Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/56/02. The contractual start date was in August 2009. The draft report began editorial review in November 2013 and was accepted for publication in September 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor John R Stradling was an acting consultant for ResMed (UK) Ltd from April 2013. Professor Mary J Morrell received research funding from ResMed (UK) Ltd as an unrestricted education grant that was awarded to collaborator Professor AS Simonds, Royal Brompton Hospital and Harefield NHS Foundation Trust.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by McMillan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Overview of sleep apnoea
Obstructive sleep apnoea (OSA) is caused by occlusion of the pharyngeal airway during sleep that results in a pause in breathing (apnoea). Each apnoea event or partial occlusion (hypopnoea) is associated with hypoxaemia, and is usually terminated by a brief arousal from sleep and an acute surge in blood pressure (BP). 1 The subsequent sleep disruption leads to symptoms of excessive daytime sleepiness in some,2 but not all, people with OSA. 3 When OSA occurs with symptoms of excessive daytime sleepiness it is termed obstructive sleep apnoea syndrome (OSAS).
The long-term implications of severe4 OSAS are considerable in middle-aged people. Daytime sleepiness impairs function and increases accident risk,5,6 with OSAS patients being two to four times more likely to have road traffic accidents (RTAs) as a result of reduced alertness while driving. 7 OSAS patients are also more likely to experience mood changes8,9 and reduced quality of life,10,11 which is often attributed to reduced social functioning and vitality. 12 In addition, there is some evidence of reduced cognitive function,13–16 although the extent of the neurocognitive deficits in patients with OSA is currently debated. 17
The cardiovascular impact of OSAS has been established using epidemiological data to show that people with OSA have a threefold increased likelihood of developing hypertension over 4 years, independent of other risk factors. 18,19 In addition, treatment trials in patients with severe OSAS have produced a 2 mmHg to 3 mmHg reduction in BP. 20–22 Untreated severe OSAS may be associated with an increased risk of stroke,23,24 cardiovascular disease25–27 and death. 28–30 However, the close association between OSAS and obesity,31 as well as other disorders that predispose to vascular disease, makes it difficult to determine the risk factors associated with OSAS. 32 This is especially true in older people, who are more likely to have comorbidities.
Obstructive sleep apnoea syndrome in older people
The prevalence of OSAS was reported to be approximately 4% in males and 2% in females in a US cohort of 602 employed men and women (30–60 years). 33 However, more recent estimates from the same cohort predict that up to 14% of males and 5% of females have OSAS. 34,35 This represents a substantial increase since 1990, in part because of the increasing prevalence of obesity36 and the ageing population. Specifically, the prevalence of OSA (in the absence of daytime sleepiness) appears to increase with age, although there is some evidence to suggest that it plateaus or decreases in the population over the age of 65 years. 37 In a study that used similar criteria to define sleep apnoea in younger and older people, prevalence was eight times higher in community-dwelling older men (65–100 years) compared with 3% in a younger population (20–44 years). 38 Table 1 reviews in detail the prevalence of OSA and OSAS in older people. The wide variation in estimates is likely to reflect the definitions used to quantify the OSA or OSAS and the different health status of the older populations studied, for example relatively healthy community-dwelling individuals or nursing home residents with comorbidity.
| Reference | n | Female (%) | Age (years) | Population | Prevalence of OSA (%) | |
|---|---|---|---|---|---|---|
| AHI (events/hour) ≥ 5 | AHI (events/hour) ≥ 10/≥ 15 | |||||
| Carskadon et al., 198139 | 40 | 55 | 62–86 | Community | 36 | – |
| Coleman et al., 198140 | 83 | 28 | 66 ± 5 | Sleep clinic | 39 | – |
| McGinty et al., 198241 | 26 | 0 | 64.4 ± 4.4 | Community | – | 62 |
| Roehrs et al., 198342 | 97 | 0 | 61–81 | Sleep clinic | 27 | – |
| Smallwood et al., 198343 | 30 | 20 | 50–80 | Community | 37 | – |
| Yesavage et al., 198544 | 41 | 0 | 69.5 ± 6.5 | Both | 73 | – |
| Hoch et al., 198645 | 56 | 52 | 69.3 ± 5.4 | Community | 5 | 4 |
| Knight et al., 198746 | 27 | NG | 75.8 ± 5.9 | Primary care | 37 | – |
| Mosko et al., 198847 | 46 | 65 | 68.7 ± 6.7 | Community | 28 | 16 |
| Ancoli-Israel et al., 198948 | 233 | 65 | 65–101 | Nursing home | 70 | – |
| Hoch and Reynolds 199049 | 105 | 53 | 60–91 | Community | 26 | 13 |
| Philips et al., 199250 | 92 | 52 | 64.2 ± 8.6 | Community | 15 | – |
| Ancoli-Israel et al., 199551 | 346 | 53 | 72.8 ± 6.1 | Community | – | 30 |
| 54 | 57 | 70.8 ± 6.2 | Community | – | 32 | |
| Bixler et al., 199852 | 75 | 0 | 65–100 | Community | 31 | 24 |
| Young et al., 200253 | 3448 | NG | 60–99 | Community | 54 | 20 |
| Endeshaw et al., 200454 | 58 | 76 | 77.7 ± 6.7 | Community | 56 | 19 |
| Haas et al., 200555 | 3643 | 52 | 70.2 ± 6.9 | Community | 46 | 20 |
| Hader et al., 200556 | 80 | 50 | 74.1 ± 6.3 | General clinic | 43 | 19 |
Aetiology of obstructive sleep apnoea syndrome in older people
The high prevalence of OSA in older people has led to debate regarding its causes and the consequences of the disease in this population. 57–59 In middle-aged people, pharyngeal occlusion occurs as a result of a reduction of pharyngeal dilator muscle tone during sleep60 coupled with excessive extraluminal pressure around the airway, produced by excessive adipose tissue. 61,62 In susceptible individuals these factors lead to airway collapse during sleep. Therefore, it is perhaps not surprising that neck circumference is a significant risk factor for OSA. 63,64 However, in older people, additional factors, such as an age-related reduction in pharyngeal muscle function65,66 and structural changes to the upper airway, increase the vulnerability to collapse. 67 Specifically, a decrease in the size of the upper airway lumen in older people,68 associated with an age-related lengthening of the pharyngeal airway in women69 and a descent of the hyoid bone,70 creates a predisposition to airway collapse.
Symptoms of obstructive sleep apnoea in older people
Older people report different levels of sleepiness and, compared with younger populations, rate their health differently for the same level of OSA severity. 71 This may be because older people have become habituated to the reduction in sleep quality that occurs as part of the normal ageing process. 72–74 Hence, older people may not suffer symptoms of daytime sleepiness as a result of the further sleep disruption caused by OSA. Alternatively, increased daytime sleepiness may be less debilitating in older people, who have different family and work demands and may have more time for daytime naps. In addition, older populations are more likely to have comorbidities which may cause sleep disruption75 and polypharmacy contributing to excessive daytime sleepiness. 76 Specifically, nocturia may disturb sleep, and there is some suggestion that OSA exacerbates nocturia. 77,78 Taken together, these factors could modify daytime sleepiness and obscure the symptoms of OSA. Therefore, although excessive sleepiness (regardless of its cause) is associated with increased all-cause mortality in older people,79 the proportion of sleepiness that is a result of OSA in older people, and hence could be modified by treatment, is unknown.
Both the ageing process80 and OSA14,15,81 are associated with a reduction in cognitive function. However, few studies have investigated the impact of OSA on cognitive function in older people. In those studies that have measured cognitive function in older people, cognitive impairment appears to be independently related to both OSA severity and increasing age, but the coexistence of these factors does not further increase dysfunction. 57,82,83 One explanation for the preservation of cognitive function in OSA patients is that neural compensation can overcome the cognitive deficits that are associated with the effects of intermittent hypoxia and/or sleep deprivation on the brain. 84 Whether or not the capacity for neural compensation is decreased in older people, who have less neural reserve, is unknown. 57 Recent data have shown that poorer sleep quality is associated with factors that may accelerate cognitive decline in older people and this finding requires further investigation. 85
With respect to the cardiovascular impact of OSA in older people, there are limited studies on the long-term consequences. Prospective observational data over 8 years86 showed that severe OSA in older people is associated with cardiovascular mortality, as it is in middle-aged people. Specifically, the cardiovascular risk in older people with untreated OSAS resulted from increased stroke and heart failure deaths. 23,25,86 However, a potential survival bias in people who have survived into older age means that they may be different in some way from younger people with OSAS. Alternatively, studies in older people with OSA may be selecting those who have developed OSA later in life.
Treatment of obstructive sleep apnoea syndrome
The evidence-based treatment of choice for moderate to severe OSAS in middle-aged patients is continuous positive airway pressure (CPAP) therapy, which modifies the cardinal symptom of excessive daytime sleepiness20 and is cost-effective. 20,87
A literature search of the PubMed and The Cochrane Library databases to September 2013 (discussed in more detail in Chapter 4, Reviews for external evidence), without language restrictions, for full articles reporting randomised controlled trials (RCTs) assessing the efficacy of CPAP treatment in OSAS, in a population with an average age of 60 years or older and the capacity to give informed consent, identified only three studies which included patients with cardiovascular conditions and compared CPAP with sham CPAP88 or no CPAP. 89,90 None of the studies was conducted in the UK or in a secondary care setting. Furthermore, they did not collect generic measures of health utility. These studies were not generalisable to the overall patient population; consequently, these studies were not used to inform the cost-effectiveness estimates in the health economic model, and which were derived solely from the results of Positive Airway Pressure in Older People: a randomised controlled trial (PREDICT).
Multiple systematic reviews and meta-analyses have assessed the efficacy of CPAP therapy; the most recent and relevant to date being the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) systematic review and economic analysis of CPAP devices for the treatment of OSAS. 20 This review concluded that CPAP is a clinically effective and cost-efficient treatment for moderate to severe OSA in well-defined middle-aged populations. It found that the majority of studies investigating the effect of CPAP treatment had enrolled patients between 44 and 58 years of age. However, it highlighted evidence gaps, with a need for trials in other patient groups, one such group being older people. It concluded that ‘clinical trials to define treatment effects at the extremes of age particularly in the elderly where cardiovascular comorbidity complicates assessment would be beneficial’. 20 Therefore, despite the high prevalence of OSA in older people, there is a paucity of evidence on the relative benefits or risks of CPAP treatment in older people. In addition, it cannot be assumed the benefits of CPAP treatment in younger populations will be replicated in older people.
Establishing the efficacy and cost-effectiveness of treatments for all common disease in older populations is a priority for health-care planners. PREDICT was an investigator-initiated project, funded by the HTA programme of the UK NIHR to address the evidence gap and enable the formulation of good-quality guidance on care for older people with OSAS.
Chapter 2 Methods
Trial design
Positive Airway Pressure in Older People: a randomised controlled trial (ISRCTN90464927) was a pragmatic, single-blinded (investigator-blinded), parallel-group, multicentre RCT of 12 months’ duration (Figure 1).
FIGURE 1.
Trial design.
All patients were randomised to receive CPAP plus best supportive care (BSC) or BSC only. The coprimary outcomes were the clinical effectiveness of CPAP in improving subjective sleepiness at 3 months and the cost-effectiveness of CPAP over the 12-month period.
Recruiting centres
Recruitment took place at secondary and tertiary care referral centres in England, Scotland and Wales, serving a variety of ethnic and social groups, and including both urban and rural areas.
At the start of the trial, patients were recruited through six secondary/tertiary care referral centres: Churchill Hospital (Oxford), Musgrove Park Hospital (Taunton), Royal Brompton Hospital (London), Royal Infirmary Edinburgh (Edinburgh), St James’s University Hospital (Leeds) and St Woolos Hospital (Newport). As the trial progressed, a further 18 centres requested to join via NIHR portfolio database; these centres were sent a feasibility questionnaire and subsequently nine further centres were opened, one of which was later closed because of recruitment difficulties. This left eight additional secondary care referral centres: Aintree Hospital (Liverpool), Blackpool Victoria Hospital (Blackpool), City General Hospital (Stoke-on-Trent), Freeman Hospital (Newcastle upon Tyne), Great Western Hospital (Swindon), Heartlands Hospital (Birmingham), New Cross Hospital (Wolverhampton) and Royal Berkshire Hospital (Reading). All centres had established sleep services where patients with OSAS are diagnosed and treated with CPAP therapy.
Ethical consideration
The trial was approved via the Integrated Research Application System (National Research Ethics Service/NHS/Health and Social Care Committees) (reference number 09/H0708/33). The trial was also approved by the local NHS Research and Development Office at each site.
Patients
Eligibility criteria
Patients were invited to participate if they were aged ≥ 65 years at the enrolment visit and had newly diagnosed OSAS. OSAS was defined as a oxygen desaturation index (ODI) at ≥ 4% desaturation threshold level for > 7.5 events/hour and an Epworth Sleepiness Scale (ESS) score of ≥ 9. Patients were not admitted to the trial if any of the following criteria applied:
-
previous exposure to CPAP therapy
-
arterial awake oxygen saturation < 90% on room air
-
forced expiratory volume in 1 second (FEV1)//forced vital capacity (FVC) ratio < 60%
-
substantial problems with sleepiness while driving (in those who are still driving)
-
currently using heavy goods vehicle or professional service vehicle driving licence
-
shift work
-
any very severe complication of OSAS such that CPAP therapy was mandatory
-
inability to give informed consent or comply with the protocol.
Screening
All patients potentially eligible to participate in the trial were identified from sleep and respiratory clinics predominantly by the principal investigator or nominated research staff member attending outpatient clinics and were initially assessed either by review of case notes or in person.
Once the diagnosis of OSAS was confirmed, based on the normal clinical practice in that centre, they were contacted by the principal investigator or nominated member of staff. Consecutively eligible patients were offered trial entry. Screening logs were kept documenting the number of patients assessed for eligibility and, if applicable, the reasons for non-inclusion.
Informed consent
Patients provided written informed consent at the enrolment visit.
Interventions
Patients were randomised to receive CPAP plus BSC or BSC alone.
Continuous positive airway pressure
Continuous positive airway pressure is the mainstay of medical treatment in middle-aged people with OSAS. CPAP machines are small electric pumps that deliver pressurised air to the upper airway via a hose and tightly fitting plastic mask that is worn over the nose and/or mouth during sleep. The air pressure acts as a pneumatic splint, opening up the airway, particularly at the pharyngeal level, thus preventing the soft tissue from collapsing. The pressure can be delivered as a fixed optimal pressure, which is usually manually set based on observation or titration during sleep. Alternatively, the pressure can be automatically adjusted, which is known as autotitrating CPAP. The autotitrating CPAP machines automatically increase and decrease the air pressure needed to maintain airway patency through the night, and hence they optimise OSA control. As the pressure delivered is adjusted by autotitrating machines, the mean pressure is often lower than that set on the fixed CPAP machines and therefore they are thought to reduce both the pressure required and associated side effects. However, it is important to note that no clinically important changes in adherence or other outcomes have been found using autotitrating CPAP versus fixed-level CPAP. 91 It has been proposed that autotitrating CPAP may benefit certain subgroups, although these have not yet been identified. 92 Serious side effects from CPAP are thought to be very rare.
There are many variations and adaptations to the delivery of CPAP therapy, such as humidification, which has been shown to prevent upper airway dryness associated with CPAP use,93 and various delivery interfaces (i.e. the type of mask). A recent systematic review94 highlighted the lack of research on the impact of different masks on adherence to treatment. Similarly, there is no evidence of increased adherence with humidified CPAP. 20
The recruitment centres were provided with identical autotitrating CPAP machines and humidification [AutoSet™, ResMed (UK) Ltd, Abingdon, Oxfordshire, UK] and a range of interfaces routinely used in clinical practice. They were asked to initiate CPAP treatment in keeping with their normal clinical practice by staff who were not involved in the trial outcome assessments or data analysis. Humidification and the choice of interface were made according to individual patient preference. At each follow-up visit, data on the hours of CPAP use, delivered pressure and any leaks were downloaded from the CPAP machine. All recruiting centres had established clinical expertise in the diagnosis and treatment of OSAS. The cost of the CPAP equipment will be discussed in Chapter 4.
Best supportive care
Best supportive care was defined as the provision of advice on minimising daytime sleepiness through sleep hygiene, using a nap/caffeine sleepiness management strategy and weight loss if appropriate. A booklet containing this information was compiled by the trial management team in conjunction with the Edinburgh and Oxford sleep centres and provided to all patients. This could also be supplemented with information routinely given at each centre.
Evidence for lifestyle modification as an efficacious treatment for OSAS is weak at present; however, lifestyle management is often recommended. 95,96 BSC was used as a comparator in the National Institute for Health and Care Excellence (NICE) HTA systematic review and economic analysis of CPAP machines for the treatment of OSAS. 20 This report showed that trials using BSC as a comparator produced results essentially identical to those from trials using subtherapeutic or sham CPAP as a comparator. Sham devices (CPAP machines that have been modified to deliver subtherapeutic pressure) have been validated as a placebo for CPAP; however, there is no consensus on the ideal comparator in sleep apnoea trials. Alternative comparators such as placebo pills or nasal dilator strips have been used, since it is argued that subtherapeutic CPAP may have an adverse impact on sleep quality. Two of our lead centres had substantial experience and expertise in using sham CPAP (Oxford and Edinburgh); however, these skills were not present across all recruiting centres and would have been difficult to establish widely. In addition, in a recent 6-month RCT, trial retention was lower in those allocated to sham CPAP. 15 BSC was, therefore, chosen as the trial comparator as it improved the simplicity of the trial delivery and was more appropriate for a multicentre design. The greater simplicity of BSC was also thought to be more suitable for a trial with a 12-month follow-up. Additionally, all patients were asked to continue with their normal medication and usual medical care for the duration of the trial.
Assessment
Both groups had identical visit schedules. Structured clinical assessments were performed at baseline and at 3 months and 12 months. Assessment visits were carried out at each local centre. Occasionally, research nurses agreed to see a patient in the patient’s own home if he/she was unable to attend the hospital. All patients received a telephone call from their centres at 1 week, 1 month and 6 months to record symptoms and side effects and to optimise CPAP adherence. Additionally, all patients completed monthly diaries recording their ESS score, functionality, quality of life, health-care usage, change in medication, caffeine and alcohol intake, frequency of exercise and any side effects.
All patients enrolled in the trial underwent a domiciliary overnight respiratory polygraphy (Embletta® GOLD™, Embla®, Amsterdam, the Netherlands) prior to treatment allocation, which was scored centrally. Domiciliary overnight pulse oximetry (Pulsox®–300i, Konica-Minolta Inc., Osaka, Japan) was performed at 3 and 12 months. Table 2 summarises the assessments completed at each time point.
| Assessment and measurement | Screening | Baseline | Week | Month | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
| Eligibility and exclusions | ✗ | ||||||||||||||
| Informed consent and enrolment | ✗ | ||||||||||||||
| Respiratory polygraphy | ✗ | ||||||||||||||
| Overnight pulse oximetry | ✗ | ✗ | |||||||||||||
| Clinical assessment visit | ✗ | ✗ | ✗ | ||||||||||||
| Telephone call | ✗ | ✗ | ✗ | ||||||||||||
| Patient diary returned via post | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Demographics | ✗ | ||||||||||||||
| Subjective sleepiness (ESS score) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Objective sleepiness (OSLER) test | ✗ | ✗ | ✗ | ||||||||||||
| Quality of life (EQ-5D) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Generic quality of life (SF-36) | ✗ | ✗ | ✗ | ||||||||||||
| Disease-specific quality of life (SAQLI) | ✗ | ✗ | ✗ | ||||||||||||
| Mood (HADS) | ✗ | ✗ | ✗ | ||||||||||||
| Functionality (TDS) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Nocturia | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||
| Mobility (TUG test) | ✗ | ✗ | ✗ | ||||||||||||
| Accidents | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||
| Cognitive function (MMSE, TMT–B, DSS test, RT) | ✗ | ✗ | ✗ | ||||||||||||
| Anthropometry | ✗ | ✗ | ✗ | ||||||||||||
| BP and resting pulse | ✗ | ✗ | ✗ | ||||||||||||
| Fasting bloods | ✗ | ✗ | ✗ | ||||||||||||
| Vascular events | ✗ | ✗ | ✗ | ||||||||||||
| Health-care usage | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Change in medication | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Caffeine and alcohol intake | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Exercise | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| CPAP compliance | ✗ | ✗ | ✗ | ||||||||||||
| CPAP side effects | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Adverse events | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
Outcome measures
Coprimary outcomes
The first coprimary outcome was the change in subjective sleepiness from baseline to 3 months, which was measured by the mean ESS score at 3 and 4 months. The ESS is the most widely used subjective severity scale in clinical and research practice. It is a self-administered short questionnaire with eight questions that requires the patient to rate his or her tendency to fall asleep in eight everyday situations using a scale of 0–3 to represent the chance of dozing, where 0 is ‘none’, 1 is ‘slight’, 2 is ‘moderate’ or 3 is ‘high’. The score is the sum of the eight questions and can range from 0 to 24; a reduction in the score represents an improvement. 97 Patients completed the ESS themselves, without input from family of friends. A standard operating procedure was provided for the administration of the questionnaire. In addition, the ESS score was measured monthly throughout the trial.
The other coprimary outcome was the cost-effectiveness and estimated health outcomes of providing CPAP plus BSC compared with BSC alone over 12 months. Health outcomes were expressed as quality-adjusted life-years (QALYs) using the European Quality of Life–5 Dimensions (EQ-5D)98 and Short Form questionnaire-6 Dimensions (SF-6D) derived from the Short Form questionnaire-36 items (SF-36)99 as an alternative scenario. Patients reported health-related quality of life (HRQoL) by filling in the EQ-5D questionnaire every month and the SF-36 at baseline, 3 and 12 months. The EQ-5D scores were valued using standard UK tariffs. Health-care resource use was recorded in the monthly diaries completed by the patients. Costs were evaluated in pounds sterling at 2012 prices from the UK NHS perspective. 100 The health economics analysis and results will be discussed in Chapter 4.
Secondary outcomes
Secondary outcomes included subjective sleepiness at 12 months, measured by the mean ESS score at months 10, 11 and 12, plus the following outcomes recorded at the 3- and 12-month assessments:
-
Objective sleepiness. This was measured by the Oxford Sleep Resistance (OSLER) test. The test measures the patient’s ability to resist sleep for up to 40 minutes. 101
-
Generic quality of life. This was assessed by the SF-36 questionnaires, which consist of 36 quality of life-related questions. Answers to questions are condensed into eight summary scores, which are further condensed into a mental component summary scale (MCS) score and a physical component summary scale (PCS) score. 102
-
Disease-specific quality of life. This was assessed using the Sleep Apnoea Quality of Life Index (SAQLI), a sleep apnoea questionnaire which included CPAP side effects. 103
-
Mood. This was assessed using the Hospital Anxiety and Depression Scale (HADS), a questionnaire with 14 questions, seven on each aspect. 104
-
Functionality. This was assessed by the Townsend Disability Scale (TDS), a nine-item questionnaire scored on a scale from 0 to 2 for each question (total 18). 105
-
Nocturia. The patients were asked if they had to pass urine and how many times per night on average.
-
Mobility. This was measured using the Timed Up and Go (TUG) test. This is the time taken in seconds to stand up from an armchair, walk 3 metres, turn, walk back to the chair and sit down. 106
-
Domestic accidents and RTAs. Domestic accidents were recorded in the case report forms (CRFs) and driving accidents were self-reported confidentially at each assessment.
-
Cognitive function was assessed using the following four tests:
-
Mini Mental State Examination (MMSE): a widely used screening tool that provides a measure of orientation, registration (immediate memory), short-term memory (but not long-term memory) and language functioning. It is scored out of 30; scores of 25–30 are considered normal. 107
-
Trail Making Test Part B (TMT–B): this gives information on visual search, scanning, speed of processing, mental flexibility and executive functions. It requires individuals to draw a line sequentially connecting 25 encircled numbers and letters on a piece of paper alternating between numbers and letters (e.g. 1, A, 2, B, 3, C, etc.). The score represents the amount of time required to complete the task, and performance decreases with increasing age and lower levels of education. 108
-
Digit Symbol Substitution (DSS) test: a coding exercise. At the top of a piece of paper is a code; each symbol in the code corresponds to a single-digit number. The individual is required to copy the code under rows of random numbers and complete as many as possible in 90 seconds. The score is the total number of correct answers completed in this time. 108
-
Simple and four-choice reaction time: a two-part test. The first part measures the time to react to a symbol appearing in a white box on a computer screen by pressing any button on a computer keyboard. The second part requires the individual to respond to the symbol appearing in any one of four white boxes at random. They have to respond using the allocated key on the keyboard, and the numbers of correct responses and errors are recorded.
-
-
Cardiovascular risk factors. These included systolic and diastolic office BP, fasting glucose, lipids and glycated haemoglobin (HbA1c).
-
New cardiovascular events. These included angina, newly diagnosed hypertension, atrial fibrillation, myocardial infarction (MI), heart failure, diabetes, stroke, transient ischaemic attack and peripheral vascular disease.
Tertiary outcomes
Treatment compliance was measured objectively by downloading data from a smart card located in the CPAP machine at the 3- and 12-month visits. The output was in a standardised, commercially available, format provided by the manufacturers of the CPAP machines. It contained data on hours and days used, pressure provided and estimated leaks. CPAP usage was recorded as the total hours used, divided by the numbers of days between the initiation of CPAP and the date of the 3- or 12-months visit. Non-users were defined as those who admitted to stopping CPAP therapy, had returned their machines or had no recorded usage data at their visits or who did not use CPAP at all in the month prior to their scheduled follow-up. Hours of usage was set to 0 hours per night in those with missing data and those who had subsequently stopped treatment.
Data collection and monitoring
Data generated by all centres were collected on CRFs, which were posted to the Oxford Respiratory Trials Unit (ORTU), the trial data co-ordinating centre, where they were entered on to a database that was created and maintained by the Medical Research Council Clinical Trials Unit (MRC CTU). The staff entering the data into the database had no part in the data collection, analysis or interpretation. All patients’ trial consent forms were reviewed and a 100% automated check was conducted for the ESS inclusion criteria. Automated data checks for consistency and date were completed for all CRFs. Data were also checked for inconsistencies in range and missing data. Missing or ambiguous data were queried with individual research nurses and resolved whenever possible. Quality control of CRF data entry was completed on a regular basis throughout the duration of the trial. Site initiation visits were organised for all sites prior to commencing recruitment and were conducted by the chief investigator, trial manager and clinical research fellow. Interim monitoring visits were completed for five centres and source data verification was completed during those visits. Eleven close-out visits were completed remotely and four centres were visited. All adverse events were reviewed by the Independent Data Monitoring Committee (IDMC).
Randomisation
Patients were randomised using a telephone computerised service provided by the MRC CTU. Allocation was physically carried out during working hours from Monday to Friday. The allocation group was indicated to the unblinded research nurse once the baseline data collection was completed.
The randomisation programme was created by the MRC CTU in accordance with its standard operating procedure and held on a secure server, access to which was confined to the CTU data manager. Allocation was on a 1 : 1 basis with a random element of 80% and stratified by disease severity (enrolment ESS score of > 13 vs. ≤ 13), functionality using the TDS score of > 1 versus ≤ 1 and recruitment centre. In the analysis, baseline ESS scores and TDS scores were entered into models as fixed-effects continuous variables. Recruiting centre was adjusted for using random effects in order to avoid dropping centres that may recruit only a single patient.
Blinding
As this was a physical device trial, the treatment allocation for the individual patients could not be concealed, although the treatment allocation could be concealed from a member of the research team completing follow-up assessments. Each centre was asked to identify a member of the research team who could be the blinded researcher and remain blinded to the treatment allocation throughout the trial. The CRFs were designed to collect blinded and unblinded data separately. Patients were discouraged from discussing their treatment allocation with the blinded research staff and the importance of maintaining blinding was highlighted in the patient information sheets (PISs). It was not possible to blind all trial staff, although the assessments were done blind wherever possible.
The trial manager and trial support staff at the co-ordinating centres in Oxford and London did not have contact with the patients. The trial statisticians analysed the results based on a treatment code, using an analysis plan that had been finalised prior to locking the database and prior to the blinded data analysis. Patients continued to see other health-care professionals unrelated to the trial for their usual medical care.
Sample size
The primary analysis was the difference between the two treatment groups in the mean change of ESS score from baseline to 3 months. The ESS is a scale from 0 to 24 and a 1-point change on the ESS is indicative of a shift in the symptom state in one domain which was considered to be the minimally clinically important difference. In the recent NICE/HTA appraisal of CPAP for OSAS in middle-aged patients,20 the effect of CPAP treatment on the difference in ESS score in middle-aged patients with mild OSA was –1.07 [standard deviation (SD) 2.4]. The inclusion criterion for this trial was in the range of moderate OSAS severity, but, since sleepiness may be less pronounced in older people, the power calculations were performed assuming a treatment response similar to that seen in mild OSAS in middle-aged patients. To detect a 1-point change in ESS score (SD of change 2.4) required 244 patients randomised in a 1 : 1 ratio with a 90% power at the two-sided 5% significance level. In shorter (less than 6 months’ duration) randomised trials with a similar design, the loss to follow-up rate was approximately 5%. Since PREDICT was of longer duration and undertaken in older people with comorbidity, it was conceivable that the loss to follow-up rate could be up to 10%. Therefore, the sample size for the trial was 270 patients (135 in each group).
Statistical methods
The statistical analysis plan was finalised and approved by the Trial Management Group (TMG). Statistical significance was tested at the 5% level for all analyses. All analyses were adjusted for the minimisation factors (enrolment ESS score of > 13 vs. ≤ 13, functionality using the TDS of > 1 vs. ≤ 1 and recruitment centre) to optimise power and reduce bias. In addition to the minimisation factors, age, sex, ODI and body mass index (BMI) were also adjusted for in an additional analysis of the primary outcome. All analyses were by intention to treat, incorporating all randomised patients who had complete data on the outcome of interest (complete-case analysis). No adjustments for multiple testing were made, but the statistical significance of the secondary outcomes was interpreted cautiously because of the large number of secondary analyses performed.
A secondary sensitivity analysis of the primary outcome was performed in order to establish proof of principle whereby patients who swapped from the BSC group to CPAP were excluded from the analysis. The effect of baseline ESS score, age, ODI and BMI and the effect of CPAP use on the primary outcome were also investigated.
All analyses and modelling were undertaken in Stata version 12.0 (StataCorp LP, College Station, TX, USA).
Descriptive statistics
All baseline data were summarised by treatment groups. Only descriptive statistics were utilised; no formal statistical comparisons were undertaken, since any differences should be the result of chance rather than bias. Categorical variables were summarised by number (n) and percentage (%) and continuous variables were summarised by mean, SD or median, 25th and 75th percentiles as appropriate.
Coprimary outcomes analysis
Subjective sleepiness
Subjective sleepiness was assessed using the ESS. The mean of the 3- and 4-month ESS score was calculated for each patient and compared with baseline. The ESS score is the sum of its eight components and, therefore, if one of the components was missing, the ESS score was set to missing. If any non-integer values were given these were included in the sum and the final ESS score rounded up or down to the next integer. Any scores obtained outside the pre-specified window of 2 to 5 months after randomisation were excluded. If either the 3- or the 4-month score was missing, the single observed score was used. If both scores were missing or outside the required time frame, the patient was excluded from the primary analysis. The difference between the randomisation ESS score and follow-up ESS score was calculated for each patient and compared between groups using a multivariable linear regression model. The analysis was adjusted for the minimisation factors as outlined previously.
Cost-effectiveness
The cost-effectiveness analysis took the perspective of the UK NHS over a time horizon of 12 months. Health outcomes were expressed in QALYs using EQ-5D and SF-6D. The analysis incorporated health-care utilisation, including inpatient and outpatient hospital visits and general practitioner (GP) visits during the trial. The cost-effectiveness analysis will be discussed in more detail in Chapter 4.
Secondary outcome analyses
Subjective sleepiness at 12 months: the mean ESS score at 10, 11 and 12 months was calculated for each patient and was taken to be the 12 month score. The same principles described for primary analysis were used for calculating the mean ESS score at 12 months. The difference between the two groups in the change in subjective sleepiness at 12 months compared with baseline was analysed using a multivariable linear regression model adjusting for the minimisation factors as outlined previously.
In addition, the changes from baseline were compared at 3 and 12 months for the following outcomes between treatment groups.
Objective sleepiness
Objective sleepiness was measured by the OSLER test. Two tests were conducted at each visit (baseline, 3 months and 12 months) and the average time taken to fall asleep at each visit was used for the analysis. Kaplan–Meier plots were used to summarise the mean time taken to fall asleep (the event of interest) at baseline, 3 months and 12 months with log-rank tests used to compare survival curves. The change from baseline in the mean time taken to fall asleep at each follow-up visit was compared between treatment groups.
Generic quality of life
Generic quality of life was assessed by the SF-36. The MCS and PCS scores were calculated. If any of the 36 questions were not answered, the MCS and PCS were set to missing along with any of the eight summary scores dependent on the missing answers.
Disease-specific quality of life
Disease-specific quality of life was assessed by the SAQLI. The score is the average of 14 sleep-related questions and, if applicable, adjusted for side effects attributable to CPAP. If any of the answers were missing, the SAQLI was also set to missing.
Mood
Mood was assessed by the HADS. The anxiety and depression summary components of the score were reported. If any of the answers were missing the relevant summary component was also set to missing.
Functionality
Functionality was assessed by the TDS. Each of the nine items of the TDS is scored 0 (yes, with no difficulty), 1 (yes, with some difficulty) or 2 (no, need help). Items are then summed to give a total score out of 18. 109 If at least one of the components was missing, the TDI was also set to missing.
Nocturia
Nocturia was assessed by the self-reported average number of times that patients get up to pass urine at night.
Mobility
Mobility was assessed by the TUG test and was measured in seconds. There is no upper time limit and the time in seconds is rounded up or down to a whole second.
The number of road and domestic accidents
The proportion of patients experiencing any accidents was analysed adjusting accident history at baseline (whether or not they had an accident at home in the month before enrolment or while driving in the 3 months before enrolment).
Cognitive function
Cogitative function was assessed using the MMSE, TMT–B, the DSS test and the simple and four-choice reaction time test. The change in the score for each of the four tests was analysed.
Cardiovascular risk factors
Cardiovascular risk factors were assessed using systolic and diastolic BPs, fasting glucose, fasting lipids and HbA1c.
New adverse cardiovascular events
These were assessed as the proportion of patients reporting any new adverse cardiovascular event at the 3- and 12-month assessment. The analysis was adjusted for the proportion of patients with any cardiovascular event at baseline.
The continuous outcomes (SF-36, SAQLI, HADS, TDS, cognitive function tests, cardiovascular risk factors, mobility test and frequency of nocturia) were analysed using multivariable regression models and adjusted for their corresponding baseline score/measurement and the minimisation factors. Non-normal (skewed) data were not an issue and could be analysed using this method because of the implications of the central limit theorem that for a large sample size the mean will be approximately normally distributed.
For binary outcomes (accident and adverse cardiovascular events) the odds of experiencing the outcome were compared between treatment groups using logistic regression. All analyses were adjusted for the minimisation factors.
Tertiary outcomes analyses
Continuous positive airway pressure usage was taken to be the mean number of hours that CPAP was used per night during follow-up (total number of hours used divided by total number of days’ follow-up). CPAP use was summarised using the median and 25th and 75th percentiles. Patients who had stopped CPAP during follow-up and were missing adherence data were assumed to have 0 hours/night usage. The number of patients stopping CPAP or swapping to CPAP from BSC was summarised along with reasons at the 3- and 12-month time points.
Sensitivity analyses
Patients who were randomised to BSC alone and who subsequently started CPAP therapy during the follow-up potentially dilute the results of the ESS score comparisons. Sensitivity analyses of the primary and secondary ESS score outcomes were performed in which BSC patients who swapped to CPAP were excluded from the analysis if CPAP therapy had been started before the visit at which the observation was recorded.
Multiple imputation analyses
Multiple imputation using chained equations was used to impute missing ESS scores over follow-up and produce an unbiased analysis under a missing at random assumption. Missing at random assumes that the probability of missing data depends only on the values of the observed data and not on the values of the missing data. The plausibility of the missing at random assumption was explored by comparing observed data in those patients with and without the outcome of interest.
All 12 ESS follow-up scores were entered into an imputation model along with the minimisation variables and the previously listed covariates (age, sex, ODI and BMI). Imputations were performed separately within treatment groups. CPAP compliance at the 3- and 12-month visits were also included in the imputation model for the CPAP group. For each treatment group, 50 imputation models were created using the ‘ice’ command in Stata. In analyses secondary to those described previously, the primary and secondary ESS score outcomes were reanalysed on the imputed data sets and the results combined using Rubin’s rules.
The missing at random assumption is untestable and may be inappropriate; therefore, the probability that ‘missing data could depend on values of the missing data’ (missing not at random) was considered. The ESS score outcomes were reanalysed on all randomised individuals under a range of ‘missing not at random’ scenarios. The aim of this technique was to determine how sensitive the observed results were to different assumptions on the unobserved outcomes in the two groups.
Exploratory analyses
Effect of continuous positive airway pressure adherence on the Epworth Sleepiness Scale score
Patients who were randomised to the CPAP group were split into tertiles by their average CPAP use in the last month of follow-up prior to the 3-month visit. Each group was compared with the BSC group in a single model on the change in the primary ESS score outcome. The minimisation variables were adjusted and a global test was used to determine whether or not the treatment effect in each of the three CPAP groups differed. A similar analysis was conducted on the secondary ESS score outcome at 12 months, splitting patients into tertiles by their average CPAP use in the last 3 months of follow-up before the 12-month visit. The effect of CPAP usage on ESS score at each time point was also modelled using multivariable fractional polynomial (FP) models110 adjusting for the minimisation variables. Since the BSC group had no compliance data, the mean change in ESS score in this group was displayed on a FP plot.
Interaction analyses
The variation of the effect of CPAP therapy compared with BSC on the primary ESS score outcome was investigated over age, BMI, ODI and ESS score at baseline. FPs were used to model the interaction between the treatment effect and each covariate, using either one or two FP transformations of the covariate of interest, whichever had the lower Akaike information criterion. 110 The minimisation variables were also adjusted for in each model with continuous variables centred about their mean. A continuous plot of the treatment effect over the original, untransformed baseline covariate was then produced with 95% confidence interval (CI). To check the plausibility of the interaction curve, the covariate was categorised at its quartiles and the treatment effect in each subgroup was estimated. These treatment effects were then plotted against the subgroup means over the continuous plot. Consistency between the results of the two analyses increases the plausibility and evidence of any treatment interaction. Disagreement between the two models may be an indicator of an erroneous FP model or a type I error of the FP approach, in which case the results of the subgroup analysis were interpreted with caution.
Analysis of monthly diaries
A longitudinal analysis of the effect of CPAP compared with BSC over the whole follow-up period was performed using the ESS scores from the monthly diaries. A multilevel model for repeated measures was used with ESS score as the response variable and patient- and month-specific random effects. A treatment-by-month interaction was added to the model to test whether or not the effect of CPAP varied over the course of follow-up. This model makes the assumption that all trial visits and monthly diaries are completed on the expected dates. An unstructured covariance matrix was used. Month was treated as a categorical variable. From the model a plot of the treatment effect and its 95% CI at each month was constructed.
Summary of changes to the protocol
The changes to the trial documents following National Research Ethics Committee (REC) approval in October 2009 are summarised below; a copy of the Statistical and health economic analysis plans is given in Appendix 1.
-
Substantial amendment SA01 (approved by the REC on 4 November 2009). Changed the version number of the PIS mentioned in the consent form to match the PIS version 2.0 already approved.
-
Substantial amendment SA02 (approved by the REC on 2 December 2009). Changed contact details, updated staff details, minor editing and formatting of the sleep diaries and added information regarding data transfer in the PIS. Clarified which ESS score measurements would be used in the analysis, quantified what was meant by a clinical diagnosis of OSAS, corrected a mistake in one of the exclusion criteria, added sections explaining the blinding in more detail and the delivery of CPAP/service provision and clarified the procedure for returning the driving questionnaire.
-
Substantial amendment SA03 (approved by the REC on 10 May 2010). Updated staff/committee details, minor editing and administrative changes. Further information added to the PIS. Standard letters inviting patients to attend their 3- and 12-month visits were introduced at the request of the participating centres. The sleep diaries were updated and information regarding Sibutramine was removed from the BSC booklet. Clarifications were required for the blinding procedure, one of the exclusion criterion, the minimisation criteria, the trial treatment, loss to follow-up and the procedure for assessing safety, quality control and adverse events section.
-
Non-substantial amendment NA04 (acknowledged by the REC on 14 May 2010). One of the minimisation criterion had been changed in error and was corrected.
-
Substantial amendment SA05 (approved by the REC on 24 February 2011). Updated staff/committee membership and contact details. Clarified that the results of the Embletta test done prior to trial enrolment were acceptable as long as they were done not more than 3 months before randomisation. Amended the coenrolment guidelines and listed the blood tests. Updated the monitoring, amendments and safety-reporting section so that it referred to a device trial rather than an investigational medicinal product trial.
-
Substantial amendment SA06 (approved by the REC on 20 June 2011). Clarified the primary and secondary outcomes, selection of centres and patients and treatment data collection. Updated the follow-up section. Corrected the sample size calculation and added information regarding the role of the IDMC and TMG.
-
Substantial amendment SA07 (approved by the REC on 8 June 2012). Clarification of how the cardiovascular risk is measured, the ESS score calculations and the analysis plan. Corrections of the statistical calculations, update of the trial manager’s details and administrative corrections.
All amendments were implemented prior to breaking of the treatment allocation code and prior to finalising the analysis plan.
Trial conduct
Trial organisation
The trial was managed and co-ordinated from the National Heart and Lung Institute, Imperial College London, Royal Brompton Hospital, London, UK (Professor Mary Morrell and Dr Alison McMillan) and the ORTU (Magda Laskawiec-Szkonter). The ORTU was also responsible for data collection and management. Statistical analysis was overseen by Professor Andrew Nunn and Daniel Bratton and conducted at the MRC CTU in London.
The Trial Steering Committee (TSC) carried overall responsibility for the safe delivery of the trial. It initially met every 6 months until it was satisfied that trial recruitment was achievable, and thereafter it met annually to provide overall supervision of the trial. The TMG was responsible for the management of the trial and met frequently. An IDMC was also appointed. Memberships of the TMG, TSC and IDMC are listed at the end of the report. A full list of the PREDICT investigators is included in Appendix 2.
Patient involvement
Two patient representatives participated in the PREDICT management; in particular, Mr Frank Govan from Oxford acted as the patient representative. He attended the TSC meetings and his feedback was very helpful in progressing the trial. For example, he raised awareness of the study to the Sleep Apnoea Trust Association, which in turn, publicised the study with their members. The protocol was discussed with Sleep Apnoea Trust Association members at their annual meeting in 2012, and we were invited to present the results at their 2014 meeting (www.sleep-apnoea-trust.org/user/image/sm52.pdf).
Members of the Welsh Sleep Apnoea Society have also supported the PREDICT study by providing publicity for the trial. In 2011, Professor Morrell was made an honorary member of the society in recognition of the research that the team was carrying out (www.welshsas.org).
Mr Govan and other patients regularly discussed the rigours of participating in research studies with the TMG. These comments have been taken into account in designing subsequent trails. Mr Govern also voted at TSC meetings, and his independent views were sought when discussing topics such as opening new trial sites, through to trial authorship.
The patients who participated in PREDICT from the London centre were invited to an annual patient and public involvement event at the Royal Brompton Hospital (once their direct involvement in the trial was over) to provide feedback on their experiences. This feedback was collated and has been used to improve the study facilities at the site, as well as trial logistics, for example increased time for travel between sites.
Trial finances
Positive Airway Pressure in Older People: a randomised controlled trial was funded by the UK NIHR HTA (project number 08/56/02). Subcontracts were established between Imperial College London, ORTU, York University or Edinburgh University and each of the recruitment centres. Trial patients’ travel expenses were paid.
Trial insurance and indemnity
The usual NHS indemnity arrangements for negligent harm were applied to the trial. Imperial College London acted as sponsor for the trial and had third-party liability insurance in accordance with all local legal requirements. The CPAP machines in the trial were covered by product warranty.
Working with industry
Continuous positive airway pressure is delivered by a specialised but widely used medical device otherwise known as a CPAP machine. For a detailed description of the type of CPAP machine used see Continuous positive airway pressure. The CPAP machine and associated equipment (masks, tubing, filters and humidification units) were supplied by ResMed (UK) Ltd, which also provided on loan the sleep diagnostic equipment (Embletta GOLD). The consumables were purchased. At the start of the trial, ResMed (UK) Ltd provided information regarding the logistics of ordering and delivering equipment to multiple centres but it had no involvement in the trial design, data collection, analysis or interpretation. At the end of the trial, ResMed (UK) provided a small financial contribution to a second joint research study day (and a trial investigators meeting), which helped cover the cost of venue hire.
At the end of the trial, any unused CPAP machines or loaned equipment were purchased or returned to ResMed (UK) Ltd. Any patients established on the autotitrating CPAP who wished to continue using it were allowed to keep the machine as a goodwill gesture from ResMed (UK) Ltd.
Positive Airway Pressure in Older People: a randomised controlled trial offers numerous examples of good practice in the industry, in which the needs of the trial are put foremost. During the first 6 months of the trial, the number of failed home respiratory polygraphy sleep studies (performed on the Embletta GOLD equipment) was higher than expected. This issue was addressed with the help of the industry and in collaborative meetings with staff at the co-ordinating centres. It became apparent there had been a technical problem in the equipment that was supplied for use in the trial. This was identified quickly and addressed by ResMed (UK) Ltd, which provided its expertise, operational and delivery infrastructure for free.
The estimated cost saving for the trial by the provision of CPAP machines and associated equipment was £122,896.00. The loan of Embletta GOLD equipment and software was approximately £103,485.00, generating a total cost saving of £226,381.00.
Chapter 3 Results
Recruitment
Recruitment took place between February 2010 and May 2012. The overall recruitment rate is shown in Figure 2. All the 12-month visits and trial exit were completed by May 2013. Although the trial was powered for 270 patients, 278 were recruited. This occurred because when approaching the target number a randomisation stop date was announced to coincidence with the end of a calendar month. Eight additional patients had completed their enrolment visit and randomisation prior to the official stop date. The TMG agreed the additional patients should be included.
FIGURE 2.
Cumulative recruitment.
The Consolidated Standards of Reporting Trials diagram shows the flow of patients through the trial (Figure 3). ‘Withdrew consent’ implies the patients withdrew from the treatment and trial, and ‘discontinued treatment’ implies the patient stopped their allocated treatment but remained in the trial. In total, 1614 individuals were screened as potential patients: of these 541 (34%) were eligible and subsequently 278 (51%) were randomised. 245 (88%) completed their 3-month follow-up and 231 (83%) completed their 12-month follow-up and the trial.
FIGURE 3.
Trial screening, enrolment, randomisation and follow-up.
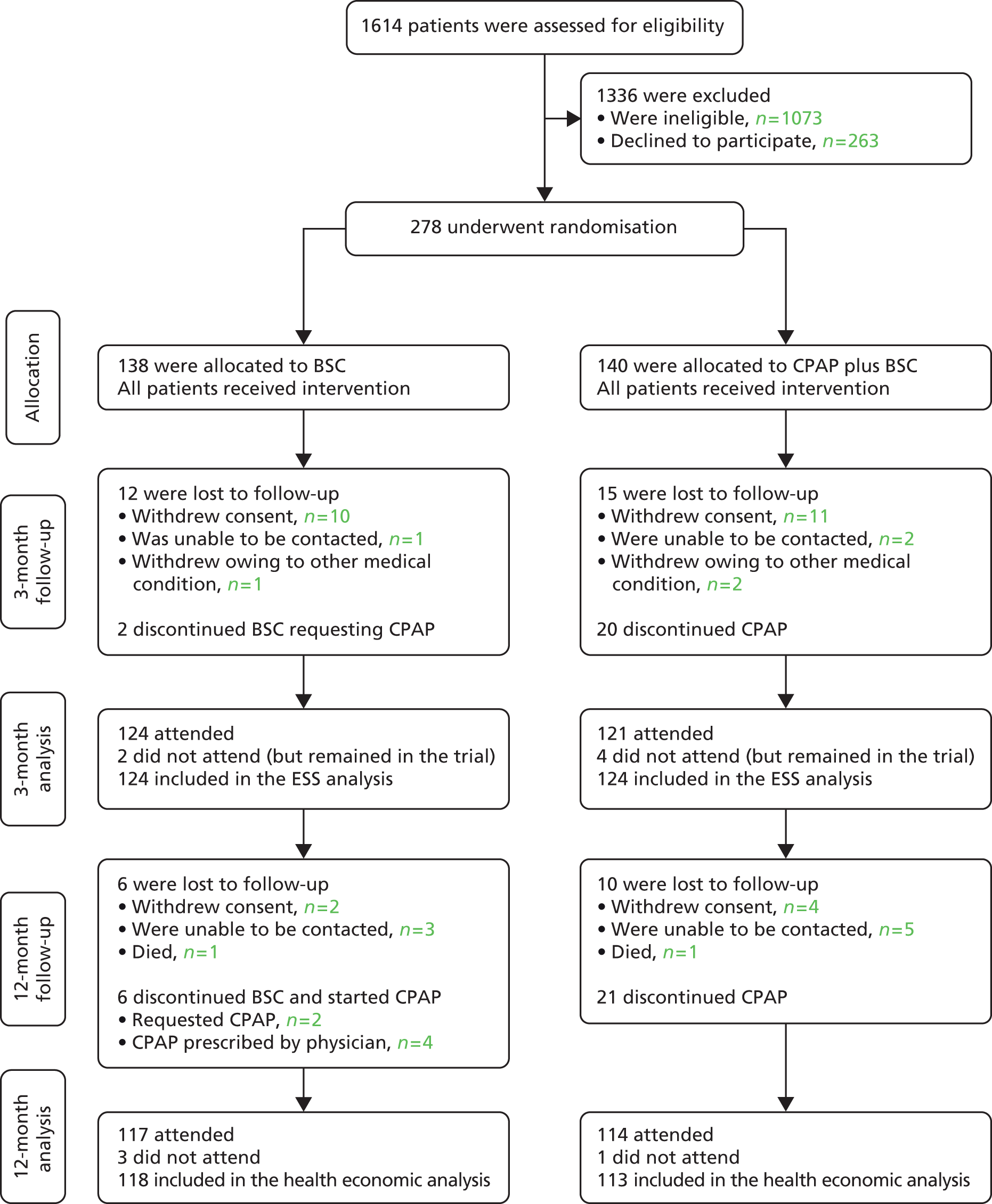
Data collected on the screening logs enabled the 1073 ineligible patients to be grouped into the following categories:
-
not meeting inclusion ODI or ESS criteria, n = 442 (41%)
-
previous exposure to CPAP, n = 79 (7%)
-
awake oxygen saturations < 90% on air or FEV1/FVC < 60%, n = 171 (16%)
-
being a professional driver, reporting sleepiness while driving, shift work or any severe symptom of OSAS for which the referring physician felt CPAP was mandatory, n = 216 (20%)
-
no information or incomplete data, n = 165 (15%).
Baseline data
In total, 278 patients were randomised; 140 to the CPAP with BSC and 138 to BSC alone. All 278 patients completed the baseline enrolment visit. The majority of patients were male (82%), with a mean age of 70 years, ranging from 65 to 89 years, and had on average moderate OSAS: ESS mean score (SD) of 11.6 (SD 3.7) and ODI 28.7 (SD 19.1) events/hour. The majority of patients were white (96%) and obese and had on average 11 years of education and normal MMSE. A total of 228 (82%) were current drivers, and 146 (53%) slept alone. Baselines demographic are shown in Table 3, clinical characteristics in Table 4, sleep characteristics in Table 5 and sleep measurements in Table 6. None of the baseline data between groups were considered different to any important degree.
| Characteristic or descriptor | BSC | CPAP | |
|---|---|---|---|
| n | 138 | 140 | |
| Age (years), median (25th–75th percentiles) | 70.3 (68.0–73.8) | 69.5 (67.1–74.1) | |
| Male sex, n (%) | 109 (79) | 120 (86) | |
| Education (years), median (25th–75th percentiles) | 11 (10–14) | 11 (10–15) | |
| MMSE, median (25th–75th percentiles) | 29 (28–30) | 29 (27–30) | |
| Current drivers, n (%) | 111 (80) | 117 (84) | |
| Ethnicity, n (%) | White | 134 (97) | 133 (95) |
| Asian | 3 (2) | 5 (4) | |
| Other | 1 (1) | 2 (1) | |
| BMI (kg/m2), mean (SD) | 33.6 (6.4) | 33.9 (5.7) | |
| Neck circumference (cm), mean (SD) | 42.6 (4.0) | 44.0 (4.4) | |
| Waist size (cm), mean (SD) | 114.1 (15.5) | 115.3 (13.6) | |
| Hip size (cm), mean (SD) | 115.7 (12.8) | 116.6 (12.1) | |
| Waist-to-hip ratio, mean (SD) | 1.0 (0.1) | 1.0 (0.1) | |
| Smoking status, n (%) | Never | 45 (33) | 42 (30) |
| Ex | 86 (62) | 92 (66) | |
| Current | 7 (5) | 6 (4) | |
| Caffeinated drinks/day, mean (SD) | 5.1 (2.7) | 5.2 (2.6) | |
| Alcoholic drinks/week, median (25th–75th percentiles) | Beer (pints) | 0 (0–2) | 0 (0–2.3) |
| Wine (glasses) | 0 (0–2) | 0 (0–2) | |
| Spirits (measure) | 0 (0–1) | 0 (0–1) | |
| Exercise frequency (defined as lasting over 10 minutes), n (%) | 5–7 times/week | 67 (49) | 69 (49) |
| 2–4 times/week | 37 (27) | 37 (26) | |
| Once/week | 9 (7) | 10 (7) | |
| < once/week | 5 (4) | 8 (6) | |
| None | 19 (14) | 16 (11) | |
| Characteristic | BSC | CPAP |
|---|---|---|
| n | 138 | 140 |
| Asthma/chronic obstructive pulmonary disease, n (%) | 34 (25) | 31 (22) |
| Other chronic lung diseases, n (%) | 13 (9) | 9 (6) |
| Ischaemic heart disease, n (%) | 49 (36) | 42 (30) |
| Hypertension, n (%) | 104 (75) | 98 (70) |
| Diabetes, n (%) | 43 (31) | 40 (29) |
| Peripheral vascular disease, n (%) | 32 (23) | 26 (19) |
| Atrial fibrillation, n (%) | 41 (30) | 28 (20) |
| Heart failure, n (%) | 11 (8) | 7 (5) |
| Cerebral vascular disease, n (%) | 19 (14) | 16 (11) |
| Systolic BP (mmHg), mean (SD) | 140.4 (20.0) | 137.7 (17.7) |
| Diastolic BP (mmHg), mean (SD) | 77.6 (12.4) | 77.7 (10.2) |
| FEV1, % predicted, mean (SD) | 84.5 (19.9) | 86.5 (19.4) |
| FVC, % predicted, mean (SD) | 5.1 (2.7) | 5.2 (2.6) |
| FEV1/FVC, mean (SD) | 83.6 (13.4) | 82.4 (12.8) |
| Nocturia (no. of times/night), mean (SD) | 2.1 (1.3) | 1.9 (1.3) |
| Incontinent overnight, n (%) | 8 (6) | 10 (7) |
| TDS, median (25th–75th percentiles) | 2.5 (1–7) | 2.5 (1–5) |
| Characteristic | BSC | CPAP | |
|---|---|---|---|
| n | 138 | 140 | |
| ESS score, mean (SD) | 11.6 (3.9) | 11.6 (3.4) | |
| OSLER (minutes), median (25th–75th percentiles) | 20.3 (9.4–37.5) | 22.4 (13.3–40.0) | |
| SAQLI, mean (SD) | 4.7 (1.2) | 4.8 (1.2) | |
| Self-reported sleep duration (hours), mean (SD) | 8.7 (1.4) | 8.5 (1.4) | |
| Sleep alone, n (%) | 71 (51) | 75 (54) | |
| Daytime nap, n (%) | 104 (75) | 107 (76) | |
| Number of naps/week, median (25th–75th percentiles) | 7 (3–7) | 7 (3–7) | |
| Duration of each nap (minutes), median (25th–75th percentiles) | 38 (25–60) | 30 (15–60) | |
| Snoring, n (%) | Yes | 127 (92) | 127 (91) |
| No | 7 (5) | 8 (6) | |
| Unknown | 4 (3) | 5 (4) | |
| Nocturnal choking, n (%) | Yes | 67 (49) | 68 (49) |
| No | 62 (45) | 55 (39) | |
| Unknown | 9 (7) | 17 (12) | |
| Witnessed apnoea, n (%) | Yes | 97 (70) | 105 (75) |
| No | 25 (18) | 19 (14) | |
| Unknown | 16 (12) | 16 (11) | |
| Measurements | BSC | CPAP |
|---|---|---|
| n | 138 | 140 |
| Time in bed (hours), mean (SD) | 8.7 (1.4) | 8.5 (1.4) |
| Apnoea Index (events/hour in bed), median (25th–75th percentiles) | 7.4 (2.7–17.3) | 7.1 (1.7–17.4) |
| Obstructive, median (25th–75th percentiles) | 6.5 (1.9–15.7) | 6.0 (1.4–15.5) |
| Central, median (25th–75th percentiles) | 0 (0–0.1) | 0 (0–0) |
| Mixed, median (25th–75th percentiles) | 0 (0–0.5) | 0 (0–0.2) |
| Hypopnea index (per hour in bed), median (25th–75th percentiles) | 18.6 (12.4–25.7) | 17.8 (11.6–28.4) |
| Total (per hour in bed), median (25th–75th percentiles) | 29.4 (18.9–46.0) | 28.1 (16.3–47.7) |
| Mean overnight O2 saturation (%), median (25th–75th percentiles) | 92.6 (90.9–93.7) | 92.6 (91.0–93.7) |
| Lowest O2 saturation (%), median (25th–75th percentiles) | 79 (73–83) | 79 (73–83) |
| Average desaturation (%), median (25th–75th percentiles) | 6.3 (5.3–7.5) | 6.3 (5.4–7.8) |
| Saturation < 90% (% of total sleep time), median (25th–75th percentiles) | 8.8 (3.3–26.3) | 8.6 (2.8–26.7) |
| ODI (> 4% events/hour), median (25th–75th percentiles) | 24.4 (15.2–39.2) | 28.1 (13.3–46.0) |
Coprimary outcomes
Subjective sleepiness
The primary outcome, the change in subjective sleepiness between groups at 3 months, is shown in Table 7. CPAP resulted in a mean change [standard error (SE)] of –3.8 (0.4) from an average (SD) of 11.5 (3.3) at baseline to 7.7 (4.0) at 3 months. BSC showed a mean change (SE) of –1.6 (0.3) from a baseline average (SD) of 11.4 (4.2) to 9.8 (4.3) at 3 months. The adjusted treatment effect at 3 months was –2.1 (95% CI –3.0 to –1.3) in favour of CPAP, which is statistically significant (p < 0.001). An additional analysis adjusting for age, sex, BMI and baseline ODI did not alter this result.
| Time assessed | BSC | CPAP |
|---|---|---|
| n randomised | 138 | 140 |
| n analysed | 124 | 124 |
| Baseline (at randomisation), mean (SD) | 11.4 (4.2) | 11.5 (3.3) |
| Month 3, mean (SD) | 9.8 (4.7) | 7.7 (4.1) |
| Month 4, mean (SD) | 9.7 (4.2) | 7.7 (4.3) |
| Mean of months 3 and 4 (SD) | 9.8 (4.3) | 7.7 (4.0) |
| Mean change from baseline (SE) | –1.6 (0.3) | –3.8 (0.4) |
| Treatment effect (95% CI), p-value | –2.1 (–3.0 to –1.3), p < 0.001 | |
Sensitivity analysis
Sensitivity analyses were performed (1) excluding two patients who swapped from BSC to CPAP prior to the 3-month assessment and (2) including all randomised patients by replacing missing values using multiple imputation. Excluding the two patients who swapped from BSC to CPAP prior to the 3-month visit resulted in a treatment effect of –2.1 (95% CI –3.0 to –1.3), p < 0.001, in favour of CPAP, identical to the primary analysis.
Results from the imputation analyses, calculating the effect of the incomplete ESS score data reported by 14 patients, estimated a change of –2.0 (95% CI –2.8 to –1.2) in favour of CPAP (p < 0.001). Once again this showed the primary result to be robust. The imputation analysis assumes missing outcomes are similar to the observed outcomes in patients with similar characteristics, but this may not be true, as missing outcomes may be better or worse than those observed. The assumption can be varied to see how sensitive the observed results are to the missing data. Figure 4 shows that observed results are not sensitive and that extreme assumptions about the missing data are needed to make any significant change to the primary analysis (i.e. a 5-point difference between missing and observed values) so any sensible assumptions about the missing data do not change the results.
FIGURE 4.
Sensitivity analysis of the primary outcome to missing data. Missing outcomes are imputed under the assumption that the average of the missing outcomes differ to the mean of the observed outcomes by an amount corresponding to each point of the x-axis. Imputation is performed for each treatment group separately.

