Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/91/36. The contractual start date was in June 2011. The draft report began editorial review in November 2014 and was accepted for publication in March 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Paul Aveyard has done ad hoc consultancy and research for the pharmaceutical industry on smoking cessation.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Blyth et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction and objectives
Smoking remains the leading preventable cause of premature deaths in the world. 1 Cigarette smokers lose about 10 years of lifespan compared with people who never smoke, although the risk of excess mortality can be considerably reduced by stopping smoking. 2 It is estimated that tobacco use was responsible for about 100 million deaths globally in the 20th century,3 and it kills about 6 million people each year in the 21st century. 1
Among adults (aged ≥ 16 years) in England, the prevalence of smoking was 20% in 2010, which was considerably lower than in 2000 (27%), and much lower than in 1980 (39%). 4,5 Two-thirds of current smokers in England report wanting to quit smoking and three-quarters have tried to do so in the past. Compared with many other countries, the percentage of former smokers among ever-smokers (former or current) in adults is relatively high in England, about 57% for men and 51% for women. 4–6
Pharmacotherapy and behavioural support are effective in helping motivated smokers to stop smoking,7–10 although relapse rates following these interventions are high. 11 Since 2001, a national network of NHS Stop Smoking Services has been established in England to provide behavioural support and pharmacotherapy to smokers who would like to quit. The English Stop Smoking Services were overseen by primary care trusts until April 2013, and have been overseen by local authorities since then. In 2010/11, 787,527 people (8% of all smokers) used NHS Stop Smoking Services, which generated 269,293 biochemically validated quitters (34% of those who set a quit date) at 4 weeks after the quit date. 12 However, about 75% of 4-week quitters go back to regular smoking after between 4 and 52 weeks. 13 The long-term success rates still make these interventions highly cost-effective14 but there is a need to find effective interventions to reduce relapse rates after the initial treatment episode.
Interventions for smoking relapse prevention
Interventions for smoking relapse prevention are generally based on the cognitive–behavioural approach to coping skills training15 and may be considered as complex health-care interventions with multiple interacting components. 16,17 For the development and evaluation of complex interventions, we need a good theoretical understanding about how the intervention causes change. 17 With the coping skill training approach, quitters are trained to anticipate situations associated with high risks of smoking relapse (such as going out with friends or feeling frustrated), and to develop skills to cope with such situations and urges to smoke again. Therefore, the effectiveness of coping skills training for relapse prevention will depend on (1) the delivery and receipt of interventions, (2) the acquiring of coping skills by quitters and (3) the use of such skills in high-risk situations. To benefit from coping skills training, quitters need to learn, practise and implement coping skills when required. 18
A Cochrane systematic review of trials of smoking relapse prevention found insufficient evidence to support the use of any specific intervention for preventing smoking relapse in short-term quitters. 19 The current smoking cessation guidelines do not recommend any specific interventions for smoking relapse prevention. 20,21 According to findings from a survey of smoking cessation professionals, the uncertain evidence base about effectiveness was an important barrier to the use of relapse prevention interventions in stop smoking practice. 22
We conducted an exploratory meta-analysis in 2009 using data from 49 trials on psychoeducational interventions for smoking relapse prevention. 23 The meta-analysis showed that coping skills training interventions significantly reduced smoking relapse in community quitters who had stopped smoking for at least 1 week at baseline [odds ratio (OR) 1.44, 95% confidence interval (CI) 1.14 to 1.81], although it was ineffective for current smokers, pregnant or postpartum quitters, hospitalised ex-smokers, forced short-term quitters and smokers with mental illness or drug abusers. 23 Therefore, it seemed that coping skills training may be effective in secured quitters who are highly motivated to remain abstinent. In addition, available evidence indicated that self-help educational materials may be as effective as interventions based on individual or group counselling for smoking relapse prevention. The pooled OR of relapse prevention associated with coping skills training was 1.46 (95% CI 1.05 to 2.05) for self-help material trials and 1.41 (95% CI 1.02 to 1.94) for counselling trials. 23 A systematic review conducted by an independent team also found that written self-help materials were efficacious for preventing smoking relapse in unaided quitters. 24
Forever Free booklets
Brandon and colleagues developed a series of eight booklets to be used as self-help materials for smoking relapse prevention and have evaluated the booklets in two randomised controlled trials in the USA. 25,26 Volunteers who had quit smoking unaided were randomised to receive either all eight booklets or only the introductory booklet. Participants who received all eight booklets had a lower rate of smoking relapse than participants who received only a single booklet (the introduction booklet). One of the two randomised studies of Forever Free booklets found that repeated mailing (high contact) was no more effective than massed mailing (low contact) of the eight booklets. 26 It has been suggested that the true effectiveness of Forever Free booklets might have been underestimated because participants in the control group received the introduction booklet that provided a summary of all relevant skills. The use of the Forever Free booklets for smoking relapse prevention was likely to be highly cost-effective [US$83–US$160 per quality-adjusted life-year (QALY) gained]. 26
Objective
Existing trials on coping skills training for smoking relapse prevention in community quitters recruited unaided quitters mainly by advertisement in newspapers and were mostly conducted in the USA. It is uncertain whether or not the results of meta-analysis23 and randomised controlled studies in the USA25,26 are generalisable to 4-week quitters who used the NHS Stop Smoking Services. A Health Technology Assessment (HTA) report recommended further research on the effectiveness of self-help interventions for smoking relapse prevention. 27
The objective of this randomised controlled study was to evaluate the clinical effectiveness and cost-effectiveness of self-help materials (Forever Free booklets) in preventing smoking relapse in 4-week quitters who have used NHS Stop Smoking Services.
Chapter 2 Methods and design
The study design was a parallel-arm pragmatic individually randomised controlled trial to evaluate the effectiveness of self-help educational material (Forever Free booklets) in preventing smoking relapse, compared with a smoking cessation booklet used currently. The trial protocol was published in an open access journal. 28
This was an open trial, without attempts to blind investigators and patients after randomisation. Because the outcome assessor and trial participants know the allocated intervention, bias may be introduced into the results. However, evidence suggested that the risk of bias may be much reduced in trials with objectively assessed outcomes. 29 In this trial, the primary outcome was biochemically verified abstinence at 12 months, which can be considered an objectively assessed outcome.
Setting
We planned to recruit 4-week quitters in NHS Stop Smoking Clinics in Norfolk, using specialist stop smoking advisors (these are specially trained stop smoking advisors who work exclusively in delivering smoking cessation advice and support). In comparison with all of England, Norfolk has a relatively high percentage of people aged 65 years and above (21.4% vs. 16.5% in 2010) and has a higher percentage of European white people (94.3% vs. 87.5% in 2009). Of those who set a quit date in 2010/11, the percentage of self-reported successful quitters was 52%, compared with an average of 49% in England. 30
After 7 months of recruitment, the number of participants recruited was under target and it was clear that we would not be able to achieve the study recruitment target from the specialist advisors in Norfolk alone. Consequently, recruitment was extended to include level 2 advisors (these are health-care professionals such as pharmacists, practice nurses or health-care assistants who are also trained in administering smoking cessation advice and support) in general practice and/or community pharmacies in Norfolk that had recruited 12 or more carbon monoxide (CO)-verified quitters in 2010/11. Participant recruitment was later further extended to include 4-week quitters from Suffolk, Hertfordshire, Lincolnshire, and Great Yarmouth and Waveney.
The investigated self-help educational materials were posted to trial participants for their use at home. At the final follow-up (12 months after quit date), participants who reported that they had not smoked during the previous 7 days were invited to have a breath CO test to confirm the self-reported status of non-smoking. The test was carried out by a researcher from the University of East Anglia (UEA) in Norwich. Study participants came to a clinic at the UEA, or a researcher visited them at home for this test.
Participant recruitment
Smokers who are motivated to quit are referred to the NHS Stop Smoking Clinics from various sources, such as by general practitioners (GPs), or are self-referred. Clients who contact the Stop Smoking Service are given an appointment for assessment with a stop smoking advisor, either individually or in group sessions. Following assessment, the client typically receives weekly behavioural support, focused on withdrawal-oriented therapy, with medication to reduce craving and withdrawal. The total contact time for each client is at least 1.5 hours from pre-quit preparation to 4 weeks after quitting. The self-reported abstinence at 4 weeks after the quit date is about 50%, and the biochemically verified abstinence ranges from 31% to 36%. 12 However, most (75%) self-reported 4-week quitters will relapse by 12 months. 13
The target population for this trial was CO-verified, 4-week quitters treated in NHS Stop Smoking Clinics who can read English and could give informed consent. According to NHS Stop Smoking Services guidance,31 the biochemically verified 4-week quitter is defined as ‘a treated smoker whose CO reading is assessed 28 days from their quit date (–3 or +14 days) and whose CO reading is less than 10 ppm (parts per million)’. That is, a face-to-face follow-up interview should be normally conducted 4 weeks from the quit date. Where a follow-up at 4 weeks is impossible, it should be conducted 25–42 days after the quit date (the Russell Standard). 31
Inclusion criteria
-
Carbon monoxide-verified 4-week quitters in the NHS Stop Smoking Service clinic who sign the consent form were eligible to participate in the trial.
Exclusion criteria
-
Pregnant quitters were excluded. The process of relapse in pregnant women is very different from non-pregnant smokers. According to the available research evidence,23 smoking relapse prevention intervention by coping skills training is ineffective for women who have stopped smoking during pregnancy.
-
We excluded quitters who were not able to read the educational material in English because the revised booklets are available in English only.
-
We excluded quitters from families at the same address, as a family member has already been included in the trial.
-
Quitters younger than 18 years were not included.
Training of stop smoking advisors for participant recruitment
For participant recruitment, stop smoking advisors introduced the study, gained consent for participation from their clients and collected baseline data. For this role, they received a half-day’s training. This consisted of:
-
outline of study, including flow diagram
-
baseline questionnaire, including group activity: completing baseline questionnaire
-
informed consent
-
spot the mistakes: demonstration by co-ordinators of how not to consent participation
-
group activity role-play: undertaking informed consent
-
distribution of study materials.
The recruitment period was 24 months, so it was important to sustain the recruiters’ interest for the duration of this period and engender their ownership of the project. Consequently, training continued throughout the whole study. Co-ordinators regularly attended the stop smoking advisors’ team meetings, where ongoing training was given to reinforce the study procedures, along with reporting of study updates. In addition, two evening refresher sessions were held, which consisted of:
-
smoking cessation research evidence
-
study updates
-
role-play demonstrating recruitment techniques
-
quiz about targeted study procedures
-
opportunity for suggestions from recruiters about how to optimise recruitment.
When recruitment was extended to include level 2 advisors, and other counties, initial training was delivered by the co-ordinators to small groups or one to one; sometimes this was then cascaded down to other advisors at the recruitment sites. All recruiters signed a training record on completion of the initial training.
Trial procedures and randomisation
Stop smoking advisors completed a study screening form for each of their clients, and introduced the study to them, by giving them a participant information sheet, containing information on the trial. Then, at the final session in the Stop Smoking Clinic (4-week post quit, when all self-reported quitters undergo a breath CO test), the advisor again explained the nature of the trial to CO-verified quitters only, answered questions from them, and invited them to participate in the trial by signing the consent form. Clients who had failed to quit were not invited to participate in the study.
After eligible quitters had signed the consent form, stop smoking advisors asked participants to complete the baseline questionnaire, which they sent to the trial co-ordinator at the UEA, along with the screening form and a copy of the consent form. On receipt of these documents, the trial co-ordinator or trial administrator randomly allocated participants to two groups (the treatment and control group), using a computerised allocation system provided by the Norwich Clinical Trials Unit (CTU). We adopted a simple randomisation process, without attempts to stratify by participant characteristics. This allocation of trial participants was concealed because the recruitment of quitters occurred before the random allocation.
As it was necessary to avoid possible information contamination across the trial arms and non-independence between members of the same family or household, we screened all eligible participants and checked their address against recruited participants to ensure that no two people from the same household were recruited. Where two people of the same family or household were found eligible and willing to participate at the same time, we randomly selected only one of them to participate in the trial. If the members of the same family used the NHS Stop Smoking Service at different times during the trial recruitment period, we included only the first family member.
We expected that many people would make repeated attempts to quit using the Stop Smoking Service during the trial period, so, to ensure that clients participated in the study only once, stop smoking advisors checked if the ‘new’ quitter had already been included in the trial. In addition, the trial co-ordinators and/or administrator also checked this to make sure a quitter was not already recruited onto the study.
Interventions investigated
The experimental intervention tested in the trial was the full pack of eight Forever Free booklets. Findings from an exploratory meta-analysis suggested that coping skills training may be effective for smoking relapse prevention in motivated quitters. 23 The results of two clinical trials in the USA suggested that a cheap intervention using Forever Free booklets25,26 may be as effective as more expensive counselling for coping skills training. Forever Free booklets include a series of eight booklets (Table 1). Booklet 1 (with 17 pages) is a brief summary of all relevant issues, including an introduction of nicotine dependence, the stages of smoking cessation, situations that are high risk for relapse, ways of coping with urges to smoke, the abstinence violation effect and ways to handle an initial slip. The remaining seven booklets provide more extensive information on important issues for relapse prevention, entitled Smoking Urges; Smoking and Weight; What if You Have a Cigarette?; Your Health; Smoking, Stress, and Mood; Lifestyle Balance; and Life without Cigarettes. The booklets can be understood by people with a reading level of fifth to sixth grade in the USA (expected reading level for children aged 10–12 years).
| Material | Contents (booklets) or headings (leaflet) |
|---|---|
| Experimental intervention: Forever Free: A Guide to Remaining Smoke Free | |
| Booklet 1: An Overview (16 pages) | About Forever Free; Seven facts about smoking and quitting; The stages of quitting; ‘Risky’ situations for ex-smokers; How to handle urges to smoke; A non-smoking lifestyle; What if you DO smoke; The most important messages |
| Booklet 2: Smoking Urges (11 pages) | What are urges?; Different types of urges; How to deal with urges to smoke; When will the urges end?; Exercises; Remember . . . ; Notes |
| Booklet 3: Smoking and Weight (15 pages) | Why a booklet on weight control after quitting?; Who gains weight?; Why do ex-smokers gain weight?; Do I have to gain weight?; Effects of smoking and weight gain on health and looks; Weight control after quitting; Exercise; Make exercise part of your day; Summary |
| Booklet 4: What if I Have a Cigarette? (7 pages) | Can’t I have just one cigarette? Be prepared for a slip; Watch out for the effects of a slip; Keep a slip from turning into a full relapse; Summary |
| Booklet 5: Your Health (11 pages) | Why this booklet?; How harmful is smoking?; What makes smoking so harmful?; What happens when you quit smoking?; Quitting smoking helps others, too; How can this information help you stay quit?; If you are smoking again |
| Booklet 6: Smoking, Stress and Mood (11 pages) | What causes stress?; What is stress?; How is stress related to smoking?; What leads up to a cigarette; So, why not smoke when stressed?; Better ways to deal with stress and negative moods |
| Booklet 7: Lifestyle Balance (15 pages) | Stress; ‘Shoulds’ versus ‘Wants’; Your daily hassles; Your ‘Shoulds’; Your ‘Wants’; Positive addictions; Summary; Pleasant events list |
| Booklet 8: Life without Cigarettes (11 pages) | Urges; Benefits of quitting; But what about my weight; If you do smoke; In closing |
| Control leaflet: Learning to Stay Stopped | |
| Learning to Stay Stopped (including covers, eight pages; recommended 10 minutes to read through) | Congratulations on stopping smoking!; Learning to stay stopped; Nicotine withdrawal; Psychological dependence; Having doubts about quitting?; Constantly thinking about smoking?; Worried about weight gain?; Bored with quitting?; Over reacting to things?; Strong cravings?; Tempting triggers?; Tired of dealing with triggers?; Smoked a cigarette? (Note: provided a short list about ‘What you can do!’ following each of the above problems) |
The original Forever Free Booklets were prepared for users in the USA. We revised and updated the booklets in places where it was judged necessary or helpful, to make the material more suitable to British users and the UK NHS. We obtained permission from the copyright holders (H Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA). Members of the Trial Steering Committee, and of the project team and three lay representatives reviewed and commented on the revised booklets. During their modification, we ensured the acceptability and understanding of the booklets to as wide a range of users as possible. The revised booklets are currently available only in English because of the considerable cost implications of translating the booklets into other languages.
After randomisation, the research team sent the corresponding self-help material by post to participants’ homes. Participants in the intervention group received a letter and all eight Forever Free booklets, which were posted by normal delivery. The first 10 intervention packages were posted by special delivery to confirm that the booklets could be posted through participants’ letter boxes; no delivery was returned, confirming that the booklets were successfully delivered. Participants in the control group received a letter and a booklet about smoking cessation, Learning to Stay Stopped. 32 The leaflet (with eight pages in total) used in the control group contains brief but comprehensive information on issues related to smoking relapse and also provides brief recommendations on how to cope with cravings and tempting triggers (see Table 1). It is commonly used in NHS Stop Smoking Services.
Sample size calculation
Without smoking relapse prevention, about 75% of CO-validated 4-week quitters would go back to regular smoking between 4 and 52 weeks. 13 An exploratory meta-analysis indicated that coping skills training interventions may reduce smoking relapse in community quitters who had stopped smoking for at least 1 week (OR 1.44, 95% CI 1.14 to 1.81). 23 Therefore, the abstinence rate of 4-week quitters at 12 months was estimated to be 25.0% in the control and 32.4% in the intervention group (based on an OR of 1.44). Assuming α = 0.05 (type 1 error) and 1 – β = 0.8 (statistical power), about 590 participants were required in each arm. 33 Assuming a dropout rate of 15%, about 700 participants were required in each arm (1400 in total).
Outcomes and data collection
At the session about 4 weeks (or 25–42 days)31 after the quit date, stop smoking advisors gathered baseline information from participants who had consented to participate in the trial. The information collected included participants’ demographic characteristics, smoking and quitting history, and level of determination and confidence to remain abstinent.
The specified primary outcome was prolonged, CO-verified smoking abstinence from months 4 to 12, with no more than five cigarettes being smoked during this period. This is in keeping with the consensus statement of the Society for Research on Nicotine and Tobacco, and the Russell Standard. 34,35 The usual definition of prolonged abstinence allows a 2-week grace period following quit day, in which lapses do not invalidate abstinence, to assess the outcome of aid to cessation trials. However, a relapse prevention intervention might prevent lapses (occasional smoking) becoming relapses (regular smoking and abandonment of the attempt to keep abstinent). Therefore, we used a 2-month grace period during which lapses to smoking do not count against achieving abstinence. As participants on enrolment were abstinent at 4 weeks after quit date, this equated to the primary outcome being prolonged abstinence from 4 months to 12 months after quit date. Following the Russell Standard, the primary outcome was prolonged abstinence from months 4 to 12, with smoking of no more than five cigarettes in total, and confirmed by CO < 10 p.p.m. at the 12-month follow-up.
The secondary outcomes were 7-day self-report abstinence at 3 months, 7-day self-report and CO-validated abstinence at 12 months post quit date. We did not specify continuous abstinence from 2 to 12 months post quit date as a secondary outcome in the trial protocol. Because continuous abstinence from 2 to 12 months was used in previous studies, we added it as an outcome measure in the stage of data analysis, in order to compare results between different studies. The continuous abstinence was defined as CO-verified smoking abstinence at 12 months and no more than five cigarettes from 2 to 12 months after the quit date.
We assessed cost-effectiveness from an NHS perspective. We collected data on the resource use associated with self-help materials (including intellectual property, adaptation, printing and postage), any additional Stop Smoking Services and cessation products at follow-up interviews. Our hypothesis was that the intervention, if effective, would improve abstinence rates, would reduce repeated use of Stop Smoking Services and might reduce use of other health care. Other resources which might be affected by the intervention were also monitored, including GP visits and hospital admissions. The European Quality of Life-5 Dimensions-3 Level (EQ-5D-3L)36 was used to estimate the benefits in terms of the QALY gain associated with each intervention during the study period.
Study participants were followed up by a researcher from the UEA 2 months after recruitment (which was 3 months after quit date) and again 11 months following recruitment (12 months after quit date). The follow-up interviews were conducted by phone and involved the researcher administering a questionnaire which asked questions about the participant’s smoking status, process variables and the use of stop smoking and other NHS services.
At 3 months after quit day (2 months after enrolment), a researcher from the UEA telephoned participants primarily to assess process measures, that is receipt, liking and use of the booklets, and to assess key skills; the booklets were intended to teach in both the intervention and the control groups. If the intervention was ineffective, this would help us to assess whether the intervention was used but people did not acquire the skills or applying the skills was ineffective in preventing relapse. This early follow-up contact was important because of the high risk of relapse during the first few months.
