Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/70/01. The contractual start date was in March 2014. The draft report began editorial review in May 2014 and was accepted for publication in November 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Disclaimer
this report contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Cooper et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Overview of cannabis use
Cannabis use may be defined as acute (occasional) or chronic, with chronic use often defined as use on most days over a period of years. 1 Cannabis dependence can develop from chronic use and is characterised by impaired control over use and difficulty in ceasing use. 1 Cannabis dependence is a recognised psychiatric diagnosis, often diagnosed via the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria and the International Classification of Diseases, 10th Revision (ICD-10). 2,3
Cannabis use has been found to exacerbate the symptoms of psychiatric disorders. 4 In one study, individuals who used cannabis regularly were found to be six times more likely to have a mood or anxiety disorder. 5 The term ‘dual diagnosis’ is used to describe individuals who have a mental health problem and also are dependent on drugs (or alcohol). 6
Epidemiology and prevalence
Cannabis is the most commonly used illicit drug worldwide. 7 In one study reporting cannabis use in European countries, use for 20 or more days per month ranged from 3.5% to 44.1%, with the figure for the UK being 3.9%. 8 In Australia, the prevalence of Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV)-defined cannabis abuse in the general population over a 12-month period has been estimated at 2.3%, whereas a national survey undertaken in the USA found that 6% of individuals who used cannabis within a 1-year period met the DSM-IV criteria for cannabis dependence. 9,10 This figure, as would be expected, varies by country. In Australia, 31.7% of individuals who used cannabis more than five times in the past year met the criteria for a cannabis use disorder. 11,12
Estimates for the prevalence of patients with a ‘dual diagnosis’ (substance abuse disorder and mental health problems) vary across sources, but it is frequently reported that over 50% of patients with mental health problems also have a substance abuse problem. 6
Impact of health problem and prognosis
The impact of cannabis use on the individual can be classed as acute or chronic. Acute effects include hyperemesis syndrome (recurrent nausea, vomiting and abdominal pain), impaired co-ordination and performance, anxiety, suicidal ideations/tendencies, impaired attention and memory and psychotic symptoms. 2,13 Chronic effects include development of cannabis dependence, cognitive impairment, pulmonary disease and malignancy of the oropharynx. 2,13 There is increasing evidence to suggest the presence of a cannabis withdrawal syndrome, with symptoms (such as dysphoric mood, disturbed sleep and gastrointestinal symptoms) beginning during the first week and continuing for several weeks following the start of abstinence. 4 In a study by Budney et al. ,14 47% of participants withdrawing from cannabis reported four or more severe symptoms, including irritability, craving and nervousness; other symptoms were less severe and included depression, restlessness and headaches.
In a cohort study comparing those seeking treatment with non-treatment seekers, those seeking treatment reported increased cannabis use and more symptoms of dependence but a more positive attitude to treatment. 15 Even when an individual has sought treatment, recovery from substance dependence is hampered by poor adherence to psychological and psychosocial treatments, with factors such as cognitive defects, personality disorder and younger age predicting low treatment adherence. 16
Measurement of disease
Cannabis abuse and dependence is diagnosed using one or more assessment criteria, the most widely used being DSM-IV and ICD-10. There are DSM-IV criteria for both substance dependence and abuse. 3 Dependence is defined as tolerance (a need for increased amounts of the substance to achieve the desired effect), withdrawal (either having withdrawal symptoms or taking another substance to avoid withdrawal symptoms), taking substance in larger amounts than intended, and having persistent desire or unsuccessful efforts to cut down use. Substance abuse is characterised by recurrent use resulting in failure to fulfil obligations, recurrent use in hazardous situations, recurrent substance-related legal problems and continued use despite recurrent social or interpersonal problems. For both sets of criteria, individuals meeting three or more criteria within a 12-month period meet the diagnosis. In 2013, the updated Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5) criteria were released. 17 In the revised criteria, there is no distinction between abuse and dependence, but a spectrum of substance use disorders. 18
Current service provision
Relevant national guidelines
Guidance from the National Institute for Health and Care Excellence (NICE) states that pharmacological interventions for chronic cannabis users are not well developed, so psychosocial interventions are the mainstay of effective treatment. 19 UK Department of Health guidelines for the treatment of chronic users recommend that clinicians should consider motivational interventions in mild cases and structured treatment with key working (when a health professional works with the individual to ensure delivery and ongoing review of care being received) in more heavy users, whereas cognitive–behavioural therapy (CBT) is recommended in cases with comorbid depression and anxiety. 20 European best practice guidance, produced by the European Monitoring Centre for Drugs and Drug Addiction, recommends the use of multidimensional family therapy for regular cannabis users, while individual sessions of CBT are stated to be possibly advantageous. 21
Management of the condition
Providing treatment to chronic users of cannabis to reduce or cease their use is a relatively recent occurrence. Until the 1980s, it was thought that chronic cannabis use did not lead to dependence and treatment was, therefore, not required. 22 Since then, research has looked into evaluating the use of a wide variety of psychological and psychosocial interventions, such as motivational interviewing (MI), CBT and contingency management (voucher incentives). 12 There is limited evidence to suggest which of the many psychological and psychosocial interventions are the most effective at reducing cannabis use.
A number of systematic reviews have been undertaken to assess the benefits of such interventions for regular cannabis users, many of which included meta-analyses. However, they all had limited scope and, therefore, did not assess all the available evidence, and several further studies have been published since. A review by Denis et al. 12 that excluded studies in populations dependent on drugs other than cannabis analysed six randomised controlled trials (RCTs) via narrative synthesis, involving interventions such as CBT and motivational enhancement therapy (MET), finding that CBT provided improved outcomes over brief interventions, whereas voucher incentives were found to enhance treatment when used in combination with other therapies. Dutra et al. 23 undertook a meta-analysis, identifying five studies assessing the use of psychological treatments (including case management, CBT and relapse prevention), finding a significant difference between outcomes for cannabis use, with a mean effect size of 0.81 [95% confidence interval (CI) 0.25 to 1.36] when comparing intervention treatments with control [which consisted of motivational enhancement, wait list control and treatment as usual (TAU)]. Other reviews have focused on specific interventions to treat regular users of cannabis. Tait et al. 24 assessed the use of internet-delivered interventions, finding that such interventions provided a significant decrease in cannabis use at post treatment [g (Hedges’ bias-corrected effect size) = 0.16, 95% CI 0.09 to 0.22; p-value < 0.001].
Previous reviews have also sought to investigate the effectiveness of such interventions across the spectrum of substance misuse, including alcohol and opioids. The review by Dutra et al. 23 reported that treatments incorporating both CBT and contingency management had the greatest effect sizes on substance use across a range of substances including cocaine, opiates and cannabis (Cohen’s d 1.02), whereas the two treatments alone had smaller effect sizes on the same group of substances (contingency management, Cohen’s d 0.58, 95% CI 0.25 to 0.90; CBT, Cohen’s d 0.28, 95% CI 0.06 to 0.51). In contrast, a review by Hunt et al. 6 included RCTs of patients with a severe mental illness and substance dependence, finding no compelling evidence to suggest a significant decrease in substance use when comparing CBT over TAU [two studies, risk ratio (RR) 1.12, 95% CI 0.44 to 2.86] or of CBT plus MI over TAU (one study, mean difference 0.19, 95% CI –0.22 to 0.60). The use of MI alone compared with usual treatment had positive effects on abstinence from alcohol (one study, RR 0.36, 95% CI 0.17 to 0.75) but no effect on other substances (one study, RR –0.07, 95% CI –0.56 to 0.42). 6 Other reviews have focused on specific interventions for ‘general’ substance misuse. Magill et al. 25 analysed 52 studies assessing the use of CBT (plus pharmacological treatments in a number of studies) on a range of substance dependences (including alcohol, cannabis, opiates and cocaine), reporting a small effect on the reduction of substance use for those studies reporting relevant outcomes (34 studies, g = 0.108, 95% CI 0.051 to 0.165; p-value < 0.005). Wood et al. 26 assessed the use of computer-delivered interventions, finding that drug prevention programmes were effective at reducing use in the mid-term (12 months) but not at post treatment. Mindfulness-based interventions have also been found to be effective for substance abuse. 27
Description of technology under assessment
Summary of interventions
This review assesses the clinical effectiveness of psychological and psychosocial interventions aimed at assisting regular cannabis users to reduce or cease their use. Only interventions delivered in an outpatient or community setting are included. A full list is provided in Chapter 3, Methods for reviewing effectiveness.
Chapter 2 Definition of the decision problem
Decision problem
The aim of this assessment was to systematically review the evidence for the clinical effectiveness of psychological and psychosocial interventions for cannabis cessation in adults who use cannabis regularly.
Population and setting
The relevant population included individuals ≥ 18 years of age who were regular users of cannabis and had participated in a study providing treatment(s) for cannabis use in a community or outpatient setting. Studies focusing specifically on treating cannabis users within prisons or the criminal justice system or in inpatient settings were excluded. Inclusion was not restricted according to level of cannabis use at baseline.
Interventions
Studies involving psychological and psychosocial interventions were included.
Relevant comparators
Comparators included other psychological and psychosocial interventions, waiting list control, TAU or no treatment (comparisons with drug treatments were excluded).
Key outcomes
The key outcomes for this review were frequency and amount of cannabis use; severity of dependence; motivation to change; level of cannabis-related problems (including medical and other); and attendance, retention and dropout rates. The results of the review were also used to formulate recommendations for future research.
Overall aims and objectives of assessment
The aims and objectives of this assessment were to systematically review the evidence for the clinical effectiveness of psychological and psychosocial interventions for cannabis cessation in people who use cannabis regularly.
Chapter 3 Assessment of clinical effectiveness
A systematic review was undertaken to evaluate the effectiveness of psychological and psychosocial interventions for cannabis cessation in adults who use cannabis regularly. The review was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (www.prisma-statement.org/). 28 The completed PRISMA checklist is presented in Appendix 1.
Methods for reviewing effectiveness
Identification of studies
The following electronic databases were searched to February 2014 for published and unpublished research evidence: MEDLINE, EMBASE, Cochrane Controlled Trials Register, Health Technology Assessment (HTA) database, Database of Abstracts of Reviews of Effects, Cochrane Database of Systematic Reviews, NHS Economic Evaluation Database, PsycINFO and Web of Science Conference Proceedings Citation Index. This included reference searching within relevant systematic reviews and included studies, contact with experts and searching clinical trials databases (https://ClinicalTrials.gov and www.controlled-trials.com) and relevant websites, including United Nations Office on Drugs and Crime (www.unodc.org), DrugScope (www.drugscope.org.uk), American Society of Addiction Medicine (www.asam.org), National Institute on Drug Abuse (www.drugabuse.gov), Canadian Centre on Substance Abuse (www.ccsa.ca), and Canadian Society of Addiction Medicine (www.csam-smca.org).
The protocol for this review is available on request from the authors.
Inclusion and exclusion criteria
Population and setting
The relevant population included participants aged ≥ 18 years, who were regular users of cannabis. Inclusion was not restricted according to level of cannabis use at baseline. The review focused on studies in a community or outpatient setting.
Studies focusing on the following subpopulations were excluded:
-
Studies in the setting of the criminal justice system, that is prisons, following release (on parole) or within the court system.
-
Studies for which the majority of participants were young people (< 18 years of age). In studies of mixed age groups, data for subgroups aged ≥ 18 years were extracted if available or, if not, then the study was included if ≥ 80% of participants were aged ≥ 18 years or, if these data were not available, where the mean age of participants was ≥ 18 years, at baseline.
-
Studies for which participants were treated in an inpatient setting, that is, the patient received treatment for regular cannabis use while occupying a hospital ward, drug rehabilitation centre or within an emergency department. Studies for which a subset of the participants were residing in inpatient psychological treatment centres were included, provided that the cannabis intervention was delivered as a standalone therapy rather than as an integrated part of psychological treatment.
-
Studies in which the intervention, or a component of the intervention, was provided to participants other than the cannabis user (e.g. parents or partners). An example of such an intervention is Multidimensional Family Therapy.
-
Studies in very specific subpopulations (such as indigenous communities or human immunodeficiency virus patients).
For studies covering abuse of more than one substance (i.e. poly-substance abuse, involving other drugs or alcohol), the following approach was taken:
-
Studies were included only if they reported cannabis-use outcomes (rather than any drug use) for the subpopulation who were cannabis users.
-
Studies were excluded if the entire study population was dependent on alcohol, cocaine, opiates, amphetamines or receiving methadone maintenance (as these are quite specific populations and less relevant to cannabis cessation).
Included interventions
Relevant interventions included a range of psychological and psychosocial interventions aiming to reduce or cease cannabis use. Combinations of therapies were included. All possible modes of delivery were included, including individual face-to-face or group sessions, plus interventions provided via the internet or telephone. Relevant interventions included:
-
CBT – an approach aiming to manage cannabis use by changing the way the participant thinks or behaves29
-
MI – a person-centred approach that aims to improve motivation to change and resolve ambivalence to change30
-
MET – a variant of MI that is manual based31
-
relapse prevention therapy – based on CBT, enables clients to cope with high-risk situations that may lead to drug taking32
-
contingency management – providing patients with tangible rewards (such as monetary vouchers) in return for a reduction or cessation in drug taking20
-
case management – a strategy to improve the co-ordination and continuity of the delivery of services to a patient33
-
mutual aid therapy – therapy in which people with similar experiences assist each other to overcome or manage their issues (e.g. Self-Management and Recovery Training)
-
other psychological and psychosocial interventions as identified within the review process.
Comparators
Comparators included other psychological and psychosocial interventions, waiting list control, TAU or no treatment. Studies comparing a psychosocial intervention with a drug treatment were excluded.
Outcomes
The key outcomes for this review were:
-
frequency and intensity of cannabis use, via self-report, with or without confirmation by biological analysis (urinalysis, hair/saliva analysis)
-
number (%) of days used, time periods of use per day, amount per day
-
number (%) reporting abstinence following intervention
-
-
severity of drug-related problems [measured via the Addiction Severity Index (ASI)]34
-
severity of dependence [measured via the Severity of Dependence Scale (SDS)]11
-
stage of change or motivation/contemplation to change [e.g. as measured by the Readiness to Change Questionnaire (RCQ)]31
-
level of cannabis-related problems – medical problems, legal problems, social and family relations, employment and support [assessed via questionnaires such as the Cannabis Problems Questionnaire (CPQ)]35
-
attendance, retention and dropout rates, measured as number of sessions attended or number (%) completing whole treatment period
-
recommendations for future research.
Included study types
Only RCTs were included in this review.
Excluded study types
The following study types were excluded:
-
non-randomised studies
-
narrative reviews, editorials, opinion pieces
-
reports written in a language other than English or published as meeting abstracts, if insufficient methodological details are reported in the abstract to allow critical appraisal of study quality and extraction of study characteristics and key outcomes.
Data extraction strategy
Titles and abstracts of citations identified by the searches were screened for potentially relevant studies by one reviewer and a 10% sample checked by a second reviewer (and a check for consistency undertaken). Full texts were screened by two reviewers. One reviewer performed data extraction for each included study. All numerical data were checked against the original article by a second reviewer and any disagreements were resolved through discussion. When studies comprised duplicate reports (parallel publications), the most recent and relevant report was used as the main source and additional reports checked for extra information. Excluded studies were tabulated (see Appendix 3).
Methods of data synthesis
Data were analysed via a narrative synthesis. As described by Popay et al. ,36 this method is based around grouping and tabulating the data in meaningful clusters, allowing results to be summarised (in the form of text and tables) to provide an overview of the direction of effect for each relevant subgroup. Within this review, studies were first divided into two main population subgroups (general cannabis users and those with a major psychiatric condition). Second, studies were categorised according to their intervention and comparison groups (e.g. CBT vs. wait list, CBT vs. MI, etc.). Third, results were tabulated for two key time points (post treatment and later follow-up). Within each study, outcomes at each time point were categorised according to whether or not they were significantly different between groups or between baseline and follow-up. Finally, summary tables were populated for each intervention/comparison. Outcomes across studies at each time point were summarised as being mainly significant, mainly not significant or mixed.
There was substantial heterogeneity between studies in terms of populations, interventions, comparators, outcome measures reported and statistics reported. To increase clarity, the main results of this review are presented in the form of an overview of the outcomes reported per study and how many showed a significant difference, as described above. Detailed numerical results per study group are not presented in the main results section, but are provided in Appendix 4 for reference. Meta-analysis was not undertaken, as this would have required restricting each analysis to studies reporting the same outcome in a consistent format with full data and it was felt that the broad results picture might have been lost.
Subgroup analyses were undertaken with regard to number of treatment sessions, group/individual treatment, high/low cannabis use at baseline, recruitment method (referral vs. voluntary), participant age and use of other substances (tobacco and alcohol) at baseline.
Quality assessment of included studies
Methodological quality of included RCTs was assessed using an adapted version of the Cochrane risk of bias assessment criteria. This tool addresses specific domains, namely sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective outcome reporting. 37 Outcome assessment was considered to be blinded if the person assessing or interviewing the participants was blinded to group allocation (although participants were not blinded and many of the data were self-reported). We made two adaptations to these criteria in order to aid quality assessment. First, we utilised the ‘5-and-20 rule’ for incomplete outcome data, as proposed by Schulz and Grimes. 38 Schulz and Grimes38 state that a lower than 5% loss in participants probably leads to little bias, while a greater than 20% loss potentially poses serious threats to validity. We therefore defined < 5% attrition as ‘low risk’, between 5% and 20% attrition as ‘intermediate risk’ and > 20% attrition as ‘high risk’. Attrition was defined as the percentage of patients not followed up at the final time point reported. The second adaptation we made to the Cochrane criteria was to add an ‘overall risk’ criterion, aiming to summarise the overall risk of studies. We categorised studies as low risk, high risk or unclear risk, determined using the following criteria. Low risk was allocated to studies where randomisation, allocation concealment, blinded outcome assessment and incomplete data were all determined to be low risk. High risk was allocated to studies deemed to have undertaken inadequate randomisation (self-selection, sequential patients, odd and even), and/or when allocation was not concealed, and/or when incomplete data were deemed to be high risk. Unclear risk was allocated to all other studies.
Patient and public involvement
In order to seek patient and public input into the review, we recruited a service user through liaison with the project’s clinical advisors, who was currently acting as a ‘service ambassador’ within their treatment service (an individual who has completed a treatment regime, ceased their primary substance use and is now involved in supporting patients at the treatment centre).
A short ‘briefing document’ using non-academic language was developed (see Appendix 5) in order to introduce the individual to the research. The briefing document included sections describing the basic principles of a systematic review, the general area in which the research is being undertaken (i.e. psychological/psychosocial treatments for regular users of cannabis) and the input required from the service user. The service user was compensated for the time spent at meetings and for travel expenses.
The review team met with the service user twice. The first meeting was scheduled once the protocol had been written. The service user provided valuable input into the following areas of the protocol:
-
an additional intervention not already identified in the protocol (mutual aid therapy)
-
two additional outcome measures that were felt to be important (daily time periods of cannabis use and contemplation to change)
-
general approval of the focus of the review.
The review team then met with the service user after the draft report had been written, when the service user had the following inputs:
-
suggested amendments to the plain English summary
-
reviewed the suggested research priorities
-
reviewed the section describing factors relevant to the NHS.
Results
Quantity of research available
The searches identified 1087 citations (1079 via database searches and eight via other sources). Of these, 919 citations were excluded at the title/abstract stage and 168 full-text articles were screened. Of these, 126 were excluded: 65 did not include relevant outcomes, 41 evaluated irrelevant populations, nine were not RCTs, five did not involve a relevant intervention, three detailed a non-relevant secondary analysis or study characteristic and three were not in English (excluded studies are listed in Appendix 3). In total, 42 articles relating to 33 RCTs were included in this review. The PRISMA flow chart is shown in Figure 1.
FIGURE 1.
Study selection process: PRISMA flow diagram.
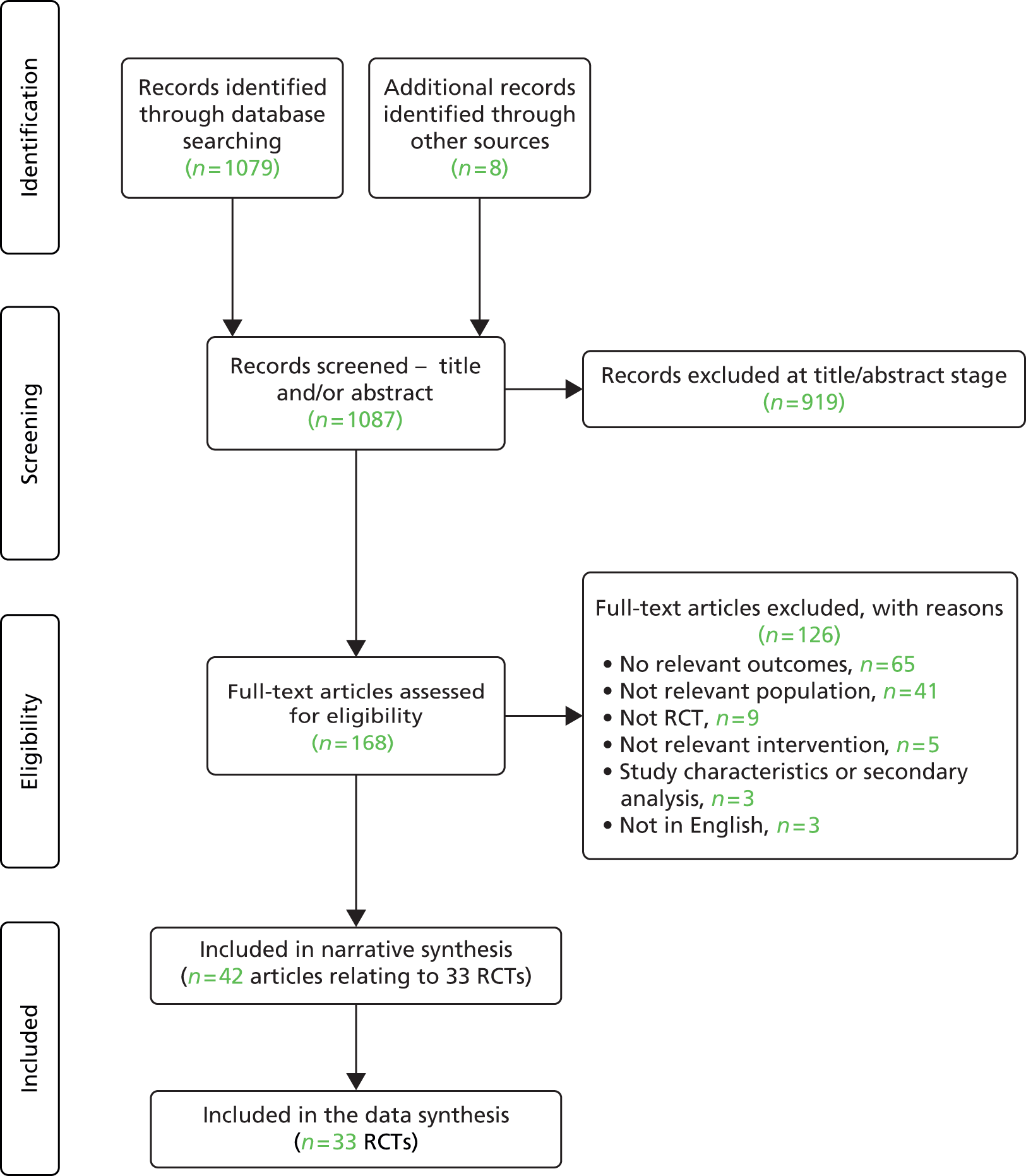
All titles and abstracts were screened for inclusion by one reviewer and a check for consistency was undertaken. A second reviewer screened approximately 10% of the references (n = 100) during the initial screening stage. No discrepancies were found.
