Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 13/06/01. The protocol was agreed in March 2013. The assessment report began editorial review in November 2013 and was accepted for publication in October 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dr Anna Mullard has received hospitality from Roche (UK) Ltd.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Greenhalgh et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Lung cancer is the most common cancer worldwide (approximately 1.61 million new cases were diagnosed in 2008) and is the second most diagnosed cancer in the UK after breast cancer (12.9% of all cancer cases). 1 It is also the most common cause of death in the UK. 1 In 2010, 42,000 people in the UK were diagnosed with lung cancer and there were 35,000 registered deaths from lung cancer. 1 The majority of cases (80%) are diagnosed in people aged over 60 years. 1
Survival rates from lung cancer are low because the majority (66%) of cases are diagnosed at a late stage when a cure is not possible. 2 Other modifying factors for survival from lung cancer include smoking status, general health, sex, race and cancer treatment. 2 Incidence rates for lung cancer differ between men and women; for men, rates have decreased by more than 45% since the late 1970s, while incidence rates for women are still increasing. 1 The Royal College of Physicians reports that survival rates from lung cancer have increased in the last 40 years. 3 However, the outlook for patients in the UK remains poor with a 1-year survival rate of 27% for women and 30% for men. At 5 years, survival in men and women is 7% and 9% respectively. 3
Table 1 illustrates recent statistics for lung cancer survival. The table is taken from Cancer Research UK’s leaflet Cancer Statistics – Key Facts. 1
| Lung cancer statistics | Males | Females | Persons | Country | Yeara |
|---|---|---|---|---|---|
| Number of new cases per year | 23,175 | 18,851 | 42,026 | UK | 2010 |
| Incidence rate per 100,000 populationb | 58.0 | 39.7 | 47.8 | UK | |
| Number of deaths per year | 19,410 | 15,449 | 34,859 | UK | 2010 |
| Mortality rate per 100,000b | 47.9 | 31.3 | 38.6 | UK | |
| 1-year survival ratec | 29.4% | 33.0% | 31.0% | England | 2005–9 |
| 5-year survival ratec | 7.8% | 9.3% | 9.0% | ||
| 10-year survival ratec | 4.9% | 5.9% | 5.3 | England and Wales | 2007 (predicted) |
The majority (86%) of lung cancers are caused by smoking and 3% by passive smoking. Other risk factors include family history, exposure to radon, air pollution and exposure to asbestos. 1
The symptoms of lung cancer may include cough, shortness of breath, coughing up phlegm with signs of blood, loss of appetite, fatigue, weight loss, and recurrent or persistent chest infection. Symptoms associated with more advanced disease include hoarseness, difficulty in swallowing, finger clubbing, swelling of the face, swelling of the neck, chest pain and shoulder pain. 4
Around 72% (approximately 20,000) of lung cancers are non-small cell lung cancers (NSCLC), which can be further classified into three histological subtypes of large-cell undifferentiated carcinoma, squamous cell carcinoma and adenocarcinoma. 5
Since the introduction of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) into clinical practice in the UK, people with non-squamous NSCLC may be further differentiated as having either EGFR-activating mutation-positive (EGFR M+) or -negative (EGFR M–) status, the latter is otherwise known as the wild type. In the UK, approximately 10% of NSCLC tumours are EGFR M+. 2 Confirmation of histological and EGFR mutation status are key drivers of treatment decisions.
Diagnosis and staging
Diagnosis
Guidelines produced by the National Institute for Health and Care Excellence (NICE; CG1216) recommend that urgent referral for chest radiography should be made when a patient presents with haemoptysis or any unexplained or persistent (lasting more than 3 weeks) symptoms, as detailed previously. If chest radiography or chest computed tomography indicates lung cancer, the patient should be urgently referred to a chest physician who will choose the most appropriate investigations for diagnosis and staging. Within the diagnostic process key issues to be addressed include histology, EGFR mutation status, disease staging, performance status (PS) and comorbid disease.
Staging
The TNM classification of malignant tumours staging system (Union for International Cancer Control7) is used to classify the size and degree of spread of NSCLC tumours. The TNM classification indicates the appropriate type of treatment (curative or palliative) and prognosis. In the TNM system, the T describes the size of the primary tumour, N describes the involvement of lymph nodes and M describes the presence of metastases. These categories can be classified further into stages. The TNM system is now in its seventh edition, having been updated in 2010. Table 2 describes the TNM staging system and illustrates the differences between the sixth and seventh editions. Table 3 describes the surgical stage groupings. Patients of interest to this appraisal are those with stage IIIB or stage IV disease, often described as patients with locally advanced or metastatic disease.
| Sixth edition | Seventh edition | |
|---|---|---|
| TNM stage | TNM stage | Descriptor |
| T1 | T1a | Maximum dimension ≤ 2 cm |
| T1b | Maximum dimension 2–3 cm | |
| T2 | T2a | Maximum dimension 3–5 cm |
| T2b | Maximum dimension > 5–7 cm | |
| T3 | Maximum dimension > 7 cm | |
| T4 | T3 | Additional nodule in same lobe |
| M1 | T4 | Additional nodule in ipsilateral different lobe |
| M1 | M1a | Additional nodules in contralateral lung or ipsilateral pleural effusion |
| M1 | M1b | Distant metastases |
| Stage | T | N | M |
|---|---|---|---|
| 0 | T1a | N0 | M0 |
| IA | T1a, T1b | N0 | M0 |
| IB | T2a | N0 | M0 |
| IIA | T1a, T1b | N1 | M0 |
| T2a | N1 | M0 | |
| T2b | N0 | M0 | |
| IIB | T2b | N1 | M0 |
| T3 | N0 | M0 | |
| IIIA | T1, T2 | N2 | M0 |
| T3 | N1, N2 | M0 | |
| T4 | N0, N1 | M0 | |
| IIIB | T4 | N2 | M0 |
| Any T | N3 | M0 | |
| IV | Any T | Any N | M1a, M1b |
Performance status
The measure of PS indicates the degree of a patient’s general well-being. The PS rating may be used when determining fitness for treatment, need for dose adjustment and a patient’s supportive care needs. The three main PS scales are the World Health Organization (WHO) PS scale,8 the Eastern Cooperative Oncology Group (ECOG) PS scale9 and the Karnofsky PS (KPS) scale. 10 The WHO PS scale is most commonly used in UK clinical practice and is described in Table 4. A WHO rating of 0 indicates that a patient is completely able to look after him/herself and a rating of 4 indicates that a patient requires substantial support.
| Scale | WHO criteria |
|---|---|
| 0 | Patient is fully active and more or less the same as before illness |
| 1 | Patient is unable to carry out heavy physical work, but can do anything else |
| 2 | Patient is up and about more than half the day; able to look after him/herself, but not well enough to work |
| 3 | Patient is in bed or sitting in a chair for more than half the day; needs some help to look after him/herself |
| 4 | Patient is in bed all the time and needs a lot of looking after |
Treatment options
The treatment options for patients with NSCLC depend on the stage of disease, disease histology, EGFR mutation status, PS, comorbidities and patient preferences. For patients with early-stage disease (stages I–II and some stage III) curative surgical resection or radiotherapy may be an option, providing the patient is medically fit. 6 A combination of radiotherapy and chemotherapy may also be an option for patients with stage I–III disease. Patients with stage III or IV disease, good PS and for whom curative treatment is not an option may initially be offered chemotherapy to improve survival, disease control and quality of life (QoL). 6 A proportion of this group of patients (33%) go on to receive further chemotherapy treatment following disease progression after first-line therapy. It is this patient group that is of relevance to this appraisal.
Epidemiology
The National Lung Cancer Audit
The National Lung Cancer Audit is part of a wider programme of national audit run by the Information Centre for Health and Social Care. The audit uses the LUngCAncerDAta database, a database that was originally developed by the Royal College of Physicians in the late 1990s. The data set use key data to describe the demographics, stage, presentation and management of patients with mesothelioma or lung cancer in England and Wales. The National Lung Cancer Audit report is published annually.
The current audit (published in 2012) reports data for patients diagnosed with lung cancer or mesothelioma first seen in 2011. 11,12 The summary report states that it represents almost all cases of lung cancer presenting to secondary care in this year. In England and Wales there were 27,649 cases of NSCLC and, of these, 19,155 were histologically confirmed. This represents a histological diagnosis rate of 70%, with the national histological diagnosis rate for all types of lung cancer reported to be 77% for all lung cancers. Of the patients diagnosed with NSCLC, approximately 57% had stage IIIB or stage IV disease. More males than females were diagnosed (15,471 compared with 12,178, respectively). There were 6698 patients with stage IIIB/IV disease who had a PS score of 0 or 1 and, of these, 55.2% received chemotherapy. Median survival for all cancer cases was 185 days (interquartile range 57–309 days) from diagnosis date. Our clinical advisors tell us that, in UK clinical practice, 25% of patients with a PS score of 0 or 1 receive second-line chemotherapy and approximately 5–15% of patients with a PS score of 2 receive second-line treatment.
Impact of lung cancer
The annual cost of lung cancer to the UK economy is estimated at £2.4B. Half of the cost of lung cancer is a result of premature deaths and time off work. Health-care costs account for a further 35%, while an additional 16% is attributable to unpaid care provided by friends and family. According to Cancer Research UK,13 each lung cancer patient is thought to cost the UK health-care system £9071 every year.
In addition to the burden of illness and effects of treatment, living with lung cancer will impact on finances, work and employment, emotional well-being and relationships with friends and family. 14
Relevant national guidelines, including National Service Frameworks
Clinical guidelines published by NICE (NICE CG1216) provide recommendations for good practice in the diagnosis and treatment of lung cancer in England and Wales. In addition, NICE has published a quality standard (NICE QS1715) that defines best practice for the care of people with lung cancer. The QS1715 states that people with stage IIIB or IV NSCLC and eligible PS scores should be offered systemic therapy (first and second line) in accordance with NICE guidance that is tailored to the pathological subtype of the tumour and individual predictive factors. 16
There are a number of NICE guidance documents that are relevant to this appraisal. These are described in Table 5.
| NICE clinical guideline/guidance | Patient group (histology/EGFR status) | Recommended treatment |
|---|---|---|
| First line | ||
| CG1216 – The diagnosis and treatment of lung cancer | All patients with NSCLC of good PS score (WHO rating 0 or 1 or Karnofsky score of 80–100) | Platinum-doublet docetaxel, gemcitabine, vinorelbine or paclitaxel. Or single agent if unable to tolerate platinum therapy |
| TA19217 – Gefitinib for the first-line treatment of locally advanced or metastatic NSCLC | EGFR M+ only | Gefitinib if provided at agreed PAS price |
| TA25818 – Erlotinib for the first-line treatment of locally advanced or metastatic EGFR M+ NSCLC | EGFR M+ only | Erlotinib if provided at the agreed PAS price |
| TA18119 – Pemetrexed for the first-line treatment of NSCLC | Confirmed adenocarcinoma or large cell (non-squamous) only | Pemetrexed + cisplatin |
| Maintenance following first line | ||
| TA19020 – Pemetrexed for the maintenance treatment of NSCLC | Non-squamous (adenocarcinoma or large cell) without disease progression after first-line platinum chemotherapy with gemcitabine, paclitaxel or docetaxel | Pemetrexed |
| Second line | ||
| CG1216 – The diagnosis and treatment of lung cancer | All NSCLC | Docetaxel monotherapy |
| TA16221 – Erlotinib for the treatment of NSCLC | All NSCLC | Erlotinib if provided at an overall treatment cost equal to that of docetaxel. It is not recommended in patients for whom docetaxel is unsuitable or contraindicated |
| TA17522 – Gefitinib for the treatment of locally advanced or metastatic NSCLC | EGFR M+ only | Gefitinib. NICE was unable to recommend the use in the NHS of gefitinib for the second-line treatment of locally advanced or metastatic NSCLC because no evidence submission was received from the manufacturer or sponsor of the technology |
| TA12423 – Pemetrexed for the treatment of NSCLC | All NSCLC | Not recommended |
First-line treatment options
The first-line chemotherapy treatment options recommended by NICE16 include platinum-based (cisplatin or carboplatin) doublet chemotherapy with docetaxel, gemcitabine, paclitaxel or vinorelbine. Pemetrexed (Alimta®, Eli Lilly & Co Ltd) plus cisplatin is an option for patients with predominantly non-squamous NSCLC. Single agents erlotinib [Tarceva®, Roche (UK) Ltd] or gefitinib (IRESSA®, AstraZeneca) are options for patients with locally advanced or metastatic EGFR M+ NSCLC. 16
Maintenance treatment options
Maintenance treatment has recently become an option for a limited group of patients. Pemetrexed as a single-agent maintenance treatment is an option for patients with locally advanced or metastatic non-squamous lung disease whose disease has not progressed following first-line chemotherapy treatment with a platinum-based doublet containing gemcitabine, paclitaxel or docetaxel. 16 NICE guidance for the use of pemetrexed as a single-agent maintenance treatment as an option for patients with locally advanced or metastatic non-squamous lung disease whose disease has not progressed following first-line chemotherapy treatment with pemetrexed plus cisplatin is currently under development.
Second-line treatment options
Current NICE recommendations for second-line treatment of NSCLC include docetaxel monotherapy or erlotinib monotherapy. Erlotinib is not recommended for the second-line treatment of locally advanced or metastatic NSCLC in patients for whom docetaxel is unsuitable (that is, in patients who are intolerant to docetaxel or in whom docetaxel is contraindicated) or for third-line treatment after docetaxel therapy. 16
Recommendation by NICE was not possible for the use of gefitinib as a second-line treatment option for patients in England and Wales, as the single technology appraisal process (2009) was terminated because no evidence submission was received from the manufacturer or sponsor of the technology. 16
Pemetrexed as a second-line treatment for locally advanced or metastatic NSCLC was not recommended by NICE.
Variation in services and/or uncertainty about best practice
Histological diagnosis
The National Lung Cancer Audit11 reports an overall histological diagnosis rate of 77% for all lung cancers. For NSCLC, the rate appears to be 70%. This means that the histological status of their disease is not tested in 30% of patients with NSCLC. Our clinical advisors tell us that some patients are too ill for treatment and thus do not undergo histological diagnosis.
Epidermal growth factor mutation testing
In clinical practice, EGFR mutation status is mostly ascertained at the same time as histological status for patients considered likely to be EGFR M+. However, clinical advice (Dr Ernie Marshall, The Clatterbridge Centre NHS Foundation Trust, Liverpool, 2013, personal communication) to the Assessment Group (AG) suggests that the EGFR testing pathway is not uniform across England and Wales. Our clinical advisors tell us that EGFR mutation testing rates are improving annually.
In the UK NHS, most patients with NSCLC have an EGFR mutation test prior to being treated for the first time, and clinicians tell us very few people need to have an EGFR mutation test before second-line treatment. The AG acknowledges that the significance of EGFR mutation status has only recently been clarified and EGFR mutation status is now increasingly being considered in the design of lung cancer trials (e.g. prospective recruitment of EGFR M+ or EGFR M– patient populations; EGFR mutation status as a stratification factor).
Description of technology under assessment
Two oral anticancer treatments, used within their licensed indications, are the focus of this review: erlotinib and gefitinib. Both are EGFR-TKIs that block the signal pathways involved in cell proliferation. The summaries of product characteristics (SPCs) for erlotinib and gefitinib are available from the Electronic Medicines Compendium. 24
Erlotinib
Erlotinib is available as film-coated tablets in 25 mg, 100 mg or 150 mg. The recommended daily dose of erlotinib is 150 mg taken at least 1 hour after food. No guidance as to duration of treatment is given. Erlotinib is licensed in the UK for the treatment of NSCLC and metastatic pancreatic cancer. The latter indication is not relevant to this review.
In the setting of NSCLC, erlotinib is licensed for use with three patient populations. In the first-line setting, erlotinib is licensed for the treatment of patients with locally advanced or metastatic NSCLC with EGFR activating mutations. The SPC25 stipulates that, prior to initiation of erlotinib therapy, people with chemotherapy-naive NSCLC should undergo EGFR mutation testing using a well-validated and robust methodology.
In the post-first-line maintenance setting, erlotinib is licensed as monotherapy for people with locally advanced or metastatic NSCLC whose disease is stable following four cycles of standard platinum-based first-line chemotherapy.
In the second-line setting, erlotinib is licensed for patients with locally advanced or metastatic NSCLC following failure of at least one prior chemotherapy.
Gefitinib
Gefitinib is available as a 250-mg film-coated tablet. The recommended dose of gefitinib is one 250-mg tablet daily. No guidance as to duration of treatment is given. It is licensed in the UK for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR-activating mutations. The licence places no restriction on where in the treatment pathway gefitinib is used. As was noted for erlotinib, the SPC26 for gefitinib stipulates that a well-validated and robust methodology is used to determine EGFR mutation status before therapy.
The Special warnings and precautions for use section of the SPC26 notes that an increased incidence of interstitial lung disease has been observed in epidemiological studies of gefitinib. Periodic liver function testing is also recommended for patients treated with gefitinib. The AG is aware that in 2003 the Food and Drug Administration (FDA) in the USA approved the use of gefitinib as a second-line treatment for patients who are refractory to platinum-based chemotherapy or docetaxel. The approval was made under the FDA’s accelerated approval regulations that allow the conditional approval of medicines based on surrogate outcomes, in this case tumour response rate (RR). The manufacturer was then required to provide the FDA with data on survival outcomes. The manufacturer has been unable to provide any data that show a positive benefit of gefitinib for survival and, consequently, the FDA (with the agreement of AstraZeneca) removed the licence for gefitinib use in the USA. 27
Current usage in the NHS
The manufacturer of erlotinib [Roche (UK) Ltd] states in its evidence submission to NICE that 70% of patients who receive second-line treatment receive erlotinib. 28
The manufacturer of gefitinib (AstraZeneca) presented in its evidence submission to NICE the number of patients receiving first-line treatment with gefitinib only. These patients are not relevant to this appraisal.
The pack costs of erlotinib and gefitinib are shown in Table 6. The costs of erlotinib to the NHS are subject to a further (confidential) discount under the patient access scheme.
| Drug | Pack size and cost |
|---|---|
| Erlotinib | 150 mg, 30-tablet pack = £1631.53. BNF list price29 September 2013 |
| Gefitinib | 250 mg, 30-tablet pack = £2167.71. BNF list price29 September 2013. NHS-discounted price available of £12,200 per patient receiving treatment beyond 60 days |
Chapter 2 Definition of the decision problem
Decision problem
The remit of this appraisal is to review and update (if necessary) the clinical effectiveness and cost-effectiveness evidence base described in NICE TA16221 and NICE TA175. 22 The key elements of the decision problem are described in Table 7.
| Interventions | Erlotinib |
| Gefitinib | |
| Patient population | Adults with locally advanced or metastatic NSCLC that has progressed following prior chemotherapya |
| Comparators | Erlotinib and gefitinib to be compared with each other and with:
|
| Outcomes |
|
| Economic analysis | The reference case stipulates that:
|
| Other considerations |
|
The AG notes that treatments given at first line will impact on treatments available to patients at disease progression. It is unlikely that any patient would be re-treated at second line with the same agent. This means that patients with EGFR M+ tumours treated at first line with a TKI (erlotinib or gefitinib) would not be treated with a TKI following disease progression.
The AG further notes that the eligible patient population for second-line erlotinib or gefitinib is small as the majority of people with EGFR M+ tumours will be diagnosed and treated with a first-line TKI, rendering them ineligible for a TKI at second line.
Overall aims and objectives of assessment
The remit of this review is to appraise the clinical effectiveness and cost-effectiveness of erlotinib and gefitinib within their licensed indications for the treatment of NSCLC that has progressed following prior chemotherapy (review of NICE technology appraisals TA16221 and TA17522).
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Search strategies
In addition to searching the two manufacturers’ submissions for relevant references, the following databases were searched for studies of erlotinib and gefitinib:
-
EMBASE (via OvidSP), from 1974 to 26 April 2013
-
MEDLINE (via OvidSP), from 1946 to 26 April 2013
-
The Cochrane Library, from inception to 28 April 2013
-
PubMed, from January 2010 to 28 April 2013.
The results were entered into an EndNote X5 (Thomas Reuters, CA, USA) library and the references were de-duplicated. Full details of the search strategies are presented in Appendix 1.
The reference lists of included trials were searched for relevant trials. Information on trials in progress was sought from oncology conference databases (American Society for Clinical Oncology and the European Society for Medical Oncology), and the ClinicalTrials.gov website was searched for ongoing trials. In addition, advice was sought from the two clinical advisors to the review and the three clinical peer reviewers.
Inclusion and exclusion criteria
Two reviewers, JG and JH, independently screened all titles and abstracts identified via searching and obtained full-paper manuscripts that were considered relevant by either reviewer (stage 1). The relevance of each study was assessed (JG and JH) according to the criteria set out in this section (stage 2). Studies that did not meet the criteria were excluded and their bibliographic details were listed alongside reasons for their exclusion. Any discrepancies were resolved by consensus and, where necessary, a third reviewer was consulted.
Study design
Only randomised controlled trials (RCTs) were included in the assessment of clinical effectiveness.
Interventions and comparators
The effectiveness of two EGFR-TKIs, erlotinib and gefitinib, within their licensed indications were assessed. Studies that compared the use of erlotinib or gefitinib with the use of docetaxel or best supportive care (BSC), or, where appropriate, with each other, were included in the review. Trials in which erlotinib was combined with other active treatments were excluded from the review.
Patient populations
Adults with locally advanced or metastatic NSCLC that has progressed following prior chemotherapy treatment were included.
Outcomes
Data on any of the following outcomes were included in the assessment of clinical effectiveness: overall survival (OS), progression-free survival (PFS), RRs, adverse events (AEs) and health-related quality of life. For the assessment of cost-effectiveness, outcomes included incremental cost per life-year (LY) gained and incremental cost per quality-adjusted life-year (QALY) gained.
Data extraction strategy
Data relating to both study design and quality were extracted by two reviewers (JG and KD) into a Microsoft Excel® spreadsheet (Microsoft Corporation, Redmond, WA, USA). Two reviewers cross-checked each other’s data extraction and where multiple publications of the same study were identified, data were extracted and it was reported as a single study.
Quality assessment strategy
The quality of clinical effectiveness studies was assessed independently by two reviewers (JG and KD) in accordance with the Centre for Reviews and Dissemination at York University’s suggested criteria. 30 All relevant information is tabulated and summarised within the text of the report. Full details and results of the quality assessment strategy for clinical effectiveness studies are reported in Appendix 2.
Methods of data synthesis
The results of the clinical data extraction and clinical study quality assessment are summarised in structured tables and as a narrative description. For patients who have progressed following prior treatment, the decision problem of interest to this review is made up of the following comparisons: the effectiveness of erlotinib and gefitinib in a population of patients with EGFR M+ tumours, the effectiveness of erlotinib and gefitinib in a population of patients with EGFR M– tumours and the effectiveness of erlotinib and gefitinib in an EGFR unknown population (i.e. whose EGFR mutation status is unknown at the time of randomisation).
Results
Quantity and quality of research available
A total of 1563 titles and abstracts were screened for inclusion in the review of clinical effectiveness and cost-effectiveness evidence. Overall, 12 relevant RCTs were identified. The process of study selection is shown in Figure 1.
FIGURE 1.
Study selection process.

Clinical effectiveness (randomised controlled trials)
A total of 12 RCTs (one of which was discussed in NICE TA162,21 namely BR.2131) were reported in 25 publications and met the criteria for inclusion into the review. The reference cited in the text refers to the primary report, and subsequent publications describing outcomes of the trials are listed by trial in Appendix 3. The AG did not find any relevant publications that were not identified by the manufacturers.
The identified trials are summarised in Table 8. A full list of publications that were excluded from the review following the application of the inclusion criteria is presented in Appendix 4. The AG also identified and assessed the quality of existing systematic reviews in order to cross-check for the identification of additional studies as well as to gain an understanding of the issues related to the combining of data in this complex clinical area. A summary and critique of relevant systematic reviews is presented in Appendix 5.
| Trial | Design | Intervention | Comparator | Patient population (EGFR M+, EGFR M– or EGFR unknown) | Retrospective EGFR subgroup data available |
|---|---|---|---|---|---|
| Gefitinib vs. erlotinib | |||||
| Kim et al.32 | Open-label, non-comparative randomised Phase II trial | Gefitinib | Erlotinib | EGFR M+ and two out of three factors associated with EGFR mutations | Yes |
| Gefitinib vs. docetaxel | |||||
| Bhatnagar et al.33 | RCT | Gefitinib | Docetaxel | EGFR unknown | No |
| INTEREST34 | Open-label Phase III RCT | Gefitinib | Docetaxel | EGFR unknown | Yes |
| ISTANA35 | Open-label Phase III RCT | Gefitinib | Docetaxel | EGFR unknown | No |
| Li et al.36 | RCT | Gefitinib | Docetaxel | EGFR unknown | No |
| SIGN37 | Open-label Phase II RCT | Gefitinib | Docetaxel | EGFR unknown | No |
| V-15-3238 | Open-label Phase III RCT | Gefitinib | Docetaxel | EGFR unknown | Yes |
| Gefitinib vs. placebo | |||||
| ISEL39 | Placebo-controlled Phase III RCT | Gefitinib + BSC | Placebo + BSC | EGFR unknown | Yes |
| Erlotinib vs. docetaxel | |||||
| DELTA40 | Open-label Phase III RCT | Erlotinib | Docetaxel | EGFR M+ and EGFR M– | Yes |
| TAILOR41 | Open-label Phase III RCT | Erlotinib | Docetaxel | EGFR M– only | Yes |
| Erlotinib vs. docetaxel/pemetrexed | |||||
| TITAN42 | Open-label Phase III RCT | Erlotinib | Docetaxel or pemetrexed | EGFR unknown | Yes |
| Erlotinib vs. placebo | |||||
| BR.2131 | Placebo-controlled Phase III RCT | Erlotinib | Placebo | EGFR unknown | Yes |
As EGFR mutation status is a key factor in this review, it is noted in Table 8 whether or not a patient’s EGFR mutation status was determined before randomisation and used as the basis for inclusion in the trial. For those trials that did not select patients based on EGFR mutation status, the final column of the table indicates whether or not any retrospective analyses of the data were conducted. It should be noted that, where retrospective EGFR subgroup analyses are available, the data are limited.
Two of the included trials, Bhatnagar et al. 33 and the Docetaxel and Erlotinib Lung Cancer Trial (DELTA),40 were only reported as conference abstracts and, therefore, limited information is available to describe these studies. The final results of the TArceva Italian Lung Optimization tRial (TAILOR)41 were published after our searches were completed; however, we have included these results in the review.
Gefitinib trials (n = 7)
Gefitinib was compared with docetaxel in six trials of patients of unknown EGFR status at the time of randomisation. 33–38 A single trial39 compared gefitinib with placebo in an EGFR unknown population.
Erlotinib trials (n = 4)
Two trials40,41 compared erlotinib with docetaxel. DELTA40 was designed to allow the assessment of treatment outcomes in EGFR M– and EGFR M+ patient populations. TAILOR41 included only patients who were known to be EGFR M–. One trial42 compared erlotinib with chemotherapy in patients whose EGFR status was unknown at the time of randomisation; the chemotherapy regimen was either docetaxel or pemetrexed depending on the treating physician’s choice. In the BR.21 trial,31 erlotinib was compared with a placebo in a population whose EGFR mutation status was unknown.
Gefitinib versus erlotinib (n = 1)
Gefitinib was compared with erlotinib in one trial32 in patients who were EGFR M+ or who were likely to be EGFR M+.
Quality assessment of the included randomised controlled trials
The results of the quality assessment exercise are presented in Appendix 2. Overall, the trials were considered to be of reasonable methodological quality.
Randomisation
Of the 10 trials reported in published papers, four32,35,36,38 did not state the methods used to randomise patients into the trial and whether or not the allocation method precluded prediction of participant assignment. One trial35 reported partial details of the randomisation method used, but stated that the treatment allocation was conducted centrally. All trials reported the number of patients randomised into the trial. Of the two trials reported in conference abstracts,33,40 only DELTA40 described the randomisation method used in the trial. Neither study reported details of allocation concealment.
Comparability across groups
All of the published trials reported the key characteristics of the participants and, with the exception of the Tarceva In Treatment of Advanced NSCLC (TITAN) trial,42 showed comparability across trial arms. The Kim et al. 32 trial was considered to be unclear on this criterion – in the trial, a ‘historical control’ was used to ascertain the efficacy of the two interventions (rather than comparing both arms) and no details are presented for the historical control group. The erlotinib and gefitinib arms of the Kim et al. 32 trial appear to be well balanced. In TAILOR,41 differences in the numbers of smokers and never-smokers, and the numbers of patients with adenocarcinoma histology were noted. In the conference abstracts (Bhatnagar et al. 33 and DELTA40) details of comparability were not presented.
Eligibility and co-interventions
All published trials specified eligibility criteria for entry into the trial. Three trials [IRESSA NSCLC Trial Evaluating REsponse and Survival versus Taxotere (INTEREST),34 Li et al. 36 and Second-line Indication of Gefitinib in NSCLC (SIGN)37] reported the use of co-medications that may have had an effect on trial outcomes. In all cases these were corticosteroids and/or antiemetics administered as premedications prior to intravenous (i.v.) chemotherapy. It is likely that the remaining trials also used these premedications but did not report this use in the publication. In the conference abstracts, limited details of inclusion criteria were reported and neither of the abstracts noted the use of comedications. 33,40
Blinding
The reporting of blinding procedures across the 10 published trials was poor. Two of the 10 published trials were placebo controlled31,39 and were stated as being ‘double blind.’ It is clear from the IRESSA Survival Evaluation in Lung cancer (ISEL)39 trial that both patients and investigators were blinded as to treatment allocation, although it is unclear whether the investigators were treatment administrators or outcome assessors, or both. We have assumed that, in the BR.21 trial,31 the patients, administrators and outcome assessors were blinded to treatment allocation, although this is not explicitly stated. Neither ISEL39 nor BR.2131 reported any testing of the blinding procedures.
The remaining eight published trials were open label. In trials in which the interventions in the trial arms are very different (e.g. i.v. infusion vs. orally administered), it is not always possible to blind patients or administrators to the treatments received. It should be possible, however, to employ procedures whereby outcome assessment is conducted in a blinded fashion or where unblinded assessment is verified by independent blinded assessment. Few details of any blinding procedures were reported in the publications of the included trials. It is noted in TAILOR41 that two independent radiologists, masked to treatment assignment, carried out post-hoc reviews of all the scans of responding patients, and in V-15-3238 the primary overall RR results that were based on investigator judgement were generally consistent with those obtained from independent response evaluation committee assessment. However, it is unknown whether or not any of the remaining trials employed similar blinding protocols.
Both of the trials33,40 reported as conference abstracts appear to be open label and neither of the trials report details of any blinding procedures used. 40,41
Patient withdrawals
The 10 trials reported as published papers all appear to have included more than 80% of randomised patients in the final analysis. Reasons for patient dropouts were clearly reported. However, this aspect of the trials is not reported in the two conference abstracts. 33,41
Intention-to-treat analysis
All but one of the trials (Li et al. 36) reported in the published papers state that an intention-to-treat (ITT) analysis was conducted. However, this aspect of the trials is not reported in the two conference abstracts. 33,40
Outcomes
None of the trials appeared to have reported fewer outcomes than were proposed in the methods section of the published paper, although the two trials reported as conference abstracts cannot be assessed on this criterion. 33,40
In addition, the AG highlights the following aspects of the included studies that have not been discussed within the remit of the quality assessment exercise:
-
TITAN42 – the trial was terminated early because of slow recruitment.
-
Kim et al. 32 – the trial used a historical control (no details were provided) to assess the relative clinical effectiveness of erlotinib and gefitinib.
-
TAILOR41 – several protocol changes were made to TAILOR, including a change of primary end point.
-
SIGN37 – the trial was not powered to formally test outcomes.
Trial characteristics
The characteristics of the included trials are presented in Table 9. All of the trials were published between 2005 and 2013. Five trials were conducted internationally, one exclusively in multiple centres in Italy (TAILOR41) and six in Asian countries: South Korea, India, the People’s Republic of China and Japan [IRESSA as Second-line Therapy in Advanced NSCLC – KoreA (ISTANA)35 and Kim et al. ,32 Bhatnagar et al. ,33 Li et al. ,36 DELTA40 and V-15-32,38 respectively]. Of the trials conducted in Asia, three were multicentred. 35,38,40 With the exception of the Li et al. 36 trial, all trial results were published in English. The Li et al. 36 paper was translated from Mandarin Chinese to English by a translation service contracted by the AG. The number of randomised patients ranged from 3033 to 1692. 39 Inclusion and exclusion criteria used in the included studies are shown in Appendix 6.
| Trial | Type of trial | Intervention | Comparator | Number patients | Location | Median follow-up | Trial support | Treatment crossover |
|---|---|---|---|---|---|---|---|---|
| Gefitinib vs. erlotinib | ||||||||
| Kim et al. 201232 | Open-label, non-comparative randomised Phase II | Gefitinib 250 mg daily | Erlotinib 150 mg daily | N = 96; gefitinib, n = 48; erlotinib n = 48 | South Korea | 16.3 months | IN-SUMG Foundation for Medical Research | At the discretion of each physician |
| Gefitinib vs. docetaxel | ||||||||
| aBhatnagar et al. 201233 | RCT | Gefitinib 250 mg daily | Docetaxel 75 mg/m2 every 3 weeks | N = 30 | India | 2 years | NS | NS |
| INTEREST 200834 | Open-label Phase III non-inferiority RCT | Gefitinib 250 mg daily | Docetaxel 75 mg/m2 every 3 weeks | N = 1466; gefitinib, n = 733; docetaxel, n = 733 | Europe, Asia and the Americas | 7.6 months | AstraZeneca | Gefitinib arm: n = 28 (4%) EGFR-TKI; n = 225 (31%) docetaxel; n = 112 (15%) other chemotherapy |
| Docetaxel arm: n = 4 (1%) docetaxel; n = 268 (37%) EGFR-TKI; n = 74 (10%) other chemotherapy | ||||||||
| ISTANA 201035 | Open-label Phase III RCT | Gefitinib 250 mg daily | Docetaxel 75 mg/m2 every 3 weeks | N = 161; gefitinib, n = 82; docetaxel, n = 79 | Korea | 13 months | AstraZeneca | Gefitinib arm: 24.7% received no further systemic chemotherapy apart from further EGFR-TKIs (2.5% gefitinib/erlotinib), 22.2% received no treatment, 29.6% received docetaxel and 44.4% received other chemotherapy |
| Docetaxel arm: 67.1% received an EGFR-TKI and 6.6% received other chemotherapy | ||||||||
| Li et al. 201036 | RCT | Gefitinib 250 mg daily | Docetaxel 75 mg/m2 every 3 weeks | N = 98; gefitinib, n = 50; docetaxel, n = 48 | People’s Republic of China | NS | NS | NS |
| SIGN 200637 | Open-label Phase II RCT | Gefitinib 250 mg daily | Docetaxel 75 mg/m2 every 3 weeks | N = 141; gefitinib, n = 68; docetaxel, n = 73 | Europe, South America and the Middle East | 9.2 months (gefitinib), 9.4 months (docetaxel) | AstraZeneca | NS |
| V-15-32 200838 | Open-label Phase III non-inferiority RCT | Gefitinib 250 mg daily | Docetaxel 60 mg/m2 every 3 weeks | N = 490; gefitinib, n = 245; docetaxel, n = 244b | Japan | 21 months | AstraZeneca | Crossover was greater than initially expected, and differences in the number and types of patients who received these post-study treatments complicated interpretation of survival results |
| Gefitinib vs. placebo | ||||||||
| ISEL 200539 | Placebo-controlled double-blind Phase III RCT | Gefitinib 250 mg daily | Placebo + BSC | N = 1692; gefitinib, n = 1129; placebo, n = 563 | Europe, Asia, Central and South America, Australia and Canada | 7.2 months | AstraZeneca | Placebo arm: 3% received gefitinib. All subsequent treatments for NSCLC were well balanced between the treatment groups. The protocol allowed for up to 15% crossover to gefitinib |
| Erlotinib vs. docetaxel | ||||||||
| aDELTA 201340 | Open-label Phase III RCT | Erlotinib 150 mg daily | Docetaxel 60 mg/m2 every 3 weeks | N = 301; erlotinib, n = 150; docetaxel, n = 151 | Japan | NS | Japanese National Hospital Organization | NS |
| TAILOR 201341 | Open-label Phase III RCT | Erlotinib 150 mg daily | Docetaxel 75 mg/m2 | N = 222; erlotinib, n = 112; docetaxel, n = 110 | Italy | 33 months | Italian Agency for Drug Administration | No crossover allowed |
| Erlotinib arm: seven participants crossed over | ||||||||
| Docetaxel arm: four participants crossed over. Third-line treatment with pemetrexed/GEM/VIN | ||||||||
| Erlotinib vs. docetaxel/pemetrexed | ||||||||
| TITAN 201242 | Open-label Phase III RCT | Erlotinib 150 mg daily | Docetaxel or pemetrexed dosing at discretion of the investigator | N = 424; erlotinib, n = 203; chemotherapy, n = 221 | International | Erlotinib: 27.9 months, docetaxel/pemetrexed: 24.8 months | Hoffmann F – La Roche, Basel, Switzerland | Erlotinib arm: 25% antimetabolites, 23% docetaxel or PAX |
| Chemotherapy arm: 12% antimetabolites, 23% TKIs, 5% switch to docetaxel, 7% switch to pemetrexed | ||||||||
| Erlotinib vs. placebo | ||||||||
| BR.21 200531 | Placebo-controlled Phase III RCT | Erlotinib 150 mg daily | Placebo | N = 731; erlotinib, n = 488; placebo, n = 243 | International | NS | Supported in part by a grant from OSI Pharmaceuticals | Erlotinib arm: 8 (1.6%) |
| Placebo arm: 18 (7.4%) received other EGFR inhibitors after study medication discontinued | ||||||||
Two of the trials were Phase II,32,37 while ISTANA,35 ISEL,39 DELTA,40 TAILOR,41 TITAN,42 V-15-32,38 INTEREST34 and BR.2131 were all Phase III trials. The phase of the Bhatnagar et al. 33 and Li et al. 36 trials is unknown. Seven of the trials were funded solely, or in part, by pharmaceutical companies,31,34,35,37–39,42 three were funded by research grants32,40,41 and the funding source for two trials33,36 is not known.
