Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 13/38/01. The protocol was agreed in August 2013. The assessment report began editorial review in February 2014 and was accepted for publication in August 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Whiting et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Objective
The overall objective of this project was to summarise the evidence on the clinical effectiveness and cost-effectiveness of viscoelastic (VE) devices to assist with the diagnosis, management and monitoring of haemostasis disorders during and after cardiac surgery, trauma-induced coagulopathy or post-partum haemorrhage (PPH). We defined the following research questions to address the review objective:
-
How do clinical outcomes differ among patients who are tested with VE devices during or after cardiac surgery compared with those who are not tested?
-
Where there were no data on one of more of the VE devices we evaluated the accuracy of that or those VE device(s) for the prediction of relevant clinical outcomes (e.g. transfusion requirement) during or after cardiac surgery.
-
-
How do clinical outcomes differ among patients with coagulopathy induced by trauma who are tested with VE devices compared with those who are not tested?
-
Where there were no data on one of more of the VE devices we evaluated the accuracy of that or those VE device(s) for the prediction of relevant clinical outcomes (e.g. transfusion requirement) in patients with trauma-induced coagulopathy.
-
-
How do clinical outcomes differ among patients with PPH who are tested with VE devices compared with those who are not tested?
-
Where there were no data on one of more of the VE devices we evaluated the accuracy of that or those VE device(s) for the prediction of relevant clinical outcomes (e.g. transfusion requirement) in patients with PPH.
-
-
What is the cost-effectiveness of VE devices during or after cardiac surgery?
-
What is the cost-effectiveness of VE devices in patients with trauma-induced coagulopathy?
-
What is the cost-effectiveness of VE devices in patients with trauma-induced PPH?
Chapter 2 Background and definition of the decision problem(s)
Population
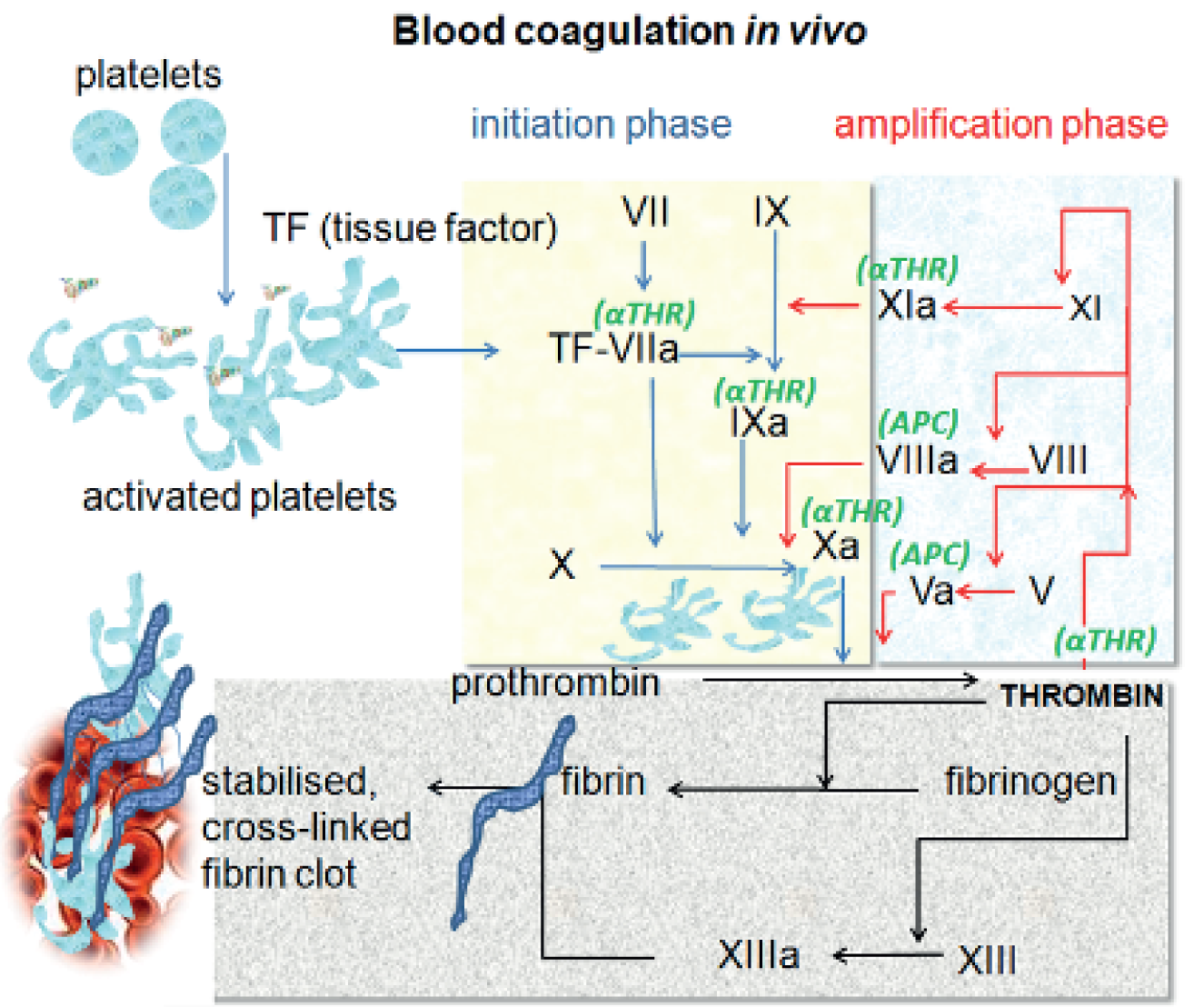
This assessment focuses on three patient groups at high risk of bleeding identified by the National Institute for Health and Care Excellence (NICE) as clinical priority areas: those undergoing cardiac surgery, those who have experienced trauma, and women with PPH. Patients undergoing cardiac surgery commonly present with bleeding complications, which can have a negative impact on their clinical outcome in terms of increased perioperative and post-operative morbidity and mortality. Bleeding can occur either as a result of the surgery/injury itself or because of acquired coagulation abnormalities as a result of the surgery, trauma or PPH. Coagulopathy occurs when the normal clotting mechanism (haemostasis) is interrupted, impairing the blood’s ability to clot. The normal clotting process starts with platelets which, combined with a number of clotting proteins, go through a series of steps to produce a solid fibrin clot (Figure 1). If any of these steps are interrupted this may result in prolonged or excessive bleeding. Although coagulopathy can be caused by genetic disorders such as haemophilia, it can also occur following injury, as occurs in perioperative or trauma-induced coagulopathy. The underlying mechanism of coagulopathy can include hyperfibrinolysis (markedly enhanced fibrinolytic activity), hypofibrinogenaemia [fibrogen (FIB) deficiency], thrombocytopenia (low levels of platelets), factor deficiency and heparin effect. 1 There are several factors that increase the risk of coagulopathy during surgery. In cardiac surgery, the use of heparin to prevent clotting while on cardiopulmonary bypass (CPB), pre-operative anticoagulation medication, the dilution, activation and consumption of coagulation factors, and the use of CPB machines – which may result in acquired platelet dysfunction, hypothermia (body temperature < 35 °C) – and hyperfibrinolysis are all associated with an increased risk of coagulopathy. 2 In major trauma, the following are associated with an increased risk of coagulopathy: consumption of coagulation factors and platelets during clot formation in an attempt to prevent loss of blood through damaged vessels; dilution of whole blood as a consequence of red cell transfusion; hormonal and cytokine-induced changes; hypoxia, acidosis and hypothermia, which predispose to further bleeding; and ongoing bleeding. 3 During pregnancy there are marked changes in haemostasis, with FIB deficiency thought to be the major coagulation abnormality associated with bleeding in PPH. 4
FIGURE 1.
Blood coagulation in vivo (https://commons.wikimedia.org/wiki/File:Coagulation_in_vivo.png). APC, activated protein C; αTHR, antithrombin; TF, tissue factor.
The populations at risk of bleeding for the patient groups considered in this assessment present a significant burden to the UK NHS. There were 36,702 cardiac surgery cases (based on Specialised Services National Definitions Set),5 based on Hospital Episode Statistics (HES) data. 6 There are approximately 20,000 major trauma cases in England every year,7 and injuries account for > 700,000 hospital admissions each year. 8 The incidence of major obstetric haemorrhage is 3.7/1000 births in the UK. 9
Patients with substantial bleeding usually require transfusion and/or re-operation. Cardiothoracic surgery (i.e. cardiac and thoracic surgery) uses 5% of all donated blood in the UK,10 and the proportion of patients requiring re-operation for bleeding is estimated at 2–8% of cardiac surgery patients. 11 Table 1 summarises the number of patients undergoing various cardiac surgeries in Scotland over a 2-year period, and shows the proportion of these patients who received a blood transfusion and the number of red blood cell (RBC) units per episode transfused. 12 The increased morbidity and mortality associated with bleeding following surgery has been shown to be related to both blood transfusion and re-operation for bleeding. 11 Patients with a diagnosis of trauma-induced coagulopathy on admission to hospital have a three- to fourfold greater mortality risk and it is independently associated with increased transfusion requirements, organ injury, septic complications and longer critical care stays. 3 Trauma is the leading cause of death and disability in adults aged < 36 years around the world,13 and haemorrhage is the cause of 40% of all trauma deaths in the UK. 14 PPH is one of the major causes of maternal mortality. There were 14 direct deaths from obstetric haemorrhage (nine from PPH) from 2006 to 2008, accounting for 9% of all maternal deaths in this period. 9
| Procedure | No. of episodes | % episodes transfused | RBC units/episode transfused |
|---|---|---|---|
| Coronary replacement operations (minus revisions) | 2359 | 47.9 | 1.6 |
| Heart and lung transplant | 8 | 75.0 | 11.3 |
| Revision coronary replacement operations | 29 | 44.8 | 2.1 |
| Valves and adjacent structures | 758 | 54.5 | 2.5 |
Red blood cell transfusion is independently associated with a greater risk of both infection (respiratory, wound infection or septicaemia) and ischaemic post-operative morbidity, hospital stay, increased early (30-day post operative) and late mortality (up to and > 1 year post operative) and hospital costs. 15 It is therefore important to appropriately treat the coagulopathy and reduce the blood loss thus reducing the requirement for blood transfusion and reducing the risks of transfusion-related adverse events and saving costs. 2 Knowledge of the exact cause of the bleed allows treatment to be tailored to the cause of the coagulopathy rather than replacing blood loss with transfusion. For example, if thrombocytopenia is identified as the cause of the bleed this can be treated by platelet transfusion. 16 Furthermore, the cost of donor blood has increased and availability has reduced and there is also the risk of blood-borne infection. 10
Intervention technologies
ROTEM delta point-of-care analyser
The ROTEM® Delta (TEM International GmbH, Munich, Germany; www.rotem.de) is a point-of-care (POC) analyser, which uses thromboelastometry, a VE method, to test for haemostasis in whole blood. It was previously known as rotational TEG or ROTEG. 6 It is performed near the patient during surgery or when admitted following trauma. It is used to assist with the diagnosis, management and monitoring of haemostasis disorders, during and after surgery, which are associated with high blood loss. It is an integrated all-in-one system and analyses the coagulation status of a blood sample to differentiate between surgical bleeding and a haemostasis disorder. 17 It uses a combination of five assays to characterise the coagulation profile of a citrated whole blood sample (Table 2). Initial screening is performed using the INTEM and EXTEM assays; if these are normal then it is an indication that surgical bleeding rather than coagulopathy is present. The use of different assays allows for rapid differential diagnosis between different haemostasis defects and anticoagulant drug effects. 17 Training in how to use the technology is required but specialist laboratory staff are not needed.
| Assay | Activator/inhibitor | Role |
|---|---|---|
| INTEM | Ellagic acid (contact activator) | Assessment of clot formation, fibrin polymerisation and fibrinolysis via the intrinsic pathway |
| EXTEM | Tissue factor | Assessment of clot formation, fibrin polymerisation and fibrinolysis via the extrinsic pathway. Not influenced by heparin. EXTEM is also the base activator for FIBTEM and ABTEM |
| HEPTEM | Ellagic acid + heparinase | Assessment of clot formation in heparinased patients. INTEM assay performed in the presence of heparinase; the difference between HEPTEM and INTEM confirms the presence of heparin |
| FIBTEM | Tissue factor + platelet antagonist | Assessment of FIB status allows detection of FIB deficiency or fibrin polymerisation disorders |
| APTEM | Tissue factor + fibrinolysis inhibitor (aprotonin) | In vitro fibrinolysis inhibition: fast detection of lysis when compared with EXTEM |
| Na-TEM | None | Non-activated assay. Can be used to run custom haemostasis tests |
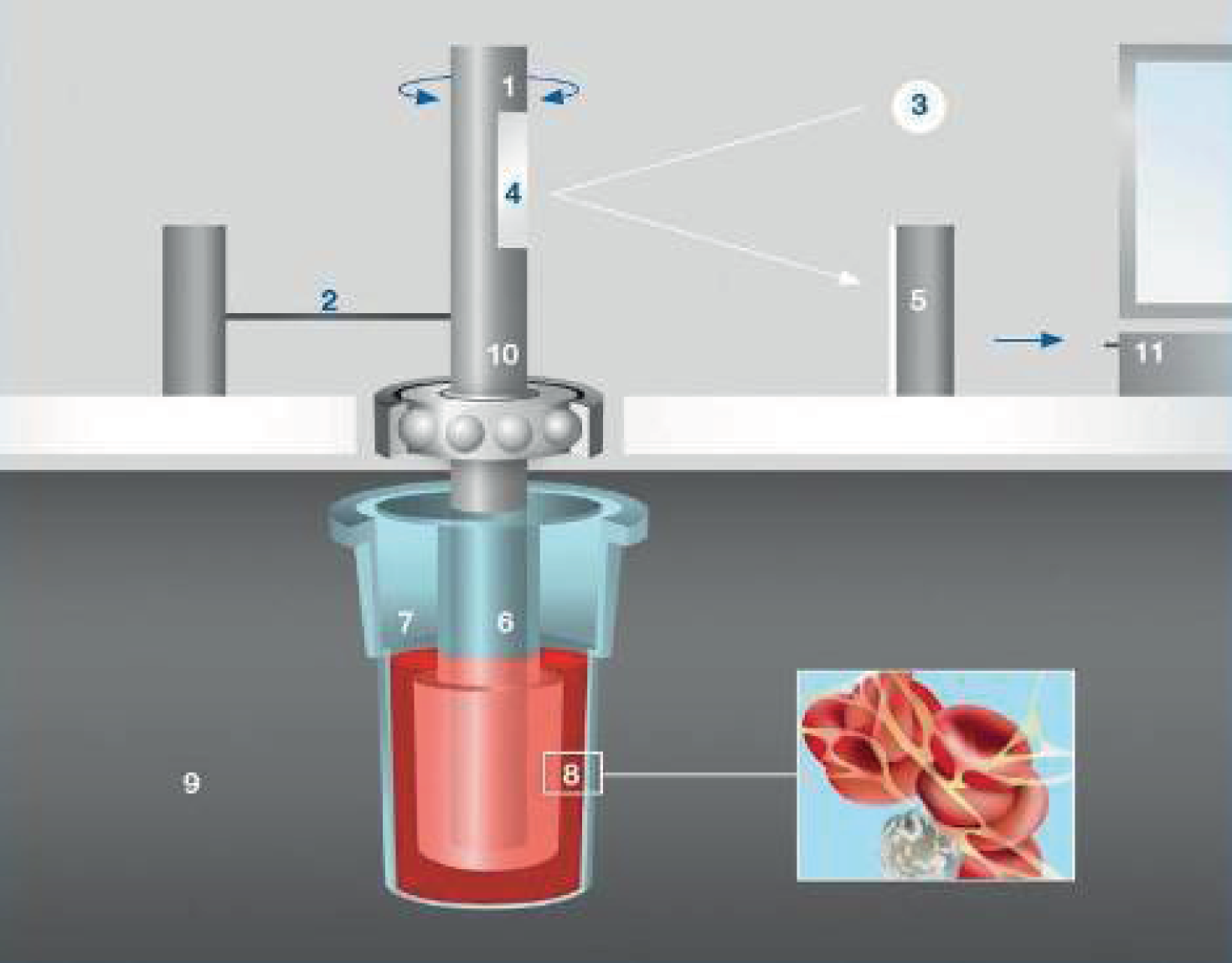
Figure 2 shows the ROTEM system. A 340-µl blood sample, which has been anticoagulated with citrate, is placed into the disposable cuvette (sample cup) (7), using an electronic pipette. A disposable sensor pin (6) is attached to the shaft, which is connected with a thin spring (2), and slowly oscillates back and forth (1), suspended in the blood sample. The signal from the pin is transmitted via an optical detector system (3–5). The test is started by adding the reagents described above. Although the typical test temperature is 37 °C, different temperatures can be selected, for example for patients with hypothermia. Although the blood remains liquid the movement is unrestricted, as the blood starts clotting, the clot restricts the rotation of the pin with increasing resistance as the firmness of the clot increases. This is measured by the ROTEM system and translated to the output, which consist of graphical displays and numerical parameters.
FIGURE 2.
ROTEM system. 18 1, Oscillating axis; 2, counterforce spring; 3, light beam from LED; 4, mirror; 5, detector (electronic camera); 6, sensor pin; 7, cuvette with blood sample; 8, fibrin strands and platelet aggregates; 9, heated cuvette holder; 10, ball bearing; and 11, data processing unit. Reproduced with permission from ROTEM®.
The graphical output of results produced by the ROTEM system is shown in Figure 3. A separate graphical display is produced for each reagent by an integrated computer. Numerical values for each of the following are also calculated and presented below the graph. Initial results are available within 5–10 minutes and full qualitative results are available in 20 minutes:
Clotting time (CT) – time from adding the start reagent until the blood starts to clot. A prolonged CT indicates abnormal clot formation.
Clot formation time (CFT) – time from CT until a clot firmness of 20-mm point has been reached and an α-angle, which is the angle of tangent between 2 and the curve. These measures indicate the speed at which the clot is forming and are mainly influenced by platelet function but are also affected by FIB and coagulation factors.
Amplitude 10 minutes after CT (A10) – used to predict maximum clot firmness (MCF) at an earlier stage and so allows earlier therapeutic decisions.
Maximum clot firmness – the greatest vertical amplitude of the trace. A low MCF value suggests decreased platelet numbers or function, decreased FIB levels of fibrin polymerisation disorders or low factor XIII activity.
Maximum lysis (ML) – fibrinolysis is detected by ML of > 15% or by better clot formation in APTEM compared with EXTEM.
Thromboelastography
The ROTEM system is a variant of the traditional thromboelastography (TEG) method developed by Hartert in 1948. 20 The two techniques are very similar, and other recent reviews have evaluated them as a single intervention class. 12,21,22 Like ROTEM, TEG is a VE method and provides a graphical representation of the clotting process. TEG is used in the TEG® 5000 analyser (Haemonetics Corporation, Niles, IL, USA; www.haemonetics.com). The rate of fibrin polymerisation and the overall clot strength is assessed. 1 Like ROTEM, TEG is able to provide an analysis of platelet function, coagulation proteases and inhibitors, and the fibrinolytic system within 30 minutes, or within 15 minutes if the rapid assay is used (Figure 4). The nomenclature used in TEG differs from that used in ROTEM; differences are summarised in Table 3. The practical differences between TEG and ROTEM are that TEG uses a torsion wire rather than the optical detector used in ROTEM to measure the clot formation, and, although the movement in ROTEM is initiated with the pin, with TEG it is initiated from the cuvette. 1 The assays used in TEG also differ (see Table 3). 24,25 The platelet mapping function means that TEG is able to measure platelet function, which cannot be assessed using ROTEM. Sample size requirements do not differ substantially between TEG and ROTEM; TEG uses a 360-µl blood sample compared with the 340-µl sample used in ROTEM. 25
| Assay | Activator/inhibitor | Role |
|---|---|---|
| Kaolin | Kaolin | Assessment of clot formation, fibrin polymerisation and fibrinolysis via the intrinsic pathway |
| Heparinase | Kaolin + heparinase | Assessment of clot formation in heparinased patients (both unfractionated and low molecular weight) |
| Platelet mapping | ADP arachidonic acid | To assess platelet function and monitor antiplatelet therapy (e.g. aspirin) |
| Rapid TEG | Kaolin + tissue factor | Extrinsic pathway test. Provides more rapid results than standard kaolin assay (mean 20 minutes vs. 30 minutes for standard TEG with initial results in < 1 minute) |
| Functional FIB assay | Lyophilised tissue factor + platelet inhibitor | Partitions clot strength (MA) into contributions from platelets and contribution from fibrin |
| Native | None | Non-activated assay. Can be used to run custom haemostasis tests |
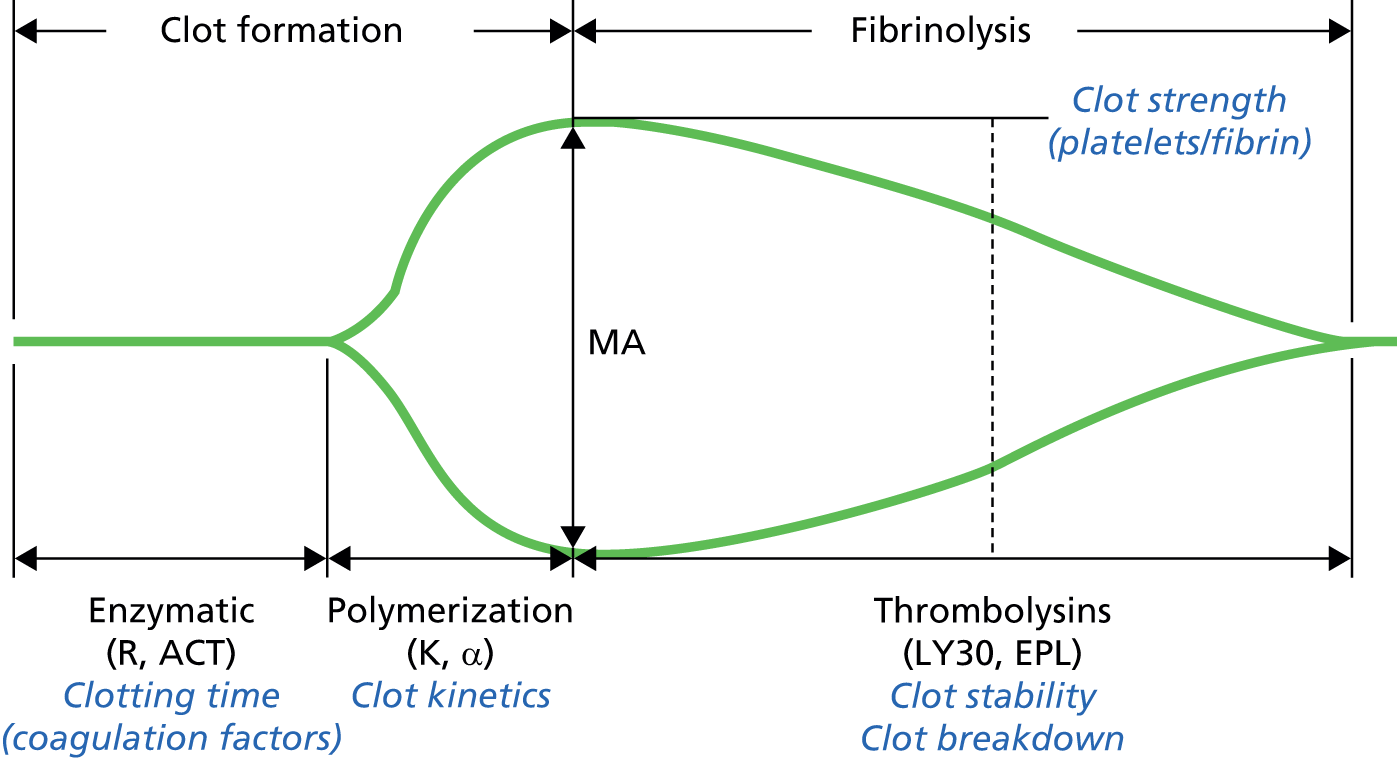
FIGURE 4.
Thromboelastography: analysis and interpretation of results. 23 ACT, activated clotting time; EPL, estimated per cent lysis; LY30, lysis at 30 minutes; MA, maximum altitude; R, clotting time. Reproduced with permission from TEG®.
Sonoclot coagulation and platelet function analyser
Another method that uses viscoelastometry to measure coagulation is the Sonoclot® coagulation and platelet function analyser (Sienco Inc., Arvada, CO, USA). This analyser was first introduced in 1975 by von Kualla et al. 26 It provides information on the haemostasis process, including coagulation, fibrin gel formation, fibrinolysis, and, like TEG, is also able to assess platelet function. The Sonoclot process is similar to ROTEM and TEG; although Sonoclot is able to use either a whole blood or plasma sample, citrated blood samples can be used but are not required. 27 A hollow, open-ended disposable plastic probe is mounted on the transducer head. The test sample (blood or plasma) is added to the cuvette containing the reagents. A similar volume to ROTEM and TEG is used: 330–360 µl. As with ROTEM, it is the probe that moves within the sample; however, rather than moving horizontally the probe moves up and down along the vertical axis. As the sample starts to clot, changes in impedance to movement are measured. Like TEG and ROTEM, Sonoclot produces a qualitative graphical display of the clotting process and also produces quantitative results of activated clotting time (ACT), the clot rate and the platelet function (Figure 5 and Table 4). 24 However, the measure of ACT produced by Sonoclot reflects initial fibrin formation whereas the equivalent measures produced by TEG and ROTEM reflects a more developed and later stage of initial clot formation. 24 Most information on clot formation is available after 15 minutes. If details on platelet function are required this may take up to 20–30 minutes. 27
| Assay | Activator/inhibitor | Role |
|---|---|---|
| SonACT | Celite | Large-dose heparin management without aprotonin |
| kACT | Kaolin | Large-dose heparin management with/without aprotonin |
| aiACT | Celite + clay | Large-dose heparin management with aprotonin |
| gbACT+ | Glass beads | Overall coagulation and platelet function assessment for use on non-heparinased patients |
| H-gbACT+ | Glass beads + heparinase | Overall coagulation and platelet function assessment in presence of heparin |
| Native | None | Non-activated assay. Can be used to run custom haemostasis tests |
FIGURE 5.
Sonoclot analysis and interpretation of results.

Comparison of viscoelastic testing devices
This report refers to the three technologies – ROTEM, TEG and Sonoclot – as a class as ‘viscoelastic testing POC coagulation testing devices’ or ‘VE devices’; however, data from each device are analysed separately. Table 5 provides an overview of the different terms used by each device to refer to the different test outputs. This table also summarises the factors affecting clot formation at each stage and the different therapeutic options.
| Development of clot | Factors affecting clot28 | Therapeutic options | ROTEM | TEG | Sonoclot |
|---|---|---|---|---|---|
| Measurement period | NA | NA | RT | – | – |
| Initial clot/fibrin formation | Factor XII and XI activity; reflective of intrinsic pathway if activators not used | Administration of plasma, coagulation factors, FIB or platelets | CT | R or ACT | ACT |
| Development of clot or rapidity of clot formation | Factor II and VIII activity; PLT and function, thrombin, FIB, HCT | CFT and α-angle (α) | Kinetics (k) and α-angle (α) | CR | |
| Maximum clot strength | FIB, platelet count and function, thrombin, factor XIII activity, HCT | MCF | MA | PEAK (peak amplitude) | |
| Time to maximum clot strength | MCF-t | TMA | Time to shoulder (P1); time to peak (P2); time from shoulder to peak (P2–P1) | ||
| Amplitude (at set time) | A5, A10 . . . | A (A5, A10 . . .) | |||
| Clot elasticity | MCE | G | – | ||
| ML | Fibrinolysis | Antifibrinolytic drugs and additional measures, such as administration of FIB or platelets | ML | – | R1, R2, R3 |
| CLR | – | ||||
| Lysis at fixed time | Lysis in 30, 45, 60 minutes (LY30, LY45, LY60) | Lysis in 30, 60 minutes (LY30, LY60) | |||
| Time to lysis | CLT (10% from MCF) | CLT ‘(2-mm drop from MA)’ instead of ‘TTL (2-mm drop from MA)’ | |||
| Platelet function | Platelet function | Platelets | _ | Platelet function | PF |
Platelet function tests
Viscoelastic tests are often performed in combination with platelet function tests in patients receiving antiplatelet drugs, such as aspirin and clopidogrel (Plavix®, Bristol-Myers Squibb and Sanofi-aventis). Although light transmission aggregometry in platelet-rich plasma is the gold standard test for platelet function, a number of rapid near-patient tests are available. 29 One of the most commonly used is the platelet function analyser (PFA) 100 (Dade-Behring, Marburg, Germany). 30 A more recently developed test, which is commonly used in combination with ROTEM, is the Multiplate® analyser (Roche, Rotkreuz, Switzerland), a near-patient test designed to detect platelet dysfunction. 31 It uses whole blood and is based on the principle of impedance platelet aggregometry. It has a turnaround time of 10 minutes and can process up to 30 tests per hour. As mentioned above, both TEG and Sonoclot can run specific platelet mapping assays – the TEG platelet mapping assay and glass bead-activated test (gbACT)+ assay for Sonoclot. However, some centres prefer to use a separate platelet function test, such as the Multiplate analyser instead of these assays. Tem International GmbH, the manufacturer of ROTEM, has recently introduced a new platelet module that is run in conjunction with the ROTEM delta. It measures platelet aggregation in whole blood samples using impedance aggregometry.
Comparator: standard laboratory tests for coagulopathy
The comparator for this technology appraisal is a combination of clinical judgement and standard laboratory tests (SLTs). Standard laboratory coagulation analyses include the following:
Prothrombin time (PT) – also used to derive the measures prothrombin ratio (PR) and international normalised ratio (INR). A measure of the extrinsic pathway of coagulation. The PT is the time it takes plasma to clot after the addition of tissue factor. The PR is the PT for a patient, divided by the result for control plasma. The INR is the ratio of a patient’s PT to a normal (control sample) raised to the power of the international sensitivity index (ISI) value for the analytical system used. The ISI value indicates how a particular batch of tissue factor compares to an international reference tissue factor.
Activated partial thromboplastin time (aPTT) – measures the ‘intrinsic’ or contact activation pathway and the common coagulation pathway. An activated matrix (e.g. silica, celite, kaolin, ellagic acid) and calcium are mixed into the plasma sample, and the time the sample takes to clot is measured.
Activated clotting/coagulation time – based on ability of whole blood to form a visible fibrin monomer in a glass tube. Used to measure heparin anticoagulation.
Platelet count (PLT) – in general, a low PLT is associated with an increased risk of bleeding. It is a purely quantitative measure and cannot detect pre-existing, drug-induced or perioperatively acquired platelet dysfunction. 2
Plasma FIB concentration – a number of assays are available to assess plasma FIB levels. The Clauss fibrinogen assay is the most common and is based on the thrombin CT. Diluted plasma is clotted with a high concentration of thrombin at 37 °C and the CT is measured. The result is compared with a calibration curve prepared by clotting a series of dilutions of a reference plasma sample of known FIB concentration to give a result in grams per litre. Most laboratories use an automated method in which clot formation is considered to have occurred when the optical density of the mixture has exceeded a certain threshold. 32
These tests have a number of limitations for prediction and detection of perioperative coagulopathy, as they were not developed to predict bleeding or guide coagulation management in a surgical setting. They are performed at a standardised temperature of 37 °C, which limits the detection of coagulopathies induced by hypothermia. 1 The aPTT and INR tests affect only the initial formation of thrombin in plasma without the presence of platelets or other blood cells. These tests are also not able to provide any information regarding clot formation over time or on fibrinolysis and so they cannot detect hyperfibrinolysis. They generally take between 40 and 90 minutes from taking the blood sample to give a result; this turnaround time may be so long that it does not reflect the current state of the coagulation system when the results are reported. 2
Care pathway
Current care pathway
The exact care pathway and use of SLTs before, during and after surgery will vary according to the specific type of surgery. Some centres routinely screen all patients pre operatively for coagulation disorders using SLTs, such as the PT and aPTT tests. 33 However, UK guidelines published in 200834 do not recommend routine coagulation tests to predict perioperative bleeding risk in unselected patients before surgery. Instead, pre-operative testing should only be considered in patients at risk of a bleeding disorder, for example those with liver disease, family history of inherited bleeding disorder, sepsis, diffuse intravascular coagulation, pre-eclampsia, cholestasis and those at risk of vitamin K deficiency. 33
It is generally recommended that patients stop taking anticoagulant medications (clopidogrel warfarin and aspirin) a number of days before surgery to reduce the risk of bleeding during surgery. 10,35 In the event of emergency surgery, this may not be possible, in which case coagulation testing should be performed. 33 If the surgery involves CPB then heparin may be administered prophylactically to reduce the risk of clotting while on CPB. 35 It is essential to monitor heparin anticoagulation if this has been administered. An initial ACT test should be performed after the first surgical incision and be repeated at regular intervals during surgery. 36 Standard coagulation tests (PLT, FIB concentration, PT, aPTT) are most commonly used to assess the coagulation status of patients who are experiencing high blood loss during surgery. However, these generally take too long to give a result that can inform treatment decisions. Instead, decisions on how to treat the bleed have to be based largely on clinical judgement. The same tests are used after surgery to monitor coagulation status.
If bleeding occurs, surgical intervention may be needed or packed RBCs are transfused if required. This is generally to maintain a haemoglobin concentration of > 6 g/dl during CPB and 8 g/dl after CPB or according to other requirements as indicated by national guidelines. Other therapeutic options depending on laboratory test results include FIB concentrate (bleeding patients with abnormal FIB), fresh frozen plasma (FFP) (if after transfusion of packed erythrocytes new laboratory results were not available and/or bleeding did not stop after FIB administration), prothrombin complex concentrate (PCC) (abnormal aPTT), antithrombin concentrate (when ACT analyses not controlled by heparin alone), desmopressin (suspected platelet dysfunction), platelet concentrates (low PLT),35 cryoprecipitate, antifibrinolytic drugs and tranexamic acid. If bleeding continues despite these treatments then additional treatment options include factor XIII concentrate and activated recombinant factor VII or factor VIIa, although not licensed for use in the UK. 10,35 Heparin dose adjustments may be made to try and control the bleeding.
Role of viscoelastic testing in the care pathway
Viscoelastic testing can be repeatedly performed during and after surgery and so can provide a dynamic picture of the coagulation process during these periods. The role of VE testing in the care pathway is unclear. It could be used either as an add-on test, in which case it would be performed as well as SLTs, or it could be as replacement test in which case SLTs would no longer be needed.
If VE testing does not prevent the need for SLTs and provides complementary findings then it should be performed in addition to any laboratory coagulation tests already recommended for specific populations. However, if the SLTs do not offer any supplementary information to that provided by VE testing then there should no longer be a need for standard tests and VE testing should replace some or all of the SLTs. VE tests offer two key potential benefits over SLTs: the shorter timescale in which they are able to provide results and the additional information on the clotting process that they offer compared with standard tests. It is hypothesised that by providing additional information and quicker results requirements for blood components/products could be targeted and so the patient is not subjected to risks associated with unnecessary transfusion. Time in theatre, resource use, length of stay (LoS) in a critical care unit, length of hospital stay, blood component/product usage and the associated costs may therefore be reduced.
Chapter 3 Assessment of clinical effectiveness
A systematic review was conducted to summarise the evidence on the clinical effectiveness of VE POC testing to assist with the diagnosis, management and monitoring of haemostasis. Systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care37 and NICE Diagnostic Assessment Programme manual. 38 We developed a protocol for the review and the protocol was registered on the PROSPERO database (CRD42013005623).
Systematic review methods
Search strategy
Search strategies were based on index test (ROTEM delta, TEG and Sonoclot), as recommended in the CRD guidance for undertaking reviews in health care37 and the Cochrane Handbook for Diagnostic Test Accuracy Reviews. 39
Candidate search terms were identified from target references, browsing database thesauri [e.g. MEDLINE (MeSH) and EMBASE (EMTREE)], existing reviews identified during the rapid appraisal process and initial scoping searches. These scoping searches were used to generate test sets of target references, which informed text mining analysis of high-frequency subject-indexing terms using EndNote X4 reference management software (Thomson Reuters, CA, USA). Strategy development involved an iterative approach testing candidate text and indexing terms across a sample of bibliographic databases and aimed to reach a satisfactory balance of sensitivity and specificity.
Search strategies were developed specifically for each database, and the keywords associated with ROTEM, TEG, thromboelastometry and Sonoclot were adapted according to the configuration of each database.
Primary clinical effectiveness searches
Primary searches were undertaken for randomised controlled trials (RCTs) in TEG, thromboelastometry and Sonoclot, and these searches were limited with an objectively derived study design filter, where appropriate.
The following databases were searched for relevant studies from inception to December 2013:
-
MEDLINE (OvidSP): 1946–September week 3 2013
-
MEDLINE In-Process & Other Non-Indexed Citations and Daily Update (OvidSP): up to 26 September 2013
-
EMBASE (OvidSP): 1974–30 September 2013
-
BIOSIS Previews (Web of Knowledge): 1956–26 September 2013
-
Science Citation Index (SCI) (Web of Science): 1970–26 September 2013
-
Conference Proceedings Citation Index (CPCI-S) (Web of Science): 1990–26 September 2013
-
Cochrane Database of Systematic Reviews (CDSR) (Internet): Issue 10, October 2013
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Internet): Issue 10, October 2013
-
Database of Abstracts of Reviews of Effects (DARE) (Internet): Issue 4, October 2013
-
Health Technology Assessment (HTA) Database (Internet): Issue 4, October 2013
-
Latin American and Caribbean Health Sciences Literature (LILACS) (Internet): http://regional.bvsalud.org/php/index.php?lang=en
-
International Network of Agencies for Health Technology Assessment (INAHTA): up to 27 September 2013, www.inahta.org/
-
NIHR HTA Programme (Internet): up to 27 September 2013
-
Aggressive Research Intelligence Facility (ARIF) (Internet): 1996–27 September 2013, www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/index.aspx
-
Medion (Internet): up to 27 September 2013, www.mediondatabase.nl/
-
International Prospective Register of Systematic Reviews (PROSPERO) (Internet): up to 27 September 2013, www.crd.york.ac.uk/prospero/
Completed and ongoing trials were identified by searches of the following resources:
-
National Institutes of Health ClinicalTrials.gov (Internet): up to 27 September 2013, www.clinicaltrials.gov/
-
metaRegister of Current Controlled Trials (mRCT) (Internet): up to 27 September 2013, www.controlled-trials.com/
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (Internet): up to 26 September 2013, www.who.int/ictrp/en/
Electronic searches were undertaken for the following conference abstracts:
-
International Society on Thrombosis and Haemostasis (ISTH) (Internet): 2009, 2011, www.isth.org/?PastMeetings
-
American Society of Anesthetists (ASA) (Internet): 2009–13, www.asaabstracts.com/strands/asaabstracts/search.htm;jsessionid=FF1E2F6EA4FF34468F5594FA255F3423
-
European Association of Cardiothoracic Anaesthesiologists (EACTA) (Internet): 2009–13
-
2013: www.applied-cardiopulmonary-pathophysiology.com/acp-2–2013.html
-
2012: www.applied-cardiopulmonary-pathophysiology.com/acp-supp1–2012.html
-
2011: Searched via publisher’s website
-
2010: www.applied-cardiopulmonary-pathophysiology.com/fileadmin/downloads/acp-2010–1/10_abstracts.pdf
-
Viscoelastic testing in post-partum haemorrhage and trauma
A second series of focused searches were undertaken without a study design filter to identify relevant references reporting TEG, thromboelastometry and Sonoclot in PPH or trauma response.
The following databases were searched for relevant studies from inception to December 2013:
-
MEDLINE (OvidSP): 1946–September 2013 week 3
-
MEDLINE In-Process & Other Non-Indexed Citations and Daily Update (OvidSP): up to 26 September 2013
-
EMBASE (OvidSP): 1974–5 November 2013.
No restrictions on language or publication status were applied. All search strategies are presented in Appendix 1. The main EMBASE strategy for each search was independently peer reviewed by a second information specialist, using the Canadian Agency for Drugs and Technologies (CADTH) Peer Review Checklist. 40 Identified references were downloaded in EndNote X4 software for further assessment and handling. References in retrieved articles and the websites set up by the manufacturers of ROTEM delta and Sonoclot were also screened for additional references. The manufacturers of ROTEM and Sonoclot, and clinical experts, submitted references of relevant publications for consideration for inclusion in the review. The final list of included papers was checked on PubMed for retractions, errata and related citations. 41–43
Inclusion and exclusion criteria
Inclusion criteria for each of the three clinical review questions are summarised in Table 6. Studies that fulfilled these criteria were eligible for inclusion in the review.
| Question | ||||||
|---|---|---|---|---|---|---|
| 1. Clinical outcomes in cardiac surgery | Prediction in cardiac surgery | 2. Clinical outcomes in trauma-induced coagulopathy | 2a. Prediction in trauma-induced coagulopathy | 3. Clinical outcomes in PPH | 3a. Prediction in PPH | |
| Participants | Adult (age ≥ 18 years) patients undergoing cardiac surgery | Adult (age ≥ 18 years) with clinically suspected coagulopathy induced by trauma | Women with PPH | |||
| Index test | VE devices (ROTEM, TEG or Sonoclot) alone or combined with platelet testing (e.g. Multiplate test) or SLTs | VE devices (ROTEM, TEG or Sonoclot) | VE devices (ROTEM, TEG or Sonoclot) or SLTs | VE devices (ROTEM, TEG or Sonoclot) | VE devices (ROTEM, TEG or Sonoclot) or SLTs | VE devices (ROTEM, TEG or Sonoclot) |
| Comparators | No testing, SLTs or other VE device | Any other VE device or none | No testing, SLTs or other VE device | Any other VE device or none | No testing SLTs or other VE device | Any other VE device or none |
| Reference standard | NA | Patient-relevant outcomes, e.g. transfusion, bleeding | NA | Patient-relevant outcomes, e.g. transfusion, bleeding | NA | Patient-relevant outcomes, e.g. transfusion, bleeding |
| Outcomes | Any reported outcomes. We anticipate that outcomes will include post-operative mortality, bleeding and transfusion outcomes, complications and re-intervention outcomes | Sufficient data to construct a 2 × 2 table of test performance | Any reported outcomes. We anticipate that outcomes will include post-operative mortality, bleeding and transfusion outcomes, complications and re-intervention outcomes | Sufficient data to construct a 2 × 2 table of test performance or prediction model data | Any reported outcomes. We anticipate that outcomes will include post-operative mortality, bleeding and transfusion outcomes, complications and re-intervention outcomes | Sufficient data to construct a 2 × 2 table of test performance or prediction model data |
| Study design | RCTsa | Diagnostic cohort studies/prediction studies | RCTsa | Diagnostic cohort/prediction studies | RCTsa | Diagnostic cohort/prediction studies |
Inclusion screening and data extraction
Two reviewers (MW and PW) independently screened the titles and abstracts of all reports identified by searches, and any discrepancies were discussed and resolved by consensus. Full copies of all studies that were deemed to be potentially relevant were obtained and the same two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus. Details of studies excluded at the full-paper screening stage are presented in Appendix 4.
Studies cited in materials provided by the manufacturers of ROTEM, TEG or Sonoclot were first checked against the project reference database, in EndNote X4; any studies not already identified by our searches were screened for inclusion following the process described above.
Data were extracted on the following: participant characteristics; study design; inclusion and exclusion criteria; details of VE test and/or test parameters evaluated; details of SLTs, where applicable; details of outcomes assessed [main outcomes were bleeding outcomes, transfusion outcomes, hospital/intensive care unit (ICU) stay, re-operation and mortality]; results. Data were extracted by one reviewer, using a piloted, standard data extraction form and checked by a second (MW and PW); any disagreements were resolved by consensus. Full data extraction tables are provided in Appendix 2.
Quality assessment
The methodological quality of included RCTs was assessed using the Cochrane Risk of Bias Tool. 44 Prediction studies were assessed for methodological quality using QUADAS-2. 45 Risk of bias assessments were undertaken by one reviewer and checked by a second reviewer (MW and PW), and any disagreements were resolved by consensus.
The results of the risk of bias assessments are summarised and presented in tables and graphs in the results of the systematic review, and are presented in full, by study, in Appendix 3.
Methods of analysis/synthesis
We provided a narrative synthesis involving the use of text and tables to summarise data to show differences in study designs, population, VE device and potential sources of bias for each of the studies being reviewed. Studies were organised by research question addressed (study population), outcome and VE device. Where possible, meta-analysis was used to derive summary effect estimates. All meta-analyses were performed using the MetaExcel (Epigear International) add on for Microsoft Excel version 1997–2003 (Microsoft Corporation, Redmond, WA, USA).
Randomised controlled trials comparing viscoelastic testing with no testing
Meta-analysis was used to estimate summary effect sizes for outcomes evaluated in multiple studies for which sufficient data were reported. Data were reported only in an appropriate format to permit pooling for dichotomous data. Summary relative risks (RRs), together with 95% confidence intervals (CIs) were estimated using DerSimonian and Laird random-effects models. Heterogeneity was investigated visually using forest plots and statistically using the I2- and Q-statistics. Data were pooled for all VE devices combined and stratified according to VE device; if no difference based on VE device was found then a summary estimate was calculated comparing VE testing irrespective of VE device to no testing. Where multiple sets of data were reported for the same outcome for a single study, for example pre-operative, post-operative and total number of patients transfused, a single data set was selected. The data set relating to the largest number of participants or latest time point was selected.
For continuous outcomes, data were not reported in a sufficiently similar format to permit pooling. Only a small number of studies reported data as means and standard deviations (SDs) or CIs, which would have allowed calculations of mean differences, and there were insufficient studies reporting data in this format to pool data. Most studies reported data as medians [some with interquartile ranges (IQRs)] and some reported p-values for comparisons of the differences between medians, usually estimated using the Mann–Whitney or Wilcoxon rank sum tests. Some studies reported only medians, with no measure of distribution around the median or estimation of the significance of the difference between groups. We summarised the results for continuous outcomes in a table showing the measure of effect reported in the study (mean or median with associated SD, CI, IQR or range), the effect estimate in the VE testing and in the control group, and any reported p-value for the comparison between the two groups.
Prediction studies
Prediction studies provided data in a variety of formats:
-
Logistic regression models for the association of the VE test parameter and the outcome (reference standard) under investigation, adjusted for a range of other variables. From these studies, we selected the adjusted odds ratio (OR) and associated 95% CI as the measure to use in the analysis.
-
Crude (unadjusted) ORs with associated 95% CIs for the association of the VE test parameter and the outcome (reference standard) under investigation. We selected these as the measure to use in the analysis.
-
Two-by-two data for the association of the VE test parameter (index test) with the outcome (reference standard) under investigation. We used these data to calculate crude ORs and associated 95% CIs.
-
Sensitivity and specificity data for the VE test parameter for the prediction of the outcome (reference standard) under investigation. If these studies also reported data on the number of participants with and without the outcome, these data were used to calculate a 2 × 2 table from which ORs were derived, as described above. If this information was not provided then sensitivity and specificity were used to calculate ORs; for these studies it was not possible to calculate associated CIs.
-
Area under the [receiver operating characteristic (ROC)] curve (AUC) for the VE test parameter for the prediction of the outcome (reference standard) under investigation. Some studies reported crude (unadjusted) AUCs; others used regression models to adjust the AUC for various other variables. If both were reported the adjusted values were selected, otherwise crude (unadjusted) AUCs together with 95% CIs were selected.
Data were not sufficiently similar to permit pooling for any of the outcomes for any of the population groups for the prediction studies; studies differed in the variables adjusted for in the regression models and the VE test parameters evaluated. For outcomes evaluated in more than two studies, forest plots were used to display adjusted and crude (unadjusted) ORs or AUCs, together with 95% CIs for individual studies. A narrative summary of the results was provided.
Investigation of heterogeneity
There was no evidence of statistical heterogeneity between studies included in the meta-analyses, and the numbers of studies included in most analyses were small. Formal statistical investigation of heterogeneity for these analyses was therefore not appropriate. The following variables were considered as possible explanations for differences between studies in the narrative synthesis: patient demographics (age, gender, surgery type), type of VE device (ROTEM, TEG, Sonoclot), time point of surgery (during surgery only, during and after surgery, and risk of bias domains).
Results of the assessment of clinical effectiveness
The literature searches of bibliographic databases identified 8960 references. After initial screening of titles and abstracts, 78 were considered to be potentially relevant and ordered for full-paper screening. No additional papers were ordered, based on screening of papers provided by test manufacturers, conference abstract hand-searching or screening references of included studies; all studies cited in documents supplied by the test manufacturers, identified through reference screening or conference abstract screening, had already been identified by bibliographic database searches. Figure 6 shows the flow of studies through the review process, and Appendix 4 provides details, with reasons for exclusions, of all publications excluded at the full-paper screening stage.
FIGURE 6.
Flow of studies through the review process. CCT, controlled clinical trial.
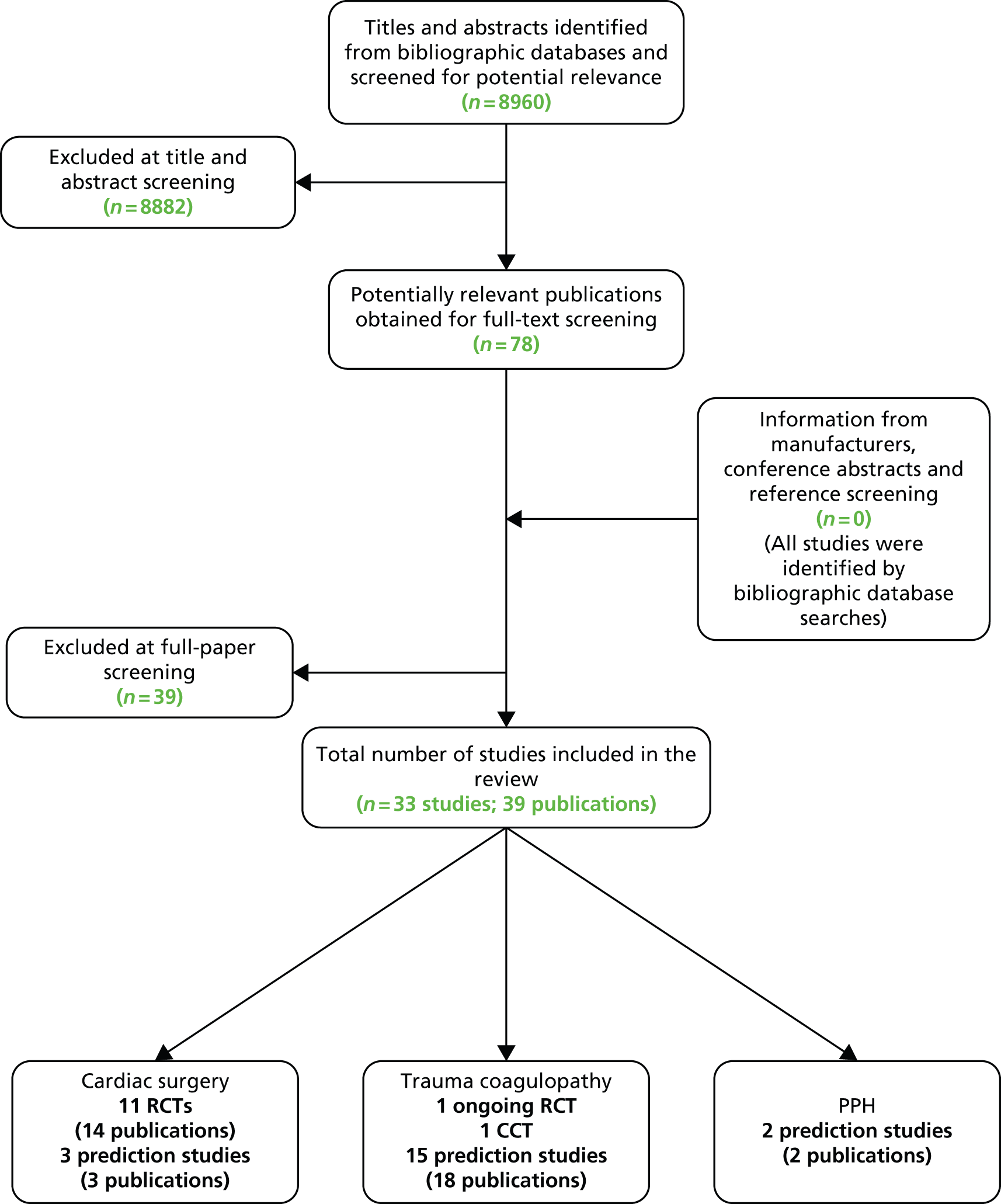
Based on the searches and inclusion screening described above, 39 publications of 33 studies were included in the review. We included 11 RCTs (13 publications)35,46–57 evaluating ROTEM and TEG in cardiac surgery patients; as no RCTs evaluating Sonoclot were identified, we also included three prediction studies58–60 that evaluated Sonoclot. We included one ongoing RCT,61,62 one controlled clinical trial (CCT)63 and 15 prediction studies (18 publications)64–81 in trauma patients, and two prediction studies82,83 in women with PPH.
Full details of the characteristics of study participants, study inclusion and exclusion criteria, VE test used and results are reported in the data extraction tables presented in Appendix 2. The results of the risk of bias assessments are presented in Appendix 3.
How do clinical outcomes differ among patients who are tested with viscoelastic devices during or after cardiac surgery compared with those who are not tested?
We included 11 RCTs (n = 1089, range 22–228) (13 publications)35,46–57 for the assessment of VE devices in patients undergoing cardiac surgery; six assessed TEG,46–51 four assessed ROTEM35,53–55 and one52 assessed ROTEG. ROTEG was an early name for ROTEM and so the study assessing ROTEG52 was grouped with the ROTEM studies in the analyses. Two RCTs53,55 were available only as abstracts.
Study details
The RCTs were conducted in Australia, Austria, Germany, Spain, Turkey, UK and the USA. Most included patients undergoing surgery irrespective of whether or not they had a bleeding event; however, two RCTs35,53 assessing ROTEM were restricted to patients who had experienced bleeding above a certain level (≥ 300 ml in first post-operative hour53 or bleeding from capillary beds requiring haemostatic therapy or blood loss exceeding 250 ml/hour or 50 ml/10 minutes35). A further RCT51 of TEG was restricted to patients at moderate to high risk for transfusion procedures. One RCT54 was restricted to patients undergoing aortic surgery, two RCTs46,48 included patients undergoing coronary artery bypass graft (CABG), and the remainder included patients undergoing mixed cardiac surgery. One study48 excluded patients with abnormal pre-operative conventional coagulation tests, another study46 excluded patients with pre-operative haemodynamic instability or a history of bleeding diathesis, and one study54 excluded patients with known (inherited) coagulation disorders. The majority of studies did not place any restriction on entry based on anticoagulation use, but one study48 excluded patients who had used low-molecular-weight heparin up to the day of operation. One study51 excluded patients with pre-existing hepatic or severe renal disease. Mean or median age, where reported, ranged from 53 to 72 years. The proportion of men ranged from 56% to 90%.
The ROTEM/TEG algorithms varied across studies. Six studies35,46,48,50,51,55 used an algorithm based on TEG or ROTEM alone. Two studies combined TEG with SLTs,50,51 two combined ROTEM with platelet function testing (POC in one),35 one of these also used Hepcon® (Medtronic, Minneapolis, MN, USA) to monitor heparin and protamine dosage,48 and one55 combined ROTEM with clinical evaluation. The timing of the VE test varied across studies. All except one study,50 which performed TEG on arrival at the ICU, administered multiple VE tests. Timing included baseline/before bypass/before anaesthesia, after CPB, after protamine administration, on admission to ICU and up to 24 hours post CPB in one study. 46 Four studies35,47,48,54 performed VE testing post surgery only on patients who were continuing to bleed. Four studies35,46,48,53 used an algorithm based on SLTs in the control group; all other studies stated that control groups included combinations of clinical judgements and SLTs. Further details are summarised in Table 7.
| Study details | n | Patient category | Entry restricted to excessive bleeding? | Entry restriction based on anticoagulation? | VE testing algorithm | Control | Timing of VE test |
|---|---|---|---|---|---|---|---|
| Ak (2009)46 | 228 | CABG | No | No | TEG | Clinician judgement including SLTs | Before anaesthesia, after CPB, 15 minutes after protamine, admission to ICU, 6 and 24 hours post CPB |
| Avidan (2004)48 | 102 | CABG | No | Yes – no coagulation medication < 72 hours of surgery | TEG combined with Hepcon platelet function testing and ACT | SLTs algorithm | Five minutes and 1 hour post CPB, 20 minutes post protamine, 2 hours post surgery if bleeding |
| Girdauskas (2010)54 | 56 | Aortic surgery | No | No | ROTEM | Clinician judgement including SLTs | Rewarming phase of CPB, before chest closure, on ICU in case of increased bleeding. Repeat ROTEM also performed 15 minutes after administration of coagulation products |
| Kultufan Turan (2006)52 | 40 | CABG or valve surgery | No | Unclear | ROTEG | Routine transfusion therapy and SLTs | Pre operation, 1 hour post operation |
| Nuttall (2001)50 | 92 | Mixed cardiac surgery | No | No | TEG combined with PT, aPTT, PLTs and FIB concentration | Clinician judgement with or without SLTs | On arrival in ICU |
| aPaniagua (2011)53 | 22 | Mixed cardiac surgery | Yes (≥ 300 ml in first post-operative hour) | NR | ROTEM | SLTs | NR |
| aRauter (2007)55 | 213 | Mixed cardiac surgery | No | NR | ROTEM + clinical signs | Routine management including SLTs | NR |
| Royston (2001)49 | 60 | Mixed cardiac surgery | No | No | TEG | Clinician judgement including SLTs | Prior to surgery, at bypass 10–15 minutes after protamine |
| Shore-Lesserson (1999)51 | 107 | Mixed cardiac surgery | Moderate to high risk for transfusion procedures | No | TEG + PLT + FIB | SLTs algorithm | Baseline, during rewarming on CPB, after protamine |
| Weber (2012)35 | 100 | Mixed cardiac surgery | Yes – bleeding from capillary beds or blood loss > 250 ml/hour or 50 ml/10 minutes | Yes – pre-operative antiplatelet therapy stopped > 6 days before surgery | ROTEM + POC testing for platelet function | SLTs algorithm | Unclear; appears to be before weaning off CPB, after protamine, for ongoing bleeding |
| Westbrook (2009)47 | 69 | Mixed cardiac surgery | No | No | TEG | Clinician judgement including SLTs | Prior to surgery, at bypass, 10–15 minutes after protamine |
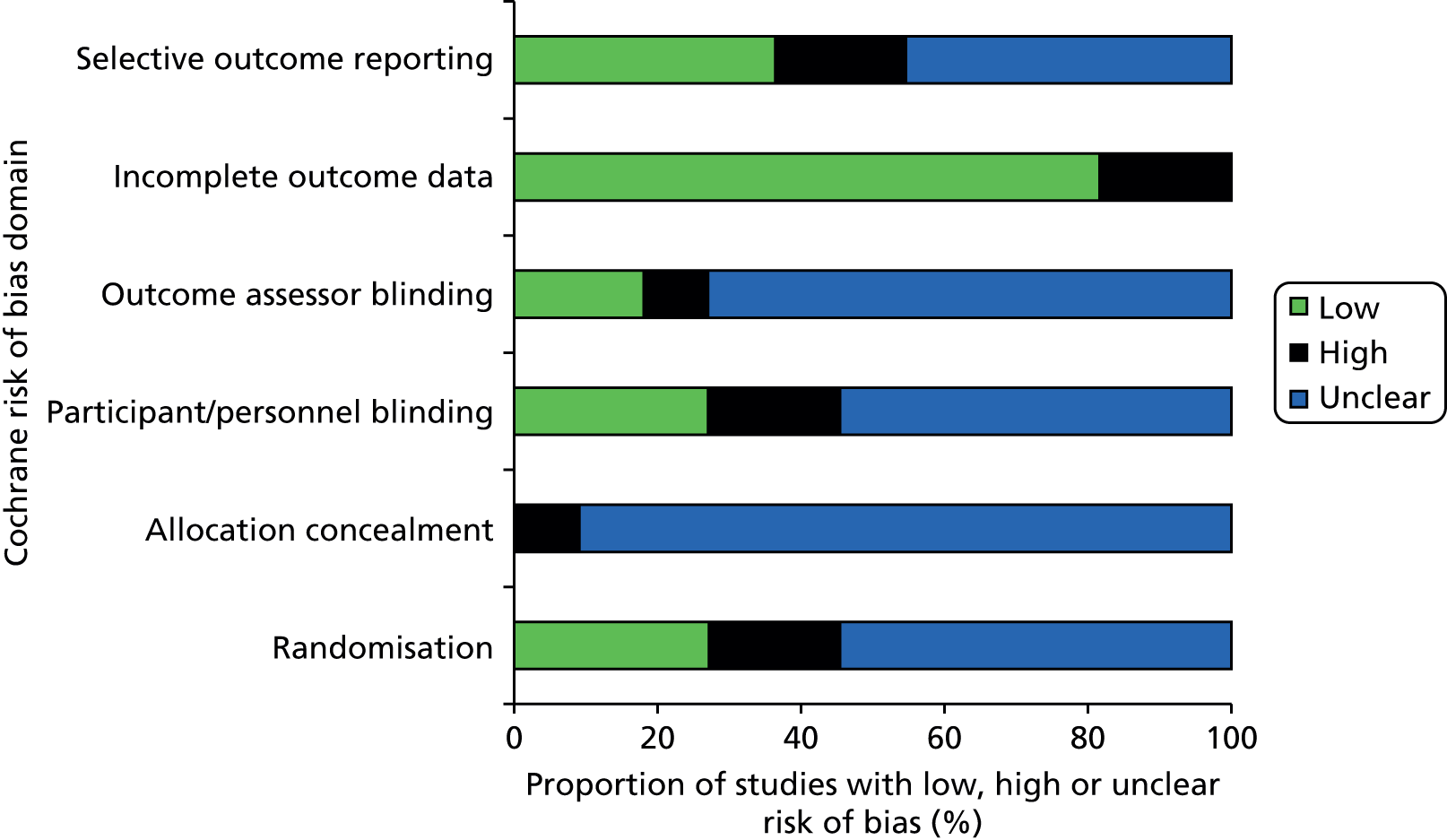
Risk of bias assessment
There were a number of methodological issues with the RCTs included in this assessment. Only three35,51,54 of the 11 RCTs35,46–55 were rated as ‘low’ risk of bias with respect to their randomisation procedures. The trials were generally poorly reported; all were rated as ‘unclear’ or ‘high’ risk of bias on at least 50% of the assessed criteria. Allocation concealment and blinding were particularly poorly reported. Only one study50 reported sufficient information to assess risk of bias in relation to allocation concealment and this study was considered to have a ‘high’ risk of bias on this criterion. This study50 moved four patients initially randomised to the algorithm group to the control group, and so allocation was not concealed for these patients.
Five of the 11 RCTs46–48,52,55 reported details of blinding of study participants and personnel; only three46,47,52 of these were rated as ‘low’ risk of bias. In one of these studies46 the anaesthesiologist who performed the transfusion was blinded to the patient’s group assignments, in one47 the surgeons were blinded to the method of haemostasis assessment, and in the third52 the physician in charge of ROTEG and ICU physician were blinded. The other two studies48,55 explicitly stated that they were unblinded. Only three RCTs48,50,55 reported details on blinding of outcome assessors. Two RCTs48,50 were rated as ‘low’ risk of bias: one48 reported that outcomes were recorded by staff in the recovery unit who were unaware of group allocation; the other50 stated that surgeons and anaesthesiologists were not aware of group allocation at the time the decision on whether or not to transfuse was made. The third RCT55 reported that it was unblinded.
Inclusion of all study participants in analyses was the only notable area of methodological strength, with all but three trials rated as ‘low’ risk of bias for the completeness of outcome data criteria. 35,46,48,50–54 The results of risk of bias assessments are summarised in Table 8 and Figure 7; full risk of bias assessments for each study are provided in Appendix 3.
| Study | Risk of bias | |||||
|---|---|---|---|---|---|---|
| Randomisation | Allocation concealment | Participant and personnel blinding | Outcome assessor blinding | Incomplete outcome data | Selective outcome reporting | |
| Ak (2009)46 | ? | ? | ☺ | ? | ☺ | ☺ |
| Avidan (2004)48 | ? | ? | ☹ | ☺ | ☺ | ? |
| Girdauskas (2010)54 | ☺ | ? | ? | ? | ☺ | ☺ |
| Kultufan Turan (2006)52 | ? | ? | ☺ | ? | ☺ | ☺ |
| Nuttall (2001)50 | ☹ | ☹ | ? | ☺ | ☺ | ? |
| Paniagua (2011)53 | ? | ? | ? | ? | ☺ | ☹ |
| Rauter (2007)55 | ? | ? | ☹ | ☹ | ☹ | ☹ |
| Royston (2001)49 | ☹ | ? | ? | ? | ☹ | ? |
| Shore-Lesserson (1999)51 | ☺ | ? | ? | ? | ☺ | ? |
| Weber (2012)35 | ☺ | ? | ? | ? | ☺ | ☺ |
| Westbrook (2009)47 | ? | ? | ☺ | ? | ☹ | ? |
FIGURE 7.
Proportion of studies fulfilling each risk of bias criteria for RCTs evaluating VE devices in patients undergoing cardiac surgery.
Results
Red blood cell transfusion
All but one49 of the included RCTs evaluated RBC transfusion as either a continuous or dichotomous outcome. Eight RCTs35,46,47,50,51,53–55 evaluated RBC transfusion within 24–48 hours as a continuous outcome (Table 9). All RCTs35,46–55 reported less volume of RBC transfusion in the VE algorithm group than in the control group but this was statistically significant in only three35,50,55 (two of ROTEM35,55 and one of TEG50); one RCT53 did not report on the statistical significance of the difference.
Six RCTs35,46,48,51,52,54 provided dichotomous data on the number of patients who received an RBC transfusion in each intervention group. The summary RR was 0.88 (95% CI 0.80 to 0.96), suggesting a significant beneficial effect of the VE testing algorithm in reducing the number of patients who received an RBC transfusion (Figure 8). There was no evidence of heterogeneity across studies (I2 = 0%). Summary estimates were similar when stratified according to VE device: RR 0.86 (95% CI 0.72 to 1.02) for the three RCTs that evaluated TEG46,48,51 and 0.88 (95% CI 0.78 to 1.00) for the three RCTs that evaluated ROTEM35,54 and ROTEG. 52
| Study | Data available | Intervention results | Control results | p-value for difference between groupsa |
|---|---|---|---|---|
| RBC transfusion (units unless otherwise stated) within 24–48 hours | ||||
| Ak (2009);46 TEG | Median (IQR) | 1 (0–1) | 1 (1–2) | 0.599 |
| Nuttall (2001);50 TEG | Median (range) | 2 (0–9) | 3 (0–70) | 0.039 |
| Shore-Lesserson (1999);51 TEG | Mean (SD) | 354 (487) ml | 475 (593) ml | 0.12 |
| Westbrook (2009);47 TEG | Total | 14 | 33 | 0.12 |
| Girdauskas (2010);54 ROTEM | Median (IQR) | 6 (2–13) | 9 (4–14) | 0.20 |
| Paniagua (2011);53 ROTEM | Mean | 3.8 | 6.4 | NR |
| Rauter (2007);55 ROTEM | Mean | 0.8 | 1.3 | < 0.05 |
| Weber (2012);35 ROTEM | Median (IQR) | 3 (2–6) | 5 (4–9) | < 0.001 |
| Any blood component transfusion (units) | ||||
| Ak (2009);46 TEG | Median (IQR) | 2 (1–3) | 3 (2–4) | 0.001 |
| Westbrook (2009);47 TEG | Total | 37 (NR) | 90 (NR) | NR |
| Girdauskas (2010);54 ROTEM | Median (IQR) | 9 (2–30) | 16 (9–23) | 0.02 |
| FFP transfusion (units, unless stated) at 12–48 hours | ||||
| Ak (2009);46 TEG | Median (IQR) | 1 (1–1) | 1 (1–2) | 0.001 |
| Nuttall (2001);50 TEG | Median (range) | 2 (0–10) | 4 (0–75) | 0.005 |
| Royston (2001);49 TEG | Total | 5 | 16 | < 0.05 |
| Shore-Lesserson (1999);51 TEG | Mean | 36 (142) ml | 217 (463) ml | < 0.04 |
| Westbrook (2009);47 TEG | Total | 22 | 18 | NR |
| Kultufan Turan (2006);52 ROTEG | Mean (SD) | 2.80 (0.95) | 2.70 (1.46) | 0.403 |
| Girdauskas (2010);54 ROTEM | Median (IQR) | 3 (0–12) | 8 (4–18) | 0.01 |
| Paniagua (2011);53 ROTEM | Total | 3.1 | 3.4 | NR |
| Rauter (2007);55 ROTEM | Total | 0 | 4 | NR |
| Weber (2012);35 ROTEM | Median (IQR) | 0 (0–3) | 5 (3–8) | < 0.001 |
| FIB (g) transfusion at 24–48 hours | ||||
| Girdauskas (2010);54 ROTEM | Median (IQR) | 2 (2–3) | 2 (2–3) | 0.70 |
| Rauter (2007);55 ROTEM | Total | 31 | 30 | NR |
| Weber (2012);35 ROTEM | Median (IQR) | 2 (0–4) | 2 (0–6) | 0.481 |
| Platelet transfusion (units, unless otherwise stated) transfusion at 12–48 hours | ||||
| Ak (2009);46 TEG | Median (IQR) | 1 (1–1) | 1 (1–2) | 0.001 |
| Nuttall (2001);50 TEG | Median (range) | 6 (0–18) | 6 (0–144) | 0.0001 |
| Royston (2001);49 TEG | Total | 1 | 9 | < 0.05 |
| Shore-Lesserson (1999);51 TEG | Mean (SD) | 34 (94) ml | 83 (160) ml | 0.16 |
| Westbrook (2009);47 TEG | Total | 5 | 15 | NR |
| Girdauskas (2010);54 ROTEM | Median (IQR) | 1 (0–4) | 2 (1–3) | 0.70 |
| Paniagua (2011);53 ROTEM | Total | 0.50 | 1.57 | < 0.05 |
| Rauter (2007);55 ROTEM | Total | 0 | 0 | NR |
| Weber (2012);35 ROTEM | Median (IQR) | 2 (0–2) | 2 (0–5) | 0.010 |
| PCC (international units) transfusion at 24–48 hours | ||||
| Girdauskas (2010);54 ROTEM | Median (IQR) | 0 (0–2000) | 3000 (2000–3000) | < 0.001 |
| Rauter (2007);55 ROTEM | Total | 3000 | 13600 | NR |
| Weber (2012);35 ROTEM | Median (IQR) | 0 (0–1800) | 1200 (0–1800) | 0.155 |
FIGURE 8.
Forest plot showing RRs (95% CI) for number of patients receiving RBC transfusion in VE groups compared with control groups in cardiac patients.