Planned exploratory analysis
Exploratory analysis were planned to investigate the effect of CPAP use and age, BMI, ESS score and ODI at baseline on the primary ESS score outcome. Patients were split into tertiles by their average CPAP use in the last month of follow-up prior to their 3-month visit. The analysis by CPAP use is shown in Table 8. The change in ESS score between baseline and 3 months in those who used CPAP the most (third tertile) was 11.4 at baseline and 6.1 at 3 months. This resulted in a treatment effect of –3.7 (95% CI –4.8 to –2.6), p < 0.001, compared with BSC.
| Time assessed | BSC | CPAP | ||
|---|---|---|---|---|
| First tertile | Second tertile | Third tertile | ||
| n | 124 | 38 | 37 | 41 |
| Mean usage (hours/night), (minimum–maximum) | – | 0 (0–0) | 1.9 (0.001–4.6) | 6.4 (4.6–8.6) |
| Baseline ESS score, mean (SD) | 11.4 (4.2) | 10.6 (3.0) | 12.0 (3.9) | 11.4 (2.7) |
| ESS score month 3, mean (SD) | 9.8 (4.3) | 8.0 (3.9) | 9.0 (4.5) | 6.1 (2.7) |
| Change, mean (SD) | –1.6 (2.9) | –2.6 (3.9) | –3.1 (4.3) | –5.3 (3.4) |
| Treatment effect (95% CI) | – | –1.3 (–2.4 to 0.1) | –1.3 (–2.4 to –0.1) | –3.7 (–4.8 to –2.6) |
| p-value | – | 0.032 | 0.034 | < 0.001 |
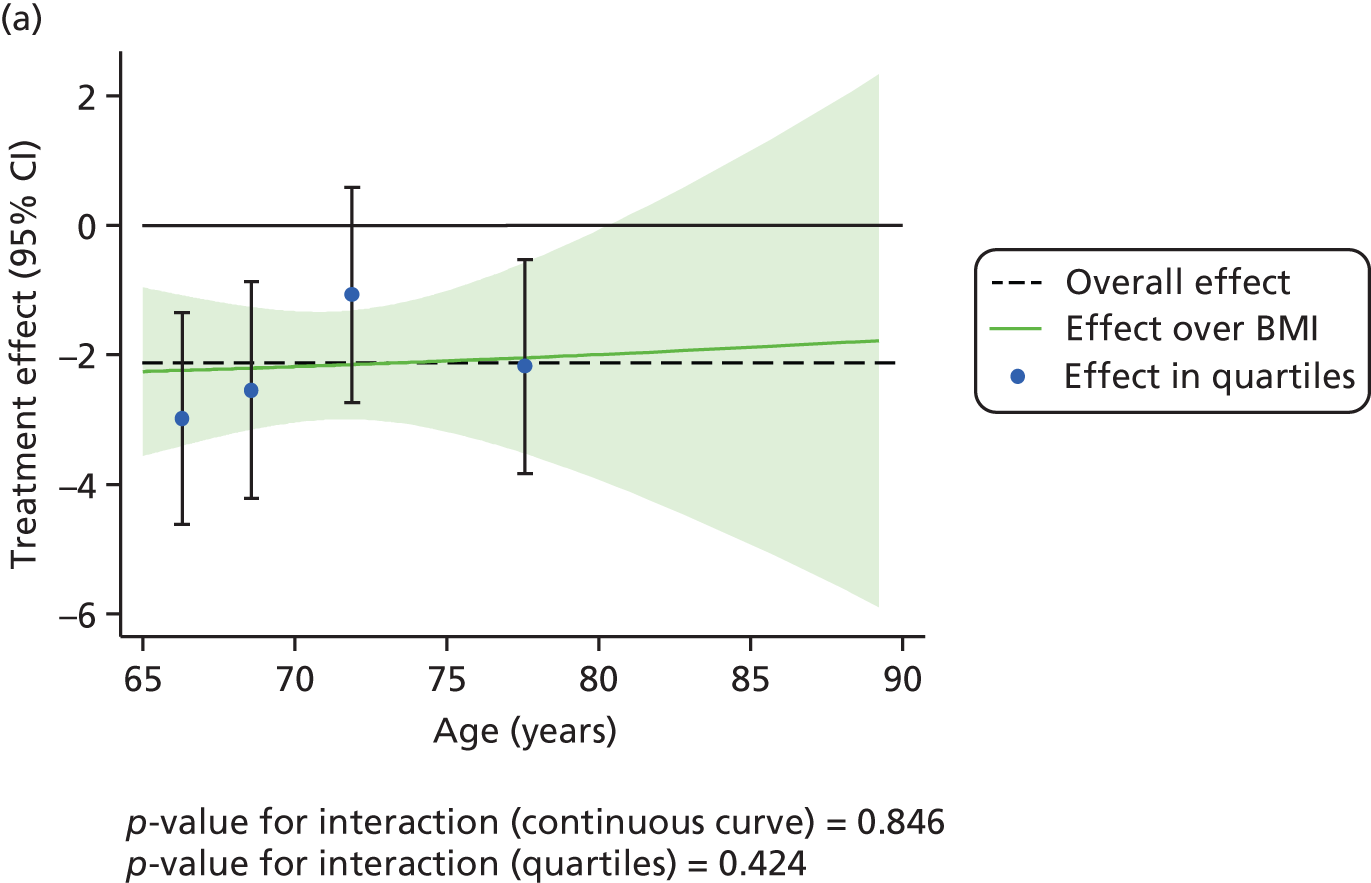
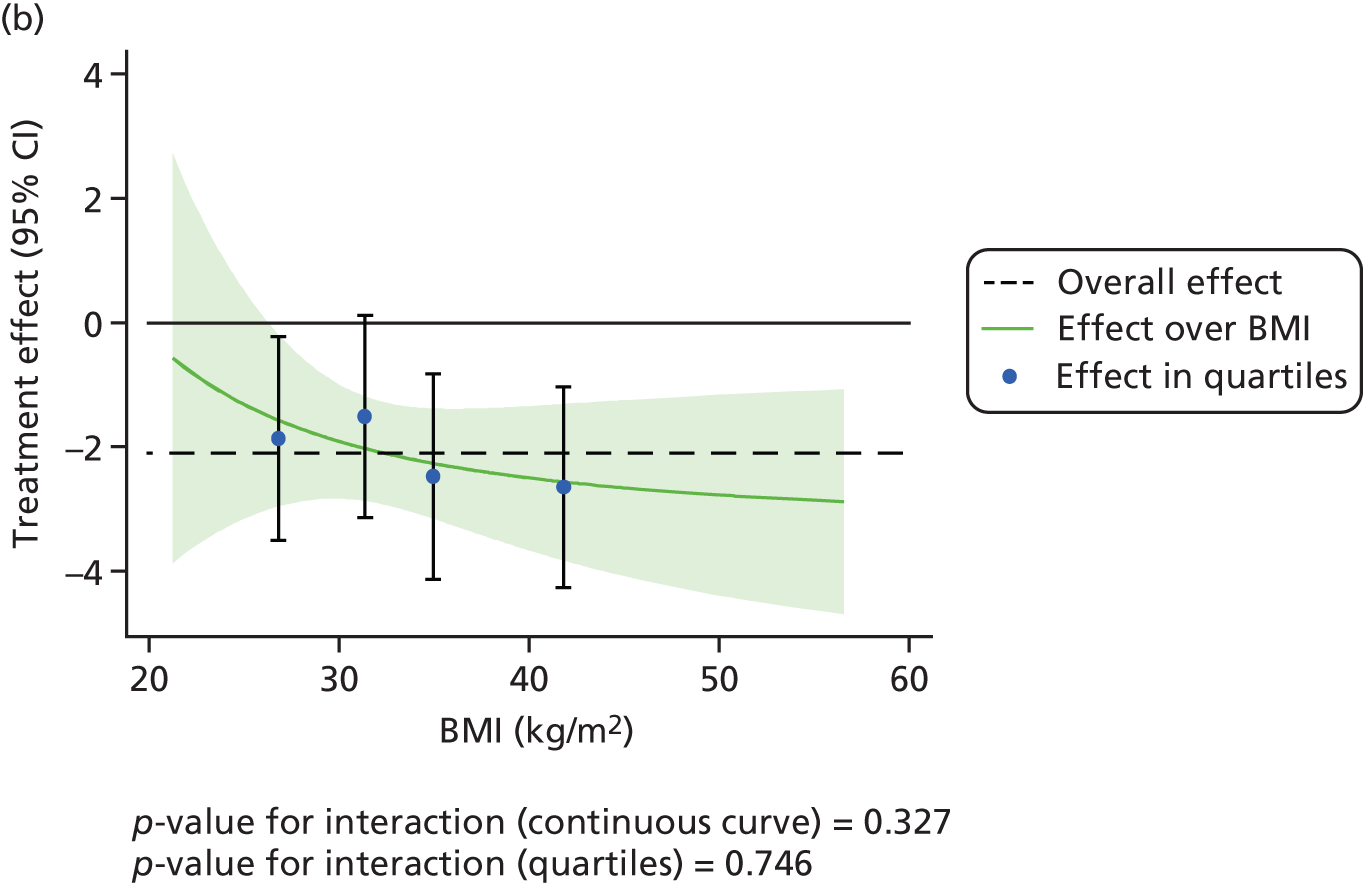
The effect of CPAP therapy compared with BSC on the primary ESS score outcome was also assessed separately over age, BMI, ESS score and ODI at baseline using FPs and is shown in Figure 5. The treatment effect was larger in patients with higher ESS score at baseline (p < 0.001).
FIGURE 5.
Interactions between primary treatment effect on ESS score and age, BMI, baseline ESS score and ODI. (a) Treatment effect over age; (b) treatment effect over BMI; (c) treatment effect over baseline ESS score; and (d) treatment effect over ODI.


Cost-effectiveness
The primary outcome of cost-effectiveness is shown in Table 9. There was small difference between those treated with CPAP and those treated with BSC. The average cost per patient was £1363 (95% CI £1121 to £1606) for those allocated to CPAP and £1389 (95% CI £1116 to £1662) for those receiving BSC. Overall, the cost accrued by the CPAP group was, on average, £35 (95% CI –£390 to £321) lower than in the BSC group, a difference which was not statistically significant. The results were not sensitive to different assumptions regarding the missing data. However, the results were sensitive to the assumptions used to cost CPAP treatment. This is discussed in Chapter 4.
| Cost-effectiveness | BSC | CPAP |
|---|---|---|
| Costs of CPAP treatment | 0 | £201 |
| Costs of health-care resource use, mean (SE) | £1389 (£139) | £1363 (£123) |
| EQ-5D QALYs, mean (SE) | 0.666 (0.020) | 0.680 (0.021) |
| SF-6D QALYs, mean (SE) | 0.658 (0.008) | 0.678 (0.007) |
| CPAP versus BSC | ||
| Difference in costs, mean (SE, 95% CI) | –£35 (£180, –£390 to £321) | |
| Difference in EQ-5D QALYs, mean (SE, 95% CI) | 0.005 (0.020, –0.034 to 0.044) | |
| Difference in SF-6D QALYs, mean (SE, 95% CI) | 0.018 (0.008, 0.003 to 0.034) | |
During the trial follow-up, the BSC group gained 0.666 (95% CI 0.627 to 0.705) QALYs using EQ-5D and 0.658 (95% CI 0.643 to 0.673) QALYs using SF-6D; the CPAP group gained 0.680 (95% CI 0.638 to 0.722) QALYs using EQ-5D and 0.678 (95% CI 0.664 to 0.691) QALYs using SF-6D. The QALY difference between the CPAP and the BSC groups was 0.005 (95% CI –0.034 to 0.044) QALYs using the EQ-5D and 0.018 (95% CI 0.003 to 0.034) QALYs using the SF-6D.
Overall, the probability that the intervention was cost-effective at the threshold conventionally used in the NHS of £20,000 per QALY gained was 0.61 using EQ-5D QALYs and 0.96 using SF-6D QALYs.
Secondary outcomes
Subjective sleepiness
The change in subjective sleepiness, as measured by the mean ESS score of months 10, 11 and 12, is shown in Table 10. CPAP resulted in a mean change (SD) of –4.2 (SD 4.1) in ESS score, from an average of 11.4 (SD 3.4) at baseline to 7.2 (SD 3.6) at 12 months. BSC showed a change of –2.1 (SD 3.6), from a baseline of 11.3 (SD 4.0) to 9.2 (SD 4.0) at 12 months. The difference between the two groups at 12 months was –2.0 (95% CI –2.8 to –1.2) in favour of CPAP, which was statistically significant (p < 0.001). A sensitivity analysis excluding eight patients who swapped from BSC to CPAP was performed but this did not alter the conclusion; the difference between the two groups was –2.1 (95% CI –3.0 to –1.3; p < 0.001), in favour of CPAP.
| Timed assessed | BSC | CPAP |
|---|---|---|
| n randomised | 138 | 140 |
| n analysed | 122 | 116 |
| ESS score baseline (at randomisation), mean (SD) | 11.3 (4.0) | 11.4 (3.4) |
| ESS score month 10, mean (SD) | 9.3 (4.3) | 7.3 (4.1) |
| ESS score month 11, mean (SD) | 9.6 (4.4) | 7.2 (4.1) |
| ESS score month 12, mean (SD) | 9.0 (4.1) | 7.0 (3.8) |
| Mean of months 10, 11 and 12, mean (SD) | 9.2 (4.0) | 7.2 (3.6) |
| Mean change from baseline (SD) | –2.1 (3.6) | –4.2 (4.1) |
| Treatment effect (95% CI), p-value | –2.0 (–2.8 to –1.2), p < 0.001 | |
Continuous positive airway pressure reduced subjective sleepiness at 3 months; the effect was maintained at 12 months and was statistically significant (p < 0.001). This is shown graphically in Figure 6. Similarly, the effect was larger in patients with greater CPAP use. The analysis by CPAP use is given in Table 11.
FIGURE 6.
The treatment effect of CPAP compared with BSC care on subjective sleepiness. Adjusted treatment effects of CPAP and BSC and their 95% CI on the mean ESS score of months 3 and 4 (coprimary outcome) and of months 10, 11 and 12 (secondary outcome). Lower scores indicate an improvement.

| Descriptor | BSC | CPAP | ||
|---|---|---|---|---|
| First tertile | Second tertile | Third tertile | ||
| n | 122 | 52 | 30 | 30 |
| Mean usage (hours/night) (minimum–maximum) | – | 0 (0–0) | 2.3 (0.002–4.4) | 6.3 (4.5–8.9) |
| Baseline ESS score, mean (SD) | 11.3 (4.0) | 11.2 (3.5) | 11.4 (4.0) | 11.8 (2.6) |
| ESS score months 10,11 and 12, mean (SD) | 9.2 (4.0) | 8.1 (3.9) | 7.3 (3.5) | 5.6 (2.6) |
| Change, mean (SD) | –2.1 (3.6) | –3.0 (4.4) | –4.2 (3.4) | –6.2 (3.3) |
| Treatment effect (95% CI) | – | –1.0 (–2.0 to 0.1) | –2.0 (–3.2 to –0.7) | –3.6 (–4.9 to –2.4) |
| p-value | – | 0.063 | 0.002 | < 0.001 |
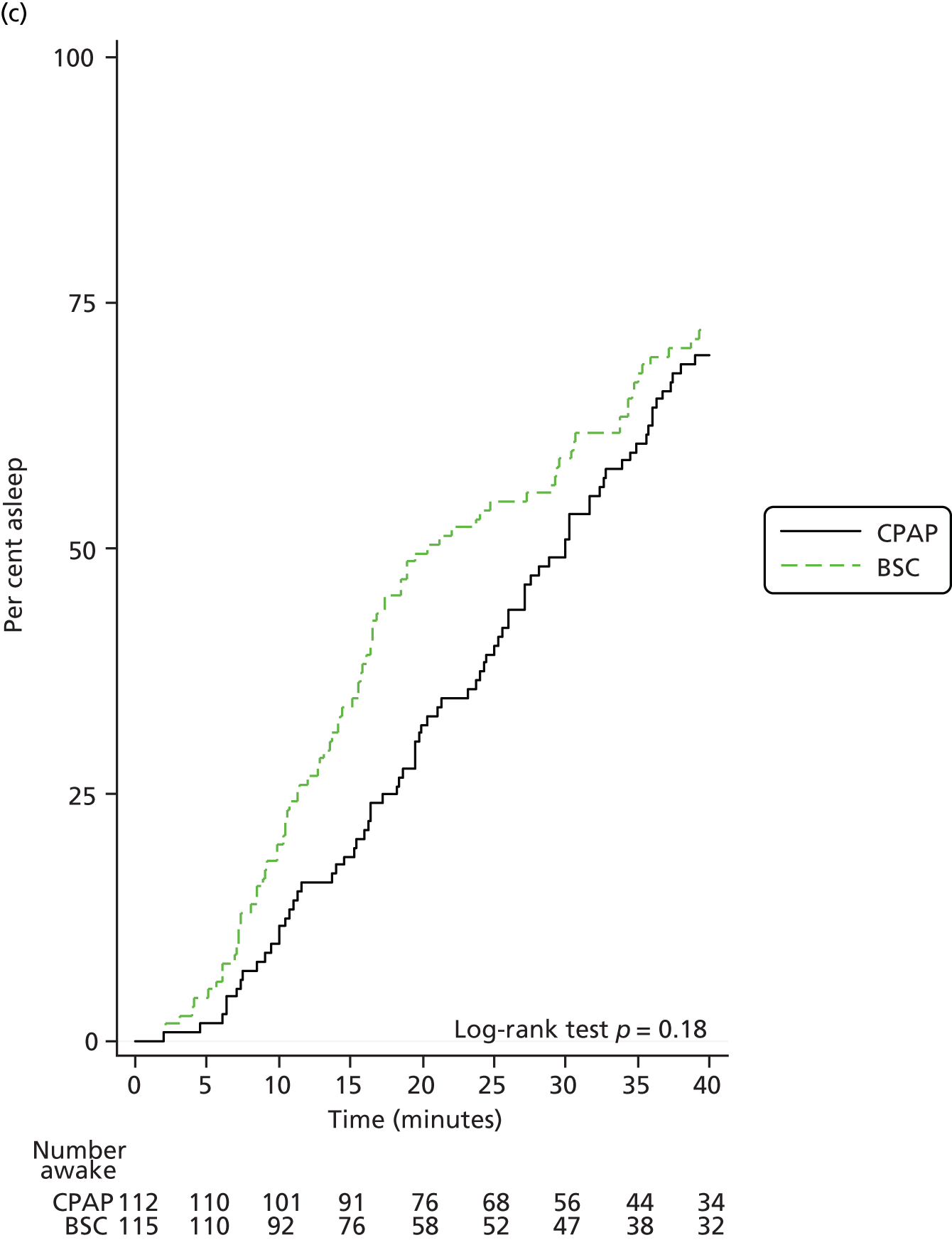
Objective sleepiness
Sleepiness was also measured objectively using the OSLER test at 3 and 12 months. The mean time to fall asleep is shown in Tables 12 and 13 (3 and 12 months, respectively). The difference between groups was statistically significant at 3 months (p = 0.024) in favour of CPAP but less so at 12 months (p = 0.058). The mean time for patients to fall asleep is also shown in Kaplan–Meier plots in Figure 7.
| Time assessed | BSC | CPAP | Treatment effect (minutes), (95% CI) | p-value |
|---|---|---|---|---|
| n | 121 | 116 | 2.8 (0.4 to 5.2) | 0.024 |
| Baseline, mean time to sleep (minutes) (SD) | 21.5 (13.4) | 23.6 (12.7) | ||
| Month 3, mean time to sleep (minutes) (SD) | 22.8 (13.9) | 27.3 (12.4) | ||
| Mean change from baseline 3 months (SD) | 1.3 (10.8) | 3.6 (10.6) |
| Time assessed | BSC | CPAP | Treatment effect (minutes), (95% CI) | p-value |
|---|---|---|---|---|
| n | 115 | 110 | 2.6 (–0.1 to 5.3) | 0.058 |
| Baseline, mean time to sleep (minutes) (SD) | 21.4 (13.3) | 24.5 (12.7) | ||
| Month 12, mean time to sleep (minutes) (SD) | 23.8 (13.4) | 27.8 (11.6) | ||
| Mean change from baseline at 12 months (SD) | 2.4 (11.9) | 3.3 (13.2) |
FIGURE 7.
Kaplan–Meier plot of average time taken to fall asleep for each patient at baseline, 3 months and 12 months. (a) Baseline; (b) 3-month visit; and (c) 12-month visit.


Quality of life and mood
Generic quality of life was assessed using the SF-36 (version 1) at 3 and 12 months. Raw scores were analysed with factor loadings obtained from Jenkinson et al. 102 The difference between groups in the energy/vitality domain was statistically significant at 3 months (p = 0.001) and 12 months (p = 0.004) in favour of CPAP. The MCS score was also statistically significant at 3 months (p = 0.046) but not at 12 months (p = 0.22). The physical functioning score was also statistically significant at 12 months (p = 0.033) in favour of CPAP but not at 3 months (p = 0.16). The difference between the two groups on each summary score at the 3- and 12-month visits is shown in Figure 8.
FIGURE 8.
Short Form questionnaire-36 items treatment effects at 3 and 12 months. Adjusted treatment effects and their 95% CI, CPAP versus BSC, on the MCS, the PCS and the eight individual components at 3 and 12 months. Higher score indicate an improvement.