The dosage of erlotinib and gefitinib was consistent with the recommended licensed dose (150 mg or 250 mg, respectively) across the trials in which those treatments were used. Of the nine trials in which docetaxel was a comparator,33–38,40–42 seven trials33–37,41,42 treated patients with 75 mg/m2 every 3 weeks and two trials38,40 treated patients with 60 mg/m2 every 3 weeks, this being the standard dose used in Japan. Median follow-up (when reported) ranged between 7.2 months39 and 33 months. 41 Information regarding post-progression treatments was not reported in four trials. 33,36,37,40
Patient characteristics
Patient characteristics are presented in Table 10. Details of individual trial inclusion and exclusion criteria are presented in Appendix 6. The median patient age (when reported) ranged between 49 and 61 years. With the exception of the Kim et al. 32 trial, the majority of patients were male (when reported). With the exception of the Li et al. 36 trial, the majority of patients were considered to have stage IV disease (when reported). The main histological type across trials was adenocarcinoma; however, the ratio of adenocarcinoma to other histological subtypes varied. For example, approximately 90% of patients in the Kim et al. 32 trial and 77% in V-15-3238 had adenocarcinoma, while lower rates were reported in BR.2131 and TITAN42 (both approximately 50%). In the main, the majority of patients had received a single prior chemotherapy treatment; however, in ISEL39 and BR.2131 approximately half of the patients had received two previous chemotherapy treatments.
| Trial | Median age, years (range) | % male | Stage IIIB (%) | Stage IV (%) | Histology, adenocarinoma/squamous (%) | Previous treatment | PS | Ethnicity | Smoking status (%) |
|---|---|---|---|---|---|---|---|---|---|
| Gefitinib vs. erlotinib | |||||||||
| Kim et al. 201232 | 60 (37–83) | 14.6 | 14.6 | 72.9 | Adenocarinoma, 91.7; squamous, 6.3 | Placebo treatment = 96.9% | ECOG score of:
|
Koreana | Current/former, 8.3; never, 91.7 |
| 56 (32–81) | 14.6 | 10.4 | 70.8 | Adenocarinoma, 89.6; squamous, 6.3 | Placebo treatment = 100% | ECOG score of:
|
Koreana | Current/former, 4.2; never, 95.8 | |
| Gefitinib vs. docetaxel | |||||||||
| bBhatnagar et al. 201233 | NR | NR | NR | NR | NR | NR | ECOG score of 0 to 2 | Indiana | NR |
| NR | NR | NR | NR | NR | NR | ECOG score of 0 to 2 | Indiana | NR | |
| INTEREST 200834 | 61 (27–84) | 63.6 | At diagnosis: 25 | At diagnosis: 52.9 | Adenocarinoma, 53.9; squamous, 25.2 | 1 = 84.4%; 2 = 15.3%; 3 = 0.3% | WHO score of:
|
White, 75%; Asian, 21%; black, 1.4%; other, 2.6% | Ever, 79.8; never, 20.2 |
| 60 (20–84) | 66.6 | At diagnosis: 28.8 | At diagnosis: 52.3 | Adenocarinoma, 54.8; squamous, 24 | 1 = 83.2%; 2 = 16.8%; 3 = 0 | WHO score of:
|
White, 73.7%; Asian, 23.1%; black, 1.6%; other, 1.6% | Ever, 79.6; never, 20.5 | |
| ISTANA 201035 | 57 (21–74) | 67.1 | 13.4 (LA) | 86 (Met) | Adenocarinoma, 65.9; squamous, 20.7 | 1 (placebo-doublet) | WHO score of:
|
Korean and East Asian | Ex, 62.2; regular, 1.2; never, 36.6 |
| 58 (20–73) | 57 | 17.7 | 82.3 | Adenocarinoma, 69.6; squamous, 13.9 | 1 (placebo-doublet) | WHO score of:
|
Korean and East Asian | Ex, 54.4; regular, 0; never, 45.6 | |
| Li et al. 201036 | 50.7 | 60 | 58 | 42 | Adenocarinoma, 56; squamous, 44 | CIS + GEM/VIN; or GEM/VIN monotherapy | KPS score of ≥ 70 | Chinese | NR |
| 48.2 | 60 | 60 | 40 | Adenocarinoma, 56; squamous, 44 | CIS + GEM/VIN; or GEM/VIN monotherapy | KPS score of ≥ 70 | Chinese | NR | |
| SIGN 200637 | 63 (34–85) | 69 | NR | 60 | NR | 1 = 97.1% | WHO score of:
|
Caucasian 41.2%; Hispanic 48.5%; oriental 4.4%; other 5.9% | Yes, 67.6; no, 26.5; unknown, 5.9 |
| 59.5 (29–83) | 51 | NR | 56 | NR | 1 = 98.6% | WHO score of:
|
Caucasian, 43.8%; black, 2.7%; Hispanic, 39.7%; oriental, 5.5%; other, 8.2% | Yes, 67.1; no, 24.7; unknown, 8.2 | |
| V-15-3238 | ≤ 64 = 56.3%; ≥ 65 = 43.7% | 61.6 | 19.2 | 64.9 | Adenocarinoma, 78.4; squamous, 15.1 | 1: 86.5%; 2: 13.5% | WHO score of:
|
Japanesea | Ever, 71; never, 29 |
| ≤ 64: 55.3%; ≥ 65: 44.7% | 61.9 | 20.5 | 61.5 | Adenocarinoma, 77; Squamous 16.8 | 1: 82.4%; 2: 17.2% | WHO score of:
|
Japanesea | Ever, 64.3; never, 35.7 | |
| Gefitinib vs. placebo | |||||||||
| ISEL 200539 | 62 (28–90) | 67 | 21 (LA) | 79 (Met) | Adenocarinoma, 45; squamous, 35 | 0 = 1 person; 1 = 49%; 2 = 50%; ≥ 3 = 1% | WHO score of:
|
White, 75%; Asian, 21%; black, 1%; other, 4% | Habitual, 17; occasional, 1; ex, 60; never, 22 |
| 61 (31–87) | 67 | 20 (LA) | 80 (Met) | Adenocarinoma, 45; squamous, 33 | 0 = 1 person; 1 = 49%; 2 = 50%; ≥ 3 = 1% | WHO score of:
|
White, 77%; Asian, 19%; black, 1%; other, 4% | Habitual, 16; occasional, 1; ex, 60; never, 22 | |
| Erlotinib vs. docetaxel | |||||||||
| bDELTA 201340 | NR | NR | NR | NR | NR | NR | ECOG score of 0 to 2 | Japanesea | NR |
| NR | NR | NR | NR | NR | NR | ECOG score of 0 to 2 | Japanesea | NR | |
| TAILOR 201341 | 66 (40–81) | 71 | NR | NR | Adenocarinoma, 63; squamous, 28 | 1 = 92% | ECOG score of:
|
White, 99%; Asian, 1% | Current/former, 83; never, 17 |
| 67 (35–83) | 66 | NR | NR | Adenocarinoma, 75; squamous, 21 | 1 = 93% | ECOG score of:
|
White, 99%; Asian, 1% | Current/former, 73; never, 27 | |
| Erlotinib vs. docetaxel/pemetrexed | |||||||||
| TITAN 201242 | 59 (36–80) | 79 | 20 | 80 | Adenocarinoma, 47; squamous, 38 | Placebo–doublet: PAX/GEM/docetaxel/VIN | ECOG score of:
|
Caucasian, 85%; Asian, 14%; other, 1% | Present, 56; past, 29; never, 15 |
| 59 (22–79) | 72 | 23 | 77 | Adenocarinoma, 52; squamous, 35 | Placebo–doublet: PAX/GEM/docetaxel/VIN | ECOG score of:
|
Caucasian, 86%; Asian,12%; other, 2% | Present, 51; past, 29; never, 20 | |
| Erlotinib vs. placebo | |||||||||
| BR.21 200531 | 62 (34–87) | 64.5 | NR | NR | Adenocarinoma, 50.4; squamous, 29.5 | 1 = 50.6%; ≥ 2 = 49.4% | ECOG score of:
|
Asian, 12.9%; other, 87.1% | Current/ever, 73.4; unknown, 5.3; never, 21.3 |
| 59 (32–89) | 65.8 | NR | NR | Adenocarinoma, 49; squamous, 32.1 | 1 = 50.2%; ≥ 2 = 49.8% | ECOG score of:
|
Asian, 12.2%; other, 87.8% | Current/ever, 77; unknown, 5.8; never, 17.3 | |
In terms of PS, the majority of patients were assessed to have an ECOG score of 0 or 1 or a WHO score of 0 or 1. 32,34,35,38,41 Up to one-third of patients in the TITAN,42 ISEL39 and SIGN37 trials were considered to have a PS score of 2 (ECOG or WHO). The patients in the Li et al. 36 trial were KPS scores of 70 or greater, and the two conference abstracts (Bhatnagar et al. 33 and DELTA40) report that patients had ECOG scores of 0 to 2.
The trial populations included in TAILOR41 and the Kim et al. 32 trial were tested for EGFR mutation status before entry into the trial. In the TAILOR41 trial, only those who were EGFR M– were randomised. The patients recruited to the Kim et al. 32 trial were those who were EGFR M+ or who had two out of three factors associated with EGFR mutations (female, never-smoker and adenocarcinoma histology). DELTA40 included patients who were EGFR M–, but it is unclear if EGFR status was ascertained at the time of randomisation.
Six,32,33,35,36,38,40 of the 12 trials were conducted in East Asia and, therefore, exclusively included patients of East Asian ethnicity. With the exception of SIGN,37 the patients in the remaining trials were predominantly white/Caucasian. When reported, the percentage of never-smokers ranged across the trials from approximately 17%42 to 94%. 32
Assessment of effectiveness
The AG’s assessment of effectiveness is based on the following patient groups:
-
previously treated adult patients with locally advanced or metastatic NSCLC and who exhibit EGFR-activating mutations (referred to as EGFR M+ population)
-
previously treated adult patients with locally advanced or metastatic NSCLC and who do not exhibit EGFR-activating mutations (referred to as EGFR M– population)
-
previously treated adult patients with locally advanced or metastatic NSCLC and for whom EGFR mutation status is unknown or indeterminate (referred to as EGFR unknown population).
Epidermal growth factor mutation-positive population
Six trials reported subgroup data on EGFR M+ patients. Kim et al. ,32 V-15-3238 and TITAN42 reported subgroup data in the main paper. BR.21,31,43 ISEL39,44 and INTEREST34,45 reported subgroup data in a separate publication.
Overall survival
Four trials reported OS, one trial reported only the number of events39,44 and three presented hazard ratios (HRs). 31,34,42 The HRs were not statistically significant for any of the comparisons described. Table 11 summarises the results.
| Study name | % of deaths in intervention arm (number of events/number randomised) | % of deaths in control arm (number of events/number randomised) | Median OS (months) | HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Gefitinib vs. docetaxel | |||||
| INTEREST34 | 72.73 (32/44 over both arms) | 14.2 vs. 16.6 | 0.83 (0.41 to 1.67) | 0.60 | |
| Gefitinib vs. BSC | |||||
| ISEL39 | 33.33 (7/21) | 0.60 (3/5) | NR | NR | NR |
| Erlotinib vs. docetaxel/pemetrexed | |||||
| TITAN42 | NR | NR | 19.3 vs. NR | 1.19 (0.12 to 11.49) | 0.88 |
| Erlotinib vs. BSC | |||||
| BR.2131 | NR | NR | 10.9 vs. 8.3 | 0.55 (0.25 to 1.19) | 0.12 |
Progression-free survival
Four trials reported limited data for PFS (Table 12). Kim et al. 32 reported median PFS and ISEL39 reported the number of events in each arm. TITAN42 found no statistically significant difference between erlotinib and docetaxel/pemetrexed. Only INTEREST34 found a statistically significant difference in PFS favouring gefitinib [HR 0.16, 95% confidence interval (CI) 0.05 to 0.49].
| Study name | % of patients who progressed in intervention arm (number of events/number randomised) | % of patients who progressed in control arm (number of events/number randomised) | Median PFS (months) | HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Gefitinib vs. docetaxel | |||||
| INTEREST34 | NR | NR | 7.0 vs. 4.1 | 0.16 (0.05 to 0.49) | 0.001 |
| Gefitinib vs. BSC | |||||
| ISEL39 | 52.38 (11/21) | 0.80 (4/5) | NR | NR | NR |
| Gefitinib vs. erlotinib | |||||
| Kim et al.32 | NR | NR | 11.9 over both arms | NR | NR |
| Erlotinib vs. docetaxel/pemetrexed | |||||
| TITAN42 | NR | NR | NR | 0.71 (0.13 to 3.97) | NR |
Response rate
Five trials reported data on RR (Table 13). The three trials that presented data separately by treatment32,34,38 found that gefitinib appears to be favoured compared with docetaxel or erlotinib. However, patient numbers in the trials are small, and only one study34 presented a p-value of 0.04 to indicate that the difference between gefitinib and docetaxel was statistically significant. Two studies31,39 presented RRs for gefitinib versus BSC and erlotinib versus BSC of 37.50%39 and 26.67% respectively. 31
| Study name | RR in intervention arm (%) (number responded/number randomised) | RR in control arm (%) (number responded/number randomised) | Overall RR (%) (number responded/number randomised) | p-value |
|---|---|---|---|---|
| Gefitinib vs. docetaxel | ||||
| INTEREST34 | 42.11 (8/19) | 21.05 (4/19) | NR | 0.04 |
| V-15-3238 | 66.67 (6/9) | 45.45 (5/11) | NR | NR |
| Gefitinib vs. BSC | ||||
| aISEL39 | NR | NR | 37.50 (6/16) | NR |
| Gefitinib vs. erlotinib | ||||
| Kim et al.32 | 66.70 (NR) | 62.50 (NR) | 76.47 (13/17) | NR |
| Erlotinib vs. BSC | ||||
| BR.2131 | NR | NR | 26.67 (4/15) | 0.035 |
Epidermal growth factor mutation-negative population
Five trials reported subgroup data on EGFR M– patients. 31,32,34,37,42 The DELTA trial included patients with and without activating mutations and whose EGFR status was known prior to their randomisation into the trial. TAILOR41 included only patients who were known to be EGFR M–.
Trials of gefitinib are included here for completeness only.
Overall survival
Six trials reported data for OS, although ISEL39 reported only the number of events in each trial arm (Table 14). The other five trials31,34,40–42 reported HRs; however, these were not statistically significant for any of the comparisons described.
| Study name | % of deaths in intervention arm (number of events/number randomised) | % of deaths in control arm (number of events/number randomised) | Median OS (months) | HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Gefitinib vs. docetaxel | |||||
| INTEREST34 | 84.98 (215/253 over both arms) | 6.4 vs. 6.0 | 1.02 (0.78 to 1.33) | 0.91 | |
| Gefitinib vs. BSC | |||||
| ISEL39 | 70.45 (93/132) | 64.91 (37/57) | NR | NR | NR |
| Erlotinib vs. docetaxel | |||||
| TAILOR41 | NR | NR | 5.4 vs. 8.2 | 1.37 (1.00 to 1.89) (adjusted) 1.28 (0.95 to 1.96) (unadjusted) |
0.05 0.10 |
| DELTA40 | NR | NR | 9.0 vs. 9.2 | 0.98 (0.69 to 1.39) | 0.914 |
| Erlotinib vs. docetaxel/pemetrexed | |||||
| TITAN42 | NR | NR | 6.6 vs. 4.4 | 0.85 (0.59 to 1.22) | 0.37 |
| Erlotinib vs. BSC | |||||
| BR.2131 | NR | NR | 7.9 vs. 3.3 | 0.74 (0.52 to 1.05) | 0.09 |
Progression-free survival
Six trials reported PFS (Table 15),32,34,39–42 although ISEL39 reported only the number of events in each treatment group and Kim et al. 32 reported PFS for EGFR M– patients overall rather than for each treatment group separately. Two trials reported HRs that were not statistically significant. 34,42 In two other trials,40,41 PFS was statistically significantly greater in the docetaxel arm than in the erlotinib arm [HR 1.39, 95% CI 1.06 to 1.82 (unadjusted), and HR 1.44, 95% CI 1.08 to 1.92 (adjusted)].
| Study name | % of deaths in intervention arm (number of events/number randomised) | % of deaths in control arm (number of events/number randomised) | Median PFS (months) | HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Gefitinib vs. docetaxel | |||||
| INTEREST34 | NR | NR | 1.7 vs. 2.6 | 1.24 (0.94 to 1.64) | 0.14 |
| Gefitinib vs. BSC | |||||
| ISEL39 | 84.09 (111/132) | 85.96 (49/57) | NR | NR | NR |
| Gefitinib vs. erlotinib | |||||
| Kim et al.32 | NR | NR | 2.8 months overall | NR | NR |
| Erlotinib vs. docetaxel | |||||
| TAILOR41 | NR | NR | 2.4 vs. 2.9 | 1.41 (1.05 to 1.89) (adjusted); 1.39 (1.06 to 1.82) (unadjusted) | 0.02; 0.01 |
| DELTA40 | NR | NR | 1.3 vs. 2.9 | 1.44 (1.08 to 1.92) | 0.013 |
| Erlotinib vs. docetaxel/pemetrexed | |||||
| TITAN42 | 90.67 (68/75) | 79.73 (59/74) | NR | 1.25 (0.88 to 1.78) | 0.20 |
Response rate
Five trials reported data on RR (Table 16). Only one trial34 reported a p-value (p = 0.37) indicating that there was no statistically significant difference between the groups. One other trial41 reported a p-value (p = 0.003) indicating that there was a statistically significant difference in RR, favouring docetaxel.
| Study name | RR in intervention arm (%) (number responded/number randomised) | RR in control arm (%) (number responded/number randomised) | Overall RR (%) (number responded/number randomised) | p-value |
|---|---|---|---|---|
| Gefitinib vs. docetaxel | ||||
| INTEREST34 | 6.60 (7/106) | 9.76 (12/123) | NR | 0.37 |
| Gefitinib vs. BSC | ||||
| aISEL39 | NR | NR | 2.59 (3/116) | NR |
| Gefitinib vs. erlotinib | ||||
| Kim et al.32 | NR | NR | 25.00 (8/32) | NR |
| Erlotinib vs. docetaxel | ||||
| TAILOR41 | 3 (3/100) | 15.46 (15/97) | NR | 0.003 |
| Erlotinib vs. BSC | ||||
| BR.2131 | NR | NR | 6.93 (7/101) | NR |
Overall population: epidermal growth factor mutation status unknown
Four trials33,35–37 considered the overall population without distinguishing between patients’ EGFR mutation status. There are no data available from the Bhatnagar et al. 33 study as this study is published as an abstract only; the AG contacted the authors and asked for additional study data, but no reply was received.
Eight trials reported data for the overall population and also performed subgroup analyses based on EGFR mutation status. 31,32,34,35,37–39,42 TAILOR41 reported overall population data which comprised EGFR M– patient data only.
Overall survival
Eight trials reported data on OS for the overall population (Table 17). 31,34–40,42 Five trials34–38 compared gefitinib with docetaxel. A median survival of 7.1 months for gefitinib and 6.9 months for docetaxel were the only data available from Li et al. 36 The other four trials presented HRs, but no statistically significant differences between the interventions were noted.
| Study name | % of deaths in intervention arm (number of events/number randomised) | % of deaths in control arm (number of events/number randomised) | Median OS (months) | HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Gefitinib vs. docetaxel | |||||
| INTEREST34 | 82.02 (593/723) | 81.13 (576/710) | 7.6 vs. 8 | PP: 1.02 (0.91 to 1.15) | 0.47 |
| ITT: 1.015 (0.901 to 1.143) | NS | ||||
| ISTANA35 | 81.71 (67/82) | 74.68 (59/79) | 14.1 vs. 12.2 | 0.87 (0.61 to 1.24) | 0.437 |
| Li et al.36 | NR | NR | 7.1 vs. 6.9 | NR | NR |
| SIGN37 | NR | NR | 7.5 vs. 7.1 | 0.97 (0.61 to 1.52) | 0.88 |
| V-15-3238 | 63.67 (156/245) | 61.48 (150/244) | 11.5 vs. 14 | 1.12 (0.89 to 1.40) | 0.33 |
| Gefitinib vs. BSC | |||||
| ISEL39 | NR | NR | 5.6 vs. 5.1 | 0.89 (0.77 to 1.02) | 0.087 |
| Erlotinib vs. docetaxel | |||||
| DELTA40 | NR | NR | 14.8 vs. 12.2 | 0.91 (0.68 to 1.22) | 0.527 |
| Erlotinib vs. docetaxel/pemetrexed | |||||
| aTITAN42 | NR | NR | 5.3 vs. 5.5 | 0.96 (0.78 to 1.19) | 0.73 |
| Erlotinib vs. BSC | |||||
| BR.2131 | 77.46 (378/488) | 86.01 (209/243) | 6.7 vs. 4.7 | 0.70 (0.58 to 0.85) | < 0.001 |
No statistically significant difference in survival was reported between gefitinib and BSC,39 between erlotinib and docetaxel40 or between erlotinib and docetaxel/pemetrexed. 42
BR.2131 found a statistically significant difference in OS, favouring erlotinib over BSC (HR 0.7.0, 95% CI 0.58 to 0.85). However, the authors presented only adjusted analyses, no details were presented describing the unadjusted analyses.
Progression-free survival
Nine trials reported data for PFS (Table 18). Four studies compared gefitinib with docetaxel. 34,35,37,38 In ISTANA,35 PFS was statistically significantly longer in the gefitinib arm than in the docetaxel arm (HR 0.729, 90% CI 0.533 to 0.988); however, if using a 95% CI as was planned in the published paper, the CI would range from 0.51 to 1.05 and the difference in PFS is no longer statistically significant. The other three trials34,37,38 found no statistically significant differences in PFS between the groups.
| Study name | % of deaths in intervention arm (number of events/number randomised) | % of deaths in control arm (number of events/number randomised) | Median PFS (months) | HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Gefitinib vs. docetaxel | |||||
| INTEREST34 | 82.02 (593/723) | 76.62 (544/710) | 2.2 vs. 2.7 | 1.04 (0.93 to 1.18) | NR |
| ISTANA35 | 74.39 (61/82) | 74.68 (59/79) | 3.3 vs. 3.4 | 0.729a (0.533 to 0.988) (unadjusted) | 0.0441 |
| 0.634a (0.459 to 0.875) (adjusted) | 0.0134 | ||||
| SIGN37 | NR | NR | 3.0 vs. 3.4 | 0.94 (0.64 to 1.39) | 0.76 |
| V-15-3238 | 90.00 (180/200) | 84.49 (158/187) | 2.0 vs. 2.0 | 0.90 (0.72 to 1.12) | 0.335 |
| Gefitinib vs. BSC | |||||
| ISEL39 | NR | NR | 3.0 vs. 2.6 | 0.82 (0.73 to 0.92) | 0.0006 |
| Gefitinib vs. erlotinib | |||||
| Kim et al.32 | NR | NR | 4.9 vs. 3.1 | NR | NR |
| Erlotinib vs. docetaxel | |||||
| DELTA40 | NR | NR | 2.0 vs. 3.2 | 1.22 (0.97 to 1.55) | 0.092 |
| Erlotinib vs. docetaxel/pemetrexed | |||||
| TITAN42 | 92.61 (188/203) | 83.26 (184/221) | 6.3 weeks vs. 8.6 weeks | 1.19 (0.97 to 1.46) | 0.089 |
| Erlotinib vs. BSC | |||||
| BR.2131 | 92.21 (450/488) | 95.47 (232/243) | 2.2 vs. 1.8 | 0.61 (0.51 to 0.74) | < 0.001 |
Neither TITAN42 nor DELTA40 found any statistically significant differences between erlotinib and docetaxel/pemetrexed or between erlotinib and docetaxel. In BR.2131 a statistically significant difference in PFS favouring erlotinib compared with BSC was reported (HR 0.61, 95% CI 0.51 to 0.74); the authors of BR.2131 presented the results of adjusted analyses only. ISEL39 found a statistically significant difference in PFS favouring gefitinib compared with BSC (HR 0.82; 95% CI 0.73 to 0.92); the authors only presented adjusted analyses. The only data that were available from the head-to-head comparison of gefitinib compared with erlotinib was a median PFS of 4.9 versus 3.1 months. 32
Response rate
Nine trials reported data for RR (Table 19). Five of these compared gefitinib with docetaxel; the RR in the gefitinib arm ranged from 9.10% to 28.10% and the RR in the docetaxel arm ranged from 7.60% to 18.75%. INTEREST34 and V-15-3238 both reported odds ratios, although only V-15-3238 found a statistically significant difference between the two groups favouring gefitinib over docetaxel. In addition, one trial found a statistically significant difference in RR favouring gefitinib when compared with BSC. 39
| Study name | RR in intervention arm (%) (number responded/number randomised) | RR in control arm (%) (number responded/number randomised) | Overall RR: odds ratio (95% CI) | p-value |
|---|---|---|---|---|
| Gefitinib vs. docetaxel | ||||
| INTEREST34 | 9.10 (NR) | 7.60 (NR) | 1.22 (0.82 to 1.84) | 0.33 |
| ISTANA35 | 28.10 (NR) | 7.60 (NR) | NR | NR |
| Li et al.36 | 22.44 (11/49) | 18.75 (9/48) | NR | NR |
| SIGN37 | 13.24 (9/68) | 13.70 (10/73) | NR | NR |
| V-15-3238 | 22.50 (45/200) | 12.80 (24/187) | 2.14 (1.21 to 3.78) | 0.009 |
| Gefitinib vs. BSC | ||||
| aISEL39 | 8.00 (77/959) | 1.00 (6/480) | 7.28 (3.10 to 16.90) | < 0.0001 |
| Gefitinib vs. erlotinib | ||||
| Kim et al.32 | 47.92 (23/48) | 39.58 (19/48) | NR | NR |
| Erlotinib vs. docetaxel/pemetrexed | ||||
| TITAN42 | 7.88 (16/203) | 6.33 (14/221) | NR | NR |
| Erlotinib vs. BSC | ||||
| BR.2131 | 8.90 (NR) | Less than 1 (NR) | NR | NR |
Meta-analysis and network meta-analysis
Meta-analysis can be used to integrate the results of multiple trials which directly compare one specific treatment with another to produce an overall estimate of treatment effect size. Network meta-analysis can be used to compare effect sizes of treatments which have not previously been directly compared in a RCT using a common treatment comparator. After careful consideration of the clinical evidence available, the AG concluded that it would be inappropriate to use meta-analysis or network meta-analysis to investigate the treatment effects of erlotinib or gefitinib. The AG has identified several clinical and methodological weaknesses in the available clinical data which preclude use of quantitative synthesis methods.
First, the major weakness is the lack of available clinical data describing the key patient populations. There are no reliable OS or PFS data available for the comparison of erlotinib or gefitinib with any comparator in patients who are EGFR M+ and who have been previously treated. The AG agrees with the manufacturer of gefitinib, which states in its manufacturer’s submission that ‘All options for meta-analysis (direct, indirect and multiple treatment comparison) have been explored, however, all options were limited by heterogeneity in important clinical factors and ultimately such analyses were deemed more likely to increase rather than reduce uncertainty’. 46
For the EGFR M– population, median OS and PFS data are available from four trials. 31,34,40,41 As DELTA40 is made up of Japanese patients for whom there no patient characteristics data are available, the AG could not include the results from this trial in a network meta-analysis. The AG does not consider that INTEREST,34 BR.2131 and TAILOR41 include patient populations that are sufficiently similar to be included in a network meta-analysis. To illustrate: both TAILOR41 and INTEREST34 included a higher proportion of patients (93% and 89%, respectively) with PS score 0 or 1 than BR.2131 (70%); TAILOR41 and INTEREST34 included mainly patients who had received only one prior chemotherapy (92% and 84%, respectively) whereas this applied to only 50% of participants in BR.2131 (50%); and TAILOR41 has a higher rate of adenocarcinoma patients (70%) than either INTEREST34 (54%) or BR.2131 (50%).
There are survival data available from eight trials that included patients whose EGFR mutation status was unknown at the time of analysis, that is the trials included both EGFR M+ and EGFR M– status patients. 31,34–39,42 A higher proportion of patients in the ISEL39 trial (50%) than in the other trials had received more than one prior treatment, although it is difficult to know exactly how many prior treatments patients in Li et al. 36 and ISTANA35 had undergone. It is therefore uncertain whether or not the patients in ISEL39 are sufficiently similar to those in the other trials. In three trials ethnicity is a key differentiator (ISTANA,35 South Korean patients; Li et al. ,36 Chinese patients; V-15-32,38 Japanese patients) and the AG considers that including all Asian trials in a network meta-analysis may not yield relevant results for a non-Asian population. The remaining two trials31,42 compared erlotinib with BSC and pemetrexed and/or docetaxel. The AG considers that the patients in TITAN42 are different from the patients in BR.21,31 as in TITAN42 100% of patients had received a single prior chemotherapy while in BR.2131 50% of patients had received two or more prior chemotherapies. In addition, outcome data were not reported separately for docetaxel- and pemetrexed-treated patients in TITAN,42 and the AG notes that it has not been proved that docetaxel and pemetrexed are clinically equivalent when used in this patient population. For the assessment of PFS, data are available from eight trials;31,34,35,37–40,42 no HR was reported in Kim et al. 32 The arguments outlined above for three trials35,38,39 for the assessment of OS are valid again here. Further, the Kim et al. 32 trial is made up of Korean patients and the AG would not include this trial in a network meta-analysis designed to inform treatment pathways for patients in England and Wales. The arguments against using data from TITAN42 and BR.2131 in a network meta-analysis are valid again here for the assessment of PFS.
In addition to the lack of comparable clinical data available from the included trials, the AG also considers that a number of the trials used statistical methods that prohibit inclusion of the trial results in a network meta-analysis. To this end, the AG examined the methods of analyses and investigated the suitability of the Cox proportional hazards models employed; details are provided in Table 20. Specifically, for the EGFR unknown populations, the Kaplan–Meier plot crosses for six trials. 34,35,37–39,42 This is a sufficient condition to reject proportionality and means that the assumption behind the Cox proportional hazards model is violated, rendering the HR difficult to interpret. Crossing of Kaplan–Meier curves may be expected for small trials with few events. However, four of these trials are large with sample sizes ranging from 424 to 1692. 34,38,39,42 In addition, the AG has previously stated2 that Kaplan–Meier plots of PFS for erlotinib and gefitinib have a different pattern to those relating to third-generation drugs in first-line studies, and it appears that Kaplan–Meier plots of PFS for several second-line trials exhibit similar differences in patterns. The proportional hazards assumption may therefore be invalid for all PFS comparisons between TKIs and standard chemotherapy. The AG considers that the use of conventional (Cox) proportional hazards methods to estimate HRs in trials of erlotinib and gefitinib compared with any other drug is problematic and that the HR results may not be accurate and should be viewed with caution. The AG concludes that conducting a network meta-analysis using data from these trials may produce unreliable results.
| Trial | Adjusted/unadjusted analysis presented | Cox proportional hazards model suitable | Statistical analysis |
|---|---|---|---|
| Gefitinib vs. docetaxel | |||
| INTEREST34 | Unadjusted for OS | K-M plot crosses for OS | ‘We used an unadjusted Cox proportional hazards model to estimate the overall survival HR and CI in the per-protocol population’35 |
| Adjusted and per-protocol for PFS | No K-M plot for PFS | To estimate the OS HR and CI in the per-protocol population, an unadjusted Cox proportional hazards model was used to estimate the HR for PFS in the evaluable-for-response population (patients in the per-protocol population with unidimensional disease according to RECIST) a Cox proportional hazards model with adjustment for sex, racial origin, histology, PS, smoking history, previous regimens, previous platinum and previous paclitaxel was used | |
| ISTANA35 | Unadjusted and adjusted presented for OS and PFS | K-M plot crosses for OS and PFS | To compare the treatment groups, an unadjusted Cox proportional hazards model was used to analyse PFS and OS (two-sided test at the 5% significance level, 95% CI). Supportive analyses using a Cox proportional hazards model were conducted with adjustment for gender, histology, smoking history, stage and performance status were also conducted |
| SIGN37 | Adjusted for OS and PFS | K-M plot crosses for OS and PFS | ‘Overall and progression-free survival were analysed using a proportional hazards model that allowed for the effect of treatment and the covariates above (PS, sex and smoking history)’38 |
| Li et al.36 | NR | Yes | No details presented |
| V-15-3238 | Unadjusted and adjusted presented (PFS-reported population) | K-M plot crosses for OS and PFS | Supportive analyses in the per-protocol population were conducted using a Cox regression model with covariate adjustment for sex, PS, tumour type, smoking history, number of prior chemotherapy regimens, age at random assignment, time from diagnosis to random assignment and best response to prior chemotherapy |
| Bhatnagar et al.33 | NR | NR | Abstract only |
| Gefitinib vs. BSC | |||
| ISEL39 | Adjusted for OS. Unclear for PFS | K-M plot crosses for OS and time to treatment failure near to the top of the plot | A stratified log-rank test was used in the primary analysis of survival. The stratification factors were: sex, histology, PS, smoking history, number of previous regimens and reason for previous chemotherapy failure. A Cox’s regression analysis was also conducted as a supportive analysis. This used a covariate adjustment for the same factors as the log-rank test |
| Gefitinib vs. erlotinib | |||
| Kim et al.32 | Unadjusted PFS. No OS | Yes | ‘A univariate analysis revealed that adenocarcinoma and activating EGFR mutation status were significant factors associated with longer PFS. A multivariate analysis revealed that adenocarcinoma histology was the only independent predictor affecting prolongation of PFS’32 |
| Erlotinib vs. docetaxel | |||
| TAILOR41 | Unadjusted and adjusted reported for OS and PFS | Yes. Schoenfeld residuals considered | ‘Time-to-event data were analysed by the K-M method. Cox proportional hazards model was used to adjust the treatment effect for histology, smoking habit’41 |
| TITAN42 | Unadjusted for both OS and PFS | K-M plot crosses towards the tail for PFS. K-M plot crosses in the middle for OS | Adjusted analyses included in appendices but primary are unadjusted |
| DELTA40 | NR | NR | Abstract only |
| Erlotinib vs. BSC | |||
| BR.2131 | Yes | Yes | In order to adjust for treatment effect and to identify prognostic factors for PFS and OS, exploratory forward stepwise regression analyses using the Cox model were conducted. Covariates explored included EGFR expression, stratification factors (except centre), sex, age, race or ethnic group, prior radiotherapy, histological subtype of cancer and smoking status |
Finally, the AG notes that some trials report unadjusted and adjusted analyses, whereas others report only unadjusted or only adjusted analyses. This may be a form of selective reporting; for example, one set of outcomes is reported rather than the other so as to maximise the apparent effectiveness of one of the interventions. It is not sensible to combine adjusted and unadjusted results, as they may not be directly comparable. In particular, the unadjusted estimate from a Cox proportional hazards model is attenuated towards the null value, so heterogeneity is likely to be introduced when adjusted and unadjusted results are combined again, rendering results from a network meta-analysis difficult to interpret. For the EGFR unknown results, three trials report adjusted analyses only for OS31,32,39 and four only for PFS. 31,34,37,39 In BR.21,31 erlotinib is statistically significantly more effective than BSC in terms of both OS and PFS, and in ISEL39 gefitinib is statistically significantly more effective than BSC.
In summary, the AG considers that because of the clinical and statistical weaknesses identified in the available clinical data, it would be inappropriate to carry out any meta-analysis or network meta-analysis to assess treatment effects of erlotinib or gefitinib in any patient population after progression following chemotherapy.
Quality of life
Quality of life data are presented in 10 trials for the overall EGFR unknown population and are summarised in Table 21. QoL data from TAILOR41 and DELTA40 are not yet available.
| Trial | Number of respondents | Measurement tool | Author summary |
|---|---|---|---|
| Gefitinib vs. docetaxel | |||
| INTEREST34 | Gefitinib, n = 490; docetaxel, n = 476 | FACT-L every 3 weeks until treatment discontinuation | Significantly more patients had sustained a clinically relevant improvement in QoL with gefitinib than with docetaxel |
| ISTANA35 | Gefitinib, n = 68; docetaxel, n = 66 | FACT-L every 3 weeks | Similar proportions of patients in each treatment group experienced an improvement |
| SIGN37 | Gefitinib, n = 85%; docetaxel, n = 87% | FACT-L every 3 weeks until treatment discontinuation | Mean FACT-L score change from baseline to end point were similar for both groups |
| Li et al.36 | NR | The improvements of symptoms and QoL were focused on the observation of cough, shortness of breath, chest tightness, fatigue and KPS scores | The improvement rate of symptoms and QoL for the patients in the gefitinib group was higher than that in the docetaxel group, resulting in a significant difference in the two groups |
| V-15-3238 | Gefitinib, n = 185; docetaxel, n = 173 | FACT-L questionnaire at baseline and every 4 weeks during study treatment until week 12 | Gefitinib showed statistically significant benefits compared with docetaxel in QoL improvement rates but there were no significant differences between treatments in lung cancer symptoms improvement rates |
| Bhatnagar et al.33 | NR | NR | Improvement in QoL for gefitinib patients |
| Gefitinib vs. BSC | |||
| ISEL39 | Paper states that about 85% of patients completed the FACT-L | FACT-L questionnaire every 4 weeks | In the overall population, changes in QoL were similar in the gefitinib and BSC groups |
| Gefitinib vs. erlotinib | |||
| Kim et al.32 | NR | EORTC QLQ-C30-Version 3.0 | There was no significant difference in QoL between the two arms |
| Erlotinib vs. docetaxel | |||
| TAILOR41 | NR | NR | NR |
| TITAN42 | Completion rates were around 90% at the baseline visit and remained above 80% | FACT-L, version 4 at baseline, every 3 weeks until week 48, and every 12 weeks thereafter until disease progression or the end of the study | There was no statistically significant difference in the time to symptom progression (or time to deterioration) in QoL in the two treatment groups |
| DELTA40 | NR | NR | NR |
| Erlotinib vs. BSC | |||
| BR.2131 | Compliance was 87% at baseline and more than 70% during treatment | QLQ-C30 every 4 weeks | Significant improvement in global QoL for erlotinib patients compared with BSC |
Gefitinib
Six trials compared gefitinib and docetaxel. The results of four of these studies favoured gefitinib,33,34,36,38 although no data were available from Bhatnagar et al. 33 to confirm their conclusions. Two studies found no statistically significant differences between gefitinib and docetaxel. 35,37 One trial compared gefitinib to BSC,39 and changes in QoL were similar in the two groups. In the comparison of erlotinib and gefitinib32 no statistically significant difference in QoL was noted.