Any blood component transfusion
Three RCTs46,47,54 evaluated any blood component transfusion as a continuous outcome (see Table 9). All three46,47,54 reported less volume of any blood component transfusion in the VE algorithm group than in the control group. This was statistically significant in two (one ROTEM54 and one TEG46); the third RCT54 did not report on the statistical significance of the difference.
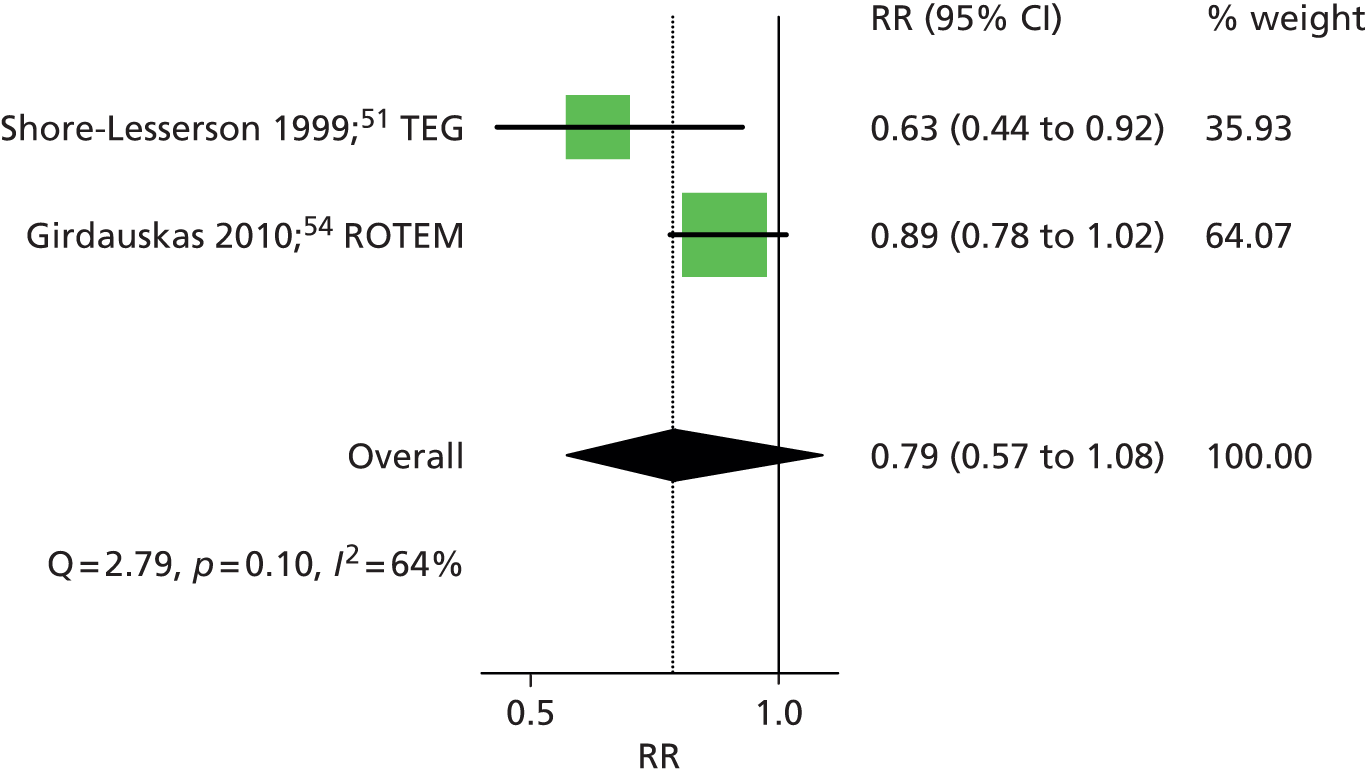
Two RCTs51,54 provided dichotomous data on the number of patients who received any blood component (defined as any blood component in one and allogeneic blood component in the other) transfusion in each intervention group. One54 assessed ROTEM (RR 0.89, 95% CI 0.78 to 1.02) and the other51 assessed TEG (RR 0.63, 95% CI 0.44 to 0.92). The summary RR was 0.79 (95% CI 0.57 to 1.08), suggesting a beneficial effect of the VE testing algorithm in reducing the number of patients who received any blood component transfusion, although this did not reach statistical significance (Figure 9). There was some evidence of heterogeneity across studies (I2 = 64%).
FIGURE 9.
Forest plot showing RRs (95% CI) for number of patients receiving any blood component transfusion in VE groups compared with control groups in cardiac patients.
Factor VIIa transfusion
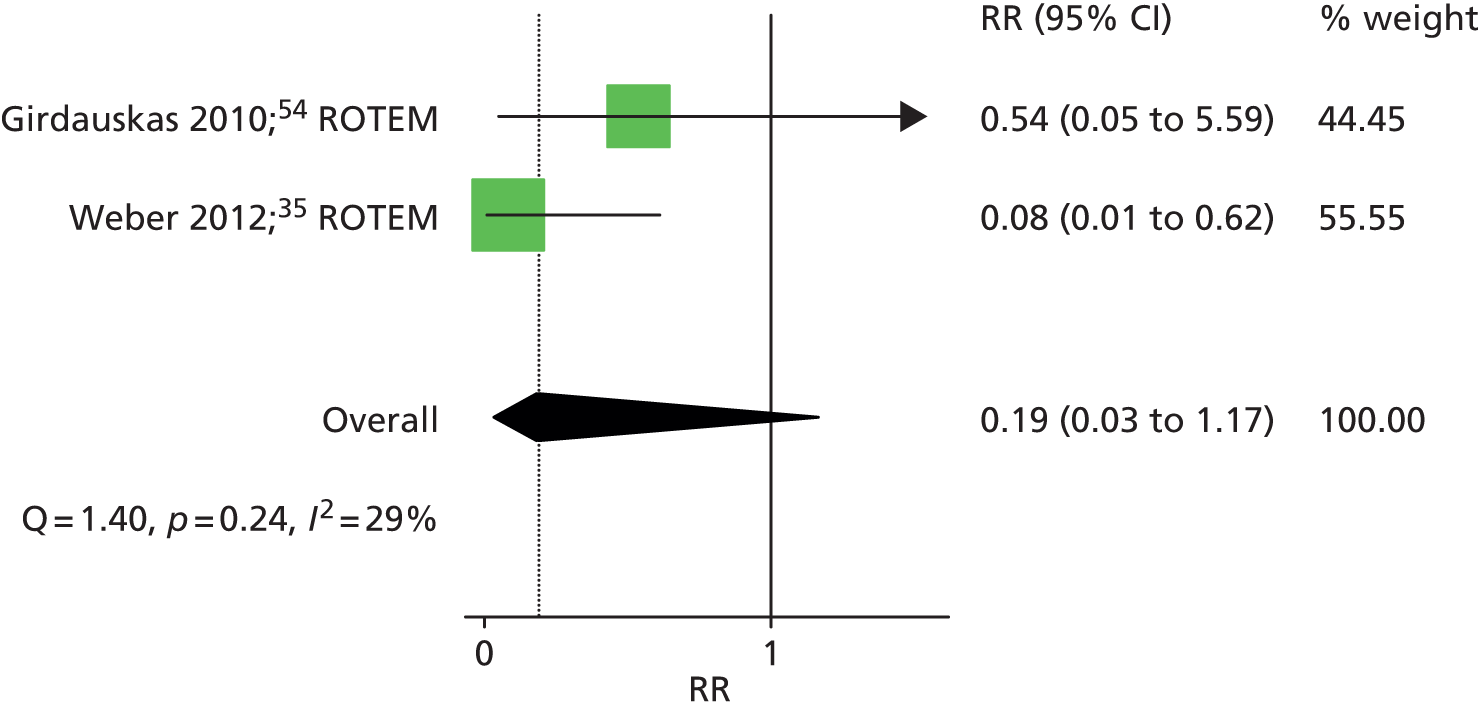
Two RCTs35,54 that assessed ROTEM provided dichotomous data on the number of patients who received a factor VIIa transfusion in each intervention group. The summary RR was 0.19 (95% CI 0.03 to 1.17), suggesting a beneficial effect of the ROTEM testing algorithm, although this difference did not reach statistical significance (p > 0.05) (Figure 10). There was little evidence of heterogeneity across studies (I2 = 29%).
FIGURE 10.
Forest plot showing RRs (95% CI) for number of patients receiving any factor VIIa transfusion in VE groups compared with control groups in cardiac patients.
Fresh frozen plasmas transfusion
All of the included RCTs35,46–55 evaluated FFP transfusion as either a continuous or dichotomous outcome. Ten RCTs35,46,47,49–55 evaluated RBC transfusion within 24–48 hours as a continuous outcome (see Table 9). All but two RCTs47,52 reported less volume of FFP transfusion in the VE algorithm group than in the control group; this was statistically significant in six35,46,49–51,54 (two of ROTEM35,54 and four of TEG46,49–51); three RCTs47,53,55 did not report on the statistical significance of the difference.
Five RCTs35,46,48,51,54 provided dichotomous data on the number of patients who received an FFP transfusion in each intervention group, all but one48 of which also reported continuous data. The summary RR was 0.47 (95% CI 0.35 to 0.65), suggesting a significant beneficial effect of the VE testing algorithm in reducing the number of patients who received an FFP transfusion (Figure 11). There was no evidence of heterogeneity across studies (I2 = 0%). However, the study by Avidan et al. 48 appeared to be an outlying result, with a RR of 5.0; however, the CI was very wide (95% CI 0.25 to 101.61). This was due to the very small number of events (two in the intervention arm and zero in the control arm). Removal of this study48 from the meta-analysis had very little impact on the summary estimate (RR 0.47, 95% CI 0.37 to 0.61). Summary estimates were similar when stratified according to VE device: RR 0.52 (95% CI 0.20 to 1.35) for the three RCTs that evaluated TEG46,48,51 and 0.46 (95% CI 0.34 to 0.63) for the two RCTs35,54 that evaluated ROTEM.
FIGURE 11.
Forest plot showing RRs (95% CI) for number of patients receiving FFP transfusion in VE groups compared with control groups in cardiac patients.

Fibrinogen transfusion
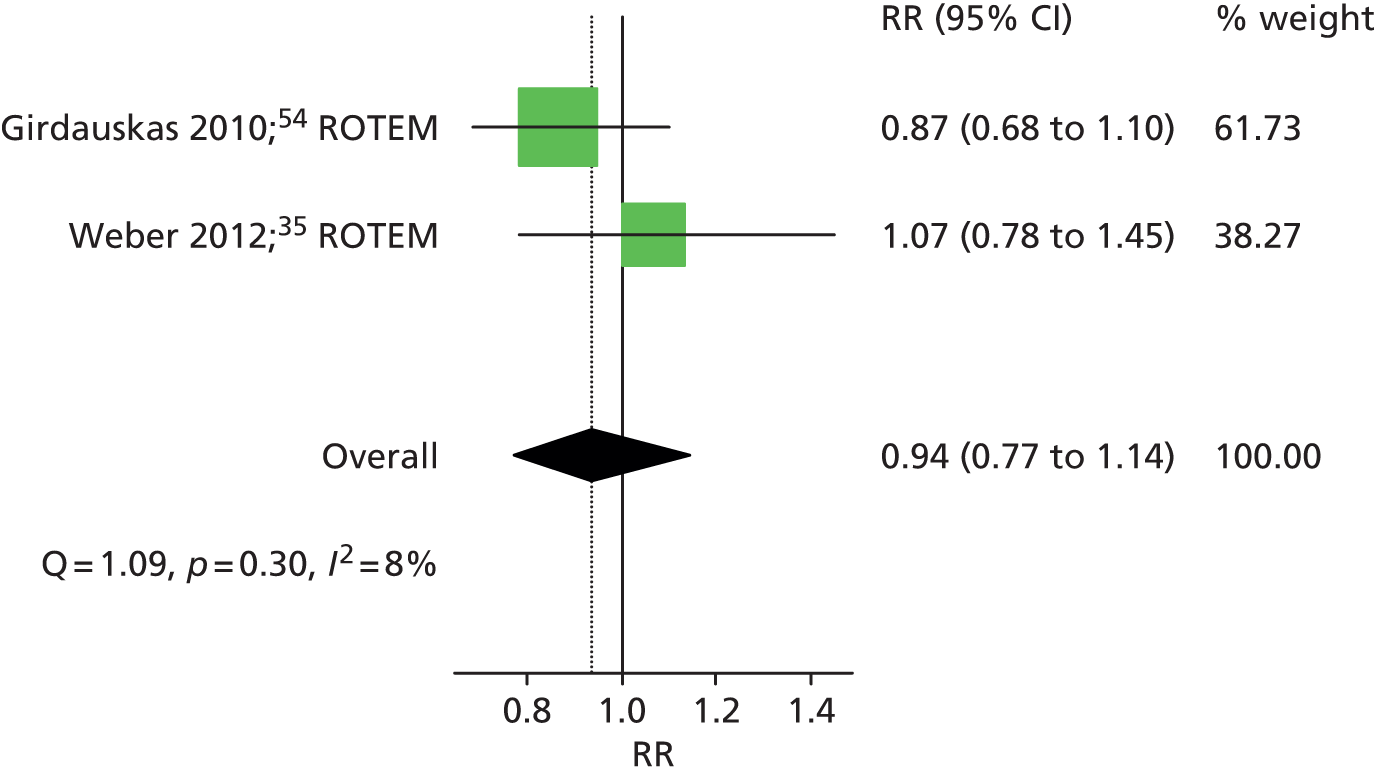
Three RCTs35,54,55 evaluated any FIB transfusion as a continuous outcome (see Table 9). All three35,54,55 reported no difference between the VE algorithm group compared with the control group in the volume of FIB transfused. Two of these RCTs35,54 also provided dichotomous data on the number of patients who received a FIB transfusion in each intervention group. The summary RR was 0.94 (95% CI 0.77 to 1.14), suggesting no difference between the treatment groups (Figure 12).
FIGURE 12.
Forest plot showing RRs (95% CI) for number of patients receiving FIB transfusion in VE groups compared with control groups in cardiac patients.
Platelet transfusion
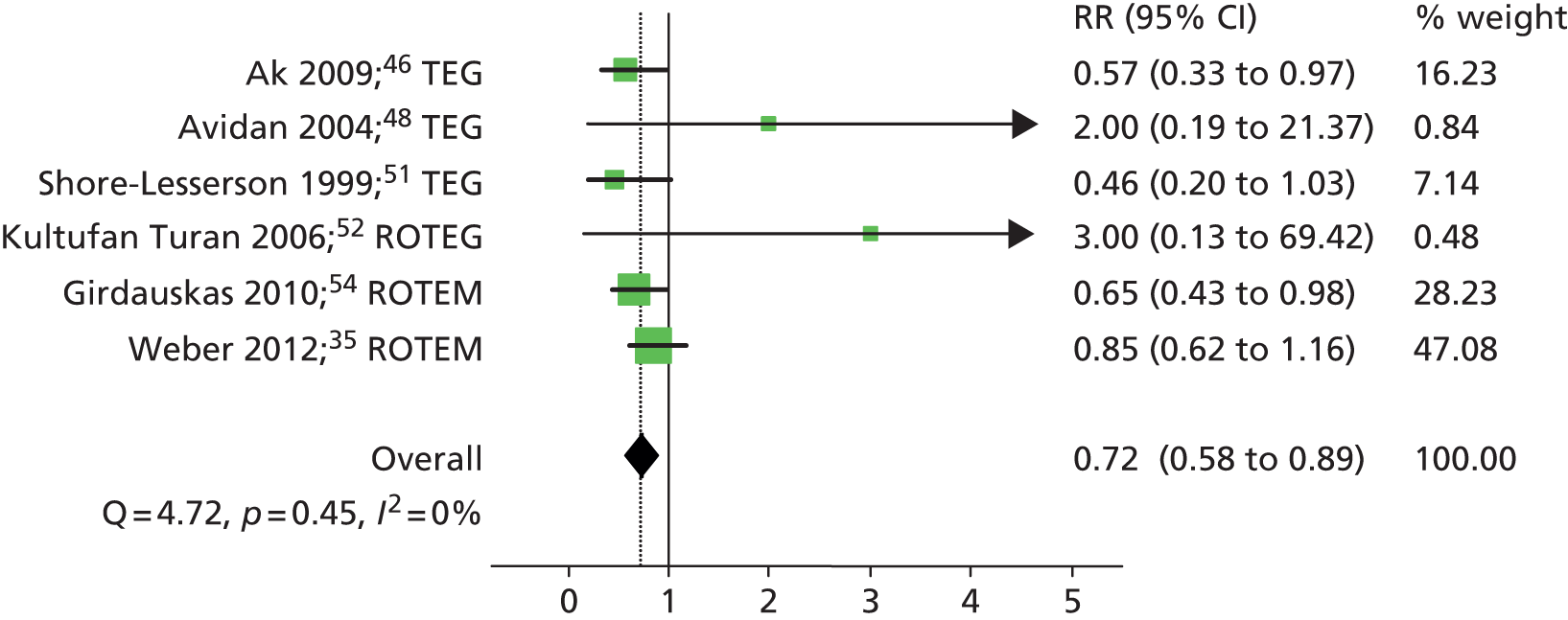
All of the included RCTs35,46–55 evaluated platelet transfusion as either a continuous or dichotomous outcome. Nine RCTs35,46,47,49–51,53–55 evaluated platelet transfusion within 24–48 hours as a continuous outcome (see Table 9). All RCTs reported less volume of platelet transfusion in the VE algorithm group compared with the control group but this was statistically significant in only five RCTs35,46,49,50,54 (two of ROTEM35,54 and three of TEG46,49,50); two RCTs47,55 did not report on the statistical significance of the difference.
Six RCTs35,46,48,51,52,54 provided dichotomous data on the number of patients who received a platelet transfusion in each intervention group. The summary RR was 0.72 (95% CI 0.58 to 0.89), suggesting a significant beneficial effect of the VE testing algorithm in reducing the number of patients who received a platelet transfusion (Figure 13). There was no evidence of heterogeneity across studies (I2 = 0%). Summary estimates were similar when stratified according to VE device: RR 0.56 (95% CI 0.36 to 0.86) for the three RCTs46,48,51 that evaluated TEG and 0.78 (95% CI 0.60 to 1.00) for the three RCTs35,52,54 that evaluated ROTEM and ROTEG.
FIGURE 13.
Forest plot showing RRs (95% CI) for number of patients receiving platelet transfusion in VE groups compared with control groups in cardiac patients.
Prothrombin complex concentrate transfusion
Three RCTs35,54,55 evaluated any PCC transfusion as a continuous outcome (see Table 9). All three RCTs35,54,55 reported less volume of PCC transfusion in the VE algorithm group than in the control group but this was statistically significant in only one RCT (p < 0.001);54 one RCT55 did not report on the statistical significance of the difference.
Two of these RCTs35,54 also provided dichotomous data on the number of patients who received a PCC transfusion in each intervention group. The summary RR was 0.39 (95% CI 0.08 to 1.95), suggesting no difference between the treatment groups (Figure 14).
FIGURE 14.
Forest plot showing RRs (95% CI) for number of patients receiving PCC transfusion in VE groups compared with control groups in cardiac patients.

Bleeding
Nine RCTs35,46–52,54 evaluated bleeding, generally measured as mediastinal tube drainage, as a continuous outcome (Table 10). The majority reported less bleeding in the VE intervention group; however, only two studies35,50 reported a statistically significant difference in bleeding between the two groups.
| Study | Data available | Intervention results | Control results | p-value for difference between groups |
|---|---|---|---|---|
| Bleeding/mediastinal tub drainage (ml) at 12-/24-hour follow-up | ||||
| Ak (2009);46 TEG | Mean (SD) | 480.5 (351.0) | 591.4 (339.2) | 0.087 |
| Avidan (2004);48 TEG | Median (IQR) | 755 (606–975) | 850 (688–1095) | > 0.05 |
| Nuttall (2001);50 TEG | Median (range) | 590 (240–2335) | 850 (290–10,190) | 0.019 |
| Royston (2001);49 TEG | Median (IQR) | 470 (295–820) | 390 (240–820) | NR |
| Shore-Lesserson (1999);51 TEG | Mean (SD) | 702 (500) | 901 (847) | 0.27 |
| Westbrook (2009);47 TEG | Median (IQR) | 875 (755–1130) | 960 (820–1200) | 0.437 |
| Kultufan Turan (2006);52 ROTEG | Mean (SD) | 837.5 (494.1) | 711.10 (489.2) | 0.581 |
| Girdauskas (2010);54 ROTEM | Median (IQR) | 890 (600–1250) | 950 (650–1400) | 0.50 |
| Weber (2012);35 ROTEM | Median (IQR) | 600 (263–875) | 900 (600–1288) | 0.021 |
| Length of ICU stay (hours) | ||||
| Ak (2009);46 TEG | Mean (SD) | 23.3 (5.7) | 25.3 (11.2) | 0.099 |
| Westbrook (2009);47 TEG | Median (IQR) | 29.4 (14.3–56.4) | 32.5 (22.0–74.5) | 0.369 |
| Girdauskas (2010);54 ROTEM | Mean (SD) | 175.2 (218.4) | 194.4 (201.6) | 0.6 |
| Weber (2012);35 ROTEM | Median (IQR) | 21 (18–31) | 24 (20–87) | 0.019 |
| Length of hospital stay (days) | ||||
| Ak (2009);46 TEG | Mean (SD) | 6.2 (1.1) | 6.3 (1.4) | 0.552 |
| Westbrook (2009);47 TEG | Median (IQR) | 9 (7–13) | 8 (7–12) | > 0.05 |
| Girdauskas (2010);54 ROTEM | Mean (SD) | 16.6 (16.4) | 17.0 (14.8) | 0.80 |
| Weber (2012);35 ROTEM | Median (IQR) | 12 (9–22) | 12 (9–23) | 0.718 |
Re-operation
Seven RCTs35,46,48–51,54 provided dichotomous data on the number of patients who required re-operation to investigate bleeding in each intervention group. The summary RR was 0.72 (95% CI 0.41 to 1.26), suggesting a beneficial effect of the VE testing algorithm in reducing the number of patients requiring re-operation, however, this difference was not statistically significant (Figure 15). There was no evidence of heterogeneity across studies (I2 = 0%). Summary estimates were similar when stratified according to VE device: RR 0.75 (95% CI 0.31 to 1.83) for the five RCTs46,48–51 that evaluated TEG and 0.69 (95% CI 0.33 to 1.44) for the two RCTs35,54 that evaluated ROTEM.
FIGURE 15.
Forest plot showing RRs (95% CI) for number of patients requiring re-operation in VE groups compared with control groups in cardiac patients.

Surgical source of bleeding identified on re-operation
Four RCTs46,50,51,54 provided dichotomous data on the number of patients in whom a surgical source of bleeding was identified on re-operation in each intervention group. The summary RR was 1.04 (95% CI 0.42 to 2.57), suggesting no difference between the intervention groups (Figure 16). There was very little evidence of heterogeneity across studies (I2 = 3%). One RCT assessed ROTEM54 and reported a RR of 0.86 (95% CI 0.26 to 2.87); the summary estimate for the three RCTs assessing TEG50,51,54 was similar at 0.99 (95% CI 0.18 to 5.36).
FIGURE 16.
Forest plot showing RRs (95% CI) for number of patients in whom a surgical source of bleeding was identified on re-operation in VE groups compared with control groups in cardiac patients.

Length of intensive care unit stay
Four RCTs35,46,47,54 evaluated the length of ICU stay as a continuous outcome (see Table 10). All studies35,46,47,54 reported shorter stays in the VE group than in the control group but this difference was statistically significant in only one study. 35
Length of hospital stay
Four RCTs35,46,47,54 evaluated the length of hospital stay as a continuous outcome (see Table 10). All studies35,46,47,54 reported that durations of stay were similar in the two treatment groups; none reported a statistically significant difference between groups.
Adverse events
One study35 reported on adverse events, including acute renal failure, sepsis and thrombotic complications. All were reduced in the ROTEM group compared with SLTs but differences were not statistically significant for individual outcomes. When the compound outcome of any adverse event was considered this was found to be significantly reduced in the ROTEM group compared with SLTs (RR 0.21, 95% CI 0.08 to 0.57).
Mortality
Four RCTs46,49,51,54 provided dichotomous data on the number of deaths (within 24 hours,51 48 hours,49 in hospital54 or ‘early mortality’46) in each intervention group. The summary RR was 0.87 (95% CI 0.35 to 2.18), suggesting no difference between the intervention groups (Figure 17). There was no evidence of heterogeneity across studies (I2 = 0%). One RCT assessed ROTEM54 and reported a RR of 0.86 (95% CI 0.26 to 2.87); the summary estimate for the three RCTs46,49,51 assessing TEG was similar at 0.88 (95% CI 0.21 to 3.66). An additional RCT35 provided data on 6-month mortality. This study35 reported significantly reduced mortality in the VE testing group at 6 months compared with the SLT group (RR 0.20, 95% CI 0.05 to 0.87).
FIGURE 17.
Forest plot showing RRs (95% CI) for number of deaths in VE groups compared with control groups in cardiac patients.

Other reported outcomes
Data were also reported on the following outcomes but each were assessed in only one or two studies, and so are not discussed in detail here: cryoprecipitate use, desmopressin treatment, dialysis-dependent renal failure, duration of ventilation, factor VIIa, fresh blood transfusion, intubation time, need for additional protamine, non-RBC balance, post-operative confusion, reinfusion, reintubation, stroke, time to stop bleeding, total heparin dose, total protamine dose, total ventilation time, time to extubation and tranexamic acid use. Full results can be found in Appendix 2.
Summary
Pooled estimates from each of the meta-analyses are summarised in Table 11. Overall, there was a significant reduction in RBC transfusion, platelet transfusion and FFP transfusion in VE testing groups compared with control. There was no significant difference between groups in terms of any blood component transfusion, factor VIIa transfusion or PCC transfusion, although data suggested a beneficial effect of the VE testing algorithm but these outcomes were evaluated in only two studies. There was no difference between groups in terms of FIB transfusion. Continuous data on blood component/product use, although inconsistently reported across studies, supported these findings; the only blood component/product that was not associated with a reduced volume of use in the VE testing group was FIB. There was a suggestion that bleeding was reduced in the VE testing groups but this was statistically significant in only two of the nine RCTs that evaluated this outcome. Clinical outcomes (re-operation, surgical cause of bleed on re-operation and mortality) did not differ between groups. There was some evidence of reduced bleeding and ICU stay in the VE testing groups compared with control but this was not consistently reported across studies. There was no difference in length of hospital stay between groups. There was no apparent difference between ROTEM or TEG for any of the outcomes evaluated.
| Outcome | Summary RR (95% CI) | No. of studies | Heterogeneity |
|---|---|---|---|
| Blood component/product use | |||
| RBC transfusion | 0.88 (0.80 to 0.96) | 6 | Q = 4.47, p = 0.48, I2 = 0% |
| Any blood component transfusion | 0.79 (0.57 to 1.08) | 2 | Q = 2.79, p = 0.10, I2 = 64% |
| Platelet transfusion | 0.72 (0.58 to 0.89) | 6 | Q = 4.47, p = 0.48, I2 = 0% |
| FFP transfusion | 0.47 (0.35 to 0.65) | 5 | Q = 4.72, p = 0.45, I2 = 0% |
| Factor VIIa transfusion | 0.19 (0.03 to 1.17) | 2 | Q = 1.40, p = 0.24, I2 = 29% |
| FIB transfusion | 0.94 (0.77 to 1.14) | 2 | Q = 1.09, p = 0.30, I2 = 8% |
| PCC transfusion | 0.39 (0.08 to 1.95) | 2 | Q = 10.23, p = 0.00, I2 = 90% |
| Clinical outcomes | |||
| Re-operation | 0.72 (0.41 to 1.26) | 7 | Q = 3.49, p = 0.74, I2 = 0% |
| Surgical cause of bleed on re-operation | 1.04 (0.42 to 2.57) | 4 | Q = 3.09, p = 0.38, I2 = 3% |
| Mortality | 0.87 (0.35 to 2.18) | 4 | Q = 1.26, p = 0.74, I2 = 0% |
How well do viscoelastic devices predict relevant clinical outcomes during or after cardiac surgery?
As none of the RCTs evaluated the Sonoclot VE test, we included lower levels of evidence for this device. Three prediction studies58–60 that evaluated Sonoclot were included in the review; two of these studies59,60 also evaluated TEG and so provided a direct comparison between these two devices. Baseline data from these studies are summarised in Table 12; full details of the studies are provided in Appendix 2.
| Study details | n | Patient category | Entry restricted to excessive bleeding? | Entry restriction based on anticoagulation? | VE test | Conventional tests | Outcome/reference standard |
|---|---|---|---|---|---|---|---|
| aBischof (2009)58 | 300 | Mixed cardiac surgery | No | Yes – no anticoagulant medication | Sonoclot | None | Bleeding > 800 ml 4 hours after surgery |
| Nuttall (1997)59 | 82 | Mixed cardiac surgery | No | No | Sonoclot, TEG | Bleeding time, platelet MPV, plasma FIB concentration, PLT, PT, aPTT, platelet HCT | Bleeding; subjective evaluation by anaesthesiologist and surgeon 10 minutes after protamine administration |
| Tuman (1989)60 | 42 | Mixed cardiac patients | High risk for transfusion procedures | Yes – no anticoagulant or antiplatelet medications 7 days before surgery | Sonoclot, TEG | ACT, PT, PTT, PLT and FIB | Bleeding; chest tube drainage greater than 150 ml/hour for 2 consecutive hours or > 300 ml/hour in 1 hour during the first 8 hours after surgery |
Study details
The cardiac prediction studies were conducted in Switzerland and the USA. All included patients undergoing mixed cardiac surgery irrespective of whether or not they had a bleeding event. One study58 excluded patients with a known coagulopathy and another excluded patients with abnormal pre-operative coagulation studies;60 both of these studies58,60 excluded patients receiving anticoagulant medication and one study60 also excluded patients on antiplatelet medications. Mean or median age, where reported, ranged from 63 to 65 years. The proportion of men ranged from 61% to 69%.
One of the studies58 evaluated Sonoclot alone and provided data on the accuracy of various different parameters to predict bleeding within 4 hours of surgery. One study59 evaluated Sonoclot, TEG and conventional laboratory tests and also provided data on the accuracy of different parameters of each of these tests for predicting bleeding based on a subjective evaluation by the anaesthesiologist and surgeon 10 minutes after protamine administration. The third study60 evaluated Sonoclot, TEG and standard laboratory testing and provided data on the accuracy of each test as a whole to predict bleeding in the first 8 hours after surgery.
Risk of bias and applicability assessment
Three studies58–60 used a predictive accuracy approach to assess the ability of VE POC testing devices to predict outcomes in patients undergoing cardiac surgery. The main areas of concern with regard to these studies were the participant selection process, which was unclear in all cases, and the applicability to the objectives of this assessment of the way in which VE testing was applied. Two58,59 of the three studies58–60 were rated as having ‘high’ applicability concerns for the index test because they assessed the predictive ability of selected individual parameters of VE testing, rather than assessing the device as a whole, or reporting data for all assays and parameters measured by the device. The results of QUADAS-2 assessments are summarised in Table 13; full QUADAS-2 assessments for each study are provided in Appendix 3.
Results
All three studies58–60 provided data that allowed calculation of ORs for the prediction of bleeding in patients who tested positive on a particular test or test parameter (Sonoclot, TEG or SLTs) compared with those who tested negative (Figure 18). Positive results on conventional tests, TEG and Sonoclot were all associated with an increased risk of bleeding with no clear differences according to test. Nuttall et al. 59 evaluated individual components of each of the tests separately, and found that all of the parameters investigated, with the exception of one TEG and one Sonoclot parameter, were associated with a significant (p < 0.05) increased risk of bleeding. Two of the SLTs (PT and aPTT) showed higher ORs than other parameters, but CIs overlapped with other SLTs and TEG and Sonoclot parameters. Bischof et al. 58 also evaluated individual test components but evaluated only the Sonoclot test; a direct comparison between Sonoclot and TEG or SLTs was therefore not possible from the results of this study. All three Sonoclot parameters showed a strong positive relationship with bleeding. Tuman et al. 60 was potentially the most informative study, as it evaluated each test class as a whole, that is, it evaluated a positive ‘TEG’ result rather looking at individual components of the TEG, similarly it evaluated SLTs as a class and Sonoclot as a whole. This study found that a positive TEG or Sonoclot result were both highly predictive of bleeding. However, the study60 was very small and CIs were wide. The limited data suggested that TEG results were more predictive than Sonoclot, but CIs overlapped. The SLTs performed less well and were not predictive of bleeding; this study60 was performed in 1989 and so may not be reflective of current practice.
FIGURE 18.
Forest plot showing ORs (95% CI) for prediction of bleeding by VE devices and SLTs in cardiac patients. A60, amplitude 60 minutes after clotting time; CR, clot rate; k, kinetics; MA, maximum amplitude; MPV, maximum platelet volume; NR, not reported; PF, platelet function; PTT, partial thromboplastin time; R, clotting time; R1, rate of fibrin monomer formation; R2, fibrinogenesis and platelet interaction; R3, rate of platelet mediated clot contraction.

How do clinical outcomes differ among patients with coagulopathy induced by trauma who are tested with viscoelastic devices compared with those who are not tested?
We identified one ongoing RCT62 that is comparing TEG (rapid assay) with conventional coagulation testing [INR, partial thromboplastin time (PTT), FIB, D-dimer] in adults with blunt or penetrating trauma who are likely to require a transfusion of RBCs within 6 hours from admission, as indicated by clinical assessment. 61,62 Additional information on this trial was provided by the study authors in the form of the study protocol. 62 The following outcomes are being evaluated in this study: quality and quantity of blood components transfused (packed RBCs, FFP, cryoprecipitate and apheresis platelets); patterns of transfusion ratios of RBC/FFP; haemorrhage-related deaths specified as very early mortality (< 2 hours post injury) and early mortality; late mortality; cessation of coagulopathic bleeding; multiple organ failure (MOF). Results from this study are not yet available. As no other RCTs were identified, we therefore considered lower levels of evidence for this objective. One CCT,63 reported only as an abstract, was included. This study63 compared a rapid-TEG-guided protocol with a standard transfusion protocol in adult trauma patients requiring massive transfusion (> 12 RBC units in 24 hours or > 4 units in 4 hours); both groups also included a near-patient haematocrit (HCT) assay. This study63 did not report numerical or statistical outcome data. It stated that there were no statistically significant differences between groups for death, acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome (SIRS), multisystem organ failure, sepsis, deep-vein thrombosis (DVT), stroke, acute coronary syndrome or days to discharge. There was a non-significant trend towards reduced pneumonia, days on the ventilator and ICU-days, and a trend towards increasing platelet use in the TEG-treated group. Baseline data from these studies are summarised in Table 14; full details of the studies are provided in Appendix 2. No other studies with a concurrent control group were identified for the trauma population.
| Study details | n | Patient category | Entry restricted to excessive bleeding? | Entry restriction based on anti-coagulation? | VE testing algorithm | Control | Timing of VE test | Outcomes assessed |
|---|---|---|---|---|---|---|---|---|
| Messenger (2011)63 | 50 | Mixed trauma | Yes – patients requiring massive transfusion (> 12 RBC units in 24 hours or > 4 units in 4 hours) | NR | TEG-guided protocol and HCT assay | Treatment according to institutional massive transfusion protocol including HCT assay | NR | Death, ARDS, SIRS, multisystem organ failure, sepsis, DVT, stroke, acute coronary syndrome, days to discharge, pneumonia, days on ventilator, ICU-days, platelet use |
| Moore (ongoing)62 | Ongoing | Mixed trauma | Yes – likely to require transfusion of RBC within 6 hours | No | TEG (rapid TEG) | INR, PTT, FIB, D-dimer | On hospital admission (usually within an hour), twice within first 6 hours post injury, 12 and 24 hours post injury | Quality and quantity of blood components transfused (packed RBCs, FFP, cryoprecipitate and apheresis platelets), patterns of transfusion ratios of RBC/FFP, haemorrhage-related deaths specified as very early mortality (< 2 hours post injury) and early mortality; late mortality; cessation of coagulopathic bleeding; MOF |
Risk of bias and applicability assessment
As the RCT has not yet been published it was not possible to assess the risk of bias in this study. 61 Details on this risk of bias assessment for the CCT are reported in Appendix 3. The CCT63 was rated as high risk of bias for randomisation and concealment of treatment allocation, as it was not a randomised study. It was rated unclear for all other domains as insufficient information were reported to make a judgement on these.
How well do viscoelastic devices predict relevant clinical outcomes in patients with coagulopathy induced by trauma?
As there were insufficient data from studies that evaluated differences in clinical outcomes between VE tested and untested populations, we included lower levels of evidence for this objective. Fifteen prediction studies64–81 (18 publications; n = 4217) were included for this objective. Nine studies64,67,69,71–74,77,81 evaluated TEG, four of these71,74,77,79,80 also evaluated SLTs; the other six studies evaluated ROTEM,65,66,68,70,75,76 with four65,68,70,76 also evaluating SLTs. No studies of Sonoclot were identified. None of the studies evaluated both TEG and ROTEM in the same patients. Baseline data from these studies are summarised in Table 15; full details of the studies are provided in Appendix 2.
| Study details | n | Patient category | Entry restriction based on anticoagulation? | VE test | Conventional test(s) | Outcome/reference standard | Variables controlled for in multivariate analysis (if used) |
|---|---|---|---|---|---|---|---|
| Cotton (2011)73 | 272 | Mixed trauma | NR | TEG | None | Massive transfusion (≥ 10 units PRBC in 6 hours) RBC transfusion (any within 6 hours) |
Age (years), gender, blunt mechanism of injury, race, ED systolic blood pressure, ED heart rate, positive FAST examination |
| Davenport (2011)70,78 | 300 | Mixed trauma | Yes – excluded patients taking anticoagulation medication | ROTEM | PR | FFP transfusion (any within 12 hours) Massive transfusion (> 10 units RBC within 12 hours) RBC transfusion (any within 12 hours) |
No multivariate analysis |
| Holcomb (2012)74 | 1974 | Mixed trauma | No | TEG | Plasma FIB, PLT, PT, aPTT, INR | Massive transfusion (≥ 10 units RBC within 6 hours) Massive transfusion of cryoprecipitate (≥ 20 units within 6 hours) Massive transfusion of plasma (≥ 6 units within 6 hours) Massive transfusion of platelets (≥ 2 apheresis units within 6 hours) Substantial bleeding (receiving first RBC unit within 2 hours of ED arrival and at least 5 RBC; transfusion or death from haemorrhage within 4 hours of ED arrival) |
Age (years), sex, mechanism of injury, base deficit, weighted, revised trauma score, and ISS |
| Ives (2012)72 | 118 | Mixed trauma | NR | TEG | None | Plasma transfusion; platelet transfusion; RBC transfusion; death | Packed RBCs in 24 hours > 10 units |
| Jeger (2012)77,79 | 76 | Mixed trauma | NR | TEG | aPTT, INR, plasma FIB, thrombin time | Any blood component transfusion within 24 hours | No multivariate analysis |
| Kaufmann (1997)64 | 69 | Blunt trauma | No | TEG | None | Any blood component transfusion within 24 hours | No multivariate analysis |
| aKorfage (2011)75 | 142 | Mixed trauma | No | ROTEM | None | Any blood component transfusion within 48 hours | Study reports that predictive values were determined using multinomial regression analyses, but it is not clear which variables were included in the final model |
| Kunio (2012)67 | 69 | Traumatic brain injury | Yes – patients taking clopidogrel or warfarin ≤ 30 days of admission excluded | TEG | None | Death; neurosurgical intervention | No multivariate analysis |
| Leemann (2010)65 | 53 | Blunt trauma | NR | ROTEM | aPTT, INR, PLT | Massive transfusion (≥ 10 units PRBC within 24 hours) | Haemoglobin ≤ 10 g/dl |
| Nystrup (2011)71 | 89 | Mixed trauma | NR | TEG | aPTT, INR | Death within 30 days | Age and ISS |
| Pezold (2012)80 | 80 | Mixed trauma | NR | TEG | aPTT, INR | Coagulation-related mortality (death after receiving a massive transfusion ≥ 10 PRBC units) within 6 hours Massive transfusion (≥ 10 units PRBC within 6 hours) |
No multivariate analysis |
| Schöchl (2011)76,81 | 323 | Mixed trauma | NR | ROTEM | Platelet count, aPTT, plasma FIB | Massive transfusion (≥ 10 RBC units within 24 hours) | No multivariate analysis |
| aSchöchl (2011)68,76 | 88 | Traumatic brain injury | NR | ROTEM | aPTT | Death | No multivariate analysis |
| aTapia (2012)69 | 230 | Mixed trauma | NR | TEG | None | Death within 30 days | No multivariate analysis |
| Tauber (2011)66 | 334 | Blunt trauma | NR | ROTEM | None | Death within 24 hours | No multivariate analysis |
Study details
The prediction studies in trauma patients were conducted in the UK, the USA, Switzerland, the Netherlands, Denmark and Austria. The majority included mixed trauma patients but three studies64–66 were restricted to patients with blunt trauma and two studies67,68 were restricted to patient with traumatic brain injury. One study69 excluded patients with traumatic brain injury, and one study65 excluded patients with isolated head injury. None of the studies restricted inclusion based on bleeding. One study70 excluded patients who had previously taken anticoagulant medication and another study67 excluded patients who had recently taken clopidogrel or warfarin. Mean or median age, where reported, ranged from 33 to 49 years. The proportion of men ranged from 59% to 82%. Mean injury severity score (ISS), reported in 11 studies,64–67,70–74,77,80 ranged from 12 to 34. Mean Glasgow Coma Scale scores ranged from 11 to 14 but were reported in only six studies. 65–67,72–74
All studies64–81 performed VE testing on admission. Three studies64,71,72 evaluated TEG as a whole with a positive result based on a combination of different TEG parameters. A further two studies66,69 assessed the presence of hyperfibrinolysis on TEG and ROTEM, which appeared to be based on more than one test parameter; however, exact details on how hyperfibrinolysis was defined were not provided. All other studies assessed individual components of the TEG or ROTEM separately. SLTs (aPTT, INR, plasma FIB, PLT and PT) were each evaluated separately. Outcomes assessed in the studies included any blood component transfusion, FFP transfusion, massive transfusion, massive transfusion of cryoprecipitate, massive transfusion of plasma, massive transfusion of platelets, plasma transfusion, platelet transfusion, RBC transfusion, bleeding, neurosurgical intervention and death. Six studies65,71–75 used multiple logistic regression models to estimate ORs for the association of individual TEG or ROTEM parameters or SLTs, with the outcomes of interest controlled for various factors, such as RBCs transfusion, age, sex, mechanism of injury, trauma/injury severity, haemoglobin levels and race. Other studies reported 2 × 2 data on the number of patients with a positive and negative test results, who did and did not have the outcome of interest,64,66,67,69,70,72 sensitivity and specificity but without sufficient data to populate 2 × 2 tables,68,71,76,77 and AUC for the ROC curve. 68,71,76
Risk of bias and applicability assessment
All of the studies64–77,80 that assessed the ability of VE testing devices to predict outcomes in trauma patients used a predictive accuracy or prediction modelling approach. The main areas of concern with regard to these studies were the process of participant selection and the applicability to the objectives of this assessment of the way in which both VE testing and the reference standard were applied. With two exceptions,73,74 all studies were rated as ‘high’ or ‘unclear’ risk of bias in the participant selection process, usually because of poor reporting or inappropriate exclusion of particular groups of patients. Ten of the 15 studies were rated as having ‘high’ applicability concerns for the index test because they assessed the predictive ability of selected individual components of VE testing rather than assessing the device as a whole or reporting data for all assays and parameters measured by the device;65,67,68,70,73–77,80 two further studies66,69 were rated as having ‘unclear’ applicability because, although the testing VE device was specified, no details of the assay(s) used or parameters measured were reported. Ten studies64,65,70,72–77,80 were rated as having ‘high’ applicability concerns with respect to the reference standard, where the reference standard was one or more measure(s) of transfusion requirements, because it was unclear whether or not the decision to transfuse was informed by VE testing results, this also resulted in an ‘unclear’ risk of bias rating with respect to the reference standard. In practice, the results of VE testing would inform the decision to transfuse, a situation that gives rise to the paradox that this type of study cannot have both ‘low’ risk of bias and ‘low’ applicability with respect to the reference standard; if the reference standard is applied as it would be in clinical practice, the study will necessarily be subject to incorporation bias. The remaining five studies66–69,71 were rated as ‘low’ applicability concerns because they reported objective reference standards (e.g. mortality). The results of QUADAS-2 assessments are summarised in Table 16 and Figure 19; full QUADAS-2 assessments for each study are provided in Appendix 3.
| Study | Risk of bias | Applicability concerns | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |||
| Cotton (2011)73 | ☺ | ☹ | ? | ☺ | ☺ | ☹ | ☹ | ||
| Davenport (2011)70 | ☹ | ☹ | ? | ☺ | ☺ | ☹ | ☹ | ||
| Holcomb (2012)74 | ☺ | ? | ? | ☺ | ☺ | ☹ | ☹ | ||
| Ives (2012)72 | ☹ | ☺ | ☺ | ? | ☹ | ☺ | ☺ | ☺ | ☹ |
| Jeger (2012)77 | ☹ | ☹ | ☺ | ☺ | ☺ | ☹ | ☹ | ||
| Kaufman (1997)64 | ? | ☺ | ☺ | ☺ | ☺ | ☺ | ☹ | ||
| Korfage (2011)75 | ? | ? | ? | ☺ | ? | ☹ | ☹ | ||
| Kunio (2012)67 | ? | ☺ | ☺ | ☺ | ☹ | ☹ | ☺ | ||
| Leeman (2010)65 | ☹ | ☺ | ? | ☺ | ☹ | ☹ | ☹ | ||
| Nystrup (2011)71 | ☹ | ? | ☺ | ☺ | ☺ | ☺ | ☺ | ||
| Pezold (2012)80 | ☹ | ☹ | ? | ☺ | ☺ | ☹ | ☹ | ||
| Schöchl (2011)76 | ☹ | ☹ | ? | ☺ | ☺ | ☹ | ☹ | ||
| Schöchl (2011)68 | ? | ☹ | ☺ | ☺ | ☹ | ☹ | ☺ | ||
| Tapia (2012)69 | ☹ | ? | ☺ | ☹ | ? | ? | ☺ | ||
| Tauber (2011)66 | ? | ☺ | ☺ | ☺ | ☹ | ? | ☺ | ||
FIGURE 19.
Proportion of studies fulfilling each QUADAS-2 criteria for prediction studies evaluating VE devices in patients with coagulopathy induced by trauma.

