapy: ischaemic/thrombotic events, unadjusted HRs. AA, arachidonic acid.
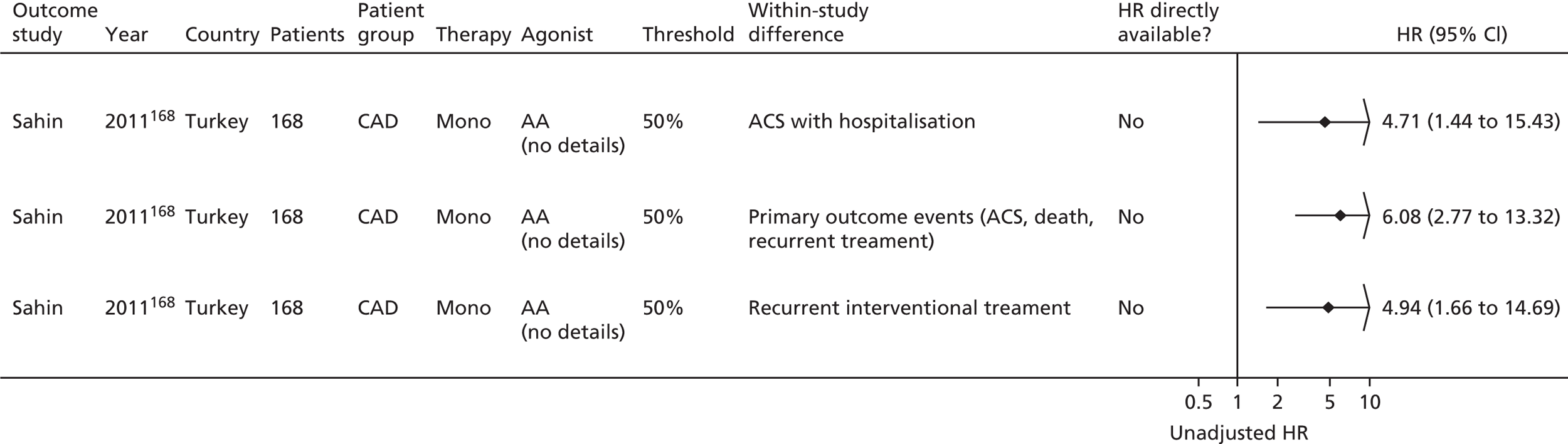
Thus, it appears that more events occurred consistently in the resistant arm; however, this is based on only three studies,117,168,174 two of which117,174 did not report all relevant data clearly.
Summary: thromboelastography
Only three studies were identified in this category,117,168,174 two with a stable117,168 and one with an acute174 disease population. Two of the three were in abstract form,168,174 so there is a lack of detail on specific patient characteristics.
There was a lack of detail in reporting of quality criteria, making overall judgements about risk of bias difficult. Lack of detail related in particular to blinding and level of compliance. The same threshold was used across the three studies. No adjusted outcome statistics were reported.
Two studies reported deaths168,174 and three reported ischaemic/thrombotic events. Only one study for each outcome could be presented in a forest plot. 168 There was too little evidence to draw any conclusions for risk of death. The direction of effect was consistent for ischaemic/thrombotic events (more events in the resistant group), but this was based on few studies and there were some reporting issues. There were differences in study populations and types of outcome measures reported (for ischaemic/thrombotic events). No adjusted measures were reported and there were no studies reporting MACEs or bleeding events.
Despite the heterogeneity, the direction of prognostic effect appears to be largely consistent with more events occurring in aspirin-resistant patients (ORs and HRs usually > 1). This suggests that TEG is a potential prognostic factor, but this is only a qualitative judgement on the evidence available and is based on very few studies; meta-analysis was not possible as there was only one study in the forest plots, and therefore a firm quantitative conclusion regarding whether or not TEG is prognostic is not currently possible.
Summary: thromboelastography
-
Three studies were identified (two with stable, one with an acute disease population).
-
The threshold used was consistent (50%).
-
A lack of detail in reporting of quality criteria, particularly around blinding and details (and implications) of compliance, hampered an overall risk-of-bias assessment.
-
Heterogeneity in outcomes, patient groups and types of reported statistics, and the fact that only one study presented data suitable for use in a forest plot, meant that meta-analysis was not possible.
-
No adjusted results were presented, and thus it is not possible to ascertain the additional prognostic value of the test over other prognostic factors.
-
No conclusions could be drawn regarding risk of death in resistant and sensitive groups.
-
Despite clinical heterogeneity between studies, there was an overall consistent trend for more events to occur in the ‘aspirin-resistant’ group for ischaemic/thrombotic events; however, this was based on one study only.
-
No studies reported MACEs or bleeding events.
Miscellaneous tests
The population and test characteristics are presented in Tables 67 and 68. There was a large amount of heterogeneity across the studies in terms of PFTs and populations. No two studies used both the same PFT and the same treatment after the PFT (i.e. monotherapy or dual therapy; see Table 67), therefore each study needs to be considered on its own.
| Study/country | Number of patients | Age (years) | Therapy | Main underlying condition | Selected other population details | Due to undergo vascular intervention? | Aspirin dose/frequency | Duration of aspirin therapy (prior to PFT) | Percentage aspirin resistant | Derivation of threshold/comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Flow cytometry | ||||||||||
| Frelinger 2009,76 USA | 700 (555 eligible for analysis) | Mean 60.7 (SEM 0.44) | Mono (32% dual during follow-up) | CAD | Smokers: 22% current (72% prior) Diabetes: 27% |
No | 81 or 325 mg/day | At least 3 days | 8.1 | No details |
| Monotherapy at time of PFT, dual during follow-up | ||||||||||
| Flow cytometry | ||||||||||
| Kaminska 2007,196 Poland | 27 (42 total sample; 27 on aspirin, 15 controls) | With diabetes (n = 12): mean 61.5 (SD 6.0) Without diabetes (n = 15): mean 60.7 (SD 6.7) |
Mono (dual during follow-up) | UA/ACS/PPCI | Smokers: 37% Diabetes: n = 12 (44.4%) |
No details | 75–150 mg/day | All patients on aspirin previously. Duration of therapy not stated | 18.5 | Aspirin resistance was associated with presence of platelet aggregates with blood induced with AA |
| Surgicutt II | ||||||||||
| Buchanan 2000,152 Canada | 516 (289 eligible for analysis) | Resistant (n = 158): mean 60.8 (SD 9.3) Sensitive (n = 131): mean 61.4 (SD 8.8) |
Mono | CABG | Ex-smokers: 63.1% of resistant group (n = 158); 78.8% of sensitive group (n = 131) Diabetes: 19.6% of resistant group (n = 158); 20.6% of sensitive group (n = 131) |
No details | 325 mg/day | 2 weeks. Bleeding times also recorded when not taking aspirin for at least 1 week | 54.7 | Coefficient of variation between on and off aspirin bleeding times ≤ 26% classified as aspirin resistant |
| Apact II platelet aggregometer | ||||||||||
| Stejskal 2006,198 Czech Republic | 103 | 64 (SD 13) | Mono | ACS | Smokers: 21% active or past smokers Diabetes: 33% |
No details | 100 mg/day | At least 3 days | 55.3 | Aspirin resistant if spontaneous aggregation was > 5% and if the slope of the aggregation curve after CPG induction was above 53%/minute |
| Platelet reactivity test | ||||||||||
| Grotemeyer 1993,154 Germany | 180 (174 eligible for analysis) | Mean 58 (SD 15) | Mono | CVD/stroke | 76 patients (7 who subsequently had an event, 69 who did not have an event) | No details | 500 mg three times a day | 24 months | 34.5 | Platelet reactivity value > 1.25 |
| Impact-R® (cone and platelet analyser test) | ||||||||||
| Monotherapy at time of PFT and during follow-up | ||||||||||
| Schwammenthal 2008,125 Israel | 105 (79 eligible for analysis) | Mean 63 (SD 12) | Mono (3.8% on dual at baseline and follow-up) | CVD/stroke | Current smokers: n = 20 (19%) Past smokers: n = 9 (27.6%) Diabetes: n = 28 (26.7%) |
No | 100 mg/day (55%), 325 mg/day (45%) | After stroke onset (at least 6 hours prior to PFT). 40% on chronic aspirin therapy (> 1 week prior to stroke) | 46.8 | Partial response 20–39% aggregation, good response < 20% aggregation |
| Monotherapy at time of PFT, dual during follow-up | ||||||||||
| Spectre 2011,93 Israel | 63 (54 eligible for analysis) | Mean 59.7 | Mono at time of PFT (dual during follow-up, post PCI) | PPCI | Smokers: 30% Diabetes: 24% |
Yes | 100 mg/day | Previous long-term aspirin use in 73% | 66.7 | Percentage surface coverage of adherent platelets. Upper tertile 1.95 (SEM 0.35), middle tertile 1.24 (SEM 0.1), lower tertile 3.3 (SEM 0.65) |
| Study | Details of kit (manufacturer) | Anticoagulant (concentration) | Agonist (concentration) | Time since last aspirin dose |
|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||
| Flow cytometry | ||||
| Frelinger 200976 | Flow cytometry (FACSCalibur™ flow cytometer, BD Biosciences, Franklin Lakes, NJ, USA) | No details | No details | No details |
| Monotherapy at time of PFT, dual during follow-up | ||||
| Flow cytometry | ||||
| Kaminska 2007196 | Flow cytometry | No details | AA Collagen |
Up to 24 hours |
| Surgicutt II | ||||
| Buchanan 2000152 | Surgicutt II bleeding time device (ITC Commercial Group, Piscataway, NJ, USA) | No details | No details | First testing: up to 24 hours Repeat testing: when each patient had not taken aspirin for a minimum of 7 days |
| Apact II platelet aggregometer | ||||
| Stejskal 2006198 | Apact II platelet aggregometer (Labitec GmbH, Ahrensburg, Germany) | No details | CPG 3 µM concentration | Up to 24 hours |
| Platelet reactivity test | ||||
| Grotemeyer 1993154 | Platelet reactivity test (newly developed modification – reference cited233) | No details | EDTA | 12 hours |
| Impact-R® (cone and platelet analyser test) | ||||
| Monotherapy at time of PFT and during follow-up | ||||
| Schwammenthal 2008125 | Impact-R® (cone and platelet analyser test) | No details | AA (1.6 mM) | At least 6 hours before blood sampling |
| Monotherapy at time of PFT, dual during follow-up | ||||
| Spectre 201193 | Impact-R® (cone and platelet analyser test) | Sodium citrate | AA (0.32 mM) | Up to 24 hours |
As it was not possible to compare directly across tests or to usefully summarise results, there has been no discussion on the quality of studies and no presentation of results. For reference, the main quality characteristics are presented in Tables 69–72 and extracted results can be found in the webpage linked to the report (http://medweb4.bham.ac.uk/NIHR_Aspirin_Resistance/).
| Domain 1: patient selection | Was a consecutive or random sample of patients enrolled? | Was patient selection independent of patient outcomes? | Were reasons for any posteligibility exclusions provided? |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Flow cytometry | |||
| Frelinger 200976 | Consecutive | Yes | Stated that less than 3% of eligible patients declined participation (reason not given) |
| Monotherapy at time of PFT, dual during follow-up | |||
| Flow cytometry | |||
| Kaminska 2007196 | No details | Yes | No details |
| Surgicutt II | |||
| Buchanan 2000152 | Consecutive | Yes | 15% of those recruited withdrew from the study; 28% of those who continued were excluded as a result of non-compliance |
| Apact II platelet aggregometer | |||
| Stejskal 2006198 | No details | Yes | No details |
| Platelet reactivity test | |||
| Grotemeyer 1993154 | Consecutive | Yes | No details |
| Impact-R® (cone and platelet analyser test) | |||
| Monotherapy at time of PFT and during follow-up | |||
| Schwammenthal 2008125 | Consecutive | Yes | No details |
| Monotherapy at time of PFT, dual during follow-up | |||
| Spectre 201193 | Consecutive | Yes | No details |
| Domain 2: PFT | If a threshold was used, was it prespecified? | How was the threshold derived (e.g. literature cut-off, based on study data)? | Is the undertaking and interpretation of the index test blinded to the patient characteristics (including clinical outcomes)? |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Flow cytometry | |||
| Frelinger 200976 | No details (appears to be a threshold as OR calculated) | No details | No details |
| Monotherapy at time of PFT, dual during follow-up | |||
| Flow cytometry | |||
| Kaminska 2007196 | Not explicitly stated; assumed that if there is any aggregation patients are classed as aspirin resistant | No details | No details |
| Surgicutt II | |||
| Buchanan 2000152 | Yes; coefficient of variation between on and off aspirin bleeding times > 26% is an aspirin responder Coefficient of variation between on and off aspirin bleeding times ≤ 26% classified as aspirin resistant |
A pilot study with healthy volunteers was performed to determine the reproducibility of the Surgicutt II bleeding time test as performed by the BRAT study nurses and technicians, and to determine the biological variability of the bleeding times over 10 weeks | No details |
| Apact II platelet aggregometer | |||
| Stejskal 2006198 | Yes; a patient was considered to be an ‘aspirin responder’ (without aspirin resistance) if spontaneous aggregation was below 5% | No details | No details |
| Platelet reactivity test | |||
| Grotemeyer 1993154 | Yes (platelet reactivity value > 1.25) | Stated that patients arbitrarily subdivided (author’s own reference cited234) | No details |
| Impact-R® (cone and platelet analyser test) | |||
| Monotherapy at time of PFT and during follow-up | |||
| Schwammenthal 2008125 | Yes; partial response 20–39% vs. good response < 20% | Literature cited149 | Unclear; treating physicians and the investigators evaluating the patients were blinded to the results of the platelet function studies |
| Monotherapy at time of PFT, dual during follow-up | |||
| Spectre 201193 | Yes, but no specific values; tertiles of percentage surface coverage of adherent platelets | Tertiles | No details |
| Domains 3 and 4: outcomes and study attrition | Were the outcomes of interest clearly defined in advance? | Were the outcome results interpreted without knowledge of the results of the PFT? | What was the proportion of missing data? (State reasons for loss to follow-up or differences in those who completed or were lost) |
|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | |||
| Flow cytometry | |||
| Frelinger 200976 | Yes | Yes; all clinical outcome data obtained by research personnel blinded to results of PFTs | 127/682 lost to follow-up (for MACE outcome) |
| Monotherapy at time of PFT, dual during follow-up | |||
| Flow cytometry | |||
| Kaminska 2007196 | No – the paper did not focus on clinical outcomes; it was focused on the results of the PFTs | Yes | The paper states that one person (out of 27) was lost to follow-up at 6 months as a result of MI; this is the same person who is recorded as having the clinical event of MI |
| Surgicutt II | |||
| Buchanan 2000152 | Yes | Yes; outcome assessors unaware of aspirin responder status | 227/516 lost to follow-up at 2 years (withdrawal or exclusion because of non-compliance) |
| Apact II platelet aggregometer | |||
| Stejskal 2006198 | Yes (broadly) | No details | Appeared to be no loss to follow-up |
| Platelet reactivity test | |||
| Grotemeyer 1993154 | Yes | No details | 6/180 lost to follow-up |
| Impact-R® (cone and platelet analyser test) | |||
| Monotherapy at time of PFT and during follow-up | |||
| Schwammenthal 2008125 | Yes | Yes; treating physicians and the investigators evaluating the patients were blinded to the results of the platelet function studies | Follow-up data were available for 81/105 patients (77%) |
| Monotherapy at time of PFT, dual during follow-up | |||
| Spectre 201193 | Yes | No details | 7/63 lost to follow-up at 6 months |
| Domain 5: confounding | Are confounders accounted for in the design or analysis (e.g. adjustment, stratification)? | If there is an adjusted outcome measure (e.g. OR, HR), what were the factors that were adjusted for? | If a HR was presented, was the proportional hazards assumption met? | Was compliance measured? | How was compliance measured? | Level of compliance |
|---|---|---|---|---|---|---|
| Monotherapy at time of PFT and during follow-up | ||||||
| Flow cytometry | ||||||
| Frelinger 200976 | Design: N/A Analysis: yes (OR) |
Sex, TIMI risk score, aspirin dose, platelet count, BMI, use of clopidogrel, statins and oral hypoglycaemic agents | N/A | Not specifically | By TxB2 levels | ‘Two patients had serum TXB2 levels in the range observed for aspirin-free healthy controls, and their platelet function was therefore consistent with aspirin noncompliance. Because “resistance” cannot be distinguished from noncompliance, these subjects were not excluded from follow-up’ |
| Monotherapy at time of PFT, dual during follow-up | ||||||
| Flow cytometry | ||||||
| Kaminska 2007196 | Design: N/A Analysis: no |
N/A | N/A | No details | No details | No details |
| Surgicutt II | ||||||
| Buchanan 2000152 | Design: N/A Analysis: no |
N/A | N/A | Yes | A blood sample was collected at the time of each bleeding time test and processed for a platelet TxA2 determination as a measure of patient compliance. Non-compliance resulted in the exclusion of any patient data from the study analysis | 28% (148 participants) were excluded from the data analysis as a result of non-compliance |
| Apact II platelet aggregometer | ||||||
| Stejskal 2006198 | Design: N/A Analysis: no |
N/A | No details | No details | No details | No details |
| Platelet reactivity test | ||||||
| Grotemeyer 1993154 | Design: N/A Analysis: yes (HR) |
Model 1: age, responder/non-responder, risk factors present/absent, previous smoking, pre-existing vascular diseases, therapy with diuretics Model 2: responder/non-responder, risk factors present/absent, previous smoking, pre-existing vascular diseases, therapy with diuretics Model 3: responder/non-responder, accumulation of risk factors, previous smoking, accumulation of pre-existing vascular diseases, therapy with diuretics |
No details | Yes | Patients who had stopped taking aspirin were excluded | Four patients did not comply |
| Impact-R® (cone and platelet analyser test) | ||||||
| Monotherapy at time of PFT and during follow-up | ||||||
| Schwammenthal 2008125 | Design: N/A Analysis: yes (for OR) |
Age, NIHSS, diabetes | N/A | No details | No details | No details |
| Monotherapy at time of PFT, dual during follow-up | ||||||
| Spectre 201193 | Design: N/A Analysis: yes (for HR) |
‘Variables chosen for inclusion into the model were those that tended to be associated with event-free survival on univariate analysis and age’ | No details | No details | No details | No details |
Population and test characteristics
Seven studies76,93,125,152,154,196,198 were identified in this category.
In two studies76,196 the test performed was flow cytometry. In two other studies93,125 the test performed was Impact-R® (cone and platelet analyser test). Other tests were bleeding time by Surgicutt II (one study),152 cationic propyl gallate-induced aggregation (one study)198 and the ‘platelet reactivity test’ (one study). 154
Populations comprised patients with CVD/stroke (two studies),125,154 CAD (one study)76 and ACS (one study),198 and those undergoing non-urgent CABG152 and PPCI. 93 There was one study196 in patients with UA/ACS undergoing PPCI. One study included only patients with a first and recent MI,196 and no other studies reported how long patients had had their primary underlying condition for.
In four studies125,152,154,198 it appeared that patients were exclusively on monotherapy both at the time of the PFT and during follow-up. In one study,125 around 4% of patients were on dual therapy (+ clopidogrel) at the time of the PFT. Given the small proportion on dual therapy, these studies have been included in the ‘monotherapy’ category.
In a further study,76 patients were on monotherapy at the time of the PFT, and around 32% went on to additionally receive clopidogrel at some point during follow-up. It is possible that not all studies have reported where a proportion of patients commenced additional therapies during follow-up.
In two studies93,196 patients were on monotherapy at the time of the PFT and all were on dual therapy (+ clopidogrel) during follow-up. These studies have been listed separately, as the addition of clopidogrel therapy in all patients may affect the rate of events, and may also be a reflection of underlying population differences compared with the other studies.
Comedications across studies, where reported, included statins, COX-2 antagonists, heparin, warfarin, beta-blockers, ACE inhibitors, calcium channel blockers, diuretics, insulin, oral hypoglycaemics and antidepressants.
Non-steroidal anti-inflammatory drugs were not permitted (or had to be discontinued within a certain time period) in two studies. 196,198 One study76 stated that 10% of patients were on NSAIDs, and there were no details on NSAIDs in the remaining studies.
The number of participants in the studies ranged from 27 to 700 (see Table 67). Where reported, mean ages of patients ranged from 58 to 64 years, with most means around the early 60s. There were more men than women across all studies, with proportions of men ranging from 52% to 86%. The proportion of patients with diabetes ranged from 20% to 44%, and that of smokers from 19% to 37% (where reported; see Table 67). All studies were conducted in hospital settings.
The dose of aspirin ranged between 75 mg/day and 500 mg/day. Details were variable across studies regarding the length of time patients had been receiving aspirin therapy, with some noting a minimum period, and some whether patients were chronic or first time users (see Table 67). No study stated whether aspirin was provided in enteric or plain form, though one study93 noted that aspirin was in chewable form.
The main study characteristics were listed in Table 67. Note that in some studies baseline characteristics have been reported only according to resistant/sensitive groups or groups with/without diabetes, rather than for the total study population.
Studies noted that there were at least 6 hours (one study125) and 12 hours (one study154) between aspirin dose and PFT. Five other studies93,147,152,196,198 stated that there were up to 24 hours between aspirin dose and PFT (see Table 68).
Studies with more than one test
Fourteen studies undertook more than one PFT (Table 73); however, there were very few data that could be compared given the differences in reported outcomes and outcome statistics. Thus, data from only four studies99,108,112,162 have been presented in forest plots (Figures 55–58). These included the two studies that compared most PFTs. 99,162 The unadjusted OR was the most frequently reported statistic and thus provided most information.
| Study | LTA | VerifyNow® Aspirin | PFA-100® | TxA2 | WBA | TEG | Other | Outcomes reported |
|---|---|---|---|---|---|---|---|---|
| Addad108 | ✓ | ✓ | MACEs | |||||
| Frelinger76 | ✓ | ✓ | ✓ (flow cytometry) | MACEs, death | ||||
| Gluckman99 | ✓ | ✓ | ✓ | ✓ | Ischaemic/thrombotic events | |||
| Kaminska196 | ✓ | ✓ (flow cytometry) | Death, ischaemic/thrombotic events | |||||
| Linnemann112 | ✓ | ✓ | MACEs, ischaemic/thrombotic events | |||||
| Lordkipanidzé162 | ✓ | ✓ | ✓ | ✓ | ✓ | MACEs | ||
| Majeed117 | ✓ | ✓ | Ischaemic/thrombotic events | |||||
| Miyata164 | ✓ | ✓ | MACEs | |||||
| Modica187 | ✓ (PA-200) | ✓ | MACEs | |||||
| Payne147 | ✓ | ✓ (flow cytometry) | Death, ischaemic/thrombotic events | |||||
| Schwammenthal125 | ✓ | ✓ (Impact-R®) | Ischaemic/thrombotic events | |||||
| Sobol186 | ✓ | ✓ | Death | |||||
| Spectre93 | ✓ | ✓ (Impact-R®) | MACEs | |||||
| Tan174 (abstract) | ✓ | ✓ | Death, ischaemic/thrombotic events |
FIGURE 55.
Study comparing PFA-100® and thromboxane metabolite measurement (Addad108). RAVE, recurrent acute vascular event.

FIGURE 56.
Study comparing PFA-100®, thromboxane metabolite measurement, VerifyNow® Aspirin and WBA (Gluckman99). s, seconds.

FIGURE 57.
Study comparing PFA-100®, thromboxane metabolite measurement, LTA and WBA (Lordkipanidzé162).

FIGURE 58.
Study comparing PFA-100® and LTA (Linnemann112). PTA, percutaneous transluminal angioplasty; s, seconds; TEA, thoracic epidural analgesia.
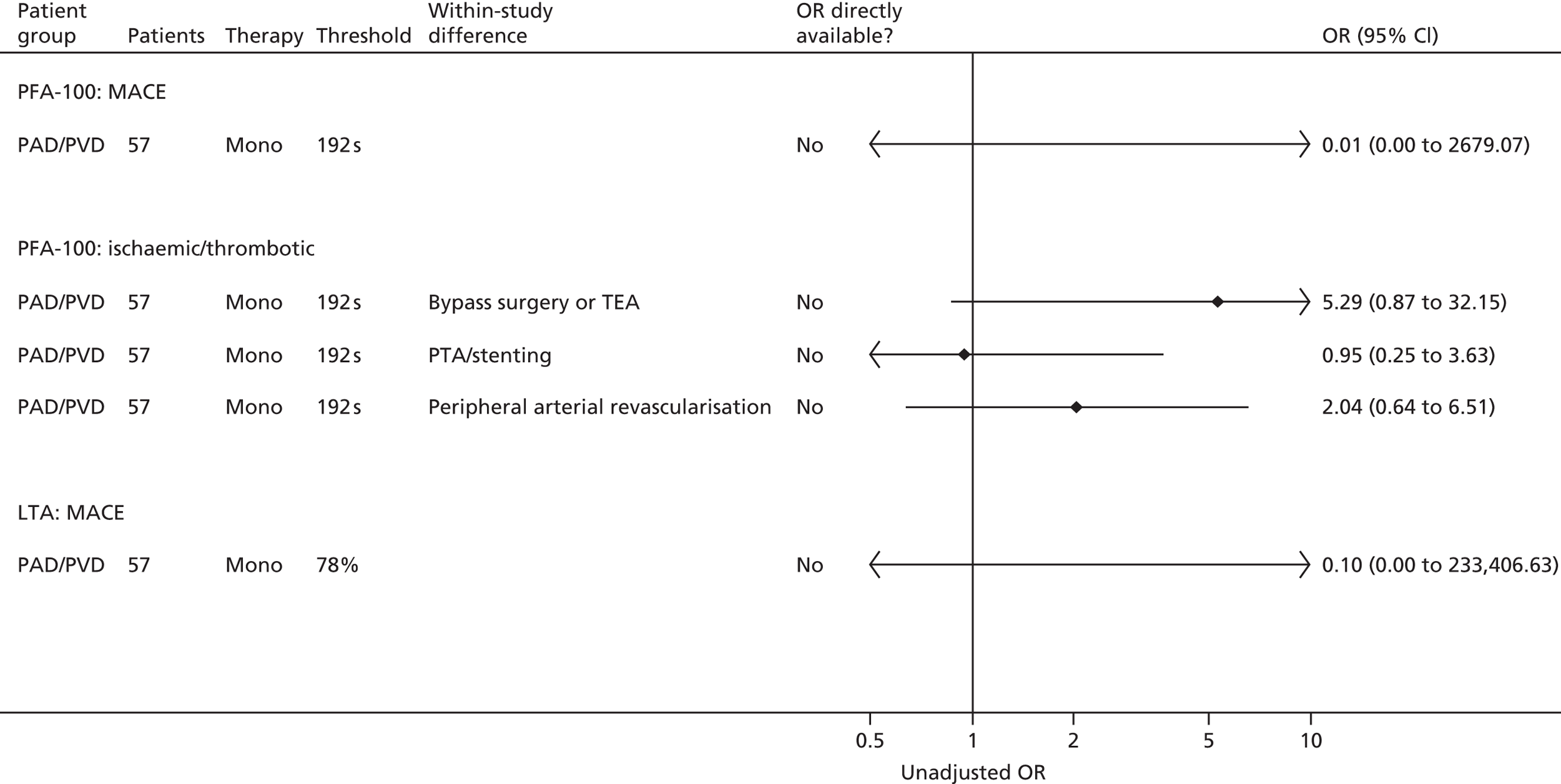
Note that data from all studies, on all reported outcomes and reported or calculable outcome statistics, are presented in the main results sections.
Within-study comparisons
The study by Addad et al. 108 used two tests, PFA-100® and thromboxane; there is a lack of consistency both within and between the different tests in terms of direction of effect and statistical significance. For example, when comparing the first tertile with the second and third (as a threshold) tertiles, there are more events in the resistant group with PFA-100®, and more events in the sensitive group with a thromboxane test. Gluckman et al. 99 compared four tests; again there is no consistency across tests in terms of how many individuals are classified as resistant or sensitive. Patients classified as resistant by a thromboxane PFT are, for example, more likely to have an event (compared with the sensitive group) than those classified as resistant by WBA, where there is no difference in event rate between resistant and sensitive. This lack of consistency in direction of effect is further demonstrated by the studies by Lorkipanidzé et al. 162 and Linnemann et al. 112 Clearly, the choice of test and threshold will influence whether an individual is classified as resistant or sensitive.
Between-study comparisons
Given the inconsistency within studies, the added heterogeneity between studies and the limited number of data, a comparison across studies of the direction of effect for individual PFTs is not feasible.
Dual therapy
The tests identified for assessing platelet function in patients on dual therapy (aspirin plus a second antiplatelet agent) are (i) LTA induced by arachidonic acid, (ii) VerifyNow® Aspirin, (iii) measurement of urinary or serum/plasma 11-dehydro-TxB2 concentrations, (iv) PFA-100®, (v) WBA induced by arachidonic acid, (vi) TEG and (vii) other miscellaneous tests. Table 3 identified the studies that have used these tests in a dual-therapy population.
The original intention was to report and analyse these studies in a similar way to the studies in patients receiving monotherapy with aspirin. However, the finding of limited evidence of the prognostic utility of platelet function testing related to aspirin monotherapy led to the decision not to undertake such analyses in dual-therapy studies.
Data on the population and test characteristics, along with quality characteristics of the studies in patients undergoing platelet function testing while receiving dual therapy, were, however, extracted and are included in the data extraction database (see Appendix 4). Should the need for these studies to be analysed exist in the future, this work can build on the data already collected.
Studies in patients with diabetes
No studies in a solely diabetic population were included in this review.
Prognostic models
The methods of this systematic review allowed for the inclusion of available prognostic models in which a PFT is one of multiple prognostic factors predicting clinical outcomes in a population of interest.
No such models were identified.
Systematic reviews
Fifteen systematic reviews were identified that met the initial inclusion criteria (Table 74). 203–217 On more detailed scrutiny, two reviews did not link the results of PFTs to clinical outcomes (one review on pharmacogenetics203 and one on the role of PFTs in guiding clinical practice which did not provide prospective follow-up of clinical outcomes204). A further review205 was predominantly focused on patients with renal insufficiency and therefore is not discussed further.
| Review | Searches up to | Research question | Systematic review methodology | Number of included studies | Population | Monotherapy only or monotherapyand dual therapy included | PFTs | Prospective follow-up of clinical outcomes | Aspirin resistance/sensitivity linked to clinical outcomes? |
|---|---|---|---|---|---|---|---|---|---|
| Included systematic reviews | |||||||||
| Musallam 2011212 | No details | Mechanisms, laboratory evaluation, clinical impact and management of resistance to aspirin and clopidogrel therapy | MEDLINE and PubMed search; no language/publication year restrictions; citation checking. No further methodological details | Reviews by Krasopoulos (2008)209 and Snoep (2007)217 discussed, also nine studies described individually | Patients with cardiovascular disease | Not specified | Not specified | Yes | Yes |
| Cañivano Petreñas 2010215 (Spanish) | November 2008 | Prevalence, epidemiology, mechanism of action and clinical consequences of aspirin resistance | PubMed, EMBASE and other databases searched up to 2008. Citation checking. Some details on selection criteria. No further methodological details | 16 studies linking aspirin with clinical outcomes | Patients at high risk of cardiovascular events | Not specified | Any PFT as long as methods defined | Yes | Yes |
| Velkovic 2009206 (conference abstract) | No details | Platelet function test options, and the epidemiology, aetiology and management of antiplatelet agent low response | PubMed search described as ‘systematic’. No further methodological details | 31 aspirin and 53 dual-therapy studies included, but citations not listed | Patients undergoing endovascular procedures | Aspirin, clopidogrel or both | Not specified | Unclear, but longer term outcomes are mentioned | Yes |
| Crescente 2008207 | October 2007 | Prevalence of non-responders to aspirin, and clinical and methodological factors that can influence it and its possible association with vascular outcomes | Details of search strategy in PubMed only, citation checking. Exclusion criteria listed. No further methodological details | Eight studies linking aspirin with clinical outcomes | Patients taking aspirin for primary or secondary prevention of vascular events | Monotherapy only | PFA-100® only | Yes | Yes |
| Ferguson 2008210 | November 2007 | Variability of antiplatelet resistance, factors associated with resistance, causes and approaches to overcoming resistance | Search strategy for PubMed only. No further methodological details | States that only prospective, controlled trials included; 17 trials identified (appears to include prospective cohorts) Results from four and seven studies respectively described as linking clinical outcomes to aspirin and clopidogrel resistance |
Patients with CAD | Aspirin and/or clopidogrel | Not specified | Yes | Yes |
| Pusch 2008216 | March 2008 | Prevalence of aspirin resistance and its association with clinical outcome; treatment approaches | Search strategy for MEDLINE, PubMed and The Cochrane Library, citation checking. Some detail on selection criteria. No further methodological details | LTA: 11 studies TxB2: 5 studies PFA-100®: 15 studies WBA and Ultegra RPFA-ASA system: 4 studies Total of 35 studies |
Patients taking aspirin for primary or secondary prevention of vascular events | Not specified | Not specified | Yes | Yes |
| Reny 2008208 | July 2007 | Clinical predictive value of PFA-100® in aspirin-treated cardiovascular patients | Details of search strategy, study selection, data extraction and methods of analysis (see Critical appraisals for further details) | 15 studies (eight prospective and seven non-prospective) | Patients with symptomatic atherosclerosis | Not specified | PFA-100® only | Yes | Yes |
| Sofi 2008211 | May 2007 | Residual platelet reactivity in coronary heart disease patients in relation to the occurrence of adverse coronary events during follow-up | Details of search strategy, study selection, data extraction and methods of analysis (see full quality appraisal for further details) | 11 prospective studies | Patients with coronary heart disease | Not specified | Not specified | Yes | Yes |
| Krasopoulos 2008209 | Unclear (‘to present’) | Relationship between aspirin resistance and clinical outcomes in patients with cardiovascular disease | Details of search strategy, study selection, quality assessment and methods of analysis (see full quality appraisal for further details) | 20 studies described as prospective included | Patients prescribed aspirin as antithrombotic therapy | Mono or dual | Not specified | Yes | Yes |
| Snoep 2007217 | October 2006 | Relationship of laboratory aspirin resistance to risk of cardiovascular recurrent events | Details of search strategy, study selection, quality assessment and methods of analysis (see full quality appraisal for further details) | 15 full-text articles and one abstract | Patients with established CAD, CVD or PAD | Not specified | Not specified | Yes | Yes |
| Wong 2004213 | January 2003 | Evidence for aspirin resistance in patients with atherosclerosis (mechanism, prevalence, definition, clinical outcomes) | Details of search strategy (MEDLINE) only; citation checking. No further methodological details. Mainly narrative approach | Five studies described narratively in terms of resistance and clinical outcomes | Patients with atherosclerosis | Not specified | Not specified | Yes | Yes |
| Howard 2002214 | February 2002 | Significance of aspirin resistance in vascular patients | Details of search strategy (MEDLINE) only up to 2002; citation checking. No further methodological details. Mainly narrative approach | Five studies described narratively in terms of resistance and clinical outcomes | Not prespecified. Patients with cardiovascular disease, CVD and PVD | Not specified | Not specified | Yes | Yes |
| Less relevant reviews in the current context | |||||||||
| Verschuren 2012203 | May 2011 | Pharmacogenetics: genetic markers linked to platelet activity or clinical outcomes | Details of search strategy in MEDLINE only (included citation checking). Few details on selection criteria. No further methodological details | None specifically looking at resistance and clinical outcomes | Patients with cardiovascular disease | Not specified | Not specified | Yes | No |
| El-Menyar 2010205 | February 2009 | Risk of stent thrombosis in patients with renal insufficiency | MEDLINE, Scopus and EBSCOhost searched. Limited details on search strategy, but no other methodological details | One study only cited for dual antiplatelet non-responsiveness in this population | All patients with chronic renal insufficiency after PCI | Not specified | Not specified | Yes | Yes |
| Dickinson 2008204 | No details | Role of PFTs in guiding surgical practice | Search strategy for MEDLINE and PubMed only. No further methodological details | Three studies referred to narratively | Patients undergoing surgery | Not specified | TEG, VerifyNow® Aspirin, PFA-100®, ACT, Sonoclot® (Sienco®, Arvada, CO), CSA in search terms | No | No |
The remaining 12 reviews all included relevant populations and at least some primary studies where the results of a PFT were linked to clinical outcomes.
One review was in abstract form only;206 the authors were contacted for further details but no response was obtained.
Most reviews did not restrict inclusion of studies by type of PFT, except two,207,208 both of which focused on the PFA-100® test only.
Only four reviews specified whether studies with patients receiving monotherapy or dual therapy were included, three including both monotherapy and dual therapy studies206,209,210 and one stipulating monotherapy only. 207
Few reviews provided details on whether only prospective or also retrospective studies were included (four studies provided this information208–211), but it appears that many included both, in some cases separating them in analysis.
All the reviews reported details of at least one database search (as per the inclusion criterion for this report). However, most presented only limited additional details on methodological aspects. Searches were limited to PubMed or MEDLINE and citation checking in half the reviews. 206,207,210,212–214 Five reviews supplied no further methodological details beyond the basic search strategy,206,210,212–214 with a further three reporting limited details on study inclusion or exclusion criteria. 207,215,216 Four reviews reported more comprehensive methodological details208,209,211,217 and these papers were critically appraised using the Assessment of Multiple Systematic Reviews (AMSTAR) checklist.
The reviews included between 5 and 84 studies (not restricted by PFT), or between 5 and 31 studies where restriction to monotherapy studies was made clear. The review with the highest number of included studies, both overall and monotherapy, was Velkovic and Coulthard206 a conference abstract with no listing of citations. By PFT, the highest number of studies identified by any review was 15 PFA-100® studies. 208,216 By comparison, the current review found 57 distinct monotherapy studies overall46,76,86,88,90,92,93,95,99,105,108–110,112,113,115–118,121,123,125,127,128,132,133,135,137,138,142,144–155,159,162–164,166,168,169,171,174,186,187,189,193,195,196,198,201,202 (and 21 monotherapy studies using the PFA-100® test76,99,108,109,112,115,116,118,123,127,132,135,137,138,144,145,150,162,186,187,189,193), despite using more stringent inclusion criteria including a restriction to prospective studies only. Including mono-, dual- and triple-therapy studies, 21 of the studies identified in this review46,127,132,134,135,137–143,145,148–154,198 were also included in the seven previous reviews207–209,211,215–217 which provided any information on exclusion criteria (see Table 75). Conversely, of the studies included by at least one other review, the current review excluded 16 at the full-text stage38,235–249 and seven at the title and abstract stage,250–256 as they did not meet the inclusion criteria. Three studies were included in other reviews which were not identified by the search strategy; one was subsequently excluded as it was a cross-sectional study,256 while the correct citation was not identifiable for the other two studies. 219,257
Critical appraisals
Four reviews208,209,211,217 reporting more comprehensive methodological details were critically appraised using the AMSTAR checklist. Significant information was nevertheless found to be lacking from all four publications which would enable a complete assessment of the validity of their conclusions.
Krasopoulos et al. ’s209 review focused on the relationship between aspirin resistance and clinical outcomes in patients with cardiovascular disease, and identified 20 studies totalling 2930 patients. The paper provided no details of a protocol or whether or not the research question and inclusion criteria were prespecified. As no search strategy was presented, it was difficult to gauge whether or not the strategy was likely to have included all potentially relevant studies; thus, it was not possible to determine whether the reduction from the 36,573 articles identified by the initial search to 320, using only the term ‘aspirin resistance’, was appropriate or had resulted in the omission of relevant studies. It appeared that only a small proportion of identified articles (57/320) were reviewed independently, but that each of the authors reviewed and tabulated data from every included paper. Four bibliographic databases were searched, and citations (from 210 papers) were reviewed. Nevertheless, there was no mention of searching grey/unpublished literature (including ongoing trials or conference abstracts) or contacting experts. Although a list of included studies was provided, one was not provided for the excluded studies.
Although the authors presented the characteristics of included studies, there were no details on thresholds (for determining resistance) or on follow-up time, and limited information on the use of a prospective or retrospective study design, all of which may have influenced the event rate in the two groups. A further concern is that though the method of establishing compliance was considered, the authors considered that aspirin status measured in hospital could be assured independent of non-compliance, and further stated that it is unlikely that patients would subsequently become non-compliant, which seems an unwarranted assumption when there might be a long follow-up period. Furthermore, as it appeared that both prospective and retrospective studies had been included in the review, compliance with treatment in hospital may not reflect previous compliance with treatment.
The authors assessed included studies using a quality rating, but it was unclear how this was derived and which specific criteria were considered, and therefore it was not possible to make a judgement on the robustness of the quality assessment, or whether their assessment was correct that the few studies (3/20) rated as not having a low risk of bias were insignificant in affecting overall results. A high level of heterogeneity was evident but a fixed-effects model was used incorrectly. Given the high level of heterogeneity (potentially due to study design, study quality, underlying disease, follow-up time, type of PFT, threshold and level of compliance), the exploration of heterogeneity was limited. This, coupled with a failure to differentiate adjusted and non-adjusted results, reduced confidence in the reported conclusion that aspirin-resistant patients are at greater risk of long-term cardiovascular morbidity [OR of any cardiovascular event 3.85, 95% CI 3.08 to 4.80; p < 0.001 overall, or OR 3.53, 95% CI 2.66 to 4.68; p < 0.001 in monotherapy (14 studies)].
The review by Reny et al. 208 considered the clinical predictive value of only one PFT, PFA-100®, in aspirin-treated cardiovascular patients, and found seven non-prospective and eight prospective studies, incorporating 1466 and 1227 patients respectively. Again, no details were provided about the existence of a protocol or whether or not the research question was prespecified, though inclusion criteria were clearly stated. Three databases were searched, and reference lists of studies and conference abstracts were also examined. However, only English-language studies were searched for. Study selection and data extraction were conducted independently by two reviewers, and disagreements were resolved by discussion among all authors. Lists of included studies and studies excluded at full-text stage, were provided.
The authors’ assessment of study quality was extremely limited. Whether or not assessment was blinded for biologists and clinicians was considered (though ill-defined), and results were found to be similar in studies whose reports explicitly mentioned blinding compared with those that did not mention the use of blinded assessments. However, the issue of blinded assessment appeared to be the only mention of study quality considered consistently. The discussion considered some other methodological limitations of the included studies, for example lack of assessment of patient compliance (on which no data were provided by individual study), sample size and non-evaluation of von Willebrand factor. However, it was apparent that none of these assessments of methodological rigour and scientific quality were specified a priori.
Prospective and non-prospective studies were considered separately. However, adjusted and non-adjusted results were not distinguished. The heterogeneity of the prospective studies was assessed, and found to be non-significant using a random-effects model. The difference in thresholds used in the studies was recognised and discussed. Perhaps as a result of the small number of (prospective) studies, subgroup analysis was restricted to narrative discussion, and then focused on the prevalence of non-responders as opposed to the relationship with cardiovascular events. A more detailed exploration of heterogeneity was lacking and would have been desirable in interpreting the review’s findings. Publication bias was considered with regard to non-prospective studies, but the funnel plot for prospective studies was not discussed.
The authors concluded that high residual platelet reactivity (i.e. non-responder status) in cardiovascular patients treated with aspirin was associated with recurrent ischaemic events. In the prospective studies they included the OR for the recurrence of an ischaemic event in aspirin non-responders relative to aspirin responders, which was 2.1 (95% CI 1.4 to 3.4, p < 0.001). Although the authors acknowledged the potential impact of dosage and threshold on this association, other important caveats should be noted in light of the limited information concerning study quality in the papers incorporated in this review. Finally, it was unclear if patients in any of the studies were on aspirin alone or on dual or triple therapy.
Snoep et al. 217 found 16 studies in their review of the relationship between laboratory aspirin resistance and the risk of cardiovascular recurrent events. In a comprehensive, as well as a priori, search strategy, four bibliographic databases were searched, with no language restrictions, and reference lists were searched and authors contacted. However, although the search terms were listed as available from the authors, they were not presented, and there was no sample search strategy, so it was difficult to assess the appropriateness of the search strategy. Although meeting abstracts were included, there were no details regarding ongoing trials or other sources of unpublished studies. Selection, quality assessment and data extraction of studies were all independently performed by two reviewers, with disagreements resolved by consensus and discussion with a third party. A list of included, but not of excluded, studies was provided. The authors stated that a funnel plot did not suggest the existence of publication bias, but did not present it in the publication.
The authors stated that the following quality criteria were assessed: control for confounders, measurement of exposure, completeness of follow-up and blinding, and, for case–control studies, matching and case definition. There was no formal scoring system. Overall findings on the quality of all included studies were not presented. Some quality findings were reported in the discussion section, but it was uncertain if this was extensive enough to capture any potential implications of quality for the results. Furthermore, while it was stated that studies were excluded because of insufficient quality during study selection, there were no details on a quality threshold. The only reference to compliance was to note that patient adherence to treatment was assessed in only three of the studies.
The review found that 15 of the 16 included studies revealed an adverse association between laboratory aspirin resistance and occurrence of cardiovascular events, with a pooled OR across all cardiovascular outcomes of 3.8 (95% CI 2.3 to 6.1). A random-effects model was used, which is appropriate as there was evidence of heterogeneity. There was no discussion of any attempt to discriminate between adjusted and non-adjusted studies. There was some subgroup analysis by type of outcome. Given the high level of heterogeneity (potentially owing to study design, study quality, underlying disease, follow-up time, type of test, threshold and level of compliance), a more detailed exploration of heterogeneity would have been appropriate. Again, a further concern was that it was unclear if patients in any of the studies were on dual or triple therapy as opposed to aspirin monotherapy.
Sofi et al. 211 conducted a meta-analysis on the relationship between residual platelet reactivity in coronary heart disease patients and the occurrence of adverse coronary events, and found 11 prospective studies comprising 1952 patients. Though the aim of the study was clearly stated, as were the inclusion criteria, again there was no reference to a protocol or to a priori published research objectives. Data were independently extracted by two reviewers, and disagreements were resolved by discussion with a third investigator. However, whether or not two reviewers also independently selected studies for inclusion was unclear. Four bibliographic databases and citations from relevant original studies and review articles were searched. No detail on whether or not grey literature was searched was provided. Only included studies, and not excluded studies, were listed. Publication bias was assessed using a funnel plot of effect size against standard error. The authors report that the funnel plot was broadly symmetrical, and so consistent with the conclusion that there was no publication bias; however, the plot was not presented in the publication.
No assessment of study quality was provided. The authors did consider the heterogeneity of the included studies in the discussion, and noted that most of the studies did not systematically assess adherence to aspirin therapy or adjust for confounding factors in the multivariate statistical models (6 out of the 11 included studies reported statistical data not adjusted for potential confounders).
The authors documented a significantly increased relative risk of adverse clinical events for patients with residual platelet reactivity on aspirin treatment (relative risk 3.11, 95% CI 1.88 to 5.15; p < 0.0001). A random-effects model was used, appropriately, as there was significant heterogeneity (I2 reported). Subgroup analyses were performed according to relevant specific variables (duration of follow-up, aspirin dosage, PFT and patient characteristics), and in each case the risk of clinical recurrences increased. In addition, the association remained statistically significant even after the exclusion of studies that reported only crude unadjusted data (relative risk 3.19, 95% CI 1.97 to 5.19; p < 0.00001). Even so, a major caveat for this review related to the lack of data on study quality, and the fact that it was unclear if patients in any of the studies were on dual or triple therapy.
All four reviews found a positive association between aspirin non-responder status and likelihood of adverse cardiovascular outcomes, despite their differences in precise research question, range of included studies (Table 75) and primary outcome measures. However, although the four critically appraised reviews were those with the most detailed methodological information from the relevant reviews identified, all had important deficiencies, variously:
-
a lack of a rigorous and transparent approach to quality assessment
-
insufficient comprehensiveness and a failure to account for the complexity of the field by not considering the effect of different PFTs, thresholds, etc.
-
not distinguishing between adjusted and non-adjusted statistical data
-
uncertainty regarding whether patients were on aspirin monotherapy or if those on dual therapy were merged in the analysis
-
uncertainty over whether included studies were prospective or retrospective in design
-
failure to account for the effect of non-compliance.
In this context, caution must be exercised in interpreting the findings from these previous reviews.
| Study | Cañivano Petreñas 2010215 | Crescente 2008207 (PFA-100® only) | Pusch 2008216 | Krasopoulos 2008209 | Reny 2008208 (PFA-100® only) | Sofi 2008211 | Snoep 2007217 | Current review |
|---|---|---|---|---|---|---|---|---|
| Andersen 2002244 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Excluded at full-text stage |
| Atiemo 2008245 | ✓ | ✓ | ✓ | Excluded at full-text stage | ||||
| Berroushot 2006246 | ✓ | ✓ | ✓ | Excluded at full-text stage | ||||
| Borna 2005225 | ✓ | ✓ | Excluded at title and abstract stage | |||||
| Bruno 2004148 | ✓ | ✓ | ||||||
| Buch 2007134 | ✓ | ✓ | ||||||
| Buchanan 2000152 | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Chen 2004242 | ✓ | ✓ | ✓ | ✓ | Excluded at full-text stage | |||
| Chen 2005243 | ✓ | ✓ | Excluded at full-text stage | |||||
| Cheng 2005255 | ✓ | Excluded at title and abstract stage | ||||||
| Christiaens 2008127 | ✓ | ✓ | ||||||
| Cornelissen 2006254 | ✓ | Excluded at title and abstract stage | ||||||
| Cotter 200446 | ✓ | ✓ | ✓ | ✓ | ||||
| Cuisset 2006143 | ✓ | ✓ | ||||||
| Eikelboom 2002151 | ✓ | ✓ | ✓ | ✓ | ||||
| Faraday 2004241 | ✓ | ✓ | Excluded at full-text stage | |||||
| Fuchs 2006138 | ✓ | ✓ | ||||||
| Geisler 2008256 | ✓ | Not identified in current search. Hard copy retrieved and excluded | ||||||
| Gianetti 2006141 | ✓ | ✓ | ✓ | |||||
| Grotemeyer 1993154 | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Grundmann 2003248 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Excluded at full-text stage | |
| Gulmez 2007253 | ✓ | Excluded at title and abstract stage | ||||||
| Gum 2001219 | ✓ | Does not appear to refer to relevant citation | ||||||
| Gum 2003149 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Gurbel 2003257 | ✓ | Does not appear to refer to relevant citation | ||||||
| Hobikoglu 2007135 | ✓ | ✓ | ✓ | |||||
| Hobikoglu 2005240 | ✓ | ✓ | ✓ | ✓ | Excluded at full-text stage | |||
| Lev 2006239 | ✓ | ✓ | Excluded at full-text stage | |||||
| Linden 2007252 | ✓ | Excluded at title and abstract stage | ||||||
| Malek 2007238 | ✓ | ✓ | ✓ | Excluded at full-text stage | ||||
| Marcucci 2006139 | ✓ | ✓ | ✓ | ✓ | ||||
| McCabe 2005249 | ✓ | Excluded at full-text stage | ||||||
| Mueller 1997153 | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Ohmori 2006142 | ✓ | ✓ | ||||||
| Pamukcu 2005251 | ✓ | ✓ | Excluded at title and abstract stage | |||||
| Pamukcu 2006140 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Pamukcu 2007137 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Poston 2006247 | ✓ | ✓ | ✓ | ✓ | Excluded at full-text stage | |||
| Poulsen 2007132 | ✓ | ✓ | ✓ | ✓ | ||||
| Poulsen 2007132 | ✓ | Excluded at title and abstract stage | ||||||
| Sambola 2004145 | ✓ | ✓ | ||||||
| Stejskal 2006198 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Tantry 200538 | ✓ | Excluded at full-text stage | ||||||
| Valles 2007237 | ✓ | Excluded at full-text stage | ||||||
| Yilmaz 2005236 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Excluded at full-text stage | |
| Zhang 2005235 | ✓ | ✓ | Excluded at full-text stage | |||||
| Ziegler 2002150 | ✓ | ✓ | ✓ |
Ongoing studies
The searches of research registers identified 65 potentially relevant records of apparent ongoing studies (see Chapter 4, Searches for the sources searched and Quantity of research available, earlier in this chapter, for the identification of these records). Copies of these records were obtained and the review selection criteria (see Chapter 4, Study selection) were applied, except for the criterion for publication type, which was ignored because it was irrelevant. Key difficulties with this selection process were the absences of information in the records given that these were not protocols for studies but only brief outlines of study aims with limited associated methodological information provided. Furthermore, it was frequently unclear whether the same record was in more than one register or some of the studies in older records had actually been completed and published.
Despite these difficulties, it was possible to identify a number of records that met the selection criteria258–262 and also records that potentially relate to fully published articles. 263,264
However, for the reasons mentioned, the selection process could not provide definitive sets of included and excluded records in regard to ongoing studies, and as such no lists are given in this report.
Therefore, this section only serves to indicate that there are a number of ongoing studies which may add further data in the future.
Relevant studies identified after the search cut-off dates
After the searches were undertaken for this review, further relevant studies continued to be published. 265–267 Although the authors of this report were aware of these publications, these have not been included in the above analysis to prevent the introduction of bias. A robust approach would be to update all the searches; however, given the magnitude and complexity of this project, this was beyond the resources available. An alternative approach would be to discuss the sensitivity of the review findings to the more recent subjectively identified evidence. However, given the heterogeneity of included studies, even within subgroups, and the absence of the ability to undertake any pooled analyses, this approach was considered to be of limited benefit.
Chapter 6 Economic analysis
This section has two aims: (i) to review systematically the published evidence relating to the cost-effectiveness of platelet function testing in patients on aspirin therapy with established cardiovascular disease, CVD or diabetes; and (ii) to assess the cost-effectiveness of platelet function testing plus change in treatment for patients with established CAD on aspirin therapy compared with no testing and no change in treatment, from a NHS and Personal Social Services (PSS) perspective. For this aim, a speculative economic model was developed.
The methods and findings of the systematic review are presented first followed by those of the speculative economic model.
Systematic review of cost-effectiveness studies
Methods
The broad methods of this systematic review were similar to those presented in Chapter 4, and thus only key details are given here.
Search strategy
Searches for economic studies were run on MEDLINE, EMBASE and NHS EED using, where appropriate, relevant terms for economic studies along with terms for clinical populations and PFTs. Examples of these strategies can be found in Appendix 2. The yield of articles from these searches was supplemented with any further economic evaluations and cost studies identified during screening of the search yield in the prognostic/diagnostic utility review.
Study selection
Two reviewers independently screened titles and abstracts for relevance. All potentially relevant articles were obtained for scrutiny against the full selection criteria, with any disagreements resolved by discussion. The criteria were:
-
Study design Cost–consequence analysis, cost-effectiveness analysis, cost–benefit analysis, cost–utility analysis, cost studies.
-
Population Patients aged ≥ 18 years on aspirin (as monotherapy or in combination with other antiplatelet agents), with established cardiovascular disease, CVD or diabetes. Studies with mixed populations were included as long as data for relevant patients were extractable.
-
Intervention Aspirin-specific platelet function assay or global PFT where patients are receiving aspirin as the only antiplatelet therapy. The list of eligible assays has been outlined previously in Table 2.
-
Comparator No assessment of aspirin resistance or current practice.
-
Outcome Cost-effectiveness, cost estimates, utilisation estimates, quality-of-life estimates.
Data extraction and quality assessment strategy
Data on the following, where available, were extracted from included studies by one health economics reviewer and checked by another:
-
study characteristics, such as study question, form of economic analysis, population, interventions, comparators, perspective, time horizon and form of modelling used
-
clinical effectiveness and cost parameters, such as effectiveness data, health state valuations (utilities), resource use data, unit cost data, price year, discounting and key assumptions
-
results and sensitivity analyses.
Studies were quality assessed using tools as part of the data extraction process, namely the Consensus on Health Economic Criteria list268 for economic evaluations and the checklist by Philips et al. 269 for model-based analyses.
Results
A total of 387 records were identified from the searches and, following the removal of duplicates, there were 299 unique records. No additional articles were identified from the process of the systematic review of prognostic/diagnostic utility studies.
None of the records was deemed relevant to this economic review and, as such, no hard copies were obtained for scrutiny against the inclusion criteria for the review.
A flow diagram presenting the process of selecting studies can be found in Figure 59.
FIGURE 59.
Flow diagram showing study selection for the economic evaluations review.