Incidence of grade 3 or 4 adverse events
In 9 of the 12 studies,31,32,34,35,37–39,41,42 grade 3 and 4 AEs were presented for the overall population only (Table 22). In the remaining three trials, only limited AE data are reported; DELTA40 and the Bhatnagar et al. 33 trial are reported in abstract format only and therefore do not describe AEs, and the investigators in the Li et al. 36 trial did not provide detailed AE data.
| Study | BSC % (n/N) | Docetaxel % (n/N) | Erlotinib % (n/N) | Gefitinib % (n/N) |
|---|---|---|---|---|
| Fatigue | ||||
| TITAN42 | NA | 0.45 (0.5/111.8) | 0 (0/196) | NA |
| SIGN37 | NA | 4.23 (3/71) | NA | 5.88 (4/68) |
| INTEREST34 | NA | 8.95 (64/715) | NA | 4.39 (32/729) |
| Kim et al.32 | NA | NA | 0 (0/48) | 0 (0/48) |
| ISTANA35 | NA | 3.95 (3/76) | NA | 1.23 (1/81) |
| V-15-3238 | NA | 2.51 (6/239) | NA | 0.41 (1/244) |
| BR.2131 | 23.14 (56/242) | NA | 18.97 (92/485) | NA |
| ISEL39 | 2.67 (15/562) | NA | NA | 3.20 (36/1126) |
| TAILOR41 | NA | 9.62 (10/104) | 5.61 (6/107) | NA |
| Diarrhoea | ||||
| TITAN42 | NA | 0 (0/111.8) | 2.55 (5/196) | NA |
| SIGN37 | NA | 4.23 (3/71) | NA | 2.94 (2/68) |
| INTEREST34 | NA | 3.08 (22/715) | NA | 2.47 (18/729) |
| Kim et al.32 | NA | NA | 0 (0/48) | 0 (0/48) |
| ISTANA35 | NA | 0 (0/76) | NA | 1.23 (1/81) |
| V-15-3238 | NA | 0.84 (2/239) | NA | 2.05 (5/244) |
| BR.2131 | 0.62 (1.5/242) | NA | 5.77 (28/485) | NA |
| ISEL39 | 0.89 (5/562) | NA | NA | 2.75 (31/1126) |
| TAILOR41 | NA | 1.92 (2/104) | 2.80 (3/107) | NA |
| FN | ||||
| TITAN42 | NA | 0.89 (1/111.8) | 0 (0/196) | NA |
| SIGN37 | NA | 2.82 (2/71) | NA | 0 (0/68) |
| INTEREST34 | NA | 10.07 (72/715) | NA | 1.23 (9/729) |
| Kim et al.32 | NA | NA | 0 (0/48) | 0 (0/48) |
| ISTANA35 | NA | 0 (0/76) | NA | 0 (0/81) |
| V-15-3238 | NA | 7.11 (17/239) | NA | 0.82 (2/244) |
| BR.2131 | 0 (0/242) | NA | 0 (0/485) | NA |
| ISEL39 | 0 (0/562) | NA | NA | 0 (0/1126) |
| TAILOR41 | NA | 3.85 (4/104) | 0 (0/107) | NA |
| Hair loss | ||||
| TITAN42 | NA | 0.45 (0.5/111.8) | 0 (0/196) | NA |
| SIGN37 | NA | 0 (0/71) | NA | 0 (0/68) |
| INTEREST34 | NA | 0 (0/715) | NA | 0 (0/729) |
| Kim et al.32 | NA | NA | 0 (0/48) | 0 (0/48) |
| ISTANA35 | NA | 0 (0/76) | NA | 0 (0/81) |
| V-15-3238 | NA | 0 (0/239) | NA | 0 (0/244) |
| BR.2131 | 0 (0/242) | NA | 0 (0/485) | NA |
| ISEL39 | 0 (0/562) | NA | NA | 0 (0/1126) |
| TAILOR41 | NA | 14.42 (15/104) | 0 (0/107) | NA |
| Nausea/vomiting | ||||
| TITAN42 | NA | 0.45 (0.5/111.8) | 0.51 (1/196) | NA |
| SIGN37 | NA | 2.82 (2/71) | NA | 2.94 (2/68) |
| INTEREST34 | NA | 2.38 (17/715) | NA | 0.96 (7/729) |
| Kim et al.32 | NA | NA | 0 (0/48) | 0 (0/48) |
| ISTANA35 | NA | 0 (0/76) | NA | 0 (0/81) |
| V-15-3238 | NA | 5.02 (12/239) | NA | 3.69 (9/244) |
| BR.2131 | 2.69 (6.5/242) | NA | 5.98 (29/485) | NA |
| ISEL39 | 0.71 (4/562) | NA | NA | 1.95 (22/1126) |
| TAILOR41 | NA | 2.88 (3/104) | 0.93 (1/107) | NA |
| Neutropenia | ||||
| TITAN42 | NA | 0.89 (1/111.8) | 0 (0/196) | NA |
| SIGN37 | NA | 40.85 (29/71) | NA | 1.47 (1/68) |
| INTEREST34 | NA | 56.78 (406/715) | NA | 2.06 (15/729) |
| Kim et al.32 | NA | NA | 0 (0/48) | 0 (0/48) |
| ISTANA35 | NA | 0 (0/76) | NA | 0 (0/81) |
| V-15-3238 | NA | 73.64 (176/239) | NA | 8.20 (20/244) |
| BR.2131 | 0 (0/242) | NA | 0 (0/485) | NA |
| ISEL39 | 0 (0/562) | NA | NA | 0 (0/1126) |
| TAILOR41 | NA | 20.19 (21/104) | 0 (0/107) | NA |
| Rash | ||||
| TITAN42 | NA | 0 (0/111.8) | 4.59 (9/196) | NA |
| SIGN37 | NA | 2.82 (2/71) | NA | 2.94 (2/68) |
| INTEREST34 | NA | 0.56 (4/715) | NA | 2.06 (15/729) |
| Kim et al.32 | NA | NA | 10.42 (5/48) | 2.08 (1/48) |
| ISTANA35 | NA | 1.32 (1/76) | NA | 6.17 (5/81) |
| V-15-3238 | NA | 0.42 (1/239) | NA | 0.41 (1/244) |
| BR.2131 | 0 (0/242) | NA | 9.07 (44/485) | NA |
| ISEL39 | 0.18 (1/562) | NA | NA | 1.60 (18/1126) |
| TAILOR41 | NA | 0 (0/104) | 14.02 (15/107) | NA |
Each study reported AEs in different ways. ISEL39 reported AEs that occurred in more than 5% of either treatment group or with a difference of at least 3% between treatment groups. TITAN42 reported those that occurred in at least 2% of patients in either group. V-15-3238 reported the most common AEs, which were considered to be those that occurred in more than 10% of the study population or that occurred with more than a 5% difference between treatments. Two studies34,37 reported AEs that occurred in more than 10% in either group. ISTANA35 reported the most common AEs, which were considered to be those occurring in at least 10% of patients in either treatment group. Three studies31,32,41 simply reported AEs, and it was unclear if the data presented by the authors included all of the AEs that occurred during the trial.
In the Bhatnagar et al. 33 trial it was reported that gefitinib had a more favourable tolerability profile than docetaxel. In DELTA,40 patients in the erlotinib arm experienced more rash and leucopenia than patients in the docetaxel arm. In the Li et al. trial37 the incidence of rash was higher in the gefitinib group than in the docetaxel group (p = 0.0296) but the incidence of other side effects was similar in both groups.
The AG considers that the AEs reported appear to be consistent with the information available for erlotinib, gefitinib and docetaxel in the SPCs. 24
Summary of clinical results
Epidermal growth factor mutation-positive population
-
No trials were identified that were conducted in a population of solely EGFR M+ patients. Limited EGFR mutation status data were retrospectively derived from relatively small subgroup analyses of RCTs that included patients of unknown EGFR mutation status at the time of randomisation.
-
Four studies reported OS outcomes,31,34,39,42 none of which was statistically significantly different for any of the comparisons described.
-
Five studies reported PFS,31,32,34,39,42 but only one trial36 found a statistically significant improvement for any comparison considered, and the results favoured gefitinib over docetaxel.
Epidermal growth factor mutation-negative population
-
Key data were derived from results of TAILOR41 and DELTA40 trials.
-
EGFR mutation status data were retrospectively derived from subgroup analyses in BR.21,31,43 Kim et al. ,32 TITAN,42 INTEREST,34,45 and ISEL. 39,44
-
OS outcome: no statistically significant differences were noted for OS for either erlotinib or gefitinib compared with any treatment.
-
PFS outcome: TAILOR41 and DELTA40 reported a statistically significant benefit of docetaxel compared with erlotinib. No statistically significant PFS benefit was reported from subgroup data.
-
RR: patients in the docetaxel arm of TAILOR41 had statistically significantly higher RRs than patients in the erlotinib arm.
Epidermal growth factor mutation unknown: overall population
-
Data were available from 11 trials31–41 carried out in populations in which EGFR mutation status was not a factor in the recruitment process (or in which overall trial results were presented).
-
OS outcome: the only statistically significant OS benefit for any treatment was reported in BR.2131 (erlotinib vs. placebo). However, this finding was based on an adjusted rather than an unadjusted analysis of the data.
-
PFS outcome:
-
Gefitinib versus docetaxel – only one of the four trials (ISTANA35) reported a statistically significant benefit of gefitinib.
-
Gefitinib versus BSC – gefitinib was reported to have a statistically significant benefit. 39
-
Erlotinib versus placebo (BR.2131) – a statistically significant PFS benefit of erlotinib was reported (in an adjusted analysis).
-
-
RR: of the trials reporting RRs,31,32,34–39,41 two noted significant differences in favour of gefitinib when compared with docetaxel38 and BSC. 39
Meta-analysis and network meta-analysis
For clinical and methodological reasons, no meta-analysis or network meta-analysis was conducted by the AG.
Quality of life
Where reported, the QoL data were derived from the EGFR unknown patients (overall population, i.e. the data are not specific to the EGFR mutation status of patients). All of the 12 trials included in this review measured QoL. However, the QoL outcomes from TAILOR41 and DELTA40 are not yet available.
Adverse events
Adverse events were reported for the overall population, that is the data are not specific to the EGFR mutation status of patients, with the exception of TAILOR. 41 Details of the AEs reported in Bhatnagar et al. ,33 Li et al. 36 and DELTA40 were limited. The AG considers that the AEs reported, despite inconsistencies across trials, appear to be consistent with the information available for erlotinib, gefitinib and docetaxel in the SPCs. 24
Discussion of clinical results
Erlotinib
Clinical evidence supporting the previously published NICE guidance TA16221 (erlotinib for the treatment of NSCLC) issued in 2008 was based on the results of a single RCT, the BR.2131 trial, that compared erlotinib with placebo. At the time of the appraisal of erlotinib in NICE TA162,21 no direct evidence comparing erlotinib with docetaxel was available and, in the evidence submission to NICE, the manufacturer of erlotinib presented an indirect treatment comparison in which docetaxel was compared with BSC and pemetrexed. The Appraisal Committee (AC) did not consider the indirect treatment comparison to be robust and concluded that it was difficult to reach a decision as to the effectiveness of erlotinib compared with docetaxel. NICE guidance (TA16221) states that erlotinib is recommended, within its licensed indication, as an alternative to docetaxel as a second-line treatment option for patients with NSCLC only on the basis that it is provided by the manufacturer at an overall treatment cost (including administration, AEs and monitoring costs) equal to that of docetaxel. The patient access scheme was then superseded to a simple discount patient access scheme following the publication of NICE TA227. 47 The price of erlotinib relevant to the NHS now is that of the list price minus the simple discount, as noted in the latest version of NICE TA162. 21
Since the publication of NICE TA162,21 three developments are worthy of note. First, the results of one RCT comparing erlotinib with chemotherapy (TITAN42) in a population of patients with unknown EGFR status have been published. The chemotherapy comparator was docetaxel or pemetrexed according to the treating physician’s choice. Pemetrexed is licensed as a second-line treatment but is not recommended by NICE and therefore was not listed as a comparator in the decision problem for this appraisal. No statistically significant differences between erlotinib and chemotherapy were reported. The authors of the published paper42 note that the choice of either docetaxel or pemetrexed was at the treating physician’s discretion and treatments were, therefore, not randomised. In addition, pemetrexed and docetaxel were not always available in all centres. For these reasons, the trial investigators published only outcomes for chemotherapy (i.e. aggregated), as the results of efficacy of erlotinib versus docetaxel and erlotinib versus pemetrexed were considered unreliable.
Second, the patent for docetaxel has expired. Docetaxel is now available generically at a considerably reduced price (less than 10% of its previous list price). 48 To date, NICE has not issued any statement suggesting that this lower price of docetaxel necessitates any change to the recommendations set out in NICE TA162. 21
Third, clinical practice has also changed since the publication of NICE TA162,21 with the identification of EGFR mutation status as a prognostic factor. Erlotinib is an EGFR-TKI and is licensed as a first-line treatment for patients with EGFR M+ tumours and as a second-line treatment for locally advanced or metastatic NSCLC regardless of EGFR mutation status. As noted previously, the majority of patients in clinical practice in England and Wales have their tumours histologically tested at diagnosis and prior to first-line treatment. Patients who are likely to have EGFR M+ tumours are also tested for activating mutations. Patients who test positive for EGFR-activating mutations are treated at first line with a TKI (either erlotinib or gefitinib), while those who are EGFR M– are treated with third-generation platinum-doublet chemotherapy or monotherapy. On progression, EGFR M+ patients are not re-treated with an EGFR-TKI and therefore receive docetaxel in line with current NICE guidance. 21 The AG is aware that some patients in the NHS are given platinum-doublet chemotherapy after first-line EGFR-TKI; however, this treatment pathway is not standard UK clinical practice. Patients who are EGFR M– are offered erlotinib or docetaxel. In summary, increased significance of EGFR mutation status in lung cancer treatment raises questions about how to treat both EGFR M+ and EGFR M– patients.
Two recent trials (TAILOR41 and DELTA40) were both designed to compare the effectiveness of erlotinib versus docetaxel in EGFR M– patients. The results of TAILOR41 are reported in a published paper, while the results of DELTA40 are presently available only as a conference abstract from the American Society for Clinical Oncology in 2013. Since TAILOR41 provides key data on the effectiveness of erlotinib compared with docetaxel in the EGFR M– population, further consideration of the trial and its relevance to clinical practice in England and Wales is warranted here.
TAILOR41 was conducted in 52 hospitals in Italy and randomised patients to receive erlotinib (n = 112) or docetaxel (n = 110). While OS was not statistically significantly different between the two arms, there was a statistically significant benefit of docetaxel over erlotinib for PFS. The QoL data are not yet available.
TAILOR41 has attracted a number of criticisms. First, the primary objective of the trial was changed at the first planned interim analysis. According to the published paper,41 the trial was initially designed to assess the effects of docetaxel and erlotinib according to the biomarkers of EGFR amplification and protein expression, and KRAS mutations. When, after masked efficacy analysis, these biomarkers were found to have no effect, the independent monitoring and safety committee recommended that the primary objective of the trial be changed to a comparison of efficacy between erlotinib and docetaxel with a primary end point of OS.
Second, TAILOR41 employed two regimens of docetaxel administration, either 75 mg/m2 every 3 weeks or weekly infusions of 35 mg/m2. The AG notes that this latter regimen would not be used in clinical practice in England and Wales.
Third, the fitness of the patients in TAILOR41 is an important consideration. The patient population consisted of a majority of patients with an ECOG PS score of 0 or 1 and only 7% with a PS score of 2. This is unlikely to reflect patients in the NHS, in which a higher proportion of PS 2 patients would be treated in routine clinical practice. The AG is aware that PS is a prognostic factor in NSCLC and poorer PS is linked to poorer outcomes. However, the AG notes that the patient population in TAILOR41 may reflect future populations of patients seen in clinical practice in England and Wales as treatment for NSCLC continues to evolve. In modern clinical practice, patients are diagnosed earlier and treated more aggressively than in the past, which means that future patients may be fitter at second line than those currently receiving second-line treatments in England and Wales.
Fourth, there are differences in other important prognostic factors between the treatment arms of TAILOR. 41 There are differences in patient characteristics (docetaxel vs. erlotinib): never-smokers (27% vs. 17%), squamous cell (21% vs. 28%) and adenocarcinoma (75.55% vs. 63.00%). All of these differences have been identified as possible modifiers of trial outcome in favour of docetaxel. 48
In its submission to NICE, the manufacturer of erlotinib has questioned the low rates of haematological toxicity in the docetaxel arm of TAILOR41 [febrile neutropenia (FN) grade 3 or 4 = 4%, neutropenia grade 3 or 4 = 21%] in comparison with the INTEREST34 trial (FN grade 3 or 4 = 10%, neutropenia grade 3 or 4 = 58%) and the JMEI49 trial (FN grade 3 or 4 = 13%, neutropenia grade 3 or 4 = 40%). The manufacturer questions whether or not these low rates are related to the fitter patient population or the use of weekly treatment schedules. The AG considers that there may be another explanation, the increased clinical awareness of docetaxel-related AEs. Docetaxel has been used in the NHS for many years and it is likely that these related AEs are currently better managed and/or more frequently avoided than in the past.
In summary, it is open to debate how far TAILOR41 reflects clinical practice in England and Wales and, therefore, whether or not the trial results are likely to be mirrored in a UK clinical population. TAILOR41 is a large, high-quality RCT in a population of patients who do not have activating EGFR mutations. The trial is very relevant to patients in the UK, as it compares two lung cancer treatments that are currently recommended by NICE for the post-progression treatment of patients with NSCLC.
The specific details of DELTA40 are as yet unavailable and so it is not possible to assess how far the Japan-based trial reflects clinical practice in England and Wales.
Gefitinib
In 2009, NICE was unable to recommend the use of gefitinib in the NHS for the second-line treatment of locally advanced or metastatic NSCLC because no evidence submission was received from the manufacturer or sponsor of the technology. 22
The marketing authorisation for gefitinib granted by the European Medicines Agency50 was based on the results of the first-line IRESSA Pan-Asia Study51 trial and second-line INTEREST34 trial. Supporting trials included ISEL,39 SIGN,37 V-15-3238 and ISTANA. 35 The European Medicines Agency’s European Public Assessment Report52 reports that concerns were raised by the scientific advisory group about the data submitted by AstraZeneca (London, UK) in support of the licensing application for gefitinib. In particular, the advisory group noted a large number of missing data with respect to EGFR mutation status and considered that this aspect should have been controlled for by the design and conduct of the clinical studies. In this respect, the clinical studies presented were considered by the European Medicines Agency52 to be inadequate. Three new trials of gefitinib have been published since 2009, which was when the European Medicines Agency52 considered the application. The three trials were conducted in small populations of patients, Kim et al. 33 (vs. erlotinib), Li et al. 36 (vs. docetaxel) and Bhatnagar et al. 33 (vs. docetaxel), and the new data they provide are not sufficiently robust to permit recommendation of a change in clinical practice.
The AG notes, as does the manufacturer of gefitinib, that in clinical practice in England and Wales patients with EGFR M+ NSCLC should be diagnosed and treated appropriately (with a TKI) at first line. As noted above, patients who go on to second-line treatment will not be re-treated with the same therapy. It is likely, therefore, that the number of patients treated with gefitinib after progression will be limited to a very small minority who were not treated with a TKI at first line, perhaps as a result of lack of diagnostic facilities.
Meta-analysis and network meta-analysis
In view of the paucity of relevant data, the AG was unable to conduct either a meta-analysis or network meta-analysis in respect of the efficacy of treatments for patients with known EGFR M+, EGFR M– or EGFR unknown NSCLC.
The majority of the clinical evidence lies with the trials that included patients with NSCLC who were of unknown mutation status. Unfortunately, a number of issues precluded any comparison of the available data for patients with NSCLC of unknown mutation status, the issues were both clinical (differences in patient populations) and methodological (adjusted vs. unadjusted outcome data, Cox proportional hazards violations). However, even if the comparison could have been carried out, given the increased significance of EGFR mutation testing, its relevance to the current decision problem and to modern clinical practice is questionable.
From the 12 included RCTs, the most reliable evidence is from a study of the EGFR M– population. For this group of patients, the results of TAILOR41 demonstrate that there is a statistically significant benefit of docetaxel over erlotinib for PFS; however, there is no statistically significant OS benefit demonstrated in this trial.
Chapter 4 Assessment of cost-effectiveness
This chapter presents a review of the published cost-effectiveness literature describing the use of erlotinib and gefitinib as treatments for patients with NSCLC who have progressed following prior chemotherapy. The AG notes that neither of the manufacturers included a cost-effectiveness review as part of its manufacturer’s submission. The AG also provides a critique of the economic model (erlotinib vs. BSC) submitted by Roche (UK) Ltd. The AG notes that AstraZeneca did not submit an economic model as part of their evidence supporting the use of gefitinib.
Systematic review of existing cost-effectiveness evidence
Methods of cost-effectiveness review
Full details of the main search strategy conducted by the AG and the proposed methods for selecting clinical and economic evidence are presented in detail in Chapter 3, Methods for reviewing effectiveness. The AG did not use specific economics-related search terms in the main strategy, as all of the potential references were scanned for references containing economic evidence. For the selection of cost-effectiveness evidence, AB and SB independently screened all economics-related titles/abstracts identified via searching and obtained full-paper manuscripts of all relevant references. The relevance of each study was then assessed (by AB and SB) according to the specific inclusion and exclusion criteria shown in Table 23. Data were extracted (AB and SB) and summarised in structured tables and as a narrative description.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Intervention | Erlotinib or gefitinib | – |
| Study design | Full EE | Methodological, editorial, commentary, cost analysis, etc. |
| Type of paper | Full paper | Abstract |
In the NHS in England and Wales (and in countries elsewhere in the world), docetaxel is commonly used to treat patients with NSCLC who have progressed after chemotherapy and is, therefore, described as a relevant comparator to erlotinib and gefitinib in published economic evaluations (EEs). Recently, the price of docetaxel has fallen29 substantially as a result of the expiry of the manufacturer’s patent. The AG discussed whether or not to exclude papers that presented data using the higher docetaxel price. The AG decided to include these papers but to highlight in the discussion section that the results of EEs that only include docetaxel at its higher price are of limited relevance to this appraisal.
Until recently, patients who required post-progression treatment for NSCLC were treated as a homogeneous group. However, clinical practice is now changing and there is growing awareness that a patient’s EGFR mutation status can affect treatment outcomes. With this in mind, the AG discussed excluding papers that did not consider how EGFR mutation status can affect patient outcomes and the treatment options available. However, on reflection, the AG decided not to exclude these papers but to highlight in the discussion that the results of EEs that only include patients with EGFR unknown status should be treated with caution.
Quantity of included evidence
From the main search, the AG identified 44 potentially relevant economic papers for inclusion in the review of economic evidence. Of these, 16 papers were considered for inclusion after stage 1 screening. Of these 16 papers, 10 were then excluded from the review and six were included in the review at stage 2. The reasons for excluding 10 papers are listed in Table 24.
| Reference | Reason for exclusion |
|---|---|
| Bongers53 | Abstract |
| Bongers54 | Systematic reviewa |
| Borget55 | Focus is on a ‘strategy’ not an individual drug |
| Capri56 | Not a full EE |
| Cuileanu57 | Abstract |
| Horgan58 | No outcome data |
| Horgan59 | Cost–consequence analysis – not a full EE |
| Laurendeau60 | Abstract |
| Nguyen61 | Abstract |
| Thongsprasert62 | Abstract – full-text study included in review |
From the systematic review by Bongers et al. ,54 a further four papers were identified for inclusion in the AG’s review. This finding alerted the AG to the fact that the main search had not picked up all of the relevant published economic studies available. The AG then carried out further searching using a combination of the following broad search terms to identify papers in MEDLINE and The Cochrane Library: erlotinib, gefitinib, lung cancer and cost. This additional generic search identified one more relevant paper by Vergnenegre et al. 63
In summary, the AG considered 11 papers to be eligible for inclusion in the review and these are listed in Table 25.
| Reference | Title |
|---|---|
| Araujo64 | An economic analysis of erlotinib, docetaxel or pemetrexed and best supportive care as second- or third-line treatment of non-small cell lung cancer |
| Asukai65 | Cost-effectiveness analysis of pemetrexed versus docetaxel in the second-line treatment of non-small cell lung cancer in Spain: results for the non-squamous histology population |
| Bradbury66 | Economic analysis: randomised placebo-controlled clinical trial of erlotinib in advanced non-small cell lung cancer |
| Holmes67 | A cost-effectiveness analysis of docetaxel in the second-line treatment of non-small cell lung cancer |
| Thongsprasert68 | Cost–utility and budget impact analyses of gefitinib in second-line treatment for advanced non-small cell lung cancer from a Thai payer perspective |
| Cromwell69 | Erlotinib or docetaxel for second-line treatment of non-small cell lung cancer |
| Cromwell70 | Erlotinib or best supportive care for third-line treatment of advanced non-small cell lung cancer: a real-world cost-effectiveness analysis |
| Lewis71 | Cost-effectiveness of erlotinib versus docetaxel for second-line treatment of advanced non-small cell lung cancer in the United Kingdom |
| Leighl72 | Economic analysis of the TAX317 trial: docetaxel versus best supportive care as second-line therapy of advanced non-small cell lung cancer |
| Carlson73 | Comparative clinical and economic outcomes of treatments for refractory non-small cell lung cancer (NSCLC) |
| Vergnenegre63 | Cost-effectiveness of second-line chemotherapy for non-small cell lung cancer |
Quality of included evidence
The AG made the decision not to quality assess the papers included in the review of cost-effectiveness evidence. This decision was made because none of the 11 studies is directly relevant to UK health-care decision-making as they do not use the off-patent price of docetaxel. Additionally, none of the studies consider the confirmed EGFR mutation status of the patient when assessing post-progression treatments.
Cost-effectiveness review: results
Relevant data were extracted from the 11 eligible papers (Table 26). These papers were published between 2002 and 2013; seven papers63,65,66,68–71 were published from 2010 onwards. All of the papers described full EEs using cost minimisation analysis (n = 164), cost-effectiveness analysis (n = 665,67,69,70,72) and/or cost–utility analysis (n = 663–65,68,71,73) techniques. All but one study70 used cost per QALY gained or cost per LY gained as the measure of cost-effectiveness. The results of six studies64,65,67,68,71,73 were derived from use of an economic model: one study63 conducted an economic analysis alongside a RCT and the remaining four studies66,69,70,72 conducted retrospective reviews of costs and/or benefits. Four studies66,69,70,72 were carried out from a Canadian NHS perspective, two67,71 from that of the UK NHS perspective, one73 from the US perspective, three63–65 from a European perspective and one68 from a Thai payer perspective. None of the studies had a time horizon of longer than 3 years. The authors of two studies69,70 had not received any financial support from the pharmaceutical industry.
| Study | Method of EE | Measure of cost-effectiveness | Study design/model | Year published | Perspective | Time horizon | Discounting | Funding body |
|---|---|---|---|---|---|---|---|---|
| Araujo64 | CMA and CUA | Cost per LY gained, cost per QALY gained | Markov-type model | 2008 | Portuguese NHS | 24 months with the option to consider 36 months | 5% for costs and benefits | Pharma |
| Asukai65 | CEA and CUA | Cost per LY gained, cost per QALY gained | Markov model | 2010 | Spanish health-care system | 36 months (lifetime) | 3% for costs and benefits | Pharma |
| Bradbury66 | CEA | Cost per LY gained | Retrospective analysis of direct medical costs AND published clinical trial data | 2010 | Canadian public health-care system | Maximum of 18 months | No discounting applied (few patients remained on study post-12 months) | Pharma |
| Holmes67 | CEA | Cost per LY gained | Decision-analytic model | 2004 | UK NHS | 2 years | Discounting was not applied | Pharma |
| Thongprasert68 | CUA | Cost per QALY gained | Markov model | 2012 | (Thai) Comptroller General’s Department, Ministry of Finance for the Civil Servant Medical Benefit Scheme | 2 years | 3% | Pharma |
| Cromwell69 | CEA | Cost per unit change in OS, cost per unit change in PFS | Retrospective review of medical records (costs and outcomes) of patients who had received treatment | 2011 | British Columbia Health Care System | Data were collected between September 2005 and March 2008 (31 months) | N/A | Public |
| Cromwell70 | CEA | Cost per QALY gained | Retrospective review of medical records (costs and outcomes) of patients who had received treatment vs. historical controls | 2012 | British Columbia Health Care System | Controls: April 2002 and March 2004 (2 years) | N/A | Public |
| Intervention: April 2004 and November 2006 (32 months) | ||||||||
| Lewis71 | CUA | Cost per QALY gained | Heath-state transition model | 2010 | UK NHS | 2 years | 3.5% was applied for year 2 of the analysis | Pharma |
| Leighl72 | CEA | Cost per QALY gained | Retrospective economic analysis of a clinical trial | 2002 | Canada’s public health-care system | Less than 1 year | Discounting was not applied as median duration of survival < 12 months in both arms | Public and pharma |
| Carlson73 | CUA | Cost per QALY gained | Decision-analytic model | 2008 | US-payer perspective | 2 years | Costs and benefits were discounted at 3% | Pharma |
| Vergnenegre63 | CUA | Cost per LY gained, cost per QALY gained | Economic analysis alongside a RCT | 2011 | French-payer perspective | 34 months | 3% discount rate used for costs | Pharma |
The 19 comparisons described in the 11 economic studies included one or more of the following interventions: erlotinib, docetaxel, pemetrexed and BSC. The most common comparison was erlotinib versus docetaxel (n = 564,68,69,71,73). Other comparisons were erlotinib versus BSC (n = 364,66,70), pemetrexed versus docetaxel (n = 463,65,68,73), docetaxel versus BSC (n = 363,67,72), erlotinib versus pemetrexed (n = 264,73), pemetrexed versus BSC (n = 163) and gefitinib versus docetaxel (n = 168). The populations described in the EEs appeared to have similar patient characteristics, namely previously treated stage III–IV patients with advanced NSCLC. The clinical data used in the EEs were derived mainly from relevant published RCT data: TAX31774 (docetaxel vs. BSC), JMEI49 (pemetrexed vs. docetaxel), BR.2131 (erlotinib vs. placebo) and INTEREST34 (gefitinib vs. docetaxel). The source of the clinical data described in two studies was patient medical records. The paper by Nafees et al. 75 provided the source of the QALY values in two papers. 63,73
The outcome data (e.g. QALY values and LYs gained) used in the evaluations were variable as a result of the assumptions employed (Table 27). To illustrate, the average total QALY value accrued over the time horizon of the models associated with each of the drugs used in the studies range was as follows: erlotinib (0.17468 to 0.42075), docetaxel (0.16068 to 0.42073), pemetrexed (0.17168 to 0.52065). In addition, the AG notes that Araujo et al. 64 assume that erlotinib, docetaxel and pemetrexed yield equivalent LYs (0.77 years), Thongprasert et al. 68 assume that the gain in LYs is equivalent when comparing docetaxel and pemetrexed (0.97 years) and when comparing erlotinib and gefitinib (0.96 years), and Carlson et al. 73 assume that the gain in LYs for erlotinib, docetaxel and pemetrexed is equivalent (0.77 years).
| Study | Comparison (intervention vs. comparator) | Characteristics of population | Details of prior treatments | Clinical outcomes | Clinical data source | Total benefits |
|---|---|---|---|---|---|---|
| Araujo64 | Erlotinib vs. BSC | Advanced or metastatic NSCLC, stage IIIA, IIIB or IV (hypothetical cohort) | Failed at least one prior treatment | Median OS, mean OS, PFS | NICE TAX31773 (docetaxel vs. BSC) | QALYs: erlotinib = 0.250, BSC = 0.186, docetaxel = 0.225, pemetrexed = 0.241 |
| Erlotinib vs. docetaxel | JMEI49 (pemetrexed vs. docetaxel) | LY gained: erlotinib = 0.77, BSC = 0.62, docetaxel = 0.77, pemetrexed = 0.77 | ||||
| Erlotinib vs. pemetrexed | BR.2130 (erlotinib vs. placebo) | |||||
| Asukai65 | Pemetrexed vs. docetaxel | Stage IIIB or IV patients with NSCLC with predominantly non-squamous histology | Previously undergone a course of chemotherapy | Median OS, PFS and tumour response | Post-hoc retrospective subgroup analysis of the JMEI49 trial (pemetrexed vs. docetaxel) | QALYs: pemetrexed = 0.52, docetaxel = 0.42, difference = 0.1 |
| LY gained: pemetrexed = 1.03, docetaxel = 0.89, difference = 0.14 | ||||||
| Bradbury66 | Erlotinib vs. placebo | Advanced NSCLC | Previously treated | Median OS and mean OS | BR.2130 (erlotinib vs. placebo) | Median OS: erlotinib = 6.7 months, placebo = 4.7 months, HR = 0.70, p < 0.001, difference = 2.0 months (0.16 years) |
| Mean OS: erlotinib = 9.0 months, placebo = 7.4 months, HR = not reported, difference = 1.6 months (0.13 years) | ||||||
| Holmes67 | Docetaxel vs. BSC | Second-line treatment of NSCLC | Prior treatment with a platinum containing chemotherapy regime (no taxanes) | Mean OS calculated using an AUC analysis | NICE TAX31773 (docetaxel vs. BSC) | LY gained: docetaxel = 8.89 months, BSC = 5.16 months, difference = 3.82 months (0.32 years) |
| Thongprasert68 | Gefitinib vs. docetaxel | Advanced NSCLC patients with stage III–IV (hypothetical cohort – based on INTEREST trial) | After one or two previous platinum-based chemotherapy regimens | OS and PFS – assumed erlotinib and gefitinib had the same mean OS/PFS | INTEREST34 (gefitinib vs. docetaxel) – data used for gefitinib/erlotinib and docetaxel | OS (years): docetaxel = 0.97, gefitinib = 0.96, erlotinib = 0.96, pemetrexed = 0.97. Difference gefitinib vs. docetaxel = 0.013, difference erlotinib vs. docetaxel = 0.013, difference pemetrexed vs. docetaxel = 0 |
| Erlotinib vs. docetaxel | JMEI49 (pemetrexed vs. docetaxel) – data used for pemetrexed | QALYs: docetaxel = 0.160, gefitinib = 0.174, erlotinib = 0.174, pemetrexed = 0.171. Difference gefitinib vs. docetaxel = 0.014, difference erlotinib vs. docetaxel = 0.014, difference pemetrexed vs. docetaxel = 0.011 | ||||
| Pemetrexed vs. docetaxel | ||||||
| Cromwell70 | Erlotinib vs. docetaxel | Stage IIIb/IV advanced NSCLC | Previously treated patients | Mean and median OS and PFS and 1-year OS | British Columbia Cancer Agency medical records68 | Mean OS (95% CI): erlotinib = 311 days (264 to 344 days), docetaxel = 310 days (248 to 333 days), difference = 1 day |
| AUC analysis | Mean PFS (95% CI): erlotinib = 64 days (61 to 66 days), docetaxel = 75 (43 to 77 days), difference = –11 days | |||||
| 1-year OS: erlotinib = 36%, docetaxel = 32.4% | ||||||
| Cromwell71 | Erlotinib vs. BSC | Stage IIIb/IV advanced NSCLC | Patients who had progressed after second-line treatment | Mean and median OS | British Columbia Cancer Agency medical records68 | Mean OS (95% CI): erlotinib = 291 days (233 to 349 days), BSC = 181 days (141 to 222 days), difference = 110 days |
| PTD and 1-year OS | Mean PTD days (95% CI): erlotinib = 195 days (148 to 242 days), BSC = 105 days (82 to 129 days), difference = 90 days | |||||
| AUC analysis | 1 year OS: erlotinib = 36%, docetaxel = 32.4% | |||||
| Lewis71 | Erlotinib vs. docetaxel | Stage III/IV patients with advanced NSCLC | One or more prior chemotherapy treatments | Mean OS | NICE TAX31773 (docetaxel vs. BSC) | QALY progression free health state: erlotinib = 0.150, docetaxel = 0.104 |
| Mean PFS | BR.2130 (erlotinib vs. placebo) EQ-5D scores (general population – visual analogue method) | QALY progression free health state: erlotinib = 0.088, docetaxel = 0.102 | ||||
| Utility scores | Total QALY: erlotinib = 0.238, docetaxel = 0.206, difference = 0.032 | |||||
| Leighl72 | Docetaxel vs. BSC | Stage IIIB or IV patients with advanced NSCLC | Previously treated with cisplatin-based chemotherapy | Mean OS. Survival data analysed using log-rank test | NICE TAX31773 (docetaxel vs. BSC) | Mean OS months (95% CI): docetaxel = 9.10 (7.51 to 10.69), BSC = 7.11 (5.60 to 8.62), p = 0.07 |
| Carlson73 | Erlotinib vs. docetaxel | > 60-year-old patients with advanced stage III–IV NSCLC | Failed at least one platinum-based chemotherapy | Mean PFS and mean OS | BR.2130 (erlotinib vs. placebo) | Mean OS: erlotinib, docetaxel, pemetrexed = 0.75 years |
| Erlotinib vs. pemetrexed | Assumed PFS and OS were the same for all three drugs | NICE TAX31773 (docetaxel vs. BSC) | Mean PFS: erlotinib, docetaxel, pemetrexed = 0.34 years | |||
| TAX 320 (docetaxel vs. BSC) | ||||||
| Pemetrexed vs. docetaxel | AE rates and utility scores | JMEI49 (pemetrexed vs. docetaxel) | QALY: erlotinib = 0.42, docetaxel = 0.41, pemetrexed = 0.41 | |||
| Published literature and Nafees EQ-5D study74 | ||||||
| Vergnenegre63 | Docetaxel vs. BSC | Patients with stage IIIB or IV NSCLC | Failed after first-line cisplatin-based chemotherapy | Median PFS and median OS | Le Groupe Français de Pneum Cancérologie 2005–6 study61 | Objective RRs: docetaxel = 10.7%, pemetrexed = 12.0% |
| Pemetrexed vs. BSC | Objective RR | Nafees EQ-5D study74 | Median PFS: docetaxel = 2.8 months, pemetrexed = 2.5 months | |||
| Docetaxel vs. pemetrexed | Utility scores | Median OS: docetaxel = 8 months, pemetrexed = 6.4 months | ||||
| QALY: docetaxel = 0.42, pemetrexed = 0.41 |
Cost data were mainly derived from relevant national sources of published cost information (Table 28), for example Spanish reference database (BOT database of pharmaceutical prices),65 Portuguese ministerial dispatch report,64 Ontario Case Costing Acute Inpatient Database69 and British National Formulary (BNF). 67 Costs were typically categorised as drug, drug administration and/or monitoring and treatment of AEs. The publication year differed by no more than 3 years from the base-cost year used in the studies.
| Study | Types of costs | Cost data sources | Cost year/currency | Costs |
|---|---|---|---|---|
| Araujo64 | Chemotherapy drugs, AEs, medical consultations, laboratory costs, complementary exams, concomitant medications, procedures and hospital stays | Grupos de Diagnosticos Homogeneos (ministerial dispatch no. 110-A/2007), hospital analytical accounting reports, Infarmed, Institute of IT and Financial Management (IGIF) database. Cost of erlotinib was supplied by Roche (UK) Ltd and the cost of pemetrexed was estimated through the price supplied by two hospital pharmacies. Cost of docetaxel was taken from the IGIF database | Prices obtained from 2006 and 2007 data were updated to 2008 prices using an annual inflation rate of approximately 3%/EUR | Total cost per patient: erlotinib = €26,478, BSC = €16,112, docetaxel = €29,262, pemetrexed = €32,762 |
| Asukai65 | Chemotherapy (drug and administration), AE treatment, BSC and one-off terminal/palliative care | Spanish reference database BOT was used for medication prices. Hospital treatment costs and laboratory tests were sourced from the Oblikue and Costs/Base de datos de costes sanitarios (SOIKOS) databases. Other costs were obtained from two IMS Health reports | 2007/EUR | Total cost per patient: pemetrexed = €34,677, docetaxel = €32,343 |
| Bradbury66 | Chemotherapy treatment, diagnostic tests, outpatient visits, concomitant medications, management of treatment-related toxicity, hospitalisations, radiation therapy and red-blood-cell transfusions | Costs were obtained from PPS Pharma Publication, Ontario Case Costing Acute Inpatient Database, individual patient trial data and Canadian Blood Service | 2007/CA$ | Mean cost per patient: erlotinib = CA$16,487, placebo = CA$4184 |
| Holmes67 | Docetaxel, drug administration and co-drug. Cost offsets (mean additional costs in the BSC group for radiotherapy and morphine use) and toxicity treatment costs were included in a sensitivity analysis | BNF and Unit Costs of Health and Social Care | 2000–01/GBP | Mean net cost per patient: docetaxel = £4432, BSC = £0.00 |
| Thongprasert68 | Direct medical costs: drug acquisition costs, drug administration and monitoring, and AE management | Drug and Medical Supply Information Center, standard cost list for health technology assessment, prices of Services of Health Facilities under the Ministry of Public Health (HITAP) | 2010 – converted to US$ using exchange rate of 30.28 THB = 1US$ (Bank of Thailand website)/THB | Total cost per patient: docetaxel = US$6483, gefitinib = US$6237, erlotinib = US$8229, pemetrexed = US$9092 |
| Cromwell69 | CTX drugs, radiation therapy, physician appointments, diagnostic tests and hospital admission | Drug costs from PPS Pharma Publication, hospital costs per diem from the Ontario Case Costing Acute Inpatient Database, transfusion costs from Canadian Blood Services, other costs from medical opinion and trial database | 2009/CA$ | Mean overall cost/patient (range): erlotinib = CA$35,708 (CA$32,241–CA$39,174), docetaxel = CA$32,817 (CA$27,940–CA$37,693), difference = CA$2891 |
| Cromwell70 | CTX drugs, radiation therapy, physician appointments, diagnostic tests and hospital admission | Provincial Medical Services Plan, provincial PharmaCare plan, home and community care and hospital-specific mean case costs | 2009/CA$ | Mean overall cost/patient (range): erlotinib = CA$34,326 (CA$6569–CA$99,370); BSC = CA$23,224 (CA$1095–CA$78,775) |
| Lewis71 | Monthly medical resource utilisation, treatment-related AEs and drug administration costs for three health states were agreed upon by a panel of lung cancer clinicians | Unit costs from BNF (2006) and PSSRU (2008) | 2009/GBP | Lifetime per patient costs: erlotinib = £13,730, docetaxel = £13,956 |
| Leighl72 | Outpatients assessments, chemotherapy administration, hospitalisation, radiation therapy, community-based nursing and supportive care, and miscellaneous items | Costs derived from trial data, hospital medical records as well as other facilities at which care was received. All physician services were based on the 1999 Ontario Health Insurance Plan fee schedule | Canadian dollars/1999 | Average cost per patient arm in NICE TAX317: docetaxel (75 mg/m2) = CA$17,738.96 BSC = CA$6935.04 |
| Carlson73 | Drug utilisation, drug administration, hospital inpatient admission, outpatient appointments AE treatments | Wholesale drug acquisition costs from First Data Bank I online database, medical services from CMS physicians fee schedule and inpatient prospective payment system, disease progression from a Kaiser Permanente study | 2007/US$ | Total cost (US$): erlotinib = $36,977, docetaxel = $39,104, pemetrexed = $43,795 |
| Vergnenegre63 | CTX drugs, drug administration, supportive treatment, hospitalisation for any reason, outpatient follow-up attendance, medical transport and grade 3 or 4 AE management costs | Costs were derived from national tariffs for diagnosis-related groups and national fees for ambulatory care, provided by French Ministry of Health and the national health insurer. Drug administration, follow-up and AE costs are an average of 2006, 2007 and 2008 tariffs | 2009/EUR | Total cost: docetaxel = €13,714 ± €7387, pemetrexed = €16,802 ± €7852. Authors compared docetaxel with BSC and pemetrexed with BSC, and assumed costs and benefits of BSC were equal to zero |
The costs estimated and employed in the EEs differ because of the assumptions made by the authors. For example, total costs per patient for erlotinib range from CA$16,487 to CA$35,708. 69 In Vergnenegre et al. ,63 the costs of BSC are assumed to equal zero while in Leighl et al. 72 the average cost of care in the BSC group was CA$6935.04. Costs and benefits were discounted at a 3%, 3.5% or a 5% discount rate, although some studies69,71,72 did not use discounting despite estimating costs and benefits over a time period greater than 12 months.