Results
Red blood cell transfusion
Three studies70,72,73 (two of TEG,72,73 one of ROTEM and SLTs70) evaluated the ability of VE devices to predict RBC transfusion (Figure 20). One used an end point of any RBC transfusion within 12 hours,70 one within 6 hours73 and one72 did not specify the time point. A positive result on each of the parameters assessed, with the exception of CT on ROTEM, was associated with an increased risk of RBC transfusion. There were no clear differences between ROTEM parameters or ROTEM and SLTs in the one study70 that reported multiple evaluations.
FIGURE 20.
Forest plot showing ORs (95% CI) for prediction of RBC transfusion by VE devices and SLTs in trauma patients. a, Adjusted OR based on multivariate analysis. EPL, estimated per cent lysis.
Any blood component transfusion
Three studies evaluated the ability of VE devices to predict any blood component transfusion (Figure 21). 64,75,77 Two evaluated TEG64,77 and one evaluated ROTEM;75 one of the studies of TEG77 also evaluated SLTs. The time frame for transfusion was within 24 hours in two studies64,77 and within 48 hours in the third. 75 A positive result on each of the parameters assessed was associated with an increased risk of any blood component transfusion; an overall TEG results suggesting the patient was hypercoagulable was associated with a decreased risk of transfusion (OR 0.14, 95% CI 0.03 to 0.76). One of the studies77 did not provide sufficient data to calculate CIs and so the significance of the ORs from this study could not be assessed. The other two studies64,75 both reported statistically significant (p < 0.05) associations for all parameters assessed. An overall TEG result indicating that the patient was hypocaoguable was found to be associated with the greatest increased risk of transfusion, but CIs were very wide (OR 180.00, 95% CI 14.15 to 2289.13). ORs for individual TEG, ROTEM or SLTs were much smaller, ranging from 2.50 to 15.26.
FIGURE 21.
Forest plot showing ORs (95% CI) for prediction of any blood component transfusion by VE devices and SLTs in trauma patients. a, Adjusted OR based on multivariate analysis. MA, maximum amplitude.
Massive transfusion
Six studies evaluated the ability of VE devices to predict massive RBC transfusion. 65,70,73,75,76,80 Three evaluated TEG73,74,80 and three evaluated ROTEM;65,70,76 all but one73 also evaluated SLTs. All used a threshold of ≥ 10 units of RBC transfused to define massive transfusion but the time frame within which this had to occur ranged from 6 hours to 24 hours. Three studies65,73,74 provided data as adjusted ORs for at least one of the VE test components; a further study70 provided data that permitted calculation of ORs (Figure 22). The other two studies76,80 provided data only on AUC for the ROC curve, together with 95% CIs (Figure 23). A positive result on each of the parameters assessed was associated with an increased risk of massive transfusion; however, this difference was not statistically significant for some of the ROTEM parameters and SLTs. There were no clear differences between ROTEM, TEG or SLTs, or individual test parameters in terms of ability to predict massive transfusion. AUCs, where reported, were between 0.70 and 0.92, with no clear differences between ROTEM, TEG or SLTs.
FIGURE 22.
Forest plot showing ORs (95% CI) for prediction of massive transfusion by VE devices and SLTs in trauma patients. a, Adjusted OR based on multivariate analysis. LY30, lysis at 30 minutes; MA, maximum amplitude.

FIGURE 23.
Forest plot showing AUCs (95% CI) of ROC curves for prediction of massive transfusion by VE devices and SLTs in trauma patients.
Mortality
Seven studies66–69,71,72,80 evaluated the association of the results of VE devices with mortality. Five studies67,69,71,72,80 evaluated TEG; two studies66,68 evaluated ROTEM; and three studies68,71,80 evaluated SLTs. Two studies66,72 defined mortality as death within 24 hours, one67 as death in hospital, two69,71 as death within 30 days, one68 did not provide a definition, and one80 restricted its definition of mortality to coagulation-related mortality [death after receiving a massive transfusion of ≥ 10 packed red blood cell (PRBC) units].
Two studies71,72 provided data as adjusted ORs; three further studies66,67,69 provided data that permitted calculation of ORs and associated CIs (Figure 24). The other two studies68,80 provided data on AUC only for the ROC curve, together with 95% CIs; these data were also reported in one of the studies71 that reported adjusted ORs (Figure 25). A positive result assessed was associated a statistically significant increased risk of death for most parameters assessed. The only exceptions were two parameters that were associated with a decreased risk of death, although this difference was not statistically significant: the presence of moderate hyperfibrinolysis (OR 0.76, 95% CI 0.09 to 6.20)66 and an overall TEG result suggesting that a patient was hypocoagulable (OR 0.23, 95% CI 0.03 to 1.91). 72 Three studies66,69,72 that evaluated a ROTEM or TEG result indicating the presence of hyperfibrinolysis showed the strongest association with death, with ORs ranging from 25 to 147, although CIs were wide. AUCs were between 0.63 and 0.93, with no clear differences between ROTEM, TEG or SLTs.
FIGURE 24.
Forest plot showing ORs (95% CI) for prediction of death by VE devices and SLTs in trauma patients. a, Adjusted OR based on multivariate analysis. EPL, estimated per cent lysis; MA, maximum amplitude; NR, not reported.

FIGURE 25.
Forest plot showing AUCs (95% CI) of ROC curves for prediction of death by VE devices and SLTs in trauma patients. MA, maximum amplitude; NR, not reported.

Other outcomes
Data were also reported on the following outcomes, but each outcome was assessed only in single studies and so are not discussed in detail here: FFP transfusion, massive transfusion of cryoprecipitate, massive transfusion of plasma, massive transfusion of platelets, plasma transfusion, platelet transfusion, substantial bleeding and neurosurgical intervention. Full results can be found in Appendix 2.
Summary
Fifteen studies provided data on the accuracy of TEG or ROTEM for the prediction of transfusion-related outcomes and death in trauma patients; eight studies also provided data on the accuracy of SLTs. The studies generally found that a positive result on each of the TEG or ROTEM parameters or on SLTs was associated with an increased risk of transfusion (RBC, any blood component and massive transfusion) and death. There was no clear difference between ROTEM, TEG or SLTs. However, none of the studies provided a direct comparison between TEG and ROTEM. An overall TEG result suggesting that a patient was hypocoagulable was the strongest predictor of any blood component transfusion. The presence of hyperfibrinolysis was the strongest predictor of mortality.
How do clinical outcomes differ among patients with post-partum haemorrhage who are tested with viscoelastic devices compared with those who are not tested?
No studies were identified that compared clinical outcomes among patients with PPH who were tested with VE devices compared with those who were not tested.
How well do viscoelastic devices predict relevant clinical outcomes in patients with post-partum haemorrhage?
As no studies evaluated differences in clinical outcomes between VE-tested and untested populations, we included lower levels of evidence for this objective. Two prediction studies82,83 were included in the review (n = 245). Both studies were available only as abstracts. Baseline data from these studies are summarised in Table 17; full details of the studies are provided in Appendix 2.
| Study details | n | Population | Entry restriction based on anticoagulation? | VE test | Conventional tests | Outcome/reference standard |
|---|---|---|---|---|---|---|
| Bolton (2011)82 | 66 | Major obstetric haemorrhage (≥ 1500 ml) | NR | ROTEM | None | Coagulopathy requiring treatment FFP transfusion; platelet transfusion (threshold and time point NR) |
| Lilley (2013)83 | 179 | PPH (≥ 1000 ml) | NR | ROTEM | Clauss fibrinogen | RBC transfusion (≥ 4 units or any transfusion) Invasive procedure |
Study details
The studies82,83 were both conducted in the UK. One study83 included women with PPH defined as ≥ 1000 ml blood loss, the other82 included women with major obstetric haemorrhage defined as ≥ 1500 ml blood loss. Neither study provided data on restriction based on previous anticoagulation therapy or information on the mean age of study participants.
One study83 evaluated the MCF based on FIBTEM on ROTEM; this study also evaluated a SLTs (Clauss fibrinogen). The other study only evaluated ROTEM but did not provide any further details on what aspects of the ROTEM test were evaluated or whether or not data related to individual components or the test as a whole. The outcomes evaluated in the studies varied: one assessed the prediction of coagulopathy requiring treatment, FFP transfusion and platelet transfusion;82 the other assessed the prediction of RBC transfusion and invasive procedures. 83
Risk-of-bias and applicability assessment
As with the trauma studies, the main areas of concern with regard to the two prediction studies82,83 conducted in patients with PPH were the applicability to the objectives of this assessment of the way in which both VE testing and the reference standard were applied. One study83 was rated as having ‘high’ applicability concerns for the index test because it assessed the predictive ability of selected individual parameters of the FIBTEM assay on the ROTEM device, rather than assessing the device as a whole, or reporting data for all assays and parameters measured by the device; the other study82 was rated as having ‘unclear’ applicability because, although it assessed the ROTEM device, no details of the assay(s) used were reported. Both studies were rated as having ‘high’ applicability concerns with respect to the reference standard because it was unclear whether or not the decision to transfuse was informed by ROTEM results, this also resulted in an ‘unclear’ risk of bias rating with respect to the reference standard. 82,83 In practice, the results of ROTEM testing would inform the decision to transfuse, a situation that gives rise to the paradox that this type of study cannot have both ‘low’ risk of bias and ‘low’ applicability with respect to the reference standard; if the reference standard is applied as it would be in clinical practice, the study will necessarily be subject to incorporation bias. The results of QUADAS-2 assessments are summarised in Table 18; full QUADAS-2 assessments for each study are provided in Appendix 3.
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Bolton (2011)82 | ? | ? | ? | ☺ | ☹ | ? | ☹ |
| Lilley (2013)83 | ☺ | ? | ? | ☺ | ☺ | ☹ | ☹ |
Results
Both studies82,83 provided data that allowed calculation of ORs for the prediction of outcomes in patients who tested positive on ROTEM compared with those who tested negative (Figure 26). The study83 that evaluated ROTEM and SLTs reported data in a format that allowed calculation of ORs only for the ROTEM parameter (MCF based on FIBTEM analysis) for the prediction of any RBC transfusion. There was a strong positive relationship between this parameter and RBC transfusion (OR 41.54, 95% CI 9.01 to 191.59). Data for other outcomes and for the SLT (Clauss fibrinogen) were reported as AUC for the ROC curve; these were very similar for Clauss fibrinogen and for MCF based on ROTEM (FIBTEM). 83 CIs were not presented and so formal comparisons of AUCs was not possible.
FIGURE 26.
Forest plot showing ORs (95% CI) for prediction of specified outcomes by ROTEM in women with PPH. NR, not reported.

The other study82 reported that a positive ROTEM result was associated with coagulopathy requiring treatment (OR 168.0, 95% CI 15.6 to 1814. 7). This study82 also evaluated FFP transfusion and platelet transfusion; data were available to calculate ORs for these outcomes but not associated CIs. The ROTEM results were also predictive of both these outcomes but the significance of the association was unclear. The size of the OR was smaller than for the association with coagulopathy requiring treatment (OR 76 for FFP transfusion and 19 for platelet transfusion). 82
Summary
Only two studies82,83 were identified that evaluated VE devices in patients with PPH. Both provided data on the accuracy of ROTEM for the prediction of outcomes; one also evaluated a SLT (Clauss fibrinogen). Both studies82,83 showed that ROTEM results were associated with the outcomes evaluated (RBC transfusion, invasive procedures, coagulopathy requiring treatment, FFP transfusion and platelet transfusion). The study that evaluated both ROTEM and Clauss fibrinogen reported similar results for both tests but did provide CIs to accompany effect estimates.
Chapter 4 Assessment of cost-effectiveness
This chapter explores the cost-effectiveness of VE POC testing to assist with the diagnosis, management and monitoring of haemostasis.
Review of economic analyses of viscoelastic testing
Search methods
Searches were undertaken to identify cost-effectiveness studies of VE POC testing. As with the clinical effectiveness searching, the main EMBASE strategy for each set of searches was independently peer reviewed by a second information specialist, using the CADTH Peer Review Checklist. 40 Search strategies were developed specifically for each database and searches took into account generic and other product names for the intervention. All search strategies are reported in Appendix 1.
The following databases were searched for relevant studies from inception to November 2013:
-
MEDLINE (OvidSP): 1946–October 4 week 2013
-
MEDLINE In-Process & Other Non-Indexed Citations and Daily Update (OvidSP): up to 5 November 2013
-
EMBASE (OvidSP): 1974–5 November 2013
-
NHS Economic Evaluation Database (EED) (Wiley): Issue 4, October 2013
-
EconLit (EBSCOhost): 1990–1 September 2013
-
Health Economic Evaluation Database (HEED) (Wiley): up to 7 November 2013, http://onlinelibrary.wiley.com/book/10.1002/9780470510933
-
IDEAS via Research Papers in Economics (REPEC) (Internet): up to 7 November 2013, http://repec.org/
Identified references were downloaded in EndNote X4 software for further assessment and handling. References in retrieved articles were checked for additional studies.
Inclusion criteria
Cost minimisation and cost-effectiveness studies that evaluated the use of TEG, ROTEM or Sonoclot compared with a control group (either concurrent or historical) consisting of no-testing, clinical judgement or SLTs were eligible for inclusion. Studies in children were excluded.
Quality assessment
Full cost-effectiveness studies were appraised using the Drummond checklist. 84
Results
The searches identified 331 records, of which five studies12,85–88 fulfilled the inclusion criteria; two85,86 were available only as conference abstracts (Figure 27). Three studies86–88 were conducted in cardiac patients, one85 in patients undergoing liver transplant, and one12 in both cardiac and liver transplant patients. One study12 was a formal cost-effectiveness analysis of VE devices in cardiac and liver transplant patients. The other four studies85–88 were cost-minimisation studies performed alongside a retrospective before-and-after study.
FIGURE 27.
Flow of studies through the health-economic review process.

Cost-effectiveness analyses
The only formal cost-effectiveness analysis was conducted for the Scottish NHS. 12,89 This report12,89 assessed the cost-effectiveness of VE in cardiac and liver transplant patients, and the model was based, to a large extent, on an earlier study by Davies et al. 90 This study90 did not fulfil the inclusion criteria (as it did not study one of the listed devices) but was, nevertheless, very informative for the current assessment; it assessed the costs and effects of various methods of minimising perioperative allogeneic blood transfusion, with cardiac patients as a subpopulation. The resulting model took into account the relationship between blood component/product use and related complications and adverse events.
A detailed summary of both of these studies12,89,90 and a quality checklist based on Drummond et al. 84 are provided in Appendices 5 and 6. Both studies12,89,90 were in general of good quality. However, they did not completely address our research questions. The study by Davies et al. 90 did not consider the use of VE testing but did model the SLTs group. The Scottish report12 used most of the approach seen in Davies et al. ’s study,90 including most input parameters. The structure of our model was also largely based on these two studies12,89,90 and we used them as main source of input data. As the Scottish study12,89 did not include a probabilistic sensitivity analysis (PSA), we added this to the analysis. In addition, although the Scottish study12 considered both short-term (up to 1 month) and long-term (up to 1 year) effects of mortality, it failed to capture any difference between 1-month and 1-year mortality. However, recent data suggest that the effects of transfusion on mortality are not just short term, with differences in mortality reported up to and beyond 1 year. 15
The cost-effectiveness of VE testing in cardiac surgery patients was assessed in the Scottish NHS report,12 but trauma patients with suspected coagulopathy were not included in the study. Furthermore, a PSA was not performed. Although the structure of our model was largely based on these two studies12,89,90 and we have used them as main source of input data, when possible, for the cardiac population, the values of the input parameters were updated using more recent literature and a PSA was added.
Cost-minimisation studies
The four cost-minimisation studies all measured and costed the volume of blood transfused before the introduction of a VE device, and compared this with volumes and costs of blood transfused after the VE device was introduced. Three studies85,87,88 evaluated ROTEM and one86 evaluated TEG. All four studies85–88 found that costs were reduced as a result of the introduction of a VE device. As these were not full cost-effectiveness studies, a formal quality appraisal was not performed.
One study of ROTEM87 showed that, after the introduction of ROTEM, the cumulative average monthly costs of all blood components/products decreased from €66 to €45 (–32%) and the average monthly costs for ROTEM were €1.58. Two other studies85,88 – one in liver transplant patients85 and one in cardiac patients88 – also reported that an algorithm incorporating ROTEM reduced costs, but neither reported a detailed breakdown of cost-savings of transfusion or the costs of the ROTEM device.
The study that evaluated TEG86 concluded that its use in cardiac surgery reduced costs. However, no numerical data were presented, and data on the effect measure used were not provided.
Model structure and methodology
This section describes the de novo model used to evaluate the cost-effectiveness of ROTEM, TEG and Sonoclot (VE devices) compared with SLTs (no VE devices) to assist with the diagnosis, management and monitoring of haemostasis in the patient populations of interest: cardiac surgery patients and trauma patients with suspected coagulopathy. There were insufficient data from the effectiveness review to construct a model for the assessment VE devices in women with PPH (see Chapter 3, How well do viscoelastic devices predict relevant clinical outcomes in patients with post-partum haemorrhage?). The models were constructed in Microsoft Excel.
Cardiac surgery
We adopted the model structure used by the Health Technology Assessment (HTA) undertaken for NHS Scotland in 2008,12 which was largely based on a cost-effectiveness study of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion by Davies et al. 90 As these studies were undertaken in 2008 and 2006, respectively, more recent data sources were used to update the input parameters of the model wherever possible.
Our model is based on a decision tree that starts with the choice of strategy to be followed, that is, VE device (ROTEM, TEG or Sonoclot) or SLTs. Within each strategy, patients then either do or do not receive a transfusion.
Red blood cell transfusion, where it occurs, may be associated with adverse events or complications. The complications included in the model were those considered in Davies et al. 90 and the Scottish HTA. 12 Most complications are a consequence of RBC transfusion, although some were modelled as a consequence of any transfusion.
Complications were categorised as (1) complications related to surgery and/or transfusion or (2) transfusion-related complications. Complications related to surgery and/or transfusion included in the model were renal dysfunction, myocardial infarction, stroke, thrombosis, excessive bleeding requiring re-operation, wound complications and septicaemia. Transfusion-related complications included transfusion-associated graft-versus-host disease, complications related to the administration of an incorrect blood component, haemolytic transfusion reactions (acute or delayed), post-transfusion purpura (PTP), transfusion-related acute lung injury (TRALI) and febrile reaction. In addition, we assumed that patients may also experience transfusion-transmitted infections. Transfusion-transmitted infections include bacterial contamination, variant Creutzfeldt–Jakob disease (vCJD), hepatitis A virus (HAV), malaria, human T-cell lymphotropic virus (HTLV), human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV). The model structure is shown in Figure 28.
FIGURE 28.
Cost-effectiveness model structure.

The model’s time horizons were set to 1 month and 1 year because the benefits of a reduction in RBC transfusion were considered to have occurred within this time frame. At 1 month, the model reflects the period of hospitalisation and accordingly captures the impact of complications related to surgery and blood loss, transfusion-related complications and infection caused by bacterial contamination. It should be noted that, as in Davies et al. ’s study,90 bacterial contamination is the only transfusion-transmitted infection that was assumed to occur during the hospitalisation period. For other transfusion-transmitted infections included in the model, a time horizon of 1 year was considered more appropriate, as these infections do not usually manifest themselves immediately. Discounting was not necessary, as the longest time horizon was set at 1 year. Costs were estimated from the perspective of the NHS in England and Wales. Consequences were expressed in life-years (LYs) gained and quality-adjusted life-years (QALYs). QALY weights (utilities) were assigned to adverse events to express their consequences. Sensitivity analysis relating to extended time periods would have been undertaken had there been potential to impact on results and conclusions.
Patients with coagulopathy induced by trauma
The model for trauma patients has largely the same structure as the model in cardiac surgery patients. The only difference relates to the ‘surgery- and/or transfusion-related complications’, which were replaced with ‘trauma- and/or transfusion-related complications’ – ARDS and MOF.
Model input parameters
This section describes the input parameters used in the model for the cardiac and trauma populations and how we estimated their values. Whenever possible, parameters were estimated from our systematic review (see Chapter 3). If systematic review data were not available, model input parameters were derived from various sources including Davies et al. 90 and the Scottish HTA reports. 12 When standard errors (SEs) were not reported, estimates for the PSA assumed a 95% CI with limits deviating 20% from the mean, as we assumed that this would represent a reasonable range of variation.
Cardiac surgery
Probability of red blood cell transfusion
We estimated the baseline risk of having a transfusion based on the number of transfusions in the SLTs group in the cardiac surgery trials included in the effectiveness review (Figure 29). This analysis was based on the studies by Ak et al. ,46 Avidan et al. ,48 Shore-Lesserson et al. 51 and Kultufan Turan et al. 52 We excluded two studies,35,54 as we did not think that the patients included in these studies were representative of general cardiac surgery patients; one study54 enrolled only high-risk patients (aortic surgery requiring hypothermic circulatory arrest, including urgent and emergency surgery) and the other study35 was restricted to patients with excessive bleeding. We used a double arcsine transformation before pooling data using the MetaXL (Epigear International, Wilston, QLD, Australia) add-on for Excel. 91 The summary estimate for the probability of RBC transfusion from these four studies46,48,51,52 was 0.592 (95% CI 0.528 to 0.654). The RR of RBC transfusion in cardiac surgery patients whose blood was tested with VE devices compared with SLTs was reported in Chapter 3 (see Results).
FIGURE 29.
Forest plot showing the probability of RBC transfusion (95% CI) in control groups in cardiac surgery trials.

To estimate the probability of RBC transfusion for the three VE strategies a RR was applied to the baseline prevalence of RBC transfusion. The effectiveness review found no evidence of a difference in the RR of RBC transfusion between studies that assessed ROTEM and those that assessed TEG (see Chapter 3, Results). We therefore applied the summary RR for RBC transfusion estimated for all studies for the ROTEM and TEG models. Limited data suggested that the accuracy of Sonoclot in predicting clinical outcomes may be similar to that of TEG. We therefore also assumed that this summary RR could be applied in the Sonoclot model. The baseline prevalence of RBC transfusion in patients who received SLTs and the RR for the three VE devices can be seen in Table 19. A beta and a normal distribution, respectively, were assigned for the PSA.
| Technology | Mean value | Distribution | Distribution parameters | Source |
|---|---|---|---|---|
| Baseline risk of RBC transfusion in SLTs group | Base case: 0.592 | Normala (probability of RBC transfusion) | µ = 0.592; σ = 0.03 | See Chapter 3, Results |
| RR: ROTEM, TEG and Sonoclot | Base-case RR = 0.88 | Normalb | µ = 0.88; σ = 0.04 |
Complications related to surgery and transfusion
Complications included in the model relating to surgery and/or transfusion were renal dysfunction, myocardial infarction, stroke, thrombosis (any type, such as DVT or peripheral vascular thrombosis), excessive bleeding requiring re-operation, wound complications and septicaemia. The only one of these complications evaluated by the RCTs included in the effectiveness review (see Chapter 3, Results) was re-operation to investigate bleeding. As with the probability of transfusion, we excluded two studies35,54 from this analysis, as the patients included in these studies were representative of general cardiac surgery patients. The summary estimate for the probability of re-operation from the remaining five studies46,48–51 was 0.053 (95% CI 0.029 to 0.084) (Figure 30). The summary RR for the difference in transfusion risk for patients who received VE testing compared with SLTs was also taken from the clinical effectiveness review (see Table 11).
FIGURE 30.
Forest plot showing the probability of re-operation for bleeding (95% CI) in control groups in cardiac surgery trials.
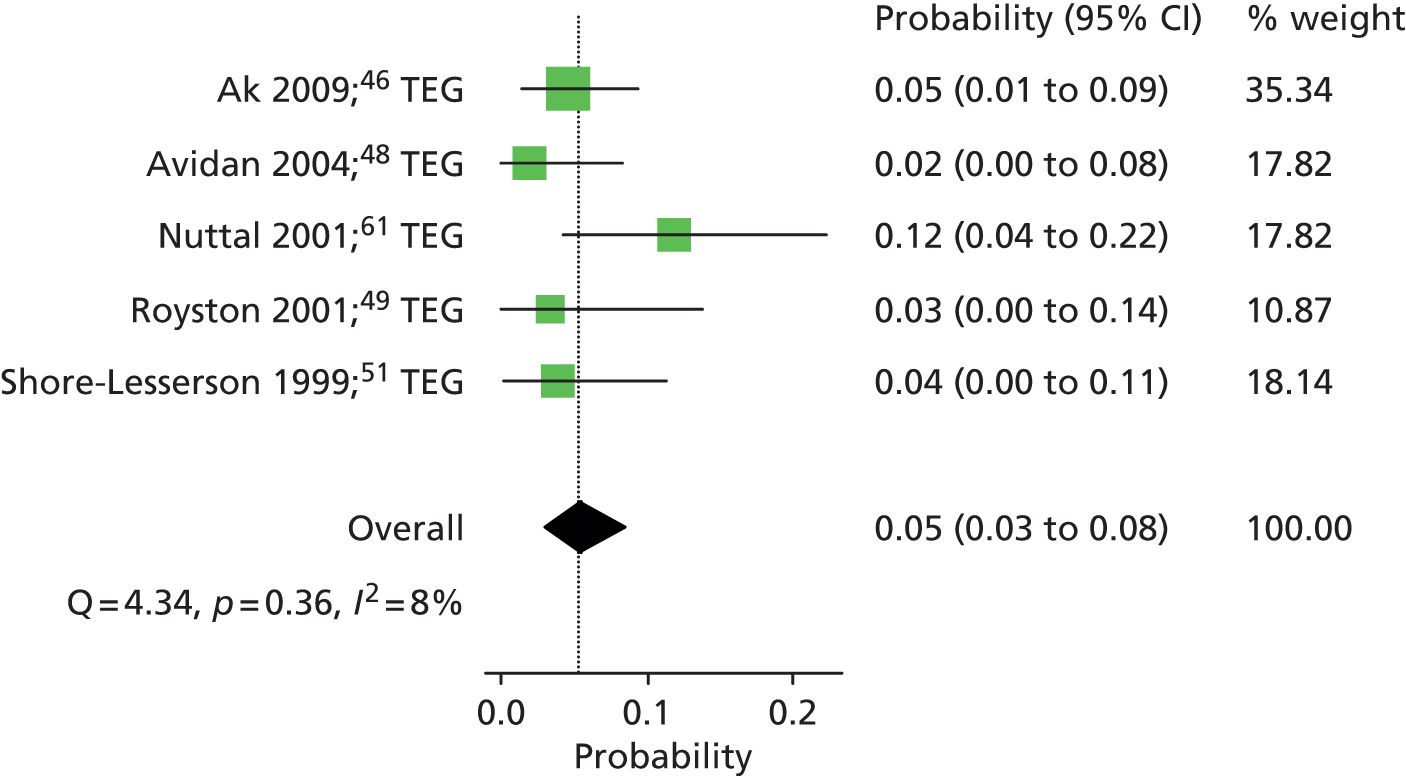
Data on the other complications were limited and we therefore assumed that there was no difference in the direct risk of having a complication between those tested with VE devices and those tested with SLTs as in Davies et al. 90 However, the risk of complications in each testing strategy was influenced indirectly by the different RBC transfusion rates associated with each strategy. The probabilities of experiencing complications related to surgery and/or transfusion, and the probability distributions for the PSA are shown in Table 20. The probability of experiencing septicaemia was sourced, as in the Scottish study,15,89 from Karkouti et al. 92 However, the population in this study92 was not representative of our population, as it included only patients who received four or more units of RBC within 1 day of surgery (i.e. patients with massive bleed). As this estimate was judged to be too high, our model used the estimate in Karkouti et al. 92 reduced by an arbitrary factor of 0.5.
| Type of complication | Mean value | Distribution | Distribution parametersa | Source |
|---|---|---|---|---|
| Renal dysfunction | 0.03 | Normal | µ = 0.03; σ = 0.003 | Davies (2006)90 |
| Myocardial infarction | 0.03 | µ = 0.03; σ = 0.003 | ||
| Stroke | 0.01 | µ = 0.01; σ = 0.001 | ||
| Thrombosis | 0.03 | µ = 0.03; σ = 0.003 | ||
| Excessive bleeding re-operation: | ||||
| Baseline risk SLTs | 0.053 | Normal | µ = 0.053; σ = 0.019 | See Chapter 3, Risk of bias assessment |
| RR VE devices | 0.72 | Log-normal | µ = 0.72; σ = 0.285 | |
| Wound complications | 0.07 | Normal | µ = 0.07; σ = 0.007 | Davies (2006)90 |
| Septicaemia | 0.0207 [0.0414 from Karkouti (2006)92] | Beta | α = 38; β = 917 | Karkouti (2006)92 and assumption |
Transfusion-related complications
The trials included in the clinical effectiveness review did not report data on transfusion-related complications, therefore, data on the probabilities of experiencing transfusion-related complications were based on reports from the UK Serious Hazards of Transfusion (SHOT). 93 The SHOT observations were first corrected for participation in the SHOT survey, as was done in Davies et al. ;90 this was 96% in 200194 and 99% in 2004. 95 As we used data dating back to 2000 (after the start of leucodepletion), we used an average of 98% participation. We assumed that, as in the Davies report,90 the total number of transfused patients per year is around 800,000. 96 Therefore, the probabilities shown in Table 21 are calculated in the following steps:
-
estimate the average number of complications per year over the available number of years (for some complications data were available from 2000 to 2012, for others only 2012 data were available)
-
divided by 800,000 to get number per transfused patient
-
divided by 0.98 to correct for survey participation.
Probabilities of experiencing a transfusion-related complication were reported as the risk per patient transfused (see Table 21).
| Type of complication | Probability (mean value) | Distribution | Distribution parametersa | Source | |
|---|---|---|---|---|---|
| Transfusion-associated graft-versus-host disease | 0.00000021 | Normal | µ = 0.00000021; σ = 0.000000022 | UK SHOT93 | |
| Incorrect blood component | 0.0003 | µ = 0.00030; σ = 0.00003086 | |||
| Haemolytic transfusion reactions | |||||
| Acute | 0.000011 | µ = 0.000011; σ = 0.00000112 | |||
| Delayed | 0.00004 | µ = 0.00004; σ = 0.000004125 | |||
| PTP | 0.0000015 | µ = 0.0000015; σ = 0.000000156 | |||
| TRALI | 0.000023 | µ = 0.000023; σ = 0.0000024 | |||
| Febrile reaction | 0.0003 | µ = 0.0003; σ = 0.000030751 | |||
Transfusion-transmitted infections
The probabilities of experiencing transfusion-transmitted infections were also taken from the UK SHOT report93 using the same method of calculation as for transfusion-related complications (Table 22). These were also reported as the risk per patient transfused.
| Type of infection | Probability (mean value) | Distribution | Distribution parameters | Source |
|---|---|---|---|---|
| Bacterial contamination | 0.000002657 | Normal | µ = 0.000002657; σ = 0.000000271 | UK SHOT93 |
| vCJD | 0.000000319 | µ = 0.000000319; σ = 0.000000033 | ||
| HAV | 0.000000213 | µ = 0.000000213; σ = 0.000000022 | ||
| Malaria | 0.000000106 | µ = 0.000000106; σ = 0.000000011 | ||
| HTLV | 0.000000213 | µ = 0.000000213; σ = 0.000000022 | ||
| HIV | 0.000000106 | µ = 0.000000109; σ = 0.000000011 | ||
| HBV | 0.000000531 | µ = 0.000000531; σ = 0.000000054 | ||
| HCV | 0 | NA | NA |
Mortality
At 1 month, we estimated the risk of mortality in the SLTs group, based on the number of deaths reported in Murphy et al.,15 as this study was based on a large sample (n = 8598) of a population that matched our target population. Murphy et al. 15 reported a 1-month mortality of 0.4% for non-transfused patients and 4.3% for transfused patients (note that these numbers were taken from the survival curves presented). Using the transfusion percentage applied in the current model (59.2%; see Table 19), this would yield an overall (transfused or not) 1-month mortality of 2.7%.
Several different complications can occur with transfusion and one would expect the mortality to vary by complication. However, it was assumed that the mortality of all transfused patients (essentially the sum of mortalities owing to each complication and no complication) was fixed at 4.3%, as seen in Murphy et al. 15 Therefore, in order to obtain a 4.3% mortality rate in the transfused group, we used a calibration procedure. What this meant is that where reliable estimates were available, a specific mortality estimate was applied to each complication (e.g. see example TRALI or graft-versus-host disease in Table 23). For the remaining complications and for no complications the mortality value was calculated so that the total mortality added up to 4.3%. This mortality value was calculated to be 4.28% (see Table 23).
For the transfusion-transmitted infections (except bacterial contamination), the 1-month mortality was assumed to be zero, as these infections were assumed to manifest themselves after the hospitalisation period. Mortality rates for various transfusion-related complications and bacterial contamination were derived from the SHOT survey. 93 Exceptions were ‘incorrect blood component’, ‘delayed haemolytic transfusion reactions’ and ‘febrile reaction’. For these, the SHOT survey reported mortality rates of (close to) zero. Implementing this in the model would imply that having such a complication would actually prevent mortality; we therefore disregarded the SHOT mortality rates for these complications. Therefore, the calibration procedure was used to calculate the mortality for all surgery and/or transfusion complications, transfusion (but without complications and ‘incorrect blood component’), ‘delayed haemolytic transfusion reactions’ and ‘febrile reaction’.
In order to estimate the mortality for VE testing, we assumed that any mortality benefit from VE testing resulted from fewer patients receiving a transfusion. This meant that the 1-month mortality for each patient group (not transfused, transfused without complications, transfused with complications) in the VE group was assumed to be the same as in the SLTs group.
At 1 year the mortality in the SLTs group was also estimated using data from Murphy et al. ,15 which reported a 1-year mortality of 1.2% for non-transfused patients and 7.8% for transfused patients. For the non-transfused patients, a 0.4% mortality at 1 month and a 1.2% mortality at 1 year yielded a mortality rate for between 1 and 12 months of (1.2% – 0.4%)/(100% – 0.4%) = 0.8%. Similarly, for the transfused patients a mortality rate for between 1 and 12 months was calculated as (7.8% – 4.3%)/(100% – 4.3%) = 3.66%.
As for 1-month mortality, the 1-year mortality for each subgroup of patients in the VE group was assumed to be the same as in the SLTs group. All of the mortality rates used in the model for the cardiac surgery population are summarised in Table 23.
| Type of complication or infection | 1 month | 1 year | |||
|---|---|---|---|---|---|
| Mean probability (SD)a (SLTs and VE) | Source | Mean probability (SD) (SLTs and VE) | Source | ||
| No transfusion | 0.0040 (0.0004) | Murphy (2007)15 | 0.0080 (0.0008) | Murphy (2007)15 | |
| Transfusion and no complications | 0.0428 (0.0043) | Calibration | 0.0366 (0.0037) Calibration |
||
| Renal dysfunction | |||||
| Myocardial infarction | |||||
| Stroke | |||||
| Thrombosis | |||||
| Excessive bleeding re-operation | |||||
| Wound complications | |||||
| Septicaemia | |||||
| Transfusion-associated graft-versus-host disease | 1 | SHOT93 | |||
| Incorrect blood component | 0.0428 (0.0043) | Calibration | |||
| Haemolytic transfusion reactions | Acute | 0.111 (0.0113) | SHOT93 | ||
| Delayed | 0.0428 (0.0043) | Calibration | |||
| PTP | 0.0667 (0.0068) | SHOT93 | |||
| TRALI | 0.0938 (0.0095) | ||||
| Febrile reaction | 0.0428 (0.0043) | Calibration | |||
| Bacterial contamination | 0.2750 (0.0280) | SHOT93 | |||
| vCJD | NA | Assumption | |||
| HAV | |||||
| Malaria | |||||
| HTLV | |||||
| HIV | |||||
| HBV | |||||
| HCV | |||||
Health benefits
Health benefits were expressed in terms of LYs and QALYs gained at 1 month and 1 year. For the calculation of the LYs, patients were assumed to die in the middle of the period when death occurred. Thus, for patients who died in month 1, we distinguish between those who die halfway through the hospitalisation period and those who die halfway between hospital discharge and end of the month. For patients who survived the first month but died subsequently, it was assumed that death occurred halfway between month 1 and month 12 (i.e. at 6.5 months).
Life-years were then valued with different utilities, depending on the health state of the patient. We followed the approach used in the Davies et al. 90 and Scottish HTA reports. 12 Except for stroke, we used utility values from the 1996 Health Survey for England:97
-
During the hospitalisation period the value for the health state associated with ‘limiting long-standing illness’ (0.64) was used.
-
For the period between hospital discharge and 1 month, the mean utility value associated with the health state ‘non-limiting long-standing illness’ (0.88) was used.
-
For months 1 to 12, the mean utility value associated with the health state ‘no long-standing illness’ (0.93) was used, except for patients with transfusion associated infection for whom the mean utility value associated with the health state ‘non-limiting long-standing illness’ (0.88) was used.
For patients with a stroke, we used a utility value of 0.64 from a study Luengo-Fernandez et al. 98 for hospital discharge to month 12. The utilities used in the model are summarised in Table 24.
| Health states | Mean utility value | Distribution | Distribution parameters |
|---|---|---|---|
| From surgery to hospital discharge | |||
| All patients | 0.64 | Beta | α = 0.7898; β = 0.4443 |
| From hospital discharge to 1 month | |||
| All patients except stroke | 0.88 | Beta | α = 2.9799; β = 0.4063 |
| Stroke patientsa | 0.64 | Normal | µ = 0.64; σ = 0.0653 |
| Months 1–12 (after surgery and hospital discharge) | |||
| All patients except stroke and transmitted infections | 0.93 | Beta | α = 5.6187; β = 0.4229 |
| Stroke | 0.64 | Normal | µ = 0.64; σ = 0.0653 |
| Transmitted infections | 0.88 | Beta | α = 2.9799; β = 0.4063 |
Costs
Short-term (1 month) and long-term (1 year) costs were considered in the model. Short-term costs included the following four groups: (1) pre-operative and perioperative costs of transfusion; (2) costs of blood components/products; (3) test costs for the identification of patients at risk of bleeding during or after transfusion; and (4) costs related to complications as a result of surgery and blood loss, transfusion-related complications and infections due to bacterial contamination. Long-term costs included those related to the other transfusion-transmitted infections, that is, vCJD, HAV, malaria, HTLV, HIV, HBV and HCV, and disabling stroke.
Pre-operative and perioperative costs of transfusion
Pre-operative and perioperative costs of transfusion were taken from the Davies report90 and inflated to 2013 prices99 (Table 25). These included blood group tests, screening, cross-matching, additional allogeneic blood matching and those related to the use of transfusion sets. These costs inflated to 2013 prices can be seen in Table 25.
| Type of service | Cost (£) | Source |
|---|---|---|
| Pre-operative costs of allogeneic blood per transfusion | 27.97 | Davies (2006)90 |
| Perioperative costs of transfusion services: | ||
| Additional allogeneic blood match | 0.65 | |
| Use of transfusion sets | 3.21 |
Cost of blood components
We included three types of blood components in the model. The prices for standard RBCs, adult platelets and clinical FFP were obtained from the NHS Blood and Transplant Service price list 2013–14,100 and these are £122.09, £208.09 and £27.98, respectively.
Data on units of blood transfused (Table 26) were obtained from Shore-Lesserson et al. 51 Although several other studies also provided information on this parameter, most provided data on the median rather than mean units of blood transfused per patient enrolled in the study. We needed to estimate the average number of units of blood per transfused patient; however, all RCTs reported the mean or median number of units of blood per patient enrolled in the study. It was possible to use these data to calculate the average volume of blood transfused per transfused patient only for studies that reported this information as a mean rather than median value, and that also provided data on the proportion of patients in the study who received a transfusion. The only study able to provide the required information was the study by Shore-Lesserson et al. 51 This study provided data on the volume of blood transfused in millilitres per patient enrolled in the study. To estimate the number of units of blood transfused per transfused patient we divided this number by 300 (the number of millilitres of blood in one unit) and then divided this number by the proportion of patients who received a transfusion.
For example, for RBCs Shore-Lesserson et al. 51 reported an average transfusion of 475 ml per patient (transfused or not) for the SLTs group. This is equivalent to 1.58 units (475/300). The proportion of patients in the SLTs arm who received a transfusion was 59% and so the average number of units per transfused patient was 1.58/0.59 = 2.65. The mean number of units of RBC transfused for patients in the VE group was slightly higher than in the SLTs group, whereas the units of FFP and platelets were lower. This might suggest that VE testing leads to some substitution of one blood component by another.
| Technology | Mean value | Distributionb | Distribution parameters | Source |
|---|---|---|---|---|
| ROTEM, TEG and Sonoclot | ||||
| RBC | 2.84 | Gamma | α = 180.03; β = 4.73 | Shore-Lesserson et al.51 |
| FFP | 0.29 | α = 62.14; β = 1.40 | ||
| Adult platelet pack | 0.27 | α = 28.12; β = 2.88 | ||
| SLTs | ||||
| RBC | 2.66 | Gamma | α = 94.46; β = 8.23 | Shore-Lesserson et al.51 |
| FFP | 1.21 | α = 53.75; β = 6.78 | ||
| Adult platelet pack | 0.47 | α = 17.34; β = 8.17 | ||
Cost of viscoelastic devices
To estimate acquisition costs of the different VE devices, we assumed that four channel devices were used. This is because, at the time of writing, this is the only version that is available for all three devices (ROTEM, TEG and Sonoclot). It should be noted that these are more expensive than one- and two-channel versions, which are available for TEG and Sonoclot. Each of the manufacturers quoted a number of extra cost items in addition to the cost of the device itself. Only those extras that were available (and comparable) for the three devices, were included in the acquisition costs in order to maintain consistency. After-care and training costs were also included, although the equivalency of these between devices was difficult to assess. As in the Scottish HTA12 we assumed that a machine would be used for 3 years (the total acquisition cost are then divided by three to obtain the cost per year). An important variable in the estimation of equipment costs per test is the number of tests per device per year. In the Scottish report,12 an assumption was made that 200 tests would be undertaken per year. However, experts indicted values much higher, ranging from 600 to 8000 per year (with the 8000 performed on an eight-channel machine). We have therefore assumed that, on average, 500 tests are performed per centre per year. It should be noted though, that here by ‘test’ we mean a set of assays. Thus, if, for one patient, at one time, three assays are run simultaneously, this is regarded as one single test. Table 27 presents the estimated equipment costs for ROTEM, TEG and Sonoclot.
| Cost component | ROTEM | TEG | Sonoclot |
|---|---|---|---|
| Four-channel device | 24,950 | 20,000 | 14,950 |
| Connectivity kit | 4078 | Included in device cost | Included in device cost |
| Software/database commander | 2415 | ||
| Printer | 126 | ||
| Trolley | 1015 | 750 | |
| Total device cost | 32,584 | 20,000 | 15,700 |
| Years of use | 3 | 3 | 3 |
| Total cost ROTEM plus extras per year | 10,861 | 6667 | 5233 |
| After-care cost per year | 1750 | 2000 | 933 |
| Training cost per year (advanced) | 725 | 0 | 0 |
| Total cost ROTEM per year | 13,336 | 8677 | 6633 |
| No. of tests per year with the four-channel device | 500 | 500 | 500 |
| Material cost per test | 26.67 | 17.33 | 13.27 |
The number of VE tests conducted on each patient in the RCTs included in the systematic reviews varied from one to six; five studies35,47–49,51 reported that patients received three tests, with three of these studies35,48,54 performing more tests if patients continued to bleed (see Table 7). We therefore assumed that each patient was tested three times in total during and after surgery. To estimate the total average cost of each VE test, the estimated equipment cost of (see Table 27) has to be added to the cost of a basic test, which has to be defined. The assays that can potentially be used by ROTEM, TEG and Sonoclot are described in Tables 2–4, respectively. Only three35,53,54 of the five studies35,52–55 that assessed ROTEM reported on the assays used. One study54 used INTEM, HEPTEM, FIBTEM and APTEM, one study35 used EXTEM, INTEM, FIBTEM and HEPTEM, and the third53 used EXTEM and FIBTEM. For the model, we assumed that INTEM, EXTEM, FIBTEM and HEPTEM would be used. Five studies46–49,51 of the six studies46–51 that used TEG provided details on the assays used: all ran standard kaolin assays, with and without heparinase. We therefore assumed a basic kaolin and heparinase test for TEG. As there were no RCTs of Sonoclot we did not have data on the assays that might be used in practice. We assumed that the gbACT and the kaolin-activated clotting/coagulation time assay (kACT) would be used, as these are similar to the assays selected for ROTEM and TEG; the kACT assay can be used for high-dose heparin management. It should be noted that in clinical practice various other combinations of assays may be used, depending on the patient. The total cost of a test for the cardiac surgery model is summarised in Table 28.
| Basic test | Cost (£) |
|---|---|
| ROTEM INTEM | 1.13 |
| ROTEM EXTEM | 1.22 |
| ROTEM FIBTEM | 2.22 |
| ROTEM HEPTEM | 2.43 |
| Cup and pin (×4) | 3.15 × 4 |
| Equipment cost | 26.67 |
| Total cost ROTEM test | 46.27 |
| Kaolin vial | 2.72 |
| Heparinase cup and pin | 8.75 |
| Plain cup and pin | 5.45 |
| Equipment cost | 17.33 |
| Total cost TEG test | 34.25 |
| gbACT | 2.20 |
| kACT | 2.20 |
| Equipment cost | 12.33 |
| Total cost Sonoclot test | 16.73 |
Cost of standard laboratory tests
As described in Chapter 2 (see Comparator: standard laboratory tests for coagulopathy), the comparator for this technology appraisal is a combination of clinical judgement and SLTs. SLTs generally include the following five tests: PT (also used to derive the measures PR and INR), aPTT, PLT, plasma FIB concentration (PFC) and ACT. The total cost per set of SLTs inflated to 2013 prices99 was taken from the Scottish HTA12 and was equal to £26 for FIB concentration, PT, PC, ACT and aPTT combined.
Hospitalisation costs
Four studies35,46,47,54 included in the systematic review reported the mean length of hospital stay of patients undergoing cardiac surgery. However, these studies reported data inconsistently, in a format that did not permit pooling, making it difficult to produce a summary estimate across studies. As more contemporary, UK-specific data were available on LoS we selected these data for inclusion in the model. The average length of hospital stay was sourced from the HES 2012–2013,101 which reports a mean stay of 10.53 days per patient undergoing cardiac surgery. The cost per day (inflated to 2013 prices) was £198 for patients without complication, according to Davies et al. 90 As none of the studies including the effectiveness review reported significant differences between VE groups and SLTs in terms of length of hospital stay, we assumed equal average length of hospital stay for each of the different strategies. This assumption is conservative towards the VE testing groups, as you would expect patients with complications to have a longer hospital stay than those without.
To estimate the costs associated with complications and infections due to bacterial contamination during the hospitalisation period, we assumed that the days of hospitalisation were valued at different unit costs, depending on the type of event experienced. For example, where a patient experienced renal dysfunction, it was assumed an overall mean LoS of 10.53 days where 5.68 days were valued at £335 (Table 29) and the remaining 3.88 days were valued at £198. When a certain complication had an associated LoS longer than 10.53 days (e.g. wound complications) it was assumed that the overall LoS was the period of hospitalisation associated with the complication, and the days were valued at the unit cost of the corresponding complication (e.g. 12 days in the case of wound complications, each day valued at £245). In the case of bacterial contamination we assumed an additional hospitalisation period of 8.4 days, each day valued at £212.
Finally, as described above, patients who died were assumed to die in the middle of the hospitalisation period (including patients requiring re-operation). Thus, patients experiencing a complication were assumed to die in the middle of the period for which the complication lasted and only the cost corresponding to the complication was used (e.g. for renal dysfunction 2.84 days valued at £335 each day). When the cause of death was a re-operation, it was assumed that patients survived half of the hospitalisation period but the total cost of the re-operation was considered.
It should be noted that, as in the Davies et al. 90 and Scottish studies,12 costs of ICU stay were not considered and thus the total costs may be underestimated. Four RCTs35,46,47,54 included in the effectiveness review evaluated the length of ICU stay and all reported shorter stays in the VE group compared with control (although this difference was only statistically significant in one study35). Thus, we may expect that if the costs of ICU stay had been included that the results would be more favourable for the VE-tested group.
| Health states | Mean (SDa) LoS in days | Mean (SDa) cost (£) per day (inflated to 2013 prices) |
|---|---|---|
| Renal dysfunction | 5.68 (0.57) | 335 (34.18) |
| Myocardial infarction | 8.91 (0.90) | 198 (20.20) |
| Stroke | 8.76 (0.89) | 270 (27.55) |
| Thrombosis | 3.32 (0.33) | 319 (32.55) |
| Excessive bleeding re-operation | 0.13 (0.01) | 2922 (298.19) |
| Wound complications | 12.00 (1.22) | 245 (25.00) |
| Septicaemia | 7.00 (0.71) | 271 (27.65) |
| Transfusion-associated graft-versus-host disease | 6.80 (0.69) | 1173 (119.69) |
| Incorrect blood component | 11.90 (1.21) | 212 (21.63) |
| Haemolytic transfusion reactions | ||
| Acute | 11.90 (1.21) | 818 (83.47) |
| Delayed | 11.90 (1.21) | 818 (83.47) |
| PTP | 2.50 (0.25) | 818 (83.47) |
| TRALI | 1.98 (0.20) | 1173 (119.69) |
| Febrile reaction | 1.00 (0.10) | 998 (101.84) |
| Bacterial contamination | 8.40 (0.85) | 212 (21.63) |
Costs between hospital discharge and 1 year after surgery
Long-term costs (during months 1 and 12 after cardiac surgery) as a result of all transfusion-transmitted infections, with the exception of bacterial contamination, were included in the model. The number and the duration of hospitalisations and outpatient visits associated with each type of infection and the corresponding unit costs were obtained from the Davies et al. report90 (Table 30). For HAV, HBV, HCV and HIV we assumed two acute hospitalisations and three outpatient visits during the first 12 months after surgery. For malaria and HTLV we assumed two acute hospitalisations with no outpatient visits. For the costs of stroke, we used recently published estimates of costs based on UK data in the first year after a stroke. The first study reported costs of £8302 (exchange rate 1US$ = £0.64)102 and the second of £9248,103 yielding an average of £8775. Finally, patients were assumed to die in the middle of the period between months 1 and 12 after hospitalisation.
| Health states | Mean (SDa) LoS | Mean (SDa) cost (£) per day | |
|---|---|---|---|
| vCJD | 0 | NA | |
| HAV | Acute hospitalisation (×2) | 5.10 (0.52) | 475 (48.47) |
| Outpatient visit (×3) | 1.00 (0.10) | 266 (27.14) | |
| Malaria | Hospitalisation (×2) | 3.40 (0.34) | 475 (48.47) |
| Outpatient visit (×0) | 1.00 (0.10) | 266 (27.14) | |
| HTLV | Hospitalisation (×2) | 1.00 (0.10) | 598 (61.02) |
| Outpatient visit (×0) | 1.00 (0.10) | 266 (27.14) | |
| HIV | Hospitalisation (×2) | 6.97 (0.71) | 598 (61.02) |
| Outpatient visit (×3) | 1.00 (0.10) | 966 (98.57) | |
| HBV | Chronic hospitalisation (×2) | 7.40 (0.75) | 475 (48.47) |
| Outpatient visit (×3) | 1.00 (0.10) | 266 (27.14) | |
| HCV | Chronic hospitalisation (×2) | 3.50 (0.35) | 341 (34.79) |
| Outpatient visit (×3) | 1.00 (0.10) | 266 (27.14) | |
Patients with coagulopathy induced by trauma
The model in patients with coagulopathy induced by trauma was based on the model that we developed for patients undergoing cardiac surgery. The difference between the models relates to the surgery- and/or transfusion-related complications, which we have replaced with trauma- and/or transfusion-related complications, that is, ARDS and MOF. Where possible, we have used trauma-specific data as inputs to the model. Where these data were not available, including the impact of VE testing on the various parameters, we have used the same input values as for the cardiac surgery population.
Probability of red blood cell transfusion
We estimated the baseline risk of RBC transfusion for the SLTs group using data from the studies included in the effectiveness review (see Chapter 3, How well do viscoelastic devices predict relevant outcomes in patients with coagulopathy induced by trauma?) that reported data on the proportion of patients who received an RBC transfusion. We used a random-effects model to estimate the mean proportion of patients who received an RBC transfusion. This gave a summary estimate of 0.321 (95% CI 0.209 to 0.444) (Figure 31). As there were no data comparing the proportion of transfused patients in a trauma population who received VE testing compared with those who received SLTs, we applied the same RR as in the cardiac surgery population. These data are summarised in Table 31.
FIGURE 31.
Forest plot showing RBC transfusion rates (95% CI) in trauma patients.