Characteristics of included studies
The 33 studies included in this review were undertaken in a range of countries: the USA (13 studies39–51), Australia (seven studies52–58), Germany (three studies59–61), Brazil (two studies62,63), Canada (two studies64,65), Switzerland (two studies66,67), Denmark (one study68), Ireland (one study69) and worldwide (two studies, one utilising internet-based interventions70 and the other undertaken in a number of locations worldwide71) (Tables 1 and 2).
| Study (country, mode of recruitment) | Interventions (number of sessions) | Number of cannabis users | Inclusion criteria: age (years) | Mean age at BL (years) (range) | Level of cannabis use/dependencea | Mean cannabis use at BL | Additional exclusion criteria | Mean alcohol and tobacco use at BL | Key outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabis use | Severity of dependence | Cannabis-related problems | Session attendance | |||||||||
| Babor 200439 and Litt 200572 (USA, voluntary and referral) | CBT/MET/CaseM (9); MET (2); wait list | 450 | ≥ 18 | 36 (18–62) | High use: DSM-IV cannabis dependence; cannabis used ≥ 40 out of 90 days | 27 days/month | Psychiatric conditions; other drug use | Total drinks in last 90 days: 47–59 | Yes | Yes | Yes | Yes |
| Budney 201142 and ClinicalTrials.gov 201373 (USA, voluntary) | CBT/MET/voucher (9); computer-delivered CBT/MET + brief therapist + voucher (9); MET (2) | 45 | 18–65 | 35 (NR) | High use: DSM-IV cannabis abuse or dependence and used cannabis ≥ 40 of previous 90 days | NR | Other drug use | NR | Yes | |||
| Budney 200641 (USA, voluntary and referral) | CBT (14); CBT/vouchers (14); vouchers | 60 | ≥ 18 | 33 (NR) | High use: MET DSM-IV cannabis dependence and used cannabis in past 30 days | 26 days/month | Psychiatric conditions; other drug use | Mean days alcohol use in past 30 days = 6–8 days | Yes | Yes | Yes | |
| Budney 200040 and Moore 200374 (USA, voluntary) | CBT/MET (14); MET (4); CBT/MET/vouchers (14) | 60 | ≥ 18 | 32 (NR) | High use: DSM-III-R classification for cannabis dependence; cannabis use in previous 30 days | 23 days/month | Psychiatric conditions; other drug use | Days of alcohol use past 30 days: 2.7–7.0 days | Yes | Yes | Yes | Yes |
| Copeland 200153 (Australia, voluntary) | CBT (6); MI (1); wait list | 229 | ≥ 18 | 32 (NR) | High use: DSM-IV cannabis dependence | NR | Other drug use | NR | Yes | Yes | Yes | Yes |
| de Dios 201243 (USA, voluntary) | MI/meditation (2); AO | 39 | 18–29 | 23 (NR) | Low use: ≥ 3 times past month | 18 days/month | Psychiatric conditions; other drug use | NR | Yes | Yes | ||
| Fernandes 201062 (Brazil, voluntary) | Tele-brief motivational intervention (1) written cannabis information | 1744 | NR | 25 (11–NR) | NR | NR | NR | NR | Yes | |||
| Fischer 201275 and Fischer 201364 (Canada, voluntary) | Brief MI (1); written cannabis information; therapist general health MI (1); written general health information | 134 | 18–28 | 20 (NR) | Low use: used for > 1 year, at least 12 of past 30 days | 24 days/month | NR | NR | Yes | |||
| Gates 201255 (Australia, voluntary) | Tele-CBT/MI (4); wait list | 160 | ≥ 16 | 36 (NR) | Low use: ≥ 1 use cannabis in last month | NR | Psychiatric conditions; other drug use | Nicotine 90-day use: 57.6–59. Alcohol: 90-day use: 20.1–25.9 | Yes | Yes | Yes | Yes |
| Gmel 201367 (Switzerland, voluntary) | Brief MI (1); AO | 378 | 19–20 | 20 (19–20) | NR | 7–9 days/month | NR | NR | Yes | |||
| Grenyer 199756 (Australia, NR) | SEDP (16); MI (1) | 40 | NR | 34 (NR) | High use: DSM-IV cannabis dependence | NR | Other drug use | NR | Yes | Yes | ||
| Hoch 201460 (Germany, referral) | CBT/MET/PPS (10); wait list | 385 | ≥ 16 | 27 (16–63) | Low use: ≥ 9 days/month | 20 days/month | Psychiatric conditions; other drug use | Alcohol: mean 0.2 litres/day. Tobacco: 78–82% used daily | Yes | Yes | Yes | Yes |
| Hoch 201259 and Hoch 200876 (Germany, voluntary and referral) | CBT/MET/PPS (10); wait list | 122 | ≥ 16 | 24 (16–44) | High use: DSM-IV cannabis dependence/abuse 89% | NR | Psychiatric conditions; other drug use | Alcohol dependence: 30% | Yes | Yes | Yes | |
| Humeniuk 201271 (worldwide, referral) | Brief MI (1); wait list | 395 | 16–62 | 31 (NR) | NR | NR | NR | NR | Yes | |||
| Jungerman 200763 (Brazil, NR) | CBT/MI/RP (4) (3 months); CBT/MI/RP (4) (1 month); wait list | 160 | ≥ 18 | 32 (18–58) | Low use: ≥ 13 days/month | 26–28 days/month | Psychiatric conditions; other drug use | Alcohol: 10–11% of prior 90 days | Yes | Yes | Yes | Yes |
| Kadden 200744 and Litt 200877 (USA, voluntary) | CBT/MET (9); CaseM (9); CBT/MET/vouchers (9); vouchers | 240 | ≥ 18 | 33 (NR) | High use: DSM-IV cannabis dependence | NR | Psychiatric conditions; other drug use | ASI alcohol score 0.10 | Yes | Yes | Yes | Yes |
| Lee 201346 (USA, referral) | Brief MI (1); AO | 212 | 18–25 | 20 (NR) | Low use: ≥ 5 days/month | 16–17 days/month | NR | NR | Yes | Yes | Yes | |
| Lee 201045 (USA, referral) | Internet-based personalised feedback (1); AO | 341 | 17–19 | 18 (NR) | Low use: any use | 3 days/month | NR | NR | Yes | Yes | ||
| Litt 201347 (USA, voluntary) | CBT/MET/vouchers (homework) (9); CBT/MET/vouchers (abstinence) (9); CaseM (9) | 215 | ≥ 18 | 33 (NR) | High use: DSM-IV cannabis dependence | 24 days/month | Psychiatric conditions; other drug use | NR | Yes | Yes | Yes | |
| Rooke 201370 (worldwide, voluntary) | Internet-based CBT/MI (6); internet-based written cannabis information | 230 | ≥ 18 | 31 (NR) | Low use: ≥ 1 day/month | 21 days/month | Psychiatric conditions; other drug use | NR | Yes | Yes | Yes | |
| Sobell 200965 (Canada, voluntary and referral) | CBT/MI (4) (group); CBT/MI (4) (individual) | 17 | ≥ 18 | 32 (NR) | Low use: ‘not severe dependence’ | 27 days/month | Psychiatric conditions; other drug use | NR | Yes | |||
| Stein 201148 (USA, voluntary) | MI (2); AO | 332 | 18–24 | 21 (NR) | Low use: ≥ 1 day/month | 17 days/month | Other drug use | NR | Yes | Yes | ||
| Stephens 200751 (USA, voluntary) | MI/personalised feedback (1); cannabis education (1); wait list | 188 | ≥ 18 | 32 (18–57) | High use: ≥ 15 days/month | 26 days/month | Psychiatric conditions; other drug use | Alcohol use on 1.8 days per week | Yes | Yes | Yes | Yes |
| Stephens 2000,50 Lozano 200678 and DeMarce 200579 (USA, voluntary) | CBT/RP/social support (14); MI (2); wait list | 291 | ≥ 18 | 34 (NR) | High use: DSM-III-R cannabis dependence | 25 days/month | Psychiatric conditions; other drug use | NR | Yes | Yes | Yes | Yes |
| Stephens 199449 (USA, voluntary) | CBT/RP (10); social support group (10) | 212 | ≥ 18 | 32 (18–65) | High use: ≥ 17 days/month | 27 days/month | Psychiatric conditions; other drug use | NR | Yes | Yes | Yes | |
| Tossmann 201161 (Germany, voluntary) | Internet-based counselling; wait list | 1292 | NR | 25 (NR) | High use: ‘any use’, 92% DSM-IV cannabis dependent at BL | NR | NR | NR | Yes | Yes | ||
| Study (country, mode of recruitment) | Interventions (number of sessions) | Number of cannabis users | Inclusion criteria: age (years) | Mean age at BL (years) (range) | Level of cannabis use/dependencea | Mean cannabis use at BL | Inclusion criteria: psychiatric condition | Additional exclusion criteria | Mean alcohol and tobacco use at BL | Key outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabis use | Severity of dependence | Cannabis-related problems | Session attendance | ||||||||||
| Baker 200652 (Australia, referral) | CBT/MI + TAU (10); TAU | 73 | ≥ 15 | 29 (15–61) | Low use: ≥ 4 days/month | 5–8 days/month | ICD-10 psychotic disorder | NR | NR | Yes | Yes | ||
| Bonsack 201166 (Switzerland, referral) | CBT/MI + TAU (6); TAU | 62 | 18–35 | 26 (18–35) | High use: 82% cannabis dependent | 23 days/month | ICD-10 psychotic disorder | Other drug use | NR | Yes | Yes | Yes | |
| Edwards 200654 (Australia, referral) | CBT/MI + TAU (10); psychoeducation (non-cannabis) + TAU (10) | 47 | 15–29 | 21 (NR) | Low use: 49% DSM-IV cannabis dependent | 8 days/month | DSM-IV psychotic disorder | NR | 2.2% DSM-IV diagnosed alcohol dependence | Yes | Yes | Yes | |
| Hjorthoj 201368 and 201280 (Denmark, referral) | CBT/MI + TAU (24); TAU | 103 | 17–42 | 27 (NR) | High use: ICD-10 cannabis dependence/abuse | 15 days/month | ICD-10 schizophrenia | NR | NR | Yes | Yes | ||
| Kay-Lambkin 201158 (Australia, voluntary and referral) | CBT/MI (10); computer-delivered CBT/MI + brief therapist (10); PCT (10) | 109 | ≥ 16 | 40 (17–70) | Low use: ≥ 4 days/month | NR | DSM-IV major depressive disorder, BDI-II ≥ 17 | Psychotic conditions | NR | Yes | Yes | ||
| Kay-Lambkin 200957 (Australia, voluntary and referral) | CBT/MI (10); computer-delivered CBT/MI + brief therapist (10); brief MI (1) | 43 | ≥ 16 | 35 (18–61) | Low use: ≥ 4 days/month | NR | DSM-IV major depressive disorder, BDI-II ≥ 17 | NR | NR | Yes | Yes | ||
| Madigan 201369 (Ireland, voluntary and referral) | CBT/MI (group) (12); TAU | 88 | 16–65 | 28 (NR) | High use: DSM-IV cannabis dependence | NR | DSM-IV schizophrenia, psychosis, major depressive or bipolar disorder | NR | NR | Yes | Yes | ||
Population
General or psychiatric
The included studies can be broadly categorised into those that sought to treat the ‘general cannabis users population’ (26 studies;39–51,53,55,56,59–63,65,67,70,71,75 see Table 1) and those that sought to treat patients with a ‘dual diagnosis’ (patients with both a psychiatric condition and cannabis use, seven studies;52,54,57,58,66,68,69 see Table 2). Among the psychiatric studies, two studies52,66 included participants with schizophrenia, psychosis or bipolar disorder (via ICD-10 criteria), one study68 included those with schizophrenia spectrum diagnosis (via ICD-10 criteria), two studies54,69 included those with psychosis (via DSM-IV criteria) and two studies57,58 included those with major depressive disorder (via DSM-IV criteria and a score ≥ 17 on the Beck Depression Inventory II).
The included studies recruited a total of 8168 participants; 7643 were involved in general population studies, whereas 525 were recruited into the psychiatric studies (participant numbers were not reported in one study,52 in which the participant numbers at follow-up were used to calculate total number of participants). The studies of the former grouping tended to restrict the inclusion of patients, with 15 studies39–41,43,44,47,49–51,55,59,60,63,65,70 excluding patients with a psychiatric condition and other drug dependencies and four studies42,48,53,56 excluding only patients with other drug dependencies. One psychiatric study excluded participants who had other drug dependences. 66
Recruitment
In order to recruit participants, the studies treating the general population most frequently used voluntary recruitment methods, that is, participants responded to advertisements (16 studies40,42–44,47–51,53,55,61,62,67,70,75), with fewer studies employing a referral mechanism (four studies45,46,60,71) or a combination of voluntary recruitment and referrals (four studies39,41,59,65); recruitment methods could not be ascertained for two studies. 56,63 Conversely, the psychiatric studies all employed referral mechanisms (four studies52,54,66,68) or a combination of referral and voluntary recruitment methods (three studies57,58,69). Therefore, the ‘general population’ studies mostly involved self-selected participants who may have been more motivated to cease use than the average cannabis user.
Age
The majority of studies employed participant age study inclusion criteria, bar three. 56,61,62 Participants were included if they were aged 18–19 years or over (19 studies39–44,46–51,53,63,65–67,70,75), aged 16–17 years or over (nine studies45,55,57–60,68,69,71) or aged 15 years or over (two studies52,54). Twelve studies also included an upper age limit; this was in the twenties (six studies43,46,48,54,67,75), thirties to forties (two studies66,68) or sixties (three studies42,69,71), while one study used an age range of 17–19 years45 and one used a range of 19–20 years. 67 At baseline, the mean age of participants across studies was 29 years (all studies, range for mean age 18–40 years, median 32 years; general population studies, range 18–36 years, median 32 years; psychiatric studies, range 21–40 years, median 28 years).
Cannabis use or dependence at baseline
Thirty of the included studies specified criteria for the level of cannabis use at study inclusion. 39–61,63,65,66,68,69,70,75 These criteria varied by study, with eight studies44,47,53,56,65,68,69,71 utilising dichotomous criteria [patients meeting the DSM-IV, Diagnostic and Statistical Manual of Mental Disorders Three (Revised) (DSM-III-R) or ICD-10 criteria for cannabis dependence or cannabis abuse], 18 studies43,45,46,48–52,54,55,57–60,63,66,70,75 selecting an inclusion point on a continuous scale of cannabis use (ranging from 1 to 20 or more days of use of cannabis per month) and four studies39–42 using a combination of both. Therefore, we classified studies into those for which the inclusion criteria for cannabis use or dependence were deemed to be ‘low’ and those for which they were deemed to be ‘high’. ‘High use’ was defined as a study inclusion criterion or population baseline measurement in which ≥ 80% of participants met the DSM or International Classification of Diseases criteria for cannabis dependence or abuse, and/or an inclusion criterion specifying that all participants used cannabis on at least 50% of days over a specified time period. Thirteen studies treating the general population included participants with ‘high’ use39–42,44,47,49–51,53,56,59,61 and 10 with ‘low’ use,43,45,46,48,55,60,63,65,70,75 and baseline use could not be determined for three studies. 62,67,71 Of those treating the psychiatric population, three studies66,68,69 included only participants with high use, whereas four52,54,57,58 included low-use participants.
Other substance use
Participants’ use of other substances at baseline was seldom reported by the studies; studies that did report this did not do so in a consistent manner. Overall, 10 studies reported alcohol use at baseline39–41,44,51,54,55,59,60,63 and two also reported tobacco use;55,60 the remaining 23 studies did not report this baseline measurement. Of the studies reporting alcohol use, five reported the average proportion of participants’ drinking days over a specified period,40,41,55,63,66 two reported average drinks per day over a specified period,51,60 two reported the proportion of participants who were deemed to meet the DSM criteria for alcohol dependence54,59 and one reported participants’ ASI score. 39
Comparators
Of the 26 ‘general population’ studies, 11 tested two or more interventions (with no inactive control arm, although some included an active control such as education),40–42,44,47,49,56,62,65,70,75 10 tested a single intervention against an inactive control [wait list or assessment only (AO)]43,45,46,48,55,59–61,67,71 and five tested more than one active intervention against an inactive control. 39,50,51,53,63 The general population studies utilised wait list (10 studies50,51,53,55,59–61,63,71) or AO (five studies43,45,46,48,67) as inactive controls. Of the ‘psychiatric’ studies, four tested a single intervention against a TAU control52,66,68,69 and three tested two or more active interventions with no inactive control. 54,57,58 TAU consisted of antipsychotic medication and psychiatric condition monitoring, plus self-help material in one study and a psychosocial intervention in two studies.
Interventions
The included interventions varied considerably. Single interventions consisted of multiple and overlapping components. In the following summary, we have classed studies by their ‘main’ intervention, which we have defined as either CBT or MI or contingency management. If a study consists of multiple intervention arms or multicomponent interventions consisting of CBT or MI, we have classed the ‘main’ intervention as CBT. The majority of general population studies (15 studies39–42,44,47,49,50,53,55,59,60,63,65,70) evaluated CBT as their main intervention, or a variation thereof. Of the 15 studies, three studies55,59,60 compared CBT with a wait list control; eight40–42,44,47,49,65,70 compared CBT with MI, a variation of CBT or another intervention; and four39,50,53,63 compared CBT with both a wait list control arm and another arm consisting of MI, a variation of CBT or another intervention. Five of the 15 studies also assessed contingency management, alone and/or in combination with CBT. 40–42,44,47 Of the 15 studies, 12 assessed the use of therapist-delivered CBT, whereas three42,55,70 assessed the use of computer- or telephone-delivered treatment (one42 of which tested therapist-delivered CBT against computer-delivered). Duration of CBT treatment ranged considerably, from 4 weeks63 to 1850 weeks. The majority of interventions involved weekly (or near weekly) sessions, with the notable exceptions of Hoch et al. 59 (two sessions per week over 5 weeks), one treatment arm of Budney et al. 40 (four sessions over 14 weeks) and Babor et al. 39 (two arms: nine sessions over 12 weeks and two sessions over 5 weeks). Nine studies assessed the use of a motivational intervention but not CBT;43,45,46,48,51,62,67,71,75 two45,62 of these assessed computer- or telephone-delivered treatment. Two general population studies did not involve MI or CBT components; Tossman et al. 61 provided internet-based counselling, whereas Grenyer et al. 56 provided supportive–expressive dynamic psychotherapy.
The psychiatric population studies evaluated the use of CBT (seven studies). 52,54,57,58,66,68,69 Five studies utilised therapist-delivered interventions,52,54,66,68,69 the remainder (two studies)57,58 assessed the use of computer-delivered CBT compared with therapist-delivered CBT. Length of treatment varied: in four studies treatment lasted 10 weeks,52,54,57,58 in one study 12 weeks69 and in two studies 24 weeks. 66,68 All CBT sessions were delivered on a weekly basis, with the notable exception of Bonsack et al. 66 (four to six sessions over 24 weeks).
No studies were found that assessed the efficacy of mutual aid therapy.
Outcomes
All of the included RCTs measured the effect of the intervention(s) on participants’ cannabis use; however, the way in which this was measured varied greatly by study. For example, studies measured point abstinence rates, abstinence over a specified period, frequency of cannabis use per day over a specified period and number of cannabis-using days over a specified period. Thirteen studies39,40,44,50,51,53–56,59,60,63,70 measured participants’ severity of cannabis dependence (measured via self-report using various instruments, most frequently using the SDS or ASI). 11,34 Fifteen studies39–41,44–47,49–51,53,55,60,63,66 measured participants’ number of cannabis-related problems [measured using various instruments, most frequently the Marijuana Problems Scale (MPS)]. 35 Twenty-five studies measured participants’ use of the intervention or session attendance. 39–41,43,44,47–55,57–61,63,66,68,69,70,77
Risk of bias in included studies
Table 3 summarises the risk of bias for each of the included studies. Most studies used an appropriately generated randomisation sequence, with 21 studies being deemed ‘low risk’, 10 ‘unclear risk’ and two ‘high risk’. Allocation concealment followed a similar pattern. No studies blinded study participants to group allocation and we deemed this form of blinding to be impossible for the interventions under review. As many of the outcome measures were self-reported, outcomes were deemed to have been blinded if the outcome assessors were blinded to group allocation. This form of blinding was poorly reported; in 18 studies, blinding of outcome assessment was unclear or unreported. 40,44–46,49,50,52,55,56,58–63,66,75,81 Participant attrition was well reported but high, ranging from 6% to 79% (mean 30.2%, median 25.5%); 22 studies were rated as high risk for this attribute (with attrition of > 20% at the final follow-up time point). Regarding overall risk, 24 studies40,41,43,48,49,52–55,57–63,65,67–69,70,71,75,81 were deemed to be ‘high risk’, in nine studies39,44–47,50,51,56,66 the risk was unclear and no studies were deemed to be ‘low risk’. In the general population subgroup, 18 studies40,41,43,48,49,53,55,59–62,63,65,67,70,71,75,81 were deemed to be at high risk of bias, whereas in eight studies39,44–47,50,51,56 the risk was unclear. In the psychiatric population studies, six52,54,57,58,68,69 were deemed to be at high risk and in one study66 the risk was unclear. Twenty-one of the studies40,41,43,48,49,53–55,57–63,65,67–69,75,81 were deemed to be at high risk owing to incomplete outcome data (high level of attrition) and three studies52,70,71 were deemed to be at high risk owing to poor random sequence generation or allocation concealment.
| Author and year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data (% attrition)a | Selective reporting | Overall riskb |
|---|---|---|---|---|---|---|---|
| Babor 200439 and Litt 200572 | Low risk | Unclear risk | Not possible | High risk | Intermediate risk (17) | Low risk | Unclear risk |
| Baker 200652 | High risk | High risk | Not possible | Unclear risk | Intermediate risk (20) | Low risk | High risk |
| Bonsack 201166 | Low risk | Low risk | Not possible | Unclear risk | Intermediate risk (13) | Low risk | Unclear risk |
| Budney 201181 and ClinicalTrials.gov 201373 | Unclear risk | Unclear risk | Not possible | Unclear risk | High risk (39) | Unclear risk | High risk |
| Budney 200641 | Low risk | Unclear risk | Not possible | High risk | High risk (28) | Low risk | High risk |
| Budney 200040 and Moore 200374 | Low risk | Unclear risk | Not possible | Unclear risk | High risk (25) | Low risk | High risk |
| Copeland 200153 | Unclear risk | Low risk | Not possible | Low risk | High risk (26) | Low risk | High risk |
| de Dios 201243 | Unclear risk | Low risk | Not possible | Low risk | High risk (27) | Low risk | High risk |
| Edwards 200654 | Low risk | Low risk | Not possible | Low risk | High risk (30) | Low risk | High risk |
| Fernandes 201062 | Low risk | Unclear risk | Not possible | Unclear risk | High risk (70) | Low risk | High risk |
| Fischer 201275 and Fischer 201364 | Unclear risk | Unclear risk | Not possible | Unclear risk | High risk (46) | Low risk | High risk |
| Gates 201255 | Low risk | Unclear risk | Not possible | Unclear risk | High risk (31) | Low risk | High risk |
| Gmel 201367 | Low risk | Low risk | Not possible | Low risk | High risk (21) | Low risk | High risk |
| Grenyer 199756 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unreported | Unclear risk | Unclear risk |
| Hjorthoj 201368 and Hjorthoj 201280 | Low risk | Low risk | Not possible | Low risk | High risk (34) | Low risk | High risk |
| Hoch 201460 | Low risk | Low risk | Not possible | Unclear risk | High risk (79) | Low risk | High risk |
| Hoch 201259 and Hoch 200876 | Low risk | Low risk | Not possible | Unclear risk | High risk (27) | High risk | High risk |
| Humeniuk 201271 | Low risk | High risk | Not possible | High risk | Intermediate risk (14) | Low risk | High risk |
| Jungerman 200763 | Low risk | Low risk | Not possible | Unclear risk | High risk (38) | Low risk | High risk |
| Kadden 200744 and Litt 200877 | Low risk | Low risk | Not possible | Unclear risk | Intermediate risk (17) | Low risk | Unclear risk |
| Kay-Lambkin 201158 | Unclear risk | Unclear risk | Not possible | Unclear risk | High risk (41) | Low risk | High risk |
| Kay-Lambkin 200957 | Unclear risk | Low risk | Not possible | Low risk | High risk (24) | Low risk | High risk |
| Lee 201346 | Low risk | Low risk | Not possible | Unclear risk | Intermediate risk (17) | Low risk | Unclear risk |
| Lee 201045 | Low risk | Low risk | Not possible | Unclear risk | Intermediate risk (6) | Low risk | Unclear risk |
| Litt 201347 | Low risk | Low risk | Not possible | High risk | Intermediate risk (15) | Low risk | Unclear risk |
| Madigan 201369 | Low risk | Unclear risk | Not possible | Low risk | High risk (42) | Low risk | High risk |
| Rooke 201370 | High risk | High risk | Not possible | Low risk | High risk (46) | Low risk | High risk |
| Sobell 200965 | Low risk | Unclear risk | Not possible | Low risk | High risk (21) | Low risk | High risk |
| Stein 201148 | Unclear risk | Unclear risk | Not possible | Low risk | High risk (21) | Low risk | High risk |
| Stephens 200751 | Low risk | Low risk | Not possible | High risk | Intermediate risk (17) | Low risk | Unclear risk |
| Stephens 2000,50 Lozano 200678 and DeMarce 200579 | Unclear risk | Low risk | Not possible | Unclear risk | Intermediate risk (10) | Low risk | Unclear risk |
| Stephens 199449 | Unclear risk | Unclear risk | Not possible | Unclear risk | High risk (21) | Low risk | High risk |
| Tossmann 201161 | Low risk | Low risk | Not possible | Unclear risk | High risk (84) | Low risk | High risk |
Assessment of effectiveness
Overview of effectiveness section
Results are presented for each intervention/comparator category (e.g. CBT vs. wait list, CBT vs. brief MI, etc.). An overall summary of results is provided in Tables 4 and 5. This is followed by more detailed results for each intervention/comparator category (see Tables 6–23). Owing to the large number of studies and the variability in outcomes and data format, detailed numerical results are not presented here. Instead, this section provides an overview of the outcomes reported per study and how many showed a significant difference, both between intervention groups and in terms of changes from baseline, at different follow-up time points. Full extracted data per study are provided in Appendix 4.