Disease-specific quality of life was measured using the SAQLI, a sleep apnoea-specific questionnaire which also incorporates side effects associated with CPAP. Both groups showed an improvement but the effect was greater in the CPAP group at 3 months (p = 0.005) and 12 months (p = 0.001).
Mood was assessed using the HADS, which was summarised into an anxiety score and a depression score. Both groups showed a reduction in their score at 3 and 12 months but the difference between groups at either time point was not statistically significant.
The SF-36, SAQLI and HADS scores are shown in Tables 14 and 15 (3 and 12 months, respectively).
| Outcome | BSC | CPAP | Treatment effect (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Month 3, mean (SD) | n | Baseline, mean (SD) | Month 3, mean (SD) | |||
| SF-36 | ||||||||
| Bodily pain | 125 | 59.9 (26.8) | 60.5 (26.4) | 123 | 61.9 (28.4) | 61.4 (26.9) | –0.7 (–5.6 to 4.2) | 0.78 |
| Energy/vitality | 123 | 45.8 (22.0) | 47.0 (22.5) | 121 | 49.9 (20.5) | 56.6 (20.9) | 6.4 (2.7 to 10.2) | 0.001 |
| General health | 124 | 55.9 (21.8) | 55.3 (22.1) | 123 | 56.5 (23.4) | 57.7 (22.1) | 1.8 (–1.5 to 5.0) | 0.29 |
| Mental health | 125 | 76.7 (14.7) | 77.7 (16.8) | 123 | 76.2 (17.2) | 80.4 (15.4) | 2.8 (–0.1 to 5.6) | 0.062 |
| Physical functioning | 124 | 54.9 (29.0) | 55.0 (29.5) | 121 | 58.2 (26.3) | 60.6 (27.5) | 2.6 (–1.1 to 6.3) | 0.16 |
| Role emotional | 125 | 72.3 (39.2) | 72.8 (37.0) | 122 | 76.8 (38.3) | 78.7 (34.8) | 3.0 (–4.3 to 10.3) | 0.41 |
| Role physical | 122 | 40.4 (42.2) | 44.5 (40.8) | 122 | 53.1 (40.9) | 53.5 (41.8) | 2.0 (–6.4 to 10.4) | 0.64 |
| Social functioning | 125 | 73.7 (27.8) | 72.0 (29.0) | 123 | 76.5 (25.8) | 80.1 (25.1) | 6.0 (1.1 to 11.0) | 0.017 |
| MCS | 118 | 51.2 (9.9) | 51.5 (10.0) | 119 | 51.9 (10.1) | 54.1 (8.9) | 1.9 (0.0 to 3.8) | 0.046 |
| PCS | 118 | 31.0 (13.6) | 31.7 (15.1) | 119 | 34.2 (13.8) | 34.2 (14.3) | –0.2 (–2.5 to 2.1) | 0.84 |
| SAQLI | ||||||||
| – | 119 | 4.7 (1.2) | 5.0 (1.1) | 121 | 4.8 (1.2) | 5.3 (1.1) | 0.3 (0.1 to 0.5) | 0.005 |
| HADS | ||||||||
| Anxiety | 125 | 5.5 (3.7) | 4.9 (3.5) | 123 | 5.3 (4.0) | 4.2 (3.4) | –0.5 (–1.1 to 0) | 0.064 |
| Depression | 124 | 4.4 (3.0) | 4.3 (2.9) | 123 | 4.5 (2.8) | 4.0 (2.9) | –0.4 (–0.9 to 0.1) | 0.17 |
| Outcome | BSC | CPAP | Treatment effect (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Month 12, mean (SD) | n | Baseline, mean (SD) | Month 12, mean (SD) | |||
| SF-36 | ||||||||
| Bodily pain | 117 | 60.2 (26.3) | 59.2 (27.2) | 114 | 61.2 (28.4) | 60.5 (26.9) | 0.1 (–5.4 to 5.5) | 0.98 |
| Energy/vitality | 116 | 46.6 (21.8) | 48.4 (22.6) | 112 | 49.4 (20.4) | 56.7 (21.4) | 6.1 (1.9 to 10.3) | 0.004 |
| General health | 116 | 56.0 (21.4) | 54.8 (22.0) | 114 | 55.9 (23.6) | 57.8 (21.8) | 2.6 (–1.1 to 6.4) | 0.17 |
| Mental health | 117 | 76.7 (14.5) | 78.0 (18.0) | 113 | 76.3 (17.9) | 79.7 (17.2) | 1.9 (–1.7 to 5.4) | 0.30 |
| Physical functioning | 117 | 55.5 (28.5) | 54.3 (29.1) | 113 | 57.2 (26.3) | 60.7 (29.1) | 4.7 (0.4 to 9.1) | 0.033 |
| Role emotional | 117 | 72.6 (38.8) | 72.9 (38.9) | 113 | 76.4 (38.8) | 79.6 (33.5) | 5.1 (–3.4 to 13.7) | 0.24 |
| Role physical | 116 | 41.8 (41.7) | 42.2 (42.0) | 112 | 52.5 (41.2) | 50.4 (42.8) | 3.0 (–6.6 to 12.6) | 0.54 |
| Social functioning | 117 | 73.1 (26.9) | 74.3 (29.2) | 114 | 76.2 (26.2) | 78.9 (25.3) | 2.6 (–2.9 to 8.1) | 0.35 |
| MCS | 114 | 51.1 (9.8) | 52.0 (10.4) | 108 | 52.1 (10.2) | 53.9 (9.4) | 1.4 (–0.8 to 3.6) | 0.22 |
| PCS | 114 | 31.3 (13.2) | 30.9 (15.3) | 108 | 33.8 (13.9) | 33.7 (14.9) | 0.6 (–2.2 to 3.4) | 0.68 |
| SAQLI | ||||||||
| – | 114 | 4.7 (1.2) | 5.1 (1.1) | 113 | 4.8 (1.2) | 5.5 (1.1) | 0.4 (0.2 to 0.6) | 0.001 |
| HADS | ||||||||
| Anxiety | 117 | 5.5 (3.6) | 4.5 (3.5) | 114 | 5.2 (3.9) | 4.1 (3.5) | –0.2 (–0.9 to 0.5) | 0.58 |
| Depression | 116 | 4.4 (3.0) | 4.2 (3.2) | 114 | 4.6 (2.9) | 3.9 (3.1) | –0.4 (–1.0 to 0.3) | 0.23 |
Functionality
The average TDS was higher at 3 and 12 months than at baseline in both groups. The difference between the groups at 3 months (p = 0.21) and 12 months (p = 0.89) was not statistically significant.
Nocturia
The frequency of nocturia appeared to decrease in both groups. The difference between the groups at 3 months (p = 0.64) and 12 months (p = 0.74) was not statistically significant.
Mobility
There was no change in the average TUG test time in the CPAP group, while there was a slight increase in the BSC group at 3 months. The difference was –0.8 seconds (95% CI –1.4 to –0.1 seconds); this difference of just under 1 second was statistically significant (p = 0.029) in favour of CPAP at 3 months. By 12 months the difference between the groups had reduced to –0.1 seconds (95% CI –0.9 to 0.7 seconds) in favour of CPAP, but this was not statistically significant (p = 0.80).
Accidents
More self-reported domestic accidents were reported at each follow-up assessment than at baseline in both groups. The difference between the groups was not statistically significant (p = 0.28) at 3 months or 12 months (p = 0.11). Very few RTAs were reported in both treatment groups at each visit. The difference in the overall number of accidents between groups was not statistically significant at 3 months (p = 0.36) or at 12 months (p = 0.20).
The results for functionality, nocturia, mobility and accidents are shown in Tables 16 and 17.
| Outcome | BSC | CPAP | Treatment effect (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Month 3, mean (SD) | n | Baseline, mean (SD) | Month 3, mean (SD) | |||
| TDS | 118 | 4.0 (4.5) | 4.7 (4.8) | 120 | 3.6 (4.0) | 3.9 (4.1) | –0.4 (–1.0 to 0.2) | 0.21 |
| Nocturia (times/night) | 123 | 2.1 (1.3) | 1.8 (1.2) | 121 | 1.9 (1.3) | 1.7 (1.2) | 0.1 (–0.2 to 0.3) | 0.64 |
| TUG test (seconds) | 117 | 12.0 (4.5) | 12.5 (5.3) | 117 | 11.4 (4.6) | 11.3 (3.9) | –0.8 (–1.4 to –0.1) | 0.029 |
| Domestic accidents n of patients with event(s), n (%) | 124 | 12 (9.7) | 14 (11.2) | 121 | 6 (5.0) | 18 (14.9) | 1.53 (0.71 to 3.31) | 0.28 |
| Driving accidents n of patients with event(s), n (%) | 88 | 2 (2.3) | 1 (1.1) | 81 | 1 (1.2) | 0 | – | – |
| All accidents n patients with event(s), n (%) | 124 | 13 (10.5) | 15 (12.1) | 121 | 7 (5.8) | 18 (14.9) | 1.42 (0.67 to 3.03) | 0.36 |
| Outcome | BSC | CPAP | Treatment effect (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Month 3, mean (SD) | n | Baseline, mean (SD) | Month 12, mean (SD) | |||
| TDS | 115 | 4.0 (4.3) | 4.8 (5.2) | 115 | 3.7 (4.1) | 4.2 (4.5) | –0.1 (–0.9 to 0.8) | 0.89 |
| Nocturia (times/night) | 116 | 2.1 (1.3) | 1.8 (1.1) | 113 | 1.9 (1.3) | 1.6 (1.4) | 0 (–0.2 to 0.3) | 0.74 |
| TUG test (seconds) | 107 | 11.7 (4.2) | 12.0 (4.6) | 108 | 11.5 (4.7) | 11.8 (4.5) | –0.1 (–0.9 to 0.7) | 0.80 |
| Domestic accidents, n of patients with event(s) (%) | 117 | 12 (10) | 18 (15) | 113 | 6 (5) | 9 (8) | 0.49 (0.21 to 1.18) | 0.11 |
| Driving accidents, n of patients with event(s) (%) | 77 | 2 (3) | 1 (1) | 73 | 1 (1) | 2 (3) | – | – |
| All accidents, n of patients with event(s) (%) | 117 | 13 (11) | 19 (16) | 113 | 6 (5) | 11 (10) | 0.59 (0.26 to 1.32) | 0.20 |
Cognitive function
Cognitive function was assessed at 3 and 12 months with the following tests: MMSE, TMT–B, the DSS test and simple and four-choice reaction time test. The results are shown in Tables 18 and 19. The difference between the groups was not statistically significant for any of the four tests at 3 or 12 months.
| Outcome measure | BSC | CPAP | Treatment effect (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Month 3, mean (SD) | n | Baseline, mean (SD) | Month 3, mean (SD) | |||
| MMSE | 123 | 28.5 (2.1) | 28.7 (1.8) | 120 | 28.2 (2.1) | 28.3 (2.1) | –0.2 (–0.6 to 0.2) | 0.25 |
| DSS test | 123 | 38.7 (11.1) | 39.6 (11.6) | 119 | 37.5 (11.9) | 39.5 (11.2) | 0.8 (–0.9 to 2.5) | 0.36 |
| TMT–B (seconds) | 123 | 117.9 (58.9) | 108.6 (49.7) | 117 | 117.7 (55.0) | 109.7 (42.7) | 0.7 (–7.2 to 8.5) | 0.87 |
| Simple reaction time test | ||||||||
| Mean time (seconds) | 95 | 382.9 (111.5) | 394.4 (129.2) | 102 | 379.5 (85.4) | 380.4 (89.9) | –12.8 (–39.9 to 14.3) | 0.35 |
| Four-choice reaction time | ||||||||
| Number of correct answers | 100 | 38.3 (2.3) | 38.5 (1.9) | 102 | 38.6 (2.3) | 38.6 (1.9) | –0.1 (–0.5 to 0.4) | 0.82 |
| Mean time for correct answers (seconds) | 100 | 680.8 (207.9) | 666.6 (181.4) | 102 | 682.3 (155.9) | 699.4 (174.2) | 32.0 (–0.7 to 64.8) | 0.055 |
| Outcome measure | BSC | CPAP | Treatment effect (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Month 12, mean (SD) | n | Baseline, mean (SD) | Month 12, mean (SD) | |||
| MMSE | 116 | 28.5 (2.0) | 28.5 (1.7) | 113 | 28.3 (2.0) | 28.5 (1.9) | 0.1 (–0.3 to 0.5) | 0.65 |
| DSS test | 116 | 39.4 (10.4) | 40.6 (11.3) | 113 | 37.2 (11.7) | 40.0 (10.7) | 1.1 (–0.6 to 2.7) | 0.22 |
| TMT–B (seconds) | 115 | 113.7 (55.8) | 107.6 (47.2) | 111 | 119.9 (57.9) | 116.6 (54.9) | 6.2 (–3.4 to 15.8) | 0.21 |
| Simple reaction time test | ||||||||
| Mean time (seconds) | 99 | 379.4 (108.1) | 388.1 (108.1) | 98 | 376.2 (84.6) | 370.0 (94.6) | –16.4 (–39.1 to 6.2) | 0.16 |
| Four-choice reaction time | ||||||||
| Number of correct answers | 100 | 38.5 (2.1) | 38.4 (2.5) | 99 | 38.6 (2.5) | 38.7 (1.7) | 0.3 (–0.2 to 0.8) | 0.26 |
| Mean time for correct answers (seconds) | 100 | 681.9 (204.2) | 688.4 (215.7) | 99 | 678.8 (204.2) | 688.1 (166.0) | 1.8 (–33.6 to 37.2) | 0.92 |
Cardiovascular risk factors
The cardiovascular risk factors at 3 and 12 months are shown in Tables 20 and 21, respectively. CPAP reduced total cholesterol at 3 months compared with BSC by –0.2 mmol/l (95% CI –0.3 mmol/l to 0.0 mmol/l) (p = 0.048). This was driven by a reduction in low-density liproprotein (LDL) cholesterol of –0.15 mmol/l (95% CI –0.29 mmol/l to –0.01 mmol/l) (p = 0.042). At 12 months the average total and LDL cholesterol were lower than at baseline in both groups and although the CPAP group had a further reduction in total and LDL cholesterol from the 3-month assessment, the difference between groups at 12 months was not statistically significant; total cholesterol (p = 0.51) and LDL cholesterol (p = 0.29).
| Outcome | BSC | CPAP | Treatment effect (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Month 3, mean (SD) | n | Baseline, mean (SD) | Month 3, mean (SD) | |||
| Systolic BP (mmHg) | 123 | 141.3 (19.8) | 137.4 (16.3) | 120 | 137.5 (18.1) | 136.3 (15.9) | 0.7 (–2.5 to 3.8) | 0.69 |
| Diastolic BP (mmHg) | 123 | 78.2 (12.6) | 76.4 (11.0) | 120 | 77.2 (10.2) | 76.1 (10.0) | 0.1 (–1.9 to 2.2) | 0.91 |
| Total cholesterol (mmol/l) | 117 | 4.6 (1.1) | 4.6 (1.1) | 114 | 4.6 (1.1) | 4.5 (1.0) | –0.2 (–0.3 to 0) | 0.048 |
| HDL (mmol/l) | 116 | 1.29 (0.39) | 1.28 (0.36) | 110 | 1.18 (0.29) | 1.18 (0.31) | –0.02 (–0.06 to 0.02) | 0.44 |
| LDL (mmol/l) | 108 | 2.63 (0.87) | 2.64 (0.91) | 102 | 2.69 (0.98) | 2.56 (0.89) | –0.15 (–0.29 to –0.01) | 0.042 |
| Triglycerides (mmol/l) | 115 | 1.61 (0.88) | 1.59 (0.77) | 108 | 1.75 (0.88) | 1.76 (1.00) | 0.06 (–0.08 to 0.20) | 0.38 |
| Glucose (mmol/l) | 119 | 6.2 (2.2) | 6.2 (1.9) | 112 | 6.3 (1.9) | 6.3 (2.0) | 0.1 (–0.3 to 0.5) | 0.54 |
| HbA1c (mmol/mol) | 111 | 46.6 (11.7) | 47.2 (12.3) | 109 | 46.2 (11.2) | 46.5 (11.6) | –0.3 (–1.6 to 1.1) | 0.70 |
| Outcome | BSC | CPAP | Treatment effect (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Month 12, mean (SD) | n | Baseline, mean (SD) | Month 12, mean (SD) | |||
| Systolic BP (mmHg) | 116 | 141.7 (20.3) | 135.5 (17.3) | 113 | 138.0 (18.2) | 137.5 (15.6) | 3.7 (0.2 to 7.3) | 0.04 |
| Diastolic BP (mmHg) | 116 | 78.5 (12.9) | 76.2 (12.0) | 113 | 77.8 (10.6) | 76.2 (9.9) | 0.2 (–2.1 to 2.5) | 0.84 |
| Total cholesterol (mmol/l) | 108 | 4.6 (1.1) | 4.5 (1.0) | 109 | 4.6 (1.1) | 4.4 (1.1) | –0.1 (–0.3 to 0.1) | 0.51 |
| HDL (mmol/l) | 106 | 1.28 (0.39) | 1.25 (0.37) | 106 | 1.19 (0.28) | 1.18 (0.30) | 0.01 (–0.03 to 0.06) | 0.57 |
| LDL (mmol/l) | 101 | 2.61 (0.88) | 2.55 (0.93) | 100 | 2.66 (0.97) | 2.50 (0.94) | –0.09 (–0.26 to 0.08) | 0.29 |
| Triglycerides (mmol/l) | 105 | 1.62 (0.90) | 1.59 (0.79) | 106 | 1.75 (0.87) | 1.74 (0.96) | 0.06 (–0.10 to 0.22) | 0.48 |
| Glucose (mmol/l) | 110 | 6.3 (2.2) | 6.4 (2.4) | 108 | 6.2 (1.8) | 6.3 (1.8) | 0.0 (–0.4 to 0.4) | 0.93 |
| HbA1c (mmol/mol) | 104 | 46.6 (11.8) | 47.7 (14.9) | 102 | 46.5 (11.2) | 46.8 (12.5) | –0.9 (–3.1 to 1.4) | 0.45 |
At 12 months there was a reduction in the systolic BP in the BSC group not seen in the CPAP group, which led to a difference between the groups of 3.7 mmHg (95% CI 0.2 mmHg to 7.3 mmHg) in favour of BSC, which was statistically significant (p = 0.040).
Cardiovascular events
New self-reported cardiovascular events were documented at 3 and 12 months and are shown in Tables 22 and 23. Atrial fibrillation was the predominant event, with more events being recorded in the BSC group, although overall the difference between groups was not statistically significant at 3 or 12 months (p = 0.48, p = 0.72).
| Adverse cardiovascular event | BSC | CPAP | Odds ratio (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Month 3, mean (SD) | n | Baseline, mean (SD) | Month 12, mean (SD) | |||
| MI, n (%) | 124 | 23 (19) | 0 | 121 | 21 (17) | 1 (1) | ||
| Stroke, n (%) | 124 | 4 (3) | 0 | 121 | 2 (2) | 0 | ||
| Transient ischaemic attack, n (%) | 124 | 12 (10) | 1 (1) | 121 | 15 (12) | 0 | ||
| New angina, n (%) | 124 | 32 (26) | 0 | 120 | 29 (24) | 1 (1) | ||
| New atrial fibrillation, n (%) | 124 | 37 (30) | 4 (3) | 121 | 24 (20) | 5 (4) | ||
| New peripheral vascular disease, n (%) | 124 | 3 (2) | 0 | 121 | 1 (1) | 0 | ||
| All adverse cardiovascular events, n (%) | 124 | 60 (48) | 5 (4) | 121 | 56 (46) | 7 (6) | 1.54 (0.47 to 5.06) | 0.48 |
| Adverse cardiovascular event | BSC | CPAP | Odds ratio (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Month 12, mean (SD) | n | Baseline, mean (SD) | Month 12, mean (SD) | |||
| MI, n (%) | 117 | 21 (18) | 0 | 114 | 22 (19) | 3 (3) | ||
| Stroke, n (%) | 117 | 3 (3) | 0 | 114 | 1 (1) | 0 | ||
| Transient ischaemic attack, n (%) | 117 | 9 (8) | 2 (2) | 114 | 12 (11) | 1 (1) | ||
| New angina, n (%) | 117 | 27 (23) | 3 (3) | 113 | 26 (23) | 2 (2) | ||
| New atrial fibrillation, n (%) | 117 | 32 (27) | 14 (12) | 114 | 22 (19) | 7 (6) | ||
| New peripheral vascular disease, n (%) | 117 | 3 (3) | 0 | 114 | 1 (1) | 1 (1) | ||
| All adverse cardiovascular events, n (%) | 117 | 56 (48) | 17 (15) | 114 | 51 (45) | 14 (12) | 0.87 (0.40 to 1.88) | 0.72 |
Tertiary outcome
Of the 140 patients randomised to CPAP treatment, 120 (86%) reported that they were still using CPAP at 3 months and 99 (71%) at 12 months. Actual CPAP data were obtained in 117 patients at 3 months, with median usage of 1.52 hours/night [interquartile range (IQR) 0.19 to 5.12 hours], and 102 patients at 12 months, with median usage of 2.22 hours/night (IQR 0.10 to 5.09 hours). Assuming zero usage in those patients with missing data and who stopped treatment during follow-up gave a more conservative estimate of median CPAP use of 1.33 hours/night (IQR 0.13 to 5.0 hours) at 3 months and 1.26 hours/night (IQR 0.04 to 4.45) at 12 months. CPAP usage data are shown in Table 24.
| Time assessed | Over first 3 months | Over 12 months |
|---|---|---|
| n randomised | 140 | 140 |
| n (%) analysed | 117 (84%) | 102 (73%) |
| Median use (mean hours/night) (IQR) | 01.52 (00.19–05.12) | 02.22 (00.10–05.09) |
| Using CPAP > 4 hours/night, n/N (%) | 41/117 (35) | 36/102 (35) |
| Missing data and stopped CPAP, n/N (%) | 7/140 (5) | 12/140 (9) |
| Median use (hours/night) (IQR)a | 01.33 (00.13–05.00) | 01.26 (00.04–04.45) |
Serious adverse events
There were 37 serious adverse events, of which 15 (in 12 patients) occurred in the CPAP group and 22 (in 13 patients) in the BSC group. They included two deaths, one in the CPAP group and one in the BSC group. All events were independently classified as unrelated to the trial. There was no suggestion of clinically important harm from CPAP use.
Self-reported side effects
This trial involved the use of an approved medical device which is the mainstay of treatment for OSAS in middle-aged populations. Therefore, the TSC did not expect any serious adverse events or adverse reactions of relevance to the device. Nonetheless, CPAP is associated with common side effects which were reported by the patients. The side effects were independently classified into categories, as suggested by the IDMC and TSC, and presented in Table 25. Treatment side effects are also incorporated in the SAQLI questionnaire.
| Side effect | BSC | CPAP |
|---|---|---|
| n | 138 | 140 |
| Possibly related to OSAS, n (%) | ||
| Daytime sleepiness/morning headaches/snoring/raised haematocrit | 4 (3) | 2 (1) |
| Cardiac dysrhythmias (e.g. atrial fibrillation) | 5 (4) | 1 (1) |
| Other cardiovascular events (e.g. stroke, transient ischaemic attack, heart failure, angina) | 5 (4) | 2 (1) |
| RTAs | 1 (1) | – |
| Probably related to CPAP, n (%) | ||
| Interface-related issues (e.g. claustrophobia, dislike of mask, leaking air, red/watery eyes, sore skin, pressure uncomfortable) | – | 33 (24) |
| Upper airway problems (e.g. dry mouth, runny or stuffy nose, sinus problems, nose bleeds) | – | 47 (34) |
| Abdominal bloating, n (%) | – | 4 (3) |
| Anxiety/dyspnoea related to CPAP | – | 4 (3) |
| General inconvenience or intolerance of CPAP or accident using the CPAP machine | – | 4 (3) |
| Possibly related to either OSAS or CPAP | ||
| Disturbed sleep (e.g. insomnia, noisy equipment) | – | 2 (1) |
| Social issues (e.g. partner disturbed, inconvenience) | – | 2 (1) |
| Probably unrelated, n (%) | ||
| Lower respiratory problems (e.g. cough, bronchitis, worsening asthma, pneumonia, ‘chest infection’) | 5 (4) | 6 (4) |
| Incidental medical conditions | 19 (14) | 21 (21) |
| Accidents (unrelated to sleepiness) | – | 2 (1) |
| Upper respiratory tract infection | – | 7 (5) |
Chapter 4 Health economics
The evaluation of the cost-effectiveness evidence of CPAP for the treatment of OSAS in people aged 65 years and over comprises a systematic review of the existing cost-effectiveness evidence on CPAP, within-trial analysis based on individual patient data collected during the 12 months of the PREDICT, and decision-analytic modelling to extrapolate to a lifetime time horizon and incorporating relevant external evidence.
Systematic review of existing cost-effectiveness evidence on continuous positive airway pressure
This systematic review provides an overview of the existing cost-effectiveness evidence, as well as an assessment of the quality and relevance of the data from the perspective of the UK NHS, on whether or not CPAP is a cost-effective treatment for patients aged ≥ 65 years. Appendix 3 reports the methods and detailed results of the systematic review, including summary data and extraction tables of the relevant studies. An overall summary of the cost-effectiveness evidence and key areas of uncertainty is provided below. The findings from the review provide the basis for the development of a new decision-analytic model reported in Economic model.
The systematic review on the existing cost-effectiveness evidence on CPAP for the treatment of OSAS found 10 relevant studies. Most studies used a Markov model to synthesise the available evidence;20,111–115 health states for cardiovascular events and RTAs were typically included. The characterised health effects of CPAP included lower risk of RTAs,20,111–117 work accidents,116 cardiovascular events20,112–114,116,117 and diabetes,116 and direct improvements in HRQoL from reduced sleepiness (all 10 studies). The improvement in HRQoL was estimated by converting other measures of quality of life118 or daytime sleepiness20,114 into health utilities, obtained directly from patients in before-and-after studies111–113,115,119 or from assumptions. 116,117 All studies concluded that CPAP is a cost-effective treatment for patients with OSAS. In general, the cost-effectiveness results were robust to alternative assumptions on parameter inputs with the exception of the health utility gain from treatment with CPAP.
The studies share two key limitations for the current decision problem:
-
None examined the cost-effectiveness of CPAP in patients aged ≥ 65 years.
-
All relied on indirect evidence to estimate the health utility benefit from treatment.
Although CPAP is likely to be beneficial in older people (as discussed in Chapter 1), the magnitude of such benefits cannot be inferred from the estimates obtained from younger populations. CPAP may be at least as cost-effective in older people, given their greater baseline risk of cerebrovascular events and the greater prevalence of age-related cognitive dysfunction, which CPAP could improve. On the other hand, CPAP may be less cost-effective, since older people generally suffer from other conditions which may affect sleep, such as Parkinson’s disease, which are not affected by CPAP. In addition, CPAP may be less effective in reducing BP in older people given their reduced acute BP response to each arousal from sleep. Finally, older people are likely to drive less often than working-age people (or shorter distances) and therefore have a lower risk of RTAs.
The limitations of the studies discussed above support a de novo analysis of the cost-effectiveness of CPAP, particularly focused on older people, and the integration of health utility evidence reported directly from patients in PREDICT. The analysis has two components, a within-trial analysis based on PREDICT which examines the cost-effectiveness of CPAP over 1 year and a decision model extrapolating to the patients’ lifetime and integrating external evidence.
Within-trial economic evaluation
Methods
Individual patient data from PREDICT were used to estimate health outcomes, costs and cost-effectiveness of CPAP in addition to BSC compared with BSC alone over 12 months. Costs were evaluated in pounds sterling at a 2012 price base from the UK NHS perspective. Health outcomes were expressed as QALYs. All analyses and modelling were undertaken in Stata 12.0.
Health outcomes
Health outcomes were expressed in QALYs using EQ-5D in the base case and SF-6D as an alternative scenario. Patients reported HRQoL by filling in EQ-5D questionnaires every month and using the SF-36 at baseline and at the 3-month and 12-month visits. EQ-5D scores are valued using the UK tariff. 120 SF-36 was translated into SF-6D using the Brazier et al. 121 algorithm. Patients who died had their HRQoL set to zero from the date of death. QALYs for each patient were calculated as the area under the curve, following the trapezium rule. 122 Differences in mean QALYs between the two patient groups were adjusted for HRQoL scores at baseline. 123
Resource use and costs
Health-care resource use was recorded in the monthly diaries filled in by patients. Information recorded in the diaries included information on medication initiated or discontinued, GP visits, nurse visits, telephone calls to the GP and to NHS Direct, ambulance use, visits to the accident and emergency department, outpatient appointments, hospital overnight admissions, emergency admissions and total number of nights in hospital over the past month. It was assumed that resource use associated with the RTAs or home accidents that were recorded during the trial would have been recorded in the monthly diary. Table 26 shows the unit costs applied to each resource use item in order to calculate the total cost per patient. Medication was not included in the total costs because the large majority of these were low-cost generics, use of which was unlikely to change the results.
| Health-care resource | Unit cost | Reference/comments |
|---|---|---|
| Visits to the GP | £43 | PSSRU unit costs 2012; 10.8b General practitioner124 |
| Home visits from the GP | £110 | PSSRU unit costs 2012; 10.8b General practitioner124 |
| Visits to nurse | £58/hour | PSSRU unit costs 2012; 10.1 Community nurse.124 Assumed 15 minutes appointment |
| Home visit from nurse | £70/hour | PSSRU unit costs 2012; 10.1 Community nurse.124 Assumed 30 minutes for appointment and travel |
| Telephone call to GP | £26 | PSSRU unit costs 2012; 10.8b General practitioner124 |
| Calls to NHS Direct | £28 | £25.53 (2007–8)125 inflated using inflating indices in PSSRU 2012124 |
| Ambulancea | £292 | £263 PSSRU 2008 inflated using inflating indices in PSSRU 2012126 |
| A&E visits | £108 | NHS Reference Costs 2011–12 – A&E not leading to admitted127 |
| Outpatient clinic | £106 | NHS Reference Costs 2011–12 – Total outpatient attendances127 |
| Hospital overnight admissions | £585 | NHS Reference Costs 2011–12 – Non-elective (short stay) HRG data127 |
| Emergency admissions | £157 | NHS Reference Costs 2011–12 – A&E leading to admitted127 |
Costs of continuous positive airway pressure treatment
The costs used in the cost-effectiveness analysis to estimate the average cost of treatment applied are shown in Table 27. The analysis followed an intention-to-treat strategy; therefore, only the patients allocated to CPAP were assumed to incur the cost of treatment. Patients allocated to BSC who switched to CPAP treatment were assumed not to incur the cost of treatment. Humidification was optional. No data were collected on the number of masks each patient received; therefore, the base case assumes that 90% of patients received one mask and 10% received two masks. The filter was supplied with the machine and it was assumed that it was changed every 6 months. The cost of filters was calculated as the average cost between the normal and the hypoallergenic filter. Patients who discontinue CPAP were assumed to return the machine (together with the humidifier, if applicable) to be re-used by another patient. The device’s useful life was assumed to be 7 years for the CPAP machine and the humidifier, 1 year for the masks and 6 months for the air filters. This was based on the assumptions made for the previous cost-effectiveness analyses of CPAP reported in the systematic review and discussions with the clinical team. Therefore, treatment costs were expressed as an annual equivalent cost using the public sector discount rate of 3.5% for machines with a lifetime greater than 1 year. 99,129 These assumptions were varied in the sensitivity analysis.
| Item | Unit cost | Machine life |
|---|---|---|
| CPAP machine | ||
| CPAP machine S9 AutoSet™ | £430 | 7 years |
| Humidifier H5i™ and climate line | £165 | 7 years |
| Masks | ||
| Mirage Quattro™ full-face mask | £120 | 1 year |
| Mirage Liberty™ | £125 | 1 year |
| Mirage Swift™ | £89 | 1 year |
| Mirage Micro™ nasal mask | £80 | 1 year |
| Filters | ||
| Air filter (S9™), pack of 50 | £8 | 6 months |
| Air filter, hypoallergenic (S9™), pack of 50 | £50 | 6 months |
Missing data
Data were missing or incomplete if patient failed to return a questionnaire, provided a partially complete questionnaire or was lost to follow-up (censored). Missing data at baseline were imputed with mean imputation. 130 Unanswered questions on resource use in the returned questionnaires were assumed to indicate that no resource use had taken place during that month. The remaining missing data were imputed, using multiple imputation with chained equations and predictive mean matching. 131 This approach assumed that data were missing at random, i.e. that the value of the missing data on costs and/or HRQoL could be predicted from the non-missing data. The multiple-imputed data sets constituted the data set used for the base case. Appendix 3 provides more details on the strategy employed to deal with missing data. Assumptions were varied in the sensitivity analysis.
Base-case analysis
The base-case analysis followed an intention-to-treat strategy, whereby patients were analysed according to allocation, irrespective of compliance with treatment. The cost-effectiveness of CPAP was evaluated by comparing the costs and QALYs in the two patient groups at 1 year, using conventional decision rules and estimating incremental cost-effectiveness ratios (ICERs) as appropriate. 132 The base-case analysis used EQ-5D to calculate QALYs whereas SF-6D was used in an alternative scenario. The probability that CPAP was cost-effective under the thresholds used by NICE (£20,000 and £30,000 per QALY gained) was calculated with semi-parametric bootstrapping. 133,134
Sensitivity analysis
A number of alternative scenarios were considered in which the assumptions used as part of the base-case results were varied. These analyses were undertaken to assess the robustness of the base-case results to alternative assumptions. Table 28 summarises the sensitivity analyses. For each element, the position in the base-case analysis is outlined, alongside the alternative assumption applied. These analyses were conducted for both the base-case scenario with QALYs calculated with EQ-5D and the alternative scenario with QALYs calculated from SF-6D.
| Scenario | Element | Position in base-case analysis | Variation in the sensitivity analysis |
|---|---|---|---|
| 1 | Costs of CPAP | The costs of the CPAP machine and the humidifier are annuitised over 7 years. Yearly replacement for masks. Filters replaced every 6 months | Frequent replacement scenario:135 CPAP machine annuitised over 3 years. Masks replaced every 3 months. Filters replaced monthly |
| 2 | Time horizon | The time horizon for costs corresponds to the lifetime of the CPAP machine (7 years) | CPAP is assumed to be used for 1 year and discarded after that; therefore, the cost of the machine is not annuitised |
| 3 | Missing data | Missing data assumed to be missing at random | Complete-case analysis – missing data assumed to be missing completely at random |
| 4 | Missing data | Missing data assumed to be missing at random | Missing data imputed with mean interpolation |
| 5 | Missing data | Missing data assumed to be missing at random | Individuals with missing data have 25% greater costs or experience 25% lower HRQoL |
Subgroup analysis
The aim of the subgroup analysis was to identify patient subgroups where the intervention was potentially more or less cost-effective than in the overall patient population. The clinical effectiveness presented in Chapter 3 showed that CPAP reduced daytime sleepiness and that the treatment effect was significantly larger in patients with a higher baseline ESS score. No other treatment interactions were found. Therefore, subgroup analysis is presented for patients with more or less severe daytime sleepiness, as measured by ESS score at baseline. The same cut-off points as those used for the stratification in the randomisation process (ESS score of < 13 and ESS score of ≥ 13) were used to define less severe (ESS score of < 13) and more severe (ESS score of ≥ 13) OSAS. Each of the subgroups were analysed independently with the same analytic model used for the overall population.
Results
Health-related quality of life
The EQ-5D health utility values for each treatment group are shown in Table 29. The proportion of patients who answered the EQ-5D questionnaire at each month varied between 100% (CPAP group at baseline) and 66% (CPAP group at months 10 and 11). The proportion of missing data was similar across treatment groups. Figure 9 shows the observed EQ-5D values over the trial for both patient groups. No clear pattern emerged. The CPAP group had greater mean EQ-5D scores at baseline and at months 2, 4–7, 9 and 12 (and lower scores in the other months); however, the differences were not statistically significant. Therefore, no clear treatment effect emerges from the comparison of EQ-5D scores between groups.
| Month | BSC | CPAP | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | Mean | SD | n | % | Mean | SD | |
| 0 | 136 | 98.55 | 0.680 | 0.242 | 140 | 100.00 | 0.693 | 0.249 |
| 1 | 116 | 84.06 | 0.687 | 0.246 | 112 | 80.00 | 0.684 | 0.280 |
| 2 | 100 | 72.46 | 0.685 | 0.258 | 98 | 70.00 | 0.700 | 0.292 |
| 3 | 121 | 87.68 | 0.704 | 0.251 | 121 | 86.43 | 0.672 | 0.301 |
| 4 | 101 | 73.19 | 0.679 | 0.259 | 97 | 69.29 | 0.692 | 0.284 |
| 5 | 101 | 73.19 | 0.660 | 0.267 | 97 | 69.29 | 0.671 | 0.328 |
| 6 | 109 | 78.99 | 0.663 | 0.255 | 103 | 73.57 | 0.677 | 0.295 |
| 7 | 105 | 76.09 | 0.652 | 0.271 | 97 | 69.29 | 0.687 | 0.276 |
| 8 | 104 | 75.36 | 0.683 | 0.268 | 88 | 62.86 | 0.668 | 0.311 |
| 9 | 109 | 78.99 | 0.650 | 0.275 | 95 | 67.86 | 0.682 | 0.287 |
| 10 | 102 | 73.91 | 0.694 | 0.254 | 92 | 65.71 | 0.647 | 0.318 |
| 11 | 99 | 71.74 | 0.647 | 0.286 | 92 | 65.71 | 0.656 | 0.310 |
| 12 | 119 | 86.23 | 0.680 | 0.264 | 115 | 82.14 | 0.689 | 0.301 |
FIGURE 9.
European Quality of Life-5 Dimensions health utility values over the trial. Figure shows mean (as a square or lozenge) and the 95% CI (as lines).

The SF-6D health utility values are shown in Table 30 and Figure 10. The proportion of patients who answered the SF-36 questionnaire was higher than for the EQ-5D; this may have been related to the questionnaire being administered at the 3-month and 12-month clinic visit, while the EQ-5D was returned monthly by post. There was also less variability in the SF-6D health utility values than that observed in the EQ-5D. This may also have been related to the administration of the questionnaire or to the differences in the scoring algorithm. The difference between treatment groups was non-statistically significant for the SF-6D.
| Month | BSC | CPAP | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | Mean | SD | n | % | Mean | SD | |
| 0 | 137 | 99.28 | 0.659 | 0.092 | 138 | 98.57 | 0.661 | 0.088 |
| 3 | 125 | 90.58 | 0.661 | 0.088 | 123 | 87.86 | 0.681 | 0.087 |
| 12 | 118 | 85.51 | 0.653 | 0.096 | 113 | 80.71 | 0.679 | 0.111 |
FIGURE 10.
Short Form questionnaire-6 Dimensions health utility values over the trial. Figure shows mean (as a square or lozenge) and the 95% CI (as lines).
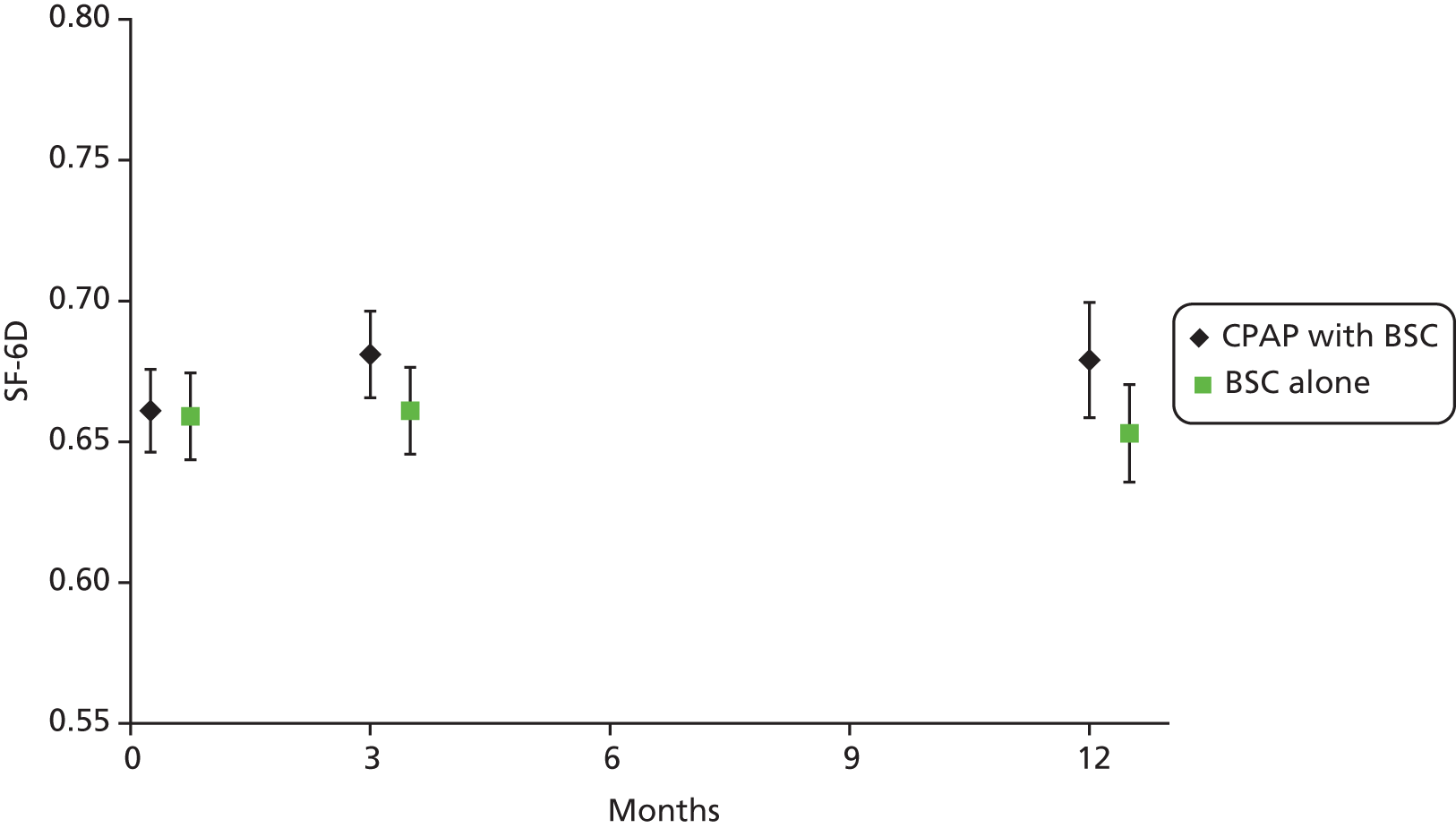
Health-care resource use and costs
The number and proportion of returned questionnaires on health-care resource are shown in Table 31. Baseline refers to the resource use in the month prior to enrolment. The proportion of returned questionnaires was similar across treatment groups and varied between 100% and 66%.
| Month | BSC | CPAP | ||
|---|---|---|---|---|
| n | % | n | % | |
| Baseline | 138 | 100.00 | 140 | 100.00 |
| 1 | 118 | 85.51 | 116 | 82.86 |
| 2 | 101 | 73.19 | 99 | 70.71 |
| 3 | 122 | 88.41 | 120 | 85.71 |
| 4 | 102 | 73.91 | 96 | 68.57 |
| 5 | 101 | 73.19 | 98 | 70.00 |
| 6 | 109 | 78.99 | 105 | 75.00 |
| 7 | 106 | 76.81 | 96 | 68.57 |
| 8 | 106 | 76.81 | 92 | 65.71 |
| 9 | 109 | 78.99 | 95 | 67.86 |
| 10 | 102 | 73.91 | 93 | 66.43 |
| 11 | 99 | 71.74 | 93 | 66.43 |
| 12 | 120 | 86.96 | 116 | 82.86 |
The resources used by each patient group [average number of times (SD) the resource was used] over the 12-month follow-up period are shown in Table 32. The estimates were obtained prior to multiple imputation of missing data. The group allocated to BSC alone had more contacts with the NHS but the differences are non-statistically significant. The most frequent contact was visits to GP (CPAP 6.93 vs. BSC 7.27 visits per patient). The second most frequent NHS contact were visits to the nurse (CPAP 3.36 vs. BSC 4.78 visits per patient), followed by outpatient appointments (CPAP 2.83 vs. BSC 3.65 per patient). Hospital overnight admissions were rare (CPAP 0.42 vs. BSC 0.55 per patient). There were 93 patients in the CPAP group who initiated medication (386 items) and 62 who discontinued medication (197 items), whereas in the BSC group 92 patients initiated medication (444 items) and 57 discontinued medication (192 items).
| Type | BSC | CPAP | ||
|---|---|---|---|---|
| Average per patient | SD | Average per patient | SD | |
| Any contact with NHS | 7.30 | 1.69 | 6.70 | 1.72 |
| Visit to GP | 7.27 | 3.20 | 6.93 | 2.94 |
| GP home visit | 0.32 | 0.67 | 0.18 | 0.47 |
| Visit to nurse | 4.78 | 3.23 | 3.36 | 2.16 |
| Nurse home visit | 0.27 | 0.80 | 0.14 | 0.51 |
| GP telephone call | 0.92 | 1.07 | 0.54 | 0.78 |
| NHS direct telephone call | 0.21 | 0.50 | 0.06 | 0.27 |
| Ambulance | 0.22 | 0.47 | 0.17 | 0.46 |
| Accident and emergency | 0.42 | 0.68 | 0.32 | 0.55 |
| Outpatient clinic | 3.65 | 2.48 | 2.83 | 1.99 |
| Patients who stayed in hospital overnight at least once | 0.42 | 0.64 | 0.33 | 0.57 |
| Hospital overnight admissions | 0.55 | 1.30 | 0.42 | 1.01 |
| Emergency admissions | 0.23 | 0.47 | 0.19 | 0.44 |
| Nights in hospital | 2.15 | 4.85 | 1.11 | 2.62 |
The health-care resource costs over the trial are shown in Table 33. These costs refer to the resource use data presented in Table 32 and multiplied by the relevant unit costs (see Table 26). On average, patients randomised to CPAP incurred less cost, but the difference was not statistically significant. The highest costs were those related to outpatient appointments, hospital overnight admissions and visits to the GP. The SD shows how much dispersion there was around the average cost; in some cost categories the SD was greater than the mean (e.g. GP home visit, nurse home visit), indicating that there was a large variability in the costs incurred by each patient.
| Type | BSC alone | CPAP | ||
|---|---|---|---|---|
| Average (£) | SD (£) | Average (£) | SD (£) | |
| Visit to GP | 312.62 | 137.71 | 298.03 | 126.56 |
| GP home visit | 35.57 | 73.41 | 20.26 | 51.98 |
| Visit to nurse | 69.27 | 46.78 | 48.79 | 31.39 |
| Nurse home visit | 9.35 | 27.85 | 5.04 | 17.69 |
| GP telephone call | 24.05 | 27.72 | 14.12 | 20.38 |
| NHS Direct telephone call | 5.95 | 13.94 | 1.55 | 7.51 |
| Ambulance | 64.79 | 136.53 | 50.76 | 133.72 |
| Accident and emergency | 45.40 | 73.27 | 34.23 | 59.88 |
| Outpatient clinic | 386.84 | 263.18 | 300.25 | 210.43 |
| Hospital overnight admissions | 321.17 | 757.68 | 246.78 | 590.14 |
| Emergency admissions | 35.82 | 74.40 | 30.57 | 68.88 |
| Total costs associated with health-care resource use | 1311 | 1009.44 | 1050 | 830.21 |
Costs of CPAP treatment
The average costs of CPAP treatment per patient (including the respective components) are shown in Table 34. All patients allocated to the intervention received a standardised CPAP machine (S9 AutosetTM ResMed (UK) Ltd, Abingdon, Oxfordshire, UK) and a mask. Eighty-two patients (59%) also received a humidifier. Patients who discontinued CPAP were assumed to return the machine (together with the humidifier, if relevant). Only eight patients allocated to the BSC group switched to CPAP treatment. Since the analysis follows intention to treat, these patients are assumed not to incur the costs of CPAP treatment. Given the small number of patients switching to CPAP treatment, this is unlikely to affect the results. The average cost of CPAP treatment was estimated at £201.14 per patient per year.
| Item | Cost element | Number | Average cost per patient (£) |
|---|---|---|---|
| A | Annual equivalent cost of CPAP machine | – | 70.32 |
| B | Annual equivalent cost of humidifier | – | 26.98 |
| C | Number (and proportion) of patients who received a humidifier | 82 (59%) | – |
| D | Average annual equivalent cost of humidifier per patient ( = B × C) | – | 15.81 |
| E | Average annual equivalent cost per patient ( = A + D) | – | 86.13 |
| F | Average cost of masks | – | 104 |
| G | Average cost of masks assuming (10% of patients received 2) ( = 1.1 × F) | – | 114 |
| H | Average cost per filter | – | 0.58 |
| I | Average cost of filters per patient per year (2 filters per year) ( = 2 × H) | – | 1.16 |
| Average cost of CPAP treatment per patient ( = E + G + I) | – | 201.14 |
Cost-effectiveness analysis
The cost-effectiveness results for PREDICT are presented in Table 35. The analysis was conducted post multiple imputation and included the costs of CPAP treatment (£201.14) for those patients allocated to the CPAP group. Detailed costs and HRQoL post imputation are given in Appendix 3. The average cost per patient was £1363 (95% CI £1121 to £1606) for those allocated to CPAP and £1389 (95% CI £1116 to £1662) for those allocated to BSC alone. The accrued cost was, on average, –£35 (95% CI –£390 to £321) lower for those allocated to CPAP. The average QALYs obtained from EQ-5D health utilities were 0.680 (95% CI 0.638 to 0.722 QALYs) for the CPAP group and 0.666 (95% CI 0.627 to 0.705 QALYs) for those allocated to BSC alone. The average QALYs obtained from SF-6D health utilities for were 0.678 (95% CI 0.664 to 0.691 QALYs) for the CPAP group and 0.658 (95% CI 0.643 to 0.673 QALYs) for those allocated to BSC alone. The CPAP group experienced more EQ-5D QALYs [0.005 (95% CI –0.034 to 0.044 QALYs)] and more SF-6D QALYs [0.018 (95% CI 0.003 to 0.034 QALYs)]. The improvements in QALYs were small, albeit statistically significant, for the SF-6D. The improvement of 0.005 QALYs is equivalent to 2 days in full health and the improvement of 0.018 QALYs is equivalent to 7 days. Overall, CPAP appeared to have improved health outcomes as well as reduced overall costs to the NHS. Therefore, CPAP with BSC dominated BSC alone. In these situations, it is not appropriate to present ICERs. 132
| Treatment group | Costs | EQ-5D QALYs | SF-6D QALYs | |||
|---|---|---|---|---|---|---|
| Average (£) | SE | Average | SE | Average | SE | |
| CPAP | 1363 | £123 | 0.680 | 0.021 | 0.678 | 0.007 |
| BSC | 1389 | £139 | 0.666 | 0.020 | 0.658 | 0.008 |
| Incremental costs and QALYs | ||||||
| CPAP with BSC – BSC alone | –35 | £180 | 0.005 | 0.020 | 0.018 | 0.008 |
The cost-effectiveness planes for the base case with EQ-5D QALYs and the alternative scenario with SF-6D QALYs are shown in Figure 11a and b, respectively. The lines represent the cost-effectiveness thresholds conventionally used in the NHS (£20,000 per additional QALY gained). The simulations for the cost-effectiveness results using EQ-5D QALYs were evenly spread across the four quadrants while the simulations using SF-6D QALYs were mostly concentrated on the eastern quadrants, indicating that there was considerable uncertainty as to CPAPs health benefits, particularly those captured by EQ-5D, and whether or not it was cost-saving. The small improvement in health outcomes was more certain with SF-6D QALYs.
FIGURE 11.
Cost-effectiveness plane for the overall population. (a) EQ-5D QALYs; and (b) SF-6D QALYs.
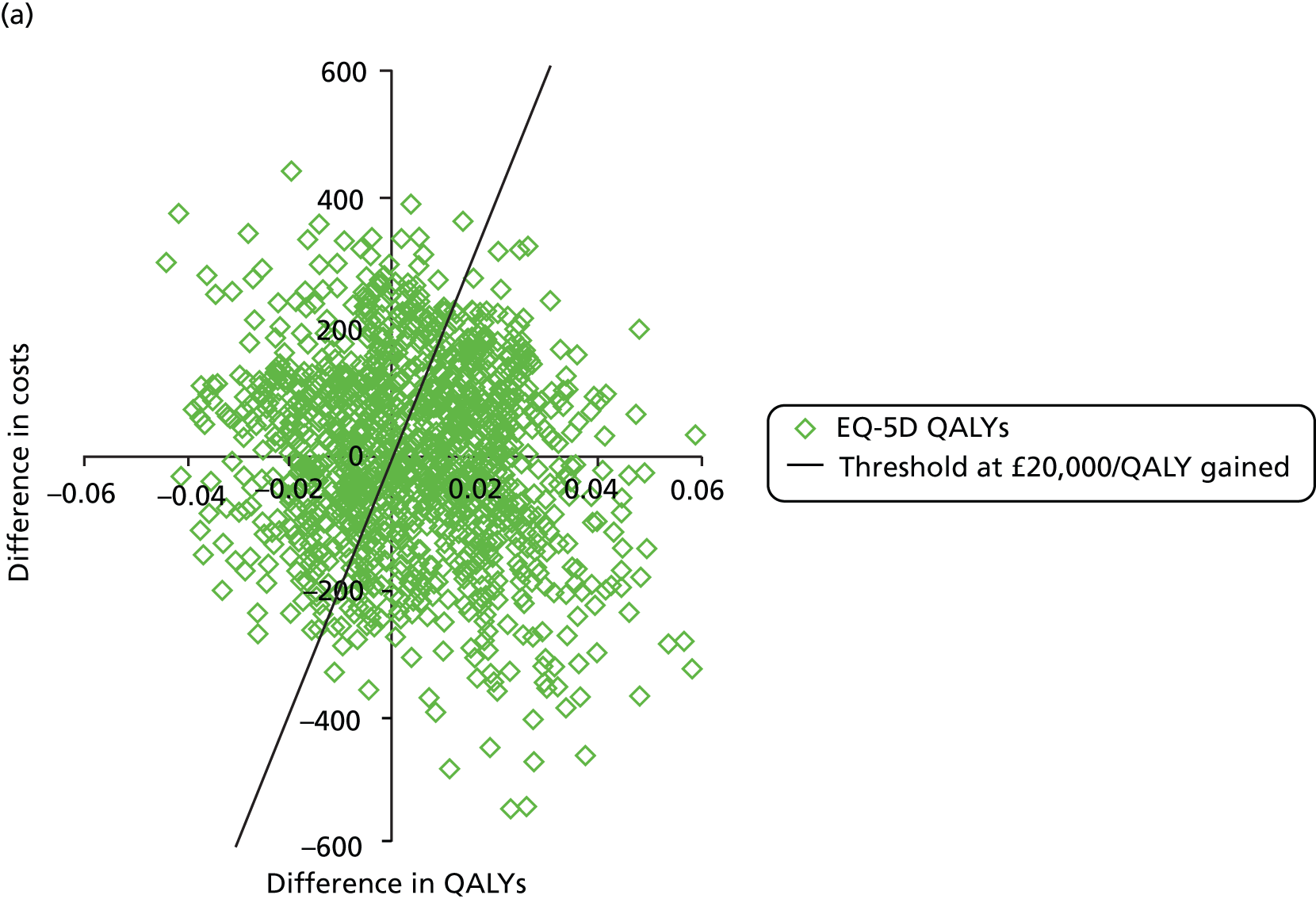

The cost-effectiveness acceptability curve for the base case (EQ-5D QALYs) and its alternative scenario with SF-6D QALYs is shown in Figure 12. The probability that the intervention was cost-effective at the thresholds conventionally used by NHS is 0.61 per QALY gained for the base case and 0.96 for the scenario with SF-6D QALYs. In the base case, the probability that CPAP was cost-effective plateaus at 0.60 as the cost-effectiveness threshold increased. This occurred because of the uncertainty around the improvement in EQ-5D QALYs observed during the trial, reflected in the 95% CI of –0.034 to 0.044. In contrast, the probability that CPAP was cost-effective was above 0.9 across the full range of cost-effectiveness thresholds with SF-6D QALYs. This is consistent with the scatter pattern in the cost-effectiveness plane (see Figure 11).
FIGURE 12.
Cost-effectiveness acceptability curve.
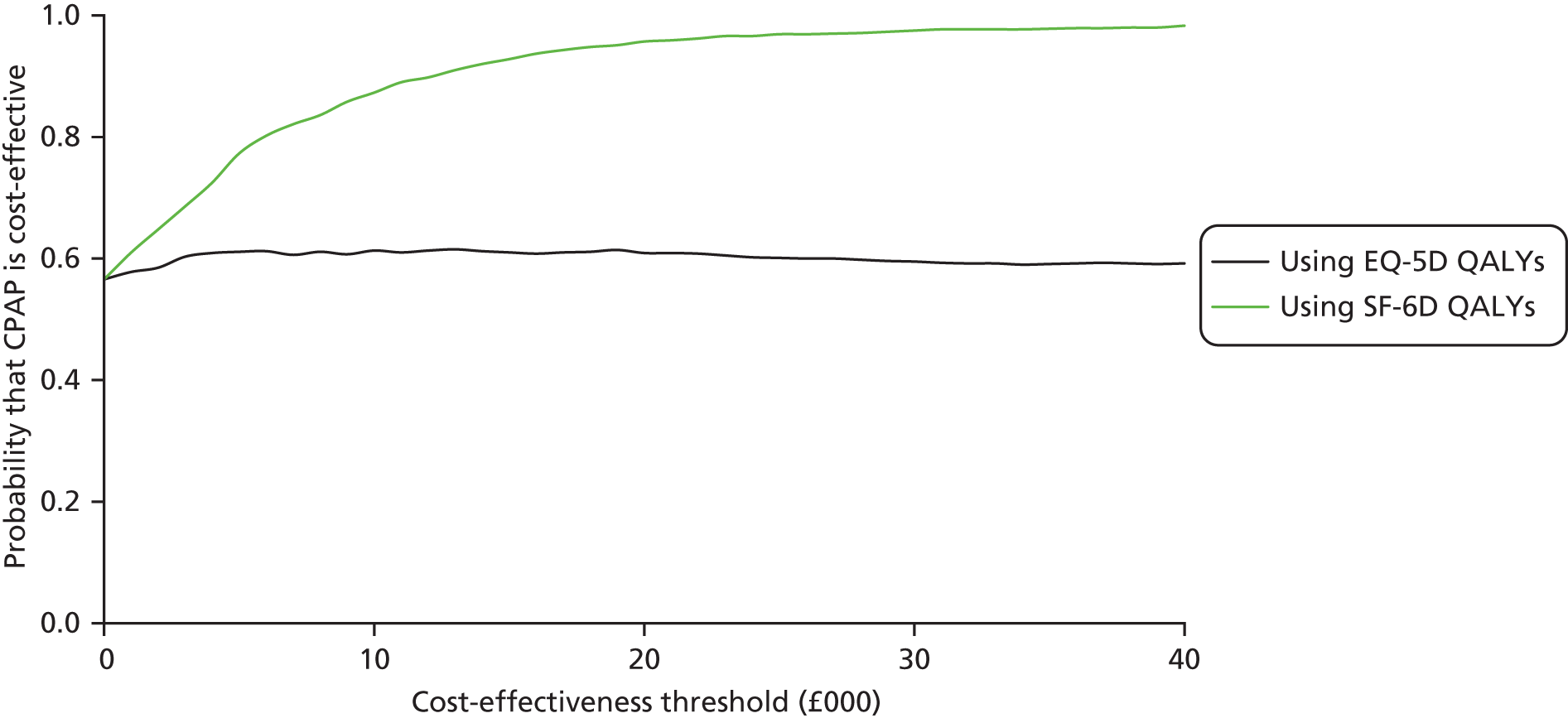
Subgroup analysis
The results for the subgroups defined according to ESS score at baseline are shown in Table 36. There were 184 patients with ESS score of < 13 (CPAP, n = 88; BSC, n = 96) and 94 patients with ESS score of ≥ 13 (CPAP, n = 52; BSC, n = 42). For the less severe OSAS subgroup, CPAP appears to be less costly and provide fewer QALYs than BSC; therefore, the ICER for BSC alone versus CPAP was £1118 per QALY gained for the base case with EQ-5D QALYs. CPAP dominated in the SF-6D QALYs scenario because it was associated with lower costs and greater QALYs; therefore, an ICER was not calculated. For the more severe OSAS subgroup, CPAP dominated BSC alone because it was associated with lower costs and better health outcomes in both the base case with EQ-5D QALYs and the scenario with SF-6D QALYs.
| Treatment group | Costs | EQ-5D QALYs | SF-6D QALYs | |||
|---|---|---|---|---|---|---|
| Average (£) | SE (£) | Average | SE | Average | SE | |
| Subgroup ESS score of < 13 | ||||||
| CPAP | 1393 | 162 | 0.677 | 0.027 | 0.675 | 0.009 |
| BSC | 1440 | 179 | 0.684 | 0.023 | 0.660 | 0.10 |
| Incremental costs and QALYs | ||||||
| CPAP with BSC – BSC alone | –19 | 231 | –0.017 | 0.025 | 0.018 | 0.010 |
| Subgroup ESS score of ≥ 13 | ||||||
| CPAP | 1313 | 188 | 0.686 | 0.035 | 0.682 | 0.012 |
| BSC | 1274 | 212 | 0.624 | 0.037 | 0.654 | 0.012 |
| Incremental costs and QALYs | ||||||
| CPAP with BSC – BSC alone | 17 | 270 | 0.049 | 0.032 | 0.018 | 0.012 |
There was considerable uncertainty in these results because in both subgroups, similarly to the overall population, the differences in costs were small and not statistically significant, –£19 (95% CI –£475 to £438) for the less severe OSAS subgroup and £17 (95% CI –£520 to £555) for the more severe OSAS subgroup. The differences in QALYs were also small and not statistically significant. The less severe OSAS subgroup experienced a difference in EQ-5D QALYs of –0.017 (95% CI –0.066 to 0.033 QALYs) and in SF-6D QALYs of 0.018 (95% CI –0.002 to 0.038 QALYs). The more severe OSAS subgroup experienced positive differences for both EQ-5D and SF-6D QALYs, although not statistically significant, at 0.049 (95% CI –0.014 to 0.111) for EQ-5D QALYs and 0.018 (95% CI –0.006 to 0.043) for SF-6D QALYs. Thus, it is difficult to draw definite conclusions from this analysis given the level of uncertainty around the results. The EQ-5D QALYs appeared to follow the improvement in ESS score, which is more pronounced in the more severe OSAS subgroups (see Table 8). The improvement in SF-6D QALYs was similar in the two subgroups, although the differences in QALYs between CPAP and BSC alone were small across subgroups.
The cost-effectiveness planes for more severe (ESS score at baseline of ≥ 13) and less severe OSAS (ESS score at baseline of < 13) are shown in Figure 13a and c, and b and d, respectively. As with Figure 11, results for EQ-5D QALYs are shown in Figure 13a and c and for SF-6D QALYs are shown in Figure 13b and d. The axis scales are the same to facilitate comparison. The results differed depending on ESS score at baseline and HRQoL instrument used to obtain QALYs. In the less severe OSAS population, the simulations for EQ-5D QALYs were mostly spread across the western quadrants, indicating decrements in health outcomes. The simulations for SF-6D QALYs were concentrated in the eastern quadrants, indicating positive gains in health. In the more severe OSAS population, most of the simulations were located in the eastern quadrants for both EQ-5D and SF-6D QALYs. In other words, we are more confident that CPAP was cost-effective in the more severe OSAS subgroup.
FIGURE 13.
Cost-effectiveness plane for population subgroups. (a) ESS score of < 13 EQ-5D; (b) ESS score of < 13 SF-6D; (c) ESS score of ≥ 13 EQ-5D; and (d) ESS score of ≥ 13 SF-6D.
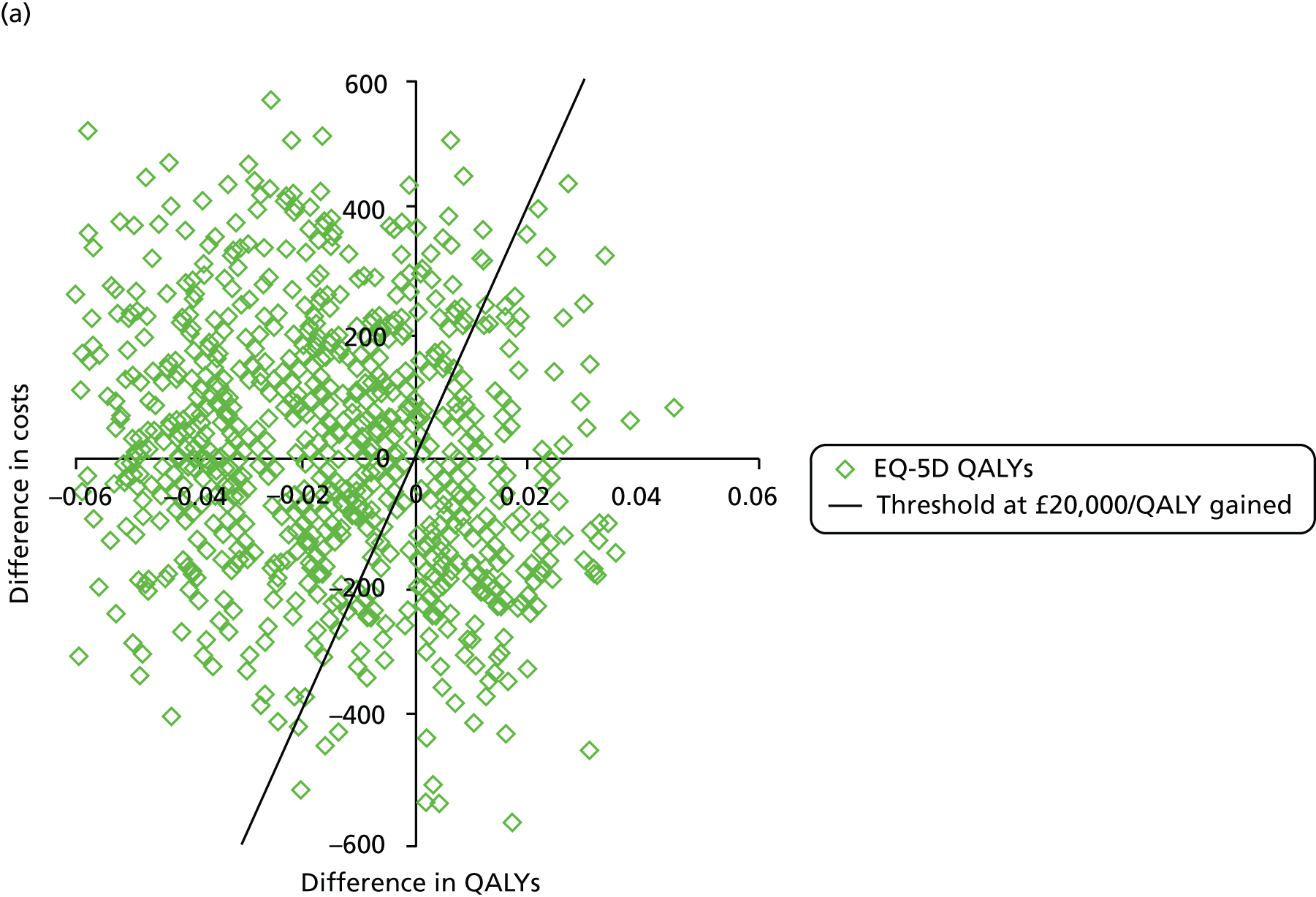