The second and final follow-up telephone call (conducted by a researcher from the UEA) took place at 12 months after quit day (11 months after enrolment), to assess the primary and secondary outcomes and to assess coping skills acquired. Participants who met the self-report criteria for at least 7-day abstinence were invited to attend a local centre to prove this by exhaled CO. People came to a clinic at the UEA or a researcher visited them at home for this test. To optimise CO test rates, we offered a shopping voucher (valued at £20) to each of the participants who attended the CO test at 12 months. If participants found it difficult to travel to the UEA, then researchers arranged to visit them at home, at a time convenient to the participant, including at evenings and weekends (46% of Norfolk CO tests were undertaken at participants’ homes). At non-Norfolk sites, local testing was arranged for the CO tests: at stop smoking shops in Lincolnshire, in participants’ homes in Suffolk and by local primary care research network nurses in Hertfordshire.
When arranging CO tests, if participants were willing but unable to commit to a time during the follow-up phone call, researchers phoned participants at a later date. If contact was not successfully made by phone, researchers sent a letter to participants inviting them to phone the research team to make a CO test appointment. When an appointment was successfully booked, participants were sent a letter confirming the appointment arrangements. To minimise non-attendance at CO test appointments, participants were sent a postcard a few days before the appointment and then phoned on the morning of the appointment. Travelling expenses were reimbursed to participants and a £20 ‘Love2shop’ shopping voucher (for use in many high-street shops) was given, in recognition of their time.
To optimise follow-up, researchers recorded all phone call attempts to participants (ranged from one to seven phone calls), and tried different days and varying times of days to be able to talk to participants, including evenings and Saturdays. Researchers also sent text messages to participants to arrange follow-ups, which also helped to increase follow-up response. If researchers were unsuccessful in contacting a participant by phone, they would then post the questionnaire, with a stamped addressed envelope, to the participant’s home. If there was no response within 2 weeks, then a second questionnaire would be posted; 5.3% of 3-month questionnaires were received by post and 6.8% of 12-month questionnaires. When completed postal questionnaires were received, the participants were posted a brief letter of thanks from the research team.
Data management and analysis methods
Paper data were held in accordance with the Data Protection Act 1998. 37 The questionnaire data were identified by a unique study number, securely held in a locked filing cabinet, in a locked research office, in a corridor of research/academic offices with restricted swipe access; participants’ personal information was kept in a separate locked filing cabinet. Two Microsoft Excel® databases (2013; Microsoft Corporation, Redmond, WA, USA) were created for the study by the Norwich CTU, one containing participants’ personal information and the other questionnaire data. Data entry was carried out by the UEA research team, and a 10% data entry check was undertaken. Data analyses were conducted using Stata software (Stata/IC for Windows, StataCorp LP, College Station, TX, USA).
The comparison of smoking abstinence rates (and any other binary outcomes) between the two trial groups was carried out using OR and its 95% CI as the measure of treatment effect, although estimated risk ratio was also reported for the primary end point. Participants who declined biochemical verification or who did not respond to follow-up were classified as smokers. However, participants who died or were known to have moved away were excluded from the numerator and denominator. Because there were no significant differences in missing data between the groups, we did not use imputation methods to assess the robustness of primary analyses. 38
We planned to compare survival curves of the intervention and control arm, by using data on time to the first event of smoking relapse. This secondary analysis was not conducted because of inadequate reporting of time the first relapse occurred by a large proportion of relapse participants.
We used exploratory subgroup analyses and logistic regression analyses with interaction terms to investigate possible effect-modifying variables, including age, gender, socioeconomic status, level of nicotine dependence, number of prior quit attempts and use of pharmacological interventions.
The analysis of mediating variables examined hypothesised mechanisms of the intervention. The effectiveness of coping skills training for relapse prevention depends on (1) the adequate delivery and receipt of the booklets, (2) the acquisition of coping skills by quitters and (3) the application of such skills in high-risk situations. This intervention mechanism suggests certain important process variables that should be investigated in the trial. At the follow-up interviews, we asked trial participants if they had received the booklets and if they had read the booklets (and how much time spent and how many booklets looked at). We then investigated whether or not the use of booklets helped the acquisition of coping skills by the participants, in terms of improved capability to identify risky situations and to know more appropriate ways of handling urges to smoke again. We then asked the trial participants whether or not they had actually applied the skills learnt from the booklets. Finally, we invited the trial participants to give an overall assessment of the usefulness of the booklets. We also planned to use more complex methods for the exploratory mediation analysis according to MacKinnon and Fairchild39 but did not because there were no differences in the primary or any secondary outcomes between the treatment and control group.
Qualitative process evaluation
We conducted a qualitative process evaluation using data collected as part of the trial telephone follow-up interviews and a further qualitative substudy of in-depth data collection. Methods used for the qualitative process evaluation are described in Chapter 6.
Economic evaluation methods
Using data from the trial, we calculated the mean incremental cost for those in the intervention arm (Forever Free booklets), compared with the control arm. As part of a cost–utility analysis, the incremental QALY gain associated with the intervention was estimated, based on EQ-5D data collected in the trial. Chapter 7 provides more details about the economic evaluation methods used in the trial.
Ethical arrangements
No adverse effects or harm on target population or society was expected from the intervention. We provided sufficient information for 4-week quitters to consider whether or not they would like to participate in the trial and recruited only those who signed the consent form. Data on individual participants remained strictly confidential. Only the project researchers directly involved in the trial had access to participants’ personal data during the study. Research ethical approval was granted by East of England Research Ethics Committee (11/EE/0091, approval received on 20 April 2011), and any protocol amendments were approved by the Research Ethics Committee.
Project management
A Trial Steering Committee was established to provide overall supervision for the study on behalf of the National Institute for Health Research (NIHR) HTA programme. The trial was overseen by a trial management group, based at the UEA, including all co-investigators. The trial management group met every 3 months to monitor and manage the trial and to review an ongoing Consolidated Standards of Reporting Trials statement (including data on recruitment, intervention and follow-up). The trial project team, consisting of the trial co-ordinators, project administrator and chief investigator, met weekly to monitor the day-to-day progress and management of the trial.
No adverse effects were expected to be associated with the educational booklets for smoking relapse prevention. This trial posed no substantial risks to participants and there would be no cause to propose stopping rules for premature closure of the trial. In addition, this trial is open and unblinded. After a discussion with the NIHR HTA programme, we decided not to have a separate data monitoring committee.
Three members of the public contributed to the revision of the Forever Free booklets, and one lay representative was on the trial steering committee.
Chapter 3 Main results
Participant flow
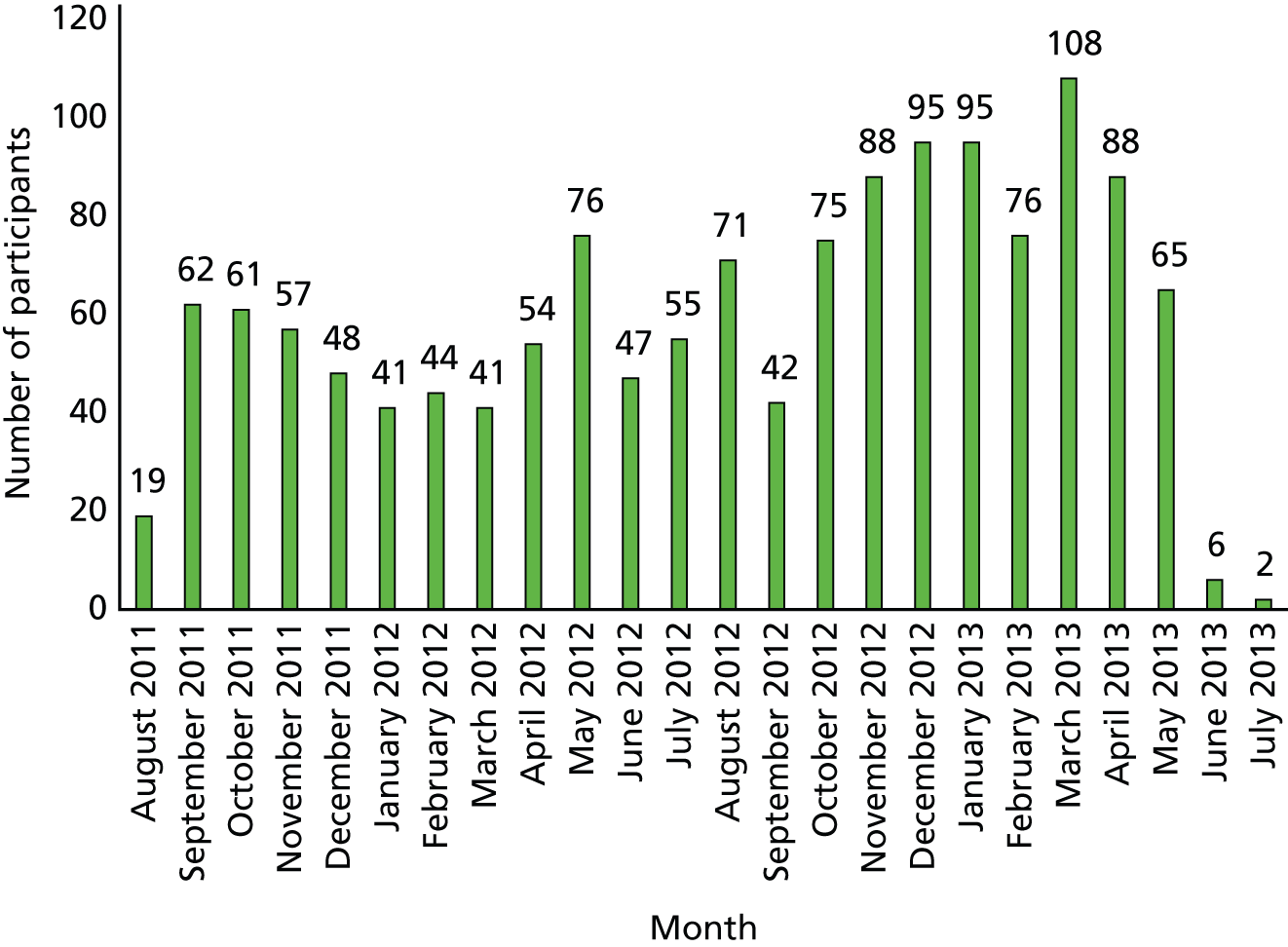
The study started in June 2011. We used 2 months to revise and print the Forever Free booklets, and to arrange trainings for stop smoking advisors who were involved in the recruitment of 4-week quitters. Participant recruitment started in August 2011. We initially recruited CO-validated 4-week quitters from the core Stop Smoking Clinics (level 3 stop smoking advisors) in Norfolk. By January 2012, we realised that the recruitment rate was slower than expected. From May 2012, the participant recruitment was expanded to level 2 stop smoking advisors and to new sites in Norfolk, Suffolk, Hertfordshire, Lincolnshire, and Great Yarmouth and Waveney. By June 2013, the recruitment target was achieved and the trial included a total of 1416 short-term quitters. The follow-up of participants, including CO test, was completed by July 2014.
Table 2 shows the number of participants by area and service type, and Figure 1 shows the number of participants recruited by month.
| Area | Specialist service | General practice | Health trainer | Pharmacy | Total |
|---|---|---|---|---|---|
| Norfolk | 1040 | 97 | 57 | 14 | 1208 |
| Suffolk | – | 57 | – | 18 | 75 |
| Hertfordshire | – | 68 | – | 7 | 75 |
| Lincolnshire | 51 | – | – | – | 51 |
| Great Yarmouth and Waveney | 7 | – | – | – | 7 |
| Total | 1098 | 222 | 57 | 39 | 1416 |
FIGURE 1.
Number of participants recruited by month.
The participant flow diagram is shown in Figure 2. From August 2011 to June 2013, there were a total of 1959 short-term quitters who were considered potentially eligible by stop smoking advisors. The number of eligible quitters who declined to participant in the studies was 370 (19%), for various reasons. Before randomisation, 173 (9%) were excluded for the following reasons: pregnancy (n = 26), family member in study (n = 71), unable to read (n = 41), prior participant (n = 34) and other (n = 1).
FIGURE 2.
Participant flow diagram. a, unable to read – literacy or disability (including dyslexia, learning difficulties and poor eyesight) = 20, limited English = 19, no further details = 2.
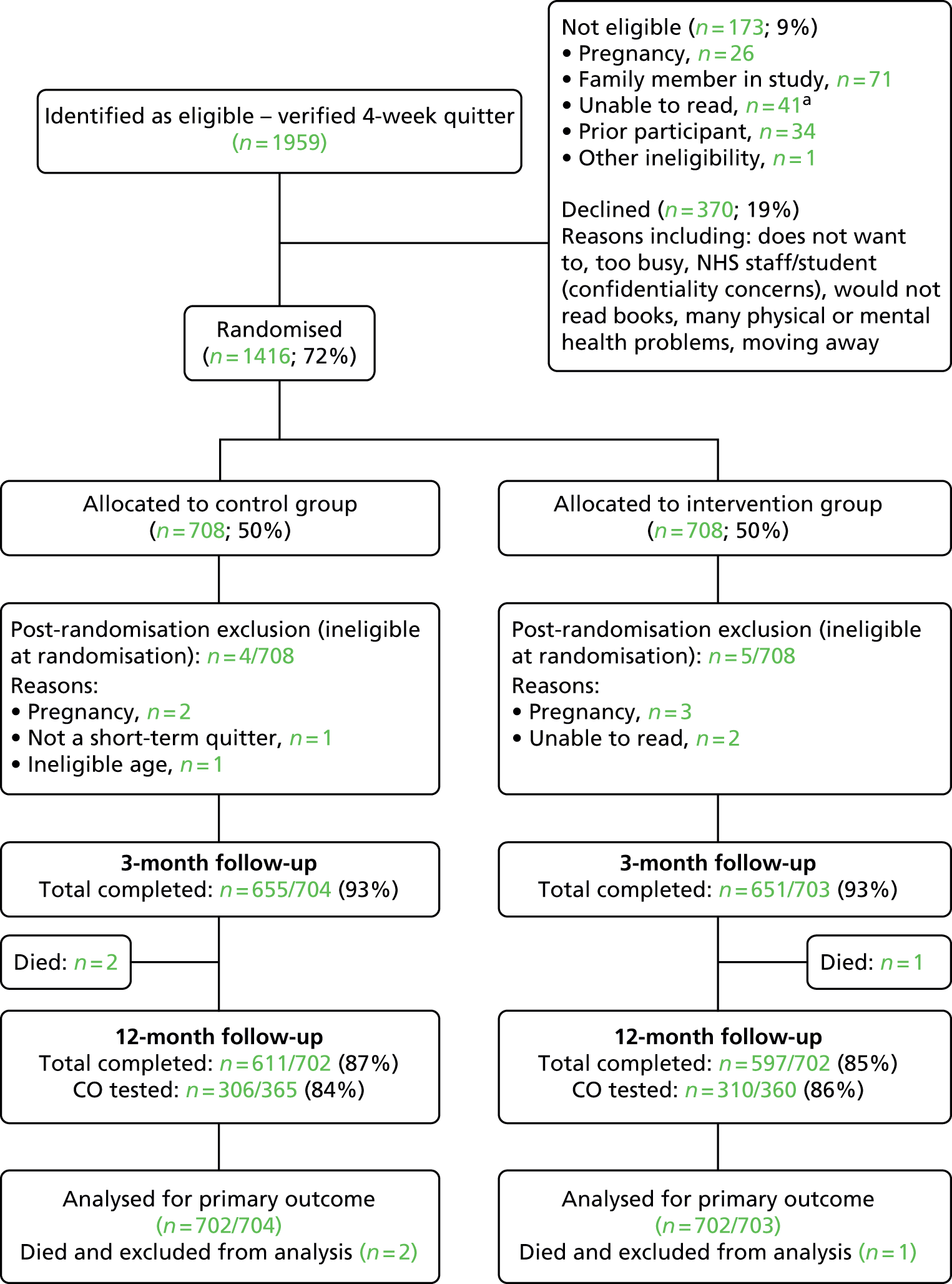
We randomly allocated 1416 short-term quitters to the intervention and the control group. After randomisation, four participants in the control group and five in the intervention group were found to be ineligible because of pregnancy at baseline, inability to read or ineligible age at randomisation. In addition, three participants in the intervention group and none in the control group withdrew from the study because of illness or other reasons (these three participants were included in analysis, with the assumption that they had started smoking again).
The follow-up rate was 93% in both the intervention (651 out of 703) and the control group (655 out of 704), at the 3-month follow-up. Two participants in the control group and one in the intervention group died before the 12-month follow-up, and these three participants were excluded from further analyses. The follow-up rate was 85% (597 out of 702) in the intervention group and 87% (611 out of 702) in the control group at the final 12-month follow-up (11 months after randomisation). The median interval between the CO verification before randomisation and the 3-month follow-up was 60 days [mean 60.6 days, standard deviation (SD) 7.6 days], and the mean interval between the CO test before randomisation and the 12-month follow-up was 334 days (mean 335.6 days, SD 11.4 days).
At the final follow-up, 360 participants in the intervention group and 365 in the control group reported abstinence in the previous 7 days and eligibility for a CO test. Verification tests were carried out for 616 of these participants, 86% in the intervention and 84% in the control group, while 109 participants declined or were unable to have the test.
The baseline characteristics of participants
The main demographic characteristics at baseline are presented in Table 3. The participants in the two groups were comparable at baseline in terms of age, sex, marital status, ethnic origin, education, employment status, English being the first language and receipt of free prescriptions. The percentage of participants who were unemployed was 10% and more than half (56%) were in receipt of free prescriptions.
| Demographic characteristic | Intervention (n = 703) | Control (n = 704) |
|---|---|---|
| Age (years), mean (SD) | 47.8 (14.1) | 47.9 (13.6) |
| Sex (female), n (%) | 381 (54.2) | 360 (51.1) |
| Marital status, n (%) | ||
| Married/living with partner | 444 (63.2) | 423 (60.1) |
| Separated/divorced | 110 (15.6) | 114 (16.2) |
| Single | 118 (16.8) | 138 (19.6) |
| Other/unknown | 31 (4.4) | 29 (4.1) |
| Ethnic origin, n (%) | ||
| European white | 690 (98.2) | 695 (98.7) |
| Other | 11 (1.6) | 8 (1.1) |
| Unknown | 2 (0.3) | 1 (0.1) |
| English the first language, n (%) | ||
| Yes | 681 (96.9) | 674 (95.7) |
| No | 11 (1.6) | 16 (2.3) |
| Unknown | 11 (1.6) | 14 (2.0) |
| Employment status, n (%) | ||
| In paid employment | 372 (52.9) | 368 (52.3) |
| Unemployed | 70 (10.0) | 71 (10.1) |
| Looking after the home | 53 (7.5) | 51 (7.2) |
| Retired | 144 (20.5) | 142 (20.2) |
| Full-time student | 9 (1.2) | 8 (1.1) |
| Other | 55 (7.8) | 64 (9.1) |
| Education level, n (%) | ||
| Degree or equivalent | 109 (15.5) | 105 (14.9) |
| A level or equivalent | 123 (17.5) | 115 (16.3) |
| GCSE or equivalent | 246 (35.0) | 234 (33.2) |
| Other | 89 (12.7) | 86 (12.2) |
| None | 129 (18.3) | 153 (21.7) |
| Unknown | 10 (1.4) | 8 (1.1) |
| Free prescription, n (%) | ||
| Yes | 400 (56.9) | 392 (55.7) |
| No | 298 (42.4) | 299 (42.5) |
| Unknown | 5 (0.7) | 13 (1.8) |
Table 4 shows smoking behaviours before the latest quit attempt. Denominators used for categorical variables were the number of participants who responded to these questions. The percentage of participants living with a smoking partner was slightly higher in the control group (29.7%) than in the intervention group (25.9%). Participants in the intervention and the control group were comparable in terms of cigarettes per day before quitting, first cigarette after waking up and previous quit attempts. Most of the participants (89%) had previously attempted to quit smoking at least once and many (27%) had attempted to quit at least four times. About 80% of participants had managed to sustain at least 4 weeks’ abstinence in their previous longest abstinence attempts and more than 40% had been abstinent for at least 6 months in their longest attempt (see Table 4).
| Smoking history variable | Intervention | Control |
|---|---|---|
| Cigarettes per day before quitting, mean (SD) | 19.9 (9.5) | 20.4 (10.2) |
| First cigarette after waking up, n (%) | n = 702 | n = 703 |
| Within 5 minutes | 295 (42.0) | 298 (42.4) |
| 6–30 minutes | 306 (43.6) | 292 (41.5) |
| > 30 minutes | 101 (14.4) | 113 (16.1) |
| Living with a smoking partner | 114 of 440 (25.9) | 124 of 418 (29.7) |
| Any previous quit attempts | 625 of 702 (89.0) | 629 of 704 (89.4) |
| Number of previous quit attempts, n (%) | n = 610 | n = 612 |
| One | 187 (30.7) | 173 (28.3) |
| Two | 171 (28.0) | 154 (25.2) |
| Three | 94 (15.4) | 112 (18.3) |
| Four or more | 158 (25.9) | 173 (28.3) |
| Longest time managed to stay quit before, n (%) | n = 603 | n = 603 |
| < 1 week | 40 (6.6) | 46 (7.6) |
| 1–4 weeks | 80 (13.3) | 68 (11.3) |
| > 4 weeks to 6 months | 224 (37.2) | 225 (37.3) |
| > 6 months to 12 months | 96 (15.9) | 111 (18.4) |
| > 12 months | 163 (27.0) | 153 (25.4) |
The reasons given for quitting were similar in the two groups (Table 5). Participants were allowed to select more than one reason. Health concern was the most common reason for quitting, including worry about participants’ own future health (70%), already affected health (55%) or family’s health (33%). Cost consideration was also a common reason given by participants (48%). Other reasons included ‘smoking sets a bad example to children’ (37%), ‘because I don’t like being addicted’ (32%) and ‘smoking is antisocial’ (21%).
| Quitting variable | Intervention | Control |
|---|---|---|
| Reasons for quitting (multiple reasons allowed), n (%) | n = 703 | n = 704 |
| Because my health is already suffering | 380 (54.1) | 393 (55.8) |
| Because smoking costs too much | 341 (48.5) | 331 (47.0) |
| For my family’s health | 240 (34.1) | 223 (31.7) |
| Smoking is antisocial | 147 (20.9) | 145 (20.6) |
| I am worried about my future health | 491 (69.8) | 495 (70.3) |
| Other people are pressurising me to | 91 (12.9) | 69 (9.8) |
| Because I don’t like being addicted | 228 (32.4) | 222 (31.5) |
| Smoking sets a bad example to children | 265 (36.8) | 259 (36.8) |
| Importance of giving up smoking at this attempt, n (%) | n = 701 | n = 699 |
| Desperately important | 344 (49.1) | 343 (49.1) |
| Very important | 322 (45.9) | 327 (46.8) |
| Quite important | 34 (4.9) | 28 (4.0) |
| Not all that important | 1 (0.1) | 1 (0.1) |
| Determination to give up smoking at this attempt, n (%) | n = 700 | n = 700 |
| Extremely determined | 483 (69.0) | 470 (67.1) |
| Very determined | 193 (27.6) | 214 (30.6) |
| Quite determined | 24 (3.4) | 16 (2.3) |
| Not all that determined | 0 (0.0) | 0 (0.0) |
| Chances of quitting for good at this attempt, n (%) | n = 701 | n = 699 |
| Extremely high | 273 (38.9) | 271 (38.8) |
| Very high | 305 (43.5) | 309 (44.2) |
| Quite high | 117 (16.7) | 115 (16.5) |
| Not very high | 5 (0.7) | 4 (0.6) |
| Low | 1 (0.1) | 0 (0.0) |
| Very low | 0 (0.0%) | 0 (0.0%) |
Most participants considered that it was extremely or very important to give up smoking (95%) and were extremely or very determined to give up smoking at this attempt (97%). The majority of participants (83%) considered that they had an extremely or very high chance of giving up smoking forever at this attempt. There were no noticeable differences in the perceived importance, stated determination and perceived chances of giving up smoking between the two groups (Table 5).
