Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 13/51/01. The protocol was agreed in September 2013. The assessment report began editorial review in April 2014 and was accepted for publication in October 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Westwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Objective
The overall objective of this project is to assess the clinical effectiveness and cost-effectiveness of high-sensitivity troponin (Tn) assays for the management of adults presenting with acute chest pain, in particular for the early (within 4 hours of presentation) rule-out of acute myocardial infarction (AMI). The following research questions were defined to address the review objectives:
-
What is the clinical effectiveness of new, high-sensitivity troponin [high-sensitivity cardiac troponin (hs-cTn)] assays (used singly or in series) compared with conventional diagnostic assessment, for achieving early discharge within 4 hours of presentation, when AMI is excluded without increase in adverse outcomes?
-
What is the accuracy of new, hs-cTn assays (used singly or in series, such that results are available within 3 hours of presentation) for the diagnosis of AMI in adults with acute chest pain?
-
What is the accuracy of new, hs-cTn assays (used singly or in series, such that results are available within 3 hours of presentation) for the prediction of major adverse cardiac events (MACEs) (cardiac death, non-fatal AMI, revascularisation or hospitalisation for myocardial ischaemia) during 30-day follow-up in adults with acute chest pain?
-
What is the cost-effectiveness of using new, hs-cTn assays (used singly or in series, such that results are available within 3 hours of presentation) compared with the current standard of serial Tn T and/or I testing on admission and at 10–12 hours post admission?
Chapter 2 Background and definition of the decision problem(s)
Population
The primary indication for this assessment is the early rule-out of AMI and consequent early discharge in people presenting with acute chest pain and suspected, but not confirmed, non-ST segment elevation myocardial infarction (NSTEMI). The assessment will also consider the potential effects of early diagnosis of AMI and of reduced specificity of testing.
Acute coronary syndrome (ACS) is the term used to describe a spectrum of conditions caused by coronary artery disease (CAD) [also known as coronary heart disease (CHD) or ischaemic heart disease]. ACS arises when atheromatous plaque ruptures or erodes, leading to vasospasm, thrombus formation and distal embolisation, obstructing blood flow through the coronary arteries. It incorporates three distinct conditions: unstable angina (UA), ST segment elevation myocardial infarction (STEMI) and NSTEMI. CAD and AMI are a significant health burden in the UK, with Office for National Statistics (ONS) mortality data for 2011 showing 23,705 deaths from AMI and 64,435 deaths from ischaemic heart disease; AMI accounted for approximately 5% of all deaths recorded in 2011, and ischaemic heart disease accounted for approximately 13%. 1
People with ACS usually present with chest pain, and chest pain has been reported as the most common cause of hospital admissions in the UK;2 Hospital Episode Statistics (HES) for 2011–12 show 243,197 emergency admissions for chest pain, accounting for approximately 5% of all emergency admissions. 3 However, many people presenting with acute chest pain will have non-cardiac underlying causes, such as gastro-oesophageal disorders, muscle pain, anxiety or stable ischaemic heart disease. A 2003 study4 on the impact of cardiology guidelines on the diagnostic classification of people with ACS in the UK reported that the majority of people admitted to hospital with chest pain have either no ischaemic heart disease or stable ischaemic heart disease. HES for 2011–12 are consistent with this observation, showing diagnoses of AMI in 47,783 emergency admissions and UA in 32,369 admissions; this represents approximately 20% and 13% of emergency admissions with chest pain, respectively. 3 Accurate and prompt differentiation of ACS (in particular AMI), stable CAD and other causes of chest pain is therefore vital to ensure appropriate and timely intervention when required and to avoid unnecessary hospital admissions.
ST segment elevation myocardial infarction can usually be diagnosed on presentation by electrocardiogram (ECG), hence the main diagnostic challenge in the investigation of suspected ACS is the detection or rule-out of NSTEMI. Investigation of ACS can also involve identification of people with UA (CAD with worsening symptoms, but no evidence of myocardial necrosis).
Since the development of protein biomarkers of myocardial damage in the 1980s, the number of biomarker assays available has proliferated, cardiac specificity has increased, and the role of biomarkers in the diagnostic work-up of acute chest pain has expanded. Cardiac biomarkers are becoming increasingly sensitive and recent European Society of Cardiology (ESC) and American College of Cardiology (ACC) guidelines5,6 enable AMI to be diagnosed with any rise and/or fall of Tn to above the laboratory reference range. This has resulted in fewer people being classified as having UA with no myocardial damage, and more people being classified as having NSTEMI. 7 The most recent 2 years of HES show that the number of emergency department (ED) attendances where the first recorded investigation was a cardiac biomarker rose from 13,743 in 2010–11 to 28,379 in 2011–12. 3 Cardiac troponins I and T (cTnI and cTnT), together with cardiac troponin C (cTnC), form the troponin–tropomyosin complex, which is responsible for regulating cardiac muscle contraction. cTnI and cTnT are used clinically as markers of cardiomyocyte necrosis, indicative of AMI. Tn assays are intended for use in conjunction with clinical history-taking and ECG monitoring as, although specificity is high, Tns may also be elevated in many other conditions, including myocarditis, congestive heart failure (HF), severe infections, renal disease and chronic inflammatory conditions of the muscle or skin. Standard biochemical diagnosis of NSTEMI is based on elevation of the cardiac biomarker Tn above the 99th percentile of the reference range for the normal population. 8 Elevated Tn levels have been shown to be associated with an increased risk of adverse cardiac outcomes. 9 However, the optimal sensitivity of standard Tn assays for AMI occurs several hours after the onset of symptoms;10 this is reflected in current clinical guidelines,11,12 which recommend cTnI or cTnT testing at initial hospital assessment and again at 10–12 hours after the onset of symptoms. As the majority of people presenting with chest pain do not have NSTEMI, for which presentation is within a few hours of symptom onset, delayed biomarker measurement may result in unnecessary periods of extended observation or hospitalisation and associated costs. The development of cardiac biomarkers that can be used at an earlier stage without reduction in sensitivity is, therefore, desirable.
Intervention technologies
The development of hs-cTn assays means that it is possible to detect lower levels of Tn in the blood. Current generations of commercially available assays have analytical sensitivities up to 100 times greater than was the case for early Tn assays (1 ng/l vs. 100 ng/l). 13 Use of these high-sensitivity assays enable the detection of small changes in cTn levels, and may enable AMI to be ruled out at an earlier time after the onset of acute chest pain. Use of the hs-cTn assays has the potential to facilitate earlier discharge for people with normal cTn levels and earlier intervention for those with elevated levels of cTn. The recommended definition of a hs-cTn assay uses two criteria:13,14
-
The total imprecision, coefficient of variation (CV), of the assay should be ≤ 10% at the 99th percentile value of a healthy reference population.
-
The limit of detection (LoD) of the assay should be such as to allow measurable concentrations to be attainable for at least 50% (ideally > 95%) of healthy individuals.
A number of high-sensitivity cardiac troponin I and cardiac troponin T (hs-cTnI and hs-cTnT) assays are currently available for use in the NHS in England and Wales; all are designed for use in clinical laboratory settings.
Abbott ARCHITECT high-sensitivity troponin I assay
The Abbott ARCHITECT® hs-cTnI STAT assay (Abbott Laboratories, Chicago, IL, USA) can be used with the Abbott ARCHITECT® i2000SR and i1000SR analysers (Abbott Laboratories). The assay is a quantitative, chemiluminescent microparticle immunoassay (CMIA) for serum or plasma samples. Results are available within 16 minutes. The ARCHITECT hs-cTnI STAT assay can detect cTnI in 96% of the reference population, and has a recommended 99th percentile cut-off of 26.2 ng/l, with a CV of 4%. 15 The assay is CE marked and available to the NHS.
AccuTnI+3 troponin I assay (Beckman Coulter)
The AccuTnI+3 hs-cTnI assay is approved for use on both the Beckman Coulter Access 2 and DxI analysers (Brea, CA, USA) and has recently received CE mark approval. The assay is a quantitative, two-site paramagnetic particle chemiluminescent sandwich immunoassay for serum or plasma samples. The AccuTnI+3 assay has a recommended 99th percentile cut-off of 40 ng/l, with a CV of < 10%. 16 A recent conference abstract reported data suggesting that the assay can detect cTnI in 88% of the reference population when used on the Access II analyser and in 58% of the reference population when used on the DxI analyser. 17 The same study17 reported a difference in the 99th centile upper reference limit between the two analysers (41 ng/l for the Access II and 34 ng/l for the DxI).
Roche Elecsys high-sensitivity troponin T assay
The Roche Elecsys® cTnT-hs (high-sensitive troponin T assay) and Roche Elecsys® cTnT-hs STAT assays (Roche Diagnostics GmbH, Mannheim, Germany) can be used on the Roche Elecsys® 2010 analyser (Roche Diagnostics GmbH) and the cobas Modular Analytics e series immunoassay analysers, e411 platform. The assay is a quantitative, sandwich electrochemiluminescence immunoassay (ECLIA) for serum and plasma samples. Results are available within 18 minutes with the standard assay and within 9 minutes if the STAT assay is used. Both versions of the assay can detect cTnT in 61% of the reference population and have a recommended 99th percentile cut-off of 14 ng/l, with a CV of < 10%. 18 Both versions of the assay are CE marked and available to the NHS.
A summary of the product properties of hs-cTnI and hs-cTnT assays available to the NHS in England and Wales is provided in Table 1.
| Manufacturer | System | Assay | LoD (ng/l) | LoB (ng/l) | 99th percentile (ng/l)a | CV at 99th percentilea | Turnaround time (minutes)a | CE marked |
|---|---|---|---|---|---|---|---|---|
| Abbott Diagnostics | ARCHITECT | STAT hs-cTnI | 1.1 to 1.9 | 0.7 to 1.3 | 26.2 | 4% | 16 | ✓ |
| Beckman Coulter | Access and UniCel DxI | AccuTnI+3 | 10 | < 10 | 40.0 | < 10% | 13 | ✓ |
| Roche | Elecsys | cTnT-hs | 5 | 3 | 14 | < 10% | 18 | ✓ |
| Roche | Elecsys | cTnT-hs STAT | 5 | 3 | 14 | < 10% | 9 | ✓ |
The hs-cTn assays can be used as single diagnostic tests, or in combination with other cardiac biomarkers, for example heart fatty acid binding protein (H-FABP) and copeptin. The use of combinations of cardiac biomarkers may increase sensitivity, when a positive result on either test is considered to be indicative of AMI, although this increase may be achieved at the expense of decreased specificity. Conversely, if a positive result on both tests is required before AMI is diagnosed, increased specificity and reduced sensitivity are likely. It is currently unclear which, if any, of the available cardiac biomarkers could add clinical benefit if used in combination with hs-cTnI and hs-cTnT, compared with hs-cTnI and hs-cTnT alone. A recent systematic review reported some data for combination testing, but none of the identified studies of Tns combined with other biomarkers used high-sensitivity methods. 7 Retrospective analysis of data from one arm of a randomised controlled trial (RCT) by the same authors provided some indication that the use of H-FABP in combination with hs-cTn, on admission, may increase sensitivity for AMI without decreasing specificity. 19 This increase was equivalent to the sensitivity achieved by serial hs-cTn testing on admission and at 90 minutes. 19 However, these tests are not readily available for analytical platforms in routine use in the NHS and discussions at the scoping stage of this assessment concluded that practical applications of H-FABP and copeptin assays and evidence for their effectiveness are not yet sufficiently developed to justify their inclusion.
This assessment will consider hs-cTn assays used singly or in series, up to 4 hours after the onset of chest pain or up to 4 hours after presentation (as reported); for serial Tn measurements, both data on change in Tn levels and peak Tn will be considered (as reported).
Comparator
The comparator for this technology appraisal is the current UK standard of serial TnT and/or I testing (using any method not defined as a hs-cTn test) on admission and at 10–12 hours after the onset of symptoms. 11
Care pathway
Diagnostic assessment
The assessment of patients with suspected ACS is described in the National Institute for Health and Care Excellence (NICE) clinical guideline 95 (CG95)11 ‘Chest pain of recent onset: Assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin’. The guideline11 specifies that initial assessment should include a resting 12-lead ECG along with a clinical history, a physical examination and biochemical marker analysis. For people in whom a regional ST segment elevation or presumed new left branch bundle block is seen on ECG, management should follow NICE clinical guideline 167 (CG167)20 ‘The acute management of AMI with ST segment elevation’. People without persistent ST-elevation changes on ECG [i.e. with suspected non-ST segment elevation acute coronary syndrome (NSTE-ACS)], should receive further investigation using cardiac biomarkers, with the aim of distinguishing NSTEMI from UA. NICE CG9511 makes the following recommendations on the use of cardiac biomarkers:
-
Take a blood sample for cTnI or cTnT on initial assessment in hospital. These are the preferred biochemical markers to diagnose AMI.
-
Take a second blood sample for cTnI or cTnT measurement 10–12 hours after the onset of symptoms.
-
Do not use biomarkers such as natriuretic peptides and high-sensitivity C-reactive protein to diagnose an ACS.
-
Do not use biomarkers of myocardial ischaemia (such as ischaemia-modified albumin) as opposed to markers of necrosis when assessing people with acute chest pain.
-
Take into account the clinical presentation, from the time of onset of symptoms and the resting 12-lead ECG findings, when interpreting Tn measurements.
Clinical guideline 9511 recommends that a diagnosis of NSTEMI should be made using the universal definition of AMI. 8 However, the third universal definition of AMI has been updated since the publication of CG95. 21 The most recent version states that AMI is defined as ‘The detection of a rise and/or fall of cardiac biomarker values (preferably cardiac Tn) with at least one value above the 99th percentile upper reference limit and with at least one of the following: symptoms of ischaemia, new or presumed new significant ST segment T wave changes or new left branch bundle block, development of pathological Q waves in the ECG, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality, or identification of an intracoronary thrombus by angiography or autopsy’.
The Scottish Intercollegiate Guidelines Network guideline 93 (SIGN 93)12 provides similar recommendations on the diagnostic work-up of people with suspected ACS, stating:
-
immediate assessment with a 12-lead ECG
-
repeat 12-lead ECG if there is diagnostic uncertainty or change in clinical status, and at discharge
-
serum Tn measurement on arrival at hospital
-
repeat serum Tn measurement 12 hours after the onset of symptoms
-
Tn concentrations should not be interpreted in isolation but with regard to clinical presentation.
Guidelines from the ESC22 on the diagnostic assessment of people with a suspected NSTE-ACS are consistent with those of NICE and SIGN, but additionally acknowledge the use of high-sensitivity Tn assays and make recommendations on a fast-track rule-out protocol. The guidelines22 state that hs-cTn assays have a negative predictive value (NPV) of > 95% for AMI on admission; including a second sample of hs-cTn at 3 hours can increase this to 100%.
Management/treatment
The NICE clinical guideline 94 (CG94), ‘Unstable angina and NSTEMI: The early management of unstable angina and non-STEMI’,23 provides recommendations on the management of people with suspected NSTE-ACS. The guideline23 states that initial treatment should include a combination of antiplatelet (aspirin, clopidogrel and glycoprotein IIb/IIIa inhibitors) and antithrombin therapy, and should take into account contraindications, risk factors and the likelihood of percutaneous coronary intervention. SIGN 9312 makes similar recommendations. It is recommended that people with a diagnosis of NSTEMI, who are assessed as being at low risk of future complications, receive conservative treatment with aspirin and/or clopidogrel, or aspirin in combination with ticagrelor. People at a higher risk of future complications should be offered coronary angiography (within 96 hours of admission), with subsequent coronary revascularisation by percutaneous coronary intervention or coronary artery bypass grafting where indicated. 23 Additional testing to quantify inducible ischaemia may also be used, before discharge, to identify those who may need further intervention23 and SIGN 9312 also recommends functional testing to identify people at higher risk. SIGN 9312 states that people in whom an elevated Tn level is not observed may be discharged for further follow-up according to clinical judgement and, in some cases, the results of ischaemia testing. 12
Longer-term follow-up of people who have had an AMI is described in full in NICE clinical guideline 48 (CG48)24 ‘Secondary prevention in primary and secondary care for patients following a myocardial infarction’. This includes recommendations on lifestyle changes, cardiac rehabilitation programmes, drug therapy [including a combination of angiotensin-converting enzyme (ACE) inhibitors, aspirin, beta-blockers and statins], and further cardiological assessment to determine whether coronary revascularisation is required.
Chapter 3 Assessment of clinical effectiveness
A systematic review was conducted to summarise the evidence on the clinical effectiveness of hs-cTn assays for the early rule-out or diagnosis of AMI in people with acute chest pain. Systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care25 and the NICE Diagnostics Assessment Programme manual. 26,27
Systematic review methods
Search strategy
Search strategies were based on intervention (high-sensitivity Tn assays) and target condition, as recommended in the CRD guidance for undertaking reviews in health care25 and the Cochrane Handbook for Diagnostic Test Accuracy Reviews. 27
Candidate search terms were identified from target references, browsing database thesauri [e.g. MEDLINE medical subject heading (MeSH) and EMBASE Emtree], existing reviews identified during the rapid appraisal process and initial scoping searches. These scoping searches were used to generate test sets of target references, which informed text mining analysis of high-frequency subject-indexing terms using EndNote X4 reference management software (Thomson Reuters, CA, USA). Strategy development involved an iterative approach testing candidate text and indexing terms across a sample of bibliographic databases, aiming to reach a satisfactory balance of sensitivity and specificity.
The following databases were searched for relevant studies from 2005 to October 2013:
-
MEDLINE (OvidSP): 2005–2013/10/wk1.
-
MEDLINE In-Process Citations and Daily Update (OvidSP): up to 2013/10/1.
-
EMBASE (OvidSP): 2005–2013/10/10.
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley): Cochrane Library Issue 10 2005–2013/10/11.
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley): Cochrane Library Issue 9 2005–2013/10/11.
-
Database of Abstracts of Reviews of Effects (DARE) (Wiley): Cochrane Library Issue 3 2005–July 2013.
-
Health Technology Assessment (HTA) Database (Wiley): Cochrane Library Issue 3 2005–July 2013.
-
Science Citation Index (SCI) (Web of Science): 2005–2013/10/14.
-
Conference Proceedings Citation Index – Science (CPCI) (Web of Science): 2005–2013/10/14.
-
Latin American and Caribbean Health Sciences Literature (LILACS) (Internet): 2005–2013/10/11 (http://regional.bvsalud.org/php/index.php?lang = en).
-
International Network of Agencies for Health Technology Assessment (INAHTA) Publications (Internet): 2005–2013/10/15 (www.inahta.org/).
-
BIOSIS Previews (Web of Knowledge): 2005–2013/10/11.
-
National Institute for Health Research (NIHR) Health Technology Assessment programme (Internet): 2005–2013/10/14.
-
Aggressive Research Intelligence Facility (ARIF) database (Internet): 2005–2013/10/16 (www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/index.aspx).
-
Medion database (Internet): 2005–2013/10/16 (www.mediondatabase.nl/).
-
PROSPERO (International Prospective Register of Systematic Reviews) (Internet): up to 2013/10/10 (www.crd.york.ac.uk/prospero/).
Completed and ongoing trials were identified by searches of the following resources (2005 to October 2013):
-
National Institutes of Health (NIH) ClinicalTrials.gov (Internet): up to 2013/10/1 (www.clinicaltrials.gov/).
-
Current Controlled Trials (CCT) (Internet): up to 2013/10/10 (www.controlled-trials.com/).
-
World Health Organization International Clinical Trials Registry Platform (ICTRP) (Internet): up to 2013/10/10 (www.who.int/ictrp/en/).
No restrictions on language or publication status were applied. Date restrictions were applied based on expert advice on the earliest appearance of literature of high-sensitivity Tn assays. Searches took into account generic and other product names for the intervention. The main EMBASE strategy for each set of searches was independently peer reviewed by a second Information Specialist, using the Canadian Agency for Drugs and Technologies in Health (CADTH) Peer Review Checklist. 28 Search strategies were developed specifically for each database and the keywords associated with high-sensitivity Tn T/I were adapted according to the configuration of each database. Full search strategies are reported in Appendix 1.
Electronic searches were undertaken for the following conference abstracts (selected based on advice from expert committee members):
-
American Heart Association (AHA) Scientific Sessions (Internet): 2009–13 (http://my.americanheart.org/professional/Sessions/ScientificSessions/Archive/Archive-Scientific-Sessions_UCM_316935_SubHomePage.jsp).
-
American Association for Clinical Chemistry (AACC) (Internet): 2009–13 (www.aacc.org/resourcecenters/meet_abstracts_archive/abstracts_archive/annual_meeting/Pages/default.aspx#).
-
European Society of Cardiology (ESC) (Internet): 2009–13 (http://spo.escardio.org/abstract-book/search.aspx).
Identified references were downloaded in EndNote X4 software for further assessment and handling. References in retrieved articles were checked for additional studies. The final list of included papers was checked on PubMed for retractions, errata and related citations. 29–31
Inclusion and exclusion criteria
Inclusion criteria for each of the clinical effectiveness questions are summarised in Table 2. Studies that fulfilled these criteria were eligible for inclusion in the review.
| Question | What is the accuracy of hs-cTn assays (used singly or in series, such that results are available within 3 hours of presentation) for the diagnosis of AMI in adults with acute chest pain? | What is the effectiveness of hs-cTn assays (used singly or in series) compared with conventional diagnostic assessment, for achieving successful early discharge of adults with acute chest pain within 4 hours of presentation? |
|---|---|---|
| Participants | Adults (≥ 18 years) presenting with acute ‘pain, discomfort or pressure in the chest, epigastrium, neck, jaw, or upper limb without an apparent non-cardiac source’32 attributable to a suspected, but not proven, AMI | |
| Setting | Secondary or tertiary care | |
| Interventions (index test) | Any hs-cTnT or hs-cTnI test,a listed in Table 1, hs-cTn assays (used singly or in series,b such that results were available within 3 hours of presentation) | |
| Comparators | Any other hs-cTn test, as specified above, or no comparator | Tn T or I measurement on presentation and 10–12 hours after the onset of symptoms |
| Reference standard | Universal definition of AMI, including measurement of Tn T or I (using any method not defined as a hs-cTn test) on presentation and 10–12 hours after the onset of symptoms in ≥ 80% of the populationc or occurrence of MACE (any definition used in identified studies) during 30-day follow-up | NA |
| Outcomesd | Test accuracy (the numbers of TP, FN, FP and TN test results) | Early discharge (≤ 4 hours after initial presentation) without MACE during follow-up, incidence of MACE during follow-up, re-attendance at or re-admission to hospital during follow-up, time to discharge, patient satisfaction or HRQoL measures |
| Study design | Diagnostic cohort studies | RCTs (CCTs) will be considered if no RCTs are identified) |
Inclusion screening and data extraction
Two reviewers (MW and PW) independently screened the titles and abstracts of all of the reports identified by searches, and any discrepancies were discussed and resolved by consensus. Full copies of all of the studies that were deemed potentially relevant were obtained and the same two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus. Details of studies excluded at the full paper screening stage are presented in Appendix 4.
Studies cited in materials provided by the manufacturers of hs-cTn assays were first checked against the project reference database, in EndNote X4; any studies not already identified by our searches were screened for inclusion following the process described above.
Data were extracted on the following: study details, inclusion and exclusion criteria, participant characteristics (demographic characteristics and cardiac risk factors), target condition (NSTEMI or AMI), details of the hs-cTnT or hs-cTnI test (manufacturer, timing, and definition of positive diagnostic threshold), details of reference standard [manufacturer, timing, diagnostic threshold for conventional Tn T or I testing, clinical and imaging components of the reference standard, method of adjudication (e.g. two independent clinicians)] and test performance outcome measures [numbers of true-positive (TP), false-positive (FP), false-negative (FN) and true-negative (TN) test results]. Data were extracted by one reviewer, using a piloted, standard data extraction form and checked by a second (MW and PW); any disagreements were resolved by consensus. Full data extraction tables are provided in Appendix 2.
Quality assessment
The methodological quality of included diagnostic test accuracy (DTA) studies was assessed using QUADAS-2. 33 Quality assessments was undertaken by one reviewer and checked by a second (MW and PW); any disagreements were resolved by consensus.
The results of the quality assessments are summarised and presented in tables and graphs in the results of the systematic review and are presented in full, by study, in Appendix 3.
Methods of analysis/synthesis
Sensitivity and specificity were calculated for each set of 2 × 2 data, and plotted in receiver operating characteristic (ROC) space. The bivariate/hierarchical summary receiver operating characteristic (HSROC) model was used to estimate summary sensitivity and specificity with 95% confidence intervals (CIs) and prediction regions around the summary points, and to derive HSROC curves for meta-analyses involving four or more studies. 34–36 This approach allows for between-study heterogeneity in sensitivity and specificity, and for the trade-off (negative correlation) between sensitivity and specificity commonly seen in diagnostic meta-analyses. For meta-analyses with fewer than four studies we estimated separate pooled estimates of sensitivity and specificity, using random-effects logistic regression. 37 Heterogeneity was assessed visually using summary receiver operating characteristic (SROC) plots and statistically using the variance of logit (sensitivity) and logit (specificity), where ‘logit’ indicates the logistic function: the smaller these values, the less heterogeneity between studies. Summary positive and negative likelihood ratios (LR+ and LR–) were derived from the summary estimates of sensitivity and specificity. Analyses were performed in Stata 10 (StataCorp LP, College Station, TX, USA), mainly using the metandi command. For analyses that would not run in Stata we used MetaDiSc version 1.4 (freeware, available to download from www.hrc.es/investigacion/metadisc_en.htm). 38
Analyses were conducted separately for each of the three hs-cTn assays. Analyses were stratified according to whether the study evaluated the prediction of AMI or MACE, timing of collection of blood sample for testing, and the threshold used to define a positive hs-cTn result. We investigated possible sources of heterogeneity using stratified analyses based on the following variables:
-
population – studies included mixed populations compared with those that excluded patients with STEMI
-
age > 70 years compared with age ≤ 70 years
-
patients with pre-existing CAD at baseline compared with patients without pre-existing CAD
-
time from symptom onset to presentation < 3 hours compared with > 3 hours
-
time from symptom onset to presentation < 6 hours compared with > 6 hours
-
low to moderate pre-test probability of disease compared with high pre-test probability of disease.
Stratified analyses were conducted for all time points and thresholds for which sufficient data were available. To investigate the influence of risk of bias on the studies, we restricted analyses to studies conducted in patients at low or unclear risk of bias for the two QUADAS items considered to have the greatest potential to have introduced bias into these studies: the item on patient spectrum (1) and the item on patient flow (4). As the focus of this review was the diagnosis of NSTEMI, we conducted these analyses in studies that excluded patients with STEMI. We used SROC plots to display summary estimates from the various primary and stratified analyses.
We compared the accuracy of the three different hs-cTn assays by tabulating summary estimates from analyses for common time points and thresholds assessed for all assays. Only one study39 provided a direct comparison of all three assays. Estimates of sensitivity, specificity, and LR+ and LR– for each assay derived from this study were included in the summary tables.
Results of the assessment of clinical effectiveness assessment
The literature searches of bibliographic databases identified 6766 references. After initial screening of titles and abstracts, 261 were considered to be potentially relevant and ordered for full paper screening; of these, 35 were included in the review. 19,40–72 All potentially relevant studies cited in documents supplied by the test manufacturers had already been identified by bibliographic database searches. One additional study73 was identified from hand-searching of conference abstracts, and two additional studies39,74 were identified from information supplied by clinical experts. Figure 1 shows the flow of studies through the review process, and Appendix 4 provides details, with reasons for exclusions, of all of the publications excluded at the full paper screening stage.
FIGURE 1.
Flow of studies through the review process.

Overview of included studies
Based on the searches and inclusion screening described above, 37 publications19,39,40–74 of 18 studies19,39,40,42,44,46,48,49,51,54,55,57,58,63,64,67,70,73 were included in the review; the results sections of this report cites studies using the primary publication and, where this is different, the publication in which the referenced data were reported. Fifteen studies19,39,40,42,44,46,49,51,54,55,57,58,64,67,70 reported accuracy data for the Roche Elecsys hs-cTnT assay, four studies39,48,58,63 reported accuracy data for the Abbott ARCHITECT hs-cTnI assay, and two studies39,73 reported accuracy data for the Beckman Coulter Access hs-cTnI assay; two studies39,58 reported data for more than one assay. No RCTs or current controlled trials (CCTs) were identified; no studies provided data on the effects on patient-relevant outcomes of management based on hs-cTn assays within 4 hours of presentation compared with management based on standard cTn assays at presentation and after 10–12 hours. All studies included in the systematic review were diagnostic cohort studies, which reported data on the diagnostic or prognostic accuracy hs-cTn assays.
Thirteen19,39,40,42,44,48,49,51,55,57,64,67,73 of the 18 included studies19,39,40,42,44,46,48,49,51,54,55,57,58,63,64,67,70,73 were conducted in Europe (two in the UK19,67), four were conducted in Australia and New Zealand,46,54,58,63 and one was conducted in the USA. 70 Thirteen39,40,42,46,48,49,51,54,55,57,63,64,70 of the 18 included studies19,39,40,42,44,46,48,49,51,54,55,57,58,63,64,67,70,73 reported receiving some support from test manufacturers, including supply of assay kits; two studies58,73 did not report any information on funding.
Full details of the characteristics of study participants, study inclusion and exclusion criteria, and hs-cTn assay used and reference standard, and detailed results are reported in the data extraction tables presented in Appendix 2.
Study quality
The main potential sources of bias in the 18 studies19,39,40,42,44,46,48,49,51,54,55,57,58,63,64,67,70,73 included in this assessment relate to patient spectrum and patient flow. There were also concerns regarding the applicability of the patient population and the reference standard in some of the included studies. The results of QUADAS-2 assessments are summarised in Table 3 and Figure 2; full QUADAS-2 assessments for each study are provided in Appendix 3. A summary of the risks of bias and applicability concerns within each QUADAS-2 domain is provided in Table 3.
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Aldous (2011)54 | ☺ | ☺ | ☺ | ☹ | ☹ | ☺ | ☹ |
| Aldous (2012)46 | ☹ | ☺ | ☺ | ☺ | ☺ | ☺ | ☹ |
| Body (2011)67 | ? | ☺ | ☺ | ☺ | ☹ | ☺ | ☹ |
| Christ (2010)57 | ☺ | ☺ | ? | ☺ | ☹ | ☺ | ☹ |
| Collinson (2013)19 | ☺ | ☺ | ☺ | ☹ | ☹ | ☺ | ☺ |
| Cullen (2013)63 | ☺ | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ |
| Eggers (2012)44 | ? | ☺ | ? | ☹ | ☹ | ☺ | ☹ |
| Freund (2011)49 | ☺ | ☺ | ☺ | ☺ | ☹ | ☺ | ☹ |
| Hoeller (2013)39 | ☺ | ☺ | ☺ | ☹ | ☹/☺ | ☺ | ☺ |
| Keller (2011)48 | ☺ | ☺ | ☺ | ☹ | ☹ | ☺ | ☹ |
| Kurz (2011)55 | ? | ☺ | ☺ | ☺ | ☹ | ☺ | ☹ |
| Lippi (2012)73 | ☺ | ☹ | ? | ? | ☹ | ☺ | ☹ |
| Melki (2011)51 | ☹ | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ |
| Parsonage (2013)58 | ? | ☺ | ☺ | ? | ☹ | ☺ | ☹ |
| Saenger (2010)70 | ? | ☺ | ? | ? | ☹ | ☺ | ☹ |
| Sanchis (2012)42 | ☹ | ☺ | ? | ☺ | ☹ | ☺ | ☺ |
| Santalo (2013)40 | ☺ | ☺ | ? | ☺ | ☺ | ☺ | ? |
| Sebbane (2013)64 | ? | ☺ | ☺ | ☹ | ☺ | ☺ | ☹ |
FIGURE 2.
Summary of QUADAS-2 results for studies of hs-cTn assays.

Patient spectrum
Three studies42,46,51 were rated as ‘high risk of bias’ for patient selection and a further six44,55,58,64,67,70 were rated as ‘unclear risk of bias’. Most studies rated as ‘unclear risk of bias’ did not provide sufficient details to make a judgement on whether appropriate steps were taken to minimise bias when enrolling patients into the study. 44,58,64,67,70 In one study,55 a large number of patients were not enrolled because of ‘technical reasons’ that were not fully defined and so it was not possible to judge whether these constituted inappropriate exclusions; this study55 was also judged as unclear risk of bias for this domain. One study46 enrolled patients presenting only between 05.30 and 20.00 and so patients who presented outside these hours were excluded; as these patients may differ in their presenting characteristics (e.g. time from symptom onset) this was considered to introduce a potential bias into the study. A further study51 stated that consecutive patients were enrolled except for temporary interruptions of the study as a result of high work load in the coronary care unit. This was also considered to have the potential to lead to the inclusion of a different spectrum of patients than if consecutive patients had been enrolled. The last study42 judged at ‘high risk of bias’ for patient enrolment excluded certain patient groups, including those with a Tn elevation in any two serial determinations, a prior diagnosis of ischaemic heart disease, structural heart disease, concomitant HF or significant bradyarrhythmia.
Although this assessment included studies that enrolled both mixed populations (i.e. when the target condition was any AMI) and studies restricted to populations in which patients with STEMI were excluded (i.e. target condition NSTEMI), the primary focus was the population of patients with STEMI excluded. Studies not restricted to this specific patient group were therefore considered to have high concerns regarding applicability. Seven studies19,40,44,46,51,55,64 were restricted to patients in whom STEMI had been excluded; an additional study39 enrolled a mixed population but also presented data for patients in whom STEMI had been excluded. Three of these studies44,51,55 were restricted to patients admitted to coronary care/chest patients units and so were considered to represent patients with more severe disease. A further study19 had strict inclusion criteria, which resulted in the inclusion of a very-low-risk population. These four studies19,44,51,55 were not considered to be representative of patients with chest pain presenting to the ED, who are the main focus of this assessment, and so were also rated as having high concerns regarding applicability. Therefore, only four studies39,40,46,75 (one39 only for a subset of data) were considered to have low concerns regarding the applicability of the included patients.
Index test
All but one of the studies19,39,40,42,44,46,48,49,51,54,55,57,58,63,64,67,70 were rated as ‘low risk of bias’ for the index test, as all reported data for at least one threshold that was prespecified [generally the 99th centile threshold, LoD or limit of blank (LoB) threshold]. The study73 that was rated as high risk of bias on this domain assessed the accuracy of the Beckman Coulter Access hs-cTnI assay at a single threshold which was derived from the ROC curve. As the reference standard (diagnosis of AMI or MACE) was interpreted after the high-sensitivity Tn test, blinding was not considered important for these studies. Inclusion criteria were very tightly defined in terms of the high-sensitivity Tn assays in which we were interested and so all studies were considered to have low concerns regarding the applicability of the index test.
Reference standard
Six studies40,42,44,55,70,71 were rated as unclear risk of bias for reference standard. In five studies,39,41,43,54,56 this was because it was unclear whether the diagnosis of AMI/MACE was made without knowledge of the high-sensitivity Tn results. Two studies71,74 reported as abstracts provided insufficient details on how the diagnosis of AMI was made, including whether adjudicators were blinded to the high-sensitivity Tn results, to judge whether an appropriate reference standard had been used. No studies were rated as high risk of bias for this domain, as these would not have fulfilled the inclusion criteria for the review. In our review question, we specified that an appropriate reference standard had to include a standard Tn measurement at baseline and at 10–12 hours after the onset of symptoms in 80% of the population. 11 Only five studies19,39,42,51,63 met this criterion for standard Tn measurement and were judged to have low concerns regarding the applicability of the reference standard; all but one of the remaining studies44,46,48,49,54,55,57,58,64,67,70,73 were judged at high risk of bias, the other study did not provide exact details on the timing of the standard Tn assay. 39
Patient flow
Six studies19,39,44,48,54,64 were considered at high risk of bias for patient flow and a further three studies58,70,73 were considered at unclear risk of bias. In all cases this was related to withdrawals from the study; verification bias was not considered to be a problem in any of the studies. The three studies58,70,71 that were rated as unclear risk of bias were reported only as abstracts and did not provide sufficient details to judge whether there were any withdrawals in the study. The studies judged at high risk of bias on this domain generally excluded patients for whom samples or high sensitive Tn results were not available.
Diagnostic accuracy of the Roche Elecsys high-sensitivity cardiac troponin T assay
Study details
Fifteen diagnostic cohort studies,19,39,40,42,44,46,49,51,54,55,57,58,64,67,70 reported in 34 publications,19,39,40–47,49–62,64–68,70–72,74 provided data on the diagnostic performance of the Roche Elecsys hs-cTnT assay. Fourteen19,39,40,44,46,49,51,54,55,57,58,64,67,70 of the 15 studies19,39,40,42,44,46,49,51,54,55,57,58,64,67,70 in this section assessed the accuracy of the Roche Elecsys hs-cTnT assay for the detection of AMI, and the remaining study42 assessed accuracy for the prediction of MACE within 30 days of the index presentation. Eight studies19,39,40,44,46,51,55,64 provided data specific to the population of interest for this assessment; participants with STEMI were excluded (i.e. the target condition was NSTEMI rather than any AMI).
All 14 of the studies19,39,40,44,46,49,51,54,55,57,58,64,67,70 that assessed accuracy for the detection of AMI reported data on the diagnostic performance of a single sample taken on presentation. All but one of the studies19,39,40,44,46,49,51,55,57,58,64,67,70 reported data for the 99th centile for the general population; the remaining study54 reported data for a ROC-derived threshold of 9.5 ng/l. Studies additionally assessed the diagnostic performance of a LoD/LoB threshold (5 ng/l or 3 ng/l) in a single sample taken on presentation,39,46,53,54,67 of a single sample taken 1–3 hours after presentation,46,51 and/or the diagnostic performance of a specified change in, or peak value of, hs-cTnT level over the initial 3 hours from presentation. 39,40,46,58,70 Table 4 provides summary estimates of the diagnostic performance of all combinations of population, diagnostic threshold and hs-cTnT test timing, which were assessed by more than one study. For analyses based on NSTEMI patients only when sufficient data were available, sensitivity analyses that excluded studies rated as ‘high risk of bias’ on one or more QUADAS domains were also reported. When combinations were assessed by a single study, diagnostic performance estimates derived from that study alone are provided. Key results used in the cost-effectiveness modelling conducted for this assessment are highlighted in bold text. Full results (including numbers of TP, FP, FN and TN test results) for all studies and all data sets are provided in Appendix 2 (see Study results).
| Grouping | Population | Risk of bias | n | Sensitivity (%) | Specificity (%) | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| Presentation samples | |||||||
| Any thresholda | All | Mixed | 14 | 88 (84 to 91) | 82 (77 to 86) | 4.88 (3.84 to 6.21) | 0.14 (0.11 to 0.19) |
| All | Low/unclear risk of bias on patient spectrum | 13 | 86 (83 to 89) | 82 (77 to 87) | 4.89 (3.76 to 6.35) | 0.16 (0.14 to 0.20) | |
| All | Low/unclear risk of bias on patient flow | 11 | 90 (87 to 93) | 80 (77 to 84) | 4.69 (3.88 to 5.66) | 0.12 (0.09 to 0.16) | |
| All | Low/unclear risk of bias on patient spectrum and patient flow | 8 | 89 (85 to 92) | 80 (74 to 85) | 4.49 (3.47 to 5.80) | 0.14 (0.11 to 0.18) | |
| 99th centile threshold | All | Mixed | 13 | 89 (85 to 92) | 82 (77 to 86) | 4.94 (3.84 to 6.39) | 0.13 (0.10 to 0.19) |
| Mixed | Mixed | 8 | 89 (86 to 91) | 81 (76 to 85) | 4.64 (3.73 to 5.76) | 0.14 (0.11 to 0.17) | |
| STEMI excluded | Mixed | 6 | 88 (78 to 93) | 84 (74 to 90) | 5.41 (3.40 to 8.63) | 0.15 (0.08 to 0.26) | |
| STEMI excluded | Low/unclear risk of bias on patient spectrum | 4 | 81 (75 to 86) | 85 (70 to 93) | 5.33 (2.65 to 10.72) | 0.22 (0.17 to 0.29) | |
| STEMI excluded | Low/unclear risk of bias on patient flow | 3 | 92 (88 to 94) | 79 (76 to 82) | 4.38 (3.02 to 6.11) | 0.10 (0.05 to 0.22) | |
| STEMI excluded | Low/unclear risk of bias on patient spectrum and patient flow | 140 | 89 (81 to 94) | 71 (66 to 76) | 3.11 (2.55 to 3.79) | 0.15 (0.08 to 0.28) | |
| Age ≤ 70 years | High risk for patient flow | 1 19 , 39 , 53 – 73 | 88 (78 to 94) | 86 (83 to 89) | 6.24 (5.03 to 7.74) | 0.14 (0.07 to 0.28) | |
| Age > 70 years | High risk for patient flow | 1 19 , 39 , 53 – 73 | 97 (92 to 99) | 49 (44 to 55) | 1.91 (1.71 to 2.14) | 0.05 (0.02 to 0.18) | |
| Patients with pre-existing CAD | High risk for patient flow | 1 19 , 39 , 53 – 73 | 93 (85 to 97) | 60 (55 to 65) | 2.32 (2.02 to 2.68) | 0.12 (0.05 to 0.26) | |
| Patients without pre-existing CAD | High risk for patient flow | 1 19 , 39 , 47 – 73 | 94 (88 to 97) | 82 (79 to 85) | 5.18 (4.36 to 6.16) | 0.07 (0.04 to 0.16) | |
| Mixed; low to moderate pre-test probability | Low | 1 49 | 89 (70 to 97) | 85 (79 to 89) | 5.79 (4.16 to 8.06) | 0.13 (0.04 to 0.41) | |
| Mixed; high pre-test probability | Low | 1 49 | 94 (77 to 99) | 66 (50 to 79) | 2.78 (1.75 to 4.41) | 0.09 (0.02 to 0.45) | |
| Symptom onset < 3 hours | 1 study high risk for patient flow | 2 39 , 67 – 73 | 78 (71 to 83) | 84 (81 to 86) | 4.88 (3.91 to 5.74) | 0.26 (0.18 to 0.39) | |
| Symptom onset > 3 hours | 1 study high risk for patient flow | 2 39 , 67 – 73 | 94 (92 to 96) | 77 (75 to 79) | 4.09 (3.33 to 5.70) | 0.08 (0.05 to 0.11) | |
| Symptom onset < 6 hours | Low | 167 | 83 (74 to 89) | 83 (79 to 86) | 4.80 (3.80 to 6.08) | 0.21 (0.14 to 0.32) | |
| Symptom onset > 6 hours | Low | 167 | 94 (78 to 99) | 81 (75 to 86) | 4.99 (3.66 to 6.81) | 0.07 (0.02 to 0.34) | |
| LoD (< 5 ng/l) | All | Mixed | 3 | 96 (94 to 98) | 41 (39 to 44) | 1.63 (0.34 to 7.07) | 0.10 (0.07 to 0.17) |
| All; outlying study conducted in patients aged > 70 years removed | Mixed | 2 | 95 (92 to 97) | 54 (51 to 58) | 2.06 (1.40 to 2.64) | 0.09 (0.07 to 0.17) | |
| Age > 70 years | High risk for patient flow | 119,39,53–73 | 100 (95 to 100) | 1 (0 to 3) | 1.01 (0.99 to 1.03) | 0.45 (0.02 to 8.56) | |
| STEMI excluded | High risk for patient spectrum | 146 | 93 (89 to 96) | 58 (55 to 62) | 2.20 (2.00 to 2.50) | 0.11 (0.07 to 0.19) | |
| LoB (< 3 ng/l) | All | Mixed | 3 | 98 (95 to 99) | 40 (38 to 43) | 1.63 (1.24 to 1.86) | 0.05 (0.02 to 0.21) |
| STEMI excluded | High risk for patient spectrum | 1 46 | 95 (92 to 98) | 48 (44 to 51) | 1.83 (1.70 to 1.97) | 0.10 (0.05 to 0.18) | |
| Mixed; symptom onset < 3 hours | Low | 167 | 99 (94 to 100) | 64 (57 to 69) | 2.73 (2.31 to 3.23) | 0.01 (0.00 to 0.16) | |
| Mixed; symptom onset > 3 hours | Low | 167 | 99 (91 to 100) | 33 (28 to 38) | 1.47 (1.36 to 1.59) | 0.03 (0.00 to 0.47) | |
| Mixed; symptom onset < 6 hours | Low | 167 | 100 (96 to 100) | 34 (30 to 39) | 1.52 (1.41 to 1.64) | 0.01 (0.00 to 0.22) | |
| Mixed; symptom onset > 6 hours | Low | 167 | 100 (84 to 100) | 33 (27 to 40) | 1.47 (1.31 to 1.65) | 0.06 (0.00 to 0.91) | |
| 1–3 hours after presentation | |||||||
| 1–3 hours after presentation, 99th centile threshold | STEMI excluded | High risk for patient spectrum | 246,51 | 95 (92 to 97) | 80 (77 to 82) | 4.75 (3.98 to 5.23) | 0.06 (0.00 to 0.63) |
| Multiple samples | |||||||
| 99th centile threshold (peak) and Δ20% (presentation to 3 hours) | All | High risk for patient spectrum | 146–50 | 50 (43 to 56) | 94 (92 to 96) | 8.40 (6.10 to 11.60) | 0.54 (0.47 to 0.62) |
| 99th centile (peak) threshold or Δ20% (presentation to 3 hours) | All | High risk for patient spectrum | 146–50 | 97 (94 to 99) | 65 (61 to 68) | 2.80 (2.50 to 3.10) | 0.04 (0.02 to 0.10) |
| 99th centile (peak) threshold and Δ20% (presentation to 2 hours) | STEMI excluded | Low | 1 46 – 50 | 50 (43 to 56) | 94 (92 to 96) | 8.42 (6.11 to 11.60) | 0.54 (0.47 to 0.62) |
| 99th centile (peak) threshold or Δ20% (presentation to 2 hours) | STEMI excluded | Low | 1 46 – 50 | 97 (94 to 99) | 65 (61 to 68) | 2.76 (2.50 to 3.05) | 0.04 (0.02 to 0.10) |
| Peak above 99th centile | All | Mixed | 246,50–58 | 94 (91 to 97) | 84 (82 to 86) | 5.88 (3.56 to 10.24) | 0.07 (0.04 to 0.11) |
| On presentation (30 minutes after arrival), and at 2, 4 and 6–8 hours or until discharge: Δ20% | STEMI excluded | Low | 140 | 99 (94 to 100) | 66 (61 to 72) | 2.94 (2.50 to 3.47) | 0.01 (0.00 to 0.15) |
| On presentation and at 1 hour: Δ17% | STEMI excluded | High risk for patient flow | 139,65 | 60 (51 to 69) | 72 (69 to 75) | 2.15 (1.77 to 2.60) | 0.55 (0.44 to 0.70) |
| On presentation and at 2 hours: Δ30% | STEMI excluded | High risk for patient flow | 140,52 | 64 (52 to 74) | 84 (80 to 87) | 3.97 (3.05 to 5.17) | 0.43 (0.31 to 0.59) |
| On presentation and at 3 hours: Δ8 ng/l | Mixed | Low | 170 | 95 (89 to 98) | 95 (91 to 97) | 19.19 (10.31 to 35.72) | 0.05 (0.02 to 0.12) |
| Prediction of MACE | |||||||
| On presentation, LoB threshold | STEMI excluded | Low | 142 | 85 (74 to 92) | 46 (41 to 51) | 1.58 (1.37 to 1.81) | 0.33 (0.18 to 0.59) |
Presentation samples
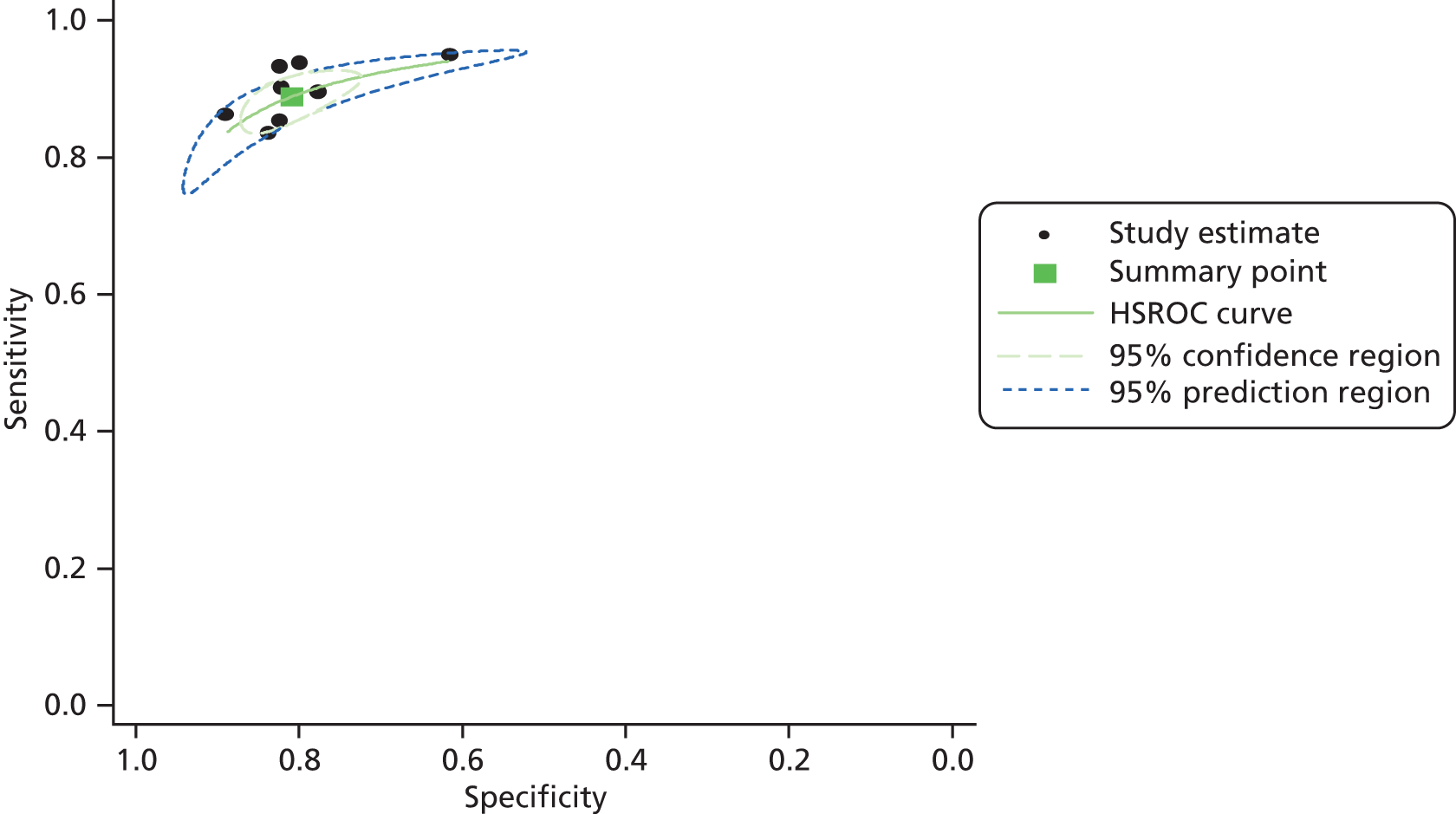
The summary estimates of sensitivity and specificity, where the diagnostic threshold was defined as the 99th centile for the general population, were 89% (95% CI 85% to 92%) and 82% (95% CI 77% to 86%), based on data from 13 studies;19,39,40,44,46,49,51,54,57,58,64,68,70 the SROC curve for this analysis is shown in Figure 3. The LR+ and LR– were 4.94 (95% CI 3.84 to 6.39) and 0.13 (95% CI 0.10 to 0.19), respectively. These estimates were similar when the analysis was restricted to studies that excluded participants with STEMI; summary estimates of sensitivity and specificity were 88% (95% CI 78 to 93%) and 84% (95% CI 74 to 90%), respectively (SROC curve shown in Figure 4) and the LR+ and LR– were 5.41 (95% CI 3.40 to 8.63) and 0.15 (95% CI 0.08 to 0.26), respectively, based on six studies. 19,40,44,46,51,64 The only study40 conducted in a population which excluded participants with STEMI, which was rated as ‘low or unclear risk of bias’ on all QUADAS domains, reported similar sensitivity and negative LR (see Table 4) to the summary estimates, but lower estimates of specificity [71% (95% CI 66% to 76%)] and LR+ [3.11 (95% CI 2.55 to 3.79)]. Results were also similar when the analysis was restricted to eight studies39,41,49,54,57,58,67,70 with a mixed population (i.e. where the target condition was any AMI); summary estimates of sensitivity and specificity were 89% (95% CI 86% to 91%) and 81% (95% CI 76% to 85%), respectively (SROC curve shown in Figure 5) and the LR+ and LR– were 4.64 (95% CI 3.73 to 5.76) and 0.14 (95% CI 0.11 to 0.17), respectively. Based on these data, it is unlikely that hs-cTnT testing on a single admission sample, using the 99th centile diagnostic threshold, would be considered adequate for either rule-out or rule-in of any AMI or NSTEMI. Although there was little apparent variation in the estimates of test performance derived from the three meta-analyses described above, the result of the second analysis (studies that excluded participants with STEMI) was selected to inform our cost-effectiveness analyses, as it best matched the main population of interest for this assessment (i.e. the target condition was NSTEMI rather than any AMI). The approach of, where possible, selecting data based on a population that excluded STEMI rather than a mixed population to inform cost-effectiveness modelling was applied throughout.
Limited data were identified on additional clinical subgroups (age > 70 years vs. ≤ 70 years,39,53 without pre-existing CAD compared with pre-existing CAD,39,46 and high pre-test probability compared with low to moderate pre-test probability (determined by clinical judgement based on cardiovascular risk factors, type of chest pain, physical findings and ECG abnormalities49). None of these studies excluded participants with STEMI. The study that stratified participants by age39,52 reported a higher estimate of sensitivity [97% (95% CI 92% to 99%)] and a lower estimate of LR– [0.05 (95% CI 0.02 or 0.18)] in participants > 70 years of age than for patients ≤ 70 years of age [88% (95% CI 78% to 94%) and 0.14 (95% CI 0.07 to 0.28), respectively]; the estimates of sensitivity and LR– for people > 70 years of age were also higher and lower, respectively, than the corresponding summary estimates derived from all 13 studies19,39,40,44,46,49,51,52,57,58,64,67,70 that used the 99th centile diagnostic threshold. A similar pattern was apparent for people with a high pre-test probability compared with those with a low to moderate pre-test probability49 and for participants without pre-existing CAD compared with those with pre-existing CAD39,47 (see Table 4). As with the age stratification, the estimates of sensitivity and LR– were higher and lower, respectively, than the corresponding summary estimates derived from all 13 studies19,39,40,44,46,49,51,54,57,58,64,67,70 which used the 99th centile diagnostic threshold, for people with a high pre-test probability and for people without pre-existing CAD. Figure 6 illustrates the variation in performance characteristics of a single admission sample, using the 99th centile diagnostic threshold, when used in different clinical subgroups. These data provide some indication that hs-cTnT testing on a single admission sample, using the 99th centile diagnostic threshold, may be adequate for rule-out of AMI in certain selected populations [older people (≥ 70 years), those without pre-existing CAD, and people classified by clinical judgement as having a high pre-test probability].
FIGURE 6.
Receiver operating characteristic space plot for the Roche Elecsys hs-cTnT assay using the 99th centile threshold and a presentation sample in different clinical subgroups.

Time from onset of chest pain to presentation was inconsistently reported across studies; when reported, the median time from onset ranged from 2.7 hours to 8.25 hours. Full details of all information reported is provided in Appendix 2 (see Baseline study details). Two studies39,67 specifically investigated variation in test performance according to time from symptom onset to presentation. Both of these studies39,67 were conducted in a mixed population (i.e. the target condition was any AMI). Study participants were stratified by presentation before or after 3 hours,39,67 and before or after 6 hours. 67 Summary estimates for the 3-hour stratification indicated that a presentation sample using the 99th centile threshold had higher sensitivity [94% (95% CI 92% to 96%)] and lower specificity [77% (95% CI 75% to 79%)] for any AMI, when used to assess people presenting at > 3 hours after the onset of chest pain than when used to assess early presenters [sensitivity 78% (95% CI 71% to 83%) and specificity 84% (95% CI 81% to 86%)] (see Table 4). The LR– was also lower when the test was used in people presenting after 3 hours from the onset of chest pain [0.08 (95% CI 0.05 to 0.11)] than in early presenters [0.26 (95% CI 0.178 to 0.39)]. Test performance in people presenting after 6 hours from the onset of chest pain was similar to that observed in people presenting after 3 hours (see Table 4). Figure 7 illustrates the variation in performance characteristics of a single admission sample, using the 99th centile diagnostic threshold, when used in people presenting at different times from the onset of chest pain. These data provide some indication that hs-cTnT testing on a single admission sample, using the 99th centile diagnostic threshold, may be adequate for rule-out of AMI when people present after 3 hours from the onset of chest pain, but that longer delays in presentation did not appear to further improve rule-out performance.
FIGURE 7.
Receiver operating characteristic space plot for the Roche Elecsys hs-cTnT assay using the 99th centile threshold and a presentation sample in people presenting at different times after symptom onset.

Five studies39,46,53,54,57,67 considered the performance of a presentation sample using a threshold equivalent to the LoD (5 ng/l) or LoB (3 ng/l) of the assay for the diagnosis of AMI. Three studies39,46,53,54 reported data for the 5 ng/l threshold; one of these studies39,52 reported data at this threshold only for participants > 70 years of age. When this study39,52 was excluded, the summary estimates of sensitivity and specificity were 95% (95% CI 92% to 97%) and 54% (95% CI 51% to 58%), respectively, and the LR+ and LR– were 2.06 (95% CI 1.40 to 2.64) and 0.09 (95% CI 0.07 to 0.17), respectively (see Table 4). Three studies reported data for the 3 ng/l threshold. 42,46,67 The summary estimates of sensitivity and specificity derived from these studies were 98% (95% CI 95% to 99%) and 40% (95% CI 38% to 43%), respectively, and the LR+ and LR– were 1.63 (95% CI 1.24 to 1.86) and 0.05 (95% CI 0.02 to 0.21), respectively (see Table 4). Only one study46 was conducted in a population that excluded people with STEMI; however, estimates of test performance from this study were similar to the summary estimates. For the 3-ng/l threshold, sensitivity and specificity derived from this study were 95% (95% CI 92% to 98%) and 48% (95% CI 44% to 51%), respectively, and the LR+ and LR– were 1.83 (95% CI 1.70 to 1.97) and 0.10 (95% CI 0.05 to 0.18), respectively (see Table 4). 46 For the 5-ng/l threshold, sensitivity and specificity derived from this study were 93% (95% CI 89% to 96%) and 58% (95% CI 55% to 62%), respectively, and the LR+ and LR– were 2.20 (95% CI 2.00 to 2.50) and 0.11 (95% CI 0.07 to 0.19), respectively (see Table 4). 46 These data provide some indication that hs-cTnT testing on a single admission sample may be adequate to rule out any AMI or NSTEMI, where a lower diagnostic threshold (5 ng/l or 3 ng/l) is used.
Subsequent samples
The summary estimates of sensitivity and specificity, where the diagnostic threshold was defined as the 99th centile for the general population but the sample was taken 1–3 hours after presentation, were 95% (95% CI 92% to 97%) and 80% (95% CI 77% to 82%), based on data from two studies. 46,51 The LR+ and LR– were 4.75 (95% CI 3.98 to 5.23) and 0.06 (95% CI 0.00 to 0.63), respectively (see Table 4). Both of these studies46,51 were conducted in populations that excluded people with STEMI. Unsurprisingly, these data indicate a similar improvement in rule-out performance to that seen when the test is used only in people presenting > 3 hours after the onset of chest pain.
