Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/71/01. The contractual start date was in August 2013. The draft report began editorial review in March 2014 and was accepted for publication in March 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Royle et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
The commissioning brief notes that diabetic retinopathy (DR) is the major cause of sight loss in the working age population in the UK, and that people with diabetes are 25 times more likely than the general population to go blind. Pan-retinal photocoagulation (PRP) by laser treatment is the standard intervention for patients with proliferative diabetic retinopathy (PDR), and it has been shown to reduce the risk of severe vision loss by 50%.
In some areas, such as Newcastle District, diabetes may no longer be the leading cause of blindness in the population of working age, because of the success of the screening and treatment programmes. 1 A review by Wong et al. (2009)2 concluded that rates of progression to PDR have fallen over recent times because of earlier identification and treatment of retinopathy, and improved control of blood glucose and blood pressure (BP). This is supported by a recent paper from Wisconsin. 3
Nevertheless, DR remains common. A Liverpool study by Younis et al. (2003)4 reported prevalences of any DR and PDR to be 46% and 4%, respectively in type 1 diabetes, and 25% and 0.5% in type 2 diabetes, although the prevalence will vary with mean duration of diabetes, with higher proportions of those with longer duration having DR. Conversely, an increasing incidence of type 2 diabetes would reduce the overall proportion with DR because more people with short duration would be entering the pool.
Introduction to diabetic retinopathy
The Royal College of Ophthalmologists (RCOphth) guidelines define DR as:5
Diabetic retinopathy is a chronic progressive, potentially sight-threatening disease of the retinal microvasculature associated with the prolonged hyperglycaemia and other conditions linked to diabetes mellitus such as hypertension.
And continues:
Diabetic retinopathy is a potentially blinding disease in which the threat to sight comes through two main routes: growth of new vessels leading to intraocular haemorrhage and possible retinal detachment with profound global sight loss, and localised damage to the macula/fovea of the eye with loss of central visual acuity.
Reproduced with permission from The Royal College of Ophthalmologists. Guidelines for Diabetic Retinopathy. 2012. URL: www.rcophth.ac.uk/page.asp?section=451§ionTitle=Clinical+Guidelines (accessed 24 September 2013). 5
Diabetic retinopathy is due to damage to the retina, particularly to its blood vessels, caused by raised blood glucose levels. The earliest changes tend to affect the capillaries, starting with dilatation.
The next stage is closure of some capillaries leading to loss of blood flow (non-perfusion) to part of the retina. If large areas of the retina are deprived of their blood supply they may be seen as paler areas. Smaller areas of ischaemia may be detected only by fluorescein angiography (FA). In this investigation, a dye is injected into a vein and passes through the blood vessels which can then be seen, thereby revealing areas without blood flow.
Non-perfusion due to capillary occlusions is the most important feature of DR, as it leads to other changes.
Capillary closure is associated with two other features: cotton wool spots and blot haemorrhages.
Cotton wool spots are so called because they appear as greyish white patches in the retina instead of the usual red colour. They are areas where blood flow has ceased. There are usually only a few, but if many (more than 6–10 in one eye) develop, it may be a sign of rapidly developing serious retinopathy.
Haemorrhages come in different sizes and shapes, referred to as ‘dot and blot’. Multiple large haemorrhages are a bad sign and indicate large areas of non-perfusion. They may herald proliferative retinopathy.
Microaneurysms appear as small red dots in the retina. These are due to dilated capillaries. Small ones may not be visible with the ophthalmoscope but are revealed by FA. With ophthalmoscopy, it may not be possible to distinguish microaneurysms from small haemorrhages.
Damage to arteries also occurs, with thickening of the walls of the artery and narrowing of the lumen, and sometimes blockage (occlusion) of the artery, thereby reducing blood flow to parts of the retina.
There are also changes in the retinal veins, such as dilatation, and sometimes looping. Loops are usually related to areas of capillary non-perfusion. Venous beading can occur and is one of the signs of severe non-proliferative diabetic retinopathy (NPDR). The walls of the veins may be thickened. Retinal vein thrombosis may follow – this is known as retinal vein occlusion.
Abnormal new retinal blood vessels may develop and are the most serious manifestation of DR. This is called ‘neovascularisation’. Because these vessels are new, their presence is referred to as indicating ‘proliferative’ retinopathy. The new abnormal vessels are fragile and are more liable to bleed, causing haemorrhages. If they bleed into the vitreous, a gel-like structure that fills the eye, the result is called vitreous haemorrhage. They may also lead to the formation of fibrous scar tissue that can put traction on the retina, leading to tractional retinal detachments. Rarely, they may regress spontaneously.
Exudates are yellowish white patches, initially small specks but may later form larger plaques. They are usually near the macula, the most sensitive part of the eye, and are associated with areas of oedema. They contain lipid deposits.
Retinopathy takes years to develop. It is not seen at diagnosis of type 1 diabetes. If seen at diagnosis of type 2 diabetes, it is an indication that the patient had undiagnosed diabetes for years.
Retinopathy may go through several stages. The first stage is called non-proliferative diabetic retinopathy (NPDR), previously known as background DR. It is very common and most people with long-standing diabetes will have it. The features include microaneurysms, haemorrhages, hard exudates and occasional cotton wool spots. Progression is variable and the changes may regress. The prognosis for mild NPDR is good, but some patients will progress to the more serious forms of NPDR, macular oedema (MO) (maculopathy) and proliferative retinopathy. The presence of multiple cotton wool spots and widespread retinal haemorrhages may indicate that proliferative retinopathy is developing. Large blot haemorrhages are usually followed by new vessels within a few months.
Maculopathy refers to visual loss due to MO (fluid leaking out of blood vessels into the macula, making it swell). It can occur in the absence of proliferative retinopathy, especially in type 2 diabetes. Maculopathy can lead to gradual visual deterioration from increasing oedema, although it can also resolve spontaneously.
About half of the people with proliferative retinopathy also have MO, but it can occur at earlier stages without PDR.
For a useful description, see www.nei.nih.gov/health/diabetic/retinopathy.asp.
Classification of diabetic retinopathy
Classification and severity grading of DR have historically been based on ophthalmoscopically visible signs of increasing severity, ranked into a stepwise scale from no retinopathy through various stages of non-proliferative or pre-proliferative disease to advanced proliferative disease.
Two different approaches to classification have emerged: (1) those used in ophthalmology, covering the full range of retinopathy, based on the Airlie House/Early Treatment Diabetic Retinopathy Study (ETDRS) classification and (2) those used in population screening.
There are various methods of classifying DR. As one protocol referee noted, no grading system is ideal for all purposes. Older studies used the ETDRS modification of the Airlie House classification and this is said to be the gold standard for classifying DR. 6
The commissioning brief refers to R2, which comes from the classification used by the English National Diabetic Retinal Screening Programme:7
-
R0 No retinopathy.
-
R1 Background – microaneurysms, retinal haemorrhages, with/without any exudate. This is broadly equivalent to the ETDRS mild NPDR stage.
-
R2 Pre-proliferative – multiple blot haemorrhages, intraretinal microvascular abnormalities (IRMAs). Moderate NPDR, referable to Ophthalmology.
-
R3 PDR.
The Scottish Diabetic Retinopathy Screening Service classification is shown in Table 1, and is slightly more detailed. 8
| Stage | Description | Action required |
|---|---|---|
| Retinopathy | ||
| R0 | No retinopathy anywhere | Routine rescreening at 12 months |
| R1 | Mild background retinopathy | Rescreen at 12 months |
| R2 | Background retinopathy requiring monitoring for progression | Rescreen at 6 months |
| R3 | Background retinopathy sufficient to require referral | Refer to Ophthalmology, probably for surveillance rather than laser treatment |
| R4 | Proliferative retinopathy | Refer to Ophthalmology, probably for laser treatment |
| Maculopathy | ||
| M0 | No features predictive of maculopathy | Rescreen 12 months |
| M1 | Any hard exudates within one to two DDs of the centre of the macula | Rescreen in 6 months |
| M2 | Any hard exudates or blot haemorrhages within one disc radius of the centre of the macula | Refer to Ophthalmology, probably for surveillance rather than laser treatment |
One problem with these classifications is that, for our purposes, the key category (R2 England or R3 Scotland), is too broad, as we are interested in the groups with severe NPDR or very severe NPDR. Another problem is that the term ‘pre-proliferative’ is sometimes used as synonymous with non-proliferative, but this usage implies that all NPDR progress to PDR, which is not the case.
There are problems with published studies because some authors talk simply of ‘moderate’ or ‘mild’ DR and do not provide sufficient data to determine the more detailed grading – as used by ETDRS. In this review, when studies have not used an accepted classification such as ETDRS, we have tried to extract enough details to allocate patients or studies to a classification as below, so that results can be expressed in terms of defined risk and features.
-
Mild to moderate NPDR:
-
intraretinal haemorrhage in fewer than four quadrants
-
microaneurysms
-
hard exudation
-
MO
-
abnormalities in the foveal avascular zone
-
-
moderate to severe NPDR:
-
mild/moderate intraretinal haemorrhage in four quadrants
-
cotton wool spots
-
venous beading
-
IRMAs
-
-
severe NPDR (4–2–1 rule) (one of the following):
-
severe intraretinal haemorrhage in four quadrants
-
venous beading in two quadrants
-
IRMA in one quadrant
-
-
very severe NPDR (two of the above)
-
proliferative diabetic retinopathy with or without high-risk characteristics (HRCs) (any three of the following):
-
presence of neovessels
-
location of the neovessels (at the optic nerve)
-
size of the neovessels: if at the optic nerve [neovascularisation of the disc (NVD)] ≥ ¼–⅓ disc area if elsewhere in the retina [neovascularisation of the retina elsewhere (outside the disc) (NVE) ≥ ½ of the disc area (if both NVD and NVE present, classified based on neovessels at the disc)
-
presence of pre-retinal haemorrhage or vitreous haemorrhage.
-
About half of patients with severe or very severe NPDR will progress to PDR within a year.
The descriptions used in the ETDRS9 are attached as Appendix 1.
Treatment of diabetic retinopathy
Laser treatment is not usually administered to people with NPDR. However, the commissioning brief from the Health Technology Assessment (HTA) programme poses the question whether:
intervention with pan-retinal laser treatment earlier in the disease, during the pre-proliferative stage (Level R2), may be more beneficial in terms of preventing loss of vision, given the detrimental and potentially irreversible effects of PDR if treatment is not obtained or is delayed.
Pan-retinal photocoagulation is sometimes referred to as scatter photocoagulation.
In considering treatment for retinopathy, three issues need to be considered:
-
The risk of visual loss without treatment.
-
The risk of visual loss with treatment.
-
The adverse effects of treatment. Laser treatment is a destructive process that can cause loss of peripheral vision in order to preserve the more important central vision.
Laser photocoagulation has been of great benefit to many people with PDR but in most cases has been better for preserving vision than restoring it, though it can improve vision, for example in eyes that have vitreous haemorrhages.
Two key studies of PRP were published in the 1980s: the ETDRS9 and the Diabetic Retinopathy Study (DRS). 10 The ETDRS9 recruited people with NPDR and people with PDR but without HRCs, and one aim was to determine when PRP should be used. In the EDTRS,9 laser treatment reduced the risk of moderate visual loss (a loss of three ETDRS lines) by 50%, but visual acuity (VA) improved in only 3% of patients. These studies9,10 are described in Chapter 2.
Laser has adverse side effects. Foveal burns, visual field defects, retinal fibrosis and laser scars have been reported. Ability to drive can be affected. Hence laser treatment is not undertaken lightly, and to extend it to people with NPDR would require careful consideration.
Treatment of DR has been based largely on the results of the ETDRS9 and DRS. 10 A small non-randomised study reported that laser treatment in people with type 1 diabetes at the severe NPDR stage reduced visual loss, compared with waiting to treat at the PDR stage, but this difference did not reach statistical significance. 11 A Swedish study by Stenkula (using xenon arc photocoagulation) also reported benefit from treating severe NPDR in a trial of PRP with one eye randomised to treatment. 12
One consideration is that if PDR is being detected earlier and treated more effectively than at the time of the landmark trials, notably ETDRS9 in the early 1980s, then any marginal benefit of treating at the NPDR stage may now be less.
We are aware that PRP is usually used when people reach the proliferative stage, but also that there is some variation in how it is applied. Some ophthalmologists may start with sparse very scattered PRP, with further lasering if the retinopathy progresses. Others may start with full mid-peripheral PRP. A third approach might be to laser only areas of mid-peripheral ischaemia as seen on FA.
It may be used when patients have high-risk features, such as new vessels, or earlier, at severe NPDR stage. There are also different stages of PDR, with some patients being classified as ‘high-risk PDR’ (HR-PDR), and one protocol referee argued that laser was mainly of benefit in PDR with high-risk characteristics (HRC-PDR) and not all PDR.
Figure 1 (reproduced with permission) from a review by Neubauer and Ulbig (2007)13 outlines current practice. Since the landmark studies DRS10 and ETDRS,9 new laser devices have been introduced.
FIGURE 1.
Algorithm for pan-retinal scatter coagulation of the retina. Reproduced from Neubauer and Ulbig13 with permission from S. Karger AG, Basel.
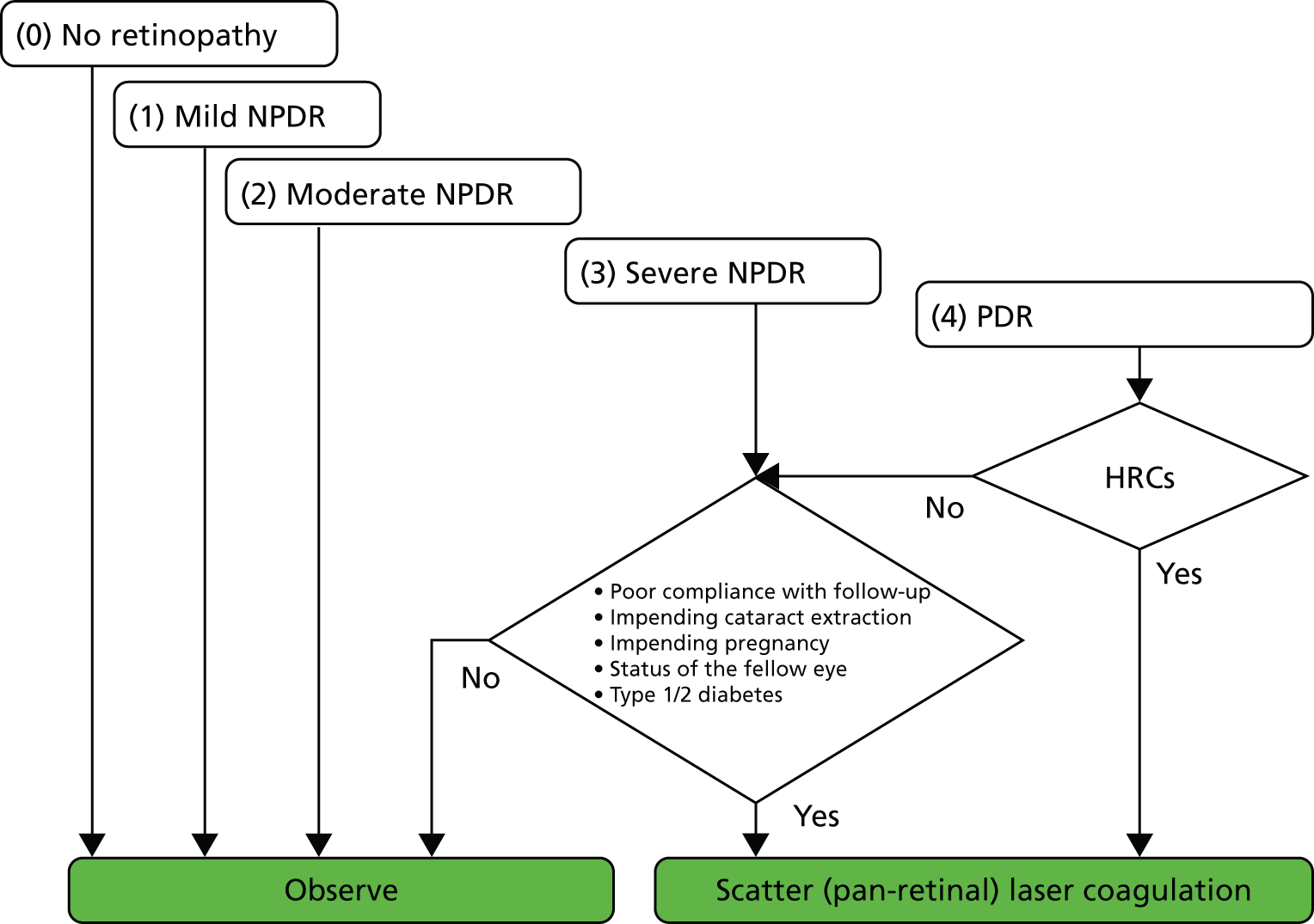
Argon and krypton lasers use ionised gas as the lasing medium, while the tunable dye laser uses a liquid solution. Neodynium-doped yttrium aluminium garnet (Nd:YAG) and diode lasers are both solid-state lasers that utilise crystals and semiconductors, respectively. The solid-state lasers are becoming the preferred option owing to their portability and ability to deliver laser in continuous and pulse mode.
Most people now use the pattern scan (PSC) or multi-spot lasers, rather than the argon laser, because they are faster and less painful. Some centres still use argon. With the traditional single-spot laser, treatment of large areas of the retina is time-consuming, can be uncomfortable for patients because of the length of time required, and there is a risk that, if the patient moves, laser may be mis-directed. With the pattern lasers, a number of spots can be applied simultaneously with one press of the foot pedal. In theory, this could be up to 56 with the PAtterned SCAnner Laser (PASCAL) system (developed by OptiMedica Corp, Santa Clara, CA, but now marketed by Topcon Corporation – Topcon UK, Newbury, Berkshire) but in practice smaller numbers are often used.
Other multi-spot lasers include the Valon TT (Valon Lasers, Vantaa, Finland), the Array LaserLink (Lumenis, Yokneam, Israel), the Navilas (OD-OS GmbH, Teltow, Germany) and the Quantel Supraspot (Quantel Medical, Cedex, France). However, most studies published used the PASCAL system. 14
The multi-spot system is much more comfortable for patients, and may reduce the number of sessions required.
The sub-threshold diode laser has been introduced though mainly for diabetic macular oedema (DMO)15,16 but has not spread much into use in PDR, possibly because for PRP it requires more sessions and more burns. It allows very short [millisecond (ms)] pulses of laser, shorter than conventional laser, sometimes called ‘micro-pulsed’. The sub-threshold refers to the visibility of burn spots. Photocoagulation is started with very low parameters, increased till a laser spot is seen, after which the power is reduced until the spot is just not seen – sub-threshold – and then the whole treatment is done at that level. But it is important to note that this is for treating the macula where the power used is much lower than that used in PRP.
We also note that in Japan a more selective approach to laser therapy is used, with targeting based on FA, so that only ischaemic areas are lasered. 17 This is a more restrictive approach than traditional PRP. Hence this review will need to classify methods of laser treatment.
Guidelines
Current guidelines from the RCOphth5 state that:
Mild and moderate DR does not require treatment, but patients should be monitored annually and advised to maintain as good diabetes control as possible.
Severe NPDR requires closer monitoring, usually every 6 months, in ophthalmology clinics, by clinical examination and digital photography. The aim is to detect progression to PDR.
In patients with very severe NPDR, PRP is considered in order to reduce progression in the following groups:
in older patients with type 2 diabetes
where monitoring of DR is difficult because of poor attendance or obscured retinal view
before cataract surgery, because that may be associated with progression
if vision has been lost in the other eye.
Reproduced with permission from The Royal College of Ophthalmologists. Guidelines for Diabetic Retinopathy. 2012. URL: www.rcophth.ac.uk/page.asp?section=451§ionTitle=Clinical+Guidelines (accessed 24 September 2013). 5
In patients with PDR, urgent PRP is recommended.
The guidelines note that some ophthalmologists treat at NPDR stages:
10.3.1 Earlier treatment: Recognition that earlier laser prevents progression to high risk retinopathy, and that PDR has higher risk of blindness was reported in both DRS and ETDRS (LEVEL 1). However the balance of risks with laser modalities available at that time meant that laser intervention was recommended only when retinopathy approached high risk PDR. With modern laser techniques, PRP is often done before the development of PDR.
The RCOphth include, in the guidelines, a useful table (Table 2) comparing the different classifications.
| ETDRS | NSC | SDRGS | AAO International | RCOphth |
|---|---|---|---|---|
| 10 none | R0 none | R0 none | No apparent retinopathy | None |
| 20 microaneurysms only | R1 background | R1 mild background | Mild NPDR | Low risk |
| 35 mild NPDR | Moderate NPDR | |||
| 43 moderate NPDR | R2 pre-proliferative | R2 moderate BDR | High risk | |
| 47 moderately severe NPDR | ||||
| 53A–D severe NPDR | R3 severe BDR | Severe NPDR | ||
| 61 mild PDR | R3 proliferative | R4 PDR | PDR | PDR |
| 65 moderate PDR | ||||
| 71, 75 HR-PDR | ||||
| 81, 85 advanced PDR |
The SIGN diabetes guideline18 recommends that ‘Patients with severe or very severe NPDR should receive close follow-up or laser photocoagulation’.
Neither the National Institute for Health and Care Excellence (NICE) diabetes guideline on type 1 diabetes19 nor on type 2 diabetes20 covers laser therapy.
Other treatment options
Control of blood glucose and blood pressure
Good control of blood glucose [aiming at glycated haemoglobin (HbA1c) no greater than 7% (53 mmol/mol)], BP (aiming at 130/80 mmHg) and blood triglycerides reduces the risk of retinopathy, though in those who have some retinopathy and poor glycaemic control, too rapid restoration of good control may worsen retinopathy, usually temporarily.
Intravitreal drugs
In recent years, two groups of drugs for intravitreal use have become available. These are:
-
Steroids, including triamcinolone, the long-acting dexamethasone implant (Ozurdex, Allergan) and the longer-acting fluocinolone implant (Iluvien, Alimera).
-
The ‘anti-vascular endothelial growth factor (VEGF)’ drugs, bevacizumab, pegaptanib, ranibizumab and aflibercept. These inhibit the action of VEGF or bind it. Aflibercept also blocks placental growth factor. VEGF increases vascular permeability and promote the growth of abnormal new vessels (neovascularisation).
The long-acting steroids have significant adverse effects, notably causation or acceleration of cataracts in the eye, and also raised intraocular pressure (IOP) that can lead to glaucoma. They are unlikely to be much used at such an early stage as NPDR because of the risk of cataract formation, but may have a role in pseudophakic patients, or in patients with DMO that does not respond to anti-VEGF treatment. The short-acting steroid, triamcinolone, is not licensed for use in the eye, but has been widely used.
The rationale for using the anti-VEGF drugs in PDR and NPDR has been summarised by the Diabetic Retinopathy Clinical Research Network (DRCRN). 21 First, they note reports of raised VEGF in ocular fluid from patients with active new vessel formation compared with no rise in patients with NPDR or inactive PDR, suggesting that VEGF stimulates neovascularisation. 22 Second, they report a number of observational studies which report that anti-VEGF drugs cause regression of PDR, albeit temporarily because the effects last only a few weeks, making repeat injection necessary, as has been shown in the treatment of DMO.
The anti-VEGF drugs have fewer adverse effects, and would probably be more acceptable at early stages than the steroids. They do have to be given by injection into the eye. In DMO, these injections are given monthly initially, but reducing in frequency thereafter. Nevertheless, anti-VEGF treatment places a significant burden on both patients and the NHS. In addition, it is, at least in DMO, only successful in about 30–50% of patients (defining success as a gain of 10 or more letters in VA). 23 Lastly, as experience is gained on the use of anti-VEGF in DR it is possible that initially unrecognised side effects may be apparent, as it was the case in age-related macular degeneration (AMD), where accumulating evidence suggests a possible effect of anti-VEGF on the development of retinal pigment epithelial atrophy. 24
The anti-VEGF drugs are now being used in combination with PRP, and we review the evidence on that in Chapter 4.
Fenofibrate
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial was primarily a study to see if the lipid-lowering agent fenofibrate could reduce macrovascular and microvascular events in type 2 diabetes. 25 However, a sub-study within FIELD25 recruited 1012 patients to a retinopathy study. The primary outcome in the main study was need for laser therapy (3.4% on fenofibrate vs. 4.9% on placebo) but the sub-study used retinal photography to assess progression of retinopathy or development of MO. The hazard ratio at 6 years for MO was 0.69 [95% confidence interval (CI) 0.54 to 0.87] in the fenofibrate group compared with placebo. The effect of fenofibrate did not seem related to changes in blood lipid levels.
The ACCORD (Action to Control Cardiovascular Risk in Diabetes) Eye Study reported a reduction of 40% in the risk of progression of retinopathy over a 4-year follow-up in patients on fenofibrate and a statin compared with those on a statin alone. 26 This was associated with a decrease in serum triglycerides. Lowering cholesterol does not appear to affect progression, as shown in the CARDS (Collaborative Atorvastatin Diabetes Study) trial of atorvastatin. 27
Preliminary searches have identified some evidence on the use of fenofibrate eye drops but such use appears to be at an early stage. The drops seem to have been patented and piloted, but not yet trialled in humans, though some work in rats suggests efficacy in arresting neovascularisation. 28 In Australia, oral fenofibrate has been approved for slowing the progression of retinopathy in type 2 diabetes. 29
Fenofibrate seems to be little used in the UK for retinopathy.
This review includes only drugs that are administered directly into the eye.
What do clinical guidelines say?
This section outlines the recommendations from five clinical guidelines, from England, Scotland, Canada, Australia and the USA, on laser photocoagulation on the treatment of NPDR and PDR.
England, Canada, USA and Australia produced separate guidelines for DR, whereas Scotland devoted one section in the Management of Diabetes guideline to the management of DR. Table 3 summarises the recommendations.
| Guideline | Mild to moderate NPDR | Severe/very severe NPDR | Non-HR-PDR | High-risk/proliferative PDR | Advanced/severe PDR |
|---|---|---|---|---|---|
| RCOphth [RCOphth UK (England)]5 | No treatment indicated | Early PRP if retinopathy approaches the proliferative stage in specific patient groups (see text above) | Not covered in guidelines | Full scatter PRP | Vitrectomy with intravitreal anti-VEGF injection is recommended if PRP seems ineffective |
| SIGN (Scotland)18 | Not covered in guidelines | Close follow-up or PRP | Not covered in guidelines | PRP | With VH too severe for PRP consider vitrectomy |
| COS (Canada)30 | Not covered in guidelines | Not covered in guidelines | Not covered in guidelines | PRP | With VH consider vitrectomy and anti-VEGF |
| AAO (USA)31 | No treatment indicated | Early PRP in specific patient groups (see text above) | Early PRP in specific patient groups (see text above) | PRP | Consider vitrectomy |
| NHMRC Australia32 | Not covered in guidelines | Early PRP in specific patient groups (see text above) | Not covered in guidelines | PRP | Consider vitrectomy if unresponsive to PRP |
As recommended in the ETDRS, laser treatment is not considered in any of the guidelines for patients with DR at stages up to and including moderate NPDR. However, only the RCOphth UK and the American Academy of Ophthalmology (AAO) guidelines actually state this in their guidelines.
Consideration of early PRP in conjunction with close follow-up at the severe stage of NPDR is recommended in England, Scotland, America and Australia but not in Canada, where the guidelines consider only the treatment of PDR. While the Scottish Intercollegiate Guidelines Network (SIGN) guidelines18 very generally recommend the consideration of PRP in patients with severe or very severe NPDR, the RCOphth UK reserved the treatment to patients approaching the proliferative stage and only in certain patient groups, i.e. older patients with type 2 diabetes, in patients in whom retinal view is difficult or examination is difficult, in patients who cannot be followed up closely, in patients in whom one eye has already been lost to PDR, and, generally, before cataract surgery. The AAO31 and the National Health and Medical Research Council (NHMRC) Australia32 do not seem to subcategorise the severe NPDR stage but list the same patient groups for consideration. They further include pregnancy (AAO31) and renal disease (NHMRC32) under the medical conditions for consideration. The AAO31 states further that partial PRP is not recommended and that, consequently, if PRP is indicated, full PRP should be performed. The AAO31 also includes the non-HR-PDR stage into this recommendation.
Pan-retinal photocoagulation is recommended for PDR with HRCs (AAO,31 NHMRC,32 COS30) and PDR with any new vessels (RCOphth UK and SIGN). The AAO,31 RCOphth UK5 and the Australian NHMRC32 stress the urgency of such treatment in their guidelines.
Anti-vascular endothelial growth factor and steroids
None of the guidelines includes anti-VEGF as a treatment option for NPDR/PDR either alone or in combination with laser photocoagulation. However, three out of the five guidelines recommend anti-VEGF for the treatment of DMO. The RCOphth UK and the AAO recommend consideration of anti-VEGF either with or without combination laser therapy, and the RCOphth classes it as ‘the new gold standard of therapy . . .’ for DMO. The Canadian guidelines, however, recommend anti-VEGF prior to PRP only for PDR with DMO. They further recommend anti-VEGF treatment before vitrectomy. The Australian guidelines recognise that anti-VEGF treatment for the management of PDR with DMO is already widely in use but say that anti-VEGF for PDR prior to laser treatment or vitrectomy lacks evidence from randomised controlled trials (RCTs).
Although anti-VEGF drugs need to be administered frequently, slow-release steroid implants have the advantage of lasting longer. The American guidelines state that intravitreal steroids might be considered in combination with PRP in patients with combined moderate NPDR and DMO. Similarly, the Australian guidelines suggest consideration of steroids in PDR with DMO in certain patient groups. Overall, recommendations to use steroids are very cautious and the Canadian Ophthalmological Society (COS), which does not include intravitreal steroids in their guidelines, reports that studies investigating the use of steroids produced conflicting results. The SIGN guidelines do not recommend any pharmacological treatment for the management of any form of PDR owing to lack of convincing evidence.
Decision problem
The commissioning brief gave the background to the topic as follows:
Diabetic retinopathy (DR) is the major cause of sight loss in the working age population in the UK and people with diabetes are 25 times more likely than the general population to go blind. Panretinal photocoagulation (PRP) by laser treatment is the standard intervention for patients with high risk progressive* diabetic retinopathy (PDR) and it has been shown to reduce the risk of severe vision loss for eyes at risk by 50%. However, an intervention with pan-retinal laser treatment earlier in the disease, during the preproliferative stage, may be more beneficial in terms of preventing loss of vision, given the detrimental and potentially irreversible effects of PDR if treatment is not obtained or is delayed.
*The term used in the brief, but presumed to mean proliferative.
The key question for this review is about the timing of PRP – would there be advantages in PRP at the severe NPDR stage, rather than waiting till PDR develops?
Population: people with NPDR.
Interventions: PRP at the NPDR stage. All variants of PRP will be included. Drug–laser combinations using an anti-VEGF drug or an injected steroid will be included.
Comparator: PRP delayed till PDR develops. This may be at the HR-PDR stage but may be used in early PDR.
Outcomes: the primary outcome is visual loss with central and peripheral loss described separately when data permit.
Secondary outcomes include the need for further treatment, and adverse effects such as the development of DMO after PRP, peripheral visual loss, quality of life (QoL), ability to drive, colour vision.
Chapter 2 The landmark trials: Diabetic Retinopathy Study and Early Treatment Diabetic Retinopathy Study
Methods
Literature searches and study selection
The search question posed in the commissioning brief was:
What is the clinical and cost-effectiveness of pan-retinal laser treatment in the management of non-proliferative (pre-proliferative) diabetic retinopathy (NPDR)?
The patient groups specified were those with early stages of NPDR (Level R2) versus the control or comparator treatment of PRP at PDR (Level R3), in any appropriate setting.
Our scoping searches gave a very low retrieval of studies that would be relevant to this search question, but did show that there were recent developments in types of laser and in the use of laser and drug combinations. Therefore, in the draft protocol we proposed a wider scope for this Technology Assessment Report than had been envisaged in the commissioning brief. This was approved by the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC) after being supported by the external referees. The decision problem was subsequently expanded to become:
Treatment of non-proliferative diabetic retinopathy: a review of pan-retinal photocoagulation, other forms of laser treatment, and combinations of photocoagulation and anti-VEGF drugs or inject steroids.
However, the broader searches revealed that there were no RCTs that compared patients at the NPDR level to those at later stages of PRP. Indeed, the most relevant and largest study done addressing the timing of PRP laser in the treatment of DR, the ETDRS, grouped together patients with moderate to severe NPDR and early PDR, and did not report outcomes on these groups separately.
Therefore, it seemed likely that a trial to address the original research question was needed, and, in order to inform a future study on PRP treatment of patients at the NPDR stage, we decided to further broaden the searches to capture all forms of current laser and topical drug treatment of DR at any stage, and explore if these newer treatments could be applied to patients at the NPDR stage.
The databases MEDLINE, EMBASE and The Cochrane Library were searched for previous systematic reviews or meta-analyses relevant to our search question (see Appendix 2 for search strategies). There were 94 potentially relevant records downloaded and the full text of five articles was examined by two reviewers (PR, NW). The most relevant review was one by Mohamed et al. (2007). 33 Although this was a useful review, its objective was to review the best evidence for primary and secondary intervention in the management of DR, including DMO, which was a lot broader than our review, so did not address our specific research question. Also, the searches were performed in May 2007, so it was several years out of date.
We searched for RCTs for the treatment of DR. We separated the results into three categories in order to provide evidence for each of the different aspects of our decision problem (Appendix 2 shows the details of the search strategies and Figure 2 shows the flow diagram for RCTs searches).
-
Trials of:
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for identifying RCTs included in Chapters 2–4.
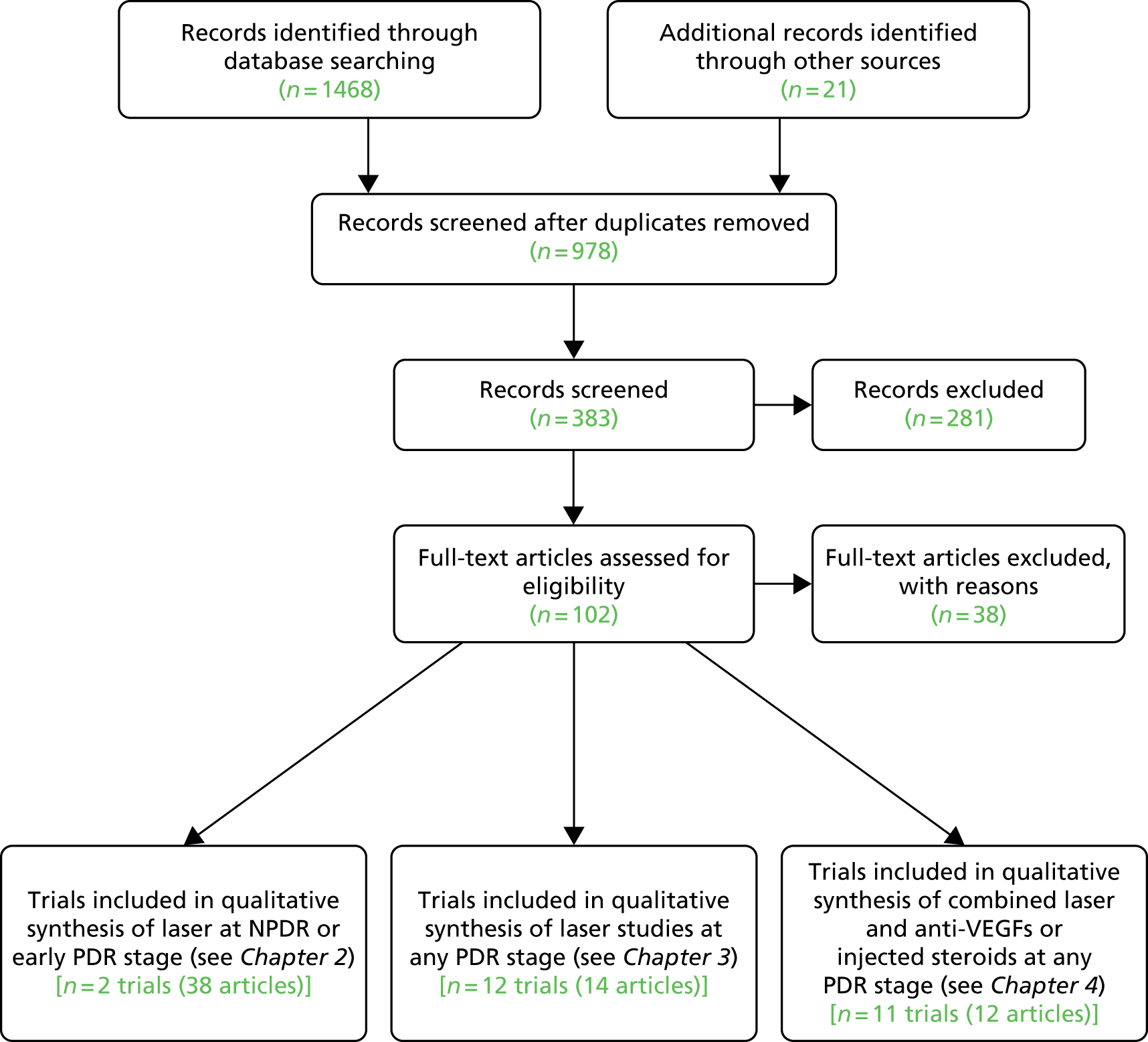
From the 102 full-text papers assessed, independently checked against the inclusion criteria by two reviewers (PR/NW), 22 references relevant to category 1 above were identified. Upon reading the full text of these references, it became evident that all were papers arising from two large RCTs, the DRS and the ETDRS, each producing many papers. Further searches were done to search specifically for publications arising from the DRS and ETDRS, and reference lists were checked, in order to obtain all the relevant papers from these two trials; this resulted in an additional 18 articles.
The excluded papers were retained and were assessed for inclusion criteria relevant to category 2 and 3 searches above, and are reviewed in Chapters 3 and 4.
Data extraction and quality assessment
The data extractions, and quality assessments (based on the Cochrane Collaboration’s risk of bias tool34), of the two trials were carried out by one reviewer (PR) and checked by a second (NW). The final number of papers reviewed was 14 from the DRS and 24 from the ETDRS.
The flow of studies is shown in Figure 2.
The Diabetic Retinopathy Study
Background
Laser photocoagulation had become widely used in the management of DR by the early 1970s in the USA. However, there was a lack of good-quality evidence supporting the risk and benefits of this procedure. Therefore, in 1971, the National Eye Institute (NEI) funded the DRS35 to evaluate photocoagulation treatment for PDR.
Study design
The DRS was a randomised, controlled clinical trial involving 15 clinical centres. A total of 1758 patients were enrolled between 1972 and 1975. Patient follow-up was completed in 1979.
The main aim of the DRS was to determine whether photocoagulation helps prevent severe visual loss (SVL) from PDR, and whether a difference exists in the efficacy and safety of argon versus xenon photocoagulation for PDR. Another objective was to obtain information on the natural history and clinical course of proliferative retinopathy.
Patients were eligible if they had best corrected visual acuity (BCVA) of 20/100 or better in each eye, and the presence of PDR in at least one eye or severe non-proliferative retinopathy in both eyes. Both eyes had to be suitable for photocoagulation. The eye to be treated was chosen randomly.
The baseline VA of the enrolled patients was equal to or better than 20/20 in approximately half of the eyes. Patients were predominantly white and had a mean age of 42.6 years; approximately 45% were classified as juvenile-onset diabetics, and there were slightly more men than women.
The principal end point was SVL, which was considered to have occurred if VA was less than 5/200 at two or more consecutively completed 4-month follow-up visits.
Quality assessment
The DRS was a high-quality trial with a low risk of bias, as shown in Table 4. The details of the design, methods and baseline results of the DRS were extensively reported in DRS report no. 6 (DRS #6). 36
| Adequate sequence generation | Adequate allocation concealment | Adequate masking | Incomplete outcome data assessed | Free of selective outcome reporting | Free of other biases (e.g. similarity at baseline, power assessment) |
|---|---|---|---|---|---|
| Yes (randomisation schedules were created by the co-ordinating centre for each clinical centre and were designed to balance both the number of right and left eyes assigned to treatment and the number assigned to argon and xenon) | Yes (the co-ordinating centre sent the treatment allocation form to each clinic in a sealed envelopes) | Yes [the protocol specified that the individual who measured VA should be unaware of (‘masked’ with regard to) the identity of the eye assigned to treatment and VA at previous visits] | Yes (the number of patients who completed specified visits up to 5 years was reported in table 3 of DRS #810) | Yes (all prespecified outcomes reported) | Yes (percentage of patients with specified baseline characteristics for 14 different variables, and yielded no significant difference at 5% level. Sample size calculations set recruitment goal at 800 patients in each treatment group) |
Treatment
One eye of each patient was randomly assigned to immediate photocoagulation and the other to follow-up without treatment, regardless of the course followed by either eye. The eye chosen for photocoagulation was randomly assigned to argon laser or to xenon arc photocoagulation. Treatment was usually completed in one or two sittings. Both treatment techniques included extensive scatter photocoagulation (PRP) and focal treatment of new vessels on the surface of the retina.
The argon treatment technique specified 800–1600 scatter burns, 500 µm in size, 0.1-second duration and direct treatment of new vessels whether on or within one disc diameter (DD) of the optic disc (NVD) or outside this area (NVE). The xenon technique was similar, but scatter burns were fewer in number, generally of longer duration, and stronger, and direct treatment was applied only to NVE on the surface of the retina. Focal treatment was also applied to microaneurysms or NVE lesions thought to be causing MO. Those treated with argon could have flat or elevated NVE treated.
Follow-up visits were planned at 4-month intervals for a minimum follow-up of 5 years, where follow-up treatment was applied as needed. BCVA was measured in both eyes by masked techniques before treatment and at 4-month intervals after treatment.
The DRS data were reviewed every 3 months by the Data Monitoring Committee for evidence of adverse and beneficial treatment effects.
Results (before protocol change)
In 1975 after an average of only 15 months of follow-up (range 0–38 months), the 2-year incidence of blindness was 16.3% in untreated eyes but only 6.4% in treated eyes. 37 Therefore, photocoagulation had reduced the 2-year risk of blindness by about 60%. This finding was unexpected and highly statistically significant. These beneficial effects were noted to some degree in all stages of DR included in the study.
Protocol change
On the basis of these results a decision was made in 1976 (more than 3 years before the planned termination of the study) to consider photocoagulation treatment for the initially untreated eyes, which now, or in the future, would fulfil any one of the following criteria, referred to as eyes with HRCs:
-
Moderate or severe new vessels on or within one DD of the optic disc.
-
Mild new vessels on or within one DD of the optic disc if fresh vitreous or pre-retinal haemorrhage is present.
-
Moderate or severe new vessels elsewhere (NVE), if fresh vitreous or pre-retinal haemorrhage is present, and if the area of new vessels was half the disc area or more.
Photocoagulation techniques were modified when treatment was carried out in eyes initially assigned to the untreated control groups after the 1976 protocol change. Argon treatment was preferred, and to decrease the risk of VA loss, many DRS investigators divided scatter treatment into two or more episodes, days or weeks apart.
Evidence of recovery before protocol change
Although the principal goal of photocoagulation treatment is to prevent visual loss, not to improve vision, there were eyes with some evidence of recovery, defined as VA ≥ 5/200 at any subsequent visit at 1, 2 or 3 consecutively completed follow-up visits. The percentage of eyes with some evidence of recovery at each visit were 28.6%, 12.2% and 7.7% in untreated eyes compared with 48.8%, 28.6% and 20.8% in treated eyes, respectively. Therefore, it appeared that recovery of VA was more frequent in treated than untreated eyes.
Harms
Some harmful effects of treatment were also found, including moderate losses of VA and constriction of peripheral visual field, which were greater in the xenon treated group than the argon group. The loss in sharp, central vision was temporary in some patients but persisted in others. However, DRS physicians believed that these harmful effects of photocoagulation in eyes with moderate or severe retinopathy were outweighed by the reduced risk of SVL without treatment at these stages.
Results after the protocol change
Additional follow-up after the DRS protocol change confirmed previous reports that, by 24 months, photocoagulation reduces the risk of SVL by 50% or more.
Cumulative rates of SVL for argon and xenon groups combined up to 72 months’ follow-up are shown in Table 5 (adapted from table 2, DRS #810). Although the risk of SVL in untreated eyes increases from 14% at 24 months to 36.7% at 72 months, it can be seen that over this time period the treatment effect was consistent (ranging between 56% and 59%).
| Follow-up (months) | Treated | Untreated | Reduction of SVL (%) |
|---|---|---|---|
| 8 | 0.7 | 1.2 | 41.7 |
| 24 | 6.2 | 14.0 | 55.7 |
| 36 | 9.0 | 21.7 | 58.5 |
| 48 | 11.6 | 27.8 | 58.3 |
| 60 | 13.9 | 33.0 | 57.9 |
| 72 | 16.6 | 36.7 | 54.8 |
The 24-month data in Table 5 differ slightly from that presented earlier (prior to the protocol change), as 43% of the 2-year visits and all of the 4-year visits included were carried out after the 1976 protocol change. All eyes are classified in the group to which they were originally randomly assigned, ignoring treatment of control eyes.
The treatment effect was somewhat greater in the xenon group than in the argon group (data not shown), but its statistical significance was borderline, and its clinical importance was outweighed by the greater harmful treatment effects observed with the xenon technique used in the DRS.
Occurrence of severe visual loss in eyes classified according to baseline severity
As patients enrolled in DRS had a broad range of severity of DR, it was important to evaluate results for different stages. Table 6 (taken from table 2, DRS #1438) shows the cumulative 2- and 4-year rates of SVL by eyes grouped by their severity of retinopathy at baseline and treatment assignment.
| Severity of retinopathy | Rate | Treated | Untreated | Reduction of SVL (%) | ||
|---|---|---|---|---|---|---|
| SVL (%) | No. at risk | SVL (%) | No. at risk | |||
| NPDR | 2 year | 2.8 | 303 | 3.2 | 297 | 12.5 |
| 4 year | 4.3 | 188 | 12.8 | 183 | 66.4 | |
| Proliferative without HRCs | 2 year | 3.2 | 615 | 7.0 | 603 | 54.3 |
| 4 year | 7.4 | 390 | 20.9 | 332 | 64.6 | |
| Proliferative with HRCs | 2 year | 10.9 | 570 | 26.2 | 473 | 58.4 |
| 4 year | 20.4 | 324 | 44.0 | 238 | 53.6 | |
| All eyes | 2 year | 6.2 | 1489 | 14.0 | 1378 | 55.7 |
| 4 year | 12.0 | 903 | 28.5 | 754 | 57.9 | |
It can be seen that the treatment effect in Table 6 is substantial (except for the group without PDR at 2 years) and fairly uniform across all subgroups at both 2 and 4 years, with reductions of SVL by from 54% to 65%.
The rate of SVL for untreated eyes with proliferative retinopathy with HRCs after 24 months of follow-up is about 26% and is reduced to 11% in treated eyes. However, in eyes with proliferative retinopathy without HRCs, the untreated rate at 2 years is much lower (7.0%), and although the beneficial treatment effects are substantial (a 54% reduction in SVL), the risks without treatment are smaller, and so the harmful effects of treatment need to be given more weight than for eyes with a higher risk.
In eyes with severe NPDR the risk of SVL without photocoagulation treatment at 2 years is low (3.2%) and reduces to 2.8% (a reduction of 12.5%) only with treatment, so the risks of treatment become even more important.
Harms: argon and xenon
Decreases of VA of one or more lines and constriction of peripheral visual field due to treatment were also observed in some eyes. These changes were sometimes due to an increase in MO, and sometimes the reduction in VA was temporary. In others, the changes persisted. The changes in visual field are important because they may mean that patients can no longer meet the requirements for driving.
Visual fields were measured using the Goldman method, wherein normal fields range from 50° (superiorly) to 90° (temporally). The DRS group defined modest visual field loss as a reduction from over 30° up to 45°, and 30° or less as severe.
The UK legal requirement is VA of 6/12 (measured in metres) or better (this is equivalent to 20/40 using measurements in feet) and with regards to visual field, to have a binocular visual field of 120 ° horizontally (in the horizontal axis) and no significant defect within the central 20 °, horizontally or vertically (above or below the horizontal meridian).
These harmful effects were more frequent and more severe following the DRS xenon technique; 50% of xenon-treated eyes suffered some loss of visual field compared with 5% of the argon-treated eyes. It was also estimated that a persistent VA decrease of one line was attributable to treatment in 19% of xenon-treated eyes and a persistent decrease of two or more lines in an additional 11%. Comparable estimates for the argon group were 11% and 3%, respectively.
Xenon photocoagulation has been discontinued.
Macular oedema in the Diabetic Retinopathy Study patients (DRS #12)
The DRS39 was not designed to evaluate the effect of photocoagulation in eyes with MO. Although focal treatment was carried out in those eyes with MO assessment, its direct effect cannot be determined because it was always combined with scatter treatment.
The loss of VA associated with scatter photocoagulation observed soon after treatment was especially prominent in eyes with pre-existing MO. It was also associated with the intensity of treatment. It was suggested that reducing MO by focal photocoagulation before initiating scatter treatment and dividing scatter treatment into multiple sessions with less-intense burns may decrease the risk of the visual loss associated with photocoagulation. 39
Summary
Results of the DRS showed that photocoagulation reduced the 2-year incidence of SVL by more than half in eyes with PDR, both with and without HRCs. However, in eyes with NPDR, where the 2-year risk of SVL in the untreated control group was low at 3.2%, photocoagulation only reduced the risk to 2.8%. Therefore, in patients with NPDR the harmful effects of photocoagulation assume more importance. Some of the harmful effects of treatment for some patients included a moderate loss of VA and a narrowing of the visual field.
Implications of Diabetic Retinopathy Study findings for treatment of early proliferative or severe non-proliferative retinopathy
The DRS concluded that in the eyes with PDR and HRCs the risk of SVL without treatment substantially outweighs the risks of photocoagulation, and prompt treatment is usually advisable. However, as the DRS findings result from a comparison between prompt treatment versus no treatment, they did not provide evidence on the relative value of prompt treatment versus deferral of treatment in the earlier stages of DR. They recommended careful follow-up for changes with DR and when non-proliferative changes are present, the follow-up visits should be at frequent intervals. 37
Finally, their conclusions stated:
Demonstration that prompt treatment of eyes with early proliferative or severe nonproliferative retinopathy is better than no treatment does not mean that prompt treatment is superior to deferral of treatment until progression occurs. 37
They called for a randomised trial to examine when best to apply PRP.
Early Treatment Diabetic Retinopathy Study
Background
The ETDRS was a multicentre, randomised clinical trial designed to evaluate argon laser photocoagulation in the management of patients with non-proliferative or early PDR. It was supported by the NEI and arose from results of the DRS, which had shown that laser photocoagulation was effective in reducing the rate of SVL from an advanced stage of DR. 9,40
Purpose and aims
The three principal clinical questions of ETDRS were:
-
When in the course of DR is it most effective to initiate photocoagulation therapy?
-
Is photocoagulation effective in the treatment of MO?
-
Is aspirin effective in altering the course of DR?
This summary will focus on the first of these questions. Our main interest is between early scatter treatment of eyes with moderate to severe NPDR or PDR without HRCs and deferral of scatter treatment unless PDR with HRCs develops.
Initially, patients were also assigned randomly to aspirin (650 mg per day) or placebo. However, aspirin was not found to have an effect on retinopathy progression, so patients assigned to aspirin were pooled with those assigned to placebo.
Quality assessment
The ETDRS was a high-quality trial with a low risk of bias as shown in Table 7.
| Adequate sequence generation | Adequate allocation concealment | Maskinga | Incomplete outcome data assessed | Free of selective outcome reporting | Free of other biases (e.g. similarity at baseline, power assessment) |
|---|---|---|---|---|---|
| Yes (co-ordinating centre staff assigned patients randomly) | Yes (sealed mailer from the central co-ordinating centre) | Partial/unclear? (masking of outcome assessors ‘Fundus Photograph Reading Center Staff did not have knowledge of the assigned photo-coagulation strategy’, ‘Visual acuity examiners were masked from treatment assignment’) | Yes (90–95% of expected follow-up visits were completed for first 3 years; 80–90% completed for follow-up longer than 3 years Of the 130,908 expected VA scores, all but 1.5% were available) |
Yes (all prespecified outcomes reported) | Yes (groups were well balanced for all characteristics, except a significantly greater proportion in the full scatter group had higher diastolic BP; power calculations performed) |
Patient recruitment
Recruitment of eligible patients began in December 1979 and was completed in July 1985. The 3711 patients accepted for the study, from 22 clinical centres in the USA, were followed through to 1989. Recruitment ended with 98% of the goal of 4000 patients enrolled. By study end, 706 patients had died, and, of the 2971 patients known to be alive, 164 did not have a final eye examination but all but 11 had some sort of final check.
Patient eligibility
To be eligible for the ETDRS, patients had to be aged between 18 and 70 years and to have DR in both eyes. Each eye had to meet either of the following eligibility criteria:
-
No MO, VA of 20/40 or better and moderate or severe non-proliferative or early proliferative retinopathy, or
-
MO, VA of 20/200 or better and mild, moderate or severe non-proliferative retinopathy or early proliferative retinopathy.
Methods for assessing outcome variables
Best corrected visual acuity was measured with logarithmic VA charts at baseline and each subsequent follow-up visit, scheduled at 4-month intervals. A standardised protocol for the collection of VA measurements was used in all clinical centres.
Stereoscopic 30° colour photographs were taken of seven standard fields at baseline, 4 months, 1 year after entry and yearly thereafter. All fundus photographs were graded according to a standardised procedure by the Fundus Photograph Reading Center staff, who had no knowledge of treatment assignments and clinical data.
Definitions of diabetic retinopathy
The ETDRS adopted the DRS definitions of severe NPDR and HR-PDR and defined moderate NPDR (see table in Appendix 1). Subsequently, the ETDRS developed a more detailed scale, which provided further subdivisions within both the NPDR and the PDR categories. 6
Assessment of severity of retinopathy and macular oedema
Fundus Photograph Reading Center staff, without knowledge of treatment assignments and clinical data, followed a standardised procedure to grade fundus photographs and fluorescein angiographs for individual lesions and DR.
Randomisation procedure
To obtain information on the appropriate timing of scatter photocoagulation, one eye of each patient in the ETDRS was assigned randomly to early photocoagulation (either mild or full scatter) and the other to deferral of photocoagulation, with follow-up scheduled every 4 months and photocoagulation to be performed promptly if HR-PDR developed.
All eyes chosen for early photocoagulation were further randomised to one of two scatter photocoagulation techniques (full or mild). Full scatter involved 1200–1600 burns in two sessions, mild scatter 400–650 burns in one session. Eyes also with MO were assigned randomly to one of two timing strategies for focal photocoagulation (immediate or delayed), so that for these eyes there were four strategies of early photocoagulation.
Three categories were defined on the basis of retinopathy severity and the presence or absence of MO at baseline, and the type of photocoagulation differed for each category.
Less severe retinopathy was defined as eyes with mild to moderate non-proliferative retinopathy, and more severe retinopathy as eyes with severe non-proliferative or early PDR.
-
Category 1: eyes without MO Eyes in this category had moderate to severe non-proliferative or early proliferative retinopathy.
Eyes randomised to immediate photocoagulation were further randomised to full or mild scatter.
In the deferred arm, eyes were followed up at 4-monthly intervals and received photocoagulation if PDR with HRC-PDR developed.
In both arms, delayed focal photocoagulation was initiated during follow-up if clinically significant macular oedema (CSMO) developed (i.e. MO that involved or threatened the centre of the macula).
Ideally, the trial would have separated NPDR from PDR, but this was not done.
-
Category 2: eyes with MO and less severe retinopathy Eyes in this category had MO and mild to moderate NPDR.
Early photocoagulation for these eyes consisted of (1) immediate focal photocoagulation to treat the MO, which was seen as a greater threat to vision than the retinopathy, with scatter photocoagulation (with further randomisation to mild or full) added if severe non-proliferative or early proliferative retinopathy developed during follow-up and (2) immediate scatter photocoagulation (with further randomisation to mild or full), with focal photocoagulation delayed for at least 4 months.
Eyes assigned to delayed focal photocoagulation received treatment at the 4-month visit if the oedema had not improved clinically and the VA score had not increased by five or more letters by that time. Focal photocoagulation was initiated at the 8-month visit if the oedema was not substantially improved, as demonstrated by either a return of an initially thickened macular centre to normal thickness or improvement in VA score by 10 or more letters. At and after the 12-month visit, initiation of focal photocoagulation was required for all eyes assigned to early PRP if they had CSMO and had not yet received focal photocoagulation. So focal was not given if the MO improved.
In the deferred arm, eyes were followed up at 4-monthly intervals and received scatter photocoagulation if HRC-PDR developed. They could receive focal photocoagulation if CSMO developed. Note that this group could only receive scatter PRP if HRC-PDR developed, whereas the early treatment arm could have PRP if they progressed to severe NPDR, early PDR or HRC-PDR.
-
Category 3: eyes with MO and more severe retinopathy Eyes in this category had MO and severe non-proliferative or early PDR.
Early photocoagulation for these eyes consisted of (1) immediate focal and scatter photocoagulation (with random allocation to mild or full) or (2) immediate scatter photocoagulation (randomisation to mild or full), with focal photocoagulation delayed for at least 4 months. The same procedure as described above for initiating focal photocoagulation at or after 4 months was used.
In the deferred arm, eyes were followed up at 4-monthly intervals and received photocoagulation if HRC-PDR developed.
Thus, in each of the three categories there are four different randomly allocated strategies for the timing and extent of early photocoagulation. All eyes received scatter (mild or full) originally, and if the retinopathy progressed to HRC-PDR, the mild scatter group received full scatter. Eyes that had MO, or developed it, received full focal photocoagulation treatment. (Approximately 85% of eyes with MO at baseline eventually received focal photocoagulation compared with only 40% of eyes without MO at baseline.)
In the deferred arms, the initial protocol specified that full scatter be given if HRC-PDR developed. The protocol was modified in 1985 to allow focal photocoagulation if CSMO was present. This was because the data had by then shown that focal photocoagulation reduced visual loss in eyes with CSMO.
Early Treatment Diabetic Retinopathy Study photocoagulation technique
Argon laser was chosen for photocoagulation in the ETDRS. The photocoagulation treatment techniques used were based on those used in the DRS and on the clinical experience of the ETDRS investigators.
Major features of the scatter and focal photocoagulation techniques used in the ETDRS are shown in the table in Appendix 3.
Full scatter Full scatter treatment consisted of a spot size of 500 µm and exposure time of 0.1 second, used with power adjusted to obtain moderately intense white burns that do not spread to become appreciably larger than 500 µm. It was estimated that a total of 1200–1600 burns were required to complete the full scatter treatment. The protocol specified that division of scatter treatment be applied in two or more episodes, in the hope of reducing the incidence of adverse treatment effects. If applied in two episodes, these were to be no less than 2 weeks apart; if in three or more episodes, these must be at least 4 days apart. No more than 900 scatter burns were to be applied in a single episode, and the initial treatment session was to be completed within 5 weeks.
Mild scatter Mild scatter treatment involved a spot size, exposure time and intensity the same as for full scatter treatment, in order to produce burns of the same strength. Burns were placed at least one burn diameter apart and scattered uniformly across the same zone of retina as specified or full scatter, using 400–650 burns, usually applied at a single episode.
Focal photocoagulation Focal photocoagulation for MO consisted of the application of argon laser burns to focal lesions (such as leaking microaneurysms as determined by FA or areas of retinal ischaemia) located between 500 and 3000 µm from the centre of the macula.
Definition of terms used in the Early Treatment Diabetic Retinopathy Study
A definition of the terms as used in the ETDRS studies is given in Table 8.
| SVL | VA < 5/200 at two consecutive follow-up visits (scheduled at 4-month intervals) |
| Moderate visual loss | Loss of 15 or more letters between baseline and follow-up visit, equivalent to a doubling of the visual angle (i.e. 20/20 to 20/40 or 20/50 to 20/100) |
| MO | Thickening of the retina within one DD of the centre of the macula: and/or hard exudates ≥ standard photograph 3 in a standard 30-degree photographic field centred on the macula (field 2), with some hard exudates within one DD of the centre of the macula |
| CSMO | Retinal thickening at or within 500 µm of the centre of the macula; and/or hard exudates at or within 500 µm of the centre of the macula, if associated with thickening of the adjacent retina. A zone or zones of retinal thickening one disc area or larger, any part of which is within one DD of the centre of the macula |
| NVD | New vessels on the disc or retina within one DD of the disc margin, or located in the vitreous any distance anterior to this area, determined by grading fundus photographs |
| NVE | New vessels ‘elsewhere’ (outside the area defined for NVD), determined by grading fundus photographs |
End points
The primary end point for assessment of early photocoagulation was the development of SVL. This was defined as VA < 5/200 at two consecutive follow-up visits (scheduled at 4-month intervals). BCVA was measured at 6 weeks and 4 months after randomisation. The procedure was repeated every 4 months thereafter.
Other end points evaluated included either severe visual loss or vitrectomy (SVLV), and change between baseline and follow-up visits in visual field, colour vision or retinopathy. Visual fields were assessed by Goldman perimetry and identification of scotomas.
Study power
Power calculations for the primary end point of SVL assumed that 10% of eyes assigned to deferral would develop SVL within 5 years. With 2000 eyes assigned to the deferral group and their 2000 fellow eyes assigned to early photocoagulation, a 40% reduction in the rate of SVL could be detected with 98% power.
Statistical methods
Comparisons of end points expressed as proportions of events were made with two-sample tests of equality of proportions. Comparisons of continuous variables were based on the two-sample z-test of equality of means.
Because multiple end points in the different groups were compared several times for the Data Monitoring Committee, a 0.01 level of probability was used for the primary end points rather than 0.05. Observed z-values of ± 2.58 or more extreme (corresponding to a 0.01 level for a single test of significance) were considered statistically significant. 9
Baseline characteristics
The baseline characteristics of the ETDRS patients, by assignment of scatter photocoagulation are shown in Table 9.
| Characteristics | Mild scatter (n = 1868) | Full scatter (n = 1843) | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age at entry (years) | < 30 | 300 | 16 | 326 | 18 |
| 30–49 | 611 | 33 | 557 | 30 | |
| ≥ 50 | 957 | 51 | 960 | 52 | |
| Sex (male) | 1063 | 57 | 1033 | 56 | |
| Race (white) | 1440 | 77 | 1394 | 76 | |
| Type 1 diabetes | 558 | 30 | 572 | 31 | |
| Duration of diabetes (years) | < 10 | 312 | 17 | 298 | 16 |
| 10–19 | 1085 | 58 | 1034 | 56 | |
| ≥ 20 | 471 | 25 | 511 | 28 | |
| Per cent desirable weight | ≥ 120 | 768 | 41 | 773 | 42 |
| SBP (mmHg) | ≥ 130 | 1215 | 65 | 1233 | 67 |
| ≥ 160 | 357 | 19 | 392 | 21 | |
| Diastolic BP (mmHg) | ≥ 85 | 691 | 37 | 760 | 41a |
| ≥ 90 | 478 | 26 | 583 | 32b | |
| History of cardiovascular disease | 884 | 47 | 928 | 50 | |
| Cigarettes/day ≥ 6 | 842 | 45 | 799 | 43 | |
| Severity of retinopathy | Level ≤ 35 (mild NPDR) | 316 | 17 | 288 | 16 |
| Level 43 (moderate NPDR) | 452 | 24 | 459 | 25 | |
| Level 47 (moderately severe NPDR) | 477 | 26 | 482 | 26 | |
| Level 53a–d (severe NPDR) | 245 | 13 | 231 | 13 | |
| Level 53e (very severe NPDR) | 50 | 3 | 53 | 3 | |
| Level 61 (mild PDR) | 169 | 9 | 169 | 9 | |
| Level 65 (moderate PDR) | 153 | 8 | 155 | 8 | |
| Level 71 (HR-PDR) | 6 | < 1 | 6 | < 1 | |
| For patients enrolled before September 1983 | HbA1c ≥ 10% | 566 | 42 | 556 | 42 |
| Serum cholesterol | ≥ 240 mg/100 ml (6.2 mmol/l) | 495 | 36 | 470 | 35 |
| Low-density lipoprotein | Cholesterol ≥ 160 mg/100 ml (4.1 mmol/l) | 318 | 25 | 346 | 27 |
Of the 3711 patients randomised, 56% were male, 52% were between 50 and 70 years of age, 57% had a duration of diabetes between 10 and 19 years, and 30% were classified as having type 1 diabetes.
By today’s standards, control of blood glucose, BP and cholesterol would not be considered satisfactory; 19% had systolic blood pressure (SBP) of 160 mmHg or more, and 42% had HbA1c of 10% or more; 36% had total cholesterol level over 6.2 mmol/l. The mean HbA1c was over 12%.
Groups were well balanced for all characteristics, except that a significantly greater proportion in the full scatter group had higher diastolic BP.
In 75% of ETDRS patients both eyes belonged to the same baseline category. Within each baseline category there were no large differences in mean VA scores between groups of eyes assigned to various strategies for early photocoagulation and eyes assigned to deferral of photocoagulation. Randomised treatment groups were comparable. Adherence to the assigned strategy for photocoagulation at the initial treatment session was reviewed and found to be over 98% for application of the assigned scatter and/or focal photocoagulation.
Results
Severe visual loss
All eyes in ETDRS had low rates of SVL, whether they received early photocoagulation (2.6%) or were in the deferral group (3.7%) at 5 years.
The relative risk (RR) of SVL for the entire period of follow-up in eyes assigned to early photocoagulation (including all strategies) compared with eyes assigned to deferral photocoagulation was 0.77 (99% CI 0.56 to 1.06), calculated using a Cox proportional hazards model with retinopathy severity and presence or absence of MO at baseline as covariates.
The RRs of SVL with photocoagulation compared with deferral for all baseline retinopathy categories when all photocoagulation strategies are compared are summarised in Table 10. It can be seen from the CIs that in none of the categories was the RR statistically significant.
| Baseline retinopathy category | RR |
|---|---|
| 1. Eyes without MO | 1.37 (99% CI 0.67 to 2.77) |
| 2. Eyes with MO and less severe retinopathy | 0.59 (99% CI 0.32 to 1.09) |
| 3. Eyes with MO and more severe retinopathy | 0.70 (99% CI 0.44 to 1.11) |
| All baseline categories combined | 0.77 (99% CI 0.56 to 1.06) |
Data for the development of SVL for all baseline categories are shown in Table 11, which gives estimates of RR in each of the categories. Analyses for the 5-year follow-up period demonstrated no statistically significant differences between any of the strategies for early photocoagulation and deferral within each category.
| Baseline retinopathy category | Photocoagulation treatment strategy | ||||
|---|---|---|---|---|---|
| Early full scatter | Early mild scatter | Deferral | |||
| Immediate focal | Delayed focal | Immediate focal | Delayed focal | ||
| 1. No MO | |||||
| 1-year rate (%) | 0.2 | 0.3 | 0.2 | ||
| 3-year rate (%) | 1.8 | 1.8 | 0.9 | ||
| 5-year rate (%) | 2.7 | 2.6 | 2.2 | ||
| No. of eyes | 583 | 590 | 1179 | ||
| RR (99% CI) | 1.24 (0.52 to 2.98) | 1.49 (0.65 to 3.39) | |||
| 2. MO and less severe retinopathy | |||||
| 1-year rate (%) | – | 0.3 | 0.1 | ||
| 3-year rate (%) | 0.3 | 0.9 | 0.3 | 0.9 | 1 |
| 5-year rate (%) | 1 | 0.9 | 2.2 | 1.2 | 2.9 |
| No. of eyes | 362 | 356 | 365 | 365 | 1429 |
| RR (99% CI) | 0.43 (0.13 to 1.44) | 0.43 (0.13 to 1.44) | 0.75 (0.29 to 1.91) | 0.74 (0.29 to 1.88) | |
| 3. MO and more severe retinopathy | |||||
| 1-year rate (%) | 1.5 | 1.2 | 0.7 | 0.4 | 1.1 |
| 3-year rate (%) | 2.6 | 2.8 | 3.5 | 2.4 | 3.8 |
| 5-year rate (%) | 4.7 | 3.8 | 4 | 4.1 | 6.5 |
| No. of eyes | 272 | 270 | 276 | 272 | 1103 |
| RR (99% CI) | 0.78 (0.68 to 1.62) | 0.59 (0.26 to 1.34) | 0.74 (0.35 to 1.57) | 0.68 (0.31 to 1.46) | |
The eyes assigned to full scatter showed a trend towards a greater treatment effect than eyes assigned to mild scatter in the first two categories. The RR of SVL for the entire period of follow-up for all categories combined in eyes assigned to early full scatter compared with eyes assigned to deferral was 0.69 (99% CI 0.45 to 1.05); in eyes assigned to mild scatter the RR was 0.84 (99% CI 0.57 to 1.25); so neither early or full scatter showed a significant decrease in RR, but full was slightly better than mild at preventing SVL.
Both the severity of retinopathy and the presence of MO at baseline were both significantly associated with the development of SVL. The RR (adjusting for the presence of MO) for the development of SVL for eyes with more severe retinopathy compared with eyes with less severe retinopathy was 2.41 (99% CI 1.73 to 3.37). Similarly, the RR (adjusting for severity of retinopathy) for the development of SVL for eyes with MO compared with eyes without MO was 1.73 (99% CI 1.17 to 2.57).
Causes of severe visual loss in the Early Treatment Diabetic Retinopathy Study
Severe visual loss developed in 257 eyes (219 persons); however, 17 of these 257 eyes with SVL had insufficient follow-up and were not included in the analysis. Of the 240 eyes left for analysis, 149 eyes (127 persons) did not recover to 5/200 or better at any visit (persistent SVL) and VA improved in 91 eyes. 41
The most common cause of SVL was vitreous or pre-retinal haemorrhage, occurring in 125 (52.1%) of the 240 eyes included in the analysis. The second and third most common causes were MO (13.8%), and macular or retinal detachment (7.1%).
When patients with persistent SVL were compared with patients without persistent SVL, they were found to have higher mean levels of HbA1c (10.4% vs. 9.7%; p = 0.001) and higher levels of cholesterol (244.1 vs. 228.5 mg/dl; p = 0.0081) at baseline. 41
The low frequency of SVL in ETDRS is probably due to the use of PRP as soon as HR-PDR developed, and to vitrectomy when required.
Severe visual loss: subgroup analysis of type 1 versus type 2 diabetes
Patients were categorised into type 1 and type 2 diabetes in order to conduct a subgroup analysis of the ETDRS data to determine whether the effects of photocoagulation on SVL in patients differed by type of diabetes. 42
The benefit of early photocoagulation for SVL was statistically significantly greater in patients with type 2 diabetes than in those with type 1 diabetes. (Cox regression for SVL: interaction of early photocoagulation and type of diabetes; p = 0.0003). However, the reduction was small and the risk was low in the deferral group in which only 3.7% developed SVL. (Note that the definition used was truly severe – very low levels of vision). Also, because of the high correlation between age and type of diabetes, a subgroup analysis by age showed similar results. The results varied amongst the categories, and according to outcome. In patients with mild to moderate NPDR at baseline, a small benefit of laser in reducing SVLV was seen in both types of diabetes with no interaction between laser treatment and type of diabetes. In patients with more severe retinopathy (severe NPDR or early PDR) there was no difference in SVLV in type 1 diabetes between early and deferred laser, but a large difference in type 2, partly because they had much poorer outcomes than those with type 1. 42
If we use progression to HRC-PDR as the outcome, statistically significant benefit is seen in both types of diabetes. If we use reduction in VA, there is a large difference between early and deferred laser in patients with type 2 diabetes and clinically significant MO who had severe NPDR or early PDR at baseline but little in patients with type 1. If we look only at those who did not have CSMO at baseline, there is no difference in type 2 between early and deferred groups.
If we use legal blindness (defined in ETDRS as VA worse than 20/100), patients with type 2 diabetes again show a significant difference between early and deferred groups, whereas no difference is seen in type 1, but the frequency of this outcome was much higher in type 2.
The difference between the types of diabetes may be due to chance. As the ETDRS authors stated, many analyses were done and chance could lead to ‘statistically significant’ results. They show this quite neatly by doing a subgroup analysis on date of birth, which showed a statistically significant interaction. 42
Vitrectomy
The initial ETDRS protocol said that vitrectomy should be done after SVL had occurred, but this was changed after the results of the Diabetic Retinopathy Vitrectomy Study appeared in 1985, and earlier vitrectomy was performed, either 1 month after detection or as soon as progressive retinal detachment occurred. 43 This meant that vitrectomy was performed in many ETDRS patients who had not developed SVL.
Vitrectomy was performed at least once in 208 (243 eyes) of the 3711 patients (the overall vitrectomy numbers suggest that about 18% of eyes had more than one vitrectomy.) At baseline, eyes undergoing vitrectomy were more likely to have severe non-proliferative or worse retinopathy. Also, there were no differences in the mean VA scores or percentages with clinically significant MO. It appears that all patients who had vitrectomy, did so after developing HRC-PDR, on average 21 months before vitrectomy. About 20% had SVL before vitrectomy. 44
The majority of patients undergoing vitrectomy had type 1 diabetes. The indications for vitrectomy were either vitreous haemorrhage (53.9%) or retinal detachment with or without vitreous haemorrhage (46.1%).
The cumulative rates of vitrectomy were 3.9% and 2.2% in the deferred and early groups, respectively, so this outcome was about as common as SVL.
The 5-year vitrectomy rates for eyes grouped by their initial photocoagulation assignment were 2.1% of eyes assigned to early full scatter photocoagulation group, 2.5% of eyes assigned to the early mild scatter group, and 4.0% of eyes assigned to the deferral group (based on ETDRS #1744 – ETDRS #99 gives a figure of 3.9% for the deferred group).
Comparison of eyes assigned to deferral of photocoagulation with eyes assigned to early photocoagulation showed no statistically significant difference in post-vitrectomy VA results; however, it should be noted that because they all developed HR-PDR before vitrectomy, most (88%) had had PRP, most with full scatter. After vitrectomy, results in immediate and deferred groups were similar – the outcome of surgery was not affected by delaying PRP. Also, there was no statistically significant difference between eyes that received either less than full scatter or no photocoagulation compared with eyes that received full scatter photocoagulation. 44
Severe visual loss or vitrectomy
The ETDRS #740 (the design paper) does not mention vitrectomy as an outcome. However, the final analysis used as one outcome, the combination of SVL and vitrectomy (SVLV), based on the reasoning that vitrectomy had saved an unknown number of eyes from SVL, and because vitrectomy could be considered an indicator of vitreous haemorrhage that had failed to clear.
The RR of SVLV at end of follow-up for eyes assigned to early photocoagulation compared with eyes assigned to deferred photocoagulation was statistically significant at 0.67 (99% CI 0.52 to 0.87). 9
The RRs of SVLV by baseline categories were:
-
no MO = 0.78 (99% CI 0.47 to 1.29)
-
MO and less severe retinopathy = 0.55 (99% CI 0.33 to 0.94)
-
MO and more severe retinopathy = 0.68 (99% CI 0.47 to 0.99).
So, once again, eyes in category 1 had a lower reduction in RR than eyes in the MO groups.
The VA immediately before vitrectomy was 5/200 or worse in 67%, but afterwards only about 28% were left with such poor vision. About 20% had VA better than 20/40 at 3 years, so vitrectomy was highly beneficial in most.
Development of high-risk characteristics proliferative diabetic retinopathy
Results for the development of HRC-PDR by baseline retinopathy category and photocoagulation strategy are shown in Table 12.
| Baseline retinopathy category | Early full scatter | Early mild scatter | Deferral | ||
|---|---|---|---|---|---|
| Immediate focal | Delayed focal | Immediate focal | Delayed focal | ||
| No MO | |||||
| 5-year rate (%) | 18.8 | 26.9 | 38.5 | ||
| RR (99% CI) | 0.41 (0.31 to 0.55) | 0.64 (0.51 to 0.61) | |||
| No. of eyes | 583 | 590 | 1179 | ||
| MO and less severe retinopathy | |||||
| 5-year rate (%) | 13.7 | 8.5 | 21.4 | 16.6 | 26.7 |
| RR (99% CI) | 0.52 (0.36 to 0.75) | 0.27 (0.16 to 0.44) | 0.81 (0.59 to 1.11) | 0.56 (0.39 to 0.80) | |
| No. of eyes | 362 | 356 | 365 | 365 | |
| MO and more severe retinopathy | |||||
| 5-year rate (%) | 28.8 | 26.3 | 40.3 | 46.7 | 61.3 |
| RR (99% CI) | 0.36 (0.26 to 0.49) | 0.34 (0.25 to 0.47) | 0.59 (0.46 to 0.77) | 0.67 (0.53 to 0.87) | |
| No. of eyes | 272 | 270 | 276 | 272 | |
Compared with deferral of photocoagulation, early photocoagulation reduced the rate of progression to HR-PDR in each baseline category (Mantel–Cox test: p < 0.001 for each strategy of early photocoagulation compared with deferral, except for immediate focal and mild scatter photocoagulation in eyes with MO and less severe retinopathy; p = 0.09). The reduction was greater in eyes with full scatter than mild scatter, essentially similar for all categories.
The RRs are adjusted for retinopathy severity and the presence or absence of MO.
The deferral arms afforded the possibility to determine the natural history of retinopathy by examining the 5-year rate of progression to the HR-PDR stage. The risks of progression in the deferral arms were 38.5% in the eyes with no MO and more severe retinopathy, 26.7% in eyes with MO and less severe retinopathy and 61.3% in eyes with MO and more severe retinopathy.