Despite variations in the methods employed and reporting of results across the studies, five of the six studies that assessed erlotinib compared with chemotherapy or BSC favoured erlotinib;64,66,70,71,72 the authors of the remaining study69 concluded that erlotinib and docetaxel were equal in terms of costs and benefits. Two studies67,72 comparing docetaxel versus BSC concluded that docetaxel was cost-effective. In another study68 gefitinib was preferred to docetaxel, and, of the two studies comparing pemetrexed versus docetaxel, one study favoured docetaxel63 and the other favoured pemetrexed. 65
Cost-effectiveness review: discussion of study methods and results
It is clear from the methods and results reported in the published cost-effectiveness literature that the conclusions drawn are very dependent on the assumptions made by the investigators and the data sources employed in the EEs (Table 29). These differ from evaluation to evaluation. Each EE must therefore be judged on its own merits and any attempt to make summary statements about different comparisons in terms of cost-effectiveness is meaningless.
| Study | Cost-effectiveness results | Sensitivity analysis | Conclusions |
|---|---|---|---|
| Araujo64 | Cost/QALY gained: erlotinib vs. BSC = €161,742, erlotinib vs. docetaxel = erlotinib dominates, erlotinib vs. pemetrexed = erlotinib dominates | Sensitivity analyses undertaken generate results similar to the base case | Use of erlotinib instead of docetaxel or pemetrexed could contribute to annual savings for the Portuguese NHS and a gain in QALYs |
| Cost/LY gained: erlotinib vs. BSC = €70,424, erlotinib vs. docetaxel = erlotinib reduces costs, erlotinib vs. pemetrexed = erlotinib reduces costs | |||
| Asukai65 | Cost/QALY gained: pemetrexed vs. docetaxel = €23,967 | Model is most sensitive to variation in OS. The PSA results show that pemetrexed has a 62% likelihood of having a QALY below €30,000 and a 77% likelihood of having a cost per LY gained below €30,000 | In the Spanish setting, pemetrexed for the second-line treatment of patients with NSCLC other than predominantly squamous cell histology is indicated as a cost-effective chemotherapy option compared with the standard docetaxel, based on its superior OS benefit and toxicity profile |
| Cost/LY gained: pemetrexed vs. docetaxel = €17,225 | |||
| Bradbury66 | Cost/LY gained: erlotinib vs. placebo = CA$94,638 | Magnitude of the survival benefit was the main influence on the size of the ICER | Authors conclude that erlotinib for patients with previously treated advanced NSCLC is marginally cost-effective and that the use of molecular predictors of benefit for targeted agents may help identify more or less cost-effective subgroups for treatment |
| Subgroup analyses: cost/LY gained (never-smokers) = CA$39,487, cost/LY gained (high EGFR gene copy number) = CA$33,353 | Subgroup analyses revealed that erlotinib may be more cost-effective in never-smokers or patients with high EGFR gene copy number | ||
| Holmes67 | Cost/LY gained: docetaxel vs. BSC = £13,863 | Sensitivity analysis showed that the number of treatment cycles per patient had most influence on the cost/LY gained | Authors conclude that docetaxel 75 mg/m2 in 3-weekly cycles is a cost-effective second-line treatment from the perspective of the UK NHS for pre-treated NSCLC in terms of survival gains made for a reasonable increase in costs |
| Thongprasert68 | Cost/QALY gained: gefitinib vs. docetaxel = gefitinib dominates, erlotinib vs. docetaxel = US$124,703, pemetrexed vs. docetaxel = US$237,150 | Sensitivity analyses showed that varying docetaxel cost and the duration of docetaxel treatment had the greatest effect on cost-effectiveness | Authors conclude that gefitinib is a dominant cost-saving strategy compared with docetaxel for the second-line treatment of advanced NSCLC from the Thai payer perspective |
| Cromwell69 | Costs and benefits were not significantly different between the two groups, it was not possible to calculate a meaningful ICER | Univariate SA could not be performed as SA results in either a numerator or a denominator of zero | Erlotinib = docetaxel in terms of costs and benefits. Choice of treatment should depend on patient preferences |
| Cromwell70 | Cost per LY gained: erlotinib vs. BSC = CA$36,838, incremental mean OS = 110 days, incremental mean cost = CA$11,102 | Univariate SA (from varying total treatment costs) yielded ICERs ranging from CA$21,300/LY gained to CA$51,700/LY gained. Other parameters varied included mean drug cost/patient and hospital cost/patient | Analyses suggest that erlotinib may be an effective and cost-effective third-line treatment for advanced NSCLC compared with BSC |
| Lewis71 | Cost per QALY gained: erlotinib vs. docetaxel = –£7106, net monetary benefit = £1181, incremental benefit = 0.032, incremental cost = –£226 | Sensitivity analyses showed the robustness of the baseline analysis, i.e. that erlotinib was cost-effective compared with docetaxel | From a health-economics perspective, for the treatment of patients with relapsed stage III–IV in the UK, erlotinib has advantages over docetaxel |
| Leighl72 | Cost per LY gained: docetaxel (75 mg/m2) vs. BSC = CA$31,776 | In univariate SA, cost-effectiveness ratios were most sensitive to changes in survival ranging from CA$18,374 to CA$117,434 with 20% variation in survival at recommended (75 mg/m2) dose | Authors concluded that the estimated cost per LY gained is within an acceptable range of health-care expenditures |
| Carlson73 | Cost per QALY gained: erlotinib vs. docetaxel = erlotinib dominates, erlotinib vs. pemetrexed = erlotinib dominates, pemetrexed vs. docetaxel = US$1,743,359 | Estimates of treatment duration were among the most influential parameters in the SA, others were time in PFS, drug costs and values of some health-state utilities. In the PSA, erlotinib was cost-saving in 65% and 87% of the simulations compared with docetaxel and pemetrexed, respectively | Results of the study suggest that erlotinib in the treatment of refractory NSCLC in the USA is less costly than alternative treatments and may lead to a slight improvement in QALYs |
| Vergnenegre63 | Cost per QALY gained: docetaxel vs. BSC = €32,652, pemetrexed vs. BSC = €40,980 | SA showed that the price of pemetrexed would need to fall by 30% to balance the cost per QALY values in each arm | Second-line treatment for NSCLC is more cost-effective with docetaxel than with pemetrexed. Both strategies have acceptable cost-effectiveness ratios compared with commonly used and reimbursed regimes for advanced NSCLC |
| Cost per LY gained: docetaxel vs. BSC = €15,545, pemetrexed vs. BSC = €22,798 |
Of the 19 comparisons considered in the 11 published studies, 13 included docetaxel as a comparator. The AG notes that the patent on docetaxel has expired and docetaxel is now available in its generic form at a cost that is less than 10% of its previous list price. 29 The AG therefore considers that the incremental cost-effectiveness ratios (ICERs) estimated in these 13 comparisons are now of limited value to decision-makers in the UK NHS. Of the six remaining comparisons, three included pemetrexed as a comparator [pemetrexed vs. BSC (n = 163) and pemetrexed vs. erlotinib (n = 264,73)]. Again, the AG considers that the results of these studies cannot be used directly to inform decision-making in the UK as pemetrexed is not recommended by NICE for the second-line treatment of patients with NSCLC in the UK NHS. The remaining three studies64,66,69 focused on the comparison of erlotinib with BSC. However, as none of the studies report ICERs for an EGFR M+ or EGFR M– patient population, the AG considers that the estimated ICERs are useful only when making treatment decisions for patients whose EGFR status is unknown, as the EGFR mutation status of this patient group can influence treatment choices. In addition, the AG is of the opinion that, although BSC is a valid comparator for a small population of patients with NSCLC, docetaxel is a more appropriate comparison for patients in the UK NHS.
The AG concludes that the results of the systematic review are of limited value to decision-makers in the UK NHS. This is a result of (1) relatively recent changes in the price of docetaxel and (2) the increased significance of EGFR mutation testing for patients with NSCLC. The AG does not summarise or draw conclusions from any other manufacturer’s submission used in previous NICE appraisals of erlotinib and/or gefitinib as these submissions were written at a time when it was not possible to take into account these aforementioned changes. The AG anticipates that future EEs in this complex clinical area will make use of the most up-to-date clinical effectiveness and cost data available.
Critique of the economic analyses submitted by manufacturers
The manufacturer of gefitinib (AstraZeneca) did not include any cost-effectiveness analyses in its submission. The objective of its manufacturer’s submission was to demonstrate the clinical benefit of gefitinib therapy in EGFR M+ patients with NSCLC following prior chemotherapy.
The manufacturer of erlotinib [Roche (UK) Ltd] states in its manufacturer’s submission that it does ‘. . . not believe it is possible to demonstrate [that] erlotinib is cost-effective compared to docetaxel following the availability of generic docetaxel at less than 10% of the list price of docetaxel in NICE TA162’. 28 The manufacturer’s base-case analysis therefore compares erlotinib and BSC in patients whose EGFR mutation status is unknown and who are unsuitable for docetaxel or who have previously received docetaxel. In a separate subgroup analysis, the manufacturer considers erlotinib versus BSC for patients with EGFR M– tumours. The AG provides a summary and critique of the EE presented in the manufacturer’s submission submitted by Roche (UK) Ltd.
The AG notes that the manufacturer of erlotinib [Roche (UK) Ltd] has not compared the cost-effectiveness of erlotinib with gefitinib. In the UK NHS, patients who have EGFR M+ tumours are likely to have received either erlotinib or gefitinib as a first-line treatment and it is, therefore, unlikely that this group of patients would be re-treated with a EGFR-TKI as part of second-line treatment. The manufacturer, therefore, has not carried out an EE for this group of patients. Furthermore, as gefitinib does not have a licence for patients who have EGFR M– tumours, the manufacturer has not carried out an EE comparing erlotinib with gefitinib for this patient population.
Review of Roche (UK) Ltd economic model: erlotinib versus best supportive care
The ERG assessed the economic model submitted by Roche using NICE’s reference case checklist (Table 30).
| NICE reference case requirements | Reference case | Does the de novo EE match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | Partial. Docetaxel was not considered. The manufacturer stated that they do not believe it would be possible to demonstrate that erlotinib is cost-effective compared with docetaxel following the availability of generic docetaxel. No comparison with gefitinib |
| Comparators | Therapies routinely used in the NHS, including technologies currently regarded as best practice | |
| Perspective on costs | NHS and Personal Social Services | Yes |
| Perspective on outcomes | All health effects on individuals | Yes |
| Type of EE | Cost-effectiveness analysis | Yes |
| Synthesis of evidence on outcomes | Based on a systematic review | N/A – only evidence from BR.2131 was used |
| Measure of health benefits | QALYs | Yes |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | Yes |
| Source of preference data for valuation of changes in HRQoL | Representative sample of general public | No. Source of preference data not specified |
| Discount rate | An annual rate of 3.5% on both costs and QALYs | Yes |
| Equity weighting | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes |
Overview of submitted manufacturer’s submission
The manufacturer developed a de novo economic model using data from the BR.2131 trial. In the base-case analysis, the manufacturer compares erlotinib versus BSC using ITT data from the BR.2131 trial. In a separate subgroup analysis, the manufacturer compares erlotinib versus BSC in an EGFR M– patient population only, this patient group was identified retrospectively. 43
The developed model is a partitioned survival model with three health states (a structure that has been used in many previous NICE oncology technology appraisals, including TA162,21 TA22747 and TA29576). The model projects PFS and OS independently with the proportion of patients in the progressed health state over time being the proportion of patients alive but not in the PFS health state.
The model structure is shown in Figure 2. All patients enter the model in the PFS health state and in each month can either progress to a worse health state [i.e. from PFS to progressed disease (PD) or from PD to death] or remain in the same health state. The model has been developed in Microsoft Excel and has a 1-week cycle length.
FIGURE 2.
Schema of manufacturer’s model. PD, progressed disease.
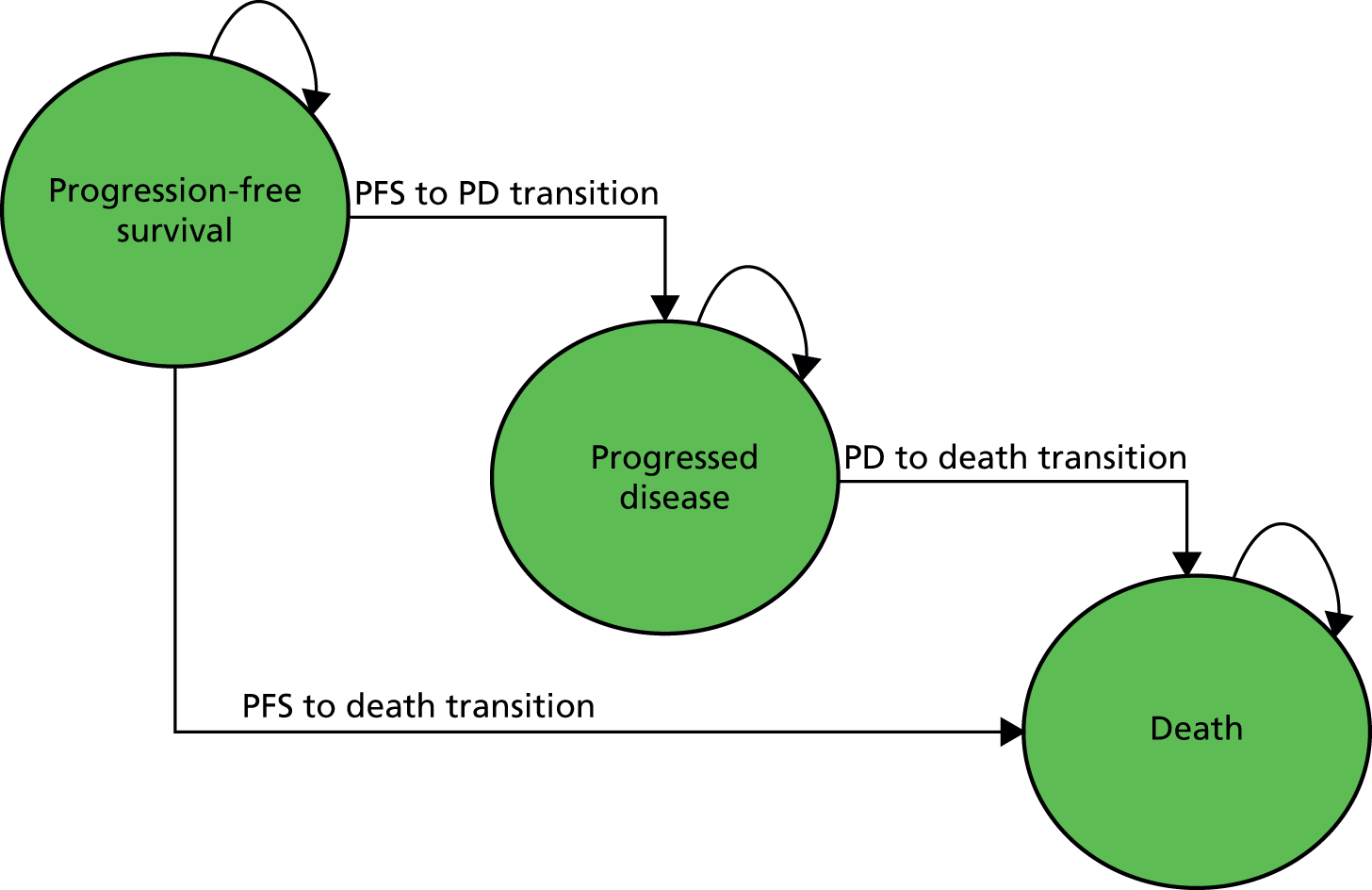
Population
The population was assumed to be the same as that recruited to the BR.2131 trial, that is patients 18 years of age or older with an ECOG PS score of between 0 and 3 and who had documented pathological evidence of NSCLC. Patients in this trial had to have received one or two regimens of combination chemotherapy and not be eligible for further chemotherapy. The only baseline population characteristic used in the model was age (61.4 years in both arms).
Interventions and comparators
The manufacturer believes that, following the availability of generic docetaxel at less than 10% of the previous list price, it is not possible to demonstrate that erlotinib is cost-effective when compared with docetaxel. The manufacturer has, therefore, only presented an analysis comparing erlotinib (maximum of one 150-mg tablet per day until disease progression) with BSC. In addition, the AG notes that the manufacturer did not compare the cost-effectiveness of erlotinib with gefitinib.
Perspective, time horizon and discounting
The EE is undertaken from the perspective of the NHS and Personal Social Services Research Unit (PSSRU). Outcomes are expressed in terms of LYs gained and QALYs gained. The time horizon is set at 6 years and, in line with the NICE Guide to the Methods Technology Appraisal,77 both costs and benefits are discounted at 3.5%.
Treatment effectiveness and extrapolation
Data from BR.2132 were used to estimate PFS and OS.
Progression-free survival
No extrapolation of PFS data was required as, by 18 months, all patients on BSC had progressed and only two patients using erlotinib remained free of progression. These two patients were assumed to have progressed at the next cycle.
Overall survival
Cumulative hazards were calculated and plotted for both arms. A linear trend was observed for both arms indicating that, although different, the rate of death in each arm remained constant over time. Based on factors including visual inspection and small patient numbers, week 70 and week 78 were chosen as the time points at which extrapolation should begin for erlotinib and BSC, respectively.
Health-related quality of life
The manufacturer extracted utility values from the published appraisal of crizotinib for the treatment of previously treated NSCLC associated with a lymphoma kinase fusion gene (NICE TA29678). The manufacturer selected and applied the pooled chemotherapy (pemetrexed or docetaxel) values to both the erlotinib and BSC arms of the model. The manufacturer considers this to be a conservative assumption, as QoL data from BR.2131 showed that erlotinib improved QoL as regards time to deterioration of key symptoms of cough, dyspnoea and pain compared with BSC.
The manufacturer notes that the patient population in PROFILE 100779 (described in TA29678) is anaplastic lymphoma kinase positive and that the utility values from this population are relatively high for patients with NSCLC. Furthermore, the patient group in PROFILE 100779 was younger and less fit than those patients enrolled in the BR.2131 trial.
The source of the utility values used in the model is presented in Table 31.
| State | Utility value | Standard error | Source |
|---|---|---|---|
| PFS | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | TA29678 |
| PD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | TA29678 |
Resources and costs
Erlotinib acquisition costs
The model assumes that erlotinib is dispensed in packs of 30 tablets (150 mg) every 4 weeks. The cost calculation takes into account the treatment duration by using data taken from BR.2131 (mean duration = 9.57 weeks). In BR.2131 19% of patients had some form of dose reduction; the effect of this is assessed in a sensitivity analysis. The cost used in the model includes the simple confidential discount agreed during TA16221 and TA25818 (Table 32).
| Cost | Value | 95% CI | Source |
|---|---|---|---|
| Pharmacy costs per pack of erlotinib dispensed | £18.20 (12 minutes of pharmacy time at £91/hour) | £9.28 to £27.12a | Millar,80 PSSRU,81 manufacturer’s submission section 4.528 |
| Erlotinib drug costs | 30 tablets × 150 mg = £1631.53,b 30 tablets × 100 mg: £1324.14,b 30 tablets × 25–50 mg: £378.33b | N/A | BNF, September 201329 list price, manufacturer’s submission table 12, section 4.528 |
Supportive care costs
The supportive care resources described in the manufacturer’s submission are in line with those used in TA162,21 which were elicited from an expert panel and updated using NHS Reference Costs (2011/12),82 PSSRU (2011),81 BNF (2012)48 and the electronic market information tool (eMit). 83 It is noted that the supportive care costs applied to the PD health state are considerably higher than those employed in recent appraisals of advanced NSCLC because in this model the high-cost end-of-life phase is not shown as a separate element.
These costs, which are displayed in Table 33, have been applied in the model at each weekly cycle.
| Supportive care costs | Included elements (per month) | Value (weekly) | |
|---|---|---|---|
| Visits and hospitalisation | Tests, procedures and medications | ||
| PFS BSC cost (including monitoring) | Hospital stay episode (2.5% points) | Blood count (all points × 0.75) | £84.67 |
| Cancer nurse (20% points × one visit) | Palliative radiotherapy (12.5% points × 1) | ||
| Palliative care nurse (30% points × one visit) | Computed tomography (30% points × 0.75) | ||
| Palliative care physician (7.5% points × one visit) | Radiography (all points × 0.75) | ||
| OP attendance (0.75 visits) | Biochemistry (all points × 0.75) | ||
| GP visit (10% points × one visit) | |||
| PD BSC cost | Hospital stay episode (30% points) | Blood count (all points × 1) | £220.34 |
| Palliative radiotherapy (20% points × 1) | |||
| Cancer nurse (10% points × one visit) | Computed tomography (5% points × 0.75) | ||
| Radiography (30% points × 0.75) | |||
| Palliative care nurse (20% points × one visit) | Biochemistry (all points × 0.75) | ||
| Home oxygen (20% points × 1) | |||
| Palliative care physician (80% points × two visits) | Steroids (dexamethasone) (50% points 0.5 mg × 160) | ||
| Non-steroidal anti-inflammatory drugs (aspirin) (30% points 200 mg × 60) | |||
| OP attendance (one visit) | Morphine (75% of patients 60 mg × 7) | ||
| GP visit (28% points × one visit) | Bisphosphonate (ibandronic acid) (7.5% points 5 mg × 28) | ||
Adverse events
Adverse event rates were taken from BR.2131 and only those AEs for which the cumulative percentage across both arms was greater than 5% were included in the manufacturer’s model. The assumed costs for treating each AE were based on resource use elicited from an expert panel and previously used in NICE TA162. 21 Costs were taken from NHS Reference Costs (2011/12),82 PSSRU (2012),84 BNF (2012)48 and eMit83 and are displayed in Table 34.
| AE | Included elements | Value |
|---|---|---|
| Rash | Outpatient attendance, oral tetracycline | £275.36 |
| Anorexia | Dietitian, steroids (dexamethasone) | £76.85 |
| Nausea and vomiting | Hospital stay, outpatient attendance, GP visit, Macmillan nurse, domperidone, steroids (dethamethasone), blood count, biochemistry | £387.59 |
| Diarrhoea | Hospital stay, outpatient attendance, GP visit, loperamide, stool culture | £584.81 |
| Infection | Hospital stay, emergency room, blood count | £1813.65 |
| Fatigue | GP visit, Macmillan nurse | £4.29 |
Cost-effectiveness results
The base-case incremental results generated by the manufacturer’s model are presented in Table 35. The ICER for the comparison of erlotinib with BSC in patients with NSCLC whose EGFR mutation status is unknown and who have progressed after prior chemotherapy treatment, is £51,036 per QALY gained and £35,593 per LY gained. Disaggregated costs for the target population are presented in Table 36.
| Technologies | Total costs (£) | Total LY gained | Total QALYs | Incremental costs (£) | Incremental LY gained | Incremental QALYs | ICER per QALY gained (£) |
|---|---|---|---|---|---|---|---|
| BSC | 5993 | 0.656 | 0.432 | – | – | – | – |
| Erlotinib | 13,522 | 0.867 | 0.579 | 7529 | 0.212 | 0.148 | 51,036 |
| Element | Cost (£) | Increment (£) | Absolute increment (£) | Absolute increment (%) | |
|---|---|---|---|---|---|
| Erlotinib | BSC | ||||
| Drug | Commercial-in-confidence information has been removed | 0 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Pharmacy | Commercial-in-confidence information has been removed | 0 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| AEs | Commercial-in-confidence information has been removed | 113 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| PFS BSC | Commercial-in-confidence information has been removed | 1020 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| PD BSC | Commercial-in-confidence information has been removed | 4860 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Total | 13,522 | 5993 | 7529 | 7529 | 100 |
Sensitivity analyses
The manufacturer carried out a large number of one-way sensitivity analyses. A tornado diagram is included in the manufacturer’s submission (figure 27, page 67). The one-way sensitivity analysis results for the five changes that have the largest impact on cost-effectiveness are displayed in Table 37.
| Change from base case | Lower ICER estimate (difference from base-case ICER) | Higher ICER estimate (difference from base-case ICER) |
|---|---|---|
| Use of the Nafees75 utility values for PFS and PD | – | £61,317 |
| Variation (± 20%) from the base case of PFS utility | £44,900 (–£6136) | £59,116 (£8080) |
| Erlotinib dose reduction in 19% of patients and PFS cost reduction by 50% | £44,121 (–£6915) | – |
| Reduction of PFS costs (–50%) for the erlotinib arm | £45,565 (–£5471) | – |
| Variation (± 20%) from the base case of PD utility | £47,997 (–£3039) | £54,487 (£3451) |
Probabilistic sensitivity analysis (PSA) was undertaken (5000 iterations of the model) by the manufacturer. A scatterplot (incremental cost vs. QALY) and a cost-effectiveness acceptability curve are included in the manufacturer’s submission (p. 70) and reproduced in Figures 3 and 4.
FIGURE 3.
Probabilistic sensitivity analysis scatterplot erlotinib vs. BSC (diagonal line = £30,000 per QALY gained).
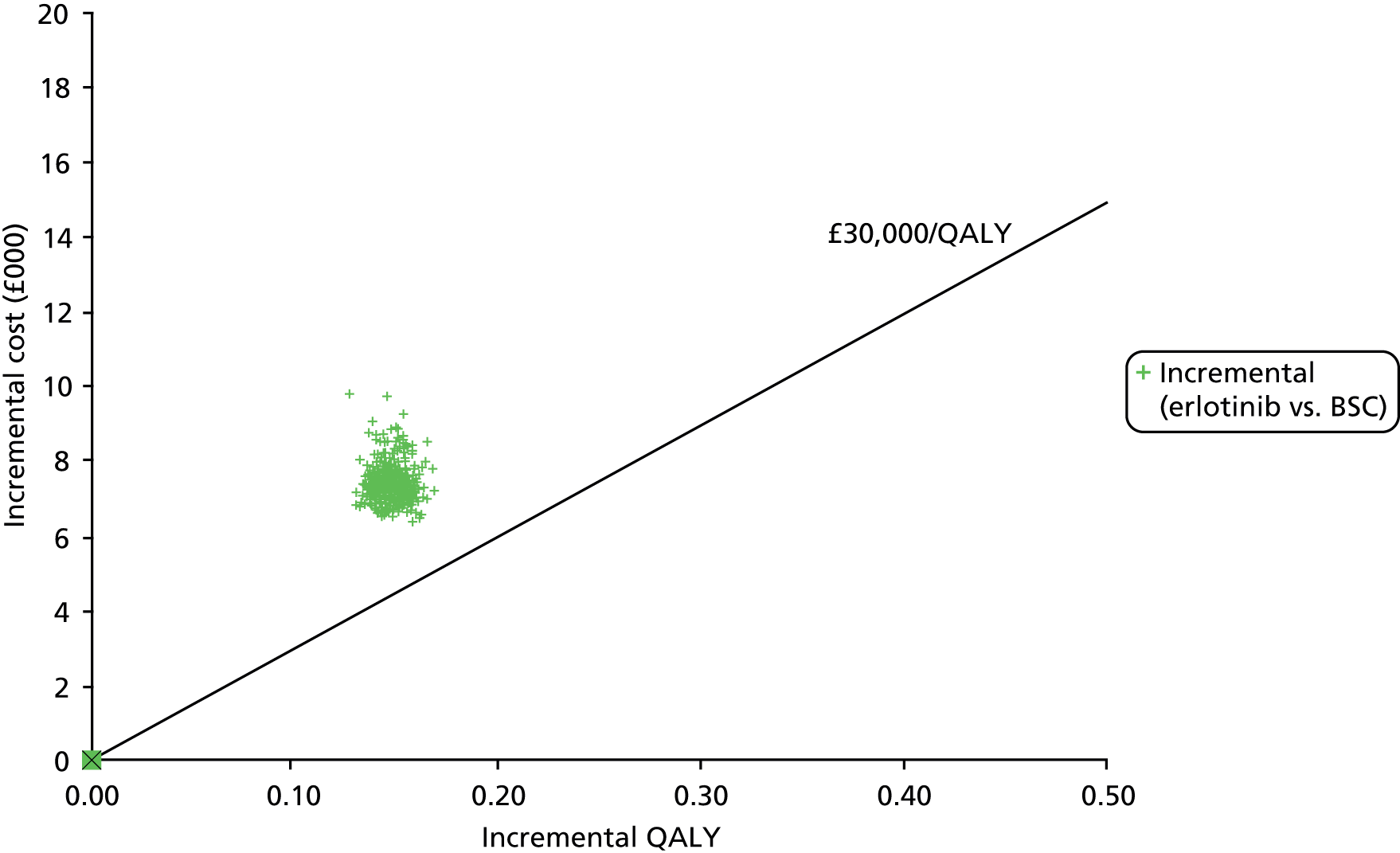
FIGURE 4.
Cost-effectiveness acceptability curve.
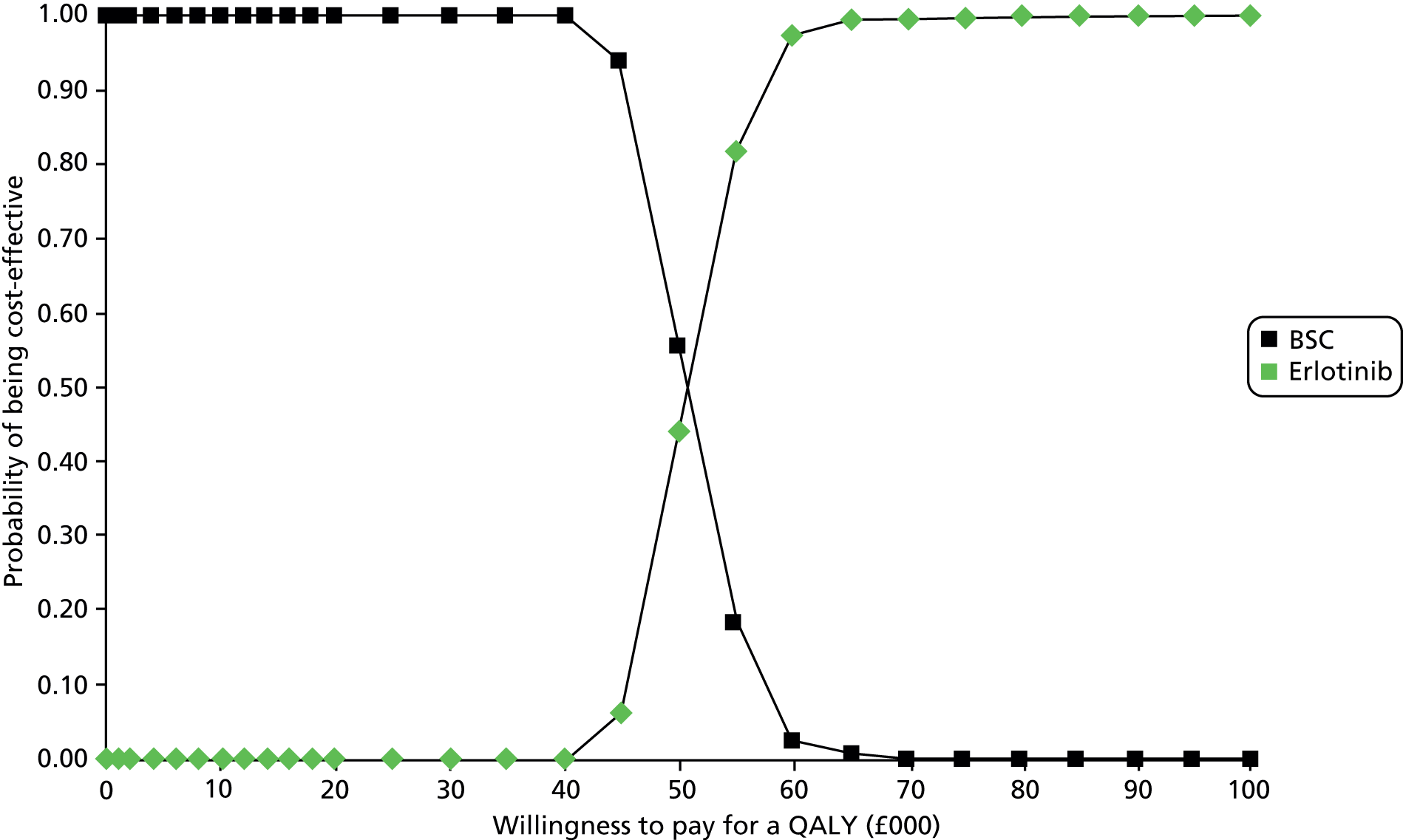
The results of the PSA are displayed in Table 38. The PSA ICER is estimated to be £50,825 per QALY gained, which is only £211 less than the base-case deterministic ICER of £51,036 per QALY gained.
| Technologies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER/QALY gained (£) | Difference from base-case ICER (£) |
|---|---|---|---|---|---|---|
| BSC | 5775 | 0.431 | – | – | – | – |
| Erlotinib | 13,265 | 0.578 | 7490 | 0.147 | 50,825 | –211 |
The PSA results show that there is a 0% probability that erlotinib is cost-effective at a willingness-to-pay threshold of £30,000 per QALY gained. However, at a threshold of £60,000 per QALY gained there is a 40% probability that erlotinib is cost-effective, and at a threshold of £65,000 per QALY gained erlotinib is cost-effective in approximately 76% of all scenarios.
Subgroup analysis
The manufacturer undertook a separate subgroup analysis for the EGFR M– population of the BR.2131 trial using data from the publication by Zhu et al. 43 The ICER for this group was £58,579 per QALY gained, a value which is approximately 14% higher than the base-case ICER. The QALY gain comes entirely from the PFS health state. The manufacturer advises that the results from this analysis, which are displayed in Table 39, should be interpreted with caution because of the limitations of the available data.
| Technologies | Total costs (£) | Total LY gained | Total QALYs | Incremental costs (£) | Incremental LY gained | Incremental QALYs | ICER per QALY gained (£) |
|---|---|---|---|---|---|---|---|
| BSC | 6362 | 0.682 | 0.447 | – | – | – | – |
| Erlotinib | 13,853 | 0.850 | 0.574 | 7490 | 0.168 | 0.128 | 58,579 |
Critique of the submitted model
The AG notes that, as well as not analysing the cost-effectiveness of erlotinib compared with docetaxel, the manufacturer did not carry out an analysis of the cost-effectiveness of erlotinib compared with gefitinib. This critique therefore focuses on the manufacturer’s analysis of the cost-effectiveness of erlotinib compared with BSC that is presented in the manufacturer’s submission. A detailed examination of model formulae and calculations has not been carried out.
The economic model submitted by the manufacturer was of a structure used in many previous oncology technology appraisals. The presented evaluation was based on data from one RCT (BR.2131). This trial recruited a population of patients with NSCLC and of unknown EGFR status; however, treatment pathways have evolved and currently patients who have EGFR M+ disease would not generally be given a EGFR-TKI as a second-line treatment, as they would already have received a TKI as a first-line therapy.
The manufacturer carried out a wide range of sensitivity analyses. The biggest impact on the size of the cost per QALY ICER (an increase of £10,281) resulted when utility values from Nafees et al. 75 replaced values from PROFILE 100779 in the manufacturer’s base-case analysis.
The AG has several concerns about the use of PROFILE 100779 values in the base-case analysis, namely:
-
These values have not been published, peer reviewed or validated.
-
There is no information on the coverage of patients within the trial completing the survey (i.e. at which time point and at which stage of treatment) so no assessment can be made of the potential for bias in any overall averages obtained.
-
The crude averages incorporate the effects of treatment-related AEs, which relate to another treatment given to younger but less fit patients with a different type of NSCLC.
In the manufacturer’s economic model, the social tariff algorithm used to calculate European Quality of Life-5 Dimensions (EQ-5D) scores is unknown. As the predominant data source in the PROFILE 100779 trial is the USA, it would not be surprising if the US tariff, which gives consistently higher scores than the UK tariff, had been used.
Figure 5 shows the relationship between health state scores using UK and US tariffs. When this conversion is applied to the PROFILE 100779 utility scores, the PFS average (US tariff) changes. The Nafees et al. 75 model gives 0.653 for stable disease PFS and 0.673 for responder PFS. Similarly, the PD average US tariff utility changes and compares closely with the Nafees et al. 75 PD utility.
FIGURE 5.
Relationship between health-state scores using UK and US tariffs.

One further point which, in this case, is likely to have only a minor impact on the size of the cost per QALY ICER relates to the cost of a hospital pharmacist’s time, which is used to estimate erlotinib administration costs. A value of £91 per hour (PSSRU 201181) has been used in the model but the most up-to-date value is £67 (PSSRU 201284).
In view of these issues, and to allow all therapy options to be compared using a consistent framework, the AG has developed a de novo cost-effectiveness model.
Assessment Group de novo economic model
This section describes the de novo cost-effectiveness model developed by the AG.
Methods
Assessment perspective
Costs and outcomes are assessed from the perspective of the UK NHS and PSSRU. Wider indirect costs and benefits (e.g. loss of productivity, value of informal care and impact on utility of patient’s family) are not considered.
Relevant patient populations
Three distinct populations are modelled as follows:
-
Previously treated adult patients with locally advanced or metastatic NSCLC and who exhibit EGFR-activating mutations (referred to as ‘EGFR M+ population’)
-
Previously treated adult patients with locally advanced or metastatic NSCLC and who do not exhibit EGFR-activating mutations (referred to as ‘EGFR M– population’)
-
Previously treated adult patients with locally advanced or metastatic NSCLC and for whom EGFR mutation status is unknown or indeterminate (referred to as ‘EGFR unknown population’)
Treatment options to be evaluated
Four pharmaceutical products are currently licenced for use in these populations:
-
Erlotinib and docetaxel may be used for treating patients in all three populations.
-
Gefitinib may be used only for patients with disease that exhibits EGFR-activating mutations.
-
Pemetrexed may be used only for patients with predominantly non-squamous disease following platinum-doublet chemotherapy as a first-line treatment. Pemetrexed was appraised as a second-line treatment for patients with NSCLC but not approved by NICE, and it is not within the scope of the current reappraisal.
Additionally, it is generally considered that a patient is unlikely to be re-treated with the same agent that was used as a first-line therapy. This constraint should, therefore, be considered as a limiting consideration when interpreting the cost-effectiveness results in each of the above populations.
Time horizon
A lifetime perspective is taken in the model, which projects all patient events and costs to a maximum of 5 years, at which time it is assumed that all patients will have died.
Mid-cycle correction
Treatment costs (drug and administration) are costed according to the number of patients progression free on the expected date of administration (when treatment is subject to specific cycle length) and to the date when a new pack of medication would be required for oral treatments. All other costs and QALYs estimates are based on PFS/OS mid-cycle corrected data, with the exception of terminal-care costs and QALYs, to which a more complex correction was applied to reflect costs and utilities in the 2 weeks prior to death.
Discount rates (costs and benefits)
In the base-case analysis both costs and outcomes are discounted at 3.5% per annum in line with NICE guidance. 78 Sensitivity analyses are reported for discount rates of 0% and 6%.