Multiple samples
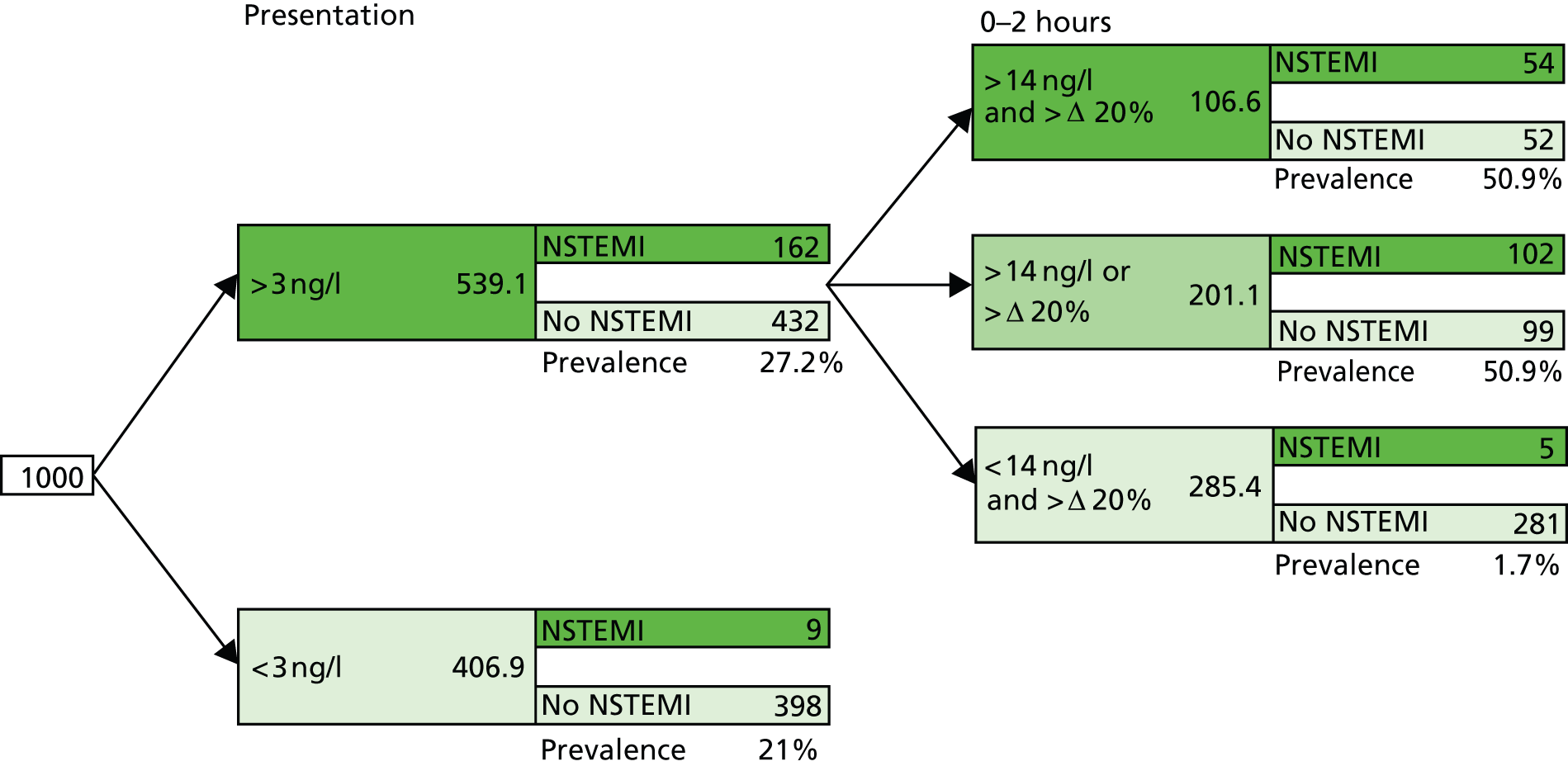
Six studies39,40,46,50,52,58,65,70 (data reported in multiple publications) provided data on the performance of a variety of diagnostic strategies involving multiple sampling, most commonly involving a combination of a peak hs-cTn value above the 99th centile diagnostic threshold and a 20% change in hs-cTnT over 2 or 3 hours following presentation (see Table 4). Figure 8 shows the results of these studies plotted in ROC space. One study46,50 reported data for this combination over 2 hours in a population that excluded people with STEMI, and this study46,50 was used in cost-effectiveness modelling. It is important to give full consideration to the optimal way of interpreting combination data of this type. As can be seen from the values reported in Table 4, a positive result from the ‘AND’ combination (defined as both a peak value above the 99th centile AND a change of > 20% over 2 hours) provides the optimum rule-in performance [LR+ 8.42 (95% CI 6.11 to 11.60)]; conversely, a negative result from the ‘OR’ combination (defined as both no value above the 99th centile AND a change of < 20% over 2 hours) provides the optimum rule-out performance [LR– 0.04 (95% CI 0.02 to 0.10)]. Where a patient has a negative result from the ‘AND’ combination/positive result from the ‘OR’ combination (defined as either a peak value above the 99th centile OR a change of > 20% over 2 hours), further investigation is likely to be needed. This optimal interpretation strategy is illustrated in Figure 9, along with a potential initial rule-out step, based on a presentation sample below the LoB threshold (3 ng/l); this strategy is included in cost-effectiveness modelling. Figure 9 shows the application of this two-stage approach to a theoretical cohort of 1000 people presenting with symptoms suggestive of ACS (STEMI excluded); the estimated number of people with AMI and a negative test result who would be erroneously discharged based on this testing strategy is 14 (nine at the first stage and five at the second stage). The prevalence of NSTEMI was estimated to be 17%, based on data from three studies40,46,64 conducted in populations that excluded people with STEMI. Four studies were excluded from the estimate of prevalence because they were considered to have unrepresentative populations: three studies44,51,55 were conducted in coronary care unit populations and one study76 was conducted in a low-risk population. It was assumed that the diagnostic performance of ‘AND’/’OR’ combinations of peak values of hs-cTnT and change over 2 hours, using the 99th centile diagnostic threshold, are the same for people in whom NSTEMI is not ruled out by the initial test (hs-cTnT > LoB) as for the initial population; this was because no test performance data were available for the combination of initial hs-cTnT test using the LoB diagnostic threshold followed by combined peak hs-cTnT and change over 2 hours using the 99th centile threshold.
FIGURE 8.
Receiver operating characteristic space plot of the Roche Elecsys hs-cTnT assay using multiple sampling strategies.

FIGURE 9.
Testing pathway for the Roche Elecsys hs-cTnT assay used in cost-effectiveness modelling.
Prognostic accuracy
One study42 assed the performance of a presentation sample at the LoB (3 ng/l) threshold for the prediction of MACE within 30 days of the index presentation. The results of this study indicate that a positive test was a poor predictor of occurrence of MACE and a negative test was not adequate to rule out MACE within 30 days (see Table 4).
Diagnostic accuracy of the Abbott ARCHITECT high-sensitivity cardiac troponin I assay
Study details
Four diagnostic cohort studies39,48,58,63 provided data on the diagnostic performance of the Abbott ARCHITECT hs-cTnI assay. Three of these studies39,48,58 assessed the accuracy of the Abbott ARCHITECT hs-cTnI assay for the detection of AMI, and the remaining study63 assessed accuracy for the prediction of MACE within 30 days of the index presentation. None of the studies in this section provided data specific to the population of interest for this assessment; participants with STEMI excluded (i.e. the target condition was NSTEMI rather than any AMI). All four studies39,48,58,63 were conducted in mixed populations. Full details of the baseline characteristics of study populations, including baseline cardiac risk factors, are provided in Appendix 2 (see Baseline study details).
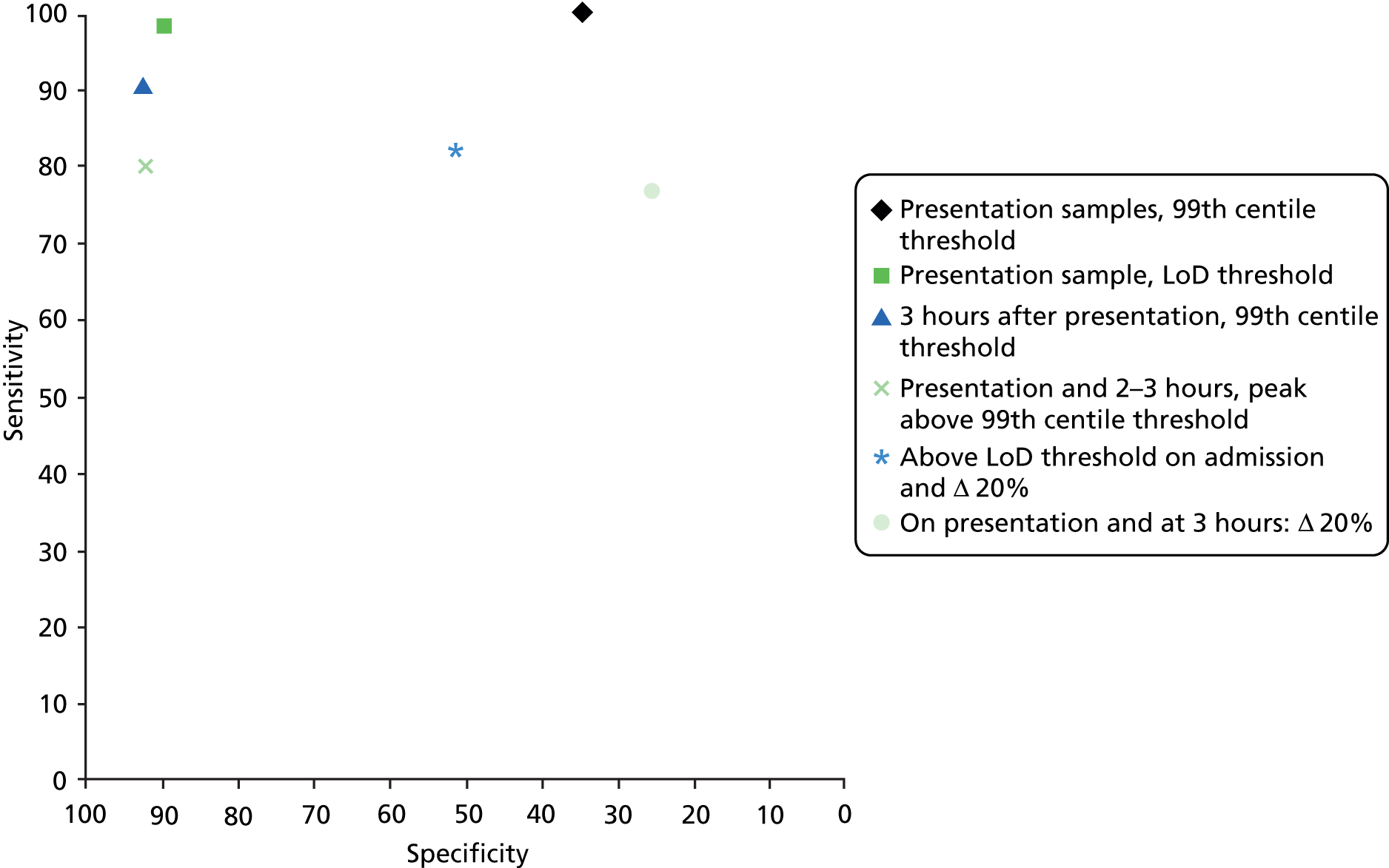
Where a single diagnostic threshold was used to define a positive test result for AMI, all studies in this section39,48,58,63 reported data for the 99th centile for the general population and a single sample taken at presentation. Table 5 provides summary estimates of diagnostic performance for this testing strategy. All other combinations of diagnostic threshold and hs-cTnI test timing were assessed by only one study. Figure 10 shows the diagnostic performance of all testing strategies assessed plotted in ROC space. Diagnostic performance estimates derived from these studies are also provided. Key results used in the cost-effectiveness modelling conducted for this assessment are highlighted in bold text. Full results (including numbers of TP, FP, FN and TN test results) for all studies and all data sets are provided in Appendix 2 (see Study results).
| Grouping | Population | Risk of bias | n | Sensitivity (%) | Specificity (%) | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| Prediction of AMI | |||||||
| Presentation samples, 99th centile threshold | Mixed | Mixed | 3 39 , 48 , 58 | 80 (77 to 83) | 93 (92 to 94) | 11.47 (9.04 to 16.19) | 0.22 (0.16 to 0.27) |
| Presentation sample, LoD threshold | Mixed | High risk for patient flow | 1 48 | 100 (98 to 100) | 35 (32 to 38) | 1.54 (1.47 to 1.62) | 0.01 (0.00 to 0.08) |
| 3 hours after presentation, 99th centile threshold | Mixed | High risk for patient flow | 1 48 | 98 (96 to 99) | 90 (88 to 92) | 10.16 (8.38 to 12.31) | 0.02 (0.01 to 0.05) |
| Presentation and 2–3 hours, peak above 99th centile threshold | Mixed | Unclear risk for patient spectrum and flow | 158 | 91 (81 to 96) | 93 (91 to 95) | 12.94 (9.74 to 17.19) | 0.09 (0.04 to 0.23) |
| Above LoD threshold on admission and Δ20% | Mixed | High risk for patient flow | 148 | 82 (78 to 86) | 52 (49 to 55) | 1.73 (1.59 to 1.88) | 0.34 (0.26 to 0.43) |
| On presentation and at 3 hours, Δ20% | Mixed | High risk for patient flow | 148 | 77 (72 to 82) | 26 (23 to 29) | 1.04 (0.97 to 1.12) | 0.87 (0.69 to 1.11) |
| Prediction of MACE | |||||||
| Presentation samples, 99th centile threshold | Mixed | High risk for patient flow for one study | 239,63 | 88 (85 to 91) | 93 (91 to 94) | 12.57 (8.88 to 15.35) | 0.13 (0.06 to 0.28) |
FIGURE 10.
Receiver operating characteristic space plot of the Abbott ARCHITECT hs-cTnT assay.
Presentation samples
Summary estimates of sensitivity and specificity based on a diagnostic threshold defined as the 99th centile for the general population were 80% (95% CI 77% to 83%) and 93% (95% CI 92% to 94%), based on data from three studies. 39,48,58 The LR+ and LR– were 11.47 (95% CI 9.04 to 16.19) and 0.22 (95% CI 0.16 to 0.27), respectively. All three studies39,48,58 were conducted in a mixed population (i.e. where the target condition was any AMI). Based on these data, it is unlikely that hs-cTnI testing on a single admission sample, using the 99th centile diagnostic threshold, would be considered adequate for rule-out of any AMI, but a positive test result may be useful in ruling in AMI.
No studies reported clinical subgroup data, or data on the performance of the test in people presenting at different times after symptom onset for the Abbott ARCHITECT hs-cTnI assay.
One study48 also considered the performance of a presentation sample using the LoD of the assay as the threshold for diagnosing AMI. This study48 provided estimates of sensitivity and specificity of 100% (95% CI 98% to 100%) and 35% (95% CI 32% to 38%), respectively, and the LR+ and LR– were 1.54 (95% CI 1.47 to 1.62) and 0.01 (95% CI 0.00 to 0.08), respectively (see Table 5). These data provide some indication that hs-cTnI testing on a single admission sample may be adequate to rule out any AMI, where a lower diagnostic threshold (the LoD of the assay) is used.
Subsequent samples
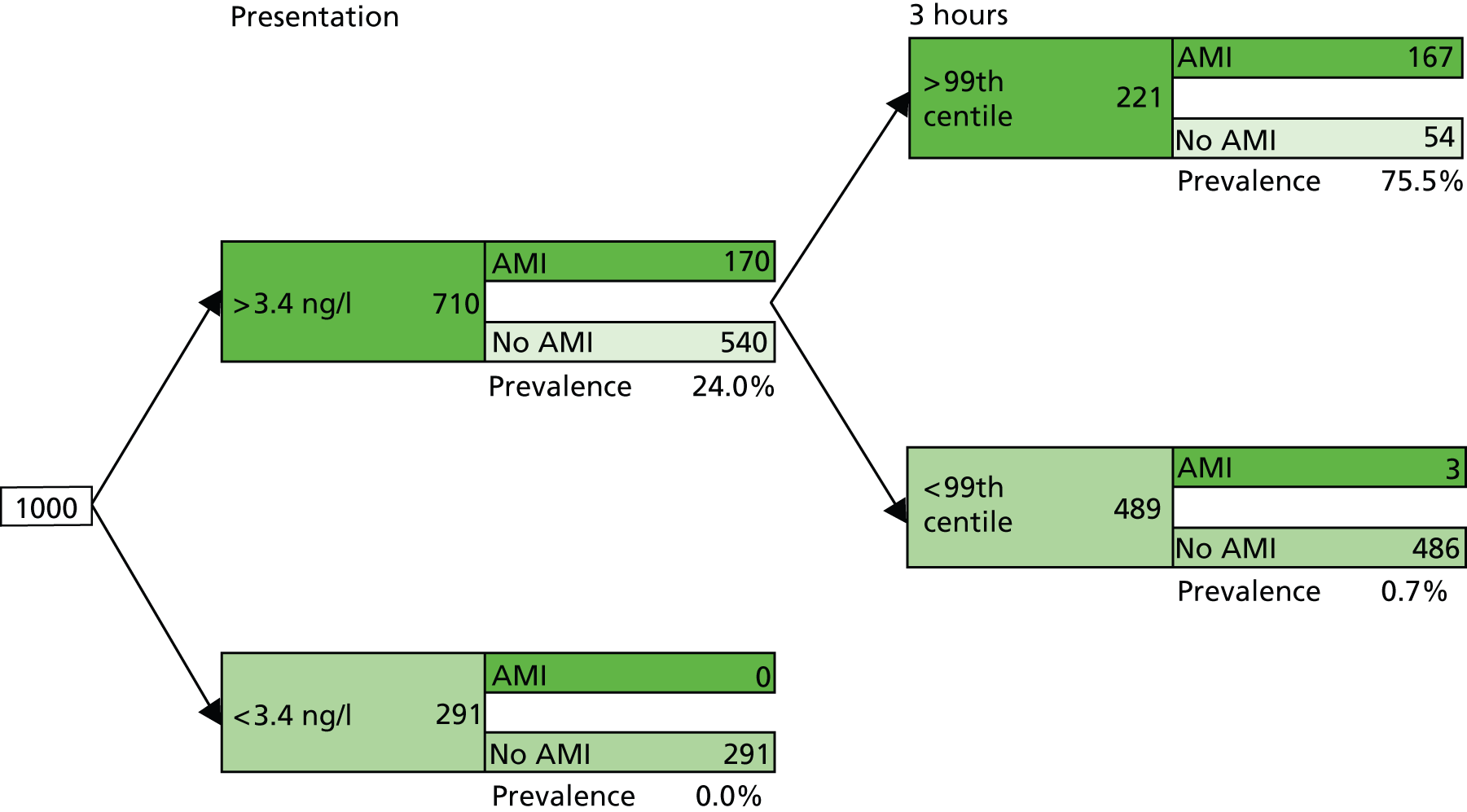
One study58 assessed the performance of hs-cTnI testing on a sample taken 3 hours after presentation, where the diagnostic threshold was defined as the 99th centile for the general population. The summary estimates of sensitivity and specificity, derived from this study, were 98% (95% CI 96% to 99%) and 90% (95% CI 88% to 92%). The LR+ and LR– were 10.16 (95% CI 8.38 to 12.31) and 0.02 (95% CI 0.01 to 0.08), respectively (see Table 5). These data provide some indication that a sample taken at 3 hours after presentation may be informative, at the 99th centile threshold, for both rule-out and rule-in of AMI.
Multiple samples
Two studies48,58 provided data on the performance of a variety of diagnostic strategies involving multiple sampling (see Table 5). None of these strategies appeared to offer a performance advantage over testing based on a single sample. Figure 11 illustrates our proposed optimal testing pathway for the Abbott ARCHITECT hs-cTnI assay; this strategy is included in cost-effectiveness modelling. As with Figure 9, which presents the Roche Elecsys hs-cTnT optimal strategy, Figure 11 shows the application of this two-stage approach to a theoretical cohort of 1000 people presenting with symptoms suggestive of ACS (STEMI excluded), with a prevalence of NSTEMI of 17%; the estimated number of people with AMI and a negative test result who would be erroneously discharged based on this testing strategy is three (zero at the first stage and three at the second stage). It was assumed that the diagnostic performance of hs-TnI using the 99th centile diagnostic threshold on a sample taken 3 hours after presentation is the same for people in whom NSTEMI is not ruled out by the initial test (hs-cTnI > LoD) as for the initial population; this was because no test performance data were available for the combination of initial hs-cTnI test using the LoD diagnostic threshold followed by 3-hour hs-cTnI and using the 99th centile threshold.
FIGURE 11.
Testing pathway for the Abbott ARCHITECT hs-cTnI assay used in cost-effectiveness modelling.
Prognostic accuracy
One study39,63 assessed the performance of a presentation sample at the 99th centile for the prediction of MACE within 30 days of the index presentation. The results of this study39,63 indicate that a positive test may be helpful in predicting the occurrence of MACE, whereas a negative test is not adequate to rule out MACE within 30 days (see Table 5).
Diagnostic accuracy of the Beckman Coulter Access high-sensitivity cardiac troponin I assay
Study details
Two diagnostic cohort studies,39,73 reported in three publications,39,64,73 provided data on the diagnostic performance of the Beckman Coulter Access hs-cTnI assay. Both studies assessed a precommercial version of the assay and both reported accuracy data for the diagnosis of AMI (any AMI65,73 or NSTEMI39). No study assessed the performance of the Beckman Coulter Access hs-cTnI assay for the prediction of MACE within 30 days of the index admission. The diagnostic performance estimates, for all combinations of diagnostic threshold and test timing assessed by included studies, are summarised in Table 6. Figure 12 shows the diagnostic performance of all testing strategies assessed, plotted in ROC space.
| Grouping | Population | Risk of bias | n | Sensitivity (%) | Specificity (%) | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| Prediction of AMI | |||||||
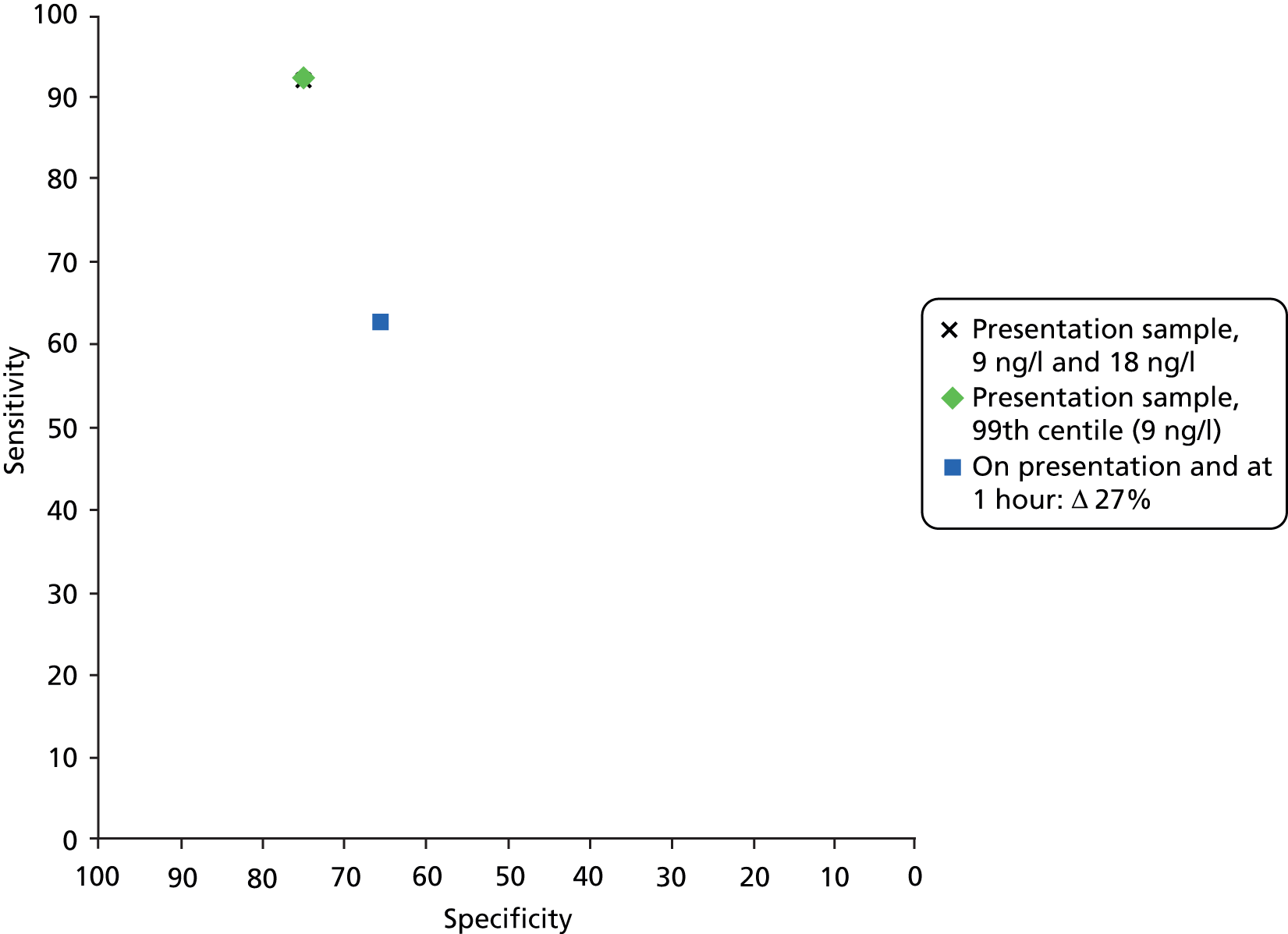
| Presentation sample, 9 ng/l and 18 ng/l | All | High risk for patient flow on one study | 239,73 | 92 (88 to 95) | 75 (72 to 77) | 3.68 (2.46 to 4.48) | 0.11 (0.07 to 0.16) |
| Presentation sample, 99th centile (9 ng/l) | Mixed | High risk for patient flow | 1 39 | 92 (88 to 95) | 75 (72 to 78) | 3.67 (3.26 to 4.13) | 0.11 (0.07 to 0.17) |
| On presentation and at 1 hour: Δ27% | STEMI excluded | High risk for patient flow | 139,63 | 63 (53 to 71) | 66 (63 to 69) | 1.85 (1.55 to 2.21) | 0.56 (0.44 to 0.72) |
FIGURE 12.
Receiver operating characteristic space plot of the Beckman Coulter Access hs-cTnI assay.
Presentation samples
Both studies16,39 assessed the diagnostic performance of a single sample taken at presentation. One study39 used the 99th centile for the general population as the diagnostic threshold. This study39 was considered to be the most relevant to our assessment and was used to inform cost-effectiveness analyses; this was the only testing strategy modelled for the Beckman Coulter Access hs-cTnI assay and, for a theoretical cohort of 1000 people presenting with symptoms suggestive of ACS (STEMI excluded) with a prevalence of NSTEMI of 17%, the estimated number of people with AMI and a negative test result who would be erroneously discharged based on this testing strategy is 14. However, it should be noted that the Beckman Coulter hs-cTnI assay evaluated in this study39 was described as ‘an investigational prototype’; the 99th centile (9 ng/l), described as ‘according to the manufacturer’, differs from the 99th centile given in the current product information leaflet (40 ng/l). 16 The estimates of sensitivity and specificity derived from this study were 92% (95% CI 88% to 95%) and 75% (95% CI 72% to 78%), respectively, and the LR+ and LR– were 3.67 (95% CI 3.26 to 4.13) and 0.11 (95% CI 0.07 to 0.17), respectively (see Table 6). The summary estimates, for the two studies16,39 combined, were very similar (see Table 6).
No studies reported clinical subgroup data, or data on the performance of the test in people presenting at different times after symptom onset, for the Beckman Coulter Access hs-cTnI assay.
Subsequent samples
Neither of the studies reported data for single samples taken at time points other than presentation.
Comparative diagnostic accuracy of the Roche Elecsys high-sensitivity troponin T assay, the Abbott ARCHITECT high-sensitivity troponin I assay and the Beckman Coulter Access high-sensitivity troponin I assay
Only one study39 provided data for a direct comparison of the diagnostic performance of all thee hs-cTn assays in the same population. These data were for the use of the 99th centile threshold in a sample taken at presentation. This was also the only time point and threshold assessed for each study by individual included studies. As can be seen from Tables 7 and 8, below, the summary estimates of the performance of each test, derived from all studies reporting data for this threshold, were similar to estimates derived from the direct comparison study alone.
| Assay | Indirect comparison | Direct comparison39 | |||
|---|---|---|---|---|---|
| n | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| Beckman Coulter Access hs-cTnI | 2 | 92 (88 to 95) | 75 (72 to 77) | 92 (88 to 98) | 75 (72 to 78) |
| Abbott ARCHITECT hs-cTnI | 3 | 80 (77 to 83) | 93 (92 to 94) | 77 (72 to 82) | 93 (91 to 94) |
| Roche Elecsys hs-cTnT | 13 | 89 (84 to 91) | 82 (77 to 86) | 90 (86 to 92) | 78 (76 to 79) |
| Assay | Indirect comparison | Direct comparison39 | |||
|---|---|---|---|---|---|
| n | LR+ | LR– | LR+ | LR– | |
| Beckman Coulter Access hs-cTnI | 2 | 3.32 (2.46 to 4.48) | 0.11 (0.07 to 0.16) | 3.68 (3.27 to 4.14) | 0.11 (0.07 to 0.17) |
| Abbott ARCHITECT hs-cTnI | 3 | 12.10 (9.04 to 16.19) | 0.21 (0.16 to 0.27) | 10.42 (8.49 to 12.79) | 0.25 (0.20 to 0.30) |
| Roche Elecsys hs-cTnT | 13 | 4.96 (3.84 to 6.96) | 0.14 (0.10 to 0.19) | 4.02 (3.65 to 4.43) | 0.13 (0.10 to 0.18) |
Selection of diagnostic strategies for inclusion in cost-effectiveness modelling
Diagnostic strategies for each hs-cTn assay were selected for inclusion in cost-effectiveness modelling based on optimal diagnostic performance as indicated by data from the systematic review. In addition, wherever possible, data from studies that excluded patients with STEMI (i.e. where the target condition was NSTEMI) were preferentially selected.
Chapter 4 Assessment of cost-effectiveness
This chapter explores the cost-effectiveness of hs-cTn assays (used singly or in series, up to 4 hours from the onset of chest pain/presentation) compared with the current standard of serial Tn T and/or I testing on admission and at 10–12 hours after the onset of symptoms for the early rule-out of AMI in people with acute chest pain.
Review of economic analyses of high-sensitivity cardiac troponin assays
Search strategy
Searches were undertaken to identify cost-effectiveness studies of high-sensitivity TnT/I. As with the clinical effectiveness searching, the main EMBASE strategy for each set of searches was independently peer reviewed by a second Information Specialist, using the Canadian Agency for Drugs and Technologies in Health (CADTH) Peer Review Checklist. 28 Search strategies were developed specifically for each database and keywords associated with high-sensitivity TnT/I were adapted according to the configuration of each database. Full search strategies are reported in Appendix 1.
The following databases were searched for relevant studies from 2005 to October 2013:
-
MEDLINE (OvidSP): 2005–2013/10/wk1.
-
MEDLINE In-Process & Other Non-Indexed Citations and Daily Update (OvidSP): up to 2013/10/1.
-
EMBASE (OvidSP): 2005–2013/10/17.
-
NHS Economic Evaluation Database (NHS EED) (Wiley): Cochrane Library Issue 3 2005 to July 2013.
-
Health Economic Evaluation Database (HEED) (Wiley): 2005–2013/10/18.
-
EconLit (EBSCO): 2005–2013/09/01.
-
Science Citation Index (SCI) (Web of Science): 2005–2013/10/21.
-
Conference Proceedings Citation Index – Science (CPCI) (Web of Science): 2005–2013/10/21.
-
Research Papers in Economics (REPEC) (Internet): up to 2013/10/21 http://repec.org/.
Identified references were downloaded in EndNote X4 software for further assessment and handling.
References in retrieved articles were checked for additional studies.
Inclusion criteria
Studies reporting a full economic analysis, which related explicitly to the cost-effectiveness of hs-cTn or standard cTn (with cTn implying either cTnI or cTnT) testing, with survival and/or quality-adjusted life-years (QALYs) as an outcome measure, were eligible for inclusion. Specifically, one of the strategies had to include cTn testing. Studies that reported only a cost-analysis of cTn testing were not included in the review.
Quality assessment
Full cost-effectiveness studies were appraised using the Drummond checklist. 77
Results
The literature search identified 152 reports. After initial screening of titles and abstracts, five reports7,19,78–80 were considered to be potentially relevant: two full papers79,80 and three HTA reports. 7,19,78 Two additional reports81,82 were provided by a clinical expert: a Canadian optimal use report82 (similar to an HTA report) and an abstract81 that was referred to in this report. All seven identified reports7,19,78–82 fulfilled inclusion criteria based on full-text assessment. The seven publications related to five studies. Figure 13 shows the flow of studies through the review process, Table 9 lists the study details and the results of the quality assessment are shown in Table 10.
FIGURE 13.
Flow of studies through the review process. CA, conference abstract; HTA, Health Technology Assessment; JA, journal article.

| Study details | Goodacre et al.78 and Fitzgerald et al.79 | Vaidya et al.81 | Thokala et al.80 and Goodacre et al.7 | CADTH report82 | Collinson et al.19 |
|---|---|---|---|---|---|
| Population | People presenting to hospital with chest pain attributable to suspected but not proven AMI, and no other potentially serious alternative pathology or comorbidity | Patients presenting to the hospital with chest pain | Patients attending hospital with symptoms suggesting MI, but a normal or non-diagnostic ECG, and no major comorbidities requiring hospital treatment | 65-year-old patients presenting to an ED with ischaemic chest pain, without ST segment elevation ECG, who require cTn testing for diagnosis of NSTEMI | Patients presenting to hospital with symptoms suggestive of MI but with no diagnostic ECG changes (ST deviation > 1 mm or T-wave inversion > 3 mm), no known history of CHD and no major comorbidities requiring inpatient treatment |
| Time horizon | Lifetime | Lifetime | Lifetime | Lifetime | Lifetime |
| Objective | Estimate the cost-effectiveness of the point-of-care panel in terms of mean costs and QALYs accrued compared with standard care | Assess the cost-effectiveness of a hs-CTnT assay, alone or combined with the H-FABP assay in comparison with the conventional cTn (cTnT) assay for the diagnosis of AMI | Estimate the incremental cost per QALY of delayed Tn testing compared with presentation testing and no testing to determine which diagnostic strategy should be recommended | To investigate the cost-effectiveness of hs-cTnT and hs-cTnI assays compared with each other, as well as with cTnI assays in patients with suspected ACS symptoms in the ED | Assess the cost-effectiveness of measuring a combination of biomarkers compared with measurement of cTn alone |
| Source of effectiveness information | Data from within the trial up to 3 months, and beyond this, lifetime costs and QALY estimates were used from a previous economic evaluation | No information | Sensitivity and specificity were taken from the meta-analysis as reported in the 2013 Goodacre report;7 the RATPAC trial19 was used for sampling patient characteristics; Mills et al.83 for risk of re-infarction and death, Polanczyk et al.84 for life expectancy of patients with MI and re-MI | Sensitivity and specificity from review performed in same report. Proportion UA and mortality estimated based on published studies, and one unpublished study. Utility decrements based on published study | Sensitivity and specificity data derived from data from the HTA (RATPAC) itself, short-term survival and probability of re-infarction based on Mills et al.83 Source for long-term survival and QALYs not specified |
| Comparators | Diagnostic assessment using the point-of-care biochemical marker panel Conventional diagnostic assessment without the panel |
Conventional cTnT hs-cTnT hs-cTnT combined with H-FABP |
No biochemical testing: discharge all patients without treatment (hypothetical) Standard Tn assay measured at presentation using the 10% CV as the threshold for positivity Standard Tn assay measured at presentation using the 99th percentile threshold High-sensitivity Tn assay measured at presentation using the 99th percentile threshold Standard Tn assay measured at presentation and 10 hours after symptom onset using the 99th percentile threshold |
hs-cTnT hs-cTnI cTnI |
No testing: discharge all patients without treatment hs-cTn at presentation: discharge home if test is negative or admit to hospital for Tn testing at 10–12 hours if positive hs-cTn and a combination of cytoplasmic or neurohormone biomarkers at presentation: discharge home if both tests are negative or admit to hospital for Tn testing at 10–12 hours if either test is positive hs-cTn at presentation and at 90 minutes as in the RATPAC protocol: discharge home if both tests are negative or admit to hospital for Tn testing at 10–12 hours if either test is positive Standard Tn testing at 10–12 hours |
| Unit costs | Microcosting study within RATPAC; PSSRU unit costs | No information | Admission and treatment were based on the national tariff Lifetime costs for MI patients were taken from Ward et al.85 The price of a Tn test was taken from the 2011 Goodacre report78 |
Costs of hospital admission were based on the Ontario Case Costing Initiative database and the Ontario Schedule of Benefits for Physician Services Costs of ED visits were based on a hospital in Southwestern Ontario and the Ontario Schedule of Benefits Unit prices of cTn tests were based on information provided by the manufacturers |
Hospital stay and treatment for MI based on NHS reference cost, biochemical testing based on Goodacre et al.78 |
| Measure of benefit | QALY | AMI survivor | QALY | QALY | QALYs |
| Study type | Trial-based economic evaluation up to 3 months, decision tree lifetime. Cost–utility analysis | Model-based cost-effectiveness and cost–utility study | Model-based cost–utility analysis | Model-based cost–utility analysis | Model-based cost–utility study |
| Model assumptions | 2-hour delay between sampling and results available 4 hours after presentation at ED patients moves to inpatient department 1 hour delay between presentation and start biomarker sampling After short term (test-treatment-outcome), progress only depends on whether or not patient had MI, and whether or not this was treated |
No information | 10 hours’ Tn testing has perfect sensitivity and specificity (as it is the reference standard) 2-hour delay from the time at which sampling could be performed to results available For presentation testing strategies: decision made within 1 hour of results available For 10 hours testing strategies: decision made according to scenario applied Diagnostic strategy influences outcomes only among patients with MI |
Non-NSTEMI patients are further classified into UA or non-ACS, with consequences for costs and outcome There is a small survival benefit (RR 1.01) of treating early compared with treating late (presentation testing vs. standard testing) |
10 hours’ Tn testing has perfect sensitivity and specificity (as it is the reference standard) Presentation blood tests taken in ED and results available and decision made within 2 hours of sampling For testing at 10–12 hours delays according to scenario used |
| Perspective | NHS | Health care | NHS | Publicly funded health care system | NHS in England and Wales |
| Discount rate | Not mentioned | No information | Nothing mentioned | 5% discount rate applied to costs and QALYs | Nothing mentioned |
| Uncertainty around cost-effectiveness ratio expressed | ICE plane, probability of strategy being dominated/cost-effective | CEACs (not shown in abstract) | CEACs for PSA results, per scenario | As reported in outcomes of one-way sensitivity analyses, and also (for PSA) in CEACs | CEACs |
| Sensitivity analysis | PSA | One way and probabilistic | One-way sensitivity analyses, scenario analyses (doctor on demand, twice-daily ward round, and once-daily ward round), and PSA | Secondary analysis using cTnI instead of cTnT, scenario analysis (doctor on demand, once-daily ward round, twice-daily ward round) and PSA | |
| Outcome (cost and LYs/QALYs) per comparator | Empirical 3 months: point of care £1217, QALY 0.158 SC £1006, QALY 0.161 For the model, no outcomes per comparator were reported |
No information | For doctor-on-demand scenario, per 1000 patients without known CAD: No testing £965,994 QALY 26,227 Presentation standard Tn, 10% CV £1,560,361 QALY 26,345 Presentation standard Tn, 99th percentile £1,609,760 QALY 26,352 Presentation hs-trop, 99th percentile £1,806,910 QALY 26,279 10 hours Tn £2,016,540 QALY 26,286 |
cTnI US$2,018 QALY 8.1385 hs-cTnI US$2,082 QALY 3.1389 hs-cTnT US$2,186 QALY 8.1399 |
For doctor-on-demand scenario, per 1000 patients: No testing £965,994 QALY 26,227 hs-cTnT at presentation £1,581,263 QALY 26,349 hs-cTnT at presentation and 90 min £1,715,526 QALY 26,354 hs-cTnT and H-FABP at presentation £1,682,362 QALY 26,359 10-hour Tn £2,016,540 QALY 26,386 |
| Summary of incremental analysis | Empirical 3 months: Increment point of care vs. SC £211 QALY –0.00282 Probability point of care cost-effective at £20,000/QALY = 0.4% Decision model 3 months: Increment point of care vs. SC £169 QALY –0.002 Probability point of care cost-effective at £20,000/QALY = 22.3% Decision model lifetime: Increment point of care vs. SC £329 QALY –0.087 Probability point of care cost-effective at £20,000/QALY = 33.6% |
hsTnT vs. cTnT: incremental €111 and 16–17 lives per 1000 AMI ICER 3748 €/QALY hsTnT + H-FABP vs. cTnT: incremental €178 ICER 5717 €/QALY |
For doctor-on-demand scenario: Presentation standard Tn 10% CV vs. no testing: £5030/QALY Presentation standard Tn 99th percentile vs. presentation standard Tn 10% CV: £6518/QALY Presentation hs-Tn 99th percentile vs. presentation standard Tn 99th percentile: £7487/QALY 10 hours’ Tn vs. presentation hs-Tn 99th percentile: £27,546/QALY |
cTnI reference hs-cTnI incremental costs US$64, incremental QALYs 0.000352 dominated (by extension) hs-cTnT incremental costs US$168, incremental QALYs 0.001408 ICER US$119,377/QALY |
No testing – reference strategy hs-cTnT compared with no testing ICER £5012/QALY hs-cTnT at presentation and at 90 minutes: dominated hs-cTnT and H-FABP compared with hs-cTnT at presentation: ICER £11,026/QALY (as reported but the correct number should be 10,871) 10-hour Tn compared with Hs-cTnT and H-FABP: ICER £12,090/QALY Conclusion: if a rapid-rule out strategy with a sensitivity of 95% (and specificity of around 90%) would be available, then a 10-hour Tn strategy does not seem cost-effective |
| Goodacre et al.78 and Fitzgerald et al.79 | Vaidya et al.81 | Thokala et al.80 and Goodacre et al.7 | CADTH report82 | Collinson et al.19 | |
|---|---|---|---|---|---|
| Study design | |||||
| The research question is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The economic importance of the research question is stated | ✓ | ✗ | ✓ | ✓ | ✓ |
| The viewpoint(s) of the analysis are clearly stated and justified | ✓ | ✓ | ✓ | ✓ | ✓ |
| The rationale for choosing alternative programmes or interventions compared is stated | ✓ | ✗ | ✓ | ✓ | ✓ |
| The alternatives being compared are clearly described | ✓ | ✓ | ✓ | ✓ | ✓ |
| The form of economic evaluation used is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The choice of form of economic evaluation is justified in relation to the questions addressed | ✓ | ✓ | ✓ | ✓ | ✓ |
| Data collection | |||||
| The source(s) of effectiveness estimates used are stated | ✓ | ✗ | ✓ | ✓ | ✓ |
| Details of the design and results of effectiveness study are given (if based on a single study) | ✓ | ✗ | ✓ | ✓ | ✓ |
| Details of the methods of synthesis or meta-analysis of estimates are given (if based on a synthesis of a number of effectiveness studies) | ✓ | ✗ | ✓ | ✓ | ✓ |
| The primary outcome measure(s) for the economic evaluation are clearly stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| Methods to value benefits are stated | ✓ | ✗ | ✓ | ✓ | ✗ |
| Details of the subjects from whom valuations were obtained were given | ✓ | ✗ | ✗ | ✗ | ✗ |
| Productivity changes (if included) are reported separately | NA | ✗ | NA | NA | NA |
| The relevance of productivity changes to the study question is discussed | NA | ✗ | NA | NA | NA |
| Quantities of resource use are reported separately from their unit costs | ✓ | ✗ | ✗ | ✗ | ✗ |
| Methods for the estimation of quantities and unit costs are described | ✓ | ✗ | ✓ | ✓ | ✓ |
| Currency and price data are recorded | ✓ | ✗ | ✓ | ✓ | ✓ |
| Details of currency of price adjustments for inflation or currency conversion are given | ✓ | ✗ | ✗ | ✗ | ✗ |
| Details of any model used are given | ✓ | ✗ | ✓ | ✓ | ✓ |
| The choice of model used and the key parameters on which it is based are justified | ✓ | ✗ | ✓ | ✓ | ✓ |
| Analysis and interpretation of results | |||||
| Time horizon of costs and benefits is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The discount rate(s) is stated | ✗ | ✗ | ✗ | ✓ | ✗ |
| The choice of discount rate(s) is justified | NA | ✗ | NA | ✓ | NA |
| An explanation is given if costs and benefits are not discounted | ✗ | ✗ | ✗ | NA | ✗ |
| Details of statistical tests and CIs are given for stochastic data | ✓ | ✗ | ✓ | ✓ | ✓ |
| The approach to sensitivity analysis is given | ✓ | ✗ | ✓ | ✓ | ✓ |
| The choice of variables for sensitivity analysis is justified | ✓ | ✗ | ✓ | ✓ | ✓ |
| The ranges over which the variables are varied are justified | ✓ | ✗ | ✓ | ✓ | ✓ |
| Relevant alternatives are compared | ✓ | ✓ | ✓ | ✓ | ✓ |
| Incremental analysis is reported | ✓ | ✗ | ✓ | ✓ | ✓ |
| Major outcomes are presented in a disaggregated as well as aggregated form | ✓ | ✗ | ✓ | ✓ | ✓ |
| The answer to the study question is given | ✓ | ✓ | ✓ | ✓ | ✓ |
| Conclusions follow from the data reported | ✓ | ✓ | ✓ | ✓ | ✓ |
| Conclusions are accompanied by the appropriate caveats | ✓ | ✗ | ✓ | ✓ | ✓ |
Goodacre (2011)78 and Fitzgerald (2011)79
This study was based on the multicentre, pragmatic controlled trial ‘Randomised Assessment of Treatment using Panel Assay of Cardiac Markers’ (RATPAC). An economic evaluation was undertaken to assess the cost-effectiveness of management based on testing with a panel of point-of-care cardiac markers compared with management without point-of-care panel assessment. The included population consisted of patients presenting to hospital with chest pain attributable to suspected, but not proven, AMI and no other potentially serious alternative pathology or comorbidity. The analysis was performed from an NHS perspective, using trial data to estimate the mean costs per patient of chest pain-related care and the mean number of QALYs accrued by patients in each arm of the trial, with a time horizon of 3 months. In addition, a decision-analytic model was constructed to duplicate (validate) trial results and extrapolate results to a longer time horizon.
Resource-use data were collected for all patients. Cost and outcome data were collected using patient notes and self-completed questionnaires. Unit prices were based partly on a microcosting study on a sample of patients, partly on a study previously undertaken by the investigators, and partly on purchase price and national unit costs. QALYs were calculated based on European Quality of Life-5 Dimensions (EQ-5D) measurements. In a sensitivity analysis, productivity costs were included as reported by the patients.
As it was anticipated that the trial would have limited power to detect a difference in major adverse events, the decision-analytic model was intended to explore whether uncertainty around the effect of the intervention upon the major adverse event rate could influence the potential cost-effectiveness of the intervention. The model used trial data to estimate costs and QALYs up to 3 months. Beyond this, lifetime cost and QALYs were estimated from a previous study. 86 It was assumed that patients who had died at 3 months would accrue no further costs or QALYs. Those who had survived non-fatal myocardial infarction (MI) would accrue costs and QALYs associated with CHD (estimated at £10,079 and 6.829, respectively). Those without CHD were assigned zero costs and 20 QALYs.
Empirical results showed that the point-of-care test strategy was dominated by standard care, which delivered slightly more QALYs at a lower cost. The probability that point-of-care testing would be more cost-effective than standard care at a willingness-to-pay threshold of £20,000 per QALY was < 1%. The decision-analytic model again resulted in higher costs and less effect for the point-of-care panel assay compared with standard care, also when extrapolated to lifetime survival. The probability of the point-of-care panel assay being cost-effective for the 3-month and lifetime model was 22.3% and 33.6%, respectively.
The main conclusion was that point-of-care panel assay testing is unlikely to be considered cost-effective in the NHS, with an 89% probability that standard care was dominant. Cost-effectiveness was mainly driven by differences in mean cost, with point estimates suggesting that, per patient, point-of-care panel assessment was £211 more expensive than standard care.
Vaidya (2012)81
This study aimed to assess the cost-effectiveness of a hs-TnT assay, alone or in combination with the H-FABP assay in comparison with the conventional cTnT assay for the diagnosis of AMI in patients presenting to hospital with chest pain. A decision-analytic model was developed to perform both a cost–utility analysis (cost per QALY gained) and a cost-effectiveness analysis [cost per life-year (LY) gained and cost per AMI averted], using a health-care perspective and a lifetime time horizon. One-way and probabilistic sensitivity analyses (PSAs) were conducted.
The incremental cost-effectiveness ratio (ICER) for hs-TnT compared with conventional cTnT was €3748 per QALY gained. For hs-cTnT in combination with H-FABP compared with conventional cTnT the ICER was €5717 per QALY gained. For LY and AMI averted, no ICERs were reported in the abstract. The PSA showed the hs-TnT assay to be the preferable strategy, with a probability of > 90%, at a ceiling ratio of €4800 per QALY. This led to the conclusion that the hs-TnT assay is very cost-effective relative to the conventional cTnT assay. Combining hs-TnT with H-FABP did not seem to offer any additional economic or health benefit over the hs-TnT test alone.
Goodacre (2013)7 and Thokala (2012)80
This study aimed to estimate the cost-effectiveness of using alternative biomarker strategies to diagnose MI, and using biomarkers, computed tomography coronary angiography (CTCA) and exercise ECG to risk-stratify Tn-negative patients. As the second aim was outside the scope of this review, we have summarised only the analysis that compares the biomarker strategies for diagnosing MI, referred to in the HTA report as ‘the diagnostic phase model’. The different diagnostic strategies were applied to a hypothetical cohort of patients attending the ED with suspected, but not proven, ACS. Patient characteristics were defined using data from the RATPAC trial,87 as well as patients’ arrival times during the day at the ED. The model assigned each patient a probability of re-infarction or death depending on their characteristics and whether or not they had treatment. The model took a lifetime time horizon. The economic perspective was that of the NHS in England and Wales.
The following strategies were applied to each patient:
-
no testing – discharge all patients without treatment (hypothetical)
-
standard Tn assay measured at presentation using the 10% CV as the threshold for positivity
-
standard Tn assay measured at presentation using the 99th percentile threshold
-
high-sensitivity Tn assay measured at presentation using the 99th percentile threshold
-
standard Tn assay measured at presentation and 10 hours after symptom onset using the 99th percentile threshold.
Blood tests at presentation were assumed to be taken in the ED, and so a decision could be made within 1 hour of the test results becoming available. For the 10–12 hours’ Tn measurement, three different scenarios were tested:
-
‘doctor-on-demand’ scenario, with medical staff available 24 hours a day to make a disposition decision within 1 hour of the results being available
-
twice-daily ward round scenario, with medical staff only available at twice daily ward rounds to make disposition decisions
-
once-daily ward round scenario, with medical staff only available at a once daily ward round to make disposition decisions.
Sensitivity and specificity estimates for the presentation Tn tests were obtained by performing meta-analysis of estimates from individual primary studies included in the accompanying review. The 10-hour Tn test was assumed to have perfect sensitivity and specificity as it was the reference standard for the review. This implies that FPs of the hs-Tn testing at presentation will still be discharged home after the 10- to 12-hour Tn test but FNs will be discharged home without treatment. The ‘discharge without testing or treatment’ by definition has perfect specificity, but a sensitivity of 0%.
The risk of re-infarction and death for patients with MI was based on a study by Mills et al. 83 Life expectancy of patients with MI, and MI with re-infarction, was estimated from Polanczyk et al. ,88 whereas the utility of patients with MI was based on Ward et al. 85 The utility of patients with re-infarction was estimated by using a multiplicative factor of 0.8 for patients with MI (expert opinion). Patients without MI were assigned the life expectancy and utility scores of the general population. Lifetime costs for patients with MI were based on Ward et al. 85 One-way sensitivity analyses were performed, as well as a PSA. In a secondary analysis, a strategy was added that involved alternative biomarkers in combination with the presentation Tn testing.
The results showed that measuring a 10-hour Tn level in all patients was the most effective strategy (ICER £27,546–103,560). However, at a threshold of £30,000 per QALY, the optimal strategy in all but one scenario was measurement of high-sensitivity Tn at presentation, with a 10-hour Tn test if positive and discharge home if negative (ICER £7487–17,191 per QALY). The exception was a scenario involving patients without known CAD and a doctor available on demand to discharge the patient, where, using the £30,000 per QALY threshold, the strategy of measuring a 10-hour Tn level in all patients was optimal (ICER of £27,546 per QALY). Sensitivity analyses showed the optimal strategy to vary with different levels of sensitivity and timing of the tests.
The report concluded that the additional costs that are likely to be incurred by measuring a 10-hour Tn level, compared with a presentation high-sensitivity Tn level, are unlikely to represent a cost-effective use of NHS resources in most of the scenarios tested.
Canadian Agency for Drugs and Technologies in Health optimal use report
This report82 aimed to determine the cost-effectiveness of hs-cTnT and hs-cTnI assays compared with each other, as well as with cTnI assays in patients with suspected ACS symptoms in the ED. For this purpose, three comparators were considered: hs-cTnT, hs-cTnI and cTnI. As cTnT is no longer available in Canada, it was not taken into account in the analysis. The target population consisted of 65-year-old patients presenting to the ED, without ST segment elevation, who required cTn testing for diagnosis of NSTEMI. For the economic evaluation, a decision tree was constructed, which calculated lifetime cost per QALY from the perspective of a publicly funded health-care system.
The model consisted of a short-term part, which had a time horizon of 1 year, and a long-term part. The short-term part incorporated the testing and treatment procedures and short-term outcomes. Patients were tested at presentation at the ED and, if they were not admitted to hospital after the first test, they were tested again after 6 hours. When the patient was admitted after the first test, treatment was said to be initiated early, and when a patient was admitted after the second test, treatment was late. One-year mortality depended on whether a patient had NSTEMI and whether they were treated early, treated late, or untreated (in the case of FN test results). Those not suffering from NSTEMI were further stratified into UA or not having ACS (non-ACS). The annual probability of death in the long-term part of the model was dependent on patient age, sex, and whether or not they had suffered a NSTEMI, UA or did not have any type of ACS in the short-term part of the model.
The sensitivity and specificity for each cTn test at presentation to the ED was derived from the systematic review which was also part of this study. In the model, patients with a negative cTn test at presentation were assumed to be observed and have a second cTn test 6 hours later. After the second cTn test, 90% of these FNs were assumed to become TPs.
Short-term mortality rates and relative risks (RRs) for treated/non-treated were taken from published clinical studies and one non-referenced study. The RR for late treatment compared with early treatment was derived from expert opinion. Long-term mortality rates were taken from published clinical studies, and one non-referenced study. QALYs were calculated by incorporating an age-specific utility decrement for patients with NSTEMI. A number of one-way sensitivity analyses were performed, as well as a PSA.
The base-case results indicated that hs-cTnI was dominated by hs-cTnT, when compared with cTnI, at an ICER of US$119,377 per QALY. The PSA showed that, for willingness-to-pay thresholds of up to US$124,000, cTnI had the highest probability of being cost-effective. For thresholds > US$124,000, hs-cTnT had the highest probability of being cost-effective. The hs-cTnI test was not likely to be cost-effective for any value of the threshold.
The authors concluded that hs-cTnT would be considered the most cost-effective testing strategy if willingness to pay for a QALY is US$119,377 or more, otherwise cTnI would be the most cost-effective test. However, there was a lot of uncertainty in results when model assumptions were changed.
Collinson (2013)19
This study used the decision tree developed in the related HTA by Goodacre et al. 7 to compare the cost-effectiveness of five diagnostic strategies to a hypothetical cohort of patients presenting to hospital with symptoms suggestive of MI but with no diagnostic ECG changes, no known history of CHD and no major comorbidities requiring inpatient treatment. Essentially, this was a substudy of the point-of-care arm of the RATPAC trial. All methods and model inputs were identical to the study by Thokala et al. 80 and the HTA report by Goodacre et al. ,7 but with slightly different strategies applied to the cohort of patients:
-
No testing – discharge all patients without treatment (theoretical ‘zero’ option)
-
high-sensitivity cTnT at presentation – discharge home if test is negative or admit to hospital for Tn-testing at 10–12 hours if positive
-
high-sensitivity cTnT and H-FABP at presentation – discharge home if both tests are negative or admit to hospital for Tn testing at 10–12 hours if either test is positive
-
high-sensitivity cTnT at presentation and at 90 minutes as in the RATPAC protocol – discharge home if both tests are negative or admit to hospital testing at 10–12 hours if either test is positive
-
standard Tn testing at 10–12 hours (current standard as per NICE guidelines).
The difference with the other studies is in the addition of H-FABP in the third strategy and in the second high-sensitive Tn test at 90 minutes in the fourth strategy. In a secondary analysis, cTnT was replaced by cTnI. Sensitivity and specificity of presentation biochemical testing were estimated using data from within the study (RATPAC). Standard Tn testing at 10–12 hours was assumed to have perfect sensitivity and specificity as this was again the reference standard.
At the £20,000 per QALY threshold, 10-hour Tn testing was cost-effective (£12,090 per QALY) in the doctor-on-demand scenario, but not in the other scenarios (once-daily ward round and twice-daily ward rounds), when high-sensitivity cTnT and H-FABP measurement at presentation was cost-effective. At the £30,000 per QALY threshold, 10-hour Tn testing was cost-effective in the doctor-on-demand scenario and twice-daily ward rounds scenario (£24,600 per QALY), whereas the TnT and H-FABP measurement at presentation strategy was cost-effective (£14,806 per QALY) in the once-daily ward round scenario. Secondary analysis using cTnI instead of cTnT showed that cTnI testing at presentation and at 90 minutes was cost-effective in all three scenarios at the £20,000 per QALY threshold, and in two of the scenarios at the £30,000 per QALY threshold, with 10-hour Tn being cost-effective only in the doctor-on-demand scenario (£24,327 per QALY). The overall conclusion was that 10-hour Tn testing is likely to be cost-effective compared with rapid rule-out strategies only if patients can be discharged as soon as a negative result is available and a £30,000 per QALY threshold is used.
Summary of studies included in the cost-effectiveness review
Most of the studies identified in this review have found that the question of whether hs-Tn testing is cost-effective cannot be answered unequivocally. In favour of hs-Tn testing, the abstract by Vaidya et al. 81 concluded that hsTnT testing is ‘very cost-effective’ and the study by Goodacre et al. 7 concluded that ‘the optimal strategy in all but one scenario was high-sensitivity Tn at presentation, with a 10 hour Tn test if positive and discharge home if negative’ (p. xv). The other papers reported ICERs that were considerably higher and with substantial uncertainty. The accuracy of high-sensitivity tests and the efficiency of decision-making based on test results were important drivers of cost-effectiveness.
Model structure and methodology
Troponin tests considered in the model
The health-economic analysis will estimate the cost-effectiveness of different Tn testing methods for diagnosing or ruling out NSTEMI, in patients presenting at the ED with suspected NSTE-ACS, who have no major comorbidities requiring hospitalisation (e.g. as HF or arrhythmia) and in whom STEMI has been ruled out. Those diagnosed with NSTEMI will then be admitted to the hospital for AMI treatment and those diagnosed as without NSTEMI can be discharged without AMI treatment and further hospital stay. AMI treatment might include aspirin, statins and ACE inhibitors and consideration of coronary revascularisation for high-risk cases. 7 Initiating AMI treatment for NSTEMI will reduce the probability of MACEs, particularly cardiac death and re-infarction.
Standard serial Tn testing, for patients with acute chest pain attributable to possible ACS, does not achieve optimal sensitivity in detecting AMI until 10–12 hours after onset of symptoms. Waiting for 10–12 hours after symptom onset is burdensome for patients and induces additional health-care costs. Therefore, various alternatives have been proposed, using more sensitive Tn tests, for the early rule-out of NSTEMI (within the 4-hour NHS ED target). 89
Two hs-cTn assays (Roche Elecsys hs-cTnT and Abbott ARCHITECT hs-cTnI) are currently used in NHS laboratories in England and Wales. One additional assay (Beckman Coulter hs-cTnI) was listed in the scope for this assessment, pending CE marking. However, each of these tests can be used at different time points and with different diagnostic thresholds, resulting in multiple possible strategies for each test. Whether or not a test strategy was included in the economic model was decided based on optimal diagnostic performance, given the available evidence on accuracy for a population with STEMI ruled out, and on applicability in clinical practice (see Results of the assessment of clinical effectiveness assessment, above). The test strategies evaluated in the model are:
-
Standard Tn at presentation and at 10–12 hours (reference standard).
-
Roche Elecsys hs-cTnT at presentation: 99th centile threshold.
-
Roche Elecsys hs-cTnT (optimal strategy): LoB threshold at presentation followed by 99th centile threshold peak within 3 hours and/or Δ20% (compared with presentation test) at 1–3 hours (see Figure 9).
-
Abbott ARCHITECT hs-cTnI at presentation: 99th centile threshold.
-
Abbott ARCHITECT hs-cTnI (optimal strategy): LoD threshold at presentation, followed by 99th centile threshold at 3 hours (see Figure 11).
-
Beckman Coulter hs-cTnI at presentation: 99th centile threshold.
-
No testing, discharge all patients without testing or treatment (only in sensitivity analyses). A Tn test may not be indicated when clinical judgement assesses the probability that a patient is experiencing an AMI as low. Therefore, consistent with the protocol, this hypothetical strategy is included in sensitivity analyses wherein the AMI prevalence is varied.
In the base case, it was assumed that standard Tn had perfect sensitivity and specificity (reference case) for diagnosing AMI. Using this assumption, all patients testing positive on a hs-cTn test but negative on the standard Tn would be classified as FPs. This implies that their risk for adverse events would be the same as for those patients testing negative on both the hs-cTn test and the standard Tn, and that they ought to be discharged home without further immediate treatment. However, recent evidence has shown that patients with a negative standard Tn, but a positive hs-cTn, may be at higher risk for adverse events than patients who test negative on both the standard and the high-sensitive Tn (Goodacre S, Medstar Washington Hospital Center, Washington, DC, USA; Lipinski M, University of Sheffield, Sheffield, UK: 2014, personal communication). A secondary analysis was therefore performed, which attributed a higher risk of adverse events to a proportion of patients testing FP with the hs-cTn test.
Based on the available evidence, two analyses were performed:
-
Base-case analysis.
-
Secondary analysis, assuming that FPs in the hs-cTn testing strategies do not have the same risk for adverse events as TNs. Instead, these patients were assigned a higher risk for (re-)infarction and death, to reflect the idea that when the hs-cTn test gives a positive result, in some cases this must be caused by a disease process, whether or not the strict definition of AMI is met. The risk of adverse events in patients with positive hs-cTn but a negative standard Tn is higher than the patients testing negative on both the hs-cTn test and the standard Tn, but lower than risk of adverse events in patients diagnosed with NSTEMI (i.e. both positive hs-cTn and standard Tn).
Model structure
This assessment uses the HTA report by Goodacre et al. 7 as a starting point for cost-effectiveness modelling. The Goodacre report compared the cost-effectiveness of several diagnostic strategies for ACS. The assessment group received the health-economic model (in SIMUL8 2011, Simul8 Corporation, Boston, MA, USA) that this HTA was based on, and this model was used as a starting point to develop a de novo model (in Microsoft Excel 2003: Microsoft Corporation, Redmond, WA, USA) adapted to better fit the scope of the current assessment. In the health-economic model the mean expected costs and QALYs were calculated for each alternative strategy. These long-term consequences were estimated based on the accuracy of the different testing strategies followed by AMI treatment or discharge from the hospital without AMI treatment for patients presenting at the ED with suspected NSTE-ACS, including patients with NSTEMI and patients without NSTEMI, who are further subdivided into ‘No ACS, no UA’ and ‘Unstable angina’. For this purpose a decision tree and a Markov model were developed. The decision tree was used to model the 30-day outcomes after presentation, based on test results and the accompanying treatment decision. These outcomes consisted of ‘No ACS, no UA’, ‘Unstable angina’, ‘Non-fatal AMI (untreated)’, ‘Non-fatal AMI (treated)’ and ‘Death’. The decision tree is shown in Figure 14.