Table 13 shows the development of HRC-PDR in eyes assigned to deferral by baseline retinopathy severity level. It can been seen that the risk of progression increases steadily with severity of retinopathy at baseline, with 5-year rates increasing from 15.5% in eyes with mild NPDR, to 56% in eyes with severe NPDR, up to 74.5% in eyes with moderate proliferative retinopathy.
| Baseline retinopathy severity (level) | No. of eyes | Cumulative rate (%) of high-risk proliferative retinopathy: 5 years |
|---|---|---|
| Level ≤ 35 (mild NPDR) | 609 | 15.5 |
| Level 43 (moderate NPDR) | 906 | 26.5 |
| Level 47 (moderately severe NPDR) | 938 | 39.4 |
| Level 53a–d (severe NPDR) | 500 | 56.0 |
| Level 53e (very severe NPDR) | 92 | 71.3 |
| Level 61 (mild PDR) | 339 | 63.8 |
| Level ≥ 65 (moderate PDR) | 327 | 74.7 |
| Total | 3711 | 40.7 |
In all categories, the 5-year risk of HRC-PDR was lowest in eyes that had full scatter PRP and highest in the deferred group. Full scatter reduced HRC-PDR by 50% and mild scatter by 25% compared with the deferred group.
Results after lens extraction
Lens surgery was performed on 205 patients (270 eyes) of the 3711 patients in the ETDRS, during follow-up that ranged from 4 to 9 years. Those having surgery were more likely to be white, older and have type 1 diabetes. Most of the lens surgery was done because of cataract; however, some may have been performed because of lens opacity that developed during or after vitrectomy. 45
Eyes assigned to early photocoagulation were more likely than eyes assigned to deferral of photocoagulation to have received scatter and/or focal photocoagulation before lens surgery. However, 64.8% of eyes assigned to deferral of photocoagulation also had scatter and/or focal photocoagulation before lens surgery.
A large proportion of all operated-on eyes had improved VA postoperatively. Eyes assigned to early photocoagulation had a trend towards a better VA outcome after lens surgery than eyes assigned to deferral, but this was not statistically significant (p = 0.04).
Moderate visual loss
Percentages of eyes in which moderate visual loss occurred are shown for each baseline category in Table 14 for up to 5 years of follow-up. Moderate visual loss in the deferred groups was commoner at 5 years in the two MO groups (prevalence 30.2% and 32.1%) than in category 1 with no MO (17.6%).
| Baseline retinopathy category | Early full scatter | Early mild scatter | Deferral | ||
|---|---|---|---|---|---|
| Immediate focal | Delayed focal | Immediate focal | Delayed focal | ||
| No MO | |||||
| 6-week rate (%) | 3.1a | 0.8 | 0.4 | ||
| 4-month rate (%) | 3.8a | 1.0 | 0.6 | ||
| 1-year rate (%) | 7.5b | 4.3 | 3.6 | ||
| 2-year rate (%) | 10.8a | 8.3 | 5.9 | ||
| 3-year rate (%) | 13.6 | 12.1 | 9.8 | ||
| 5-year rate (%) | 15.5 | 13.3 | 17.6 | ||
| No. of eyes | 583 | 590 | 1179 | ||
| MO and less severe retinopathy | |||||
| 6-week rate (%) | 1.4 | 4.5 | 1.6 | 3.0 | 1.6 |
| 4-month rate (%) | 2.5 | 9.7a | 2.2 | 6.4 | 3.8 |
| 1-year rate (%) | 5.3 | 15.9a | 3.7a | 10.5 | 8.6 |
| 2-year rate (%) | 7.6a | 19.1 | 8.9a | 15.1 | 16.6 |
| 3-year rate (%) | 11.2a | 23.1 | 12.2a | 19.0 | 21.1 |
| 5-year rate (%) | 22.4 | 29.8 | 19.5a | 21.8b | 30.2 |
| No. of eyes | 362 | 356 | 365 | 365 | 1429 |
| MO and more severe retinopathy | |||||
| 6-week rate (%) | 7.7a | 7.8a | 7.6a | 5.9b | 1.7 |
| 4-month rate (%) | 12.2b | 11.2 | 4.8 | 10.1 | 6.5 |
| 1-year rate (%) | 16.2 | 16.9 | 12.7 | 13.6 | 15.5 |
| 2-year rate (%) | 21.1 | 20.0 | 15.3b | 21.5 | 22.2 |
| 3-year rate (%) | 23.6 | 20.9 | 20.7 | 23.3 | 27.1 |
| 5-year rate (%) | 26.2 | 24.1 | 24.1 | 25.7 | 32.1 |
| No. of eyes | 272 | 270 | 276 | 272 | 1103 |
It can been seen that for all baseline categories full scatter photocoagulation appeared to have an adverse effect on moderate visual loss at both the 6-week and 4-month follow-up visits. This effect was also seen to a lesser extent with mild scatter. For eyes without MO there was a statistically significant effect of higher moderate visual loss in eyes for full scatter up to 2 years. At 3 and 5 years there was a no significant difference in eyes with photocoagulation compared with deferral.
In eyes with MO and less severe retinopathy the increase in moderate visual loss was statistically significant at 4 months, and 1 year for eyes with full scatter, but at 2, 3 and 5 years there were no significant differences. At the 5-year follow-up there was a statistically significant decrease in moderate visual loss in eyes with mild scatter and a non-significant decrease in eyes with full scatter. Eyes with immediate focal photocoagulation appeared to show a statistically significant beneficial effect of early photocoagulation for all follow-up points, beginning with the first year.
In eyes with MO and more severe retinopathy, there was a significant increase in moderate visual loss at 6 weeks for all strategies of photocoagulation. At 4 months this was seen only for eyes with immediate focal and full scatter. The only other significant difference was a lower rate at 2 years for eyes with immediate focal and mild scatter.
The summary of ETDRS #99 notes that scatter photocoagulation was not effective in reducing moderate visual loss in patients with MO.
Visual field
The cumulate distribution of visual field scores obtained using the Goldman 1/4e test object at baseline, 4- and 48-month visits showed no difference in distributions of visual field between categories of assigned strategies at baseline. 9 The Goldman method is less sensitive than methods used today.
By the 4-month visit, eyes assigned to deferral of photocoagulation showed no significant change in scores compared with baseline. By contrast, at 4 months all three baseline categories of eyes assigned to immediate full scatter photocoagulation had significantly greater loss of visual field than eyes assigned to deferral (p < 0.001). Eyes with mild scatter also showed a lower loss of visual field. There was a statistically significant difference (p < 0.001) between the loss of visual field between eyes assigned to immediate full and immediate mild scatter. So mild scatter may be less effective, but has fewer adverse effects.
The visual field worsened in all groups from baseline to 4 years. The scores for eyes assigned to immediate full scatter remained significantly (p < 0.001) worse than for eyes assigned to deferral. This reflects the harm done by PRP.
Colour vision
Colour vision was measured using the Farnsworth–Munsell 100 hue test at baseline, and at 8-month and 4-year follow-up visits. There was significant impairment of colour vision at baseline, with 50% of the ETDRS population having colour vision scores worse than 95% of the normal population. Colour vision is a macular function so should not be affected by PRP to the peripheral retina, but might be affected by focal or grid laser for MO.
Eyes with more severe retinopathy, both without and with MO, showed no significant difference at any visit between eyes assigned to any strategy of early photocoagulation and eyes assigned to deferral. All of the eyes with MO and more severe retinopathy assigned to early photocoagulation had scatter photocoagulation as part of their initial treatment.
However, for eyes with less severe retinopathy and MO assigned to immediate focal and delayed scatter photocoagulation, there was less loss of colour vision at the 4-year visit (p < 0.001) comparing the combination of both groups of eyes assigned to immediate focal with eyes assigned to deferral.
Summary and conclusions
Severe visual loss
The primary end point of the ETDRS was the development of SVL. The 5-year RR of SVL for eyes assigned to early photocoagulation (combining all strategies for photocoagulation) compared with deferral for all baseline categories combined was 0.77 (99% CI 0.56 to 1.06). Thus, it was shown that early photocoagulation reduces the risk of SVL by about 23%, but the 99% CI overlapped with no difference.
When analysed by baseline retinopathy category it was shown that eyes with MO and less severe retinopathy had a lower RR of 0.59 (99% CI 0.32 to 1.09) and eyes with MO and more severe retinopathy showed a RR 0.70 (99% CI 0.44 to 1.11), respectively. Eyes with no MO and more severe retinopathy had a higher RR of 1.37 (99% CI 0.67 to 2.77) but the CIs were wide.
Severe visual loss or vitrectomy
The combined end point of SVLV showed a 33% reduction with early photocoagulation compared with deferral, with a RR of 0.67 (99% CI 0.52 to 0.97). As noted above, about 20% of eyes that had vitrectomy had SVL before vitrectomy but the rest did not, and many improved thereafter.
High-risk proliferative retinopathy
Early photocoagulation resulted in a significant reduction in the rate of developing high-risk proliferative retinopathy compared with deferral of photocoagulation. Strategies for photocoagulation that included immediate full scatter reduced the rate of developing high-risk proliferative retinopathy by approximately 50%, whereas strategies that included immediate mild scatter reduced that rate by approximately 25%.
When eyes assigned to deferral were stratified according to baseline retinopathy, the rate of progression to the high-risk stage generally increased as the retinopathy increased.
Harms
There were some harmful effects associated with early scatter photocoagulation. Adverse effects of moderate visual loss were shown more frequently at 6 weeks and 4 months compared with eyes assigned to deferral, but this loss was not shown in any group by the 3-year follow-up.
There was evidence of a significant loss of visual field in all groups at 4 years and this was worse for eyes assigned to full scatter. Also, colour vision showed some reduction at 4 years in the category of eyes with less severe retinopathy and MO assigned to immediate focal and delayed scatter photocoagulation.
The Early Treatment Diabetic Retinopathy Study conclusions and recommendations
Data from the ETDRS demonstrated that early photocoagulation reduced the risk of developing SVL, and the risk of progression of retinopathy. However, the rates of SVL were low in both the early photocoagulation and deferral groups, and statistical significance using 99% CIs was obtained for SVLV but not for DVL alone.
When making the decision whether to initiate scatter photocoagulation, the side effects must be carefully considered. For most eyes that have not yet reached the high-risk proliferative stage, these side effects of scatter photocoagulation must be balanced with the possible small benefit of early photocoagulation in reducing the risk of SVL. 9
The ETDRS recommended that:
Provided careful follow-up can be maintained, scatter photocoagulation is not recommended for eyes with mild or moderate non-proliferative retinopathy. When retinopathy is more severe, scatter photocoagulation should be considered and usually should not be delayed if the eye has reached the high-risk proliferative stage.
Discussion
Rates of progression to SVL in ETDRS were low. They might be even lower now, with tighter control of blood glucose, BP and lipids. Tighter control of metabolic factors especially glycaemia can also slow progression. There was a fear that if HbA1c is reduced too quickly, retinopathy may temporarily worsen, usually, but not always, temporarily – the ‘glycaemic re-entry’ phenomenon. 46 This phenomenon may date from the days when patients were left poorly controlled on oral agents for years and then started on insulin, and is less common now. However, it is still seen in pregnancy if that stimulates a rapid improvement in control – a dramatic drop in HbA1c may be associated with a deterioration in retinopathy.
The diagnosis of sight-threatening retinopathy may be a powerful motivating factor.
The differences were more marked in progression to HR-PDR, so perhaps with longer follow-up the SVL differences would have increased, though not if they were carefully monitored and PRP given once HRC appeared. However, it should be borne in mind that in category 2 (MO and less severe retinopathy) the deferred group could receive PRP only once they reached HR-PDR, whereas the early photocoagulation groups could have ‘rescue’ PRP from the severe NPDR stage onwards. So there was some imbalance in application of rescue laser.
As reported in Table 10, the RR for progression to SVL in category 1 eyes (i.e. eyes without MO) was 1.37 (99% CI 0.67 to 2.77), i.e. the early PRP group did worse, though not statistically significantly, than in categories 2 and 3, which all had MO at baseline. It is likely that if this group was removed from the combined analysis, the overall RR would have been less than the 0.77 (99% CI 0.56 to 1.06) and the primary end point result would have been statistically significant. This might suggest that treating MO avoided SVL more than treating retinopathy. However, as reported above, the main cause of SVL was vitreous or pre-retinal haemorrhage (52%), with MO well behind at 14%, followed by macular or retinal detachment (7.0%).
The reason for the MO groups doing better than category 1 may simply be that they had a higher risk of visual loss and so more to gain.
The level of vision used for the primary outcome (less than 5/200) was very low – any trial nowadays would try to preserve vision at better levels, for example at 20/200. The ETDRS definition of moderate visual loss was defined in ETDRS #99 as loss of 15 letters or more between baseline and follow-up, which would apply today.
Pautler (2010)47 noted the clear recommendation from the ETDRS group against PRP in eyes with mild or moderate NPDR, but commented that the recommendations for severe NPDR and early PDR were much less clear – only that PRP should be considered. He suggests that ‘This cautious wording may have led physicians away from treating this group of eyes’.
Pautler (2010)47 suggests that PRP might be used in severe NPDR and early PDR in the following situations:
-
bilateral DR approaching HR-PDR
-
poor compliance with follow-up
-
poor glycaemic control
-
type 1 diabetes (despite the ETDRS result showing greater effect in type 2 diabetes)
-
DMO (but treating the DMO first)
-
previous SVL in the other eye
-
pregnancy
-
rubeosis (new vessels in the iris)
-
large area of new vessels outside the macula.
He also suggests factors that might lead to postponement of PRP in eyes with severe NPDR or early PDR such as past laser harm in the other eye, good glycaemic control, no DMO, low-risk of visual loss in the fellow eye and patient preference.
In the ETDRS, PRP was applied to all midperipheral retina, whether ischaemic or not. Currently, using wide-angle FA, areas of retinal ischaemia can be adequately identified. Laser photocoagulation could be applied selectively to areas of retinal ischaemia, potentially reducing side effects of this treatment, such as visual field defects, as in the Japanese trial17 described in Chapter 3.
The risk of progressing to HRC-PDR was reduced more than that of SVL. The reduction in the risk of progression to HRC-PDR is not unexpected. PRP treatment ablates much of the retina. As it appears that retinal ischaemia drives the VEGF response required for the development and support of neovascularisation, following laser treatment there would be little chance for PDR to occur. In the ETDRS, patients were followed at 4-monthly intervals (unless a problem such as vitreous haemorrhage occurred). It is unknown whether similar results would still be observed if the trial would have allowed closer follow-up so that HR-PDR could have been treated more promptly.
One of the possible side effects of PRP that could have a negative impact in the QoL of patients undergoing this treatment is the development of peripheral visual field defects. Depending on their severity, peripheral visual field defects may prevent individuals from driving. Delaying PRP until it is clearly needed – for instance, until neovessels develop – may give individuals extra years of maintaining driving standards and better QoL. About 20% of people may not meet Driver and Vehicle Licensing Agency (DVLA) driving standards after bilateral PRP. 48
The ETDRS established two groups, based on fundus examination, for the evaluation of treatment effects: (1) severe NPDR and early PDR and (2) HR-PDR. However, the presence or absence of neovascularisation clearly determines a different stage of disease, as visual loss occurs as a direct result of the neovascularisation process in most cases. This is illustrated by the fact that over half of people in the ETDRS who experienced SVL did so as a result of vitreous or pre-retinal haemorrhage. Thus, it might have been more appropriate to evaluate the effectiveness of treating with PRP at early PDR (less than HRCs) stage when compared with treating when HR-PDR characteristics had developed. A third group with severe NPDR could have also been included. Having severe NPDR and early PDR together made this group somewhat heterogeneous.
Decision problem revisited
The ETDRS was a very good quality and detailed study. However, it was conducted several decades ago, and one question is whether new developments since the time of the ETDRS have changed the balance of benefits and harms.
These developments include:
-
Improvements in diabetes care, with better control of blood glucose, BP and lipids.
-
Changes in laser treatment, arising from advances in laser technologies, different regimens and better targeting of laser therapy. There has been a trend to ‘lighter’ laser treatment with the aim of causing fewer adverse effects but retaining the same effectiveness.
-
The advent of new drugs for DMO, which may also affect retinopathy, and, more importantly for our purposes, are being used in combination with laser photocoagulation in DMO, partly to reduce the adverse effects. Patients with both DMO and PDR will be expected to receive both PRP and anti-VEGFs, and the latter may affect the PDR.
-
Advances in imaging, such as optical coherence tomography (OCT), which may make detection of DMO more reliable.
Given these changes, the next questions are:
-
If it was decided to start PRP at the NPDR stage, based on the results of ETDRS, what sort of laser treatment would be used? Pattern lasers?
-
If PRP was given earlier, should it be targeted at areas of retinal ischaemia, as detected by wide-angle FA, or given by conventional PRP that ablates the whole mid/peripheral retina, both perfused and non-perfused areas (NPAs)? (The same question could apply to PRP for PDR.)
-
Should drug treatment, mainly with the anti-VEGF drugs, or perhaps with intravitreal steroids, also be used in combination with PRP?
These questions are addressed in the Chapters 3 and 4.
Another issue is whether modern techniques of measuring DR and MO might also affect staging of retinopathy, and aid selection of people for PRP. This might be done both by determining who is at most risk of progression to HR-PDR, and who is at most risk from damage by PRP. This might ensure that PRP is given to the people who will most benefit. It is known that eyes with MO before PRP are more likely to have a reduction in VA after PRP,9,49 and, as has been pointed out by Browning (2005)50/Browning et al. (2004)51 and Massin et al. (2006),52 ophthalmologists often have difficulty detecting MO. Browning et al. (2008)53 also reported that the probability of MO being detected by OCT, but not by clinical examination (stereoscopic slit-lamp examination), increased as the retinopathy became more severe. The advent of OCT with its very good sensitivity for detecting retinal thickening should lead to better detection of MO and consequent tailoring of laser treatment to the needs of the individual eye.
Chapter 3 Laser studies: efficacy and safety
Aim of the chapter
The evidence from ETDRS suggests that treatment of severe NPDR and early PDR was more effective in reducing future visual loss than waiting to treat at HR-PDR stage, but there are weaknesses in the evidence. Only SVLV reached statistical significance. ETDRS did not provide results separately for severe NPDR and early PDR. The primary end point was SVL which was uncommon in all groups, and as defined was very severe. The reduction in the development of HRC-PDR in eyes treated with PRP earlier might have been expected to lead to further reductions in visual loss with longer follow-up.
So one question for policy-makers is whether the evidence is deemed sufficient to recommend PRP at NPDR and early PDR stage, or whether further research is necessary, which might include separating NPDR and early PDR.
However, the balance of risk and harm, and costs, may have changed since the advent of new laser technologies and treatment regimes. These may be as effective but have fewer adverse effects. So recommendation for treatment or for further research would need to take account of changes in:
-
laser machines
-
more modern regimens. It is necessary to consider both type of laser and the ways in which they are used – number of burns, number of sessions, selective versus PRP
-
more accurate diagnosis aided by imaging devices such as OCT and wide-angle cameras, that were not available at the time of the DRS and ETDRS
-
metabolic control.
In this chapter we review some laser studies from more recent times. The main aim is to identify which machines and regimens would be used now, either in treatment or research. Preliminary searches showed that none of the newer trials addressed our primary question of the optimum timing of PRP, and we therefore decided to use studies of laser photocoagulation at later stages and see what could be extrapolated from these.
A feature of trends in laser photocoagulation is that it tended to use less intense laser burns, and may be more targeted, for example treating only areas of peripheral ischaemia detected using wide-angle FA, with fewer adverse effects. One question which then arises is whether it has become less effective.
Modified ETDRS (mETDRS) direct/grid photocoagulation as used for DMO was described by the DRCRN (DRCRnet) as being targeted only at areas of thickened retina, areas of retinal non-perfusion and leaking microaneurysms using a smaller laser spot (50 µm) and less intense burn end point (grey) in order to balance therapeutic effect and adverse effects. 54
Most people now use pattern lasers for PRP, rather than the argon laser, because they are faster and less painful, but there is still sparse use of argon.
The sub-threshold diode laser is less destructive than the argon laser, depending on how it is applied. If at sub-threshold level then it would be expected to cause less damage than argon applied at threshold levels. If the diode was applied with a micropulse mode (reducing the temperature of the tissue – less thermal effect so less damage. Photocoagulation with the diode laser is reported to damage only the outer retinal layers and the choroid, whereas the argon laser damages both inner and outer retina and choroid. 55
The sub-threshold diode laser has been introduced in the treatment of DMO, but has not spread much into use, possibly because for PRP, it requires more sessions and more burns. 15
We also note that in Japan, a more selective approach to laser therapy is used, with targeting based on FA, so that only ischaemic areas are lasered. 17 This is a more restrictive approach than traditional PRP.
Methods
Inclusion and exclusion criteria for Chapters 3 and 4
We used the same approach for laser trials (this chapter) and drug–laser combinations (see Chapter 4).
Inclusion criteria
Type of studies
-
For comparing effectiveness of different types of laser treatment and of the combination of lasers and anti-VEGF and steroid injections, we looked for RCTs.
-
For assessing adverse events, we also included observational studies.
-
Publication year 2000 or later, in order to reflect current practice.
-
We included studies at any stage of retinopathy because of a dearth of laser studies at NPDR stage. For effectiveness in terms of visual state, we preferred a minimum duration of 6 months, but we included trials with follow-up of 3 months or more, because regression of neovascularisation can be seen 2–3 months after PRP. We also included non-trial studies of shorter duration for data on adverse effects.
Types of participants
-
Patient groups – type 1 and type 2 diabetes, with NPDR or PDR, being treated with laser photocoagulation.
Follow-up
-
For effectiveness, studies with a minimum follow-up period of 6 months were included.
-
For safety, shorter duration trials were also included.
Outcomes
-
Visual acuity; progression and regression of retinopathy; contrast sensitivity.
-
Adverse effects in eye – pain, cataract, raised IOP, vitreous bleeds, need for vitrectomy.
-
Number of treatments and hence visits required.
-
We were not interested in outcomes not evident to patients such as retinal or central macular thickness (CMT), or angiogram results, which are more guides to treatment than outcomes.
Exclusion criteria
-
Studies of treatment of DMO were excluded for assessing laser efficacy, as PRP is not used for DMO. However, they could be included for assessing the efficacy of drugs if they reported effects on DR (NPDR or PDR). Studies with fewer than 20 eyes were excluded.
Search strategy
The databases MEDLINE, EMBASE and The Cochrane Library were searched using the search strategies detailed in Chapter 2 and Appendix 2. The databases were searched from their inception until August 2013 and then auto-alerts were run until February 2014. However, for this section, only studies published since 2000 were included, as we were interested in recent laser methods and drug developments. In practice, this applied only to laser trials, as there were no drug-plus-laser studies before 2000.
Identification of studies
Titles and abstracts of the records retrieved were checked against the inclusion criteria by two independent reviewers (NW/PR). Any studies definitely or possibly fulfilling the inclusion criteria were retrieved in full and checked for final inclusion by two reviewers independently (NW/PR). There was no need for discussions with a third reviewer.
Data extraction strategy
Data were extracted into a predesigned data extraction form. Data were extracted by one reviewer (PR/DS/KF) and checked by a second reviewer (KF/DS/PA).
Quality assessment strategy
The risk of bias or quality of RCTs was assessed using the Cochrane risk of bias tool, including the following items:
-
adequacy of sequence generation
-
allocation concealment
-
masking (patients, doctors, outcome assessors)
-
adequacy of handling of incomplete outcome data
-
selective reporting
-
presence of other bias (e.g. lack of similarity at baseline, inadequate power)
-
funding source and authors conflict of interest.
The quality assessment was done by one reviewer (DS/KF) and checked by a second reviewer (KF/DS/PA).
Results
Results of the searches
A total of 978 records were retrieved by the searches. The titles and abstracts were screened for inclusion and exclusion. Based on titles and abstracts, 102 were considered possible inclusions and full texts of these were obtained. Out of these, 38 were included in Chapter 2, and 38 were excluded because of not meeting the inclusion criteria outlined above. Seventeen were excluded as they were published pre-2000; the reasons for exclusion of the remaining 21 studies are given in Table 15. For the sake of brevity the trials will simply be referred to by the name of the first author and publication year.
| Study ID | Reason for exclusion |
|---|---|
| Bandello 200556 | Patients have DMO only |
| Bressler 201357 | Patients received focal/grid laser only |
| Brown 200358 | Not a RCT |
| Cardillo 200859 | Patients have DMO only |
| Chappelow 201260 | Not a RCT |
| Cho 200961 | Superseded by later report |
| DRCRN 200962 | Not a RCT |
| Gurelik 200463 | Not a RCT |
| Lee 200064 | Not a RCT |
| Lee 201065 | Not a RCT |
| Luttrull 200866 | Not a RCT |
| Mason 200867 | Not a RCT |
| Muqit 201368 | Not a RCT |
| Neubauer 200713 | Not a RCT |
| Shimura 200549 | Not a RCT |
| Sivaprasad 201215 | Not a RCT |
| Summanen 201269 | Not a RCT |
| Venkatesh 201170 | Patients have DMO only |
| Vujosevic 201071 | Patients have DMO only |
| Writing committee for DRCRN 200754 | Patients have DMO only |
| Zucchiatti 200972 | Not a RCT |
We included 12 RCTs (in 14 articles) published after 2000 to assess the efficacy and safety of new laser technologies in patients with DR, though most had PDR. These are reviewed in this chapter.
Also included were 11 RCTs (published in 12 articles) that used anti-VEGFs or injectable steroids on their own or in combination with laser and compared it against laser. These are reviewed in Chapter 4.
Trials of laser photocoagulation (published after 2000)
Diabetic retinopathy
The studies included groups at different stages:
-
PDR: this included five studies (Bandello 2001;73 Muqit 2010/11;74–76 Muqit 2013;77 Muraly 2011;78 Tewari 200055). Of these, Muqit 2010/11,74–76 Muqit 201377 and Muraly 201178 included newly diagnosed PDR.
-
One study (Bandello 200173) included HR-PDR patients, in which HR-PDR was defined as PDR with two to four HRCs, i.e. new vessels at disc greater than ¼ to ⅓ of disc area or vitreous or pre-retinal haemorrhage associated with less extensive new vessels at disc, or with new vessels elsewhere of half of the disc area or more in size.
-
One study (Al-Hussainy 200879) included patients with PDR (n = 17), central retinal vein occlusion (CRVO; n = 2) and ocular ischaemic syndrome (definition not given; n = 1) who were undergoing PRP for the first time.
-
Shimura (2003)80 included patients with severe NPDR or early PDR without visual disturbances.
-
Two studies (Mirshahi 2013;81 Nagpal 201082) included patients with bilaterally symmetrical very severe NPDR or PDR.
-
One study (Japanese Society of Ophthalmic Diabetology 201217) included patients with pre-proliferative diabetic retinopathy (PPDR) (the definition of PPDR was not clear – patients with no previous laser and multiple non-perfusion areas larger than one disc area on FA were included).
-
One study (Salman 201183) included patients with NPDR with CSMO, or PDR.
-
One study (Suto 200884) included patients with severe NPDR or early PDR of similar severity in both eyes and a similar cataract grade in both eyes.
Other patient characteristics
Table 16 provides details of number of patients and eyes treated in the included studies. In most of the studies, patients were receiving laser for the first time. The ages of participants ranged between 26 and 86 years.
| Study (author and year) | No. of patients | No. of eyes | Details of previous treatment |
|---|---|---|---|
| Al-Hussainy 200879 | 20 | 20 | PRP for first time |
| Bandello 200173 | 50 | 65 | Patients with previous history of treatment excluded |
| Japanese Society of Ophthalmic Diabetology 201217 | 69 | 69 | Patients with no previous photocoagulation were included |
| Mirshahi 201381 | 33 | 66 | Patients with previous history of laser excluded |
| Muqit 2010/1174–76 | 22 | 36 | Treatment-naive patients |
| Muqit 201377 | 24 | 30 | Treatment-naive patients |
| Muraly 201178 | 50 | 100 | PRP for first time |
| Nagpal 201082 | 60 | 60 | Patients with previous history of laser and anti-VEGFs excluded |
| Salman 201183 | 120 | 120 | Patients with previous history of laser and anti-VEGFs excluded |
| Shimura 200380 | 36 | 72 | Not clear |
| Suto 200884 | 29 | 58 | Not clear |
| Tewari 200055 | 25 | 50 | Patients with previous history of laser excluded |
Six trials included only patients with type 2 diabetes (Japanese Society of Ophthalmic Diabetology 2012;17 Mirshahi 2013;81 Salman 2011;83 Shimura 2003;80 Suto 2008;84 Tewari 200055) and one included only patients with type 1 diabetes (Bandello 200173). Two studies included patients with either type of diabetes (Muqit 2010/11;74–76 Muqit 201377). Three studies (Al-Hussainy 2008;79 Nagpal 2010;82 Muraly 201178) did not report the type of diabetes.
Baseline VA was reported in logMAR scale (Bandello 2001;73 Japanese Society of Ophthalmic Diabetology 2012;17 Mirshahi 2013;81 Muqit 2011;76 Salman 201183) or in letters (ETDRS) (Muqit 201074) or in Snellen scale (Muraly 2011;78 Nagpal 2010;82 Tewari 200055). Four studies did not report baseline VA (Al-Hussainy 2008;79 Muqit 2013;77 Shimura 2003;80 Suto 200884). VA ranged from 0.02 to 1.0 logMAR in seven studies; 77–79 letters in one study and 6/6 (20/20) to 6/60 (20/200) in three studies. Details of previous treatments were not reported in two studies (Shimura 2003;80 Tewari 200055). In the remaining studies, patients with previous histories of treatment with laser, drugs or surgery were excluded. In some, patients were receiving laser for the first time (see Table 16).
Co-morbidities were not reported consistently. In Bandello 2001,73 26–35% of patients had CSMO. In the Japanese Society study,17 there were 70–84% with cataract, 64–71% with hypertension (HTN) and 31–42% with nephropathy. The remaining studies did not report comorbidities.
Follow-up
Four studies (Muqit 2010;74 Muqit 2013;77 Salman 2011;83 Shimura 200380) had less than 6 months of follow-up (Table 17). In four studies, (Mirshahi 2013;81 Muraly 2011;78 Nagpal 2010;82 Tewari 200055), patients were followed up for 6 months. One study (Suto 200884) followed patients for 12 months. Patients were followed up for 18 months in Muqit 2011. 76 The follow-up period ranged from 6 to 45 months in Al-Hussainy 2008. 79 In one study (Japanese study of Ophthalmology 201217) the follow-up period ranged between 6 and 60 months. In Bandello 2001,73 the average follow-up period was around 22 months.
| Studies | Follow-up |
|---|---|
| Al-Hussainy 200879 | 6–45 months |
| Bandello 200173 | 22.4 ± 9.7 months in the light PRP group; 21.6 ± 9.3 months in the classic PRP group |
| Japanese Society of Ophthalmic Diabetology 201217 | 6–60 months |
| Mirshahi 201381 | 6 months |
| Muqit 2010/1174–76 | 12 weeks – Muqit 2010;74 18 months – Muqit 201176 |
| Muqit 201377 | 12 weeks |
| Muraly 2010–1178 | 6 months |
| Nagpal 201082 | 6 months |
| Salman 201183 | Average 9–10.8 weeks |
| Shimura 200380 | 12 weeks |
| Suto 200884 | 12 months |
| Tewari 200055 | 6 months |
Intervention (details of laser)
Table 18 gives details of types of lasers used in the included RCTs.
| Pattern laser trials | |||
|---|---|---|---|
| Author and year | PSC experimental arm(s) | Comparator regimen | Notes |
| Muqit 2010/1174–76 | 20-ms multi-spot single session | 100-ms multi-session single-spot PRP | Patients with active PDR underwent top-up PRP treatment with 20-ms pulse PSC photocoagulation using ETDRS guidelines. Laser used was frequency-doubled Nd:YAG solid-state laser, 532 nm, both arms |
| Muqit 201377 | 1. TRP – single session, 20 ms, 1500–2500 grey-white burns 2. MT-PRP, light grey barely visible burns |
SI-PRP, 2500 grey-white burns | Frequency-doubled 532-nm Nd:YAG solid-state laser consisting of a modified slit lamp and optical system All arms used the PASCAL laser (Topcon Medical Laser Systems, Santa Clara, CA) |
| Muraly 201178 | PSC (OptiMedica, Santa Clara, CA): single sitting of PRP. Usually 30 ms, 2100–3900 burns | Conventional laser using 532-nm frequency-doubled Nd:YAG (Carl Zeiss Meditec, Dublin, California): two to three sittings of PRP | Both methods adjusted to achieve mild to moderate (grey to grey-white) retinal burn |
| Nagpal 201082 | 20-ms PSC (OptiMedica Corp, Santa Clara, CA) 950–1100 spots | GLX (532 nm) (Iridex Corp, Mountain View, CA) single-spot slit-lamp delivery PRP, 500–700 burns | Both lasers performed in two sittings |
| Salman 201183 | PASCAL (OptiMedica, Silicon Valley, CA) 20 ms, 200 µm, 1000 burns | Conventional laser, 700 burns; 532 nm green-light diode-pumped solid state (Novus Spectra, Lumenis, USA) | Two groups, one having PRP, the other having focal or grid for CMSO |
| Other trials | |||
| Author and year | Experimental/intervention | Standard/experimental/no treatment | Notes |
| Al-Hussainy 200879 | Short exposure (0.02 seconds or 20 ms) high-energy scatter PRP | Conventional exposure (100 ms or 0.1 seconds) PRP, power sufficient to produce a visible grey-white burn | 532-nm, frequency-doubled Nd:YAG laser (manufacturer not given) |
| Bandello 200173 | Light PRP on non-perfused peripheral and midperipheral areas | Conventional PRP on non-perfused peripheral and midperipheral areas | All eyes treated with 920 argon lasers (Argon Coherent Medical, Palo Alto, CA or Argon Ophtalas, Biophysic Médical, Clermont Ferrand, France) monochromatic green light using three types of contact lenses. Fluorescein angiograms were done to show perfusion |
| Japanese Society of Ophthalmic Diabetology 201217 | Selective PRP to NPA in PPDR | No photocoagulation till PDR develops | Details of type of laser not given |
| Mirshahi 201381 | Single-spot short duration (20 ms) PRP | Conventional (100 ms) PRP | In both, conventional continuous-wave, frequency-doubled Nd:YAG 532-nm photocoagulation used (Novus Varia, Lumenis, USA) |
| Shimura 200380 | Weekly PRP | Biweekly PRP | PRP scatter laser in four sessions. Krypton red laser (Nidek, Gamagori, Japan) |
| Suto 200884 | PRP then cataract surgery (PRP-first group) | Cataract surgery then PRP (surgery-first group) | Multicolour laser (Coherent Inc., Palo Alto, CA) and a quadraspheric fundus laser lens used. All treatment done using a yellow mainly or red krypton if cataract present |
| Tewari 200055 | Diode laser (810 nm, Microlase, Keeler Inc., UK) | Argon laser (514 nm, Novus 2000, Coherent) | Scatter photocoagulation performed in two to four sittings |
Five studies (Muqit 2010/11;74,76 Muqit 2013;77 Muraly 2011;78 Nagpal 2010;82 Salman 201183) studied the efficacy of pattern photocoagulation (PSC) used in different ways, i.e. in duration or form or sittings.
Quality assessment/risk of bias
Not all studies gave enough details to assess risk of bias. In that case, we categorised them as ‘unclear’. See Table 19 for details.
| Study (author and year) | Adequate sequence generation | Adequate allocation concealment | Masking | Incomplete outcome data assessed | Free of selective outcome reporting | Free of other biases (e.g. similarity at baseline, power assessment) |
|---|---|---|---|---|---|---|
| Al-Hussainy 200879 | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Bandello 200173 | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk |
| Japanese Society of Ophthalmology 201217 | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk |
| Mirshahi 201381 | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk |
| Muqit 2010/1174–76 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Muqit 201377 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Muraly 201178 | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk |
| Nagpal 201082 | Unclear | Unclear | Unclear | Unclear | Low risk | Low risk |
| Salman 201183 | Unclear | Unclear | Unclear | Unclear | Unclear | Low risk |
| Shimura 200380 | Unclear | Unclear | Unclear | Unclear | Low risk | Unclear |
| Suto 200884 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Tewari 200055 | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk |
Allocation
In seven studies (Bandello 2001;73 Japanese Society of Ophthalmic Diabetology 2012;17 Mirshahi 2013;81 Muqit 2010;74 Muqit 2013;77 Suto 2008;84 Tewari 200055), the method of randomisation was adequate, for example computer-generated random numbers (Bandello 2001),73 random number tables (Japanese Society of Ophthalmic Diabetology 2012),17 through hospital pharmacy’s centralised service (Mirshahi 2013),81 permuted blocks (Muqit 2010;74 Muqit 2013;77 Suto 200884) and tossing a coin (Tewari 200055). In the remaining studies (Al-Hussainy 2008;79 Muraly 2011;78 Nagpal 2010;82 Salman 2011;83 Shimura 200380), the randomisation procedure was either not reported or reported inadequately. In three studies (Muqit 2010;74 Muqit 2013;77 Suto 200884) the allocation concealment was adequate. None of the other studies reported on allocation concealment.
Masking
The included studies were checked to see whether both assessors and patients were masked to study treatment. The masking of the former is more important than the latter. Only five studies (Al-Hussainy 2008;79 Bandello 2001;73 Muqit 2010;74,75 Muqit 2013;77 Suto 200884) gave sufficient description of masking while the remaining studies had no information regarding this. In Al-Hussainy (2008),79 patients were masked to the order and the initial site of the treatment (superior or inferior, which was chosen at random). However, it was not clear whether assessors were masked to the treatment.
In Bandello (2001),73 all the post-treatment controls were performed in each centre by an investigator unaware of the treatment group of patients. The post-treatment controls involved complete examination, a series of retinal photographs, and fluorescein angiograph, and this was done on 6 and 12 weeks and every 3 months in the first year and, after 12 months, done every 6 monthly. In Muqit 2010,74 two graders masked to the treatment assessed fundus photographs and fundus FA at baseline at the final visit to grade PDR activity.
In Muqit (2013),77 participants were masked to single-session treatment allocation but the investigator was not masked. After laser treatment, a masked assessor used a questionnaire to assess pain responses. In addition, two masked retina specialists independently assessed PDR grade.
In Suto (2008),84 masked ophthalmologists assessed the disease stage using the ETDRS classification.
Incomplete outcome data
There was adequate description about incomplete data except in two studies (Salman 2011;83 Shimura 200380).
Free of selective reporting
All studies except Salman (2011)83 reported all prespecified and predefined outcomes. In Salman (2011),83 only narrative information was provided on complications and pain. In addition, there was no information on intra- and post-procedure pain.
Free of other biases
In most studies, the baseline characteristics were comparable between treatment groups. In Salman (2011),83 baseline VA was slightly different in the two treatment groups (0.31 vs. 0.6 logMAR). In Shimura (2003),80 baseline characteristics of patients were not given.
Funder/conflict of interest
Funding was not clear in some studies (Al-Hussainy 2008;79 Bandello 2001;73 Mirshahi 2013;81 Muraly 2011;78 Tewari 200055). However, it appears these studies were funded by the affiliated academic’s institution. One study (Muqit 2010/1174–76) was funded by the manufacturer of the PSC laser (OptiMedica Corporation, Santa Clara, CA, USA). One study (Muraly 201178) reported that the authors had no conflict of interest. In Muqit 2010/11,74–76 one author was an employee of OptiMedica Corporation and one author had received financial support from the same company. In the remaining studies, this information was not available.
Details of studies
The studies are each described narratively below and summarised in table format in Table 20.
| Study | Participants and baseline values | Intervention | Outcomes and ocular safety | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID: Al-Hussainy 200879 Aim: To compare short exposure, high-energy laser settings with conventional settings, using a 532-nm, frequency-doubled, Nd:YAG laser and assess the patients in terms of pain experienced and effectiveness of treatment Design: Prospective, masked, randomised study Follow-up: 6–45 months Proportions completing the study: 100% Reasons for withdrawal (brief): n/a |
n: 20 PDR = 17; CRVO = 2; ocular ischaemia = 1 Inclusion criteria: Patients undergoing PRP for first time Exclusion criteria: NR Age (years): Mean 62 (range 26–76) Sex: NR Diabetes type: NR HbA1c: NR Baseline VA: NR Baseline CMT: NR Baseline DR stage: PDR = 17 (not clear which grade of PDR) Previous laser or intravitreal drug treatment: All patients undergoing PRP for first time Ocular comorbidities: NR |
Group 1: Treatment A, the superior or inferior retina was treated with approximately 500 shots of scatter laser photocoagulation using ‘conventional’ laser parameters: spot size 300 µm, exposure 0.1 seconds, power sufficient to cause blanching of retina Group 2: The remaining hemi-retina was treated with treatment B with approximately 500 burns of spot size 300 µm and parameters of higher power but shorter duration (0.02 seconds) to give a similar visible laser end point Description of type of laser and delivery: Scatter PRP, frequency-doubled, Nd:YAG laser, with a wavelength of 532 nm |
Pain: Treatment A (0.1 seconds)Treatment B (0.02 seconds)p-valuePain response (cm on the VAS)5.111.405< 0.001SD2.8610.951 Resolution of neovascularisation on and around the optic disc (NVD) and new vessels elsewhere (NVE): 18/20 patients with follow-up ranging from 6 to 45 months |
Treatment A (0.1 seconds) | Treatment B (0.02 seconds) | p-value | Pain response (cm on the VAS) | 5.11 | 1.405 | < 0.001 | SD | 2.861 | 0.951 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment A (0.1 seconds) | Treatment B (0.02 seconds) | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain response (cm on the VAS) | 5.11 | 1.405 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD | 2.861 | 0.951 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Bandello 200173 Aim: To verify whether a PRP performed using low levels of argon green laser energy (light PRP) has the same efficacy as conventional PRP using argon green wavelengths (classic PRP) in eyes with HR-PDR Design: RCT Follow-up: Light 22.4 months (± 9.7); classic 21.6 (± 9.3) Proportions completing the study: 100% Reasons for withdrawal: None |
n: 65 eyes with HR-PDR of 50 consecutive patients Inclusion criteria: Age at least 18 years, BCVA of 0.4 or more, PDR with two to four HRCs (new vessels at disc greater than ¼ to ⅓ DA or vitreous or pre-retinal haemorrhage associated with less extensive new vessels at disc, or with new vessels elsewhere ½ DA or more in size) Exclusion criteria: VH obscuring more than 20% of the fundus, maculopathy reducing the BCVA to below 0.4, tractional retinal detachment, media clarity inadequate to permit completion of laser PRP, and previous laser treatment Age (years): 48–57 (SD 11–13) Sex: 37–38% female Diabetes type: Insulin dependent HbA1c: NR Baseline VA: BCVA (logMAR) 0.12 to 0.14 (SD 0.13 to 0.15) Baseline CMT: NR Baseline DR stage: PDR Previous laser or intravitreal drug treatment: No Ocular comorbidities: 26.5–35.5% CSMO |
Group 1: In eyes selected for light PRP, the operator tried to obtain a very light grey biomicroscopic effect on the retina. The energy used was the lowest capable of producing a result on the retinal tissue. The target corresponded to the grade 1 of L’Esperance scale (barely visible, blanching of pigment epithelium), median power 235 mW, spot size 500 µm, spot number 2748 ± 468, mean session number 3.5 ± 1.3 Group 2: In eyes selected for classic PRP, the treatment target was the classic burn (figure 2) corresponding to grade 3 of L’Esperance scale (opaque, dusky, grey-white, off-white). Median power 420 mW, spot size 500 µm, spot number 2080 ± 320, mean session number 8.7 ± 2.1 Description of type of aser and delivery: Classic and light PRP using monochromatic green light |
Mean logMAR BCVA Light PRPClassic PRPp-valueBaseline0.12 ± 0.130.14 ± 0.150.493Final0.18 ± 0.250.27 ± 0.300.231 Regression of HR-PDR (end of follow-up) Light PRPClassic PRPp-valueNo HRCs31/34 eyes (91%)30/31eyes (97%)0.615UnchangedTwo eyes (6%)–WorseningOne eye (3%)One eye (3%)Light PRPClassic PRPp-valueMedian power (mW) (range)235 (100–540)420 (200–950)< 0.001Spot size (µm)500500Spot number2748 ± 4682080 ± 320< 0.001Mean session number3.5 ± 1.38.7 ± 2.1< 0.001Total mean session no.7.4 ± 2.49.9 ± 2.2< 0.001 Twenty-six eyes (76%) in the light PRP and 14 (45%) in the classic PRP group, whose retinal photographs and fluorescein angiographies at the 3-month control showed no new vessels regression, underwent further treatment (p = 0.012) Side effects Light PRPClassic PRPp-value (Fisher’s exact test)VH06 (19%)0.009CD03 (10%)0.103Troublesome pain1 (3%)4 (13%)0.184Neurotrophic keratopathy02 (6%)0.224Appearance or worsening of CSMO1 (3%)7 (23%)0.023 |
Light PRP | Classic PRP | p-value | Baseline | 0.12 ± 0.13 | 0.14 ± 0.15 | 0.493 | Final | 0.18 ± 0.25 | 0.27 ± 0.30 | 0.231 | Light PRP | Classic PRP | p-value | No HRCs | 31/34 eyes (91%) | 30/31eyes (97%) | 0.615 | Unchanged | Two eyes (6%) | – | Worsening | One eye (3%) | One eye (3%) | Light PRP | Classic PRP | p-value | Median power (mW) (range) | 235 (100–540) | 420 (200–950) | < 0.001 | Spot size (µm) | 500 | 500 | Spot number | 2748 ± 468 | 2080 ± 320 | < 0.001 | Mean session number | 3.5 ± 1.3 | 8.7 ± 2.1 | < 0.001 | Total mean session no. | 7.4 ± 2.4 | 9.9 ± 2.2 | < 0.001 | Light PRP | Classic PRP | p-value (Fisher’s exact test) | VH | 0 | 6 (19%) | 0.009 | CD | 0 | 3 (10%) | 0.103 | Troublesome pain | 1 (3%) | 4 (13%) | 0.184 | Neurotrophic keratopathy | 0 | 2 (6%) | 0.224 | Appearance or worsening of CSMO | 1 (3%) | 7 (23%) | 0.023 | |||||||||||||||||||||||
| Light PRP | Classic PRP | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 0.12 ± 0.13 | 0.14 ± 0.15 | 0.493 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Final | 0.18 ± 0.25 | 0.27 ± 0.30 | 0.231 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Light PRP | Classic PRP | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No HRCs | 31/34 eyes (91%) | 30/31eyes (97%) | 0.615 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unchanged | Two eyes (6%) | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Worsening | One eye (3%) | One eye (3%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Light PRP | Classic PRP | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median power (mW) (range) | 235 (100–540) | 420 (200–950) | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spot size (µm) | 500 | 500 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spot number | 2748 ± 468 | 2080 ± 320 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean session number | 3.5 ± 1.3 | 8.7 ± 2.1 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total mean session no. | 7.4 ± 2.4 | 9.9 ± 2.2 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Light PRP | Classic PRP | p-value (Fisher’s exact test) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VH | 0 | 6 (19%) | 0.009 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CD | 0 | 3 (10%) | 0.103 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Troublesome pain | 1 (3%) | 4 (13%) | 0.184 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Neurotrophic keratopathy | 0 | 2 (6%) | 0.224 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance or worsening of CSMO | 1 (3%) | 7 (23%) | 0.023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Japanese Society of Ophthalmic Diabetology 201217 Aim: To determine whether the S-PC of NPAs in PPDR is effective at preventing the development of PDR. The VA course in patients who received the S-PC was also evaluated Design: RCT – multicentre Follow-up: Ranged from 6 to 60 (average 34.4, SD 18.00) months Of the 69 patients, 36 patients (23 in non-photocoagulation; 13 in photocoagulation) completed the 36-months’ follow-up Proportions completing the study: 77% (53/69) Reasons for withdrawal: 10 stopped coming to the hospital, three switched hospitals, one developed SVL due to central retinal artery occlusion, one died, and one developed an allergy to fluorescein |
n: 69 patients Inclusion criteria: Patients with PPDR treatment in 2004–8 in the participating institution with no previous photocoagulation and multiple NPA larger than one DA on FA images Exclusion criteria: Clear FA images not obtainable due to opaque media; unable to perform FA due to conditions like allergy; history of intraocular surgery (but those with ≥ 3 years after cataract surgery included); those with severe PPDR requiring PRP Age (years): 59.3–60.8 (SD 8.1 to 10.9) Sex (female): 19–31% female Diabetes type: Type 2 HbA1c (%): 7.5–7.7 (SD 1.5 to 1.7) Baseline VA (logMAR): 0.02–0.03 (SD 0.12 to 0.22) Baseline CMT: NR Baseline DR stage: PPDR Previous laser or intravitreal drug treatment: No PC Ocular comorbidities: Cataract: 70–84%; HTN: 64–71%; nephropathy: 31–42%; posterior vitreous detachment: 3–14% |
Group 1: Non-PC group (no photocoagulation) Group 2: S-PC group (PC group) Description of type of laser and how delivered: S-PC of NPAs |
Development of PDR in 69 patientsOverall 18/69 (26%)Non-PC groupPC groupp-value15/37 (41%)3/32 (95)0.003Development of PDR in 36 patientsNon-PC groupPC groupp-value12/23 (52%)2/13 (15%)0.03Photocoagulation in 36 patientsNo. of coagulation spots for the initial coagulation92–365 (mean 233)Additional coagulation performed17 patients (54%) either once or twice (mean 1.1 times)No. of additional coagulation spots128 to 372/session (mean 224) performed 6–30 months after initial coagulation (mean 14.6 months)VA in 36 patients after 36 months (logMAR)Non-PC groupPC groupp-value0.12, SD 0.430.14, SD 0.330.86VA change in 36 patients (logMAR)Non-PC groupPC groupp-value0.11, SD 0.470.11, SD 0.270.97A decrease of ≥ 0.2Non-PC groupPC groupp-value2/23 (9%)3/13 (23%)0.24Reason for decrease of VA in non-photocoagulation – cataract progression (n = 1), VH (n = 1); reason in photocoagulation – cataract progression (n = 1), MO (n = 2)Severe VA loss (defined by the ETDRS as corrected VA of < 0.025)n = 1 with VH in the non-photocoagulation group | Development of PDR in 69 patients | Overall 18/69 (26%) | Non-PC group | PC group | p-value | 15/37 (41%) | 3/32 (95) | 0.003 | Development of PDR in 36 patients | Non-PC group | PC group | p-value | 12/23 (52%) | 2/13 (15%) | 0.03 | Photocoagulation in 36 patients | No. of coagulation spots for the initial coagulation | 92–365 (mean 233) | Additional coagulation performed | 17 patients (54%) either once or twice (mean 1.1 times) | No. of additional coagulation spots | 128 to 372/session (mean 224) performed 6–30 months after initial coagulation (mean 14.6 months) | VA in 36 patients after 36 months (logMAR) | Non-PC group | PC group | p-value | 0.12, SD 0.43 | 0.14, SD 0.33 | 0.86 | VA change in 36 patients (logMAR) | Non-PC group | PC group | p-value | 0.11, SD 0.47 | 0.11, SD 0.27 | 0.97 | A decrease of ≥ 0.2 | Non-PC group | PC group | p-value | 2/23 (9%) | 3/13 (23%) | 0.24 | Reason for decrease of VA in non-photocoagulation – cataract progression (n = 1), VH (n = 1); reason in photocoagulation – cataract progression (n = 1), MO (n = 2) | Severe VA loss (defined by the ETDRS as corrected VA of < 0.025) | n = 1 with VH in the non-photocoagulation group | ||||||||||||||||||||||||||||||||||||||||||||||
| Development of PDR in 69 patients | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall 18/69 (26%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-PC group | PC group | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15/37 (41%) | 3/32 (95) | 0.003 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Development of PDR in 36 patients | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-PC group | PC group | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12/23 (52%) | 2/13 (15%) | 0.03 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Photocoagulation in 36 patients | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. of coagulation spots for the initial coagulation | 92–365 (mean 233) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional coagulation performed | 17 patients (54%) either once or twice (mean 1.1 times) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. of additional coagulation spots | 128 to 372/session (mean 224) performed 6–30 months after initial coagulation (mean 14.6 months) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VA in 36 patients after 36 months (logMAR) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-PC group | PC group | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0.12, SD 0.43 | 0.14, SD 0.33 | 0.86 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VA change in 36 patients (logMAR) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-PC group | PC group | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0.11, SD 0.47 | 0.11, SD 0.27 | 0.97 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A decrease of ≥ 0.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-PC group | PC group | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2/23 (9%) | 3/13 (23%) | 0.24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reason for decrease of VA in non-photocoagulation – cataract progression (n = 1), VH (n = 1); reason in photocoagulation – cataract progression (n = 1), MO (n = 2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severe VA loss (defined by the ETDRS as corrected VA of < 0.025) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n = 1 with VH in the non-photocoagulation group | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Mirshahi 201381 Aim: To compare pain score of single-spot short duration time (20 ms) PRP with conventional (100 ms) PRP in DR Design: Randomised double-masked, placebo-controlled, single-centre trial Follow-up: 6 months Proportions completing the study: Recruited 76 eyes from 38 patients. Five patients did not complete the follow-up period, and were excluded Reasons for withdrawal: Five patients did not complete the follow-up period |
n: 66 eyes from 33 patients Inclusion criteria: Patients with bilaterally symmetrical very severe non-PDR or PDR Exclusion criteria: Any type of glaucoma, rubeosis iridis, significant media opacity, any history of intraocular surgery except cataract surgery, CSMO, CMT > 300 µm by OCT and marked leakage in FA, previous retinal laser therapy, and patients with combined retinal detachment, uncontrolled HTN (BP ≥ 180/110 mmHg), and poor glycaemic control (HbA1c ≥ 10 mg/dl) Age (years): 58.9 (SD 7.8) Sex (female): 48% Diabetes type: Type 2 (all) HbA1c (mg/dl): 8.23 (SD 1.18) Baseline VA: Conventional group: 0.40 ± 0.24 Short-time group: 0.37 ± 0.27 p = 0.718 Baseline CMT: Conventional: 255 ± 33.7 µm Short-time: 266 ± 33.8 µm; p = 0.334 Baseline DR stage: Non-PDR (n = 18); PDR (n = 15) Previous laser or intravitreal drug treatment: See Exclusion criteria Ocular comorbidities: See Exclusion criteria |
Group 1: Single-spot short duration time (20 ms) PRP Group 2: Conventional (100 ms) PRP Description of type of laser and delivery: A conventional continuous-wave, frequency-doubled Nd:YAG (532 nm) retinal photocoagulator (Novus Varia, Lumenis, USA) with spot size of 200 μm was used in both laser types. Pulse duration of 20 ms was used for the short time PRP and 100 ms for the conventional PRP. Laser energy was adjusted to achieve moderate whitening on the retina. Laser was done in a single session, but five patients in the conventional group did not tolerate the SS-PRP, and the PRP was completed in the next week |
Outcomes: Conventional groupShort-time groupp-valuePain score (VAS)7.5 ± 1.141.75 ± 0.870.000Mean pre-laser BCVA (logMAR)0.40 ± 0.240.37 ± 0.270.718Mean post-laser BCVA (logMAR)0.44 ± 0.280.32 ± 0.220.144Additional PRP (patients)430.68Efficacy of treatment (%)87.990.90.66PRP sessions (single/two)28/533/none0.02ComplicationsNoneNone |
Conventional group | Short-time group | p-value | Pain score (VAS) | 7.5 ± 1.14 | 1.75 ± 0.87 | 0.000 | Mean pre-laser BCVA (logMAR) | 0.40 ± 0.24 | 0.37 ± 0.27 | 0.718 | Mean post-laser BCVA (logMAR) | 0.44 ± 0.28 | 0.32 ± 0.22 | 0.144 | Additional PRP (patients) | 4 | 3 | 0.68 | Efficacy of treatment (%) | 87.9 | 90.9 | 0.66 | PRP sessions (single/two) | 28/5 | 33/none | 0.02 | Complications | None | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Conventional group | Short-time group | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain score (VAS) | 7.5 ± 1.14 | 1.75 ± 0.87 | 0.000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean pre-laser BCVA (logMAR) | 0.40 ± 0.24 | 0.37 ± 0.27 | 0.718 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean post-laser BCVA (logMAR) | 0.44 ± 0.28 | 0.32 ± 0.22 | 0.144 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional PRP (patients) | 4 | 3 | 0.68 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Efficacy of treatment (%) | 87.9 | 90.9 | 0.66 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PRP sessions (single/two) | 28/5 | 33/none | 0.02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Complications | None | None | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Muqit 2010/1174–76 Aim: To compare a single-spot, 100-ms PRP across three sessions and multi-spot PSC 20-ms PRP in a single session (aimed to test the non-inferiority of SS-PRP compared with MS-PRP) Design: Randomised trial Follow-up: 12 weeks (but follow-up data for 18 months in Muqit 2011 Manchester PSC Study Report 476) Completion rate: 100% Reasons for withdrawal: n/a |
n: 40 eyes of 24 patients (one eye from each group discarded so 38 eyes) Inclusion criteria: Patients aged > 18 years, newly diagnosed PDR; either type 1 or type 2 diabetes; ETDR VA 35–85 letters; mean CRT < 300 µm with absence of intraretinal and/or subretinal fluid; adequate pupil dilatation and clear media to perform laser photocoagulation, digital photography and OCT; ability to perform accurate Humphrey visual field test Exclusion criteria: Recent (last 6 months) or ongoing poor glycaemic control; HbA1c > 10 mg/dl; uncontrolled HTN (BP ≥ 180/100 mmHg); history of CRF or renal transplant for DN; lens opacity/cataract; any previous surgical or laser treatment to the study or fellow eye; planned YAG peripheral iridotomy; previous laser photocoagulation or macular laser treatment to the study or fellow eye; history of DMO in study or fellow eye; any previous ocular condition that may be associated with a risk of MO; active eyelid or adnexal infection; previous retinal treatment – laser, drug or surgery; planned intraocular surgery within 1 year Age (years): Mean 56; range 29–60 Sex (female): 34% (13 eyes) Diabetes type: Type 1 and type 2 HbA1c (%): Mean 8.2; range 5.5–10.0 Baseline VA: SS-PRP group – 77 letters (SD 9.9); MS-PRP group – 79 letters (SD 7.9) Baseline CMT: 240 µm in SS-PRP group; 242 µm in MS-PRP group; range 182–297 µm Baseline DR stage: Mild (8 eyes); moderate (23 eyes); severe (7 eyes) Previous laser or intravitreal drug treatment: None Ocular comorbidities: HTN (19 eyes) |
Group 1: 20 eyes underwent multi-spot, 20-ms SS-PRP using 5.5 and 4.4 multi-spot arrays Group 2: 20 eyes underwent single-spot, 100-ms MS-PRP in three sessions across a period of 4 weeks Description of type of laser and delivery: The PSC laser is a frequency-doubled, Nd:YAG, solid-state laser with a wavelength of 532 nm. Photocoagulation is applied in a rapid pattern array with a pulse duration of 10–20 ms. In both groups, threshold laser photocoagulation treatment was titrated to, and designated by, a mild grey-white burn (between grade 2+ and 3+) according to ETDRS guidelines. All eyes received 1500 burns performed under topical anaesthesia. The mean power used for SS-PRP was significantly higher at 277 mW (range, 200–456 mW; p < 0.001) than MS-PRP (143 mW; range, 104–188 mW). The mean fluence for SS-PRP was 4.2 J/cm2 (range, 3–7 J/cm2), significantly lower than that for MS-PRP (11.2 J/cm2; range, 8–15 J/cm2; p < 0.001). The total PRP treatment time for 1500 burns was significantly shorter using multi-spot SS-PRP than with single-spot MS-PRP [SS-PRP, mean, 5.04 minutes (SD 1.5 minutes); vs. MS-PRP, mean, 59.3 minutes (SD 12.7 minutes); p < 0.001] |
Outcomes:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Muqit 2010/11 (Manchester PSC Study Report 4)74–76 Aim: To quantify the 20-ms PSC PRP ablation dosage required for regression of PDR, and to explore factors related to long-term regression Design: Retrospectively studied a cohort of patients who participated in a RCT, the Manchester PSC Study Follow-up: 18 months Completion rate: 36 patients included for analysis Reasons for withdrawal: No one withdrew; however, some patients failed to comply with follow-up appointments since completion of MAPASS (Please note this study includes patients from Muqit 201074) |
n: 36 eyes of 22 patients Inclusion criteria: Newly diagnosed PDR; aged more than 18 years; type 1 or type 2 diabetes; ETDRS VA between 35 and 85 letters; mean CRT of less than 300 μm; adequate pupil dilatation and clear media to perform laser photocoagulation, digital photography and OCT scans; ability to perform accurate Humphrey visual field test; if both eyes eligible then both eyes randomised as per protocol and treated independently Exclusion criteria: Last 6 months or ongoing poor glycaemic control. HbA1c > 10 mg/dl; uncontrolled HTN; history of chronic renal failure or renal transplant for DR; lens opacity/cataract that could influence vision and results; any previous surgical or laser treatment in study or fellow eye; planned YAG peripheral iridotomy; previous laser photocoagulation or macular laser treatment to study eye or fellow eye; history of DMO in study or fellow eye; any previous ocular condition that may be associated with a risk of MO; active lid or adnexal infection; previous retinal treatment: laser, drug or surgery; planned intraocular surgery within 1 year Age (average): Group 1, 42 years; group 2, 42 years; group 3, 47 years; (range 29–70) Sex: Nine eyes female Diabetes type: Type 1 (24 eyes) and type 2 (12 eyes) HbA1c (%): Range 5.5–10 Baseline VA (logMAR): Group 1, 0.04 (SD 0.04); Group 2, 0.17 (SD 0.17); Group 3, 0.14 (SD 0.12) Baseline CMT: NR Baseline DR stage: Grade 1 (7 eyes); grade 2 (21 eyes); grade 3 (7 eyes) Previous laser or intravitreal drug treatment: Treatment-naive patients Ocular comorbidities: NR |
Please see above for details of two different interventions After this, all patients with active PDR underwent top-up PRP treatment using 20-ms pulse PSC photocoagulation using ETDRS guidelines. Threshold top-up PRP treatment was titrated to and designated by a mild grey-white burn (between grade 2+ and 3+) according to ETDRS guidelines at MREH. All treatments performed in a single session under topical 0.4% oxybuprocaine hydrochloride, using 20-ms PSC 5 × 5, 4 × 4, 3 × 3 or 2 × 2 multi-spot arrays. Burn distribution for top-up PRP treatment involved one burn width apart, greater than two DDs temporal to the fovea, no closer than one row within the arcades, and burn placement as close to the ora serrata as possible Patients categorised into three groups according to baseline grade of PDR: Group 1 (grade 1), Group 2 (grade 2) and Group 3 (grade 3) |
Outcomes: Group 1 (n = 8)Group 2 (n = 21)Group 3 (n = 7)p-valueVA (logMAR)No changeNo changeNo changeNRMean power of PRP213 mW (range 104–350, SD 96)220 mW (range 116–482, SD 101)291 mW (range 140–398, SD 59)NRComplete disease regression6/8 (75%) eyes14/21 (67%) eyes3/7 (43%) eyesNRMean time to regression5.8 months (range 3–10)11 months (range 3–19)17 monthsNRTotal no. of laser spots, mean (range)2187 (1500–3450)3998 (1500–8364)6924 (4097–9234)Group 2 vs. 1: p = 0.012;Group 3 vs. 1: p = 0.012Average sessions1–346NRLaser dosimetry required to produce complete PDR disease regression2416 burns (n = 6)3902 (n = 14)5446Laser ablation required to achieve complete regressionMean 264 mm2, SD 95 (181–416 mm2)471 mm2, SD 264 (181–698 mm2)657 mm2, SD 258 (494–954 mm2)Group 2 vs. 1: p = 0.036Group 3 vs. 1: p = 0.0091Fitness to drive – patients who had undergone bilateral PRP treatmentN = 14; questioned about the status of fitness to drive according to DVLA UK requirements. All patients underwent independent VA and binocular VF testing within 6 months of the final study visit. 13/14 patients reported passing the DVLA standard driving standards. One patient failed because of suboptimal VA level, despite a satisfactory binocular VF test Ocular safety:
|
Group 1 (n = 8) | Group 2 (n = 21) | Group 3 (n = 7) | p-value | VA (logMAR) | No change | No change | No change | NR | Mean power of PRP | 213 mW (range 104–350, SD 96) | 220 mW (range 116–482, SD 101) | 291 mW (range 140–398, SD 59) | NR | Complete disease regression | 6/8 (75%) eyes | 14/21 (67%) eyes | 3/7 (43%) eyes | NR | Mean time to regression | 5.8 months (range 3–10) | 11 months (range 3–19) | 17 months | NR | Total no. of laser spots, mean (range) | 2187 (1500–3450) | 3998 (1500–8364) | 6924 (4097–9234) | Group 2 vs. 1: p = 0.012; Group 3 vs. 1: p = 0.012 |
Average sessions | 1–3 | 4 | 6 | NR | Laser dosimetry required to produce complete PDR disease regression | 2416 burns (n = 6) | 3902 (n = 14) | 5446 | Laser ablation required to achieve complete regression | Mean 264 mm2, SD 95 (181–416 mm2) | 471 mm2, SD 264 (181–698 mm2) | 657 mm2, SD 258 (494–954 mm2) | Group 2 vs. 1: p = 0.036 Group 3 vs. 1: p = 0.0091 |
Fitness to drive – patients who had undergone bilateral PRP treatment | N = 14; questioned about the status of fitness to drive according to DVLA UK requirements. All patients underwent independent VA and binocular VF testing within 6 months of the final study visit. 13/14 patients reported passing the DVLA standard driving standards. One patient failed because of suboptimal VA level, despite a satisfactory binocular VF test | |||||||||||||||||||||||||||||||||||||||||||||||
| Group 1 (n = 8) | Group 2 (n = 21) | Group 3 (n = 7) | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VA (logMAR) | No change | No change | No change | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean power of PRP | 213 mW (range 104–350, SD 96) | 220 mW (range 116–482, SD 101) | 291 mW (range 140–398, SD 59) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Complete disease regression | 6/8 (75%) eyes | 14/21 (67%) eyes | 3/7 (43%) eyes | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean time to regression | 5.8 months (range 3–10) | 11 months (range 3–19) | 17 months | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total no. of laser spots, mean (range) | 2187 (1500–3450) | 3998 (1500–8364) | 6924 (4097–9234) | Group 2 vs. 1: p = 0.012; Group 3 vs. 1: p = 0.012 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average sessions | 1–3 | 4 | 6 | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laser dosimetry required to produce complete PDR disease regression | 2416 burns (n = 6) | 3902 (n = 14) | 5446 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laser ablation required to achieve complete regression | Mean 264 mm2, SD 95 (181–416 mm2) | 471 mm2, SD 264 (181–698 mm2) | 657 mm2, SD 258 (494–954 mm2) | Group 2 vs. 1: p = 0.036 Group 3 vs. 1: p = 0.0091 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fitness to drive – patients who had undergone bilateral PRP treatment | N = 14; questioned about the status of fitness to drive according to DVLA UK requirements. All patients underwent independent VA and binocular VF testing within 6 months of the final study visit. 13/14 patients reported passing the DVLA standard driving standards. One patient failed because of suboptimal VA level, despite a satisfactory binocular VF test | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Muqit 2013 BJO (PETER PAN Study)77 Aim: To evaluate the short-term effects on macular thickness of a standard intensity PSC TRP and reduced fluence/MT-PRP compared with SI-PRP Design: Prospective randomised clinical trial Follow-up: 12 weeks (4 and 12 weeks) Proportions completing the study: 100% Reasons for withdrawal: n/a (Short-duration trial – therefore useful for adverse effects but, no effectiveness) |
n: 30 eyes of 24 patients (10 eyes/arm) Inclusion criteria: Patients aged > 18 years of age with newly diagnosed treatment-naive PDR; type 1 or 2 diabetes; ETDRS VA between 35 and 85 letters; mean CRT of < 300 µm; adequate pupil dilatation and clear media to perform laser photocoagulation, digital photography and OCT scans; ability to perform accurate Humphrey visual field test Exclusion criteria: Poor glycaemic control (HbA1c > 10 mg/dl); uncontrolled HTN (BP ≥ 180/100 mmHg); chronic renal failure or renal transplant for diabetic nephropathy; lens opacity/cataract; planned yttrium aluminium garnet peripheral iridotomy; treatment (medical, surgical, pan-retinal or macular laser) within the last 12 months; history of MO in study eye; any previous condition associated with a risk of MO; systemic treatment toxic to the retina or risk of MO; active lid or adnexal infection; planned intraocular surgery within 6 months; vitreomacular traction on FD-OCT scan; ≥ grade 2 epiretinal membrane on FD-OCT scan Age (years): 42–45 (SD 8.8–15.9) Sex (female): 20–40% Diabetes type: Type 1 (70–90%); type 2 (10–30%) HbA1c (%): 8.4–8.6 (SD 1.1 to 1.5) Baseline VA: NR Baseline CMT: NR Baseline DR stage: Mild PDR (n = 14; moderate PDR (n = 8); severe PDR (n = 8) Previous laser or intravitreal drug treatment: None Comorbidities: No patients with HTN, cardiovascular disease or nephropathy |
Group 1: TRP (single session, multi-spot 20-ms TRP) Group 2: Minimally traumatic PRP Group 3: Standard intensity PRP Description of type of laser and delivery: The PSC photocoagulation system is a frequency-doubled 532-nm Nd:YAG solid-state laser (Topcon Medical Laser Systems, Santa Clara, CA) that consists of a modified slit lamp and optical system, which telecentrically images the surface of a multimode step index optical fibre through a two-axis scanner |
PDR activity (12 weeks)TRPMT-PRPSI-PRPp-valuePartial regression60%50%70%No difference between groupsComplete regression10%20%20%No change30%20%Worsening10% One eye in each group (n = 3) required additional 1500 burns of their respective laser at 4 weeks) VA (at 12 weeks)ComparisonDifferencep-valueTRP vs. SI-PRP1.3 letters, SD 11; 95% CI –6.55 to 9.150.717TRP vs. MT-PRP0.7 letters, SD 8.4, 95% CI –5.33 to 6.730.799MT-PRP vs. SI-PRP0.6 letters, SD 7.2 95% CI –4.53 to 5.730.797 Pain response (12 weeks): TRPSI-PRPMT-PRPDescription of pain:n = 1 Sharpn = 2n = 3 Shootingn = 2 Stabbingn = 1 Burningn = 1 Throbbingn = 1n = 1 Electric shock-liken = 1n = 1Mean NPS score1.7 SD 2.33.1 SD 2.70.5 SD 1.3(All categorised as mild pain; no pain in 80% of patients in the MT-PRP group)Mean VAS score immediately post laser15.7 SD 23.732.4 SD 24.23.5 SD 7.8(All categorised as mild)NPS, numerical pain score.No significant differences for pain responses between TRP vs. MT-PRP (NPS, p = 0.05; VAS, p = 0.19) and TRP vs. SI-PRP (NPS, p = 0.12; VAS, p = 0.07). MT-PRP had significantly less pain than SI-PRP (NPS, p = 0.001; VAS, p = 0.005).