Discussion
No economic evaluations or cost studies were found during the search for literature on the cost and cost-effectiveness of platelet function testing in patients with established cardiovascular disease or CVD. This is surprising given the amount of research identified by the systematic review of prognostic/diagnostic utility (see Chapter 4) and the degree of debate around this topic.
Economic modelling
This section provides a detailed description of the speculative economic model developed to estimate the cost-effectiveness of platelet function testing with the option of a change in treatment, compared with the current approach of no platelet function testing and no change in treatment. The model considers how being classified as aspirin resistant based on a test of platelet function and subsequently adding or changing treatment may lead to a reduced risk of experiencing a MACE, but may also increase the risk of major bleeding. Owing to the large amount of clinical uncertainty identified by the systematic review of prognostic and diagnostic utility (see Chapter 5), this economic model evaluates a hypothetical PFT and hypothetical change of treatment in patients with existing cardiovascular disease or CVD, considers whether or not such a test would be cost-effective and investigates the main factors affecting cost-effectiveness. An overview of the key characteristics of the cost-effectiveness analysis is shown in Box 1.
Intervention Hypothetical aspirin-specific PFT or global PFT where patients are receiving aspirin as the only antiplatelet therapy at the time of testing, followed by a hypothetical change of treatment if, based on the test, patients are defined as aspirin resistant. Possible treatment options are (1) increase dose, (2) increase frequency (split dose), (3) combination therapy, (4) change treatment (alternative monotherapy).
Comparator No assessment of platelet function and no change of treatment (current practice).
Population Cohort of patients with stable CAD who are receiving aspirin as the sole antiplatelet agent (monotherapy); 66% male and aged 60 years.
Time frame Lifetime time horizon; 1-year time cycle.
Perspective NHS/PSS.
Effects MACEs and major bleeds.
Costs Costs associated with platelet function testing, changing antiplatelet therapy and treating patients who have experienced a fatal or non-fatal MACE or major bleed.
Outcomes Mortality, quality of life, QALYs.
Assessment of cost-effectiveness Cost per additional QALY gained.
QALY, quality-adjusted life-year.
Methods
Model description
A speculative economic model developed as a decision tree combined with a Markov model was built in TreeAge Pro® (TreeAge Software, Inc., Williamstown, MA, USA ) to estimate the cost-effectiveness of platelet function testing with the option of change in treatment compared with no testing and no change in treatment (current treatment). The population considered was patients with stable CAD on aspirin monotherapy. Based on clinical judgement the patient cohort was assumed to be aged 60 years and 66% male, in keeping with studied populations included in this report. The time horizon of the model was patient lifetime and the model was therefore run for 41 years. Mortality data were weighted to take into account the greater proportion of men with the disease. A time cycle of 1 year was chosen, as it was felt that a shorter and more detailed time period would not be required owing to the speculative nature of the model and data inputs. The model structure is shown in Figure 60.
FIGURE 60.
Decision tree and Markov model used to assess the cost-effectiveness of platelet function testing plus change of treatment.
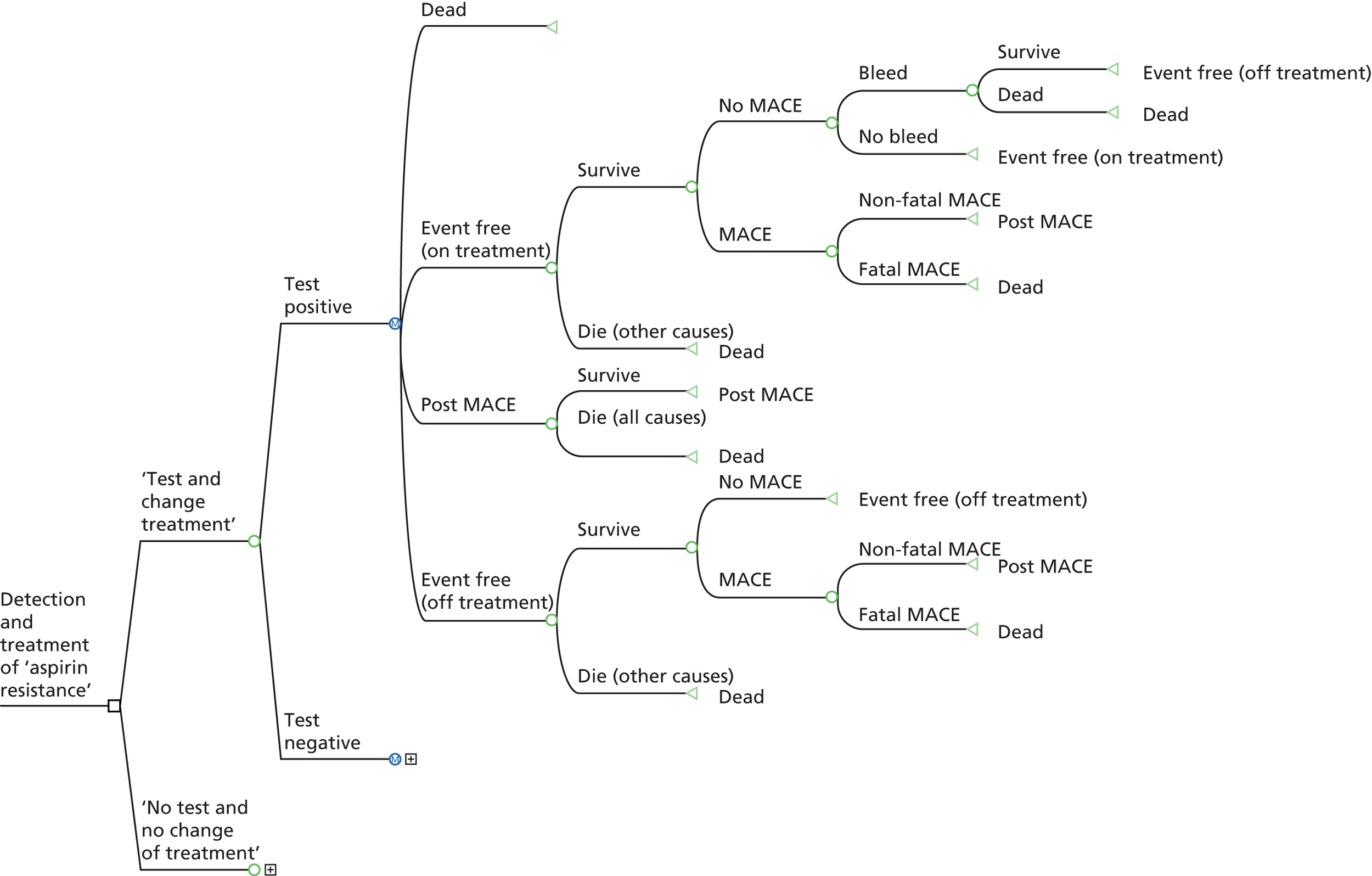
In the decision tree, for both treatment options of testing with a change in treatment and no testing and no change in treatment (current treatment), the cohort was separated at a chance node based on whether patients were identified as aspirin resistant or aspirin sensitive. The Markov model followed on from each of these branches. Therefore, patients identified as aspirin resistant followed one model pathway, while those identified as aspirin sensitive followed a separate model pathway. However, the subsequent model pathways were the same for all test and treatment options.
The entire cohort began in the state ‘event free (on treatment)’. From this health state, patients moved along a pathway where they could remain in this health state, die from other causes or experience a fatal or non-fatal MACE or major bleed. Depending on the events experienced during this initial pathway, patients moved to the subsequent health states ‘post event’ after a non-fatal MACE, ‘event free (off treatment)’ after suffering a non-fatal major bleed and having antiplatelet therapy discontinued, or ‘dead’, or remained in the health state ‘event free (on treatment)’. Once in a ‘post event’ health state, the patient either remained in this state or died, with the mortality risk higher as a result of having suffered a previous MACE. The pathway for ‘event free (off treatment)’ was the same as for those who were event free and on treatment, but with adjustments to event rates taking into account whether or not treatment was being taken.
The intervention option assumed that all patients received a hypothetical PFT with a one-off cost, and, if defined as aspirin resistant, had their treatment changed with an associated additional cost. The treatment change could decrease the risk of a MACE but could also increase the risk of a major bleed. If a major bleed occurred, all antiplatelet therapy was discontinued and the risk of a future MACE increased to a higher level. Those who were defined as aspirin sensitive did not have their treatment changed. In the current treatment option, there was no testing (although the differentiation between aspirin resistant and aspirin sensitive was maintained) and aspirin monotherapy was continued, unless a major bleed caused a discontinuation of treatment. In the base case, the model assumed that those defined as aspirin resistant had an overall average raised risk of MACEs and an overall average benefit from a change in treatment.
The model was designed to estimate costs, from the perspective of the NHS and PSS, and outcomes in terms of quality-adjusted life-years (QALYs) gained by each arm of the model, with costs and QALYs accumulated depending on the transitions between health states. The difference between the costs, incidence of health outcomes and impact on quality of life and mortality between treatment options was used to estimate the incremental costs and effects of applying a hypothetical PFT and treatment change. The model also attempted to incorporate uncertainty in model parameters by incorporating probability distributions for the majority of input parameters. All costs were for a price year of 2011–12, and were inflated to this price year where appropriate. Costs and QALYs were discounted at a rate of 3.5% per annum.
Estimation of model parameters
The parameters and sources used in the model are summarised in Tables 76–78. Given the range of data required to populate the model, a variety of approaches were used to identify parameter estimates. First, systematic reviews were undertaken to identify parameter estimates reported in the existing literature. However, as described in Chapter 5 and in Systematic review of cost-effectiveness studies, the prognostic and diagnostic utility data available were associated with heterogeneity and uncertainty and no relevant published economic evaluations were identified. Several parameter estimates were sourced from clinical papers, for example the risk of major bleeding, and utility values for health states were obtained from previous modelling studies in cardiovascular disease. However, as a result of the clinical uncertainty in this area, the majority of the data in this model are based on expert opinion and assumptions. As the model is speculative, the base-case result aims to present cost-effectiveness for a hypothetical test and treatment strategy which reduces the risk of MACEs in aspirin-resistant patients. The values used in the sensitivity analysis aim to test all of the assumptions and present different scenarios to show when a test and change in treatment may or may not be cost-effective.
| Probabilities | Value | Distribution | Source |
|---|---|---|---|
| Testing positive for aspirin resistance | 0.225 | Beta α = 2, β = 6.89 |
Estimate from clinical review (see Tables 4, 15, 26, 37, 48, 58 and 67) |
| MACE (aspirin sensitive) (1 year) | 0.05 | Beta α = 2, β = 38 |
Assumption from expert opinion |
| Proportion of MACEs as stroke | 0.2 | Beta α = 2, β = 8 |
Assumption from expert opinion |
| Fatal MACE | 0.09 | Beta α = 2, β = 20.2 |
Assumption from expert opinion |
| Major bleed (1 year) | 0.001 | Beta α = 2, β = 1998 |
Antithrombotic Trialists’ Collaboration (2009)4 |
| Death from a major bleed | 0.125 | Beta α = 2, β = 14 |
Assumption from expert opinion |
| Relative risks | |||
| Relative risk applied to baseline MACE risk to calculate risk if aspirin resistant (note the reciprocal was applied to the baseline risk) | 0.66 | Beta α = 3.88, β = 2 |
Assumption from expert opinion |
| Relative risk of a major bleed with a change in treatment | 1.4 | Beta (reciprocal value of 0.71 used) α = 5, β = 2 |
Assumption using data from Eikelboom (2012)5 |
| Relative risk of impact of change in treatment on MACE | 0.8 | α = 8, β = 2 | Assumption from expert opinion |
| Standardised mortality ratio | |||
| Post MACE | 2.7 | Log normal σ = 0.036 |
Bronnum-Hansen (2001)270,271 |
| Variable | Cost (£) | Source |
|---|---|---|
| Test for aspirin resistance | 50 | Assumption (minimum cost) |
| Antiplatelet therapy (aspirin) (annual) | 11 | BNF (2013)272 |
| Hypothetical additional treatment if aspirin resistant (annual) | 30 | BNF (2013)272 |
| Major bleed | 4287 | Weighted cost of GI bleed from NHS reference costs 2011–12273 (75%) and acute stroke cost (25%) |
| Acute MI | 5487 | Robinson (2005)274 |
| Acute stroke | 11,020 | Youman (2003)275 |
| Long-term MI (annual) | 2196 | Robinson (2005)274 |
| Long-term stroke (annual) | 2721 | Youman (2003)275 |
| Fatal MI | 2359 | Greenhalgh (2011)276 |
| Fatal stroke | 9326 | Greenhalgh (2011)276 |
| Event/health state | Value | Beta distribution | Source |
|---|---|---|---|
| Post MI | 0.88 | α = 285.94, β = 38.99 | Cooper (2008)277 |
| Acute MI | 0.76 (distribution for decrement of 0.12) |
α = 2, β = 14.67 | Ward (2007)278 |
| Post stroke | 0.63 | α = 91.15, β = 53.53 | Ward (2007)278 |
| Acute stroke | 0.55 (distribution for decrement of 0.08) |
α = 2, β = 23 | Cooper (2008)277 |
| One-off disutility of a bleed | –0.1426 | α = 2, β = 12.03 | Greenhalgh (2011)276 |
Many of the clinical parameters in the model were not known with any certainty, therefore values were estimated after discussion with clinical experts in the study team, and wide probability distributions were applied to these estimates to reflect the extent of uncertainty. The clinical parameter values can be found in Table 76.
The base-case value for the proportion defined as aspirin resistant varied considerably between tests and studies. Given this high variation across studies included in the prognostic utility review, an arbitrary value of 22.5% was used in the base case, in keeping with the range of values identified. This parameter was tested across a wide range of values in the sensitivity analysis. The annual risk of a MACE in clinically stable patients who were defined as aspirin sensitive and on aspirin was assumed to be 5%. An overall baseline risk of MACEs in aspirin-resistant patients was difficult to determine from the prognostic review, therefore an assumption was made for the base case that this risk would be, on average, higher. A base case value of 7.5% was chosen (assumption from expert opinion). In order to vary this value in both the deterministic and probabilistic sensitivity analysis (PSA), the risk of a MACE in aspirin-sensitive patients was divided by a relative risk to give the higher risk estimate for aspirin-resistant patients. For the base case a relative risk of 0.66 was used to obtain a risk of 7.5%. The model was constructed to allow an assumption of no association between the result of the PFT and the risk of MACEs (and relative risk is equal to 1), with a hypothesis that either platelet function as measured by the test is not related to the clinical outcome, the test does not discriminate well or there is little difference in the risks between those defined as aspirin resistant and those defined as aspirin sensitive. In the PSA, this relative risk was sampled from a distribution in two stages. A uniform distribution was used to determine a number between 0 and 1. For all values between 0 and a certain proportion, x, sampling would be from a beta distribution around the relative risk of 0.66. For values sampled between x and 1, the risk of MACEs in aspirin-resistant and aspirin-sensitive patients would be the same, and the relative risk would be 1. Therefore the smaller the fixed proportion x, the more likely there would not be an increased risk of events in patients defined as aspirin resistant.
A hypothetical change in treatment for aspirin-resistant patients was assumed to have some effect in the base-case model, and a relative risk of 0.8 was used (assumption from expert opinion). As described previously for the risk of MACEs in aspirin-resistant patients, in the PSA the distribution of the relative risk for treatment effect was constructed so that a value of no effect could be applied for a fixed proportion, and an assumption of no effect of a change in treatment could be tested. The annual risk of major bleeding due to antiplatelet therapy in aspirin-sensitive patients was set at 0.01% (assumption from expert opinion). To account for a change in antiplatelet therapy, and a possible increase in major bleeds, a relative risk was applied to increase this risk of bleeding. As the change in treatment was hypothetical, a value of 1.4 was chosen, taking into account evidence on the impact of adding clopidogrel to aspirin or doubling the dose of an antiplatelet agent. 5 If an aspirin-resistant or aspirin-sensitive patient was taken off all antiplatelet therapy as a result of a major bleed, the patient’s risk of a further bleeding event (in an ‘off treatment’ health state) was assumed to be zero. Although there will be a very small risk of a major bleed in reality, this will be lower than the aspirin estimate of 0.01%, and is therefore considered to be negligible with regards to impact on the model results.
It was estimated that 9% of major bleeds would result in death, from the assumption that 1 in 10 events would be fatal. Probability of death from a major bleed was assumed to be 12.5%, assuming that 25% of major bleeds were intracranial haemorrhage (ICH) and that half of these were fatal.
Sex-specific life tables were used to determine the probability of death for all ages. The risk of death was adjusted to ensure there was no double counting of cardiovascular disease deaths, using data from the Office for National Statistics on the proportion of deaths by cardiovascular disease causes. 279 The model assumed that there was an increased risk of death once in the postevent health state and this was applied to the probability of death.
No published data were available on the cost of any of the PFTs, and as the test in the model was deemed to be hypothetical, an arbitrary cost of £50 was used, with alternative higher costs included in the sensitivity analysis. The cost of a change in treatment was also unknown as there are a number of potential changes in treatment that could be made, which may increase costs greatly or not at all. In the base-case analysis, the cost of generic clopidogrel (£30 a year, 75 mg once daily) in addition to aspirin was used. This was varied in the sensitivity analysis to consider the approximate cost of adding a newer branded drug such as prasugrel (£600 a year, assuming a body weight of > 60 kg and a daily dose of 10 mg) or ticagrelor (£702 a year, assuming 90 mg twice daily). The cost of aspirin for a year (£11) applied to all patients in the model except for those who had all antiplatelet treatment discontinued. All drug costs were obtained from the British National Formulary (BNF). 272 The acute and long-term costs of stroke were obtained from a UK study collecting primary data,275 and acute and long-term costs of MI were obtained from a previous cardiovascular disease model. 274 The source of fatal stroke and MI costs was an economic modelling study concerning other antiplatelet therapy for cardiovascular disease. 276 The exact split between stroke and MI events is not known, therefore an estimate of 20% stroke and 80% MI was used, and costs weighted accordingly. The cost of a major bleed assumed that 75% of bleeds were GI and 25% were ICH. NHS reference costs273 provided the cost of a GI bleed, and the cost of an acute stroke was assumed for an ICH. No long-term costs were attributed to bleeds, and this underestimated the cost; however, it was assumed that 50% of those who suffered an ICH died, and the initial risk of a bleed was very low. The perspective adopted for the model was that of the NHS/PSS, and a price year of 2011–12 was applied. Unit costs used in the model can be found in Table 77.
Outcomes were measured in QALYs. Age-related general population utilities from the European Quality of Life-5 Dimensions (EQ-5D) provided the baseline values for quality of life,280 with quality of life decreasing with increasing age. Utility values were given for all health states. When a MACE occurred within the model, a utility for an acute event was applied for the first year, with further, slightly higher utility applied in the postevent health state. The lower utility value for an acute health state was applied as a decrease in utility which was subtracted from the postevent utility value. The weighting of stroke to MI events used for MACE costs was also utilised here. Values were applied multiplicatively; therefore, the value for the state of the clinical event was multiplied by the value for the age. Where a major bleed occurred, a one-off reduction in utility was applied. The utility values were obtained from previous studies,276–278 and entered into the model as beta distributions (see Table 78).
Model assumptions
Owing to the speculative nature of this model, a number of assumptions were made regarding the model structure and input parameters. In the base case it was assumed that, based on a hypothetical test of platelet function, patients could be defined as aspirin resistant (having insufficient platelet function inhibition) or aspirin sensitive, and that patients defined as aspirin resistant have a higher average risk of MACEs than those defined as aspirin sensitive. This higher risk could, on average, be subsequently reduced with a hypothetical change in treatment. MACEs only included fatal and non-fatal stroke and MI, with the assumption that MI was the event occurring 80% of the time. Recurrent events were not included in the model, and once a MACE occurred, the patient moved into a postevent state with a lower quality of life and ongoing costs. Furthermore, it was assumed that a change in antiplatelet therapy would increase the risk of a major bleed (assumed to be a GI bleed or ICH). If a major bleed of this type occurred anywhere in the model, a one-off cost and reduction in quality of life was applied and all antiplatelet therapy ceased, thus increasing the risk of a MACE in the future. The model did not take into account the possible ongoing costs or additional reduction in quality of life from the starting state of stable CAD; however, this applied to all treatment options. The cost of a change in treatment was assumed to be the additional cost of clopidogrel in the base case, with newer, more expensive drugs included in the sensitivity analysis. Although these drugs are more appropriate for acute coronary syndromes rather than stable disease, it was felt that these prices would be a reasonable approximation of a newer drug which may be available for stable patients.
Assessment of cost-effectiveness
The analysis was designed to generate the cost per additional QALY gained of performing a PFT and modifying treatment based on the test findings. In order to ensure consistency in the model, an additional arm (test and no change of treatment) was also included in the economic model. However, this is not a feasible clinical option, because if no change in treatment is intended based on the test result, then there is no reason to test. As the only difference between this arm and the standard care (no test and no change of treatment) arm is the cost of the test, it served as a consistency check for the functioning of the model. Results for this option are not presented in this report.
Where available, data were entered into the model as distributions in order to fully incorporate the uncertainty around parameter values so that a PSA could be undertaken. The PSA was run with 1000 simulations and cost-effectiveness planes and acceptability curves were produced. Owing to the speculative nature of the model and the large amount of uncertainty around many of the model variables, beta distributions were applied to those parameters where neither a reasonable point estimate nor an actual distribution was known. The beta distributions were constructed in order to represent the greatest amount of uncertainty and were presented in Tables 76 and 78. The currently accepted, lower National Institute for Health and Care Excellence threshold of £20,000 per QALY gained is used to assess cost-effectiveness. 281
Deterministic sensitivity analysis
A sensitivity analysis was performed to determine the impact of changing key parameters on the model results. Therefore, many of the model parameters were subject to one- and two-way sensitivity analysis, using hypothetical increases or decreases, to determine the key drivers of the model results. Parameters varied included the costs of the test and change in treatment, event costs, the probability of testing positive for aspirin resistance, changes to the risk of MACEs with aspirin resistance, fatality rate and composition of MACEs (in terms of proportions of stroke and MI) and reduction in risk with a change in treatment. Parameters associated with major bleeding were also varied. A ‘worst-case scenario’ was also run, which assumed that those who were aspirin resistant did not have a higher risk of MACEs, a change in treatment was not effective, the test cost was £1000, the change in treatment cost £702 a year and the risk of major bleeding increased by a relative risk of 2.8.
Results
This section presents the results of the cost-effectiveness analysis.
The base-case results shown in Table 79 indicate that the intervention of ‘test and change treatment’ is cost-effective, and ‘no test and no change of treatment’ (standard care) is absolutely dominated, i.e. is more costly and less effective. This analysis assumed that for aspirin-resistant patients, there was an overall higher average risk of MACEs and an overall average reduction in MACE risk with a change in treatment. Results from the PSA show that although there is a wide spread of points, the majority are in the south-east quadrant of the cost-effectiveness plane (Figure 61), indicating dominance of the ‘test and change treatment’ strategy. The cost-effectiveness acceptability curve (Figure 62) also shows that the intervention has a high probability of being cost-effective at all willingness-to-pay thresholds.
| Strategy | Mean cost (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (cost per QALY) |
|---|---|---|---|---|---|
| ‘No test and no change of treatment’ | 13,256 | 9.6607 | |||
| ‘Test and change treatment’ | 12,940 | –316 | 9.7370 | 0.0763 | Dominant |
FIGURE 61.
Cost-effectiveness plane of ‘test and change treatment’ vs. ‘no test and no change in treatment’: base-case analysis.

FIGURE 62.
Cost-effectiveness acceptability curve of ‘test and change treatment’ vs. ‘no test and no change in treatment’: base-case analysis.

Sensitivity analysis
Tables 80 and 81 present the results of one-way sensitivity analysis of key model parameters, where each variable was varied with all other parameters fixed at base-case values.
| Results | Cost difference vs. standard care (£) | QALY difference vs. standard care | ICER for ‘test and change treatment’ (cost per QALY gained) (£) |
|---|---|---|---|
| Base-case result | –316 | 0.0763 | Dominant |
| Sensitivity analysis | |||
| Testing positive for aspirin resistance | |||
| 0.5 | –763 | 0.1696 | Dominant |
| 0.1 | –116 | 0.0346 | Dominant |
| 0.01 | 34 | 0.0034 | 9951 |
| MACE risk if aspirin resistant | |||
| 0.05 (no change) | –263 | 0.0704 | Dominant |
| 0.10 (risk doubled) | –328 | 0.0754 | Dominant |
| Fatal MACEs | |||
| 0.18 (doubled) | –278 | 0.0858 | Dominant |
| 0.045 (halved) | –335 | 0.0715 | Dominant |
| Proportion of MACEs as stroke | |||
| 0.4 (doubled) | –348 | 0.0821 | Dominant |
| 0.5 (equal split) | –364 | 0.0850 | Dominant |
| Major bleed on aspirin | |||
| 0.01 (risk increased tenfold) | –267 | 0.0617 | Dominant |
| Death from a major bleed | |||
| 0.25 (doubled) | –317 | 0.0754 | Dominant |
| Relative risk of a major bleed with a change in treatment | |||
| 2.8 (risk doubled) | –305 | 0.0726 | Dominant |
| 1.2 (risk reduced) | –317 | 0.0768 | Dominant |
| Relative risk of a MACE with change in treatment | |||
| 0.9 | –94 | 0.0357 | Dominant |
| 1 | 108 | –0.0008 | Dominated by no test and no change of treatment |
| No change in MACE risk if aspirin resistant and no reduced risk of MACEs with change in treatment | 121 | –0.0010 | Dominated by no test and no change of treatment |
| Results | Cost difference vs. standard care (£) | QALY difference vs. standard care | ICER for ‘test and change treatment’ (cost per QALY gained) (£) |
|---|---|---|---|
| Base-case result | –316 | 0.0763 | Dominant |
| Sensitivity analysis | |||
| Cost of test for aspirin resistance | |||
| £100 (doubled) | –266 | 0.0763 | Dominant |
| £500 (increased tenfold) | 134 | 0.0763 | 1760 |
| £1000 (high value) | 634 | 0.0763 | 8314 |
| Cost of hypothetical additional treatment | |||
| £600 (annual cost of prasugrel) | 870 | 0.0763 | 11,410 |
| £702 (annual cost of ticagrelor) | 1083 | 0.0763 | 14,192 |
| All event costs increased by 50% | |||
| All events | –529 | 0.0763 | Dominant in all cases |
| Major bleed | –314 | 0.0763 | |
| Acute MI | –345 | 0.0763 | |
| Acute stroke | –330 | 0.0763 | |
| Long-term MI | –444 | 0.0763 | |
| Long-term stroke | –356 | 0.0763 | |
| Fatal MI | –317 | 0.0763 | |
| Fatal stroke | –317 | 0.0763 | |
| All event costs decreased by 50% | |||
| All events | –100 | 0.0763 | Dominant in all cases |
| Major bleed | –318 | 0.0763 | |
| Acute MI | –287 | 0.0763 | |
| Acute stroke | –301 | 0.0763 | |
| Long-term MI | –185 | 0.0763 | |
| Long-term stroke | –275 | 0.0763 | |
| Fatal MI | –314 | 0.0763 | |
| Fatal stroke | –314 | 0.0763 | |
| Time horizon (years) | |||
| 5 | –50 | 0.0059 | Dominant |
| 10 | –168 | 0.0191 | Dominant |
| 20 | –304 | 0.0495 | Dominant |
Most of the clinical variables did not change the overall results where the ‘test and change treatment’ was cost-effective and dominant over standard care (see Table 80). Decreasing the percentage that test positive for aspirin resistance to only 1% meant that the intervention was no longer dominant, but was still cost-effective at approximately £10,000 per QALY gained. Neither changing the nature of MACEs in terms of proportions of strokes and MIs and percentage of fatal MACEs, nor changing the risk and fatality rate from bleeds, had any impact on the results. Reducing the effectiveness of the treatment change (in terms of preventing MACE) did change the magnitude of the cost and QALY differences; however, only an ineffective treatment changed the result in favour of standard treatment. This was more apparent when it was assumed that there was no baseline increased risk of MACEs and no impact of testing and treating.
Table 81 presents the sensitivity analysis around the cost parameters and time horizon of the model. Increasing or decreasing all of the event costs, or each one separately by 50%, did not change the overall result. However, the model was sensitive to the cost of a PFT, although the ‘test and change treatment’ option was still cost-effective even at a value of £1000 per test. The cost of the hypothetical treatment also had an impact, with the costs of newer, branded antiplatelet therapies giving incremental cost-effectiveness ratios (ICERs) of over £10,000 per QALY but still below the £20,000-per-QALY threshold. Decreasing the time horizon of the model to 5, 10 and 20 years did reduce the cost and QALY differences, but ‘test and change treatment’ was still cost-effective.
Table 82 shows the results of the ‘worst-case scenario’ where, in the ‘test and change treatment’ option, costs of the PFT and a change in treatment were higher, those who were aspirin resistant did not have a higher risk of MACEs than those who were aspirin sensitive, a change in treatment was not effective and the risk of major bleeding was higher. Here, the ‘test and change treatment’ option is dominated by standard care, and costs £2585 more per patient with a loss of 0.0043 QALYs.
| Strategy | Mean cost (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (cost per QALY) |
|---|---|---|---|---|---|
| ‘No test and no change of treatment’ | 12,445 | 9.8073 | |||
| ‘Test and change treatment’ | 15,030 | 2585 | 9.8030 | –0.0043 | Dominated by no test and no change of treatment |
Two-way sensitivity analyses were also undertaken to assess the impact of changing two key variables at the same time (Table 83). The cost of the PFT and change in treatment were both increased at the same time, which changed the result from dominance to a positive ICER. Once both test and treatment costs were in the region of £600–700 each, then the intervention was no longer cost-effective, assuming a £20,000-per-QALY threshold.
| Cost of test (£) | Cost of change in treatment (£) | Cost difference vs. standard care (£) | QALY difference vs. standard care | ICER for ‘test and change treatment’ (cost per QALY gained) (£) |
|---|---|---|---|---|
| 50 | 30 | –316 | 0.0763 | Dominant |
| 125 | 125 | –43 | 0.0763 | Dominant |
| 250 | 250 | 342 | 0.0763 | 4276 |
| 375 | 375 | 727 | 0.0763 | 9090 |
| 500 | 500 | 1112 | 0.0763 | 13,904 |
| 625 | 625 | 1498 | 0.0763 | 18,719 |
| 750 | 750 | 1883 | 0.0763 | 23,533 |
| 875 | 875 | 2268 | 0.0763 | 28,346 |
| 1000 | 1000 | 2653 | 0.0763 | 33,160 |
Finally, PSA was undertaken where a variable related to the presence of a reduction in MACE risk due to a change in treatment was altered. In the base case, it was assumed there would be some impact of treatment. However, the model was constructed so that, in a PSA, the variable for risk reduction could hold the value 1 (no change) for a given proportion, x, with the assumption that for this proportion x of patients, the treatment had no impact on MACE risk. The first PSA set this value x to 0.5 and the results can be seen in Figures 63 and 64. This analysis reduces the probability of ‘test and change treatment’ being cost-effective at the £20,000-per-QALY threshold to 48% – no longer a cost-effective option. A further PSA was run with 50% of patients getting no benefit from treatment and the cost of a change in treatment set to £702 a year, and this further reduced the probability of cost-effectiveness to 24% (Figures 65 and 66). Finally, a PSA was undertaken to consider a scenario where 50% of all patients defined as aspirin resistant had no increase in MACE risk and 50% of all those defined as aspirin resistant do not benefit from a change in treatment (Figures 67 and 68). Again, the ‘test and change treatment’ option is not cost-effective, and has a 50% probability of being cost-effective at £20,000 per QALY.
FIGURE 63.
Cost-effectiveness plane of ‘test and change treatment’ vs. ‘no test and no change in treatment’: 50% of patients have no change in MACE risk with a change in treatment.

FIGURE 64.
Cost-effectiveness acceptability curve of ‘test and change treatment’ vs. ‘no test and no change in treatment’: 50% of patients have no change in MACE risk with a change in treatment.
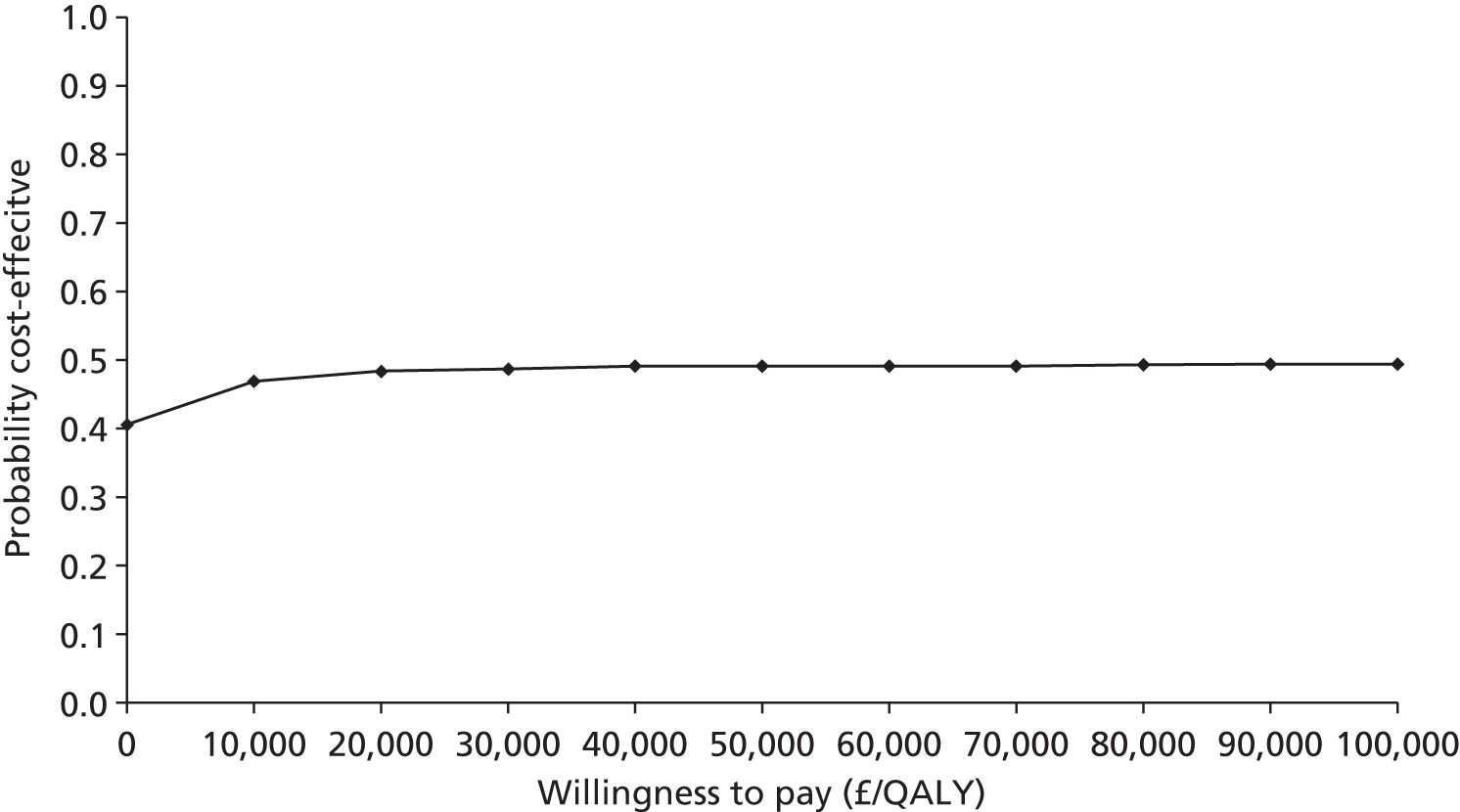
FIGURE 65.
Cost-effectiveness plane of ‘test and change treatment’ vs. ’no test and no change in treatment’: 50% of patients have no change in MACE risk with a change in treatment and change in treatment costs are £702.

FIGURE 66.
Cost-effectiveness acceptability curve of ‘test and change treatment’ vs. ‘no test and no change in treatment’: 50% of patients have no change in MACE risk with a change in treatment and change in treatment costs are £702.

FIGURE 67.
Cost-effectiveness plane of ‘test and change treatment’ vs. ‘no test and no change in treatment’: 50% of patients do not have elevated MACE risk and 50% have no change in MACE risk with a change in treatment.

FIGURE 68.
Cost-effectiveness acceptability curve of ‘test and change treatment’ vs. ‘no test and no change in treatment’: 50% of patients do not have elevated MACE risk and 50% have no change in MACE risk with a change in treatment.