FIGURE 14.
Decision tree structure.

The long-term consequences in terms of costs and QALYs were estimated using a Markov cohort model (Figure 15) with a lifetime time horizon (60 years). The cycle time was 1 year, except for the first cycle, which was adjusted to 335.25 days (365.25–30) to ensure that the decision tree period (30 days) and the first cycle combined summed to 1 year. The following health states were included:
-
‘No acute coronary syndrome and no unstable angina (no ACS, no UA)’
-
‘Unstable angina’
-
‘Post AMI (treated and untreated)’
-
‘Post AMI with re-infarction’
-
‘Death’.
FIGURE 15.
Markov model structure. a, During the first year post AMI, a distinction is made between treated and untreated AMI.
Model parameters
Estimates for the model input parameters were retrieved from the literature and by consulting experts for unpublished data. Accuracy estimates were derived from the systematic review component of this assessment (see Results of the assessment of clinical effectiveness assessment, above).
Transition probabilities
An overview of transition probabilities is provided in Table 11.
| Parameter | Estimate | SE/95% CI | Distribution | Source |
|---|---|---|---|---|
| Decision tree (short term) | ||||
| NSTEMI prevalencea | 0.170 | 0.028 | Beta | Santalo (2013),40 Aldous (2012),46 Sebbane (2013),64 APACE39 |
| Proportion of UA (of all non-NSTEMI patients) | 0.160 | 0.038 | Beta | CADTH (2013)82 |
| Decision tree (30 day) probabilities | ||||
| Mortality (30 day) treated AMI | 0.097 | 0.012 | Beta | Pope (2000)90 |
| Mortality (30 day) untreated AMI | 0.105 | 0.069 | Beta | Pope (2000)90 |
| Mortality (30 day) treated UA | 0.021 | 0.005 | Beta | Pope (2000)90 |
| Mortality (30 day) no ACS | b | – | Fixed | ONS91 |
| Markov model (long term) | ||||
| AMI incidence | c | – | Fixed | British Heart Foundation92 |
| Annual re-infarction (treated)d | 0.023 | 0.001 | Beta | Smolina (2012)93 |
| RR re-infarction (untreated vs. treated)e | 2.568 | 1.366 to 5.604 | Log-normal | Mills (2011)83 |
| Annual mortality no ACS | b | – | Fixed | ONS91 |
| Annual mortality post MId | 0.066 | 0.000 | Beta | Smolina (2012)93 |
| Annual mortality post re-infarctiond | 0.142 | 0.002 | Beta | Smolina (2012)93 |
| HR mortality (UA vs. NSTEMI) | 0.781 | 0.581 to 1.053 | Log-normal | Allen (2006)94 |
| RR mortality (untreated vs. treated)d | 1.877 | 0.951 to 4.239 | Log-normal | Mills (2011)83 |
| Secondary analysis (adjusted RR for patients tested FP) | ||||
| OR AMIf | 0.840 | 0.578–1.235 | Log-normal | Goodacre S, Lipinski M, personal communication; Lipinski et al.95 |
| OR deathf | 0.649 | 0.465–0.901 | Log-normal | Goodacre S, Lipinski M, personal communication; Lipinski et al.95 |
| Proportion of AMIg | 0.105 | 0.011 | Beta | Goodacre S, Lipinski M, personal communication; Lipinski et al.95 |
| Proportion of deathg | 0.114 | 0.011 | Beta | Goodacre S, Lipinski M, personal communication; Lipinski et al.95 |
| RR AMIf,h | 0.855 | 0.602–1.197 | Log-normal | Goodacre S, Lipinski M, personal communication; Lipinski et al.95 |
| RR deathf,h | 0.676 | 0.500–0.911 | Log-normal | Goodacre S, Lipinski M, personal communication; Lipinski et al.95 |
Decision tree
The proportions of patients testing positive or negative (and thus commencing AMI treatment or being discharged from the hospital) were based on the estimated accuracy of the testing strategies considered (Table 12) and the estimated prevalence of NSTEMI in the UK [17.0% with standard error (SE) 2.8%; see Table 11]. 39,40,46,64 This prevalence was higher than that derived from the RATPAC trial78 and used in the Goodacre model,7 because the RATPAC study population was a low-risk population. 79,87 The proportion of TPs, FPs, FNs and TNs were calculated as follows:
-
TP = NSTEMI prevalence × sensitivity
-
FP = (1 – NSTEMI prevalence) × (1 – specificity)
-
FN = NSTEMI prevalence × (1 – sensitivity)
-
TN = (1 – NSTEMI prevalence) × specificity.
Subsequently, the proportions of patients who receive AMI treatment (TP + FP), and who are discharged without AMI treatment (TN + FN) were calculated. These results are listed in Table 13.
| Strategy | Sensitivity (SE)a | Specificity (SE)a | Distribution | Source |
|---|---|---|---|---|
| Serial standard Tn testing | 1.00 (–) | 1.00 (–) | Fixed | Assumption |
| Roche Elecsys hs-cTnT (99th centile at presentation) | 0.88 (0.04) | 0.84 (0.04) | Multivariate normal | Chapter 3 |
| Roche Elecsys hs-cTnT (optimal strategy)b | 0.93 (0.02)c | 0.82 (0.01)c | Multivariate normal | Chapter 3 |
| Abbott ARCHITECT hs-cTnI (99th centile at presentation) | 0.80 (0.02) | 0.93 (0.00) | Multivariate normal | Chapter 3 |
| Abbott ARCHITECT hs-cTnI (optimal strategy)d | 0.98 (0.01)c | 0.94 (0.01)c | Multivariate normal | Chapter 3 |
| Beckman Coulter hs-cTnI (99th centile) | 0.92 (0.02) | 0.75 (0.01) | Multivariate normal | Chapter 3 |
| No Tn teste | 0.00 (–) | 1.00 (–) | Fixed | Assumption |
| Strategy | TP | FP | FN | TN | PPV | NPV | LR+ | LR– |
|---|---|---|---|---|---|---|---|---|
| Serial standard Tn testing | 0.17 | 0.00 | 0.00 | 0.83 | 1.00 | 1.00 | 1.00 | 0.00 |
| Roche Elecsys hs-cTnT (99th centile at presentation) | 0.15 | 0.13 | 0.02 | 0.70 | 0.53 | 0.97 | 5.41 | 0.15 |
| Roche Elecsys hs-cTnT (optimal strategy) | 0.16 | 0.15 | 0.01 | 0.68 | 0.51 | 0.98 | 5.05 | 0.09 |
| Abbott ARCHITECT hs-cTnI (99th centile at presentation) | 0.14 | 0.06 | 0.03 | 0.77 | 0.70 | 0.96 | 11.47 | 0.21 |
| Abbott ARCHITECT hs-cTnI (optimal strategy) | 0.17 | 0.05 | 0.00 | 0.78 | 0.76 | 1.00 | 15.67 | 0.02 |
| Beckman Coulter hs-cTnI (99th centile) | 0.16 | 0.21 | 0.01 | 0.62 | 0.43 | 0.98 | 3.67 | 0.11 |
| No Tn testa | 0.00 | 0.00 | 0.17 | 0.83 | 0.00 | 0.83 | 0.00 | 1.00 |
After treatment, TP patients in the decision tree were allocated to ‘Non-fatal AMI (treated)’ and FP patients were further subdivided between ‘No ACS, no UA’ and ‘Unstable angina’ (based on the proportion of UA among non-NSTEMI patients; see Table 11). After being discharged, TN patients were also subdivided between ‘No ACS, no UA’ and ‘Unstable angina’, whereas FN patients were allocated to ‘Non-fatal AMI (untreated)’. The proportions of FNs, reported in Table 13, can be considered as the proportions of AMIs that would have been missed when assuming that standard Tn testing has perfect accuracy. Finally, to calculate the total number of deaths in the decision tree, the probability of 30-day mortality was assigned based on abovementioned subdivision (see Table 11). It was assumed that UA is always correctly diagnosed, hence the mortality probability for treated UA was used.
Markov model
The age-dependent AMI incidence in the UK92 was used to model the occurrence of AMI for patients in the health states ‘No ACS’ and ‘Unstable angina’. It was assumed that all AMIs in the Markov trace are diagnosed correctly and thus receive treatment. For patients in the ‘Post-MI’ health state, the probability of re-infarction after treated AMI was retrieved from a UK record linkage study (n = 387,452), which assessed long-term survival and recurrence after AMI. 93 For the current assessment the probabilities for females and males were weighted according to the estimated proportion of females and males in the population (males = 58.1%7). The re-infarction probability for the ‘Post-MI with re-infarction’ health state is equal to the re-infarction probability for the ‘Post-MI’ health state. The re-infarction RR for people with untreated AMI compared with treated AMI was calculated from a recent study by Mills et al. 83 based on patients with a Tn concentration of 5–19 ng/l. This RR was assumed only for the first year after presentation at ED, after which no increased risk was assumed (i.e. RR = 1.0 for untreated vs. treated AMI after year 1).
Age-dependent mortality from the general population was used for patients in the ‘No ACS, no UA’ health state. 91 For the ‘Post-MI’ and ‘Post-MI with re-infarction’ health states, mortality was extracted from the record linkage study. 93 Again, the study by Mills et al. 83 was used to calculate the mortality RR for untreated AMI compared with treated AMI for the first year, after which an RR of 1.0 was used. Finally, a multivariate adjusted mortality hazard ratio (HR) for UA compared with NSTEMI was retrieved from a study by Allen et al. 94 to calculate mortality after UA.
All input parameters for the Markov model are reported in Table 11.
Health-state utilities
Age-dependent utility scores, from the UK general population, were calculated for patients in the ‘No ACS, no UA’ health state based on a linear regression model. 85 These age-dependent utility scores from the general population were combined with age-dependent disutilities for AMI82 to calculate utilities for the ‘Post-MI’ health states (with or without re-infarction). Utility scores for the ‘Unstable angina’ health state were calculated based on Post-MI utility scores and a utility increment of 0.010 (Table 14). 85
| Parameter | Estimate | SE | Distribution | Source |
|---|---|---|---|---|
| No ACS, no UA | ||||
| Intercept | 1.060 | 0.029 | Normal | Ward et al.85 |
| Disutility for age | 0.004 | 0.001 | Normal | Ward et al.85 |
| Post-MI [disutility compared with no ACS by age (years)] | ||||
| Age = 45 | 0.060 | 0.001 | Normal | CADTH82 |
| Age = 55 | 0.051 | 0.001 | Normal | CADTH82 |
| Age = 65 | 0.025 | 0.001 | Normal | CADTH82 |
| Age = 75 | 0.007 | 0.001 | Normal | CADTH82 |
| UA | ||||
| Utility increment compared with AMI | 0.010 | 0.042 | Normal | Ward et al.85 |
Resource use and costs
Test-specific resource use consisted of the number of tests performed and the duration of hospital stay (hours) before discharge/AMI treatment (Table 15).
| Parameter | Estimate | SE/range | Distribution | Source |
|---|---|---|---|---|
| Number of tests | ||||
| Serial standard Tn testing | 2.00 | – | Fixed | Assumption |
| Roche Elecsys hs-cTnT (99th centile at presentation) | 1.00 | – | Fixed | Assumption |
| Roche Elecsys hs-cTnT (optimal strategy) | 1.60 | 0.02 | Betaa | Chapter 3 |
| Abbott ARCHITECT hs-cTnI (99th centile at presentation) | 1.00 | – | Fixed | Assumption |
| Abbott ARCHITECT hs-cTnI (optimal strategy) | 1.71 | 0.02 | Betaa | Chapter 3 |
| Beckman Coulter hs-cTnI (99th centile) | 1.00 | – | Fixed | Assumption |
| No Tn testb | 0.00 | – | Fixed | Assumption |
| Hospital stay (hours) before discharge/AMI treatmentb | ||||
| Serial standard Tn testing | 14 | 13–15 | Beta PERT | Assumption |
| Roche Elecsys hs-cTnT (99th centile at presentation) | 3 | – | Fixed | Assumption |
| Roche Elecsys hs-cTnT optimal strategy (patients with AMI ruled out on first test) | 3 | – | Fixed | Assumption |
| Roche Elecsys hs-cTnT optimal strategy (patients receiving both tests) | 5 | 4–6 | – | Assumption |
| Abbott ARCHITECT hs-cTnI (99th centile at presentation) | 3 | – | Fixed | Assumption |
| Abbott ARCHITECT hs-cTnI optimal strategy (patients with AMI ruled out on first test) | 3 | – | Fixed | Assumption |
| Abbott ARCHITECT hs-cTnI optimal strategy (patients receiving both tests) | 6 | – | Fixed | Assumption |
| Beckman Coulter hs-cTnI (99th centile at presentation) | 3 | – | Fixed | Assumption |
| No Tn testb | 0 | – | Fixed | Assumption |
Health-state costs (Table 16) were mainly retrieved from previous economic evaluations conducted in the UK. 85,97 Health-state costs for the ‘Unstable angina’, ‘Post-MI’ and ‘Post-MI with re-infarction’ consisted of costs for three 15-minute general practitioner consultations and medication costs. 85 For the first year in the ‘Unstable angina’ health state, costs for clopidogrel (for 60%) and hospitalisation (for 50%) were added to this. The first year costs for both ‘Post-MI’ health states were based on resource data from the Nottingham Heart Attack Register. 97
| Parameter | Estimate (£) | SE/range (£) | Distribution | Source |
|---|---|---|---|---|
| Health-state costs | ||||
| No ACS, no UA first year | 0 | – | Fixed | Assumption |
| No ACS, no UA subsequent year | 0 | – | Fixed | Assumption |
| UA first yeara | 548 | – | Fixed | Ward et al.85 |
| UA subsequent yeara | 213 | – | Fixed | Ward et al.85 |
| Post-MI first yeara,b | 5835 | 488 | Gamma | Palmer et al.97 |
| Post-MI subsequent yearsa,b | 213 | – | Fixed | Ward et al.85 |
| Event costs | ||||
| Costs of fatal AMIa | 1451 | – | Fixed | Ward et al.85 |
| AMI treatment costs | 3436 | – | Fixed | Department of Health98 |
| Unit prices | ||||
| Hospital stay costs (per hour)c | 27 | – | Fixed | Department of Health98 |
| Test costsa | 20 | 18–26 | Beta PERT | Goodacre et al.,7 Thokal et al.80 |
Additionally, costs of fatal events, retrieved from a UK economic evaluation,85 were accumulated for all fatal AMIs. For this purpose, it was assumed that all 30-day deaths after ‘true’ NSTEMI were due to a fatal AMI event. In addition, AMI treatment costs were calculated based on the national tariff for non-elective AMI without complications [Healthcare Resource Group (HRG) code: EB10Z]. 98 To calculate the hospital stay costs for patients, based on the number of hours before the test results become available, non-elective NHS reference costs for the general medical ward were used (HRG code: EB01Z). 98 For this purpose, it was assumed that doctors were available on demand, and the time to discharge was delayed because of time between arrival at the ED and start of first sampling (1 hour) and the time between sampling and the results being available (2 hours). In the case of multiple testing, the 1-hour delay between arrival at the ED and start of sampling was applied to only the first test; however, this also affected the timing of the second test if applicable. The 2-hour delay before test results become available applies to all tests performed. Incorporating these time delays effectively implies that only tests at presentation and tests performed 1 hour after presentation could inform decisions within the NHS 4-hour ED target. All other multiple testing strategies, as well as standard Tn testing at 10–12 hours, would require a transfer from the ED to the general ward (patients are transferred to the general ward 4 hours after presentation at the ED). Finally, the test costs include panel (including reagent, machine and maintenance), calibration and quality control costs. Depending on the annual number of panels, the test costs varied between £16.18 and £21.33, for annual rates of testing of 1500 and 3000, respectively. 80 Based on clinical expert input, the average test costs were estimated to be £20 (2011 price level). 7,80
Overview of main model assumptions
The main assumptions in the health-economic analyses were:
-
Serial Tn testing (comparator) has perfect accuracy (sensitivity = 1.0 and specificity = 1.0).
-
For the Roche Elecsys hs-cTnT and Abbott ARCHITECT hs-cTnI optimal strategies it was assumed that the sensitivity and specificity for the subpopulation not discharged after the presentation test is equal to the sensitivity and specificity for the initial group (presenting at the ED).
-
The life expectancy, quality of life and costs for FP patients is, in the base-case analysis, equal to the life expectancy, quality of life and costs of TN patients. This assumption was amended in the secondary and sensitivity analyses.
-
In contrast with AMIs occurring during the decision tree period, all AMIs (either first or re-infarction) occurring in the Markov trace are diagnosed correctly and thus treated.
-
UA is always correctly diagnosed and thus treated.
-
The re-infarction probability for the ‘Post-MI with re-infarction’ health state is equal to the re-infarction probability for the ‘Post-MI’ health state.
-
The increased ‘Post-MI’ re-infarction and mortality probabilities for untreated AMI were assumed to last 1 year: afterwards a RR of 1.0 was applied (for untreated vs. treated AMI).
-
There is no additional benefit of starting treatment early, so treatment effect for high-sensitive strategies is equal to treatment effect for standard Tn strategy.
-
All 30-day deaths (after presentation at the ED) are due to fatal AMI events and will receive the associated costs.
Model analyses
Expected costs, LYs and QALYs were estimated for all Tn testing methods. Discount rates of 3.5% and a half-cycle correction were applied for both costs and effects. Incremental cost and QALYs for each strategy compared with standard Tn, and compared with the next best alternative, were calculated. The ICER was then calculated by dividing the incremental costs by the incremental QALYs. PSAs (10,000 simulations) were performed, and cost-effectiveness acceptability curves (CEACs) and cost-effectiveness acceptability frontiers (CEAFs) were constructed. Although CEACs can be used to illustrate decision uncertainty, the option with the highest probability of being cost-effective may not necessarily be the most cost-effective option according to the expected values. Moreover, CEAFs can be used to illustrate the decision uncertainty surrounding the most cost-effective option. 100
Secondary analysis
For the base case, it was assumed that patients who tested negative on standard Tn and positive on hs-cTn tests would experience life expectancy and quality of life equal to TN patients. This assumption is, however, debatable, as unpublished data (Goodacre S, Lipinski M, personal communication) show that patients with a negative standard Tn test and positive hs-cTn test have an increased risk of (re-)infarction and mortality compared with those who test negative on both standard Tn and hs-cTn tests. Although this risk was not as high as in patients with both positive standard Tn and positive hs-cTn tests, it could still be considered prognostically important. Therefore, in this secondary analysis the risk of re-infarction and mortality was adjusted for patients who tested FP (see Table 11). It was assumed that for this proportion of patients, the relative treatment benefit would be equal to that for TP patients. As the prevalence of this ‘higher risk subgroup’ is likely to be the same for all comparators, it was assumed that this proportion was equal to the lowest proportion of FP patients for all hs-cTn tests (see Table 13). This ‘higher risk subgroup’ was assumed to be treated for all hs-cTn tests (as they tested positive with these tests) and untreated for the standard Tn test (as they tested negative with this test), thus affecting the probability of adverse outcomes and treatment costs. In addition, the post-MI utility and health-state costs were used for this ‘higher-risk subgroup’.
Sensitivity analysis
For both the base case and the secondary analysis, the following one-way sensitivity analyses were performed to assess the impact of model assumptions and input parameters on the estimated outcomes:
Model assumptions:
-
The assumption that the increased post AMI re-infarction and mortality probabilities for untreated AMI lasts for only 1 year was replaced by the assumption that these probabilities would remain elevated for a lifetime.
-
The assumption that a doctor will be available on demand and thus that a decision could be made immediately (as in the base case) was replaced with an assumed delay (1, 2 or 3 hours) before a doctor is available and a decision could be made.
-
As for the previous sensitivity analysis, except that the delay (1, 2 or 3 hours) applies only once patients are transferred to the general ward 4 hours after presentation (no delay in the ED).
-
A total delay of 1.5 hours is assumed (includes delay from the time at which sampling could be performed to the time at which results became available and delay between arrival at hospital and Tn assessment commencing) rather than assuming a total delay of 3 hours (base case).
-
AMI treatment costs are applied for patients who tested FP rather than using no treatment costs, as assumed in the base-case analysis.
-
In addition to the health-state costs of UA during the first year, the AMI treatment costs are also applied for patients with UA (during the first year), rather than assuming no additional treatment costs.
Model input parameters (varied to lower and upper boundary of the 95% CI unless stated otherwise):
-
test costs [test costs was varied over a wider range (£5–40) than the 95% CI]
-
AMI treatment costs (±25%)
-
post-MI first-year health-state costs
-
utility increment for UA compared with AMI
-
post-MI disutility compared with no ACS
-
mortality (30 day) treated AMI (decision tree)
-
mortality (30 day) untreated AMI (decision tree)
-
annual re-infarction (after initial AMI)
-
RR re-infarction (untreated vs. treated AMI)
-
annual post-MI mortality
-
annual post-MI mortality after re-infarction
-
HR mortality (UA vs. NSTEMI)
-
RR mortality (untreated vs. treated AMI).
Subgroup analysis
For both the base case and the secondary analysis, a number of subgroup analyses were performed. The main subgroup analyses were based on age- and sex-dependent re-infarction probabilities, mortality probabilities (for all health states), AMI incidence and quality of life, and could be applied to all test strategies. Accuracy was thus assumed to be subgroup independent (equal to the base case values). The following subgroups were identified:
-
Sex.
-
Age (45, 55, 65, 75 and 85 years).
-
People with a history of previous NSTEMI. For this purpose, a proportion of 0% UA was assumed and the probabilities for the initial ‘Post-MI’ health state were used for the ‘No ACS, no UA’ health state and the probabilities for ‘Post-MI with re-infarction’ were used for the ‘Post-MI’ and ‘Post-MI with re-infarction’ health states. This subgroup analysis was performed for only the base case, as for the secondary analysis this would lead to lower mortality probabilities for FP patients than TN patients (which seems implausible).
-
Subgroups with varying AMI prevalence (1%, 5%, 10%, 20%, 30%). In these analyses the no-testing strategy was included as a comparator, as a Tn test may not be indicated when clinical judgement assesses that the probability that a patient is experiencing an AMI is low. For the no-testing strategy it is assumed that patients will be discharged immediately.
It should be noted that the main subgroup analyses (described above) differ from the subgroups described in the systematic review component of this assessment (see Chapter 3, Presentation samples), for which specific accuracy and prevalence data were available. Additional subgroup analyses were performed based on these subgroup-specific accuracy data. However, these analyses could be performed for only the Roche Elecsys hs-cTnT assay at presentation sample, using the 99th centile diagnostic threshold, compared with standard Tn testing; no subgroup-specific accuracy data were available for the other two hs-cTn assays. The following subgroups were considered:
-
age ≤ 70 years and age > 70 years
-
patients with pre-existing CAD and patients without pre-existing CAD
-
symptom onset at < 3 hours before presentation and symptom onset at ≥ 3 hours before presentation.
The subgroups with high pre-test probability and low-to-moderate pre-test probability were not considered, as the prevalence data for these subgroups were unknown.
Results of cost-effectiveness analyses
This section describes the results using probabilistic analyses for the base-case analysis and the secondary analysis. In addition, the sensitivity analyses (deterministic) and subgroup analyses are described (these deterministic analyses are also presented in tabulated form in Appendices 5–9.
Base-case analysis
The base-case analysis includes six test strategies. Tables 17 and 18 show the probabilistic results of this analysis. Standard Tn testing was both most effective (15.101 LYs, 11.730 QALYs) and most expensive (£2697). The Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was least effective (15.076 LYs, 11.712 QALYs) and least expensive (£2253). Compared with standard Tn testing, hs-cTn testing resulted in ICERs ranging between £90,725 and £24,019 savings per QALY lost.
| Strategy | LYs (95% CI) | Compared with standard Tn |
|---|---|---|
| Abbott ARCHITECT hs-cTnI (99th centile at presentation) | 15.076 (14.321 to 15.764) | –0.024 |
| Roche Elecsys hs-cTnT (99th centile at presentation) | 15.085 (14.332 to 15.770) | –0.016 |
| Beckman Coulter hs-cTnI (99th centile at presentation) | 15.090 (14.338 to 15.774) | –0.010 |
| Roche Elecsys hs-cTnT optimal strategy | 15.091 (14.340 to 15.776) | –0.009 |
| Abbott ARCHITECT hs-cTnI optimal strategy | 15.098 (14.351 to 15.780) | –0.003 |
| Standard Tn | 15.101 (14.356 to 15.781) |
| Strategy | Costs, £ (95% CI) | QALYs (95% CI) | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott ARCHITECT hs-cTnI (99th centile at presentation) | £2253 (£1702 to £2877) | 11.712 (10.312 to 13.157) | –£444 | –0.018 | £24,019 | ||||
| Roche Elecsys hs-cTnT (99th centile at presentation) | £2296 (£1731 to £2936) | 11.718 (10.319 to 13.165) | –£401 | –0.012 | £33,247 | Abbott ARCHITECT hs-cTnI (99th centile at presentation) | £42 | 0.006 | Extendedly dominated |
| Beckman Coulter hs-cTnI (99th centile at presentation) | £2324 (£1755 to £2971) | 11.723 (10.323 to 13.172) | –£373 | –0.008 | £48,337 | Abbott ARCHITECT hs-cTnI (99th centile at presentation) | £71 | 0.011 | £6600 |
| Roche Elecsys hs-cTnT (optimal strategy) | £2422 (£1846 to £3077) | 11.723 (10.326 to 13.171) | –£275 | –0.007 | £38,528 | Beckman Coulter hs-cTnI (99th centile at presentation) | £98 | 0.001 | Extendedly dominated |
| Abbott ARCHITECT hs-cTnI (optimal strategy) | £2491 (£1908 to £3148) | 11.728 (10.328 to 13.177) | –£206 | –0.002 | £90,725 | Beckman Coulter hs-cTnI (99th centile at presentation) | £167 | 0.005 | £30,631 |
| Standard Tn | £2697 (£2113 to £3359) | 11.730 (10.334 to 13.179) | Abbott ARCHITECT hs-cTnI (optimal strategy) | £206 | 0.002 | £90,725 | |||
Comparisons based on the next best alternative showed that for willingness-to-pay values of < £6600 per QALY, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, would be cost-effective. For thresholds between £6600 and £30,631 per QALY, the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective; above £30,631 per QALY the Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective. Standard Tn becomes cost-effective at a threshold of £90,725 or higher (see Table 18).
At willingness-to-pay thresholds of £20,000 and £30,000 per QALY, the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, had probabilities of being cost-effective of 47% and 35%, respectively. Although the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective at a willingness-to-pay threshold of £30,000 per QALY, the Abbott ARCHITECT hs-cTnI optimal strategy had the highest probability of being cost-effective (35%) at this threshold (Figures 16 and 17).
FIGURE 16.
Cost-effectiveness acceptability curve and incremental cost-effectiveness plane (incremental costs and QALYs compared with standard Tn) for base-case analysis.
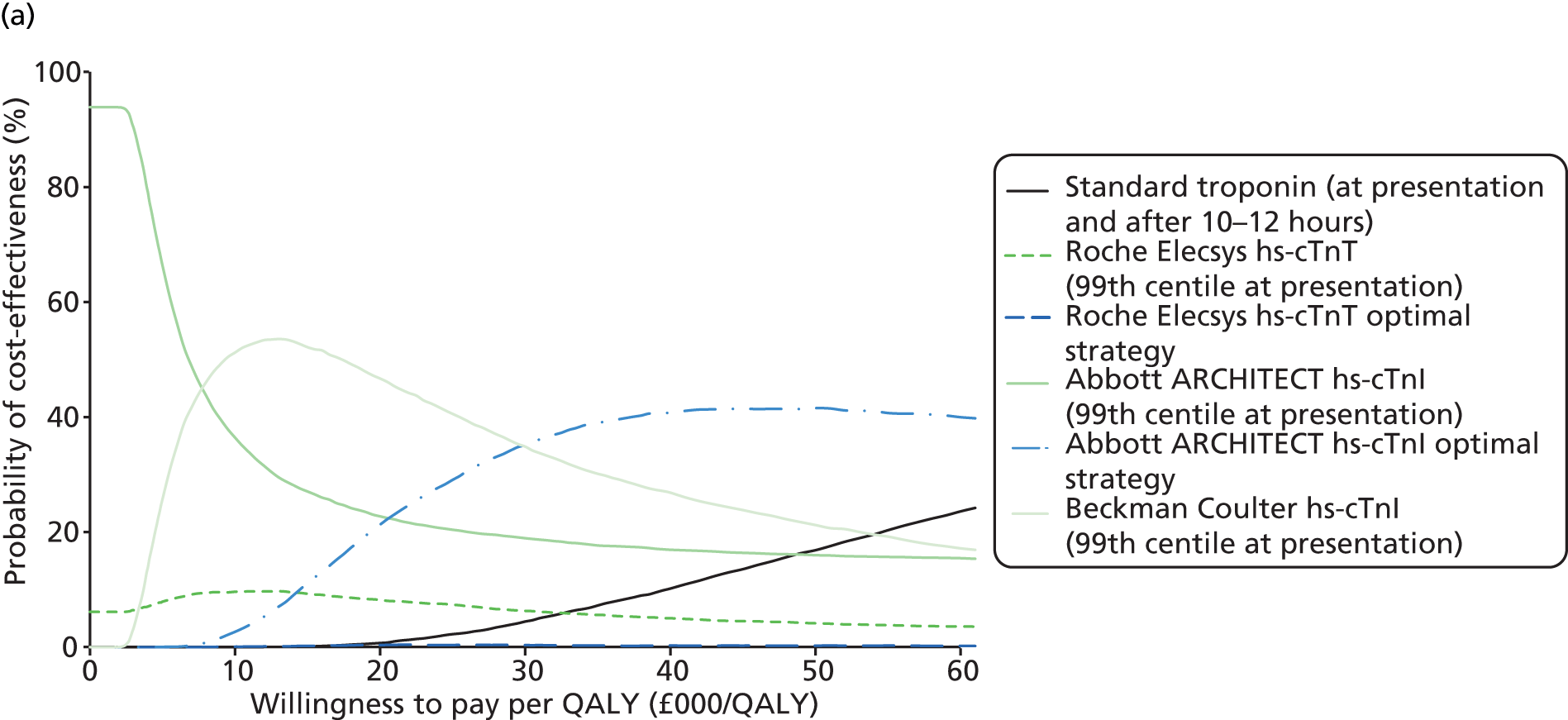

FIGURE 17.
Cost-effectiveness acceptability frontier for base-case analysis.

Secondary analysis
The secondary analysis includes the same six test strategies. This analysis assumed that in a proportion of patients with a FP hs-cTn test (i.e. positive hs-cTn test and a negative standard Tn test), there is prognostic significance [i.e. it is associated with an increased risk of adverse events (mortality and re-infarction)].
Standard Tn testing was least effective (14.785 LYs, 11.464 QALYs) and most expensive (£3058). The Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was the least effective hs-cTn test strategy (14.833 LYs, 11.501 QALYs) and, overall, the least expensive strategy (£2781). The Abbott ARCHITECT hs-cTnI optimal strategy was most effective (14.855 LYs, 11.518 QALYs). Standard Tn testing was dominated by all hs-cTn testing strategies.
Comparisons based on the next best alternative showed that for willingness-to-pay values of < £13,623 per QALY, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective. For thresholds between £13,623 and £14,562 per QALY, the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, was cost-effective; above £14,562 per QALY the Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective (Tables 19 and 20).
| Strategy | LYs (95% CI) | Compared with standard Tn |
|---|---|---|
| Abbott ARCHITECT hs-cTnI (99th centile at presentation) | 14.833 (14.104 to 15.487) | 0.048 |
| Roche Elecsys hs-cTnI (99th centile at presentation) | 14.837 (14.111 to 15.491) | 0.052 |
| Beckman Coulter hs-cTnI (99th centile at presentation) | 14.839 (14.114 to 15.488) | 0.054 |
| Roche Elecsys hs-cTnT (optimal strategy) | 14.843 (14.119 to 15.494) | 0.058 |
| Abbott ARCHITECT hs-cTnI (optimal strategy) | 14.855 (14.129 to 15.502) | 0.070 |
| Standard Tn | 14.785 (14.061 to 15.436) |
| Strategy | Costs (95% CI) | QALYs (95% CI) | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott ARCHITECT hs-cTnI (99th centile at presentation) | £2781 (£2247 to £3388) | 11.501 (10.087 to 12.918) | –£277 | 0.037 | Dominant | ||||
| Roche Elecsys hs-cTnT (99th centile at presentation) | £2823 (£2271 to £3442) | 11.504 (10.092 to 12.920) | –£235 | 0.040 | Dominant | Abbott ARCHITECT hs-cTnI (99th centile at presentation) | £42 | 0.003 | £13,623 |
| Beckman Coulter hs-cTnI (99th centile at presentation) | £2851 (£2299 to £3477) | 11.506 (10.093 to 12.923) | –£207 | 0.042 | Dominant | Roche Elecsys hs-cTnT (99th centile at presentation) | £28 | 0.001 | Extendedly dominated |
| Roche Elecsys hs-cTnT (optimal strategy) | £2949 (£2390 to £3579) | 11.509 (10.095 to 12.926) | –£109 | 0.045 | Dominant | Roche Elecsys hs-cTnT (99th centile at presentation) | £126 | 0.004 | Extendedly dominated |
| Abbott ARCHITECT hs-cTnI (optimal strategy) | £3018 (£2446 to £3659) | 11.518 (10.103 to 12.936) | –£39 | 0.054 | Dominant | Roche Elecsys hs-cTnT (99th centile at presentation) | £196 | 0.013 | £14,562 |
| Standard Tn | £3058 (£2485 to £3708) | 11.464 (10.053 to 12.869) | Abbott ARCHITECT hs-cTnI (optimal strategy) | £39 | –0.054 | Dominated | |||
At willingness-to-pay thresholds of £20,000 and £30,000 per QALY, the Abbott ARCHITECT hs-cTnI optimal strategy had the highest probability of being cost-effective (53% and 67%, respectively; Figures 18 and 19).
FIGURE 18.
Cost-effectiveness acceptability curve and incremental cost-effectiveness plane (incremental costs and QALYs compared with standard Tn) for secondary analysis.

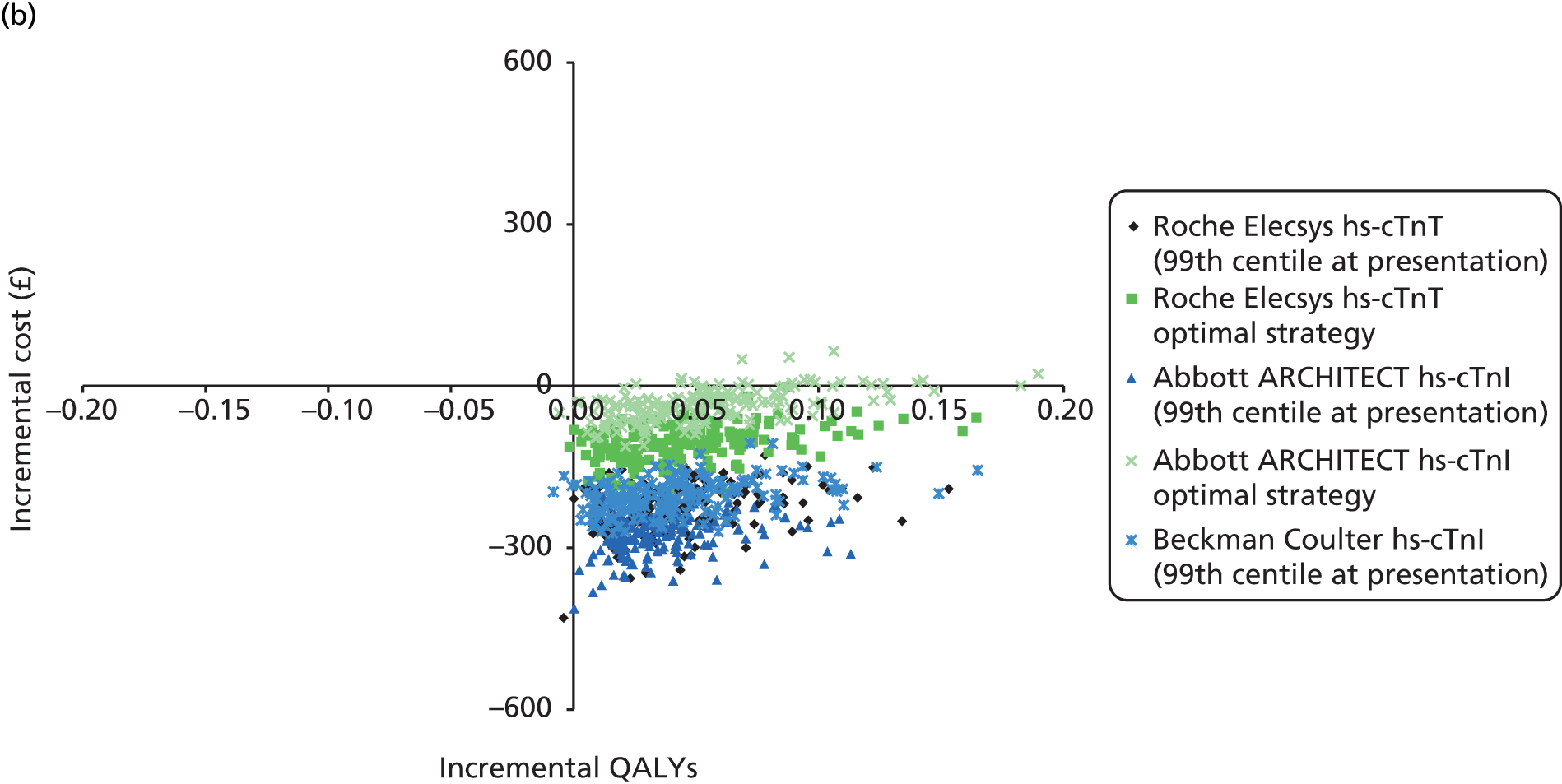
FIGURE 19.
Cost-effectiveness acceptability frontier for secondary analysis.

Sensitivity analysis
The deterministic analysis for the base-case analysis is presented in Appendix 5. When it was assumed that the post-MI re-infarction and mortality probabilities would remain elevated for untreated AMI for a life-time period, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £1642 per QALY, at which point the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, became cost-effective up to a threshold of £7602 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds between £7602 and £26,532 per QALY. Standard Tn testing was cost-effective for thresholds of > £26,532 per QALY. Consistent with the base-case analysis, all ‘no doctor on demand’ sensitivity analyses (1, 2 or 3 hours) showed that the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between approximately £8000 and £40,000 per QALY. Similarly, where the total delay decreased to 1.5 hours (and assuming availability of a doctor on demand), the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between £7778 and £29,653 per QALY, at which point the ARCHITECT hs-cTnI optimal strategy became cost-effective. Adding AMI treatment costs for the patients with a FP test substantially impacted upon the results: standard Tn testing was cost-effective for all threshold values of > £16,050 per QALY. Adding AMI treatment costs to the UA health state for the first year had a negligible impact on the incremental outcomes.
The following input parameters had a noticeable impact on the estimated cost-effectiveness: 30-day mortality for treated and untreated AMI (decision tree) and the mortality RR for treated AMI compared with untreated AMI (Markov trace). Varying the remaining parameters did not have a substantial impact on the results (i.e. the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between approximately £10,000 and £35,000 per QALY).
The deterministic analysis for the secondary analysis is presented in Appendix 6. When assuming that the post-AMI re-infarction and mortality probabilities would remain elevated for untreated AMI for a life-time period, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £1853 per QALY, at which point the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, became cost-effective up to a threshold of £2017 per QALY. The Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between £2017 and £5889 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds of > £5889 per QALY. For all ‘no doctor-on-demand’ sensitivity analyses, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £18,000 per QALY for 1, 2 and 3 hours’ delay. The Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between £18,000 and £19,000, £20,000 and £22,000 per QALY in case of 1, 2 and 3 hours’ delay, respectively. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for higher thresholds. Similarly to the deterministic base case, for which the total delay decreased to 1.5 hours (assuming availability of a doctor on demand), the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of below £14,956, at which point the ARCHITECT hs-cTnI optimal strategy became cost-effective. Adding AMI treatment costs for all patients with a FP test gave similar results to the deterministic analysis: the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for all threshold values of < £15,508 per QALY, at which point the Abbott hs-cTnI optimal strategy became the preferred option. Adding AMI treatment costs to the ‘Unstable angina’ health state for the first year had a negligible impact on the incremental outcomes.
The following input parameters had a noticeable impact on the estimated cost-effectiveness of the secondary analysis: increased test cost (of £40 per test), 30-day mortality for treated and untreated AMI (decision tree), and the re-infarction and mortality RR for treated AMI compared with untreated AMI (Markov trace). Varying the remaining parameters did not have a substantial impact on the results.
Subgroup analysis
Additional analyses were performed for subgroups based on age, sex, people with a history of previous NSTEMI, and AMI prevalence. These deterministic subgroup analyses (for the base case) analysis are presented in Appendix 7. Consistent with the base-case analyses, analyses based on age and sex subgroups indicated that, up to an age of 75 years, the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between approximately £10,000 and £35,000 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for higher thresholds up to £115,000–170,000, at which point standard Tn testing became cost-effective. For females aged > 85 years, the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between £15,793 and £74,597 per QALY; the Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds between £74,597 and £259,592 per QALY, and standard Tn testing was cost-effective for thresholds of £259,592 per QALY and higher. For males aged > 85 years, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £28,711 per QALY; the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds between £28,711 and £143,225 per QALY and the Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds between £143,225 and £503,476 per QALY, at which point standard Tn testing became cost-effective. The results for the subgroup with a history of previous NSTEMI were almost identical to the base-case analysis.
For subgroup analyses considering AMI prevalence, no testing was included as additional comparator. For an AMI prevalence of 1%, the no-testing strategy was cost-effective up to thresholds of £27,409 per QALY, at which point the Beckman Coulter hs-cTnI (99th centile) test became cost-effective up to a threshold of £447,934 per QALY. For an AMI prevalence of 5–20%, the no-testing strategy was cost-effective up to thresholds of £8759–11,703 per QALY, at which point the Beckman Coulter hs-cTnI (99th centile) test became cost-effective up to thresholds of £32,042–97,709 per QALY. For an AMI prevalence of 30%, the no-testing strategy was cost-effective up to a threshold of £8431 per QALY, at which point the Beckman Coulter hs-cTnI (99th centile) test became cost-effective up to a threshold of £24,745 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds between £24,745 and £70,942 per QALY.
In addition, cost-effectiveness estimates for the subgroups, described in Chapter 3 (see Presentation samples), based on subgroup-specific accuracy and prevalence, are reported in Appendix 9 (only comparing the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, and standard Tn testing). The results of these analyses indicated that differences in accuracy and AMI prevalence between subgroups had a substantial impact on the cost-effectiveness of the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, compared with standard Tn testing (ICER range: £22,111–355,571; deterministic base case: £41,233).
The deterministic subgroup analyses for the secondary analysis are presented in Appendix 8. For females aged 45 years and males aged 45 or 55 years, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £16,023–17,836 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy became cost-effective for higher thresholds. For females aged 55 or 65 years and males aged 65 or 75 years, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £13,064–16,994 per QALY. From this threshold up to £18,999–25,149 per QALY, the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, was most cost-effective. The Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for higher thresholds. For females aged 75 or 85 years, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective up to thresholds of £12,392£21,140 per QALY, at which point the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, became cost-effective up to thresholds of £16,407–26,911 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy became cost-effective for thresholds of > £24,020–45,709 per QALY. For males aged 85 years, the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was cost-effective for thresholds of < £66,418 per QALY. The Abbott ARCHITECT hs-cTnI optimal strategy became cost-effective for higher thresholds.
For subgroup analyses considering AMI prevalence, no testing was included as additional comparator. For an AMI prevalence of 1%, the no-testing strategy was cost-effective up to a threshold of £4563 per QALY, at which point the Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, became cost-effective up to a threshold of £109,991 per QALY, where the Abbott ARCHITECT hs-cTnI optimal strategy became cost-effective. Similarly, for AMI prevalences of 5% and 10% the thresholds were £5209 and £35,574, and £5820 and £22,684, respectively. For a AMI prevalences of 20% and 30%, the Abbott ARCHITECT hs-cTnI optimal strategy was cost-effective for thresholds of > £16,319 and £15,410, respectively.
In contrast with the base-case analysis (described above), the subgroup-specific accuracy and prevalence (only comparing the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, and standard Tn testing) did not have an important impact on the cost-effectiveness (see Appendix 9). The Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, was dominant for all subgroups.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
All 18 studies19,39,40,42,44,46,48,49,51,54,55,57,58,63,64,67,70,73 (37 publications19,39,40–74) included in the systematic review assessed the accuracy of one or more hs-cTn tests for the diagnosis of any AMI or for NSTEMI. There were no controlled trials comparing clinical outcomes in people assessed using hs-cTn tests to those assessed using conventional Tn assays. The majority (15/18) of the included studies reported data for the Roche Elecsys hs-cTnT assay; four studies39,48,58,63 reported data for the Abbott ARCHITECT hs-cTnI assay and two studies39,73 reported data for precommercial versions of the Beckman Coulter Access hs-cTnI assay. Not all of the included studies reported data on accuracy for the diagnosis of NSTEMI (i.e. for a population that excluded people with STEMI), which was the target population for this assessment. However, where data were available for both any AMI (population with symptoms suggestive of ACS) and NSTEMI (population which excluded people with STEMI), estimates of test performance were generally similar (see Tables 4 and 6).
When diagnosis was based on a single sample taken at presentation, using the 99th centile for the general population as the diagnostic threshold, positive LRs derived from summary estimates of sensitivity and specificity indicated that neither the Roche Elecsys hs-cTnT assay nor the Beckman Coulter Access hs-cTnI would be adequate to rule in a diagnosis of NSTEMI. The LR+ for the Roche Elecsys hs-cTnT assay was 5.41 (95% CI 3.40 to 8.63) and the LR+ for the Beckman Coulter Access hs-cTnI was 3.67 (95% CI 3.26 to 4.13). By contrast, the LR+ for the Abbott ARCHITECT hs-cTnI assay, in a population that did not exclude STEMI, was 11.47 (95% CI 9.04 to 16.19), indicating that a positive test using this assay may have some utility in confirming a diagnosis of AMI. The corresponding LR–s indicated that a negative test result on a single sample taken at presentation, using the 99th centile for the general population as the diagnostic threshold, would not be adequate to rule out NSTEMI using any of the three assays assessed. LR– was 0.15 (95% CI 0.08 to 0.26) for the Roche Elecsys hs-cTnT, 0.11 (95% CI 0.07 to 0.17) for the Beckman Coulter Access hs-cTnI, and 0.22 (95% CI 0.16 to 0.27) for the Abbott ARCHITECT hs-cTnI assay. Although these LRs are fairly low, the consequences of missing an AMI are so great that a test needs to be able to rule out an AMI with a very high degree of certainty. It should be noted that the Beckman Coulter hs-cTnI assay evaluated in the APACE study39 was described as ‘an investigational prototype’; the 99th centile (9 ng/l), described as ‘according to the manufacturer’, differs from the 99th centile given in the current product information leaflet (40 ng/l),16 and from values reported in a conference abstract, (41 ng/l for the Access II analyser and 34 ng/l for the DxI analyser). 17 When a hypothetical cohort of 1000 people is considered, assuming a prevalence of NSTEMI of 17% [derived from studies included in our systematic review (see Chapter 3, Presentation samples)] the estimated number of people with AMI and a negative test result who would be erroneously discharged based on this testing protocol is 20 for the Roche Elecsys hs-cTnT assay, 14 for the Beckman Coulter Access hs-cTnI assay, and 34 for the Abbott ARCHITECT hs-cTnI assay.
Some limited data were available on the diagnostic performance of the Roche Elecsys hs-cTnT assay in clinical subgroups, using a single sample taken at presentation and the 99th centile diagnostic threshold. These data indicated a lower LR– when the test is used in certain population groups [e.g. people aged > 70 years LR– 0.05 (95% CI 0.02 to 0.18); people without pre-existing CAD LR– 0.07 (95% CI 0.04 to 0.16)] and with a high pre-test probability (determined by clinical judgement based on cardiovascular risk factors, type of chest pain, physical findings and ECG abnormalities; LR– 0.09, 95% CI 0.02 to 0.45). Using the hypothetical cohort of 1000 people described above, the estimated number of people with AMI and a negative test result who would be erroneously discharged if the test were used to rule out AMI in these selected populations is five for people aged > 70 years, 10 for people without pre-existing CAD, and 10 for people with a clinical assessment of high pre-test probability. When the performance of the Roche Elecsys hs-cTnT assay was assessed in a population restricted to people who presented at > 3 hours after the onset of symptoms, a similar fall in the LR– was observed (LR– 0.08, 95% CI 0.05 to 0.11); the estimated number of people with AMI and a negative test result who would be erroneously discharged if the test were used to rule out AMI in this populations is 10.
We constructed optimal testing strategies for the Roche Elecsys hs-cTnT assay (see Figure 9 and Chapter 3, Diagnostic accuracy of the Roche Elecsys hs-cTnT assay, Multiple samples) and for the Abbott ARCHITECT hs-cTnI assay (see Figure 11 and Chapter 3, Diagnostic accuracy of the Abbott ARCHITECT hs-cTnI assay, Multiple samples). Both strategies use a two-step process, which provides two potential opportunities to rule out AMI and hence to discharge patients within the 4-hour window specified in the scope for this assessment. This potential is conditional upon the achievement of short (< 1 hour) turnaround times for hs-cTn testing, as recommended by the joint National Academy of Clinical Biochemistry and IFCC guidelines on Tn testing101 and in line with clinical opinion; a study of 1355 ED physicians in the USA indicated that 75% believed that the results of Tn testing should be available to them within 45 minutes. 102 The initial step for both the Abbott ARCHITECT hs-cTnI optimal strategy and Roche Elecsys hs-cTnT optimal strategy was based on the use of an LoB (3 ng/l) diagnostic threshold in a sample taken at presentation and was selected for optimal rule-out potential (low LR–), regardless of poor rule-in performance. For the Roche Elecsys hs-cTnT optimal strategy, the second step involves an additional sample taken 2– 3 hours after admission and was selected to provide the best possible combination of rule-out and rule-in performance. Using the hypothetical cohort of 1000 people previously described, the initial step of the proposed Roche Elecsys hs-cTnT optimal strategy would result in discharge of 407 people, nine of whom would have been erroneously discharged with AMI. The second step of this strategy involves a combination of testing on admission and after 2 hours, where a negative result is defined as both no sample above the 99th centile AND a change of < 20% over 2 hours and provides the optimum rule-out performance (LR– 0.04, 95% CI 0.02 to 0.10); conversely, a positive result is defined as both a peak value above the 99th centile AND a change of > 20% over 2 hours and provides the optimum rule-in performance (LR+ 8.42, 95% CI 6.11 to 11.60). Application of the rule-out component of the second step would result in discharge of a further 286 people, five of whom would have been erroneously discharged. For the proposed Abbott ARCHITECT hs-cTnI optimal strategy, the initial rule-out step would result in discharge of 291 people, all of whom would have been appropriately discharged. The second step of this strategy involves repeat testing on a sample taken 3 hours after admission, using the 99th centile diagnostic threshold. Application of the rule-out component of the second step would result in discharge of a further 486 people, three of whom would have been erroneously discharged. Available data on the Beckman Coulter hs-TnI assay were insufficient to support construction of an optimal testing strategy.
Cost-effectiveness
The review of economic analyses of hs-cTn (i.e. either hs-cTnI or hs-cTnT) testing for the early rule-out of AMI in people with acute chest pain found four HTA reports, two full papers and one abstract. Based on all of these publications, it can be said that, in general, the question of whether hs-cTn testing is cost-effective cannot yet be answered unequivocally. The majority of papers reported substantial ICERs, with considerable uncertainty. In particular, the accuracy of high-sensitivity tests, as well as the efficiency of decision-making based on test results, were found to be important drivers of cost-effectiveness.
In our health-economic analysis, the cost-effectiveness of different testing strategies – involving hs-cTn for the early rule-out of AMI in people with acute chest pain presenting to the ED with suspected ACS and STEMI ruled out – was assessed. All analyses had the same comparator: standard Tn testing at 10–12 hours, which is considered the reference standard and therefore was assumed to have perfect sensitivity and specificity. In addition to the base-case analysis, given some evidence that FPs compared with this reference standard also have a poor prognosis, a secondary analysis was conducted, which assumed an increased adverse event risk for patients with FP hs-cTn tests. A number of subgroup and sensitivity analyses were also performed.
In the base-case analysis, standard Tn testing was both most effective and most costly. Strategies considered cost-effective depending upon ICER thresholds were Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold (thresholds of < £6597), Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold (thresholds between £6597 and £30,042), Abbott ARCHITECT hs-cTnI optimal strategy (LoD threshold at presentation, followed by 99th centile threshold at 3 hours) (thresholds between £30,042 and £103,194), and the standard Tn test (thresholds of > £103,194). The Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, and the Roche Elecsys hs-cTnT optimal strategy [LoB threshold at presentation followed by 99th centile threshold and/or Δ20% (compared with presentation test) at 1–3 hours] were extendedly dominated in this analysis (one of the more effective strategies was better value in that the ICER was lower).
In the secondary analysis, which assumed that a proportion of FPs in the hs-cTn testing strategies had an increased risk of adverse events, standard Tn was least effective and most costly and, therefore, a dominated strategy. The most effective strategy here was the Abbott ARCHITECT hs-cTnI optimal strategy. The Roche Elecsys hs-cTnT optimal strategy was extendedly dominated (one of the more effective strategies was better value in that the ICER was lower), as was the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, in this analysis. Strategies considered cost-effective were Abbott ARCHITECT hs-cTnI assay at presentation, using the 99th centile diagnostic threshold (thresholds of < £12,217), Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold (thresholds between £12,217 and £14,992), and Abbott ARCHITECT hs-cTnI optimal strategy (thresholds of > £14,992).
Sensitivity analyses showed that, in general, there were no major changes in the relative cost-effectiveness of strategies. That is, dominancy and order of relative cost-effectiveness were comparable, although the ICERs were different. Exceptions included assuming that the increased 30-day mortality for treated MI compared with untreated MI applied to a lifetime (instead of only during the first year after presentation at ED), which meant that standard Tn could be cost-effective from a threshold of ≥ £26,352. The same assumption applied to the secondary analysis meant that the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, was no longer extended dominated but was considered cost-effective at thresholds of between £2017 and £5889. Another sensitivity analysis that resulted in substantial changes was assigning AMI treatment costs to patients who tested FP. In the base case, under this assumption, standard Tn became cost-effective at an ICER threshold of £20,000 (ICER £16,050 compared with the Abbott ARCHITECT hs-cTnI optimal strategy). In the secondary analysis, however, assigning treatment costs to FP patients did not have an impact on the position of standard Tn; it was still dominated by another strategy (i.e. less effective and more costly).
Subgroup analyses (with non-subgroup specific accuracy data) for the base case showed that ICERs compared with the next best strategy were slightly higher for males at all ages. Also, for both females and males, ICERs increased with age. In addition, from ages ≥ 55 years (base case 53 years), the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, became extendedly dominated. In the subgroup with previous NSTEMI, again the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, was extendedly dominated, and ICERs are slightly higher than in the whole group. Subgroup analysis based on MI prevalence (including a no-testing strategy) indicated that only when MI prevalence is as low as 1% (base case 17%) was the no-testing strategy considered cost-effective up to an ICER threshold of £27,409, after which the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, strategy takes over. The higher the prevalence, the lower the point at which the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, strategy became cost-effective (i.e. £11,703 for prevalence 5%, £9740 for prevalence 10%, and £6597 for 17%).
For the secondary analysis, again, the ICERS for males were slightly higher than for females. For the various age categories, results were rather diffuse, but, as in the base case, ICERs appeared to increase with age. There did not appear to be a substantial difference between the MI prevalence subgroups [i.e. the no-testing strategy was cost-effective only up to rather modest ICER thresholds (£4563–7109) for all values of prevalence].
The subgroup analyses using subgroup-specific accuracy and prevalence could be performed for only the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, as there were no subgroup data on Beckman Coulter hs-cTnI and Abbott ARCHITECT hs-cTnI assays. The comparator was the standard Tn at 10–12 hours, which was assumed to have perfect sensitivity and specificity. For the base case, the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, was always less costly and less effective, but ICERs were more favourable for the following subgroups compared with their counterparts: age ≤ 70 years, with pre-existing CAD, and symptom onset at < 3 hours. For the secondary analysis, the standard Tn was dominated by the Roche Elecsys hs-cTnT assay at presentation, using the 99th centile diagnostic threshold, overall, as this test was both less costly and more effective. However, the subgroups that rendered the highest savings per QALY gained were consistent with the base-case analysis (i.e. age ≤ 70 years, with pre-existing CAD, and symptom onset at < 3 hours). Although data are lacking, it seems likely that these differences between subgroups can be extrapolated, at least partly, to the other tests considered in the base-case analysis.
Strengths and limitations of assessment
Clinical effectiveness
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Because of the known difficulties in identifying test accuracy studies using study design-related search terms,103 search strategies were developed to maximise sensitivity at the expense of reduced specificity. Thus, large numbers of citations were identified and screened, relatively few of which met the inclusion criteria of the review.
The possibility of publication bias remains a potential problem for all systematic reviews. Considerations may differ for systematic reviews of test accuracy studies. It is relatively simple to define a positive result for studies of treatment, for example a significant difference between the treatment and control groups that favours treatment. This is not the case for test accuracy studies, which measure agreement between index test and reference standard. It would seem likely that studies finding greater agreement (high estimates of sensitivity and specificity) will be published more often. In addition, test accuracy data are often collected as part of routine clinical practice, or by retrospective review of records; test accuracy studies are not subject to the formal registration procedures applied to RCTs and are therefore more easily discarded when results appear unfavourable. The extent to which publication bias occurs in studies of test accuracy remains unclear; however, simulation studies have indicated that the effect of publication bias on meta-analytic estimates of test accuracy is minimal. 104 Formal assessment of publication bias in systematic reviews of test accuracy studies remains problematic and reliability is limited. 27 We did not undertake a statistical assessment of publication bias in this review. However, our search strategy included a variety of routes to identify unpublished studies and resulted in the inclusion of a number of conference abstracts.
Clear inclusion criteria were specified in the protocol for this review, a copy of which is available on the PROSPERO website (registration number CRD42013005939). The eligibility of studies for inclusion is therefore transparent. In addition, we have provided specific reasons for exclusion for all of the studies which were considered potentially relevant at initial citation screening and were subsequently excluded on assessment of the full publication (see Appendix 4). The review process followed recommended methods to minimise the potential for error and/or bias;25 studies were independently screened for inclusion by two reviewers, and data extraction and quality assessment were done by one reviewer and checked by a second (MW and PW). Any disagreements were resolved by consensus.
Studies included in this review were assessed for risk of bias and applicability using the QUADAS-2 tool developed by the authors33 and recommended by the Cochrane Collaboration. 27 QUADAS-2 is structured into four key domains covering participant selection, index test, reference standard, and the flow of patients through the study (including timing of tests). Each domain is rated for risk of bias (low, high or unclear); the participant selection, index test and reference standard domain are, also, separately rated for concerns regarding the applicability of the study to the review question (low, high or unclear). The results of the QUADAS-2 assessment are reported, in full, for all included studies in Appendix 3 and are summarised in Chapter 3 (see Study quality). The main potential sources of bias in the studies included in this assessment were related to patient spectrum and patient flow (QUADAS domains 1 and 4). Reporting of the participant selection process was frequently unclear; a further study55 was rated as unclear for this domain as a large number of patients were not enrolled as a result of ‘technical reasons’ that were not fully defined and so it was not possible to judge whether or not these comprised inappropriate exclusions. The most common feature of studies rated as ‘high risk of bias’ for patient selection was the inclusion of participants based on staffing or work flow considerations; for example, participants were excluded if they presented at night or during busy periods. 42,46,51 All ratings of ‘high risk of bias’ for patient flow were due to high proportions of withdrawals. There were also concerns regarding the applicability of the patient population and the reference standard in some of the included studies. The main area of concern, with respect to population, was for studies that enrolled mixed populations (i.e. when the target condition was any AMI); because the primary focus of this assessment was the diagnosis of NSTEMI in populations where patients with STEMI were excluded (i.e. target condition NSTEMI), the primary focus was the population of patients with STEMI excluded, mixed population studies that were not restricted to this specific patient group were considered to have high concerns regarding applicability. However, as noted above (see Clinical effectiveness), where data were available for both any AMI (mixed population) and NSTEMI (population which excluded people with STEMI), estimates of test performance were generally similar. In accordance with current NICE guidance,11 our review question specified that an appropriate reference standard had to include a standard Tn measurement at baseline and at 10–12 hours after the onset of symptoms in 80% of the population. Although studies generally included a baseline and a second, later, standard Tn measurement, only five19,39,42,51,63 met the specific timing criterion for the second standard Tn measurement; studies that did not meet this criterion were classified as having high concerns regarding applicability.