Smoking relapse results
Prolonged carbon monoxide-verified smoking abstinence
The primary outcome was prolonged smoking abstinence (with no more than five cigarettes) from months 4 to 12, and CO-verified at the 12-month follow-up. The proportion of prolonged abstinence was 36.9% in the intervention group and 38.6% in the control group (Table 6). There was no statistically significant difference between the intervention and control groups (OR 0.93, 95% CI 0.75 to 1.15).
| End point | Intervention, n/N (%) | Control, n/N (%) | OR (95% CI); p-value |
|---|---|---|---|
| Smoking abstinence | |||
| Primary outcome: prolonged abstinence from 4 to 12 months (CO-validated at 12 months) | 259 of 702 (36.9) | 271 of 702 (38.6) | 0.930 (0.749 to 1.154); p = 0.509 |
| Continuous abstinence from 2 to 12 months (CO-validated at 12 months) | 217 of 702 (30.9) | 238 of 702 (33.9) | 0.872 (0.697 to 1.091); p = 0.231 |
| CO-validated 7-day smoking abstinence at 12 months | 309 of 702 (44.0) | 305 of 702 (43.4) | 1.023 (0.829 to 1.264); p = 0.830 |
| Smoking relapse | |||
| 7-day self-reported smoking at 3 months | 145 of 703 (20.6) | 147 of 704 (20.9) | 0.985 (0.761 to 1.274); p = 0.906 |
| 7-day self-reported smoking at 12 months | 342 of 702 (48.7) | 337 of 702 (48.0) | 1.029 (0.835 to 1.269); p = 0.789 |
Secondary smoking outcomes
The 7-day self-report point prevalence of smoking was, on average, 21% at 3 months and 48% at 12 months, and there were no statistically significant differences between the intervention and control groups (see Table 6). CO-verified smoking abstinence at 12 months included participants who self-reported smoking abstinence that was validated by CO tests. The CO-verified smoking abstinence at 12 months was 44%, and again there was no difference between the two groups. We also calculated continuous CO-validated smoking abstinence from months 2 to 12, after excluding any self-reported smoking (even only a puff). The continuous smoking abstinence was 31% in the treatment group and 34% in the control group, and there was no significant difference between the two groups (see Table 6).
Interactions between treatment effect and patient-level variables
We explored the association between treatment effect and patient-level variables by logistic regression analysis with interaction terms. Ratio of ORs were used to indicate whether or not the effect of the treatment was modified by participant characteristics. The treatment effect was not statistically significantly associated with participant characteristics at baseline (Table 7).
| Baseline variable | Ratio of ORs (95% CI); p-value of interaction |
|---|---|
| Age | 0.994 (0.978 to 1.010); p = 0.448 |
| Sex (1, female; 0, male) | 1.364 (0.885 to 2.103); p = 0.160 |
| Education (1, none or GCSE; 0, A level/degree) | 1.017 (0.629 to 1.646); p = 0.944 |
| Marital status (1, yes; 0, other) | |
| Married or living with a partner | 0.912 (0.581 to 1.433); p = 0.690 |
| Single | 1.221 (0.688 to 2.169); p = 0.495 |
| Separated or divorced | 1.385 (0.744 to 2.579); p = 0.304 |
| Employment status (1, yes; 0, other) | |
| In paid employment | 0.925 (0.600 to 1.426); p = 0.724 |
| Looking after the home | 2.127 (0.907 to 4.989); p = 0.083 |
| Retired | 1.011 (0.593 to 1.722); p = 0.969 |
| Unemployed | 1.533 (0.693 to 3.391); p = 0.291 |
| Free prescription (1, yes; 0, no) | 1.052 (0.686 to 1.615); p = 0.816 |
| Stop smoking advisor who recruited quitters: core services vs. other | 0.663 (0.390 to 1.128); p = 0.130 |
| Living with a smoking partner (1, yes; 0, no) if married or living with a partner | 0.555 (0.297 to 1.039); p = 0.066 |
| Number of cigarettes per day before quitting | 0.991 (0.969 to 1.014); p = 0.436 |
| Time of smoking the first cigarette after waking (1, within 5 minutes; 0, ≥ 5 minutes) | 1.082 (0.696 to 1.683); p = 0.726 |
| Any previous quit attempt (1, yes; 0, no) | 0.868 (0.441 to 1.707); p = 0.681 |
| Longest time managed to stay quit before (1, < 4 weeks; 0, ≥ 4 weeks), among those with previous attempts | 1.243 (0.688 to 2.245); p = 0.471 |
Association between smoking abstinence and baseline variables
Smoking abstinence and demographic variables at baseline
The results of logistic regression analysis between smoking abstinence at 12 months (the primary end point) and demographic variables are shown in Table 8. The smoking abstinence was not statistically significantly associated with sex, education and receipt of free prescription. However, age was statistically significantly associated with smoking abstinence at 12 months (p = 0.011). For example, the prevalence of smoking abstinence was 42% in participants aged ≥ 50 years and 35% in those aged < 50 years. Marital status was also significantly associated with smoking relapse (p = 0.003). The prevalence of smoking abstinence was 33% in participants who were single, 30% in those separated or divorced and 41% in people who cohabited. There was also a strong association with employment status (p < 0.001). Of participants in paid employment, 38% remained smoking free, compared with 35% of participants looking after the home, 41% of retired people, 26% of unemployed participants and only 18% of full-time students.
| Baseline demographic characteristic | n | OR (95% CI) | p-value |
|---|---|---|---|
| Age | 1404 | 1.010 (1.002 to 1.0183) | p = 0.011 |
| Sex (1, female; 0, male) | 1404 | 0.960 (0.774 to 1.192) | p = 0.712 |
| Free prescription (1, yes; 0, no) | 1387 | 0.861 (0.692 to 1.071) | p = 0.179 |
| Educational level | |||
| None | 1211 | 1.00 | p = 0.744a |
| GCSE | 1.010 (0.744 to 1.372) | ||
| A level | 1.012 (0.707 to 1.450) | ||
| Degree | 1.172 (0.813 to 1.691) | ||
| Marital status | |||
| Single | 1402 | 1.00 | p = 0.003a |
| Married or living with a partner | 1.412 (1.052 to 1.894) | ||
| Separated or divorced | 0.861 (0.584 to 1.269) | ||
| Employment status | |||
| In paid employment | 1404 | 1.00 | p< 0.001a |
| Looking after the home | 0.880 (0.572 to 1.353) | ||
| Retired | 1.138 (0.860 to 1.505) | ||
| Unemployed | 0.570 (0.379 to 0.886) | ||
| Full-time student | 0.356 (0.101 to 1.250) | ||
Smoking abstinence at 12 months and smoking-related variables at baseline
Table 9 shows the results of logistic regression analyses to investigate the association between smoking abstinence at 12 months (the primary end point) and smoking-related variables at baseline. Smoking abstinence at 12 months was not associated with stated reasons for quitting, stated importance, stated determinations or perceived chances of staying off cigarette for good (see Table 9).
| Smoking-related variable | n | OR (95% CI) | p-value |
|---|---|---|---|
| Stop smoking advisor: core services vs. other | 1404 | 1.361 (1.043 to 1.774) | p = 0.023 |
| Living with a smoking partner (1, yes; 0, no) | 856 | 0.730 (0.536 to 0.995) | p = 0.046 |
| Number of cigarettes per day before quitting | 1403 | 0.993 (0.982 to 1.005) | p = 0.246 |
| Number of cigarettes per day before quitting (0, < 10; 1, ≥ 10) | 1403 | 0.606 (0.447 to 0.820) | p = 0.001 |
| Time of first cigarette after waking up | |||
| Within 5 minutes | 1403 | 1.00 | p = 0.005a |
| 6–30 minutes | 1.320 (1.042 to 1.673) | ||
| > 30 minutes | 1.631 (1.186 to 2.244) | ||
| Any previous quit attempt (1, yes; 0, no) | 1403 | 0.570 (0.406 to 0.799) | p = 0.001 |
| Number of previous quit attempts | |||
| None | 1371 | 1.00 | p = 0.001a |
| One | 0.701 (0.479 to 1.027) | ||
| Two | 0.593 (0.402 to 0.876) | ||
| Three | 0.581 (0.379 to 0.891) | ||
| Four or more | 0.445 (0.300 to 0.661) | ||
| Longest time managed to stay quit before | |||
| None | 1355 | 1.00 | p < 0.001a |
| < 1 week | 0.720 (0.422 to 1.228) | ||
| 1–4 weeks | 0.542 (0.341 to 0.861) | ||
| > 4 weeks to 6 months | 0.406 (0.278 to 0.592) | ||
| > 6 months to 12 months | 0.702 (0.461 to 1.071) | ||
| > 12 months | 0.765 (0.519 to 1.128) | ||
| Stated important of quitting (from 1, desperately important, to 4, not all that important) | 1397 | 0.932 (0.775 to 1.122) | p = 0.459 |
| Stated determination to quit (from 1, extremely determined, to 4, not all that determined) | 1397 | 0.878 (0.715 to 1.077) | p = 0.212 |
| Perceived chance of successfully quitting (from 1, extremely high, to 6, very low) | 1404 | 1.000 (0.998 to 1.001) | p = 0.765 |
| Stated reasons for quitting | |||
| Because my health is already suffering | 1404 | 1.042 (0.838 to 1.294) | p = 0.713 |
| Because smoking costs too much | 0.882 (0.711 to 1.095) | p = 0.256 | |
| For my family’s health | 0.981 (0.780 to 1.234) | p = 0.870 | |
| Smoking is antisocial | 1.293 (0.995 to 1.681) | p = 0.055 | |
| I am worried about my future health | 1.032 (0.815 to 1.307) | p = 0.794 | |
| Other people are pressurising me to | 1.093 (0.780 to 1.533) | p = 0.605 | |
| Because I don’t like being addicted | 1.141 (0.906 to 1.436) | p = 0.262 | |
| Smoking sets a bad example to children | 0.994 (0.795 to 1.243) | p = 0.961 |
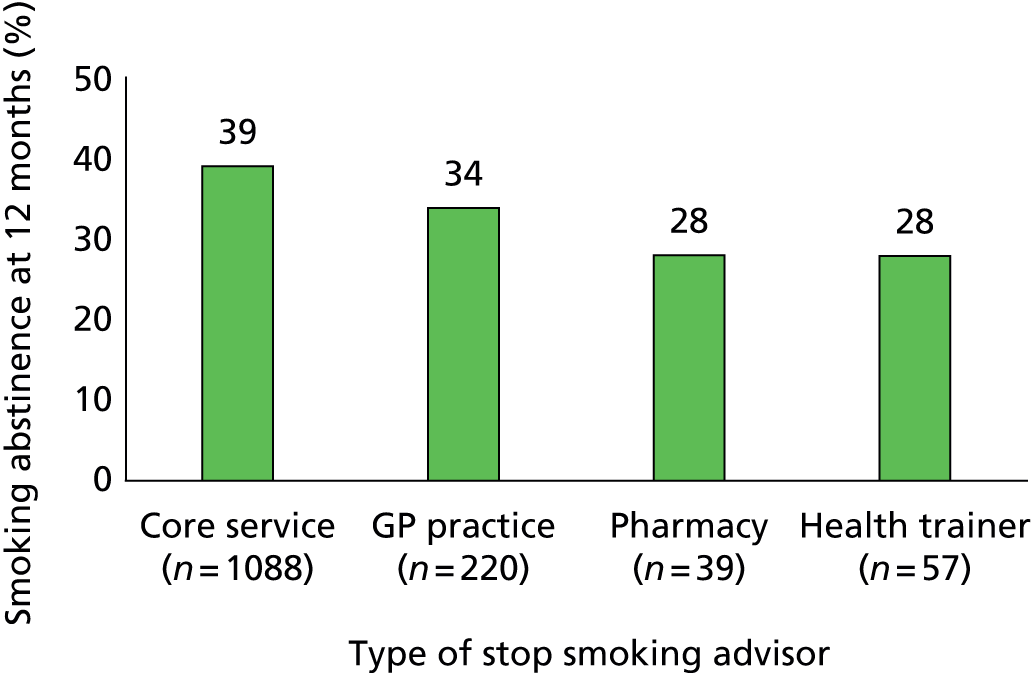
Quitters who were treated by specialist level 3 advisors were less likely to return to smoking than those recruited from other types of Stop Smoking Services (p = 0.023). The prevalence of smoking abstinence was 39% in quitters from core services, 34% from GP practice and 28% from pharmacies and health trainers (Figure 3).
FIGURE 3.
Smoking abstinence at 12 months by type of stop smoking advisors who recruited quitters.
Although smoking abstinence at 12 months was positively associated with cohabitating with a partner (see Table 8), the percentage of smoking abstinence was lower for people living with a partner who smoked than for those who did not (see Table 9). The percentage of smoking abstinence at 12 months was 36% for participants living with a smoking partner and 43% for participants living with non-smokers (p = 0.046).
Time until the first cigarette after waking was statistically significantly associated with smoking relapse (p = 0.001). The percentage of smoking abstinence was 33% in participants who smoked the first cigarette within 5 minutes after waking up, 40% in participants who had the first cigarette between 6 and 30 minutes after waking up and 45% in those who had the first cigarette more than 30 minutes after waking up. When the absolute number of cigarettes smoked per day before the current attempt was examined, there was no statistically significant association between the number of cigarettes per day and the smoking abstinence at 12 months (p = 0.246). However, smoking abstinence was statistically significantly higher in participants who smoked fewer than 10 cigarettes per day before quitting (48%) than in those who smoked 10 or more cigarettes (36%) (p = 0.001).
Previous quit attempt was associated with increased smoking relapse (see Table 9). The smoking abstinence prevalence at 12 months was 36% in participants with any previous quit attempt and 50% in participants for whom the current quit attempt was the first quit attempt (p = 0.001). In addition, there was a tendency that the more previous quit attempts, the higher the risk of smoking relapse at the current attempts. The percentage of smoking abstinence was 41% in participants who had only one previous quit attempt, 37% in participants who had two or three previous quit attempts and 31% for those with four or more previous quit attempts (Figure 4). Among participants who had previous attempts, there was a clear linear association between smoking abstinence at 12 months and the longest time they had previously managed to stay quit (see Table 9).
FIGURE 4.
Smoking abstinence at 12 months and number of previous quit attempts.

Chapter 4 Process and mediating variables
Educational booklets-related variables
Table 10 shows whether or not participants still had and had read the booklets. At the 3-month follow-up, 89% of the intervention group and 79% of the control group reported receiving the booklets or leaflet. The difference between the two groups was statistically significant (p < 0.001). The percentage of participants who reported that they still possessed the booklets was statistically significantly higher in the treatment group than in the control group at the 3-month (83% vs. 62%) and 12-month follow-ups (49% vs. 35%).
| Booklet-related variable | Intervention group | Control group | p-value |
|---|---|---|---|
| Booklets received at 3 months | 628/703 (89.3) | 554/704 (78.7) | p < 0.001 |
| Still had booklets at follow-up, n/N (%) | |||
| At 3 months | 580/703 (82.5) | 437/704 (62.1) | p < 0.001 |
| At 12 months | 343/702 (48.9) | 242/702 (34.5) | |
| Had read the booklets (reported at follow-up), n/N (%) | |||
| 2–3 months | 495/703 (70.4) | 485/704 (68.9) | p = 0.535 |
| 4–12 months | 189/702 (26.9) | 144/702 (20.5) | p = 0.005 |
| How helpful was the booklet, n/N (%) | |||
| Reported at 3 months | N = 703 | N = 704 | |
| Unhelpful/missing | 299 (42.5) | 304 (43.2) | p = 0.805 |
| Somewhat helpful | 203 (28.9) | 250 (35.5) | |
| Very helpful | 201 (28.6) | 150 (21.3) | |
| Reported at 12 months | N = 702 | N = 702 | |
| Unhelpful/missing | 290 (41.3) | 294 (41.9) | p = 0.829 |
| Somewhat helpful | 215 (30.6) | 259 (36.9) | |
| Very helpful | 197 (28.1) | 149 (21.2) | |
At the 3-month follow-up, about 70% of the participants reported that they had read the booklets (or the leaflet) and there was no significant difference between the two groups (p = 0.535). By 12 months, only 27% in the treatment group and 21% in the control group reported having read the booklets or leaflet between 4 and 12 months, and the difference between the groups was statistically significant (p = 0.005).