Cost-effectiveness discussion
The results of the cost-effectiveness analysis for the base-case values indicate that if a PFT were available that was able to accurately identify individual patients who, while receiving aspirin therapy, were at higher risk of an adverse clinical outcome than other patients, and a subsequent change in treatment reduced the risk of MACEs in such individuals, this would be a highly cost-effective strategy, as long as the costs of testing and a change in treatment were not excessively high. This result was robust with regards to most model variables. However, this result changes when values of important (and currently unknown) variables are varied. Within the sensitivity analyses, results were sensitive to the proportion defined as ‘aspirin resistant’, and the availability of an effective treatment alternative for patients on aspirin discovered to be at an elevated risk of MACEs. Factors that adversely affect the cost-effectiveness of testing and changing therapy include only a very small proportion of patients being defined as ‘aspirin resistant’, if the cost of testing is high and a change in treatment is costly and/or not highly effective. This is exemplified by the ‘worst-case scenario’ sensitivity analysis, which demonstrates that if an expensive test cannot identify individuals at a higher risk of clinical events and a costly change in treatment does not reduce the risk of MACEs but increases the risk of bleeding, then the ‘test and change treatment’ option results in much higher costs and a reduction in QALYs, and is therefore not cost-effective.
A key strength of this analysis is that this is the first economic model to consider the cost-effectiveness of testing for and treating patients defined as ‘aspirin resistant’, and it can illustrate the key variables that have an impact on the results. Although few good-quality data currently exist to populate the model, a model structure exists for reanalysis once additional data become available. The main limitation is the highly speculative nature of the model and the uncertainty around parameter values resulting from the absence of evidence. This has been addressed where possible by the deterministic sensitivity analysis that has been undertaken and by applying the greatest amount of uncertainty around many of the model parameter values. However, as most distributions have been assumed, there should be caution in interpreting the results of the PSA, as there may be biases in quantifying the uncertainty and the direction of this bias is unknown.
The model assumes that patients at elevated risk of cardiovascular outcomes can be accurately identified by a test, i.e. that PFTs are perfectly accurate in discriminating those patients with and without ‘abnormal’ platelet function and that ‘abnormal’ platelet function is associated with a risk of cardiovascular outcomes. This assumption was, in part, explored in sensitivity analyses. The preferred approach in economic models for diagnostic testing is to consider test performance in terms of sensitivity and specificity, requiring prevalence data and a reference standard test, which do not exist for PFTs. In the absence of information, a perfect test has been assumed, and the probability of being ‘aspirin resistant’ varied in the sensitivity analysis.
The model structure may also be viewed as somewhat simplistic, with no recurrence of clinical events and no increase in the risk of events over time. However, if the model had included both features, a reasonable ‘test and change treatment’ strategy is likely to appear even more cost-effective. This model is for stable CAD patients only, and an additional model was not constructed for ACS patients on dual therapy. As this patient group is likely to have an even higher risk of MACEs, the results are likely to have shown similar levels of cost-effectiveness.
Summary
-
Currently, there is no existing economic evidence on the cost or cost-effectiveness of platelet function testing for ‘aspirin resistance’, which is surprising considering the amount of research identified by the systematic review of prognostic/diagnostic utility and the degree of debate around this topic.
-
This is the first model to attempt to estimate the cost-effectiveness of a ‘test and change treatment’ strategy for ‘aspirin resistance’.
-
The model is highly speculative owing to the heterogeneity and uncertainty around the prognostic/diagnostic utility of PFTs available to populate the model and contains a number of assumptions.
-
If a PFT can accurately identify patients at higher risk of adverse clinical outcomes while receiving aspirin therapy as the sole antiplatelet agent, and patients have an effective treatment change, then a ‘test and change treatment’ option is very likely to be cost-effective. Conversely, if a PFT cannot identify these patients, and a treatment change is not effective in reducing MACE risk, then a ‘test and change treatment’ strategy is not cost-effective.
-
The parameters with the greatest impact on model results are the proportion of patients who are correctly identified as having a high risk of adverse clinical outcomes, the effectiveness of a change in treatment if ‘aspirin resistant’, the cost of a test and the cost of a change in treatment.
-
The model requires more robust data on the association between a designation of ‘aspirin resistance’ and the risk of adverse clinical outcomes, PFTs that can accurately define patients as ‘aspirin resistant’ and appropriate alternative therapy options for those at higher risk of adverse clinical outcomes.
Chapter 7 Discussion
Summary of results: prognostic utility review
Monotherapy
For assessment of the prognostic utility of PFTs in patients receiving aspirin as a monotherapy (at the time of testing), a majority of patients in all studies included were considered to have stable disease. There was considerable heterogeneity in other population characteristics (e.g. smoking, diabetes), as well as differences relating to treatment (e.g. aspirin dose), tests and testing procedures used, threshold for classifying patients as ‘aspirin resistant’, range and definition of clinical outcomes recorded and length of patient follow-up. There was a lack of detail in reporting of quality criteria and no study reported all the items considered to be important. Lack of detail related in particular to blinding (of those undertaking PFTs to patient characteristics, or blinding of outcome assessors), loss-to-follow-up information and level of compliance with aspirin treatment. There was no consistent reporting of outcome statistics (e.g. OR and HR, adjusted and unadjusted). Given the above, pooling of data for each type of test was deemed to be inappropriate. As such, the summary results below are based on non-statistical assessment of trends across studies.
The data show variability in prognostic effect sizes across the studies. In most assessments of association between a PFT designation of ‘aspirin resistance’ and clinical events, there are more studies with effect estimates above 1 (i.e. the risk of adverse clinical outcome in a group of patients designated ‘aspirin resistant’ is greater than that in a group designated ‘aspirin sensitive’), but this was not uniform.
Many of the included studies contain very small numbers of patients and outcome events. This can lead to extreme prognostic effect estimates that arise merely by chance; however, the uncertainty about the estimates will be reflected by extremely wide CIs. Studies with extremely wide CIs covering values well above and below the null effect (e.g. intervals spanning ORs or HRs from close to zero to over 20) are essentially providing very little, if any, useful information. Therefore, caution should be applied when focusing on the estimates from such studies, and rather the focus should be on the wide CIs that reflect the large uncertainty.
Overall, there is a possible trend suggestive of more clinical events occurring in those groups of patients designated ‘aspirin resistant’, with some results in some studies showing statistical significance; this is the case across the majority of tests (LTA, VerifyNow® Aspirin, PFA-100®, thromboxane metabolite measurement), to a lesser extent for TEG, and with data for WBA not allowing many conclusions to be drawn. This trend is also fairly consistent across some outcomes (i.e. death, MACEs and ischaemic thrombotic events) irrespective of test, though the direction of effect is not always consistent for different thresholds applied to the data from the same study. There are very limited data on bleeding events and thus no inference could be drawn.
The results suggest that PFTs (specifically LTA, VerifyNow® Aspirin, PFA-100®, thromboxane metabolite measurement and TEG) may have some prognostic value as they are fairly consistently associated with elevated risk of cardiovascular events (MACEs or death). However, as meta-analysis was not possible, no firm quantitative conclusions can be drawn as to their prognostic value. Given that the effect sizes for an association with clinical events are relatively small and highly uncertain, a determination of the diagnostic utility of PFTs (for an individual, determining if they are at higher risk of a clinical event) was not possible in this report.
Dual-therapy studies
The tests identified for assessing platelet function in patients on dual therapy (aspirin plus a second antiplatelet agent at the time of the PFT) are (i) LTA, (ii) VerifyNow® Aspirin, (iii) measurement of urinary or serum/plasma 11-dehydro-TxB2 concentrations, (iv) PFA-100®, (v) WBA, (vi) TEG and (vii) other miscellaneous tests (see Chapter 5, Dual therapy). The original intention was to report and analyse these studies in a similar way to the studies in patients receiving monotherapy with aspirin. As a result of the complex nature of the searches and study selection process, and also issues around reporting, studies on dual-therapy patients were included and data extraction undertaken in parallel with that for monotherapy studies. As it remains unclear whether ‘aspirin resistance’ is a distinct biological entity, with specific underlying mechanisms, or signals a more general platelet hyperactivity state in which aspirin is simply insufficient to inhibit platelet responses on its own,14,44,74 the interplay between aspirin and a second antiplatelet agent in terms of platelet inhibition is an area of intense research. While clinical trials have demonstrated that the addition of a second antiplatelet agent results in more pronounced platelet inhibition, and consequently reduced risk of MACEs and increased risk of bleeding,5,22 it remains unknown whether or not poor platelet response to one agent is linked to poor response to the other. For example, a number of studies have shown that ‘aspirin-resistant’ patients had normal responses to clopidogrel;195,282,283 conversely, a number of studies have reported concomitant resistance to both aspirin and clopidogrel. 98,142,284 Given the high variability and the limited evidence of prognostic utility found in patient cohorts taking aspirin alone, it was decided not to analyse the results in populations exposed to dual antiplatelet therapy, as this introduced an extra unpredictable variable. The data on ‘aspirin resistance’ (but not ‘clopidogrel resistance’) have, however, been extracted from the included studies, and are available for analysis (see Appendix 4).
Strengths and limitations of available evidence and the prognostic utility review
Considerations on the volume of evidence identified
Within the limited parameters of the review, it became clear that there was an underestimate of the number of studies available based on the initial scoping exercise. It had been assumed that any new studies identified would have been subsequent to the previous systematic reviews; however,
-
Not all the studies had been published since the previous systematic reviews, and thus the previous systematic reviews were not sufficiently comprehensive (see Chapter 5, Systematic reviews).
-
As a more thorough approach to identifying the evidence was undertaken, this revealed a surprisingly large number of studies which scoping could not easily predict. This is exemplified by the large number of hard-copy articles which were retrieved in order to make selection judgements. This was often driven by the fact that the nature of the studies did not easily convey that relevant information for this report was available.
-
It became clear that the nature of the studies, in terms of both the populations and the interventions, had also altered. Thus, more patients with acute rather than stable disease were included, particularly patients with ACS, acute stroke and patients undergoing PCIs.
The nature of the included populations also drove a change in the nature of the interventions, with many of the later studies including populations treated with aspirin plus at least one other antiplatelet agent.
A stepwise approach to analysis of the included studies was planned and this aided management of the large number of included studies.
Considerations on the reporting of available evidence
The availability of a larger-than-expected volume of evidence has already been discussed above, as has the unrepresentativeness of most existing systematic reviews. A further salient point is the amount of unreported evidence relevant to this report. One-third of the articles meeting the inclusion criteria for the prognostic/diagnostic review did not report the association between PFT results and subsequent clinical outcomes, despite both being measured in the study being reported. Although reporting of this association might not have been within the aims of these studies and was thus, understandably, not reported as a priority, it suggests that a large number of data were not accessible to this review. It was beyond the scope of this project to ascertain the availability of these data for analysis. Lack of reporting may also relate to results not being reported for all thresholds for a designation of ‘aspirin resistance’, and/or time points for outcome measurement, changes in antiplatelet treatment during the course of a study and items relevant to quality assessment (see Monotherapy).
Considerations on the analysis
Tests are measured on a continuous scale for each patient. However, the studies identified by our review focused predominantly on comparing test-positive (‘aspirin-resistant’) and test-negative (‘aspirin-sensitive’) groups, with ‘resistant’ and ‘sensitive’ groups defined by dichotomising the continuous test by a chosen threshold value. This was not surprising, and indeed the data extraction and meta-analysis strategy was planned to synthesise results for each threshold reported where possible. However, the chosen threshold value was often highly variable from study to study, and this was a major reason why meta-analysis was not deemed sensible. Some studies presented results for three or four categories (e.g. based on tertiles or quartiles). It is now recognised that it is better to analyse continuous variables on a continuous scale. Dichotomisation285/categorisation reduces power to detect genuine prognostic factors, and misses the opportunity to examine non-linear trends and the underlying prognostic association across the entire range of the factor’s values. For meta-analysis, availability of individual patient data (IPD) would help synthesise linear and non-linear trends across multiple studies of the same factor. 286,287 Though the collection, cleaning and synthesis of IPD can be time-consuming and expensive,288 it eases many problems with meta-analysis and avoids the complexity of extracting and dealing with multiple, variable and often selectively reported thresholds from published reports. However, an IPD analysis in the context of the current report would have required the consistent within- and between-study collection of all possible patient, test and outcome effect modifiers and a substantial proportion of study authors to make these data available. This was beyond the scope of this report and would likely be hampered by a number of factors, as outlined in the research recommendations (see Recommendations for future research).
Thus, pooling could only be considered for dichotomous data for each test. An array of data extraction and validated analysis methods allowed us to directly and indirectly obtain effect sizes and CIs from the included studies (see Chapter 4, Prognostic ability: unadjusted and adjusted odds ratios and hazard ratios). This led to substantially more information being available than would otherwise have been the case. Sometimes only rounded percentages of events were reported in each group (rather than exact numbers) and so only approximate numbers were obtainable.
However, as a result of poor or incomplete reporting, many studies did not allow suitable results to be extracted or calculated. In addition, even when the same types of effect sizes could be obtained from multiple studies of the same test, there was a vast amount of clinical and methodological heterogeneity across studies in important factors, as outlined above (see Monotherapy).
It was therefore decided that pooled results would be difficult to interpret meaningfully. The extracted results were therefore summarised on forest plots (but without pooled results). These plots provide the effect estimate and its CI for each study, alongside key clinical and methodological information such as the patient group, outcome, sample size, threshold level and agonist. The absence of the ability to pool studies meant that examinations of publication bias (e.g. funnel plots) were also not possible, which was unfortunate as publication bias is a known issue in prognosis research. 289–292
Consideration on the usefulness of prognostic factors
Prognostic factors are defined as measurable characteristics associated with the risk of a subsequent outcome in people with a given disease or health condition. A recent series on prognosis research293–296 discusses how a single prognostic factor rarely predicts individual outcome risk accurately, and usually does not suitably discriminate between high-risk and low-risk individuals, as in this report. This is why prognostic models are developed, as they utilise multiple prognostic factors in combination to improve individual risk prediction accuracy and to better discriminate the underlying risk across individuals. No prognostic models were identified that aimed to utilise multiple tests with multiple prognostic factors for the purpose of predicting individual outcome risk in patients on aspirin monotherapy for prevention of cardiovascular events.
It should be noted that prognostic factors do have a broad array of potential uses in both clinical practice and health research. 293–296 For instance, they help to define disease at diagnosis; they aid the design and analysis of trials; they are confounders to consider in observational studies and unbalanced trials; and they are the building blocks of risk prediction models. The designation of ‘aspirin resistance’ based on a PFT is a potential prognostic factor, but it should ideally be considered in conjunction with any other factors identified as prognostic of future adverse clinical outcomes. Thus, even if there was certainty around the prognostic utility of PTFs, there is a need to ascertain if any other factors have prognostic utility and, if so, ideally a prognostic model should be developed and validated to aid treatment management.
Summary of the economic evaluation
Currently, there is no existing economic evidence on the cost or cost-effectiveness of platelet function testing for ‘aspirin resistance’, which might seem surprising considering the amount of research identified by the systematic review of prognostic/diagnostic utility, the positive findings of many existing systematic reviews of prognostic utility of PFTs in this area and the degree of debate around this topic. This report presents the first model to attempt to estimate the cost-effectiveness of a ‘test and change treatment’ strategy using platelet function testing to define an at-risk population. The model is highly speculative owing to the large degree of heterogeneity and uncertainty around the prognostic utility of PFTs, and it contains numerous assumptions. This has been addressed, where possible, by the deterministic sensitivity analysis and also by taking into account the uncertainty around many of the model parameter values. In addition, further analyses have been presented to show scenarios where platelet function testing for ‘aspirin resistance’ and a change in treatment would not be cost-effective. The model structure may also be viewed as somewhat simplistic, with no recurrence of clinical events and no increase in the risk of events over time, but as the model is speculative, with few data to populate it, there would be little value in making the model unnecessarily complex at this stage.
Assuming that a PFT can accurately identify patients at higher risk of adverse clinical outcomes while receiving aspirin therapy as the sole antiplatelet agent, and patients changed to an effective treatment, then a ‘test and change treatment’ option is very likely to be cost-effective. Conversely, if a PFT cannot identify these patients, and a treatment change is not effective in reducing adverse clinical outcome (MACE) risk, then a ‘test and change treatment’ strategy is not cost-effective. The parameters with the greatest impact on model results are the proportion of patients who are correctly identified as having high risk of clinical outcome, the effectiveness of a change in treatment if designated ‘aspirin resistant’, the cost of a test and the cost of a change in treatment. The accuracy of testing, the additional risk of an adverse outcome associated with a designation of ‘aspirin resistance’ and the effectiveness of a change in therapy are the most uncertain parameters. The model requires more robust data on all of these aspects.
Recommendations for future research
The heterogeneity and methodological problems identified in this review are similar to other attempted meta-analyses of prognostic factor studies. Attempting to conduct and draw inferences from systematic reviews of prognostic factor studies is difficult. 293 Primary prognostic factor studies are often poorly designed, inappropriately analysed and poorly/selectively reported. 289–292,297–304 This leads to confusion about whether or not factors are genuinely prognostic, with the play of chance and selective reporting typically leading to overoptimism in the prognostic effect sizes seen in the literature.
Primary studies evaluating the prognostic ability of tests should focus on standardising how the tests are measured and conducting large, protocol-driven studies with statistical analysis plans that aim to examine prognostic and predictive ability. Guidelines for those planning and undertaking a prognostic factor study have been suggested and should be used, to ensure higher standards of study quality, design and analysis than are currently observed. 305,306 This is important in the current context as many studies included in the prognostic utility review had other primary aims (e.g. effectiveness of an intervention).
A prospective rather than retrospective design is preferable as it enables clear inclusion criteria, more complete baseline and follow-up data, and greater standardisation of diagnostic and therapeutic procedures, as well as ensuring that the primary factors and outcomes can be specified in advance, reducing the potential for data dredging and thus type I errors and selective reporting. This is especially important in larger studies aiming to replicate earlier exploratory prognostic factor findings, and these should incorporate a suitable sample size calculation to ensure adequate power to detect an important prognostic effect, if one exists. Statistical analysis methods can be improved by analysing continuous factors on their continuous scale, thereby avoiding the use of arbitrary threshold levels to categorise them; by considering non-linear relationships; and by including multivariable analyses that assess a factor’s prognostic value over existing prognostic factors. 293
Future research should ideally concentrate on developing and validating prognostic models that utilise multiple prognostic factors in combination. For an individual with a given state of health (start point), a prognostic model converts the combination of predictor values to an estimate of the risk of experiencing a specific end point within a specific time period. Ideally, this produces an estimate of the absolute risk (absolute probability) of experiencing the end point. This is the information that clinicians require to make decisions, rather than reliance on the result of a single PFT. One option is to use IPD from high-quality, prospective, primary studies that use very similar clinical populations and measure similar tests, patient characteristics (prognostic factors) and outcomes. 295 Having IPD from multiple studies offers a natural opportunity to increase sample size and essentially develop a prognostic model within a meta-analysis framework. 307 Variation in model accuracy across studies, and its causes, can be explored. Additionally, such collaborative efforts encourage consensus towards a single, well-developed and validated prognostic model, rather than a number of competing and non-validated models for the same clinical problem, championed by each group separately.
However, in the current context, an IPD meta-analysis would be hampered by substantial heterogeneity across studies, even if only a single PFT at a time was considered in a well-defined population. In particular, heterogeneity relates to test thresholds used, variability in test methods, timing of test and length of follow-up, and range and definition of clinical events. A large number of variables would likely need to be considered (e.g. smoking status, age, sex, prior events, disease severity, diabetes, mean platelet volume, etc.), even allowing for the fact that not all will be found to have a significant effect. Given that a minimum of 10 events per variable is considered necessary in a prognostic model, the frequently low event rates in studies limit the number of variables that can be assessed. An IPD analysis may also be biased, depending on which study authors make their data available and the different methodological quality of the studies.
Given the above, using IPD from existing studies could render a useful prognostic model improbable to build. Therefore, one or more new prospective cohort studies may be a more feasible option. A primary study could incorporate the following elements, the majority of which have not been appropriately considered in existing studies:
-
several tests performed in the same individuals (at present there are very few studies looking at more than one or two tests)
-
standardisation of laboratory methods
-
tests performed in duplicate to assess variability
-
repeat tests to assess variability over time
-
sufficiently large sample size to allow for sufficient events in both resistant and sensitive groups
-
sufficiently long follow-up for relevant clinical outcomes to occur
-
use of standardised criteria for individual outcomes, and also individual reporting of all outcomes contributing to composite outcome measures
-
rigorous assessment of adherence
-
robust methodological quality
-
analyses could be undertaken for different test thresholds.
The above should be applied to one or more well-described cardiovascular/cerebrovascular population(s) and should also consider current treatment guidelines with regard to antiplatelet therapy for the given population.
The aim of a primary study would be to (i) identify what tests (variables) have a prognostic association with future outcomes, both on their own (unadjusted) and independent of other prognostic factors (adjusted); and (ii) develop a prognostic model that utilises the identified prognostic tests and variables in combination, in order to predict absolute risk of adverse outcomes for future individuals.
Before initiating such a study, a group of clinical and methodological leaders in the field should meet and agree criteria such as the tests to be measured, the relevant clinical populations and timing of test measurement, how the tests are measured, and what outcomes are relevant and how these are measured.
If more than one new cohort study is to be undertaken on a similar population, ideally this should be done in collaboration, ensuring similar protocols, methodology and factors/tests to be recorded, with agreement to pool IPD once each individual study is completed. This would ensure reduced heterogeneity across studies and the opportunity to then simultaneously develop and validate a model.
Once these issues have been addressed it may be possible to undertake a ‘test–treat trial’ using a prognostic model to tailor antiplatelet therapy to individuals.
Chapter 8 Conclusions
The current report has failed to demonstrate a convincing association between ‘aspirin resistance’, as defined by a PFT, and clinical outcome, on any test and in any outcome, despite the existence of a vast number of studies which have sought to clarify this association. The issues surrounding potential inaccessibility of relevant data and the heterogeneity across all of the study parameters have been discussed. The implications of a designation of ‘aspirin resistance’ based on a PFT remain uncertain and, thus, so does the question of how best to define ‘aspirin resistance’. Until a definitive study has been undertaken to answer this question, which must include some measure of adherence to therapy, platelet function testing has no demonstrable clinical utility.
Although evidence indicates that some tests may have some prognostic value, methodological and clinical heterogeneity of studies and different approaches to analyses create confusion and inconsistency in prognostic results, and prevented a quantitative summary of their prognostic effect. Large, protocol-driven and adequately powered primary studies are needed, using standardised and agreed methods of measurements to evaluate the prognostic ability of each test in the same population(s). For PFTs to inform individual risk prediction, and thus be useful for clinical decision-making, it is likely that they need to be considered in combination and alongside other prognostic factors, within a prognostic model.
Acknowledgements
The authors thank the following:
Simon Stevens for his invaluable administrative support, excellent organisational skills and heroic efforts to source articles.
Jo Hine for additional administrative support.
Steve Watson, Paul Harrison and Gregory Y H Lip for clinical and platelet function testing guidance.
Pelham Barton for economic modelling guidance.
All the people who kindly gave their time to help translate articles.
Contribution of authors
Janine Dretzke wrote and edited sections of the report and undertook study selection, data extraction and quality assessment for the prognostic review.
Richard D Riley devised, led and executed the statistical analysis, extracted statistical data for the prognostic review and wrote sections of the report.
Marie Lordkipanidzé provided detailed knowledge of the PFTs, their utilisation and interpretation of their results, undertook study selection for the prognostic review and wrote the background section of the report.
Susan Jowett led the economic section of the report, contributed to all parts of the economic review and development of the economic model and associated analysis, and wrote sections of the report.
Jennifer O’ Donnell undertook study selection, data extraction and quality assessment for the prognostic review, and contributed to several sections of the report.
Joie Ensor contributed to the plan for statistical analysis and interpretation of the data, extracted statistical data for the prognostic review, managed the flow of statistical evidence and contributed to several sections of the report.
Eoin Moloney contributed to all parts of the economic review and development of the economic model and associated analysis, and wrote sections of the report.
Malcolm Price contributed to the plan for statistical analysis and interpretation of the data and extracted statistical data for the prognostic review.
Smriti Raichand contributed to the development of the protocol and undertook study selection.
James Hodgkinson undertook data extraction and an appraisal of existing systematic reviews.
Susan Bayliss contributed to deriving the search strategies and ran the searches in electronic databases.
David Fitzmaurice contributed to all parts of the project, undertook study selection, provided clinical insight, wrote sections of the report and takes responsibility for overall content.
David Moore led the review section of the report, contributed to all parts of the project, undertook study selection, compiled the report, and wrote and edited sections of the report.
Publications
Dretzke J, Riley R, Lordkipanidzé M, Jowett S, O’Donnell J, Ensor J, et al. Protocol for a systematic review of the diagnostic and prognostic utility of tests currently available for the detection of aspirin resistance in patients with established cardiovascular or cerebrovascular disease. Syst Rev 2013;2:16.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Scarborough P, Bhatnagar P, Wickramasinghe K, Smolina K, Mitchell C, Rayner M. Coronary Heart Disease Statistics 2010. London: British Heart Foundation; 2010.
- Jack DB. One hundred years of aspirin. Lancet 1997;350:437-9. http://dx.doi.org/10.1016/S0140-6736(97)07087-6.
- Prescriptions Dispensed in the Community: England, Statistics for 2001 to 2011. Leeds: Health and Social Care Information Centre; 2011.
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60. http://dx.doi.org/10.1016/S0140-6736(09)60503-1.
- Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e89-11.
- Bell AD, Roussin A, Cartier R, Chan WS, Douketis JD, Gupta A, et al. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines Executive Summary. Can J Cardiol 2011;27:208-21. http://dx.doi.org/10.1016/j.cjca.2010.12.033.
- American Diabetes Association . Standards of medical care in diabetes – 2013. Diabetes Care 2013;36:11-66. http://dx.doi.org/10.2337/dc13-S011.
- Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care 2010;33:1395-402. http://dx.doi.org/10.2337/dc10-0555.
- Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov 2010;9:154-69. http://dx.doi.org/10.1038/nrd2957.
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation 2010;121:e46-215. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.192667.
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517-84. http://dx.doi.org/10.1161/STR.0b013e3181fcb238.
- Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg 2007;45:1185-91. http://dx.doi.org/10.1016/j.jvs.2007.02.004.
- Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, Collet JP, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2851-906. http://dx.doi.org/10.1093/eurheartj/ehr211.
- Lordkipanidzé M. Advances in monitoring of aspirin therapy. Platelets 2012;23:526-36. http://dx.doi.org/10.3109/09537104.2012.711865.
- Awtry EH, Loscalzo J. Aspirin. Circulation 2000;101:1206-18. http://dx.doi.org/10.1161/01.CIR.101.10.1206.
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971;231:232-5. http://dx.doi.org/10.1038/newbio231232a0.
- Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J Clin Invest 1975;56:624-32. http://dx.doi.org/10.1172/JCI108132.
- Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest 1982;69:1366-72. http://dx.doi.org/10.1172/JCI110576.
- Laine L. Review article: gastrointestinal bleeding with low-dose aspirin – what’s the risk?. Aliment Pharmacol Ther 2006;24:897-908. http://dx.doi.org/10.1111/j.1365-2036.2006.03077.x.
- Bertrand OF, Larose E, Rodes-Cabau J, Gleeton O, Taillon I, Roy L, et al. Incidence, predictors, and clinical impact of bleeding after transradial coronary stenting and maximal antiplatelet therapy. Am Heart J 2009;157:164-9. http://dx.doi.org/10.1016/j.ahj.2008.09.010.
- Suh JW, Mehran R, Claessen BE, Xu K, Baber U, Dangas G, et al. Impact of in-hospital major bleeding on late clinical outcomes after primary percutaneous coronary intervention in acute myocardial infarction: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. J Am Coll Cardiol 2011;58:1750-6. http://dx.doi.org/10.1016/j.jacc.2011.07.021.
- Patrono C, Andreotti F, Arnesen H, Badimon L, Baigent C, Collet JP, et al. Antiplatelet agents for the treatment and prevention of atherothrombosis. Eur Heart J 2011;32:2922-32. http://dx.doi.org/10.1093/eurheartj/ehr373.
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999-3054. http://dx.doi.org/10.1093/eurheartj/ehr236.
- Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011;124:2458-73. http://dx.doi.org/10.1161/CIR.0b013e318235eb4d.
- Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Ettinger SM, et al. 2011 ACCF/AHA Focused Update of the Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;123:2022-60. http://dx.doi.org/10.1161/CIR.0b013e31820f2f3e.
- Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, et al. Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501-55. http://dx.doi.org/10.1093/eurheartj/ehq277.
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124:e574-651. http://dx.doi.org/10.1161/CIR.0b013e31823ba622.
- Michelson AD, Cattaneo M, Eikelboom JW, Gurbel P, Kottke-Marchant K, Kunicki TJ, et al. Aspirin resistance: position paper of the Working Group on Aspirin Resistance. J Thromb Haemost 2005;3:1309-11. http://dx.doi.org/10.1111/j.1538-7836.2005.01351.x.
- Cattaneo M. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler Thromb Vasc Biol 2004;24:1980-7. http://dx.doi.org/10.1161/01.ATV.0000145980.39477.a9.
- Position Paper on the Regulatory Requirements for the Authorization of Low-Dose Modified Release ASA Formulations in the Secondary Prevention of Cardiovascular Events. London: European Medicines Agency; 2002.
- Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther 2008;118:18-35. http://dx.doi.org/10.1016/j.pharmthera.2008.01.001.
- Grove EL, Storey RF, Wurtz M. Platelet function testing in atherothrombotic disease. Curr Pharm Des 2012;18:5379-91. http://dx.doi.org/10.2174/138161212803251862.
- FitzGerald GA. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am J Cardiol 1991;68:11B-15B. http://dx.doi.org/10.1016/0002-9149(91)90379-Y.
- Crescente M, Di Castelnuovo A, Iacoviello L, De Gaetano G, Cerletti C. PFA-100 closure time to predict cardiovascular events in aspirin-treated cardiovascular patients: a meta-analysis of 19 studies comprising 3,003 patients. Thromb Haemost 2008;99:1129-31. http://dx.doi.org/10.1160/TH08-03-0130.
- Chan MV, Armstrong PC, Papalia F, Kirkby NS, Warner TD. Optical multichannel (optimul) platelet aggregometry in 96-well plates as an additional method of platelet reactivity testing. Platelets 2011;22:485-94. http://dx.doi.org/10.3109/09537104.2011.592958.
- Santilli F, Rocca B, De Cristofaro R, Lattanzio S, Pietrangelo L, Habib A, et al. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: implications for aspirin ‘resistance’. J Am Coll Cardiol 2009;53:667-77. http://dx.doi.org/10.1016/j.jacc.2008.10.047.
- Lordkipanidzé M, Pharand C, Schampaert E, Turgeon J, Palisaitis DA, Diodati JG. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J 2007;28:1702-8. http://dx.doi.org/10.1093/eurheartj/ehm226.
- Tantry US, Bliden KP, Gurbel PA. Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J Am Coll Cardiol 2005;46:1705-9. http://dx.doi.org/10.1016/j.jacc.2005.05.090.
- Gurbel PA, Bliden KP, Dichiara J, Newcomer J, Weng W, Neerchal NK, et al. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation 2007;115:3156-64. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.675587.
- Faraday N, Yanek LR, Mathias R, Herrera-Galeano JE, Vaidya D, Moy TF, et al. Heritability of platelet responsiveness to aspirin in activation pathways directly and indirectly related to cyclooxygenase-1. Circulation 2007;115:2490-6. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.667584.
- Harrison P, Segal H, Blasbery K, Furtado C, Silver L, Rothwell PM. Screening for aspirin responsiveness after transient ischemic attack and stroke: comparison of 2 point-of-care platelet function tests with optical aggregometry. Stroke 2005;36:1001-5. http://dx.doi.org/10.1161/01.STR.0000162719.11058.bd.
- Frelinger AL, Furman MI, Linden MD, Li Y, Fox ML, Barnard MR, et al. Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1- and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance. Circulation 2006;113:2888-96. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.596627.
- Rocca B, Petrucci G. Variability in the responsiveness to low-dose aspirin: pharmacological and disease-related mechanisms. Thrombosis 2012;2012. http://dx.doi.org/10.1155/2012/376721.
- Lordkipanidzé M, Pharand C, Palisaitis DA, Diodati JG. Aspirin resistance: truth or dare. Pharmacol Ther 2006;112:733-43. http://dx.doi.org/10.1016/j.pharmthera.2006.05.011.
- Schwartz KA, Schwartz DE, Ghosheh K, Reeves MJ, Barber K, DeFranco A. Compliance as a critical consideration in patients who appear to be resistant to aspirin after healing of myocardial infarction. Am J Cardiol 2005;95:973-5. http://dx.doi.org/10.1016/j.amjcard.2004.12.038.
- Cotter G, Shemesh E, Zehavi M, Dinur I, Rudnick A, Milo O, et al. Lack of aspirin effect: aspirin resistance or resistance to taking aspirin?. Am Heart J 2004;147:293-300. http://dx.doi.org/10.1016/j.ahj.2003.07.011.
- Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet 2011;378:1231-43. http://dx.doi.org/10.1016/S0140-6736(11)61215-4.
- Peace A, McCall M, Tedesco T, Kenny D, Conroy RM, Foley D, et al. The role of weight and enteric coating on aspirin response in cardiovascular patients. J Thromb Haemost 2010;8:2323-5. http://dx.doi.org/10.1111/j.1538-7836.2010.03997.x.
- Katona E, Kovacs E, Bereczky Z, Balogh L, Homorodi N, Haramura G, et al. Development of reference methods for evaluating the effect of aspirin and for comparison with routinely used methods; the lack of aspirin resistance in healthy volunteers. J Thromb Haemost 2011;9:15-9.
- Schwartz KA, Schwartz DE, Barber K, Reeves M, De Franco AC. Non-compliance is the predominant cause of aspirin resistance in chronic coronary arterial disease patients. J Transl Med 2008;6. http://dx.doi.org/10.1186/1479-5876-6-46.
- Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 2001;345:1809-17. http://dx.doi.org/10.1056/NEJMoa003199.
- Capone ML, Sciulli MG, Tacconelli S, Grana M, Ricciotti E, Renda G, et al. Pharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjects. J Am Coll Cardiol 2005;45:1295-301. http://dx.doi.org/10.1016/j.jacc.2005.01.045.
- Wilner KD, Rushing M, Walden C, Adler R, Eskra J, Noveck R, et al. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J Clin Pharmacol 2002;42:1027-30. http://dx.doi.org/10.1177/009127002401102858.
- Van Ryn J, Kink-Eiband M, Kuritsch I, Feifel U, Hanft G, Wallenstein G, et al. Meloxicam does not affect the antiplatelet effect of aspirin in healthy male and female volunteers. J Clin Pharmacol 2004;44:777-84. http://dx.doi.org/10.1177/0091270004266623.
- Maree AO, Curtin RJ, Dooley M, Conroy RM, Crean P, Cox D, et al. Platelet response to low-dose enteric-coated aspirin in patients with stable cardiovascular disease. J Am Coll Cardiol 2005;46:1258-63. http://dx.doi.org/10.1016/j.jacc.2005.06.058.
- Angiolillo DJ. Antiplatelet therapy in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2007;14:124-31. http://dx.doi.org/10.1097/MED.0b013e32807f2ad9.
- Dichiara J, Bliden KP, Tantry US, Hamed MS, Antonino MJ, Suarez TA, et al. The effect of aspirin dosing on platelet function in diabetic and nondiabetic patients: an analysis from the aspirin-induced platelet effect (ASPECT) study. Diabetes 2007;56:3014-19. http://dx.doi.org/10.2337/db07-0707.
- Watala C, Boncler M, Gresner P. Blood platelet abnormalities and pharmacological modulation of platelet reactivity in patients with diabetes mellitus. Pharmacol Rep 2005;57:42-58.
- Watala C, Golanski J, Pluta J, Boncler M, Rozalski M, Luzak B, et al. Reduced sensitivity of platelets from type 2 diabetic patients to acetylsalicylic acid (aspirin) – its relation to metabolic control. Thromb Res 2004;113:101-13. http://dx.doi.org/10.1016/j.thromres.2003.12.016.
- Pulcinelli FM, Biasucci LM, Riondino S, Giubilato S, Leo A, Di Renzo L, et al. COX-1 sensitivity and thromboxane A2 production in type 1 and type 2 diabetic patients under chronic aspirin treatment. Eur Heart J 2009;30:1279-86. http://dx.doi.org/10.1093/eurheartj/ehp097.
- Watala C, Pluta J, Golanski J, Rozalski M, Czyz M, Trojanowski Z, et al. Increased protein glycation in diabetes mellitus is associated with decreased aspirin-mediated protein acetylation and reduced sensitivity of blood platelets to aspirin. J Mol Med (Berl) 2005;83:148-58. http://dx.doi.org/10.1007/s00109-004-0600-x.
- Kobzar G, Mardla V, Samel N. Short-term exposure of platelets to glucose impairs inhibition of platelet aggregation by cyclooxygenase inhibitors. Platelets 2011;22:338-44. http://dx.doi.org/10.3109/09537104.2010.535931.
- Pascale S, Petrucci G, Dragani A, Habib A, Zaccardi F, Pagliaccia F, et al. Aspirin-insensitive thromboxane biosynthesis in essential thrombocythemia is explained by accelerated renewal of the drug target. Blood 2012;119:3595-603. http://dx.doi.org/10.1182/blood-2011-06-359224.
- Dillinger JG, Sideris G, Henry P, Bal dit Sollier C, Ronez E, Drouet L. Twice daily aspirin to improve biological aspirin efficacy in patients with essential thrombocythemia. Thromb Res 2012;129:91-4. http://dx.doi.org/10.1016/j.thromres.2011.09.017.
- Dragani A, Pascale S, Recchiuti A, Mattoscio D, Lattanzio S, Petrucci G, et al. The contribution of cyclooxygenase-1 and -2 to persistent thromboxane biosynthesis in aspirin-treated essential thrombocythemia: implications for antiplatelet therapy. Blood 2010;115:1054-61. http://dx.doi.org/10.1182/blood-2009-08-236679.
- Henry P, Vermillet A, Boval B, Guyetand C, Petroni T, Dillinger JG, et al. 24-hour time-dependent aspirin efficacy in patients with stable coronary artery disease. Thromb Haemost 2011;105:336-44. http://dx.doi.org/10.1160/TH10-02-0082.
- Lordkipanidzé M, Pharand C, Schampaert E, Palisaitis DA, Diodati JG. Heterogeneity in platelet cyclooxygenase inhibition by aspirin in coronary artery disease. Int J Cardiol 2011;150:39-44. http://dx.doi.org/10.1016/j.ijcard.2010.02.025.
- Perneby C, Wallen NH, Rooney C, Fitzgerald D, Hjemdahl P. Dose- and time-dependent antiplatelet effects of aspirin. Thromb Haemost 2006;95:652-8.
- Di Minno G. Aspirin resistance and platelet turnover: a 25-year old issue. Nutr Metab Cardiovasc Dis 2011;21:542-5. http://dx.doi.org/10.1016/j.numecd.2011.04.002.
- Grove EL, Hvas AM, Mortensen SB, Larsen SB, Kristensen SD. Effect of platelet turnover on whole blood platelet aggregation in patients with coronary artery disease. J Thromb Haemost 2011;9:185-91. http://dx.doi.org/10.1111/j.1538-7836.2010.04115.x.
- Capodanno D, Patel A, Dharmashankar K, Ferreiro JL, Ueno M, Kodali M, et al. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv 2011;4:180-7. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.110.960187.
- Rocca B, Santilli F, Pitocco D, Mucci L, Petrucci G, Vitacolonna E, et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost 2012;10:1220-30. http://dx.doi.org/10.1111/j.1538-7836.2012.04723.x.
- Spectre G, Arnetz L, Ostenson CG, Brismar K, Li N, Hjemdahl P. Twice daily dosing of aspirin improves platelet inhibition in whole blood in patients with type 2 diabetes mellitus and micro- or macrovascular complications. Thromb Haemost 2011;106:491-9. http://dx.doi.org/10.1160/TH11-04-0216.
- Wurtz M, Grove EL. Interindividual variability in the efficacy of oral antiplatelet drugs: definitions, mechanisms and clinical importance. Curr Pharm Des 2012;18:5344-61. http://dx.doi.org/10.2174/138161212803251925.
- Grove EL, Hvas AM, Johnsen HL, Hedegaard SS, Pedersen SB, Mortensen J, et al. A comparison of platelet function tests and thromboxane metabolites to evaluate aspirin response in healthy individuals and patients with coronary artery disease. Thromb Haemost 2010;103:1245-53. http://dx.doi.org/10.1160/TH09-08-0527.
- Frelinger AL, Li Y, Linden MD, Barnard MR, Fox ML, Christie DJ, et al. Association of cyclooxygenase-1-dependent and -independent platelet function assays with adverse clinical outcomes in aspirin-treated patients presenting for cardiac catheterization. Circulation 2009;120:2586-96. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.900589.
- Cattaneo M. Laboratory detection of ‘aspirin resistance’: what test should we use (if any)?. Eur Heart J 2007;28:1673-5. http://dx.doi.org/10.1093/eurheartj/ehm232.
- Raichand S, Moore D, Riley R, Lordkipanidzé M, Dretzke J, O’Donnell J, et al. Protocol for a systematic review of the diagnostic and prognostic utility of tests currently available for the detection of aspirin resistance in patients with established cardiovascular or cerebrovascular disease. Syst Rev 2013;2. http://dx.doi.org/10.1186/2046-4053-2-16.
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427-37. http://dx.doi.org/10.7326/0003-4819-144-6-200603210-00010.
- Altman DG. Prognostic models: a methodological framework and review of models for breast cancer. Cancer Invest 2009;27:235-43. http://dx.doi.org/10.1080/07357900802572110.
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. http://dx.doi.org/10.1002/(SICI)1097-0258(19981230)17:24%3C2815::AID-SIM110%3E3.0.CO;2-8.
- Perneger TV. Estimating the relative hazard by the ratio of logarithms of event-free proportions. Contemp Clin Trials 2008;29:762-6. http://dx.doi.org/10.1016/j.cct.2008.06.002.
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004;23:1351-75. http://dx.doi.org/10.1002/sim.1761.
- Kip KE, Hollabaugh K, Marroquin OC, Williams DO. The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol 2008;51:701-7. http://dx.doi.org/10.1016/j.jacc.2007.10.034.
- Ozben S, Ozben B, Tanrikulu AM, Ozer F, Ozben T. Aspirin resistance in patients with acute ischemic stroke. J Neurol 2011;258:1979-86. http://dx.doi.org/10.1007/s00415-011-6052-7.
- Lee SP, Park KW, Shin DH, Lee HY, Kang HJ, Koo BK, et al. Efficacy of predicting thrombotic events with combination of dual point-of-care testing (POCT) after drug-eluting stent implantation for coronary heart disease: results from the CILON-T randomized trial POCT substudy. J Atheroscler Thromb 2011;18:914-23. http://dx.doi.org/10.5551/jat.9290.
- Feher A, Pusch G, Harang G, Gasztonyi B, Papp E, Werling D, et al. Aspirin resistance in cerebrovascular patients. Int J Cardiol 2011;152:111-12. http://dx.doi.org/10.1016/j.ijcard.2011.07.028.
- Chiu FC, Wang TD, Lee JK, Shih FY, Lin JW, Huang CH, et al. Residual platelet reactivity after aspirin and clopidogrel treatment predicts 2-year major cardiovascular events in patients undergoing percutaneous coronary intervention. Eur J Intern Med 2011;22:471-7. http://dx.doi.org/10.1016/j.ejim.2011.02.021.
- van der Loo B, Braun J, Koppensteiner R. On-treatment function testing of platelets and long-term outcome of patients with peripheral arterial disease undergoing transluminal angioplasty. Eur J Vasc Endovasc Surg 2011;42:809-16. http://dx.doi.org/10.1016/j.ejvs.2011.08.014.
- Amoah V, Smallwood A, Worrall AP, Lovatt T, Armesilla AL, Nevill AM, et al. Poor aspirin response in diabetic patients presenting with acute coronary syndromes: results using a near patient test. Thromb Res 2011;128:196-9. http://dx.doi.org/10.1016/j.thromres.2011.04.003.
- Kim HJ, Lee JM, Seo JH, Kim JH, Hong DM, Bahk JH, et al. Preoperative aspirin resistance does not increase myocardial injury during off-pump coronary artery bypass surgery. J Korean Med Sci 2011;26:1041-6. http://dx.doi.org/10.3346/jkms.2011.26.8.1041.
- Spectre G, Mosseri M, Abdelrahman NM, Briskin E, Bulut A, Loncar S, et al. Clinical and prognostic implications of the initial response to aspirin in patients with acute coronary syndrome. Am J Cardiol 2011;108:1112-18. http://dx.doi.org/10.1016/j.amjcard.2011.06.013.
- Grdinic A, Vojvodic D, Djukanovic N, Colic M, Grdinic AG, Ignjatovic V, et al. PCI and clopidogrel: antiplatelet responsiveness and patient characteristics. Acta Cardiol 2011;66:333-40.
- Abumiya T, Houkin K. Association of recurrent cerebral infarction with adenosine diphosphate- and collagen-induced platelet aggregation in patients treated with ticlopidine and/or aspirin. J Stroke Cerebrovasc Dis 2011;20:319-23. http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2010.01.013.
- Angiolillo DJ, Bernardo E, Zanoni M, Vivas D, Capranzano P, Malerba G, et al. Impact of insulin receptor substrate-1 genotypes on platelet reactivity and cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 2011;58:30-9. http://dx.doi.org/10.1016/j.jacc.2011.02.040.
- Kuliczkowski W, Greif M, Gasior M, Kaczmarski J, Pres D, Polonski L. Effects of platelet and inflammatory system activation on outcomes in diabetic patients with ST segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Kardiol Pol 2011;69:531-7.
- Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Harmsze AM, Hackeng CM, et al. High on-treatment platelet reactivity to both aspirin and clopidogrel is associated with the highest risk of adverse events following percutaneous coronary intervention. Heart 2011;97:983-90. http://dx.doi.org/10.1136/hrt.2010.220491.
- Gluckman TJ, McLean RC, Schulman SP, Kickler TS, Shapiro EP, Conte JV, et al. Effects of aspirin responsiveness and platelet reactivity on early vein graft thrombosis after coronary artery bypass graft surgery. J Am Coll Cardiol 2011;57:1069-77. http://dx.doi.org/10.1016/j.jacc.2010.08.650.
- Ko YG, Suh JW, Kim BH, Lee CJ, Kim JS, Choi D, et al. Comparison of 2 point-of-care platelet function tests, VerifyNow Assay and Multiple Electrode Platelet Aggregometry, for predicting early clinical outcomes in patients undergoing percutaneous coronary intervention. Am Heart J 2011;161:383-90. http://dx.doi.org/10.1016/j.ahj.2010.10.036.
- Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, ten Berg JM, Hackeng CM. High on-aspirin platelet reactivity as measured with aggregation-based, cyclooxygenase-1 inhibition sensitive platelet function tests is associated with the occurrence of atherothrombotic events. J Thromb Haemost 2010;8:2140-8. http://dx.doi.org/10.1111/j.1538-7836.2010.04017.x.
- Smit JJ, van Werkum JW, ten Berg J, Slingerland R, Ottervanger JP, Heestermans T, et al. Prehospital triple antiplatelet therapy in patients with acute ST elevation myocardial infarction leads to better platelet aggregation inhibition and clinical outcome than dual antiplatelet therapy. Heart 2010;96:1815-20. http://dx.doi.org/10.1136/hrt.2010.201889.
- Campo G, Fileti L, de Cesare N, Meliga E, Furgieri A, Russo F, et al. Long-term clinical outcome based on aspirin and clopidogrel responsiveness status after elective percutaneous coronary intervention: a 3T/2R (tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel) trial substudy. J Am Coll Cardiol 2010;56:1447-55. http://dx.doi.org/10.1016/j.jacc.2010.03.103.
- Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, et al. High on-treatment platelet reactivity by more than one agonist predicts 12-month follow-up cardiovascular death and non-fatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting. Thromb Haemost 2010;104:279-86. http://dx.doi.org/10.1160/TH10-01-0007.
- Chu JW, Wong CK, Chambers J, Wout JV, Herbison P, Tang EW. Aspirin resistance determined from a bed-side test in patients suspected to have acute coronary syndrome portends a worse 6 months outcome. QJM 2010;103:405-12. http://dx.doi.org/10.1093/qjmed/hcq038.
- Eshtehardi P, Windecker S, Cook S, Billinger M, Togni M, Garachemani A, et al. Dual low response to acetylsalicylic acid and clopidogrel is associated with myonecrosis and stent thrombosis after coronary stent implantation. Am Heart J 2010;159:891-8. http://dx.doi.org/10.1016/j.ahj.2010.02.025.
- Campo G, Valgimigli M, Frangione A, Luccarelli S, Cangiano E, Cavazza C, et al. Evaluation of platelet inhibition by tirofiban in patients stratified according to aspirin and clopidogrel responsiveness: the 3T/2R (Tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel). J Am Coll Cardiol 2010;55:255-6. http://dx.doi.org/10.1016/j.jacc.2009.08.052.
- Addad F, Chakroun T, Abderazek F, Ben-Farhat M, Hamdi S, Dridi Z, et al. Response variability to aspirin and one-year prediction of vascular events in patients with stable coronary artery disease. J Thromb Thrombolysis 2010;29:108-13. http://dx.doi.org/10.1007/s11239-009-0335-1.
- Aksu H, Ozer O, Unal H, Hobikoglu G, Norgaz T, Buturak A, et al. Significance of mean platelet volume on prognosis of patients with and without aspirin resistance in settings of non-ST-segment elevated acute coronary syndromes. Blood Coagul Fibrinolysis 2009;20:686-93. http://dx.doi.org/10.1097/MBC.0b013e32833161ac.
- Thomson VS, John B, George P, Joseph G, Jose J. Aspirin resistance in Indian patients with coronary artery disease and cardiovascular events. J Postgrad Med 2009;55:252-6. http://dx.doi.org/10.4103/0022-3859.58927.
- Cuisset T, Cayla G, Frere C, Quilici J, Poyet R, Gaborit B, et al. Predictive value of post-treatment platelet reactivity for occurrence of post-discharge bleeding after non-ST elevation acute coronary syndrome. Shifting from antiplatelet resistance to bleeding risk assessment?. Eurointervention 2009;5:325-9. http://dx.doi.org/10.4244/51.
- Linnemann B, Prochnow S, Mani H, Schwonberg J, Lindhoff-Last E. Variability of non-response to aspirin in patients with peripheral arterial occlusive disease during long-term follow-up. Ann Hematol 2009;88:979-88. http://dx.doi.org/10.1007/s00277-009-0708-8.
- Kempfert J, Anger K, Rastan A, Krabbes S, Lehmann S, Garbade J, et al. Postoperative development of aspirin resistance following coronary artery bypass. Eur J Clin Invest 2009;39:769-74. http://dx.doi.org/10.1111/j.1365-2362.2009.02175.x.
- Ivandic BT, Sausemuth M, Ibrahim H, Giannitsis E, Gawaz M, Katus HA. Dual antiplatelet drug resistance is a risk factor for cardiovascular events after percutaneous coronary intervention. Clin Chem 2009;55:1171-6. http://dx.doi.org/10.1373/clinchem.2008.115089.
- Foussas SG, Zairis MN, Tsirimpis VG, Makrygiannis SS, Patsourakos NG, Adamopoulou EN, et al. The impact of aspirin resistance on the long-term cardiovascular mortality in patients with non-ST segment elevation acute coronary syndromes. Clin Cardiol 2009;32:142-7. http://dx.doi.org/10.1002/clc.20293.
- Boncoraglio GB, Bodini A, Brambilla C, Corsini E, Carriero MR, Parati EA. Aspirin resistance determined with PFA-100 does not predict new thrombotic events in patients with stable ischemic cerebrovascular disease. Clin Neurol Neurosurg 2009;111:270-3. http://dx.doi.org/10.1016/j.clineuro.2008.11.001.
- Majeed F, Kop WJ, Poston RS, Kallam S, Mehra MR. Prospective, observational study of antiplatelet and coagulation biomarkers as predictors of thromboembolic events after implantation of ventricular assist devices. Nat Clin Pract Cardiovasc Med 2009;6:147-57. http://dx.doi.org/10.1038/ncpcardio1441.
- Bevilacqua S, Alkodami AA, Volpi E, Cerillo AG, Berti S, Glauber M, et al. Risk stratification after coronary artery bypass surgery by a point-of-care test of platelet function. Ann Thorac Surg 2009;87:496-502. http://dx.doi.org/10.1016/j.athoracsur.2008.05.038.
- Saw J, Densem C, Walsh S, Jokhi P, Starovoytov A, Fox R, et al. The effects of aspirin and clopidogrel response on myonecrosis after percutaneous coronary intervention: a BRIEF-PCI (Brief Infusion of Intravenous Eptifibatide Following Successful Percutaneous Coronary Intervention) trial substudy. JACC Cardiovasc Interv 2008;1:654-9. http://dx.doi.org/10.1016/j.jcin.2008.08.017.
- Gori AM, Marcucci R, Paniccia R, Giusti B, Fedi S, Antonucci E, et al. Thrombotic events in high risk patients are predicted by evaluating different pathways of platelet function. Thromb Haemost 2008;100:1136-45.
- Cha JK, Jeon HW, Kang MJ. ADP-induced platelet aggregation in acute ischemic stroke patients on aspirin therapy. Eur J Neurol 2008;15:1304-8. http://dx.doi.org/10.1111/j.1468-1331.2008.02306.x.
- Gurbel PA, Antonino MJ, Bliden KP, Dichiara J, Suarez TA, Singla A, et al. Platelet reactivity to adenosine diphosphate and long-term ischemic event occurrence following percutaneous coronary intervention: a potential antiplatelet therapeutic target. Platelets 2008;19:595-604. http://dx.doi.org/10.1080/09537100802351065.
- Campo G, Valgimigli M, Frangione A, Tebaldi M, Ferrari R. Prognostic value of serial platelet reactivity measurements on long-term clinical outcome in patients with ST-elevation myocardial infarction undergoing primary PCI. J Thromb Haemost 2008;6:1824-6. http://dx.doi.org/10.1111/j.1538-7836.2008.03112.x.
- Gori AM, Marcucci R, Migliorini A, Valenti R, Moschi G, Paniccia R, et al. Incidence and clinical impact of dual nonresponsiveness to aspirin and clopidogrel in patients with drug-eluting stents. J Am Coll Cardiol 2008;52:734-9. http://dx.doi.org/10.1016/j.jacc.2008.05.032.
- Schwammenthal Y, Tsabari R, Shenkman B, Schwartz R, Matetzky S, Lubetsky A, et al. Aspirin responsiveness in acute brain ischaemia: association with stroke severity and clinical outcome. Cerebrovasc Dis 2008;25:355-61. http://dx.doi.org/10.1159/000118382.
- Aradi D, Konyi A, Palinkas L, Berki T, Pinter T, Tahin T, et al. Thienopyridine therapy influences late outcome after coronary stent implantation. Angiology 2008;59:172-8. http://dx.doi.org/10.1177/0003319707304572.
- Christiaens L, Ragot S, Mergy J, Allal J, Macchi L. Major clinical vascular events and aspirin-resistance status as determined by the PFA-100 method among patients with stable coronary artery disease: a prospective study. Blood Coagul Fibrinolysis 2008;19:235-9. http://dx.doi.org/10.1097/MBC.0b013e3282f9ade8.
- Gengo FM, Rainka M, Robson M, Gengo MF, Forrest A, Hourihane M, et al. Prevalence of platelet nonresponsiveness to aspirin in patients treated for secondary stroke prophylaxis and in patients with recurrent ischemic events. J Clin Pharmacol 2008;48:335-43. http://dx.doi.org/10.1177/0091270007313324.
- Jacopo G, Elisabetta V, Silverio S, Massimiliano M, Sergio B, Grazia AM, et al. Identification of platelet hyper-reactivity measured with a portable device immediately after percutaneous coronary intervention predicts in stent thrombosis. Thromb Res 2007;121:407-12. http://dx.doi.org/10.1016/j.thromres.2007.04.009.
- Blindt R, Stellbrink K, de Taeye A, Muller R, Kiefer P, Yagmur E, et al. The significance of vasodilator-stimulated phosphoprotein for risk stratification of stent thrombosis. Thromb Haemost 2007;98:1329-34.
- Foussas SG, Zairis MN, Patsourakos NG, Makrygiannis SS, Adamopoulou EN, Handanis SM, et al. The impact of oral antiplatelet responsiveness on the long-term prognosis after coronary stenting. Am Heart J 2007;154:676-81. http://dx.doi.org/10.1016/j.ahj.2007.06.013.
- Poulsen TS, Kristensen SR, Korsholm L, Haghfelt T, Jorgensen B, Licht PB, et al. Variation and importance of aspirin resistance in patients with known cardiovascular disease. Thromb Res 2007;120:477-84. http://dx.doi.org/10.1016/j.thromres.2006.10.022.
- Chen WH, Cheng X, Lee PY, Ng W, Kwok JY, Tse HF, et al. Aspirin resistance and adverse clinical events in patients with coronary artery disease. Am J Med 2007;120:631-5. http://dx.doi.org/10.1016/j.amjmed.2006.10.021.
- Buch AN, Singh S, Roy P, Javaid A, Smith KA, George CE, et al. Measuring aspirin resistance, clopidogrel responsiveness, and postprocedural markers of myonecrosis in patients undergoing percutaneous coronary intervention. Am J Cardiol 2007;99:1518-22. http://dx.doi.org/10.1016/j.amjcard.2007.01.023.
- Hobikoglu GF, Norgaz T, Aksu H, Ozer O, Erturk M, Destegul E, et al. The effect of acetylsalicylic acid resistance on prognosis of patients who have developed acute coronary syndrome during acetylsalicylic acid therapy. Can J Cardiol 2007;23:201-6. http://dx.doi.org/10.1016/S0828-282X(07)70744-4.
- Bliden KP, Dichiara J, Tantry US, Bassi AK, Chaganti SK, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate?. J Am Coll Cardiol 2007;49:657-66. http://dx.doi.org/10.1016/j.jacc.2006.10.050.
- Pamukcu B, Oflaz H, Onur I, Oncul A, Ozcan M, Umman B, et al. Clinical relevance of aspirin resistance in patients with stable coronary artery disease: a prospective follow-up study (PROSPECTAR). Blood Coagul Fibrinolysis 2007;18:187-92. http://dx.doi.org/10.1097/MBC.0b013e328040c115.
- Fuchs I, Frossard M, Spiel A, Riedmuller E, Laggner AN, Jilma B. Platelet function in patients with acute coronary syndrome (ACS) predicts recurrent ACS. J Thromb Haemost 2006;4:2547-52. http://dx.doi.org/10.1111/j.1538-7836.2006.02239.x.
- Marcucci R, Paniccia R, Antonucci E, Gori AM, Fedi S, Giglioli C, et al. Usefulness of aspirin resistance after percutaneous coronary intervention for acute myocardial infarction in predicting one-year major adverse coronary events. Am J Cardiol 2006;98:1156-9. http://dx.doi.org/10.1016/j.amjcard.2006.05.041.
- Pamukcu B, Oflaz H, Oncul A, Umman B, Mercanoglu F, Ozcan M, et al. The role of aspirin resistance on outcome in patients with acute coronary syndrome and the effect of clopidogrel therapy in the prevention of major cardiovascular events. J Thromb Thrombolysis 2006;22:103-10. http://dx.doi.org/10.1007/s11239-006-8952-4.
- Gianetti J, Parri MS, Sbrana S, Paoli F, Maffei S, Paradossi U, et al. Platelet activation predicts recurrent ischemic events after percutaneous coronary angioplasty: a 6 months prospective study. Thromb Res 2006;118:487-93. http://dx.doi.org/10.1016/j.thromres.2005.10.011.
- Ohmori T, Yatomi Y, Nonaka T, Kobayashi Y, Madoiwa S, Mimuro J, et al. Aspirin resistance detected with aggregometry cannot be explained by cyclooxygenase activity: involvement of other signaling pathway(s) in cardiovascular events of aspirin-treated patients. J Thromb Haemost 2006;4:1271-8. http://dx.doi.org/10.1111/j.1538-7836.2006.01958.x.
- Cuisset T, Frere C, Quilici J, Barbou F, Morange PE, Hovasse T, et al. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost 2006;4:542-9. http://dx.doi.org/10.1111/j.1538-7836.2005.01751.x.
- Morawski W, Sanak M, Cisowski M, Szczeklik M, Szczeklik W, Dropinski J, et al. Prediction of the excessive perioperative bleeding in patients undergoing coronary artery bypass grafting: role of aspirin and platelet glycoprotein IIIa polymorphism. J Thorac Cardiovasc Surg 2005;130:791-6. http://dx.doi.org/10.1016/j.jtcvs.2005.02.041.
- Sambola A, Heras M, Escolar G, Lozano M, Pino M, Martorell T, et al. The PFA-100 detects sub-optimal antiplatelet responses in patients on aspirin. Platelets 2004;15:439-46. http://dx.doi.org/10.1080/69537100412351272550.
- Stejskal D, Proskova J, Lacnak B, Horalik D, Hamplova A, Oral I, et al. Use of assessment of aggregation of thrombocytes induced by cationic propyl gallate to estimate recurrence of cardiovascular complications. Vnitr Lek 2004;50:591-9.
- Payne DA, Jones CI, Hayes PD, Thompson MM, London NJ, Bell PR, et al. Beneficial effects of clopidogrel combined with aspirin in reducing cerebral emboli in patients undergoing carotid endarterectomy. Circulation 2004;109:1476-81. http://dx.doi.org/10.1161/01.CIR.0000121739.05643.E6.
- Bruno A, McConnell JP, Cohen SN, Tietjen GE, Wallis RA, Gorelick PB, et al. Serial urinary 11-dehydrothromboxane B2, aspirin dose, and vascular events in blacks after recent cerebral infarction. Stroke 2004;35:727-30. http://dx.doi.org/10.1161/01.STR.0000117097.76953.A6.
- Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol 2003;41:961-5. http://dx.doi.org/10.1016/S0735-1097(02)03014-0.
- Ziegler S, Maca T, Alt E, Speiser W, Schneider B, Minar E. Monitoring of antiplatelet therapy with the PFA-100 in peripheral angioplasty patients. Platelets 2002;13:493-7. http://dx.doi.org/10.1080/0953710021000057866.
- Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation 2002;105:1650-5. http://dx.doi.org/10.1161/01.CIR.0000013777.21160.07.
- Buchanan MR, Schwartz L, Bourassa M, Brister SJ, Peniston CM. BRAT Investigators . Results of the BRAT study – a pilot study investigating the possible significance of ASA nonresponsiveness on the benefits and risks of ASA on thrombosis in patients undergoing coronary artery bypass surgery. Can J Cardiol 2000;16:1385-90.
- Mueller MR, Salat A, Stangl P, Murabito M, Pulaki S, Boehm D, et al. Variable platelet response to low-dose ASA and the risk of limb deterioration in patients submitted to peripheral arterial angioplasty. Thromb Haemost 1997;78:1003-7.
- Grotemeyer KH, Scharafinski HW, Husstedt IW. Two-year follow-up of aspirin responder and aspirin non responder. A pilot-study including 180 post-stroke patients. Thromb Res 1993;71:397-403. http://dx.doi.org/10.1016/0049-3848(93)90164-J.
- Sørensen PS, Agerskov AL, Gormsen J, Pedersen H, Marquardsen J, Petersson H, et al. Prognostic value of in vitro measurements of platelet aggregability and fibrinolytic activity in patients with reversible cerebral ischemic attacks. Eur Neurol 1983;22:437-41. http://dx.doi.org/10.1159/000115599.
- Tang FK, Lin LJ, Hua N, Lu H, Qi Z, Tang XZ. Earlier application of loading doses of aspirin and clopidogrel decreases rate of recurrent cardiovascular ischemic events for patients undergoing percutaneous coronary intervention. Chin Med J 2012;125:631-8.
- Breet NJ, Sluman MA, van Berkel MAJPJ, van Werkum JW, Bouman HJ, Harmsze AM, et al. Effect of gender difference on platelet reactivity. Neth Heart J 2011;19:451-7. http://dx.doi.org/10.1007/s12471-011-0189-y.
- Milicic D, Lovric D, Skoric B, Narancic-Skoric K, Gornik I, Sertic J. Platelet response to standard aspirin and clopidogrel treatment correlates with long-term outcome in patients with acute ST-elevation myocardial infarction. Int J Cardiol 2011;153:227-9. http://dx.doi.org/10.1016/j.ijcard.2011.09.055.
- De Boni A, De Riva V, Galloni E, Perini F. Residual platelet activity and clinical failure of antithrombotic prophylaxis in acute cerebral ischaemic stroke. Riv Ital Med Labor 2011;7:163-9. http://dx.doi.org/10.1007/s13631-011-0024-8.
- Marcucci R, Gori AM, Paniccia R, Giusti B, Cordisco A, Balzi D, et al. High-on treatment platelet reactivity by different stimuli is a determinant of 1 year mortality in acute coronary syndrome patients: Data from AMI-florence 2 study. J Thromb Haemost 2011;9:546-7.
- Marcucci R, Gori AM, Paniccia R, Giusti B, Cordisco A, Balzi D, et al. High-on clopidogrel platelet reactivity is a determinant of 1-year mortality in acute coronary syndrome patients: Data from AMI-florence 2 study. J Thromb Haemost 2011;9.
- Lordkipanidzé M, Diodati JG, Schampaert E, Palisaitis DA, Pharand C. Lack of clinical predictiveness of six major platelet function tests used to assess aspirin response in patients with stable coronary artery disease. J Thromb Haemost 2011;9.
- Spectre G, Mosseri M, Abdelrahman NM, Briskin E, Bulut A, Loncar S, et al. Dynamic response to aspirin in patients with acute coronary syndrome, clinical and prognostic implications. J Thromb Haemost 2011;9. http://dx.doi.org/10.1016/j.amjcard.2011.06.013.
- Miyata S, Kada A, Nagatsuka K. Clinical impact of aspirin ‘resistance’ on the secondary prevention of atherothrombosis. J Thromb Haemost 2011;9.
- Kaymaz C, Tokgoz HC, Tanboga IH, Poci N, Aktemur T, Kirca N, et al. Can we translate the high and low on-treatment platelet reactivity to early, mid and long term risks for ischemic and bleeding events following the stent placement. Eur Heart J 2011;32.
- Orta KK, Kocas C, Yildiz A, Abaci O, Okcun B, Coskun U, et al. Long-term follow-up of the patients with Aspirin resistant end stage kidney disease. Eur Heart J 2011;32:322-3.
- Kaymaz C, Tanboga IH, Tokgoz HC, Can MM, Poci N, Aktemur T, et al. Comparison of multiplate, VerifyNow and PFA-100 in the assessment of on-treatment platelet reactivity in patients treated with aspirin and clopidogrel following coronary stenting. Eur Heart J 2011;32.
- Sahin DY, Koc M, Cayli M, Sen O, Kalkan GY, Kanadasi M, et al. Frequency of aspirin resistance in patients with chronic ischemic heart disease in Cukurova region and the relationship between aspirin resistance and adverse clinical events. Int J Cardiol 2011;147. http://dx.doi.org/10.1016/S0167-5273(11)70427-2.
- Zanow J, Eckart A, Ludewig S, Losche W. Frequency and clinical significance of the ‘non-response’ to acetylsalicylic acid in patients with peripheral occlusive artery disease. Vasomed 2010;22.
- Ryu D-S, Hong C-K, Sim Y-S, Kim C-H, Jung J-Y, Joo J-Y. Anti-platelet drug resistance in the prediction of thromboembolic complications after neurointervention. J Korean Neurosurg Soc 2010;48:319-24. http://dx.doi.org/10.3340/jkns.2010.48.4.319.
- Lee J-H, Cha J-K, Lee SJ, Ha S-W, Kwon SU. Addition of cilostazol reduces biological aspirin resistance in aspirin users with ischaemic stroke: a double-blind randomized clinical trial. Eur J Neurol 2010;17:434-42. http://dx.doi.org/10.1111/j.1468-1331.2009.02837.x.
- Angiolillo D, Bernardo E, Zanoni M, Capranzano P, Malerba G, Trabetti E, et al. Impact of IRS-1 genotypes on platelet reactivity and cardiovascular outcomes in type 2 diabetes mellitus patients with coronary artery disease. J Am Coll Cardiol 2010;55. http://dx.doi.org/10.1016/j.jacc.2011.02.040.
- Bouman HJ, van Werkum JW, Smit JJ, Breet NJ, Postma S, Heestermans T, et al. Predictive value of various platelet function tests on ST-segment resolution and clinical outcome in stemi patients randomized to either dual or triple antiplatelet therapy: the onTIME2 platelet function substudy. J Am Coll Cardiol 2010;55. http://dx.doi.org/10.1016/S0735-1097(10)61949-3.
- Tan C, Lin J, Chen W, Lin R, Chen S. Elevated serum ubiquitin and ubiquitin conjugates: A potential biomarker for aspirin resistance in acute myocardial infarction patients. Arterioscler Thromb Vasc Biol 2010;30:e219-20.
- Toth G, Pride GL, Novakovic R, Matevosyan K, Sarode R, Welch BG. Thromboembolic complications and response to antiplatelet therapy in patients undergoing endovascular stenting for aneurysm treatment. Stroke 2010;41:e265-6.
- Bobesc E, Rado M, Datc G, Dobrean D, Dok BF. The effects of endothelial dysfunction, platelets hyperactivity, oxidative stress biomarkers on ejection fraction, wall motion score index and prognosis in patients with acute coronary syndromes. Eur J Echocardiogr 2010;11:ii122-3.
- Bobescu E, Radoi M, Datcu G, Dobreanu D, Doka B. Correlation between endothelial dysfunction, platelets hyperactivity, oxidative stress biomarkers and cumulative effects on prognosis in patients with acute coronary syndromes. Eur Heart J Suppl 2010;12.
- Ripley AW, Narang J, Fifi J, Bennett HL. Aspirin and clopidogrel resistance in patients requiring endovascular neurological intervention. J Neurosurg Anesthesiol 2010;22.
- Kuliczkowski W, Kaczmarski J, Greif M, Polonski L, Gasior M, Serebruany V, et al. Thrombin induced aggregation and 6-months outcome in patients with diabetes and STEMI treated with primary PCI. Eur Heart J 2010;31.
- Bobescu E, Radoi M, Datcu G, Dobreanu D, Donea M, Doka B, et al. Evaluation of platelets hyperactivity, hypercoagulability status and oxidative stress biomarkers and outcomes in patients with acute coronary syndromes. Eur Heart J 2010;31:971-2.
- Fateh-Moghadam S, Mueller E, Ziemer S, Dietz R, Gawaz M, Bocksch W. Responsiveness to higher maintenance dose of dual antiplatelet therapy and its impact on stent thrombosis. Eur Heart J 2010;31.
- Ko YG, Suh JW, Kim JS, Choi DH, Hong MK, Jang YS, et al. Prognostic significance of dual hyporesponsiveness to aspirin and clopidogrel measured by verifynow assay in the patients undergoing percutaneous coronary intervention. Eur Heart J 2010;31:970-1.
- Tokgoz HC, Tanboga IH, Can MM, Bezgin T, Akgun T, Turkyilmaz E, et al. Does platelet response to clopidogrel and/or aspirin as assessed by multiplate analyser predict short and long-term outcome following coronary stenting?. Eur Heart J 2010;31.
- Colic M, Calija B, Babic T, Sarenac D, Ljubic M, Spasojevic B, et al. Exaggerated platelet hypo reactivity increases bleeding risk as measured by multiplate impedance aggregometry test after coronary stenting. Eur Heart J 2010;31.
- Catakoglu AB, Aytekin S, Celebi H, Sener M, Kurtoglu H, Demiroglu C, et al. The influence of aspirin resistance on non-fatal coronary events following percutaneous coronary interventions. Arch Med Sci 2009;5:531-8.
- Sobol AB, Mochecka A, Selmaj K, Loba J. Is there a relationship between aspirin responsiveness and clinical aspects of ischemic stroke?. Adv Clin Exp Med 2009;18:473-9.
- Modica A, Karlsson F, Mooe T. The impact of platelet function or C-reactive protein, on cardiovascular events after an acute myocardial infarction. Thromb J 2009;7. http://dx.doi.org/10.1186/1477-9560-7-12.
- Angiolillo D, Bernardo E, Capranzano P, Sabate M, Jimenez-Quevedo P, Ferreiro JL, et al. Prognostic implications of high platelet reactivity as defined by multiple agonists in diabetes mellitus patients on dual antiplatelet therapy. J Am Coll Cardiol 2009;53.
- Harrison P, Silver L, Segal H, Syed A, Mehta Z, Rothwell P. Long term prognostic value of aspirin non-responsiveness and platelet reactivity after acute vascular events: a population-base cohort study. J Thromb Haemost 2009;7.
- Range G, Richard P, Chassaing S, Belle L, Thuaire C, Cazaux P, et al. Impact of low response to aspirin and/or clopidogrel in 1001 patients undergoing coronary stenting: 1-month results of the VERIFRENCHY registry. Eur Heart J 2009;30. http://dx.doi.org/10.1016/j.acvd.2014.03.004.
- Milicic D, Lovric D, Skoric B, Samardzic J, Gornik I, Narancic SK, et al. Effects of lower platelet aspirin and clopidogrel response on long-term outcomes in patients with acute STEMI. Eur Heart J 2009;30. http://dx.doi.org/10.1016/j.ijcard.2011.09.055.
- Vaduganathan M, Guthikonda S, Nure B, Siqueiros-Garcia A, Mangalpally KR, Paranilam J, et al. Point-of-care measurement of aspirin responsiveness predicts long-term clinical outcomes in patients undergoing elective percutaneous coronary intervention. Am J Cardiol 2009;104. http://dx.doi.org/10.1016/j.amjcard.2009.08.501.
- Silver LE, Harrison P, Segal H, Syed A, Mehta Z, Rothwell PM. Long-term prognostic value of aspirin non-responsiveness and platelet reactivity after acute vascular events: a population-based prospective cohort study. Cerebrovasc Dis 2009;27.
- Kim S, Lee DH, Lim HK, Kwon SU, Choi CG, Suh DC. Change of platelet reactivity to antiplatelet therapy after stenting procedure for cerebral artery stenosis: Verifynow antiplatelet assay before and after stenting. Neuroradiology 2009;51.
- Eikelboom JW, Hankey GJ, Thom J, Bhatt DL, Steg PG, Montalescot G, et al. Incomplete inhibition of thromboxane biosynthesis by acetylsalicylic acid: determinants and effect on cardiovascular risk. Circulation 2008;118:1705-12. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.768283.
- Kaminska M, Osada J, Dabrowska M, Kloczko J, Kramkowski K, Musial WJ. The assessment of antiplatelet activity of aspirin among survivors of myocardial infarction treated with PCI in six months observation – preliminary results. Pol Prz Kardiol 2007;9:335-41.
- Gurbel PA, Bliden KP, Navickas I, Cohen E, Tantry US. Post-coronary intervention recurrent ischemia in the presence of adequate platelet inhibition by dual antiplatelet therapy: What are we overlooking?. J Thromb Haemost 2007;5:2300-1. http://dx.doi.org/10.1111/j.1538-7836.2007.02749.x.
- Stejskal D, Vaclavik J, Lacnak B, Proskova J. Aspirin resistance measured by cationic propyl gallate platelet aggregometry and recurrent cardiovascular events during 4 years of follow-up. Eur J Intern Med 2006;17:349-54. http://dx.doi.org/10.1016/j.ejim.2006.01.006.
- Sambu N, Radhakrishnan A, Dent H, Calver AL, Corbett S, Gray H, et al. Personalised antiplatelet therapy in stent thrombosis: observations from the Clopidogrel Resistance in Stent Thrombosis (CREST) registry. Heart 2012;98:706-11. http://dx.doi.org/10.1136/heartjnl-2011-301164.
- Lee DH, Kim HS, Kim SM, Kwon SU, Suh DC. Change of platelet reactivity to antiplatelet therapy after stenting procedure for cerebral artery stenosis: VerifyNow antiplatelet assay before and after stenting. Neurointervention 2012;7:23-6. http://dx.doi.org/10.5469/neuroint.2012.7.1.23.
- Feng XR, Liu ML, Liu F, Tian QP, Fan Y, Liu QZ. Aspirin response and related factors in aged patients. Chung-Hua Hsin Hsueh Kuan Ping Tsa Chih 2011;39:925-8.
- Eskandarian R, Darabian M. Impact of aspirin resistance on one year’s prognosis in chronic stable angina in Semnan – Iran. Atheroscler Suppl 2011;12. http://dx.doi.org/10.1016/S1567-5688(11)70811-X.
- Verschuren JJ, Trompet S, Wessels JA, Guchelaar HJ, de Maat MP, Simoons ML, et al. A systematic review on pharmacogenetics in cardiovascular disease: is it ready for clinical application?. Eur Heart J 2012;33:165-75. http://dx.doi.org/10.1093/eurheartj/ehr239.
- Dickinson KJ, Troxler M, Homer-Vanniasinkam S. The surgical application of point-of-care haemostasis and platelet function testing. Br J Surg 2008;95:1317-30. http://dx.doi.org/10.1002/bjs.6359.
- El-Menyar A, Hussein H, Al Suwaidi J. Coronary stent thrombosis in patients with chronic renal insufficiency. Angiology 2010;61:297-303. http://dx.doi.org/10.1177/0003319709344574.
- Velkovic J, Coulthard A. The drugs don’t work: low response to antiplatelet agents in patients undergoing endovascular procedures. J Med Imaging Radiat Oncol 2009;53.
- Crescente M, Di Castelnuovo A, Iacoviello L, Vermylen J, Cerletti C, De Gaetano G. Response variability to aspirin as assessed by the platelet function analyzer (PFA)-100. A systematic review. Thromb Haemost 2008;99:14-26. http://dx.doi.org/10.1160/TH07-08-0530.
- Reny JL, de Moerloose P, Dauzat M, Fontana P. Use of the PFA-100 closure time to predict cardiovascular events in aspirin-treated cardiovascular patients: a systematic review and meta-analysis. J Thromb Haemost 2008;6:444-50. http://dx.doi.org/10.1111/j.1538-7836.2008.02897.x.
- Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin ‘resistance’ and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ 2008;336:195-8. http://dx.doi.org/10.1136/bmj.39430.529549.BE.
- Ferguson AD, Dokainish H, Lakkis N. Aspirin and clopidogrel response variability: review of the published literature. Tex Heart Inst J 2008;35:313-20.
- Sofi F, Marcucci R, Gori AM, Abbate R, Gensini GF. Residual platelet reactivity on aspirin therapy and recurrent cardiovascular events – a meta-analysis. Int J Cardiol 2008;128:166-71. http://dx.doi.org/10.1016/j.ijcard.2007.12.010.
- Musallam KM, Charafeddine K, Bitar A, Khoury M, Assaad S, Beresian J, et al. Resistance to aspirin and clopidogrel therapy. Int J Lab Hematol 2011;33:1-18. http://dx.doi.org/10.1111/j.1751-553X.2010.01268.x.
- Wong S, Appleberg M, Ward CM, Lewis DR. Aspirin resistance in cardiovascular disease: a review. Eur J Vasc Endovasc Surg 2004;27:456-65. http://dx.doi.org/10.1016/j.ejvs.2003.12.025.
- Howard PA. Aspirin resistance. Ann Pharmacother 2002;36:1620-4. http://dx.doi.org/10.1345/aph.1C013.
- Canivano PL, Garcia YC. Resistance to aspirin: prevalence, mechanisms of action and association with thromboembolic events. A narrative review. Farm Hosp 2010;34:32-43. http://dx.doi.org/10.1016/j.farma.2009.08.002.
- Pusch G, Feher G, Kotai K, Tibold A, Gasztonyi B, Feher A, et al. Aspirin resistance: focus on clinical endpoints. J Cardiovasc Pharmacol 2008;52:475-84. http://dx.doi.org/10.1097/FJC.0b013e31818eee5f.
- Snoep JD, Hovens MM, Eikenboom JC, Van Der Bom JG, Huisman MV. Association of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events: a systematic review and meta-analysis. Arch Intern Med 2007;167:1593-9. http://dx.doi.org/10.1001/archinte.167.15.1593.
- Mansour K, Taher AT, Musallam KM, Alam S. Aspirin resistance. Adv Hematol 2009;2009. http://dx.doi.org/10.1155/2009/937352.
- Gum PA, Kottke-Marchant K, Poggio ED, Gurm H, Welsh PA, Brooks L, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 2001;88:230-5. http://dx.doi.org/10.1016/S0002-9149(01)01631-9.
- Andersen LA, Gormsen J. Platelet aggregation and fibrinolytic activity in transient cerebral ischemia. Acta Neurol Scand 1976;55:76-82. http://dx.doi.org/10.1111/j.1600-0404.1977.tb05628.x.
- Fritsma GA, Ens GE, Alvord MA, Carroll AA, Jensen R. Monitoring the antiplatelet action of aspirin. JAAPA 2001;14:57-8.
- Jilma B. Platelet function analyzer (PFA-100): a tool to quantify congenital or acquired platelet dysfunction. J Lab Clin Med 2001;138:152-63. http://dx.doi.org/10.1067/mlc.2001.117406.
- Favaloro EJ. Clinical application of the PFA-100. Curr Opin Hematol 2002;9:407-15. http://dx.doi.org/10.1097/00062752-200209000-00004.
- Escolar G, Cases A, Vinas M, Pino M, Calls J, Cirera I, et al. Evaluation of acquired platelet dysfunctions in uremic and cirrhotic patients using the platelet function analyser (PFA 100TM): Influence of hematocrit elevation. Haematologica 1999;84:614-19.
- Borna C, Lazarowski E, van Heusden C, Ohlin H, Erlinge D. Resistance to aspirin is increased by ST-elevation myocardial infarction and correlates with adenosine diphosphate levels. Thromb J 2005;3. http://dx.doi.org/10.1186/1477-9560-3-10.
- Sathiropas P. Detection of small inhibitory effects on acetylsalicylic acid (ASA) by platelet impedance aggregometry in whole blood. Thromb Res 1988;51:55-62. http://dx.doi.org/10.1016/0049-3848(88)90282-4.
- Friend M, Vucenik I, Miller M. Platelet responsiveness to aspirin in patients with hyperlipidemia. BMJ 2003;326:82-3. http://dx.doi.org/10.1136/bmj.326.7380.82.
- Korpanty A, Ravindra S, Yassin J, Frenkel EP. Sensitivity of whole blood aggregation, platelet rich plasma, and platelet function analyzer in detection aspirin effect on platelet function. J Am Soc Hematol 2002;100.
- Korpanty A, Frenkel EP, Sarode R. An approach to the assessment of platelet function: comparison of optical platelet rich and impedance whole blood aggregation methods. Thromb Hemost 2005;11:25-3. http://dx.doi.org/10.1177/107602960501100103.
- Ivandic B, Giannitis E, Schlick P, Staritz P, Katus H, Hohlfeld T. Determination of aspirin responsiveness by use of whole blood platelet aggregometry. Clin Chem 2007;53:614-19. http://dx.doi.org/10.1373/clinchem.2006.081059.
- Poston RS, White C, Gu J, Brown J, Gammie J, Pierson RN, et al. Aprotinin shows both hemostatic and antithrombotic effects during off-pump coronary artery bypass grafting. Ann Thorac Surg 2006;81:104-10. http://dx.doi.org/10.1016/j.athoracsur.2005.05.085.
- Haude M, Hafner G, Jablonka A, Rupprecht HJ, Prellwitz W, Meyer J, et al. Guidance of anticoagulation after intracoronary implantation of Palmaz-Schatz stents by monitoring prothrombin and prothrombin fragment 1 + 2. Am Heart J 1995;130:228-38. http://dx.doi.org/10.1016/0002-8703(95)90433-6.
- Grotemeyer KH. The platelet-reactivity-test – a useful ‘by-product’ of the blood-sampling procedure?. Thromb Res 1991;61:423-31. http://dx.doi.org/10.1016/0049-3848(91)90656-H.
- Grotemeyer KH. Effects of acetylsalicylic acid in stroke patients. Evidence of nonresponders in a subpopulation of treated patients. Thromb Res 1991;63:587-93. http://dx.doi.org/10.1016/0049-3848(91)90085-B.
- Zhang Y, Liang J, Zhou YJ, Yuan H, Zhang YZ, Dong L. Chung-Hua Hsin Hsueh Kuan Ping Tsa Chih 2005;33:695-9.
- Yilmaz MB, Balbay Y, Caldir V, Ayaz S, Guray Y, Guray U, et al. Late saphenous vein graft occlusion in patients with coronary bypass: possible role of aspirin resistance. Thromb Res 2005;115:25-9. http://dx.doi.org/10.1016/j.thromres.2004.07.004.
- Valles J, Santos MT, Fuset MP, Moscardo A, Ruano M, Perez F, et al. Partial inhibition of platelet thromboxane A2 synthesis by aspirin is associated with myonecrosis in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2007;99:19-25. http://dx.doi.org/10.1016/j.amjcard.2006.07.058.
- Malek LA, Spiewak M, Filipiak KJ, Grabowski M, Szpotanska M, Rosiak M, et al. Persistent platelet activation is related to very early cardiovascular events in patients with acute coronary syndromes. Kardiol Pol 2007;65:40-5.
- Lev EI, Patel RT, Maresh KJ, Guthikonda S, Granada J, DeLao T, et al. Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J Am Coll Cardiol 2006;47:27-33. http://dx.doi.org/10.1016/j.jacc.2005.08.058.
- Hobikoglu GF, Norgaz T, Aksu H, Ozer O, Erturk M, Nurkalem Z, et al. High frequency of aspirin resistance in patients with acute coronary syndrome. Tohoku J Exp Med 2005;207:59-64. http://dx.doi.org/10.1620/tjem.207.59.
- Faraday N, Braunstein JB, Heldman AW, Bolton ED, Chiles KA, Gerstenblith G, et al. Prospective evaluation of the relationship between platelet–leukocyte conjugate formation and recurrent myocardial ischemia in patients with acute coronary syndromes. Platelets 2004;15:9-14. http://dx.doi.org/10.1080/09537100310001644006.
- Chen W-H, Lee P-Y, Ng W, Tse H-F, Lau C-P. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol 2004;43:1122-6. http://dx.doi.org/10.1016/j.jacc.2003.12.034.
- Chen W-H, Lee P-Y, Ng W, Kwok JYY, Cheng X, Lee SWL, et al. Relation of aspirin resistance to coronary flow reserve in patients undergoing elective percutaneous coronary Intervention. Am J Cardiol 2005;96:760-3. http://dx.doi.org/10.1016/j.amjcard.2005.04.056.
- Andersen K, Hurlen M, Arnesen H, Seljeflot I. Aspirin non-responsiveness as measured by PFA-100 in patients with coronary artery disease. Thromb Res 2002;108:37-42. http://dx.doi.org/10.1016/S0049-3848(02)00405-X.
- Atiemo AD, Ng’Alla LS, Vaidya D, Williams MS. Abnormal PFA-100 closure time is associated with increased platelet aggregation in patients presenting with chest pain. J Thromb Thrombolysis 2008;25:173-8. http://dx.doi.org/10.1007/s11239-007-0045-5.
- Berrouschot J, Schwetlick B, von Twickel G, Fischer C, Uhlemann H, Siegemund T, et al. Aspirin resistance in secondary stroke prevention. Acta Neurol Scand 2006;113:31-5. http://dx.doi.org/10.1111/j.1600-0404.2005.00419.x.
- Poston RS, Gu J, Brown JM, Gammie JS, White C, Nie L, et al. Endothelial injury and acquired aspirin resistance as promoters of regional thrombin formation and early vein graft failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg 2006;131:122-30. http://dx.doi.org/10.1016/j.jtcvs.2005.08.058.
- Grundmann K, Jaschonek K, Kleine B, Dichgans J, Topka H. Aspirin non-responder status in patients with recurrent cerebral ischemic attacks. J Neurol 2003;250:63-6. http://dx.doi.org/10.1007/s00415-003-0954-y.
- McCabe DJ, Harrison P, Mackie IJ, Sidhu PS, Lawrie AS, Purdy G, et al. Assessment of the antiplatelet effects of low to medium dose aspirin in the early and late phases after ischaemic stroke and TIA. Platelets 2005;16:269-80. http://dx.doi.org/10.1080/09537100400020567.
- Poulsen TS, Jørgensen B, Korsholm L, Licht PB, Haghfelt T, Mickley H. Prevalence of aspirin resistance in patients with an evolving acute myocardial infarction. Thromb Res 2007;119:555-62. http://dx.doi.org/10.1016/j.thromres.2006.04.005.
- Pamukcu B, Oflaz H, Nisanci Y. The role of platelet glycoprotein IIIa polymorphism in the high prevalence of in vitro aspirin resistance in patients with intracoronary stent restenosis. Am Heart J 2005;149:675-80. http://dx.doi.org/10.1016/j.ahj.2004.10.007.
- Linden MD, Furman MI, Frelinger AL, Fox ML, Barnard MR, Li Y, et al. Indices of platelet activation and the stability of coronary artery disease. J Thromb Haemost 2007;5:761-5. http://dx.doi.org/10.1111/j.1538-7836.2007.02462.x.
- Gulmez O, Yildirir A, Kaynar G, Konas D, Aydinalp A, Ertan C, et al. Effects of persistent platelet reactivity despite aspirin therapy on cardiac troponin I and creatine kinase-MB levels after elective percutaneous coronary interventions. J Thromb Thrombolysis 2007;25:239-46. http://dx.doi.org/10.1007/s11239-007-0067-z.
- Cornelissen J, Kirtland S, Lim E, Goddard M, Bellm S, Sheridan K, et al. Biological efficacy of low against medium dose aspirin regimen after coronary surgery: analysis of platelet function. Thromb Haemost 2006;95:476-82.
- Cheng X, Chen WH, Lee PY, Ng W, Kwok YY, Lau CP. Prevalence, profile, predictors, and natural history of aspirin resistance measured by the Ultegra Rapid Platelet Function Assay-ASA in patients with coronary heart disease. Circulation 2005;111.
- Geisler T, Kapp M, Gohring-Frischholz K, Daub K, Dosch C, Bigalke B, et al. Residual platelet activity is increased in clopidogrel- and ASA-treated patients with coronary stenting for acute coronary syndromes compared with stable coronary artery disease. Heart 2008;94:743-7. http://dx.doi.org/10.1136/hrt.2006.100891.
- Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003;107:2908-13. http://dx.doi.org/10.1161/01.CIR.0000072771.11429.83.
- ClinicalTrials.gov . Aspirin Resistance and Prognosis of Patients With Critical Limb Ischaemia n.d. http://ClinicalTrials.gov/show/NCT01104441 (accessed 2 April 2012).
- ClinicalTrials.gov . Dual Antiplatelet Therapy in Patients With Aspirin Resistance Following Coronary Artery Bypass Grafting n.d. http://ClinicalTrials.gov/show/NCT01159639 (accessed 2 April 2012).
- ClinicalTrials.gov . Aspirin Responsiveness and Outcome in Coronary Artery Bypass Graft (CABG) Surgery n.d. http://ClinicalTrials.gov/show/NCT01174862 (accessed 2 April 2012).
- ClinicalTrials.gov . Resistance to Aspirin and Or Clopidogrel Among Patients With PAD n.d. http://ClinicalTrials.gov/show/NCT00262561 (accessed 2 April 2012).
- ClinicalTrials.gov . The Study on Profile and Genetic Factors of Aspirin Resistance (ProGEAR Study) n.d. http://clinicaltrials.gov/ct2/show/study/NCT00250380 (accessed 2 April 2012).
- ClinicalTrials.gov . Evaluation and Comparison of Several Point-of-Care Platelet Function Tests in Predicting Clinical Outcomes in Clopidogrel Pre-Treated Patients Undergoing Elective PCI n.d. http://ClinicalTrials.gov/show/NCT00352014 (accessed 2 April 2012).
- ClinicalTrials.gov . Preoperative Aspirin and Postoperative Antiplatelets in Coronary Artery Bypass Grafting: The PAPA CABG Study n.d. https://clinicaltrials.gov/show/NCT00330772 (accessed 2 April 2012).
- Reny JL, Berdague P, Poncet A, Barazer I, Nolli S, Fabbro-Peray P, et al. Antiplatelet drug response status does not predict recurrent ischemic events in stable cardiovascular patients: results of the ADRIE study. Circulation 2012;125:3201-10. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.085464.
- Collet J-P, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. New Engl J Med 2012;367:2100-9. http://dx.doi.org/10.1056/NEJMoa1209979.
- Yamane K, Taniguchi R, Watanabe S, Kawato M, Shirakawa R, Higashi T, et al. Impact of platelet reactivity on long-term clinical outcomes and bleeding events in Japanese patients receiving antiplatelet therapy with aspirin. J Atheroscler Thromb 2012;19:1142-53. http://dx.doi.org/10.5551/jat.14100.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics 2006;24:355-71. http://dx.doi.org/10.2165/00019053-200624040-00006.
- Bronnum-Hansen H, Davidsen M, Thorvaldsen P. Long-term survival and causes of death after stroke. Stroke 2001;32:2131-6. http://dx.doi.org/10.1161/hs0901.094253.
- Bronnum-Hansen H, Jorgensen T, Davidsen M, Madsen M, Osler M, Gerdes LU, et al. Survival and cause of death after myocardial infarction: the Danish MONICA study. J Clin Epidemiol 2001;54:1244-50. http://dx.doi.org/10.1016/S0895-4356(01)00405-X.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- NHS Reference Costs: Financial Year 2011 to 2012. London: Department of Health; 2012.
- Robinson M, Palmer S, Sculpher MJ, Philips Z, Ginnelly L, Bowens A, et al. Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision analytic modelling. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9270.
- Youman P, Wilson K, Harraf F, Kalra L. The economic burden of stroke in the United Kingdom. Pharmacoeconomics 2003;21:43-50. http://dx.doi.org/10.2165/00019053-200321001-00005.
- Greenhalgh J, Bagust A, Boland A, Martin SC, Oyee J, Blundell M, et al. Clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events (review of Technology Appraisal No. 90): a systematic review and economic analysis. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15310.
- Cooper A, Nherera L, Calvert N, O’Flynn N, Turnbull N, Robson J, et al. Clinical Guidelines and Evidence Review for Lipid Modification: Cardiovascular Risk Assessment and the Primary and Secondary Prevention of Cardiovascular Disease. London: National Collaborating Centre for Primary Care and Royal College of General Practitioners; 2008.
- Ward S, Lloyd JM, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11140.
- Statistical Bulletin: Deaths Registered in England and Wales, 2012. Newport: Office for National Statistics; 2012.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- The Guidelines Manual. London: NICE; 2012.
- Lordkipanidzé M, Diodati JG, Schampaert E, Palisaitis DA, Pharand C. Prevalence of unresponsiveness to aspirin and/or clopidogrel in patients with stable coronary heart disease. Am J Cardiol 2009;104:1189-93. http://dx.doi.org/10.1016/j.amjcard.2009.06.025.
- Fontana P, Nolli S, Reber G, de Moerloose P. Biological effects of aspirin and clopidogrel in a randomized cross-over study in 96 healthy volunteers. J Thromb Haemost 2006;4:813-19. http://dx.doi.org/10.1111/j.1538-7836.2006.01867.x.
- Cuisset T, Frere C, Quilici J, Morange PE, Camoin L, Bali L, et al. Relationship between aspirin and clopidogrel responses in acute coronary syndrome and clinical predictors of non response. Thromb Res 2009;123:597-603. http://dx.doi.org/10.1016/j.thromres.2008.04.003.
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006;25:127-41. http://dx.doi.org/10.1002/sim.2331.
- Riley RD, Lambert PC, Abo G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340:410-14. http://dx.doi.org/10.1136/bmj.c221.
- Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol 1999;28:1-9. http://dx.doi.org/10.1093/ije/28.1.1.
- Altman DG, Trivella M, Pezzella F, Harris AL, Pastorino U, Auget J-L, et al. Advances in Statistical Methods for the Health Sciences. Boston, MA: Birkhauser; 2006.
- Kyzas PA, Loizou KT, Ioannidis JPA. Selective reporting biases in cancer prognostic factor studies. J Natl Cancer Inst 2005;97:1043-55. http://dx.doi.org/10.1093/jnci/dji184.
- Hemingway H, Riley RD, Altman DG. Ten steps towards improving prognosis research. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4184.
- Riley RD, Abrams KR, Sutton AJ, Lambert PC, Jones DR, Heney D, et al. Reporting of prognostic markers: current problems and development of guidelines for evidence-based practice in the future. Br J Cancer 2003;88:1191-8. http://dx.doi.org/10.1038/sj.bjc.6600886.
- Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using ‘optimal’ cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994;86:829-35. http://dx.doi.org/10.1093/jnci/86.11.829.
- Riley RD, Hayden JA, Steyerberg EW, Moons KG, Abrams K, Kyzas PA, et al. Prognosis research strategy (PROGRESS) 2: prognostic factor research. PLOS Med 2013;10. http://dx.doi.org/10.1371/journal.pmed.1001380.
- Hemingway H, Croft P, Perel P, Hayden JA, Abrams K, Timmis A, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.e5595.
- Steyerberg EW, Moons KG, van der Windt DA, Hayden JA, Perel P, Schroter S, et al. Prognosis research strategy (PROGRESS) 3: prognostic model research. PLOS Med 2013;10. http://dx.doi.org/10.1371/journal.pmed.1001381.
- Hingorani AD, Windt DA, Riley RD, Abrams K, Moons KG, Steyerberg EW, et al. Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.e5793.
- Rifai N, Altman DG, Bossuyt PM. Reporting bias in diagnostic and prognostic studies: time for action. Clin Chem 2008;54:1101-3. http://dx.doi.org/10.1373/clinchem.2008.108993.
- Simon R, Sobin LH. TNM Online. John Wiley & Sons; 2003.
- Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al. A systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma. Health Technol Assess 2003;7. http://dx.doi.org/10.3310/hta7050.
- Kyzas PA, Denaxa-Kyza D, Ioannidis JP. Almost all articles on cancer prognostic markers report statistically significant results. Eur J Cancer 2007;43:2559-79. http://dx.doi.org/10.1016/j.ejca.2007.08.030.
- Altman DG. BMJ 2001;323:224-8. http://dx.doi.org/10.1136/bmj.323.7306.224.
- Malats N, Bustos A, Nascimento CM, Fernandez F, Rivas M, Puente D, et al. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol 2005;6:678-86. http://dx.doi.org/10.1016/S1470-2045(05)70315-6.
- Hemingway H, Philipson P, Chen R, Fitzpatrick NK, Damant J, Shipley M. Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLOS Med 2010;7. http://dx.doi.org/10.1371/journal.pmed.1000286.
- Sauerbrei W. Prognostic factors. Confusion caused by bad quality design, analysis and reporting of many studies. Adv Otorhinolaryngol 2005;62:184-200.
- Altman DG, Lyman GH. Methodological challenges in the evaluation of prognostic factors in breast cancer. Breast Cancer Res Treat 1998;52:289-303. http://dx.doi.org/10.1023/A:1006193704132.
- Simon R, Altman DG. Statistical aspects of prognostic factor studies in oncology. Br J Cancer 1994;69:979-85. http://dx.doi.org/10.1038/bjc.1994.192.
- Debray TP, Moons KG, Ahmed I, Koffijberg H, Riley RD. A framework for developing, implementing, and evaluating clinical prediction models in an individual participant data meta-analysis. Stat Med 2013;32:3158-80. http://dx.doi.org/10.1002/sim.5732.
- Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. http://dx.doi.org/10.1016/j.jclinepi.2005.02.022.
Appendix 1 Search strategies: prognostic/diagnostic utility review
Database: MEDLINE (Ovid) (1946 to April week 3, 2012)
-
((ASA or aspirin or acetylsalicylic or anti platelet or antiplatelet or anti-platelet) adj2 (respons$ or non-respons$ or respond$ or non-respond$ or resistance or resist$)).mp
-
(platelet adj (response or respond$ or reactivity)).mp.
-
1 or 2
-
exp Aspirin/
-
exp Drug Resistance/
-
4 and 5
-
3 or 6
-
(platelet function adj (analys$ or analyz$)).mp.
-
(platelet function adj (assay$ or test$)).mp.
-
Platelet Function Tests/
-
PFA-100.mp.
-
PlateletWorks.mp.
-
Platelet Mapping.mp.
-
Impact Cone.mp.
-
platelet analyser$.mp.
-
platelet analyzer$.mp.
-
multiplate.mp.
-
aggregometry.mp.
-
LTA.mp.
-
AA-induced LTA.mp.
-
lumiaggregometry.mp.
-
WBA.mp.
-
ULTEGRA assay.mp.
-
Impact-R.mp.
-
TRAP-6.mp.
-
TEG.mp.
-
s-TEG.mp.
-
thromboelastometry.mp.
-
ROTEM.mp.
-
VerifyNow.mp.
-
Verify-Now.mp.
-
VN-RPFA.mp.
-
VASP.mp.
-
VASP-P.mp.
-
platelet reactivity index.mp.
-
vasodilator-stimulated phosphoprotein phosphorylation assay$.mp.
-
T-Guide.tw.
-
T Guide.ti,ab.
-
xylum clot signature analyser.mp.
-
xylum clot signature analyzer.mp.
-
ASA test$.mp.
-
ASA assay$.mp.
-
AA-induced LTA.mp.
-
exp Platelet Count/ or platelet counting.mp.
-
thrombelastography.mp. or Thrombelastography/
-
thrombotic status analyser$.mp.
-
thrombotic status analyzer$.mp.
-
or/8-47
-
exp Cardiovascular Diseases)
-
exp Cerebrovascular Disorders/
-
exp Diabetes Mellitus/
-
or/49-51
-
48 and 52
-
(predict$ or prognos$).mp.
-
48 and 54
-
7 or 53
-
7 or 55
-
56 or 57
-
exp animals/ not humans/
-
58 not 59
Database: MEDLINE (Ovid) In-Process & Other Non-Indexed Citations (25 April 2012)
-
((ASA or aspirin or acetylsalicylic or anti platelet or antiplatelet or anti-platelet) adj2 (respons$ or non-respons$ or respond$ or non-respond$ or resistance or resist$)).mp.
-
(platelet adj (response or respond$ or reactivity)).mp.
-
1 or 2
-
(platelet function adj (analys$ or analyz$)).mp.
-
(platelet function adj (assay$ or test$)).mp.
-
PFA-100.mp.
-
PlateletWorks.mp.
-
Platelet Mapping.mp.
-
Impact Cone.mp.
-
platelet analyser$.mp.
-
platelet analyzer$.mp.
-
multiplate.mp.
-
aggregometry.mp.
-
LTA.mp.
-
AA-induced LTA.mp.
-
lumiaggregometry.mp.
-
WBA.mp.
-
ULTEGRA assay.mp.
-
Impact-R.mp.
-
TRAP-6.mp.
-
TEG.mp.
-
s-TEG.mp.
-
thromboelastometry.mp.
-
ROTEM.mp.
-
VerifyNow.mp.
-
Verify-Now.mp.
-
VN-RPFA.mp.
-
VASP.mp.
-
VASP-P.mp.
-
platelet reactivity index.mp.
-
vasodilator-stimulated phosphoprotein phosphorylation assay$.mp.
-
T-Guide.tw.
-
T Guide.ti,ab.
-
xylum clot signature analyser.mp.
-
xylum clot signature analyzer.mp.
-
ASA test$.mp.
-
ASA assay$.mp.
-
AA-induced LTA.mp.
-
exp Platelet Count/ or platelet counting.mp.
-
thrombelastography.mp. or Thrombelastography/
-
thrombotic status analyser$.mp.
-
thrombotic status analyzer$.mp.
-
or/4-42
-
(cardiovascular or cerebrovascular or diabetes).mp.
-
43 and 44
-
(predict$ or prognos$).mp.
-
43 and 46
-
3 or 45
-
3 or 47
-
48 or 49
Database: EMBASE (Ovid) (1980 to week 16, 2012)
-
((ASA or aspirin or acetylsalicylic or anti platelet or antiplatelet or anti-platelet) adj2 (respons$ or non-respons$ or respond$ or non-respond$ or resistance or resist$)).mp
-
(platelet adj (response or respond$ or reactivity)).mp.
-
1 or 2
-
exp acetylsalicylic acid/
-
exp drug resistance/
-
4 and 5
-
3 or 6
-
(platelet function adj (analys$ or analyz$)).mp.
-
(platelet function adj (assay$ or test$)).mp.
-
PFA-100.mp.
-
PlateletWorks.mp.
-
Platelet Mapping.mp.
-
Impact Cone.mp.
-
platelet analyser$.mp.
-
platelet analyzer$.mp.
-
multiplate.mp.
-
aggregometry.mp.
-
LTA.mp.
-
AA-induced LTA.mp.
-
lumiaggregometry.mp.
-
WBA.mp.
-
ULTEGRA assay.mp.
-
Impact-R.mp.
-
TRAP-6.mp.
-
TEG.mp.
-
s-TEG.mp.
-
thromboelastometry.mp.
-
ROTEM.mp.
-
VerifyNow.mp.
-
Verify-Now.mp.
-
VN-RPFA.mp.
-
VASP.mp.
-
VASP-P.mp.
-
platelet reactivity index.mp.
-
vasodilator-stimulated phosphoprotein phosphorylation assay$.mp.
-
T Guide.mp.
-
T-Guide.mp.
-
xylum clot signature analy$.mp.
-
ASA tests$.mp.
-
ASA assay$.mp.
-
AA-induced LTA.mp.
-
platelet counting.mp.
-
thrombelastography.mp. or exp thromboelastography/
-
thrombotic status analy$.mp.
-
or/8-44
-
exp cardiovascular disease/
-
exp cerebrovascular disease/
-
exp diabetes mellitus/
-
or/46-48
-
45 and 49
-
(predict$ or prognos$).mp.
-
45 and 51
-
7 or 50
-
7 or 52
-
53 or 54
-
exp animal/ not human/
-
55 not 56
Database: The Cochrane Library (Wiley) (Cochrane Central Register of Controlled Trials 2012, issue 4 of 12)
#1 (ASA or aspirin or acetylsalicylic or anti next platelet or antiplatelet) near/2 (respon* or non next respon* or resist*)
#2 (platelet) near/1 (response or respond* or reactivity)
#3 (#1 OR #2)
#4 MeSH descriptor Aspirin explode all trees
#5 MeSH descriptor Drug Resistance explode all trees
#6 (#4 AND #5)
#7 (#3 OR #6)
#8 (platelet function) near/1 (analys* or analyz*)
#9 (platelet function) near/1 (assay* or test*)
#10 PFA-100
#11 PlateletWorks
#12 Platelet next Mapping
#13 Cone
#14 platelet next analy*
#15 multiplate
#16 aggregometry
#17 LTA
#18 lumiaggregometry
#19 WBA
#20 ULTEGRA
#21 Impact-R
#22 TRAP-6
#23 TEG
#24 thromboelastometry
#25 ROTEM
#26 VerifyNow
#27 Verify-Now
#28 VN-RPFA
#29 VASP
#30 VASP-P
#31 platelet next reactivity next index
#32 vasodilator next stimulated next phosphoprotein
#33 T next Guide
#34 xylum next clot
#35 (ASA) next (test* or assay*)
#36 AA next induced
#37 platelet next counting
#38 thromboelastography
#39 thrombotic next status next analy*
#40 (#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39)
#41 MeSH descriptor Cardiovascular Diseases explode all trees
#42 MeSH descriptor Cerebrovascular Disorders explode all trees
#43 MeSH descriptor Diabetes Mellitus explode all trees
#44 (#41 OR #42 OR #43)
#45 (#40 AND #44)
#46 predict* or prognos*
#47 (#40 AND #46)
#48 (#7 OR #45)
#49 (#7 OR #47)
#50 (#48 OR #49)
Conference proceedings searches
Database: Science Citation Index (Web of Knowledge) (searched 1 May 2012)
Title= (aspirin resistance)
Refined by: Web of Science Categories=( PERIPHERAL VASCULAR DISEASE OR CARDIAC CARDIOVASCULAR SYSTEMS ) AND Document Type=( MEETING ABSTRACT )
Timespan=All Years. Databases=SCI-EXPANDED.
Lemmatization=On
Title=(platelet function test*)
Refined by: Web of Science Categories=( PERIPHERAL VASCULAR DISEASE OR CARDIAC CARDIOVASCULAR SYSTEMS ) AND Document Type=( MEETING ABSTRACT )
Timespan=All Years. Databases=SCI-EXPANDED.
Database: Conference Proceedings Citation Index (Web of Knowledge) (searched 1 May 2012)
Title=(aspirin resistance)
Refined by: Web of Science Categories=( CARDIAC CARDIOVASCULAR SYSTEMS OR PERIPHERAL VASCULAR DISEASE ) AND Document Type=( MEETING ABSTRACT OR PROCEEDINGS PAPER )
Timespan=All Years. Databases=CPCI-S.
Title=(platelet function test*)
Refined by: Web of Science Categories=( CARDIAC CARDIOVASCULAR SYSTEMS OR PERIPHERAL VASCULAR DISEASE ) AND Document Type=( MEETING ABSTRACT OR PROCEEDINGS PAPER )
Timespan=All Years. Databases=CPCI-S.
Appendix 2 Search strategies for economic studies
Database: MEDLINE (Ovid) (1946 to April week 4, 2012)
-
((ASA or aspirin or acetylsalicylic or anti platelet or antiplatelet or anti-platelet) adj2 (respons$ or non-respons$ or respond$ or non-respond$ or resistance or resist$)).mp.
-
(platelet adj (response or respond$ or reactivity)).mp.
-
1 or 2
-
exp Aspirin/
-
exp Drug Resistance/
-
4 and 5
-
3 or 6
-
(platelet function adj (analys$ or analyz$)).mp.
-
(platelet function adj (assay$ or test$)).mp.
-
Platelet Function Tests/
-
PFA-100.mp.
-
PlateletWorks.mp.
-
Platelet Mapping.mp.
-
Impact Cone.mp.
-
platelet analyser$.mp.
-
platelet analyzer$.mp.
-
multiplate.mp.
-
aggregometry.mp.
-
LTA.mp.
-
AA-induced LTA.mp.
-
lumiaggregometry.mp.
-
WBA.mp.
-
ULTEGRA assay.mp.
-
Impact-R.mp.
-
TRAP-6.mp.
-
TEG.mp.
-
s-TEG.mp.
-
thromboelastometry.mp.
-
ROTEM.mp.
-
VerifyNow.mp.
-
Verify-Now.mp.
-
VN-RPFA.mp.
-
VASP.mp.
-
VASP-P.mp.
-
platelet reactivity index.mp.
-
vasodilator-stimulated phosphoprotein phosphorylation assay$.mp.
-
T-Guide.tw.
-
T Guide.ti,ab.
-
xylum clot signature analyser.mp.
-
xylum clot signature analyzer.mp.
-
ASA test$.mp.
-
ASA assay$.mp.
-
AA-induced LTA.mp.
-
exp Platelet Count/ or platelet counting.mp.
-
thrombelastography.mp. or Thrombelastography/
-
thrombotic status analyser$.mp.
-
thrombotic status analyzer$.mp.
-
or/8-47
-
exp Cardiovascular Diseases/
-
exp Cerebrovascular Disorders/
-
exp Diabetes Mellitus/
-
or/49-51
-
48 and 52
-
(predict$ or prognos$).mp.
-
48 and 54
-
7 or 53
-
7 or 55
-
56 or 57
-
economics/
-
exp “costs and cost analysis”/
-
cost of illness/
-
exp health care costs/
-
economic value of life/
-
exp economics medical/
-
exp economics hospital/
-
economics pharmaceutical/
-
exp "fees and charges"/
-
(econom$ or cost or costs or costly or costing or price or pricing or pharmacoeconomic$).tw.
-
(expenditure$ not energy).tw.
-
(value adj1 money).tw.
-
budget$.tw.
-
decision support techniques/
-
markov.mp.
-
exp models economic/
-
decision analysis.mp.
-
cost benefit analysis/
-
or/59-76
-
58 and 77
Database: EMBASE (Ovid) (1980 to week 17, 2012)
-
((ASA or aspirin or acetylsalicylic or anti platelet or antiplatelet or anti-platelet) adj2 (respons$ or non-respons$ or respond$ or non-respond$ or resistance or resist$)).mp.
-
(platelet adj (response or respond$ or reactivity)).mp.
-
1 or 2
-
exp acetylsalicylic acid/
-
exp drug resistance/
-
4 and 5
-
3 or 6
-
(platelet function adj (analys$ or analyz$)).mp.
-
(platelet function adj (assay$ or test$)).mp.
-
PFA-100.mp.
-
PlateletWorks.mp.
-
Platelet Mapping.mp.
-
Impact Cone.mp.
-
platelet analyser$.mp.
-
platelet analyzer$.mp.
-
multiplate.mp.
-
aggregometry.mp.
-
LTA.mp.
-
AA-induced LTA.mp.
-
lumiaggregometry.mp.
-
WBA.mp.
-
ULTEGRA assay.mp.
-
Impact-R.mp.
-
TRAP-6.mp.
-
TEG.mp.
-
s-TEG.mp.
-
thromboelastometry.mp.
-
ROTEM.mp.
-
VerifyNow.mp.
-
Verify-Now.mp.
-
VN-RPFA.mp.
-
VASP.mp.
-
VASP-P.mp.
-
platelet reactivity index.mp.
-
vasodilator-stimulated phosphoprotein phosphorylation assay$.mp.
-
T Guide.mp.
-
T Guide.mp.
-
xylum clot signature analy$.mp.
-
ASA tests$.mp.
-
ASA assay$.mp.
-
AA-induced LTA.mp.
-
platelet counting.mp.
-
thrombelastography.mp. or exp thromboelastography/
-
thrombotic status analy$.mp.
-
or/8-44
-
exp cardiovascular disease/
-
exp cerebrovascular disease/
-
exp diabetes mellitus/
-
or/46-48
-
45 and 49
-
(predict$ or prognos$).mp
-
45 and 51
-
7 or 50
-
7 or 52
-
53 or 54
-
cost benefit analysis/
-
cost effectiveness analysis/
-
cost minimization analysis/
-
cost utility analysis/
-
economic evaluation/
-
(cost or costs or costed or costly or costing).tw.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
(technology adj assessment$).tw.
-
decision support.mp.
-
markov.mp.
-
exp statistical model/
-
decision analysis.mp.
-
exp “cost benefit analysis”/
-
or/56-68
-
55 and 69
Database: The Cochrane Library (Wiley) (NHS Economic Evaluation Database) (2012, issue 2 of 4)
(Search date 4 May 2012.)
#1 (ASA or aspirin or acetylsalicylic or anti next platelet or antiplatelet) near/2 (respon* or non next respon* or resist*)
#2 (platelet) near/1 (response or respond* or reactivity)
#3 (#1 OR #2)
#4 MeSH descriptor Aspirin explode all trees
#5 MeSH descriptor Drug Resistance explode all trees
#6 (#4 AND #5)
#7 (#3 OR #6)
#8 (platelet function) near/1 (analys* or analyz*)
#9 (platelet function) near/1 (assay* or test*)
#10 PFA-100
#11 PlateletWorks
#12 Platelet next Mapping
#13 Cone
#14 platelet next analy*
#15 multiplate
#16 aggregometry
#17 LTA
#18 lumiaggregometry
#19 WBA
#20 ULTEGRA
#21 Impact-R
#22 TRAP-6
#23 TEG
#24 thromboelastometry
#25 ROTEM
#26 VerifyNow
#27 Verify-Now
#28 VN-RPFA
#29 VASP
#30 VASP-P
#31 platelet next reactivity next index
#32 vasodilator next stimulated next phosphoprotein
#33 T next Guide
#34 xylum next clot
#35 (ASA) next (test* or assay*)
#36 AA next induced
#37 platelet next counting
#38 thromboelastography
#39 thrombotic next status next analy*
#40 (#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39)
#41 MeSH descriptor Cardiovascular Diseases explode all trees
#42 MeSH descriptor Cerebrovascular Disorders explode all trees
#43 MeSH descriptor Diabetes Mellitus explode all trees
#44 (#41 OR #42 OR #43)
#45 (#40 AND #44)
#46 predict* or prognos*
#47 (#40 AND #46)
#48 (#7 OR #45)
#49 (#7 OR #47)
#50 (#48 OR #49)
Appendix 3 Sensitivities and specificities
As outlined in Chapter 4, Diagnostic/predictive accuracy, there are limitations with regard to assessment of the diagnostic utility of PFTs in the context of prediction of future adverse clinical outcomes in aspirin-treated patients.
Sensitivities and specificities were rarely reported by studies included in this report. Where data were available to calculate these metrics, they were extracted. In both cases the reference standard for calculations was the occurrence, or not, of a named clinical outcome. The presence of these data is mentioned where relevant in the main results section of this report (see Chapter 5). As studies frequently reported more than one clinical outcome, there was the possibility of using multiple reference standards. In such cases, sensitivities and specificities are presented for each outcome. It should be noted that the occurrence of a clinical outcome is not necessarily indicative of a poor response to aspirin therapy, as the outcome might also occur by chance or as a result of other factors, such as poor adherence to therapy.
This appendix reports a speculative analysis of diagnostic utility for each group of PFTs in patients receiving aspirin as a monotherapy at the time of platelet function testing for each clinical outcome. Brief methods are outlined below prior to presentation of the results.
Methods
The diagnostic/predictive accuracy of each test relates to the absolute scale and summarises its ability to accurately classify which patients go on to experience clinically important outcomes. For test results classed as ‘positive’ or ‘negative’ (or ‘high’ or ‘low’) and linked to a binary outcome at a particular time, the data required to complete a 2 × 2 table were sought (Table 84).
| Test result | Number of patients with an event | Number of patients with no events |
|---|---|---|
| Test positive (aspirin resistant) | TP (a) | FP (b) |
| Test negative (aspirin sensitive) | FN (c) | TN (d) |
When available, the 2 × 2 table allowed estimation of the sensitivity [a/(a + c)] and specificity [b/(b + d)] of the test, with CIs, and (for cohort studies) the prevalence of the outcome (a + c)/(b + d). Sensitivity is defined as the probability of correctly classifying those patients who ultimately experience an event as test positive, and specificity as the probability of correctly classifying those who do not go on to experience an event as test negative. If studies provided a 2 × 2 table with one or both groups having a zero cell, then a continuity correction of 0.5 was added to all cells in order to work out the variance and CI for sensitivity and specificity. 308 CIs were derived on the logit scale, and then transformed back to the original sensitivity and specificity scale.
Though most studies reported dichotomised test results (e.g. ‘positive’ vs. ‘negative’), sometimes results were presented by three or more groups of test results (e.g. ‘high’, ‘normal’, ‘low’). In this situation, to allow greater consistency across studies, the groupings were collapsed where possible to form a dichotomy again, and allow for the generation of tables that compared two groups (e.g. a table for ‘high’ vs. ‘normal’ or ‘low’, and one for ‘high’ or ‘normal’ vs. ‘low’).
Owing to the clinical and methodological heterogeneity between studies, pooling of data was determined to be inappropriate even in subgroups of studies employing the same PFT. However, data are presented in this report in forest plots (without the summary estimate), along with some relevant study characteristics highlighting heterogeneity.
Results
In all sections below, given the uncertainty around the prognostic utility findings presented in the main body of this report, the sensitivities and specificities do not meaningfully contribute to the report.
Light transmission aggregometry: death
FIGURE 69.
Light transmission aggregometry, monotherapy: death, sensitivity. AA, arachidonic acid.