| Comparison | Number of studies, number randomised (number of patients followed up), cannabis use categorisation high n, low n | Intervention (n sessions) | Computer (n sessions) | Individual or group, duration | Key findings |
|---|---|---|---|---|---|
| CBT vs. wait list | Six studies,39,50,53,59,60,63 n = 1265 (997), high 4, low 2 | CBT (4–14 sessions) | Wait list | Five individual/one group, 5–18 weeks | CBT (4–14) significantly better than wait list: post treatment (five of five studies with data39,50,59,60,63) and at 9 months (one of one study39). Significant change from baseline: CBT, post treatment (four of four studies39,50,59,60) and at 6–9 months (three of three studies39,59,60). Wait list, post treatment (two of two studies59,60) |
| CBT vs. brief MI | Four studies,39,40,50,53 n = 707 (581), high 4 | CBT (6–14 sessions) | MI/MET (1–4 sessions) | Three individual/one both, 6–18 weeks | CBT (6–14) vs. MI (one to four): mixed results. Of four studies, two studies40,50 showed CBT better on some outcomes while two studies39,53 showed few between-group differences (post treatment and at 9–16 months). Significant change from baseline: CBT and MI, post treatment (three studies39,40,50) and 9–16 months (two studies39,53) |
| SEDP vs. brief MI | One study,56 n = 40 (40), high 1 | SEDP (16 sessions) | MI (1 session) | NR, NR | SEDP (16 sessions) significantly better than MI (1 session): post treatment (one study,56 limited outcomes) |
| CBT vs. other | Four studies,44,49,63,65 n = 462 (365), high 2, low 2 | CBT (4–10 sessions) | Various | Two individual/one group/one both, 4–12 weeks | CBT vs. social support group or case management: no significant difference, post treatment or 14–15 months (two of two studies49,77). Significant change from baseline, all groups, post treatment and 14–15 months (two of two studies44,49). Group vs. individual: one small study65 favours individual vs. group CBT-4 (limited data) |
| Computer-/tele-CBT vs. other | Three studies,55,61,70 n = 1682 (481), high 1,61 low 2 | Computer-/tele-CBT (4–6 sessions) | Wait list or education | Three individual, 3–7 weeks | Tele-CBT significantly better than wait list: most outcomes, post treatment and at 3 months (one of one study55). Internet-delivered CBT/counselling significantly better than wait list/education: most outcomes at 3 months (two of two studies61,70). Significant change from baseline: all groups, post treatment and at 3 months (two of two studies55,70) |
| Brief MI vs. wait list or AO | 10 studies,39,43,45,46,48,50,51,53,67,71 n = 2437 (2288), high 4, low 6 | MET/MI (1 or 2 sessions) | Wait list or AO | Nine individual/one group, 1–5 weeks | Brief MI vs. wait list/AO: some significant differences. Brief MI significantly better on some outcomes but not all, post treatment (five of five studies39,43,48,50,51) and at 3–9 months (seven of seven studies43,45,46,48,53,67,71). Significant change from baseline: post treatment and at 3–6 months, brief MI (two of two studies39,48), wait list/AO (two of two studies48,71) |
| Brief MI vs. other | Three studies,51,62,75 n = 2002 (754), high 1, low 2 | MI or tele-MI (1 session) | Cannabis or health education | Three individual, 1 week | Brief MI vs. other: mixed results, limited data. Brief MI better than education control on some but not all outcomes, post treatment (one of one study51) and at 3–12 months (two51,62 of three studies51,62,70). Significant change from baseline: brief MI and education control at 3 months (one of one study75) |
| Contingency management vs. other | Five studies,40–42,44,47 n = 680 (581), high 5 | Voucher (abstinence), CBT + voucher | CBT (9–14 sessions), MET (2–4 sessions), other | Five individual, 8–14 weeks | Post treatment: CBT + voucher or voucher alone better than CBT or MET (three of three studies40,42,44). Maintained for CBT + voucher, not voucher alone: CBT + voucher better than CBT or voucher at 14–15 months (two of two studies41,44). Significant change from baseline: all groups post treatment and at 14–15 months (three of three studies41,44,47) |
| Comparison | Number of studies, number randomised (number of patients followed up), cannabis use categorisation high n, low n | Intervention (n sessions) | Computer (n sessions) | Individual or group, duration | Key findings |
|---|---|---|---|---|---|
| CBT + TAU vs. TAU | Four studies,52,66,68,69 n = 326 (254), high 3, low 1 | CBT (6–24 sessions) + TAU | TAU | Three individual/one group, 10–26 weeks | CBT + TAU vs. TAU: few significant differences post treatment. No significant difference at 10–12 months (four of four small studies,52,66,68,69 limited data). Little significant change from baseline: no change (two studies52,69), change on one outcome in both groups (one study66), post treatment and at 12 months |
| CBT vs. other | Three studies,54,57,58 n = 199 (197), low 3 | CBT or computer-delivered CBT (10 sessions) | Education (10 sessions), CBT (10 sessions), PCT (10 sessions), brief MI (1 session) | Three individual, 10–12 weeks | CBT vs. psychoeducation (10 sessions): no significant difference post treatment or at 9 months; both groups improved from baseline (one study,54 limited data). Computer-based CBT vs. CBT or PCT (10 sessions): no significant difference post treatment; (one study,58 limited data). Computer-based CBT or CBT (10 sessions) better than brief MI (1 session) at 12 months; all improved from baseline (one study,57 limited data) |
Outcomes reported
Outcomes reported in most studies could be classified into four main groups: cannabis use, severity of dependence, cannabis-related problems and level of attendance or compliance with the intervention(s). Cannabis use outcomes included point abstinence rates, abstinence over a specified period, number of days using cannabis or number of days abstinent (over a specified period), amount of cannabis use per day and number of periods of use per day (e.g. of four daily periods). For session attendance, seven studies41,44,47,49,54,57,58 reported significance levels between study groups; this was non-significant in all cases.
Subgroup analyses: effect of intervention and population characteristics
The effect of intervention and population characteristics on results was also examined to assess whether or not any patterns could be observed in terms of which studies showed positive results. Findings are described within each intervention/comparator category and an overview provided in Subgroup analyses: effect of intervention and population characteristic.
Studies in general population of cannabis users
Cognitive–behavioural therapy compared with wait list control
Description of studies
Six studies39,50,53,59,60,63 (n = 1265 randomised, 997 followed up) compared CBT (4–14 sessions) with wait list control (Tables 6 and 7). Session attendance ranged from 60% to 72% (not reported in three studies59,60,63). Five studies39,53,59,60,63 provided individual CBT sessions and one50 provided group sessions. CBT interventions also incorporated other strategies including case management (one study),39 psychosocial problem-solving (two studies)59,60 and a social support group (one study). 50 Participants were classified as having high baseline use/dependence in four studies39,50,53,59 and low use/dependence in two studies. 60,63 Two studies were conducted in the USA,39,50 two in Germany,59,60 one in Australia53 and one in Brazil. 63
| Comparison | Number of studies, number randomised (number followed up), categorisation high n, low n | Intervention (n sessions) | Computer (n sessions) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|---|
| CBT vs. wait list | Six studies39,50,53,59,60,63 (see Table 7), n = 1265 (997), high 4,39,50,53,59 low 260,63 | CBT (4–14 sessions), some CBT included: CaseM (1), PPS (2), social support (1) | Wait list | Five individual, one group, 5–18 weeks | Significant difference: five studies:39,50,59,60,63 CBT significantly better than wait list on most outcomes: | Significant change: four studies:39,50,59,60 significant improvement baseline to post treatment on most outcomes, CBT group (two studies39,50) or both groups (two studies59,60) | 6–9 months | Significant difference: one study:53 CBT-6 significantly better than wait list on most outcomes at 9 months:
|
Significant change: three studies:39,59,60 significant improvements on most outcomes in CBT group from baseline to 6 months (two studies59,60) or to 9 months (one study39) |
| Study, country, cannabis use, recruitment, mean age (range) | Intervention (number of sessions) (mean number of sessions attended), number randomised (followed up) | Computer, number randomised (followed up) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|
| Babor 200439 and Litt 200572 (MTP), USA, high use (DSM-IV 100%), voluntary + referral, 36 years (18–62 years) | CBT/MET/CaseM (9) (6.5), n = 156 (133) | Wait list, n = 148 (137) | Individual, 12 weeks | Significant difference:
|
Significant change:
|
9 months | Significant change:
|
|
| Copeland 2001,53 Australia, high use (DSM-IV 96%), voluntary, 32 years (≥ 18 years) | CBT (6) (4.2), n = 78 (58) | Wait list, n = 69 (51) | Individual, 6 weeks | 9 months | Significant difference:
|
|||
No significant difference:
|
||||||||
| Hoch 201460 (CANDIS-II), Germany, low use (ICD-10 56%), referral, 27 years (16–63 years) | CBT/MET/PPS (10) (NR), n = 255 (166) | Wait list, n = 130 (106) | Individual, 12 weeks | Significant difference:
(All p < 0.001) (d = –0.7, 95% CI –2.9 to 2.1) |
Significant change:
|
6 months | Significant change:
|
|
| (All groups except amount/week, CBT only) | (Data for CBT only) | |||||||
| Hoch 201259 and Hoch 200876 (CANDIS), Germany, high use (DSM-IV 89%), voluntary + referral, 24 years (16–44 years) | CBT/MET/PPS (10) (NR), n = 90 (79) | Wait list, n = 32 (31) | Individual, 5–8 weeks | Significant difference:
|
Significant change:
|
6 months | Significant change:
|
|
| Jungerman 2007,63 Brazil, low use (≥ 13 day/month), NR, 32 years (18–58 years) | CBT/MI/RP (4) (NR), n = 52 (27) | Wait list, n = 52 (35) | Individual, 12 weeks | Significant difference:
|
||||
No significant difference:
|
||||||||
| Stephens 2000,50 Lozano 200678 and DeMarce 2005,79 USA, high use (DSM-III-R 98%), voluntary, 34 years (≥ 18 years) | CBT/RP/social support group (14) (8.4), n = 117 (95) | Wait list, n = 86 (79) | Group, 18 weeks | Significant difference:
|
Significant change:
|
Main results
Five studies39,50,59,60,63 reported post-treatment (5–18 weeks) outcomes. All five reported significantly better results for CBT (4–14 sessions) than for wait list on most outcomes, including cannabis use (significant in all five studies), severity of dependence (significant in four39,50,59,60 out of five studies) and cannabis problems (significant in three39,50,60 out of four studies39,50,59,60 reporting this). In addition, four studies39,50,59,60 reported change from baseline to post treatment; all four reported significant improvements from baseline on most outcomes, for the CBT groups (two studies39,59) or for both the CBT and wait list groups (two studies50,60). Effect sizes at 12 weeks (based on data from two studies39,60) ranged from 0.4 to 1.1 for cannabis use outcomes and from 0.9 to 1.6 for severity of dependence.
Only one study53 reported between-group data at a later follow-up point than post treatment (because, in most studies with a wait list comparison, the wait list group began treatment when other groups completed theirs and so could not be followed for longer). This study reported significantly better results for CBT (6 sessions) than wait list on most outcomes at 9 months post baseline (7.5 months after end of treatment), including cannabis use, severity of dependence and cannabis problems. Three studies39,59,60 reported significant improvements from baseline to 6 months (two studies59,60) or 9 months (one study39), for the CBT group (wait list groups were not followed for this long).
Effects of intervention characteristics
All six studies reported mainly positive findings so there were no clear differences in results according to population or intervention differences. 39,50,53,59,60,63 All durations of CBT (4–14 sessions) appeared effective; there were slightly fewer significant effects in the study of four-session CBT,63 but this may have been owing to the smaller number of participants in this study. The one study of group CBT50 (14 sessions) had similar positive outcomes to the individual CBT studies.
Effects of population characteristics
In terms of baseline cannabis use/dependence, three studies classed as high use39,50,59 all showed significant effects post treatment, while of the two studies classed as low use, one60 showed significant effects on all outcomes and the other63 on some but not all outcomes. This may indicate slightly less effectiveness in participants with lower baseline use, or may be simply a result of the smaller number of participants in the latter study. 63 Two studies50,53 used voluntary recruitment, one60 used referrals, and two39,59 used a combination (for one63 this was not reported); all studies showed significant effects regardless of recruitment method. Mean age ranged from 24 years to 36 years and there were no clear differences in effects according to age.
Cognitive–behavioural therapy or psychotherapy compared with brief motivational interviewing
Description of studies
Four studies39,40,50,53 (n = 707 randomised, 581 followed up) compared CBT (6–14 sessions) with brief MI/MET (1–4 sessions) (Tables 8 and 9). Three studies39,40,53 provided individual CBT sessions, whereas one50 compared group CBT with individual MET. CBT interventions also included case management (one study)39 and a social support group (one study). 50 One further study, reported only in abstract form, compared supportive–expressive dynamic psychotherapy (16 sessions, not reported whether individual or group) with brief MI (1 session). 56 Attendance within the CBT or psychotherapy arm of the studies ranged from 60% to 72% (not reported in two studies40,56). Owing to the brief nature of the MI arms, only one study39 reported attendance for this intervention (mean 1.6 sessions attended from a total of 2). Participants were classified as having high baseline use in all five studies. Three studies were conducted in the USA39,40,50 and two in Australia. 53,56
| Comparison | Number of studies, number randomised (number followed up), categorisation high n, low n | Intervention (number of sessions) | Computer (number of sessions) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|---|
| CBT vs. brief MI | Four studies39,40,50,53 (see Table 9), n = 707 (581), high 4 | CBT (6–14 sessions) | MI/MET (1–4 sessions) | Three individual, one group vs. individual, 6–18 weeks | Mixed results: one study:39 CBT-9 significantly better than MET-2 on most outcomes (one study).39 Two studies:40,50 no significant difference on most outcomes (CBT-14 vs. MET-2/4); one study40 had few participants | Significant change: three studies:39,40,50 significant improvement baseline to post treatment on most outcomes, CBT and MI groups | 9–16 months | Mixed results: two studies:39,53 CBT-6/9 significantly better than MET-1/2 on some outcomes but not others at 9 and 15 months. One study:50 no significant difference on most outcomes for CBT-14 vs. MET-2 at 16 months | Significant change: two studies:39,50 significant improvement from baseline on most outcomes in CBT and MI groups at 9–16 months |
| Supportive–expressive dynamic psychotherapy vs. brief MI | One study56 n = 40 (40), high | Supportive–expressive dynamic psychotherapy (16 sessions) | MI (1 session) | NR, NR | Significant difference (limited data): one study:56 psychotherapy-16 better than brief MI-1, limited outcomes |
| Study, country, cannabis use, recruitment, mean age (range) | Intervention (number of sessions) (mean number of sessions attended), number randomised (followed up) | Computer (number of sessions), number randomised (followed up) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|
| Babor 200439 and Litt 200572 (MTP), USA, high use (DSM-IV 100%), voluntary + referral, 36 years (18–62 years) | CBT/MET/CaseM (9) (6.5), n = 156 (133) | MET (2) (1.6), n = 146 (128) | Individual, 12 weeks | Significant difference:
|
Significant change:
|
9 months, 15 months | Significant difference:
|
Significant change:
|
No significant difference:
|
||||||||
No significant difference:
|
||||||||
| Mechanism: CBT-9 and MET-2 increased coping skills relative to wait list (no difference between CBT and MET). Increase in coping skills reduced cannabis use |
Significant difference:
|
|||||||
No significant difference:
|
||||||||
| Budney 2000,40 USA, high use (DSM-III-R 100%), voluntary, 32 years (≥ 18 years) | CBT/MET (14) (NR), n = 20 (15) | MET (4) (NR), n = 20 (16) | Individual, 14 weeks | No significant difference:
|
Significant change:
|
|||
| Copeland 2001,53 Australia, high use (DSM-IV 96%), voluntary, 32 years (≥ 18 years) | CBT (6) (4.2), n = 78 (58) | MI (1), n = 82 (61) | Individual, 6 weeks | 9 months | Significant difference:
|
|||
No significant difference:
|
||||||||
| Grenyer 199756 (abstract), Australia, high use (DSM-IV 100%), NR, 34 years (NR) | SEDP (16) (NR), n = 20 (20) | MI (1), n = 20 (20) | NR, NR, 16 sessions | Significant difference:
|
||||
| Stephens 2000,50 Lozano 200678 and DeMarce 2005,79 USA high use (DSM-III-R 98%), voluntary, 34 years (≥ 18 years) | CBT/RP/social support (14) (8.4) (group), n = 117 (95) | MI (2) (NR) (individual), n = 88 (75) | Group vs. individual, 18 weeks | No significant difference:
|
Significant change:
|
16 months | No significant difference:
|
Significant change:
|
Main results
Overall, the comparison of longer durations of CBT with brief MI/MET showed mixed results; however, both interventions provided improvements from baseline. Three CBT studies reported between-group data post treatment (at 12–18 weeks). 39,40,50 Of these, one study39 reported that nine-session CBT was significantly better than two-session MET on most outcomes (including cannabis use, dependence and problems). Conversely, two studies40,50 reported no significant difference on any outcomes between CBT (14 sessions) and MET (2 or 4 sessions), although one study40 involved few participants, which may impact on significance levels. Three CBT studies39,40,50 reported change from baseline to post treatment; all three reported significant improvements on most outcomes for both the CBT and MI groups. One study investigating possible mechanisms for changes in cannabis use reported that participants in both the 9-session CBT and 2-session MET groups increased their coping skills relative to wait list with no significant difference between CBT and MET, and that this increase in coping skills was associated with reduction in cannabis use. 39 Effect sizes at 12 weeks (based on data from one study39) ranged from 0.4 to 0.5 for both cannabis use and severity of dependence outcomes.
One further study56 reported that 16-session dynamic psychotherapy was significantly better than one-session MI; however, limited outcomes were reported (i.e. percentage abstinent, severity of symptoms).
Results for later follow-ups were again mixed. Three studies of CBT reported between-group data at later follow-ups. Two of these studies39,53 reported that CBT (6 or 9 sessions) was significantly better than MET (1 or 2 sessions) on some outcomes (some cannabis use, dependence) but not other outcomes (some cannabis use, cannabis problems) at 9 and 15 months’ follow-up. The third study50 reported no significant difference on most outcomes for CBT plus social support (14 group sessions) compared with MET (2 individual sessions) at 16 months’ follow-up. The study of dynamic psychotherapy did not report later follow-up data. Two studies39,50 reported change from baseline at follow-up (9–16 months), both finding significant improvements on most outcomes in both the CBT and brief MI groups. Effect sizes at 9 months (based on data from one study39) ranged from 0.3 to 0.5 for both cannabis use and severity of dependence outcomes.
Effects of intervention characteristics
In terms of number of sessions, this section compares four studies of CBT (6–14 sessions) with briefer MI/MET treatments (1–4 sessions). As described above, some studies showed better results for CBT than MI (one39 post treatment, two at later follow-ups39,53), whereas others showed no significant differences (two40,50 post treatment, one at later follow-ups50). When CBT gave better outcomes, this may be owing to the nature of the CBT treatment, or the fact that more sessions were provided, or a combination of the two. In terms of group compared with individual treatments, one study50 showed little difference between group CBT plus social support and individual MI (although both groups improved from baseline), whereas studies of individual CBT compared with MI showed mixed results, as described above. 39,40,53
Effects of population characteristics
It was not possible to assess the effects of baseline cannabis use/dependence, as all studies were classified as high use. In terms of recruitment method, three CBT studies used voluntary recruitment40,50,53 and showed mixed results, whereas the one study39 using a combination of voluntary recruitment and referrals showed mostly significant effects; however, no studies used referrals only, so the significance of this is not clear. It was not possible to assess effects of participant age, as all studies in this grouping had a similar mean age (32–36 years).
Cognitive–behavioural therapy compared with other interventions (or different cognitive–behavioural therapy format or duration)
Description of studies
Four studies44,49,63,65 (n = 462 randomised, 365 followed up) compared CBT (4–10 sessions) with another intervention (social support group,49 case management sessions44) or compared individual with group CBT65 or CBT over different durations (Tables 10 and 11). 63 Two studies44,49 reported overall session attendance (of both interventions), ranging from 58% to 76%, with both studies reporting no significant differences in attendance between the two interventions (session attendance not reported in two studies63,65). Participants were classified as having high baseline use in two studies44,49 and low use in two studies. 63,65 Two studies were conducted in the USA,44,49 one in Canada65 and one in Brazil. 63
| Comparison | Number of studies, number randomised (followed up), categorisation high n, low n | Intervention (number of sessions) | Computer (number of sessions) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|---|
| CBT vs. other intervention (or different format or duration) | Four studies,44,49,63,65 (see Table 11), n = 462 (365), high 2,44,49 low 263,65 | CBT (4–10 sessions) | Social support group, CaseM, group vs. individual CBT, CBT over different durations | Two individual, one group, one group vs. individual, 4–12 weeks | Mixed results: one study:49 no significant difference between group CBT-10 and social support group-10. One study:44 no significant difference between CBT-9 and nine CaseM sessions. One study:65 favours individual vs. group CBT-4 (one outcome, small study). One study:63 CBT-4 over 12 weeks vs. 4 weeks: favours 12 weeks on some outcomes, not others | Significant change: three studies:44,49,65 significant improvement baseline to post treatment on most outcomes in both groups | 12–15 months | No significant difference: one study:49 no significant difference between group CBT-10 and social support group-10 at 15 months. One study:44 no significant difference between CBT-9 and nine CaseM sessions at 14 months. One study:65 no difference between individual vs. group CBT-4 at 12 months (one outcome, small study) | Significant change: three studies:44,49,65 significant improvement from baseline on most outcomes in both groups at 14–15 months |
| Study, country, cannabis use, recruitment, mean age (range) | Intervention (number of sessions) (mean number of sessions attended), number randomised (followed up) | Computer (number of sessions) (mean number of sessions attended) number randomised (followed up) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|
| Jungerman 2007,63 Brazil, low use (≥ 13 day/month), NR, 32 years (18–58 years) | CBT/MI/RP (4) (NR) (over 12 weeks), n = 52 (35) | CBT/MI/RP (4) (NR) (over 4 weeks), n = 56 (37) | Individual, 4–12 week | Significant difference (favours 12 weeks):
|
||||
No significant difference:
|
||||||||
| Kadden 200744 and Litt 2008,77 USA, high use (DSM-IV 100%), voluntary, 33 years (≥ 18 years) | CBT/MET (9) (5.2a), n = 61 (55) | CaseM (9) (5.2a), n = 62 (54) | Individual, 9 weeks | No significant difference:
|
Significant change:
|
14 months | No significant difference:
|
Significant change:
|
| Sobell 2009,65 Canada, low use (not severe) voluntary + referral, 32 years (≥ 18 years) | CBT/MI (4) (NR) (individual), n = NR (8) | CBT/MI (4) (NR) (group), n = NR (9) | Individual vs. group, NR, four sessions | No significant difference:
|
Significant change:
|
12 months | No significant difference:
|
Significant change:
|
| Stephens 1994,49 USA, high use (≥ 17 day/month), voluntary, 32 years (18–65 years) | CBT/RP (10) (7.6b), n = 106 (80) | Social support group (10) (7.6b), n = 106 (87) | Group, 12 weeks | No significant difference:
|
Significant change:
|
15 months | No significant difference:
|
Significant change:
|
Main results
One study49 reported no significant difference between 10 sessions of group CBT and 10 sessions of group social support, either post treatment or at 15 months’ follow-up. A further study44 reported no significant difference between 9 sessions of CBT and 9 sessions of case management (help with problems of daily living possibly related to cannabis use), either post treatment or at 14 months’ follow-up. However, both studies reported significant improvements from baseline in both groups, which were maintained after 14–15 months. The other two studies63,65 compared CBT format or duration and are discussed below.
Effects of intervention characteristics
One study65 compared four sessions of individual with group CBT; however, only 17 cannabis users were analysed and only one relevant outcome reported (days abstinent). Both groups improved from baseline and this was maintained at 12 months. Results non-significantly favoured individual CBT post treatment but this effect was not maintained at 12 months. Another study63 compared four sessions of CBT over either a 12- or a 4-week period. Post-treatment results significantly favoured 12-week treatment on some outcomes (i.e. dependence, cannabis problems) but not cannabis use outcomes.
Effects of population characteristics
Studies in this category were too heterogeneous in terms of interventions/comparators to allow meaningful assessment of the effects of population characteristics.