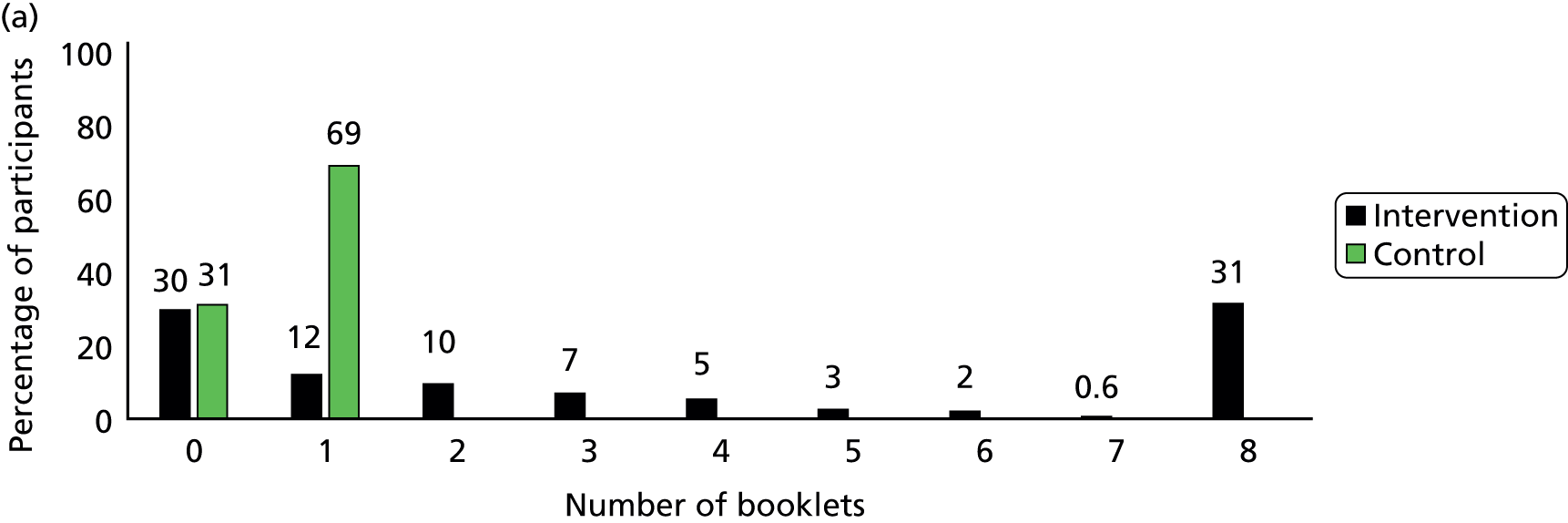
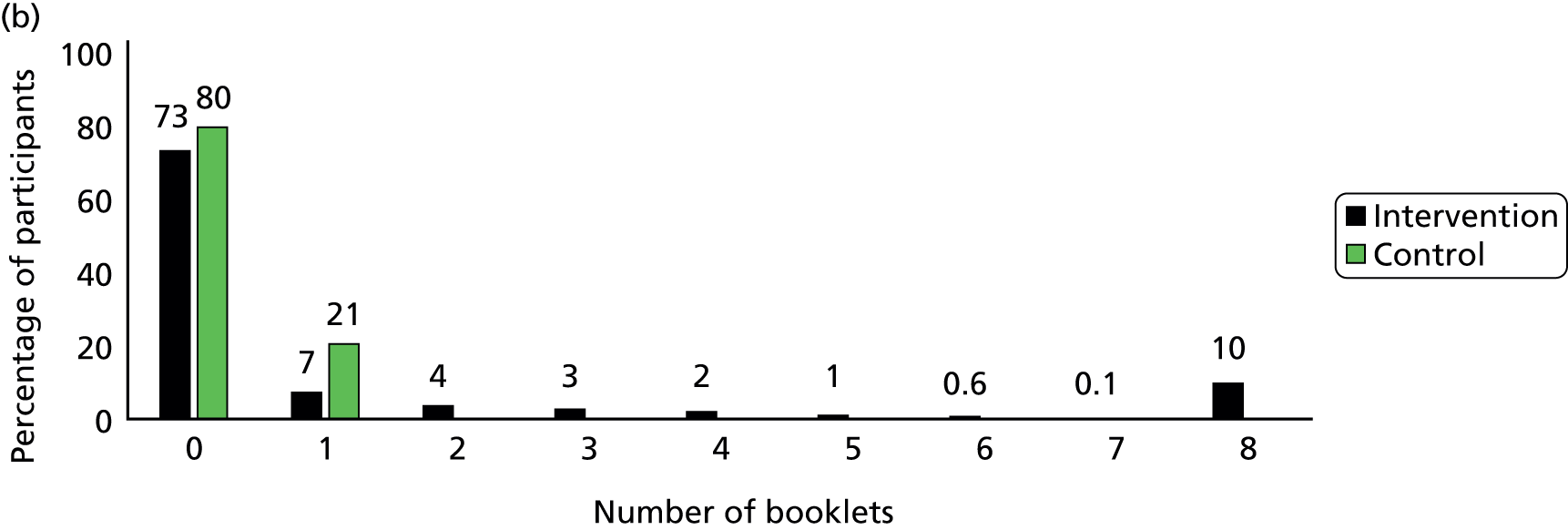
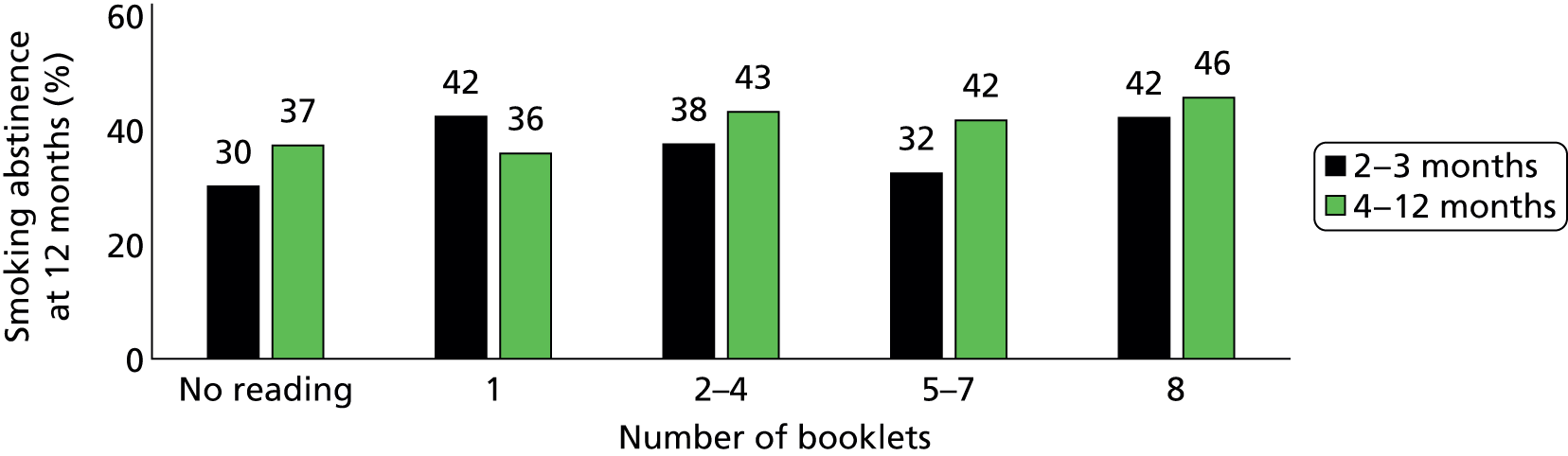
Participants in the intervention group reported spending more time reading the booklets than did control group participants (Figure 5). The percentage of participants who spent more than 30 minutes on reading the booklets was 43% in the intervention group and only 9% in the control group by the 3-month follow-up, and it was 18% and 7% respectively between 4 and 12 months. In the intervention group, 58% of participants reported they had read more than one of the eight booklets by 3 months and only 20% had done so between 4 and 12 months (Figure 6). The percentage of participants who read all eight booklets in the intervention group was 31% by 3 months and 10% between 4 and 12 months.
FIGURE 5.
Time spent on reading the educational booklets. (a) Between 2 and 3 months; (b) between 4 and 12 months. Note, percentages have been rounded.
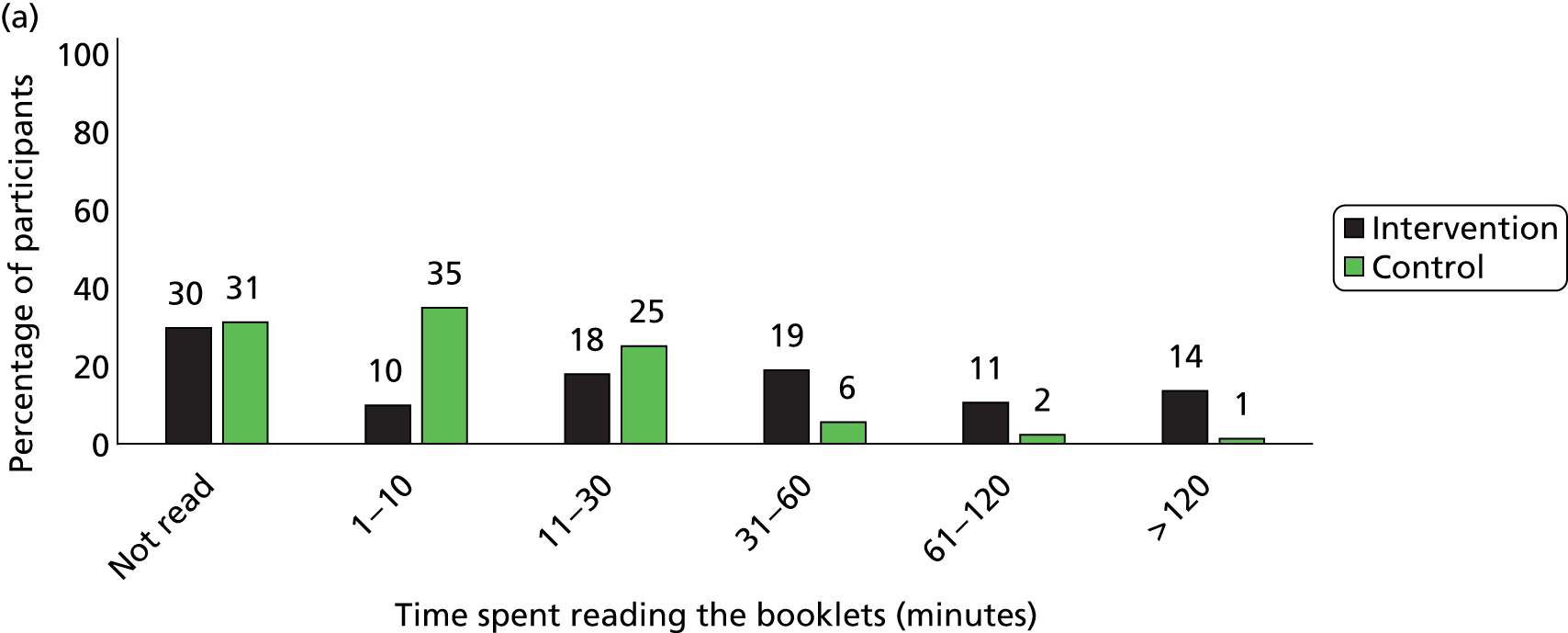
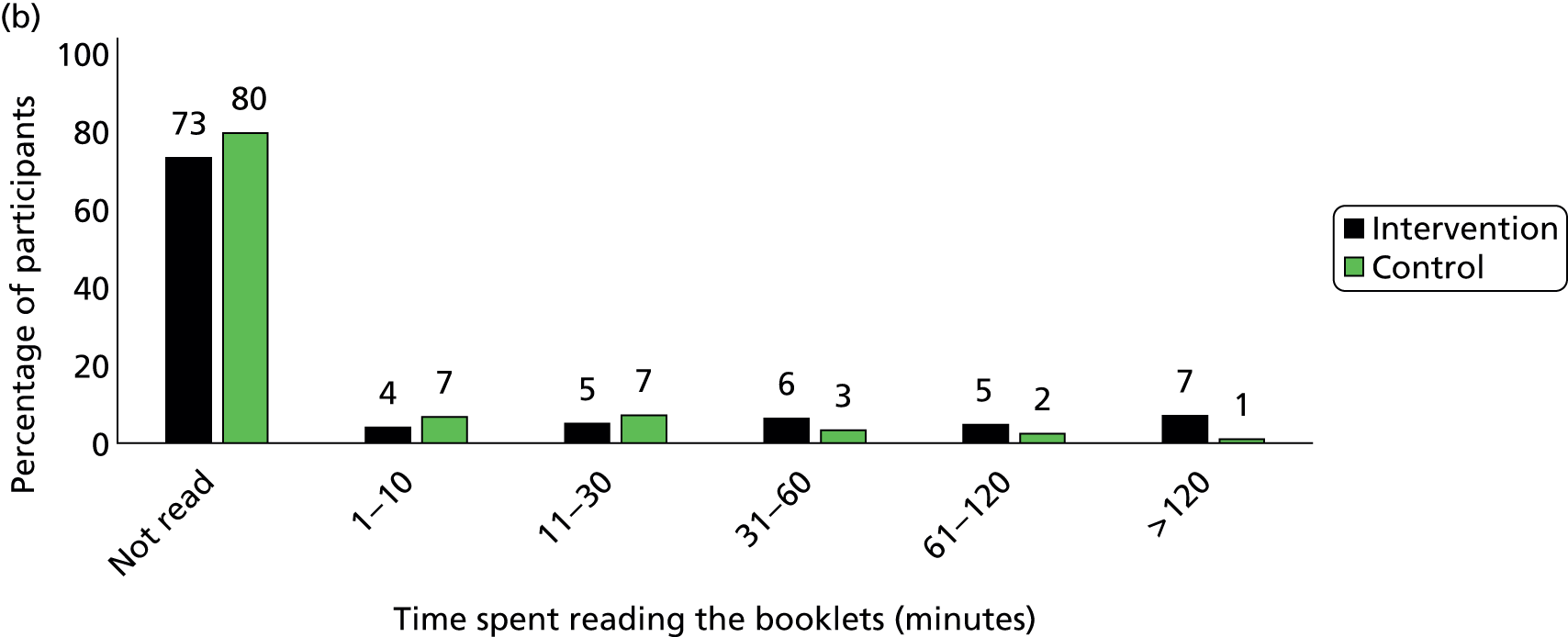
FIGURE 6.
Number of booklets that participants had read. (a) Between 2 and 3 months; (b) between 4 and 12 months.
The percentage of participants who considered the booklets unhelpful (or unclear) was similar between the treatment and control group (see Table 10). Compared with the control group, the percentage of participants who considered the booklets very helpful was somewhat higher in the intervention group (28% vs. 21% at 12 months), while the percentage of those who considered the booklets somewhat helpful was slightly lower in the intervention group (31% vs. 37% at 12 months).
We asked the participants if reading the booklets meant that they were more able to identify situations in which the risk of relapse was higher. The proportion of participants reporting that they knew much more about which situations might lead to relapse was 26% in the intervention group and 18% in the control group at the 3-month follow-up, and 25% and 21% respectively at the 12-month follow-up. The proportion of participants who reported that reading the booklets taught them no more than they knew already was lower in the treatment group at the 3-month follow-up (48% vs. 53%; p = 0.04), but there was no difference between the groups at the 12-month follow-up (Table 11). Between the treatment and control group, there were no significant differences in the proportion of participants who reported that reading the booklets taught them more about ways to handle urges to smoke at 3 and 12 months (see Table 11).
| Knowledge about risky situations and ways of handling urges | Intervention group | Control group | p-value |
|---|---|---|---|
| Knew more about relapse risky situations, n (%) | |||
| Reported at 3 months | N = 703 | N = 704 | |
| No/not sure | 335 (47.7) | 374 (53.1) | p = 0.040 |
| A little more | 187 (26.6) | 204 (29.0) | |
| Much more | 181 (25.8) | 126 (17.9) | |
| Reported at 12 months | N = 702 | N = 702 | |
| No/not sure | 340 (48.4) | 341 (48.6) | p = 0.957 |
| A little more | 180 (25.6) | 212 (30.2) | |
| Much more | 182 (25.9) | 149 (21.2) | |
| Knew more about ways of handling urges, n/N (%) | |||
| Reported at 3 months | N = 703 | N = 704 | |
| No/not sure | 342 (48.7) | 373 (53.0) | p = 0.104 |
| A little more | 193 (27.5) | 193 (27.4) | |
| Much more | 168 (23.9) | 138 (19.6) | |
| Reported at 12 months | N = 702 | N = 702 | |
| No/not sure | 344 (49.0) | 346 (49.3) | p = 0.915 |
| A little more | 190 (27.1) | 220 (31.3) | |
| Much more | 168 (23.9) | 136 (19.4) | |
Coping strategies and activities
When participants were asked what things they knew they could do to cope with urges to smoke, the percentage of all participants who reported one or more strategies was 87% at the 3-month follow-up and 66% at the 12-month follow-up, and there was no significant difference between the two groups (Table 12). The number of coping strategies that participants reported they knew is shown in Figure 7, which reveals no significant difference between the groups at both the 3-month follow-up (p = 0.798) and the 12-month follow-up (p = 0.453).
| Coping strategy | Intervention group | Control group | p-value |
|---|---|---|---|
| Knew at least one thing that could be done to handle urges, n/N (%) | |||
| At 3 months | 608/703 (86.5) | 611/704 (86.8) | p = 0.867 |
| At 12 months | 447/702 (63.7) | 460/702 (65.5) | p = 0.468 |
| Knew at least one behavioural activity that could be used to handle urges, n/N (%) | |||
| At 3 months | 465/703 (66.2) | 470/704 (66.8) | p = 0.807 |
| At 12 months | 325/702 (46.3) | 339/702 (48.3) | p = 0.454 |
| Knew at least one mental exercise that could be used to handle urges, n/N (%) | |||
| At 3 months | 303/703 (43.1) | 275/704 (39.1) | p = 0.124 |
| At 12 months | 201/702 (28.6) | 194/702 (27.6) | p = 0.678 |
| Knew both behavioural and mental things that could be used to handle urges, n/N (%) | |||
| At 3 months | 160/703 (22.8) | 134/704 (19.0) | p = 0.086 |
| At 12 months | 79/702 (11.3) | 73/702 (10.4) | p = 0.606 |
| Ever attempted to do something to cope with urges, n/N (%) | |||
| At 3 months | 580/703 (82.5) | 585/704 (83.1) | p = 0.768 |
| At 12 months | 420/702 (59.8) | 431/702 (61.4) | p = 0.548 |
FIGURE 7.
Percentage (%) of participants who reported things they knew to handle urges, by number of things reported and treatment group. (a) At 3 months; (b) at 12 months.


Behavioural coping strategies were more frequently reported than mental scoping skills. At the 3-month follow-up, the percentage of participants who reported at least one behavioural coping strategy was 67%, compared with 41% who reported at least one mental coping strategy. Only 21% of participants reported both behavioural and mental coping strategies at the 3-month follow-up (see Table 12).
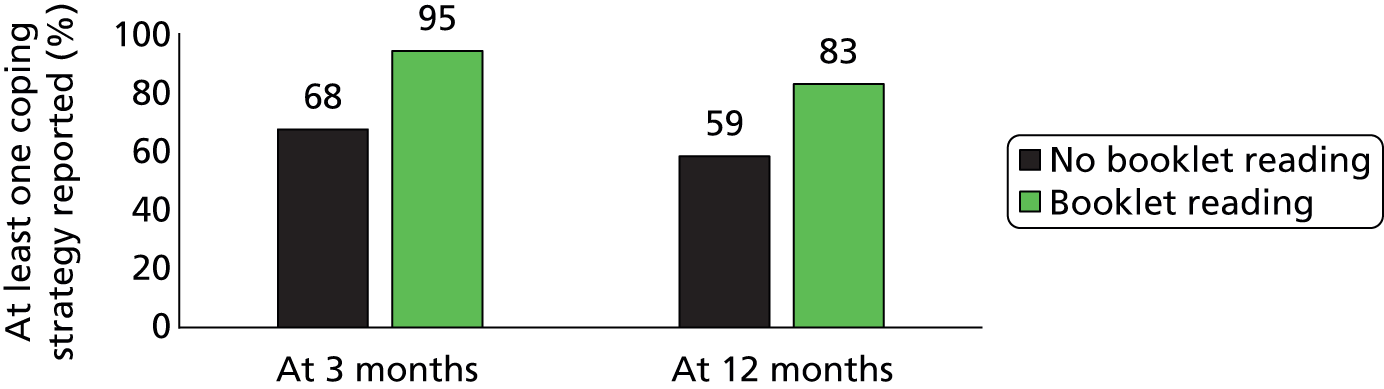
People who reported reading the booklets tended to report knowing more strategies to cope with urges (Figure 8). For example, at the 12-month follow-up, the percentage of participants who knew one or more coping strategy was 83% in those who had read the booklets and 59% in those who had not.
FIGURE 8.
Reporting of coping strategies and booklet reading.
About 83% of all participants by 3 months and 61% between 4 and 12 months reported enacting a strategy to handle urges to smoke, with no significant differences between groups (see Table 12). The percentage of participants who tried to do something was positively associated with any booklet reading, knowing more about risky situations and knowing more about ways of handling urges (Figure 9). However, there were no significant differences in attempts to do something to handle urges between reading of one and more booklets, and between knowing little more and much more about risky situations and ways of handling urges (see Figure 9).
FIGURE 9.
Ever tried to do something to handle urges and booklet reading, and changes in knowledge on risky situations and ways of handling urges.