FIGURE 70.
Light transmission aggregometry, monotherapy: death, specificity. AA, arachidonic acid.

Light transmission aggregometry: major adverse cardiac events
FIGURE 71.
Light transmission aggregometry, monotherapy: MACEs, sensitivity. AA, arachidonic acid.

FIGURE 72.
Light transmission aggregometry, monotherapy: MACEs, specificity. AA, arachidonic acid.
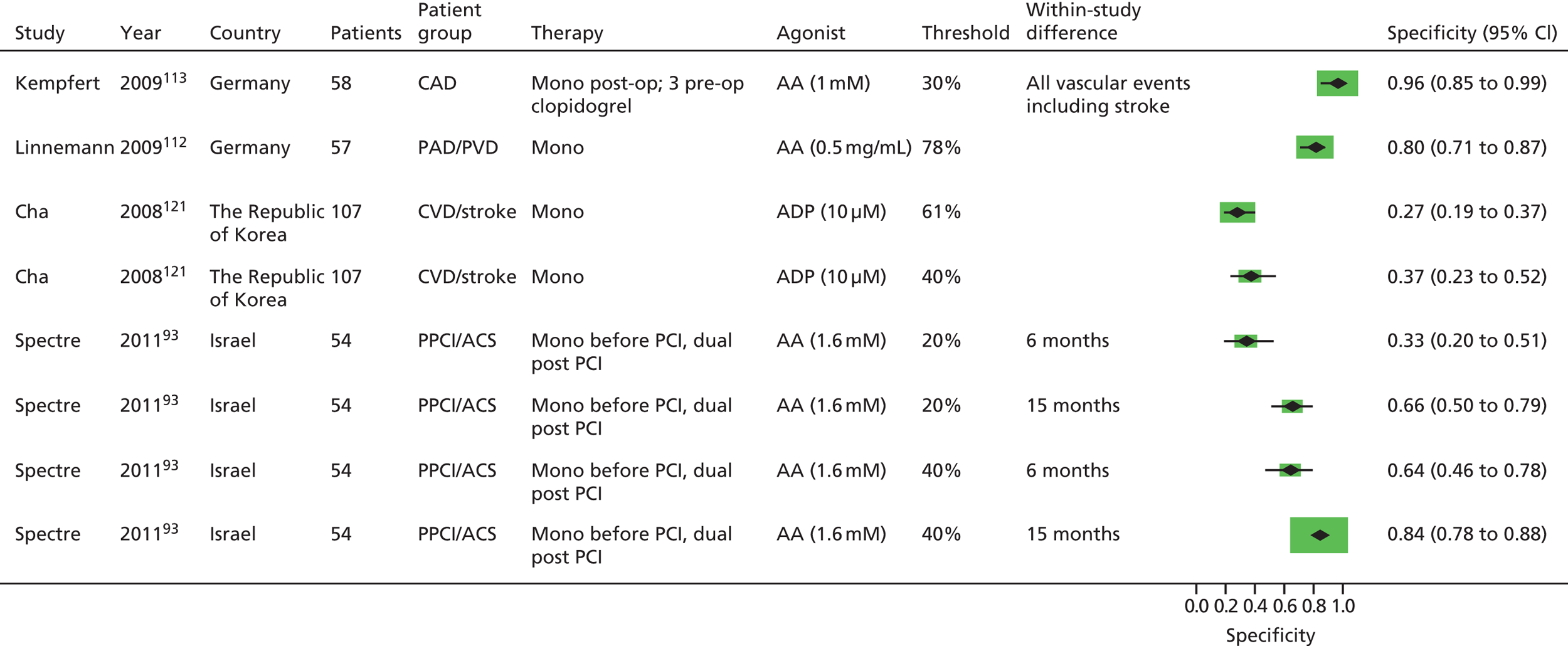
Light transmission aggregometry: ischaemic/thrombotic events
FIGURE 73.
Light transmission aggregometry, monotherapy: ischaemic/thrombotic events, sensitivity. AA, arachidonic acid.
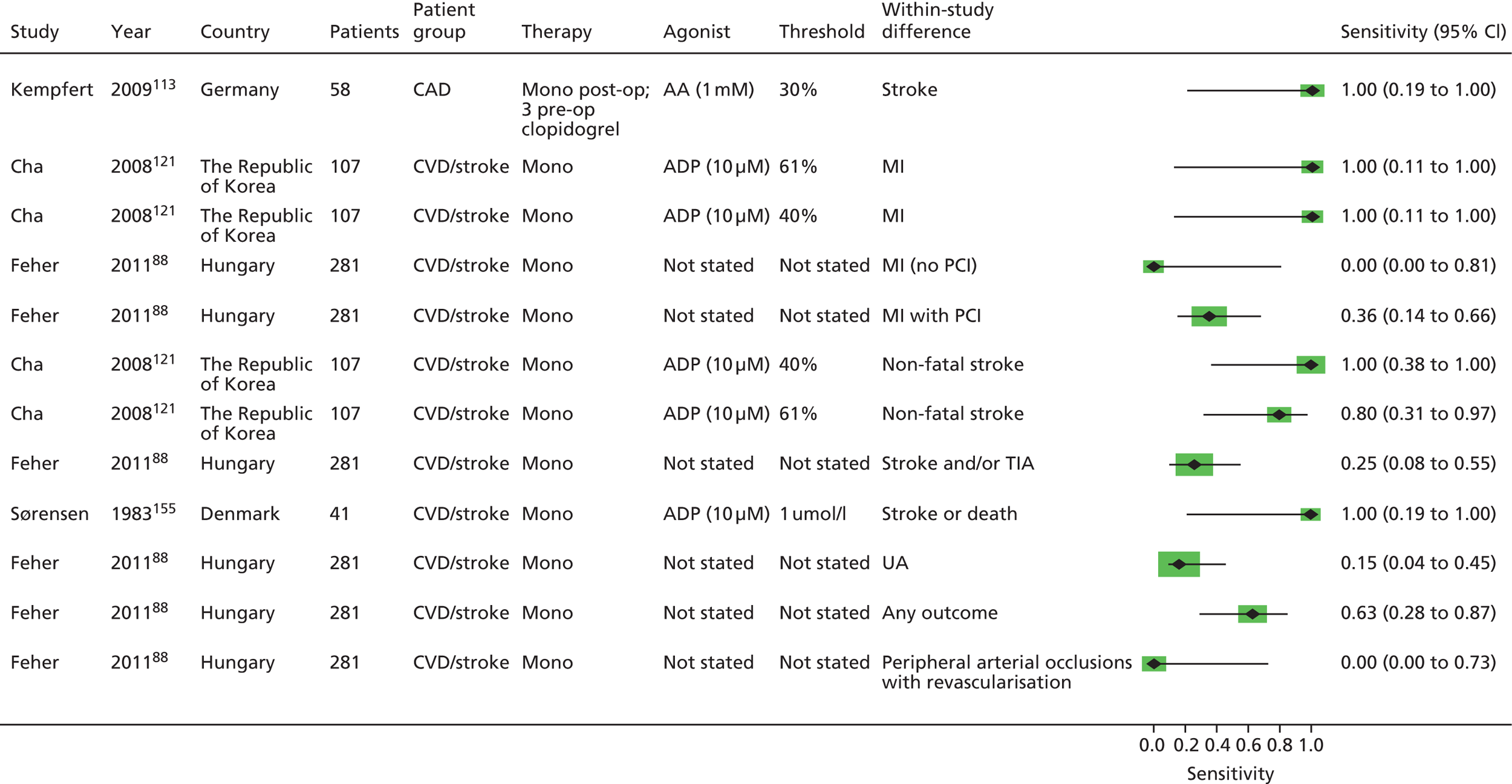
FIGURE 74.
Light transmission aggregometry, monotherapy: ischaemic/thrombotic events, specificity. AA, arachidonic acid.

VerifyNow® Aspirin: death
FIGURE 75.
VerifyNow® Aspirin, monotherapy: death, sensitivity.
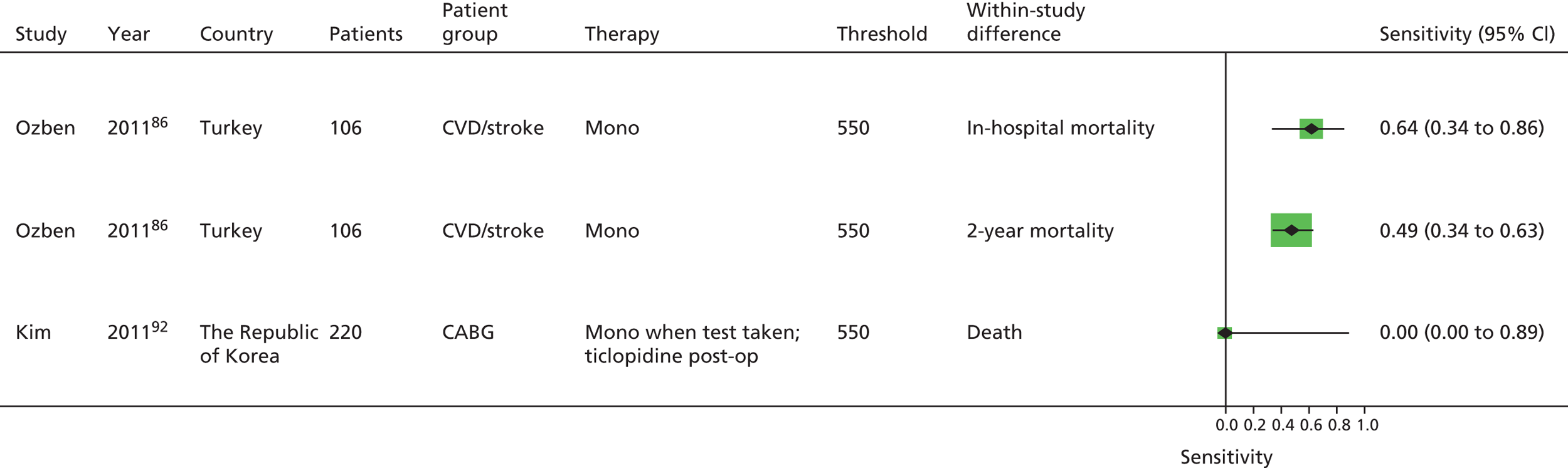
FIGURE 76.
VerifyNow® Aspirin, monotherapy: death, specificity.
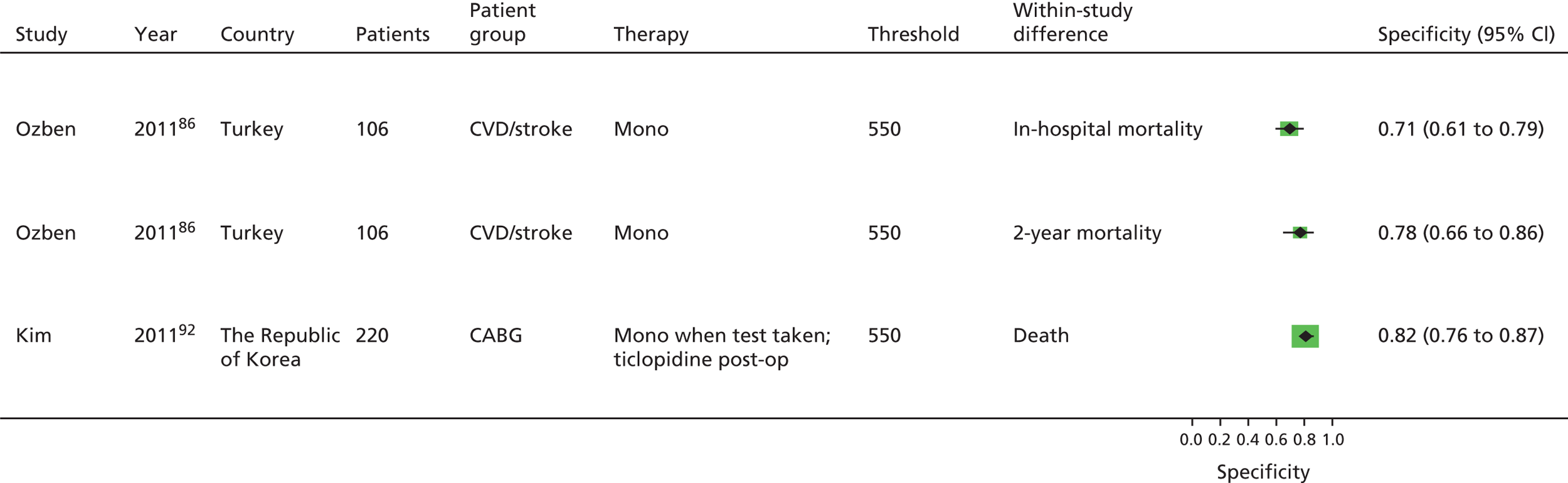
VerifyNow® Aspirin: major adverse cardiac events
FIGURE 77.
VerifyNow® Aspirin, monotherapy: MACEs, sensitivity.

FIGURE 78.
VerifyNow® Aspirin, monotherapy: MACEs, specificity.
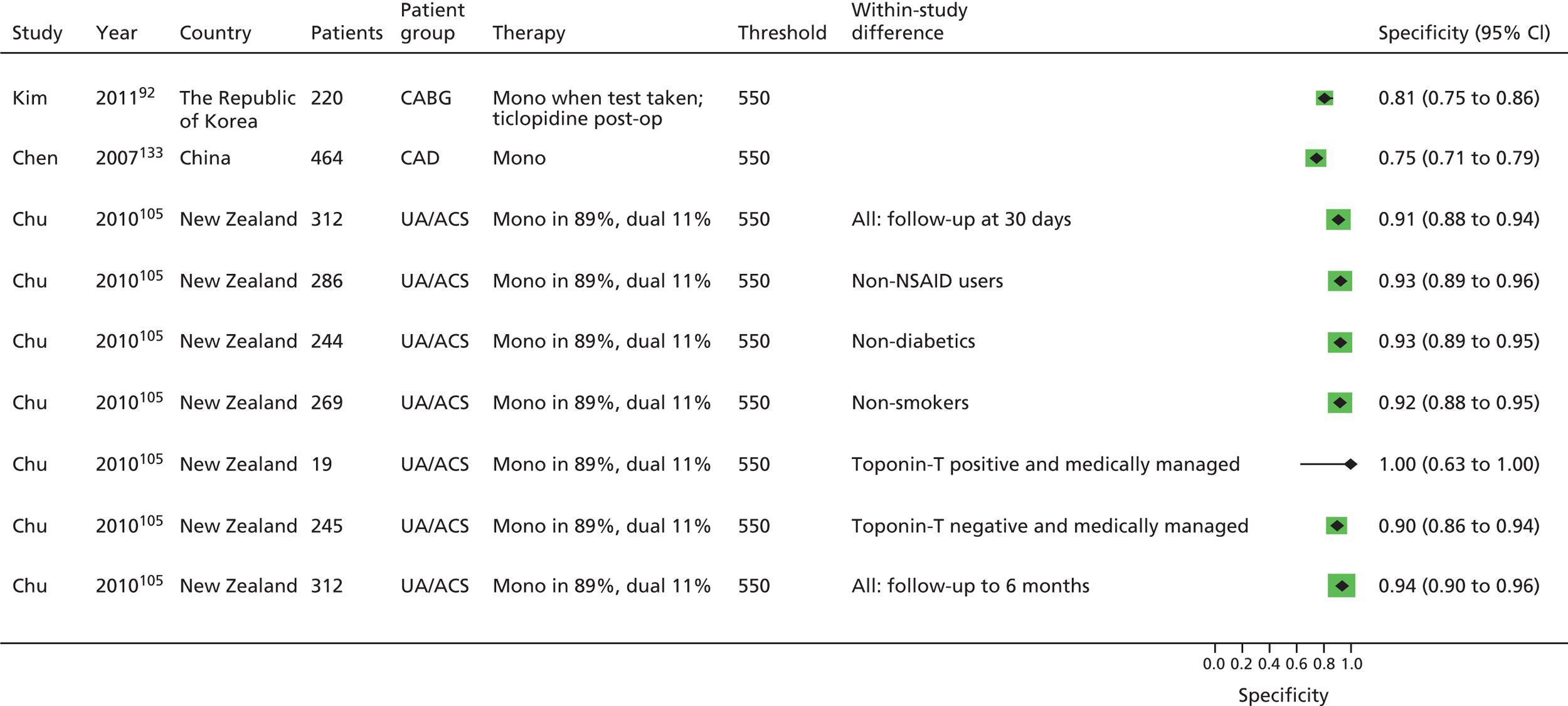
VerifyNow® Aspirin: ischaemic/thrombotic events
FIGURE 79.
VerifyNow® Aspirin, monotherapy: ischaemic/thrombotic events, sensitivity.

FIGURE 80.
VerifyNow® Aspirin, monotherapy: ischaemic/thrombotic events, specificity.

VerifyNow® Aspirin: bleeding events
FIGURE 81.
VerifyNow® Aspirin, monotherapy: bleeding events, sensitivity.

FIGURE 82.
VerifyNow® Aspirin, monotherapy: bleeding events, specificity.
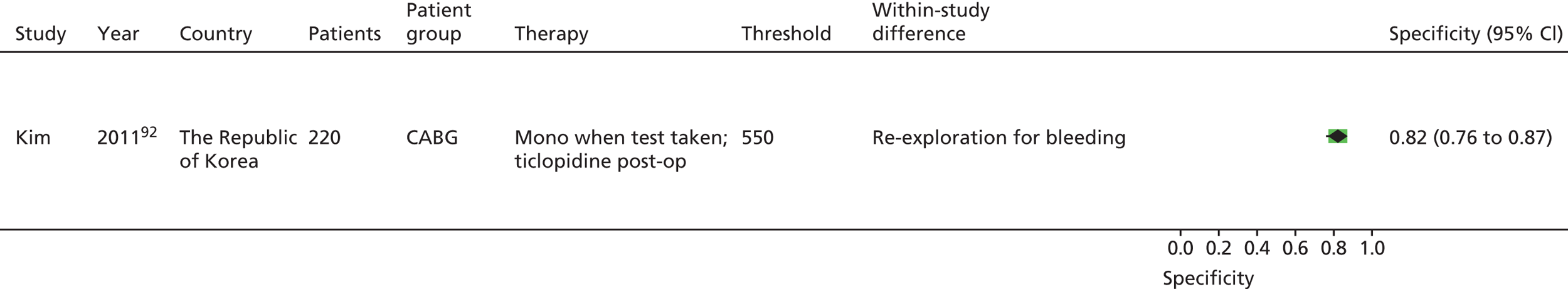
Thromboxane metabolite measurement: death
FIGURE 83.
Thromboxane metabolite measurement, monotherapy: death, sensitivity.

FIGURE 84.
Thromboxane metabolite measurement, monotherapy: death, specificity.
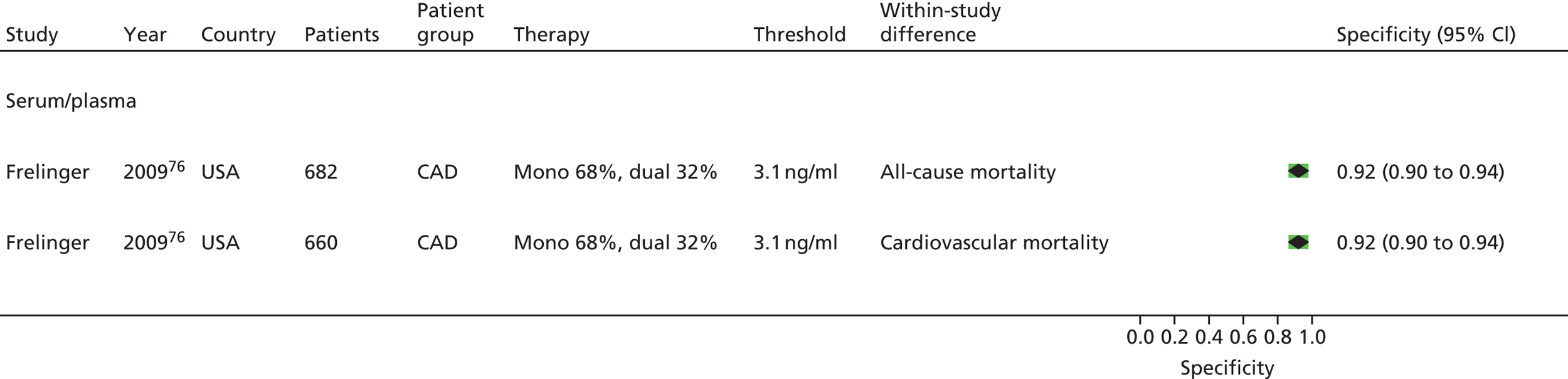
Thromboxane metabolite measurement: major adverse cardiac events
FIGURE 85.
Thromboxane metabolite measurement, monotherapy: MACEs, sensitivity. RAVE, recurrent acute vascular event.

FIGURE 86.
Thromboxane metabolite measurement, monotherapy: MACEs, specificity. RAVE, recurrent acute vascular event.
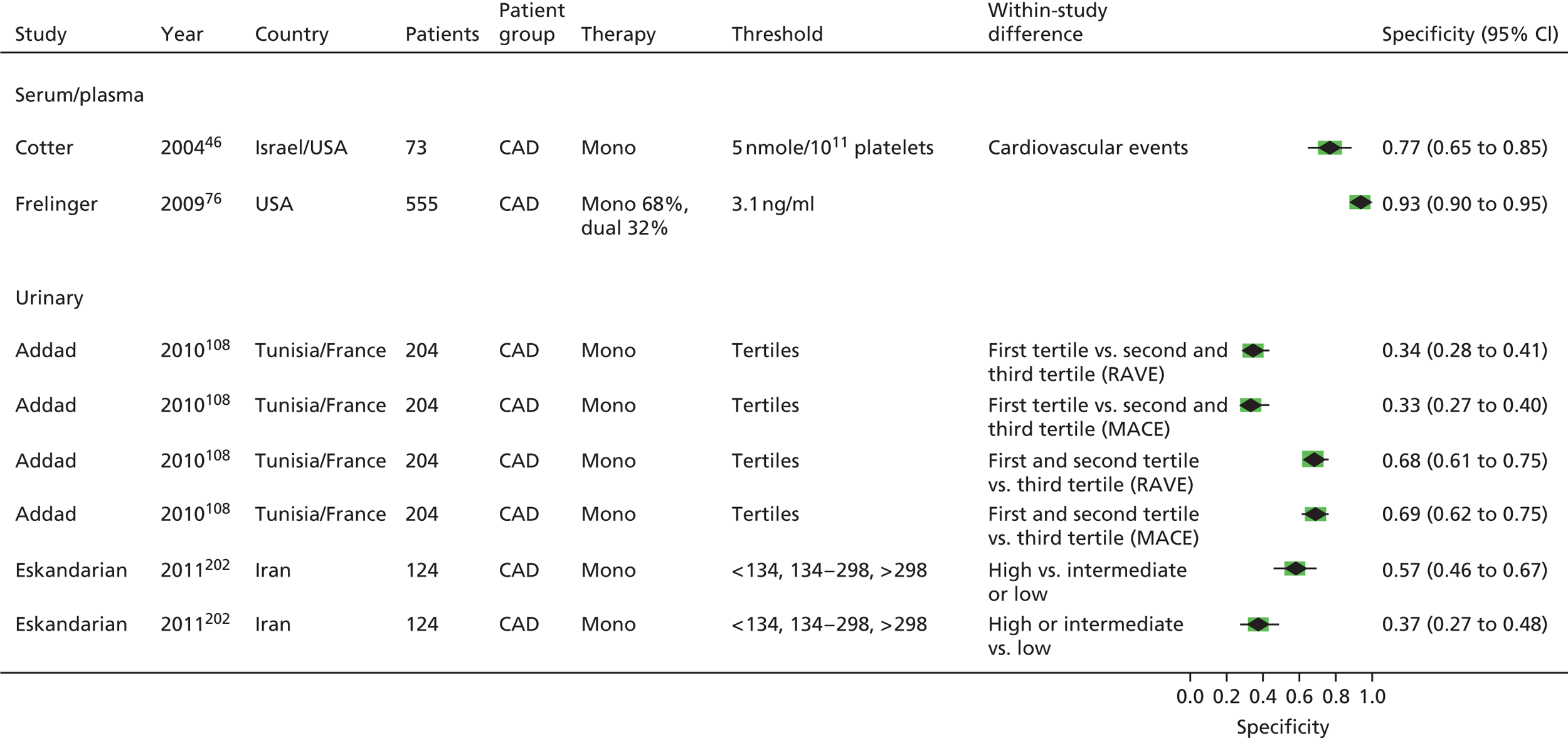
Thromboxane metabolite measurement: ischaemic/thrombotic events
FIGURE 87.
Thromboxane metabolite measurement, monotherapy: ischaemic/thrombotic events, sensitivity.

FIGURE 88.
Thromboxane metabolite measurement, monotherapy: ischaemic/thrombotic events, specificity.

PFA-100®: death
FIGURE 89.
PFA-100®, monotherapy: death, sensitivity. s, seconds.
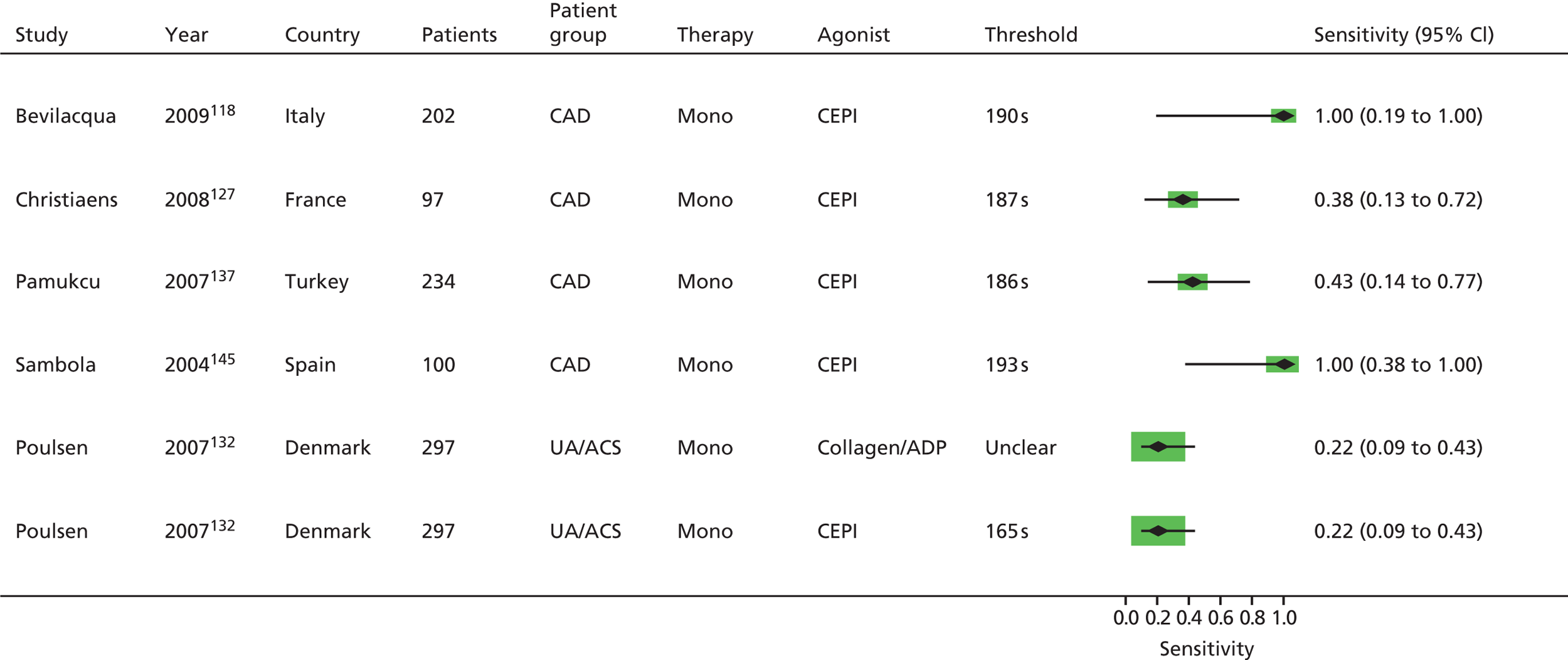
FIGURE 90.
PFA-100®, monotherapy: death, specificity. s, seconds.
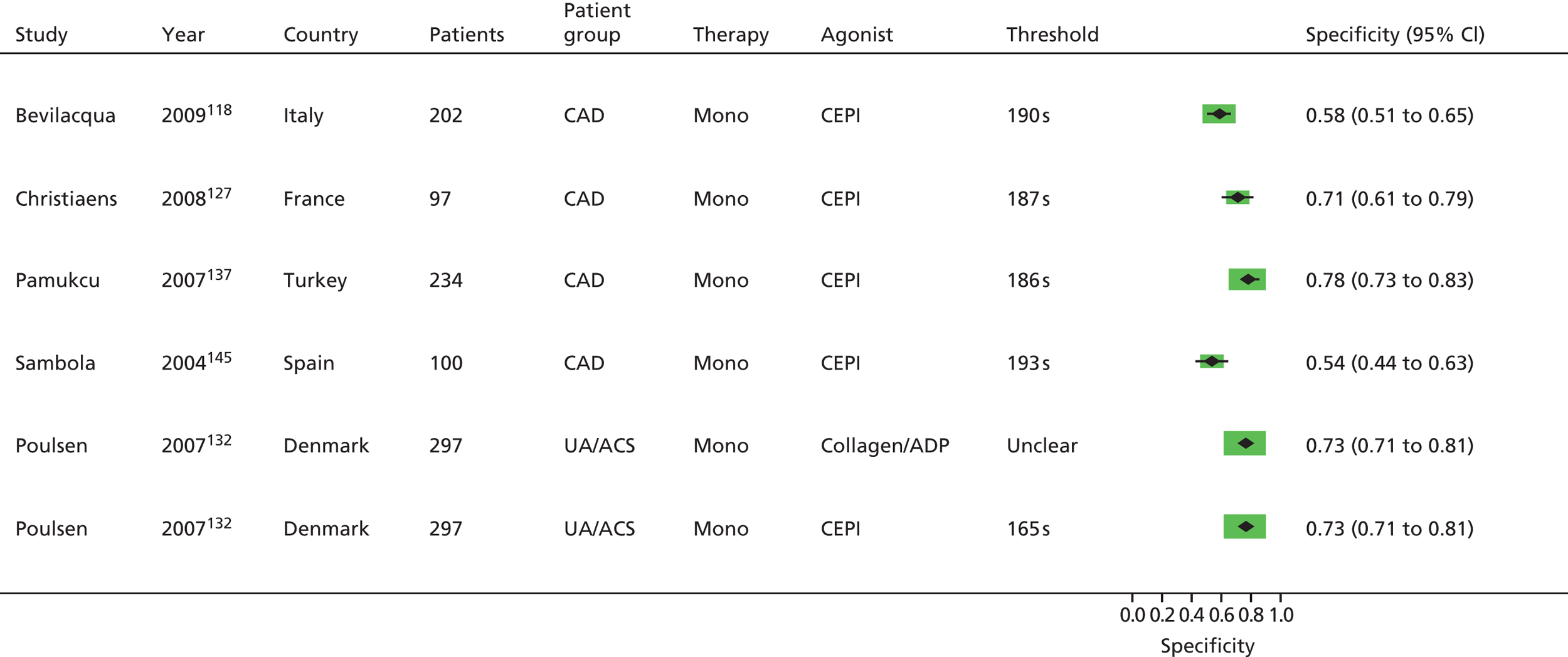
PFA-100®: major adverse cardiac events
FIGURE 91.
PFA-100®, monotherapy: MACEs, sensitivity. RAVE, recurrent acute vascular event; s, seconds.
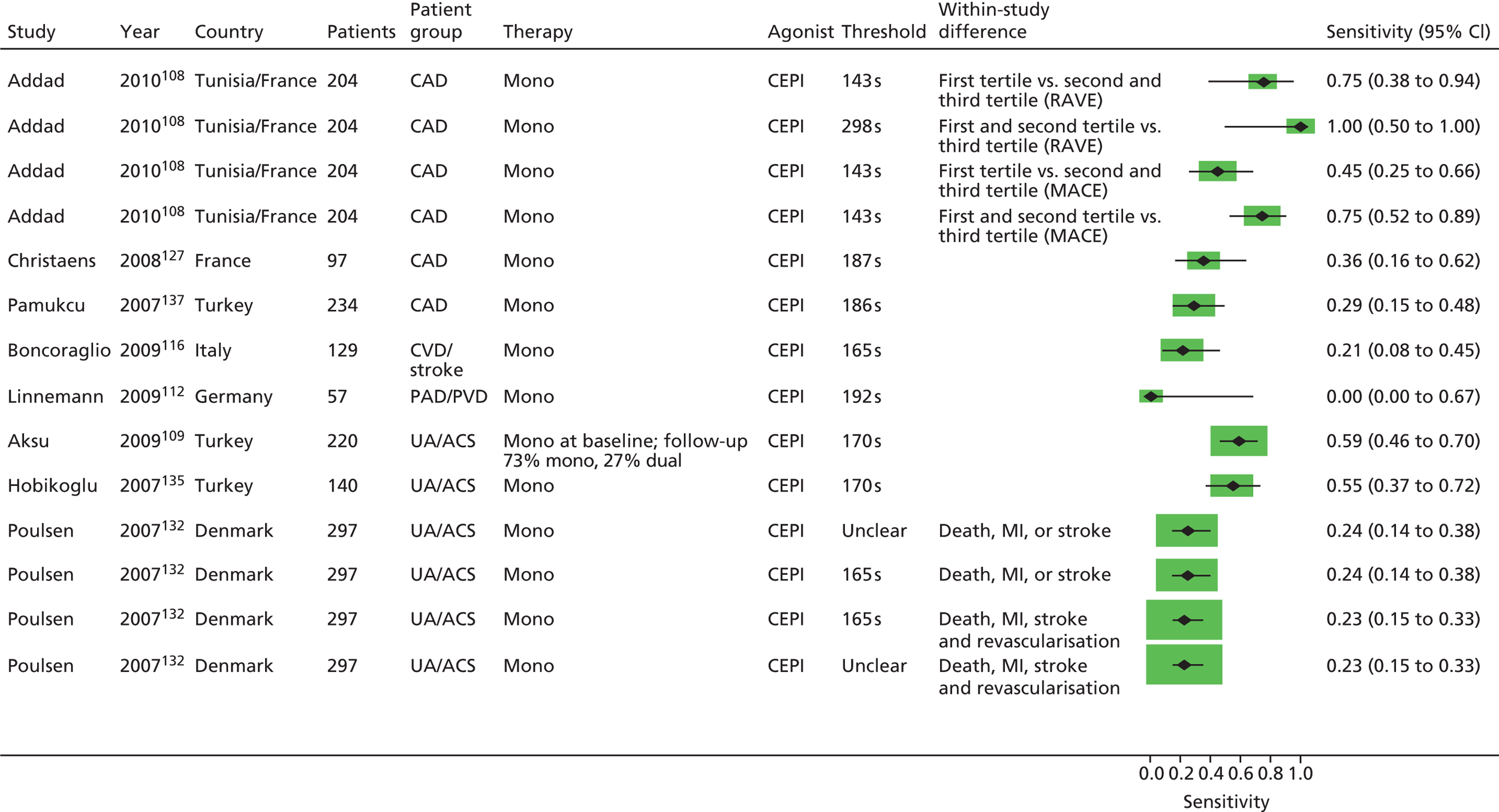
FIGURE 92.
PFA-100®, monotherapy: MACEs, specificity. RAVE, recurrent acute vascular event; s, seconds.
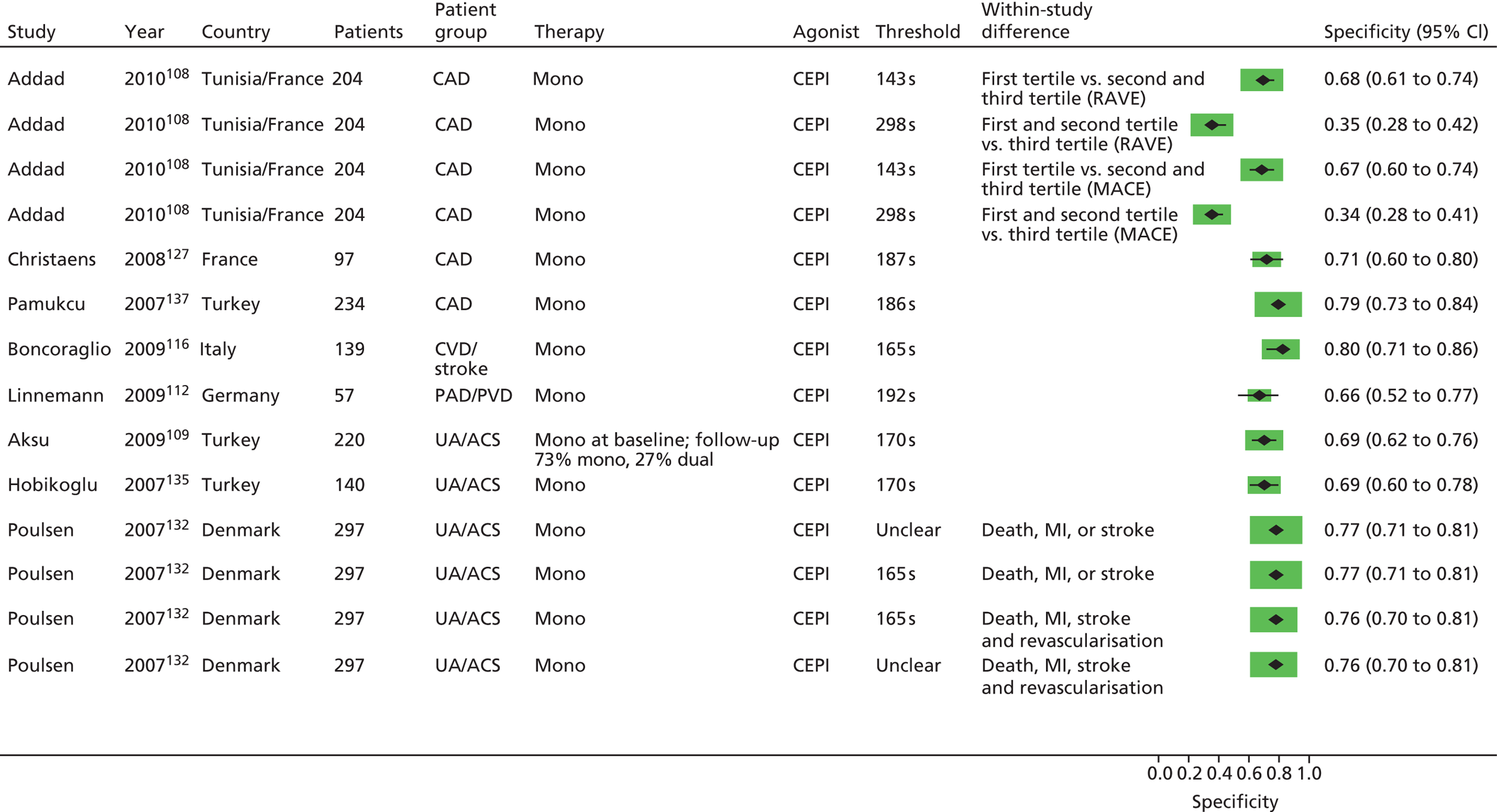
PFA-100®: ischaemic/thrombotic events
FIGURE 93.
PFA-100®, monotherapy: ischaemic/thrombotic events, sensitivity. PTA, percutaneous transluminal angioplasty; s, seconds; TEA, thoracic epidural analgesia.