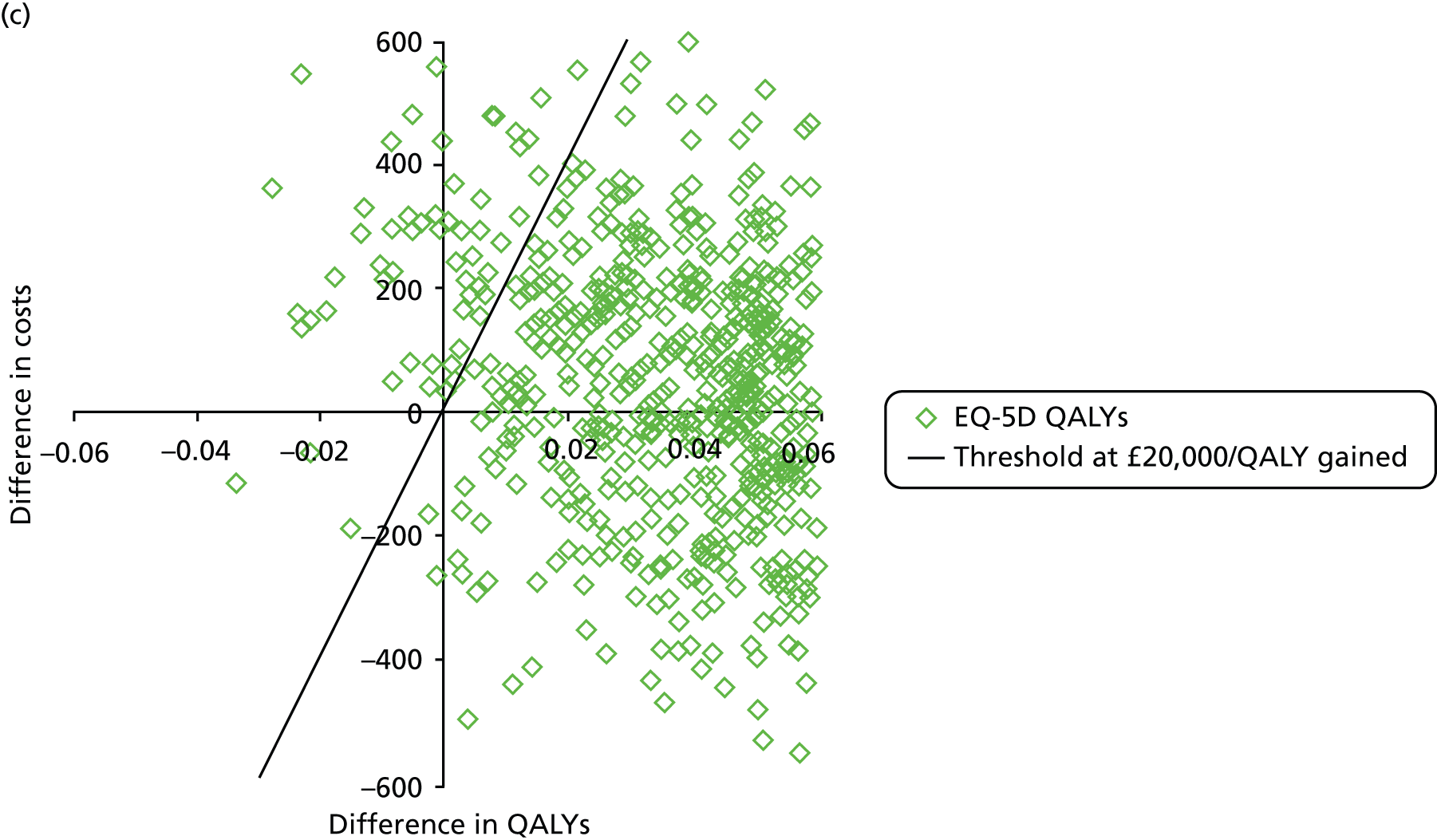

The cost-effectiveness acceptability curves for the subgroups are shown in Figure 14. The certainty around the cost-effectiveness of the intervention depended on the HRQoL instrument and the subgroup considered. In the less severe OSAS subgroup, the probability that CPAP was cost-effective decreased as the cost-effectiveness threshold increased for EQ-5D QALYs but increased for SF-6D QALYs. This reflected the distribution of the differences in QALYs presented in Figure 13. Using EQ-5D QALYs, the mean difference was close to zero but the majority of the simulations returned a decrement in QALYs between the treatment groups. In the cost-effectiveness acceptability curve, that decrement was compensated by the decrease in costs. However, as the threshold increased, the value placed on QALYs increased in relation to the costs savings. Therefore, for higher threshold values, CPAP was less likely to be cost-effective. Using SF-6D QALYs, the majority of simulations returned a gain in QALYs between treatment groups. This was because, as the threshold increased, the gains were valued, and the probability that CPAP was cost-effective increases. In the more severe OSAS group, the probability that CPAP was cost-effective increased as the threshold increased. This reflected the cost-effectiveness plane in Figure 13, where most of the simulations, for both EQ-5D and SF-6D QALYs, returned gains in health.
FIGURE 14.
Cost-effectiveness acceptability curves for less severe OSAS population. (a) ESS score of < 13; and (b) ESS score of ≥ 13.
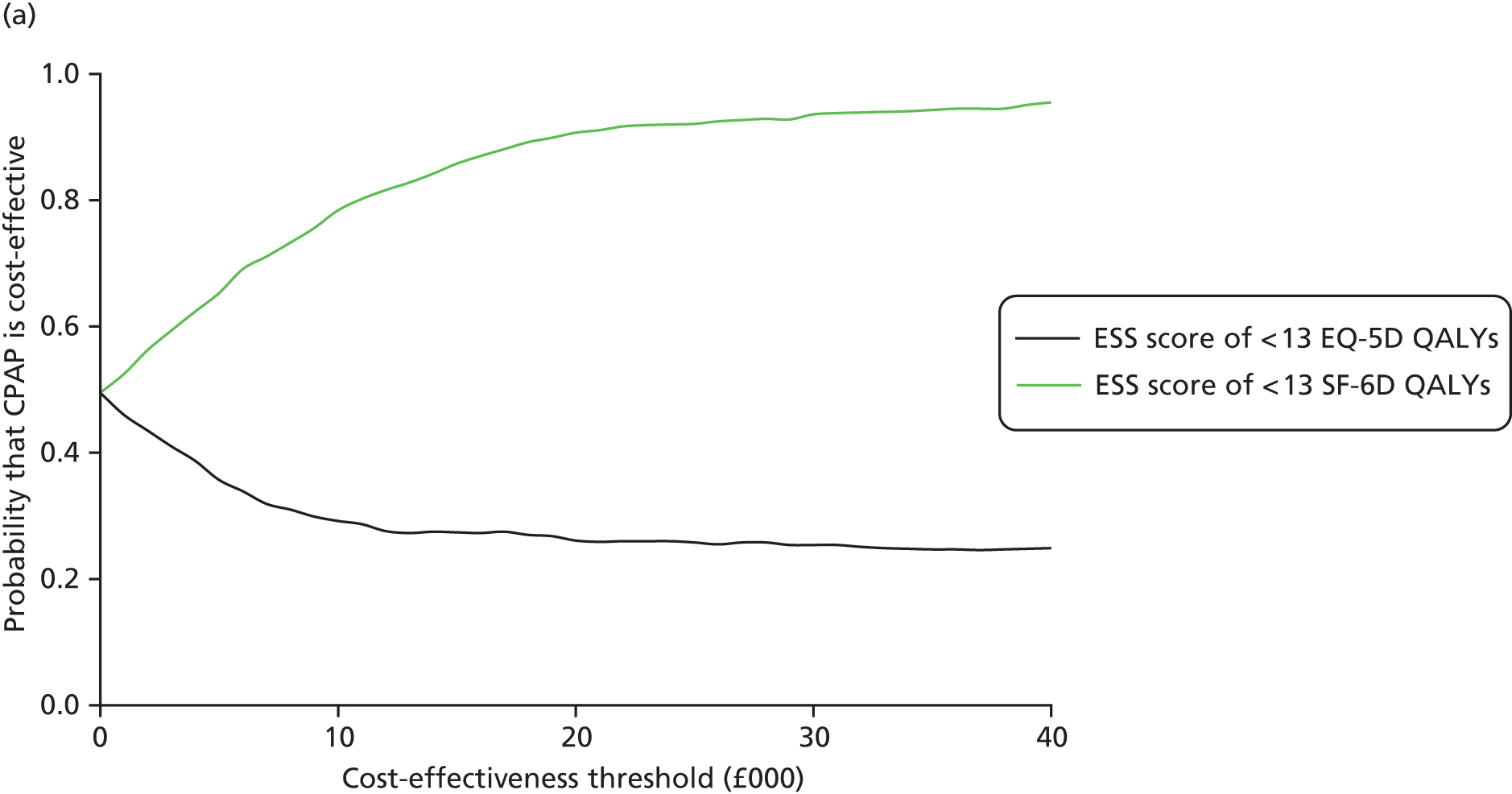
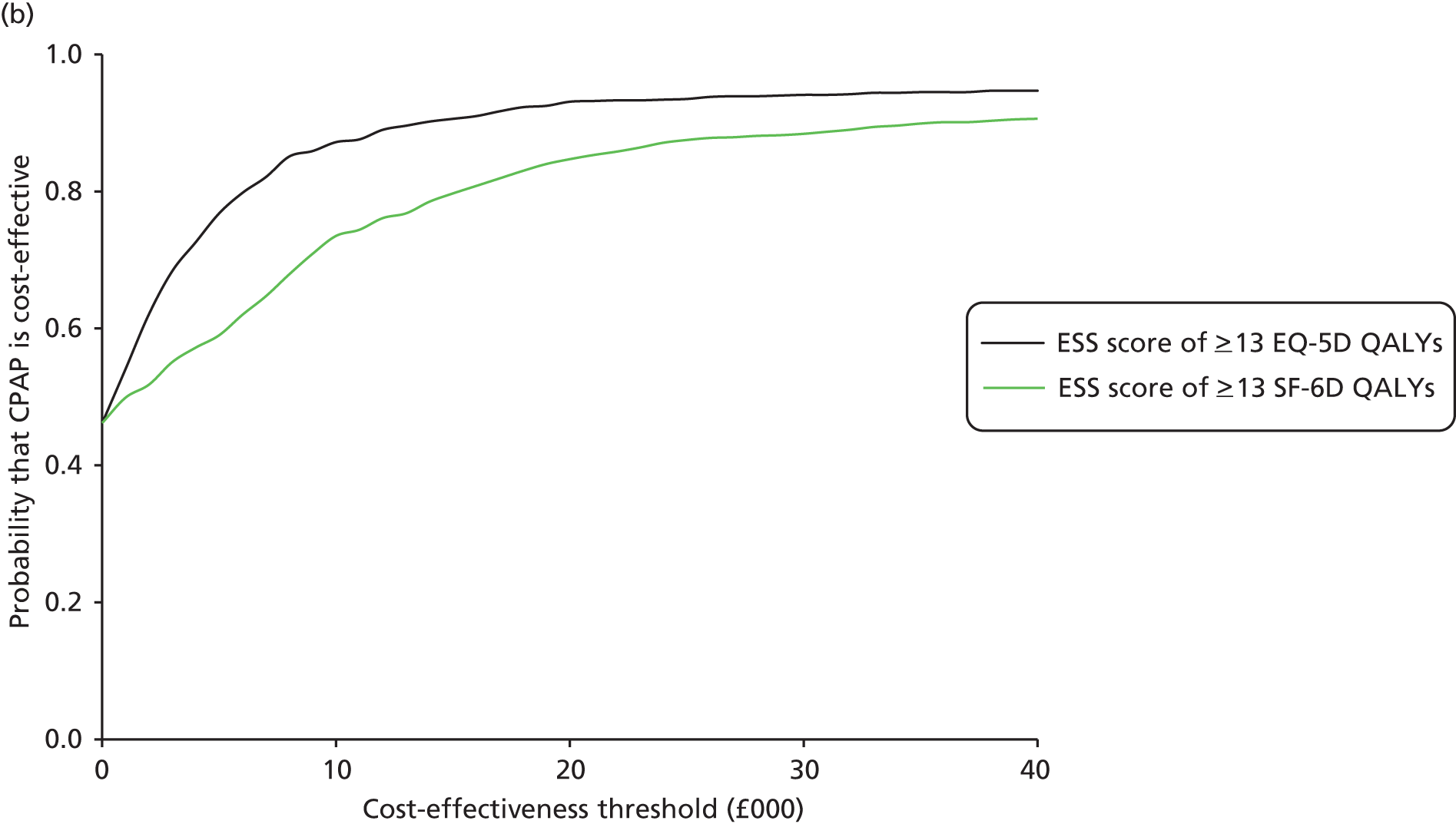
Sensitivity analysis
A selection of the results for the sensitivity analysis is presented in Table 37 (see Appendix 3 for additional information). The cost differences and QALY differences were small and close to zero in the majority of analyses, and this resulted in variable ICERs. Therefore, while the results were relatively consistent in terms of costs and QALYS, the cost-effectiveness conclusions were mixed. In two scenarios with EQ-5D QALYs and three with SF-6D, QALYs CPAP dominated BSC alone. In four scenarios with EQ-5D QALYs and three with SF-6D QALYs, CPAP had a positive ICER. In two of the scenarios with EQ-5D QALYs, the ICER was above the conventional thresholds of cost-effectiveness used by NICE of £20,000 and £30,000 per QALY gained.
| Scenario | Difference in costs | Difference in EQ-5D QALYs | ICER/EQ-5D QALYs (£) | SF-6D QALYs | ICER/SF-6D QALYs (£) | Probability that CPAP is cost-effective at £20,000/QALY gained | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average (£) | SE (£) | Average | SE | Average | SE | EQ-5D QALYs | SF-6D QALYs | |||
| Base case | –35 | 180 | 0.005 | 0.020 | D | 0.018 | 0.008 | D | 0.61 | 0.96 |
| 1: Frequent replacement scenario (CPAP costs = £608.95) | 373 | 180 | 0.005 | 0.020 | 74,600 | 0.018 | 0.008 | 20,722 | 0.25 | 0.46 |
| 2: CPAP used for 1 year (CPAP costs = £710.16) | 474 | 180 | 0.005 | 0.020 | 94,800 | 0.018 | 0.008 | 26,333 | 0.20 | 0.30 |
| 3A: Complete-case analysis (EQ-5D QALYs) | –258 | 258 | 0.010 | 0.029 | D | NA | NA | NA | 0.75 | NA |
| 3B: Complete-case analysis (SF-6D QALYs) | –201 | 247 | NA | NA | NA | 0.015 | 0.013 | D | NA | 0.91 |
| 4: Mean interpolation | 43 | 201 | 0.006 | 0.018 | 7167 | 0.014 | 0.007 | 3071 | 0.55 | 0.81 |
| 5A: Missing not at random (costs) | –27 | 201 | 0.005 | 0.020 | D | 0.018 | 0.008 | D | 0.62 | 0.96 |
| 5B: Missing not at random (QALYs) | –35 | 180 | –0.006 | 0.19 | 5833 | 0.012 | 0.009 | D | 0.42 | 0.85 |
Economic model
The ICER for CPAP was above the conventional thresholds of cost-effectiveness in scenarios 1 and 2, which assumed a higher treatment cost for CPAP. Scenario 1 assumed a greater frequency in the replacement of the machine and in the use of the consumables and scenario 2 assumed that the CPAP machine was used for only 1 year then discarded. In scenario 1, the CPAP and humidifier were assumed to be replaced every 3 years, the masks every 3 months and the filters monthly. The average cost of CPAP per patient per year tripled in scenario 1 from £201.14 in the base case to £608.94 and also more than tripled in scenario 2 to £710.16. This increase in the costs of CPAP resulted in an increase in the average difference in total costs, from –£35 (95% CI –£390 to £321) in the base case to £373 (95% CI £17 to £729) in scenario 1 and to £474 (95% CI £119 to £830) in scenario 2. The ICER for scenario 1 using EQ-5D QALYs was £74,600 and for scenario 2 was £47,800; for the same scenarios using SF-6D QALYs, it was £94,800 and £26,333, respectively. These results suggested that the cost-effectiveness of CPAP was highly sensitive to the costs of CPAP treatment.
Scenario 3 used only the data from patients who completed all questionnaires on HRQoL and costs. It assumed that these data were missing completely at random; that is, that the probability that data were missing was independent of both the observed and unobserved data. CPAP dominated BSC alone.
In scenario 4, missing data were imputed with the average of the observed data for each patient. The mean difference in costs changed slightly to £43 (95% CI –£353 to £440) while the differences in QALYs remained unchanged. Nevertheless, the change in the mean difference in costs between treatment groups resulted in a positive ICER of £7167 per EQ-5D QALY gained and £3071 per SF-6D QALY gained. This showed how small differences in costs or QALYs had a large impact on the results. Note that this scenario should be considered with caution, as it assumed that patients’ costs and HRQoL were expected to remain constant over the trial, which may not be the case.
The ‘missing not at random’ scenarios explored how the results changed if missing data on HRQoL and costs were assumed to be systematically different from that of patients with similar characteristics but who returned the questionnaires. The difference in costs changed very slightly, to –£26 (95% CI –£422 to £369), with little impact on the results. The difference in EQ-5D QALYs changed more noticeably from 0.005 (95% CI –0.034 to 0.044) to –0.006 (95% CI –0.044 to 0.031) whereas the difference in SF-6D QALYs changed slightly from 0.018 (95% CI 0.003 to 0.034) to 0.012 (95% CI –0.006 to 0.03). As a result, assuming that patients with missing data experienced lower HRQoL reduced the probability that CPAP was cost-effective from 0.61 to 0.42 for EQ-5D QALYs and from 0.96 to 0.85 for SF-6D QALYs.
Introduction
The results of the within-trial analysis suggested that CPAP may be a cost-effectiveness alternative to BSC alone. However, there was a question of whether or not all of the relevant outcomes of CPAP treatment were captured within the 12-month follow-up period. For example, a potential long-term benefit of CPAP is the reduction in the risk of cardiovascular events [e.g. stroke and coronary heart disease (CHD)]. No difference in the incidence of these events was observed in PREDICT; however, RCTs, such as PREDICT, are unlikely to capture differences in the incidence of such rare events. In order to extrapolate results beyond the period of follow-up and to incorporate relevant external evidence, it is common to employ a decision model. Other studies examining the cost-effectiveness of CPAP have considered the effect of CPAP in reducing the risk of cardiovascular events, diabetes, and work and RTAs using evidence from a variety of sources (see Systematic review of existing cost-effectiveness evidence on continuous positive airway pressure). McDaid et al. 20 used intermediate outcomes such as reductions in BP and cholesterol to predict long-term risks of cardiovascular events using published risk models. Therefore, by employing a decision model, differences in BP and other risk factors observed within the trial could be translated into differences in the incidence of events, which in turn have an impact on costs and QALYs. Furthermore, if there does exist relevant external evidence, it can be brought to bear on the cost-effectiveness analysis so that it synthesises all of the available evidence.
For these reasons, a decision model was employed to formally assess the cost-effectiveness of CPAP for the treatment of OSAS in older patients over a lifetime time horizon. Health outcomes were expressed in QALYs. Costs took the NHS and Personal Social Services perspectives, expressed in pounds sterling at a 2011–12 price base. Both costs and health outcomes were discounted at a 3.5% annual discount rate, in line with the NICE reference case.
The cost-effectiveness of CPAP was evaluated by comparing the additional costs of CPAP in combination with BSC, with its additional benefits in terms of improvement in HRQoL. HRQoL was measured by both EQ-5D and SF-6D. The results are presented for both measures, but it should be noted that EQ-5D QALYs constituted the primary outcome of PREDICT. The cost-effectiveness of CPAP was estimated using conventional decision rules and reported as an ICER if applicable. 132 All results were probabilistic in that input parameters were entered as probability distributions and Monte Carlo simulation was used to propagate the uncertainty over 10,000 simulations. The probabilistic results were translated into cost-effectiveness acceptability curves and probabilities that CPAP was cost-effective under conventional thresholds used by NICE of £20,000 and £30,000 per QALY gained. 136 Subgroup analysis is presented for patients with more or less severe daytime sleepiness: ESS score of < 13 and ESS score of ≥ 13 (as for the within-trial analysis). Each of the subgroups were analysed independently in the same model.
Reviews for external evidence
Systematic review of the clinical effectiveness of continuous positive airway pressure
A systematic review was conducted to identify any additional external evidence on the effectiveness of CPAP in this population to supplement the data collected in PREDICT. The inclusion criteria were RCTs comparing CPAP with sham CPAP, BSC or usual care, and dental devices in patients with an average age of 60 years or older with OSAS and capacity to give informed consent.
Three studies were identified that met the inclusion criteria from 3560 unique titles (see Appendix 3 for details of the review). The three studies included patients with cardiovascular conditions and compared CPAP therapy with sham CPAP88 or no CPAP89,90 for OSAS in the secondary care setting. None of the studies was conducted in the UK and none collected generic measures of health utility. The primary outcome was left ventricular ejection fraction in Egea et al.,88 baroreflex sensitivity in Ruttanaumpawan et al. 89 and a number of neurological, quality of life, sleep-related and mortality outcomes in Parra et al. 90 Two studies88,89 reported BP at baseline and at follow-up; however, both these studies focused on patients with chronic heart failure and their follow-up was short, at 3 and 1 months, respectively. In the Egea et al. study,88 no statistically significant differences were found in BP. In the Ruttanaumpawan et al. study,89 the reduction in average systolic BP at 1 month was statistically significant but the reduction in average diastolic BP was not.
The results of these three studies are difficult to generalise to the overall patient population with OSAS and aged 60 years and older, given their focus in patients with concomitant cardiovascular disease. Egea et al. 88 and Ruttanaumpawan et al. 89 included only patients with chronic heart failure and Parra et al. 90 included only patients who had had an ischaemic stroke. These patients are likely to be a smaller proportion of those with OSAS. In PREDICT, 18 (6%) of patients had chronic heart failure and 8 (3%) had had a stroke. If the effect of CPAP was systematically different for these patients, the effect of CPAP observed in the identified studies is not generalisable to the overall patient population. Consequently, these studies were not used to inform the effectiveness estimates in the model, and these were derived solely from the results of PREDICT.
Road traffic accidents
Another potential long-term benefit of CPAP is the reduction in RTAs. RCTs are unlikely to capture reductions in the rate of RTAs given their infrequency. For example, the rate of RTAs in drivers aged 60–69 years was 96 per 100,000. 137 Therefore, reviews of observational studies were examined for evidence in reductions in the rate of RTAs in older OSAS patients. Four systematic reviews were identified. 138–141 All of the studies included in these reviews evaluated the risk of RTAs in patients whose average age was below 60 years. Given the small number of events recorded in the trial and paucity of published evidence in this patient population, RTAs are not included in the model.
Linking intermediate outcomes to final events
Intermediate outcomes such as BP or blood cholesterol can be used to predict the risk of final events using published risk models. There is a large number of risk models published in the literature. The Framingham risk model for cardiovascular disease was selected because it has been extensively validated and was used in a prior evaluation of CPAP. 20,142,143 The Framingham risk model is based on the Framingham cohort, a large prospective cohort of US men and women aged 30 to 74 years and validated in multiple populations. 143 It calculates risk of fatal and non-fatal cardiovascular events for an individual within a certain age range based on smoking status, BP, blood cholesterol, diabetes status and whether or not there is electrocardiographic evidence of left ventricular hypertrophy. The limitation of the Framingham risk model is that it has not been validated for patients aged 75 years and older.
A summary of the treatment effects observed in PREDICT for intermediate outcomes is shown in Table 38. The effects on intermediate outcomes were small, generally not statistically significant and somewhat inconsistent. CPAP appeared to increase BP, which would increase the risk for cardiovascular events, but decreased total cholesterol and increases high-density lipoprotein cholesterol, which in turn would decrease the risk. Given the small size, uncertainty and inconsistency in the direction of effect, these outcomes were be included only as a scenario analysis.
| Outcome | Treatment effect (95% CI) |
|---|---|
| Systolic BP (mmHg) | 3.7 (0.2 to 7.3) |
| Diastolic BP (mmHg) | 0.2 (–2.1 to 2.5) |
| Total cholesterol (mmol/l) | –0.1 (–0.3 to 0.1) |
| HDL cholesterol (mmol/l) | 0.01 (–0.03 to 0.06) |
| LDL cholesterol (mmol/l) | –0.09 (–0.26 to 0.08) |
| Triglycerides (mmol/l) | 0.06 (–0.10 to 0.22) |
Adherence to continuous positive airway pressure therapy
Adherence to CPAP may affect the clinical effectiveness and cost-effectiveness of treatment. PREDICT recorded adherence to CPAP at 3 months and at 1 year from treatment initiation in terms of proportion of patients who reported using CPAP and average hourly usage per night (see Chapter 3, Tertiary outcome; Table 24). Of the 140 patients randomised to CPAP treatment, 120 (86%) at 3 months and 99 (71%) at 12 months reported that they were still using CPAP.
A recent systematic review on adherence to CPAP was used to extrapolate this parameter over time. 144 In a first stage, the studies included in the review were examined for their relevance in this patient population and whether or not data were provided on the proportion of patients using CPAP at one or more time periods from treatment initiation. Three studies evaluated compliance with CPAP use in patients with an average age of over 65 years: Russo-Magno et al. 145 in patients with an average age of 73 years, Bravata et al. 146 in patients with an average age of 66 years and Woehrle et al. 147 presented subgroup analysis in patients aged 60–70 years and patients 70 years of age and older. 145–147 However, none of these three studies reported the proportion of patients using CPAP at specified time points from treatment initiation. In a second stage, the studies included in the systematic review were examined for evidence on adherence at specified time points from treatment initiation. Two studies reported the proportion of patient using CPAP: McArdle et al. 148 examined CPAP use in yearly intervals up to 5 years in 1103 patients aged 43–58 years (average 50 years), Sin et al. 149 examined CPAP use in patients with an average age of 47.1 years at 2 weeks, 4 weeks, 3 months and 6 months. The reduction in adherence over time in McArdle et al. 148 was used to extrapolate the adherence at 1 year observed in PREDICT by assuming that adherence in the PREDICT patient population was reduced by the same proportion as the adherence in McArdle et al.
Final model
A cohort Markov model was developed to evaluate the cost-effectiveness of CPAP compared with BSC alone. The model tracked the health outcomes (health utility values, deaths) and costs over the cohort’s lifetime. In the base case, the model consisted of two health states: OSAS (treated with CPAP or treated with BSC alone) and death (Figure 15). Cycle length was 1 year. CPAP was assumed to improve HRQoL and reduce NHS costs as observed in PREDICT (for more details see Within-trial economic evaluation). Adherence in the first year was obtained from PREDICT and assumed to deteriorate over time, as reported in McArdle et al. 148 The estimated of adherence used in the economic model is presented in Table 39. Adherence in McArdle et al. reduced by 10% in year 2, by 1% in year 3 and by 5% in year 4. 148 Applying these reductions to the adherence at 1 year observed in PREDICT yielded an adherence of 63.9% at year 2, 63.3% at year 3 and 60.1% at year 4. After year 4, adherence was assumed to remain constant.
| Adherence | PREDICT | McArdle et al.148 | Reduction | Decision model |
|---|---|---|---|---|
| 1 year | 71% | 84% | – | 71% |
| 2 years | – | 74% | 84% – 74% = 10% | 71% × 90% = 63.9% |
| 3 years | – | 73% | 74% – 73% = 1% | 63.9% × 99% = 63.3% |
| 4 years | – | 68% | 73% – 68% = 5% | 63.3% × 95% = 60.1% |
In a scenario analysis, the model included four health states (OSAS, OSAS post CHD, OSAS post stroke and death) and two events (CHD and stroke). In this scenario, CPAP not only improved HRQoL and reduced NHS costs but also changed the risk for cardiovascular events. The patient cohort started in the OSAS state. At each 1-year cycle, patients were at risk of CHD and stroke. Following CHD or stroke, patients moved to the state post CHD or post stroke, respectively. The states of post CHD and post stroke had an elevated risk of death. The Framingham risk equation was used to link the effect of CPAP on intermediate outcomes (systolic BP, cholesterol) to cardiovascular events.
FIGURE 15.
Diagram of model structure for the scenario analysis.
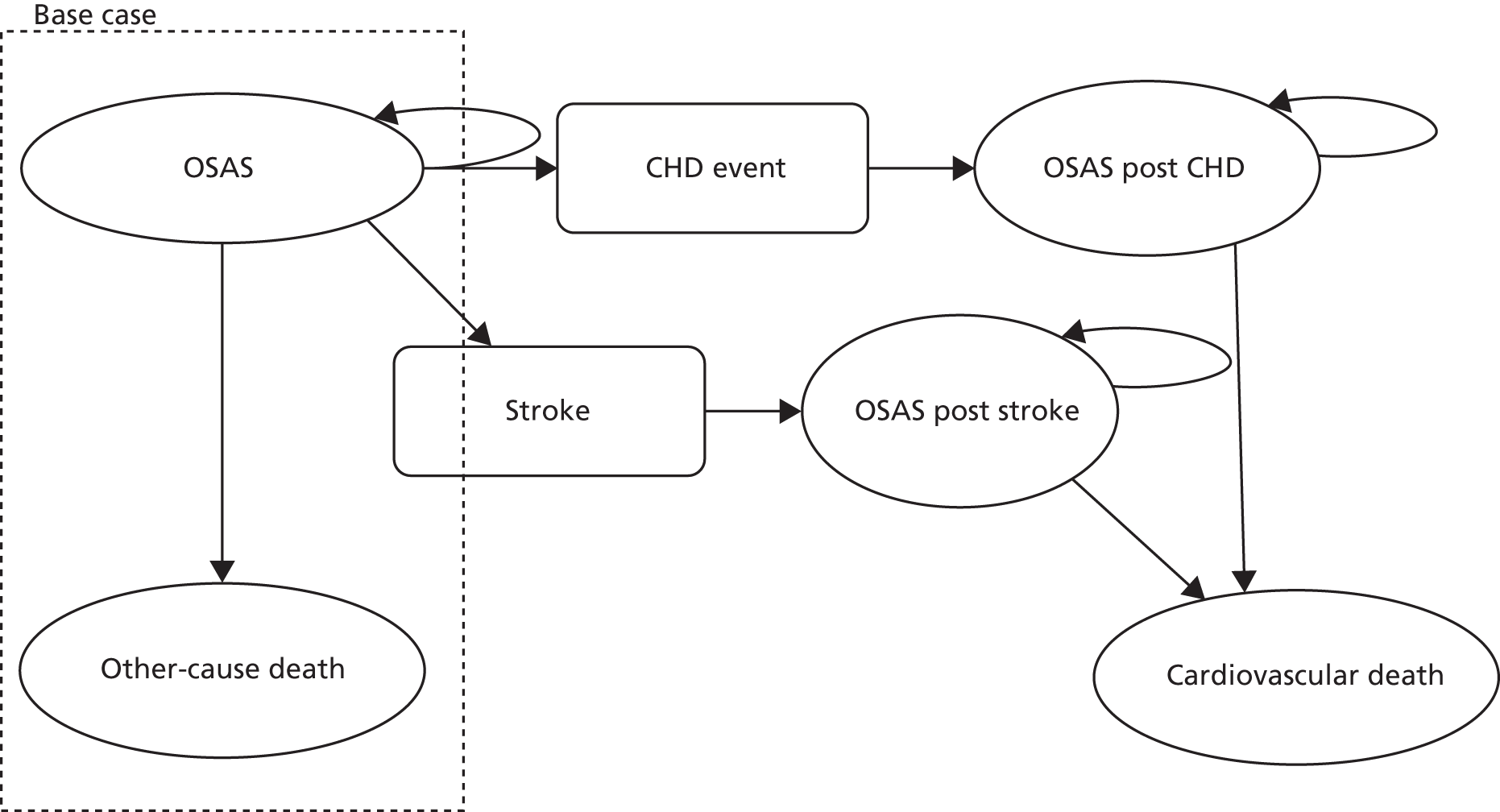
Health-related quality of life and quality-adjusted life-years
Health-related quality of life was expressed in terms of QALYs by quality adjusting the period of time for which the average patient was alive within the model using an appropriate health utility value. Table 40 shows the health utility values used in the model. Health utility in the OSAS state corresponded to the average baseline EQ-5D in the patients that took part in PREDICT. The effect of CPAP during the first year corresponded to the improvement in EQ-5D QALYs observed in the trial at 1 year, adjusted for baseline, as presented in Table 35. The effect in subsequent years was adjusted for adherence assuming that reductions in adherence had a proportional impact on treatment effect. For example, a reduction in compliance of 10% from 31% to 27.9% reduced the effect of CPAP in health utility by 10% from 0.005 to 0.0045. The health utility decrements associated with age, CHD and stroke were obtained from the catalogue of EQ-5D scores developed by Sullivan et al. 150
| Health state or event | Mean | SD or SE | Distribution | Source | ||
|---|---|---|---|---|---|---|
| EQ-5D | SF-6D | EQ-5D | SF-6D | |||
| OSAS untreated | 0.687 | 0.660 | 0.245 | 0.090 | Beta | PREDICT |
| CPAP effect in year 1 | 0.005 | 0.018 | 0.020 | 0.008 | Normal | PREDICT |
| CPAP effect in year 2 | 0.0045 | 0.0162 | 0.020a | 0.008a | Normal | Calculated (effect × 90%) |
| CPAP effect in year 3 | 0.0045 | 0.0160 | 0.020a | 0.008a | Normal | Calculated (effect × 89%) |
| CPAP effect in year 4 | 0.0042 | 0.0152 | 0.020a | 0.008a | Normal | Calculated (effect × 85%) |
| Age decrement | 0.0003 | NA | 0.0002 | NA | Normal | Sullivan et al.150 |
| For the scenario with cardiovascular events | ||||||
| Stroke decrement | 0.1009 | 0.0123 | Normal | Acute cerebrovascular disease150 | ||
| CHD decrement | 0.0557 | 0.0112 | Normal | Acute MI150 | ||
Cardiovascular events
In the scenario including cardiovascular events, the Framingham risk equations were employed to estimate the risk of events from the intermediate outcomes recorded in PREDICT. 20,143 The characteristics of the patient cohort for the scenario with cardiovascular events are presented in Table 41. These correspond to the average patient characteristics at baseline in PREDICT. Table 42 presents the probability of a cardiovascular event based on the characteristics of the cohort at baseline. The probability was calculated for subgroups of patients by sex, smoking status and diabetes using only those patients with complete data for these variables at baseline. The risk for the PREDICT patient population was a weighted average of the risks for each subgroup, weighted by their relative proportions in the population.
| Variables | Mean | SD |
|---|---|---|
| Age (years) | 70.59 | 4.66 |
| Systolic BP (mmHg) | 139.01 | 18.92 |
| Total cholesterol, mmol/l (mg/dl) | 4.58 (177.02) | 1.04 (40.35) |
| HDL cholesterol, mmol/l (mg/dl) | 1.24 (31.00) | 0.34 (13.11) |
| Sex | Male | Female | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Smoking status | Yes | Yes | No | No | Yes | Yes | No | No | – |
| Diabetes | Yes | No | No | Yes | Yes | No | No | Yes | – |
| Number of patients | 5 | 8 | 102 | 56 | 0 | 0 | 15 | 5 | – |
| 1-year probability of cardiovascular events | |||||||||
| CHD | 0.053 | 0.041 | 0.027 | 0.036 | NA | NA | 0.012 | 0.024 | 0.030 |
| Stroke | 0.010 | 0.006 | 0.003 | 0.005 | NA | NA | 0.002 | 0.006 | 0.004 |
| Death from CHD | 0.013 | 0.011 | 0.006 | 0.007 | NA | NA | 0.001 | 0.007 | 0.006 |
| Death from cardiovascular disease | 0.014 | 0.011 | 0.006 | 0.008 | NA | NA | 0.003 | 0.008 | 0.007 |
All-cause death
In the model, patients are at risk of death at every cycle. Patients in the OSAS state experience an age-dependent risk of death, obtained from interim lifetables for England and Wales for the years 2009–11. 151 For the scenario including cardiovascular events, the age-dependent risk of death from causes other than cardiovascular was estimated using a cause elimination approach. Patients in the post-stroke or post-CHD OSAS group (who survived the cardiovascular event) were then at an elevated risk of death as in the McDaid et al. analysis, with a relative risk of 3.2 (95% CI 2.67 to 3.83)152 for the post-CHD state and a relative risk of 2.3 (95% CI 2.0 to 2.7) for the post-stroke state. 153
Resource use and costs
Resource use and costs can be split into two components: (1) those related to CPAP therapy and (2) those related to cardiovascular events, only applicable in the scenario including cardiovascular events. The within-trial analysis estimated that patients allocated to CPAP accrued on average £35 (95% CI –£390 to £321) lower costs (Table 43). This cost difference included the costs of CPAP and the costs associated with any health-care resource use. Therefore, this difference in costs was applied to the hypothetical patient cohort in the OSAS state. As with the improvement in health utility, the cost difference was adjusted for reduced adherence over time. For the scenario including cardiovascular events, costs associated with cardiovascular events were obtained from the McDaid et al. report and inflated to 2011–12 prices. 20,124,154
| Parameter | Cost | Distribution | Source | |
|---|---|---|---|---|
| Mean | SE | |||
| Costs related to CPAP | ||||
| Costs in the OSAS state untreated | £1389 | £139 | Gamma | PREDICT, within-trial analysis (see Table 10) |
| Effect of CPAP on costs | –£35 | £180 | Normal | PREDICT, within-trial analysis (see Table 10) |
| Costs related to cardiovascular events | ||||
| Cost of fatal CHD event | ||||
| Price year 2004–05 | 3021 | 367 | Normal | McDaid et al.,20 Briggs et al.154 |
| Inflated to 2011–12 | 3716 | 451 | ||
| Cost of non-fatal CHD event | ||||
| Price year 2004–05 | 9997 | 429 | Normal | McDaid et al.,20 Briggs et al.154 |
| Inflated to 2011–12 | 12,296 | 528 | ||
| Ongoing cost of CHD | ||||
| Price year 2004–05 | 751 | 117 | Normal | McDaid et al.,20 Briggs et al.154 |
| Inflated to 2011–12 | 924 | 144 | ||
| Inflation index from 2004–05 to 2011–12 | 285.7/232.3 = 1.23 | NA | NA | PSSRU124 |
| Acute cost of stroke (year 1) | ||||
| Price year 2004–05 | 9067 | 294 | Normal | McDaid et al.,20 Vergel et al.155 |
| Inflated to 2011–12 | 11,152 | 362 | ||
| Ongoing cost of stroke (year 2 and beyond) | ||||
| Price year 2004–05 | 2392 | 282 | Normal | McDaid et al.,20 Vergel et al.155 |
| Inflated to 2011–12 | 2942 | 347 | ||
Patient population
The model followed a hypothetical patient cohort that corresponded to the patients in PREDICT, that is patients aged 65 years and over with OSAS (see Table 41 for the patients’ characteristics at baseline).
Sensitivity analyses
The sensitivity analyses conducted in the model-based analysis are summarised in Table 44. All analyses were fully probabilistic. The sensitivity analyses aimed to explore the robustness of the results to the main assumptions and parameter inputs. Therefore, scenario analyses were performed for the effect of CPAP on cardiovascular risk outcomes and the cost of CPAP therapy. Univariate sensitivity analysis was conducted to the cost of the CPAP machine.
| Scenario | Element | Position in base-case analysis | Variation in the sensitivity analysis |
|---|---|---|---|
| 1 | CPAP used for 1 year | The costs of the CPAP machine and the humidifier are annuitised over 7 years. Yearly replacement for masks. Filters replaced every 6 months | CPAP is assumed to be used for 1 year and discarded after that; therefore, the cost of the machine is not annuitised. CPAP therapy costs £710.16 per patient |
| 2 | Cardiovascular effects with CPAP direct effects | Changes in the risk of cardiovascular events are not included in the base-case model | CPAP changes the risk of cardiovascular events as predicted by the Framingham risk equation through its effect on BP and cholesterol as observed in the PREDICT clinical trial. CPAP increases health outcomes and reduces costs are reduced in PREDICT |
| 3 | Cardiovascular effects only | Changes in the risk of cardiovascular events are not included in the base-case model | CPAP changes the risk of cardiovascular events as predicted by the Framingham risk equation through its effect on BP and cholesterol as observed in the PREDICT clinical trial. The direct effect of CPAP on costs and QALYs is not considered |
Results
Base case
The results for the base case are presented in Table 45. CPAP reduced the average costs per patient by –£369 and improved health outcomes by 0.051 EQ-5D QALYs. The improvement for SF-6D QALYs was 0.182. Since CPAP reduced costs and improved health outcomes, it dominated BSC alone and an ICER is not calculated.
| Treatment | Average costs | Average EQ-5D QALYs | Average SF-6D QALYs |
|---|---|---|---|
| CPAP | £15,887 | 8.046 | 7.862 |
| BSC | £16,216 | 7.994 | 7.680 |
| Incremental costs and QALYs | |||
| CPAP with BSC – BSC alone | –£329 | 0.051 | 0.182 |
The distribution of average costs and average QALYs over the 10,000 simulations conducted for the probabilistic sensitivity analysis are shown in Figure 16. Similarly to the within-trial analysis, there was considerable uncertainty around the results using EQ-5D QALYs. In the analysis with SF-6D QALYs, most simulations were located in the eastern quadrants; the large majority were below the cost-effectiveness threshold, represented by the diagonal line.
FIGURE 16.
Cost-effectiveness plane for the model-based base-case analysis. (a) EQ-5D; and (b) SF-6D.


The probability that CPAP was cost-effective over a range of cost-effectiveness thresholds is shown in Figure 17. The uncertainty around the results observed in the cost-effectiveness planes above (see Figure 16) is reflected in the probability that the CPAP was cost-effective. In the EQ-5D analysis, the scatter of the simulations translated into a curve plateauing at 0.6. The SF-6D analysis indicated a greater probability that the CPAP was cost-effective across the range of thresholds. The probability that CPAP was cost-effective at the conventional thresholds used by NICE of £20,000 and £30,000 per QALY gained was 0.62 for EQ-5D QALYs and 0.95 and 0.97, respectively, for SF-6F QALYs. These results were consistent with those of the within-trial analysis.
FIGURE 17.
Cost-effectiveness acceptability curves for model-based base-case analysis.