| Technology | Mean value | Distribution | Distribution parameters | Source |
|---|---|---|---|---|
| Baseline risk: SLTs | 0.321 | Normala (probability of RBC transfusion) | µ = 0.321; σ = 0.056 | Estimated |
| RR: ROTEM, TEG and Sonoclot | RR = 0.88 | Normalb | µ = 0.88; σ = 0.08c | See Chapter 3 (Figure 8) and assumption |
Complications related to trauma and/or transfusion
We included the two main reported complications that can occur as a result of trauma, which also show a relationship with transfusion. 104 These complications are ARDS and MOF. Although other complications may also be relevant, they were not reported in the studies in trauma patients who were included in the systematic review (see Chapter 3, How well do viscoelastic devices predict relevant clinical outcomes in patients with coagulopathy induced by trauma?).
Estimates for the incidence of ARDS were obtained from a study by Chaiwat et al. 105 of 14,070 trauma patients conducted in the USA. This study105 reported an overall incidence of ARDS of 4.6%. It also allowed calculation of the data on ARDS related to transfusion, as it provided the incidence in patients who did not receive a transfusion, that is, 1.7%, which allowed us to estimate the proportion of patients with ARDS among those who received a transfusion as 15.5%. For MOF, no studies were found that either provided estimates or allowed direct calculation of incidence for those transfused. However, the overall incidence of MOF in trauma patients is higher than that of ARDS, ranging from 15% to 25%, which is three to five times higher than the ARDS incidence. 106–109 Assuming that the same ratio applies for the incidence in the transfused patients, an estimate of about 45–75% MOF would follow, with a simple average of 60%. However, the only trauma study retrieved on MOF,109 which reported the transfusion rate, found this rate to be double (45.8%) that of the ARDS study by Chaiwat et al. 105 (21%). Therefore, it might be suggested that an MOF incidence rate of 30% is a more realistic assumption; however, it is clear that this assumption is very uncertain.
Transfusion-related complications
The probability of transfusion-related complications was assumed to be the same as that for the cardiac surgery patients (see Table 21).
Transfusion-transmitted infections
The probability of transfusion-transmitted infections was assumed to be the same as that for the cardiac surgery population (see Table 22). This is likely to be an underestimation, as patients with trauma receive on average more units of blood than cardiac surgery patients (see Tables 26 and 33), increasing the exposure to various donors.
Mortality
At 1 month, we estimated the baseline risk of mortality for the SLTs group using the same method used to estimate the baseline risk of RBC transfusion. We identified studies included in the effectiveness review (see Chapter 3, How well do viscoelastic devices predict relevant clinical outcomes in patients with coagulopathy induced by trauma?) that reported data on 30-day or in-hospital mortality; if the time frame for mortality was not reported, it was assumed to be longer-term (i.e. up to 30 day) mortality. We then used a random-effects model to estimate the mean 1-month mortality in the SLTs group of 15.7% (95% CI 11.7% to 20.1%) (Figure 32).
FIGURE 32.
Forest plot showing overall 1-month mortality rates (95% CI) in trauma patients.
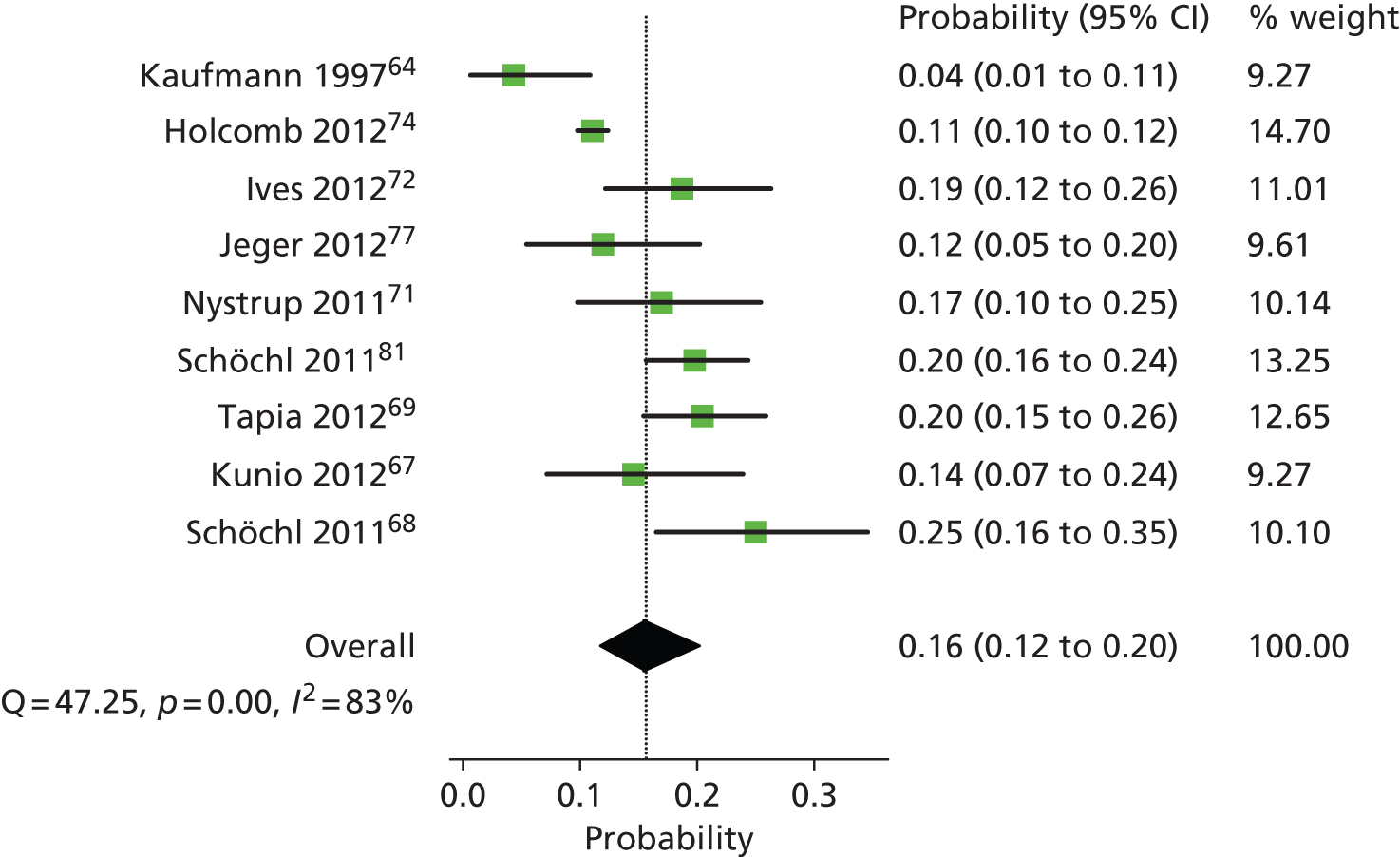
As for the cardiac patients, we aimed to assign 1-month mortality rates to transfused and non-transfused patients, such that the overall mortality rate would be equal to 15.7%. We were able to retrieve one study110 that reported mortality rates separately for transfused and non-transfused. This study included 1172 trauma patients who were admitted to an ICU, of whom 67% received a transfusion. In-hospital mortality was reported for patients who received a blood transfusion (21.4%) and those who did not (6.5%), showing that mortality was 3.3 times higher among patients who received a transfusion. It should be noted that the number of days in hospital for transfused patients was also higher (18.6 vs. 9), so the mortality rates are less easy to interpret than if mortality had been reported for a fixed time period (e.g. 30 days). The severity of the trauma was also more severe in these patients than might be seen in a general trauma population (mean ISS = 24); however, as similar data were not available in a general trauma population, this was the best estimate available.
Thus we assumed that the ratio of mortality for transfused (Morttrans) to non-transfused (Mortnot trans) was 3.3. Therefore, the goal was to estimate mortality rates such that the weighted average of these yielded an overall mortality of 15.7% – the mean mortality in the SLTs group derived from the systematic review, that is, 32% Morttrans + 68% Mortnot trans = 15.7%. From this, it follows that mortality was 9.1% in patients who did not receive a transfusion and 29.8% in those that did.
We then estimated mortality for the two trauma- and/or transfusion-related complications: ARDS and MOF. As none of the papers included in the systematic review reported on the incidence of ARDS or MOF and their associated mortality rates, we estimated these data from other sources. We estimated the probability of mortality in patients with ARDS from a trial in ARDS patients that reported a mortality rate of 83/385 = 21.6%. 111 We pooled data from two studies106,107 to estimate the mortality rate in patients with MOF (Table 32): a 12-year prospective study of 339 patients with post-injury MOF,106 and a prediction modelling study of 104 trauma patients of whom 21 developed MOF. 107 This yielded an overall MOF mortality rate of 26.2%.
| Study | No. dead | No. MOF | Mean |
|---|---|---|---|
| Dewar (2013)107 | 5 | 21 | 0.238 |
| Ciesla (2005)106 | 90 | 342 | 0.263 |
| Overall mean (inverse variance) | 0.262 | ||
| SE | 0.023 | ||
One-month mortality rates for transfusion-related complications and transfusion-transmitted infections were derived when possible from the SHOT survey,93 and, as in the cardiac surgery population, it was assumed that all infections apart from bacterial contamination would only manifest themselves after 1 month, implying a zero mortality rate in the first month.
However, as we calculated earlier, the overall mortality in the transfused group had to be 29.8% in order to achieve an overall mortality of 15.7% after 1 month. The ARDS and MOF mortality that we estimated from published studies were lower than this, which would imply that having ARDS or MOF lowers the mortality rate. As this is clinically implausible, we were confronted with a consistency issue caused by using data from various studies all with slightly different populations and ways of reporting. Any way of dealing with this issue involves arbitrary choices. We made the decision that all complication mortality rates that were below the overall mortality rate for the transfused patients would become part of a calibration similar to that applied in the cardiac population. De facto this means that only the mortality rate for transfusion-associated graft-versus-host disease (which is ‘1’) was not included in the calibration. The calibration procedure itself meant that all other transfusion-related mortality parameters were set to x, and a value of x was sought such that the transfusion mortality was 29.8%.
As in the cardiac population, the 1-month mortality for each subgroup of patients in the VE group (Table 33) was assumed to be the same as in the SLTs group, implying that any mortality benefit in the VE group was due to fewer patients being transfused.
| Type of complication or infection | 1 month (SLTs and VE) | ||
|---|---|---|---|
| Mean value (SDa) | Source | ||
| No transfusion | 0.091 (0.009) | Bochicchio (2008)110 and calibration | |
| Transfusion and no complications | 0.296 (0.030) | Calibration | |
| MOF | |||
| ARDS | |||
| Transfusion-associated graft-versus-host disease | 1 | SHOT93 | |
| Incorrect blood component | 0.296 (0.030) | Calibration | |
| Haemolytic transfusion reactions | Acute | ||
| Delayed | |||
| PTP | |||
| TRALI | |||
| Febrile reaction | |||
| Bacterial contamination | |||
| vCJD | NA | Assumption | |
| HAV | |||
| Malaria | |||
| HTLV | |||
| HIV | |||
| HBV | |||
| HCV | |||
For mortality of between 1 and 12 months after trauma, few data were available. One study112 was identified, which reported 3% mortality for this period. However, no information was identified on how this mortality is distributed over transfused and non-transfused patients. We therefore applied the same ratio as for 1-month mortality (3.3). Now we need to solve 32% Morttrans + 68% Mortnot trans = 3.0%. This yielded a mortality in the non-transfused of 1.7% and mortality in the transfused of 5.7%. These values were assumed to apply to both the SLTs and VE group.
Health benefits
The calculation of LYs was carried out in the same way as for the cardiac surgery patients. For the calculation of QALYs, we explored trauma-specific utilities. We used a review paper by Hofhuis and Spronk113 to identify relevant studies on utilities in trauma patients. This paper113 lists various studies in trauma patients reporting on health-related quality of life.
We selected studies that reported a mean European Quality of Life-5 Dimensions (EQ-5D) utility. Most studies collected EQ-5D data only 2–7 years after the trauma. Only one study,114 collected EQ-5D utilities 12–18 months after trauma. This study114 included patients with severe trauma (ISS scores ≥ 16) and reported a mean utility of 0.69 (SE 0.016) in these patients 12–18 months after the trauma. None of the studies reported utilities for the period of hospitalisation and shortly afterwards. We therefore assumed the same utility for the period of hospitalisation as for the cardiac population during hospitalisation, that is, 0.64, and a utility of 0.69, the value obtained in trauma patients at 12–18 months, after discharge. It is likely that these utility values are overestimations. It is reasonable to assume that trauma patients will have a worse quality of life than cardiac patients. However, no published data were available to show how much lower that utility should be. Similarly, the value of 0.69 was derived from a group of patients evaluated 12–18 months after the trauma; it is likely that the utility would be worse closer to the event. We opted for conservative estimates as lower utility values would have resulted in larger QALY gains for VE testing.
For patients with ARDS we used the results of a prospective cohort study that measured quality-adjusted survival in 200 patients in the first year after ARDS. 115 This study115 reported utilities of 0.60 (SE 0.01) and 0.64 (SE 0.01) at 6 months and 1 year after onset of ARDS, respectively. We applied the first value to the period of 1 month, and the latter to the period between months 1 and 12. As with the utility values for the general trauma population, these values, especially that for the period of hospitalisation, are likely to be an overestimation, as the utility would be expected to be worse closer to the trauma. Additionally, patients with a long stay on the ventilator would be expected to have utility values close to 0 while on the ventilator, and this is not taken into account in these values. However, in the absence of more reliable estimates of utilities for these patients we adopted these conservative values.
We were unable to find similar data for patients with MOF and so applied the same utilities as for patients with ARDS, based on the assumption that both complications are similar in their severity. For patients with transfusion-related complications, we assumed that after discharge, as in the cardiac population, the utility would be equivalent to patients without complications. We assumed that the additional disutility from having a transfusion-related infection was estimated by multiplying the utility of trauma patients having no transfusion complications or infection with the utility applied in the cardiac population for patients with infections. 116 Table 34 summarises the utilities used in the trauma model.
| Health states | Mean utility value | Distribution | Distribution parameters | Source |
|---|---|---|---|---|
| During hospitalisation | ||||
| All patients except transfusion and trauma complications | 0.64 | Beta | α = 0.7898; β = 0.4443 | Assumed same as cardiac population |
| Transfusion and trauma complications | 0.60 | Normal | µ = 0.60; σ = 0.091 | Angus (2001)115 |
| From hospital discharge to 1 month | ||||
| All patients except transfusion and trauma complications | 0.69 | Normal | µ = 0.69; σ = 0.1056 | Holtslag (2007)114 |
| Transfusion and trauma complications | 0.60 | Normal | µ = 0.60; σ = 0.091 | Angus (2001)115 |
| Months 1–12 (after surgery and hospital discharge) | ||||
| All patients except transfusion and trauma complications or transfusion-transmitted infection | 0.69 | Normal | µ = 0.69; σ = 0.1056 | Holtslag (2007)114 |
| Transfusion and trauma complications | 0.64 | µ = 0.64; σ = 0.0979 | Angus (2001)115 | |
| Transfusion-transmitted infection | 0.69 × 0.88 = 0.61 | µ = 0.61; σ = 0.0933 | Holtslag (2007)114 and Davies (2006)90 | |
Costs
Similar to the model in cardiac surgery patients, the trauma model also considered short (1 month) and long-term (1 year) costs. Short-term costs included the following four groups: (1) peritrauma costs of transfusion; (2) costs of blood components; (3) test costs for the identification of patients at risk of bleeding during or after transfusion; and (4) costs related to complications due to surgery and blood loss, transfusion-related complications and infection due to bacterial contamination. Long-term costs included those related to the other transfusion-transmitted infections (i.e. vCJD, HAV, malaria, HTLV, HIV, HBV and HCV).
Peritrauma costs of transfusion
We applied the same pre-operative and perioperative costs of transfusion as for the cardiac surgery population, under the assumption that tests that are undertaken pre-operatively in the cardiac population, such as cross-matching, are now done while the patient receives trauma care (see Cardiac surgery, Table 25, above).
Cost of blood components
As with the cardiac surgery population, we included three types of blood components in the model: standard RBCs, adult platelets and clinical FFP. We used data from the only two trauma studies71,72 in the effectiveness review that reported volumes of blood components used to estimate the average number of units transfused per transfused patient (Table 35). As both studies71,72 included a similar number of patients, a simple average was taken to estimate the number of units transfused per patient. This was adjusted by the proportion of patients who received a transfusion to give an estimate per transfused patient. To estimate the number of units for the VE testing strategy, we calculated the ratio of units transfused among cardiac patients tested with VE device (2.84) to the number of units transfused among cardiac patients tested with SLTs (2.65), based on the study by Shore-Lesserson et al. ,51 that is 1.07, and assumed that this would also be applicable to the trauma population.
| Study | RBC | FFP | Platelets |
|---|---|---|---|
| Ives (2012)72 | 9.5 | 10.9 | 3.3 |
| Nystrup (2011)71 | 3.4 | 2.2 | 1.6 |
| Average units per patient | 6.45 | 6.55 | 2.45 |
| Average units per transfused patient SLTs group | 20.09 | 20.40 | 7.63 |
| Ratio of units transfused among VE-tested patients compared with SLTs-tested patients (cardiac surgery population) | 1.07 | 0.24 | 0.57 |
| Average units per transfused patient VE group | 21.50 | 4.90 | 4.35 |
Cost of viscoelastic devices
In line with the study protocol of the ongoing RCT in trauma patients62 we assumed that each patient was tested five times. In addition, we assumed that the acquisition costs would be the same as in the cardiac population, as the material costs of the device would be the same and we again assumed that 500 tests would be performed per year.
The only difference in costs in terms of device was for the types of assays used to define a basic test (Table 36). We assumed that trauma patients would not be tested using the heparin assays. Therefore, for ROTEM we assumed that a basic test would consist of INTEM, EXTEM and FIBTEM; this was similar to the assays evaluated in the predictive accuracy studies included in the systematic review. For Sonoclot we assumed that patients would just receive a basic gbACT. For TEG, we assumed that the regular kaolin test would be replaced by the rapid TEG assay, as this was used by almost all of the predictive accuracy studies included in the systematic review and is also the assay used in the ongoing RCT. 61,62
| Basic test | Cost (£) |
|---|---|
| ROTEM INTEM | 1.13 |
| ROTEM EXTEM | 1.22 |
| ROTEM FIBTEM | 2.22 |
| Cup and pin (×3) | 3.15 × 3 |
| Equipment cost | 26.67 |
| Total cost ROTEM test | 40.69 |
| Rapid TEG | 11.25 |
| Plain cup and pin | 5.45 |
| Equipment cost | 17.33 |
| Total cost TEG test | 34.03 |
| gbACT | 2.20 |
| Equipment cost | 12.33 |
| Total cost Sonoclot test | 14.53 |
Cost of standard laboratory tests
These were assumed to be the same as for the cardiac population. The costs for SLTs for the cardiac population were based on a general battery of coagulation tests and it is likely that similar tests would be run for trauma patients.
Hospitalisation costs
Data on length of hospital stay for trauma patients were taken from the only two trauma studies71,72 included in the effectiveness review that reported on this parameter. One study72 reported a mean stay of 10.8 days and the other71 reported 10.3 days, which gives a simple average of 10.55 in-hospital days. Of these days, on average 4.9 were spent on the ICU. 72 For the ICU costs we assumed costs per day of £1173, based on National Schedule of Reference Costs – Years 2012–13. 117 For hospital stay beyond the stay in ICU, it was difficult to define a cost per day, as trauma patients can have a wide variety of injuries and may thus be admitted to various departments. As we were unable to define a more reliable estimate, we assumed the same per-day unit costs as for the cardiac surgery patients.
For patients with ARDS, we used data from Angus et al.,111 who reported an ICU LoS of 18.8 days, whereas hospital LoS was 26.8 days. For patient with MOF, we used data from Dewar et al. 107 who reported an ICU LoS of 19.1 days. No data were reported on overall LoS, so we assumed that after ICU discharge the patient spent the same amount of time in regular care as the trauma patients with ARDS (i.e. 26.8 – 18.8 = 8 days).
As the incidence of MOF and ARDS is high, and their mean length of ICU stay and hospital stay was much longer than the overall mean length of ICU stay, we estimated the length of ICU and hospital stay for patients who did not experience either MOF or ARDS, so that the overall mean length of hospital stay was 10.55 days and the mean length of ICU stay was 4.9 days. This gave ICU and hospital lengths of stay for patients without ARDS or MOF as estimated at 2.2 days and 7.4 days, respectively.
We had no data on how transfusion-related complications and bacterial infection would affect LoS. We therefore assumed the same LoS for these complications as for cardiac surgery patients and the same unit costs per day. Although patients remained in ICU for their trauma, we did not apply any hospital costs for complications, as we assumed that the level of care was already such that the marginal resource use because of the complications was relatively small. Once patients were no longer on the ICU, we applied the per-day costs for complications in the same way that we did for cardiac patients.
Costs between hospital discharge and 1 year after surgery
Long-term costs (during months 1 and 12 after trauma) due to all transfusion-transmitted infections, with the exception of bacterial contamination, were included in the model in the same way as for the cardiac population.
Sensitivity and scenario analyses
Probabilistic sensitivity analysis
The impact of statistical uncertainties regarding the model’s input parameters was explored through PSA. PSA results were presented in the cost-effectiveness plane for all the technologies compared. Cost-effectiveness acceptability curves (CEACs) were used to determine the probability of a strategy being considered cost-effective given a threshold incremental cost-effectiveness ratio (ICER). The probability distributions used in the PSA are listed in the tables presented above (see Model structure and methodology and Model input parameters).
Expected value of perfected information analysis
For the trauma model, we explored the value of information associated with the model uncertainty by estimating the expected value of perfect information (EVPI), which is the amount the decision-maker should be willing to pay to eliminate all uncertainty in the decision. The decision is made based on the expected net monetary benefit given current information, that is the technology with the highest expected net monetary benefit is chosen as optimal. The EVPI per patient was calculated as the average of the maximum net benefits across all PSA outcomes (expected net benefit of perfect information) minus the maximum average net benefit for the different technologies (expected net benefit given current information). Additional research might be justified when the expected net benefit for future patients, defined as the population EVPI, exceeds the expected costs of additional research. Therefore, the per-patient EVPI is multiplied by the population size to give the population EVPI. This is then summed over the lifetime for which the research recommendation is expected to be valid, discounted at 3.5% to give the net present value. 118 We selected a period of 5 years for this value. For the trauma model, a potential population of 16,825 adult patients in the UK was assumed, based on data from the National Audit Office. 119 This was calculated as follows:
-
total number of major trauma (ISS ≥ 16) patients in England was approximately 20,000 per year
-
number who die before they get to hospital is 2400
-
proportion aged ≤ 15 years is 4.4%.
Note that this provides an upper limit of the potential population, as SLTs and VE testing will probably not be indicated for the whole trauma population. We distinguished two approaches to the population EVPI depending on whether or not the problem to be addressed was which of the four different strategies should be recommended, or whether or not to recommend VE testing (e.g. ROTEM) instead of SLTs. In the former case, all four technologies were included in the EVPI estimation. For the latter situation we compared only ROTEM, as it the most expensive strategy.
Scenario analyses
Scenario analyses were performed to investigate the influence of number of years of machine usage, number of tests performed per year, number of tests per patient, RR of be probability of transfusion, baseline prevalence of RBC transfusion, units of blood component transfused, 1-month mortality, and the probability of experiencing complications related to trauma and/or transfusion (trauma model only). We only performed these analyses for the most expensive VE device (ROTEM) as if the results were cost-effective for this device then they would also be cost-effective for the other devices (TEG and Sonoclot).
Number of years of machine usage
The base case assumed that the hospital would use the VE device for 3 years. In this scenario, we increased the time that the hospital would use the device for to 5 years. Increasing the number of years for which the machine would be used affects only the cost of ROTEM, reducing it from £2588 to £2562 for the cardiac model, and from £6973 to £6929 for the trauma model.
Number of tests per year
The usage of the machine determines the material cost of a VE test: the higher the number of tests per machine per year, the lower the material cost (and therefore the higher the likelihood of being cost-effective). In the base case we assumed that on average, 500 tests would be run on each VE device per year. In the sensitivity analysis, we reduced the number of tests per year to 200, the value used in the Scottish HTA report. 12 We used iterative analysis to investigate the minimum number of tests per device year that would need to be performed for the VE devices to be considered cost-saving and cost-effective (ICERs of £0 and £30,000, respectively).
Number of tests per patient
In the base-case scenario, we assumed that each patient was tested three times in the cardiac surgery population and five times in the trauma population, based on the testing protocols used in the included RCTs. However, clinical experts suggested that, in practice, the number of tests performed per patient may be lower. In this scenario we therefore investigated the effects of changing the number of tests per patients. For the cardiac surgery population we reduced the number of tests so that non-transfused patients were tested once and transfused patients twice. For the trauma population we assumed that non-transfused patients would be tested two times and transfused patients would be tested three times. Reducing the number of tests per patients reduces the costs of both VE testing and SLTs.
Combination of assays
For the base case, we assumed that the number of times a patient was tested and the assays used were the same as in the RCTs included in the systematic review. Clinical experts suggested that the assays modelled for ROTEM and TEG may not be those used in clinical practice. Therefore, we explored the effects of varying the ROTEM and TEG assays and the number of times a patient was tested. This impacts only on the costs of the test, not on their effectiveness. Different scenarios were not explored for the Sonoclot device.
Three scenarios were defined for the cardiac population:
-
Three sets of tests performed:
-
Test 1: four assays (EXTEM/FIBTEM/INTEM/HEPTEM on ROTEM; rapid TEG/FIB/standard kaolin/heparinase on TEG)
-
Tests 2 and 3: two assays (EXTEM/FIBTEM on ROTEM; rapid TEG/FIB on TEG).
-
-
Three sets of tests performed, all with two assays (INTEM/HEPTEM on ROTEM; TEG: standard kaolin/heparinase).
-
Two sets of tests performed, all with two assays (INTEM/HEPTEM on ROTEM; TEG: standard kaolin/heparinase).
Three similar scenarios were defined for the trauma population:
-
Five sets of tests performed:
-
Test 1: four assays (EXTEM/FIBTEM/INTEM/HEPTEM on ROTEM; rapid TEG/FIB/standard kaolin/heparinase on TEG)
-
Tests 2–5: all with two assays (EXTEM/FIBTEM on ROTEM; rapid TEG/FIB on TEG).
-
-
Five sets of tests performed: all with single assays (EXTEM on ROTEM; rapid TEG).
-
Three sets of tests performed: all with single assays (EXTEM on ROTEM; rapid TEG).
Viscoelastic testing as an add-on to standard laboratory test
In the base-case scenario, we assumed that VE testing would be used as a replacement for SLT. However, it is possible that in clinical practice at least some of the VE tests will be performed in addition to SLTs, thus increasing the costs of the VE scenarios. Therefore, we investigated the impact of using VE testing as an add-on to SLTs in a separate scenario.
Relative risk of the probability of transfusion
The base-case scenario in both the cardiac and trauma models was based on the summary RR of RBC transfusion equal to 0.88 (95% CI 0.80 to 0.96) estimated in the systematic review (see Figure 8). We investigated the effects of this replacing 0.88 with the lower and upper limits of the CI. For the trauma population we assumed that the RR of RBC transfusion was equivalent to that in the cardiac surgery population. We conducted additional analyses to investigate the validity of the assumption. We used iterative analysis to investigate the minimum RR that would be needed for VE devices to be considered cost-saving and cost-effective (ICERs of £0 and £30,000, respectively). For this analysis, we assumed that equal blood volumes would be transfused in the VE-tested and SLTs groups.
Baseline prevalence of red blood cell transfusion
We varied the baseline prevalence of RBC transfusion by selecting one value lower than the base case and one value higher. For the lower estimate in cardiac surgery patients, we used the estimate from Murphy et al. ,15 which evaluated all patients who underwent cardiac surgery at the Bristol Royal Infirmary between 1996 and 2003 (n = 8598). This study15 reported a probability of RBC transfusion of 0.429. We did not have a reliable estimate for a higher prevalence of RBC transfusion in cardiac surgery patients and so selected an arbitrary value of 1.5 times the base-case value, equivalent to a probability of RBC transfusion of 0.89 in the SLTs group. For the trauma model, we did not identify any reliable sources for estimates of RBC transfusion in these patients. The baseline prevalence used in the trauma model (0.321) was estimated from studies included in the systematic review and had an accompanying 95% CI of 0.209 to 0.444. We investigated the effects of replacing the value in the base case with the upper (0.444) and lower (0.209) confidence limits around this estimate. As estimates in the trauma population were considered to be more uncertain, we conducted additional analyses in this population. We used iterative analysis to investigate the minimum baseline prevalence of RBC transfusion that would be required for VE devices to be considered cost-saving and cost-effective (ICERs of £0 and £30,000, respectively). For this analysis, we assumed that equal blood volumes would be transfused in the VE-tested and SLTs groups. We repeated this analysis for a RR of RBC transfusion in VE-tested patients compared with SLTs-tested patients of 0.95 (vs. 0.88 used in the base-case analysis), as the estimates of RR was uncertain in this population.
Units of blood component transfused
The estimates for the average units of blood transfused per transfused patient for the base case for both trauma and cardiac surgery were derived from studies included in the systematic review (see Table 26). In both the cardiac surgery and trauma populations, the number of units of RBC transfused for patients in the VE group was slightly higher than in the SLTs group, whereas the number of units of FFP and platelets were lower. We investigated the effects of changing the average units of blood transfused so that the average number of units transfused was the same in the SLTs and VE-testing groups.
Probability of experiencing complications related to trauma and/or transfusion (trauma model only)
The mean probability of experiencing ARDS and MOF included in the model were 0.155 and 0.30, respectively. In this scenario, we investigated the effect of reducing and increasing these probabilities by half; we replaced the base-case values by 0.0775 (ARDS) and 0.15 (MOF), and 0.2325 (ARDS) and 0.45 (MOF). We also investigated the effect of reducing the probability of complications related to trauma and/or transfusion, transfusion-related complications and transfusion-related infections to zero.
One-month mortality
For the base case in both the cardiac surgery and trauma populations, the 1-month mortality for transfused patients was calibrated to obtain an overall 1-month mortality figure: in the cardiac surgery patients, this was equal to 0.027 overall (the value reported by Murphy et al. ;15 see Table 23) and 0.0428 in the transfused patients (see Table 23); in the trauma population, the overall mortality figure was 0.157, and 0.296 in the transfused patients. We investigated the halving and doubling the mortality in the transfused patients (and making associated changes to the non-transfused, such that overall mortality remained the same); we replaced the base-case value with 0.0214 and 0.0642 in the cardiac surgery model, and with 0.1483 and 0.4450 in the trauma model.
Model assumptions
The assumptions used in the model are summarised below (Box 1):
ROTEM, TEG and Sonoclot were assumed to be equally effective.
Complications related to surgery and/or transfusion, transfusion-related complications and infection caused by bacterial contamination were assumed to occur during the hospitalisation period.
For the transfusion-transmitted infections (except bacterial contamination), 1-month mortality was assumed to be zero, as these infections were assumed to manifest themselves after the hospitalisation period.
Patients were assumed to die in the middle of the period where death occurred.
We assumed that four-channel VE devices were used.
Only those extra items that were available (and comparable) for the three devices, were included in the acquisition costs. After-care and training costs were also included.
We assumed 3 years of machine usage.
We assumed that, on average, 500 tests were performed per machine per year.
We assumed equal average length of hospital stay for the VE and SLTs groups.
For HAV, HBV, HCV and HIV, we assumed two acute hospitalisations and three outpatient visits during the first 12 months after surgery. For malaria and HTLV we assumed two acute hospitalisations with no outpatient visits.
Cardiac surgery populationWe assumed that there was no difference in the risk of having a complication between those tested with VE devices and those tested with SLTs (except for the probability of re-operation), except due to transfusion.
The probability of experiencing septicaemia was sourced from Karkouti et al. 92 but reduced by an arbitrary factor of 0.5.
The mortality associated with ‘incorrect blood component’, ‘delayed haemolytic transfusion reactions’, ‘febrile reaction’, all surgery and/or transfusion complications, and patients with transfusion but without complications was estimated using the calibration procedure described above (see Cardiac surgery, Transfusion-transmitted infections).
We assumed that any mortality benefit from VE testing resulted from fewer patients receiving a transfusion, which meant that the 1-month mortality for each patient group (not transfused, transfused without complications, transfused with complications) in the VE group was assumed to be the same as in the SLTs group.
The 1-year mortality for patients in each category (not transfused, transfused without complications, transfused with complications) for the VE group was assumed to be the same as in the SLTs group.
A basic test for ROTEM was defined as a combination of the INTEM, EXTEM, FIBTEM and HEPTEM assays. A basic test for TEG was defined as a standard kaolin assay and a heparinase assay. A basic test for Sonoclot was a combination of the gbACT and kACT would be used for this population.
It was assumed that each patient is tested three times in total during and after surgery.
For parameters for which SE were not reported, estimates for the PSA assumed a 95% CI with limits deviating 20% from the mean.
Trauma populationFor the proportion of patients who received VE testing compared with the ones who received SLTs, we applied the same RR as in the cardiac surgery population.
An MOF incidence rate of 30% was assumed.
The probability of transfusion-related complications and the probability of transfusion-transmitted infections were assumed to be the same as for cardiac surgery patients.
The ratio between mortality for transfused and non-transfused patients was assumed to be the same as in the Bochicchio et al. 110 study.
We assumed that all of the complication mortality rates that were below the overall mortality rate for transfused patients were part of a calibration, resulting in equal probabilities.
The 1-month and 1-year mortality for patients in each category (not transfused, transfused without complications, transfused with complications) for the VE group was assumed to be the same as in the SLTs group.
For the period of hospitalisation and the period from discharge to 1 month we assumed the same utility as for the cardiac population during hospitalisation.
We applied the same pre-operative and perioperative costs of transfusion as for the cardiac surgery population.
To estimate the number of units of blood transfused for the VE-testing strategy, we estimated the ratio of units transfused in the VE group and the units transfused in the SLTs group found in the cardiac group, and applied this to the SLTs trauma volumes.
A basic test for ROTEM was defined as a combination of the INTEM, EXTEM and FIBTEM assays. The rapid TEG assay was considered as the basic test for TEG. A basic test for Sonoclot was the gbACT assay.
We assumed that each patient was tested five times.
For parameters for which SE was not reported, estimates for the PSA assumed a 95% CI with limits deviating 30% from the mean.
Results of cost-effectiveness analyses
Base-case results for model in cardiac surgery patients
The base-case results from the analysis reported as LYs, QALYS and costs per technology for patients undergoing cardiac surgery are summarised in Table 37.
| Outcome | SLTs | ROTEM | TEG | Sonoclot |
|---|---|---|---|---|
| LY | 0.9624 | 0.9660 | 0.9660 | 0.9660 |
| QALY | 0.8726 | 0.8773 | 0.8773 | 0.8773 |
| Cost (£) | 2631 | 2588 | 2552 | 2499 |
| Incremental QALYs vs. SLTs | 0.0047 | 0.0047 | 0.0047 | |
| ICs vs. SLTs (£) | –43 | –79 | –132 |
Under the assumptions made above (see Model structure and methodology), all of the VE technologies dominated SLTs. As the same treatment effects were assumed for each VE testing device, effectiveness (measured using LYs and QALYs) was the same for each device. The cost of Sonoclot was lower than that of ROTEM or TEG and so this device was associated with greater cost-savings (£132) than TEG (£79) or ROTEM (£43).
Note that, in general, when four strategies are compared, a full incremental analysis should be performed. As is clear from Table 38, this would result in the simple conclusion that Sonoclot dominates all other options. Such an approach would be helpful to address the question of which of the four different testing strategies should be recommended. However, if the actual question is whether or not to recommend VE testing instead of SLTs then the pairwise comparisons against SLT shown here are more informative.
The total cost of testing per patient undergoing cardiac surgery for the four technologies included in the base-case analysis was £139 for ROTEM, £103 for TEG, £78 for SLTs, and £50 for Sonoclot. Other outputs of interest from the base-case analysis were overall 1-month and 1-year mortality, the percentage of patients experiencing surgery and/or transfusion complications, the percentage of patients experiencing transfusion-related complications, the percentage of patients experiencing transfusion-transmitted infections, transfusion costs and hospitalisation costs. These are summarised in Table 38. Note that for these outputs there is no difference between the three VE devices. These results show that, compared with SLTs, the use of VE devices is associated with less mortality, a reduced probability of experiencing complications, less transfusion and fewer hospitalisation costs. The probability of experiencing transfusion-transmitted infections was very low (almost zero) in both groups but was lower in the VE group.
| Outcome | VE | SLTs |
|---|---|---|
| 1-month mortality (%) | 2.4 | 2.7 |
| 1-year mortality (%) | 4.6 | 5.1 |
| Percentage surgery and/or transfusion complications | 11.9 | 14.4 |
| Percentage transfusion-related complications | 0.04 | 0.04 |
| Percentage transfusion-transmitted infections | 0.00 | 0.00 |
| Transfusion costs (£) | 231 | 290 |
| Hospitalisation costs (£) | 2174 | 2213 |
Results of the probabilistic sensitivity analyses in cardiac surgery patients
The impact of the statistical uncertainties in the model was investigated in the PSA. As the model assumed differences in technology costs between only the three VE technologies, the scatterplot of the PSA outcomes in the cost-effectiveness plane was not very informative (Figure 33).
FIGURE 33.
Cost-effectiveness plane with PSA outcomes for all technologies in cardiac surgery patients.

The CEACs for each technology are shown in Figure 34. PSA confirmed that SLTs is the strategy with the lowest probability of being cost-effective. This is to be expected, as the base-case scenario suggested that all three of the VE devices were both cheaper and more effective than SLTs. The CEACs for ROTEM, TEG and Sonoclot are very close together, especially at higher ceiling ratios, which would be expected, as the only difference between the three strategies assumed in the model was a difference in technology cost. At lower ceiling ratios, larger differences were observed, as Sonoclot was the cheapest technology in our model and so had the highest probability of being cost-effective.
FIGURE 34.
Cost-effectiveness acceptability curves for all technologies in cardiac surgery patients.

The information presented in Figure 34 is helpful to address the question of which of the four different testing strategies should be recommended. However, as mentioned earlier, if the actual question is whether or not to recommend VE testing instead of SLTs, then pairwise comparisons may be more informative. The deterministic pairwise results are presented in Table 37. The CEACs in Figures 35–37 illustrate the difference between ROTEM, TEG or Sonoclot and SLTs in terms of the probability of being cost-effective. At a cost-effectiveness threshold of £30,000 per QALY, the probability of cost-effectiveness for each of the three VE technologies was 0.79 for ROTEM (the most expensive device), 0.82 for TEG, and 0.87 for Sonoclot (the cheapest device). At higher thresholds, the cost-effectiveness probabilities converged to around 0.8 for all technologies.
FIGURE 35.
Cost-effectiveness acceptability curves: ROTEM vs. SLTs (cardiac surgery).

FIGURE 36.
Cost-effectiveness acceptability curves: TEG vs. SLTs (cardiac surgery).
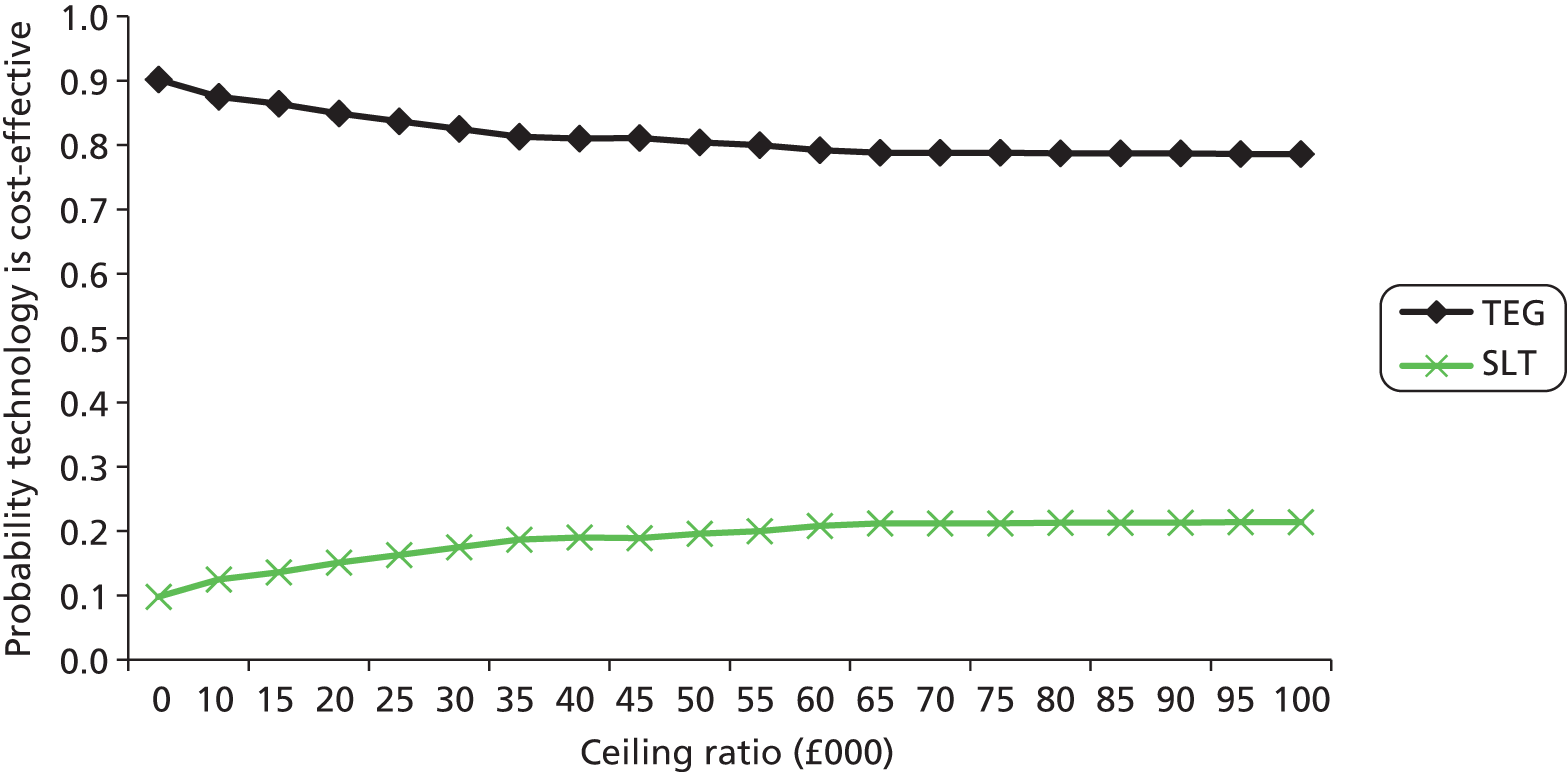
FIGURE 37.
Cost-effectiveness acceptability curves: Sonoclot vs. SLTs (cardiac surgery).

Results of scenario analyses in cardiac surgery patients
All scenario analyses suggested that ROTEM remained cost-saving (Table 39). CEACs for all analyses (not shown) were similar to those in Figure 35. The only two exceptions were the number of tests run on each device per year, and using VE testing as an add-on to SLTs rather than a replacement. After reducing the number of tests run on each device from 500 to 200, ROTEM no longer dominated SLTs, and an ICER of £16,487 is found (see Table 39 and Figure 38). At a cost-effectiveness threshold of £30,000 per QALY, the probability of cost-effectiveness for ROTEM was 0.62. As the cost-effectiveness threshold increased, the probability of cost-effectiveness for ROTEM converged to around 0.70. We estimated, using iterative analysis, that if all other parameters in the model remain unchanged, the costs of ROTEM and SLTs would be equal if 326 tests were run on ROTEM each year. At this level the ICER would be £0. If number of tests per year is reduced to 152 then the ICER is around £30,000. When VE testing is performed in addition to SLTs rather than as a replacement, the ICER of ROTEM + SLTs compared with SLTs becomes £7487. Table 40 presents the results for the various assay combinations explored. Note that in these combinations TEG is now the more costly VE test. However, in all of the scenarios VE testing is still dominant compared with SLTs.
FIGURE 38.
Cost-effectiveness acceptability curves ROTEM vs. SLTs: scenario based on 200 tests per year.
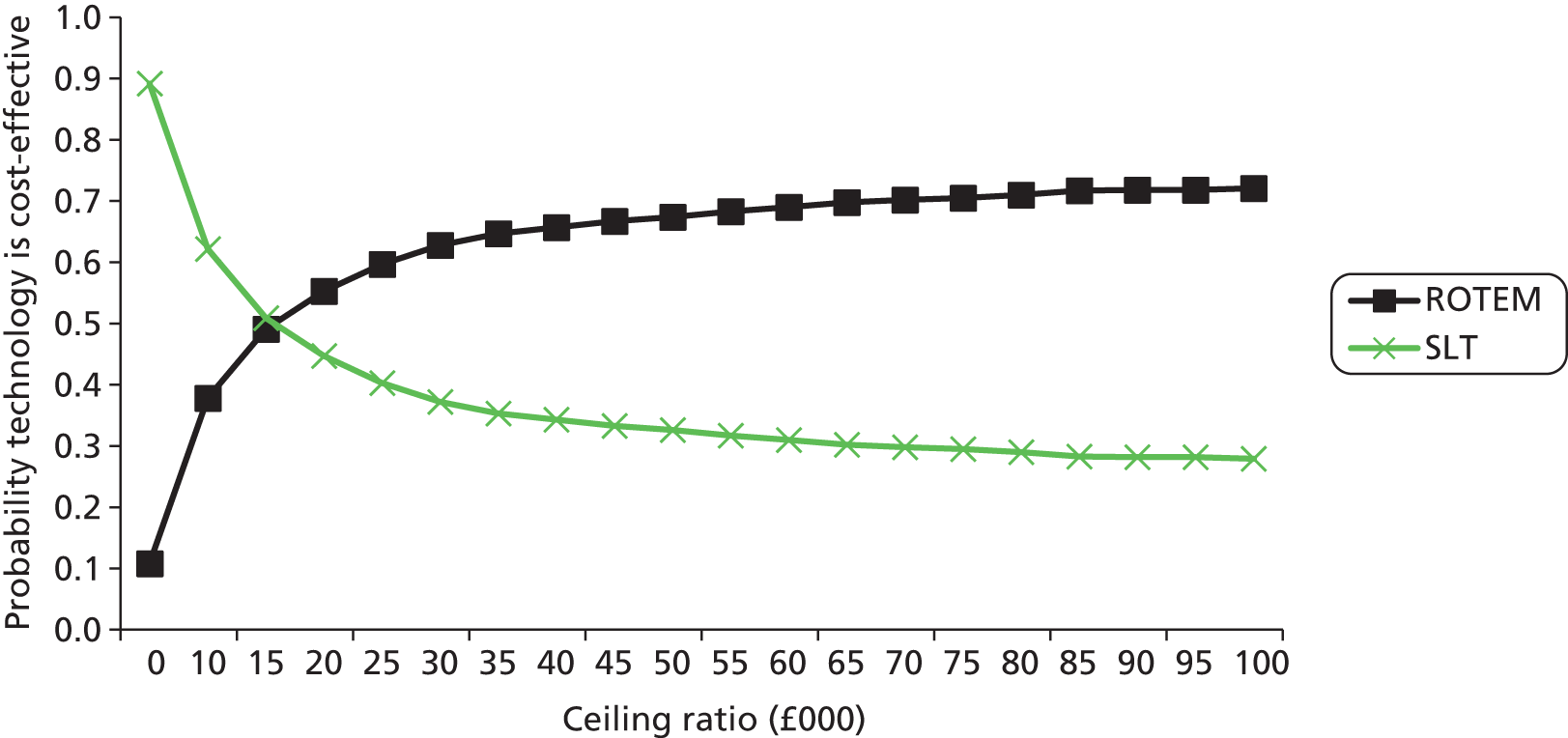
| Scenario | ROTEM | SLTs | Incremental QALY | IC (£) | ICER | ||||
|---|---|---|---|---|---|---|---|---|---|
| LYs | QALYs | Cost (£) | LYs | QALYs | Cost (£) | ||||
| Base case | 0.9660 | 0.8773 | 2588 | 0.9624 | 0.8726 | 2631 | 0.0047 | –43 | Dominance |
| 5 years’ machine usage | 0.9660 | 0.8773 | 2562 | 0.9624 | 0.8726 | 2631 | 0.0047 | –69 | Dominance |
| 200 tests per year | 0.9660 | 0.8773 | 2708 | 0.9624 | 0.8726 | 2631 | 0.0047 | 77 | £13,679 |
| No. of tests per patient decreased (1, no transfusion; 2, transfusion) | 0.9660 | 0.8773 | 2519 | 0.9624 | 0.8726 | 2620 | 0.0047 | –101 | Dominance |
| VE testing add-on to SLT | 0.9660 | 0.8773 | 2666 | 0.9624 | 0.8726 | 2620 | 0.0047 | 35 | £7487 |
| RR transfusion = 0.80 (lower limit) | 0.9684 | 0.8804 | 2554 | 0.9624 | 0.8726 | 2631 | 0.0078 | –77 | Dominance |
| RR transfusion = 0.96 (upper limit) | 0.9636 | 0.8742 | 2621 | 0.9624 | 0.8762 | 2631 | 0.0016 | –10 | Dominance |
| Lower probability of transfusion (0.429) | 0.9733 | 0.8867 | 2486 | 0.9707 | 0.8833 | 2501 | 0.0034 | –14 | Dominance |
| Higher probability of transfusion (0.890) | 0.9527 | 0.8601 | 2773 | 0.9473 | 0.8530 | 2868 | 0.0070 | –95 | Dominance |
| Equal volumes of blood components transfused | 0.9660 | 0.8773 | 2612 | 0.9624 | 0.8726 | 2631 | 0.0047 | –18 | Dominance |
| Calibrated 1-month mortality (0.0214) | 0.9768 | 0.8870 | 2601 | 0.9747 | 0.8837 | 2646 | 0.0033 | –45 | Dominance |
| Calibrated 1-month mortality (0.0642) | 0.9552 | 0.8676 | 2574 | 0.9501 | 0.8616 | 2616 | 0.0060 | –41 | Dominance |
| Additional scenarios cardiac surgery patients | Technology | LYs | QALYs | Cost (£) | Incremental QALY vs. SLT | IC vs. SLT | ICER vs. SLT |
|---|---|---|---|---|---|---|---|
| Base case | ROTEM | 0.9660 | 0.8773 | 2588 | 0.0047 | –43 | Dominance |
| TEG | 0.9660 | 0.8773 | 2552 | 0.0047 | –79 | ||
| Sonoclot | 0.9660 | 0.8773 | 2499 | 0.0047 | –132 | ||
| SLT | 0.9624 | 0.8726 | 2631 | ||||
| Assay scenario 1: three sets of test performed – first time four assays; next, two tests, two assays | ROTEM | 0.9660 | 0.8773 | 2568 | 0.0047 | –63 | Dominance |
| TEG | 0.9660 | 0.8773 | 2575 | 0.0047 | –56 | ||
| Sonoclot | 0.9660 | 0.8773 | 2499 | 0.0047 | –132 | ||
| SLT | 0.9624 | 0.8726 | 2631 | ||||
| Assay scenario 2: three sets of test performed, each time two assays | ROTEM | 0.9660 | 0.8773 | 2559 | 0.0047 | –72 | Dominance |
| TEG | 0.9660 | 0.8773 | 2543 | 0.0047 | –87 | ||
| Sonoclot | 0.9660 | 0.8773 | 2499 | 0.0047 | –132 | ||
| SLT | 0.9624 | 0.8726 | 2631 | ||||
| Assay scenario 3: two sets of test performed, each time two assays | ROTEM | 0.9660 | 0.8773 | 2522 | 0.0047 | –98 | Dominance |
| TEG | 0.9660 | 0.8773 | 2512 | 0.0047 | –108 | ||
| Sonoclot | 0.9660 | 0.8773 | 2482 | 0.0047 | –138 | ||
| SLT | 0.9624 | 0.8726 | 2620 |
Base-case results for model in patients with coagulopathy induced by trauma
The base-case results from the analysis reported as LYs, QALYs and costs per technology for patients with coagulopathy induced by trauma are summarised in Table 41.
| Outcome | SLTs | ROTEM | TEG | Sonoclot |
|---|---|---|---|---|
| LY | 0.8343 | 0.8425 | 0.8425 | 0.8425 |
| QALY | 0.5644 | 0.5713 | 0.5713 | 0.5713 |
| Cost (£) | 7661 | 6973 | 6940 | 6842 |
| Incremental QALYs vs. SLTs | 0.0069 | 0.0069 | 0.0069 | |
| ICs vs. SLTs (£) | –688 | –721 | –818 |
All of the VE technologies dominated SLTs. As with the cardiac surgery model, the cost of Sonoclot was lower than that of ROTEM or TEG, and so this device was associated with greater cost-savings (£818) than TEG (£721) or ROTEM (£688). The total cost of testing per trauma patient for the four technologies was £203 for ROTEM, £170 for TEG, £130 for SLTs, and £73 for Sonoclot (£84). Other intermediate outcomes are summarised in Table 42.
| Outcome | VE device | SLTs |
|---|---|---|
| 1-month mortality (%) | 14.9 | 15.7 |
| 1-year mortality (%) | 17.3 | 18.2 |
| Percentage trauma and/or transfusion complications | 12.9 | 14.6 |
| Percentage transfusion-related complications | 0.02 | 0.02 |
| Percentage transfusion-transmitted infections | 0.00 | 0.00 |
| Transfusion costs (£) | 1045 | 1491 |
| Hospitalisation costs (£) | 5724 | 6040 |
Results of the probabilistic sensitivity analyses in patients with coagulopathy induced by trauma
The impact of statistical uncertainties in the model was investigated in the PSA. The scatterplot of the PSA outcomes in the cost-effectiveness plane (Figure 39) did not show clear preference for any one of the VE technologies.
FIGURE 39.
Cost-effectiveness plane with PSA outcomes for all technologies in trauma population.
The CEACs for each strategy are shown in Figure 40. The PSA confirmed that SLTs was the strategy with the lowest probability of being cost-effective (0.022 at most). This is to be expected, as the base-case scenario suggested that all three of the VE devices were both cheaper and more effective than SLTs. As with the cardiac surgery model, the CEACs for ROTEM, TEG and Sonoclot were very close together, which would be expected as the only difference between the three strategies assumed in the model was a difference in technology cost. At lower ceiling ratios, larger differences were observed as Sonoclot was the cheapest technology in our model.
FIGURE 40.
Cost-effectiveness acceptability curves for all technologies in trauma population.

A comparison of ROTEM with SLTs found a cost-effectiveness probability equal to 0.96 for ROTEM for a ceiling ratio equal to £0 (see CEAC in Figure 41). As the ceiling ratio increased, the CEAC for ROTEM converged to 0.87. A similar pattern was observed for TEG and Sonoclot (CEACs not shown).
FIGURE 41.
Cost-effectiveness acceptability curves: ROTEM vs. SLTs trauma population.

Results of the expected value of perfect information analysis
The population EVPI results are presented in Figure 42. This shows that, at a cost-effectiveness threshold of £30,000 per QALY, the population EVPI when all four technologies are considered was £25,017,471, whereas the population EVPI when only ROTEM and SLTs were compared was more than 22 times lower at £1,263,131. This huge difference in EVPI is to be expected, given that there is little uncertainty whether or not any one of the VE devices is superior to SLTs, but much uncertainty as to which of three devices is the optimal device. This is illustrated in the results of the PSA (see Figures 37–39).
FIGURE 42.
Population EVPI in trauma model (all technologies and ROTEM vs. SLTs only).