Telephone- or internet-based cognitive–behavioural therapy or counselling compared with wait list or other interventions
Description of studies
Three studies55,61,70 (n = 1682 randomised, 481 followed up) compared telephone- or internet-based interventions with wait list or education controls (Tables 12 and 13). Interventions included telephone-delivered CBT,55 internet-delivered CBT70 and internet-delivered counselling. 61 Participants were classified as having high baseline use in one study61 and low use in two studies. 55,70 Two studies reported session attendance for the CBT arm of the study, reporting mean attendances of 83%55 and 58%. 70 Two studies were conducted in Australia55,70 and one in Germany. 61
| Comparison | Number of studies, number randomised (followed up), categorisation high n, low n | Intervention (number of sessions) | Computer | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|---|
| Computer-/tele-CBT vs. wait list or other | Three studies55,61,70 (see Table 13), n = 1682 (481), high 1,61 low 255,70 | Tele-CBT (4 sessions), internet-delivered CBT (6 sessions) or internet counselling (50 days) | Wait list or written information | Three individual, 3–7 weeks | Significant difference (mostly): one study:55 tele-CBT-4 better than wait list on most outcomes. One study:70 internet-delivered CBT-6 better than written information on some outcomes, not others | Significant change: two studies:55,70 significant improvement baseline to post treatment on most outcomes in both groups | 3 months | Significant difference: one study:55 tele-CBT-4 better than wait list on most outcomes at 3 months. One study:70 internet-delivered CBT-6 better than written material, most outcomes, 3 months. One study:61 internet counselling better than wait list on most outcomes at 3 months | Significant change: two studies:55,70 significant improvement from baseline on most outcomes in both groups at 3 months |
| Study, country, cannabis use, recruitment, mean age (range) | Intervention (number of sessions) (mean number of sessions attended), number randomised (followed up) | Computer, number randomised (followed up) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|
| Gates 2012,55 Australia, low use (98% SDS ≥ 3), voluntary, 36 years (≥ 16 years) | Tele-CBT/MI (4) (3.3), n = 79 (54) | Wait list, n = 81 (72) | Individual, 3 weeks | Significant difference:
|
Significant change:
|
3 months | Significant difference:
|
Significant change:
|
No significant difference (borderline):
|
||||||||
No significant difference (borderline):
|
||||||||
No significant difference:
|
||||||||
| Rooke 2013,70 Australia, UK, USA, other, low use (≥ 1 day/month), voluntary, 31 years (≥ 18 years) | Internet-based CBT/MI (6) (3.5), n = 119 (76) | Written cannabis information, n = 111 (73) | Individual, 6 weeks | Significant difference:
|
Significant change:
|
3 months | Significant difference:
|
Significant change:
|
No significant difference:
|
No significant difference (borderline):
|
|||||||
| Tossmann 2011,61 Germany, high use (DSM-IV 92%), voluntary, 25 years (NR) | Internet-based counselling (50 days), n = 863 (100) | Wait list, n = 429 (106) | Individual, 7 weeks | 3 months | Significant difference:
|
Main results
One study55 reported significantly better results for four sessions of telephone-delivered CBT than wait list control on most outcomes post treatment (i.e. dependence, cannabis problems, some cannabis use outcomes), with some effects maintained at 3 months (i.e. dependence and problems, not cannabis use). Both the telephone-delivered CBT and wait list groups showed improvements from baseline post treatment and at 3 months. Another study70 compared six sessions of internet-based CBT with written cannabis information. Results post treatment significantly favoured internet-delivered CBT on some outcomes (i.e. some cannabis use) but not others (i.e. abstinence, dependence), while all outcomes (i.e. cannabis use, dependence) were significant or borderline significant in favour of internet-delivered CBT at 3 months; both the internet-delivered CBT and control groups showed improvements from baseline post treatment and at 3 months. Effect sizes of 0.3 were observed for cannabis use outcomes post treatment and at 3 months. 70 A further study61 reported better outcomes for 50-day internet-based counselling than for wait list control at 3-month follow-up, on the limited outcomes reported (i.e. cannabis use, self-efficacy).
Effects of intervention characteristics
The three studies were too heterogeneous in their interventions and comparators to allow meaningful assessment of the effects of other intervention characteristics.
Effects of population characteristics
In terms of baseline cannabis use/dependence, the study classed as high use61 showed slightly more positive effects than the two studies classed as low use,55,70 but this comparison is based on limited data. All three studies used voluntary recruitment and all showed some positive results. Mean age ranged from 25 years to 36 years; however, the effect of age could not be meaningfully assessed owing to the small number of studies in this category.
Brief motivational interviewing compared with wait list or assessment only
Description of studies
Ten studies39,43,45,46,48,50,51,53,67,71 (n = 2437 randomised, 2288 followed up) compared a brief intervention (1 or 2 sessions of MET, MI or personalised feedback) with wait list or AO (Tables 14 and 15). One study assessed a internet-based intervention (personalised feedback). 45 One study provided a group MI session67 and the other nine provided individual sessions. Eight studies39,43,46,48,50,51,53,71 reported session attendance, ranging from 80% to 100%. Those interventions involving 1 session did not have a markedly increased attendance compared with those involving more than 1 session [mean attendance across studies: 1-session interventions (four studies) – 91%; 2 or more session interventions (four studies) – 88%]. Participants were classified as having high baseline use in four studies39,50,51,53 and low use in six studies. 43,45,46,48,67,71 Seven studies were conducted in the USA,39,43,45,46,48,50,51 one in Australia,53 one in Switzerland67 and one across four countries. 71
| Comparison | Number of studies, number randomised (followed up), categorisation high n, low n | Intervention (number of sessions) | Computer | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|---|
| Brief MI vs. wait list or AO | 10 studies,39,43,45,46,48,50,51,53,67,71 (see Table 15), n = 2437 (2288), high 4,39,50,51,53 low 643,45,46,48,67,71 | MET, MI or personalised feedback (1 or 2 sessions, one internet-based study) | Wait list or AO | Nine individual, one group, 1 session or 2–5 weeks | Some significant difference | Significant change: | 3–9 months | Some significant difference: | Significant change (most): |
Three studies (low use):43,45,46 better on some outcomes, not others
|
Three studies: significant improvement from baseline at 3–6 months on most outcomes in both groups (two studies)48,71 or MI group (one study)39 One study:45 no significant change after internet-based PF at 3–6 months |
| Study, country, cannabis use, recruitment, mean age (range), category | Intervention (number of sessions) (mean number of sessions attended), number randomised (followed up) | Computer, number randomised (followed up) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|
| Babor 200439 and Litt 200572 (MTP), USA, high use (DSM-IV 100%). voluntary + referral, 36 years (18–62 years) | MET (2) (1.6), n = 146 (128) | Wait list, n = 148 (137) | Individual, 5 weeks | Significant difference:
|
Significant change:
|
9 months | Significant change:
|
|
No significant difference:
|
||||||||
| Copeland 2001,53 Australia, high use (DSM-IV 96%), voluntary, 32 years (≥ 18 years) | MI (1) (88% attended), n = 82 (61) | Wait list, n = 69 (51) | Individual, 1 session | 9 months | Significant difference:
|
|||
No significant difference:
|
||||||||
| de Dios 2012,43 USA, low use (≥ 3 day/month), voluntary, 23 years (18–29 years), female only | MI/meditation (2) (2), n = 22 (17) | AO, n = 12 (10) | Individual, 2 week | Significant difference:
|
3 months | Significant difference:
|
||
No significant difference:
|
No significant difference:
|
|||||||
| Gmel 2013,67 Switzerland, low use (any), voluntary, 20 years (19–20 years), men only | Brief MI (1); 50% telephone, booster session at 3 months, n = 174 (NR) | AO, n = 204 (NR) | Group, 1 session | 6 months | No significant difference (MI vs. AO):
|
|||
No significant difference (MI +/– booster session):
|
||||||||
| Humeniuk 2012,71 Australia, USA, Brazil, India, low use (high use excluded), referral, 31 years (16–62 years) | Brief MI (1) (100% attendance), n = 212 (NR) | Wait list, n = 183 (NR) | Individual, 1 session | 3 months | Significant difference:
|
Significant change:
|
||
| Lee 2013,46 USA, low use (≥ 5 day/month) referral, 20 years (18–25 years), students | Brief MI (1) (55% in person, 30% written, 15% none), n = 106 (87) | AO, n = 106 (94) | Individual, 1 session | 3 months, 6 months | Significant difference:
|
|||
| No significant difference: | ||||||||
| No significant difference: | ||||||||
| Lee 2010,45 USA, low use (any), referral, 18 years (17–19 years), students | Internet-based personalised feedback (1) (NR), n = 171 (162) | AO, n = 170 (162) | Individual, 1 session | 3 months, 6 months | No significant difference:
|
No significant change:
|
||
Significant difference (subgroup):
|
||||||||
No significant difference:
|
||||||||
| Stein 2011,48 USA, low use (DSM-IV 40%), voluntary, 21 years (18–24 years), female only | MI (2) (1.7), n = 163 (NR) | AO, n = 169 (NR) | Individual, 4 weeks | Significant difference (subgroup):
|
Significant change:
|
3 months, 6 months | Significant difference:
|
Significant change:
|
No significant difference:
|
No significant difference:
|
Significant change:
|
||||||
| Stephens 2007,51 USA, high use (≥ 15 day/month), voluntary, 32 years (18–57 years) | MI/personalised feedback (1) (89% attendance), n = 62 (58) | Wait list, n = 64 (62) | Individual, 1 session, follow-up at 7 weeks | Significant difference:
|
||||
No significant difference:
|
||||||||
| Stephens 2000,50 Lozano 200678 and DeMarce 2005,79 USA, high use (DSM-III-R 98%), voluntary, 34 years (≥ 18 years) | MI (2) (86% attendance both), n = 88 (75) | Wait list, n = 86 (79) | Individual, 4 weeks | Significant difference:
|
Significant change:
|
Main results
Five studies reported between-group data post treatment and the results showed a mixed picture with some significant effects. 39,43,48,50,51 One study50 (with high baseline use) reported significantly better results for two-session MET than wait list on all outcomes (cannabis use, dependence, problems), whereas four studies39,43,48,51 (two high,39,51 two low use43,48) reported that MI/MET (one or two sessions) gave significantly better results than wait list or AO on some outcomes (i.e. most cannabis use outcomes, dependence) but not others (i.e. some cannabis use outcomes, problems). Three studies reported change from baseline to post treatment, all of which reported significant improvements on most outcomes in both groups (two studies)48,50 or in the MI group (one study). 39
Two studies reported effect sizes at post treatment and one at a later follow-up point. 39,46 Effect sizes (Cohen’s d) at post treatment ranged from 0.29 to 0.60 for cannabis use outcomes and were 0.33 for dependence symptoms. 39 Another study reported effect sizes as RRs, where the effect size for cannabis use outcomes ranged from 0.76 to 0.99 at post treatment (3 months) and was 0.90 for cannabis problems, whereas at follow-up (6 months) the RR ranged from 1.03 to 1.11 and was 1.15 for cannabis problems. 46
At later follow-ups, seven studies reported mixed between-group results, again with some significant effects. 43,45,46,48,53,67,71 At 3 months, two studies48,71 (both low baseline use) reported significantly better results for MET/MI on the single outcome reported (cannabis use), whereas three studies43,45,46 (all low use) showed better results for MET/MI on some outcomes (some cannabis use) but not others (some cannabis use, problems). At 6 months, four studies45,46,48,67 (all low use) reported no significant differences between MET/MI and wait list/AO, while at 9 months one study53 (high use) reported better results for MET/MI on some outcomes (some cannabis use, dependence, problems). Three studies reported significant improvements from baseline to 3–6 months on most outcomes in both groups (two studies)48,71 or in the MI group (one study),39 whereas one study45 reported no significant change following internet-based personalised feedback at 3–6 months.
Effects of intervention characteristics
There was no obvious difference in results between studies of one-session or two-session MI/MET, with most studies showing mixed results both post treatment (a single 1-session study51 compared with four 2-session studies39,43,48,50) and at later follow-ups (five 1-session studies45,46,53,67,71 compared with two 2-session studies43,48). The one study67 using a group intervention (1-session MI) showed no significant effect at 6 months on the single outcome reported (cannabis days of use); it is unclear whether this reflects the group delivery or other factors (only a single session was provided and results were not measured earlier than 6 months).
Effects of population characteristics
In terms of baseline cannabis use/dependence, at post treatment one study50 with high baseline use reported better results for MET on all outcomes, whereas four studies39,43,48,51 (two high, two low use) reported better results for MI/MET on some outcomes but not others. At later follow-ups, results were mixed, both among the one study53 with high use and the six43,45,46,48,67,71 with low use. Therefore (based on the post-treatment data) studies with high baseline use/dependence may have been slightly more likely to show significant effects, but there is little strong evidence for this.
There was no clear difference in results according to recruitment method; the three studies45,46,71 recruiting participants via referral and the one39 recruiting via referral and voluntary methods all showed mixed results which were not obviously different from the other six studies43,48,50,51,53,67 using voluntary recruitment. Mean age ranged from 18 years to 36 years. Five studies43,45,46,48,67 assessed relatively young populations (mean age 18–23 years, upper age range in teens or twenties); these studies were all classed as low baseline use. There were no clear differences in effects according to age, with the five studies43,45,46,48,67 of younger populations showing mixed results in a similar manner to other studies.
In addition, two studies with low baseline use reported subgroup effects. 45,48 One study45 reported no significant difference between internet-based personalised feedback and control across all participants, but a significant difference for participants with a higher contemplation to change use or a family history of drug problems. Another study,48 in which participants were not seeking treatment for their cannabis use, reported no significant difference between brief MI and AO across all participants, but significant differences for those with a desire to cease use.
Brief motivational interviewing compared with other interventions
Description of studies
Three studies51,62,75 (n = 2002 randomised, 754 followed up) compared a brief intervention (one session of MI or telephone MI) with education controls (regarding cannabis or general health) (Tables 16 and 17). All MI sessions were individual (not group). One study51 reported session attendance, in which 89% of participants attended a MI session and 94% attended a ‘cannabis education’ session. Participants were classified as having high baseline use in one study51 and low use in two studies. 62,75 Studies were conducted in the USA,51 Canada75 and Brazil. 62
| Comparison | Number of studies, number randomised (followed up), categorisation high n, low n | Intervention (number of sessions) | Computer (number of sessions) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|---|
| Brief MI vs. other intervention | Three studies51,62,75 (see Table 17), n = 2002 (754), high 1,51 low 262,75 | MI or tele-MI (1 session) | Cannabis or health education | Three individual, 1 session | Mixed results, limited data: one study:51 MI-1 significantly better than education on most outcomes, not all | NR | 3–12 months | Mixed results: two studies of MI-1 vs. education: MI-1 significantly better on some outcomes, not others (one study51); no significant difference (one study75); 3/6 months and 12 months. One study:62 tele-MI-1 better than education on one outcome (6 months) | Limited data: one study:75 significant improvement in MI-1 and education groups (3 months) |
| Study, country, cannabis use, recruitment, mean age (range) | Intervention (number of sessions) (mean number of sessions attended), number randomised (followed up) | Computer, number randomised (followed up) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|
| Fernandes 2010,62 Brazil, low use (NHSDA 88% dependent), voluntary, 25 years (11–NR years) | Tele-brief MI (1) (NR), n = 873 (262) | Written cannabis information, n = 871 (262) | Individual, 1 session | 6 months | Significant difference:
|
|||
| Fischer 201275 Fischer 2013,64 Canada, low use (≥ 12 day/month), voluntary, 20 years (18–28 years) | Brief MI (1) (NR), n = 25 (23) | Three education controls, n = 109 (90) | Individual, 1 session | 3 months, 12 months | No significant difference:
|
Significant change:
|
||
No significant change:
|
||||||||
No significant difference:
|
||||||||
| Stephens 2007,51 USA, high use (≥ 15 day/months), voluntary, 32 years (18–57 years) | MI/personalised feedback (1) (89% attended), n = 62 (58) | Cannabis education (1) (94% attended), n = 62 (59) | Individual, 1 session, follow-up at 7 weeks | Significant difference:
|
6 months, 12 months | Significant difference:
|
||
No significant difference:
|
||||||||
No significant difference:
|
Significant difference:
|
|||||||
No significant difference:
|
Main results
One study51 of MI (1 session) compared with education control reported significantly better results for MI on some outcomes (i.e. some cannabis use outcomes, dependence) but not other outcomes (i.e. some cannabis use outcomes, cannabis problems), both post treatment and at 6 and 12 months’ follow-up. Another study75 reported no significant differences between 1-session MI and education after 3 and 12 months (only cannabis use outcomes were reported); however, all groups significantly improved from baseline. A further study62 reported that one-session telephone MI was significantly better than education control on a single outcome (% abstinent) after 6 months, with an odds ratio of 1.6 (95% CI 1.2 to 2.0).
Effects of intervention and population characteristics
There were too few studies in this category to allow meaningful assessment of the effects of other study characteristics.
Contingency management (vouchers for abstinence) versus other interventions
Description of studies
Five studies40–42,44,47 (n = 680 randomised, 581 followed up) compared contingency management (vouchers for abstinence assessed via urine tests), alone or in combination with CBT, with other interventions (Tables 18 and 19). One study also assessed computer-based CBT plus contingency management. 42 Comparators included CBT40,41,44 (9–14 sessions), MET40,42 (2–4 sessions), case management44,47 (9 sessions) and CBT plus vouchers for completed CBT homework. 47 Three studies reported session attendance; attendance at the CBT plus voucher arms of the studies was reported as 61%47 and 69%41. Attendance in the ‘other’ arms of the trials was reported as 67% (case management intervention)47 and 63% (CBT intervention without vouchers). 41 One study44 reported attendance across both arms (58%). All interventions were individual (not group). Participants were classified as having high baseline use in all five studies and all five studies were conducted in the USA.
| Comparison | Number of studies, number randomised (followed up), categorisation high n, low n | Intervention (number of sessions) | Computer (number of sessions) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|---|
| Contingency management (vouchers for abstinence) vs. other | Five studies40–42,44,47 (see Table 19), n = 680 (581), high 5 | CBT + voucher, compCBT + voucher, voucher alone (all vouchers for negative urine tests) | CBT (9–14, MET (2–4), CaseM (9). CBT + voucher (for CBT homework) | Five individual, 8–14 weeks | Significant difference (some): CBT + voucher or voucher alone better than CBT alone: three studies:40,42,44 CBT + voucher better than CBT or MET alone (some outcomes). Two studies: voucher alone better than CBT (two studies)41,44 or case management (one study)44 (some outcomes). One study:42 no significant difference between CBT + voucher and compCBT + voucher (one outcome) | Significant change: three studies:40,41,44 significant improvement baseline to post treatment on most outcomes in all groups | 14–15 months | Significant difference (some): CBT + voucher better than CBT or voucher alone: two studies:41,44 CBT + voucher better than CBT or voucher alone (some outcomes) at 14–15 months. Vouchers alone: good results post treatment but declined during follow-up; CBT + voucher better at later follow-ups41,44 One study:47 CBT + voucher (abstinence) better than CBT + voucher (CBT homework), some outcomes, 5–8 months. Mechanism: two studies:44,47 long-term abstinence predicted by abstinence during treatment, coping skills and self-efficacy | Significant change (some): three studies:41,44,47 significant improvement from baseline at 14–15 months on some/most outcomes (all groups) |
| Study, country, cannabis use, recruitment, mean age (range) | Intervention (number of sessions) (number of sessions attended), number randomised (followed up) | Computer (number of sessions) (mean number of sessions attended) number randomised (followed up) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|
| Budney 201142 (abstract) ClinicalTrials.gov 2013,73 USA, high use (DSM-IV 100%), voluntary, 35 years (18–65 years) | CBT/MET/voucher (9) (NR), n = 29 (16) CompCBT/MET/brief therapist/voucher (9) (NR), n = 30 (21) |
MET (2) (NR), n = 16 (9) | Individual, 12 weeks | Significant difference (CBT + voucher or compCBT + voucher better than MET):
|
12 months | Significant difference:
|
||
No significant difference (CBT + voucher vs. compCBT + voucher):
|
||||||||
| Budney 2006,41 USA, high use (DSM-IV 100%), voluntary + referral 33 years (≥ 18 years) | CBT/voucher (14) (9.6a), n = 30 (26) Voucher (14), n = 30 (24) |
CBT (14) (8.8a), n = 30 (26) | Individual,14 weeks | Significant difference (voucher better than CBT):
|
Significant change:
|
15 months | Significant or nearly significant difference (CBT + voucher better than either alone):
|
Change maintained post treatment:
|
No significant difference (CBT + voucher vs. other groups):
|
||||||||
No significant difference (voucher vs. CBT):
|
Change not maintained post treatment:
|
|||||||
No significant difference (all groups):
|
No significant difference (all groups):
|
|||||||
| Budney 200040 and Moore 2003,74 USA, high use (DSM-III-R 100%), voluntary, 32 years (≥ 18 years) | CBT/MET/voucher (14) (NR), n = 20 (14) | CBT/MET (14) (NR), n = 20 (15) MET (4) (NR), n = 20 (16) |
Individual, 14 weeks | Significant difference (CBT + voucher better than CBT or MET):
|
Significant change:
|
|||
No significant difference (all groups):
|
||||||||
Additional outcome data:
|
||||||||
| Kadden 200744 and Litt 2008,77 USA, high use (DSM-IV 100%), voluntary, 33 years (≥ 18 years) | CBT/MET/voucher (9) (5.2), n = 63 (59) Voucher, n = 54 (50) |
CBT/MET (9) (5.2), n = 61 (55) CaseM (9) (5.2), n = 62 (54) |
Individual, 9 weeks | Significant difference (voucher vs. CaseM):
|
Significant change:
|
14 months | Significant difference:
|
Significant change:
|
No significant difference:
|
No significant difference:
|
|||||||
| Mechanism: short-term abstinence predicted abstinence during treatment; long-term abstinence predicted by coping skills and self-efficacy | ||||||||
| Litt 2013,47 USA, high use (DSM-IV 100%), voluntary, 33 years (≥ 18 years) | CBT/MET/voucher (abstinence) (9) (5.5b), n = 73 (66) | CaseM (9) (6.0), n = 71 (65) CBT/MET/voucher (homework) (9) (5.7b), n = 71 (65) |
Individual, 8 weeks | 5–8 months, 14 months | Significant difference (CBT + abstinence voucher better than CBT + homework voucher):
|
Significant change:
|
||
No significant difference (CBT + voucher vs. CaseM):
|
||||||||
No significant difference (any groups):
|
Main results
Results post treatment differed from those at later follow-up. Four studies40–42,44 reported between-group data post treatment; results favoured either CBT plus vouchers or vouchers alone over CBT alone. Three studies40,42,44 reported better results (on some outcomes only) for CBT plus vouchers than for CBT or MET alone. In addition, two studies reported better results (again on some outcomes only) for vouchers alone than CBT alone (two studies)41,44 or case management alone (one study). 44 One study41 assessed continuous abstinence for ≥ 6 weeks and reported an odds ratio of 6.0 (95% CI 1.7 to 21.0) for vouchers alone compared with CBT and an odds ratio of 4.1 (95% CI 1.2 to 14.4) for CBT plus vouchers compared with CBT alone.
Later follow-ups indicated that positive results were maintained for combined treatment with CBT plus vouchers. However, the beneficial short-term results for vouchers alone were less likely to be maintained long term. Three studies41,44,47 reported between-group data at 14–15 months. Two studies41,44 reported better results for CBT plus vouchers than for either CBT or vouchers alone (on some outcomes) at 14–15 months’ follow-up. Significant improvements from baseline were reported on some or most outcomes in all groups post treatment (three studies40,41,44) and at 14–15 months’ follow-up (three studies41,44,47).
Two further studies made other comparisons. One study42 reported no significant difference between CBT plus voucher and computer-based CBT plus voucher post treatment (however, only one outcome – weeks of continuous abstinence – was reported). Another study47 reported that CBT plus voucher (for abstinence) gave better results than CBT plus voucher (for CBT homework) on some but not all outcomes at 5–8 months’ follow-up.
Two studies44,47 investigated potential mechanisms for changes in cannabis use and reported that long-term abstinence was predicted by abstinence during treatment and by increases in coping skills and self-efficacy. A further analysis of two studies40,74 assessed ability to maintain abstinence, reporting that 54% of participants achieved at least 2 weeks’ continuous abstinence at any point and, of these, 24% lapsed to cannabis use within 1 month, 46% within 3 months and 71% within 6 months.
Effects of intervention and population characteristics
It was difficult to assess the effects of study characteristics within this category as all studies were similar in many aspects of their design. All five studies40–42,44,47 provided individualised treatment, all were classified as high baseline use, all used either voluntary recruitment (four studies40,42,44,47) or a combination of voluntary and referrals (one study41) and the mean age was 32–35 years in all five studies.