Subgroup analyses
The cost-effectiveness results for the subgroup populations defined according to ESS score at baseline are shown in Table 46. In the less severe OSAS subgroup (ESS score of < 13 at baseline), the use of CPAP treatment reduced overall costs by £201. The effect on QALYs was dependent on the measure used: EQ-5D QALYs were reduced by 0.169 but SF-6D QALYs were increased by 0.181. Therefore, the ICER for the less severe OSAS subgroup using EQ-5D QALYs was £1189 per QALY gained. CPAP dominated in the SF-6D QALYs scenario. In the more severe OSAS subgroup, the use of CPAP treatment increased overall costs by £176 and both EQ-5D and SF-6D QALYs were increased. The ICERs were £360 per EQ-5D QALY gained and £967 per SF-6D QALY gained. These results were similar but in greater order of magnitude to the results of the within-trial analysis.
| Treatment | Average costs | Average EQ-5D QALYs | Average SF-6D QALYs |
|---|---|---|---|
| Subgroup ESS score of < 13 | |||
| CPAP | £16,019 | 7.823 | 7.861 |
| BSC | £16,221 | 7.992 | 7.679 |
| Incremental costs and QALYs | |||
| CPAP with BSC – BSC alone | –£201 | –0.169 | 0.181 |
| Subgroup ESS score of ≥ 13 | |||
| CPAP | £16,396 | 8.483 | 7.860 |
| BSC | £16,216 | 7.994 | 7.678 |
| Incremental costs and QALYs | |||
| CPAP with BSC – BSC alone | £176 | 0.489 | 0.182 |
The cost-effectiveness planes for both population subgroups and by instrument used for obtaining QALYs, EQ-5D or SF-6D are shown in Figure 18. The same scale was used across the four plots to facilitate comparisons. In analysis of the less severe OSAS subgroup (ESS score of < 13 at baseline), using EQ-5D, there was considerable uncertainty on how CPAP affected costs and QALYs. In the analysis using SF-6D, some uncertainty around the effect on costs remained, but CPAP appeared to improve health outcomes across most of the simulations. In the more severe OSAS subgroup (ESS score of ≥ 13 at baseline), there was some degree of certainty that CPAP improved health outcomes, in terms of both EQ-5D and SF-6D. The uncertainty around the impact on costs remained.
FIGURE 18.
Cost-effectiveness plane for subgroup populations. (a) ESS score of < 13 EQ-5D; (b) ESS score of < 13 SF-6D; (c) ESS score of ≥ 13 EQ-5D; and (d) ESS score of ≥ 13 SF-6D.
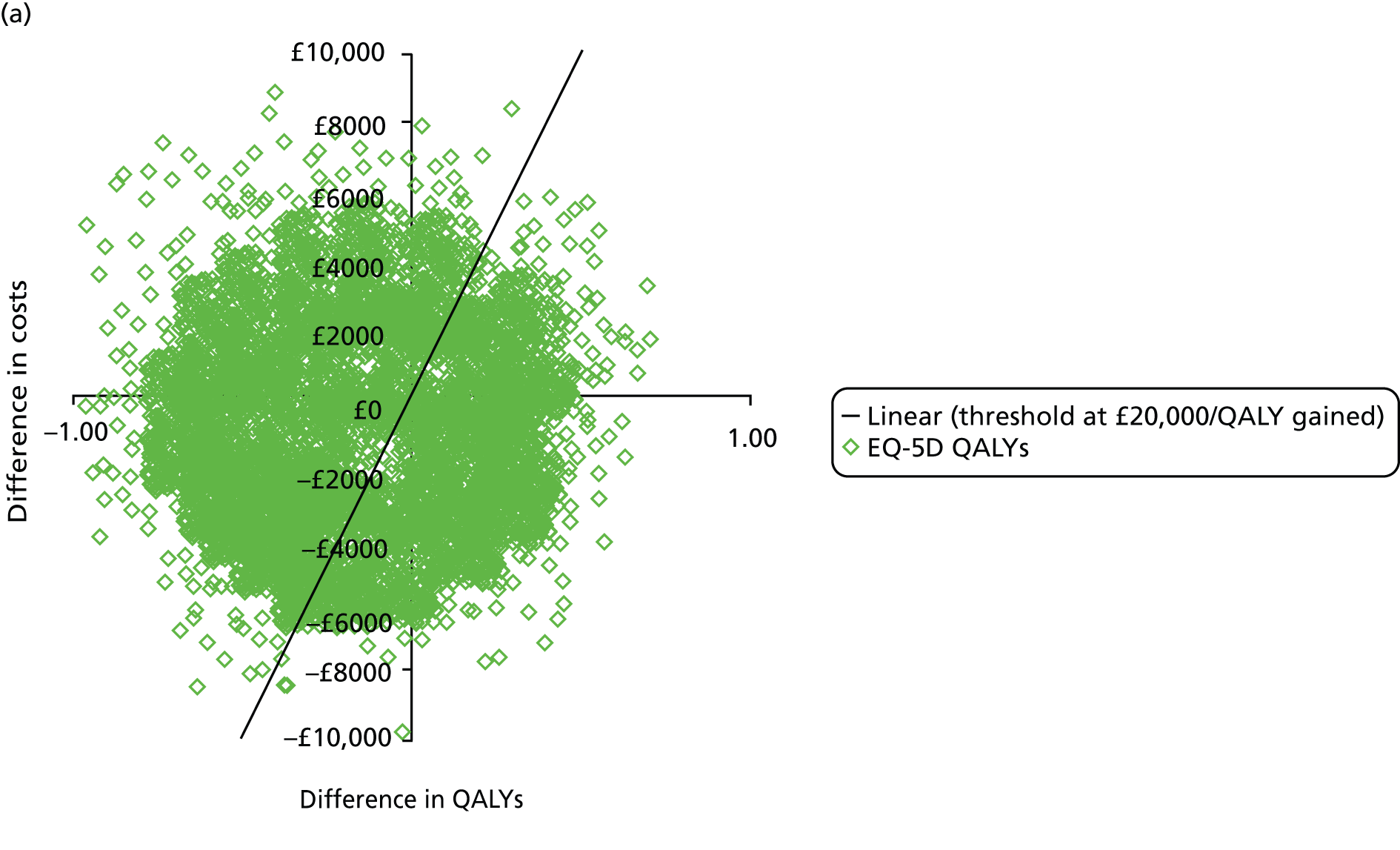
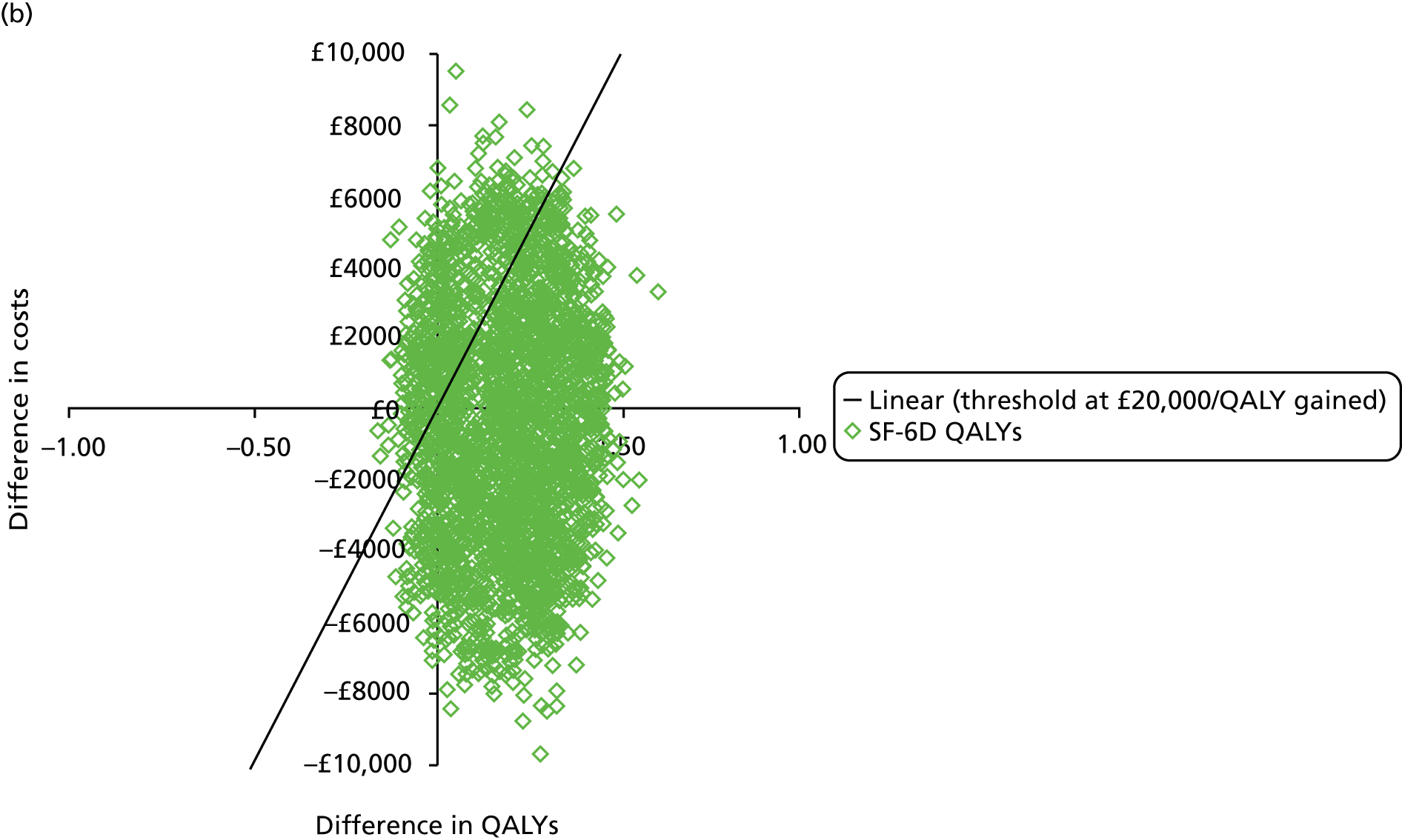
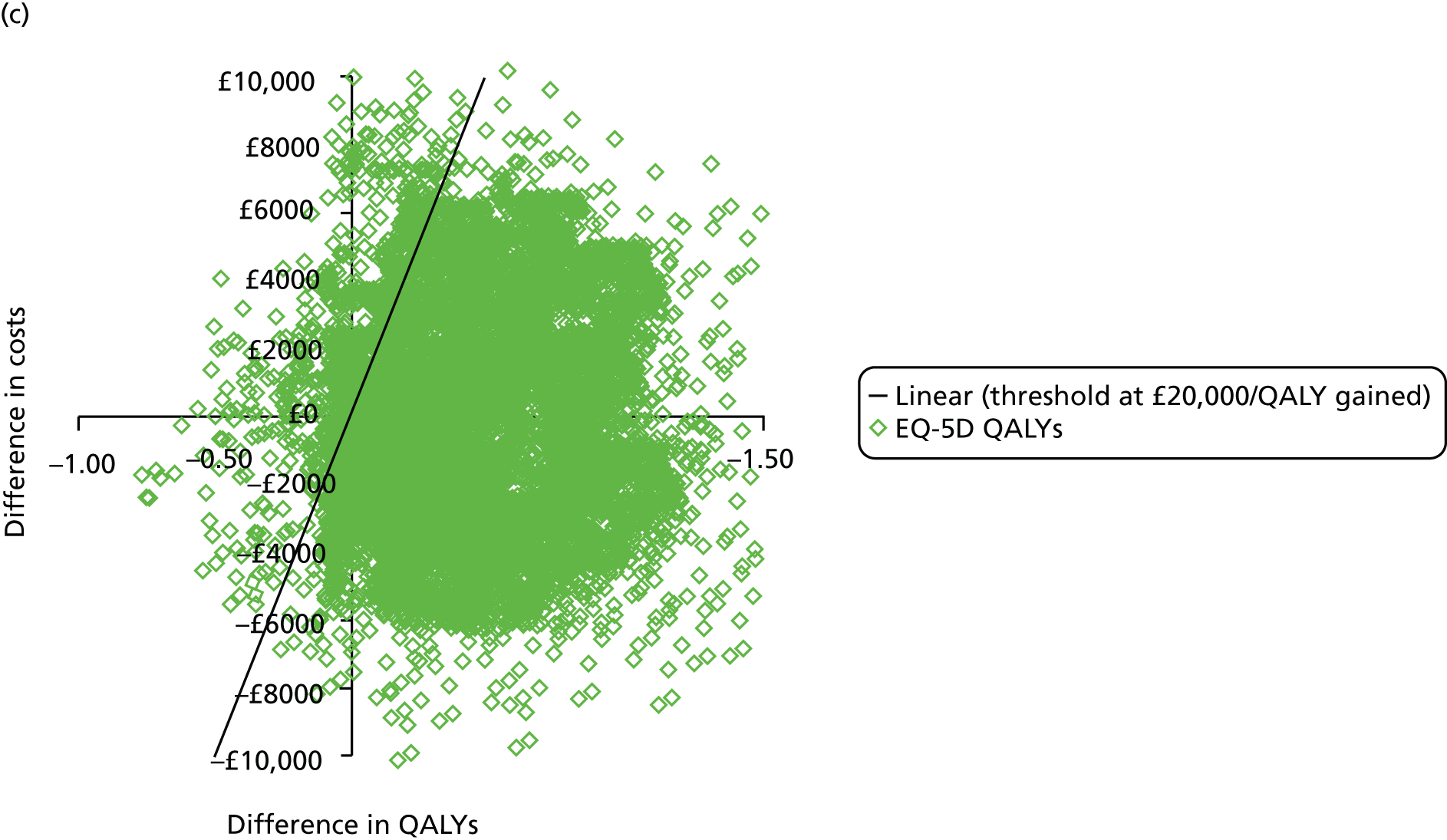

The cost-effectiveness acceptability curve over a range of cost-effectiveness thresholds for both subgroups is shown in Figure 19. The curves were similar to the within-trial analysis. In the less severe OSAS subgroup, the probability that CPAP was cost-effective at £20,000 per QALY gained was 0.28 for the analysis with EQ-5D QALYs and 0.89 for the analysis with SF-6D QALYs. In the more severe OSAS subgroup, the probability that CPAP was cost-effective was 0.91 for the analysis with EQ-5D QALYs and 0.83 for the analysis with SF-6D QALYs.
FIGURE 19.
Cost-effectiveness acceptability curve for the subgroup (a) ESS score of < 13; and (b) ESS score of ≥ 13.


Sensitivity analysis
The results of the scenario analysis are shown in Table 47. See Appendix 3 for more detailed results including average costs and QALYs for each treatment, cost-effectiveness planes and cost-effectiveness acceptability curves. In scenario 1, the costs of CPAP treatment were increased to £710.16 because of more frequent replacement of the machine and consumables. Therefore, the difference in costs in the first year increased from –£35 to £474 (see Table 37). This cost difference was extrapolated over the patients’ lifetime in the model to £4785 (from –£329) in the base case. The probability that CPAP was cost-effective reduced from 0.62 to 0.20 when using EQ-5D QALYs and from 0.95 to 0.31 when using SF-6D QALYs. This was consistent with the findings of the within-trial analysis.
| Scenario | Difference in average costs | Difference in average EQ-5D QALYs | ICER/EQ-5D QALYs | Difference in average SF-6D QALYs | ICER/SF-6D QALYs | Probability that CPAP is cost-effective at £20,000/QALY gained | |
|---|---|---|---|---|---|---|---|
| EQ-5D QALYs | SF-6D QALYs | ||||||
| Base case | –£329 | 0.051 | D | 0.182 | D | 0.62 | 0.95 |
| 1. CPAP used for 1 year ( = £710.16) | £4785 | 0.051 | £94,404 | 0.182 | £26,599 | 0.20 | 0.31 |
| 2. Cardiovascular effects with effect of CPAP on costs and QALYs | –£327 | 0.022 | D | 0.139 | D | 0.58a | 0.92a |
| 3. Cardiovascular effects only | –£10 | –0.024 | £401 | –0.023 | £427 | 0.27a | 0.28a |
Scenario 2 included the effect of CPAP on cardiovascular risk predicted by the Framingham risk equations. The impact of including cardiovascular outcomes was very small. The difference in QALYs was reduced from 0.051 to 0.022 when using EQ-5D QALYs and from 0.182 to 0.139 when using SF-6D QALYs.
In scenario 3, only cardiovascular effects were considered; the cost and QALY difference observed at 12 months in PREDICT were not included. Over the patients’ lifetime, the differences in costs and QALYs were very small and uncertain (–£10; EQ-5D QALYs, –0.024; SF-6D QALYs, –0.023). This reflects the small effect of CPAP in diastolic BP and cholesterol observed in the trial.
The impact of the cost of the CPAP machine on the EQ-5D ICERs and in the probability that CPAP is cost-effective is shown in Figure 20. Note that the cost of the CPAP machine was annuitised over 7 years using a discount rate of 3.5%. Therefore, if the cost of the CPAP machine was doubled from £430 to £860, the annuitised cost of the machine increased from £70.32 to £140.65. This cost should be added to the annuitised cost of the humidifier (£26.98 multiplied by the proportion of people who received humidifier, 0.59 = £15.81) and the cost of consumables (£115.16) to give a final therapy cost of £271.62. In this analysis, the ICER for CPAP was above £20,000 per QALY gained and the probability that CPAP was cost-effective was below 0.50 for a machine cost of £1290 (three times the base-case cost of £430). These results indicated that a key driver of cost-effectiveness was whether or not patients returned the machine rather than the cost of the machine itself.
FIGURE 20.
Impact of the unit cost of the CPAP machine on the probability that the CPAP therapy is cost-effective.
Discussion of the assessment of the cost-effectiveness
Continuous positive airway pressure treatment appeared to be a cost-effective alternative to BSC alone. CPAP decreased costs by a small amount and improved health outcomes. However, the differences in costs and health outcomes between the treatment groups were small and uncertain. The subgroup analysis by level of sleepiness suggested that CPAP was more likely to be cost-effective in the more severe OSAS subgroup of patients. These results were consistent across the within-trial and model-based analyses.
The key drivers of cost-effectiveness were the costs of CPAP treatment and the benefits of CPAP on HRQoL. Some patients may fail to return the machine following treatment discontinuation. In these patients, the CPAP machine is a sunk cost, as it does not benefit the patient and cannot be reissued to another patient. If CPAP machines are not fully utilised over the lifetime of the machine, on average, the cost per patient of CPAP treatment increases. In the extreme scenario that all patients discontinued treatment after 1 year and did not return the machine, the probability that CPAP was cost-effective at £20,000 per QALY gained was 0.20 for EQ-5D QALYs and 0.30 for SF-6D QALYS. The implication for clinical practice is that patients should be encouraged to return the CPAP machine if they cease to use it.
The benefits in HRQoL were small when measured by either EQ-5D or SF-6D, but the SF-6D results may seem more pronounced as the difference was statistically significant. The smaller amount of variability with the SF-6D compared with the EQ-5D may be related to the data collection process and/or the instrument itself. The EQ-5D was collected every month through a sleep diary, which was filled in by the patient at home and sent by post. The SF-36, from which SF-6D is derived, was collected less often during a clinic visit at baseline and at 3 months and 12 months. This may have influenced the reporting of HRQoL by patients, although this would have been the case for both the CPAP and the BSC groups. In addition, the EQ-5D and SF-6D questionnaires have some differences. The SF-6D uses 11 questions from the SF-36 health status measure divided over six health domains: pain (six levels), mental health (five levels), physical functioning (six levels), social functioning (five levels), role limitations (four levels) and vitality (five levels). 121 The EQ-5D comprises five questions, with three levels each, on mobility, self-care, usual activities, pain, and anxiety and depression. 156 The dimensions in SF-6D, particularly vitality, may render the SF-6D more sensitive to changes in sleepiness and sleep quality. PREDICT is the first trial to collect SF-6D and EQ-5D following treatment of OSAS; however, some studies have compared SF-36 with EQ-5D and found that SF-36 was more sensitive to the impact of CPAP on HRQoL. 153,155
The impact of including cardiovascular outcomes on the cost-effectiveness of CPAP appeared to be negligible. This reflected the small difference in BP and cholesterol between patient groups observed in PREDICT. The increase in systolic BP would increase the risk of cardiovascular outcomes whereas the change in cholesterol would decrease it. Overall, and on balance, the change in these intermediate outcomes resulted in a small decrease in costs and QALYs. A limitation of this assessment was the incorporation of the impact of CPAP on cardiovascular outcomes. The Framingham risk equations have not been validated in a population older than 74 years of age. Therefore, the risk of cardiovascular outcomes may not have been correctly estimated. The direction of the bias is unclear and would have depended on whether the risk was under- or overestimated and on the effect of BP versus cholesterol on overall cardiovascular risk. CPAP would have been favoured if the combined effect of CPAP on BP and cholesterol decreased overall cardiovascular risk and the risk was overestimated or if the combined effect increased overall risk and risk was underestimated. Nonetheless, the impact of this bias was likely to be small, given the small change in costs and QALYs observed for the scenario with cardiovascular effects.
The cost-effectiveness analysis entailed three components to ensure appropriate consideration of all the relevant evidence: a systematic review of previous economic evaluations, a within-trial analysis using individual patient data collected in PREDICT and a model-based analysis incorporating the data collected in PREDICT with relevant external evidence. A systematic review on the clinical effectiveness of CPAP in older people confirmed that PREDICT was the sole source of evidence in this patient population. As it is considered that the CPAP treatment effects recorded in younger patients were not generalisable to older patients, this meant that PREDICT was the sole source of evidence of the treatment effects of CPAP included in the model-based analysis. Uncertainty around the cost-effectiveness results was quantified with a range of scenarios and sensitivity analyses in the within-trial and model-based analyses.
Areas of uncertainty included whether or not the savings in health-care costs are sustained over time and the differences between EQ-5D and SF-6D QALYs. The savings in health-care costs are small and uncertain; however, on average, these were enough to offset the cost of CPAP treatment. The savings may reflect the effect of CPAP on health-care resource use. Patients on CPAP may experience fewer adverse effects caused by their OSAS than patients on BSC alone and use the NHS less often as a result. Alternatively, the average saving per patient may be as result of chance and an artefact of the trial being underpowered to detect differences in costs.
The differences in the improvements in QALYs observed with EQ-5D and SF-6D were another area of uncertainty and a key driver of cost-effectiveness. As discussed, these differences may be related to the failure of the questionnaires in capturing the impact of sleepiness on quality of life or to the differences in the frequency and setting of the administration. Future research should explore the differences between EQ-5D and SF-6D as well as how different methods to collect HRQoL data impact on the results.
Chapter 5 Discussion
Main findings
Positive Airway Pressure in Older People: a randomised controlled trial was designed to assess the clinical efficacy of CPAP in older people with OSAS at 3 months and its cost-effectiveness over 12 months. CPAP improved sleepiness after 3 months by 2.1 points on the ESS compared with BSC. The beneficial effects were maintained at 12 months, and the magnitude of the improvements was similar to those seen in middle-aged patients with equivalent disease severity. 20 This subjective improvement in sleepiness was corroborated by the improvement in objective sleepiness at 3 months.
Continuous positive airway pressure also improved quality of life, both generic and disease-specific. CPAP-related improvement was statistically significant for the QALYs calculated with the SF-6D but not with the EQ-5D, equating to 1 week and 2 days, respectively. The CPAP group also accrued marginally lower health-care costs than BSC alone over 12 months. Overall, the economic benefit of CPAP was linked to the reduced health-care usage, offsetting the cost of the equipment, making it a cost-effective alternative to BSC for the treatment of OSAS in older people. The discrepancy between the two QALY measures could be a result of the EQ-5D being a less sensitive measure of the changes in health status attributed to sleepiness than the SF-36 (from which the SF-6D is derived). 12
Additional findings
Secondary outcomes related to cognitive function did not show any difference between the two groups despite reductions in sleepiness in the CPAP group. However, the baseline cognitive scores were often within the age-adjusted normative range, which may have resulted in a ceiling effect. Cognitive dysfunction is well recognised in middle-aged OSAS patients14,157 and is potentially linked to changes in brain morphology. 158 However, the impact of OSAS on cognitive function, separate from its effects on sleepiness and vigilance, is debated. 159,160 In older people with OSAS, the benefits of CPAP could be reduced because the capacity for neuronal recovery is less, owing to a combination of neurodegeneration associated with ageing and the life-long effects of OSAS. 57
The cardiovascular risk factors showed a small reduction in total cholesterol at 3 months, which was driven by a reduction in the LDL component. These findings are similar to those in a more severe and sleepier OSAS population, following 1 month of treatment with CPAP. 161 CPAP resulted in no improvement in BP. In the BSC group there was a small improvement in the systolic BP at 12 months. This finding echoes the results of a recent RCT of cardiovascular risk in mild asymptomatic patients162 in which CPAP usage, more specifically low usage, seemed to slightly raise BP. We speculate this could be a result of the BSC group following the BSC advice more closely.
Other secondary outcomes which showed no statistically significant difference between the two groups at 3 and 12 months were mood, frequency of nocturia and accidents. Interestingly, the patients in this trial had a relatively low prevalence of depression compared with a recent study. 8 We speculate that the expected lack of improvement in nocturia with CPAP may have been because of the multifactorial nature of this symptom in older people. 163
Comparison with other trials
A review of the clinical effectiveness of CPAP therapy in older people revealed three RCTs (from a possible 3560 titles) assessing the efficacy of CPAP treatment in OSAS patients with an average age of 60 years or older and the capacity to give informed consent. These studies included patients with cardiovascular conditions and compared CPAP therapy with sham CPAP88 or no CPAP. 89,90 None of the studies assessed daytime sleepiness or collected generic measures of health-care usage and they were not conducted in a secondary-care setting. The primary outcomes were left ventricular ejection fraction,88 baroreflex sensitivity,89 a number of neurological, quality-of-life and sleep-related effects and mortality. 90 Two studies reported BP at baseline and at follow-up;88,89 however, both of these studies focused on patients with chronic heart failure and their follow-up was short, at 3 months and 1 month, respectively. In the Egea et al. 88 study, no statistically significant differences were found in BP. In the Ruttanaumpawan et al. 89 study, the reduction in average systolic BP at 1 month was statistically significant but not the reduction in average diastolic BP. Overall, the results of these three studies are difficult to generalise to PREDICT, given their focus in patients with concomitant cardiovascular disease. Egea et al. 88 and Ruttanaumpawan et al. 89 included only patients with chronic heart failure and Parra et al. 90 included only patients who had had an ischaemic stroke.
Treatment adherence
The CPAP adherence was low at 3 and 12 months, which is likely to have diluted any treatment effect between the groups. 164 Indeed, exploratory analyses revealed that the treatment effect was larger in patients with more frequent CPAP use. The mean CPAP usage and the percentage of patients using CPAP at 12 months were similar to another UK RCT, albeit one of a shorter duration in patients with minimally symptomatic OSA. 162
The CPAP machines used in PREDICT were autoadjusting and, based on previous studies, it is unlikely that the autoadjusting machines were the cause of less frequent CPAP use. 165–167 On the other hand, we adopted a clinical approach to initiating and managing CPAP treatment, which may have resulted in a less frequent CPAP use, compared with a more intensive trial protocol. 15 However, with the approach that was adopted in this study, we have ensured that the PREDICT outcomes reflect clinical practice in the UK, which in turn has strengthened the validity and applicability of the health economic assessment. An additional factor that may have contributed to the low frequency of CPAP use in older people is reduced social support. We do not know how many patients were married, a factor that has been reported to be associated with increased CPAP compliance;168 however, just over half the patients slept alone.
Strengths and weaknesses
Positive Airway Pressure in Older People: a randomised controlled trial was designed as a pragmatic trial, recruiting older OSAS patients with comorbidity from geographically diverse areas throughout the UK. The findings are, therefore, relevant to what would be seen in clinical practice. Additionally, one of the unique elements of the trial design was the simultaneous cost-effectiveness evaluation, as well as the analysis of clinical effectiveness, measured over a relatively long time period. Finally, the high follow-up rates of patients attending at 3 and 12 months (over 80%) was impressive considering the duration of the trial.
A possible limitation of the trial was that sham CPAP was not used; therefore, as it was a physical device trial, the treatment allocation for the individual patients could not be concealed. The treatment allocation was concealed as far as possible from the member of the research team completing follow-up assessments. However, we reasoned that any placebo effect there might have been in the CPAP group would be expected to have disappeared by 12 months and that patients using CPAP might have expected an improvement but they would not have known by how much. Moreover, the results of the OSLER test and the observation of a therapeutic dose–response relationship between the treatment effect and CPAP use support a real effect.
Generalisability
With respect to generalisability, PREDICT did not focus on asymptomatic older people with OSA, and, although it could be argued that the patients studied had a relatively low mean ESS score at baseline, they were sufficiently symptomatic to seek treatment. At the other end of the disease spectrum, the exclusion of highly symptomatic OSAS patients in whom CPAP was considered mandatory is likely to have diminished the effect size. Patients with a higher baseline ESS score had a greater treatment effect in the exploratory analysis. Equally, the marginal improvement in cost-effectiveness was greater in the more symptomatic patients.
Continuous positive airway pressure prescribed for the symptom of excessive sleepiness due to OSAS in older people is more effective than BSC alone and no more expensive than BSC. The beneficial treatment effect is greater in patients with a higher ESS score prior to treatment and additionally in those the patients who used the CPAP treatment more.
Implications for health care
Clinical guidelines play an important role in improving health care for people with long-term conditions; however, it is well recognised they often fail to address the effects of comorbidity and polypharmacy. 169 There is also an inequality of research in older people with OSAS170 and PREDICT addresses this. The high-quality data from this trial will add to the knowledge of age-related changes, improve the generalisability of research findings and help inform best practice in the clinical management of a population that is growing older. The results of this study clearly support the use of CPAP for the treatment of OSAS in older people.
Implication for future research
Adherence to treatment is a recognised concern, particularly in multimorbid patients; despite this, few studies have investigated how adherence could be promoted. Suggested research priorities for future research are:
-
To focus on the optimisation of CPAP delivery, especially in older patients. Can changes in health-care delivery improve adherence? Stratifying older patients with OSAS according to comorbidities and social factors to assess the clinical effectiveness and cost-effectiveness of CPAP treatment could further inform the delivery of care. Given uncertainty surrounding use of EQ-5D further work could be undertaken to assess quality-of-life measures this group of patients.
-
To define patient-centred outcomes for treatment of OSAS in women and in ethnic groups, both of whom are currently under-represented in clinical trials.
-
To identify potential biomarkers sleepiness and cognitive function that would enable early detection, which could be used in studied to inform when in the disease cycle treatment is needed to avert central nervous system sequalae.
-
To explore the hypothesis that OSA in different groups may have different causes anatomically and physiologically, with different consequences.
This last point remains to be explored and may be fundamental to the understanding and targeting of treatment of the disorder.
Conclusion
-
PREDICT has been the longest and most comprehensive controlled treatment trial in older OSAS patients to date, assessing both the therapeutic and economic impact of CPAP treatment.
-
PREDICT has addressed the lack of research in older people with OSAS.
-
The results of PREDICT clearly show that CPAP reduces symptoms of excessive daytime sleepiness in older patients with OSAS, as it does in middle-aged populations, and that these clinical benefits are associated with reduced health-care utilisation.
Acknowledgements
The PREDICT team would also like to thank all the patients and carers who gave their time to be part of this trial. ResMed (UK) Ltd for their donation of the CPAP machines and loan of sleep diagnostic equipment. Members of PREDICT IDMC: Professor Tim Peto (Chairperson), Professor John Gibson and Professor David Wright. The TSC: Professor Walter McNicholas (Chairperson), Professor Sir Neil Douglas and Dr Ian Smith (independent members), Daniel J Bratton, Professor Robert J Davies, Dr Mark Elliot, Mr Frank Govan, Dr Melissa Hack, Magda Laskawiec-Szkonter, Dr Alison McMillan, Professor Mary J Morrell, Professor Andrew J Nunn, Dr Justin Pepperell, Dr Renata L Riha, Professor Mark Sculpher, Professor Anita Simonds, Professor John Stradling and Dr John Starr. Trial management: Magda Laskawiec-Szkonter. Data entry: Assunta Sabia and Jack Quaddy and referring clinicians in every area for supporting PREDICT.
PREDICT investigators
Principal investigators
Dr Martin Allen, Dr Dev Banerjee, Dr Chris Davies, Dr Lee Dowson, Dr Mark Elliott, Dr Melissa Hack, Dr Alison McMillan, Dr John O’Reilly, Dr Mohammed Paracha, Dr Justin Pepperell, Dr Renata Riha, Professor Anita Simonds, Dr Andrew Stanton, Professor John Stradling and Dr Sophie West.
Research staff
Birmingham
Kerryanne James, Sarah Manney and Matthew Nicholls.
Blackpool
Jules Chadwick, Kate O’Reilly, Judith Saba and Gemma Swarbrick.
Edinburgh
Lizzie Hill, Donna Fairley and Marjorie Vennelle.
Leeds
Craig Armstrong, Clair Favager and Sue Watts.
Liverpool
Stephen Emegbo and Pam Parry.
London
Dr Martin Glasser, Lydia Paniccia, Luxumi Sridharan and Dr Neil Ward.
Newcastle
Peter Close, Lyndsay Rostron and Therese Small.
Newport
Clare Acreman, Sarah Mitchell and Jeanette Richards.
Oxford
Isabel Chabata, Nicky Crosthwaite, Tara Harris, Debby Nicoll and Barbara Winter.
Reading
Jacqui Webb.
Stoke on Trent
Andrew Bain, Nathalie Bryan and Ann Cooper.
Swindon
Sam Backway and Sue Meakin.
Taunton
Dawn Redwood and Tania Wainwright.
Wolverhampton
Jillian Andrews, Lucy Reynolds and Louise Spragg.
Contribution of authors
Alison McMillan drafted the first and subsequent versions of this report, with supervision by Mary J Morrell and the other authors, who reviewed and approved the final submitted report.
Mary J Morrell and Renata L Riha were co-chief investigators for the trial, and designed the trial with Robert J Davies.
Andrew J Nunn and Daniel J Bratton undertook the statistical analyses. Rita Faria and Susan Griffin carried out the health economic analysis and wrote Chapter 4.
All authors participated in data interpretation.
Publications
McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respiratory Medicine 2014;2:804–12.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014;383:736-47. http://dx.doi.org/10.1016/S0140-6736(13)60734-5.
- Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 1999;353:2100-5. http://dx.doi.org/10.1016/S0140-6736(98)10532-9.
- Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab 2005;90:4510-15. http://dx.doi.org/10.1210/jc.2005-0035.
- American Academy of Sleep Medicine Task Force . Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89.
- Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med 1999;340:847-51. http://dx.doi.org/10.1056/NEJM199903183401104.
- Masa JF, Rubio M, Findley LJ. Habitually sleepy drivers have a high frequency of automobile crashes associated with respiratory disorders during sleep. Am J Respir Crit Care Med 2000;162:1407-12. http://dx.doi.org/10.1164/ajrccm.162.4.9907019.
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001;56:508-12. http://dx.doi.org/10.1136/thorax.56.7.508.
- Douglas N, Young A, Roebuck T, Ho S, Miller BR, Kee K, et al. Prevalence of depression in patients referred with snoring and obstructive sleep apnoea. Intern Med J 2013;43:630-4. http://dx.doi.org/10.1111/imj.12108.
- McCall WV, Harding D, O’Donovan C. Correlates of depressive symptoms in patients with obstructive sleep apnea. J Clin Sleep Med 2006;2:424-6.
- Moyer C. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med 2001;2:477-91. http://dx.doi.org/10.1016/S1389-9457(01)00072-7.
- Baldwin CM, Griffith KA, Nieto FJ, O’Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep 2001;24:96-105.
- Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res 1997;6:199-204. http://dx.doi.org/10.1046/j.1365-2869.1997.00043.x.
- Engleman H, Joffe D. Neuropsychological function in obstructive sleep apnoea. Sleep Med Rev 1999;3:59-78. http://dx.doi.org/10.1016/S1087-0792(99)90014-X.
- Twigg GL, Papaioannou I, Jackson M, Ghiassi R, Shaikh Z, Jaye J, et al. Obstructive sleep apnea syndrome is associated with deficits in verbal but not visual memory. Am J Respir Crit Care Med 2010;182:98-103. http://dx.doi.org/10.1164/rccm.200901-0065OC.
- Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012;35:1593-602. http://dx.doi.org/10.5665/sleep.2226.
- Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep 2013;36:203-20. http://dx.doi.org/10.5665/sleep.2374.
- Gozal D. CrossTalk proposal: the intermittent hypoxia attending severe obstructive sleep apnoea does lead to alterations in brain structure and function. J Physiol 2013;591:379-81. http://dx.doi.org/10.1113/jphysiol.2012.241216.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378-84. http://dx.doi.org/10.1056/NEJM200005113421901.
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000;283:1829-36. http://dx.doi.org/10.1001/jama.283.14.1829.
- McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnea–hypopnea syndrome: a systematic review and economic analysis. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13040.
- Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med 2007;167:757-64. http://dx.doi.org/10.1001/archinte.167.8.757.
- Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012;8:587-96. http://dx.doi.org/10.5664/jcsm.2170.
- Redline S. Obstructive sleep apnea–hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010;182:1332-3. http://dx.doi.org/10.1164/ajrccm.182.10.1332b.
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034-41. http://dx.doi.org/10.1056/NEJMoa043104.
- Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation 2010;122:352-60. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.901801.
- Monahan K, Redline S. Role of obstructive sleep apnea in cardiovascular disease. Curr Opin Cardiol 2011;26:541-7. http://dx.doi.org/10.1097/HCO.0b013e32834b806a.
- McNicholas WT. Cardiovascular outcomes of CPAP therapy in obstructive sleep apnea syndrome. Am J Physiol Regul Integr Comp Physiol 2007;293:R1666-70. http://dx.doi.org/10.1152/ajpregu.00401.2007.
- Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31:1071-8.
- Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31:1079-85.
- Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000132.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015-21. http://dx.doi.org/10.1001/jama.284.23.3015.
- Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 2010;7:677-85. http://dx.doi.org/10.1038/nrcardio.2010.145.
- Young . The occurrence of sleep disordered breathing among middle age adults. N Engl J Med 1993;328:1230-5.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. http://dx.doi.org/10.1093/aje/kws342.
- Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J 2009;33:907-14. http://dx.doi.org/10.1183/09031936.00180108.
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults. 1999–2008. JAMA 2010;303:235-41. http://dx.doi.org/10.1001/jama.2009.2014.
- Ancoli-Israel S, Gehrman P, Kripke DF, Stepnowsky C, Mason W, Cohen-Zion M, et al. Long-term follow-up of sleep disordered breathing in older adults. Sleep Med 2001;2:511-16. http://dx.doi.org/10.1016/S1389-9457(00)00096-4.
- Bixler E. Effects of age on sleep apnea in men. Am J Respir Crit Care Med 1998;157:144-8. http://dx.doi.org/10.1164/ajrccm.157.1.9706079.
- Carskadon M, Dement W. Respiration during sleep in the aged human. J Gerontol 1981;36:420-30. http://dx.doi.org/10.1093/geronj/36.4.420.
- Coleman RM, Miles LE, Guilleminault CC, Zarcone VP, van den Hoed J, Dement WC. Sleep-wake disorders in the elderly: polysomnographic analysis. J Am Geriatr Soc 1981;29:289-96.
- McGinty D, Littner M, Beahm E, Ruiz-Primo E, Young E, Sowers J. Sleep related breathing disorders in older men: a search for underlying mechanisms. Neurobiol Aging 1982;3:337-50. http://dx.doi.org/10.1016/0197-4580(82)90022-7.
- Roehrs T, Zorick F, Sicklesteel J, Wittig R, Roth T. Age-related sleep-wake disorders at a sleep disorder center. J Am Geriatr Soc 1983;31:364-70.
- Smallwood RG, Vitiello MV, Giblin EC, Prinz PN. Sleep apnea: relationship to age, sex, and Alzheimer’s dementia. Sleep 1983;6:16-22.
- Yesavage J, Bliwise D, Guilleminault C, Carskadon M, Dement W. Preliminary communication: intellectual deficit and sleep-related respiratory disturbance in the elderly. Sleep 1985;8:30-3.
- Hoch CC, Reynolds CF, Kupfer DJ, Houck PR, Berman SR, Stack JA. Sleep-disordered breathing in normal and pathologic aging. J Clin Psychiatry 1986;47:499-503.
- Knight H, Millman R, Gur R, Saykin AJ, Doherty JU, Pack AI. Clinical significance of sleep apnea in the elderly. Am Rev Respir Dis 1987;136:845-50. http://dx.doi.org/10.1164/ajrccm/136.4.845.
- Mosko S, Dickel M, Ashurst J. Night-to-night variability in sleep apnea and sleep-related periodic leg movements in the elderly. Sleep 1988;11:340-8.
- Ancoli-Israel S, Parker L, Sinaee R, Fell RL, Kripke DF. Sleep fragmentation in patients from a nursing home. J Gerontol 1989;44:18-21. http://dx.doi.org/10.1093/geronj/44.1.M18.
- Hoch CC, Reynolds CF. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep 1990;6:502-11.
- Phillips B, Berry D, Schmitt F, Magan LK, Gerhardstein DC, Cook YR. Sleep-disordered breathing in the healthy elderly. Clinically significant?. Chest 1992;101:345-9. http://dx.doi.org/10.1378/chest.101.2.345.
- Ancoli-Israel S, Klauber M, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med 1995;152:1946-9. http://dx.doi.org/10.1164/ajrccm.152.6.8520760.
- Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 1998;157:144-8. http://dx.doi.org/10.1164/ajrccm.157.1.9706079.
- Young T, Shahar E, Nieto F, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002;162:893-900. http://dx.doi.org/10.1001/archinte.162.8.893.
- Endeshaw YW, Johnson TM, Kutner MH, Ouslander JG, Bliwise DL. Sleep-disordered breathing and nocturia in older adults. J Am Geriatr Soc 2004;52:957-60. http://dx.doi.org/10.1111/j.1532-5415.2004.52264.x.
- Hass D, Foster G, Nieto J, Redline S, Resnick HE, Robbins JA, et al. Age-dependent associations between sleep disordered breathing and hypertension. Circulation 2005;111:614-21. http://dx.doi.org/10.1161/01.CIR.0000154540.62381.CF.
- Hader C, Schroeder A, Hinz M, Micklefield GH, Rasche K. Sleep disordered breathing in the elderly: comparison of women and men. J Physiol Pharmacol 2005;56:85-91.
- Ayalon L, Ancoli-Israel S, Drummond SP. Obstructive sleep apnea and age: a double insult to brain function?. Am J Respir Crit Care Med 2010;182:413-19. http://dx.doi.org/10.1164/rccm.200912-1805OC.
- Weaver TE, Chasens ER. Continuous positive airway pressure treatment for sleep apnea in older adults. Sleep Med Rev 2007;11:99-111. http://dx.doi.org/10.1016/j.smrv.2006.08.001.
- Young T. Sleep-disordered breathing in older adults: is it a condition distinct from that in middle aged adults?. Sleep 1996;19:529-30.
- Dempsey JA. Pathophysiology of sleep apnoea. Physiol Rev 2010;90:47-112. http://dx.doi.org/10.1152/physrev.00043.2008.
- Horner RL. Factors Influencing the Adequacy of the Upper Air Way as a Conduit for Airflow in Man. London: University of London; 1990.
- Horner RL, Mohiaddin RH, Lowell DG, Shea SA, Burman ED, Longmore DB, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J 1989;2:613-22.
- Davies RJ, Stradling JR. Cephalometric measurements in snorers, non-snorers, and patients with sleep apnoea. Thorax 1991;46. http://dx.doi.org/10.1136/thx.46.12.938.
- Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J 1990;3:509-14.
- Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo YL, et al. The influence of aging on pharyngeal collapsibility during sleep. Chest 2007;131:1702-9. http://dx.doi.org/10.1378/chest.06-2653.
- Worsnop C, Kay A, Kim Y, Trinder J, Pierce R. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol 2000;88:1831-9.
- Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol 2008;104:1618-24. http://dx.doi.org/10.1152/japplphysiol.00045.2008.
- Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J 1997;10:2087-90. http://dx.doi.org/10.1183/09031936.97.10092087.
- Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med 2006;119.
- Kollias I, Krogstad O. Adult craniocervical and pharyngeal changes – a longitudinal cephalometric study between 22 and 42 years of age. Part I: morphological craniocervical and hyoid bone changes. Eur J Orthod 1999;21:333-44. http://dx.doi.org/10.1093/ejo/21.4.333.
- Morrell MJ, Finn L, McMillan A, Peppard PE. The impact of ageing and sex on the association between sleepiness and sleep disordered breathing. Eur Respir J 2012;40:386-93. http://dx.doi.org/10.1183/09031936.00177411.
- Browne HA, Adams L, Simonds AK, Morrell MJ. Sleep apnoea and day time function in the elderly –what is the impact of arousal frequency?. Respir Med 2003;97:1102-8. http://dx.doi.org/10.1016/S0954-6111(03)00142-2.
- Boselli M, Parrino L, Smerieri A, Terzano MG. Effect of age on EEG arousals in normal sleep. Sleep 1998;21:351-7.
- Mathur R, Douglas NJ. Frequency of EEG arousals from nocturnal sleep in normal subjects. Sleep 1995;18:330-3.
- Gagnon JF, Bedard MA, Fantini ML, Petit D, Panisset M, Rompré S, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology 2002;59:585-9. http://dx.doi.org/10.1212/WNL.59.4.585.
- Willcox SM, Himmelstein DU, Woolhandler S. Inappropriate drug prescribing for the community-dwelling elderly. JAMA 1994;272:292-6. http://dx.doi.org/10.1001/jama.1994.03520040054040.
- Bing MH, Jennum P, Moller LA, Mortensen S, Lose G. Obstructive sleep apnea in a Danish population of men and women aged 60–80 years with nocturia. J Clin Sleep Med 2012;8:515-20. http://dx.doi.org/10.5664/jcsm.2144.
- Bloom HG, Ahmed I, Alessi CA, Ancoli-Israel S, Buysse DJ, Kryger MH, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc 2009;57:761-89. http://dx.doi.org/10.1111/j.1532-5415.2009.02220.x.
- Empana JP, Dauvilliers Y, Dartigues JF, Ritchie K, Gariepy J, Jouven X, et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke 2009;40:1219-24. http://dx.doi.org/10.1161/STROKEAHA.108.530824.
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci 2004;5:87-96. http://dx.doi.org/10.1038/nrn1323.
- Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS). Sleep 2000;23:S102-8.
- Mathieu A, Mazza S, Decary A, Massicotte-Marquez J, Petit D, Gosselin N, et al. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients. Sleep Med 2008;9:112-20. http://dx.doi.org/10.1016/j.sleep.2007.03.014.
- Cohen-Zion M, Stepnowsky C, Marler, Shochat T, Kripke DF, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc 2001;49:1622-7. http://dx.doi.org/10.1111/j.1532-5415.2001.49270.x.
- Ancoli-Israel S, Ayalon L. Diagnosis and treatment of sleep disorders in older adults. Am J Geriatr Psychiatry 2006;14:95-103. http://dx.doi.org/10.1097/01.JGP.0000196627.12010.d1.
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol 2013;70:1537-43. http://dx.doi.org/10.1001/jamaneurol.2013.4258.
- Martínez-García MA, Campos-Rodríguez F, Catalan-Serra P, Soler-Cataluña JJ, Almeida-Gonzalez C, De la Cruz Morón I, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med 2012;186:909-16. http://dx.doi.org/10.1164/rccm.201203-0448OC.
- Weatherly HL, Griffin SC, McDaid C, Durée KH, Davies RJ, Stradling JR, et al. An economic analysis of continuous positive airway pressure for the treatment of obstructive sleep apnea-hypopnea syndrome. Int J Technol Assess Health Care 2009;25:26-34. http://dx.doi.org/10.1017/S0266462309090047.
- Egea CJ, Aizpuru F, Pinto JA, Ayuela JM, Ballester E, Zamarron C, et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Med 2008;9:660-6. http://dx.doi.org/10.1016/j.sleep.2007.06.018.
- Ruttanaumpawan P, Gilman MP, Usui K, Floras JS, Bradley TD. Sustained effect of continuous positive airway pressure on baroreflex sensitivity in congestive heart failure patients with obstructive sleep apnea. J Hypertens 2008;26:1163-8. http://dx.doi.org/10.1097/HJH.0b013e3282fb81ed.
- Parra O, Sanchez-Armengol A, Bonnin M, Arboix A, Campos-Rodríguez F, Pérez-Ronchel J, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J 2011;37:1128-36. http://dx.doi.org/10.1183/09031936.00034410.
- Rosen CL, Auckley D, Benca R, Foldvary-Schaefer N, Iber C, Kapur V, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep 2012;35:757-67. http://dx.doi.org/10.5665/sleep.1870.
- Haniffa M, Lasserson TJ, Smith I. Interventions to improve compliance with continuous positive airway pressure for obstructive sleep apnoea. Cochrane Database Syst Rev 2004;4.
- Martins De Araujo MT, Vieira SB, Vasquez EC, Fleury B. Heated humidification or face mask to prevent upper airway dryness during continuous positive airway pressure therapy. Chest 2000;117:142-7. http://dx.doi.org/10.1378/chest.117.1.142.
- de Andrade RG, Piccin VS, Nascimento JA, Viana FM, Genta PR, Lorenzi-Filho G. Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. J Bras Pneumol 2014;40:658-68. http://dx.doi.org/10.1590/S1806-37132014000600010.
- Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev 2001;1. http://dx.doi.org/10.1002/14651858.CD002875.
- Araghi MH, Chen YF, Jagielski A, Choudhury S, Banerjee D, Hussain S, et al. Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta-analysis. Sleep 2013;36:1553-62. http://dx.doi.org/10.5665/sleep.3056.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540-5.
- EuroQol – a new facility for the measurement of health-related quality of life . The EuroQol Group. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. http://dx.doi.org/10.1016/S0167-6296(01)00130-8.
- The Green Book. Appraisal and Evaluation in Central Government. London: Treasury Guidance; 2011.
- Bennett LS, Stradling JR, Davies RJ. A behavioural test to assess daytime sleepiness in obstructive sleep apnoea. J Sleep Res 1997;6:142-5. http://dx.doi.org/10.1046/j.1365-2869.1997.00039.x.
- Jenkinson CLR, Wright L, Coulter A. The UK SF-36: An Analysis and Interpretation Manual. University of Oxford: Health Services Research Unit; 1996.
- Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med 1998;158:494-503. http://dx.doi.org/10.1164/ajrccm.158.2.9712036.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- McGee MA, Johnson AL, Kay DW. The description of activities of daily living in five centres in England and Wales: the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Age Ageing 1998;27:605-13. http://dx.doi.org/10.1093/ageing/27.5.605.
- Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142-8.
- Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry 1983;40. http://dx.doi.org/10.1001/archpsyc.1983.01790060110016.
- Lezak MD. Neuropsychological Assessment. Oxford: Oxford University Press; 1995.
- Townsend P. Poverty in the United Kingdom: A Survey of Household Resources and Standards of Living. London: Penguin Books; 1979.
- Royston P, Sauerbrei W. A new approach to modelling interactions between treatment and continuous covariates in clinical trials by using fractional polynomials. Stat Med 2004;23:2509-25. http://dx.doi.org/10.1002/sim.1815.
- Ayas NT, FitzGerald JM, Fleetham JA, White DP, Schulzer M, Ryan CF, et al. Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Arch Intern Med 2006;166:977-84. http://dx.doi.org/10.1001/archinte.166.9.977.
- Guest JF, Helter MT, Morga A, Stradling JR. Cost-effectiveness of using continuous positive airway pressure in the treatment of severe obstructive sleep apnoea/hypopnoea syndrome in the UK. Thorax 2008;63:860-5. http://dx.doi.org/10.1136/thx.2007.086454.
- Mar J, Rueda J, Durán-Cantolla J, Schechter C, Chilcott J. The cost-effectiveness of nCPAP treatment in patients with moderate-to-severe obstructive sleep apnoea. Eur Respir J 2003;21:515-22. http://dx.doi.org/10.1183/09031936.03.00040903.
- Sadatsafavi M, Marra CA, Ayas NT, Stradling J, Fleetham J. Cost-effectiveness of oral appliances in the treatment of obstructive sleep apnoea – hypopnoea. Sleep Breath 2009;13:241-52. http://dx.doi.org/10.1007/s11325-009-0248-4.
- Tan MCY, Ayas NT, Mulgrew A, Cortes L, FitzGerald JM, Fleetham JA, et al. Cost-effectiveness of continuous positive airway pressure therapy in patients with obstructive sleep apnea–hypopnea in British Columbia. Can Respir J 2008;15:159-65.
- Gander P, Scott G, Mihaere K, Scott H. Societal costs of obstructive sleep apnoea syndrome. NZ Med J 2010;123:13-2.
- Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep 2011;34:695-709. http://dx.doi.org/10.5665/sleep.1030.
- Chilcott J, Clayton E, Chada N, Hanning CD, Kinnear W, Waterhouse JC. Nasal Continuous Positive Airways Pressure in the Management of Sleep Apnoea. Leicester: Trent Institute for Health Services Research; 2000.
- Tousignant P, Cosio MG, Levy RD, Groome PA. Quality adjusted life years added by treatment of obstructive sleep apnea. Sleep 1994;17:52-60.
- Dolan P, Gudex C, Kind P, Williams A. The time trade-off method: results from a general population study. Health Econ 1996;5:141-54. http://dx.doi.org/10.1002/(SICI)1099-1050(199603)5:2<141::AID-HEC189>3.0.CO;2-N.
- Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference-based single index from the UK SF-36 Health Survey. J Clin Epidemiol 1998;51:1115-28. http://dx.doi.org/10.1016/S0895-4356(98)00103-6.
- Torrance GW, Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care 1989;5:559-75. http://dx.doi.org/10.1017/S0266462300008461.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Curtis L. Unit Costs of Health and Social Care 2012 2012.
- UK Parliament . Daily Hansard – Written Answers 2009. www.publications.parliament.uk/pa/cm200809/cmhansrd/cm090223/text/90223w0053.htm#09022423005854 (accessed 26 October 2013).
- Curtis L. Unit Costs of Health and Social Care 2008 2008.
- Department of Health . NHS Reference Costs: Financial Year 2011 to 2012 2012. www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (accessed 26 October 2013).
- ResMed (UK) Ltd Hospital Price List. Abingdon: ResMed; 2013.
- Drummond M, Sculpher M, Torrance G, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005;172:1363-70. http://dx.doi.org/10.1164/rccm.200412-1631SO.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Johannesson M, Weinstein MC. On the decision rules of cost-effectiveness analysis. J Health Econ 1993;12:459-67. http://dx.doi.org/10.1016/0167-6296(93)90005-Y.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40. http://dx.doi.org/10.1002/(SICI)1099-1050(199707)6:4<327::AID-HEC282>3.0.CO;2-W.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Philips Respironics . 2012 Medicare Guidelines for CPAP – Mask and Accessory Replacement 2012. www.respironics.vgm.com/files/Medicare-Guidelines-Replacement-2012.pdf (accessed 26 October 2013).
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- UK Department for Transport . Drivers in Reported Accidents by Gender, Number Injured, Road User Type and Age, Great Britain, 2011 2012. www.gov.uk/government/uploads/system/uploads/attachment_data/file/10137/ras20002.xls (accessed 26 October 2013).
- Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, Petridou ET. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev 2011;15:301-10. http://dx.doi.org/10.1016/j.smrv.2010.10.002.
- Ellen RL, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Son-Hing M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med 2006;2:193-200.
- Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med 2009;5:573-81.
- Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep 2010;33:1373-80.
- Matheny M, McPheeters ML, Glasser A, Mercaldo N, Weaver RB, Jerome RN, et al. Systematic review of cardiovascular disease risk assessment tools. Rockville, MD: US Agency for Healthcare Research and Quality; 2011.
- Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991;121:293-8. http://dx.doi.org/10.1016/0002-8703(91)90861-B.
- Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev 2011;15:343-56. http://dx.doi.org/10.1016/j.smrv.2011.01.003.
- Russo-Magno P, O’Brien A, Panciera T, Rounds S. Compliance with CPAP therapy in older men with obstructive sleep apnea. J Am Geriatr Soc 2001;49:1205-11. http://dx.doi.org/10.1046/j.1532-5415.2001.49238.x.
- Bravata DM, Concato J, Fried T, Ranjbar N, Sadarangani T, McClain V, et al. Autotitrating continuous positive airway pressure for patients with acute transient ischemic attack: a randomized feasibility trial. Stroke 2010;41:1464-70. http://dx.doi.org/10.1161/STROKEAHA.109.566745.
- Woehrle H, Graml A, Weinreich G. Age- and gender-dependent adherence with continuous positive airway pressure therapy. Sleep Med 2011;12:1034-6. http://dx.doi.org/10.1016/j.sleep.2011.05.008.
- McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:1108-14. http://dx.doi.org/10.1164/ajrccm.159.4.9807111.
- Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea population-based study. Chest 2002;121:430-5. http://dx.doi.org/10.1378/chest.121.2.430.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. http://dx.doi.org/10.1177/0272989X11401031.
- Office for National Statistics . Interim Lifetables, England and Wales, 2010–2012 2013. www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2010–2012/stbilt2012.html (accessed 26 October 2013).
- Rosengren A, Wilhelmsen L, Hagman M, Wedel H. Natural history of myocardial infarction and angina pectoris in a general population sample of middle-aged men: a 16-year follow-up of the Primary Prevention Study, Göteborg, Sweden. J Intern Med 1998;244:495-50. http://dx.doi.org/10.1111/j.1365-2796.1998.00394.x.
- Dennis MS, Burn J, Sandercock P, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke 1993;24:796-800. http://dx.doi.org/10.1161/01.STR.24.6.796.
- Briggs A, Mihaylova B, Sculpher M, Hall A, Wolstenholme J, Simoons M, et al. Cost effectiveness of perindopril in reducing cardiovascular events in patients with stable coronary artery disease using data from the EUROPA study. Heart 2007;93:1081-6. http://dx.doi.org/10.1136/hrt.2005.086728.
- Vergel YB, Palmer S, Asseburg C, Fenwick E, de Belder M, Abrams K, et al. Is primary angioplasty cost effective in the UK? Results of a comprehensive decision analysis. Heart 2007;93:1238-43. http://dx.doi.org/10.1136/hrt.2006.111401.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. http://dx.doi.org/10.3109/07853890109002087.
- Engleman HM. Sleep. 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax 2004;59:618-22. http://dx.doi.org/10.1136/thx.2003.015867.
- Morrell MJ, Jackson ML, Twigg GL, Ghiassi R, McRobbie DW, Quest RA, et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax 2010;65:908-14. http://dx.doi.org/10.1136/thx.2009.126730.
- Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP). Brain Res Bull 2003;61:87-92. http://dx.doi.org/10.1016/S0361-9230(03)00068-6.
- Rosenzweig I, Williams SC, Morrell MJ. CrossTalk opposing view: the intermittent hypoxia attending severe obstructive sleep apnoea does not lead to alterations in brain structure and function. J Physiol 2013;591:383-5. http://dx.doi.org/10.1113/jphysiol.2012.241224.
- Robinson GV, Stradling JR, Davies RJO. Sleep·6: obstructive sleep apnoea/hypopnoea syndrome and hypertension. Thorax 2004;59:1089-94. http://dx.doi.org/10.1136/thx.2003.015875.
- Craig SE, Kohler M, Nicoll D, Bratton DJ, Nunn A, Davies R, et al. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax 2012;67:1090-6. http://dx.doi.org/10.1136/thoraxjnl-2012-202178.
- Guilleminault C, Lin CM, Goncalves MA, Ramos E. A prospective study of nocturia and the quality of life of elderly patients with obstructive sleep apnea or sleep onset insomnia. J Psychosom Res 2004;56:511-15. http://dx.doi.org/10.1016/S0022-3999(04)00021-2.
- Aloia MS, Ilniczky N, Di Dio P, Perlis ML, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults with sleep apnea. J Psychosom Res 2003;54:71-6. http://dx.doi.org/10.1016/S0022-3999(02)00548-2.
- Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs. standard continuous positive airway pressure therapy. Chest 2005;127:2085-93. http://dx.doi.org/10.1378/chest.127.6.2085.
- Senn O, Brack T, Matthews F, Russi EW, Bloch KE. Randomized short-term trial of two autoCPAP devices versus fixed continuous positive airway pressure for the treatment of sleep apnea. Am J Respir Crit Care Med 2003;168:1506-11. http://dx.doi.org/10.1164/rccm.200304-542OC.
- Massie CA, McArdle N, Hart RW, Schmidt-Nowara WW, Lankford A, Hudgel DW, et al. Comparison between automatic and fixed positive airway pressure therapy in the home. Am J Respir Crit Care Med 2003;167:20-3. http://dx.doi.org/10.1164/rccm.200201-022OC.
- Gagnadoux F, Le Vaillant M, Goupil F, Pigeanne T, Chollet S, Masson P, et al. Influence of marital status and employment status on long-term adherence with continuous positive airway pressure in sleep apnea patients. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0022503.
- Hughes LD, McMurdo MET, Guthrie B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing 2013;42:62-9. http://dx.doi.org/10.1093/ageing/afs100.
- McMurdo MET, Roberts H, Parker S, Wyatt N, May H, Goodman C, et al. Improving recruitment of older people to research through good practice. Age Ageing 2011;40:659-65. http://dx.doi.org/10.1093/ageing/afr115.
- Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnoea. Hypertension 2007;50:417-23. http://dx.doi.org/10.1161/HYPERTENSIONAHA.106.085175.
- HeartStats. 2009.
- Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke 1996;27:373-80.
- Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry 2003;74:317-21.
- Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 2008;52:818-27.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53.
- Barbe F, Sunyer J, de la Pena A, Pericas J, Mayoralas LR, Anto JM. Effect of continuous positive airway pressure on the risk of road accidents in sleep apnoea patients. Respiration 2007;74:44-9.
- O’Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med 2009;179:1159-64.
- Meara E, White C, Cutler DM. Trends in medical spending by age, 1963–2000. Health Aff (Millwood) 2004;23:176-83.
- Hogan C, Lunney J, Gabel J, Lynn J. Medicare beneficiaries’ costs of care in the last year of life. Health Aff (Millwood) 2001;20:188-95.
- Stuart B, Lloyd J, Zhao L, Kamal-Bahl S. Obesity, disease burden, and prescription spending by community-dwelling Medicare beneficiaries. Curr Med Res Opin 2008;24:2377-87.
- Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke 1996;27:1459-66.
- Kauf TL, Velazquez EJ, Crosslin DR, Weaver WD, Diaz R, Granger CB. The cost of acute myocardial infarction in the new millennium: evidence from a multinational registry. Am Heart J 2006;151:206-12.
- Qureshi AI, Suri MF, Nasar A, Kirmani JF, Ezzeddine MA, Divani AA. Changes in cost and outcome among US patients with stroke hospitalized in 1990 to 1991 and those hospitalized in 2000 to 2001. Stroke 2007;38:2180-4.
- Blincoe LJ, Seay A, Zaloshnja E, Miller T, Romano E, Luchter S. The Economic Impact of Motor Vehicle Crashes, 2000. Washington, DC: U.S. Department of Transportation, National Highway Traffic Safety Administration; 2002.
- Chakravorty I, Cayton R, Szczepura A. Health utilities in evaluating intervention in the sleep apnoea/hypopnoea syndrome. Eur Respir J 2002;20:1233-8.
- Jenkinson S, Stradling J, Petersen S. How should we evaluate health status? A comparison of three methods in patients presenting with obstructive sleep apnoea. Qual Life Res 1998;7:95-100.
- Graham JD, Thompson KM, Goldie SJ, Segui-Gomez M, Weinstein MC. The cost effectiveness of air bags by seating position. JAMA 1997;278:1418-25.
- Williams GR. Incidence and characteristics of total stroke in the United States. BMC Neurol 2001;1.
- Greenberg-Dotan S, Reuveni H, Simon-Tuval T, Oksenberg A, Tarasiuk A. Gender differences in morbidity and health care utilization among adult obstructive sleep apnea patients. Sleep 2007;30:1173-80.
- Sarasin FP, Gaspoz JM, Bounameaux H. Cost-effectiveness of new antiplatelet regimens used as secondary prevention of stroke or transient ischemic attack. Arch Intern Med 2000;160:2773-8.
- McDaid CGS, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers S. The Continuous Positive Airway Pressure for the Treatment of Obstructive Sleep Apnoea–Hypopnoea Syndrome: A Systematic Review and Economic Analysis. York: CRD/CHE Technology Assessment Group (Centre for Reviews and Dissemination/Centre for Health Economics), University of York; 2007.
- Pickard AS, Johnson JA, Feeny DH. Responsiveness of generic health-related quality of life measures in stroke. Qual Life Res 2005;14:207-19.
- Bradley CJ, Kroll J, Holmes-Rovner M. The health and activities limitation index in patients with acute myocardial infarction. J Clin Epidemiol 2000;53:555-62.
- Mahoney EM, Jurkovitz CT, Chu H, Becker ER, Culler S, Kosinski AS. Cost and cost-effectiveness of an early invasive vs conservative strategy for the treatment of unstable angina and non-ST-segment elevation myocardial infarction. JAMA 2002;288:1851-8.
- The Economic Burden of Unintentional Injury in Canada. n.d.
- Vodden K, Smith D, Meng R. The Social Cost of Motor Vehicle Crashes in Ontario. Ontario, ON: Safety Research Office, Safety Policy Branch; 1994.
- Mercer GW, Halabisky L. Proceedings of Traffic Safety and Auto Engineering Stream of the World Congress on Whiplash-Associated Disorders. British Columbia: Insurance Corporation of British Columbia; 1999.
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001;56:508-12.
- Mazza S, Pépin JL, Naëgelé B, Rauch E, Deschaux C, Ficheux P. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J 2006;28:1020-8.
- Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep 2004;27:453-8.
- Lindberg E, Carter N, Gislason T, Janson C. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med 2001;164:2031-5.
- Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B. Population based study of sleep disordered breathing as a risk factor for hypertension. Arch Intern Med 1997;157:1746-52.
- MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990;335:765-74.
- Pankow W, Lies A, Friedrich W. Sleep-disordered breathing and hypertension. N Engl J Med 2000;343:966-7.
- Mar J, Rodriguez-Artalejo F. Which is more important for the efficiency of hypertension treatment: hypertension stage, type of drug or therapeutic compliance?. J Hypertens 2001;19:149-55.
- Cassel W, Ploch T, Becker C, Dugnus D, Peter JH, von Wichert P. Risk of traffic accidents in patients in sleepdisordered breathing: reduction with nasal CPAP. Eur Respir J 1996;9:2606-11.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics 2014;32:1157-70. http://dx.doi.org/10.1007/s40273-014-0193-3.
- Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006;3. http://dx.doi.org/10.1002/14651858.CD001106.pub2.
- Marshall NS, Barnes M, Travier N, Campbell AJ, Pierce RJ, McEvoy RD, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax 2006;61:430-4. http://dx.doi.org/10.1136/thx.2005.050583.
- Rao M, Rajda G, Uppuluri S, Beck GR, Liu L, Bisognano JD. The role of continuous positive airway pressure in the treatment of hypertension in patients with obstructive sleep apnoea-hypoapnea syndrome: a review of randomised trials. Rev Recent Clin Trials 2010;5:35-42. http://dx.doi.org/10.2174/157488710790820526.
- Egea C, Aizpuru F, Pinto J, Ayuela J, Ballester E, Zamarron C, et al. Cardiac Function After CPAP Therapy in Chronic Heart Failure and Sleep Apnoea n.d.
Appendix 1 Statistical and health economic analysis plans
Appendix 2 PREDICT investigators and memberships of committees
Trial steering committee
Professor Walter McNicholas (Chairperson), Professor Sir Neil Douglas and Dr Ian Smith (independent members), Daniel Bratton, Professor Robert Davies, Dr Mark Elliot, Mr Frank Govan, Dr Melissa Hack, Magda Laskawiec-Szkonter, Dr Alison McMillan, Professor Mary Morrell, Professor Andrew Nunn, Dr Justin Pepperell, Dr Renata Riha, Professor Mark Sculpher, Professor Anita Simonds, Professor John Stradling and Dr John Starr.
Independent data monitoring committee
Chairperson Professor Tim Peto, Professor John Gibson and Professor David Wright.
Trial management
Magda Laskawiec-Szkonter (ORTU).
Data entry
Jack Quaddy and Assunta Sabia (ORTU).
Research staff by centre
Birmingham (Heartlands Hospital)
Dr Dev Banerjee, Kerryanne James, Sarah Manney and Matthew Nicholls.
Blackpool (Victoria Hospital)
Dr Mohammed Paracha, Jules Chadwick, Kate O’Reilly, Judith Saba and Gemma Swarbrick.
Edinburgh (Royal Infirmary Edinburgh)
Dr Renata Riha, Lizzie Hill, Donna Fairley and Marjorie Vennelle.
Leeds (St James’ University Hospital)
Dr Mark Elliott, Craig Armstrong, Clair Favager and Sue Watts.
Liverpool (Aintree Hospital)
Dr John O’Reilly, Stephen Emegbo and Pam Parry.
London (Royal Brompton Hospital)
Professor Mary J Morrell, Professor Anita Simonds, Dr Martin Glasser, Lydia Paniccia, Luxumi Sridharan, Dr Alison McMillan and Dr Neil Ward.
Newcastle (Freeman Hospital)
Dr Sophie West, Peter Close, Lyndsay Rostron and Therese Small.
Newport (St Woolos Hospital)
Dr Melissa Hack, Clare Acreman, Sarah Mitchell and Jeanette Richards.
Oxford (Churchill Hospital)
Professor John Stradling, Isabel Chabata, Nicky Crosthwaite, Tara Harris, Debby Nicoll and Barbara Winter.
Reading (Royal Berkshire Hospital)
Dr Chris Davies and Jacqui Webb.
Stoke-on-Trent (City General Hospital)
Dr Martin Allen, Andrew Bain, Nathalie Bryan and Ann Cooper.
Swindon (Great Western Hospital)
Dr Andrew Stanton, Sam Backway and Sue Meakin.
Taunton (Musgrove Park Hospital)
Dr Justin Pepperell, Dawn Redwood and Tania Wainwright.
Wolverhampton (New Cross Hospital)
Dr Lee Dowson, Jillian Andrew, Lucy Reynolds and Louise Spragg.
Appendix 3 Appendix to the economic chapter
Systematic review of existing cost-effectiveness evidence
Methods
Systematic searches of the literature were conducted to identify potentially relevant studies for inclusion in the assessment of cost-effectiveness of CPAP against any comparator in the treatment of OSAS. Full details of the search strategies are reported in Appendix 4. The search strategies were based on those conducted by McDaid et al. 20 for the HTA report on CPAP devices for the treatment of OSAS. Since the systematic review of existing cost-effectiveness evidence in McDaid et al. 20 included studies up to 2006, our searches were run for studies published from January 2006 to April 2012. The economic evaluations included in the systematic review in McDaid et al. 20 were also included in the current systematic review. 111,113,118,119
The systematic review included full economic evaluations that compared two or more options and considered both costs and consequences (including cost-effectiveness, cost–utility and cost–benefit analyses). Titles and abstracts were assessed independently by two reviewers for inclusion and any discrepancies were resolved by consensus. Data were extracted by one reviewer using a standardised data extraction form and checked for accuracy by a second reviewer. The information is summarised within the text, alongside a detailed critique of the included studies and their relevance to the UK NHS. The findings from the review provide the basis for the development of a new decision model.
Results
Figure 21 presents a flow diagram summarising the identification and selection of studies. A total of 3560 unique records were identified from the systematic literature searches, of which seven subsequently met the inclusion criteria. 20,111,112,114–117 In addition, three additional studies were identified from the previous HTA on the subject. 113,118,119 Table 48 reports a brief summary of the studies. More detailed data extraction summary tables are presented at the end of this section.
FIGURE 21.
Flow diagram showing number of studies identified and included in the review of cost-effectiveness of CPAP.