We identified one recently published systematic review which included an assessment of the accuracy of hs-cTn assays for the diagnosis of AMI and prediction of MACE. 7 This review, by Goodacre et al. ,7 also evaluated standard cTn assays (alone and in combination with other cardiac biomarkers) and the diagnostic accuracy of other cardiac biomarkers, as well as including prediction modelling studies, all of which were outside the scope of this assessment. Our systematic review represents an advance on Goodacre et al. ,7 as it provides a more up-to-date and comprehensive assessment of the performance of hs-cTn assays. Although the Goodacre review7 was published in 2013, search dates were reported as 1995 to November 2010; hence it included only two studies,57,72 which met the definition of a hs-cTn assay used in our assessment. Both of these studies57,72 assessed the diagnostic performance of the Roche Elecsys hs-cTnT assay when applied to a single sample taken at presentation, using the 99th centile diagnostic threshold, and neither excluded participants with STEMI. Both studies57,72 were also included in our systematic review and one study57 contributed data to our summary estimates (based on a total of 15 studies) of the performance of the Roche Elecsys hs-cTnT assay for the diagnosis of any AMI at this threshold studies; the other72 was an early publication of the APACE study, the most recent publication that contributed data to our main analysis (accuracy for the diagnosis of NSTEMI), which included a total of six studies. 39 The summary estimate of sensitivity derived from our systematic review was lower (88% for both any AMI or NSTEMI analyses) than that reported by the Goodacre review (96% for any AMI),7 and our summary estimate of specificity was higher (82% for any AMI and 84% for NSTEMI) than that reported by the Goodacre review7 (72% for any AMI). A more recent systematic review, published as a conference abstract, reported summary estimates of the sensitivity and specificity of hs-cTn on an admission sample of 88% and 82%, respectively, based on data from 17 studies. 105 This review pooled data from different hs-cTn assays in one analysis and also included data from some studies that assessed assays that do not meet the definition of a hs-cTn assay used in our assessment. Despite these limitations, the summary estimates of sensitivity and specificity matched the summary estimates from our review for the performance of the Roche Elecsys hs-cTnT assay for the diagnosis of any AMI; this is unsurprising, as 13 of the 17 studies included in the analysis assed the Roche Elecsys hs-cTnT assay, using the 99th centile diagnostic threshold. 105 Our assessment represents an advance on both of these systematic reviews in that we provide up-to-date estimates of the diagnostic performance of assays meeting a strict definition for hs-cTn, which are stratified by hs-cTn assay type, diagnostic threshold and timing of the Tn test.
We believe that our assessment provides information of direct relevance to UK clinical practice as we focus on the performance of hs-cTn within the 4-hour time window corresponding to the target for NHS EDs, which specifies that ‘no one should be waiting more than four hours in the ED from arrival to admission, transfer or discharge’. 89 Furthermore, we have used the data from our systematic review to propose strategies for how hs-cTn assays might be applied and interpreted in order to maximise diagnostic performance. These strategies were devised with consideration to test timing, diagnostic threshold and interpretation of combinations of multiple test results. One limitation of this approach is that our estimates of the effectiveness and cost-effectiveness of the proposed two-step strategies require the assumption that the diagnostic performance of the second step is the same when used in people in whom NSTEMI is not ruled out by the first step as it is when used in the whole population (see Chapter 3, Diagnostic accuracy of the Roche Elecsys hs-cTnT assay, Multiple samples, and Diagnostic accuracy of the Abbott ARCHITECT hs-cTnI assay, Multiple samples). This assumption was necessary because no combined test performance data were available for the proposed strategies. However, it can be argued that the assumption is reasonable as the first step in both strategies focuses on rule-out performance and thus has a low LR+. This means that there is a relatively small change in the prevalence of AMI between the first and second steps (17–27% for the Roche Elecsys hs-cTnT optimal strategy and 17–24% for the Abbott ARCHITECT hs-cTnI optimal strategy).
Our assessment was less comprehensive for the Abbott ARCHITECT hs-cTnI assay and the Beckman Coulter hs-cTnI assay than for the Roche Elecsys hs-cTnT, because available data were limited for these two assays.
Cost-effectiveness
Our cost-effectiveness analysis is the most comprehensive to date in terms of the number of relevant hs-cTn test strategies for the early rule-out of AMI in people presenting to the ED with acute chest pain and suspected ACS. Moreover, the de novo probabilistic model was based on one previously developed for a published and peer reviewed HTA. 80 This model was also used in a later assessments on the cost-effectiveness of biomarkers in patients with suspected ACS. 19 For the present analysis, a number of adjustments were made to the model, but most of the assumptions were maintained.
The model was also informed by a comprehensive, high-quality, systematic review of DTA. Additional parameters were either those from the original HTA model, or any of the further assessments, or, where necessary, were based on a pragmatic literature review. Such a review is standard practice in economic modelling given the large number of parameters required and we expect that the review has delivered the most relevant information given that it focused on identifying the most recent large UK-based studies.
As in any economic model, a number of major and minor assumptions had to be made. It is important to understand the impact of these assumptions in order to interpret correctly the results of the model. The impact of most assumptions has been explored in sensitivity and secondary analyses. However, one major assumption that was maintained throughout all analyses was the conservative assumption of no health benefit of early treatment in the hs-cTn strategies compared with ‘late’ treatment in the standard cTn strategy. Although many experts believe that there must be a benefit, at least to some extent, of treating patients early, there is no evidence to support or quantify a timing effect, as yet. In addition, there may well also be adverse effects associated with early treatment also (e.g. the risk of bleeding, unnecessary percutaneous coronary interventions, etc.). The Canadian HTA report82 identified in the economic review (see Chapter 4, Canadian Agency for Drugs and Technologies in Health optimal use report) did include an advantage for early treatment compared with late treatment, based on one study,106 which investigated the effect of a 36-hour treatment delay. The RR found in this study106 was then recalculated, assuming a constant effect of timing on treatment benefit, to a RR of 1.035 of mortality for a treatment delay of 6 hours compared with early treatment, was again adjusted to 1.01 based on expert opinion. Any possible adverse effect of early treatment was not considered in this analysis. A similar approach would have been possible in the present model, but, in our view, this would not be informative, given the level of uncertainty underlying this final estimate. Therefore, it was decided to leave out a possible effect of timing of treatment. This could be considered a conservative approach but even this is uncertain.
The assumption that standard Tn, as the reference standard, has perfect sensitivity and specificity was also maintained throughout all analyses. Although a simplification, given that the actual reference standard is standard Tn plus clinical information, this approach is consistent with previous modelling and incorporation of the effect of clinical information to the hs-cTn test would be very difficult, given the current lack of data. To some extent, clinical judgement might already be incorporated into the modelling because, for the effect of treatment (RR for re-infarction and mortality), the study performed by Mills et al. 83 was used. In this study,83 not all patients with negative tests results were left untreated; we might therefore speculate that, where patients who tested negative were treated, this was because of clinical judgement. However, we cannot be certain that the observations from this trial reflect the true contribution of clinical judgement. On the other hand, there is recent evidence that the prognostic performance of standard Tn testing may be imperfect. For example, a negative Tn test might assess correctly that a patient is not experiencing a NSTEMI, but some patients with negative test results may still benefit from treatment. To take this possibility into account, a secondary analysis was performed, which resulted in the standard Tn strategy being dominated by the hs-cTn testing strategies. In other words, it seems reasonable to conclude that not only might hs-cTn be cost-effective, it might also be more effective than standard Tn.
Another assumption, which was varied in sensitivity analysis, with a rather substantial impact on results, was how to attribute costs of treatment to patients testing FP in the hs-cTn treatment strategies. In the base-case analysis, FP patients were assigned survival, quality of life, and costs of TN patients (i.e. they were basically assumed not to be treated). However, if hs-cTn assays were incorporated in clinical practice, patients with a positive result would be treated, at least up to the point where it is discovered they were FP. Therefore, in a sensitivity analysis, FP patients were assigned treatment costs as if they were TP, but mortality and quality of life as if they were TN. For the base case, this would change results quite dramatically, as the hs-cTn strategies would become more expensive but not more effective, whereas for the standard Tn nothing would change. For the secondary analysis (some hs-cTn FPs need and get treatment) things are different, as in this case treatment costs would be incurred for a proportion of patients (5%) but these patients would also receive the benefits of treatment. This approach had a very limited effect on results, in terms of strategies that were cost-effective. In our opinion, the secondary analysis, which assigns treatment costs to all FPs, but also assumes that some of these patients benefit treatment, is the most plausible scenario.
Uncertainties
Clinical effectiveness
The performance of any test that uses the 99th centile for the general population as the diagnostic threshold will be dependent upon the characteristics of the reference population from which this value was derived. Although the product information leaflet for the Abbott ARCHITECT hs-cTnI assay recommends that ‘each laboratory should verify that the 99th centile is transferable to its population or establish its own 99th centile’,15 test accuracy data included in the assessment are predominantly based on the 99th centiles for the three assays (Roche Elecsys hs-cTnT, Abbott ARCHITECT hs-cTnI, Beckman Coulter hs-cTnI) as reported by their respective manufacturers. 15,16,18 The 99th centile for the Roche Elecsys hs-cTnT was reported as being derived from a study population of 616 apparently healthy volunteers and blood donors, with an age range of 20–71 years and equal proportions of males and females;107 no further details were reported. The 99th centile for the Abbott ARCHITECT hs-cTnI assay was described as being derived from a study of ‘1,531 apparently healthy individuals in a US population with normal levels of BNP, HbA1c, and estimated GFR values’. 15 Although a 2012 ‘in press’ reference for this study was given in the APACE study,39 we were not able to identify any corresponding publication. It should also be noted that the Beckman Coulter hs-cTnI assay evaluated in the APACE study39 was described as ‘an investigational prototype’; the 99th centile (9 ng/l), described as ‘according to the manufacturer’, differs from the 99th centile given in the current product information leaflet (40 ng/l). 16 The product information leaflet describes this value as being derived from general practice samples obtained from London, UK, and the surrounding area; samples were from 1000 people aged > 40 years, with approximately equal numbers of males and females, and samples from people with abnormal urea and electrolytes, liver function tests, glucose or NT-proBNP (N-terminal pro-β-natiuretic peptide), were excluded. 16 Expected values, and hence diagnostic thresholds, derived from groups of healthy volunteers may have limited applicability to the population in whom hs-cTn testing would be applied in practice, for example with respect to age range. Data provided in the product information leaflets for the Roche Elecsys hs-cTnT assay and the Abbott ARCHITECT hs-cTnI assay both indicated that 99th centile values differed between males and females; the Abbott ARCHITECT hs-cTnI assay reported values of 15.6 ng/l and 34.2 ng/l for females and males, respectively,15 and the Roche Elecsys hs-cTnT assay reported values of 10.0 ng/l and 14.2 ng/l for females and males, respectively. 18 Despite this, we were unable to identify any data on whether the diagnostic performance of tests varies according to sex, when a single common diagnostic threshold is used for both males and females; the effectiveness of using sex-specific diagnostic thresholds therefore remains uncertain. Similarly, we were unable to identify any data on the diagnostic performance of hs-cTn assays when used in people with impaired renal function.
Differences in the populations used to derive the 99th centile diagnostic threshold, and hence in the Tn level at which this threshold set, may also affect the ability of an assay to achieve the first point of the accepted definition of a hs-cTn assay (i.e. a CV of ≤ 10% at the 99th centile for the general population). A standardised definition of the required reference population would be useful in ensuring a ‘level playing field’ for classification of assays as ‘high sensitive’ and would aid comparisons between tests.
We identified some data on the diagnostic performance of hs-cTn testing in clinically important subgroups (older people,39,53 and people with and without pre-existing CAD). 39,47 However, these data were very limited and were available only for the Roche Elecsys hs-cTnT assay. Therefore, there remains some uncertainty about how the diagnostic performance of individual hs-cTn assays may vary in clinically relevant subgroups, as well as what may constitute the optimal testing strategy in these groups.
A significant limitation of this assessment follows from the design of the primary studies included in the systematic review. The objective of these studies was to evaluate the diagnostic performance of hs-cTn assays when compared with a reference standard based on the universal definition of AMI endorsed by the ESC, the ACC, the AHA and the World Heart Federation (WHF). 8,21,22 The scope for this assessment did not include studies that evaluated the use of hs-cTn testing in combination with other tests, thus, studies that assessed the combined accuracy of a clinical risk score and a hs-cTn test used together would have been excluded; however, we did not identify any studies that were excluded on this basis. Studies assessing the diagnostic performance of a hs-cTn test alone, in which participants were subgrouped by clinical risk, met our inclusion criteria and were included in the systematic review. We identified only one study of this type,49 which, as described above (see Clinical effectiveness), indicated that the rule-out performance of hs-cTnT testing may be improved if the test is used in a population with high clinically determined pre-test probability. There remains uncertainty around how hs-cTn testing would perform if used, as it would be in clinical practice, in combination with a clinical assessment of pre-test probability (with or without formal risk scoring). Full assessment of the independent predictive value of hs-cTn testing requires multivariable prediction modelling.
A final area of uncertainty exists with respect to the clinical significance of a ‘FP’ hs-cTn result [i.e. does a positive hs-cTn result imply a clinically important change in cardiac risk, when a diagnosis of AMI is not confirmed (based on standard Tns and the universal definition)]? Re-adjudication of the final diagnosis, using later hs-cTn measurements in place of the conventional Tn results, can provide some insight into this issue. The most recent publication from the APACE study39 reported that when hs-cTnT results (including a 6-hour time point) were included in the reference standard diagnosis, this resulted in 131 participants being classified as having had a small AMI, which would have been classified as ‘no AMI’ where adjudication was based on standard Tn results.
Cost-effectiveness
The main uncertainties for the cost-effectiveness analysis lie in the model assumptions, particularly regarding the effect of actual clinical practice in terms of both other diagnostic information and treatment given this information. Although many of these assumptions have been varied in one-way sensitivity analysis, the precise implication of FN test results, where patients are discharged without essential treatment, or of FP test results, where patients stay in hospital and may receive unnecessary interventions, is unknown.
It should also be emphasised that the uncertainty resulting from the abovementioned assumptions was not parameterised in the model and is therefore not reflected in the PSAs or in the CEACs.
Chapter 6 Conclusions
Implications for service provision
We propose the use of two-step testing strategies to optimise the diagnostic performance of hs-cTn testing. There is evidence to suggest that undetectable levels of Tn (below the LoB/LoD of the assay) on presentation, measured using the Roche Elecsys hs-cTnT assay or the Abbott ARCHITECT hs-cTnI assay, may be sufficient to rule out NSTEMI in people presenting with symptoms that are suggestive of ACS. There is also evidence to suggest that a further rule-out step may be possible, within the 4-hour NHS ED target. For the Abbott ARCHITECT hs-cTnI assay, this second rule-out step would be based on a Tn level below the 99th centile in a sample taken 3 hours after presentation. For the Roche Elecsys hs-cTnT assay, the second rule-out step would be based on a Tn level below the 99th centile in all samples and a change in Tn level of < 20% between presentation and 2 hours. There is insufficient evidence to determine an optimal testing strategy for the Beckman Coulter hs-cTnI assay. There is some limited evidence to suggest that a Tn level below the 99th centile on presentation, measured using the Roche Elecsys hs-cTnT assay, may be sufficient to rule out NSTEMI in some groups (people aged > 70 years, people without pre-existing CAD and people with a clinically determined high pre-test probability).
When considering the base-case analysis it appears that the Beckman Coulter hs-cTnI assay at presentation, using the 99th centile diagnostic threshold, would be the cost-effective strategy, given an ICER threshold of £20,000–30,000. However, both cost and QALY differences between the strategies were small. This means that within the hs-cTn testing strategies, ICERs can change substantially especially with small changes in either costs or QALYs. Therefore, it is difficult to be confident that other hs-cTn strategies might not be cost-effective.
Overall, the model does not provide strong evidence to prefer one hs-cTn testing strategy over another. Results do, however, indicate that hs-cTn testing in general may be cost-effective compared with standard Tn testing. This becomes more likely if one assumes that hs-cTn testing detects some patients who require treatment despite their testing negative with standard Tn, as shown in the secondary analysis. In particular, the Abbott ARCHITECT hs-cTnI optimal strategy, which involves multiple testing and varying diagnostic thresholds, may be promising. The main issue, with regard to service provision, if implementation of a hs-cTn testing strategy is considered, is the balance between the likely reduction in cost and the risk of a reduction in effectiveness, albeit possibly small.
Suggested research priorities
Diagnostic cohort studies are needed to evaluate fully the performance of our proposed optimal testing strategies in a clinical setting.
If adoption of the Beckman Coulter hs-cTnI is to be considered, further studies are needed to evaluate fully the diagnostic accuracy of this test at the thresholds currently recommended by the manufacturer and to inform the development of an optimal testing strategy.
Further diagnostic cohort studies, or subgroup analyses of existing data sets, are needed to explore fully possible variation in the accuracy of hs-cTn assays and the optimal testing strategies for these assays in relevant demographic and clinical subgroups: sex; age; ethnicity; renal function; previous CAD; and previous AMI.
It is important to explore further the effects of clinical judgement (assessment of pre-test probability) on the diagnostic performance of hs-cTn testing. This could be achieved by assessing the combined diagnostic accuracy of risk scoring tools, such as TIMI or GRACE, and hs-cTn tests, or by assessing the accuracy of hs-cTn testing in subgroups stratified by pre-test probability.
Multivariable prediction modelling studies may be useful to assess the independent prognostic value of a positive hs-cTn test result, in the context of other clinical risk factors and tests.
As most of the uncertainties in the economic model were caused by assumptions relating to clinical effectiveness, this type of research would also facilitate economic analyses of hs-cTn testing.
Acknowledgements
The authors acknowledge the clinical advice and expert opinion provided by Dr Rick Body, Consultant in Emergency Medicine and Honorary Lecturer in Cardiovascular Medicine, Manchester Royal Infirmary/University of Manchester; Dr Nick Mills, Reader and Consultant Cardiologist, University of Edinburgh; Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield; Professor Adam Timmis, Professor of Clinical Cardiology, Queen Mary University London and St Bartholomew’s Hospital, London; Professor Paul Collinson, Consultant Chemical Pathologist, St George’s Healthcare NHS Trust; Mr Alan Reid, UKNEQAS cardiac biomarkers scheme organiser and principal clinical scientist, Southern General Hospital, Glasgow; and Mr Thomas James, Advanced Nurse Practitioner, Emergency Department, Wirral University Teaching Hospital NHS Trust. We would also like to thank Gill Worthy and Robert Wolff, KSR Ltd, for their assistance in checking data extraction for the systematic review, and Steve Ryder, KSR Ltd, for assistance with peer review of the economic models. The authors also would like to thank the lay members of the NICE Diagnostics Advisory Committee and assessment subgroup for providing input on the patients’ perspective at key stages of the assessment process. The development of ‘Cost-effectiveness model of diagnostic strategies for suspected acute coronary syndrome (ACS)’, which formed the starting point for the cost-effectiveness modelling included in this assessment, was funded by an NIHR HTA grant (HTA 09/22/21).
Contribution of authors
Marie Westwood and Penny Whiting planned and performed the systematic review and interpretation of evidence.
Thea van Asselt, Bram Ramaekers, Praveen Thokala and Manuela Joore, planned and performed the cost-effectiveness analyses and interpreted results.
Nigel Armstrong contributed to planning and interpretation of cost-effectiveness analyses, acquisition of input data and conducted model peer review.
Janine Ross devised and performed the literature searches and provided information support to the project.
Johan Severens and Jos Kleijnen provided senior advice and support to the systematic review and cost-effectiveness analyses, respectively.
All parties were involved in drafting and/or commenting on the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Office for National Statistics (ONS) . Death Registration Summary Tables – England and Wales, 2011 2012. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77–276695 (accessed 28 August 2013).
- Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart 2005;91:229-30. http://dx.doi.org/10.1136/hrt.2003.027599.
- Health and Social Care Information Centre (HSCIC) . Hospital Episode Statistics, Admitted Patient Care – England 2011–12: Primary Diagnosis, 4 Characters Table 2012. www.hscic.gov.uk/catalogue/PUB08288 (accessed 28 August 2013).
- Collinson PO, Rao AC, Canepa-Anson R, Joseph S. Impact of European Society of Cardiology/American College of Cardiology guidelines on diagnostic classification of patients with suspected acute coronary syndromes. Ann Clin Biochem 2003;40:156-60. http://dx.doi.org/10.1258/000456303763046085.
- Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2003;24:28-66. http://dx.doi.org/10.1016/S0195-668X(02)00618-8.
- Antman E, Anbe D, Armstrong P, Bates E, Green L, Hand M, et al. ACC/AHA Guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2004;110:e82-29. http://dx.doi.org/10.1016/j.jacc.2004.07.014.
- Goodacre S, Thokala P, Carroll C, Stevens JW, Leaviss J, Al Khalaf M, et al. Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17010.
- Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173-95. http://dx.doi.org/10.1016/j.jacc.2007.09.011.
- Ebell MH, White LL, Weismantel D. A systematic review of troponin T and I values as a prognostic tool for patients with chest pain. J Fam Pract 2000;49:746-53.
- Ebell MH, Flewelling D, Flynn CA. A systematic review of troponin T and I for diagnosing acute myocardial infarction. J Fam Pract 2000;49:550-6.
- National Institute for Health and Care Excellence (NICE) . Chest Pain of Recent Onset: Assessment and Diagnosis of Recent Onset Chest Pain or Discomfort of Suspected Cardiac Origin. 2010. www.nice.org.uk/nicemedia/live/12947/47938/47938.pdf (accessed 9 July 2013).
- SIGN 93. Acute Coronary Syndromes. A National Clinical Guideline. Edinburgh: SIGN; 2013.
- Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem 2009;55:1303-6. http://dx.doi.org/10.1373/clinchem.2009.128363.
- Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54-61. http://dx.doi.org/10.1373/clinchem.2011.165795.
- Architect STAT High Sensitive Troponin-I: Package Insert. Longford: Abbott; 2012.
- Access Immunoassay Systems: AccuTnI+3 Instructions for Use. Galway: Beckman Coulter; 2013.
- Gaze D, Hodges-Savola C, Holmes D, Faye S, Tubman J, Collinson P. Determining the 99th percentile reference interval for the Beckman Cardiac Troponin I assays. Paper presented at IFCC WorldLab; 22–26 Jun 2014; Istanbul: Turkey. Clin Chem Lab Med 2014;52.
- Troponin T hs/Troponin T hs STAT Immunoassay: Product Information. Mannheim: Roche; 2012.
- Collinson PO, Gaze DC, Thokala P, Goodacre S. Randomised assessment of treatment using panel assay of cardiac markers: contemporary biomarker evaluation (RATPAC CBE). Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17150.
- Myocardial Infarction with ST-segment Elevation: The Acute Management of Myocardial Infarction with ST-segment Elevation. Manchester: NICE; 2013.
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. http://dx.doi.org/10.1093/eurheartj/ehs184.
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999-3054. http://dx.doi.org/10.1093/eurheartj/ehr236.
- Unstable Angina and NSTEMI: The Early Management of Unstable Angina and Non-ST-Segment-Elevation Myocardial Infarction. Manchester: NICE; 2010.
- MI – Secondary Prevention: Secondary Prevention in Primary and Secondary Care for Patients Following a Myocardial Infarction. London: NICE; 2007.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health care. York: University of York; 2009.
- Diagnostics Assessment Programme Manual. Manchester: NICE; 2011.
- Cochrane Diagnostic Test Accuracy Working Group . Handbook for Diagnostic Test Accuracy Reviews 2009. http://srdta.cochrane.org/handbook-dta-reviews (accessed 29 August 2013).
- CADTH Peer Review Checklist for Search Strategies. Ottawa: CADTH; 2013.
- Wright K, McDaid C. Is the retraction of journal articles in electronic journals and databases consistent and timely? A case study n.d.
- Wright K, McDaid C. Reporting of article retractions in bibliographic databases and online journals. J Med Libr Assoc 2011;99:164-7. http://dx.doi.org/10.3163/1536-5050.99.2.010.
- Royle P, Waugh N. Should systematic reviews include searches for published errata?. Health Info Libr J 2004;21:14-20. http://dx.doi.org/10.1111/j.1471-1842.2004.00459.x.
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007;50:e1-157. http://dx.doi.org/10.1016/j.jacc.2007.02.013.
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PMM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. http://dx.doi.org/10.1016/j.jclinepi.2005.02.022.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. http://dx.doi.org/10.1093/biostatistics/kxl004.
- Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A, et al. An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol 2008;61:1095-103. http://dx.doi.org/10.1016/j.jclinepi.2007.09.013.
- Riley RD, Abrams KR, Sutton AJ, Lambert PC, Thompson JR. Bivariate random-effects meta-analysis and the estimation of between-study correlation. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186/1471-2288-7-3.
- Zamora J, Abraira V, Nuriel A, Khan KS, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6.
- Hoeller R, Rubini Gimenez M, Reichlin T, Twerenbold R, Zellweger C, Moehring B, et al. Normal presenting levels of high-sensitivity troponin and myocardial infarction. Heart 2013;99:1567-72. http://dx.doi.org/10.1136/heartjnl-2013-303643.
- Santalo M, Martin A, Velilla J, Povar J, Temboury F, Balaguer J, et al. Using high-sensitivity troponin T: the importance of the proper gold standard. Am J Med 2013;126:709-17. http://dx.doi.org/10.1016/j.amjmed.2013.03.003.
- Aldous S, Pemberton C, Richards AM, Troughton R, Than M. High-sensitivity troponin T for early rule-out of myocardial infarction in recent onset chest pain. Emerg Med J 2012;29:805-10. http://dx.doi.org/10.1136/emermed-2011-200222.
- Sanchis J, Bardaji A, Bosch X, Loma-Osorio P, Marin F, Sanchez PL, et al. Usefulness of high-sensitivity troponin T for the evaluation of patients with acute chest pain and no or minimal myocardial damage. Am Heart J 2012;164:194-200. http://dx.doi.org/10.1016/j.ahj.2012.05.015.
- Haaf P, Drexler B, Reichlin T, Twerenbold R, Reiter M, Meissner J, et al. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation 2012;126:31-40. http://dx.doi.org/10.1161/CIRCULATIONAHA.112.100867.
- Eggers KM, Venge P, Lindahl B. High-sensitive cardiac troponin T outperforms novel diagnostic biomarkers in patients with acute chest pain. Clin Chim Acta 2012;413:1135-40. http://dx.doi.org/10.1016/j.cca.2012.03.011.
- Reiter M, Twerenbold R, Reichlin T, Benz B, Haaf P, Meissner J, et al. Early diagnosis of acute myocardial infarction in patients with pre-existing coronary artery disease using more sensitive cardiac troponin assays. Eur Heart J 2012;33:988-97. http://dx.doi.org/10.1093/eurheartj/ehr376.
- Aldous SJ, Richards M, Cullen L, Troughton R, Than M. Diagnostic and prognostic utility of early measurement with high-sensitivity troponin T assay in patients presenting with chest pain. CMAJ 2012;184:E260-8. http://dx.doi.org/10.1503/cmaj.110773.
- Potocki M, Reichlin T, Thalmann S, Zellweger C, Twerenbold R, Reiter M, et al. Diagnostic and prognostic impact of copeptin and high-sensitivity cardiac troponin T in patients with pre-existing coronary artery disease and suspected acute myocardial infarction. Heart 2012;98:558-65. http://dx.doi.org/10.1136/heartjnl-2011-301269.
- Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011;306:2684-93. http://dx.doi.org/10.1001/jama.2011.1896.
- Freund Y, Chenevier-Gobeaux C, Bonnet P, Claessens Y-E, Allo J-C, Doumenc B, et al. High-sensitivity versus conventional troponin in the emergency department for the diagnosis of acute myocardial infarction. Crit Care 2011;15. http://dx.doi.org/10.1186/cc10270.
- Aldous SJ, Richards AM, Cullen L, Than MP. Early dynamic change in high-sensitivity cardiac troponin T in the investigation of acute myocardial infarction. Clin Chem 2011;57:1154-60. http://dx.doi.org/10.1373/clinchem.2010.161166.
- Melki D, Lind S, Agewall S, Jernberg T. Diagnostic value of high sensitive troponin T in chest pain patients with no persistent ST-elevations. Scand Cardiovasc J 2011;45:198-204. http://dx.doi.org/10.3109/14017431.2011.565792.
- Reichlin T, Irfan A, Twerenbold R, Reiter M, Hochholzer W, Burkhalter H, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation 2011;124:136-45. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.023937.
- Reiter M, Twerenbold R, Reichlin T, Haaf P, Peter F, Meissner J, et al. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J 2011;32:1379-89. http://dx.doi.org/10.1093/eurheartj/ehr033.
- Aldous SJ, Florkowski CM, Crozier IG, Elliott J, George P, Lainchbury JG, et al. Comparison of high sensitivity and contemporary troponin assays for the early detection of acute myocardial infarction in the emergency department. Ann Clin Biochem 2011;48:241-8. http://dx.doi.org/10.1258/acb.2010.010219.
- Kurz K, Giannitsis E, Becker M, Hess G, Zdunek D, Katus HA. Comparison of the new high sensitive cardiac troponin T with myoglobin, h-FABP and cTnT for early identification of myocardial necrosis in the acute coronary syndrome. Clin 2011;100:209-15. http://dx.doi.org/10.1007/s00392-010-0230-y.
- Hochholzer W, Reichlin T, Stelzig C, Hochholzer K, Meissner J, Breidthardt T, et al. Impact of soluble fms-like tyrosine kinase-1 and placental growth factor serum levels for risk stratification and early diagnosis in patients with suspected acute myocardial infarction. Eur Heart J 2011;32:326-35. http://dx.doi.org/10.1093/eurheartj/ehq429.
- Christ M, Popp S, Pohlmann H, Poravas M, Umarov D, Bach R, et al. Implementation of high sensitivity cardiac troponin T measurement in the emergency department. Am J Med 2010;123:1134-42. http://dx.doi.org/10.1016/j.amjmed.2010.07.015.
- Parsonage W, Cullen L, Greenslade J, Tate J, Ungerer J, Hammett C, et al. Comparison of highly sensitive troponin I and T results in the diagnosis of acute myocardial infarction. Paper presented at 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention; 9–11 March 2013; San Francisco: CA. J Am Coll Cardiol 2013;61. http://dx.doi.org/10.1016/S0735-1097(13)60229-6.
- Collinson P, Gaze D, Thokala P, Goodacre S. To examine the diagnostic accuracy of highly sensitive troponin assays using diagnosis based on the universal definition of myocardial infarction in the unselected emergency room population. Presented at the Europen Society of Cardiology Congress 2012, Munich, Germany, 25–29 August 2012. Eur Heart J 2012;33.
- Body R, Burrows G, Cook G, Carley SD, France M, Jarvis J, et al. High sensitivity troponin: validation and subsequent audit of a novel ‘rule out’ cut-off. Paper presented at College of Emergency Medicine Autumn Conference 2011, Gateshead, UK 21–23 September 2011. Emerg Med J 2011;28. http://dx.doi.org/10.1136/emermed-2011-200617.1.
- Melki D, Lind S, Agewall S, Jernberg T. High sensitive troponin T rules out myocardial infarction 2 hours from admission in chest pain patients. Paper presented at the American College of Cardiology’s 59th Annual Scientific Session and i2 Summit: Innovation in Intervention, Atlanta, GA, 14–16 March 2010. J Am Coll Cardiol 2010;55. http://dx.doi.org/10.3109/14017431.2011.565792.
- Aldous S, Florkowski C, George P, Than M, Crozier I. High sensitivity troponin assays predict major adverse events at 2 years and at levels below the 99th percentile. Paper presented at the American College of Cardiology’s 59th Annual Scientific Session and i2 Summit: Innovation in Intervention, Atlanta, GA, 14–16 March 2010. J Am Coll Cardiol 2010;55.
- Cullen L, Mueller C, Parsonage WA, Wildi K, Greenslade JH, Twerenbold R, et al. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol 2013;62:1242-9. http://dx.doi.org/10.1016/j.jacc.2013.02.078.
- Sebbane M, Lefebvre S, Kuster N, Jreige R, Jacques E, Badiou S, et al. Early rule out of acute myocardial infarction in ED patients: value of combined high-sensitivity cardiac troponin T and ultrasensitive copeptin assays at admission. Am J Emerg Med 2013;31:1302-8. http://dx.doi.org/10.1016/j.ajem.2013.04.033.
- Irfan A, Reichlin T, Twerenbold R, Meister M, Moehring B, Wildi K, et al. Early diagnosis of myocardial infarction using absolute and relative changes in cardiac troponin concentrations. Am J Med 2013;126:781-8. http://dx.doi.org/10.1016/j.amjmed.2013.02.031.
- Reiter M, Twerenbold R, Reichlin T, Mueller M, Hoeller R, Moehring B, et al. Heart-type fatty acid-binding protein in the early diagnosis of acute myocardial infarction. Heart 2013;99:708-14. http://dx.doi.org/10.1136/heartjnl-2012-303325.
- Body R, Carley S, McDowell G, Jaffe AS, France M, Cruickshank K, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011;58:1332-9. http://dx.doi.org/10.1016/j.jacc.2011.06.026.
- Aldous SJ, Florkowski CM, Crozier IG, George P, Mackay R, Than M. High sensitivity troponin outperforms contemporary assays in predicting major adverse cardiac events up to two years in patients with chest pain. Ann Clin Biochem 2011;48:249-55. http://dx.doi.org/10.1258/acb.2010.010220.
- Keller T, Zeller T, Echevarria FO, Tzikas S, Baldus S, Bickel C, et al. High sensitive troponin I dynamic improves early diagnosis of acute myocardial infarction. Eur Heart J 2011;32.
- Saenger AK, Korpi-Steiner NL, Bryant SC, Karon BS, Jaffe AS. Utilization of a high sensitive troponin T assay optimizes serial sampling in the diagnosis of acute myocardial infarction compared to multiple contemporary troponin assays. Circulation 2010;122.
- Freund Y, Chenevier-Gobeaux C, Goulet H, Claessens Y, Bonnet P, Allo J, et al. Comparison of high-sensitivity cardiac troponin concentrations versus conventional troponin for the diagnosis of myocardial infarction in the emergency department. Ann Emerg Med 2010;56. http://dx.doi.org/10.1016/j.annemergmed.2010.06.524.
- Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858-67. http://dx.doi.org/10.1056/NEJMoa0900428.
- Lippi G, Cervellin G, Salvagno M, Montagnana R, Musa R, Aloe G. Brain natriuretic peptide does not improve the diagnostic performance of high-sensitive troponin for the early diagnosis of myocardial infarction. Paper presented at the AACC Annual Meeting, Los Angeles, CA, 15–19 July 2012. Clin Chem 2012;58:A4-8.
- Body R, Carley SD, McDowell G, Nuttall M, Wibberley C, France M, et al. Use of low level high sensitivity troponin to rule out acute myocardial infarction in the emergency department. Eur Heart J Suppl 2010;12:F111-12.
- Irfan A, Reichlin T, Twerenbold R, Wildi K, Mueller C. Determinants of early changes in high-sensitive troponin levels among patients with non-acute myocardial infarction cause of chest pain. J Am Coll Cardiol 2013;61. http://dx.doi.org/10.1016/S0735-1097(13)60232-6.
- Park SR, Kang YR, Seo MK, Kang MK, Cho JH, An YJ, et al. Clinical predictors of incomplete ST-Segment resolution in the patients with acute ST segment elevation myocardial infarction. Korean Circ 2009;39:310-16. http://dx.doi.org/10.4070/kcj.2009.39.8.310.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Goodacre S, Bradburn M, Fitzgerald P, Cross E, Collinson P, Grey A, et al. The RATPAC (randomised assessment of treatment using panel assay of cardiac markers) trial: a randomized controlled trial of point-of-care cardiac markers in the emergency department. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15230.
- Fitzgerald P, Goodacre SW, Cross E, Dixon S. Cost-effectiveness of point-of-care biomarker assessment for suspected myocardial infarction: the randomized assessment of treatment using panel assay of cardiac markers (RATPAC) trial. Acad Emerg Med 2011;18:488-95. http://dx.doi.org/10.1111/j.1553-2712.2011.01068.x.
- Thokala P, Goodacre SW, Collinson PO, Stevens JW, Mills NL, Newby DE, et al. Cost-effectiveness of presentation versus delayed troponin testing for acute myocardial infarction. Heart 2012;98:1498-503. http://dx.doi.org/10.1136/heartjnl-2012-302188.
- Vaidya A, Severens JL, Bongaerts BWC, Cleutjens KBJM, Nelemans PJ, Hofstra L. Use of high-sensitive troponin T assay for the early diagnosis of acute myocardial infarction in chest pain patients: an economic evaluation. Med Decis Making 2012;32. http://dx.doi.org/10.1186/1471-2261-14-77.
- High-Sensitivity Cardiac Troponin for the Rapid Diagnosis of Acute Coronary Syndrome in the Emergency Department: a Clinical and Cost-effectiveness Evaluation. Ottawa: CADTH; 2013.
- Mills NL, Churchhouse AMD, Lee KK, Anand A, Gamble D, Shah ASV, et al. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA 2011;305:1210-16. http://dx.doi.org/10.1001/jama.2011.338.
- Wodniecki J, Jachec W, Szczurek-Katanski K, Wilczek K, Kawecki D, Tarnawski R, et al. Troponin T: is it a marker of restenosis after transluminal percutaneous angioplasty in unstable angina patients?. Pol Arch Med Wewn 1999;101:33-7.
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11140.
- Oluboyede Y, Goodacre S, Wailoo A. Cost-effectiveness of chest pain unit care in the NHS. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-174.
- Goodacre SW, Bradburn M, Cross E, Collinson P, Grey A, Hall AS, et al. The Randomised Assessment of Treatment using Panel Assay of Cardiac Markers (RATPAC) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Heart 2011;97:190-6. http://dx.doi.org/10.1136/hrt.2010.203166.
- Polanczyk CA, Kuntz KM, Sacks DB, Johnson PA, Lee TH. Emergency department triage strategies for acute chest pain using creatine kinase-MB and troponin I assays: a cost-effectiveness analysis. Ann Intern Med 1999;131:909-18. http://dx.doi.org/10.7326/0003-4819-131-12-199912210-00002.
- The NHS Plan: A Plan for Investment, a Plan for Reform. London; 2000.
- Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163-70. http://dx.doi.org/10.1056/NEJM200004203421603.
- Office for National Statistics (ONS) . Interim Life Tables, England &Amp; Wales, 1980–82 to 2010–12 2013. www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2010–2012/rft-ew.xls (accessed 10 January 2014).
- British Heart Foundation . Heart Statistics: Morbidity, Incidence n.d. www.bhf.org.uk/research/heart-statistics/morbidity/incidence.aspx (accessed 10 January 2014).
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes 2012;5:532-40. http://dx.doi.org/10.1161/CIRCOUTCOMES.111.964700.
- Allen LA, O’Donnell CJ, Camargo CA, Giugliano RP, Lloyd-Jones DM. Comparison of long-term mortality across the spectrum of acute coronary syndromes. Am Heart J 2006;151:1065-71. http://dx.doi.org/10.1016/j.ahj.2005.05.019.
- Lipinski MJ, Baker NC, Escárcega RO, Torguson R, Chen F, Aldous SJ, et al. Comparison of conventional and high-sensitivity troponin in patients with chest pain: a collaborative meta-analysis. Am Heart J 2015;169:6-16. http://dx.doi.org/10.1016/j.ahj.2014.10.007.
- Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690-1. http://dx.doi.org/10.1001/jama.280.19.1690.
- Palmer S, Sculpher M, Philips Z, Robinson M, Ginnelly L, Bakhai A, et al. A Cost-Effectiveness Model Comparing Alternative Management Strategies for the Use of Glycoprotein IIB IIA Antagonists in the Non-ST-Elevation Acute Coronary Syndrome. n.d. www.nice.org.uk/nicemedia/live/11469/32434/32434.pdf (accessed 10 January 2014).
- Department of Health (DoH) . PbR Tariff Information Spreadsheet for 2012–13 2012. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/216214/dh_133578.xls (accessed 17 January 2014).
- Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2011.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97. http://dx.doi.org/10.1111/j.1524-4733.2008.00358.x.
- Apple FS, Jesse RL, Newby LK, Wu AHB, Christenson RH, Cannon CP, et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical Issues for biochemical markers of acute coronary syndromes. Clin Chem 2007;53:547-51. http://dx.doi.org/10.1373/clinchem.2006.084715.
- Novis DA, Jones BA, Dale JC, Walsh MK. Biochemical markers of myocardial injury test turnaround time: a College of American Pathologists Q-Probes study of 7020 troponin and 4368 creatine kinase-MB determinations in 159 institutions. Arch Pathol Lab Med 2004;128:158-64.
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011;64:602-7. http://dx.doi.org/10.1016/j.jclinepi.2010.07.006.
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. http://dx.doi.org/10.1016/j.jclinepi.2005.01.016.
- Lipinski MJ, Baker NC, Escarcega RO, Torguson R, Epstein S, Aldous SJ, et al. Comparison of conventional and high-sensitivity troponin in patients presenting to the emergency department with chest pain: a collaborative meta-analysis. J Am Coll Cardiol 2014;63. http://dx.doi.org/10.1016/S0735-1097(14)60016-4.
- Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, Faxon DP, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009;360:2165-75. http://dx.doi.org/10.1056/NEJMoa0807986.
- Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:254-61. http://dx.doi.org/10.1373/clinchem.2009.132654.
- Meune C, Balmelli C, Twerenbold R, Reichlin T, Reiter M, Haaf P, et al. Patients with acute coronary syndrome and normal high-sensitivity troponin. Am J Med 2011;124:1151-7. http://dx.doi.org/10.1016/j.amjmed.2011.07.032.
- Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol 2001;38:2114-30. http://dx.doi.org/10.1016/S0735-1097(01)01702-8.
- Giannitsis E, Becker M, Kurz K, Hess G, Zdunek D, Katus HA. High-sensitivity cardiac troponin T for early prediction of evolving non-ST segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem 2010;56:642-50. http://dx.doi.org/10.1373/clinchem.2009.134460.
- Ahmed W, Schlett CL, Uthamalingam S, Truong QA, Koenig W, Rogers IS, et al. Single resting hsTnT level predicts abnormal myocardial stress test in acute chest pain patients with normal initial standard troponin. JACC Cardiovasc Imaging 2013;6:72-8. http://dx.doi.org/10.1016/j.jcmg.2012.08.014.
- Aldous S, Florkowski C, Crozier I, Elliott J, George P, Lainchbury J, et al. Most patients with acute myocardial infarction have raised high sensitivity troponin on presentation outperforming conventional assays. Paper presented at New Zealand Annual Scientific Meeting of the Cardiac Society of Australia and New Zealand, Rotorua, NZ 25–27 June 2010. Heart Lung Circ 2010;19.
- Aldous S, Florkowski C, Crozier I, George P, Than M. High sensitivity troponin outperforms conventional assays in predicting 2 year adverse events. Paper presented at the New Zealand Annual Scientific Meeting of the Cardiac Society of Australia and New Zealand, Rotorua, NZ, 25–27 June 2010. Heart Lung Circ 2010;19.
- Aldous SJ. High sensitivity troponin outperforms contemporary assays in predicting major adverse cardiac events up to two years in patients with chest pain. Ann Clin Biochem 2011;48:249-55. http://dx.doi.org/10.1258/acb.2010.010220.
- Aldous SJ, Florkowski C, George P, Than M, Crozier I. High sensitivity troponin out-performs conventional troponin assays for prediction of major adverse cardiac events at 2 years. Paper presented at the European Society of Cardiology, ESC Congress, Stockholm, Sweden, 28 August–1 September 2010. Eur Heart J 2010;31:650-1. http://dx.doi.org/10.1258/acb.2010.010220.
- Aldous SJ, Richards AM, Troughton R, Than M. ST2 has diagnostic and prognostic utility for all-cause mortality and heart failure in patients presenting to the emergency department with chest pain. J Card Fail 2012;18:304-10. http://dx.doi.org/10.1016/j.cardfail.2012.01.008.
- Aldous SJ, Richards M, Cullen L, Troughton R, Than M. A 2-hour thrombolysis in myocardial infarction score outperforms other risk stratification tools in patients presenting with possible acute coronary syndromes: comparison of chest pain risk stratification tools. Am Heart J 2012;164:516-23. http://dx.doi.org/10.1016/j.ahj.2012.06.025.
- Aldous S, Pemberton C, Troughton R, Than M, Richards AM. Heart fatty acid binding protein and myoglobin do not improve early rule out of acute myocardial infarction when highly sensitive troponin assays are used. Resuscitation 2012;83:E27-8. http://dx.doi.org/10.1016/j.resuscitation.2011.09.031.
- Alexandra AM, Leopoldo BA, Briseno de la Cruz J, Julio SZ, Hector GP, Carlos MS. Usefulness of copeptin in the early diagnosis of acute coronary syndrome in the cardiovascular emergency department. Paper presented at the 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, San Francisco, CA, 9–11 March 2013. J Am Coll Cardiol 2013;61.
- Arenja N, Reiter M, Twerenbold R, Reichlin T, Potocki M, Breidthardt T, et al. Early diagnosis of acute myocardial infarction in patients with diagnostic uncertainty using more sensitive cardiac troponin assays. Eur Heart J 2010;31. http://dx.doi.org/10.1093/eurheartj/ehr033.
- Bahrmann P, Bahrmann A, Christ M, Bertsch T, Sieber CC. Performance of high-sensitivity troponin T in the early diagnosis of non-ST-elevation myocardial infarction in elderly patients presenting to an emergency department. Eur Heart J 2012;33.
- Bahrmann P, Bahrmann A, Breithardt O-A, Daniel WG, Christ M, Sieber CC, et al. Additional diagnostic and prognostic value of copeptin ultra-sensitive for diagnosis of non-ST-elevation myocardial infarction in older patients presenting to the emergency department. Clin Chem Lab Med 2013;51:1307-19. http://dx.doi.org/10.1515/cclm-2012-0401.
- Bahrmann P, Christ M, Bahrmann A, Rittger H, Heppner HJ, Achenbach S, et al. A 3-hour diagnostic algorithm for non-ST-elevation myocardial infarction using high-sensitivity cardiac troponin T in unselected older patients presenting to the emergency department. J Am Med Dir Assoc 2013;14:409-16. http://dx.doi.org/10.1016/j.jamda.2012.12.005.
- Bahrmann P, Heppner H-J, Christ M, Bertsch T, Sieber C. Early detection of non-ST-elevation myocardial infarction in geriatric patients by a new high-sensitive cardiac troponin T assay. Aging Clin Exp Res 2012;24:290-4. http://dx.doi.org/10.3275/7927.
- Balmelli C, Meune C, Twerenbold R, Reichlin T, Rieder S, Drexler B, et al. Comparison of the performances of cardiac troponins, including sensitive assays, and copeptin in the diagnostic of acute myocardial infarction and long-term prognosis between women and men. Am Heart J 2013;166:30-7. http://dx.doi.org/10.1016/j.ahj.2013.03.014.
- Balmelli C, Reiter M, Reichlin T, Twerenbold R, Haaf P, Irfan A, et al. Direct comparison of high-sensitive and sensitive cardiac troponin assays for risk stratification in patients with acute chest pain. Eur Heart J 2011;32.
- Beyrau R, Braun S, Dolci A, Freidank H, Giannitsis E, Handy B, et al. Multicentre evaluation of a high sensitive Elecsys (R) troponin T assay. Clin Chem 2009;55. http://dx.doi.org/10.1016/j.cca.2010.12.034.
- Bhardwaj A, Truong QA, Peacock WF, Yeo KTJ, Storrow A, Thomas S, et al. A multicenter comparison of established and emerging cardiac biomarkers for the diagnostic evaluation of chest pain in the emergency department. Am Heart J 2011;162:276-98. http://dx.doi.org/10.1016/j.ahj.2011.05.022.
- Bhardwaj A, Truong QA, Storrow A, Peacock WF, Lee HK, Yeo K-TJ, et al. Serial measurement of unbound free fatty acids adds to highly sensitive troponin I in the diagnostic evaluation of chest pain. J Am Coll Cardiol 2011;57. http://dx.doi.org/10.1016/S0735-1097(11)60936-4.
- Biasillo G, Biasucci LM, Bona RD, Dato I, Leo M, Gustapane M, et al. High sensitivity troponin assay for early diagnosis of acute coronary syndrome in a real-world situation: comparison between HS-TNT, LOCI HS-TNI, HS-TNI ROCHE and conventional TNT. Paper presented at the American College of Cardiology’s 59th Annual Scientific Session and i2 Summit: Innovation in Intervention, Atlanta, GA, 14–16 March 2010. J Am Coll Cardiol 2010;55.
- Biasucci LM, Biasillo G, Della Bona R, Leo M, Gustapane M, Dato I, et al. Value of high sensitivity troponin assays in non selected patients with chest pain admitted to emergency department. Circulation 2010;122.
- Biasucci LM, Dellabona R, Leo M, Biasillo G, Zaninotto M, Plebani M, et al. High sensitivity troponin assays for early diagnosis of acute coronary syndrome in a real-world situation: comparison between four different high-sensitivity assays and conventional Troponin-T. Eur Heart J Suppl. 2010;12.
- Biasucci LM, Della Bona R, Biasillo G, Leo M, Gustapane M, Gentiloni Silveri N, et al. Cardiac troponins for early diagnosis of acute coronary syndrome in a real-world situation: Comparison between high sensitivity and conventional assays. Paper presented at the European Society of Cardiology Congress, Stockholm, Sweden, 28 August–1 September 2010. Eur Heart J 2010;31.
- Biasucci LM, Della Bona R, Biasillo G, Leo M, Zaninotto M, Plebani M, et al. Role of MPO for early management of patients with chest pain in Emergency Department: comparison with Conventional TnT and hs-Troponin Assay. Eur Heart J Suppl 2010;12:F71-2.
- Biasucci LM, Gustapane M, Della Bona R, Biasillo G, Leo M, Dato I, et al. Myeloperoxidase has no role in diagnosis of Acute Coronary Syndromes in subjects with chest pain in emergency department. Paper presented at the 60th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, New Orleans, LA, 2–5 April 2011. J Am Coll Cardiol 2011;57. http://dx.doi.org/10.1016/S0735-1097(11)61022-X.
- Biener M, Mueller M, Vafaie M, Katus H, Giannitsis E. Diagnostic performance of rising, falling, or rising and falling kinetic changes of high-sensitivity cardiac troponin t in an unselected emergency department population. Paper presented at the 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, San Francisco, CA, 9–11 March 2013. J Am Coll Cardiol 2013;61. http://dx.doi.org/10.1016/S0735-1097(13)60241-7.
- Biener M, Mueller M, Vafaie M, Keller T, Blankenberg S, White HD, et al. Usefulness of a 3-hour compared to a 6-hour blood sampling protocol using high-sensitivity cardiac troponin T for rule-out and rule-in of non-STEMI in an unselected emergency department population. Eur Heart J 2012;33.
- Biener M, Mueller M, Vafaie M, Keller T, Blankenberg S, White HD, et al. Comparison of a 3-hour versus a 6-hour sampling-protocol using high-sensitivity cardiac troponin T for rule-out and rule-in of non-STEMI in an unselected emergency department population. Int J Cardiol 2013;167:1134-40. http://dx.doi.org/10.1016/j.ijcard.2012.09.122.
- Bethesda, MD: National Library of Medicine (US); 2006.
- Body R, Carley S, Burrows G, Pemberton P, MacKway-Jones K. Combining heart fatty acid binding protein and high sensitivity troponin in the emergency department. Paper presented at the 14th International Conference on Emergency Medicine, Dublin, Ireland, 27–30 June 2012. Acad Emerg Med 2012;19:748-9.
- Body R, Carley SD, Burrows G, MacKway-Jones K. The Manchester acute coronary syndromes (MACs) decision rule to reduce unnecessary admissions for cardiac chest pain: derivation and external validation. Paper presented at the 14th International Conference on Emergency Medicine, Dublin, Ireland, 27–30 June 2012. Acad Emerg Med 2012;19. http://dx.doi.org/10.1136/heartjnl-2014-305564.
- Body R, Carley S, McDowell G. High-sensitivity troponin < 3 ng/L ruled out acute myocardial infarction. Ann Intern Med 2012;156:JC2-9. http://dx.doi.org/10.7326/0003-4819-156-4-201202210-02009.
- Braga F, Dolci A, Cavallero A, Ghezzi A, Infusino I, Milano M, et al. Evaluation of the sensitivity of two highly sensitive troponin assays for early detection of non ST-elevation myocardial infarction (NSTEMI)]. Biochim Clin 2011;35:186-9.
- Braga F, Dolci A, Cavallero A, Ghezzi A, Infusino I, Milano M, et al. Evaluation of the sensitivity of two highly sensitive troponin assays for early detection of non ST-elevation myocardial infarction (NSTEMI). Clin Chem Lab Med 2011;49.
- Bronze L, Monteiro M, Dias A, Almeida L, Marques P, Simoes C, et al. High sensitivity cardiac troponin as a screening tool in a general emergency department. Paper presented at the 25th Annual Congress of the European Society of Intensive Care Medicine, Lisbon, Portugal, 13–17 October 2012. Intensive Care Med 2012;38:S172-3.
- Brown AM, Sease KL, Robey JL, Shofer FS, Hollander JE. The impact of B-type natriuretic peptide in addition to troponin I, creatine kinase-MB, and myoglobin on the risk stratification of emergency department chest pain patients with potential acute coronary syndrome. Ann Emerg Med 2007;49:153-63. http://dx.doi.org/10.1016/j.annemergmed.2006.08.024.
- Buccelletti F, Galiuto L, Marsiliani D, Iacomini P, Mattogno P, Carroccia A, et al. Comparison of diagnostic accuracy between three different rules of interpreting high sensitivity troponin T results. Intern Emerg Med 2012;7:365-70. http://dx.doi.org/10.1007/s11739-012-0787-8.
- Buhl D, Schnuriger B, Henzi B, Exadaktylo A, Windecke S, Zimmerman H. Standard troponin versus high-sensitive troponin in emergency department patients a one month experience in a tertiary emergency department. Paper presented at Annual Assembly of the Swiss Society of Clinical Chemistry and Tri-National Congress of Laboratory Medicine, Zurich, Switzerland 2–4 November 2011. Clin Chem Lab Med 2011;49.
- Cardillo MT, Biasucci LM, Zaninotto M, Gentiloni Silveri N, Biasillo G, Mion M, et al. Chest pain patients with false positive hs-TnT in emergency department have the same one year risk of MACE as those who were hospitalized for acute coronary syndromes. Paper presented at ESC Congress 2012, Munich, Germany 25–29 August 2012. Eur Heart J 2012;33.
- Carmo GAL, Calderaro D, Caramelli B. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation 2013;127. http://dx.doi.org/10.1161/CIRCULATIONAHA.112.136044.
- Ceriani E, Rusconi AM, Gruppo di Autoformazione M. Highly sensitive troponin and diagnostic accuracy in acute myocardial infarction. Intern Emerg Med 2012;7:471-3. http://dx.doi.org/10.1007/s11739-012-0814-9.
- Charpentier S, Maupas-Schwalm F, Cournot M, Elbaz M, Ducasse JL, Lauque D. Rapid non ST elevation myocardial infarction rule out with combination of copeptin and troponin in the emergency department. Paper presented at Annual Meeting of the Society for Academic Emergency Medicine, Boston, MA 1–5 June 2011. Acad Emerg Med 2011;18.
- Chenevier-Gobeaux C, Meune C, Freund Y, Wahbi K, Claessens Y-E, Doumenc B, et al. Influence of age and renal function on high-sensitivity cardiac troponin T diagnostic accuracy for the diagnosis of acute myocardial infarction. Am J Cardiol 2013;111:1701-7. http://dx.doi.org/10.1016/j.amjcard.2013.02.024.
- Collinson P, Gaze D, Thokala P, Goodacre S. The diagnostic accuracy of novel biomarkers of myocardial injury in the unselected emergency room population. Eur Heart J 2012;33. http://dx.doi.org/10.1136/heartjnl-2013-304716.
- Collinson P, Gaze D, Thokala P, Goodacre S. Economic analysis of the randomised assessment of treatment using panel assay of cardiac markers-contemporary biomarker evaluation study (RATPAC CBE). Paper presented at ESC Congress, Munich, Germany 25–29 August 2012. Eur Heart J 2012;33.
- Collinson P, Goodacre S, Gaze D, Grey A, Team RR. Very early diagnosis of chest pain by point-of-care testing: comparison of the diagnostic efficiency of a panel of cardiac biomarkers compared with troponin measurement alone in the RATPAC trial. Heart 2012;98:312-18. http://dx.doi.org/10.1136/heartjnl-2011-300723.
- Collinson PO, Gaze DC, Bainbridge K, Morris F, Morris B, Price A, et al. Utility of admission cardiac troponin and ‘Ischemia-modified albumin’ measurements for rapid evaluation and rule out of suspected acute myocardial infarction in the emergency department. Emerg Med J 2006;23:256-61. http://dx.doi.org/10.1136/emj.2005.028241.
- Collinson PO, Goodacre S, Bradburn M, Fitzgerald P, Cross L, Grey A, et al. The RATPAC trial (randomised assessment of treatment using panel assay of cardiac markers): a randomised controlled trial of point-of-care cardiac markers in the emergency department. Paper presented at the ESC Congress, Stockholm, Sweden, 28 August–1 September 2010. Eur Heart J 2010;31.
- Costabel JP, Conde D. A new algorithm in the chest pain unit using the high-sensitivity troponin T. Am J Emerg Med 2013;31:993-4. http://dx.doi.org/10.1016/j.ajem.2013.03.002.
- Cullen LA, Greenslade JH, Brown AFT, Hammett CJK, Than MP, Chu KH, et al. Comparison of 2 and 6 hour time intervals in the diagnosis of acute myocardial infarction. Paper presented at the European Society of Cardiology, ESC Congress, Paris, France, 27–31 August 2011. Eur Heart J 2011;32.
- Dawson C, Benger JR, Bayly G. Serial high-sensitivity troponin measurements for the rapid exclusion of acute myocardial infarction in low-risk patients. Emerg Med J 2013;30:593-4. http://dx.doi.org/10.1136/emermed-2012-201574.
- Diercks D, Mumma BE, Peacock WF, Hollander JE, Safdar B, Mahler SA, et al. Incremental value of objective cardiac testing in addition to physician impression and serial contemporary troponin measurements in women. Paper presented at the American Heart Association, Scientific Sessions and Resuscitation Science Symposium, Los Angeles, CA, 3–6 November 2012. Circulation 2012;126.
- Drexler B, Reichlin T, Schaub N, Twerenbold R, Reiter M, Steuer S, et al. Growth-differentiation factor-15 in the early diagnosis and risk stratification of patients with acute chest pain. Paper presented at the European Society of Cardiology, ESC Congress, Paris, France, 27–31 August 2011. Eur Heart J 2011;32. http://dx.doi.org/10.1373/clinchem.2011.173310.
- Engel G, Rockson SG. Rapid diagnosis of myocardial injury with troponin T and CK-MB relative index. Mol Diagn Ther 2007;11:109-16. http://dx.doi.org/10.1007/BF03256230.
- Escabi-Mendoza J, Lopez-Mas AJ, Espinell N. Utility of point of care troponin measurement for the diagnosis of acute non-ST elevation myocardial infarction in the emergency department. Paper presented at the 13th Congress of Chest Pain Centers, Las Vegas, NV, 27–30 April 2010. Crit Pathw Cardiol 2010;9:175-6. http://dx.doi.org/10.1097/HPC.0b013e3181f18901.
- Figiel L, Kasprzak JD, Peruga J, Lipiec P, Drozdz J, Krzeminska-Pakula M, et al. Heart-type fatty acid binding protein: a reliable marker of myocardial necrosis in a heterogeneous group of patients with acute coronary syndrome without persistent ST elevation. Kardiol Pol 2008;66:253-9.
- Freund Y, Chenevier-Gobeaux C, Bonnet P, Claessens YE, Allo JC, Doumenc B, et al. Combination of high sensitive troponin and copeptin for rule out of acute myocardial infarction. Paper presented at the European Society of Cardiology Congress, Paris, France, 27–31 August 2011. Eur Heart J 2011;32.
- Freund Y, Chenevier-Gobeaux C, Leumani F, Doumenc B, Claessens Y, Cosson C, et al. Concomitant measurement of copeptin and high sensitivity troponin for fast and reliable rule out of acute myocardial infarction. Paper presented at the American College of Emergency Physicians, San Francisco, CA, 15–16 October 2011. Ann Emerg Med 2011;58. http://dx.doi.org/10.1016/j.annemergmed.2011.06.061.
- Giannitsis E, Kehayova T, Vafaie M, Katus HA. Combined testing of high-sensitivity troponin T and copeptin on presentation at prespecified cutoffs improves rapid rule-out of non-ST-segment elevation myocardial infarction. Clin Chem 2011;57:1452-5. http://dx.doi.org/10.1373/clinchem.2010.161265.
- Giavarina D. Copeptin and high sensitive troponin for a rapid rule out of acute myocardial infarction?. Clin Lab 2012;58:359-60.
- Giavarina D, Carta M, Fortunato A, Wratten ML, Hartmann O, Soffiati G. Copeptin and high sensitive troponin for a rapid rule out of acute myocardial infarction?. Clin Lab 2011;57:725-30.
- Gimenez MR, Hoeller RH, Zellweger C, Wildi K, Wasila M, Haaf P, et al. Rapid rule out of acute myocardial infarction using undetectable levels of high-sensitive cardiac troponin T. Eur Heart J 2012;33. http://dx.doi.org/10.1016/j.ijcard.2013.06.049.
- Gimenez MR, Hoeller R, Wildi K, Twerenbold R, Moehring B, Wasila M, et al. Normal levels of high-sensitive cardiac troponin I at presentation in patients with acute chest pain. Eur Heart J 2012;33.
- Gustapane M, Biasucci LM, Cardillo MT, Biasillo G, Della Bona R, Caroli A, et al. Is hs-tnt cut-off level for acute coronary syndromes age dependent? Paper presented at the American Heart Association 2012 Scientific Sessions and Resuscitation Science Symposium, Los Angeles, CA, 3–6 November 2012. Circulation 2012;126.
- Gustapane M, Biasucci LM, Cardillo MT, Biasillo G, Zaninotto M, Mion M, et al. Which is the better cut-off for high sensitivity troponin T in emergency room?. Eur Heart J 2012;33.