Studies in populations with psychiatric conditions
Cognitive–behavioural therapy plus treatment as usual compared with treatment as usual
Description of studies
Four studies52,66,68,69 (n = 326 randomised, 254 followed up) compared CBT (6–24 sessions) plus TAU with TAU alone (Tables 20 and 21). TAU generally consisted of antipsychotic medication and psychiatric condition monitoring. In addition, in one study52 a self-help book on substance abuse was provided, and two studies68,69 explicitly stated that a psychosocial intervention was provided to participants receiving TAU. In terms of the study interventions, two studies52,68 provided individual CBT sessions, one66 individual plus optional group sessions, and one69 group sessions. Three studies reported session attendance: two reported percentage of participants attending all sessions (85%66 and 67%68), while one study69 reported the proportion attending more than 1 session (46%, 12-session intervention). Participants were classified as having high baseline use in three studies66,68,69 and low use in one study. 52 One study was conducted in Switzerland,66 one in Denmark,68 one in Ireland69 and one in Australia. 52
| Comparison | Number of studies, number randomised (followed up), categorisation high n, low n | Intervention (number of sessions) | Computer | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|---|
| CBT + TAU vs. TAU | Four studies,52,66,68,69 (see Table 21), n = 326 (254), high 3,66,68,69 low 152 | CBT (6–24 sessions) + TAU | TAU | Three individual, one group, 10–26 weeks | Few significant differences: four studies:52,66,68,69 CBT (6–24 sessions) + TAU significantly better than TAU alone on one outcome, not others (note: low participant numbers may affect p-values; in one study only 46% CBT group attended any sessions69) | Little significant change: one study:66 significant improvement in both groups (single outcome). Two studies:52,69 no significant improvement | 10–12 months | No significant difference (almost): four studies: 52,66,68,69 no significant difference between CBT (6–24 sessions) + TAU and TAU alone at 10–12 months, except quality of life in one study69 (note: low numbers may affect p-values; in one study only 46% CBT group attended any sessions69) | Little significant change one study:66 significant improvement in both groups at 12 months (single outcome). Two studies:52,69 no significant improvement at 12 months |
| Study, country, cannabis use, recruitment, mean age (range) | Intervention (number of sessions) (number of sessions attended), number randomised (followed up) | Computer, number randomised (followed up) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|
| Baker 2006,52 Australia, low use (≥ 4 day/month), referral, 29 years (15–61 years) | CBT/MI + TAU (10) (NR), n = NR (39) | TAU, n = NR (34) | Individual, 10 weeks | Significant difference:
|
No significant change:
|
12 months | No significant difference:
|
No significant change:
|
No significant difference:
|
||||||||
| Bonsack 2011,66 Switzerland, high use (DSM-IV 82%), referral, 26 years (18–35 years) | CBT/MI + TAU (6) (5.1), n = 30 (22) | TAU, n = 32 (27) | Individual (+ optional group), 6 months | Significant difference:
|
Significant change:
|
12 months | No significant difference:
|
Significant change:
|
No significant difference:
|
||||||||
| Hjorthoj 201368 and Hjorthoj 2012,80 Denmark, high use (ICD-10 100%), referral, 27 years (17–42 years) | CBT/MI + TAU (24) (16), n = 52 (38) | TAU, n = 51 (30) | Individual, 6 months | No significant difference (borderline):
|
10 months | No significant difference:
|
||
No significant difference:
|
||||||||
| Madigan 2013,69 Ireland, high use (DSM-IV 100%), voluntary + referral, 28 years (16–65 years) | CBT/MI (12) (46% attended ≥ 1), n = 59 (42) | TAU, n = 29 (22) | Group, 12 weeks | Significant difference:
|
No significant change:
|
12 months | Significant difference:
|
No significant change:
|
No significant difference:
|
No significant difference:
|
Main results
Results indicated little effect of CBT plus TAU over TAU alone in this population; however, data were limited in that the numbers of analysed participants per study were relatively low (22–42 per group), which may affect significance levels. Furthermore, in one study, only 46% of the CBT group attended any sessions. 69 In addition, the provision of psychosocial and other interventions in the control groups as part of TAU may potentially have reduced any difference in outcomes between groups, although two52,68 of three52,68,69 studies showed no changes from baseline in either group.
All four studies52,66,68,69 reported between-group data post treatment, each reporting a significantly better result for CBT plus TAU than TAU on a single outcome only and not on other outcomes. Outcomes with significant or near-significant effects in one study each were: joints per week, joints per month, number of days used and quality of life; however, there were no significant differences in days used, days abstinent or per cent abstinent. Other outcomes (i.e. severity of dependence, cannabis problems) were not reported in any study. At 10–12 months’ follow-up, these four studies52,66,68,69 reported no significant differences between CBT plus TAU and TAU alone on any cannabis use outcomes; however, one study69 reported a significant difference in quality of life. Two studies52,69 reported no significant improvements from baseline in any group either post treatment or at 12 months, while one study66 reported a significant improvement in both groups on the single relevant outcome reported (joints per week).
Effects of intervention characteristics
There were no clear differences in results according to number of CBT sessions (6–24) or group compared with individual treatment.
Effects of population characteristics
There were no clear differences in results for the one study52 classed as low baseline use/dependence compared with the three66,68,69 with high use. In terms of recruitment method, three studies recruited via referrals only52,66,68 and one via using a combination of voluntary recruitment and referrals;69 all four showed little effect of CBT plus TAU over TAU alone. The effect of age on results could not be assessed as the mean age was similar across studies (26–29 years).
Cognitive–behavioural therapy compared with other interventions
Description of studies
Three studies54,57,58 (n = 199 randomised, 197 followed up) assessed CBT (one study;54 10 sessions) or computer-based CBT with brief weekly therapist input (two studies;57,58 10 sessions) (Tables 22 and 23). Comparators included psychoeducation (10 sessions; non-cannabis-based),54 person-centred therapy (PCT; 10 sessions)58 or brief MI (1 session). 57 Sessions were individualised (not group). Session attendance was reported as 76%,54 53%58 and 76%82 for the CBT interventions. For comparators, 84% attended all psychoeducation sessions,54 54% attended all sessions for PCT58 and 87% participants attended all brief MI sessions. 82 Participants were classified as having low baseline use in all studies and all studies were conducted in Australia.
| Comparison | Number of studies, number randomised (followed up), categorisation high n, low n | Intervention (number of sessions) | Computer (number of sessions) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|---|
| CBT vs. other | Three studies,54,57,58 (see Table 23), n = 199 (197), low use (3) | CBT (10 sessions), compCBT + brief therapist (10 sessions) | Psychoeducation-10, CBT-10, PCT-10, brief MI-1 | Three individual, 10–12 weeks | No significant difference: one study:54 CBT vs. psychoeducation (10 sessions + TAU): no significant difference. One study:58 CBT or compCBT vs. PCT; compCBT vs. CBT or PCT: no significant difference | Significant change: one study:54 significant change, CBT and psychoeducation (single outcome) | 9–12 months | No significant difference: one study:54 CBT vs. psychoeducation (10 sessions + TAU): no significant difference at 9 months. Significant difference: one study:57 CBT-10 or compCBT-10 significantly better than MI-1 (single outcome) | Significant change: one study:54 significant change, CBT and psychoeducation at 9 months (single outcome). One study:57 significant change across groups at 12 months (single outcome) |
| Study, country, cannabis use, recruitment, mean age (range) | Intervention (number of sessions) (number of sessions attended), number randomised (followed up) | Computer (number of sessions), (mean number of sessions attended), number randomised (followed up) | Individual or group, duration | Post-treatment difference between groups | Post-treatment change from baseline | Follow-up | Follow-up difference between groups | Follow-up change from baseline |
|---|---|---|---|---|---|---|---|---|
| Edwards 200654 (CAP), Australia, low use (DSM-IV 49%), referral, 21 years (15–29 years) | CBT/MI + TAU (10), (7.6a), n = 23 (22) | Psychoeducation (non-cannabis) + TAU (10) (8.4a), n = 24 (23) | Individual, 12 weeks | No significant difference:
|
Significant change:
|
9 months | No significant difference:
|
Change maintained after treatment end:
|
| Kay-Lambkin 2011,58 Australia, low use (≥ 4 day/month), voluntary + referral, 40 years (17–70 years) | compCBT/MI/brief therapist (10) (5.3b), total n = 109 (NR) | PCT (10) (5.4b), CBT/MI (10) (6.1b), (n = 109) | Individual, 10 weeks | No significant difference (CBT + compCBT vs. PCT):
|
||||
No significant difference (compCBT vs. CBT + PCT):
|
||||||||
| Kay-Lambkin 2009,57 Australia, low use (≥ 4 day/month), voluntary + referral, 35 years (18–61 years) | compCBT/MI/brief therapist (10) (7.6a), total n = NR (43) | Brief MI (1), CBT/MI (10) (8.7a), (n = NR) | Individual, 10 weeks | 12 months | Significant difference (CBT + compCBT vs. MI):
|
Significant change:
|
Main results
Two studies reported results post treatment. One study54 reported no significant differences between CBT and psychoeducation (10 sessions each) post treatment or at 9 months’ follow-up, but numbers of analysed participants were low; however, both groups showed a significant improvement from baseline, post treatment and at 9 months (based on one relevant outcome, number of days used). Another study58 reported no significant difference between the three types of 10-session therapy (CBT, computer-delivered CBT with brief therapist input, or PCT) post treatment. Individual group comparisons were not reported, only the following comparisons: CBT or computer-delivered CBT compared with PCT, and computer-delivered CBT compared with CBT or PCT. Changes from baseline were not reported for this study.
A further study57 reported that 10 sessions of either CBT or computer-delivered CBT with brief therapist input (analysed together) was significantly better than 1-session MI at 12 months’ follow-up, and that there was a significant improvement from baseline across groups at 12 months; however, only one relevant outcome (mean use per day) was reported for this study.
Effects of intervention and population characteristics
Intervention and comparator groups were too heterogeneous to allow meaningful assessment of the effects of other study characteristics on results.
Subgroup analyses: effect of intervention and population characteristics
This section provides a summary of the possible effects of intervention and population characteristics on results. These are covered in each of the intervention/comparator categories above and are summarised here.
Number of sessions, and comparison of longer cognitive–behavioural therapy, compared with shorter motivational interviewing
Two sets of data imply that longer courses of CBT may be somewhat more effective than shorter courses of MI, but findings were mixed. First, four studies directly compared CBT (6–14 sessions) against brief MI (1–4 sessions). 39,40,50,53 Of these, some showed better results for CBT than MI (one39 post treatment, two at later follow-ups39,53), whereas others showed no significant differences (two40,50 post treatment, one at later follow-ups50). Second, studies comparing brief MI with wait list showed some significant effects, but these were not as positive overall as results for CBT compared with wait list which were significant on nearly all outcomes. The somewhat better results for CBT than MI could be due to the nature of CBT treatment, the fact that more sessions were provided, or a combination of the two.
Within six studies of CBT compared with wait list,39,50,53,59,60,63 all durations of CBT (4–14 sessions) appeared effective. There were slightly fewer significant effects in the study of four-session CBT,63 but this may have been related to the small number of participants. One study63 compared four sessions of CBT over either a 4- or 12-week period; post-treatment results significantly favoured 12-week treatment on some but not all outcomes. Within studies of brief MI compared with wait list, there was no clear difference between studies of one-session or two-session MI, with both durations showing mixed results.
Group or individual treatment
Of the 33 included studies, 2739–48,51–55,57–63,66,68,70,71,75 provided individualised treatments, whereas three49,67,69 provided group treatment and two50,65 compared group with individual treatment (not reported for one). 56 There were insufficient group treatment studies within most intervention/comparator categories to meaningfully compare group with individual treatment. Within studies of CBT compared with wait list, the one study of group CBT50 had similar positive outcomes to the five individual CBT studies. Within studies of CBT compared with brief MI, one study50 showed little benefit of group CBT compared with individual MI, and studies of individual CBT compared with MI showed mixed results. Within studies of brief MI compared with wait list, studies of individual MI showed mixed results, whereas the one study67 of group MI showed no significant effect; however only 1 session of MI was provided and results were not measured until 6 months and then only on one outcome (cannabis days of use). One study65 directly compared individual with group CBT (4 sessions each), but only 17 cannabis users were analysed and only one relevant outcome reported (days abstinent); results non-significantly favoured individual CBT post treatment, but this effect was not maintained at 12 months. Overall, group treatment may possibly be less effective than individual treatment, but this is based on very limited data.
High compared with low baseline cannabis use/dependence
The impact of high or low baseline cannabis use/dependence could be assessed to some extent within certain intervention/comparator categories: CBT compared with wait list, computer-delivered/telephone-delivered CBT compared with other, and brief MI compared with wait list. Within each of these categories, studies with low baseline use43,45,46,48,55,60,63,67,70,71 appeared slightly less likely to show significant differences on all outcomes than studies of high use. 39,50,51,53,59,61 However, this difference was not substantial or conclusive. This potential difference could be owing to the interventions having a greater effect on participants with higher baseline use/dependence, either because a greater effect could be demonstrated or because these participants may have been more motivated to reduce use; however, this conclusion should be treated with caution.
In addition, two studies with low baseline use reported subgroup effects. 45,48 One study45 reported no significant difference between internet-based personalised feedback and the control group across all participants, but a significant difference for participants with a higher contemplation to change use or a family history of drug problems. Another study,48 in which participants were not seeking treatment for their cannabis use, reported no significant difference between brief MI and AO across all participants, but significant differences for those with a desire to cease use.
Recruitment method (voluntary compared with referrals)
Among the 26 general population studies,39–51,53,55,56,59–63,65,67,70,71,75 1640,42–44,47–50,51,53,55,61,62,67,70,75 used voluntary recruitment via advertisement, while four45,46,60,71 used referrals and four39,41,59,65 used a combination of voluntary and referrals (not reported for two studies56,63). Results for the general population studies showed no clear difference in results according to recruitment method, although this comparison is based on limited data. No clear difference was observed within studies of CBT compared with wait list (two voluntary,50,53 one referral,60 two combination;39,59 all positive results) or within studies of brief MI compared with wait list (six voluntary,43,48,50,51,53,67 three referral,45,46,71 one combination;39 all mixed results). Nevertheless, it should be noted that the majority of the general population studies recruited volunteers via advertisement and, therefore, may reflect a more motivated group when compared with the ‘average’ cannabis user.
In contrast to the general population studies, all seven studies in psychiatric populations recruited patients via referral (four studies52,54,66,68) or a combination of referral and voluntary methods (three studies57,58,69). Across four studies of CBT plus TAU compared with TAU in psychiatric populations, three studies52,66,68 used referrals and one69 used a combination of voluntary and referrals; all four showed little difference between CBT plus TAU and TAU alone. Comparisons within the other three studies54,57,58 were too heterogeneous to assess the effects of recruitment method.
Participant age
Within most intervention/comparator categories, mean ages were similar across studies, so the effect of age could not be meaningfully assessed. This was true for comparisons of CBT compared with wait list (mean age 24–36 years), CBT compared with brief MI (mean age 32–36 years), telephone-/internet-delivered CBT (mean age 25–36 years) and CBT plus TAU vs. TAU (mean age 26–29 years). For brief MI compared with wait list (10 studies39,43,45,46,48,50,51,53,67,71), mean age ranged from 18 years to 36 years. Five studies43,45,46,48,67 assessed relatively young populations (mean age 18–23 years, upper age range in teens or twenties) and these studies were all classed as low baseline use/dependence. There were no clear differences in effects according to age, with the five studies of younger populations showing mixed results in a similar manner to other studies.
Chapter 4 Discussion
Statement of principal findings
General population studies
Of 26 studies39–51,53,55,56,59–63,65,67,70,71,75 assessing the general population of cannabis users (7643 randomised participants), 1640,42–44,47–50,51,53,55,61,62,67,70,75 recruited via advertisement and eight39,41,45,46,59,60,65,71 via referrals or both. Baseline use/dependence was high in 13 studies39–42,44,47,49–51,53,56,59,61 and low in 10. 43,45,46,48,55,60,63,65,70,75 Across six studies39,50,53,59,60,63 of CBT (4–14 sessions) compared with wait list, CBT was significantly better on most outcomes (cannabis use, severity of dependence, cannabis problems) post treatment (in all five studies39,50,59,60,63 with data) and at 9 months (in the one study53 with later follow-up). Four studies39,40,50,53 comparing CBT (6–14 sessions) with briefer MI/MET (1–4 sessions) gave mixed results, with two studies40,50 showing better results for CBT post treatment and at 9–16 months, while two further studies39,53 showed few between-group differences; both CBT and MI gave significant improvements from baseline. In one small study,56 supportive–expressive dynamic psychotherapy (16 sessions) gave significant improvements over one-session MI. One study49 of CBT compared with social support group (10 sessions each) and another44 of CBT compared with case management (nine sessions each) showed no significant differences between groups but all groups significantly improved from baseline with changes maintained at 14–15 months. One study each of telephone-delivered CBT, internet-delivered CBT and internet-delivered counselling all showed significant improvements over wait list or education control post treatment and at 3 months. 55,61,70
Ten studies39,43,45,46,48,50,51,53,67,71 assessing brief MI/MET (one or two sessions) compared with wait list or AO gave mixed results, with brief MI appearing significantly better on some outcomes but not others, post treatment and at 3–9 months. Results were similar for three studies51,62,75 comparing brief MI against education controls. Five studies40–42,44,47 assessed contingency management (monetary vouchers for abstinence). Vouchers alone and CBT plus vouchers gave better results than CBT or MET alone post treatment (three studies40,42,44), while at 14–15 months positive results were maintained for CBT plus vouchers but less so for vouchers alone (two studies41,44).
Psychiatric population studies
Seven studies52,54,57,58,66,68,69 (525 randomised participants) assessed psychiatric populations (schizophrenia, psychosis, bipolar disorder or major depression); all recruited via referrals or referrals plus advertisements. Baseline use/dependence was high in three studies66,68,69 and low in four. 52,54,57,58 Across four studies52,66,68,69 assessing CBT (6–24 sessions) plus TAU compared with TAU alone, there were few significant between-group differences post treatment and none at 10–12 months (small studies; limited data), with little change from baseline in either group. Two studies54,58 reported no significant difference between different types of 10-session therapy (one compared CBT, computer-delivered CBT and PCT; the other compared CBT and psychoeducation), although the latter reported significant improvements from baseline in both groups (limited data). 54 A further study reported improvements for 10-session CBT or computer-delivered CBT over single-session MI at 12 months’ follow-up on one outcome (daily cannabis use). 57
Strengths and limitations of the assessment
Strengths
This report systematically reviews the evidence for a range of psychological and psychosocial treatments for regular users of cannabis. Thirty-three studies were included and the scope of the review is inclusive, covering a wide range of populations, interventions and outcomes. The majority of previous reviews in this topic area have restricted their scope to a subtype of intervention or population, or are not specific to cannabis users. 6,33 The present review has included all psychosocial or psychological interventions undertaken in the adult, community-dwelling population of cannabis users and has only included RCTs, ensuring that only the highest quality available evidence has been included. Robust methods were used, including a search methodology with wide scope, grey literature searching and contact with clinical experts in the area. Data were double-checked for accuracy.
The included studies reported a heterogeneous set of data; in many cases, similar outcome measures (e.g. cannabis use) were reported in different ways. Narrative synthesis was used to analyse and explore the data. Results are presented for each intervention/comparator category (e.g. CBT vs. wait list or CBT vs. MI) and by population (general vs. psychiatric). This allows studies with similar comparisons and populations to be analysed together, to provide an overview of the direction of effects for each category at different time points. This approach also minimises loss of data, as any meta-analysis would have been restricted to studies reporting the same outcome in a consistent format and reporting full data [including standard deviations (SDs), etc.].
Limitations
There was substantial heterogeneity between studies in terms of their populations, interventions, comparators, outcome measures and data format, and limited time was available to conduct this systematic review short report. Therefore, results are presented as an overview of the outcomes reported per study and how many showed a significant difference, both between intervention groups and in terms of changes from baseline, at different follow-up time points. Detailed numerical results per group are not presented in the main results section (these are provided in Appendix 4) and meta-analysis was not undertaken. This approach has the limitations that (1) it was not possible to present effect sizes for outcomes and (2) data were not pooled across studies. However, the narrative synthesis approach was thought to provide benefits in terms of interpretability as described above. In addition, owing to time constraints, we were unable to include studies written in languages other than English.
Only RCTs were included in this review. Although this ensures that only the highest quality of evidence is included in the synthesis, it does potentially result in informative studies being rejected. Nine potentially relevant articles were excluded owing to this;83–91 however, most of these studies would have been rejected for other exclusion criteria (four of the studies included a population comprising people aged < 18 years). 84,88,90,91
We excluded studies that were undertaken within the criminal justice setting (e.g. studies undertaken within the court system, prison, or while study participants are on parole). We also excluded studies that treated individuals other than the cannabis user (e.g. a family member). Although risking the exclusion of potentially valid trials, excluding these populations reduced the many sources of heterogeneity. This review focused on community-delivered interventions. Interventions carried out within the criminal justice setting are unlikely to be replicable when delivered to cannabis users outside that setting, owing to differences in recruitment, intervention delivery and outcome assessment.
The studies included in this review utilised a variety of recruitment methods, involving voluntary recruitment, referral by a health-care professional or a combination of both. Studies in the general population of cannabis users mostly used voluntary recruitment methods (most often via advertisements); therefore, these may have reflected more motivated populations and may not be generalisable to all cannabis users. Conversely, this may reflect practice in that psychological interventions are likely to be provided to those willing to receive them. In addition, the included studies recruited cannabis users with varying frequencies of cannabis use at baseline.
Results obtained from the psychiatric population studies may have been affected by provision of TAU to both groups, which in two studies included psychosocial interventions. 68,69 Although these presumably focused on the psychiatric condition rather than cannabis use, they may indirectly have affected cannabis outcomes in both groups. Another study provided a self-help book on substance abuse as part of TAU. 52
The following topics are outside the scope of this systematic review, but could form aspects of future work in this area. Although this short report focused on treatment of adult cannabis users, there is a large amount of literature on treatment of cannabis use in adolescents, including the effects of preventative strategies as well as interventions involving families or schools. In addition, assessment of the effects of factors such as therapist type and treatment fidelity, which are important factors when considering psychosocial interventions, may form a part of future reviews.
Assessment of factors relevant to the National Health Service and other parties
It would be important to consider the following points relating to implementation of any psychosocial intervention for cannabis use.
Intervention delivery
-
Availability of CBT and other treatments within the NHS, and which type of health professional would provide these. Department of Health guidance suggests that psychosocial interventions for substance misuse may be delivered by a key worker (when a health professional works with the individual to ensure delivery and ongoing review of care being received) with the required competencies or by a drug worker or psychologist. 20
-
UK guidelines also emphasise the importance of person-centred care, consideration of family and carer involvement, links between services to avoid loss of contact and importance of key working. 19,20 They also advise that treatment and information should be accessible to people with disabilities and to those who do not speak or read English. 19
Patient identification
-
How cannabis users would be identified/diagnosed and referred for treatment (e.g. via a general practitioner, social worker or a variety of routes).
-
The level of cannabis use or dependence at which treatment is required.
-
Whether all patients with a particular level of use or dependence would be referred or, for example, only those who wished to receive a psychosocial intervention and/or those expressing a desire to reduce or cease use (note that the majority of data for ‘general population’ studies involved participants voluntarily responding to advertisements). Existing UK guidelines advise that service users should be allowed to make informed decisions about their treatment in partnership with their health professionals. 19
-
Other relevant interventions exist that do not explicitly target cannabis users, but are aimed at individuals with addictive behaviours. Although no relevant RCTs of such therapies were identified in this review, they may be worthy of further consideration or research. For example, mutual aid therapies (such as Self-Management and Recovery Training) involve people with similar experiences assisting each other to overcome or manage their issues. Department of Health guidance advises that self-help and mutual aid groups should be recommended for all drug misusers seeking to achieve and maintain abstinence. 20 Other interventions not within the scope of this review may increase the effectiveness of psychosocial or psychological interventions for cannabis cessation. For example, nicotine replacement therapy may increase the ability of regular cannabis users to reduce their use of the drug, although a recently conducted pilot study found that this was not the case for a group of 12 cannabis-dependent individuals. 92
Comparison of results from this review with relevant national guidelines
-
Existing UK guidelines from NICE advise that CBT should not be offered routinely for treatment of cannabis abuse. Department of Health guidance advises that brief motivational interventions may be considered in mild cases of cannabis use, whereas more heavily dependent users may require structured treatment with key working. 19,20 Our review found that CBT appeared effective for routine treatment of cannabis abuse, but it was unclear how much more effective it was than briefer interventions.