|
PDR activity (12 weeks) | TRP | MT-PRP | SI-PRP | p-value | Partial regression | 60% | 50% | 70% | No difference between groups | Complete regression | 10% | 20% | 20% | No change | 30% | 20% | Worsening | 10% | VA (at 12 weeks) | Comparison | Difference | p-value | TRP vs. SI-PRP | 1.3 letters, SD 11; 95% CI –6.55 to 9.15 | 0.717 | TRP vs. MT-PRP | 0.7 letters, SD 8.4, 95% CI –5.33 to 6.73 | 0.799 | MT-PRP vs. SI-PRP | 0.6 letters, SD 7.2 95% CI –4.53 to 5.73 | 0.797 | TRP | SI-PRP | MT-PRP | Description of pain: | n = 1 | Sharp | n = 2 | n = 3 | Shooting | n = 2 | Stabbing | n = 1 | Burning | n = 1 | Throbbing | n = 1 | n = 1 | Electric shock-like | n = 1 | n = 1 | Mean NPS score | 1.7 SD 2.3 | 3.1 SD 2.7 | 0.5 SD 1.3 | (All categorised as mild pain; no pain in 80% of patients in the MT-PRP group) | Mean VAS score immediately post laser | 15.7 SD 23.7 | 32.4 SD 24.2 | 3.5 SD 7.8 | (All categorised as mild) | NPS, numerical pain score. No significant differences for pain responses between TRP vs. MT-PRP (NPS, p = 0.05; VAS, p = 0.19) and TRP vs. SI-PRP (NPS, p = 0.12; VAS, p = 0.07). MT-PRP had significantly less pain than SI-PRP (NPS, p = 0.001; VAS, p = 0.005). |
|||||||||||||||||||||||||||||
| PDR activity (12 weeks) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRP | MT-PRP | SI-PRP | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Partial regression | 60% | 50% | 70% | No difference between groups | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Complete regression | 10% | 20% | 20% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No change | 30% | 20% | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Worsening | 10% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VA (at 12 weeks) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Comparison | Difference | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRP vs. SI-PRP | 1.3 letters, SD 11; 95% CI –6.55 to 9.15 | 0.717 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRP vs. MT-PRP | 0.7 letters, SD 8.4, 95% CI –5.33 to 6.73 | 0.799 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MT-PRP vs. SI-PRP | 0.6 letters, SD 7.2 95% CI –4.53 to 5.73 | 0.797 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRP | SI-PRP | MT-PRP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description of pain: | n = 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sharp | n = 2 | n = 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shooting | n = 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stabbing | n = 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Burning | n = 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Throbbing | n = 1 | n = 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric shock-like | n = 1 | n = 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean NPS score | 1.7 SD 2.3 | 3.1 SD 2.7 | 0.5 SD 1.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (All categorised as mild pain; no pain in 80% of patients in the MT-PRP group) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean VAS score immediately post laser | 15.7 SD 23.7 | 32.4 SD 24.2 | 3.5 SD 7.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (All categorised as mild) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NPS, numerical pain score. No significant differences for pain responses between TRP vs. MT-PRP (NPS, p = 0.05; VAS, p = 0.19) and TRP vs. SI-PRP (NPS, p = 0.12; VAS, p = 0.07). MT-PRP had significantly less pain than SI-PRP (NPS, p = 0.001; VAS, p = 0.005). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Muraly 201178 Aim: To describe the results of a single session of PSC vs. multiple sessions of conventional laser in cases of PDR Design: Prospective study. Two eyes of an individual patient were randomly assigned: one for a single session of PRP using PSC and the other for multiple sessions of conventional laser Follow-up: 6 months (days 1, 7 and 30 and then every month for the next 6 months) Completion rate: All patients were followed up Reason for withdrawals: None Retreatment criteria: (1) if new vessels appear active; (2) appearance of new vessel; and (3) increase in frequency and extent of VH since the last visit Pain determined based on the patients response: mild (no facial expression during laser and no complain of pain during the procedure); moderate (facial expression and complained of pain during the procedure); severe (patients not able to tolerate pain during the procedure, requiring a break of few minutes before continuing or requiring peribulbar or retrobulbar injection) All patients with moderate or severe pain received oral analgesic after the procedure |
n: 100 eyes of 50 patients with PDR on whom PRP was done for the first time Inclusion criteria: PDR with two to four high-risk characters (new vessel at the disc of at least ⅓ to ¼ DA or vitreous or pre-retinal haemorrhages associated with less extensive new vessel at the disc, or with new vessels elsewhere ½ DA or more in size). Only patients with early PDR and HR-PDR in both eyes were included in the study; age at least 18 years or more; BCVA of 6/60 or better Exclusion criteria: DMO excluded; VH obscuring view; patients with vision in only one eye or those in whom only one eye had PDR/high-risk PDSR; maculopathy; tractional retinal detachment; media clarity inadequate to perform a complete laser; previous laser; poor follow-up; and uncontrolled high BP, blood sugar or nephropathy Age (years): Mean 58.24 (range 32–78 for men; 33–70 for women) Sex: 32% female Diabetes type: NR; only reported that ‘39 were diabetic and 11 had both diabetes and HTN’ HbA1c: NR Baseline VA: Right eye 20/20 to 20/300; left eye 20/20 to 20/1200 Baseline CMT: NR Baseline DR stage: Early PDR (10 eyes) and HR-PDR (40 eyes) Previous laser or intravitreal drug treatment: Patients with previous laser were excluded Ocular comorbidities: NR |
Group 1: PSC: single sitting of PRP via PSC, power and describe the results of a single session of PSC vs. multiple sessions of conventional laser in cases of PDR Group 2: Conventional laser: two to three sittings of PRP via conventional laser, power and duration were adjusted to obtain mild to moderate (grey to grey-white) retinal burn. The laser spot diameter in air was 200 mm Description of type of laser and delivery: The following instruments were used:
|
Outcomes: PSC laserConventional laserp-valueMean power (mW)439 (275–950)192.8 (125–300)NRDuration (ms)20–30200NRMean total no. of spots2795 (2100–3892)1414 (1200–1672)NRMean time taken (minutes)10.4 (6–16)29 (20–29)0.0001Pain, n (%): Mild40 (80)0 (0)– Moderate10 (20)11 (22)0.291 Severe025 (50)– Very severe014 (28)–Retreatment: Yes5 (10)14 (28)0.006 No45 (90)36 (72)–1 month: Regressed NVE and NVD45 (90)32 (64)– Persisting NVE and NVD5 (10)12 (24)0.841 VH fresh0(0)6 (12)–6 months: Regressed NVE and NVD49 (98)44 (88)– Persisting NVE and NVD0(0)3 (6)0.933 VH old1 (2)3 (6)– |
PSC laser | Conventional laser | p-value | Mean power (mW) | 439 (275–950) | 192.8 (125–300) | NR | Duration (ms) | 20–30 | 200 | NR | Mean total no. of spots | 2795 (2100–3892) | 1414 (1200–1672) | NR | Mean time taken (minutes) | 10.4 (6–16) | 29 (20–29) | 0.0001 | Pain, n (%): | Mild | 40 (80) | 0 (0) | – | Moderate | 10 (20) | 11 (22) | 0.291 | Severe | 0 | 25 (50) | – | Very severe | 0 | 14 (28) | – | Retreatment: | Yes | 5 (10) | 14 (28) | 0.006 | No | 45 (90) | 36 (72) | – | 1 month: | Regressed NVE and NVD | 45 (90) | 32 (64) | – | Persisting NVE and NVD | 5 (10) | 12 (24) | 0.841 | VH fresh | 0(0) | 6 (12) | – | 6 months: | Regressed NVE and NVD | 49 (98) | 44 (88) | – | Persisting NVE and NVD | 0(0) | 3 (6) | 0.933 | VH old | 1 (2) | 3 (6) | – | |||||||||||||||||||||
| PSC laser | Conventional laser | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean power (mW) | 439 (275–950) | 192.8 (125–300) | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Duration (ms) | 20–30 | 200 | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean total no. of spots | 2795 (2100–3892) | 1414 (1200–1672) | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean time taken (minutes) | 10.4 (6–16) | 29 (20–29) | 0.0001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain, n (%): | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mild | 40 (80) | 0 (0) | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Moderate | 10 (20) | 11 (22) | 0.291 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severe | 0 | 25 (50) | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Very severe | 0 | 14 (28) | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Retreatment: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yes | 5 (10) | 14 (28) | 0.006 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No | 45 (90) | 36 (72) | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Regressed NVE and NVD | 45 (90) | 32 (64) | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Persisting NVE and NVD | 5 (10) | 12 (24) | 0.841 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VH fresh | 0(0) | 6 (12) | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Regressed NVE and NVD | 49 (98) | 44 (88) | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Persisting NVE and NVD | 0(0) | 3 (6) | 0.933 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VH old | 1 (2) | 3 (6) | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Nagpal 201082 Aim: To compare the efficacy, collateral damage, and convenience of PRP for PDR or severe NPDR using a 532-nm GLX vs. a multi-spot 532-nm PSC Design: Prospective RCT. One eye of each patient was randomised to undergo PRP with the GLX and other eye with the PSC Follow-up: At months 1, 3 and 6 Completion rate: 100% Reasons for withdrawal: n/a |
n: 60 patients with bilaterally symmetrical PDR or severe NPDR Inclusion criteria: Bilaterally symmetrical PDR or severe non-proliferative DR Exclusion criteria: Patients with a history of previous laser treatments and/or intravitreal injections in either eye, eyes with a pre-treatment BCVA of < 6/24, patients with media opacities such as significant cataract, corneal opacity, or VH obscuring fundus details in either eye, eyes with diabetic maculopathy, other coincidental ocular disorders, such as glaucoma, uveitis, retinitis pigmentosa, myopia > –6 D, retinal degenerations and dystrophies, optic disc pathologies, present or past Age (years): Age range 45–61 (mean age 52) Sex: 26 female; 34 male Diabetes type: NR HbA1c: NR Baseline VA: (Best corrected) 6/6–6/12: 27 patients (GLX) and 28 (PSC); 6/18–6/24: 33 patients (GLX) and 32 (PSC) Baseline CMT: NR Baseline DR stage: 31 patients proliferative DR, 29 severe NPDR Previous laser or intravitreal drug treatment: Excluded Ocular comorbidities: MO, CSMO; other, e.g. cataract, glaucoma – excluded |
Group 1: GLX single-spot slit-lamp delivery PRP, two sittings: total of 500–700 laser spots in each sitting, spot size of 200 µm, pulse duration of 200 ms, power was adjusted to achieve grade 3 burns, spots placed at one spot distance with a Mainster 165 PRP lens Group 2: PSC two sittings: 950–1100 laser spots in each sitting, spot size of 200 µm, pulse duration of 20 ms, power was adjusted to achieve grade 3 burns, spots placed at one spot distance with a Mainster 165 PRP lens, used 5 × 5 square grid, thereby applying 25 spots simultaneously |
Length per sitting: PSCGLXp-valueAverage1.43 minutes per sitting4.53 minutes per sitting0.008 Pain: PSCGLXp-valueVASRange 0–1, average 0.33Range 3–9, average 4.60.007 VA: Laser6/6–6/126/18–6/24p-valueGLX (no. of subjects):Difference between post-treatment visual acuities between the two groups was not statistically significant. The change in VA was not statistically significant (p = 0.508) Pre laser2733 Post laser3129PSC (no. of subjects): Pre laser2832 Post laser3426 Macular thickness: No evidence of change in macular thickness was seen in any of the eyes Regression: Comparable regression in eyes treated with either modality Complications: None of the eyes developed any complication Six patients required laser augmentation because of fresh neovascularisation (NVE) fronds: GLX group = 4; PSC group = 2 Retinal sensitivity: PSCGLXp-valueCentral 15°25.08 dB (range, 20.56–27.62 dB)23.16 dB (range, 19.31–27.37 dB)Difference was not statistically significant (zone A; p = 0.26; zone B; p = 0.09)15–30°22.08 dB (range, 8.25–23.88 dB)17.14 dB (range, 6.93–23.25 dB) |
PSC | GLX | p-value | Average | 1.43 minutes per sitting | 4.53 minutes per sitting | 0.008 | PSC | GLX | p-value | VAS | Range 0–1, average 0.33 | Range 3–9, average 4.6 | 0.007 | Laser | 6/6–6/12 | 6/18–6/24 | p-value | GLX (no. of subjects): | Difference between post-treatment visual acuities between the two groups was not statistically significant. The change in VA was not statistically significant (p = 0.508) | Pre laser | 27 | 33 | Post laser | 31 | 29 | PSC (no. of subjects): | Pre laser | 28 | 32 | Post laser | 34 | 26 | PSC | GLX | p-value | Central 15° | 25.08 dB (range, 20.56–27.62 dB) | 23.16 dB (range, 19.31–27.37 dB) | Difference was not statistically significant (zone A; p = 0.26; zone B; p = 0.09) | 15–30° | 22.08 dB (range, 8.25–23.88 dB) | 17.14 dB (range, 6.93–23.25 dB) | |||||||||||||||||||||||||||||||||||||||||||||||||
| PSC | GLX | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average | 1.43 minutes per sitting | 4.53 minutes per sitting | 0.008 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSC | GLX | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VAS | Range 0–1, average 0.33 | Range 3–9, average 4.6 | 0.007 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Laser | 6/6–6/12 | 6/18–6/24 | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GLX (no. of subjects): | Difference between post-treatment visual acuities between the two groups was not statistically significant. The change in VA was not statistically significant (p = 0.508) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pre laser | 27 | 33 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Post laser | 31 | 29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSC (no. of subjects): | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pre laser | 28 | 32 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Post laser | 34 | 26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSC | GLX | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Central 15° | 25.08 dB (range, 20.56–27.62 dB) | 23.16 dB (range, 19.31–27.37 dB) | Difference was not statistically significant (zone A; p = 0.26; zone B; p = 0.09) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15–30° | 22.08 dB (range, 8.25–23.88 dB) | 17.14 dB (range, 6.93–23.25 dB) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Salman 201183 Aim: To compare the safety and efficacy of PSC laser photocoagulation in comparison with the conventional laser photocoagulation in the treatment of DR Design: Prospective randomised case series (120 patients divided into four groups. Operator chose whether or not to do PSC, based on the random distribution by computer system) Follow-up: B1, mean 10.8 weeks (SD = 5.6); B2, mean 9.0 weeks (SD = 4.5) Completion rate: 100% Reasons for withdrawal: n/a (Follow-up less than 6 months; group A is DMO only) |
n: 120 procedures of 120 patients (60 with PDR) Inclusion criteria: Patients with type 2 DR with need for laser for (NPDR) with (CSMO), focal or diffuse maculopathy or for PDR Exclusion criteria: Patients with ischaemic maculopathy, previous laser or intravitreal injection, vitrectomy or associated retinal diseases as retinal vein occlusion Age (years): Mean age 48.9 (SD 9.3, range 41–86) Sex: 72 (60%) male; 48 (40%) female Diabetes type: Type 2 HbA1c: NR Baseline VA: Group B1: mean VA logMAR (minimal angle resolution): 0.31 (SD = 0.23), Snellen equivalent: 6/12 Group B2: mean VA logMAR (minimal angle resolution): 0.6 (SD = 0.61), Snellen equivalent: 6/24 Baseline CMT: NR Baseline DR stage: Groups B1 and B2: PDR Previous laser or intravitreal drug treatment: Excluded Ocular comorbidities: Not mentioned for PDR group |
Group A1: Patients undergoing focal or modified grid macular laser photocoagulation for NPDR (with MO) using conventional laser (The Novus Spectra, which is a 532 nm green-light diode-pumped solid-state (DPSS) photocoagulator (Lumenis) with treatment durations: 10–3000 ms, spot size from 50 µm up to 500 µm and power from 50 mW up to 2500 mW Group A2: Patients undergoing focal or modified grid macular laser photocoagulation for NPDR (with MO) using PSC laser photocoagulation Group B1: Patients undergoing pan laser photocoagulation (PRP) for PDR using conventional (DPSS) laser photocoagulation PSC was used for additional fill-in PRP in 10/30 procedures of group B1 in which conventional laser photocoagulation was not successful in controlling the neovascularisation Group B2: Patients undergoing PRP for PDR using PSC laser photocoagulation |
For treatment of PDR: Success rate (regression of neovascularisation, and no further treatment planned): B2 = 28/30 vs. B1 = 20/30 (p < 0.05) Nine out of ten procedures with additional PSC were successful with regression of neovascularisation at their latest follow-up visit. One eye needed further laser. Three patients had needed a sub-Tenon’s anaesthetic for their conventional laser session, but none of them required it for their PSC procedure B2: 14 (46%) performed in single session, 16 (54%) in two sessions None of the eyes with SS-PRP developed any complications, regression noted in all, with no further treatment planned at their last follow-up visit No complications related to laser treatment were noted in any patient Post-laser VA logMAR: B1 = 0.3 (SD = 0.27) Snellen equivalent: 6/12 B2 = 0.53 (SD = 0.61) Snellen equivalent: 6/18 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Shimura 200380 Aim: To investigate the alterations of macular thickness during and after PRP in patients with severe DR and good vision, and to compare the outcomes of weekly and biweekly treatments Design: Prospective, comparative interventional case series Follow-up: 12 weeks Completion rate: Unclear Reasons for withdrawal: Seven eyes of four patients (four eyes in the weekly treated group and three eyes in the biweekly) who developed MO and reduced VA were excluded because the time course of macular thickening in those eyes was monotonically increasing and different from that in the remaining patients (Follow-up less than 6 months) |
n: 36 diabetic patients with severe non-proliferative or early PDR without visual disturbances Inclusion criteria: Diabetic patients with symmetrical, severe non-proliferative or early PDR without visual disturbances, VA of 20/20 or better Exclusion criteria: Clinically significant MO Age (years): 55.6 ± 7.8 (range 35–68) Sex (% female): 42 (15/36) Diabetes type: Type 2 HbA1c: 8.7 ± 1.4 mg/dl Baseline VA: NR Baseline CMT: Weekly group: 191 ± 9.9 µm Biweekly: 191 ± 10.7 µm p = 0.814 Baseline DR stage: Severe non-proliferative or early PDR Previous laser or intravitreal drug treatment: NR Ocular comorbidities: No history of any other ocular disease except refractive errors was reported |
Group 1: Weekly PRP treatment Group 2: Biweekly PRP treatment Description of type of laser and delivery: PRP scatter treatment completed in four sessions. The size of the spots on the retina was 200–500 µm, and the duration of the application was 0.15–0.2 seconds. The initial burns were non-confluent and placed > 2 DDs from the fovea out to the equator. A krypton red laser was used, resulting in a grey retina. Each spot was produced by a 120- to 180-mW exposure. The number of spots in each session was around 500 |
Additional focal laser treatment for retinal neovascularisation at 16 weeks: Weekly group: 15 eyes Biweekly: 13 There was a significant difference of the initial HbA1c levels between patients who did not need additional treatments (p = 0.0031) VA: After the completion of PRP, the VA was maintained in 89% (32 of 36 eyes) of eyes treated weekly and in 92% (33 of 36 eyes) treated biweekly |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Suto 200884 Aim: To evaluate the outcomes in patients with DR and cataract who had PRP first and cataract surgery second in one eye, and cataract surgery followed by PRP in the fellow eye Design: RCT Follow-up: 12 months Proportions completing the study: 100% Reasons for withdrawal (brief): n/a |
n: 29 patients Inclusion criteria: Untreated severe non-proliferative or early PDR of similar severity in both eyes and a similar cataract grade in both eyes and who were at least 50 years old Exclusion criteria: Age < 50 years, high lens opacity, loss of zonular fibres, pupillary anomalies synechias, history of serious coexistent ocular disease (e.g. glaucoma, optic atrophy, ocular tumour), pseudoexfoliation syndrome, use of topical or systemic steroids or non-steroidal anti-inflammatory drug during previous months, corneal opacities, history of uveitis or retinal vein occlusion, previous ocular trauma or intraocular surgery, severe refractive error, decrease in HbA1c by ≥ 3% from 3 months, serious renal, hepatic, endocrine, pulmonary, cardiac neurological, rheumatic, psychiatric, or cerebral dysfunction, alcohol or drug abuse problems, allergy to fluorescein, posterior capsule rupture during surgery, Nd:YAG laser capsulotomy for posterior capsule opacification during observation period Age (mean ± SD), years: 66.0 ± 7.5 Sex (female): 69 (20/29) Diabetes type: Type 2 HbA1c (mean%): 7.4 ± 1.0 Baseline VA: NR Baseline DR stage: Severe non-proliferative or early proliferative diabetic based on ETDRS scale: Cataract first: Level 53: n = 22; level 61: n = 7 PRP first: Level 53: n = 21; level 61: n = 8 Previous laser or intravitreal drug treatment: None |
Group 1: PRP was performed before cataract surgery in one eye (PRP-first group). Cataract surgery was performed 1 to 3 months after the final preoperative PRP session in the PRP-first eye Group 2: PRP was performed after cataract surgery in the contralateral eye (surgery-first group). Cataract surgery was performed within 4 days Description of type of laser and delivery: A multicolour laser and a quadraspheric fundus laser lens were used. The laser parameters conditions were 200 µm, 0.12 to 0.16 W, and 0.2–0.4 seconds in the PRP-first group and 200 µm, 0.08 to 0.12 W, and 0.2–0.4 seconds in the surgery-first group. All treatment was done using a yellow mainly or red krypton if cataract was higher When macular lesions were observed, they were treated before PRP |
Outcomes: ParameterPRP firstSurgery firstp-valueBCVA 20/40 or better, n (%)20 (69.0)28 (96.6)0.012MO severity, n Absent66 146 21012 395Rate of progression of MO, n (%):16 (51.7)8 (27.6)0.033 Focal treatment0.527 Before surgery30 After surgery148DR severity, based on ETDRS scale: Level 531613 Level 611010 Level 6536Rate of progression or retinopathy, n (%)8 (27.6)12 (41.4)0.270 Risk of progression of MO higher in the PRP-first group than in the surgery-first group (RR = 2.0; 95% CI 1.49 to 2.51) |
Parameter | PRP first | Surgery first | p-value | BCVA 20/40 or better, n (%) | 20 (69.0) | 28 (96.6) | 0.012 | MO severity, n | Absent | 6 | 6 | 1 | 4 | 6 | 2 | 10 | 12 | 3 | 9 | 5 | Rate of progression of MO, n (%): | 16 (51.7) | 8 (27.6) | 0.033 | Focal treatment | 0.527 | Before surgery | 3 | 0 | After surgery | 14 | 8 | DR severity, based on ETDRS scale: | Level 53 | 16 | 13 | Level 61 | 10 | 10 | Level 65 | 3 | 6 | Rate of progression or retinopathy, n (%) | 8 (27.6) | 12 (41.4) | 0.270 | |||||||||||||||||||||||||||||||||||||||||||||
| Parameter | PRP first | Surgery first | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BCVA 20/40 or better, n (%) | 20 (69.0) | 28 (96.6) | 0.012 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MO severity, n | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Absent | 6 | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | 4 | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 10 | 12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 9 | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rate of progression of MO, n (%): | 16 (51.7) | 8 (27.6) | 0.033 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Focal treatment | 0.527 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Before surgery | 3 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| After surgery | 14 | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DR severity, based on ETDRS scale: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Level 53 | 16 | 13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Level 61 | 10 | 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Level 65 | 3 | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rate of progression or retinopathy, n (%) | 8 (27.6) | 12 (41.4) | 0.270 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Tewari 200055 Aim: To compare treatment with diode infrared and argon green scatter photocoagulation for PDR Design: Prospective randomised trial Follow-up: 6 months Completion rate: Unclear Reasons of withdrawal: n/a |
n: 50 eyes of 25 patients Inclusion criteria: Patients with bilateral PDR Exclusion criteria: Patients who had previously received photocoagulation; who had hypertensive retinopathy, vascular block, or hazy media; and those in whom laser delivery was difficult Age: Mean 56.25 (SD 8.2) years Sex: NR Diabetes type: Type 2 HbA1c: NR Baseline VA: 6/9 to 6/60 (better than 20/30 to 20/200) in argon group and 6/6 to 6/60 (20/20 to 20/200) in diode group Baseline CMT: NR Baseline DR stage: Bilateral PDR Previous laser or intravitreal drug treatment: NR Ocular comorbidities: NR |
Group 1: Argon laser: spot size: 200–500 µm, duration 0.1–0.2 seconds, mean no. of spots 1694 ± 234 Group 2: Diode laser: spot size: 200–500 µm, duration 0.2–0.3 seconds, mean no. of spots 1439 ± 206 Description of type of laser and delivery: Diode infrared (810 nm) and argon green (514 nm) scatter photocoagulation done in two to four sittings; laser power varied between 0.2 and 0.4 W |
Outcomes: Argon laserDiode laserRegression of neovascularisation18/25 eyes (72%)16/25 eyes (64%)VH2/25 eyes (8%)2/25 eyes (8%)Lager augmentation requirement8/25 eyes (32%)12/25 eyes (48%) Mean VA: Argon laserDiode laserBefore laser treatment4.16 ± 2.773.22 ± 2.05Six weeks after laser photocoagulation5.04 ± 3.034.56 ± 2.56Final after 6 months4.76 ± 2.833.62 ± 2.12 Constriction of the peripheral visual field (I-4-e) in both groups Contrast sensitivity: Diode vs. argon (p = 0.06; Student’s t-test) Pain: Argon laserDiode laserp-value7 (28%)23 (92%)< 0.001 χ2 |
Argon laser | Diode laser | Regression of neovascularisation | 18/25 eyes (72%) | 16/25 eyes (64%) | VH | 2/25 eyes (8%) | 2/25 eyes (8%) | Lager augmentation requirement | 8/25 eyes (32%) | 12/25 eyes (48%) | Argon laser | Diode laser | Before laser treatment | 4.16 ± 2.77 | 3.22 ± 2.05 | Six weeks after laser photocoagulation | 5.04 ± 3.03 | 4.56 ± 2.56 | Final after 6 months | 4.76 ± 2.83 | 3.62 ± 2.12 | Argon laser | Diode laser | p-value | 7 (28%) | 23 (92%) | < 0.001 χ2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Argon laser | Diode laser | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Regression of neovascularisation | 18/25 eyes (72%) | 16/25 eyes (64%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VH | 2/25 eyes (8%) | 2/25 eyes (8%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lager augmentation requirement | 8/25 eyes (32%) | 12/25 eyes (48%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Argon laser | Diode laser | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Before laser treatment | 4.16 ± 2.77 | 3.22 ± 2.05 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Six weeks after laser photocoagulation | 5.04 ± 3.03 | 4.56 ± 2.56 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Final after 6 months | 4.76 ± 2.83 | 3.62 ± 2.12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Argon laser | Diode laser | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 (28%) | 23 (92%) | < 0.001 χ2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Al-Hussainy 2008
This trial compared short and standard exposures. 79 Al-Hussainy (2008)79 used a scatter PRP frequency-doubled Nd:YAG laser with a wavelength of 532 nm with oxybuprocaine 0.4% analgesia. The laser was given either as short (0.02 seconds) exposure, high-energy laser or longer (0.1 seconds) laser with approximately 500 burns of spot size 300 µm to get similar effects. The conventional laser was performed in the superior or inferior retina while the remaining hemi-retina was treated with the short exposure laser. It was not clear whether the two lasers were given in the same eye of the same patient. In 18 out of 20 patients with follow-up ranging between 6 and 45 months, there was resolution of neovascularisation on and around the optic disc (NVD) and new vessels elsewhere (NVE). In the remaining two cases (one with CRVO and the other with PPDR), there was no resolution. Vitreous haemorrhage developed in the patient with CRVO, which meant no further treatment could be given. The patient with PPDR had no NVD/NVE at baseline and did not develop it during follow-up. Visual analogue scale (VAS) was used to measure the pain response. Pain response was significantly lower in the group receiving a short exposure high-energy laser than for those receiving conventional laser (1.4 cm vs. 5.11 cm on VAS; p < 0.001). On retinal photographs, no obvious differences were seen in photocoagulation reactions between the two lasers.
Bandello 2001
In Bandello (2001),73 a low-energy power (light PRP) laser application was compared against the conventional classic PRP in a trial in 65 eyes. The aim was ablation of non-perfused peripheral and mid-peripheral NPAs. Light was argon laser aiming at a very light grey effect, using the lowest energy possible with the target burnt areas corresponded to the grade 1 of L’Esperance scale (barely visible, blanching of pigment epithelium), whereas in the latter the target resembled the classic burn, i.e. grade 3 of L’Esperance scale (opaque, dusky, grey-white, off-white). Both lasers were delivered using monochromatic green light through contact lenses. The authors found no difference between the two treatments in terms of mean change in BCVA (0.06 vs. 0.13 logMAR). (LogMAR is on scale of 0–1, with lower better.) LogMAR increased from 0.12 to 0.18 in light and 0.14 to 0.27 in standard.
(Note that the visibility of burns varies amongst different people. A whitish scar may appear in a very pigmented person with the same power that would give only a very faint scar in a blond individual.)
Slightly more patients in the classic PRP group showed regression of HRCs than in the light PRP group (97% vs. 91%); however, the difference was not statistically significant (p = 0.615). Improvement in PDR was considered if there was a reduction of one or more HRCs. One eye in each group showed worsening of retinopathy. In the light PRP group, at the 12-months follow-up one eye had an increase in HRCs therefore was given additional classic PRP group. Median power in the classic PRP group was significantly greater than in the light PRP (420 mW, range 200–950 mW vs. 235 mW, range 100–540 mW; p < 0.001). In both forms of laser, spots were 500 µm in size. The spot numbers were significantly greater in the light PRP group {2748 [standard deviation (SD) 468] vs. 2080 (SD 320); p < 0.001}. The mean number of sessions and total sessions was significantly higher in the classic PRP group than in the light PRP group [8.7 (SD 2.1) vs. 3.5 (SD 1.3); p < 0.001; total: 9.9 (SD 2.2) vs. 7.4 (SD 2.4); p < 0.001]. More patients in the classic PRP group than in the light PRP group complained of troublesome pain (13% vs. 3%, p = 0.184). Significantly more of patients in the classic PRP group developed vitreous haemorrhage (19% vs. 0%; p = 0.009) and appearance or worsening of CSMO (23% vs. 3%; p = 0.023) than in the light PRP group. Furthermore, slightly more patients in the classic PRP group than in the light PRP group developed other complications including choroidal detachment (CD) (10% vs. 0%; p = 0.103) and neurotrophic keratopathy (6% vs. 0%; p = 0.224).
So the lighter laser technique appeared almost as effective but with fewer adverse effects. The authors attribute the lower rates of complications, such as vitreous haemorrhage in the lighter group, to the lower energy used, resulting in reduced heat absorption.
Japanese Society of Ophthalmic Diabetology 2012
Targeted retinal photocoagulation (TRP) treats only areas of retinal non-perfusion.
The aim of this RCT was to compare selective photocoagulation (S-PC) with no photocoagulation in patients with NPDR. 17 In Japan, most ophthalmologists use S-PC in which NPAs are identified by FA and treated at the NPDR stage. (The paper uses the term PPDR. Those with PPDR severe enough to be deemed to require PRP were excluded.)
This study17 compared S-PC (S-PC group) of NPAs only in PPDR, with no photocoagulation till PDR developed (non-photocoagulation group). In the S-PC group, the photocoagulation spots on the retina were of 400–500 µm with a space of approximately one photocoagulation spot between the two. In the non-photocoagulation group, patients were followed up and no interventions were given until they developed PDR, when PRP was performed. It is not clear how many were so treated. PDR was defined as any so includes early PDR as defined by ETDRS. If MO developed, patients could have laser if the ophthalmologist decided it was needed.
Only 69 patients were recruited, all with type 2 diabetes. PPDR is not defined.
The study17 was stopped early because more PDR developed in the non-photocoagulation arm. About one-third of the patients had not reached the 3-year follow-up, and 16 dropped out (more in the photocoagulation arm).
Change in VA after 36 months was not different between the two groups (0.11, SD 0.47, logMAR in non-photocoagulation group vs. 0.11, SD 0.27, in photocoagulation group; p = 0.97). Slightly more patients in the S-PC group [3/13 (23%)] than in the non-photocoagulation group [2/23 (9%)] lost VA of ≥ 0.2 logMAR; however, the difference was not statistically significant (p = 0.24). Reasons for decreases in VA in the non-photocoagulation group were cataract progression (n = 1) and vitreous haemorrhage (n = 1), and in the S-PC group cataract progression (n = 1) and MO (n = 2). One patient in the non-photocoagulation group had SVL (defined by the ETDRS as corrected VA of < 0.25) due to vitreous haemorrhage. Significantly more patients in the non-photocoagulation group developed PDR, defined as new vessels on FA, than those in the S-PC group. If only those with 3-year follow-up are included, the incidence of PDR was 52% (12/23) in the no-photocoagulation group and 15% (2/13) in the photocoagulation group (p = 0.03).
The mean number of spots during the initial photocoagulation in 36 patients was 233 (range 92–365). In 54% patients (n = 17) additional coagulation was performed either once or twice (mean 1.1 times). The mean number of additional coagulation spots was 224 (range 128–372 per session), which was performed between 6 and 30 months (mean 14.6 months) after initial photocoagulation.
In summary, early PRP at the PPDR stage reduced progression to PDR but was not associated with any difference in mean VA at 36 months. The effects on visual fields or driving vision were not reported. This was a small study17 but might be worth repeating with larger numbers. However, it might be argued that might be unethical because of the results.
Literature searches by the authors found little evidence on the use of this method. They reported another study from Japan (in Japanese) by Shimizu et al. (1989),85 which was a non-randomised comparison in eyes with PPDR but only 20% had S-PC – the rest had PRP.
They concluded that this method was used only in Japan.
Mirshahi 2013
This study compared different exposures. 81 A continuous-wave, frequency-doubled Nd:YAG (532 nm) retinal photocoagulation with spot size of 200 µm was used. The difference between the two arms was only in the duration of the exposure: single-spot short duration (20 ms) PRP and conventional (100 ms) PRP. The energy of the laser was adjusted to achieve moderate whitening on the retina. The conventional laser required a mean power of 273 ± 107 mW, while the short-duration laser required 721 ± 406 mW to achieve moderate whitening on the retina. In the former, an average of 1218 ± 441 spots was performed and an average of 2125 ± 503 spots in the latter. Photocoagulation was usually performed in a single sitting, but in five patients in the conventional group the treatment had to be completed in the following week. There was no difference in change in VA between the two treatments: single-spot short duration (20 ms) PRP against conventional (100 ms) PRP (p > 0.05). All short PRP was performed in single sessions, whereas five of the conventional laser was performed in two sessions and remainder (28) in single sessions. The difference between the two in terms of sessions was significant (p = 0.02). Three patients in the short PRP group and four patients in the conventional group required additional PRP (p = 0.68). The pain score was significantly lower in patients receiving single-spot short duration (20 ms) PRP than in those receiving conventional (100 ms) PRP [1.75 (SD 0.87) vs. 7.5 (SD 1.14) on VAS; p < 0.001]. No complications were seen with single-spot short duration PRP.
In summary, the short exposure laser performed in single sitting was found to be significantly less painful and as effective as the conventional laser.
Muqit 2010/11
The MAnchester PSC study (MAPASS) (Muqit 2010;74,75 Muqit 201176) compared multi-spot 20-ms single-session PRP (SS-PRP) using 5.5 and 4.4 multi-spot arrays, given in a single session, with single-spot 100-ms multiple-session PRP (MS-PRP) given in three sessions over 4 weeks. 74–76 Patients (40 eyes of 24 patients; in analysis 38 eyes included) had newly diagnosed PDR, described as being in three grades:
-
Mild PDR Less than Standard Airlie House photograph 10A (SAH10A) mean logMAR 0.04.
-
Moderate PDR Neovascularisation away from disc (NVE) greater than half DD and/or NVD greater than Airlie House 10A, mean logMAR 0.17.
-
Severe PDR Multiple NVE and or NVD, mean logMAR 0.14.
The laser used was PSC a frequency-doubled Nd:YAG solid-state laser with a wavelength of 532 nm. In both groups, the threshold laser photocoagulation treatment was titrated to, and designated by, a mild grey-white burn (between grades 2 and 3) according to ETDRS guidelines. All eyes received 1500 burns performed under topical anaesthesia.
Outcomes in Muqit (2010)74,75 included:
-
Central subfield retinal thickness.
-
Mean change in VA At 12 weeks, VA increased by four letters (SD six letters) from baseline in the SS-PRP compared with the MS-PRP group.
-
PDR grade at 12 weeks No significant difference between the treatment groups in terms of effect on PDR activity.
-
Adverse events Numerical pain score (NPS) within 1 hour and mean numerical headache score 1 month after treatment. The mean NPS immediately after laser was higher in the conventional exposure group than in the short exposure group [2.4 (SD 2.3) vs. 4.9 (SD 3.3)]. Patients in the former group categorised pain as moderately severe in intensity, and those in the latter group as mild. At 1 month, the mean numerical headache score was significantly lower in the short exposure group than in the conventional group [1.5 (SD 2.7) vs. 3.2 (SD 3.5); diff. 95% CI 3.7 to 0.3; p = 0.045]. The median duration of photophobia was significantly lower in the short exposure group (3 hours vs. 72 hours; p < 0.001). The effect of the laser on driving and other activities was similar in both treatment groups. No other immediate or short-term ocular complications were reported.
Initial follow-up was only 12 weeks, but follow-up after the initial trial was in routine care, for 1–2 years, range 15–19 months according to grade.
In Muqit (2011),76 data from a cohort of patients from Muqit (2010)74,75 were used to quantify the 20-ms PSC ablation required for regression of PDR.
Patients were grouped according to their baseline PDR (Group 1 – grade 1, Group 2 – grade 2, Group 3 – grade 3). Numbers in groups were quite small (8–14).
Twenty eyes received multi-spot, 20-ms single-session PRP, while the remaining 20 eyes underwent single-spot, 100-ms multiple sessions PRP (three sessions across a period of 4 weeks). Only 36 eyes of 22 patients were included in the analysis because one patient died shortly after completing the MAPASS trial and another patient was lost to follow-up. The mean power of the laser ranged from 104 to 482 mW across groups. Patients with grade 1 PDR (Group 1) received the lowest mean power laser (213 mW, range 104–350 mW), followed by those with grade 2 PDR (Group 2) (220 mW, range 116–482 mW) and patients with grade 3 PDR (Group 3) (291 mW, range 140–398 mW). Similarly, Group 1 had the lowest mean total number of spots (2187, range 1500–3450 mW), followed by Group 2 (3988, range 1500–8364 mW) and finally Group 3 (6924, range 4097–9234 mW). The difference was significant in comparisons of Group 2 versus Group 1 (p = 0.012) and Group 3 versus Group 1 (p = 0.012). The average number of sessions ranged from one to three in Group 1, four in Group 2 and six in Group 3.
Outcomes in Muqit (2011)76 included:
-
complete PDR regression – no leakage on WF-FA (wide-field Optos FA) and/or disappearance of neovascular (NV) complexes
-
VA – no significant changes at final follow-up.
Safety end points were:
-
DMO
-
vitreous haemorrhage
-
tractional retinal detachment.
At the end of follow-up, there was no significant change in VA within and between the groups (only SD reported, no p-value reported). The study76 also reported that cataract surgery (n = 1), vitrectomy (n = 1) and top-up PRP did not affect VA. Patients who underwent bilateral PRP treatment (n = 14) with mean burns of over 4000, were questioned about the status of fitness to drive according to DVLA UK requirement. All patients underwent testing within 6 months of the final study visit. Out of 14 patients, 13 passed the DVLA standard driving standards, and one failed because of suboptimal VA level, despite having a satisfactory binocular visual field test.
Complete regression was seen in 75% (6/8 eyes) in Group 1; 67% (14/21) in Group 2; and 3/7 eyes in Group 3. In Group 1 complete disease regression occurred at a mean time of 5.8 months (range 3-10 months). In Groups 2 and 3, the mean times to regression were 11 months (range 3-19 months) and 17 months, respectively. The mean laser ablation required to achieve complete PDR regression in Groups 1, 2 and 3 were 264 mm2 (SD 95 mm2; range 181–416 mm2); 471 mm2 (SD 264 mm2; range 181–698 mm2); and 657 mm2 (SD 258 mm2; range 494–954 mm2), respectively.
No complications were seen with a 20-ms PSC laser. There were also no reports of unexpected adverse or serious adverse events in the study. 76 None of the patients showed signs of intraretinal/subretinal haemorrhage or blood vessel damage from 20-ms PRP burns and no indirect laser-related ocular complications. However, there were reports of seven vitreous haemorrhage and one tractional retinal detachment associated with elevated/forward NVD greater than the SAH10A. Three eyes developed uncomplicated, complete posterior vitreous detachment. Within 6 months after completing the study, DMO developed in three eyes but was unrelated to top-up PRP treatments, and in one patient it was related to pregnancy.
Muqit et al. (2011)76 concluded that SS-PRP was as safe and effective as MS-PRP. PDR was improved in 74% of eyes in the SS-PRP group and 53% of eyes in the MS-PRP group (difference not statistically significant).
Muqit 2013
Muqit et al. (2013)77 is a pilot study (PETER PAN study) of 30 eyes of 24 patients with newly diagnosed treatment-naive PDR, 35–85 letters (6/60 or better), excluding DMO or any other cause MO, compared three arms:
-
Standard intensity PSC PRP Grey-white burn, single session, 2500 burns, 20 ms. SI-PRP.
-
A minimally traumatic or reduced fluence PSC PRP (MT-PRP) Single-session, titrated to produce grey-white burn then power reduced to produce light grey barely visible burn. 2500 burns, 20 ms. Lower power used – compared with TRP and SI-PRP.
-
SI-TRP Standard intensity PSC targeted laser to treat areas of retinal capillary non-perfusion. 1500–2500 burns, 20 ms covering area of capillary non-perfusion (Optos wide-angle directed). The aim of targeting is to avoid damage (scarring) to well-perfused areas. See earlier study from same group for details of targeted approach. 68
One aim was to avoid causing MO.
At the end of 12 weeks, the changes in VA were not significantly different (but note that they had only eight patients in each group) between the treatment groups [TRP vs. SI-PRP 1.3 letters (SD 11 letters), 95% CI 6.55 to 9.15 letters; p = 0.717; TRP vs. MT-PRP 0.7 letters (SD 8.4 letters), 95% CI 5.33 to 6.73 letters; p = 0.799; MT-PRP vs. SI-PRP 0.6 letters (SD 7.2 letters) 95% CI 4.53 to 5.73 letters; p = 0.797]. At 12 weeks there was no significant difference amongst the three treatment groups (TRP; MT-PRP; SI-PRP) in terms of PDR activity. Seventy per cent of patients in the SI-PRP group, 60% in the TRP group and 50% in the MT-PRP group had partial regression of PDR activity. Similar proportions of patients (20%) in the MT-PRP and SI-PRP groups had complete regression of their PDR activity, whereas only 10% of patients in the TRP group had complete regression. PDR worsened in about 10% of patients in the SI-PRP but in none in the other groups. In 30% in the TRP group and 20% in the MT-TRP there was no change in their retinopathy. Measures like description of pain, mean NPS and VAS were used to report the effect of lasers on pain. During 12 weeks’ follow-up, mean NPS was greater in the SI-PRP group [3.1 (SD 2.7)] than in the other two groups [1.7 (SD 2.3) in TRP; 0.5 (SD 1.3) in MT-PRP]. NPS was significantly lower in the MT-PRP group than the SI-PRP group (p = 0.001) but not against the TRP group (p = 0.05). Pain was categorised as mild in all of the groups. In the MT-PRP group, 80% of patients had no pain. Similarly, mean VAS score was significantly lower in the MT-PRP group than in the SI-PRP group [3.5 (SD 7.8) vs. 32.4 (SD 24.2); p = 0.005] but not against the TRP group [3.5 (SD 7.8) vs. 15.7 (SD 23.7); p = 0.19]. Again pain was categorised as mild in all groups.
There were no ocular complications or adverse events during the immediate or short-term follow-up after treatment. Wide-angle imaging was done using the Optos device. There were also no signs of intraretinal haemorrhage or blood vessel compromise at the locations of TRP, MT-PRP or SI-PRP burns. Both MT-PRP and SI-PRP produced less retinal thickening than SI-PRP.
In summary, the three methods PSC were not significantly different from each other in terms of change in VA and regression of PDR activity. Pain appeared to be significantly lower with MT-PRP and TRP than with SI-PRP.
Muraly 2011
This study compared the efficacy of single-session PSC against two to three sessions of conventional laser in 100 eyes of 50 patients with PDR or HR-PDR in both eyes. 78 One eye was randomised to PSC laser, whereas the other eye of the same patient received conventional laser (a 532-nm frequency-doubled Nd:YAG) in two to three sittings. The intervals between sessions were not reported.
At 1- and 6-month follow-up, more patients in the PSC laser group had regression of NVE and NVD than in the conventional laser group (90% vs. 64% 1 month; 98% vs. 88% 6 months). Fluorescein angiograms were performed. Fewer patients in the PSC group had persisting NVE and NVD at 1 month and none at 6 months (10% vs. 24% 1 month; 0% vs. 6% 6 months). At 1 month, six patients in the conventional laser group developed fresh vitreous haemorrhage but none in the PSC group did so. One patient in the PSC group and three patients in the other group had old vitreous haemorrhage at 6 months’ follow-up. The mean power of the laser was greater for pattern laser PSC than that of the conventional laser (439 mW, range 275–950 mW vs. 192.8 mW, range 125–300 mW; p-value not reported). The duration of the PSC laser was usually 30 ms, but ranged from 20 to 200 ms, whereas that of the conventional laser was 200 ms. The mean time taken to perform the laser was significantly greater for the conventional photocoagulation than for the PSC treatment (29 minutes vs. 10.4 minutes; p < 0.0001). More patients receiving PSC photocoagulation had only mild pain compared with those receiving conventional laser (mild – 80% vs. 0%). Many of those receiving the latter had severe to very severe pain, whereas no one in the PSC laser group complained of severe to very severe pain (severe – 50% vs. 0%; very severe – 28% vs. 0%). BCVA was not reported.
Muraly et al. (2011)78 concluded that photocoagulation with a single PSC session was as effective as conventional laser.
The Muraly study (2011)78 was criticised by Jojo and Mohamed (2012)86 (who thought that the PSC group had had more extensive treatment than the conventional arm), but Muraly et al. (2012)87 noted that expansion after conventional laser treatment equalised the affected area.
Nagpal 2010
Nagpal et al. (2010)82 compared the efficacy of single-spot 532-nm solid-state green laser (GLX) against a multi-spot 532-nm PSC in patients with bilaterally symmetrical PDR or severe NPDR. One eye was randomised to receive PSC photocoagulation, whereas the other eye of the same patient received GLX. Both lasers were completed in two sittings in each eye with an interval of 7 days between the two sittings. The durations were 20 ms for PSC and 200 ms for GLX, with spot size of 250 µm in both. PSC photocoagulation involved 950–1100 spots and GLX 500–700 laser spots. There was no significant difference in the post-laser VA between the GLX and the PSC group. In addition, the difference in the change in VA between the two groups was not statistically significant (p = 0.0508). The average length per sitting for PSC was 1.43 minutes and for GLX was 4.53 minutes. The difference between the two was statistically significant (p = 0.008). Regression in retinopathy was comparable between the two groups but no data were reported. The effect of GLX and PSC on retinal sensitivity was not statistically significant different between the two groups [central 15° (Zone A): 25.08 dB, range 20.56–27.26 dB with PSC vs. 23.16 dB, range 19.31–27.37 dB with GLX; p = 0.26] [15–30° (Zone B): 22.08 dB, range 8.25–23.88 dB with PSC vs. 17.14 dB, range 6.93–23.25 dB with GLX; p = 0.09]. Mean VAS score was significantly lower in the PSC than with the GLX (average 0.33, range 0–1 vs. average 4.6, range 3–9; p = 0.007).
In summary, PSC photocoagulation was quicker and caused significantly less pain than GLX while giving similar results in regression in retinopathy and change in VA.
Salman 2011
Salman et al. (2011)83 compared the safety and efficacy of PSC against conventional laser photocoagulation (Novus Spectra 532-nm green light; treatment duration of 10–3000 ms, spot size from 50 to 500 µm, power from 50 to 2500 mW) in 120 patients either with NPDR and CSMO or, PDR.
There were four groups of 30 patients (A1, A2, B1 and B2) in the study. 83 Groups A1 and A2 included patients with NPDR and CSMO, whereas those in groups B1 and B2 had PDR. Patients in Group A1 and A2 underwent focal or modified grid macular laser photocoagulation for NPDR (as stated by the authors but presumably it was for the MO). Group A1 patients received conventional laser, whereas patients in Group A2 received PSC laser photocoagulation. Patients in group B1 and B2 underwent PRP, the former with conventional diode-pumped sold-state (DPSS) laser and the latter with the PSC laser.
In Group A1, the mean power of the conventional laser used was 100 mW (SD 20.5 mW) and an average of 85 (SD 76.6) burns were performed. The mean power of the conventional laser was 215 mW (SD 51.3 mW), with an average of 700 (SD 201.1) burns performed in Group 2. Patients in Group A2 received PSC photocoagulation, the mean power of which was 332 mW (SD 105.5 mW) and an average of 145 (SD 92.2) burns were performed. Those in Group B2 also received PSC photocoagulation, mean of 1090 (SD 410.4) burns with a mean power of 410 mW (SD 115.2 mW). The power of the laser used was significantly higher with the PSC than the conventional laser (p < 0.001) because the former is given for a relatively shorter duration.
Follow-up at 12 months included BCVA, photography and FA.
Visual acuity did not change significantly following laser in any groups. More patients receiving PSC achieved success, i.e. regression of neovascularisation and no further treatment planned, than those patients receiving the conventional laser (28/30 vs. 20/30; p < 0.05).
In Group B2 (PSC), 46% (14) had a single session and 54% (16) had two sessions. None of the patients in the single-session PRP group developed any complications and all had regression of their retinopathy. Also none of the patients needed further treatment plan at his/her last follow-up visit. There were also no reports of complications related to laser treatment. When asked to rate the pain following laser on a scale between 0 and 5, with ‘5’ being very severe pain, patients rated PSC as 0.61 and standard laser as 2.72.
The authors concluded that PSC was safe, rapid and effective but required higher power because of shorter exposure time.
Shimura 2003
In this study, the efficacy of pan-retinal scatter photocoagulation used either as weekly or biweekly treatment was compared in 36 patients with severe NPDR or non-HR-PDR but good vision (baseline VA of 20/20 or better) before laser therapy. 80 A krypton red laser was used. One eye of the patient was treated weekly, whereas the other eye of the same patient was treated biweekly. All had four sessions. In each session, around 500 spots were performed, with each spot of 200–500 µm in diameter and the duration of the exposure ranging from 0.15 to 0.2 seconds. The order of the treatments was nasal followed by inferior, superior and, finally, temporal. Slightly more patients in the biweekly PRP group maintained their VA at 16 weeks than the eyes treated with the weekly PRP (92% vs. 89%). At 16 weeks, 15 eyes in the weekly group and 13 eyes in the biweekly group received additional focal laser treatment for retinal neovascularisation.
Suto 2008
Suto et al. (2008)84 compared PRP before cataract surgery (PRP-first group) and after cataract surgery (surgery first group) in patients with bilateral cataract and severe NPDR or early PDR. 84 One eye had PRP first, and the other eye of the same patient had PRP after cataract surgery. In the PRP-first group, cataract surgery was done 1–3 months after the final PRP session. In the surgery-first group, cataract surgery was performed within 4 days. The laser treatment involved making spot size of 200 µm, 0.12–0.16 W, and 0.2–0.4 seconds in the PRP-first group and 200 µm, 0.08–0.12 W, and 0.2–0.4 seconds in the surgery-first group. At 12 months’ follow-up, significantly more eyes in the surgery-first group had a BCVA of 20/40 or better than in the PRP-first group (96.6% vs. 69%; p = 0.012). There was no significant difference in the rate of progression of DR (27.6% in PRP-first vs. 41.4% in surgery-first group; p = 0.270). Worsening of MO (assessed by FA, not by OCT) was twice as likely in the PRP-first group as in the surgery-first group (RR = 2.0; 95% CI 1.49 to 2.51). In summary, the authors found that patients in the surgery-first group had better VA than those in the PRP-first group. There was no difference in the rate of progression of DR in the two groups, but progression of MO was commoner in the PRP-first group. This was presumably related to the higher power required.
Tewari 2000
Tewari et al. (2000)55 compared diode (810 nm) and argon laser (514 nm) PRP in 25 patients with bilateral PDR. One eye received diode laser (810 nm) treatment and the other eye received argon laser (514 nm) treatment, each done in two to four sittings. 55 Around 200–500-µm spots were applied, with exposure ranging from 0.1 to 0.2 seconds with the argon laser, and 0.2–0.3 seconds with the diode laser. The power of the laser varied between 0.2 and 0.4 W to give ‘moderate intensity’ burns. At 6 months’ follow-up, VA (as measured by reciprocals of Snellen) in the argon group reduced from 4.16 (SD 2.77) to 4.76 (SD 2.83), and from 3.22 (SD 2.05) to 3.62 (SD 2.12) in the diode laser group. The difference in change in VA between the two groups was not significant. There was no statistical difference between the two groups in regression of neovascularisation (72% vs. 64%; p = not reported; only states not statistically significant). There was reduction in contrast sensitivity (Cambridge low-contrast gratings) in both groups (diode, from 169.2 ± 59.3 to 142.2 ± 49.2; argon, from 164.6 ± 56.2 to 142.2 ± 49.2). Vitreous haemorrhage developed in two eyes (8%) in each group. Slightly more eyes in the diode laser group required laser augmentation than those in the argon laser group (48% vs. 32%). Significantly more patients receiving diode laser complained of pain than those receiving argon laser (92% vs. 28%; p < 0.001). In summary, there is no difference in efficacy between argon and diode laser. Pain appeared to be significantly lower with the argon laser. The authors of the study55 concluded that diode laser is an appropriate alternative to the argon laser to perform scatter laser in DR, though they recommend a study with longer follow-up than their 6 months.
Summary
All of the studies included above are RCTs, most of them with low risk of bias. Findings include:
-
Some had only small numbers of patients, and follow-up was often short.
-
The majority of the patients included in these studies had PDR, with a few with very severe NPDR.
-
There were five trials of PSC photocoagulation against conventional PRP. One study compared the efficacy of PSC laser given in three different ways – TRP, MT-PRP or SI-PRP. 77 The efficacy of PSC photocoagulation was also compared if given as multi-spot 20-ms single session against single-spot 100 multi-session in one study. 75,78
-
One study compared threshold diode and argon laser. 55 No study compared PSC with sub-threshold diode laser. One study compared light (grade 1 of L’Esperance scale) with standard PRP (grade 3). 73 Other studies examined different ways of giving standard PRP.
-
In most studies, patients were followed for at least 6 months. We included four trials with follow-up period of less than 6 months, mainly to look at adverse events.
-
It appeared that most of the studies were funded by academic institutions. One trial was funded by a PSC laser manufacturer.
-
VA – At the end of follow-up, in all studies there was no significant difference amongst laser types in terms of change in VA.
-
Retinopathy – Scatter PRP was found to cause resolution of neovascularisation on and around the optic disc (NVD) and new vessels elsewhere (NVE). Trials of modern methods of PRP, light, PSC and diode either showed no difference in improvement in retinopathy (three trials) or reported better results with PSC (two trials).
The Japanese approach of selective PRP aimed at ischaemic areas only, in PPDR delayed progression to PDR, with only 15% of the selective PRP group developing PDR compared with 52% of those receiving no photocoagulation (p = 0.03).
There are also no differences between PRP performed before or after cataract surgery in progression of DR. However, patients in the surgery-first group had better VA than those in the PRP-first group. Progression of MO was significantly higher in the PRP-first group.
Adverse events
Pain was more common with conventional photocoagulation than with PSC. Pain was significantly less in patients receiving short exposure laser, less in those receiving light PRP, and in those receiving PSC compared with GLX. In a study comparing different types of PSC photocoagulation, pain was higher in SI-PRP followed by TRP and MT-PRP (Muqit 201377). However, pain was mild in nature. Pain was more common with diode laser than argon laser.
Discussion of randomised controlled trial evidence
The general conclusion from the trials reviewed above is that modern methods of laser photocoagulation are as effective as conventional lasers, but it is not possible to say that they are more effective. Multi-spot photocoagulation has advantages, such as reduced pain and faster treatment, making treatment less onerous for patients. Pain relates mainly to the power and duration of the laser used and as modern laser technologies use shorter-duration laser pulses and are probably given with less intensity (not looking for a white laser mark but more for grey mark) then they are less painful. Less intense and confluent PRP may be slightly less effective but has fewer side effects. Shorter pulses are as effective but cause less pain.
The published evidence base is somewhat limited because of the small size and short duration of some studies.
Bandello et al. (1993)88 suggest that the advantages of the diode laser include compact size, no need for a cooling system, low price, high transmission of laser through cataract and vitreous haemorrhages.
Diode laser treatment was reviewed by Neubauer and Ulbig (2007),13 who report that its advantages include small size and lower cost. They report no difference in progression of PDR, but more pain.
In another narrative review, mainly of use in DMO, Sivaprasad and Dorin (2012)15 argue that sub-threshold diode laser micropulse photocoagulation (SDM) is as effective as, but less destructive than, standard focal/grid photocoagulation. Standard is taken to be the mETDRS approach, which is aimed only at areas of thickened retina and non-perfusion, and leaking microaneurysms, using burns with smaller areas and less-intense heat (grey). In micropulse lasering, the laser is used in a series of short pulses, with the theory being that this allows the retinal tissue to recover a bit between pulses, thereby causing less thermal damage and subsequent scarring.
The authors found little evidence for the use of SDM for PRP, but the main one being a retrospective case series (see Luttrull et al. 200866 reviewed later). In DMO, Sivaprasad and Dorin (2012)15 report five RCTs of:
-
SDM versus conventional continuous-wave 514-nm argon laser threshold photocoagulation (two trials)89,90
-
three trials of SDM versus mETDRS: two with 514-nm argon laser, the other unspecified.
These trials and the observational studies are reported as confirming the advantages of SDM, but more when the high-density version of SDM is used, rather than the normal density one. In the trial by Lavinsky et al. (2011),91 twice as many eyes gained 15 or more letters with HD-SDM than with mETDRS.
Sivaprasad and Dorin (2012)15 ponder on why there has been little uptake of SDM, and suggest the following reasons:
-
SDM has been undergoing a slow evolution.
-
Micropulse lasering has been being refined.
-
The arrival of the anti-VEGF drugs has taken attention away from laser treatments.
-
Lasers with micropulse emission were not available.
-
The optimum dosing remains to be determined.
-
The laser used is the 810-nm infrared diode, which has not been as popular as the 514-/532-nm green lasers.
Subthreshold diode micropulse laser photocoagulation is said by Muqit et al. (2013)77 to produce clinically undetectable laser lesions requiring a high number of laser treatments. The source cited is Luttrull (2008),66 already mentioned.
Data on adverse effects from observational studies
It is important to review the evidence on the adverse effects of laser photocoagulation for three reasons. The first is the usual one, in that we need to balance the benefits of photocoagulation against the harms, especially when considering administering PRP at earlier stages of retinopathy. The second is that the types of laser treatment have been changing, with a trend towards lighter laser treatment, and the harms seen in the landmark trials such as ETDRS, may not be a good guide to the harms seen in modern laser therapy. Lighter may mean many things, such as a less-white burn but also a laser applied in a different manner than before (for instance, with shorter duration pulses compared with longer ones).
Third, modern imaging methods such as OCT make it easier to detect MO, allowing that to be treated first, so preventing worsening of MO after PRP.
Although it could be argued that the most useful data on harms might come from RCTs, a counter argument may be that RCTs may not reflect routine care. A systematic review in 2001 found evidence that being in a trial improves care (a ‘trial effect’),92 though a later Cochrane review in 2008 did not confirm this finding. 93
We decided to review non-RCT evidence on adverse effects. Although, in what follows, we are primarily concerned with adverse effects, we also provide some data on efficacy. This is partly because adverse effects might be reduced by much lighter laser treatment, but at the cost of reduced effectiveness. Lack of efficacy could be considered as an adverse event. However, for efficacy we rely mainly on the RCT evidence presented above.
Searches for evidence of adverse effects of lasers from non-randomised controlled trial studies
Searches additional to those for RCTs were done to find any non-RCT studies concerning laser photocoagulation in DR at any stage. Searches of MEDLINE and EMBASE (as shown in Appendix 2, Search strategies, section f) were run and downloaded into EndNote version 7 (Thomson Reuters, CA, USA).
In addition, the EndNote database created from previous searches for PRP and lasers (see Appendix 2, Search strategies, sections a–e) was searched using the following keywords: (adverse or risk* or harm* or side effect* or safety or pain or visual loss or complication*) and (laser or photocoagulation or panretinal or pan-retinal or scatter or PRP).
A systematic review, by Fong et al. (2007),94 which covered the earlier literature on safety of lasers was found. Given that and our focus on the safety of currently used lasers, we limited our selection of articles to those published from the year 2000 onwards.
The searches described above resulted in 137 unique references, of which 84 were excluded on the basis of title and abstract or publication date. The full papers for the remaining 53 references were obtained and checked for inclusion by two authors (PR/NW). Articles that were letters to the editor or comments or editorials were excluded. Also, articles that were RCTs and had been already reviewed in this chapter were excluded.
As we were interested in studies with outcomes that were reported by patients, we excluded outcomes such as change in retinal thickness, as it is been shown that it is not well correlated with visual loss. (The DRCRN group noted only modest correlation between VA and retinal thickness after focal laser photocoagulation for DMO. 95)
This left 19 studies49,60,62,65,66,68,72,96–107 remaining, of which six68,96–100 were excluded for reasons shown in Table 21. Of the remaining 13 studies, 1049,60,62,65,66,72,101–104 were data extracted and included in Table 22. Three studies were dealt with narratively. 105–107
| Study ID | Reason for exclusion |
|---|---|
| Du 201196 | No patient-reported outcomes – reports very sensitive measures of retinal function that might not be detected by patients |
| Maeshima 200497 | No patient-reported outcomes |
| Muqit 201368 | No patient-reported outcomes |
| Raman 201098 | Patients did not have DR |
| Shimura 200999 | No patient-reported outcomes |
| Wang 2014100 | No patient-reported outcomes – reports a method of measuring VFs |
| Study | Participants and baseline values | Description of intervention and laser | Ocular safety outcomes |
|---|---|---|---|
| Study ID: Chappelow 201260 Aim: To evaluate the efficacy of PRP performed via the PSC vs. argon laser PRP Design: Retrospective comparative case series Follow-up: PSC = 313 days; argon = 410 days |
n: 82 eyes of 82 patients Description of DR: Newly diagnosed HR-PDR Age (mean), years: ≈56 Diabetes type: Argon green laser: 33% NIDDM, 67% IDDM; PSC: 20% NIDDM, 80% IDDM |
Group 2: PSC = 41 |
|
| Study ID: DRCRN 200962 Aim: To compare the effects of single-sitting vs. four-sitting PRP with argon laser on MO in subjects with severe non-proliferative or early proliferative DR with relatively good VA and no or mild centre-involved MO Design: Non-randomised, prospective, multicentred clinical trial Follow-up: 34 weeks Proportions completing the study: At 34 weeks visit completion was 88% in the one-sitting group and 82%, in the four-sitting group |
n: 155 eyes of 155 people Description of DR: 144 of the 155 subjects had fundus photographs that could be graded by the Reading Center; 73 eyes (51%) were classified as having non-proliferative retinopathy, 50 eyes (35%) had non-high-risk retinopathy PDR and 21 eyes (15%) had HR-PDR (but not considered to have HR-PDR by the enrolling ophthalmologist). MO considered to be present on baseline photographs in 27 eyes (18%) Age (median), years: 55 Sex: 49% female Diabetes type: 22% type 1, 78% type 2 HbA1c (median): Group 1 = 7.7%; Group 2 = 8.2% Baseline VA: 20/25 (mean letter score 83) |
One-sitting regimen: 1200–1600 burns Four-sitting regimen: Spread over 12 weeks, with each sitting separated by 4 weeks (±4 days), ≈300 burns in each of the first two sittings and investigator judgement for number of burns for the third and fourth sittings, as long as the total for the four sittings was between 1200 and 1600 burns. The burn characteristics were argon laser using 200-µm spot size; exposure 0.1 second recommended, 0.05–0.20 second allowed; intensity, standard mild white retinal burns; distribution, edges at least one burn width apart, no closer than one row within the arcades, no closer than two DDs temporal to the fovea; extent, arcades ≈3000 µm from the macular centre to at least the equator; wavelength, green or yellow (red could be used if VH was present precluding use of green or yellow) Group 1: One-sitting PRP (n = 84) Group 2: Four sittings of PRP (n = 71) |
VA 3 day visit: VA was slightly worse in the one-sitting than in the four-sitting group; median change from baseline in letter score of –3 and –1, respectively (p = 0.005) Weeks 4 and 17: Median VA changes from baseline in letter score of –1 in each group at both visits (p = 0.37 and 0.66, respectively) Week 34, VA was slightly worse in the four-sitting group, with a median change from baseline in letter score of 0 vs. –2 (p = 0.006) 7% in the one-sitting group and 9% in the four-sitting group had VA of 10 or more letters worse than baseline (p = 0.75) VH: A VH reducing acuity by 10 or more letters from baseline occurred in two eyes in each group between the 17- and 34-week visits MO was commoner in the four-sitting group Conclusion: Results suggest that clinically meaningful differences in VA are unlikely following application of PRP in one sitting compared with four sittings in subjects in this cohort |
| Study ID: Kaiser 2000101 Aim: To describe the clinical features and complications of DR 1 year after PRP for PDR in a tertiary care centre between 1985 and 1995 Design: Retrospective cohort study – data collection from medical records Follow-up: 1 year (treated between 1985 and 1995) |
n: 297 eyes of 186 patients Description of DR: PDR not previously treated with PRP Age (median), years: 55 Sex: 43% male Diabetes type: 25% using insulin, 75% NIDDM |
In general, eyes were treated according to guidelines summarised in the ETDRS. Patients diagnosed with CSMO at the time of initial PRP were either first treated with the appropriate focal or grid macular laser therapy, after which PRP was initiated, or PRP and focal treatment were instituted simultaneously. PRP was completed with an argon laser placing 500-mm spots. The average number of burns placed during the PRP sessions was 1806 spots per treated eye | VH was present in 44% of eyes at baseline. New VH developed in 37% of eyes during the first year after PRP Vitrectomy was performed in 10% of eyes from baseline through the 1-year follow-up visit Traction retinal detachment was present in 4% of eyes at baseline and newly developed in 6% of eyes during follow-up Repeat PRP treatment was performed in 107 (39%) of eyes after initial treatment. Most of these eyes (75 eyes) had just one additional treatment within the first year after initiation of the first PRP treatment Conclusion: The data from this study are applicable to many patients treated in a tertiary treatment centre and hence useful for counselling patients on the possible complications of PRP, e.g. to prepare them for the possibility of needing at least one additional laser treatment during the first year after completion of their initial PRP |
| Study ID: Kovacic 2012102 Aim: To compare two PRP techniques for effectiveness of treatment of PDR and to determine which has fewer complications of MO and VA Design: Comparative case series – patients were divided into two groups according to the types of laser treatment Follow-up: 6 months |
n: 180 eyes Description of DR: Type 1 diabetic patients with PDR and incipient papillary neovascularisation (papillary neovasculates or within one papillary diameter from papillae), which is a high-risk indicator for serious sight loss Age (mean years): Group 1 = 41.6; Group 2 = 37.7 Sex: 50.8% male Diabetes type: All type 1 HbA1c: NR |
Blue–green argon laser. Pan-retinal (mild scatter) photocoagulation was performed in two different ways: Group 1: n = 87 eyes. CPRP was performed with ‘mild-scatter’ technique number 650 spots with 500 µm, exposition 0.5 second, two optic DD from macula to pre-equatorial (15° to 80°) Group 2: n = 93 eyes. PPRP technique, 650 spots, 500 µm in diameter, exposition 0.5 second, five optic DD from the centre of the macula (40°–105°) |
MO Before therapy MO in 48.4% eyes with CPRP and 52.9% eyes PPRP (p = 0.54) After 1 week The CPRP group has 1.6 times more MO than in the PPRP group (p = 0.010) After 3 and 6 months No statistically significant difference between treatment with CPRP and PPRP (p = 0.389 and p = 0.166). MO at 6 months = 18.3% in CPRP and 10.3% in PPRP VA Before therapy Mean VA (SD) in CPRP group = 0.75 ± 0.22, PPRP group = 0.72 ± 0.22, (p = 0.025 for difference) After 1 week Mean VA in CPRP group = 0.60 ± 0.29, PPRP group = 0.67 ± 0.21 (p = 0.081) After 3 months Mean VA for CPRP group = 0.69 ± 0,27, PPRP group = 0.73 ± 0.22 (p = 0.749) After 6 months Mean VA CPRP group = 0.7 ± 0.26, PPRP group = 0.71 ± 0.26 (p = 0.71) Conclusion: VA deteriorated 1 week after treatment with CPRP and PPRP as a result of pre-existing or worsening MO. After 3–6 months of beginning of treatment, MO and VA were not changed significantly. VA was slightly better in patients treated with PPRP |
| Study ID: Lee 201065 Aim: To investigate changes in macular thickness after PRP in patients with severe DR without MO and to compare the VA outcomes Design: Prospective cohort study Follow-up: 12 months |
n: 60 eyes in 30 patients Description of DR: Severe non-proliferative or early proliferative DR. VA in each eye was ≥ 0.8 with no MO as determined by clinical examination and OCT Age (mean), years: 60 HbA1c (mean): 7.6 mg/dl |
An argon green laser was used, with a retinal spot size of 500 m and an intensity of 200–500 mW until a grey burn spot was evident. The duration of the application was 0.1 second and the total number of spots was 1200–1600 During the 12-month follow-up period, additional PRP was required in 6/60 eyes (10%) because of residual retinal neovascularisation. PRP treatment was designed to be completed in four sessions |
VA: The mean ± SD of the VA measurements, converted to logarithm of the minimal angle of resolution, was 0.02 ± 0.11 before PRP and 0.04 ± 0.11, 0.04 ± 0.07, 0.02 ± 0.07, and 0.04 ± 0.08 at 1, 3, 6 and 12 months after PRP, respectively. None of the follow-up measurements differed significantly from the baseline VA before PRP (> 0.05 for each) Conclusion: Biweekly PRP (1200–1600 spots) had no effect on VA in patients with severe DR without MO, determined by clinical and OCT examination, although mild macular thickening frequently persists. PRP was performed safely and without visual loss for patients in whom MO was not detected by OCT examination before PRP |
| Study ID: Luttrull 200866 Aim: To report the VA and clinical outcomes of a pilot study of SDM PRP for treatment of DR Design: Retrospective chart review of all patients undergoing PRP for DR between April 2000 and February 2003 Follow-up: Median of 1 year |
n: 99 eyes of 63 patients Description of DR: Severe non-proliferative retinopathy or any degree of PDR Age (mean), years: 60.5 Sex: 62% male Diabetes type: 41% type 1, 59% type 2 HbA1c: NR |
SDM PRP: An 810-nm diode laser photo-coagulator was used in its MicroPulse operating mode. All patients were treated with a 500-µm aerial spot size, 0.20-second pulse envelope duration, with an initial 2.0 W power setting. Within the laser pulse envelope, a 15% duty cycle, each delivering a train of 100 sequential laser pulses of 300 ms ‘on’ time separated by 1700 ms ‘off’ time, was employed for each patient. Treatment sessions per eye ranged from one to six (with a median of two sessions per eye). All SDM PRP was performed by a single surgeon | VH: Probability of VH at 1 year following initial SDM PRP for PDR was 12.5% (Kaplan–Meier survival analysis) Vitrectomy: Probability of undergoing vitrectomy following SDM PRP for PDR was 14.6% at 12 months (Kaplan–Meier survival analysis) Complications of SDM PRP: No complications were observed i.e. no visible laser lesions, laser-induced chorioretinal scarring, no patient complained of postoperative pain or loss of VA, accommodation, night vision, or VF; postoperative inflammation was not observed in any eye; no eye demonstrated worsening of MO following SDM PRP, and no eye without MO developed MO postoperatively Conclusion: SDM PRP minimised retinal damage and treatment complications in the management of high-risk non-proliferative and PDR. Visual loss was prevented with a low rate of VH and vitrectomy postoperatively |
| Study ID: Shimura 200549 Aim: To compare macular thickness and BCVA before and after PRP in patients with severe DR and good VA to identify factors that predict postoperative visual function Design: Prospective, non-comparative, interventional case series Follow-up: 24 weeks |
n: 64 eyes Description of DR: Severe non-proliferative or early PDR. VA of each eye was 20/20 or better with no CSMO. The absence of CSMO was diagnosed by at least three independent clinicians Age (mean), years: 58.8 Sex: 36 male; 28 female Diabetes type: All patients HbA1c: 8.4% (mean) |
PRP performed four times at 2-week intervals. Size of the spots on the retina was 200–500 µm, duration of the application was 0.15–0.2 seconds, using the Volk Super Quad 160 fundus laser lens and a krypton red laser mounted on a slit lamp No. of spots in each session ≈500; total no. of burns after the four sessions was ≈2000 |
VA Patients divided into three groups based on changes in VA over 24-week observation period
Conclusion: For eyes with severe DR and good VA, PRP did not affect postoperative VA in more than 80% of patients at 24 weeks |
| Study ID: Tinley 2009103 Aim: To evaluate the outcomes of routine, single-session, indirect PRP for PDR, to examine adverse events related to indirect laser within the first 8 weeks of treatment, and to compare results with the UK NDRLTA Design: Retrospective study – review of case notes of patients treated from 2000 and 2006 Follow-up: 8 weeks (and 9 months’ final follow-up) |
n: 107 eyes of 107 patients Description of DR: Patients with PDR undergoing indirect PRP in theatre. For patients undergoing bilateral simultaneous treatment, the eye with the worse VA was included. Patients who had received prior PRP treatment were excluded Age (mean), years: 54.5 Sex: 29% female Diabetes type: 35.5% type 1, 64.5% type 2 |
Single-session indirect PRP. Indirect laser was performed using the Lumenis Novus 2000 machine and a 20- or 30-D lens. Typical laser settings were 200–300 mW power on continuous mode, with burn duration adjusted to produce moderately intense burns, spaced approximately one burn width apart, and extending to the far retinal periphery. No. of burns not given | 15 patients (14.0%) returned with adverse events within the first 8 weeks of indirect PRP 10 events were mild or transient, resolving within a further 2 months but five were persistent and visually significant, with three requiring vitrectomy during the study period Conclusion: The incidence of significant PRP-induced adverse events was low after indirect PRP and the outcomes were not inferior to the outpatient-based UK NDRLTA. Therefore, for patients with PDR, routine, single-session, indirect PRP in an operating theatre setting is an acceptable and well-tolerated alternative to slit lamp-based treatment. (However it is presumably higher cost.) |
| Study ID: Velez-Montoya 2010104 Aim: To analyse the safety profile of the PSC photocoagulator laser Design: Retrospective case series (review of the clinical records of all laser sessions that were performed using the PSC system from November 2007 to July 2008) Follow-up: 8 months |
n: 1301 cases Description of DR: 80% of treatments in patients with severe non-proliferative or PDR, most with some type of media opacities (cataract or VH grade I or 2) Exclusions: All incomplete records and those involving MPC treatments |
All sessions were conducted by two surgeons with a PSC photo-coagulator, a 532-nm double-frequency Nd:YAG laser. For PRP, the 2 × 2, 3 × 3, 4 × 4 and 5 × 5 arrays were most commonly used, but changes were made during treatment between these different patterns according to experience and patient characteristics All burns were applied one burn width apart |
Retinal bleeding in 17 cases (1.3%) All cases had a clinical diagnosis of PDR. 12 patients had a previous history of photocoagulation. The mean follow-up time was 42 days, during which complete resolution of the bleeding was observed without further consequences and without need for additional treatment CD in two cases (0.15%) Both cases had a clinical diagnosis of PDR with poor metabolic control The detachments occurred 5 and 7 days after laser application. The follow-up times were 56 and 44 days, after which the cases resolved satisfactorily after treatment Exudative retinal detachment in one case (0.07%) Patient had poor metabolic control and a history of multiple previous laser treatments. The follow-up time was 45 days, during which the patient received periocular steroids and topical NSAIDs. The retinal detachment resolved without further complications and the final BCVA was 20/40 Conclusion: The PSC photocoagulation system has a low incidence of complications |
| Study ID: Zucchiatti 200972 (meeting abstract) Aim: To evaluate the safety and efficacy of the PSC photocoagulator system in the treatment of PDR Design: A retrospective review of cases between December 2006 and May 2008 Follow-up: 11.5 months average (6–19 months range) |
n: 26 eyes of 21 patients Description of DR: Naive severe NPDR or PDR; 7 eyes (27%) had severe NPDR, 16 eyes (62%) had early PDR, 3 eyes (11%) had HR-PDR |
Full PRP was performed using the PSC system. On average, 3.9 sessions were required for a complete PRP. Parameters: mean power was 822 mW, exposure time of 20 ms, spot size was 200–400 µm. In 21 eyes the 5 × 5 pattern was used (81%), and the 3 × 3 pattern was applied in five eyes (19%). An average of 880 spots were delivered in each session | No major side effects were registered in most eyes. One eye only developed a choroidal detachment after receiving 2600 spots at higher power a single session, which resolved spontaneously in 1 week Conclusion: Retinal photocoagulation using PSC system has comparable efficacy to published results with conventional photocoagulation |
Description of non-randomised controlled evidence for safety of lasers
Three studies were concerned with multi-spot pattern photocoagulation,60,72,104 which delivers a group of burns in a pattern, with one application (by depressing the foot control), rather than the standard one burn at a time. So patients are less likely to become fatigued and uncomfortable. However, the number of spots that can be delivered at the same time is limited by pain, increasing if more than four spots at a time are used. All used the PASCAL laser from Topcon.
Chappelow (2012)60 compared the efficacy of the PASCAL laser PSC with standard argon laser PRP in 82 eyes (41 eyes in each group) of patients newly diagnosed with HR-PDR in a retrospective case series, with mean follow-up times of 313 and 410 days, respectively. There was a higher incidence of vitreous haemorrhage and pars plana vitrectomy (PPV) in the PSC group than in the argon group (37% vs. 24% and 24% vs. 12%, respectively) but both these differences were not statistically significant. Also, 10% of the PSC group had neovascularisation of the iris (NVI) and 5% neovascular glaucoma (NVG), whereas none in the argon group was reported. However, the study60 was not adequately powered to detect a significant difference in these outcomes – it would have needed five times as many eyes for that. The incidence of vitreous haemorrhage was much higher than in other studies. This may reflect reduced efficacy of PSC but incidence was also high in the argon group. The authors also noted that the comparison was between a standard argon laser with which they were very experienced, and a PSC system that was newly introduced.
Velez-Montoya (2010)104 analysed the safety profile of PSC laser in a retrospective review of 1301 cases with a follow-up of 8 months. Approximately 80% of patients had severe NPDR or PDR, most with some type of media opacities. The most common complication reported was retinal bleeding in 17 cases (1.3%), followed by choroidal detachment in two cases (0.15%) and exudative retinal detachment in one case. They concluded that the PSC photocoagulation system has a low incidence of complications.
Zucchiatti (2009)72 (meeting abstract only) in a retrospective cases series of 26 eyes of 21 patients evaluated the safety and efficacy of the PSC photocoagulator system in the treatment of PDR in patients with severe NPDR or PDR. Seven eyes (27%) had severe NPDR, 16 eyes (62%) had early PDR, three eyes (11%) had HR-PDR in an average follow-up of 11.5 months. They found no major side effects.
The latter two studies72,104 find that PSC has few adverse effects.
The variation in adverse effects amongst the different studies may be due to differences in study designs, differing severity of PDR and differing methods of photocoagulation.
As noted earlier, the most common problem after PRP is MO.
The two following studies,49,65 along with the DRCRN study62 in the next section, looked at changes in VA in patients with severe NPDR or early PDR without MO.
Lee et al. (2010)65 conducted a prospective cohort study to investigate VA and changes in macular thickness after biweekly PRP with argon green laser, designed to be completed in four sessions. The cohort included 60 eyes in 30 patients with severe NPDR or early PDR without MO, as determined by clinical examination and OCT. None of the VA measurements at 1-, 3-, 6- and 12-month follow-up differed significantly from baseline. Therefore, for patients in whom MO was not detected by an OCT examination before PRP, PRP was performed safely and without visual loss.
A prospective case series was conducted by Shimura et al. (2005)49 in 64 eyes of 64 consecutive patients with severe non-proliferative or early PDR and good VA, and with no CSMO detected by ophthalmoscopy. PRP was performed four times at 2-week intervals using a krypton red laser. The patients were divided into three groups based on changes in VA after the 24 weeks’ observation period. In Group A, 54 eyes (84%) had VA that remained at the preoperative level; in Group B (three eyes), VA decreased < 2 lines, but returned to preoperative VA, and in the remaining seven eyes in Group C the VA decreased slightly (< 2 lines) at 2 weeks but then continued to decrease to < 20/60 at the 24 weeks’ examination. As no vitreous haemorrhage and cataract progression were observed in Groups B and C, it was assumed that the cause of the visual loss was probably from MO. As MO was assessed by ophthalmoscopy, it is possible that some may have been missed.
One-sitting photocoagulation versus four sittings pan-retinal photocoagulation
A prospective, non-randomised observational study sponsored by the DRCRN compared a single-sitting of PRP (n = 84 eyes) versus PRP delivered over four sittings (n = 71) on the development of MO. 62 The study62 was not randomised because many members of the Network did not consider delivering PRP in one session to be safe, because of the risk of MO. So some operators gave all PRP in a single session, and the others in four sessions. The 155 eyes (of 155 patients) had severe NPDR or early PDR with relatively good VA and no or mild centre-involved MO. The completion rate at the 34 weeks’ follow-up was 88% in the one-sitting group and 82% in the four-sitting group. A green or yellow argon laser was used, unless vitreous haemorrhage was present – when red could be used.
At the three-day visit VA was slightly worse in the one-sitting group than the four-sitting group, but, by week 34, VA was slightly worse in the four-sitting group, with a median change from baseline in letter score of 0 versus –2, respectively (p = 0.006). A vitreous haemorrhage reducing acuity by 10 or more letters from baseline occurred in two eyes in each group between the 17- and 34-week visits. The results of this study62 indicated no clinically meaningful differences in VA between the application of PRP in one sitting compared with four sittings. This would be more convenient for patients and reduce costs, though some might prefer several shorter sessions to one long one. However, it would need a large randomised trial to confirm the results.
Real-world clinical setting
A retrospective cohort study by Kaiser et al. (2000)101 looked at complications after 1 year in eyes treated with PRP for PDR. The study101 was conducted in a tertiary care centre between 1985 and 1995, and included 297 eyes of 186 patients who had not been previously treated with PRP. Eyes were treated generally according to guidelines summarised in the ETDRS, and patients diagnosed with CSMO at the time of initial PRP were either first treated with the focal or grid macular laser therapy before PRP, or were simultaneously treated with PRP and focal laser.
During the first year after PRP, new vitreous haemorrhage developed in 37% of eyes, vitrectomy was performed in 10% of eyes, and traction retinal detachment was newly developed in 6% of eyes, and repeat PRP treatment was performed in 39% of eyes.
Such data, set in a large tertiary treatment centre, could be useful for preparing patients on likelihood of possible complications of PRP. However, the high vitreous haemorrhage and repeat treatment rates suggest a lack of efficacy. Although meeting our cut-off of publication after 2000, it does reflect practice as far back as 1985, so may be less useful as a guide to current methods.
Comparing two different techniques of delivering pan-retinal photocoagulation
Kovacic et al. (2012)102 reported the results of a case series comparing two different PRP techniques in type 1 diabetic patients with PDR and incipient papillary neovascularisation. One group (n = 87 eyes) underwent central classical pan-retinal photocoagulation (CPRP) and the other group (n = 93 eyes) received PPRP. They were followed up for 6 months.