- Haaf P, Drexler B, Reichlin T, Twerenbold R, Reiter M, Meissner J, et al. Mid-regional pro-adrenomedullin as a novel predictor of mortality in patients with acute chest pain. Paper presented at the European Society of Cardiology Congress, Paris, France, 27–31 August 2011. Eur Heart J 2011;32:733-4.
- Haaf P, Drexler B, Reichlin T, Twerenbold R, Reiter M, Meissner J, et al. High sensitive cardiac troponin in the distinction of acute myocardial infarction from acute cardiac non-coronary disease. Eur Heart J 2011;32.
- Haaf P, Drexler B, Reichlin T, Twerenbold R, Reiter M, Meissner J, et al. High-Sensitivity Cardiac Troponin in the Distinction of Acute Myocardial Infarction From Acute Cardiac Noncoronary Artery Disease Response. Circulation 2013;127:E355-6. http://dx.doi.org/10.1161/CIRCULATIONAHA.112.145201.
- Haaf P, Twerenbold R, Reiter M, Reichlin T, Wildi K, Zellweger C, et al. Absolute and relative changes in cardiac troponin T and I measured with three high-sensitive cardiac troponin assays in emergency department patients with non-coronary chest pain. Eur Heart J 2012;33.
- Haaf P, Wildi K, Reiter M, Zellweger C, Twerenbold R, Hoeller R, et al. Accuracy of high-sensitive cardiac troponins for long-term mortality. Eur Heart J 2012;33.
- Haltern G, Peiniger S, Bufe A, Reiss G, Gulker H, Scheffold T. Comparison of usefulness of heart-type fatty acid binding protein versus cardiac troponin T for diagnosis of acute myocardial infarction. Am J Cardiol 2010;105:1-9. http://dx.doi.org/10.1016/j.amjcard.2009.08.645.
- Heinisch C, Twerenbold R, Reiter M, Reichlin T, Meissner J, Arenja N, et al. Early diagnosis of acute myocardial infarction using the combination of a high-sensitive cardiac troponin assays and copeptin: insights from a multicenter study. Paper presented at the European Society of Cardiology Congress, Stockholm, Sweden, 28 August–1 September 2010. Eur Heart J 2010;31:51-2.
- Hochholzer W, Reichlin T, Twerenbold R, Stelzig C, Hochholzer K, Meissner J, et al. Incremental value of high-sensitivity cardiac troponin T for risk prediction in patients with suspected acute myocardial infarction. Clin Chem 2011;57:1318-26. http://dx.doi.org/10.1373/clinchem.2011.162073.
- Hochholzer W, Twerenbold R, Reichlin T, Meissner J, Reiter M, Heinisch C, et al. Use of a novel high-sensitivity troponin assay for risk stratification in patients with acute chest pain: insights from an international multicenter study. Eur Heart J 2010;31.
- Hoeller R, Gimenez MR, Wildi K, Haaf P, Zellweger C, Twerenbold R, et al. Rapid rule out of acute myocardial infarction using undetectable levels of high-sensitive cardiac troponin I. Eur Heart J 2012;33.
- Hoeller R, Gimenez MR, Zellweger C, Wildi K, Wasila M, Haaf P, et al. Undetectable levels of high-sensitive cardiac troponin I at presentation in patients with acute chest pain. Eur Heart J 2012;33. http://dx.doi.org/10.1016/j.ijcard.2013.06.049.
- Ilva T, Lund J, Porela P, Mustonen H, Voipio-Pulkki L-M, Eriksson S, et al. Early markers of myocardial injury: cTnI is enough. Clin Chim Acta 2009;400:82-5. http://dx.doi.org/10.1016/j.cca.2008.10.005.
- Inoue K, Suwa S, Ohta H, Itoh S, Maruyama S, Masuda N, et al. Heart fatty acid-binding protein offers similar diagnostic performance to high-sensitivity troponin T in emergency room patients presenting with chest pain. Circ J 2011;75:2813-20. http://dx.doi.org/10.1253/circj.CJ-11-0598.
- Irfan A, Reichlin T, Twerenbold R, Reiter M, Balmelli C, Meissner J, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Eur Heart J 2011;32.
- Irfan A, Reichlin T, Twerenbold R, Reiter M, Winkler K, Meissner J, et al. Prevalence and determinants of elevated high-sensitive cardiac troponin T levels among patients with non-cardiac cause of chest pain. Paper presented at the European Society of Cardiology Congress, Paris, France, 27–31 August 2011. Eur Heart J 2011;32:99-100. http://dx.doi.org/10.1016/j.amjmed.2011.10.031.
- Irfan A, Reichlin T, Twerenbold R, Wildi K, Mueller C. Combination of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Paper presented at the 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, San Francisco, CA. J Am Coll Cardiol 2013;61. http://dx.doi.org/10.1016/S0735-1097(13)60233-8.
- Irfan A, Reichlin T, Twerenbold R, Wildi K, Mueller C. The prognostic value of absolute and relative changes in cardiac troponin concentrations among non-acute myocardial infarction patients. Paper presented at the 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, San Francisco, CA. J Am Coll Cardiol 2013;61. http://dx.doi.org/10.1016/S0735-1097(13)60236-3.
- Jairam S, Jones P, Samaraie L, Chataline A, Davidson J, Stewart R. Clinical diagnosis and outcomes for Troponin T ‘positive’ patients assessed by a high sensitivity compared with a 4th generation assay. Emerg Med Australas 2011;23:490-501. http://dx.doi.org/10.1111/j.1742-6723.2011.01446.x.
- Januzzi JL, Bamberg F, Lee H, Truong QA, Nichols JH, Karakas M, et al. High-sensitivity troponin T concentrations in acute chest pain patients evaluated with cardiac computed tomography. Circulation 2010;121:1227-34. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.893826.
- Januzzi JL, Bamberg F, Lee H, Truong QA, Nichols J, Karakas M, et al. Correlates of high sensitivity troponin T concentrations in chest pain patients with and without acute coronary syndrome. Circulation 2009;120.
- Januzzi JL, Zakroysky P, Quynh T, Woodard P, Pope JH, Hauser T, et al. Performance of two sensitive troponin I assays for the evaluation of suspected ACS: results from the multicenter rule out myocardial infarction using computer assisted tomography (ROMICAT) II biomarker sub-study. J Am Coll Cardiol 2013;61. http://dx.doi.org/10.1016/S0735-1097(13)60230-2.
- Jia C-Y, Wang L, Mao Z-G, Zhang J-L, Zhang L. Sichuan Da Xue Xue Bao. Yi Xue Ban/Journal of Sichuan University; 2009.
- Kagawa Y, Toyofuku M, Masaoka Y, Muraoka Y, Okimoto T, Otsuka M, et al. Comparison of heart-type fatty acid binding protein and sensitive troponin for the diagnosis of early acute myocardial infarction. Int J Cardiol 2013;166:347-51. http://dx.doi.org/10.1016/j.ijcard.2011.10.080.
- Karakas M, Januzzi JL, Meyer J, Lee H, Schlett CL, Truong QA, et al. Copeptin does not add diagnostic information to high-sensitivity troponin T in low- to intermediate-risk patients with acute chest pain: results from the rule out myocardial infarction by computed tomography (ROMICAT) study. Clin Chem 2011;57:1137-45. http://dx.doi.org/10.1373/clinchem.2010.160192.
- Kavsak PA, Hill SA, Bhanich Supapol W, Devereaux PJ, Worster A. Biomarkers for predicting serious cardiac outcomes at 72 hours in patients presenting early after chest pain onset with symptoms of acute coronary syndromes. Clin Chem 2012;58:298-302. http://dx.doi.org/10.1373/clinchem.2011.172064.
- Kavsak PA, MacRae AR, Newman AM, Lustig V, Palomaki GE, Ko DT, et al. Effects of contemporary troponin assay sensitivity on the utility of the early markers myoglobin and CKMB isoforms in evaluating patients with possible acute myocardial infarction. Clin Chim Acta 2007;380:213-16. http://dx.doi.org/10.1016/j.cca.2007.01.001.
- Kavsak PA, Worster A, You JJ, Oremus M, Shortt C, Phan K, et al. Ninety-minute vs 3-h performance of high-sensitivity cardiac troponin assays for predicting hospitalization for acute coronary syndrome. Clin Chem 2013;59:1407-10. http://dx.doi.org/10.1373/clinchem.2013.208595.
- Kavsak P, Bhargava R, Lustig V, MacRae AR, Palomaki GE, Vandersluis R, et al. Assessing the requirement for the six-hour interval between specimens in the American Heart Association case definition for AMI, using a highly sensitive troponin assay. Clin Chem 2005;51:A22-3.
- Kavsak P, Hill SA, You JJ, Oremus M, Devereaux P, Jaffe AS, et al. Early serial measurements of myeloperoxidase, a sensitive cardiac troponin I and high-sensitivity cardiac troponin T in patients presenting within 6 hours of chest pain onset. Clin Biochem 2012;45. http://dx.doi.org/10.1016/j.clinbiochem.2012.07.043.
- Kavsak P, MacRae AR, Yerna M, Jaffe AS. Evaluation of a high sensitivity cardiac troponin I assay in an Acute Coronary Syndrome population. Clin Chem 2008;54.
- Kavsak P, Worster A, Devereaux P, Heels-Ansdell D, Guyatt GH, Opie J, et al. A clinically sensitive cardiac troponin I assay (AccuTnI) versus the high sensitive cardiac troponin T assay to predict early serious cardiac outcomes in patients with potential acute coronary syndrome. Paper presented at the 2011 Meeting of the Canadian Society of Clinical Chemists, Vancouver, Canada, 4–8 June 2011. Clin Biochem 2011;44.
- Kavsak P, Wang X, Ko D, MacRae AR, Jaffe AS. Early detection of change in concentration by a research high sensitivity troponin I (hs-cTNI) assay and health outcomes at 30 days in a chest pain population. Clin Biochem 2010;43.
- Keene D, Silva RD, Cooper I, Cooper J, Bokhari A, Wassif W. Clinical significance of high sensitivity troponin T: results of six month follow up data from an unselected population at initial presentation to hospital. Paper presented at the 8th International Congress of Update in Cardiology and Cardiovascular Surgery, Antalya, Turkey, 1–4 March 2012. Int J Cardiol 2012;155:S182-3. http://dx.doi.org/10.1016/S0167-5273(12)70440-0.
- Keller T, Ojeda F, Zeller T, Tzikas S, Bickel C, Baldus S, et al. Early rule-out of myocardial infarction is facilitated by soluble FMS-like tyrosine kinase-1. Circulation 2011;124.
- Keller T, Tzikas S, Echevarria FO, Zeller T, Baldus S, Bickel C, et al. High sensitive troponin I versus multiple marker for early diagnosis of myocardial infarction. Eur Heart J 2011;32:422-3.
- Keller T, Tzikas S, Zeller T, Czyz E, Lillpopp L, Roth A, et al. Copeptin improves early diagnosis of acute myocardial infarction. Circulation 2009;120. http://dx.doi.org/10.1016/j.jacc.2010.01.029.
- Keller T, Tzikas S, Zeller T, Czyz E, Lillpopp L, Ojeda FM, et al. Copeptin improves early diagnosis of acute myocardial infarction. J Am Coll Cardiol 2010;55:2096-106. http://dx.doi.org/10.1016/j.jacc.2010.01.029.
- Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med 2009;361:868-77. http://dx.doi.org/10.1056/NEJMoa0903515.
- Kelly AM. Performance of a sensitive troponin assay in the early diagnosis of acute myocardial infarction in the emergency department. Emerg Med Australas 2011;23:181-5. http://dx.doi.org/10.1111/j.1742-6723.2011.01388.x.
- Khan DA, Sharif MS, Khan FA. Diagnostic performance of high-sensitivity troponin T, myeloperoxidase, and pregnancy-associated plasma protein A assays for triage of patients with acute myocardial infarction. Korean J Lab Med 2011;31:172-8. http://dx.doi.org/10.3343/kjlm.2011.31.3.172.
- Khoo R, Kandiban P, Saw S, Sethi S. Comparative study: analytical and clinical performance of Advia Centaur troponin I: Ultra assay and Elecsys troponin T assay. Clin Chem 2008;54.
- Kitamura M, Hata NH, Takayama TT, Hirayama AH, Ogawa MO, Yamashina AY, et al. High-sensitivity troponin T for earlier diagnosis of acute coronary syndrome with initially negative rapid-troponin T test: subanalysis of HsTnT-iNET study focused on coronary angiographic findings. Eur Heart J 2012;33.
- Kobayashi N, Hata N, Seino Y, Kume N, Shinada T, Tomita K, et al. Matrix metalloproteinase-9 is a sensitive and specific biomarker for the earliest stage acute coronary syndrome: comparison with high sensitivity troponin T. Eur Heart J 2011;32.
- Kobayashi N, Hata N, Kume N, Shinada T, Tomita K, Shirakabe A, et al. Soluble lectin-like oxidized LDL receptor-1 and high-sensitivity troponin T as diagnostic biomarkers for acute coronary syndrome. Improved values with combination usage in emergency rooms. Circ J 2011;75:2862-71. http://dx.doi.org/10.1253/circj.CJ-11-0724.
- Koenig W, Bamberg F, Lee H, Truong QA, Nichols JH, Trischler G, et al. High-sensitivity troponin reliably excludes acute coronary syndrome in patients with acute chest pain: results from the rule out myocardial infarction by computed tomography (ROMICAT) study. Circulation 2008;118:S637-S637.
- Lacnak B, Stejskal D, Jedelsky L, Karpisek M, Sprongl L. Utilisation of Glykogen Phosphorylase BB measurement in the diagnosis of acute coronary syndromes in the event of chest pain. Vnitr Lek 2007;53:1164-9.
- Lee KK, Mills NL, Churchhouse AMD, Anand A, Gamble D, Shah A, et al. Implementation of a sensitive troponin I assay reduces death and recurrent myocardial infarction in patients with suspected acute coronary syndrome. Heart 2011;97. http://dx.doi.org/10.1136/heartjnl-2011-300198.5.
- Lindahl B, James S, Johnston N, Venge P. A new high-sensitive cardiac troponin I assay allows identification of the vast majority of patients with acute coronary syndromes. Eur Heart J 2009;30. http://dx.doi.org/10.1016/j.jacc.2009.05.051.
- Lippi G, Cervellin G. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation 2013;127. http://dx.doi.org/10.1161/CIRCULATIONAHA.112.128603.
- Lippi G, Cervellin G, Robuschi E, Salvagno GL, Montagnana M, Aloe R, et al. Comparison of high sensitivity and contemporary troponin I immunoassays for the early detection of acute myocardial infarction in the emergency department. Ann Clin Biochem 2012;49:205-6. http://dx.doi.org/10.1258/acb.2011.011211.
- Lippi G, Mattiuzzi C, Cervellin G. Critical review and meta-analysis on the combination of heart-type fatty acid binding protein (H-FABP) and troponin for early diagnosis of acute myocardial infarction. Clin Biochem 2013;46:26-30. http://dx.doi.org/10.1016/j.clinbiochem.2012.10.016.
- Lotze U, Lemm H, Heyer A, Mueller K. Combined determination of troponin T high sensitive and copeptin for early rule out of acute myocardial infarction: first experience in a general emergency department. Paper presented at the European Society of Cardiology Congress, Paris, France, 27–31 August 2011. Eur Heart J 2011;32.
- Lotze U, Lemm H, Heyer A, Muller K. Combined determination of highly sensitive troponin T and copeptin for early exclusion of acute myocardial infarction: first experience in an emergency department of a general hospital. Vasc Health Risk Manag 2011;7:509-15. http://dx.doi.org/10.2147/VHRM.S21753.
- Macrae AR, Kavsak PA, Lustig V, Bhargava R, Vandersluis R, Palomaki GE, et al. Assessing the requirement for the 6-hour interval between specimens in the American Heart Association Classification of Myocardial Infarction in Epidemiology and Clinical Research Studies. Clin Chem 2006;52:812-18. http://dx.doi.org/10.1373/clinchem.2005.059550.
- Mair J, Ploner T, Hammerer-Lercher A, Schratzberger P, Griesmacher A, Pachinger O. High-sensitive cardiac troponin T (hs-cTnT) assay is not superior to a previous CTNT assay generation for the diagnosis of acute myocardial infarctions in a real-world emergency department. Paper presented at the Osterreichische Kardiologische Gesellschaft Annual Conference, Salzburg, Austria, 25–28 May 2011. J Kardiol 2011;18.
- Mair J, Ploner T, Hammerer-Lercher A, Schratzberger P, Griesmacher A, Pachinger O. High-sensitive cardiac troponin T (hs-cTnT) assay is not superior to a previous cTnT assay generation for the diagnosis of acute myocardial infarctions in a real-world emergency department. Eur Heart J 2011;32.
- Matsui S, Ishii J, Kawai T, Hattori T, Hattori K, Okumura M, et al. Diagnostic and prognostic value of serum concentration of high-sensitivity cardiac troponin T relative to heart-type fatty acid-binding protein in the early hours of suspected acute coronary syndrome. Paper presented at the American Heart Association’s Scientific Sessions, Orlando, FL, 12–16 November 2011. Circulation 2011;124.
- Mazhar J, Pera V, Siddiqui A, Bouwhuis J, Du Toit S, Devlin G. Clinical impact of high sensitive troponin T (HsTnT) on diagnosis of acute coronary syndrome (ACS). Paper presented at the Cardiac Society of Australia and New Zealand Annual Scientific Meeting and the International Society for Heart Research Australasian Section Annual Scientific Meeting, Perth, Australia, 11–14 August 2011. Heart Lung Circ 2011;20. http://dx.doi.org/10.1016/j.hlc.2011.05.070.
- Melanson SE, Bonaca M, Scirica B, Sabatine M, Morrow DA, Jarolim P. Prospective evaluation of the prognostic implications of low level elevation of cardiac troponin using a new highly-sensitive assay for cardiac troponin I: results from the MERLIN-TIMI 36 trial. Clin Chem 2008;54.
- Melki D, Lind S, Agewall S, Jernberg T. Excellent early risk stratification using troponins and N-terminal pro-brain natriuretic peptides in chest pain patients but no additional value of high-sensitive troponin T. Paper presented at the 60th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, New Orleans, LA, 2–5 April 2011. J Am Coll Cardiol 2011;57. http://dx.doi.org/10.1016/S0735-1097(11)61080-2.
- Melki D, Lind S, Agewall S, Jernberg T. Prognostic value of combining high sensitive troponin T and N-terminal pro B-type natriuretic peptide in chest pain patients with no persistent ST-elevation. Clin Chim Acta 2012;413:933-7. http://dx.doi.org/10.1016/j.cca.2012.02.008.
- Menhofer D, Sandhofer A, Hammerer-Lercher A, Mair J. Copeptin in everyday clinical practice of an emergency department. Paper presented at the Jahrestagung 2013 der Osterreichischen Kardiologischen Gesellschaft, Salzburg, Austria, 5–8 June 2013. Wien Klin Wochenschr 2013;125:S9-10.
- Meune C, Balmelli C, Marxer T, Meissner J, Twerenbold R, Reiter M, et al. High-sensitive troponin, B-type natriuretic peptide and coronary angiogram findings in patients with non ST-segment elevation acute coronary syndrome. Int J Cardiol 2011;153:335-7. http://dx.doi.org/10.1016/j.ijcard.2011.09.038.
- Meune C, Balmelli C, Twerenbold R, Reiter M, Reichlin T, Ziller R, et al. Utility of 14 novel biomarkers in patients with acute chest pain and undetectable levels of conventional cardiac troponin. Int J Cardiol 2013;167:1164-9. http://dx.doi.org/10.1016/j.ijcard.2012.03.117.
- Meune C, Zuily S, Wahbi K, Claessens Y-E, Weber S, Chenevier-Gobeaux C. Combination of copeptin and high-sensitivity cardiac troponin T assay in unstable angina and non-ST-segment elevation myocardial infarction: a pilot study. Arch Cardiovasc Dis 2011;104:4-10. http://dx.doi.org/10.1016/j.acvd.2010.11.002.
- Mikkel MS, Martin LN, Thode J, Steen IH, Iversen K, Clemmensen P, et al. High-sensitivity cardiac troponins, copeptin and heart-type fatty acid-binding protein in a single sample multi-marker approach at admission for the diagnosis of acute myocardial infarction. Paper presented at the 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, San Francisco, CA, 9–11 March 2013. J Am Coll Cardiol 2013;61.
- Mikkel MS, Martin LN, Thode J, Steen IH, Iversen K, Clemmensen P, et al. Absolute delta and peak HS-CTN values are superior to relative delta values in diagnosing acute myocardial infarction in an unselected chest pain population. Paper presented at the 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, San Francisco, CA, 9–11 March 2013. J Am Coll Cardiol 2013;61.
- Mikkel MS, Nielsen M, Thode J, Hansen S, Iversen K, Clemmensen P, et al. Usefulness of creatine-kinase myocardial band in the diagnosis of acute myocardial infarction after the advent of high-sensitivity cardiac troponins. Paper presented at the 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, San Francisco, CA, 9–11 March 2013. J Am Coll Cardiol 2013;61.
- Mills NL, Churchhouse AMD, Anand A, Gamble D, MacLeod M, Graham C, et al. Clinical outcome and sensitive troponin I assay in patients with suspected acute coronary syndrome. Heart 2010;96. http://dx.doi.org/10.1016/s0735-1097(10)61158-8.
- Mills NL, Churchhouse AMD, Anand A, Gamble D, MacLeod M, Graham C, et al. Clinical outcome and sensitive troponin I assay in patients with suspected acute coronary syndrome. Paper presented at the American College of Cardiology’s 59th Annual Scientific Session and i2 Summit: Innovation in Intervention, Atlanta, GA, 14–16 March 2010. J Am Coll Cardiol 2010;55.
- Mills NL, Lee KK, McAllister DA, Churchhouse AMD, MacLeod M, Stoddart M, et al. Implications of lowering threshold of plasma troponin concentration in diagnosis of myocardial infarction: cohort study. Br Med J 2012;344. http://dx.doi.org/10.1136/bmj.e1533.
- Mingels AM, Joosen IA, Versteylen MO, Laufer EM, Winkens MH, Wildberger JE, et al. High-sensitivity cardiac troponin T: risk stratification tool in patients with symptoms of chest discomfort. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0035059.
- Moehring B, Twerenbold R, Wildi K, Haaf P, Hoeller R, Gimenez MR, et al. Prospective evaluation of the safety of the 2011 ESC guidelines for rapid rule-out of NSTEMI using a novel high sensitive assay for troponin I. Eur Heart J 2012;33.
- Moehring B, Twerenbold R, Wildi K, Haaf P, Reichlin T, Arenja N, et al. Prospective evaluation of the diagnostic accuracy of the novel ESC 2011 guidelines for rapid rule-out of NSTEMI using high sensitive cardiac troponin T. Eur Heart J 2012;33.
- Montagnana M, Lippi G, Danese E, Salvagno GL, Cervellin G, Guidi GC. Serum concentration of neopterin on admission does not improve the diagnostic performance of highly-sensitive troponin I. Clin Chem Lab Med 2012;50:747-8. http://dx.doi.org/10.1515/cclm.2011.829.
- Morrow DA, Antman EM. Evaluation of high-sensitivity assays for cardiac troponin. Clin Chem 2009;55:5-8. http://dx.doi.org/10.1373/clinchem.2008.117218.
- Nagurney JT, Brown DFM, Chae C, Chang Y, Chung WG, Cranmer H, et al. The sensitivity of cardiac markers stratified by symptom duration. J Emerg Med 2005;29:409-15. http://dx.doi.org/10.1016/j.jemermed.2005.05.011.
- Bethesda, MD: National Library of Medicine (US); 2010.
- Naroo GY, Mohamed Ali S, Butros V, Al Haj A, Mohammed I, Alosert M, et al. Elevated heart-type fatty acid-binding protein predicts early myocardial injury and aids in the diagnosis of non-ST elevation myocardial infarction. Hong Kong J Emerg Med 2009;16:141-7.
- Ngan Kee L, Bell D, Crooke M, Cooke R, Mann S. How the new high-sensitivity troponin T test will change our diagnosis of myocardial infarction. Paper presented at the New Zealand Annual Scientific Meeting of the Cardiac Society of Australia and New Zealand, Rotorua, New Zealand, 25–27 June 2010. Heart Lung Circ 2010;19:S25-6.
- Noad R, Donnelly P, Higginson D, Trinnick T, Duly E, McMechan S, et al. Evaluation of a new highly sensitive troponin assay in suspected ACS. Ir J Med Sci 2010;179.
- Normann J, Mueller M, Biener M, Vafaie M, Katus HA, Giannitsis E. Effect of older age on diagnostic and prognostic performance of high-sensitivity troponin T in patients presenting to an emergency department. Am Heart J 2012;164:698-705. http://dx.doi.org/10.1016/j.ahj.2012.08.003.
- Nusier MK, Ababneh BM. Diagnostic efficiency of creatine kinase (CK), CKMB, troponin T and troponin I in patients with suspected acute myocardial infarction. J Health Sci 2006;52:180-5. http://dx.doi.org/10.1248/jhs.52.180.
- Olivieri F, Galeazzi R, Giavarina D, Testa R, Abbatecola AM, Ceka A, et al. Aged-related increase of high sensitive Troponin T and its implication in acute myocardial infarction diagnosis of elderly patients. Mech Ageing Dev 2012;133:300-5. http://dx.doi.org/10.1016/j.mad.2012.03.005.
- Orsborne C, Body R. How sensitive is high-sensitivity troponin in the emergency department? A systematic review and meta-analysis. Paper presented at the 14th International Conference on Emergency Medicine, Dublin, Ireland, 27–30 June 2012. Acad Emerg Med 2012;19.
- Paoloni R, Kumar P, Janu M. Pilot study of high-sensitivity troponin T testing to facilitate safe early disposition decisions in patients presenting to the emergency department with chest pain. Intern Med J 2010;40:188-92. http://dx.doi.org/10.1111/j.1445-5994.2009.01962.x.
- Perego F, Dipaola F, Gruppo di Autoformazione M. Does a lower diagnostic threshold of sensitive plasma troponin I assay improve clinical outcomes of patients with chest pain?. Intern Emerg Med 2011;6:559-60. http://dx.doi.org/10.1007/s11739-011-0718-0.
- Plebani M, Mion M, Novello E, Zaninotto M, Babuin L, Iliceto S. Clinical performance of a new high-sensitivity cardiac troponin I assay. Clin Chem 2009;55.
- Ploner T, Mair J, Pachinger O, Schratzberger P, Hammerer-Lercher A, Griesmacher A. Myocardial infarction diagnosis in the emergency department: highly sensitive cardiac Troponin T (hs-cTnT) is not superior to the 4th cTnT-Test Generation. Wien Klin Wochenschr 2011;123:A59-60.
- Popp S, Herdtle S, Pohlmann H, Poravas M, Umarov D, Bach R, et al. High-sensitive troponin T measurement for patients with acute chest pain: improvement of diagnostics? Paper presented at the 30th International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium, 9–12 March 2010. Crit Care 2010;14:S90-1. http://dx.doi.org/10.1016/j.amjmed.2010.07.015.
- Potocki M, Reichlin T, Twerenbold R, Ernst S, Winkler K, Reiter M, et al. Copeptin and sensitive cardiac troponin in the early diagnosis of acute myocardial infarction in patients with preexisting coronary artery disease. Paper presented at the European Society of Cardiology Congress, Paris, France, 27–31 August 2011. Eur Heart J 2011;32:90-1.
- Pracon R, Kruk M, Jakubczak B, Demkow M, Bilinska ZT. Superior early diagnostic performance of a sensitive cardiac troponin assay as compared to a standard troponin test in the diagnosis of acute myocardial infarction. Kardiol Pol 2012;70:131-8.
- Rajdl D, Hromadka M, Trefil L, Racek J, Pechman V, Cech J, et al. Diagnostic sensitivity of high sensitivity troponin in patients with acute myocardial infarction. Clin Chem Lab Med 2011;49.
- Ray P, Freund Y, Chenevier-Gobeaux C, Allo JC, Boddaert J, Riou B. Diagnostic performance of high-sensitivity troponin in emergency elderly patients. Paper presented at the 7th Congress of the EUGMS, Malaga, Spain, 28–30 September 2011. Eur Geriatr Med 2011;2:S119-20. http://dx.doi.org/10.1186/cc10270.
- Reichlin T, Mueller C. Serial changes in highly sensitive cardiac troponin improve the early diagnosis of acute myocardial infarction. Evid Based Med 2012;17. http://dx.doi.org/10.1136/ebmed-2012-100672.
- Reichlin T, Schindler C, Twerenbold R, Hochholzer W, Haaf P, Irfan A, et al. Clinical application of high sensitive troponin T in the diagnosis of acute myocardial infarction. Paper presented at the European Society of Cardiology Congress, Paris, France, 27–31 August 2011. Eur Heart J 2011;32. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.023937.
- Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012;172:1211-18. http://dx.doi.org/10.1001/archinternmed.2012.3698.
- Reichlin T, Twerenbold R, Hochholzer W, Reiter M, Meissner J, Arenja N, et al. Change in incidence of acute myocardial infarction and other cardiac disorders associated with the clinical introduction of high-sensitive cardiac troponin assays. Paper presented at the European Society of Cardiology Congress, Stockholm, Sweden, 28 August–1 September 2010. Eur Heart J 2010;31.
- Reichlin T, Twerenbold R, Hochholzer W, Reiter M, Meissner J, Potocki M, et al. Clinical use of a high-sensitive cardiac troponin assay in patients with suspected myocardial infarction. Paper presented at the European Society of Cardiology Congress, Stockholm, Sweden, 28 August–1 September 2010. Eur Heart J 2010;31.
- Reichlin T, Twerenbold R, Reiter M, Steuer S, Bassetti S, Balmelli C, et al. Introduction of high-sensitivity troponin assays: impact on myocardial infarction incidence and prognosis. Am J Med 2012;125:1205-13. http://dx.doi.org/10.1016/j.amjmed.2012.07.015.
- Rubini Gimenez M, Hoeller RH, Zellweger C, Wildi K, Wasila M, Haaf P, et al. Rapid rule out of acute myocardial infarction using undetectable levels of high-sensitive cardiac troponin T. Paper presented at ESC Congress 2012, Munich, Germany, 25–29 August 2012. Eur Heart J 2012;33. http://dx.doi.org/10.1016/j.ijcard.2013.06.049.
- Rudolph V, Keller T, Schulz A, Echevarria FO, Rudolph T, Bickel C, et al. Myeloperoxidase as early diagnostic and prognostic marker of acute coronary syndrome: a head-to-head comparison with high sensitive troponin I. Paper presented at the 60th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, New Orleans, LA, 2–5 April 2011. J Am Coll Cardiol 2011;57. http://dx.doi.org/10.1016/S0735-1097(11)61021-8.
- Rudolph V, Keller T, Schulz A, Echevarria FO, Rudolph T, Bickel C, et al. Myeloperoxidase as early diagnostic and prognostic marker of acute coronary syndrome: a head-to-head-comparison with high sensitive troponin I. Eur Heart J 2011;32. http://dx.doi.org/10.1016/s0735-1097(11)61021-8.
- Rudolph V, Keller T, Schulz A, Ojeda F, Rudolph TK, Tzikas S, et al. Diagnostic and prognostic performance of myeloperoxidase plasma levels compared with sensitive troponins in patients admitted with acute onset chest pain. Circ Cardiovasc Genet 2012;5:561-8. http://dx.doi.org/10.1161/CIRCGENETICS.111.962290.
- Samaraie L, Jairam S, Chataline A, Stewart R, White H, Sawtell F, et al. Comparison of high sensitivity and 4th generation troponin assays in an inpatient clinical cohort. Paper presented at the New Zealand Annual Scientific Meeting of the Cardiac Society of Australia and New Zealand, Rotorua, New Zealand, 25–27 June 2010. Heart Lung Circulation 2010;19:S30-1.
- Scharnhorst V, Krasznai K, van’t Veer M, Michels R. Rapid detection of myocardial infarction with a sensitive troponin test. Am J Clin Pathol 2011;135:424-8. http://dx.doi.org/10.1309/AJCPA4G8AQOYEKLD.
- Schaub N, Reichlin T, Meune C, Twerenbold R, Haaf P, Hochholzer W, et al. Markers of plaque instability in the early diagnosis and risk stratification of acute myocardial infarction. Clin Chem 2012;58:246-56. http://dx.doi.org/10.1373/clinchem.2011.172940.
- Schoos MM, Nielsen ML, Thode J, Hansen SI, Iversen K, Clemmensen P, et al. High-sensitivity cardiac troponins, copeptin and heart-type fatty acid-binding protein in a single sample multi-marker approach at admission for the diagnosis of acute myocardial infarction. J Am Coll Cardiol 2013;61. http://dx.doi.org/10.1016/S0735-1097(13)60190-4.
- Schoos MM, Nielsen M, Thode J, Hansen S, Iversen K, Clemmensen P, et al. Usefulness of creatine-kinase myocardial band in the diagnosis of the acute myocardial infarction after the advent of high-sensitivity cardiac troponins. J Am Coll Cardiol 2013;61. http://dx.doi.org/10.1016/S0735-1097(13)60240-5.
- Schreiber DH, Agbo C, Wu AHB. Short-term (90 min) diagnostic performance for acute non-ST segment elevation myocardial infarction and 30-day prognostic evaluation of a novel third-generation high sensitivity troponin I assay. Clin Biochem 2012;45:1295-301. http://dx.doi.org/10.1016/j.clinbiochem.2012.06.005.
- Sethi A, Bajaj A, Bahekar A, Bhuriya R, Khosla S. Diagnostic accuracy of high sensitivity troponin: a meta-analysis. Paper presented at the 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention; 9–11 March 2013; San Francisco, CA. J Am Coll Cardiol 2013;61. http://dx.doi.org/10.1016/S0735-1097(13)60227-2.
- Shand JA, Howe A, Connelly M, Menown IB, McKeown P, McEneaney DJ. Absolute change in highly sensitive troponin T concentration 2 hours after an initial sample out-performs other highly sensitive troponin diagnostic models for the early diagnosis of myocardial infarction. Paper presented at the American Heart Association 2012 Scientific Sessions and Resuscitation Science Symposium, Los Angeles, CA, 3–6 November 2012. Circulation 2012;126.
- Shortt CR, Worster A, Hill SA, Kavsak PA. Comparison of hs-cTnI, hs-cTnT, hFABP and GPBB for identifying early adverse cardiac events in patients presenting within six hours of chest pain-onset. Clin Chim Acta 2013;419:39-41. http://dx.doi.org/10.1016/j.cca.2013.01.008.
- Spanuth E, Ivandic B, Giannitsis E. High sensitivity troponin for detection of myocardial infarction: comparison of troponin T high sensitive (TNT HS) and pathfast CTNI. Clin Chem Lab Med 2011;49.
- Spasic-Obradovic G, Radakovic A, Obradovic S. High sensitivity (HS) troponin T stat assay diagnostic value in patients with chest pain and underlying chronic disease. Clin Chem Lab Med 2011;49.
- Stengaard C, Sorensen JT, Ladefoged SA, Christensen EF, Lassen JF, Erik Botker H, et al. The value of prehospital and inhospital high-sensitivity troponin t analysis for rapid rule in and rule out of patients with acute myocardial infarction. Paper presented at the American Heart Association 2012 Scientific Sessions and Resuscitation Science Symposium, Los Angeles, CA, 3–6 November 2012. Circulation 2012;126. http://dx.doi.org/10.1016/j.amjcard.2013.06.026.
- Tajsic M, Andric T, Jarai R, Schwarz MA, Kitzbrecht J, Koch J, et al. Copeptin as a part of the dual biomarker strategy for early diagnosis of NSTEMI-WILCOP study. Paper presented at the Jahrestagung 2013 der Osterreichischen Kardiologischen Gesellschaft, Salzburg, Austria, 5–8 June 2013. Wien Klin Wochenschr 2013;125:S8-9.
- Tajsic M, Jarai R, Kitzbrecht J, Koch J, Wojta J, Huber K. Copeptin as a part of the dual biomarker strategy for early diagnosis of NSTEMI: Wilcop study. Paper presented at the 62nd Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, San Francisco, CA, 9–11 March 2013. J Am Coll Cardiol 2013;61. http://dx.doi.org/10.1016/S0735-1097(13)60189-8.
- Tajsic M, Jarai R, Wojta J, Huber K. Diagnostic relevance of copeptin in addition to high-sensitivity troponin in patients with acute chest pain: preliminary results of the Wilcop registry. Paper presented at the Osterreichische Kardiologische Gesellschaft Jahrestagung, Salzburg, Austria, 30 May–2 June 2012. J Kardiol 2012;19:127-8.
- Tajsic M, Simon R, Jarai R, Schwarz MA, Kitzbrecht J, Koch J, et al. Relevance of copeptin for employment of dual biomarker strategy in diagnosis of ACS: WILCOP study. Paper presented at the Jahrestagung 2013 der Osterreichischen Kardiologischen Gesellschaft, Salzburg, Austria, 5–8 June 2013. Wien Klin Wochenschr 2013;125:s12-13.
- Tamimi W, Alothaim A, Alhodab A, Dafterdar R. Evaluation of Troponin I in patients with acute myocardial infarction in the emergency department. J Clin Diagn Res 2010;4:3170-5.
- Tanaka T, Sohmiya K-I, Kitaura Y, Takeshita H, Morita H, Ohkaru Y, et al. Clinical evaluation of point-of-care-testing of heart-type fatty acid-binding protein (H-FABP) for the diagnosis of acute myocardial infarction. J Immunoassay Immunochem 2006;27:225-38. http://dx.doi.org/10.1080/15321810600734919.
- Than M, Cullen L, Aldous S, Parsonage WA, Reid CM, Greenslade J, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol 2012;59:2091-8. http://dx.doi.org/10.1016/j.jacc.2012.02.035.
- Thelin J, Borna C, Erlinge D, Ohlin B. The combination of high sensitivity troponin T and copeptin facilitates early rule-out of ACS: a prospective observational study. BMC Cardiovasc Disord 2013;13. http://dx.doi.org/10.1186/1471-2261-13-42.
- Thomas JJ, Taylor L, Camp T, Ghaemmaghami C. Delta measurements, using an ultrasensitive troponin I assay, reliably diagnose acute coronary syndromes and predict adverse cardiac events within 30 days of ED visits. Ann Emerg Med 2007;50. http://dx.doi.org/10.1016/j.annemergmed.2007.06.121.
- Thomas JJ, Taylor L, Camp T, Ghaommaghami C. Delta measurements of an ultrasensitive troponin I assay reliably diagnose the full spectrum of acute coronary syndromes and predict adverse cardiac events within 30 days of ED visits. Circulation 2007;116.
- Truong QA, Bayley J, Hoffmann U, Bamberg F, Schlett CL, Nagurney JT, et al. Multi-marker strategy of natriuretic peptide with either conventional or high-sensitivity troponin-T for acute coronary syndrome diagnosis in emergency department patients with chest pain: from the ‘Rule Out Myocardial Infarction using Computer Assisted Tomography’ (ROMICAT) trial. Am Heart J 2012;163:972-9. http://dx.doi.org/10.1016/j.ahj.2012.03.010.
- Truong QA, Hoffmann U, Bayley J, Schlett CL, Bamberg F, Nagurney JT, et al. Adding natriuretic peptides to either conventional or high-sensitivity troponin-T improves reclassification of acute coronary syndrome diagnosis in emergency department patients with chest pain: from the ROMICAT trial. Paper presented at the American Heart Association’s Scientific Sessions, Orlando, FL, 12–16 November 2011. Circulation 2011;124. http://dx.doi.org/10.1016/j.ahj.2012.03.010.
- Twerenbold R, Reiter M, Heinisch C, Reichlin T, Arenja N, Socrates T, et al. Early diagnosis of acute myocardial infarction using sensitive cardiac troponin assays in patients with kidney disease. Eur Heart J 2010;31:649-50.
- Twerenbold R, Reiter M, Reichlin T, Heinisch C, Meissner J, Arenja N, et al. Early diagnosis of acute myocardial infarction using sensitive cardiac troponin assays in women. Eur Heart J 2010;31.
- Twerenbold R, Reiter M, Reichlin T, Meissner J, Heinisch C, Socrates T, et al. Direct comparison of three high-sensitive cardiac troponin assays in the early diagnosis of acute myocardial infarction -insights from a multicenter study. Paper presented at the European Society of Cardiology Congress, Stockholm, Sweden, 28 August–1 September 2010. Eur Heart J 2010;31.
- Twerenbold R, Reichlin T, Reiter M, Haaf P, Drexler B, Arenja N, et al. Copeptin in combination with high-sensitive cardiac troponin for early risk stratification in acute chest pain. Paper presented at the European Society of Cardiology Congress, Paris, France, 27–31 August 2011. Eur Heart J 2011;32:953-4.
- Twerenbold R, Reichlin T, Reiter M, Haaf PH, Wildi K, Potocki M, et al. Early diagnosis of acute myocardial infarction in patients with kidney disease using more sensitive cardiac troponin assays. Paper presented at the ESC Congress, Munich, Germany, 25–29 August 2012. Eur Heart J 2012;33:910-11.
- Bethesda, MD: National Library of Medicine (US); 2013.
- Comparison Between the New Highly Sensitive Troponin T and the Conventional Troponin T Test in Elderly Patients. Bethesda, MD: National Library of Medicine (US); 2013.
- Van Wijk S, Jacobs L, Eurlings LW, van Kimmenade R, Lemmers R, Broos P, et al. Troponin T Measurements by High-Sensitivity vs. Conventional Assays for Risk Stratification in Acute Dyspnea. Clin Chem 2012;58:284-92. http://dx.doi.org/10.1373/clinchem.2011.175976.
- Vasikaran SD, Macdonald SPJ, Sikaris KA. High-sensitivity cardiac troponin assays for risk stratification and for the diagnosis of acute myocardial infarction. Ann Clin Biochem 2012;49:209-10. http://dx.doi.org/10.1258/acb.2012.012058.
- Veljkovic K, Hill S, Bhanich-Supapol W, Worster A, Kavsak P. Incorporating both absolute and relative change in high-sensitivity cardiac troponin T concentrations for an early diagnosis of myocardial infarction. Clin Biochem 2012;45. http://dx.doi.org/10.1016/j.clinbiochem.2012.07.074.
- Venge P, James S, Jansson L, Lindahl B. Clinical performances of two high-sensitive cardiac troponin I assays. Clin Chem 2008;54. http://dx.doi.org/10.1373/clinchem.2008.106500.
- Venge P, James S, Jansson L, Lindahl B. Clinical performance of two highly sensitive cardiac troponin I assays. Clin Chem 2009;55:109-16. http://dx.doi.org/10.1373/clinchem.2008.106500.
- Venge P, Ohberg C, Flodin M, Lindahl B. Early and late outcome prediction of death in the emergency room setting by point-of-care and laboratory assays of cardiac troponin I. Am Heart J 2010;160:835-41. http://dx.doi.org/10.1016/j.ahj.2010.07.036.
- Weber M, Bazzino O, Navarro Estrada JL, de Miguel R, Salzberg S, Fuselli JJ, et al. Improved diagnostic and prognostic performance of a new high-sensitive troponin T assay in patients with acute coronary syndrome. Am Heart J 2011;162:81-8. http://dx.doi.org/10.1016/j.ahj.2011.04.007.
- Weber M, Moellmann H, Nef H, Mayer S, Liebetrau C, Woelken M, et al. Diagnostic and prognostic value of high sensitive troponin T in patients with acute coronary syndromes. Eur Heart J 2009;30.
- Wildi KS, Twerenbold R, Reiter M, Moehring B, Haaf P, Reichlin T, et al. Direct comparison of absolute and relative changes in high-sensitive cardiac troponin I in the early diagnosis of AMI. Paper presented at the ESC Congress, Munich, Germany, 25–29 August 2012. Eur Heart J 2012;33:909-10.
- Wong PSC, Rao G, Innasimuthu A, Saeed Y, Robinson A, Robinson D. Validation of a prediction score to distinguish patients with acute myocardial infarction from other causes of raised troponin T. Paper presented at the European Society of Cardiology, ESC Congress; 28 August–1 September 2010; Stockholm: Sweden. Eur Heart J 2010;31.
- Worster A, Krizmanich W, Preyra IJ, Kavsak P. A comparison of high-sensitivity cardiac troponin I assay with the current sensitive cardiac troponin I test in the emergency department. Paper presented at the 2013 CAEP/ACMU; 1–5 June 2013; Vancouver: Canada. Can J Emerg Med 2013;15.
- Zahid M, Good CB, Singla I, Sonel AF. Clinical significance of borderline elevated troponin I levels across different assays in patients with suspected acute coronary syndrome. Am J Cardiol 2009;104:164-8. http://dx.doi.org/10.1016/j.amjcard.2009.03.012.
- Zahid M, Good CB, Sonel AF. Clinical significance of borderline elevated troponin-1 levels across different assays. Circulation 2008;118. http://dx.doi.org/10.1016/j.amjcard.2009.03.012.
- Zellweger C, Wildi K, Reichlin T, Haaf P, Hoeller R, Rubini Moehring M, et al. Diagnostic and prognostic value of copeptin in patients with acute chest pain and diabetes. Paper presented at the ESC Congress, Munich, Germany, 25–29 August 2012. Eur Heart J 2012;33:205-6.
- Zuily S, Chenevier-Gobeaux C, Claessens Y-E, Wahbi K, Weber S, Meune C. High diagnostic performance of a high-sensitivity cardiac troponin T assay in patients with suspected acute coronary syndrome. Int J Cardiol 2011;146:115-16. http://dx.doi.org/10.1016/j.ijcard.2010.09.084.
Appendix 1 Literature search strategies
Clinical effectiveness search strategies
MEDLINE (OvidSP): 1946 to 2013/10/Week 1
Searched: 11 October 2013
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs).ti,ab,ot. (229)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni).ti,ab,ot. (99)
-
((troponin t or tnt or ctnt or tropt or trop t) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (563)
-
((troponin I or tni or ctni or tropI or trop I) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (349)
-
(troponin$ adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (769)
-
(troponin$ adj5 (architect or elecsys or accutni or accu-tni or access or unicel)).ti,ab,hw,ot. (66)
-
or/1-6 (1215)
-
troponin t/ or troponin I/ or (60304-72-5 or 77108-40-8).rn. (8642)
-
(sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive).ti,ab,ot. (4,878,300)
-
8 and 9 (4209)
-
7 or 10 (4559)
-
chest pain/ (9293)
-
((chest or thorax or thoracic) adj2 (pain$ or discomfort or tight$ or pressure)).ti,ab,ot,hw. (28,602)
-
exp myocardial ischemia/ (357,748)
-
(acute adj2 coronary adj2 syndrome$).ti,ab,ot. (16,495)
-
(preinfarc$ Angina$ or pre infarc$ Angina$).ti,ab,ot. (285)
-
Unstable angina$.ti,ab,ot. (10,718)
-
((heart$ or myocardi$ or cardiac or coronary) adj2 (preinfarc$ or infarc$ or attack$ or arrest$ or occlusion$ or isch?emia$)).ti,ab,ot. (194,088)
-
(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI).ti,ab,ot. (53,168)
-
or/12-19 (444,673)
-
11 and 20 (2503)
-
animals/ not (animals/ and humans/) (3,957,888)
-
21 not 22 (2336)
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP): up to 2013/10/01; MEDLINE Daily Update (OvidSP): up to 2013/10/01
Searched: 11 October 2013
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs).ti,ab,ot. (32)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni).ti,ab,ot. (9)
-
((troponin t or tnt or ctnt or tropt or trop t) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (62)
-
((troponin I or tni or ctni or tropI or trop I) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (29)
-
(troponin$ adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (99)
-
(troponin$ adj5 (architect or elecsys or accutni or accu-tni or access or unicel)).ti,ab,hw,ot. (3)
-
or/1-6 (125)
-
troponin t/ or troponin I/ or (60304-72-5 or 77108-40-8).rn. (5)
-
(sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive).ti,ab,ot. (388,942)
-
8 and 9 (3)
-
7 or 10 (127)
-
chest pain/ (13)
-
((chest or thorax or thoracic) adj2 (pain$ or discomfort or tight$ or pressure)).ti,ab,ot,hw. (1742)
-
exp myocardial ischemia/ (170)
-
(acute adj2 coronary adj2 syndrome$).ti,ab,ot. (1544)
-
(preinfarc$ Angina$ or pre infarc$ Angina$).ti,ab,ot. (3)
-
Unstable angina$.ti,ab,ot. (378)
-
((heart$ or myocardi$ or cardiac or coronary) adj2 (preinfarc$ or infarc$ or attack$ or arrest$ or occlusion$ or isch?emia$)).ti,ab,ot. (8220)
-
(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI).ti,ab,ot. (4224)
-
or/12-19 (12,386)
-
11 and 20 (76)
-
animals/ not (animals/ and humans/) (1462)
-
21 not 22 (76)
EMBASE (OvidSP): 1974 to 2013/10/10
Searched: 11 October 2013
-
“high sensitivity cardiac troponin T”/ or high sensitivity troponin t assay/ (12)
-
“high sensitivity cardiac troponin I”/ or high sensitivity troponin i assay/ (3)
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs).ti,ab,ot. (565)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni).ti,ab,ot. (190)
-
((troponin t or tnt or ctnt or tropt or trop t) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (1052)
-
((troponin I or tni or ctni or tropI or trop I) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (598)
-
(troponin$ adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (1478)
-
(troponin$ adj5 (architect or elecsys or accutni or accu-tni or access or unicel)).ti,ab,hw,ot. (106)
-
or/1-8 (2142)
-
troponin t/ or troponin I/ or (60304-72-5 or 77108-40-8).rn. (18,661)
-
(sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive).ti,ab,ot,hw. (6,591,905)
-
10 and 11 (9505)
-
9 or 12 (10,097)
-
thorax pain/ (44,504)
-
((chest or thorax or thoracic) adj2 (pain$ or discomfort or tight$ or pressure)).ti,ab,ot,hw. (64,208)
-
acute coronary syndrome/ (24,295)
-
(acute adj2 coronary adj2 syndrome$).ti,ab,ot,hw. (34,428)
-
exp heart muscle ischemia/ (73,551)
-
exp heart infarction/ (266,027)
-
exp Unstable-Angina-Pectoris/ (16,552)
-
(preinfarc$ Angina$ or pre infarc$ Angina$).ti,ab,ot,hw. (374)
-
Unstable angina$.ti,ab,ot. (14,593)
-
((heart$ or myocardi$ or cardiac or coronary) adj2 (preinfarc$ or infarc$ or attack$ or arrest$ or occlusion$ or isch?emia$)).ti,ab,ot,hw. (406,203)
-
(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI).ti,ab,ot,hw. (85,655)
-
or/14-24 (498,902)
-
13 and 25 (6007)
-
animal/ (1,890,932)
-
animal experiment/ (1,720,343)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (5,825,865)
-
or/27-29 (5,825,865)
-
exp human/ (15,014,990)
-
human experiment/ (317,206)
-
or/31-32 (15,016,431)
-
30 not (30 and 33) (4,642,837)
-
26 not 34 (5642)
-
limit 35 to yr=“2005 -Current” (4374)
-
remove duplicates from 36 (4282)
Cochrane Database of Systematic Reviews (CDSR) (Wiley), Issue 10/October, up to 2013/10/11; Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley), Issue 9/September, 2013; Database of Abstracts of Reviews of Effects (DARE) (Wiley), Issue 3/July, 2013; Health Technology Assessment Database (HTA) (Wiley), Issue 3/July:2013; NHS Economic Evaluation Database (NHS EED) (Wiley), Issue 3/July, 2013
Searched: 11 October 2013
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs):ti,ab,kw (5)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni):ti,ab,kw (5)
-
((troponin t or tnt or ctnt or tropt or trop t) near/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive)):ti,ab,kw (12)
-
((troponin I or tni or ctni or tropI or trop I) near/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive)):ti,ab,kw (10)
-
(troponin* near/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive)):ti,ab,kw (27)
-
(troponin* near/5 (architect or elecsys or accutni or accu-tni or access or unicel)):ti,ab,kw (2)
-
#1 or #2 or #3 or #4 or #5 or #6 (42)
-
MeSH descriptor: [Troponin T] this term only (265)
-
MeSH descriptor: [Troponin I] this term only (309)
-
#8 or #9 (543)
-
(sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive):ti,ab,kw (170,016)
-
#10 and #11 (236)
-
#7 or #12 (249)
-
MeSH descriptor: [Chest Pain] this term only (335)
-
((chest or thorax or thoracic) near/2 (pain* or discomfort or tight* or pressure)):ti,ab,kw (1793)
-
(acute near/2 coronary near/2 syndrome*):ti,ab,kw (1678)
-
MeSH descriptor: [Myocardial Ischemia] explode all trees (20,427)
-
(preinfarc* Angina* or pre infarc* Angina*):ti,ab,kw (90)
-
(Unstable angina*):ti,ab,kw (1818)
-
((heart* or myocardi* or cardiac or coronary) near/2 (preinfarc* or infarc* or attack* or arrest* or occlusion* or isch?emia*)):ti,ab,kw (16,156)
-
(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI):ti,ab,kw (4740)
-
#14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 (28,923)
-
#13 and #22 from 2005 to 2013 (114)
CDSR search retrieved 0 references; CENTRAL search retrieved 108 references; DARE search retrieved 2 references; HTA search retrieved 1 references; NHS EED search retrieved 3 references.
Science Citation Index – Expanded (SCI) (Web of Science): 1970–2013/10/14; Conference Proceedings Citation Index (CPCI-S) (Web of Science): 1990–2013/10/14
Searched: 14 October 2013
Databases = SCI-EXPANDED, CPCI-S, CCR-EXPANDED, IC Timespan = 2005–13
-
228 TS=(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs)
-
90 TS=(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni)
-
1438 TS=((troponin* or tnt or ctnt or tropt or tni or ctni or tropI or “trop t” or “trop I”) NEAR/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or “high performance” or ultrasensitive))
-
1470 #3 OR #2 OR #1
-
13,963 TS=((chest or thorax or thoracic) NEAR (pain* or discomfort or tight* or pressure))
-
19,298 TS=(acute NEAR/2 coronary NEAR/2 syndrome*)
-
393 TS=(preinfarc* angina* or pre infarc* angina)
-
5481 TS=unstable angina*
-
115,395 TS=((heart* or myocard* or cardiac or coronary) NEAR/2 (preinfarc* or infarc* or attack* or arrest* or occlusion* or isch?emia*))
-
40,133 TS=(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI)
-
155,342 #10 OR #9 OR #8 OR #7 OR #6 OR #5
-
835 #11 AND #4
Latin American and Caribbean Health Sciences (LILACS): 1982–2013/09/24 (http://regional.bvsalud.org/php/index.php?lang=en)
Searched: 14 October 2013
| Terms | Records |
|---|---|
| (Troponin$ or MH:D05.750.078.730.825.925 or MH:D12.776.210.500.910.925 or MH:D12.776.220.525.825.925 or MH:D05.750.078.730.825.962 or MH:D12.776.210.500.910.962 or MH:D12.776.220.525.825.962 or MH:D05.750.078.730.825 or MH:D12.776.210.500.910 or MH:D12.776.220.525.825 or Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs or Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni) | 247 |
| Total | 247 |
International Network of Agencies for Health Technology Assessment (INAHTA): up to 2013/10/15 (www.inahta.org/Search2/?pub=1)
Searched: 15 October 2013
| Search term | Results |
|---|---|
| Troponin | 9 |
| Elecsys | 2 |
| Architect | 0 |
| Accutni | 0/1 |
| unicel | 0 |
| Total | 11 |
BIOSIS Previews (Web of Knowledge): 1956–2013/10/11
Searched: 14 October 2013
-
Databases=BIOSIS Previews Timespan=2005-2013
-
266 TS=(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs)
-
114 TS=(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni)
-
1055 TS=((troponin* or tnt or ctnt or tropt or tni or ctni or tropI or “trop t” or “trop I”) NEAR/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or “high performance” or ultrasensitive))
-
1095 #3 OR #2 OR #1
-
7468 TS=((chest or thorax or thoracic) NEAR (pain* or discomfort or tight* or pressure))
-
11,149 TS=(acute NEAR/2 coronary NEAR/2 syndrome*)
-
196 TS=(preinfarc* angina* or pre infarc* angina)
-
3025 TS=unstable angina*
-
62,717 TS=((heart* or myocard* or cardiac or coronary) NEAR/2 (preinfarc* or infarc* or attack* or arrest* or occlusion* or isch?emia*))
-
28,931 TS=(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI)
-
83,999 #10 OR #9 OR #8 OR #7 OR #6 OR #5
-
628 #11 AND #4
National Institute for Health Research Health technology Assessment (Internet) (www.hta.ac.uk/) up to 2013/10/14
Searched: 14 October 2013
Browsed with Troponin terms – six results.
Aggressive Research Intelligence Facility (Internet): 1996–2013/10/16 (www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/databases/index.aspx)
Searched: 16 October 2013
| Search terms | Quick search |
|---|---|
| Troponin* | 21 |
| Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs | 0 |
| Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni | 0 |
| Total | 21 |
Medion database: up to 2013/10/16 (www.mediondatabase.nl/)
Searched: 16 October 2013
Searched: in ‘Whole Database’
| Search term in ‘topics’ | Results |
|---|---|
| Troponin | 0 |
| Troponins | 0 |
| Total | 0 |
PROSPERO (International Prospective Register of Systematic Reviews) (Internet): up to 2013/10/10 (www.crd.york.ac.uk/prospero/)
Searched: 10 October 2013
Searched: in ‘All fields’
| Terms | Records |
|---|---|
| Troponin* | 8 |
| Total | 8 |
Clinicaltrials.gov (Internet) (http://clinicaltrials.gov/ct2/search/advanced)
Searched: 14 October 2013
Advanced search option – search terms box.
| Search terms | Condition | Intervention | Records |
|---|---|---|---|
| Troponin% AND (sensitiv% OR hs OR early OR initial OR rapid OR present% OR ultra OR high performance OR ultrasensitive OR elecsys OR architect OR accutni OR access OR unicel) | 186 | ||
| Troponin% | 109 | ||
| (Hstnt OR hs-tnt OR hsctnt Or hs-ctnt OR tnt-hs OR tnths OR ctnths OR ctnt-hs OR Hstni OR hs-tni OR hsctni OR hs-ctni OR tni-hs OR tnihs OR ctnihs OR ctni-hs OR ctni-ultra OR accutni OR accu-tni) | 17 | ||
| Total | 312 |
metaRegister of Controlled Trials (mRCT) (Internet) (www.controlled-trials.com/)
Searched: 10 October 2013
| Search terms | Results |
|---|---|
| (troponin* AND (sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive)) | 333 |
| Total | 333 |
WHO International Clinical Trials Registry Platform (ICTRP) (Internet) (www.who.int/ictrp/en/)
Searched: 10 October 2013
Advanced search option
Date of registration limited to 01/01/2005 to 10/10/2013
| Title | Condition | Intervention | Records |
|---|---|---|---|
| Troponin OR Troponins | 67 | ||
| Troponins | 2 | ||
| Troponin | This search does not work – the results are irrelevant and do not contain the word troponin in the intervention field | ||
| Total | 69 |
American Heart Association: Scientific Sessions (http://my.americanheart.org/professional/Sessions/ScientificSessions/Archive/Archive-Scientific-Sessions_UCM_316935_SubHomePage.jsp)
Searched: 29 October 2013
2013: Conference not yet taken place at time of searching
2012: http://circ.ahajournals.org/content/vol126/21_MeetingAbstracts
2011: http://circ.ahajournals.org/content/vol124/21_MeetingAbstracts
2010: http://circ.ahajournals.org/content/vol122/21_MeetingAbstracts
2009: http://circ.ahajournals.org/content/120/21/2152.full.pdf
| Keyword | 2013 | 2012 | 2011 | 2010 | 2009 | Total |
|---|---|---|---|---|---|---|
| Troponin* | N/A | 138 | 131 | 109 | 1 | 379 |
American Association for Clinical Chemistry (www.aacc.org/resourcecenters/meet_abstracts_archive/abstracts_archive/annual_meeting/Pages/default.aspx#)
Searched: 29 October 2013
2013 Abstracts from: Clinical Chemistry, 59(S10):A1–295
www.aacc.org/events/Annual_Meeting/abstracts/Documents/AACC_13_AbstractBook_Complete.pdf
2012 Abstracts from: Clinical Chemistry, 58(S10):a1–A264
www.aacc.org/events/annualmtgdirectory/Documents/AACC_12_AbstractBook-Final-Complete.pdf
2011 Abstracts from: Clinical Chemistry, 57 (S10): A1–A235
www.aacc.org/events/annualmtgdirectory/documents/AACC_11_FullAbstract.pdf
2010 Abstracts from: Clinical Chemistry, 57 (6 Suppl): A1–276
www.aacc.org/events/annualmtgdirectory/Pages/2010PosterAbstracts.aspx#
2009 19–23 July, Chicago, IL, www.abstractsonline.com/viewer/searchAdvanced.asp?MKey={CA6D749E-BE20-4F85-899B-8A84E2268F72}&AKey={B08F832C-9D23-4F0B-96C3-3FA22F3D94A1}
| Keyword | 2013 | 2012 | 2011 | 2010 | 2009 | Totals |
|---|---|---|---|---|---|---|
| Troponin | 48 | 21 | 32 | 40 | 29 | 170 |
European Society of Cardiology (http://spo.escardio.org/abstract-book/search.aspx)
Searched: 29 October 2013
| Keyword | 2013 | 2012 | 2011 | 2010 | 2009 | Total |
|---|---|---|---|---|---|---|
| Troponin | 52 | 51 | 61 | 51 | 25 | 240 |
| Troponins | 2 | 1 | 2 | 1 | 2 | 8 |
Additional searches
Results sorted by Link Ranking (www.ncbi.nlm.nih.gov/pubmed/)
Searched: 10 December 2013
Nine of the included publications were not indexed on PubMed. Indexed publications were checked for errata and comments. For each reference, the first 20 references were retrieved by carrying out a Related Citations search using PubMed’s similarity matching algorithm. These records were downloaded for screening. All related citations were checked against the EndNote Library to remove duplicates, and only new unique references were imported and screened = 58 records.
| Reference | PMID | Result retrieved |
|---|---|---|
| Santalo40 | 23764266 | 20/131 |
| Aldous41 | 22109535 | 20/145 |
| Sanchis42 | 22877804 | 20/203 |
| Haaf43 | 22623715 | 20/203 |
| Eggers44 | 22456003 | 20/145 |
| Reiter45 | 22044927 | 20/280 |
| Aldous46 | 22291171 | 20/277 |
| Potocki47 | 22337952 | 20/304 |
| Keller48 | 22203537 | 20/300 |
| Meune108 | 22014790 | 20/252 |
| Freund49 | 21663627 | 20/142 |
| Aldous50 | 21784766 | 20/254 |
| Melki51 | 21428843 | 20/210 |
| Reichlin52 | 21709058 | 20/162 |
| Reiter53 | 21362702 | 20/261 |
| Aldous54 | 21441390 | 20/251 |
| Kurz55 | 20852870 | 20/207 |
| Hochholzer56 | 21138939 | 20/138 |
| Christ57 | 20932502 | 20/201 |
| Parsonage58 | Not in PubMed | |
| Collinson59 | Not in PubMed | |
| Body60 | Not in PubMed | |
| Melki61 | Not in PubMed | |
| Aldous62 | Not in PubMed | |
| Cullen63 | 23583250 | 20/133 |
| Sebbane64 | 23816196 | 20/131 |
| Irfan65 | 23870791 | 20/134 |
| Collinson19 | 23597479 | 20/275 |
| Reiter66 | 23514979 | 20/155 |
| Body67 | 21920261 | 20/192 |
| Aldous68 | 21441393 | 20/174 |
| Keller69 | Not in PubMed | |
| Collinson58 | Not in PubMed | |
| Saenger70 | Not in PubMed | |
| Lippi73 | Not in PubMed | |
| Hoeller39 | 23604180 | 20/107 |
| Total | 640 | |
| Following duplicate removal, number of records screened | 58 | |
Cost-effectiveness searches
MEDLINE (OvidSP): 1946 to 2013/10/Week 1
Searched: 18 October 2013
-
economics/ (27,116)
-
exp “costs and cost analysis”/ (182,544)
-
economics, dental/ (1866)
-
exp “economics, hospital”/ (19,403)
-
economics, medical/ (8578)
-
economics, nursing/ (3879)
-
economics, pharmaceutical/ (2605)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (427,344)
-
(expenditure$ not energy).ti,ab. (17,552)
-
(value adj1 money).ti,ab. (22)
-
budget$.ti,ab. (17,208)
-
or/1-11 (551,693)
-
((energy or oxygen) adj cost).ti,ab. (2752)
-
(metabolic adj cost).ti,ab. (798)
-
((energy or oxygen) adj expenditure).ti,ab. (16,662)
-
or/13-15 (19,503)
-
12 not 16 (547,348)
-
letter.pt. (803,396)
-
editorial.pt. (334,975)
-
historical article.pt. (299,710)
-
or/18-20 (1,423,597)
-
17 not 21 (519,320)
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs).ti,ab,ot. (229)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni).ti,ab,ot. (99)
-
((troponin t or tnt or ctnt or tropt or trop t) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (563)
-
((troponin I or tni or ctni or tropI or trop I) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (349)
-
(troponin$ adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (769)
-
(troponin$ adj5 (architect or elecsys or accutni or accu-tni or access or unicel)).ti,ab,hw,ot. (66)
-
or/23-28 (1215)
-
troponin t/ or troponin I/ or (60304-72-5 or 77108-40-8).rn. (8642)
-
(sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive).ti,ab,ot. (4,878,300)
-
30 and 31 (4209)
-
29 or 32 (4559)
-
chest pain/ (9293)
-
((chest or thorax or thoracic) adj2 (pain$ or discomfort or tight$ or pressure)).ti,ab,ot,hw. (28,602)
-
exp myocardial ischemia/ (357,748)
-
(acute adj2 coronary adj2 syndrome$).ti,ab,ot. (16,495)
-
(preinfarc$ Angina$ or pre infarc$ Angina$).ti,ab,ot. (285)
-
Unstable angina$.ti,ab,ot. (10,718)
-
((heart$ or myocardi$ or cardiac or coronary) adj2 (preinfarc$ or infarc$ or attack$ or arrest$ or occlusion$ or isch?emia$)).ti,ab,ot. (194,088)
-
(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI).ti,ab,ot. (53,168)
-
or/34-41 (444,673)
-
33 and 42 (2503)
-
animals/ not (animals/ and humans/) (3,957,888)
-
43 not 44 (2336)
-
limit 45 to yr=“2005 -Current” (1457)
-
22 and 46 (43)
Costs filter: Centre for Reviews and Dissemination. NHS EED Economics Filter: MEDLINE (Ovid) monthly search York: Centre for Reviews and Dissemination; 2010.