Mediating variables and smoking abstinence at 12 months
The mediating variables were not associated with the educational materials used in general, and the analyses of associations between mediating variables and the smoking outcome were therefore conducted without taking into account participants’ treatment group. The results of the logistic regression analyses to investigate the association between smoking abstinence at 12 months and mediating variables are presented in Table 13. Smoking abstinence at 12 months was less common in people who had not read booklets by 3 months than in those who did, although there was no significant association between smoking abstinence and booklet reading between 4 and 12 months (p = 0.493). The percentage of smoking abstinence at 12 months was 41% in participants who read the booklets, compared with 30% for those who did not, by the 3-month follow-up, and it was 39% and 37% respectively between 4 and 12 months (Figure 10).
| Mediating variable | OR (95% CI); p-value | |
|---|---|---|
| 2–3 months | 4–12 months | |
| Any reading of booklets | 1.625 (1.274 to 2.072); p < 0.001 | 1.092 (0.849 to 1.406); p = 0.493 |
| Time spent on reading booklets | 1.073 (1.001 to 1.151); p = 0.048 | 1.020 (0.943 to 1.104); p = 0.615 |
| Number of booklets being looked at | 1.035 (0.996 to 1.075); p = 0.080 | 1.047 (0.989 to 1.109); p = 0.117 |
| Know more about risky situations | 1.186 (1.037 to 1.356); p = 0.013 | 1.484 (1.299 to 1.696); p < 0.001 |
| Know more about ways of handling urges | 1.146 (1.002 to 1.310); p = 0.046 | 1.462 (1.276 to 1.675); p < 0.001 |
| No. of coping strategies reported | 1.076 (0.989 to 1.170); p = 0.088 | 1.375 (1.246 to 1.517); p < 0.001 |
| Ever tried to do something to handle urges | 1.730 (1.275 to 2.347); p < 0.001 | 3.109 (2.445 to 3.954); p < 0.001 |
FIGURE 10.
Smoking abstinence at 12 months by booklet reading status.

There was no clear dose–response relationship among participants who had read any booklets (Figures 11 and 12). The results of multiple logistic regression analyses are shown in Table 14. Smoking abstinence at 12 months was statistically significantly associated with booklet reading between 2 and 3 months (p < 0.001), but not with booklet reading between 4 and 12 months (p = 0.759), which indicated that the use of education materials at an early stage may be important. After including the variable of any booklet reading in analyses, there was no significant association between smoking abstinence and any reading of booklets during months 4–12, time spent on reading or the number of booklets read (see Table 14).
FIGURE 11.
Smoking abstinence at 12 months by time spent on reading booklets.

FIGURE 12.
Smoking abstinence at 12 months by number of booklets read.
| Booklet reading | OR (95% CI); p-value | |
|---|---|---|
| 2–3 months | 4–12 months | |
| Any reading (binary) | 1.850 (1.323 to 2.587); p < 0.001 | 1.083 (0.650 to 1.806); p = 0.759 |
| Time on reading (ordinal) | 0.931 (0.827 to 1.049); p = 0.240 | 0.927 (0.778 to 1.103); p = 0.392 |
| Number of booklets read | 1.012 (0.960 to 1.068); p = 0.650 | 1.077 (0.989 to 1.174); p = 0.088 |
Smoking abstinence at 12 months was higher in participants who reported knowing more about risky situations or knowing more ways to handle urges because they had read the booklets (see Table 13). However, there was no significant difference in smoking abstinence at 12 months between knowing a little more and knowing much more (Figures 13 and 14). The proportion of smoking abstainers was higher in those who reported knowing more strategies to cope (see Table 13), although there was no clear dose–response relationship between knowing only one and knowing more than one strategy (Figure 15).
FIGURE 13.
Smoking abstinence at 12 months by knowing more about risky situations.
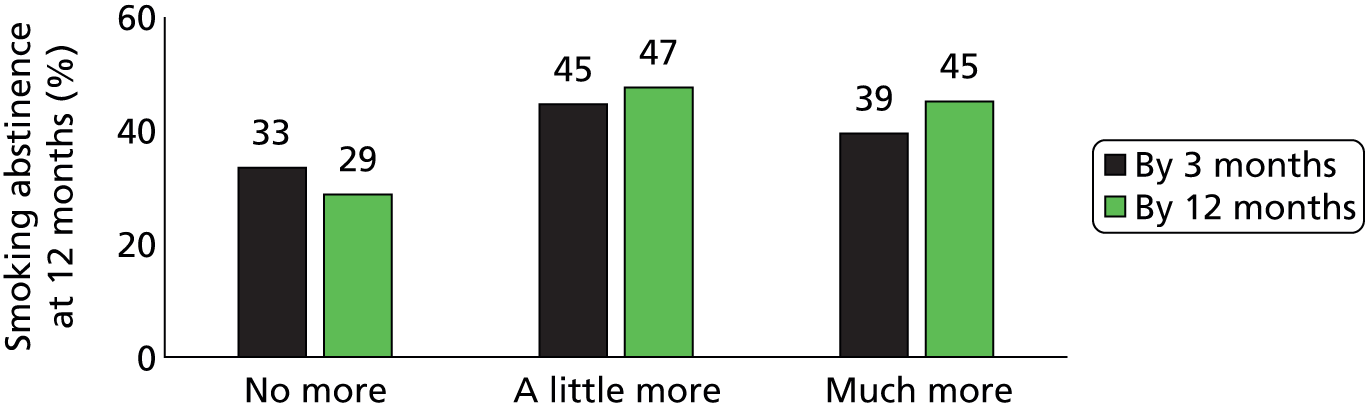
FIGURE 14.
Smoking relapse at 12 months by knowing more about ways of handling urges.

FIGURE 15.
The number of coping strategies known and smoking abstinence at 12 months.

Participants who reported doing something to handle urges to smoke were less likely to relapse by 12 months than were people who had no strategy to cope with urges (see Table 13). Of participants who reported that they had tried to handle urges between 4 and 12 months, 48% remained smoking free by 12 months, compared with 23% of those who did not report a strategy (Figure 16). The difference in smoking abstinence between no attempt and any attempts from 4 to 12 months (48% vs. 23%) was greater than between no attempt and any attempts from 2 to 3 months (40% vs. 28%).
FIGURE 16.
Smoking abstinence at 12 months and attempts to do something to handle urges.

Further analysis was conducted by using the following categories: participants who reported no strategy to handle urges, participants who reported using strategies only at the 3-month follow-up, participants who reported strategies only at the 12-month follow-up and participants who reported using strategies at both the 3-month and the 12-month follow-ups (Table 15). The proportion of smoking abstainers was lowest among participants who reported using no strategies or attempts to control urges at all (19%) and highest among participants who had attempted by the 3-month and 12-month follow-up (48%). Participants who reported any attempts by only the 3-month follow-up had a smoking relapse rate slightly higher than those who reported no attempts at all (24% vs. 19%). These results indicated that the prevention of smoking relapse needs continued efforts to do something to handle urges.
| Attempt | n/N | Smoking abstinence, % (95% CI) |
|---|---|---|
| No attempt at all | 30/159 | 18.9 (13.5 to 25.8) |
| Only by 3-month follow-up | 95/394 | 24.1 (20.1 to 28.6) |
| Only by 12-month follow-up | 37/83 | 44.6 (34.1 to 55.6) |
| At both 3- and 12-month follow-up | 368/768 | 47.9 (44.4 to 51.5) |
Chapter 5 Use of additional smoking cessation interventions
Any use of additional cessation interventions
At the 3- and 12-month follow-up interviews, participants were asked whether or not they had used smoking cessation aids following their initial use of the NHS Stop Smoking Service at baseline. There were no significant differences in the use of additional stop smoking interventions between the treatment and the control groups (Table 16).
| Additional stop smoking interventions | Treatment group | Control group | p-value |
|---|---|---|---|
| Any contacts with Stop Smoking Clinics, n/N (%) | |||
| During 2–3 months | 406/703 (57.8) | 434/704 (61.7) | p = 0.136 |
| During 4–12 months | 105/702 (15.0) | 107/702 (15.2) | p = 0.881 |
| Any use of stop smoking medications, n/N (%) | |||
| During 2–3 months | 510/703 (72.6) | 532/704 (75.6) | p = 0.196 |
| During 4–12 months | 155/702 (22.1) | 180/702 (25.6) | p = 0.118 |
| Any use of varenicline (Champix®, Pfizer), n/N (%) | |||
| During 2–3 months | 282/703 (40.1) | 275/704 (39.1) | p = 0.687 |
| During 4–12 months | 38/702 (5.4) | 41/702 (5.8) | p = 0.728 |
| Any use of bupropion (Zyban®, GSK), n/N (%) | |||
| During 2–3 months | 1/703 | 4/704 | –a |
| During 4–12 months | 2/702 | 2/702 | –a |
| Any use of NRT products, n/N (%) | |||
| During 2–3 months | 244/703 (34.7) | 267/704 (37.9) | p = 0.210 |
| During 4–12 months | 122/702 (17.4) | 143/702 (20.4) | p = 0.152 |
| Use of other educational materials, n/N (%) | |||
| During 2–3 months | 124/703 (17.6) | 142/704 (20.2) | p = 0.225 |
| During 4–12 months | 68/702 (9.7) | 62/702 (8.8) | p = 0.581 |
| Any use of electronic cigarettes, n/N (%)b | |||
| During 2–3 months | 18/703 (2.6) | 19/704 (2.7) | p = 0.871 |
| During 4–12 months | 43/702 (6.1) | 56/702 (8.0) | p = 0.175 |
In total, 60% of participants contacted an NHS Stop Smoking Clinic in the 2 months after they were proven abstinent at week 4; 15% did so from months 4 to 12. Most people (74%) continued using cessation medication in the 2 months after randomisation; 24% did so between 4 and 12 months after randomisation. Specifically, 40% of participants used varenicline and 36% used nicotine replacement therapies (NRTs) by the 3-month follow-up. Only 6% of participants used varenicline and 19% used NRT between 4 and 12 months after randomisation. In general, the use of cessation aids decreased over time. The exception is the use of electronic cigarettes, which increased from 2.6% between 2 and 3 months to 7.1% between 4 and 12 months.
Additional cessation treatments and smoking abstinence at 12 months
Table 17 compares the results of prolonged abstinence between 4 and 12 months by the use of additional cessation interventions. In general, the use of cessation interventions between 2 and 3 months was associated with increased smoking abstinence by 12 months, while the use of cessation treatments between 4 and 12 months was associated with a lower percentage of prolonged smoking abstinence. Of the participants who contacted stop smoking advisors, 42% achieved prolonged smoking abstinence during the first 2 months after randomisation, compared with 31% of those without such contact (p < 0.001). However, only 25% of participants who contacted stop smoking advisors remained abstinent between 4 and 12 months, compared with 40% of participants who did not have such contact (see Table 17). These results indicated that additional smoking cessation treatments were more likely to be used during months 4–12 by relapsed participants than by those who were still smoking free.
| Additional stop smoking interventions | Not used | Used | p-value |
|---|---|---|---|
| Any contacts of Stop Smoking Clinics, n/N (%) | |||
| During 2–3 months | 176/565 (31.2) | 354/839 (42.2) | p < 0.001 |
| During 4–12 months | 478/1192 (40.1) | 52/212 (24.5) | p < 0.001 |
| Any stop smoking medications, n/N (%) | |||
| During 2–3 months | 106/363 (29.2) | 424/1041 (40.7) | p < 0.001 |
| During 4–12 months | 440/1069 (41.2) | 90/335 (26.9) | p < 0.001 |
| Any use of varenicline, n/N (%) | |||
| During 2–3 months | 310/847 (36.6) | 220/557 (39.5) | p = 0.273 |
| During 4–12 months | 520/1325 (39.3) | 10/79 (12.7) | p < 0.001 |
| Any use of NRT, n/N (%) | |||
| During 2–3 months | 318/894 (35.6) | 212/510 (41.6) | p = 0.026 |
| During 4–12 months | 450/1139 (39.5) | 80/265 (30.2) | p = 0.005 |
| Any use of bupropion, n/N (%) | |||
| During 2–3 months | 529/1399 (37.8) | 1/5 | –a |
| During 4–12 months | 529/1400 (37.8) | 1/4 | –a |
| Use of other educational materials, n/N (%) | |||
| During 2–3 months | 412/1138 (36.2) | 118/266 (44.4) | p = 0.013 |
| During 4–12 months | 467/1274 (36.7) | 63/130 (48.5) | p = 0.008 |
| Any use of electronic cigarettes, n/N (%)b | |||
| During 2–3 months | 517/1368 (37.8) | 13/36 (36.1) | p = 0.837 |
| During 4–12 months | 503/1305 (38.5) | 27/99 (27.3) | p = 0.026 |
The numbers of participants exhibiting 7-day point smoking abstinence (CO-validated) at 12 months by additional cessation treatments are shown in Table 18. The point smoking abstinence at 12 months tended to be slightly higher among participants who contacted stop smoking advisors (49% vs. 43%) or used any cessation medications (46% vs. 43%) during months 4–12, although the difference was statistically non-significant (see Table 18). Clearly, the use of additional cessation treatments between 4 and 12 months helped some relapsed participants quit again before the 12-month follow-up.
| Additional stop smoking interventions | Not used | Used | p-value |
|---|---|---|---|
| Any contacts of Stop Smoking Clinics, n/N (%) | |||
| During 2–3 months | 207/565 (36.6) | 407/839 (48.5) | p < 0.001 |
| During 4–12 months | 510/1192 (42.8) | 104/212 (49.1) | p = 0.090 |
| Any stop smoking medications, n/N (%) | |||
| During 2–3 months | 122/363 (33.6) | 492/1041 (47.3) | p < 0.001 |
| During 4–12 months | 460/1069 (43.0) | 154/335 (46.0) | p = 0.344 |
| Any use of varenicline, n/N (%) | |||
| During 2–3 months | 362/847 (42.7) | 252/557 (45.2) | p = 0.355 |
| During 4–12 months | 577/1325 (43.6) | 37/79 (46.8) | p = 0.567 |
| Any use of NRT products, n/N (%) | |||
| During 2–3 months | 364/894 (40.7) | 250/510 (49.0) | p = 0.003 |
| During 4–12 months | 495/1139 (43.5) | 119/265 (44.9) | p = 0.669 |
| Any use of electronic cigarettes, n/N (%)a | |||
| During 2–3 months | 601/1368 (43.9) | 13/36 (36.1) | p = 0.350 |
| During 4–12 months | 580/1305 (44.4) | 34/99 (34.3) | p = 0.051 |
| Use of other educational materials, n/N (%) | |||
| During 2–3 months | 477/1138 (41.9) | 137/266 (51.5) | p = 0.005 |
| During 4–12 months | 540/1274 (42.4) | 74/130 (56.9) | p = 0.001 |
Chapter 6 A qualitative process evaluation of use of self-help materials for relapse prevention within the context of the SHARPISH trial
This chapter reports on qualitative data collected as part of the trial follow-up questionnaire, and in-depth data collected as part of a further qualitative substudy. Process evaluation of randomised controlled trials involving qualitative methods is increasingly recognised as important to informing and assisting with interpretation of trial outcomes. 40,41 In the case of the Self-Help And Relapse Prevention In Smoking for Health (SHARPISH) trial, we sought to explore patient-reported experiences of using the self-help materials (the control leaflet or the intervention booklets), focusing on the response of individuals to the intervention being tested. 42 This has enabled us to understand the variations in ways in which the materials were actually used and the extent to which the materials were engaged with.
Methods
A thematic content analysis of open-ended responses to questionnaire follow-up questions collected at 3 months and 12 months was undertaken. A further in-depth qualitative substudy was undertaken across three core areas of recruitment:
-
study 1a: qualitative interviews with participants who had and had not relapsed in the control and intervention arms of the SHARPISH trial at 12-month follow-up
-
study 1b: a focus group with a selected sample of smoking cessation professionals
-
study 1c: focus groups with trial participants.
For study 1a, a purposive sample of participants already recruited to the SHARPISH trial was selected. The sample aimed to achieve maximum variation across trial participants by accessing demographic data collected as part of the trial to ensure that there was a spread of representation. A sample size of approximately 40 participants was the target recruitment. Study 1b recruited a convenience sample of professionals to take part in a focus group. A focus group was undertaken with core service staff (specialist advisors), who recruited 73% of the participants to the trial. Study 1c purposefully recruited a further subsample of trial participants to take part in focus groups. The aim was to sample approximately 20 patients in total, consisting of four focus groups of approximately five patients per group.
The interview guide was developed in consultation with lay representatives, and the focus group topic guide was developed following analysis of the qualitative interview data.
There were two key aims of the analysis of the interview and focus group data:
-
to give an explanatory context for trial findings
-
to explore patient experience of use of self-help materials.
A thematic content analysis43 approach was used to code all data in transcripts that was relevant to exploring the use of the self-help booklets and the leaflet. Transcripts were read through line by line, and sections of text were coded for meaning. Codes were mostly descriptive, but some interpretative codes were assigned. Following a grounded theory approach,44 initial open coding was organised into higher-level (axial) coding, and interpretations reported here are made on the basis of the axial coding scheme (Figure 17). Coding was undertaken primarily by one researcher (CN). Two independent researchers undertook independent coding on a total of 25% of the transcripts. Coding was discussed at team meetings until consensus on categories was reached. Final analysis was presented to lay representatives for verification and feedback. In this chapter, italic text has been used to show coding that arose from the interviews.
FIGURE 17.
Summary of qualitative coding. CBT, cognitive–behavioural therapy; SSS, Stop Smoking Service.

Data from studies 1b and 1c were used to triangulate the qualitative data and assist with validation of and consensus on the qualitative analysis. Focus groups were also used as an opportunity to feed back trial findings to participants and stop smoking professionals, and to gather feedback and views on the trial outcome.
Analysis of trial open-ended questionnaire data
All available qualitative comments to 12-month follow-up questionnaire responses were extracted. Responses were coded in answer to the questions:
-
Do you have any other comments about the booklet(s)?
-
What were the main things you learnt from reading the booklet(s)?
Comments were initially coded into considerations of the booklets as being helpful or as being generally unhelpful. A total of 426 responses were coded under the helpful code, in comparison with a total of 284 coded as unhelpful. This coding includes those randomised to both the intervention and control groups, so in the context of this analysis participants were commenting on the written material received (i.e. either the control leaflet or the intervention booklets). Given this, we see that just over half of the trial sample (710 people) gave a further open-ended comment about the self-help materials received and, of those, 60% considered the written material to be helpful.
This corresponds well with trial process mediating variables, finding that those who reported reading the intervention booklets self-reported higher levels of strategies for coping with urges and had lower relapse rates than those who reported not having read the booklets in the intervention group.
Among those answering the open-ended question inviting further comment on the booklets who indicated that they had found them unhelpful, a total of 142 participants indicated that they were unaware of or were unable to recall the content of the self-help information received. A smaller but possibly important section of the sample (68 participants) suggested that the written materials did not contain any new or novel information that they had not heard before.
Study 1a: qualitative interviews with individual trial participants
Demographics
In total, 43 qualitative interviews were undertaken as part of study 1a (Table 19). Interviews were audio-recorded and transcribed in full. The recruitment target was exceeded because of purposive sampling to ensure coverage of participants across all trial areas.
| Smoking status | Intervention | Control |
|---|---|---|
| Abstinent | 15 | 11 |
| Relapsed | 8 | 9 |
Secondary sampling characteristics
-
Age (three groups: older, mid, younger. Cut-off points based on trial age distribution):
-
older, > 55 years, n = 18
-
mid, 30–54 years, n = 23
-
younger, 18–29 years, n = 2.
-
-
Sex:
-
women, n = 23
-
men, n = 20.
-
-
Employment status:
-
employed or self-employed, n = 25
-
retired, n = 9
-
unemployed, n = 2
-
‘other’, n = 7.
-
-
Level of prior nicotine dependence (self-report of number of cigarettes smoked per day at trial baseline):
-
heavy, ≥ 30 per day, n = 3
-
moderate, = 11–29 per day, n = 29
-
light, ≤ 10 per day, n = 11.
-
-
History of smoking (number of prior quit attempts):
-
one or two, n = 20
-
three or more, n = 21
-
never tried before, n = 1.
-
(one person missing data).
-
-
Use of pharmacological interventions (NRT, varenicline – self-report at baseline) – all used something apart from one person (willpower alone).
-
Service attendance (core specialist Stop Smoking Service or other community-based stop smoking advisor:
-
core service, n = 31
-
other community-based advisor, n = 12.
-
-
Area (recruitment area):
-
Norfolk, n = 37
-
Hertfordshire, n = 3
-
Suffolk, n = 2
-
Lincolnshire, n = 1.
-
This purposive sample was representative of the study population as a whole in terms of secondary sampling characteristics: gender, employment, use of pharmacological interventions, service attendance and area of recruitment. We specifically sought to oversample from those who reported continued abstinence at follow-up, since this was the primary trial outcome studied.
There was slight over-representation of older people (> 55 years), lighter smokers (≤ 10 per day) and those with a greater number of prior quit attempts (≥ 3) – probably a function of age.
Findings
Of the 43 interviews fully transcribed, 23 participants were recruited from the trial intervention group and were probed during interviews around their use of the self-help booklets. Questions were open ended to be non-leading, but prompts asked participants to think about how they had used the booklets as well as their general impressions of them, their perceived usefulness and suggestions for possible improvements to the materials.
Participants gave general comments that were classified as broadly positive or negative. Further comments were coded to explore perceptions of the content, perceived impact, use and possible improvements related to the booklets (see Figure 17).