Model design
The decision model (Figure 6) is conceptually straightforward, involving two health states prior to death (progression free after second-line chemotherapy, post progression). Therapy is treated as an extended event, given over several cycles (usually of 3 weeks’ duration). However, orally administered treatments (erlotinib and gefitinib) are given continuously until the disease progresses, and treatment is assumed to be coterminous with the duration of the PFS state.
FIGURE 6.
Conceptual model of second-/third-line decision model, indicating health states (rectangles), events/procedures (ovals) and transitions (arrows).
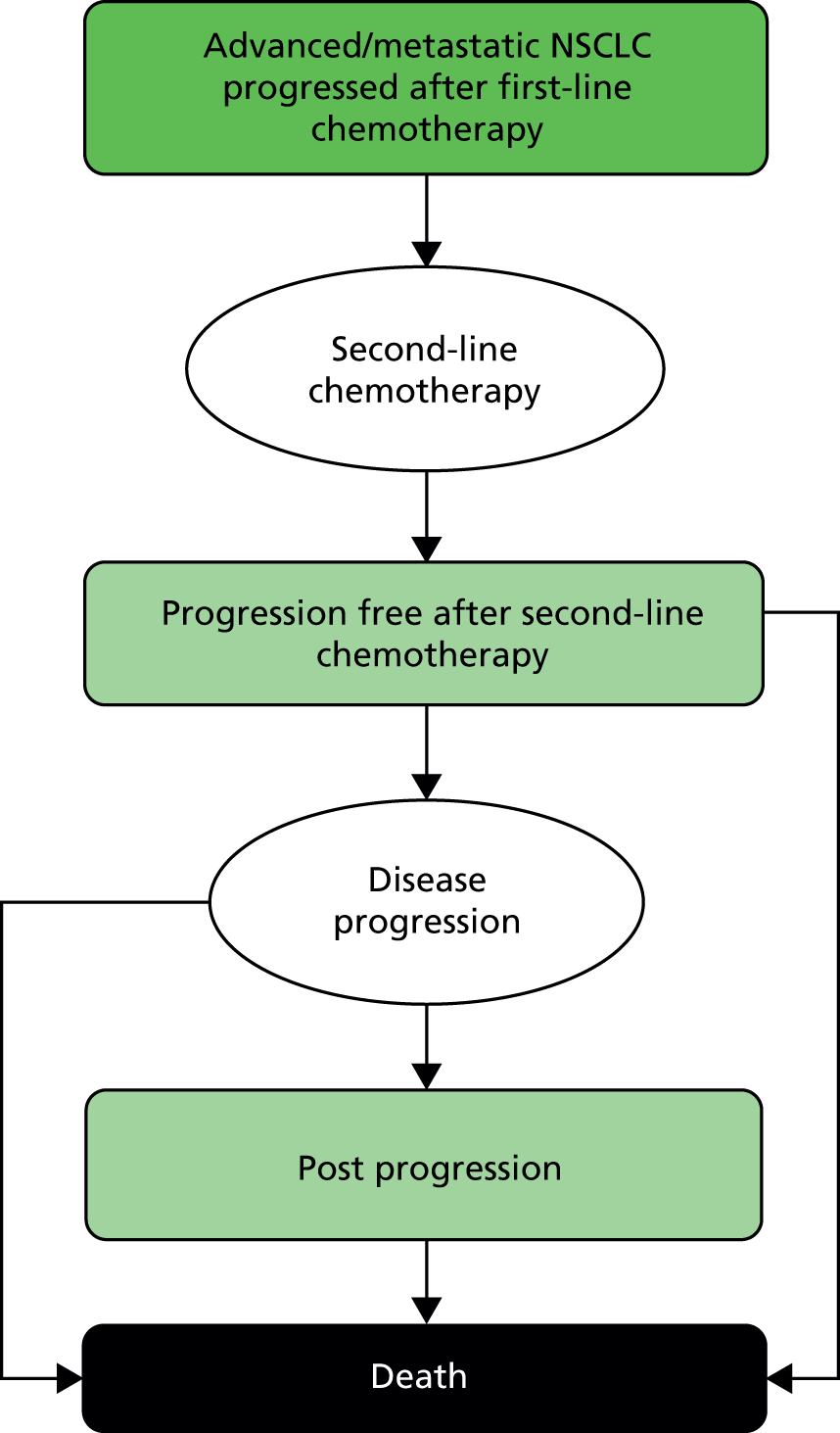
Disease progression after second-line therapy is treated as an event, resulting in one of two transitions, either to a period of post-progression survival (PPS), which eventually results in death, or to immediate death. Further lines of therapy are possible but are not modelled explicitly, as the proportion of patients receiving subsequent active treatments is small in the UK. Instead, additional resources and utility effects are included in the post-progression health state to represent average usage.
The model is implemented as a Microsoft Excel workbook, using macro-programming to perform PSA to assess the relative probabilities of cost-effectiveness between the available second-line treatments.
Ideally, the model should be driven by evidence from clinical trials relating to each of the model’s health states: the duration of PFS when patients receive second-line treatment and the duration of PPS when patients receive only BSC. Unfortunately, the only outcomes routinely reported for clinical trials are PFS and OS. Thus the model can only be populated indirectly by inferring the probable experience of patients in the intermediate states. This leads to potentially serious difficulties and inconsistencies in model implementation. In particular, the normal practice of treating PFS and OS as independent variables is naive, since PFS is a major component of OS. Not recognising this easily leads to situations where deriving an estimate for PPS by subtracting estimated PFS from estimated OS leads to erroneous negative values at some point during the simulation period. The modeller has to exercise great care at every stage of model development, calibration and use so as to guard against producing nonsensical results.
Synthesis of outcome data: progression-free survival and overall survival
Epidermal growth factor mutation-positive population
No clinical trials have been identified which compare second-line treatments in a population of only patients with EGFR-activating mutations.
Epidermal growth factor mutation-negative population
Clinical effectiveness data for this patient group are restricted to TAILOR,41 which compares erlotinib with docetaxel. Published Kaplan–Meier survival curves were digitised by the AG to provide source data for projecting the full cohort experience until death. Both PFS and OS curves exhibited forms inconsistent with the standard parametric functions routinely featured in commercially available statistical software. All such functions assume that a single continuous disease and treatment process is in effect throughout the duration of the trial, resulting in gradual smooth changes in event risk and survival outcomes from randomisation until the outcome event (progression/death for PFS or death for OS). The Kaplan–Meier curves from TAILOR41 show clearly that this assumption is invalid, with quite different behaviour exhibited over different periods of the trial in both patient groups.
The natural history of untreated advanced/metastatic lung cancer is generally straightforward, involving a high but constant risk of disease progression and death within a short time period (usually best represented as a Poisson process, i.e. an exponential survival function). However, when short-term interventions are applied to patients, the normal disease dynamic is distorted, typically into three time periods: an initiation period (prior to treatments achieving full efficacy), an efficacious period (when different treatments may show divergent risk of progression/death) and a loss of efficacy period (when the natural course of progressive disease is reasserted).
Examination by the AG of the cumulative hazard plots for the trial data indicated that a three-phase spline model (with two ‘knot’ points) closely represents the published trial results and outperforms any of the standard parametric functions conventionally employed. In the first phase, event risks are very similar in both trial arms. In the second phase, patients in both trial arms are subject to increased risk of an event (progression or death) and at different levels of risk corresponding to differential treatment efficacy, so that the survival curves diverge. In the final phase, event risks reduce substantially in both arms. In addition, the transitions between phases appear to occur at the same time from randomisation in both treatment arms. The event risk within each phase was found to conform closely to a constant (equivalent to an exponential survival function) in both treatment arms. The main structural difference between statistical models for the two treatments occurs in the final phase. For PFS the event risk remains higher in the erlotinib arm, suggesting that PFS outcomes continue to diverge indefinitely, whereas in the OS comparison the long-term mortality risk stabilises at the same level once all patients have suffered disease progression, thus suggesting that for the remainder of patients’ lifetimes survival prognosis is unrelated to previous treatments.
Figures 7 and 8 demonstrate the correspondence between TAILOR42 data and the AG’s projective models. The calibrated models were only used to project PFS and OS during and beyond the third phase to maximise the use of the unadjusted trial data. In all cases projection was commenced at the same value of the estimated remaining PFS or OS to avoid introducing bias from projecting different proportions of patient experience subject to different degrees of modelling error. For PFS projection began at the point when 30% of patients were estimated to be event-free and for OS projection began at 41%. Details of the AG’s model parameters, estimates and standard errors are provided in Appendix 7.
FIGURE 8.
Three-phase projective spline models fitted to OS data from TAILOR.
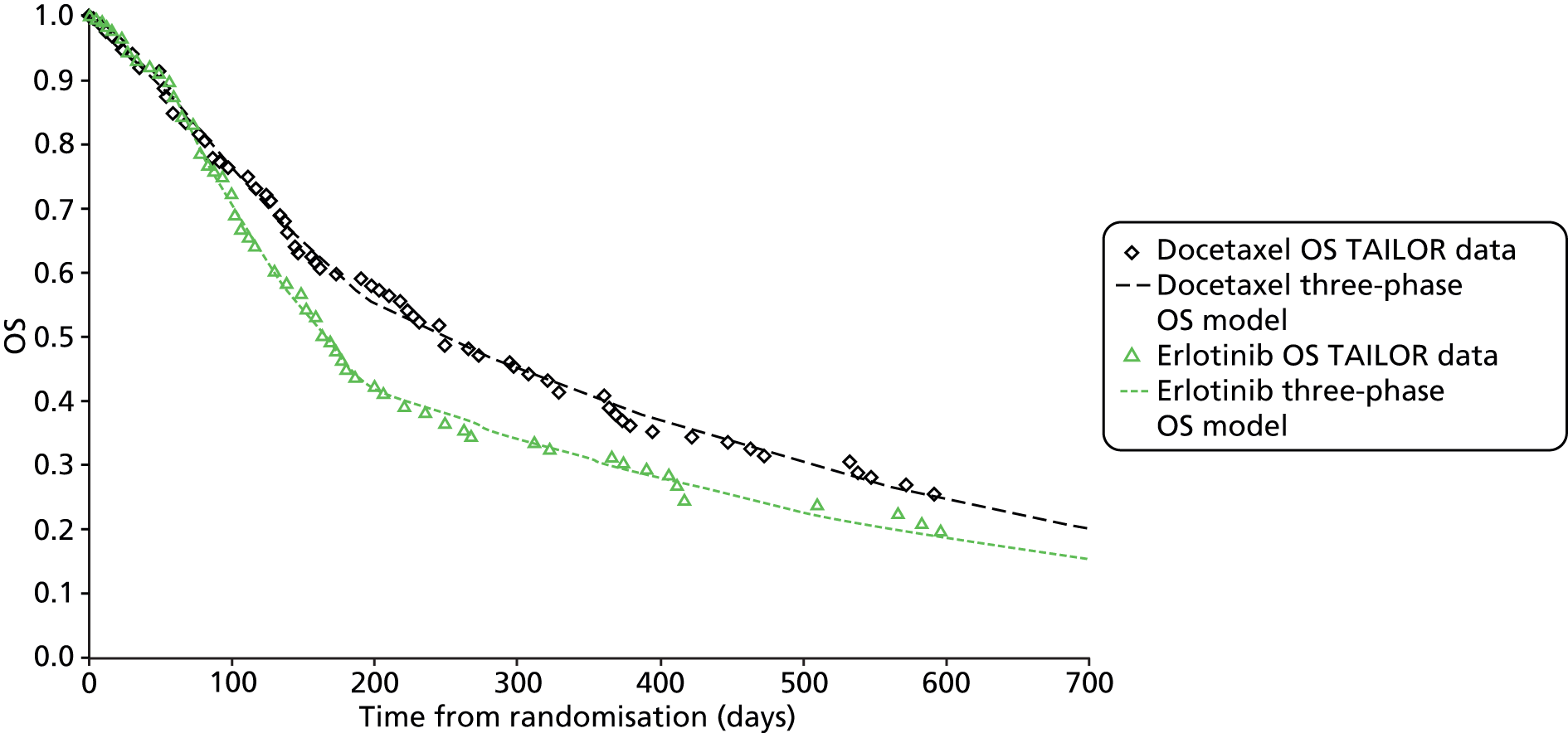
Epidermal growth factor mutation status unknown population
Clinical effectiveness data for this patient group are restricted to the BR.2131 trial. The manufacturer’s model included detailed Kaplan–Meier analysis data, which provided the source data for projecting the full cohort experience until death. Both PFS and OS curves exhibited similar forms to those observed in TAILOR. 41 Therefore, a similar three-phase spline model (with two ‘knot’ points) was employed for analysis of the BR.2131 data. The transitions between phases (‘knot’ points) in the two trial arms occur at different points between the first two phases but at a common time point between phases 2 and 3. The event risk within each phase was found to conform closely to a constant (equivalent to an exponential survival function) in both treatment arms. In both OS and PFS models the long-term event risk (phase 3) exhibits the same hazard rate in both arms of the trial.
In these circumstances a simplified model formulation could be focused on the final long-term period (phase 3), recognising that accurate Kaplan–Meier data are available into the final period and should be applied directly, which limits the need for projection of missing data to a short final period. A single exponential long-term model was calibrated for a single hazard parameter, and separate constant parameters for each treatment arm which together correspond to the separation between the survival curves at the second ‘knot’ point (296 days).
Figures 9 and 10 show the correspondence between the trial data and the late-stage projective models. These calibrated models were only used to project PFS and OS during and beyond the third phase to maximise the use of the unadjusted trial data. In all cases projection was commenced at the same value of the estimated remaining PFS or OS to avoid introducing bias from projecting different proportions of patient experience subject to different degrees of modelling error. For PFS projection began at the point when 5% of patients were estimated to be event-free and for OS at 25%. Details of the model parameters, estimates and standard errors are provided in Appendix 7.
Synthesis of outcome data: RRs to second-line chemotherapy
The Nafees et al. 75 multivariate utility model (which is used in the AG model) includes two levels of response to therapy as predictive variables: ‘responder’ (either complete or partial response) and ‘stable disease’ (neither response nor disease progression). Estimates for these variables were obtained by pooling reported responses described in published clinical trials relevant to each population: 15 trials31,34,37–39,42,49,54,74,85–91 involving patients undifferentiated by mutation status and only one trial each for the EGFR M+ population32 and the EGFR M– population. 41 The Kim et al. trial32 included 35% of patients with confirmed EGFR M+ status and also patients with a high probability of EGFR-activating mutations on the basis of other patient characteristics. The parameter values obtained are shown in Table 40.
| Patient population and treatment | Responders (%) | Stable disease (%) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| EGFR M+ population | ||||
| Erlotinib | 39.6 | 26.4 to 53.6 | 27.1 | 15.6 to 40.4 |
| Gefitinib | 47.9 | 34.1 to 61.9 | 30.2 | 27.8 to 32.6 |
| EGFR M– population | ||||
| Docetaxel | 15.5 | 9.0 to 23.3 | 28.9 | 20.3 to 38.2 |
| Erlotinib | 3.0 | 0.6 to 7.1 | 23.0 | 15.3 to 31.7 |
| EGFR unknown population | ||||
| BSC/placebo | 1.2 | 0.5 to 2.1 | 30.8 | 26.8 to 35.0 |
| Docetaxel | 8.5 | 7.2 to 9.9 | 36.2 | 33.1 to 39.3 |
| Erlotinib | 8.7 | 6.8 to 10.7 | 29.8 | 26.6 to 33.0 |
Synthesis of outcome data: adverse events
The costs and disutilities of treatment-related AEs are limited in the model to seven major categories, (using the results of a multivariate model by Nafees et al. 75 described in detail in Health valuation estimation): diarrhoea, fatigue, FN, hair loss, nausea/vomiting, neutropenia and skin rash.
The reported incidences of grade 3 and 4 AEs in all published second-line chemotherapy trials were pooled to obtain estimates of the proportion of patients suffering each event during treatment. No attempt was made to carry out a more sophisticated meta-analysis as reporting of AEs was often incomplete and lacking consistency. Table 41 details the incidence rates obtained for each second-line chemotherapy agent.
| AE incidence rates | Diarrhoea | Fatigue | FN | Hair loss | Nausea/vomiting | Neutropenia | Skin rash |
|---|---|---|---|---|---|---|---|
| BSC/placebo | |||||||
| Mean (%) | 0.7 | 11.0 | 0.0 | 0.0 | 1.8 | 0.0 | 0.1 |
| 95% CI | 0.3 to 1.4 | 9.0 to 13.1 | 0.0 to 0.2 | 0.0 to 0.2 | 1.1 to 2.8 | 0.0 to 0.2 | 0.0 to 0.4 |
| Docetaxel | |||||||
| Mean (%) | 2.1 | 7.4 | 7.6 | 1.1 | 2.9 | 46.7 | 0.5 |
| 95% CI | 1.5 to 2.9 | 6.2 to 8.6 | 6.4 to 8.8 | 0.6 to 1.6 | 2.1 to 3.7 | 44.4 to 48.9 | 0.3 to 0.9 |
| Gefitinib | |||||||
| Mean (%) | 2.3 | 2.9 | 0.4 | 0.0 | 1.6 | 1.4 | 1.7 |
| 95% CI | 1.7 to 2.9 | 2.3 to 3.6 | 0.2 to 0.7 | 0.0 to 0.1 | 1.1 to 2.1 | 1.0 to 1.9 | 1.2 to 2.3 |
| Erlotinib | |||||||
| Mean (%) | 3.7 | 9.9 | 0.0 | 0.0 | 3.4 | 0.0 | 8.1 |
| 95% CI | 2.6 to 4.9 | 8.1 to 11.8 | 0.0 to 0.2 | 0.0 to 0.2 | 2.4 to 4.6 | 0.0 to 0.2 | 6.5 to 9.9 |
These values were used to model treatments in the EGFR M+ population (where no relevant clinical trial has been undertaken) and in the EGFR unknown population. For the EGFR M– population, the AE incidence rates reported in TAILOR41 have been used directly, as shown in Table 42.
| Treatment arm | Diarrhoea | Fatigue | FN | Hair loss | Nausea/vomiting | Neutropenia | Skin rash |
|---|---|---|---|---|---|---|---|
| Docetaxel | |||||||
| Mean (%) | 1.9 | 9.6 | 6.35a | 14.4 | 2.9 | 20.2 | 0.0 |
| 95% CI | 0.2 to 5.3 | 4.8 to 15.9 | 1.8 to 13.5 | 8.4 to 21.8 | 0.6 to 6.8 | 13.1 to 28.4 | 0.0 to 2.4 |
| Erlotinib | |||||||
| Mean (%) | 2.8 | 5.6 | 0.0 | 0.0 | 0.9 | 0.0 | 14.0 |
| 95% CI | 0.6 to 6.7 | 2.1 to 10.7 | 0.0 to 2.4 | 0.0 to 2.4 | 0.0 to 3.4 | 0.0 to 2.4 | 8.1 to 21.2 |
Active treatment cost estimation
Second-line active treatment doses for docetaxel were calculated individually on the basis of the patient’s body surface area. Calculations are carried out separately for males and females, and a weighted average cost is obtained using the relative proportions of recorded deaths91 from malignant neoplasm of trachea, bronchus and lung in England and Wales in 2012 (55.2% males, 44.8% females).
Two sources are available as options to provide unit costs relating to the purchase of drugs: the list prices of erlotinib, gefitinib, docetaxel (generic) and dexamethasone shown in the BNF29 (July 2013), and the prices reported in eMit83 produced by the Commercial Medicines Unit of the Department of Health for docetaxel and dexamethasone. The eMit provides estimated mean product prices for generic medicines drawn from information from about 95% of NHS trusts. For both erlotinib and gefitinib, patient access schemes prices have been agreed with the Department of Health and are shown in Table 43, which summarises the unit cost data employed in the estimation of chemotherapy acquisition costs.
| Product | Vial content | BNF price29 (£) | eMit price84 (£) |
|---|---|---|---|
| Mean | Mean | ||
| Docetaxela | 20 mg | 138.33 | 7.93 |
| 80 mg | 454.53 | 32.40 | |
| 140 mg | 900.00 | 39.13 | |
| Gefitinibb | Per patient | 12,200 | 12,200 |
| Erlotinib | 30 × 150 mg | 1631.53 | 1631.53 |
| NHS discount | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
| Dexamethasonea | 50 × 2 mg | 6.96 | 1.80 |
Docetaxel costs are estimated per 21-day cycle (including the costs of required co-medication). The oral medications (erlotinib and gefitinib) are costed on the basis of whole-pack costs incurred whenever previous supplies are exhausted. As part-used packs cannot be reused when treatment is discontinued, some wastage is unavoidable. The AG’s base-case analysis is carried out using the eMit83 prices for docetaxel and co-medication, with BNF29 prices used in a sensitivity analysis. Where a discounted price for a patented drug is available across the whole NHS, the appropriate discount is applied in all analyses. The estimated drug cost per cycle to the NHS of each second-line treatment is shown in Table 44.
| Second-line treatment | Estimated cost – BNF 66 prices29 (£) | Estimated cost – eMit prices84 (£) | ||
|---|---|---|---|---|
| Per cycle | Per patient | Per cycle | Per patient | |
| Docetaxel | 922.81a | N/A | 44.88a | N/A |
| Erlotinib | Commercial-in-confidence information has been removedb | N/A | Commercial-in-confidence information has been removedb | N/A |
| Gefitinib | N/A | 12,200 | N/A | 12,200 |
It is assumed that treatment continues until disease progression or death. Time-to-off-treatment data for erlotinib from the BR.2131 trial were analysed and compared with PFS data but were not found to be statistically significantly different.
The unit costs employed for chemotherapy administration, based on NHS Reference Costs 2011/12,82 are shown in Table 45. On clinical advice, docetaxel is assumed always to be administered in a day-case setting and oral medication packs are issued as part of a nurse-led outpatient visit.
| Treatment setting | HRG code | Description | Mean (£) | Standard errora (£) |
|---|---|---|---|---|
| Day-case unit | SB12Z | Simple parenteral chemotherapy at first attendance | 203.16 | 7.47 |
| Day-case unit | SB15Z | Subsequent doses of chemotherapy | 283.89 | 10.14 |
| Outpatient visit | NCLFUSFF 370 | Medical oncology | 106.00 | 10.60a |
Health state cost estimation
Costs have been estimated relating to patient monitoring and supportive care in three health states: in PFS (either during or following second-line treatment), post progression when no active treatment is received and for terminal care (assumed to last, on average, for 14 days).
In PFS patients are expected to receive regular consultant-led outpatient consultations and periodic diagnostic tests (chest radiography, computed tomography and electrocardiogram). During PPS patients are assumed to have been discharged to community-based supportive care where care is provided by the patient’s general practitioner (in surgery or at home) and community nursing staff. In the terminal phase, care is likely to be more intensive, with the package varying by the chosen setting.
Table 46 details the mean volumes of each resource assumed and Table 47 summarises the unit costs used with the relevant sources. More detailed information describing cost assumptions is presented in the publication by Brown et al. 2
| Resource | PFS | PPS | Terminal care | Source |
|---|---|---|---|---|
| Outpatient visit | 9.61 pa | – | – | Big Lung Trial92 |
| Chest radiography | 6.79 pa | – | – | Big Lung Trial92 |
| CT (chest) | 0.62 pa | – | – | Big Lung Trial92 |
| CT (other) | 0.36 pa | – | – | Big Lung Trial92 |
| ECG | 1.04 pa | – | – | Big Lung Trial92 |
| Hospital/hospice episode | – | – | 8.93 days | Average stay for non-elective long-stay IP episode plus average IP excess days for HRG DZ17 A – NHS Reference Costs 2011/1282 |
| Community nurse visit | 26 visits (20 minutes) pa | 52 visits (20 minutes) pa | 28 hours (2 hours per day) | Appendix 1 of NICE Guideline CG81,93 Marie Curie report94 |
| Clinical nurse specialist | 12 hours contact time pa | 52 hours contact time pa | – | Appendix 1 of NICE Guideline CG8193 |
| GP surgery | 12 consultations pa | – | – | Appendix 1 of NICE Guideline CG8193 |
| GP home visit | – | 26 visits pa | 7 visits (alternate days) | Marie Curie report94 |
| Therapist visit | – | 26 hours pa | – | Appendix 1 of NICE Guideline CG8193 |
| Macmillan nurse | – | – | 50 hours | Marie Curie report94 |
| Drugs/equipment | – | – | As required | Marie Curie report94 |
| Location of terminal care | – | – | Hospital 55.8%, hospice 16.9%, home 27.3% | Office for National Statistics death registration summary tables 5.2 and 1291 |
| Resource | Unit cost (£) | Source |
|---|---|---|
| Outpatient follow-up visit | £113.17 | NHS Reference Costs 2011–12, HRG code CLFUSFF 800 – clinical oncology82 |
| Chest radiography | £30.26 | NHS Reference Costs 2011/12, code DAPF – direct access plain film82 |
| CT (chest) | £124.99 | NHS Reference Costs 2011–12, HRG code RA12Z (2 areas with contrast)82 |
| CT (other) | £134.57 | NHS Reference Costs 2011–12, HRG code RA13Z (3 areas with contrast)82 |
| ECG | £60.73 | NHS Reference Costs 2011/12, code EA47Z – direct access ECG82 |
| Community nurse | £70.00 per hour | PSSRU Unit Costs of Health and Social Care 2012, page 175, cost per hour spent on home visits (including qualification)84 |
| Clinical nurse specialist | £91.00 per contact hour | PSSRU Unit Costs of Health and Social Care 2012, page 181, cost per contact hour (including qualification)84 |
| GP surgery visit | £43.00 | PSSRU Unit Costs of Health and Social Care 2012, page 183, cost per surgery visit (11.7 minutes, including direct care staff)84 |
| GP home visit | £110.00 | PSSRU Unit Costs of Health and Social Care 2012, page 183, cost per home visit (23.4 minutes, including travel time)84 |
| Therapist | £44.00 | PSSRU Unit Costs of Health and Social Care 2012, page 194, cost per hour (including training)84 |
| Terminal care inpatient care | £2716.53 + 0.84 excess days at £232.90 per day | NHS Reference Costs 2011/12, code DZ17 A (respiratory neoplasms with major complicating conditions) non-elective inpatient (long stay – episode/excess days)83 |
| Terminal care in hospice | 25% increase on hospital inpatient care | Assumption |
| Macmillan nurse | 66.7% of community nurse cost | Assumption |
| Drugs and equipment | £500 | Marie Curie report,94 figure of £240 increased for inflation |
Adverse event cost estimation
The costs of treating grade 3 and 4 AEs of second-line therapy are spread over 12 weeks (four cycles) and estimated using NHS Reference Costs for 2011/12,82 as follows:
Diarrhoea
It is assumed that a typical patient will have two hospital admissions during second-line treatment, corresponding to health research group (HRG) code FZ48C (malignant general abdominal disorders of length of stay 1 day or less) as a non-elective short-stay episode, each costing £525.38.
Fatigue
It is assumed that a typical patient will have one hospital admission during second-line treatment, corresponding to HRG code WA17X (other admissions related to neoplasms with intermediate complicating conditions) as a non-elective long-stay episode of 5 to 7 days costing £2233.40.
Febrile neutropenia
The NICE Decision Support Unit report on the cost of FN95 has been updated for current NHS Reference Costs. 83 This assumes 1.4 episodes per patient during the second-line treatment. The estimated cost per patient is £7066.63.
Hair loss
It is assumed that there are no hospital episodes related to this AE and no direct costs are incurred.
Nausea/vomiting
It is assumed that a typical patient will have two hospital admissions during second-line treatment, corresponding to HRG code FZ48C (malignant general abdominal disorders of length of stay 1 day or less) as a non-elective short-stay episode, each costing £525.38.
Neutropenia (non-febrile)
It is assumed that 10% of patients will experience two episodes of neutropenia requiring hospital treatment during second-line treatment. The cost per episode is £866.61 and is estimated from the weighted average of mean costs for HRG codes WA02W (disorders of immunity without human immunodeficiency virus/acquired immune deficiency syndrome with complicating condition) and PA48A (blood cell disorders with complicating condition) across non-elective long and short-stay episodes, and day-case admissions.
Skin rash
It is assumed that a typical patient will have one additional outpatient consultation for this condition during second-line treatment. A weighted-average NHS reference cost of £109.77 is used, based on codes 370 (Medical oncology) and 800 (Clinical oncology), for both consultant-led and non-consultant-led visits.
Health valuation estimation
Ideally, the utility of patients with NSCLC should be informed by data obtained directly from the relevant patient population relating to their perceived condition at all phases of the treatment pathway covered by the economic model. Unfortunately, this is practically and ethically impractical for patients suffering advanced disease with severe symptoms (arising from either the natural course of the disease or related to treatments received) and who have generally very limited remaining life expectancy. Few clinical trials have attempted to collect patient health utility data, and RRs are generally poor as few patients continue to complete questionnaires as their condition worsens. We identified, via a comprehensive literature search, very few studies describing relevant utility data for use in our model.
An observation study conducted in the Netherlands96 between 1999 and 2002 attempted to obtain such data (using the EQ-5D instrument) from patients with NSCLC treated between 2004 and 2007 and surviving to 2008. Unfortunately, this patient sample is not representative of the populations considered in the AG’s model (patients with locally advanced and/or metastatic NSCLC), since only 44% of patients had received any chemotherapy, only 41% had stage III/IV disease and only 14% had local/regional or metastatic recurrent disease at the time of the survey. Clearly the results of the observation study are dominated by patients who were diagnosed at an early stage and had successful surgery, thus potentially biasing numeric estimates of utility towards higher values.
One clinical trial with relevant data compared two radiotherapy regimens for poor-prognosis patients with NSCLC in 13 Dutch radiotherapy centres. 97 Patients completed EQ-5D questionnaires initially weekly, and then 2-weekly until death, enabling EQ-5D utility scores to be estimated. Responses were obtained on 83% of occasions, allowing the temporal trend in patient utility to be characterised. Some data from the published results have been used in the AG’s model.
The only alternative to direct measurement of patient symptoms for estimating utility is via a structured sample of the general public valuing a set of typical patient scenarios, representing the range of likely conditions experienced by patients with NSCLC during their remaining lifetime. Two such recent studies have been identified. Doyle et al. 98 recruited 101 volunteers from the general public in the London area, who were asked to value six typical health states experienced by advanced NSCLC patients, using the standard gamble method. This allowed estimation of a mean utility value for patients with stable disease on treatment, as well as the incremental effect of response to treatment and also the incremental disutility of three common symptoms (cough, dyspnoea and pain). Although promising, this study provides only limited results which are insufficient to populate all the health states and important AEs which are required to populate the current model.
The utility scheme which has been adopted for use in the AG’s model is that described in a paper published in 2008 by Nafees et al. 75 This also uses the standard gamble method and employed 100 volunteers from the UK general population. In this case a more extensive set of scenarios were used (17 specific disease health states plus two anchor states), developed with the help of a panel of oncologists and designed specifically to address a range of the most common severe AEs experienced by advanced NSCLC patients undergoing second-line treatment for metastatic cancer. A mixed-model analysis yielded simultaneous utility estimates for three health states (responding to treatment, stable disease and progressive disease) together with incremental disutility values for seven common serious grade 3 and 4 AEs – diarrhoea, fatigue, FN, hair loss (alopecia), nausea/vomiting, neutropenia and rash. The range of AEs in the Nafees et al. 75 model is sufficient to cover all the major problems experienced with current treatments.
Applying the treatment-specific AE incidence rates (see Tables 41 and 42) and treatment RRs (see Table 40) to the Nafees et al. 75 utility model yields a full set of health-state utilities for each treatment option as shown in Table 48. The utility for the terminal period (last 2 weeks of life) was obtained by use of results reported for average EQ-5D scores relative to the time prior to death (figure 3 in the van den Hout et al. 2006 study97 of palliative radiotherapy in patients with NSCLC).
| Second-line therapy | PFS | PPS (> 2 weeks prior to death) | Terminal period (2 weeks) |
|---|---|---|---|
| EGFR M– population (TAILOR trial41) | |||
| Docetaxel | 0.6225 | 0.4734 | 0.2488 |
| Erlotinib | 0.6450 | 0.4734 | 0.2488 |
| EGFR M– population (wild-type subgroup of BR.21 trial31) and EGFR unknown population (BR.21 trial31) | |||
| Erlotinib | 0.6351 | 0.4734 | 0.2488 |
| BSC | 0.6353 | 0.4734 | 0.2488 |
Modelling assumptions
Following disease progression it is assumed that subsequent experience of health care (and associated health and social costs) and QoL is broadly equivalent for all patients and are independent of previous treatments received.
No explicit disutility adjustment is included to reflect differences in patient preferences and experience of i.v. therapy versus oral therapy versus BSC, beyond that implicit in differences in AE incidence rates.
Sensitivity analysis
For each modelled scenario, univariate sensitivity analysis was performed for all model parameters using lower and upper CIs, and these are reported in the form of a torpedo diagram indicating the 20 variables most influential on the size of the deterministic ICER. In addition, a PSA was carried out and through a probabilistic ICER, a scatterplot of replication incremental costs and QALYs and cost-effectiveness acceptability curves.
Beta distributions are employed in both univariate sensitivity analyses and PSA for parameters involving proportions (RRs, AE rates, sex mix, place of death and proportion of PFS which are fatal). For all other parameters, normal distributions are used.
The manufacturer of erlotinib proposed in their submission an exploratory analysis comparing erlotinib with BSC in a subgroup43 of BR.2131 trial patients. The AG has, therefore, applied data for this subgroup to their model as a further sensitivity analysis.
Results
Epidermal growth factor mutation-positive population
In the absence of any relevant clinical trial evidence in this population there is no reliable basis on which to assess the cost-effectiveness of available treatments.
The AG has considered carefully the evidence submitted by the manufacturer of gefitinib, but it concludes that the information made available to the AG in the manufacturer’s submission does not allow any formal decision modelling to be undertaken. This is because, at the very least, compatible PFS data and treatment RRs would be required in addition to OS estimates to allow a decision model to be populated.
Epidermal growth factor mutation-negative population
Erlotinib versus docetaxel
Deterministic results from the main EGFR M– model based on data from TAILOR41 are summarised in Table 49. It should be noted that the rate of FN and the estimation of its costs were a key point of debate during the assessment process (see Appendix 8). Several iterations of the cost-effectiveness results for the EGFR M– patient population were produced in response to particular needs; the results presented in Table 49 are those presented at the NICE final AC meeting. The calculations are based on a rate of FN of 6.35%. This figure is derived from the subgroup of patients in TAILOR41 who received 3-weekly treatments of docetaxel (the treatment regime used in clinical practice in England and Wales).
| Cost-effectiveness result | Docetaxel | Erlotinib | Incremental | |||
|---|---|---|---|---|---|---|
| Survival (mean) | Years | Months | Years | Months | Years | Months |
| PFS | 0.409 | 4.91 | 0.287 | 3.45 | 0.122 | 1.46 |
| PPS | 0.731 | 8.77 | 0.641 | 7.70 | 0.089 | 1.07 |
| Terminal | 0.038 | 0.46 | 0.038 | 0.46 | 0.000 | 0.00 |
| OS | 1.178 | 14.13 | 0.967 | 11.60 | 0.211 | 2.53 |
| QALYs | Undiscounted | Discounted | Undiscounted | Discounted | Undiscounted | Discounted |
| PFS | 0.2537 | 0.2526 | 0.1853 | 0.1850 | 0.0684 | 0.0676 |
| PPS | 0.3459 | 0.3311 | 0.3036 | 0.2920 | 0.0423 | 0.0392 |
| Terminal | 0.0095 | 0.0092 | 0.0095 | 0.0093 | 0.0000 | 0.0001 |
| OS | 0.6091 | 0.5930 | 0.4984 | 0.4863 | –0.1107 | –0.1067 |
| Costs | Undiscounted | Discounted | Undiscounted | Discounted | Undiscounted | Discounted |
| 2L Tx acquisition | £342 | £340 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| 2L administration | £2314 | £2305 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| 2L Tx AEs | £585 | £585 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| PFS BSC | £1531 | £1524 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| PPS BSC | £5148 | £4928 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Terminal care | £3917 | £3820 | – | – | – | – |
| Total costs | £13,837 | £13,504 | £14,302.08 | £14,049.00 | £465 | £545 |
| ICER | ||||||
| Cost per QALY gained | –£5112 (discounted) for erlotinib vs. docetaxel (dominated) | |||||
| Net benefit | ||||||
| £ per patient (£30,000 per QALY) | –£3746 per patient for erlotinib vs. docetaxel (dominated) | |||||
Erlotinib is dominated by docetaxel in the EGFR M– population, yielding a reduced mean survival and fewer QALYs while also involving a greater net cost of treatment. Univariate sensitivity analysis (Figure 11) for the deterministic base case indicates that the use of generic docetaxel in place of the branded product is the major factor in establishing docetaxel as the preferred option. The incidence rate of FN has a larger influence on the estimated ICER than other model parameters, but for none of model parameters is the known parameter uncertainty sufficient to alter the conclusion that erlotinib is dominated by docetaxel in the EGFR M– population. The only model input which could alter this conclusion is the incidence rate of FN in docetaxel-treated patients; this is considered in Appendix 8.
FIGURE 11.
Univariate sensitivity analysis: erlotinib vs. docetaxel second-line treatment in the EGFR M– population from TAILOR – 20 most influential parameters. DC, day case; Doc, docetaxel; Erl, erlotinib; GP, general practitioner.

A PSA (Figure 12) and the cost-effectiveness acceptability curve (Figure 13) yield a similar result: an estimated ICER of –£7709 per QALY gained, indicating that for any cost-effectiveness threshold greater than £0 per QALY there is a probability greater than 99% that erlotinib is less cost-effective than docetaxel.
FIGURE 12.
Probabilistic sensitivity analysis: scatterplot of the base-case analysis for erlotinib vs. docetaxel second-line treatment in the EGFR M– population using evidence from TAILOR. 41

FIGURE 13.
Cost-effectiveness acceptability curves for the comparison of erlotinib vs. docetaxel second-line treatment in the EGFR M– population using evidence from TAILOR. 41

Erlotinib versus best supportive care
The manufacturer of erlotinib submitted a sensitivity analysis of its main economic analysis of the population of unknown EGFR status (see next section), using survival data from a post-hoc reanalysis42 of the results of the BR.2131 trial. This analysis restricts attention to those patients who were confirmed not to have EGFR-activating mutations, that is only EGFR M– (or EGFR wild-type) disease. Inevitably the source data43 are less reliable than the main ITT analysis of BR.2131 results because of the risk of imbalance in baseline patient characteristics and the reduced sample size.
In order to replicate this sensitivity analysis, the AG has carried out a similar exercise using the same outcome data applied to the AG model structure. Figures 14 and 15 show the trajectories fitted to the trial data to populate the decision model.
FIGURE 14.
Projective models fitted to PFS data from the EGFR M– subgroup of the BR.2131 trial. WT, wild type.