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP): up to 2013/10/01, MEDLINE daily update: up to 2013/10/01
Searched: 18 October 2013
-
economics/ (2)
-
exp “costs and cost analysis”/ (87)
-
economics, dental/ (0)
-
exp “economics, hospital”/ (8)
-
economics, medical/ (0)
-
economics, nursing/ (0)
-
economics, pharmaceutical/ (1)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (39,821)
-
(expenditure$ not energy).ti,ab. (1172)
-
(value adj1 money).ti,ab. (4)
-
budget$.ti,ab. (1822)
-
or/1-11 (41,689)
-
((energy or oxygen) adj cost).ti,ab. (218)
-
(metabolic adj cost).ti,ab. (67)
-
((energy or oxygen) adj expenditure).ti,ab. (911)
-
or/13-15 (1160)
-
12 not 16 (41,354)
-
letter.pt. (24,293)
-
editorial.pt. (14,525)
-
historical article.pt. (68)
-
or/18-20 (38,878)
-
17 not 21 (40,906)
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs).ti,ab,ot. (32)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni).ti,ab,ot. (9)
-
((troponin t or tnt or ctnt or tropt or trop t) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (62)
-
((troponin I or tni or ctni or tropI or trop I) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (29)
-
(troponin$ adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot. (99)
-
(troponin$ adj5 (architect or elecsys or accutni or accu-tni or access or unicel)).ti,ab,hw,ot. (3)
-
or/23-28 (125)
-
troponin t/ or troponin I/ or (60304-72-5 or 77108-40-8).rn. (5)
-
(sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive).ti,ab,ot. (388,942)
-
30 and 31 (3)
-
29 or 32 (127)
-
chest pain/ (13)
-
((chest or thorax or thoracic) adj2 (pain$ or discomfort or tight$ or pressure)).ti,ab,ot,hw. (1742)
-
exp myocardial ischemia/ (170)
-
(acute adj2 coronary adj2 syndrome$).ti,ab,ot. (1544)
-
(preinfarc$ Angina$ or pre infarc$ Angina$).ti,ab,ot. (3)
-
Unstable angina$.ti,ab,ot. (378)
-
((heart$ or myocardi$ or cardiac or coronary) adj2 (preinfarc$ or infarc$ or attack$ or arrest$ or occlusion$ or isch?emia$)).ti,ab,ot. (8220)
-
(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI).ti,ab,ot. (4224)
-
or/34-41 (12,386)
-
33 and 42 (76)
-
animals/ not (animals/ and humans/) (1462)
-
43 not 44 (76)
-
limit 45 to yr=“2005 -Current” (75)
-
22 and 46 (4)
Costs filter: Centre for Reviews and Dissemination. NHS EED Economics Filter: MEDLINE (Ovid) monthly search. York: Centre for Reviews and Dissemination; 2010.
EMBASE (OvidSP): 1974 to 2013/10/17
Searched: 18 October 2013
-
health-economics/ (33,273)
-
exp economic-evaluation/ (205,882)
-
exp health-care-cost/ (197,503)
-
exp pharmacoeconomics/ (169,588)
-
or/1-4 (471,813)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (590,127)
-
(expenditure$ not energy).ti,ab. (23,360)
-
(value adj2 money).ti,ab. (1320)
-
budget$.ti,ab. (23,595)
-
or/6-9 (613,918)
-
5 or 10 (885,833)
-
letter.pt. (844,056)
-
editorial.pt. (449,323)
-
note.pt. (587,506)
-
or/12-14 (1,880,885)
-
11 not 15 (799,169)
-
(metabolic adj cost).ti,ab. (876)
-
((energy or oxygen) adj cost).ti,ab. (3163)
-
((energy or oxygen) adj expenditure).ti,ab. (19,981)
-
or/17-19 (23,208)
-
16 not 20 (794,101)
-
“high sensitivity cardiac troponin T”/ or high sensitivity troponin t assay/ (12)
-
“high sensitivity cardiac troponin I”/ or high sensitivity troponin i assay/ (3)
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs).ti,ab,ot. (571)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni).ti,ab,ot. (193)
-
((troponin t or tnt or ctnt or tropt or trop t) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (1059)
-
((troponin I or tni or ctni or tropI or trop I) adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (602)
-
(troponin$ adj2 (sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive)).ti,ab,ot,hw. (1489)
-
(troponin$ adj5 (architect or elecsys or accutni or accu-tni or access or unicel)).ti,ab,hw,ot. (106)
-
or/22-29 (2155)
-
troponin t/ or troponin I/ or (60304-72-5 or 77108-40-8).rn. (18,726)
-
(sensitiv$ or hs or early or initial or rapid or present$ or ultra or high performance or ultrasensitive).ti,ab,ot,hw. (6,601,404)
-
31 and 32 (9548)
-
30 or 33 (10,144)
-
thorax pain/ (44,662)
-
((chest or thorax or thoracic) adj2 (pain$ or discomfort or tight$ or pressure)).ti,ab,ot,hw. (64,388)
-
acute coronary syndrome/ (24,412)
-
(acute adj2 coronary adj2 syndrome$).ti,ab,ot,hw. (34,558)
-
exp heart muscle ischemia/ (73,666)
-
exp heart infarction/ (266,475)
-
exp Unstable-Angina-Pectoris/ (16,570)
-
(preinfarc$ Angina$ or pre infarc$ Angina$).ti,ab,ot,hw. (374)
-
Unstable angina$.ti,ab,ot. (14,604)
-
((heart$ or myocardi$ or cardiac or coronary) adj2 (preinfarc$ or infarc$ or attack$ or arrest$ or occlusion$ or isch?emia$)).ti,ab,ot,hw. (406,847)
-
(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI).ti,ab,ot,hw. (85,913)
-
or/35-45 (499,787)
-
34 and 46 (6035)
-
animal/ (1,890,937)
-
animal experiment/ (1,721,607)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (5,828,979)
-
or/48-50 (5,828,979)
-
exp human/ (15,032,575)
-
human experiment/ (317,393)
-
or/52-53 (15,034,016)
-
51 not (51 and 54) (4,644,866)
-
47 not 55 (5669)
-
limit 56 to yr=“2005 -Current” (4401)
-
remove duplicates from 57 (4309)
-
21 and 58 (129)
Costs filter: Centre for Reviews and Dissemination. NHS EED Economics Filter: EMBASE (Ovid) weekly search. York: Centre for Reviews and Dissemination; 2010.
NHS Economic Evaluation Database (NHS EED) (Wiley) Issue 3/July:2013
Searched: 11 October 2013
-
(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs):ti,ab,kw (5)
-
(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or) accutni or accu-tni):ti,ab,kw (5)
-
((troponin t or tnt or ctnt or tropt or trop t) near/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive)):ti,ab,kw (12)
-
((troponin I or tni or ctni or tropI or trop I) near/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive)):ti,ab,kw (10)
-
(troponin* near/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive)):ti,ab,kw (27)
-
(troponin* near/5 (architect or elecsys or accutni or accu-tni or access or unicel)):ti,ab,kw (2)
-
#1 or #2 or #3 or #4 or #5 or #6 (42)
-
MeSH descriptor: [Troponin T] this term only (265)
-
MeSH descriptor: [Troponin I] this term only (309)
-
#8 or #9 (543)
-
(sensitiv* or hs or early or initial or rapid or present* or ultra or high performance or ultrasensitive):ti,ab,kw (170,016)
-
#10 and #11 (236)
-
#7 or #12 (249)
-
MeSH descriptor: [Chest Pain] this term only (335)
-
((chest or thorax or thoracic) near/2 (pain* or discomfort or tight* or pressure)):ti,ab,kw (1793)
-
(acute near/2 coronary near/2 syndrome*):ti,ab,kw (1678)
-
MeSH descriptor: [Myocardial Ischemia] explode all trees (20,427)
-
(preinfarc* Angina* or pre infarc* Angina*):ti,ab,kw (90
-
(Unstable angina*):ti,ab,kw (1818)
-
((heart* or myocardi* or cardiac or coronary) near/2 (preinfarc* or infarc* or attack* or arrest* or occlusion* or isch?emia*)):ti,ab,kw (16,156)
-
(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI):ti,ab,kw (4740)
-
#14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 (28,923)
-
#13 and #22 from 2005 to 2013 (114)
NHS EED search retrieved three references.
Health Economic Evaluation Database (HEED) (Internet): up to 2013/10/18 (http://onlinelibrary.wiley.com/book/10.1002/9780470510933)
Searched: 18 October 2013
Compound search, (all data), unable to limit by date
Troponin*
AND
sensitiv* OR hs OR early OR initial OR rapid OR present OR ultra OR high performance OR ultrasensitive OR elecsys OR architect OR accutni OR access OR unicel
N=20
Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs or Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra
N=0
Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni
N=0
EconLit (EBSCO) 1990–2013/09/01
Searched: 18 October 2013
Search modes – Boolean/Phrase
S1 TX Troponin* (0)
S2 TX Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs (0)
S3 TX Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni (0)
Science Citation Index Expanded (SCI) (Web of Science): 1970–2013/10/21, Conference Proceedings Citation Index (CPCI-S) (Web of Science): 1990–2013/10/21
Searched: 21 October 2013
-
622,444 TS=(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or budget*)
-
10,144 TS=(expenditure* not energy)
-
952 TS=(value NEAR money)
-
626,873 #3 OR #2 OR #1
-
22,383 TS=((energy or oxygen) NEAR cost)
-
1804 TS=(metabolic NEAR cost)
-
12,974 TS=((energy or oxygen) NEAR expenditure)
-
35,684 #7 OR #6 OR #5
-
602,398 #4 NOT #8
-
230 TS=(Hstnt or hs-tnt or hsctnt or hs-ctnt or tnt-hs or tnths or ctnths or ctnt-hs)
-
91 TS=(Hstni or hs-tni or hsctni or hs-ctni or tni-hs or tnihs or ctnihs or ctni-hs or ctni-ultra or accutni or accu-tni)
-
1442 TS=((troponin* or tnt or ctnt or tropt or tni or ctni or tropI or “trop t” or “trop I”) NEAR/2 (sensitiv* or hs or early or initial or rapid or present* or ultra or “high performance” or ultrasensitive))
-
1474 #12 OR #11 OR #10
-
14,001 TS=((chest or thorax or thoracic) NEAR (pain* or discomfort or tight* or pressure))
-
19,324 TS=(acute NEAR/2 coronary NEAR/2 syndrome*)
-
393 TS=(preinfarc* angina* or pre infarc* angina)
-
5486 TS=unstable angina*
-
115,562 TS=((heart* or myocard* or cardiac or coronary) NEAR/2 (preinfarc* or infarc* or attack* or arrest* or occlusion* or isch?emia*))
-
40,195 TS=(MI or ACS or STEMI or NSTE-ACS or NSTEACS or nonSTEMI or NSTEMI or AMI or UAP or OMI)
-
155,582 #19 OR #18 OR #17 OR #16 OR #15 OR #14
-
839 #20 AND #13
-
32 #21 AND #9
Databases = SCI-EXPANDED, CPCI-S, CCR-EXPANDED, IC Timespan = 2005–2013.
Research Papers in Economics (REPEC) up to 2013/10/21 (http://econpapers.repec.org/scripts/search/search.asp?pg=-1)
Searched: 21 October 2013
Advanced search
| Free text search | Results | Total |
|---|---|---|
| Troponin | 0/2 | 0 |
| Troponins | 0/1 | 0 |
Appendix 2 Data extraction tables
Baseline study details
| Study details | Selection criteria | Participant details | Test manufacturer |
|---|---|---|---|
| Aldous (2012)41,46,50 Country: New Zealand Funding: Funded by the National Heart Foundation of New Zealand and assay reagents were provided by the manufacturer (Roche). One author declared personal funding from Abbott Recruitment: November 2007 to December 2010 Number of participants: 939,46 38541 |
Inclusion criteria: Adults (≥ 18 years) with symptoms suggestive of cardiac ischaemia (acute chest, epigastric, neck, jaw or arm pain or discomfort or pressure without an apparent non-cardiac source) Exclusion criteria: ST segment elevation on ECG;46 unable to provide informed consent; would not be available to follow-up Patient category: NSTEMI46 Mixed41 |
Median age (IQR), years: 65 (56–76) Male (%): 60 White (%): 89 Previous CAD (%): 52 Previous family history (%): 60 Previous revascularisation (%): 30 Diabetes (%): 17 Smoking (%): 61 Hypertension (%): 61 Dyslipidaemia (%): 58 Median BMI (IQR), kg/m2: 28 (25–31) Median (IQR) time to presentation (hours): 6.3 (3.3–13.3) |
Roche |
| Aldous (2011)54,62,68 Country: New Zealand Funding: Manufacturers (Roche and Abbott) supplied assays. The study was funded by a New Zealand National Heart Foundation grant Recruitment: November 2006 to April 2007 Number of participants: 332 |
Inclusion criteria: Consecutive patients presenting to the ED with chest pain; participants were eligible for inclusion if the attending clinician had sufficient suspicion of ACS that serial Tns and ECGs were considered necessary Exclusion criteria: < 18 years; samples not stored for both time points (on admission and at 6–24 hours) Patient category: Mixed |
Median age (IQR), years: 64 (53–74) Male (%): 60 White (%): 85 Previous CAD (%): 54 Previous family history (%): 40 Diabetes (%): 16 Smoking (%): 45 Hypertension (%): 46 Dyslipidaemia (%): 38 Median (IQR) time to presentation (hours): 4.0 (2.0 to 8.6) |
Roche |
| Body (2011)60,67,74 Country: UK Funding: Central Manchester NHS Trust Recruitment: January 2006 to February 2007 Number of participants eligible (enrolled): 1004 (703) |
Inclusion criteria: Presenting to ED with chest pain; age > 25 years and chest pain within previous 24 hours that initial treating physician suspected may be cardiac in nature Exclusion criteria: Renal failure requiring dialysis, trauma with suspected myocardial contusion, or another medical condition mandating hospital admission or if they did not consent to and provide a blood sample for use by the research team Patient category: Mixed |
Mean age (SD), years: 59 (14) Male (%): 61 Kidney disease (%): 1 Previous AMI (%): 24 Previous family history (%): 48 Previous revascularisation (%): 20 Diabetes (%): 18 Smoking (%): 31 Dyslipidaemia (%): 48 Median time to presentation (hours): 3.5 |
Roche |
| Christ (2010)57 Country: Germany Funding: hs-cTnT test kits were provided by Roche Recruitment: 7 September 2009 to 21 September 2009 Number of participants: 137 |
Inclusion criteria: Consecutive patients with acute chest pain of possible coronary origin presenting to the emergency department Exclusion criteria: NR Patient category: Mixed |
Mean age (SD), years: 66 (16) Male (%): 64 Previous AMI (%): 32 Previous CAD (%): 34 Previous family history (%): 12 Previous revascularisation (%): 24 Diabetes (%): 22 Smoking (%): 22 Hypertension (%): 66 Dyslipidaemia (%): 35 Mean BMI (SD), kg/m2: 28 (5) Time to presentation (hours): 0–2, 36%; 2–6, 22%; 6–24, 33%; > 24, 20% |
Roche |
| Collinson (2013)19,58,59 Country: UK Funding: UK Health Technology Assessment programme Study name: Point-of-care arm of the RATPAC study Recruitment: February 2007 to June 2008 Number of participants: 850 |
Inclusion criteria: Patients presenting to the ED with chest pain attributable to suspected, but not proven, AMI Exclusion criteria: ECG changes diagnostic for AMI or high-risk ACS (> 1 mm ST deviation, or > 3 mm inverted T waves); known CAD with prolonged (> 1 hour) or recurrent typical cardiac-type pain; proven or suspected serious non-cardiac pathology (e.g. pulmonary embolism); comorbidity or social problems requiring hospital admission even if AMI ruled out; obvious non-cardiac cause of chest pain (e.g. pneumothorax or muscular pain); presentation > 12 hours after most significant episode of pain Patient category: NSTEMI |
Median age (IQR), years: 54 (44 to 64) Male (%): 60 Previous AMI (%): 40 Previous family history (%): Previous revascularisation (%): 1 Diabetes (%): 8 Smoking (%): 28 Hypertension (%): 35 Dyslipidaemia (%): 24 Median (IQR) time to presentation (hours): 8.25 (5.17 to 12.30) |
Roche |
| Cullen (2013)63 Countries: New Zealand and Australia Funding: The manufacturers (Abbott, Roche and Siemens) provided partial funding Study name: ADAPT study (ACTRN12611001069943) Recruitment: November 2007 to February 2011 Number of participants: 1635 |
Inclusion criteria: Prospectively recruited adults with at least 5 minutes of possible cardiac symptoms in accordance with the AHA case definitions (acute chest, epigastric, neck, jaw or arm pain; or discomfort or pressure without a clear non-cardiac source) Exclusion criteria: Pregnancy; unable or unwilling to consent; recruitment inappropriate (e.g. terminal illness); transfer from another hospital; follow-up considered impossible (e.g. homeless patients) Patient category: Mixed |
Mean age (SD), years: 59 (13) Male (%): 60 Previous AMI (%): 24 Previous family history (%): 57 Previous revascularisation (%): 8 Diabetes (%): 15 Smoking (%): 18 Hypertension (%): 52 Dyslipidaemia (%): 57 Mean (SD) time to presentation (hours): 22.3 (60.5) |
Abbott |
| Eggers (2012)44 Country: Sweden Funding: Swedish Society of Medicine and the Selander Foundation Study name: FASTER 1-study and FAST II study Recruitment: May 2000 (FAST II), October 2002 (FASTER I) to March 2001 (FAST II), August 2003 (FASTER I) Number of participants eligible (enrolled): 495 (360) |
Inclusion criteria: Chest pain with ≥ 15-minute duration within the last 24 hours (FAST II-study), or the last 8 hours (FASTER I-study). Analysis restricted to patients with symptom onset < 8 hours Exclusion criteria: ST segment elevation on the admission 12-lead ECG, leading to immediate reperfusion therapy or its consideration was used as exclusion criterion Patient category: NSTEMI |
Median age (IQR), years: 67 (58–76) Male (%): 66 Previous AMI (%): 38 Previous revascularisation (%): 18 Diabetes (%): 18 Smoking (%): 18 Hypertension (%): 43 Dyslipidaemia (%): 38 Delay < 4 hours (%): 40 |
Roche |
| Freund (2011)49,71 Country: France Funding: Assay kits for the study were provided by the manufacturers (Roche) Recruitment: August 2005 to January 2007 No. of participants: 317 |
Inclusion criteria: Consecutive adults (> 18 years) presenting to the ED with chest pain suggestive of ACS (onset or peak within the previous 6 hours) Exclusion criteria: Patients with acute kidney failure requiring dialysis were excluded Patient category: Mixed (13 were STEMI and 32 NSTEMI) |
Mean (SD): 57 (17) Male (%): 65 Previous CAD (%): 26 Previous family history (%): 32 Diabetes (%): 14 Smoking (%): 40 Hypertension (%): Dyslipidaemia (%): 36 |
Roche |
| Hoeller (2011)39,43,45,47,52,53,56,63,65,66,72 Countries: Switzerland, Spain, USA and Germany Funding: Swiss National Science Foundation, Swiss Heart Foundation, Department of Internal Medicine of the University Hospital Basel, Roche, Siemens, Abbott, Brahms, nanosphere, and 8sense Study name: APACE trial (NCT00470587) Recruitment: April 2006 to August 2011 Number of participants: 2245 |
Inclusion criteria: Consecutive adults presenting to the ED with symptoms suggestive of AMI (e.g. acute chest pain, angina pectoris at rest, other thoracic sensations) within an onset or peak within the last 12 hours Exclusion criteria: Terminal kidney failure requiring dialysis Patient category: Mixed |
Median age (IQR), years: 62 (50–75) Male (%): 69 Previous AMI (%): 24 Previous CAD (%): 34 Previous family history (%): 43 Previous revascularisation (%): 24 Diabetes (%): 18 Smoking (%): 61 Hypertension (%): 64 Dyslipidaemia (%): 45 Median BMI (IQR), kg/m2: 27 (24–30) Presenting < 3 hours from symptom onset (%): 24 |
Roche, Abbott, Beckman Coulter |
| Keller (2011)48,69 Country: Germany Funding: Abbott Diagnostics provided study funding Recruitment: January 2007 to December 2008 Number of participants: 1818 |
Inclusion criteria: Consecutive adults (18–85 years) presenting to three chest pain units with chest pain suggestive of ACS Exclusion criteria: Major surgery or trauma within the previous 4 weeks; pregnancy; intravenous drug abuse; anaemia (haemoglobin < 10 g/dl) Patient category: Mixed |
Mean age (SD), years: 61 (14) Male (%): 66 Previous CAD (%): 36 Previous family history (%): 32 Diabetes (%): 16 Smoking (%): 24 Hypertension (%): 74 Dyslipidaemia (%): 73 Mean BMI (SD), kg/m2: 28 (5) |
Abbott |
| Kurz (2011)55 Country: Germany Funding: Investigators were supported by Roche diagnostics and assay kits were also provided by the manufacturer Recruitment: May 2008 to December 2008 Number of participants: 94 |
Inclusion criteria: Consecutive patients admitted to a chest pain unit with symptoms suggestive of ACS Exclusion criteria: ST segment elevation; severe kidney dysfunction (glomerular filtration rate < 60 ml/minute/1.73 m2); patients undergoing percutaneous coronary intervention during follow-up sampling Patient category: NSTEMI |
Mean age (SD), years: 66 (11) Male (%): 71 Previous AMI (%): 37 Previous CAD (%): 50 Previous family history (%): 32 Previous revascularisation (%): 17 Diabetes (%): 31 Smoking (%): 22 Hypertension (%): 78 Dyslipidaemia (%): 65 Median symptom onset (IQR, minutes): 358 (152–929) BMI (95% CI/range/IQR): 28 (4) |
Roche |
| Lippi (2012)73 Country: Italy Funding: NR Recruitment: NR Conference abstract only Number of participants: 57 |
Inclusion criteria: Consecutive patients presenting to the ED with chest pain, within 3 hours of the onset of pain Exclusion criteria: None reported Patient category: Mixed |
No participant details reported | Beckman |
| Melki (2011)51,61 Country: Sweden Funding: Partially supported by a grant from Roche Diagnostics, who also provided reagents. Also supported by the Swedish Heart and Lung Foundation and National Board of Health and Welfare Recruitment: August 2006 to January 2008 Number of participants: 233 |
Inclusion criteria: Patients admitted to a coronary care unit with chest pain or other symptoms suggestive of ACS within 12 hours of admission Exclusion criteria: Patients with persistent ST segment elevation Patient category: NSTEMI |
Median age (IQR), years: 65 (55–76) Male (%): 67 Previous AMI (%): 30 Previous revascularisation (%): 21 Diabetes (%): 23 Smoking (%): 17 Hypertension (%): 50 Mean symptom onset (95% CI/range/IQR, hours): 5 (3–8) |
Roche |
| Parsonage (2013)58 Country: Australia Funding: NR Recruitment: NR Conference abstract only Number of participants: 737 |
Inclusion criteria: Patients with symptoms of possible ACS Exclusion criteria: None reported Patient category: Mixed |
Mean age (IQR): 54 (44–65) Male (%): 60 |
Abbott, Roche |
| Saenger (2010)70 Country: USA Funding: Two authors declared individual funding from manufacturers (one from Roche diagnostics and one from Beckman Coulter and Abbott) Recruitment: NR Conference abstract only Number of participants: 288 |
Inclusion criteria: Patients presenting to the ED with symptoms suggestive of AMI Exclusion criteria: None reported Patient category: Mixed Details: NSTEMI 19%, STEMI 15% |
No further participant details reported | Roche |
| Sanchis (2012)42 Country: Spain Funding: Supported by a grant from Roche Diagnostics Study name: PITAGORAS study Recruitment: NR Number of participants: 446 |
Inclusion criteria: Patients presenting to the ED with chest pain of possible coronary origin and onset of pain within the previous 24 hours Exclusion criteria: Exclusion criteria: persistent ST segment elevation on ECG; Tn elevation in any of two serial determinations (at arrival and 6–8 hours later); prior diagnosis of ischaemic heart disease by either the finding of significant stenosis in a prior coronary angiogram or previously documented AMI; left bundle branch block or other non-interpretable ECG or inability to performance exercise test; structural heart disease different from ischaemic heart disease; concomitant HF or significant bradyarrhythmia (< 55 beats/minute) or tachyarrhythmia (> 110 beats/minute) at admission Patient category: NSTEMI |
Mean age (SD), years: 60 (12) Male (%): 59 Previous family history (%): 14 Diabetes (%): 20 Smoking (%): 25 Hypertension (%): 54 Dyslipidaemia (%): 46 |
Roche |
| Santaló (2013)40 Country: Spain Funding: Reagents and logistical support were provided by Roche diagnostics Study name: TUSCA study Recruitment: NR Number of participants: 358 |
Inclusion criteria: Adult (> 18 years) described as presenting with acute coronary syndromes and symptom duration ≥ 5 minutes; population included 174 people with a final diagnosis of non-acute coronary syndromes Exclusion criteria: Exclusion criteria: ST segment elevation; new left bundle branch block; pre-admission thrombolytic therapy; defibrillation or cardioversion before sampling; pregnancy; renal failure requiring dialysis; UA within 2 months; coronary artery bypass graft within 3 months Patient category: NSTEMI |
Mean age (range), years: 69 (27–93) Male (%): 68 Previous CAD (%): 35 Diabetes (%): 26 Hypertension (%): 62 Presentation within 3 hours: 46.2% |
Roche |
| Sebbane (2013)64 Country: France Funding: Study funded by the hospital, with assay reagents supplied by the manufacturers Recruitment: December 2009 to November 2011 Number of participants: 248 |
Inclusion criteria: Adults presenting to the ED with chest pain of recent (within 12 hours of presentation) Exclusion criteria: Traumatic causes of chest pain. STEMI was defined by the persistent elevation of the ST segment of at least 1 mm in two contiguous ECG leads or by the presence of a new left bundle branch block with positive cardiac enzyme results. Patients with STEMI were excluded from the analysis for our review Patient category: NSTEMI (data also reported for mixed AMI but not extracted) |
Median age (IQR), years: 61 (48–75) Male (%): 63 |
Roche |
Index test and reference standard details
| Study details | High-sensitivity Tn details (ng/l) | Reference standard details | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Manufacturer | LoD | 99th centile | CV | Target condition | Time frame | Reference standard | Standard Tn | Observer | |
| Aldous (2012) 41 , 46 , 50 | Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 13 | NSTEMI | NR | ACC109 | Conventional Tns were measured using Abbott Diagnostics TnI (LoD 10 ng/l, 99th centile 28 ng/l, CV < 10% at 32 ng/l, decision threshold 30 ng/l) Timing: On presentation, and at 2 hours and 6–12 hours |
Diagnoses on admission and at follow-up were independently adjudicated by one cardiologist, who was blinded to hs-cTnT results |
| Aldous (2011) 54 , 62 , 68 | Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 13 | AMI | NR | Joint ESC, ACC, AHA and WHF8 | Conventional Tns were measured using Abbott Diagnostics TnI 2 (LoD 10 ng/l, 99th centile 28 ng/l, CV < 10% at 32 ng/l) Change (rise or fall) in TnI 2, or no change but no clear alternative cause of Tn elevation, were considered indicative of AMI Timing: On presentation and at follow-up (6–24 hours) |
Final diagnoses were adjudicated independently by cardiologists, blinded to patient history and hs-cTnT |
| Body (2011) 60 , 67 , 74 | Roche Elecsys hs-cTnT | NR | 14 | < 10% at 9 | AMI | 12 hours | Joint ESC, ACC, AHA and WHF8 | Rise or fall of cTnT, or both, above the 99th percentile (10 ng/l) in the appropriate clinical context For patients with modest elevations of cTnT (< 0.1 ng/ml) at baseline, an absolute difference of at least 20 ng/l on serial sampling was considered to represent a significant rise, fall, or both based on the analytical performance of the cTnT assay Timing: At least 12 hours after the onset of the most significant symptoms |
Two independent investigators, who had all clinical, laboratory, and imaging data available for review, but who were blinded to hs-cTnT levels |
| Christ (2010) 57 | Roche Elecsys hs-cTnT | 3 | 14 | < 10% at 13 | AMI | NR | Joint ESC, ACC, AHA and WHF8 | Myocardial necrosis was diagnosed on the basis of a rising and/or falling cTnT pattern > 20% or < 20% compared with the cTnT levels admission) with at least one value above the 99th percentile and an imprecision of < 10% Myocardial necrosis not related to AMI was defined as a typical rise and fall of cTnT levels without clinical evidence of CAD, and cardiac pain without necrosis was defined as a typical patient history and clinical signs of cardiac pain without increased levels of cTnT UA was diagnosed when a patient had normal Tn levels and typical angina at rest or exercise, or a cardiac catheterisation result compatible with the diagnosis cTnT cut-off level of 0.04 µg/l Timing: At presentation and about 6 hours at discretion of physician |
Two independent consultants |
| Collinson (2013) 19 , 58 , 59 | Roche Elecsys hs-cTnT | 3 | 14 | < 10% at 13 | NSTEMI | NR | Joint ESC, ACC, AHA and WHF8 | Conventional Tns were measured using one of the following methods: Siemens cTnI Ultra (LoD 6 ng/l, 99th centile 40 ng/l, CV 10% at 30 ng/l) Abbott cTnI (LoD 10 ng/l, 99th centile 12 ng/l, CV 10% at 32 ng/l) Beckman AccuTnI (LoD 10 ng/l, 99th centile 40 ng/l, CV 10% at 60 ng/l) Roche cTnT (LoD 10 ng/l, 99th centile 10 ng/l, CV 10% at 30 ng/l) Timing: On presentation and at 10–12 hours |
An initial working diagnosis was recorded by the senior ED clinician and reviewed by two independent clinicians; all were blind to hs-cTnT results |
| Cullen (2013) 63 | Abbott ARCHITECT hs-cTnI STAT | 1.2 | 26.2 | < 5% at 26.2 | MACE | 30 days | MACE | NR | Adjudication of all cardiac end points was made by two cardiologists, with consultation of a third cardiologist in case of disagreement |
| Eggers (2012) 44 | Roche Elecsys hs-cTnT | 3 | 14 | < 10% at 13 | NSTEMI | NR | Joint ESC, ACC, AHA and WHF8 | cTnI (Stratus CS: Siemens Healthcare Diagnostics, Deerfield, IL, USA). Non-STEMI defined as: cTnI above the 99th percentile of 0.07 µg/l at least at one measurement together with a ≥ 20% rise and/or fall and an absolute change ≥ 0.05 µg/l within 24 hours To allow for the calculation of relative changes, cTnI was set to 0.02 µg/l (i.e. a concentration below the lowest level of detection) when reported as 0.00 or 0.01 µg/l Timing: Eight time points during the first 24 hours following enrolment |
NR |
| Freund (2011) 49 , 71 | Roche Elecsys hs-cTnT | 3 | 14 | < 10% at 14 | AMI | 30 days | Joint ESC, ACC, AHA and WHF8 | cTnI (Siemens Healthcare Diagnostica Inc., NewarK, USA or Access analyser Beckman Coulter Inc., Brea, USA). Threshold for Siemens assay 140 ng/l, CV 10% Threshold for Beckman assay 60 ng/l, CV 10% Timing: On presentation and at 3–9 hours if needed |
Two independent ED physicians, who were blinded to hs-cTnT results. Disagreements were adjudicated by a third ED physician |
| Hoeller (2011) APACE 39 , 47 , 52 , 53 , 56 , 65 | Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 13 | AMI | NR | Joint ESC, ACC, AHA and WHF8 | Conventional Tns were measured using Roche cTnT fourth generation assay (CV < 10% at 35 ng/l), Beckman Coulter Accu cTnI (CV < 10% at 60 ng/l), or Abbott Axsym cTnI ADV (CV < 10% at 160 ng/l). A positive test was defined as change ≥ 30% of 99th centile or 10% CV level, within 6 to 9 hours Timing: On presentation and at 6–9 hours |
Final diagnoses were adjudicated by two independent cardiologists blind to hsTnT results. When there was disagreement, a third cardiologist was consulted |
| APACE 72 | 2 | ||||||||
| APACE 39 , 65 | Beckman (pre-commercial assay) | 2 | 9 | < 10% at 9 | AMI and NSTEMI | 30 days | |||
| APACE 63 | Abbott ARCHITECT hs-cTnI STAT | 1.2 | 26.2 | < 5% at 26.2 | AMI | 30 days | |||
| APACE 39 | MACE | NA | Adjudication of all cardiac end points was made by two cardiologists, with consultation of a third cardiologist in case of disagreement | ||||||
| Keller (2011) 48 | Abbott ARCHITECT hs-cTnI STAT | 3.4 | 24–30 for this study population | 10% at 5.2 | AMI | 30 days | Joint ESC, ACC, AHA and WHF8 | Conventional serial Tn T or I (no further details) Timing: On presentation and at 3 and 6 hours |
Final diagnosis adjudicated by two independent cardiologists, with disagreements referred to a third cardiologist; all three were blinded to hs-TnI results |
| Kurz (2011) 55 | Roche Elecsys hs-cTnT | 3 | 13.5 | 8% at 10 | NSTEMI | 24 hours | Joint ESC, ACC, AHA and WHF8 | Fourth generation cTnT (Roche Elecsys, Mannheim, Germany) LoD 10 ng/l, diagnostic threshold 30 ng/l Diagnosis of NSTEMI required elevated cTnT concentration in at least one of the consecutive samples collected within 24 hours of the index event Timing: On presentation, at 6 hours and at least one sample between presentation and 6 hours |
NR |
| Lippi (2012) 73 | Beckman Coulter prototype hs-cTnI (hs-Accu-TnI) | 2.1 | 8.6 | NR | AMI | NR | AMI (unclear method) | NR | NR |
| Melki (2011) 51 , 61 | Roche Elecsys hs-cTnT | 2 | 14 | < 10% at 13 | NSTEMI | NR | Joint ESC, ACC, AHA and WHF8 | Conventional Tn Roche fourth generation TnT (LoD 10 ng/l, 10% CV at 35 ng/l), or Beckman Coulter Access AccuTnI (LoD 10 ng/l, 99th centile 40 ng/l, CV < 10% at 60 ng/l Timing: On presentation and 9 to 12 hours later |
Final diagnosis determined by the individual cardiologist, then adjudicated by two independent evaluators; all three were blinded to hs-TnT results |
| Parsonage (2013) 58 | Abbott ARCHITECT hs-cTnI STAT | NR | 26.2 | NR | AMI | NR | AMI (unclear method) | NR Timing: On admission and > 6 hours after presentation |
Final diagnosis was adjudicated by two independent cardiologists |
| Saenger (2010) 70 | Roche Elecsys hs-cTnT | NR | 14 | NR | AMI | NR | AMI (unclear method) | NR | NR |
| Sanchis (2012) 42 | Roche Elecsys hs-cTnT | 3 | 14 | < 10% at 14 | MACE | 30 days | MACE | NR | NR |
| Santaló (2013) 40 | Roche Elecsys hs-cTnT | NR | 14 | 10% at 9.3 | NSTEMI | NR | National Academy of Clinical Biochemistry and International Federation of Clinical Chemistry Committee101 | Roche cTnT; NSTEMI was defined as cTnT > 10 ng/l and ΔcTnT > 20% Timing: 30 minutes after arrival and at 2, 4 and 6–8 hours or until discharge |
Final diagnosis was made by an adjudication committee |
| Sebbane (2013) 64 | Roche Elecsys hs-cTnT | 5 | 14 | < 10% at 13 | NSTEMI | NR | Joint ESC, ACC, AHA and WHF8 | cTnI measured using the Access2 analyser (Access Immunosystem, Beckman Instruments, France). The LoD was < 10 ng/l and the decision threshold was 40 ng/l Timing: Convention cardiac Tn (cTnI) on presentation, 6 hours later and beyond as needed |
Two independent ED physicians, blinded to hs-cTnT results |
Study results
| Study details | Tn assay | Timing | Threshold (ng/l) | Target condition | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldous (2011) 54 | Roche | On presentation | 14 | AMI | 92 | 36 | 18 | 186 | 83 (75 to 89) | 84 (78 to 88) | 5.1 (3.7 to 6.9) | 0.2 (0.13 to 0.3) |
| 5 | 106 | 131 | 4 | 91 | 96 (90 to 98) | 41 (35 to 48) | 1.6 (1.4 to 1.8) | 0.1 (0.04 to 0.25) | ||||
| 13 | 92 | 38 | 18 | 184 | 83 (75 to 89) | 83 (77 to 87) | 4.8 (3.6 to 6.5) | 0.2 (0.13 to 0.31) | ||||
| 15 | 93 | 29 | 17 | 193 | 84 (76 to 90) | 87 (82 to 91) | 6.4 (4.5 to 9) | 0.18 (0.12 to 0.28) | ||||
| Aldous (2012) 41 | Roche | On presentation | 14 | AMI | 74 | 54 | 8 | 249 | 90 (81 to 95) | 82 (77 to 86) | 5 (3.9 to 6.4) | 0.12 (0.07 to 0.24) |
| 0–1 hours after presentation | 14 | 77 | 63 | 5 | 240 | 93 (86 to 97) | 79 (74 to 83) | 4.5 (3.6 to 5.6) | 0.08 (0.04 to 0.19) | |||
| 0–2 hours after presentation | 14 | 78 | 67 | 4 | 236 | 95 (87 to 98) | 78 (73 to 82) | 4.3 (3.4 to 5.3) | 0.07 (0.03 to 0.17) | |||
| On presentation and at 2 hours | 14 and no change | 78 | 74 | 4 | 229 | 95 (87 to 98) | 75 (70 to 80) | 3.9 (3.1 to 4.7) | 0.07 (0.03 to 0.18) | |||
| < 14 and Δ20% | 49 | 81 | 33 | 222 | 60 (49 to 70) | 73 (68 to 78) | 2.2 (1.7 to 2.9) | 0.55 (0.42 to 0.72) | ||||
| 14 and Δ20% | 46 | 23 | 36 | 280 | 56 (45 to 66) | 92 (89 to 95) | 7.2 (4.7 to 11.2) | 0.48 (0.37 to 0.61) | ||||
| 14 or Δ20% | 81 | 131 | 1 | 172 | 98 (93 to 100) | 57 (51 to 62) | 2.3 (2 to 2.6) | 0.03 (0.01 to 0.16) | ||||
| Aldous (2012) 46 | Roche | On presentation | 14 | NSTEMI | 181 | 134 | 24 | 600 | 88 (83 to 92) | 82 (79 to 84) | 4.8 (4.1 to 5.7) | 0.15 (0.1 to 0.21) |
| On presentation | 5 | 192 | 305 | 13 | 429 | 93 (89 to 96) | 58 (55 to 62) | 2.2 (2 to 2.5) | 0.11 (0.07 to 0.19) | |||
| On presentation | 3 | 196 | 383 | 9 | 351 | 95 (92 to 98) | 48 (44 to 51) | 1.8 (1.7 to 2) | 0.1 (0.05 to 0.18) | |||
| 2 hours after presentation | 14 | 189 | 149 | 16 | 585 | 92 (87 to 95) | 80 (77 to 82) | 4.5 (3.9 to 5.2) | 0.1 (0.06 to 0.16) | |||
| 5 | 196 | 340 | 9 | 394 | 95 (92 to 98) | 54 (50 to 57) | 2.1 (1.9 to 2.2) | 0.09 (0.05 to 0.16) | ||||
| 3 | 201 | 424 | 4 | 310 | 98 (95 to 99) | 42 (39 to 46) | 1.7 (1.6 to 1.8) | 0.05 (0.02 to 0.13) | ||||
| Data from: Aldous (2011)50 | 0–2 hours after presentation | Peak 14 | 189 | 149 | 11 | 590 | 94 (90 to 97) | 80 (77 to 83) | 4.7 (4 to 5.4) | 0.07 (0.04 to 0.13) | ||
| 14 and Δ20% | 99 | 43 | 101 | 696 | 50 (43 to 56) | 94 (92 to 96) | 8.4 (6.1 to 11.6) | 0.54 (0.47 to 0.62) | ||||
| 14 or Δ20% | 195 | 260 | 5 | 479 | 97 (94 to 99) | 65 (61 to 68) | 2.8 (2.5 to 3.1) | 0.04 (0.02 to 0.1) | ||||
| Body (2011) 68 | Roche | On presentation | 3 | AMI | 130 | 378 | 0 | 195 | 100 (96 to 100) | 34 (30 to 38) | 1.5 (1.4 to 1.6) | 0.01 (0 to 0.18) |
| On presentation | 14 | 111 | 101 | 19 | 472 | 85 (78 to 90) | 82 (79 to 85) | 4.8 (4 to 5.8) | 0.18 (0.12 to 0.27) | |||
| On presentation: symptom onset < 3 hours | 3 | 79 | 89 | 0 | 156 | 99 (94 to 100) | 64 (57 to 69) | 2.7 (2.3 to 3.2) | 0.01 (0 to 0.16) | |||
| On presentation: symptom onset < 3 hours | 14 | 63 | 42 | 13 | 203 | 82 (72 to 89) | 83 (78 to 87) | 4.8 (3.6 to 6.4) | 0.21 (0.13 to 0.35) | |||
| On presentation: symptom onset > 3 hours | 3 | 51 | 221 | 0 | 107 | 99 (91 to 100) | 33 (28 to 38) | 1.5 (1.4 to 1.6) | 0.03 (0 to 0.47) | |||
| On presentation: symptom onset > 3 hours | 14 | 47 | 59 | 4 | 269 | 91 (81 to 96) | 82 (77 to 86) | 5.1 (4 to 6.5) | 0.11 (0.04 to 0.26) | |||
| On presentation: symptom onset < 6 hours | 3 | 105 | 253 | 0 | 133 | 100 (96 to 100) | 34 (30 to 39) | 1.5 (1.4 to 1.6) | 0.01 (0 to 0.22) | |||
| On presentation: symptom onset < 6 hours | 14 | 87 | 66 | 18 | 320 | 83 (74 to 89) | 83 (79 to 86) | 4.8 (3.8 to 6.1) | 0.21 (0.14 to 0.32) | |||
| On presentation: symptom onset > 6 hours | 3 | 25 | 125 | 0 | 62 | 98 (84 to 100) | 33 (27 to 40) | 1.5 (1.3 to 1.6) | 0.06 (0 to 0.91) | |||
| On presentation: symptom onset > 6 hours | 14 | 24 | 35 | 1 | 152 | 94 (78 to 99) | 81 (75 to 86) | 5 (3.7 to 6.8) | 0.07 (0.02 to 0.34) | |||
| Christ (2010) 57 | Roche | On presentation | 14 | AMI | 19 | 45 | 1 | 72 | 93 (74 to 98) | 61 (52 to 70) | 2.4 (1.9 to 3.1) | 0.12 (0.02 to 0.55) |
| Christ (2010) 57 | Roche | On presentation | 14 | AMI | 20 | 92 | 0 | 25 | 100 (81 to 100) | 22 (15 to 30) | 1.25 (1.11 to 1.40) | 0.11 (0.01 to 1.74) |
| Collinson (2013) 19 | Roche | On presentation | 14 | NSTEMI | 53 | 33 | 14 | 733 | 79 (68 to 87) | 96 (94 to 97) | 18 (12.6 to 25.7) | 0.22 (0.14 to 0.35) |
| On presentation and at 1.5 hours | Peak 14 | NSTEMI | 57 | 43 | 11 | 736 | 83 (73 to 90) | 94 (93 to 96) | 14.9 (11 to 20.3) | 0.18 (0.1 to 0.3) | ||
| Cullen (2013) 63 | Abbott | On presentation and at 2 hours | 26.2 on admission and at 2 hours | MACE | 227 | 96 | 20 | 1292 | 92 (88 to 95) | 93 (92 to 94) | 13.2 (10.9 to 16.1) | 0.09 (0.06 to 0.13) |
| Eggers (2012) 44 | Roche | On presentation | 14 | NSTEMI | 101 | 59 | 27 | 173 | 79 (71 to 85) | 74 (68 to 80) | 3.1 (2.4 to 3.9) | 0.29 (0.2 to 0.4) |
| 45.7 | NSTEMI | 65 | 11 | 63 | 221 | 51 (42 to 59) | 95 (91 to 97) | 10.3 (5.7 to 18.5) | 0.52 (0.43 to 0.62) | |||
| Freund (2011) 49 | Roche | On presentation | 14 | AMI | 42 | 48 | 3 | 224 | 92 (81 to 97) | 82 (77 to 86) | 5.2 (4 to 6.8) | 0.09 (0.03 to 0.25) |
| On presentation: low/moderate pre-test probability | 20 | 36 | 2 | 200 | 89 (70 to 97) | 85 (79 to 89) | 5.8 (4.2 to 8.1) | 0.13 (0.04 to 0.41) | ||||
| On presentation: high pre-test probability | 22 | 12 | 1 | 24 | 94 (77 to 99) | 66 (50 to 79) | 2.8 (1.7 to 4.4) | 0.09 (0.02 to 0.45) | ||||
| Hoeller (2011) 39 | Roche | On presentation | 14 | AMI | 398 | 363 | 46 | 1265 | 90 (86 to 92) | 78 (76 to 80) | 4 (3.6 to 4.4) | 0.13 (0.1 to 0.18) |
| On presentation: symptom onset < 3 hours | 14 | 79 | 63 | 28 | 335 | 74 (65 to 81) | 84 (80 to 87) | 4.6 (3.6 to 6) | 0.31 (0.23 to 0.43) | |||
| On presentation: symptom onset ≥ 3 hours | 14 | 318 | 300 | 18 | 931 | 95 (92 to 96) | 76 (73 to 78) | 3.9 (3.5 to 4.3) | 0.07 (0.05 to 0.11) | |||
| Beckman | On presentation | 9 | 209 | 231 | 18 | 693 | 92 (88 to 95) | 75 (72 to 78) | 3.7 (3.3 to 4.1) | 0.11 (0.07 to 0.17) | ||
| Abbott | 26.2 | 240 | 93 | 71 | 1163 | 77 (72 to 81) | 93 (91 to 94) | 10.4 (8.4 to 12.7) | 0.25 (0.2 to 0.3) | |||
| Data from: Reichlin (2009)74 | Roche | On presentation | 2 | 123 | 512 | 0 | 83 | 100 (97 to 100) | 14 (11 to 17) | 1.2 (1.1 to 1.2) | 0.03 (0.00 to 0.46) | |
| Abbott | 10 | 116 | 77 | 7 | 518 | 94 (89 to 98) | 87 (84 to 90) | 7.3 (5.9 to 9.0) | 0.07 (0.03 to 0.13) | |||
| Data from: Reiter (2011)53 | Roche | On presentation: > 70 years only | 5 | 98 | 305 | 0 | 3 | 99 (95 to 100) | 1 (0 to 3) | 1 (1 to 1) | 0.45 (0.02 to 8.56) | |
| On presentation: > 70 years only | 14 | 96 | 157 | 2 | 151 | 97 (92 to 99) | 49 (44 to 55) | 1.9 (1.7 to 2.1) | 0.05 (0.02 to 0.18) | |||
| On presentation: ≤ 70 years | 14 | 54 | 87 | 7 | 533 | 88 (78 to 94) | 86 (83 to 88) | 6.2 (5 to 7.7) | 0.14 (0.07 to 0.28) | |||
| Data from: Potocki (2012)47 | On presentation: with pre-existing CAD | 14 | 73 | 142 | 5 | 213 | 93 (85 to 97) | 60 (55 to 65) | 2.3 (2 to 2.7) | 0.12 (0.05 to 0.26) | ||
| On presentation: without pre-existing CAD | 14 | 100 | 114 | 6 | 517 | 94 (88 to 97) | 82 (79 to 85) | 5.2 (4.4 to 6.2) | 0.07 (0.04 to 0.16) | |||
| Data from: Hochholzer (2011)56 | On presentation | 11 | 129 | 177 | 3 | 454 | 97 (93 to 99) | 72 (68 to 75) | 3.5 (3.1 to 3.9) | 0.04 (0.01 to 0.1) | ||
| On presentation | 11 | NSTEMI | 90 | 177 | 3 | 454 | 96 (90 to 99) | 72 (68 to 75) | 3.4 (3 to 3.9) | 0.05 (0.02 to 0.14) | ||
| Data from: Irfan (2013)65 | On presentation and at 1 hour | Δ17% | 65 | 202 | 43 | 520 | 60 (51 to 69) | 72 (69 to 75) | 2.1 (1.8 to 2.6) | 0.55 (0.44 to 0.7) | ||
| Beckman | Δ27% | 68 | 245 | 40 | 477 | 63 (53 to 71) | 66 (63 to 69) | 1.9 (1.6 to 2.2) | 0.56 (0.44 to 0.72) | |||
| Data from: Reichlin (2011)52 | Roche | On presentation and at 2 hours | Δ30% | 43 | 84 | 24 | 439 | 64 (52 to 74) | 84 (80 to 87) | 4 (3 to 5.2) | 0.43 (0.31 to 0.59) | |
| Data from: Cullen (2013)63 | Abbott | 26.2 on admission and at 2 hours | MACE | 129 | 62 | 27 | 691 | 82 (76 to 88) | 92 (90 to 93) | 10 (7.8 to 12.8) | 0.19 (0.14 to 0.27) | |
| Keller (2011) 48 | Abbott | On presentation | 3.4 | AMI | 282 | 633 | 0 | 345 | 100 (98 to 100) | 35 (32 to 38) | 1.5 (1.5 to 1.6) | 0.01 (0 to 0.08) |
| 30 | AMI | 232 | 77 | 50 | 901 | 82 (77 to 86) | 92 (90 to 94) | 10.4 (8.3 to 12.9) | 0.19 (0.15 to 0.25) | |||
| 3 hours after presentation | 3.4 | AMI | 282 | 959 | 0 | 19 | 100 (98 to 100) | 2 (1 to 3) | 1 (1 to 1) | 0.09 (0.01 to 1.46) | ||
| 30 | AMI | 277 | 94 | 5 | 884 | 98 (96 to 99) | 90 (88 to 92) | 10.2 (8.4 to 12.3) | 0.02 (0.01 to 0.05) | |||
| On presentation and at 3 hours | Δ20% | AMI | 218 | 723 | 64 | 255 | 77 (72 to 82) | 26 (23 to 29) | 1 (1 to 1.1) | 0.87 (0.69 to 1.11) | ||
| 3.4 on admission and Δ20% | AMI | 254 | 454 | 54 | 498 | 82 (78 to 86) | 52 (49 to 55) | 1.7 (1.6 to 1.9) | 0.34 (0.26 to 0.43) | |||
| 30 after 3 hours and Δ20% | AMI | 187 | 34 | 110 | 929 | 63 (57 to 68) | 96 (95 to 97) | 17.6 (12.5 to 24.7) | 0.38 (0.33 to 0.45) | |||
| 30 after 3 hours and Δ20% to in patients < 30 ng/l on admission | AMI | 52 | 26 | 4 | 869 | 92 (82 to 97) | 97 (96 to 98) | 31.1 (21.2 to 45.7) | 0.08 (0.03 to 0.2) | |||
| Kurz (2011) 55 | Roche | On presentation | 9.5 | NSTEMI | 38 | 11 | 8 | 37 | 82 (69 to 90) | 77 (63 to 86) | 3.5 (2.1 to 5.9) | 0.24 (0.13 to 0.44) |
| 14 | NSTEMI | 16 | 7 | 10 | 24 | 61 (42 to 77) | 77 (60 to 88) | 2.6 (1.3 to 5.2) | 0.51 (0.3 to 0.85) | |||
| Within 3 hours of presentation | 14 | NSTEMI | 26 | 7 | 0 | 23 | 98 (84 to 100) | 76 (58 to 87) | 4.1 (2.2 to 7.6) | 0.02 (0 to 0.38) | ||
| On presentation and within 3 hours | 14 and Δ20% | NSTEMI | 11 | 27 | 15 | 3 | 43 (26 to 61) | 11 (4 to 27) | 0.5 (0.3 to 0.8) | 5.08 (1.8 to 14.37) | ||
| Lippi (2012) 73 | Beckman | On presentation | 18 | AMI | 9 | 17 | 0 | 31 | 95 (66 to 99) | 64 (50 to 76) | 2.7 (1.8 to 4) | 0.08 (0.01 to 1.17) |
| Melki (2011) 51 | Roche | On presentation | 14 | NSTEMI | 112 | 21 | 2 | 98 | 98 (93 to 99) | 82 (74 to 88) | 5.5 (3.7 to 8) | 0.03 (0.01 to 0.09) |
| 2 hours after presentation | 14 | NSTEMI | 114 | 25 | 0 | 94 | 100 (96 to 100) | 79 (71 to 85) | 4.7 (3.3 to 6.6) | 0.01 (0 to 0.09) | ||
| Parsonage (2013) 58 | Abbott | On presentation | 26.2 | AMI | 45 | 34 | 6 | 652 | 88 (76 to 94) | 95 (93 to 96) | 17.4 (12.4 to 24.5) | 0.13 (0.06 to 0.27) |
| On presentation and at 2 hours | 26.2 peak | AMI | 47 | 48 | 4 | 638 | 91 (81 to 96) | 93 (91 to 95) | 12.9 (9.7 to 17.2) | 0.09 (0.04 to 0.23) | ||
| Roche | On presentation | 14 | AMI | 44 | 75 | 7 | 611 | 86 (74 to 93) | 89 (86 to 91) | 7.8 (6.1 to 9.9) | 0.16 (0.08 to 0.31) | |
| On presentation and at 2 hours | 14 peak | AMI | 48 | 82 | 3 | 604 | 93 (83 to 98) | 88 (85 to 90) | 7.8 (6.3 to 9.6) | 0.08 (0.03 to 0.21) | ||
| Saenger (2010) 70 | Roche | On presentation | 14 | AMI | 92 | 38 | 6 | 152 | 93 (87 to 97) | 80 (74 to 85) | 4.6 (3.5 to 6.2) | 0.08 (0.04 to 0.17) |
| On presentation and at 3 hours | Δ 8 | AMI | 94 | 9 | 4 | 181 | 95 (89 to 98) | 95 (91 to 97) | 19.2 (10.3 to 35.7) | 0.05 (0.02 to 0.12) | ||
| Sanchis (2012) 42 | Roche | On presentation | 3 | MACE | 53 | 207 | 9 | 177 | 85 (74 to 92) | 46 (41 to 51) | 1.6 (1.4 to 1.8) | 0.33 (0.18 to 0.59) |
| On presentation and 6–8 hours | 3 | MACE | 57 | 234 | 5 | 150 | 91 (82 to 96) | 39 (34 to 44) | 1.5 (1.3 to 1.7) | 0.22 (0.1 to 0.5) | ||
| 14 | MACE | 21 | 42 | 41 | 342 | 34 (24 to 46) | 89 (85 to 92) | 3.1 (2 to 4.8) | 0.74 (0.62 to 0.89) | |||
| Santaló (2013) 40 | Roche | On presentation | 14 | NSTEMI | 71 | 80 | 8 | 199 | 89 (81 to 94) | 71 (66 to 76) | 3.1 (2.5 to 3.8) | 0.15 (0.08 to 0.28) |
| On presentation and at 2–4 and 6–8 hours or until discharge | Δ 20% | NSTEMI | 79 | 94 | 0 | 185 | 99 (94 to 100) | 66 (61 to 72) | 2.9 (2.5 to 3.5) | 0.01 (0 to 0.15) | ||
| Sebbane (2013) 64 | Roche | On presentation, or sample taken during pre-hospital management | 14 | NSTEMI | 19 | 25 | 6 | 142 | 75 (56 to 88) | 85 (79 to 89) | 4.9 (3.2 to 7.5) | 0.29 (0.15 to 0.58) |
| 18 | NSTEMI | 19 | 17 | 6 | 150 | 75 (56 to 88) | 90 (84 to 93) | 7.2 (4.4 to 11.8) | 0.28 (0.14 to 0.54) |
Appendix 3 QUADAS-2 assessments
Study: Aldous (2011)54
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Consecutive adults presenting to the ED with chest pain were eligible for inclusion | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Unselected chest pain population AMI diagnoses may have included both NSTEMI and STEMI | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT on admission and after 6 hours. Data reported for admission, for four thresholds No details of interpretation reported. One threshold was derived from ROC analysis; primary analysis based on 99th centile |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard diagnosis of AMI based on joint ECS and ACC criteria and included serial conventional cTnI (10- to 12-hour time point not specified) Determination of diagnosis was made blind to hs-TnT results |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| Participants for whom stored samples were not available at both time points were excluded | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: High |
Study: Aldous (2012)46
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Patients presenting to the ED between 05.30 and 20.00 hours, and with chest pain | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: High |
| B. APPLICABILITY | |
| Patients with ST segment elevation excluded | |
| Do the included patients match the question? | Concerns: Low |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT Data reported for multiple thresholds based on predetermined properties of the assay Frozen samples used, unclear whether interpretation of index test was blind to reference standard |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard final diagnosis of AMI, based on ACC criteria and including the results of serial conventional cTnI (10- to 12-hour time point not specified), but blinded to hs-TnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: Low |
Study: Body (2011)67
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Prospective enrolment of patients; unclear if consecutive | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Mixed chest pain | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT. Threshold 99th percentile cut point and LoD. Blinding not reported; objective test interpreted prior to reference standard so unlikely to have been influenced by knowledge of reference standard | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Thorgeson criteria; time point not specified. Clinicians were blinded to hs-Tn | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| 301 patients were excluded prior to enrolment; all patients enrolled included in 2 × 2 table | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: Low |
Study: Christ (2010)57
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Retrospective analysis of consecutive patients presenting to ED with chest pain | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Patients with general chest pain symptoms, includes participants with a final diagnosis of STEMI | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT. Threshold 99th percentile cut point. Blinding not reported; retrospective analysis and so disease status may have been known when interpreting results. However, objective test and so unlikely to have been influenced by knowledge of disease state | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Joint ESC and ACC criteria; time point not specified. Unclear whether clinicians were blinded to hs-Tn. A second consensus diagnosis incorporating hs-Tn was also made and so clinicians may have been aware of the result for the first consensus diagnosis based only on standard Tn | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| No dropouts reported, all included patients accounted for in flow diagram and numbers suggest that Tn results were available for all | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: Low |
Study: Collinson (2013)19
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Participants with chest pain and suspected AMI; study uses subgroup of one arm of an RCT. Patients at high risk of NSTEMI excluded | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Chest pain patients excluding those with diagnostic ECG changes | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT on admission and at 90 minutes Reference standard (final diagnosis) determined after hs-TnT Threshold based on assay characteristics including 99th centile |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard diagnosis of AMI based on joint ESC and ACC criteria and included serial conventional cTnT or cTnI (10- to 12-hour time point specified) Determination of diagnosis was made blind to hs-TnT results |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| 1125 enrolled, 25 no samples collected, 250 samples taken but study samples not collected | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: High |
Study: Cullen (2013)63
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Consecutively recruited adults presenting to the ED with cardiac symptoms | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Unselected chest pain population AMI diagnoses may have included both NSTEMI and STEMI | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Abbott ARCHITECT hs-STAT TnI; threshold was 99th centile Frozen samples were used, but laboratory technicians were blinded to patient data |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| 30-day MACE, adjudicated blind to index tests, but with access to clinical records, ECG and conventional Tn results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| No patients were lost to 30-day follow-up. Procedure for adjudication of 30-day MACE was the same in all cases, but investigations undergone by individual patients varied | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | No |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: Low |
Study: Eggers (2012)44
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Unclear whether consecutive or random patients were enrolled. | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Non-STEMI patients with chest pain presenting to coronary care/chest pain unit | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT. Threshold 99th percentile cut point and 95% specificity value. Blinding not reported; objective test interpreted prior to reference standard so unlikely to have been influenced by knowledge of reference standard | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Joint ESC and ACC criteria; time point not specified. Unclear whether clinicians were blinded to high-sensitivity troponin. A second consensus diagnosis | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| Only 360 patients out of 495 who fulfilled inclusion criteria had all biochemical tests performed and were included in the analysis; reasons for not performing tests were not reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: High |
Study: Freund (2011)49
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Consecutive adults presenting to the ED with chest pain (onset or peak within previous 6 hours). Patients with acute kidney failure requiring dialysis were excluded | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Unselected ED chest pain population, includes participants with a final diagnosis of STEMI; data also presented for subgroups with low-moderate and with high pre-test probability | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT on admission and at 3–9 hours if available. Reference standard (final diagnosis) adjudicated by two independent physicians after acute episode. Threshold was 99th centile | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard final diagnosis, based on joint ESC and ACC criteria and included conventional cTnI on admission and at 3–9 hours if needed (10- to 12-hour time point not specified). Clinicians adjudicating final diagnosis were blind to hs-TnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: Low |
Study: Hoeller (2013)39
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Patients presenting to the ED with symptoms suggestive of AMI. Consecutive patients with hs-TnT measurements available were included | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Unselected chest pain population AMI diagnoses may have included both NSTEMI and STEMI | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche hs-TnT, Beckman Coulter Hs-AccuTnI and Abbott ARCHITECT hs-TnI on admission Reference standard probably made later than admission; 99th centiles for assays used as diagnostic thresholds (some publications also reported data for ROC-derived thresholds) |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard final diagnosis of AMI, ESC criteria and included cTn assays (0 and 6 hours). Unclear whether those adjudicating final diagnosis were blind to hs-TnI/hs-TnT results in all cases, some publications reported blinding | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes/no |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| 2245 participants were included in the trial, 2072 were included in the hs-TnT analysis, 1151 were included in the hs-TnI (Beckman) analysis, and 1567 were included in the hs-TnI (Abbott) analysis Most exclusions were because hsTn measurements were not available |
|
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: High |
Study: Keller (2011)48
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Consecutive patients presenting to chest pain units | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| General chest pain populations, some participants had a final diagnosis of STEMI | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Abbott Architect STAT hs-TnI, on admission and at 3 hours. Reference standard (final diagnosis) was adjudicated after hs-TnI testing. Thresholds based on test properties, appeared to be prespecified | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard diagnosis of AMI based on joint ESC and ACC criteria and included serial conventional cTnT (10- to 12-hour time point not specified) Determination of diagnosis was made blind to hs-TnT results |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| None of the analyses included all study participants (558 or 867 participants missing) | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: High |
Study: Kurz (2011)55
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Consecutive patients admitted to a chest pain unit. 206 Patients not included owing to ‘technical reasons’ (not fully defined, e.g. venepuncture not possible) | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Appears to be an unselected chest pain population, STEMI excluded. Second publication110 is for a retrospectively selected subgroup of participants with a diagnosis of NSTEMI or UA. Patients were admitted to chest pain units | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT, data reported for admission, 3- and 6-hour samples (6-hour data not extracted) Reference standard Tn testing occurred after hs-TnT. Threshold was prespecified for data extracted from Giannitsis et al.,110 but not from Kurz et al.55 (low risk of bias for Giannitsis et al.110 data) |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard diagnosis of AMI based on joint ESC and ACC criteria and included serial conventional cTnT (10- to 12-hour time point not specified) Unclear whether determination of diagnosis was made blind to hs-TnT results |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: Low |
Study: Lippi (2012)73
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Consecutive patients presenting to the ED with chest pain of recent onset (< 3 hours) No exclusion criteria reported |
|
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Unselected chest pain population AMI diagnoses may have included both NSTEMI and STEMI | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Beckman Coulter HS-AccuTnI on admission. Reference standard final diagnosis (AMI); probably made later than admission hs-TnI. Threshold derived from ROC analysis | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: High |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard final diagnosis of AMI, criteria for diagnosis not reported Unclear whether those adjudicating final diagnosis were blind to hs-TnI |
|
| Is the reference standard likely to correctly classify the target condition? | Unclear |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| No withdrawals reported | |
| Did all patients receive a reference standard? | Unclear |
| Did patients receive the same reference standard? | Unclear |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: Unclear |
Study: Melki (2011)51
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Recruitment described as ‘consecutive except for temporary interruptions of the study due to high work load in the coronary care unit’ | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: High |
| B. APPLICABILITY | |
| Chest pain patients admitted to chest pain unit, excluding ST segment elevation | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT on admission and at 2 hours. Reference standard (final diagnosis) determined after hs-TnT testing. Threshold based on assay characteristics, appears predetermined | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard diagnosis of AMI based on joint ESC and ACC criteria and included serial conventional cTnT or cTnI (9- to 12-hour time point specified) Determination of diagnosis was made blind to hs-TnT results |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: Low |
Study: Parsonage (2013)58
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Prospective studies; no further details on recruitment | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Unselected chest pain population AMI diagnoses may have included both NSTEMI and STEMI | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT and Abbott ARCHITECT hs-STAT TnI. Threshold was 99th centile Index test occurred before adjudication of final diagnosis |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard diagnosis of AMI (criteria unclear) and included serial conventional cTnI (10- to 12-hour time point not specified). Determination of diagnosis was made blind to hs-TnT and hs-TnI results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| Patients appear to be missing from the analyses, as 2 × 2 data (derived from reported sensitivity and specificity estimates and total number of AMI) do not match reported number of test positives | |
| Did all patients receive a reference standard? | Unclear |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: Unclear |
Study: Saenger (2010)70
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| No details on how patients were selected. No exclusion criteria reported | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| No exclusion criteria reported, reference standard was AMI (diagnosis method not specified),diagnoses included STEMI | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT on admission and after 3 hours. Data reported for admission and 0–3 hours. No details of interpretation reported. Threshold for Δ value derived from ROC analysis; 99th centile also used | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Reference standard diagnosis of AMI (no details reported) | |
| Is the reference standard likely to correctly classify the target condition? | Unclear |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| No withdrawals reported | |
| Did all patients receive a reference standard? | Unclear |
| Did patients receive the same reference standard? | Unclear |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: Unclear |
Study: Sanchis (2012)42
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Patients excluded owing to Tn elevation in any of two serial determinations (at arrival and 6–8 hours later) and prior diagnosis of ischaemic heart disease. No details on how patients were selected for the study | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: High |
| B. APPLICABILITY | |
| Selected low-risk population | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT on admission and at 6–8 hours (data reported for admission and peak values). Reference standard (30-day composite) occurred after testing. Thresholds were reported as prespecified | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Composite 30-day end point of AMI, death and revascularisation Not clear whether those adjudicating AMI were aware of hs-TnT results |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| All participants appeared to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: Low |
Study: Santalo (2013)40
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Consecutive adult patients presenting to the ED | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Appears to be an unselected ED chest pain population | |
| Do the included patients match the question? | Concerns: Low |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT on admission and at 2, 4 and 6–8 hours or until discharge (data reported for admission and Δ values). Unclear whether hs-TnT interpreted blind to cTnT | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Final diagnosis adjudicated by committee, based on Roche cTnT at admission and 2, 4 and 6–8 hours or until discharge (10- to 12-hour time point not specified). NSTEMI defined as cTnT > 10 ng/l and ΔcTnT > 20%; also 99th centile. Unclear whether adjudicators were blinded to hs-TnT | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: Unclear |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: Low |
Study: Sebbane (2013)62
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| No details on how patients were selected for inclusion | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Unselected cohort of adult patients presenting with chest pain of recent onset (within 12 hours) | |
| Do the included patients match the question? | Concerns: Low |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Roche Elecsys hs-TnT on admission or from sample taken during pre-hospital management. Final diagnosis adjudicated 1 month after acute episode. Optimal diagnostic thresholds were determined using within-study ROC analyses; 99th centile also reported | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Diagnosis determined by two independent ED physicians, based on joint ESC and ACC criteria. Reference standard included cTnI taken on admission, at 6 hours and beyond, as needed (10- to 12-hour time point not specified). Physicians had access to serial cTnI results, but were blinded to hs-TnT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| 54 patients were excluded from the analyses because of missing data, including lack of copeptin, hs-cTnT, and cTnI measurements | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: High |
Appendix 4 Table of excluded studies with rationale
To be included in the review, studies had to fulfil the following criteria:
Population Adults (≥ 18 years) presenting with acute ‘pain, discomfort or pressure in the chest, epigastrium, neck, jaw, or upper limb without an apparent non-cardiac source’ attributable to a suspected, but not proven, AMI or ACS.