FIGURE 94.
PFA-100®, monotherapy: ischaemic/thrombotic events, specificity. PTA, percutaneous transluminal angioplasty; s, seconds; TEA, thoracic epidural analgesia.

Whole-blood aggregometry: death
FIGURE 95.
Whole-blood aggregometry, monotherapy: death, sensitivity. AA, arachidonic acid.

FIGURE 96.
Whole-blood aggregometry, monotherapy: death, specificity. AA, arachidonic acid.

Whole-blood aggregometry: major adverse cardiac events
FIGURE 97.
Whole-blood aggregometry, monotherapy: MACEs, sensitivity.

FIGURE 98.
Whole-blood aggregometry, monotherapy: MACEs, specificity.

Whole-blood aggregometry: ischaemic/thrombotic events
FIGURE 99.
Whole-blood aggregometry, monotherapy: ischaemic/thrombotic events, sensitivity. AA, arachidonic acid.

FIGURE 100.
Whole-blood aggregometry, monotherapy: ischaemic/thrombotic events, specificity. AA, arachidonic acid.

Thromboelastography: death
FIGURE 101.
Thromboelastography, monotherapy: death, sensitivity.
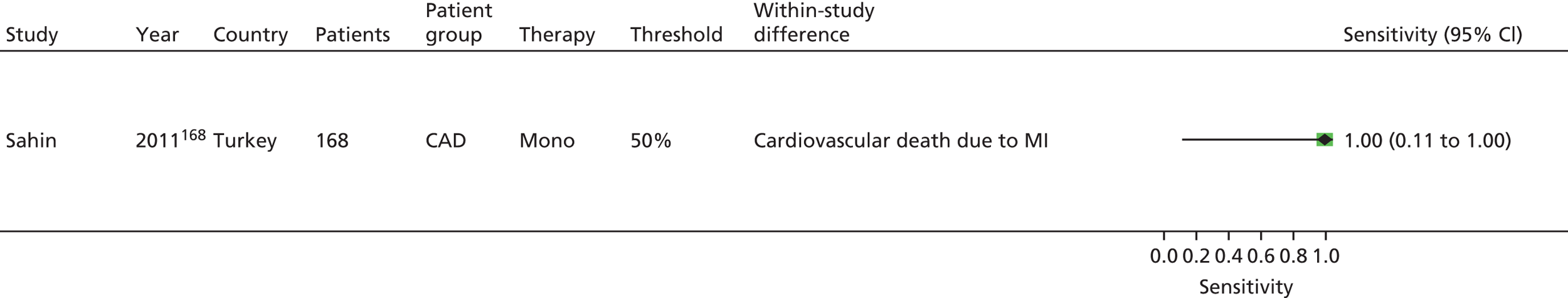
FIGURE 102.
Thromboelastography, monotherapy: death, specificity.