Before therapy, 48.4% eyes in CPRP group and 52.9% eyes in PPRP group had MO (p = 0.54 for difference). After 6 months there was no statistically significant difference between the groups (p = 0.166) for the percentage with MO after treatment, i.e. CPRP (18.3%) and PPRP (10.3%).
Visual acuity deteriorated 1 week after treatment with CPRP and PPRP owing to pre-existing or worsening MO, but after 6 months there was no statistically significant difference between groups (p = 0.71) in the mean VA.
Subthreshold diode micropulse pan-retinal photocoagulation for treatment of diabetic retinopathy
Luttrull et al. (2008)66 studied the VA and clinical outcomes in a pilot study of SDM PRP for severe NPDR or any degree of PDR. The study66 was a retrospective chart review of 99 eyes of 63 patients with severe NPDR or any degree of PDR diabetic undergoing SDM PRP between April 2000 and February 2003. Of the 99 eyes, 45 had severe NPDR or low-risk PDR. For severe NPDR, success was taken to be absence of progression to PDR. About 60% of patients had type 2 diabetes. Patients were offered SDM PRP on the grounds that it was less likely to cause retinal damage or scarring, but might need more treatments than conventional PRP.
The median follow-up period was 1 year. The number of treatment sessions per eye ranged from one to six (with a median of two sessions per eye). All were performed by a single surgeon.
The term ‘burn’ is not used, but an average of 1696 ‘laser applications’ were delivered at the first session, and a mean total of 3003. Luttrull et al. (2008)66 mention an absence of observable laser lesions and scarring at time of treatment or during follow-up. The implication is that no ‘burns’ were created, and that no retinal scarring was caused. Vitreous haemorrhage developed in 17 eyes (21%). Pain during the procedure was not reported, but the authors comment that SDM PRP was ‘well tolerated’. The authors comment that the value of the study66 is limited by small numbers, short follow-up, lack of controls, and lack of detailed tests of retinal function. They recommend a RCT.
The probability of vitreous haemorrhage at 1 year following initial SDM PRP for PDR was 12.5% (Kaplan–Meier survival analysis) and the probability of undergoing vitrectomy was 14.6% at 12 months. No complications were observed. The authors concluded that visual loss was prevented with a low rate of vitreous haemorrhage and vitrectomy postoperatively.
Single-session indirect pan-retinal photocoagulation
Tinley and Gray (2009)103 evaluated the outcomes of routine, single session, indirect PRP for PDR. They explain that although indirect laser treatment is usually limited to patients with special needs, who may not tolerate slit-lamp treatment, in their centre they use it routinely for PDR, on the grounds that it provides easier access to the peripheral retina and is more comfortable for patients.
The aim was to examine adverse events related to indirect laser within the first 8 weeks of treatment, and to compare results with the UK National Diabetic Retinopathy Laser Treatment Audit (NDRLTA). 108
Tinley and Gray (2009)103 carried out a retrospective review of case notes of 107 eyes (of 107 patients) undergoing indirect PRP between 2000 and 2006. The initial follow-up period was 8 weeks, and then a 9 months’ final follow-up. Fifteen patients (14.0%) returned with adverse events within the first 8 weeks of indirect PRP; five events were persistent and visually significant, with three requiring vitrectomy during the study period.
It was concluded that the incidence of significant PRP-induced adverse events was low after indirect PRP and the outcomes were not inferior to the outpatient-based UK NDRLTA. Hence single-session indirect PRP can be a suitable alternative to slit lamp-based treatment. They performed it in an operating theatre, which would be more expensive, but it can be done in an outpatient department.
Indirect PRP can be used in eyes where the view of the fundus is hazy.
Other studies reporting adverse events
Natesh et al. (2011)105 performed 883 single-session PRPs over a 2-year period using the PSC photocoagulator, and reported one patient symptomatic of choroidal detachment (CD) and worsening MO (the laser parameters for the PRP were 2700 burns at 200 mW, a duration of 30 ms, and a spot size of 200 mm with a fluence of 19 joules (J)/cm2. However, they acknowledge that that incidence of CD may have been a little higher owing to unreported, subclinical and self-limiting cases. They concluded that, although PSC PRP is superior to conventional PRP in terms of patient comfort, laser control, laser precision, and safety, there is still a risk of CD and worsening of MO, and patients should be counselled on these risks before undergoing PSC PRP. This study105 provides further support for the single-session approach.
Kapoor et al. (2010)106 reported an unusual case of acute angle closure glaucoma following argon laser PRP, which was initially mistaken for a viral illness (patients can present with ocular pain, nausea and vomiting).
Pain of photocoagulation
Pain is a common side effect of laser photocoagulation but was not reported in most of the studies in Table 22. Richardson and Waterman (2009)107 undertook a national survey of all ophthalmic units within the UK in late 2006 to explore the effects of pain on the procedure within clinical practice.
They achieved a 77% response rate (111 questionnaires), and the vast majority of responses (96%) were from doctors. A large proportion (79%) of units saw up to 20 cases a week and most patients received up to five sessions of PRP.
Overall, 88% of the practitioners said that PRP could be painful, and 99% said that pain has a negative effect on the delivery of the therapy; however, 67% did not have a pain protocol and 80% said no analgesia was routinely given for PRP.
It was thought that once present, pain can significantly affect the number and strength of burns delivered and it can indirectly increase the number of sessions required to complete the therapy. Also it could delay treatment therefore extending patient stay. A small number of respondents thought it could potentially be a reason for a lack of compliance. Therefore, the effects of pain could lead to under-treatment and hence to accelerated sight loss in people with PDR.
The authors did comment that, as for all surveys, the results need to be viewed with caution, as, with all survey findings, they are what clinicians say they do, which may not be the same as what they actually do.
The RCOphth guideline recommends that when applying repeat laser therapy it is important to try to avoid the previously treated areas, as pain may be felt by patients who have had previous laser treatment if the new laser burns encroach on the previously treated areas, especially in the horizontal meridian.
Pain can be prevented by anaesthesia delivered through the sub-Tenon’s capsule, but this blocks the eye for hours and it has to be patched because of the risk of dryness. There is also a risk of subconjunctival haemorrhage and infection, and some authorities have argued that with modern multi-spot PRP, anaesthesia is not required for most patients. 109
Discussion
Data on the incidence of adverse events comes from a mixture of different types of lasers and different methods of delivering the lasers, different levels of severity of DR, RCTs and non-randomised study designs, different follow-up times, and different methods of measuring outcomes.
In some studies, such as Chappelow et al. (2012)60 the higher incidence of adverse effects, such as vitreous haemorrhage, may reflect lower efficacy of the PSC photocoagulation.
Pan-retinal photocoagulation destroys retinal tissue, and this leads to symptoms due to the loss of function of the burned areas, including peripheral visual field defects, reduced night vision, reduced colour vision, and decreased contrast sensitivity. Fong et al. (2007)94 noted that visual field defects could occur in up to 50% of treated patients, depending on intensity of PRP and level of testing.
Macular oedema
The most common complication of PRP is the exacerbation of MO. Bressler et al. (2011)110 cites unpublished data from the ETDRS:
. . . in patients with such edema involving the center of the macula at baseline, an evaluation that was performed 4 months after baseline panretinal photocoagulation showed that 19% of the patients lost approximately two or more lines on a visual-acuity chart, including 11% who lost approximately three or more lines.
The DRCRN (Protocol for NCT01489189) also reports that in the ETDRS, which was performed prior to the advent of OCT, 16% of eyes that underwent full PRP (1200–1600 spots) were noted to have MO on stereoscopic fundus photographs by 4 months compared with only 12% in eyes that did not have PRP (FL Ferris, MD, unpublished data, 17 June 2008 – cited in Protocol). 21
Browning (2005)50 reported that ophthalmologists without OCT support were liable to miss some cases of MO. Browning notes that after PRP for NPDR or PDR, eyes with MO are twice as likely (18% vs. 9%) to lose two or more lines of VA 6 weeks post treatment as eyes with no MO.
Yang et al. (2001)111 also report that OCT is more sensitive than clinical examination (slit-lamp biomicroscopy) in detecting DMO.
The advent of OCT means that MO can be detected and treated with focal laser before administering PRP. Lee et al. (2010)65 noted that in the past decade, OCT had become much more available, and that it is more sensitive than clinical examination for detecting DMO, especially when macular thickness was 201–300 µm. In past decades, PRP may have made undiagnosed MO worse, rather than causing MO de novo.
Fong et al. (2007)54 concluded (from the ETDRS) that moderate visual loss occurs and appears to be more common in eyes with pre-existing MO. The RCOphth guidelines advise treating maculopathy either at the same time or prior to peripheral scatter retinal photocoagulation (PRP). 5
The risk of MO is why PRP is usually given over two to four sessions, along with the problem of patients getting tired when spots had to be administered one at a time.
However, there is some evidence that the risk may be less with modern methods.
Lee et al. (2010)65 reported a slight increase in macular thickness after PRP but no reduction in VA, which they attributed to exclusion of pre-PRP MO by OCT, giving PRP biweekly, and reducing the total number of spots from 2000 to 1200–1600.
Driving
The RCOphth guideline notes that, after full PRP, about 40–50% of patients have some reduction in visual fields, which may have implications for fitness to drive, but that the risk is lower with more modern regimens that have smaller and lighter burns.
Mackie 1995 applied the UK DVLA criteria to 100 consecutive patients who had had bilateral PRP with the argon laser, and found that 19% failed the driving criteria because of loss of visual field due to PRP. 112
Vernon et al. (2009)113 identified a group of patients who had had bilateral PRP, with small burn size, about 10 years before (in 1988–90) and obtained results from 25 of them. Of those (17) who drove, only two had stopped – neither because of failure to meet the DVLA rules – and nearly all of the rest confirmed having passed the driving vision test after PRP. Hulbert and Vernon (1996)114 writing in 1992, argue that smaller burns [300 microns (µm) rather than 500 µm] gave a better chance of being able to retain a driving licence.
In a modelling exercise, Davies (1999)115 concluded that the pattern of PRP could be adjusted in order to preserve driving vision, and that small burns (200 µm) would cause less loss of peripheral vision than large ones (500 µm). However, Quinn (1999)116 was concerned that distributing burns in such a way as to preserve driving vision might leave untouched ischaemic areas, leading to neovascularisation, and suggested a randomised trial.
Driving at night may be a particular problem. Fong et al. (2007)94 noted that after PRP, 38% of people reported worsened night driving and 60% worsened dark adaptation.
An excellent patient information leaflet notes that one in five people are aware of some loss of peripheral vision after PRP, and 3% have to stop driving because their peripheral vision has been reduced. 117
People who have laser treatment to both eyes, or to one eye if they only have sight in one eye, are required in the UK to inform the DVLA. 118
Other adverse events
The RCOphth guidelines list other adverse effects, though some are rare. 5 They include:
-
some loss of contrast sensitivity
-
loss of central vision due to inadvertent laser application to the foveal and parafoveal regions; the RCOphth guidelines recommend constant checking that the laser is not hitting the fovea
-
reduction of VA
-
a possible reduction in accommodative power
-
some dimness of vision
-
some loss of colour vision
-
rare complications, such as corneal burns, raised IOP or angle closure, pre-retinal or subretinal fibrosis, and tractional retinal detachment.
Informing patients
Adverse events should be carefully considered before deciding when to give PRP and patients should be made aware of them. There is usually some hesitation in performing PRP on diabetic patients with less severe retinopathy, especially in eyes with good vision. 49,80 The RCOphth guideline notes that scatter PRP can cause transient worsening or development of MO and that patients should be warned of this and also of the possibility of vitreous and subhyaloid haemorrhages. 5
Chapter 4 Combined laser and drug studies
Aims
Following laser therapy, MO may arise either de novo, or through exacerbation of prior MO. This can lead to temporary reduction in VA. It has been suggested that drugs like anti-VEGFs and steroids may have a place in reducing MO and vision loss when used in combination with PRP. If they do reduce the risk of DMO, they may make it safer to administer PRP in a single session.
Methods
The comprehensive search done for laser trials for Chapter 3 also provided trials for this chapter. We selected studies that had investigated the efficacy of drug and laser combination in patients with NPDR or PDR. Because the main interest was reduction in adverse effects, we relaxed the minimum duration rule of 6 months.
Results
The details of the search strategies are given in Chapter 3. We included 11 trials (published in 12 papers119–130) that compared the efficacy of anti-VEGFs or injectable steroids used in conjunction with laser in patients with DR.
Seven studies (Cho 2010;119 DRCRN 2011;120 Ernst 2012;121 Filho 2011122,123 with Lucena 2013; Mirshahi 2008;124 Preti 2013;125 Tonello 2008126), as shown in Table 23, compared the efficacy of anti-VEGFs used in combination with laser in patients with DR. Cho et al. (2010)119 and DRCRN (2011)120 also used intravitreal triamcinolone acetonide (IVTA). Three trials (Cho 2010;119 DRCRN 2011;120 Preti 2013125) aimed to assess the effect of anti-VEGF in combination with laser in reducing adverse events such as DMO, and three trials (Tonello 2008;126 Filho 2011;122,123 Mirshahi 2008124) were more concerned with added therapeutic benefits of anti-VEGFs in combination with laser. One trial (Ernst 2012121) compared the efficacy of anti-VEGF alone with PRP alone in laser-naive patients.
Six studies (Cho 2010;119 DRCN 2011;120 Maia 2009;127 Mirshahi 2010;128 Shimura 2006;129 Unoki 2009130), as shown in Table 24, compared the efficacy of triamcinolone as an adjunctive therapy to laser in patients with DR.
Baseline characteristics of included studies
Tables 23 and 24 give details of baseline characteristics of the participants in the included studies.
| Study (author and year) | No. of patients | No. of eyes | Follow-up | Comparisons | DR |
|---|---|---|---|---|---|
| Cho 2010119 | 76 | 46 eyes with CSMO, 45 eyes without CSMO | 3 months | (i) PRP (ii) PRP + IVB (iii) PRP + IVTA |
Very severe NPDR and HRC-PDR |
| DRCRN 2011120 | 319 | 345 | 14 weeks (primary outcome); 56 weeks for safety | (i) Sham injection + focal/grid laser + PRP (ii) 0.5 mg ranibizumab + focal/grid laser + PRP (iii) 4 mg TA + focal/grid laser + PRP |
Severe NPDR or PDR and centre involving DMO |
| Ernst 2012121 | 10 | 20 | 12 months | (i) PRP (ii) IVB |
Severe NPDR and PDR |
| Filho 2011122,123 | 40 | 29 | 48 weeks | (i) PRP (ii) PRP + IVR |
HR-PDR |
| Mirshahi 2008124 | 40 | 80 | 16 weeks | (i) Laser + IVB (ii) Laser + sham |
HR-PDR |
| Preti 2013125 | 35 | 70 | 6 months | (i) PRP (ii) PRP + IVB |
HR-PDR |
| Tonello 2008126 | 22 | 30 | 16 weeks | (i) PRP (ii) PRP + IVB |
HR-PDR |
| Study (author and year) | No. of patients | No. of eyes | Follow-up | Comparisons | DR |
|---|---|---|---|---|---|
| Cho 2010119 | 76 | 46 eyes with CSMO, 45 eyes without CSMO | 3 months | (i) PRP (ii) PRP + IVB (iii) PRP + IVTA |
Very severe NPDR and HR-PDR |
| DRCRN 2011120 | 319 | 345 | 14 weeks (primary outcome); 56 weeks for safety | (i) Sham injection + focal/grid laser + PRP (ii) 0.5 mg ranibizumab + focal/grid laser + PRP (iii) 4 mg TA + focal/grid laser + PRP |
Severe NPDR or PDR and centre involving DMO |
| Maia 2009127 | 22 | 44 | 12 months | (i) PRP + macular laser (ii) PRP + macular laser + IVTA |
PDR with CSMO |
| Mirshahi 2010128 | 18 | 36 | 6 months 18 months complications |
(i) IVTA + PRP + MPC (injected eye) (ii) PRP + MPC (control eye) |
HR-PDR with CSMO |
| Shimura 2006129 | 10 | 20 | 24 weeks | (i) Sub-Tenon’s TA + PRP (ii) PRP only |
Severe NPDR and early PDR |
| Unoki 2009130 | 41 | 82 | 6 months | (i) PRP only (ii) PSTA (posterior sub-Tenon’s triamcinolone injection 20 mg) + PRP |
Severe NPDR or PDR (plus could have CSMO but only if present in both eyes) |
Study design
All the included studies were RCTs.
Diabetic retinopathy
-
Anti-VEGFs:
-
Steroids:
-
one study (Cho 2010119) – very severe NPDR and HR-PDR
-
one study (DRCRN 2011120) – severe NPDR or PDR and DMO
-
one study (Maia 2009127) – PDR and CSMO
-
one study (Mirshahi 2010128) – HR-PDR with CSMO
-
one study (Shimura 2006129) – severe NPDR and early PDR
-
one study (Unoki 2009130) – severe NPDR or PDR (plus could have CSMO but only if present in both eyes).
-
Quality assessment/risk of bias
Not all studies gave enough details to assess risk of bias. In that case, we categorised them as ‘unclear’. See Tables 25 and 26.
| Study (author and year) | Adequate sequence generation | Adequate allocation concealment | Masking | Incomplete outcome data assessed | Free of selective outcome reporting | Free of other biases (e.g. similarity at baseline, power assessment) |
|---|---|---|---|---|---|---|
| Cho 2010119 | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk |
| DRCRN 2011120 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ernst 2012121 | Unclear | Unclear | Unclear | Unclear | Unclear | Low risk |
| Filho 2012 and Lucena 2013122,123 | Unclear | Unclear – sealed envelopes | Low risk | Low risk | Low risk | Low risk |
| Mirshahi 2008124 | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk |
| Preti 2013125 | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk |
| Tonello 2008126 | Low risk | Unclear – opaque envelopes | Low risk | Low risk | Low risk | Low risk |
| Study (author and year) | Adequate sequence generation | Adequate allocation concealment | Masking | Incomplete outcome data assessed | Free of selective outcome reporting | Free of other biases (e.g. similarity at baseline, power assessment) |
|---|---|---|---|---|---|---|
| Cho 2010119 | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk |
| DRCRN 2011120 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Maia 2009127 | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Mirshahi 2010128 | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk |
| Shimura 2006129 | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk |
| Unoki 2009130 | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk |
Allocation
Four studies (Cho 2010;119 Ernst 2012;121 Preti 2013;125 Mirshahi 2008124) only reported that patients were randomised. No details on allocation concealment were reported.
In two studies (Filho 2012;122,123 Tonello 2008126), participants were allocated in groups of two and a technician was asked to pick one of the two identical opaque envelopes, which is a less secure method than central randomisation.
Mirshahi (2010)128 used block randomisation method to allocate participants into intervention and control group. However, there was no detail on allocation concealment.
The allocation method was not clear in Shimura (2006)129 and Maia (2009). 127
In DRCRN (2011),120 central randomisation was provided via the DRCRN website. In Unoki (2009),130 participants were randomised using a stratification method. The stratification was done according to BCVA.
Masking of outcome assessments
Five studies (Cho 2010;119 Ernst 2012;121 Mirshahi 2008;124 Mirshahi 2010;128 Preti 2013;125 Shimura 2006129) had no details on masking. In three studies (Filho 2012;122,123 Tonello 2008;126 Maia 2009127) investigators were masked. In DRCRN (2011)120 the staff measuring VA and the OCT technician were masked to allocation. Unoki (2009)130 did not mask participants and investigators to study allocation. However, technicians measuring VA and OCT and statistician analysing the data were masked to study allocation.
Incomplete outcome data
Four studies (Cho 2010;119 Shimura 2006;129 Tonello 2008;126 Maia 2009127) followed up all participants. One study (Ernst 2012121) reported that five patients were lost to follow-up but reasons were not given. However, all five patients were excluded from the analysis.
Four studies (DRCRN 2011;120 Filho 2012;122,123 Mirshahi 2010;128 Preti 2013125) gave adequate description of withdrawals and lost to follow-up.
Free of selective reporting
All studies reported prespecified outcomes.
Free of other biases
In all studies, baseline characteristics between intervention and control groups were similar. Three studies (Maia 2009;127 DRCRN 2011;120 Unoki 2009130) gave information regarding power calculation. Unoki (2009)130 reported that for 80% power, a total of 40 eyes in each treatment group was required. In Maia (2009),127 it was reported that a total of 18 eyes of 18 patients was required for 80% power. However, after adjusting for a 20% lost to follow-up, the sample size required to reach the same study power increased to 22 eyes. In DRCRN (2011),120 it was reported that for 90% power, a total of 364 eyes were needed to ascertain a difference in mean VA from baseline to 14 weeks between different interventions. For the primary outcome, an intention-to-treat analysis was done, which included all the randomised eyes. However, 19 eyes were excluded from one site, as the baseline imputed values of central subfield thickness was < 250 µm in 63% of eyes. For missing data, last observation carried forward method was used. For other outcomes (safety), missing data were not imputed.
Funder/conflict of interest
Three studies (Cho 2010;119 Mirshahi 2010;128 Shimura 2006129) appeared to be funded by an academic institution. DRCRN was funded by the US National Institutes of Health. In two studies (Ernst 2012;121 Filho 2012122,123) the funding source was not clear. Shimura (2006)129 gave no details on conflict of interest.
Four studies (Filho 2012;122,123 Maia 2009;127 Preti 2013;125 Tonello 2008126) were part funded by research organisations and in three authors declared no conflict of interest. Tonello et al. (2008)126 gave details (Filho – CNPq; Maia 2009 – part supported by CAPES; Preti 2013 – São Paulo Research Foundation; Tonello 2008 – supported partly by the Foundation to support Education, Research and Assistance, Clinics Hospital, Faculty of Medicine of Ribeirao Preto). Unoki (2009)130 was supported by a grant from the Scientific Research from the Japanese Government.
The studies are described narratively below and a detailed summary given in Table 27.
| Study | Participants and baseline values | Intervention | Outcomes and ocular safety | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID: Cho 2010119 Aim: To compare and evaluate the efficacy and safety of IVTA and IVB as adjunctive treatments to PRP in reducing PRP-related short-term vision loss and MO Design: Prospective, comparative, interventional case series: the enrolled eye randomly received PRP with IVTA (IVTA group) or PRP with IVB (IVB group) or PRP only (PRP group); in patients with bilateral eyes eligible for the study, one eye of each patient was randomly chosen to receive PRP with IVTA or PRP with IVB or PRP only and the other eye received the other procedure Follow-up: 3 months Proportions completing the study: NR Reasons for withdrawal: NR |
n: 46 eyes with CSMO and 45 eyes without CSMO of 76 patients Inclusion criteria: Patients age ≥ 18 years, very severe NPDR to HR-PDR, and Snellen BCVA of ≥ 0.3 Exclusion criteria: Systemic and diastolic BPs of ≥ 180 and 110 mmHg, HbA1c levels exceeding 9.5%, chronic renal failure, major surgery within 1 month, previous systemic steroid or anti-VEGF treatment, patients with ocular conditions other than DR study (e.g. retinal vein occlusion, uveitis or other ocular inflammatory disease, NVG, etc.), patients with a history of treatment for DMO in the previous 3 months, previous PRP or focal/grid laser photocoagulation, previous intraocular surgery, uncontrolled glaucoma Age: IVTA group: Mean age was 50.8 ± 36.8 years IVB group: 50.9 ± 46.0 years PRP group: 51.0 ± 26.0 years Sex (female%): With CSMO: IVTA group: 31.25 IVB group: 37.5 PRP group: 35.71 Without CSMO: IVTA group: 28.57 IVB group: 33.33 PRP group: 31.25 Diabetes type: NR HbA1c: With CSMO: IVTA group: 6.91% (SD = 0.21) IVB group: 7.81 (0.32) PRP group: 7.31 (0.41) Without CSMO: IVTA group: 7.12% (SD = 0.13) IVB group: 7.21 (0.23) PRP group: 7.18 (0.09) Baseline VA: IVTA group: mean BCVA (logMAR) 0.27 ± 0.25 IVB group: 0.28 ± 0.31 PRP group: 0.26 ± 0.28 Baseline CMT: IVTA group: mean CMT 343.89 ± 330.79 µm IVB group: 328.02 ± 335.29 µm PRP group: 326.27 ± 315.17 µm Baseline DR stage: Very severe NPDR to HR-PDR Previous laser or intravitreal drug treatment: See Exclusion criteria Ocular comorbidities: See Exclusion criteria |
Group 1: PRP Group 2: IVB + PRP Group 3: IVTA + PRP Description of type of laser and delivery: Scatter laser treatment using a 532-nm argon green laser (Visulas 532s/LSL 532s Laser Slit Lamp; Carl Zeiss Meditec) at three time points (1-week intervals). Focal/grid laser photocoagulation was performed at the time of initiation PRP in patients with CSMO Description of anti-VEGF treatment: IVB (1.25 mg/0.05 ml) was given about 1 week prior to initial PRP with a 30-gauge needle with topical anaesthesia Description of steroid treatment: IVTA (4 mg/0.1 ml) was given 1 day after first session of PRP with a 30-gauge needle with topical anaesthesia |
Mean BCVA (logMAR): PRP groupIVTA groupIVB groupp-valueMean BCVA (logMAR)All eyes with PRPBaseline0.26–1 month0.29–PRP group – vs. baseline: p = 0.031IVTA and IVB groups – vs. baseline: not significantBetween group difference: not significant3 months0.29–IVTA and IVB groups – vs. baseline: not significantBetween group difference: not significantEyes with CSMEIVTA group – significant improvement of BCVA during 3 months; significant difference in mean BCVA between the IVTA and IVB groupsEyes without CSMOPRP group – significant worsening of BCVA CMT: PRP groupIVTA groupIVB groupp-valueCMTAll eyes with PRPSignificant decreases in CMT during the follow-up period in both IVTA and IVB groupsEyes with CSMOSignificant decreases in CMT in both IVTA and IVB groupsEyes without CSMOBaseline: 209.75 ± 27.47 µm1 month: 259.00 ± 58.28 µm3 months: 276.14 ± 47.38 µmPRP group: 1 month p = 0.023; 3 months p = 0.011IVTA and IVB groups: no significant changesNo significant difference in CMT at 1 month and 3 months between IVTA and IVB groups Proportion of eyes with visual gain ≥ 0.1 logMAR: PRP groupIVTA groupIVB groupp-valueEyes with CSMOProportion of eyes with visual gain ≥ 0.1 logMAR was significantly higher in IVTA than in IVB and PRP. Visual gain ≥ 0.2 logMAR was significantly higher in IVTA than in PRPEyes without CSMOProportions of eyes with visual gain ≥ 0.1 logMAR was significantly higher in IVTA and IVB than in PRP. Proportions of eyes with visual gain ≥ 0.2 logMAR was not statistically different among three groups Proportion of eyes with visual loss ≥ 0.1 logMAR and visual loss ≥ 0.2 logMAR: PRP groupIVTA groupIVB groupp-valueEyes with CSMOProportion of eyes with visual loss ≥ 0.1 logMAR and visual loss ≥ 0.2 logMAR was significantly higher in PRP than IVTAEyes without CSMOProportions of eyes with visual loss ≥ 0.1 logMAR and visual loss ≥ 0.2 logMAR was significantly higher in PRP than in IVTA and IVB Proportion of eyes with a decrease/increase in CMT: PRP groupIVTA groupIVB groupp-valueEyes with CSMOProportion of eyes with a decrease in CMT was significantly lower in PRP than in IVTA and IVB. Proportion of eyes with a decrease in CMT was significantly higher in IVTA than in IVBEyes without CSMOProportion of eyes with an increase in CMT was significantly higher in PRP than in IVTA and IVB PRP group: Four patients with increased proliferation and development of VH during follow-up Injection-related complications:
|
PRP group | IVTA group | IVB group | p -value | Mean BCVA (logMAR) | All eyes with PRP | Baseline | 0.26 | – | 1 month | 0.29 | – | PRP group – vs. baseline: p = 0.031 IVTA and IVB groups – vs. baseline: not significant Between group difference: not significant |
3 months | 0.29 | – | IVTA and IVB groups – vs. baseline: not significant Between group difference: not significant |
Eyes with CSME | IVTA group – significant improvement of BCVA during 3 months; significant difference in mean BCVA between the IVTA and IVB groups | Eyes without CSMO | PRP group – significant worsening of BCVA | PRP group | IVTA group | IVB group | p -value | CMT | All eyes with PRP | Significant decreases in CMT during the follow-up period in both IVTA and IVB groups | Eyes with CSMO | Significant decreases in CMT in both IVTA and IVB groups | Eyes without CSMO | Baseline: 209.75 ± 27.47 µm 1 month: 259.00 ± 58.28 µm 3 months: 276.14 ± 47.38 µm |
PRP group: 1 month p = 0.023; 3 months p = 0.011 IVTA and IVB groups: no significant changes No significant difference in CMT at 1 month and 3 months between IVTA and IVB groups |
PRP group | IVTA group | IVB group | p-value | Eyes with CSMO | Proportion of eyes with visual gain ≥ 0.1 logMAR was significantly higher in IVTA than in IVB and PRP. Visual gain ≥ 0.2 logMAR was significantly higher in IVTA than in PRP | Eyes without CSMO | Proportions of eyes with visual gain ≥ 0.1 logMAR was significantly higher in IVTA and IVB than in PRP. Proportions of eyes with visual gain ≥ 0.2 logMAR was not statistically different among three groups | PRP group | IVTA group | IVB group | p-value | Eyes with CSMO | Proportion of eyes with visual loss ≥ 0.1 logMAR and visual loss ≥ 0.2 logMAR was significantly higher in PRP than IVTA | Eyes without CSMO | Proportions of eyes with visual loss ≥ 0.1 logMAR and visual loss ≥ 0.2 logMAR was significantly higher in PRP than in IVTA and IVB | PRP group | IVTA group | IVB group | p-value | Eyes with CSMO | Proportion of eyes with a decrease in CMT was significantly lower in PRP than in IVTA and IVB. Proportion of eyes with a decrease in CMT was significantly higher in IVTA than in IVB | Eyes without CSMO | Proportion of eyes with an increase in CMT was significantly higher in PRP than in IVTA and IVB | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PRP group | IVTA group | IVB group | p -value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean BCVA (logMAR) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All eyes with PRP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 0.26 | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 0.29 | – | PRP group – vs. baseline: p = 0.031 IVTA and IVB groups – vs. baseline: not significant Between group difference: not significant |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months | 0.29 | – | IVTA and IVB groups – vs. baseline: not significant Between group difference: not significant |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eyes with CSME | IVTA group – significant improvement of BCVA during 3 months; significant difference in mean BCVA between the IVTA and IVB groups | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eyes without CSMO | PRP group – significant worsening of BCVA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PRP group | IVTA group | IVB group | p -value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CMT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All eyes with PRP | Significant decreases in CMT during the follow-up period in both IVTA and IVB groups | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eyes with CSMO | Significant decreases in CMT in both IVTA and IVB groups | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eyes without CSMO | Baseline: 209.75 ± 27.47 µm 1 month: 259.00 ± 58.28 µm 3 months: 276.14 ± 47.38 µm |
PRP group: 1 month p = 0.023; 3 months p = 0.011 IVTA and IVB groups: no significant changes No significant difference in CMT at 1 month and 3 months between IVTA and IVB groups |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PRP group | IVTA group | IVB group | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eyes with CSMO | Proportion of eyes with visual gain ≥ 0.1 logMAR was significantly higher in IVTA than in IVB and PRP. Visual gain ≥ 0.2 logMAR was significantly higher in IVTA than in PRP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eyes without CSMO | Proportions of eyes with visual gain ≥ 0.1 logMAR was significantly higher in IVTA and IVB than in PRP. Proportions of eyes with visual gain ≥ 0.2 logMAR was not statistically different among three groups | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PRP group | IVTA group | IVB group | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eyes with CSMO | Proportion of eyes with visual loss ≥ 0.1 logMAR and visual loss ≥ 0.2 logMAR was significantly higher in PRP than IVTA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eyes without CSMO | Proportions of eyes with visual loss ≥ 0.1 logMAR and visual loss ≥ 0.2 logMAR was significantly higher in PRP than in IVTA and IVB | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PRP group | IVTA group | IVB group | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eyes with CSMO | Proportion of eyes with a decrease in CMT was significantly lower in PRP than in IVTA and IVB. Proportion of eyes with a decrease in CMT was significantly higher in IVTA than in IVB | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eyes without CSMO | Proportion of eyes with an increase in CMT was significantly higher in PRP than in IVTA and IVB | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: DRCRN 2011120 Aim: To assess the efficacy of intravitreal injection of ranibizumab or TA in patients with DR and DMO treated with focal/grid laser Design: Phase 3, randomised, multicentre, clinical trial. 48 clinical sites in the USA Follow-up: Primary outcome (14 weeks); safety (56 weeks). Proportions completing the study: 14 weeks – sham (n = 118 eyes, 96%), ranibizumab (n = 103, 91%), triamcinolone (n = 105, 96%); 56 weeks – sham (n = 111, 90%), ranibizumab (n = 95, 84%), triamcinolone (n = 93, 85%) Reasons for withdrawal (brief): Death before 14 weeks = 4 (1%); death after 14 weeks = 4 (1%) – All deaths not related to study treatment |
n: 345; 26 (8%) with two study eyes Inclusion criteria: Patients aged 18 years or more with type 1 or type 2 diabetes and with severe NPDR or PDR and centre-involving DMO and BCVA ETDRS score of ≥ 24 Exclusion criteria: Previous history of extensive and unnecessary treatment with PRP; treatment of DMO prior to 4 months of the study; treatment of steroid-induced increase IOP; glaucoma; IOP ≥ 25 mmHg Age (years): Mean 55, SD 12; median, 25th to 75th percentile [sham: 54, 45 to 61; ranibizumab: 57, 48 to 64); triamcinolone: 58, 49 to 64)] Sex: 40% female; [sham: 36%; ranibizumab: 42%; triamcinolone: 40%] Diabetes type: Type 1 (11 to 16%) or type 2 diabetes (82 to 87%) or uncertain (2 to 6%); [type 1 – sham: 16%; ranibizumab: 12%; triamcinolone: 11%]; [type 2 – sham: 82%; ranibizumab: 82%; triamcinolone: 87%]; [uncertain – sham: 2%; ranibizumab: 6%; triamcinolone: 2%] HbA1c (%): Median 7.9 to 8.1; [sham: 7.9, 7 to 9.6; ranibizumab: 8.1, 7.1 to 9.9; triamcinolone: 8.1, 7 to 9.7 Baseline BCVA: Mean 64 ± 15 (approximately 20/50) Baseline CMT: Mean 392 ± 151 µm Baseline DR stage: Based on investigator assessment – severe NPDR (18% of eyes); PDR (82% of eyes) Based on reading centre assessment – moderately severe NPDR or less severe NPDR (20% of eyes); severe NPDR (5% of eyes); PDR (75% of eyes including 35% with HR-PDR or level 71–75) Previous laser or intravitreal drug treatment: Scatter photocoagulation: 13–18%; no. of previous treatment for DMO: 65–66%; previous laser for DMO: 29–33%; previous IVTA for DMO: 1–8%; previous vitrectomy for DMO: 0–2%; previous peribulbar triamcinolone for DMO: 0–1%; previous anti-VEGF for DMO: 1–5% Ocular comorbidities: DMO – predominantly focal 17–30%, neither predominantly focal or diffuse 13–22%, predominantly diffuse 55–62% |
Group 1: Sham injection + focal/grid laser + PRP (n = 123) Group 2: 0.5 mg ranibizumab + focal/grid laser + PRP (n = 113) Group 3: 4 mg TA + focal/grid laser + PRP (n = 109) Description of type of laser and delivery: After baseline treatment with sham or ranibizumab or triamcinolone, patients received focal/grid laser between 3 and 10 days. Following focal/grid laser, all patients also received PRP immediately, or on the next day, but no later than 14 days from the day of baseline treatment. PRP included giving 1200–1600 burns either in one session or in three sessions However, all PRP sessions should be completed within 49 days after allocation In the study, laser that could produce automated patterns, e.g. PSC laser could be used Description of anti-VEGF/steroid treatment: All patients were prepared by applying povidone–iodine preparation on the conjunctiva Treating investigators decided whether to give antibiotics before or after injection. One group of patients received intravitreal injection of 0.5 mg ranibizumab (Lucentis), while another group of patients received intravitreal injection of 4 mg TA (Trivaris) |
Before the primary outcome (14-week visit):
Change in BCVA from baseline to 14 weeks study visit: Sham + laser (n = 123)Ranibizumab + laser (n = 113)Triamcinolone + laser (n = 109)Difference compared against shamLetter scoreMean change from baseline–4 SD 14+ 1 SD 11+ 2 SD 11vs. ranibizumab: +5.6, 95% CI 2.2 to 9.0; p < 0.001vs. triamcinolone: +6.7, 95% CI 3.2 to 10.1; p < 0.001Median (25th to 75th percentile)–2 (–8, +3)+ 2 (–3, +7)+ 1 (–3, +8)Distribution of change, n (%)≥ 15-letter gain5 (4)8 (7)11 (10)NR14- to 10-letter gain5 (4)13 (12)13 (12)NR9- to 5-letter gain12 (10)20 (18)13 (12)NRSame + 4 letters54 (44)53 (47)50 (46)NR5- to 9-letter loss19 (15)9 (8)11 (10)NR10- to 14-letter loss10 (8)2 (2)8 (7)NR≥ 15-letter loss18 (15)8 (7)3 (3)NRDifference in proportion with ≥ 10-letter improvement from sham (95% CI)–+10 (+1 to +20)+14 (+4 to +25)–RR for comparison with sham12.79, 95% CI 1.33 to 5.87; p = 0.0023.58, 95% CI 1.69 to 7.61; p < 0.001Difference in proportion with ≥ 10 letter worsening from sham, 95% CI––13 (–24 to –3)–13 (–23 to –3)RR for comparison with sham10.4, 95% CI 0.19 to 0.87; p = 0.0080.44, 95% CI 0.21 to 0.91; p = 0.01 Change in VA from baseline to week 14 among subgroups: Sham, ranibizumab, triamcinolone (n)Sham + laserRanibizumab + laserTriamcinolone + laserPrevious treatmentNo80, 75, 72–4 ± 13+2 ± 12+2 ± 11Yes43, 38, 37–5 ± 14+1 ± 9+3 ± 11Baseline VA letter score≥ 6666, 64, 61–5 ± 12–1 ± 90 ± 7≤ 6557, 49, 48–2 ± 16+4 ± 13+5 ± 13Baseline central subfield thickness< 40076, 68, 65–3 ± 13+1 ± 9+2 ± 9≥ 40046, 44, 42–6 ± 15+2 ± 14+2 ± 12DR severityNPDR37, 26, 21–2 ± 100 ± 10–2 ± 8PDR82, 83, 84–5 ± 10+2 ± 12+3 ± 11No. of PRP sittingsOne sitting49, 38, 41–2 ± 12+2 ± 7+2 ± 12Multiple sittings73, 73, 66–5 ± 14+1 ± 13+2 ± 10PRP automated pattern usedYes36, 21, 21–4 ± 12+2 ± 130 ± 10No86, 90, 86–4 ± 14+1 ± 11+3 ± 11Baseline HbA1c (%)< 861, 46, 48–3 ± 11+3 ± 100 ± 11≥ 859, 57, 55–6 ± 160 ± 12+4 ± 10 After 14 weeks:
Sham + laser (n = 111)Ranibizumab + laser (n = 95)Triamcinolone + laser (n = 93)Difference compared against shamLetter scoreMean change from baseline–6, SD 17–4, SD 21–5, SD 16vs. ranibizumab:+1.9, 95% CI –3.7 to 7.5; p = 0.44vs. triamcinolone: +1.2, 95% CI –4.4 to +6.8; p = 0.63Median (25th to 75th percentile)–3 (–11, +4)+1 (–12, +8)–3 (–12, +3)Distribution of change, n (%)≥ 1-letter gain6 (5)12 (13)7 (8)NR14- to 10-letter gain9 (8)10 (11)5 (5)NR9- to 5-letter gain9 (8)12 (13)8 (9)NRSame + 4 letters39 (35)27 (28)31 (33)NR5- to 9-letter loss16 (14)8 (8)14 (15)NR10- to 14-letter loss8 (7)8 (8)8 (9)NR≥ 15-letter loss24 (22)18 (19)20 (22)NRDifference in proportion with ≥ 10 letter improvement from sham, 95% CI–+ 8 (–4 to + 20)+ 0.2 (–10 to + 10)–RR for comparison with sham12.00, 95% CI 1.04 to 3.87; p = 0.021.22, 95% CI 10.57 to 2.63; p = 0.55Difference in proportion with ≥ 10 letter worsening from sham, 95% CI––1 (–15 to + 13)+ 2 (–13 to + 16)RR for comparison with sham10.95, 95% CI 0.58 to 1.55; p = 0.821.04, 95% CI 0.64 to 1.69; p = 0.85 Major ocular adverse events: Up to 14-week visit Sham + laser (n = 133)Ranibizumab + laser (n = 116)Triamcinolone + laser (n = 115)Endophthalmitis01 (0.9)0Ocular vascular event000Retinal detachment4 (3)1 (1)1 (1)Vitrectomy1 (1)01 (1)VH16 (12)6 (5)7 (6)Elevated IOP/glaucoma (mmHg)Increase ≥ 103 (2)020 (17)Increase ≥ 302 (2)05 (4)Initiation of IOP-lowering medication2 (2)02 (2)No. of eyes meeting one or more of the above3 (2)020 (17)Glaucoma surgery000Cataract surgeryPhakic at baselinen = 120n = 93n = 105No. of eyes with cataract surgery000 One case of endophthalmitis was related to ranibizumab injection All had tractional detachment except in two eyes which had unspecified detachment (one before 14 weeks and one after 14 weeks) All vitrectomies were done for PDR After 14–56 weeks visit Sham + laser (n = 131)Ranibizumab + laser (n = 111)Triamcinolone + laser (n = 112)Endophthalmitis000Ocular vascular event000Retinal detachment4 (3)5 (5)1 (1)Vitrectomy17 (13)8 (7)7 (6)VH28 (21)25 (23)20 (18)Elevated IOP/glaucoma (mmHg)Increase ≥ 10 from baseline6 (5)6 (5)10 (9)Increase ≥ 304 (3)4 (4)4 (4)Initiation of IOP-lowering medication at any visit after the 14-week visit7 (5)5 (5)17 (15)No. of eyes meeting one or more of the above11 (8)7 (6)20 (18)IOP-lowering medication at 56 week visit3(2)4 (4)9 (9)Glaucoma surgery01 (1)1 (1)Cataract surgeryPhakic at 14 weeksn = 119n = 91n = 102No. of eyes with cataract surgery2 (2)3 (3)6 (6) |
Sham + laser (n = 123) | Ranibizumab + laser (n = 113) | Triamcinolone + laser (n = 109) | Difference compared against sham | Letter score | Mean change from baseline | –4 SD 14 | + 1 SD 11 | + 2 SD 11 | vs. ranibizumab: +5.6, 95% CI 2.2 to 9.0; p < 0.001 vs. triamcinolone: +6.7, 95% CI 3.2 to 10.1; p < 0.001 |
Median (25th to 75th percentile) | –2 (–8, +3) | + 2 (–3, +7) | + 1 (–3, +8) | Distribution of change, n (%) | ≥ 15-letter gain | 5 (4) | 8 (7) | 11 (10) | NR | 14- to 10-letter gain | 5 (4) | 13 (12) | 13 (12) | NR | 9- to 5-letter gain | 12 (10) | 20 (18) | 13 (12) | NR | Same + 4 letters | 54 (44) | 53 (47) | 50 (46) | NR | 5- to 9-letter loss | 19 (15) | 9 (8) | 11 (10) | NR | 10- to 14-letter loss | 10 (8) | 2 (2) | 8 (7) | NR | ≥ 15-letter loss | 18 (15) | 8 (7) | 3 (3) | NR | Difference in proportion with ≥ 10-letter improvement from sham (95% CI) | – | +10 (+1 to +20) | +14 (+4 to +25) | – | RR for comparison with sham | 1 | 2.79, 95% CI 1.33 to 5.87; p = 0.002 | 3.58, 95% CI 1.69 to 7.61; p < 0.001 | Difference in proportion with ≥ 10 letter worsening from sham, 95% CI | – | –13 (–24 to –3) | –13 (–23 to –3) | RR for comparison with sham | 1 | 0.4, 95% CI 0.19 to 0.87; p = 0.008 | 0.44, 95% CI 0.21 to 0.91; p = 0.01 | Sham, ranibizumab, triamcinolone (n) | Sham + laser | Ranibizumab + laser | Triamcinolone + laser | Previous treatment | No | 80, 75, 72 | –4 ± 13 | +2 ± 12 | +2 ± 11 | Yes | 43, 38, 37 | –5 ± 14 | +1 ± 9 | +3 ± 11 | Baseline VA letter score | ≥ 66 | 66, 64, 61 | –5 ± 12 | –1 ± 9 | 0 ± 7 | ≤ 65 | 57, 49, 48 | –2 ± 16 | +4 ± 13 | +5 ± 13 | Baseline central subfield thickness | < 400 | 76, 68, 65 | –3 ± 13 | +1 ± 9 | +2 ± 9 | ≥ 400 | 46, 44, 42 | –6 ± 15 | +2 ± 14 | +2 ± 12 | DR severity | NPDR | 37, 26, 21 | –2 ± 10 | 0 ± 10 | –2 ± 8 | PDR | 82, 83, 84 | –5 ± 10 | +2 ± 12 | +3 ± 11 | No. of PRP sittings | One sitting | 49, 38, 41 | –2 ± 12 | +2 ± 7 | +2 ± 12 | Multiple sittings | 73, 73, 66 | –5 ± 14 | +1 ± 13 | +2 ± 10 | PRP automated pattern used | Yes | 36, 21, 21 | –4 ± 12 | +2 ± 13 | 0 ± 10 | No | 86, 90, 86 | –4 ± 14 | +1 ± 11 | +3 ± 11 | Baseline HbA1c (%) | < 8 | 61, 46, 48 | –3 ± 11 | +3 ± 10 | 0 ± 11 | ≥ 8 | 59, 57, 55 | –6 ± 16 | 0 ± 12 | +4 ± 10 | Sham + laser (n = 111) | Ranibizumab + laser (n = 95) | Triamcinolone + laser (n = 93) | Difference compared against sham | Letter score | Mean change from baseline | –6, SD 17 | –4, SD 21 | –5, SD 16 | vs. ranibizumab:+1.9, 95% CI –3.7 to 7.5; p = 0.44 vs. triamcinolone: +1.2, 95% CI –4.4 to +6.8; p = 0.63 |
Median (25th to 75th percentile) | –3 (–11, +4) | +1 (–12, +8) | –3 (–12, +3) | Distribution of change, n (%) | ≥ 1-letter gain | 6 (5) | 12 (13) | 7 (8) | NR | 14- to 10-letter gain | 9 (8) | 10 (11) | 5 (5) | NR | 9- to 5-letter gain | 9 (8) | 12 (13) | 8 (9) | NR | Same + 4 letters | 39 (35) | 27 (28) | 31 (33) | NR | 5- to 9-letter loss | 16 (14) | 8 (8) | 14 (15) | NR | 10- to 14-letter loss | 8 (7) | 8 (8) | 8 (9) | NR | ≥ 15-letter loss | 24 (22) | 18 (19) | 20 (22) | NR | Difference in proportion with ≥ 10 letter improvement from sham, 95% CI | – | + 8 (–4 to + 20) | + 0.2 (–10 to + 10) | – | RR for comparison with sham | 1 | 2.00, 95% CI 1.04 to 3.87; p = 0.02 | 1.22, 95% CI 10.57 to 2.63; p = 0.55 | Difference in proportion with ≥ 10 letter worsening from sham, 95% CI | – | –1 (–15 to + 13) | + 2 (–13 to + 16) | RR for comparison with sham | 1 | 0.95, 95% CI 0.58 to 1.55; p = 0.82 | 1.04, 95% CI 0.64 to 1.69; p = 0.85 | Sham + laser (n = 133) | Ranibizumab + laser (n = 116) | Triamcinolone + laser (n = 115) | Endophthalmitis | 0 | 1 (0.9) | 0 | Ocular vascular event | 0 | 0 | 0 | Retinal detachment | 4 (3) | 1 (1) | 1 (1) | Vitrectomy | 1 (1) | 0 | 1 (1) | VH | 16 (12) | 6 (5) | 7 (6) | Elevated IOP/glaucoma (mmHg) | Increase ≥ 10 | 3 (2) | 0 | 20 (17) | Increase ≥ 30 | 2 (2) | 0 | 5 (4) | Initiation of IOP-lowering medication | 2 (2) | 0 | 2 (2) | No. of eyes meeting one or more of the above | 3 (2) | 0 | 20 (17) | Glaucoma surgery | 0 | 0 | 0 | Cataract surgery | Phakic at baseline | n = 120 | n = 93 | n = 105 | No. of eyes with cataract surgery | 0 | 0 | 0 | Sham + laser (n = 131) | Ranibizumab + laser (n = 111) | Triamcinolone + laser (n = 112) | Endophthalmitis | 0 | 0 | 0 | Ocular vascular event | 0 | 0 | 0 | Retinal detachment | 4 (3) | 5 (5) | 1 (1) | Vitrectomy | 17 (13) | 8 (7) | 7 (6) | VH | 28 (21) | 25 (23) | 20 (18) | Elevated IOP/glaucoma (mmHg) | Increase ≥ 10 from baseline | 6 (5) | 6 (5) | 10 (9) | Increase ≥ 30 | 4 (3) | 4 (4) | 4 (4) | Initiation of IOP-lowering medication at any visit after the 14-week visit | 7 (5) | 5 (5) | 17 (15) | No. of eyes meeting one or more of the above | 11 (8) | 7 (6) | 20 (18) | IOP-lowering medication at 56 week visit | 3(2) | 4 (4) | 9 (9) | Glaucoma surgery | 0 | 1 (1) | 1 (1) | Cataract surgery | Phakic at 14 weeks | n = 119 | n = 91 | n = 102 | No. of eyes with cataract surgery | 2 (2) | 3 (3) | 6 (6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sham + laser (n = 123) | Ranibizumab + laser (n = 113) | Triamcinolone + laser (n = 109) | Difference compared against sham | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Letter score | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean change from baseline | –4 SD 14 | + 1 SD 11 | + 2 SD 11 | vs. ranibizumab: +5.6, 95% CI 2.2 to 9.0; p < 0.001 vs. triamcinolone: +6.7, 95% CI 3.2 to 10.1; p < 0.001 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median (25th to 75th percentile) | –2 (–8, +3) | + 2 (–3, +7) | + 1 (–3, +8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Distribution of change, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≥ 15-letter gain | 5 (4) | 8 (7) | 11 (10) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14- to 10-letter gain | 5 (4) | 13 (12) | 13 (12) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9- to 5-letter gain | 12 (10) | 20 (18) | 13 (12) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Same + 4 letters | 54 (44) | 53 (47) | 50 (46) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5- to 9-letter loss | 19 (15) | 9 (8) | 11 (10) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10- to 14-letter loss | 10 (8) | 2 (2) | 8 (7) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≥ 15-letter loss | 18 (15) | 8 (7) | 3 (3) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Difference in proportion with ≥ 10-letter improvement from sham (95% CI) | – | +10 (+1 to +20) | +14 (+4 to +25) | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RR for comparison with sham | 1 | 2.79, 95% CI 1.33 to 5.87; p = 0.002 | 3.58, 95% CI 1.69 to 7.61; p < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Difference in proportion with ≥ 10 letter worsening from sham, 95% CI | – | –13 (–24 to –3) | –13 (–23 to –3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RR for comparison with sham | 1 | 0.4, 95% CI 0.19 to 0.87; p = 0.008 | 0.44, 95% CI 0.21 to 0.91; p = 0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sham, ranibizumab, triamcinolone (n) | Sham + laser | Ranibizumab + laser | Triamcinolone + laser | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Previous treatment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No | 80, 75, 72 | –4 ± 13 | +2 ± 12 | +2 ± 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yes | 43, 38, 37 | –5 ± 14 | +1 ± 9 | +3 ± 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline VA letter score | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≥ 66 | 66, 64, 61 | –5 ± 12 | –1 ± 9 | 0 ± 7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 65 | 57, 49, 48 | –2 ± 16 | +4 ± 13 | +5 ± 13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline central subfield thickness | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 400 | 76, 68, 65 | –3 ± 13 | +1 ± 9 | +2 ± 9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≥ 400 | 46, 44, 42 | –6 ± 15 | +2 ± 14 | +2 ± 12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DR severity | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NPDR | 37, 26, 21 | –2 ± 10 | 0 ± 10 | –2 ± 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDR | 82, 83, 84 | –5 ± 10 | +2 ± 12 | +3 ± 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. of PRP sittings | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| One sitting | 49, 38, 41 | –2 ± 12 | +2 ± 7 | +2 ± 12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Multiple sittings | 73, 73, 66 | –5 ± 14 | +1 ± 13 | +2 ± 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PRP automated pattern used | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yes | 36, 21, 21 | –4 ± 12 | +2 ± 13 | 0 ± 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No | 86, 90, 86 | –4 ± 14 | +1 ± 11 | +3 ± 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline HbA1c (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 8 | 61, 46, 48 | –3 ± 11 | +3 ± 10 | 0 ± 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≥ 8 | 59, 57, 55 | –6 ± 16 | 0 ± 12 | +4 ± 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sham + laser (n = 111) | Ranibizumab + laser (n = 95) | Triamcinolone + laser (n = 93) | Difference compared against sham | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Letter score | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean change from baseline | –6, SD 17 | –4, SD 21 | –5, SD 16 | vs. ranibizumab:+1.9, 95% CI –3.7 to 7.5; p = 0.44 vs. triamcinolone: +1.2, 95% CI –4.4 to +6.8; p = 0.63 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median (25th to 75th percentile) | –3 (–11, +4) | +1 (–12, +8) | –3 (–12, +3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Distribution of change, n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≥ 1-letter gain | 6 (5) | 12 (13) | 7 (8) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14- to 10-letter gain | 9 (8) | 10 (11) | 5 (5) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9- to 5-letter gain | 9 (8) | 12 (13) | 8 (9) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Same + 4 letters | 39 (35) | 27 (28) | 31 (33) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5- to 9-letter loss | 16 (14) | 8 (8) | 14 (15) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10- to 14-letter loss | 8 (7) | 8 (8) | 8 (9) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≥ 15-letter loss | 24 (22) | 18 (19) | 20 (22) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Difference in proportion with ≥ 10 letter improvement from sham, 95% CI | – | + 8 (–4 to + 20) | + 0.2 (–10 to + 10) | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RR for comparison with sham | 1 | 2.00, 95% CI 1.04 to 3.87; p = 0.02 | 1.22, 95% CI 10.57 to 2.63; p = 0.55 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Difference in proportion with ≥ 10 letter worsening from sham, 95% CI | – | –1 (–15 to + 13) | + 2 (–13 to + 16) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RR for comparison with sham | 1 | 0.95, 95% CI 0.58 to 1.55; p = 0.82 | 1.04, 95% CI 0.64 to 1.69; p = 0.85 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sham + laser (n = 133) | Ranibizumab + laser (n = 116) | Triamcinolone + laser (n = 115) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Endophthalmitis | 0 | 1 (0.9) | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ocular vascular event | 0 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Retinal detachment | 4 (3) | 1 (1) | 1 (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitrectomy | 1 (1) | 0 | 1 (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VH | 16 (12) | 6 (5) | 7 (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Elevated IOP/glaucoma (mmHg) | Increase ≥ 10 | 3 (2) | 0 | 20 (17) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Increase ≥ 30 | 2 (2) | 0 | 5 (4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Initiation of IOP-lowering medication | 2 (2) | 0 | 2 (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. of eyes meeting one or more of the above | 3 (2) | 0 | 20 (17) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glaucoma surgery | 0 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cataract surgery | Phakic at baseline | n = 120 | n = 93 | n = 105 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. of eyes with cataract surgery | 0 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sham + laser (n = 131) | Ranibizumab + laser (n = 111) | Triamcinolone + laser (n = 112) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Endophthalmitis | 0 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ocular vascular event | 0 | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Retinal detachment | 4 (3) | 5 (5) | 1 (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitrectomy | 17 (13) | 8 (7) | 7 (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VH | 28 (21) | 25 (23) | 20 (18) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Elevated IOP/glaucoma (mmHg) | Increase ≥ 10 from baseline | 6 (5) | 6 (5) | 10 (9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Increase ≥ 30 | 4 (3) | 4 (4) | 4 (4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Initiation of IOP-lowering medication at any visit after the 14-week visit | 7 (5) | 5 (5) | 17 (15) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. of eyes meeting one or more of the above | 11 (8) | 7 (6) | 20 (18) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IOP-lowering medication at 56 week visit | 3(2) | 4 (4) | 9 (9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glaucoma surgery | 0 | 1 (1) | 1 (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cataract surgery | Phakic at 14 weeks | n = 119 | n = 91 | n = 102 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. of eyes with cataract surgery | 2 (2) | 3 (3) | 6 (6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Ernst 2012121 (note to editor) Aim: To investigate IVB injections alone without prior PRP Design: Prospective, paired-eye, randomised pilot study. The right eye was randomly assigned to treatment with PRP or IVB, and the left eye received the other treatment Follow-up: 12 months Proportion completing the study: 10/15 (67%) Reasons for withdrawal: Unable to ascertain the reasons these patients left |
n: 15 patients enrolled, 10 followed up (20 eyes) Inclusion criteria: Patients with type 2 diabetes and symmetric untreated severe NPDR or PDR Exclusion criteria: MO or prior intraocular surgery Age: Average 53 ± 9 years Sex (%female): 70 (7/10) Diabetes type: Type 2 HbA1c: NR Baseline VA: IVB group: –0.12 ± 0.22 PRP group: –0.14 ± 0.23 Baseline CMT: NR Baseline DR stage: Five patients had PDR and five had severe NPDR Previous laser or intravitreal drug treatment: NR Ocular comorbidities: MO – none CSMO – other e.g. cataract, glaucoma |
Group 1: IVB Group 2: PRP Description of type of laser and delivery: Treated in two sessions, with a third session only if there was angiographic evidence of NV activity at month 4 Description of anti-VEGF treatment: 2.5 mg (0.1 ml) (Avastin, Genentech, San Francisco, CA, USA) were injected every 2 months during the study |
IVBPRPp-valueBCVA (logMAR)–0.14 ± 0.19–0.17 ± 0.10Difference to baselineIVB: 0.52PRP: 0.64Final CMT197 ± 17 µm243 ± 49 µm0.012NV leakage completely resolved4/5 eyes with PDR1/5 eyes with PDR0.11PDR developmentNone of 10 eyes with severe NPDRComplicationsnone2/10 eyes VH, 3/10 MO | IVB | PRP | p-value | BCVA (logMAR) | –0.14 ± 0.19 | –0.17 ± 0.10 | Difference to baseline IVB: 0.52 PRP: 0.64 |
Final CMT | 197 ± 17 µm | 243 ± 49 µm | 0.012 | NV leakage completely resolved | 4/5 eyes with PDR | 1/5 eyes with PDR | 0.11 | PDR development | None of 10 eyes with severe NPDR | Complications | none | 2/10 eyes VH, 3/10 MO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IVB | PRP | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BCVA (logMAR) | –0.14 ± 0.19 | –0.17 ± 0.10 | Difference to baseline IVB: 0.52 PRP: 0.64 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Final CMT | 197 ± 17 µm | 243 ± 49 µm | 0.012 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NV leakage completely resolved | 4/5 eyes with PDR | 1/5 eyes with PDR | 0.11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDR development | None of 10 eyes with severe NPDR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Complications | none | 2/10 eyes VH, 3/10 MO | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Filho 2011122 Aim: To evaluate the effects of PRP compared with PRP plus intravitreal injection of 0.5 mg of ranibizumab (IVR) in patients with HR-PDR Design: Prospective randomised trial Follow-up: 48 weeks Proportions completing the study: 72.5% (29/40) Reasons for withdrawal: PRPplus group: 2 patients died, lost to follow-up: 2 before week 16, 1 before week 32 (week 16: n = 17), (week 32: n = 16), (week 48: n = 15) PRP group: one patient excluded because of tractional retinal detachment, one patient excluded because of VH, two patients died, lost to follow-up: two patients before week 16 (week 16: n = 16), (week 32: n = 15), (week 48: n = 14) |
n: 40 patients enrolled (5 patients with one eye and 35 patients with PDR in both eyes), 29 followed up Inclusion criteria: HR-PDR according to ETDRS definition (classification symptoms given in text) Exclusion criteria: Prior laser treatment or vitrectomy, history of thromboembolic event including myocardial infarction or cerebral vascular accident; major surgery within the prior 6 months or planned within the next 28 days; uncontrolled HTN; known coagulation abnormalities or current use of anticoagulative medication other than aspirin; any condition affecting documentation Age (mean): PRP: 63.3 ± 2.5 PRPplus: 50.5 ± 3.0 (p = 0.0036) Sex (% female): PRP: 36% (5/14) PRPplus: 40% (6/15) Diabetes type: NR HbA1c (mean ± SD): PRP: 9.3 ± 1.2 PRPplus: 9.4 ± 0.8 Baseline VA: NR Baseline CMT: NR Baseline DR stage: HR-PDR Previous laser or intravitreal drug treatment: See Exclusion criteria Ocular comorbidities: See Follow-up and Exclusion criteria |
Group 1: PRP Group 2: PRP plus (PRP plus 0.5 mg IVR) Description of type of laser and delivery: Performed in two sessions (at week 0 and week 2) according to ETDRS guidelines. 600–800 500-µm spots were performed per session. CSMO treated with macular focal-grid laser at the time of the first PRP session. Patients could be retreated with focal laser or PRP at the 16- and 32-week study visits Description of anti-VEGF treatment: Intravitreal injection of 0.5 mg (0.05 ml) of ranibizumab was performed approximately 60 minutes after the completion of the first PRP session (at week 0) using 29-gauge needle inserted through the inferotemporal pars plana 3.0–3.5 mm posterior to the limbus using topical proparacaine drops under sterile conditions. Patients could be retreated with focal laser or ranibizumab at the 16- and 32-week study visits |
OutcomeWeekPRP (difference to baseline)PRPplus (difference to baseline)Between-groups p-value (Wilcoxon)FLA16–2.4 ± 0.4 (0.0003)–3.9 ± 0.4 (< 0.0001)0.054832–3.1 ± 1.0 (0.0067)–5.2 ± 0.6 (< 0.0001)0.088748–2.9 ± 1.3 (0.0083)–5.8 ± 0.7 (< 0.0001)0.0291BCVA160.06 ± 0.03 (0.0625)0.00 ± 0.01 (0.5000)0.1142320.08 ± 0.03 (0.0078)–0.01 ± 0.02 (0.5000)0.0216480.08 ± 0.03 (0.0078)0.00 ± 0.02 (0.5000)0.0243CSMT1634.4 ± 29.6 (0.0085)–14.2 ± 36.9 (0.0188)0.47063254.7 ± 36.8 (0.0025)–13.6 ± 41.8 (0.0820)0.18974818.1 ± 9.4 (0.0043)–14.7 ± 39.1 (0.0698)0.7106 All patients in the PRPplus group were retreated with IVR, and all patients in the PRP group received an additional 500-µm laser spots at weeks 16 and 32 No serious drug-related adverse events were observed in the 20 eyes (20 patients) treated with ranibizumab Minor local transient adverse events: Subconjunctival haemorrhage and foreign body sensation in 25% (5/20), 29.4% (5/17) and 31.25% (5/16) of patients at baseline, week 16 and week 32 |
Outcome | Week | PRP (difference to baseline) | PRPplus (difference to baseline) | Between-groups p-value (Wilcoxon) | FLA | 16 | –2.4 ± 0.4 (0.0003) | –3.9 ± 0.4 (< 0.0001) | 0.0548 | 32 | –3.1 ± 1.0 (0.0067) | –5.2 ± 0.6 (< 0.0001) | 0.0887 | 48 | –2.9 ± 1.3 (0.0083) | –5.8 ± 0.7 (< 0.0001) | 0.0291 | BCVA | 16 | 0.06 ± 0.03 (0.0625) | 0.00 ± 0.01 (0.5000) | 0.1142 | 32 | 0.08 ± 0.03 (0.0078) | –0.01 ± 0.02 (0.5000) | 0.0216 | 48 | 0.08 ± 0.03 (0.0078) | 0.00 ± 0.02 (0.5000) | 0.0243 | CSMT | 16 | 34.4 ± 29.6 (0.0085) | –14.2 ± 36.9 (0.0188) | 0.4706 | 32 | 54.7 ± 36.8 (0.0025) | –13.6 ± 41.8 (0.0820) | 0.1897 | 48 | 18.1 ± 9.4 (0.0043) | –14.7 ± 39.1 (0.0698) | 0.7106 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome | Week | PRP (difference to baseline) | PRPplus (difference to baseline) | Between-groups p-value (Wilcoxon) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FLA | 16 | –2.4 ± 0.4 (0.0003) | –3.9 ± 0.4 (< 0.0001) | 0.0548 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32 | –3.1 ± 1.0 (0.0067) | –5.2 ± 0.6 (< 0.0001) | 0.0887 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48 | –2.9 ± 1.3 (0.0083) | –5.8 ± 0.7 (< 0.0001) | 0.0291 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BCVA | 16 | 0.06 ± 0.03 (0.0625) | 0.00 ± 0.01 (0.5000) | 0.1142 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32 | 0.08 ± 0.03 (0.0078) | –0.01 ± 0.02 (0.5000) | 0.0216 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48 | 0.08 ± 0.03 (0.0078) | 0.00 ± 0.02 (0.5000) | 0.0243 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CSMT | 16 | 34.4 ± 29.6 (0.0085) | –14.2 ± 36.9 (0.0188) | 0.4706 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32 | 54.7 ± 36.8 (0.0025) | –13.6 ± 41.8 (0.0820) | 0.1897 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48 | 18.1 ± 9.4 (0.0043) | –14.7 ± 39.1 (0.0698) | 0.7106 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Lucena 2013123 (same study population as Filho 2011) Aim: To compare pain related to intravitreal injection and PRP in the management of patients with high-risk proliferative diabetic retinopathy Design: Randomised trial, open label Follow-up: 16 weeks Proportions completing the study: 76% (31/40), 17 patients from PRPplus, 14 from PRP were evaluated for pain scores Reasons for withdrawal: NR, see Filho 2011122 |
n: 40 patients enrolled (five patients with one eye and 35 patients with PDR in both eyes), 31 patients assessed Inclusion criteria: Patients with HR-PDR, according to ETDRS (classification symptoms given in text) Exclusion criteria: Prior laser treatment or vitrectomy in the study eye; history of thromboembolic event; major surgery within the prior 6 months or planned within the next 28 days; uncontrolled HTN; known coagulation abnormalities or current use of anticoagulation medication other than aspirin; any condition affecting documentation or follow-up Age, mean ± SD (years): PRP: 63.5 ± 8.9 PRPplus: 51.1 ± 11.3 (p = 0.0018) Sex (% female): PRP: 36% (5/14) PRPplus: 41% (7/17) Diabetes type: NR HbA1c mean ± SD (%): PRP: 9.3 ± 1.1 PRPplus: 9.1 ± 0.8 (p = 0.5391) Baseline VA: NR Baseline CMT: NR Baseline DR stage: HR-PDR Previous laser or intravitreal drug treatment: See exclusion criteria Ocular comorbidities: See exclusion criteria |
Group 1: PRP Group 2: PRP plus IVR Description of type of laser and delivery: PRP was performed in two sessions (at week 0 and week 2) according to ETDRS guidelines. 600–800 500-µm spots were performed per session, at the discretion of the treating investigator. If patients had CSMO) macular focal/grid laser was performed during first PRP session. Patients could be retreated with focal/grid laser at the 16- and 32-week study visits. If both eyes were eligible for the study, the eye with best VA was included Description of anti-VEGF treatment: Intravitreal injection of 0.5 mg (0.05 ml) of ranibizumab was performed approximately 60 minutes after the completion of the first PRP session (at week 0) using 29-gauge needle inserted through the inferotemporal pars plana 3.0–3.5 mm posterior to the limbus using topical proparacaine drops under sterile conditions. Patients could be retreated with focal laser or ranibizumab at the 16- and 32-week study visits |
PRP plusPRPp-valueMean pain (±SEM)4.7 ± 2.1 (12/17 patients pain score = 0)60.8 ± 7.8 (minimal score for one patient = 10.5)< 0.0001 | PRP plus | PRP | p-value | Mean pain (±SEM) | 4.7 ± 2.1 (12/17 patients pain score = 0) | 60.8 ± 7.8 (minimal score for one patient = 10.5) | < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PRP plus | PRP | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean pain (±SEM) | 4.7 ± 2.1 (12/17 patients pain score = 0) | 60.8 ± 7.8 (minimal score for one patient = 10.5) | < 0.0001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Mirshahi 2008124 Aim: To evaluate the additional therapeutic effect of a single intravitreal injection of bevacizumab (Avastin) on standard laser treatment in HRC. PDR patients in terms of regression of retinal neovascularisation Design: A prospective, fellow-eye sham controlled clinical trial. Fellow eyes of each case were randomly assigned to receive Avastin or sham Follow-up: 16 weeks Proportions completing the study: 100% Reasons for withdrawal: n/a |
n: 80 eyes – 40 bilateral HRC-PDR patients (Avastin: 40 eyes; sham: 40 eyes) Inclusion criteria: Patients with bilateral HRC-PDR type 2 diabetes according to DRS criteria (criteria given in text) with or without MO Exclusion criteria: Patients with uncontrolled HTN, recent (in the past 6 months) myocardial infarction or cerebrovascular accident, uncontrolled glaucoma, a history of any kind of retinal photocoagulation, or a diagnosis of tractional retinal detachment Age (mean): 52 years (range: 39–68) Sex (female%): 70 (28/40) Diabetes type: Type 2 HbA1c (mean): 8.4% Baseline VA: NR Baseline CMT: NR Baseline DR stage: HR-PDR Previous laser or intravitreal drug treatment: See Exclusion criteria Ocular comorbidities: 26 cases with CSMO that patients received MPC for |
Group 1: Standard laser treatment and focal or grid for CSMO + bevacizumab (Avastatin) (single injection 1.25 mg) Group 2: Standard laser treatment and focal or grid for CSMO + sham Description of type of laser and delivery: Treatment according to ETDRS protocol, i.e. PRP (1200–1500 spots, 200-ms duration, ½ spot size apart) and focal or grid MPC for CSMO. PRP was completed in three sessions, 1 week apart Description of anti-VEGF treatment: 1.25 mg (0.05 cubic centimetres) bevacizumab (Genentech Inc.) on the first session of their laser treatment (following laser therapy). Under topical anaesthesia observing sterile conditions. First we did an anterior chamber paracentesis and then intravitreal injection was carried out through supratemporal pars plana Sham treatment: Fellow eye injection was mimicked with a needleless syringe |
Regression: GroupsWeek 6 follow-upWeek 16 follow-upComplete regression, n (%)Partial regression, n (%)None, n (%)Complete regression, n (%)Partial regression, n (%)None, n (%)Avastin (n = 40)35 (87.5)5 (12.5)010 (25)28 (70)2 (5)Sham (n = 40)10 (25)26 (65)4 (10)10 (25)26 (65)4 (10) Difference between groups at 6 weeks (p < 0.005) No clinical evidence of post injection uveitis, endophthalmitis, haemorrhage, and/or change in lens status was observed in follow-ups, no arterial embolic event In the subgroup of Avastin-treated cases, recurrence of PDR in univariate analysis was associated with HTN, female gender, and higher HbA1c levels, but in logistic regression analysis, only HbA1c maintained a significant relationship |
Groups | Week 6 follow-up | Week 16 follow-up | Complete regression, n (%) | Partial regression, n (%) | None, n (%) | Complete regression, n (%) | Partial regression, n (%) | None, n (%) | Avastin (n = 40) | 35 (87.5) | 5 (12.5) | 0 | 10 (25) | 28 (70) | 2 (5) | Sham (n = 40) | 10 (25) | 26 (65) | 4 (10) | 10 (25) | 26 (65) | 4 (10) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Groups | Week 6 follow-up | Week 16 follow-up | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Complete regression, n (%) | Partial regression, n (%) | None, n (%) | Complete regression, n (%) | Partial regression, n (%) | None, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Avastin (n = 40) | 35 (87.5) | 5 (12.5) | 0 | 10 (25) | 28 (70) | 2 (5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sham (n = 40) | 10 (25) | 26 (65) | 4 (10) | 10 (25) | 26 (65) | 4 (10) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Preti 2013125 Aim: To compare the efficacy of therapy with PRP and IVB injections vs. PRP alone in patients with HR-PDR (HR-PDR) Design: RCT. Patients who met the inclusion criteria had the first eye randomised to the SG that received PRP with IVB injections and the second eye assigned to the CG, which received only PRP Follow-up: 6 months Proportions completing the study: 83% (35/42) Reasons for withdrawal: Development of VH |
n: 84 eyes of 42 patients – 7 patients later removed from study owing to VH, 35 patients (70 eyes) assessed Inclusion criteria: ≥ 18 years, HR-PDR (HR-PDR) in both eyes with or without DMO and BCVA ≥ 20/200 Exclusion criteria: Pretreatment for DR (laser, intraocular medication, surgery), pre-retinal and VH, changes in the vitreous–retinal interface, external eye infection, prior thromboembolic events, SBPs and DBPs of ≥ 180 and 110 mmHg, A1c levels exceeding 15%, chronic renal failure, major surgery within 1 month, previous treatment with antivascular agents Age (mean): 56 years (range 43–73) Sex (% female): 40 (14/35) Diabetes type: Type 2 HbA1c (mean%): 9.1 Baseline VA (mean BCVA in logMAR): SG group: 0.38 (range –0.01 to 1.00) CG group: 0.24 (range –0.02 to 0.88) Baseline CMT (mean foveal thickness): SG: 295 µm (range 179–600) CG: 305.5 µm (range 171–578) p = 0.82 Baseline macular volume (mm3): SG: 8582.49 (range 6016–13,880) CG: 8730.11 (range 6470–14,189) p = 0.87 Baseline DR stage: HR-PDR Previous laser or intravitreal drug treatment: See Exclusion criteria Ocular comorbidities: 37.2% (n = 13) had DMO in one eyes and 32.4% (n = 12) had DMO in both eyes |
Group 1: PRP + IVB (SG) Group 2: PRP group (CG) Description of type of laser and delivery: Patients underwent 3 episodes (weekly for 3 weeks) of PRP in both eyes according to ETDRS methods using double-frequency Nd:YAG laser (523 nm Alcon, Opthalas 432 EyeLite Laser Photocoagulator, CA, USA) through the 160 Mainster OMRA-PRP lens (Volk Corp Mentor, OH, USA) 300–500 shots per episode, 500-µm-sized burns, exposure time 0.1–0.2 ms and moderate intensity (200–500 mW). If concomitant DMO existed it was treated at the time of PRP Description of anti-VEGF treatment: two IVB injections (1.25 mg/0.05 ml), PRP initiated 1 week after injection, second injection administered after third PRP session, plus 5% topical povidone |
Comparison between CG and SG: CGSGp-valueVA––No statistically significant difference between CG and SGFoveal thickness (μm)1 month335.86 ± 145.92276.66 ± 81.300.0133 months316.54 ± 137.70296.49 ± 124.230.286 months364.83 ± 196.01328.17 ± 150.580.45Macular volume (μm3)1 month9347.09 ± 2095.608663.60 ± 1301.240.0333 months9211.97 ± 2030.178617.60 ± 1726.200.0066 months9574 ± 2751.889033.06 ± 1991.390.11Patients with DMO in both eyesVANo statistically significant difference at any time pointFoveal thickness (μm)1 month451.60 ± 145.38341.33 ± 73.990.0473 months400.67 ± 142.05394.92 ± 133.480.606 months480.17 ± 224.10395.17 ± 160.540.32Macular volume (mm3)No statistically significant difference in macular volume at any follow-up period Comparison of CG to baseline and SG to baseline: CGSGp-valueBCVA (reduction relative to baseline)1 month0.31 ± 0.25vs. baseline – CG = 0.001; SG, no statistically significant difference3 months0.30 ± 0.24vs. baseline – CG = 0.01; SG, no statistically significant difference6 months0.34 ± 0.29vs. baseline – CG = 0.02; SG, no statistically significant differenceFoveal thickness (μm) (increase relative to baseline)1 month335.88 ± 145.92vs. baseline – CG = 0.001; SG, no statistically significant difference3 months316.54 ± 137.71vs. baseline – CG = 0.143; SG, no statistically significant difference6 months364.83 ± 196.01vs. baseline – CG = 0.042; SG, no statistically significant differenceMacular volume (increase relative to baseline)1 month9347.10 ± 2095.60vs. baseline – CG < 0.001; SG, no statistically significant difference3 months9211.97 ± 2030.17vs. baseline – CG = 0.001; SG, no statistically significant difference6 months9574.00 ± 2751.88vs. baseline – CG = 0.003; SG, no statistically significant differencePatients with DMO in both eyesBCVABaseline0.24 ± 0176 months0.42 ± 0.31Difference in CG from baseline to 6 months; p = 0.033SG – no statistically significant differences None of the patients experienced complications such as ocular HTN, lens opacity progression, anterior chamber reaction, arterial thromboembolic events |
CG | SG | p-value | VA | – | – | No statistically significant difference between CG and SG | Foveal thickness (μm) | 1 month | 335.86 ± 145.92 | 276.66 ± 81.30 | 0.013 | 3 months | 316.54 ± 137.70 | 296.49 ± 124.23 | 0.28 | 6 months | 364.83 ± 196.01 | 328.17 ± 150.58 | 0.45 | Macular volume (μm3) | 1 month | 9347.09 ± 2095.60 | 8663.60 ± 1301.24 | 0.033 | 3 months | 9211.97 ± 2030.17 | 8617.60 ± 1726.20 | 0.006 | 6 months | 9574 ± 2751.88 | 9033.06 ± 1991.39 | 0.11 | Patients with DMO in both eyes | VA | No statistically significant difference at any time point | Foveal thickness (μm) | 1 month | 451.60 ± 145.38 | 341.33 ± 73.99 | 0.047 | 3 months | 400.67 ± 142.05 | 394.92 ± 133.48 | 0.60 | 6 months | 480.17 ± 224.10 | 395.17 ± 160.54 | 0.32 | Macular volume (mm3) | No statistically significant difference in macular volume at any follow-up period | CG | SG | p-value | BCVA (reduction relative to baseline) | 1 month | 0.31 ± 0.25 | vs. baseline – CG = 0.001; SG, no statistically significant difference | 3 months | 0.30 ± 0.24 | vs. baseline – CG = 0.01; SG, no statistically significant difference | 6 months | 0.34 ± 0.29 | vs. baseline – CG = 0.02; SG, no statistically significant difference | Foveal thickness (μm) (increase relative to baseline) | 1 month | 335.88 ± 145.92 | vs. baseline – CG = 0.001; SG, no statistically significant difference | 3 months | 316.54 ± 137.71 | vs. baseline – CG = 0.143; SG, no statistically significant difference | 6 months | 364.83 ± 196.01 | vs. baseline – CG = 0.042; SG, no statistically significant difference | Macular volume (increase relative to baseline) | 1 month | 9347.10 ± 2095.60 | vs. baseline – CG < 0.001; SG, no statistically significant difference | 3 months | 9211.97 ± 2030.17 | vs. baseline – CG = 0.001; SG, no statistically significant difference | 6 months | 9574.00 ± 2751.88 | vs. baseline – CG = 0.003; SG, no statistically significant difference | Patients with DMO in both eyes | BCVA | Baseline | 0.24 ± 017 | 6 months | 0.42 ± 0.31 | Difference in CG from baseline to 6 months; p = 0.033 SG – no statistically significant differences |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CG | SG | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VA | – | – | No statistically significant difference between CG and SG | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Foveal thickness (μm) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 335.86 ± 145.92 | 276.66 ± 81.30 | 0.013 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months | 316.54 ± 137.70 | 296.49 ± 124.23 | 0.28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 364.83 ± 196.01 | 328.17 ± 150.58 | 0.45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Macular volume (μm3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 9347.09 ± 2095.60 | 8663.60 ± 1301.24 | 0.033 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months | 9211.97 ± 2030.17 | 8617.60 ± 1726.20 | 0.006 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 9574 ± 2751.88 | 9033.06 ± 1991.39 | 0.11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients with DMO in both eyes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VA | No statistically significant difference at any time point | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Foveal thickness (μm) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 451.60 ± 145.38 | 341.33 ± 73.99 | 0.047 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months | 400.67 ± 142.05 | 394.92 ± 133.48 | 0.60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 480.17 ± 224.10 | 395.17 ± 160.54 | 0.32 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Macular volume (mm3) | No statistically significant difference in macular volume at any follow-up period | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CG | SG | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BCVA (reduction relative to baseline) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 0.31 ± 0.25 | vs. baseline – CG = 0.001; SG, no statistically significant difference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months | 0.30 ± 0.24 | vs. baseline – CG = 0.01; SG, no statistically significant difference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 0.34 ± 0.29 | vs. baseline – CG = 0.02; SG, no statistically significant difference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Foveal thickness (μm) (increase relative to baseline) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 335.88 ± 145.92 | vs. baseline – CG = 0.001; SG, no statistically significant difference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months | 316.54 ± 137.71 | vs. baseline – CG = 0.143; SG, no statistically significant difference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 364.83 ± 196.01 | vs. baseline – CG = 0.042; SG, no statistically significant difference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Macular volume (increase relative to baseline) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 9347.10 ± 2095.60 | vs. baseline – CG < 0.001; SG, no statistically significant difference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months | 9211.97 ± 2030.17 | vs. baseline – CG = 0.001; SG, no statistically significant difference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 9574.00 ± 2751.88 | vs. baseline – CG = 0.003; SG, no statistically significant difference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Patients with DMO in both eyes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BCVA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 0.24 ± 017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 0.42 ± 0.31 | Difference in CG from baseline to 6 months; p = 0.033 SG – no statistically significant differences |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Tonello 2008126 (same SG as Filho and Lucena) Aim: To evaluate the effects of PRP compared with PRP plus IVB on BCVA and total area of FLA from active new vessels (NVs) in patients with HR-PDR Design: RCT, patients randomly assigned to receive PRP (PRP group) or PRP plus Follow-up: 16 weeks Proportions completing the study: 100% Reasons for withdrawal: (Two patients presented with conditions that might have affected documentation or follow-up and were excluded) |
n: 22 patients, 30 eyes, HR-PDR was identified in one eye of 14 patients and in both eyes of 8 patients (eye with worse VA was selected to receive PRP + IVB) Inclusion criteria: Patients were included if they had HR-PDR, which was defined according to the guidelines by the ETDRS (criteria given in text) Exclusion criteria: History of prior laser treatment or vitrectomy in the study eye; history of any thromboembolic event; major surgery within the prior 6 months or planned within the next 28 days; uncontrolled HTN; known coagulation abnormalities or current use of anticoagulative medication other than aspirin, any condition affecting documentation or follow-up Age (mean): PRPplus: 54.06 years ± 11.74 PRP: 51.4 years ± 10.65 Sex (%female): PRPplus: 26.7 (4/15) PRP: 46.6 (7/15) Diabetes type: NR HbA1c (mean%): PRPplus: 9.56 ± 1.50 PRP: 9.67 ± 1.49 Baseline VA (BCVA logMAR): PRPplus: 0.26 (20/40) PRP: 0.26 (20/40) Baseline CMT: NR Baseline DR stage: HR-PDR Previous laser or intravitreal drug treatment: See Exclusion criteria Ocular comorbidities: Nuclear sclerosis; three eyes in the PRP group, two eyes in the PRPplus group, some degree of macular non-perfusion (type 1–30) three eyes in the PRP group, four eyes in the PRPplus group |
Group 1: PRP Group 2: PRP plus IVB Description of type of laser and delivery: PRP performed at two time points – weeks 1 and 3 according to ETDRS guidelines), 600–800 500-µm spots per episode Description of anti-VEGF treatment: Intravitreal injection of 1.5 mg (0.06 ml) bevacizumab was administered about 60 minutes after the completion of the second PRP session (week 3) injected into the vitreous cavity via a 29.5-gauge needle inserted through the inferotemporal pars plana 3.0–3.5 mm posterior to the limbus using topical proparacaine drops under sterile conditions |
Study periodPRPplusPRPBCVA (logMAR ± SEM)Active NVs (mm2 ± SEM)BCVA (logMAR ± SEM)Active NVs (mm2 ± SEM)Baseline0.26 ± 0.0711.15 ± 2.240.26 ± 0.0515.31 ± 4.81Week 40.28 ± 0.050.62 ± 0.530.27 ± 0.0714.73 ± 4.82Week 90.29 ± 0.040.67 ± 0.250.31 ± 0.0614.26 ± 4.57Week 160.29 ± 0.044.46 ± 1.090.31 ± 0.0613.58 ± 4.29 No serious drug-related adverse events were observed in the 15 eyes (15 patients) treated with bevacizumab No significant changes in lens status or IOP were observed in any of the 15 injected eyes during the 16-week follow-up period Minor local transient adverse events related to the treatment procedure, such as subconjunctival haemorrhage and foreign body sensation, were reported in seven (47%) and two (13.5%) patients, respectively |
Study period | PRPplus | PRP | BCVA (logMAR ± SEM) | Active NVs (mm2 ± SEM) | BCVA (logMAR ± SEM) | Active NVs (mm2 ± SEM) | Baseline | 0.26 ± 0.07 | 11.15 ± 2.24 | 0.26 ± 0.05 | 15.31 ± 4.81 | Week 4 | 0.28 ± 0.05 | 0.62 ± 0.53 | 0.27 ± 0.07 | 14.73 ± 4.82 | Week 9 | 0.29 ± 0.04 | 0.67 ± 0.25 | 0.31 ± 0.06 | 14.26 ± 4.57 | Week 16 | 0.29 ± 0.04 | 4.46 ± 1.09 | 0.31 ± 0.06 | 13.58 ± 4.29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study period | PRPplus | PRP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BCVA (logMAR ± SEM) | Active NVs (mm2 ± SEM) | BCVA (logMAR ± SEM) | Active NVs (mm2 ± SEM) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 0.26 ± 0.07 | 11.15 ± 2.24 | 0.26 ± 0.05 | 15.31 ± 4.81 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Week 4 | 0.28 ± 0.05 | 0.62 ± 0.53 | 0.27 ± 0.07 | 14.73 ± 4.82 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Week 9 | 0.29 ± 0.04 | 0.67 ± 0.25 | 0.31 ± 0.06 | 14.26 ± 4.57 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Week 16 | 0.29 ± 0.04 | 4.46 ± 1.09 | 0.31 ± 0.06 | 13.58 ± 4.29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Laser in combination with anti-vascular endothelial growth factors
Cho 2010
The aim of the study119 was to assess and compare the efficacy and safety of IVTA and intravitreal bevacizumab (IVB) for reducing PRP-related short-term vision loss and MO, in patients ranging from having very severe NPDR to HR-PDR. One group of patients received only PRP (30 eyes), the second group received IVB plus PRP (31) and the third group received IVTA plus PRP (30). Scatter laser was given to all patients at three different time points, with 1-week intervals using a 532-nm argon green laser (Visulas 532s/LSL). Each patient received between 300 and 500 spots (500-µm spot) per episode at a power that caused blanching of the retina. If both eyes had HR-PDR, PRP was delivered in each eye on the same day. In eyes with CSMO, focal/grid photocoagulation was also performed at the time of initiation of PRP. In those receiving PRP and IVB, bevacizumab 1.25 mg/0.05 ml was injected about 1 week before the initiation of PRP. In the group receiving PRP and IVTA, triamcinolone 4 mg/0.1 ml was injected 1 day after the first session of PRP. All patients were followed up for 3 months.