Setting Secondary or tertiary care.
Index test Abbott ARCHITECT (STAT hs-cTnI); Beckman Coulter Access and Unicel DxI (accuTnI+ 3); Roche Elecsys (cTnT-hs or cTnT-hs STAT); results available within 3 hours.
Reference standard Universal definition of AMI, including measurement of Tn T or I (using any method not defined as a hs-cTn test) on presentation and 10–12 hours after the onset of symptoms in ≥ 80% of the population or occurrence of MACE (any definition used in identified studies) during 30-day follow-up.
Outcome Sufficient data to construct 2 × 2 table of test performance.
The table below summarises studies that were screened for inclusion, based on full-text publication, but which did not fulfil one or more of the above criteria. Studies were assessed sequentially against criteria; as soon as a study had failed, based on one of the criteria, it was not assessed against subsequent criteria. The table shows which of the criteria each study fulfilled (‘Yes’) and on which item it failed (‘No’).
| Study details | Primary study | Population | Setting | Index test | Reference standard | Outcome |
|---|---|---|---|---|---|---|
| Ahmed (2013)111 | Yes | Yes | Yes | Yes | No | |
| Aldous (2010)112 | Yes | Yes | Yes | Yes | Unclear | No |
| Aldous (2010)113 | Yes | Yes | Yes | Yes | Unclear | No |
| Aldous (2012)114 | No | |||||
| Aldous (2010)115 | Yes | Yes | Yes | Unclear | No | |
| Aldous (2012)116 | Yes | Yes | Yes | Unclear | Yes | No |
| Aldous (2012)117 | Yes | Yes | Yes | No | ||
| Aldous (2012)118 | No | |||||
| Aldous (2012)54 | No | |||||
| Alexandra (2013)119 | Yes | Yes | Yes | No | ||
| Arenja (2010)120 | Yes | Yes | Yes | Yes | No | |
| Bahrmann (2012)121 | Yes | No | ||||
| Bahrmann (2013)122 | Yes | No | ||||
| Bahrmann (2013)123 | Yes | No | ||||
| Bahrmann (2012)124 | Yes | Yes | Yes | Yes | No | |
| Balmelli (2013)125 | Yes | Yes | Yes | Yes | Unclear | No |
| Balmelli (2011)126 | Yes | Yes | Yes | Yes | Unclear | No |
| Beyrau (2009)127 | Yes | No | ||||
| Bhardwaj (2011)128 | Yes | Yes | Yes | No | ||
| Bhardwaj (2011)129 | Yes | Yes | Yes | No | ||
| Biasillo (2010)130 | Yes | Yes | Yes | Unclear | No | |
| Biasucci (2010)131 | Yes | Yes | Yes | Yes | No | |
| Biasucci (2010)132 | Yes | Yes | Yes | Yes | No | |
| Biasucci (2010)133 | Yes | Yes | Yes | Yes | No | |
| Biasucci (2010)134 | Yes | Yes | Yes | Yes | No | |
| Biasucci (2011)135 | Yes | Yes | Yes | Yes | No | |
| Biener (2013)136 | Yes | Unclear | Yes | Unclear | Unclear | No |
| Biener (2012)137 | Yes | Yes | Yes | Unclear | Unclear | No |
| Biener (2013)138 | Yes | Yes | Yes | No | ||
| Biosite (2006)139 | Yes | Yes | Yes | No | ||
| Body (2012)140 | Yes | Yes | Yes | Unclear | Unclear | No |
| Body (2012)141 | Yes | Yes | Yes | Yes | Yes | No |
| Body (2012)142 | No | |||||
| Braga (2011)143 | Yes | Yes | Yes | Yes | Yes | No |
| Braga (2011)144 | Yes | Yes | Yes | Yes | Yes | No |
| Bronze (2012)145 | Yes | Yes | Yes | Yes | No | |
| Brown (2007)146 | Yes | Yes | Yes | No | ||
| Buccelletti (2012)147 | Yes | Yes | Yes | Yes | No | |
| Buhl (2011)148 | Yes | No | ||||
| Cardillo (2012)149 | Yes | Yes | Yes | Yes | Yes | No |
| Carmo (2013)150 | No | |||||
| Ceriani (2012)151 | No | |||||
| Charpentier (2011)152 | Yes | Yes | Yes | No | ||
| Chenevier-Gobeaux (2013)153 | No | |||||
| Collinson (2012)154 | Yes | Yes | Yes | No | ||
| Collinson (2012)155 | Yes | Yes | Yes | No | ||
| Collinson (2012)156 | Yes | Yes | Yes | No | ||
| Collinson (2006)157 | Yes | Yes | Yes | No | ||
| Collinson (2010)158 | Yes | Yes | Yes | No | ||
| Costabel (2013)159 | No | |||||
| Cullen (2011)160 | Yes | Yes | Yes | No | ||
| Dawson (2013)161 | Yes | No | ||||
| Diercks (2012)162 | Yes | Yes | Yes | No | ||
| Drexler (2011)163 | Yes | Yes | Yes | Unclear | Unclear | No |
| Engel (2007)164 | Yes | Yes | Yes | No | ||
| Escabi-Mendoza (2010)165 | Yes | Yes | Yes | No | ||
| Figiel (2008)166 | Yes | No | ||||
| Fitzgerald (2011)79 | Yes | Yes | Yes | No | ||
| Freund (2011)167 | Yes | Yes | Yes | Unclear | Unclear | No |
| Freund (2011)168 | Yes | Yes | Yes | Unclear | Unclear | No |
| Giannitsis (2010)110 | Yes | No | ||||
| Giannitsis (2011)169 | Yes | Yes | Yes | Unclear | Unclear | No |
| Giavarina (2012)170 | No | |||||
| Giavarina (2011)171 | Yes | Yes | Yes | No | ||
| Gimenez (2012)172 | Yes | Yes | Yes | No | ||
| Gimenez (2012)173 | Yes | Yes | Yes | No | ||
| Goodacre (2011)80 | Yes | Yes | Yes | No | ||
| Goodacre (2013)7 | No | |||||
| Goodacre (2011)87 | Yes | Yes | Yes | No | ||
| Gustapane (2012)174 | Yes | Yes | Yes | Unclear | No | |
| Gustapane (2012)175 | Yes | Yes | Yes | Unclear | No | |
| Haaf (2011)176 | Yes | Yes | Yes | Unclear | Unclear | No |
| Haaf (2011)177 | Yes | Yes | Yes | Unclear | Unclear | No |
| Haaf (2013)178 | No | |||||
| Haaf (2012)179 | Yes | Yes | Yes | Yes | No | |
| Haaf (2012)180 | Yes | Yes | Yes | Yes | No | |
| Haltern (2010)181 | Yes | Yes | Yes | No | ||
| Heinisch (2010)182 | Yes | Yes | Yes | Unclear | Unclear | No |
| Hochholzer (2011)183 | Yes | Yes | Yes | Yes | No | |
| Hochholzer (2010)184 | Yes | Yes | Yes | Yes | No | |
| Hoeller (2012)185 | Yes | Yes | Yes | Yes | No | |
| Hoeller (2012)186 | Yes | Yes | Yes | Yes | No | |
| Ilva (2009)187 | Yes | Yes | No | |||
| Inoue (2011)188 | Yes | Yes | Yes | Yes | No | |
| Irfan (2011)189 | Yes | Yes | Yes | Unclear | Unclear | No |
| Irfan (2011)190 | Yes | Yes | Yes | Unclear | No | |
| Irfan (2013)191 | Yes | Yes | Yes | Yes | Unclear | No |
| Irfan (2013)192 | Yes | Yes | Yes | Yes | Unclear | No |
| Jairam (2011)193 | Yes | No | ||||
| Januzzi (2010)194 | Yes | Yes | Yes | No | ||
| Januzzi (2009)195 | Yes | Yes | Yes | Yes | No | |
| Januzzi (2013)196 | Yes | Yes | Yes | No | ||
| Jia (2009)197 | Yes | Yes | Yes | No | ||
| Kagawa (2013)198 | Yes | Yes | Yes | No | ||
| Karakas (2011)199 | Yes | Yes | Yes | Yes | No | |
| Kavsak (2012)200 | Yes | Yes | Yes | Unclear | Unclear | No |
| Kavsak (2007)201 | Yes | Yes | Yes | No | ||
| Kavsak (2013)202 | Yes | Yes | Yes | Unclear | No | |
| Kavsak (2005)203 | Yes | Yes | Yes | No | ||
| Kavsak (2012)204 | Yes | Yes | Yes | Yes | Unclear | No |
| Kavsak (2008)205 | Yes | No | ||||
| Kavsak (2011)206 | Yes | Yes | Yes | Yes | No | |
| Kavsak (2010)207 | Yes | Yes | Yes | Yes | Yes | No |
| Keene (2012)208 | Yes | Yes | Yes | Yes | No | |
| Keller (2011)209 | Yes | Yes | Yes | Yes | Unclear | No |
| Keller (2011)210 | Yes | Yes | Yes | Yes | Unclear | No |
| Keller (2009)211 | Yes | Yes | Yes | No | ||
| Keller (2010)212 | Yes | Yes | Yes | No | ||
| Keller (2009)213 | Yes | Yes | Yes | No | ||
| Kelly (2011)214 | Yes | Yes | Yes | No | ||
| Khan (2011)215 | Yes | Yes | Yes | Yes | No | |
| Khoo (2008)216 | Yes | Unclear | Yes | No | ||
| Kitamura (2012)217 | Yes | Yes | Yes | No | ||
| Kobayashi (2011)218 | Yes | Yes | Yes | Unclear | No | |
| Kobayashi (2011)219 | Yes | Yes | Yes | Yes | No | |
| Koenig (2008)220 | Yes | Yes | Yes | Yes | No | |
| Lacnak (2007)221 | Yes | Yes | Yes | Unclear | No | |
| Lee (2011)222 | Yes | Yes | Yes | Unclear | No | |
| Lindahl (2009)223 | Yes | No | ||||
| Lippi (2013)224 | No | |||||
| Lippi (2012)225 | No | |||||
| Lippi (2013)226 | No | |||||
| Lotze (2011)227 | Yes | Yes | Yes | No | ||
| Lotze (2011)228 | Yes | Yes | Yes | Yes | No | |
| Macrae (2006)229 | Yes | Yes | Yes | No | ||
| Mair (2011)230 | Yes | No | ||||
| Mair (2011)231 | Yes | No | ||||
| Matsui (2011)232 | Yes | Yes | Yes | Unclear | Unclear | No |
| Mazhar (2011)233 | Yes | Yes | Yes | Unclear | No | |
| Melanson (2008)234 | Yes | Yes | Yes | No | ||
| Melki (2011)235 | Yes | Yes | Yes | Yes | No | |
| Melki (2011)236 | Yes | Yes | Yes | No | ||
| Menhofer (2013)237 | Yes | No | ||||
| Meune (2011)238 | Yes | Yes | Yes | Yes | Yes | No |
| Meune (2011)108 | Yes | Yes | Yes | Yes | Yes | No |
| Meune (2013)239 | Yes | Yes | Yes | Yes | No | |
| Meune (2011)240 | Yes | Yes | Yes | Yes | No | |
| Mikkel (2013)241 | Yes | Yes | Yes | Yes | Unclear | No |
| Mikkel (2013)242 | Yes | Yes | Yes | No | ||
| Mikkel (2013)243 | Yes | Yes | Yes | No | ||
| Mills (2010)244 | Yes | Yes | Yes | Unclear | No | |
| Mills (2010)245 | Yes | Yes | Yes | Unclear | No | |
| Mills (2012)246 | Yes | Yes | Yes | Yes | No | |
| Mingels (2012)247 | Yes | No | ||||
| Moehring (2012)248 | Yes | Yes | Yes | Yes | No | |
| Moehring (2012)249 | Yes | Yes | Yes | Yes | Unclear | No |
| Montagnana (2012)250 | Yes | Yes | Yes | Yes | Yes | No |
| Morrow (2009)251 | No | |||||
| Nagurney (2005)252 | Yes | Yes | Yes | No | ||
| Nanosphere (2010)253 | Yes | Yes | Yes | Unclear | Yes | No |
| Naroo (2009)254 | Yes | Yes | Yes | No | ||
| Ngan (2010)255 | Yes | Yes | Yes | Yes | Yes | No |
| Noad (2010)256 | Yes | Yes | Yes | Unclear | Unclear | No |
| Normann (2012)257 | Yes | Yes | Yes | Yes | No | |
| Nusier (2006)258 | Yes | Yes | Yes | No | ||
| Olivieri (2012)259 | Yes | Yes | Yes | No | ||
| Orsborne (2012)260 | No | |||||
| Paoloni (2010)261 | Yes | Yes | Yes | Unclear | No | |
| Perego (2011)262 | Yes | |||||
| Plebani (2009)263 | Yes | Yes | Yes | No | ||
| Ploner (2011)264 | Yes | No | No | |||
| Popp (2010)265 | Yes | Yes | Yes | Yes | No | |
| Potocki (2011)266 | Yes | Yes | Yes | No | ||
| Pracon (2012)267 | Yes | Yes | Yes | No | ||
| Rajdl (2011)268 | Yes | Yes | Yes | Yes | Unclear | No |
| Ray (2011)269 | Yes | Yes | Yes | Unclear | Unclear | No |
| Reichlin (2012)270 | No | |||||
| Reichlin (2011)271 | Yes | Yes | Yes | Unclear | Unclear | No |
| Reichlin (2012)272 | Yes | Yes | Yes | Yes | No | |
| Reichlin (2010)273 | Yes | Yes | Yes | Unclear | No | |
| Reichlin (2010)274 | Yes | Yes | Yes | Unclear | No | |
| Reichlin (2012)275 | Yes | Yes | Yes | Yes | No | |
| Rubini Gimenez (2012)276 | Yes | Yes | Yes | Yes | Unclear | No |
| Rudolph (2011)277 | Yes | Yes | Yes | Unclear | Unclear | No |
| Rudolph (2011)278 | Yes | Yes | Yes | Unclear | Unclear | No |
| Rudolph (2012)279 | Yes | Yes | Yes | No | ||
| Samaraie (2010)280 | Yes | Yes | Yes | Unclear | No | |
| Scharnhorst (2011)281 | Yes | Yes | Yes | No | ||
| Schaub (2012)282 | Yes | Yes | Yes | Yes | Yes | No |
| Schoos (2013)283 | Yes | Yes | Yes | Yes | Unclear | No |
| Schoos (2013)284 | Yes | Yes | Yes | Yes | Unclear | No |
| Schreiber (2012)285 | Yes | Yes | Yes | No | ||
| Sethi (2013)286 | No | |||||
| Shand (2012)287 | Yes | Yes | Unclear | Unclear | No | |
| Shortt (2013)288 | No | |||||
| Spanuth (2011)289 | Yes | Yes | Yes | Unclear | Unclear | No |
| Spasic-Obradovic (2011)290 | Yes | Yes | Yes | Yes | No | |
| Stengaard (2012)291 | Yes | Yes | Yes | Unclear | Unclear | No |
| Tajsic (2013)292 | Yes | Yes | Yes | Unclear | Unclear | No |
| Tajsic (2013)293 | Yes | Yes | Yes | Unclear | Unclear | No |
| Tajsic (2012)294 | Yes | Yes | Yes | Unclear | No | |
| Tajsic (2013)295 | Yes | Yes | Yes | Unclear | Unclear | No |
| Tamimi (2010)296 | Yes | Yes | Yes | No | ||
| Tanaka (2006)297 | Yes | Yes | Yes | No | ||
| Than (2012)298 | Yes | Yes | Yes | No | ||
| Thelin (2013)299 | Yes | Yes | Yes | Yes | No | |
| Thomas (2007)300 | Yes | No | ||||
| Thomas (2007)301 | Yes | No | ||||
| Truong (2012)302 | Yes | Yes | Yes | No | ||
| Truong (2011)303 | Yes | Yes | No | Unclear | Unclear | No |
| Twerenbold (2010)304 | Yes | Yes | Yes | Yes | Unclear | No |
| Twerenbold (2010)305 | Yes | Yes | Yes | Yes | Unclear | No |
| Twerenbold (2010)306 | Yes | Yes | Yes | Yes | Unclear | No |
| Twerenbold (2011)307 | Yes | Yes | Yes | No | ||
| Twerenbold (2012)308 | Yes | Yes | Yes | Yes | No | |
| University of Edinburgh (2013)309 | Yes | Yes | Yes | Unclear | No | |
| University of Erlangen (2013)310 | Yes | Yes | Yes | Unclear | Unclear | |
| Van Wijk (2012)311 | Yes | Yes | Yes | Yes | No | |
| Vasikaran (2012)312 | No | |||||
| Veljkovic (2012)313 | Yes | Yes | Yes | Yes | Unclear | No |
| Venge (2008)314 | Yes | No | ||||
| Venge (2009)315 | Yes | No | ||||
| Venge (2010)316 | Yes | No | ||||
| Weber (2011)317 | Yes | No | ||||
| Weber (2009)318 | Yes | No | ||||
| Wildi (2012)319 | Yes | Yes | Yes | No | ||
| Wong (2010)320 | Yes | No | Yes | No | ||
| Worster (2013)321 | Yes | No | Yes | Yes | No | No |
| Zahid (2009)322 | Yes | Yes | Yes | Unclear | Unclear | No |
| Zahid (2008)323 | Yes | Yes | Yes | No | ||
| Zellweger (2012)324 | Yes | Yes | Yes | Yes | No | |
| Zuily (2011)325 | Yes | Yes | Yes | Yes | No |
Appendix 5 Sensitivity analyses (base case)
Deterministic base case
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2257 | 11.734 | –£440 | –0.015 | £28,870 | ||||
| Roche 99th centile | £2301 | 11.740 | –£396 | –0.010 | £41,233 | Abbott 99th centile | £44 | 0.006 | £7777 |
| Beckman 99th centile | £2327 | 11.743 | –£370 | –0.006 | £58,988 | Roche 99th centile | £26 | 0.003 | £7777 |
| Roche strategy | £2426 | 11.744 | –£271 | –0.006 | £48,559 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2493 | 11.748 | –£204 | –0.002 | £124,391 | Beckman 99th centile | £167 | 0.005 | £35,904 |
| Standard Tn | £2697 | 11.749 | Abbott strategy | £204 | 0.002 | £124,391 | |||
Increased re-infarction and mortality risk for no treatment (vs. treated) = lifetime (instead of only during the first year after presentation at emergency department)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2257 | 11.677 | –£440 | –0.072 | £6112 | ||||
| Roche 99th centile | £2301 | 11.704 | –£396 | –0.045 | £8731 | Abbott 99th centile | £44 | 0.027 | Extendedly dominated |
| Beckman 99th centile | £2327 | 11.720 | –£370 | –0.030 | £12,493 | Abbott 99th centile | £69 | 0.042 | £1642 |
| Roche strategy | £2426 | 11.723 | –£271 | –0.026 | £10,284 | Beckman 99th centile | £99 | 0.003 | Extendedly dominated |
| Abbott strategy | £2493 | 11.741 | –£204 | –0.008 | £26,352 | Beckman 99th centile | £167 | 0.022 | £7602 |
| Standard Tn | £2697 | 11.749 | Abbott strategy | £204 | 0.008 | £26,352 | |||
No doctor on demand, but average waiting time before doctor becomes available is increased with 1, 2 or 3 hours
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Waiting time for doctor/decision pending delay = 1 hour(s) | |||||||||
| Abbott 99th centile | £2285 | 11.734 | –£440 | –0.015 | £28,869 | ||||
| Roche 99th centile | £2329 | 11.740 | –£396 | –0.010 | £41,232 | Abbott 99th centile | £44 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2355 | 11.743 | –£370 | –0.006 | £58,987 | Abbott 99th centile | £70 | 0.009 | £7776 |
| Roche strategy | £2470 | 11.744 | –£255 | –0.006 | £45,643 | Beckman 99th centile | £115 | 0.001 | Extendedly dominated |
| Abbott strategy | £2541 | 11.748 | –£184 | –0.002 | £112,580 | Beckman 99th centile | £186 | 0.005 | £40,072 |
| Standard Tn | £2725 | 11.749 | Abbott strategy | £184 | 0.002 | £112,580 | |||
| Waiting time for doctor/decision pending delay = 2 hour(s) | |||||||||
| Abbott 99th centile | £2313 | 11.734 | –£440 | –0.015 | £28,868 | ||||
| Roche 99th centile | £2357 | 11.740 | –£396 | –0.010 | £41,231 | Abbott 99th centile | £44 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2383 | 11.743 | –£370 | –0.006 | £58,987 | Abbott 99th centile | £70 | 0.009 | £7776 |
| Roche strategy | £2515 | 11.744 | –£239 | –0.006 | £42,727 | Beckman 99th centile | £132 | 0.001 | Extendedly dominated |
| Abbott strategy | £2588 | 11.748 | –£165 | –0.002 | £100,769 | Beckman 99th centile | £205 | 0.005 | £44,240 |
| Standard Tn | £2754 | 11.749 | Abbott strategy | £165 | 0.002 | £100,769 | |||
| Waiting time for doctor/decision pending delay = 3 hour(s) | |||||||||
| Abbott 99th centile | £2342 | 11.734 | –£440 | –0.015 | £28,868 | ||||
| Roche 99th centile | £2386 | 11.740 | –£396 | –0.010 | £41,231 | Abbott 99th centile | £44 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2411 | 11.743 | –£370 | –0.006 | £58,986 | Abbott 99th centile | £70 | 0.009 | £7775 |
| Roche strategy | £2559 | 11.744 | –£223 | –0.006 | £39,811 | Beckman 99th centile | £148 | 0.001 | Extendedly dominated |
| Abbott strategy | £2636 | 11.748 | –£146 | –0.002 | £88,957 | Beckman 99th centile | £225 | 0.005 | £48,408 |
| Standard Tn | £2782 | 11.749 | Abbott strategy | £146 | 0.002 | £88,957 | |||
Doctor on demand at emergency department, but average waiting time before doctor becomes available in the general ward is increased with 1, 2 or 3 hours (discharge to general ward after 4 hours after presenting at emergency department)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Waiting time for doctor/decision pending delay = 1 hour(s) | |||||||||
| Abbott 99th centile | £2258 | 11.734 | –£468 | –0.015 | £30,665 | ||||
| Roche 99th centile | £2302 | 11.740 | –£424 | –0.010 | £44,080 | Abbott 99th centile | £44 | 0.006 | £7776 |
| Beckman 99th centile | £2327 | 11.743 | –£398 | –0.006 | £63,347 | Roche 99th centile | £26 | 0.003 | £7776 |
| Roche strategy | £2443 | 11.744 | –£282 | –0.006 | £50,541 | Beckman 99th centile | £115 | 0.001 | Extendedly dominated |
| Abbott strategy | £2513 | 11.748 | –£212 | –0.002 | £129,290 | Beckman 99th centile | £186 | 0.005 | £40,072 |
| Standard Tn | £2725 | 11.749 | Abbott strategy | £212 | 0.002 | £129,290 | |||
| Waiting time for doctor/decision pending delay = 2 hour(s) | |||||||||
| Abbott 99th centile | £2259 | 11.734 | –£495 | –0.015 | £32,459 | ||||
| Roche 99th centile | £2302 | 11.740 | –£451 | –0.010 | £46,927 | Abbott 99th centile | £44 | 0.006 | £7776 |
| Beckman 99th centile | £2328 | 11.743 | –£425 | –0.006 | £67,705 | Roche 99th centile | £26 | 0.003 | £7776 |
| Roche strategy | £2460 | 11.744 | –£294 | –0.006 | £52,522 | Beckman 99th centile | £132 | 0.001 | Extendedly dominated |
| Abbott strategy | £2534 | 11.748 | –£220 | –0.002 | £134,189 | Beckman 99th centile | £205 | 0.005 | £44,240 |
| Standard Tn | £2754 | 11.749 | Abbott strategy | £220 | 0.002 | £134,189 | |||
| Waiting time for doctor/decision pending delay = 3 hour(s) | |||||||||
| Abbott 99th centile | £2260 | 11.734 | –£522 | –0.015 | £34,254 | ||||
| Roche 99th centile | £2303 | 11.740 | –£478 | –0.010 | £49,774 | Abbott 99th centile | £44 | 0.006 | £7775 |
| Beckman 99th centile | £2329 | 11.743 | –£453 | –0.006 | £72,064 | Roche 99th centile | £26 | 0.003 | £7775 |
| Roche strategy | £2477 | 11.744 | –£305 | –0.006 | £54,504 | Beckman 99th centile | £148 | 0.001 | Extendedly dominated |
| Abbott strategy | £2554 | 11.748 | –£228 | –0.002 | £139,089 | Beckman 99th centile | £225 | 0.005 | £48,408 |
| Standard Tn | £2782 | 11.749 | Abbott strategy | £228 | 0.002 | £139,089 | |||
Total delay of 1.5 hours
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2214 | 11.734 | –£440 | –0.015 | £28,871 | ||||
| Roche 99th centile | £2258 | 11.740 | –£396 | –0.010 | £41,234 | Abbott 99th centile | £44 | 0.006 | £7778 |
| Beckman 99th centile | £2284 | 11.743 | –£370 | –0.006 | £58,989 | Roche 99th centile | £26 | 0.003 | £7778 |
| Roche strategy | £2359 | 11.744 | –£296 | –0.006 | £52,933 | Beckman 99th centile | £75 | 0.001 | Extendedly dominated |
| Abbott strategy | £2422 | 11.748 | –£233 | –0.002 | £142,108 | Beckman 99th centile | £138 | 0.005 | £29,653 |
| Standard Tn | £2655 | 11.749 | Abbott strategy | £233 | 0.002 | £142,108 | |||
Myocardial infarction treatment costs added for patients that were tested false-positive
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2456 | 11.734 | –£241 | –0.015 | £15,824 | ||||
| Abbott strategy | £2671 | 11.748 | –£26 | –0.002 | £16,050 | Abbott 99th centile | £215 | 0.014 | £15,797 |
| Standard Tn | £2697 | 11.749 | Abbott strategy | £26 | 0.002 | £16,050 | |||
| Roche 99th centile | £2760 | 11.740 | £63 | –0.010 | Dominated | Standard Tn | £63 | –0.010 | Dominated |
| Roche strategy | £2947 | 11.744 | £251 | –0.006 | Dominated | Standard Tn | £251 | –0.006 | Dominated |
| Beckman 99th centile | £3038 | 11.743 | £341 | –0.006 | Dominated | Standard Tn | £341 | –0.006 | Dominated |
Myocardial infarction treatment costs added to first year of unstable angina
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2703 | 11.734 | –£440 | –0.015 | £28,870 | ||||
| Roche 99th centile | £2747 | 11.740 | –£396 | –0.010 | £41,233 | Abbott 99th centile | £44 | 0.006 | £7777 |
| Beckman 99th centile | £2773 | 11.743 | –£370 | –0.006 | £58,988 | Roche 99th centile | £26 | 0.003 | £7777 |
| Roche strategy | £2872 | 11.744 | –£271 | –0.006 | £48,559 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2940 | 11.748 | –£204 | –0.002 | £124,391 | Beckman 99th centile | £167 | 0.005 | £35,904 |
| Standard Tn | £3144 | 11.749 | Abbott strategy | £204 | 0.002 | £124,391 | |||
Test costs
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Test costs = £5 | |||||||||
| Abbott 99th centile | £2240 | 11.734 | –£425 | –0.015 | £27,856 | ||||
| Roche 99th centile | £2284 | 11.740 | –£381 | –0.010 | £39,624 | Abbott 99th centile | £44 | 0.006 | £7778 |
| Beckman 99th centile | £2310 | 11.743 | –£355 | –0.006 | £56,526 | Roche 99th centile | £26 | 0.003 | £7778 |
| Roche strategy | £2400 | 11.744 | –£265 | –0.006 | £47,439 | Beckman 99th centile | £90 | 0.001 | Extendedly dominated |
| Abbott strategy | £2466 | 11.748 | –£199 | –0.002 | £121,624 | Beckman 99th centile | £156 | 0.005 | £33,550 |
| Standard Tn | £2665 | 11.749 | Abbott strategy | £199 | 0.002 | £121,624 | |||
| Test costs = £40 | |||||||||
| Abbott 99th centile | £2278 | 11.734 | –£460 | –0.015 | £30,150 | ||||
| Roche 99th centile | £2322 | 11.740 | –£416 | –0.010 | £43,264 | Abbott 99th centile | £44 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2348 | 11.743 | –£390 | –0.006 | £62,097 | Abbott 99th centile | £70 | 0.009 | £7776 |
| Roche strategy | £2458 | 11.744 | –£279 | –0.006 | £49,972 | Beckman 99th centile | £111 | 0.001 | Extendedly dominated |
| Abbott strategy | £2528 | 11.748 | –£210 | –0.002 | £127,886 | Beckman 99th centile | £180 | 0.005 | £38,878 |
| Standard Tn | £2737 | 11.749 | Abbott strategy | £210 | 0.002 | £127,886 | |||
Acute myocardial infarction treatment costs
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| AMI treatment costs = £2577 | |||||||||
| Abbott 99th centile | £2119 | 11.734 | –£415 | –0.015 | £27,188 | ||||
| Roche 99th centile | £2154 | 11.740 | –£380 | –0.010 | £39,551 | Abbott 99th centile | £34 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2174 | 11.743 | –£360 | –0.006 | £57,307 | Abbott 99th centile | £55 | 0.009 | £6096 |
| Roche strategy | £2272 | 11.744 | –£262 | –0.006 | £46,877 | Beckman 99th centile | £98 | 0.001 | Extendedly dominated |
| Abbott strategy | £2333 | 11.748 | –£201 | –0.002 | £122,710 | Beckman 99th centile | £159 | 0.005 | £34,223 |
| Standard Tn | £2534 | 11.749 | Roche strategy | £201 | 0.002 | £122,710 | |||
| AMI treatment costs = £4295 | |||||||||
| Abbott 99th centile | £2394 | 11.734 | –£466 | –0.015 | £30,551 | Abbott 99th centile | £53 | 0.006 | £9458 |
| Roche 99th centile | £2448 | 11.740 | –£413 | –0.010 | £42,914 | Roche 99th centile | £32 | 0.003 | £9458 |
| Beckman 99th centile | £2479 | 11.743 | –£381 | –0.006 | £60,669 | Beckman 99th centile | £100 | 0.001 | Extendedly dominated |
| Roche strategy | £2579 | 11.744 | –£281 | –0.006 | £50,240 | Beckman 99th centile | £174 | 0.005 | £37,586 |
| Abbott strategy | £2654 | 11.748 | –£207 | –0.002 | £126,073 | Roche strategy | £207 | 0.002 | £126,073 |
| Standard Tn | £2860 | 11.749 | |||||||
Post-myocardial infarction health-state costs
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Post-MI health-state costs (first year) = £6791 | |||||||||
| Abbott 99th centile | £2393 | 11.734 | –£443 | –0.015 | £29,024 | ||||
| Roche 99th centile | £2438 | 11.740 | –£398 | –0.010 | £41,387 | Abbott 99th centile | £45 | 0.006 | £7931 |
| Beckman 99th centile | £2464 | 11.743 | –£371 | –0.006 | £59,142 | Roche 99th centile | £26 | 0.003 | £7931 |
| Roche strategy | £2563 | 11.744 | –£272 | –0.006 | £48,713 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2632 | 11.748 | –£204 | –0.002 | £124,545 | Beckman 99th centile | £167 | 0.005 | £36,059 |
| Standard Tn | £2836 | 11.749 | Abbott strategy | £204 | 0.002 | £124,545 | |||
| Post-MI health-state costs (first year) = £4879 | |||||||||
| Abbott 99th centile | £2121 | 11.734 | –£438 | –0.015 | £28,715 | ||||
| Roche 99th centile | £2164 | 11.740 | –£395 | –0.010 | £41,078 | Abbott 99th centile | £43 | 0.006 | £7623 |
| Beckman 99th centile | £2189 | 11.743 | –£369 | –0.006 | £58,834 | Roche 99th centile | £25 | 0.003 | £7623 |
| Roche strategy | £2288 | 11.744 | –£271 | –0.006 | £48,405 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2355 | 11.748 | –£204 | –0.002 | £124,237 | Beckman 99th centile | £166 | 0.005 | £35,750 |
| Standard Tn | £2558 | 11.749 | Abbott strategy | £204 | 0.002 | £124,237 | |||
Utility difference between unstable angina and acute myocardial infarction
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Utility difference between UA and AMI = 0.12 | |||||||||
| Abbott 99th centile | £2257 | 11.779 | –£440 | –0.015 | £28,870 | ||||
| Roche 99th centile | £2301 | 11.785 | –£396 | –0.010 | £41,233 | Abbott 99th centile | £44 | 0.006 | £7777 |
| Beckman 99th centile | £2327 | 11.788 | –£370 | –0.006 | £58,988 | Roche 99th centile | £26 | 0.003 | £7777 |
| Roche strategy | £2426 | 11.789 | –£271 | –0.006 | £48,559 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2493 | 11.793 | –£204 | –0.002 | £124,391 | Beckman 99th centile | £167 | 0.005 | £35,904 |
| Standard Tn | £2697 | 11.794 | Abbott strategy | £204 | 0.002 | £124,391 | |||
| Utility difference between UA and AMI = –0.10 | |||||||||
| Abbott 99th centile | £2257 | 11.581 | –£440 | –0.015 | £28,870 | ||||
| Roche 99th centile | £2301 | 11.587 | –£396 | –0.010 | £41,233 | Abbott 99th centile | £44 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2327 | 11.590 | –£370 | –0.006 | £58,988 | Abbott 99th centile | £70 | 0.009 | £7777 |
| Roche strategy | £2426 | 11.591 | –£271 | –0.006 | £48,559 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2493 | 11.595 | –£204 | –0.002 | £124,391 | Beckman 99th centile | £167 | 0.005 | £35,904 |
| Standard Tn | £2697 | 11.597 | Abbott strategy | £204 | 0.002 | £124,391 | |||
Myocardial infarction disutility
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| MI disutility = –0.059 (age = 45 years); –0.050 (age = 55 years); –0.024 (age = 65 years); –0.006 (age = 75+ years) | |||||||||
| Abbott 99th centile | £2257 | 11.735 | –£440 | –0.015 | £28,832 | ||||
| Roche 99th centile | £2301 | 11.741 | –£396 | –0.010 | £41,178 | Abbott 99th centile | £44 | 0.006 | £7767 |
| Beckman 99th centile | £2327 | 11.744 | –£370 | –0.006 | £58,910 | Roche 99th centile | £26 | 0.003 | £7767 |
| Roche strategy | £2426 | 11.745 | –£271 | –0.006 | £48,495 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2493 | 11.749 | –£204 | –0.002 | £124,227 | Beckman 99th centile | £167 | 0.005 | £35,857 |
| Standard Tn | £2697 | 11.751 | Abbott strategy | £204 | 0.002 | £124,227 | |||
| MI disutility = –0.061 (age = 45 years); –0.052 (age = 55 years); –0.026 (age = 65 years); –0.008 (age = 75+ years) | |||||||||
| Abbott 99th centile | £2257 | 11.733 | –£440 | –0.015 | £28,908 | ||||
| Roche 99th centile | £2301 | 11.738 | –£396 | –0.010 | £41,287 | Abbott 99th centile | £44 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2327 | 11.742 | –£370 | –0.006 | £59,066 | Abbott 99th centile | £70 | 0.009 | £7787 |
| Roche strategy | £2426 | 11.742 | –£271 | –0.006 | £48,623 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2493 | 11.746 | –£204 | –0.002 | £124,556 | Beckman 99th centile | £167 | 0.005 | £35,952 |
| Standard Tn | £2697 | 11.748 | Abbott strategy | £204 | 0.002 | £124,556 | |||
Mortality (30-day) treated acute myocardial infarction (decision tree)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Mortality (30-day) treated AMI = 0.120 | |||||||||
| Abbott 99th centile | £2219 | 11.710 | –£432 | –0.010 | £41,819 | ||||
| Roche 99th centile | £2260 | 11.714 | –£391 | –0.007 | £60,062 | Abbott 99th centile | £41 | 0.004 | £10,692 |
| Beckman 99th centile | £2284 | 11.716 | –£367 | –0.004 | £86,264 | Roche 99th centile | £24 | 0.002 | £10,692 |
| Roche strategy | £2383 | 11.717 | –£268 | –0.004 | £70,874 | Beckman 99th centile | £99 | 0.000 | Extendedly dominated |
| Abbott strategy | £2448 | 11.719 | –£203 | –0.001 | £182,781 | Beckman 99th centile | £164 | 0.003 | £52,200 |
| Standard Tn | £2651 | 11.721 | Abbott strategy | £203 | 0.001 | £182,781 | |||
| Mortality (30-day) treated AMI = 0.074 | |||||||||
| Abbott 99th centile | £2295 | 11.758 | –£448 | –0.020 | £22,206 | ||||
| Roche 99th centile | £2342 | 11.765 | –£401 | –0.013 | £31,543 | Abbott 99th centile | £47 | 0.007 | Extendedly dominated |
| Beckman 99th centile | £2369 | 11.770 | –£374 | –0.008 | £44,952 | Abbott 99th centile | £75 | 0.012 | £6277 |
| Roche strategy | £2469 | 11.771 | –£274 | –0.007 | £37,076 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2538 | 11.776 | –£205 | –0.002 | £94,345 | Beckman 99th centile | £169 | 0.006 | £27,519 |
| Standard Tn | £2743 | 11.778 | Abbott strategy | £205 | 0.002 | £94,345 | |||
Mortality (30-day) untreated acute myocardial infarction (decision tree)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Mortality (30-day) untreated AMI = 0.240 | |||||||||
| Abbott 99th centile | £2227 | 11.707 | –£470 | –0.042 | £11,153 | ||||
| Roche 99th centile | £2282 | 11.723 | –£415 | –0.027 | £15,623 | Abbott 99th centile | £55 | 0.016 | Extendedly dominated |
| Beckman 99th centile | £2314 | 11.732 | –£383 | –0.017 | £22,042 | Abbott 99th centile | £88 | 0.025 | £3528 |
| Roche strategy | £2414 | 11.734 | –£282 | –0.015 | £18,271 | Beckman 99th centile | £100 | 0.002 | Extendedly dominated |
| Abbott strategy | £2490 | 11.745 | –£207 | –0.005 | £45,686 | Beckman 99th centile | £176 | 0.013 | £13,697 |
| Standard Tn | £2697 | 11.749 | Abbott strategy | £207 | 0.005 | £45,686 | |||
| Mortality (30-day) untreated AMI = 0.000 | |||||||||
| Abbott 99th centile | £2280 | 11.755 | –£417 | 0.006 | Dominant | ||||
| Roche 99th centile | £2316 | 11.753 | –£381 | 0.004 | Dominant | Abbott 99th centile | £35 | –0.002 | Dominated |
| Beckman 99th centile | £2336 | 11.752 | –£361 | 0.002 | Dominant | Abbott 99th centile | £56 | –0.003 | Dominated |
| Roche strategy | £2434 | 11.751 | –£263 | 0.002 | Dominant | Abbott 99th centile | £154 | –0.004 | Dominated |
| Abbott strategy | £2496 | 11.750 | –£201 | 0.001 | Dominant | Abbott 99th centile | £215 | –0.005 | Dominated |
| Standard Tn | £2697 | 11.749 | Abbott 99th centile | £417 | –0.006 | Dominated | |||
Annual re-infarction probability (after initial acute myocardial infarction)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Annual re-infarction (after initial AMI) = 0.26 | |||||||||
| Abbott 99th centile | £2286 | 11.722 | –£440 | –0.015 | £28,543 | ||||
| Roche 99th centile | £2330 | 11.728 | –£397 | –0.010 | £40,757 | Abbott 99th centile | £44 | 0.006 | £7704 |
| Beckman 99th centile | £2356 | 11.731 | –£371 | –0.006 | £58,299 | Roche 99th centile | £26 | 0.003 | £7704 |
| Roche strategy | £2455 | 11.732 | –£272 | –0.006 | £47,995 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2523 | 11.736 | –£204 | –0.002 | £122,916 | Beckman 99th centile | £167 | 0.005 | £35,493 |
| Standard Tn | £2727 | 11.737 | Abbott strategy | £204 | 0.002 | £122,916 | |||
| Annual re-infarction (after initial AMI) = 0.19 | |||||||||
| Abbott 99th centile | £2227 | 11.746 | –£440 | –0.015 | £29,218 | ||||
| Roche 99th centile | £2270 | 11.752 | –£396 | –0.009 | £41,738 | Abbott 99th centile | £44 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2296 | 11.755 | –£370 | –0.006 | £59,719 | Abbott 99th centile | £70 | 0.009 | £7856 |
| Roche strategy | £2395 | 11.756 | –£271 | –0.006 | £49,157 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2463 | 11.760 | –£204 | –0.002 | £125,955 | Beckman 99th centile | £167 | 0.005 | £36,342 |
| Standard Tn | £2666 | 11.761 | Abbott strategy | £204 | 0.002 | £125,955 | |||
Relativer risk re-infarction (untreated compared with treated)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| RR re-infarction (untreated vs. treated) = 5.15 | |||||||||
| Abbott 99th centile | £2259 | 11.730 | –£438 | –0.019 | £22,555 | ||||
| Roche 99th centile | £2302 | 11.737 | –£395 | –0.012 | £32,258 | Abbott 99th centile | £43 | 0.007 | £5999 |
| Beckman 99th centile | £2327 | 11.741 | –£370 | –0.008 | £46,195 | Roche 99th centile | £25 | 0.004 | £5999 |
| Roche strategy | £2426 | 11.742 | –£271 | –0.007 | £38,009 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2493 | 11.747 | –£204 | –0.002 | £97,530 | Beckman 99th centile | £166 | 0.006 | £28,076 |
| Standard Tn | £2697 | 11.749 | Abbott strategy | £204 | 0.002 | £97,530 | |||
| RR re-infarction (untreated vs. treated) = 1.28 | |||||||||
| Costs | QALYs | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |
| Abbott 99th centile | £2256 | 11.736 | –£441 | –0.013 | £33,518 | ||||
| Roche 99th centile | £2300 | 11.741 | –£397 | –0.008 | £47,838 | Abbott 99th centile | £44 | 0.005 | Extendedly dominated |
| Beckman 99th centile | £2326 | 11.744 | –£371 | –0.005 | £68,404 | Abbott 99th centile | £70 | 0.008 | £9086 |
| Roche strategy | £2425 | 11.744 | –£272 | –0.005 | £56,324 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2493 | 11.748 | –£204 | –0.001 | £144,162 | Beckman 99th centile | £167 | 0.004 | £41,666 |
| Standard Tn | £2697 | 11.749 | Abbott strategy | £204 | 0.001 | £144,162 | |||
Annual post-myocardial infarction mortality
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Annual post-MI mortality = 0.068 | |||||||||
| Abbott 99th centile | £2248 | 11.715 | –£440 | –0.015 | £28,843 | ||||
| Roche 99th centile | £2292 | 11.721 | –£396 | –0.010 | £41,191 | Abbott 99th centile | £44 | 0.006 | £7777 |
| Beckman 99th centile | £2318 | 11.724 | –£370 | –0.006 | £58,924 | Roche 99th centile | £26 | 0.003 | £7777 |
| Roche strategy | £2417 | 11.725 | –£271 | –0.006 | £48,508 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2485 | 11.729 | –£204 | –0.002 | £124,247 | Beckman 99th centile | £167 | 0.005 | £35,869 |
| Standard Tn | £2688 | 11.731 | Abbott strategy | £204 | 0.002 | £124,247 | |||
| Annual post-MI mortality = 0.065 | |||||||||
| Abbott 99th centile | £2266 | 11.753 | –£440 | –0.015 | £28,897 | ||||
| Roche 99th centile | £2309 | 11.758 | –£396 | –0.010 | £41,275 | Abbott 99th centile | £44 | 0.006 | £7777 |
| Beckman 99th centile | £2335 | 11.762 | –£370 | –0.006 | £59,053 | Roche 99th centile | £26 | 0.003 | £7777 |
| Roche strategy | £2434 | 11.762 | –£271 | –0.006 | £48,610 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2502 | 11.766 | –£204 | –0.002 | £124,538 | Beckman 99th centile | £167 | 0.005 | £35,940 |
| Standard Tn | £2706 | 11.768 | Abbott strategy | £204 | 0.002 | £124,538 | |||
Annual mortality post myocardial infarction after re-infarction
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Annual mortality post-MI with re-infarction = 0.137 | |||||||||
| Abbott 99th centile | £2258 | 11.737 | –£440 | –0.015 | £28,946 | ||||
| Roche 99th centile | £2302 | 11.742 | –£396 | –0.010 | £41,341 | Abbott 99th centile | £44 | 0.006 | £7797 |
| Beckman 99th centile | £2328 | 11.746 | –£370 | –0.006 | £59,144 | Roche 99th centile | £26 | 0.003 | £7797 |
| Roche strategy | £2427 | 11.746 | –£271 | –0.006 | £48,687 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2494 | 11.750 | –£204 | –0.002 | £124,721 | Beckman 99th centile | £167 | 0.005 | £35,999 |
| Standard Tn | £2698 | 11.752 | Abbott strategy | £204 | 0.002 | £124,721 | |||
| Annual mortality post-MI with re-infarction = 0.146 | |||||||||
| Abbott 99th centile | £2256 | 11.731 | –£440 | –0.015 | £28,795 | ||||
| Roche 99th centile | £2300 | 11.737 | –£396 | –0.010 | £41,126 | Abbott 99th centile | £44 | 0.006 | £7758 |
| Beckman 99th centile | £2325 | 11.740 | –£370 | –0.006 | £58,835 | Roche 99th centile | £26 | 0.003 | £7758 |
| Roche strategy | £2424 | 11.741 | –£271 | –0.006 | £48,433 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2492 | 11.745 | –£204 | –0.002 | £124,067 | Beckman 99th centile | £167 | 0.005 | £35,812 |
| Standard Tn | £2696 | 11.746 | Abbott strategy | £204 | 0.002 | £124,067 | |||
Hazard ratio mortality (unstable angina compared with non-ST segment elevation myocardial infarction)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| HR mortality (UA vs. NSTEMI) = 1.053 | |||||||||
| Abbott 99th centile | £2205 | 11.558 | –£440 | –0.015 | £28,870 | ||||
| Roche 99th centile | £2249 | 11.564 | –£396 | –0.010 | £41,233 | Abbott 99th centile | £44 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2274 | 11.567 | –£370 | –0.006 | £58,988 | Abbott 99th centile | £70 | 0.009 | £7777 |
| Roche strategy | £2374 | 11.568 | –£271 | –0.006 | £48,559 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2441 | 11.572 | –£204 | –0.002 | £124,391 | Beckman 99th centile | £167 | 0.005 | £35,904 |
| Standard Tn | £2645 | 11.573 | Abbott strategy | £204 | 0.002 | £124,391 | |||
| HR mortality (UA vs. NSTEMI) = 0.581 | |||||||||
| Abbott 99th centile | £2306 | 11.898 | –£440 | –0.015 | £28,870 | ||||
| Roche 99th centile | £2349 | 11.904 | –£396 | –0.010 | £41,233 | Abbott 99th centile | £44 | 0.006 | £7777 |
| Beckman 99th centile | £2375 | 11.907 | –£370 | –0.006 | £58,988 | Roche 99th centile | £26 | 0.003 | £7777 |
| Roche strategy | £2474 | 11.908 | –£271 | –0.006 | £48,559 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2542 | 11.912 | –£204 | –0.002 | £124,391 | Beckman 99th centile | £167 | 0.005 | £35,904 |
| Standard Tn | £2746 | 11.913 | Abbott strategy | £204 | 0.002 | £124,391 | |||
Relative risk mortality (untreated vs. treated acute myocardial infarction)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| RR mortality (untreated vs. treated AMI) = 3.908 | |||||||||
| Abbott 99th centile | £2224 | 11.709 | –£472 | –0.040 | £11,771 | ||||
| Roche 99th centile | £2280 | 11.724 | –£417 | –0.025 | £16,467 | Abbott 99th centile | £56 | 0.015 | £3759 |
| Beckman 99th centile | £2313 | 11.733 | –£384 | –0.017 | £23,212 | Roche 99th centile | £33 | 0.009 | £3759 |
| Roche strategy | £2414 | 11.734 | –£283 | –0.015 | £19,250 | Beckman 99th centile | £100 | 0.002 | Extendedly dominated |
| Abbott strategy | £2490 | 11.745 | –£207 | –0.004 | £48,054 | Beckman 99th centile | £176 | 0.012 | £14,443 |
| Standard Tn | £2697 | 11.749 | Abbott strategy | £207 | 0.004 | £48,054 | |||
| RR mortality (untreated vs. treated AMI) = 0.901 | |||||||||
| Abbott 99th centile | £2272 | 11.746 | –£425 | –0.003 | £128,875 | ||||
| Roche 99th centile | £2310 | 11.747 | –£387 | –0.002 | £186,080 | Abbott 99th centile | £38 | 0.001 | Extendedly dominated |
| Beckman 99th centile | £2333 | 11.748 | –£364 | –0.001 | £268,237 | Abbott 99th centile | £61 | 0.002 | £31,275 |
| Roche strategy | £2431 | 11.748 | –£266 | –0.001 | £219,979 | Beckman 99th centile | £98 | 0.000 | Extendedly dominated |
| Abbott strategy | £2495 | 11.749 | –£202 | 0.000 | £570,869 | Beckman 99th centile | £162 | 0.001 | £161,425 |
| Standard Tn | £2697 | 11.749 | Abbott strategy | £202 | 0.000 | £570,869 | |||
Appendix 6 Sensitivity analyses (secondary analysis)
Deterministic secondary analysis
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2789 | 11.530 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2832 | 11.532 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2858 | 11.532 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2957 | 11.535 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3025 | 11.543 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,047 |
| Standard Tn | £3064 | 11.493 | Abbott strategy | £39 | –0.050 | Dominated | |||
Increased re-infarction and mortality risk for no treatment (vs. treated) = lifetime (instead of only during the first year after presentation at emergency)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2789 | 11.473 | –£286 | 0.089 | Dominant | ||||
| Roche 99th centile | £2833 | 11.496 | –£242 | 0.113 | Dominant | Abbott 99th centile | £43 | 0.023 | £1853 |
| Beckman 99th centile | £2858 | 11.509 | –£217 | 0.126 | Dominant | Roche 99th centile | £26 | 0.013 | £2017 |
| Roche strategy | £2957 | 11.515 | –£118 | 0.131 | Dominant | Beckman 99th centile | £99 | 0.006 | Extendedly dominated |
| Abbott strategy | £3025 | 11.537 | –£50 | 0.154 | Dominant | Beckman 99th centile | £167 | 0.028 | £5889 |
| Standard Tn | £3075 | 11.383 | Abbott strategy | £50 | –0.154 | Dominated | |||
No doctor on demand, but average waiting time before doctor becomes available is increased with 1, 2 or 3 hours
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Waiting time for doctor/decision pending delay = 1 hour(s) | |||||||||
| Abbott 99th centile | £2817 | 11.530 | –£275 | 0.036 | Dominant | ||||
| Roche 99th centile | £2861 | 11.532 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | £17,587 |
| Beckman 99th centile | £2887 | 11.532 | –£206 | 0.039 | Dominant | Roche 99th centile | £26 | 0.000 | Extendedly dominated |
| Roche strategy | £3002 | 11.535 | –£91 | 0.042 | Dominant | Roche 99th centile | £141 | 0.003 | Extendedly dominated |
| Abbott strategy | £3073 | 11.543 | –£20 | 0.050 | Dominant | Roche 99th centile | £212 | 0.011 | £18,628 |
| Standard Tn | £3093 | 11.493 | Abbott strategy | £20 | –0.050 | Dominated | |||
| Waiting time for doctor/decision pending delay = 2 hour(s) | |||||||||
| Abbott 99th centile | £2846 | 11.530 | –£275 | 0.036 | Dominant | ||||
| Roche 99th centile | £2890 | 11.532 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | £17,586 |
| Beckman 99th centile | £2915 | 11.532 | –£206 | 0.039 | Dominant | Roche 99th centile | £26 | 0.000 | Extendedly dominated |
| Roche strategy | £3047 | 11.535 | –£74 | 0.042 | Dominant | Roche 99th centile | £157 | 0.003 | Extendedly dominated |
| Abbott strategy | £3121 | 11.543 | £0 | 0.050 | Dominant | Roche 99th centile | £232 | 0.011 | £20,326 |
| Standard Tn | £3121 | 11.493 | Abbott strategy | £0 | –0.050 | Dominated | |||
| Waiting time for doctor/decision pending delay = 3 hour(s) | |||||||||
| Abbott 99th centile | £2875 | 11.530 | –£275 | 0.036 | Dominant | ||||
| Roche 99th centile | £2918 | 11.532 | –£231 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | £17,584 |
| Beckman 99th centile | £2944 | 11.532 | –£206 | 0.039 | Dominant | Roche 99th centile | £26 | 0.000 | Extendedly dominated |
| Roche strategy | £3092 | 11.535 | –£58 | 0.042 | Dominant | Roche 99th centile | £174 | 0.003 | Extendedly dominated |
| Standard Tn | £3149 | 11.493 | Roche 99th centile | £231 | –0.039 | Dominated | |||
| Abbott strategy | £3169 | 11.543 | £20 | 0.050 | £390 | Roche 99th centile | £251 | 0.011 | £22,024 |
Doctor on demand at emergency department, but average waiting time before doctor becomes available in the general ward is increased with 1, 2 or 3 hours (discharge to general ward after 4 hours after presenting at emergency department)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Waiting time for doctor/decision pending delay = 1 hour(s) | |||||||||
| Abbott 99th centile | £2790 | 11.530 | –£303 | 0.036 | Dominant | ||||
| Roche 99th centile | £2834 | 11.532 | –£259 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | £17,587 |
| Beckman 99th centile | £2859 | 11.532 | –£234 | 0.039 | Dominant | Roche 99th centile | £26 | 0.000 | Extendedly dominated |
| Roche strategy | £2975 | 11.535 | –£118 | 0.042 | Dominant | Roche 99th centile | £141 | 0.003 | Extendedly dominated |
| Abbott strategy | £3046 | 11.543 | –£47 | 0.050 | Dominant | Roche 99th centile | £212 | 0.011 | £18,628 |
| Standard Tn | £3093 | 11.493 | Abbott strategy | £47 | –0.050 | Dominated | |||
| Waiting time for doctor/decision pending delay = 2 hour(s) | |||||||||
| Abbott 99th centile | £2791 | 11.530 | –£330 | 0.036 | Dominant | ||||
| Roche 99th centile | £2835 | 11.532 | –£286 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | £17,586 |
| Beckman 99th centile | £2860 | 11.532 | –£261 | 0.039 | Dominant | Roche 99th centile | £26 | 0.000 | Extendedly dominated |
| Roche strategy | £2992 | 11.535 | –£129 | 0.042 | Dominant | Roche 99th centile | £157 | 0.003 | Extendedly dominated |
| Abbott strategy | £3066 | 11.543 | –£55 | 0.050 | Dominant | Roche 99th centile | £232 | 0.011 | £20,326 |
| Standard Tn | £3121 | 11.493 | Abbott strategy | £55 | –0.050 | Dominated | |||
| Waiting time for doctor/decision pending delay = 3 hour(s) | |||||||||
| Abbott 99th centile | £2792 | 11.530 | –£357 | 0.036 | Dominant | ||||
| Roche 99th centile | £2836 | 11.532 | –£313 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | £17,584 |
| Beckman 99th centile | £2862 | 11.532 | –£288 | 0.039 | Dominant | Roche 99th centile | £26 | 0.000 | Extendedly dominated |
| Roche strategy | £3010 | 11.535 | –£140 | 0.042 | Dominant | Roche 99th centile | £174 | 0.003 | Extendedly dominated |
| Abbott strategy | £3087 | 11.543 | –£63 | 0.050 | Dominant | Roche 99th centile | £251 | 0.011 | £22,024 |
| Standard Tn | £3149 | 11.493 | Abbott strategy | £63 | –0.050 | Dominated | |||
Total delay of 1.5 hours
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2746 | 11.530 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2790 | 11.532 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2815 | 11.532 | –£207 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2890 | 11.535 | –£132 | 0.042 | Dominant | Abbott 99th centile | £144 | 0.006 | Extendedly dominated |
| Abbott strategy | £2953 | 11.543 | –£69 | 0.050 | Dominant | Abbott 99th centile | £207 | 0.014 | £14,956 |
| Standard Tn | £3022 | 11.493 | Abbott strategy | £69 | –0.050 | Dominated | |||
Myocardial infarction treatment costs added for patients that were tested false-positive
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2841 | 11.530 | –£224 | 0.036 | Dominant | ||||
| Abbott strategy | £3056 | 11.543 | –£9 | 0.050 | Dominant | Abbott 99th centile | £215 | 0.014 | £15,507 |
| Standard Tn | £3064 | 11.493 | £0 | 0.000 | Abbott strategy | £9 | –0.050 | Dominated | |
| Roche 99th centile | £3144 | 11.532 | £80 | 0.039 | £2065 | Abbott strategy | £89 | –0.011 | Dominated |
| Roche strategy | £3331 | 11.535 | £267 | 0.042 | £6360 | Abbott strategy | £275 | –0.008 | Dominated |
| Beckman 99th centile | £3421 | 11.532 | £356 | 0.039 | £9142 | Abbott strategy | £365 | –0.011 | Dominated |
Myocardial infarction treatment costs added to first year of unstable angina
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £3212 | 11.530 | –£275 | 0.036 | Dominant | ||||
| Roche 99th centile | £3256 | 11.532 | –£231 | 0.039 | Dominant | Abbott 99th centile | £43 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £3281 | 11.532 | –£205 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £3381 | 11.535 | –£106 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3449 | 11.543 | –£38 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,048 |
| Standard Tn | £3487 | 11.493 | Abbott strategy | £38 | –0.050 | Dominated | |||
Test costs
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Test costs = £5 | |||||||||
| Abbott 99th centile | £2772 | 11.530 | –£260 | 0.036 | Dominant | ||||
| Roche 99th centile | £2816 | 11.532 | –£217 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2841 | 11.532 | –£191 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2931 | 11.535 | –£101 | 0.042 | Dominant | Abbott 99th centile | £159 | 0.006 | Extendedly dominated |
| Abbott strategy | £2997 | 11.543 | –£35 | 0.050 | Dominant | Abbott 99th centile | £225 | 0.014 | £16,260 |
| Standard Tn | £3032 | 11.493 | Abbott strategy | £35 | –0.050 | Dominated | |||
| Test costs = £40 | |||||||||
| Abbott 99th centile | £2810 | 11.530 | –£295 | 0.036 | Dominant | ||||
| Roche 99th centile | £2854 | 11.532 | –£252 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | £17,586 |