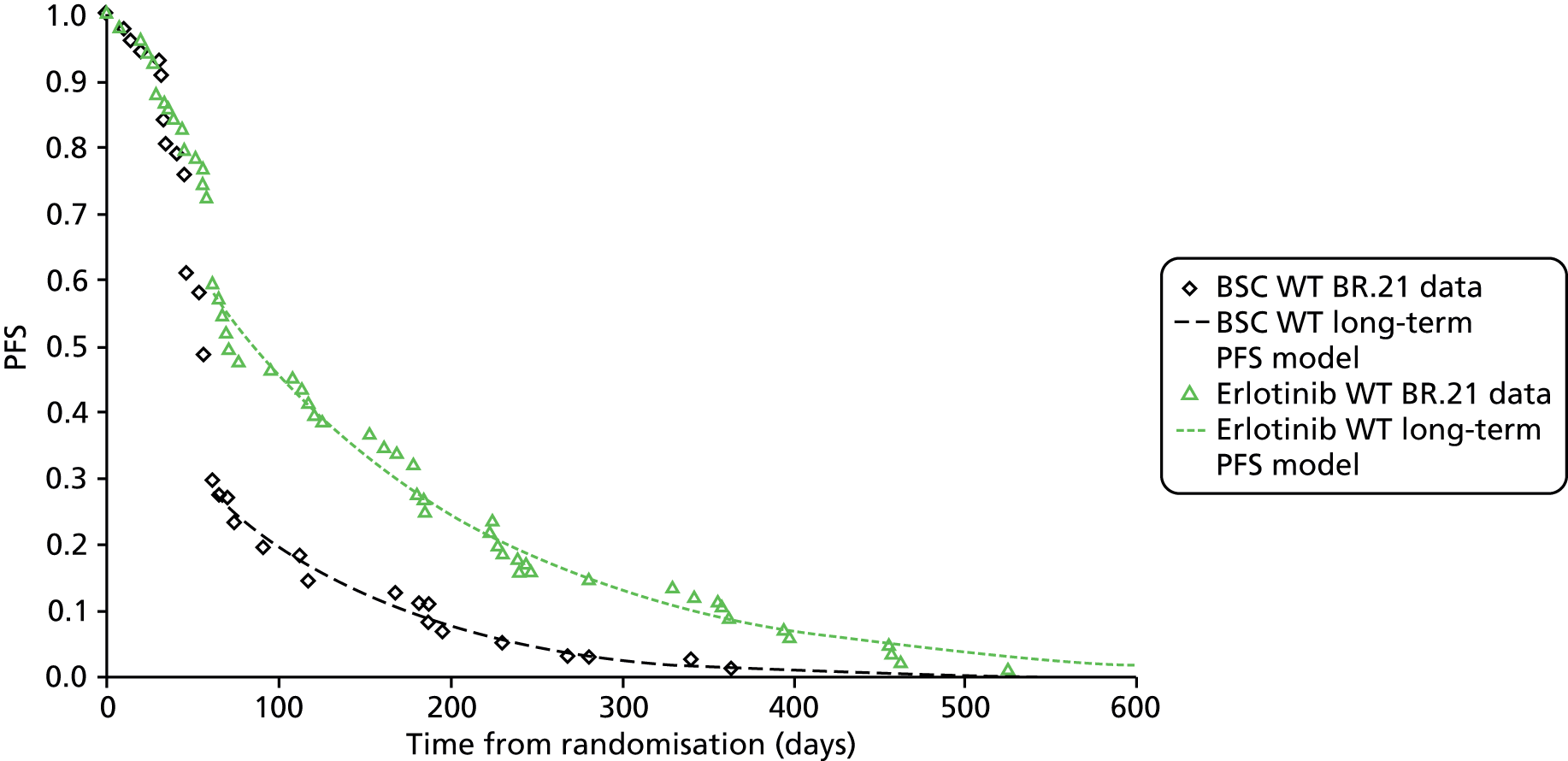
FIGURE 15.
Projective models fitted to OS data from the EGFR M– subgroup of the BR.2131 trial. WT, wild type.
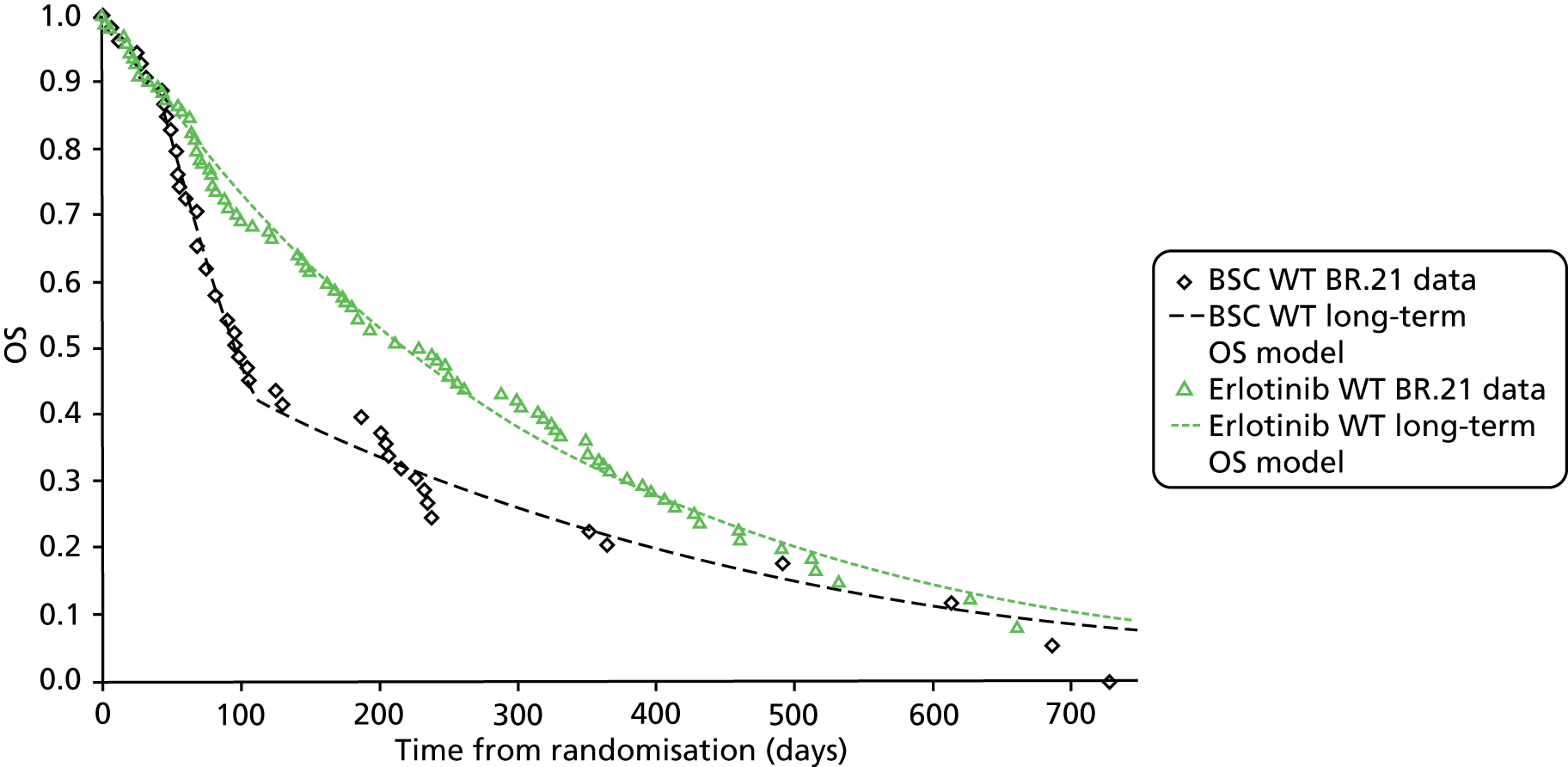
Deterministic results from the EGFR M– model based on subgroup EGFR M– data43 from the BR.2131 trial are summarised in Table 50. The estimated mean OS advantage of using erlotinib rather than BSC is 2.2 months, all of which occurs prior to disease progression. The corresponding gain in mean discounted QALYs is 0.116 per patient. The estimated ICER of £54,686.73 per QALY gained is above the range normally considered cost-effective. The results of univariate sensitivity analyses are summarised in Figure 16, indicating that these results are most affected by projective survival model parameters (especially for the OS model), utility model parameters and the incidence of key AEs.
| Cost-effectiveness result | BSC | Erlotinib | Incremental (erlotinib vs. BSC) | |||
|---|---|---|---|---|---|---|
| Survival (mean) | Years | Months | Years | Months | Years | Months |
| PFS | 0.223 | 2.670 | 0.407 | 4.884 | 0.184 | 2.213 |
| PPS | 0.416 | 4.987 | 0.415 | 4.976 | –0.001 | –0.011 |
| Terminal | 0.038 | 0.452 | 0.038 | 0.453 | 0.000 | 0.001 |
| OS | 0.676 | 8.109 | 0.859 | 10.313 | 0.184 | 2.204 |
| QALYs | Undiscounted | Discounted | Undiscounted | Discounted | Undiscounted | Discounted |
| PFS | 0.1414 | 0.1413 | 0.2585 | 0.2575 | 0.1171 | 0.1163 |
| PPS | 0.1967 | 0.1911 | 0.1963 | 0.1911 | –0.0005 | 0.0001 |
| Terminal | 0.0094 | 0.0093 | 0.0094 | 0.0093 | 0.0001 | 0.0000 |
| OS | 0.3475 | 0.3416 | 0.4641 | 0.4579 | 0.1167 | 0.1163 |
| Costs | Undiscounted | Discounted | Undiscounted | Discounted | Undiscounted | Discounted |
| 2L Tx acquisition | £0.00 | £0.00 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| 2L administration | £0.00 | £0.00 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| 2L Tx AEs | £533.70 | £533.31 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| PFS BSC | £827.93 | £827.33 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| PPS BSC | £2961.94 | £2878.02 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Terminal care | £3882.90 | £3836.73 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| ICER | ||||||
| Total costs | £8206.46 | £8075.39 | £14,596.93 | £14,436.92 | £6390.47 | £6361.53 |
| Cost per QALY gained | £54,686.73 for erlotinib vs. BSC | |||||
FIGURE 16.
Univariate sensitivity analysis: erlotinib vs. BSC second-line treatment in the EGFR M– population subgroup of the BR.2131 trial – 20 most influential parameters. Erl, erlotinib.
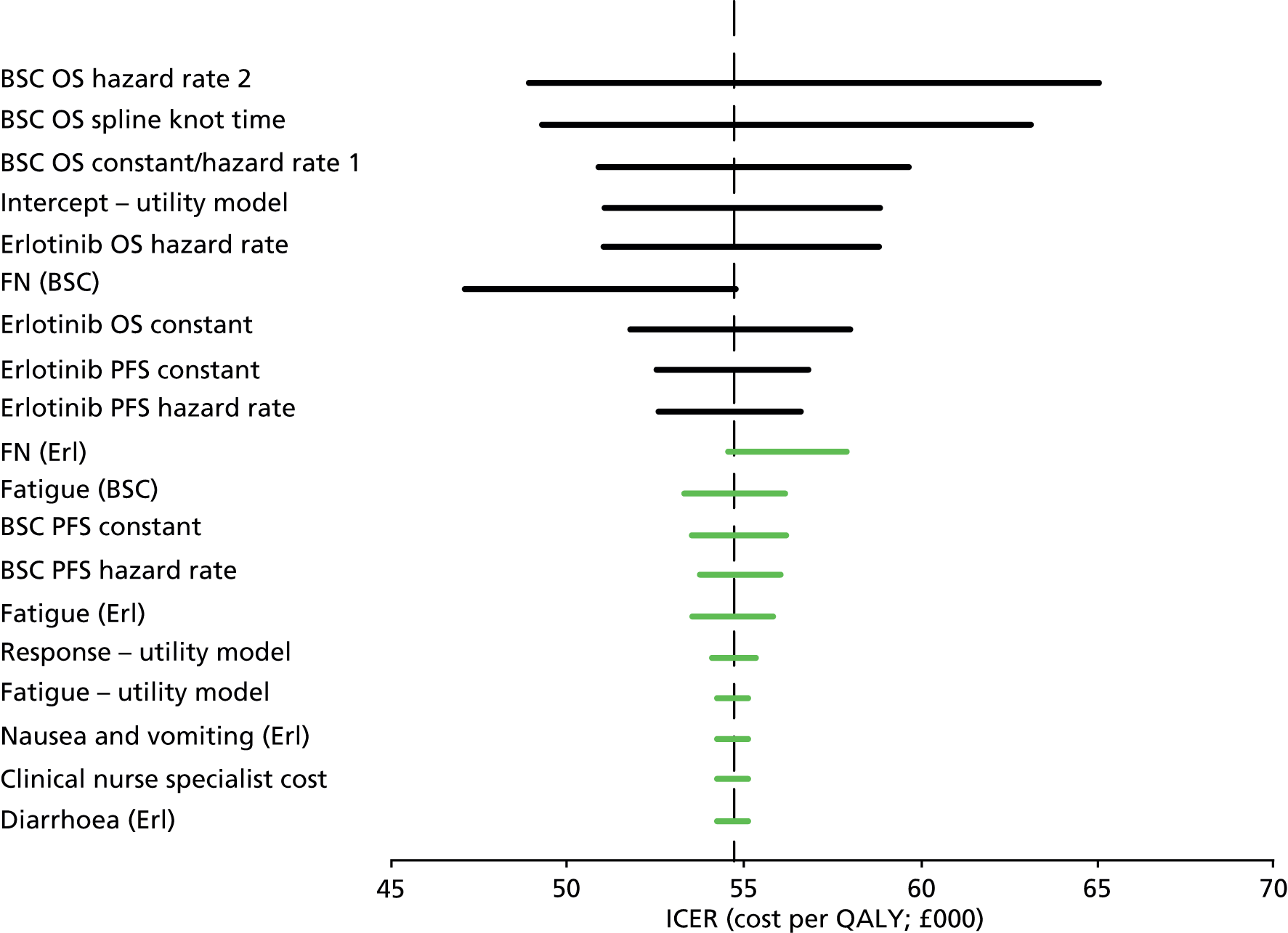
Probabilistic sensitivity analysis incorporating uncertainty in all model parameters indicates a slightly lower estimated ICER of £54,184 per QALY gained. Examination of the PSA scatterplot (Figure 17) and the cost-effectiveness acceptability curves (Figure 18) indicate strong general confidence that erlotinib exhibits a high ICER when compared with BSC in this subgroup (0% of simulations favour erlotinib at a willingness-to-pay threshold of £30,000 per QALY gained, and 12% at £50,000 per QALY gained).
FIGURE 17.
Probabilistic sensitivity analysis: scatterplot of the base-case cost-effectiveness analysis for erlotinib vs. BSC second-line treatment in the EGFR M– subgroup of the BR.2131 trial.

FIGURE 18.
Cost-effectiveness acceptability curves for the comparison of erlotinib vs. BSC second-line treatment in the EGFR M– subgroup from the BR.2131 trial.
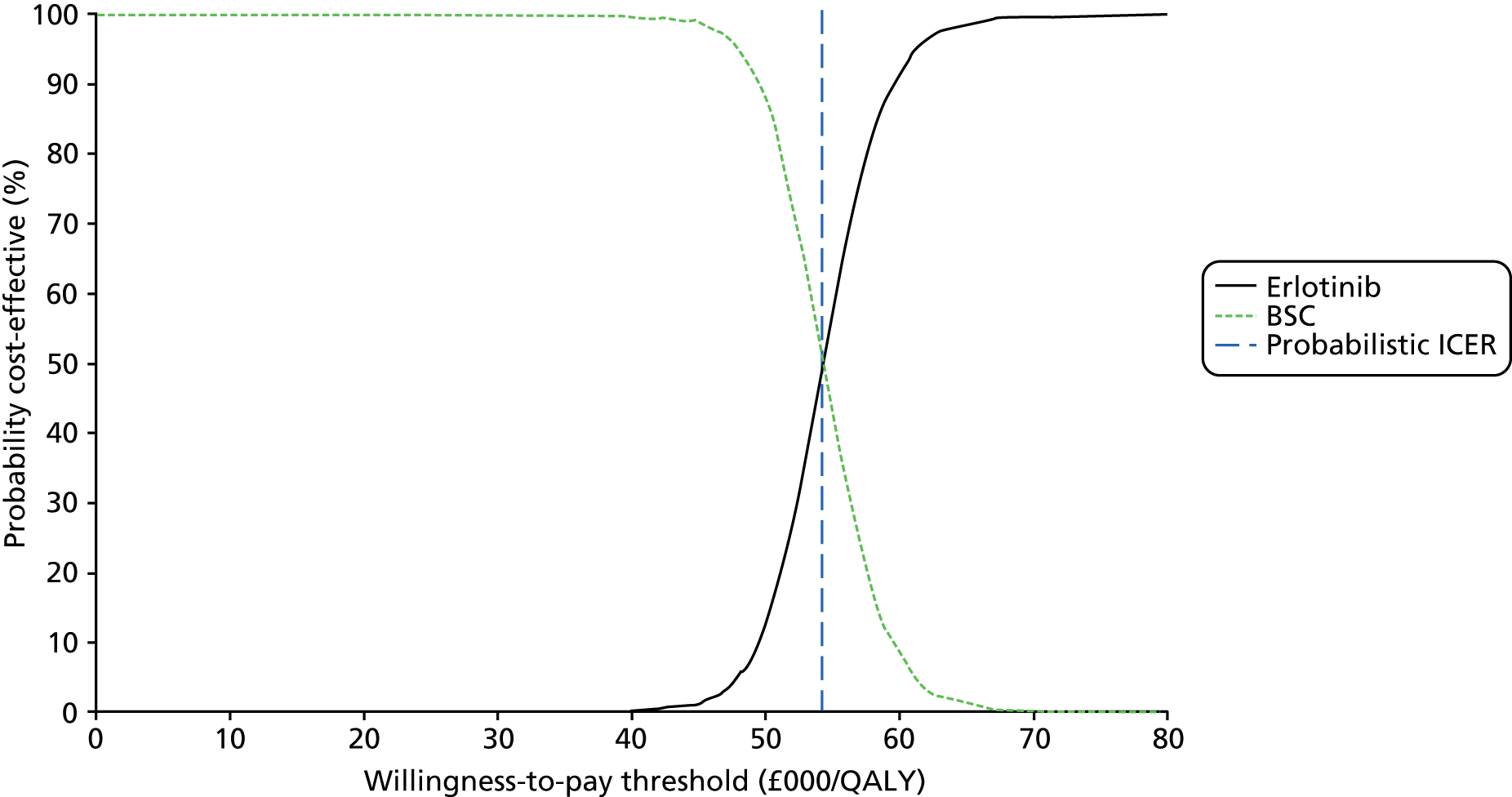
Epidermal growth factor mutation status unknown population
Deterministic results from the EGFR status unknown model based on data from the BR.2131 trial are summarised in Table 51. The estimated survival advantage of using erlotinib rather than BSC is 2.1 months, of which 1.7 months is prior to disease progression. The corresponding gain in mean discounted QALYs is 0.103 per patient. The overall incremental cost per patient is higher for erlotinib use (£6314 discounted), primarily because of the acquisition cost of erlotinib (£5677 discounted). The estimated ICER of £61,132 per QALY gained is well beyond the range normally considered cost-effective. The results of univariate sensitivity analyses are summarised in Figure 19, indicating that these results are unaffected by uncertainty in almost all model parameters. The only exceptions are the intercept parameter value in the Nafees et al. 75 utility model (i.e. the baseline NSCLC population utility value in patients with stable disease) and the incidence of FN when docetaxel is used.
| Cost-effectiveness result | BSC | Erlotinib | Incremental (erlotinib vs. BSC) | |||
|---|---|---|---|---|---|---|
| Survival (mean) | Years | Months | Years | Months | Years | Months |
| PFS | 0.235 | 2.815 | 0.374 | 4.490 | 0.140 | 1.675 |
| PPS | 0.403 | 4.831 | 0.439 | 5.267 | 0.036 | 0.435 |
| Terminal | 0.038 | 0.458 | 0.038 | 0.454 | 0.000 | –0.004 |
| OS | 0.675 | 8.104 | 0.851 | 10.211 | 0.176 | 2.106 |
| QALYs | Undiscounted | Discounted | Undiscounted | Discounted | Undiscounted | Discounted |
| PFS | 0.1490 | 0.1488 | 0.2376 | 0.2369 | 0.0886 | 0.0881 |
| PPS | 0.1906 | 0.1869 | 0.2077 | 0.2023 | 0.0171 | 0.0153 |
| Terminal | 0.0095 | 0.0094 | 0.0094 | 0.0093 | –0.0001 | –0.0001 |
| OS | 0.3491 | 0.3452 | 0.4548 | 0.4484 | 0.1057 | 0.1033 |
| Costs | Undiscounted | Discounted | Undiscounted | Discounted | Undiscounted | Discounted |
| 2L Tx acquisition | £0.00 | £0.00 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| 2L administration | £0.00 | £0.00 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| 2L Tx AEs | £562.53 | £561.77 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| PFS BSC | £873.25 | £872.06 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| PPS BSC | £2853.14 | £2798.84 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Terminal care | £3938.22 | £3900.12 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Total costs | £8227.15 | £8132.79 | £14,610.64 | £14,446.38 | £6383.49 | £6313.59 |
| ICER | ||||||
| Cost per QALY gained | £61,161.81 for erlotinib vs. BSC | |||||
FIGURE 19.
Univariate sensitivity analysis: erlotinib vs. BSC second-line treatment in the EGFR unknown subgroup of the BR.2131 trial – 20 most influential parameters. Erl, erlotinib.
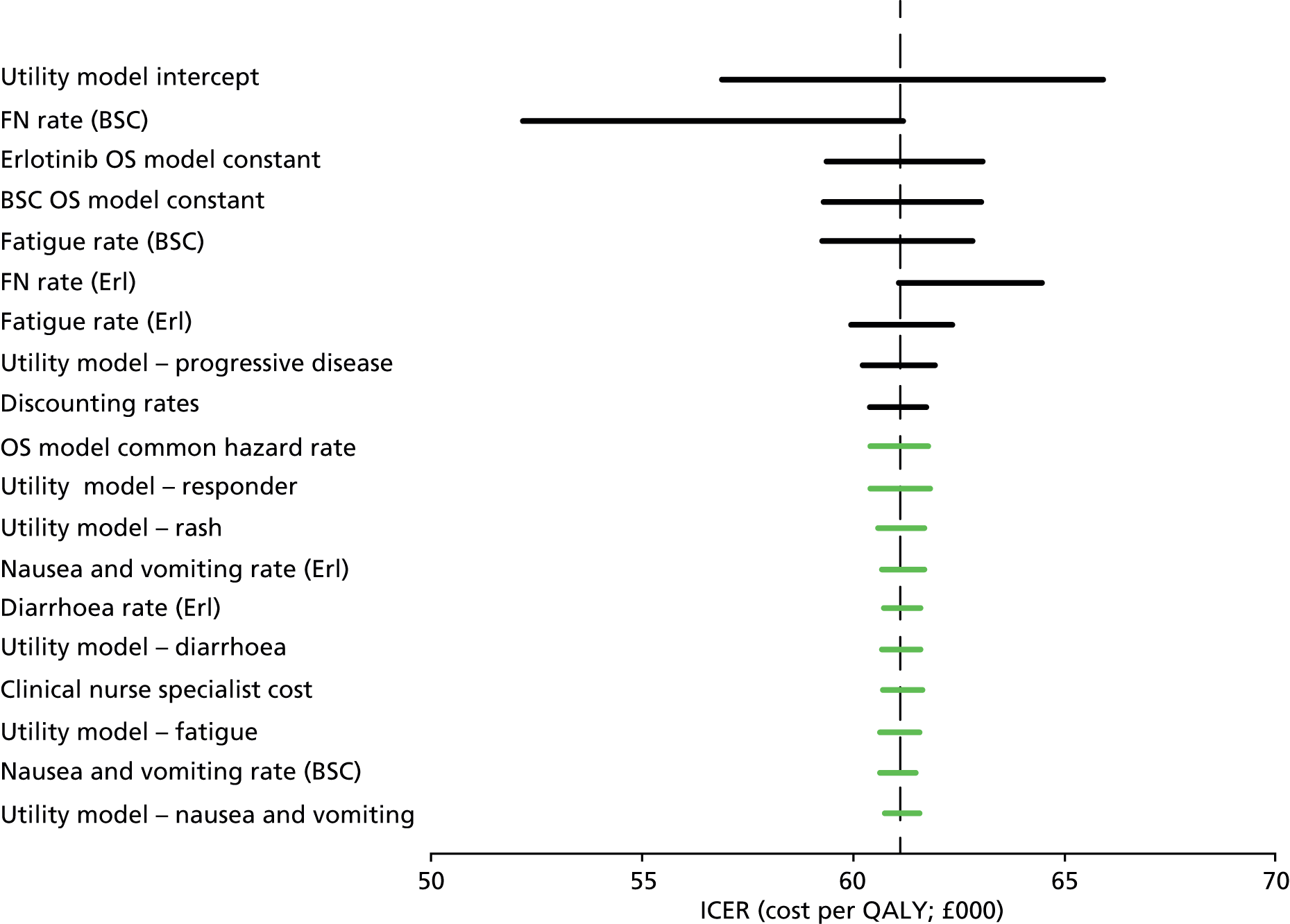
Probabilistic sensitivity analysis incorporating uncertainty in all model parameters indicates a slightly lower estimated ICER of £59,973 per QALY gained. Examination of the PSA scatterplot (Figure 20), and the cost-effectiveness acceptability curves (Figure 21) indicate strong general confidence that erlotinib is not more cost-effective than BSC in this population (0% of simulations favour erlotinib at a willingness-to-pay threshold of £30,000 per QALY gained).
FIGURE 20.
Probabilistic sensitivity analysis: scatterplot of the base-case cost-effectiveness analysis for erlotinib vs. BSC second-line treatment in the EGFR unknown population from the BR.2131 trial.
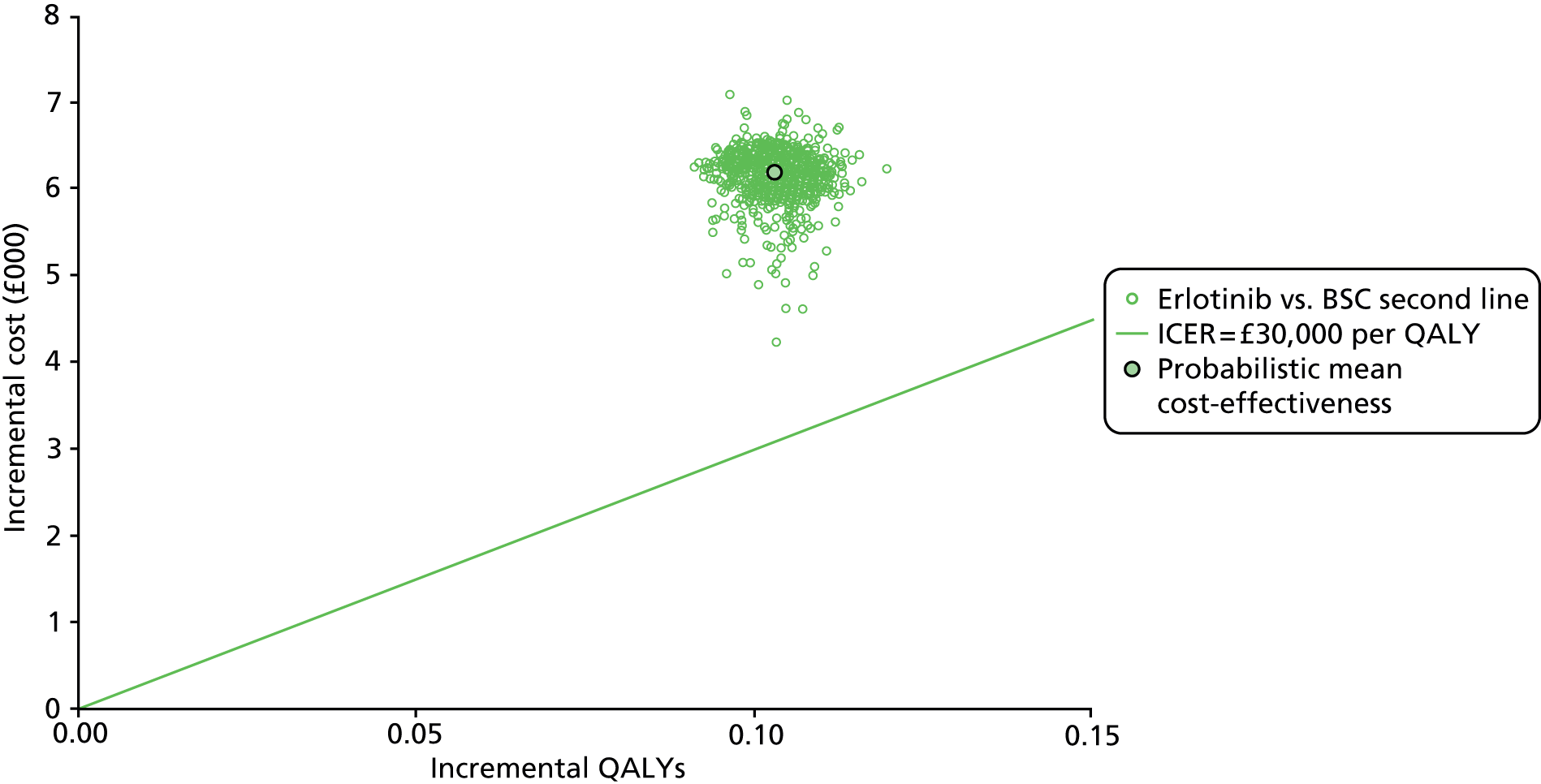
FIGURE 21.
Cost-effectiveness acceptability curves for the base case for erlotinib vs. BSC second-line treatment of NSCLC in the EGFR unknown population using results from the BR.2131 trial.
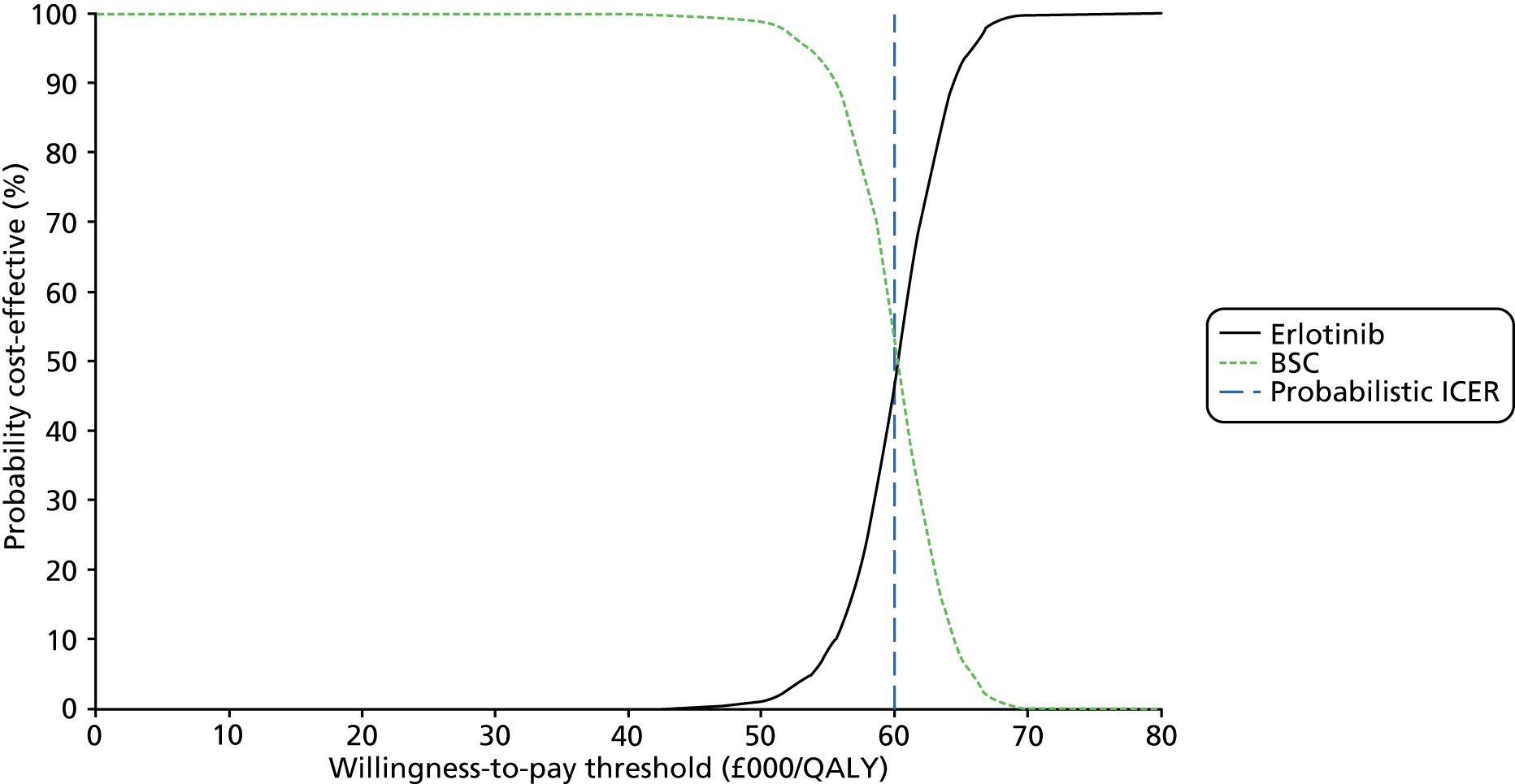
Summary and discussion of Assessment Group model results
The very weak evidence base for comparative second-line treatments, especially in subgroups defined by EGFR-TKI activating mutation status, has severely restricted the AG’s ability to assess the relative cost-effectiveness of all potential treatments and comparators indicated in appraisal scope.
In the absence of reliable RCT data comparing second-line treatments in a population with confirmed EGFR-activating mutations, no cost-effectiveness analysis could be undertaken. This is a serious information deficit that urgently requires remedy. In particular, this problem prevents any consideration of gefitinib as a potential post-progression treatment, as gefitinib is licensed for use only in patients with activating mutations. The AG is aware that current treatments for patients who have EGFR M+ disease are evolving and include the use of platinum-doublet chemotherapy after progression following EGFR-TKI treatments; however, no robust data are available for use in this appraisal.
TAILOR41 comparing the effectiveness of docetaxel monotherapy and erlotinib is the only RCT with data currently available in a population with confirmed disease and lacking EGFR-activating mutations. Cost-effectiveness analysis using data from this trial indicates that erlotinib is dominated by docetaxel in the EGFR M– population yielding a reduced mean survival and fewer QALYs while involving a greater net cost of treatment. When additional studies are published for the EGFR M– population, it will become clearer whether this result is confirmed or brought into question.
A significant survival benefit for docetaxel may be translated into good cost-effectiveness over erlotinib (erlotinib is dominated by docetaxel, ICER = –£5112 per QALY gained), on the basis that generic docetaxel is priced at the level corresponding to that currently paid by the NHS. If published list prices are substituted, docetaxel looks much less attractive.
A subgroup analysis of the BR.2131 trial comparing erlotinib with BSC in those patients without EGFR-activating mutations confirms that erlotinib generates survival advantages, but at high cost, so that the estimated ICER is high for the EGFR M– population (£54,687 per QALY gained).
In the case of patients who are eligible for second-line therapy but for whom definitive determination of EGFR mutation status is not available for any reason, cost-effectiveness analysis based on the whole of the BR.2131 trial cohort also yields a high ICER value for the EGFR unknown population (£61,132 per QALY gained).
Thus, on the basis of the clinical-effectiveness data currently useable for economic analysis, it does not appear that second-line erlotinib for NSCLC is an attractive option in the EFGR M– or EGFR unknown populations, and at present there are no sources of effectiveness data on which to base an assessment of erlotinib compared with any other option in those patients with confirmed EGFR-activating mutations. The absence of suitable head-to-head trials in the era of EGFR mutation testing is therefore the main limitation on the economic analyses that could be carried out by the AG.
The analyses described here do not take into account the issue of patient experience and preferences in the delivery of second-line treatment, in particular, that oral therapy is widely preferred by patients and clinicians to treatments delivered intravenously. This affects only the comparison made between erlotinib and docetaxel in the EGFR M– population. One possible approach to dealing with this concern is to include an additional utility increment applied only to erlotinib in the analysis to represent the reduction in pain, anxiety and disruption to everyday activities from switching to an oral treatment. There is no objective way to measure such an effect at present. However, a sensitivity analysis can be carried out by assessing the effect of the maximum possible patient health utility increment on the estimated ICER. This is achieved by setting the additional increment at the level which corresponds to returning a patient to the average QoL experienced in the general population at the equivalent mean age (utility about 0.8). This requires raising the EQ-5D score by 0.155, which reduces the incremental loss of QALYs slightly but leaves erlotinib still dominated by docetaxel. This extreme sensitivity analysis indicates that any realistic assessment of utility advantage due to oral therapy is very unlikely to alter the relative cost-effectiveness of erlotinib and docetaxel in the EGFR M– population.
Assessment of factors relevant to the NHS and other parties
This review has highlighted that a key development since TA16221 in 2009 has been the expiration of the patent for docetaxel. This means that generic versions of docetaxel are now available in England and Wales at a substantially reduced cost to the NHS. In TA162,21 NICE recommends the use of docetaxel and erlotinib as second-line treatments for patients with NSCLC. Erlotinib is currently recommended only on the basis that it is provided by the manufacturer at an overall treatment cost equal to that of docetaxel. Docetaxel is now available at 10% of its original list price. Clearly, this reduced price of docetaxel has resource implications that are relevant to the NHS, NICE and the manufacturer of erlotinib. In particular, the results of the AG’s cost-effectiveness analysis comparing erlotinib with docetaxel show that docetaxel is more cost-effective than erlotinib in an EGFR M– patient population.
Recent advances in lung cancer diagnosis and treatments have revealed that expected clinical benefit from available lung cancer treatments can be positively or negatively affected by a patient’s EGFR mutation status. The AG therefore considers it imperative that EGFR mutation tests are routinely available for all NSCLC patients at the time of diagnosis, prior to treatment. The NHS is making every effort to offer timely EGFR mutation tests to patients with NSCLC across England and Wales, however clinical expert opinion is that EGFR mutation tests are not currently routinely available in all centres because of the unavailability of testing facilities and inconclusive results.
In patient populations in which docetaxel is preferred to erlotinib from a cost-effectiveness perspective, there are concerns that this represents a backwards step in patient treatment options. Docetaxel is administered as an i.v. infusion, which means that patients are required to attend hospital as a day-case to receive this treatment. Replacing erlotinib (oral therapy) with docetaxel (i.v. therapy) has major implications not only for NHS resource use and staff, but also in terms of patient preference.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness results
Epidermal growth factor mutation-positive population
No trials were identified that were conducted in a population of solely EGFR M+ patients. The EGFR M+ data for this population were retrospectively derived from subgroup analyses of RCTs that included patients of unknown EGFR mutation status at the time of randomisation. 31,32,34,38,39,42 The outcome data described in these analyses are based on small patient numbers. The outcomes reported are diverse and, in many cases, are limited by poor reporting and lack of statistical power.
Epidermal growth factor mutation-negative population
The clinical effectiveness data available for the EGFR M– population were derived from a RCT that randomised only EGFR M– patients41 and a RCT that was designed to assess clinical outcomes in an EGFR M– population. 40 In addition, EGFR mutation status data were retrospectively derived from BR.21,31 Kim et al. ,32 TITAN,42 INTEREST34 and ISEL;39 however, the subgroup data suffered from the same limitations described previously for the EGFR M+ population. The AG is aware that gefitinib is not licensed for patients with EGFR M– and so the INTEREST34 and ISEL39 trials are included in this group for completeness only. No statistically significant differences were noted for OS for either erlotinib or gefitinib compared with any treatment. For PFS, a statistically significant benefit of docetaxel compared with erlotinib was noted in both TAILOR41 and DELTA. 40 The RR in TAILOR41 was statistically significantly greater for the docetaxel arm of the trial than for the erlotinib arm.
Epidermal growth factor mutation status unknown: overall population
The overall population is made up of trial populations in which EGFR mutation status was not a factor in the recruitment process (or where overall trial results were presented). The data from 11 trials were included in this assessment (TAILOR41 reported only EGFR M– population data). For OS, only BR.2131 reported a statistically significant benefit of any treatment (favouring erlotinib compared with placebo); however, the AG notes that this finding was based on an adjusted rather than an unadjusted analysis of the data.
For PFS, when gefitinib was compared with docetaxel, only one of the four trials (ISTANA35) reported a statistically significant benefit for gefitinib (using 90% CI). When compared with BSC, gefitinib was reported to have a statistically significant benefit in the ISEL39 trial. When erlotinib was compared with placebo in BR.21,31 a statistically significant PFS benefit of erlotinib was reported (in an adjusted analysis). The head-to-head comparison of erlotinib and gefitinib32 did not report HRs for the PFS.
The AG was unable to compare data from any of the trials for any patient population or treatment via meta-analysis or network meta-analysis.
Cost-effectiveness results
The AG developed a de novo economic model for the specific purpose of this Multiple Technology Appraisal and carried out several cost-effectiveness analyses.