| First author (year) | Perspective | Country | Population | Comparators | Results |
|---|---|---|---|---|---|
| Chilcott (2000)118 | Third-party payer | UK | Middle-age patients referred to sleep clinic | No treatment | ICER = £3200/QALY |
| Tousignant (2003)119 | Third-party payer | Canada | Middle-aged patients (average age = 57 years) | No treatment | ICER between CAN$3397/QALY and CAN$9792/QALY; ICER without three outliers = CAN$18,637/QALY |
| Mar (2003)113 | Third-party payer | Spain | 50-year-old male with moderate to severe OSAS, defined by AHI > 30 events/hour and ESS score of > 10 | No treatment | ICER 5-years = €7861/QALY; ICER lifetime = €4938/QALY |
| Ayas (2006)111 | Third-party payer and societal | USA | Patients with moderate to severe OSAS (AHI ≥ 15 events/hour), aged between 25 and 54 years, drivers | No treatment | Third-party payer ICER = US$3354/QALY; societal ICER = US$314/QALY |
| Tan (2008)115 | Third-party payer and societal | USA | Patients with moderate to severe OSAS, aged between 30 and 59 years, drivers | No treatment | Third-party payer ICER = CAN$3626/QALY; societal ICER = CAN$2979/QALY |
| Guest (2008)112 | Third-party payer | UK | 55-year-old patient with severe OSAS (AHI > 30 events/hour) and ESS score of ≥ 12 | No treatment | CPAP dominates no treatment. Probablilty that CPAP is cost-efficient at £20,000/QALY is 0.99 |
| McDaid (2009)20 | Third-party payer | England and Wales | 50-year-old male | Dental devices and no treatment | ICER = £3899/QALY; Probability cost-efficient at £20,000/QALY = 0.80 |
| Sadatsafavi (2009)114 | Third-party payer | USA | Patients with moderate to severe OSAS (AHI ≥ 15 events/hour), aged between 25 and 64 years | Dental devices and no treatment | ICER = US$27,540/QALY |
| Gander (2010)116 | Societal | New Zealand | Patients between 30 and 59 years | No treatment | ICER = NZ$506.79/QALY |
| Pietzsch (2011)117 | Third-party payer | USA | 50-year-old male with moderate to severe OSAS | No treatment | ICER = US$16,172/QALY over 10 years and US$15,915/QALY for lifetime |
All studies except Gander et al. 116 evaluated the cost-effectiveness of CPAP from a health-care or third-party payer perspective. Gander et al. 116 estimated the economic burden of OSAS from a societal perspective and, as a secondary analysis, presented an estimate of the ICER for treating OSAS compared with no treatment. Three studies were conducted in the UK,20,112,118 3 in the USA,111,114,117 two in Canada,115,119 one in New Zealand116 and one in Spain. 113
All studies compared CPAP with no treatment, and two also compared CPAP with dental devices. 20,114 Gander et al. 116 bundled CPAP together with dental devices and surgery under the treatment intervention. Pietzsch et al. 117 evaluated both the diagnostic options and CPAP treatment compared with no treatment. The time horizon employed was 5-years in six studies. 111,113–115,118,119 A lifetime horizon was used in three studies. 20,113,117 Alternative time horizons were used by three studies: 1 year in Gander et al.,116 10 years in Pietzsch et al. 117 and 14 years in Guest et al. 112
In six studies, the patient population consisted of a cohort of middle-age men with moderate to severe OSAS. 20,112,113,117–119 Three studies presented results for a patient cohort with moderate to severe OSAS, which were calculated from averaging the results from six patient subgroups defined by sex and age. In Ayas et al. 111 the cohort was aged between 25 and 54 years; in Tan et al. 115 the cohort was aged between 30 and 59 years; and in Sadatsafavi et al. 114 the cohort was aged between 25 and 64 years. Gander et al. 116 included patients aged between 30 and 59 years with any level of OSAS severity. Only McDaid et al. 20 and Pietzsch et al. 117 conducted subgroup analysis. Subgroup populations were defined by age, sex and disease severity by McDaid et al. 20 and by age, sex and disease prevalence by Pietzsch et al. 117 Note that no study looked specifically at patients aged 60 years and over.
In order to synthesise the available evidence and extrapolate over the chosen time horizon, most studies employed a decision-analytic model. A Markov model was employed in six studies. 20,111–115 Gander et al. 116 used a decision tree. Pietzsch et al. 117 used a decision tree for the diagnostics pathway and a Markov model for subsequent treatment decisions. McDaid et al. 20 used the structure first proposed by Mar et al. 113 of a Markov model with four health states [OSAS event-free, post stroke, post CHD and dead] and three events (CHD, stroke and RTAs). Guest et al. 112 used a similar model, but including a health state following the RTA event. Tan et al. 115 used the same model as Ayas et al.,111 which has an event-free OSAS state and six health states post RTA, corresponding to increasing levels of injury or death. Sadatsafavi et al. 114 used the same structure for the RTA component of the model but included post-MI and post-stroke health states. The Markov model in Pietzsch et al. 117 had five health states: event-free, hypertension, post MI, post stroke and dead. Patients were at risk of developing clinical hypertension and of experiencing an MI, stroke and RTA. The decision tree in Gander et al. 116 modelled the long-term consequences of increased costs owing to diabetes, cardiovascular disease, RTAs and work accidents in the untreated patients. Tousignant et al. 119 quality-adjusted the life expectancy of the patients included in the study, estimated based on Canadian lifetables, to calculate the cost-effectiveness of CPAP over a lifetime horizon. Chilcott et al. 118 synthesised data obtained from a review of the literature.
In line with the various model structures employed, treatment effectiveness was incorporated across the studies differently. A lower risk of RTAs was considered by eight studies. 20,111–117 Six studies included a reduction in cardiovascular events. 20,112–114,116,117 Gander et al. 116 included a reduction in the risk of diabetes and in the risk of work accidents. McDaid et al. 20 was the only study which incorporated a reduction in daytime sleepiness, as measured by ESS scores. Most studies based the effectiveness estimates in observational data on patients with moderate to severe OSAS. Seven studies assumed that CPAP reduces the risk of the various events to that of the general population and took the baseline risk of events in the untreated patients from observational studies. 111–117 McDaid et al. 20 obtained data on the effectiveness of CPAP in reducing ESS score and BP from a bivariate meta-analysis of RCTs and on the reduction in RTAs from observational evidence.
All studies included the benefits of CPAP in terms of HRQoL. Chilcott118 converted SF-36 data into health utility weights using the Brazier et al. 121 algorithm. Tousignant et al. 119 obtained health utility values before and after CPAP treatment directly from patients receiving CPAP with a standard gamble exercise, although the before treatment scores were valued retrospectively. Mar et al. 113 administered the EQ-5D instrument to patients before and after CPAP treatment; these values were also used by Guest et al. 112 Ayas et al. 111 and Tan et al. 115 sourced health utility weights from Chakravroty et al.,171 which evaluated HRQoL before and after treatment with a standard gamble exercise. McDaid et al. 20 developed a mapping function relating ESS score with EQ-5D and SF-6D, in which a reduction by 1 point in the ESS score was found to correspond to an improvement in health utility of 0.01. Sadatsafavi et al. 114 used this relationship in their model. Gander et al. 116 assumed a QALY gain of 5.4, based on data from the Sleep Alliance and Tousignant et al.,119 although it is unclear how this estimate was obtained. Pietzsch et al. 117 used data from the US Medical Expenditure Panel Survey to estimate that age- and sex-specific HRQoL in terms of EQ-5D was reduced by 16% in untreated OSAS patients and by 7% in OSAS patients treated with CPAP.
Across all studies, the results of the cost-effectiveness analysis led the authors to conclude that CPAP was a cost-effective treatment for patients with OSAS. The ICERs were lower than the typical willingness to pay for an additional QALY used in each of the countries considered, and in Guest et al. 112 CPAP was found to be associated with reduced costs and increased health benefits compered with no treatment. In general, the ICERs were robust to alternative assumptions on parameter inputs with the exception of the HRQoL gain from treatment with CPAP. HRQoL gain due to CPAP treatment had the greatest impact on the ICER for all studies that included this parameter in their sensitivity analysis. 20,111,113–115,117
Data extraction tables
| Study details | McDaid et al. (2009)20 |
|---|---|
| Economic evaluation type | Cost–utility analysis |
| Intervention | CPAP |
| Comparator(s) | Dental devices |
| Conservative management (no treatment) | |
| Outcomes | Primary outcome: incremental cost per QALY gained |
| Currency (year) | Pounds sterling (2005) |
| Study design | Markov model |
| Perspective | Third-party payer (NHS and Personal Social Services) |
| Setting | England and Wales |
| Patient population | Base case: 50-year-old male. Base-case results were estimated as the weighted average of the results according to average ESS score (mild – ESS score = 7, moderate – ESS score = 13 or severe – ESS score = 16) |
| Subgroup analyses were conducted by sex, OSAS severity (as measured by ESS) and other relevant baseline characteristics. | |
| Time horizon | Lifetime |
| Model structure | Four health states:
|
Three types of events:
|
|
| Cycle length: 1 year | |
| The model records the ESS score of the patient cohort as time progresses | |
| Treatment effectiveness and sources | CPAP and dental devices:
|
| Resources used and costs and sources | Cost of the interventions included: cost of the devices, staff time, and overheads |
| Cost associated with cardiovascular events | |
| Cost of RTA | |
| Published sources and manufacturer’s submission on auto-CPAP device | |
| HRQoL and sources | Health outcomes expressed in terms of QALYs
|
| Compliance | Long-term compliance was based on an observational study. Patients discontinuing treatment were assumed to return immediately to the levels of ESS score, BP and utility associated with no treatment |
| Mortality | All-cause mortality obtained from UK lifetables adjusted for deaths due to stroke and CHD |
| Relative risk of death for patients experiencing stroke were adjusted upwards using factors published in the literature | |
| Adverse events from treatment | None |
| Subgroup analysis | Subgroup analysis by baseline severity of OSAS as measured by ESS score:
|
Subgroup analysis by age:
|
|
| Discounting | 3.5% for costs and health outcomes |
| Results | Base case (males aged 50 years):
|
Scenario (females aged 50 years)
|
|
| Assessment of uncertainty | All results are presented probabilistic |
Scenario analysis:
|
|
| Conclusions | CPAP is more cost-effective than dental devices and conservative management for patients with OSAS |
| Issues in the generalisability to younger or older populations, particularly because of comorbidities | |
| Key cost-effectiveness drivers | HRQoL benefit associated with the reduction in ESS score as a result of CPAP |
| Rate of RTAs | |
| Uncertainties |
|
| Conflicts of interest | None |
| Study details | Pietzsch et al. (2011)117 |
|---|---|
| Economic evaluation type | Cost–utility analysis |
| Intervention | Diagnostics: full-night polysomnography, split-night polysomnography and unattended portable home monitoring |
| Treatment: CPAP | |
| Comparator(s) | No diagnostic technology |
| No treatment | |
| Outcomes | Incremental costs per QALY |
| Currency (year) | US dollar (2008) |
| Study design | Decision tree (for diagnostic strategies) and Markov model (for treatment) |
| Perspective | Third-party payer |
| Setting | USA |
| Patient population | Base case: 50-year-old male with a prevalence of moderate to severe OSAS of 50% |
| Sensitivity analyses considered women, alternative ages and alternative levels of OSAS prevalence | |
| Time horizon | 10-years and lifetime |
| Model structure | The decision tree splits the cohort into four groups:
|
The Markov model has five health states:
|
|
Death can occur as a result of:
|
|
Events:
|
|
| Treatment effectiveness and sources | Cardiovascular:
|
RTAs:
|
|
| Resources used and costs and sources | Direct health-care costs only:
|
| HRQoL and sources | QALYs HRQoL values were derived from the self-reported health of participants in the Medical Expenditure Panel Survey, as measured by EQ-5D:
|
| Mortality | All-cause mortality obtained from US lifetables adjusted for deaths due to stroke and MI |
| Compliance | Assumption that 10.2% of patients decline therapy, as observed in a study with 353 patients diagnosed with moderate OSAS7 CPAP compliance stabilises after 4 years at 68%, as observed in a study of long-term CPAP compliance113 |
| Adverse events from treatment | None |
| Subgroup analysis | None |
| Discounting | 3% for costs and benefits |
| Results | CPAP therapy:
|
Diagnostics strategies:
|
|
| Assessment of uncertainty | Scenarios tested:
|
| Conclusions | CPAP is more cost-effective than dental devices and conservative management for patients with OSAS Issues in the generalisability to patients with mild disease or higher than average baseline risks for cardiovascular events |
| Key cost-effectiveness drivers | CPAP costs and HRQoL benefits from treatment |
| Uncertainties | Generalisability of costs across jurisdictions Assumptions around the effectiveness of CPAP |
| Conflicts of interest | None |
| Study details | Ayas et al. (2006)111 |
|---|---|
| Economic evaluation type | Cost–utility analysis |
| Intervention | CPAP |
| Comparator(s) | Conservative management (no treatment) |
| Outcomes | Primary outcome: incremental cost per QALY |
| Currency (year) | US dollar (2003) |
| Study design | Markov model |
| Perspective | Third-party payer (US) and societal |
| Setting | USA |
| Patient population | Base case:
|
| Time horizon | 5-year |
| Model structure | Markov model:
|
| Treatment effectiveness And sources |
|
| Resources used and costs and sources | Base case: direct health-care costs
|
Scenario – societal perspective:
|
|
| HRQoL and sources |
|
| Compliance |
|
| Mortality | US lifetables |
| Adverse events from treatment | None included |
| Subgroup analysis | None |
| Discounting | 3% for costs and health outcomes |
| Results | Base case (third-party payer): ICER for CPAP was $3354/QALY Societal: ICER for CPAP was $314/QALY |
| Assessment of uncertainty | Univariate sensitivity analysis:
|
| Conclusions | CPAP is a cost-effective use of health-care resources from both the health-care payer and the societal perspective |
| Key cost-effectiveness drivers | HRQoL benefit from CPAP therapy |
| Uncertainties |
|
| Conflicts of interest | None |
| Study details | Sadatsafavi et al. (2009)114 |
|---|---|
| Economic evaluation type | Cost–utility analysis |
| Intervention | CPAP |
| Comparator(s) | Dental devices Conservative management (no treatment) |
| Outcomes | Primary outcome: incremental cost per QALY gained |
| Currency (year) | US dollar (2004) |
| Study design | Markov model |
| Perspective | Third-party payer (US) |
| Setting | USA |
| Patient population | Base case: patients with moderate to severe OSAS (AHI ≥ 15 events per hour) |
| Results obtained from a weighted average of results stratified by age (25–34, 35–44, 45–54 and 55–64 years) and sex | |
| Time horizon | 5 years |
| Model structure | Four health states:
|
| Events: MI, stroke and RTA | |
| Cycle length: 1 year | |
| Treatment effectiveness and sources | CPAP and dental devices reduce risk of cardiovascular events and RTAs
|
| Resources used and costs and sources | Base case: third-party payer perspective:
|
| HRQoL and sources | Health outcomes expressed in terms of QALYs
|
| Compliance | Assumed that compliance is equal for dental devices and CPAP. McArdle et al.:148 adherence of 84% at 1 year and 68% at 5 years |
| Mortality | US lifetables |
| Adverse events from treatment | None |
| Subgroup analysis | None |
| Discounting | 3% for costs and health outcomes |
| Results |
|
| Assessment of uncertainty | ICER robust to scenarios considered except:
|
| Conclusions | CPAP is more cost-effective than dental devices and conservative management for patients with OSAS |
| Key cost-effectiveness drivers | Gain in HRQoL associated CPAP and dental devices |
| Effect of CPAP and dental devices on RTAs and cardiovascular events | |
| Uncertainties | HRQoL benefits associated with CPAP and dental devices |
| Compliance to therapy | |
| Conflicts of interest | None |
| Study details | Tan et al. (2008)115 |
|---|---|
| Economic evaluation type | Cost–utility analysis |
| Intervention | CPAP |
| Comparator(s) | Conservative management (no treatment) |
| Outcomes | Primary outcome: incremental cost per QALY |
| Currency (year) | Canadian dollar (2005) |
| Study design | Markov model |
| Perspective | Third-party payer (US) and societal |
| Setting | USA |
| Patient population | Base case:
|
| Time horizon | 5 year |
| Model structure | As per Ayas et al.111 |
| Treatment effectiveness and sources | As per Ayas et al.111 |
| The annual probability of RTA in individuals without OSAS was determined using RTA data for 1997 from the Insurance Corporation of British Colombia. These were assumed to apply to patients treated with CPAP | |
| Resources used and costs and sources | Base case: third-party payer perspective:
|
| HRQoL and sources | As per Ayas et al.111 |
| Compliance | As per Ayas et al. 111 |
| Mortality | Canadian lifetables |
| Adverse events from treatment | None included |
| Subgroup analysis | None |
| Discounting | 3% for costs and health outcomes |
| Results | Base case (third-party payer): ICER for CPAP was CAN$3626/QALY Societal: ICER for CPAP was CAN$2979/QALY |
| Assessment of uncertainty | Univariate sensitivity analysis:
|
| Conclusions | CPAP is a cost-effective use of health-care resources from both the health-care payer and the societal perspectives |
| Key cost-effectiveness drivers | HRQoL benefit from CPAP therapy |
| Uncertainties |
|
| Conflicts of interest | None |
| Study details | Guest et al. (2008)112 |
|---|---|
| Economic evaluation type | Cost–utility analysis |
| Intervention | CPAP |
| Comparator(s) | Conservative management (no treatment) |
| Outcomes | Expected costs and QALYs with CPAP and no treatment Expected percentage of patients surviving at 14 years Expected percentage of event-free surviving patients at 14 years |
| Currency (year) | UK pound sterling (2005) |
| Study design | Markov model |
| Perspective | Third-party payer (NHS) |
| Setting | UK |
| Patient population | 55-year-old patient with severe OSAS as defined by an AHI > 30 (events/hour) and daytime sleepiness (ESS score of ≥ 12) |
| Time horizon | 14 years |
| Model structure | Health states:
|
|
|
| Cycle length: 1 year | |
| Patients post stroke can no longer drive | |
| Treatment effectiveness and sources |
|
| Resources used and costs and sources | Third-party perspective: direct health-care costs only:
|
| HRQoL and sources. | Health outcomes expressed in terms of QALYs. HRQoL weights were obtained from a Spanish study reporting EQ-5D values before and after CPAP treatment:113
|
| Compliance | Assumed that 74% of patients are compliant during the first year of treatment, that 3.8% of patients discontinue after second year, and the discontinuation rate declines exponentially over the remaining time horizon |
| Mortality | Not reported other from cardiovascular event, stroke or RTA |
| Adverse events from treatment | None |
| Subgroup analysis | None |
| Discounting | 3.5% for costs and health outcomes |
| Results |
|
| Assessment of uncertainty | Probabilistic sensitivity analysis Threshold analysis:
|
| Conclusions | CPAP is less costly and more effective than conservative management for patients with OSAS after a minimum of 2 years of treatment |
| Key cost-effectiveness drivers | From the results presented, compliance and cardiovascular benefits from CPAP. However, results for alternative assumptions around the HRQoL gain from CPAP were not presented |
| Uncertainties | Benefits from CPAP in terms of HRQoL gain, reduction in cardio/cerebrovascular events and reduction in RTAs Compliance over time |
| Conflicts of interest | The study was sponsored by ResMed (UK) Ltd, manufacturers of a CPAP device |
| Study details | Gander et al. (2010)116 |
|---|---|
| Economic evaluation type | Combination of cost of illness, cost–benefit and cost–utility analysis |
| Intervention | CPAP |
| Comparator(s) | No treatment |
| Outcomes | Total costs, costs per category |
| Incremental costs per QALY | |
| Currency (year) | New Zealand dollar (not reported) |
| Study design | Decision tree |
| Perspective | Societal |
| Setting | New Zealand |
| Patient population | Patients with OSAS aged between 30 and 59 years. Prevalence obtained from the population in the Wellington region was 5.61% (95% CI 2.62% to 8.60%) |
| Time horizon | 1 year |
| Model structure | Decision tree starting at when patients develop symptoms:
|
| Treatment effectiveness and sources |
|
|
|
|
|
| Resources used and costs and sources | Costs were categorised as direct health-care, direct non-health care, indirect and intangible costs:
|
| HRQoL and sources | Cost–utility analysis assumes a per case QALY gain of 5.4 (0.10 to 8.00) and that 20% of patients are treated |
| The source of QALY gain is Sleep Alliance and Tousignant et al. (1994) | |
| Compliance | Not considered |
| Mortality | Not considered |
| Adverse events from treatment | None |
| Subgroup analysis | None |
| Discounting | None required (time horizon = 1 year) |
| Results |
|
| Assessment of uncertainty | Monte Carlo simulations to obtain 95% CIs |
| Conclusions | Treating OSAS is a cost-effective use of resources |
| Key cost-effectiveness drivers | OSAS prevalence is a key determinant of total costs |
| Uncertainties | Generalisability of input parameters, mostly referring to a severe population, to the overall OSAS population |
| Conflicts of interest | None |
| Study details | Mar et al. (2003)113 |
|---|---|
| Economic evaluation type | Cost–utility analysis |
| Intervention | Nasal CPAP |
| Comparator(s) | No treatment |
| Outcomes | Incremental costs per QALY |
| Currency (year) | Euro (2000) |
| Study design | Markov model |
| Perspective | Third-party payer |
| Setting | Spain |
| Patient population | 50-year-old male patient with moderate to severe OSAS, defined by an AHI > 30 (events/hour) and ESS score of > 10 |
| Time horizon | 5 years and lifetime |
| Model structure | Four health states:
|
Three events:
|
|
| Cycle length of 1 year | |
| Treatment effectiveness and sources | Patients with untreated OSAS are at an increased risk of stroke, CHD and RTA. Patients with treated OSAS are at the same risk as the general population For the calculation of relative risks for CHD and stroke, authors assumed that patients with AHI > 30 have an increase in diastolic arterial pressure of 3.6 mmHg, based on Young et al.203 and applying the relationship presented by MacMahon et al.204
|
| Resources used and costs and sources | Costs included were those of diagnosis and treatment of OSAS and the costs attributable to cardiovascular morbidity |
| HRQoL and sources. | HRQoL with and without treatment:
|
| Compliance | 10% dropout rate from treatment in the first year. This had an impact on costs but not on health outcomes |
| Mortality | Mortality from events
|
| Adverse events from treatment | Not considered |
| Subgroup analysis | None |
| Discounting | 3% for both costs and effects |
| Results |
|
| Assessment of uncertainty | Univariate sensitivity analysis:
|
| Conclusions | Treatment of OSAS with nasal CPAP is a cost-effective use of resources The key clinical benefit from treatment is the improvement in HRQoL, which is also where the evidence base is strongest |
| Key cost-effectiveness drivers | HRQoL benefit from treatment |
| Uncertainties | Generalisability to patients with less severe OSAS, since estimates of effectiveness and HRQoL benefit were obtained from patients with severe OSAS Treatment effectiveness and HRQoL benefit, owing to the observational nature of the data used |
| Conflicts of interest | None |
| Study details | Chilcott et al. (2000)118 – from McDaid et al. (2009)20 |
|---|---|
| Economic evaluation type | Cost–utility analysis |
| Intervention | Nasal CPAP |
| Comparator(s) | No treatment |
| Outcomes | Incremental costs per QALY |
| Currency (year) | UK Pound sterling (not reported) |
| Study design | Review synthesis. Effectiveness estimates based on results from two before-and-after studies |
| Perspective | Third-party payer |
| Setting | UK |
| Patient population | Patients referred to a sleep clinic. Typically middle-aged |
| Time horizon | 5 years |
| Model structure | Not applicable |
| Treatment effectiveness and sources | Three studies12 using before and after initiation of CPAP |
| Resources used and costs and sources | Resources related with investigation, diagnostic, treatment and follow-up. Unit costs and resource use reported separately |
| HRQoL and sources. | Health outcomes expressed in terms of QALYs The gain in HRQoL was 0.12 QALYs (95% CI 0.09 to 0.16 QALYs) over 1 year HRQoL values generated via SF-36 survey using the Brazier et al.121 algorithm. Societal preferences were applied using time trade-off and standard gamble |
| Compliance | 10% dropout rate from treatment in the first year. This had an impact on costs but not on health outcomes |
| Mortality | – |
| Adverse events | – |
| Subgroup analysis | None |
| Discounting | 6% for costs and 1.5% for health benefits |
| Results | CPAP was estimated to be more costly and more effective than no treatment. The ICER for CPAP at 1 year was £8300/QALY and at year 5 was £3200/QALY Small differences in clinical effectiveness and cost were found when comparing CPAP with dental devices but these were not explicitly quantified |
| Assessment of uncertainty | Univariate sensitivity analysis for impact of: time horizon, costs of investigation for CPAP, long term costs of maintenance, follow-up and other health-care costs, improved mortality, improved morbidity and discount rate |
| Conclusions | Treatment with CPAP was found to be as cost-effective as other commonly funded treatments. The results for CPAP over dental devices were likely to be highly uncertain |
| Key cost-effectiveness drivers | – |
| Uncertainties | ICER was sensitive to time horizon |
| Conflicts of interest | – |
| Study details | Tousignant et al. (1994)119 |
|---|---|
| Economic evaluation type | Cost–utility analysis |
| Intervention | Nasal CPAP |
| Comparator(s) | No treatment |
| Outcomes | Incremental costs per QALY |
| Currency (year) | Canadian dollars (1989?) |
| Study design | Cross-sectional study |
| Perspective | Third-party payer |
| Setting | UK |
| Patient population | Patients attending a hospital sleep clinic (n = 19, mean age 57 years, SE = 10 years) and who had been receiving CPAP treatment for an average of 9 months |
| Time horizon | Lifetime |
| Model structure | Not applicable |
| Treatment effectiveness and sources | CPAP improves HRQoL. Improvement in HRQoL expressed in utility, measured with standard gamble, and QALYs |
| Resources used and costs and sources | Third-party perspective:
|
| HRQoL and sources. | Health benefits expressed in terms of QALYs HRQoL weights obtained directly from patients via standard gamble The mean utility score for the pre-treatment state was 0.63 (± 0.29) and for the CPAP-treated state was 0.87 (± 0.17) |
| Compliance | Not considered |
| Mortality | Patient life expectancy estimated using Canadian lifetables |
| Adverse events | Not considered |
| Subgroup analysis | None |
| Discounting | 5% costs and health benefits |
| Results | High estimate: ICER = CAN$279/QALY Low estimate: ICER = CAN$3523/QALY Analysis without three outliers: ICER +CAN$18,637/QALY |
| Assessment of uncertainty | Scenario analysis: alternative estimate of CPAP costs and excluding three outliers with high treatment effects |
| Conclusions | CPAP is likely to be a cost-effective intervention in the Canadian context |
| Key cost-effectiveness drivers | Not considered |
| Uncertainties | Costs of CPAP; impact of outliers in the estimate of the improvement in HRQoL |
| Conflicts of interest | None |
Strategy to handle missing data
The strategy to handle missing data for the within-trial cost-effectiveness analysis followed three stages as proposed by Faria et al. :208 (1) descriptive analysis of the missing data to inform the assumption on the missing data mechanism, (2) choice of strategy to handle missing data in accordance with the assumption made for the missing data mechanism and (3) sensitivity analysis.
Descriptive analysis of the missing data
Amount of missing data by trial group at each follow-up period
The percentage of patients with missing data on costs or health utility values was 62.2% (n = 173); that is only 37.8% (n = 105) of patients have complete data on costs or health utility values. The percentage of patients who answered the EQ-5D questionnaire at each month varied between 100% (CPAP group at baseline) and 65.71% (CPAP group at months 10 and 11). The percentage of returned questionnaires on resource use is similar across treatment groups and varied between 100% and 66%.
Missing data patterns
Figures 22–24 show the missing data pattern for EQ-5D, SF-6D and costs at each month. The pattern of missing data is non-monotonic; that is patients with missing data on one month may have complete data on a subsequent month. The pattern of missing data is different across the different months. Given that costs and QALYs are cumulative quantities, missing data at one time point has an impact in the quantities estimated for the entire trial duration.
FIGURE 22.
Missing EQ-5D data.
FIGURE 23.
Missing SF-6D data before.
FIGURE 24.
Missing data on costs.