The primary outcome measure was mean BCVA, which worsened significantly in the PRP group at both 1 (0.26 to 0.29 logMAR; p = 0.031) and 3 months (0.26–0.29; p = 0.030) follow-up. (The logMAR scale ranges from 0 to 1.0, with 0 best.) In contrast, there was no significant change in BCVA in the IVTA and IVB groups. In eyes with CSMO, there was significant improvement in BCVA during 3 months’ follow-up only in the IVTA group. In eyes without CSMO, vision significantly worsened only in the PRP group.
The secondary outcome was gain in vision of ≥ 0.1 logMAR. In eyes with CSMO, significantly more eyes in the IVTA group (75%) than in the IVB (38%) and PRP (7%) groups achieved this. Similarly, more eyes in the IVTA group (38%) than in the PRP group (none) gained vision of ≥ 0.2 logMAR (< 0.05). More eyes in the PRP group lost vision of ≥ 0.1 (57%) or ≥ 0.2 logMAR (29%) than in the IVTA group (none). The corresponding figures for the bevacizumab group were 31% and 13% (all rounded to whole figures).
In eyes without CSMO, significantly higher proportions of eyes in the IVTA (43%) and IVB (35%) groups gained ≥ 0.1 logMAR than in the PRP group (6%). However, the proportion of eyes gaining vision of ≥ 0.2 logMAR was not statistically different between IVTA and IVB groups. The proportion of eyes losing vision of ≥ 0.2 logMAR was significantly higher in the PRP group (38%) than in the other two groups (none) (p < 0.05).
Patients in the IVTA and IVB groups had significant reduction in CMT in eyes with CSMO. In eyes without CSMO, CMT increased significantly only in the PRP group.
Four patients in the PRP group had progression of PDR, including vitreous haemorrhage, compared with none in the IVTA and IVB groups. In the IVTA group one eye had cataract progression and four eyes had increased IOP compared with none in the IVB group.
The authors conclude that both IVTA and IVB may be effective adjunctive treatments to PRP, minimising the risk of PRP-induced MO and visual loss. However, the study119 was short term.
The Diabetic Retinopathy Clinical Research Network (2011)
This was a high-quality study. 120 The aim of the study120 was to compare the efficacy of intravitreal injection of ranibizumab (Lucentis) or triamcinolone acetonide (TA) (Trivaris) in participants aged 18 years or more with severe NDPR (according to investigator assessment: severe NPDR in 18% of study eyes; according to the reading centre assessment: moderately severe NPDR or less severe NPDR in 20% of study eyes and severe NPDR in 5% of eyes) or PDR (according to investigator assessment, PDR in 82% of study eyes; according to the reading centre assessment: PDR in 75% of study eyes and out of this 35% had HR-PDR or level 71 or 75) and DMO treated with focal or grid laser. Those participants with previous history of extensive treatment with laser were excluded, as were participants with previous treatment for DMO, glaucoma, and increased IOP due to steroids and, lastly, those with IOP more than 25 mmHg. Patients were recruited from March 2007 to June 2009.
Initially, only one eye of each participant was included in the study. 120 Eyes were randomised to three different interventions. One group received sham injection (pressing a needleless syringe against the conjunctiva) at baseline and at 4 weeks, another group received intravitreal injection of 0.5 mg ranibizumab at baseline and 4 weeks and the third group received intravitreal injection of 4 mg TA at baseline and sham injection at 4 weeks. A later amendment to the protocol was made to include both eyes of the participant. During randomisation, patients could then receive one of three different treatment strategies. The first strategy involved injecting the better-seeing eye (BSE) with sham and injecting the worse-seeing eye (WSE) with ranibizumab or triamcinolone. The second strategy involved injection of ranibizumab or triamcinolone in the BSE and sham injection in the WSE. The third group included those patients with the same VA in both eyes. In this group, the right eye was categorised as the BSE and the other eye as the WSE.
Following randomisation, at baseline patients either received sham or intravitreal injection of ranibizumab or triamcinolone. All patients then received focal or grid laser for DMO between 3 and 10 days after baseline treatment. After this, patients could receive the first session of PRP immediately, or on the next day, but no later than 14 days after the baseline treatment. PRP included giving 1200–1600 burns in one session or in up to three sessions. All the sessions of PRP had to be completed within 49 days from the day of allocation. If patients lost vision by 10 or more letters following PRP due to worsening of MO then additional PRP treatment was postponed by 2 weeks or until the risk of MO reduced. The primary outcome measure was change in VA from baseline to 14 weeks. After this period, patients could receive additional treatment for their MO and retinopathy but only as part of their standard treatment. Hence, participants would not receive intravitreal injection of anti-VEGFs, steroids or focal/grid laser. Safety outcomes were measured at 56 weeks.
The 14-week visit was completed by 96% (118 eyes), 91% (103 eyes) and 96% (105 eyes) of eyes in the sham, ranibizumab and triamcinolone groups, respectively. The corresponding figures for 56 weeks completion rate were 90% (11 eyes), 84% (95 eyes) and 85% (93 eyes), respectively. Adherence to allocated therapies was very good.
During the 14 weeks’ period, none of the eyes required additional treatment for their DMO. All eyes completed their PRP sessions except one eye in the sham, two eyes in the ranibizumab and two eyes in the triamcinolone arms. Slightly more eyes in the sham group completed PRP session in one sitting: 49 eyes (40%) in the sham, 38 eyes (34%) in the ranibizumab and 41 eyes (38%) in the triamcinolone. There were no significant differences in the number of eyes requiring additional PRP: ranibizumab arm (21 eyes or 19%) than in the triamcinolone (24 eyes or 23%) and sham (29 eyes or 24%).
The number of eyes requiring additional treatment for DMO (part of standard care) was significantly lower in the ranibizumab (44%; p = 0.004) and triamcinolone (42%; p = 0.004) arms than in the sham arm (59%).
At 14 weeks, the mean change in VA from baseline was +1 ± 11 in the ranibizumab arm, + 2 ± 11 in the triamcinolone arm and –4 ± 14 in the sham arm. The difference was statistically significant in both ranibizumab (p < 0.001) and triamcinolone arm (p < 0.001) compared with the sham arm. The proportion of patients gaining ≥ 15 letters was 10% in the triamcinolone arm, 7% in the ranibizumab arm and 7% in the sham arm. The proportion of patients gaining 10–14 letters was similar in the triamcinolone and the ranibizumab arm (12%) but only 4% in the sham arm. The proportion of patients losing letters was greater in the sham arm than in both ranibizumab and triamcinolone arms. The authors also reported changes in VA according to different subgroups – previous treatment for DMO, baseline VA (≥ 66 or ≤ 65), baseline central subfield thickness (< 400 or ≥ 400), DR (NPDR or PDR), diffuse vs. focal oedema, number of PRP sittings (one or multiple), PRP automated pattern use and baseline HbA1c levels (< 8 or ≥ 8%). In all subgroups, there was improvement (p-values not given) in vision in both triamcinolone and ranibizumab compared with the sham arm.
At 56 weeks, mean changes in VA letter score were similar: –6 (SD 17), –4 (SD 21) and –5 (SD 16) in the sham, ranibizumab and triamcinolone arms, respectively. During the follow-up period from baseline to week 14, there was one case of endophthalmitis, in the ranibizumab arm. There were no ocular vascular events. More eyes in the sham arm developed retinal detachment than in the other groups (n = 4, 3% vs. n = 1, 1% in ranibizumab and triamcinolone). Only one eye had vitrectomy in the sham arm. More eyes in the sham arm (n = 16 or 12%) had vitreous haemorrhage than in the ranibizumab (n = 6 or 5%) or triamcinolone (n = 7 or 6%) arm. More eyes (n = 20 or 17%) in the triamcinolone arm developed increased IOP of ≥ 10 mmHg from baseline than in the sham arm (n = 3 or 2%), and two developed IOP ≥ 30 mmHg. No eyes in the ranibizumab arm had increment of IOP ≥ 10 mmHg. No eyes had surgery for glaucoma or cataract.
Between 14 weeks and 56 weeks, there were slightly more cases of retinal detachment in the ranibizumab group (n = 5 or 5%) followed by sham (n = 4 or 3%) and triamcinolone (n = 1 or 1%). More eyes in the sham arm (n = 17 or 13%) had vitrectomy than in the ranibizumab (n = 8 or 7%) or triamcinolone arm (n = 7 or 6%). The number of eyes with vitreous haemorrhage was similar in the sham (n = 28 or 21%) and in the ranibizumab arm (n = 25 or 23%) but lower in the triamcinolone arm (n = 20 or 18%). Slightly more eyes in the triamcinolone arm had increments of IOP of ≥ 10 mmHg from baseline than the other two arms (9% vs. 5% rani vs. 5% sham). The number of eyes with an increment of IOP of ≥ 30 mmHg was similar in all groups (≈4%). Significantly more eyes in the triamcinolone group (15%) received IOP-lowering medication at any visit after the 14-week visit than in the sham (5%) or ranibizumab group (5%). Similarly, more eyes in the triamcinolone arm received IOP lowering medication at 56 weeks visits than in other groups (9% vs. 2% sham vs. 4% ranibizumab). One eye each in the ranibizumab and triamcinolone groups had glaucoma surgery. Slightly more eyes receiving triamcinolone had cataract surgery (6% vs. 2% sham vs. 3% ranibizumab).
There was no significant difference amongst the groups in vascular events: 4 (4%) patients in the sham arm, 8 (7%) patients in the ranibizumab and 4 (3%) in the triamcinolone arm. No vascular events in the ranibizumab arm occurred at less than 3 weeks after the injection.
The authors concluded that both ranibizumab and triamcinolone are efficacious and safe in patients receiving focal/grid laser and PRP.
Ernst 2012
The aim of this paired-eye pilot study121 was to investigate the efficacy of IVB alone in patients with type 2 diabetes with PDR (five patients) and severe NPDR (five patients), without MO, and naive to PRP. Patients were followed up every 2 months for 12 months. One eye of each patient had 2.5 mg (0.1 ml) of bevacizumab injected every 2 months while the other eye received PRP in two sessions. At month 4, if there was evidence of NV activity, then patients also received a third session of PRP.
In both groups, there was no significant change in mean BCVA (logMAR) from baseline [IVB –0.12 (SD 0.22) to –0.14 (SD 0.19); p = 0.52. PRP –0.14 (SD 0.23) to –0.17 (SD 0.10); p = 0.64]. Patients in the IVB group had significantly lower mean CMT than those in the PRP group [197 (SD 17 µm) vs. 243 (SD 49 µm); p = 0.012]. None of the eyes with severe NPDR developed PDR. NV leakage completely resolved in 4/5 eyes with PDR in the IVB group and 1/5 eyes with PDR in the PRP group. However, the difference between the two was not statistically significant (p = 0.11). No complications were reported in the IVB group but 2/10 eyes and 3/10 eyes in the PRP group developed vitreous haemorrhage and MO, respectively.
The authors concluded that IVB was effective in PDR and severe NPDR but that a larger study was needed to confirm this finding.
Filho 2011
The aim of the study122,123 was to compare the effects of PRP against PRP plus intravitreal injection of 0.5 mg ranibizumab (IVR) in patients with HR-PDR. Of the initial 40 recruits, 29 were followed up for 48 weeks. The lasers were performed in two sessions, at week 0 and week 2. In each session, around 600–800 spots, of 500-µm size, were performed. In eyes with CSMO, focal/grid laser was given at the time of initiation of the first PRP session. During FA, if active new vessels were seen then patients were allowed to be retreated with 500 spots (500-µm size) per quadrant of active new vessels. At the 16 and 32 weeks study visits, if CSMO was present, patients were retreated with focal/grid laser if more spots were possible. Ranibizumab 0.5 mg (0.05 ml) was given approximately 60 minutes after the first PRP session. At the 16- and 32-weeks study visits, patients in the IVR groups could be re-treated with ranibizumab if active new vessels were seen.
The primary outcome was total area (mm3) of fluorescein leakage (FLA) from active NV but BCVA was also measured. At 48 weeks, the FLA reduction was significantly greater in the PRP group than in the PRP-plus-IVR group [–5.8 (SD 0.7) vs. –2.9 (SD 1.3); p = 0.0291] but the reduction was not statistically significant different at the 16 weeks (0.054) and 32 weeks (0.08) visits.
There was a reduction of BCVA of 0.6–0.08 logMAR compared with baseline in the PRP group at all three visits, but no change in the PRP plus group at any time points. The difference between the two groups was significant at 32 (0.021) and 48 weeks (0.024) study visits. CSMT increased by 20% from baseline in the PRP group but fell by 5% in the PRP-plus-IVR group. The difference between the two groups was not statistically significant.
There were no reports of serious drug-related adverse events (uveitis, endophthalmitis or change in lens or IOP) in the 20 eyes treated with IVR. There were reports of minor local transient (resolved within 1 week) adverse events related to procedures – subconjunctival haemorrhage and foreign body sensation in 5/20 (5%), 5/17 (29.4%) and 5/16 (31.25%) at week 0, 16 and 32, respectively.
A separate paper (Lucena 2013123) reported the results for pain. Pain score in each patient was measured using a 100-degree VAS. The mean pain score was significantly lower in the PRP plus group than in the PRP group [4.7 standard error of mean (SEM) 2.1 vs. 60.8 SEM 7.8; p < 0.0001]. The intensity of pain was also measured. In the PRP plus group, the intensity of pain score was 0 (meaning no pain at all during intravitreal injection) in 12/17 patients, whereas the pain intensity score in the PRP group was comparatively high. In the PRP group, only one patient had pain intensity score of 10.5, whereas in the remaining patients, the score was more than 30.The difference between the two groups was statistically significant (p < 0.0001).
The authors concluded that adding IVR after PRP appeared to protect against the modest VA loss and macular swelling observed in eyes treated with PRP alone.
Mirshahi 2008
The aim of the study124 was to find out additional benefits of a single intravitreal injection of bevacizumab on standard laser treatment in 40 patients with bilateral HR-PDR. Patients were followed up for 16 weeks. The fellow eye of each patient was randomly assigned to receive IVB or a sham injection. All patients received standard PRP, with 1200–1500 spots performed about half-spot size apart, 200 ms in duration and completed in three sessions, 1 week apart. In eyes with CSMO, focal or grid macular photocoagulation (MPC) was performed (not clear when but presumably at the initiation of the first PRP session). The PRP-plus-IVB group received intravitreal injection of 1.25 mg (0.05 cc) of bevacizumab following the first PRP session. The PRP-plus-sham group had a needleless syringe pressed against the eye (also after the PRP session). If required, additional PRP was performed. The primary outcome was regression, defined as complete (no leakage at 2-minute image), partial (decrease of leakage at 2-minute image compared with baseline image) and none. At 6 weeks, a significantly higher proportion of patients in the IVB group than in the sham group (87.5% vs. 25%; p < 0.005) showed complete regression on angiography, and all showed some regression. However, at 16 weeks the proportion of patients achieving complete regression was similar in both groups (25%). The proportion of patients showing partial regression was slightly greater in the IVB group than in the sham group at 16 weeks (70% vs. 65%). Only about 5% of patients in the IVB group showed no regression, whereas the corresponding figure for sham group was 10%.
There were no reports of post-injection uveitis, endophthalmitis or haemorrhage, and no change in lens status or arterial embolic events. Univariate analysis of subgroup of IVB-treated patients showed that recurrence of PDR was associated with HTN and patient characteristics such as female gender and high HbA1c levels. However, in logistic regression, only HbA1c level was found to be a significant factor.
The authors concluded that a single injection of IVB considerably but temporarily improved the short-term response to scatter PRP in HR-PDR, but the effect was short-lived, as many of the eyes showed rapid recurrence.
Preti 2013
The aim of the study125 was to compare the efficacy of PRP used on its own (control group) or in combination with IVB (study group) in 35 patients (70 eyes) with HR-PDR. Patients were followed up for 6 months. One eye was randomised to the study group and the fellow eye to the control group. All patients received three episodes (1 week apart) of PRP using double-frequency Nd:YAG laser with 300–500 shots per episode, a burn of 500 µm in size, exposure time of 0.1–0.2 seconds and in moderate intensity (200–500 mW). If patient had DMO, then it was treated at the time of initiation of PRP. The study group also received two intravitreal injection of 1.25 mg (0.05 ml) – one dose received 1 week before the first PRP session and the second injection received after the third PRP session.
There was no statistically significant difference in VA between the study and control group, in either the whole group or the bilateral DMO subgroup (12 patients). There were greater reductions in foveal thickness in the study group than in the control group, significantly at 1 month but not significantly different at 3 months (p = 0.28) and 6 months (p = 0.45). There were no reports of complications such as ocular HTN, lens opacity, progression, anterior chamber reaction or arterial thromboembolic events. The authors report a secondary outcome of change in VA from baseline as showing a significant decline in the control group but no change in the IVB group – it is not clear how this fits with the primary outcome showing no difference in VA between groups.
The authors concluded that in HRC-PDR, using IVB injections as adjuvant treatment to PRP reduces the VA deterioration compared with PRP alone. This was based only on secondary outcomes.
Tonello 2008
The aim of the study126 was to compare the effects of PRP used on its own or in combination with IVB in 22 patients (30 eyes) with HR-PDR. None had CSMO. Patients were followed up for 16 weeks. Patients with HR-PDR in only one eye (14) were randomised but in eight patients with bilateral HR-PDR, the eye with worse VA was selected to receive PRP plus IVB and the other eye received PRP alone. So the study126 was only partly a RCT. PRP was given in two sessions – week 1 and week 3 – performing 600–800 spots of 500-µm size per episode. Intravitreal injection of 1.5 mg (0.06 ml) bevacizumab was administered 60 minutes after completing the second PRP session (week 3).
At no time point was there significant change in BCVA from baseline in either groups (IVB – baseline 0.26 to week 16 0.29 logMAR; PRP – baseline 0.26 to week 16 0.31 logMAR) or between the groups.
In the IVB group, there was significant reduction in the total area of leakage from active NVs compared with the PRP group at all time points (weeks 4 and 9, ±1; week 16, ±2; p < 0.001).
There were no reports of serious drug-related complications in the 15 eyes (of 15 patients) treated with IVB, but only minor local transient adverse events that were related to the injection including subconjunctival haemorrhage (seven patients) and foreign body sensation (two patients).
The authors concluded that in the short-term, the adjunctive use of IVB with PRP was associated with a greater reduction in the area of active leaking NVs than PRP alone in patients with HR-PDR, but no difference in BCVA.
Adding intravitreal bevacizumab to laser: observational study
The efficacy of IVB in preventing PRP-induced macular thickening and visual dysfunction in eyes with HRC-PDR was investigated by Mason et al. (2008). 67
They divided 60 eyes of 30 patients non-randomly into two groups. The patients had VA of 20/30 or better and no eye had CSMO. One group had 1.25 mg of IVB 1 week before initiation of PRP treatment and the control group had PRP treatment only. OCT was performed before all treatments and at each follow-up examination.
After 24 weeks the BCVA as measured by the mean (SD) LogMAR in the control group increased from 0.069 (0.076) at baseline to 0.149 (0.113) at 24 weeks; by contrast, the IVB group decreased from 0.073 (0.071) at baseline to 0.039 (0.054) at 24 weeks. The mean change in BCVA between each group from baseline to 24 weeks was statistically significant (p ≤ 0.0001). Therefore, this study67 indicates that a single IVB injection given before standard PRP may help prevent PRP-induced visual loss in eyes with HRC-PDR and good vision. However, this would need to be further tested in a larger randomised study with longer follow-up.
Laser in combination with steroids
These studies119,120,127–130 are described narratively below and a detailed summary is given in Table 28 [note that two studies, Cho et al. (2010)119 and DRCRN (2011),120 both of which have an anti-VEGF and a triamcinolone arm, have been summarised in Table 27 so will not be repeated in Table 28].
| Study | Participants and baseline values | Intervention | Outcomes and ocular safety | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID: Maia 2009127 Aim: To evaluate the potential adjunctive effects of IVTA in combination with laser photocoagulation (pan-retinal and macular) treatment for the management of patients with both PDR and CSMO Design: Randomised trial, one eye (per patient) was randomly assigned to receive one IVTA (IVTA group), and fellow eyes received no additional treatment (CG) Follow-up: 12 months Proportions completing the study: 86% (19/22) Reasons for withdrawal (brief): 3 patients (CG) developed vitreous and/or pre-retinal haemorrhage, and data were excluded from analyses of 6-, 9- and 12-month data for BCVA and OCT variables |
n: 22 patients with bilateral treatment naive moderate PDR and CSMO Inclusion criteria: New vessels elsewhere ≥ 0.5 DA in one or more quadrants or new vessels on or within 1 DD of the optic disc < 0.25 to 0.33 DA, and CSMO by biomicroscopic evaluation in both eyes, ‘symmetric’ disease (defined for this study as the presence of the aforementioned characteristics [ETDRS severity level 65] in the absence of HR-PDR in each eye), and CMT greater than 250 µm on OCT evaluation Exclusion criteria: Aphakia, cataract surgery in the past 12 months or anticipated need for cataract surgery in the next 12 months, history of glaucoma, loss of vision as a result of other causes, systemic corticosteroid therapy, severe systemic disease other than diabetes, and any condition affecting follow-up or documentation (including pre-retinal or VH) Age (mean ± SD): 61.9 ± 5.3 Gender (female%): 54.5 (12/22) Diabetes type: type 2 HbA1c (% ± SD): 9.6 ± 1.4 Baseline VA [BCVA (logMAR)] IVTA group: 0.44 CG: 0.38 p = 0.019 Baseline CMT: IVTA: 360 µm Control: 331 µm p = 0.148 Baseline DR stage: Moderate PDR and CSMO Previous laser or intravitreal drug treatment: NR |
Group 1: PRP + macular laser Group 2: PRP + macular laser + IVTA Description of type of laser and delivery: Bilateral laser photocoagulation consisting of full scatter PRP treatment performed in three episodes (at weeks 1, 2 and 3) according to ETDRS guidelines; 400–600 500-µm spots per session, at the discretion of the treating investigator. Macular (focal and/or grid) laser photocoagulation performed in each eye at the first laser treatment episode (at week 1) Description of steroid treatment: One intravitreal injection of 4.0 mg (0.1 ml) of TA performed approximately 60 minutes after the completion of the third session of PRP (week 3) using topical anaesthesia |
IVTA (mean ± SD)Control (mean ± SD)p-valuelogMAR BCVA (Snellen equivalent)Baseline0.44 ± 0.17 (20/50–2)0.38 ± 0.17 (20/50–1)0.019Month 10.23 ± 0.13 (≈20/32–2)0.44 ± 0.15 (20/50–2)< 0.001Month 30.17 ± 0.09 (≈20/32–1)0.38 ± 0.13 (20/50–2)< 0.001Month 60.14 ± 0.09 (20/25–2)0.30 ± 0.14 (20/40)< 0.001Month 90.14 ± 0.08 (20/25–2)0.34 ± 0.17 (20/40–2)< 0.001Month 120.12 ± 0.07 (20/25–1)0.32 ± 0.16 (20/40–1)< 0.001CMT (µm)Baseline360.05 ± 84.85331.68 ± 78.880.148Month 1254.32 ± 27.06351.64 ± 76.86< 0.001Month 3239.18 ± 27.62308.05 ± 55.81< 0.001Month 6239.32 ± 26.48290.16 ± 58.02< 0.001Month 9232.37 ± 22.89301.26 ± 55.04< 0.001Month 12236.37 ± 16.14265.74 ± 27.27< 0.001Total macular volume (mm3)Baseline8.59 ± 1.688.44 ± 1.510.483Month 17.57 ± 0.898.30 ± 1.22< 0.001Month 37.39 ± 0.778.01 ± 0.96< 0.001Month 67.33 ± 0.697.83 ± 0.82< 0.001Month 97.33 ± 0.688.01 ± 0.66< 0.001Month 127.32 ± 0.607.78 ± 0.59< 0.001 IOP 1 month: significant increase in mean IOP in eyes in IVTA compared with eyes in control (p = 0.012) IVTA group: mean increase in IOP = 3.2 mmHg (range 2–11) Cataract surgery after 12 months: 6 eyes in ITVA group (27.3% of eyes) (p = 0.02) Additional macular laser photocoagulation: IVTA: 2 of 22 (9.1%) eyes Control: 13 of 22 (59.1%) eyes p < 0.001 No clinical evidence of uveitis, endophthalmitis, or ocular toxicity was observed. No serious drug-related adverse events in the IVTA group. Three patients (CG) developed vitreous and/or pre-retinal haemorrhage, and data were excluded from analyses |
IVTA (mean ± SD) | Control (mean ± SD) | p-value | logMAR BCVA (Snellen equivalent) | Baseline | 0.44 ± 0.17 (20/50–2) | 0.38 ± 0.17 (20/50–1) | 0.019 | Month 1 | 0.23 ± 0.13 (≈20/32–2) | 0.44 ± 0.15 (20/50–2) | < 0.001 | Month 3 | 0.17 ± 0.09 (≈20/32–1) | 0.38 ± 0.13 (20/50–2) | < 0.001 | Month 6 | 0.14 ± 0.09 (20/25–2) | 0.30 ± 0.14 (20/40) | < 0.001 | Month 9 | 0.14 ± 0.08 (20/25–2) | 0.34 ± 0.17 (20/40–2) | < 0.001 | Month 12 | 0.12 ± 0.07 (20/25–1) | 0.32 ± 0.16 (20/40–1) | < 0.001 | CMT (µm) | Baseline | 360.05 ± 84.85 | 331.68 ± 78.88 | 0.148 | Month 1 | 254.32 ± 27.06 | 351.64 ± 76.86 | < 0.001 | Month 3 | 239.18 ± 27.62 | 308.05 ± 55.81 | < 0.001 | Month 6 | 239.32 ± 26.48 | 290.16 ± 58.02 | < 0.001 | Month 9 | 232.37 ± 22.89 | 301.26 ± 55.04 | < 0.001 | Month 12 | 236.37 ± 16.14 | 265.74 ± 27.27 | < 0.001 | Total macular volume (mm3) | Baseline | 8.59 ± 1.68 | 8.44 ± 1.51 | 0.483 | Month 1 | 7.57 ± 0.89 | 8.30 ± 1.22 | < 0.001 | Month 3 | 7.39 ± 0.77 | 8.01 ± 0.96 | < 0.001 | Month 6 | 7.33 ± 0.69 | 7.83 ± 0.82 | < 0.001 | Month 9 | 7.33 ± 0.68 | 8.01 ± 0.66 | < 0.001 | Month 12 | 7.32 ± 0.60 | 7.78 ± 0.59 | < 0.001 | ||||||||||||||||||||||||||||||
| IVTA (mean ± SD) | Control (mean ± SD) | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| logMAR BCVA (Snellen equivalent) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 0.44 ± 0.17 (20/50–2) | 0.38 ± 0.17 (20/50–1) | 0.019 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 1 | 0.23 ± 0.13 (≈20/32–2) | 0.44 ± 0.15 (20/50–2) | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 3 | 0.17 ± 0.09 (≈20/32–1) | 0.38 ± 0.13 (20/50–2) | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 6 | 0.14 ± 0.09 (20/25–2) | 0.30 ± 0.14 (20/40) | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 9 | 0.14 ± 0.08 (20/25–2) | 0.34 ± 0.17 (20/40–2) | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 12 | 0.12 ± 0.07 (20/25–1) | 0.32 ± 0.16 (20/40–1) | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CMT (µm) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 360.05 ± 84.85 | 331.68 ± 78.88 | 0.148 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 1 | 254.32 ± 27.06 | 351.64 ± 76.86 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 3 | 239.18 ± 27.62 | 308.05 ± 55.81 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 6 | 239.32 ± 26.48 | 290.16 ± 58.02 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 9 | 232.37 ± 22.89 | 301.26 ± 55.04 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 12 | 236.37 ± 16.14 | 265.74 ± 27.27 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total macular volume (mm3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 8.59 ± 1.68 | 8.44 ± 1.51 | 0.483 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 1 | 7.57 ± 0.89 | 8.30 ± 1.22 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 3 | 7.39 ± 0.77 | 8.01 ± 0.96 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 6 | 7.33 ± 0.69 | 7.83 ± 0.82 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 9 | 7.33 ± 0.68 | 8.01 ± 0.66 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Month 12 | 7.32 ± 0.60 | 7.78 ± 0.59 | < 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Mirshahi 2010128 Aim: To compare the therapeutic effectiveness of combined IVTA plus PRP and MPC vs. PRP and MPC alone in patients with HR-PDR and CSMO Design: Prospective, controlled trial, one eye of each patient was selected to undergo IVTA injection based on block randomisation (matched for age, duration of MO, and status of glycaemic control) Follow-up: 6 months, 24 months for complications Proportions completing the study: 78% (18/23) Reasons for withdrawal (brief): Dense VH (two patients), tractional retinal detachment (one patient), loss of future follow-up (two patients) |
n: 23 patients Inclusion criteria: HR-PDR, VA between 20/200 and 20/30, diffuse and subfoveal CSMO, CMT more than 230 µm measured by OCT Exclusion criteria: History of prior laser treatment, existence of tractional retinal detachment, history of glaucoma, corticosteroid-responder patients, patients with active ocular surface disease and VH, hazy media that interferes with PRP, patients with one eye and a history of amblyopia, strabismus and anisometropia Age: 55.6 ± 6.5 years Diabetes type: Type 2 HbA1c: 9.93 ± 1.2% Baseline VA [BCVA (logMAR)]: IVTA: 0.46 ± 0.29 Control: 0.56 ± 0.27 Baseline CMT (µm): IVTA: 319.2 ± 79.1 Control: 345.9 ± 100.6 p = 0.65 Baseline DR stage: HR-PDR |
Group 1: IVTA injection 1 week before initial PRP and MPC (injected eye) Group 2: PRP and MPC (control eye) Description of type of laser and delivery: PRP was performed in three sessions at weekly intervals. MPC was performed in the first session of PRP in both groups with grid photocoagulation Description of steroid treatment: 4 mg triamcinolone was injected into the vitreous body 3.5 mm posteriorly from the inferotemporal of corneal limbus at 5 o’clock using a 27-gauge needle 1 week before PRP using topical anaesthesia |
BCVA, CMT, IOP:VariablesFollow-up intervalsIVTA eyes (n = 18)Control eyes (n = 18)p-valueLogMAR BCVABaseline0.46 ± 0.290.56 ± 0.270.321 month0.50 ± 0.420.61 ± 0.250.044 months0.46 ± 0.340.56 ± 0.260.146 months0.39 ± 0.290.55 ± 0.330.08CMT, µmBaseline319.2 ± 79.1345.9 ± 100.60.651 month260.5 ± 78.5a322.2 ± 90.30.014 monthsNDNDND6 months279.5 ± 76.9b316.3 ± 105.70.36IOP, mmHgBaseline15.72 ± 2.3216.11 ± 2.160.481 month18.56 ± 2.09c17.50 ± 1.50d0.134 months17.18 ± 2.24e16.41 ± 2.060.476 months16.71 ± 1.89f16.35 ± 1.660.52 Mean ± SD Remarkable difference within each group compared with baseline value: ap = 0.024, bp = 0.06, cp = 0.001, dp = 0.001, ep = 0.026, fp = 0.045 Standardised change in macular thickening (%): SCMT in IVTA eyesSCMT in control eyes1 month6 months1 month6 monthsMean29.416.85.665.03SD52.255.831.547.4Minimum–61.3–100.0–45.9–100.17Maximum97.795.745.881.5 Three IVTA and four control eyes experienced recurrence of MO necessitating retreatment for five eyes During the subsequent year after the regular visits, two IVTA eyes and one control eye had significant cataract progression in which surgical treatment was needed. Non-clearing VH (one eye control group) and tractional retinal detachment (two eyes control group) |
Variables | Follow-up intervals | IVTA eyes (n = 18) | Control eyes (n = 18) | p-value | LogMAR BCVA | Baseline | 0.46 ± 0.29 | 0.56 ± 0.27 | 0.32 | 1 month | 0.50 ± 0.42 | 0.61 ± 0.25 | 0.04 | 4 months | 0.46 ± 0.34 | 0.56 ± 0.26 | 0.14 | 6 months | 0.39 ± 0.29 | 0.55 ± 0.33 | 0.08 | CMT, µm | Baseline | 319.2 ± 79.1 | 345.9 ± 100.6 | 0.65 | 1 month | 260.5 ± 78.5a | 322.2 ± 90.3 | 0.01 | 4 months | ND | ND | ND | 6 months | 279.5 ± 76.9b | 316.3 ± 105.7 | 0.36 | IOP, mmHg | Baseline | 15.72 ± 2.32 | 16.11 ± 2.16 | 0.48 | 1 month | 18.56 ± 2.09c | 17.50 ± 1.50d | 0.13 | 4 months | 17.18 ± 2.24e | 16.41 ± 2.06 | 0.47 | 6 months | 16.71 ± 1.89f | 16.35 ± 1.66 | 0.52 | SCMT in IVTA eyes | SCMT in control eyes | 1 month | 6 months | 1 month | 6 months | Mean | 29.4 | 16.8 | 5.66 | 5.03 | SD | 52.2 | 55.8 | 31.5 | 47.4 | Minimum | –61.3 | –100.0 | –45.9 | –100.17 | Maximum | 97.7 | 95.7 | 45.8 | 81.5 | ||||||||||||||||||||||||||
| Variables | Follow-up intervals | IVTA eyes (n = 18) | Control eyes (n = 18) | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LogMAR BCVA | Baseline | 0.46 ± 0.29 | 0.56 ± 0.27 | 0.32 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 0.50 ± 0.42 | 0.61 ± 0.25 | 0.04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 months | 0.46 ± 0.34 | 0.56 ± 0.26 | 0.14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 0.39 ± 0.29 | 0.55 ± 0.33 | 0.08 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CMT, µm | Baseline | 319.2 ± 79.1 | 345.9 ± 100.6 | 0.65 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 260.5 ± 78.5a | 322.2 ± 90.3 | 0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 months | ND | ND | ND | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 279.5 ± 76.9b | 316.3 ± 105.7 | 0.36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IOP, mmHg | Baseline | 15.72 ± 2.32 | 16.11 ± 2.16 | 0.48 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 18.56 ± 2.09c | 17.50 ± 1.50d | 0.13 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 months | 17.18 ± 2.24e | 16.41 ± 2.06 | 0.47 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 16.71 ± 1.89f | 16.35 ± 1.66 | 0.52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SCMT in IVTA eyes | SCMT in control eyes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 6 months | 1 month | 6 months | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean | 29.4 | 16.8 | 5.66 | 5.03 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD | 52.2 | 55.8 | 31.5 | 47.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum | –61.3 | –100.0 | –45.9 | –100.17 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Maximum | 97.7 | 95.7 | 45.8 | 81.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Shimura 2006129 Aim: To evaluate prospectively the efficacy of a single sub-Tenon’s capsule injection of TA against PRP-induced macular thickening and visual disturbance in patients with severe DR and good vision Design: Prospective controlled study, the eye injected with TA was determined randomly just before injection, and the uninjected eye served as the control Follow-up: 24 weeks Proportions completing the study: 100% Reasons for withdrawal (brief): n/a |
n: 20 eyes of 10 patients Inclusion criteria: Symmetrical severe NPDR and early PDR, parafoveal thickness more than 300 µm; good VA in both eyes Exclusion criteria: IOP > 16 mmHg after 1 month of treatment with topical 0.1% dexamethasone Age (mean ± SD): 63.0 ± 8.2 years Gender (female%): 50 (5/10) Diabetes type: Type 2 HbA1c: 7.3 ± 0.76% Baseline VA [BCVA(logMAR)]: IVTA: 0.055 ± 0.07 Control: 0.065 ± 0.07 p = 0.386 Baseline CMT: IVTA: 235.5 ± 37.5 µm Control: 233.7 ± 39.8 µm p = 0.352 Parafoveal thickness: IVTA: 388.4 ± 41.8 µm Control: 388.0 ± 43.3 µm p = 0.455 Baseline DR stage: Severe non-proliferative and early PDR Previous laser or intravitreal drug treatment: None of the patients had a history of retinal photocoagulation or ocular surgery Ocular comorbidities: No clinically significant MO in either eye. No history of glaucoma, ocular HTN, or both. Cataract surgery, without complication in both eyes (n = 3), cataract in both eyes (n = 7) |
Group 1: Sub-Tenon’s capsule injection of 20 mg TA (triamcinolone) + PRP Group 2: PRP only Description of type of laser and delivery: PRP was performed four times at 2-week intervals in both eyes, size of the spots on the retina was 200–500 µm, and the duration was 0.15–0.2 seconds with a krypton red laser using topical anaesthesia. The number of spots in each session was approximately 500 Description of steroid treatment: 1 week before starting the first PRP session (–1 week), 0.5 ml (20 mg) TA was injected through the sub–Tenon’s capsule space reaching the posterior pole in one eye |
Foveal thickness, parafoveal thickness and BCVA (individual results for 10 patients also available): IVTA groupControl groupDifference at 24 weeks (p-value)–1 week0 weekDifference to baseline24 weeks–1 week0 weekDifference to baseline24 weeksAlteration of foveal thicknessMean235.5204.4p = 0.029235.3233.7234.4p = 0.4843120.0063SD37.521.938.639.840.268.2Alteration of parafoveal thicknessMean388.4290.5p < 0.0001302.2388387.6p = 0.499394.4SD41.816.616.143.345.635.8Alteration of logMAR VAMean0.0550.02p = 0.1630.0850.0650.07p = 0.4550.240.0063SD0.0720.0420.1060.0710.0790.133 –1 week: before laser and IVTA treatment 0 week: after IVTA but before laser treatment |
IVTA group | Control group | Difference at 24 weeks ( p -value) | –1 week | 0 week | Difference to baseline | 24 weeks | –1 week | 0 week | Difference to baseline | 24 weeks | Alteration of foveal thickness | Mean | 235.5 | 204.4 | p = 0.029 | 235.3 | 233.7 | 234.4 | p = 0.484 | 312 | 0.0063 | SD | 37.5 | 21.9 | 38.6 | 39.8 | 40.2 | 68.2 | Alteration of parafoveal thickness | Mean | 388.4 | 290.5 | p < 0.0001 | 302.2 | 388 | 387.6 | p = 0.499 | 394.4 | SD | 41.8 | 16.6 | 16.1 | 43.3 | 45.6 | 35.8 | Alteration of logMAR VA | Mean | 0.055 | 0.02 | p = 0.163 | 0.085 | 0.065 | 0.07 | p = 0.455 | 0.24 | 0.0063 | SD | 0.072 | 0.042 | 0.106 | 0.071 | 0.079 | 0.133 | ||||||||||||||||||||||||||||||||||||||||||||
| IVTA group | Control group | Difference at 24 weeks ( p -value) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| –1 week | 0 week | Difference to baseline | 24 weeks | –1 week | 0 week | Difference to baseline | 24 weeks | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alteration of foveal thickness | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean | 235.5 | 204.4 | p = 0.029 | 235.3 | 233.7 | 234.4 | p = 0.484 | 312 | 0.0063 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD | 37.5 | 21.9 | 38.6 | 39.8 | 40.2 | 68.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alteration of parafoveal thickness | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean | 388.4 | 290.5 | p < 0.0001 | 302.2 | 388 | 387.6 | p = 0.499 | 394.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD | 41.8 | 16.6 | 16.1 | 43.3 | 45.6 | 35.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alteration of logMAR VA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean | 0.055 | 0.02 | p = 0.163 | 0.085 | 0.065 | 0.07 | p = 0.455 | 0.24 | 0.0063 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD | 0.072 | 0.042 | 0.106 | 0.071 | 0.079 | 0.133 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study ID: Unoki 2009130 Aim: To evaluate the efficacy of a single posterior sub-Tenon’s capsule injection of TA (PSTA) before PRP Design: Randomised, contralateral eye, controlled open-label trial Follow-up: 6 months Proportions completing the study: 98% Reasons for withdrawal (brief): Lost to follow-up |
n: 82 eyes (41 patients) Inclusion criteria: 20 years or older of either gender with type 1 or type 2 diabetes; severe NPDR or PDR with clear ocular media and no other disease in either eye. The patients could have CSMO as defined by the ETDRS only if it was present in both eyes Exclusion criteria: A history of pan-retinal or focal photocoagulation; a history of vitrectomy; presence of VH; signs of vitreomacular traction; periocular or intraocular steroid within the past 6 months; poorly controlled diabetes (HbA1c > 10%); a history of glaucoma or ocular HTN; BCVA that differed between eyes by more than two Snellen lines Age (mean ± SD): 60.1 (11.5) Gender (female%): 44% Diabetes type 2%: 98% HbA1c %: 8.2 (SD 2.1) Baseline VA [BCVA (logMAR)]: 0.13 (0.33) Baseline foveal thickness(µm): 269.0 (114.9) Baseline DR stage n (%): Severe NPDR = 29 (71%) PDR = 12 (29%) Ocular comorbidities: CSMO in 17 (41%) |
Group 1: Single PSTA 20 mg before PRP Group 2: No injections before PRP Description of type of laser and delivery: The PRP was performed four times at 2-week intervals in both eyes. The spot size on the retina was 200–300 µm, the power of the laser was 150–200 mW, and the duration of the application was 0.2 seconds. The number of spots in each session was approximately 400, so the total number of burns after completion of the four sessions was approximately 1600. Topical anaesthesia was used in all cases, and all patients were treated as outpatients. If CSMO were present in both eyes at baseline, focal or grid laser therapy was performed at the first session of PRP Description of steroid treatment: Posterior sub-Tenon’s capsule injection under topical anaesthesia, 20 mg of TA (Kenacort: Bristol-Myers Squibb, New York) in a volume of 0.5 ml was injected in the inferotemporal quadrant; this was done 1 week before the first PRP session |
LogMAR BCVA, retinal thickness, IOP: Mean change at 6 months from baselinePSTA + PRPPRP onlyp-value between groupsLogMAR BCVA (all patients)Improvement of 0.072 (SD 0.028)Worsening of 0.010 (SD 0.029)0.04LogMAR BCVA in eyes without CSMOImprovement of 0.04 (SD 0.13)Worsening of 0.020 (SD 0.12)LogMAR BCVA in eyes with CSMOImprovement of 0.12 (SD 0.22)Worsening of 0.00 (SD 0.25)Foveal thicknessLessening of 9.7 (SD 85.6) µmIncrease of 32.8 (SD 82.8) µm0.04IOPThere were no significant differences in IOP either between baseline and each follow-up point within the same group, or between the two groups at each follow-up pointOther complicationsNo other injection-related complications, including cataract progression, were observed |
Mean change at 6 months from baseline | PSTA + PRP | PRP only | p-value between groups | LogMAR BCVA (all patients) | Improvement of 0.072 (SD 0.028) | Worsening of 0.010 (SD 0.029) | 0.04 | LogMAR BCVA in eyes without CSMO | Improvement of 0.04 (SD 0.13) | Worsening of 0.020 (SD 0.12) | LogMAR BCVA in eyes with CSMO | Improvement of 0.12 (SD 0.22) | Worsening of 0.00 (SD 0.25) | Foveal thickness | Lessening of 9.7 (SD 85.6) µm | Increase of 32.8 (SD 82.8) µm | 0.04 | IOP | There were no significant differences in IOP either between baseline and each follow-up point within the same group, or between the two groups at each follow-up point | Other complications | No other injection-related complications, including cataract progression, were observed | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean change at 6 months from baseline | PSTA + PRP | PRP only | p-value between groups | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LogMAR BCVA (all patients) | Improvement of 0.072 (SD 0.028) | Worsening of 0.010 (SD 0.029) | 0.04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LogMAR BCVA in eyes without CSMO | Improvement of 0.04 (SD 0.13) | Worsening of 0.020 (SD 0.12) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LogMAR BCVA in eyes with CSMO | Improvement of 0.12 (SD 0.22) | Worsening of 0.00 (SD 0.25) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Foveal thickness | Lessening of 9.7 (SD 85.6) µm | Increase of 32.8 (SD 82.8) µm | 0.04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IOP | There were no significant differences in IOP either between baseline and each follow-up point within the same group, or between the two groups at each follow-up point | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other complications | No other injection-related complications, including cataract progression, were observed | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cho 2010
Please see above for details of this study.
Diabetic Retinopathy Clinical Research Network 2011
Please see above for details of this study.
Maia 2009
The aim of the study127 was to determine the adjunctive effects of IVTA in combination with PRP and MPC in 22 patients with PDR and CSMO. Patients were followed up for 12 months. One group of patients received PRP and macular laser, whereas the second group received PRP, macular laser and IVTA. All patients received bilateral full scatter laser (each eye sequentially on the same day) in three sessions – weeks 1, 2 and 3 – consisting of 400–500 500-µm-size spots performed per session. In addition, all patients received macular (focal/grid) laser photocoagulation in each eye at the time of the initiation of the first PRP session. At the end of the third PRP session, one eye per patient was randomly assigned to receive IVTA (IVTA group) and the fellow eye of the same patient to receive no additional treatment (control group). IVTA treatment was a single intravitreal injection of 4 mg/0.1 ml of triamcinolone (Kenalog 40) 60 minutes after the third PRP session. During follow-up visits, if patients still presented with CSMO and treatable lesions on FA, then additional macular (grid and/or focal) laser treatments were given. In patients with focal leaks of greater than 500 µm from the centre of the macula, treatment was given.
There was significant improvement in mean BCVA in the IVTA group at all time points (p < 0.001) compared with those in the control group. At 12 months’ follow-up, mean BCVA in the IVTA group was 0.12 (SD 0.07) compared with 0.44 (SD 0.17) at baseline. The corresponding figures in the control group were 0.32 (SD 0.16) and 0.38 (SD 0.17), respectively. 12/19 (63.1%) eyes in the IVTA group and 2/19 (10.5%) eyes in the control group had improvements of two to three ETDRS lines. The difference between the two groups was statistically significant (p < 0.001). There was also significant reduction in mean CMT and total macular volume (TMV) in the IVTA group compared with the control group at all time points (p < 0.001). However, there was a significant increase in mean IOP in the IVTA group compared with the control group at 1 month (p = 0.12) but no difference at other time points. Mean IOP increase in the IVTA group was 3.2 mmHg, range 2–11 mmHg. Four eyes had to be treated with anti-glaucoma drops for 4–6 weeks as their IOP was greater than 24 mmHg.
All patients were phakic bilaterally, and, at 12 months’ follow-up, cataract surgery was indicated in six eyes receiving IVTA and laser (27.3% of the IVTA group; p = 0.02). Significantly (p < 0.001) more eyes in the control group received additional macular laser due to persistent MO – 2/22 (9.1%) eyes in the IVTA group and 13/22 (59.1%)
There were no reports of uveitis or endophthalmitis. Three patients in the control group developed vitreous and/or pre-retinal haemorrhage.
The authors concluded that IVTA in combination with laser improved vision and reduced macular thickness in patients with moderate PDR with CSMO, but at the cost of some raised IOP and cataract development. In view of the small numbers in their study,127 they advocated a large RCT.
Mirshahi 2010
The aim of the study128 was to compare the benefits of IVTA in combination with PRP and MPC (injected eye) against the combination of PRP and MPC alone (control eye) in 18 patients with type 2 diabetes and bilateral HR-PDR and CSMO. One eye of each patient was randomised as the study eye and the fellow eye as control. The primary and secondary outcome measures were measured at 1, 4 and 6 months. Patients were then followed up for a maximum of 18 months for any possible complications or for any additional treatment. PRP was performed in three sessions at weekly intervals. MPC was performed at the time of initiation of the first PRP session. One week before the first PRP session, 4 mg TA was injected.
Mean HbA1c was poor, at 9.93%.
At 1-month follow-up, mean VA was significantly greater in patients in the injected eye than in the control eye (20/50 vs. 20/66; p = 0.04). However, the difference was not significant at all other time points (4 months 20/58 vs. 20/73; p = 0.14; 6 months 20/50 vs. 20/70; p = 0.08). Similarly, mean change of VA was not statistically significant at 6 months follow-up (IVTA –0.05 ± 0.22 vs. control –0.008 ± 0.29; p = 0.56).
The reduction in CMT was significantly greater in the IVTA group than in the control group at 1 month (p = 0.01) but not significant at 6 months (p = 0.36). In the IVTA group, IOP was increased at 1 month [18.56 ± 2.09 mmHg; baseline: 15.72 (SD 2.32) mmHg], falling thereafter at 6 months, still high compared with the baseline value [16.71 (SD 1.89) mmHg]. In three eyes, IOP was treated.
Three eyes in the IVTA group and four eyes in the control group had persistent MO. Out of these, five eyes had to be re-treated. In the subsequent year after the regular visits, two eyes in the IVTA group and one eye in the control group had significant cataract progression requiring surgery. In the control group, one eye had non-clearing vitreous haemorrhage and two had tractional retinal detachment, respectively.
The authors concluded that their study128 showed no benefit on vision from adding IVTA to laser photocoagulation.
Shimura 2006
The aim of the study129 was to investigate the efficacy of a single sub-Tenon’s capsule injection, rather than intravitreal, of IVTA for reducing PRP-induced macular thickening and visual disturbance. In 10 type 2 diabetes patients with severe DR and good vision, one eye of the eye received IVTA injection while the fellow eye of the same patient served as a control. The IVTA group received 0.5 ml (20 mg) TA injections 1 week before the first PRP session. All patients received PRP in both eyes for four sessions at 2 weeks’ interval. Approximately 500 spots of 200- to 500-µm-size spots, exposure duration of 0.15–0.2 seconds was performed using a krypton red laser. Patients were followed up for 24 weeks.
Mean VA in the IVTA group improved initially [from 0.055 (SD 0.072) logMAR – week –1 to 0.02 (SD 0.04) at week 0] but remained unchanged after PRP. The final VA was 0.085 ± 0.11 logMAR, which was significantly (p = 0.0063) better than the control eye [0.24 (SD 0.13)]. In the control eye, VA reduced significantly following the PRP (week 0 to week 8) but stabilised after week 8.
In the IVTA group, foveal thickness reduced significantly following IVTA injection and before PRP (p = 0.029). However, foveal thickness increased following PRP therapy up to week 6 and gradually started to subside. In the control group, there was significant increment in foveal thickness, which never returned to the baseline level.
No IOP results are given, but the authors comment that no increases in IOP were seen – giving TA by the sub-Tenon’s capsule route may be less likely to cause this, though the effect on MO may be less than with intravitreal injection.
The authors concluded that a single injection of TA prior to PRP has beneficial effects for preventing PRP-induced foveal thickness and visual loss in patients with severe DR and good vision. They recommend a larger trial, perhaps giving TA 5 weeks before PRP.
Unoki 2009
This is a 6-month RCT involving patients recruited from Kyoto University Hospital between July 2006 and October 2007. 130 It was designed to evaluate the efficacy of a single posterior sub-Tenon’s capsule injection of triamcinolone acetonide (PSTA) before PRP versus PRP with no injection.
The primary end point of the trial, for which a sample size calculation was reported, was change in BCVA at 6 months compared with that at baseline using the logarithm of the minimum angle of resolution (logMAR). The secondary end points were changes in retinal thickness and IOP.
The trial included 82 eyes of 41 patients with bilateral severe NPDR (71%) or PDR (29%). The patients could have clinically significant MO as defined by the ETDRS, but only if it was present in both eyes. At baseline, 41% of patients recruited had CSMO. Nearly all (98%) had type 2 diabetes and the mean age was 60 years.
One eye of each patient was randomly allocated to one of two treatment arms (PTSA injection + PRP vs. PRP + no injection), which meant the fellow eye was automatically allocated to the other arm. The trial was open label to the patients and investigators, but the OCT technicians and statistical analysers were masked to the treatment assignment.
The PTSA injection consisted of 20 mg of TA (Kenacort) in a volume of 0.5 ml. It was injected 1 week before the first PRP session. Eyes in the control group received no injections.
The PRP treatment was performed four times at 2-week intervals in both eyes. The spot size on the retina was 200–300 µm, the power of the laser was 150–200 mW, and the duration of the application was 0.2 seconds. The total number of burns after completion of the four sessions was approximately 1600. If CSMO were present in both eyes at baseline, focal or grid laser therapy was performed at the first session of PRP.
The mean change in logMAR BCVA at 6 months from baseline in the PTSA + PRP group showed an improvement of 0.072 (SD 0.028) in the PSTA group and a worsening of 0.010 (SD 0.029) in the control group (PRP + no injection). This difference was statistically significant (p = 0.04).
The changes in foveal thickness between groups at 6 months were also statistically significant (p = 0.04), showing a lessening of 9.7 (SD 85.6) µm in the PTSA group and an increase of 32.8 (SD 82.8) µm in the control group.
There were no differences in IOP between groups at any of the time points (baseline, and 1, 3 and 6 months) measured. Also, there was no cataract progression observed, or any other injection-related complications.
The authors concluded that PSTA before PRP appears to be beneficial in preventing PRP-induced visual loss in eyes with DR by reducing the chance of macular thickening. The authors did point out a potential source of bias between the groups, in that focal/grid laser was performed in all with CSMO regardless of treatment assignment, and it was not possible to perform focal/grid laser in a standardised condition for each individual eye with CSMO.
Summary
For anti-VEGF the evidence from these mainly small trials is fairly consistent – the five bevacizumab and the two ranibizumab trials suggest that one or two injections can reduce the risk of PRP-induced MO. The trials are short term, but that is not a problem because the MO provoked by PRP is a short-term effect in the few months after PRP.
One question that cannot be answered by these trials is whether it would be as effective to treat with anti-VEGF drugs only if MO developed after PRP, given that many patients do not develop it. Treating prophylactically means that many would be treated unnecessarily, with cost implications. Indeed, even treating only those that do develop DMO might not be cost-effective if it is temporary with no long-term effects.
One study (DRCRN 2011120) found both ranibizumab and triamcinolone to be effective and safe up to 14 weeks. The same group of patients were followed up for up to 56 weeks but, without using study drugs, mainly to look at safety data, and found no difference between the two treatments. The authors, however, question whether there is a place for the use of anti-VEGFs and steroids long term.
Only one study (Cho 2010119) included patients with NPDR. The trial (Ernst 2012121) that compared bevacizumab alone with PRP alone concluded that the drug might be slightly better, but would need five to six injections over the first year compared with two laser sessions, and probably more in later years, as the effect of the anti-VEGFs is temporary.
The strength of the evidence base is that we have a set of RCTs. The limitations are their small size, and, for our purposes, that most patients had HR-PDR rather than severe NPDR.
In three trials, triamcinolone showed benefit in reducing the risk of MO after PRP and improving BCVA in patients with CSMO, but in another (Mirshahi 2008124) it did not. However, IVTA increased IOP, a well-known side effect of steroids. Triamcinolone given via the sub-Tenon’s capsule did not (Unoki 2009130). However, in one RCT, sub-Tenon’s capsule administration was reported to be less effective in reducing MO than the intravitreal route. 59
Given the higher risk of adverse effects, anti-VEGF treatment might be preferable to steroids, though cost would need to be considered.
However, the question of whether anti-VEGF drugs should be used prophylactically remains open, as trials of the two (or three) strategies would be necessary, these being:
-
no prophylaxis – treat only those people who develop MO after PRP
-
routine prophylaxis – anti-VEGF before PRP
-
no VEGF treatment – use focal laser if MO develops.
As will be reported in Chapter 8, a considerable amount of research is under way on the combination of PRP and anti-VEGF drugs.
Chapter 5 Systematic review of existing cost-effectiveness evidence
Introduction
This chapter reports a systematic review of existing economic evaluations (including model-based economic evaluations) of the use of PRP and/or anti-VEGF medication for patients with moderate and/or severe NPDR or early and/or severe PDR. The aim was to review the available literature including existing models and to identify any suitable data (e.g. costs, utilities and transition probabilities) to help inform our economic model.
Methods
The systematic search including searches of the following electronic databases: MEDLINE OVID (1946 to 12 September 2013), EMBASE (1974 to 12 September 2013), and the meeting abstracts database in the Web of Science (1900 to 24 October 2013). The search terms included economic and QoL terms cross referenced with DR terms. The search was limited to studies published in English Language and Humans. The search strategy was developed with input from an Information Specialist (PR). Details of the search strategies are provided in Appendix 2.
Citations and abstracts from each of the electronic online databases were exported into a citation software package (EndNote) and any duplicate citations were removed. Two reviewers (PA and HM) independently reviewed titles and abstracts to identify potentially relevant papers. There was no need for discussions with a third reviewer.
All abstracts were then read for relevance and were considered relevant to this review if they met the following inclusion criteria:
-
The study is a full economic analysis on the treatment (laser and/or medication) for DR; or
-
The study conducted a partial economic analysis (costs or effects) on the treatment (laser and/or medication) for DR (e.g. costing studies or QoL studies).
Abstracts that may provide useful information for the economic model (such as costs, utilities and transition probabilities) were further retained but not included in this review. These abstracts also included studies that specifically related to the treatment for DMO, treatment for AMD and of screening for DR. These studies were categorised as:
-
The study contains useful information for DR on: adverse events/complications, disutilities, and/or natural history, incidence or prevalence (UK based).
-
The study contains useful information on costs and/or effects for DR (the study does not have to be treatment related).
-
The study discusses a model-based long-term economic analysis of screening for DR.
For the relevant abstracts, we obtained the relevant full-text articles. The reference lists of retrieved articles in category A or category B were checked for potentially relevant papers that met the inclusion criteria. A data extraction form was developed to capture the main characteristics associated with the relevant studies identified.
For any studies that were classed as full economic evaluations, we critically appraised them against the framework on quality assessment for economic evaluation studies developed by the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) group. 131 The CHEERS framework sets out best practice for reporting economic evaluations under six main categories: title and abstract, introduction, methods, results, discussion and other. If the studies included any model-based economic evaluations, they were further critically appraised using the framework on quality assessment for economic modelling developed by Philips et al. (2004). 132 The framework developed by Philips et al. (2004)132 sets out best practice for reporting on decision-analytic models used in economic evaluations under the dimensions of structure, data and consistency.
Results
The electronic database search identified 2556 potentially relevant citations. After removing duplicates, 1896 potential abstracts remained. After reviewing the published titles and abstracts of the remaining studies, no studies presented a full economic evaluation (including model-based evaluation) on the treatment (laser and/or medication treatment) for DR (category A). Six studies provided a partial economic analysis on the treatment (laser and/or medication) for DR (category B). One of the studies was excluded because it was a letter/reply to an article that was already included in category B. 133 In total, five articles134–138 were retained for data extraction. Figure 3 depicts a flow diagram of the abstracts identified and number of studies included in the cost-effectiveness review.
FIGURE 3.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for cost-effectiveness studies.
Out of the 1890 abstracts that were excluded, 234 abstracts were retained as they appeared to contain some useful information for the model. Sixty-six abstracts contained useful information for DR with regards to adverse events, disutilities, incidence and prevalence (category C); 147 abstracts had useful information on the costs and/or effects for DR (category D); and 21 abstracts were model-based long-term economic analyses looking at screening for DR. Six of the included abstracts in category D related to cost-effectiveness studies which were treatment related (laser treatment/anti-VEGF medication) for other eye diseases (hence, they were not included in category A).
As no studies were found to be a full economic evaluation or model-based economic evaluations (category A), we did not assess the quality of these articles using either the CHEERS framework131 or the Philips et al. (2004) checklist. 132
None of the studies looked at the costs associated with DR and treatment (laser and/or medication). Five studies134–138 focused on health outcomes associated with laser and/or medication treatment for patients with DR. Three papers were on QoL: Scanlon et al. (2006)134 used qualitative interviews, Tsilimbaris et al. (2013)135 used a vision-specific QoL measure, and Wirostko et al. (2011)136 used conjoint analysis. We also found one study on patient satisfaction and the patient–provider relationship (Mozaffarieh 2005137) and one study on anxiety levels (Trento 2006138).
The five studies134–138 were conducted in different countries: UK, Crete, Canada, Austria and Italy. The smallest sample size was 20 patients135 and the largest sample size was 259 patients. 138 Three studies assessed patients who were undergoing laser treatment for the first time. 134,137,138
The main limitations of the studies were the small sample sizes;135 and the short durations of follow-up. 134,136 Most patients were generally satisfied with laser treatment. 134,137 The study by Tsilimbaris et al. (2013)135 found that laser treatment did not have a significant impact on patients vision-related QoL; laser treatment was experienced by patients as an event that generates anxiety. 138 Finally, patients preferred the attributes which were associated with improving vision or preventing further vision loss. 136 The studies are described in more detail below.
Scanlon et al. (2006)134 present data from 227 qualitative interviews with 156 patients. Interviews were conducted both pre-laser and post-laser treatment; 54% were PDR patients and 46% were MO patients. The interviews were conducted across four eye clinics in the UK. Our interest is mainly in the PDR patients. There were three groups of patients:
-
Group 1 The first treatment group, of newly diagnosed patients coming for their first laser treatment. This included 27 PDR pre-treatment and 19 PDR post-treatment patient interviews.
-
Group 2 The follow-up group were patients coming to a normally scheduled follow-up after their initial treatment. Six of the 11 interviews were in PDR patients.
-
Group 3 The multiple treatment group consisted of patients already having had multiple treatments for their eye condition and who were returning for clinical follow-up visits or for additional treatment. There were 50 PDR pre-treatment and 21 PDR post-treatment interviews.
All of Group 1 and a subset of the Group 3 patients were interviewed before their treatment in the clinic and then by telephone again 2 weeks after their treatment. Both Group 2 and Group 3 patients were asked to recall symptoms before their first and after their laser treatment. The responses from all three groups were combined for the qualitative analysis.
Amongst Group 1 PDR patients, the most frequently reported symptoms prior to the first laser treatment were blurred vision (44%), short-sightedness (44%), and difficulty with poor lighting (41%). After photocoagulation their most frequently reported symptoms were short-sightedness (37%), blurred vision (21%) and flickering spots before their eyes (21%).
Responses in Group 2 were not reported separately for PDR and MO. For all of 11 Group 2 responders, blurred vision was both the most frequently reported symptom (55%) that they recalled having just prior to their first treatment, and the most frequent visual disturbance (50%) when they returned for their clinic follow-up visit. In Group 3 PDR patients, the most commonly reported symptoms were blurred vision, difficulty with poor lighting, difficulty with night vision and flickering spots before eyes.
Before and after photocoagulation differences varied amongst groups. Group 1 PDR patients had few comments about the pre–post differences, with the most common comments were ‘Problem with reading/watching TV since treatment’ and ‘Not comfortable driving at night since treatment’. Group 3 PDR patients noted changes more often, mostly detrimental, after their laser treatment in their functional status or role limitations. The most common comments on pre–post differences in their functional status or role limitations were ‘Problem with reading/watching TV since treatment’, ‘No change in limitations since treatment’, ‘Some improvement in limitations since treatment’ and ‘Not comfortable driving at all since treatment’.
In terms of comments about satisfaction with various aspects of laser treatment, there were a high number of expressions of the expectation that the treatment would arrest the progression of their eye disease even with those having had multiple treatments. Most participants indicated that they would elect to have the laser treatment again if their doctor felt it was necessary, even though the treatment had less of an impact than they hoped for, and expectations about the treatment were basically met for the majority of the participants. In Group 1 PDR patients the most frequent comment was ‘Would have liked more information before treatment’ followed by ‘Felt treatment not as bad as expected’. Many patients going into their first treatments expected that the treatment would take care of their eye problem and they would not require repeated treatments.
Amongst Group 1 and 3 PDR patients under the theme of ‘Feelings or satisfaction with treatment after laser’ the most frequent comment was ‘Would choose laser treatment again if needed’. When asked about the effect of treatment on QoL, the most frequent comment was ‘My quality of life has not changed since treatment’. Therefore, it seems that the majority of patients registered no change in their QoL.
One of the main weaknesses of the study134 was that follow-up interviews were conducted only 2 weeks post laser treatment, which was too soon to reflect the more beneficial long-term palliative effect from the laser treatment.
Tsilimbaris et al. (2013)135 from Crete used the NEI 25-Item Visual Function Questionnaire (VFQ-25) tool at the beginning and at least 1 month after the completion of PRP, in patients with type 2 diabetes and bilateral PDR that was treated with PRP. Their study135 was small consisting of 20 patients (12 men and 8 women) with a mean age of 65 years. They excluded patients who had had laser treatment before. PRP involved an average of 2140 laser spots, spot size of 200 µm, per eye, in multiple consecutive sessions. Mean energy of the spots was 252 mW and duration 200 ms. The NEI VFQ-25 consists of 25 vision-targeted questions representing 11 vision-related subscales, plus an additional single-item general health rating question. Each item is scored in a scale of 0–100; a higher score indicates greater vision-related QoL. The overall composite score is calculated by averaging across the subscale scores.
The mean composite score before laser treatment was 71.9 (SD 14.8) and after treatment it was 70.6 (SD 17.2) which was not significantly different (p = 0.748, paired t-test). The authors also found that none of the subscale scores had a statistically significant effect, before or after laser treatment. There was an increase in the composite score post-laser treatment in 11 patients, there was no difference in one patient, and there was a decrease in eight patients. The treatment intensity, as indicated by the mean number of laser spots per eye each patient received, had no correlation with the pre-treatment to post-treatment change in the VFQ-25 composite score (Spearman’s r = 0.104; p = 0.670). So PRP was well tolerated by the patients and did not seem to affect the patients’ vision-related QoL.
One weakness of this study135 was that any patients having complications such intravitreal haemorrhage were excluded from the final analysis. The authors do not report how many patients were excluded for this reason.
Wirostko et al. (2011)136 assessed patient preferences for the different DR treatments (anti-VEGF, focal laser, pan-retinal laser or steroid treatment) using a technique called conjoint analysis in three centres in Canada. Preferences were sought from 161 patients: PDR – 25%; DMO – 31%; both PDR and DMO – 26%; no PDR or DMO – 18%. Of these patients, 49% (n = 79) were treated with laser only; 3% (n = 5) with injection (either steroid and/or anti-VEGF); 22% (n = 36) with both laser and injection; and 25% (n = 41) were treatment naive. The conjoint analysis survey involved patients making trade-offs among 11 DR attributes. The 11 attributes assessed were derived from a literature review and in consultation with three DR specialists, which included mode of administration, required number of office visits, treatment-related pain, the chance of improving central vision, and the risk of adverse events. Each attribute was described using two to three levels that represented the full range of possibilities across the four treatments. Utilities were generated for every level of each attribute and then ordinary least squares regression was used to calculate the final set of utilities for the attribute levels. The utilities were summed for different treatment profiles (based on the respective combinations of attribute levels) to determine which treatment would be preferred.
Of the 11 attributes, those affecting visual functioning were considered the most important such as improving VA and reducing adverse events (i.e. chance of cataracts) and those attributes not directly affecting vision such as administration or treatment-related pain were considered to be less important. Fifty-two per cent patients would prefer treatment by anti-VEGF compared with 20%, 17% and 11% with steroid, focal laser and pan-retinal laser, respectively. Patients who developed PDR, 46% preferred to be treated with anti-VEGF compared with 27%, 17% and 10% who would prefer to be treated with steroid, focal laser and pan-retinal laser, respectively. Preferences did not vary greatly by previous treatment experience, age or type of DR. Overall, the patient population were generally satisfied with the laser treatment that was provided.
In terms of limitations of the study,136 the authors noted that the cost of treatment was not included as an attribute; a one-year time horizon was not long enough to capture all the effects of laser treatment and the sample did not represent the full range of patients with DR.