Content of Forever Free booklets
There were some very positive comments about the design and content of the booklets. Specifically mentioned without prompting was information given on the physiology of stopping smoking and the physical process of craving. In addition, participants spontaneously volunteered that identifying smoking triggers and suggestions for distraction strategies were useful when smoking cravings were felt.
One participant discussed remembering the information given about the physical impact of stopping smoking, and how he found this useful and motivating:
It was a glance through and there was one bit that I did read which I found quite interesting was the effects when you stop smoking . . . what happens after 1 day . . . 2 days after a week.
Was it like sort of a chart?
Like a chart sort of thing.
Yes.
And it goes down you know after I forget what it was now but after so many days nicotine leaves the body . . . um, and then sense taste and smell starts to improve . . . and then it gets to the point of uh your chances of a heart attack become the same as a non-smoker . . . and after so long, you know, and that’s a target to go for.
Participant number 0766: male, aged 54 years, abstinent, intervention group
The frequency of coding around this area, and other more technical aspects of stopping smoking, seemed to suggest that participants valued scientific information that reaffirmed their reasons for stopping smoking, and provided motivation to keep maintaining abstinence for a certain period of time (e.g. to reach the point at which chances of a heart attack become the same as for a non-smoker). Others commented specifically on concrete and practical content of the booklets that they felt able to implement themselves, such as the suggestions for distraction strategies:
I wouldn’t say there was any one – thing – only, only the, the, the – if you, you start getting the urge then to try and keep yourself, erm – keep your, your mind occupied and, and [coughs] and so to divert it away from – erm from just thinking about cigarettes.
Yeah – so when you had an urge, you know or when you felt like you really wanted to smoke, you used some of what it suggested? . . .
As I say the drinking, the drinking plenty of, something I never used to do, drink plenty of water and . . . and just general keep try, general keep me, keep myself a bit busy.
Participant number 0677: male, aged 75 years, relapsed, intervention group
Participants generally felt that the booklets were ‘well designed’ and ‘authoritative’. The first booklet, giving an overview of relapse prevention, was particularly mentioned more frequently than the other booklets. The booklet focusing on weight and controlling weight gain was also mentioned. However, some participants felt that the booklets were ‘overly simplistic or contained too much information’. Others could not remember specific details about the booklets, and 6 of the 23 intervention group participants reported that they felt that the booklets contained no new information – ‘nothing that I didn’t know already’:
What was in the booklets is . . . it was more geared up to somebody that, but then I still think that people who are smoking know all of the coping strategies to get through it and things, maybe it just reiterates it a bit more, but . . . it was teaching your granny to suck eggs.
Participant number 0748: female, aged 39 years, abstinent, intervention group
This point was specifically mentioned in the context of the advice that had already been received via the Stop Smoking Service:
Most – as I said – I’d already received from [name] or from my advisor.
Participant number 0702: male, aged 33 years, relapsed, intervention group
Perceived impact
There was a mixture of responses as to the perceived longer-term impact of the booklets. Some reported that the booklets were depressing or actually worked counter to their aim by reminding the participant of smoking:
I’ll be very honest and I’ll say after I read the first, I started to read it, I got in, er I flicked through a few books.
Yeah, yeah.
I got depressed.
Really.
So yeah, I, I felt like a cigarette after I read it.
Participant number 0666: male, aged 65 years, abstinent, intervention group
However, 12 of the 23 intervention group participants reported retaining the booklets, with a view to ‘keep referring back’ to them. Others who had relapsed ‘planned to use the booklets for a future quit attempt’.
Many participants reported ‘sharing them with others’. One focus group participant also corroborated this, reporting that she referred to the booklets in her own work within the health service and found it particularly useful to signpost patients towards sections of the booklets of relevance to them, such as the information on weight loss or dietary changes. Twelve of the intervention group participants reported this, saying that they allowed partners or family members to read them.
Uh so I read that quite seriously, the others I glanced through, my son was up and he said ‘what are you doing dad?’ and I told him and he said ‘oh I’ll have a go at them’.
Yes.
So he took them . . . so I didn’t really read through them very much but he took them and he stopped.
Participant number 0720: male, aged 69 years, abstinent, intervention group
Four participants reported quite forcefully that the booklets were ‘not for me’. The data here gave a sense that, while the booklet format and content may be particularly suited to some people, there were others for whom the booklets were definitely not suited:
It wasn’t pitched too high or too low, I think it was just right for somebody, but personally not for me, and in fact that’s why I only read the first couple I thought ‘well . . . if they’re all like that’ . . . you know, not for me, but they’re still in the cupboard, I’ve not just ditched them.
Participant number 0748: female, aged 39 years, abstinent, intervention group
Use of the booklets
As with the perceived impact, participants described different degrees of engagement. Some participants reported ‘flicking through the booklets, not reading all the booklets’ or reading them once but never referring back to them:
Yeah I was given the booklets, yeah I read through most of them but ummm, yeah, and then they were put out of the way, and I haven’t looked at ‘em since, to be honest! [Laughs.]
Participant number 0638: male, aged 59 years, abstinent, intervention group
However, 12 of the 23 intervention group participants reported that they did refer back to the booklets as a strategy to help them cope with urges to smoke:
And it’s basically, if I come across a, you know, a weak, weak moment or whatever . . .
You would look at the booklets?
Yeah, yeah, yeah I would go through yes . . . almost definitely, distinctly feel, as I say, I’ll see how I get on this weekend, but I, er, definitely think I’ll, er, I’ll have to, I’ll need to refer back to them I think . . .
Yeah, yeah. And did you keep looking at them every day, or did you put them aside for a few weeks and come back to them?
No, I, er, I wouldn’t say I looked at them every day, I, two or three times, at least two or three times a week, I would look through them.
Participant number 0677: male, aged 75 years, relapsed, intervention group
Others reported referring back to the booklets to reaffirm their motivation to stay abstinent, and to praise themselves by reminding themselves of how long it had been since the initial quit attempt:
But then obviously I went back to them and then obviously . . . yes, I already had it open because, yes I did, cos every now and then I would I’d just look at it just to remind myself that, you know, now you know where I am now, where I was even like 6 months ago . . .
Participant number 0723: female, aged 45 years, abstinent, intervention group
Suggestions for possible improvement to the materials
Many other suggestions were given by individual participants for possible ways in which the self-help booklets might be improved, including those listed below:
-
an abbreviated version/needs to be more succinct
-
addressing smoking marijuana
-
content needs to be more targeted towards certain age groups
-
information would be better as a ‘slow drip’
-
more graphic or explicit material on the harms of smoking
-
more information on downsides of NRT
-
more information on health consequences
-
more information on positive health improvements of staying stopped
-
more information on NRT and other stop smoking aids
-
more information on side effects of stopping smoking
-
more visual impact
-
need information on electronic cigarettes
-
would have been good to have had them at first contact with stop smoking advisor
-
electronic booklets would be preferable, as could flick to relevant information easily
-
would be good as PDFs (portable document format files) for an e-book reader
-
would use a mobile phone app
-
would use an app
-
would use the internet for support booklets.
Frequent suggestions were for electronic versions of the self-help booklets in PDF format, as an app or available as part of internet-based support. Suggestions about the timing of the information were also illuminating, as some reported feeling overwhelmed by the quantity of information received all at once or that the information would be better received as a ‘slow drip’, delivered over a longer period of time. These comments link to other comments around the desire for more ‘succinct’ or ‘abbreviated versions’ of the booklets.
Use of the control leaflet
For comparison between the trial groups, participants from the control group were also asked about their use of the self-help relapse prevention leaflet Learning to Stay Stopped. Comments in relation to this leaflet were, unsurprisingly, briefer. However, revealingly, many of the inductively derived codes were similar to the coding reported above for the intervention booklets. In terms of the leaflet content, participants generally reported that the leaflets did not contain anything they had not already learnt themselves, possibly via their stop smoking advisor:
To be honest, I’ve been trying to stop for so long, to be honest, it didn’t have anything in it that I didn’t already know.
Participant number 0697: female, aged 46 years, relapsed, control group
Although there was a roughly even split among control group participants between those who generally reported that they found the leaflet ‘useful’ and those who found it ‘not useful’, many of the more in-depth comments were more negative, and there was a perception that the information was not relevant to me:
Um, I wouldn’t say to me, personally, they were useful . . . um, I feel they’d be much more helpful to somebody younger or somebody that hasn’t smoked for as long.
Participant number 0779: female, aged 44 years, relapsed, control group
Many comments about use of the control leaflet related to the fact that the leaflet was not frequently referred back to, or was read once but not again:
I couldn’t pick out I really couldn’t, yes it’s just it’s one of those, one of those pieces of literature you read . . . and you digest and then you think it’s not, it doesn’t interest me what it’s saying, so you just put it out of your mind, so to be honest I didn’t actually gain anything. I didn’t go back to it. I didn’t do anything with it.
Participant number 0727: female, aged 53 years, abstinent, control group
Study 1b: feedback from professionals
A short group discussion was held with cessation professionals to feed back initial study findings. Twelve stop smoking advisors attended this team meeting (including advisors and team leaders/managers). This brief informal feedback meeting was used as an opportunity to gain feedback from stop smoking professionals on the trial findings and to glean views on the self-help materials used as part of the trial.
The stop smoking professionals commented that they liked the booklets and that they looked professionally produced. Booklets were passed around at the group discussion but no detailed comments were given with regard to the specific content of the booklets.
Professionals reported that they felt a slight sense of disappointment that the trial had found that the booklets were not effective. However, they were reassured that the trial had been robustly carried out and were interested in feedback from qualitative interview data about participant use of the Stop Smoking Services.
The stop smoking advisors were pleased and interested that the overall relapse prevention rates had been better than the national average and were interested in how the trial rates compared with Norfolk generally and with national figures. They suggested that the reduced relapse rates could possibly be a result of the follow-up provided by the research team and advisors raising the subject of relapse with participants as part of the recruitment process, and in particular asking them to sign forms. The normal protocol in the service was to congratulate smokers on quitting and remind them not to have even one puff in the future. It was thought that the focus on relapse prevention as the trial was recruiting may have had an effect in itself in improving relapse rates in both trial groups. As a practical implication of this possibility, the advisors suggested that the service could consider providing patients with a contract for longer-term support to help encourage continued abstinence from smoking or to encourage relapsed smokers to return to the service where relapse occurred.
Study 1c: findings from focus group discussions with participants
Following completion of recruitment to the qualitative interview project, two focus groups were arranged with trial participants. The aim of these participant focus groups was to feed back initial study findings as well as to stimulate group discussion and further feedback on both the intervention and control materials. We initially aimed to run four focus groups, across both trial and intervention group participants, and to capture the views of those who had remained abstinent and those who relapsed back to smoking. However, we found it difficult to gain agreement from those who had relapsed to attend focus groups. Therefore, we ran two focus groups with those who had remained abstinent from smoking, further capturing the views of 10 trial participants, across the control and intervention groups.
Demographically, those participating in the focus groups were broadly similar to those participating in the trial for all parameters with the exception that younger participants (18–29 years) were not represented.
Both focus groups were audio-recorded, although transcription was not undertaken, as data were used for verification of the qualitative analysis.
In general, findings from the focus groups supported the qualitative analysis reported (see Study 1a: qualitative interviews with individual trial participants, Findings). Participants reported mixed responses to the intervention booklets, with some reporting having read and engaged with the materials, and others reporting not reading the booklets and not retaining them for future reference. Of note, two of the sample of 10 focus group participants reported never having seen either the control leaflet or the intervention booklets. This is perhaps significant given the small numbers of focus group attendees, although it may be a reflection of recall difficulty, as some participants had entered the trial 2 or 3 years previously.
Those attending the focus groups generally felt that the key to maintaining abstinence from smoking was centred on internal motivation and a real drive to want to succeed. Prompts were given about the usefulness of support and self-help materials, but the consensus was that no booklet or leaflet would be sufficient to prevent relapse if the internal motivation to stay quit was not sufficiently strong. However, once quit, and with a strong desire to stay quit, participants did report that it was useful to be able to refer to information about the benefits of staying abstinent from smoking, and some suggested that looking back at self-help materials assisted them with maintaining motivation to stay quit. However, there was a general feeling that the form of self-help information could just as easily be a brief leaflet and that the more extended support offered via the intervention booklets was superfluous.
As in the in-depth qualitative interview results, focus group participants reported that they did not gain any significant new information, or at least at this time point could not recall any information, from the self-help materials in comparison with the help and advice they had received from the Stop Smoking Service. Participants spoke with fondness about their individual stop smoking advisors. They reported a sense of increased motivation as a result of the regular initial meetings with stop smoking advisors, and a sense of reward when they managed to maintain the quit and had this validated via a CO test with the stop smoking advisor. In this sense, there was a general feeling that self-help support could be a useful adjunct to the advice received during the one-to-one appointments with stop smoking advisors, but that the one-to-one support, combined with the pharmacological support on prescription, was the key element to their long-term successful quit attempt. The focus group participants echoed the interviews when they reported enjoying the extended contact with the research team and the follow-up CO test at 12 months. They suggested that this continued contact could be an addition to the Stop Smoking Service, and that others might be encouraged to maintain a long-term quit attempt knowing that a follow-up appointment would be arranged. Additionally, a number of focus group participants reported sharing the intervention booklets with others (family members or friends), and that others had managed to stop smoking while also referring to these booklets (see Study 1a: qualitative interviews with individual trial participants, Findings).
As focus groups were conducted following completion of the trial follow-up, for some participants the initial quit date had been nearly 3 years previously. Interestingly, 8 out of the 10 participants attending the focus group discussion reported that they had initially quit using varenicline as an aid. Focus group participants were very keen to discuss varenicline and the consensus was that it worked remarkably well for the initial cessation attempt.
Conclusions
The qualitative process analysis clearly shows a mixed response to self-help booklets for the prevention of smoking relapse. Specifically with regard to the intervention booklets, participants seem to be either very motivated and to have really engaged with the booklets, as demonstrated by the positive comments, or to have disliked the booklets and in some cases not read them at all. For those reporting negative feelings towards the booklets, the overall sense was that the booklets were not useful and did not offer any particularly new or novel insights to what was already known about smoking relapse prevention. This may be a result of the detailed advice that was received on a one-to-one basis via stop smoking advisors. Indeed, participants were often able to recall advice delivered face to face but found it more difficult to recall the content of the trial intervention booklets.
The self-help booklets were relevant and attractive to some, but not to all. This suggests that careful targeting of self-help materials to those most receptive to the self-help approach may be a way forward for smoking relapse prevention. Resources are wasted on those who do not enjoy reading or who find engaging with self-help materials unhelpful.
The self-help intervention booklets were experienced as particularly unhelpful by some participants, who reported that they did not wish to be reminded of smoking. Again, this suggests that careful targeting of intervention materials is appropriate, as some individuals may do better if they are able to distract themselves during times of craving by focusing on something completely unrelated to smoking. For others, it was helpful specifically to read the booklets, reinforce their own reasons for quitting and remind themselves of the health benefits of staying stopped. It is potentially important for future service provision that these differences in personality traits and approach be established by stop smoking advisors in order that relevant advice and possible reference to self-help materials can be appropriately tailored.
The qualitative process evaluation data revealed that in some cases neither intervention nor control materials were received by individuals. Similarly, a small minority of participants reported difficulty with reading the study materials. Although recruitment attempted to screen for reading difficulties in order to exclude participants who were unable to read or had difficulty with reading, clearly a number of participants may have slipped through this screening net.
Many participants in the qualitative study, whether abstinent or relapsed, reported retaining and sometimes referring back to the intervention booklets. Others reported their intentions of using the booklets in future quit attempts. While there is likely to be some positive respondent bias impacting on these reports, a potential benefit of self-help relapse prevention materials is that, if retained, they can be reused and referred back to in future quit attempts. Conceptualising addiction to tobacco as a chronic relapsing/remitting condition, and the task of achieving abstinence as a process or a cycle, suggests that the longer-term impact of self-help materials may be important in supporting eventual abstinence. Although this longer-term follow-up is beyond the scope of this study, recording data on use of self-help materials over time is an area for future research in the field of monitoring of smoking behaviour.
Chapter 7 Economic evaluation methods and results
Overview
In order to estimate the cost-effectiveness of the intervention booklets compared with the control leaflet, a within-trial cost–utility analysis was undertaken. In the base-case analysis, costs were estimated from the perspective of the NHS and the EQ-5D was used to estimate QALYs. Participants were randomised at the 4-week quit point (baseline data were taken at this point) and followed up 2 months later (at the 3-month follow-up point) and 11 months later (at the 12-month follow-up point).
Methods
Estimating costs
The study team monitored the levels of resource use associated with the intervention booklets (including intellectual property, adaptation, printing and postage; this was also done for the control booklet. Additionally, at the 3- and 12-month follow-up points, participants were asked to report how many of the following items of resource use they had received:
-
NHS Stop Smoking Clinic visits
-
NHS Stop Smoking Clinic phone calls
-
stop smoking aids (medication and materials)
-
GP visits
-
hospital admissions (and total number of days admitted, if applicable).
At the 3-month follow-up participants were asked about usage in the 2 months since randomisation, and at the 12-month follow-up they were asked about usage the last 9 months. Together this enabled usage to be estimated over the 11 months since the 4-week quit date (baseline data were taken at this point).
In relation to the medication (if applicable) participants were also asked whether or not they paid for this themselves. In the base-case analysis,45 as the UK National Institute for Health and Care Excellence methods guide46 recommends costs be calculated from the perspective of the NHS and Personal Social Services, participant-borne medication costs were excluded. They were, however, included in a subsequent sensitivity analysis. 45
Costs were assigned to each of the aforementioned items of resource use, where these were estimated at 2012/13 financial year levels. Discounting was not undertaken, as the follow-up time was less than 1 year. To estimate per-participant costs for the intervention and control booklets, the total cost was simply divided by the number of participants in the arm in question.
Overall per-participant costs were estimated by summing the cost associated with each reported resource item, plus the estimated cost of the intervention or control booklets (depending on the arm to which the participant was allocated). Hereafter, this base-case analysis is referred to as the NHS perspective. When participant medication costs are added, this is referred to as the NHS and participant medication costs.
Measuring outcomes
To estimate levels of health-related quality of life, participants were asked to complete the EQ-5D-3L36 at baseline (the 4-week quit point), 2 months later (at the 3-month follow-up point) and 11 months later (at the 12-month follow-up point). Responses were then converted into a utility score (a scale where death is equal to 0 and full health 1) using the York A1 tarrif. 47 The area under the curve method48 was then used to estimate the QALY score over the 11-month period since baseline data were collected at the 4-week quit date (the EQ-5D score was assumed to change linearly), enabling a cost–utility analysis to be undertaken.
Missing data assumptions
If a participant reported that he or she had a contact with the NHS Stop Smoking Clinics but did not report the number of visits/phone calls, then the average value for those who reported these values was used. Similarly, if a participant reported visiting visited his or her GP, or having been admitted to hospital, but did not report the associated number of visits/days, the average value for those who reported these values was used. If a respondent reported taking a particular medication, but did not report the associated number of weeks, then the number of weeks was assumed to be equivalent to the average for those who responded. If the participant did not report paying or otherwise for any of the medication he or she reported having taken, it was assumed the NHS would have paid for it. All other missing data were left as such and corresponding individuals were excluded from the analysis.
Analysis
The intention-to-treat strategy was used where patients were analysed within the group to which they were allocated, regardless of, for example, whether or not they had used the booklet(s) in question. A complete-case analysis approach49 was also adopted, whereby participants were included only if complete cost and outcome data at all time points were available. In order to estimate the mean incremental cost and incremental effect (QALY gain) associated with the intervention, compared with that for the control group, regression analysis was undertaken. The system of seemingly unrelated regression equations50 was used as it has been argued that such methods are generally robust to skewed data and to allow for any correlation between costs and effects. The cost and QALY regressions were ran simultaneously, with particular baseline demographic and smoking descriptive variables being included (as covariates) only if all participants (for whom cost and effect data were available) had complete data for the covariate in question and there was an a priori expectation that the covariate might be associated with cost and/or QALY scores.
With regard to the interpretation of incremental cost and incremental effect data from the regression, assuming that dominance45 did not occur, that is, that neither intervention nor control was on average both more costly and less effective, the incremental cost-effectiveness ratio (mean incremental cost/mean QALY gain)46 was calculated. However, if either the incremental cost and/or incremental effect were negative, owing to the potential for misinterpretation, the incremental net benefit51 would be calculated at the cost-effectiveness threshold (λ) value of £20,000 per QALY. 46 Additionally, in order to estimate the level of uncertainty associated with the decision as to whether or not the intervention was cost-effective, we used the non-parametric bootstrap technique52 (with 5000 replications sampled with replacement) to estimate the probability that each intervention was cost-effective according to the cost-effectiveness acceptability curve (CEAC). 53 The probability of the intervention being cost-effective was specifically estimated at the λ of £20,000 per QALY.