Results of scenario analysis in patients with coagulopathy induced by trauma
All scenario analyses outlined above (see Scenario analyses) suggested that ROTEM remained cost-saving (Tables 43 and 44). CEACs and population EVPI curves for all analyses (not shown) were very similar to those shown in Figures 39 and 40. The iterative analysis performed to estimate the number of tests per year such that ROTEM would still be cost-saving suggested a break-even value of 81 tests per year; at this level the ICER was £0. When the number of tests per year was reduced to 65 the ICER was approximately £30,000.
Threshold analysis on the combined effect of a reduction in the percentage transfused and the blood volumes transfused, where we assumed that equal volumes of blood were transfused in the VE-testing and SLTs groups, showed that at a RR of transfusion of ≥ 0.9822 ROTEM was no longer cost-saving (ICER was 0). When the RR of transfusion increased to 0.9874, the ICER of ROTEM compared with SLTs was £30,000. Table 44 presents the results for the various assay combinations explored, and with these combinations TEG is now the more costly VE test, but still dominant compared with SLTs.
Reducing baseline transfusion risk in the SLTs group, assuming that equal volumes of blood were transfused in the VE testing and SLTs group, showed that ROTEM was no longer cost-saving at a transfusion rate of 5%, and the ICER was £30,000 for a transfusion rate of 4%. This compares to a transfusion rate of 32% used in the base-case analysis. We repeated the analysis but increased the RR of RBC transfusion from 0.88 to 0.95. For this analysis, the ICER was > £30,000 for a transfusion rate of ≤ 8%. After reducing the probability of complications related to trauma and/or transfusion, transfusion-related complications and transfusion-related infection to zero, ROTEM remained cost-saving, with a reduction in costs of £372.
| Scenario | ROTEM | SLTs | Incremental QALY | IC | ICER | ||||
|---|---|---|---|---|---|---|---|---|---|
| LYs | QALYs | Cost (£) | LYs | QALYs | Cost (£) | ||||
| Base case | 0.8425 | 0.5713 | 6973 | 0.8343 | 0.5644 | 7661 | 0.0069 | –688 | Dominance |
| 5 years’ machine usage | 0.8425 | 0.5713 | 6929 | 0.8343 | 0.5644 | 7661 | 0.0069 | –731 | |
| 200 tests per year | 0.8425 | 0.5713 | 7173 | 0.8343 | 0.5644 | 7661 | 0.0069 | –488 | |
| No. of tests per patient decreased (2, no transfusion; 3 transfusion) | 0.8425 | 0.5713 | 6862 | 0.8343 | 0.5644 | 7591 | 0.0069 | –729 | |
| VE testing add-on to SLT | 0.8425 | 0.5713 | 7103 | 0.8343 | 0.5644 | 7661 | 0.0069 | –558 | |
| RR transfusion = 0.80 (lower limit) | 0.8480 | 0.5759 | 6668 | 0.8343 | 0.5644 | 7661 | 0.0115 | –993 | |
| RR transfusion = 0.96 (upper limit) | 0.8370 | 0.5667 | 7278 | 0.8343 | 0.5644 | 7661 | 0.0023 | –383 | |
| Lower probability of transfusion SLTs group (0.209) | 0.8636 | 0.5889 | 5802 | 0.8582 | 0.5844 | 6224 | 0.0045 | –422 | |
| Higher probability of transfusion SLTs group (0.444) | 0.8194 | 0.5520 | 8259 | 0.8080 | 0.5425 | 9238 | 0.0095 | –979 | |
| Equal volumes of blood components transfused | 0.8425 | 0.5713 | 7240 | 0.8343 | 0.5644 | 7661 | 0.0069 | –421 | |
| Probability experiencing ARDS (0.0775) and MOF (0.15) | 0.8420 | 0.5731 | 5814 | 0.8337 | 0.5665 | 6344 | 0.0066 | –530 | |
| Probability experiencing ARDS (0.2325) and MOF (0.45) | 0.8430 | 0.5695 | 8132 | 0.8349 | 0.5624 | 8977 | 0.0071 | –846 | |
| Calibrated 1-month mortality (0.1483) | 0.8823 | 0.5969 | 7144 | 0.8794 | 0.5935 | 7855 | 0.0034 | –711 | |
| Calibrated 1-month mortality (0.4450) | 0.8028 | 0.5457 | 6801 | 0.7891 | 0.5354 | 7466 | 0.0104 | –664 | |
| Additional scenarios trauma patients | Technology | LYs | QALYs | Cost (£) | Incremental QALY vs. SLT | IC (£) vs. SLT | ICER vs. SLT |
|---|---|---|---|---|---|---|---|
| Base case | ROTEM | 0.8425 | 0.5713 | 6973 | 0.0069 | –688 | Dominance |
| TEG | 0.8425 | 0.5713 | 6940 | 0.0069 | –721 | ||
| Sonoclot | 0.8425 | 0.5713 | 6842 | 0.0069 | –818 | ||
| SLTs | 0.8343 | 0.5644 | 7661 | ||||
| Assay scenario 1: five sets of test performed – first time, four assays; next, four tests, two assays | ROTEM | 0.8425 | 0.5713 | 6961 | 0.0069 | –699 | Dominance |
| TEG | 0.8425 | 0.5713 | 6970 | 0.0069 | –690 | ||
| Sonoclot | 0.8425 | 0.5713 | 6842 | 0.0069 | –818 | ||
| SLTs | 0.8343 | 0.5644 | 7661 | 0.8343 | |||
| Assay scenario 2: five sets of test performed, each time two assays | ROTEM | 0.8425 | 0.5713 | 6925 | 0.0069 | –736 | Dominance |
| TEG | 0.8425 | 0.5713 | 6912 | 0.0069 | –748 | ||
| Sonoclot | 0.8425 | 0.5713 | 6842 | 0.0069 | –818 | ||
| SLTs | 0.8343 | 0.5644 | 7661 | 0.8343 | |||
| Assay scenario 3: three sets of test performed, each time two assays | ROTEM | 0.8425 | 0.5713 | 6863 | 0.0069 | –746 | Dominance |
| TEG | 0.8425 | 0.5713 | 6855 | 0.0069 | –753 | ||
| Sonoclot | 0.8425 | 0.5713 | 6813 | 0.0069 | –796 | ||
| SLTs | 0.8343 | 0.5644 | 7609 | 0.8343 |
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
All completed RCTs identified by our systematic review were conducted in patients undergoing cardiac surgery. Pooled estimates, derived from meta-analyses of dichotomous data, indicated that VE testing (TEG or ROTEM) was associated with significant reductions in the numbers of patients receiving RBC transfusion, platelet transfusion and FFP transfusion, compared with a SLTs-based strategy. There were no significant differences between the VE testing and SLTs in terms of factor VIIa transfusion, any blood component transfusion or PCC transfusion; although data suggested a beneficial effect associated with VE testing, these outcomes were evaluated in only two studies. 35,54 There was no apparent difference in the rates of FIB transfusion between patients managed using VE testing and those managed using SLTs. Continuous data on blood component/product use, although inconsistently reported across studies, supported these findings; the only blood component/product that was not associated with a reduced volume of use in the VE testing group was FIB. There were no apparent differences in clinical outcomes (re-operation, surgical cause of bleed on re-operation and mortality) between patients managed using VE testing and those managed using SLTs. There was some evidence of reduced bleeding35,50 and ICU stay35 in the VE testing groups compared with SLTs groups, but this was not consistently reported across studies. There was no apparent difference in the length of hospital stay between groups. All meta-analyses, with the exception of factor VIIa transfusion, FIB transfusion and PCC transfusion, which included only studies of ROTEM, included both studies of TEG and studies of ROTEM; summary estimates were similar when stratified by VE device, thus, there was no evidence to indicate a difference in effectiveness between the two devices. However, it should be noted that none of the included RCTs reported a direct comparison between TEG and ROTEM.
As none of the RCTs described above evaluated the Sonoclot VE test, we included lower levels of evidence for this device. Three prediction studies that evaluated Sonoclot were included in the review,61,62,120 two of these also evaluated TEG and SLTs, enabling a direct comparison between the two devices and between VE devices and SLTs. 61,62 Data reported by the three studies in this group were not suitable for meta-analyses. All three studies61,62,120 used measures of bleeding as the reference standard or as the dependent variable in multivariable models. Positive results on conventional tests, TEG and Sonoclot were generally associated with an increased risk of bleeding with no clear differences according to test. The limited available data do not suggest a significant difference in the ability of Sonoclot and TEG to predict bleeding; however, there were insufficient data to rule out a difference in the overall clinical effectiveness of these two devices. No studies reported any data comparing Sonoclot and ROTEM.
With the exception of one small, non-RCT,65 all studies conducted in trauma patients or women with PPH included in our systematic review were prediction studies. These studies either reported the predictive accuracy of different VE device parameters and/or SLTs with a reference standard consisting of clinical outcome or measure of transfusion requirements. These studies generally found that a positive result on each of the TEG or ROTEM parameters or on SLTs was associated with an increased risk of transfusion (RBC, any blood product and massive transfusion) and death. There was no clear difference between ROTEM, TEG or SLTs. However, none of the studies provided a direct comparison between TEG and ROTEM. An overall TEG result suggesting that a patient was hypocoagulable was the strongest predictor of any blood component transfusion. The presence of hyperfibrinolysis was the strongest predictor of mortality. No studies of the Sonoclot device were identified that fulfilled inclusion criteria for the either the trauma or PPH populations.
A previous Cochrane review,21 last updated in 2011, evaluated the effectiveness of transfusion strategies guided by VE devices in patients with severe bleeding. This review21 concluded that there was no evidence that TEG or ROTEM improved morbidity or mortality and that, although transfusion strategies guided by VE devices appeared to reduce the amount of bleeding, the clinical implications of this remained uncertain. Our systematic review differs from the Cochrane review21 on a number of key points. The Cochrane review21 was not restricted to any specific clinical groups – as a result, it included one study of patients undergoing liver surgery, as well as eight RCTs of patients undergoing cardiac surgery, all of which were also included in our review. Our review represents an advance on the Cochrane review21 in that it identified three further RCTs35,53,55 conducted in patients undergoing cardiac surgery. In addition, because the Cochrane review21 was restricted to RCTs, it did not include any studies assessing Sonoclot, whereas we were able to include some limited data on this device. A key difference in approach between our systematic review and the Cochrane review21 was in the handling of continuous data. The Cochrane review21 converted median values to means in order to allow pooled estimates to be generated, even though the Cochrane handbook includes a specific recommendation that this approach should not be used; the Cochrane Handbook (section 7.7.3.6) states that ‘Ranges are very unstable and, unlike other measures of variation, increase when the sample size increases. They describe the extremes of observed outcomes rather than the average variation. Ranges should not be used to estimate SDs. One common approach has been to make use of the fact that, with normally distributed data, 95% of values will lie within 2 × SD either side of the mean. The SD may therefore be estimated to be approximately one-quarter of the typical range of data values. This method is not robust and we recommend that it should not be used’. 121 We do not believe that this approach can be justified and have therefore reported individual study results in forest plots and summarised findings in a narrative synthesis. Finally, we noted two specific errors in data extraction in the Cochrane review. 21 First, the study by Westbrook et al. 47 was included in a meta-analysis of the proportion of patients undergoing surgical re-intervention for exploration of bleeding; data from this study47 had been erroneously extracted from the baseline characteristics table that reported the number of patients in each arm who were undergoing a repeat cardiac surgical intervention. Second, meta-analyses of the proportion of patients undergoing FFP transfusion and the proportion of patients undergoing platelet transfusion, which were reported in the Cochrane review,21 included data derived from a graph reported in Nuttall et al. 50 The graph recorded the numbers of patients, in each arm of the trial, who received FFP only, platelets only, or platelets and FFP and/or cryoprecipitate;50 this means that the graph cannot be used to derive either the total number of patients who received FFP or the total number who received platelets. The Cochrane review21 appeared to have extracted the numbers of patients receiving FFP and the numbers of patients receiving platelets as though these were the total numbers of patients receiving each blood component. As more patients in the control (SLTs) arm received multiple blood components,50 this error had the effect of producing a RR which favoured the control group, a result which was in the opposite direction to all three of the other studies included in the meta-analysis. 21 A systematic review conducted for a Health Technology Assessment (HTA) report, published in 2008, included studies of VE devices in cardiac surgery, but did not restrict inclusion by study design;91 the two RCTs included in this assessment, which met the inclusion criteria for our review,48,50 were also included in both our review and the Cochrane review. 21 The Health Technology Assessment report92 concluded that, assuming 200 tests per annum, the use of VE devices appeared to be clinically effective and cost-effective, reducing the need for inappropriate transfusions, decreasing blood component requirements and reducing the number of deaths, complications and infections. The results of our systematic review are consistent with previous reviews21,91 in that they suggest that the use of VE devices may be a clinically effective approach to the management haemostasis in patients undergoing cardiac surgery.
We are not aware of any previous systematic reviews assessing the effectiveness of VE devices for the management of patients with trauma-induced coagulopathy or PPH. A Cochrane Diagnostic Test Accuracy protocol has recently been published with the title ‘Thromboelastography (TEG) and thromboelastometry (ROTEM) for trauma-induced coagulopathy in adult trauma patients with bleeding’. 22
Cost-effectiveness
We assessed the cost-effectiveness of VE devices in two key populations: patients undergoing cardiac surgery and patients with trauma acquired coagulopathies. There were insufficient data from the clinical effectiveness review to construct a model to assess the cost-effectiveness of VE devices in women with PPH. There were no data on the clinical effectiveness of Sonoclot; we therefore assumed that the TEG- and ROTEM-based estimates used in the model would also be applicable to Sonoclot; thus the same health effect estimates were used for all three VE devices.
The cost-effectiveness model suggested that VE testing is cost-saving and more effective than standard laboratory testing in cardiac surgery patients. The per-patient cost-saving was slightly smaller for ROTEM (£43) than for TEG (£79) and Sonoclot (£132). This finding was entirely dependent on material costs, which are slightly higher for ROTEM in the base case. When other combinations of assays are assumed, TEG could be slightly less cost-saving than ROTEM. When all of the uncertainties included in the model were taken into account, at a cost-effectiveness threshold of £30,000 per QALY, the probability of cost-effectiveness for each of the three VE technologies was 0.79 for ROTEM (the most expensive device), 0.84 for TEG and 0.87 for Sonoclot (the cheapest device). At higher thresholds, probabilities converged to around 0.8 for all technologies. Scenario analyses were used to assess the potential impact of changing various input values for the model. In these scenarios the results remained largely unchanged. Only when the number of tests performed per machine per year was VE testing was no longer cost-saving when the number of tests performed per machine was fewer than 326. When this number was 152, the ICER was around £30,000.
For the trauma population, the per-patient cost-savings due to VE testing were more substantial, amounting to £688 for ROTEM compared with SLTs, £721 for TEG and £818 for Sonoclot. A comparison of the most expensive technology, ROTEM, with SLTs found a cost-effectiveness probability equal to 0.96 for ROTEM for a ceiling ratio of £0. As the ceiling ratio increased, this probability converged on 0.87. The increased cost savings observed for the trauma compared with the cardiac population were primarily due to the higher blood volumes that are transfused in the trauma patients. Scenario analyses constructed to assess the impact of various parameters showed similar results. Given the lack of effectiveness data in trauma patients, the current results should be regarded as indicative of the potential cost-effectiveness of VE testing only in trauma patients.
Strengths and limitations of assessment
Clinical effectiveness
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Because of the known difficulties in identifying test accuracy studies using study design-related search terms,122 and potential need to include non-RCTs and prediction modelling studies, search strategies were developed to maximise sensitivity at the expense of reduced specificity. Thus, large numbers of citations were identified and screened, many of which did not meet the inclusion criteria of the review.
The possibility of publication bias remains a potential problem for all systematic reviews. Publication bias was not formally assessed in this review because, for RCTs, the number of studies was too small for such an assessment to be meaningful and, for prediction studies, there is no reliable method of assessing publication bias. However, our search strategy included a variety of routes to identify unpublished studies and resulted in the inclusion of a number of conference abstracts and the identification of one ongoing RCT. 62 Considerations may differ for systematic reviews of test accuracy studies. It is relatively simple to define a positive result for studies of treatment, for example a significant difference between the treatment and control groups which favours treatment. This is not the case for test accuracy studies, which measure agreement between index test and reference standard, or prediction modelling studies, which measure the extent to which a particular test result is predictive of outcome(s) once other potentially predictive variables have been adjusted for. However, it would seem likely that studies finding greater agreement between the index test and reference standard (high estimates of sensitivity and specificity) or that the index test is a significant, independent predictor of outcome will be published more often.
Clear inclusion criteria were specified in the protocol for this review and the one protocol modification that occurred during the assessment has been documented in the methods section of this report (see Table 6). The eligibility of studies for inclusion is therefore transparent. In addition, we have provided specific reasons for excluding all of the studies considered potentially relevant at initial citation screening (see Appendix 4). The review process followed recommended methods to minimise the potential for error and/or bias;37 studies were independently screened for inclusion by two reviewers, and data extraction and quality assessment were undertaken by one reviewer and checked by a second (MW and PW). Any disagreements were resolved by consensus.
Studies included in this review were assessed for risk of bias using published tools appropriate to study design and/or the type of data extracted. Studies that provided data on the accuracy of VE testing to predict clinical outcomes and/or transfusion requirements were assessed using the QUADAS-2 tool. 45 QUADAS-2 is structured into four key domains covering participant selection, index test, reference standard and the flow of patients through the study (including timing of tests). Each domain is rated for risk of bias (low, high or unclear); the participant selection, index test and reference standard domain are also separately rated for concerns regarding the applicability of the study to the review question (low, high or unclear). Although designed specifically for this type of study, QUADAS-2 was also considered the best option for assessment of the prediction modelling studies. This was because the prediction modelling studies included in this assessment are unusual in that they generally present the results of several multivariable models for each outcome/dependent variable; a separate model is needed for each VE testing parameter or SLTs, as parameters and tests frequently measure the same or similar coagulation properties and cannot be considered independent. In addition, studies aimed to assess the ability of individual VE testing parameters or SLTs to predict the occurrence of very short-term outcomes. For these reasons, the studies were considered to have more in common with diagnostic accuracy studies than with classic prognostic/prediction modelling studies. RCTs were assessed using Cochrane’s tool for assessing risk of bias in randomised trials. 44 The results of the risk of bias and QUADAS-2 assessments are reported, in full, for all included studies (see Appendix 3) and in summary in the results [see Chapter 3, Risk of bias assessment, How do clinical outcomes differ among patients with coagulopathy induced by trauma who are tested with viscoelastic devices compared with those who are not tested? (Risk of bias and applicability assessment), How well do viscoelastic devices predict relevant clinical outcomes in patients with post-partum haemorrhage? (Risk of bias and applicability assessment); Tables 8, 16 and 18; and Figures 5 and 17].
Although we identified 11 RCTs35,46–57 that compared the effectiveness of VE testing with a SLTs-based approach for the management of haemostasis in patients undergoing cardiac surgery, the potential to produce summary effect estimates was limited by the wide variety of outcomes reported and a lack of standardisation of the way in which these were measured. The assessment of heterogeneity was limited by the relatively small number of studies that contributed to each meta-analysis. The Q-statistic has limited power to detect heterogeneity when the number of studies included in a meta-analysis is low. No summary estimates of continuous data (e.g. duration of hospital or ICU stay, or volume of blood component/product transfused) were possible as the majority of these data were appropriately reported as medians with range or IQR. Pooling only those studies that reported continuous outcomes as mean ± SD would be unrepresentative of the group as a whole, and would be likely to result in greater weight being given to studies that reported data as mean ± SD without consideration of whether or not these data were normally distributed.
At the start of this assessment the role of VE testing in the care pathway was considered to be unclear; it could be used either as an add-on to, or replacement for SLTs. Three of the RCTs included in our systematic review compared the effectiveness of VE testing combined with SLTs (two studies using TEG50,51 and one using ROTEM55) to SLTs alone, that is, these studies provided data on the add-on value of VE testing. For all outcomes assessed, the results of these studies were consistent with those of studies that compared VE testing alone with SLTs. These findings indicate that performing SLTs in addition to VE testing is unlikely to give further benefit over that provided by VE testing alone. VE testing can therefore be regarded as a replacement for SLTs.
All of the studies conducted in trauma patients or women with PPH included in this review have considerable limitations with respect to their ability to address the overall aim of assessing the clinical effectiveness of VE devices for assessment of haemostasis in these patient groups. With the exception of one small, non-RCT,63 all studies in these patient groups were prediction studies, which either reported the predictive accuracy of different SLTs and/or VE device parameters for which the reference standard was a clinical outcome or measure of transfusion requirements, or the results of prediction models, for which each test or parameter was modelled separately, as described above, with clinical outcome or transfusion requirement as the dependent variable. When the reference standard or dependent variable in the model was a measure of transfusion requirements, it is not possible for studies to be rated as both ‘low risk of bias’ and ‘low applicability concerns’ with respect to the reference standard. This is because, in order for such a study to reflect clinical practice and be rated ‘as low applicability concerns,’ the decision to transfuse would need to be made with knowledge of the test results; however, there is then an inevitable risk of incorporation bias leading to a rating of ‘high risk of bias.’ The need for a separate model for each VE testing parameter or SLTs, as described above, creates a further problem in that prediction studies cannot adequately assess the overall predictive performance of VE devices compared with SLTs as they would be used in practice. Finally, any type of prediction study is suboptimal, in that these studies can only ever provide an indication of the ability of VE testing or SLTs to predict clinical outcomes or transfusion requirements. These studies cannot provide information on how interventions and subsequent clinical outcomes may differ according to whether or not a POC VE testing or SLTs-based strategy is used; these data can be derived only from controlled trials.
Cost-effectiveness
Our study can be regarded as an important update of the cardiac surgery aspect of the evaluation undertaken for NHS Scotland,12 and is the first cost-effectiveness analysis of VE devices in trauma patients. It was informed by an up-to-date, high-quality systematic review that included a number of RCTs published since the NHS Scotland evaluation. 12 We also added a PSA to the model in order to assess the simultaneous impact of the various uncertainties. A further strength of our model is that we included longer-term mortality data than those that were included in previous evaluations, which included mortality up to only 1 month. Our cardiac surgery model used data based on a large study by Murphy et al. ,15 which showed that the effects of transfusion on mortality continued up to and beyond 1 year. Similar data were not available for the trauma population. We therefore had to make some assumptions for this population. We extrapolated the ratio of mortality in transfused to non-transfused patients found in a study that provided this information up to hospital discharge to 1-year follow-up and then applied these data to the overall mortality rate for this period from another study in trauma patients. It would be expected that a RR at hospital discharge is too high at 1 year; the study in cardiac patients showed that the difference in mortality between transfused and non-transfused patients decreased over time. Scenario analyses showed that changing the ratio of mortality in transfused patients compared with non-transfused patients did not affect results. We might reasonably assume that, given that mortality is low between 1 month and 1 year, this would also be the case if we had made similar changes to 1-year mortality.
The main outcome used in the economic models was the proportion of patients at risk of RBC transfusion. From this, it was possible to impute other effects, such as units of blood transfused, adverse events, complications, changes to quality of life and overall survival. This is consistent with the only cost-effectiveness study in the field, the Scottish HTA report. 12,89 It is also consistent with the study by Davies et al. ,90 on which the Scottish HTA report12 was based, in which costs and effects of methods of minimising perioperative allogeneic RBC transfusion were assessed for cardiac patients as a subpopulation. In order to estimate the mortality for VE testing, we assumed that any mortality benefit from VE testing resulted only from fewer patients receiving an RBC transfusion. However, literature suggests that both whether or not a patient is transfused, and the number of units transfused, may be predictive of mortality. 123 Thus it is possible that differential mortality between VE and SLTs could result from reasons other than simple differential rates of transfusion, such as reduced volume transfused or differential transfusion of other blood components, for example FFP and platelets. This could potentially have resulted in an underestimation of the benefits of VE testing on mortality than currently accounted for in the model. Conversely, the mortality estimate was derived from the study by Murphy et al. ,15 which was based on observational data. It may therefore be suggested that differences in mortality between transfused and non-transfused could have arisen because of confounding, as those patients who were transfused may have been more ill than those who were not. This could have had the effect of biasing the model in favour of VE testing. However, Murphy et al. 15 argue against this explanation as prognostic factors were well balanced across transfused and non-transfused groups, mean nadir HCT was similar between groups, adjustment for confounding did not alter observed effects, and effects were observed within strata of patients with and without important risk factors. Thus we consider it reasonable to assume that any bias we introduced by using the observed mortality data for our model is limited. This decision is supported by the fact that the RR of mortality for ROTEM and TEG was 0.90 in our model, which was almost identical to the RR estimated in the systematic review of RCTs (0.87).
A strength of our study was the detailed consultation with manufacturers regarding the costs of each VE device. This was important as each device is available with different numbers of channels and runs different assays, which are not directly comparable between devices. We decided which assays and number of tests to model based on the combination of assays and numbers of tests used in the trials so that the costs included in the model correspond to the source of the effectiveness data. However, it is unclear whether or not the results found in the trials would also be applicable to different assay combinations and numbers of tests used in clinical practice. We found that varying the number of tests, which could also be a proxy for assay combinations, did not alter the conclusions in terms of cost-effectiveness. The length of time that a machine is used for and the average number of tests run per machine per year influences the material cost of a test. However, scenario analysis showed that the number of tests had to be very low before VE testing was no longer cost-effective.
A major limitation of both models was the lack of data on the effectiveness of the Sonoclot device. None of the RCTs included in our review assessed this device. As the only difference in the models was the costs of the devices, and Sonoclot was the cheapest device, Sonoclot was the most likely to be cost-effective. However, this should be interpreted with extreme caution as a result of the lack of evidence.
There were no data on the clinical effectiveness of any of the VE devices in trauma patients. We therefore assumed equivalent clinical effectiveness to the cardiac surgery population. Clinical experts were consulted regarding their views on the validity of this assumption. They indicated that patients undergoing (elective) cardiac surgery are likely to differ from trauma patients, which may affect the relative effectiveness of the VE devices. Specifically, it was noted that trauma patients are likely to have higher blood loss and therefore have greater blood transfusion requirements. We were able to estimate the baseline risk of RBC transfusion in trauma patients from the predictive accuracy studies included in the systematic review, but these studies could not inform the RR of transfusion in patients who were and were not tested with a VE device. There was general agreement that an assumption of equivalent clinical effectiveness in terms of the RR of RBC transfusion between the cardiac surgery and trauma populations was a reasonable assumption, given the lack of other reliable data. Although this assumption may be clinically problematic, scenario analysis indicated that if the RR of RBC transfusion was as high as 0.98, VE testing would still be cost-saving in this population. This compares with a value of 0.88 derived from the systematic review of cardiac surgery patients and used in the base-case analysis.
The 1-year time horizon used by our model could be regarded as a further limitation. However, we would argue that extrapolation over a longer time horizon is unnecessary. This is because at 1 year all VE devices were shown to be both more effective and cheaper than SLTs, and with little uncertainty (probabilities of at least 0.68 of being cost-effective); effectiveness would only increase and costs would be likely to decrease over a lifetime. The expected increase in effectiveness is based on the avoidance of transfusions supported by Murphy et al. ,15 who showed that transfusion continues to increase mortality beyond 1 year. In addition, long-term complications, such as stroke, which are likely to be avoided by fewer transfusions, would also imply lower cost.
Where possible, we used cardiac surgery and trauma-specific utility and cost estimates in our models. However, for some of the short-term utility parameters we were unable to find trauma-specific data. We made the conservative assumption that during the first month trauma patients would have the same utility as that of cardiac surgery patients. Given that many trauma patients spent quite some time on an ICU, often being ventilated, the true utility is likely to be lower. In addition, we had no good data on costs of a hospital stay once trauma patients leave the ICU. This is related to the fact that these patients may go to a wide variety of departments, depending on the type of trauma (e.g. brain trauma or mainly orthopaedic trauma). We therefore made the assumption that costs per day would also be the same as for cardiac patients; it was unclear whether or not this was likely to be an overestimation or underestimation. However, given that these utilities and costs apply to only a very short time period, they are unlikely to have influenced whether or not VE testing was cost-effective.
We conducted an EVPI for the trauma population, as we felt that there less evidence and therefore greater uncertainty for this population. This showed that it may be worth spending money on further primary research given that, when comparing all four technologies (ROTEM, TEG, Sonoclot and SLTs) the population EVPI was around £25M for an ICER of £30,000. However, the EVPI should be interpreted with caution, given that the value when comparing only a single VE device (ROTEM) with SLTs was 22 times lower at just over £1.25M. This would suggest that there is relatively little uncertainty as to whether or not ROTEM would be cost-effective in comparison with SLTs. This is inconsistent with the evidence, as the data to inform the trauma model was derived from trials conducted in cardiac surgery patients. The full uncertainty associated with this limitation, as well as other assumptions, may not have been captured by this analysis.
Uncertainties
Clinical effectiveness
The results of our systematic review are consistent with previous reviews,21,89 in that they suggest that the use of VE devices may be a clinically effective approach to the management haemostasis in patients undergoing cardiac surgery. Our results indicate that the use of VE devices may be associated with a reduction in transfusion rates; however, whether or not this reduction represents a decrease in inappropriate transfusions and whether or not it translates into changes in important clinical outcomes (e.g. duration of ICU/hospital stay, morbidity and mortality) remains less clear. Studies included in our review provided some indication that the use of VE devices may be associated with a reduction in the duration of ICU stay. However, data were not considered suitable for meta-analyses and only one study35 showed a statistically significant decrease in the length of ICU stay for patients managed using an algorithm based on a VE device compared with those managed using an algorithm based on SLTs; this study35 restricted inclusion to patients who were bleeding from capillary beds or had blood loss > 250 ml/hour or 50 ml/10 minutes.
The existence of a link between the use of VE devices and clinical outcome is even more uncertain when these devices are used in the management of trauma patients or women with PPH. Studies in trauma patients or women with PPH included in our review consistently indicated a link between a positive test result (VE device of SLTs) and transfusion outcomes or mortality. However, we did not identify any completed RCTs in these patient groups, although we did identify one ongoing RCT62 (recruitment has reached 105 participants out of a target of 120) and additional information on this study was provided by the authors in the form of the study protocol. As described in the above (see Strengths and limitations of assessment), prediction studies cannot provide information on how interventions and subsequent clinical outcomes may differ according to whether or not a POC VE testing or SLTs-based strategy is used. Further, in contrast with the RCTs conducted in patients undergoing cardiac surgery, they cannot provide data on how transfusion rates may differ according to whether or not the decision to transfuse is based on the use of a VE device or on SLTs. Our systematic review included one small (n = 50) controlled clinical trial63 that compared the effectiveness of an ‘institutional massive transfusion protocol’ (details not reported) to a TEG-guided protocol (details not reported) for the management of trauma patients. This study63 was published only as a conference abstract and no numerical data were reported; however, the results section stated that there were no statistically significant differences, or trends towards differences, between groups in mortality, ARDS, SIRS, MOF, sepsis, cardiovascular events, or duration of hospitalisation; a trend towards reduced pneumonia, reduced days on ventilation and reduced duration of ICU stay in the TEG-guided group was reported. 63 We did not include studies of VE devices with a historical control group in our review, as it is not possible to attribute any observed differences between groups in these studies solely to the introduction of the VE device. One such study,124 from a German level I trauma centre, reported reductions in the annual use of transfusion products from 2002 to 2010 (PRBC –33%, FFP –79%, platelet concentrates –65%) following the introduction of an algorithm for coagulation management in trauma patients based on POC ROTEM combined with calculated goal-directed therapy with FIB and PCC; the number of study participants was unclear, but approximately 250 trauma patients per year were treated in the emergency room. The study protocol provided by the authors of the ongoing trial64 also reported the results of a before-and-after study, conducted in their institution. Although much smaller than the German study, this study64 had the advantage of assessing two immediately consecutive populations: before (n = 34) and after (n = 34) rapid TEG was added to the institution’s massive transfusion protocol; unlike the German study, this implies that rapid TEG was the only change to management strategy. Results from this study64 indicated that patients managed with a protocol that included rapid TEG had more effective resuscitation than those managed using the standard massive transfusion protocol; median improvement in lactate from presentation to 6 hours was 2% for the standard massive transfusion protocol and 44% for the standard massive transfusion protocol + rapid TEG, and median improvement in pH from presentation to 6 hours was 1% for the standard massive transfusion protocol and 2% for the standard massive transfusion protocol + rapid TEG. 125 Rates of transfusion of all blood components were consistently less after the introduction of rapid TEG, but differences did not reach statistical significance. 125 Finally, mortality fell from 65% to 29% (p = 0.04) after the introduction of rapid TEG. 125 Taken together, the results of these studies could be considered to indicate that further investigation of the clinical utility of VE devices in trauma patients and women with PPH is warranted.
There is currently a lack of adequate information on the potential role of VE devices in the early detection of hyperfibrinolysis and any consequent effects on clinical outcomes, and this is an area that may particularly warrant further investigation. Data from the CRASH-2 trial indicate that greatest survival benefit from antifibrinolytic therapy in trauma patients is seen with very early (< 1 hour after injury) intervention. 126 There are also some published data indicating that the risk of death from bleeding increases at levels of clot lysis < 7.5% (at 30 minutes post maximum clot strength) which is generally regarded as normal. 127,128 The ROTEM FIBTEM assay and the TEG functional FIB assay use a reagent that is specific for the fibrin polymerisation process, which declines more rapidly than FIB levels as measured in the laboratory. 129 This adds the potential to detect the pathology at an earlier stage in its evolution to the time gained from using POC testing compared with laboratory-based testing. 130,131 A small observational study, which did not meet the criteria for inclusion in our systematic review, reported that primary fibrinolysis, as diagnosed by TEG, occurred < 1 hour post injury in 18% of a series of severely injured patients requiring massive transfusion, and was associated with increased blood component requirements, coagulopathy and haemorrhage-related death. VE devices therefore have the potential to provide a sufficiently timely and sensitive method of detecting fibrinolysis to enable optimally effective intervention. FIB is also thought to play a major role in the evolution of PPH and can be an early predictor of severity;129 however, data in this population are even more sparse than for trauma. Neither of the two PPH studies included in review82,83 reported hyperfibrinolysis as an outcome, although one83 did evaluate the ROTEM FIBTEM assay.
The extent to which VE devices may be considered to be clinically equivalent remains uncertain. As outlined in the background section of this report (see Tables 2–4), the range of parameters measured differs between the three devices included in this assessment (TEG, ROTEM and Sonoclot). Despite these differences, the available data provide no strong evidence of a difference in clinical effectiveness between TEG and ROTEM; however, it should be noted that there is no strong evidence that the devices are equivalent, as there were no studies providing a direct comparison between the two devices. Data on Sonoclot were very sparse, limited to three studies59,60,120 in the cardiac surgery population; data from two of these studies,59,60 which provided a direct comparison with TEG, did not suggest a significant difference in the ability of the two devices to predict bleeding.
Issues of training requirements and implementation are outside the scope of this assessment; however, a 2010 published report132 of studies undertaken by the UK National External Quality Assessment Scheme (NEQAS) for Blood Coagulation on the use of TEG and ROTEM devices in operating theatres has indicated that there may be some areas of concern. The published article132 reported the results of a series of four quality assurance studies, with up to 18 TEG users and 10 ROTEM users involved in testing two samples per study. The samples were normal plasmas, factor VIII or XI deficient samples, or normal plasmas spiked with heparin. The precision of the tests varied greatly for both devices, with coefficients of variances ranging from 7.1% to 39.9% for TEG and 7.0% to 83.6% for ROTEM. 132 Some centres returned results that were judged to be sufficiently different from those obtained by other participants to predict alterations in patient management decisions. 132 Based on these findings, it would seem that staff training requirements are likely to be an important consideration for the implementation of these devices. A UK study,133 published in 2009, compared users’ experience of TEG and ROTEM over a 1-week period; the study133 included seven consultant anaesthetists, one consultant haematologist, one associate specialist anaesthetist and two senior trainee anaesthetists, all of whom were trained by the manufacturers. The summary of the opinions of study participants suggested that the TEG training programme was preferred, and that better service support was provided for this device. 133 However, this is a very small study133 and may not be reflective of current experience in the NHS.
Cost-effectiveness
Substantial uncertainties around the cost-effectiveness of VE devices for the identification and management of coagulopathies remain, particularly with respect to the trauma population. The main uncertainties in the cost-effectiveness analyses follow directly from those described for the review of clinical effectiveness. Uncertainties are caused by lack of clinical effectiveness data for Sonoclot in the cardiac surgery population, and by a lack of clinical effectiveness data for any of the VE devices in the trauma and PPH populations. Once the results of the ongoing RCT,62 and any future RCTS, in the trauma population become available, our trauma model can readily be updated.
Other uncertainties pertain particularly to the trauma patients. As well as a requirement for data on the clinical effectiveness of VE testing in this population, this also includes data on trauma-specific costs and utilities. The influence of RBC transfusion on longer-term mortality (beyond in hospital mortality) in trauma patients is also unclear.
Chapter 6 Conclusions
Implications for service provision
For patients undergoing cardiac surgery, there was evidence from RCTs that VE testing (TEG or ROTEM) may be effective in reducing the numbers of patients receiving RBC transfusion, platelet transfusion and FFP transfusion, compared with a SLTs-based management strategy. Trial data also indicated that VE testing, compared with SLTs, may be associated with a reduction in the number of patients receiving factor VIIa transfusion, any blood component transfusion or PCC transfusion, but for these outcomes, differences did not reach statistical significance. There was no apparent difference in the rates of FIB transfusion between patients managed using VE testing and those managed using SLTs. The available data did not support an improvement in clinical outcomes (re-operation, surgical cause of bleed on re-operation and mortality) or length of hospital stay for patients managed using VE testing compared with those managed using SLTs. There was some evidence of reduced bleeding and reduced length of ICU stay for patients managed with VE testing compared with those managed using SLTs, but this was not consistently reported across studies. There was no evidence to indicate a difference in clinical effectiveness between the TEG and ROTEM devices, on any measure. No data were identified on the clinical effectiveness of Sonoclot. The limited available data on the ability of Sonoclot and TEG to predict bleeding (as opposed to clinical effectiveness) did not indicate a significant difference between the two devices. There was no evidence to indicate that performing SLTs in addition to VE testing gave any further benefit over that provided by VE testing alone. VE testing can therefore be regarded as a replacement for SLTs.
The base-case results of the cost-effectiveness analysis indicated that VE testing is cost-saving and more effective than SLTs in patients undergoing cardiac surgery. The per-patient cost-saving was slightly smaller for ROTEM (£43) than for TEG (£79) and Sonoclot (£132). However, this is based on the assumption that the effectiveness of Sonoclot is the same as that of TEG and ROTEM in the absence of data on the clinical effectiveness of this device. This finding was entirely dependent on material costs, which were slightly higher for ROTEM in the base-case analysis. When alternative assay combinations were modelled then TEG could be more costly than ROTEM. Scenario analyses, used to assess the potential impact of varying the way in which VE devices were used, did not alter the overall conclusion that VE testing is cost-saving.
There was no evidence on the clinical effectiveness of VE testing, using any device, in trauma patients or women with PPH. Available data generally indicated that a positive result on each of the TEG or ROTEM parameters or on SLTs was predictive of transfusion (RBC, any blood component and massive transfusion) and death. This implies a potential for improved intervention based on VE testing; however, there were no data showing that the use of VE devices could change outcomes. There were no clear differences between ROTEM, TEG or SLTs. No studies of the Sonoclot device were identified that fulfilled inclusion criteria for the either the trauma or PPH populations.
Cost-effectiveness analyses indicated that the per-patient cost-savings attributed to VE testing were more substantial for the trauma population than for patients undergoing cardiac surgery. This finding was primarily a result of the much higher blood volumes that are transfused in trauma patients. As with the cardiac surgery population, scenario analyses did not alter the overall conclusion that VE testing is cost-saving. However, given the potentially problematic assumption that the clinical effectiveness of VE testing is the same in trauma patients as it is in cardiac surgery patients, these results should be regarded as indicative of the potential cost-effectiveness of VE testing only in trauma patients.
Suggested research priorities
The clinical effectiveness and cost-effectiveness of VE testing in trauma patients and women with PPH remains uncertain. Clinical trials are urgently required in these populations in order to assess the effectiveness of VE testing compared with management based on SLTs. Outcomes assessed should include, but may not be limited to, bleeding outcomes, transfusion rates, volumes transfused, duration of hospital/ICU stay and mortality. The trauma model included in this assessment could readily be adapted to utilise data from such trials. It is also likely that the model structure could be adapted for women with PPH, as there is no reason to believe that effect categories would be substantially different.
No studies providing data on the clinical effectiveness of Sonoclot were identified in any of the populations considered by this assessment (patients undergoing cardiac surgery, trauma patients and women with PPH). Therefore, if the adoption of Sonoclot were to be considered, trials of this device would have high priority.
This assessment found no evidence to support any difference in clinical effectiveness between the three VE devices considered (ROTEM, TEG and Sonoclot). However, there was no strong evidence of equivalent clinical effectiveness between the devices for any of the populations considered (patients undergoing cardiac surgery, trauma patients and women with PPH). This was because no trial reported a direct comparison between VE devices. Trials comparing more than one VE device with SLTs would therefore be particularly useful.
None of the studies included in the clinical effectiveness review reported follow-up of participants to assess the potential effects of different testing regimens on longer-term, transfusion-related complications and mortality. Future trials should include longer-term follow-up, beyond the initial hospital episode, with a view to informing improved cost-effectiveness modelling.
Acknowledgements
The authors acknowledge the clinical advice and expert opinion provided by Dr Andrew Ronald, Consultant Anaesthetist and Honorary Senior Clinical Lecturer, Aberdeen Royal Infirmary; Dr Sarah Haynes, Autologous Transfusion Lead Scientist, Wythenshawe Hospital, Manchester; Dr Niall O’Keeffe, Consultant in Cardiac Anaesthesia and Intensive Care, Central Manchester University Hospital NHS Foundation Trust; Dr Seema Agarwal, Consultant in Cardiothoracic Anaesthesia, Liverpool Heart and Chest Hospital NHS Foundation Trust; Dr Laura Green, Consultant Haematologist and Honorary Senior Clinical Lecturer, Barts Health NHS Trust; and Mr Simon Davidson, Clinical Scientist, Royal Brompton and Harefield NHS Foundation Trust. We would also like to thank Diana Hilmer for help with the literature searches. Finally, the authors would like to thank the lay members of the NICE Diagnostics Advisory Committee and Assessment Subgroup for providing input on the patients’ perspectives at key stages of the assessment process.
Contributions of authors
Penny Whiting and Marie Westwood planned and performed the systematic review and interpretation of evidence.
Maiwenn Al and Isaac Corro Ramos planned and performed the cost-effectiveness analyses and interpreted results.
Steve Ryder and Nigel Armstrong contributed to planning and interpretation of cost-effectiveness analyses, acquisition of input data and conducted model peer review.
Kate Misso and Janine Ross devised and performed the literature searches and provided information support to the project.
Johan Severens and Jos Kleijnen provided senior advice and support to the systematic review and cost-effectiveness analyses, respectively.
All parties were involved in drafting and/or commenting on the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Data sharing statement
Data are included as appendices to the report.
References
- Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol 2005;27:81-90. http://dx.doi.org/10.1111/j.1365-2257.2005.00681.x.
- Weber CF, Klages M, Zacharowski K. Perioperative coagulation management during cardiac surgery. Curr Opin Anaesthesiol 2013;26:60-4. http://dx.doi.org/10.1097/ACO.0b013e32835afd28.
- Curry NS, Davenport RA, Hunt BJ, Stanworth SJ. Transfusion strategies for traumatic coagulopathy. Blood Rev 2012;26:223-32. http://dx.doi.org/10.1016/j.blre.2012.06.004.
- Solomon C, Collis RE, Collins PW. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br J Anaesth 2012;109:851-63. http://dx.doi.org/10.1093/bja/aes361.
- Specialised Services National Definitions Set. London: NHS Specialist Services; 2009.
- Hospital Episode Statistics 2011–2012. Leeds: HSCIC; n.d.
- New Major Trauma Centres to Save Up to 600 Lives Every Year. London: DoH; 2012.
- Health Protection in the 21st Century: Understanding the Burden of Disease; Preparing for the Future. London: HPA; 2005.
- Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011;118:1-203. http://dx.doi.org/10.1111/j.1471-0528.2010.02847.x.
- Dunning J, Versteegh M, Fabbri A, Pavie A, Kolh P, Lockowandt U, et al. Guideline on antiplatelet and anticoagulation management in cardiac surgery. Eur J Cardiothorac Surg 2008;34:73-92. http://dx.doi.org/10.1016/j.ejcts.2008.02.024.
- Vivacqua A, Koch CG, Yousuf AM, Nowicki ER, Houghtaling PL, Blackstone EH, et al. Morbidity of bleeding after cardiac surgery: is it blood transfusion, reoperation for bleeding, or both?. Ann Thorac Surg 2011;91:1780-90. http://dx.doi.org/10.1016/j.athoracsur.2011.03.105.
- Craig J, Aguiar-Ibanez R, Bhattacharya S, Downie S, Duffy S, Kohli H, et al. The Clinical and Cost Effectiveness of Thromboelastography/Thromboelastometry: Health Technology Assessment Report 11. Glasgow: NHS Health Improvement Scotland; 2008.
- Hess JR, Lindell AL, Stansbury LG, Dutton RP, Scalea TM. The prevalence of abnormal results of conventional coagulation tests on admission to a trauma center. Transfusion 2009;49:34-9. http://dx.doi.org/10.1111/j.1537-2995.2008.01944.x.
- Frith D, Goslings JC, Gaarder C, Maegele M, Cohen MJ, Allard S, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost 2010;8:1919-25. http://dx.doi.org/10.1111/j.1538-7836.2010.03945.x.
- Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544-52. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.698977.
- Tanaka KA, Bolliger D, Vadlamudi R, Nimmo A. Rotational thromboelastometry (ROTEM)-based coagulation management in cardiac surgery and major trauma. J Cardiothorac Vasc Anesth 2012;26:1083-93. http://dx.doi.org/10.1053/j.jvca.2012.06.015.
- Welcome to ROTEM®. Munich, Germany: Tem Innovations GmbH; 2013.
- How it Works: Thromboelastometry (TEM®). Munich, Germany: Tem Innovations GmbH. c.; 2013.
- How it Works: ROTEM® Analysis. Munich, Germany: Tem Innovations GmbH.; 2013.
- Hartert H. Blutgerinningsstudien mit der thrombelastographie, einem neuen untersuchingsverfahren. Klin Wochenschr 1948;26:577-83. http://dx.doi.org/10.1007/BF01697545.
- Afshari A, Wikkelso A, Brok J, Moller AM, Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev 2011;3. http://dx.doi.org/10.1002/14651858.CD007871.pub2.
- Hunt H, Hyde C, Stanworth S, Curry N, Perel P, Woolley T, et al. Thromboelastography (TEG) and thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding Diagnostic Test Accuracy Protocol. Cochrane Database Syst Rev 2013;2. http://dx.doi.org/10.1002/14651858.CD010438.pub2.
- TEG® 5000: Hemostasis Analyzer System: The New Standard of Care in Hemostasis Management Internet. Braintree, MA: Haemonetics Corporation; 2013.
- Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg 2008;106:1366-75. http://dx.doi.org/10.1213/ane.0b013e318168b367.
- Omert LA, Popovsky M. Thromboelastography versus thromboelastometry: time for a fair appraisal. Anaesthesiology 2012;116:970-1. http://dx.doi.org/10.1097/ALN.0b013e31824a878d.
- von Kaulla KN, Ostendorf P, von Kaulla E. The impedance machine: a new bedside coagulation recording device. J Med 1975;6:73-88.
- Sonoclot® Coagulation & Platelet Function Analyzer with Graphics Printer. For In Vitro Diagnostic Use: Operator’s Manual. For Firmware Version 4.0. Sonoclot Analyzer, DP-2951, User Manual 020–1001 Rev. 4.0.1. Arvada, CO: Sienco Inc.; n.d.
- McMichael MA, Smith SA. Viscoelastic coagulation testing: technology, applications, and limitations. Vet Clin Pathol 2011;40:140-53. http://dx.doi.org/10.1111/j.1939-165X.2011.00302.x.
- Paniccia R, Antonucci E, Maggini N, Romano E, Gori AM, Marcucci R, et al. Assessment of platelet function on whole blood by multiple electrode aggregometry in high-risk patients with coronary artery disease receiving antiplatelet therapy. Am J Clin Pathol 2009;131:834-42. http://dx.doi.org/10.1309/AJCPTE3K1SGAPOIZ.
- Practical Haemostasis.com . Platelet Function Testing: PFA-100 2013. www.practical-haemostasis.com/Platelets/platelet_function_testing_pfa100.html (accessed 25 July 2013).
- Multiplate. The New Standard for Platelet Diagnostics. Munich: Verum Diagnostica GmbH; 2013.
- Mackie IJ, Kitchen S, Machin SJ, Lowe GD. Guidelines on fibrinogen assays. Br J Haematol 2003;121:396-404. http://dx.doi.org/10.1046/j.1365-2141.2003.04256.x.
- Van Veen JJ, Spahn DR, Makris M. Routine preoperative coagulation tests: an outdated practice?. Br J Anaesth 2011;106:1-3. http://dx.doi.org/10.1093/bja/aeq357.
- Chee YL, Crawford JC, Watson HG, Greaves M. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. British Committee for Standards in Haematology. Br J Haematol 2008;140:496-504. http://dx.doi.org/10.1111/j.1365-2141.2007.06968.x.
- Weber CF, Gorlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anaesthesiology 2012;117:531-47. http://dx.doi.org/10.1097/ALN.0b013e318264c644.
- Rodriguez E, Ferguson TB. Hemostatic Monitoring for Cardiothoracic Surgery Patients 2008. www.medscape.org/viewarticle/576461 (accessed 17 July 2013).
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009.
- Diagnostics Assessment Programme manual. Manchester: NICE; 2011.
- Handbook for DTA Reviews. Cochrane; 2009.
- CADTH Peer Review Checklist for Search Strategies. Ottawa: CADTH; 2013.
- Royle P, Waugh N. Should systematic reviews include searches for published errata?. Health Info Libr J 2004;21:14-20. http://dx.doi.org/10.1111/j.1471-1842.2004.00459.x.
- Wright K, McDaid C. Is the retraction of journal articles in electronic journals and databases consistent and timely? A case study. Madrid Spain: Cochrane Colloquium, Cochrane; 2011.
- Wright K, McDaid C. Reporting of article retractions in bibliographic databases and online journals. J Med Libr Assoc 2011;99:164-7. http://dx.doi.org/10.3163/1536-5050.99.2.010.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Ak K, Isbir CS, Tetik S, Atalan N, Tekeli A, Aljodi M, et al. Thromboelastography-based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg 2009;24:404-10. http://dx.doi.org/10.1111/j.1540-8191.2009.00840.x.
- Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastograph (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ 2009;18:277-88. http://dx.doi.org/10.1016/j.hlc.2008.08.016.
- Avidan MS, Alcock EL, Da Fonseca J, Ponte J, Desai JB, Despotis GJ, et al. Comparison of structured use of routine laboratory tests or near-patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth 2004;92:178-86. http://dx.doi.org/10.1093/bja/aeh037.
- Royston D, von Kier S. Reduced haemostatic factor transfusion using heparinase-modified thrombelastography during cardiopulmonary bypass. Br J Anaesth 2001;86:575-8. http://dx.doi.org/10.1093/bja/86.4.575.