Mozaffarieh et al. (2005)137 from Vienna assessed short-term treatment satisfaction after initial photocoagulation, and long-term satisfaction taking into account the patients’ final expectations of their vision, in 105 patients undergoing first photocoagulation treatment for DMO (n = 49) or PDR (n = 56) between June 2002 and March 2004. Patients were informed of the benefits and adverse effects of laser photocoagulation, and were told that the main aim of treatment was to avoid further visual deterioration and blindness. The argon laser was used. To assess overall patient satisfaction with laser treatment, all patients completed the Diabetes Treatment Satisfaction Questionnaire (DTSQ), scores ranged from 0 to 36; a higher score indicates greater satisfaction. Patients’ degree of satisfaction in relation to VA results was assessed using a Likert scale.
Nine months after initial photocoagulation, 25% of patients reported improvement in VA, 71% reported no change in vision, and 4% reported deterioration in vision. Level of satisfaction as assessed by the DTSQ was high (mean score 29.6); 46.4% of patients with PDR scored 31 or higher on the DTSQ. Overall, using the Likert scale about 70% of the patients were completely satisfied, even though only 9% of these patients reported an improvement in VA. A further 21% were partially satisfied and 10% were dissatisfied with the results of treatment.
The authors emphasise the need to set realistic expectations by explaining, as they did, that the main aim of photocoagulation is to avoid further visual deterioration and that treatment may not necessarily improve their eyesight.
Trento et al. (2006)138 from four centres in Northern Italy used four questionnaires – the Hospital Anxiety and Depression Scale (HADS), Family Apgar-List of Threatening Experiences (FA-LTE), State–Trait Anxiety Inventories 1 and 2 (STAI-1 and STAI-2) – to assess the anxiety associated with laser treatment in patients with sight-threatening diabetic retinopathy (STDR). They recruited two groups of patients: 131 waiting for laser treatment and 128 control subjects waiting for screening or other non-intervention visits. Scatter and/or focal-grid photocoagulation was performed by argon green laser. Most patients had type 2 diabetes and 80% of the laser group had previous photocoagulation, compared with only 1.5% of the comparison group.
High anxiety scores were detected by HADS, STAI-1 and STAI-2 among patients waiting for photocoagulation. Overall, scores for people waiting for laser treatment were higher than for control subjects, with the exception of FA-LTE. After adjusting for centre, gender, previous laser treatment and schooling, HADS and STAI-1 remained significantly lower among control subjects. Anxiety was not reduced by having had previous photocoagulation. However, there were differences amongst the centres. All four centres provided written material about DR and photocoagulation, but differed in facilities. Centre B provided further information about retinopathy and laser treatment in a relaxing setting, and had lower anxiety rates than Centre C, which had what the authors describe as an unpleasant setting and high patient throughput.
Discussion
The cost-effectiveness search highlighted only five studies134–138 that were partial economic analyses looking at the treatment (laser and/or medication) for patients with DR. None of the studies looked at the cost of treatment and the cost of follow-up, and focused only on the health outcomes associated with treatment.
For the economic model, the two most useful studies found in the search in terms of the health outcomes are the studies by Tsilimbaris et al. (2013)135 and Wirostko et al. (2011). 136
The Tsilimbaris study135 used a vision-related instrument (NEI VFQ-25) to measure vision-related QoL. The NEI VFQ-25 is a 25-item self-administered questionnaire, which assesses visual health and the impact this has on daily activities and QoL. 139 The questionnaire measures difficulty with near and distance vision activities, driving difficulties, limitations in social and role functioning, lack of independence due to vision, mental health symptoms caused by vision, peripheral and colour vision, and eye pain. The scoring is done in a two-stage process: (1) each item is scored on a scale of 0 (lowest score) to 100 (highest score), where a higher score represents better functioning; and (2) items within each subscale are averaged together (there are 11 subscales in total for the VFQ-25). To obtain the combined score for the questionnaire, the average of the subscales (excluding the general health rating question) is undertaken. Averaging across the subscales scores rather than individual items gives equal weight to each subscale.
The VFQ-25 has been used in various studies where they have used mapping methods to predict European Quality of Life-5 Dimensions (EQ-5D) utility scores (which are needed in order to estimate quality-adjusted life-years (QALYS), the recommended utility measure for NICE. For example, Browne et al. (2012)140 predicted EQ-5D and Short Form questionnaire-6 Dimensions (SF-6D) utility values from the VFQ-25 for glaucoma patients using three types of models: ordinary least squares, Tobit regression and censored least absolute deviations, and the models were compared using the root-mean-square error and the mean absolute error, whereas, Payakachat et al. (2009)141 also used the same three models to predict EQ-5D utility scores from VFQ-25 for patients with age-related macular degeneration. These models can be used for mapping questionnaires for any patient group.
The Wirostko study136 used a technique called conjoint analysis or discrete choice experiments (DCEs). This technique is based on the premise that any intervention can be described by its characteristics (or attributes) and that the extent to which an individual values an intervention depends on the level of these attributes. 142 There are five main steps in conducting a DCE study: (1) identify the key attributes; (2) assigning levels to each of the key attributes; (3) developing the different choice scenarios; (4) establishing the preferences; and (5) data analysis. This method can help to elicit health-state utility values, which can be used to calculate QALYs as long as information on QoL and survival has been incorporated into the design of the DCEs.
The main limitations of the use of these two studies for our economic model are: the study by Tsilimbaris et al. (2013)135 did not include a generic preference-based measure such as the EQ-5D or SF-12 or SF-6D; and in the study by Wirostko et al. (2011)136 the cost of treatment was not included as an attribute. Chapter 7 describes the economic model for patients with moderate/severe NPDR.
Chapter 6 Prevalence, progression and implications for modelling
Rationale for modelling
The main source of data for the effects of administering PRP at the severe NPDR stage, rather than waiting till PDR develops, is the ETDRS. 9 Much has changed in diabetes care over the decades since ETDRS, including improvements in diabetic control. As noted in the Chapter 1, the prevalence of serious retinopathy has declined in recent decades.
Mean HbA1c (the best measure of glycaemic control) has improved considerably since the 1980s. The recent Wisconsin paper comparing the Wisconsin Diabetes Registry Study (WDRS) and Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR)143 cohorts of people with type 1 diabetes, diagnosed in the periods 1987–92 and 1979–80,3 reported that mean HbA1c in the WDRS cohort was 8.0% and in WESDR 9.3%. Interestingly, it should be noted that 48% of the WDRS cohort were on continuous subcutaneous insulin infusion (CSII) (insulin pumps) compared with less than 2% of the WESDR cohort. The 48% is far higher than the proportion of people with type 1 diabetes on CSII in the UK. A survey of Scottish Health Boards in March 2013 reported that only 3.5% of people over the age of 18 years with type 1, were on CSII. 144
Nordwall et al. (2004)144 reported that in Sweden, in patients with type 1 diabetes diagnosed under the age of 15 in the years 1961–85, the frequency of severe retinopathy (defined as need laser treatment) had declined from 47% after 25 years in the cohort diagnosed 1961–5, to 24% in the 1971–5 cohort. The peak age of diagnosis would be 10–12 years, so the 25-year follow-up would take them to 35–40 years of age. However, there was less reduction in background DR with 80% having that (presumably mild NPDR) at 25 years’ duration.
The Wong et al. (2009)2 meta-analysis divided studies of progression to PDR and SVL according to time period, before and after 1985 (when ETDRS results were published). They reported a big drop – 19.5% – with PDR at 4 years in former period, 2.6% in latter, and SVL at 4 years 9.7% in 1975–85 versus 3.2% in 1986–2008.
Ten-year rates for PDR were 11.5% versus 6.6%. For SVL, they were 6.0% versus 2.6%.
The mixes of types of diabetes in the two periods were different: 71% had type 1 in studies from latter period versus 48% in earlier one.
The people at highest risk of retinopathy are those with poor glycaemic control. Unfortunately, improvement in mean HbA1c may conceal the fact that a significant proportion is still poorly controlled. The Scottish Diabetes Survey 2011 (Table 29) reported that those with type 1 have a greater proportion with poor control – 37%. 143 Only 15% of people with type 2 had such poor control. However, because there are far more people with type 2 than type 1, most (77%) people with HbA1c over 9.0% had type 2 diabetes.
| HbA1c | Type 1 | Type 2 | |
|---|---|---|---|
| < 7.5% | 22% | 62% | |
| 7.5–9.0% | 41% | 23% | |
| > 9.0% | 37% | 15% | |
| Number with A1c > 9% | 9000 | 30,000 | % with T2 77% |
So overall, 17% of Scottish patients had HbA1c of over 9%. They are at highest risk of retinopathy, and the ETDRS results from a group with poor control (42% had HbA1c over 10%) should be applicable to them.
The National Diabetes Audit for England and Wales145 reported that over 18.1% of people with type 1 diabetes and 7.2% of people with type 2 diabetes have poor glycaemic control (HbA1c > 10%) (Table 30).
| HbA1c | Type 1 diabetes | Type 2 diabetes |
|---|---|---|
| < 48 mmol/mol (6.5%) | 6.5% | 26.2% |
| 6.5–7.4% | 20.5% | 39.6% |
| 7.5–10.0% | 54.9% | 27.0% |
| > 10.0% | 18.1% | 7.25% |
Model run 1
So our first run uses ETDRS data,9 applicable to those with poor glycaemic control. If early PRP at severe NPDR stage compared with delaying PRP till PDR develops is not cost-effective in this group, it is unlikely to be cost-effective in lower-risk groups.
Model run 2
The costing assumes that conventional argon laser is used, given over at least two sessions to reduce the risk of PRP-associated MO. In a sensitivity analysis, we test the effect in this cohort of replacing conventional argon laser with PSC laser given in one session, combined with a single injection of IVB to reduce the risk of DMO. In effect this run merely changes some costs, but also creates a more convenient scenario for patients.
Types of diabetes
One finding from ETDRS was that early PRP was more beneficial in patients with type 2 diabetes than those with type 1. 42 It was also noted that in the deferred group progression to HR-PDR was faster in type 1 than type 2, and that early PRP reduced the development of HR-PDR less in type 1: 3–40% versus 50% reduction in type 2.
One excellent source of data on progression of retinopathy in type 1 is the Diabetes Control and Complications Trial (DCCT) study, especially with the Epidemiology of Diabetes Interventions and Complications (EDIC) extension to give a further 18 years of follow-up. The DCCT data come from a cohort of type 1 diabetes only, mean age at end of DCCT/entry into EDIC 33 years. 145,147
However, the figures for progression give insufficient detail. Table 31 gives the prevalence of different levels of retinopathy for the conventional group. There was an increase in prevalence of severe NPDR of 22.9%, but we cannot say where the patients in that group came from.
| Retinopathy | At end of DCCT | EDIC year 18 |
|---|---|---|
| None | 17.3% | 4.7% |
| Microaneurysms only | 32.1% | 26.8% |
| Mild NPDR | 28.5% | 18.3% |
| Moderate NPDR | 14.3% | 19.6% |
| Severe NPDR | 7.8% | 30.7% |
Severe visual loss was rare in the DCCT and so we cannot model that.
Other studies of progression in type 1 diabetes include the Wisconsin (WESDR) study, which reported that, in those with no retinopathy or NPDR at baseline, 37% had developed PDR by 14 years of follow-up. 148
In type 2 diabetes, the equivalent study to DCCT was the United Kingdom Prospective Diabetes Study (UKPDS). However, very few patients progressed to severe disease. Stratton et al. (2001)149 reported that 37% had some retinopathy at diagnosis. Of these, 29% progressed by two ETDRS scale steps or more over 6 years, or laser photocoagulation or vitreous haemorrhage. HbA1c predicted progression: 18% in lowest tertile of HbA1c to 40% in top band (HbA1c 7.5% and over). SBP was a weaker predictor: 26% lowest third, 36% highest. Smoking reduced the risk of progression by about half. So the UKPDS data is not suitable for our purposes.
Harris Nwanyanwu et al. (2013)150 reported progression in people in a large managed care network in the USA (which sounds population based but may not be because many people do not have insurance). They followed 4617 people with NPDR (no details of stages given but said to be newly diagnosed) to see how many developed PDR for a mean on 1.7 years, during which time 6.7% progressed to PDR. An important finding was that for every 1% increase in HbA1c, there was a 14% increase in the risk of progressing from NPDR to PDR.
Other studies reporting progression include the Blue Mountains Study,151 wherein 4.1% of people with NPDR progressed to PDR over 5 years.
Jones et al. (2012)152 from Norfolk provide data from a cohort of over 20,000 screened up to 14 times (Norwich was one of the pilot screening sites). Amongst those with background (mild and moderate NPDR?) 23% developed PPDR and 6% developed PDR after 1 year. Their classification was a simpler version of ETDRS. After 10 years, those with background DR had 56% progression to PPDR and 11% to PDR. But this may be an underestimate because referred patients were removed from the screening system. The data provide background to pre-proliferative progression rates.
Transition probabilities
The Markov model in Chapter 7 uses transition probabilities for the progression through various stages of DR. Most clinical studies present information in the form of progression rates at a specified time. These rates were converted to transition probabilities using the formula below, where r is the progression rate and t is time:
Where progression rates were not available from the literature, we converted the probability of the event over a period of time to a constant rate using the formula below:
Then, the calculated rate was used, as above, to derive the transition probability.
This section reports on the sources of the progression rates of DR, the progression of CSMO and SVL, and methods used to derive the transition probabilities. These transition probabilities were derived from the literature and in consultation with clinical experts. We report on the progression rates used from the literature and the transition probabilities (progression and regression) calculated for the progression to various stages of DR. The limitations are discussed later. Ideally, we would have found data on progression and where relevant, regression to and from each stage, but many studies combined stages, for example jumping from moderate NPDR to HR-PDR. Table 32 summarises the transition probabilities for the usual care and intervention arms, respectively. Tables 33 and 34 summarise the post-treatment transition probabilities for the usual care and intervention arms, respectively.
| Moderate NPDR | Severe NPDR | Severe NPDR and CSMO ± VI | Early PDR | Early PDR and CSMO ± VI | HR-PDR | HR-PDR and CSMO ± VI | Severe PDR | Severe PDR and CSMO ± VI | SVL | |
|---|---|---|---|---|---|---|---|---|---|---|
| Moderate NPDR | a | 0.0221 | 0.0281 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe NPDR | 0 | 0 | 0.0061 | 0.0214 | 0 | 0 | 0 | 0 | 0 | |
| Severe NPDR and CSMO ± VI | 0 | 0 | 0 | 0.0548 | 0 | 0 | 0 | 0 | 0 | |
| Early PDR | 0 | 0 | 0 | 0 | 0.0061 | 0.0717 | 0.0778 | 0 | 0 | 0 |
| Early PDR and CSMO ± VI | 0 | 0 | 0 | 0 | 0 | 0.1434 | 0.1555 | 0 | 0 | 0.0258 |
| HR-PDR | 0 | 0 | 0 | 0 | 0 | 0 | 0.0061 | 0.0459 | 0.0520 | 0 |
| HR-PDR and CSMO ± VI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0918 | 0.1039 | 0.0535 |
| Severe PDR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0061 | 0.0535 |
| Severe PDR and CSMO ± VI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0535 |
| SVL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | a |
| Moderate NPDR | Severe NPDR | Severe NPDR and CSMO ± VI | Early PDR | Early PDR and CSMO ± VI | High-risk/moderate PDR | High-risk/moderate PDR and CSMO ± VI | Severe PDR | Severe PDR and CSMO ± VI | Severe visual loss | |
|---|---|---|---|---|---|---|---|---|---|---|
| HR-PDR PT | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0 | 0.0061 | 0.0459 | 0.0520 | 0 |
| HR-PDR and CSMO ± VI PT | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0 | 0.0918 | 0.1039 | 0.0252 |
| Severe PDR PT | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0 | 0.0061 | 0.0252 |
| Severe PDR and CSMO ± VI PT | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0 | 0.0252 |
| Moderate NPDR | Severe NPDR | Severe NPDR and CSMO ± VI | Early PDR | Early PDR and CSMO ± VI | High-risk/moderate PDR | High-risk/moderate PDR and CSMO ± VI | Severe PDR | Severe PDR and CSMO ± VI | Severe visual loss | |
|---|---|---|---|---|---|---|---|---|---|---|
| Severe NPDR PT | 0.0036 | 0 | 0.0061 | 0.0171 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe NPDR and CSMO PT | 0.0036 | 0.0036 | 0 | 0.0342 | 0.0464 | 0 | 0 | 0 | 0 | 0 |
| Early PDR PT | 0.0036 | 0.0036 | 0.0036 | 0 | 0.0061 | 0.0186 | 0.0247 | 0 | 0 | 0 |
| Early PDR and CSMO PT | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0 | 0.0284 | 0.0494 | 0 | 0 | 0.0092 |
| HR-PDR PT | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0 | 0.0061 | 0.0459 | 0.0520 | 0 |
| HR-PDR and CSMO ± VI PT | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0 | 0.0918 | 0.1039 | 0.0252 |
| Severe PDR PT | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0 | 0.0061 | 0.0252 |
| Severe PDR and CSMO ± VI PT | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0.0036 | 0 | 0.0252 |
Rates of progression (usual care)
In the UK, the current standard of care is to initiate PRP when the severity level of DR reaches HRCs. 42
Moderate non-proliferative diabetic retinopathy
Progression rates for people who progressed from moderate NPDR to severe NPDR were obtained from a population-based study from Melbourne by McCarty et al. (2003). 153 These authors provided information on the 5-year probability (2 of 10) of developing severe NPDR for people categorised as moderate NPDR, at baseline. We converted this probability to a to a 6-month transition probability of 0.0221 to be used in the model. For people who progressed to severe NPDR and CSMO with/without visual impairment, we used the transition probability of progressing from moderate NPDR to severe NPDR in addition to the progression rate reported by Klein et al. (1998)148 for people developing CSMO. Klein et al. (1998)143 reported a 14-year progression rate of 17% (95% CI 14.1% to 19.9%) for people developing CSMO. From this information, we derived a transition probability of 0.0281 for people progressing from moderate NPDR to severe NPDR and CSMO.
Severe non-proliferative diabetic retinopathy
The progression rate for people developing severe NPDR and CSMO with/without visual impairment was obtained from Klein et al. (1998). 148 These authors reported a 14-year progression rate of 17% (95% CI 14.1% to 19.9%) for people developing CSMO. From this, we calculated a 6-month transition probability of 0.0061 for progressing from severe NPDR to developing severe NPDR with CSMO. Progression rate for people developing early PDR was taken from ETDRS #12. 6 These authors reported the 5-year progression rate to early PDR for people categorised as severity level 53, at baseline. From this, we derived a 6-month transition probability of 0.0214 for people developing early PDR from baseline severe NPDR.
Severe non-proliferative diabetic retinopathy and clinically significant diabetic macular oedema with/without visual impairment
For the progression rate to early PDR and CSMO from the severe NPDR and CSMO health state, Pautler (2010)47 suggested that people with DMO and PDR are at greater risk of developing more severe retinopathy than people with PDR alone. Owing to the lack of information on progression rates in the literature for people developing more severe retinopathy from the severe NPDR and CSMO health state, we assumed that the progression rate is twice that of severe NPDR developing more severe retinopathy. We derived a 6-month transition probability of 0.0548 for people with baseline severe NPDR and CSMO with/without visual impairment and progressing to early PDR and CSMO with/without visual impairment.
Early proliferative diabetic retinopathy
The progression rate for people developing early PDR and CSMO with/without visual impairment was taken from Klein et al. (1998). 148 Information on the progression rate to HR-PDR from baseline early PDR was obtained from ETDRS #18. 154 The authors reported a 5-year cumulative progression rate of 74.4% (95% CI 69.8% to 79.4%) to HR-PDR for people categorised as early PDR, at baseline. From this, we derived a 6-month transition probability of 0.0717 for people progressing to HR-PDR from baseline early PDR. For people progressing to HR-PDR and CSMO with/without visual impairment we used the progression rate of developing HR-PDR in addition to the progression rate for people developing CSMO. From this information, we derived a transition probability of 0.0778 for people progressing from early PDR to HR-PDR and CSMO.
Early proliferative diabetic retinopathy and clinically significant diabetic macular oedema with/without visual impairment
For the progression rates to more severe retinopathy from the early PDR and CSMO health state, Pautler (2010)47 suggested that people with DMO and PDR are at greater risk of developing more severe retinopathy than people with PDR alone. Owing to the paucity of information on progression rates for people developing more severe retinopathy from the early PDR and CSMO health state, we assumed that the progression rate is twice that of early PDR developing more severe retinopathy. We derived a 6-month transition probability of 0.1434 and 0.1555 for people developing HR-PDR and HR-PDR and CSMO, respectively, from baseline early PDR and CSMO with/without visual impairment. For progression to SVL, the transition probability was derived from information taken from DRS #14. 38 These authors presented a 4-year progression rate of 20.9% to SVL for untreated eyes categorised as proliferative without HRCs. We derived a 6-month transition probability of 0.0258 for people progressing to SVL from early PDR and CSMO.
High-risk proliferative diabetic retinopathy
The cumulative progression rate for people developing HR-PDR and CSMO with/without visual impairment from baseline HR-PDR was obtained from Klein et al. (1998). 148 From this, we calculated a transition probability of 0.0061 for people developing HR-PDR and CSMO. For people progressing to severe PDR, information was obtained from the McCarty et al. (2003). 153 These authors reported a 5-year transition probability for people treated for PDR and remaining in the PDR health state. At the 5-year follow-up, three out of eight people remained in the PDR health state. We assumed this transition probability for people developing severe PDR. The 5-year transition probability was converted to an annual rate and then re-converted to a 6-month transition probability of 0.0459. For people progressing to severe PDR and CSMO with/without visual impairment we used the progression rate of developing severe PDR in addition to the transition probability for people developing CSMO. The progression rate for people developing severe PDR was obtained from the study by McCarty et al. (2003)153 in addition to the progression rate for people developing CSMO. From these studies, we derived a 6-month transition probability of 0.0520 of progressing to severe PDR and CSMO with/without visual impairment from baseline HR-PDR.
High-risk proliferative diabetic retinopathy and clinically significant diabetic macular oedema with/without visual impairment
For the progression rates to more severe retinopathy from the HR-PDR and CSMO health state, Pautler (2010)47 suggested that people with DMO and PDR are at greater risk of developing more severe retinopathy than people with PDR alone. Owing to the lack of information on the progression rates for people developing more severe retinopathy from the HR-PDR and CSMO health state, we assumed that the progression rate is twice that of HR-PDR developing more severe retinopathy. From this information, we derived a 6-month transition probability of 0.0918 for people developing severe PDR from baseline HR-PDR and CSMO. For progression to SVL, the transition probability was derived from information taken from DRS #14. 38 These authors presented a 4-year progression rate of 44.0% to SVL for untreated eyes categorised as proliferative with HRCs. We derived a 6-month transition probability of 0.0535 for people progressing to SVL from early PDR and CSMO.
Severe proliferative diabetic retinopathy
The cumulative progression rate for people developing severe PDR and CSMO with/without visual impairment from baseline severe PDR was taken from the study by Klein et al. (1998). 143 From this study,143 we estimated a 6-month transition probability of 0.0061 for developing severe PDR and CSMO. For progression to SVL, the transition probability was derived from information taken from DRS #14. 38 These authors presented a 4-year progression rate of 44.0% to SVL for untreated eyes categorised as proliferative with HRCs (so not quite at severe stage). We derived a 6-month transition probability of 0.0535 for people progressing to SVL from severe PDR.
Severe proliferative diabetic retinopathy and clinically significant diabetic macular oedema with/without visual impairment
For progression to SVL, the transition probability was derived based on information obtained from DRS #14. 38 These authors presented a 4-year progression rate of 44.0% to SVL for untreated eyes categorised as proliferative with HRCs. We derived a 6-month transition probability of 0.0535 for people progressing to SVL from severe PDR and CSMO with/without visual impairment.
High-risk proliferative diabetic retinopathy post treatment
For people developing HR-PDR and CSMO from HR-PDR at baseline, we derived a transition probability based on information obtained from Klein et al. (1998). 148 From this, we calculated a transition probability of 0.0061 for people developing HR-PDR and CSMO. For the progression to severe PDR, information was obtained from McCarty et al. (2003). 153 These authors reported a 5-year transition probability for people treated for PDR and remaining in the PDR health state. We assumed that this was the progression rate for people developing severe PDR. The 5-year transition probability was converted to an annual rate and then re-converted to a 6-month transition probability. For the progression rate to severe PDR and CSMO, we derived a transition probability from the McCarty et al. (2003)153 study in addition to the transition probability of developing CSMO. From this, we calculated a 6-month transition probability of 0.0520 for people progressing to severe PDR and CSMO after treatment for HR-PDR. Information on the progression to SVL was taken from DRS #14. 38 These authors presented a 4-year progression rate of 20.4% to SVL for treated eyes categorised as proliferative with HRCs. From this progression rate, we derived a 6-month transition probability of 0.0258 for people developing SVL after treatment for HR-PDR.
High-risk and clinically significant diabetic macular oedema with/without visual impairment post treatment
The progression rate for people that received treatment for HR-PDR and CSMO with/without visual impairment to more severe health states were taken from the literature. For people progressing to severe PDR, we derived a transition probability based on information reported by McCarty et al. (2003). 153 The 5-year transition probability was converted to an annual rate and then re-converted to a 6-month transition probability of 0.0459. We assumed that people with PDR and CSMO are twice as likely to develop more severe retinopathy than people with PDR alone. We estimated a 6-month transition probability of 0.0918 for people developing severe PDR from baseline HR-PDR and CSMO. For people progressing to severe PDR and CSMO, we derived a transition probability based on information reported by McCarty et al. (2003)153 in addition to a transition probability of developing CSMO. Also, we assumed that people with PDR and CSMO are twice as likely to develop more severe retinopathy than people with PDR alone. The derived transition probability of 0.1039 is for progression to severe NPDR and CSMO from baseline HR-PDR and CSMO. Progression rates to SVL were obtained from DRS #14. 38 These authors reported a 4-year progression rate of 20.4% to SVL for people treated for PDR with HRCs. We derived a 6-month transition probability of 0.0252 to SVL for eyes in the HR-PDR and CSMO post-treatment health state.
Severe proliferative diabetic retinopathy post treatment
The progression rates for people that received treatment for severe PDR and progressing to more severe retinopathy health states were taken from the literature. For people progressing to severe PDR and CSMO, we derived a transition probability from the Klein et al. (1998). 148 Progression rates to SVL were obtained from DRS #14. 38 These authors presented a 4-year progression rate of 20.4% to SVL for people treated for PDR with HRCs. From this, we derived a 6-month transition probability of 0.0252 to SVL for eyes in the severe PDR post-treatment health state.
Severe proliferative diabetic retinopathy and clinically significant diabetic macular oedema with/without visual impairment post treatment
The progression rates for people that received treatment for severe PDR and CSMO and progressing to more severe retinopathy were obtained from the DRS #14. 38 From this study,38 these authors presented a 4-year progression rate of 20.4% to SVL for people treated for PDR with HRCs. From this, we derived a 6-month transition probability of 0.0252 to SVL for eyes in the severe PDR post-treatment health state.
Rates of regression (usual care)
Regression rates for people following treatment for DR were obtained from Klein et al. (2008),155 who reported a 25-year cumulative rate of improvement in DR following laser treatment of 18% (95% CI 14.1% to 19.9%). We assumed this improvement to all regression health states, and converted this rate to a 6-month transition probability of 0.0036 to be used in the model.
Rates of progression (intervention: early pan-retinal photocoagulation)
People who have moderate NPDR are monitored, and then treated with PRP when they progress to the severe NPDR stage.
All transitions in the intervention arm were the same as the usual care arm except for the transitions that are listed below.
Severe non-proliferative diabetic retinopathy post treatment
The progression rate for people that received treatment for severe NPDR to more severe health states were taken from the literature. The progression rate to severe NPDR and CSMO with/without visual impairment was derived from the Klein et al. (1998)148 study. These authors reported a 14-year cumulative progression rate of 17% (95% CI 14.1% to 19.9%) for developing CSMO. From this, we estimated a 6-month transition probability of 0.0061 for progressing to severe NPDR and CSMO. The progression rate for people developing early PDR was taken from ETDRS #12. 6 We assumed that PRP reduces the progression rate to early PDR by 20%. These authors reported a 5-year progression rate of 21.6% to early PDR for people categorised as severity level 53. From this, we derived a 6-month transition probability of 0.0171.
Severe non-proliferative diabetic retinopathy and clinically significant diabetic macular oedema with/without visual impairment post treatment
For the progression rate to early PDR from the severe NPDR and CSMO post-treatment health state, we obtained information from the ETDRS #12. 6 These authors reported a 5-year progression rate of 21.6% for people developing early PDR from severe NPDR. Here, assumed that PRP will reduce the progression rate to early PDR by 20%. Additionally, we assumed that the progression rate to early PDR in people with CSMO, is twice that of severe NPDR without DMO. We derived a transition probability of 0.0342 for people developing early PDR from severe NPDR and CSMO post-treatment health state. For people developing early PDR and CSMO, we obtained information from the ETDRS #12. 6 These authors reported a 5-year progression rate of 21.6% for people developing early PDR from severe NPDR. Here, we assumed that PRP will reduce the progression rate to early PDR by 20%. Additionally, we assumed that people with PDR and CSMO are twice as likely to progress to more severe retinopathy than people with PDR but without CSMO. We derived a transition probability of 0.0463 for people progressing to early PDR and CSMO from the severe NPDR and CSMO post-treatment health state.
Early proliferative diabetic retinopathy post treatment
For people developing early PDR and CSMO, we derived a transition probability of 0.0061 from the 14-year progression rate of CSMO obtained from Klein et al. (1998). 148 For the progression to HR-PDR, the ETDRS #99 reported results on the 5-year progression rate of 18.8% to HR-PDR for people treated for moderate-severe NPDR or early PDR. We derived a 6-month transition probability of 0.0186 for people developing HR-PDR. The progression rate to HR-PDR and CSMO was obtained from the ETDRS #9 study. 9 These authors reported a 5-year progression rate of 18.8% to HR-PDR for eyes treated for moderate-severe NPDR. In addition, we added the derived transition probability of developing CSMO based on the progression rate reported by Klein et al. (1998). 148 From this we derived a 6-month transition probability of 0.0247 for the progression to HR-PDR and CSMO for eyes treated for early PDR.
Early proliferative diabetic retinopathy and clinically significant diabetic macular oedema with/without visual impairment post treatment
For the progression to HR-PDR, the ETDRS #99 reported results on the 5-year progression rate of 28.8% to HR-PDR for people treated for CSMO and more severe (severe NPDR or early PDR) retinopathy. We derived a 6-month transition probability of 0.0284 for people progressing to HR-PDR having received treatment for early PDR and CSMO. To calculate the progression rate to HR-PDR and CSMO, we obtained information on the progression rates from the ETDRS #99 and Klein et al. (1998)148 studies. Also, we assumed that early PRP reduces the progression rate to HR-PDR and CSMO by 20%. Additionally, we assumed that the progression is twice that of early PDR developing more severe retinopathy. From this, we estimated a transition probability of 0.0494 for people progressing to HR-PDR and CSMO from the early PDR and CSMO post-treatment health state. The progression rate to SVL was obtained from DRS #14. 38 These authors presented a 4-year progression rate of 7.4% to SVL for people treated for PDR without HRCs. From this, we derived a 6-month transition probability of 0.0092 to SVL for eyes treated for PDR without HRCs.
Rates of regression (intervention)
Regression rates for people following treatment for DR were obtained from Klein et al. (2008),155 who reported a 25-year cumulative regression rate of 18% (95% CI 14% to 21%) for improvement in DR following laser treatment. We assumed this improvement to all health states, and converted this rate to a 6-month transition probability of 0.0036 to be used in the model.
Limitations
We encountered a number of problems. Ideally, we would like to have had good population-based and recent data on progression rates, and hence transition probabilities, along the retinopathy pathway: moderate NPDR > severe NPDR > early PDR > HR-PDR > severe PDR > SVL.
However, many studies jumped stages, for example giving only progression from NPDR to HR-PDR.
Some of the studies with the most detailed information – such as the DRS,38 ETDRS6,154 and WESDR143,155 (discussed in earlier chapters) – are now somewhat out of date. A meta-analysis by Wong et al. (2009)2 showed that progression rates are now much lower than in decades past, which they attribute to improved control of blood glucose, BP and lipids, and to better eye care, with earlier identification of retinopathy through screening and better treatment. The 10-year incidence of PDR was 11.5% in the period 1975–85, and 6.6% in the period 1986–2008. For SVL, the corresponding figures were 6.0% and 2.6%.
Inevitably, data on progression may always be out of date because it takes 20 years to collect 20-year progression data, by which time advances in care may have reduced the risk.
The studies found provided useful information on progression rates, but varied in terms of the study population, sample size included, categories of diabetes, classification of DR, length of follow-up, outcome measures and clinical end points. These characteristics of the studies can have an impact on the transition probabilities used to model disease progression.
In our model, there were limitations which we must acknowledge. Firstly, the model was populated with transition probabilities derived from various sources. From these studies, authors may have followed up a cohort of people with type 1 diabetes only,11,143,155 or type 2 diabetes149 or a mixed group of type 1 and type 2. 38,153 Additionally, studies may have included people with DR in one eye only and both eyes. Scanlon et al. (2013)156 reported that people with DR in both eyes are likely to progress to more severe retinopathy compared with people with retinopathy in one eye.
Secondly, in the baseline analysis, the progression rates were obtained from the ETDRS,6,9,154 where the clinical end points were mainly progression to HR-PDR, vitrectomy or SVL. Hence, we had to seek information from other studies to derive transition probabilities of progressing to less severe health states. For example, progression from moderate NPDR to severe NPDR information was obtained from McCarty et al. (2003). 153 These authors followed up 121 diabetics. At baseline, majority of the people included in this study had no retinopathy or mild NPDR. Ten and eight people had moderate NPDR and PDR, respectively. Owing to the small sample size of people with moderate NPDR at baseline, this may not be an accurate representation of the progression rate to more severe retinopathy. Information on the progression rate to early PDR following treatment for severe NPDR was not available from the literature. Most studies assessed the impact of PRP in reducing the progression rate to HR-PDR or SVL.
Additionally, some studies (McCarty 2003153) did not differentiate between the severity level of PDR at baseline. Hence, in some cases, we assumed that the progression rate for people with early PDR, HR-PDR or severe PDR was the same for progressing to more severe health states. This may have the impact of underestimating/overestimating the progression rates, as we would expect an individual with early PDR to progress to more severe health states at a slower rate than an individual with HR-PDR.
Thirdly, due to the paucity of information on the progression rates for people with DR and CSMO developing more severe retinopathy, we assumed the progression rate is twice the progression rate for a person with DR alone. Paulter (2010)47 suggested that people with PDR and DMO are likely to progress to more severe retinopathy compared with people without PDR. However, this progression was not quantified by Paulter (2010). 47 The impact of this assumption on our results may lead to an under/overestimation of the progression rates.
Fourthly, the progression rates were mainly obtained from studies pre-1998, and the management of diabetes, DR and blood glucose management has improved owing to a better understanding of the retinopathy disease process. 157
The various uncertainties may lead to underestimating/overestimating the progression rates used in the model.
However, the largest uncertainty arises not from uncertainties over progression rates, but from the absence of recent data on the benefits of PRP at severe NPDR or early PDR compared with waiting till HR-PDR, using modern laser methods and adjuvant anti-VEGF treatment.
Chapter 7 Model for assessing cost-effectiveness of pan-retinal photocoagulation for non-proliferative diabetic retinopathy
Introduction
We built a Markov model in order to determine whether offering PRP treatment to patients with severe NPDR is cost-effective compared with delaying treatment till the PDR stages. This chapter describes the structure of the model, the inputs into the model, the assumptions made, the different scenarios that have been evaluated, the main results and sensitivity analyses.
Model structure
To assess the cost-effectiveness of early treatment versus delaying treatment of PRP, a Markov (state-transition) model was developed using Microsoft Excel version 2013 (Microsoft Corporation, Redmond, WA, USA). A Markov model was the most appropriate choice because progression of DR can evolve over time and during this time, patients can move to different stages of DR (health states) or can die.
The economic model was developed by determining the different clinical pathways for patients presenting with moderate NPDR through to irreversible severe vision loss and blindness (and to death). We have used information from the systematic review on the clinical effectiveness, but most notably the DRS (see Chapter 2), and the ETDRS (see Chapter 2), and from expert opinion to develop the different clinical pathways.
There are two treatment arms within the model:
-
Current practice (usual care) Patients are observed until they progress to the HR-PDR health state (and onwards) when they receive PRP.
-
Early PRP (intervention) Patients receive PRP once they progress to the severe NPDR health state.
Figures 4 and 5 show the model structure for people receiving current practice (usual care) and Figures 6 and 7 show the model structure for people receiving early PRP (intervention arm). Health states in the model structure are shown in the ovals, the arrows represent the transitions that patients can make in the model, the recurring arrows show that patients can stay in that same health state for more than one cycle, and death is an absorbing health state.
FIGURE 4.
Current practice (usual care) progression. PT, post treatment; VI, visual impairment.

FIGURE 5.
Current practice (intervention) regression. PT, post treatment; VI, visual impairment.
FIGURE 6.
Early PRP (intervention) progression. PT, post treatment; VI, visual impairment.

FIGURE 7.
Early PRP (usual care) regression. PT, post treatment; VI, visual impairment.
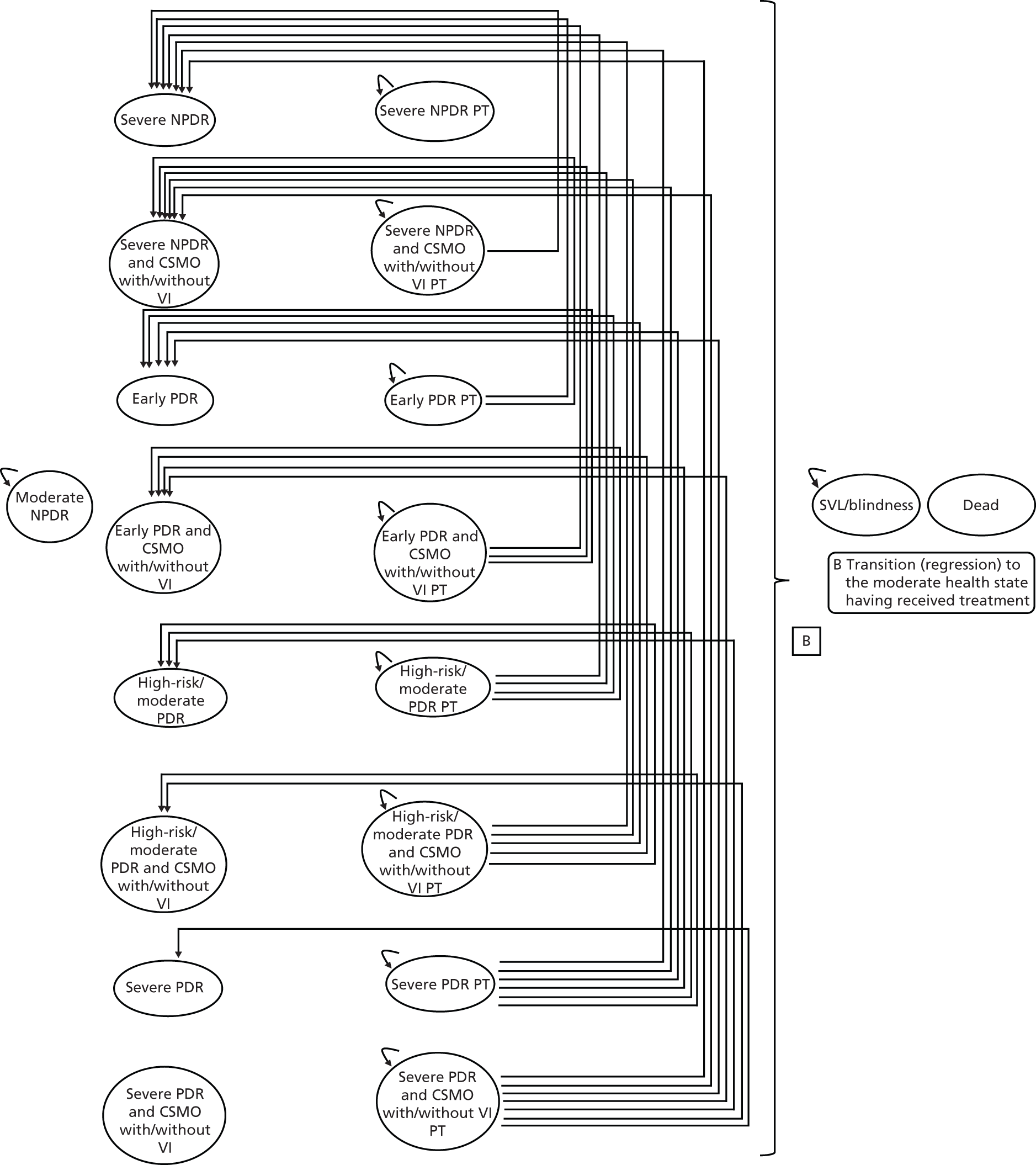
Table 35 lists the different health states for the model. The four post-treatment health states for severe NPDR, severe NPDR and CSMO with/without visual impairment, early PDR and early PDR and CSMO with/without visual impairment do not apply to the usual care arm.
| Health state | Post-treatment health state |
|---|---|
| Moderate NPDR | |
| Severe NPDR | Severe NPDR PT |
| Severe NPDR and CSMO with/without VI | Severe NPDR and CSMO with/without VI PT |
| Early PDR | Early PDR PT |
| Early PDR and CSMO with/without VI | Early PDR and CSMO with/without VI PT |
| HR-PDR | HR-PDR PT |
| HR-PDR and CSMO with/without VI | HR-PDR and CSMO with/without VI PT |
| Severe PDR | Severe PDR PT |
| Severe PDR and CSMO with/without VI | Severe PDR and CSMO with/without VI PT |
| SVL/blindness | |
| Death |
The model starts by assigning a cohort of 1000 patients presenting with moderate NPDR at an ophthalmology clinic. The model assumes that people progress through all stages of DR: moderate NPDR > severe NPDR > early PDR > HR-PDR > severe PDR > SVL.
In the first cycle, patients can either stay in the moderate NPDR health state or progress to either severe NPDR health state or to severe NPDR and CSMO with/without visual impairment health state or die from diabetes-related disease or from other causes. In the intervention arm, those patients in the severe NPDR health state or the severe NPDR and CSMO with/without visual impairment health state, receive treatment and at the end of the cycle they move to the corresponding post-treatment health state.
In the second cycle, patients in the usual care arm can stay in either the moderate or severe NPDR health states or progress to early PDR or early PDR and CSMO with/without visual impairment or die from diabetes-related disease or from other causes. In the next cycle, the patients can stay in either the moderate or severe NPDR or early PDR health states or progress to HR-PDR or HR-PDR and CSMO with/without visual impairment health states or die (note that the patients in the early PDR and CSMO with/without visual impairment can also progress to the SVL/blindness health state because of DMO). When the patient moves to the HR-PDR or HR-PDR and CSMO with/without visual impairment health states, they receive treatment and at the end the cycle they move to the corresponding post-treatment health state. Once the patients enter the post-treatment health states, they can either stay in this health state or progress to one of the more severe health states, regress back to earlier stages of the disease, or die. Patients can stay in the post-treatment health state for more than one cycle.
In the intervention arm, in the second cycle and onwards, patients can stay in the moderate NPDR health state or progress to either severe NPDR or severe NPDR and CSMO with/without visual impairment or die. For those patients who were in the severe NPDR health states in the second cycle, in the third cycle they can progress to early PDR or early PDR and CSMO with/without visual impairment and so forth. When the patients progress to one of these health states (i.e. severe NPDR or early PDR) they will receive treatment and at the end the cycle they move to the corresponding post-treatment health state. For those patients who received treatment in the previous cycle, they start in the post-treatment health state and they can either stay in this health state or progress to one of the more severe health states, regress back to earlier stages of the disease, or die. Patients can stay in the post-treatment health state for more than one cycle.
The cycle length for each model was set to 6 months5 and transitions between each health state occur at the end of each cycle. The transitions that can be made from each health state for the usual care and intervention arms are highlighted in Appendix 4, Table 49. A number of assumptions were made in the model:
-
Patients progress through all stages of DR.
-
People can have advanced DR with no symptoms.
-
Treatment can lead to regression back to earlier stages (i.e. to less severe health states having received treatment).
-
Patients cannot regress from the SVL/blindness health state.
-
DMO can occur at most stages of DR.
-
DMO can lead to visual impairment in the absence of PDR.
-
In people with DR and CSMO, the latter is usually treated first.
-
In people with DR and CSMO, treatment might improve one but not the other.
-
PRP might precipitate DMO.
-
Proportion of patients in severe PDR or severe PDR and CSMO with/without visual impairment health states may also develop vitreous haemorrhage or pre-retinal haemorrhage, or both.
Base-case analysis
As DR is a bilateral disease, we have assumed that the model is a two-eye model and that the severity level is the same in each eye. For the base-case analysis, we have adopted a 30-year time horizon. A hypothetical cohort of 1000 diabetic patients with a starting age of 50 years were followed. We adopted a starting age of 50 years for the economic model, as this is the mean age of patients with DR. Treatment of retinopathy is in secondary care. The analysis is conducted from the perspective of the NHS and Personal Social Services (PSS). All costs are in pounds sterling (£) in 2012–13 prices. Health outcomes were measured in QALYs. Results are expressed as incremental cost per QALY gained. An annual discount rate of 3.5% is applied to both costs and outcomes.
Model inputs
Transition probabilities
For the base-case analysis, transition probabilities were based on data derived from ETDRS, as this was the main source of data for the effects of administering PRP at the severe NPDR or early PDR stages (ETDRS did not report results for NPDR and early PDR separately) rather than waiting till HR-PDR develops. Chapter 6 details the literature used and assumptions made for deriving these transition probabilities and Tables 32–34 show the transition probabilities that have been used in the base-case analysis.
Utilities
Most of the health-state utility values for DR are based on different VA ranges. Although there are a few studies that have health-state values by the different DR severity levels, for example no retinopathy, background retinopathy, STDR, blindness,158 background retinopathy, proliferative diabetic retinopathy, MO, severe vision loss/blindness,159 and no retinopathy, non-STDR, STDR, blindness,160 they do not provide enough detail for the different DR severity levels we need for the model. The most useful paper was by Ting et al. (2007)157 who developed a Markov model of a novel DR prognostic device for DR progression. They had utility values for the following health states: no DR, microaneurysm, mild NPDR, moderate NPDR, severe NPDR, PDR, maculopathy and blind. The utility values used by Ting et al. (2007)157 were a weighted average based on two papers: Brown et al. (1999)161 who provided utility values (time-trade off values) for a range of visual acuities associated with DR, and Fong et al. (2002)162 who provided information on the range of VA for different stages of DR and data from the WSE was used. However, we have not used the values from Ting et al. (2007)157 in our model because of two reasons: (1) we could not replicate their results; and (2) the utility values looked suspiciously high, for example a patient with no DR had a utility value of 0.8402 – this was very similar to the utility value for someone who was at a more advanced stage of DR, i.e. severe NPDR (0.8182) and PDR (0.8137).
We have used the same two studies that were used in Ting et al. (2007)157 to estimate the health-state utility values for our economic model: Brown et al. (1999)161 and Fong et al. (2002). 162 In addition, for patients with MO, we have used utility values from a study by Smith et al. (2008),163 who estimated utility values for vision loss in a community-based population with type 2 diabetes.
In summary, Fong et al. (2002)162 reported the number (distribution) of people by three VA ranges (≥ 20/40, < 20/40 and > 20/200, ≤ 20/200) for the BSE for no/minimal retinopathy, background/mild NPDR, moderate/severe NPDR and PDR. Smith et al. (2008)163 reported the number of people by five VA ranges (≥ 20/20, 20/25 to 20/35, 20/40, 20/50 to 20/70, ≤ 20/80) for MO, which we then grouped into the same three VA ranges as Fong et al. (2002). 162
Using the five VA levels from Brown et al. (1999)161 we have linked these to the three VA levels in Fong et al. (2002)162 in order to calculate an overall utility value for that VA group. Then, using the number of patients, we have estimated weighted utility values for the three severity levels: moderate/severe NPDR, PDR and MO. For patients who have MO, a disutility of –0.03 was applied to the utility value for MO obtained from Brown et al. (1999). 161 This value was the minimum QALY loss associated with acuity loss of least 20/30 in one eye and was based on a paper by Rein et al. (2011)164 who estimated the cost-effectiveness of three screening strategies for patients with no or early DR. For patients who move to a retinopathy health state with CSMO, the utility value for that health state was based on an average of the value of that DR health state and MO.
The utility value for severe vision loss/blindness was a weighted average of the two groups in the Brown et al. (1999)161 paper for VA range 20/200 to 20/400 and for counting fingers to hand motion. The utility values for the two arms are shown in the Table 36. We have assumed that the pre-treatment utility values are the same as the post-treatment utility values for any health state. The benefits result from a re-distribution amongst health states.
| Health state | Usual care arm | Intervention arm |
|---|---|---|
| Moderate NPDR | 0.7915 | 0.7915 |
| Severe NPDR | 0.7915 | 0.7915 |
| Severe NPDR and CSMO | 0.7365 | 0.7365 |
| Early PDR | 0.7047 | 0.7047 |
| Early PDR and CSMO | 0.6930 | 0.6930 |
| HR-PDR | 0.7047 | 0.7047 |
| HR-PDR and CSMO | 0.6930 | 0.6930 |
| Severe PDR | 0.7047 | 0.7047 |
| Severe PDR and CSMO | 0.6930 | 0.6930 |
| Severe NPDR PT | 0.7915 | |
| Severe NPDR and CSMO PT | 0.7365 | |
| Early PDR PT | 0.7047 | |
| Early PDR and CSMO PT | 0.6930 | |
| HR-PDR PT | 0.7047 | 0.7047 |
| HR-PDR and CSMO PT | 0.6930 | 0.6930 |
| Severe PDR PT | 0.7047 | 0.7047 |
| Severe PDR and CSMO PT | 0.6930 | 0.6930 |
| SVL/blindness | 0.6218 | 0.6218 |
Resource use and costs
Resource-use information for each of the health states was based on information from the RCOphth guidelines5 and from expert clinical opinion. These eye appointments consist of the examination being conducted using a slit-lamp ophthalmoscope and the appointment will also include VA tests, administering of eye drops, and check of current treatments. Table 37 shows the number of ophthalmology and monitoring visits for each 6-month cycle.
| Health state | No. of ophthalmology and monitoring visits |
|---|---|
| Moderate NPDR | 1 |
| Severe NPDR/severe NPDR PT/severe NPDR and CSMO with/without VI/severe NPDR and CSMO with/without VI PT | 1 |
| Early PDR/early PDR PT/early PDR and CSMO with/without VI/early PDR and CSMO with/without VI PT | 1.5 |
| HR-PDR/HR-PDR PT/HR-PDR and CSMO with/without VI/HR-PDR and CSMO with/without VI PT | 2 |
| Severe PDR/severe PDR PT/severe PDR and CSMO with/without VI/severe PDR and CSMO with/without VI PT | 2 |
| Severe vision loss/blindness | 0.5 |
For patients who receive PRP treatment we have assumed that both eyes will be treated at the same time and PRP treatment will be given over two sessions, to reduce the risk of DMO. Patients who also have DMO will receive focal laser first for both eyes and also an OCT test will also be undertaken. These two treatments have been costed as separate visits.
For the different items of resource use, the associated unit costs are presented are in pounds sterling (£) in 2012–13 prices. We have used national reference costs where possible for items such as clinic visits, laser treatment, surgery and tests165 (Table 38).
| Resource use (HRG code) | National average unit cost (lower–upper quartile) | Source |
|---|---|---|
| First Ophthalmology clinic visit (WF01B) | £106 (£87 to £124) | NHS reference costs 2012–13165 |
| Monitoring clinic visit (WF01A) | £80 (£67 to £89) | |
| PRP laser (OP BZ22B) | £131 (£69 to £145) | |
| Focal laser (OP BZ22B) | £131 (£69 to £145) | |
| OCT (OP BZ23Z) | £117 (£93 to £133) | |
| Vitrectomy surgery (DC BZ22B) | £989 (£589 to £1304) | |
| Annual cost of blindnessa | £1483 | Mitchell 2012166 |
The annual cost of blindness was obtained from a study by Mitchell et al. (2012)166 who looked at the cost-effectiveness of ranibizumab in treatment of DMO causing visual impairment. The annual cost of blindness comprised the following costs incurred by the NHS such as low-vision aids, low-vision rehabilitation (occupational health therapist), community care, depression and hip fracture/replacement, which were outlined in a previous costing study on blindness by Meads et al. (2006). 167 The costs were in pounds sterling (£) in 2010 prices and have been inflated to 2012–13 prices using the Hospital and Community Health Services Index. 168
Complications
Some patients who receive PRP may develop complications. For the model we have assumed that a proportion of people (see below) who receive PRP will develop MO or, less often, vitreous haemorrhage for one cycle only.
Data on precipitation of DMO by PRP was obtained from the ETDRS #9,9 which found that a third of eyes without MO at baseline who were assigned to early photocoagulation received focal photocoagulation when clinically significant MO developed during the 5-year follow-up.
A proportion of patients in the severe PDR or the severe PDR and CSMO with/without visual impairment may also develop vitreous or pre-retinal haemorrhage after PRP. The Diabetic Retinopathy Vitrectomy Study Report #1169 found that vitreous haemorrhage occurred frequently in eyes treated for severe PDR or very severe PDR. Over a 2-year follow-up period, 25% of the people had undergone vitrectomy.
We have assumed that in the same cycle that patients receive PRP, they have a chance of developing either DMO or vitreous haemorrhage, and within this same cycle the patient would receive treatment: patients who get MO would get focal laser and many patients who get vitreous haemorrhage receive vitrectomy surgery (some vitreous haemorrhages may resolve). We have added in the appropriate cost and a disutility value of –0.03164 has been included for that one cycle.
Mortality
Age-specific mortality rates used in the model were based on the UK general population lifetime tables from the Office for National Statistics (ONS). 170 Using the ONS data, the average probability of death for males and females were combined. As the cohort ages, mortality rates generally increase throughout the time horizon in the model. To reflect the higher mortality rates of people with diabetes than those with no diabetes we have used a weighted average of two all-cause mortality rates, which were based on UK population data to obtain a mortality multiplier: (1) Soedamah-Muthu et al. (2006)171 estimated the all-cause mortality rate in patients with type 1 diabetes compared with a non-diabetic population from the UK general practice research database; and (2) Mulnier et al. (2006)172 estimated the all-cause mortality rate in patients with type 2 diabetes in a large cohort selected from the general practice research database.
In addition, we have also included another mortality multiplier for people with diabetes with advanced retinopathy versus all people with diabetes. These mortality multipliers by severity level were obtained from two further papers:
-
Cusick et al. (2005)173 followed up a cohort of type 1 and type 2 diabetic patients from 1980 to 1985 to assess the association between diabetic complications and mortality in the ETDRS. The authors reported hazard ratios for people with moderate NPDR, severe NPDR, mild PDR and moderate/high PDR compared with a reference group of no/mild retinopathy using Cox proportional hazard models. The authors adjusted for age, sex, statistically significant baseline characteristics (p < 0.05) and all other diabetic complications and presented separate ratios for type 1 and type 2 diabetic patients. From these results, we calculated an average weighted mortality rates (hazard rates) by severity of DR for type 1 and type 2 diabetes combined. The mortality hazard ratios for people with moderate NPDR, severe NPDR, mild PDR and moderate/high PDR were 1.118, 1.422, 0.992 and 1.705, respectively, compared with diabetics with no/mild retinopathy.
-
Klein et al. (1999)174 followed up two groups of patients depending on when their diabetes was diagnosed (younger-onset or older-onset patients) to investigate the association of ocular disease and mortality. The authors reported age and sex adjusted hazard ratios for people with severe visual impairment (loss) compared with a reference group without visual impairment. For the model, we calculated an average weighted mortality hazard ratio of 3.321 for people with SVL.
Measuring cost-effectiveness
Using the Markov model we have calculated for a cohort of patients the expected quality-adjusted survival based on their likelihood of surviving each cycle, their expected health-state utility value, and their expected costs. We have adopted a 30-year time horizon and the starting age for the patient cohort is 50 years. The analysis is conducted from the perspective of the NHS and PSS. Cost-effectiveness was measured in terms of the incremental cost per QALY gained [incremental cost-effectiveness ratio (ICER)]. Discount rates of 3.5% were applied to both costs and outcomes.
We present both deterministic and probabilistic results. For the probabilistic analysis, the gamma distribution was used for costs and the beta distribution was used for utility values. 175 As the values for both costs and utilities used in the model were means or weighted averages an assumption was made for the standard error in order to calculate the alpha and beta values that are required for the probabilistic sensitivity analysis. For example, for utilities the standard error was assumed to be 0.1 of the mean value176 and for the variation in mean cost, a coefficient of variation of 0.1 of the mean value was used to obtain the standard errors. 177
To represent the uncertainty in the parameters used in the model and to illustrate sampling uncertainty, we undertook probabilistic sensitivity analyses using 1000 simulations. For the PSA to reflect the amount and pattern of the variation, results were calculated by selecting random values from each distribution. We used a gamma distribution for costs and beta distributions for utilities and transition probabilities. These bootstrapped simulations were plotted along the cost-effectiveness plane. Each point on the cost-effectiveness plane is a simulation from the probabilistic analysis. The cost-effectiveness plot represents the uncertainty surrounding the incremental costs and QALYs for the two arms that are being compared. In addition, these simulations were also used to obtain the cost-effectiveness acceptability curves (CEACs), which illustrate the effect of sampling uncertainty, in which individual model parameters were sampled from the appropriate probability distribution. CEACs were presented using a willingness-to-pay (WTP) threshold from £0 to £50,000.
Scenario and sensitivity analyses
As mentioned earlier for the base-case analysis, two treatment arms were modelled using progression data from the ETDRS studies:
-
Current practice (usual care) Patients are observed until they progress to the HR-PDR health state (and onwards) when they receive PRP.
-
Early PRP (intervention) Patients receive PRP once they progress to the severe NPDR health state.
Sensitivity analyses were conducted by altering base-case inputs to the model. Several types of scenario and sensitivity analyses were explored. Using ETDRS data for the transition probabilities, the following scenario analyses were conducted, and inputs for the scenario and sensitivity analyses are shown in Table 39:
-
PRP and anti-VEGF drugs for DR (laser and drugs) This scenario is the same as for the base-case analysis, that is, patients receive PRP for DR and focal laser for DMO. However, the difference is that patients also receive one round of anti-VEGF injections (two injections, one for each eye), in addition to their PRP treatment, in order to prevent or reduce the presence of MO after PRP. We used the cost of ranibizumab for one scenario and for the other scenario the cost of bevacizumab. We also included the cost of administering the drug.
-
PRP for DR and anti-VEGF drugs for DMO (laser and drugs for DMO) This scenario is the same as the base-case analysis, the only difference is that instead of patients receiving focal laser they get can now receive anti-VEGF medication for their DMO. We have assumed for those patients receiving anti-VEGF treatment, they have eight injections in the first 6 months (four injections for each eye). For the intervention arm, these patients then move to the corresponding post-treatment health state, where they receive six injections (three injections for each eye) and if they stay in the post-treatment state for each future cycle, they receive two further injections (one in each eye). For the usual care arm, these patients who remain in that health state they receive six injections (three injections for each eye) and if they stay in the same health state for each future cycle, they receive two further injections (one in each eye). For each visit, we have also included the cost of administering the anti-VEGF treatment. We used the cost of ranibizumab for one scenario and for the other scenario the cost of bevacizumab.
-
For the intervention arm, we have assumed that PRP treatment will start at the severe NPDR stage Patients with severe NPDR and CSMO have focal laser first. In this sensitivity analysis, we start treatment at the early PDR or early PDR and CSMO.
-
We have assumed in the base-case analysis that PRP treatment will be administered over two sittings (in total, four laser treatments for the two eyes) In this sensitivity analysis, we vary this assumption by using one sitting (two laser treatments for two eyes) and four sittings (eight laser treatments for two eyes). We have assumed that the risk of DMO remains the same.
-
In the base-case analysis, the cost of blindness did not include any residential (home care) costs In this sensitivity analysis, we add in the cost of residential care to the annual cost of blindness.
-
In the base-case analysis, we have used Brown et al. (1999)161 to estimate health-state utilities using the time-trade off method In this sensitivity analysis we used health-state utility values from Lloyd et al. (2008). 181 The authors in this paper used EQ-5D to derive utility values. We know from previous work that generic measures such as the EQ-5D are insensitive to changes that are significant to patients. 182 As mentioned in the previous chapter, one further source of data for DR progression (transition probabilities) is used in this scenario analysis.
-
In the base-case analysis, PRP was administered over two sittings In another sensitivity analysis, we have assumed that PRP will be administered in one sitting and one round of anti-VEGF medication will also be administered, as PRP laser may exacerbate DMO.
| Unit costs | ||
|---|---|---|
| Resource use (HRG code) | National average unit cost (lower–upper quartile) | Source |
| Anti-VEGF – ranibizumab | £742 | BNF178 |
| Anti-VEGF – bevacizumab | £50 to £100 | NICE179,180 |
| Administration of anti-VEGF medications (WF01A) | £80 (£67 to 89) | NHS reference costs 2012–13165 |
| Annual cost of blindness – including residential (home) care | £6972 | Mitchell 2012166 |
Results
We present here the cost-effectiveness deterministic and probabilistic results for usual care (current practice) versus intervention (early PRP).
Base-case analysis: cost-effectiveness results
For the base-case analysis we compared the cost-effectiveness of administering PRP treatment to patients with severe NPDR compared with delaying PRP treatment till the HR-PDR stages. Using data from the ETDRS for progression rates, a time horizon of 30 years and with a starting age of 50 years for the patient cohort, Table 40 shows the deterministic (undiscounted and discounted) and probabilistic (discounted) results.
| Costs and QALYs | Usual care (current practice) | Intervention (early PRP) |
|---|---|---|
| Deterministic – undiscounted | ||
| Total mean costs | £5426 | £3770 |
| Total mean QALYs | 10.3879 | 10.6306 |
| Incremental costs | –£1657 | |
| Incremental QALYs | 0.2427 | |
| ICER (cost per QALY gained) | Dominated | |
| Deterministic – discounted | ||
| Total mean costs | £3853 | £2753 |
| Total mean QALYs | 7.8236 | 7.9572 |
| Incremental costs | –£1101 | |
| Incremental QALYs | 0.1337 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic – discounted | ||
| Total mean costs | £3858 | £2746 |
| Total mean QALYs | 7.8332 | 7.9624 |
| Incremental costs | –£1112 | |
| Incremental QALYs | 0.1292 | |
| ICER (cost per QALY gained) | Dominated | |
For all scenarios, the cost for the usual care arm (i.e. delaying treatment till HR-PDR stages) was more costly than the intervention arm, and the mean QALYs were also lower (discounted deterministic results: incremental costs –£1101, incremental QALYs 0.1337). The ICER for usual care was dominated by the intervention; that is, offering PRP treatment to patients with severe NPDR was cheaper and more effective than delaying PRP treatment till the HR-PDR stages.
Figure 8 shows the cost-effectiveness plane for usual care vs. intervention (early PRP). The graph clearly shows that the cost for intervention arm is much lower than the usual care arm. There is some uncertainty on the effect of early PRP, as the QALYs are scattered over the bottom two quadrants of the cost-effectiveness plane; however, as the majority of these iterations (58%) are in the south-east quadrant of the plane, this makes the intervention slightly more cost-effective. Figure 9 shows the CEAC, for a WTP threshold from £0 to £50,000 per QALY. If a decision-maker is willing to pay between £20,000 and £30,000 per QALY, early PRP is likely to be 60% more cost-effective than usual care.
FIGURE 8.
Cost-effectiveness plane: usual care (current practice) vs. intervention (early PRP).
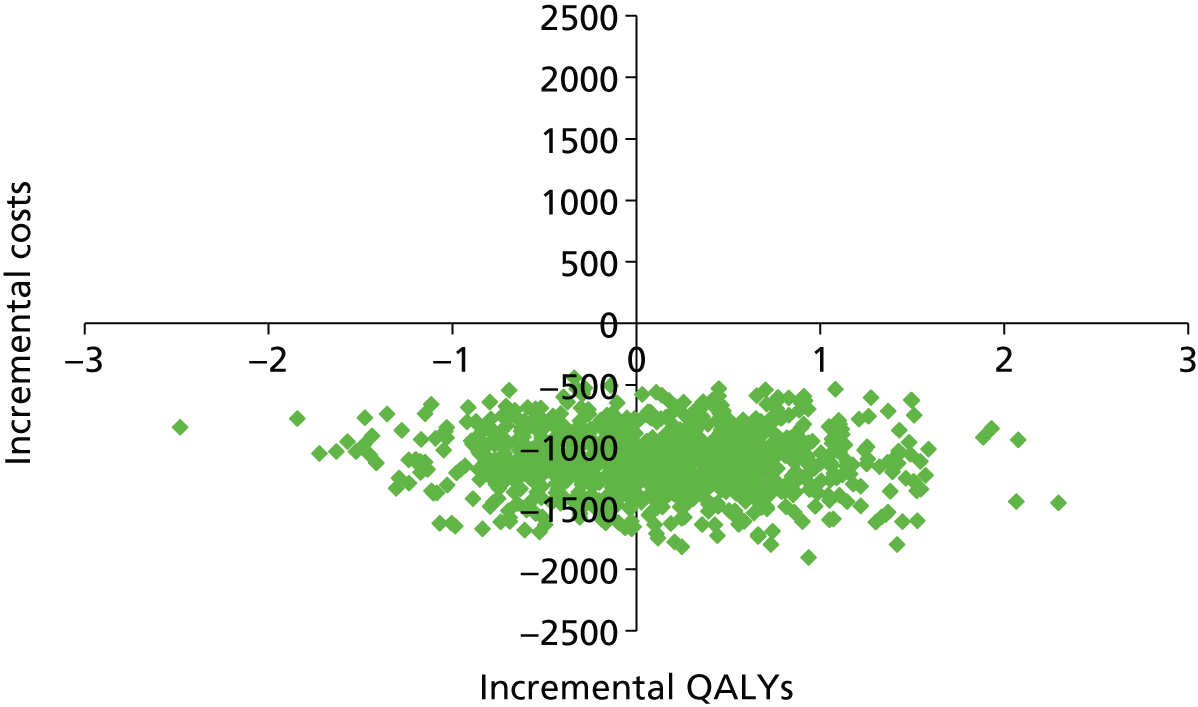
FIGURE 9.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (early PRP). INT, intervention; UC, usual care.
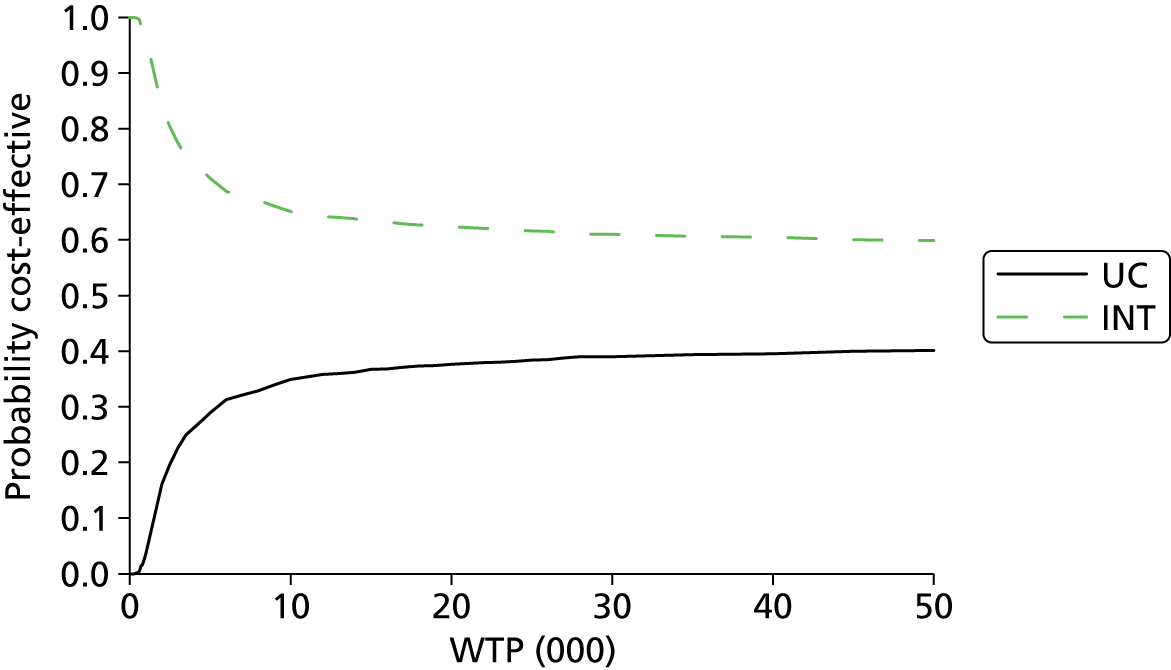
Sensitivity analysis: cost-effectiveness results
(a) Pan-retinal photocoagulation and anti-vascular endothelial growth factor drugs for diabetic retinopathy (laser and drugs)
In this scenario, patients also receive one round of anti-VEGF injections (two injections, one for each eye) in addition to PRP treatment for DR (and focal laser for DMO), in order to prevent or reduce the presence of MO after PRP. We have used the cost of ranibizumab for one scenario and for the other scenario we have used the cost of bevacizumab.
Table 41 presents the discounted results for the deterministic and probabilistic analyses.
| Costs and QALYs | Usual care (current practice) | Intervention (early PRP) |
|---|---|---|
| Deterministic – ranibizumab | ||
| Total mean costs | £4396 | £4546 |
| Total mean QALYs | 7.8236 | 7.9572 |
| Incremental costs | £150 | |
| Incremental QALYs | 0.1337 | |
| ICER (cost per QALY gained) | £1122 | |
| Probabilistic – ranibizumab | ||
| Total mean costs | £4396 | £4538 |
| Total mean QALYs | 7.8332 | 7.9624 |
| Incremental costs | £141 | |
| Incremental QALYs | 0.1292 | |
| ICER (cost per QALY gained) | £1094 | |
| Deterministic – bevacizumab | ||
| Total mean costs | £3933 | £3016 |
| Total mean QALYs | 7.8236 | 7.9572 |
| Incremental costs | –£917 | |
| Incremental QALYs | 0.1337 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic – bevacizumab | ||
| Total mean costs | £3931 | £3010 |
| Total mean QALYs | 7.8242 | 7.9552 |
| Incremental costs | –£921 | |
| Incremental QALYs | 0.1310 | |
| ICER (cost per QALY gained) | Dominated | |
The results in Table 41 show that when one round of anti-VEGF medication ranibizumab is given in addition to PRP at an extra cost of £742 for each injection in each eye plus the administration cost, even though the intervention is slightly more effective, it is also slightly more costly. The incremental cost per QALY gained ratio is £1122 (deterministic results). When one round of anti-VEGF medication bevacizumab is given in addition to PRP at an extra cost of £75 for each injection in each eye plus the administration cost, early PRP is still cheaper and more effective than usual care, that is, intervention dominates usual care (in line with the base-case results).
Figure 10 shows the cost-effectiveness plane if ranibizumab is used as the anti-VEGF medication. Although the iterations are split across the four quadrants of the cost-effectiveness plane, the majority of the iterations are in the north-east quadrant (38%), indicating that the intervention is more costly and more effective than usual care. Figure 11 shows the CEAC for using ranibizumab as the anti-VEGF medication and for a threshold £20,000–30,000, early PRP is approximately 55% more cost-effective than usual care. When bevacizumab is used as the anti-VEGF medication, the iterations fall in the bottom two quadrants (57.5% of iterations are in the south-east quadrant), even though it is cheaper, there is still some uncertainty around its effectiveness (Figure 12) and if a decision-maker is willing to pay between £20,000 and £30,000 per QALY, there is a 60% probability that early PRP is more cost-effective than usual care (Figure 13), in line with the base-case results.
FIGURE 10.
Cost-effectiveness plane: usual care (current practice) vs. intervention (early PRP) for ranibizumab.

FIGURE 11.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (early PRP) for ranibizumab. INT, intervention; UC, usual care.

FIGURE 12.
Cost-effectiveness plane: usual care (current practice) vs. intervention (early PRP) for bevacizumab.

FIGURE 13.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (early PRP) for bevacizumab. INT, intervention; UC, usual care.

(b) Pan-retinal photocoagulation for diabetic retinopathy and anti-vascular endothelial growth factor drugs for diabetic macular oedema (laser and drugs)
In this scenario, patients also receive PRP for DR and anti-VEGF drugs for DMO instead of focal laser. We have used the cost of ranibizumab for one scenario and for the other scenario we have used the cost of bevacizumab. Table 42 presents the discounted results for the deterministic and probabilistic analyses and Figures 14–17 present the cost-effectiveness planes and the CEACs.
| Costs and QALYs | Usual care (current practice) | Intervention (early PRP) |
|---|---|---|
| Deterministic – ranibizumab | ||
| Total mean costs | £22,803 | £14,373 |
| Total mean QALYs | 7.8236 | 7.9572 |
| Incremental costs | –£8430 | |
| Incremental QALYs | 0.1337 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic – ranibizumab | ||
| Total mean costs | £22,843 | £14,285 |
| Total mean QALYs | 7.8332 | 7.9624 |
| Incremental costs | –£8558 | |
| Incremental QALYs | 0.1292 | |
| ICER (cost per QALY gained) | Dominated | |
| Deterministic – bevacizumab | ||
| Total mean costs | £5474 | £4373 |
| Total mean QALYs | 7.8236 | 7.9572 |
| Incremental costs | –£1101 | |
| Incremental QALYs | 0.1337 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic – bevacizumab | ||
| Total mean costs | £5462 | £4377 |
| Total mean QALYs | 7.8242 | 7.9552 |
| Incremental costs | –£1085 | |
| Incremental QALYs | 0.1310 | |
| ICER (cost per QALY gained) | Dominated | |
FIGURE 14.
Cost-effectiveness plane: usual care (current practice) vs. intervention (early PRP) for ranibizumab.
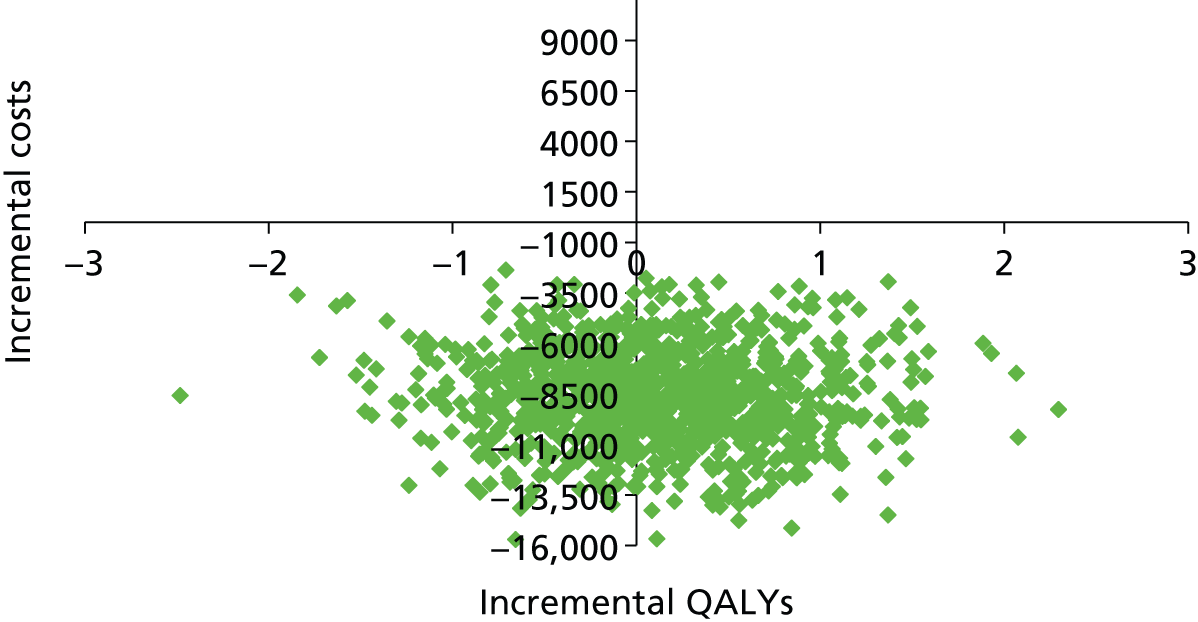
FIGURE 15.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (early PRP) for ranibizumab. INT, intervention; UC, usual care.