Association between missingness and baseline variables
Association between missingness and baseline variables
Logistic regressions were conducted to establish whether or not baseline variables were predictors of missingness at 1%, 5% and 10% of statistical significance. The statistically significant baseline variables for missing costs were HbA1c (p < 0.01), heart rate (p < 0.05), SF-6D (p < 0.1), total cholesterol (p < 0.1) and triglycerides (p < 0.1). The statistically significant baseline variables for missing EQ-5D values were HbA1c (p < 0.01), health rate (p < 0.05), sex (p < 0.05) and ESS score (p < 0.1).
Association between missingness and observed outcomes
Logistic regressions were conducted to establish whether or not missing costs or health utility values at month 3 (which were collected in a clinic visit rather than by postal questionnaire) were predictors of missingness in subsequent months. Costs and health utility values at month 3 were statistically significant predictors of subsequent missingness of costs at month 4 (p < 0.1), month 8 (p < 0.05) and month 11 (p < 0.05) and predictors of missingness of EQ-5D at month 4 (p < 0.1), month 8 (p < 0.1) and month 11 (p < 0.1).
These analyses suggest that some baseline variables and some observed costs and EQ-5D values may be predictors of missingness of unobserved costs and health utility values. Therefore, data are unlikely to be missing completely at random. The base-case assumption is that data are missing at random and depend on baseline variables as well as observed costs and EQ-5D values. Given that it is impossible to know whether or not data are missing not at random from the observed data, sensitivity analysis tests the impact of departures from the missing at random assumption.
Choice of method to handle missing data
There are two broad types of methods that fit with the assumption that data are missing at random: multiple imputation and likelihood-based methods. Multiple imputation with chained equations was chosen because it is straightforward to implement and can deal with the non-normal distributions of costs and health utility values.
A number of imputation models were tested and their performance assessed by comparing the distribution of imputed with observed values. The model that produced the most similar distribution used linear regression to impute missing health utilities and log-transformed costs at each month over 63 imputations with predictive mean matching. The explanatory variables were the observed log-transformed costs and health utilities at each month, cardiovascular variables at baseline (angina, hypertension, previous heart attack, diabetes, atrial fibrillation, health rate), prognostic factors at baseline (smoking status, age, sex, blood glucose, HbA1c, total cholesterol, triglycerides) and ESS score at each month.
Figures 25 and 26 compare the distribution of QALYs and total costs before (_mj = 0) and in the imputed data sets (_mj = 1 to 10). Only the first 10 multiple-imputed data sets are shown here. The distribution of QALYs and total costs is similar between the original and multiple-imputed data sets.
FIGURE 25.
Distribution of QALYs post imputation.
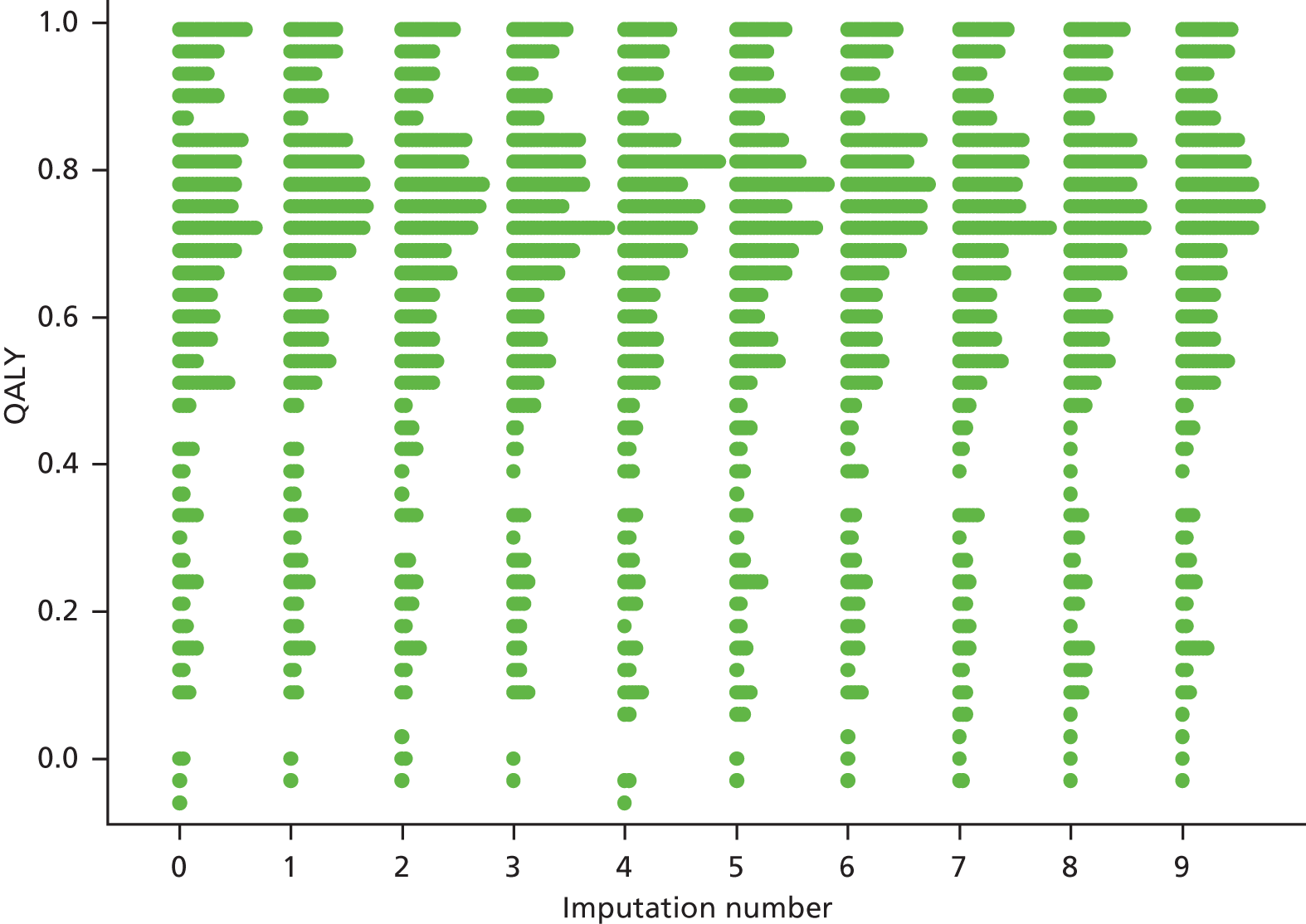
FIGURE 26.
Distribution of total costs post imputation.

Table 59 compares the average costs per patient for each month between the original and multiple-imputed data sets. The average costs are similar between the data sets.
| Time period | CPAP with BSC | BSC alone | ||||||
|---|---|---|---|---|---|---|---|---|
| Original | Imputed | Original | Imputed | |||||
| Mean (£) | SD (£) | Mean (£) | SD (£) | Mean (£) | SD (£) | Mean (£) | SD (£) | |
| Month 1 | 79 | 159 | 84 | 166 | 116 | 218 | 125 | 231 |
| Month 2 | 94 | 323 | 100 | 319 | 88 | 157 | 107 | 234 |
| Month 3 | 90 | 223 | 97 | 239 | 111 | 253 | 120 | 269 |
| Month 4 | 66 | 153 | 80 | 213 | 130 | 423 | 134 | 393 |
| Month 5 | 71 | 135 | 74 | 150 | 95 | 216 | 100 | 213 |
| Month 6 | 85 | 168 | 94 | 190 | 97 | 217 | 105 | 223 |
| Month 7 | 82 | 159 | 90 | 173 | 84 | 187 | 88 | 188 |
| Month 8 | 71 | 170 | 84 | 212 | 117 | 292 | 122 | 296 |
| Month 9 | 82 | 137 | 95 | 169 | 96 | 182 | 101 | 188 |
| Month 10 | 94 | 223 | 107 | 273 | 118 | 401 | 118 | 375 |
| Month 11 | 80 | 143 | 93 | 175 | 109 | 221 | 115 | 226 |
| Month 12 | 156 | 545 | 165 | 571 | 150 | 498 | 153 | 506 |
| Total | 1050 | 1163 | 1311 | 1389 | ||||
Table 60 compares the average EQ-5D and SF-6D per patient for each follow-up questionnaire between the original and multiple-imputed data sets. The point estimates and SDs are similar between original and imputed data sets.
| Item | CPAP with BSC | BSC alone | ||||||
|---|---|---|---|---|---|---|---|---|
| Original | Imputed | Original | Imputed | |||||
| EQ-5D | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Month 1 | 0.684 | 0.280 | 0.688 | 0.270 | 0.687 | 0.246 | 0.676 | 0.255 |
| Month 2 | 0.700 | 0.292 | 0.699 | 0.289 | 0.685 | 0.258 | 0.680 | 0.257 |
| Month 3 | 0.672 | 0.301 | 0.667 | 0.300 | 0.704 | 0.251 | 0.699 | 0.255 |
| Month 4 | 0.692 | 0.284 | 0.691 | 0.279 | 0.679 | 0.259 | 0.677 | 0.257 |
| Month 5 | 0.671 | 0.328 | 0.674 | 0.314 | 0.660 | 0.267 | 0.659 | 0.270 |
| Month 6 | 0.677 | 0.295 | 0.662 | 0.295 | 0.663 | 0.255 | 0.644 | 0.272 |
| Month 7 | 0.687 | 0.276 | 0.698 | 0.266 | 0.652 | 0.271 | 0.638 | 0.272 |
| Month 8 | 0.668 | 0.311 | 0.655 | 0.300 | 0.683 | 0.268 | 0.660 | 0.284 |
| Month 9 | 0.682 | 0.287 | 0.693 | 0.274 | 0.650 | 0.275 | 0.647 | 0.277 |
| Month 10 | 0.647 | 0.318 | 0.660 | 0.295 | 0.694 | 0.254 | 0.672 | 0.273 |
| Month 11 | 0.656 | 0.310 | 0.673 | 0.293 | 0.647 | 0.286 | 0.639 | 0.299 |
| Month 12 | 0.689 | 0.301 | 0.693 | 0.291 | 0.680 | 0.264 | 0.684 | 0.260 |
| SF-6D | ||||||||
| Month 3 | 0.681 | 0.087 | 0.679 | 0.087 | 0.661 | 0.088 | 0.662 | 0.087 |
| Month 12 | 0.679 | 0.111 | 0.681 | 0.107 | 0.653 | 0.113 | 0.654 | 0.115 |
Sensitivity analysis to departures from missing at random assumption
Sensitivity analysis was conducted to the missing at random assumption (see next section for more details) by analysing the data as (1) complete-case analysis (assuming missing completely at random), (2) imputing data with mean interpolation and (3) assuming that individuals with missing data have 25% greater costs or 25% lower health utility values than what is predicted by the multiple imputation procedure (assuming missing not at random). The results are presented in the next section.
Detailed results for the sensitivity analysis
Scenario 1: frequent replacement of continuous positive airway pressure device and consumables
The base case makes a number of assumptions on the lifetime of the devices and the frequency that consumables, such as filters and masks, need replacing. However, there is some uncertainty around these parameters. The device or the consumables may need replacing earlier, which will drive up the costs. Therefore, the sensitivity analysis explores the impact of a worst-case scenario for costs, in which the lifetime of the CPAP device and humidifier is assumed to be 3 years, the masks are replaced every 3 months and the filters replaced monthly. This assumption corresponds to the maximum yearly CPAP supplies reimbursed by Medicare, a large US health insurer. 136 Table 61 presents the costs of CPAP treatment for the worst-case scenario. The costs of CPAP treatment increased from £201.14 in the base-case scenario to £608.94 in the worst-case scenario, mostly as a result of the increased number of masks.
| Item | Cost element | Number | Average cost per patient (£) |
|---|---|---|---|
| A | Annual equivalent cost of CPAP device | – | 153.48 |
| B | Annual equivalent cost of humidifier | – | 58.89 |
| C | Number (and proportion) of patients who received a humidifier | 82 (59%) | – |
| D | Average annual equivalent cost of humidifier per patient (= B × C) | – | 34.50 |
| E | Average annual equivalent cost per patient (= A + D) | – | 197.98 |
| F | Average cost of masks | – | 104 |
| G | Average cost of masks assuming (patients received four masks) (= 4 × F) | – | 414 |
| H | Average cost per filter | – | 0.58 |
| I | Average cost of filters per patient per year (12 filters per year) (= 12 × H) | – | 6.96 |
| Average cost of CPAP treatment per patient (= E + G + I) | – | 608.94 |
Table 62 presents the cost-effectiveness results for scenario 1. Increasing the costs of CPAP from £201.14 to £608.94 per patient reverses the difference in costs from –£35 (95% CI –£390 to £321) to £373 (95% CI £17 to £729). The ICER is £74,600 per EQ-5D QALY and £20,722 per SF-6D QALY.
| Intervention | Costs | EQ-5D QALYs | SF-6D QALYs | |||
|---|---|---|---|---|---|---|
| Average | SE | Average | SE | Average | SE | |
| CPAP with BSC | £1771 | £123 | 0.680 | 0.021 | 0.678 | 0.007 |
| BSC alone | £1389 | £139 | 0.666 | 0.020 | 0.658 | 0.008 |
| Incremental costs and QALYs | ||||||
| CPAP with BSC – BSC alone | £373 | £180 | 0.005 | 0.020 | 0.018 | 0.008 |
Figure 27 presents the cost-effectiveness acceptability curve for the scenario 1. The probability that the CPAP is cost-effective is considerably lower than in the base-case analysis; it reduced from 0.61 to 0.25 for the base case with EQ-5D QALYs and from 0.96 to 0.46 for the base case with SF-6D QALYs. These results suggest that the greater costs associated with the CPAP treatment itself may deem the intervention not cost-effective.
FIGURE 27.
Cost-effectiveness acceptability curve for scenario 1.

Scenario 2: continuous positive airway pressure is used for 1 year
The base case assumes that the CPAP and humidifier devices have a lifetime of 7 years and can be reused across patients. Scenario 2 assumes that the CPAP and humidifier devices are used for 1 year only. Therefore, their costs are not annuitised and all the costs of CPAP treatment are incurred in the 1 year. Table 63 shows the costs of CPAP treatment for this scenario. The cost of CPAP treatment increased from £201.14 in the base case to £710.16 in scenario 2.
| Item | Cost element | Number | Average cost per patient (£) |
|---|---|---|---|
| A | Cost of CPAP device | – | 430 |
| B | Cost of humidifier | – | 165 |
| C | Number (and proportion) of patients who received a humidifier | 82 (59%) | – |
| D | Average cost of humidifier per patient ( = B × C) | – | 96.64 |
| E | Average cost per patient ( = A + D) | – | 595 |
| F | Average cost of masks | – | 104 |
| G | Average cost of masks assuming (10% received 2 masks) ( = 1.1 × H) | – | 114 |
| H | Average cost per filter | – | 0.58 |
| I | Average cost of filters per patient per year (2 filters per year) ( = 2 × H) | – | 1.16 |
| Average cost of CPAP treatment per patient ( = E +G + I) | – | 710.16 |
Table 64 presents a summary of the cost-effectiveness results for scenario 2 (see Appendix 3 for detailed results). As with scenario 1, the difference in costs between treatment groups is reduced because of the increase in the costs of CPAP to £474 (95% CI £119 to £830). The ICER is £94,800 per EQ-5D QALYs and £26,333 per SF-6D QALY gained.
| Intervention | Costs (£) | EQ-5D QALYs | SF-6D QALYs | |||
|---|---|---|---|---|---|---|
| Average | SE | Average | SE | Average | SE | |
| CPAP with BSC | 1873 | 124 | 0.680 | 0.021 | 0.678 | 0.007 |
| BSC alone | 1389 | 139 | 0.666 | 0.020 | 0.658 | 0.008 |
| Incremental costs and QALYs | ||||||
| CPAP with BSC – BSC alone | 474 | 180 | 0.005 | 0.020 | 0.018 | 0.008 |
Figure 28 presents the cost-effectiveness acceptability curve for the scenario 2, 1-year time horizon. The probability that the intervention is cost-effective is 0.20 for the base case (EQ-5D QALYs) and 0.30 for the scenario with SF-6D QALYs.
FIGURE 28.
Cost-effectiveness acceptability curve for scenario 2.
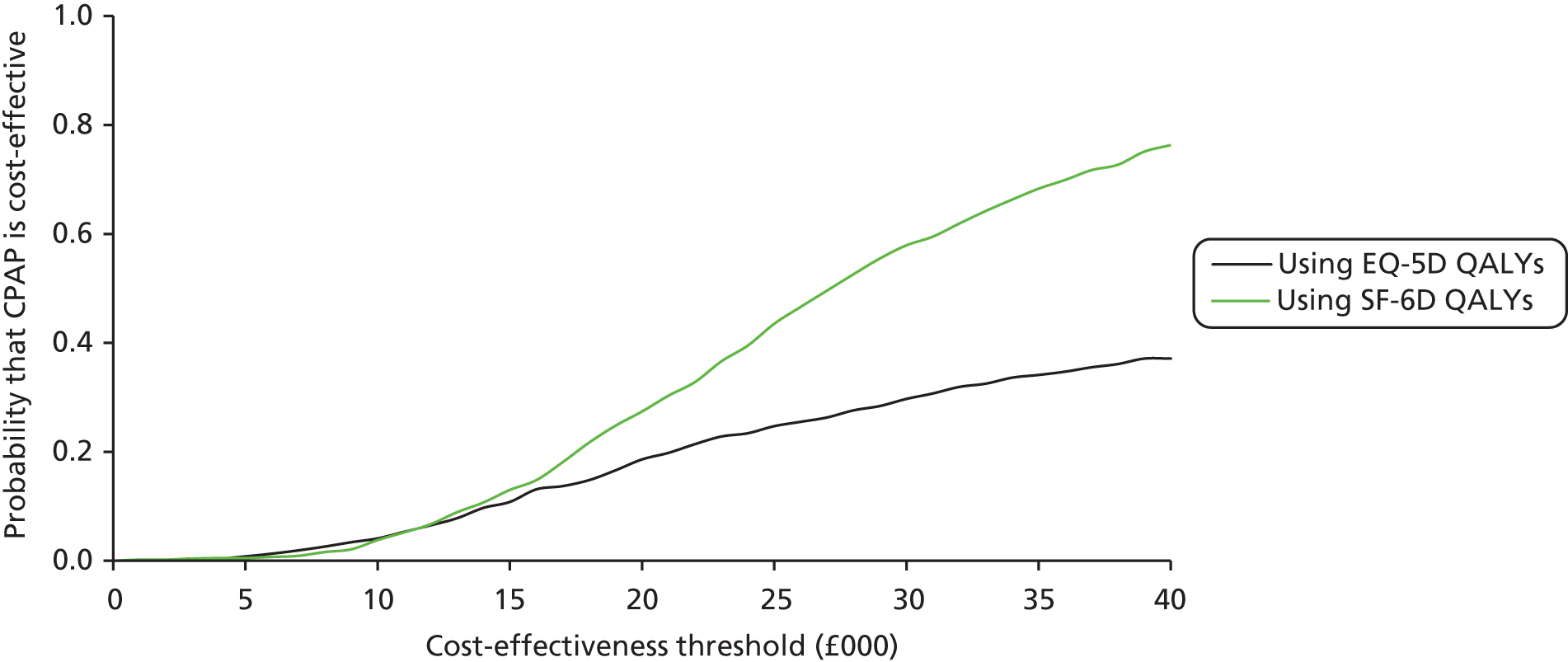
Scenario 3: complete-case analysis
This scenario uses only the data from patients who completed all questionnaires on HRQoL and resource use (for costs). This assumes that data are missing completely at random, that is that the probability that data are missing is independent of both the observed and unobserved data. Therefore, the patients who completed all questionnaires are assumed to be representative of all patients. Complete-case analysis will result in biased estimates of the effect of the intervention on costs and QALYs if data are not missing completely at random. In addition, the estimates will be inefficient because not all data are used, as patients who returned some of the questionnaires are removed from the analysis. The complete-case sample for the scenario with EQ-5D QALYs consists of 59 patients (43%) allocated to BSC alone and 48 (34%) allocated to CPAP with BSC. The complete-case sample for the scenario with SF-6D QALYs consists of 61 patients (44%) allocated to BSC alone and 52 patients (37%) allocated to CPAP with BSC.
Table 65 presents the cost-effectiveness results for the complete-case scenario. The cost-effectiveness results are broadly similar to the base case. CPAP appears to be cost-saving but the difference is not statistically significant (complete-case scenario with EQ-5D QALYs, 95% CI –£769 to £252; SF-6D QALYs, 95% CI –£690 to £288). The group allocated to CPAP experienced more QALYs but the difference is not statistically significant (complete-case scenario with EQ-5D QALYs, 95% CI –0.048 to 0.067; SF-6D QALYs, –0.010 to 0.040). As a result, CPAP with BSC dominates BSC alone.
| Intervention | Costs (£) | QALYs | ||
|---|---|---|---|---|
| Average | SD | Average | SD | |
| Complete case for EQ-5D | ||||
| CPAP with BSC | 1146 | 1170 | 0.686 | 0.278 |
| BSC alone | 1382 | 1499 | 0.698 | 0.233 |
| Incremental costs and QALYs | ||||
| CPAP with BSC – BSC alone | –258 | 258 | 0.010 | 0.029 |
| Complete case for SF-6D | ||||
| CPAP with BSC | 1169 | 1151 | 0.673 | 0.097 |
| BSC alone | 1354 | 1481 | 0.670 | 0.093 |
| Incremental costs and QALYs | ||||
| CPAP with BSC – BSC alone | –201.04 | 247 | 0.015 | 0.013 |
Figure 29 shows the cost-effectiveness acceptability curve for the complete-case scenario. CPAP appears highly likely to be cost-effective over a range of cost-effectiveness thresholds. The probability that the intervention is cost-effective at a threshold of £20,000 per QALY gained is 0.75 for the scenario with EQ-5D QALYs and 0.91 for the scenario with SF-6D QALYs.
FIGURE 29.
Cost-effectiveness acceptability curve for complete-case scenario.