-
In terms of people with co-existing psychiatric conditions, NICE and Department of Health guidelines suggest that CBT should be considered for treatment of cannabis users with comorbid depression and anxiety disorders. 19,20 Our review found that studies with people suffering from psychological conditions appeared to show less promising effects of CBT,52,54,57,58,66,68,69 but we focused on studies of CBT aimed at treating the cannabis abuse rather than the psychological condition and these studies may have been confounded by both groups receiving TAU for the psychological condition (including psychosocial treatments in some cases). 52,66,68,69
Chapter 5 Conclusions
Implications for service provision
This systematic review has identified a disparate evidence base that differed most notably in the nature and length of the interventions, the comparator groups, the populations studied (which differed in cannabis use at baseline as well as presence or absence of a psychiatric condition) and the outcomes measured (differing in metrics used, statistics reported and follow-up periods). Studies recruited participants using either voluntary or direct referral methods – studies utilising voluntary recruitment methods identified a self-selecting population, which may not be representative of real-world cannabis users. 39–44,47–50,53,55,61,62,67,70,75 In addition, 24 of the studies were deemed to be at high risk of bias, mostly owing to high attrition rates. 40,41,43,48,49,52–55,57–63,65,67–71,75,81 No studies were deemed to be at low risk of bias.
In light of the above, it is difficult to make definite conclusions regarding the effectiveness of the included psychological and psychosocial interventions. Based on the available evidence, CBT (4–14 sessions) appeared to significantly improve outcomes post treatment (cannabis use, severity of dependence, cannabis problems) compared with wait list in the general population of cannabis users, but only one53 of the six39,50,53,59,60,63 studies reported outcomes at a follow-up time point significantly after the treatment period elapsed. Studies of brief MI/MET (one or two sessions) gave mixed results, with some improvements over wait list, whereas some comparisons were not significant. Comparisons of CBT (6–14 sessions) with briefer MI/MET (1–4 sessions) also gave mixed results, with longer courses of CBT providing some improvements over MI. Significant effects were maintained in two39,53 of the three39,50,53 studies reporting later follow-ups. Courses of other types of therapy (social support group, case management, supportive–expressive dynamic psychotherapy) gave similar improvements to CBT based on limited data. Limited data indicated that telephone- or internet-based interventions may also be effective. Contingency management (vouchers for abstinence) gave promising results in the short term; at later follow-ups vouchers in combination with CBT gave better results than either vouchers or CBT alone. There were insufficient data to assess the effects of group compared with individual treatment.
In populations with psychiatric conditions (schizophrenia, psychosis, bipolar disorder or major depression), CBT appeared to have little effect over TAU, but this was based on four small studies with limited data. 52,66,68,69 Results may potentially have been affected by provision of TAU in both groups and the fact that patients were referred rather than volunteering for treatment. Other studies reported no significant difference between 10 sessions of CBT, computer-delivered CBT, PCT or psychoeducation, but improvements for 10-session CBT or computer-delivered CBT over single-session MI.
Included studies were heterogeneous and most were considered at high risk of bias. Based on the available evidence, courses of CBT and (to a lesser extent) one or two sessions of MI improved outcomes in a self-selected population of cannabis users. There is some evidence that CBT (6–14 sessions) may be more effective than briefer MI interventions, although results were mixed. Contingency management may also enhance long-term outcomes in combination with CBT. Results of CBT for cannabis cessation in psychiatric populations were less promising, but may have been affected by provision of TAU in both groups and the referred populations.
Suggested research priorities
The highest priority area for future research should be to identify the number and frequency of sessions required to provide reductions in cannabis use, which was unclear from the identified evidence. CBT (4–14 sessions) gave improved outcomes over wait list in general population studies, whereas briefer MI-based interventions (one or two sessions) appeared to have some effectiveness, although results were mixed. CBT also appeared to give somewhat better results than briefer MI-based interventions; however, it was unclear to what extent CBT outcomes were better. Future studies may wish to assess further the effectiveness of shorter courses of therapy. This could include brief interventions (e.g. one or two sessions). Alternatively, because studies of four- or six-session CBT seemed to have similar effectiveness to studies with 10–14 sessions, further assessment of four- to six-session CBT may be worthwhile. Relative cost-effectiveness of longer and shorter interventions may also be useful to assess. If shorter interventions are as efficacious and more cost-effective than longer ones, the former could be made more widely available.
The following areas for future research do not have as high priority, but are important nonetheless. They do not have any order of importance in relation to each other.
Interventions
Future studies may wish to test other interventions in addition to CBT. The use of contingency management (vouchers for abstinence) improved long-term outcomes when added to CBT. Future studies assessing CBT (and/or brief interventions) may also wish to include a group receiving CBT and contingency management. In addition, mutual aid therapies and self-help groups (for which no RCTs were identified in this review) may be worthy of future study. Other treatments not within the scope of this review, such as nicotine replacement therapy, could be assessed in conjunction with psychological and psychosocial interventions in order to increase effectiveness. All studies should aim to report the included interventions in sufficient detail to allow replication.
The current review has identified that CBT may be effective when delivered to cannabis-dependent individuals, but effectiveness has not been demonstrated when such treatment is provided to patients with psychological comorbidities. This lack of effectiveness for the dually diagnosed population confirms findings from a previous review. 6 These findings suggest that patients with a dual diagnosis may require separate treatments for their substance abuse and psychological problem. Alternatively, if this lack of effectiveness in the dually diagnosed population was due to the participant themselves being unable to respond to the CBT treatment owing to their psychological condition, further research may be necessary to identify interventions that are effective in such populations.
Populations
Future studies may also wish to consider the potential effects of recruitment method. Most existing general population studies recruited via advertisement. Studies using other methods of recruitment may be more generalisable to a clinical setting. Conversely, a somewhat selected study population may be reasonable (e.g. those wishing to cease use and/or willing to receive the intervention) as current guidance suggests that these populations would be the most relevant to receive psychosocial interventions (as opposed to, for example, users expressing no desire to cease use).
The included studies did not report the effect of the psychopharmacology of cannabis. Recent findings indicate that certain strains of cannabis containing high levels of cannabidiol are associated with less cognitive impairment and positive therapeutic potential in psychosis and other disorders (such as Parkinson’s disease). 93–96 This pharmacological factor could be an important modulating factor in treatment outcomes and, therefore, should be taken into account in future studies.
Outcomes
Outcomes reported in most studies could be classified into four main groups: (1) level of attendance, (2) cannabis use, (3) severity of dependence and (4) cannabis-related problems. Cannabis use covered a range of specific outcomes including point abstinence rates, abstinence over a specified period, number of days using cannabis or number of days abstinent (over a specified period), amount of cannabis use per day, and number of periods of use per day (e.g. of four daily periods). All the above outcomes showed significant effects in at least some studies. Future studies may wish to consider which outcomes have been most commonly used in existing studies making similar comparisons, to improve comparability between studies. In terms of the above outcomes, level of cannabis use can be difficult to measure owing to the different levels of active ingredients in different cannabis products. Abstinence is a frequently used outcome but may not be desirable or attainable for all users. Severity of dependence addresses the impact on a person’s life rather than focusing on quantities of use. Cannabis-related problems may also be a useful measure but in populations with other issues (such as psychiatric conditions) it may be difficult to distinguish between the causes of problems. Patient preference for different types of psychological intervention may also be useful to assess.
Methodology
Future studies should carefully consider trial methodology. The studies included in this review utilised a range of comparison groups, including active treatments (e.g. a variation of CBT or MI), less specific controls for time and attention (e.g. cannabis education) and inactive controls (wait list or AO). We recommend that wait list controls are included as a group in future studies, even those comparing different types or durations of active treatments, to indicate whether or not active treatments are effective when compared with no treatment (as well as with each other).
In addition, it would be useful to consider carefully future study designs for populations with psychiatric conditions. If TAU (for the psychiatric condition) is provided to all groups, this should be reflective of current clinical practice and consideration should be given to whether or not it may confound the study intervention (e.g. whether or not it includes psychosocial interventions). It may also be worth considering whether or not a single psychosocial intervention can be tailored to address jointly both the psychiatric condition and cannabis use.
The studies included in this review followed up participants over various time periods, ranging, overall, between 12 weeks and 16 months. Future studies should aim to follow-up patients over the long term; wait list controls with long-term follow-up are also valuable to assess fully long-term effects of treatment; however, this needs to be balanced against ethics considerations and acceptability to trial participants.
Acknowledgements
Many thanks to our clinical advisors for providing support and advice for this review:
Mr Mick Holmes, Team Leader, Sheffield Adult Treatment Service.
Mr Matt Knight, Manager (Alcohol and Drugs), Public Health England.
Dr Olawale Lagundoye, Consultant in Addiction Psychiatry, Sheffield Health and Social Care.
Thanks to the following for reviewing the draft report:
Dr Chris Carroll (Reader in HTA), School of Health and Related Research (ScHARR).
Dr Edward Day (Senior Clinical Lecturer), Kings College London.
Dr Alun George (Clinical Lead), Leeds East/North East Community Drug Treatment Service.
Thanks also to Philip Preece for providing service user input into the review protocol and final report and to Gill Rooney, Project Administrator, at ScHARR for providing administrative support and preparing and formatting the report.
About the School of Health and Related Research
The ScHARR is one of the nine departments that constitute the Faculty of Medicine, Dentistry and Health at the University of Sheffield, Sheffield, UK. ScHARR specialises in health services and public health research, the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the NIHR HTA programme on behalf of a range of policy-makers, including the NICE. ScHARR-TAG is part of a wider collaboration of a number of units from other regions including Southampton Health Technology Assessment Centre (SHTAC), University of Southampton, Southampton, UK; Aberdeen HTA Group, University of Aberdeen, Aberdeen, UK; Liverpool Reviews and Implementation Group (LRiG), University of Liverpool, Liverpool, UK; Peninsular Technology Assessment Group (PenTAG), University of Exeter, Exeter, UK; the NHS Centre for Reviews and Dissemination, University of York, York, UK; Warwick Evidence, University of Warwick, Coventry, UK; the BMJ Group and Kleijnen Systematic Reviews.
Contributions of authors
Katy Cooper and Robin Chatters carried out the systematic review and quality assessment of the studies.
Eva Kaltenthaler provided methodological input.
Ruth Wong carried out the literature searches.
All authors contributed to the drafting of the report.
Data sharing statement
All data available are included as an appendix to the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet 2009;374:1383-91. http://dx.doi.org/10.1016/S0140-6736(09)61037-0.
- ICD-10: International Statistical Classification of Diseases and Related Health Problems. Geneva: World Health Organization; 2000.
- Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press Inc.; 2000.
- Danovitch I, Gorelick DA. State of the art treatments for cannabis dependence. Psychiatr Clin North Am 2012;35:309-26. http://dx.doi.org/10.1016/j.psc.2012.03.003.
- Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med 2006;36:1447-60. http://dx.doi.org/10.1017/S0033291706008361.
- Hunt GE, Siegfried N, Morley K, Sitharthan T, Cleary M, Hunt GE, et al. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database of Syst Rev 2013;10. http://dx.doi.org/10.1002/14651858.cd001088.pub3.
- World Drug Report 2013. New York, NY: United Nations; 2013.
- Prevalence of Daily Cannabis Use in the European Union and Norway. Lisbon: EMCDDA; 2012.
- Grant BF, Pickering R. The relationship between cannabis use and DSM-IV cannabis abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 1998;10:255-64. http://dx.doi.org/10.1016/S0899-3289(99)00006-1.
- Swift W, Hall W, Teesson M. Cannabis use and dependence among Australian adults: results from the National Survey of Mental Health and Wellbeing. Addiction 2001;96:737-48. http://dx.doi.org/10.1046/j.1360-0443.2001.9657379.x.
- Swift W, Copeland J, Hall W. Choosing a diagnostic cut-off for cannabis dependence. Addiction 1998;93:1681-92. http://dx.doi.org/10.1046/j.1360-0443.1998.931116816.x.
- Denis C, Lavie E, Fatseas M, Auriacombe M, Denis C, Lavie E, et al. Psychotherapeutic interventions for cannabis abuse and/or dependence in outpatient settings. Cochrane Database of Syst Rev 2006;3.
- Winstock AR, Ford C, Witton J. Assessment and management of cannabis use disorders in primary care. BMJ 2010;340:800-4. http://dx.doi.org/10.1136/bmj.c1571.
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction 1999;94:1311-22. http://dx.doi.org/10.1046/j.1360-0443.1999.94913114.x.
- van der Pol P, Liebregts N, de Graaf R, Korf DJ, van den Brink W, van Laar M. Facilitators and barriers in treatment seeking for cannabis dependence. Drug Alcohol Depend 2013;133:776-80. http://dx.doi.org/10.1016/j.drugalcdep.2013.08.011.
- Brorson HH, Ajo AE, Rand-Hendriksen K, Duckert F. Drop-out from addiction treatment: a systematic review of risk factors. Clin Psychol Rev 2013;33:1010-24. http://dx.doi.org/10.1016/j.cpr.2013.07.007.
- Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press Inc.; 2013.
- Substance-Related and Addictive Disorders. American Psychiatric Press Inc.; 2013.
- NICE Clinical Guideline 51: Drug Misuse: Psychosocial Interventions. London, UK: NICE; 2007.
- Department of Health (England) . Drug Misuse and Dependence: UK Guidelines on Clinical Management 2007.
- European Monitoring Centre for Drugs and Drug Addiction . Best Practice Portal: Treatment Options for Cannabis Users 2014. www.emcdda.europa.eu/best-practice/treatment/cannabis-users (accessed 6 February 2014).
- McRae AL, Budney AJ, Brady KT, McRae AL, Budney AJ, Brady KT. Treatment of marijuana dependence: a review of the literature. J Subst Abuse Treat 2003;24:369-76. http://dx.doi.org/10.1016/S0740-5472(03)00041-2.
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008;165:179-87. http://dx.doi.org/10.1176/appi.ajp.2007.06111851.
- Tait RJ, Spijkerman R, Riper H. Internet and computer based interventions for cannabis use: a meta-analysis. Drug Alcohol Depend 2013;133:295-304. http://dx.doi.org/10.1016/j.drugalcdep.2013.05.012.
- Magill M, Ray LA. Cognitive-behavioral treatment with adult alcohol and illicit drug users: a meta-analysis of randomized controlled trials. J Stud Alcohol Drugs 2009;70:516-27. http://dx.doi.org/10.15288/jsad.2009.70.516.
- Wood SK, Eckley L, Hughes K, Hardcastle KA, Bellis MA, Schrooten J, et al. Computer-based programmes for the prevention and management of illicit recreational drug use: a systematic review. Addict Behav 2014;39:30-8. http://dx.doi.org/10.1016/j.addbeh.2013.09.010.
- Chiesa A, Serretti A. Are mindfulness-based interventions effective for substance use disorders? A systematic review of the evidence. Subst Use Misuse 2013;49:492-51. http://dx.doi.org/10.3109/10826084.2013.770027.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000097.
- NHS Choices . Cognitive Behavioural Therapy 2012. www.nhs.co.uk/conditions/cognitive-behavioural-therapy/Pages/Introduction.aspx (accessed 6 February 2014).
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: Guilford Press; 1991.
- Smedslund G, Berg RC, Hammerstrom KT, Steiro A, Leiknes KA, Dahl HM, et al. Motivational interviewing for substance abuse. Cochrane Database Syst Rev 2011;5. http://dx.doi.org/10.1002/14651858.cd008063.pub2.
- Maddock CBM. Interventions for cannabis misuse. Adv Psychiatr Treat 2006;12:432-9. http://dx.doi.org/10.1192/apt.12.6.432.
- Hesse M, Vanderplasschen W, Rapp R, Broekaert E, Fridell M. Case management for persons with substance use disorders. Cochrane Database Syst Rev 2007;17. http://dx.doi.org/10.1002/14651858.cd006265.pub2.
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis 1980;168:26-33. http://dx.doi.org/10.1097/00005053-198001000-00006.
- Copeland J, Gilmour S, Gates P, Swift W. The Cannabis Problems Questionnaire: factor structure, reliability, and validity. Drug Alcohol Depend 2005;80:313-19. http://dx.doi.org/10.1016/j.drugalcdep.2005.04.009.
- Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews 2006. www.lancaster.ac.uk/shm/research/nssr/research/dissemination/publications/NS_Synthesis_Guidance_v1.pdf (accessed 6 February 2014).
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359:781-5. http://dx.doi.org/10.1016/S0140-6736(02)07882-0.
- Babor TF, Carroll K, Christiansen K, Donaldson J, Herrell J, Kadden R, et al. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol 2004;72:455-66. http://dx.doi.org/10.1037/0022-006X.72.3.455.
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL, Budney AJ, Higgins ST, et al. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol 2000;68:1051-61. http://dx.doi.org/10.1037/0022-006X.68.6.1051.
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive–behavioral therapy for cannabis dependence. J Consult Clin Psychol 2006;74:307-16. http://dx.doi.org/10.1037/0022-006X.74.2.307.
- Budney AJ, Stanger C, Costello P, Fearer S, Walker DD, Bickel WK. Computer-Assisted Delivery of Behavioral Treatment for Cannabis Use Disorders: Preliminary Results from a Controlled Trial and Implications for Dissemination 2011. www.cpdd.org/Pages/Meetings/CPDD11AbstractBook.pdf (accessed 6 February 2014).
- de Dios MA, Herman DS, Britton WB, Hagerty CE, Anderson BJ, Stein MD, et al. Motivational and mindfulness intervention for young adult female marijuana users. J Subst Abuse Treat 2012;42:56-64. http://dx.doi.org/10.1016/j.jsat.2011.08.001.
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav 2007;32:1220-36. http://dx.doi.org/10.1016/j.addbeh.2006.08.009.
- Lee CM, Neighbors C, Kilmer JR, Larimer ME. A brief, web-based personalized feedback selective intervention for college student marijuana use: a randomized clinical trial. Psychol Addict Behav 2010;24:265-73. http://dx.doi.org/10.1037/a0018859.
- Lee CM, Kilmer JR, Neighbors C, Atkins DC, Zheng C, Walker DD, et al. Indicated prevention for college student marijuana use: a randomized controlled trial. J Consult Clin Psychol 2013;81:702-9. http://dx.doi.org/10.1037/a0033285.
- Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: randomized trial of contingency management and self-efficacy enhancement. Addict Behav 2013;38:1764-75. http://dx.doi.org/10.1016/j.addbeh.2012.08.011.
- Stein MD, Hagerty CE, Herman DS, Phipps MG, Anderson BJ. A brief marijuana intervention for non-treatment-seeking young adult women. J Subst Abuse Treat 2011;40:189-98. http://dx.doi.org/10.1016/j.jsat.2010.11.001.
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: a test of the relapse prevention model. J Consult Clin Psychol 1994;62:92-9. http://dx.doi.org/10.1037/0022-006X.62.1.92.
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol 2000;68:898-90. http://dx.doi.org/10.1037/0022-006X.68.5.898.
- Stephens RS, Roffman RA, Fearer SA, Williams C, Burke RS. The Marijuana Check-up: promoting change in ambivalent marijuana users. Addiction 2007;102:947-57. http://dx.doi.org/10.1111/j.1360-0443.2007.01821.x.
- Baker A, Bucci S, Lewin TJ, Kay-Lambkin F, Constable PM, Carr VJ, et al. Cognitive-behavioural therapy for substance use disorders in people with psychotic disorders: randomised controlled trial. Br J Psychiatry 2006;188:439-48. http://dx.doi.org/10.1192/bjp.188.5.439.
- Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive–behavioral interventions for cannabis use disorder. J Subst Abuse Treat 2001;21:55-64. http://dx.doi.org/10.1016/S0740-5472(01)00179-9.
- Edwards J, Elkins K, Hinton M, Harrigan SM, Donovan K, Athanasopoulos O, et al. Randomized controlled trial of a cannabis-focused intervention for young people with first-episode psychosis. Acta Psychiatr Scand 2006;114:109-17. http://dx.doi.org/10.1111/j.1600-0447.2006.00783.x.
- Gates PJ, Norberg MM, Copeland J, Digiusto E. Randomized controlled trial of a novel cannabis use intervention delivered by telephone. Addiction 2012;107:2149-58. http://dx.doi.org/10.1111/j.1360-0443.2012.03953.x.
- Grenyer BF, Solowij N, Peters R. Brief versus intensive psychotherapy for cannabis dependence. NIDA Research Monograph 1997;174.
- Kay-Lambkin FJ, Baker AL, Lewin TJ, Carr VJ. Computer-based psychological treatment for comorbid depression and problematic alcohol and/or cannabis use: a randomized controlled trial of clinical efficacy. Addiction 2009;104:378-88. http://dx.doi.org/10.1111/j.1360-0443.2008.02444.x.
- Kay-Lambkin FJ, Baker AL, Kelly B, Lewin TJ. Clinician-assisted computerised versus therapist-delivered treatment for depressive and addictive disorders: a randomised controlled trial. Med J Aust 2011;195:S44-50.
- Hoch E, Noack R, Henker J, Pixa A, Hofler M, Behrendt S, et al. Efficacy of a targeted cognitive–behavioral treatment program for cannabis use disorders (CANDIS). Eur Neuropsychopharmacol 2012;22:267-80. http://dx.doi.org/10.1016/j.euroneuro.2011.07.014.
- Hoch E, Buhringer G, Pixa A, Dittmer K, Henker J, Seifert A, et al. CANDIS treatment program for cannabis use disorders: Findings from a randomized multi-site translational trial. Drug Alcohol Depend 2014;134:185-93. http://dx.doi.org/10.1016/j.drugalcdep.2013.09.028.
- Tossmann HP, Jonas B, Tensil MD, Lang P, Strüber E. A controlled trial of an internet-based intervention program for cannabis users. Cyberpsychol Behav Social Network 2011;14:673-9. http://dx.doi.org/10.1089/cyber.2010.0506.
- Fernandes S, Ferigolo M, Benchaya MC, Moreira TC, Pierozan PS, Mazoni CG, et al. Brief Motivational Intervention and telemedicine: a new perspective of treatment to marijuana users. Addict Behav 2010;35:750-5. http://dx.doi.org/10.1016/j.addbeh.2010.03.001.
- Jungerman FS, Andreoni S, Laranjeira R. Short term impact of same intensity but different duration interventions for cannabis users. Drug Alcohol Depend 2007;90:120-7. http://dx.doi.org/10.1016/j.drugalcdep.2007.02.019.
- Fischer B, Dawe M, McGuire F, Shuper PA, Capler R, Bilsker D, et al. Feasibility and impact of brief interventions for frequent cannabis users in Canada. J Subst Abuse Treat 2013;44:132-8. http://dx.doi.org/10.1016/j.jsat.2012.03.006.
- Sobell LC, Sobell MB, Agrawal S. Randomized controlled trial of a cognitive-behavioral motivational intervention in a group versus individual format for substance use disorders. Psychology Addict Behav 2009;23:672-83. http://dx.doi.org/10.1037/a0016636.
- Bonsack C, Gibellini MS, Favrod J, Montagrin Y, Besson J, Bovet P, et al. Motivational intervention to reduce cannabis use in young people with psychosis: a randomized controlled trial. Psychother Psychosom 2011;80:287-97. http://dx.doi.org/10.1159/000323466.
- Gmel G, Gaume J, Bertholet N, Fluckiger J, Daeppen JB. Effectiveness of a brief integrative multiple substance use intervention among young men with and without booster sessions. J Subst Abuse Treat 2013;44:231-40. http://dx.doi.org/10.1016/j.jsat.2012.07.005.
- Hjorthoj CR, Fohlmann A, Larsen AM, Gluud C, Arendt M, Nordentoft M. Specialized psychosocial treatment plus treatment as usual (TAU) versus TAU for patients with cannabis use disorder and psychosis: the CapOpus randomized trial. Psychol Med 2013;43:1499-510. http://dx.doi.org/10.1017/S0033291712002255.
- Madigan K, Brennan D, Lawlor E, Turner N, Kinsella A, O’Connor JJ, et al. A multi-center, randomized controlled trial of a group psychological intervention for psychosis with comorbid cannabis dependence over the early course of illness. Schizophr Res 2013;143:138-42. http://dx.doi.org/10.1016/j.schres.2012.10.018.
- Rooke S, Copeland J, Norberg M, Hine D, McCambridge J. Effectiveness of a self-guided web-based cannabis treatment program: randomized controlled trial. J Med Internet Res 2013;15. http://dx.doi.org/10.2196/jmir.2256.
- Humeniuk R, Ali R, Babor T, Souza-Formigoni ML, de Lacerda RB, Ling W, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction 2012;107:957-66. http://dx.doi.org/10.1111/j.1360-0443.2011.03740.x.
- Litt MD, Kadden RM, Stephens RS. Marijuana Treatment Project Research Group . Coping and self-efficacy in marijuana treatment: results from the marijuana treatment project. J Consult Clin Psychol 2005;73:1015-25. http://dx.doi.org/10.1037/0022-006X.73.6.1015.