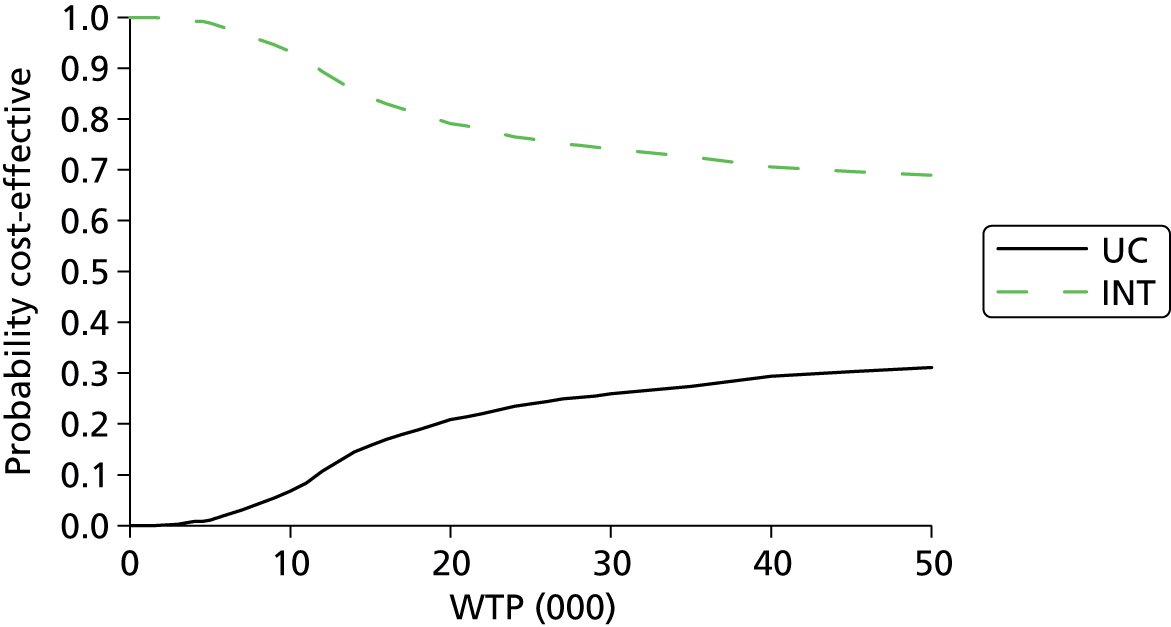
FIGURE 16.
Cost-effectiveness plane: usual care (current practice) vs. intervention (early PRP) for bevacizumab.
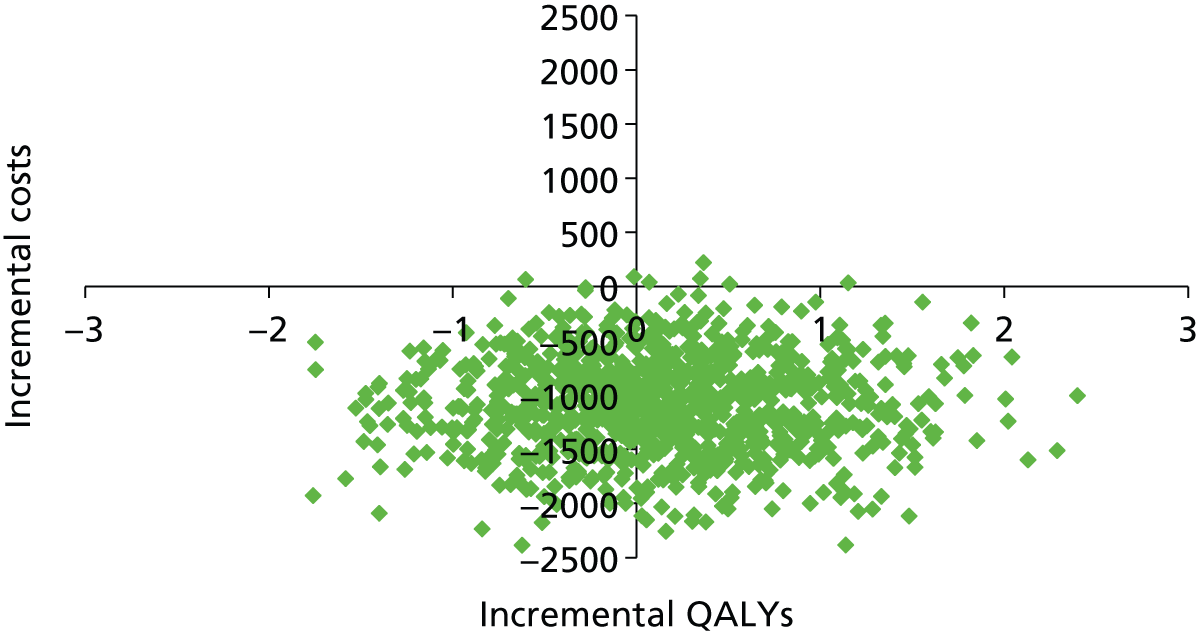
FIGURE 17.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (early PRP) for bevacizumab. INT, intervention; UC, usual care.
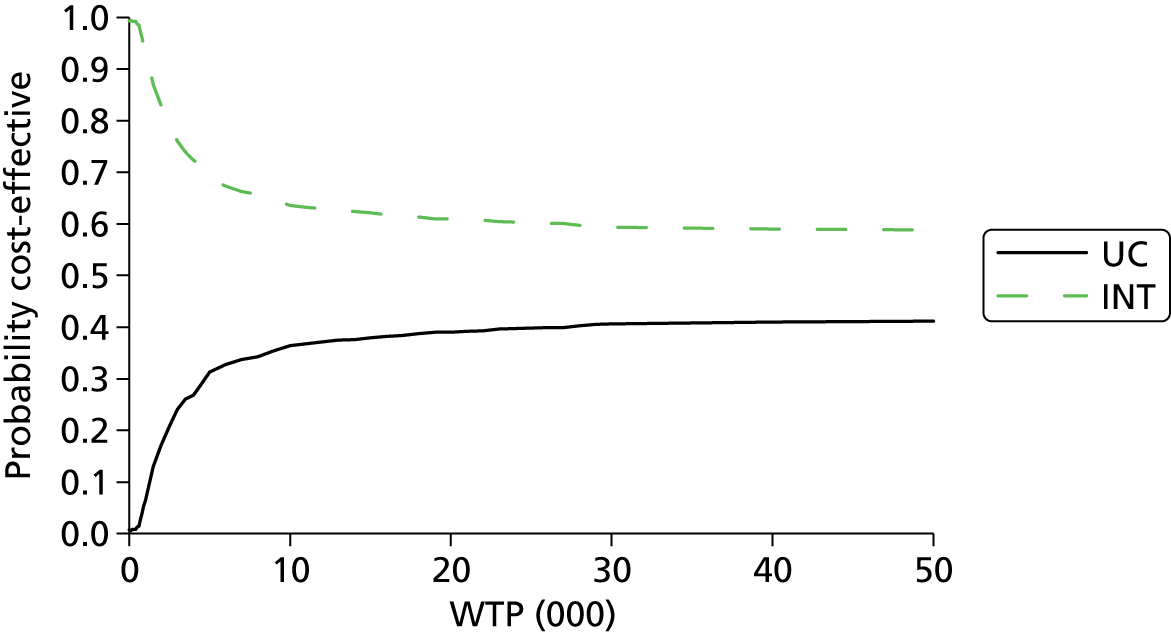
The results in Table 42 show that using anti-VEGF medication (either ranibizumab or bevacizumab) for DMO and PRP for DR, for each scenario, the costs of the intervention arm are lower than with the usual care arm, and also there are more QALYs gained in the intervention arm (early PRP) compared with usual care. That is, early PRP dominates usual care. The corresponding cost-effectiveness planes are shown in Figures 14 and 16, where the majority of iterations are in the bottom two quadrants of the plane (approximately 58% of iterations are in the south-east quadrants). If ranibizumab is used to treat DMO, the corresponding CEAC shows that if the decision-maker is willing to pay £20,000 per QALY, there is nearly an 80% probability that the intervention is more cost-effective, and if they are willing to pay £30,000 per QALY then the probability that intervention is more cost-effective than usual care is 75% (see Figure 15). Whereas if bevacizumab is used for DMO, and if a decision-maker is willing to pay between £20,000 and £30,000 per QALY, there is a 60% probability that early PRP is more cost-effective than usual care (see Figure 17), which is in line with the base-case results. Note that these results assume multiple injections of anti-VEGF agents to treat DMO, in contrast with single injections to reduce the risk of DMO. So the cost-effectiveness depends on costs of anti-VEGF avoided.
(c) Pan-retinal photocoagulation treatment starts at early proliferative diabetic retinopathy
In this scenario, patients receive PRP for DR at the early PDR or early PDR and CSMO with/without visual impairment stages.
Table 43 presents the discounted results for the deterministic and probabilistic analyses for patients receiving PRP at the early PDR stage. For both scenarios, intervention is only slightly cheaper than usual care by approximately £120; however, the intervention is more effective. Owing to these small differences, the intervention has been found to dominate usual care.
| Costs and QALYs | Usual care (current practice) | Intervention (early PRP) |
|---|---|---|
| Deterministic | ||
| Total mean costs | £3853 | £3725 |
| Total mean QALYs | 7.8236 | 7.8645 |
| Incremental costs | –£128 | |
| Incremental QALYs | 0.0409 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic – ranibizumab | ||
| Total mean costs | £3858 | £3738 |
| Total mean QALYs | 7.8332 | 7.8787 |
| Incremental costs | –£120 | |
| Incremental QALYs | 0.0454 | |
| ICER (cost per QALY gained) | Dominated | |
Figure 18 shows the cost-effectiveness plane for usual care versus intervention (early PRP at the early PDR stage). The graph clearly shows the uncertainty as the iterations are scattered across the four quadrants. However, the majority of the iterations are in the south-east quadrant (34.3%), which emphasises the dominance (albeit small) of intervention over usual care. This uncertainty is also shown in the CEAC (Figure 19), if the decision-maker is willing to pay between £10,000 and £50,000 per QALY, early PRP at the early PDR stage is likely to be no more cost-effective than usual care.
FIGURE 18.
Cost-effectiveness plane: usual care (current practice) vs. intervention (early PRP at early PDR stage).

FIGURE 19.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (early PRP at early PDR stage). INT, intervention; UC, usual care.
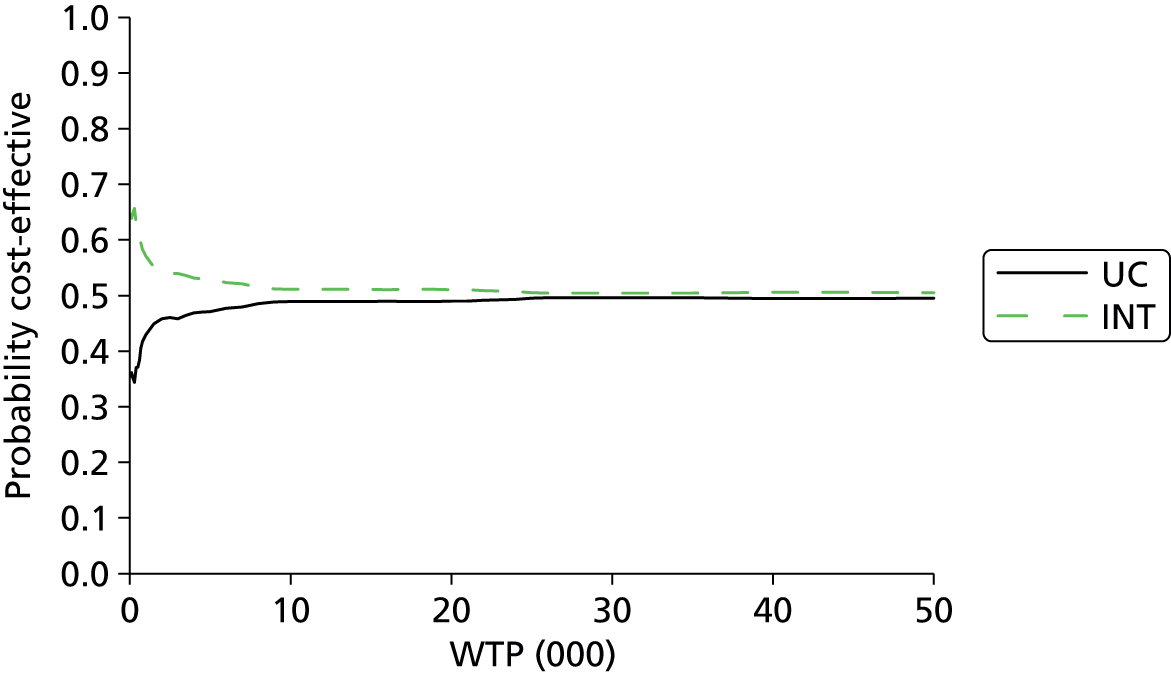
(d) Pan-retinal photocoagulation treatment in one or four sittings
We have assumed in the base-case analysis that PRP treatment will be administered over two sittings (in total, four laser treatments for the two eyes). In this sensitivity analysis, we have varied this assumption by using one sitting (two laser treatments for two eyes) and four sittings (eight laser treatments for two eyes). We have assumed that the risk of DMO remains the same.
Table 44 shows the results when PRP is administered in either one or four sittings. For each scenario, the costs of the intervention arm are lower than the usual care arm and also there are more QALYs gained in the intervention arm (early PRP) than with usual care. That is, the intervention (early PRP) dominates usual care. When PRP is administered over four sittings compared with the one sitting, the difference in incremental costs falls by almost a half. Figure 20 shows the cost-effectiveness plane if PRP is administered in one sitting and Figure 21 shows the cost-effectiveness plane if PRP is administered over four sittings. Both graphs show that most the iterations fall in the bottom two quadrants (approximately 55% of iterations are in the south-east quadrants); even though it is cheaper there is still some uncertainty around its effectiveness. If a decision-maker is willing to pay between £20,000 and £30,000 per QALY, then there is a 55–60% probability that the intervention is more cost-effective than usual care (Figures 22 and 23), in line with the base-case results.
| Costs and QALYs | Usual care (current practice) | Intervention (early PRP) |
|---|---|---|
| Deterministic – one sitting | ||
| Total mean costs | £3762 | £2452 |
| Total mean QALYs | 7.8236 | 7.9572 |
| Incremental costs | –£1310 | |
| Incremental QALYs | 0.1337 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic – one sitting | ||
| Total mean costs | £3765 | £2450 |
| Total mean QALYs | 7.8431 | 7.9482 |
| Incremental costs | –£1316 | |
| Incremental QALYs | 0.1051 | |
| ICER (cost per QALY gained) | Dominated | |
| Deterministic – four sittings | ||
| Total mean costs | £4035 | £3353 |
| Total mean QALYs | 7.8236 | 7.9572 |
| Incremental costs | –£682 | |
| Incremental QALYs | 0.1337 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic – four sittings | ||
| Total mean costs | £4026 | £3346 |
| Total mean QALYs | 7.8416 | 7.9519 |
| Incremental costs | –£680 | |
| Incremental QALYs | 0.1103 | |
| ICER (cost per QALY gained) | Dominated | |
FIGURE 20.
Cost-effectiveness plane: usual care (current practice) vs. intervention (early PRP) – one sitting for PRP.
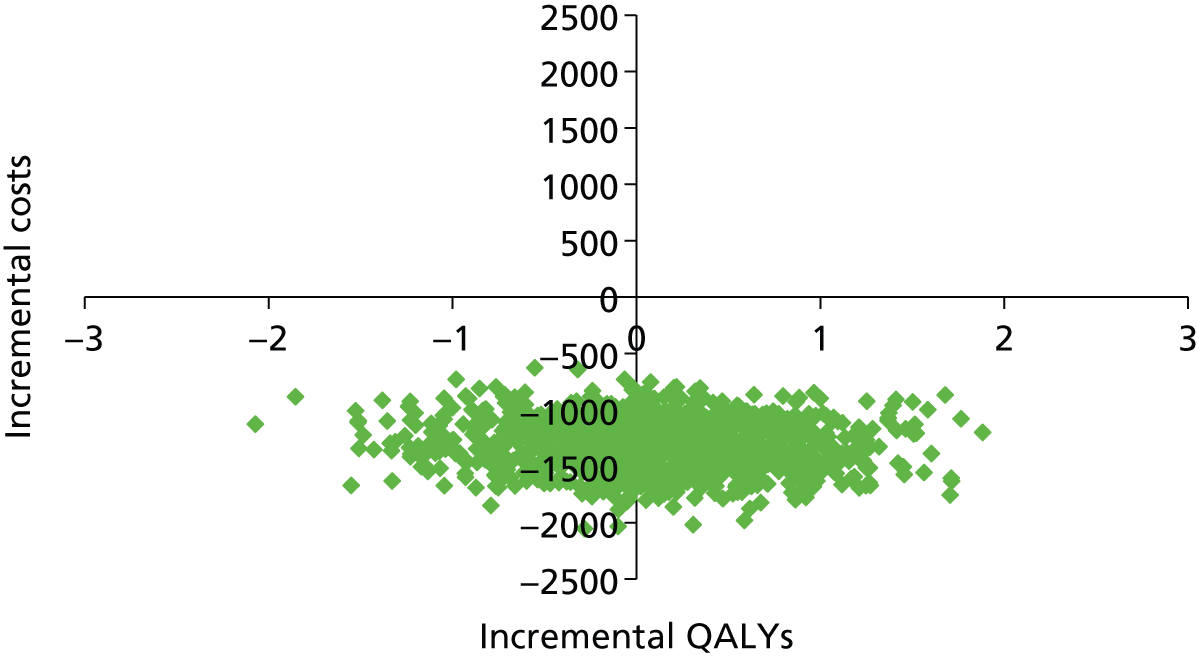
FIGURE 21.
Cost-effectiveness plane: usual care (current practice) vs. intervention (early PRP) – four sittings for PRP. INT, intervention; UC, usual care.

FIGURE 22.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (early PRP) – one sitting for PRP.
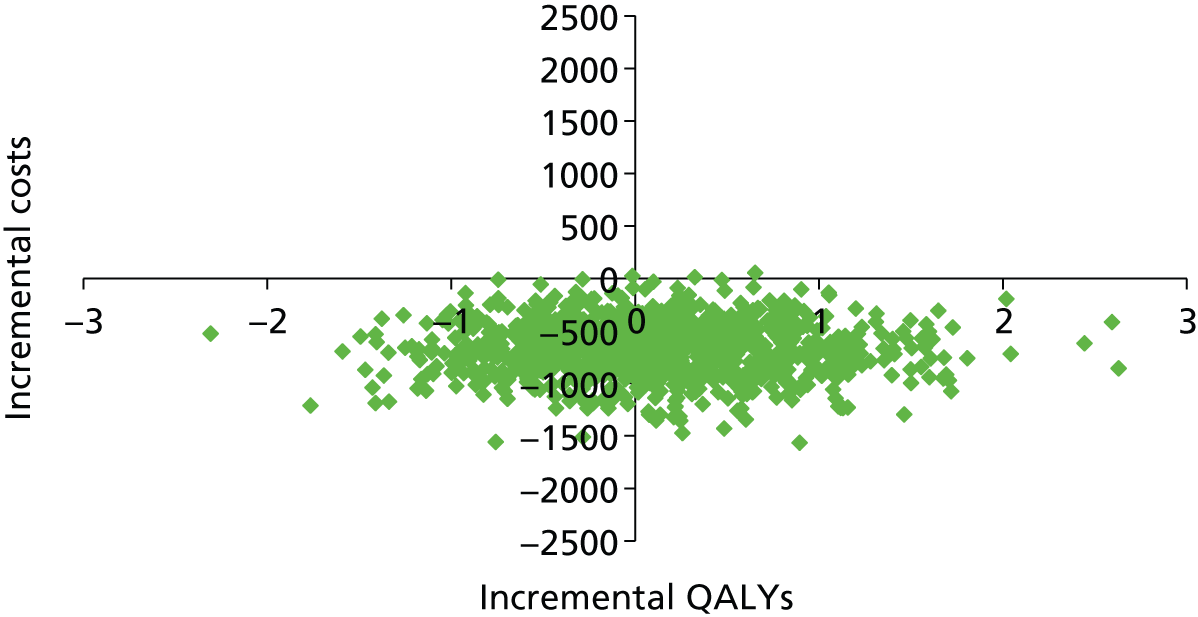
FIGURE 23.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (early PRP) – four sittings for PRP. INT, intervention; UC, usual care.

(e) Including residential (home care) costs into the annual cost of blindness
In the base-case analysis, the cost of blindness did not include any residential (home care) costs. In this sensitivity analysis, we add in the cost of residential care to the annual cost of blindness.
Table 45 presents the discounted results for the deterministic and probabilistic analyses for usual care versus intervention inclusive of the residential care costs in the annual cost of blindness. For both scenarios, intervention is cheaper than usual care and is more effective. That is, the intervention (early PRP) dominates usual care. Figure 24 shows the cost-effectiveness plane and the graph clearly shows that the intervention is cheaper; however, there is uncertainty in the effectiveness, as the iterations are scattered across the bottom two quadrants [the majority of the iterations are in the south-east quadrant (54.8%)]. This uncertainty is also shown in the CEAC (Figure 25), if the decision-maker is willing to pay £10,000 per QALY then there is a 66% probability that the intervention is more cost-effective than usual care; however, the cost-effectiveness falls slightly if the decision-maker has a higher threshold (at £30,000 per QALY the intervention is 58% more cost-effective).
| Costs and QALYs | Usual care (current practice) | Intervention (early PRP) |
|---|---|---|
| Deterministic | ||
| Total mean costs | £4951 | £3135 |
| Total mean QALYs | 7.8236 | 7.9572 |
| Incremental costs | –£1816 | |
| Incremental QALYs | 0.1337 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic | ||
| Total mean costs | £4942 | £3134 |
| Total mean QALYs | 7.8386 | 7.9396 |
| Incremental costs | –£1.808 | |
| Incremental QALYs | 0.1010 | |
| ICER (cost per QALY gained) | Dominated | |
FIGURE 24.
Cost-effectiveness plane: usual care (current practice) vs. intervention (annual cost of blindness inclusive of residential care costs).

FIGURE 25.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (annual cost of blindness inclusive of residential care costs). INT, intervention; UC, usual care.

(f) Using health-state utilities from Lloyd et al. (2008)
In the base-case analysis, we have used utilities from Brown et al. (1999)161 to estimate health-state utilities using the time-trade off method. In this sensitivity analysis we have used health-state utility values from Lloyd et al. (2008). 181
Table 46 presents the discounted results for the deterministic and probabilistic analyses for usual care versus intervention when using health-state utility values from Lloyd et al. (2008). 181 For both scenarios, intervention is cheaper than usual care and is more effective. That is, the intervention (early PRP) dominates usual care. The utility values for both arms are approximately 1.3 QALYs lower than the base-case analysis. Figure 26 shows the cost-effectiveness plane and the graph clearly shows that the intervention is cheaper; however, there is uncertainty in the effectiveness as the iterations are scattered across the bottom two quadrants [the majority of the iterations are in the south-east quadrant (58.9%)].
| Costs and QALYs | Usual care (current practice) | Intervention (early PRP) |
|---|---|---|
| Deterministic | ||
| Total mean costs | £3853 | £2753 |
| Total mean QALYs | 6.4845 | 6.6040 |
| Incremental costs | –£1101 | |
| Incremental QALYs | 0.1195 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic | ||
| Total mean costs | £3851 | £2749 |
| Total mean QALYs | 6.4925 | 6.6144 |
| Incremental costs | –£1102 | |
| Incremental QALYs | 0.1220 | |
| ICER (cost per QALY gained) | Dominated | |
FIGURE 26.
Cost-effectiveness plane: usual care (current practice) vs. intervention (using health-state utilities from Lloyd et al. 2008181).
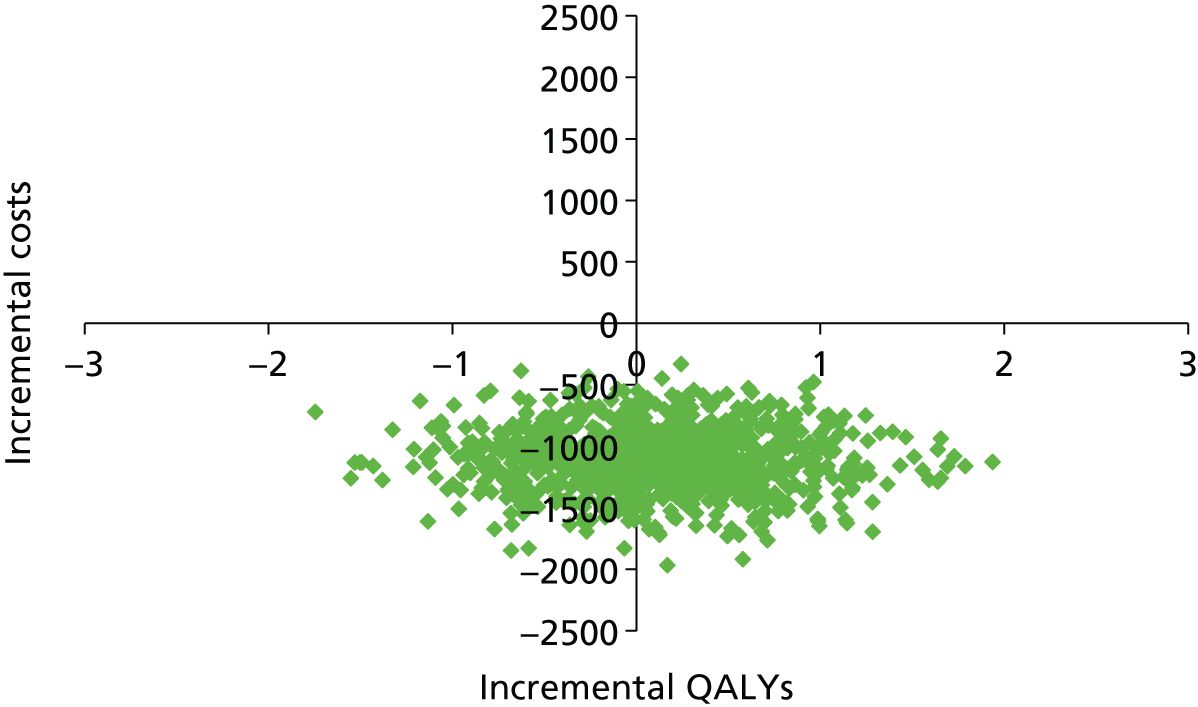
If a decision-maker is willing to pay between £20,000 and £30,000 per QALY, then the probability is around 60% that the intervention is more cost-effective than usual care (Figure 27; see also Figure 23), in line with the base-case results.
FIGURE 27.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (using health-state utilities from Lloyd et al. 2008181). INT, intervention; UC, usual care.

(g) Pan-retinal photocoagulation treatment in one sitting plus anti-vascular endothelial growth factor drugs
In this scenario, patients have PRP administered in just one sitting. To reduce or prevent the presence of MO after PRP, one round of anti-VEGF medication (two injections, one for each eye) will also be administered. We have used the cost of ranibizumab for one scenario and for the other scenario the cost of bevacizumab has been used. Table 47 presents the discounted results for the deterministic and probabilistic analyses.
| Costs and QALYs | Usual care (current practice) | Intervention (early PRP) |
|---|---|---|
| Deterministic – ranibizumab | ||
| Total mean costs | £4305 | £4245 |
| Total mean QALYs | 7.8236 | 7.9572 |
| Incremental costs | –£60 | |
| Incremental QALYs | 0.1337 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic – ranibizumab | ||
| Total mean costs | £4294 | £4237 |
| Total mean QALYs | 7.8370 | 7.9674 |
| Incremental costs | –£57 | |
| Incremental QALYs | 0.1304 | |
| ICER (cost per QALY gained) | Dominated | |
| Deterministic – bevacizumab | ||
| Total mean costs | £3842 | £2716 |
| Total mean QALYs | 7.8236 | 7.9572 |
| Incremental costs | –£1126 | |
| Incremental QALYs | 0.1337 | |
| ICER (cost per QALY gained) | Dominated | |
| Probabilistic – bevacizumab | ||
| Total mean costs | £3840 | £2712 |
| Total mean QALYs | 7.8206 | 7.9626 |
| Incremental costs | –£1128 | |
| Incremental QALYs | 0.1420 | |
| ICER (cost per QALY gained) | Dominated | |
Table 47 shows that when PRP is administered in one sitting in addition to one round of anti-VEGF ranibizumab or bevacizumab, early PRP is still cheaper and more effective than usual care, that is, intervention dominates usual care (in line with the base-case results). There was a greater cost difference between the two arms when bevacizumab was used.
Figure 28 shows the cost-effectiveness plane if ranibizumab is used as the anti-VEGF medication and PRP is administered in one sitting. Although the iterations are split across the four quadrants of the cost-effectiveness plane, the majority of the iterations are in the south-east quadrant (34%), indicating that the intervention is cheaper and more effective than usual care. Figure 29 shows the CEAC for using ranibizumab as the anti-VEGF medication and when PRP is administered in one sitting, and for a threshold of £0–50,000, there is a 60% probability that early PRP is more cost-effective than usual care. When bevacizumab is used as the anti-VEGF medication, the iterations fall in the bottom two quadrants (58.3% of iterations are in the south-east quadrant) (Figure 30), and if a decision-maker is willing to pay between £20,000 and £30,000 per QALY there is again a 60% probability that early PRP is more cost-effective than usual care (Figure 31), in line with the base-case results.
FIGURE 28.
Cost-effectiveness plane: usual care (current practice) vs. intervention (early PRP) for PRP in one sitting and one round of ranibizumab.
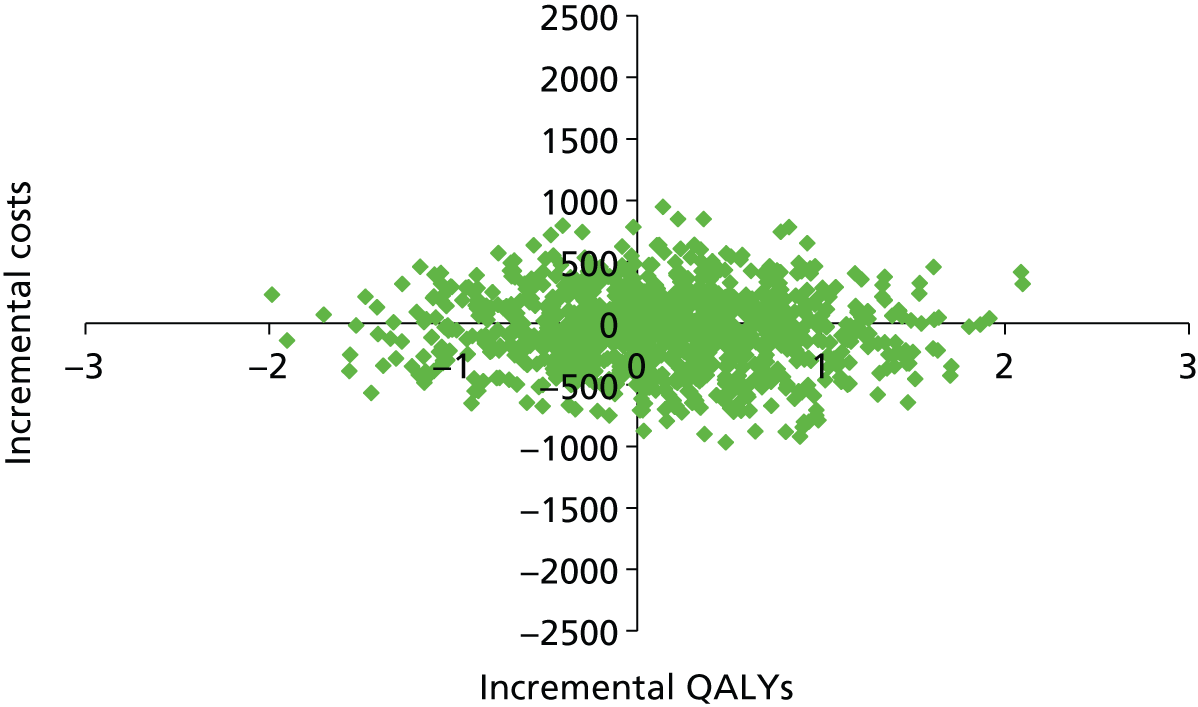
FIGURE 29.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (early PRP) for PRP in one sitting and one round of ranibizumab. INT, intervention; UC, usual care.
FIGURE 30.
Cost-effectiveness plane: usual care (current practice) vs. intervention (early PRP) for PRP in one sitting and one round of bevacizumab.

FIGURE 31.
Cost-effectiveness acceptability curve: usual care (current practice) vs. intervention (early PRP) for PRP in one sitting and one round of bevacizumab. INT, intervention; UC, usual care.

Finally, we conducted some sensitivity analyses around the assumption that people with DMO and NPDR are at a greater risk of developing more severe retinopathy than people with NPDR alone. Owing to the lack of information, we assumed that the progression rate is twice that of people with severe NPDR alone developing more severe retinopathy (see Chapter 6). In the sensitivity analyses we varied this assumption by using values of 0.5, 1, 3 and 4, but we found that intervention (early PRP) still dominated usual care by being cheaper and more effective.
Discussion
We have built a Markov (state-transition) model to assess the cost-effectiveness of observing patients until they progress to HR-PDR states where they receive PRP (usual care) versus patients receiving PRP treatment once they progress to the severe NPDR health states (intervention).
For the base-case analysis, we have adopted a 30-year time horizon. A hypothetical cohort of 1000 diabetic patients with a starting age of 50 years were followed. The cycle length for each model was set to 6 months, and transitions between each health state occur at the end of cycle. The analysis was conducted from the perspective of the NHS and PSS. The main source of data for the transition probabilities was the ETDRS studies. 9 Information on mortality rates for patients with diabetes and complications associated with PRP were obtained from the literature. Health-state utilities data were obtained from peer-reviewed published studies for DR and health outcomes were measured in QALYs. The majority of unit costs were obtained from the NHS reference costs database. 165 All costs are in pounds sterling (£) in 2012–13 prices. Results are expressed as incremental cost per QALY gained. An annual discount rate of 3.5% was applied to both costs and QALYs. We ran the model deterministically and probabilistically with 1000 iterations. We undertook various sensitivity analyses. These bootstrapped iterations were plotted onto cost-effectiveness planes and they were also used to calculate the CEACs. The CEACs were presented using a WTP threshold from £0 to £50,000.
Methods and summary of findings
For the base-case analysis, offering PRP treatment to patients with severe NPDR compared with delaying PRP treatment till the HR-PDR stages was cheaper and more effective, that is, the intervention was dominant. Treating earlier with PRP laser, at a cost of £131 per eye, meant that fewer people in the intervention arm than in the usual care arm progressed to more advanced stages of DR. Delaying treatment for patients in the usual care arm meant that more of them progressed to the higher stages of DR such as severe PDR health states including the severe vision loss/blindness state as compared with the intervention arm.
The annual cost of blindness excluding residential care cost was £1483, which partly explains the higher costs associated with the usual care arm than the intervention arm. Evidence from the cost-effectiveness plane showed that although costs were lower for the intervention arm, there was uncertainty in the effectiveness arm; as the majority of these iterations were in the south-east quadrant of the cost-effectiveness plane, this made the intervention slightly more cost-effective. The CEAC plane indicated that if a decision-maker is willing to pay £20,000–30,000 per QALY then treating earlier at the severe NPDR stage was more cost-effective than usual care (delaying treatment till HR-PDR stages).
A number of sensitivity analyses were undertaken to determine the cost-effectiveness of various options; the majority of results were in line with the base-case analyses. For example, assuming that the effectiveness remained the same, when adding the cost of one round of anti-VEGF injections (two injections, one for each eye) in addition to PRP treatment for patients with DR, when using bevacizumab at a cost of £75 per eye (plus administration costs), intervention dominated usual care. When ranibizumab was used as the anti-VEGF injection at a cost of £742 per eye (plus administration costs), even though it was effective, it was also more expensive. This additional cost of treating earlier is clearly shown in the increase in costs. This uncertainty was also highlighted in the cost-effectiveness plane, where the bootstrapped iterations moved up along the vertical axis (incremental cost axis). However, for both anti-VEGF medications, the CEACs showed that the intervention was slightly more cost-effective than usual care.
In another sensitivity analysis instead of patients receiving focal laser for their DMO, they received anti-VEGF medication (in total, patients would receive seven injections for each eye (14 injections in total) in their first year plus the administration costs), in line with the base-case results intervention dominated usual care. The main cost driver here was cost of the anti-VEGF drug; if ranibizumab was used the cost difference between the two arms was over £8000, this cost difference between the two arms falls to £1000 if bevacizumab was used. The CEACs showed that if a decision-maker was willing to pay £20,000 per QALY, there is an 80% probability that the intervention was more cost-effective than usual care if ranibizumab was used, whereas if bevacizumab was used the probability falls to 60% (see Figures 15 and 17).
In the base-case analysis we had assumed that PRP was administered over two sittings (four laser treatments for two eyes), we varied this assumption in the sensitivity analysis, where we had assumed that the risk of DMO remained the same and PRP was administered over one (two laser treatments for two eyes) or four sittings (eight laser treatments for two eyes); again, the results were in line with the base-case analysis, that is, intervention dominated care. When the cost of residential care (at a cost of approximately £5000) was added to the annual cost of blindness, assuming that the effectiveness remained the same, the cost difference between the two arms increased approximately by £700 as compared with the base-case analysis. However, the results were still in line with base-case analysis, as the intervention still dominated usual care.
When we assumed that patients would receive PRP treatment in the intervention arm in the early PDR stages as opposed to the severe NPDR stages (base-case analysis), the intervention was found to dominate usual care. Looking at this in more detail, the differences between the two arms was negligible – costs were approximately £100 lower in the intervention arm and there was a QALY gain of around 0.04 more QALYs in the intervention arm. The bootstrapped iterations were scattered across all four quadrants in the cost-effectiveness plane and the CEAC found that treating at the early PDR stage is likely to dominate usual care. One reason for this may be due to the data that we used for the progression rates, as the ETDRS did not report results separately for NPDR and early PDR.
In the final sensitivity analysis we used health-state utilities from the Lloyd et al. (2008)181 paper, as opposed to the Brown et al. (1999)161 paper. The utility values in the base-case analysis were based on patients with DR elicited using the time-trade off method. 161 In the sensitivity analysis, utilities were elicited from patients with DR using the EQ-5D measure. 181 The EQ-5D measure is the preferred measure by NICE when eliciting utility values. 183 However, we know from previous work that generic measures such as the EQ-5D are insensitive to changes that are significant to patients in vision-related studies;182 in addition to the EQ-5D, vision-related measures such as the NEI VFQ-25139 and the Vision and Quality of Life (VisQoL) questionnaires184,185 could be included in studies/trials to measure utilities. This may partly explain why the utility values in the paper that used EQ-5D181 were a lot lower than those in the paper that used the time-trade off measure. 161 However, the overall direction and magnitude of results in the sensitivity analysis remained the same as the base-case analysis, and intervention dominated usual care.
Strengths and limitations
Although we undertook a thorough search for cost-effectiveness studies of the use of PRP and/or anti-VEGF medication for patients with moderate or severe NPDR or early PDR we could not identify any economic evaluations or modelling-based studies (see Chapter 5). The Markov model built here is novel, as it considers using PRP treatment at an earlier stage of DR as opposed to current practice (treat when a patient reaches HR-PDR stage). The model also contains more detailed health states differentiated by the different severity levels for DR than previous studies, which have focused on screening for DR and not necessarily PRP treatment for DR. 60,158,159 The model also considers using PRP laser in combination with anti-VEGF medication.
However, the model does have a number of limitations:
-
Firstly, we populated the model with progression data mainly from the ETDRS trial. 9 Although this information from the ETDRS was useful, it is now dated – patients in the ETDRS had poorer glycaemic control, and the treatment of diabetes and laser treatment since the ETDRS studies were published have been greatly improved. More recent studies have since been conducted, but no trial has addressed the timing questions.
-
Secondly, in the sensitivity analyses where the treatment for retinopathy was with the use of PRP laser and/or anti-VEGF treatment, we have assumed that the treatment effect, in terms of progressing to more severe retinopathy was the same as PRP treatment alone, as the effect of the anti-VEGF drugs is temporary, and we have found no evidence that their temporary effect increase the effect of PRP.
-
Thirdly, in the model, we have assumed that patients can develop adverse events, the most important of which is MO, as a result of receiving PRP treatment for DR. We obtained these proportions based on information on from the ETDRS. 9 Although these studies are useful, these complication rates may not be accurate because laser treatment since then has improved (see Chapter 3). Owing to these changes, we expect that the rate of adverse events from PRP treatment would have decreased over time; although we believe that this would not have an overall impact on the magnitude and direction of cost-effectiveness ratio. Another side effect of PRP laser is pain; however, costs of pain treatment such as using a simple analgesia (e.g. paracetamol) are negligible and are unlikely to have a significant impact on the cost-effectiveness ratio. Also, in terms of complications, in the economic model we have not taken into account any adverse events due to focal laser or any adverse effects of anti-VEGF treatment. We know that there are few side effects from anti-VEGF treatment (see Chapter 4) and, again, this would not have any significant impact on the magnitude and direction of cost-effectiveness ratio.
-
Fourthly, in the model we have assumed that the costs of PRP laser and focal laser are the same, and these unit costs were obtained from the NHS reference costs database. 165 However, although the reference costs may be the same, PRP takes much longer to do than focal or grid photocoagulation, and more sessions are required – focal/grid can be done in one session. To get a more accurate picture of these costs it would have been better to have carried out ‘bottom-up costing’ and then analyses with different types of laser (i.e. multi-spot, argon) and their associated costs, including local anaesthetics for multi-spot. We believe having more accurate costs for the different types of laser will not alter the magnitude and direction of the ICER. For the anti-VEGF treatment we obtained costs for bevacizumab from NICE179 for two NHS hospital trusts and for ranibizumab from the British National Formulary. 178 The cost differential between the two anti-VEGF treatments was about £650. Even though we used ranibizumab at the current list price, in the economic model the intervention (early PRP) was cost-effective compared with usual care (current practice); however, if we were to use the discounted price (which is confidential), the intervention should become even more cost-effective.
-
Fifthly, in terms of utility estimates that we have used in the economic model, the literature search conducted in Chapter 5 did not identify any studies with health-state utility values by the detailed severity levels that we have in our economic model. We used the studies by Fong et al. (2002)162 and Smith et al. (2008)163 to characterise the different VA levels into health states. Using this information we were then able to link these health states levels to the health-state utilities values for patients with DR as reported in the Brown et al. (1999)161 paper. There were some drawbacks with this method, as the Fong et al. (2002)162 paper reported only two diabetic severity levels that were applicable to our model: moderate/severe NPDR and PDR; hence, in our model we have the same utility value for a patient with early PDR as someone who has severe PDR. Likewise, Smith et al. (2008)163 reported only MO; we did not have any information on whether this was clinically significant or not, and whether there was any visual impairment.
-
We also applied a utility decrement of 0.03 to patients who move to a state with CSMO. This may be a conservative assumption based on data from a previous screening study for DR. 164 However, our literature review conducted in Chapter 5 did not highlight any further data on disutilities associated with progressing through all the different stages of DR.
-
Sixthly, we did not include in the model the impact on patients of losing the ability to drive.
-
Seventhly, we did not include an analysis of systemic treatment aimed at improving glycaemic and BP control. A patient with an HbA1c of 10%, a BP of 150/100 mmHg and renal problems is at much higher risk of progression to visual loss than one who achieves excellent metabolic and BP control.
-
Finally, in the economic model we have not differentiated by gender or whether the patient had type 1 or type 2 diabetes owing to the insufficient information, such as progression rates and utility values, which we had available for the model. Although, some subgroup analyses could be done using some of the available literature, there is not enough evidence on the use of early PRP treatment in these patients and this will provide even further uncertainty to the cost-effectiveness estimates.
Other potential issues that may affect the cost-effectiveness
In the modelling, we do not include corticosteroid injections (steroids). The rationale for this is that we would want to include only short-acting ones with the aim of reducing the risk of post-PRP. No one is going to use long-acting steroids in this situation. So that means that the steroids licensed for DMO – long-acting dexamethasone and fluocinolone – would not be considered. That leaves only triamcinolone, and we should note that the preservative-free form of that used in the DRCRN trials, Trivaris, is no longer in production. It was made by Allergan who also make Ozurdex. So we are left with Kenalog. That is not licensed for use in the eye. It was designed for use in joints, but was widely used for eye conditions before the anti-VEGFs arrived.
However in Chapter 4, we report more adverse effects with triamcinolone (raised IOP) than with the anti-VEGFs. Its other advantages over the anti-VEGFs might be that is much less expensive than ranibizumab and aflibercept, though not than bevacizumab, that it lasts longer (3–4 months or more) and so requires fewer injections, and that in DMO it may work in patients in whom an anti-VEGF has failed. The anti-VEGFs are a considerable advance in DMO but they produce good results (gain of 10 or more letters) in only around half of eyes. So there may still be an occasional place for triamcinolone in the UK and we have retained the triamcinolone trials in Chapter 4. It is also worth noting that in many countries, the licensed anti-VEGFs are unlikely to be affordable.
The factors that would determine the cost-effectiveness of adjuvant anti-VEGF treatment include:
-
The frequency of development of CSMO after PRP. This appears to be less frequent with modern ‘lighter’ laser methods than was seen in ETDRS, as noted in some of the studies in Chapter 3. For example, in Bandello et al. (2001),75 the proportion of patients with worsening CSMO was significantly greater in the classical PRP group than in the light PRP group (23% vs. 3%; p = 0.023).
-
Compared with older studies, the better detection of MO by OCT compared with clinical examination.
-
The usually temporary nature of MO after PRP, meaning that the disutility would also be temporary (and not influential if modelling involves 6-month cycles because it would usually have resolved before that time point?). However, as noted earlier, the visual loss is not always temporary, at least in older studies such as DRS and ETDRS. 9,10
-
The utility weight given to modest changes in VA. The effect of a small change may have profound impact in QoL if it means that patients have to stop driving.
-
The disutility of pain, though that is transient, and a more important economic factor might be the disutility of less effective PRP if it could not be completed owing to pain. As reported earlier (see Chapter 4), pain may be reduced by administration of an anti-VEGF a week before PRP. 123 However administration of subconjunctival or sub-Tenon’s anaesthesia should remove pain.
-
The cost of anti-VEGF treatment, both drug cost (high with ranibizumab, modest with bevacizumab) and administration. If using ranibizumab at list price (the discounted price is confidential), the cost might be in the region of £900.
-
The cost of PRP, if anti-VEGF cover allowed it to be given in one session rather than several. Patients could have sub-Tenon’s anaesthesia, then the anti-VEGF injection, and then PRP, and then be seen in 3 months. This would be more convenient for them. It might also be safer to have one visit in hospitals where appointments may be postponed and treatment delayed, increasing the risk of vitreous haemorrhage before treatment is completed.
-
Any adverse effects of anti-VEGF treatment. In Chapter 4, it was found that anti-VEGF mainly caused transient adverse events related mainly to injection such as subconjunctival haemorrhage or foreign body sensation. The incidence of serious drug-related adverse events like endophthalmitis, uveitis, vitreous haemorrhage or retinal detachment was minimal. In DRCRN (2011),120 there was one case of endophthalmitis in the ranibizumab group. In the same study,120 more patients in the sham group had retinal detachment (3% vs. 1%) and vitreous haemorrhage (12% vs. 5%) than those in the ranibizumab group. None of the studies found that anti-VEGF drug caused thromboembolic adverse events. The overall pain score was also significantly lower in the group receiving PRP plus anti-VEGF than in those receiving PRP only (Lucena 2013123 – VAS score 4.7 vs. 60.8).
-
The net gain in QALYs, taking into account reduced disutility from CSMO; any disutility from adverse effects including the injection into the eye itself; and possibly reduced disutility from fewer PRP sessions.
Comparisons with other studies
No studies were identified that had considered the cost-effectiveness of early treatment with PRP for severe NPDR, and, therefore, appropriate comparisons with other existing studies were not possible. For example, the cost-effectiveness studies of DR, which have been identified, assessed the cost-effectiveness of various screening strategies in a cohort of people where the starting point was people who diabetic with no or early retinopathy,158,164 whereas in our model the starting point was patients with moderate NPDR, or studies that assessed the cost-effectiveness of various treatment options for DMO,166,186,187 whereas our model primarily focused on treating patients who had severe NPDR.
Chapter 8 Discussion and research needs
Statement of principal findings
-
The DRS10 confirmed that laser photocoagulation was effective in reducing the risk of visual loss due to PDR.
-
The ETDRS9 addressed the timing question, and reported that earlier PRP, at severe NPDR and early PDR stages, was more effective then postponing PRP till later PDR. However, it did not report results separately for early PDR and severe NPDR, and the difference in the primary outcome of SVL did not quite reach statistical significance at the ETDRS level of 99% CIs: RR 0.77, 99% CI 0.56 to 1.06.
-
A composite outcome of SVL and vitrectomy did give a statistically significant result: RR 0.67, 99% CI 0.52 to 0.97.
-
There is currently insufficient evidence to recommend PRP for NPDR.
-
There has been a trend towards lighter methods of PRP, where lighter refers to using lasers at lower power and producing less severe burns. PSC: The multi-spot laser allow more spots to be delivered in one application, hence shortening the procedure time, but more spots are needed as short-duration burns do not spread. The multi-spot lasers are also of shorter duration (10–20 ms as compared with 100 ms in older systems).
-
Laser devices that can apply patterns of spots have advantages over single-spot devices. Newer lasers have other features that allow parameters to be controlled. However, in terms of visual outcomes, there is a lack of robust data favouring one laser over another, and there are no data to demonstrate superiority of any laser technology compared with argon laser, which was the laser used in the ETDRS. The SDM system shows promise in focal laser for DMO but there are very few data on use in PRP.
-
There is accumulating evidence that combining PRP with either an anti-VEGF drug or triamcinolone may reduce the risk of PRP-associated MO. Whether this improves long-term results is not known. There would be advantages if it allowed PRP to be done in one session.
-
There are uncertainties around the cost-effectiveness of PRP at NPDR or early PDR stage, but, in a range of analyses, earlier PRP either dominates delaying PRP till HR-PDR stage or is cost-effective with quite low ICERs.
-
The uncertainty around the economic analysis and the limitations for the economic model indicate the need for more research. The results from the economic model should be treated with caution.
The deferred arms in the studies may not reflect routine care, where delays in treatment may result in patients having vitreous bleeds and presenting as emergencies. Even without delays, treatment at advanced PDR stage can have complications such as vitreous haemorrhage and tractional retinal detachment due to contraction of NV tissue. This would not be a problem with treatment at less severe stages.
Strengths and limitations
The strengths of this report include:
-
wide-ranging searches for evidence
-
extension of the original scope to include current laser technologies and new drug treatments, to add value to the review and make it more useful for planning future research
-
a thorough review of adverse events of PRP, based on both RCTs and observational studies, and including outcomes important to patients, such as ability to drive
-
recommendations of the specific research questions, underpinned by a thorough presentation of research currently under way
-
a new economic model of DR.
The limitations included:
-
Dependence on one trial, the ETDRS, for the critical issue of timing of PRP – whether to use it at NPDR stage. The ETDRS was carried out in the 1980s, before OCT was available, and it did not give results separately for NPDR and early PDR.
-
Few trials comparing argon laser with newer laser technologies. So no data to show whether modern laser technologies are superior to the argon laser used in ETDRS.
-
A lack of data on the most effective use of anti-VEGF drugs in combination with lasers – should they be given prophylactically to all or only to those who develop DMO after PRP?
-
A lack of good, up-to-date, long-term data on progression and regression of retinopathy after treatment.
Research completed but not yet published in full
Several studies of combination treatment with anti-VEGF drugs and laser have been reported as abstracts. Inevitably details are sparse, till full publication.
In two studies, the anti-VEGF used was pegaptanib:
-
In a small, three-armed trial, Estudillo and Gonzalez (2013)188 [the same trial seems to have been reported by Gonzalez (2013)189 alone but with slightly different figures] randomised 20 eyes to pegaptanib alone (three initial injections then repeats every 12 weeks; eight patients), pegaptanib (three initial injections only) plus selective laser treatment (eight), and standard PRP alone (four). The third group did better in BCVA after 12 months.
-
Leal et al. (2013)190 randomised 22 patients with HR-PDR into two arms in what was described as an exploratory Phase II study. One arm had standard PRP as per DRS. The other arm had ‘progressive PRP’ plus pegaptanib, The progressive PRP starts with the DRS third ring, followed if need be by further laser treatment moving inwards. (Full details are not given in the abstract but this sounds like the lighter PRP technique reported by Madeira et al. 2009191 in an abstract from a EURETINA meeting.) They call it external ring photocoagulation, and the aim appears to be to minimise visual field defect and the risk of MO, and have less effect on night vision (argon green laser was used).
Eyes that received the combined treatment lost on average only one letter of BCVA compared with six in the PRP-alone group.
Two abstracts reported results with bevacizumab:192,193
-
Preti et al. (2013)192 randomised both eyes of 23 patients with HRC-PDR. One eye was treated with PRP plus bevacizumab (number of injections not given) and the other with PRP alone. No visual outcome results are given, only choroidal thickness.
-
Another abstract from the same group with 30 patients appears to have come from the same study. 193 It reports no significant change in BCVA in either group.
Four studies reported on the use of ranibizumab;194–197 three are by-products of DMO trials:194–196
-
Lohmann et al. (2013)194 reported that in the RELATION trial of ranibizumab + laser versus laser alone for DMO (so focal or grid laser, not PRP), a subgroup of 27 patients had PDR at baseline. Of those (20) in the combined group, eight (40%) showed regression of PDR, whereas none in the laser-alone group did. They conclude that anti-VEGF treatment may be effective in PDR.
-
Ehrlich et al. (2013)195 report findings from the RISE and RIDE trials in which patients with DMO were randomised to ranizumab (two doses) or sham injections. Patients could have macular laser or PRP if required. Secondary outcomes included progression of DR, expressed in ETDRS stages, vitreous haemorrhage and need for PRP. Regression by 36 months was commoner in the ranibizumab arms (3-step improvement 3% in sham arm, 15% and 13% in ranibizumab arms). Progression to PDR was reported in 34% in the sham arm, compared with 13% and 15% in the ranibizumab arms.
-
The DRCRN carried out a RCT of IVR and triamcinolone for DMO, and in this abstract report effects amongst those with DR, outcomes being progression from NPDR to PDR, occurrence of vitreous haemorrhage, need for PRP or worsening of DR by at least two levels. 196 The advantage of this study is large numbers. In eyes without PDR at baseline (n = 538), the drug arms had lower (4%) progression than the sham injection plus laser arm (10%). (Note that laser was for DMO, so focal or grid, not PRP.) In eyes with PDR at baseline (n = 254), more (20%) progressed in the non-drug arm than in the drug arms (2–9%). The authors cautiously comment that ‘These results suggest that use of these drugs to prevent worsening of DR is worth further investigation’.
-
In a group of patients with bilateral PDR, Ferraz et al. (2013)197 compared PRP alone, given in three sessions as per ETDRS guidelines, with PRP plus ranibizumab at weeks 1 and 4. Eyes were randomly allocated to ranibizumab or a sham injection. Results were reported separately for those with and without CSMO at baseline. In those with CSMO, after 6 months’ follow-up, BCVA improved by four letters in the combined group and decreased by five letters in the PRP-alone group. In those without CSMO, VA improved by eight letters in the combined group and was unchanged in the PR-alone group. Vitreous haemorrhage was less common in the combined group (10%) than the PRP-alone group (23%).
Ongoing or recently completed research searches
Sources searched for ongoing or recently completed research were ClinicalTrials.gov, WHO (World Health Organization) Clinical Trials Registry Platform Search Portal, Current Controlled Trials, UK Clinical Trials Gateway, EU Clinical Trials Register, and UK Clinical Research Network Study Portfolio.
Searches were performed on 16 January 2014 using keywords: diabetic retinopathy AND (laser OR photocoagulation OR PRP OR scatter).
Studies selected were those in which the main condition being studied was DR (we excluded those that included patients with DMO only) and the intervention included PRP photocoagulation alone or in combination with another intervention, such as an intravitreal drug, and where the results had not been found published in full or as a meeting abstract. We selected only RCTs, apart from one observational study, which we considered to be investigating an outcome-important patients’ QoL (i.e. being able to maintain a UK driving licence).
We included 16 studies in total: 15 RCTS and the one observational study. Eight studies include anti-VEGF drugs (one each with aflibercept, bevacizumab, and pegaptinib and five with ranibizumab) either given alone or as adjunctive therapy to PRP and seven involve different methods of laser delivery. All have a Clinical ClinicalTrials.gov Identifier (and will be identified by this number) apart from one that was identified in the UK Clinical Research Network Study Portfolio database (UKCRN ID 13472). Studies are briefly summarised in more detail in Table 48, and also described below.
| Title and sponsors | Aim and study design | Main inclusion criteria | Intervention | Primary outcome and key dates |
|---|---|---|---|---|
| Treatment with Intravitreal Aflibercept Injection for Proliferative Diabetic Retinopathy, The A.C.T. Study Sponsor: Ophthalmic Consultants of Long Island Collaborator: Regeneron Pharmaceuticals NCT01813773 |
To assess the safety of IAI in the treatment of proliferative diabetic retinopathy by evaluating the incidence and severity of adverse events Randomised open-label study, single centre, pilot study Estimated enrolment: 20 |
Retinal neovascularisation secondary to DR, BCVA in the study eye better than 20/320 using an ETDRS chart | Arm 1: IAI (2 mg) every 4 weeks. Following the 5 initial injections between day 1 and week 16, this group will continue to receive IAI every 4 weeks, beginning week 20, through week 48 Arm 2: IAI (2 mg) every 8 weeks. Following the 5 initial injections between day 1 and week 16, this group will receive IAI every 8 weeks, beginning week 24, through week 48 |
Incidence and severity of adverse events of IAI in the treatment of PDR Time frame: 52 weeks Study start date: March 2013 Estimated study completion date: March 2015 Estimated primary completion date: December 2014 |
| Effect of Adjunctive Intravitreal Bevacizumab Before Panretinal Photocoagulation in Macular Thickness and Retinal Nerve Fiber Layer Thickness Sponsor: Kyungpook National University NCT01504724 |
To investigate the effect of adjunctive IVB before PRP compared with only PRP on CMT and retinal nerve fibre layer thickness in patients with severe diabetic retinopathy without MO Randomised, parallel assignment, open label Enrolment: 30 |
Patients with severe NPDR or early PDR without MO, BCVA of 20/25 or better. Patients followed up for at least 6 months after the first PRP | Arm 1: IVB group. Patients were treated with IVB injections approximately within 1 week before the first PRP. Then patients had PRP, which was done in three sessions at weeks 0, 1 and 2 according to ETDRS guidelines. The superior, inferior, and nasal and temporal areas were treated sequentially Arm 2: Only PRP group. Patients had PRP, which was done in three sessions at weeks 0, 1 and 2 according to ETDRS guidelines. The superior, inferior, and nasal and temporal areas were treated sequentially |
CMT measured by Cirrus HD OCT Time frame: 6 months after first PRP session This study has been completed Study start date: August 2011 Study completion date: August 2013 Primary completion date: August 2012 (Final data collection date for primary outcome measure) |
| Macugen for Proliferative Diabetic Retinopathy Study With Extended Dosing (M-PDRS ED) Sponsor: Valley Retina Institute Collaborator: Pfizer NCT01486771 |
To investigate if intravitreal injections of pegaptanib will induce the regression of PDR and reduce the need for retinal photocoagulation Pilot study, randomised, factorial assignment, open label Enrolment: 30 |
Active PDR with HRCs as defined by the DRS; ETDRS VA score ≥ 24 letters (approximately 20/320) and ≤ 85 letters (approximately 20/20) by the ETDRS VA protocol at the screening visit; eyes with mild pre-retinal haemorrhage or mild VH that does not interfere with clear visualisation of the macula and optic disc; evaluating physician believes that PRP can be safely withheld for 3 weeks | Arm 1: IV Macugen Q6. Receive 3 intravitreal pegaptanib injections at 6-week intervals, then 3 additional injections at 12-week intervals Arm 2: IV Mac Q6. Receive selective laser photocoagulation after 3 intravitreal pegaptanib injections Arm 3: PRP. Will act as the control group, subjects in this group will receive standard PRP (mETDRS protocol) |
Efficacy of intravitreal pegaptanib sodium injections in inducing regression of HR-PDR (as determined by percentage of eyes without treatment failure) using standard PRP as the control arm Time frame: 54 weeks Study start date: November 2007 Estimated study completion date: February 2014 Estimated primary completion date: August 2013 (final data collection date for primary outcome measure) |
| Efficacy and Safety of Lucentis® Monotherapy Compared With Lucentis® Plus Panretinal Photocoagulation (PRP) and PRP in the Treatment of Patients With High Risk Proliferative Diabetic Retinopathy Sponsor: José Cunha-Vaz, Association for Innovation and Biomedical Research on Light and Image NCT01280929 |
To demonstrate superiority of one of the treatment arms over a 12-month treatment period in the regression of neovascularisation Prospective, randomised, multicentre, open-label Phase II study Estimated enrolment: 54 |
HR-PDR eye, BCVA at baseline > 20/320 in the study eye, clear ocular media and adequate pupillary dilatation to permit good quality fundus photography, IOP < 21 mmHg, type 1 or type 2 diabetes | Arm 1: PRP at month 0 that can be repeated after month 3 Arm 2: Intravitreous injection of ranibizumab every 4 weeks at month 0, month 1 and month 2 that can be repeated after month 3 Arm 3: Combination treatment of ranibizumab intravitreous injections plus PRP (2 weeks ± 1 week after injection), at month 0, month 1 and month 2 that can be repeated after month 3 |
Regression of neovascularisation Time frame: 12-month treatment Start date: September 2010 Estimated study completion date: December 2013 |
| Multicenter 12 Months Clinical Study to Evaluate Efficacy and Safety of Ranibizumab Alone or in Combination with Laser Photocoagulation vs. Laser Photocoagulation Alone in Proliferative Diabetic Retinopathy (PRIDE) Sponsor: Novartis Pharmaceuticals Collaborators: Cologne Image Reading Center & Laboratory (CIRCL) Spranger Laboratories NCT01594281 |
To assess the efficacy and safety of ranibizumab alone or in combination with laser treatment vs. laser treatment alone in proliferative diabetic retinopathy Randomised, parallel, single-blind (outcomes assessor), Phase IV study Estimated enrolment: 120 |
Proliferative diabetic retinopathy, BCVA ≥ 20 ETDRS letters, diabetes with HbA1c ≤ 12% (= 107 mmol/mol) | Arm 1: Ranibizumab Arm 2: Laser photocoagulation Arm 3: Ranibizumab and laser photocoagulation |
Change of area of neovascularisations as measured by FA Time frame: 12 months Study start date: December 2012 Estimated study completion date: April 2015 (final data collection date for primary outcome measure) |
| Prospective, Randomized, Multicentre, Open-label, Phase II/III Study to Assess Efficacy and Safety of Ranibizumab 0.5 mg Intravitreal Injections Plus Panretinal Photocoagulation (PRP) vs. PRP in Monotherapy in the Treatment of Subjects With High Risk Proliferative Diabetic Retinopathy (PROTEUS) Sponsor: Association for Innovation and Biomedical Research on Light and Image NCT01941329 |
To compare the efficacy and safety of ranibizumab 0.5 mg intravitreal injections plus PRP vs. PRP alone in the regression of the neovascularisation area in patients with high-risk proliferative diabetic retinopathy over a 12-month treatment period Prospective, randomised, multicentre, open-label, Phase II/III study Estimated enrolment: 94 |
High-risk proliferative diabetic retinopathy, neovascularisation in the disc ≥ ¼ DA or neovascularisation elsewhere ≥ ½ DA, NVE < ½ DA + vitreous and/or pre-retinal haemorrhage and/or rubeosis; NVD < ¼ DA + vitreous and/or pre-retinal haemorrhage and/or rubeosis, BCVA at baseline ≥ 24 ETDRS letters score (approximate Snellen equivalent 20/320) | Arm 1: Ranibizumab + PRP. 3 intravitreous injections of ranibizumab combined with standard PRP (2 ± 1 weeks after injection), at months 0, 1 and 2, which can be repeated after month 3, with always at least 1 month of interval between injections Arm 2: PRP monotherapy. PRP between months 0 and 2, with 1 mandatory laser session in month 0 and more laser sessions as needed until month 2 to complete the PRP treatment. After completing the PRP treatment, PRP sessions can be repeated from months 3 to 11 |
Regression of neovascularisation Time frame: 12-month treatment Study start date: January 2014 Estimated study and primary completion date: July 2015 |
| PASCAL Laser vs. ETDRS Laser Associated with Intravitreal Ranibizumab (IVR) vs. Only IVR for Proliferative Diabetic Retinopathy Sponsor: University of São Paulo NCT02005432 |
To evaluate the effects on retinal morphophysiology of full scatter single target PRP vs. full scatter multiple target PRP (both combined with intravitreous injections of ranibizumab) vs. IVR alone in patients with PDR Randomised, parallel assignment, single blind (outcomes assessor) Enrolment: 31 |
Presence of PDR, presence of retinal neovascularisation, defined as active neovessels (fine retinal vessels with saccular dilatations or extremities covered with blood or associated with recurrent VH) with VA better than 20/800 and with no previous laser treatment | Arm 1: SS-PRP arm. PRP single shoot (ETDRS – green diode laser) + 0.05 ml IVR Arm 2: MS-PRP arm. Multiple shoot panphotocoagulation (PASCAL) plus IVR Arm 3: IVR arm. Only IVR |
Mean change in the total area of active retinal neovessels, as measured by FA leakage area, in mm2 Time frame: Baseline to week 48 Study start date: February 2012 Estimated primary completion date: November 2014 |
| Prompt Panretinal Photocoagulation vs. Intravitreal Ranibizumab with Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy Sponsor: DRCRN Collaborators: NEI, Genentech NCT01489189 |
To determine if VA outcomes at 2 years in eyes with PDR that receive anti-VEGF therapy with deferred PRP are non-inferior to those in eyes that receive standard prompt PRP therapy Randomised, parallel assignment, open label Estimated enrolment: 316 |
Diagnosis of diabetes (type 1 or type 2), and meets at least all of the following ocular criteria: presence of PDR which the investigator intends to manage with PRP alone but for which PRP can be deferred for at least 4 weeks in the setting of IVR, in the investigator’s judgement, electronic-ETDRS VA letter score > 24 (approximate Snellen equivalent 20/320) on the day of randomisation, media clarity, pupillary dilatation | Arm 1: Intravitreal injection of 0.5 mg ranibizumab (at baseline and up to every 4 weeks using defined retreatment criteria) with deferred PRP (PRP is deferred until failure/futility criteria for intravitreal injection are met) Arm 2: Prompt PRP alone at baseline (full session completed within 56 days) |
Mean change in VA from baseline to 2 years Time frame: 2 years (primary outcome) Total follow-up: 5 years Study start date: March 2012 Estimated study completion date: December 2017 Estimated primary completion date: December 2014 (final data collection date for primary outcome measure) |
| Incidence of MO After Panretinal Photocoagulation (PRPC) Performed in a Single Session Versus Four Sessions in Diabetic Patients Sponsor: Centre Hospitalier Universitaire Dijon NCT01766362 |
To show that PRPC performed in a single session using a PASCAL laser leads to better management of the disease, a saving of time and better comfort for both patient and doctor Randomised, parallel, open-label, Phase III study Estimated enrolment: 90 |
Diabetes type 1 or 2, severe non-proliferative or early proliferative diabetic retinopathy (according to the Alfediam classification); CMT ≤ 350 µm according to spectralis OCT | Arm 1: PRPC using PASCAL laser – a single session Arm 2: PRPC using PASCAL laser – four sessions, with every session spaced out by a month |
CMT at 9 months after the start of PRPC using PASCAL laser Time frame: 9 months Study start date: October 2011 Estimated primary completion date: October 2013 |
| Safety and Effectiveness Study of 532 nm Laser Subthreshold Panretinal Photocoagulation for Severe NPDR Sponsor: Sun Yat-sen University NCT01759121 |
To evaluate the therapeutic efficacy of 532-nm laser partially sub-threshold PRP with PASCAL end point management function for severe NPDR Randomised, parallel controlled, double-blind (subject, outcomes assessor), clinical trial Estimated enrolment: 84 |
Diagnosis of severe NPDR, age: 45–80 years, BCVA ≥ 20/100, myopia ≤ –6 degree (–6D), no previous PRP, and no major ocular surgery within 3 months | Arm 1: Traditional-PRP (T-PRP): 532-nm short-pulse PRP with PASCAL function Arm 2: Sub-threshold PRP (S-PRP): 532-nm partially subthreshold short-pulse PRP with PASCAL Endpoint Management function |
Change of BCVA; probability of VH Time frame: 1 year Study start date: December 2012 Estimated study completion date: July 2014 |
| Safety and Efficacy of Single-session, Low-Fluence Panretinal Photocoagulation for Proliferative Diabetic Retinopathy Sponsor: Asociación para Evitar la Ceguera en México NCT01737957 |
To determine the safety and efficacy of a single session of low-fluence PRP when compared with full-fluence PRP Randomised, single-blind study Estimated enrolment: 60 |
Type 1 or type 2 diabetes, proliferative diabetic retinopathy | Arm 1: Low-fluence PRP in a single session with 532-nm green laser (other name: PUREPOINT laser) Arm 2: Full-fluence PRP in two sessions with 532-nm laser (other name: PUREPOINT laser) |
Measurement of macular thickness changes by spectral domain OCT Time frame: Baseline, 1 week, 6 weeks, 12 weeks, 16 weeks Study start date: November 2012 Estimated study completion date: March 2013 |
| Morphological and Functional Retinal Changes Following Retinal Photocoagulation Using a Semiautomated Patterned Scanning Laser System in Proliferative Retinopathy or Macular Edema Secondary to Diabetes Mellitus or Retinal Vein Occlusion Sponsor: Medical University of Vienna NCT00682240 |
To measure imaging of retinal morphological changes with time secondary to laser treatment as assessed with high-definition OCT Randomised, crossover assignment, open label Estimated enrolment: 80 |
Patients with either retinopathy/maculopathy secondary to diabetes type 1 or 2 with medical indication for segmental or pan-retinal laser coagulation or with necessity for completion of previous incomplete laser photocoagulation, patients with PDR requiring pan-retinal laser treatment (Arms 1, 2 and 3), patients with MO requiring central focal or grid laser treatment (Arm 4) | Arm 1: A single-session pan-retinal laser treatment using the PASCAL laser system Arm 2: Multi-session pan-retinal laser treatment using the PASCAL laser system Arm 3: Multi-session pan-retinal laser treatment according to the conventional protocol, using a conventional laser system Arm 4: Patients with persistent central or para-central DMO receiving focal or grid laser treatment |
Retinal morphological changes with time secondary to laser treatment as assessed with OCT Time frame: 2007–14 Study start date: October 2007 Estimated study completion date: December 2015 Estimated primary completion date: June 2015 |
| Panretinal Photocoagulation for Diabetic Retinopathy with PASCAL Laser Sponsor: Federal University of São Paulo NCT01304225 |
To investigate the clinical efficacy and safety of the PASCAL laser for DR. Patients with proliferative or severe non-proliferative retinopathy will be treated with PRP utilising different treatment strategies Randomised, parallel assignment, single blind (subject) Estimated enrolment: 60 |
Proliferative or severe NPDR (type 1 or type 2), BCVA of 20/50 or better | Arm 1: 100-ms single-shot. PRP utilising 100-ms pulse duration, moderate intensity burns, in a single-shot fashion Arm 2: 20-ms multiple-shot. PRP utilising 20-ms pulse duration, moderate intensity burns, in a multiple-shot fashion Arm 3: 20-ms multiple-shot, barely visible. PRP utilising 20-ms pulse duration, barely visible intensity burns, in a multiple-shot fashion |
Incidence of SVL after 1 year [SVL is defined as VA worse or equal to 5/200 in two consecutive visits (DRS/ETDRS primary outcome)] Time frame: 1 year Study start date: September 2010 Estimated study completion date: March 2013 |
| PASCAL Pan Retinal Photo-Stimulation in Pre-Proliferative Diabetic Retinopathy: a Safety and Efficacy Study Sponsor: Central Manchester University Hospitals NHS Trust (CMFT) Funder: Optos Plc, OptiMedica Corporation UKCRN ID 13472 |
To find if treating patients using a single session of lower intensity laser (PASCAL® Pan Retinal PhotoStimulation, PRPhS), at an earlier stage in DR (during the severe NPDR stage) when the abnormal new vessels are not developed, will prevent diabetic patients developing PDR Randomised, single-centre, pilot, feasibility study Global sample size: 30 |
1. Treatment-naive severe NPDR 2. ETDRS VA equivalent to 35 letters or better 3. Any of the following: extensive (> 20) intraretinal haemorrhages in each of 4 quadrants; definite venous beading in 2+ quadrants; prominent IRMA in 1+ quadrant 4. Mean CRT of ≤ 300 µm as measured by Deep Range Imaging OCT scans |
Arm 1: Patients will be treated with the normal parameters used in PASCAL laser Arm 2: Patients will be treated with a lower intensity than normal, using the Endpoint Management system (a new software from the PASCAL laser, which allows operator to decrease the intensity of the burns (invisible burns), showing some landmarks with normal intensity; the area which has been treated can be seen) Arm 3: Patients will be observed, such as is being done in clinic routinely |
Primary outcomes not specified Closure date: 3 March 2015 |
| Comparison of Two Laser Therapy Methods for PDR Sponsor: Shahid Beheshti Medical University NCT01232179 |
A comparison between results and side effects of treatment of proliferative diabetic retinopathy by conventional and extended targeted PRP Randomised, double-blind, Phase II trial |
Patients with PDR suitable for photocoagulation therapy, pupil with dilatation ≥ 6 mm, no previous treatment with anti-VEGF drugs 3 months before study, no previous vitroretinal surgery or laser therapy | Arm 1: Conventional PRP Arm 2: Targeted PRP: 1200–1600 spots in far periphery retina, anterior equator |
No leakage in widefield FA Time frame: 3 months after laser therapy Study start date: October 2010 Estimated primary completion date: April 2011 |
| A Prospective Clinical Study of the Effects of Panretinal Photocoagulation Delivered With a Multi-spot Photocoagulator on Retinal Sensitivity and Driving Eligibility in Patients With Diabetic Retinopathy Sponsor: Moorfields Eye Hospital NHS Foundation Trust Collaborator: Insulin Dependent Diabetes Trust NCT01383772 |
To determine the risk of failing the VA criteria to hold a driving licence following retinal laser treatment delivered with a multi-spot photocoagulator Observational, cohort study Estimated enrolment: 100 |
Patients of either sex aged 18 years or over, diabetes (type 1 or type 2), BCVA ≥ 6/60 in both eyes, requiring full bilateral PRP, no previous laser treatment | Patients will undergo baseline VA testing and complete a QoL questionnaire. All patients will receive their laser treatment as part of standard clinical care via the multi-spot photocoagulator, which will require approximately four 20-minute sessions. At 6 months following the completion of treatment the patients will undergo repeat VA testing as conducted at baseline and complete a QoL questionnaire | Risk of failing VA criteria to hold a driving licence Time frame: VAs at baseline and at 6 months Study start date: June 2012 Estimated study completion date: December 2013 Estimated primary completion date: August 2013 |
NCT01813773 is a small randomised pilot study assessing the incidence and severity of adverse events with two different dosing regimens of intravitreal aflibercept injections for PDR. Both arms of the study have five initial injections between day 1 and week 16; Arm 1 will then continue to receive IAI every 4 weeks, beginning week 20, through week 48; and Arm 2 will receive intravitreal aflibercept injections every 8 weeks, beginning week 24, through week 48.
We also note that the Efficacy and Mechanism Evaluation (EME) programme is to fund a trial called CLARITY, which will look at the clinical effectiveness and cost-effectiveness of aflibercept used in combination with PRP for PDR. 198,199 CLARITY is short for ‘Clinical efficacy and mechanistic evaluation of aflibercept for proliferative diabetic retinopathy’.
NCT01504724 is a recently completed 6-month randomised study of 30 patients (60 eyes) looking at the effect of adjunctive IVB before PRP versus PRP only on CMT in patients with severe DR without MO. Patients had weekly PRP treatments in three sessions, and then were randomly assigned to either the IVB group (who had adjunctive IVB within 1 week before first PRP) or a control group who had only PRP (done in three sessions at weeks 0, 1 and 2 according to ETDRS guidelines).
NCT01486771 is a randomised three-arm pilot study, which aims to investigate if intravitreal injections of pegaptanib will induce the regression of proliferative diabetic retinopathy and reduce the need for retinal photocoagulation in patients with active PDR with HRCs. Arm 1 patients will receive three intravitreal pegaptanib injections at 6-week intervals, then three additional injections at 12-week intervals; Arm 2 will receive selective laser photocoagulation after three intravitreal pegaptanib injections; and Arm 3 will act as the control group and receive standard PRP (mETDRS protocol).
NCT01280929 is randomised, multicentre, open-label, three-arm Phase II study, with an estimated enrolment of 54 patients, looking at the efficacy and safety of IVR injections monotherapy compared with ranibizumab plus PRP and PRP alone, in the treatment of patients with HR-PDR. The primary outcome is regression of neovascularisation at 12 months.
NCT01594281 is another three-arm study assessing the efficacy and safety of ranibizumab alone or in combination with laser treatment versus laser treatment alone in patients with proliferative diabetic retinopathy. This Phase IV study plans to enrol 120 patients, and the primary outcome is the change of area of neovascularisation as measured by FA at 12 months.
NCT01941329 is a randomised, multicentre, open-label, Phase II/III study, with an estimated enrolment of 94 patients. The study aims to assess efficacy and safety of IVR plus PRP versus PRP in monotherapy in the treatment of subjects with HR-PDR over a 12-month treatment period. The primary outcome is regression of neovascularisation.
NCT02005432 is a three-arm pilot study that compares two different methods of laser (PASCAL laser vs. ETDRS laser) both with IVR versus IVR alone in patients with PDR. Arm 1 consists of single shoot PRP (ETDRS) plus 0.05 ml IVR; Arm 2 is multiple shoot PRP (PASCAL) plus IVR; and Arm 3 is IVR only. The primary outcome is the mean change from baseline in the total area of active retinal neovessels, as measured by FA leakage area at 48 weeks.