An additional threshold analysis45 was also conducted to cater for the possibility that the control group within this study may have received more than usual care – provision of a single leaflet containing key messages for smoking relapse prevention. We therefore estimated what the incremental cost of the intervention would have been on the basis of the intervention (Forever Free booklets) cost alone and then assessed what QALY gain would be necessary, given such a cost, in order for the cost per QALY to fall below the cost-effectiveness threshold (λ) value of £20,000 per QALY. 46
Results
Response rates
The number of people who completed the EQ-5D-3L questionnaire at baseline (the 4-week quite point), the 3-month follow-up and 12-month follow-up was 682 (97.0%), 644 (91.6%) and 574 (81.7%) respectively in the intervention group and 666 (94.7%), 646 (91.9%) and 597 (84.9%), respectively, in the control group. Based on these data, QALY scores could be calculated for 537 (76.4%) intervention participants and 530 (75.4%) control participants. In relation to costs, the response rate varied across resource items – the number who completed both the resource item questions at 3 and 12 months varied between 555 (78.9%) for the intervention group and 574 (81.7%) for the control group in relation to hospital admissions, and 570 (81.1%) and 585 (83.2%) respectively for stop smoking medication. Overall costs were available for 549 (78.1%) and 570 (81.1%) participants respectively. Finally, a total of 521 (74.1%) intervention participants and 528 (75.1%) control participants completed all three EQ-5D questionnaires and each of the 3 and 12-month follow-up cost questionnaires, this group constitutes the complete-case group on which all subsequent analyses are based.
Costs
The estimated costs associated with the provision of the intervention and control booklets are outlined in Tables 20 and 21. Levels of other items of resource use, as reported by participants, are given in Tables 22 and 23. It can be seen that the levels of resource use are broadly similar across groups. The unit costs which were assigned to these resource items, along with details of the associated sources and assumptions, are outlined in Table 24. This enabled the overall (unadjusted) costs (from the perspective of the NHS), along with the total cost for each component item of resource use, to be calculated (see Table 22). The mean overall (unadjusted) NHS costs were estimated to be higher in the control group. However, it should be noted that this difference can largely be accounted for by hospital admission costs and the fact that one participant in the control group reported spending 98 days in hospital.
| Component part | Total cost (£) | Per-participant cost (£) |
|---|---|---|
| Copyright (booklets previously used in the USA)a | 1562.50 | 2.22 |
| Revisions to the booklets | 2500.00 | 3.56 |
| Printing costs | 8661.60 | 12.32 |
| Postage (£2.68 per pack, sent to 703 participants) | 1884.04 | 2.68 |
| Total | 14,608.14 | 20.78 |
| Component part | Total cost (£) | Per-participant cost (£) |
|---|---|---|
| Purchase of booklets, including printing | 217.50 | 0.31 |
| Postage (0.36p, sent to 704 participants) | 253.44 | 0.36 |
| Total | 470.94 | 0.67 |
| Item | Levels of resource use | Mean cost (£) | ||
|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |
| NHS Stop Smoking Clinic visits | 2.25 | 2.29 | 31.45 | 32.12 |
| NHS stop smoking phone call | 0.27 | 0.31 | 0.95 | 1.09 |
| Stop smoking medication (NHS perspective) | 110.35 | 117.07 | ||
| Stop smoking medication (NHS and individual perspective) | 134.71 | 143.99 | ||
| NHS Quit kit | 0.04 | 0.05 | 0.04 | 0.05 |
| GP visits | 4.55 | 4.56 | 168.53 | 168.86 |
| Hospital admissions (days) | 0.63 | 0.97 | 221.67 | 338.08 |
| Provision of intervention booklets | 20.78 | – | ||
| Provision of control booklets | – | – | – | 0.67 |
| Overall costs (NHS perspective) | – | – | 553.78 | 657.95 |
| Overall costs (NHS and participant medication costs) | – | – | 578.14 | 684.87 |
| Item | Levels of resource use, per participant (weeks) | Mean cost (£, NHS perspective) | Mean cost (£, NHS and individual medication perspective) | |||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| Bupropion | 0.03 | 0.04 | 0.63 | 0.80 | 0.63 | 0.80 |
| Varenicline | 2.94 | 3.08 | 49.74 | 52.12 | 49.74 | 52.12 |
| Nicotine gum | 0.63 | 0.53 | 1.92 | 2.08 | 4.03 | 3.44 |
| Nicotine patches | 2.13 | 2.39 | 19.78 | 23.41 | 24.84 | 27.15 |
| Nicotine microtabs | 0.57 | 0.72 | 2.98 | 2.27 | 4.54 | 5.76 |
| Nicotine lozenges | 1.34 | 1.23 | 4.38 | 4.19 | 10.04 | 9.24 |
| Nicotine inhalator | 1.70 | 2.11 | 18.17 | 21.18 | 24.93 | 30.95 |
| Nicotine nasal spray | 0.05 | 0.11 | 0.81 | 0.85 | 0.81 | 1.77 |
| Nicotine mouth spray | 1.30 | 1.09 | 11.95 | 10.16 | 15.14 | 12.75 |
| Item | Estimated unit cost (£) |
|---|---|
| NHS Stop Smoking Clinic visita | 14.00 |
| NHS stop smoking phone callb | 3.50 |
| Bupropion (weekly cost)c | 19.24 |
| Varenicline (weekly cost)c | 16.93 |
| Nicotine gum (weekly cost)c | 6.44 |
| Nicotine patches (weekly cost)c | 11.64 |
| Nicotine microtabs (weekly cost)c | 8.00 |
| Nicotine lozenges (weekly cost)c | 7.52 |
| Nicotine inhaler (weekly cost)c | 14.69 |
| Nicotine nasal spray (weekly cost)c | 16.21 |
| Nicotine mouth spray (weekly cost)c | 11.66 |
| NHS Quit kit (weekly cost)d | 1.00 |
| GP visite | 37.00 |
| Hospital admission – cost per dayf | 349.12 |
Outcomes
European Quality of Life-5 Dimensions-3-Level scores at baseline, 3-month follow up and 12-month follow-up are given in Table 25. Again, it can be seen that these scores are broadly similar in the two groups; this also applies to the QALY scores, which were calculated from the EQ-5D-3L scores.
| Intervention group | Baseline | 3-month follow-up | 12-month follow-up | QALY |
|---|---|---|---|---|
| Intervention | 0.832 (0.256) | 0.825 (0.251) | 0.814 (0.254) | 0.753 (0.204) |
| Control | 0.821 (0.254) | 0.821 (0.242) | 0.806 (0.255) | 0.747 (0.196) |
Analysis
The results of the seemingly unrelated regression are shown in Table 26, where it can be seen that there was no significant difference between either the overall cost (NHS perspective) or the QALY score between the two groups. As the incremental cost and QALY gain were both (slightly) negative, we estimated the mean incremental net benefit [at the (λ) value of £20,000 per QALY] to be £74.79. This positive value would indicate that the intervention was estimated to be cost-effective. However, the CEAC shows that there is a large uncertainty associated with this result, as the intervention has only a 64.4% probability of being cost-effective at this level, that is there would be a 35.6% chance of making the wrong decision by choosing to implement the intervention.
| Independent variable | Dependent variable | ||
|---|---|---|---|
| Costs (NHS perspective, £), (95% CI) | Costs (NHS and participant medication costs, £), (95% CI) | QALY score, (95% CI) | |
| Treatment group (intervention vs. control) | –84.49 (–280.96 to 111.98) | –87.89 (–284.33 to 108.54) | 0.000 (–0.017 to 0.016) |
| EQ-5D baseline score | –1269.74 (–1686.84 to –852.64) | –1254.09 (–1671.13 to –837.05) | 0.536 (0.501 to 0.517) |
| Gender (female vs. male) | –38.84 (–246.21 to 168.53) | –55.33 (–262.67 to 152.01) | 0.014 (–0.003 to 0.031) |
| Age at baseline | –1.28 (–11.17 to 8.61) | –1.06 (–10.94 to 8.83) | –0.001 (–0.001 to 0.000) |
| Employment status | |||
| Unemployed vs. in paid employment | –109.21 (–471.25 to 252.83) | –103.44 (–465.43 to 258.54) | –0.052 (–0.022 to –0.082) |
| Looking after the home vs. in paid employment | –119.07 (–532.29 to 294.15) | –136.70 (–549.86 to 276.46) | –0.001 (–0.035 to 0.034) |
| Retired vs. in paid employment | 253.92 (–80.63 to 588.48) | 250.67 (–83.84 to 585.18) | –0.031 (–0.059 to –0.003) |
| Other vs. in paid employment | 465.08 (122.81 to 807.35) | 472.28 (130.06 to 814.50) | –0.026 (–0.054 to 0.003) |
| Number of cigarettes per day before quitting | |||
| 11–20 vs. ≤ 10 | 191.73 (–95.46 to 478.92) | 188.33 (–98.82 to 475.48) | –0.004 (–0.028 to 0.020) |
| > 20 vs. ≤ 10 | 307.69 (–17.79 to 633.19) | 311.61 (–13.82 to 637.05) | –0.020 (–0.047 to 0.008) |
| Constant | 1496.82 (827.66 to 2165.98) | 1509.78 (840.71 to 2178.85) | 0.353 (0.297 to 0.409) |
The results of the sensitivity analysis (NHS and participant medication costs) were virtually identical to that of the base case (see Table 26), although the slightly lower mean incremental cost resulted in a slightly higher incremental net benefit (£78.20) and probability of cost-effectiveness (66.1%).
The cost of providing the Forever Free booklets was estimated to be £20.78. Threshold analysis indicated that, in order for the cost per QALY to fall below the threshold of £20,000 per QALY, a QALY gain > 0.0010 would be required. Taking account of the fact that the area under the curve technique is used estimate the QALY, a utility gain of 0.0013 would thereby be required for the cost per QALY to fall below that threshold.
Summary
A complete-case within-trial analysis was conducted, with costs estimated from an NHS viewpoint. Based on regression analysis, it was estimated that there was no significant difference in mean costs or mean QALY scores between the intervention and control groups. Although the estimated mean incremental net benefit was positive (£74.79), the probability of cost-effectiveness was estimated to be only 64.4% according to the CEAC (based on λ = £20,000 per QALY). Coupled with the aforementioned effectiveness results, this would suggest that we are not able to conclude that the provision of the intervention booklets is cost-effective.
Chapter 8 Discussion
This randomised controlled trial compared the effect of a set of eight revised Forever Free booklets and a control leaflet on the prevention of smoking relapse in 4-week quitters who used NHS Stop Smoking Services. The trial randomised 1407 eligible 4-week quitters, and the follow-up rate was 93% at the 3-month follow-up and 86% at the 12-month follow-up. The trial results indicate that there were no significant differences between the treatment and the control group in terms of the primary end point (36.9% vs. 38.6%) and any secondary smoking outcomes at 12 months after quit date (see Table 6). There was no evidence that people in the intervention group developed more skills and responses believed to be necessary to prevent relapse than those in the control group. Our qualitative study also found mixed results, with participants either liking or strongly disliking the intervention booklets.
Comparison with other relevant evidence
According to findings from two systematic reviews, self-help educational booklets were effective in preventing smoking relapse in unaided quitters. 23,24 For example, the pooled OR of self-help booklets for smoking relapse prevention in unaided quitters was 1.52 (95% CI 1.15 to 2.01),24 based on data from three randomised trials. 25,26,57 Specifically, two of the three trials included in the meta-analysis25,26 found that a set of eight Forever Free booklets was more effective than a single introduction booklet for the prevention of smoking relapse in unaided quitters. However, results from the current trial indicate that the full set of Forever Free booklets was no more effective than a single leaflet in short-term quitters who received behavioural support to stop smoking (Figure 18). To be comparable with the outcome reported in other trials, the outcome used in Figure 18 was self-reported smoking prevalence at the end of the follow-up. The difference in the OR for self-reported smoking prevalence between the current trial and the previous studies is statistically significant (p = 0.03).
FIGURE 18.
Results of the current and previous trials: self-reported point smoking prevalence.
It is worth considering possible explanations for the different findings in the present study compared with the two previous studies using a very similar intervention. 25,26 Whereas the present study found no outcome differences between the intervention and control conditions, the previous studies both reported statistically significant group differences among recent unaided quitters. The following key differences between the studies might provide insight into the discrepant findings.
-
The original studies were conducted in the USA more than 10 years ago, whereas the present study was conducted in the UK. Aside from the other differences noted below, it is possible that cultural differences interfered with the efficacy of the booklet, even though surface structure adaptations were enacted.
-
The booklets were originally developed to aid self-quitters in place of more intensive face-to-face treatment, and the previous studies involved those smokers. In contrast, the present study used the booklets as an extension of an intensive face-to-face intervention provided by the NHS Stop Smoking Clinics. Indeed, the abstinence rates achieved in this study exceeded even the typical rates reported for NHS clinics. Thus, smokers in the present study may have received unusually high-quality treatment, which may have rendered the booklets superfluous. That said, it is worth noting that many participants still had no strategy to deal with urges to smoke. Previous research found little effects of the booklets among (1) individuals who had been abstinent for more that 3 months at the time of enrolment,25 (2) telephone quitline callers who had received NRT58 and (3) pregnant women from higher income groups. 59 This pattern suggests that the booklets have greatest effect among those receiving suboptimal assistance with their efforts to quit smoking.
-
The present study achieved outstanding follow-up rates by using a series of strategies to keep participants engaged in the study. Moreover, follow-up assessments were inclusive and conducted by phone or in person. In contrast, the previous studies collected follow-up data via brief mailed questionnaires with the goal of minimising possible reactance effects. It is possible that follow-up procedures used in the present study enhanced the treatment effect, thereby further limiting the additional effect of the intervention.
-
The eight booklets were originally developed to be distributed to smokers over the course of year, with the goal of maintaining their motivation to quit throughout this period. However, one study found that the booklets were equally efficacious when delivered all at once. 26 For this reason, we sent participants all eight booklets at once in the present study. However, in retrospect, this bulk mailing immediately after the NHS intervention may have increased the tendency for respondents to feel the intervention was somewhat redundant. Perhaps it may have been better to have delivered this intervention as a series of mailings over the follow-up period, when they might have served to both maintain abstinence motivation and remind participants of key messages from counselling.
The self-reported abstinence at 12 months was on average 52% and the continuous CO-validated smoking abstinence from months 2 to 12 was on average 32% (95% CI 30% to 35%) in the current study (see Table 6). These secondary smoking end points may be more comparable to the smoking outcome used in other longer-term studies of 4-week quitters in NHS Stop Smoking Services. 13,60,61 The prevalence of both continuous CO-validated abstinence and self-reported abstinence by 12 months was significantly higher in the current study than the estimates from other studies (Table 27).
| Study | Design | Number of quitters and recruitment time/location | Main characteristics of quitters | Smoking abstinence (95% CI) |
|---|---|---|---|---|
| Ferguson13 |
|
|
|
|
| Bauld60 |
|
|
|
|
| Snuggs61 |
|
|
|
|
| SHARPISH (the current trial) |
|
|
|
|
It is interesting to explore possible reasons for differences in smoking abstinence across these studies of 4-week quitters in NHS Stop Smoking Services. All studies in Table 27 recruited CO-validated 4-week quitters in NHS Stop Smoking Services. The average age and sex of the participants were similar across studies, although there may be some differences in other participant characteristics. Compared with the rest of England, Norfolk has a relatively high proportion of people aged ≥ 65 years (21.4% vs. 16.5% in 2010) and a higher percentage of European white people (94.3% vs. 87.5% in 2009). The current trial excluded pregnant women, quitters who were not able to read educational materials in English and those in prisons. It is likely that the two observational studies may be more inclusive, by including all CO-validated 4-week quitters who gave consent to participate in the study. Although some participant characteristics at baseline are associated with the risk of smoking relapse, it is not always possible to compare all participant characteristics across the studies because of differences in ways of measuring and reporting.
The study by Ferguson et al. 13 and the study by Bauld60 were observational studies, and the study by Snuggs et al. 61 was a single-arm pilot trial of text messaging for relapse prevention. No additional supports were provided to study participants in the two observational studies. In the pilot study by Snuggs et al. ,61 mobile phone text messages for relapse prevention were sent to study participants weekly for 12 weeks and then fortnightly for up to 6 months. Owing to the single-arm design and the large percentage of participants lost to follow-up (32%), it was unclear whether or not smoking abstinence was improved by the intensive text messaging intervention. In comparison, the SHARPISH trial was a randomised controlled study of a self-help intervention for smoking relapse prevention. All participants in the current study received a telephone follow-up interview at 3 months after quit date. Participants in the treatment group received a set of eight Forever Free booklets, and participants in the control group of the current trial also received a leaflet for smoking relapse prevention. We may therefore presume that the telephone follow-up intervention at 3 months and the brief educational leaflet might have reduced smoking relapse at 12 months. Indeed, anecdotal evidence from our qualitative study suggested that participants enjoyed participating in the study and were aware that they would be contacted by a researcher, which may have had a motivating effect on smoking abstinence. However, previous evidence summarised in a Cochrane systematic review19 indicated that extended interventions were not effective for preventing smoking relapse. A more recently published pilot study also reported that smoking abstinence at 52 weeks was not increased by extended interactive voice response telephony in aided quitters. 62
Participants lost to follow-up were counted as being relapsed in the three studies. The percentage of participants who were lost to follow-up was 15% in the current study, compared with 22% in Bauld et al. ,60 27% in Ferguson et al. 13 and 32% in Snuggs et al. 61 If some participants who were lost to follow-up remained abstinent, the low rate of loss to follow-up in the current study (15%) may have contributed to a high-rate of smoking abstinence at the follow-up.
In comparison with other published trials, our study is the only one to have included a detailed qualitative process evaluation. This enables us to interpret the trial findings considering the participant perspective of reported use of the intervention and control materials.
In terms of cost-effectiveness, we are aware of only one other paper that has assessed the cost-effectiveness of a similar intervention. 26 Brandon et al. 26 estimated only the cost of the intervention (a one-off mailing of the booklets compared with repeated mailing/letters) and included QALY estimates based on assumptions about how changes in quit rates would translate into QALYs. Their estimates of the intervention costs (US$21.15 to US$43.94, year 2000 price estimates) were comparable to ours, but there were differences in the mean incremental QALY gain. Brandon et al. 26 estimated the mean incremental QALY gain to be between 0.2561 and 0.2741, where these differences are likely to largely arise because, in contrast to our study, the booklets were found to be effective at increasing quit rates.
Generalisability
The results of the trial may not be generalisable to pregnant women, people younger than 18 years and those who are not able to read English as these groups were excluded. In addition, 98% of the study participants are European white, and the trial results may not be generalisable to ethnic minority quitters.
Of the 1959 eligible 4-week quitters, 19% (n = 370) declined study participation for various reasons (see Figure 2). Short-term quitters who consented to participate in the study may be systematically different from other eligible quitters who declined. Using data routinely collected by the Norfolk Stop Smoking Service, we examined the comparability of study participants and other short-term quitters in Norfolk. Data extraction from the Stop Smoking Service database was undertaken by the information technology department at the Norfolk Community Health and Care Trust. We obtained data on all episodes in which a CO-verified quit was recorded, for the period 1 August 2011 (commencement of SHARPISH recruitment) to 31 May 2013 (close of SHARPISH to recruitment), a total of 22 months. These data must be viewed with a certain amount of caution as improving data recording and data quality was an ongoing process during the period of the trial and it is acknowledged that the records held on the database for this period are not absolutely complete.