Thromboelastography: ischaemic/thrombotic events
FIGURE 103.
Thromboelastography, monotherapy: ischaemic/thrombotic events, sensitivity.
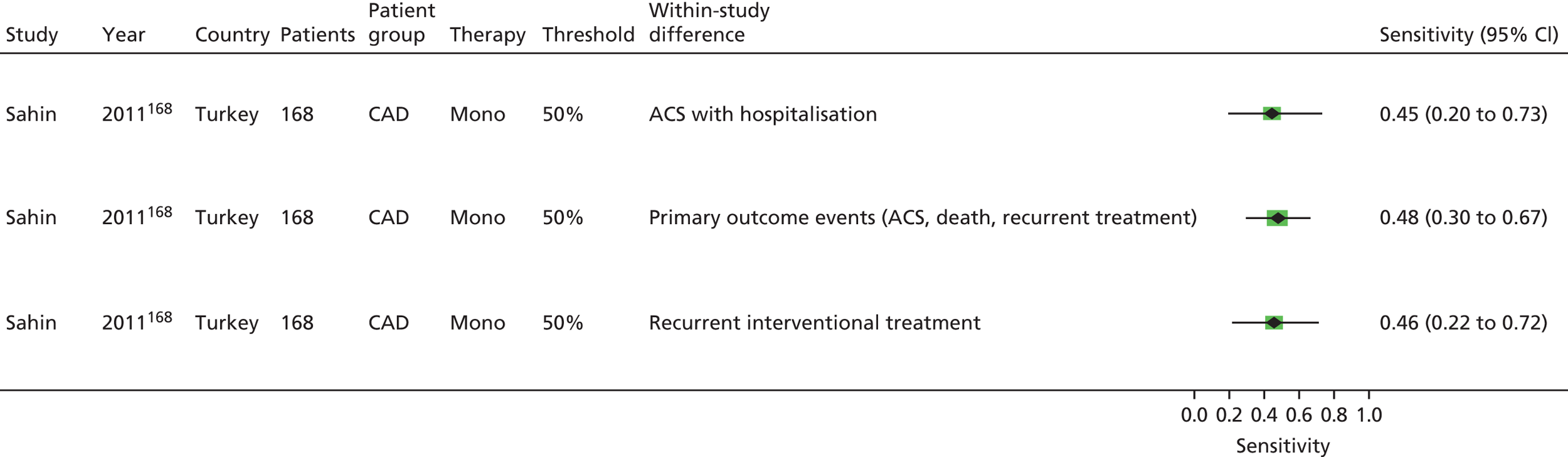
FIGURE 104.
Thromboelastography, monotherapy: ischaemic/thrombotic events, specificity.
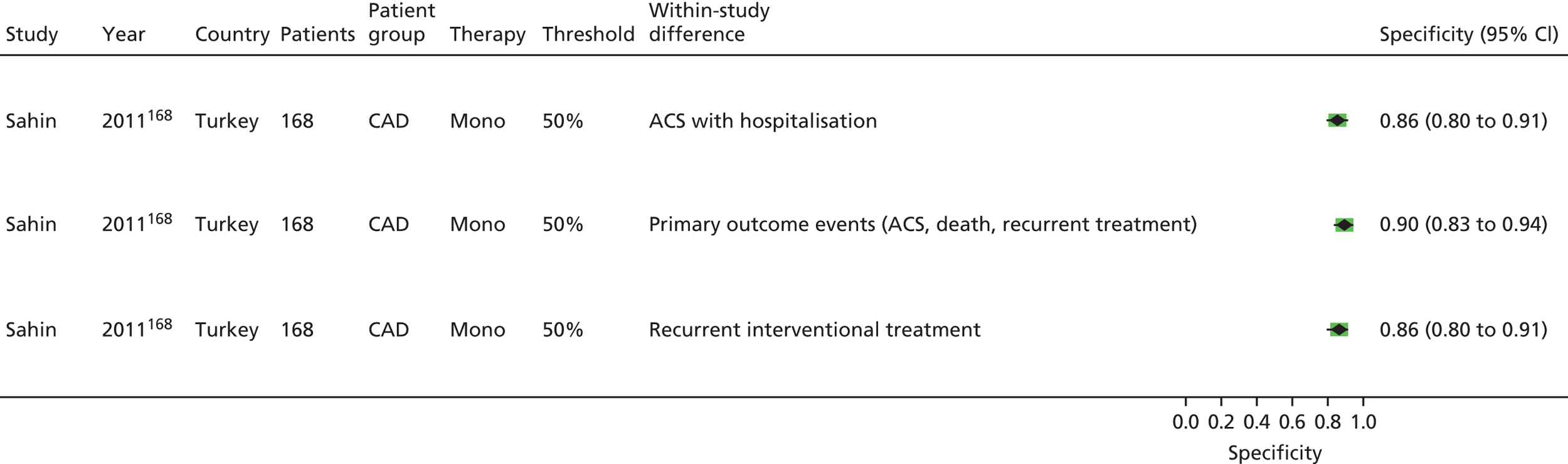
Appendix 4 Supplementary data
Owing to the extensive nature of the data extracted from the included studies for this project, it was deemed unfeasible to adequately present all the data in this report (even as appendices). The results section of the report contains, where necessary, details of the studies, including the populations studied, test characteristics and quality-related features, and data for key outcomes are presented in illustrative forest plots. However, this only applies for studies on patients being treated with aspirin as a single antiplatelet agent (monotherapy), and even so some data are omitted, including the numerical information that was used to produce the forest plots. Furthermore, data from studies in patients treated with dual (and triple) therapy, with aspirin as one of the agents, is completely omitted.
In the interest of transparency, the authors wish for all extracted and analytical data to be available to readers of this report. The data has been made available through a web portal which can be accessed via the following URL: http://medweb4.bham.ac.uk/NIHR_Aspirin_Resistance/
The data files available include Microsoft Excel spreadsheets of extracted data and statistical analysis, and specific data files used in Stata (StataCorp LP, College Station, TX, USA) to produce the forest plots presented in this report.
Below is an outline of the web portal indicating the files available for online viewing.
Project title: The prognostic utility of tests of platelet function for the detection of ‘aspirin resistance‘ in patients with established cardiovascular or cerebrovascular disease: a systematic review and economic evaluation
Project ref.: 10/36/02
Please find below links to the various data files relating to the project.
Data extraction tables
Data tables in Excel for all included studies where patients were receiving aspirin monotherapy at the time of platelet function testing. These data relate to studies reported in Chapter 5 (section Monotherapy) of the HTA report.
Monotherapy-Included studies
Data tables in Excel for all included studies where patients were receiving aspirin dual therapy at the time of platelet function testing. These data relate to studies relevant to Chapter 5, Dual therapy in the HTA report.
Dual therapy-Included studies
Stata data files
Light transmission aggregometry for monotherapy studies
The following links relate to the Stata data files used in the analysis of studies using light transmission aggregometry (LTA) to measure platelet function in patients receiving aspirin monotherapy at the time of platelet function testing.
LTA-monotherapy-all outcomes-unadjusted hazard ratios
LTA-monotherapy-all outcomes-adjusted hazard ratios
LTA-monotherapy-all outcomes-unadjusted odds ratios
LTA-monotherapy-all outcomes-adjusted odds ratios
LTA-monotherapy-all outcomes-sensitivity and specificity
VerifyNow for monotherapy studies
The following links relate to the Stata data files used in the analysis of studies using VerifyNow to measure platelet function in patients receiving aspirin monotherapy at the time of platelet function testing.
VerifyNow-monotherapy-all outcomes-unadjusted hazard ratios
VerifyNow-monotherapy-all outcomes-adjusted hazard ratios
VerifyNow-monotherapy-all outcomes-unadjusted odds ratios
VerifyNow-monotherapy-all outcomes-adjusted odds ratios
VerifyNow-monotherapy-all outcomes-sensitivity and specificity
Thromboxane measurement for monotherapy studies
The following links relate to the Stata data files used in the analysis of studies using thromboxane to measure platelet function in patients receiving aspirin monotherapy at the time of platelet function testing.
Thromboxane-monotherapy-all outcomes-unadjusted hazard ratios
Thromboxane-monotherapy-all outcomes-adjusted hazard ratios
Thromboxane-monotherapy-all outcomes-unadjusted odds ratios
Thromboxane-monotherapy-all outcomes-adjusted odds ratios
Thromboxane-monotherapy-all outcomes-sensitivity and specificity
PFA-100 for monotherapy studies
The following links relate to the Stata data files used in the analysis of studies using PFA-100 to measure platelet function in patients receiving aspirin monotherapy at the time of platelet function testing.
PFA-100-monotherapy-all outcomes-unadjusted hazard ratios
PFA-100-monotherapy-all outcomes-adjusted hazard ratios
PFA-100-monotherapy-all outcomes-unadjusted odds ratios
PFA-100-monotherapy-all outcomes-adjusted odds ratios
PFA-100-monotherapy-all outcomes-sensitivity and specificity
Whole blood aggregometry for monotherapy studies
The following links relate to the Stata data files used in the analysis of studies using whole blood aggregometry to measure platelet function in patients receiving aspirin monotherapy at the time of platelet function testing.
Whole Blood Aggregometry-all outcomes-unadjusted hazard ratios
Whole Blood Aggregometry-all outcomes-adjusted hazard ratios
Whole Blood Aggregometry-all outcomes-unadjusted odds ratios
Whole Blood Aggregometry-all outcomes-adjusted odds ratios
Whole Blood Aggregometry-all outcomes-sensitivity and specificity
Thromboelastography for monotherapy studies
The following links relate to the Stata data files used in the analysis of studies using TEG to measure platelet function in patients receiving aspirin monotherapy at the time of platelet function testing.
TEG-all outcomes-unadjusted hazard ratios
TEG-all outcomes-adjusted hazard ratios
TEG-all outcomes-unadjusted odds ratios
TEG-all outcomes-adjusted odds ratios
TEG-all outcomes-sensitivity and specificity
Appendix 5 List of unobtainable articles for prognostic/diagnostic utility systematic review
Barbano G, De Matteis F. [The thromboelastogram in the post-infarct period.] Atti Soc Ital Cardiol 1962;22:Comunicazioni 90–1.
Bogutskii BV, Ezhova VA, Shibanova ZN. [Thrombelastographic studies in patients with incipient cerebral atherosclerosis undergoing complex treatment.] Vopr Kurortol Fizioter Lech Fiz Kult 1971;36:504–7.
Bujold E, Tapp S, Giguere Y. Aspirin resistance and adverse pregnancy outcomes. Neuroendocrinol Lett 2011;32:369–70.
Fauknerova M, Osmancik P, Spacek M, Kejst L, Kalvach P. Aggregometry in secondary prevention of stroke. Aspirin resistance. Ceska Slov Neurol Neurochir 2011;74:527–32.
Fronescu E, Vilcu A. [Thromboelastographic investigations in atherosclerosis.] Med Interna (Bucur) 1962;14:1199–206.
Goelian P. Resistances to antiplatelet agents. Rev Francoph Orthopt 2010;3:12–16.
Gritsiuk AI. [Diagnosis of the pre-thrombotic state in cardiovascular diseases.] Vrach Delo 1971;3:8–14.
Haas T. Point of care diagnostic: thromboelastometry (ROTEM®). Wien Klin Wochenschr 2010;122(Suppl. 5):19–20.
Kwon SU. Overcome Biochemical Aspirin Resistance Through Cilostazol Combination (ARCC). Stroke Trials Registry, Internet Stroke Center; 2007. URL: www strokecenter org/trials
Laguta PS, Katkova OV, Dobrovol’skii AB, Titaeva EV, Deev AD, Panchenko EP. Aspirin resistance in patients with stable ischemic heart disease. Kardiologiia 2010;50:4–11.
Liu L, Yang F, Li M, Hou H-J, Liu Y-H, Chen G-H, et al. Evaluation of the efficacy of anti-platelet aggregation drugs in patients using thromboelastograph after percutaneous transluminal angioplasty and stenting. Chin J Cerebrovasc Dis 2012;9:67–71.
Manus J-M. Aspirin resistance: How to detect it. Actual Pharm 2005;440:7.
Nidhinandana S, Changchit S. Prevalence of aspirin resistance in stroke patients in Phramongkutklao Hospital. J Med Assoc Thai 2010;93(Suppl. 6):51–4.
Okeahialiam BN, Ikeme AC. Suspected incidence of aspirin resistance. West Afr J Med 2010;29:129.
Petricevic M, Biocina B, Konosic S, Gasparovic H, Siric F, Burcar I. Early post coronary artery bypass grafting platelet hyperactivity, assessed by whole blood impedance aggregometry, indicates dual antiplatelet therapy. Heart Surg Forum 2011;14:S115.
Pregowski J, Przyluski J, Karcz M, Norwa-Otto B, Kruk M, Kalinczuk L, et al. Relation of subacute stent thrombosis and resistance to acetylsalicylic acid and clopidogrel in patients with acute coronary syndrome. Insights from the ANIN Myocardial Infarction Registry. Postepy Kardiol Interwencyjnej 2010;6:154–60.
Sinzinger H, Kritz H, Berent R, Schmid P, Steinbrenner D. Increased inflammatory activity rather than platelet function predicts events during acetylsalicylic acid (ASA) therapy for secondary prevention – a 10 years follow-up. Proceedings of the 20th International Congress on Thrombosis, Athens, 25–28 June 2008. pp. 87–91.
Tereshchenko OI. [Thrombelastogram in patients with coronary arteriosclerosis and auricular fibrillation.] Vrach Delo 1974;0:78–9.
Tulecki L, Gburek T. Aspirin resistance after cardiosurgical operations – Current review. Pol Prz Chir 2006;78:1193–204.
Wong S, Lewis D. Resistance to antiplatelet therapy: fact or fiction? N Z Med J 2005;118:U1459.
Appendix 6 Excluded articles for prognostic/diagnostic utility systematic review
| Article | Reason for exclusion |
|---|---|
| Abderrazek F, Chakroun T, Addad F, Dridi Z, Gerotziafas G, Gamra H, et al. The GPIIIa PlA polymorphism and the platelet hyperactivity in Tunisian patients with stable coronary artery disease treated with aspirin. Thromb Res 2010;125:e265–8 | D |
| Abuzahra M, Pillai M, Caldera A, Hartley WB, Gonzalez R, Bobek J, et al. Comparison of higher clopidogrel loading and maintenance dose to standard dose on platelet function and outcomes after percutaneous coronary intervention using drug-eluting stents. Am J Cardiol 2008;102:401–3 | C |
| Ahmed N, Meek J, Davies GJ. Plasma salicylate level and aspirin resistance in survivors of myocardial infarction. J Thromb Thrombolysis 2010;29:416–20 | D |
| Ahn SG, Lee SH, Sung JK, Kim JY, Yoon J. Intra-individual variability of residual platelet reactivity assessed by the VerifyNow-P2Y12 assay in patients with clopidogrel resistance after percutaneous coronary intervention. Platelets 2011;22:305–7 | C |
| Ajjan R, Storey RF, Grant PJ. Aspirin resistance and diabetes mellitus. Diabetologia 2008;51:385–90 | A |
| Aleil B, Jacquemin L, De PF, Zaehringer M, Collet JP, Montalescot G, et al. Clopidogrel 150 mg/day to overcome low responsiveness in patients undergoing elective percutaneous coronary intervention: results from the VASP-02 (Vasodilator-Stimulated Phosphoprotein-02) randomized study. JACC Cardiovasc Interv 2008;1:631–8 | C |
| Aleil B, Meyer N, Cazenave JP, Mossard JM, Gachet C. High stability of blood samples for flow cytometric analysis of VASP phosphorylation to measure the clopidogrel responsiveness in patients with coronary artery disease. Thromb Haemost 2005;94:886–7 | C, D |
| Alessi M-C, Cuisset T, Quilici J, Cohen W, Fourcade L, Grosdidier C, et al. Benefit of tailored therapy with high clopidogrel maintenance dose according to CYP2C19 genotypes in clopidogrel non responders undergoing coronary stenting for ACS. J Thromb Haemost 2011;9:48 | D |
| Alessi M-C, Cuisset T, Quilici J, Grosdidier C, Fourcade L, Gaborit B, et al. High post-treatment platelet reactivity and impaired clinical prognosis but adequate response to thienopyridine in elderly patients with unstable coronary disease. J Thromb Haemost 2011;9:791 | B, C |
| Alexander W, Price MJ, Mega JL. Platelet reactivity: The GRAVITAS trial. P T 2011;36:47 | B, C |
| Alexopoulos D, Plakomyti T-E, Xanthopoulou I. Variability and treatment of high on-prasugrel platelet reactivity in patients with initial high on-clopidogrel platelet reactivity. Int J Cardiol 2012;154:333–4 | A |
| Alfonso F, Angiolillo DJ. Platelet function assessment to predict outcomes after coronary interventions: hype or hope? J Am Coll Cardiol 2006;48:1751–4 | A |
| Almsherqi ZA, McLachlan CS, Sharef SM. More on: enhanced antiplatelet effect of clopidogrel in patients whose platelets are least inhibited by aspirin: a randomized cross-over trial. J Thromb Haemost 2006;4:1638–9 | A |
| Althoff TF, Fischer M, Knebel F, Langer E, Ziemer S, Baumann G. Elevated residual platelet reactivity to adenosine diphosphate and arachidonic acid in patients after myocardial infarction compared to patients after elective coronary stenting. Eur Heart J 2009;30:330 | D |
| Altman R, Luciardi HL, Muntaner J, Herrera RN. The antithrombotic profile of aspirin. Aspirin resistance, or simply failure? Thromb J 2004;2:1–8 | A |
| Ambrus JL, Ambrus CM, Akhter S. Aspirin ‘allergy’ and resistance. J Am Coll Cardiol 2004;44:939–40 | A |
| Anand SS. Vascular viewpoint. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Eikelboom JW, Hirsh J, Weitz J, Johnston M, Yi Q, Yusuf S. Circulation 2002;105:1650–5 | A |
| Andersen K, Hurlen M, Arnesen H, Seljeflot I. Aspirin non-responsiveness as measured by PFA-100 in patients with coronary artery disease. Thromb Res 2002;108:37–42 | D |
| Andreassi MG, Adlerstein D, Coceani M, Shehi E, Vecoli C, Sampietro T, et al. High-risk single-nucleotide polymorphisms (SNPs) and genetic score on recurrent cardiovascular events following ischaemic heart disease. Eur Heart J 2011;32:947 | B, C |
| Angiolillo DJ, Alfonso F. Platelet function testing and cardiovascular outcomes: steps forward in identifying the best predictive measure. Thromb Haemost 2007;98:707–9 | A |
| Angiolillo DJ, Bernardo E, Sabate M, Jimenez-Quevedo P, Costa MA, Palazuelos J, et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 2007;50:1541–7 | C |
| Angiolillo DJ, Firstenberg MS, Price MJ, Tummala PE, Hutyra M, Welsby IJ, et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA 2012;307:265–74 | C |
| Angiolillo DJ. Applying platelet function testing in clinical practice: What are the unmet needs? JAMA 2011;306:1260–1 | A |
| Angiolillo DJ. Tackling the diabetic platelet: is high clopidogrel dosing the answer? J Thromb Haemost 2006;4:2563–5 | A |
| Ankolekar S, Fox S, May J, Bath P. Assessing the efficacy of antiplatelet agents using remote testing of platelet P-selectin: preliminary observations from the triple antiplatelets for reducing dependency after ischaemic stroke (TARDIS) platelet sub study. Platelets 2010;21:395 | D |
| Ann S-G, Lee S-H, Sung JK, Lee J-W, Youn Y-J, Kim J-Y, et al. High post-clopidogrel platelet reactivity assessed by VerifyNow P2Y12 assay doses not predict stent thrombosis in acute coronary syndromes. J Am Coll Cardiol 2011;57(Suppl. 1):E929 | C |
| Anon. A mainstay drug underperforms. But a bedside test makes it easier to spot ‘aspirin resistance’. Heart Advisor 2004;7:3 | A |
| Anon. Aspirin resistance studied. US Pharm 1995;20:98 | A |
| Anon. Aspirin resistance: a worry? Johns Hopkins Medical Letter: Health After 50 2005;16:1–2 | A |
| Anon. I’ve been taking aspirin daily for 25 years, and clopidogrel for several years. In spite of this, I’ve had a number of heart attacks. You reported recently on people who can’t benefit from aspirin, and I think I may be one of them. What should I do? Heart Advisor 2004;7:8 | A |
| Anon. The importance of ‘aspirin resistance’. Aust J Pharm 2009;90:77 | A |
| Antoniucci D. Editorial comment: No role for triple antiplatelet therapy? J Am Coll Cardiol 2011;57:290–1 | A |
| Aradf D, Rideg O, Vorobcsuk A, Horvath IG, Komocsi A. Impact of cytochrome P450 2C19 and ABCB1 genotypes on post-clopidogrel platelet reactivity and clinical outcome. J Am Coll Cardiol 2010;56(Suppl. 1):B1–2 | C |
| Aradi D, Komocsi A, Vorobcsuk A, Rideg O, Tokes-Fuzesi M, Magyarlaki T, et al. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: systematic review and meta-analysis. Am Heart J 2010;160:543–51 | B, C |
| Aradi D, Komocsi A. Platelet function monitoring in patients on clopidogrel: what should we learn from GRAVITAS? Platelets 2012;23:167–76 | A |
| Aradi D, Rideg O, Vorobcsuk A, Magyarlaki T, Magyari B, Konyi A, et al. Justification of 150 mg clopidogrel in patients with high on-clopidogrel platelet reactivity. Eur J Clin Invest 2012;42:384–92 | C |
| Aradi D, Tokes-Fuzesi M, Paska T, Komocsi A. Monitoring the efficacy of antiplatelet therapy: all methods are equal, but some methods are more equal than others? Am Heart J 2008;155:e33 | A |
| Aradi D, Vorobcsuk A, Pinter T, Konyi A, Magyari B, Horvath IG, et al. Doubling the maintenance dose of clopidogrel in patients with high post-clopidogrel platelet reactivity after percutaneous coronary intervention: the DOSER randomized, placebo-controlled trial. Eur Heart J 2010;31:970 | B, C |
| Arai T, Endo A, Ikeda Y, Matsubara Y, Ogawa S, Ohono Y, et al. Impact of chronic kidney disease on platelet reactivity in patients with drug-eluting stent implantation. Catheter Cardiovasc Interv 2009;73:S77 | C, D |
| Araullo MLC. Aspirin resistance among patients with recurrent non-cardioembolic stroke detected by rapid platelet function analyzer. J Stroke Cerebrovasc Dis 2008;17:2–8 | D |
| Arazi HC, Doiny DG, Torcivia RS, Grancelli H, Waldman SV, Nojek C, et al. Impaired anti-platelet effect of aspirin, inflammation and platelet turnover in cardiac surgery. Interact Cardiovasc Thorac Surg 2010;10:863–7 | D |
| Ari H, Aradi D, Komocsi A, Price MJ, Cuisset T, Hazarbasanov D, et al. Efficacy and safety of platelet function-guided antiplatelet therapy: systematic review and meta-analysis. Int J Cardiol 2012;155:S49–50 | C |
| Artang R, Jensen E, Pedersen F, Frandsen NJ. Thrombelastography in healthy volunteers, patients with stable angina and acute chest pain. Thromb Res 2000;97:499–503 | D |
| Ashbrook M, Schwatz J, Heroux A, Escalante V, Jeske W, Walenga J, et al. Platelet function in patients with a left ventricular assist device. J Thromb Haemost 2011;9:344 | D |
| Awidi A, Saleh A, Dweik M, Kailani B, Abu-Fara M, Nabulsi R, et al. Measurement of platelet reactivity of patients with cardiovascular disease on-treatment with acetyl salicylic acid: a prospective study. Heart Vessels 2011;26:516–22. [Erratum published in Heart Vessels 2011;26:523] | D |
| Aydinalp A, Atar I, Altin C, Gulmez O, Atar A, Acikel S, et al. Platelet function analysis with two different doses of aspirin. Turk Kardiyol Dernegi Ars 2010;38:239–43 | D |
| Aydinalp A, Atar I, Gulmez O, Atar A, Acikel S, Bozbas H, et al. The clinical significance of aspirin resistance in patients with chest pain. Clin Cardiol 2010;33:E1–7 | D |
| Bach R, Jung F, Kohsiek I, Ozbek C, Spitzer S, Scheller B, et al. Factors affecting the restenosis rate after percutaneous transluminal coronary angioplasty. Thromb Res 1994;74(Suppl. 1):55–67 | D |
| Balduini CL, Noris P, Bertolino G, Previtali M. Heparin modifies platelet count and function in patients who have undergone thrombolytic therapy for acute myocardial infarction. Thromb Haemost 1993;69:522–3 | D |
| Baluda VP, Lakin KM, Pabvlishchuk SA, Deianov I, Nedelkovskii I. [Aspirin sensitivity of healthy persons and patients.] Kardiologiia 1981;21:55–7 | C, D |
| Banti A, Mosialos L, Zarifis J, Kaprinis I, Vassara C, Kiskinis D, et al. Pharmacological resistance to clopidogrel (PRC) status is modified in time after percutaneous coronary intervention. A prospective study with repeated point of care monitoring with multiplate analyser. J Thromb Haemost 2009;7:587 | C |
| Barbano G, Solera L. [On the control of anticoagulant therapy in myocardial infarct. Thromboelastography and quick’s time: considerations and comparisons.] Minerva Med 1964;55:612–18 | B |
| Barkagan ZS, Chernenko VF. [The significance of thromboelastography in obliterating diseases of the arteries of the extremities.] Khirurgiia (Mosk) 1969;45:60–5 | B |
| Barragan P, Paganelli F, Camoin-Jau L, Bourguet N, Boulay-Moine D, Moulard M, et al. Validation of a novel ELISA-based VASP whole blood assay to measure P2Y12-ADP receptor activity. Thromb Haemost 2010;104:410–11 | C |
| Basili S, Pignatelli P, Carnevale R, Di SS, Loffredo L, Violi F. PFA-100 A reliable tool to evaluate patients compliance to aspirin therapy. J Thromb Haemost 2009;7:883 | D |
| Bates ER, Lau WC. Controversies in antiplatelet therapy for patients with cardiovascular disease. Circulation 2005;111:e267–71 | A |
| Beard K, Folia C, Liovas A. Platelet function tests are highly variable when used to identify patients resistant to clopidogrel. Value Health 2010;13:A342 | B, C |
| Beer JH. Aspirin resistance for clinicians. Curr Hematol Rep 2004;3:149–50 | A |
| Behr T, Kuch B, Behr W, von Scheidt W. Optimizing of thienopyridine therapy by multiple electrode platelet aggregometry in clopidogrel low responders undergoing PCI. Clin Res Cardiol 2011;100:907–14 | C |
| Beigel R, Herscovici R, Fefer P, Hod H, Matetzky S. The anti-platelet effect of prasugrel as compared with clopidogrel in ST-elevation acute myocardial infarction patients undergoing primary angioplasty. J Am Coll Cardiol 2012;59(Suppl. 1):E480 | C |
| Berent R, Sinzinger H. ‘Aspirin – resistance’? A few critical considerations on definition, terminology, diagnosis, clinical value, natural course of atherosclerotic disease, and therapeutic consequences. Vasa 2011;40:429–38 | A |
| Berger CT, Wolbers M, Meyer P, Daikeler T, Hess C. High incidence of severe ischaemic complications in patients with giant cell arteritis irrespective of platelet count and size, and platelet inhibition. Rheumatology 2009;48:258–61 | C |
| Berger PB. Resistance to antiplatelet drugs: is it real or relevant? Catheter Cardiovasc Interv 2004;62:43–5 | A |
| Berglund U, Wallentin L. Effects of ticlopidine on platelet function and on coronary insufficiency in patients with angina pectoris. Adv Prostaglandin Thromboxane Leukot Res 1985;13:277–80 | D |
| Berkowitz SD, Frelinger AL, III, Hillman RS. Progress in point-of-care laboratory testing for assessing platelet function. Am Heart J 1998;136:S51–65 | A |
| Bernal ME, Saban RJ. [Aspirin resistance.] Med Clin 2005;124:30–6 | A |
| Bernlochner I, Byrne RA, Kastrati A, Sibbing D. The future of platelet function testing to guide therapy in clopidogrel low and enhanced responders. Exp Rev Cardiovasc Ther 2011;9:999–1014 | A |
| Bertrand OF, Rodes-Cabau J, Rinfret S, Larose E, Bagur R, Proulx G, et al. Impact of final activated clotting time after transradial coronary stenting with maximal antiplatelet therapy. Am J Cardiol 2009;104:1235–40 | C |
| Bezborod’ko BN, Batrak AA. [Thromboelastographic study of whole blood and oxalate plasma in patients with coronary arteriosclerosis in the ischemic stage.] Ter Arkh 1971;43:53–5 | C |
| Bhatt DL. Aspirin resistance: more than just a laboratory curiosity. J Am Coll Cardiol 2004;43:1127–9 | A |
| Bilsel T, Akbulut T, Yesilcimen K, Terzi S, Sayar N, Dayi SU, et al. Single high-dose bolus tirofiban with high-loading-dose clopidogrel in primary coronary angioplasty. Heart Vessels 2006;21:102–7 | C |
| Biondi-Zoccai G, Lotrionte M. Aspirin resistance in cardiovascular disease. BMJ 2008;336:166–7 | A |
| Bisdas T, Haverich A, Teebken OE. Letter by Bisdas, et al. regarding article, ‘Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting: the clopidogrel after surgery for coronary artery disease (CASCADE) trial’. Circulation 2011;124:e194–6 | A |
| Blatt PM, Roberts HR. Letter: Platelet-function tests: predictive value. New Engl J Med 1975;293:611 | A |
| Bochenek T, Wilczynski M, Wita K, Lelek M, Trusz-Gluza M, Bochenek A. [Evaluation of platelet response to antiplatelet drugs. An important clue for cardiologists and cardiosurgeons.] Kardiol Pol 2007;65:1266–9 | A |
| Bocks M, Majahalme N, Russell M. Aspirin non-responsiveness, genetic polymorphisms in platelet aggregation, and the risk of thrombus formation in children with congenital heart disease. Catheter Cardiovasc Interv 2010;76:S14–15 | B |
| Bonello L, Camoin-Jau L, Armero S, Com O, Arques S, Burignat-Bonello C, et al. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. Am J Cardiol 2009;103:5–10 | C |
| Bonello L, Camoin-Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008;51:1404–11 | C |
| Bonello L, Camoin-Jau L, Dignat-George F, Paganelli F. A threshold of platelet reactivity for ischaemic events? Eur Heart J 2008;29:2185–6 | A |
| Bonello L, Harouri K, Sabatier F, Camoin-Jau L, Arnaud L, Baumstarck-Barrau K, et al. Level of ADP receptor P2Y12 blockade during percutaneous coronary intervention predicts the extent of endothelial injury, assessed by CEC measurement. Eur Heart J 2010;31:976 | B, C |
| Bonello L, Lemesle G, De Labriolle A, Roy P, Steinberg DH, Pinto Slottow TL, et al. Impact of a 600 mg loading dose of clopidogrel on 30-day outcome in unselected patients undergoing percutaneous coronary intervention. Am J Cardiol 2008;102:1318–22 | C |
| Bonello L, Paganelli F, Arpin-Bornet M, Auquier P, Sampol J, Dignat-George F, et al. Vasodilator-stimulated phosphoprotein phosphorylation analysis prior to percutaneous coronary intervention for exclusion of postprocedural major adverse cardiovascular events. J Thromb Haemost 2007;5:1630–6 | C |
| Bonello L, Pansieri M, Mancini J, Bonello R, Maillard L, Barnay P, et al. High on-treatment platelet reactivity after prasugrel loading dose and cardiovascular events after percutaneous coronary intervention in acute coronary syndromes. J Am Coll Cardiol 2011;58:467–73 | C |
| Bonten T, Snoep JD, Assendelft WJ, van der Meer V, Zwaginga JJ, Rosendaal FR, et al. Aspirin am or pm? A randomized cross-over trial. J Thromb Haemost 2011;9:334–5 | A |
| Boos CJ, Lip GY. Platelet activation and cardiovascular outcomes in acute coronary syndromes. J Thromb Haemost 2006;4:2542–3 | A |
| Bouman HJ, Breet NJ, Van Werkum JW, Zwart B, Ten CH, Hackeng CM, et al. Platelet reactivity in patients with coronary stent thrombosis. Eur Heart J 2010;31:973 | D |
| Bouman HJ, van Werkum JW, Breet NJ, Ten CH, Hackeng CM, ten Berg JM. A case–control study on platelet reactivity in patients with coronary stent thrombosis. J Thromb Haemost 2011;9:909–16 | D |
| Bradbury J. Aspirin resistance may increase death risk in some patients with heart disease. Lancet 2002;359:1128 | A |
| Brar S, ten Berg J, Marcucci R, Price MJ, Valgimigli M, Kim H-S, et al. Impact of clopidogrel on-treatment platelet reactivity on stent thrombosis after percutaneous coronary intervention: results from a collaborative meta-analysis of individual participant data. J Am Coll Cardiol 2011;57(Suppl. 1):E1632 | C |
| Brar SS, ten Berg J, Marcucci R, Price MJ, Valgimigli M, Kim HS, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol 2011;58:1945–54 | C |
| Braunwald E, Angiolillo D, Bates E, Berger PB, Bhatt D, Cannon CP, et al. Antiplatelet therapy and platelet function testing. Clin Cardiol 2008;31(Suppl. 1):I36. [Erratum published in Clin Cardiol 2009;32:111] | A |
| Breet NJ, Bouman HJ, van Werkum JW, Ruven H, Hackeng CM, Berg JMT. First prospective comparison between platelet function tests in prediction of clinical outcome in 1100 patients undergoing coronary stent placement: Do point-of-care platelet function assays predict clinical outcomes in clopidogrel pretreated patients undergoing elective PCI (POPular study). Circulation 2010;120:2153 | C |
| Breet NJ, De Jong C, Bos WJW, van Werkum JW, Bouman HJ, Kelder JC, et al. The impact of renal function on platelet reactivity and clinical outcome in patients undergoing percutaneous coronary intervention with stenting. Eur Heart J 2011;32:187 | C |
| Breet NJ, Pittens CAC, Bouman HJ, van Werkum JW, ten Berg JM, Hackeng CM. The effect of platelet reactivity on infarct related artery patency in patients with ST-elevation myocardial infarction. J Thromb Haemost 2009;7:590 | C |
| Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Hackeng CM, ten Berg JM. The relationship between platelet reactivity and infarct-related artery patency in patients presenting with a ST-elevation myocardial infarction. Thromb Haemost 2011;106:331–6 | D |
| Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2010;303:754–62. [Errata published in JAMA 2010;303:1257, JAMA 2011;305:2174, JAMA 2011;305:2172–3] | C |
| Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, ten Berg JM, Hackeng CM. Do not adjust the platelet count in light transmittance aggregometry when predicting thrombotic events after percutaneous coronary intervention. J Thromb Haemost 2010;8:2326–8 | C |
| Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, ten Berg JM, Hackeng CM. Both peak and late aggregation are capable of identifying patients at risk for atherothrombotic events. Thromb Haemost 2011;105:197–9 | C |
| Breet NJ. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2011;305:2174 | C |
| Briongos FS, Salido-Tahoces L, de Juan-Baguda J, Marti-Sanchez D. [Multiple arterial thrombosis and resistance to conventional antiaggregation.] Rev Esp Cardiol 2011;64:836–7 | A |
| Brister SJ, Buchanan MR. Aspirin ‘resistance’ and its impact on cardiovascular morbidity and mortality: it is real, clinically relevant and should be measured. Heart 2009;95:1223–4 | A |
| Brizzio M, Shaw R, Sperling J, Grau J, Zapolanski A. Low responsiveness to clopidogrel therapy as a possible predictor for early failure of coronary percutaneous intervention requiring surgical revascularization. Innov Technol Tech Cardiothorac Vasc Surg 2011;6:190 | B |
| Brizzio ME, Zapolanski A, Shaw RE, Collins M, Bosticco B, Sperling JS. The utilization of platelet inhibition tests in patients who receive preoperative clopidogrel reduces the waiting time for elective coronary surgery. Innov Technol Tech Cardiothorac Vasc Surg 2010;5:193 | B |
| Broussalis E, Killer-Oberpfalzer M. Is multiple electrode platelet aggregometry beneficial in the management of carotid artery stenting? Intervent Neuroradiol 2011;17:210 | B, C |
| Buchanan MR. Acetylsalicylic acid resistance and clinical outcome – the Hobikoglu study is worth noting. Can J Cardiol 2007;23:207–8 | A |
| Bugnicourt JM, Roussel B, Garcia PY, Canaple S, Lamy C, Godefroy O. Aspirin non-responder status and early neurological deterioration: a prospective study. Clin Neurol Neurosurg 2011;113:196–201 | D |
| Buonamici P, Marcucci R, Migliorini A, Gensini GF, Santini A, Paniccia R, et al. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol 2007;49:2312–17 | C |
| Cairns JA, Eikelboom J. The pursuit of clinically relevant measures of platelet function after antiplatelet drug therapy. J Am Coll Cardiol 2008;52:1978–80 | A |
| Calviere L, Voisin S, Coulon G, Viguier A, Sie P, Larrue V. Prevalence of low responsiveness to clopidogrel in patients with stroke or TIA. Cerebrovasc Dis 2011;31:259 | D |
| Camilleri E, Jacquin L, Paganelli F, Bonello L. Personalized antiplatelet therapy: review of the latest clinical evidence. Curr Cardiol Rep 2011;13:296–302 | A |
| Camoin-Jau L, Bonello L, Armero S, Com O, Barragan P, Paganelli F, et al. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. J Thromb Haemost 2009;7:589 | C |
| Campo G, Ferraresi P, Marchesini J, Bernardi F, Valgimigli M. Relationship between paraoxonase Q192R gene polymorphism and on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention. J Thromb Haemost 2011;9:2106–8 | C |
| Campo G, Parrinello G, Ferraresi P, Lunghi B, Tebaldi M, Miccoli M, et al. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol 2011;57:2474–83 | C |
| Campo G, Valgimigli M, Gemmati D, Percoco G, Tognazzo S, Cicchitelli G, et al. Value of platelet reactivity in predicting response to treatment and clinical outcome in patients undergoing primary coronary intervention: insights into the STRATEGY Study. J Am Coll Cardiol 2006;48:2178–85 | C |
| Can MM, Tanboga IH, Turkyilmaz E, Karabay CY, Akgun T, Koca F, et al. The risk of false results in the assessment of platelet function in the absence of antiplatelet medication: comparison of the PFA-100, multiplate electrical impedance aggregometry and verify now assays. Thromb Res 2010;125:e132–7 | D |
| Cao J, Li XL, Fan L, Ye L. Single nucleotide polymorphisms of Ho-1 and Cox-1 are associated with complete aspirin resistance defined by light transmittance aggregation in the elderly. Heart 2011;97(Suppl. 3):A188 | D |
| Capanni M, Prisco D, Antonucci E, Chiarugi L, Boddi V, Abbate R, et al. The pre-procedural platelet state predicts clinical recurrence after coronary angioplasty. Int J Clin Lab Res 1999;29:145–9 | C |
| Capodanno D, Patel A, Dharmashankar K, Ferreiro JL, Ueno M, Kodali M, et al. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv 2011;4:180–7 | D |
| Caron N, Rivard GE, Michon N, Morin F, Pilon D, Moutquin JM, et al. Low-dose ASA response using the PFA-100 in women with high-risk pregnancy. J Obstet Gynaecol Can 2009;31:1022–7 | B, D |
| Carrie D, Garcia C, Gratacap MP, Voisin S, Payrastre B, Sie P. Assessment of platelet response to clopidogrel through measurement of ADP-induced Akt phosphorylation. J Thromb Haemost 2009;7:1411–13 | C, D |
| Casassus F, Leroux L, James C, Sidibe S, Desplantes A, Naibo D, et al. Response to prasugrel in ST elevation myocardial infarction patients: Is it as rapid as we expected? J Am Coll Cardiol 2011;58(Suppl. 1):B43–4 | B, C |
| Cassiano O, Mairano C. [Considerations and research on the relations between chronic peripheral arteriopathy and blood coagulation.] Minerva Cardioangiol 1963;11:510–15 | B |
| Cattaneo M. [Tailored treatment with clopidogrel based on the results of platelet function tests should not be implemented, in the absence of clear indications on the methodology to be used.] G Ital Cardiol 2011;12:172–3 | B, C |
| Cattaneo M. Laboratory detection of ‘aspirin resistance’: what test should we use (if any)? Eur Heart J 2007;28:1673–5 | A |
| Cayla G, Macia JC, Rabesandratana H, Roubille F, Gervasoni R, Pasquie JL, et al. Flow cytometric assessment of vasodilator-stimulated phosphoprotein: prognostic value of recurrent cardiovascular events after acute coronary syndromes. Arch Cardiovasc Dis 2008;101:743–51 | C |
| Cepelakova H, Cepelak V, Sova J. [Prognostic significance of some coagulation changes in myocardial infarct.] Vnitr Lek 1973;19:478–86 | B |
| Chen H. Randomized comparison of adjunctive Naoxintong versus standard maintenance dose clopidogrel in patients with CYP2C19*2 gene mutation: results of the ABNJC-CYP2C19*2 (Adjunctive Buchang Naoxintong Jiaonang Versus Standard Maintenance Dose Clopidogrel in Patients with CYP2C19*2 Gene Mutation) randomized study. Int J Cardiol 2011;152:S26 | B, C |
| Chen L, Bracey AW, Radovancevic R, Cooper JR, Jr, Collard CD, Vaughn WK, et al. Clopidogrel and bleeding in patients undergoing elective coronary artery bypass grafting. J Thorac Cardiovasc Surg 2004;128:425–31 | C, D |
| Chen W-H, Lee P-Y, Ng W, Kwok JYY, Cheng X, Lee SWL, et al. Relation of aspirin resistance to coronary flow reserve in patients undergoing elective percutaneous coronary Intervention. Am J Cardiol 2005;96:760–3 | D |
| Chen W-H, Lee P-Y, Ng W, Tse H-F, Lau C-P. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol 2004;43:1122–6 | D |
| Chen W-H, Lee P-Y. Reply to ‘comments in response to low-dose aspirin increases aspirin resistance in patients with coronary artery disease’. Am J Med 2006;119:287–8 | A |
| Chen YW, Lee KY, Li CH, Lin YY, Yang CD, Hsieh CF, et al. Difference in platelet function test could predict prognosis in the acute stage of ischemic stroke. Cerebrovasc Dis 2011;31:281–2 | D |
| Chen ZQ. Influence of Ranitidine on gastrointestinal hemorrhage and thrombosis caused by dual antiplatelet therapy after PCI in patients with coronary heart disease. Cardiology 2010;117:48–9 | C |
| Chiesa FT. [Thromboelastography in coronary heart disease: evaluations provided of the blood coagulation situation; indications for therapy.] G Gerontol 1971;19:800–4 | B |
| Choi DH, Suh JW, Park KW, Kang HJ, Kim HS. Assessment of the bioequivalence of brand and biogeneric formulations of abciximab for the treatment of acute coronary syndrome: a prospective, open-label, randomized, controlled study in Korean patients. Clin Ther 2009;31:1804–11 | B, C |
| Cholette JM, Mamikonian L, Alfieris GM, Blumberg N, Lerner NB. Aspirin resistance following pediatric cardiac surgery. Thromb Res 2010;126:200–6 | B |
| Chooi JL, Vesamia SA, Gama CA, Szecowka-Harte SR, Brown V. Thromboelastography in prediction of blood loss after cardiac surgery. Eur J Anaesthesiol 2011;28:84 | D |
| Chow SL, Cheung RJ. Aspirin resistance: a growing concern. Formulary 2006;41:192–201 | A |
| Christiaens L, Allal J, Brizard A, Macchi L. Aspirin resistance: a reality? Med Ther 2003;9:181–6 | A |
| Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost 2010;8:148–56 | B, C, D |
| Clappers N, van Oijen MG, Sundaresan S, Brouwer MA, Te Morsche RH, Keuper W, et al. The C50T polymorphism of the cyclooxygenase-1 gene and the risk of thrombotic events during low-dose therapy with acetyl salicylic acid. Thromb Haemost 2008;100:70–5 | C |
| Cocozza M, Milani M, Picano T, Oliviero U, Russo N, Coto V. Antiaggregatory effects of picotamide in long-term treatment: a 2-year, double-blind placebo-controlled trial. Vasc Med 1997;2:292–5 | B |
| Cohen AH, Doiny D, Waldman SV, Spampinato TR, Grancelli H, Miriuka S, et al. Association between reduced aspirin inhibition and platelet turnover in cardiac surgery. Eur Heart J 2009;30:916–17 | D |
| Cohen M, Merino A, Hawkins L, Greenberg S, Fuster V. Clinical and angiographic characteristics and outcome of patients with rest-unstable angina occurring during regular aspirin use. J Am Coll Cardiol 1991;18:1458–62 | C |
| Collet JP, Cayla G, Cuisset T, Elhadad S, Range G, Vicaut E, et al. Randomized comparison of platelet function monitoring to adjust antiplatelet therapy versus standard of care: rationale and design of the assessment with a double randomization of a fixed dose versus a monitoring-guided dose of aspirin and clopidogrel after DES implantation, and treatment interruption versus continuation, 1 year after stenting (ARCTIC) study. Am Heart J 2011;161:5–12 | A |
| Collet J-P, Hulot JS, Pena ANA, Vilard ERIC, Esteve JB, Cayla G, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: impact on clinical events and on. Eur Heart J 2009;30:906 | B, C |
| Combescure C, Fontana P, Mallouk N, Berdague P, Labruyere C, Barazer I, et al. Clinical implications of clopidogrel non-response in cardiovascular patients: a systematic review and meta-analysis. J Thromb Haemost 2010;8:923–33 | C |
| Conti CR. Is aspirin resistance a risk factor? Clin Cardiol 2005;28:163–4 | A |
| Cordina SM, Vazquez G, Suri MF, Lakhsminarayan K, Qureshi Z. Does aspirin failure increase the chances of recurrent stroke and/or death among patients with ischemic stroke: a pooled analysis of two prospective trials. Stroke 2010;41:e214 | C |
| Cotton JM, Worrall AM, Hobson AR, Smallwood A, Amoah V, Dunmore S, et al. Individualised assessment of response to clopidogrel in patients presenting with acute coronary syndromes: a role for short thrombelastography? Cardiovasc Ther 2010;28:139–46. [Erratum published in Cardiovasc Ther 2010;28:254. Rajendra R (corrected to Raghuraman RP)] | C |
| Cowley MJ, Kuritzky L. Developments in antiplatelet therapy for acute coronary syndromes and considerations for long-term management. Curr Med Res Opin 2009;25:1477–90 | C |
| Cox D. Aspirin resistance: a nebulous concept. J Clin Pharmacol Pharm 2010;1:39–47 | A |
| Crescente M, Di Castelnuovo A, Iacoviello L, De Gaetano G, Cerletti C. PFA-100 closure time to predict cardiovascular events in aspirin-treated cardiovascular patients: a meta-analysis of 19 studies comprising 3,003 patients. Thromb Haemost 2008;99:1129–31 | A |
| Cubero JM. Candy study: minor myocardial damage in patients with type 2 diabetes mellitus and dual hypo-responsiveness to aspirin and clopidogrel with Xience V stent implantation. J Am Coll Cardiol 2012;59(Suppl. 1):E488 | D |
| Cuisset T, Frere C, Quilici J, Gaborit B, Castelli C, Poyet R, et al. Predictive values of post-treatment adenosine diphosphate-induced aggregation and vasodilator-stimulated phosphoprotein index for stent thrombosis after acute coronary syndrome in clopidogrel-treated patients. Am J Cardiol 2009;104:1078–82 | C |
| Cuisset T, Frere C, Quilici J, Morange PE, Mouret JP, Bali L, et al. Glycoprotein IIb/IIIa inhibitors improve outcome after coronary stenting in clopidogrel nonresponders: a prospective, randomized study. JACC Cardiovasc Interv 2008;1:649–53 | C |
| Cuisset T, Frere C, Quilici J, Morange PE, Nait-Saidi L, Carvajal J, et al. Benefit of a 600 mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol 2006;48:1339–45 | C |
| Cuisset T, Frere C, Quilici J, Morange PE, Nait-Saidi L, Mielot C, et al. High post-treatment platelet reactivity is associated with a high incidence of myonecrosis after stenting for non-ST elevation acute coronary syndromes. Thromb Haemost 2007;97:282–7 | C |
| Cuisset T, Frere C, Quilici J, Poyet R, Bali L, Morange PE, et al. Glycoprotein IIbIIIa inhibitors improve clinical outcome after coronary stenting in clopidogrel non responders: a prospective, randomized study. Arch Cardiovasc Dis Suppl 2010;2:13 | C |
| Cuisset T, Hamilos M, Sarma J, Sarno G, Wyffels E, Vanderheyden M, et al. Relation of low response to clopidogrel assessed with point-of-care assay to periprocedural myonecrosis in patients undergoing elective coronary stenting for stable angina pectoris. Am J Cardiol 2008;101:1700–3 | D |
| Cuisset T, Morange P-E, Alessi M-C. High residual platelet reactivity and thrombotic events. JAMA 2011;306:2561 | A, B, C |
| Cuisset T, Quilici J, Fugon L, Cohen W, Roux P, Gaborit B, et al. Non-adherence to aspirin in patients undergoing coronary stenting: negative impact of comorbid conditions and implications for clinical management. Arch Cardiovasc Dis 2011;104:306–12 | D |
| Cuisset T, Quilici J, Grosdidier C, Fourcade L, Gaborit B, Pankert M, et al. Comparison of platelet reactivity and clopidogrel response in patients ≤ 75 years versus > 75 years undergoing percutaneous coronary intervention for non-ST-segment elevation acute coronary syndrome. Am J Cardiol 2011;108:1411–16 | C |
| Curzen N, Sambu N. Interventional cardiology: Antiplatelet therapy in percutaneous coronary intervention: is variability of response clinically relevant? Heart 2011;97:1433–40 | A |
| Dai Y, Lee A, Critchley LA, White PF. Does thromboelastography predict postoperative thromboembolic events? A systematic review of the literature. Anesth Analg 2009;108:734–42 | B, C |
| Dalal AR, D’Souza S, Shulman MS. Brief review: coronary drug-eluting stents and anesthesia. Can J Anaesth 2006;53:1230–43 | C |
| Dalal JJ, Bloom A, Henderson AH. Transmyocardial platelet behavior in CAD. Circulation 1981;63:969–70 | B, C, D |
| Dalal JJ, Penny WJ, Saunders KC, Sheridan DJ, Bloom AL, Henderson AH. Platelet counts and aggregates in coronary artery disease. Eur Heart J 1982;3:107–13 | C, D |
| Dale J, Myhre E, Loew D. Bleeding during acetylsalicylic acid and anticoagulant therapy in patients with reduced platelet reactivity after aortic valve replacement. Am Heart J 1980;99:746–52 | C |
| Dalen JE. Aspirin resistance: is it real? Is it clinically significant? Am J Med 2007;120:1–4 | A |
| Dalen JE. Is aspirin resistance due to noncompliance? Arch Intern Med 2008;168:550 | A |
| Dalen M, Van Der Linden J, Lindvall G, Ivert T. Correlation between point-of-care platelet function testing and bleeding after coronary artery surgery. Scand Cardiovasc J 2012;46:32–8 | D |
| D’Andrea D, Furbatto F, Boccalatte M, Scarpelli M. Use of point-of-care testing to assess optimal antiplatelet therapy when a nasogastric tube is the only way of administration. Int J Cardiol 2011;147:S67 | D |
| Darcy MD, Kanterman RY, Kleinhoffer MA, Vesely TM, Picus D, Hicks ME, et al. Evaluation of coagulation tests as predictors of angiographic bleeding complications. Radiology 1996;198:741–4. | C |
| Davlouros PA, Arseniou A, Hahalis G, Chiladakis J, Mazarakis A, Damelou A, et al. Timing of clopidogrel loading before percutaneous coronary intervention in clopidogrel-naive patients with stable or unstable angina: a comparison of two strategies. Am Heart J 2009;158:585–91 | C |
| Dawson J, Quinn TJ, Rafferty M, Ray G, Walters MR, Lees KR. Aspirin resistance in patients with recent stroke: a case–control study. Cerebrovasc Dis 2009;27:45 | D |
| De Gaetano G, Cerletti C. Aspirin resistance: a revival of platelet aggregation tests? J Thromb Haemost 2003;1:2048–50 | A |
| De Gaetano G. Aspirin resistance in diabetic patients. Diabetes Care 2004;27:1244–5 | A |
| De Matteis F, Barbano G. [The thrombelastogram in coronary disease.] Minerva Med 1963;54:194–202 | B, D |
| de Miguel CA, Cuellas RC, Diego NA, Samaniego LB, Alonso RD, Fernandez VF, et al. Post-treatment platelet reactivity predicts long-term adverse events better than the response to clopidogrel in patients with non-ST-segment elevation acute coronary syndrome. Rev Esp Cardiol 2009;62:126–35 | C |
| de Miguel Castro A, Diego Nieto A, Perez de Prado A. Letter by de Miguel Castro, et al. regarding article, ‘cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up’. Circulation 2009;120:e98 | A |
| De Miguel Castro A, Nieto AD, Perez de Prado A. Letter by De Miguel Castro, et al. regarding article, ‘Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the gauging responsiveness with a VerifyNow P2Y12 assay: impact on Thrombosis and Safety (GRAVITAS) trial’. Circulation 2012;125:e570 | A |
| De Rosa R, Galasso G, Piscione F, Santulli G, Iaccarino G, Piccolo R, et al. Increased risk of cardiovascular events associated with the GPIIIA PlA2 polymorphism. Cardiovasc Res 2010;87:S74–5 | C |
| Dekhtiar’ AL, Synovets AS. [Thromboelastogram data in patients with thromboobliterating diseases of the vessels.] Vrach Delo 1967;5:45–7 | C, D |
| Deliargyris EN, Boudoulas H. Aspirin resistance. Hell J Cardiol 2004;45:1–5 | A |
| Dengler R, Lichtinghagen R, Schumacher H, Weissenborn K, Worthmann H. C-reactive protein and its pharmacologic reduction as predictor of outcome in the early treatment with aspirin and extended-release dipyridamole versus low dose aspirin alone for TIA/ischemic stroke within 24 hour of symptom-onset (EARLY-Trial). Stroke 2010;41:e261 | C |
| D’Erasmo E, Aliberti G, Celi FS, Vecci E, Mazzuoli G. [Sequential evaluation of platelet count and mean platelet volume during myocardial infarction.] Medicina 1988;8:58–60 | B, D |
| D’Erasmo E, Aliberti G, Celi FS, Vecci E, Romagnoli E, Mazzuoli G. [Changes in several blood platelet parameters in cerebral infarct.] Recenti Prog Med 1988;79:12–14 | B, C, D |
| D’Erasmo E. [Relationship between number and volume of circulating blood platelets in cerebral and myocardial infarct.] Recenti Prog Med 1990;81:675–6 | A, C, D |
| Dhatariya KK. Aspirin for everyone over 50? Don’t forget aspirin resistance. BMJ 2005;331:161 | A |
| Di Giovanni V, Agrifoglio G. [Thrombelastographic observations in the postoperative period and its value in the prevention of thrombophletitic complications.] Minerva Chir 1963;18:299–311 | B, D |
| Di Minno G, Violi F. Aspirin resistance and diabetic angiopathy: back to the future. Thromb Res 2004;113:97–9 | A |
| Di Sciascio G, Patti G, Pasceri V, Gatto L, Colonna G, Montinaro A, et al. Effectiveness of in-laboratory high-dose clopidogrel loading versus routine pre-load in patients undergoing percutaneous coronary intervention: results of the ARMYDA-5 PRELOAD (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) randomized trial. J Am Coll Cardiol 2010;56:550–7 | C |
| Dietrich GV, Schueck R, Menges T, Kiesenbauer NP, Fruehauf AC, Marquardt I. Comparison of four methods for the determination of platelet function in whole blood in cardiac surgery. Thromb Res 1998;89:295–301 | B, D |
| Dmoszynska-Giannopoulou A, Pluta A, Kowalewski J. [Platelet function and lipid disorders in diabetics.] Pol Arch Med Wewn 1979;62:293–7 | B, D |
| Dmoszynska-Giannopoulou A. [Function and coagulative activity of blood platelets in patients with attacks of transient cerebral ischemia.] Polski Tygodnik Lekarski 1984;39:1569–71 | B, D |
| Doraiswamy VA. Letter by Doraiswamy regarding article, ‘Intensifying platelet inhibition with tirofiban in poor responders to aspirin, clopidogrel, or both agents undergoing elective coronary intervention: results from the double-blind, prospective, randomized Tailoring Treatment with Tirofiban in Patients Showing Resistance to Aspirin and/or Resistance to Clopidogrel study’. Circulation 2010;121:e235 | A |
| Drouet L, Bal dit SC, Henry P. [The basis of platelets: platelets and atherothrombosis: an understanding of the lack of efficacy of aspirin in peripheral arterial disease (PAD) and diabetic patients.] Drugs 2010;70(Suppl 1.):9–14 | A |
| Drouet L. [Is aspirin resistance a reality?] Rev Med Interne 2004;25:101–3 | A |
| Drzewoski J, Watala C. Is aspirin resistance a real problem in people with type 2 diabetes? Diabetes Care 2004;27:1245–6 | D |
| d’Udekem Y. The devastating consequences of thrombosis of systemico pulmonary shunts and the blind administration of aspirin. Thromb Res 2010;126:163 | A |
| Dyszkiewicz-Korpanty AM, Kim A, Burner JD, Frenkel EP, Sarode R. Comparison of a rapid platelet function assay – Verify Now Aspirin – with whole blood impedance aggregometry for the detection of aspirin resistance. Thromb Res 2007;120:485–8 | B, D |
| Eikelboom J, Oldgren J, Reilly P, Yusuf S, Wallentin L, Ezekowitz M, et al. No evidence of platelet activation in patients with atrial fibrillation who are treated with dabigatran: a substudy of the rely trial. J Thromb Haemost 2011;9:346 | D |
| Eikelboom J. Aspirin resistance: does it exist and is it an important clinical problem? Clin Adv Haematol Oncol 2006;4:268–70 | A |
| Eikelboom JW, Emery J, Hankey GJ. The use of platelet function assays may help to determine appropriate antiplatelet treatment options in a patient with recurrent stroke on baby aspirin: against. Stroke 2010;41:2398–9 | A |
| Eikelboom JW, Hankey GJ. Aspirin resistance: a new independent predictor of vascular events? J Am Coll Cardiol 2003;41:966–8 | A |
| El Ghannudi S, Ohlmann P, Jesel L, Radulescu B, El Adraa E, Crimizade U, et al. Impaired inhibition of P2Y(12) by clopidogrel is a major determinant of cardiac death in diabetes mellitus patients treated by percutaneous coronary intervention. Atherosclerosis 2011;217:465–72 | C |
| El Ghannudi S, Ohlmann P, Meyer N, Wiesel ML, Radulescu B, Chauvin M, et al. Impact of P2Y12 inhibition by clopidogrel on cardiovascular mortality in unselected patients treated by percutaneous coronary angioplasty: a prospective registry. JACC Cardiovasc Interv 2010;3:648–56 | C |
| El Ghannudi S, Ohlmann P, Wiesel ML, Radulescu B, Bareiss P, Chauvin M, et al. Impact of platelet poor responsiveness to clopidogrel on cardiovascular outcome in type 2 Diabetes mellitus patients treated by percutaneous coronary angioplasty. Eur Heart J 2009;30:194 | B, C |
| El Ghannudi S, Ohlmann P, Wiesel ML, Radulescu B, Bareiss P, Chauvin M, et al. Platelet reactivity assessed by flow cytometric VASP phosphorylation is an independent predictor of death and cardiovascular death in unselected patients undergoing PCI. Eur Heart J 2009;30:192–3 | B, C |
| Lu TQ, Mu DJ, Tang YD, Chen J, Yuan JQ, Wu YJ, et al. The clopidogrel response is associated with clinical outcomes of the CHD patients who underwent elective PCI. Cardiology 2010;117:58–9 | B |
| Elwood P, Morgan G. Aspirin resistance: what is the risk of cardiovascular morbidity? BMJ 2008;336:291 | A |
| Elwood PC, Beswick A, Pickering J, McCarron P, O’Brien JR, Renaud SR, et al. Platelet tests in the prediction of myocardial infarction and ischaemic stroke: evidence from the Caerphilly Prospective Study. Br J Haematol 2001;113:514–20 | C |
| Elwood PC, Renaud S, Beswick AD, O’Brien JR, Sweetnam PM. Platelet aggregation and incident ischaemic heart disease in the Caerphilly cohort. Heart 1998;80:578–82 | B |
| Elwood PC, Renaud S, Sharp DS, Beswick AD, O’Brien JR, Yarnell JW. Ischemic heart disease and platelet aggregation. The Caerphilly Collaborative Heart Disease Study. Circulation 1991;83:38–44 | B |
| Eng M, Hudson PA, Endemann S, Barker C, Williams M, Levisay J, et al. Low on-treatment reactivity with clopidogrel does not significantly impact bleeding complications in patients receiving dual anti-platelet therapy. J Am Coll Cardiol 2011;57(Suppl. 1):E1635 | C |
| Epimakhova OV, Sumarokov AV. [Functional activity of thrombocytes in myocardial diseases.] Kardiologiia 1983;23:32–5 | B, C, D |
| Erlinge D, Berg JT, Foley D, Angiolillo D, Brown P, Wagner H, et al. Prasugrel 5 mg in low body weight patients reduces platelet reactivity to a similar extent as prasugrel 10 mg in higher body weight patients: results from the feather trial. J Am Coll Cardiol 2012;59(Suppl. 1):E341 | C |
| Esposito G. Responsiveness to P2Y12 receptor inhibitors. Curr Opin Cardiol 2011;26(Suppl. 1):S31–7 | A |
| Eto K, Ochiai M, Isshiki T, Takeshita S, Terakura M, Sato T, et al. Platelet aggregability under shear is enhanced in patients with unstable angina pectoris who developed acute myocardial infarction. Jpn Circ J 2001;65:279–82 | D |
| Ettinger MG. Thromboelastographic studies in cerebral infarction. Stroke 1974;5:350–4 | B, D |
| Lindhoff-Last E. Non-compliance and genetic polymorphisms: when patients do not respond to ASA or clopidogrel. Dtsch Apoth Ztg 2010;150:59–60 | A |
| Fang H. The effect of CYP2C19*17 on platelet response and clinical outcomes in patients taking clopidogrel: a meta-analysis. Ther Drug Monit 2011;33:479 | C |
| Faraday N, Braunstein JB, Heldman AW, Bolton ED, Chiles KA, Gerstenblith G, et al. Prospective evaluation of the relationship between platelet–leukocyte conjugate formation and recurrent myocardial ischemia in patients with acute coronary syndromes. Platelets 2004;15:9–14 | D |
| Farsad F, Safara E, Bakhshandeh H, Golpira R, Zavarehee A, Hashemian F. Assessment of clopidogrel resistance by platelet function test: optical aggregometry in ischemic heart disease patients undergoing coronary intervention. J Am Pharm Assoc 2011;51:218 | B, C |
| Fattorutto M, Pradier O, Schmartz D, Ickx B, Barvais L. Does the platelet function analyser (PFA-100) predict blood loss after cardiopulmonary bypass? Br J Anaesth 2003;90:692–3 | B, D |
| Fefer P, Beigel R, Shenkman B, Gannot S, Varon D, Savion N, et al. Evaluation of platelet response to different clopidogrel dosing regimens in patients with acute coronary syndrome in clinical practice. J Am Coll Cardiol 2012;59(Suppl. 1):E484 | B, C, D |
| Feher G, Feher A, Pusch G, Koltai K, Tibold A, Gasztonyi B, et al. Clinical importance of aspirin and clopidogrel resistance. World J Cardiol 2010;2:171–86 | A |
| Feher G, Koltai K, Papp E, Alkonyi B, Solyom A, Kenyeres P, et al. Aspirin resistance: possible roles of cardiovascular risk factors, previous disease history, concomitant medications and haemorrheological variables. Drugs Aging 2006;23:559–67 | D |
| Feher G, Pusch G, Szapary L. Optical aggregometry and aspirin resistance. Acta Neurol Scand 2009;119:139 | A |
| Ferraris VA, Ferraris SP, Joseph O, Wehner P, Mentzer RM, Jr. Aspirin and postoperative bleeding after coronary artery bypass grafting. Ann Surg 2002;235:820–7 | D |
| Ferreiro JL, Sibbing D, Angiolillo DJ. Platelet function testing and risk of bleeding complications. Thromb Haemost 2010;103:1128–35 | A |
| Fisher M, Johns T. Letter by Fisher and Johns regarding article, ‘Variable platelet response to aspirin and clopidogrel in atherothrombotic disease’. Circulation 2007;116:e521 | A |
| Fisher M, Knappertz V. Comments in response to ‘low-dose aspirin increases aspirin resistance in patients with coronary artery disease’. Am J Med 2006;119:287–8 | A |
| Fitscha P, Kaliman J, Sinzinger H, Peskar BA. Follow-up of in-vivo platelet function (platelet half-life, thromboxane B2) in patients with coronary heart disease. Vasa 1984;13:338–42 | D |
| Fitscha P, Sinzinger H. [Defects in the prostaglandin system. VII. (Generalized, inherited [?]) cyclooxygenase defect.] Wien Klin Wochenschr 1988;100:711–15 | A |
| FitzGerald GA. Parsing an enigma: the pharmacodynamics of aspirin resistance. Lancet 2003;361:542–4 | A |
| Focks JJ, van Oijen MG, Lanas A, Verheugt F, Brouwer MA. Co-administration of proton pump inhibitors and clopidogrel: a systematic review on laboratory and clinical endpoints. Gastroenterology 2011;140(Suppl. 1):S395 | B, C |
| Fong J, Cheng-Ching E, Hussain MS, Katzan I, Gupta R. Predictors of biochemical aspirin and clopidogrel resistance in patients with ischemic stroke. J Stroke Cerebrovasc Dis 2011;20:227–30 | D |
| Fontana P, Berdague P, Castelli C, Nolli S, Barazer I, Fabbro-Peray P, et al. Clinical predictors of dual aspirin and clopidogrel poor responsiveness in stable cardiovascular patients from the ADRIE study. J Thromb Haemost 2010;8:2614–23 | D |
| Fontana P, Reber G, de Moerloose P. Assessing aspirin responsiveness using the Verify Now Aspirin assay. Thromb Res 2008;121:581–2 | B, C, D |
| Fontana P, Remones V, Reny JL, Aiach M, Gaussem P. P2Y1 gene polymorphism and ADP-induced platelet response. J Thromb Haemost 2005;3:2349–50 | B, C, D |
| Fontana P, Reny JL. Laboratory-defined aspirin resistance and recurrent cardiovascular events. Arch Intern Med 2008;168:549–50 | A |
| Foo F, Oldroyd KG. Clinical value of antiplatelet therapy in patients with acute coronary syndromes and in percutaneous coronary intervention. Biomarkers Med 2011;5:9–30 | A |
| Forestier F, Belisle S, Contant C, Harel F, Janvier G, Hardy JF. [Reproducibility and interchangeability of the Thromboelastograph, Sonoclot and Hemochron activated coagulation time in cardiac surgery.] Can J Anaesth 2001;48:902–10 | D |
| Fosburgh D. Aspirin and clopidogrel resistance. Mayo Clin Proc 2006;81:989 | A |
| Freedman JE. The aspirin resistance controversy: clinical entity or platelet heterogeneity? Circulation 2006;113:2865–7 | A |
| Frelinger AL, III, Michelson AD. Clopidogrel linking evaluation of platelet response variability to mechanism of action. J Am Coll Cardiol 2005;46:646–7 | A |
| Frelinger AL, III. Platelet reactivity with prolonged aspirin treatment – steady going at 2 year. Circ J 2010;74:1077–8 | A |
| Frelinger AL, III, Furman MI, Linden MD, Li Y, Fox ML, Barnard MR, et al. Response to letter regarding article, ‘Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1- and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance’. Circulation 2007;115:e46 | A |
| Frere C, Cuisset T, Gaborit B, Alessi MC, Hulot JS. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non-ST acute coronary syndrome. J Thromb Haemost 2009;7:1409–11 | B, C, D |
| Frere C, Cuisset T, Quilici J, Camoin L, Carvajal J, Morange PE, et al. ADP-induced platelet aggregation and platelet reactivity index VASP are good predictive markers for clinical outcomes in non-ST elevation acute coronary syndrome. Thromb Haemost 2007;98:838–43 | C |
| Frere C, Cuisset T, Quilici J, Gaborit B, Poyet R, Bali L, et al. Predictive ability of post treatment ADP-induced aggregation and VASP index on stent thrombosis after acute coronary syndrome in clopidogrel-treated patients: a prospective study. J Thromb Haemost 2009;7:585 | B, C |
| Freynhofer M, Brozovic I, Bruno V, Leherbauer L, Djurkovic M, Jarai R, et al. Routine determination of platelet reactivity in patients on long-term dual antiplatelet therapy: The WILMAA Registry. Eur Heart J Suppl 2010;12:F65 | C, D |
| Freynhofer MK, Brozovic I, Bruno V, Farhan S, Vogel B, Jakl G, et al. Multiple electrode aggregometry and vasodilator stimulated phosphoprotein-phosphorylation assay in clinical routine for prediction of postprocedural major adverse cardiovascular events. Thromb Haemost 2011;106:230–9 | C |
| Friend M, Vucenik I, Miller M. Platelet responsiveness to aspirin in patients with hyperlipidaemia. BMJ 2003;326:82–3 | D |
| Fritsma GA, Ens GE, Alvord MA, Carroll AA, Jensen R. Monitoring the antiplatelet action of aspirin. JAAPA 1961;14:57–8 | B, D |
| Fukushima K, Kobayashi Y, Kitahara H, Iwata Y, Kuroda N, Ooyama M, et al. Effect of 450 mg loading dose of clopidogrel on platelet function in Japanese patients undergoing coronary stent placement. Heart Vessels 2010;25:182–6 | C |
| Gabriel SA, Beteli CB, Tanighuchi RS, Tristao CK, Gabriel EA, Job JR. Aspirin resistance and atherothrombosis. Rev Bras Cir Cardiovasc 2007;22:96–103 | A |
| Gaglia MA, Torguson R, Pakala R, Sardi G, Xue Z, Suddath WO, et al. Patients with acute coronary syndromes undergoing percutaneous coronary intervention have higher levels of on-treatment platelet reactivity. Circulation 2011;124(Suppl. 1):A9814 | C, D |
| Gaglia MA, Torguson R, Sardi G, Xue Z, Suddath WO, Kent KM, et al. Patients with acute coronary syndrome have higher levels of on-treatment platelet reactivity. J Am Coll Cardiol 2011;58(Suppl. 1):B48–9 | C, D |
| Gaglia MA, Torguson R, Xue Z, Pakala R, Gonzalez MA, Ben-Dor I, et al. Periprocedural myocardial infarction and high on-treatment platelet reactivity. J Am Coll Cardiol 2011;57(Suppl. 1):E1897 | C |
| Gajos GP, Undas A, Rostoff P, Nessler J, Piwowarska W. Responsiveness to clopidogrel after percutaneous coronary intervention can be improved with polyunsaturated omega-3 fatty acids especially in patients with CYP 2C19 loss-of-function polymorphism. Eur Heart J 2010;31:202 | D |
| Galvani M. High postclopidogrel platelet reactivity in non-ST-elevation acute coronary syndrome treated with stenting: a clue for adverse prognosis? J Thromb Haemost 2006;4:536–8 | A |
| Gao F, Wang ZX, Men JL, Ren J, Wei MX. Effect of polymorphism and type II diabetes on aspirin resistance in patients with unstable coronary artery disease. Chin Med J 2011;124:1731–4 | D |
| Gao L, Li YJ, Chen KY. [Effects of supplementing qi and activating blood circulation method on platelet aggregation rate, adhesion rate and thromboxane B2 level in patients with stable angina pectoris and intolerable to aspirin.] Zhongguo Zhong Xi Yi Jie He Za Zhi 2008;28:300–3 | B |
| Garcia-Rinaldi R. Thromboprophylaxis with antiplatelet agents prevents cerebral thromboembolism in patients with mechanical aortic prostheses. Stroke 2011;42:e345 | B |
| Gasparovic H, Petricevic M, Biocina B. Letter by Gasparovic, et al. regarding article, ‘Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting: the clopidogrel after surgery for coronary artery disease (CASCADE) trial’. Circulation 2011;124:e193–6 | A |
| Gasparovic H, Petricevic M, Biocina B. Reduction of thienopyridine-associated bleeding using multiple electrode whole-blood aggregometry. Ann Thorac Surg 2011;92:778–9 | A |
| Gavrilov AO, Shilov AM. [Characteristics of the thromboelastogram indices of the arterial and venous blood acute myocardial infarct.] Probl Gematol Pereliv Krovi 1979;24:27–9 | C, D |
| Gawaz M, Seyfarth M, Muller I, Rudiger S, Pogatsa-Murray G, Wolf B, et al. Comparison of effects of clopidogrel versus ticlopidine on platelet function in patients undergoing coronary stent placement. Am J Cardiol 2009;87:332–6 | C, D |
| Geara AS, Azzi N, Bassil C, El-Sayegh S. Aspirin resistance in hemodialysis patients. Int Urol Nephrol 2012;44:323–5 | D |
| Geisler T, Anders N, Paterok M, Langer H, Stellos K, Lindemann S, et al. Platelet response to clopidogrel is attenuated in diabetic patients undergoing coronary stent implantation. Diabetes Care 2007;30:372–4 | C, D |
| Geisler T, Gawaz M, Steinhubl SR, Bhatt DL, Storey RF, Flather M. Current strategies in antiplatelet therapy – does identification of risk and adjustment of therapy contribute to more effective, personalized medicine in cardiovascular disease? Pharmacol Ther 2010;127:95–107. [Erratum published in Pharmacol Ther 2010;128:385] | A |
| Geisler T, Gawaz M. Editorial on ‘Residual platelet reactivity is an independent predictor of myocardial injury in acute myocardial infarction patients on antiaggregant therapy’ by Marcucci, et al. Thromb Haemost 2007;98:705–6 | A |
| Geisler T, Grass D, Bigalke B, Stellos K, Drosch T, Dietz K, et al. The Residual Platelet Aggregation after Deployment of Intracoronary Stent (PREDICT) score. J Thromb Haemost 2008;6:54–61 | C |
| Geisler T, Htun P, Fateh-Moghadam S, Bischofs C, Mueller K, Bigalke B, et al. Responsiveness to clopidogrel adversely influences outcome in patients with chronic kidney disease undergoing coronary interventions. Eur Heart J 2010;31:341 | C |
| Geisler T, Kapp M, Gohring-Frischholz K, Daub K, Dosch C, Bigalke B, et al. Residual platelet activity is increased in clopidogrel- and ASA-treated patients with coronary stenting for acute coronary syndromes compared with stable coronary artery disease. Heart 2008;94:743–7 | D |
| Geisler T, Langer H, Wydymus M, Gohring K, Zurn C, Bigalke B, et al. Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. Eur Heart J 2006;27:2420–5 | C |
| Geisler T, Zurn C, Paterok M, Gohring-Frischholz K, Bigalke B, Stellos K, et al. Statins do not adversely affect post-interventional residual platelet aggregation and outcomes in patients undergoing coronary stenting treated by dual antiplatelet therapy. Eur Heart J 2008;29:1635–43 | C |
| Geisler T, Zurn C, Simonenko R, Rapin M, Kraibooj H, Kilias A, et al. Early but not late stent thrombosis is influenced by residual platelet aggregation in patients undergoing coronary interventions. Eur Heart J 2010;31:59–66 | C |
| Ghosh K, Shetty S, Mota L. Aspirin resistance in patients with coronary artery disease – which test to use in routine management? Blood Coagul Fibrinolysis 2008;19:324–6 | D |
| Gibbings G. No easy way to identify aspirin resistance. Practitioner 2008;252:6 | A |
| Gibson CM, Ly HQ, Murphy SA, Ciaglo LN, Southard MC, Stein EB, et al. Usefulness of clopidogrel in abolishing the increased risk of reinfarction associated with higher platelet counts in patients with ST-elevation myocardial infarction (results from CLARITY-TIMI 28). Am J Cardiol 2006;98:761–3 | A |
| Giugliano RP, McCabe CH, Sequeira RF, Frey MJ, Henry TD, Piana RN, et al. First report of an intravenous and oral glycoprotein IIb/IIIa inhibitor (RPR 109891) in patients with recent acute coronary syndromes: results of the TIMI 15A and 15B trials. Am Heart J 2000;140:81–93 | C |
| Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, et al. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am J Cardiol 2009;103:806–11 | C |
| Giusti B, Saracini C, Vestrini A, Magi A, Marcucci R, Rossi L, et al. Genetic variants besides CYP2C19*2 polymorphism are associated with major adverse cardiovascular events in high risk vascular patients on dual antiplatelet therapy. Eur Heart J 2010;31:156 | C |
| Glauser J, Emerman CL, Bhatt DL, Peacock WF. Platelet aspirin resistance in ED patients with suspected acute coronary syndrome. Am J Emerg Med 2010;28:440–4 | B |
| Gluszek J, Posadzy-Malaczynska A, Tykarski A, Pupek-Musialik D, Gracz M, Kara-Perz H. Acetylsalicylic acid (aspirin) test for the diagnosis of renovascular hypertension. Clin Invest Med 1997;20:171–5 | D |
| Golanski J, Chizynski K, Golanski R, Watala C. [Application of platelet function analyzer (PFA-100TM) and whole blood aggregometry to monitor blood platelet sensitivity to acetylsalicylic acid (aspirin). Is it possible to reliably monitor antiplatelet treatment using routine laboratory diagnostic methods?] Pol Arch Med Wew 2000;104:355–61 | D |
| Goodman T, Ferro A, Sharma P. Pharmacogenetics of aspirin resistance: a comprehensive systematic review. Br J Clin Pharmacol 2008;66:222–32 | D |
| Gordon B, Biss T, Butt T, McDiarmid A, Oezalp F, Pillay T, et al. Platelet mapping thromboelastography for individualized antiplatelet treatment after implantation of the HeartWare ventricular assist device. J Heart Lung Transplant 2011;30(Suppl. 1):S208–9 | D |
| Gorog DA, Douglas H, Ahmed N, Lefroy DC, Davies GJ. Coronary angioplasty enhances platelet reactivity through von Willebrand factor release. Heart 2003;89:329–30 | B, D |
| Gratsianskii NA. [Antiplatelet therapy in coronary heart disease. Some problems and achievements.] Kardiologiia 2010;50:4–21 | A |
| Gray EN, Cheema FH. Letter to the editor. ASAIO J 2008;54:138 | A |
| Green D. Prevention of thromboembolism after spinal cord injury. Sem Thromb Hemost 1991;17:347–50 | A |
| Greenbaum AB, Grines CL, Bittl JA, Becker RC, Kereiakes DJ, Gilchrist IC, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J 2006;151:689 | C |
| Greer DM. Aspirin and antiplatelet agent resistance: implications for prevention of secondary stroke. CNS Drugs 2010;24:1027–40 | A |
| Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Comparison of methods to evaluate aspirin-mediated platelet inhibition after percutaneous intervention with stent implantation. Platelets 2011;22:188–95 | D |
| Gritsiuk AI, Sopina NV. [Blood coagulation and fibrinolytic system in patients with marked coronary arteriosclerosis.] Kardiologiia 1970;10:9–16 | C, D |
| Grotemeyer KH. Effects of acetylsalicylic acid in stroke patients. Evidence of nonresponders in a subpopulation of treated patients. Thromb Res 1991;63:587–93 | D |
| Grove EL, Hvas AM, Johnsen HL, Hedegaard SS, Pedersen SB, Mortensen J, et al. A comparison of platelet function tests and thromboxane metabolites to evaluate aspirin response in healthy individuals and patients with coronary artery disease. Thromb Haemost 2010;103:1245–53 | D |
| Grove EL, Hvas AM, Kristensen SD. Aspirin resistance: myth or major problem? Scand J Clin Lab Invest 2008;68:257–9 | A |
| Grove EL, Hvas AM, Kristensen SD. Platelet function testing in atherothrombotic disease: steps forward in managing resistance to antiplatelet therapy. Kardiol Pol 2008;66:478–9 | A |
| Grundmann K, Jaschonek K, Kleine B, Dichgans J, Topka H. Aspirin non-responder status in patients with recurrent cerebral ischemic attacks. J Neurol 2003;250:63–6 | D |
| Guan SY, Han YL, Li Y, Guo L, Yang BS, Wang SL, et al. [Effects of intensive antiplatelet therapy for patients with high on-treatment platelet reactivity after coronary stent implantation.] Chung-Hua Hsin Hsueh Kuan Ping Tsa Chih 2012;40:25–9 | C |
| Guha S, Mookerjee S, Guha P, Sardar P, Deb S, Roy PD, et al. Antiplatelet drug resistance in patients with recurrent acute coronary syndrome undergoing conservative management. Indian Heart J 2009;61:348–52 | D |
| Gupta MC, Dhamija JP, Mathur DS, Sharma BM. Role of propranolol and aspirin in decreasing platelet aggregation in occlusive cerebrovascular diseases. J Assoc Physicians India 1983;31:565–7 | D |
| Gupta S, Gupta MM. Aspirin and clopidogrel resistance – a myth or reality: an update. Indian Heart J 2008;60:245–53 | A |
| Gupta S, Gupta VK, Dhamija RK, Kela AK. Platelet aggregation patterns in normotensive and hypertensive subjects. Indian J Physiol Pharmacol 2002;46:379–82 | B |
| Gupta S. When aspirin doesn’t work. Time 2002;159:83 | A |
| Gurbel P, Bliden KP, Tantry U. Evidence that pre-existent variability in platelet response to ADP accounts for ‘clopidogrel resistance’: a rebuttal. J Thromb Haemost 2007;5:1087–8 | A |
| Gurbel P. Aspirin – Scope and limitations. Br J Cardiol 2010;17(Suppl. 1):S8–9 | A |
| Gurbel PA, Antonino MJ, Bliden KP, Dichiara J, Suarez TA, Singla A, et al. Platelet reactivity to adenosine diphosphate and long-term ischemic event. J Thromb Haemost 2009;7:572 | C |
| Gurbel PA, Bilden KP, Tantry US. Diagnostics for aspirin resistance. Mol Diagn Ther 2008;12:55–6 | A |
| Gurbel PA, Bliden KP, Antonino MJ, Gesheff T, Cummings CC, Dubois BV, et al. Time dependence of clopidogrel loading effect: platelet activation versus platelet aggregation. Thromb Res 2012;129:1–2 | C |
| Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol 2005;46:1820–6 | C |
| Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003;107:2908–13 | C |
| Gurbel PA, Bliden KP, Kreutz RP, Dichiara J, Antonino MJ, Tantry US. The link between heightened thrombogenicity and inflammation: pre-procedure characterization of the patient at high risk for recurrent events after stenting. Platelets 2009;20:97–104 | C |
| Gurbel PA, Bliden KP, Samara W, Yoho JA, Hayes K, Fissha MZ, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol 2005;46:1827–32 | D |
| Gurbel PA, Bliden KP, Zaman KA, Yoho JA, Hayes KM, Tantry US. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation 2005;111:1153–9 | C |
| Gurbel PA, Bliden KP. Durability of platelet inhibition by clopidogrel. Am J Cardiol 2003;91:1123–5 | C |
| Gurbel PA, Jeong Y-H, Mahla E, Bliden K, Tantry U. The association of preoperative platelet function testing and bleeding in patients undergoing elective coronary artery bypass grafting. J Am Coll Cardiol 2012;59(Suppl. 1):E1455 | B, D |
| Gurbel PA, Mahla E, Antonino MJ, Tantry US. Response variability and the role of platelet function testing. J Invasive Cardiol 2009;21:172–8 | A |
| Gurbel PA, Mahla E, Tantry US. Peri-operative platelet function testing: the potential for reducing ischaemic and bleeding risks. Thromb Haemost 2011;106:248–52 | A |
| Gurbel PA, Tantry US. Platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents? Circulation 2012;125:1276–87 | A |
| Gurbel PA, Tantry US, Shuldiner AR. The worry about clopidogrel ‘nonresponsiveness’: identification and treatment in the post-percutaneous coronary intervention patient. JACC Cardiovasc Interv 2009;2:1102–4 | A |
| Gurbel PA, Tantry US. Acceptance of high platelet reactivity as a risk factor: now, what do we do about it? JACC Cardiovasc Interv 2010;3:1008–10 | A |
| Gurbel PA, Tantry US. An initial experiment with personalized antiplatelet therapy: the GRAVITAS trial. JAMA 2011;305:1136–7 | A |
| Gurbel PA, Tantry US. Antiplatelet resistance – fact or myth? Am Heart Hosp J 2009;7:50–7 | A |
| Guthikonda S, Kleiman NS. Is aspirin resistance valid? Future Cardiol 2006;2:1–4 | A |
| Ha SJ, Woo JS, Kim WS, Kim SJ, Kim W, Kim MK, et al. The effect of cilostazol adding or clopidogrel doubling on platelet function with diabetes mellitus and coronary artery disease on dual antiplatelet therapy. Eur Heart J 2010;31:975 | C, D |
| Hackeng CM, Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJT, et al. Prospective comparison of platelet function tests in predicting clinical outcome in 1069 patients undergoing coronary stent implantation: the popular study. Pathophysiol Haemost Thromb 2010;37:A129 | C |
| Halushka MK, Halushka PV. Why are some individuals resistant to the cardioprotective effects of aspirin? Could it be thromboxane A2? Circulation 2002;105:1620–2 | A |
| Hankey GJ, Eikelboom JW. Aspirin resistance. BMJ 2004;328:477–9 | A |
| Harding S, Johnston L, Michel J, Ramanathan A, La FA, Sasse A, et al. High on treatment platelet reactivity is common and differs among ethnic groups. Heart Lung Circul 2011;20:S34–5 | D |
| Harmsze AM, van Werkum JW, Hackeng CM, Ruven HJ, Kelder JC, Bouman HJ, et al. The influence of CYP2C19*2 and *17 on on-treatment platelet reactivity and bleeding events in patients undergoing elective coronary stenting. Pharmacogenet Genomics 2012;22:169–75 | C |
| Harmsze AM, van Werkum JW, Souverein PC, Breet NJ, Bouman HJ, Hackeng CM, et al. Combined influence of proton-pump inhibitors, calcium-channel blockers and CYP2C19*2 on on-treatment platelet reactivity and on the occurrence of atherothrombotic events after percutaneous coronary intervention. J Thromb Haemost 2011;9:1892–901 | C |
| Harmsze AM, van Werkum JW, ten Berg JM, Ruven HJT, Hackeng CM, Tjoeng MM, et al. Effect of genetic variants on the effectiveness of clopidogrel. Pharm Weekbl 2011;146:79–84 | B, C |
| Harrison P, Keeling D. Platelet hyperactivity and risk of recurrent thrombosis. J Thromb Haemost 2006;4:2544–6 | A |
| Harrison P, Mumford A. Screening Tests of Platelet Function: Update on their Appropriate Uses for Diagnostic Testing. New York, NY: Thieme; 2009 | A |
| Harrison P. The influence of citrate concentrations on PFA-100 closure times, platelet hyper-reactivity and aspirin monitoring. Thromb Res 2010;126:e137–8 | D |
| Hennekens CH, Schror K, Weisman S, FitzGerald GA. Terms and conditions: semantic complexity and aspirin resistance. Circulation 2004;110:1706–8 | A |
| Henry P, Drouet L. Resistances to antiplatelet agents. Arch Mal Coeur Vaiss Prat 2010;16:14–21 | A |
| Hezard N, Tessier-Marteau A, Macchi L. New insight in antiplatelet therapy monitoring in cardiovascular patients: from aspirin to thienopyridine. Cardiovasc Hematol Disord Drug Targets 2010;10:224–33 | A |
| Hillarp A, Lethagen S, Mattiasson I. Aspirin resistance is not a common biochemical phenotype explained by unblocked cyclooxygenase-1 activity. J Thromb Haemost 2003;1:196–7 | A |
| Hillegass WB, Zoghbi G, Brott BC, Kilgore ML, Chapman GD, Misra VK. Cost-effectiveness of new oral antiplatelet agents and testing strategies during early invasive management of acute coronary syndrome patients. Circulation 2011;124(Suppl. 1):A18228 | B |
| Hillman RS. Platelet aspirin resistance detection and validation. J Am Coll Cardiol 2006;47:2565 | A |
| Hjemdahl P. Platelet reactivity, exercise, and stable coronary artery disease. Eur Heart J 1995;16:1017–19 | A |
| Hjemdahl P. Should we monitor platelet function during antiplatelet therapy? Heart 2008;94:685–7 | A |
| Ho WK, Hankey GJ, Eikelboom JW. Is there a role for laboratory testing to identify patients at risk of aspirin treatment failure? Blood Coagul Fibrinolysis 2004;15:129–30 | A |
| Hobikoglu GF, Norgaz T, Aksu H, Ozer O, Erturk M, Nurkalem Z, et al. High frequency of aspirin resistance in patients with acute coronary syndrome. Tohoku J Exp Med 2005;207:59–64 | D |
| Hochholzer W, Trenk D, Bestehorn HP, Fischer B, Valina CM, Ferenc M, et al. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol 2006;48:1742–50 | C |
| Hochholzer W, Trenk D, Frundi D, Blanke P, Fischer B, Andris K, et al. Time dependence of platelet inhibition after a 600 mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation 2005;111:2560–4 | C |
| Hodges JS. Incorrect p value in: comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2010;303:1257 | A |
| Hoffmann S. Do platelet function assays predict clinical outcomes in clopidogrel pretreated patients undergoing elective PCI? Herz 2010;35:50 | A |
| Hokimoto S, Mizobe M, Chitose T, Kaikita K, Nakagawa K, Ogawa H. Impact of CYP2C19 polymorphism and diabetes mellitus on platelet reactivity and clinical outcomes in patients following coronary stent placement. Circulation 2011;124(Suppl. 1):A15054 | B, C |
| Hokimoto S, Ogawa H. Is it safe to use a proton pump inhibitor with clopidogrel? A comparison of clopidogrel with or without rabeprazole in Japan. Gastroenterology 2010;138(Suppl. 1):S498 | C |
| Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA 2011;306:2704–14 | B, C |
| Hong DM, Jeon YS, Kim JH, Lim TW, Lim YJ, Bahk JH, et al. Preoperative platelet response to collagen is associated with myocardial injury after off-pump coronary bypass graft in patients taking aspirin. Korean J Anesth 2010;58:129–35 | D |
| Hong JM, Shin DH, Lee JS. Comparison of platelet aggregation with thienopyridines in acute cerebral ischemia. Cerebrovasc Dis 2011;31:262–3 | B |
| Horiuchi H, Ikeda T, Taniguchi R, Kita T, Kimura T, Hisanori H. Characterization of the antiplatelet effect of aspirin at enrollment and after a 2-year follow-up in the real clinical setting in Japan. J Mol Cell Cardiol 2010;48(Suppl. 1):S115 | D |
| Houel R, Mazoyer E, Kirsch M, Boval B, Drouet L, Loisance DY. Resistance to aspirin after external ventricular assist device implantation. J Thorac Cardiovasc Surg 2003;126:1636–7 | D |
| Hovens MM, Snoep JD, Eikenboom JC, Van Der Bom JG, Mertens BJ, Huisman MV. Prevalence of persistent platelet reactivity despite use of aspirin: a systematic review. Am Heart J 2007;153:175–81 | D |
| Htun P, Fateh-Moghadam S, Bischofs C, Banya W, Muller K, Bigalke B, et al. Low responsiveness to clopidogrel increases risk among CKD patients undergoing coronary intervention. J Am Soc Nephrol 2011;22:627–33 | C |
| Hu DY, Sun YH. [The current concept of aspirin resistance.] Chung-Hua Hsin Hsueh Kuan Ping Tsa Chih 2006;34:1057–8 | A |
| Huang Y, Gueyffier F, Boissel JP, Nony P. Can poor-compliance be considered as an important cause for aspirin resistance? An ‘in-silico’ approach. Fundam Clin Pharmacol 2010;24:62 | A |
| Huczek Z, Filipiak KJ, Kochman J, Michalak M, Roik M, Grabowski M, et al. Medium on-treatment platelet reactivity to ADP is favorable in patients with acute coronary syndromes undergoing coronary stenting. Platelets 2011;22:521–9 | C |
| Huczek Z, Filipiak KJ, Kochman J, Michalak M, Roik M, Opolski G. Variable response in platelet reactivity to 600 mg clopidogrel-loading dose influences prognosis in ST-segment elevation myocardial infarction. J Am Coll Cardiol 2009;53:A428 | B, C |
| Huczek Z, Filipiak KJ, Kochman J, Piatkowski R, Grabowski M, Roik M, et al. Baseline platelet reactivity in acute myocardial infarction treated with primary angioplasty – influence on myocardial reperfusion, left ventricular performance, and clinical events. Am Heart J 2007;154:62–70 | C |
| Huczek Z, Filipiak KJ, Kochman J, Piatkowski R, Grabowski M, Roik M, et al. Prognostic significance of platelet function in the early phase of ST-elevation myocardial infarction treated with primary angioplasty. Med Sci Monit 2008;14:CR144–51 | C |
| Hulot J-S, Collet J-P, Cayla G, Silvain J, Allanic F, Bellemain-Appaix A, et al. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post-myocardial infarction patients. Circ Cardiovasc Interv 2011;4:422–8 | C |
| Husstedt IW, Grotemeyer KH, Evers S, Staschewski F, Wertelewski R. Progression of distal symmetric polyneuropathy during diabetes mellitus: clinical, neurophysiological, haemorheological changes and self-rating scales of patients. Eur Neurol 1997;37:90–4 | D |
| Ibrahim K. Increased rate of stent thrombosis due to clopidogrel resistance in patients in therapeutic hypothermia after sudden cardiac death. Eur Heart J 2011;32:252 | C |
| Iijima R, Ndrepepa G, Mehilli J, Bruskina O, Schulz S, Schomig A, et al. Relationship between platelet count and 30-day clinical outcomes after percutaneous coronary interventions. Pooled analysis of four ISAR trials. Thromb Haemost 2007;98:852–7 | C |
| Ikeda T, Taniguchi R, Watanabe S, Kawato M, Kondo H, Shirakawa R, et al. Characterization of the antiplatelet effect of aspirin at enrollment and after 2-year follow-up in a real clinical setting in Japan. Circ J 2010;74:1227–35 | D |
| Inoue Y, Shimizu M, Hasuda T, Uehara Y, Nakae S, Kubota T, et al. Effect of platelet aggregation inhibition by aspirin and clopidogrel in post stenting patients. J Mol Cell Cardiol 2010;48(Suppl. 1):S173 | D |
| Isbir S, Ak K, Aksoy N. Platelet function tests predict bleeding and thrombotic events after off-pump coronary bypass grafting. Eur J Cardiothorac Surg 2005;28:514–15 | A |
| Israelian LA, Lubnin AI, Tseitlin AM. [Thromboelastography as a method for preoperative assessment of hemostasis state in neurosurgical patients on long term aspirin therapy.] Anesteziol Reanimatol 2011;4:27–32 | C |
| it-Mokhtar O, Bonello L, Benamara S, Paganelli F. High on treatment platelet reactivity. Heart Lung Circ 2012;21:12–21 | A |
| Itoh T, Nakai K, Ono M, Hiramori K. Can the risk for acute cardiac events in acute coronary syndrome be indicated by platelet membrane activation marker P-selectin? Coron Artery Dis 1995;6:645–50 | B |
| Ivandic BT, Kurz K, Keck F, Staritz P, Lehrke S, Katus HA, et al. Tirofiban optimizes platelet inhibition for immediate percutaneous coronary intervention in high-risk acute coronary syndromes. Thromb Haemost 2008;100:648–54 | C |
| Ivanova NA. [Some indices of the blood coagulation system and thrombus-formation in patients with diabetes mellitus.] Kardiologiia 1971;11:121–5 | C, D |
| Izaguirre-Avila R, de la Pena-Diaz A, Barinagarrementeria-Aldatz F, Gonzalez-Pacheco H, Ramirez-Gutierrez AE, Ruiz-Sandoval JL, et al. Effect of clopidogrel on platelet aggregation and plasma concentration of fibrinogen in subjects with cerebral or coronary atherosclerotic disease. Clin Appl Thromb Hemost 2002;8:169–77 | B, C |
| Jackson AN, Hume AL. Aspirin for CV prevention – for which patients? J Fam Pract 2011;60:518–23 | A |
| Jafri SM, Riddle JM, Raman SB, Goldstein S. Altered platelet function in patients with severe congestive heart failure. Henry Ford Hosp Med J 1986;34:156–9 | D |
| Jaremo P, Milovanovic M, Lindahl TL, Richter A. Elevated platelet density and enhanced platelet reactivity in stable angina pectoris complicated by diabetes mellitus type II. Thromb Res 2009;124:373–4 | D |
| Jeon HW, Cha JK. Factors related to progression of middle cerebral artery stenosis determined using transcranial Doppler ultrasonography. J Thromb Thrombolysis 2008;25:265–9 | D |
| Jeon SB, Song HS, Kim BJ, Kim HJ, Kang DW, Kim JS, et al. Biochemical aspirin resistance and recurrent lesions in patients with acute ischemic stroke. Eur Neurol 2010;64:51–7 | D |
| Jeong YH, Hwang JY, Kim IS, Park Y, Hwang SJ, Lee SW, et al. Adding cilostazol to dual antiplatelet therapy achieves greater platelet inhibition than high maintenance dose clopidogrel in patients with acute myocardial infarction: results of the adjunctive cilostazol versus high maintenance dose clopidogrel in patients with AMI (ACCEL-AMI) study. Circ Cardiovasc Interv 2010;3:17–26 | C, D |
| Jeong Y-H, Hwang S-J, Park Y, Kim I-S, Kwak CH, Hwang J-Y. Cytochrome 2C19 polymorphism and response to adjunctive cilostazol versus high maintenance-dose clopidogrel in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol 2010;55(Suppl. 1):A130 | C, D |
| Jeong Y-H, Kim I-S, Choi B-R, Kwak CH, Hwang J-Y. The optimal threshold of high post-treatment platelet reactivity could be defined by a point-of-care VerifyNow P2Y12 assay. Eur Heart J 2008;29:2186–7 | C |
| Jeong Y-H, Kim I-S, Park Y, Yun S-E. Platelet inhibition by adjunctive cilostazol vs. high maintenance-dose clopidogrel in patients with acute myocardial infarction according to cytochrome P450 2C19 genotype. J Thromb Haemost 2011;9:48–9 | D |
| Jeong Y-H, Koh J-S, Kwon TJ, Park Y, Kim I-S. Impact of the cytochrome P450 2C19*3 polymorphism on platelet reactivity and adverse clinical events in patients with acute myocardial infarction. J Thromb Haemost 2011;9:793 | B, C |
| Jeong Y-H, Kwon TJ, Tantry US, Park Y, Hwang S-J, Bliden KP, et al. Correlation between platelet reactivity and type of post-discharge bleeding events in PCI-treated patients: results of the ACCEL-BLEED study. J Am Coll Cardiol 2011;58(Suppl. 1):B221 | B, C |
| Jeong Y-H, Lee K, Yoo S-Y, Park J-H, Suh J, Park K-H. Accelerated inhibition of platelet aggregation, inflammation and myonecrosis by adjunctive cilostazol loading in patients with acute coronary syndrome: the results of the ACCEL-LOADING-ACS multicenter randomized trial. J Am Coll Cardiol 2012;59(Suppl. 1):E455 | C |
| Jeong Y-H, Lee K, Yoo S-Y, Park J-H, Suh J, Park KH. Multicenter randomized trial evaluating the efficacy of cilostazol on inhibition of platelet aggregation, inflammation and myonecrosis in ACS patients undergoing PCI: the results of accel-loading-ACS (accelerated inhibition of platelet aggregation, inflammation and myonecrosis by adjunctive cilostazol loading in patients with acute coronary syndrome) trial. Am J Cardiol 2012;109(Suppl. 1):1S–2S | C |
| Jeong YH, Park Y, Bliden KP, Tantry US, Gurbel PA. High platelet reactivity to multiple agonists during aspirin and clopidogrel treatment is indicative of a global hyperreactive platelet phenotype. Heart 2012;98:343–4 | D |
| Jeong YH, Tantry US, Bliden KP, Gurbel PA. Cilostazol to overcome high on-treatment platelet reactivity in Korean patients treated with clopidogrel and calcium-channel blocker. Circ J 2011;75:2534–6 | A |
| Jeong YH, Tantry US, Kim IS, Koh JS, Kwon TJ, Park Y, et al. Effect of CYP2C19*2 and *3 loss-of-function alleles on platelet reactivity and adverse clinical events in East Asian acute myocardial infarction survivors treated with clopidogrel and aspirin. Circ Cardiovasc Interv 2011;4:585–94 | C |
| Jeong YH, Tantry UT, Jung TJ, Park YH, Kim IS, Bliden KA, et al. Impact of the cytochrome P450 2C19*3 polymorphism on platelet reactivity and adverse clinical events in patients with acute myocardial infarction. Eur Heart J 2011;32:508 | C |
| Jeong Y-H, Yongwhi P, In-Suk K, Tae JK, Jin-Yong H. The influence of genetic polymorphism and drug–drug interaction on antiplatelet effect in patients treated with long-term dual antiplatelet therapy. J Thromb Haemost 2011;9:75–6 | D |
| Jha AK. Using aspirin resistance to predict long-term cardiovascular outcomes. J Clin Outcomes Manage 2007;14:491 | A |
| Jian C, Lin L, Li F. Prevalence and risk factors for aspirin resistance in older patients with cardiovascular disease. Heart 2011;97(Suppl. 3):A101 | D |
| Jilma B, Fuchs I. Aspirin. Methods to assess aspirin resistance. J Thromb Haemost 2004;2:337–8 | A |
| Jilma B, Fuchs I. Detecting aspirin resistance with the platelet function analyzer (PFA-100). Am J Cardiol 2001;88:1348–9 | A |
| Jilma B. Synergistic antiplatelet effects of clopidogrel and aspirin detected with the PFA-100 in stroke patients. Stroke 2003;34:849–54 | A |
| Jilma B. Therapeutic failure or resistance to aspirin. J Am Coll Cardiol 2004;43:1332–3 | A |
| Johns A, Fisher M, Knappertz V. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur Heart J 2006;27:1754–5 | A |
| Johnson GJ, Sharda AV, Rao GH, Ereth MH, Laxson DD, Owen WG. Measurement of shear-activated platelet aggregate formation in non-anticoagulated blood: utility in detection of clopidogrel-aspirin-induced platelet dysfunction. Clin Appl Thromb Hemost 2012;18:140–9 | D |
| Jukema JW, Collet J-P, De Luca L. Antiplatelet therapy in patients with ST-elevation myocardial infarction undergoing myocardial revascularisation: beyond clopidogrel. Curr Med Res Opin 2012;28:203–11 | C |
| Jungmayr P. Cardiovascular morbidity: clinical relevance of aspirin resistance. Dtsch Apoth Ztg 2008;148:43–4 | A |
| Kabbani SS, Watkins MW, Ashikaga T, Terrien EF, Holoch PA, Sobel BE, et al. Platelet reactivity characterized prospectively: a determinant of outcome 90 days after percutaneous coronary intervention. Circulation 2001;104:181–6 | C |
| Kabbani SS, Watkins MW, Ashikaga T, Terrien EF, Sobel BE, Schneider DJ. Usefulness of platelet reactivity before percutaneous coronary intervention in determining cardiac risk one year later. Am J Cardiol 2003;91:876–8 | C |
| Kakouros N, Kickler TS, Laws K, Rade JJ. Hematocrit significantly affects the results of the verifynow assay in patients receiving dual antiplatelet therapy. Circulation 2011;124(Suppl. 1):A17082 | D |
| Kakuliia MS, Panchenko VM. [Functional status of thrombocytes in patients with acute forms of ischemic heart disease.] Klin Med (Mosk) 1987;65:88–92 | B, C, D |
| Kalyanasundaram A, Berger PB. Routine platelet testing should not be performed on all patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv 2010;3:284–7 | A |
| Kang HS, Kwon BJ, Kim JE, Han MH. Preinterventional clopidogrel response variability for coil embolization of intracranial aneurysms: clinical implications. Am J Neuroradiol 2010;31:1206–10 | C |
| Kapoor JR. Enteric coating is a possible cause of aspirin resistance. J Am Coll Cardiol 2008;52:1276–7 | A |
| Karabulut H, Toraman F, Evrenkaya S, Goksel O, Tarcan S, Alhan C. Clopidogrel does not increase bleeding and allogenic blood transfusion in coronary artery surgery. Eur J Cardiothorac Surg 2004;25:419–23 | C |
| Kasotakis G, Pipinos II, Lynch TG. Current evidence and clinical implications of aspirin resistance. J Vasc Surg 2009;50:1500–10 | A |
| Kaymaz C, Tanboga IH, Tokgoz HC, Poci N, Can MM, Kirca N, et al. Clinical, procedural, cardiometabolic and laboratory correlates of platelet reactivity to clopidogrel and aspirin on multiplate analyser in patients with coronary stenting. Eur Heart J 2011;32:752–3 | D |
| Kerenyi A, Soltesz P, Veres K, Szegedi G, Muszbek L. Monitoring platelet function by PFA-100 closure time measurements during thrombolytic therapy of patients with myocardial infarction. Thromb Res 2005;116:139–44 | D |
| Kim C-H, Park K-W, Jeon K, Kang S-H, Kim K-H, Lee H-Y, et al. Decreased response to clopidogrel is a risk factor for cardiovascular events after stent implantation: Cross verify study. J Am Coll Cardiol 2010;55(Suppl. 1):A51 | C |
| Kim H-L, Suh J-W, Lee S-P, Oh I-Y, Kang H-J, Koo B-K, et al. No legacy effect of six months’ treatment of cilostazol on the long-term prognosis of patients who underwent drug-eluting stent implantation: 2-year follow-up of the cilon-t trial. J Am Coll Cardiol 2012;59(Suppl. 1):E1404 | C |
| Kim IS, Choi BR, Jeong YH, Kwak CH, Kim S. The CYP2C19*2 and CYP2C19*3 polymorphisms are associated with high post-treatment platelet reactivity in Asian patients with acute coronary syndrome. J Thromb Haemost 2009;7:897–9 | B, C, D |
| Kim IS, Jeong YH, Kang MK, Koh JS, Park Y, Hwang SJ, et al. Correlation of high post-treatment platelet reactivity assessed by light transmittance aggregometry and the VerifyNow P2Y12 assay. J Thromb Thrombolysis 2010;30:486–95 | C |
| Kim J-Y, Yoon J. Aspirin and clopidogrel resistance in drug eluting stent era. Korean Circ J 2007;37:135–47 | A |
| Kim KE, Woo KS, Goh RY, Quan ML, Cha KS, Kim MH, et al. Comparison of laboratory detection methods of aspirin resistance in coronary artery disease patients. Int J Lab Hematol 2010;32:50–5 | D |
| Kim MH, Yu LH, Kim JH, Park SY, Park TH, Cha KS, et al. Initial loading therapy of cilostazol improved antiplatelet responsiveness in patient with percutaneous coronary intervention. J Am Coll Cardiol 2010;55(Suppl. 1):A208 | D |
| King A, Bath PM, Markus HS. Clopidogrel versus dipyridamole in addition to aspirin in reducing embolization detected with ambulatory transcranial Doppler: a randomized trial. Stroke 2011;42:650–5 | C |
| King A, Bath PMW, Markus H. What is the best antiplatelet regimen in acute carotid stenosis? A randomised trial comparing clopidogrel versus dipyridamole in addition to aspirin on asymptomatic embolisation. Cerebrovasc Dis 2010;29:160–1 | C |
| Kirtane AJ, Parise H, Witzenbichler B, Weisz G, Rinaldi M, Neumann F-J, et al. Does platelet function testing add significant incremental risk stratification to unselected patients undergoing des implantation? The adapt-des study. J Am Coll Cardiol 2012;59(Suppl. 1):E292 | C |
| Kirtane AJ, Rinaldi M, Parise H, Witzenbichler B, Weisz G, Neumann F-J, et al. Impact of point-of-care platelet function testing among patients with and without acute coronary syndromes undergoing PCI with drug-eluting stents: An ADAPT-des substudy. J Am Coll Cardiol 2012;59(Suppl. 1):E291 | C |
| Kirtane AJ, Stuckey T, Parise H, Witzenbichler B, Weisz G, Rinaldi M, et al. Impact of point-of-care platelet function testing among diabetic and non-diabetic patients undergoing PCI with drug-eluting stents: an adapt-des substudy. J Am Coll Cardiol 2012;59(Suppl. 1):E268 | B |
| Kleiman NS. Could clopidogrel’s platelet inhibition be enhanced by an increased loading dose and eptifibatide? Nat Clin Pract Cardiovasc Med 2005;2:344–5 | A |
| Kliger C, Babaev A, Shah B, Feit F, Slater J, Attubato M. Dual antiplatelet therapy responsiveness in patients undergoing percutaneous revascularization for peripheral arterial occlusive disease. J Am Coll Cardiol 2012;59(Suppl. 1):E2049 | D |
| Kobune K, Inoue M, Morikawa M, Tsuboi M, Takanami Y, Iwane Y, et al. Effects of combination therapy with low-dose aspirin and warfarin on platelet functions after heart valve replacement. Res Comm Chem Pathol Pharmacol 1991;74:153–65 | D |
| Koerner H, Derveaux C, Alexandrou M, Graeber S, Roth C, Papanagiotou P, et al. Do clopidogrel nonresponders have an increased risk of adverse events during supra-aortal angioplasty and stenting? Stroke Res Treat 2012;2012:904534 | C |
| Konorza TFM. TAILORED: tailored clopidogrel loading doses according to platelet reactivity monitoring decrease early stent thrombosis. Herz 2009;34:74 | C |
| Kooistra MP, van Es A, Marx JJ, Hertsig ML, Struyvenberg A. Low-dose aspirin does not prevent thrombovascular accidents in low-risk haemodialysis patients during treatment with recombinant human erythropoietin. Nephrol Dial Transplant 1994;9:1115–20 | A |
| Koscielny J, Aslan T, Meyer O, Kiesewetter H, Jung F, Mrowietz C, et al. Use of the platelet reactivity index by Grotemeyer, platelet function analyzer, and retention test Homburg to monitor therapy with antiplatelet drugs. Semin Thromb Hemost 2005;31:464–9 | B, D |
| Koscielny J, Meyer O, Kiesewetter H, Jung F, Latza R. [Platelet reactivity index by Grotemeyer.] Hamostaseologie 2004;24:207–10 | A |
| Kottke BA. The ultimate solution to the problem of aspirin resistance. J Am Coll Cardiol 2008;52:1276 | A |
| Koul S, Andell P, Martinsson A, Smith JG, Schersten F, Harnek J, et al. Upstream clopidogrel followed by pre-PCI prasugrel gives rapid platelet inhibition and represents a feasible option in STEMI patients undergoing primary PCI. J Am Coll Cardiol 2012;59(Suppl. 1):E526 | C |
| Kour D, Tandon VR, Kapoor B, Mahajan A, Parihar A, Smotra S. Aspirin resistance. JK Science 2006;8:116–17 | A |
| Kralisz P, Dobrzycki S, Nowak K, Kochman W, Gajewska-Bachorzewska H, Gugala K, et al. Platelet inhibition by increased tirofiban dosing during primary coronary angioplasty for ST elevation myocardial infarction. Kardiol Pol 2004;60:459–67 | C |
| Kramer MC, Nieuwland R, Sturk A, de Winter RJ. [Routine platelet aggregation inhibition not useful when using acetylsalicylic acid or clopidogrel.] Ned Tijdschr Geneeskd 2009;153:190–5 | A |
| Kratzer MA. Platelet function analyzer (PFA)–100 closure time in the evaluation of platelet disorders and platelet function: a rebuttal. J Thromb Haemost 2006;4:1845–6 | A |
| Kremneva LV, Shalaev SV. [Resistance to desaggregants: causes, clinical implication, methods of diagnosis and correction.] Ter Arkh 2008;80:89–95 | A |
| Krishna V, Diamond GA, Kaul S. Do platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents? The role of platelet reactivity and genotype testing in the prevention of atherothrombotic cardiovascular events remains unproven. Circulation 2012;125:1288–303 | A |
| Kronish IM, Rieckmann N, Shimbo D, Davidson KW. Letter by Kronish, et al. regarding article, ‘Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1- and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance’. Circulation 2007;115:e45 | A |
| Kubik MM, Richardson SG. A serial study of platelet reactivity throughout the first six months after myocardial infarction: its modification by sulphinpyrazone. Postgrad Med J 1987;63:351–6 | B |
| Kudaravalli J, Deshpande N, Avanapu SR, Vijaya LG, Nagaveni. Evaluation of aspirin resistance in elderly people in rural population in India. Der Pharm Lett 2011;3:5–9 | D |
| Kuliczkowski W, Halawa B, Korolko B, Mazurek W. Aspirin resistance in ischaemic heart disease. Kardiol Pol 2005;62:14–25 | D |
| Kusunoki M, Kimura K, Nagatsuka K, Isaka Y, Uyama O, Yoneda S, et al. Platelet hyperaggregability in ischemic cerebrovascular disease and effects of aspirin. Thromb Haemost 1982;48:117–19 | D |
| Kuzniatsova N, Shantsila E, Blann AD, Tse HF, Lip GYH. Is increased arterial stiffness related to aspirin responsiveness in patients with coronary artery disease: a possible mechanism for increased cardiovascular events in aspirin non-responders? Eur Heart J 2011;32:253 | D |
| Kwok CS, Loke YK. Critical overview on the benefits and harms of aspirin. Pharmaceuticals 2010;3:1491–506 | C |
| Kwon SU, Lee J-H, Cha J-K, Lee SJ, Ha S-W. Addition of cilostazol reduce biochemical aspirin resistance in aspirin users with ischemic stroke. Stroke 2009;40:e270 | D |
| Lakkis N, Dokainish H, Abuzahra M, Tsyboulev V, Jorgensen J, De Leon AP, et al. Reticulated platelets in acute coronary syndrome: a marker of platelet activity. J Am Coll Cardiol 2004;44:2091–3 | A, C, D |
| Lam H, Chan CK, Yip SF, Yam PW, Chui KL, Chan YH, et al. Monocyte platelet aggregate act as platelet function assay and prognostic marker for acute myocardial infarction. J Am Coll Cardiol 2011;58(Suppl. 1):B95 | C |
| Lam W, Gill JB, Trask RV, Mallavarapu CT, Rocha-Singh KJ, Mikell FL, et al. Comparative 30-day economic and clinical outcomes of platelet glycoprotein IIb/IIIa inhibitor use during elective percutaneous coronary intervention: Prairie ReoPro Versus Integrilin Cost Evaluation (PRICE) trial. Am Heart J 2001;141:402–9 | C |
| Lancaster G. Aspirin resistance, an emerging, often overlooked, factor in the management of patients with coronary artery disease. Clin Cardiol 2006;29:334 | A |
| Lanza GA, Scalone G, Battipaglia I, Barone L, Coviello I, Milo M, et al. Evidence of increased platelet reactivity in the first six months after acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2011;57(Suppl. 1):E1093 | C |
| Lassar TA, Simon DI, Croce K. Optimizing antiplatelet therapy following percutaneous coronary intervention: clinical pathways for platelet function testing. Rev Cardiovasc Med 2011;12(Suppl. 1):S23–33 | A |
| Latour JG, Theroux P, Bourassa MG. Sulfinpyrazone decreases epinephrine-induced platelet aggregation after myocardial infarction. Am J Cardiol 1982;50:938–44 | B |
| Lee K, Lee S-W, Lee J-W, Kim S-Y, Youn Y-J, Ahn M-S, et al. The significance of clopidogrel low-responsiveness on stent thrombosis and cardiac death assessed by the VerifyNow P2Y12 assay in patients with acute coronary syndrome within 6 months after drug-eluting stent implantation. Korean Circ J 2009;39:512–18 | C |
| Lee KH, Lee SH, Lee JW, Sung JK, Wang HS, Youn YJ, et al. The significance of clopidogrel low-responsiveness assessed by a point-of-care assay in acute coronary syndrome patients undergoing coronary stenting. Eur Heart J 2009;30:328 | C |
| Lee SJ, Kim JG, Ko Y, Lee BH. Clopidogrel-induced platelet inhibition may predict early ischemic complications following stent-assisted angioplasty for symptomatic cerebral atherosclerotic disease. Stroke 2011;42:e96 | C |
| Lee SP, Bae JW, Park KW, Rha SW, Bae JH, Suh JW, et al. Inhibitory interaction between calcium channel blocker and clopidogrel. Efficacy of cilostazol to overcome it. Circ J 2011;75:2581–9 | C |
| Lee S-P, Suh J-W, Park KW, Lee H-Y, Kang H-J, Koo B-K, et al. Study design and rationale of ‘Influence of Cilostazol-based triple anti-platelet therapy on ischemic complication after drug-eluting stent implantation (CILON-T)’ study: a multicenter randomized trial evaluating the efficacy of Cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease. Trials 2010;11:87 | A |
| Legrand V, Barbato E, Chenu P, Wijns W. Platelet reactivity after ASA and clopidogrel treatment assessed with VerifyNow point-of-care analysis in patients with stable coronary artery disease undergoing percutaneous coronary intervention. Eurointervention 2010;6(Suppl. H):12 | D |
| Lemesle G, Mokhtar OA, Armero S, Mancini J, Bonello C, Tahirou I, et al. Relationship between platelet reactivity inhibition and major bleeding in patients undergoing percutaneous coronary intervention. Cardiovasc Revasc Med 2010;11:207 | C |
| Leone G, Valori VM, Sandric S, Cudillo L, Bizzi B. Platelet activation and thromboembolism in patients with mitral valve prolapse. Thromb Res 1982;28:831–5 | D |
| Lev EI, Patel RT, Maresh KJ, Guthikonda S, Granada J, DeLao T, et al. Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J Am Coll Cardiol 2006;47:27–33 | D |
| Lev EI. Aspirin resistance transient laboratory finding or important clinical entity? J Am Coll Cardiol 2009;53:678–80 | A |
| Levine PL. Editorial. Platelet-function tests: predictive value. New Engl J Med 1975;292:1346–7 | A |
| Li JS, Eric Y, Berezny KY, Bokesch PM, Takahashi M, Graham J, et al. Dosing of clopidogrel for platelet inhibition in infants and young children: primary results of the Platelet Inhibition in Children On cLOpidogrel (PICOLO) Trial. Circulation 2008;117:553–9 | B, C |
| Li Y, Han Y, Wang S, Li C, Mai X, Jing Q, et al. Effects of laboratory test guided, individually tailored antiplatelet therapy after percutaneous coronary artery intervention. Circulation 2010;122:e67 | C |
| Lian J, Azmi S, Navaratnam P. Gaps and unmet needs in antiplatelet therapies for acute coronary syndrome (ACS) and chronic coronary heart disease (CHD). Value Health 2011;14:A47 | C |
| Lievykh AE, Mamchur VI, Rodionova VV, Bespala DV. Thiotriazolin enhances antiaggregatory activity of acetylsalicilic acid and reduces its gastrotoxicity in patients with coronary heart disease. Basic Clin Pharmacol Toxicol 2011;109:100 | D |
| Lim E, Carballo S, Cornelissen J, Ali ZA, Grignani R, Bellm S, et al. Dose-related efficacy of aspirin after coronary surgery in patients with Pl(A2) polymorphism (NCT00262275). Ann Thorac Surg 2007;83:134–8 | D |
| Liu L, Lin Z, Shen Z, Zhang G, Li S, Cao P. Platelet hyperfunction exists in both acute non-haemorrhagic and haemorrhagic stroke. Thromb Res 1994;75:485–90 | B, D |
| Liu P-Y, Hsu L-J, Lee P-T, Lee C-H, Chen J-Y, Li Y-H, et al. Clopidogrel hyporesponsiveness, interacting with diabetes mellitus, predicts 2-year cardiovascular outcome among Taiwanese population underging percutaneous coronary intervention. J Am Coll Cardiol 2011;58(Suppl. 1):B114 | C |
| Liusov VA. [State of the blood coagulation system in patients with myocardial infarct and chest pain and the changes in it during the use of anticoagulants and fibrinolysin.] Kardiologiia 1967;7:29–35 | D |
| Lo PR, D’Amico T, Montana M, Canino B, Romano A, Caimi G. Platelet activation markers in long-term follow-up of young subjects with acute myocardial infarction. Clin Hemorheol Microcirc 2006;35:527–8 | C |
| Loew D, Vinazzer H. Influence of simultaneous administration of low-dose heparin and acetylsalicylic acid on blood coagulation and platelet functions. Haemostasis 1974;3:319–28 | B, D |
| Lordkipanidze M, Pharand C, Diodati JG. Comparison of different methods of measurement of aspirin resistance: using the appropriate statistic (reply). Eur Heart J 2008;29:138–9 | A |
| Lotrionte M, Biondi-Zoccai GG, Agostoni P, Sheiban I. Comparison of different methods of measurement of aspirin resistance: using the appropriate statistic. Eur Heart J 2008;29:138–9 | A |
| Lu YL, Chen YD, Lu SZ. [Effects of intensive antiplatelet therapy in patients with high platelet aggregability after percutaneous coronary intervention.] Chung-Hua Hsin Hsueh Kuan Ping Tsa Chih 2007;35:793–6 | C |
| Lutsep H. Update on selecting and adjusting antiplatelet therapy for prevention of noncardiogenic, recurrent ischemic stroke. Exp Rev Cardiovasc Ther 2011;9:1295–303 | A |
| Luxembourg B, Mani H, Toennes SW, Strubel G, Klaeffling C, Daemgen-von Brevern G, et al. Factitious anticoagulant-resistance as a cause of recurrent arterial bypass graft occlusions. Thromb Haemost 2007;97:1046–8 | A |
| Luzak B, Boncler M, Rywaniak J, Wilk R, Stanczyk L, Czyz M, et al. The effect of a platelet cholesterol modulation on the acetylsalicylic acid-mediated blood platelet inhibition in hypercholesterolemic patients. Eur J Pharmacol 2011;658:91–7 | D |
| Luzak B, Boncler M, Rywaniak J, Wilk R, Stanczyk L, Rysz J, et al. The effect of platelet and plasma oxLDL and platelet cholesterol on the ASA-mediated blood platelet inhibition and platelet protein acetylation in atorvastatin-treated hypercholesterolemic patients. J Thromb Haemost 2009;7:349 | D |
| Ly HQ, Kirtane AJ, Murphy SA, Buros J, Cannon CP, Braunwald E, et al. Association of platelet counts on presentation and clinical outcomes in ST-elevation myocardial infarction (from the TIMI Trials). Am J Cardiol 2006;98:1–5 | A |
| Lynch DR, Jr, Khan FH, Vaidya D, Williams MS. Persistent high on-treatment platelet reactivity in acute coronary syndrome. J Thromb Thrombolysis 2012;33:267–73 | D |
| Macchi L, Petit E, Brizard A, Gil R, Neau JP. Aspirin resistance in vitro and hypertension in stroke patients. J Thromb Haemost 2004;2:2051–3 | D |
| Madan M, Berkowitz SD, Christie DJ, Smit AC, Sigmon KN, Tcheng JE. Determination of platelet aggregation inhibition during percutaneous coronary intervention with the platelet function analyzer PFA-100. Am Heart J 2002;144:151–8 | B |
| Madsen EH, Gehr NR, Johannesen NL, Schmidt EB, Kristensen SR. Platelet response to aspirin and clopidogrel in patients with peripheral atherosclerosis. Platelets 2011;22:537–46 | D |
| Madsen EH, Saw J, Kristensen SR, Schmidt EB, Pittendreigh C, Maurer-Spurej E. Long-term aspirin and clopidogrel response evaluated by light transmission aggregometry, VerifyNow, and thrombelastography in patients undergoing percutaneous coronary intervention. Clin Chem 2010;56:839–47 | D |
| Madsen EH, Schmidt EB, Gehr N, Johannesen NL, Kristensen SR. Testing aspirin resistance using the Platelet Function Analyzer-100: some methodological caveats and considerations. J Thromb Haemost 2008;6:386–8 | D |
| Magd AKA, Elsawy E, Ramzy A, Ragy H, Okasha N. Is there a platelet rebound effect to stopping clopidogrel? Am J Cardiol 2009;104(Suppl. 1):173D | C |
| Maiorov I. [The status of the blood anticoagulation system in angina pectoris and myocardial infarct.] Kardiologiia 1968;8:24–30 | B |
| Malanowicz W. [A preliminary evaluation of thromboelastography in coronary heart diseases.] Pol Arch Med Wewn 1966;36:351–6 | B, D |
| Malek LA, Bilinska ZT, Sitkiewicz D, Klopotowski M, Witkowski A, Ruzyllo W. Platelet reactivity on aspirin, clopidogrel and abciximab in patients with acute coronary syndromes and reduced estimated glomerular filtration rate. Thromb Res 2010;125:67–71 | D |
| Malek LA, Ruzyllo W. Bleeding and drug resistance – pitfalls of contemporary antiplatelet and antithrombotic treatment in cardiology. Postepy Kardiol Interwencyjnej 2008;4:74–9 | A |
| Malek LA, Spiewak M, Filipiak KJ, Grabowski M, Szpotanska M, Rosiak M, et al. Persistent platelet activation is related to very early cardiovascular events in patients with acute coronary syndromes. Kardiol Pol 2007;65:40–5 | D |
| Malmstrom RE, Ostergren J, Jorgensen L, Hjemdahl P, CASTOR investigators. Poor performance with a platelet counting technique to monitor clopidogrel inhibitory effects in the point-of-care setting. Thromb Res 2010;125:473–4 | C, D |
| Mamelov I. [Thrombelastographic data in diseases of the peripheral vessels.] Vestn Khir Im I I Grek 1966;97:76–7 | B, C |
| Mandava P, Anderson J, Dalmeida W, Kent TA. Platelet inhibition may explain results with abciximab In the treatment of acute ischemic stroke. Cerebrovasc Dis 2009;27:16 | B, C, D |
| Mangiacapra F, Barbato E, Patti G, Gatto L, Vizzi V, Ricottini E, et al. Point-of-care assessment of platelet reactivity after clopidogrel to predict myonecrosis in patients undergoing percutaneous coronary intervention. JACC Cardiovasc Interv 2010;3:318–23 | C |
| Mangiacapra F, Barbato E. Individual variability of response to antiplatelet therapy is an important determinant of adverse clinical outcome. High Blood Press Cardiovasc Prev 2010;17:121–30 | A |
| Mangiacapra F, Barbato E. Residual platelet reactivity: predicting short- and long-term clinical outcome in patients undergoing percutaneous coronary revascularization. Biomarkers Med 2010;4:421–34 | A |
| Mangiacapra F, De Bruyne B, Muller O, Trana C, Ntalianis A, Bartunek J, et al. High residual platelet reactivity after clopidogrel: extent of coronary atherosclerosis and periprocedural myocardial infarction in patients with stable angina undergoing percutaneous coronary intervention. JACC Cardiovasc Interv 2010;3:35–40 | C |
| Mangiacapra F, Muller O, Trana C, Ntalianis A, Wijns W, De Bruyne B, et al. High platelet reactivity after clopidogrel correlates with the extent of coronary atherosclerosis and predicts peri-procedural outcome in patients with stable angina undergoing PCI. Eur Heart J 2009;30:199 | C |
| Mangiacapra F, Patti G, Barbato E, Peace AJ, Ricottini E, Vizzi V, et al. A therapeutic window for platelet reactivity for patients undergoing elective percutaneous coronary intervention: results of the ARMYDA-PROVE (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty Platelet Reactivity for Outcome Validation Effort) study. JACC Cardiovasc Interv 2012;5:281–9 | C |
| Mangiacapra F, Patti G, Gatto L, Nusca A, Vizzi V, Ricottini E, et al. Inadequate platelet inhibition with clopidogrel measured by a point-of-care assay and poorer peri-procedural outcome in diabetic patients undergoing percutaneous coronary intervention. Eur Heart J 2009;30:784 | C |
| Mangiacapra F, Patti G, Peace A, Gatto L, Ricottini E, Vizzi V, et al. Validating ARMYDA-PRO/bleeds: A therapeutic window in platelet reactivity for patients undergoing elective percutaneous coronary intervention. G Ital Cardiol 2011;12(Suppl. 3):e8 | C |
| Mangiacapra F, Patti G, Peace A, Gatto L, Vizzi V, Ricottini E, et al. Comparison of platelet reactivity and periprocedural outcomes in patients with versus without diabetes mellitus and treated with clopidogrel and percutaneous coronary intervention. Am J Cardiol 2010;106:619–23 | C |
| Mani H, Lindhoff-Last E. [Diagnosis, causes, relevance of a complex phenomenon. Resistance to aspirin and clopidogrel.] Pharm Unserer Zeit 2009;38:342–50 | A |
| Mani H, Lindhoff-Last E. Response to Mortensen, et al. ‘Do the Behring Coagulation Timer (BCT) and the Platelet Function Analyzer PFA-100 identify the same patients as being aspirin non-responder?’ Platelets 2007;18:391–2 | A |
| Marcucci R, Gensini GF. High on-clopidogrel platelet reactivity and risk of MACE after PCI with stent implantation. Intervent Cardiol 2010;2:619–21 | A |
| Marcucci R, Giusti B, Gori AM, Paniccia R, Saracini C, Vestri A, et al. Cardiovascular death and nonfatal MI in ACS patients are predicted by residual platelet reactivity to ADP in the absence of CYP2C19*2 allele: beyond genetic screening. Eur Heart J 2009;30:197 | C |
| Marcucci R, Gori AM, Giusti B, Paniccia R, Fabbri A, Cordisco A, et al. High on treatment platelet reactivity in acute coronary syndrome patients: an acute-phase reaction? Pathophysiol Haemost Thromb 2010;37:A132 | D |
| Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, et al. Response to letter regarding article, ‘cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up’. Circulation 2009;120:e99 | A |
| Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation 2009;119:237–42 | C |
| Marcucci R, Paniccia R, Gori AM, Fabbri A, Antonucci E, Casola G, et al. Natural history of high on treatment platelet reactivity by ADP in patients with ACS undergoing PCI. J Thromb Haemost 2011;9:546 | C, D |
| Maree AO, Fitzgerald DJ. Variable platelet response to aspirin and clopidogrel in atherothrombotic disease. Circulation 2007;115:2196–207 | A |
| Maree AO, Jneid H, Fitzgerald DJ. Aspirin resistance and atherothrombotic disease. J Am Coll Cardiol 2006;48:846–7 | A |
| Martin JF, Bath PM, Burr ML. Mean platelet volume and myocardial infarction. Lancet 1992;339:1000–1 | A |
| Masik MG, Potapov BA, Kropel’nitskaia MV. [The status of the blood coagulation system during treatment of patients with diabetes mellitus.] Vrach Delo 1969;10:21–4 | C, D |
| Matetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Goldenberg I, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004;109:3171–5. [Erratum published in Circulation 2011;124:e459. Note: Bienart, Roy (corrected to Beinart, Roy)] | C |
| McCabe DJ, Harrison P, Machin SJ, Watt H, Brown MM. Measurement of the antiplatelet effects of aspirin in cerebrovascular disease. Stroke 2004;35:e146–7 | A |
| McCabe DJ, Harrison P, Mackie IJ, Sidhu PS, Lawrie AS, Purdy G, et al. Assessment of the antiplatelet effects of low to medium dose aspirin in the early and late phases after ischaemic stroke and TIA. Platelets 2005;16:269–80 | D |
| McCabe DJ, Harrison P, Sidhu PS, Brown MM, Machin SJ. Circulating reticulated platelets in the early and late phases after ischaemic stroke and transient ischaemic attack. Br J Haematol 2004;126:861–9 | C |
| McClure MW, Berkowitz SD, Sparapani R, Tuttle R, Kleiman NS, Berdan LG, et al. Clinical significance of thrombocytopenia during a non-ST-elevation acute coronary syndrome. The platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy (PURSUIT) trial experience. Circulation 1999;99:2892–900 | C |
| McGill D, McGuiness J, Lloyd J, Ardlie N. Platelet function and exercise-induced myocardial ischaemia in coronary heart disease patients. Thromb Res 1989;56:147–58 | B, D |
| McKee SA, Sane DC, Deliargyris EN. Aspirin resistance in cardiovascular disease: a review of prevalence, mechanisms, and clinical significance. Thromb Haemost 2002;88:711–15 | A |
| Meade TW, Cooper JA, Miller GJ. Platelet counts and aggregation measures in the incidence of ischaemic heart disease (IHD). Thromb Haemost 1997;78:926–9 | B |
| Medeiros FB, de Andrade AC, Angelis GA, Conrado VC, Timerman L, Farsky P, et al. Bleeding evaluation during single tooth extraction in patients with coronary artery disease and acetylsalicylic acid therapy suspension: a prospective, double-blinded, and randomized study. J Oral Maxillofac Surg 2011;69:2949–55 | D |
| Mehta AB, Mardikar HM, Shah S. Clopidogrel resistance: chemical curiosity or clinical enigma? Indian Heart J 2007;59:116–17 | A |
| Mehta H, Price MJ. Time to surgery and adverse events for patients receiving clopidogrel therapy. J Am Coll Cardiol 2011;57(Suppl. 1):E1939 | C |
| Mendez A, Huber AR. Aspirin resistance – a complex but serious phenomenon. J Kardiol 2010;17:68–9 | B, D |
| Mendolicchio GL, Zavalloni D, Corrada E, Rossi M, Marconi M, Bacci M, et al. Resistance to clopidogrel detected by real time evaluation of platelet thrombus formation. J Thromb Haemost 2009;7:896–7 | C |
| Mendolicchio GL. Efficacy assessment of antiplatelet therapy in acute coronary syndrome by time-volume analysis of platelet thrombus formation. Arterioscler Thromb Vasc Biol 2010;30:e223 | C |
| Mengistu AM, Mayer J, Boldt J, Rhm KD, Suttner SW. Usefulness of monitoring platelet function by multiple electrode aggregometry in primary coronary artery bypass surgery. J Cardiothorac Vasc Anesth 2011;25:42–7 | C, D |
| Merlini PA, Rossi M, Menozzi A, Buratti S, Brennan DM, Moliterno DJ, et al. Thrombocytopenia caused by abciximab or tirofiban and its association with clinical outcome in patients undergoing coronary stenting. Circulation 2004;109:2203–6 | C |
| Mezzano D, Quiroga T, Pereira J. The Level of Laboratory Testing Required for Diagnosis or Exclusion of a Platelet Function Disorder Using Platelet Aggregation and Secretion Assays. New York, NY: Thieme; 2009 | A |
| Michelson AD. Aspirin resistance. Pathophysiol Haemost Thromb 2006;35:5–9 | A |
| Michelson AD. Evaluation of platelet function by flow cytometry. Pathophysiol Haemost Thromb 2006;35:67–82 | A |
| Michelson AD. Platelet function testing in cardiovascular diseases. Circulation 2004;110:e489–93 | A |
| Michelson AD. Platelet function testing in cardiovascular diseases. Hematology 2005;10(Suppl. 1):132–7 | A |
| Migliorini A, Valenti R, Giuliani G, Buonamici P, Cerisano G, Carrabba N, et al. Prognostic impact of nonresponsivness platelet residual reactivity to clopidogrel therapy in patients with left main disease treated with coronary drug-eluting stent implantation. Eur Heart J 2009;30:847–8 | B, C |
| Milovanovic M, Fransson E, Hallert C, Jaremo P. Atrial fibrillation and platelet reactivity. Int J Cardiol 2010;145:357–8 | D |
| Mishra P, Kotwal J, Dutta V, Singh N, Kotwal A. Prevalence of aspirin and clopidogrel resistance in cardiac patients. Indian J Hematol Blood Transfus 2011;27:236 | D |
| Moerenhout CM, Claeys MJ, Haine S, Miljoen H, Bosmans JM, Vertessen F, et al. Clinical relevance of clopidogrel unresponsiveness during elective coronary stenting: experience with the point-of-care platelet function assay-100 C/ADP. Am Heart J 2010;159:434–8 | C |
| Mokhtar OA, Lemesle G, Armero S, Mancini J, Bonello C, Tahirou I, et al. Relationship between platelet reactivity inhibition and non-CABG related major bleeding in patients undergoing percutaneous coronary intervention. Thromb Res 2010;126:e147–9 | C |
| Montalescot G, Sideris G, Meuleman C, Bal-Dit-Sollier C, Lellouche N, Steg PG, et al. A randomized comparison of high clopidogrel loading doses in patients with non-ST-segment elevation acute coronary syndromes: the ALBION (Assessment of the Best Loading Dose of Clopidogrel to Blunt Platelet Activation, Inflammation and Ongoing Necrosis) trial. J Am Coll Cardiol 2006;48:931–8 | C |
| Morel O, Bernhard N, Desprez D, Grunebaum L, Freyssinet JM, Toti F, et al. Residual prothrombotic status in low responder patients to clopidogrel identified by Vasodilator-Stimulated Phosphoprotein Phosphorylation (VASP) analysis? Thromb Haemost 2008;99:448–51 | C, D |
| Morel O, El Ghannudi S, Jesel L, Radulescu B, Meyer N, Wiesel ML, et al. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol 2011;57:399–408 | C |
| Morel O, Faure A, Ohlmann P, Jesel L, Desprez D, Grunebaum L, et al. Impaired platelet responsiveness to clopidogrel identified by flow cytometric vasodilator-stimulated phosphoprotein (VASP) phosphorylation in patients with subacute stent thrombosis. Thromb Haemost 2007;98:896–9 | C |
| Moriyama Y, Toyohira H, Saigenji H, Shimokawa S, Taira A, Nakamura K. [Influence of acute aortic dissection, on platelet function second department of surgery, department of pharmacy: preliminary report.] Nippon Geka Gakkai Zasshi 1995;96:725 | D |
| Morris A, Aliprandi-Costa B, Brieger D. Platelet function analysis: a comparison of methods. Int J Cardiol 2010;145:167–8 | C |
| Mortensen J, Poulsen TS, Refsgaard J, Kristensen SD. Do the Behring Coagulation Timer and the Platelet Function Analyzer-100 identify the same patients as being aspirin non-responders? Platelets 2007;18:389–90 | A |
| Motoda C, Ueda H, Hayashi Y, Toyofuku M, Okimoto T, Otsuka M, et al. Impact of platelet reactivity to adenosine diphosphate before implantation of drug-eluting stents on subsequent adverse cardiac events in patients with stable angina. Circ J 2012;76:641–9 | C |
| Motoyoshi K. Platelet function 2 weeks following stent implantation and its relation with factors for stent thrombosis. With special reference to the difference between silorimus-eluting and bare metal stents. Teikyo Med J 2007;30:111–20 | D |
| Mueller C, Neumann FJ, Hochholzer W, Trenk D, Zeller T, Perruchoud AP, et al. The impact of platelet count on mortality in unstable angina/non-ST-segment elevation myocardial infarction. Am Heart J 2006;151:1214–17 | C |
| Muir AR, McMullin MF, Patterson C, McKeown PP. Associations between methods of measurement of aspirin resistance and their temporal variations in patients with ischaemic heart disease. Heart 2008;94:A137 | D |
| Muller-Schunk S, Linn J, Peters N, Spannagl M, Deisenberg M, Bruckmann H, et al. Monitoring of clopidogrel-related platelet inhibition: correlation of nonresponse with clinical outcome in supra-aortic stenting. Am J Neuroradiol 2008;29:786–91 | C |
| Murray S. Does sex affect how patients respond to ASA? CMAJ 2006;174:773 | A |
| Musumeci G, Valgimigli M, Sirbu V, Aprile A, Matiashvili A, Trivisonno A, et al. Correlation between stent strut coverage and platelet reactivity to predict outcome after coronary stenting. Eur Heart J 2009;30:674 | D |
| Myers RI. The variability of platelet response to aspirin and clopidogrel: revisiting the Caprie, Cure, Credo, and Match trials. Proc (Bayl Univ Med Cent) 2005;18:331–6 | A |
| Mylotte D, Foley D, Kenny D. Platelet function testing: methods of assessment and clinical utility. Cardiovasc Hematol Agents Med Chem 2011;9:14–24 | A |
| Nagata Y, Kurokawa K, Inomata J, Aburatani I, Maruyama M, Usuda K. Usefulness of the platelet aggregately index in predicting atherothrombotic event in patient undergoing coronary intervention. Am J Cardiol 2011;107(Suppl. 1):29A | C |
| Naidech AM, Bernstein RA, Levasseur K, Bassin SL, Bendok BR, Batjer HH, et al. Platelet dysfunction is associated with worse outcome after intracerebral hemorrhage. Stroke 2009;40:e234 | B |
| Naidech AM, Bernstein RA, Levasseur K, Bassin SL, Bendok BR, Batjer HH, et al. Platelet activity and outcome after intracerebral hemorrhage. Ann Neurol 2009;65:352–6 | D |
| Naidech AM, Jovanovic B, Liebling S, Garg RK, Bassin SL, Bendok BR, et al. Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke 2009;40:2398–401 | B |
| Nava C, Recaldin E, Franchi F. [Hemocoagulation in myocardial infarct (thromboelastographic findings).] Acta Gerontol (Milano) 1967;17:146–68 | B |
| Navarro-Nunez L, Pastor F, Lozano M, Marin F, Hurtado J, Roldan V, et al. Comparison of four tests to assess platelet reactivity in patients under dual antiplatelet therapy. J Thromb Haemost 2009;7:570 | D |
| Nazarian SM, Thompson JB, Gluckman TJ, Laws K, Jani JT, Kickler TS, et al. Clinical and laboratory factors associated with shear-dependent platelet hyper-reactivity in patients on chronic aspirin therapy. Thromb Res 2010;126:379–83 | D |
| Neubauer H, Kaiser AF, Endres HG, Kruger JC, Engelhardt A, Lask S, et al. Tailored antiplatelet therapy can overcome clopidogrel and aspirin resistance – the BOchum CLopidogrel and Aspirin Plan (BOCLA-Plan) to improve antiplatelet therapy. BMC Med 2011;9:3 | D |
| Nezami N, Nargabad ON, Ghorashi S. Letter by Nezami, et al. regarding article, ‘Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness With a VerifyNow P2Y12 Assay: Impact on Thrombosis and Safety (GRAVITAS) trial’. Circulation 2012;125:e569 | A |
| Nishikawa M, Isshiki T, Ogawa H, Kimura T, Yokoi H, Nanto S, et al. Consistent and potent platelet inhibition of prasugrel irrespective of CYP2C19 polymorphism in Japanese patients with coronary artery disease. J Thromb Haemost 2011;9:336 | C, D |
| Nitta M, Yano H, Endo T, Tsukahara K, Hibi K, Kimura K, et al. Contributing factors to high on-treatment residual platelet reactivity in patients with acute coronary syndromes. J Am Coll Cardiol 2012;59(Suppl. 1):E520 | C |
| Noren I, Garde A. Value of blood coagulation tests in ischemic cerebral disease. Eur Neurol 1986;25:330–8 | D |
| Nowakowski P. In reply to the comment on antiplatelet treatment monitoring. Anest Intens Ter 2007;39:164–5 | A |
| Ntalianis A, Cuisset T, Hamilos M, Muller O, Mangiacapra F, Trana C, et al. PCI-related myocardial infarction in patients with renal insufficiency reloaded with clopidogrel: an open-label multicenter registry. Eur Heart J 2011;32:1039 | C |
| Ntalianis A, Trana C, Muller O, Mangiacapra F, Peace A, Hamilos M, et al. Safety and efficacy of reloading doses of clopidogrel and aspirin before elective PCI to optimize periprocedural outcome in elderly. Eur Heart J 2010;31:221 | D |
| Obergfell A, Strotmann J, Bonz A, Bauersachs J, Ertl G, Walter U, et al. Impaired platelet responses to clopidogrel and ticlopidine in a patient with recurrent coronary stent stenosis. Thromb Haemost 2004;92:1446–7 | A |
| O’Brien JR. Platelet function tests and thrombosis. Adv Exp Med Biol 1972;34:43–54 | A |
| O’Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009;374:989–97 | C |
| Oestreich JH, Steinhubl SR, Ferraris SP, Akers WS. High residual platelet reactivity on standard clopidogrel maintenance dose predicts increased responsiveness to the double-standard dose in an assay-dependent manner. Thromb Haemost 2011;105:927–30 | C, D |
| Oh I-Y, Park KW, Kang S-H, Park JJ, Na S-H, Kang H-J, et al. Association of cytochrome P450 2C19*2 polymorphism with clopidogrel response variability and cardiovascular events in Koreans treated with drug-eluting stents. Heart 2012;98:139–44 | C |
| Oh I-Y, Yoon C-H, Park S-J, Park K-W, Lee H-Y, Cho H-J, et al. Cytochrome P450 2C19 loss-of-function polymorphism and cardiovascular events in patients treated with drug-eluting stent. J Am Coll Cardiol 2010;55(Suppl. 1):A113 | C |
| Ohlmann P, El Ghannudi S, Wiesel M-L, Radulescu B, Bareiss P, Chauvin M, et al. Platelet reactivity assessed by flow cytometric vasp phosphorylation is an independent predictor of death and cardiovascular death in unselected patients undergoing PCI. Am J Cardiol 2009;104(Suppl. 1):171D | C |
| Okada K, Tsukahara K, Endo T, Hibi K, Uchino K, Umemura S, et al. Carriage of reduced-function CYP2C19 allele is an independent predictor of periprocedual myocardial infarction in patients with non ST-segment elevation acute coronary syndromes. J Am Coll Cardiol 2012;59(Suppl. 1):E528 | C |
| Oliveira DC, Silva RF, Silva DJ, Lima VC. Aspirin resistance: fact or fiction. Arq Bras Cardiol 2010;95:e91–4 | A |
| Oliver WC, Kroening BJ, Waldo S, Diegel R, Brommer C, Schroeder D, et al. Coagulation tests predict bleeding in the intensive care unit after cardiac surgery. Transfusion 2010;50:180A–1A | B, C, D |
| O’Malley T, Langhorne P, Elton RA, Stewart C. Platelet size in stroke patients. Stroke 1995;26:995–9 | C, D |
| Oshima S, Noda K, Fukushima H, Nakamura S, Taniguchi I, Kugimiya F, et al. Platelet reactivity after thienopyridine treatment: assessment with screen filtration pressure method and drug-eluting stent thrombosis. J Am Coll Cardiol 2010;55(Suppl. 1):A171 | C |
| Osmancik PP, Bednar F, Pavkova L, Tousek P, Stros P, Jirasek K. Higher platelet activity is present in patients with restenosis after percutaneous coronary intervention compared with patients with an occlusion of coronary artery bypass graft. Blood Coagul Fibrinolysis 2008;19:807–12 | C, D |
| Ozben B, Tanrikulu AM, Ozben T, Caymaz O. Aspirin resistance in hypertensive patients. J Clin Hypertens 2010;12:714–20 | D |
| Paikin J, Eikelboom JW, Brons S, Rokoss MJ, Valettas N, Velianou JL, et al. Distribution of response to clopidogrel therapy using vasodilator stimulated phosphoprotein assay in patients undergoing primary percutaneous coronary intervention for ST segment elevation myocardial infarction. Can J Cardiol 2011;27(Suppl. 1):S182–3 | B, C |
| Paniccia R, Antonucci E, Maggini N, Romano E, Lucarini L, Rossi L, et al. Platelet reactivity in stable atherosclerotic disease patients on chronic antiplatelet treatment. J Thromb Haemost 2009;7:599–600 | D |
| Papathanasiou A, Goudevenos J, Tselepis AD. Resistance to aspirin and clopidogrel: possible mechanisms, laboratory investigation, and clinical significance. Hellenic J Cardiol 2007;48:352–63 | A |
| Papp E, Havasi V, Bene J, Komlosi K, Czopf L, Magyar E, et al. Glycoprotein IIIA gene (PlA) polymorphism and aspirin resistance: is there any correlation? Ann Pharmacother 2005;39:1013–18 | D |
| Park D-W, Kim Y-G, Park G-M, Hwang K-W, Kwon C-H, Jang J-Y, et al. Differential prognostic implication of a point-of-care platelet function test in acute coronary syndrome versus stable angina after percutaneous coronary intervention. Am J Cardiol 2012;109(Suppl. 1):77–8 | C |
| Park D-W, Kim Y-G, Park G-M, Hwang KW, Kwon CH, Jang JY, et al. Differential prognostic implication of a point-of-care platelet function test in patients with acute coronary syndrome versus stable angina and who undergoing percutaneous coronary intervention. Circulation 2011;124(Suppl. 1):A15095 | B, C |
| Park D-W, Lee S-W, Yun S-C, Song H-G, Ahn J-M, Lee J-Y, et al. A point-of-care platelet function assay and C-reactive protein for prediction of major cardiovascular events after drug-eluting stent implantation. J Am Coll Cardiol 2011;58:2630–9 | C |
| Park JH, Kim JS, Ahn C-M, Hong SJ, Choi JW, Ahn KJ, et al. A prospective multi-center study exploring method of clopidogrel pre-treatment undergoing conventional coronary angiogram in angina patients. Am J Cardiol 2012;109(Suppl. 1):136S | C, D |
| Park KW, Jeon KH, Kang SH, Oh IY, Cho HJ, Lee HY, et al. Clinical outcomes of high on-treatment platelet reactivity in Koreans receiving elective percutaneous coronary intervention (from results of the CROSS VERIFY study). Am J Cardiol 2011;108:1556–63 | C |
| Parodi G, Bellandi B, Venditti F, Carrabba N, Valenti R, Migliorini A, et al. Residual platelet reactivity, bleedings, and adherence to treatment in patients having coronary stent implantation treated with prasugrel. Am J Cardiol 2012;109:214–18 | C |
| Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A, Giusti B, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA 2011;306:1215–23 | C |
| Parodi G, Said K, Elfaramawy A, Hassan M, Marcucci R, Bellandi B, et al. Effectiveness of bivalirudin therapy in patients with high residual platelet activity after clopidogrel loading dose in patients undergoing elective percutanous coronary intervention: Six-month results from the Antithrombotic Regimens and Outcome (ARNO) trial. J Am Coll Cardiol 2010;56(Suppl. 1):B28 | C |
| Pastor-Perez FJ, Rivera J, Hurtado JA, Navarro L, Ruiz-Nodar JM, Roldan V, et al. Residual platelet activation: too many methods for an accurate definition in clinical practice. Eur Heart J 2009;30:195 | D |
| Patrono C. Aspirin resistance: definition, mechanisms and clinical read-outs. J Thromb Haemost 2003;1:1710–13 | A |
| Patti G, Nusca A, Mangiacapra F, Gatto L, D’Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol 2008;52:1128–33 | C |
| Patti G, Nusca A. Influence of platelet reactivity on outcome of patients with acute myocardial infarction undergoing primary angioplasty. Circ J 2011;75:2050–1 | A |
| Patti G, Pasceri V, Vizzi V, Ricottini E, Di Sciascio G. Usefulness of platelet response to clopidogrel by point-of-care testing to predict bleeding outcomes in patients undergoing percutaneous coronary intervention (from the Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty-Bleeding Study). Am J Cardiol 2011;107:995–1000 | C |
| Payne DA, Jones CI, Hayes PD, Bell PRF, Goodal AH, Naylor AR. Effect of low-dose (75 mg) clopidogrel on platelet reactivity, ADP variability, and clopidogrel resistance when given before carotid surgery. The Vascular Society Yearbook 2005. London: The Vascular Society of Great Britain and Ireland; 2005. p. 48 | D |
| Peace A, McCall M, Tedesco T, Kenny D, Conroy RM, Foley D, et al. The role of weight and enteric coating on aspirin response in cardiovascular patients. J Thromb Haemost 2010;8:2323–5 | D |
| Peace AJ, Egan K, Kavanagh GF, Tedesco AF, Foley DP, Dicker P, et al. Reducing intra-individual variation in platelet aggregation: implications for platelet function testing. J Thromb Haemost 2009;7:1941–3 | B, D |
| Perez de Prado A, Cuellas C, Diego A, de Miguel A, Fernandez-Vazquez F. Platelet reactivity and stent thrombosis: still some issues to solve. J Am Coll Cardiol 2009;54:666–7 | A |
| Perez de Prado A, Cuellas C, Diego A, de Miguel A, Samaniego B, Alonso-Orcajo N, et al. Influence of platelet reactivity and response to clopidogrel on myocardial damage following percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndrome. Thromb Res 2009;124:678–82 | C |
| Petr R, Motovska Z, Marinov L, Bilkova D, Widimsky P. Resistance to clopidogrel assessed by VASP phosphorylation is a negative prognostic factor in patients undergoing elective PCI for stable coronary artery disease: Analysis from laboratory substudy of Prague 8 trial. J Am Coll Cardiol 2010;55(Suppl. 1):A98 | C |
| Pham JP, Ueno M, Tello-Montoliu A, Ferreiro JL, Tomasello SD, Dharmashankar K, et al. Impact of gastric acid-suppressing therapies on platelet reactivity in patients with coronary artery disease treated with clopidogrel: results of a pharmacodynamic study. J Am Coll Cardiol 2011;58:1396–8 | A |
| Piedade PR, Gagliardi RJ, Damiani IT, Nassar Junior AP, Fuzaro MM, Sanvito WL. [Platelet aggregation test: application in the control of antiplatelet aggregation in the secondary prevention of stroke.] Arq Neuropsiquiatr 2003;61:764–7 | C |
| Pierri H, Wajngarten M, Chamone D, Giannini SD, Ramires JA, Bellotti G, et al. [Behavior of the platelet number and aggregation in elderly patients with coronary disease subjected to stress.] Arq Bras Cardiol 1988;51:451–3 | C, D |
| Pittens CA, Bouman HJ, van Werkum JW, ten Berg JM, Hackeng CM. Comparison between hirudin and citrate in monitoring the inhibitory effects of P2Y12 receptor antagonists with different platelet function tests. J Thromb Haemost 2009;7:1929–32 | B, C, D |
| Pliutto AM. [Changes of certain indicators of blood coagulation in patients with coronary arteriosclerosis and hypertensive disease.] Lab Delo 1974;8:465–8 | B, C, D |
| Poston R, Gu J, Brown J, Gammie J, White C, Manchio J, et al. Hypercoagulability affecting early vein graft patency does not exist after off-pump coronary artery bypass. J Cardiothorac Vasc Anesth 2005;19:11–18 | D |
| Poston R, Gu J, Manchio J, Lee A, Brown J, Gammie J, et al. Platelet function tests predict bleeding and thrombotic events after off-pump coronary bypass grafting. Eur J Cardiothorac Surg 2005;27:584–91 | D |
| Poston RS, Gu J, Brown JM, Gammie JS, White C, Nie L, et al. Endothelial injury and acquired aspirin resistance as promoters of regional thrombin formation and early vein graft failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg 2006;131:122–30 | D |
| Poston RS, Gu J, White C, Jeudy J, Nie L, Brown J, et al. Perioperative management of aspirin resistance after off-pump coronary artery bypass grafting: possible role for aprotinin. Transfusion 2008;48(Suppl.):39S–46S | D |
| Postula M, Tarchalska-Krynska B, Filipiak KJ, Kosior D, Serafin A, Huczek Z, et al. Factors responsible for ‘aspirin resistance’ – can we identify them? Kardiol Pol 2010;68:403–11 | D |
| Poulsen TS, Kastrup A, Mickley H. Is aspirin resistance or female gender associated with a high incidence of myonecrosis after nonurgent percutaneous coronary intervention? J Am Coll Cardiol 2005;45:635–6 | A |
| Poulsen TS, Kristensen SR, Atar D, Mickley H. A critical appraisal of the phenomenon of aspirin resistance: a review. Cardiology 2005;104:83–91 | A |
| Pouplard C, Blicq E, Regina S, De Labriolle A, Charbonnier B, Gruel Y. Relation between cytochrome P450 2C19 681 G>A polymorphism, platelet responsiveness to ADP and clinical outcome after elective percutaneous coronary intervention in patients treated with clopidogrel. J Thromb Haemost 2009;7:1054 | C |
| Powers ER, Fowler A, Thieling C, Diaz-Gonzales V, Devlin M, Fernandes V, et al. Point-of-care testing of Platelet Function at the time of percutaneous coronary intervention identifies patients at risk for early post-procedure ischemia. J Am Coll Cardiol 2009;53:A69 | D |
| Preisman S, Kogan A, Itzkovsky K, Leikin G, Raanani E. Modified thromboelastography evaluation of platelet dysfunction in patients undergoing coronary artery surgery. Eur J Cardiothorac Surg 2010;37:1367–74 | D |
| Preisman S, Kogan A, Itzkovsky K, Leikin G, Raanani E. The risk of postoperative bleeding in patients receiving clopidogrel can be predicted using modified bedside thromboelastography. Interact Cardiovasc Thorac Surg 2009;9:S107 | D |
| PRICE Investigators. Comparative 30-day economic and clinical outcomes of platelet glycoprotein IIb/IIIa inhibitor use during elective percutaneous coronary intervention: Prairie ReoPro versus Integrilin Cost Evaluation (PRICE) trial. Am Heart J 2001;141:402–9 | C |
| Price M, Lillie E, Angiolillo D, Teirstein P, Berger P, Tanguay JF, et al. Platelet reactivity on clopidogrel therapy and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent pharmacodynamic analysis of the GRAVITAS trial. Eur Heart J 2011;32:507–8 | B, C |
| Price MJ, Angiolillo DJ, Teirstein PS, Lillie E, Manoukian SV, Berger PB, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation 2011;124:1132–7 | B, C |
| Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097–105. [Erratum published in JAMA 2011;305:2174. Note: Stillablower, Michael E (corrected to Stillabower, Michael E)] | C |
| Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J 2008;29:992–1000 | C |
| Price MJ, Nayak KR, Barker CM, Kandzari DE, Teirstein PS. Predictors of heightened platelet reactivity despite dual-antiplatelet therapy in patients undergoing percutaneous coronary intervention. Am J Cardiol 2009;103:1339–43 | C |
| Price MJ. ‘A threshold of platelet reactivity for ischaemic events?’ and ‘The optimal threshold of high post-treatment platelet reactivity could be defined by a point-of care VerifyNow P2Y12 assay’: reply. Eur Heart J 2008;29:2187 | A |
| Price MJ. Should routine platelet testing be done on all patients undergoing percutaneous coronary intervention? The evidence base for platelet function testing in patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv 2010;3:277–83 | A |
| Price MJ. The evidence base for platelet function testing in patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv 2010;3:277–83. [Erratum published in Circ Cardiovasc Interv 2010;3:e18] | A |
| Prieto R, Martlnez-Selles M, Fernandez-Aviles F. Essential thrombocytemia and acute coronary syndrome: clinical profile and association with other thromboembolic events. Acute Card Care 2008;10:116–20 | D |
| Qayyum R, Kral BG, Yanek LR, Vaidya D, Nyquist PA, Mathias R, et al. Greater post-aspirin residual platelet aggregation is associated with increased risk of incident acute coronary syndromes in healthy individuals. Circulation 2011;124(Suppl. 1):A16658 | B |
| Qureshi H, Ahmed A, Dhillon JK. Evaluation of clinical and cost effectiveness of thromboelastography (TEG) in cardiac surgery. Transfus Altern Transfus Med 2010;11:38 | D |
| Rade JJ, Gluckman TJ, McLean RC, Thompson JB, Thiemann DR, Laws K, et al. Aspirin-insensitive platelet hyper-reactivity and thromboxane generation are independent risk factors for early vein graft occlusion after coronary artery bypass surgery. J Am Coll Cardiol 2009;53:A334 | D |
| Raman S, Jilma B. Time lag in platelet function inhibition by clopidogrel in stroke patients as measured by PFA-100. J Thromb Haemost 2004;2:2278–9 | D |
| Randall M, Storey RF, Venables GS, Gaines PA, Cleveland TJ. Variability in patient response to clopidogrel and effects on outcome from carotid stenting. J Neurol Neurosurg Psychiatry 2010;81:e69–70 | C, D |
| Range G, Thuaire C, Richard P, Chassaing S, Belle L, Cazaux P, et al. Clinical impact of response to both aspirin and clopidogrel in 1001 patients undergoing coronary stenting: the one-year results of the multicenter Verifrenchy study. Eur Heart J 2010;31:154 | D |
| Ranjadayalan K, Umachandran V, Timmis AD, Gutteridge CN. Platelet size and outcome after myocardial infarction. Lancet 1992;339:625 | B |
| Ranucci M, Baryshnikova E, Soro G, Ballotta A, De Benedetti D, Conti D, et al. Multiple electrode whole-blood aggregometry and bleeding in cardiac surgery patients receiving thienopyridines. Ann Thorac Surg 2011;91:123–9 | C |
| Ray MJ, Walters DL, Bett N, Cameron J, Wood P, Aroney C. Point-of-care testing shows clinically relevant variation in the degree of inhibition of platelets by standard-dose abciximab therapy during percutaneous coronary intervention. Catheter Cardiovasc Interv 2004;62:150–4 | B, C |
| Reed GW, Cannon CP. Personalized therapy following drug-eluting stenting using platelet function testing and C-reactive protein. J Am Coll Cardiol 2011;58:2640–1 | A |
| Ren Y, Chen Y, Zhao M, Chen J, Chen L. Comparative study of aspirin and clopidogrel in high risk ACS. Heart 2010;96:A142–3 | D |
| Ren YH, Yang TS, Wang Y, Gai LY, Liu HB, Chen L, et al. Evaluation of triple anti-platelet therapy by modified thrombelastography in patients with acute coronary syndrome. Chin Med J 2008;121:850–2 | D |