- Nuttall GA, Oliver WC, Santrach PJ, Bryant S, Dearani JA, Schaff HV, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anaesthesiology 2001;94:773-81. http://dx.doi.org/10.1097/00000542-200105000-00014.
- Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg 1999;88:312-19. http://dx.doi.org/10.1097/00000539-199902000-00016.
- Kultufan Turan S, Aydinli B, Ayik I, Yagar S, Kazanci D, Karadeniz U, et al. The role of rotational thromboelastgraphy on decision of blood transfusion in open heart surgery. Göğüs-Kalp-Damar Anestezi Ve Yoğun Bakim Dernegi Dergisi 2006;12:154-9.
- Paniagua P, Koller T, Requena T, Gil JM, Campos JM, Galan J. Randomized controled trial to evaluate postoperative coagulation management with bed-side trombelastometry (Rotem) compared with a transfusion protocol based on laboratory meausurments in bleeding patients after cardiac surgery: preliminary data. Paper presented at the European Anaesthesiology Congress (EUROANAESTHESIA); 11–14 June 2011; Amsterdam: Netherlands. Eur J Anaesthesiol 2011;28. http://dx.doi.org/10.1097/00003643-201106001-00301.
- Girdauskas E, Kempfert J, Kuntze T, Borger MA, Enders J, Fassl J, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117-24. http://dx.doi.org/10.1016/j.jtcvs.2010.04.043.
- Rauter M, Kastenbauer T, Schwarz S, Fitzgerald R. Reduced number of red blood cell transfusions in cardiac surgery due to perioperative coagulation testing by thromboelastometry. Acta Anaesthesiol Scand 2007;51.
- Shore-Lesserson L, Manspeizer HE, Francis S, DePerio M. Intraoperative thromboelastography (TEG) reduces transfusion requirements. Anesth Analg 1998;46.
- Shore-Lesserson L, Manspeizer HE, Francis S, Deperio M. Thromboelastography decreases transfusion requirements after cardiac surgery. Anaesthesiology 1998;89. http://dx.doi.org/10.1097/00000542-199809060-00038.
- Bischof D, Graves K, Zollinger A, Hofer CK. Predictive value of sonoclot and routine coagulation tests for postoperative bleeding in patients undergoing cardiac surgery. Paper presented at the 22nd Annual Congress of the European Society of Intensive Care Medicine (ESICM); 11–14 Jan 2009; Vienna: Austria. Intensive Care Med 2009;35.
- Nuttall GA, Oliver WC, Ereth MH, Santrach PJ. Coagulation tests predict bleeding after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1997;11:815-23. http://dx.doi.org/10.1016/S1053-0770(97)90112-9.
- Tuman KJ, Spiess BD, McCarthy RJ, Ivankovich AD. Comparison of viscoelastic measures of coagulation after cardiopulmonary bypass. Anesth Analg 1989;69:69-75. http://dx.doi.org/10.1213/00000539-198907000-00013.
- Comparison of rapid thrombelastography and conventional coagulation testing for haemostatic resuscitation in trauma. Bethesda (MD): National Library of Medicine (US); 2013.
- Moore E. A Prospective, Randomized Comparison of Rapid Thrombelastography (R-TEG) and Conventional Coagulation Testing for Guiding the Diagnosis and Hemostatic Resuscitation of Trauma Patients at Risk for Post-Injury Coagulopathy (NCT 01536496). Denver, CO: Denver Health and Hospital Authority; n.d.
- Messenger BM, Craft RM, Carroll RC, Daley BJ, Enderson B, Snider CG. TEG-guided massive transfusion in trauma patients. Paper presented at the Annual Meeting of the International Anesthesia Research Society (IARS); 21–24 May 2011; Vancouver: Canada. Anesth Analg 2011;112.
- Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma 1997;42:716-22. http://dx.doi.org/10.1097/00005373-199704000-00023.
- Leemann H, Lustenberger T, Talving P, Kobayashi L, Bukur M, Brenni M, et al. The role of rotation thromboelastometry in early prediction of massive transfusion. J Trauma 2010;69:1403-8. http://dx.doi.org/10.1097/TA.0b013e3181faaa25.
- Tauber H, Innerhofer P, Breitkopf R, Westermann I, Beer R, El Attal R, et al. Prevalence and impact of abnormal ROTEM assays in severe blunt trauma: results of the ‘Diagnosis and Treatment of Trauma-Induced Coagulopathy (DIA-TRE-TIC) study’. Br J Anaesth 2011;107:378-87. http://dx.doi.org/10.1093/bja/aer158.
- Kunio NR, Differding JA, Watson KM, Stucke RS, Schreiber MA. Thrombelastography-identified coagulopathy is associated with increased morbidity and mortality after traumatic brain injury. Am J Surg 2012;203:584-8. http://dx.doi.org/10.1016/j.amjsurg.2011.12.011.
- Schöchl H, Solomon C, Redl H, Bahrami S. Rotational thromboelastometry in patients with severe traumatic brain injury: possible risk stratification?. Shock 2011;36.
- Tapia NM, Chang AL, Norman MA, Welsh FJ, Scott BG, Wall MJ, et al. Hyperfibrinolysis on thromboelastogram (TEG) predicts mortality in massively transfused trauma patients. Paper presented at the 98th Annual Clinical Congress of the American College of Surgeons, Surgical Forum 2012 Clinical Congress; 30 September–4 October 2012; Chicago: US. J Am Coll Surg 2012;215. http://dx.doi.org/10.1016/j.jamcollsurg.2012.06.153.
- Davenport R, Manson J, De’Ath H, Platton S, Coates A, Allard S, et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med 2011;39:2652-8. http://dx.doi.org/10.1097/CCM.0b013e3182281af5.
- Nystrup KB, Windelov NA, Thomsen AB, Johansson PI. Reduced clot strength upon admission, evaluated by thrombelastography (TEG), in trauma patients is independently associated with increased 30-day mortality. Scand J Trauma Resusc Emerg Med 2011;19. http://dx.doi.org/10.1186/1757-7241-19-52.
- Ives C, Inaba K, Branco BC, Okoye O, Schöchl H, Talving P, et al. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg 2012;215:496-502. http://dx.doi.org/10.1016/j.jamcollsurg.2012.06.005.
- Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma Inj Infect Crit Care 2011;71:407-17. http://dx.doi.org/10.1097/TA.0b013e31821e1bf0.
- Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department experience with 1974 consecutive trauma patients. Ann Surg 2012;256:476-86. http://dx.doi.org/10.1097/SLA.0b013e3182658180.
- Korfage AR, Greuters S, Brinkman AC, Viersen V, Boer C. The incidence proportion and predictability of transfusion requirements in trauma patients in the context of fast access to trauma care. Paper presented at the European Anaesthesiology Congress (EUROANAESTHESIA) 2011; 11–14 June 2011; Amsterdam: Netherlands. Eur J Anaesthesiol 2011;28. http://dx.doi.org/10.1097/00003643-201106001-00616.
- Schöchl H, Cotton B, Inaba K, Nienaber U, Fischer H, Voelckel W, et al. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care 2011;15. http://dx.doi.org/10.1186/cc10539.
- Jeger V, Willi S, Liu T, Yeh DD, De Moya M, Zimmermann H, et al. The rapid TEG alpha-angle may be a sensitive predictor of transfusion in moderately injured blunt trauma patients. Sci World J 2012. http://dx.doi.org/10.1100/2012/821794.
- Davenport R, Coates A, Allard S, Pasi J, MacCallum P, Stanworth S, et al. Trauma haemorrhage: evolving role of ROTEM for predicting transfusion requirements. Shock 2009;32. http://dx.doi.org/10.1097/CCM.0b013e3182281af5.
- Jeger V, Willi S, Liu T, Omert L, Orr A, Popovsky MA, et al. Comparison of point-of-care thrombelastography versus conventional coagulation tests in the emergency department management of trauma. Paper presented at 31st International Symposium on Intensive Care and Emergency Medicine; 22–25 October 2011; Brussels: Belgium. Crit Care 2011;15.
- Pezold M, Moore EE, Wohlauer M, Sauaia A, Gonzalez E, Banerjee A, et al. Viscoelastic clot strength predicts coagulation-related mortality within 15 minutes. Surgery 2012;151:48-54. http://dx.doi.org/10.1016/j.surg.2011.06.023.
- Schöchl H, Solomon C, Redl H, Bahrami S. Rotational thromboelastometric (ROTEM) test results in the emergency room predicts massive transfusion in major trauma patients. Shock 2011;36.
- Bolton S, Harkness M, Thompson S. ROTEM thromboelastometry identifies blood product requirements during major obstetric haemorrhage. Paper presented at the Annual Meeting of the Obstetric Anaesthetists’ Association; 26–27 May 2011; Edinburgh: UK. Int J Obstet Anesth 2011;20.
- Lilley GJ, Burkett-St Lawrent DA, Collins PW, Collis RE. A prospective study to evaluate early clauss fibrinogen and fibtem as predictors for major obstetric haemorrhage. Paper presented at the Annual Meeting of the Obstetric Anaesthetists’ Association; 23–24 May 2013; Bournemouth: UK. Int J Obstet Anesth 2013;22.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Gorlinger K, Dirkmann D, Muller-Beisenhirtz H, Paul A, Hartmann M, Saner F. Thromboelastometry-based perioperative coagulation management in visceral surgery and liver transplantation: Experience of 10 years and 1105 LTX. Paper presented at 16th Annual International Congress of the International Liver Transplantation Society; 16–19 June 2010; Hong Kong: China. Liver Transpl 2010;16.
- Qureshi H, Ahmed A, Dhillon JK. Evaluation of clinical and cost effectiveness of thromboelastography (TEG) in cardiac surgery. Paper presented at 11th Annual NATA Symposium; 8–9 April 2010; Barcelona: Spain. Transfus Altern Transfus Med 2010;11.
- Spalding GJ, Hartrumpf M, Sierig T, Oesberg N, Kirschke CG, Albes JM. Cost reduction of perioperative coagulation management in cardiac surgery: value of ‘bedside’ thrombelastography (ROTEM). Eur J Cardiothorac Surg 2007;31:1052-7. http://dx.doi.org/10.1016/j.ejcts.2007.02.022.
- Gorlinger K, Fries D, Dirkmann D, Weber CF, Hanke AA, Schöchl H. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus Med Hemother 2012;39:104-13. http://dx.doi.org/10.1159/000337186.
- Craig J, Aguiar-Ibanez R, Bhattacharya S, Downie S, Duffy S, Kohli H, et al. Amendment to Health Technology Assessment Report 11: The Clinical and Cost Effectiveness of Thromboelastography/Thromboelastometry. Glasgow: NHS Health Improvement Scotland; 2009.
- Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10440.
- Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health 2013;67:974-8. http://dx.doi.org/10.1136/jech-2013-203104.
- Karkouti K, Yau TM, Rensburg Av, McCluskey SA, Callum J, Wijeysundera DN, et al. The effects of a treatment protocol for cardiac surgical patients with excessive blood loss on clinical outcomes. Vox Sang 2006;91:148-56. http://dx.doi.org/10.1111/j.1423-0410.2006.00813.x.
- Serious Hazards of Transfusion: Annual SHOT Report 2012. Manchester: SHOT; 2012.
- Murphy MF, Edbury C, Wickenden C. Survey of the implementation of the recommendations in the Health Services Circular 1998/224 ‘Better Blood Transfusion’. Transfus Med 2003;13:121-5. http://dx.doi.org/10.1046/j.1365-3148.2003.00431.x.
- Murphy MF, Howell C. Survey of the implementation of the recommendations in the Health Service Circular 2002/009 ‘Better Blood Transfusion’. Transfus Med 2005;15:453-60. http://dx.doi.org/10.1111/j.1365-3148.2005.00621.x.
- The National Blood Service: Report by the Comptroller and Auditor General. HC 6 Session 2000–2001: 20 December 2000. London: National Audit Office; 2000.
- Prescott-Clarke P, Primatesta P. Health Survey for England ‘96. Volume 1: Findings. London: Stationery Office; 1998.
- Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology 2013;81:1588-95. http://dx.doi.org/10.1212/WNL.0b013e3182a9f45f.
- Consumer Price Inflation, August 2013. London: ONS; 2013.
- NHS Blood and Transplant Service . NHS Blood and Components: Price List 2013 2014 2013. http://hospital.blood.co.uk/library/pdf/price_list_2013_14.pdf (accessed 17 December 2013).
- Hospital Episode Statistics 2012–2013. Leeds: HSCIC; n.d.
- Luengo-Fernandez R, Grey AM, Rothwell PM. A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke 2012;43:3343-51. http://dx.doi.org/10.1161/STROKEAHA.112.667204.
- McConnachie A, Walker A, Robertson M, Marchbank L, Peacock J, Packard CJ, et al. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur Heart J 2014;35:290-8. http://dx.doi.org/10.1093/eurheartj/eht232.
- Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care 2013;17. http://dx.doi.org/10.1186/cc12685.
- Chaiwat O, Lang JD, Vavilala MS, Wang J, MacKenzie EJ, Jurkovich GJ, et al. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anaesthesiology 2009;110:351-60. http://dx.doi.org/10.1097/ALN.0b013e3181948a97.
- Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed?. Arch Surg 2005;140:432-8. http://dx.doi.org/10.1001/archsurg.140.5.432.
- Dewar DC, Tarrant SM, King KL, Balogh ZJ. Changes in the epidemiology and prediction of multiple-organ failure after injury. J Trauma Acute Care Surg 2013;74:774-9. http://dx.doi.org/10.1097/TA.0b013e31827a6e69.
- Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg 1997;132:620-4. http://dx.doi.org/10.1001/archsurg.1997.01430300062013.
- Johnson JL, Moore EE, Kashuk JL, Banerjee A, Cothren CC, Biffl WL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg 2010;145:973-7. http://dx.doi.org/10.1001/archsurg.2010.216.
- Bochicchio GV, Napolitano L, Joshi M, Bochicchio K, Meyer W, Scalea TM. Outcome analysis of blood product transfusion in trauma patients: a prospective, risk-adjusted study. World J Surg 2008;32:2185-9. http://dx.doi.org/10.1007/s00268-008-9655-0.
- Angus DC, Clermont G, Linde-Zwirble WT, Musthafa AA, Dremsizov TT, Lidicker J, et al. Healthcare costs and long-term outcomes after acute respiratory distress syndrome: a phase III trial of inhaled nitric oxide. Crit Care Med 2006;34:2883-90. http://dx.doi.org/10.1097/01.CCM.0000248727.29055.25.
- Laupland KB, Svenson LW, Grant V, Ball CG, Mercado M, Kirkpatrick AW. Long-term mortality outcome of victims of major trauma. Injury 2010;41:69-72. http://dx.doi.org/10.1016/j.injury.2009.06.006.
- Hofhuis JG, Spronk PE. Health-related quality of life and influence of age after trauma: an overview. J Trauma Acute Care Surg 2014;76:549-56. http://dx.doi.org/10.1097/TA.0b013e3182a9d105.
- Holtslag HR, van Beeck EF, Lindeman E, Leenen LP. Determinants of long-term functional consequences after major trauma. J Trauma 2007;62:919-27. http://dx.doi.org/10.1097/01.ta.0000224124.47646.62.
- Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1389-94. http://dx.doi.org/10.1164/ajrccm.163.6.2005123.
- Ara R, Brazier J. Comparing EQ-5D scores for comorbid health conditions estimated using 5 different methods. Med Care 2012;50:452-9. http://dx.doi.org/10.1097/MLR.0b013e318234a04a.
- NHS Reference Costs 2012–2013. London: DH; 2013.
- Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C. Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-beta and glatiramer acetate for multiple sclerosis. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8270.
- Major Trauma Care in England. Report by the Comptroller and Auditor General (HC 213; Session 2009–2010). London: National Audit Office; 2010.
- Lewandrowski K. Managing utilization of new diagnostic tests. Clin Leader Manag Rev 2003;17:318-24.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2011.
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011;64:602-7. http://dx.doi.org/10.1016/j.jclinepi.2010.07.006.
- Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med 2006;34:1608-16. http://dx.doi.org/10.1097/01.CCM.0000217920.48559.D8.
- Gorlinger K, Dirkmann D, Lendemans S, Husmann B, Waydhas C, Piepenbrink K, et al. Massive bleeding algorithms: experience of a German level I trauma center. Haematology Society of Australia and New Zealand (HSANZ)/ Australia and New Zealand Society of Blood Transfusion (ANZSBT)/Australasian Society of Thrombosis and Haemostasis (ASTH) International Society of Hematology (ISH) Annual Scientific Meeting 2011, Sydney, NSW Australia, 30 October–2 November 2011. Conference Publication. Transfusion Medicine 2012.
- Kashuk J, Moore E, Wohlauer M, Johnson J, Pezold M, Lawrence J, et al. Initial experiences with point of care (POC) rapid thrombelastography (TEG) for management of life threatening postinjury coagulopathy (PIC). Paper presented at 33rd Annual Conference on Shock; 12–15 June 2010; Portland: US. Shock 2010;33:27-8.
- Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23-32. http://dx.doi.org/10.1016/S0140-6736(10)60835-5.
- Kutcher ME, Cripps MW, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Criteria for empiric treatment of hyperfibrinolysis after trauma. J Trauma Acute Care Surg 2012;73:87-93. http://dx.doi.org/10.1097/TA.0b013e3182598c70.
- Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schöchl H, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg 2012;73:365-70. http://dx.doi.org/10.1097/TA.0b013e31825c1234.
- de Lange NM, Lance MD, De Groot R, Beckers EAM, Henskens YM, Scheepers HCJ. Obstetric hemorrhage and coagulation: an update. Thromboelastography, thromboelastometry, and conventional coagulation tests in the diagnosis and prediction of postpartum hemorrhage. Obstet Gynecol Surv 2012;67:426-35. http://dx.doi.org/10.1097/OGX.0b013e3182605861.
- Kandala S, Natarajan A, Dean MN, Maynard S, Lucas DN, Rao K. Thromboelastography versus routine laboratory testing in the obstetric unit. Association of Anaesthetists of Great Britain and Ireland’s Annual Congress, Harrogate, UK, 22–24 September 2010. Anaesthesia 2010;65:1247-8.
- Jeger V, Zimmermann H, Exadaktylos AK. Can RapidTEG accelerate the search for coagulopathies in the patient With multiple injuries?. J Trauma Inj Infect Crit Care 2009;66:1253-7. http://dx.doi.org/10.1097/TA.0b013e31819d3caf.
- Kitchen DP, Kitchen S, Jennings I, Woods T, Walker I. Quality assurance and quality control of thromboelastography and rotational thromboelastometry: the UK NEQAS for Blood Coagulation Experience. Semin Thromb Hemost 2010;36:757-63. http://dx.doi.org/10.1055/s-0030-1265292.
- Jackson GN, Ashpole KJ, Yentis SM. The TEG vs the ROTEM thromboelastography/thromboelastometry systems. Anaesthesia 2009;64:212-15. http://dx.doi.org/10.1111/j.1365-2044.2008.05752.x.
- Brohi K. Diagnosis and management of coagulopathy after major trauma. Br J Surg 2009;96:963-4. http://dx.doi.org/10.1002/bjs.6691.
- Carroll RC, Craft RM, Langdon RJ, Clanton CR, Snider CC, Wellons DD, et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res 2009;154:34-9. http://dx.doi.org/10.1016/j.trsl.2009.04.001.
- Chevannes C, Harrod I, Bhalla A, Barclay P, Mallaiah S. Fast rotational thromboelastometry evaluation in major obstetric haemorrhage. Br J Anaesth 2012;109. http://dx.doi.org/10.1111/anae.12859.
- Craft R, Carroll R, Langdon R, Clanton C, Snider C, Lawson C, et al. Point-of-care thrombelastography assays for coagulopathy in trauma patients. Transfusion 2008;48. http://dx.doi.org/10.1016/j.trsl.2009.04.001.
- Curry N, Raja A, Hughes T, Black J, Pullinger R, Davenport R, et al. A prospective observational study evaluating changes in coagulation parameters in trauma admissions. Paper presented at 50th Annual Scientific Meeting of the British Society for Haematology; 19–21 April 2010; Edinburgh: UK. Br J Haematol 2010;149.
- Dietrich GV, Schueck R, Menges T, Kiesenbauer NP, Fruehauf A-C, Marquardt I. Comparison of four methods for the determination of platelet function in whole blood in cardiac surgery. Thromb Res 1998;89:295-301. http://dx.doi.org/10.1016/S0049-3848(98)00020-6.
- Ducloy-Bouthors AS, Bauters A, Tournoys A, Wibaut B, Richart P. ROTEM in obstetric: near patient-test as predictor of post-partum hemorrhage. Paper presented at 15th WFSA World Congress of Anaesthesiologists; 25–30 March 2012; Predio Ferial de Buenos Aires: Argentina. Br J Anaesth 2012;108:191-2.
- Ducloy-Bouthors AS, Bauters A, Wibaut B, Tournoys A, Barre C, Pilla SC, et al. Fibtem: Early predictor of severe pph and related hypofibrinogenemia. Paper presented at the 20th FIGO World Congress of Gynecology and Obstetrics; 7–12 October 2012; Rome: Italy. Int J Gynaecol Obstet 2012;119.
- Pivalizza EG. Comparison of sonoclot and hemacron act’s during cardiac surgery. Anesth Analg 1997;84.
- Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma Inj Infect Crit Care 2008;64:S64-S8. http://dx.doi.org/10.1097/TA.0b013e318160772d.
- Forestier F, Belisle S, Contant C, Harel F, Janvier G, Hardy J-F. Reproducibility and interchangeability of the Thromboelastograph, Sonoclot and Hemochron activated coagulation time in cardiac surgery. Can J Anaesth 2001;48:902-10. http://dx.doi.org/10.1007/BF03017358.
- Grassetto A, De Nardin M, Ganzerla B, Geremia M, Saggioro D, Ni ES, et al. ROTEM (R)-guided coagulation factor concentrate therapy in trauma: 2-year experience in Venice, Italy. Crit Care 2012;16. http://dx.doi.org/10.1186/cc11322.
- Hagemo JS, Gaarder C, Naess PA, Viksmoen Y, Heier HE. Comparing TEG and RoTEM as point-of-care devices in trauma patients. Paper presented at 31st International Congress of the International Society of Blood Transfusion in Joint Cooperation with the 43rd Congress of the DGTI; 26 June–1 July 2010; Berlin: Germany. Vox Sang 2010;99.
- Huissoud C, Carrabin N, Audibert F, Levrat A, Massignon D, Berland M, et al. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. BJOG 2009;116:1097-102. http://dx.doi.org/10.1111/j.1471-0528.2009.02187.x.
- Jeong Y-H, Bliden K, Antonino MJ, Tantry US, Gurbel PA. First comparison of thromboelastography versus light transmittance aggregometry to define high platelet reactivity. J Am Coll Cardiol 2011;57. http://dx.doi.org/10.1016/S0735-1097(11)61506-4.
- Johansson PI. Standard coagulation tests versus viscoelastic POC monitoring. Wien Klin Wochenschr 2010;122:9-10.
- Karlsson O, Henriksson BA, Jeppsson A, Hellgren M. Coagulopathies early in postpartum haemorrhage; thromboelastography and haemostatic laboratory analyses. Paper presented at 5th International Symposium on Women’s Health Issues in Thrombosis and Haematosis; 1–3 February 2013; Vienna: Austria. Thromb Res 2013;131. http://dx.doi.org/10.1016/S0049-3848(13)70115-4.
- Levrat A, Gros A, Rugeri L, Inaba K, Floccard B, Negrier C, et al. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth 2008;100:792-7. http://dx.doi.org/10.1093/bja/aen083.
- Miles WS, Schiffern L, Howard D, Geller H, Lipford N. A thromboelastogram protocol reduces blood utilization in cardiac surgical intensive care unit patients. Crit Care Med 2007;35.
- Miyashita T, Kuro M. Evaluation of platelet function by Sonoclot analysis compared with other hemostatic variables in cardiac surgery. Anesth Analg 1998;87:1228-33. http://dx.doi.org/10.1097/00000539-199812000-00002.
- Newland MC, Chapin JW, Hurlbert BJ, Kennedy EM, Newland JR. Thromboelastograph and sonoclot signature monitoring of chances in blood coagulation following cardiopulmonary bypass. Anaesthesiology 1987;67. http://dx.doi.org/10.1097/00000542-198709001-00199.
- Nix CM, Bhinder R, Tan T, Carey MF. Point of care thromboelastography during massive obstetric haemorrhage in a maternity hospital. Paper presented at Annual Meeting of the Obstetric Anaesthetists’ Association; 26–27 May 2011; Edinburgh: UK. Int J Obstet Anesth 2011;20.
- Porite N, Strike E, Lacis R, Vanags I, Avots A, Harlamovs V. Thromboelastography-guided transfusion algorithm reduces haemotransfusions in cardiac surgery. Atheroscler Suppl 2004;5:25-6.
- Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost 2012;10:1342-51. http://dx.doi.org/10.1111/j.1538-7836.2012.04752.x.
- Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost 2007;5:289-95. http://dx.doi.org/10.1111/j.1538-7836.2007.02319.x.
- Schöchl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma Inj Infect Crit Care 2009;67:125-31. http://dx.doi.org/10.1097/TA.0b013e31818b2483.
- Shah B, Subramanium A, Agrawal D, Mukhopadhyay A. Thromboelastography-viscoelastic haemostatic assay (VHA) as an indicator of overt disseminated intravascular coagulation in brain injury patients within 24 hours after injury. Paper presented at 9th World Congress on Brain Injury of the International Brain Injury Association; 21–25 March 2012; Edinburgh: UK. Brain Inj 2012;26:586-7.
- Shah BA, Subramanium A, Sharma S, Agrawal D, Chhabra G, Mukhopadhyay AK. Thromboelastography-Viscoelastic Haemostatic Assay (VHA) versus routine plasma based coagulation parameters in trauma patients to detect coagulopathy within 24 hrs after injury. Paper presented at 53rd Annual Meeting of the American Society of Hematology (ASH); 10–13 December 2011; San Diego, US. Blood 2011;118.
- Shore-Lesserson L, Reich DL, De Perio M, Silvay G. Thromboelastographic assessment of platelet-rich plasmapheresis during cardiac reoperations. Anaesthesiology 1992;77. http://dx.doi.org/10.1097/00000542-199209001-00138.
- Stanworth SJ, Morris TP, Gaarder C, Goslings JC, Maegele M, Cohen MJ, et al. Reappraising the concept of massive transfusion in trauma. Crit Care 2010;14. http://dx.doi.org/10.1186/cc9394.
- Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg 2013;74:378-86. http://dx.doi.org/10.1097/TA.0b013e31827e20e0.
- Thai J, Reynolds EJ, Natalia N, Cornelissen C, Lemmens HJM, Hill CC, et al. Comparison between RapidTEG (R) and conventional thromboelastography in cardiac surgery patients. Br J Anaesth 2011;106:605-6. http://dx.doi.org/10.1093/bja/aer054.
- Traverso MJ, Koza J, Arcelus J, Walenga JM, Pifarre R. Comparison of thromblastograph and Sonoclot in predicting post-operative blood loss in cardiopulmonary bypass. FASEB J 1993;7.
- Woolley T, Midwinter M, Spencer P, Watts S, Doran C, Kirkman E. Utility of interim ROTEM (R) values of clot strength, A5 and A10, in predicting final assessment of coagulation status in severely injured battle patients. Injury 2013;44:593-9. http://dx.doi.org/10.1016/j.injury.2012.03.018.
- Yamada T, Katori N, Tanaka KA, Takeda J. Impact of Sonoclot hemostasis analysis after cardiopulmonary bypass on postoperative hemorrhage in cardiac surgery. J Anesth 2007;21:148-52. http://dx.doi.org/10.1007/s00540-006-0477-7.
- Sharma AD, Sreeram G, Erb T, Grocott HP, Slaughter TF. Leukocyte-reduced blood transfusions: perioperative indications, adverse effects, and cost analysis. Anesth Analg 2000;90:1315-23. http://dx.doi.org/10.1097/00000539-200006000-00010.
- Llewelyn CA, Hewitt PE, Knight RSG, Amar K, Cousens S, Mackenzie J, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 2004;363:417-21. http://dx.doi.org/10.1016/S0140-6736(04)15486-X.
- Unit Costs of Health and Social Care 2006. Canterbury: PSSRU, University of Kent; n.d.
Appendix 1 Literature search strategies
Clinical effectiveness searches
Randomised controlled trial searches
EMBASE (OvidSP): 1974–30 September 2013
Searched 1 October 2013.
Search strategy
-
Random$.tw. or clinical trial$.mp. or exp health care quality/ (3,200,870)
-
animal/ (1,889,848)
-
animal experiment/ (1,717,916)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (5,819,410)
-
or/2-4 (5,819,410)
-
exp human/ (14,983,864)
-
human experiment/ (316,823)
-
or/6-7 (14,985,305)
-
5 not (5 and 8) (4,638,337)
-
1 not 9 (3,047,951)
-
thromboelastography/ (4910)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw,dv. (5750)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw,dv. (45)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw,dv. (2)
-
TEG.ti,ab,ot,dv. (1769)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw,dv. (993)
-
whole blood h?emosta$ system$.ti,ab,ot,hw,dv. (2)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw,dv. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw,dv. (782)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw,dv. (778)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw,dv. (6)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw,dv. (6)
-
(Sonoclot or sono-clot).ti,ab,ot,hw,dv. (158)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw,dv. (17)
-
or/11-24 (7601)
-
10 and 25 (1163)
Trials filter:
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE (best sens). J Med Libr Assoc 2006;94:41-7.
MEDLINE (OvidSP): 1946–September 2013, week 3
Searched 27 September 2013.
Search strategy
-
randomized controlled trial.pt. or “randomized controlled trials as topic”/ (482,025)
-
controlled clinical trial.pt. (89,224)
-
random$.ti,ot. (111,186)
-
placebo.ab. (155,394)
-
drug therapy.fs. (1,753,686)
-
random$.ab. (658,632)
-
trial.ab. (299,080)
-
groups.ab. (1,263,660)
-
or/1-8 (3,415,580)
-
animals/ not (animals/ and humans/) (3,941,632)
-
9 not 10 (2,911,473)
-
Thrombelastography/ (3421)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw. (4232)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw. (24)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw. (0)
-
TEG.ti,ab,ot. (933)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw. (459)
-
whole blood h?emosta$ system$.ti,ab,ot,hw. (1)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw. (260)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw. (360)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw. (3)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw. (3)
-
(Sonoclot or sono-clot).ti,ab,ot,hw. (108)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw. (12)
-
or/12-25 (5052)
11 and 26 (1051)
Trials filter based on:
- Lefebvre C, Manheimer E, Glanville J, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2011.
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP): up to 26 September 2013 MEDLINE Daily Update (OvidSP): up to 26 September 2013
Searched 27 September 2013.
Search strategy
-
randomized controlled trial.pt. or “randomized controlled trials as topic”/ (864)
-
controlled clinical trial.pt. (38)
-
random$.ti,ot. (10,417)
-
placebo.ab. (6835)
-
drug therapy.fs. (1577)
-
random$.ab. (52,321)
-
trial.ab. (18,616)
-
groups.ab. (94,330)
-
or/1-8 (143,469)
-
animals/ not (animals/ and humans/) (1886)
-
9 not 10 (143,057)
-
Thrombelastography/ (4)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw. (114)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw. (0)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw. (0)
-
TEG.ti,ab,ot. (119)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw. (8)
-
whole blood h?emosta$ system$.ti,ab,ot,hw. (0)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw. (28)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw. (36)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw. (0)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw. (0)
-
(Sonoclot or sono-clot).ti,ab,ot,hw. (5)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw. (1)
-
or/12-25 (211)
-
11 and 26 (53)
Trials filter based on:
- Lefebvre C, Manheimer E, Glanville J, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2011.
Biosis Previews (Web of Knowledge): 1956–26 September 2013
Searched 27 September 2013.
Search strategy
#1 2539 TS= (thrombo-elastogra* or thrombelastogra* or thrombelasto-gra* or thromboelastogra*)
#2 426 TS= (thromb$ NEAR elastogra*)
#3 1 TS= (thromb* NEAR elasto-gra*)
#4 638 TS= (TEG NEAR/10 thromb*)
#5 452 TS= (haemoscope* or hemoscope* or haemonetics or hemonectics)
#6 812 TS= (whole blood hemosta* system*)
#7 191 TS= (whole blood haemosta* system*)
#8 278 TS= (ROTEM* or ROTEG*)
#9 302 TS= (thrombo-elastomet* or thrombelastomet* or thromboelastomet*)
#10 11 TS= (thromb* NEAR/2 elastom*)
#11 0 TS= (thromb* NEAR/2 elasto-m*)
#12 99 TS= (Sonoclot or sono-clot)
#13 17 TS= ((viscoelastic or visco-elastic) NEAR/3 (detection or coagulation) NEAR/3 (system* or process or test or tests or analyz* or analys* or assay* or device* or measurement*))
#14 4142 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
Science Citation Index (SCI) (Web of Science): 1970–26 September 2013; Conference Proceedings Citation Index (CPCI-S) (Web of Science): 1990–26 September 2013
Searched 27 September 2013.
Search strategy
#1 2373 TS= (thrombo-elastogra* or thrombelastogra* or thrombelasto-gra* or thromboelastogra*)
#2 26 TS= (thromb$ NEAR elastogra*)
#3 0 TS= (thromb* NEAR elasto-gra*)
#4 639 TS= (TEG NEAR/10 thromb*)
#5 321 TS= (haemoscope* or hemoscope* or haemonetics or hemonectics)
#6 285 TS= (whole blood hemosta* system*)
#7 91 TS= (whole blood haemosta* system*)
#8 403 TS= (ROTEM* or ROTEG*)
#9 458 TS= (thrombo-elastomet* or thrombelastomet* or thromboelastomet*)
#10 10 TS= (thromb* NEAR/2 elastom*)
#11 0 TS= (thromb* NEAR/2 elasto-m*)
#12 126 TS= (Sonoclot or sono-clot)
#13 29 TS= ((viscoelastic or visco-elastic) NEAR/3 (detection or coagulation) NEAR/3 (system* or process or test or tests or analyz* or analys* or assay* or device* or measurement*))
#14 3407 #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
Cochrane Database of Systematic Reviews (CDSR) (Wiley), Issue 10, October 2013; Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley), Issue 10, October 2013; Database of Abstracts of Reviews of Effects (DARE) (Wiley), Issue 4, October 2013; Health Technology Assessment Database (HTA) (Wiley), Issue 4, October 2013
Searched 5 November 2013.
Search strategy
#1 MeSH descriptor: [Thrombelastography] this term only (151)
#2 (thrombo-elastogra* or thrombelastogra* or thrombelasto-gra* or thromboelastogra*):ti,ab,kw (252)
#3 (thromb* near/2 elastogra*):ti,ab,kw (1)
#4 (thromb* near/2 elasto-gra*):ti,ab,kw (0)
#5 TEG:ti,ab (87)
#6 (haemoscope* or hemoscope* or haemonetics or hemonectics):ti,ab,kw (52)
#7 whole blood h?emosta* system*.ti,ab,kw (0)
#8 whole blood h?emo-sta* system*:ti,ab,kw (0)
#9 (ROTEM* or ROTEG):ti,ab,kw (22)
#10 (thrombo-elastomet* or thrombelastomet* or thromboelastomet*):ti,ab,kw (27)
#11 (thromb* near/2 elastom*):ti,ab,kw (4)
#12 (thromb* near/2 elasto?m*):ti,ab,kw (0)
#13 (Sonoclot or sono-clot):ti,ab,kw (12)
#14 ((viscoelastic or visco-elastic) near/3 (detection or coagulation) near/2 (system* or process or test or tests or analyz* or analys* or assay* or device* or measurement*)):ti,ab,kw (0)
#15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 (326)
-
CDSR search retrieved three references
-
CENTRAL search retrieved 313 references
-
DARE search retrieved three references
-
HTA search retrieved three references.
National Institutes of Health Clinical Trials.gov: up to 27 September 2013 (http://clinicaltrials.gov/ct2/search)
Searched 27 September 2013.
| Search terms | Records |
|---|---|
| Interventions: (thrombo-elasto* OR thrombelasto* OR thromb* elasto* OR thromboelasto* OR TEG OR haemoscope* OR hemoscope* OR haemonetics OR hemonectics OR ROTEM* OR ROTEG OR Sonoclot OR sono-clot) | 46 |
| Interventions: (“whole blood” AND (hemosta* ORhaemosta* OR hemo-sta* OR haemo-sta*) AND system*) | 0 |
| Interventions: ((viscoelastic OR visco-elastic) AND (detection OR coagulation) AND (system* OR process OR test OR tests OR analyz* OR analys* OR assay* OR device* OR measurement*)) | 1 |
| Total | 47 |
metaRegister of Current Controlled Trials (mRCT): up to 27 September 2013 (www.controlled-trials.com/)
Searched 27 September 2013.
| Search terms | Records |
|---|---|
| (thrombo-elasto* OR thrombelasto* OR thromb* elasto* OR thromboelast* OR TEG OR haemoscope* OR hemoscope* OR haemonetics OR hemonectics OR ROTEM* OR ROTEG OR Sonoclot OR sono-clot) | 69 |
| (“whole blood” AND (hemosta* ORhaemosta* OR hemo-sta* OR haemo-sta*) AND system*) | 8 |
| ((viscoelastic OR visco-elastic) AND (detection OR coagulation) AND (system* OR process OR test OR tests OR analyz* OR analys* OR assay* OR device* OR measurement*)) | 3 |
| Total | 80 |
WHO International Clinical Trials Registry Platform (ICTRP): up to 26 September 2013 (www.who.int/ictrp/en/)
Searched 26 September 2113.
| Title | Records |
|---|---|
| (thrombo-elasto* OR thrombelasto* OR thromb* elasto* OR thromboelasto* OR TEG) | 57 |
| (haemoscope* OR hemoscope* OR haemonetics OR hemonectics) | 0 |
| (ROTEM* OR ROTEG OR Sonoclot OR sono-clot) | 31 |
| (“whole blood” AND (hemosta* ORhaemosta* OR hemo-sta* OR haemo-sta*) AND system*) | 67 |
| (viscoelastic AND detection AND system*) | 0 |
| (viscoelastic AND detection AND process) | 0 |
| (viscoelastic AND detection ANDtest) | 0 |
| (viscoelastic AND detection AND tests) | 0 |
| (viscoelastic AND detection AND analyz*) | 0 |
| (viscoelastic AND detection AND analys*) | 0 |
| (viscoelastic AND detection AND assay*) | 0 |
| (viscoelastic AND detection AND device*) | 0 |
| (viscoelastic AND detection AND measurement*) | 0 |
| (visco-elastic AND detection AND system*) | 0 |
| (visco-elastic AND detection AND process) | 0 |
| (visco-elastic AND detection ANDtest) | 0 |
| (visco-elastic AND detection AND tests) | 0 |
| (visco-elastic AND detection AND analyz*) | 0 |
| (visco-elastic AND detection AND analys*) | 0 |
| (visco-elastic AND detection AND assay*) | 0 |
| (visco-elastic AND detection AND device*) | 0 |
| (visco-elastic AND detection AND measurement*) | 0 |
| (viscoelastic AND coagulation AND system*) | 0 |
| (viscoelastic AND coagulation AND process) | 0 |
| (viscoelastic AND coagulation AND test) | 0 |
| (viscoelastic AND coagulation AND tests) | 0 |
| (viscoelastic AND coagulation AND analyz*) | 0 |
| (viscoelastic AND coagulation AND analys*) | 0 |
| (viscoelastic AND coagulation AND assay*) | 0 |
| (viscoelastic AND coagulation AND device*) | 0 |
| (viscoelastic AND coagulation AND measurement*) | 0 |
| (visco-elastic AND coagulation AND system*) | 0 |
| (visco-elastic AND coagulation AND process) | 0 |
| (visco-elastic AND coagulation AND test) | 0 |
| (visco-elastic AND coagulation AND tests) | 0 |
| (visco-elastic AND coagulation AND analyz*) | 0 |
| (visco-elastic AND coagulation AND analys*) | 0 |
| (visco-elastic AND coagulation AND assay*) | 0 |
| (visco-elastic AND coagulation AND device*) | 0 |
| (visco-elastic AND coagulation AND measurement*) | 0 |
| Total | 155 |
Aggressive Research Intelligence Facility (ARIF): 1996–27 September 2013
URL: www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/databases/index.aspx
Searched 27 September 2013.
| Search terms | All indexed fields | All non-indexed text fields | Records |
|---|---|---|---|
| Thromboelastograph* | 0 | 1 | 1 |
| thrombo-elastograph* | 0 | 0 | 0 |
| Thrombelastograph* | 0 | 0 | 0 |
| thrombelasto-graph* | 0 | 0 | 0 |
| Thrombo elastograph* | 0 | 0 | 0 |
| Thromboelasto graph* | 0 | 0 | 0 |
| TEG | 0 | ERROR message | 0 |
| Haemoscope* | 0 | 0 | 0 |
| Hemoscope* | 0 | 0 | 0 |
| Haemonetics | 0 | 0 | 0 |
| Hemonectics | 0 | 0 | 0 |
| ROTEM* | 0 | 0 | 0 |
| ROTEG | 0 | 0/1 → irrelevant (osteoprotegerin) | 0 |
| thrombo-elastomet* | 0 | 0 | 0 |
| Thrombelastomet* | 0 | 0 | 0 |
| Thromboelastomet* | 0 | 0 | 0 |
| Thrombo elastomet* | 0 | 0 | 0 |
| Sonoclot | 0 | 0 | 0 |
| sono-clot | 0 | 0 | 0 |
| viscoelastic | 0 | 1 | 1 |
| visco-elastic | 0 | 0 | 0 |
| Total | 0 | 2/3 | 2 |
National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme: up to 27 September 2013
Searched 27 September 2013.
Browsed with ROTEM terms.
N = 0.
International Prospective Register of Systematic Reviews (PROSPERO): up to 27 September 2013 (www.crd.york.ac.uk/prospero/search.asp)
Searched 27 September 2013.
Search in all fields.
| Search terms | Records |
|---|---|
| thromboelastography | 0 |
| thrombo-elastography | 0 |
| Thrombelastography | 0 |
| thrombelasto-graphy | 0 |
| Thrombo elastography | 0 |
| Thromboelasto graphy | 0 |
| TEG | 2 |
| Haemoscope | 0 |
| hemoscope | 0 |
| Haemonetics | 0 |
| Hemonectics | 0 |
| ROTEM | 1 |
| ROTEG | 0 |
| thrombo-elastometry | 0 |
| Thrombelastometry | 0 |
| Thromboelastometry | 0 |
| Thrombo elastometry | 0 |
| Sonoclot | 1 |
| sono-clot | 1 |
| viscoelastic | 1 |
| visco-elastic | 1 |
| Total | 7 |
| Total after deduplication | 2 |
International Network of Agencies for Health Technology Assessment (INAHTA): up to 27 September 2013 (www.inahta.org/)
Searched 27 September 2013.
| Search term | Results |
|---|---|
| Thromboelastog* | 0 |
| Thrombelastog* | 0 |
| Thrombelastomet* | 0 |
| Thromboelastomet* | 0 |
| Rotem | 0 |
| Roteg* | 0 |
| Sonoclot | 0 |
| Haemoscope* | 0 |
| Hemoscope* | 0 |
| Haemonetics | 0 |
| Hemonetics | 0 |
| viscoelastic | 0 |
| Total | 0 |
Latin American and Caribbean Health Sciences (LILACS): up to 26 September 2013 (http://regional.bvsalud.org/php/index.php?lang=en)
Searched 27 September 2013.
| Terms | Records |
|---|---|
| (thrombelastogra$ or thromboelastogra$ or tromboelastogra$ or thrombo-elastogra$ or trombo-elastogra$ or MH:E01.370.225.625.115.830 or MH:E05.200.625.115.830 or TEG or haemoscop$ or hemoscop$ or haemonetics or hemonetics or Rotem$ or Roteg or Sonoclot or sono-clot or thromboelastomet$ or thrombelastomet$ or thrombo-elastomet$ or tromboelastomet$ or trombo-elastomet$) | 61 |
| ((viscoelastic or visco-elastic) AND (detection OR coagulation) AND (system$ OR process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)) | 0 |
| (“whole blood” AND ((haemosta$ or hemosta$) AND (system$))) | 1 |
| Total | 62 |
Spanish and Portuguese translations of MeSH terms identified using the DeCS (Health Sciences Descriptors) thesaurus: http://decs.bvs.br/I/homepagei.htm
Medion: up to 27 September 2013 (www.mediondatabase.nl/)
Searched 27 September 2013.
Searched in ‘Whole Database’.
| Search term in ‘topics’ | Results |
|---|---|
| Thromboelastograph | 0 |
| Thrombelastograph | 0 |
| Thromboelastography | 0 |
| Thrombelastography | 0 |
| Thrombelastomet* | 0 |
| Thromboelastomet* | 0 |
| Rotem | 0 |
| Roteg* | 0 |
| Sonoclot | 0 |
| Haemoscope* | 0 |
| Hemoscope* | 0 |
| Haemonetics | 0 |
| Hemonetics | 0 |
| viscoelastic | 0 |
| Total | 0 |
Post-partum haemorrhage searches
EMBASE (OvidSP): 1974–30 September 2013
Searched 1 October 2013.
Search strategy
-
animal/ (1,889,848)
-
animal experiment/ (1,717,916)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (5,819,410)
-
or/1-3 (5,819,410)
-
exp human/ (14,983,864)
-
human experiment/ (316,823)
-
or/5-6 (14,985,305)
-
4 not (4 and 7) (4,638,337)
-
thromboelastography/ (4910)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw,dv. (5750)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw,dv. (45)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw,dv. (2)
-
TEG.ti,ab,ot,dv. (1769)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw,dv. (993)
-
whole blood h?emosta$ system$.ti,ab,ot,hw,dv. (2)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw,dv. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw,dv. (782)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw,dv. (778)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw,dv. (6)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw,dv. (6)
-
(Sonoclot or sono-clot).ti,ab,ot,hw,dv. (158)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw,dv. (17)
-
or/9-22 (7601)
-
23 not 8 (6789)
-
exp obstetric haemorrhage/ (9038)
-
exp labor complication/ (131,568)
-
obstetric emergency/ (316)
-
labor stage 3/ (568)
-
exp instrumental delivery/ (64,245)
-
exp childbirth/ (47,045)
-
exp pregnancy disorder/ (421,205)
-
exp pregnancy/ (620,411)
-
exp obstetric procedure/ (335,160)
-
((postpartum or post-partum or “after birth” or afterbirth or “third stage” or “3rd stage” or “final stage” or birth or childbirth or labour or labor or perinatal$ or per-natal$ or Caesar$ or cesar$ or c-section or obstetric$ or placenta$ or parturi$ or puerpal$ or puerper$ or intra-partum$ or intrapartum$ or preeclamp$ or pre-eclamp$ or eclamp$) adj3 (haemorr$ or hemorr$ or bleed$ or blood$)).ti,ab,ot,hw. (21,313)
-
(lochia or cruenta or purulenta or Lochiorrhea$ or ((postpartum or post-partum) adj3 fluxus)).ti,ab,ot. (609)
-
or/25-35 (942,425)
-
24 and 36 (455)
MEDLINE (OvidSP): 1946–September 2013, week 3
Searched 1 October 2013.
Search strategy
-
animals/ not (animals/ and humans/) (3,941,632)
-
Thrombelastography/ (3421)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw. (4232)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw. (24)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw. (0)
-
TEG.ti,ab,ot. (933)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw. (459)
-
whole blood h?emosta$ system$.ti,ab,ot,hw. (1)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw. (260)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw. (360)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw. (3)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw. (3)
-
(Sonoclot or sono-clot).ti,ab,ot,hw. (108)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw. (12)
-
or/2-15 (5052)
-
16 not 1 (4427)
-
exp Labor, Obstetric/ (38,786)
-
exp delivery, Obstetric/ (60,793)
-
exp Obstetric Labor Complications/ (51,037)
-
exp pregnancy/ (714,444)
-
((postpartum or post-partum or “after birth” or afterbirth or “third stage” or “3rd stage” or “final stage” or birth or childbirth or labour or labor or perinatal$ or per-natal$ or Caesar$ or cesar$ or c-section or obstetric$ or placenta$ or parturi$ or puerpal$ or puerper$ or intra-partum$ or intrapartum$ or preeclamp$ or pre-eclamp$ or eclamp$) adj3 (haemorr$ or hemorr$ or bleed$ or blood$)).ti,ab,ot,hw. (13,888)
-
(lochia or cruenta or purulenta or Lochiorrhea$ or ((postpartum or post-partum) adj3 fluxus)).ti,ab,ot. (530)
-
or/18-23 (727,283)
-
17 and 24 (254)
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP): up to 30 September 2013; MEDLINE Daily Update (OvidSP): up to 30 September 2013
Searched 1 October 2013.
Search strategy
-
animals/ not (animals/ and humans/) (2696)
-
Thrombelastography/ (6)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw. (118)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw. (0)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw. (0)
-
TEG.ti,ab,ot. (122)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw. (8)
-
whole blood h?emosta$ system$.ti,ab,ot,hw. (0)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw. (29)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw. (37)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw. (0)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw. (0)
-
(Sonoclot or sono-clot).ti,ab,ot,hw. (5)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw. (1)
-
or/2-15 (215)
-
16 not 1 (214)
-
exp Labor, Obstetric/ (19)
-
exp delivery, Obstetric/ (46)
-
exp Obstetric Labor Complications/ (45)
-
exp pregnancy/ (487)
-
((postpartum or post-partum or “after birth” or afterbirth or “third stage” or “3rd stage” or “final stage” or birth or childbirth or labour or labor or perinatal$ or per-natal$ or Caesar$ or cesar$ or c-section or obstetric$ or placenta$ or parturi$ or puerpal$ or puerper$ or intra-partum$ or intrapartum$ or preeclamp$ or pre-eclamp$ or eclamp$) adj3 (haemorr$ or hemorr$ or bleed$ or blood$)).ti,ab,ot,hw. (743)
-
(lochia or cruenta or purulenta or Lochiorrhea$ or ((postpartum or post-partum) adj3 fluxus)).ti,ab,ot. (15)
-
or/18-23 (1242)
-
17 and 24 (2)
Trauma searches
EMBASE (OvidSP): 1974–30 September 2013
Searched 1 October 2013.
Search strategy
-
animal/ (1,889,848)
-
animal experiment/ (1,717,916)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (5,819,410)
-
or/1-3 (5,819,410)
-
exp human/ (14,983,864)
-
human experiment/ (316,823)
-
or/5-6 (14,985,305)
-
4 not (4 and 7) (4,638,337)
-
thromboelastography/ (4910)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw,dv. (5750)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw,dv. (45)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw,dv. (2)
-
TEG.ti,ab,ot,dv. (1769)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw,dv. (993)
-
whole blood h?emosta$ system$.ti,ab,ot,hw,dv. (2)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw,dv. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw,dv. (782)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw,dv. (778)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw,dv. (6)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw,dv. (6)
-
(Sonoclot or sono-clot).ti,ab,ot,hw,dv. (158)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw,dv. (17)
-
or/9-22 (7601)
-
23 not 8 (6789)
-
exp injury/ (1,492,208)
-
wound/ or bite wound/ or gunshot injury/ or knife cut/ or missile wound/ or stab wound/ (32,224)
-
exp blunt trauma/ (20,182)
-
multiple trauma/ (10,361)
-
exp rupture/ (76,335)
-
exp traumatic shock/ (5045)
-
exp accident/ (143,084)
-
seatbelt/ or traffic safety/ (6329)
-
seatbelt injury/ (446)
-
traffic/ or bicycle/ or exp car driving/ or dangerous goods transport/ or motorized transport/ or patient transport/ or traffic accident/ or traffic noise/ or exp traffic safety/ (91,387)
-
exp motor vehicle/ (27,510)
-
emergency/ (34,524)
-
exp emergency treatment/ (161,412)
-
emergency health service/ (66,643)
-
intensive care/ (83,265)
-
emergency medicine/ (25,513)
-
exp traumatology/ (7325)
-
paramedical personnel/ or paramedical profession/ (13,231)
-
rescue personnel/ (5523)
-
emergency nursing/ (4949)
-
emergency physician/ or emergency/ or emergency ward/ or emergency nurse practitioner/ (88,148)
-
(Trauma$ or accident$ or crash or crashed or crashes or collision$ or collide$ or smash or pile-up).ti,ab,ot. (430,019)
-
((Car$ or motorcar$ or cycle$ or cycling or bicycl$ or bike$ or motorbike$ or motorcycle$ or motor-bike$ or motor-cycle$ or vehic$ or motor$ or traffic or road or pedestrian$ or lorry or lorries or truck or trucks or van or vans or pick-up$) adj8 (injur$ or accident$ or crash$ or collide$ or collision$ or smash$ or bump$ or shunt$ or trauma$ or crush$ or compress$ or impact$)).ti,ab,ot. (167,496)
-
(multiple?trauma$ or poly?trauma$ or multiple?injur$ or complex?injur$).ti,ab,ot,hw. (4371)
-
(wound$ or injur$ or fractur$ or burn or burns or burned or scald$ or stab$ or shot$ or shoot$ or lacerat$ or gunshot$).ti,ab,ot. (1,816,547)
-
(dogbite$ or animalbite$ or bite$ or bitten).ti,ab,ot. (28,226)
-
(splenosis or splenoses).ti,ab,ot. (556)
-
(h?emothorax or h?emo-thorax or pneumothorax or pneumo-thorax).ti,ab,ot,hw. (33,993)
-
(h?emoperiton$ or h?emo-periton$ or free?fluid or intraperiton$ or retroperiton$ or intra-periton$ or retro-periton$).ti,ab,ot,hw. (230,854)
-
((spleen or splenic or liver or hepatic or abdomen or abdominal or stomach or thorax or thoracic or chest or chests) adj5 (trauma$ or injur$ or ruptur$ or bleed$ or crush$ or penetrate$ or perforat$ or blunt or force or compress$ or tear$)).ti,ab,ot,hw. (135,217)
-
mechanical trauma$.ti,ab,ot. (1571)
-
((thermal or blast or crush or avulsion or compress$) adj2 injur$).ti,ab. (11,436)
-
(open fractur$ or compound fractur$).ti,ab,ot,hw. (6437)
-
(ATLS or ALS or BLS or EMST).ti,ab,ot. (56,748)
-
Advanced life support.ti,ab,ot. (1991)
-
basic life support.ab,ti,ot. (1623)
-
((emergency or trauma or critical or casualty) adj3 (care or treat$ or unit or units or department$)).ab,ti,ot. (122,678)
-
(“emergency room” or “emergency rooms” or er or ers or “emergency department” or “emergency departments” or “casualty department” or “casualty departments” or “accident and emergency” or “accidents and emergencies” or “A&E” or “A & E”).ti,ab,ot. (175,800)
-
((trauma adj3 system$) or (life adj3 support$) or (primary adj3 survey$) or (golden adj3 hour) or (first adj3 aid$)).ab,ti,ot. (24,907)
-
(management adj3 trauma).ab,ti,ot. (3484)
-
((prehospital or pre-hospital or preclinical or pre-clinical) adj3 (care or support or treat$)).ab,ti,ot. (5298)
-
(para-medic$ or paramedic$).ab,ti,ot,hw. (20,661)
-
((emergency or critical or trauma or triage or ambulanc$) adj3 (doctor$ or crew$ or staff or team$ or technician$ or worker$ or nurs$ or specialist$)).ab,ti,ot. (17,195)
-
((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra-cran$ or inter-cran$) adj5 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).ab,ti,ot. (141,857)
-
((head or crani$ or cerebr$ or brain$ or intra-cran$ or inter-cran$) adj5 (haematoma$ or hematoma$ or haemorrhag$ or hemorrhag$ or bleed$ or pressure)).ti,ab,ot. (40,456)
-
(“diffuse axonal injury” or “diffuse axonal injuries”).ti,ab,ot. (1109)
-
((brain or cerebral or intracranial or intra-cranial) adj3 (oedema or edema or swell$)).ab,ti,ot. (15,857)
-
((spine$ or spinal) adj3 (fracture$ or injury$ or break$ or broke$)).ti,ab,ot. (36,019)
-
((head or crani$ or cerebr$ or brain$ or skull$) adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab,ot. (129,021)
-
((femur$ or femoral$) adj3 (fracture$ or injur$ or trauma$ or broke$ or break$)).ti,ab,ot. (19,819)
-
((pelvis or pelvic) adj3 (fracture$ or injur$ or trauma$ or broke$ or break$)).ti,ab,ot. (6332)
-
((crush$ or burn$) adj3 (injur$ or trauma$)).ti,ab,ot. (14,324)
-
Advanced trauma life support.ti,ab,ot. (583)
-
((emergency or trauma or critical or casualty) adj3 (center$ or centre$)).ab,ti,ot. (13,571)
-
((unconscious$ or coma$ or concuss$ or “persistent vegetative state”) adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab,ot. (3493)
-
(MVA or MVC or RTA or RTC).ti,ab,ot. (10,618)
-
exp military phenomena/ (58,197)
-
military medicine/ (26,478)
-
soldier/ (21,784)
-
(complex emergenc$ or man-made hazard$ or complex hazard$).ti,ab,ot,hw. (237)
-
(war$ or conflict or violence or fighting or genocid$ or massacre$ or mass killing$).ti,ab,ot,hw. (594563)
-
(Military or battlefield$ or battle-field$ or medevac or med-evac or “medical evacuation” or “medical evacuations” or army or armies).ti,ab,ot. (44,427)
-
or/25-86 (4,167,681)
-
24 and 87 (1620)
MEDLINE (OvidSP): 1946–September 2013, week 3
Searched 1 October 2013.
Search strategy
-
animals/ not (animals/ and humans/) (3,941,632)
-
Thrombelastography/ (3421)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw. (4232)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw. (24)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw. (0)
-
TEG.ti,ab,ot. (933)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw. (459)
-
whole blood h?emosta$ system$.ti,ab,ot,hw. (1)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw. (260)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw. (360)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw. (3)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw. (3)
-
(Sonoclot or sono-clot).ti,ab,ot,hw. (108)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw. (12)
-
or/2-15 (5052)
-
16 not 1 (4427)
-
exp “Wounds and Injuries”/ (689,154)
-
exp Accidents/ (138,755)
-
Seat Belts/ (3324)
-
exp Motor Vehicles/ (14,903)
-
Emergencies/ (33,912)
-
exp Emergency Treatment/ (93,377)
-
exp Emergency Medical Services/ (94,125)
-
Intensive Care/ (15,220)
-
Traumatology/ (2101)
-
emergency medical technicians/ (4761)
-
Emergency Nursing/ (5484)
-
exp Emergency Service, Hospital/ (48,577)
-
(Trauma$ or accident$ or crash or crashed or crashes or collision$ or collide$ or smash or pile-up).ti,ab,ot. (319,055)
-
((Car$ or motorcar$ or cycle$ or cycling or bicycl$ or bike$ or motorbike$ or motorcycle$ or motor-bike$ or motor-cycle$ or vehic$ or motor$ or traffic or road or pedestrian$ or lorry or lorries or truck or trucks or van or vans or pick-up$) adj8 (injur$ or accident$ or crash$ or collide$ or collision$ or smash$ or bump$ or shunt$ or trauma$ or crush$ or compress$ or impact$)).ti,ab,ot. (124,403)
-
(multiple?trauma$ or poly?trauma$ or multiple?injur$ or complex?injur$).ti,ab,ot,hw. (2912)
-
(wound$ or injur$ or fractur$ or burn or burns or burned or scald$ or stab$ or shot$ or shoot$ or lacerat$ or gunshot$).ti,ab,ot. (1,430,203)
-
(dogbite$ or animalbite$ or bite$ or bitten).ti,ab,ot. (23,110)
-
(splenosis or splenoses).ti,ab,ot. (457)
-
(h?emothorax or h?emo-thorax or pneumothorax or pneumo-thorax).ti,ab,ot,hw. (22,295)
-
(h?emoperiton$ or h?emo-periton$ or free?fluid or intraperiton$ or retroperiton$ or intra-periton$ or retro-periton$).ti,ab,ot,hw. (129,878)
-
((spleen or splenic or liver or hepatic or abdomen or abdominal or stomach or thorax or thoracic or chest or chests) adj5 (trauma$ or injur$ or ruptur$ or bleed$ or crush$ or penetrate$ or perforat$ or blunt or force or compress$ or tear$)).ti,ab,ot,hw. (99,682)
-
mechanical trauma$.ti,ab,ot. (1206)
-
((thermal or blast or crush or avulsion or compress$) adj2 injur$).ti,ab. (9177)
-
(open fractur$ or compound fractur$).ti,ab,ot,hw. (3113)
-
(ATLS or ALS or BLS or EMST).ti,ab,ot. (38,646)
-
((emergency or trauma or critical or casualty) adj3 (care or treat$ or unit or units or department$)).ab,ti,ot. (86,241)
-
(“emergency room” or “emergency rooms” or er or ers or “emergency department” or “emergency departments” or “casualty department” or “casualty departments” or “accident and emergency” or “accidents and emergencies” or “A&E” or “A & E”).ti,ab,ot. (127,879)
-
((trauma adj3 system$) or (life adj3 support$) or (primary adj3 survey$) or (golden adj3 hour) or (first adj3 aid$)).ab,ti,ot. (19,387)
-
(management adj3 trauma).ab,ti,ot. (2663)
-
((prehospital or pre-hospital or preclinical or pre-clinical) adj3 (care or support or treat$)).ab,ti,ot. (3931)
-
(para-medic$ or paramedic$).ab,ti,ot,hw. (5379)
-
((emergency or critical or trauma or triage or ambulanc$) adj3 (doctor$ or crew$ or staff or team$ or technician$ or worker$ or nurs$ or specialist$)).ab,ti,ot. (13,364)
-
((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra-cran$ or inter-cran$) adj5 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).ab,ti,ot. (105,502)
-
((head or crani$ or cerebr$ or brain$ or intra-cran$ or inter-cran$) adj5 (haematoma$ or hematoma$ or haemorrhag$ or hemorrhag$ or bleed$ or pressure)).ti,ab,ot. (29,545)
-
(“diffuse axonal injury” or “diffuse axonal injuries”).ti,ab,ot. (792)
-
((brain or cerebral or intracranial or intra-cranial) adj3 (oedema or edema or swell$)).ab,ti,ot. (11,773)
-
((spine$ or spinal) adj3 (fracture$ or injury$ or break$ or broke$)).ti,ab,ot. (27,922)
-
((head or crani$ or cerebr$ or brain$ or skull$) adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab,ot. (95,593)
-
((femur$ or femoral$) adj3 (fracture$ or injur$ or trauma$ or broke$ or break$)).ti,ab,ot. (15,098)
-
((pelvis or pelvic) adj3 (fracture$ or injur$ or trauma$ or broke$ or break$)).ti,ab,ot. (4724)
-
((crush$ or burn$) adj3 (injur$ or trauma$)).ti,ab,ot. (11,152)
-
Advanced trauma life support.ti,ab,ot. (458)
-
((emergency or trauma or critical or casualty) adj3 (center$ or centre$)).ab,ti,ot. (10,437)
-
((unconscious$ or coma$ or concuss$ or “persistent vegetative state”) adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab,ot. (2590)
-
(MVA or MVC or RTA or RTC).ti,ab,ot. (8602)
-
exp Military Personnel/ (23,530)
-
War/ (18,376)
-
Military Medicine/ (25,794)
-
(complex emergenc$ or man-made hazard$ or complex hazard$).ti,ab,ot,hw. (203)
-
(war$ or conflict or violence or fighting or genocid$ or massacre$ or mass killing$).ti,ab,ot,hw. (364,591)
-
(Military or battlefield$ or battle-field$ or medevac or med-evac or “medical evacuation” or “medical evacuations” or army or armies).ti,ab,ot. (36,008)
-
or/18-68 (2,862,142)
-
17 and 69 (699)
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP): up to 30 September 2013; MEDLINE Daily Update (OvidSP): up to 30 September 2013
Searched 1 October 2013.
Search strategy
-
animals/ not (animals/ and humans/) (2696)
-
Thrombelastography/ (6)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw. (118)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw. (0)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw. (0)
-
TEG.ti,ab,ot. (122)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw. (8)
-
whole blood h?emosta$ system$.ti,ab,ot,hw. (0)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw. (29)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw. (37)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw. (0)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw. (0)
-
(Sonoclot or sono-clot).ti,ab,ot,hw. (5)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw. (1)
-
or/2-15 (215)
-
16 not 1 (214)
-
exp “Wounds and Injuries”/ (606)
-
exp Accidents/ (142)
-
Seat Belts/ (1)
-
exp Motor Vehicles/ (13)
-
Emergencies/ (21)
-
exp Emergency Treatment/ (128)
-
exp Emergency Medical Services/ (113)
-
Intensive Care/ (31)
-
Traumatology/ (2)
-
emergency medical technicians/ (2)
-
Emergency Nursing/ (2)
-
exp Emergency Service, Hospital/ (62)
-
(Trauma$ or accident$ or crash or crashed or crashes or collision$ or collide$ or smash or pile-up).ti,ab,ot. (26,819)
-
((Car$ or motorcar$ or cycle$ or cycling or bicycl$ or bike$ or motorbike$ or motorcycle$ or motor-bike$ or motor-cycle$ or vehic$ or motor$ or traffic or road or pedestrian$ or lorry or lorries or truck or trucks or van or vans or pick-up$) adj8 (injur$ or accident$ or crash$ or collide$ or collision$ or smash$ or bump$ or shunt$ or trauma$ or crush$ or compress$ or impact$)).ti,ab,ot. (9256)
-
(multiple?trauma$ or poly?trauma$ or multiple?injur$ or complex?injur$).ti,ab,ot,hw. (167)
-
(wound$ or injur$ or fractur$ or burn or burns or burned or scald$ or stab$ or shot$ or shoot$ or lacerat$ or gunshot$).ti,ab,ot. (134,477)
-
(dogbite$ or animalbite$ or bite$ or bitten).ti,ab,ot. (1574)
-
(splenosis or splenoses).ti,ab,ot. (19)
-
(h?emothorax or h?emo-thorax or pneumothorax or pneumo-thorax).ti,ab,ot,hw. (1069)
-
(h?emoperiton$ or h?emo-periton$ or free?fluid or intraperiton$ or retroperiton$ or intra-periton$ or retro-periton$).ti,ab,ot,hw. (5116)
-
((spleen or splenic or liver or hepatic or abdomen or abdominal or stomach or thorax or thoracic or chest or chests) adj5 (trauma$ or injur$ or ruptur$ or bleed$ or crush$ or penetrate$ or perforat$ or blunt or force or compress$ or tear$)).ti,ab,ot,hw. (4052)
-
mechanical trauma$.ti,ab,ot. (57)
-
((thermal or blast or crush or avulsion or compress$) adj2 injur$).ti,ab. (504)
-
(open fractur$ or compound fractur$).ti,ab,ot,hw. (248)
-
(ATLS or ALS or BLS or EMST).ti,ab,ot. (1434)
-
((emergency or trauma or critical or casualty) adj3 (care or treat$ or unit or units or department$)).ab,ti,ot. (6953)
-
(“emergency room” or “emergency rooms” or er or ers or “emergency department” or “emergency departments” or “casualty department” or “casualty departments” or “accident and emergency” or “accidents and emergencies” or “A&E” or “A & E”).ti,ab,ot. (10,753)
-
((trauma adj3 system$) or (life adj3 support$) or (primary adj3 survey$) or (golden adj3 hour) or (first adj3 aid$)).ab,ti,ot. (1077)
-
(management adj3 trauma).ab,ti,ot. (204)
-
((prehospital or pre-hospital or preclinical or pre-clinical) adj3 (care or support or treat$)).ab,ti,ot. (282)
-
(para-medic$ or paramedic$).ab,ti,ot,hw. (261)
-
((emergency or critical or trauma or triage or ambulanc$) adj3 (doctor$ or crew$ or staff or team$ or technician$ or worker$ or nurs$ or specialist$)).ab,ti,ot. (830)
-
((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra-cran$ or inter-cran$) adj5 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).ab,ti,ot. (6315)
-