- ClinicalTrials.gov . Development and Efficacy Test of Computerized Treatment for Marijuana Dependence 2013. https://clinicaltrials.gov/show/NCT00594659 (accessed 6 February 2014).
- Moore BA, Budney AJ. Relapse in outpatient treatment for marijuana dependence. J Subst Abuse Treat 2003;25:85-9. http://dx.doi.org/10.1016/S0740-5472(03)00083-7.
- Fischer B, Jones W, Shuper P, Rehm J. 12-month follow-up of an exploratory ‘brief intervention’ for high-frequency cannabis users among Canadian university students. Subst Abuse Treat Prev Policy 2012;7. http://dx.doi.org/10.1186/1747-597X-7-15.
- Hoch E, Noack R, Henker J, Rohrbacher H, Pixa A, Buhringer G, et al. Tailoring CBT to problem profiles of patients with cannabis use disorders. Sucht 2008;54.
- Litt MD, Kadden RM, Kabela-Cormier E, Petry NM. Coping skills training and contingency management treatments for marijuana dependence: exploring mechanisms of behavior change. Addiction 2008;103:638-48. http://dx.doi.org/10.1111/j.1360-0443.2008.02137.x.
- Lozano BE, Stephens RS, Roffman RA. Abstinence and moderate use goals in the treatment of marijuana dependence. Addiction 2006;101:1589-97. http://dx.doi.org/10.1111/j.1360-0443.2006.01609.x.
- DeMarce JM, Stephens RS, Roffman RA. Psychological distress and marijuana use before and after treatment: testing cognitive–behavioral matching hypotheses. Addict Behav 2005;30:1055-9. http://dx.doi.org/10.1016/j.addbeh.2004.09.009.
- Hjorthoj C, Fohlmann A, Mette LA, Gluud C, Arendt M, Nordentoft M. CapOpus-intervention study for cannabis use disorder in psychosis. Early Interv Psychiatry 2012;6.
- Budney AJ, Fearer S, Walker DD, Stanger C, Thostenson J, Grabinski M, et al. An initial trial of a computerized behavioral intervention for cannabis use disorder. Drug Alcohol Depend 2011;115:74-9. http://dx.doi.org/10.1016/j.drugalcdep.2010.10.014.
- Kay-Lambkin FJ, Baker AL, Kelly BJ, Lewin TJ. It’s worth a try: The treatment experiences of rural and Urban participants in a randomized controlled trial of computerized psychological treatment for comorbid depression and alcohol/other drug use. J Dual Diagn 2012;8:262-76. http://dx.doi.org/10.1080/15504263.2012.723315.
- Gaudiano BA, Weinstock LM, Miller IW. Improving treatment adherence in patients with bipolar disorder and substance abuse: Rationale and initial development of a novel psychosocial approach. J Psychiatr Pract 2011;17:5-20. http://dx.doi.org/10.1097/01.pra.0000393840.18099.d6.
- Hides LM, Elkins KS, Scaffidi A, Cotton SM, Carroll S, Lubman DI, et al. Does the addition of integrated cognitive behaviour therapy and motivational interviewing improve the outcomes of standard care for young people with comorbid depression and substance misuse?. Med J Aust 2011;195:S31-7.
- Lykke J, Oestrich I, Austin SF, Hesse M. The implementation and evaluation of cognitive milieu therapy for dual diagnosis inpatients: A pragmatic clinical trial. J Dual Diagn 2010;6:58-72. http://dx.doi.org/10.1080/15504260903498763.
- McLellan AT. A randomized controlled trial of brief cognitive–behavioral interventions for cannabis use disorder. J Subst Abuse Treat 2001;21:65-6. http://dx.doi.org/10.1016/S0740-5472(01)00196-9.
- Miller WR. It all depends. Addiction 2008;103:1819-20. http://dx.doi.org/10.1111/j.1360-0443.2008.02359.x.
- Naar-King S. Motivational interviewing in adolescent treatment. Can J Psychiatry 2011;56:651-7.
- Nery FG, Soares JC. Comorbid bipolar disorder and substance abuse: Evidence-based options. Curr Psychiatry 2011;10:57-66.
- Pokhrel P, Sussman S, Rohrbach LA, Sun P. Prospective associations of social self-control with drug use among youth from regular and alternative high schools. Subst Abuse Treat Prev Policy 2007;2. http://dx.doi.org/10.1186/1747-597X-2-22.
- Riley KJ, Rieckmann T, McCarty D. Implementation of MET/CBT 5 for adolescents. J Behav Health Serv Res 2008;35:304-14. http://dx.doi.org/10.1007/s11414-008-9111-9.
- Hill KP, Toto LH, Lukas SE, Weiss RD, Trksak GH, Rodolico JM, et al. Cognitive behavioral therapy and the nicotine transdermal patch for dual nicotine and cannabis dependence: a pilot study. Am J Addict 2013;22:233-8. http://dx.doi.org/10.1111/j.1521-0391.2012.12007.x.
- Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 2013;27:19-27. http://dx.doi.org/10.1177/0269881112460109.
- Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study. Br J Psychiatry 2010;197:285-90. http://dx.doi.org/10.1192/bjp.bp.110.077503.
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2012;2. http://dx.doi.org/10.1038/tp.2012.15.
- Chagas MH, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther 2014;39:564-6. http://dx.doi.org/10.1111/jcpt.12179.
- The PRISMA Statement. n.d.
Appendix 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist
| Section/topic | Number | Checklist item | Reported on page number |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | xxi–xxiv |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 1–3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 5 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 7–10 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 7 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 93, 94 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 9 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 9 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 8 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 9, 10 |
| Summary measures | 13 | State the principal summary measures (e.g., RR, difference in means) | N/A |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | 9 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | N/A |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | 9 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 11 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | 11–20 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 20 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | 26–72 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | N/A |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | 21, 22 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]) | 71, 72 |
| Discussion | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., health-care providers, users, and policy makers) | 73 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 74, 75 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 77–79 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | iii |
Appendix 2 Literature search strategies
The following strategy was developed for use in MEDLINE. This strategy was subsequently translated in accordance with the other databases searched.
MEDLINE search strategy
-
Substance-Related Disorders/
-
(cannabis$ or marijuana or marihuana or hashish).ab,ti.
-
1 and 2
-
exp marijuana abuse/
-
((cannabis$ or marijuana or marihuana or hashish) adj2 (misuse or abuse$ or addict$ or depend$ or disorder$ or use$)).ab,ti.
-
or/3-5
-
((cannabis$ or marijuana or marihuana or hashish) adj3 (therap$ or treatment$)).ab,ti.
-
(cessation adj2 (therap$ or treat$)).ab,ti.
-
exp psychotherapy/
-
psychotherap$.ab,ti.
-
((psychodynamic or psychosocial) adj2 (therap$ or treatment$ or intervention$ or program$)).ab,ti.
-
exp Behavior Therapy/
-
((behavio$ or cognitive$) adj3 (therap$ or treatment$ or management or intervention$ or program$)).ab,ti.
-
cbt.ab,ti.
-
exp Counseling/
-
counsel$.ab,ti.
-
exp Mind-Body Therapies/
-
((relaxation or imagery) adj2 (therap$ or technique$)).ab,ti.
-
(guided adj2 imagery).ab,ti.
-
biofeedback.ab,ti.
-
(family adj2 therap$).ab,ti.
-
(motivation$ adj3 (therap$ or interview$)).ab,ti.
-
((case or contingency) adj2 (therap$ or management)).ab,ti.
-
((coping skill$ or cbst or self control or assertive$) adj2 (training or therap$)).ab,ti.
-
aversi$ therap$.ab,ti.
-
covert sensiti?ation.ab,ti.
-
or/7-26
-
6 and 27
-
meta-analysis as topic/
-
(meta analy$ or metaanaly$).tw.
-
Meta-Analysis/
-
(systematic adj (review$1 or overview$1)).tw.
-
“Review Literature as Topic”/
-
or/29-33
-
(cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or cinhal or science citation index or bids or cancerlit).ab.
-
((reference adj list$) or bibliograph$ or hand-search$ or (relevant adj journals) or (manual adj search$)).ab.
-
((selection adj criteria) or (data adj extraction)).ab.
-
“review”/
-
37 and 38
-
comment/ or editorial/ or letter/
-
Animals/
-
Humans/
-
41 not (41 and 42)
-
40 or 43
-
34 or 35 or 36 or 39
-
45 not 44
-
28 and 46
-
Randomized controlled trials as Topic/
-
Randomized controlled trial/
-
Random allocation/
-
randomized controlled trial.pt.
-
Double blind method/
-
Single blind method/
-
Clinical trial/
-
exp Clinical Trials as Topic/
-
controlled clinical trial.pt.
-
clinical trial$.pt.
-
multicenter study.pt.
-
or/48-58
-
(clinic$ adj25 trial$).ti,ab.
-
((singl$ or doubl$ or treb$ or tripl$) adj (blind$ or mask$)).tw.
-
Placebos/
-
Placebo$.tw.
-
randomly allocated.tw.
-
(allocated adj2 random).tw.
-
or/60-65
-
59 or 66
-
Case report.tw.
-
Letter/
-
Historical article/
-
68 or 69 or 70
-
exp Animals/
-
Humans/
-
72 not (72 and 73)
-
71 or 74
-
67 not 75
-
28 and 76
Appendix 3 Table of excluded studies with rationale
| Author and year | Reason for exclusion |
|---|---|
| Azrin NH, McMahon PT, Donohue B, Besalel VA, Lapinski KJ, Kogan ES, et al. Behavior therapy for drug abuse: a controlled treatment outcome study. Behav Res Ther 1994;32:857–66 | No relevant outcomes |
| Barrowclough C, Haddock G, Beardmore R, Conrod P, Craig T, Davies L, et al. Evaluating integrated MI and CBT for people with psychosis and substance misuse: Recruitment, retention and sample characteristics of the MIDAS trial. Addict Behav 2009;34:859–66 | No relevant outcomes |
| Barrowclough C, Haddock G, Wykes T, Beardmore R, Conrod P, Craig T, et al. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial. BMJ 2010;341:c6325 | No relevant outcomes |
| Barrowclough C, Lobbanl F, Warburton J, Choudhry I, Gregg L, Wood H, et al. HELPER ReCAP: Rethinking Choices after Psychosis – a phase-specific psychological therapy for people with problematic cannabis use following a first episode of psychosis. Early Interv Psychiatry 2010;4(Suppl. 1):161 | No relevant outcomes |
| Bellack AS, Bennett ME, Gearon JS, Brown CH, Yang Y, Bellack AS, et al. A randomized clinical trial of a new behavioral treatment for drug abuse in people with severe and persistent mental illness. Arch Gen Psychiatry 2006;63:426–32 | No relevant outcomes |
| Bernstein E, Edwards E, Dorfman D, Heeren T, Bliss C. Screening and brief intervention to reduce marijuana use among youth and young adults in a pediatric emergency department. Acad Emerg Med 2009;16:1174–85 | Not relevant population |
| Bond GR, McDonel EC, Miller LD, Pensec M. Assertive community treatment and reference groups: an evaluation of their effectiveness for young adults with serious mental illness and substance abuse problems. Psychosoc Rehabil J 1991;15:31–43 | No relevant outcomes |
| Brooks AJ, Penn PE. Comparing treatments for dual diagnosis: twelve-step and self-management and recovery training. Am J Drug Alcohol Abuse 2003;29:359–83 | No relevant outcomes |
| Brown TG, Seraganian P, Tremblay J, Annis H. Process and outcome changes with relapse prevention versus 12-step aftercare programs for substance abusers. Addiction 2002;97:677–89 | No relevant outcomes |
| Buckner JD, Carroll KM. Effect of anxiety on treatment presentation and outcome: results from the Marijuana Treatment Project. Psychiatry Res 2010;178:493–500 | No relevant outcomes |
| Budney AJ, Moore BA, Rocha H. Abstinence-based vouchers delivered without psychotherapy increase abstinence during treatment for marijuana dependence. Drug Alcohol Depend 2001;63(Suppl. 1):21 | No relevant outcomes |
| Campbell AN, Nunes EV, McClure EA, Hu MC, Turrigiano E, Goldman B, et al. Characteristics of an outpatient treatment sample by primary substance of abuse. J Addict Med 2013;7:363–71 | No relevant outcomes |
| Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, et al. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J Consult Clin Psychol 2006;74:955–66 | Not relevant population |
| Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, et al. Computer-assisted delivery of cognitive–behavioral therapy for addiction: A randomized trial of CBT4CBT. Am J Psychiatry 2008;165:881–8 | No relevant outcomes |
| Carroll KM, Nich C, Lapaglia DM, Peters EN, Easton CJ, Petry NM, et al. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction 2012;107:1650–9 | Not relevant population |
| ClinicalTrials.gov. Effectiveness of a Brief Intervention for Substances Consumption Linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): A Randomized Control Trial in Chilean Primary Care. 2013. URL: http://ClinicalTrials.gov/show/NCT01573416 (accessed 6 February 2014) | No relevant outcomes |
| ClinicalTrials.gov. Study Comparing Two Types of Psychotherapy for Treating Depression and Substance Abuse. 2009. URL: http://ClinicalTrials.gov/show/NCT00108407 (accessed 6 February 2014) | Not relevant intervention |
| ClinicalTrials.gov. Integrated CBT for Cannabis Dependence With Co-occurring Anxiety Disorders. 2013. URL: https://clinicaltrials.gov/show/NCT01875796 (accessed 6 February 2014) | No relevant outcomes |
| ClinicalTrials.gov. Maximizing the Efficacy of Cognitive Behavior Therapy and Contingency Management. 2011. URL: https://clinicaltrials.gov/ct2/show/NCT00350649 (accessed 6 February 2014) | Not relevant population |
| ClinicalTrials.gov. Effect of Motivational Therapy on Schizophrenia With Cannabis Misuse. 2013. URL: https://clinicaltrials.gov/show/NCT00798109 (accessed 6 February 2014) | No relevant outcomes |
| ClinicalTrials.gov. Adapted Cognitive/Affective Remediation for Cannabis Misuse in Schizophrenia. 2011. URL: https://clinicaltrials.gov/show/NCT01292577 (accessed 6 February 2014) | No relevant outcomes |
| ClinicalTrials.gov. Screening, Brief Intervention and Referral to Treatment for Substance Abuse in Mental Health Treatment Settings. 2013. URL: https://clinicaltrials.gov/show/NCT01883791 (accessed 6 February 2014) | No relevant outcomes |
| ClinicalTrials.gov. CANDIS – Targeted Treatment for Cannabis Disorders. 2007. URL: http://ClinicalTrials.gov/show/NCT00252980 (accessed 6 February 2014) | No relevant outcomes |
| ClinicalTrials.gov. CANDIS-II: Evaluation of the Cognitive-behavioural Treatment Programme CANDIS. 2009. URL: http://ClinicalTrials.gov/show/NCT00673647 (accessed 6 February 2014) | No relevant outcomes |
| ClinicalTrials.gov. Marijuana Treatment Project – 3. 2014. URL: http://ClinicalTrials.gov/show/NCT00107588 (accessed 6 February 2014) | No relevant outcomes |
| ClinicalTrials.gov. Specialized Addiction Treatment Versus Treatment as Usual for Young Patients With Cannabis Abuse and Psychosis. 2011. URL: https://clinicaltrials.gov/ct2/show/NCT00484302 (accessed 6 February 2014) | No relevant outcomes |
| ClinicalTrials.gov. INCA – Intervention and Neuropsychology in Cannabis Abuse. 2007. URL: https://clinicaltrials.gov/ct2/show/NCT00279604 (accessed 6 February 2014) | No relevant outcomes |
| ClinicalTrials.gov. A Brief Marijuana Intervention for Adolescent Women – 1. 2013. URL: https://clinicaltrials.gov/ct2/show/NCT00227864 (accessed 6 February 2014) | No relevant outcomes |
| Copeland J, Swift W, Rees V, Copeland J, Swift W, Rees V. Clinical profile of participants in a brief intervention program for cannabis use disorder. J Subst Abuse Treat 2001;20:45–52 | No relevant outcomes |
| Diamond GS, Liddle HA, Wintersteen MB, Dennis ML, Godley SH, Tims F, et al. Early therapeutic alliance as a predictor of treatment outcome for adolescent cannabis users in outpatient treatment. Am J Addict 2006;15(Suppl. 1):26–33 | Not relevant population |
| Drapkin ML. Tate SR, McQuaid JR, Brown SA. Does initial treatment focus influence outcomes for depressed substance abusers? J Subst Abuse Treat 2008;35:343–50 | No relevant outcomes |
| Favrod J. Motivational interventions: psychosis and cannabis. Encephale 2009;35:S209–13 | Not in English |
| Fohlmann AH, Hjorthoej C, Larsen A, Nordentoft M. CapOpus. Randomized clinical trial: specialized addiction treatment (MI & CBT) versus treatment as usual for young patients with cannabis abuse and psychosis. Early Interv Psychiatry 2010;4:160 | No relevant outcomes |
| Gaudiano BA, Weinstock LM, Miller IW. Improving treatment adherence in patients with bipolar disorder and substance abuse: rationale and initial development of a novel psychosocial approach. J Psychiatr Pract 2011;17:5–20 | Not a RCT |
| Godley MD, Godley SH, Dennis ML, Funk RR, Passetti LL, Petry NM, et al. A randomized trial of assertive continuing care and contingency management for adolescents with substance use disorders. J Consult Clin Psychol 2014;82:40–51 | Not relevant population |
| Godley MD, Godley SH, Dennis ML, Funk R, Passetti LL, Godley MD, et al. Preliminary outcomes from the assertive continuing care experiment for adolescents discharged from residential treatment. J Subst Abuse Treat 2002;23:21–32 | Not relevant population |
| Goti J, Diaz R, Serrano L, Gonzalez L, Calvo R, Gual A, et al. Brief intervention in substance-use among adolescent psychiatric patients: a randomized controlled trial. Eur Child Adoles Psychiatry 2010;19:503–11 | Not relevant population |
| Granholm E, Tate SR, Link PC, Lydecker KP, Cummins KM, McQuaid J, et al. Neuropsychological functioning and outcomes of treatment for co-occurring depression and substance use disorders. Am J Drug Alcohol Abuse 2011;37:240–9 | No relevant outcomes |
| Greenfield SF, Trucco EM, McHugh RK, Lincoln M, Gallop RJ, Greenfield SF, et al. The Women’s Recovery Group Study: a Stage I trial of women-focused group therapy for substance use disorders versus mixed-gender group drug counseling. Drug Alcohol Depend 2007;90:39–47 | No relevant outcomes |
| Hawkins JD, Catalano RF Jr, Gillmore MR, Wells EA, Hawkins JD, Catalano RFJ, et al. Skills training for drug abusers: generalization, maintenance, and effects on drug use. J Consult Clin Psychol 1989;57:559–63 | Not relevant population |
| Hendricks PS, Delucchi KL, Humfleet GL, Hall SM, Hendricks PS, Delucchi KL, et al. Alcohol and marijuana use in the context of tobacco dependence treatment: impact on outcome and mediation of effect. Nicotine Tob Res 2012;14:942–51 | No relevant outcomes |
| Hendriks V, van der Schee E, Blanken P, Hendriks V, van der Schee E, Blanken P. Treatment of adolescents with a cannabis use disorder: main findings of a randomized controlled trial comparing multidimensional family therapy and cognitive behavioral therapy in The Netherlands. Drug Alcohol Depend 2011;119:64–71 | Not relevant population |
| Hendriks VM, van der Schee E, Blanken P. Multidimensional family therapy and cognitive behavioral therapy in adolescents with a cannabis use disorder: a randomised controlled study. Tijdschrift voor Psychiatrie 2013; 55:747–59 | Not in English |
| Henggeler SW, Pickrel SG, Brondino MJ. Multisystemic treatment of substance-abusing and dependent delinquents: outcomes, treatment fidelity, and transportability. Ment Health Serv Res 1999;1:171–84 | Not relevant population |
| Hides LM, Elkins KS, Scaffidi A, Cotton SM, Carroll S, Lubman DI, et al. Does the addition of integrated cognitive behaviour therapy and motivational interviewing improve the outcomes of standard care for young people with comorbid depression and substance misuse? Med J Aust 2011;195:S31–7 | Not a RCT |
| Hides L, Carroll S, Scott R, Cotton S, Baker A, Lubman D, et al. Quik fix: a randomized controlled trial of an enhanced brief motivational interviewing intervention for alcohol/cannabis and psychological distress in young people. Psychother Psychosom 2013;82:122–4 | No relevant outcomes |
| Hill KP, Toto LH, Lukas SE, Weiss RD, Trksak GH, Rodolico JM, et al. Cognitive behavioral therapy and the nicotine transdermal patch for dual nicotine and cannabis dependence: a pilot study. Am J Addict 2013;22:233–8 | Not relevant intervention |
| Hjorthoj CR, Orlovska S, Fohlmann A, Nordentoft M. Psychiatric treatment following participation in the CapOpus randomized trial for patients with comorbid cannabis use disorder and psychosis. Schizophr Res 2013;151:191–6 | No relevant outcomes |
| James W, Preston NJ, Koh G, Spencer C, Kisely SR, Castle DJ. A group intervention which assists patients with dual diagnosis reduce their drug use: a randomised controlled trial. Psychol Med 2004;34:983–90 | No relevant outcomes |
| Jerrell JM, Ridgely MS. Comparative effectiveness of three approaches to serving people with severe mental illness and substance abuse disorders. J Nerv Ment Dis 1995;183:566–76 | No relevant outcomes |
| Johnson IS, Craig T, Hinton T, King M, Major B, Marston L. Randomised controlled trial of the clinical and cost-effectiveness of a contingency management intervention for reduction of cannabis use and of relapse in early psychosis (Project record). Health Technology Assessment Database 2012. URL: www.nets.nihr.ac.uk/projects/hta/0914450 (accessed 6 February 2014) | No relevant outcomes |
| Jonas B, Tossmann P, Tensil M, Leuschner F, Struber E. Efficacy of a single-session online-intervention on problematic substance use. Sucht 2012;58:173–82 | Not in English |
| Kadden RM, Litt MD, Dion KB. Increased alcohol use following treatment for marijuana dependence. Alcohol Clin Exp Res 2004;28:146A | No relevant outcomes |
| Kay-Lambkin FJ, Baker AL, Kelly BJ, Lewin TJ. It’s worth a try: the treatment experiences of rural and Urban participants in a randomized controlled trial of computerized psychological treatment for comorbid depression and alcohol/other drug use. J Dual Diagn 2012;8:262–76 | No relevant outcomes |
| Kemp R, Harris A, Vurel E, Sitharthan T. Stop Using Stuff: Trial of a drug and alcohol intervention for young people with comorbid mental illness and drug and alcohol problems. Australas Psychiatry 2007;15:490–3 | No relevant outcomes |
| Killeen TK, Upadhyana H, Mcrae A, Waldrop A, Brown C, Brady K. Contingency management for community treatment-seeking adolescents with marijuana use disorders. Proceedings of the 70th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2008 June 14–19; San Juan, Puerto Rico, USA 2008;95. URL: www.cpdd.vcu.edu/Pages/Meetings/CPDD08AbstractBook2.pdf (accessed 6 February 2014) | Not relevant population |
| Kleber HD, Weiss RD, Anton J, George TP, Greenfield SF, Kosten TR, et al. Treatment of patients with substance use disorders: Second edition. Am J Psychiatry 2006;163(Suppl. 8):1–81 | No relevant outcomes |
| Kuper LE, Gallop R, Greenfield SF. Changes in coping moderate substance abuse outcomes differentially across behavioral treatment modality. Am J Addict 2010;19:543–9 | No relevant outcomes |
| Latimer WW, Winters KC, D’Zurilla T, Nichols M. Integrated family and cognitive–behavioral therapy for adolescent substance abusers: a stage I efficacy study. Drug Alcohol Depend 2003;71:303–17 | Not relevant population |
| Lawlor E, Madigan K, Russell V, O’Connor JJ, Turner N, Clarke M, et al. Engagement with a group-based psychological intervention for those with early phase psychosis and concurrent use of cannabis. Early Interv Psychiatry 2012;6:28 | No relevant outcomes |
| Lehman AF, Herron JD, Schwartz RP, Myers CP. Rehabilitation for adults with severe mental illness and substance use disorders. A clinical trial. J Nerv Ment Dis 1993;181:86–90 | No relevant outcomes |