| Renaud S. Risk factors for coronary heart disease and platelet functions. Adv Exp Med Biol 1984;164:129–44 | A, B |
| Renda G, Sciartilli A, De Caterina R. Aspirin resistance. Haematologica 2003;88(Suppl. 4):43–9 | A |
| Reny J-L, Berdague P, Combescure C, Nolli S, Barazer I, Fabbro-Peray P, et al. High residual platelet reactivity assessed with specific and global tests in the ADRIE study: comparative predictive values for the recurrence of ischemic events. J Thromb Haemost 2011;9:720 | D |
| Reny J-L, Fontana P, Fabbro-Peray P, Laporte S, van Werkum JW, ten Berg JM, et al. Practical use and limitations of ADP aggregation testing to predict cardiovascular events: design and preliminary results of a meta-analysis on individual patients data. J Thromb Haemost 2011;9:337–8 | C |
| Reny JL, Quere I, de Moerloose P, Fontana P. Aspirin response variability assessed with the PFA-100 device. Thromb Haemost 2008;99:968–9 | A |
| Rha JH, Kim SR, Kim SH, Kim IG, Song CS, Choi YJ, et al. Relationships between laboratory antiplatelet resistance and clinical antiplatelet failure. Cerebrovasc Dis 2009;27:49 | D |
| Ricottini E, Patti G, Vizzi V, Nusca A, Grieco D, Pasceri V, et al. A heightened platelet response to clopidogrel by point-of-care testing predicts bleeding outcomes in patients undergoing percutaneous coronary intervention. Results of armyda-bleeds (antiplatelet therapy for reduction of myocardial damage during angioplasty-bleeding study). J Am Coll Cardiol 2011;57(Suppl. 1):E1631 | C |
| Rideg O, Komocsi A, Magyarlaki T, Tokes-Fuzesi M, Miseta A, Kovacs GL, et al. Impact of genetic variants on post-clopidogrel platelet reactivity in patients after elective percutaneous coronary intervention. Pharmacogenomics 2011;12:1269–80 | C |
| Ripley AW, Narang J, Leitch S, Fifi J, Bennett H. Clopidogrel resistance in patients requiring endovascular neurological intervention. J Neurosurg Anesthesiol 2011;23:441 | C |
| Rodzynek JJ, Leautaud P, Martin T, Schoenfeld PL, Wettendorff P, Delcourt A. Detection of a procoagulant activity in acute ischaemic heart disease. Thromb Res 1984;33:355–60 | D |
| Rohatgi S, Aronow HD. Detection and management of aspirin resistance. Crit Pathw Cardiol 2004;3:177–83 | A |
| Romashov VP. [Comparative evaluation of thromboelastographic data and some biochemical indices in myocardial infarct and stenocardia patients during anticoagulant therapy.] Ter Arkh 1964;36:11–17 | B, D |
| Ronge R, Lindemann S, Gawaz M. Sex differences in platelet reactivity and response to aspirin therapy: Comment. Dtsch Med Wochenschr 2006;131:1990 | A |
| Rouvier J, Scazziota A, Altman R. Aspirin resistance. Circulation 2002;106:e200–1 | A |
| Ryan WL, Hakenkamp K. Variable response to aspirin measured by platelet aggregation and bleeding time. Lab Med 1991;22:197–202 | B, D |
| Ryu DS, Hong CK, Sim YS, Kim CH, Jung JY, Joo JY. Anti-platelet drug resistance in the prediction of thromboembolic complications after neurointervention. J Korean Neurosurg Soc 2010;48:319–24 | D |
| Ryu J, Lee Y, Lee J, Choi J, Kim K, Chang S. Efficacy of high maintenance dose of clopidogrel to overcome high post treatment platelet P2Y12 reactivity after drug eluting coronary stent implantation. Eur Heart J 2010;31:976–7 | D |
| Saad AA, Abd Elsalam AM, Kamal GM, Abou El-Ezz NF, El-Hagracy RS. Effect of cytochrome P450 3A5 polymorphism on platelet reactivity after treatment with clopidogrel in patients scheduled for percutaneous coronary intervention. Egypt Heart J 2011;63:23–31 | C |
| Sacchi S, Curci G, Peduzzi M, Barbieri U, Piccinini L. Evaluation of platelet kinetics and some coagulation parameters in diabetic retinopathy. Panminerva Med 1981;23:21–4 | D |
| Sadic BO, Tanrikulu MA, Koc M, Ozben T, Caymaz O. Aspirin resistance in patients with chronic renal failure. Eur Heart J 2010;31:966 | D |
| Sahin DY, Koc M, Cayli M, Uysal OK, Karaarslan O, Kanadasi M, et al. [The frequency of aspirin resistance by a modified thrombelastography method and its relationship with clinical and laboratory parameters in patients with stable coronary artery disease.] Turk Kardiyol Dernegi Ars 2012;40:33–40 | D |
| Sairaku A, Nakano Y, Eno S, Hondo T, Matsuda K, Kisaka T, et al. Platelet function measured using a whole blood aggregometer can predict bleeding events. J Atheroscler Thromb 2011;18:16–23 | B, C |
| Salat A, Boehm D, Pulaki S, Murabito M, Berlakovich G, Kretschmer G, et al. Possibility of checking compliance and efficacy of antiaggregatory treatment following femoro-popliteal vein bypass surgery. Thromb Res 1998;89:91–5 | D |
| Saleh N, Hansson LO, Kohut M, Nilsson T, Tornvall P. Platelet function and myocardial injury during percutaneous coronary intervention. J Thromb Thrombolysis 2002;13:69–73 | D |
| Sambu N, Dent H, Warner T, Englyst N, Leadbeater P, Hobson A, et al. Stopping clopidogrel 1 year after drug-eluting stent (DES) implantation: is it safe? J Am Coll Cardiol 2011;58(Suppl. 1):B41 | D |
| Sambu N, Radhakrishnan A, Dent H, Calver A, Corbett S, Gray H, et al. The clopidogrel resistance in stent thrombosis (CREST) registry: the case for personalized antiplatelet therapy? J Am Coll Cardiol 2011;58(Suppl. 1):B23 | D |
| Sane DC, McKee SA, Malinin AI, Serebruany VL. Frequency of aspirin resistance in patients with congestive heart failure treated with antecedent aspirin. Am J Cardiol 2002;90:893–5 | D |
| Sano K, Sugamata W, Deyama J, Uematsu M, Fujioka D, Nakamura T, et al. Usefulness of platelet retention assay for diagnosing acute coronary syndromes and predicting clinical outcome in patients with chest pain and/or shortness of breath. Circulation 2011;124(Suppl. 1):A9446 | B, C |
| Sano T, Yamazaki H. [Progress in blood platelet function tests and anticipation of thrombosis.] Nippon Rinsho 1978;36:3836–41 | A |
| Sansalvador V, Ezcurdia I. [Thromboelastographic study of the blood coagulation during the postoperative and postinfarct periods.] Rev Clin Esp 1964;92:185–8 | B, C, D |
| Saraf S, Bensalha I, Gorog DA. Antiplatelet resistance – does it exist and how to measure it? Clin Med Cardiol 2009;3:77–91 | A |
| Saraf S, Christopoulos C, Salha IB, Stott DJ, Gorog DA. Impaired endogenous thrombolysis in acute coronary syndrome patients predicts cardiovascular death and nonfatal myocardial infarction. J Am Coll Cardiol 2010;55:2107–15 | C |
| Sarafoff N, Neumann L, Morath T, Bernlochner I, Mehilli J, Schomig A, et al. Impact of calcium channel blockers on the pharmacodynamic effect and the clinical efficacy of clopidogrel after drug eluting stenting. J Am Coll Cardiol 2011;57(Suppl. 1):E1913 | B, C |
| Sarafoff N, Neumann L, Morath T, Bernlochner I, Mehilli J, Schomig A, et al. Lack of impact of calcium-channel blockers on the pharmacodynamic effect and the clinical efficacy of clopidogrel after drug-eluting stenting. Am Heart J 2011;161:605–10 | C |
| Sardella G, Calcagno S, De Carlo C, Pennacchi M, Placentino F, Stio R, et al. Pharmacodynamic effects of switching therapy in PCI patients with high on treatment platelet reactivity and genotype variation: high clopidogrel dose versus prasugrel (reset trial). J Am Coll Cardiol 2012;59(Suppl. 1):E6 | C |
| Sardi G, Zaheeruddin S, Pakala R, Carnicero AL, Torguson R, Badr S, et al. Does on-treatment platelet reactivity testing before coronary artery bypass surgery have a value in predicting in-hospital major bleeding? J Am Coll Cardiol 2012;59(Suppl. 1):E261 | C |
| Sarkiss MG, Yusuf SW, Warneke CL, Botz G, Lakkis N, Hirch-Ginsburg C, et al. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer 2007;109:621–7 | C, D |
| Sarode R, Frenkel E. And, more on: assessing aspirin responsiveness using the VerifyNow aspirin assay. Thromb Res 2008;121:587–8 | A |
| Saunders J, Nambi V, Kimball KT, Virani SS, Morrisett JD, Lumsden AB, et al. Variability and persistence of aspirin response in lower extremity peripheral arterial disease patients. J Vasc Surg 2011;53:668–75 | D |
| Saunders JT, Nambi V, Virani S, Yang E, Bergeron A, Athamneh H, et al. Twelve month persistence of poor response to aspirin in patients with peripheral arterial disease enrolled in the effect of lipid modification on peripheral arterial disease after endovascular intervention trial (ELIMIT). J Am Coll Cardiol 2010;55(Suppl. 1):A155 | D |
| Scalone G, Coviello I, Barone L, Battipaglia I, Aurigemma C, Careri G, et al. Evidence of increased platelet reactivity in the first six months after acute ST segment elevation myocardial infarction. Thromb Res 2011;128:174–8 | C, D |
| Schaefer A, Flierl U, Seydelmann N, Bauersachs J. Impaired P2Y12 inhibition contributes to increased cardiovascular events following prehospital administration of clopidogrel in patients with ST elevation myocardial infarction. Eur Heart J Suppl 2010;12:F52–3 | C |
| Schafer A, Bonz AW, Eigenthaler M, Bauersachs J. Late thrombosis of a drug-eluting stent during combined anti-platelet therapy in a clopidogrel nonresponsive diabetic patient: shall we routinely test platelet function? Thromb Haemost 2007;97:862–5 | A |
| Schafer A, Flierl U, Kossler J, Seydelmann N, Kobsar A, Stork S, et al. Early determination of clopidogrel responsiveness by platelet reactivity index identifies patients at risk for cardiovascular events after myocardial infarction. Thromb Haemost 2011;106:141–8 | C |
| Scheen AJ, Legrand D. Aspirin and clopidogrel resistance in patients with diabetes mellitus. Eur Heart J 2006;27:2900–1 | A |
| Scheinowitz M, Slottow TLP, Pakala R, Baffour R, Bonello L, Torguson R, et al. Elevated circulating endothelial progenitor cells in stent thrombosis patients is not correlated with platelet reactivity index. J Am Coll Cardiol 2009;53:A13 | B, C |
| Schneider DJ, Herrmann HC, Lakkis N, Aguirre F, Wan Y, Aggarwal A, et al. Enhanced early inhibition of platelet aggregation with an increased bolus of tirofiban. Am J Cardiol 2002;90:1421–3 | C |
| Schneider DJ. On defining aspirin resistance. J Am Coll Cardiol 2005;46:1710–11 | A |
| Schreiber TL, Macina G, Bunnell P, Tenney RD, McNulty A, Kikel M, et al. Unstable angina or non-Q wave infarction despite long-term aspirin: response to thrombolytic therapy with implications on mechanisms. Am Heart J 1990;120:248–55 | C |
| Schror K, Huber K, Hohlfeld T. Functional testing methods for the antiplatelet effects of aspirin. Biomarkers Med 2011;5:31–42 | A |
| Schror K. What is aspirin resistance? Br J Cardiol 2010;17(Suppl. 1):S5–7 | A |
| Schulz S, Sibbing D, Braun S, Morath T, Mehilli J, Massberg S, et al. Platelet response to clopidogrel and restenosis in patients treated predominantly with drug-eluting stents. Am Heart J 2010;160:355–61 | C |
| Schulz S, Sibbing D, Morath T, von Beckerath N, Mehilli J, Byrne RA, et al. Does platelet reactivity to clopidogrel effect restenosis after DES implantation? Eur Heart J 2009;30:199 | C |
| Schwartz MB, Hawiger J, Timmons S, Friesinger GC. Platelet aggregates in ischemic heart disease. Thromb Haemost 1980;43:185–8 | B |
| Schwonberg J, Linnemann B, Toennes SW, Mani H, Lindhoff-Last E. Variability of residual platelet function despite clopidogrel treatment in patients with peripheral arterial occlusive disease – a prospective study. J Thromb Haemost 2009;7:897 | B |
| Scirica BM, Cannon CP, Cooper R, Aster RH, Brassard J, McCabe CH, et al. Drug-induced thrombocytopenia and thrombosis: evidence from patients receiving an oral glycoprotein IIb/IIIa inhibitor in the Orbofiban in Patients with Unstable coronary Syndromes (OPUS-TIMI 16) trial. J Thromb Thrombolysis 2006;22:95–102 | C |
| Seidel H, Rahman MM, Scharf RE. Monitoring of antiplatelet therapy. Current limitations, challenges, and perspectives. Hamostaseologie 2011;31:41–51 | A |
| Selvaraj CL, Van De Graaff EJ, Campbell CL, Abels BS, Marshall JP, Steinhubl SR. Point-of-care determination of baseline platelet function as a predictor of clinical outcomes in patients who present to the emergency department with chest pain. J Thromb Thrombolysis 2004;18:109–15 | C |
| Serebruany V, McKenzie M, Meister A, Fuzaylov S, Gurbel P, Atar D, et al. Whole blood impedance aggregometry for the assessment of platelet function in patients with congestive heart failure (EPCOT trial). Eur J Heart Fail 2002;4:461–7 | C, D |
| Serebruany V. Lack of outcome benefit and clopidogrel ‘resistance.’ The TRITON trial challenge. Thromb Haemost 2010;103:415–18 | A |
| Serebruany VL, Goto S. The challenge of monitoring platelet response after clopidogrel. Eur Heart J 2008;29:2833–4 | A |
| Serebruany VL, McKenzie ME, Meister AF, Fuzaylov SY, Gurbel PA, Atar D, et al. Failure of platelet parameters and biomarkers to correlate platelet function to severity and etiology of heart failure in patients enrolled in the EPCOT trial. With special reference to the Hemodyne hemostatic analyzer. Whole blood impedance aggregometry for the assessment of platelet function in patients with congestive heart failure. Pathophysiol Haemost Thromb 2002;32:8–15 | D |
| Sevcikova H, Vojacek J, Bis J, Sevcik R, Maly J, Pecka M, et al. Good short-term but not long-term reproducibility of the antiplatelet efficacy laboratory assessment. Clin Appl Thromb Hemost 2012;18:174–80 | D |
| Shalaev SV. [Development of myocardial infarction in aspirin-treated unstable angina pectoris. Repeated studies of platelet aggregation and of the thromboxane-prostacyclin system.] Kardiologiia 1992;32:27–30 | D |
| Shal’nov AI. [Ischemic heart disease and blood coagulation (data on the epidemiological examination of men 40–59 years of age by thrombelastography).] Ter Arkh 1981;53:41–6 | C, D |
| Shantsila E, Lip GY. Beyond glucose levels in diabetic patients with coronary artery disease: platelet activity and non-responsiveness to antiplatelet therapy. Thromb Haemost 2008;100:7–8 | A |
| Shantsila E, Watson T, Lip GY. Aspirin resistance: what, why and when? Thromb Res 2007;119:551–4 | A |
| Sharma RK, Reddy HK, Singh VN, Sharma R, Voelker DJ, Bhatt G. Aspirin and clopidogrel hyporesponsiveness and nonresponsiveness in patients with coronary artery stenting. Vasc Health Risk Manag 2009;5:965–72 | A |
| Sharma RK, Voelker DJ, Sharma R, Reddy HK, Dod H, Marsh JD. Evolving role of platelet function testing in coronary artery interventions. Vasc Health Risk Manag 2012;8:65–75 | A |
| Sharma S, Moffat D, Christopoulous C, Klonizakis M, Wellstead D, Farrington K, et al. Global thrombotic status predicts cardiovascular events in end-stage renal failure. Eur Heart J 2010;31:971 | B, C |
| Sharma V, Kaul S, Al-Hazzani A, Prabha TS, Rao PPKM, Dadheech S, et al. Association of C3435T multi drug resistance gene-1 polymorphism with aspirin resistance in ischemic stroke and its subtypes. J Neurol Sci 2012;315:72–6 | C |
| Sharp DS, Ben-Shlomo Y, Beswick AD, Andrew ME, Elwood PC. Platelet aggregation in whole blood is a paradoxical predictor of ischaemic stroke: Caerphilly prospective study revisited. Platelets 2005;16:320–8 | C |
| Shechter M, Merz CN, Paul-Labrador MJ, Kaul S. Blood glucose and platelet-dependent thrombosis in patients with coronary artery disease. J Am Coll Cardiol 2000;35:300–7 | D |
| Shen J, Zhang RY, Zhang Q. [Impact of statins on clopidogrel platelet inhibition in patients with acute coronary syndrome or stable angina.] Chung-Hua Hsin Hsueh Kuan Ping Tsa Chih 2008;36:807–11 | D |
| Shi ZW. [Aspirin resistant concept gives no guide to clinical application.] Chung-Hua Nei Ko Tsa Chih 2008;47:269–71 | A |
| Shimbo D, Child J, Davidson K, Geer E, Osende JI, Reddy S, et al. Exaggerated serotonin-mediated platelet reactivity as a possible link in depression and acute coronary syndromes. Am J Cardiol 2002;89:331–3 | B, D |
| Shimomura H, Hokimoto S, Ogawa H. Clinical outcomes following coronary stenting in Japanese patients with and without proton pump inhibitor. J Am Coll Cardiol 2010;55(Suppl. 1):A52 | C |
| Sholokhova GI, Iakovleva IM. [Thromboelastogram in ischemic heart disease.] Kardiologiia 1973;13:130–2 | B, C, D |
| Sibbing D, Braun S, Morath T, Mehilli J, Vogt W, Schomig A, et al. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol 2009;53:849–56 | C |
| Sibbing D, Byrne RA, Bernlochner I, Kastrati A. High platelet reactivity and clinical outcome – fact and fiction. Thromb Haemost 2011;106:191–202 | A |
| Sibbing D, Byrne RA, Kastrati A. Role of platelet function testing in clinical practice: current concepts and future perspectives. Curr Drug Targets 2011;12:1836–47 | A |
| Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation 2010;121:512–18 | C |
| Sibbing D, Mayer K, Bernlochner I, Morath T, Jaitner J, Haase U, et al. Platelet function testing guided use of prasugrel in patients with high on-clopidogrel treatment platelet reactivity reduces the risk of early stent thrombosis. J Am Coll Cardiol 2012;59(Suppl. 1):E265 | C |
| Sibbing D, Morath T, Braun S, Stegherr J, Mehilli J, Vogt W, et al. Clopidogrel response status assessed with Multiplate point-of-care analysis and the incidence and timing of stent thrombosis over six months following coronary stenting. Thromb Haemost 2010;103:151–9 | C |
| Sibbing D, Schulz S, Braun S, Morath T, Stegherr J, Mehilli J, et al. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost 2010;8:250–6 | C |
| Sibbing D, Steinhubl SR, Schulz S, Schomig A, Kastrati A. Platelet aggregation and association with stent thrombosis and bleeding in clopidogrel-treated patients: initial evidence of a therapeutic window. Eur Heart J 2010;31:147 | B, C |
| Sibbing D, Taubert D, Schomig A, Kastrati A, von Beckerath N. Pharmacokinetics of clopidogrel in patients with stent thrombosis. J Thromb Haemost 2008;6:1230–2 | C, D |
| Sibbing D, von Beckerath O, Schomig A, Kastrati A, von Beckerath N. Diabetes mellitus and platelet function after administration of aspirin and a single 600 mg dose of clopidogrel. J Thromb Haemost 2006;4:2566–8 | C |
| Siegemund A, Korner I, Scholz U. Monitoring of antiplatelet agents – necessary in patients with cardiovascular diseases? J Thromb Haemost 2009;7:877 | D |
| Silagy CA, McNeil JJ, Donnan GA, Tonkin AM, Worsam B, Campion K. The PACE pilot study: 12-month results and implications for future primary prevention trials in the elderly. (Prevention with low-dose Aspirin of Cardiovascular disease in the Elderly). J Am Geriatr Soc 1994;42:643–7 | B |
| Siller-Matula J, Delle KG, Lang IM, Neunteufl T, Kozinski M, Kubica J, et al. Phenotyping versus genotyping for prediction of adverse events in clopidogrel non-responders. J Kardiol 2011;18:189 | B, C |
| Siller-Matula JM, Christ G, Lang IM, le-Karth G, Huber K, Jilma B. Multiple electrode aggregometry predicts stent thrombosis better than the vasodilator-stimulated phosphoprotein phosphorylation assay. J Thromb Haemost 2010;8:351–9 | C |
| Siller-Matula JM, Lang I, Christ G, Jilma B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J Am Coll Cardiol 2008;52:1557–63 | C |
| Siller-Matula JM, le-Karth G, Lang IM, Neunteufl T, Kozinski M, Kubica J, et al. Phenotyping vs. genotyping for prediction of clopidogrel efficacy and safety: the PEGASUS-PCI study. J Thromb Haemost 2012;10:529–42 | C |
| Siller-Matula JM, le-Karth G, Neunteufl T, Lang I, Kozinski M, Kubica J, et al. Phenotyping versus genotyping for prediction of adverse events in clopidogrel non-responders. Eur Heart J 2011;32:172 | B, C |
| Simoons ML, de Boer MJ, van den Brand MJ, van Miltenburg AJ, Hoorntje JC, Heyndrickx GR, et al. Randomized trial of a GPIIb/IIIa platelet receptor blocker in refractory unstable angina. European Cooperative Study Group. Circulation 1994;89:596–603 | C, D |
| Simpfendorfer C, Kottke-Marchant K, Lowrie M, Anders RJ, Burns DM, Miller DP, et al. First chronic platelet glycoprotein IIb/IIIa integrin blockade. A randomized, placebo-controlled pilot study of xemilofiban in unstable angina with percutaneous coronary interventions. Circulation 1997;96:76–81 | C |
| Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011;26:1336–44 | C |
| Singh A, Feit F, Bangalore S. Does triple therapy with cilostazol decrease platelet reactivity in patients undergoing percutaneous coronary intervention? A meta analysis of randomized clinical trials. J Am Coll Cardiol 2012;59(Suppl. 1):E527 | C |
| Singh A, Gupta OP, Sangha HK. Platelet function tests in acute myocardial infarction. Indian Heart J 1980;32:25–9 | B, D |
| Singh M, Shah T, Singh P, Adigopula S, Kodumuri V, Molnar J, et al. Platelet function tests predict a relationship between on-treatment platelet reactivity and clinical outcome in patients undergoing percutaneous coronary intervention. Circulation 2011;124(Suppl. 1):A12086 | B, C |
| Singh S, Kothari SS, Bahl VK. Aspirin resistance: myth or reality? Indian Heart J 2003;55:217–22 | A |
| Smit JJJ, van Werkum JW, Heestermans AACM, Postma S, Hermanides RS, Dill T, et al. Insufficient platelet aggregation inhibition by pre-hospital clopidogrel alone vs additional high dose Tirofiban in patients with acute STEMI undergoing primary PCI resulting in worse clinical outcome. Eur Heart J 2009;30:193–4 | B |
| Smith JP, Haddad EV, Boutaud O, Oram DA, Blakemore DL, Chen QX, et al. Metabolic syndrome associates with resistance to aspirin in patients with coronary artery disease. Circulation 2009;120:S1033 | D |
| Smith PS. Who can resist aspirin? Nursing 2005;35:20 | A |
| Smitherman TC, Milam M, Woo J, Willerson JT, Frenkel EP. Elevated beta thromboglobulin in peripheral venous blood of patients with acute myocardial ischemia: direct evidence for enhanced platelet reactivity in vivo. Am J Cardiol 1981;48:395–402 | B, C, D |
| Smout J, Cleanthis M, Stansby G. Comment on ‘hyperresponsiveness of platelets in ischemic stroke’ by Fateh-Moghadam et al. Thromb Haemost 2008;99:239 | A |
| Smout J, Stansby G. Aspirin resistance. Br J Surg 2002;89:4–5 | A |
| Snoep JD, Eikenboom JCJ, Zwaginga JJ, Roest M, Patrono C, Rocca B, et al. Platelet reactivity and recurrent cardiovascular events in patients with stable cardiovascular disease using aspirin: a head-to-head comparison of different tests. J Thromb Haemost 2011;9:16 | D |
| Snoep JD, Hovens MMC, Eikenboom JCJ, Van Der Bom JG, Huisman MV. Is aspirin resistance due to noncompliance? – reply. Arch Intern Med 2008;168:550 | A |
| Snoep JD, Hovens MMC, Eikenboom JCJ, Van Der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J 2007;154:221–31 | C |
| Snoep JD, Roest M, Barendrecht A, Rosendaal FR, Van Der Bom JG. High platelet reactivity and the risk of myocardial infarction in young women. J Thromb Haemost 2009;7:275 | D |
| Sobol AB, Selmaj K, Mochecka A, Kumor A, Loba J. Aspirin responsiveness in ischemic stroke patients in relation to diabetes mellitus, lipid and hemostatic profile. Adv Clin Exp Med 2010;19:593–9 | D |
| Soffer D, Moussa I, Karatepe M, Harjai KJ, Boura J, Dixon SR, et al. Suboptimal inhibition of platelet aggregation following tirofiban bolus in patients undergoing percutaneous coronary intervention for unstable angina pectoris. Am J Cardiol 2003;91:872–5 | C, D |
| Sofi F, Marcucci R, Gori AM, Giusti B, Abbate R, Gensini GF. Clopidogrel non-responsiveness and risk of cardiovascular morbidity. An updated meta-analysis. Thromb Haemost 2010;103:841–8 | C |
| Mayo Clinic. Some people are resistant to aspirin’s protective effects. Mayo Clin Health Lett 2004;22:4 | A |
| Song T-J, Lee J-B, Suh S-H, Lee K-Y. The influence of anti-platelet resistance for the development of silent ischemia after carotid artery stenting. Int J Stroke 2010;5:162 | D |
| SoRelle R. Resisting aspirin. Circulation 2002;105:e9094–5 | A |
| Sorensen EN, Snyder TA, Lindsay MJ, Moainie SL, Feller ED, Griffith BP. Seven-fold stroke reduction in Ventricular-Assist Device (VAD) patients with titrated antiplatelet therapy. J Heart Lung Transplant 2009;28(Suppl. 1):S154 | C |
| Steinhubl SR, Campbell CL. Variability in response to aspirin: do we understand the clinical relevance? J Thromb Haemost 2005;3:665–9 | A |
| Steinhubl SR, Varanasi JS, Goldberg L. Determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol 2003;42:1336–7. [Erratum published in J Am Coll Cardiol 2004;43:154. Note: Varinasi JS (corrected to Varanasi JS)] | A |
| Steinhubl SR, Varanasi JS, Goldberg L. Determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol 2003;42:1336. [Erratum published in J Am Coll Cardiol 2004;43:154] | A |
| Steinhubl SR. The illusion of ‘optimal’ platelet inhibition. JACC Cardiovasc Interv 2012;5:278–80 | A |
| Stiefelhagen P, Krasopoulos G. Aspirin resistance increases cardiovascular risk. MMW-Fortschr Med 2009;151:27 | A |
| Strano A, Davi G. Platelet function tests and coronary heart disease. Adv Exp Med Biol 1984;164:31–47 | A |
| Suchkova EN. [Blood coagulation capacity in patients with diabetes mellitus according to biochemical and thromboelastographic data.] Ter Arkh 1965;37:80–5 | C, D |
| Sudkamp M, Mehlhorn U, Reza RM, Hekmat K, Easo J, Geissler HJ, et al. Cardiopulmonary bypass copolymer surface modification reduces neither blood loss nor transfusions in coronary artery surgery. Thorac Cardiovasc Surg 2002;50:5–10 | C, D |
| Suh J-W, Kim C-H, Oh I-Y, Yoon C-H, Cho Y-S, Youn T-J, et al. The effect of tailored antiplatelet therapeutic strategy during percutaneous coronary intervention on periprocedural myonecrosis in diabetic patients: insights from the DM-verifynow study. J Am Coll Cardiol 2012;59(Suppl. 1):E1532 | C |
| Suh JW, Lee SP, Park KW, Lee HY, Kang HJ, Koo BK, et al. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J Am Coll Cardiol 2011;57:280–9 | C |
| Sung JK, Yoon YJ, Lee NS, Lee JW, Kim J-Y, Lee SH, et al. The effect of clopidogrel low-responsiveness assessed by VerifyNow P2Y12 assay on thrombotic events after implantation of drug-eluting stent. J Am Coll Cardiol 2010;55(Suppl. 1):A206 | C |
| Suslina ZA, Tanashian MM, Domashenko MA. [Resistance to antiplatelet drugs in patients with cerebrovascular disorders.] Vestn Ross Akad Med Nauk 2011;7:3–8 | A |
| Szczeklik A, Musial J, Undas A. Reasons for resistance to aspirin in cardiovascular disease. Circulation 2002;106:e181–2 | A |
| Szczeklik A, Undas A. More on: aspirin resistance. J Thromb Haemost 2004;2:1489 | A |
| Szczeklik A. Reasons for aspirin resistance. Thromb Haemost 2011;105:1124 | A |
| Szymezak J, Moreau C, Loriot MA, Durand E, Van Viet H, Desnos M, et al. High on-clopidogrel platelet reactivity: genotyping can help to optimize antiplatelet treatment. Thromb Res 2011;128:92–5 | C |
| Takatsu Y, Yui Y, Hattori R, Sakaguchi K, Susawa T, Yui N, et al. Platelet aggregation and thromboxane B2 release in patients with acute myocardial infarction – their relation to coronary patency. Jpn Circ J 1988;52:314–20 | B |
| Tanaka KA, Szlam F, Kelly AB, Vega JD, Levy JH. Clopidogrel (Plavix) and cardiac surgical patients: implications for platelet function monitoring and postoperative bleeding. Platelets 2004;15:325–32 | D |
| Tanboga IH, Tokgoz HC, Can MM, Bezgin T, Turkyilmaz E, Akgun T, et al. The polymorphisms of CYP2C19, CYP3A5 and CYP2C9 enzymes in relation to platelet response to clopidogrel as assessed by multiplate analyser and long-term clinical outcome. Eur Heart J 2010;31:1017–18 | B |
| Tanigawa T, Nishikawa M, Kitai T, Ueda Y, Okinaka T, Makino K, et al. Increased platelet aggregability in response to shear stress in acute myocardial infarction and its inhibition by combined therapy with aspirin and cilostazol after coronary intervention. Am J Cardiol 2000;85:1054–9 | D |
| Tanrikulu AM, Ozben B, Koc M, Papila-Topal N, Ozben T, Caymaz O. Aspirin resistance in patients with chronic renal failure. J Nephrol 2011;24:636–46 | D |
| Tantry US, Bliden KP, Gurbel PA. Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J Am Coll Cardiol 2005;46:1705–9 | D |
| Tantry US, Jeong Y-H, Navarese EP, Gurbel PA. Platelet function measurement in elective percutaneous coronary intervention patients: exploring the concept of a P2Y12 inhibitor therapeutic window. JACC Cardiovasc Interv 2012;5:290–2 | A |
| Tantry US, Mahla E, Gurbel PA. Aspirin resistance. Prog Cardiovasc Dis 2009;52:141–52 | A |
| Tantry US, Bliden KP, Suarez TA, Kreutz RP, Dichiara J, Gurbel PA. Hypercoagulability, platelet function, inflammation and coronary artery disease acuity: results of the Thrombotic RIsk Progression (TRIP) study. Platelets 2010;21:360–7 | D |
| Tantry US, Gurbel PA. Assessment of oral antithrombotic therapy by platelet function testing. Nat Rev Cardiol 2011;8:572–9 | A |
| Taomoto K, Ohnishi H, Kuga Y, Nakashima K, Kodama Y, Kubota H, et al. Clinical outcome and platelet function of acute stroke by global thrombosis test (GTT) after t-PA therapy. J Thromb Haemost 2011;9:84 | B, C, D |
| Tarjan J, Salamon A, Jager R, Poor F, Barczi V, Dinnyes J, et al. [The rate of acetylsalicylic acid non-respondents among patients hospitalized for acute coronary disease, previously undergoing secondary salicylic acid prophylaxis.] Orvosi Hetil 1999;140:2339–43 | D |
| Tarzia V, Paolini C, Bottio T, Rizzoli G, Spiezia L, Dal LC, et al. Perioperative hemostasis and bleeding risk in double-antiplatelet high risk patients undergoing coronary revascularization procedure. A prospective controlled study. Eur Heart J 2010;31:62 | C, D |
| Tefferi A. Overcoming ‘aspirin resistance’ in MPN. Blood 2012;119:3377–8 | A |
| Tello-Montoliu A, Jover E, Marin F, Bernal A, Lozano ML, Sanchez-Vega B, et al. Influence of CYP2C19 polymorphisms in on-treatment platelet reactivity and prognosis in an unselected population of non ST elevation acute coronary syndrome. Eur Heart J 2011;32:264 | C |
| Tello-Montoliu A, Jover E, Marin F, Bernal A, Lozano ML, Sanchez-Vega B, et al. Influence of CYP2C19 polymorphisms in platelet reactivity and prognosis in an unselected population of non ST elevation acute coronary syndrome. Rev Esp Cardiol (Engl Ed) 2012;65:219–26 | C |
| ten Berg JM. Error in a study of the comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary artery stent implantation. JAMA 2011;305:2172–3. [Erratum for JAMA 2010;303:754–62] | A |
| Thani KB, Ilapakurti M, Ang L, Prasad A, Palakodeti V, Mahmud E. Increased incidence of peri-procedural myocardial infarction in patients pre-treated with clopidogrel and undergoing percutaneous coronary intervention: role of fibrinogen and platelet reactivity. Catheter Cardiovasc Interv 2010;75:S59 | B |
| Theakos N, Dedeilias P, Argiriou M, Roussakis A, Patronis M, Bolos K. Postoperative aspirin and clopidogrel resistance in patients submitted to on-pump coronary artery bypass surgery. Interact Cardiovasc Thorac Surg 2009;9:S106 | D |
| Thomas M, Wijeyeratne Y, May J, Fox S, Heptinstall S. P-selectin is associated with subsequent atherothrombotic events in patients with recent acute coronary syndromes treated with clopidogrel. J Thromb Haemost 2011;9:743 | C |
| Tidiane AM, Ghenim R, Bongard V, Boudou N, Dumonteil N, Hammami N, et al. Assessment of dual antiplatelet responsiveness with the point-of-care device verifynow after percutaneous coronary intervention in elderly patients (> 75 years). J Am Coll Cardiol 2010;55(Suppl. 1):A171 | D |
| Tidjane AM, Ghenim R, Bongard V, Boudou N, Dumonteil N, Hammami N, et al. Natural history of dual anti-platelet responsiveness after angioplasty in elderly patients. Arch Cardiovasc Dis Suppl 2010;2:15 | D |
| Tirosh-Wagner T, Strauss T, Rubinshtein M, Tamarin I, Mishaly D, Paret G, et al. Point of care testing in children undergoing cardiopulmonary bypass. Pediatr Blood Cancer 2011;56:794–8 | B |
| Tobin WO, Kinsella JA, Collins DR, Coughlan T, O’Neill D, Egan B, et al. Enhanced ex vivo inhibition of platelet function following addition of dipyridamole to aspirin after transient ischaemic attack or ischaemic stroke: first results from the TRinity AntiPlatelet responsiveness (TrAP) study. Br J Haematol 2011;152:640–7 | D |
| Tobin WO, Kinsella JA, Collins DR, Coughlan T, O’Neill D, Feeley TM, et al. Enhanced ex vivo inhibition of platelet function after addition of dipyridamole to aspirin in ischaemic cerebrovascular disease – interim results from the trinity antiplatelet responsiveness study. J Neurol Neurosurg Psychiatry 2009;80:e1 | D |
| Tobin WO, Kinsella JA, Murphy RP, Collins DR, Coughlan T, O’Neill D, et al. Circulating reticulated platelets influence the ex vivo response to aspirin, but not dipyridamole or clopidogrel, in the early phase after TIA or ischaemic stroke – initial results from the trinity antiplatelet responsiveness (TRAP) study. Cerebrovasc Dis 2011;31:142 | D |
| Tobin WO, Kinsella JA, Murphy RP, Collins DR, Coughlan T, O’Neill D, et al. Novel longitudinal definitions of aspirin and clopidogrel ‘non-responsiveness’ on the PFA-100: results from the trinity antiplatelet responsiveness (TRAP) study. Cerebrovasc Dis 2011;31:139 | D |
| Topcuoglu MA, Arsava EM, Ay H. Antiplatelet resistance in stroke. Expert Rev Neurother 2011;11:251–63 | A |
| Tousek P, Osmancik P, Paulu P, Kocka V, Widimsky P. Clopidogrel up-titration versus standard dose in patients with high residual platelet reactivity after percutaneous coronary intervention: a single-center pilot randomised study. Int J Cardiol 2011;150:231–2 | C |
| Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, et al. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol 2008;51:1925–34 | C |
| Trenk D, Hochholzer W, Fromm MF, Zolk O, Valina CM, Stratz C, et al. No association of paraoxonase-1 Q192R genotype with antiplatelet effects of clopidogrel in patients undergoing elective coronary stent placement. Br J Clin Pharmacol 2011;72:8 | B |
| Trenk D, Hochholzer W, Fromm MF, Zolk O, Valina CM, Stratz C, et al. Paraoxonase-1 Q192R polymorphism and antiplatelet effects of clopidogrel in patients undergoing elective coronary stent placement. Circ Cardiovasc Genet 2011;4:429–36 | C |
| Trenk D, Hochholzer W, Frundi D, Stratz C, Valina CM, Bestehorn HP, et al. Impact of cytochrome P450 3A4-metabolized statins on the antiplatelet effect of a 600-mg loading dose clopidogrel and on clinical outcome in patients undergoing elective coronary stent placement. Thromb Haemost 2008;99:174–81 | C |
| Trenk D, Hochholzer W, Valina CM, Stratz C, Bestehorn H-P, Buettner HJ, et al. Clinical events in patients undergoing percutaneous coronary intervention with bare-metal stenting after discontinuation of clopidogrel. Eur Heart J 2010;31:965 | B |
| Trenk D, Hochholzer W, Valina CM, Stratz C, Bestehorn HP, Buttner HJ, et al. Increased incidence of clinical events in patients undergoing percutaneous coronary intervention with baremetal stenting after discontinuation of clopidogrel. Br J Clin Pharmacol 2010;70:5 | B, C |
| Trenk D, Neumann FJ. Aspirin resistance an underestimated risk in patients with drug-eluting stents? J Am Coll Cardiol 2008;52:740–2 | A |
| Tschopp TB, Zucker MB. Platelet function tests in disease. Annu Rev Med 1973;24:1–18 | A |
| Tsiaousis GZ, Zairis MN, Patsourakos N, Makrygiannis S, Vogiatzidis K, Gougourela E, et al. Oral proton pump inhibitors and their impact on the effectiveness of dual anti-platelet therapy during the first year after elective coronary stenting. J Am Coll Cardiol 2009;53:A335 | C |
| Tsimikas S, Leibundgut G. Post-thienopyridine platelet response, cardiovascular outcomes, and personalized therapy: en attendant Godot. JACC Cardiovasc Interv 2010;3:657–9 | A |
| Tsui PT, Lau CL, Lo YK, Chan NY, Wu CW, Choy CC, et al. Low platelet responsiveness to clopidogrel is related to target lesion revascularization in Chinese. Am J Cardiol 2008;105(Suppl. 1):25B | C, D |
| Tsui PT, Lau CL, Lo YK, Mok NS, Lau ST. Effectiveness of clopidogrel in Chinese. Int J Cardiol 2011;147:S11 | B, C |
| Tsukahara K, Kimura K, Ebina T, Kosuge M, Hibi K, Iwahashi N, et al. The relationship between high on-treatment platelet reactivity and rapid angiographic progression of non-culprit coronary lesions in patients with acute coronary syndromes. J Am Coll Cardiol 2011;57(Suppl. 1):E928 | C |
| Tsukahara K, Kimura K, Morita S, Ebina T, Kosuge M, Hibi K, et al. Impact of high-responsiveness to dual antiplatelet therapy on bleeding complications in patients receiving drug-eluting stents. Circ J 2010;74:679–85 | C |
| Turakhia MP, Murphy SA, Pinto TL, Antman EM, Giugliano RP, Cannon CP, et al. Association of platelet count with residual thrombus in the myocardial infarct-related coronary artery among patients treated with fibrinolytic therapy for ST-segment elevation acute myocardial infarction. Am J Cardiol 2004;94:1406–10 | A |
| Uchiyama S, Yamazaki M, Maruyama S, Handa M, Ikeda Y, Fukuyama M, et al. Shear-induced platelet aggregation in cerebral ischemia. Stroke 1994;25:1547–51 | D |
| Ueno M, Ferreiro JL, Tomasello SD, Tello-Montoliu A, Capodanno D, Seecheran N, et al. Impact of pentoxifylline on platelet function profiles in patients with type 2 diabetes mellitus and coronary artery disease on dual antiplatelet therapy with aspirin and clopidogrel. JACC Cardiovasc Interv 2011;4:905–12 | D |
| Ulehlova J, Slavik L, Krcova V, Galuszka J, Vaclavik J. Platelet gene polymorphisms related to acute myocardial infarction in young patients. J Thromb Haemost 2011;9:343 | D |
| Undas A, Placzkiewicz-Jankowska E, Zielinski L, Tracz W. Lack of aspirin-induced decrease in thrombin formation in subjects resistant to aspirin. Thromb Haemost 2007;97:1056–8 | D |
| Valenti R, Migliorini A, Giuliani G, Parodi G, Buonamici P, Cerisano G, et al. Nonresponsiveness to clopidogrel and long-term clinical outcome after drug-eluting stenting for unprotected left main coronary disease. Am J Cardiol 2009;104(Suppl. 1):97D | C |
| Valenti R, Migliorini A, Parodi G, Marcucci R, Buonamici P, Cerisano G, et al. Diabetes mellitus requiring insulin and high residual platelet reactivity after clopidogrel loading dose are predictors of long-term clinical outcome in patients with acute coronary syndrome: the RECLOSE2-ACS diabetes mellitus substudy. J Am Coll Cardiol 2011;58(Suppl. 1):B114 | C |
| Valenti R, Parodi G, Antoniucci D. High residual platelet reactivity and thrombotic events – reply. JAMA 2011;306:2561–2 | A, B, C |
| Valenti R, Parodi G, Migliorini A, Marcucci R, Buonamici P, Cerisano G, et al. The impact of high residual platelet reactivity after clopidogrel loading on long-term clinical outcome of patients with acute coronary syndromes receiving an invasive treatment: the RECLOSE 2-ACS trial. J Am Coll Cardiol 2011;58(Suppl. 1):B16 | C |
| Valenti R, Vergara R, Migliorini A, Carrabba N, Cerisano G, Parodi G, et al. High residual platelet reactivity after clopidogrel loading and clinical outcome after drug-eluting stenting for chronic total occlusion. G Ital Cardiol 2011;12(Suppl. 1):36S | C |
| Valgimigli M, Campo G, Ferrari R, de Cesare N, Meliga E, Vranckx P, et al. Response to letter regarding article, ‘intensifying platelet inhibition with tirofiban in poor responders to aspirin, clopidogrel, or both agents undergoing elective coronary intervention: results from the double-blind, prospective, randomized tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel study’. Circulation 2010;121:e236 | A |
| Valles J, Santos MT, Fuset MP, Moscardo A, Ruano M, Perez F, et al. Partial inhibition of platelet thromboxane A2 synthesis by aspirin is associated with myonecrosis in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2007;99:19–25 | D |
| van Werkum JW, Hackeng CM, Smit J-J, van’t Hof AWJ, Verheugt FWA, ten Berg JM. Monitoring antiplatelet therapy with point-of-care platelet function assays: a review of the evidence. Future Cardiol 2008;4:33–55 | A |
| van Werkum JW, Kleibeuker M, Mieremet N, ten Berg JM, Hackeng CM. Evaluation of the platelet response to clopidogrel with light transmittance aggregometry: peak aggregation or late aggregation? J Thromb Haemost 2007;5:884–6 | C, D |
| van Werkum JW, ten Berg JM. Platelet reactivity as a risk-factor for stent thrombosis: can this be one of the currently available appropriate methods to determine platelet reactivity? Eurointervention 2008;4(Suppl. C):11–16 | A |
| van Werkum JW, Topcu Y, Postma S, Kelder JC, Hackeng CM, ten Berg JM, et al. Effects of diabetes mellitus on platelet reactivity after dual antiplatelet therapy with aspirin and clopidogrel. Thromb Haemost 2008;99:637–9 | C, D |
| van Werkum JW, van der Stelt CA, Seesing TH, Hackeng CM, ten Berg JM. A head-to-head comparison between the VerifyNow P2Y12 assay and light transmittance aggregometry for monitoring the individual platelet response to clopidogrel in patients undergoing elective percutaneous coronary intervention. J Thromb Haemost 2006;4:2516–18 | C, D |
| van Werkum JW, van der Stelt CA, Seesing TH, ten Berg JM, Hackeng CM. The flow cytometric VASP assay can be used to determine the effectiveness of clopidogrel in patients treated with abciximab. J Thromb Haemost 2007;5:881–3 | C, D |
| Varanasi JS, Steinhubl SR. Antiplatelet effect of aspirin in patients with cerebrovascular disease. Stroke 2004;35:e144–5 | A |
| Vatakencherry A, Narang J, Kim J, Wasnick J, Langer D, Bennett H. Aspirin resistance in neurosurgical vascular bypass procedures. J Neurosurg Anesthesiol 2008;20:294–5 | D |
| Verschuren JJ, Boden H, Wessels JA, Guchelaar H-J, Schalij MJ, Jukema JW. Platelet pharmacogenetics in common clinical practice. Circulation 2011;124(Suppl. 1):A12668 | C |
| Vila PM, Zafar MU, Badimon JJ. Platelet reactivity and nonresponse to dual antiplatelet therapy: a review. Platelets 2009;20:531–8 | A |
| Violi F, Pignatelli P, Basili S. Letter by Violi et al. regarding article, ‘Association of cyclooxygenase-1-dependent and -independent platelet function assays with adverse clinical outcomes in aspirin-treated patients presenting for cardiac catheterization’. Circulation 2010;122:e429 | A |
| Violi F, Pignatelli P. Aspirin resistance. Lancet 2006;367:2059–60 | A |
| Violi F, Pignatelli P. The need for a consistent definition of ‘aspirin resistance’. J Thromb Haemost 2006;4:1618–19 | A |
| Vlachojannis GJ, Dimitropoulos G, Alexopoulos D. Clopidogrel resistance: current aspects and future directions. Hell J Cardiol 2011;52:236–45 | A |
| Volenti R, Vergara R, Migliorini A, Carrabba N, Cerisano G, Parodi G, et al. High residual platelet reactivity after clopidogrel loading and clinical outcome after drug-eluting stenting for chronic total occlusion. J Am Coll Cardiol 2010;56(Suppl. 1):B49 | C |
| von Kaulla E, von Kaulla KN. [Detection of a thrombotic tendency by means of coagulation tests (author’s transl).] MMW Munch Med Wochenschr 1974;116:1387–96 | A |
| von Pape KW, Strupp G, Bonzel T, Bohner J. Effect of compliance and dosage adaptation of long term aspirin on platelet function with PFA-100 in patients after myocardial infarction. Thromb Haemost 2005;94:889–91 | D |
| Von Pape KW, Dzijan-Horn M, Bohner J, Spannagl M, Weisser H, Calatzis A. Control of aspirin effect in chronic cardiovascular patients using two whole blood platelet function assays: PFA-100 and Multiplate. Hamostaseologie 2007;27:155–60 | D |
| Voora D, Horton J, Shah SH, Shaw LK, Newby LK. Polymorphisms associated with in vitro aspirin resistance are not associated with clinical outcomes in patients with coronary artery disease who report regular aspirin use. Am Heart J 2011;162:166–72 | C |
| Waheed R, Lockhart MK, Gopinath D, Mohammed A, Wazir S, Quealy K, et al. Prevalence of aspirin resistance in patients with peripheral arterial disease. Vasc Med 2010;15:143–4 | D |
| Walters TK, Mitchell DC, Wood RF. Low-dose aspirin fails to inhibit increased platelet reactivity in patients with peripheral vascular disease. Br J Surg 1993;80:1266–8 | D |
| Wang B. Progress of aspirin resistance. Chin J Cerebrovasc Dis 2010;7:553–6 | A |
| Wang L, Wang X, Chen F. Clopidogrel resistance is associated with long-term thrombotic events in patients implanted with drug-eluting stents. Drugs R D 2010;10:219–24 | C |
| Wang TY, Ou FS, Roe MT, Harrington RA, Ohman EM, Gibler WB, et al. Incidence and prognostic significance of thrombocytopenia developed during acute coronary syndrome in contemporary clinical practice. Circulation 2009;119:2454–62 | C |
| Wang X-D, Zhang D-F, Liu X-B, Lai Y, Qi W-G, Luo Y, et al. Modified clopidogrel loading dose according to platelet reactivity monitoring in patients carrying ABCB1 variant alleles in patients with clopidogrel resistance. Eur J Intern Med 2012;23:48–53 | C |
| Wang XD, Zhang DF, Zhuang SW, Lai Y. Modifying clopidogrel maintenance doses according to vasodilator-stimulated phosphoprotein phosphorylation index improves clinical outcome in patients with clopidogrel resistance. Clin Cardiol 2011;34:332–8 | C |
| Wang ZJ, Zhou YJ, Liu YY, Yu M, Shi DM, Zhao YX, et al. Impact of clopidogrel resistance on thrombotic events after percutaneous coronary intervention with drug-eluting stent. Thromb Res 2009;124:46–51 | C |
| Warner TD, Mitchell JA, Kirkby NS. Short thromboelastography and the identification of high platelet reactivity while on and off therapy. Heart 2012;98:679–80 | A |
| Wasowicz M, McCluskey SA, Wijeysundera DN, Yau TM, Meinri M, Beattie WS, et al. The incremental value of thrombelastography for prediction of excessive blood loss after cardiac surgery: an observational study. Anesth Analg 2010;111:331–8 | C |
| Weber AA, Zimmermann KC, Meyer-Kirchrath J, Schror K. Cyclooxygenase-2 in human platelets as a possible factor in aspirin resistance. Lancet 1999;353:900 | A |
| Weber ZA, Rodgers PT. The clinical significance of the interaction between proton pump inhibitors and clopidogrel. J Pharm Technol 2010;26:22–6 | B, C |
| Welsby IJ, Jiao K, Ortel TL, Brudney CS, Roche AM, nett-Guerrero E, et al. The kaolin-a. J Cardiothorac Vasc Anesth 2006;20:531–5 | C, D |
| Wenaweser P, Hess O. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol 2005;46:CS5–6 | D |
| Wientong P, Jongjarornprasert W, Panomvana D. Platelet aggregation and serum thromboxane B2 level after taking 60 mg/day of aspirin in type 2 diabetic Thai patients. Int J Pharm Pharm Sci 2011;3(Suppl. 3):47–50 | D |
| Willoughby SR, Stewart S, Holmes AS, Chirkov YY, Horowitz JD. Platelet nitric oxide responsiveness: a novel prognostic marker in acute coronary syndromes. Arterioscler Thromb Vasc Biol 2005;25:2661–6 | C |
| Winckers K, Poenitz V, van Oerle R, Noordermeer K, Ten CH, Nilsen DWT. A case control study on platelet response to antiplatelet therapy in survivors of late stent thrombosis. J Thromb Haemost 2009;7(S2):649 | D |
| Windelov NA, Welling KL, Ostrowski SR, Johansson PI. The prognostic value of thrombelastography in identifying neurosurgical patients with worse prognosis. Blood Coagul Fibrinolysis 2011;22:416–19 | B, C |
| Wong S, Appleberg M, Lewis DR. Antiplatelet therapy in peripheral occlusive arterial disease. ANZ J Surg 2006;76:364–72 | C |
| Wong S, Appleberg M, Ward CM, Lewis DR. Review finds further work is required to define and assess the incidence of aspirin resistance. Evid Based Cardiovasc Med 2004;8:267 | A |
| Wong S, Morel-Kopp MC, Chen Q, Appleberg M, Ward CM, Lewis DR. Overcoming aspirin resistance: increased platelet inhibition with combination aspirin and clopidogrel and high dose aspirin therapy in aspirin resistant patients with peripheral vascular disease. Thromb Haemost 2006;95:1042–3 | D |
| Woo JS, Kim W, Lee JH, Choi E-Y, Jang WS, Kim GS. Association of platelet reactivity and endothelial function and anti-inflammatory effects in patients with stable coronary artery disease. Am J Cardiol 2012;109(Suppl. 1):77S | C, D |
| Woo KS, Kim BR, Kim JE, Goh RY, Yu LH, Kim MH, et al. Determination of the prevalence of aspirin and clopidogrel resistances in patients with coronary artery disease by using various platelet-function tests. Kor J Lab Med 2010;30:460–8 | D |
| Worrall A, Armesilla A, Norell M, Khogali S, Cusack M, Smallwood A, et al. The presence of the CYP p450 C19*2 allele is associated with impaired response to clopidogrel as measured by the verifynow P2Y12 near-patient testing device in patients undergoing coronary angiography. Eur Heart J 2009;30:327 | C |
| Wurtz M, Grove EL, Wulff LN, Kaltoft AK, Tilsted HH, Jensen LO, et al. Patients with previous definite stent thrombosis have a reduced antiplatelet effect of aspirin and a larger fraction of immature platelets. JACC Cardiovasc Interv 2010;3:828–35 | D |
| Xanthopoulou I, Tsigkas G, Damelou A, Theodoropoulos KC, Kassimis G, Chouchoulis K, et al. Predictors of high on-treatment platelet reactivity early after clopidogrel loading in ST elevation myocardial infarction patients. J Am Coll Cardiol 2012;59(Suppl. 1):E485 | B |
| Xu Z-H, Jiao J-R, Yang R, Luo B-Y, Wang X-F, Wu F. Aspirin resistance: clinical significance and genetic polymorphism. J Int Med Res 2012;40:282–92 | B |
| Yahia AM, Latorre J, Gordon V, Whapham J, Malek A, Fessler RD. Thromboembolic events associated with Neuroform stent in endovascular treatment of intracranial aneurysms. J Neuroimaging 2010;20:113–17 | C |
| Yamaguchi Y, Abe T, Sato Y, Moriki T, Murata M. Point-of-care assessment after clopidogrel treatment predicts clinical outcomes of patients with cardiovascular disease: a meta-analysis of six studies. J Thromb Haemost 2011;9:544 | C |
| Yamane K, Ikeda T, Taniguchi R, Kita T, Kimura T, Horiuchi H. Characterization of the antiplatelet effect of aspirin at enrollment and after a 2-year follow-up in the real clinical setting in Japan. J Thromb Haemost 2011;9:85 | D |
| Yilmaz MB, Balbay Y, Caldir V, Ayaz S, Guray Y, Guray U, et al. Late saphenous vein graft occlusion in patients with coronary bypass: possible role of aspirin resistance. Thromb Res 2005;115:25–9 | D |
| Yong G, Rankin J, Ferguson L, Thom J, French J, Brieger D, et al. Randomized trial comparing 600- with 300-mg loading dose of clopidogrel in patients with non-ST elevation acute coronary syndrome undergoing percutaneous coronary intervention: results of the Platelet Responsiveness to Aspirin and Clopidogrel and Troponin Increment after Coronary intervention in Acute coronary Lesions (PRACTICAL) Trial. Am Heart J 2009;157:60–9 | C |
| Yoo JR, Kim SY, Kim KS, Joo S-J. Prevalence and clinical characteristics of aspirin resistance defined by impedance platelet aggregometry. Am J Cardiol 2012;109(Suppl. 1):13S | D |
| Zalewski J, Lech P, Durak M, Roslawiecka A, Gajos G, Undas A, et al. Platelet function in patients with ST-segment elevation myocardial infarction is associated with microvascular injury. Eur Heart J 2010;31:772 | D |
| Zawilska KM, Jamrozek-Jedlinska M, Duszynska M, Jedlinski I, Slomczynski M. Time-related changes of the sensitivity to anti-platelet therapy in patients with acute coronary syndrome after percutaneous coronary intervention. J Thromb Haemost 2011;9:547–8 | D |
| Zhao YJ, Zhou LJ, Li WM, Liu PD, Chen YD, Song LY. [Safety and efficacy of firebird drug-eluting stent combination tirofiban in patients with acute coronary syndrome.] Zhonghua Yi Xue Za Zhi 2008;88:2553–5 | C |
| Zhivoderov VM, Kondratchik SI. [Relation between disorders of lipid metabolism and the process of blood coagulation in myocardial infarct according to thrombelastographic data.] Ter Arkh 1975;47:68–72 | D |
| Zimmermann N, Kienzle P, Weber AA, Winter J, Gams E, Schror K, et al. Aspirin resistance after coronary artery bypass grafting. J Thorac Cardiovasc Surg 2001;121:982–4 | D |
| Zimmermann N, Kurt M, Winter J, Gams E, Wenzel F, Hohlfeld T. Detection and duration of aspirin resistance after coronary artery bypass grafting. J Thorac Cardiovasc Surg 2008;135:947–8 | D |
| Zoller H, Suss W, Gross W. [Thrombocytes, blood coagulation factors and fibrinolysis following aortocoronary venous bypass operation.] Vasa Suppl 1991;32:305–8 | D |
| Zurn CS, Geisler T, Paterok M, Gawaz M. [Influence of statins on the antiplatelet effect of clopidogrel and on cardiovascular outcome in patients after coronary intervention.] Dtsch Med Wochenschr 2008;133:817–22 | C |
| Zytkiewicz M, Gielwanowska L, Wojtasinska E, Psuja P, Zawilska K. Resistance to acetylsalicylic acid in patients after ischemic stroke. Pol Arch Med Wewn 2008;118:727–33 | D |
| Reference | Characteristic requiring further information |
|---|---|
| Chen WH, Lee PY, Ng W, Kwok JY, Cheng X, Lee SW, et al. Relation of aspirin resistance to coronary flow reserve in patients undergoing elective percutaneous coronary intervention. Am J Cardiol 2005;96:760–3 | D |
| Cho K-H, Kim J-H, Sohn S-I. The clinical significance of aspirin resistance in aspirin-taking patients with acute ischemic stroke. Int J Stroke 2010;5:311–12 | C |
| Geisler T, Mueller K, Aichele S, Stellos K, Zuern CS, Htun P, et al. Impact of inflammatory markers on platelet function and cardiovascular outcome in patients with symptomatic coronary artery disease. Eur Heart J 2010;31:156–7 | D |
| Horiuchi H, Yokoi H, Kimura T, Isshiki T, Ogawa H, Ikeda Y. More rapid and greater pharmacodynamic effects of prasugrel in Japanese patients with stable coronary artery disease (CAD) undergoing elective PCI. J Thromb Haemost 2011;9:86 | C |
| Jin L, Xu SH, Yan XW. [The effect of low dose aspirin on the platelet function in patients with acute myocardial infarction.] Chung-Hua Nei Ko Tsa Chih 1993;32:542–4 | D |
| Kojuri J, Mahmoody Y, Sabegh BZ, Jannati M, Mahboodi A, Khalili A. Dose-related effect of aspirin on laboratory-defined platelet aggregation and clinical outcome after coronary stenting. Cardiovasc Ther 2010;28:147–52 | C |
| Liu F, Liu J-C, Wang D-M, Li J, Wang L-J, Liu Y. Aspirin and clopidogrel resistance in patients with symptomatic carotid and vertebrobasilar artery stenosis. Chin J Cerebrovasc Dis 2008;5:15 | D |
| Meves SH, Overbeck U, Endres HG, Krogias C, Neubauer H. Dose-dependent effect of early antiplatelet therapy in acute ischaemic stroke. Thromb Haemost 2012;107:69–79 | D |
| Morofuji Y, So G, Hiu T, Kawakubo J, Hayashi K, Kitagawa N, et al. Preoperative analysis of platelet aggregability in carotid surgery. Neurol Surg 2011;39:459–63 | D |
| Sezer O, Stepper W, Schneider T, Welp H, Tjan TD, Sauerland C, et al. Residual platelet activity in LVAD patients with stroke. Thorac Cardiovasc Surg 2011;59:V57 | C |
| Valgimigli M. Main results of the tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel study (3T/2R). Clin Res Cardiol 2008;97:853–4 | C |
| Zhang Y, Liang J, Zhou YJ, Yuan H, Zhang YZ, Dong L. [Study on the relationship between aspirin resistance and incidence of myonecrosis after non-emergent percutaneous coronary intervention.] Chung-Hua Hsin Hsueh Kuan Ping Tsa Chih 2005;33:695–9 | D |
Appendix 7 Prognostic/diagnostic utility systematic review: articles in which outcome data are not presented in relation to platelet function test results
Below is a list of 62 articles that met the inclusion criteria for review and contained PFT results and clinical outcome data but failed to report the outcome data in relation to the test result. These articles provided no relevant information on prognostic utility of the PFT but indicate that there may be unreported relevant data.
Abumiya T, Houkin K, Morita S, Fukuhara S. Prospective study of platelet aggregation in antiplatelet therapy. Stroke 2009;40:e248.
Al-Atassi T, Lapierre H, Boodhwani M, Lam K, Forgie M, Rubens F, et al. Cerebral microembolization after bioprosthetic aortic valve replacement: an open-label study of daily warfarin + aspirin versus aspirin alone. Circulation 2011;124(Suppl.1):A14512.
Althoff TF, Fischer M, Langer E, Ziemer S, Baumann G. Sustained enhancement of residual platelet reactivity after coronary stenting in patients with myocardial infarction compared to elective patients. Thromb Res 2010;125:e190–6.
Altman R, Rivas AJ, Gonzalez CD. Bleeding tendency in dual antiplatelet therapy with aspirin/clopidogrel: Rescue of the template bleeding time in a single-center prospective study. Thromb J 2012;10:3.
Ashbrook M, Schwartz J, Heroux A, Walenga J, Jeske W, Escalante V, et al. Left ventricular assist device induced coagulation and platelet activation and effect of the current anticoagulant therapy regimen. J Am Coll Cardiol 2012;59(Suppl. 1):E880.
Atiemo AD, Ng’Alla LS, Vaidya D, Williams MS. Abnormal PFA-100 closure time is associated with increased platelet aggregation in patients presenting with chest pain. J Thromb Thrombolysis 2008;25:173–8.
Beigel R, Hod H, Fefer P, Asher E, Novikov I, Shenkman B, et al. Relation of aspirin failure to clinical outcome and to platelet response to aspirin in patients with acute myocardial infarction. Am J Cardiol 2011;107:339–42.
Beigel RS, Hod H, Shenkman B, Fefer P, Savion N, Varon D, et al. Aspirin failure is associated with worse clinical outcome but not with an inadequate platelet response to aspirin in patients with acute myocardial infarction. J Am Coll Cardiol 2010;55(Suppl. 1):A108.
Berent R, Auer J, Franklin B, Schmid P, von Duvillard SP. Platelet response to aspirin 50 and 100 mg in patients with coronary heart disease over a five-year period. Am J Cardiol 2011;108:644–50.
Berrouschot J, Schwetlick B, von Twickel G, Fischer C, Uhlemann H, Siegemund T, et al. Aspirin resistance in secondary stroke prevention. Acta Neurol Scand 2006;113:31–5.
Blanchard O, Ehrensperger E, Minuk J, Solymoss S. Antiplatelet resistance in patients with recent cerebral ischemic events. Stroke 2011;42:e346.
Bobescu E, Radoi M, Dobreanu D, Rogozea L, Doka B, Catanescu G. Drugs with effects in reduction of oxidative stress, platelets hyperactivity, hypercoagulability status and incidence of sudden death in ACS. Fundam Clin Pharmacol 2011;25:I.
Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Hackeng CM, ten Berg JM. Dual antiplatelet therapy resistance to aspirin and clopidogrel identifies patients at the highest risk of recurrent atherothrombotic events after percutaneous coronary intervention. Circulation 2010;122:A16601.
Catella-Lawson F, Kapoor S, Moretti D, De Marco S, Vigilante GJ, Cucchiara AJ, et al. Oral glycoprotein IIb/IIIa antagonism in patients with coronary artery disease. Am J Cardiol 2001;88:236–42.
Christie DJ, Kottke-Marchant K, Gorman RT. Hypersensitivity of platelets to adenosine diphosphate in patients with stable cardiovascular disease predicts major adverse events despite antiplatelet therapy. Platelets 2008;19:104–10.
Claeys MJ, Van der Planken MG, Michiels JJ, Vertessen F, Dilling D, Bosmans JM, et al. Comparison of antiplatelet effect of loading dose of clopidogrel versus abciximab during coronary intervention. Blood Coagul Fibrinolysis 2002;13:283–8.
Collet J-P, Pena A, Hulot JS, Silvain J, Barthelemy O, Beygui F, et al. Can we override clopidogrel resistance? Eur Heart J 2009;30:199.
Cuisset T, Frere C, Quilici J, Bali L, Poyet R, Morange PE, et al. Predictive value of post treatment platelet reactivity for occurrence of post-discharge bleeding after non ST elevation acute coronary syndrome. Arch Cardiovasc Dis Suppl 2010;2:1.
Djukanovic N, Todorovic Z, Obradovic S, Zamaklar-Trifunovic D, Njegomirovic S, Milic NM, et al. Abrupt cessation of one-year clopidogrel treatment is not associated with thrombotic events. J Pharmacol Sci 2011;117:12–18.
El-Atat F, Sarkar K, Kodali V, Karajgikar R, Jakkulla M, Mares A, et al. A randomized pilot trial for aggressive therapeutic approaches in aspirin-resistant patients undergoing percutaneous coronary intervention. J Invasive Cardiol 2011;23:9–13.
Etz C, Welp H, Rothenburger M, Tjan TD, Wenzelburger F, Schmidt C, et al. Analysis of platelet function during left ventricular support with the Incor and Excor system. Heart Surg Forum 2004;7:E423–7.
Ezekowitz MD, Reilly PA, Nehmiz G, Simmers TA, Nagarakanti R, Parcham-Azad K, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO study). Am J Cardiol 2007;100:1419–26.
Fateh-Moghadam S, Htun P, Tomandl B, Sander D, Stellos K, Geisler T, et al. Hyperresponsiveness of platelets in ischemic stroke. Thromb Haemost 2007;97:974–8.
Fifi JT, Hartenstein L, Ortiz RA, Niimi Y, Berenstein A. Antiplatelet drug resistance predicts thrombotic complications in patients undergoing cerebrovascular stenting. Stroke 2010;41:e282–3.
Fowler JA, Depta J, Novak E, Katzan I, Bakdash S, Kottke-Marchant K, et al. Clinical outcomes using a platelet-function guided approach for prevention of ischemic events in patients with stroke or TIA. J Am Coll Cardiol 2012;59(Suppl. 1):E1401.
Gao P, Xiong H, Zheng Z, Li L, Gao R, Hu SS. Evaluation of antiplatelet effects of a modified protocol by platelet aggregation in patients undergoing ‘one-stop’ hybrid coronary revascularization. Platelets 2010;21:183–90.
Gardner CD, Zehnder JL, Rigby AJ, Nicholus JR, Farquhar JW. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis 2007;18:787–93.
Geisler T, Mueller K, Aichele S, Bigalke B, Stellos K, Htun P, et al. Impact of inflammatory state and metabolic control on responsiveness to dual antiplatelet therapy in type 2 diabetics after PCI: prognostic relevance of residual platelet aggregability in diabetics undergoing coronary interventions. Clin Res Cardiol 2010;99:743–52. [Erratum published in Clin Res Cardiol 2010;99:769.]
Grotemeyer KH, Evers S, Fischer M, Husstedt IW. Piracetam versus acetylsalicylic acid in secondary stroke prophylaxis. A double-blind, randomized, parallel group, 2 year follow-up study. J Neurol Sci 2000;181:65–72.
Guo Z, Hasbach J, Koschinsky T. Effect of acetylsalicylic acid on renal function of type 1 diabetic patients with microalbuminuria. Diabetes Stoffwechsel 1998;7:41–7.
Gurbel PA, Bliden KP, Navickas IA, Mahla E, Dichiara J, Suarez TA, et al. Adenosine diphosphate-induced platelet-fibrin clot strength: a new thrombelastographic indicator of long-term poststenting ischemic events. Am Heart J 2010;160:346–54.
Gurbel PA, Bliden KP, Saucedo JF, Suarez TA, Dichiara J, Antonino MJ, et al. Bivalirudin and clopidogrel with and without eptifibatide for elective stenting: effects on platelet function, thrombelastographic indexes, and their relation to periprocedural infarction results of the CLEAR PLATELETS-2 (Clopidogrel with Eptifibatide to Arrest the Reactivity of Platelets) study. J Am Coll Cardiol 2009;53:648–57.
Izumi T, Miyachi S, Haraguchi K, Matsubara N, Naito T, Wakabayashi T. Ischemic complications on carotid artery stenting in the non-responder of antiplatelet agents. Intervent Neuroradiol 2011;17:199–200.
Kaymaz C, Tanboga IH, Can MM, Tokgoz HC, Sonmez K, Saglam M, et al. Gene mutations or polymorphisms in association with platelet response to aspirin and/or clopidogrel and long-term clinical outcome following coronary stenting. Eur Heart J 2011;32:244.
Kaymaz C, Tanboga IH, Tokgoz HC, Poci N, Kirca N, Aktemur T, et al. The time-dependent loss in platelet response to aspirin and clopidogrel: Implications for patient compliance to antiplatelets. Eur Heart J 2011;32:756.
Kidson-Gerber G, Weaver J, Gemmell R, Prasan AM, Chong BH. Serum thromboxane B2 compared to five other platelet function tests for the evaluation of aspirin effect in stable cardiovascular disease. Heart Lung Circ 2010;19:234–42.
Kim BJ, Lee S-W, Park S-W, Kang D-W, Kim JS, Kwon SU. Insufficient platelet inhibition is related to silent embolic cerebral infarctions after coronary angiography. Stroke 2012;43:727–32.
Kim BK, Oh SJ, Yoon SJ, Jeon DW, Ko YG, Yang JY. A randomized study assessing the effects of pretreatment with cilostazol on periprocedural myonecrosis after percutaneous coronary intervention. Yonsei Med J 2011;52:717–26.
Kwak YL, Kim JC, Choi YS, Yoo KJ, Song Y, Shim JK. Clopidogrel responsiveness regardless of the discontinuation date predicts increased blood loss and transfusion requirement after off-pump coronary artery bypass graft surgery. J Am Coll Cardiol 2010;56:1994–2002.
Lee J-Y, Park D-W, Kim Y-G, Park G-M, Hwang KW, Kwon CH, et al. Clinical implication of the aspirin resistance after drug-eluting stent implantation. Circulation 2011;124(Suppl. 1):A15074.
Lee K, Lee S-H, Lee J-W, Youn Y-J, Kim S-Y, Kim J-Y, et al. The significance of clopidogrel low-responsiveness assessed by a point-of-care assay in acute coronary syndrome patients undergoing coronary stenting. J Am Coll Cardiol 2009;53:A335–6.
Lee K, Lee S-H, Youn Y-J, Kim S-Y, Lee J-W, Kim J-Y, et al. Significance of slow-response to clopidogrel assessed by a point-of-care assay in acute coronary syndrome patients undergoing coronary stenting. Am J Cardiol 2009;103:2B.
Lordkipanidze M, Diodati JG, Palisaitis DA, Schampaert E, Turgeon J, Pharand C. Genetic determinants of response to aspirin: appraisal of 4 candidate genes. Thromb Res 2011;128:47–53.
Marcucci R, Gori AM, Paniccia R, Giusti B, Balzi D, Cordisco A, et al. High-on treatment platelet reactivity by different stimuli is a determinant of mortality in acute coronary syndrome patients: data from ami-florence 2 study. Eur Heart J 2010;31:157.
Marcucci R, Paniccia R, Consoli A, Maggini N, Miranda M, Antonucci E, et al. Monitoring of platelet function in patients with cerebral artery aneurysms undergoing endovascular treatment with stents: a review of 56 cases. J Thromb Haemost 2011;9:547.
Matetzky S, Shenkman B, Guetta V, Shechter M, Bienart R, Goldenberg I, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004;109:3171–5.
Migliorini A, Valenti R, Marcucci R, Parodi G, Giuliani G, Buonamici P, et al. High residual platelet reactivity after clopidogrel loading and long-term clinical outcome after drug-eluting stenting for unprotected left main coronary disease. Circulation 2009;120:2214–21.
Mrdovic I, Savic L, Perunicic J, Lasica R, Asanin M, Vasiljevic Z, et al. Antiplatelet therapy adjustment improved composite 30-day clinical outcome after primary percutaneous coronary intervention. Eur Heart J 2009;30:908.
Muller K, Aichele S, Herkommer M, Bigalke B, Stellos K, Htun P, et al. Impact of inflammatory markers on platelet inhibition and cardiovascular outcome including stent thrombosis in patients with symptomatic coronary artery disease. Atherosclerosis 2010;213:256–62.
Poston RS, White C, Gu J, Brown J, Gammie J, Pierson RN, et al. Aprotinin shows both hemostatic and antithrombotic effects during off-pump coronary artery bypass grafting. Ann Thorac Surg 2006;81:104–10.
Ren YH, Zhao M, Chen YD, Chen L, Liu HB, Wang Y, et al. Omeprazole affects clopidogrel efficacy but not ischemic events in patients with acute coronary syndrome undergoing elective percutaneous coronary intervention. Chin Med J 2011;124:856–61.
Rodriguez RA, Lapierre H, Boodhwani M, Lam K, Forgie M, Rubens F, et al. Effects of warfarin and aspirin versus aspirin alone on cerebral microembolization and platelet function after bioprosthetic aortic valve replacement. Can J Cardiol 2010;26:104D.
Serebruany VL, Malinin AI, Jerome SD, Lowry DR, Morgan AW, Sane DC, et al. Effects of clopidogrel and aspirin combination versus aspirin alone on platelet aggregation and major receptor expression in patients with heart failure: the Plavix Use for Treatment Of Congestive Heart Failure (PLUTO-CHF) trial. Am Heart J 2003;146:713–20.
Serebruany VL, Malinin AI, Ziai W, Pokov AN, Bhatt DL, Alberts MJ, et al. Effects of clopidogrel and aspirin in combination versus aspirin alone on platelet activation and major receptor expression in patients after recent ischemic stroke: for the Plavix Use for Treatment of Stroke (PLUTO-Stroke) trial. Stroke 2005;36:2289–92.
Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009;302:849–57.
Sobol AB, Mochecka A, Czupryniak L, Loba J. Aspirin in the treatment of ischemic stroke in type 2 diabetic patients – preliminary report. Diabetol Pol 2004;11:32–8.
Spiewak M, Malek LA, Kostrzewa G, Kisiel B, Serafin A, Filipiak KJ, et al. Influence of C3435T multidrug resistance gene-1 (MDR-1) polymorphism on platelet reactivity and prognosis in patients with acute coronary syndromes. Kardiol Pol 2009;67:827–34.
Suh J-W, Kim S-Y, Park J-S, Kim Y-S, Kang H-J, Koo B-K, et al. Comparison of triple antiplatelet therapy including triflusal and conventional dual therapy in patients who underwent drug-eluting stent implantation. Int Heart J 2009;50:701–9.
Talarico GP, Brancati M, Burzotta F, Porto I, Trani C, De Vita M, et al. Glycoprotein IIB/IIIA inhibitor to reduce postpercutaneous coronary intervention myonecrosis and improve coronary flow in diabetics: the ‘OPTIMIZE-IT’ pilot randomized study. J Cardiovasc Med 2009;10:245–51.
Tidjane MA, Voisin S, Lhermusier T, Bongard V, Sie P, Carrie D. More on: adenosine diphosphate-inducible platelet reactivity shows a pronounced age dependency in the initial phase of antiplatelet therapy with clopidogrel. J Thromb Haemost 2011;9:614–16.
Wang Z, Gao F, Men J, Ren J, Modi P, Wei M. Aspirin resistance in off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg 2012;41:108–12.
Yamamoto K, Hokimoto S, Chitose T, Morita K, Ono T, Kaikita K, et al. Impact of CYP2C19 polymorphism on residual platelet reactivity in patients with coronary heart disease during antiplatelet therapy. J Cardiol 2011;57:194–201.
Glossary
- ‘Aspirin resistant’
- Those individuals prescribed aspirin therapy classified as having insufficient inhibition of platelet reactivity (i.e. elevated platelet reactivity) based on the platelet function test and threshold specified by the authors of the relevant studies.
- ‘Aspirin sensitive’
- Those individuals prescribed aspirin therapy classified as having sufficient inhibition of platelet reactivity (i.e. low platelet reactivity) based on the platelet function test and threshold specified by the authors of the relevant studies.
- Major adverse cardiac event (MACE)
- Individual definitions vary between studies, but for the purposes of this report, this is any composite measure including death and cardiovascular events with or without ischaemic events.
- Predictive utility
- Whether or not a platelet function test with good prognostic utility is able for individual patients to distinguish between those who will and those who will not have an adverse outcome, in order to determine if treatment modification should be considered based on the test result.
- Prognostic utility
- Whether or not a platelet function test is able to distinguish between groups of patients with different average outcome risks even if it does not accurately predict individual outcome risk.
List of abbreviations
- ACE
- angiotensin-converting enzyme
- ACS
- acute coronary syndrome
- ADP
- adenosine diphosphate
- AMSTAR
- Assessment of Multiple Systematic Reviews
- ARU
- aspirin reaction unit
- CABG
- coronary artery bypass graft
- CAD
- coronary artery disease
- CEPI
- collagen/epinephrine
- CI
- confidence interval
- COX
- cyclo-oxygenase
- CVD
- cerebrovascular disease
- ET
- essential thrombocythaemia
- GI
- gastrointestinal
- GP
- general practitioner
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICH
- intracranial haemorrhage
- IPD
- individual patient data
- LEAD
- lower-extremity artery disease
- LTA
- light transmission aggregometry
- MACE
- major adverse cardiac event
- MI
- myocardial infarction
- NHS EED
- NHS Economic Evaluation Database
- NIHR
- National Institute for Health Research
- NIHSS
- National Institutes of Health Stroke Scale
- NSAID
- non-steroidal anti-inflammatory drug
- OR
- odds ratio
- PAD
- peripheral arterial disease
- PCI
- percutaneous coronary intervention
- PFA-100®
- platelet function analyser-100
- PFT
- platelet function test
- PPCI
- primary percutaneous coronary intervention
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PVD
- peripheral vascular disease
- QALY
- quality-adjusted life-year
- ROC
- receiver operating characteristic
- RR
- rate ratio
- SD
- standard deviation
- SVG
- saphenous vein graft
- TEG
- thromboelastography
- TIA
- transient ischaemic attack
- TxA2
- thromboxane A2
- TxB2
- thromboxane B2
- UA
- unstable angina
- WBA
- whole-blood aggregometry