For the EGFR M+ population, the AG was not able to carry out a cost-effectiveness analysis of available treatments, as there is an absence of relevant direct clinical trial evidence in this patient population.
For the EGFR M– population, the AG compared docetaxel with erlotinib using data from TAILOR. 41 In this comparison erlotinib was dominated by docetaxel, yielding a reduced mean survival and fewer QALYs while also involving a greater net cost of treatment. Docetaxel yielded a survival advantage over erlotinib of 2.5 months. The overall treatment cost of docetaxel was £545 lower than the cost of erlotinib. The AG estimated the size of the erlotinib versus docetaxel ICER to be –£5112 per QALY gained. However, if published list prices are used instead of eMit prices, the ICER for docetaxel versus erlotinib increases to over £57,000 per QALY gained, with a probabilistic ICER of nearly £70,000 per QALY gained.
For the EGFR M– population, the AG also compared erlotinib versus BSC in a sensitivity analysis using data from the post-hoc reanalysis of BR.2143 described in the manufacturer’s submission submitted by Roche (UK) Ltd. In this comparison, erlotinib yielded a survival advantage over BSC of 2.2 months, with an incremental QALY gain of 0.116. The overall treatment cost of erlotinib was £6362 higher than the cost of BSC. The AG estimated the size of the erlotinib versus BSC ICER to be £54,687 per QALY gained. This ICER is above the range normally accepted to be cost-effective. PSA incorporating uncertainty in all model parameters indicates a slightly lower ICER of £54,984 per QALY gained.
For the EGFR unknown population, the AG compared erlotinib and BSC using data from the BR.2131 trial. In this comparison, erlotinib yielded a survival advantage of 2.1 months, with an incremental QALY gain of 0.103. The overall treatment cost of erlotinib was £6312 higher than the cost of BSC. The AG estimated the size of the erlotinib versus BSC ICER to be £61,132 per QALY gained. This ICER is outside the range normally accepted to be cost-effective. PSA incorporating uncertainty in all model parameters indicates a slightly lower ICER of £59,973 per QALY gained.
Strengths and limitations of the assessment
A key strength of this review is that it has brought together all the available evidence relevant to the clinical effectiveness and cost-effectiveness of erlotinib and gefitinib in patients who have disease progression following prior chemotherapy. From a clinical perspective, this has enabled the AG to identify the substantial gaps in the current evidence base and to offer pertinent research recommendations. The findings of the review have also highlighted the importance of EGFR mutation status for the selection of effective treatments for patients with NSCLC. From a health economics perspective, a key strength of the review is that the current price of docetaxel has been used in the EEs carried out by the AG when appropriate. To date, there are no published cost-effectiveness analyses that have used this off-patent price of docetaxel to compare second-line treatments for patients with NSCLC. Consequently, no speculation regarding the implications of this lower price of docetaxel for the NHS is required as the AG is able to provide the AC with up-to-date and relevant cost-effectiveness information. Finally, the AG has attempted to consider the implicit benefit associated with the use of an oral therapy rather than an i.v. therapy by including an additional utility increment applied only to erlotinib in the extreme sensitivity analysis to represent the reduction in pain, anxiety and disruption to everyday activities from switching to an oral treatment. However, this failed to reverse the dominance of docetaxel over erlotinib in the EGFR M– population.
The main limitation of the assessment is the lack of clinical data available for distinct patient populations. Clearly, the gaps in the evidence base have precluded the assessment of clinical effectiveness and cost-effectiveness of relevant treatments. Specifically, the AG was unable to carry out an EE of treatments for patients with EGFR M+ tumours. A second limitation is that the evidence that is available to support the second-line use of erlotinib, gefitinib and docetaxel is mainly derived from trials that include patients whose EGFR mutation status was unknown at the time of randomisation. A final limitation is that the cost-effectiveness analyses rely on the QALY values modelled from data obtained from a sample of the general population, as highlighted by the AG, these values do not reflect directly patient experience or patients’ preference for the mode of treatment (oral vs. i.v. treatments).
Uncertainties
The results of the recent TAILOR41 trial demonstrate that docetaxel has a statistically significant PFS benefit when compared with erlotinib in a European EGFR M– population. However, a number of criticisms have been levelled at TAILOR,41 and it is as yet uncertain whether or not the reported PFS benefit seen in an Italian population would be achieved by patients in clinical practice in England and Wales.
There is much debate about the true rate of FN in patients treated with docetaxel in the UK NHS. The AG considered this issue in depth and the results can be seen in Appendix 8.
Other relevant factors
There is a clear and well-expressed argument in the manufacturer’s submission submitted by Roche (UK) Ltd that some clinicians are not in favour of a move from oral erlotinib to i.v. docetaxel for patients with NSCLC. In the manufacturer’s submission Roche (UK) Ltd states that ‘restricting funding of erlotinib on the basis of this re-review would represent a substantial backwards step in the treatment of advanced NSCLC, worsen the poor survival of people with relapsed lung cancer in the UK and remove the only treatment option available to many in this patient group. It would also have a significant impact upon the future treatment options available for UK NSCLC patients (given the fact that a significant number of technologies currently in development are designed to be combined with erlotinib)’. 28 It is not within the remit of the AG to address these concerns. The AG has instead focused on providing a systematic review of the clinical effectiveness and cost-effectiveness evidence available, and has carried out robust, relevant cost-effectiveness analyses based on its own de novo economic model.
Chapter 6 Conclusions
Implications for service provision
The largest group of patients to whom the results of this appraisal apply is the EGFR M– patient population. The results of the AG’s cost-effectiveness analysis comparing erlotinib and docetaxel in patients who have disease progression favour the use of docetaxel. Switching from an oral therapy (erlotinib) to an i.v. therapy (docetaxel) would have substantial implications for service provision for both patients and staff in the UK NHS.
Suggested research priorities
It is suggested that any future trials in this area should distinguish between patients who have EGFR M+ and EGFR M– status. To date, the evidence base supporting the use of post-progression treatments for patients with activating EGFR mutations is weak and not sufficiently robust to inform decision-making.
Even when there is a wealth of evidence available (e.g. EGFR unknown status), it is not possible to compare the results of different RCTs using quantitative methods, as the included trial populations are often very diverse. To facilitate treatment comparisons, future trials in this area must be designed to ensure that only patients who best represent patients in clinical practice are included in the trials (e.g. in terms of histology, PS, smoking status and previous treatments).
There has been recent clinician interest in the role of second-line platinum-doublet chemotherapy in EGFR M+ patients as well as manufacturer interest in the use of gefitinib post chemotherapy in the same group of patients, and both of these research areas should be investigated. It would also be valuable to research further the issues associated with re-challenge (re-challenge with EGFR-TKIs in EGFR M+ patients and re-challenge with chemotherapy in EGFR M– patients and EGFR unknown patients) after treatment failure.
Acknowledgements
For their comments on the final draft of this report, we thank Dr Clive Mulatero, senior lecturer in medical oncology, St James’s Institute of Oncology, Leeds; Dr Paula Scullin, consultant oncologist, Belfast Health and Social Care Trust; Professor Peter Clark, consultant oncologist, The Clatterbridge Centre NHS Foundation Trust; and Dr Kathleen Boyd, health economist, University of Glasgow.
Declared competing interests of the reviewers
Dr Paula Scullin has received hospitality, sponsorship to attend meetings and speaker fees from Roche (UK) Ltd and AstraZeneca. Dr Clive Mulatero has received remuneration from Roche (UK) Ltd and AstraZeneca for consultancy, attending symposia, organising education and speaker fees. He has also received research funding from both manufacturers.
Contributions of authors
Janette Greenhalgh was project lead and reviewed the clinical evidence.
Adrian Bagust undertook critical appraisal of manufacturers’ economic model and development of de novo economic model.
Angela Boland supported the review process (clinical and economics).
Kerry Dwan undertook clinical quality assessment, data extraction and was a statistical advisor.
Sophie Beale undertook economic quality assessment and data extraction.
Juliet Hockenhull undertook literature selection and data management.
Christine Proudlove was the pharmacological advisor.
Yenal Dundar undertook literature searching.
Marty Richardson undertook data extraction and was a statistical advisor.
Rumona Dickson supported the review process.
Anna Mullard was a clinical advisor.
Ernie Marshall was a clinical advisor.
All authors read and commented on draft versions of the report.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Cancer Research UK . Lung Cancer Key Facts 2013. www.cancerresearchuk.org/cancer-info/cancerstats/keyfacts/lung-cancer/ (accessed September 2013).
- Brown T, Pilkington G, Bagust A, Boland A, Oyee J, Tudur-Smith C, et al. Clinical effectiveness and cost-effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer: a systematic review and economic evaluation. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17310.
- Royal College of Physicians (RCP) . National Lung Cancer Audit: Resources 2013. www.rcplondon.ac.uk/resources/national-lung-cancer-audit (accessed September 2013).
- Cancer Research UK . Lung Cancer Symptoms 2013. www.cancerresearchuk.org/cancer-help/type/lung-cancer/about/lung-cancer-symptoms (accessed September 2013).
- Erlotinib and Gefitinib for Treating Non-Small Cell Lung Cancer that has Progressed Following Prior Chemotherapy (Review of NICE Technology Appraisals 162 and 175): Final Scope. London: NICE; 2013.
- The Diagnosis and Treatment of Lung Cancer: CG121 (Update of NICE Clinical Guideline 24). London: NICE; 2011.
- TNM. UICC; 2013.
- Cancer Research UK . CancerHelp UK, Performance Status n.d. www.cancerhelp.org.uk/about-cancer/cancer-questions/performance-status (accessed February 2011).
- Eastern Cooperative Oncology Group (ECOG) . ECOG Performance Status 2013. http://ecog.dfci.harvard.edu/general/perf_stat.html (accessed September 2013).
- Karnofskv DA, Burchenal JH, Mcleod CM. The Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949.
- National Lung Cancer Audit Report 2012. Health and Social Care Information Centre; 2012.
- Health and Social Care Information Centre . National Lung Cancer Audit: 2011 Patient Cohort 2012. www.hscic.gov.uk/searchcatalogue?productid=10043&q=title%3a%22Lung+cancer%22&infotype=0%2fAudit&sort=Relevance&size=10&page=1#top (accessed August 2013).
- Cancer Research UK . Lung Cancer UK Price Tag Eclipses the Cost of Any Other Cancer 2012. www.cancerresearchuk.org/cancer-info/news/archive/pressrelease/2012-11-07-lung-cancer-price-tag (accessed September 2013).
- Macmillan . Living With and After Cancer 2013. www.macmillan.org.uk/HowWeCanHelp/HowWeCanHelp.aspx (accessed September 2013).
- Lung Cancer for Adults (QS17). London: NICE; 2012.
- NICE Pathways – Treatment and Palliative Care for Lung Cancer. London: NICE; 2013.
- Gefitinib for the First-Line Treatment of Locally Advanced or Metastatic Non-Small Cell Lung Cancer: TA192. London: NICE; 2010.
- Erlotinib for the First-Line Treatment of Locally Advanced or Metastatic EGFR-TK Mutation-Positive Non-Small Cell Lung Cancer: TA258. London: NICE; 2012.
- Pemetrexed for the First-Line Treatment of Non-Small Cell Lung Cancer: Technology Assessment TA181. London: NICE; 2009.
- Pemetrexed for the Maintenance Treatment of NSCLC: Technology Assessment TA190. London: NICE; 2010.
- Erlotinib for the Treatment of Non-Small Cell Lung Cancer: TA162. London: NICE; 2008.
- Gefitinib for the Second-Line Treatment of Locally Advanced or Metastatic Non-Small Cell Lung Cancer: Terminated Technology Appraisal No. 175. London: NICE; 2009.
- Pemetrexed for the Treatment of Non-Small Cell Lung Cancer: TA124. London: NICE; 2007.
- electronic Medicines Compendium . EMC 2013. www.medicines.org.uk/emc/default.aspx (accessed September 2013).
- electronic Medicines Compendium . SPC– Tarceva 2013. www.medicines.org.uk/emc/medicine/16781/SPC/Tarceva+ 25mg%2c+ 100mg+and+ 150mg+Film-Coated+Tablets/ (accessed October 2013).
- electronic Medicines Compendium . SPC – IRESSA 2013. www.medicines.org.uk/emc/medicine/22104/SPC/IRESSA+ 250mg+film-coated+tablets/ (accessed October 2013).
- Federal Register . AstraZeneca Pharmaceuticals LP; Withdrawal of Approval of a New Drug Application for IRESSA 2012. www.federalregister.gov/articles/2012/04/25/2012–9944/astrazeneca-pharmaceuticals-lp-withdrawal-of-approval-of-a-new-drug-application-for-iressa (accessed October 2013).
- Roche (UK) Ltd . Erlotinib for the Treatment of Non-Small Cell Lung Cancer That Has Progressed Following Prior Chemotherapy – MTA Submission to NICE 2013.
- British National Formulary (Online). London: BMJ Group and Pharmaceutical Press; 2013.
- CRD’s Guidance for Undertaking Reviews in Healthcare: Systematic Reviews. York: CRD, University of York; 2009.
- Shepherd FA, Pereira JR, Ciuleanu T, Eng HT, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small cell lung cancer. N Engl J Med 2005;353:123-32. http://dx.doi.org/10.1056/NEJMoa050753.
- Kim ST, Uhm JE, Lee J, Sun JM, Sohn I, Kim SW, et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non-small cell lung cancer who failed previous chemotherapy. Lung Cancer 2012;75:82-8. http://dx.doi.org/10.1016/j.lungcan.2011.05.022.
- Bhatnagar AR, Singh DP, Sharma R, Kumbhaj P. Docetaxel versus gefitinib in patients with locally advanced or metastatic NSCLC pretreated with platinum-based chemotherapy. J Thorac Oncol 2012;3.
- Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. http://dx.doi.org/10.1016/S0140-6736(08)61758-4.
- Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small-cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res 2010;16:1307-14. http://dx.doi.org/10.1158/1078-0432.CCR-09-1903.
- Li H, Wang X, Hua F. [Second-line treatment with gefitinib or docetaxel for advanced non-small-cell lung cancer]. Chin J Clin Oncol 2010;37:16-8.
- Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K. Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anti-Cancer Drugs 2006;17:401-9. http://dx.doi.org/10.1097/01.cad.0000203381.99490.ab.
- Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 2008;26:4244-52. http://dx.doi.org/10.1200/JCO.2007.15.0185.
- Thatcher N, Chang A, Parikh P, Pereira JR, Ciuleanu T, Von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (IRESSA Survival Evaluation in Lung cancer). Lancet 2005;366:1527-37. http://dx.doi.org/10.1016/S0140-6736(05)67625-8.
- Okano Y, Ando M, Asami K, Fukuda M, Nakagawa H, Ibata H, et al. Randomized phase III trial of erlotinib (E) versus docetaxel (D) as second- or third-line therapy in patients with advanced non-small-cell lung cancer (NSCLC) who have wild-type or mutant epidermal growth factor receptor (EGFR): Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol: ASCO Annual Meeting Proceedings 2013;31.
- Garassino MC, Martelli O, Broggini M, Farina G, Veronese S, Rulli E, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8. http://dx.doi.org/10.1016/S1470-2045(13)70310-3.
- Ciuleanu T, Stelmakh L, Cicenas S, Miliauskas S, Grigorescu AC, Hillenbach C, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 2012;13:300-8. http://dx.doi.org/10.1016/S1470-2045(11)70385-0.
- Zhu CQ, Da Cunha Santos G, Ding K, Sakurada A, Cutz JC, Liu N, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada clinical trials group study BR.21. J Clin Oncol 2008;26:4268-75. http://dx.doi.org/10.1200/JCO.2007.14.8924.
- Hirsch FR, Varella-Garcia M, Bunn PA, Franklin WA, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol 2006;24:5034-42. http://dx.doi.org/10.1200/JCO.2006.06.3958.
- Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol 2010;28:744-52. http://dx.doi.org/10.1200/JCO.2009.24.3030.
- AstraZeneca . NICE Multiple Technology Appraisal of Erlotinib and Gefitinib for Treating Non-Small-Cell Lung Cancer That Has Progressed Following Prior Chemotherapy (Review of TA162 and TA175) 2013.
- Erlotinib Monotherapy for Maintenance Treatment of Non-Small-Cell Lung Cancer (TA227). London: NICE; 2011.
- British National Formulary (Online). London: BMJ Group and Pharmaceutical Press; 2012.
- Hanna N, Shepherd FA, Fossella FV, Pereira JR, Demarinis F, Von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. http://dx.doi.org/10.1200/JCO.2004.08.163.
- IRESSA. London: EMA; 2009.
- Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. New Engl J Med 2009;361:947-57. http://dx.doi.org/10.1056/NEJMoa0810699.
- Assessment Report for IRESSA. London: EMA; 2009.
- Bongers ML, Coupe VM, Jansma EP, Smit EF, Uyl-de Groot C. Cost-effectiveness of treatment with new agents in advanced non-small-cell lung cancer: a systematic review. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.1194.
- Bongers ML, Coupe VM, Jansma EP, Smit EF, Uyl-de Groot CA. Cost effectiveness of treatment with new agents in advanced non-small-cell lung cancer: a systematic review. Pharmacoeconomics 2012;30:17-34. http://dx.doi.org/10.2165/11595000-000000000-00000.
- Borget I, Cadranel J, Pignon JP, Quoix E, Coudert B, Westeel V, et al. Cost-effectiveness of three strategies for second-line erlotinib initiation in non-small-cell lung cancer: the ERMETIC study part 3. Eur Respir J 2012;39:172-9. http://dx.doi.org/10.1183/09031936.00201210.
- Capri S, Morabito A, Carillio G, Grossi F, Longo R, Cerea G, et al. Economic evaluation of erlotinib, docetaxel and pemetrexed as second line therapy in non-small-cell lung cancer. Pharmacoeconomics Ital Res Art 2007;9:113-24. http://dx.doi.org/10.1007/BF03320705.
- Ciuleanu TE, Dediu M, Minea LN, Baculea S, Szkultecka-Debek M. Cost-effectiveness analysis of erlotinib in the treatment of advanced non-small-cell lung cancer (NSCLC) in Romania. Value Health 2010;3. http://dx.doi.org/10.1016/S1098-3015(10)72166-5.
- Horgan AM, Bradbury PA, Amir E, Ng R, Douillard JY, Kim ES, et al. An economic analysis of the INTEREST trial, a randomized trial of docetaxel versus gefitinib as second-/third-line therapy in advanced non-small-cell lung cancer. Ann Oncol 2011;22:1805-11. http://dx.doi.org/10.1093/annonc/mdq682.
- Horgan AM, Shepherd FA, Bradbury PA, Ng R, Leighl NB. Preliminary cost-consequence analysis of the INTEREST trial, a randomized trial of docetaxel versus gefitinib as 2nd line therapy in advanced non-small-cell lung cancer (abstract no 8110). J Clin Oncol: ASCO Annual Meeting Proceedings 2008;26. http://dx.doi.org/10.1093/annonc/mdq682.
- Laurendeau C, Chouaid C, Florentin V, Duchon D’engenieres V, Detournay B. Cost-minimization analysis of second-line chemotherapy for nonsmall-cell lung cancer (NSCLC). Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.1138.
- Nguyen TTT, Yagudina R, Kulikov A. Cost-effectiveness analysis of erlotinib versus docetaxel, pemetrexed for second-line treatment of advanced non-small-cell lung cancer in Russia. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.1191.
- Thongprasert S, Permsuwan U. Cost-effectiveness and budget impact analyses of gefitinib in second-line treatment for advanced non-small cell lung cancer from Thai payer perspective. Asia Pac J Clin Oncol 2012;8:53-61. http://dx.doi.org/10.1111/j.1743-7563.2012.01528.x.
- Vergnenegre A, Corre R, Berard H, Paillotin D, Dujon C, Robinet G, et al. Cost-effectiveness of second-line chemotherapy for non-small-cell lung cancer. J Thorac Oncol 2011;6:161-8. http://dx.doi.org/10.1097/JTO.0b013e318200f4c1.
- Araujo A, Parente B, Sotto-Mayor R, Teixeira E, Almodovar T, Barata F, et al. An economic analysis of erlotinib, docetaxel, pemetrexed and best supportive care as second or third line treatment of non-small-cell lung cancer (structured abstract). Rev Port Pneumol 2008;14:803-27.
- Asukai Y, Valladares A, Camps C, Wood E, Taipale K, Arellano J, et al. Cost-effectiveness analysis of pemetrexed versus docetaxel in the second-line treatment of non-small-cell lung cancer in Spain: results for the non-squamous histology population. BMC Cancer 2010;10. http://dx.doi.org/10.1186/1471-2407-10-26.
- Bradbury PA, Tu D, Seymour L, Isogai PK, Zhu L, Ng R, et al. Economic analysis: Randomized placebo-controlled clinical trial of erlotinib in advanced non-small-cell lung cancer. J Natl Cancer Inst 2010;102:298-306. http://dx.doi.org/10.1093/jnci/djp518.
- Holmes J, Dunlop D, Hemmett L, Sharplin P, Bose U. A cost-effectiveness analysis of docetaxel in the second-line treatment of non-small-cell lung cancer. Pharmacoeconomics 2004;22:581-9. http://dx.doi.org/10.2165/00019053-200422090-00003.
- Thongprasert S, Tinmanee S, Permsuwan U. Cost-utility and budget impact analyses of gefitinib in second-line treatment for advanced non-small-cell lung cancer from Thai payer perspective. Asia Pac J Clin Oncol 2012;8:53-61. http://dx.doi.org/10.1111/j.1743-7563.2012.01528.x.
- Cromwell I, Hoek K, Melosky B, Peacock S. Erlotinib or docetaxel for second-line treatment of non-small-cell lung cancer: a real-world cost-effectiveness analysis. J Thorac Oncol 2011;6:2097-103. http://dx.doi.org/10.1097/JTO.0b013e31822f657a.
- Cromwell I, Hoek K, Malfair Taylor SC, Melosky B, Peacock S. Erlotinib or best supportive care for third-line treatment of advanced non-small-cell lung cancer: a real-world cost-effectiveness analysis. Lung Cancer 2012;76:472-7. http://dx.doi.org/10.1016/j.lungcan.2011.12.003.
- Lewis G, Peake M, Aultman R, Gyldmark M, Morlotti L, Creeden J, et al. Cost-effectiveness of erlotinib versus docetaxel for second-line treatment of advanced non-small-cell lung cancer in the United Kingdom. J Int Med Res 2010;38:9-21. http://dx.doi.org/10.1177/147323001003800102.
- Leighl NB, Shepherd FA, Kwong R, Burkes RL, Feld R, Goodwin PJ. Economic analysis of the TAX 317 trial: docetaxel versus best supportive care as second-line therapy of advanced non-small-cell lung cancer. J Clin Oncol 2002;20:1344-52. http://dx.doi.org/10.1200/JCO.20.5.1344.
- Carlson JJ, Reyes C, Oestreicher N, Lubeck D, Ramsey SD, Veenstra DL. Comparative clinical and economic outcomes of treatments for refractory non-small-cell lung cancer (NSCLC). Lung Cancer 2008;61:405-15. http://dx.doi.org/10.1016/j.lungcan.2007.12.023.
- Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103.
- Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non-small-cell lung cancer. Health Qual Life Outcom 2008;6. http://dx.doi.org/10.1186/1477-7525-6-84.
- Breast Cancer (HER2 Negative, Oestrogen Receptor Positive, Locally Advanced or Metastatic) – Everolimus (with an Aromatase Inhibitor) (TA295). London: NICE; 2013.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- TA296 Lung Cancer (Non-Small-Cell, Anaplastic Lymphoma Kinase Fusion Gene, Previously Treated) – Crizotinib: Guidance. London: NICE; 2013.
- Shaw A, Kim D-W, Nakagawa K, Seto T, Crino L, Ahn M-J, et al. Phase III Study of Crizotinib Vs. Pemetrexed or Docetaxel Chemotherapy Patients With Advanced ALK Positive NSCLC (PROFILE 1007) n.d.
- Millar DR, Corrie P, Hill M, Pulfer A. A service evaluation to compare secondary care resource use between XELOX and FOLFOX-6 regimens in the treatment of metastatic colorectal cancer (MCRC) from a UK National Health Service (NHS) perspective. Value Health 2008;11. http://dx.doi.org/10.1016/S1098-3015(10)66610-7.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2011.
- Department of Health . NHS Reference Costs 2011–2012 2013. www.gov.uk/government/uploads/system/uploads/attachment_data/file/127115/NSRC01-2011-12xls.xls (accessed September 2013).
- Department of Health . Electronic Market Information Tool (eMit) 2012. http://cmu.dh.gov.uk/electronic-market-information-tool-emit/ (accessed September 2013).
- Curtis L. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2012.
- Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol 2000;18:2354-62.
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) (corrected). J Clin Oncol 2003;21:2237-46. http://dx.doi.org/10.1200/JCO.2003.10.038.
- Karampeazis A, Voutsina A, Souglakos J, Kentepozidis N, Giassas S, Christofillakis C, et al. Pemetrexed versus erlotinib in pretreated patients with advanced non-small-cell lung cancer: a Hellenic Oncology Research Group (HORG) randomized phase 3 study. Cancer 2013;119:2754-64. http://dx.doi.org/10.1002/cncr.28132.
- Kris MG, Natale RB, Herbst RS, Lynch TJ, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small-cell lung cancer: a randomized trial. JAMA 2003;290:2149-58. http://dx.doi.org/10.1001/jama.290.16.2149.
- Lee DH, Kim SW, Suh C, Yoon HD, Yi EJ, Lee J-S, et al. Phase II study of erlotinib as a salvage treatment for non-small-cell lung cancer patients after failure of gefitinib treatment. Ann Oncol 2008;19:2039-42. http://dx.doi.org/10.1093/annonc/mdn423.
- Sun JM, Lee KH, Kim SW, Lee DH, Min YJ, Yun HJ, et al. Gefitinib versus pemetrexed as second-line treatment in patients with nonsmall cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08–01): an open-label, phase 3 trial. Cancer 2012;118:6234-42. http://dx.doi.org/10.1002/cncr.27630.
- Office for National Statistics . Death Registration Summary Tables (England and Wales, 2012) 2012. www.ons.gov.uk/ons/rel/vsob1/death-reg-sum-tables/2012/index.html (accessed September 2013).
- Maslove L, Gower NH, Spiro SG, Rudd RM, Stephens R, West P. Estimation of the additional costs of chemotherapy for patients with advanced non-small-cell lung cancer. Thorax 2005;60:564-69. http://dx.doi.org/10.1136/thx.2004.039479.
- Advanced Breast Cancer: Diagnosis and Treatment (CG81). London: NICE; 2009.
- Taylor DG, Carter S. Valuing Choice-Dying at Home: A Case for More Equitable Provision of High Quality Support for People who Wish to Die at Home. London: Marie Curie Cancer Care; 2004.
- Morgan A, Sutton A, Wailoo A. The Risk and Costs of Febrile Neutropenia in Patients with Non Small Cell Lung Cancer Treated with Docetaxel. Sheffield: NICE Decision Support Unit, ScHARR, University of Sheffield; 2007.
- Grutters J, Joore M, Wiegman E, Langendijk J, de Ruysscher D, Hochstenbag M. Health-related quality of life in patients surviving non-small-cell lung cancer. Thorax 2010;65:903-7. http://dx.doi.org/10.1136/thx.2010.136390.
- van den Hout W, Kramer G, Noordijk E, Leer JW. Cost-utility analysis of short versus long course palliative radiotherapy in patients with non-small-cell lung cancer. J Natl Cancer Inst 2006;98:1786-94. http://dx.doi.org/10.1093/jnci/djj496.
- Doyle S, Lloyd A, Walker M. Health state utility scores in advanced non-small-cell lung cancer. Lung Cancer 2008;62:374-80. http://dx.doi.org/10.1016/j.lungcan.2008.03.019.
- Bianic F, Despiegel N, Cure S, Campbell J, Wang Z, Cappelleri JC, et al. Network meta-analysis of second and third-line treatments on overall response and overall survival in patients with metastatic non-small-cell lung cancer. Eur J Cancer 2011;47:S616-17. http://dx.doi.org/10.1016/S0959-8049(11)72393-0.
- Kris M, Mok T, Kim E, Douillard JY, Fukuoka M, Thatcher N. Response and progression-free survival in 1006 patients with known EGFR mutation status in phase III randomized trials of gefitinib in individuals with non-small-cell lung cancer. Eur J Cancer Suppl 2009;7:505-6. http://dx.doi.org/10.1016/S1359-6349(09)71716-1.
- Guo J, Ma B, Zhou H, Wang Y, Zhang Y. Gefitinib for non-small-cell lung cancer: a meta analysis. Chin J Lung Cancer 2011;14:351-7. http://dx.doi.org/10.3779/j.issn.1009-3419.2011.04.09.
- Hawkins N, Scott DA, Woods BS, Thatcher N. Time to broaden our horizons; the case for network meta-analysis within relapsed nonsmall cell lung cancer (NSCLC). Ann Oncol 2008;19:996-1003.
- Jiang J, Huang L, Liang X, Zhou X, Huang R, Chu Z, et al. Gefitinib versus docetaxel in previously treated advanced non-small-cell lung cancer: a meta-analysis of randomized controlled trials. Acta Oncol 2011;50:582-8. http://dx.doi.org/10.3109/0284186X.2010.546368.
- Petrelli F, Borgonovo K, Cabiddu M, Barni S. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated nonsmall-cell lung cancer: a meta-analysis of 13 randomized trials. Clin Lung Cancer 2012;13:107-14. http://dx.doi.org/10.1016/j.cllc.2011.08.005.
- Erlotinib and Gefitinib for Treating Non-Small Cell Lung Cancer that has Progressed Following Prior Chemotherapy (Review of NICE Technology Appraisals 162 and 175). Liverpool: Liverpool Reviews and Implementation Group; 2014.
- Camps C, Massuti B, Jimenez A, Maestu I, Gomez RG, Isla D, et al. Randomized phase III study of 3-weekly versus weekly docetaxel in pretreated advanced non-small-cell lung cancer: a Spanish Lung Cancer Group trial. Ann Oncol 2006;17:467-72. http://dx.doi.org/10.1093/annonc/mdj115.
- Chen YM, Shih JF, Perng RP, Tsai CM, Whang-Peng J. A randomized trial of different docetaxel schedules in non-small-cell lung cancer patients who failed previous platinum-based chemotherapy. Chest 2006;129:1031-8. http://dx.doi.org/10.1378/chest.129.4.1031.
- Gervais R, Ducolone A, Breton JL, Braun D, Lebeau B, Vaylet F, et al. Phase II randomised trial comparing docetaxel given every 3 weeks with weekly schedule as second-line therapy in patients with advanced non-small-cell lung cancer (NSCLC). Ann Oncol 2005;16:90-6. http://dx.doi.org/10.1093/annonc/mdi018.
- Gridelli C, Gallo C, Di Maio M, Barletta E, Illiano A, Maione P, et al. A randomised clinical trial of two docetaxel regimens (weekly vs. 3 week) in the second-line treatment of non-small-cell lung cancer. The DISTAL 01 study. Br J Cancer 2004;91:1996-2004. http://dx.doi.org/10.1038/sj.bjc.6602241.
- Lilenbaum R, Rubin M, Samuel J, Boros L, Chidiac T, Seigel L, et al. A randomized phase II trial of two schedules of docetaxel in elderly or poor performance status patients with advanced non-small-cell lung cancer. J Thorac Oncol 2007;2:306-11. http://dx.doi.org/10.1097/01.JTO.0000263713.38826.8e.
- Pectasides D, Pectasides M, Farmakis D, Kostopoulou V, Nikolaou M, Gaglia A, et al. Comparison of docetaxel and docetaxel–irinotecan combination as second-line chemotherapy in advanced non-small-cell lung cancer: a randomized phase II trial. Ann Oncol 2005;16:294-9. http://dx.doi.org/10.1093/annonc/mdi053.
- Quoix E, Lebeau B, Depierre A, Ducolone A, Moro-Sibilot D, Milleron B, et al. Randomised, multicentre phase II study assessing two doses of docetaxel (75 or 100 mg/m2) as second-line monotherapy for non-small-cell lung cancer. Ann Oncol 2004;15:38-44. http://dx.doi.org/10.1093/annonc/mdh005.
- Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol 2012;30:3640-7. http://dx.doi.org/10.1200/JCO.2012.42.6932.
- Schuette W, Nagel S, Blankenburg T, Lautenschlaeger C, Hans K, Schmidt EW, et al. Phase III study of second-line chemotherapy for advanced non-small-cell lung cancer with weekly compared with 3-weekly docetaxel. J Clin Oncol 2005;23:8389-95. http://dx.doi.org/10.1200/JCO.2005.02.3739.
- Wachters FM, Groen HJ, Biesma B, Schramel FM, Postmus PE, Stigt JA, et al. A randomised phase II trial of docetaxel vs. docetaxel and irinotecan in patients with stage IIIb–IV non-small-cell lung cancer who failed first-line treatment. Br J Cancer 2005;92:15-20. http://dx.doi.org/10.1038/sj.bjc.6602268.
- Professional Organisation Statement. Multiple Technology Appraisal: Erlotinib and Gefitinib for Treating Non-Small Cell Lung Cancer that has Progressed Following Prior Chemotherapy (Review of NICE Technology Appraisals 162 and 175) [ID620]. London: RCP; 2013.
- Sharma A, Lo A, Mukherji D, Burcombe R, Sevitt T, Taylor H, et al. An analysis of rate of admissions to an inpatient facility across 3 trusts in relation to the delivery of docetaxel chemotherapy in non small cell lung cancer (NSCLC). Lung Cancer 2009;63. http://dx.doi.org/10.1016/S0169-5002(09)70018-2.
Appendix 1 Literature search strategies
MEDLINE (via OvidSP)
URL: http://ovidsp.tx.ovid.com/sp–3.15.1b/
Date range searched: 1946 to 26 April 2013.
Date searched: 26 April 2013.
Search strategy
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
randomly.ab.
-
trial.ab.
-
or/1-6
-
(animals not (humans and animals)).sh.
-
7 not 8
-
exp Carcinoma, Non-Small-Cell Lung/ or nsclc.ti,ab.
-
(non-small or non small or nonsmall).ti,ab.
-
(lung or pulmonary or bronchus or bronchogenic or bronchial or bronchoalveolar or alveolar).ti,ab.
-
(neoplasm$ or cancer$ or carcinoma$ or adenocarcinoma$ or angiosarcoma$ or chrondosarcoma$ or sarcoma$ or teratoma$ or lymphoma$ or blastoma$ or microcytic$ or carcinogenesis or tumour$ or tumor$ or metast$).ti,ab.
-
10 or (and/11-13)
-
(erlotinib or tarceva or “osi 774”).ti,ab.
-
(gefitinib or iressa or ZD 1839).ti,ab.
-
15 or 16
-
9 and 14 and 17
-
limit 18 to English language
EMBASE (via OvidSP)
URL: http://ovidsp.tx.ovid.com/sp–3.15.1b/
Date range searched: 1974 to 26 April 2013.
Date searched: 26 April 2013.
Search strategy
-
Randomized Controlled Trial/
-
Randomization/
-
Single blind procedure/
-
Double blind procedure/
-
Double blind procedure/
-
Crossover procedure/
-
Randomi?ed controlled trial$.tw.
-
random$.ti,ab.
-
placebo.ti,ab.
-
or/1-9
-
animal/ not (animal/ and human/)
-
10 not 11
-
exp lung non small cell cancer/ or nsclc.ti,ab.
-
(non-small or non small or nonsmall).ti,ab.
-
(lung or pulmonary or bronchus or bronchogenic or bronchial or bronchoalveolar or alveolar).ti,ab.
-
(neoplasm$ or cancer$ or carcinoma$ or adenocarcinoma$ or angiosarcoma$ or chrondosarcoma$ or sarcoma$ or teratoma$ or lymphoma$ or blastoma$ or microcytic$ or carcinogenesis or tumour$ or tumor$ or metast$).ti,ab.
-
13 or (and/14-16)
-
exp erlotinib/
-
(erlotinib or tarceva or “osi 774”).ti,ab.
-
exp gefitinib/
-
(gefitinib or iressa or ZD 1839).ti,ab.
-
or/18-21
-
12 and 17 and 22
-
limit 23 to English language
The Cochrane Library
URL: http://onlinelibrary.wiley.com/cochranelibrary/search/
Date range searched: inception to 28 April 2013.
Date searched: 28 April 2013.
Search strategy
#1. MeSH descriptor: [Carcinoma, Non-Small-Cell Lung] explode all trees
#2. “non-small-cell lung cancer”:ti,ab,kw (word variations have been searched)
#3. erlotinib or tarceva:ti,ab,kw (word variations have been searched)
#4. gefitinib or iressa:ti,ab,kw (word variations have been searched)
#5. #1 or #2
#6. #3 or #4
#7. #5 and #6
PubMed
URL: www.ncbi.nlm.nih.gov/pubmed
Date range searched: January 2010 to 28 April 2013.
Date searched: 28 April 2013.