((head or crani$ or cerebr$ or brain$ or intra-cran$ or inter-cran$) adj5 (haematoma$ or hematoma$ or haemorrhag$ or hemorrhag$ or bleed$ or pressure)).ti,ab,ot. (1534)
-
(“diffuse axonal injury” or “diffuse axonal injuries”).ti,ab,ot. (39)
-
((brain or cerebral or intracranial or intra-cranial) adj3 (oedema or edema or swell$)).ab,ti,ot. (551)
-
((spine$ or spinal) adj3 (fracture$ or injury$ or break$ or broke$)).ti,ab,ot. (1784)
-
((head or crani$ or cerebr$ or brain$ or skull$) adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab,ot. (5788)
-
((femur$ or femoral$) adj3 (fracture$ or injur$ or trauma$ or broke$ or break$)).ti,ab,ot. (970)
-
((pelvis or pelvic) adj3 (fracture$ or injur$ or trauma$ or broke$ or break$)).ti,ab,ot. (310)
-
((crush$ or burn$) adj3 (injur$ or trauma$)).ti,ab,ot. (712)
-
Advanced trauma life support.ti,ab,ot. (31)
-
((emergency or trauma or critical or casualty) adj3 (center$ or centre$)).ab,ti,ot. (704)
-
((unconscious$ or coma$ or concuss$ or “persistent vegetative state”) adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab,ot. (168)
-
(MVA or MVC or RTA or RTC).ti,ab,ot. (495)
-
exp Military Personnel/ (20)
-
War/ (11)
-
Military Medicine/ (7)
-
(complex emergenc$ or man-made hazard$ or complex hazard$).ti,ab,ot,hw. (12)
-
(war$ or conflict or violence or fighting or genocid$ or massacre$ or mass killing$).ti,ab,ot,hw. (24,821)
-
(Military or battlefield$ or battle-field$ or medevac or med-evac or “medical evacuation” or “medical evacuations” or army or armies).ti,ab,ot. (2894)
-
or/18-68 (200,579)
-
17 and 69 (66)
Conference proceeding searches
International Society on Thrombosis and Haemostasis (ISTH): 2009, 2011 (www.isth.org/?PastMeetings)
Searched 28 November 2013.
Searched Annual meetings abstract books for:
-
2009 – http://onlinelibrary.wiley.com/doi/10.1111/jth.2009.7.issue-s2/issuetoc
-
2010 – not available online
-
2011 – http://onlinelibrary.wiley.com/doi/10.1111/jth.2011.9.issue-s2/issuetoc
-
2012 – not available online
-
2013 – not available online.
| Year | Abstracts |
|---|---|
| 2009 | 39 |
| 2010 | n/a |
| 2011 | 49 |
| 2012 | n/a |
| 2013 | n/a |
| Total | 88 |
Terms browsed included:
-
ROTEM
-
ROTEG
-
Sonoclot
-
TEG
-
Viscoelastic
-
Visco-elastic.
American Society of Anesthesiologists (ASA): 2009–13 (www.asaabstracts.com/strands/asaabstracts/search.htm;jsessionid=FF1E2F6EA4FF34468F5594FA255F3423)
Searched 28 November 2013.
| Term | Title | Abstract |
|---|---|---|
| ROTEM | 8 | 28 |
| ROTEG | 0 | 0 |
| TEG | 8 | 43 |
| SONOCLOT | 0 | 0 |
| Viscoelastic | 1 | 7 |
| Visco-elastic | 0 | 1 |
| Subtotal | 17 | 79 |
| Total | 96 | |
European Association of Cardiothoracic Anaesthesiologists (EACTA): 2009–13
Searched 28 November 2013.
-
2013 – www.applied-cardiopulmonary-pathophysiology.com/acp-2-2013.html
-
2012 – www.applied-cardiopulmonary-pathophysiology.com/acp-supp1-2012.html
-
2011 – Searched via publisher’s website
-
2010 – www.applied-cardiopulmonary-pathophysiology.com/fileadmin/downloads/acp-2010-1/10_abstracts.pdf
| Term | 2009 | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|
| ROTEM | 0 | 0 | 0 | 1 | 0 |
| ROTEG | 0 | 0 | 0 | 0 | 0 |
| TEG | 0 | 0 | 0 | 0 | 0 |
| SONOCLOT | 0 | 0 | 0 | 0 | 0 |
| Viscoelastic | 0 | 0 | 0 | 0 | 0 |
| Visco-elastic | 0 | 0 | 0 | 0 | 0 |
| Thrombo | 0 | 0 | 1 | 1 | 0 |
| Subtotal | 0 | 0 | 1 | 2 | 0 |
| Total | 3 | ||||
Additional searches
PubMed Related Citations search undertaken for included studies
Results sorted by Link Ranking.
URL: www.ncbi.nlm.nih.gov/pubmed/
Searched 28 November 2013.
Of 42 included studies, only 21 references were indexed on PubMed.
For each reference, the first 20 references were retrieved by carrying out a Related Citations search using PubMed’s similarity matching algorithm. These records were downloaded for screening. All related citations were checked against the EndNote Library to remove duplicates, and only new unique references were imported and screened.
| Reference | PMID | Result retrieved |
|---|---|---|
| #28. Ak46 | 19583608 | 20/45 |
| #30. Avidan48 | 14722166 | 20/519 |
| #8034. Cotton73 | 21825945 | 20/249 |
| #5582. Davenport70 | 21765358 | 20/149 |
| #1107. Girdauskas54 | 20951260 | 20/221 |
| #5470. Holcomb74 | 22868371 | 20/299 |
| #5464. Ives72 | 22766227 | 20/121 |
| #7985. Jeger77 | 22547997 | 20/93 |
| #3851. Kaufmann64 | 9137263 | 20/354 |
| #8574. Kunio67 | 22425448 | 20/94 |
| #5727. Leemann65 | 21150521 | 20/125 |
| #48. Nuttall59 | 9412876 | 20/233 |
| #32. Nuttall48 | 11388527 | 20/350 |
| #498. Pezold80 | 21899867 | 20/184 |
| #31. Royston49 | 11573637 | 20/120 |
| #5707. Schöchl76 | 22078266 | 20/153 |
| #33. Shore 51 | 9972747 | 20/598 |
| #78. Tauber66 | 21705350 | 20/237 |
| #4261. Tuman60 | 2742171 | 20/195 |
| #35. Weber35 | 22914710 | 20/108 |
| #29. Westbrook47 | 19117801 | 20/202 |
| Total | 440 | |
| Following duplicate removal, no. of records screened | 101 | |
Health economics searches
EMBASE (OvidSP): 1974–5 November 2013
Searched 6 November 2013.
Search strategy
-
health-economics/ (33,331)
-
exp economic-evaluation/ (206,551)
-
exp health-care-cost/ (198,226)
-
exp pharmacoeconomics/ (170,054)
-
or/1-4 (473,269)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (592,569)
-
(expenditure$ not energy).ti,ab. (23,436)
-
(value adj2 money).ti,ab. (1327)
-
budget$.ti,ab. (23,658)
-
or/6-9 (616,419)
-
5 or 10 (889,041)
-
letter.pt. (846,057)
-
editorial.pt. (450,524)
-
note.pt. (589,815)
-
or/12-14 (1,886,396)
-
11 not 15 (802,081)
-
(metabolic adj cost).ti,ab. (878)
-
((energy or oxygen) adj cost).ti,ab. (3167)
-
((energy or oxygen) adj expenditure).ti,ab. (20,058)
-
or/17-19 (23,290)
-
16 not 20 (796,999)
-
exp animal/ (19,435,707)
-
exp animal-experiment/ (1,729,328)
-
nonhuman/ (4,161,134)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (5,024,049)
-
or/22-25 (20,785,461)
-
exp human/ (15,078,566)
-
exp human-experiment/ (317,907)
-
27 or 28 (15,080,007)
-
26 not (26 and 29) (5,706,423)
-
21 not 30 (737,003)
-
thromboelastography/ (4953)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw,dv. (5797)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw,dv. (46)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw,dv. (2)
-
TEG.ti,ab,ot,dv. (1801)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw,dv. (1003)
-
whole blood h?emosta$ system$.ti,ab,ot,hw,dv. (2)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw,dv. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw,dv. (793)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw,dv. (790)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw,dv. (7)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw,dv. (7)
-
(Sonoclot or sono-clot).ti,ab,ot,hw,dv. (159)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw,dv. (17)
-
or/32-45 (7669)
-
31 and 46 (238)
Costs filter:
- NHS EED Economics Filter: EMBASE (Ovid) Weekly Search. York: CRD; 2010.
MEDLINE (OvidSP): 1946-2013/10/wk 4
Searched 6 November 2013.
Search strategy
-
economics/ (27,117)
-
exp “costs and cost analysis”/ (182,817)
-
economics, dental/ (1866)
-
exp “economics, hospital”/ (19,436)
-
economics, medical/ (8580)
-
economics, nursing/ (3880)
-
economics, pharmaceutical/ (2607)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (428,332)
-
(expenditure$ not energy).ti,ab. (17,575)
-
(value adj1 money).ti,ab. (22)
-
budget$.ti,ab. (17,221)
-
or/1-11 (552,792)
-
((energy or oxygen) adj cost).ti,ab. (2756)
-
(metabolic adj cost).ti,ab. (800)
-
((energy or oxygen) adj expenditure).ti,ab. (16,687)
-
or/13-15 (19,533)
-
12 not 16 (548,438)
-
letter.pt. (804,607)
-
editorial.pt. (335,541)
-
historical article.pt. (299,905)
-
or/18-20 (1,425,550)
-
17 not 21 (520,378)
-
animals/ not (animals/ and humans/) (3,962,474)
-
22 not 23 (486,879)
-
Thrombelastography/ (3453)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw. (4267)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw. (24)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw. (0)
-
TEG.ti,ab,ot. (951)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw. (459)
-
whole blood h?emosta$ system$.ti,ab,ot,hw. (1)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw. (269)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw. (370)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw. (3)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw. (3)
-
(Sonoclot or sono-clot).ti,ab,ot,hw. (109)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw. (12)
-
or/25-38 (5091)
-
24 and 39 (90)
Costs filter:
- NHS EED Economics Filter: EMBASE (Ovid) Weekly Search. York: CRD; 2010.
MEDLINE In-Process and Other Non-Indexed Citations (OvidSP): up to 5 November 2013
MEDLINE Daily Update (OvidSP): up to 5 November 2013
Searched 6 November 2013.
Search strategy
-
economics/ (4)
-
exp “costs and cost analysis”/ (260)
-
economics, dental/ (0)
-
exp “economics, hospital”/ (28)
-
economics, medical/ (2)
-
economics, nursing/ (2)
-
economics, pharmaceutical/ (5)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (41,521)
-
(expenditure$ not energy).ti,ab. (1256)
-
(value adj1 money).ti,ab. (5)
-
budget$.ti,ab. (1905)
-
or/1-11 (43,580)
-
((energy or oxygen) adj cost).ti,ab. (235)
-
(metabolic adj cost).ti,ab. (70)
-
((energy or oxygen) adj expenditure).ti,ab. (974)
-
or/13-15 (1238)
-
12 not 16 (43,222)
-
letter.pt. (26,653)
-
editorial.pt. (15,882)
-
historical article.pt. (186)
-
or/18-20 (42,699)
-
17 not 21 (42,728)
-
animals/ not (animals/ and humans/) (3186)
-
22 not 23 (42,660)
-
Thrombelastography/ (6)
-
(thrombo-elastogra$ or thrombelastogra$ or thrombelasto-gra$ or thromboelastogra$).ti,ab,ot,hw. (129)
-
(thromb$ adj2 elastogra$).ti,ab,ot,hw. (0)
-
(thromb$ adj2 elasto-gra$).ti,ab,ot,hw. (0)
-
TEG.ti,ab,ot. (123)
-
(haemoscope$ or hemoscope$ or haemonetics or hemonectics).ti,ab,ot,hw. (9)
-
whole blood h?emosta$ system$.ti,ab,ot,hw. (0)
-
whole blood h?emo-sta$ system$.ti,ab,ot,hw. (0)
-
(ROTEM$ or ROTEG).ti,ab,ot,hw. (30)
-
(thrombo-elastomet$ or thrombelastomet$ or thromboelastomet$).ti,ab,ot,hw. (39)
-
(thromb$ adj2 elastom$).ti,ab,ot,hw. (0)
-
(thromb$ adj2 elasto?m$).ti,ab,ot,hw. (0)
-
(Sonoclot or sono-clot).ti,ab,ot,hw. (6)
-
((viscoelastic or visco-elastic) adj3 (detection or coagulation) adj2 (system$ or process or test or tests or analyz$ or analys$ or assay$ or device$ or measurement$)).ti,ab,ot,hw. (1)
-
or/25-38 (232)
-
24 and 39 (3)
Costs filter:
- NHS EED Economics Filter: EMBASE (Ovid) Weekly Search. York: CRD; 2010.
NHS Economics Evaluation Database (NHS EED) (Wiley): Issue 4, October 2013
Searched 5 November 2013.
Search strategy
#1 MeSH descriptor: [Thrombelastography] this term only (151)
#2 (thrombo-elastogra* or thrombelastogra* or thrombelasto-gra* or thromboelastogra*):ti,ab,kw (252)
#3 (thromb* near/2 elastogra*):ti,ab,kw (1)
#4 (thromb* near/2 elasto-gra*):ti,ab,kw (0)
#5 TEG:ti,ab (7)
#6 (haemoscope* or hemoscope* or haemonetics or hemonectics):ti,ab,kw (52)
#7 whole blood h?emosta* system*.ti,ab,kw (0)
#8 whole blood h?emo-sta* system*:ti,ab,kw (0)
#9 (ROTEM* or ROTEG):ti,ab,kw (22)
#10 (thrombo-elastomet* or thrombelastomet* or thromboelastomet*):ti,ab,kw (27)
#11 (thromb* near/2 elastom*):ti,ab,kw (4)
#12 (thromb* near/2 elasto?m*):ti,ab,kw (0)
#13 (Sonoclot or sono-clot):ti,ab,kw (12)
#14 ((viscoelastic or visco-elastic) near/3 (detection or coagulation) near/2 (system* or process or test or tests or analyz* or analys* or assay* or device* or measurement*)):ti,ab,kw (0)
#15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 (326)
NHS EED search retrieved three references.
EconLit (EBSCOhost): 1990–1 September 2013
Searched 7 November 2013.
Search strategy
S1 TX ((thrombo-elastogra* or thrombelastogra* or thrombelasto-gra* or thromboelastogra*)) OR TX (thromb* N2 elastogra*) OR TX (thromb* N2 elasto-gra*) (0)
S2 TX ((haemoscope* or hemoscope* or haemonetics or hemonectics)) OR TX whole blood h#emosta* system* OR TX whole blood h#emo-sta* system* (0)
S3 TX ((TEG or ROTEM* or ROTEG)) OR TX ((thrombo-elastomet* or thrombelastomet* or thromboelastomet* ) OR TX (thromb* N2 elastom*) (0)
S4 TX (thromb* N2 elasto#m*) OR TX ((Sonoclot or sono-clot)) OR TX (( (viscoelastic or visco-elastic) N3 (detection or coagulation) N2 (system* or process or test or tests or analyz* or analys* or assay* or device* or measurement*))) (0)
S5 s1 or s2 or s3 or s4 (0)
IDEAS via Research Papers in Economics (REPEC): up to 7 November 2013 (http://repec.org/)
Searched 7 November 2013.
| Search terms | Results |
|---|---|
| (thrombo-elastogram | thrombo-elastograph | thrombelastogram | thrombelastograph) | 0 |
| (thrombelasto-graph | thromboelastogram | thrombelasto-gram | thromboelastograph) | 0 |
| (haemoscope | hemoscope | haemonetics | hemonectics | haemoscopes | hemoscopes) | 0 |
| “whole blood haemostasis system” | “whole blood haemostatic system” | 0 |
| “whole blood hemostasis system” | “whole blood hemostatic system” | 0 |
| (TEG | ROTEM | ROTEG) | 26 |
| (thrombo-elastometry | thrombelastometry | thromboelastometry) | 0 |
| (Sonoclot | sono-clot) | 0 |
| (viscoelastic | visco-elastic) + (detection | coagulation) + (system | process | test | tests | analyz | analysis | assay | device | measurement) | 58 |
| Total | 84 |
Health Economic Evaluation Database (HEED) (Wiley): up to 7 November 2013 (http://onlinelibrary.wiley.com/book/10.1002/9780470510933)
Searched 7 November 2013.
Compound search, (all data).
| All data | All data | Results |
|---|---|---|
| thrombo-elastogra* or thrombelastogra* or thrombelasto-gra* or thromboelastogra* | – | 5 |
| thromb* | AND elastogra* OR elasto-gra* OR elastom* | 0 |
| haemoscope* or hemoscope* or haemonetics or hemonectics OR Sonoclot or sono-clot | – | 7 |
| whole blood haemosta* system* | OR whole blood hemo-sta* system* | 0 |
| TEG OR ROTEM* or ROTEG | – | 2 |
| thrombo-elastomet* or thrombelastomet* or thromboelastomet* | – | 0 |
| (viscoelastic or visco-elastic) AND (detection or coagulation) | AND (system* or process or test or tests or analyz* or analys* or assay* or device* or measurement*) | 0 |
| Total | 14 |
Appendix 2 Data extraction tables
Baseline details: cardiac surgery studies
| Study details | Selection criteria | Participant details | Intervention | Control | |
|---|---|---|---|---|---|
| Ak (2009)46 Country: Turkey Funding: NR Study design: RCT Recruitment: NR No. of participants: 228 |
Inclusion criteria: Consecutive patients undergoing elective first-time CABG Exclusion criteria: Pre-operative haemodynamic instability, malignancies, history of bleeding diathesis, use of low-molecular-weight heparin molecules until the day of operation, recent treatment (< 5 days) with a glycoprotein IIb/IIIa antagonist or clopidogrel, impaired renal function (creatinine > 2 mg/dl) and any liver disease resulting in elevated liver function tests |
Patient category: CABG Previous anticoagulation therapy: 65% of standard group and 59% of TEG group received aspirin until the day before the operation Mean age (SD) 64 (20) % male: 76 |
Test: TEG (kaolin and heparinase) Parameters: r, MA, LY30 r ≥ 14 mm then FFP (1 unit);a r ≥ 21 mm then FFP (2 units);a r ≥ 28 mm then FFP (4 units);a MA < 48 mm then platelets (1 unit); MA < 40 mm then platelets (2 units); LY30 > 7.5% then tranexamic acid Test timing t1, before induction of general anaesthesia; t2, after institution of CPB; t3, 15 minutes after administration of protamine sulphate; t4, on admission to the ICU; t5, 6 hours after CPB; and, last, t6, 24 hours after CPB |
Clinician-directed transfusion. Decision for blood component (platelet suspension and/or FFP) was determined by using the criteria obtained from abnormal conventional laboratory tests, absence of visible clots, and presence of generalised oozing-type bleeding in the surgical field. Platelet suspension was ordered if the PLT was < 100,000/µl. FFP was given if the PT was > 14 seconds or aPTT was > 1.5× normal. After complete neutralisation of systemic heparin, an additional dose of protamine sulphate was given according to the control ACT values (25 mg if the ACT was between 120 and 150 seconds or 50 mg if it was > 150 seconds). In this group, the appropriate amount of blood components was judged according to the clinical discretion of the anaesthesiologist responsible for the post-operative care of the patient. TA requirement was determined by absence of visible clots and presence of generalised oozing-type bleeding in the surgical field Test timing Same as group 1 |
|
| Study details | Selection criteria | Participant details | Intervention | Control | Comments |
| Avidan (2004)48 Country: UK Funding: Medicell UK provided TEG consumables at discounted prices, Medtronic provided consumables for the Hepcon machine, other funding was provided by the Royal College of Anaesthetists UK, and National Blood Services UK Study design: RCT Recruitment: NR No. of participants: 102 |
Inclusion criteria: Patients having elective, first-time CABG with CPB, who were treated by the same surgeon and anaesthetic team Exclusion criteria: Pre-operative abnormal clotting tests (INR > 1.5, aPTT ratio > 1.5, or PLT < 150 × 109/l) Medication affecting coagulation within 72 hours of surgery (warfarin, heparin, low-molecular-weight heparin, aspirin and clopidogrel) |
Patient category: CABG Previous anticoagulation therapy: None within 72 hours before surgery Mean age (range), years 64 (57–71) % male: 78 |
Test: TEG (heparinase) Parameters: R, α-angle, MA and LY30 The intervention (POC) protocol also included Hepcon (heparin dose response and heparin–protamine titration tests), platelet function testing [PFA-100 analyser (Dade Behring, Deerfield, IL, USA)] and ACT (Hemochron; ITC, Edison, NJ, USA). Bleeding was managed and transfusion triggers were set based on POC alone; algorithm reported as a flow chart in the paper. TEG: LY30 (> 7.5%) plus bleeding > 100 ml/hour, response 2 mU aprotinin i.v.; R (> 10 minutes) plus excessive bleeding, response 4 units FFP. RBC transfusion was triggered by haemoglobin concentration < 8 g/dl Test timing TEG after 5 minutes and after 1 hour on CPB and 20 minutes post-protamine administration and 2 hours post surgery if excessive bleeding continued |
Laboratory tests included ACT, INR, aPTT ratio and FBC. Laboratory tests were requested only for patients with increased bleeding, and investigators were blinded to POC test results. The full management algorithm was reported as a flow chart in the paper. Bleeding > 100 ml/hour in the first 24 hours after surgery, response 2 mU aprotinin and 0.4 µg/kg body weight desmopressin; still bleeding > 100 ml/hour and INR or aPTT > 1.5 × control value, response 4 units FFP; excessive bleeding persists or PLT < 50 × 109/l, response platelet transfusion. RBC transfusion was triggered by haemoglobin concentration < 8 g/dl Test timing ACT at baseline, every 30 minutes on CPB and post protamine; INR, aPTT and FBC 2 hours post surgery if excessive bleeding continued |
Data were also reported for an additional, retrospective, matched control group, which comprised patients undergoing CABG who received blood components at the individual clinician’s discretion |
| Study details | Selection criteria | Participant details | VE test | Conventional tests |
|---|---|---|---|---|
| Bischof (2009)58 Country: Switzerland Funding: NR Study design: Prediction study Recruitment: July 2007 to December 2008 No. of participants: 300 |
Inclusion criteria: Patients undergoing cardiac surgery Exclusion criteria: Known coagulopathy or anticoagulant medication |
Patient category: Any cardiac surgery Details: CPB 61%, off pump 39% Previous anticoagulation therapy: Mean age (SD, range), years: 65 (11, 27–87) % male: 69 % white: NR |
Sonoclot (GbACT): CR, PF, ACT | None |
| Study details | Selection criteria | Participant details | Intervention | Control |
|---|---|---|---|---|
| Girdauskas (2010)54 Country: Germany Funding: Not stated Study design: RCT Recruitment: July 2007 to January 2008 No. of participants: 56 |
Inclusion criteria: > 18 years undergoing aortic surgery requiring HCA, including urgent and emergency surgery Exclusion criteria: Pregnant, known (inherited) coagulation disorders (haemophilia A or B, activated protein C resistance, etc.) or were unable to give informed consent |
Patient category: Aortic surgery Previous anticoagulation therapy: Pre-operative aspirin 33% in ROTEM and 28% in control; pre-operative warfarin – one patient (4%) in each group. No patients were receiving clopidogrel or heparin Mean age (SD) 62 (16) % male: 57 |
Test: ROTEM (INTEM, HEPTM, FIBTEM and APTEM run simultaneously on four ROTEM channels) Parameters: CT or MTF CT by HEPTEM (> 260 seconds), response FFP (15 ml/kg body mass); CT by APTEM (> 120 seconds), response 3000 IU PPSB; MCF by HEPTEM ≤ 45 mm) or FIBTEM (> 8 mm), response 1 platelet concentrate; MCF by FIBTEM (< 8 mm), response 2 g FIB; MCF by APTEM or HEPTEM (> 1.5), response 3 g tranexamic acid; CT by INTEM or HEPTEM (> 1.5), response 5000 IU protamine. Average 3.6 tests per patient performed Test timing During rewarming phase of CPB, second ROTEM carried out for documentation of transfusion effect irrespective of bleeding. If no microvascular bleeding was determined, the chest was closed and further ROTEM tests were performed in the ICU only in case of increased bleeding. In cases of persistent microvascular bleeding in the operating room, ROTEM coagulation analysis was performed 15 minutes after administration of all appropriate coagulation products |
Patients in the control group received the initial transfusion in the operating room on the basis of clinical judgement (empirically) and, subsequently, on the basis of standard coagulation test results: ACT (> 160 seconds), response 5000 IU protamine; PTT > 60 seconds or INR > 1.5, response FFP (15 ml/kg body mass); platelets (< 100 000 cells/µl), response 1 platelet concentrate; FIB (< 1.2 mg/dl), response 2 g FIB; alpha-2 antiplasmin < 80%, response 3 g tranexamic acid Test timing Blood samples for laboratory tests drawn after administration of protamine |
| Study details | Selection criteria | Participant details | Intervention | Control | Comments |
|---|---|---|---|---|---|
| Kultufan Turan (2006)52 Country: Turkey Funding: Not for profit Study design: RCT Recruitment: NR No. of participants: 40 |
Inclusion criteria: Open heart surgery – either CABG or valve surgery Exclusion criteria: None stated |
Patient category: Mixed cardiac surgery Details: CABG or valve surgery Previous anti-coagulation therapy: Unclear Mean age (SD or range), years: 53 (18–78) % male: 90 |
Test: Rotational thromboelastography (ROTEG) Parameters: Performed pre operative and 1 hour post operatively Transfusion algorithm based on ROTEG. Post-operative transfusion indicated if bleeding was > 400 ml with 1 hour or > 1000 ml within 4 hours Test timing Performed preoperative and 1 hour post operatively |
Routine transfusion therapy, standard laboratory coagulation testing. Post operative transfusion indicated if bleeding was > 400 ml with 1 hour or > 1000 ml within 4 hours | Turkish language – extraction based on data in Cochrane review, paper abstract and tables |
| Study details | Selection criteria | Participant details | VE test | Conventional tests |
|---|---|---|---|---|
| Nuttall (1997)59 Country: USA Funding: NR Study design: Prediction study Recruitment: NR No. of participants: 82 |
Inclusion criteria: Adult men and women scheduled for elective cardiac surgery requiring CPB Exclusion criteria: NR |
Patient category: Mixed cardiac surgery Details: CABG and/or valve replacement or repair, or congenital heart surgery Previous anticoagulation therapy: 11 patients received pre-operative heparin, 3 received pre-operative coumadin, 18 received pre-operative aspirin Mean age (SD or range), years: 63 (NR) % male: 61 % white: NR |
TEG (NR): MA, R, R + k, α-angle, MA + 30 Sonoclot (NR): P1 (time to shoulder), P2 (time to peak), P1–P2, onset, R1, R2, R3 |
Bleeding time, platelet MPV, plasma FIB concentration, PLT, PT, aPTT, platelet HCT |
| Study details | Selection criteria | Participant details | Intervention | Control |
|---|---|---|---|---|
| Nuttall (2001)50 Country: USA Funding: Not stated Study design: RCT Recruitment: NR No. of participants: 92 |
Inclusion criteria: Adults scheduled for elective cardiac surgery requiring CPB Exclusion criteria: Pregnancy |
Patient category: Mixed cardiac surgery Details: Elective cardiac surgery requiring CPB; 58% CABG, 72% valve surgery, 37% other cardiac surgery Previous anticoagulation therapy: 34% in intervention and 15.7% in control were receiving warfarin; 42% in intervention and 45% in control receive pre-operative aspirin; 34% in intervention and 16% in control received pre-operative coumadin; 34% in intervention and 18% in control received pre-operative i.v. heparin Mean age (SD or range), years: 68 (NR) % male: 73 |
Test: TEG (NR) Parameters: MA POC algorithm incorporating: TEG – MA (< 48 mm) available in 30 minutes and PLTs (< 102 K/mm3) available in 5–10 minutes for platelet transfusion or DDAVP administration; whole blood PT (> 16.6 seconds) and aPTT (> 57 seconds) available in 3–6 minutes for FFP transfusion; FIB concentration (<144 mg/dl) available within 1 hour for cryoprecipitate transfusion Test timing Tests performed on arrival in ICU |
Transfusion based on clinician’s judgement with or without guidance from laboratory tests |
| Study details | Selection criteria | Participant details | Intervention | Control |
|---|---|---|---|---|
| Paniagua (2011)53 Country: Spain Funding: NR Study design: RCT Recruitment: NR Conference abstract only No. of participants: 22 |
Inclusion criteria: All patients scheduled to cardiac surgery with extracorporeal circulation who had major post-operative bleeding (≥ 300 ml in first post-operative hour) Exclusion criteria: NR |
Patient category: Mixed cardiac surgery Details: NR Previous anticoagulation therapy: NR Mean age (SD or range), years: NR % male: NR |
Test: ROTEM (EXTEM and FIBTEM) Parameters: MCF Hypofibrinogenaemia: MCF in ECTEM < 50 and FIBTEM < 9; thrombocytopenia MCF EXTEM < 50 and FIBTEM ≥ 9 Test timing All measurements done at time of inclusion and 10 minutes after each treatment until bleeding stopped (< 150 ml/hour) |
Standard laboratory measurements: hypofibrinogenaemia: FIB (Clauss method) < 1 g/l; thrombocytopenia PLT < 80 × 109/l Test timing All measurements done at time of inclusion and 10 minutes after each treatment until bleeding stopped (< 150 ml/hour) |
| Study details | Selection criteria | Participant details | Intervention | Control |
|---|---|---|---|---|
| Rauter (2007)55 Country: Austria Funding: Not stated Study design: RCT Recruitment: NR Conference abstract only No. of participants: 213 |
Inclusion criteria: Patients scheduled for routine on-pump cardiac surgery Exclusion criteria: NR |
Patient category: Mixed cardiac surgery Details: NR Previous anticoagulation therapy: NR Mean age (SD or range), years: NR % male: NR |
Test: ROTEM (NR) Parameters: NR ROTEM + clinical signs Test timing NR |
Routine management (aPTT, Quick, FIB, haemoglobin, clinical signs of anaemia) Test timing NR |
| Study details | Selection criteria | Participant details | Intervention | Control |
|---|---|---|---|---|
| Royston (2001)49 Country: UK Funding: NR Study design: RCT Recruitment: NR No. of participants: 60 |
Inclusion criteria: NR; appears to be patients undergoing heart surgery Exclusion criteria: NR |
Patient category: Mixed cardiac surgery Details: 10% heart transplantation, 50% revascularisation, 40% Ross procedure, multiple valve or valve and revascularisation surgery. No patients were having repeat operations Previous anticoagulation therapy: 10% were taking aspirin and/or warfarin immediately before surgery a further 50% were taking aspirin Age range, years: (21–83) % male: |
Test: TEG (with and without heparinase) Parameters: r, MA, LYS30 r > 14 mm then 1 unit FFP; r > 21 mm then 2 units FFP; r > 28 mm then 4 units FFP; MA < 48 mm then 1 platelet pool; MA < 40 mm then 2 platelet pools; LY30 > 7.5% then aprotonin Test timing Sample 1, prior to surgery; sample 2, at bypass (included heparinase); sample 3, 10–15 minutes after protamine, developed with and without heparinase |
Wishes of the clinician. SLTs performed included aPTT, PLT and FIB concentration Test timing 10–15 minutes after protamine |
| Study details | Selection criteria | Participant details | Intervention | Control |
|---|---|---|---|---|
| Shore-Lesserson (1999)51,56,57 Country: USA Funding: NR Study design: RCT Recruitment: NR No. of participants: 107 |
Inclusion criteria: Adult patients undergoing a cardiac surgical procedure that had a moderate to high risk for requiring a transfusion (single valve replacement, multiple valve replacement, combined coronary artery bypass plus valvular procedure, cardiac reoperation or thoracic aortic replacement) Exclusion criteria: Significant pre-existing hepatic disease (transaminase levels > 2 times control), renal disease requiring dialysis, or requirement for pre-operative inotropic support |
Patient category: Mixed cardiac surgery Details: Moderate transfusion risk procedures (single valve, repeat CABG) 32% High transfusion risk procedures (combined procedures, repeat valve) 66% Previous anticoagulation therapy: Patients receiving pre-operative heparin infusion and those who had taken aspirin within the past 7 days were included Mean age (SD or range), years: 66 (15) % male: 58 |
Test: TEG (heparin and non-heparin TEG) Heparinase-modified TEG R time < 50% of non-heparinase R time, response additional protamine (50 mg); PLT (< 100,000/µl) and TEG MA (< 45 mm), response if bleeding persisted 6 units platelets transfused; R time (> 20 mm), response 2 units FFP transfused if bleeding persisted; FIB (< 100 mg/dl), response 10 units cryoprecipitate if bleeding persisted; TEG evidence of fibrinolysis (LY30 > 7.5%), response 10 g ε-aminocaproic acid at clinician’s discretion if bleeding persisted Test timing Baseline: celite- and TF-activated TEG During re-warming on CPB: celite- and TF-activated TEG with heparinase After protamine administration: celite- and TF-activated TEG + heparinase-modified TEG to rule out residual heparinisation |
ACT (> 15% above baseline), response 50 mg additional protamine; PLT (< 100,000/µl), response 6 units of platelets if bleeding persisted; PT (> 150% of control), response 2 units of FFP if bleeding persisted; FIB (< 100 mg/dl), response 10 units of cryoprecipitate if bleeding persisted; persistent bleeding and failure of all previous therapies, response 10 g ε-aminocaproic acid at physician’s discretion Test timing Baseline: PLT; FIB; PT; aPTT During re-warming on CPB: PLT After protamine administration: PT, aPTT, FIB |
| Study details | Selection criteria | Participant details | VE test | Conventional tests |
|---|---|---|---|---|
| Tuman (1989)60 Country: USA Funding: NR Study design: Prediction study Recruitment: NR No. of participants: 42 |
Inclusion criteria: Adult cardiac surgical patients prospectively felt to be at high risk for excessive post-CPB bleeding. Patients were considered to be at high risk for bleeding if they were undergoing pre-operative cardiac procedures, valve replacement, ventricular or aortic arch aneurysm resection or other complex cardiac procedures Exclusion criteria: Abnormal pre-operative coagulation or liver function studies, anticoagulant or antiplatelet medications 7 days before surgery |
Patient category: Mixed cardiac surgery Details: Previous anticoagulation therapy: No patients received previous anticoagulation therapy Mean age (SD or range), years: NR % male: NR % white: NR |
Sonoclot (NR): ACT, R1, R2, PEAK and R3 TEG (NR): R, k, MA, α-value, A60 |
ACT, PT, PTT, PLT and FIB |
| Study details | Selection criteria | Participant details | Intervention | Control | Comments |
|---|---|---|---|---|---|
| Weber (2012)35 Country: Germany Funding: NR Study design: RCT Recruitment: May 2009 to April 2010 No. of participants: 100 |
Inclusion criteria: A two-stage inclusion process was used: Stage 1: patients (≥ 18 years) scheduled for elective, complex cardiothoracic surgery (combined CABG and valve surgery, double or triple valve procedures, aortic surgery or redo surgery) with CPB Stage 2: patients were enrolled in the study after heparin reversal following CPB if at least one of the following two criteria were fulfilled: (1) diffuse bleeding from capillary beds at wound surfaces requiring haemostatic therapy as assessed by the anaesthesiologist and surgeon by inspecting the operative field; (2) intraoperative or post operative (during the first 24 post-operative hours) blood loss exceeding 250 ml/hour or 50 ml/10 minutes Exclusion criteria: Pregnancy |
Patient category: Mixed cardiac surgery Details: Redo surgery 27% Combined CABG and valve surgery 44% Double valve surgery 23% Triple valve surgery 3% Aortic surgery 14% Previous anticoagulation therapy: Pre-operative antiplatelet therapy, including aspirin was stopped at least 6 days before surgery Mean age (SD), years 71 (8) % male: 62 |
Test: ROTEM (EXTEM, INTEM, FIBTEM, HEPEM) CT(s) for EXTEM (< 80 seconds), INTEM and HEPTEM (< 240 seconds), response 15 ml/kg body weight FFP particularly if CT prolongation did not respond to administration of PCC, response 20–30 IU/kg body weight (EXTEM only); A10 (mm) for all four tests, including FIBTEM (< 40 mm EXTEM and > 10 mm FIBTEM), response 25–50 mg/kg body weight in bleeding patients, platelet concentrates. In addition to ROTEM, the intervention group received aggregometric POC testing for platelet function Test timing Platelet count, FIB, aPTT and INR performed preoperatively, at admission to ICU and after 24 hours in ICU Timing of ROTEM test unclear, but appears to be after declamping of the aorta, before weaning off CPB, after protamine administration, and to guide therapy in ongoing bleeding |
Laboratory coagulation tests: PLT; haemoglobin concentration, response packed erythrocytes transfused to maintain haemoglobin concentration > 6 g/dl during CBP and > 8 g/dl after CBP, 15 ml/kg body weigh FFP if bleeding did not stop after FIB and 4 units RBC; FIB concentration (< 150–200 mg/dl), response, 25–50 mg/kg body weight in bleeding patients FIB; INR (> 1.4), response 20–30 IU/kg body weight; aPTT (> 50 seconds), response 20–30 IU/kg body weight Detailed flow chart of management algorithm reported in article Test timing Platelet count, FIB, aPTT and INR performed preoperatively, at admission to ICU and after 24 hours in ICU Serial ACT intraoperatively Additional coagulation tests if bleeding persisted after intervention |
88 patients included in the operating room and 12 in the ICU Study terminated early owing to interim analysis showing between-group difference for primary outcome (p < 0.001) |
| Study details | Selection criteria | Participant details | Intervention | Control |
|---|---|---|---|---|
| Westbrook (2009)47 Country: Australia Funding: NR Study design: RCT Recruitment: NR No. of participants: 69 |
Inclusion criteria: Patients presenting for cardiac surgery Exclusion criteria: Patients undergoing cardiac surgery with lung transplantation |
Patient category: Mixed cardiac surgery Details: Redo surgery: intervention group 6.3%, control group 2.5% Urgent presentation: intervention 9.4%, control 10% Previous anticoagulation therapy: Pre-operative aspirin: intervention 23.6%, control 23.6% Pre-operative heparin: intervention 9.38%, control 5.0% Pre-operative warfarin: intervention 15.6%, control 10,0% Pre-operative clopidogrel: intervention 6.25%, control 2.5% Mean age (SD or range), years: 63 (NR) % male: 71 |
Test: TEG (kaolin + heparinase) Parameters: R, MA, LY30 Blood components were administered in accordance with a coagulation correction protocol (reported as a flow chart), which was based on TEG measurements (R, MA, LY30) alone Test timing Where possible, patients taking clopidogrel or aspirin had platelet mapping studies before anaesthesia Plain and heparinased TEG before the bypass, in the re-warming phase and after protamine administration Additional TEG for refractory bleeding in theatre or ICU |
Blood components were given at the discretion of the attending physician, based on previous experience and standard laboratory coagulation tests (aPTT, INR, FIB level, PLT) Test timing Timing at the discretion of physicians |
Baseline details: trauma studies
| Study details | Selection criteria | Participant details | VE test | Conventional tests |
|---|---|---|---|---|
| Cotton (2011)73 Country: USA Funding: NR Study design: Prediction study Recruitment: October 2009 to February 2010 No. of participants: 272 |
Inclusion criteria: All adult (≥ 18 years) trauma patients who arrived directly from the scene and were the institution’s highest level of trauma activation Exclusion criteria: Patients who had burn wounds > 20% total body surface area, or who died within 30 minutes of arrival were excluded |
Patient category: Trauma Details: Blunt trauma 72% Previous anticoagulation therapy: NR Mean age (SD or range), years: 34 (24–50) % male: 74 % white: 50 GCS: 14 (3–15) ISS: 14 (8–25) |
TEG (rapid TEG): ACT | None |
| Davenport (2011)70,78 Country: UK Funding: Partially funded by a NIHR programme grant Study design: Prediction study Recruitment: January 2007 to June 2009 No. of participants eligible (enrolled): 325 (300) |
Inclusion criteria: All adult trauma patients (> 15 years) who met the local criteria for full trauma team activation were eligible for enrolment and recruited into the study when research personnel were present (08.00–20.00 daily) Exclusion criteria: Exclusion criteria were: arrival in the ED > 2 hours after injury; the administration of > 2000 ml of intravenous fluid before ED arrival; transfer from another hospital; and burns covering > 5% of the total body surface area |
Patient category: Trauma Details: Penetrating injuries 21% Previous anticoagulation therapy: Patients were excluded if they were taking anticoagulation therapy Mean age (range), years: 33 (23–48) % male: 82 % white: NR GCS: NR ISS: 12 (4–25) |
ROTEM (EXTEM): CA5, CT, αα-angle | PT ratio (PR) |
| Holcomb (2012)74 Country: USA Funding: Government funding Study design: Prediction study Recruitment: September 2009 to February 2011 No. of participants: 1974 |
Inclusion criteria: All adult trauma patients admitted to single unit who met the institution’s highest-level trauma activation Exclusion criteria: Younger than 18 years or admitted directly to the burn unit |
Patient category: Trauma Details: 207 had isolated traumatic brain injury no further details Previous anticoagulation therapy: 17 had pre-injury exposure to warfarin Mean age (range), years: 33 (23–49) % male: 75 % white: 54 GCS: 12 (3–15) ISS: 17 (9–26) |
TEG (rapid TEG): ACT, k time, LY30, MA, r-value, α-angle | Plasma FIB concentration, PLT, PT, aPTT, INR |
| Ives (2012)72 Country: USA Funding: Not stated Study design: Prediction study Recruitment: November 2010 to April 2011 No. of participants eligible (enrolled): 260 (118) |
Inclusion criteria: Institution’s highest tier of trauma team activation criteria (systolic blood pressure < 90 mmHg, or heart rate > 120 b.p.m., or respiratory rate < 10 breaths/minute or > 29 breaths/minute, or unresponsive to pain (excluding isolated traumatic brain injury with normal vital signs), or age > 70 years [excluding ground-level fall], or gunshot wound to chest or abdomen, or stab wound to anterior chest) Exclusion criteria: None stated |
Patient category: Trauma Details: 52% penetrating trauma Previous anticoagulation therapy: NR Mean age (range), years: 37 (8–91) % male: 77 % white: NR GCS: 11.8 (4.8) ISS: 15.9 |
TEG (kaolin): EPL, MA or clot strength | None |
| Jeger (2012)77,79 Country: Switzerland Funding: Government research grant Study design: Prediction study Recruitment: November 2009 to May 2010 No. of participants eligible (enrolled): 85 (76) |
Inclusion criteria: Patients were included if they were > 16 years and had suspected multiple injuries, and a physician with TEG experience was available Exclusion criteria: None reported |
Patient category: Trauma Details: Blunt trauma 83% Craniocerebral injury 43% Previous anticoagulation therapy: NR Mean age (SD), years: 49 (21) % male: NR % white: NR GCS: NR ISS: 18 ± 10 |
TEG (kaolin): G, k, MA, time to peak, α-angle TEG (rapid TEG): G, k, MA, time to peak, α-angle |
aPTT, INR, plasma FIB, concentration, thrombin time |
| Kaufmann (1997)64 Country: USA Funding: Non-monetary support provided by Haemoscope Corporation Study design: Prediction study Recruitment: August 1994 to January 1995 No. of participants: 69 |
Inclusion criteria: Blunt trauma patients; trauma code criteria; age > 14 years; examined using TEG Exclusion criteria: None stated |
Patient category: Blunt trauma Previous anticoagulation therapy: 3 patients had recently taken aspirin; no patients on warfarin Mean age (range), years: 40 (16–82) % male: 59 % white: NR GCS: NR ISS: 12.3 (1–75) |
TEG: r, K, α-angle, and MA | None |
| Korfage (2011)75 Country: the Netherlands Funding: NR Study design: Prediction study Recruitment: NR Conference abstract only No. of participants: 142 |
Inclusion criteria: Trauma patients admitted to the ED of VU University Medical Center, Amsterdam Exclusion criteria: None reported |
Patient category: Trauma Previous anticoagulation therapy: Five patients used anticoagulant medication Mean age (SD), years: 46 (18) % male: 61 % white: NR GCS: NR ISS: NR |
ROTEM (EXTEM): CFT | None |
| Kunio (2012)67 Country: USA Funding: Not stated Study design: Prediction study Recruitment: February 2010 to April 2011 No. of participants: 69 |
Inclusion criteria: Level 1 trauma centre; traumatic brain injury with intracranial haemorrhage on admission non-contrast head CT; ≥ 16 years Exclusion criteria: Use of clopidogrel or warfarin within 30 days of admission |
Patient category: Traumatic brain injury Previous anticoagulation therapy: Patients taking clopidogrel or warfarin within 30 days of admission excluded; 16% were taking aspirin before admission Mean age (range), years: 46 (30–64) % male: 81 % white: NR GCS: 13 (7–15) ISS: 21 (17–33) |
TEG (not stated): R | None |
| Leemann (2010)65 Country: Switzerland Funding: NR Study design: Retrospective prediction study Recruitment: January 2006 to December 2006 No. of participants: 53 |
Inclusion criteria: ISS ≥ 16 and ROTEM measurements on admission available Exclusion criteria: Isolated head injury (AIS head ≥ 3 and AIS chest, abdomen and extremity < 3); penetrating mechanism of injury |
Patient category: Blunt trauma Details: Severe traumatic brain injury present in 64% of patients Previous anticoagulation therapy: NR Mean age (SD), years: 40 (2) % male: 76 % white: NR GCS: ≤ 8 79% ISS: 31.1 ± 1.7 |
ROTEM (EXTEM and INTEM): A10, A20, CFT, MCF, α-angle | aPTT, INR, PLT |
| Messenger (2011)63 Country: Canada Funding: NR Study design: CCT Conference abstract only Recruitment: NR No. of participants: 50 |
Inclusion criteria: Adult trauma patients requiring massive transfusion (> 12 RBC units in 24 hours or > 4 units in 4 hours) Exclusion criteria: None reported |
Patient category: Mixed trauma; high risk of bleeding Previous anticoagulation therapy: NR % male: NR % white: NR GCS: NR ISS: NR |
Test: TEG-guided protocol and HCT assay Parameters: rapid TEG (r, MA, LY30) Test timing NR |
Treatment according to institutional massive transfusion protocol based on POC HCT assay Test timing NR |
| Study detail | Selection criteria | Participant details | Intervention | Control |
| Moore (ongoing)62 Country: USA Funding: Denver Health and Hospital Authority and Haemonetics Corporation Study design: RCT Recruitment: September 2010 – ongoing No. of participants: target 120 |
Inclusion criteria: Age > 18 years, admitted to Denver Health Medical Center, blunt or penetrating trauma sustained < 6 hours before admission, with ISS > 15, likely to require transfusion of RBC within 6 hours from admission as indicated by clinical assessment Exclusion criteria: Chronic liver disease (total bilirubin > 2.0 mg/dl). Advanced cirrhosis discovered on laparotomy will be a criterion for study withdrawal and exclusion of conventional coagulation or rapid TEG/TEG data from the analysis); known inherited defects of coagulation function (e.g. haemophilia, Von Willebrand disease), pregnancy |
Patient category: Previous anticoagulation therapy: n/a % male: Not yet available % white: Not yet available GCS: Not yet available ISS: Not yet available |
Test: TEG (rapid TEG) Parameters: [TEG-ACT, α-angle, K value, MA (maximum amplitude), G value (clot strength) and fibrinolysis (EPL)] Test timing Blood component therapy per usual clinical practice. The test arm involves the use of rapid-TEG to diagnose and describe post-injury coagulopathy and to guide blood component replacement per institutional algorithm. The current institutional massive transfusion protocol will be followed Test timing On hospital admission (usually within an hour), twice within first 6 hours post injury, 12 and 24 hours post injury |
INR, PTT, FIB, D-dimer Blood component therapy guided by conventional coagulation tests (aPTT, INR, FIB level, D-dimer) per usual clinical practice. The current institutional massive transfusion protocol will be followed Test timing Same as TEG |
| Nystrup (2011)71 Country: Denmark Funding: NR Study design: Retrospective prediction study Recruitment: 2006–7 No. of participants: 89 |
Inclusion criteria: Patients on the European TARN database, who had a TEG analysis performed along with the initial blood tests on admission and before any blood component/products are administered. The TARN database only includes patients with severe traumatic injuries Exclusion criteria: None reported |
Patient category: Trauma Details: Blunt trauma 85%. Cause of trauma: road traffic accident 73%; fall from height 11%; assault 11%; suicide attempt 4.5% Type of trauma: thoracic 17%; abdominal 8%; extremities 4.5%; cerebral 20%; multiple trauma 41.5%; other 9% Previous anticoagulation therapy: NR Mean age (range), years: 39 (35–43) % male: 66 % white: NR GCS: NR ISS: 21 (95% CI 19–23) |
TEG (not stated): clot strength, MA | aPTT, INR |
| Pezold (2012)80 Country: USA Funding: Government research grants Study design: Prediction study Recruitment: May 2008 to September 2010 No. of participants: 80 |
Inclusion criteria: Trauma activations, patients age > 15 years, ISS > 15, and both BD and rapid TEG obtained on arrival at the ED Exclusion criteria: None reported |
Patient category: Trauma Details: Blunt trauma 38% Penetrating trauma 62% Previous anticoagulation therapy: Mean age (SD), years: 34 (2) % male: 81 % white: NR GCS: NR ISS: 29 ± 1 |
TEG (rapid TEG): G (global measure of clot strength) dynes/cm2 | aPTT, INR |
| Schöchl (2011)76,81 Country: Austria Funding: NR Study design: Retrospective prediction study Recruitment: January 2005 to December 2010 No. of participants: 323 |
Inclusion criteria: All patients with an ISS ≥ 16, from whom blood samples were taken immediately on admission to the emergency room, were eligible for inclusion in the study Exclusion criteria: Therapy withheld because of non-survivable injuries; patient suffered from burns; patient transferred from other hospitals |
Patient category: Trauma Details: None reported Previous anticoagulation therapy: NR Mean age (range), years: 44 (26–59) % male: 79 % white: NR GCS: NR ISS: NR |
ROTEM (INTEM): CT, MCF, CFT ROTEM (EXTEM): MCF, CFT, CT ROTEM (FIBTEM): A10, MCF |
Platelet count, aPTT, plasma FIB concentration |
| Schöchl (2011)68,76 Country: Austria Funding: NR Study design: Cohort Recruitment: NR Conference abstract only No. of participants: 88 |
Inclusion criteria: Patients with isolated severe traumatic brain injury (AIS head 92 and AIS extracranial G3) at admission to the emergency room Exclusion criteria: None reported |
Patient category: Traumatic brain injury Previous anticoagulation therapy: NR Mean age (SD or range), years: NR % male: NR % white: NR GCS: NR ISS: NR |
ROTEM (FIBTEM): MCF | aPTT |
| Tapia (2012)69 Country: USA Funding: Not stated Study design: Prediction study Recruitment: January 2008 to June 2011 Conference abstract only No. of participants eligible (enrolled): 291 (230) |
Inclusion criteria: Patients on Urban Level 1 Trauma Centre database who had received ≥ 6 units RBC in first 24 hours of admission Exclusion criteria: Traumatic brain injury |
Patient category: Trauma Details: 58% had penetrating injury Previous anticoagulation therapy: Not stated Mean age (SD or range), years: NR % male: NR % white: NR GCS: NR ISS: NR |
TEG (not specified) | None |
| Tauber (2011)66 Country: Austria Funding: Not stated Study design: Prediction study Recruitment: July 2005 to July 2008 No. of participants: 334 |
Inclusion criteria: Adult polytrauma (ISS of ≥ 15 resulting from injury of at least two body regions. Isolated head injury was defined as a GCS score of ≤ 14 after blunt head trauma in patients with an AIS of 3 in any other body region) patients, who were admitted to the Level I Trauma Centre. Patients with isolated traumatic brain injury were enrolled from 2006 Exclusion criteria: Age < 18 years, with penetrating injuries, admitted to the study hospital later than 12 hours after trauma, pre-existing coagulopathy, burn injury, malignant disease, were avalanche victims, or exhibited non-head single trauma |
Patient category: Blunt trauma Details: 274 blunt polytrauma and 60 isolated brain injury Previous anticoagulation therapy: Patients who had previously received anticoagulation therapy (warfarin/platelet aggregation inhibitors n = 3) were excluded Mean age (SD or range), years: 43 (27–56) % male: 78 % white: NR GCS: 11 (6–15) ISS: 34 (24–45) |
ROTEM (FIBTEM): hyperfibrinolysis only | None |
| Tuman (1989)60 Country: USA Funding: NR Study design: Prediction study Recruitment: NR No. of participants: 42 |
Inclusion criteria: Adult cardiac surgical patients prospectively felt to be at high risk for excessive post-CPB bleeding. Patients were considered to be at high risk for bleeding if they were undergoing reoperative cardiac procedures, valve replacement, ventricular or aortic arch aneurysm resection or other complex cardiac procedures Exclusion criteria: Abnormal pre-operative coagulation or liver function studies, anticoagulant or antiplatelet medications 7 days before surgery |
Patient category: Mixed cardiac surgery Details: Previous anticoagulation therapy: No patients received previous anticoagulation therapy Mean age (SD or range), years: NR % male: NR % white: NR |
Sonoclot (NR): ACT, R1, R2, PEAK and R3 TEG (NR): R, k, MA, α-angle, A60 |
ACT, PT, PTT, PLT and FIB |
Baseline details: post-partum haemorrhage studies
| Study details | Selection criteria | Participant details | VE test | Conventional tests |
|---|---|---|---|---|
| Bolton (2011)82 Country: UK Funding: Not stated Study design: Prediction study Recruitment: NR Conference abstract only No. of participants: 66 |
Inclusion criteria: Patients with major obstetric haemorrhage (≥ 1500 ml) requiring SLTs for suspected coagulopathy Exclusion criteria: Not stated |
Patient category: PPH Previous anticoagulation therapy: NR Mean age (SD or range), years: NR |
ROTEM (NR): NR | None |
| Lilley (2013)83 Country: UK Funding: ROTEM provided by TEM international Study design: Prediction study Recruitment: April 2012 to September 2012 Conference abstract only No. of participants: 179 |
Inclusion criteria: Women with PPH ≥ 1000 ml Exclusion criteria: NR |
Patient category: PPH Previous anticoagulation therapy: NR Mean age (SD or range), years: NR |
ROTEM (FIBTEM): MCF | Clauss FIB |
Results from randomised controlled trials in cardiac surgery patients
| Study details | Outcome | Data available | VE testing arm results | Control results | RR or MD (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Ak (2009) 46 | RBC transfusion | No. of patients/no. of events | 52/114 | 60/110 | 0.8 (0.64 to 1.08) | 0.181 |
| FFP transfusion | 19/114 | 31/110 | 0.5 (0.36 to 0.99) | 0.038 | ||
| Platelet transfusion | 17/114 | 29/110 | 0.5 (0.34 to 0.97) | 0.033 | ||
| Bleeding: mediastinal blood loss > 400 ml in the first hour after surgery or > 100 ml/hour for 4 consecutive hours | 11/114 | 9/110 | 1.1 (0.52 to 2.65) | 0.700 | ||
| Need for additional protamine | 62/114 | 47/110 | 1.2 (0.97 to 1.67) | 0.080 | ||
| Tranexamic acid use | 10/114 | 21/110 | 0.4 (0.24 to 0.94) | 0.007 | ||
| Re-operation: re-exploration for bleeding | 6/114 | 5/110 | 1.1 (0.38 to 3.44) | NR | ||
| Re-thoracotomy for bleeding | 6/114 | 4/110 | 1.3 (0.43 to 4.51) | 0.574 | ||
| Surgical source of bleed (identified on re-exploration) | 6/114 | 2/110 | 2.5 (0. 60 to 10.54) | NR | ||
| Death | 3/114 | 2/110 | 1.3 (0.27 to 6.70) | NR | ||
| RBC transfusion (units transfused) | Median/IQR | 1 (0–1) | 1 (1–2) | NA | 0.599 | |
| Any blood component transfusion [total allogeneic exposure (unit)] | 2 (1–3) | 3 (2–4) | NA | 0.001 | ||
| FFP transfusion (units transfused) | 1 (1–1) | 1 (1–2) | NA | 0.001 | ||
| Platelet transfusion (units transfused) | 1 (1–1) | 1 (1–2) | NA | 0.001 | ||
| Bleeding [mediastinal test tube drainage (ml) within 12 hours] | Mean (SD) | 480.50 (351) | 591.40 (339.20) | –110.9 (–201.3 to –20.5) | 0.017 | |
| Mean length of ICU stay (hours) | 23.30 (5.70) | 25.30 (11.20) | –2.0 (–4.34 to 0.34) | 0.099 | ||
| No. of days in hospital | 6.20 (1.10) | 6.30 (1.40) | –0.1 (–0.43 to 0.23) | 0.552 | ||
| Avidan (2004) 48 | RBC transfusion within 24 hours | No. of patients/no. of events | 34/51 | 35/51 | 0.9 (0.75 to 1.27) | NR |
| FFP transfusion within 24 hours | 2/51 | 0/51 | 4.9 (0.25 to 101.61) | NR | ||
| Platelet transfusion within 24 hours | 2/51 | 1/51 | 1.6 (0.23 to 12.16) | NR | ||
| Re-operation for suspected surgical bleeding | 1/51 | 1/51 | 1.0 (0.11 to 9.30) | NR | ||
| Bleeding [blood loss in 24 hours (ml)] | Median/IQR | 755 (606–975) | 850 (688–1095) | NA | > 0.05 | |
| Girdauskas (2010) 54 | RBC transfusion within 24 hours | No. of patients/no. of events | 24/27 | 27/29 | 0.9 (0.81 to 1.12) | 0.80 |
| FFP transfusion within 24 hours | 9/27 | 25/29 | 0.3 (0.23 to 0.68) | < 0.001 | ||
| FIB within 24 hours | 21/27 | 26/29 | 0.8 (0.69 to 1.10) | 0.20 | ||
| Factor VIIa transfusion | 1/27 | 2/29 | 0.6 (0.09 to 4.55) | 0.80 | ||
| Massive transfusion (> 20 units) within 24 hours | 5/27 | 10/29 | 0.5 (0.23 to 1.37) | 0.03 | ||
| Platelet transfusion within 24 hours | 14/27 | 23/29 | 0.6 (0.44 to 0.99) | 0.03 | ||
| PCC within 24 hours | 4/27 | 26/29 | 0.1 (0.08 to 0.43) | < 0.001 | ||
| Allogeneic blood components transfused within 24 hours | 24/27 | 29/29 | 0.8 (0.78 to 1.02) | 0.06 | ||
| Re-exploration for bleeding within 24 hours | 5/27 | 7/29 | 0.7 (0.30 to 2.08) | 0.70 | ||
| Dialysis-dependent renal failure | 5/27 | 7/29 | 0.7 (0.30 to 2.08) | 0.6 | ||
| Mean length of ICU stay | 6/27 | 7/29 | 0.9 (0.37 to 2.32) | 0.80 | ||
| Post-operative confusion | 4/27 | 7/29 | 0.6 (0.23 to 1.83) | 0.50 | ||
| Reintubation | 7/27 | 5/29 | 1.4 (0.55 to 3.86) | 0.40 | ||
| Stroke | 4/27 | 3/29 | 1.3 (0.38 to 5.04) | 0.60 | ||
| Surgical source of bleed (found on re-exploration) | 4/27 | 5/29 | 0.8 (0.28 to 2.72) | 0.80 | ||
| Death in hospital | 4/27 | 5/29 | 0.8 (0.28 to 2.72) | 0.80 | ||
| RBC transfusion (perioperative transfusion within 24 hours) | Median/IQR | 6 (2–13) | 9 (4–14) | NA | 0.20 | |
| Any blood component transfusion (perioperative transfusion within 24 hours) | 9 (2–30) | 16 (9–23) | 0.02 | |||
| FFP transfusion (perioperative transfusion within 24 hours) | 3 (0–12) | 8 (4–18) | 0.01 | |||
| FIB (perioperative transfusion within 24 hours) | 2 (2–3) | 2 (2–3) | 0.70 | |||
| Platelet transfusion (perioperative transfusion within 24 hours) | 1 (0–4) | 2 (1–3) | 0.70 | |||
| PCC (perioperative transfusion within 24 hours) | 0 (0–2000) | 3000 (2000–3000) | < 0.001 | |||
| Bleeding (ml) within 24 hours | 890 (600–1250) | 950 (650–1400) | 0.50 | |||
| Mean length of ICU stay (number staying > 10 days) | Mean | 7.3 (9.1) | 8.1 (8.4) | –0.8 (–5.4 to 3.8) | 0.6 | |
| No. of days in hospital | 16.6 (16.4) | 17.0 (14.8) | –0.4 (–8.6 to 7.8) | 0.80 | ||
| Kultufan Turan (2006) 52 | RBC transfusion within 24 hours | No. of patients/no. of events | 7/20 | 12/20 | 0.6 (0.31 to 1.17) | 0.031 |
| Platelet transfusion within 24 hours | 1/20 | 0/20 | 3.0 (0.13 to 69.42) | 1.00 | ||
| Whole fresh blood transfusion within 24 hours | 3/20 | 5/20 | 0.6 (0.19 to 2.10) | 0.422 | ||
| Bleeding (post operative within 24 hours) | Mean (SD) | 837.50 (494.1) | 711.10 (489.2) | 126.4 (–178.3 to 431.1) | 0.581 | |
| FFP transfusion (units within 24 hours) | Mean (SD) | 2.80 (0.95) | 2.70 (1.46) | 0.1 (–0.66 to 0.86) | 0.403 | |
| Nuttall (2001) 50 | Surgical source of bleed | No. of patients/no. of events | 0/41 | 2/51 | 0.2 (0.01 to 5.03) | 0.50 |
| Re-operation | 0/41 | 6/51 | 0.0 (0.005 to 1.65) | 0.032 | ||
| RBC transfusion | Median/range | 2 (0, 9) | 3 (0, 70) | NA | 0.039 | |
| Platelet transfusion | Median/range | 6 (0, 18) | 6 (0–144) | 0.0001 | ||
| FFP transfusion | Median/range | 2 (0, 10) | 4 (0–75) | 0.005 | ||
| Bleeding [mediastinal tube drainage (ml) within 24 hours] | Median/range | 590 (240, 2335) | 850 (290–10,190) | 0.019 | ||
| Paniagua (2011) 53 | RBC transfusion | Mean | 3.80 | 6.40 | NA | NR |
| Platelet transfusion | Total transfused | 0.50 | 1.57 | < 0.05 | ||
| FFP transfusion | Total transfused | 3.10 | 3.40 | NR | ||
| Rauter (2007) 55 | RBC transfusion: units (intraoperative + 48 hours post operative) | Mean | 0.8 | 1.3 | NA | < 0.05 |
| FFP transfusion: units (intraoperative + 48 hours post operative) | Total transfused | 0 | 4 | NR | ||
| Platelet transfusion: units (intraoperative + 48 hours post operative) | 0 | 0 | NR | |||
| PCC: IU (intraoperative + 48 hours post operative) | 3000 | 13,600 | NR | |||
| FIB: g (intraoperative + 48 hours post operative) | 31 | 30 | NR | |||
| Royston (2001) 49 | Haemostatic blood component transfusion within 12 hours | No. of patients/no. of events | 5/30 | 10/30 | 0.5 (0.21 to 1.29) | NR |
| Re-operation (return to theatre for control of surgical bleed) within 48 hours | 1/30 | 1/30 | 1.0 (0.11 to 9.09) | NR | ||
| Death within 48 hours | 0/30 | 0/30 | 1.0 (0.02 to 48.80) | NR | ||
| FFP transfusion (units within 12 hours) | Total | 5 | 16 | NA | < 0.05 | |
| Platelet transfusion (platelet pools within 12 hours) | Total | 1 | 9 | < 0.05 | ||
| Bleeding (chest tube losses within 12 hours) | Median/IQR | 470 (295–820) | 390 (240–820) | NR | ||
| Shore-Lesserson (1999) 51 | RBC transfusion (total within 24 hours) | No. of patients/no. of events | 22/53 | 31/52 | 0.7 (0.48 to 1.03) | NR |
| RBC transfusion (post operative) | 10/53 | 16/52 | 0.6 (0.32 to 1.22) | |||
| RBC transfusion (intraoperative) | 17/53 | 23/52 | 0.7 (0.45 to 1.19) | |||
| Any blood component transfusion | 22/53 | 34/52 | 0.6 (0.44 to 0.93) | |||
| FFP transfusion (total within 24 hours) | 4/53 | 16/52 | 0.2 (0.10 to 0.70) | |||
| FFP transfusion (post operative) | 2/53 | 11/52 | 0.2 (0.06 to 0.79) | |||
| FFP transfusion (intraoperative) | 3/53 | 8/52 | 0.4 (0.12 to 1.32) | |||
| Platelet transfusion (intraoperative) | 5/53 | 8/52 | 0.6 (0.23 to 1.73) | |||
| Platelet transfusion (total within 24 hours) | 3/53 | 9/52 | 0.3 (0.11 to 1.16) | |||
| Platelet transfusion (total within 24 hours) | 7/53 | 15/52 | 0.4 (0.22 to 1.04) | |||
| Re-operation for post-operative bleeding | 0/53 | 2/52 | 0.1 (0.01 to 3.99) | |||
| Surgical source of bleed found on re-exploration | 0/53 | 1/52 | 0.3 (0.01 to 7.85) | |||
| Death | 0/53 | 2/52 | 0.1 (0.009 to 3.99) | |||
| RBC transfusion (total ml within 24 hours) | Mean (SD) | 354 (487) | 475 (593) | –121 (–329 to 87) | 0.12 | |
| Platelet transfusion (total ml within 24 hours) | 34 (94) | 83 (160) | –49 (–99 to 1) | 0.16 | ||
| FFP transfusion (total ml within 24 hours) | 36 (142) | 217 (463) | –181 (–313 to –50) | < 0.04 | ||
| Bleeding [mediastinal tube drainage + reinfusion (ml) within 24 hours] | 702 (500) | 901 (847) | –199 (–466 to 68) | 0.27 | ||
| Weber (2012) 35 | RBC transfusion (24 hours post operative) | No. of patients/no. of events | 32/50 | 41/50 | 0.7 (0.61 to 1.00) | 0.07 |
| RBC transfusion (total within 24 hours) | 42/50 | 49/50 | 0.8 (0.76 to 0.97) | 0.031 | ||
| RBC transfusion (intraoperative) | 33/50 | 45/50 | 0.7 (0.59 to 0.91) | 0.007 | ||
| Factor VIIa transfusion (total within 24 hours) | 1/50 | 12/50 | 0.1 (0.02 to 0.62) | 0.002 | ||
| Factor VIIa transfusion (24 hours post operative) | 0/50 | 4/50 | 0.1 (0.06 to 2.01) | 0.117 | ||
| Factor VIIa transfusion (intraoperative) | 1/50 | 9/50 | 0.1 (0.02 to 0.84) | 0.016 | ||
| FFP transfusion (total within 24 hours) | 20/50 | 40/50 | 0.5 (0.35 to 0.73) | < 0.001 | ||
| FFP transfusion (intraoperative) | 16/50 | 39/50 | 0.4 (0.27 to 0.64) | < 0.001 | ||
| FFP transfusion (24 hours post operative) | 7/50 | 19/50 | 0.3 (0.18 to 0.81) | 0.011 | ||
| FIB (intraoperative) | 23/50 | 26/50 | 0.8 (0.60 to 1.32) | 0.689 | ||
| FIB (total within 24 hours) | 32/50 | 30/50 | 1.0 (0.79 to 1.44) | 0.837 | ||
| FIB (24 hours post operative) | 16/50 | 14/50 | 1.1 (0.63 to 2.05) | 0.828 | ||
| Platelet transfusion (total within 24 hours) | 28/50 | 33/50 | 0.8 (0.62 to 1.16) | 0.412 | ||
| Platelet transfusion (24 hours post operative) | 23/50 | 26/50 | 0.8 (0.60 to 1.32) | 0.689 | ||
| Platelet transfusion (intraoperative) | 10/50 | 24/50 | 0.4 (0.23 to 0.79) | 0.006 | ||
| PCC (intraoperative) | 13/50 | 16/50 | 0.8 (0.45 to 1.50) | 0.66 | ||
| PCC (24 hours post operative) | 12/50 | 16/50 | 0.7 (0.41 to 1.41) | 0.504 | ||
| PCC (total within 24 hours) | 22/50 | 26/50 | 0.8 (0.57 to 1.27) | 0.433 | ||
| Desmopressin treatment (intraoperative) | 26/50 | 27/50 | 0.9 (0.677 to 1.39) | 1.0 | ||