| Beckman 99th centile | £2879 | 11.532 | –£226 | 0.039 | Dominant | Roche 99th centile | £26 | 0.000 | Extendedly dominated |
| Roche strategy | £2990 | 11.535 | –£115 | 0.042 | Dominant | Roche 99th centile | £137 | 0.003 | Extendedly dominated |
| Abbott strategy | £3060 | 11.543 | –£45 | 0.050 | Dominant | Roche 99th centile | £207 | 0.011 | £18,141 |
| Standard Tn | £3105 | 11.493 | Abbott strategy | £45 | –0.050 | Dominated | |||
Acute myocardial infarction
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| AMI treatment costs = £2577 | |||||||||
| Abbott 99th centile | £2607 | 11.530 | –£286 | 0.036 | Dominant | ||||
| Roche 99th centile | £2641 | 11.532 | –£252 | 0.039 | Dominant | Abbott 99th centile | £34 | 0.002 | £13,770 |
| Beckman 99th centile | £2661 | 11.532 | –£232 | 0.039 | Dominant | Roche 99th centile | £20 | 0.000 | Extendedly dominated |
| Roche strategy | £2759 | 11.535 | –£134 | 0.042 | Dominant | Roche 99th centile | £118 | 0.003 | Extendedly dominated |
| Abbott strategy | £2820 | 11.543 | –£73 | 0.050 | Dominant | Roche 99th centile | £179 | 0.011 | £15,751 |
| Standard Tn | £2893 | 11.493 | Abbott strategy | £73 | –0.050 | Dominated | |||
| AMI treatment costs = £4295 | |||||||||
| Abbott 99th centile | £2971 | 11.530 | –£265 | 0.036 | Dominant | ||||
| Roche 99th centile | £3024 | 11.532 | –£212 | 0.039 | Dominant | Abbott 99th centile | £53 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £3055 | 11.532 | –£181 | 0.039 | Dominant | Abbott 99th centile | £84 | 0.003 | Extendedly dominated |
| Roche strategy | £3155 | 11.535 | –£81 | 0.042 | Dominant | Abbott 99th centile | £185 | 0.006 | Extendedly dominated |
| Abbott strategy | £3230 | 11.543 | –£6 | 0.050 | Dominant | Abbott 99th centile | £259 | 0.014 | £18,698 |
| Standard Tn | £3236 | 11.493 | Abbott strategy | £6 | –0.050 | Dominated | |||
Post-myocardial infarction health-state costs
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Post MI health-state costs (first year) = £6791 | |||||||||
| Abbott 99th centile | £2970 | 11.530 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £3014 | 11.532 | –£231 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £3040 | 11.532 | –£205 | 0.039 | Dominant | Abbott 99th centile | £71 | 0.003 | Extendedly dominated |
| Roche strategy | £3139 | 11.535 | –£106 | 0.042 | Dominant | Abbott 99th centile | £170 | 0.006 | Extendedly dominated |
| Abbott strategy | £3208 | 11.543 | –£37 | 0.050 | Dominant | Abbott 99th centile | £239 | 0.014 | £17,199 |
| Standard Tn | £3245 | 11.493 | Abbott strategy | £37 | –0.050 | Dominated | |||
| Post MI health-state costs (first year) = £4879 | |||||||||
| Abbott 99th centile | £2608 | 11.530 | –£275 | 0.036 | Dominant | ||||
| Roche 99th centile | £2651 | 11.532 | –£233 | 0.039 | Dominant | Abbott 99th centile | £43 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2676 | 11.532 | –£207 | 0.039 | Dominant | Abbott 99th centile | £68 | 0.003 | Extendedly dominated |
| Roche strategy | £2775 | 11.535 | –£108 | 0.042 | Dominant | Abbott 99th centile | £167 | 0.006 | Extendedly dominated |
| Abbott strategy | £2842 | 11.543 | –£41 | 0.050 | Dominant | Abbott 99th centile | £234 | 0.014 | £16,896 |
| Standard Tn | £2883 | 11.493 | Abbott strategy | £41 | –0.050 | Dominated | |||
Utility difference between unstable angina and acute myocardial infarction
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Utility difference between UA and AMI = 0.12 | |||||||||
| Abbott 99th centile | £2789 | 11.572 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2832 | 11.575 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2858 | 11.575 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2957 | 11.578 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3025 | 11.586 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,046 |
| Standard Tn | £3064 | 11.536 | Abbott strategy | £39 | –0.050 | Dominated | |||
| Utility difference between UA and AMI = –0.10 | |||||||||
| Abbott 99th centile | £2789 | 11.385 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2832 | 11.387 | –£232 | 0.038 | Dominant | Abbott 99th centile | £44 | 0.003 | Extendedly dominated |
| Beckman 99th centile | £2858 | 11.388 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2957 | 11.391 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3025 | 11.399 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,051 |
| Standard Tn | £3064 | 11.349 | Abbott strategy | £39 | –0.050 | Dominated | |||
Myocardial infarction disutility
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| MI disutility = –0.059 (age = 45 years); –0.050 (age = 55 years); –0.024 (age = 65 years); –0.006 (age = 75 + years) | |||||||||
| Abbott 99th centile | £2789 | 11.531 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2832 | 11.534 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2858 | 11.534 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2957 | 11.537 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3025 | 11.545 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,025 |
| Standard Tn | £3064 | 11.495 | Abbott strategy | £39 | –0.050 | Dominated | |||
| MI disutility = –0.061 (age = 45 years); –0.052 (age = 55 years); –0.026 (age = 65 years); –0.008 (age = 75 + years) | |||||||||
| Abbott 99th centile | £2789 | 11.528 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2832 | 11.530 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2858 | 11.531 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2957 | 11.534 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3025 | 11.542 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,070 |
| Standard Tn | £3064 | 11.492 | Abbott strategy | £39 | –0.050 | Dominated | |||
Mortality (30-day) treated acute myocardial infarction (decision tree)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Mortality (30-day) treated AMI = 0.120 | |||||||||
| Abbott 99th centile | £2750 | 11.504 | –£269 | 0.039 | Dominant | ||||
| Roche 99th centile | £2790 | 11.504 | –£228 | 0.039 | Dominant | Abbott 99th centile | £41 | 0.000 | Dominated |
| Beckman 99th centile | £2814 | 11.502 | –£205 | 0.038 | Dominant | Abbott 99th centile | £64 | –0.002 | Dominated |
| Roche strategy | £2913 | 11.506 | –£106 | 0.041 | Dominant | Abbott 99th centile | £163 | 0.002 | Extendedly dominated |
| Abbott strategy | £2979 | 11.514 | –£40 | 0.049 | Dominant | Abbott 99th centile | £229 | 0.010 | £24,010 |
| Standard Tn | £3019 | 11.465 | Abbott strategy | £40 | –0.049 | Dominated | |||
| Mortality (30-day) treated AMI = 0.074 | |||||||||
| Abbott 99th centile | £2828 | 11.555 | –£282 | 0.033 | Dominant | ||||
| Roche 99th centile | £2875 | 11.560 | –£236 | 0.038 | Dominant | Abbott 99th centile | £47 | 0.005 | £9175 |
| Beckman 99th centile | £2902 | 11.562 | –£208 | 0.040 | Dominant | Roche 99th centile | £28 | 0.002 | £12,967 |
| Roche strategy | £3002 | 11.565 | –£109 | 0.043 | Dominant | Beckman 99th centile | £100 | 0.003 | Extendedly dominated |
| Abbott strategy | £3072 | 11.574 | –£39 | 0.051 | Dominant | Beckman 99th centile | £170 | 0.011 | £15,399 |
| Standard Tn | £3111 | 11.522 | Abbott strategy | £39 | –0.051 | Dominated | |||
Mortality (30-day) untreated acute myocardial infarction (decision tree)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Mortality (30-day) untreated AMI = 0.240 | |||||||||
| Abbott 99th centile | £2759 | 11.503 | –£294 | 0.066 | Dominant | ||||
| Roche 99th centile | £2813 | 11.515 | –£239 | 0.079 | Dominant | Abbott 99th centile | £55 | 0.012 | £4404 |
| Beckman 99th centile | £2846 | 11.521 | –£207 | 0.085 | Dominant | Roche 99th centile | £32 | 0.006 | £5228 |
| Roche strategy | £2946 | 11.525 | –£106 | 0.089 | Dominant | Beckman 99th centile | £101 | 0.004 | Extendedly dominated |
| Abbott strategy | £3022 | 11.541 | –£30 | 0.104 | Dominant | Beckman 99th centile | £176 | 0.019 | £9139 |
| Standard Tn | £3052 | 11.436 | Abbott strategy | £30 | –0.104 | Dominated | |||
| Mortality (30-day) untreated AMI = 0.000 | |||||||||
| Abbott 99th centile | £2813 | 11.551 | –£262 | 0.013 | Dominant | ||||
| Roche 99th centile | £2847 | 11.545 | –£227 | 0.007 | Dominant | Abbott 99th centile | £35 | –0.005 | Dominated |
| Beckman 99th centile | £2868 | 11.541 | –£206 | 0.003 | Dominant | Abbott 99th centile | £55 | –0.010 | Dominated |
| Roche strategy | £2966 | 11.543 | –£108 | 0.005 | Dominant | Abbott 99th centile | £153 | –0.008 | Dominated |
| Abbott strategy | £3028 | 11.546 | –£46 | 0.008 | Dominant | Abbott 99th centile | £215 | –0.005 | Dominated |
| Standard Tn | £3074 | 11.538 | Abbott 99th centile | £262 | –0.013 | Dominated | |||
Annual re-infarction probability (after initial acute myocardial infarction)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Annual re-infarction (after initial AMI) = 0.26 | |||||||||
| Abbott 99th centile | £2830 | 11.515 | –£275 | 0.036 | Dominant | ||||
| Roche 99th centile | £2873 | 11.517 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.003 | Extendedly dominated |
| Beckman 99th centile | £2899 | 11.517 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2998 | 11.520 | –£107 | 0.042 | Dominant | Abbott 99th centile | £169 | 0.006 | Extendedly dominated |
| Abbott strategy | £3066 | 11.529 | –£39 | 0.050 | Dominant | Abbott 99th centile | £237 | 0.014 | £16,867 |
| Standard Tn | £3105 | 11.478 | Abbott strategy | £39 | –0.050 | Dominated | |||
| Annual re-infarction (after initial AMI) = 0.19 | |||||||||
| Abbott 99th centile | £2747 | 11.545 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2791 | 11.547 | –£233 | 0.038 | Dominant | Abbott 99th centile | £43 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2817 | 11.548 | –£207 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2916 | 11.551 | –£108 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £2984 | 11.559 | –£40 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,241 |
| Standard Tn | £3023 | 11.509 | Abbott strategy | £40 | –0.050 | Dominated | |||
Relative risk re-infarction (untreated vs. treated)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| RR re-infarction (untreated vs. treated) = 5.15 | |||||||||
| Abbott 99th centile | £2791 | 11.525 | –£277 | 0.036 | Dominant | ||||
| Roche 99th centile | £2834 | 11.529 | –£234 | 0.041 | Dominant | Abbott 99th centile | £43 | 0.004 | £10,647 |
| Beckman 99th centile | £2859 | 11.531 | –£209 | 0.042 | Dominant | Roche 99th centile | £25 | 0.001 | Extendedly dominated |
| Roche strategy | £2958 | 11.534 | –£110 | 0.045 | Dominant | Roche 99th centile | £124 | 0.004 | Extendedly dominated |
| Abbott strategy | £3025 | 11.543 | –£43 | 0.054 | Dominant | Roche 99th centile | £192 | 0.014 | £14,126 |
| Standard Tn | £3068 | 11.489 | Abbott strategy | £43 | –0.054 | Dominated | |||
| RR re-infarction (untreated vs. treated) = 1.28 | |||||||||
| Abbott 99th centile | £2788 | 11.532 | –£275 | 0.036 | Dominant | ||||
| Roche 99th centile | £2832 | 11.533 | –£231 | 0.038 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2858 | 11.533 | –£205 | 0.038 | Dominant | Abbott 99th centile | £70 | 0.002 | Dominated |
| Roche strategy | £2957 | 11.536 | –£106 | 0.040 | Dominant | Abbott 99th centile | £169 | 0.004 | Extendedly dominated |
| Abbott strategy | £3025 | 11.544 | –£38 | 0.048 | Dominant | Abbott 99th centile | £237 | 0.012 | £19,764 |
| Standard Tn | £3063 | 11.496 | Abbott strategy | £38 | –0.048 | Dominated | |||
Annual post-myocardial infarction mortality
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Annual post-MI mortality = 0.068 | |||||||||
| Abbott 99th centile | £2779 | 11.509 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2822 | 11.511 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2848 | 11.512 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2947 | 11.514 | –£107 | 0.042 | Dominant | Abbott 99th centile | £169 | 0.006 | Extendedly dominated |
| Abbott strategy | £3015 | 11.523 | –£39 | 0.050 | Dominant | Abbott 99th centile | £237 | 0.014 | £17,036 |
| Standard Tn | £3054 | 11.472 | Abbott strategy | £39 | –0.050 | Dominated | |||
| Annual post-MI mortality = 0.065 | |||||||||
| Abbott 99th centile | £2799 | 11.551 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2843 | 11.553 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2868 | 11.553 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2968 | 11.556 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3035 | 11.565 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,059 |
| Standard Tn | £3075 | 11.515 | Abbott strategy | £39 | –0.050 | Dominated | |||
Annual mortality post-myocardial infarction after re-infarction
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Annual mortality post-MI with re-infarction = 0.137 | |||||||||
| Abbott 99th centile | £2790 | 11.532 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2834 | 11.535 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2859 | 11.535 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2958 | 11.538 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3026 | 11.546 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,091 |
| Standard Tn | £3066 | 11.496 | Abbott strategy | £39 | –0.050 | Dominated | |||
| Annual mortality post-MI with re-infarction = 0.146 | |||||||||
| Abbott 99th centile | £2788 | 11.527 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2831 | 11.529 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2857 | 11.530 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2956 | 11.533 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3024 | 11.541 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,005 |
| Standard Tn | £3063 | 11.491 | Abbott strategy | £39 | –0.050 | Dominated | |||
Hazard ratio mortality (unstable angina compared with non-ST segment elevation myocardial infarction)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| HR mortality (UA vs. NSTEMI) = 1.053 | |||||||||
| Abbott 99th centile | £2740 | 11.363 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2783 | 11.365 | –£232 | 0.038 | Dominant | Abbott 99th centile | £44 | 0.003 | Extendedly dominated |
| Beckman 99th centile | £2809 | 11.366 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2908 | 11.369 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £2976 | 11.377 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,051 |
| Standard Tn | £3015 | 11.327 | Abbott strategy | £39 | –0.050 | Dominated | |||
| HR mortality (UA vs. NSTEMI) = 0.581 | |||||||||
| Abbott 99th centile | £2835 | 11.685 | –£275 | 0.037 | Dominant | ||||
| Roche 99th centile | £2879 | 11.688 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2904 | 11.688 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £3003 | 11.691 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3071 | 11.699 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,044 |
| Standard Tn | £3111 | 11.649 | Abbott strategy | £39 | –0.050 | Dominated | |||
Relative risk mortality (untreated compared with treated acute myocardial infarction)
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| RR mortality (untreated vs. treated AMI) = 3.908 | |||||||||
| Abbott 99th centile | £2757 | 11.505 | –£274 | 0.042 | Dominant | ||||
| Roche 99th centile | £2812 | 11.516 | –£219 | 0.053 | Dominant | Abbott 99th centile | £56 | 0.012 | £4755 |
| Beckman 99th centile | £2845 | 11.522 | –£186 | 0.059 | Dominant | Roche 99th centile | £33 | 0.006 | £5714 |
| Roche strategy | £2945 | 11.526 | –£85 | 0.063 | Dominant | Beckman 99th centile | £101 | 0.004 | Extendedly dominated |
| Abbott strategy | £3022 | 11.541 | –£9 | 0.078 | Dominant | Beckman 99th centile | £177 | 0.019 | £9476 |
| Standard Tn | £3031 | 11.463 | Abbott strategy | £9 | –0.078 | Dominated | |||
| RR mortality (untreated vs. treated AMI) = 0.901 | |||||||||
| Abbott 99th centile | £2804 | 11.542 | –£276 | 0.034 | Dominant | ||||
| Roche 99th centile | £2842 | 11.540 | –£238 | 0.032 | Dominant | Abbott 99th centile | £38 | –0.002 | Dominated |
| Beckman 99th centile | £2865 | 11.537 | –£216 | 0.029 | Dominant | Abbott 99th centile | £60 | –0.004 | Dominated |
| Roche strategy | £2963 | 11.540 | –£118 | 0.032 | Dominant | Abbott 99th centile | £159 | –0.002 | Dominated |
| Abbott strategy | £3027 | 11.545 | –£54 | 0.037 | Dominant | Abbott 99th centile | £223 | 0.003 | £69,543 |
| Standard Tn | £3081 | 11.508 | Abbott strategy | £54 | –0.037 | Dominated | |||
Appendix 7 Subgroup analyses (base case)
Deterministic base case
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2257 | 11.734 | –£440 | –0.015 | £28,870 | ||||
| Roche 99th centile | £2301 | 11.740 | –£396 | –0.010 | £41,233 | Abbott 99th centile | £44 | 0.006 | £7777 |
| Beckman 99th centile | £2327 | 11.743 | –£370 | –0.006 | £58,988 | Roche 99th centile | £26 | 0.003 | £7777 |
| Roche strategy | £2426 | 11.744 | –£271 | –0.006 | £48,559 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2493 | 11.748 | –£204 | –0.002 | £124,391 | Beckman 99th centile | £167 | 0.005 | £35,904 |
| Standard Tn | £2697 | 11.749 | Abbott strategy | £204 | 0.002 | £124,391 | |||
Age and sex subgroups
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Females | |||||||||
| Age = 45 years | |||||||||
| Abbott 99th centile | £2087 | 12.853 | –£443 | –0.016 | £27,038 | ||||
| Roche 99th centile | £2132 | 12.859 | –£398 | –0.010 | £38,540 | Abbott 99th centile | £45 | 0.006 | £7414 |
| Beckman 99th centile | £2158 | 12.863 | –£372 | –0.007 | £55,060 | Roche 99th centile | £27 | 0.004 | £7414 |
| Roche strategy | £2258 | 12.864 | –£272 | –0.006 | £45,357 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2326 | 12.868 | –£204 | –0.002 | £115,910 | Beckman 99th centile | £168 | 0.005 | £33,583 |
| Standard Tn | £2530 | 12.870 | Abbott strategy | £204 | 0.002 | £115,910 | |||
| Age = 55 years | |||||||||
| Abbott 99th centile | £2093 | 10.615 | –£443 | –0.016 | £28,189 | ||||
| Roche 99th centile | £2138 | 10.620 | –£398 | –0.010 | £40,181 | Abbott 99th centile | £45 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2164 | 10.624 | –£372 | –0.006 | £57,405 | Abbott 99th centile | £71 | 0.009 | £7728 |
| Roche strategy | £2263 | 10.624 | –£272 | –0.006 | £47,288 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2332 | 10.629 | –£204 | –0.002 | £120,850 | Beckman 99th centile | £168 | 0.005 | £35,013 |
| Standard Tn | £2536 | 10.630 | Abbott strategy | £204 | 0.002 | £120,850 | |||
| Age = 65 years | |||||||||
| Abbott 99th centile | £2087 | 8.193 | –£443 | –0.015 | £29,368 | ||||
| Roche 99th centile | £2132 | 8.199 | –£398 | –0.010 | £41,866 | Abbott 99th centile | £45 | 0.006 | Extendedly dominated |
| Beckman 99th centile | £2158 | 8.202 | –£372 | –0.006 | £59,816 | Abbott 99th centile | £71 | 0.009 | £8044 |
| Roche strategy | £2258 | 8.203 | –£272 | –0.006 | £49,272 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2326 | 8.207 | –£204 | –0.002 | £125,935 | Beckman 99th centile | £167 | 0.005 | £36,479 |
| Standard Tn | £2530 | 8.208 | Abbott strategy | £204 | 0.002 | £125,935 | |||
| Age = 75 years | |||||||||
| Abbott 99th centile | £2037 | 5.640 | –£442 | –0.013 | £32,776 | ||||
| Roche 99th centile | £2082 | 5.645 | –£398 | –0.009 | £46,745 | Abbott 99th centile | £45 | 0.005 | Extendedly dominated |
| Beckman 99th centile | £2108 | 5.648 | –£371 | –0.006 | £66,808 | Abbott 99th centile | £71 | 0.008 | £8942 |
| Roche strategy | £2207 | 5.649 | –£272 | –0.005 | £55,024 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2276 | 5.652 | –£204 | –0.001 | £140,710 | Beckman 99th centile | £167 | 0.004 | £40,725 |
| Standard Tn | £2480 | 5.654 | Abbott strategy | £204 | 0.001 | £140,710 | |||
| Age = 85 years | |||||||||
| Abbott 99th centile | £1826 | 3.107 | –£437 | –0.007 | £59,890 | ||||
| Roche 99th centile | £1869 | 3.110 | –£394 | –0.005 | £85,736 | Abbott 99th centile | £43 | 0.003 | Extendedly dominated |
| Beckman 99th centile | £1894 | 3.112 | –£369 | –0.003 | £122,857 | Abbott 99th centile | £68 | 0.004 | £15,793 |
| Roche strategy | £1993 | 3.112 | –£270 | –0.003 | £101,053 | Beckman 99th centile | £99 | 0.000 | Extendedly dominated |
| Abbott strategy | £2059 | 3.114 | –£203 | –0.001 | £259,592 | Beckman 99th centile | £166 | 0.002 | £74,597 |
| Standard Tn | £2263 | 3.115 | Abbott strategy | £203 | 0.001 | £259,592 | |||
| Males | |||||||||
| Age = 45 years | |||||||||
| Abbott 99th centile | £2404 | 14.047 | –£438 | –0.015 | £28,815 | ||||
| Roche 99th centile | £2447 | 14.053 | –£395 | –0.010 | £41,214 | Abbott 99th centile | £43 | 0.006 | £7660 |
| Beckman 99th centile | £2472 | 14.056 | –£370 | –0.006 | £59,021 | Roche 99th centile | £25 | 0.003 | £7660 |
| Roche strategy | £2571 | 14.057 | –£271 | –0.006 | £48,561 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2638 | 14.061 | –£204 | –0.002 | £124,616 | Beckman 99th centile | £166 | 0.005 | £35,870 |
| Standard Tn | £2842 | 14.062 | Abbott strategy | £204 | 0.002 | £124,616 | |||
| Age = 55 years | |||||||||
| Abbott 99th centile | £2407 | 11.852 | –£438 | –0.014 | £30,338 | ||||
| Roche 99th centile | £2450 | 11.857 | –£395 | –0.009 | £43,396 | Abbott 99th centile | £43 | 0.005 | Extendedly dominated |
| Beckman 99th centile | £2476 | 11.860 | –£370 | –0.006 | £62,149 | Abbott 99th centile | £68 | 0.008 | £8059 |
| Roche strategy | £2575 | 11.861 | –£271 | –0.005 | £51,134 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2642 | 11.865 | –£204 | –0.002 | £131,231 | Beckman 99th centile | £166 | 0.004 | £37,768 |
| Standard Tn | £2845 | 11.866 | Abbott strategy | £204 | 0.002 | £131,231 | |||
| Age = 65 years | |||||||||
| Abbott 99th centile | £2371 | 9.384 | –£438 | –0.013 | £32,627 | ||||
| Roche 99th centile | £2413 | 9.389 | –£395 | –0.008 | £46,682 | Abbott 99th centile | £43 | 0.005 | Extendedly dominated |
| Beckman 99th centile | £2439 | 9.392 | –£369 | –0.006 | £66,867 | Abbott 99th centile | £68 | 0.008 | £8647 |
| Roche strategy | £2538 | 9.392 | –£270 | –0.005 | £55,011 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2605 | 9.396 | –£203 | –0.001 | £141,222 | Beckman 99th centile | £166 | 0.004 | £40,624 |
| Standard Tn | £2808 | 9.397 | Abbott strategy | £203 | 0.001 | £141,222 | |||
| Age = 75 years | |||||||||
| Abbott 99th centile | £2253 | 6.574 | –£437 | –0.011 | £39,186 | ||||
| Roche 99th centile | £2295 | 6.578 | –£394 | –0.007 | £56,106 | Abbott 99th centile | £42 | 0.004 | Extendedly dominated |
| Beckman 99th centile | £2320 | 6.581 | –£369 | –0.005 | £80,406 | Abbott 99th centile | £68 | 0.007 | £10,317 |
| Roche strategy | £2419 | 6.581 | –£270 | –0.004 | £66,133 | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2486 | 6.584 | –£203 | –0.001 | £169,919 | Beckman 99th centile | £166 | 0.003 | £48,814 |
| Standard Tn | £2689 | 6.585 | Abbott strategy | £203 | 0.001 | £169,919 | |||
| Age = 85 years | |||||||||
| Abbott 99th centile | £1940 | 3.634 | –£429 | –0.004 | £114,585 | ||||
| Roche 99th centile | £1980 | 3.635 | –£389 | –0.002 | £164,917 | Abbott 99th centile | £40 | 0.001 | Extendedly dominated |
| Beckman 99th centile | £2004 | 3.636 | –£366 | –0.002 | £237,203 | Abbott 99th centile | £63 | 0.002 | £28,711 |
| Roche strategy | £2102 | 3.636 | –£267 | –0.001 | £194,744 | Beckman 99th centile | £99 | 0.000 | Extendedly dominated |
| Abbott strategy | £2167 | 3.637 | –£203 | 0.000 | £503,476 | Beckman 99th centile | £163 | 0.001 | £143,225 |
| Standard Tn | £2369 | 3.638 | Abbott strategy | £203 | 0.000 | £503,476 | |||
Subgroup with history of previous non-ST segment elevation myocardial infarctiona
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £4643 | 5.764 | –£472 | –0.019 | £25,031 | ||||
| Roche 99th centile | £4699 | 5.771 | –£417 | –0.012 | £35,017 | Abbott 99th centile | £56 | 0.007 | Extendedly dominated |
| Beckman 99th centile | £4732 | 5.775 | –£384 | –0.008 | £49,358 | Abbott 99th centile | £89 | 0.011 | £7994 |
| Roche strategy | £4834 | 5.776 | –£281 | –0.007 | £40,639 | Beckman 99th centile | £103 | 0.001 | Extendedly dominated |
| Abbott strategy | £4910 | 5.781 | –£205 | –0.002 | £101,225 | Beckman 99th centile | £178 | 0.006 | £31,052 |
| Standard Tn | £5115 | 5.783 | Abbott strategy | £205 | 0.002 | £101,225 | |||
Myocardial infarction prevalence
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| MI prevalence = 1% | |||||||||
| No testing | £576 | 12.891 | –£439 | –0.005 | £96,456 | ||||
| Abbott 99th centile | £687 | 12.894 | –£329 | –0.001 | £366,354 | No testing | £111 | 0.004 | Extendedly dominated |
| Roche 99th centile | £690 | 12.895 | –£326 | –0.001 | £576,522 | No testing | £113 | 0.004 | Extendedly dominated |
| Beckman 99th centile | £691 | 12.895 | –£324 | 0.000 | £878,364 | No testing | £115 | 0.004 | £27,409 |
| Roche strategy | £774 | 12.895 | –£241 | 0.000 | £734,155 | Beckman 99th centile | £83 | 0.000 | Extendedly dominated |
| Abbott strategy | £813 | 12.895 | –£202 | 0.000 | £2,097,914 | Beckman 99th centile | £122 | 0.000 | £447,934 |
| Standard Tn | £1016 | 12.895 | Abbott strategy | £202 | 0.000 | £2,097,914 | |||
| MI prevalence = 5% | |||||||||
| No testing | £855 | 12.586 | –£581 | –0.023 | £25,513 | ||||
| Abbott 99th centile | £1079 | 12.604 | –£356 | –0.004 | £79,492 | No testing | £224 | 0.018 | Extendedly dominated |
| Roche 99th centile | £1092 | 12.606 | –£344 | –0.003 | £121,526 | No testing | £237 | 0.020 | Extendedly dominated |
| Beckman 99th centile | £1100 | 12.607 | –£336 | –0.002 | £181,894 | No testing | £245 | 0.021 | £11,703 |
| Roche strategy | £1187 | 12.607 | –£249 | –0.002 | £151,398 | Beckman 99th centile | £87 | 0.000 | Extendedly dominated |
| Abbott strategy | £1233 | 12.608 | –£203 | 0.000 | £420,420 | Beckman 99th centile | £133 | 0.001 | £97,709 |
| Standard Tn | £1436 | 12.609 | Abbott strategy | £203 | 0.000 | £420,420 | |||
| MI prevalence = 10% | |||||||||
| No testing | £1204 | 12.205 | –£758 | –0.046 | £16,645 | ||||
| Abbott 99th centile | £1570 | 12.242 | –£391 | –0.009 | £43,635 | No testing | £366 | 0.037 | Extendedly dominated |
| Roche 99th centile | £1596 | 12.245 | –£366 | –0.006 | £64,651 | No testing | £392 | 0.040 | Extendedly dominated |
| Beckman 99th centile | £1611 | 12.247 | –£350 | –0.004 | £94,836 | No testing | £407 | 0.042 | £9740 |
| Roche strategy | £1703 | 12.247 | –£258 | –0.003 | £78,554 | Beckman 99th centile | £92 | 0.000 | Extendedly dominated |
| Abbott strategy | £1758 | 12.250 | –£203 | –0.001 | £210,733 | Beckman 99th centile | £147 | 0.003 | £53,931 |
| Standard Tn | £1961 | 12.251 | Abbott strategy | £203 | 0.001 | £210,733 | |||
| MI prevalence = 20% | |||||||||
| No testing | £1900 | 11.443 | –£1112 | –0.091 | £12,211 | ||||
| Abbott 99th centile | £2551 | 11.516 | –£461 | –0.018 | £25,706 | No testing | £651 | 0.073 | Extendedly dominated |
| Roche 99th centile | £2603 | 11.523 | –£410 | –0.011 | £36,214 | No testing | £702 | 0.080 | Extendedly dominated |
| Beckman 99th centile | £2633 | 11.527 | –£379 | –0.007 | £51,306 | No testing | £733 | 0.084 | £8759 |
| Roche strategy | £2735 | 11.528 | –£277 | –0.007 | £42,131 | Beckman 99th centile | £102 | 0.001 | Extendedly dominated |
| Abbott strategy | £2808 | 11.532 | –£204 | –0.002 | £105,889 | Beckman 99th centile | £175 | 0.005 | £32,042 |
| Standard Tn | £3012 | 11.534 | Abbott strategy | £204 | 0.002 | £105,889 | |||
| MI prevalence = 30% | |||||||||
| No testing | £2597 | 10.681 | –£1466 | –0.137 | £10,733 | ||||
| Abbott 99th centile | £3532 | 10.791 | –£531 | –0.027 | £19,730 | No testing | £935 | 0.110 | Extendedly dominated |
| Roche 99th centile | £3610 | 10.801 | –£454 | –0.017 | £26,735 | No testing | £1012 | 0.120 | Extendedly dominated |
| Beckman 99th centile | £3655 | 10.807 | –£408 | –0.011 | £36,797 | No testing | £1058 | 0.125 | £8431 |
| Roche strategy | £3767 | 10.808 | –£296 | –0.010 | £29,991 | Beckman 99th centile | £112 | 0.001 | Extendedly dominated |
| Abbott strategy | £3858 | 10.815 | –£205 | –0.003 | £70,942 | Beckman 99th centile | £203 | 0.008 | £24,745 |
| Standard Tn | £4063 | 10.818 | Abbott strategy | £205 | 0.003 | £70,942 | |||
Appendix 8 Subgroup analyses (secondary analysis)
Deterministic secondary analysis
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Abbott 99th centile | £2789 | 11.530 | –£276 | 0.036 | Dominant | ||||
| Roche 99th centile | £2832 | 11.532 | –£232 | 0.039 | Dominant | Abbott 99th centile | £44 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £2858 | 11.532 | –£206 | 0.039 | Dominant | Abbott 99th centile | £69 | 0.003 | Extendedly dominated |
| Roche strategy | £2957 | 11.535 | –£107 | 0.042 | Dominant | Abbott 99th centile | £168 | 0.006 | Extendedly dominated |
| Abbott strategy | £3025 | 11.543 | –£39 | 0.050 | Dominant | Abbott 99th centile | £236 | 0.014 | £17,047 |
| Standard Tn | £3064 | 11.493 | Abbott strategy | £39 | –0.050 | Dominated | |||
Age and sex subgroups
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| Females | |||||||||
| Age = 45 years | |||||||||
| Abbott 99th centile | £2602 | 12.547 | –£276 | 0.042 | Dominant | ||||
| Roche 99th centile | £2647 | 12.549 | –£231 | 0.044 | Dominant | Abbott 99th centile | £45 | 0.003 | Extendedly dominated |
| Beckman 99th centile | £2673 | 12.550 | –£205 | 0.044 | Dominant | Abbott 99th centile | £71 | 0.003 | Extendedly dominated |
| Roche strategy | £2773 | 12.553 | –£105 | 0.048 | Dominant | Abbott 99th centile | £170 | 0.006 | Extendedly dominated |
| Abbott strategy | £2841 | 12.562 | –£37 | 0.057 | Dominant | Abbott 99th centile | £239 | 0.015 | £16,023 |
| Standard Tn | £2878 | 12.505 | Abbott strategy | £37 | –0.057 | Dominated | |||
| Age = 55 years | |||||||||
| Abbott 99th centile | £2605 | 10.407 | –£276 | 0.034 | Dominant | ||||
| Roche 99th centile | £2650 | 10.410 | –£231 | 0.037 | Dominant | Abbott 99th centile | £45 | 0.003 | £15,224 |
| Beckman 99th centile | £2676 | 10.410 | –£205 | 0.038 | Dominant | Roche 99th centile | £26 | 0.001 | Extendedly dominated |
| Roche strategy | £2776 | 10.413 | –£105 | 0.040 | Dominant | Roche 99th centile | £126 | 0.003 | Extendedly dominated |
| Abbott strategy | £2844 | 10.421 | –£37 | 0.048 | Dominant | Roche 99th centile | £194 | 0.011 | £17,150 |
| Standard Tn | £2881 | 10.373 | Abbott strategy | £37 | –0.048 | Dominated | |||
| Age = 65 years | |||||||||
| Abbott 99th centile | £2592 | 8.089 | –£276 | 0.025 | Dominant | ||||
| Roche 99th centile | £2637 | 8.092 | –£232 | 0.029 | Dominant | Abbott 99th centile | £45 | 0.003 | £13,064 |
| Beckman 99th centile | £2663 | 8.094 | –£205 | 0.030 | Dominant | Roche 99th centile | £26 | 0.001 | Extendedly dominated |
| Roche strategy | £2762 | 8.096 | –£106 | 0.032 | Dominant | Roche 99th centile | £126 | 0.003 | Extendedly dominated |
| Abbott strategy | £2831 | 8.103 | –£37 | 0.039 | Dominant | Roche 99th centile | £194 | 0.010 | £18,999 |
| Standard Tn | £2868 | 8.064 | Abbott strategy | £37 | –0.039 | Dominated | |||
| Age = 75 years | |||||||||
| Abbott 99th centile | £2521 | 5.618 | –£278 | 0.015 | Dominant | ||||
| Roche 99th centile | £2565 | 5.621 | –£234 | 0.019 | Dominant | Abbott 99th centile | £44 | 0.004 | £12,392 |
| Beckman 99th centile | £2592 | 5.623 | –£207 | 0.021 | Dominant | Roche 99th centile | £26 | 0.002 | £16,407 |
| Roche strategy | £2691 | 5.625 | –£108 | 0.022 | Dominant | Beckman 99th centile | £99 | 0.002 | Extendedly dominated |
| Abbott strategy | £2759 | 5.630 | –£40 | 0.028 | Dominant | Beckman 99th centile | £168 | 0.007 | £24,020 |
| Standard Tn | £2799 | 5.602 | Abbott strategy | £40 | –0.028 | Dominated | |||
| Age = 85 years | |||||||||
| Abbott 99th centile | £2250 | 3.104 | –£289 | 0.002 | Dominant | ||||
| Roche 99th centile | £2292 | 3.106 | –£247 | 0.004 | Dominant | Abbott 99th centile | £42 | 0.002 | £21,140 |
| Beckman 99th centile | £2317 | 3.107 | –£222 | 0.005 | Dominant | Roche 99th centile | £25 | 0.001 | £26,911 |
| Roche strategy | £2416 | 3.108 | –£123 | 0.006 | Dominant | Beckman 99th centile | £99 | 0.001 | Extendedly dominated |
| Abbott strategy | £2483 | 3.111 | –£56 | 0.009 | Dominant | Beckman 99th centile | £166 | 0.004 | £45,709 |
| Standard Tn | £2539 | 3.102 | Abbott strategy | £56 | –0.009 | Dominated | |||
| Males | |||||||||
| Age = 45 years | |||||||||
| Abbott 99th centile | £2958 | 13.801 | –£275 | 0.042 | Dominant | ||||
| Roche 99th centile | £3000 | 13.803 | –£233 | 0.044 | Dominant | Abbott 99th centile | £43 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £3026 | 13.803 | –£207 | 0.044 | Dominant | Abbott 99th centile | £68 | 0.002 | Dominated |
| Roche strategy | £3125 | 13.806 | –£108 | 0.047 | Dominant | Abbott 99th centile | £167 | 0.005 | Extendedly dominated |
| Abbott strategy | £3192 | 13.815 | –£41 | 0.056 | Dominant | Abbott 99th centile | £235 | 0.014 | £16,897 |
| Standard Tn | £3233 | 13.759 | Abbott strategy | £41 | –0.056 | Dominated | |||
| Age = 55 years | |||||||||
| Abbott 99th centile | £2954 | 11.689 | –£276 | 0.035 | Dominant | ||||
| Roche 99th centile | £2997 | 11.691 | –£233 | 0.037 | Dominant | Abbott 99th centile | £43 | 0.002 | Extendedly dominated |
| Beckman 99th centile | £3022 | 11.691 | –£208 | 0.037 | Dominant | Abbott 99th centile | £68 | 0.002 | Dominated |
| Roche strategy | £3121 | 11.694 | –£109 | 0.040 | Dominant | Abbott 99th centile | £167 | 0.005 | Extendedly dominated |
| Abbott strategy | £3188 | 11.702 | –£41 | 0.048 | Dominant | Abbott 99th centile | £234 | 0.013 | £17,836 |
| Standard Tn | £3230 | 11.654 | Abbott strategy | £41 | –0.048 | Dominated | |||
| Age = 65 years | |||||||||
| Abbott 99th centile | £2902 | 9.306 | –£276 | 0.026 | Dominant | ||||
| Roche 99th centile | £2945 | 9.309 | –£234 | 0.029 | Dominant | Abbott 99th centile | £43 | 0.003 | £16,877 |
| Beckman 99th centile | £2970 | 9.310 | –£209 | 0.029 | Dominant | Roche 99th centile | £25 | 0.001 | Extendedly dominated |
| Roche strategy | £3069 | 9.312 | –£110 | 0.032 | Dominant | Roche 99th centile | £124 | 0.003 | Extendedly dominated |
| Abbott strategy | £3136 | 9.319 | –£42 | 0.039 | Dominant | Roche 99th centile | £191 | 0.010 | £19,851 |
| Standard Tn | £3179 | 9.280 | Abbott strategy | £42 | –0.039 | Dominated | |||
| Age = 75 years | |||||||||
| Abbott 99th centile | £2752 | 6.560 | –£278 | 0.017 | Dominant | ||||
| Roche 99th centile | £2795 | 6.563 | –£236 | 0.019 | Dominant | Abbott 99th centile | £42 | 0.002 | £16,994 |
| Beckman 99th centile | £2819 | 6.563 | –£211 | 0.020 | Dominant | Roche 99th centile | £25 | 0.001 | Extendedly dominated |
| Roche strategy | £2918 | 6.565 | –£112 | 0.022 | Dominant | Roche 99th centile | £124 | 0.003 | Extendedly dominated |
| Abbott strategy | £2985 | 6.570 | –£45 | 0.027 | Dominant | Roche 99th centile | £191 | 0.008 | £25,149 |
| Standard Tn | £3030 | 6.543 | Abbott strategy | £45 | –0.027 | Dominated | |||
| Age = 85 years | |||||||||
| Abbott 99th centile | £2374 | 3.631 | –£283 | 0.006 | Dominant | ||||
| Roche 99th centile | £2414 | 3.631 | –£244 | 0.007 | Dominant | Abbott 99th centile | £40 | 0.001 | Extendedly dominated |
| Beckman 99th centile | £2437 | 3.631 | –£220 | 0.007 | Dominant | Abbott 99th centile | £63 | 0.001 | Extendedly dominated |
| Roche strategy | £2536 | 3.632 | –£122 | 0.007 | Dominant | Abbott 99th centile | £162 | 0.001 | Extendedly dominated |
| Abbott strategy | £2601 | 3.634 | –£57 | 0.010 | Dominant | Abbott 99th centile | £227 | 0.003 | £66,418 |
| Standard Tn | £2657 | 3.624 | Abbott strategy | £57 | –0.010 | Dominated | |||
Myocardial infarction prevalence
| Strategy | Costs | QALYs | Compared with standard Tn | Compared with next best strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | Comparator | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | |||
| MI prevalence = 1% | |||||||||
| No testing | £1072 | 12.546 | –£439 | –0.005 | £96,456 | ||||
| Abbott 99th centile | £1405 | 12.619 | –£106 | 0.068 | Dominant | No testing | £333 | 0.073 | £4563 |
| Roche 99th centile | £1407 | 12.615 | –£104 | 0.064 | Dominant | Abbott 99th centile | £2 | –0.004 | Dominated |
| Beckman 99th centile | £1408 | 12.611 | –£103 | 0.061 | Dominant | Abbott 99th centile | £3 | –0.008 | Dominated |
| Roche strategy | £1492 | 12.614 | –£20 | 0.064 | Dominant | Abbott 99th centile | £87 | –0.005 | Dominated |
| Standard Tn | £1511 | 12.550 | Abbott 99th centile | £106 | –0.068 | Dominated | |||
| Abbott strategy | £1531 | 12.620 | £20 | 0.070 | £290 | Abbott 99th centile | £126 | 0.001 | £109,991 |
| MI prevalence = 5% | |||||||||
| No testing | £1316 | 12.265 | –£581 | –0.023 | £25,513 | ||||
| Abbott 99th centile | £1747 | 12.348 | –£150 | 0.060 | Dominant | No testing | £431 | 0.083 | £5209 |
| Roche 99th centile | £1759 | 12.346 | –£137 | 0.058 | Dominant | Abbott 99th centile | £13 | –0.002 | Dominated |
| Beckman 99th centile | £1766 | 12.343 | –£130 | 0.055 | Dominant | Abbott 99th centile | £20 | –0.005 | Dominated |
| Roche strategy | £1854 | 12.346 | –£43 | 0.058 | Dominant | Abbott 99th centile | £107 | –0.002 | Dominated |
| Standard Tn | £1897 | 12.288 | Abbott 99th centile | £150 | –0.060 | Dominated | |||
| Abbott strategy | £1900 | 12.352 | £4 | 0.064 | £61 | Abbott 99th centile | £154 | 0.004 | £35,574 |
| MI prevalence = 10% | |||||||||
| No testing | £1623 | 11.913 | –£758 | –0.046 | £16,645 | ||||
| Abbott 99th centile | £2178 | 12.008 | –£203 | 0.050 | Dominant | No testing | £554 | 0.095 | £5820 |
| Roche 99th centile | £2203 | 12.008 | –£178 | 0.049 | Dominant | Abbott 99th centile | £25 | 0.000 | Dominated |
| Beckman 99th centile | £2218 | 12.006 | –£163 | 0.048 | Dominant | Abbott 99th centile | £40 | –0.002 | Dominated |
| Roche strategy | £2311 | 12.009 | –£71 | 0.051 | Dominant | Abbott 99th centile | £133 | 0.001 | Extendedly dominated |
| Abbott strategy | £2366 | 12.017 | –£15 | 0.058 | Dominant | Abbott 99th centile | £188 | 0.008 | £22,684 |
| Standard Tn | £2381 | 11.958 | Abbott strategy | £15 | –0.058 | Dominated | |||
| MI prevalence = 20% | |||||||||
| No testing | £2247 | 11.202 | –£1112 | –0.091 | £12,211 | ||||
| Abbott 99th centile | £3053 | 11.324 | –£306 | 0.031 | Dominant | No testing | £806 | 0.122 | £6625 |
| Roche 99th centile | £3104 | 11.327 | –£255 | 0.034 | Dominant | Abbott 99th centile | £51 | 0.004 | £14,063 |
| Beckman 99th centile | £3135 | 11.328 | –£224 | 0.035 | Dominant | Roche 99th centile | £30 | 0.001 | Extendedly dominated |
| Roche strategy | £3237 | 11.331 | –£122 | 0.038 | Dominant | Roche 99th centile | £132 | 0.004 | Extendedly dominated |
| Abbott strategy | £3310 | 11.340 | –£49 | 0.047 | Dominant | Roche 99th centile | £206 | 0.013 | £16,319 |
| Standard Tn | £3359 | 11.293 | Abbott strategy | £49 | –0.047 | Dominated | |||
| MI prevalence = 30% | |||||||||
| No testing | £2880 | 10.484 | –£1466 | –0.137 | £10,733 | ||||
| Abbott 99th centile | £3942 | 10.634 | –£404 | 0.013 | Dominant | No testing | £1062 | 0.149 | £7109 |
| Roche 99th centile | £4019 | 10.641 | –£327 | 0.020 | Dominant | Abbott 99th centile | £77 | 0.008 | £10,278 |
| Beckman 99th centile | £4065 | 10.645 | –£281 | 0.024 | Dominant | Roche 99th centile | £46 | 0.004 | £12,899 |
| Roche strategy | £4177 | 10.648 | –£169 | 0.027 | Dominant | Beckman 99th centile | £112 | 0.003 | Extendedly dominated |
| Abbott strategy | £4268 | 10.658 | –£78 | 0.037 | Dominant | Beckman 99th centile | £203 | 0.013 | £15,410 |
| Standard Tn | £4346 | 10.621 | Abbott strategy | £78 | –0.037 | Dominated | |||
Appendix 9 Subgroup analyses based on accuracy and acute myocardial infarction prevalence (available for only the Roche 99th centile test)
| Base case | MI prevalencea | Roche 99th centile | Standard Tn | Increments | ||||
|---|---|---|---|---|---|---|---|---|
| Costs | QALYs | Costs | QALYs | ΔCosts | ΔQALYs | ΔCosts/ΔQALYs | ||
| Base case | 17% | £2301 | 11.740 | £2697 | 11.749 | –£396 | –0.010 | £41,233 |
| Age ≤ 70 years | 28% | £3411 | 10.946 | £3853 | 10.961 | –£442 | –0.015 | £28,633 |
| Age > 70 years | 10% | £1550 | 6.274 | £1880 | 6.275 | –£330 | –0.001 | £355,571 |
| With pre-existing CAD | 20% | £2641 | 11.528 | £3012 | 11.534 | –£371 | –0.006 | £58,509 |
| Without pre-existing CAD | 16% | £2236 | 11.816 | £2592 | 11.821 | –£356 | –0.004 | £80,454 |
| Symptom onset < 3 hours | 22% | £2726 | 11.369 | £3222 | 11.391 | –£496 | –0.022 | £22,111 |
| Symptom onset > 3 hours | 13% | £1929 | 12.032 | £2277 | 12.036 | –£348 | –0.003 | £103,107 |
| Symptom onset < 3 hours | 17% | £2241 | 11.732 | £2697 | 11.749 | –£456 | –0.017 | £26,327 |
| Symptom onset > 3 hours | 17% | £2341 | 11.745 | £2697 | 11.749 | –£356 | –0.004 | £80,677 |
| Base case | 17% | £2832 | 11.532 | £3064 | 11.493 | –£232 | 0.039 | Dominant |
| Age ≤ 70 yearsb | 28% | £3839 | 10.780 | £4148 | 10.756 | –£310 | 0.024 | Dominant |
| Age > 70 yearsc | 10% | £2111 | 6.245 | £2259 | 6.222 | –£148 | 0.023 | Dominant |
| With pre-existing CAD | 20% | £3142 | 11.325 | £3359 | 11.293 | –£217 | 0.031 | Dominant |
| Without pre-existing CAD | 16% | £2778 | 11.604 | £2967 | 11.560 | –£189 | 0.044 | Dominant |
| Symptom onset < 3 hours | 22% | £3209 | 11.180 | £3556 | 11.159 | –£347 | 0.021 | Dominant |
| Symptom onset > 3 hours | 13% | £2503 | 11.806 | £2673 | 11.760 | –£171 | 0.046 | Dominant |
| Symptom onset < 3 hours | 17% | £2772 | 11.524 | £3064 | 11.493 | –£292 | 0.031 | Dominant |
| Symptom onset > 3 hours | 17% | £2873 | 11.535 | £3064 | 11.493 | –£192 | 0.042 | Dominant |
Acute myocardial infarction prevalence in subgroups
| Subgroup | Prevalence of AMI (x) | Prevalence of AMI in whole population from subgroups were derived (y) | Prevalence assuming population prevalence of 17% (multiple x*y/17) | Source |
|---|---|---|---|---|
| Age ≤ 70 years | 24% | 15% | 28% | APACE39,52 |
| Age > 70 years | 9% | 15% | 10% | APACE39,52 |
| Patients with CAD | 18% | 16% | 20% | APACE39,52 |
| Patients without CAD | 14% | 16% | 16% | APACE39,52 |
| < 3 hours from symptoms67 | 24% | 18% | 22% | APACE,39 Body (2011)67 |
| > 3 hours from symptoms67 | 14% | 18% | 13% | APACE,39 Body (2011)67 |
| < 3 hours from symptoms39 | 21% | 21% | 17% | APACE,39 Body (2011)67 |
| > 3 hours from symptoms39 | 21% | 21% | 17% | APACE,39 Body (2011)67 |
Appendix 10 National Institute for Health and Care Excellence guidance relevant to the management of suspected acute coronary syndrome
-
MI – secondary prevention: secondary prevention in primary and secondary care for patients following a myocardial infarction. NICE clinical guideline CG172 (2013). URL: http://guidance.nice.org.uk/CG172. Date for review: not stated.
-
Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. NICE clinical guideline CG95 (2010). URL: www.nice.org.uk/guidance/CG95. Reviewed March 2013, review recommended.
-
Unstable angina and NSTEMI: the early management of unstable angina and non-ST segment elevation myocardial infarction. NICE clinical guideline CG94 (2010). URL: www.nice.org.uk/guidance/CG94. Last modified November 2013.
-
BRAHMS copeptin assay to rule out myocardial infarction in patients with acute chest pain. NICE medical technology guidance MTG4 (2011). URL: http://guidance.nice.org.uk/MTG4. Date for review: not stated.
-
Myocardial infarction with ST segment elevation: the acute management of myocardial infarction with ST segment elevation. NICE clinical guideline CG167 (2013). URL: http://guidance.nice.org.uk/CG167. Date for review: not stated.
Glossary
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A mathematical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- False-negative
- Incorrect negative test result – number of diseased persons with a negative test result.
- False-positive
- Incorrect positive test result – number of non-diseased persons with a positive test result.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test whose performance is being evaluated.
- Likelihood ratio
- Likelihood ratios describe how many times more likely it is that a person with the target condition will receive a particular test result than a person without the target condition.
- Markov model
- An analytic method particularly suited to modelling repeated events, or the progression of a chronic disease over time.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Meta-regression
- Statistical technique used to explore the relationship between study characteristics and study results.
- Opportunity costs
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Publication bias
- Bias arising from the preferential publication of studies with statistically significant results.
- Quality-adjusted life-year
- A measure of health gain, used in economic evaluations, in which survival duration is weighted or adjusted by the patient’s quality of life during the survival period.
- Quality of life
- An individual’s emotional, social and physical well-being and their ability to perform the ordinary tasks of living.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity which result from varying the diagnostic threshold.
- Reference standard
- The best currently available method for diagnosing the target condition. The index test is compared against this to allow calculation of estimates of accuracy.
- Sensitivity
- Proportion of people with the target disorder who have a positive test result.
- Specificity
- Proportion of people without the target disorder who have a negative test result.
- True-negative
- Correct negative test result – number of non-diseased persons with a negative test result.
- True-positive
- Correct positive test result – number of diseased persons with a positive test result.
List of abbreviations
- AACC
- American Association for Clinical Chemistry
- ACC
- American College of Cardiology
- ACE
- angiotensin-converting enzyme
- ACS
- acute coronary syndrome
- AHA
- American Heart Association
- AMI
- acute myocardial infarction
- CAD
- coronary artery disease
- CADTH
- Canadian Agency for Drugs and Technologies in Health
- CCT
- controlled clinical trial
- CEAC
- cost-effectiveness acceptability curve
- CEAF
- cost-effectiveness acceptability frontier
- CHD
- coronary heart disease
- CI
- confidence interval
- CTCA
- computed tomography coronary angiography
- cTn
- cardiac troponin
- CV
- coefficient of variation
- DTA
- diagnostic test accuracy
- ECG
- electrocardiography/electrocardiogram
- ECLIA
- electrochemiluminescence immunoassay
- ED
- emergency department
- ESC
- European Society of Cardiology
- FN
- false-negative
- FP
- false-positive
- H-FABP
- heart fatty acid binding protein
- HES
- Hospital Episode Statistics
- HF
- heart failure
- HR
- hazard ratio
- hs-cTn
- high-sensitivity cardiac troponin
- hs-cTnI
- high-sensitivity cardiac troponin I
- hs-cTnT
- high-sensitivity cardiac troponin T
- HSROC
- hierarchical summary receiver operating characteristic
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- LoB
- limit of blank
- LoD
- limit of detection
- LR–
- negative likelihood ratio
- LR+
- positive likelihood ratio
- LY
- life-year
- MACE
- major adverse cardiac event
- MeSH
- medical subject heading
- MI
- myocardial infarction
- NICE
- National Institute for Health and Care Excellence
- NIH
- National Institutes of Health
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- NSTE-ACS
- non-ST segment elevation acute coronary syndrome
- NSTEMI
- non-ST segment elevation myocardial infarction
- ONS
- Office for National Statistics
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- RR
- relative risk
- SE
- standard error
- SIGN
- Scottish Intercollegiate Guidelines Network
- SROC
- summary receiver operating characteristic
- STEMI
- ST segment elevation myocardial infarction
- Tn
- troponin
- TN
- true-negative
- TP
- true-positive
- UA
- unstable angina
- WHF
- World Heart Federation