Search strategy
((erlotinib or tarceva or gefitinib or iressa)) AND lung cancer
Filters
Clinical trial, publication date from 2010/01/01 to 2013, humans, English
Search detail
((“erlotinib”[Supplementary Concept] OR “erlotinib”[All Fields]) OR (“erlotinib”[Supplementary Concept] OR “erlotinib”[All Fields] OR “tarceva”[All Fields]) OR (“gefitinib”[Supplementary Concept] OR “gefitinib”[All Fields]) OR (“gefitinib”[Supplementary Concept] OR “gefitinib”[All Fields] OR “iressa”[All Fields])) AND (“lung neoplasms”[MeSH Terms] OR (“lung”[All Fields] AND “neoplasms”[All Fields]) OR “lung neoplasms”[All Fields] OR (“lung”[All Fields] AND “cancer”[All Fields]) OR “lung cancer”[All Fields]) AND (Clinical Trial[ptyp] AND (“2010/01/01”[PDAT] : “2013/12/31”[PDAT]) AND “humans”[MeSH Terms] AND English[lang])
Appendix 2 Quality assessment of included studies
| Trial | Randomisation | Baseline comparability | Eligibility criteria specified | Co-interventions identified | Blinding | Withdrawals | ITT | Other outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Truly random | Allocation concealment | Number stated | Presented | Achieved | Assessors | Administration | Participants | Procedure assessed | > 80% in final analysis | Reasons stated | |||||
| aBhatnagar et al. 201233 | NS | NS | ✓ | ✗ | NS | ✓✗ | NS | NS | NS | NS | NS | NS | NS | NS | Unclear |
| BR.21 200531 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NS | ✓b | ✓b | ✓ | NS | ✓ | ✓ | ✓ | ✗ |
| aDELTA 201340 | ✓ | NS | ✓ | ✗ | NS | ✓ | NS | NS | NS | ✗ | NA | NS | NS | NS | Unclear |
| INTEREST 200834 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NS | ✗ | ✗ | NA | ✓ | ✓ | ✓ | ✗ |
| ISEL 200539 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NS | NS | ✓ | ✓ | NS | ✓ | ✓ | ✓ | ✗ |
| ISTANA 201035 | NS | NS | ✓ | ✓ | ✓ | ✓ | NS | NS | ✗ | ✗ | NA | ✓ | ✓ | ✓ | ✗ |
| cKim 201232 | NS | NS | ✓ | Unclear | Unclear | ✓ | NS | NS | ✗ | ✗ | NA | ✓ | NA | Unclear | ✗ |
| Li et al. 201036 | NS | NS | ✓ | ✓ | ✓ | ✓ | ✓ | NS | NS | NS | NS | ✓ | NS | NS | ✗ |
| SIGN 200637 | Unclear | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | NA | ✓ | ✓ | ✓ | ✗ |
| TAILOR 201341 | ✓ | ✓ | ✓ | ✓ | ✓✗d | ✓ | NS | ✓✗e | ✗ | ✗ | NA | ✓ | ✓ | ✓ | ✗ |
| TITAN 201242 | ✓ | ✓ | ✓ | ✓ | ✓✗ | ✓ | NS | ✗ | ✗ | ✗ | NA | ✓ | ✓ | ✓ | ✗ |
| V-15-32 200838 | NS | NS | ✓ | ✓ | ✓ | ✓ | NS | ✓✗f | ✗ | ✗ | NA | ✓ | ✓ | ✓ | ✗ |
Appendix 3 Table of included studies and associated publications
| Trial | Associated publications |
|---|---|
| Bhatnagar et al.33 | Bhatnagar AR, Singh DP, Sharma R, Kumbhaj P. Docetaxel versus gefitinib in patients with locally advanced or metastatic NSCLC pretreated with platinum-based chemotherapy. J Thorac Oncol 2012;3:S159 |
| BR.2131 | Shepherd FA, Pereira JR, Ciuleanu T, Eng HT, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. New Engl J Med 2005;353:123–32 |
| Bezjak A, Shepherd F, Tu D, Clark G, Santabarbara P, Pater J, et al. Symptom response in non-small-cell lung cancer (NSCLC) patients (pts) treated with erlotinib: quality of life analysis of the NCIC CTG BR.21 trial. J Clin Oncol: ASCO Annual Meeting Proceedings 2005;23:625 | |
| Bezjak A, Tu D, Seymour L, Clark G, Trajkovic A, Zukin M, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2006;24:3831–7 | |
| Zhu CQ, Da Cunha Santos G, Ding K, Sakurada A, Cutz JC, Liu N, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada clinical trials group study BR.21. J Clin Oncol 2008;26:4268–75 | |
| DELTA40 | Okano Y, Ando M, Asami K, Fukuda M, Nakagawa H, Ibata H, et al. Randomized phase III trial of erlotinib (E) versus docetaxel (D) as second- or third-line therapy in patients with advanced non-small-cell lung cancer (NSCLC) who have wild-type or mutant epidermal growth factor receptor (EGFR): Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol: ASCO Annual Meeting Proceedings 2013;31:8006 |
| INTEREST34 | Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809–18 |
| Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol 2010;28:744–52 | |
| ISEL39 | Chang A, Parikh P, Thongprasert S, Tan EH, Perng RP, Ganzon D, et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non-small-cell lung cancer: subset analysis from the ISEL study. J Thorac Oncol 2006;1:847–55 |
| Thatcher N, Chang A, Parikh P, Pereira JR, Ciuleanu T, Von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (IRESSA Survival Evaluation in Lung cancer). Lancet 2005;366:1527–37 | |
| Hirsch FR, Varella-Garcia M, Bunn Jr PA, Franklin WA, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol 2006;24:5034–42 | |
| ISTANA35 | Lee D, Kim S, Park K, Kim J, Lee J, Shin S, et al. A randomized open-label study of gefitinib versus docetaxel in patients with advanced/metastatic non-small-cell lung cancer (NSCLC) who have previously received platinum-based chemotherapy [abstract no. 8025]. J Clin Oncol: ASCO Annual Meeting Proceedings 2008;26:430 |
| Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small-cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res 2010;16:1307–14 | |
| Kim et al.32 | Kim ST, Uhm JE, Lee J, Sun JM, Sohn I, Kim SW, et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non-small-cell lung cancer who failed previous chemotherapy. Lung Cancer 2012;75:82–8 |
| Ahn J, Kim S, Ahn M, Lee J, Uhm J, Sun J, et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non-small-cell lung cancer who failed previous chemotherapy. J Clin Oncol 2010;28:7551 | |
| Li et al.36 | Li H, Wang X, Hua F. [Second-line treatment with gefitinib or docetaxel for advanced non-small-cell lung cancer]. Chin J Clin Oncol 2010;37:16–18 |
| SIGN37 | Cufer T, Vrdoljak E. Results from a Phase II, open-label, randomized study (SIGN) comparing gefitinib with docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer [abstract]. J Clin Oncol: ASCO Annual Meeting Proceedings 2005;23:629 |
| Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K. Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anti-Cancer Drugs 2006;17:401–9 | |
| TAILOR41 | Farina G, Longo F, Martelli O, Pavese I, Mancuso A, Moscetti L, et al. Rationale for treatment and study design of tailor: a randomized phase III trial of second-line erlotinib versus docetaxel in the treatment of patients affected by advanced non-small-cell lung cancer with the absence of epidermal growth factor receptor mutations. Clin Lung Cancer 2011;12:138–41 |
| Garassino MC, Martelli O, Bettini A, Floriani I, Copreni E, Lauricella C, et al. TAILOR: a phase III trial comparing erlotinib with docetaxel as the second-line treatment of NSCLC patients with wild-type (wt) EGFR. J Clin Oncol 2012;30 | |
| Garassino MC, Marabese M, Broggini M, Lauricella C, Floriani I, Martelli O, et al. Effect of tumor-specific KRAS mutational status on impact of anti-EGFR therapy in non-small-cell lung cancer (NSCLC). J Clin Oncol 2010;1:7564 | |
| aGarassino MC, Martelli O, Broggini M, Farina G, Veronese S, Rulli E, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981–8 | |
| TITAN42 | Ciuleanu T, Stelmakh L, Cicens S, Gonzlez EE. Efficacy and safety of erlotinib versus chemotherapy in second-line advanced non-small-cell lung cancer (NSCLC) with poor prognosis: the phase III TITAN study. Lung Cancer 2011;71:S44 |
| Ciuleanu T, Stelmakh L, Cicenas S, Miliauskas S, Grigorescu AC, Hillenbach C, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 2012;13:300–8 | |
| V-15-3238 | Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 2008;26:4244–52 |
| Sekine I, Ichinose Y, Nishiwaki Y, Yamamoto N, Tsuboi M, Nakagawa K, et al. Quality of life and disease-related symptoms in previously treated Japanese patients with non-small-cell lung cancer: results of a randomized phase III study (V-15-32) of gefitinib versus docetaxel. Ann Oncol 2009;20:1483–8 |
Appendix 4 Table of excluded publications with rationale
| Full reference | Reason for exclusion |
|---|---|
| Abstracts of the Chicago Multidisciplinary Symposium in Thoracic Oncology. 6–8 September 2012, Chicago, IL, USA. J Thorac Oncol 2012;7(Suppl. 4):S203–340 | Not a RCT |
| Addison CL, Ding K, Zhao H, Le Maitre A, Goss GD, Seymour L, et al. Plasma transforming growth factor alpha and amphiregulin protein levels in NCIC Clinical Trials Group BR.21. J Clin Oncol 2010;28:5247–56 | Subgroup analysis |
| Aparisi F, Sanchez A, Giner V, Munoz J, Esquerdo G, Garde J, et al. A multi-center, open, randomized, phase II study to investigate the sequential administration of docetaxel and intermittent erlotinib versus erlotinib as a second-line therapy for advanced non-small-cell lung cancer (NSCLC). Eur J Cancer 2011;47:S630 | No relevant comparator |
| Aprile G, Belvedere O, Puglisi F. From the podium to the patient: bringing the 2008 ASCO meeting to the clinic. Anti-Cancer Drugs 2008;19:941–56 | Meeting report |
| Asahina H, Oizumi S, Inoue A, Kinoshita I, Ishida T, Fujita Y, et al. Phase II study of gefitinib readministration in patients with advanced non-small-cell lung cancer and previous response to gefitinib. Oncology 2010;79:423–9 | Not a RCT |
| Augustovski F, Pichon Riviere A, Alcaraz A, Bardach A, Ferrante D, Garcia Marti S, et al. Erlotinib for the Management of Advanced Lung Cancer. Buenos Aires: Institute for Clinical Effectiveness and Health Policy (IECS); 2005 | Not a RCT |
| Augustovski F, Pichon Riviere A, Alcaraz A, Bardach A, Ferrante D, Garcia Marti S, et al. Gefitinib for Advanced Lung Cancer Treatment. Buenos Aires: Institute for Clinical Effectiveness and Health Policy (IECS); 2005 | Review |
| Bezjak A. Erlotinib improves symptoms as well as survival in NSCLC. Oncol Rep 2005;(Fall):99–100 | Review |
| Canadian Coordinating Office for Health Technology Assessment. Gefitinib for Advanced or Metastatic Non-Small-Cell Lung Cancer. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 2004 | Review |
| Canadian Coordinating Office for Health Technology Assessment. Gefitinib. Gefitinib for Inoperable or Recurrent Non-Small-Cell Lung Cancer. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 2004 | Review |
| Cella D, Herbst RS, Lynch TJ, Prager D, Belani CP, Schiller JH, et al. Clinically meaningful improvement in symptoms and quality of life for patients with non-small-cell lung cancer receiving gefitinib in a randomized controlled trial. J Clin Oncol 2005;23:2946–54 | No relevant comparator |
| Danish Centre for Evaluation and Health Technology Assessment. Health Technology Assessment of Erlotinib (Tarceva) for Palliative Treatment of Non-Small-Cell Lung Cancer – Accelerated Assessment. Copenhagen: Danish Centre for Evaluation and Health Technology Assessment (DACEHTA); 2005 | Review |
| Danish Centre for Evaluation and Health Technology Assessment. IRESSA for Non-Small-Cell Lung Cancer – Early Warningon New Health Technology. Copenhagen: Danish Centre for Evaluation and Health Technology Assessment (DACEHTA); 2002 | Non-English abstract |
| Douillard JY, Giaccone G, Horai T, Noda K, Vansteenkiste JF, Takata I, et al. Improvement in disease-related symptoms and quality of life in patients with advanced non-small-cell lung cancer (NSCLC) treated with ZD1839 (‘IRESSA’) (IDEAL 1). Proc Am Soc Clin Oncol 2002;21:299a, abstract 1195 | No relevant comparator |
| Erlotinib: new drug. Non small-cell lung cancer: like gefitinib, no established advantage. Prescrire Int 2006;15:86–9 | Review |
| Fehrenbacher L, O’Neill V, Belani CP, Bonomi P, Hart L, Melnyk O, et al. A phase II, multicenter, randomized clinical trial to evaluate the efficacy and safety of bevacizumab in combination with either chemotherapy (docetaxel or pemetrexed) or erlotinib hydrochloride compared with chemotherapy alone for treatment of recurrent or refractory non-small-cell lung cancer. J Clin Oncol: ASCO Annual Meeting Proceedings 2006;24:7062 | Not for licensed indication |
| Feld R, Sridhar SS, Shepherd FA, Mackay JA, Evans WK. Use of the epidermal growth factor receptor inhibitors gefitinib and erlotinib in the treatment of non-small-cell lung cancer: a systematic review. J Thorac Oncol 2006;1:367–76 | Review |
| Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Final results from a phase II trial of ZD1839 (‘IRESSA’) for patients with advanced non-small-cell lung cancer (IDEAL 1). J Clin Oncol: Proceed Am Soc Clin Oncol 2002;21:298a, abstract 1188 | No relevant comparator |
| Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol 2003;21:2237–46. | No relevant comparator |
| Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol 2003;21:2237–46. [Erratum published in J Clin Oncol 2004;22:4863] | Erratum |
| Gefitinib: a second look. Non-small cell lung cancer: still very disappointing. Prescrire Int 2009;18:145–7 | Review |
| Gefitinib: Disappointing. Prescrire Int 2006;15:88 | Review |
| Gridelli C, Rossi A, Venturino P, de Marinis F. Treatment, rationale, and study design of TALISMAN study: a randomized phase II open-label study of second-line erlotinib versus intermittent erlotinib dosing with docetaxel in the treatment of former-smoker men affected by recurrent squamous non-small-cell lung cancer. Clin Lung Cancer 2011;12:70–3 | No relevant comparator |
| Gridelli C, Rossi A, Venturino P, de Marinis F. Treatment, rationale, and study design of TALISMAN study: a randomized phase II open-label study of second-line erlotinib versus intermittent erlotinib dosing with docetaxel in the treatment of former-smoker men affected by recurrent squamous non-small-cell lung cancer. Clin Lung Cancer 2011;12:258 | No relevant comparator |
| Hirsch FR, Dziadziuszko R, Thatcher N, Mann H, Watkins C, Parums DV, et al. Epidermal growth factor receptor immunohistochemistry: comparison of antibodies and cutoff points to predict benefit from gefitinib in a phase 3 placebo-controlled study in advanced non-small-cell lung cancer. Cancer 2008;12:1114–21 | Not relevant patient population |
| Hong J, Kyung SY, Lee SP, Park JW, Jung SH, Lee JI, et al. Pemetrexed versus gefitinib versus erlotinib in previously treated patients with non-small-cell lung cancer. Korean J Intern Med 2010;25:294–300 | Not a RCT |
| Johnson DH, Arteaga CL. Gefitinib in recurrent non-small-cell lung cancer: an IDEAL trial? J Clin Oncol 2003;21:2227–9 | Editorial |
| Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290:2149–58 | No relevant comparator |
| Kris MG, Natale RB, Herbst RS, Lynch TJ, Prager D, Belani CP, et al. A phase II trial of ZD1839 (‘IRESSA’) in advanced non-small-cell lung cancer (NSCLC) patients who had failed platinum- and docetaxel-based regimens (IDEAL 2) [abstract]. Proc Am Soc Clin Oncol 2002;21:292a, abstract 1166 | No relevant comparator |
| Leki R, Kawahara M, Watanabe H, Takada Y, Mori K, Yana T, et al. The impact of response evaluation committee in a phase III study (V-15-32) of gefitinib versus docetaxel in Japanese patients with non-small-cell lung cancer [Abstract No. 298P]. Ann Oncol 2009;19(Suppl. 8):109–10 | No relevant outcome |
| Leki R, Kawahara M, Watanabe H, Takada Y, Mori K, Yana T, et al. The impact of response evaluation committee in a phase III study (V-15-32) of gefitinib versus docetaxel in Japanese patients with non-small-cell lung cancer. Ann Oncol 2008;19:viii109–viii10 | No relevant outcome |
| Liu G, Cheng D, Ding K, Maitre A, Liu N, Patel D, et al. Pharmacogenetic analysis of BR.21, a placebo-controlled randomized phase III clinical trial of erlotinib in advanced non-small-cell lung cancer. J Thorac Oncol 2012;7:316–22 | No relevant outcome |
| Liu G, Cheng D, Le Maitre A, Liu N, Chen Z, Seymour L, et al. EGFR and ABCG2 polymorphisms as prognostic and predictive markers in the NCIC CTG BR.21 trial of single-agent erlotinib in advanced non-small-cell lung cancer (NSCLC). J Clin Oncol 2010;28:7538 | No relevant outcome |
| Liu G, Cheng D, Le Maitre A, Liu N, Chen Z, Seymour L, et al. Genetic polymorphisms as prognostic/predictive biomarkers of single-agent erlotinib therapy in NCIC-CTG BR.21 non-small-cell lung cancer (NSCLC) trial. Pharmacoepidemiol Drug Safety 2010;19:S207 | No relevant outcome |
| Manegold C, Gatzemeier U, Kaukel E. Results from a randomised, double blind phase II trial of ZD1839 (IRESSA) as 2nd/3rd-line monotherapy in advanced non small cell lung cancer (NSCLC) (IDEAL 1). J Cancer Res Clin Oncol 2002;128(Suppl. 1):S45 | No relevant comparator |
| Morere JF, Brechot JM, Westeel V, Gounant V, Lebeau B, Vaylet F, et al. Randomized phase II trial of gefitinib or gemcitabine or docetaxel chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2 or 3 (IFCT-0301 study). Lung Cancer 2010;70:301–7 | First-line treatment |
| Murphy M, Stordal B. Erlotinib or gefitinib for the treatment of relapsed platinum pretreated non-small-cell lung cancer and ovarian cancer: a systematic review (structured abstract). Drug Resist Updates 2011;14:177–90 | Review |
| Natale RB, Skarin A, Maddox AM, Hammond LA, Thomas R, Gandara DR, et al. Improvement in symptoms and quality of life for advanced non-small-cell lung cancer patients receiving ZD1839 (‘IRESSA’) in IDEAL 2 [abstract]. Proc Am Soc Clin Oncol 2002;21:292a, abstract 1167 | No relevant comparator |
| National Horizon Scanning Centre. Erlotinib (Tarceva) for Non Small Cell Lung Cancer – Advanced or Metastatic, Maintenance after First-Line Therapy and Second Line (in Combination with Bevacizumab): Horizon Scanning Technology Briefing. Birmingham: National Horizon Scanning Centre (NHSC); 2009 | Not a RCT |
| National Institute for Health and Care Excellence (NICE). Erlotinib for the Treatment of Non-Small-Cell Lung Cancer (Structured Abstract). London: NICE; 2008. | Not a RCT |
| National Institute for Health and Care Excellence (NICE). Gefitinib for the Second-Line Treatment of Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (Terminated Appraisal). London: NICE; 2009 | Not a RCT |
| National Horizon Scanning Centre. IRESSA for NSCLC – horizon scanning review. Birmingham: National Horizon Scanning Centre (NHSC); 2002 | Review |
| Niho S. [V15-32 and INTEREST]. Japan J Lung Cancer 2009;49:944–9 | Report |
| Nishiwaki Y, Yano S, Tamura T, Nakagawa K, Kudoh S, Horai T, et al. [Subset analysis of data in the Japanese patients with NSCLC from IDEAL 1 study on gefitinib.] Gan To Kagaku Ryoho 2004;31:567–73 | No relevant comparator |
| Park K, Goto K. A review of the benefit-risk profile of gefitinib in Asian patients with advanced non-small-cell lung cancer. Curr Med Res Opin 2006;22:561–73 | Review |
| Park SJ, Kim HT, Lee DH, Kim KP, Kim SW, Suh C, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small-cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556–60 | Not a RCT |
| Reinmuth N, Thomas M. An approach to personalized medicine: the BATTLE trial. Clin Investig 2011;1:699–705 | No relevant comparator |
| Robinson DM, Keating GM, Perry CM. Erlotinib. Am J Cancer 2005;4:247–52 | Review |
| Roman PS, Leon L, Slawomir WP. Cutaneous toxicity secondary to erlotinib therapy in patients with non-small-cell lung cancer in the NCIC CTG BR.21 study: time course and correlation with survival. J Clin Oncol 2012;30:7573 | No relevant outcome |
| Rosell R, Bastus R, Olaverri A, Anton I, Blanco R, Domine M, et al. Customized chemotherapy based on BRCA1 mRNA expression and EGFR mutations in lung adenocarcinoma. Ann Oncol 2008;19:viii93 | Not a RCT |
| Rossi D, Dennetta D, Ugolini M, Catalano V, Alessandroni P, Giordani P, et al. Activity and safety of erlotinib as second- and third-line treatment in elderly patients with advanced non-small-cell lung cancer: a phase II trial. Target Oncol 2010;5:231–5 | Not a RCT |
| Sequist LV, Muzikansky A, Engelman JA. A new BATTLE in the evolving war on cancer. Cancer Discov 2011;1:14–16 | Review |
| Sim EHA, Yang IA, Fong K, Wood-Baker R, Bowman R. Gefitinib for advanced non-small-cell lung cancer. Cochrane Database Syst Rev 2007;4:CD006847 | Protocol |
| Sorlini C, Barni S, Petrelli F, Novello S, De Marinis F, De Pas TM, et al. PROSE: randomized proteomic stratified phase III study of second line erlotinib versus chemotherapy in patients with inoperable non-small-cell lung cancer (NSCLC). J Clin Oncol 2011;1 | Not relevant comparator |
| [Tyrosine kinase inhibitor erlotinib (Tarceva) improves survival of patients with multiple previous treatments]. Krankenpfl J 2004;42:158 | Non-English |
| Wheatley-Price P, Ding K, Seymour L, Clark GM, Shepherd FA. Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008;26:2350–7 | Subgroup analysis |
| Xu B, Lee D, Ranganathan A. Highlights from: The 2009 Annual Meeting of the American Society of Clinical Oncology. Clin Lung Cancer 2009;10:217–22 | Review |
| Yamamoto N, Nishiwaki Y, Negoro S, Jiang H, Itoh Y, Saijo N, et al. Disease control as a predictor of survival with gefitinib and docetaxel in a phase III study (V-15-32) in advanced non-small-cell lung cancer patients. J Thorac Oncol 2010;5:1042–7 | No relevant outcome |
| Zielinski SL, Travis K. Randomized trial of gefitinib for advanced lung cancer closed early. J Natl Cancer Inst 2005;97:712 | Not relevant patient population |
Appendix 5 Systematic reviews
Quality appraisal of identified reviews
Six systematic reviews were identified. Two were reported as conference abstracts99,100 and a third101 was a Chinese language publication with an English abstract and data extraction tables in English. These three reviews did not lend themselves well to the quality assessment exercise. In the three full-text publications the reporting quality was high. These reviews, however, pooled data from the included trials. The AG considers this pooling to be inappropriate.
| Quality criterion | aBianic et al. (2011)99 | bGuo et al. (2011)101 | Hawkins et al. (2008)102 | Jiang et al. (2011)103 | aKris et al. (2009)100 | Petrelli et al. 2012104 |
|---|---|---|---|---|---|---|
| Was the review question clearly defined in terms of population, interventions, comparators, outcomes and study designs? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Was the search strategy adequate and appropriate? | NS | ✓ | ✓ | ✓✗c | NS | ✓ |
| Were preventative steps taken to minimise bias and errors in the study selection process? | NS | NS | ✓ | NS | NS | NS |
| Were appropriate criteria used to assess the quality of the primary studies? | NS | ✓ | ✓ | ✓ | NS | ✗ |
| Were preventative steps taken to minimise bias and errors in the QA process? | NS | NS | NS | NS | NS | NS |
| Were preventative steps taken to minimise bias and errors in the data extraction process? | NS | ✓ | ✓ | ✓ | NS | NS |
| Were adequate details presented for each of the primary studies? | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Were appropriate methods used for data synthesis? Were differences between studies assessed? Were the studies pooled, and if so was it appropriate and meaningful to do so? | NS | Unclear | ✗d | ✗d | Unclear | ✗d |
| Do the authors’ conclusions accurately reflect the evidence that was reviewed? | Unclear from abstract | Unclear from abstract | ✓✗d | ✗d | Unclear from abstract | ✗d |
Table of identified systematic reviews: summary
| Review | Title | Patient population | Stated purpose and studies included | Main conclusions |
|---|---|---|---|---|
| aBianic (2011)99 | Network meta-analysis of second and third-line treatments on overall response and overall survival in patients with metastatic non-small-cell lung cancer | Metastatic NSCLC who have progressed after first-line treatment | To perform a network meta-analysis of recommended second/third-line treatments for overall response and survival in metastatic NSCLC. Included seven RCTs: JMEI, TAX317, V-15-32, INTEREST, ISTANA, ISEL and BR.21 | Evidence for second/third-line treatment effects on response is stronger than evidence for survival. The exceptions are targeted therapies – this class is likely to be the most promising source for badly needed new therapies |
| Guo (2011)101 | Gefitinib for non-small-cell lung cancer: a meta-analysis | First- and second-line NSCLC | To evaluate the clinical efficacy and safety of gefitinib for NSCLC. Meta-analysis of 13 RCTs | Gefitinib shows more superiority for NSCLC and its clinical application is worthy to be advocated |
| Hawkins (2008)102 | Time to broaden our horizons, the case for network meta-analysis within relapsed non-small-cell lung cancer (NSCLC) | Locally advanced/metastatic NSCLC who have progressed after first-line treatment | Network meta-analysis of six RCTs including SIGN, JMEI, TAX317, BR.21, INTEREST and ISEL | The analysis of the limited network suggested that docetaxel is more effective than erlotinib, whereas the analysis of the extended network suggested the opposite |
| Jiang (2011)103 | Gefitinib versus docetaxel in previously treated advanced non-small-cell lung cancer: a meta-analysis of randomized controlled trials | Previously treated NSCLC | To compare the efficacy, QoL, symptom improvement and toxicities of gefitinib with docetaxel in previously treated advanced NSCLC. Analysis of four RCTs: ISTANA, V-15-32, INTEREST and SIGN | Although similar for OS and PFS, gefitinib showed an advantage over docetaxel in terms of objective RR, QoL and tolerability. Therefore, gefitinib is an important and valid treatment option for previously treated advanced NSCLC patients |
| aKris (2009)100 | Response and progression-free survival in 1006 patients with known EGFR mutation status in Phase III randomized trials of gefitinib in individuals with non-small-cell lung cancer | NSCLC | Phase III trials of gefitinib monotherapy, focusing on patients with known EGFR mutation status | These results justify pre-treatment determination of EGFR mutation status at the time of diagnosis to select therapy with higher response and improved PFS |
| Petrelli (2012)104 | Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated nonsmall-cell lung cancer: a meta-analysis of 13 randomized trials | Previously treated or untreated EGFR M+ NSCLC | Phase II or III RCTs of gefitinib or erlotinib compared with chemotherapy, BSC or placebo. Included first-line trials and INTEREST, BR.21, ISEL and V-15-32 | Selecting patients with NSCLC for EGFR mutations and offering them an EGFR-TKI results in a better RR and progression-delaying effect than does standard chemotherapy. The performance appears similar in second-line settings in which the chance of obtaining a response is 63% higher with EGFR-TKIs |
Appendix 6 Data abstraction tables
| Trial | Key inclusion criteria | Key exclusion criteria |
|---|---|---|
| aBhatnagar 201233 |
|
|
| BR.21 200531 |
|
|
| aDELTA 201340 |
|
|
| INTEREST 200834 |
|
|
| ISEL 200539 |
|
|
| ISTANA 201035 |
|
|
| Kim 201232 |
|
|
| Li et al. 201036 |
|
|
| SIGN 200637 |
|
|
| TAILOR 201341 |
|
|
| TITAN 201242 |
|
|
| V-15-32 200838 |
|
|
Appendix 7 Details of probabilistic sensitivity analysis: survival model parameters
All survival parameters are assumed to be drawn from normal distributions. Please note that the following terms and their abbreviations have been used in Tables 58–67.
| Term | Abbreviation |
|---|---|
| Zero time hazard | S0 |
| First spline knot | S1 |
| Second spline knot | S2 |
| Hazard rate – phase 1 | R1 |
| Hazard rate – phase 2 (erlotinib) | R2E |
| Hazard rate – phase 2 (docetaxel) | R2D |
| Hazard rate – phase 3 | R3 |
| Hazard rate – phase 3 (erlotinib) | R3E |
| Hazard rate – phase 3 (docetaxel) | R3D |
| BSC intercept | B |
| Erlotinib intercept | E |
| Common hazard rate | R |
| BSC phase 1 intercept | A |
| BSC phase 1 hazard rate | BSCR1 |
| Spline knot time | S |
| BSC phase 2 hazard rate | BSCR2 |
| Parameters (monthly) | Estimate | Standard error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| S1 | 1.95859 | 0.09800 | 1.76442 | 2.15277 |
| S2 | 6.46245 | 0.14348 | 6.17816 | 6.74675 |
| R1 | 0.06972 | 0.00226 | 0.06525 | 0.07420 |
| R2E | 0.16142 | 0.00342 | 0.15465 | 0.16820 |
| R2D | 0.10000 | 0.00177 | 0.09651 | 0.10350 |
| R3 | 0.06118 | 0.00136 | 0.05849 | 0.06388 |
| Correlation | S1 | S2 | R1 | R2E | R2D | R3 |
|---|---|---|---|---|---|---|
| S1 | 1 | –0.295 | 0.608 | 0.699 | 0.171 | 0.040 |
| S2 | – | 1 | 0.008 | –0.635 | –0.434 | –0.461 |
| R1 | – | – | 1 | 0.080 | –0.436 | 0.057 |
| R2E | – | – | – | 1 | 0.551 | 0.061 |
| R2D | – | – | – | – | 1 | –0.218 |
| R3 | – | – | – | – | – | 1 |
| Parameters (monthly) | Estimate | Standard error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| S0 | 0.02216 | 0.00424 | 0.01384 | 0.03048 |
| S1 | 1.71743 | 0.01793 | 1.68229 | 1.75257 |
| S2 | 2.88616 | 0.03963 | 2.80848 | 2.96385 |
| R1 | 0.14308 | 0.00466 | 0.13395 | 0.15222 |
| R2E | 0.71455 | 0.01608 | 0.68303 | 0.74607 |
| R2D | 0.42007 | 0.00939 | 0.40167 | 0.43848 |
| R3E | 0.25035 | 0.01025 | 0.23025 | 0.27044 |
| R3D | 0.17527 | 0.00497 | 0.16554 | 0.18501 |
| Correlation | S0 | S1 | S2 | R1 | R2E | R2D | R3E | R3D |
|---|---|---|---|---|---|---|---|---|
| S0 | 1 | –0.283 | –0.003 | –0.817 | –0.050 | 0.117 | 0.017 | –0.028 |
| S1 | – | 1 | –0.259 | 0.560 | 0.673 | 0.305 | –0.039 | 0.066 |
| S2 | – | – | 1 | 0.006 | –0.552 | –0.500 | –0.451 | –0.426 |
| R1 | – | – | – | 1 | 0.100 | –0.232 | –0.033 | 0.056 |
| R2E | – | – | – | – | 1 | 0.541 | –0.098 | 0.167 |
| R2D | – | – | – | – | – | 1 | 0.147 | –0.250 |
| R3E | – | – | – | – | – | – | 1 | 0.212 |
| R3D | – | – | – | – | – | – | – | 1 |
| Parameters (weekly) | Estimate | Standard error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Intercept | 0.30686 | 0.01474 | 0.27724 | 0.33648 |
| Hazard rate | 0.04167 | 0.00036 | 0.04094 | 0.04240 |
| Correlation | Intercept | Hazard rate |
|---|---|---|
| Intercept | 1 | –0.878 |
| Hazard rate | – | 1 |
| Parameters (daily) | Estimate | Standard error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| B | 0.42445 | 0.02050 | 0.38371 | 0.46519 |
| E | –0.02941 | 0.02048 | –0.07011 | 0.01128 |
| R | 0.00320 | 0.00005 | 0.00311 | 0.00330 |
| Correlation | B | E | R |
|---|---|---|---|
| B | 1 | 0.909 | –0.935 |
| E | – | 1 | –0.972 |
| R | – | – | 1 |
| Parameters (daily) | Estimate | Standard error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| B | 1.46083 | 0.05163 | 1.35702 | 1.56464 |
| E | 0.14557 | 0.05047 | 0.04409 | 0.24705 |
| R | 0.00664 | 0.00015 | 0.00634 | 0.00694 |
| Correlation | B | E | R |
|---|---|---|---|
| B | 1 | 0.811 | –0.829 |
| E | 1 | –0.979 | |
| R | 1 |
| Parameters (monthly) | Estimate | Standard error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Erlotinib intercept | –0.00978 | 0.01237 | –0.03449 | 0.01494 |
| Erlotinib hazard rate | 0.09791 | 0.00137 | 0.09517 | 0.10065 |
| Correlation | Intercept | Hazard rate |
|---|---|---|
| Intercept | 1 | –0.856 |
| Hazard rate | – | 1 |
| Parameters (monthly) | Estimate | Standard error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| A | –0.30146 | 0.05571 | –0.41539 | –0.18752 |
| BSCR1 | 0.31157 | 0.02270 | 0.26515 | 0.35799 |
| S | 3.75313 | 0.19346 | 3.35747 | 4.14880 |
| BSCR2 | 0.07890 | 0.00414 | 0.07043 | 0.08737 |
| Correlation | A | R1 | S | R2 |
|---|---|---|---|---|
| A | 1 | –0.957 | 0.574 | 0.000 |
| BSCR1 | – | 1 | –0.708 | 0.000 |
| S | – | – | 1 | –0.466 |
| BSCR2 | – | – | – | 1 |
| Parameters (daily) | Estimate | Standard error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Erlotinib intercept | 0.15445 | 0.03923 | 0.07480 | 0.23410 |
| Erlotinib hazard rate | 0.00623 | 0.00016 | 0.00590 | 0.00655 |
| Correlation | Intercept | Hazard rate |
|---|---|---|
| Intercept | 1 | –0.882 |
| Hazard rate | 1 |
| Parameters (daily) | Estimate | Standard error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| BSC intercept | 0.65426 | 0.08620 | 0.47053 | 0.83798 |
| BSC hazard rate | 0.00959 | 0.00043 | 0.00867 | 0.01051 |
| Correlation | Intercept | Hazard rate |
|---|---|---|
| Intercept | 1 | –0.885 |
| Hazard rate | – | 1 |
Appendix 8 Rates of febrile neutropenia associated with treatment with docetaxel
See addendum 1 and addendum 2 of the AG report. 105
Evidence from published trials
Several approaches can be taken to estimate of the proportion of patients treated with docetaxel monotherapy who will experience one or more episodes of grade 3 or 4 FN as a result of treatment. A total of eight different estimated incidence rates were identified as follows.
Assessment Group base case (TAILOR)41
Four patients in TAILOR41 were reported to have experienced grade 3 or 4 FN in the docetaxel arm, all of whom were in the subgroup of 63 patients treated every 3 weeks with high-dose docetaxel (75mg/m2 body surface area). This corresponds to an incidence rate of 6.35% (95% CI 1.79% to 13.50%) and relates to the dose and frequency of docetaxel administration most commonly used in the UK.
Decision Support Unit Report94
During the first appraisal of erlotinib versus docetaxel in second-line chemotherapy for NSCLC (NICE TA16221) the Decision Support Unit was asked to investigate the incidence of FN and its associated treatment costs. They conducted a meta-analysis of reported trials and estimated the incidence as 5.95% (95% CI 5.30% to 7.70%).
TAILOR trial41 (all patients)
If no distinction is made between high dose (3-weekly) and low dose (weekly docetaxel 35mg/m2 body surface area), the FN incidence rate is 3.85% (95% CI 1.07% to 8.28%).
Other trials (pre-epidermal growth factor testing)
Data from 17 randomised clinical trials,34,37,41,42,49,64,74,85,106–115 which included 3-weekly high-dose docetaxel monotherapy as one treatment arm, were combined to provide a weighted average incidence rate. It was not possible to carry out a formal meta-analysis because of the diversity of comparators, populations and settings of these trials. The weighted average estimate is 7.3% (95% CI 6.3% to 8.3%). Heterogeneity testing of trial incidence values identified that two of the larger trials34,49 exhibited significantly higher incidence rates than the remaining 15 trials. Therefore, two weighted average values were selected for sensitivity testing, 10.8% (95% CI 8.9% to 12.8%) and 5.0% (95% CI 4.0% to 6.2%), corresponding to these distinct data subsets. The maximum estimated incidence among all 17 trials, 12.7% (95% CI 9.0% to 16.8%) was also selected for exemplification in the decision model.
Extreme sensitivity analysis
In order to explore the impact of a very high incidence rate, the value of the greatest upper confidence limit of any of these 17 trial arms was selected – 25%.
Comment on Royal College of Physicians suggested incidence rates
In the Royal College of Physicians submission document to NICE it is stated that:
‘In clinical practice, admission rates for neutropaenic sepsis and treatment complications are 25–50% with docetaxel compared to < 5% with erlotinib’. 116
Unfortunately no supporting evidence was cited for this statement. Subsequently, the Royal College of Physicians responded to the Appraisal Consultation Document citing a conference abstract by Sharma117 of an observational study of admissions in three NHS trusts to support a figure of 41%. The abstract shows that 41% is the total number of hospital admissions in second-line docetaxel treatment (9 out of 22 admissions), whereas only four of these were a result of neutropenic sepsis (i.e. 18%). In addition, it should be noted that admission rates are necessarily higher than incidence rates, as the Decision Support Unit estimated that affected patients require an average of 1.4 admissions per patient. Using this factor to adjust admission rates to incidence rates, the best estimate from the Sharma117 study is an incidence rate of 13.0% (95% CI 2.7% to 29.5%). The small numbers involved and the wide CI (which encompasses all of the eight estimates listed above) indicates that these data add nothing useful to the consideration of FN incidence rates.
Sensitivity analysis for febrile neutropenia incidence
Table 68 summarises the cost-effectiveness results for the AG-revised base case and the seven alternative FN scenarios described previously. In all cases erlotinib is not cost-effective compared with docetaxel, because the cost and utility effect of varying FN incidence is not sufficient to counteract the estimated survival advantage of docetaxel. The incremental cost is zero for a FN rate of 16.2% (equal cost, but QALY gain for docetaxel). The ICER for erlotinib versus docetaxel only exceeds £30,000 cost savings per QALY lost for docetaxel FN incidence rates above 63%.
| Scenario | FN incidence (%) | Erlotinib | Docetaxel | Incremental | ICER | |||
|---|---|---|---|---|---|---|---|---|
| Total cost | Total QALYs | Total cost | Total QALYs | Cost | QALYs | £/QALY | ||
| AG-revised base case | 6.35 | £14,049 | 0.4863 | £13,504 | 0.5930 | £545 | –0.1067 | –£5112 (dominated) |
| Decision Support Unit estimate | 5.95 | £14,049 | 0.4863 | £13,482 | 0.5931 | £567 | –0.1067 | –£5312 (dominated) |
| TAILOR trial41 (all patients) | 3.85 | £14,049 | 0.4863 | £13,365 | 0.5939 | £684 | –0.1076 | –£6353 (dominated) |
| Weighted average (all trials) | 7.26 | £14,049 | 0.4863 | £13,554 | 0.5926 | £495 | –0.1063 | –£4654 (dominated) |
| Weighted average (2 high-incidence trials) | 10.80 | £14,049 | 0.4863 | £13,749 | 0.5913 | £300 | –0.1050 | –£2854 (dominated) |
| Weighted average (15 low-incidence trials) | 5.03 | £14,049 | 0.4863 | £13,431 | 0.5934 | £618 | –0.1072 | –£5768 (dominated) |
| Maximum trial | 12.68 | £14,049 | 0.4863 | £13,853 | 0.5906 | £196 | –0.1044 | –£1876 (dominated) |
Table 69 provides an overview of the three estimated AG base-case ICERs made available to the AC during this appraisal.
| Amendment | Incremental cost | Incremental QALYs | Deterministic ICER | Probabilistic ICER |
|---|---|---|---|---|
| AG report estimate | –£1653 | –0.1076 | £15,359/QALY | £12,719/QALY |
| Amended for FN incidence rate (6.35%) (addendum 1) | –£3311 | –0.1076 | £31,039/QALY | £28,328/QALY |
| Amended for FN incidence rate and corrected FN cost calculation (addendum 2) | £545 | –0.1076 | –£5112/QALY (dominated) | –£7709/QALY (dominated) |
List of abbreviations
- AC
- Appraisal Committee
- AE
- adverse event
- AG
- Assessment Group
- BNF
- British National Formulary
- BSC
- best supportive care
- CI
- confidence interval
- DELTA
- Docetaxel and Erlotinib Lung Cancer Trial
- ECOG
- Eastern Cooperative Oncology Group
- EE
- economic evaluation
- EGFR
- epidermal growth factor receptor
- EGFR M–
- epidermal growth factor mutation negative
- EGFR M+
- epidermal growth factor mutation positive
- EGFR unknown
- epidermal growth factor mutation status unknown
- eMit
- electronic market information tool
- EQ-5D
- European Quality of Life-5 Dimensions
- FDA
- Food and Drug Administration
- FN
- febrile neutropenia
- HR
- hazard ratio
- HRG
- health research group
- ICER
- incremental cost-effectiveness ratio
- INTEREST
- IRESSA NSCLC Trial Evaluating REsponse and Survival versus Taxotere
- ISEL
- IRESSA Survival Evaluation in Lung cancer
- ISTANA
- IRESSA as Second-line Therapy in Advanced NSCLC – KoreA
- ITT
- intention to treat
- i.v.
- intravenous
- KPS
- Karnofsky Performance Status scale
- LY
- life-year
- NICE
- National Institute for Health and Care Excellence
- NSCLC
- non-small cell lung cancer
- OS
- overall survival
- PD
- progressed disease
- PFS
- progression-free survival
- PPS
- post-progression survival
- PS
- performance status
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- response rate
- SIGN
- Second-line Indication of Gefitinib in NSCLC
- SPC
- summary of product characteristics
- TAILOR
- TArceva Italian Lung Optimization tRial
- TITAN
- Tarceva In Treatment of Advanced NSCLC
- TKI
- tyrosine kinase inhibitor
- WHO
- World Health Organization
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence data removed and replaced by the statement ‘commercial-in-confidence is removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.