| Liddle HA, Dakof GA, Parker K, Diamond GS, Barrett K, Tejeda M, et al. Multidimensional family therapy for adolescent drug abuse: results of a randomized clinical trial. Am J Drug Alcohol Abuse 2001;27:651–88 | Not relevant population |
| Liddle HA, Dakof GA, Turner RM, Henderson CE, Greenbaum PE, Liddle HA, et al. Treating adolescent drug abuse: a randomized trial comparing multidimensional family therapy and cognitive behavior therapy. Addiction 2008;103:1660–70 | Not relevant population |
| Lykke J, Oestrich I, Austin SF, Hesse M. The implementation and evaluation of cognitive milieu therapy for dual diagnosis inpatients: a pragmatic clinical trial. J Dual Diagn 2010;6:58–72 | Not a RCT |
| Madigan K, Lawlor E, Brennan D, Turner N, Kinsella A, O’Connor JJ, et al. A multi-centre, randomised controlled trial of a group psychological intervention for psychosis with comorbid cannabis dependence over the early course of illness. Early Interv Psychiatry 2012;6:27 | Study characteristics or secondary analysis |
| Magill M, Barnett NP, Apodaca TR, Rohsenow DJ, Monti PM. The role of marijuana use in brief motivational intervention with young adult drinkers treated in an emergency department. J Stud Alcohol Drugs 2009;70:409–13 | Not relevant population |
| Mariani JJ, Cheng WY, Bisaga A, Sullivan M, Carpenter K, Nunes EV, et al. Comparison of clinical trial recruitment populations: treatment-seeking characteristics of opioid-, cocaine-, and cannabis-using participants. J Subst Abuse Treat 2011;40:426–30 | No relevant outcomes |
| Marsden J, Farrell M, Bradbury C, Dale-Perera A, Eastwood B, Roxburgh M, et al. Development of the Treatment Outcomes Profile. Addiction 2008;103:1450–60 | No relevant outcomes |
| Martin G, Copeland J. The adolescent cannabis check-up: randomized trial of a brief intervention for young cannabis users. J Subst Abuse Treat 2008;34:407–14 | Not relevant population |
| Martino S, Carroll KM, Nich C, Rounsaville BJ. A randomized controlled pilot study of motivational interviewing for patients with psychotic and drug use disorders. Addiction 2006;101:1479–92 | No relevant outcomes |
| McCambridge J, Strang J. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: results from a multi-site cluster randomized trial. Addiction 2004;99:39–52 | Not relevant population |
| McCambridge J, Strang J. Deterioration over time in effect of Motivational Interviewing in reducing drug consumption and related risk among young people. Addiction 2005;100:470–8 | Not relevant population |
| McCambridge J, Slym RL, Strang J. Randomized controlled trial of motivational interviewing compared with drug information and advice for early intervention among young cannabis users. Addiction 2008;103:1809–18 | Not relevant population |
| McCambridge J, Day M, Thomas BA, Strang J. Fidelity to Motivational Interviewing and subsequent cannabis cessation among adolescents. Addict Behav 2011;36:749–54 | Not relevant population |
| McCambridge J, Hunt C, Jenkins RJ, Strang J. Cluster randomised trial of the effectiveness of motivational interviewing for universal prevention. Drug Alcohol Depend 2011;114:177–84 | Not relevant population |
| McGillicuddy NB, Rychtarik RG, Duquette JA, Morsheimer ET. Development of a skill training program for parents of substance-abusing adolescents. J Subst Abuse Treat 2001;20:59–68 | Not relevant population |
| McLellan AT. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treat 2001;21:65–6 | Not a RCT |
| Miller WR. It all depends. Addiction 2008;103:1819–20 | Not a RCT |
| Miller WR, Yahne CE, Tonigan JS. Motivational interviewing in drug abuse services: a randomized trial. J Consult Clin Psychol 2003;71:754–63 | No relevant outcomes |
| Montgomery L, Petry NM, Carroll KM. Moderating effects of race in clinical trial participation and outcomes among marijuana-dependent young adults. Drug Alcohol Depend 2012;126:333–9 | No relevant outcomes |
| Moore BA, Budney AJ. Abstinence at intake for marijuana dependence treatment predicts response. Drug Alcohol Depend 2002;67:249–57 | No relevant outcomes |
| Morley KC, Sitharthan G, Haber PS, Tucker P, Sitharthan T. The efficacy of an opportunistic cognitive behavioral intervention package (OCB) on substance use and comorbid suicide risk: a multisite randomized controlled trial. J Consult Clin Psychol 2014;82:130–40 | No relevant outcomes |
| Murphy DA, Chen X, Naar-King S, Parsons JT, Adolescent TN. Alcohol and marijuana use outcomes in the Healthy Choices motivational interviewing intervention for HIV-positive youth. AIDS Patient Care Stds 2012;26:95–100 | Not relevant population |
| Nagel T, Robinson G, Condon J, Trauer T. Approach to treatment of mental illness and substance dependence in remote Indigenous communities: results of a mixed methods study. Aust J Rural Health 2009;17:174–82 | Not relevant population |
| Nery FG, Soares JC. Comorbid bipolar disorder and substance abuse: Evidence-based options. Current Psychiatry 2011;10:57–66 | Not a RCT |
| Nordentoft M, Hjorthoj C, Fohlmann A. Capopus trial: an observer-blinded RCT of specialized addiction treatment versus standard treatment for young patients with cannabis abuse and psychosis. Eur Psychiatry 2009;24:S1178 | No relevant outcomes |
| Nyamathi A, Branson C, Kennedy B, Salem B, Khalilifard F, Marfisee M, et al. Impact of nursing intervention on decreasing substances among homeless youth. Am J Addict 2012;21:558–65 | Not relevant intervention |
| Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomised trial with postpartum women. Am J Prev Med 2007;32:231–8. [Erratum published in Am J Prev Med 2007;32:549] | Not relevant population |
| Peters EN, Nich C, Carroll KM. Primary outcomes in two randomized controlled trials of treatments for cannabis use disorders. Drug Alcohol Depend 2011;118:408–16 | No relevant outcomes |
| Peters EN, Petry NM, Lapaglia DM, Reynolds B, Carroll KM. Delay discounting in adults receiving treatment for marijuana dependence. Exp Clin Psychopharmacol 2013;21:46–54 | No relevant outcomes |
| Peterson PL, Baer JS, Wells EA, Ginzler JA, Garrett SB. Short-term effects of a brief motivational intervention to reduce alcohol and drug risk among homeless adolescents. Psychol Addict Behav 2006;20:254–64 | Not relevant population |
| Phan O, Jouanne C, Monge S. A random clinical trial concerning the psychotherapy of adolescents addicted to cannabis. Ann Med Psychol (Paris) 2010;168:145–51 | No relevant outcomes |
| Pokhrel P, Sussman S, Rohrbach LA, Sun P. Prospective associations of social self-control with drug use among youth from regular and alternative high schools. Subst Abuse Treat Prev Policy 2007;2:22 | Not a RCT |
| Ramchand R, Griffin BA, Suttorp M, Harris KM, Morral A. Using a cross-study design to assess the efficacy of motivational enhancement therapy-cognitive behavioral therapy 5 (MET/CBT5) in treating adolescents with cannabis-related disorders. J Stud Alcohol Drugs 2011;72:380–9 | Not relevant population |
| Rees V, Copeland J, Swift W, Roffman R, Stephens R. Brief cognitive behavioral interventions for cannabis dependence. NIDA Research Monograph 1999;179:79 | No relevant outcomes |
| Riley KJ, Rieckmann T, McCarty D. Implementation of MET/CBT 5 for adolescents. J Behav Health Serv Res 2008;35:304–14 | Not a RCT |
| Roffman RA, Stephens RS, Simpson EE, Whitaker DL. Treatment of marijuana dependence: preliminary results. J Psychoactive Drugs 1988;20:129–37 | No relevant outcomes |
| Roffman RA, Klepsch R, Wertz JS, Simpson EE, Stephens RS. Predictors of attrition from an outpatient marijuana-dependence counseling program. Addict Behav 1993;18:553–66 | No relevant outcomes |
| Rooke SE, Gates PJ, Norberg MM, Copeland J. Applying technology to the treatment of cannabis use disorder: Comparing telephone versus Internet delivery using data from two completed trials. J Subst Abuse Treat 2014;46:78–84 | No relevant outcomes |
| Rowe C, Rigter H, Henderson C, Gantner A, Mos K, Nielsen P, et al. Implementation fidelity of Multidimensional Family Therapy in an international trial. J Subst Abuse Treat 2013;44:391–99 | Not relevant population |
| Ruehlmann A, Hoch E, Noack R, Henker J, Pixa A, Rohrbacher H, et al. Efficacy of the Manualized Cognitive-Behavioral Treatment Program Cannabis Use Disorders. Reno/Sparks, Nevada, NV: Proceedings of the 71th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2009. URL: www.cpdd.org/Pages/Meetings/CPDD09AbstractBook.pdf (accessed 6 February 2014) | Study characteristics or secondary analysis |
| Santisteban DA, Coatsworth JD, Perez-Vidal A, Kurtines WM, Schwartz SJ, LaPerriere A, et al. Efficacy of brief strategic family therapy in modifying Hispanic adolescent behavior problems and substance use. J Fam Psychol 2003;17:121–33 | Not relevant population |
| Santisteban DA, Mena MP, McCabe BE. Preliminary results for an adaptive family treatment for drug abuse in Hispanic youth. J Fam Psychol 2011;25:610–14 | Not relevant population |
| Shane P, Diamond GS, Mensinger JL, Shera D, Wintersteen MB. Impact of victimization on substance abuse treatment outcomes for adolescents in outpatient and residential substance abuse treatment. Am J Addict 2006;15(Suppl. 1):34–42 | Not relevant population |
| Smeerdijk M, Keet R, Dekker N, van RB, Krikke M, Koeter M, et al. Motivational interviewing and interaction skills training for parents to change cannabis use in young adults with recent-onset schizophrenia: a randomized controlled trial. Psychol Med 2012;42:1627–36 | Not relevant population |
| Smeerdijk M, Keet R, de HL, Barrowclough C, Linszen D, Schippers G. Feasibility of teaching motivational interviewing to parents of young adults with recent-onset schizophrenia and co-occurring cannabis use. J Subst Abuse Treat 2014;46:340–5 | Not relevant population |
| Spring B, Ferguson MJ. CALM technology-supported intervention: synopsis of evidence for an emerging class of practice tool. Transl Behav Med 2011;1:8–9 | Not relevant population |
| Stephens RS, Wertz JS, Roffman RA. Self-efficacy and marijuana cessation: a construct validity analysis. J Consult Clin Psychol 1995;63:1022–31 | No relevant outcomes |
| Stephens RS, Wertz JS, Roffman RA. Predictors of marijuana treatment outcomes: the role of self-efficacy. J Subst Abuse 1993;5:341–53 | No relevant outcomes |
| Stephens RS, Babor TF, Kadden R, Miller M, Marijuana Treatment Project Research Group. The Marijuana Treatment Project: rationale, design and participant characteristics. Addiction 2002;97(Suppl. 1):109–24 | No relevant outcomes |
| Strain EC. Single versus multiple drug focus in substance abuse clinical trials research: The devil is in the details. Drug Alcohol Depend 2003;70:131–4 | No relevant outcomes |
| Strang J, McCambridge J. Can the practitioner correctly predict outcome in motivational interviewing? J Subst Abuse Treat 2004;27:83–8 | No relevant outcomes |
| Stanger C, Budney AJ, Kamon JL. Contingency management for adolescent marijuana abuse. Proceedings of the 68th Annual Scientific Meeting of the College on Problems of Drug Dependence. Scottsdale, AZ; 2006 | Not relevant population |
| Tetzlaff BT, Kahn JH, Godley SH, Godley MD, Diamond GS, Funk RR, et al. Working alliance, treatment satisfaction, and patterns of posttreatment use among adolescent substance users. Psychol Addict Behav 2005;19:199–207 | Not relevant population |
| VanScoyoc J, Stanger C, Budney, Thostenson J. Disruptive behavior disorder influence response to contingency management among adolescent marijuana abusers. Proceedings of the 70th Annual Scientific Meeting of the College on Problems of Drug Dependence. San Juan, PR; 2008 | Not relevant intervention |
| Vendetti J, McRee B, Miller M, Christiansen K, Herrell J, Marijuana Treatment Project Research Group. Correlates of pre-treatment drop-out among persons with marijuana dependence. Addiction 2002;97(Suppl. 1):125–34 | No relevant outcomes |
| Waldron HB, Slesnick N, Brody JL, Turner CW, Peterson TR. Treatment outcomes for adolescent substance abuse at 4- and 7-month assessments. J Consult Clin Psychol 2001;69:802–13 | Not relevant population |
| Waldron HB, Turner CW, Ozechowski TJ. Profiles of drug use behavior change for adolescents in treatment. Addict Behav 2005;30:1775–96 | Not relevant population |
| Walker D, Stephens R, Rowland J, Roffman R. The influence of client behavior during motivational interviewing on marijuana treatment outcome. Addict Behav 2011;36:669–73 | No relevant outcomes |
| Werch CE, Bian H, Carlson JM, Moore MJ, Diclemente CC, Huang IC, et al. Brief integrative multiple behavior intervention effects and mediators for adolescents. J Behav Med 2011;34:3–12 | Not relevant population |
| White HR, Morgan TJ, Pugh LA, Celinska K, Labouvie EW, Pandina R, et al. Evaluating two brief substance-use interventions for mandated college students. J Stud Alcohol 2006;67:309–17 | Not relevant population |
| Winstock AR, Ford C, Witton J. Assessment and management of cannabis use disorders in primary care. BMJ 2010;30:800–4 | No relevant outcomes |
| Wittchen HU. Targeted cognitive–behavioral treatment for cannabis use disorders (CANDIS): efficacy, longterm stability, and efficiency. Eur Neuropsychopharmacol 2010;20:S206 | Study characteristics or secondary analysis |
| Worley MJ, Tate SR, Brown SA. Mediational relations between 12-Step attendance, depression and substance use in patients with comorbid substance dependence and major depression. Addiction 2012;107:1974–83 | Not relevant intervention |
| Wykes T. Cannabis use: Defining the targets for psychological treatment. Schizophr Bull 2011;37:285 | No relevant outcomes |
Appendix 4 Table of full data from included studies
| Author and year | Cannabis use (1) outcome measure, (2) absolute values (mean/median, SD/SE), (3) p-values (groups/baseline), (4) between group difference and CI | ||
|---|---|---|---|
| 4 months (post treatment) | 9 months | 15 months | |
| Babor 200439 and Litt 200572 |
|
|
|
| 15 weeks (post treatment) | 12 months | ||
| Baker 200652 |
|
|
|
| 3 months | 6 months (post treatment) | 12 months | |
| Bonsack 201166 |
|
|
|
| 14 weeks | |||
| Budney 200040 |
|
|
|
| 14 weeks (post treatment) | 12 months | ||
| Budney 200641 |
|
|
|
| 12 weeks (post treatment) | 9 months | ||
| Budney 201142 (abstract) and ClinicalTrials.gov 201373 |
|
|
|
|
|||
| 34 weeks (median) | |||
| Copeland 200153 |
|
||
| 1 month (post treatment) | 2 months | 3 months | |
| de Dios 201243 |
|
|
|
| 3 months (post treatment) | 6 months | ||
| Edwards 200654 |
|
|
|
| 6 months | |||
| Fernandes 201062 |
|
||
| 3 months (post treatment) | 12 months | ||
| Fischer 201275 and Fischer 201364 | Note: cannabis BI group is combination of brief MI and written cannabis information groups. Control group is combination of therapist general health MI-1 and written general health information | ||
|
|
||
| 4 weeks (post treatment) | 12 weeks | ||
| Gates 201255 | Both groups showed significant improvements from baseline on all outcomes (p < 0.001) at 4 and 12 weeks | ||
|
|
||
| 6 months | |||
| Gmel 201367 |
|
|
|
| 4 months | |||
| Grenyer 199756 |
|
|
|
| 4 months | 6 months (post treatment) | ||
| Hjorthoj 201368 Hjorthoj 201280 |
|
|
|
| 8–12 weeks (post treatment) | 3 months | 6 months | |
| Hoch 201259 and Hoch 200876 |
|
|
|
| 12 weeks (post treatment) | 6 months | ||
| Hoch 201460 |
|
|
|
| 3 months | |||
| Humeniuk 201271 |
|
||
| 4 months | |||
| Jungerman 200763 |
|
|
|
| 30 days (first episode of treatment after 30 days) | 14 months | ||
| Kadden 200744 Litt 200877 |
|
|
|
| 3 months (post treatment) | 12 months | ||
| Kay-Lambkin 200957 |
|
|
|
| 3 months | |||
| Kay-Lambkin 201158 |
|
||
| 3 months | 6 months | ||
| Lee 201045 |
|
|
|
| 3 months | 6 months | ||
| Lee 201346 |
|
|
|
| 2 months (post treatment) | 8 months | ||
| Litt 201347 |
|
|
|
| 3 months | 1 year | ||
| Madigan 201369 |
|
|
|
| 6 weeks (post treatment) | 3 months | ||
| Rooke 201370 |
|
|
|
| Post treatment (unknown time point) | 12 months | ||
| Sobell 200965 |
|
|
|
| 1 month (post treatment) | 3 months | 6 months | |
| Stein 201148 |
|
|
|
| 3 months | 12 months | ||
| Stephens 199449 |
|
|
|
| 1 month (post treatment) | 4 months | 16 months | |
| Stephens 200050 Lozano 200678 DeMarce 200579 |
|
|
|
| 7 weeks (post treatment) | 6 months | 12 months | |
| Stephens 200751 |
|
|
|
| 3 months | |||
| Tossmann 201161 |
|
||
| Author and year | Severity of dependence (1) outcome measure, (2) absolute values (mean/median, SD/SE), (3) p-values (groups/baseline), (4) between group difference and CI | |
|---|---|---|
| 4 months | 9 months | |
| Babor 200439 and Litt 200572 (secondary) |
|
|
| 14 weeks | ||
| Budney 200040 |
|
|
| 34 weeks (median) | ||
| Copeland 200153 |
|
|
| 3 months | 6 months | |
| Edwards 200654 |
|
|
| 4 weeks | 12 weeks | |
| Gates 201255 |
|
|
| 4 months | ||
| Grenyer 199756 |
|
|
| Post treatment | 6 months | |
| Hoch 201259 and Hoch 200876 |
|
|
| Post assessment | 6 months | |
| Hoch 201460 |
|
|
| 4 months | ||
| Jungerman 200763 |
|
|
|
||
| All follow-up points (2, 8 and 14 months) | ||
| Kadden 200744 and Litt 200877 |
|
|
| 6 weeks | 3 months | |
| Rooke 201370 |
|
|
| 4 months | 16 months | |
| Stephens 2000,50 Lozano 200678 and DeMarce 200579 |
|
|
| Author and year | Cannabis problems (1) outcome measure, (2) absolute values (mean/median, SD/SE), (3) p-values (groups/baseline), (4) between group difference and CI, (5) time point | ||
|---|---|---|---|
| 4 months | 9 months | ||
| Babor 200439 and Litt 200572 (secondary) |
|
|
|
| 14 weeks | |||
| Budney 200040 |
|
||
| 14 weeks (post treatment) | 12 months | ||
| Budney 200641 |
|
|
|
| 34 weeks (median) | |||
| Copeland 200153 |
|
||
| 4 weeks | 12 weeks | ||
| Gates 201255 |
|
|
|
| Post assessment | |||
| Hoch 201460 |
|
||
|
|||
|
|||
| 4 months | |||
| Jungerman 200763 |
|
||
|
|||
|
|||
|
|||
| 2 months (post treatment) and 14 months | |||
| Kadden 200744 and Litt 200877 |
|
||
| 3 months | 6 months | ||
| Lee 201045 |
|
|
|
| 3 months | 6 months | ||
| Lee 201346 |
|
|
|
| End of treatment | |||
| Litt 201347 |
|
||
| 3 months | 12 months | ||
| Stephens 199449 |
|
|
|
| 4 months | 16 months | ||
| Stephens 200050 Lozano 200678 DeMarce 200579 |
|
|
|
| 7 weeks | 6 months | 12 months | |
| Stephens 200751 |
|
|
|
| Author and year | Motivation to change (1) outcome measure, (2) absolute values (mean/median, SD/SE), (3) p-values (groups/baseline), (4) between group difference and CI | ||
|---|---|---|---|
| 3 months | 6 months | 12 months | |
| Bonsack 201166 |
|
|
|
| 14 weeks | |||
| Budney 200040 |
|
||
| 3 months | 6 months | ||
| Edwards 200654 |
|
|
|
| 2 months (post treatment) | 14 months | ||
| Kadden 200744 and Litt 200877 |
|
|
|
|
|||
| Post treatment | |||
| Litt 201347 |
|
||
| 7 weeks | |||
| Stephens 200751 |
|
||
| 3 months | |||
| Tossmann 201161 |
|
||
| Author and year | Attendance/compliance/dropout rates (1) outcome measure, (2) absolute values (mean/median, SD/SE), (3) p-values (groups/baseline), (4) between group difference and CI, (5) time point |
|---|---|
| Babor 200439 and Litt 200572 (secondary) |
|
|
|
| Baker 200652 | Cannabis subgroup: NR all participants:
|
| Bonsack 201166 |
|
| Budney 200040 |
|
|
|
| Budney 200641 |
|
|
|
| Copeland 200153 |
|
|
|
| de Dios 201243 |
|
| Edwards 200654 |
|
| Gates 201255 |
|
| Hjorthoj 201368 and Hjorthoj 201280 |
|
| Hoch 201259 and Hoch 200876 |
|
| Hoch 201460 |
|
| Humeniuk 201271 |
|
| Jungerman 200763 |
|
| Kadden 200744 and Litt 200877 |
|
| Kay-Lambkin 200957 |
|
|
|
| Kay-Lambkin 201158 |
|
|
|
| Lee 201346 |
|
|
|
| Litt 201347 and Litt 201347 |
|
|
|
| Madigan 201369 |
|
| Rooke 201370 |
|
| Stein 201148 |
|
| Stephens 199449 |
|
| Stephens 2000,50 Lozano 200678 and DeMarce 200579 |
|
|
|
|
|
| Stephens 200751 |
|
| Tossmann 201161 |
|
Appendix 5 Patient and public involvement: service user briefing document
Glossary
- Bipolar disorder
- A mental disorder characterised by episodes of elevated mood alternating with episodes of depression.
- Cannabis withdrawal syndrome
- Symptoms following cannabis withdrawal, including dysphoric mood, disturbed sleep and gastrointestinal symptoms.
- Case management
- A strategy to improve the co-ordination and continuity of the delivery of services to a patient.
- Cognitive–behavioural therapy
- A therapy that aims to change the way the participant thinks or behaves.
- Contingency management
- Providing clients with tangible rewards (such as monetary vouchers) to reinforce behaviour change (e.g. a reduction or cessation in drug taking).
- Dual diagnosis
- The condition of suffering from a mental illness and a substance abuse problem.
- Hyperemesis syndrome
- A disorder characterised by nausea, vomiting and abdominal pain, caused by regular cannabis use.
- Key working
- When a health professional works with the individual to ensure delivery and ongoing review of care being received.
- Major depressive disorder
- A mental disorder characterised by low mood, low self-esteem and loss of interest in normally enjoyable activities.
- Motivational enhancement therapy
- A variant of motivational interviewing that is manual based.
- Motivational interviewing
- A person-centred approach that aims to improve motivation to change and resolve ambivalence to change.
- Mutual aid therapy
- Therapy in which people with similar experiences assist each other to overcome or manage their issues (e.g. Self-Management and Recovery Training).
- Nicotine replacement therapy
- The remedial administration of nicotine to the body by means other than tobacco, to aid cessation of smoking tobacco.
- Psychosis disorder
- Generic psychiatric term for a mental state involving a loss of contact with reality.
- Relapse prevention
- Based on cognitive–behavioural therapy; enables clients to cope with high-risk situations that may lead to drug taking.
- Schizophrenia spectrum diagnosis
- Mental disorders with similar features to schizophrenia; may include hallucinations, delusions, motivational loss and withdrawal.
- Supportive–expressive dynamic psychotherapy
- Psychotherapy involving supportive techniques to put patient at ease and expressive techniques to help understand role of drugs in feelings/behaviours and other means of resolving problems.
List of abbreviations
- AO
- assessment only
- ASI
- Addiction Severity Index
- CBT
- cognitive–behavioural therapy
- CI
- confidence interval
- CPQ
- Cannabis Problems Questionnaire
- DSM
- Diagnostic and Statistical Manual of Mental Disorders
- DSM-5
- Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition
- DSM-III-R
- Diagnostic and Statistical Manual of Mental Disorders Three (revised)
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- HTA
- Health Technology Assessment
- ICD-10
- International Classification of Diseases, 10th Revision
- MET
- motivational enhancement therapy
- MI
- motivational interviewing
- MPS
- Marijuana Problems Scale
- NICE
- National Institute for Health and Care Excellence
- NRT
- nicotine replacement therapy
- PCT
- person-centred therapy
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCQ
- Readiness to Change Questionnaire
- RCT
- randomised controlled trial
- RR
- risk ratio
- ScHARR
- School of Health and Related Research
- ScHARR-TAG
- School of Health and Related Research Technology Assessment Group
- SD
- standard deviation
- SDS
- Severity of Dependence Scale
- TAU
- treatment as usual