Scenario 4: missing data imputed with interpolation
In this scenario, missing data on HRQoL and on costs are imputed with mean interpolation, that is missing data are imputed with the average observed data for each patient. This assumes that the probability that data are missing depends on the observed data but not on the unobserved values (missing at random assumption). This method has two major limitations that support its use only as a sensitivity analysis: first, it artificially reduces uncertainty by treating unobserved values as observed data, and second, it assumes the observed costs and HRQoL are representative of the unobserved data without taking into consideration other covariates, such as age or comorbidities. In addition, this method requires additional assumptions if only the baseline data were observed for some patients. Last value carried forward was conducted when no other data were observed in addition to baseline.
Table 66 shows the cost-effectiveness results for scenario 3. The results are generally similar to the base case. CPAP is associated with a small increase in costs but the difference is not statistically significant. The 95% CI is similar to that obtained for the base case (base case, 95% CI –£390 to £321; scenario 4, 95% CI –£353 to £440). In terms of QALYs, CPAP appears to increase QALYs by a small amount for both the EQ-5D and the SF-6D valuations; this difference is statistically significant for SF-6D QALYs (95% CI 0.001 to 0.028) but not for EQ-5D QALYs (95% CI –0.029 to 0.041). As a result, CPAP is associated with an ICER of £7167 per EQ-5D QALY gained and £3071 per SF-6D QALY gained.
| Intervention | Costs (£) | EQ-5D QALYs | SF-6D QALYs | |||
|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | |
| CPAP with BSC | 1421 | 1806 | 0.678 | 0.253 | 0.675 | 0.081 |
| BSC alone | 1366 | 1697 | 0.662 | 0.229 | 0.659 | 0.087 |
| Incremental costs and QALYs | ||||||
| CPAP with BSC – BSC alone | 43 | 201 | 0.006 | 0.018 | 0.014 | 0.007 |
Figure 30 presents the cost-effectiveness acceptability curve for scenario 4. The probability that the intervention is cost-effective at £20,000 per QALY gained is 0.55 for the base case with EQ-5D QALYs and 0.81 for the scenario with SF-6D QALYs.
FIGURE 30.
Cost-effectiveness acceptability curve for scenario 4.
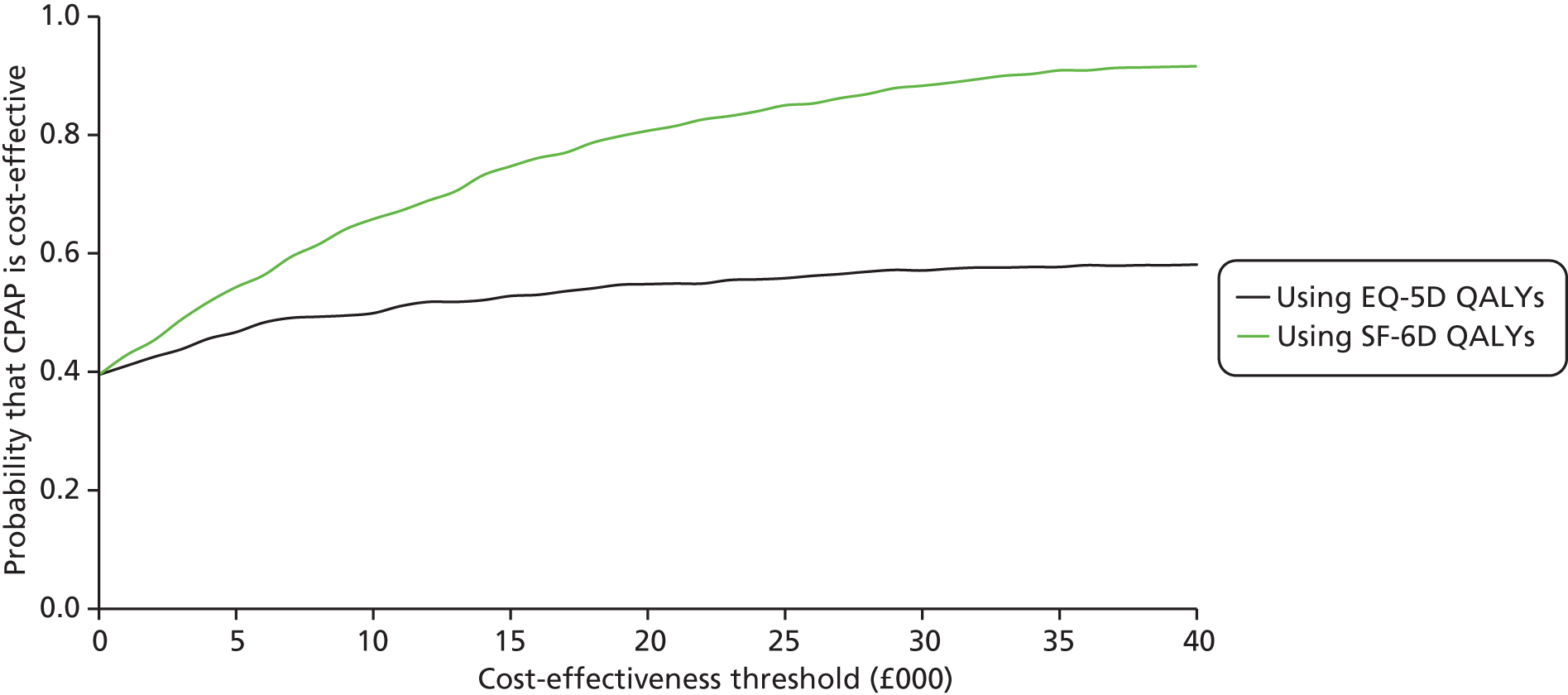
Scenario 5: testing the missing at random assumption
This scenario explores the robustness of the results to the assumption underpinning multiple imputation that the data are missing at random. The multiple-imputation procedure predicts the unobserved data from the data which were observed, assuming that the observed are representative of the unobserved data conditional on covariates. However, the costs or the HRQoL of individuals with missing data may systematically differ from that of individuals without missing data. For example, individuals lost to follow-up may have experienced fewer health benefits than those who remained in the study. It is impossible to know for certain whether or not the costs and QALYs individuals with missing data are similar to those of individuals with complete data. However, sensitivity analysis can explore the impact on the cost-effectiveness results of assuming that individuals with missing data have greater costs or lower HRQoL than that predicted by the multiple-imputation model.
Table 67 shows the results for scenario 5. The results are consistent with those of the base case. Increasing the predicted costs of the individuals with missing data by 25% results in a similar difference in costs between treatment groups as in the base case [–£26 (95% CI –£422 to £369) vs. base case –£35 (95% CI –£390 to £321)]. Therefore, CPAP dominates. Reducing the predicted HRQoL of the individuals with missing data by 25% changes the direction of effect for EQ-5D QALYs [–0.006 (95% CI –0.044 to 0.031 QALYs)] but not for SF-6D QALYs [0.012 (95% CI –0.006 to 0.030 QALYs)]. Nonetheless, these differences, as in the base case, are not statistically significant. The ICER for the EQ-5D analysis is £5833 per QALY gained. CPAP dominates BSC in the analysis with SF-6D QALYs.
| Assumption | Costs (£) | EQ-5D QALYs | SF-6D QALYs | |||
|---|---|---|---|---|---|---|
| Average | SE | Average | SE | Average | SE | |
| Patients with missing data have 25% greater costs | ||||||
| CPAP with BSC | 1462 | 140 | 0.680 | 0.021 | 0.678 | 0.007 |
| BSC alone | 1479 | 150 | 0.666 | 0.020 | 0.658 | 0.008 |
| Incremental costs and QALYs | ||||||
| CPAP with BSC – BSC alone | –27 | 201 | 0.005 | 0.020 | 0.018 | 0.008 |
| Patients with missing data have 25% less HRQoL | ||||||
| CPAP with BSC | 1462 | 140 | 0.636 | 0.021 | 0.655 | 0.008 |
| BSC alone | 1479 | 150 | 0.633 | 0.019 | 0.642 | 0.008 |
| Incremental costs and QALYs | ||||||
| CPAP with BSC – BSC alone | –35 | 180 | –0.006 | 0.019 | 0.012 | 0.009 |
Figure 31 shows the cost-effectiveness acceptability curves for scenario 5. In the scenario in which costs are increased, the probability that CPAP is cost-effective at £20,000 per QALY gained is 0.62 for the analysis with EQ-5D QALYs and 0.96 for the analysis with SF-6D QALYs. This is similar to the base case at 0.61 for EQ-5D QALYs and 0.96 for SF-6D QALYs. In the scenario in which HRQoL is decreased, the probability that CPAP is cost-effective at £20,000 per QALY gained is 0.42 for the analysis with EQ-5D QALYs and 0.85 for the analysis with SF-6D QALYs.
FIGURE 31.
Cost-effectiveness acceptability curves for scenario 5.
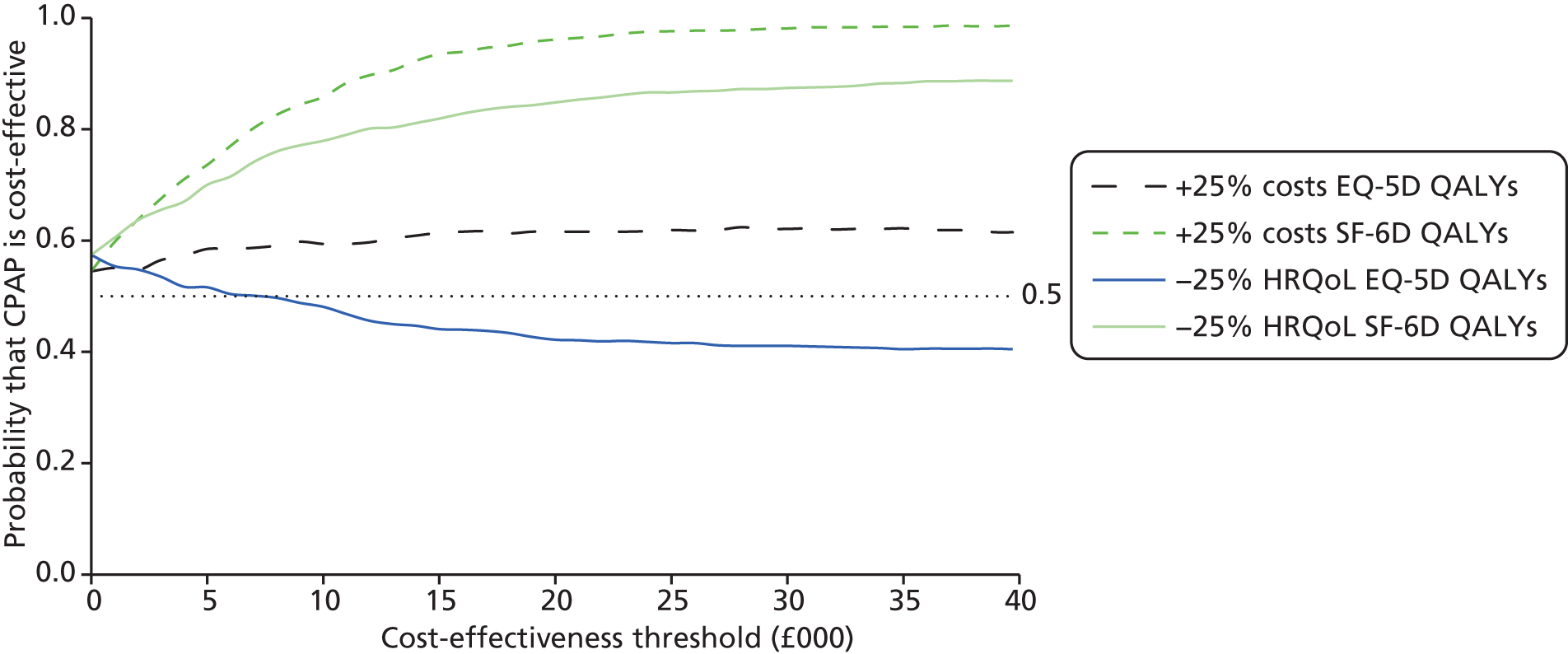
Appendix 4 Search strategies
Searches for systematic reviews and guidelines
Cochrane Database of Systematic Reviews
Searched 28 March 2012.
URL: http://onlinelibrary.wiley.com
Search strategy
#1. MeSH descriptor Sleep Apnea Syndromes explode all trees (1043)
#2. (apnea or apnoea):ti,ab (2393)
#3. (hypopnea or hypopnoea):ti,ab (612)
#4. (hypoapnea or hypoapnoea):ti,ab (2)
#5. (sahs or shs or osas or osa):ti,ab (770)
#6. (#1 OR #2 OR #3 OR #4 OR #5) (2635)
#7. MeSH descriptor Positive-Pressure Respiration explode all trees (1691)
#8. (cpap or apap or ncpap or autocpap or auto-cpap):ti,ab (1278)
#9. (positive near3 airway near3 pressure):ti,ab (1185)
#10. (#7 OR #8 OR #9) (2538)
#11. (#6 AND #10), from 2006 to 2012 (448)
Of 448 total results in Cochrane Library, nine were from the Cochrane Database of Systematic Reviews 2006 onwards.
Database of Abstracts of Reviews of Effects
Searched 28 March 2012.
URL: http://onlinelibrary.wiley.com
Search strategy
#1. MeSH descriptor Sleep Apnea Syndromes explode all trees (1043)
#2. (apnea or apnoea):ti,ab (2393)
#3. (hypopnea or hypopnoea):ti,ab (612)
#4. (hypoapnea or hypoapnoea):ti,ab (2)
#5. (sahs or shs or osas or osa):ti,ab (770)
#6. (#1 OR #2 OR #3 OR #4 OR #5) (2635)
#7. MeSH descriptor Positive-Pressure Respiration explode all trees (1691)
#8. (cpap or apap or ncpap or autocpap or auto-cpap):ti,ab (1278)
#9. (positive near3 airway near3 pressure):ti,ab (1185)
#10. (#7 OR #8 OR #9) (2538)
#11. (#6 AND #10), from 2006 to 2012 (448)
Of 448 total results in Cochrane Library, 12 were from DARE.
Health Technology Assessment Database
Searched 28 March 2012.
URL: http://onlinelibrary.wiley.com
Search strategy
#1. MeSH descriptor Sleep Apnea Syndromes explode all trees (1043)
#2. (apnea or apnoea):ti,ab (2393)
#3. (hypopnea or hypopnoea):ti,ab (612)
#4. (hypoapnea or hypoapnoea):ti,ab (2)
#5. (sahs or shs or osas or osa):ti,ab (770)
#6. (#1 OR #2 OR #3 OR #4 OR #5) (2635)
#7. MeSH descriptor Positive-Pressure Respiration explode all trees (1691)
#8. (cpap or apap or ncpap or autocpap or auto-cpap):ti,ab (1278)
#9. (positive near3 airway near3 pressure):ti,ab (1185)
#10. (#7 OR #8 OR #9) (2538)
#11. (#6 AND #10), from 2006 to 2012 (448)
Of 448 total results in Cochrane Library, seven were from HTA.
Scottish Intercollegiate Guidelines
Searched 28 March 2012.
URL: www.sign.ac.uk
Search strategy
List of guidelines checked – last update to Sleep Apnea Guideline was 2003.
National Guideline Clearinghouse
Searched 28 March 2012.
URL: www.guideline.gov/search/advanced-search.aspx
Search strategy
apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea or sahs or shs or osas or osa
Limited to 2006, 2007, 2008, 2009, 2010 and 2011.
There were 67 results online which needed to be screened manually, as they could not be downloaded.
Health Services/Technology Assessment Text
Searched 28 March 2012.
URL: www.ncbi.nlm.nih.gov/books/advanced
Search strategy
apnea OR apnoea OR hypopnea OR hypopnea OR hypopnea OR hypopnea
Results screened and details of one 2011 AHRQ guideline added to Endnote library.
Turning Research into Practice Database
Searched 28 March 2012.
URL: www.tripdatabase.com
Search strategy
(title:(apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea) AND (cpap or apap or ncpap or autocpap)) from:2006
Three guideline results screened online – all identified by Clinical Evidence search so not downloaded.
Clinical Evidence
Searched 28 March 2012.
URL: http://clinicalevidence.bmj.com
There were 12 post 2006 guidelines on sleep apnea identified. These could not be downloaded; therefore, a list with links was copied to word document ‘Guidelines from Clinical Evidence search.docx’.
Searches for trials
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>
Searched 19 March 2012 via Ovid.
Search strategy
-
exp Sleep Apnea Syndromes/ (19,930)
-
(apnea or apnoea).ti,ab. (25,874)
-
(hypopnea or hypopnoea).ti,ab. (4789)
-
(hypoapnea or hypoapnoea).ti,ab. (36)
-
sleep disordered breathing.ti,ab. (2989)
-
(sleep adj2 respirat$ disorder$).ti,ab. (201)
-
sahs.ti,ab. (338)
-
shs.ti,ab. (971)
-
osa.ti,ab. (4692)
-
osas.ti,ab. (2314)
-
osahs.ti,ab. (651)
-
or/1-11 (32,846)
-
exp positive-pressure respiration/ (18,367)
-
(positive adj3 airway adj3 pressure).ti,ab. (5712)
-
(cpap or ncpap or apap or bipap).ti,ab. (5975)
-
(c pap or bi pap or nc pap).ti,ab. (50)
-
autocpap.ti,ab. (19)
-
or/13-17 (21,531)
-
12 and 18 (5267)
-
limit 19 to yr="2006 - 2012" (2333)
-
randomized controlled trial.pt. (322,037)
-
controlled clinical trial.pt. (83,702)
-
randomized.ab. (237,867)
-
placebo.ab. (133,799)
-
drug therapy.fs. (1,509,972)
-
randomly.ab. (174,912)
-
trial.ab. (245,654)
-
groups.ab. (1,145,620)
-
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 (2,887,984)
-
20 and 29 (680)
-
limit 30 to english language (620)
EMBASE <1996 to 2012 Week 11>
Searched 19 March 2012 via Ovid.
Search strategy
-
Sleep Apnea Syndrome/ (23,594)
-
(apnea or apnoea).ti,ab. (24,479)
-
(hypopnea or hypopnoea).ti,ab. (5727)
-
(hypoapnea or hypoapnoea).ti,ab. (42)
-
Sleep Disordered Breathing/ (2654)
-
sleep disordered breathing.ti,ab. (3732)
-
(sleep adj2 respirat$ disorder$).ti,ab. (176)
-
sahs.ti,ab. (355)
-
shs.ti,ab. (1025)
-
osa.ti,ab. (6190)
-
osas.ti,ab. (2811)
-
osahs.ti,ab. (783)
-
or/1–12 (33,560)
-
positive end expiratory pressure/ (19,580)
-
(positive adj3 airway adj3 pressure).ti,ab. (5606)
-
(cpap or ncpap or apap or bipap).ti,ab. (6559)
-
(c pap or bi pap or nc pap).ti,ab. (56)
-
autocpap.ti,ab. (34)
-
or/14–18 (21,932)
-
13 and 19 (7358)
-
controlled study/ (3,116,507)
-
exp clinical trial/ (719,714)
-
outcomes research/ (65,036)
-
randomized controlled trial/ (250,869)
-
(randomized or randomised or placebo or randomly).ab. (483,327)
-
trial.ti. (89,510)
-
or/21–26 (3,706,892)
-
20 and 27 (2494)
-
limit 28 to (english language and yr=“2006 – 2012”) (1381).
Cochrane Central Register of Controlled Trials
Searched 28 March 2012.
URL: http://onlinelibrary.wiley.com
Search strategy
#1. MeSH descriptor Sleep Apnea Syndromes explode all trees (1043)
#2. (apnea or apnoea):ti,ab (2393)
#3. (hypopnea or hypopnoea):ti,ab (612)
#4. (hypoapnea or hypoapnoea):ti,ab (2)
#5. (sahs or shs or osas or osa):ti,ab (770)
#6. (#1 OR #2 OR #3 OR #4 OR #5) (2635)
#7. MeSH descriptor Positive-Pressure Respiration explode all trees (1691)
#8. (cpap or apap or ncpap or autocpap or auto-cpap):ti,ab (1278)
#9. (positive near3 airway near3 pressure):ti,ab (1185)
#10. (#7 OR #8 OR #9) (2538)
#11. (#6 AND #10), from 2006 to 2012 (448)
Of 448 total results in Cochrane Library, 395 were from CENTRAL.
Cumulative Index to Nursing and Allied Health Literature 1981 to present
Searched 19 March 2012 via EBSCOhost.
S13. (S8 and S12) Limiters - English Language; Published Date from: 20060101-20120331 (614)
S12. (S9 or S10 or S11) (4355)
S11. TI (cpap or ncpap or apap or bipap or c pap or bi pap or nc pap or autocpap) or AB(cpap or ncpap or apap or bipap or c pap or bi pap or nc pap or autocpap) (930)
S10. TI (positive N3 airway N3 pressure) or AB(positive N3 airway N3 pressure) (1119)
S9. (MH “Positive Pressure Ventilation+”) (3987)
S8. (S1 or S2 or S3 or S4 or S5 or S6 or S7) (5843)
S7. TI (sahs or shs or osa or osas or osahs) or AB(sahs or shs or osa or osas or osahs) (1237)
S6. TI (sleep N2 respirat* disorder*) or AB(sleep N2 respirat* disorder*) (36)
S5. TI (“sleep disordered breathing”) or AB(“sleep disordered breathing”) (665)
S4. TI (hypoapnea or hypoapnoea) or AB(hypoapnea or hypoapnoea) (1)
S3. TI (hypopnea or hypopnoea) or AB(hypopnea or hypopnoea) (657)
S2. TI (apnea or apnoea) or AB(apnea or apnoea) (3843)
S1. (MH “Sleep Apnea Syndromes+”) (4224)
In total there were 614 results.
Science Citation Index
1900 to 21 March 2012.
Searched 22 March 2012 via Web of Science.
Lemmatization off, 2006–12.
#14. #12 and #13
#13. TS=(random* or blind* or comparative or comparison or prospective or controlled or trial or crossover or evaluation)
#12. #6 and #11
#11. #7 or #8 or #9 or #10
#10. TS = autocpap
#9. TS = (“c pap” or “nc pap” or “bi pap”)
#8. TS = (cpap or ncpap or apap or bipap)
#7. TS = (positive same airway same pressure)
#6. #1 or #2 or #3 or #4 or #5
#5. TS = (sahs or shs or osa or osas or osahs)
#4. TS = “sleep disordered breathing”
#3. TS = (hypoapnea or hypoapnoea)
#2. TS = (hypopnea or hypopnoea)
#1. TS = (apnea or apnoea)
In total there were 1228 results.
Conference Proceedings Citation Index – Science
1990 to 21 March 2012.
Searched 22 March 2012 via Web of Science.
Lemmatization off, 2006–12.
#12. #6 and #11
#11. #7 or #8 or #9 or #10
#10. TS = autocpap
#9. TS = (“c pap” or “nc pap” or “bi pap”)
#8. TS = (cpap or ncpap or apap or bipap)
#7. TS = (positive same airway same pressure)
#6. #1 or #2 or #3 or #4 or #5
#5. TS = (sahs or shs or osa or osas or osahs)
#4. TS = “sleep disordered breathing”
#3. TS = (hypoapnea or hypoapnoea)
#2. TS = (hypopnea or hypopnoea)
#1. TS = (apnea or apnoea)
388 results.
Zetoc Conferences
1993 to 22 March 2012.
Searched 22 March 2012.
URL: www.theses.com/default.asp
Search strategy
conference: autocpap
conference: bi pap
conference: c pap
conference: nc pap
conference: bipap
conference: apap
conference: ncpap
conference: cpap
conference: positive airway pressure
Search results from 2006 onwards were downloaded for each search – a total of 103 results were retrieved.
Index to THESES
1716 to 22 March 2012.
Searched 22 March 2012.
URL: www.theses.com/default.asp
Search strategy
((apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea or sleep) and (cpap or ncpap or apap or bipap or c pap or bi pap or nc pap or autocpap)) OR ((apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea or leep) and (positive airway pressure)) OR ((sahs or shs or osa or osas or osahs) and (cpap or ncpap or apap or bipap or c pap or bi pap or nc pap or autocpap)) OR ((sahs or shs or osa or osas or osahs) and (positive airway pressure))
In total, 22 total results were retrieved – there was no facility to save search, search by year restriction or view records without inputting search string each time, and no download facility. Results reviewed, and seven 2006-onwards results were printed off as hard copies and sent to Rita.
Cost-effectiveness searches
Economic evaluations of sleep apnoea and continuous positive airway pressure
NHS Economic Evaluation Database
Searched 28 March 2012.
URL: http://onlinelibrary.wiley.com
Search strategy
#1. MeSH descriptor Sleep Apnea Syndromes explode all trees (1043)
#2. (apnea or apnoea):ti,ab (2393)
#3. (hypopnea or hypopnoea):ti,ab (612)
#4. (hypoapnea or hypoapnoea):ti,ab (2)
#5. (sahs or shs or osas or osa):ti,ab (770)
#6. (#1 OR #2 OR #3 OR #4 OR #5) (2635)
#7. MeSH descriptor Positive-Pressure Respiration explode all trees (1691)
#8. (cpap or apap or ncpap or autocpap or auto-cpap):ti,ab (1278)
#9. (positive near3 airway near3 pressure):ti,ab (1185)
#10. (#7 OR #8 OR #9) (2538)
#11. (#6 AND #10), from 2006 to 2012 (448)
Of 448 total results in Cochrane Library, 14 were from NHSEED.
EconLit
1961 to February 2012.
Searched 23 March 2012 via Ovid.
Search strategy
-
(apnea or apnoea).ti,ab.
-
(hypopnea or hypopnoea).ti,ab.
-
(hypoapnea or hypoapnoea).ti,ab.
-
sleep disordered breathing.ti,ab.
-
(sleep adj2 respirat$ disorder$).ti,ab.
-
sahs.ti,ab.
-
shs.ti,ab.
-
osa.ti,ab.
-
osas.ti,ab.
-
osahs.ti,ab.
-
or/1–10
-
(positive adj3 airway adj3 pressure).ti,ab.
-
(cpap or ncpap or apap or bipap).ti,ab.
-
(c pap or bi pap or nc pap).ti,ab.
-
autocpap.ti,ab.
-
or/12–15
-
11 and 16
-
limit 17 to yr=“2006 – 2012”
Nil results found.
Economic evaluations of sleep apnoea (any intervention)
EconPapers
Searched 28 March 2012.
URL: http://econpapers.repec.org/
Search strategy
apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea or (sleep AND disorder*)
Limited to working papers.
Seven results were scanned – none was relevant.
NHS Economic Evaluation Database
Searched 30 March 2012.
URL: http://onlinelibrary.wiley.com
Search strategy
#1. MeSH descriptor Sleep Apnea Syndromes explode all trees (1043)
#2. (apnea or apnoea):ti,ab (2393)
#3. (hypopnea or hypopnoea):ti,ab (612)
#4. (hypoapnea or hypoapnoea):ti,ab (2)
#5. (sahs or shs or osas or osa):ti,ab (770)
#6. (#1 OR #2 OR #3 OR #4 OR #5), from 2006 to 2012 (1073)
Of 1073 total results in Cochrane Library, 25 were from NHSEED.
Health Technology Assessment Database
Searched 30 March 2012.
URL: http://onlinelibrary.wiley.com
Search strategy
#1. MeSH descriptor Sleep Apnea Syndromes explode all trees (1043)
#2. (apnea or apnoea):ti,ab (2393)
#3. (hypopnea or hypopnoea):ti,ab (612)
#4. (hypoapnea or hypoapnoea):ti,ab (2)
#5. (sahs or shs or osas or osa):ti,ab (770)
#6. (#1 OR #2 OR #3 OR #4 OR #5), from 2006 to 2012 (1073)
Of 1073 total results in Cochrane Library, 36 were from HTA.
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R)
1946 to present.
Searched 30 March 2012 via Ovid.
Search strategy
-
exp Sleep Apnea Syndromes/ (19,973)
-
(apnea or apnoea).ti,ab. (25,964)
-
(hypopnea or hypopnoea).ti,ab. (4820)
-
(hypoapnea or hypoapnoea).ti,ab. (36)
-
sleep disordered breathing.ti,ab. (3003)
-
(sleep adj2 respirat$ disorder$).ti,ab. (201)
-
sahs.ti,ab. (340)
-
shs.ti,ab. (977)
-
osa.ti,ab. (4726)
-
osas.ti,ab. (2328)
-
osahs.ti,ab. (655)
-
or/1-11 (32,955)
-
economics/ (26,193)
-
exp “costs and cost analysis”/ (162,116)
-
economics, dental/ (1836)
-
exp “economics, hospital”/ (17,730)
-
economics, medical/ (8429)
-
economics, nursing/ (3855)
-
economics, pharmaceutical/ (2307)
-
(econom$ or cost or costs or costly or costing or pharmacoeconomic$).ti,ab. (380,943)
-
(value adj1 money).ti,ab. (20)
-
budget$.ti,ab. (16,542)
-
or/13-22 (494,267)
-
((energy or oxygen) adj cost).ti,ab. (2543)
-
(metabolic adj cost).ti,ab. (671)
-
((energy or oxygen) adj expenditure).ti,ab. (14,406)
-
or/24-26 (16,967)
-
23 not 27 (490,330)
-
letter.pt. (752,630)
-
editorial.pt. (302,459)
-
historical-article.pt. (280,726)
-
or/29-31 (1,322,522)
-
28 not 32 (464,959)
-
animals/ (4,889,109)
-
human/ (12,139,643)
-
34 not (34 and 35) (3,594,930)
-
33 not 36 (439,079)
-
12 and 37 (811)
-
limit 38 to (english language and yr=“2006 – 2012”) (319)
EMBASE
1996 to 2012 Week 12.
Searched 30 March 2012 via Ovid.
Search strategy
-
Sleep Apnea Syndrome/ (24,439)
-
(apnea or apnoea).ti,ab. (25,475)
-
(hypopnea or hypopnoea).ti,ab. (5996)
-
(hypoapnea or hypoapnoea).ti,ab. (43)
-
Sleep Disordered Breathing/ (2644)
-
sleep disordered breathing.ti,ab. (3906)
-
(sleep adj2 respirat$ disorder$).ti,ab. (187)
-
sahs.ti,ab. (365)
-
shs.ti,ab. (1067)
-
osa.ti,ab. (6367)
-
osas.ti,ab. (2902)
-
osahs.ti,ab. (820)
-
or/1-12 (34,818)
-
health-economics/ (13,562)
-
exp economic-evaluation/ (147,865)
-
exp health-care-cost/ (143,430)
-
14 or 15 or 16 (253206)
-
(econom$ or cost or costs or costly or costing or pharmacoeconomic$).ti,ab. (356,198)
-
(value adj2 money).ti,ab. (872)
-
budget$.ti,ab. (13,757)
-
18 or 19 or 20 (364,417)
-
17 or 21 (487,556)
-
letter.pt. (477,438)
-
editorial.pt. (310,953)
-
note.pt. (397,942)
-
23 or 24 or 25 (1,186,333)
-
22 not 26 (434,638)
-
(metabolic adj cost).ti,ab. (510)
-
((energy or oxygen) adj cost).ti,ab. (1653)
-
((energy or oxygen) adj expenditure).ti,ab. (12,628)
-
28 or 29 or 30 (14,363)
-
27 not 31 (431,837)
-
exp animal/ (680,271)
-
exp animal-experiment/ (773,680)
-
nonhuman/ (2,423,637)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (2,170,304)
-
33 or 34 or 35 or 36 (3,344,387)
-
exp human/ (7,831,966)
-
exp human-experiment/ (168,040)
-
38 or 39 (7,832,215)
-
37 not (37 and 40) (2,390,216)
-
32 not 41 (397,819)
-
13 and 42 (1267)
-
limit 43 to (english language and yr=“2006 - 2012”) (700)
| Source | Results | After de-duplication | Custom 4 field |
|---|---|---|---|
| CDSR | 20 | 0 | – |
| DARE | 12 | 1 | DARE 28/03/12 |
| HTA | 7 | 6 | HTA 28/03/12 |
| SIGN | 0 | – | – |
| National Guidelines Clearinghouse | 67 | Not downloadable | – |
| HSTAT | 1 | 1 | HSTAT 28/03/12 |
| TRIP | 3 | 0 | – |
| Clinical Evidence | 12 | Not downloadable | – |
| MEDLINE | 620 | 609 | MEDLINE and MEDLINE In-Process 09/03/12 |
| EMBASE | 1381 | 896 | EMBASE 19/03/12 |
| CENTRAL | 395 | 107 | CENTRAL 28/03/12 |
| CINAHL | 614 | 376 | CINAHL 19/03/12 |
| Science Citation Index | 1228 | 595 | Science Citation Index 22/03/12 |
| Conference Proceedings Citation Index | 388 | 271 | Conference Proceedings Citation Index 22/03/12 |
| Zetoc conferences | 103 | 65 | Zetoc conference abstracts 22/03/12 |
| Index to THESES | 7 | Not downloadable | – |
| NHSEED (sleep apnoea AND cpap) | 14 | 1 | NHSEED CPAP 28/03/12 |
| EconLit | 0 | – | – |
| EconPapers | 0 | – | – |
| NHSEED (all sleep apnoea) | 25 | 6 | NHSEED ALL SLEEP APNEA 30/03/12 |
| HTA (all sleep apnoea) | 36 | 30 | HTA ALL SLEEP APNEA 30/03/12 |
| MEDLINE (sleep apnoea cost studies) | 319 | 242 | MEDLINE and MEDLINE In-Process ALL SLEEP APNEA costs 30/03/12 |
| EMBASE (sleep apnoea cost studies) | 700 | 354 | EMBASE ALL SLEEP APNEA costs 30/03/12 |
| Totals | – | 3560 | – |
Appendix 5 Systematic review on the clinical effectiveness of continuous positive airway pressure
Methods
A systematic review on the clinical effectiveness of CPAP was conducted to identify RCTs comparing CPAP with sham CPAP, BSC/usual care and dental devices in a patient population with an average age of 60 years or over with OSAS and capacity to give informed consent. Therefore, studies in patients with dementia were excluded. The literature searches updated the searches conducted for the McDaid et al. 20 report on CPAP and dental devices but focused on the period from January 2006 to March 2012. The studies identified in this review were checked for their relevance for our systematic review.
Results
The updated searches found 4872 titles, which were added to the library with the results of the cost-effectiveness searches and de-duplicated. In total, the searches identified 3560 unique titles. Of these, 67 studies appeared to be potentially relevant for the systematic review on clinical effectiveness. Figure 32 presents the flow diagram of identification and selection of studies. The searches retrieved six potentially relevant reviews on the use of CPAP. 20,209–212 The studies included in these reviews were examined. Only one study met our inclusion criteria;88 however, since it was a conference abstract referring to another title included in the review,88 it was subsequently excluded. From the other 60 titles identified, three studies met our inclusion criteria. 88–90 The systematic review in the previous HTA report had identified 48 relevant studies out of 6325 potentially relevant titles. 20 Given that the average age across the 48 studies ranged from 44 years to 58 years, none met our inclusion criteria and therefore none was included in our review. In sum, the systematic review on clinical effectiveness of CPAP identified three relevant RCTs comparing CPAP with sham CPAP, BSC/usual care and dental devices in a patient population with an average age of 60 years or over with OSAS.
FIGURE 32.
Flow diagram showing number of studies identified and included in the review of clinical effectiveness of CPAP.
Table 69 summarises the characteristics of the studies included in the systematic review of clinical effectiveness. Full data extraction tables can be found in the section Data extraction tables.
| Egea et al. (2008)88 | Ruttanaumpawan et al. (2008)89 | Parra et al. (2011)90 | |
|---|---|---|---|
| Methods | |||
| Study design | Multicentre RCT | RCT | Multicentre RCT |
| Comparator | Sham CPAP | No CPAP | No CPAP |
| Number randomised, mean (SE) | I 35; C 38 | I 19; C 14 | I 71; C 69 |
| Treatment duration | 3 months | 1 month | 24 months |
| Primary outcomes | Left ventricular ejection fraction | Baroreflex sensitivity | Neurological, cardiovascular, quality of life and mortality outcomes |
| Patient characteristics | |||
| Age (years), mean (SE) | I 64 (0.9); C 63 (1.6) | I 59.0 (7.8); C 60.5 (10.3) | I 63.7 (9.1); C 65.5 (9.1) |
| % male | I 96; C 91 | I 94.7; C 85.7 | I 71.9; C 69.6 |
| BMI (kg/m2), mean (SE) | I 31.7(2.4); C 30.5 (1.6) | I 30.3 (5.80); C 32.3 (8.6) | I 30.2 (4.6); C 28.8 (4.0) |
| BP (systolic/diastolic, mmHg), mean (SE) | I 123 (3.7)/76 (2.3); C 126 (2.9)/75 (2.1) | I 122 (15)/66 (12); C 131 (24)/64 (14) | – |
| Disease severity [AHI, mean (SE)] | I 43 (4.4); C 41 (5.6) | I 36.2 (18.1); C 51.3 (15.6) | I 38.4 (12.6); C 38.4 (14.6) |
| Severity of sleepiness [ESS score, mean (SE)] | I 8.0 (0.7); C 7.3 (0.8) | – | I 8.3 (3.3); C 7.3 (4.1) |
| Results | |||
| Primary outcomes, mean (SE) | Baseline: I mean 28.0 (SE 1.5); C mean 28.1 (SE 1.5) | Baseline: I median 5.4 (IQR 2.2–8.3); C median 4.9 (IQR 3.1–8.7) | – |
| 3 months: I mean 30.5 (SE 0.8); C mean 28.1 (SE 1.7) | 1-month I median 7.9 (IQR 4.4–9.4); C median 4.7 (IQR 2.9–7.4) | – | |
| Other outcomes of relevance to economic evaluation | |||
| BP (systolic/diastolic, mmHg), mean (SE) | Baseline: I 123.0 (3.7)/76.3 (2.3); C 124.2(2.8)/74.8(2.1) | Baseline: I 122 (15)/67 (12); C 131 (24)/64 (14) | – |
| 3 months: I 123.0 (4.1)/75.3 (2.3); C 120.5 (2.6)/75.2 (3.1) | 1 month: I 113(12)/61(9); C 136(28)/63(12) | – | |
| ESS score, mean (SE) | Baseline: I 8.0 (0.7); C 7.1 (0.8) | – | – |
| 3 months: I 4.8 (0.6); C 5.3 (0.7) | – | – | |
| AHI, mean (SE) | – | Baseline: I 36.2 (18.1); C 51.3 (15.6) | – |
| – | 1-month: I 9.3 (8.7); C 47.4 (19.1) | – | |
The three studies included in the systematic review compared CPAP therapy with sham CPAP,88 or no CPAP89,90 for OSAS in the secondary care setting in patients with cardiovascular conditions. None of the studies was conducted in the UK. The studies varied in duration: 1 month in Ruttanaumpawan et al.,89 3 months in Egea et al. 88 and 24 months in Parra et al. 90 The primary outcome was different for each study: left ventricular ejection fraction,88 baroreflex sensitivity89 and a number of neurological, quality of life, sleep-related and mortality outcomes. 90 Common secondary outcomes examined by two or more studies were ESS score,88–90 BP88,89 and quality of life with SF-36. 88,90
Egea et al. 88 and Ruttanaumpawan et al. 89 included patients with chronic heart failure referred to the sleep clinic whose AHI was greater than 10 events/hour88 or equal or greater than 20 events/hour,89 while Parra et al. 90 included patients admitted with first ever ischaemic stroke with Apnoea–Hypopnoea Index equal or greater than 20 events/hour. 90 Egea et al. 88 included patients with Cheyne–Stokes apnoea (17% of the study population) but presented results for the subgroup of patients with confirmed OSAS. Participants were mostly male and overweight or obese, as indicated by an average BMI of at least 28 kg/m2. The AHI and ESS measures at baseline suggest that patients across the three studies suffered from moderate to severe OSAS.
Egea et al. 88 observed a statistically significant improvement in left ventricular ejection fraction and in ESS score in the CPAP group but no statistically significant differences were recorded for BP and quality of life. Results were similar for the subgroup of patients with confirmed OSAS. Ruttanaumpawan et al. 89 also observed a statistically significant improvement in baroreflex sensitivity, Apnoea–Hypopnoea Index, heart rate and systolic BP. In Parra et al.,90 the CPAP group experienced a statistically significantly higher improvement in the neurological outcomes at 1 month, which was not sustained throughout follow-up. There were no statistically significant differences in SF-36 scores at any of the data collection points (1, 3, 12 and 24 months).
Across the three studies, CPAP appears to improve sleep function, cardiovascular outcomes and quality of life in patients over 60 years of age. However, the limitations of the studies prevent definitive conclusions. First, all three studies included only patients with cardiac conditions, who are unlikely to represent all patients over 60 years of age with OSAS. Second, two studies had short follow-up and small samples sizes. Given that Parra et al. 90 found that statistically significant differences at 1 month were not sustained at longer follow-ups, there are doubts on whether or not the results observed at 1 month89 and at 3 months88 can be extrapolated over longer time horizons. Third, none of the studies collected measures of HRQoL, such as EQ-5D or SF-6D, that can be used for cost-effectiveness analysis.
Data extraction tables
| Study details | Egea et al. (2008)88 | ||||
|---|---|---|---|---|---|
| Intervention | CPAP therapy | ||||
| Comparator(s) | Sham CPAP therapy | ||||
| Study setting | Spain | ||||
| Design | Randomised multicentre controlled trial | ||||
| Duration | 3 months | ||||
| Inclusion criteria |
|
||||
| Exclusion criteria |
|
||||
| Outcomes | Primary outcome: left ventricular ejection fraction | ||||
Secondary outcomes:
|
|||||
| Pre-defined subgroups | Patients with Cheyne–Stokes apnoea defined as:
|
||||
Respiratory events were classified as:
|
|||||
| Participants: number randomised | 73 patients randomised:
|
||||
| Participants: number of withdrawals with reasons | 7 patients randomised for CPAP withdrawn:
|
||||
6 patients randomised for sham CPAP withdrawn:
|
|||||
| Baseline characteristics | Baseline characteristics (adapted from Table 1, p. 663)88 | ||||
| CPAP (n = 28) | Sham CPAP (n = 32) | ||||
| Age (years), mean (SE) | 64 (0.9) | 63 (1.6) | |||
| Sex (% male) | 96 | 91 | |||
| BMI (kg/m2), mean (SE) | 31.7 (2.4) | 30.5 (1.6) | |||
| Daily snoring (%) | 83 | 69 | |||
| Snoring three or more times/week (%) | 88 | 76 | |||
| ESS score, mean (SE) | 8.0 (0.7) | 7.3 (0.8) | |||
| Minimum SaO2 (%), mean (SE) | 76.9 (2.0) | 77.4 (2.1) | |||
| AHI (events/hour), mean (SE) | 43 (4.4) | 41 (5.6) | |||
| Systolic BP (mmHg), mean (SE) | 123 (3.7) | 126 (2.9) | |||
| Diastolic BP (mmHg), mean (SE) | 76 (2.3) | 75 (2.1) | |||
| Pretibial oedema (%) | 19 | 13 | |||
| Jugular ingurgitation (%) | 8 | 0 | |||
| Left ventricular ejection fraction (%), mean (SE) | 28.0 (0.5) | 28.1 (1.5) | |||
| Statistically significant results at p = 0.05 in bold | |||||
| Results | Results at 3 month follow-up (adapted from Table 3, page 665)88 | ||||
| Baseline | 3 months | Baseline | 3 months | ||
| Left ventricular ejection fraction (%), mean (SE) | 28.0 (1.5) | 30.5 (0.8) | 28.1 (1.5) | 28.1 (1.7) | |
| ESS score, mean (SE) | 8.0 (0.7) | 4.8 (0.6) | 7.1 (0.8) | 5.3 (0.7) | |
| Systolic BP (mmHg), mean (SE) | 123.0 (3.7) | 123.0 (4.1) | 124.2 (2.8) | 120.5 (2.6) | |
| Diastolic BP (mmHg), mean (SE) | 76.3 (2.3) | 75.3 (2.3) | 74.8 (2.1) | 75.2 (3.1) | |
| SF-36 physical, mean (SE) | 41.8 (1.8) | 45.1 (1.4) | 42.1 (1.7) | 41.3 (1.9) | |
| SF-36 mental, mean (SE) | 47.8 (2.4) | 49.9 (2.0) | 47.0 (2.3) | 49.8 (1.8) | |
| New York Heart Association (% class I–II) | 75 | 82 | 65 | 74 | |
| New York Heart Association (% class II) | 64 | 71 | 58 | 74 | |
| 6-minute walking test (m), mean (SE) | 424 (20) | 420 (19) | 394 (20) | 405 (22) | |
| Statistically significant results at p = 0.05 in bold | |||||
| Results for subgroups | Results at 3-months follow-up for the patients with obstructive apnoea or hypopnoea (adapted from Table 4, page 665)88 | ||||
| CPAP (n = 20) | Sham CPAP (n = 25) | ||||
| Baseline | 3 months | Baseline | 3 months | ||
| Left ventricular ejection fraction (%), mean (SE) | 28.8 (1.6) | 31.0 (1.6) | 27.2 (1.6) | 26.7 (1.7) | |
| ESS score, mean (SE) | 8.6 (0.8) | 5.0 (0.8) | 6.9 (5.2) | 5.2 (0.8) | |
| Systolic BP (mmHg), mean (SE) | 124.3 (4.2) | 124.3 (4.9) | 125 (2.7) | 123.4 (2.8) | |
| Diastolic BP (mmHg), mean (SE) | 75.6 (2.3) | 76.0 (2.8) | 75.8 (2.4) | 77.0 (3.7) | |
| SF-36 physical, mean (SE) | 41.4 (2.0) | 44.9 (1.8) | 42.0 (2.1) | 40.7 (2.1) | |
| SF-36 mental, mean (SE) | 46.4 (3.0) | 48.8 (2.3) | 45.8 (2.7) | 48.7 (2.2) | |
| New York Heart Association (% class I–II) | 60 | 70 | 50 | 67 | |
| 6-minute walking test (m), mean (SE) | 403 (21) | 406 (21) | 381 (23) | 393 (24) | |
| Statistically significant results at p = 0.05 in bold | |||||
| Conclusions | CPAP therapy increases left ventricular ejection fraction in patients with associated sleep-related disordered breathing and severe chronic heart failure, however, this improvement was not translated into an improvement in quality of life or cardiac function | ||||
| Limitations |
|
||||
| Conflicts of interest | None | ||||
| Study details | Rutanaumpawan et al. (2008)89 | ||||
|---|---|---|---|---|---|
| Intervention | CPAP | ||||
| Comparator(s) | Usual care for heart failure | ||||
| Study setting | Canada | ||||
| Design | RCT | ||||
| Duration | 1 month | ||||
| Inclusion criteria |
|
||||
| Exclusion criteria |
|
||||
| Outcomes |
|
||||
|
|||||
| Pre-defined subgroups |
|
||||
| Participants: Number randomised |
|
||||
| Participants: number of withdrawals with reasons |
|
||||
| Baseline characteristics | Baseline characteristics (adapted from table 1, page 1165)89 | ||||
| Selected variables | CPAP (n = 19) | Control (n = 14) | |||
| Age (years), mean (SE) | 59.0 (7.8) | 60.5 (10.3) | |||
| Sex (% male) | 94.7 | 85.7 | |||
| Cause of heart failure | |||||
| Ischaemic (%) | 63 | 64 | |||
| Non-ischaemic (%) | 37 | 36 | |||
| New York Heart Association functional class, mean (SE) | 2.4 (0.6) | 2.3 (0.4) | |||
| BMI (kg/m2), mean (SE) | 30.3 (5.8) | 32.3 (8.6) | |||
| Left ventricular ejection fraction (%), mean (SE) | 29.0 (11.4) | 30.8 (8.9) | |||
| SBP (mmHg), mean (SE) | 122 (15) | 131 (24) | |||
| Diastolic BP (mmHg), mean (SE) | 66 (12) | 64 (14) | |||
| Data expressed as means (SE). Statistically significant results at p = 0.05 in bold | |||||
| Results | Results at 1-month follow-up (adapted from tables 2 and 3 pages 1165–6)89 | ||||
| Selected variables | CPAP (n = 19) | Control (n = 14) | |||
| Baseline | 1 month | Baseline | 1 month | ||
| Sleep-related | |||||
| AHI events per hour, mean (SE) | 36.2 (18.1) | 9.3 (8.7) | 51.3 (15.6) | 47.4 (19.1) | |
| Mean SaO2 (%), mean (SE) | 94.7 (1.6) | 96.1 (1.6) | 94.3 (2.1) | 94.1 (2.0) | |
| Cardiovascular | |||||
| Left ventricular ejection fraction (%), mean (SE) | 29.0 (11.4) | 36.1 (10.6) | 30.8 (8.9) | 29.4 (8.0) | |
| Heart rate (bpm), mean (SE) | 66 (8) | 62 (8) | 66 (11) | 66 (9) | |
| SBP (mmHg), mean (SE) | 122 (15) | 113 (12) | 131 (24) | 136 (28) | |
| Diastolic BP (mmHg), mean (SE) | 67 (12) | 61 (9) | 64 (14) | 63 (12) | |
| Baroreflex sensitivity | |||||
| Slope (ms/mmHg), median (IQR) | 5.4 (2.2; 8.3) | 7.9 (4.4–9.4) | 4.9 (3.1–8.7) | 4.7 (2.9–7.4) | |
| Slope in +SBP/+R–R sequences, median (IQR) | 4.7 (2.4; 8.5) | 8.1 (4.5–12.6) | 5.5 (3.3–10.0) | 4.0 (3.2–6.9) | |
| Slope in SBP/–R–R sequences, median (IQR) | 5.4 (2.2; 8.8) | 5.6 (4.3–10.0) | 5.5 (4.1–11.2) | 5.0 (2.7–7.7) | |
| Between-groups statistically significant results at p = 0.05 in bold | |||||
| Results for subgroups | None | ||||
| Conclusions | Treatment of OSA in patients with heart failure improves baroreflex sensitivity and cardiovascular signs | ||||
| Limitations |
|
||||
| Conflicts of interest | None | ||||
| Study details | Parra et al. (2011)90 | ||
|---|---|---|---|
| Intervention | Nasal CPAP in addition to usual stroke care | ||
| Comparator(s) | Usual stroke care, in accordance with the recommendations of the Spanish Cerebrovascular Study Group of the Spanish Society of Neurology | ||
| Study setting | Spain | ||
| Design | Randomised controlled multicentre study | ||
| Duration | 24 months | ||
| Inclusion criteria |
|
||
| Exclusion criteria |
|
||
| Outcomes | Assessments at baseline, 1, 3, 12 and 24 months after stroke
|
||
| Pre-defined subgroups | None | ||
| Participants: Number randomised | 140 patients were randomised but only 126 were followed up:
|
||
| Participants: Number of withdrawals with reasons | 14 patients randomised to CPAP dropped-out because of machine discomfort | ||
| Baseline characteristics | Baseline characteristics (selected data from Table 1, page 1131)90 | ||
| CPAP (n = 57) | Control (n = 69) | ||
| Age (years), mean (SE) | 63.7 (9.1) | 65.5 (9.1) | |
| Sex (% male) | 71.9 | 69.6 | |
| BMI (kg/m2), mean (SE) | 30.2 (4.6) | 28.8 (4.0) | |
| Snoring, often or always (%) | 94.7 | 85.5 | |
| Observed apnoea at night, often or always (%) | 70.2 | 46.4 | |
| ESS score, mean (SE) | 8.3 (3.3) | 7.3 (4.1) | |
| AHI (events/hour), mean (SE) | 38.4 (12.6) | 38.4 (14.6) | |
| Physical component SF-36, mean (SE) | 42.3 (11.1) | 43.1 (7.8) | |
| Mental component SF-36, mean (SE) | 47.1 (13.3) | 48.2 (12.9) | |
| Statistically significant results at p = 0.05 in bold. | |||
| Results | Mean CPAP use was 5.3 hours (SE = 1.9 hours) per night during an average of 6.8 (SE = 0.6) nights a week | ||
| Results (adapted from table 3 page 1133)90 | |||
| CPAP (n = 57), mean (SE) | Control (n = 69), mean (SE) | ||
| Barthel index | |||
| Baseline | 75.9 (27.9) | 73.6 (27.0) | |
| 3 months | 95.0 (13.4) | 92.8 (17.8) | |
| 12 months | 95.3 (10.0) | 91.4 (17.8) | |
| 24 months | 94.3 (10.9) | 93.1 (15.8) | |
| Canadian scale | |||
| Baseline | 8.3 (1.6) | 8.0 (1.9) | |
| 3 months | 9.3 (1.0) | 9.3 (1.3) | |
| 12 months | 9.4 (1.2) | 9.4 (1.3) | |
| 24 months | 9.3 (1.3) | 9.5 (1.0) | |
| Rankin scale | |||
| Baseline | 2.3 (1.3) | 2.8 (1.3) | |
| 3 months | 1.6 (0.9) | 2.0 (1.1) | |
| 12 months | 1.6 (0.9) | 2.1 (1.2) | |
| 24 months | 1.8 (1.1) | 2.2 (1.1) | |
| SF-36 physical | |||
| Baseline | 42.6 (10.2) | 42.3 (11.8) | |
| 3 months | 44.9 (9.2) | 44.8 (11.8) | |
| 12 months | 46.7 (8.8) | 46.5 (11.7) | |
| 24 months | 45.8 (10.0) | 46.0 (9.8) | |
| SF-36 mental | |||
| Baseline | 43.3 (13.2) | 43.7 (14.1) | |
| 3 months | 46.9 (10.9) | 46.3 (14.4) | |
| 12 months | 49.1 (14.0) | 44.6 (12.8) | |
| 24 months | 47.6 (13.8) | 47.8 (12.1) | |
| Statistically significant results at p = 0.05 in bold. The overall cardiovascular event-free survival rate after 24 months was 87.7% (50 out of 57 subjects) in the CPAP group and 88.4% (61 out of 69) in the control group (p = 0.911) | |||
| Results for subgroups | None | ||
| Conclusions | Early use of CPAP in patients with a first-ever ischaemic stroke and moderate to severe OSA is associated with a significant improvement in neurological function, but this improvement is not sustained at follow-up | ||
| Limitations |
|
||
| Conflicts of interest | None | ||
Appendix 6 Detailed results of the model-based sensitivity analysis
Scenario 1: continuous positive airway pressure is used for 1 year (costs = £710.016)
The base case assumes that the CPAP and humidifier devices have a lifetime of 7 years and can be reused across patients. Scenario 2 assumes that the CPAP and humidifier devices are used for 1 year only. Therefore, their costs are not annuitised and all the costs of CPAP treatment are incurred in the 1 year. CPAP therapy costs £710.16 per patient and the difference in costs estimated from the within-trial analysis (see Appendix 3, Table 64) was £474 (95% CI £119 to £830). The model extrapolates this difference over the patient’s lifetime.
Table 73 shows the cost-effectiveness results for scenario 1. The average difference in costs is £4785 over the patient’s lifetime. The difference in EQ-5D QALYs was 0.051 and in SF-6D QALYs was 0.182; the ICERs, respectively, are £94,404 and £26,599 per QALY gained.
| Treatment | Average costs (£) | Average EQ-5D QALYs | Average SF-6D QALYs |
|---|---|---|---|
| CPAP with BSC | 21,002 | 8.041 | 7.859 |
| BSC alone | 16,216 | 7.990 | 7.678 |
| Incremental costs and QALYs | |||
| CPAP with BSC – BSC alone | 4785 | 0.051 | 0.182 |
Figure 33 presents the cost-effectiveness acceptability curve for the scenario 1. The curve has a similar shape to the curves for scenarios 1 and 2 of the within-trial sensitivity analysis (see Appendix 3, Figures 27 and 28). The probability that CPAP is cost-effective at £20,000 is 0.20 for the analysis with EQ-5D QALYs and 0.31 for SF-6D QALYs. The reduction in the probability that the intervention is cost-effective reflects the trade-off between small and uncertain gains in health and the increase in costs.
FIGURE 33.
Cost-effectiveness acceptability curve for scenario 1 (CPAP is used for 1 year at an average cost per patient of £710.16).

Scenario 2: cardiovascular effects with effect of continuous positive airway pressure on costs and quality-adjusted life-years
In scenario 2, the model includes two additional health states (post stroke and post CHD) and two additional events (stroke and CHD). The model is run for six patient subgroups defined according to their sex, smoking status and diabetes status. The final results are a weighted average of the results for each subgroup weighted by their relative proportion in the patient population participating in PREDICT. Table 74 presents the percentage of each subgroup in the overall population. Note that this calculation uses only the patients with complete data in these variables.
| Population | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Sex (= 0 male) | 0 | 0 | 0 | 0 | 1 | 1 |
| Smoking status (= 0 no smoker) | 1 | 1 | 0 | 0 | 0 | 0 |
| Diabetes (= 0 not diabetic) | 1 | 0 | 0 | 1 | 0 | 1 |
| Number of patients | 5 | 8 | 102 | 56 | 15 | 5 |
| Percentage | 2.6 | 4.2 | 53.4 | 29.3 | 7.9 | 2.6 |
Tables 75 and 76 show the cost-effectiveness results for each subgroup and for the overall population. The differences in costs and QALYs are similar to those in the base-case analysis. Overall, the cost savings are approximately £300 (vs. £329 in the base case). The difference in the results for EQ-5D and SF-6D QALYs is a result of the intrinsic variation from the probabilistic analysis. The increase in EQ-5D QALYs is 0.022 (vs. 0.051 in the base case) and the increase in SF-6D QALYs is 0.139 (vs. 0.182 in the base case). Therefore, including cardiovascular effects appears to reduce the health benefits obtained with CPAP therapy but has little impact on costs.
| Population | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Costs without CPAP (£) | 15,776 | 15,946 | 16,442 | 16,310 | 16,988 | 16,323 |
| Costs with CPAP (£) | 15,535 | 15,610 | 16,089 | 16,019 | 16,652 | 16,075 |
| Outcomes without CPAP | 6.130 | 6.604 | 7.224 | 6.839 | 7.877 | 7.244 |
| Outcomes with CPAP | 6.135 | 6.611 | 7.248 | 6.858 | 7.920 | 7.268 |
| Weights (%) | 2.62 | 4.19 | 53.40 | 29.32 | 7.85 | 2.62 |
| Difference in costs (£) | –241 | –336 | –353 | –291 | –337 | –248 |
| Difference in QALYs | 0.005 | 0.007 | 0.023 | 0.018 | 0.043 | 0.023 |
| Weighted average | ||||||
| Difference in costs (£) | –327 | |||||
| Difference in QALYs | 0.022 | |||||
| Population | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Costs without CPAP (£) | 15,779 | 15,948 | 16,440 | 16,309 | 16,987 | 16,320 |
| Costs with CPAP (£) | 15,502 | 15,593 | 16,106 | 16,036 | 16,679 | 16,061 |
| Outcomes without CPAP | 5.886 | 6.343 | 6.939 | 6.570 | 7.566 | 6.959 |
| Outcomes with CPAP | 5.994 | 6.461 | 7.082 | 6.700 | 7.735 | 7.100 |
| Weights (%) | 2.62 | 4.19 | 53.40 | 29.32 | 7.85 | 2.62 |
| Difference in costs (£) | –277 | –354 | –334 | –273 | –308 | –258 |
| Difference in QALYs | 0.108 | 0.119 | 0.143 | 0.131 | 0.168 | 0.141 |
| Weighted average | ||||||
| Difference in costs (£) | –311 | |||||
| Difference in QALYs | 0.139 | |||||
Figure 34 shows the cost-effectiveness plane for the most common subgroup, patients who are male, non-smokers and non-diabetic. The distribution of the simulations across the quadrants is similar to that of the base case. In the analysis with EQ-5D QALYs, the simulations are scattered evenly across the four quadrants. In the analysis with SF-6D QALYs, the simulations are concentrated in the eastern quadrants (better health outcomes).
FIGURE 34.
Cost-effectiveness plane for scenario 3 (subgroup population 3). (a) EQ-5D; and (b) SF-6D.


Figure 35 shows the cost-effectiveness acceptability curve for subgroup 3. The curve is similar to the base-case analysis. The probability that CPAP is cost-effective is 0.58 for EQ-5D QALYs and 0.92 for SF-6D QALYs. These results suggest that including cardiovascular effects has little impact on the results.
FIGURE 35.
Cost-effectiveness acceptability curve for scenario 2 (subgroup population 3).
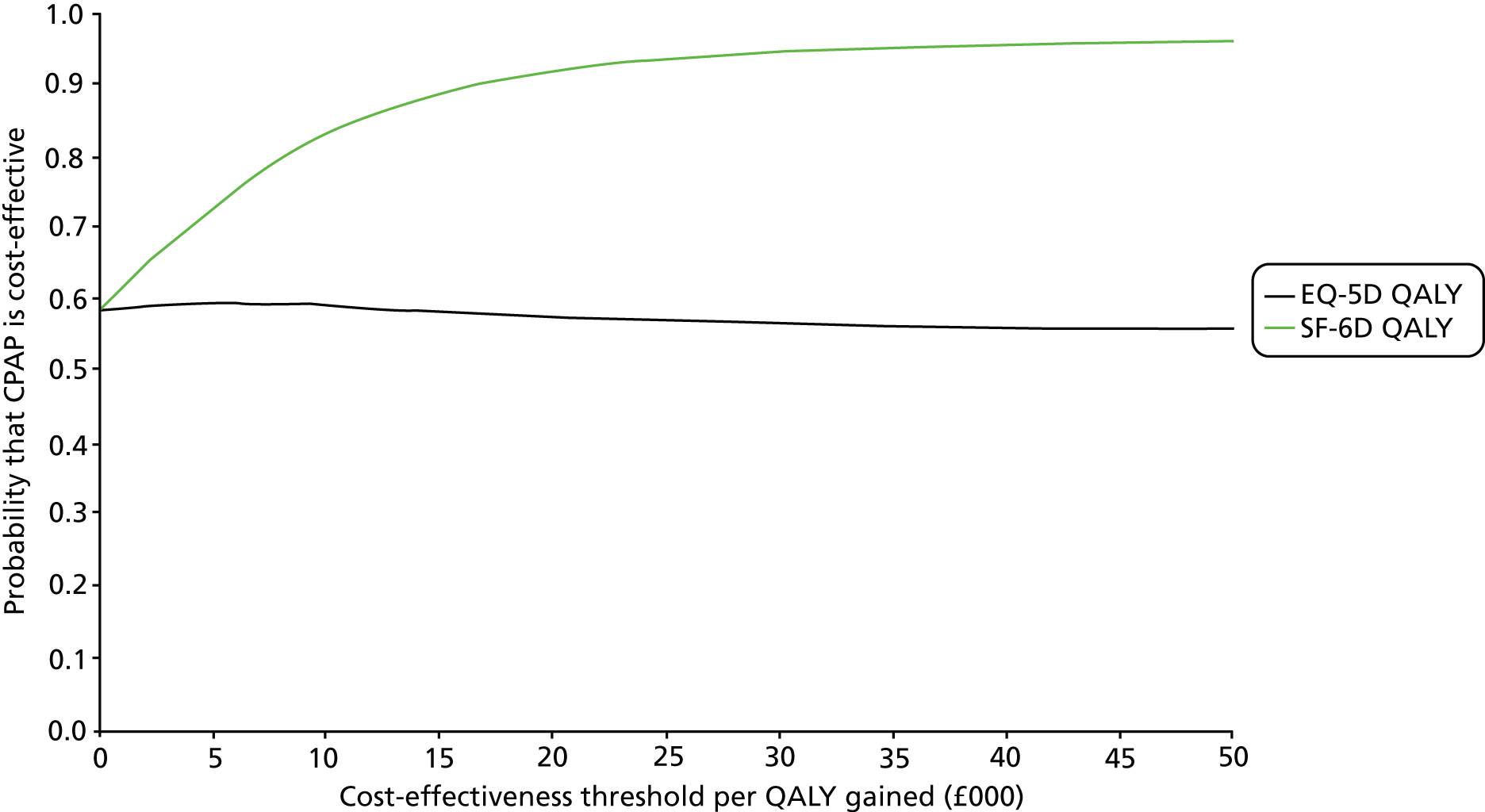
Scenario 3: cardiovascular effects without effect of continuous positive airway pressure on costs and quality-adjusted life-years
Scenario 3 uses the model with cardiovascular effects but does not consider the impact of CPAP therapy on costs and QALYs. In other words, it assumes that there is no improvement in QALYs or cost savings from CPAP therapy over 1 year. Therefore, the QALY improvement and cost saving from CPAP is set to zero. The purpose of this scenario is to test the impact of including cardiovascular effects of CPAP on costs and QALYs independent to the direct effect of CPAP. This will indicate whether or not the cardiovascular effects are a key driver of the cost-effectiveness results.
Table 77 and 78 present the cost-effectiveness results for this scenario. The difference in costs and QALYs is very small; cost savings of £10 and QALY decrement of approximately 0.023. The results are similar for EQ-5D and SF-6D QALYs because the same HRQoL parameters are used for these analyses except HRQoL at baseline (0.687 for EQ-5D and 0.660 for SF-6D).
| Population | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Costs without CPAP (£) | 15,776 | 15,944 | 16,445 | 16,310 | 16,986 | 16,322 |
| Costs w/ CPAP (£) | 15,802 | 15,909 | 16,421 | 16,309 | 17,018 | 16,380 |
| Outcomes without CPAP | 6.128 | 6.605 | 7.223 | 6.839 | 7.876 | 7.246 |
| Outcomes with CPAP | 6.095 | 6.571 | 7.200 | 6.813 | 7.864 | 7.222 |
| Weights (%) | 2.62 | 4.19 | 53.40 | 29.32 | 7.85 | 2.62 |
| Difference in costs (£) | 26 | –35 | –24 | 0 | 32 | 59 |
| Difference in QALYs | –0.033 | –0.034 | –0.023 | –0.027 | –0.012 | –0.024 |
| Weighted average | ||||||
| Difference in costs | –10 | |||||
| Difference in QALYs | –0.024 | |||||
| Population | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Costs without CPAP (£) | 15,777 | 15,944 | 16,441 | 16,308 | 16,989 | 16,321 |
| Costs with CPAP (£) | 15,802 | 15,910 | 16,417 | 16,307 | 17,021 | 16,379 |
| Outcomes without CPAP | 5.887 | 6.343 | 6.939 | 6.570 | 7.567 | 6.960 |
| Outcomes with CPAP | 5.854 | 6.311 | 6.917 | 6.544 | 7.555 | 6.937 |
| Weights (%) | 2.62% | 4.19 | 53.40 | 29.32 | 7.85 | 2.62 |
| Difference in costs (£) | 25 | –34 | –24 | –1 | 32 | 58 |
| Difference in QALYs | –0.033 | –0.032 | –0.022 | –0.026 | –0.011 | –0.023 |
| Weighted average | ||||||
| Difference in costs (£) | –10 | |||||
| Difference in QALYs | –0.023 | |||||
Figure 36 shows the cost-effectiveness planes for subgroup 3. The differences in costs and QALYs are very small; most of the simulations are between –£100 and £100 and –0.10 QALYs and 0.10 QALYs. In addition, the differences are also uncertain. Approximately one-quarter of the simulations are located in the north-east quadrant, indicating better QALYs but also increased costs; the remaining 75% of the simulations are located in the south-west quadrant, indicating worse health outcomes and lower costs.
FIGURE 36.
Cost-effectiveness plane for scenario 3 (subgroup 3). (a) EQ-5D; and (b) SF-6D.
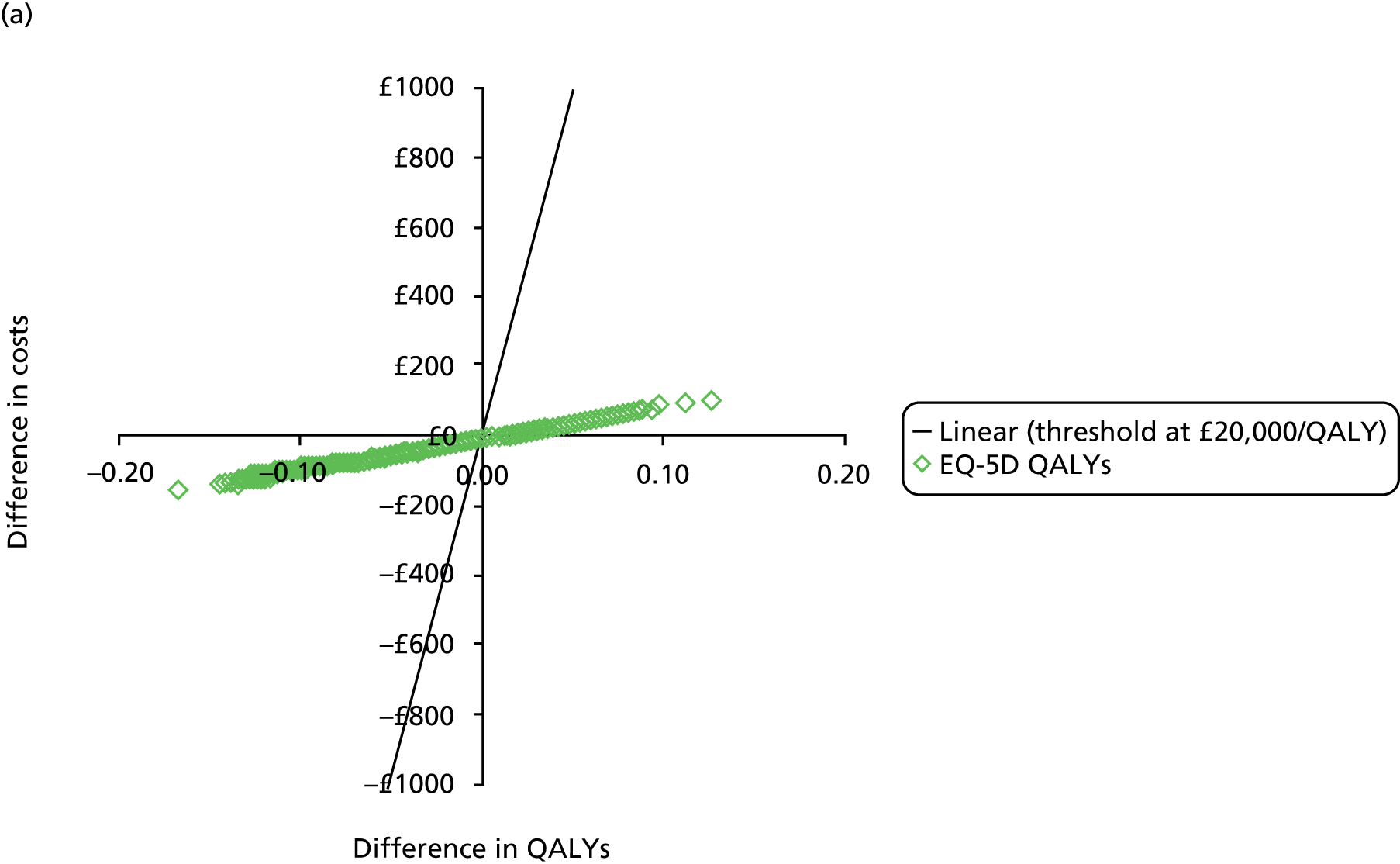

Figure 37 shows the cost-effectiveness acceptability curve for subgroup 3. At low thresholds (< £1000/QALY gained), the cost savings compensate the losses in QALYs. As the threshold increases, potential losses in health are valued more highly and the probability that CPAP is cost-effective drops to below 0.30.
FIGURE 37.
Cost-effectiveness acceptability curve for scenario 3 (subgroup 3).

List of abbreviations
- BMI
- body mass index
- BP
- blood pressure
- BSC
- best supportive care
- CHD
- coronary heart disease
- CI
- confidence interval
- CPAP
- continuous positive airway pressure
- CRF
- case report form
- DSS
- Digit Symbol Substitution
- EQ-5D
- European Quality of Life-5 Dimensions
- ESS
- Epworth Sleepiness Scale
- FEV1
- forced expiratory volume in 1 second
- FP
- fractional polynomial
- FVC
- forced vital capacity
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HbA1c
- glycated haemoglobin
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IDMC
- Independent Data Monitoring Committee
- IQR
- interquartile range
- LDL
- low-density lipoprotein
- MCS
- mental component summary scale
- MI
- myocardial infarction
- MMSE
- Mini Mental State Examination
- MRC CTU
- Medical Research Council Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- ODI
- oxygen desaturation index
- ORTU
- Oxford Respiratory Trials Unit
- OSA
- obstructive sleep apnoea
- OSAS
- obstructive sleep apnoea syndrome
- OSLER
- Oxford Sleep Resistance
- PCS
- physical component summary scale
- PIS
- patient information sheet
- PREDICT
- Positive Airway Pressure in Older People: a randomised controlled trial
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RTA
- road traffic accident
- SAQLI
- Sleep Apnoea Quality of Life Index
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- TDS
- Townsend Disability Scale
- TMG
- Trial Management Group
- TMT–B
- Trail Making Test Part B
- TSC
- Trial Steering Committee
- TUG
- Timed Up and Go