| Desmopressin treatment (total within 24 hours) | 36/50 | 35/50 | 1.0 (0.80 to 1.32) | 1.0 | ||
| Desmopressin treatment (24 hours post operative) | 10/50 | 9/50 | 1.1 (0.50 to 2.43) | 1.0 | ||
| Re-operation | 5/50 | 8/50 | 0.6 (0.24 to 1.76) | NR | ||
| Acute renal failure | 3/50 | 10/50 | 0.071 | |||
| Sepsis | 1/50 | 7/50 | 0.059 | |||
| Thrombotic complications | 0/50 | 2/50 | 0.495 | |||
| Composite adverse events (acute renal failure, sepsis, thrombotic complications, and allergic reactions) | 4/50 | 19/50 | < 0.001 | |||
| 6-month mortality | 2/50 | 10/50 | 0.013 | |||
| RBC transfusion (within 24 hours) | Median/IQR | 3 (2–6) | 5 (4–9) | NA | < 0.001 | |
| FFP transfusion (units within 24 hours) | 0 (0–3) | 5 (3–8) | < 0.001 | |||
| FIB (g within 24 hours) | 2 (0–4) | 2 (0–6) | 0.481 | |||
| Platelet transfusion (units within 24 hours) | 2 (0–2) | 2 (0–5) | 0.010 | |||
| PCC (IU within 24 hours) | 0 (0–1800) | 1200 (0–1800) | 0.155 | |||
| Bleeding (post-operative chest tube blood loss, ml, within 24 hours) | 600 (263–875) | 900 (600 1288) | 0.021 | |||
| Mean length of ICU stay (hours) | 21 (18–31) | 24 (20–87) | 0.019 | |||
| No. of days in hospital | 12 (9–22) | 12 (9–23) | 0.718 | |||
| Westbrook (2009) 47 | RBC transfusion (units) | Total no. of units | 14 | 33 | NA | 0.12 |
| Any blood component transfusion (units) | 37 | 90 | NR | |||
| FFP transfusion (units) | 22 | 18 | NR | |||
| Platelet transfusion (units) | 5 | 15 | NR | |||
| Bleeding (ml) | Median/IQR | 875 (755–1130) | 960 (820) | 0.437 | ||
| Mean length of ICU stay (hours) | 29.4 (14.3–56.4) | 32.5 (22.0–74.5) | 0.369 | |||
| No. of days in hospital | 9 (7–13) | 8 (7–12) | > 0.05 |
Results from prediction studies in cardiac surgery patients
| Study details | VE or conventional test | Test assay, parameters and threshold | Outcome/reference standard details and timing | Crude DOR (95% CI) | AUC (95% CI or SE) |
|---|---|---|---|---|---|
| Bischof (2009) 58 | Sonoclot | Glass bead activated, PF | Bleeding (> 800 ml); chest tube drainage recorded hourly for the first 4 hours after surgery | 18.6 (7.6 to 45.7) | 0.79 (0.72 to 0.87) |
| Sonoclot | Glass bead activator, ACT | 10.2 (4.7 to 21.9) | 0.76 (0.70 to 0.82) | ||
| Sonoclot | Glass bead activated, CR | 8.2 (3.9 to 17.2) | 0.72 (0.63 to 0.81) | ||
| Nuttall (1997) 59 | Conventional | Bleeding time: 5 minutes | Bleeding (subjective): anaesthesiologist and surgeon evaluated blood loss 10 minutes after protamine administration. The patient was characterised as a ‘bleeder’ if both physicians determined the surgical field was ‘wet’ (microvascular bleeding). If both physicians determined the surgical field was dry, the patient was labelled as a ‘non-bleeder.’ If there was disagreement between the physicians on the condition of the surgical field, the patient was excluded from data analysis | 4.6 (1.7 to 12.5) | 0.69 (0.07) |
| PT: 15.3 seconds | 11.0 (3.7 to 32.9) | 0.81 (0.05) | |||
| aPTT: 41.3 seconds | 11.2 (3.6 to 34.8) | 0.80 (0.04) | |||
| Platelet MPV: 7.8 fl | 5.0 (1.9 to 13.3) | 0.72 (0.06) | |||
| Platelet count: 102 K/mm3 | 6.2 (2.3 to 16.7) | 0.77 (0.06) | |||
| Platelet HCT: 0.08% | 6.4 (2.3 to 17.5) | 0.78 (0.07) | |||
| Plasma FIB concentration: 144 mg/dl | 4.2 (1.6 to 11.2) | 0.72 (0.06) | |||
| Sonoclot | R1: 16 cm/minute | 4.3 (1.4 to 13.0) | 0.68 (0.05) | ||
| P1–P2: 774 seconds | 5.1 (1.6 to 16.6) | 0.58 (0.07) | |||
| P2 (time to peak): 1182 seconds | 5.5 (1.8 to 16.8) | 0.62 (0.07) | |||
| P1 (time to shoulder): 408 seconds | 6.4 (1.8 to 22.4) | 0.59 (0.07) | |||
| R2: 5.1 cm/minute | 3.4 (1.3 to 8.8) | 0.73 (0.05) | |||
| Onset: 220 seconds | 1.3 (0.5 to 3.2) | 0.42 (0.07) | |||
| R3: –1.6 cm/minutes | 5.0 (1.9 to 13.1) | 0.30 (0.06) | |||
| TEG | MA + 30: 46 mm | 2.6 (1.0 to 6.6) | 0.64 (0.06) | ||
| R: 17 mm | 2.1 (0.8 to 5.4) | 0.59 (0.06) | |||
| MA: 48 mm | 5.3 (2.0 to 14.3) | 0.71 (0.06) | |||
| α-angle: 42° | 4.1 (1.6 to 10.7) | 0.67 (0.06) | |||
| R + k: 25 mm | 3.3 (1.3 to 8.6) | 0.67 (0.06) | |||
| Tuman (1989) 60 | Conventional | ACT, PT, PTT, PLT and FIB: abnormalities of coagulation were defined as values exceeding 20% reduction from the lowest values of the normal range (FIB, PLT), or exceeding 20% of the highest values of the normal range | Bleeding defined as chest tube drainage > 150 ml/hour for 2 consecutive hours or > 300 ml/hour in 1 hour during the first 8 hours after surgery | 0.5 (0.1 to 2.2) | NR |
| Sonoclot | ACT, R1, R2, PEAK and R3: abnormalities of coagulation were defined as values exceeding 20% reduction from the lowest values of the normal range or 20% of the highest values (ACT) | 37.2 (NR) | NR | ||
| TEG | NR, R, k, MA, α-value, A60: abnormalities of coagulation were defined as values exceeding 20% reduction from the lowest values of the normal range | 98.5 (NR) | NR |
Results from prediction studies in trauma patients
| Study details | VE or conventional test | Test assay, parameters and threshold | Outcome/reference standard details and timing | DOR (95% CI) | AUC (95% CI) | Variables adjusted for in model |
|---|---|---|---|---|---|---|
| Cotton (2011) 73 | TEG | Rapid TEG, ACT: < 105 seconds | Massive transfusion (≥ 10 units PRBC): 6 hours | 5.15 (1.36 to 19.49) | NR | Age (years), gender, blunt mechanism of injury, race, ED Systolic blood pressure, ED heart rate, positive FAST examination |
| RBC transfusion: 6 hours | 1.85 (1.07 to 3.19) | NR | ||||
| Davenport (2011) 70 | Conventional | PR: > 1.2 | RBC transfusion (any PRBC): 12 hours | 5.2 (2.1 to 13.0) | NR | Crude |
| ROTEM | EXTEM, CT: >94 seconds | 1.8 (0.9 to 3.8) | NR | Crude | ||
| EXTEM, CA5: ≤ 35 mm | 3.7 (2.0 to 7.0) | |||||
| EXTEM, α-angle: <65° | 3.9 (1.8 to 8.2) | |||||
| Conventional | PR: > 1.2 | Massive transfusion (>10 units PRBC): 12 hours | 13.2 (3.6 to 47.6) | NR | Crude | |
| ROTEM | EXTEM, CA5: ≤ 35 mm | 13.4 (3.4 to 52.5) | NR | Crude | ||
| EXTEM, CT: > 94 seconds | 3.0 (0.7 to 11.7) | |||||
| EXTEM, α-angle: < 65° | 7.5 (2.1 to 26.0) | |||||
| Conventional | PR: > 1.2 | FFP transfusion (any): 12 hours | 6.1 (2.4 to 15.4) | NR | Crude | |
| ROTEM | EXTEM, CA5: ≤ 35 mm | 3.5 (1.7 to 7.0) | NR | Crude | ||
| EXTEM, CT: > 94 seconds | 1.4 (0.6 to 3.4) | |||||
| EXTEM, α-angle: < 65° | 3.9 (1.8 to 8.7) | |||||
| Holcomb (2012) 74 | Conventional | aPTT: > 35 | Massive transfusion (continuous) ≥ 10 units RBC: 6 hours | 3.08 (1.52 to 6.26) | NR | Age, sex, mechanism of injury, base deficit, weighted, revised trauma score and ISS |
| INR: > 1.5 | 3.44 (1.75 to 6.77) | NR | ||||
| Plasma FIB concentration: < 180 | 2.03 (0.63 to 6.55) | NR | ||||
| Platelet count: < 150 | 2.39 (1.00 to 5.75) | NR | ||||
| PT: > 18 | 2.89 (1.41 to 5.95) | NR | ||||
| TEG | Rapid TEG, ACT: > 128 | 1.95 (1.08 to 3.54) | NR | |||
| Rapid TEG, k: > 2.5 | 2.48 (1.32 to 4.65) | NR | ||||
| Rapid TEG, LY30: > 3% | 1.99 (1.01 to 3.89) | NR | ||||
| Rapid TEG, MA: < 55 | 3.63 (1.81 to 6.98) | NR | ||||
| Rapid TEG, r-value: > 1.1 | 2.34 (1.21 to 4.55) | NR | ||||
| Rapid TEG, α-angle: < 56° | 8.99 (2.86 to 28.29) | NR | ||||
| Conventional | aPTT: > 35 | Massive transfusion of cryoprecipitate (continuous) ≥ 20 units: 6 hours | 2.26 (0.73 to 7.09) | NR | ||
| INR: > 1.5 | 4.25 (1.58 to 11.48) | NR | ||||
| Plasma FIB concentration: < 180 | 1.36 (0.26 to 7.01) | NR | ||||
| Platelet count: < 150 | 2.44 (0.79 to 7.55) | NR | ||||
| PT: > 18 | 2.25 (0.96 to 6.76) | NR | ||||
| TEG | Rapid TEG, ACT: > 128 | 1.83 (0.19 to 4.25) | NR | |||
| Rapid TEG, k: > 2.5 | 4.04 (1.74 to 9.36) | NR | ||||
| Rapid TEG, LY30: > 3% | 3.50 (1.47 to 8.36) | NR | ||||
| Rapid TEG, MA: < 55 | 4.71 (1.97 to 11.28) | NR | ||||
| Rapid TEG, r-value: > 1.1 | 1.81 (0.71 to 4.64) | NR | ||||
| Rapid TEG, α-angle: < 56° | 7.96 (2.20 to 18.85) | NR | ||||
| Conventional | aPTT: > 35 | Massive transfusion of plasma (continuous) ≥ 6 units: 6 hours | 3.34 (1.58 to 7.09) | NR | ||
| INR: > 1.5 | 3.72 (2.16 to 6.41) | NR | ||||
| Plasma FIB concentration: < 180 | 1.33 (0.45 to 3.99) | NR | ||||
| Platelet count: < 150 | 2.19 (1.02 to 4.72) | NR | ||||
| PT: > 18 | 3.49 (1.84 to 6.63) | NR | ||||
| TEG | Rapid TEG, ACT: > 128 | 1.63 (1.02 to 2.61) | NR | |||
| Rapid TEG, k: > 2.5 | 2.20 (1.33 to 3.65) | NR | ||||
| Rapid TEG, LY30: >3% | 1.48 (0.85 to 2.59) | NR | ||||
| Rapid TEG, MA: < 55 | 3.10 (1.77 to 5.35) | NR | ||||
| Rapid TEG, r-value: > 1.1 | 1.95 (1.15 to 3.34) | NR | ||||
| Rapid TEG, α-angle: < 56° | 6.06 (2.13 to 11.26) | NR | ||||
| Conventional | aPTT: > 35 | Massive transfusion of platelets (continuous) ≥ 2 apheresis units: 6 hours | 5.02 (2.42 to 10.44) | NR | ||
| INR: > 1.5 | 4.91 (2.68 to 9.01) | NR | ||||
| Plasma FIB concentration: < 180 | 2.44 (0.84 to 7.13) | NR | ||||
| Platelet count: < 150 | 4.01 (1.92 to 8.38) | NR | ||||
| PT: > 18 | 5.04 (2.65 to 9.59) | NR | ||||
| TEG | Rapid TEG, ACT: > 128 | 1.70 (0.99 to 2.91) | NR | |||
| Rapid TEG, k: > 2.5 | 2.45 (1.39 to 4.32) | NR | ||||
| Rapid TEG, LY30: > 3% | 2.02 (1.10 to 3.70) | NR | ||||
| Rapid TEG, MA: < 55 | 2.47 (1.32 to 4.62) | NR | ||||
| Rapid TEG, r-value: > 1.1 | 1.95 (1.06 to 3.56) | NR | ||||
| Rapid TEG, α-angle: < 56° | 6.70 (2.34 to 10.02) | NR | ||||
| Conventional | Plasma FIB concentration: < 180 | Substantial bleeding defined as (1) receiving first RBC unit within 2 hours of ED arrival and (2) at least five RBC transfusion or death from haemorrhage within 4 hours of ED arrival | 2.01 (0.68 to 5.97) | NR | ||
| PT: > 18 | 2.55 (1.59 to 4.10) | NR | ||||
| aPTT: > 35 | 2.68 (1.62 to 4.45) | NR | ||||
| INR: > 1.5 | 3.40 (1.66 to 7.00) | NR | ||||
| Platelet count: < 150 | 2.52 (1.22 to 5.25) | NR | ||||
| TEG | Rapid TEG, LY30: > 3% | 1.94 (1.16 to 3.24) | NR | |||
| Rapid TEG, MA: < 55 | 2.42 (1.41 to 4.15) | NR | ||||
| Rapid TEG, α-angle: < 56° | 2.66 (1.13 to 6.28) | NR | ||||
| Rapid TEG, k: > 2.5 | 1.75 (1.16 to 2.66) | NR | ||||
| Rapid TEG, r-value: > 1.1 | 2.52 (1.43 to 4.43) | NR | ||||
| Rapid TEG, ACT: > 128 | 1.70 (1.04 to 2.77) | NR | ||||
| Ives (2012) 72 | TEG | Kaolin, EPL: Hyperfibrinolysis defined as EPL > 15% | Death within 24 hours | 25.0 (2.8 to 221.4) | NR | Packed RBCs in 24 hours >10 U |
| Kaolin, r, K, α-angle, and MA; hypocoagulable: two or more of the following: prolonged reaction time, prolonged amplitude, and decreased angle and/or MA | 7.0 (1.7 to 29.2) | NR | ||||
| Kaolin, r, K, α-angle, and MA; hypercoagulable: short reaction time, short amplitude, increased angle and/or MA | 0.33 (0.04 to 2.7) | NR | ||||
| Kaolin, EPL: Hyperfibrinolysis defined as EPL > 15% | RBC transfusion | 42.0 | NR | |||
| Plasma transfusion | 8.3 (2.3 to 29.6) | NR | ||||
| Platelet transfusion | 7.8 (2.2 to 27.8) | NR | ||||
| Jeger (2012) 77 | Conventional | INR: > 1.2 | Any blood component transfusion (patients receiving blood components): 24 hours | 4.5 (NR) | 73 (NR) | Crude |
| INR: > 1.5 | 5.6 (NR) | 73 (NR) | Crude | |||
| Thrombin time: > 13.2 seconds | 2.5 (NR) | 53 (NR) | Crude | |||
| aPTT: > 60 seconds | 2.6 (NR) | 74 (NR) | Crude | |||
| Plasma FIB: < 3 g/l | 8.3 (NR) | 74 (NR) | Crude | |||
| TEG | Kaolin, k: > 1.7 | 3.1 (NR) | 67 (NR) | Crude | ||
| Rapid TEG, k: >1.8 minutes | 7.5 (NR) | 79 (NR) | Crude | |||
| Rapid TEG, α-angle: <74.7° | 7.0 (NR) | 77 (NR) | Crude | |||
| Rapid TEG, MA: < 59.6 mm | 8.5 (NR) | 75 (NR) | Crude | |||
| Rapid TEG, G: < 7374 d/s | 7.5 (NR) | 73 (NR) | Crude | |||
| Kaolin, α-angle: < 58.5° | 4.0 (NR) | 66 (NR) | Crude | |||
| Kaolin, MA: < 58.4 mm | 9.3 (NR) | 70 (NR) | Crude | |||
| Kaolin, time to peak: > 24.7 minutes | 3.0 (NR) | 58 (NR) | Crude | |||
| Kaolin, G: < 7073 d/s | 9.3 (NR) | 70 (NR) | Crude | |||
| Rapid TEG, time to peak: > 17.3 minutes | 4.2 (NR) | 69 (NR) | Crude | |||
| Kaufmann (1997) 64 | TEG | r, K, α-angle, and MA: Hypocoagulable defined as two or more of the following: long r and/or K time, decreased α-angle, and decreased MA | Any blood component transfusion (any components transfused from the time of presentation to the ED): 24 hours | 104.9 (8.2 to 1333.6) | NR | Crude |
| r, K, α-angle, and MA: Hypercoagulable: two or more of the following – short r and/or K time, increased α-angle, and increased MA | 0.2 (0.0 to 0.9) | NR | Crude | |||
| Korfage (2011) 75 | ROTEM | EXTEM, CFT: NR | Any blood component transfusion (‘prolonged EXTEM CFT’) ‘need for transfusion’: 48 hours | 15.26 (1.47 to 158.30) | NR | Multinomial regression analyses unclear which variables included in the final model |
| Kunio (2012) 67 | TEG | R > 9 minutes | Neurosurgical intervention (intracranial pressure monitor, ventriculostomy, craniotomy, craniectomy) | 6.8 (0.7 to 61.6) | NR | Crude |
| Death in hospital | 7.5 (1.3 to 44.8) | NR | Crude | |||
| Leemann (2010) 65 | Conventional | aPTT >36 seconds | Massive transfusion (≥ 10 units PRBC) within 24 hours | 7.75 (1.93 to 31.18) | NR | Crude |
| Platelet count <100 × 103 | 4.71 (0.77 to 28.77) | |||||
| INR > 1.2 | 10.11 (2.63 to 38.81) | |||||
| ROTEM | INTEM, MCF: abnormal (normal range 50–72 mm) | 5.63 (1.37 to 23.06) | ||||
| INTEM, A20: abnormal (normal range 50–71 mm) | 5.16 (1.01 to 26.45) | |||||
| INTEM, A10: abnormal (normal range 44–66 mm) | 11.20 (1.33 to 94.49) | |||||
| INTEM, α-angle: abnormal (normal range 70–83°) | 5.23 (0.60 to 45.67) | |||||
| EXTEM, MCF: abnormal (normal range 50–72 mm) | 3.95 (0.96 to 16.21) | |||||
| EXTEM, A20: abnormal (normal range 50–71 mm) | 4.29 (0.83 to 22.03) | |||||
| EXTEM, A10: abnormal (normal range 43–65 mm) | 4.36 (0.86 to 22.26) | |||||
| EXTEM, α-angle: abnormal (normal range 63–83°) | 2.80 (0.67 to 11.79) | |||||
| EXTEM, CFT: abnormal (normal range 34–159 seconds) | 4.38 (1.05 to 18.32) | |||||
| INTEM, MCF: abnormal (normal range 50–72 mm) | 8.47 (1.19 to 62.50) | 0.82 (0.71 to 0.94) | Haemoglobin ≤ 10 g/dl | |||
| Nystrup (2011) 71 | Conventional | aPTT: NR | Death within 30 days | 1.10 (1.00 to 1.20) | 0.78 (0.61 to 0.95) | Age and ISS |
| INR: NR | NR | 0.63 (0.44 to 0.81) | Crude | |||
| TEG | MA: maximum clot strength < 50 mm | 5.00 (1.22 to 20.45) | NR | Age and ISS | ||
| MA: NR | NR | 0.70 (0.53 to 0.86) | Crude | |||
| Pezold (2012) 80 | Conventional | aPTT | Death (NA); coagulation-related mortality (death after receiving a massive transfusion ≥ 10 PRBC units): 6 hours | NR | 0.89 (0.81 to 0.97) | Age, ISS and systolic blood pressure |
| INR | NR | 0.88 (0.80 to 0.97) | ||||
| TEG | Rapid TEG, G | NR | 0.93 (0.87 to 0.98) | |||
| Conventional | aPTT | Massive transfusion (≥ 10 units PRBC): 6 hours | NR | 0.90 (0.83 to 0.97) | ||
| INR | NR | 0.92 (0.86 to 0.98) | ||||
| TEG | Rapid TEG, G | NR | 0.89 (0.83 to 0.96) | |||
| Schöchl (2011) 68 | Conventional | aPTT | Death; overall mortality | NR | 0.76 (0.64 to 0.88) | Crude |
| ROTEM | FIBTEM, MCF | NR | 0.73 (0.59 to 0.87) | |||
| Schöchl (2011) 76 | Conventional | aPTT: ≤ 35.2 s | Massive transfusion (≥ 10 RBC units): 24 hours | 18.9 | 0.85 (0.81 to 0.89) | Crude |
| Plasma FIB: ≤ 148 mg/dl | 11.2 | 0.83 (0.78 to 0.87) | ||||
| Platelet count: ≤ 161 × 103/µl | 4.6 | 0.70 (0.65 to 0.75) | ||||
| ROTEM | INTEM, CT: ≤ 167 seconds | 5.9 | 0.71 (0.65 to 0.76) | |||
| ROTEM | INTEM, MCF: ≤ 51 mm | 6.5 | 0.78 (0.73 to 0.83) | |||
| ROTEM | FIBTEM, MCF: ≤ 7 mm | 10.6 | 0.84 (0.79 to 0.88) | |||
| ROTEM | INTEM, CFT: ≤ 111 seconds | 6.1 | 0.78 (0.73 to 0.82) | |||
| ROTEM | FIBTEM, A10: ≤ 4 mm | 8.3 | 0.83 (0.78 to 0.87) | |||
| ROTEM | EXTEM, CT: ≤ 72 seconds | 4.6 | 0.71 (0.66 to 0.76) | |||
| ROTEM | EXTEM, CFT: ≤ 147 seconds | 5.6 | 0.74 (0.68 to 0.79) | |||
| ROTEM | EXTEM, MCF: ≤ 52 mm | 5.0 | 0.76 (0.71 to 0.81) | |||
| Tapia (2012) 69 | TEG | Presence of hyperfibrinolysis; no further details | Death within 30 days | 98.7 (12.7 to 765.1) | NR | Crude |
| Tauber (2011) 66 | ROTEM | FIBTEM: fulminant hyperfibrinolysis | Death (NA); overall mortality: 24 hours | 40.2 (8.6 to 187.1) | NR | Crude |
| FIBTEM: any hyperfibrinolysis | 10.3 (4.2 to 25.4) | |||||
| FIBTEM: moderate hyperfibrinolysis | 1.1 (0.1 to 8.7) |
Results from prediction studies in patients with post-partum haemorrhage
| Study details | VE or conventional test | Test assay, parameters and threshold | Outcome/reference standard details and timing | DOR (95% CI) | AUC (95% CI) | Variables adjusted for in model |
|---|---|---|---|---|---|---|
| Bolton (2011) 82 | ROTEM | NR | Coagulopathy requiring treatment | 102.8 (9.5 to 1110.6) | NR | Crude |
| FFP transfusion | 76 (NR) | NR | ||||
| Platelet transfusion | 19.0 (NR) | NR | ||||
| Lilley (2013) 83 | Conventional | Clauss fibrinogen | RBC transfusion (any transfusion) | NR | 0.72 (NR) | Crude |
| ROTEM | FIBTEM, MCF: < 18 mm | RBC transfusion (any) | 33.7 (7.3 to 155.7) | 0.74 (NR) | ||
| Conventional | Clauss fibrinogen | RBC transfusion (≥ 4 units) | NR | 0.84 (NR) | ||
| ROTEM | FIBTEM, MCF | RBC transfusion (≥ 4 units) | NR | 0.80 (NR) | ||
| Conventional | Clauss fibrinogen | Invasive procedures | NR | 0.93 (NR) | ||
| ROTEM | FIBTEM, MCF | Invasive procedures | NR | 0.89 (NR) |
Appendix 3 Risk of bias assessments
Cochrane risk of bias assessments for randomised controlled trials in cardiac patients
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | No details on randomisation method | Unclear |
| Allocation concealment | No details on concealment of allocation | Unclear |
| Participant/personnel blinding | Transfusions were performed by the anaesthesiologist, who was blinded to the patient’s group assignment. Unclear whether or not patient was blinded but would have been unlikely to influence results | Low |
| Outcome assessor blinding | Transfusions (outcome) were performed by the anaesthesiologist, who was blinded to the patient’s group assignment | Low |
| Incomplete outcome data | No withdrawals reported, all patients randomised appear to be included in the analysis | Low |
| Selective outcome reporting | All outcomes specified in the methods reported in the results; no mention of study protocol | Low |
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | No details on randomisation method | Unclear |
| Allocation concealment | No details on concealment of allocation | Unclear |
| Participant/personnel blinding | Investigators were not blind to group allocation | High |
| Outcome assessor blinding | Blood loss into the chest tube and post-surgical blood component use were recorded by staff in the recovery unit who were unaware of group allocation | Low |
| Incomplete outcome data | No withdrawals reported, all patients randomised appear to be included in the analysis | Low |
| Selective outcome reporting | Outcomes were not specified in the methods section; no mention of study protocol | Unclear |
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | Patients were randomly assigned using a computer-generated list | Low |
| Allocation concealment | No details on concealment of allocation | Unclear |
| Participant/personnel blinding | No details on blinding | Unclear |
| Outcome assessor blinding | No details on blinding | Unclear |
| Incomplete outcome data | No withdrawals reported, all patients randomised appear to be included in the analysis | Low |
| Selective outcome reporting | All outcomes specified in the methods reported in the results; no mention of study protocol | Low |
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | No details on randomisation method | Unclear |
| Allocation concealment | No details on concealment of allocation | Unclear |
| Participant/personnel blinding | Physician in charge of ROTEG and ICU physician were blinded | Low |
| Outcome assessor blinding | No details on blinding | Unclear |
| Incomplete outcome data | No withdrawals reported; all patients randomised appear to be included in the analysis | Low |
| Selective outcome reporting | All outcomes specified in the methods reported in the results; no mention of study protocol | Low |
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | Computer-generated randomisation list with a block size of four to one of two groups. Four of the patients initially randomised to the algorithm group were converted to the control group because of unavailability of study personnel | High |
| Allocation concealment | No details on concealment of allocation | High |
| Participant/personnel blinding | No details on blinding | Unclear |
| Outcome assessor blinding | The surgeons and anaesthesiologists were not made aware of which group the patients were placed in until after they decided that the patient had abnormal bleeding after CPB and they felt the patient needed to have transfusion of non-erythrocyte components. Therefore, the people making the transfusion decisions were blinded to group designation of the patients until after the determination of abnormal bleeding after CPB | Low |
| Incomplete outcome data | Four of the patients initially randomised to the algorithm group were converted to the control group because of unavailability of study personnel. An ITT analysis was conducted for a small number of the outcomes but not all; data were extracted for the per-protocol analyses for consistency. ITT analyses reported similar results to per-protocol analyses. No additional dropouts reported | Low |
| Selective outcome reporting | Outcomes were not specified in the methods section; no mention of study protocol | Unclear |
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | No details on randomisation method | Unclear |
| Allocation concealment | No details on concealment of allocation | Unclear |
| Participant/personnel blinding | No details on blinding | Unclear |
| Outcome assessor blinding | No details on blinding | Unclear |
| Incomplete outcome data | No withdrawals reported; all patients randomised appear to be included in the analysis | Low |
| Selective outcome reporting | Abstract only; limited data reported | High |
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | No details on randomisation method | Unclear |
| Allocation concealment | No details on concealment of allocation | Unclear |
| Participant/personnel blinding | Unblinded | High |
| Outcome assessor blinding | Unblinded | High |
| Incomplete outcome data | Five patients were excluded as a result of protocol violations and were not included in the analysis | High |
| Selective outcome reporting | Outcomes were not specified in the methods section; no mention of study protocol. Abstract only, so appears that some outcomes missing and no measure of significance of results | High |
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | ‘Allocation by means of series of sealed envelopes’ – no further details. Patients who returned to theatre for control of surgical bleeding or who died within 48 hours of surgery were discarded and replaced by measurements from an additional patient | High |
| Allocation concealment | ‘Allocation by means of series of sealed envelopes’ – no further details | Unclear |
| Participant/personnel blinding | No details on blinding | Unclear |
| Outcome assessor blinding | No details on blinding | Unclear |
| Incomplete outcome data | Patients who returned to theatre for control of surgical bleeding or who died within 48 hours of surgery were discarded and replaced by measurements from an additional patient. Two patients had excessive bleeding, none died | High |
| Selective outcome reporting | Outcomes not prespecified in methods and no mention of protocol | Unclear |
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | Patients were randomly assigned in a prospective fashion, using a table of random numbers | Low |
| Allocation concealment | No details on concealment of allocation | Unclear |
| Participant/personnel blinding | No details on blinding | Unclear |
| Outcome assessor blinding | No details on blinding | Unclear |
| Incomplete outcome data | All 105 participants appear to have been included in the analyses. One patient in the control group had a surgical bleed and was excluded from the bleeding and transfusion analyses | Low |
| Selective outcome reporting | Outcomes were not specified in the methods section; no mention of study protocol | Unclear |
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | The randomisation list was computer-generated using a balanced (allocation ratio 1 : 1) block-wise (20 × 10) randomisation by the software BiAS for Windows 9.07© (Epsilon Inc., Darmstadt, Germany) | Low |
| Allocation concealment | No details on concealment of allocation | Unclear |
| Participant/personnel blinding | The attending physicians in the POC group were blinded to the results of conventional coagulation tests. Not clear whether or not conventional group were blinded to POC results | Unclear |
| Outcome assessor blinding | No details on blinding | Unclear |
| Incomplete outcome data | No withdrawals reported; all patients randomised appear to be included in the analysis | Low |
| Selective outcome reporting | All outcomes specified in the methods reported in the results; no mention of study protocol | Low |
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | No details on randomisation method | Unclear |
| Allocation concealment | No details on concealment of allocation | Unclear |
| Participant/personnel blinding | Surgeons were blinded to the method of haemostasis assessment | Low |
| Outcome assessor blinding | Decisions about the administration of blood components were based on TEG alone or SLTs alone, depending on group allocation; blinding was not explicitly reported | Unclear |
| Incomplete outcome data | No withdrawals were reported and all participants appear to have been included in the analyses | Low |
| Selective outcome reporting | Outcomes were not specified in the methods section; no mention of study protocol | Unclear |
QUADAS-2 assessments for prediction studies in cardiac patients
Study: Bischof (2009)58
| A. RISK OF BIAS | |
|---|---|
| Patients undergoing cardiac surgery. Patients with known coagulopathy or anticoagulant medication were excluded | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Limited details provided | |
| Do the included patients match the question? | Concerns: unclear |
| A. RISK OF BIAS | |
|---|---|
| Sonoclot (ACT, CR and PF), glass bead-activated and celite/clay-activated, pre-operative and post-protamine testing. Full data were reported only for post-protamine, GbACT The reference standard (bleeding) occurred after testing. No threshold was reported |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Bleeding (chest tube drainage > 800 ml in the first 4 hours after surgery), objective reference standard; unclear whether blinded to Sonoclot results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| No withdrawals were reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: low |
Study: Nuttall (1997)59
| A. RISK OF BIAS | |
|---|---|
| Adult men and women scheduled for elective cardiac surgery requiring CPB No exclusion criteria were reported |
|
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Mixed cardiac surgery | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| TEG and Sonoclot, methods described in detail Standard thresholds used Data reported only for individual TEG and Sonoclot parameters |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Bleeding: patients classified as bleeders or non-bleeders by two anaesthetists, classification was subjective. The physicians evaluating the haemostatic condition of the operative field were blinded to the results of all coagulation tests | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| If there was disagreement on whether or not the patient was a bleeder then the patient was excluded from the analysis | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: high |
Study: Tuman (1989)60
| A. RISK OF BIAS | |
|---|---|
| Adult cardiac surgical patients prospectively considered to be at high risk for excessive post-CPB bleeding Exclusion criteria: abnormal pre-operative coagulation or liver function studies; anticoagulant or antiplatelet medications within 7 days before surgery |
|
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Adult cardiac surgical patients | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| Sonoclot and TEG Interpreted before bleeding had occurred Standard prespecified thresholds used Data reported as the predictive accuracy of the whole test |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Occurrence of bleeding measured objectively; unclear whether blinded to Sonoclot and TEG results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| All patients enrolled were included in the 2 × 2 table | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: low |
Cochrane risk of bias assessments for controlled clinical trial in trauma patients
| Domain | Support for judgement | Risk of bias |
|---|---|---|
| Random sequence generation | Not randomised | High |
| Allocation concealment | Not randomised and so allocation not concealer | High |
| Participant/personnel blinding | No details on blinding reported | Unclear |
| Outcome assessor blinding | No details on blinding reported | Unclear |
| Incomplete outcome data | Numerical outcome data were not reported and so not possible to access | Unclear |
| Selective outcome reporting | Outcomes were not prespecified and so it was unclear whether only selected outcomes were reported | Unclear |
QUADAS-2 assessments for prediction studies in trauma patients
Study: Cotton (2011)73
| A. RISK OF BIAS | |
|---|---|
| Consecutive major trauma activations, adult patients | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Major trauma, no specific categories | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| Rapid TEG full data reported only for ACT, as this is the earliest result available. Reference standard (transfusion outcomes) assessed after rapid TEG. Thresholds derived from study data | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Massive transfusion reference standard or absence of any transfusion within 6 hours | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| No dropouts reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: low |
Study: Davenport (2011)70
| A. RISK OF BIAS | |
|---|---|
| Trauma patients were included only if they presented when research staff were present (0800 to 2000), i.e. not a consecutive sample | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Trauma patients including blunt and penetrating injuries | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| Three ROTEM EXTEM parameters plus PR. Each parameter analysed separately. Reference standard (transfusion) occurred after testing. ROTEM thresholds were derived from patients with normal PR values within study | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Transfusion requirements. Unclear whether transfusion requirements were determined with knowledge of ROTEM and/or PR results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| 325 patients were enrolled; 25 were missing from the analyses; 3 ROTEM sample analyses incomplete; 15 consent processes could not be completed; 7 retrospective exclusions | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: low |
Study: Holcomb (2012)74
| A. RISK OF BIAS | |
|---|---|
| All adult trauma patients admitted between September 2009 and February 2011 | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Trauma patients described as institution’s highest-level trauma activation. Injuries not described in detail except that 297 had traumatic brain injury | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| Rapid TEG assays and thresholds described but unclear how thresholds were derived | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Multiple reference standards relating to bleeding and transfusion requirements | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| No withdrawals reports | |
| Did all patients receive a reference standard? | Unclear |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: low |
Study: Ives (2012)72
| A. RISK OF BIAS | |
|---|---|
| Convenience sample; only 45% of those eligible enrolled reasons for not enrolling; remainder not reported | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Mixed trauma patients | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| TEG evaluated at standard thresholds | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| Primary outcome mortality within 24 hours. Secondary outcomes were transfusion requirements – details on timing and thresholds not reported | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low/unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low/high |
| A. RISK OF BIAS | |
|---|---|
| 5 patients did not contribute to the regression model; reasons for this were not reported | |
| Did all patients receive a reference standard? | Unclear |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: high |
Study: Jeger (2012)77
| A. RISK OF BIAS | |
|---|---|
| Prospective, non-consecutive observational study of trauma patients; patients included where a physician with TEG experience was available on admission. No exclusion criteria reported | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Trauma patients, mainly blunt trauma. | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| TEG, rapid TEG, and conventional laboratory tests. Data reported separately for each parameter. Reference standard (transfusion requirements determined after testing). Not clear whether TEG thresholds were predefined | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Transfusion requirements. Physicians were blinded to TEG results. The decision to transfuse was based on clinical evaluation and predefined thresholds for conventional laboratory coagulation tests Note: risk of bias is high for conventional laboratory tests |
|
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Nine patients were excluded as a result of technical problems and handling errors | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: low |
Study: Kaufmann (1997)64
| A. RISK OF BIAS | |
|---|---|
| Prospective study of blunt trauma patients | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Mixed blunt trauma patients; some had received aspirin | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| TEG detailed description of execution, including machine | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| Transfusion of any blood component, timing specified, decision reported to be blind to TEG result | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| All patients included in 2 × 2 table | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: low |
Study: Korfage (2011)75
| A. RISK OF BIAS | |
|---|---|
| Trauma patients admitted to an emergency department in Amsterdam | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Limited details reported | |
| Do the included patients match the question? | Concerns: unclear |
| A. RISK OF BIAS | |
|---|---|
| Data appear to have been collected for ROTEM INTEM, EXTEM and FIBTEM, plus conventional laboratory tests, but predictive data were reported only for EXTEM CFT. Reference standard (transfusion requirements) occurred after testing. No threshold was reported | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Transfusion requirements. Not clear whether or not need for transfusion was determined with knowledge of ROTEM results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| No dropouts reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: low |
Study: Kunio (2012)67
| A. RISK OF BIAS | |
|---|---|
| Methods of patient enrolment unclear | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Only patients with traumatic brain injury; no use of specified anticoagulants prior to enrolment | |
| Do the included patients match the question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| TEG; no details on assays used. State that manufacturers reference ranges used for all parameters | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: unclear |
| A. RISK OF BIAS | |
|---|---|
| Mortality and need for neurosurgical intervention | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| All patients appear to have been included in the 2 × 2 table | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: low |
Study: Leemann (2010)65
| A. RISK OF BIAS | |
|---|---|
| Retrospective review of trauma patients for whom admission ROTEM results were available. Patients with isolated head injury were excluded | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Trauma patients (excluding isolated head injury) ISS ≥ 16 | |
| Do the included patients match the question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Various ROTEM parameters, analysed individually. Reference standard (massive transfusion) occurred after testing. Thresholds appear to have been based on predefined normal reference ranges | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Massive transfusion within 24 hours | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| No dropouts reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: low |
Study: Nystrup (2011)71
| A. RISK OF BIAS | |
|---|---|
| Retrospective study of patients from a trauma registry, for whom admission TEG results were available | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Severe trauma, variety of causes and types reported | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| Limited details of TEG. Data reported only for overall clot strength and MA. Reference standard (30-day mortality) occurred after testing | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Reference standard 30-day mortality | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| No dropouts reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: low |
Study: Pezold (2012)80
| A. RISK OF BIAS | |
|---|---|
| Review of trauma activations at a single centre between May 2008 and September 2010; appears retrospective. Three patients who died from traumatic brain injury were excluded | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Trauma patients ISS > 15 | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| Rapid TEG, only one parameter reported (G, global measure of clot strength). Reference standards (outcomes) occurred after testing. Only ROC AUC data were reported | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Reference standard MT and coagulation-related mortality | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| No dropouts reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: low |
Study: Schöchl (2011)76
| A. RISK OF BIAS | |
|---|---|
| Retrospective analysis of patients admitted to a trauma centre between 2005 and 2010, for whom blood samples were taken on admission | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Trauma patients with an ISS ≥ 16 years | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| ROTEM and conventional laboratory tests. Data reported separately for each assay parameter. Blinding of interpretation unclear. Optimal thresholds derived from ROC curves | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Reference standard was MT within 24 hours in all cases | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| No dropouts reported. Retrospective study, so data likely to have been complete | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: low |
Study: Schöchl (2011)68
| A. RISK OF BIAS | |
|---|---|
| Retrospective study of patients with isolated severe traumatic brain injury | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| No details reported | |
| Do the included patients match the question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Data reported for one parameter of ROTEM (FIBTEM MCF) and aPTT only. Reference standard (mortality) occurred after testing. Only ROC AUC data reported | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| Reference standard overall mortality | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| No dropouts reported | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: low |
Study: Tapia (2012)69
| A. RISK OF BIAS | |
|---|---|
| Retrospective analysis of database patients | |
| Was a consecutive or random sample of patients enrolled? | No |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: high |
| B. APPLICABILITY | |
| No details on included patients | |
| Do the included patients match the question? | Concerns: unclear |
| A. RISK OF BIAS | |
|---|---|
| TEG; no details on how the test was performed the threshold used to who interpreted the results | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: unclear |
| A. RISK OF BIAS | |
|---|---|
| Mortality; no details on how mortality was assessed | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| Not all patients had data on TEG | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: high |
Study: Tauber (2011)66
| A. RISK OF BIAS | |
|---|---|
| Adult polytrauma patients with an ISS ≥ 15 | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Patients with non-head single trauma excluded | |
| Do the included patients match the question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| ROTEM FIBTEM and EXTEM; data extractable only for hyperfibrinolysis on FIBTEM as a predictor of early mortality. Exact details on how hyperfibrinolysis was defined were not reported Reference standard was death, which would have occurred after the index test as interpreted |
|
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: unclear |
| A. RISK OF BIAS | |
|---|---|
| Reference standard was death within 24 hours | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| All participants appear to have been included in the analyses | |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: low |
QUADAS-2 assessments for prediction studies in women with post-partum haemorrhage
Study: Bolton (2011)82
| A. RISK OF BIAS | |
|---|---|
| Not stated | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Major obstetric haemorrhage | |
| Do the included patients match the question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| ROTEM, no further details on assay, result parameter or threshold | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: unclear |
| A. RISK OF BIAS | |
|---|---|
| Coagulopathy requiring treatment, FFP transfusion and platelet transfusion, assessed according to standard criteria | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| All patients appear to have received the reference standard but little detail on patient flow | |
| Did all patients receive a reference standard? | Unclear |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: low |
Study: Lilley (2013)83
| A. RISK OF BIAS | |
|---|---|
| Consecutive patients, no further details | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: low |
| B. APPLICABILITY | |
| Women with PPH ≥ 1000 ml | |
| Do the included patients match the question? | Concerns: low |
| A. RISK OF BIAS | |
|---|---|
| ROTEM with FIBTEM assay, only MCF evaluated | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or its interpretation differ from the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| RBC transfusion and invasive procedures; no details on how these were assessed | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
| A. RISK OF BIAS | |
|---|---|
| All patients appear to have received the reference standard but little detail on patient flow | |
| Did all patients receive a reference standard? | Unclear |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: low |
Appendix 4 Table of excluded studies with rationale
| Study details | Population | VE test | Study design | Comments |
|---|---|---|---|---|
| Brohi (2009)134 | Trauma | Unclear | Not primary study | |
| Carroll (2009)135 | Trauma | TEG | DTA – outcome | TEG parameters not dichotomised |
| Chevannes (2012)136 | PPH | ROTEM | Unclear | |
| Craft 2008)137 | Trauma | TEG | Correlation | |
| Curry 2010)138 | Trauma | ROTEM | Correlation | |
| Dietrich (1998)139 | Cardiac | Unclear | Unclear | |
| Ducloy-Bouthors140 | PPH | ROTEM | Correlation | PPH case–control; insufficient data to include |
| Ducloy-Bouthors (2012)141 | PPH | ROTEM | Other/unclear comparative | PPH case–control; insufficient data to include |
| Pivalizza (1997)142 | Cardiac | Sonoclot | Correlation | |
| Plotkin (2008)143 | Trauma | TEG | Correlation regression | |
| Forestier (2001)144 | Cardiac | TEG, Sonoclot | Unclear | |
| Grassetto (2012)145 | Trauma | ROTEM | Unclear | |
| Hagemo (2010)146 | Trauma | ROTEM, TEG | Correlation | |
| Huissoud (2009)147 | PPH | TEG | Case–control | Case–control predicting PPH |
| Jeong (2011)148 | Unclear | TEG | Unclear | |
| Johansson (2010)149 | Unclear | Unclear | Not primary study | |
| Karlsson (2013)150 | PPH | TEG | Case–control | PPH case–control; insufficient data to include |
| Kashuk (2010)125 | Trauma | TEG | DTA – outcome | Wrong outcome – thrombosis |
| Levrat (2008)151 | Trauma | ROTEM | DTA – other | Hyperfibrinolysis based on laboratory tests |
| Miles (2007)152 | Cardiac | ROTEM | Unclear | |
| Miyashita (1998)153 | Cardiac | Sonoclot | DTA – other | Correlation only |
| Newland (1987)154 | Cardiac | TEG, Sonoclot | Correlation | |
| Nix (2011)155 | PPH | TEG | Case series | |
| Porite (2004)156 | Cardiac | TEG | Unclear | |
| Rourke (2012)157 | Trauma | ROTEM | DTA – other | FIB based on SLTs as outcome |
| Rugeri (2007)158 | Trauma | ROTEM | DTA – other | |
| Schöchl (2009)159 | Trauma | ROTEM | DTA – outcome; positive test only | Hyperfibrinolysis patients only (test positive on ROTEM) then looked at relationship with mortality |
| Shah (2012)160 | Trauma | TEG | Case series | |
| Shah (2011)161 | Trauma | TEG | DTA – other | |
| Shore-Lesserson (1992)162 | Cardiac | RCT of treatment | ||
| Stanworth (2010)163 | Trauma | Unclear | DTA – outcome | |
| Tanaka (2012)16 | Unclear | ROTEM | Unclear | |
| Tapia (2013)164 | Trauma | TEG | Historical control | Selected patient group; all had received massive transfusion |
| Thai (2011)165 | Cardiac | TEG | Unclear | |
| Traverso (1993)166 | Cardiac | TEG, Sonoclot | Animal study | |
| Woolley (2013)167 | Trauma | ROTEM | DTA – other | |
| Yamada (2007)168 | Cardiac | Sonoclot | Correlation |
Appendix 5 Summary of studies included in the cost-effectiveness review
| Study details | Craig (2008)12 | Davies (2006)90 |
|---|---|---|
| Time horizon | One month for the base case and 1 year for further analyses | One month for the primary analysis, and 1, 10 and 30 years for secondary analyses |
| Objective | To evaluate the clinical effectiveness and cost-effectiveness of using TEG and thromboelastometry analysers compared with SLTs/assays and clinical discretion used alone, to diagnose the cause of unexplained bleeding during or after surgery | To compare patient outcomes, resource use and costs to the NHS and NHS BTA associated with cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion |
| Source of effectiveness information/testing accuracy data | Systematic literature review | Systematic literature review |
| Comparators | 1. SLTs 2. Clinical discretion |
1. PAD 2. EPO 3. PAD plus EPO 4. ANH 5. Cell salvage plus ANH 6. Antifibrinolytics (aprotinin, tranexamic acid, ε-aminocaproic acid) 7. Fibrin sealants 8. Restrictive transfusion thresholds or protocols |
| Reference standard | SLTs | NA |
| Unit costs | Sources: NHS Department of Health, NHS Blood and Transplant Service, Davies (2006),90 Sharma (2000),169 Llewelyn (2004),170 VE manufacturer and clinical experts All costs were adjusted for inflation to reflect costs related to the year 2005–6. The 2006 PSSRU inflation indices for Hospital and Community Health Services171 were used to adjust for costs reported in price years different to 2005–6 |
Sources: NHS reference costs,117 South Manchester University Hospital Trust, NHS BTA, Department of Health reference costs,117 Chartered Institute of Public Finance and Accountancy, manufacturers of cell salvage equipment and clinical experts |
| Measure of benefit | LYs lived and QALYs | QALYs |
| Study type | Cost-effectiveness study | Cost-effectiveness study |
| Model assumptions | 1. Complications related to surgery or transfusion, transfusion-related complications and infection due to bacterial contamination occur during the hospitalisation period 2. For liver transplantation, all patients would receive transfusion 3. In cardiac surgery, probabilities of experiencing transfusion or surgical complications are the same across strategies 4. Mortality rate for patients not transfused is the same for all strategies 5. For patients with no complications or infections, a zero mortality rate during the hospitalisation period is considered 6. Half-cycle correction applied to death events 7. Utility associated to no transfusion is the same as utility associated to transfusion without adverse events 8. A 3-year contract leasing programme is arranged with the manufacturer (to include the costs of a service contract for potential repairs and replacement) 9. On average, the hospital performs 200 tests per year 10. Only one test is performed per patient not requiring transfusion, whereas for those patients requiring transfusion an intraoperative and a post-operative test are conducted 11. The set of SLTs is defined following Scottish Clinical Practice 12. The calculation of the total cost per set of SLTs considers that the costs on the ward to take the blood and record the results are the same 13. Clinical discretion blood component usage is the same as that of SLTs in cardiac surgery. 14. The costs are zero if patients are managed by means of clinical discretion. No laboratory tests or supplies are used in this scenario and any opportunity costs of labour time are negligible 15. Average length of hospital stay is the same across all strategies 16. TE tests are independent of clinical judgement 17. Some of the parameters used to populate the liver transplantation model are based on data used for the cardiac model |
1. The pathways for strategies to minimise blood loss or the need for a blood transfusion and those that rely on transfusion of allogeneic blood are identical 2. The probability of needing a blood transfusion differs between strategies 3. The risk difference between cell salvage and each of the alternative transfusion strategies is the same as the risk difference between each strategy and the control (allogeneic blood) 4. Patients treated by autologous transfusion strategies, who required a transfusion, would have an autologous transfusion first, followed by an allogeneic transfusion if necessary 5. For those strategies that did not use autologous blood, if there were insufficient data to estimate a strategy-specific probability of an adverse event, the probability for the allogeneic comparison was used to approximate the probability of the adverse event 6. The probability of IBCT for PAD transfusion is equal to the probability of IBCT of any blood transfusion 7. If an adverse event was not reported to SHOT, the probability of this event was zero 8. Transfusion-transmitted infections apply only to people having an allogeneic blood transfusion 9. Cost of allogeneic blood in the EPO strategy is equal to that of the allogeneic strategy 10. Adverse events caused by either transfusion or surgery, transfusion only and bacterial contamination would occur within 1 day of the transfusion 11. Additional annual cost for non-disabling stroke is zero 12. With the exception of bacterial contamination, transfusion-transmitted infections were assumed to be diagnosed after discharge from the index admission |
| Perspective | NHS Scotland | NHS |
| Discount rate | NA | NR |
| Uncertainty around cost-effectiveness ratio expressed | NA | The associated likelihood that cell salvage is cost-effective compared with the allogeneic blood transfusion strategy, PAD, PAD plus EPO, FSs, AFs and EPO is > 50% ANH was associated with a probability of being cost-effective compared with cell salvage of around 80% |
| Sensitivity analysis | One-way and multiway (deterministic) | Wherever possible, probability distributions were obtained from the systematic review If not available, minimum and maximum estimates were used to estimate a triangular distribution |
| Outcome (cost and LYS/QALYs) per comparator | TEG and thromboelastometry analysers is the dominant strategy | The net benefit of cell salvage was between £112 and £359 per person, compared with the allogeneic blood transfusion strategy, PAD, PAD plus EPO, FSs, AFs and EPO ANH was associated with a net benefit of £97 compared with cell salvage |
| Summary of incremental analysis | TEG and thromboelastometry analysers is the dominant strategy | Primary analysis: Incremental cost-effectiveness: all cell salvage vs. allogeneic transfusion strategies, all surgical procedures, 1-month time frame:
|
Appendix 6 Drummond assessment for studies included in the cost-effectiveness review
| Quality time | Craig (2008)12 | Davies (2006)90 |
|---|---|---|
| Study design | ||
| The research question is stated | ✓ | ✓ |
| The economic importance of the research question is stated | ✓ | ✓ |
| The viewpoint(s) of the analysis are clearly stated and justified | ✓ | ✓ |
| The rationale for choosing alternative programmes or interventions compared is stated | ✓ | ✓ |
| The alternatives being compared are clearly described | ✓ | ✓ |
| The form of economic evaluation used is stated | ✓ | ✓ |
| The choice of form of economic evaluation is justified in relation to the questions addressed | ✓ | ✓ |
| Data collection | ||
| The source(s) of effectiveness estimates used are stated | ✓ | ✓ |
| Details of the design and results of effectiveness study are given (if based on a single study) | NA | NA |
| Details of the methods of synthesis or meta-analysis of estimates are given (if based on a synthesis of a number of effectiveness studies) | ✓ | ✓ |
| The primary outcome measure(s) for the economic evaluation are clearly stated | ✓ | ✓ |
| Methods to value benefits are stated | ✓ | ✓ |
| Details of the subjects from whom valuations were obtained were given | NA | NA |
| Productivity changes (if included) are reported separately | NA | NA |
| The relevance of productivity changes to the study question is discussed | NA | NA |
| Quantities of resource use are reported separately from their unit costs | ✓ | ✓ |
| Methods for the estimation of quantities and unit costs are described | ✓ | ✓ |
| Currency and price data are recorded | ✓ | ✓ |
| Details of currency of price adjustments for inflation or currency conversion are given | ✓ | ✓ |
| Details of any model used are given | ✓ | ✓ |
| The choice of model used and the key parameters on which it is based are justified | ✓ | ✓ |
| Analysis and interpretation of results | ||
| Time horizon of costs and benefits is stated | ✓ | ✓ |
| The discount rate(s) is stated | NA | ✗ |
| The choice of discount rate(s) is justified | NA | ✗ |
| An explanation is given if costs and benefits are not discounted | ✓ | ✗ |
| Details of statistical tests and CIs are given for stochastic data | ✗ | ✓ |
| The approach to sensitivity analysis is given | Deterministic: ✓ PSA: ✗ |
Deterministic: ✗ PSA: ✓ |
| The choice of variables for sensitivity analysis is justified | Deterministic: ✓ PSA: ✗ |
Deterministic: ✗ PSA: ✓ |
| The ranges over which the variables are varied are justified | Deterministic: ✓ PSA: ✗ |
Deterministic: ✗ PSA: ✓ |
| Relevant alternatives are compared | ✓ | ✓ |
| Incremental analysis is reported | ✓ | ✓ |
| Major outcomes are presented in a disaggregated as well as aggregated form | ✓ | ✓ |
| The answer to the study question is given | ✓ | ✓ |
| Conclusions follow from the data reported | ✓ | ✓ |
| Conclusions are accompanied by the appropriate caveats | ✓ | ✓ |
Appendix 7 Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | i |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | v, vi |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes and study design (PICOS) | 1 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. web address), and, if available, provide registration information including registration number | 15 |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale | 17 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 15, 16 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 16 and Appendix 1 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 18 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 18 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made | 18 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 18 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 18 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis | 18–19 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies) | NA |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were prespecified | 19 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 19, 20 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations | Appendix 2 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see Item 12) | Appendix 3; various sections within results (p. 21) |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group; (b) effect estimates and CIs, ideally with a forest plot | Appendix 2 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including CIs and measures of consistency | Various sections within results (from p. 23) |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | NA |
| Additional analysis | 23 | Give results of additional analyses, if done [e.g. sensitivity or subgroup analyses, meta-regression (see Item 16)] | Various sections within results (from p. 23) |
| Discussion | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. health-care providers, users and policy-makers) | 89 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias), and at review level (e.g. incomplete retrieval of identified research, reporting bias) | 91 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 99, 100 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review | iii |
Glossary
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A theoretical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test that is being evaluated.
- Markov model
- An analytic method particularly suited to modelling repeated events, or the progression of a chronic disease over time.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Meta-regression
- Statistical technique used to explore the relationship between study characteristics and study results.
- Opportunity costs
- The cost of foregone outcomes that could have been achieved through alternative investments.
- Prediction study
- Study that evaluates the ability of a variable to predict an outcome.
- Publication bias
- Bias arising from the preferential publication of studies with statistically significant results.
- Quality of life
- An individual’s emotional, social and physical well-being and their ability to perform the ordinary tasks of living.
- Quality-adjusted life-year
- A measure of health gain, used in economic evaluations, in which survival duration is weighted or adjusted by the patient’s quality of life during the survival period.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity which result from varying the diagnostic threshold.
- Reference standard
- The best currently available diagnostic test, against which the index test is compared.
- Sensitivity
- Proportion of people with the target disorder who have a positive test result.
- Specificity
- Proportion of people without the target disorder who have a negative test result.
- Viscoelastic test
- A test that uses a viscoelastic method, either thromboelastometry or thromboelastography, to test for haemostasis.
List of abbreviations
- A10
- amplitude 10 minutes after clotting time
- ACT
- activated clotting/coagulation time
- aPTT
- activated partial thromboplastin time
- ARDS
- acute respiratory distress syndrome
- AUC
- area under the curve
- CABG
- coronary artery bypass graft
- CADTH
- Canadian Agency for Drugs and Technologies
- CCT
- controlled clinical trial
- CEAC
- cost-effectiveness acceptability curve
- CFT
- clot formation time
- CI
- confidence interval
- CPB
- cardiopulmonary bypass
- CRD
- Centre for Reviews and Dissemination
- CT
- clotting time
- DVT
- deep-vein thrombosis
- EACTA
- European Association of Cardiothoracic Anaesthesiologists
- EVPI
- expected value of perfect information
- FFP
- fresh frozen plasma
- FIB
- fibrinogen
- gbACT
- glass bead-activated test
- HAV
- hepatitis A virus
- HBV
- hepatitis B virus
- HCT
- haematocrit
- HCV
- hepatitis C virus
- HES
- Hospital Episode Statistics
- HIV
- human immunodeficiency virus
- HTA
- Health Technology Assessment
- HTLV
- human T-cell lymphotropic virus
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- INR
- international normalised ratio
- IQR
- interquartile range
- ISI
- international sensitivity index
- ISS
- injury severity score
- kACT
- kaolin-activated clotting/coagulation time assay
- LoS
- length of stay
- LY
- life-year
- MCF
- maximum clot firmness
- ML
- maximum lysis
- MOF
- multiple organ failure
- NICE
- National Institute for Health and Care Excellence
- NR
- not reported
- OR
- odds ratio
- PCC
- prothrombin complex concentrate
- PLT
- platelet count
- POC
- point of care
- PPH
- post-partum haemorrhage
- PR
- prothrombin ratio
- PRBC
- packed red blood cell
- PSA
- probabilistic sensitivity analysis
- PT
- prothrombin time
- PTP
- post-transfusion purpura
- PTT
- partial thromboplastin time
- QALY
- quality-adjusted life-year
- RBC
- red blood cell
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- RR
- relative risk
- SD
- standard deviation
- SE
- standard error
- SHOT
- Serious Hazards of Transfusion
- SIRS
- systemic inflammatory response syndrome
- SLT
- standard laboratory test
- TEG
- thromboelastography
- TRALI
- transfusion-related acute lung injury
- vCJD
- variant Creutzfeldt–Jakob disease
- VE
- viscoelastic
- WHO
- World Health Organization