NCT01489189 is a trial sponsored by the DRCRN (DRCRnet) which compares prompt PRP with 0.5 mg IVR with deferred PRP. The aim is to determine if VA outcomes at 2 years in eyes with IVR plus deferred PRP are non-inferior to those in eyes that receive standard prompt PRP therapy. The inclusion criteria includes patients with type 1 or 2 diabetes with PDR (with or without DMO) in which the investigator intends to manage with PRP alone, but for which PRP can be deferred for at least 4 weeks, if an intravitreal injection is given. One arm will receive 0.5 mg IVR with deferred PRP (PRP is deferred until failure/futility criteria for intravitreal injection are met) and the other will receive prompt PRP alone (with the full session completed within 56 days).
NCT01766362 aims to show that PRP performed in a single session using a PASCAL laser leads to better management of PDR, with a saving of time and better comfort for both patient and doctor. This randomised, open-label, Phase III study aims to enrol 90 patients with severe NPDR or early PDR. One arm will include a single session of PRP using PASCAL laser and the other will receive four sessions of PASCAL laser, with sessions spaced out by a month. The primary outcome is CMT at 9 months.
NCT01759121 plans to include 90 patients is an open-label trial to investigate the safety and effectiveness of 532-nm laser sub-threshold PRP with PASCAL Endpoint Management function for severe NPDR. One arm will consist of traditional PRP (532-nm short-pulse PRP with PASCAL function) compared with another arm with sub-threshold PRP (532-nm partially sub-threshold short-pulse PRP with PASCAL Endpoint Management function). The main outcomes will be the change of BCVA and the probability of vitreous haemorrhage at 1 year.
NCT01737957 aims to test the safety and efficacy of single-session, low-fluence PRP for PDR when compared with full-fluence PRP. The hypothesis is that a single-session of low-fluence PRP will be safe regarding the progression of MO and the presence of adverse events, and will efficiently induce regression of neovascularisation in patients with PDR. Patients in one arm will received low-fluence PRP in a single session with 532-nm green laser (PUREPOINT laser) and the other arm will receive full-fluence PRP in two sessions with 532-nm laser (PUREPOINT laser). The primary outcome is macular thickness changes measured by spectral domain OCT at 1, 6, 12 and 16 weeks.
NCT00682240 is a four-arm, randomised, crossover trial to assess morphological and functional retinal changes following retinal photocoagulation using PASCAL laser in proliferative retinopathy or MO secondary to diabetes or retinal vein occlusion. Arm 1 will receive a single-session of PRP using the PASCAL laser system; Arm 2 will receive multi-session PRP using PASCAL, Arm 3 will receive multi-session PRP using a conventional laser system; and in Arm 4 patients with persistent central or para-central DMO will receive focal or grid laser treatment.
NCT01304225 is trial investigating the clinical efficacy and safety of the PASCAL laser for DR in patients with PDR or severe NPDR, utilising three different treatment strategies. The main outcome is the incidence of SVL after 1 year. The comparisons include: Arm 1 with PRP using a 100-ms pulse duration, moderate intensity burns, in a single-shot fashion; Arm 2 with PRP using a 20-ms pulse duration, moderate intensity burns, in a multiple-shot fashion; and Arm 3 with PRP using a 20-ms pulse duration, barely visible intensity burns, in a multiple-shot fashion.
UKCRN ID 13472 is a randomised, three-arm pilot study with a target recruitment of 24 patients looking at the safety and efficacy of PASCAL in patients with PPDR. It aims to find whether treating patients using a single session of lower intensity laser (PASCAL® Pan Retinal PhotoStimulation, PRPhS) at an earlier stage in DR (during the severe NPDR stage) will prevent diabetic patients developing PDR. Patients in Arm 1 will be treated with the normal parameters used in PASCAL laser; Arm 2 patients will be treated with a lower intensity laser than normal, using the Endpoint Management system (a way of marking target areas with visible burns followed by reduced energy and hence non-visible spots within the target area); and patients in Arm 3 will be observed, as done routinely in clinical practice.
NCT01232179 is a 3-month Phase II trial comparing two methods of laser therapy in patients with PDR suitable for photocoagulation therapy. One arm will receive conventional PRP and the other will receive extended targeted PRP, consisting of 1200–1600 spots in the far periphery retina. The primary outcome is no leakage in wide-field FA at 3 months.
NCT01383772 is a prospective observational cohort study looking at the effects of PRP, delivered with a multi-spot photocoagulator, on retinal sensitivity and driving eligibility in patients with DR. The primary outcome is the risk of failing visual field criteria to hold a UK driving licence. Eligible patients are those with diabetes (type 1 or type 2), BCVA ≥ 6/60 in both eyes, requiring full bilateral PRP, and no previous laser treatment. The anticipated enrolment is 100 patients. All will receive the multi-spot photocoagulator laser as part of standard clinical care. Patients will undergo visual field testing and complete a QoL questionnaire at baseline and again at 6 months.
A novel development, some way away from use in humans, is topical delivery of anti-VEGFs by eye drops. Davis et al. (2014)200 have shown that bevacizumab can be delivered into the eyes of rats by this method.
The HTA programme has part-funded the Cochrane Eyes and Vision Group to produce a review of laser photocoagulation for NPDR, which will include a review of PRP and anti-VEGF drugs for NPDR, but also photocoagulation for DMO. 201
Summary of ongoing research and research needs
-
There are several trials of different anti-VEGF drugs, either against photocoagulation or in combination with it.
-
The anti-VEGF trials fall into two groups: those assessing the efficacy of anti-VEGFs given alone, and those assessing their ability to reduce adverse effects of PRP, notably MO.
-
There are several trials investigating different methods of photocoagulation for patients with DR at different stages.
-
Most concern later stages of PDR. Only five studies (NCT01504724, NCT01766362, NCT01759121, NCT01304225 and UKCRN ID 13472) specifically mention that they include patients at the NPDR stage. One involving IVB combined with PRP, and the other four are concerned with different methods of delivering laser.
-
The most relevant study to the research question in this report is the UKCRN ID 13472, which is the only one addressing the question of timing of PRP. It is a pilot that aims to recruit only eight patients per arm.
If multiple studies are done, then, to allow comparisons to be made across studies or to allow studies to be combined:
-
The studies should all have the same intermediate and final outcomes.
-
The studies should clearly state the proportions of people over time who are at the different severity levels and also what their VA is.
-
Details should be given for separately for type 1 and type 2 diabetes.
Economic studies
Long-term studies are needed, which measure the progression (and regression) of people with DR through all the different stages of retinopathy. These studies will include patients receiving treatments, such as PRP laser and/or anti-VEGF, which are administered at earlier time points, i.e. at the severe NPDR or early PDR stages as opposed to waiting till the retinopathy progresses to the HR-PDR stages.
These studies should also include health-related QoL (HRQoL) measures to enable calculation of QALYs which are needed for cost-effectiveness analyses. These could include the generic preference-based measures such as EQ-5D or SF-6D alongside some disease-specific measures such as NEI VFQ-25 and VisQol. As the generic-based measures are said to be insensitive to changes in DR progression, the disease-specific measures could be mapped onto generic measures to obtain health-state utility values which can then be used in future cost-effectiveness analyses.
Retaining ability to drive would be an important outcome, which can make the difference between being in employment or not.
The studies would also benefit from having accurate cost estimates for the treatment whether it is for the different types of PRP laser or the different anti-VEGF medications.
The economic model created here for treating patients early who have severe NPDR can be used for other treatments, such as oral fenofibrate to assess their likely cost-effectiveness in reducing retinopathy progression. In addition, this model can be extended to start at the no or mild DR stage.
Therefore, there remains a need for a trial sufficiently large to address the decision problem posed for this review. Such a trial would use best modern laser technologies. We think there is accumulating evidence that use of single-dose anti-VEGF drugs in combination with PRP reduces the adverse effects and may allow PRP to be administered in a single session, and a trial could test this hypothesis.
So the features of a trial would include:
-
Randomisation to PRP at severe NPDR and early PDR stages, reported separately, compared with deferring laser till HR-PDR stage.
-
A multi-spot laser device.
-
Randomisation to prophylactic anti-VEGF treatment to prevent MO versus treating only the minority who develop MO. There would need to be subgroups: those who have never had DMO; those who have DMO; and those who have had DMO in the past.
-
Adequate numbers, which would need to be large to have power to take account of the various factors.
-
At least 2 years’ follow-up.
Other research needs include:
-
Better and up-to-date data on progression and regression of retinopathy at each stage. This will always be somewhat problematic, as, for example, 20-year progression rates take 20 years to collect, by which time treatment and outcomes may have changed.
-
Data on the best way to administer PRP taking into account confluence, intensity and location – whether to apply to the midperipheral retina versus focusing on areas of ischaemia using wide-angle FA, as the more selective approach may limit the damage caused by laser treatment.
-
Data on optimum frequency of follow-up. This might be based on risk factors such as glycaemic control, BP and kidney function. Such factors might also influence decisions on when to administer PRP.
-
If anti-VEGFs are to be used in adjuvant treatment, when is the best time to give them? There would be resource advantages in giving at the same visit as PRP. Some patients might appreciate having everything done at once, others might find it too much.
-
Our focus was on the use of short-term, usually single, anti-VEGF treatment to reduce the risk of PRP-associated DMO. Anti-VEGF therapy could also be used longer term to reduce progression of retinopathy, but at the cost of multiple injections. The value of this is currently unclear but studies are under way.
-
An alternative use of anti-VEGF drugs would be in people needing PRP, but who have DMO. Anti-VEGF treatment could be used instead of focal laser to clear macular fluid, after which PRP would be applied, though as DMO may take some time to clear after anti-VEGF treatment, PRP might have to be given at the same time. This might require close follow-up in case the anti-VEGF caused rapid regression of neovessels and scarring that might lead to tractional retinal detachment.
-
Determining whether those with DMO but who require PRP, might benefit from a single injection of the dexamethasone implant to provide stability for several months.
Acknowledgements
Advisory panel
We thank Professor Peter Scanlon for advice on a number of issues.
We thank Rachel Court for helping to obtain journal articles.
Contribution of authors
Pamela Royle carried out all literature searches, wrote the Plain English summary, Chapter 2, the safety section of Chapter 3, part of the QoL section of Chapter 5, the section on research in progress, and formatted and edited the whole report.
Hema Mistry wrote Chapter 5, developed the economic model, carried out the cost-effectiveness analysis and wrote Chapter 7.
Peter Auguste helped to develop the economic model, drafted Chapter 6, and assisted with the cost-effectiveness analysis.
Deepson Shyangdan drafted Chapters 3 and 4.
Karoline Freeman data extracted the studies for Chapter 4.
Noemi Lois provided expert ophthalmological advice throughout and commented on drafts.
Norman Waugh drafted Chapters 1 and 8, and the scientific summary, contributed to all other chapters, and edited the final report.
Pamela Royle, Noemi Lois and Norman Waugh wrote the abstract.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Arun CS, Al-Bermani A, Stannard K, Taylor R. Long-term impact of retinal screening on significant diabetes-related visual impairment in the working age population. Diabet Med 2009;26:489-92. http://dx.doi.org/10.1111/j.1464-5491.2009.02718.x.
- Wong TY, Mwamburi M, Klein R, Larsen M, Flynn H, Hernandez-Medina M, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care 2009;32:2307-13. http://dx.doi.org/10.2337/dc09-0615.
- LeCaire TJ, Palta M, Klein R, Klein BE, Cruickshanks KJ. Assessing progress in retinopathy outcomes in type 1 diabetes: comparing findings from the Wisconsin Diabetes Registry Study and the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care 2013;36:631-7. http://dx.doi.org/10.2337/dc12-0863.
- Younis N, Broadbent DM, Harding SP, Vora JP. Incidence of sight-threatening retinopathy in Type 1 diabetes in a systematic screening programme. Diabet Med 2003;20:758-65. http://dx.doi.org/10.1046/j.1464-5491.2003.01035.x.
- The Royal College of Ophthalmologists . Guidelines for Diabetic Retinopathy 2012. www.rcophth.ac.uk/page.asp?section=451&sectionTitle=Clinical+Guidelines (accessed 24 September 2013).
- Early Treatment Diabetic Retinopathy Study Research Group . Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98:823-33. http://dx.doi.org/10.1016/S0161-6420(13)38014-2.
- NHS Screening Programmes . NHS Diabetic Eye Screening Programme 2013. http://diabeticeye.screening.nhs.uk/ (accessed 17 March 2014).
- NHS Scotland . Scottish Diabetic Retinopathy Screening Collaborative 2013 n.d. www.ndrs.scot.nhs.uk/ (accessed 17 March 2014).
- Early Treatment Diabetic Retinopathy Study Research Group . Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology 1991;98:766-85. http://dx.doi.org/10.1016/S0161-6420(13)38011-7.
- Diabetic Retinopathy Research Group . Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology 1981;88:583-600.
- Lovestam-Adrian M, Agardh CD, Torffvit O, Agardh E. Type 1 diabetes patients with severe non-proliferative retinopathy may benefit from panretinal photocoagulation. Acta Ophthalmol Scand 2003;81:221-5. http://dx.doi.org/10.1034/j.1600-0420.2003.00050.x.
- Stenkula S. Photocoagulation in diabetic retinopathy. A multicentre study in Sweden. Acta Ophthalmol Scand Suppl 1984;162:1-100.
- Neubauer AS, Ulbig MW. Laser treatment in diabetic retinopathy. Ophthalmologica 2007;221:95-102. http://dx.doi.org/10.1159/000098254.
- Clarkson D. Multi-Spot Laser Photocoagulation 2012. www.opticianonline.net/assets/getAsset.aspx?ItemID=6214 (accessed 23 March 2014).
- Sivaprasad S, Dorin G. Subthreshold diode laser micropulse photocoagulation for the treatment of diabetic macular edema. Expert Rev Med Devices 2012;9:189-97. http://dx.doi.org/10.1586/erd.12.1.
- Sivaprasad S, Elagouz M, McHugh D, Shona O, Dorin G. Micropulsed diode laser therapy: evolution and clinical applications. Surv Ophthalmol 2010;55:516-30. http://dx.doi.org/10.1016/j.survophthal.2010.02.005.
- Sato Y, Kojimahara N, Kitano S, Kato S, Ando N, . Japanese Society of Ophthalmic Diabetology: Subcommittee on the Study of Diabetic Retinopathy Treatment . Multicenter randomized clinical trial of retinal photocoagulation for preproliferative diabetic retinopathy. Jpn J Ophthalmol 2012;56:52-9. http://dx.doi.org/10.1007/s10384-011-0095-2.
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Diabetes: SIGN 116 2010. www.sign.ac.uk/pdf/sign116.pdf (accessed 24 September 2013).
- National Institute for Health and Clinical Excellence (NICE) . CG15 Type 1 Diabetes in Children, Young People and Adults. NICE Guideline 2012 n.d. www.nice.org.uk/guidance/CG15/NICEGuidance (accessed 17 March 2014).
- National Institute for Health and Clinical Excellence (NICE) . CG66 Type 2 Diabetes (partially Updated by CG87) 2008 n.d. http://guidance.nice.org.uk/CG66 (accessed 17 March 2014).
- Diabetic Retinopathy Clinical Research Network (DRCRnet) . Protocol: Prompt Panretinal Photocoagulation Versus Intravitreal Ranibizumab With Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy 2011. http://drcrnet.jaeb.org/Studies.aspx?RecID=201 (accessed 26 February 2014).
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480-7. http://dx.doi.org/10.1056/NEJM199412013312203.
- Ford JA, Lois N, Royle P, Clar C, Shyangdan D, Waugh N. Current treatments in diabetic macular oedema: systematic review and meta-analysis. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002269.
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, . CATT Research Group . Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388-98. http://dx.doi.org/10.1016/j.ophtha.2012.03.053.
- Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007;370:1687-97. http://dx.doi.org/10.1016/S0140-6736(07)61607-9.
- Chew EY, Ambrosius WT, Davis MD, Danis RP, . ACCORD Eye Study Group . Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363:233-44. http://dx.doi.org/10.1056/NEJMoa1001288.
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685-96. http://dx.doi.org/10.1016/S0140-6736(04)16895-5.
- Chen Y, Lu B, Zhang Q, Ma J. n.d.
- McCall B. World First Approval for Fenofibrate in Diabetic Retinopathy 2013. www.medscape.com/viewarticle/815213 (accessed 17 March 2014).
- Hooper P, Boucher MC, Cruess A, Dawson KG, Delpero W, Greve M, et al. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of diabetic retinopathy. Can J Ophthalmol 2012;47:S1-30. http://dx.doi.org/10.1016/j.jcjo.2011.12.025.
- American Academy of Ophthalmology . Diabetic Retinopathy PPP–updated October 2012 2008. http://one.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp--september-2008–4th-print (accessed 29 September 2013).
- Mitchell P, Foran S. Australian Government: National Health and Medical Research Council (NHMRC) . Guidelines for the Management of Diabetic Retinopathy 2008. www.nhmrc.gov.au/_files_nhmrc/publications/attachments/di15.pdf (accessed 17 March 2014).
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007;298:902-16. http://dx.doi.org/10.1001/jama.298.8.902.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed) 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Diabetic Retinopathy Study Research Group . Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol 1976;81:383-96. http://dx.doi.org/10.1016/0002-9394(76)90292-0.
- Diabetic Retinopathy Study Research Group . Diabetic retinopathy study, report number 6: design, methods, and baseline results. Invest Ophthalmol Vis Sci 1981;21:149-20.
- Diabetic Retinopathy Study Research Group . Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology 1978;85:82-106. http://dx.doi.org/10.1016/S0161-6420(78)35693-1.
- Diabetic Retinopathy Study Research Group . Indications for photocoagulation treatment of diabetic retinopathy: Diabetic Retinopathy Study Report no. 14. Int Ophthalmol Clin 1987;27:239-53. http://dx.doi.org/10.1097/00004397-198702740-00004.
- Ferris FL, Podgor MJ, Davis MD. Macular edema in Diabetic Retinopathy Study patients; Diabetic Retinopathy Study Report Number 12. Ophthalmology 1987;94:754-60. http://dx.doi.org/10.1016/S0161-6420(87)33526-2.
- Early Treatment Diabetic Retinopathy Study Research Group . Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics; ETDRS report number 7. Ophthalmology 1991;98:741-56. http://dx.doi.org/10.1016/S0161-6420(13)38009-9.
- Fong DS, Ferris FL, Davis MD, Chew EY. Causes of severe visual loss in the early treatment diabetic retinopathy study; ETDRS report no. 24. Early Treatment Diabetic Retinopathy Study Research Group. Am J Ophthalmol 1999;127:137-41. http://dx.doi.org/10.1016/S0002-9394(98)00309-2.
- Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc 1996;94:505-37.
- Diabetic Retinopathy Vitrectomy Study Research Group . Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial; Diabetic Retinopathy Vitrectomy Study Report 2. Arch Ophthalmol 1985;103:1644-52. http://dx.doi.org/10.1001/archopht.1985.01050110038020.
- Flynn HW, Jr, Chew EY, Simons BD, Barton FB, Remaley NA, Ferris FL. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. The Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1992;99:1351-7. http://dx.doi.org/10.1016/S0161-6420(92)31779-8.
- Chew EY, Benson WE, Remaley NA, Lindley AA, Burton TC, Csaky K, et al. Results after lens extraction in patients with diabetic retinopathy: early treatment diabetic retinopathy study report number 25. Arch Ophthalmol 1999;117:1600-6. http://dx.doi.org/10.1001/archopht.117.12.1600.
- Chantelau E, Kohner EM. Why some cases of retinopathy worsen when diabetic control improves. BMJ (Clin Res Ed) 1997;315:1105-6. http://dx.doi.org/10.1136/bmj.315.7116.1105.
- Pautler SE, Browning DJ. Diabetic Retinopathy: Evidence Based Management. New York: Springer; 2010.
- Williamson TH, George N, Flanagan DW, Norris V, Blamires T, Gale AG. Vision in Vehicles III. North Holland: 1991; n.d.
- Shimura M, Yasuda K, Nakazawa T, Tamai M. Visual dysfunction after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Am J Ophthalmol 2005;140:8-15. http://dx.doi.org/10.1016/j.ajo.2005.02.029.
- Browning DJ. Visual dysfunction after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Am J Ophthalmol 2005;140:127-8. http://dx.doi.org/10.1016/j.ajo.2005.03.025.
- Browning DJ, McOwen MD, Bowen RM, O’Marah TL. Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology 2004;111:712-15. http://dx.doi.org/10.1016/j.ophtha.2003.06.028.
- Massin P, Girach A, Erginay A, Gaudric A. Optical coherence tomography: a key to the future management of patients with diabetic macular oedema. Acta Ophthalmol Scand 2006;84:466-74. http://dx.doi.org/10.1111/j.1600-0420.2006.00694.x.
- Browning DJ, Fraser CM, Clark S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology 2008;115:533-9.e2. http://dx.doi.org/10.1016/j.ophtha.2007.06.042.
- Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG, . Diabetic Retinopathy Clinical Research Network . Comparison of the modified early treatment diabetic retinopathy study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol 2007;125:469-80. http://dx.doi.org/10.1001/archopht.125.4.469.
- Tewari HK, Ravindranath HM, Kumar A, Verma L. Diode laser scatter photocoagulation in diabetic retinopathy. Ann Ophthalmol 2000;32:110-12. http://dx.doi.org/10.1007/s12009-000-0027-0.
- Bandello F, Polito A, Del Borrello M, Zemella N, Isola M. ‘Light’ versus ‘classic’ laser treatment for clinically significant diabetic macular oedema. Br J Ophthalmol 2005;89:864-70. http://dx.doi.org/10.1136/bjo.2004.051060.
- Bressler SB, Qin H, Melia M, Bressler NM, Beck RW, Chan CK, et al. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol 2013;131:1033-40. http://dx.doi.org/10.1001/jamaophthalmol.2013.4154.
- Brown CD. A comparative study of panretinal photocoagulation and vitrectomy for advanced diabetic retinopathy. Mil Med 2003;168:553-5.
- Cardillo JA, Lavinsky D, Melo LAS, Dare A, Castro L, Costa RA, et al. Comparison of the Modified Early Treatment Diabetic Retinopathy Study and Normal or High Density Subthreshold Infrared Micro Pulsed Photocoagulation for Diabetic Macular Edema; 2008 n.d. www.iovs.org:.
- Chappelow AV, Tan K, Waheed NK, Kaiser PK. Panretinal photocoagulation for proliferative diabetic retinopathy: pattern scan laser versus argon laser. Am J Ophthalmol 2012;153:137-42. http://dx.doi.org/10.1016/j.ajo.2011.05.035.
- Cho WB, Oh SB, Moon JW, Kim HC. Panretinal photocoagulation combined with intravitreal bevacizumab in high-risk proliferative diabetic retinopathy. Retina 2009;29:516-22. http://dx.doi.org/10.1097/IAE.0b013e31819a5fc2.
- Brucker AJ, Qin H, Antoszyk AN, Beck RW, Bressler NM, . Diabetic Retinopathy Clinical Research Network . Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol 2009;127:132-40. http://dx.doi.org/10.1001/archophthalmol.2008.565.
- Gurelik G, Coney JM, Zakov ZN. Binocular indirect panretinal laser photocoagulation for the treatment of proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging 2004;35:94-102.
- Lee CM, Olk RJ, Akduman L. Combined modified grid and panretinal photocoagulation for diffuse diabetic macular edema and proliferative diabetic retinopathy. Ophthalmic Surg Las 2000;31:292-300.
- Lee SB, Yun YJ, Kim SH, Kim JY. Changes in macular thickness after panretinal photocoagulation in patients with severe diabetic retinopathy and no macular edema. Retina 2010;30:756-60. http://dx.doi.org/10.1097/IAE.0b013e3181c701e0.
- Luttrull JK, Musch DC, Spink CA. Subthreshold diode micropulse panretinal photocoagulation for proliferative diabetic retinopathy. Eye 2008;22:607-12. http://dx.doi.org/10.1038/sj.eye.6702725.
- Mason JO, Yunker JJ, Vail R, McGwin G. Intravitreal bevacizumab (Avastin) prevention of panretinal photocoagulation-induced complications in patients with severe proliferative diabetic retinopathy. Retina 2008;28:1319-24. http://dx.doi.org/10.1097/IAE.0b013e31818356fb.
- Muqit MMK, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, et al. Optos-guided pattern scan laser (PASCAL)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol 2013;91:251-8. http://dx.doi.org/10.1111/j.1755-3768.2011.02307.x.
- Summanen P. Scatter photocoagulation: pros and cons with old and new laser equipments. Acta Ophthalmol 2012;90.
- Venkatesh P, Ramanjulu R, Azad R, Vohra R, Garg S. Subthreshold micropulse diode laser and double frequency neodymium: YAG laser in treatment of diabetic macular edema: a prospective, randomized study using multifocal electroretinography. Photomed Laser Surg 2011;29:727-33. http://dx.doi.org/10.1089/pho.2010.2830.
- Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010;30:908-16. http://dx.doi.org/10.1097/IAE.0b013e3181c96986.
- Zucchiatti I, Veritti D, Lanzetta P, Bandello F. Efficacy and safety of PASCAL photocoagulator in the treatment of proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 2009;50:194-5.
- Bandello F, Brancato R, Menchini U, Virgili G, Lanzetta P, Ferrari E, et al. Light panretinal photocoagulation (LPRP) versus classic panretinal photocoagulation (CPRP) in proliferative diabetic retinopathy. Semin Ophthalmol 2001;16:12-8. http://dx.doi.org/10.1076/soph.16.1.12.4223.
- Muqit MM, Marcellino GR, Gray JC, McLauchlan R, Henson DB, Young LB, et al. Pain responses of Pascal 20 ms multi-spot and 100 ms single-spot panretinal photocoagulation: Manchester Pascal Study, MAPASS report 2. Br J Ophthalmol 2010;94:1493-8. http://dx.doi.org/10.1136/bjo.2009.176677.
- Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, et al. Single-session vs multiple-session pattern scanning laser panretinal photocoagulation in proliferative diabetic retinopathy: The Manchester Pascal Study. Arch Ophthalmol 2010;128:525-33. http://dx.doi.org/10.1001/archophthalmol.2010.60.
- Muqit MM, Marcellino GR, Henson DB, Young LB, Turner GS, Stanga PE. Pascal panretinal laser ablation and regression analysis in proliferative diabetic retinopathy: Manchester Pascal Study Report 4. Eye 2011;25:1447-56. http://dx.doi.org/10.1038/eye.2011.188.
- Muqit MMK, Young LB, McKenzie R, John B, Marcellino GR, Henson DB, et al. Pilot randomised clinical trial of Pascal TargETEd Retinal versus variable fluence PANretinal 20 ms laser in diabetic retinopathy: PETER PAN study. Br J Ophthalmol 2013;97:220-7. http://dx.doi.org/10.1136/bjophthalmol-2012-302189.
- Muraly P, Limbad P, Srinivasan K, Ramasamy K. Single session of Pascal versus multiple sessions of conventional laser for panretinal photocoagulation in proliferative diabetic retinopathy: a comparative study. Retina 2011;31:1359-65. http://dx.doi.org/10.1097/IAE.0b013e318203c140.
- Al-Hussainy S, Dodson PM, Gibson JM. Pain response and follow-up of patients undergoing panretinal laser photocoagulation with reduced exposure times. Eye 2008;22:96-9. http://dx.doi.org/10.1038/sj.eye.6703026.
- Shimura M, Yasuda K, Nakazawa T, Kano T, Ohta S, Tamai M. Quantifying alterations of macular thickness before and after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Ophthalmology 2003;110:2386-94. http://dx.doi.org/10.1016/j.ophtha.2003.05.008.
- Mirshahi A, Lashay A, Roozbahani M, Fard MA, Molaie S, Mireshghi M, et al. Pain score of patients undergoing single spot, short pulse laser versus conventional laser for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2013;251:1103-7. http://dx.doi.org/10.1007/s00417-012-2167-5.
- Nagpal M, Marlecha S, Nagpal K. Comparison of laser photocoagulation for diabetic retinopathy using 532-nm standard laser versus multispot pattern scan laser. Retina 2010;30:452-8. http://dx.doi.org/10.1097/IAE.0b013e3181c70127.
- Salman AG. Pascal laser versus conventional laser for treatment of diabetic retinopathy. Saudi J Ophthalmol 2011;25:175-9. http://dx.doi.org/10.1016/j.sjopt.2011.01.006.
- Suto C, Hori S, Kato S. Management of type 2 diabetics requiring panretinal photocoagulation and cataract surgery. J Cataract Refract Surg 2008;34:1001-6. http://dx.doi.org/10.1016/j.jcrs.2008.02.019.
- Shimizu Y, Kojima K. Clinical analysis of preproliferative diabetic retinopathy prognosis of photocoagulation. Folia Ophthalmol Jpn 1989;40:1635-42.
- Jojo V, Mohamed M. Re: Single session of Pascal versus multiple sessions of conventional laser for panretinal photocoagulation in proliferative diabetic retinopathy: a comparative study. Retina 2012;32:1697-8. http://dx.doi.org/10.1097/IAE.0b013e3182695b03.
- Muraly M, Limbad P, Srinivasan K, Amasamy K. Re: Single session of Pascal versus multiple sessions of conventional laser for panretinal photocoagulation in proliferative diabetic retinopathy: a comparative study. Retina 2012;32:1698-9. http://dx.doi.org/10.1097/IAE.0b013e3182695be4.
- Bandello F, Brancato R, Trabucchi G, Lattanzio R, Malegori A. Diode versus argon-green laser panretinal photocoagulation in proliferative diabetic retinopathy: a randomized study in 44 eyes with a long follow-up time. Graefes Arch Clin Exp Ophthalmol 1993;231:491-4. http://dx.doi.org/10.1007/BF00921112.
- Figueira J, Khan J, Nunes S, Sivaprasad S, Rosa A, de Abreu JF, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol 2009;93:1341-4. http://dx.doi.org/10.1136/bjo.2008.146712.
- Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol 2004;88:1173-9. http://dx.doi.org/10.1136/bjo.2003.040949.
- Lavinsky D, Cardillo JA, Melo LA, Jr, Dare A, Farah ME, Belfort R. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci 2011;52:4314-23. http://dx.doi.org/10.1167/iovs.10-6828.
- Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a ‘trial effect’. J Clin Epidemiol 2001;54:217-24. http://dx.doi.org/10.1016/S0895-4356(00)00305-X.
- Vist GE, Bryant D, Somerville L, Birminghem T, Oxman AD. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.mr000009.pub4.
- Fong DS, Girach A, Boney A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: a literature review. Retina 2007;27:816-24. http://dx.doi.org/10.1097/IAE.0b013e318042d32c.
- Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, . Diabetic Retinopathy Clinical Research Network . Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 2007;114:525-36. http://dx.doi.org/10.1016/j.ophtha.2006.06.052.
- Du B, Zhang H, Chan HHL, Wang JT, Ho PWC, Xu YS. Retinal function and morphology of severe non-proliferative diabetic retinopathy before and after retinal photocoagulation. Clin Exp Optom 2011;94:284-90. http://dx.doi.org/10.1111/j.1444-0938.2011.00585.x.
- Maeshima K, Utsugi-Sutoh N, Otani T, Kishi S. Progressive enlargement of scattered photocoagulation scars in diabetic retinopathy. Retina 2004;24:507-11. http://dx.doi.org/10.1097/00006982-200408000-00002.
- Raman R, Ananth V, Pal SS, Gupta A. Internal ophthalmoplegia after retinal laser photocoagulation. Cutan Ocul Toxicol 2010;29:203-8. http://dx.doi.org/10.3109/15569527.2010.489336.
- Shimura M, Yasuda K, Nakazawa T, Abe T, Shiono T, Iida T, et al. Panretinal photocoagulation induces pro-inflammatory cytokines and macular thickening in high-risk proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2009;247:1617-24. http://dx.doi.org/10.1007/s00417-009-1147-x.
- Wang Y, Muqit MM, Stanga PE, Young LB, Henson DB. Spatial changes of central field loss in diabetic retinopathy after laser. Optom Vis Sci 2014;91:111-20. http://dx.doi.org/10.1097/OPX.0000000000000103.
- Kaiser RS, Maguire MG, Grunwald JE, Lieb D, Jani B, Brucker AJ, et al. One-year outcomes of panretinal photocoagulation in proliferative diabetic retinopathy. Am J Ophthalmol 2000;129:178-85. http://dx.doi.org/10.1016/S0002-9394(99)00322-0.
- Kovacic Z, Ivanisevic M, Bojic L, Hrgovic Z, Lesin M, Kurelovic D. Comparing two techniques of panretinal photocoagulation on visual acuity on patients with proliferative diabetic retinopathy. Med Arh 2012;66:321-3. http://dx.doi.org/10.5455/medarh.2012.66.321-323.
- Tinley CG, Gray RH. Routine, single session, indirect laser for proliferative diabetic retinopathy. Eye 2009;23:1819-23. http://dx.doi.org/10.1038/eye.2008.394.
- Velez-Montoya R, Guerrero-Naranjo JL, Gonzalez-Mijares CC, Fromow-Guerra J, Marcellino GR, Quiroz-Mercado H, et al. Pattern scan laser photocoagulation: safety and complications, experience after 1301 consecutive cases. Br J Ophthalmol 2010;94:720-4. http://dx.doi.org/10.1136/bjo.2009.164996.
- Natesh S, Ranganath A, Harsha K, Yadav NK, Bhujang BS. Choroidal detachment after PASCAL photocoagulation. Can J Ophthalmol 2011;46. http://dx.doi.org/10.3129/i10-108.
- Kapoor B, Peh KK, Raman SV. An unusual case of acute angle closure glaucoma following argon laser pan retinal photocoagulation. BMJ Case Rep 2010. http://dx.doi.org/10.1136/bcr.12.2009.2511.
- Richardson C, Waterman H. Pain relief during panretinal photocoagulation for diabetic retinopathy: a national survey. Eye 2009;23:2233-7. http://dx.doi.org/10.1038/eye.2008.421.
- Bailey CC, Sparrow JM, Grey RH, Cheng H. The National Diabetic Retinopathy Laser Treatment Audit. II. Proliferative retinopathy. Eye 1998;12:77-84. http://dx.doi.org/10.1038/eye.1998.14.
- Stanga PE, Muqit MM. Re: Comparison of laser photocoagulation for diabetic retinopathy using 532-nm standard laser versus multispot pattern scan laser. Retina 2010;30:1749-50.
- Bressler NM, Beck RW, Ferris LF. Panretinal photocoagulation for proliferative diabetic retinopathy. N Engl J Med 2011;365:1520-6. http://dx.doi.org/10.1056/NEJMct0908432.
- Yang CS, Cheng CY, Lee FL, Hsu WM, Liu JH. Quantitative assessment of retinal thickness in diabetic patients with and without clinically significant macular edema using optical coherence tomography. Acta Ophthalmol Scand 2001;79:266-70. http://dx.doi.org/10.1034/j.1600-0420.2001.790311.x.
- Mackie SW, Webb LA, Hutchison BM, Hammer HM, Barrie T, Walsh G. How much blame can be placed on laser photocoagulation for failure to attain driving standards?. Eye 1995;9:517-25. http://dx.doi.org/10.1038/eye.1995.118.
- Vernon SA, Bhagey J, Boraik M, El-Defrawy H. Long-term review of driving potential following bilateral panretinal photocoagulation for proliferative diabetic retinopathy. Diabet Med 2009;26:97-9. http://dx.doi.org/10.1111/j.1464-5491.2008.02623.x.
- Hulbert MFG, Vernon SA. Passing the DLVC field regulations following bilateral pan-retinal photocoagulation in diabetics. Eye 1996;6:456-60. http://dx.doi.org/10.1038/eye.1992.96.
- Davies N. Altering the pattern of panretinal photocoagulation: could the visual field for driving be preserved?. Eye 1999;13:531-6. http://dx.doi.org/10.1038/eye.1999.132.
- Quinn MJ. Can altering the pattern of laser photocoagulation for proliferative diabetic retinopathy help retain visual fields for driving?. Eye 1999;13:495-6. http://dx.doi.org/10.1038/eye.1999.129.
- Whittington Health NHS. Preparing for Laser Treatment for Diabetic Retinopathy and Maculopathy 2013. www.whittington.nhs.uk/document.ashx?id = 818 (accessed 25 March 2014).
- Diabetes UK. Driving and Diabetes 2009. www.diabetes.org.uk/upload/How%20we%20help/catalogue/DrivingandDiabetes_Final_ToUpload.pdf (accessed 25 March 2014).
- Cho WB, Moon JW, Kim HC. Intravitreal triamcinolone and bevacizumab as adjunctive treatments to panretinal photocoagulation in diabetic retinopathy. Br J Ophthalmol 2010;94:858-63. http://dx.doi.org/10.1136/bjo.2009.168997.
- Diabetic Retinopathy Clinical Research Network (DRCRnet) . Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina 2011;31:1009-27. http://dx.doi.org/10.1097/IAE.0b013e318217d739.
- Ernst BJ, Garcia-Aguirre G, Oliver SC, Olson JL, Mandava N, Quiroz-Mercado H. Intravitreal bevacizumab versus panretinal photocoagulation for treatment-naive proliferative and severe nonproliferative diabetic retinopathy. Acta Opthalmol 2012;90:e573-4. http://dx.doi.org/10.1111/j.1755-3768.2011.02364.x.
- Filho JAR, Messias A, Almeida FPP, Ribeiro JAS, Costa RA, Scott IU, et al. Panretinal photocoagulation (PRP) versus PRP plus intravitreal ranibizumab for high-risk proliferative diabetic retinopathy. Acta Ophthalmol 2011;89:e567-72. http://dx.doi.org/10.1111/j.1755-3768.2011.02184.x.
- Lucena CR, Ramos Filho JA, Messias AM, da Silva JA, de Almeida FP, Scott IU, et al. Panretinal photocoagulation versus intravitreal injection retreatment pain in high-risk proliferative diabetic retinopathy. Arq Bras Oftalmol 2013;76:18-20. http://dx.doi.org/10.1590/S0004-27492013000100006.
- Mirshahi A, Roohipoor R, Lashay A, Mohammadi SF, Abdoallahi A, Faghihi H. Bevacizumab-augmented retinal laser photocoagulation in proliferative diabetic retinopathy: a randomized double-masked clinical trial. Eur J Ophthalmol 2008;18:263-9.
- Preti RC, Vasquez Ramirez LM, Ribeiro Monteiro ML, Pelayes DE, Takahashi WY. Structural and functional assessment of macula in patients with high-risk proliferative diabetic retinopathy submitted to Panretinal photocoagulation and associated intravitreal bevacizumab injections: a comparative, randomised, controlled trial. Ophthalmologica 2013;230:1-8. http://dx.doi.org/10.1159/000348605.
- Tonello M, Costa RA, Almeida FP, Barbosa JC, Scott IU, Jorge R. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthalmol 2008;86:385-9. http://dx.doi.org/10.1111/j.1600-0420.2007.01056.x.
- Maia OO, Takahashi BS, Costa RA, Scott IU, Takahashi WY. Combined laser and intravitreal triamcinolone for proliferative diabetic retinopathy and macular edema: one-year results of a randomized clinical trial. Am J Ophthalmol 2009;147:291-7.e2. http://dx.doi.org/10.1016/j.ajo.2008.08.024.
- Mirshahi A, Shenazandi H, Lashay A, Faghihi H, Alimahmoudi A, Dianat S. Intravitreal triamcinolone as an adjunct to standard laser therapy in coexisting high-risk proliferative diabetic retinopathy and clinically significant macular edema. Retina 2010;30:254-9. http://dx.doi.org/10.1097/IAE.0b013e3181b4f125.
- Shimura M, Yasuda K, Shiono T. Posterior sub-Tenon’s capsule injection of triamcinolone acetonide prevents panretinal photocoagulation-induced visual dysfunction in patients with severe diabetic retinopathy and good vision. Ophthalmology 2006;113:381-7. http://dx.doi.org/10.1016/j.ophtha.2005.10.035.
- Unoki N, Nishijima K, Kita M, Suzuma K, Watanabe D, Oh H, et al. Randomised controlled trial of posterior sub-Tenon triamcinolone as adjunct to panretinal photocoagulation for treatment of diabetic retinopathy. Br J Ophthalmol 2009;93:765-70. http://dx.doi.org/10.1136/bjo.2008.152041.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research. Value Health 2013;16:e1-5. http://dx.doi.org/10.1016/j.jval.2013.02.010.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Fenwick EK, Lamoureux EL. Effect of pan-retinal photocoagulation treatment on vision-related quality of life of patients with proliferative diabetic retinopathy. Retina 2013;33. http://dx.doi.org/10.1097/IAE.0b013e3182928b33.
- Scanlon PH, Martin ML, Bailey C, Johnson E, Hykin P, Keightley S. Reported symptoms and quality-of-life impacts in patients having laser treatment for sight-threatening diabetic retinopathy. Diabet Med 2006;23:60-6. http://dx.doi.org/10.1111/j.1464-5491.2005.01736.x.
- Tsilimbaris MK, Kontadakis GA, Tsika C, Papageorgiou D, Charoniti M. Effect of panretinal photocoagulation treatment on vision-related quality of life of patients with proliferative diabetic retinopathy. Retina 2013;33:756-61. http://dx.doi.org/10.1097/IAE.0b013e31826b0c06.
- Wirostko B, Beusterien K, Grinspan J, Ciulla T, Gonder J, Barsdorf A, et al. Patient preferences in the treatment of diabetic retinopathy. Patient Prefer Adherence 2011;5:229-37. http://dx.doi.org/10.2147/PPA.S11972.
- Mozaffarieh M, Benesch T, Sacu S, Krepler K, Biowski R, Wedrich A. Photocoagulation for diabetic retinopathy: determinants of patient satisfaction and the patient–provider relationship. Acta Ophthalmol Scand 2005;83:316-21. http://dx.doi.org/10.1111/j.1600-0420.2005.00455.x.
- Trento M, Tomelini M, Lattanzio R, Brancato R, Coggiola A, Benecchi R, et al. Perception of, and anxiety levels induced by, laser treatment in patients with sight-threatening diabetic retinopathy. A multicentre study. Diabet Med 2006;23:1106-9. http://dx.doi.org/10.1111/j.1464-5491.2006.01957.x.
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050-8. http://dx.doi.org/10.1001/archopht.119.7.1050.
- Browne C, Brazier J, Carlton J, Alavi Y, Jofre-Bonet M. Estimating quality-adjusted life years from patient-reported visual functioning. Eye 2012;26:1295-301. http://dx.doi.org/10.1038/eye.2012.137.
- Payakachat N, Summers KH, Pleil AM, Murawski MM, Thomas J, Jennings K, et al. Predicting EQ-5D utility scores from the 25-item National Eye Institute Vision Function Questionnaire (NEI-VFQ 25) in patients with age-related macular degeneration. Qual Life Res 2009;18:801-13. http://dx.doi.org/10.1007/s11136-009-9499-6.
- Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ (Clin Res Ed) 2000;320:1530-3. http://dx.doi.org/10.1136/bmj.320.7248.1530.
- Diabetes in Scotland . Scottish Diabetes Survey 2012. www.diabetesinscotland.org.uk/Publications.aspx?catId = 3 (accessed 21 March 2014).
- Nordwall M, Bojestig M, Arnqvist HJ, Ludvigsson J. Declining incidence of severe retinopathy and persisting decrease of nephropathy in an unselected population of Type 1 diabetes: the Linkoping Diabetes Complications Study. Diabetologia 2004;47:1266-72. http://dx.doi.org/10.1007/s00125-004-1431-6.
- Diabetes Control and Complications Trial Research Group . Progression of retinopathy with intensive versus conventional treatment in the diabetes control and complications trial. Ophthalmology 1995;102:647-61. http://dx.doi.org/10.1016/S0161-6420(95)30973-6.
- The Health and Social Care Information Centre (HSCIC), Diabetes UK . National Diabetes Audit 2011–2012 Report 1: Care Processes and Treatment Targets n.d. www.hqip.org.uk/assets/NCAPOP-Library/NCAPOP-2013–14/NDA-2011–2012-CareProcessesTreatmentTargets.pdf (accessed 21 March 2014).
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, . DCCT/EDIC Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643-53. http://dx.doi.org/10.1056/NEJMoa052187.
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998;105:1801-15. http://dx.doi.org/10.1016/S0161-6420(98)91020-X.
- Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001;44:156-63. http://dx.doi.org/10.1007/s001250051594.
- Harris Nwanyanwu K, Talwar N, Gardner TW, Wrobel JS, Herman WH, Stein JD. Predicting development of proliferative diabetic retinopathy. Diabetes Care 2013;36:1562-8. http://dx.doi.org/10.2337/dc12-0790.
- Cikamatana L, Mitchell P, Rochtchina E, Foran S, Wang JJ. Five-year incidence and progression of diabetic retinopathy in a defined older population: the Blue Mountains Eye Study. Eye 2007;21:465-71. http://dx.doi.org/10.1038/sj.eye.6702771.
- Jones CD, Greenwood RH, Misra A, Bachmann MO. Incidence and progression of diabetic retinopathy during 17 years of a population-based screening program in England. Diabetes Care 2012;35:592-6. http://dx.doi.org/10.2337/dc11-0943.
- McCarty DJ, Fu CL, Harper CA, Taylor HR, McCarty CA. Five-year incidence of diabetic retinopathy in the Melbourne Visual Impairment Project. Clin Experiment Ophthalmol 2003;31:397-402. http://dx.doi.org/10.1046/j.1442-9071.2003.00685.x.
- Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, et al. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report 18. Invest Ophthalmol Vis Sci 1998;39:233-52.
- Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008;115:1859-68. http://dx.doi.org/10.1016/j.ophtha.2008.08.023.
- Scanlon PH, Stratton IM, Histed M, Chave SJ, Aldington SJ. The influence of background diabetic retinopathy in the second eye on rates of progression of diabetic retinopathy between 2005 and 2010. Acta Ophthalmol 2013;91:e335-9. http://dx.doi.org/10.1111/aos.12074.
- Ting HYJ, Martin DK, Haas M. A Markov Model of Diabetic Retinopathy Progression for the Economic Evaluation of a Novel DR Prognostic Device: CHERE Working Paper 2007 14 2007. www.chere.uts.edu.au/pdf/wp2007_14.pdf (accessed 18 March 2014).
- Rodgers M, Hodges R, Hawkins J, Hollingworth W, Duffy S, McKibbin M, et al. Colour vision testing for diabetic retinopathy: a systematic review of diagnostic accuracy and economic evaluation. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13600.
- Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. Validation of the CORE diabetes model against epidemiological and clinical studies. Curr Med Res Opin 2004;20:S27-40. http://dx.doi.org/10.1185/030079904X2006.
- Rachapelle S, Legood R, Alavi Y, Lindfield R, Sharma T, Kuper H, et al. The cost-utility of telemedicine to screen for diabetic retinopathy in India. Ophthalmology 2013;120:566-73. http://dx.doi.org/10.1016/j.ophtha.2012.09.002.
- Brown MM, Brown GC, Sharma S, Shah G. Utility values and diabetic retinopathy. Am J Ophthalmol 1999;128:324-30. http://dx.doi.org/10.1016/S0002-9394(99)00146-4.
- Fong DS, Sharza M, Chen W, Paschal JF, Ariyasu RG, Lee PP. Vision loss among diabetics in a group model Health Maintenance Organization (HMO). Am J Ophthalmol 2002;133:236-41. http://dx.doi.org/10.1016/S0002-9394(01)01364-2.
- Smith DH, Johnson ES, Russell A, Hazlehurst B, Muraki C, Nichols GA, et al. Lower visual acuity predicts worse utility values among patients with type 2 diabetes. Qual Life Res 2008;17:1277-84. http://dx.doi.org/10.1007/s11136-008-9399-1.
- Rein DB, Wittenborn JS, Zhang X, Allaire BA, Song MS, Klein R, et al. The cost-effectiveness of three screening alternatives for people with diabetes with no or early diabetic retinopathy. Health Serv Res 2011;46:1534-61. http://dx.doi.org/10.1111/j.1475-6773.2011.01263.x.
- NHS Reference Costs 2012 to 2013. London: DH; 2013.
- Mitchell P, Annemans L, Gallagher M, Hasan R, Thomas S, Gairy K, et al. Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial. Br J Ophthalmol 2012;96:688-93. http://dx.doi.org/10.1136/bjophthalmol-2011-300726.
- Meads C, Hyde C, Lafuma A. How much is the cost of visual impairment: caveat emptor. PharmacoEconomics 2006;24:207-10. http://dx.doi.org/10.2165/00019053-200624020-00008.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Diabetic Retinopathy Vitrectomy Study Research Group . Two-year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Diabetic Retinopathy Vitrectomy Study (DRVS) report #1. Ophthalmology 1985;92:492-50. http://dx.doi.org/10.1016/S0161-6420(85)34002-2.
- Office for National Statistics (ONS) . UK Interim Life Tables, 1980–82 to 2008–10 2011. http://www.ons.gov.uk/ons/taxonomy/index.html?nscl = Interim+Life+Tables (accessed 18 April 2014).
- Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia 2006;49:660-6. http://dx.doi.org/10.1007/s00125-005-0120-4.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006;23:516-21. http://dx.doi.org/10.1111/j.1464-5491.2006.01838.x.
- Cusick M, Meleth AD, Agrón E, Fisher MR, Reed GF, Knatterud GL, et al. Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: early treatment diabetic retinopathy study report no. 27. Diabetes Care 2005;28:617-25. http://dx.doi.org/10.2337/diacare.28.3.617.
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol 1999;117:1487-95. http://dx.doi.org/10.1001/archopht.117.11.1487.
- Briggs A, Claxton K, Schulpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al. The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11470.
- Drummond M, McGuire A. Economic Evaluation in HealthCare: Merging Theory with Practice. Oxford: Oxford University Press; 2001.
- British National Formulary. London: BMA and RPS; 2013.
- National Institute for Health and Clinical Excellence (NICE) . Bevacizumab (Avastin) for Eye Conditions 2010. www.nice.org.uk/media/AC2/3C/BevacizumabEyeConditionsPrescopingReport.pdf (accessed 25 March 2014).
- NICE Decision Support Unit . Macular Oedema – Bevacizumab As a Comparator 2013. www.nicedsu.org.uk/Macular-oedema--bevacizumab(2804604).htm (accessed 25 March 2014).
- Lloyd A, Nafees B, Gavriel S, Rousculp MD, Boye KS, Ahmad A. Health utility values associated with diabetic retinopathy. Diabet Med 2008;25:618-24. http://dx.doi.org/10.1111/j.1464-5491.2008.02430.x.
- Malkin AG, Goldstein JE, Perlmutter MS, Massof RW. Responsiveness of the EQ-5D to the effects of low vision rehabilitation. Optom Vis Sci 2013;90:799-805. http://dx.doi.org/10.1097/OPX.0000000000000005.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 n.d. http://publications.nice.org.uk/guide-to-the-methods-of-technology-appraisal-2013-pmg9 (accessed 21 March 2014).
- Peacock S, Misajon R, Iezzi A, Richardson J, Hawthorne G, Keeffe J. Vision and quality of life: development of methods for the VisQoL vision-related utility instrument. Ophthalmic Epidemiol 2008;15:218-23. http://dx.doi.org/10.1080/09286580801979417.
- Misajon R, Hawthorne G, Richardson J, Barton J, Peacock S, Iezzi A, et al. Vision and quality of life: the development of a utility measure. Invest Ophthalmol Vis Sci 2005;46:4007-15. http://dx.doi.org/10.1167/iovs.04-1389.
- Dewan V, Lambert D, Edler J, Kymes S, Apte RS. Cost-effectiveness analysis of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2012;119:1679-84. http://dx.doi.org/10.1016/j.ophtha.2012.01.049.
- Pershing S, Enns EA, Matesic B, Owens DK, Goldhaber-Fiebert JD. Cost-effectiveness of treatment of diabetic macular edema. Ann Intern Med 2014;160:18-29. http://dx.doi.org/10.7326/M13-0768.
- Estudillo M, Gonzalez V. Proliferative Diabetic Retinopathy. A comparison of anti-VGEF therapy and laser in preserving visual function in patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 2013;54.
- Gonzalez V. The impact on vision and regression of retinal neovascularization of anti-VEGF induction in combination with quarterly anti-VEGF maintenance or selective PRP versus standard PRP. Invest Ophthalmol Vis Sci 2013;54.
- Leal S, Figueira J, Ribeiro L, Cachulo ML, Silva R, Nunes S, et al. Combination of intravitreal injection of Pegaptanib plus progressive PRP versus full PRP alone in patients with High Risk Proliferative Diabetic Retinopathy. Invest Ophthalmol Vis Sci 2013;54.
- Madeira MIA, Neves J, Abdala M. A Different Approach of Pan Retinal Photocoagulation for the Treatment of Proliferative Diabetic Retinopathy (external Ring Photocoagulation) n.d.
- Preti R, Mutti A, Vazquez L, Ferraz D, Zacharias L, Carra M, et al. Evaluation of choroidal thickness in high-risk proliferative diabetic retinopathy treated with panretinal photocoagulation associated or not with intravitreal bevacizumab injections: a 3 months, randomized, controlled and masked clinical trial. Invest Ophthalmol Vis Sci 2013;54.
- Preti RC, Ramirez LM, Barros AC, Motta AA, Morita C, Maia Junior OO, et al. Structural and functional macular evaluation in proliferative diabetic retinopathy patients treated using panretinal photocoagulation combined with intravitreal bevacizumab injections. Invest Ophthalmol Vis Sci 2012;53.
- Lohmann C, Voegeler J, Liakopoulos S, Wiedemann P, Spital G, Lang G, et al. Double-masked trial demonstrates superiority of combined ranibizumab plus laser versus laser in patients with diabetic macular edema with or without proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 2013;54.
- Ehrlich J, Domalpally A, Yau L, Hopkins J, Ip M. Effects of intravitreal ranibizumab on diabetic retinopathy severity: 36 month data from the RISE and RIDE phase III trials. Invest Ophthalmol Vis Sci 2013;54.
- Miller DG. Diabetic Retinopathy Clinical Research Network . Exploratory analysis of diabetic retinopathy worsening (progression) at 1 year and 3 years in a randomized clinical trial evaluating ranibizumab and triamcinolone. Invest Ophthalmol Vis Sci 2012;53.
- Ferraz D, Sophie R, Bittencourt M, Preti R, Vazquez L, Motta A, et al. Intravitreal ranibizumab combined with panretinal photocoagulation in patients with treatment-naive proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 2013;54.
- Medical Research Council NIHR n.d. www.nets.nihr.ac.uk/__data/assets/pdf_file/0008/66995/outcomes-feb-2013.pdf (accessed 12 March 2014).
- Centre for Health Economics and Medicines Evaluation . 2013/14/Winter/Newsletter/of/the/Centre/for/Health/Economics/and/Medicines/Evaluation/(CHEME) 2014. http://cheme.bangor.ac.uk/documents/2013_14%20Winter%20Newsletter%20CHEME.pdf (accessed 25 March 2014).
- Davis BM, Normando EM, Guo L, O’Shea P, Moss SE, Somavarapu S, et al. Topical delivery of avastin to the posterior segment of the eye in vivo using annexin A5-associated liposomes. Small 2014;10:1575-84. http://dx.doi.org/10.1002/smll.201303433.
- National Institute for Health Research (NIHR) . SR–13/101/47:/Laser/Photocoagulation/for/Non-Proliferative/Diabetic/Retinopathy 2013. www.nets.nihr.ac.uk/projects/sr/1310147 (accessed 25 March 2014).
- Early Treatment Diabetic Retinopathy Study Research Group . Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991;98:786-80. http://dx.doi.org/10.1016/S0161-6420(13)38012-9.
Appendix 1 Classification of diabetic retinopathy in the early treatment of diabetic retinopathy study
Adapted from the ETDRS Research Group. 6,40,202
| Disease severity level | Findings observable upon dilated ophthalmoscopy |
|---|---|
| Mild non-proliferative retinopathy | At least one microaneurysm, and definition not met for moderate non-proliferative retinopathy, severe non-proliferative retinopathy, early proliferative retinopathy, or high-risk proliferative retinopathy (see below) |
| Moderate non-proliferative retinopathy | Haemorrhages and/or microaneurysms ≥ standard photograph 2A;a and/or soft exudates, venous beading, or IRMAs definitely present; and definition not met for severe non-proliferative retinopathy, early proliferative retinopathy, or high-risk proliferative retinopathy (see below) |
| Severe non-proliferative retinopathy | Soft exudates, venous beading, and IRMAs all definitely present in at least two of fields 4 through 7; or two of the preceding three lesions present in at least two of fields 4 through 7 and haemorrhages and microaneurysms present in these four fields, equalling or exceeding standard photo 2A in at least one of them; or IRMAs present in each of fields 4 through 7 and equalling or exceeding standard photograph 8A in at least two of them; and definition not met for early proliferative retinopathy or high-risk proliferative retinopathy (see below) |
| Early proliferative retinopathy (i.e. proliferative retinopathy without DRS HRCs) | New vessels; and definition not met for high-risk proliferative retinopathy (see below) |
| High-risk proliferative retinopathy (proliferative retinopathy with DRS HRCs) | New vessels on or within one DD of the optic disc (NVD) ≥ standard photograph 10Aa (about ¼ to ⅓ disc area), with or without vitreous or pre-retinal haemorrhage; or vitreous and/or pre-retinal haemorrhage accompanied by new vessels, either NVD < standard photograph 10A or new vessels elsewhere (NVE) ≥ ¼ disc area |
| Less severe retinopathy | Mild or moderate non-proliferative retinopathy |
| More severe retinopathy | Severe non-proliferative or early proliferative retinopathy |
Appendix 2 Search strategies
Search strategies
In order to capture the wide range of evidence relating to PRP, other forms of laser treatment, and drug treatments in the treatment of all stages of DR, 10 separate types of search (as outlined below) were designed and performed:
-
systematic reviews and meta-analyses of PRP for DR
-
RCTs for treatment of DR
-
all studies on NPDR
-
all studies on lasers in DR
-
progression or natural history of DR
-
side effects of lasers from non-RCT studies
-
QoL in DR after PRP
-
cost-effectiveness of treatment in DR
-
ongoing or recently completed research
-
additional sources searched.
(a) Searches for previous systematic reviews or meta-analyses on the treatment of diabetic retinopathy
Ovid MEDLINE 1946 to August Week 2 2013
-
exp *Diabetic Retinopathy/
-
(diabet* and retinopathy).m_titl.
-
1 or 2
-
(systematic review or meta-analysis).tw.
-
meta-analysis.pt.
-
4 or 5
-
3 and 6
EMBASE 1980 to 2013 Week 34
-
exp *Diabetic Retinopathy/
-
(diabet* and retinopathy).m_titl.
-
1 or 2
-
(systematic review or meta-analysis).tw.
-
3 and 4
Cochrane Database of Systematic Reviews July 2013
(diabet* and retinopathy) in Title
(b) Searches for randomised controlled trials for the treatment of diabetic retinopathy
These searches included all aspects of treatment – including laser surgery and drug treatment.
Ovid MEDLINE 1946 to August Week 2 2013; Ovid MEDLINE In-Process & Other Non-Indexed Citations August 26, 2013
-
exp *Diabetic Retinopathy/
-
(diabet* and retinopathy).m_titl.
-
randomized controlled trial.pt.
-
random*.tw.
-
3 or 4
-
1 or 2
-
5 and 6
-
1 or 2
-
3 or 4
-
5 and 6
EMBASE 1974 to 2013 August 27
-
exp *diabetic retinopathy/
-
(diabet* and retinopathy).m_titl.
-
1 and 2
-
(random* adj3 trial*).tw.
-
(random* adj3 control*).tw.
-
4 or 5
-
3 and 6
The Cochrane Library
Cochrane Central Register of Controlled Trials: Issue 7 of 12, July 2013.
Search strategy “diabetic retinopathy” in title, and laser or photocoagulation or photo-coagulation in title abstract keywords in Trials”.
After deduplication, resulted in 383 in the database and 92 were selected for full text.
Additional searches for non-randomised controlled trial evidence
(c) Searches for studies on non-proliferative diabetic retinopathy
Additional searches of any study design were done to find any studies that specifically mentioned DR at the non-proliferative or pre-proliferative stage, in order to find additional information for the clinical background section or data on progression or natural history. The searches below were run and downloaded into EndNote and resulted in 928 records in the database after removal of duplicates; the full text of 59 articles was requested and further examined.
Ovid MEDLINE 1946 to August Week 2 2013; Ovid MEDLINE In-Process & Other Non-Indexed Citations August 27, 2013
-
((non-proliferative or nonproliferative or preproliferative or pre-proliferative) adj3 retinopathy).tw.
-
early retinopathy.tw.
-
NPDR.tw.
-
1 or 2 or 3
-
exp *Diabetic Retinopathy/
-
4 and 5
-
limit 6 to English language
Ovid EMBASE 1974 to 2013 August 27=569
-
((non-proliferative or nonproliferative or preproliferative or pre-proliferative) adj retinopathy).tw.
-
early retinopathy.tw.
-
NPDR.tw.
-
1 or 2 or 3
-
exp *Diabetic Retinopathy/
-
4 and 5
-
limit 6 to English language
(d) Searches for studies on laser in diabetic retinopathy
Ovid MEDLINE 1946 to August Week 2 2013, Ovid MEDLINE In-Process & Other Non-Indexed Citations August 27, 2013; EMBASE 1974 to 2013 August 27
-
exp *diabetic retinopathy/
-
diabetic retinopathy.m_titl.
-
(laser or photo-coagulation or photocoagulation or panretinal or pan-retinal or PRP).m_titl.
-
1 and 2
-
3 and 4
-
limit 5 to English language
The Cochrane Library
Cochrane Central Register of Controlled Trials: Issue 8 of 12, August 2013.
There are 79 results from 71,0762 records for your search on “(laser or photocoagulation or photo-coagulation) and diabetic retinopathy and (NPDR or non-proliferative or non-proliferative or pre-proliferative or preproliferative) in title abstract keywords in Trials”.
(e) Searches of progression of diabetic retinopathy
These searches below were done to inform the background section and the model.
EMBASE 1974 to 2013 September 19; Ovid MEDLINE 1946 to September Week 2 2013; Ovid MEDLINE In-Process & Other Non-Indexed Citations September 19, 2013
-
diabetic retinopathy.m_titl.
-
exp Disease Progression/
-
(progression or natural or course).m_titl.
-
exp Diabetic Retinopathy/
-
1 and 4
-
2 and 3 and 5
-
limit 6 to English language
Resulted in 300 records.
(f) Searches for adverse effects of lasers from non-randomised controlled trial studies
The EndNote database created from previous searches for PRP and lasers was searched using the following keywords:
(adverse or risk* or harm* or side effect* or safety or pain or visual loss or complication*) and (laser or (photocoagulation or panretinal or pan-retinal or scatter or PRP).
The results of this search were then supplemented with searches of MEDLINE and EMBASE.
Ovid MEDLINE 1946 to November Week 3 2013
-
exp Diabetic Retinopathy/
-
Laser Coagulation/ae, ct [Adverse Effects, Contraindications]
-
1 and 2
-
limit 3 to English language
EMBASE 1974 to 2014 Week 02
-
exp Diabetic Retinopathy/
-
exp laser coagulation/ae [Adverse Drug Reaction]
-
limit 1 to English language
(g) Quality of life searches for diabetic retinopathy after pan-retinal photocoagulation
Searches of existing databases created from the previous searches above were done using the keywords ‘quality of life’ and ‘laser or photocoagulation’. These records were exported into a new EndNote database and also supplemented with the following database searches.
Ovid MEDLINE In-Process & Other Non-Indexed Citations February 10, 2014; Ovid MEDLINE 1946 to January Week 5 2014; EMBASE 1974 to 2014 February 10 – Retrieved 36
-
exp “Quality of Life”/
-
quality of life.tw.
-
1 or 2
-
exp Laser Coagulation/
-
(laser or photocoagulation).tw.
-
4 or 5
-
3 and 6
-
limit 7 to English language
-
exp Diabetic Retinopathy/
-
diabetic retinopathy.mp.
-
9 or 10
-
8 and 11
-
limit 12 to English language
The resulting EndNote database had 80 references, of which 16 were selected for the section on the quality for DR after PRP.
(h) Searches for ongoing or recently completed research
Searches were done on 16 January 2014 using the keywords “diabetic retinopathy AND (laser OR photocoagulation OR PRP OR scatter)”.
Sources searched were ClinicalTrials.gov, WHO, Clinical Trials Registry Platform Search Portal, Current Controlled Trials, UK Clinical Trials Gateway, EU Clinical Trials Register, UK Clinical Research Network Study Portfolio.
Selected those where the condition being studies was diabetic retinopathy (but excluded those where it was only DMO) and the intervention included scatter or PRP laser alone or in combination with something else and where the results had not been found published in full or as a meeting abstract. Selected only those that had a RCT study design – apart from one observational study.
Also searched the Association for Research in Vision and Ophthalmology (ARVO) meeting abstracts website for recently completed RCTs from 2002 to present using the keywords (randomized and laser) or (randomized and photocoagulation) and selected those that were published between 2011 and 2013 and which were about PRP laser for DR.
(i) Cost-effectiveness search strategies
MEDLINE and EMBASE searches
-
exp quality adjusted life year/
-
quality adjusted life year.mp.
-
(QALY or QALYs).mp.
-
utilit$.mp.
-
(EuroQol or Euro Qol or Euro-Qol or EQ 5D or EQ5D or EQ-5D).mp.
-
(health utilities index or health-utilities-index or HUI).mp.
-
(SF 6D or SF6D or SF-6D).mp.
-
(short form 36 or short-form 36 or SF 36 or SF36 or SF-36).mp.
-
(quality of wellbeing or quality of well-being or QWB).mp.
-
(healthy years equivalent or hyes or hye).mp.
-
(time trade off or time trade-off or time-trade-off or TTO).mp.
-
(standard gamble or standard-gamble or SG).mp.
-
(15 D or 15D).mp.
-
((willing$ adj2 pay) or WTP).mp.
-
Health Status/
-
exp Quality of Life/
-
(quality adj2 life).mp.
-
(health state* or health status).mp.
-
(hrql or hrqol or disability$ or disutility$).mp.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
-
exp Cost-Benefit Analysis/
-
(cost effective$ or cost-effective$).mp.
-
(cost utility$ or cost-utilit$).mp.
-
(cost benefit$ or cost-benefit$).mp.
-
(willingness to pay or wtp or willingness-to-pay or willingness to accept or willingness-to-accept or net benefit or net-benefit or contingent valuation).mp.
-
(Pharmacoeconomic$ or pharmaco-economic$ or Economic analy$ or Economic evaluation$).mp.
-
(economic adj2 (evaluation$ or analy$ or study or studies)).mp.
-
(cost adj2 (evaluation$ or analy$ or study or studies or effective$ or benefit$ or utili$)).mp.
-
((markov or decision) adj2 model).mp.
-
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
-
exp cost$/
-
exp Economics/
-
cost*.tw.
-
(resource adj2 unit).mp.
-
(resource adj2 item).mp.
-
resource$.mp.
-
31 or 32 or 33 or 34 or 35 or 36
-
20 or 30 or 37
-
diabetic retinopathy.m_titl.
-
exp Diabetic Retinopathy/
-
39 or 40
-
38 and 40
-
limit 42 to English language
Web of Knowledge/Web of Science
diabetic retinopathy AND (“quality of life” or Qol or hrqol or quality adjusted life year* or QALY* or cost* or economic* or pharmacoeconomic* or model* or euro-qol or utilit* or EuroQol or Euro Qol or EQ5D or EQ-5D or SF-36 or SF36 or time trade* or TTO or standard gamble or markov or visual analog* or discrete choice or health stat* or “willingness to pay”).
(j) Additional sources searched
-
Auto-alerts Weekly auto-alerts in Ovid MEDLINE and EMBASE were run for all of the above search strategies from August 2013 to March 2014 in order to capture new studies added after the initial searches.
-
Value in Health website Also searched the website of the journal Value in Health using the search term “diabetic retinopathy” in the title or abstract for full text articles and meeting abstracts.
-
ARVO meeting abstracts Searched the ARVO website for meeting abstracts (2002 to present) indexed in the Investigative Ophthalmology & Visual Science (IOVS) journal using the keywords “diabetic retinopathy and (laser or photocoagulation or PRP)”.
-
Contact with authors Contacted authors of some published and ongoing trials for further clarification.
Appendix 3 Major features of Early Treatment Diabetic Retinopathy Study early photocoagulation
(Taken from table 2, ETDRS #7. 40)
| Scatter | Full | Mild |
|---|---|---|
| Burn characteristics | ||
| Size | 500 µm (at retina) | 500 µm (at retina) |
| Exposure | 0.1 seconds | 0.1 seconds |
| Intensity | Moderate | Moderate |
| Number | 1200–1600 | 400–650 |
| Placement | Half burn apart > 2 DDs from fovea out to equator | ≥ 1 burn apart > 2 DDs from fovea out to equator |
| No. of episodes | ≥ 2 | 1 |
| Lesion treated directly | Patches of NVE < 2 disc areas | Patches of NVE < 2 disc areas |
| Indications for follow-up treatment | Recurrent or new NVE or high-risk proliferative retinopathy | Recurrent or new NVE or high-risk proliferative retinopathy |
| Focal | Direct | Grid |
| Burn characteristics | ||
| Size | 50–100 µm | < 200 µm (at retina) |
| Exposure | 0.05–0.1 seconds | 0.05–0.1 seconds |
| Intensity | Sufficient to whiten or darken large microaneurysms | Mild |
| Number | Sufficient to satisfactorily treat all focal leaks | Sufficient to cover all areas of diffuse leakage and non-perfusion |
| Placement | 500–3000 µm from centre of fovea | Spaced > 1 burn width apart 500–3000 µm from centre of fovea |
| No. of episodes | 1 | 1 |
| Indications for follow-up treatment | Presence of CSMO and treatable lesions at ≥ 4 months | Presence of CSMO and treatable lesions at ≥ 4 months |
Appendix 4 Health-state transitions for the model (usual care and intervention)
| Usual care arm | Intervention arm |
|---|---|
Moderate NPDR will remain in that health state or progress to:
|
Moderate NPDR will remain in that health state or progress:
|
Severe NPDR will remain in that health state or progress to:
|
Severe NPDR will progress to:
|
Severe NPDR PT will remain in that health state or progress to:
|
|
Or regress to:
|
|
Severe NPDR and CSMO with/without VI will remain in that health state or progress to:
|
Severe NPDR and CSMO with/without VI will progress to:
|
Severe NPDR and CSMO with/without VI PT will remain in that health state or progress to:
|
|
Or regress to:
|
|
Early PDR will remain in that health state or progress to:
|
Early PDR will progress to:
|
Early PDR PT will remain in that health state or progress to:
|
|
Or regress to:
|
|
Early PDR and CSMO with/without VI will remain in that health state or progress to:
|
Early PDR and CSMO with/without VI will progress to:
|
Early PDR and CSMO with/without VI post treatment will remain in that health state or progress to:
|
|
Or regress to:
|
|
HR-PDR will progress to:
|
HR-PDR will progress to:
|
HR-PDR PT will remain in that health state or progress to:
|
HR-PDR PT will remain in that health state or progress to:
|
Or regress to:
|
Or regress to:
|
HR-PDR and CSMO with/without VI will progress to:
|
HR-PDR and CSMO with/without VI will progress to:
|
HR-PDR and CSMO with/without VI PT will remain in that health state or progress to:
|
HR-PDR and CSMO with/without VI PT will remain in that health state or progress to:
|
Or regress to:
|
Or regress to:
|
Severe PDR will progress to:
|
Severe PDR will progress to:
|
Severe PDR PT will remain in that health state or progress to:
|
Severe PDR PT will remain in that health state or progress to:
|
Or regress to:
|
Or regress to:
|
Severe PDR and CSMO with/without VI will progress to:
|
Severe PDR and CSMO with/without VI will progress to:
|
Severe PDR and CSMO with/without VI PT will remain in that health state or progress to:
|
Severe PDR and CSMO with/without VI PT will remain in that health state or progress to:
|
Or regress to:
|
Or regress to:
|
SVL/blindness will remain in that health state or progress
|
SVL/blindness will remain in that health state or progress
|
| Dead will remain in that health state | Dead will remain in that health state |
List of abbreviations
- AAO
- American Academy of Ophthalmology
- ACCORD
- Action to Control Cardiovascular Risk in Diabetes
- AMD
- age-related macular degeneration
- ARVO
- Association for Research in Vision and Ophthalmology
- BCVA
- best corrected visual acuity
- BP
- blood pressure
- BSE
- better-seeing eye
- CARDS
- Collaborative Atorvastatin Diabetes Study
- CD
- choroidal detachment
- CEAC
- cost-effectiveness acceptability curve
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CMT
- central macular thickness
- COS
- Canadian Ophthalmological Society
- CPRP
- central classical pan-retinal photocoagulation
- CRVO
- central retinal vein occlusion
- CSII
- continuous subcutaneous insulin infusion
- CSMO
- clinically significant macular oedema
- DCCT
- Diabetes Control and Complications Trial
- DCE
- discrete choice experiment
- DD
- disc diameter
- DMO
- diabetic macular oedema
- DPSS
- diode-pumped solid state
- DR
- diabetic retinopathy
- DRCRN
- Diabetic Retinopathy Clinical Research Network
- DRS
- Diabetic Retinopathy Study
- DTSQ
- Diabetes Treatment Satisfaction Questionnaire
- DVLA
- Driver and Vehicle Licensing Agency
- EDIC
- Epidemiology of Diabetes Interventions and Complications
- EME
- Efficacy and Mechanism Evaluation
- EQ-5D
- European Quality of Life-5 Dimensions
- ETDRS
- Early Treatment Diabetic Retinopathy Study
- FA
- fluorescein angiography
- FA-LTE
- Family Apgar-List of Threatening Experiences
- FIELD
- Fenofibrate Intervention and Event Lowering in Diabetes
- FLA
- fluorescein leakage
- GLX
- solid-state green laser
- HADS
- Hospital Anxiety and Depression Scale
- HbA1c
- glycated haemoglobin
- HR-PDR
- high-risk proliferative diabetic retinopathy
- HRC
- high-risk characteristic
- HRC-PDR
- high-risk characteristics proliferative diabetic retinopathy
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- HTN
- hypertension
- HUI
- Health Utilities Index
- ICER
- incremental cost-effectiveness ratio
- IOP
- intraocular pressure
- IOVS
- Investigative Ophthalmology & Visual Science
- IRMA
- intraretinal microvascular abnormality
- IVB
- intravitreal bevacizumab
- IVR
- intravitreal ranibizumab
- IVTA
- intravitreal triamcinolone acetonide
- MAPASS
- MAnchester PSC Study
- mETDRS
- modified ETDRS
- MO
- macular oedema
- MPC
- macular photocoagulation
- MS-PRP
- multiple-session pan-retinal photocoagulation
- MT-PRP
- minimally traumatic pan-retinal photocoagulation
- NDRLTA
- National Diabetic Retinopathy Laser Treatment Audit
- Nd:YAG
- neodymium-doped yttrium aluminium garnet
- NEI
- National Eye Institute
- NEI VFQ-25
- National Eye Institute Visual Function Questionnnaire-25
- NETSCC
- NIHR Evaluation, Trials and Studies Coordinating Centre
- NHMRC
- National Health and Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NPA
- non-perfused area
- NPDR
- non-proliferative diabetic retinopathy
- NPS
- numerical pain score
- NSC
- National Screening Committee
- NV
- neovascular
- NVD
- neovascularisation of the disc
- NVE
- neovascularisation of the retina elsewhere (outside the disc)
- NVG
- neovascular glaucoma
- NVI
- neovascularisation of the iris
- OCT
- optical coherence tomography
- ONS
- Office for National Statistics
- PASCAL
- PAtterned SCAnning Laser
- PDR
- proliferative diabetic retinopathy
- PPDR
- pre-proliferative diabetic retinopathy
- PPV
- pars plana vitrectomy
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRP
- pan-retinal photocoagulation (also sometimes called ‘scatter photocoagulation’)
- PSC
- pattern scan
- PSS
- Personal Social Services
- PSTA
- posterior sub-Tenon’s triamcinolone injection
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QWB
- quality of well-being
- RCOphth
- Royal College of Ophthalmologists
- RCT
- randomised controlled trial
- RR
- relative risk
- S-PC
- selective photocoagulation
- SAH10A
- Standard Airlie House photograph 10A
- SBP
- systolic blood pressure
- SD
- standard deviation
- SDM
- sub-threshold diode micropulse laser photocoagulation
- SDRGS
- Scottish Diabetic Retinopathy Grading Scheme
- SEM
- standard error of mean
- SF
- short form
- SF-6D
- Short Form questionnaire-6 Dimensions
- SG
- standard gamble
- SI-PRP
- standard intensity pan-retinal photocoagulation
- SIGN
- Scottish Intercollegiate Guidelines Network
- SS-PRP
- single-session pan-retinal photocoagulation
- STAI
- State–Trait Anxiety Inventory
- STDR
- sight-threatening diabetic retinopathy
- SVL
- severe visual loss
- SVLV
- severe visual loss or vitrectomy
- TA
- triamcinolone acetonide
- TMV
- total macular volume
- TRP
- targeted retinal photocoagulation
- TTO
- time trade-off
- UKPDS
- United Kingdom Prospective Diabetes Study
- VA
- visual acuity
- VAS
- visual analogue scale
- VEGF
- vascular endothelial growth factor
- VF
- visual field
- VFQ
- Visual Function Questionnaire
- VisQoL
- Vision and Quality of Life
- WDRS
- Wisconsin Diabetes Registry Study
- WESDR
- Wisconsin Epidemiologic Study of Diabetic Retinopathy
- WF-FA
- wide-field Optos FA
- WHO
- World Health Organization
- WSE
- worse-seeing eye
- WTP
- willingness to pay