There were a total of 5186 CO-verified quits recorded in the period. The number of recorded events was 4626, after excluding prisoners, persons in secure hospitals and the Royal Air Force, young people under 18 years and duplicates episodes for the same individuals. The basic characteristics of study participants and other CO-validated short-term quitters in Norfolk are shown in Table 28. The average age of study participants and non-participants was similar (median 47 years), but there were slightly (although statistically significantly) fewer male quitters in the SHARPISH study (47% vs. 51%). The study participants had a higher percentage of managerial/professional occupation (18% vs. 15%), a higher percentage of intermediate occupation (9% vs. 5%) and a lower percentage of routine/manual occupation (29% vs. 33%).
| Characteristics | Norfolk SHARPISH | Norfolk ALL minus Norfolk SHARPISH | p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 1199 | 100 | 3427 | 100 | – |
| Age, years (median) | 47 | – | 47 | – | – |
| Sex: male | 563 | 47 | 1749 | 51 | 0.015 |
| Occupationa | |||||
| Full-time student | 15 | 1 | 24 | 1 | 0.073 |
| Home carer | 87 | 7 | 195 | 6 | 0.051 |
| Intermediate | 112 | 9 | 178 | 5 | < 0.001 |
| Managerial/professional | 213 | 18 | 504 | 15 | 0.012 |
| Never worked/long-term unemployed | 75 | 6 | 249 | 7 | 0.238 |
| Retired | 236 | 20 | 600 | 18 | 0.091 |
| Routine/manual | 347 | 29 | 1123 | 33 | 0.0143 |
| Sick/disabled unable to work | 95 | 8 | 239 | 7 | 0.274 |
| Unable to code | 19 | 2 | 315 | 9 | < 0.001 |
| Treatment episode numberb | |||||
| One | 705 | 59 | 2412 | 70 | < 0.01 |
| Two | 278 | 23 | 733 | 21 | < 0.01 |
| Three or more | 178 | 15 | 321 | 9 | < 0.01 |
| Unknown | 38 | – | 0 | – | – |
| Smoking cessation medication received in first 4 weeks after quit datec | |||||
| NRT | 476 | 40 | 1880 | 54 | < 0.001 |
| Varenicline | 694 | 48 | 1484 | 43 | < 0.001 |
| Bupropion | 5 | 0.4 | 21 | 0.6 | 0.435 |
| None | 3 | 0.3 | 21 | 0.6 | 0.133 |
| Unknown | 22 | 2 | 68 | 2 | 0.747 |
The study participants were less likely to be in their first episode of treatment with the Norfolk Stop Smoking Service than other CO-verified quitters (61% vs. 70%). Compared with non-participants in Norfolk, relatively more study participants received varenicline (48% vs. 43%) and relatively fewer study participants used NRT (40% vs. 54%) in terms of smoking cessation treatment medication (see Table 28).
Therefore, it is possible that study participants may be associated with relatively more favourable longer-term abstinence outcomes than non-participants. The differences were generally small or moderate, even though such differences tended to be statistically significant because of the large sample size. The results of exploratory analyses (see Table 7) indicated that the effect of the experimental booklets was not significantly modified by participant characteristics at baseline. Therefore, the relative effect of the experimental and control booklets on smoking abstinence at 12 months estimated by the current study should be generalisable to other short-term quitters who used NHS Stop Smoking Services in Norfolk.
Strengths and limitations
The current trial successfully achieved the recruitment target, and randomised 1407 eligible CO-validated short-term quitters, which is the largest such study so far. The percentage of participants who were lost to the final follow-up at 12 months was low (15%) compared with other studies that evaluated longer-term smoking relapse outcomes in 4-week quitters who used NHS Stop Smoking Services (see Table 27). The prespecified primary outcome is prolonged (with fewer than five cigarettes from months 4 to 12) CO-validated abstinence at 12 months. Participant allocation was adequately concealed and the main characteristics of participants were well balanced at baseline. The estimated 95% CI was narrow for the primary outcome (OR 0.930, 95% CI 0.749 to 1.154), which did not include the assumed average OR (1.44). Therefore, the statistical power was adequate to detect the assumed difference in smoking outcomes if it was true. Therefore, we are confident that there is no clinically important difference in smoking outcomes at 12 months between the intervention groups.
As with practically all behavioural interventions it was not possible to blind participants and investigators to allocation for follow-up. The primary and other smoking results are unlikely to be biased because of lack of blinding,29 although bias might have been introduced in the measurement of process and mediating variables such as reported reading of educational materials and feedback on the perceived helpfulness of the educational booklets. However, these assessments also showed few differences between the intervention and the control groups and therefore are unlikely to have played a role here.
Although there were no significant differences in smoking relapse between the experimental and the control groups, the overall rate of smoking abstinence at 12 months was relatively high in the current trial compared with other studies of short-term quitters in the NHS Stop Smoking Services (see Table 27). In addition to the follow-up interview at 3 months, the participants in the control group also received a single leaflet containing key messages for smoking relapse prevention and a telephone contact at 3 months. Additional to other possible explanations, a brief leaflet may be effective to reduce smoking relapse in short-term quitters. However, we could not be certain because the current trial had not included a control group without follow-up interview at 3 months and educational leaflet.
This study included a qualitative process evaluation. This is a strength of the approach as the qualitative study allows for further interpretation of the trial findings from a participant perspective. We found mixed responses to the intervention booklets, suggesting that some participants may be more receptive to self-help information than others, although we were not able to assess openness and willingness to accept to the self-help approach (psychological characteristics) as part of the trial.
Interpretation and implications
The main participant characteristics were comparable at baseline between the intervention and the control groups. The proportion of prolonged (from 4 to 12 months) CO-validated abstinence at 12 months after quit date was 36.9% in the intervention group and 38.6% in the control group. There was no statistically significant difference between the groups (OR 0.93, 95% CI 0.75 to 1.15). In addition, there were no statistically significant differences between the groups in other secondary smoking outcomes, such as 7-day self-reported point smoking prevalence, and verified smoking abstinence at the final follow-up. Subgroup and regression analyses did not reveal moderating effects of any baseline characteristics on the efficacy of the intervention. The current trial had adequate statistical power so that the result is unlikely to be false negative. Therefore, the convincing evidence from this randomised trial does not support the use of more self-help educational materials than a single leaflet for smoking relapse prevention in short-term quitters in smokers who have received behavioural support.
To interpret the trial results, it is important to remember the hypothesised mechanisms of coping skills training for relapse prevention. Self-help educational materials could be effective if:
-
quitters have received and read the self-help educational materials
-
by reading the self-help educational materials, quitters have learnt new skills or reinforced their skills to identify risky situations and to do something to handle smoking urges
-
quitters have actually done something to avoid high risky situations and/or something to cope with smoking urges.
In addition to these points, we hypothesised that the eight Forever Free booklets were more effective than a single leaflet containing the similar but much briefer points for smoking relapse prevention (see Table 1).
In the current trial, we collected data on process variables relevant to the hypothesised mechanisms. Compared with participants in the control group, relatively more participants in the Forever Free booklet group who reported that they had received the booklets (89% vs. 79%) at the 3-month follow-up, and reported that still had the booklets at 3 months (83% vs. 62%) and at 12 months (49% vs. 35%). The differences in the reported receipt and keeping of the self-help educational material between the two groups may be because a set of eight booklets used in the treatment group was more noticeable or substantial than a single leaflet used in the control group. In addition, reporting bias could not be rule out.
The proportions of participants who reported that they had read the booklets (or the leaflet) were similar (70% vs. 69%) at the 3-month follow-up. However, only small percentages of participants in both the treatment and control group reported that they had read the self-help material from 4 to 12 months (27% vs. 21%). In addition, participants in the treatment group reported that they spent somewhat more time reading the educational material than those in the control group. The embedded qualitative study revealed occasional instances when participants reported looking up the booklets to see how to deal with difficult situations, but this was uncommon. Participants regarded much of the material as nicely presented but nothing new, and the overall impression was that it lacked impact.
There were no significant differences between the groups in the percentages of participants who reported they knew any (little or much) more about relapse risky situations (52% vs. 51%) or ways of handling urges (51% vs. 51%) by the 12-month follow-up. More importantly, there were no differences between the groups in the percentages of participants who reported that they had attempted to do something to cope with urges between 2 and 3 months (83% vs. 83%) and between 4 and 12 months (60% vs. 61%).
All study participants had received intensive behavioural support from stop smoking advisors before participating in the trial, and most of them (89%) had previously attempted to quit. In addition, after quitting smoking at 4 weeks, 60% of the participants contacted NHS stop smoking advisors during months 2–3, and 15% did so from months 4 to 12. Therefore, it is very likely that they had received information from stop smoking advisors similar to that in the Forever Free booklets. The qualitative interviews found that study participants could recall some advice received from stop smoking advisors, while they found it difficult to recall the information contained in the booklets. Nevertheless, the booklets and the advisors between them failed to give many people the skills they needed to avoid relapsing or many participants failed to apply a coping strategy where required.
Previous studies reported that smoking cessation support by specialist stop smoking advisors was associated with a higher short-term quit rate (4 weeks after the quit date) compared with support by non-specialists (for example, GPs or pharmacists). 63,64 Data from the current study revealed that the percentage of participants who reported prolonged smoking abstinence was higher among those receiving specialist core services than among those receiving non-specialist services (39% vs. 32%; p = 0.023). Further research is required to investigate this difference between specialist and non-specialist stop smoking advisors.
In agreement with evidence from previous studies,65,66 reported attempts by study participants to do something to cope with urges were associated with a lower risk of smoking relapse. Forty per cent of participants who reported they did something to cope with urges between 2 and 3 months remained smoking abstinent from months 4 to 12, compared with 28% of participants who did not. The difference in smoking abstinence was more considerable between any strategies and no strategies from months 4 to 12 (48% vs. 23%) (see Figure 16). The results of further exploratory analyses (see Table 15) revealed that the percentage of smoking abstinence from months 4 to 12 was lowest, at both the 3- and the 12-month follow-ups, among participants who reported no strategies at all (19%) and the highest among participants who attempted strategies (48%). The percentage of participants who reported smoking abstinence from months 4 to 12 was slightly higher among those who reported strategies only at the 3-month follow-up than among those who reported no strategies at all (24% vs. 19%). These results indicate that continued efforts are needed to enable quitters to help cope with smoking urges and prevent smoking relapse. The full set of eight Forever Free booklets was no more effective than a single leaflet in increasing the strategies by study participants to do something to cope with smoking urges, as there was no difference between the two trial groups in the percentage of participants who attempted to do something.
In summary, there were some small differences in the percentage of participants who recalled having had the booklets and reading them. However, there were no differences in the percentage of participants who reported that they knew any more about coping skills between the groups and no differences in reported actual strategies to handle smoking urges between the trial groups. Therefore, the risk of longer-term smoking relapse was not affected by posting the full set of revised Forever Free booklets to people who had achieved 4 weeks of abstinence with the aid of behavioural support and medication compared with a single leaflet containing a similar but briefer message for smoking relapse prevention.
Indirect evidence comparing the results of different non-randomised studies indicated that a telephone follow-up, along with a single leaflet, may reduce smoking relapse at 12 months in 4-week quitters who have used NHS Stop Smoking Services. However, in a pilot study,61 mobile phone text messages were sent to 4-week quitters weekly for 12 weeks and then fortnightly up to 6 months. Given the intensive frequency of text messaging, the smoking abstinence rate at 6 months in that study61 was still much lower than the rate of smoking abstinence at 12 months in the current trial (see Table 27). In addition, evidence from other previous studies does not support the use of extended non-pharmacological interventions for preventing smoking relapse. 19,62 Reasons for the lower risk of smoking relapse in the current trial were uncertain because of the between-study differences in design, participant characteristics at baseline and different rates of participants lost to follow-up.
Qualitative data indicated that some 4-week quitters found continued interventions for smoking relapse prevention unhelpful and even harmful, although positive feedback from study participants was also common. According to the results of exploratory quantitative analyses, there was no clear dose–response relationship between smoking relapse and the use of self-help educational material-related process variables. Therefore, it is unlikely that smoking relapse could be reduced simply by sending more self-help educational materials to short-term quitters in the NHS Stop Smoking Services.
Despite these caveats, it is important to note that most participants relapsed and many failed to have simple strategies to deal with urges to smoke and could not identify risky situations. For reasons that we can only speculate on, the booklets proved to be an unengaging intervention that participants did not attend to sufficiently to gain or improve these coping skills.
Conclusions
Implications for smoking cessation
Initially developed for, and validated on, unaided self-quitters, the full set of the revised Forever Free booklets was not found to provide additional benefit to short-term quitters who already receive intensive behavioural interventions. Compared with a single leaflet, the actual use of coping skills for smoking relapse prevention was not further improved in 4-week quitters by receiving the full set of eight revised Forever Free booklets. The risk of smoking relapse could not be reduced simply by posting more information to CO-validated 4-week quitters in NHS Stop Smoking Services. Our qualitative study confirmed that many participants felt that the content of the booklets did not significantly enhance the detailed advice and support that they had already received via the NHS Stop Smoking Service.
Recommendations for further research
-
Actual use of coping skills is associated with a lower risk of long-term smoking relapse. Further research should focus on interventions that may increase the use of coping skills when required. In particular, participants in this study reported that they had heard all the material in the booklets previously; however, when asked to identify risky situations, many could not do so and many had no strategy to combat urges to smoke. Behavioural support tends to involve smokers in the passive role of soaking up information. Finding ways to elicit and practise strategies during face-to-face support may prove more effective in preventing relapse.
-
Our qualitative study suggested that targeting of self-help interventions may be appropriate, as some seemed more receptive to this approach than others. This warrants further investigation.
-
Reasons for different longer-term smoking outcomes across different studies and among individual 4-week quitters need to be investigated. Improved understanding of variables related with smoking relapse may help develop novel interventions for smoking relapse prevention.
Acknowledgements
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health. We acknowledge the support of NIHR, through the Primary Care Research Network.
Paul Aveyard is funded by an NIHR award and the UK Centre for Tobacco Control Studies (a UK Clinical Research Collaboration Public Health Research Centre of Excellence) and received funding from the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Medical Research Council and the Department of Health.
We thank staff from NHS Norfolk and Norfolk Community Health and Care NHS Trust for providing advice on the development of the trial protocol. We thank stop smoking advisors from NHS Stop Smoking Services in Norfolk, Lincolnshire, Suffolk, Hertfordshire, and Great Yarmouth and Waveney for recruiting quitters to the study. The trial was conducted in collaboration with the Norwich CTU, whose staff provided input into the design, conduct and analysis (Tony Dyer – Randomisation and Data Management; Garry Barton – Health Economics). We thank Laura Vincent for providing administrative support, data entering and data checking and Christina-Jane Crossman-Barnes for checking aspects of the health economic analyses. Thanks three members of the public who contributed to the revision of the Forever Free booklets, and one lay representative on the trial steering committee. We thank all users of Stop Smoking Services who participated in the study.
Contribution of authors
All authors made substantial contributions to the conception or design of the trial or to the acquisition or interpretation of data for the study. All authors contributed by drafting or commenting on the draft report and approved the final version.
Annie Blyth (SHARPISH Trial Co-ordinator, Senior Research Associate) was responsible for participant recruitment, follow-up interviewing and CO testing, acquisition of data and report drafting.
Vivienne Maskrey (SHARPISH Trial Co-ordinator, Senior Research Associate) was responsible for participant recruitment, follow-up interviewing, acquisition and analysis of data and report drafting.
Caitlin Notley (Society for the Study of Addiction Research Fellow) was responsible for conception and design, acquisition and analysis of qualitative process data, drafting of qualitative investigation chapter and commenting on the draft manuscript.
Garry R Barton (Reader in Health Economics) was responsible for conception and design, analysis of economic evaluation data, drafting of economic evaluation chapter and commenting on the draft report.
Tracey J Brown (SHARPISH Trial Co-ordinator, Research Associate) was responsible for participant recruitment, follow-up interview and CO testing, acquisition of data and commenting on the draft report.
Paul Aveyard (Professor of Behaviour Medicine) was responsible for conception and design of the trial, methodological support, interpretation of results and commenting on the draft report.
Richard Holland (Professor of Public Health) was responsible for conception and design of the trial, methodological support, interpretation of results and commenting on the draft report.
Max O Bachmann (Professor of Health Services Research) was responsible for conception and design of the trial, methodological support, interpretation of results and commenting on the draft report.
Stephen Sutton (Professor of Behavioural Science) was responsible for conception and design of the trial, methodological support, interpretation of results and commenting on the draft report.
Jo Leonardi-Bee (Associate Professor) was responsible for conception and design of the trial, statistical methods support, results interpretation and commenting on the draft manuscript.
Thomas H Brandon (Professor of Psychology) was responsible for conception and design of the trial, statistical methods, interpretation of results and commenting on the draft report.
Fujian Song (SHARPISH Trial Chief Investigator, Professor in Research Synthesis and Health Services Research) was responsible for conception and design of the trial, project management, data analysis and report writing.
Trial Steering Committee
Andy McEwen (Chairperson), Hayden McRobbie, Debbie Kelly, Clive Slater, Roberta Aldred (Patient and Public Involvement), Emily DeVoto (Patient and Public Involvement), Deborah Arnott, Jacqueline Bryony, Judy Henwood and Lesley Maloney. (Note: some Trial Steering Committee members were not involved in the whole time period of the project.)
Data sharing statement
For research purposes, data from this study can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Report on the Global Tobacco Epidemic, 2013. Geneva: WHO; 2013.
- Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med 2014;370:60-8. http://dx.doi.org/10.1056/NEJMra1308383.
- Peto R, Lopez AD, Koop E, Pearson CE, Schwarz MR. Critical Issues in Global Health. San Francisco, CA: Jossey-Bass; 2001.
- Statistics on Smoking: England, 2012. Leeds: Lifestyles Statistics Team, Health and Social Care Information Centre; 2012.
- Statistics on Smoking: England, 2014. Leeds: Lifestyle Statistics Team, Health and Social Care Information Centre; 2014.
- Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet 2012;380:668-79. http://dx.doi.org/10.1016/S0140-6736(12)61085-X.
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2004;3. http://dx.doi.org/10.1002/14651858.cd000146.pub2.
- Hughes J, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2004;4. http://dx.doi.org/10.1002/14651858.cd000031.pub2.
- Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2012;10. http://dx.doi.org/10.1002/14651858.cd008286.pub2.
- West R, McNeill A, Raw M. Smokeless tobacco cessation guidelines for health professionals in England. Br Dent J 2004;196:611-18. http://dx.doi.org/10.1038/sj.bdj.4811286.
- Stapleton JA, Sutherland G, Russell MA. How much does relapse after one year erode effectiveness of smoking cessation treatments? Long-term follow up of randomised trial of nicotine nasal spray. BMJ 1998;316:830-1. http://dx.doi.org/10.1136/bmj.316.7134.830.
- West R, May S, West M, Croghan E, McEwen A. Performance of English Stop Smoking Services in first 10 years: analysis of service monitoring data. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4921.
- Ferguson J, Bauld L, Chesterman J, Judge K. The English smoking treatment services: one-year outcomes. Addiction 2005;100:59-6. http://dx.doi.org/10.1111/j.1360-0443.2005.01028.x.
- West R. The clinical significance of "small" effects of smoking cessation treatments. Addiction 2007;102:506-9. http://dx.doi.org/10.1111/j.1360-0443.2007.01750.x.
- Marlatt GA, Gordon JR, Davidson P, Davidson S. Behavioral Medicine: Changing Health Lifestyles. New York, NY: Brunner/Mazel; 1980.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. http://dx.doi.org/10.1136/bmj.321.7262.694.
- Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol 2008;57:660-80. http://dx.doi.org/10.1111/j.1464-0597.2008.00341.x.
- Hajek P, Stead LF, West R, Jarvis M, Hartmann-Boyce J, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev 2013;8. http://dx.doi.org/10.1002/14651858.cd003999.pub4.
- NICE Public Health Guidance – Smoking Cessation Services in Primary Care, Pharmacies, Local Authorities and Workplaces, Particularly for Manual Working Groups, Pregnant Women and Hard to Reach Communities. NICE Public Health Guidance 10. London: NICE; 2008.
- West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: an update. Thorax 2000;55:987-99. http://dx.doi.org/10.1136/thorax.55.12.987.
- Agboola SA, Coleman TJ, McNeill AD. Relapse prevention in UK Stop Smoking Services: a qualitative study of health professionals’ views and beliefs. BMC Health Serv Res 2009;9. http://dx.doi.org/10.1186/1472-6963-9-67.
- Song F, Huttunen-Lenz M, Holland R. Effectiveness of complex psycho-educational interventions for smoking relapse prevention: an exploratory meta-analysis. J Public Health (Oxf) 2010;32:350-9. http://dx.doi.org/10.1093/pubmed/fdp109.
- Agboola S, McNeill A, Coleman T, Leonardi Bee J. A systematic review of the effectiveness of smoking relapse prevention interventions for abstinent smokers. Addiction 2010;105:1362-80. http://dx.doi.org/10.1111/j.1360-0443.2010.02996.x.
- Brandon TH, Collins BN, Juliano LM, Lazev AB. Preventing relapse among former smokers: a comparison of minimal interventions through telephone and mail. J Consult Clin Psychol 2000;68:103-13. http://dx.doi.org/10.1037/0022-006X.68.1.103.
- Brandon TH, Meade CD, Herzog TA, Chirikos TN, Webb MS, Cantor AB. Efficacy and cost-effectiveness of a minimal intervention to prevent smoking relapse: dismantling the effects of amount of content versus contact. J Consult Clin Psychol 2004;72:797-808. http://dx.doi.org/10.1037/0022-006X.72.5.797.
- Coleman T, Agboola S, Leonardi-Bee J, Taylor M, McEwen A, McNeill A. Relapse prevention in UK Stop Smoking Services: current practice, systematic reviews of effectiveness and cost-effectiveness analysis. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14490.
- Song F, Holland R, Barton GR, Bachmann M, Blyth A, Maskrey V, et al. Self-help materials for the prevention of smoking relapse: study protocol for a randomized controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-69.
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601-5. http://dx.doi.org/10.1136/bmj.39465.451748.AD.
- Statistics on NHS Stop Smoking Services: England, April 2010–March 2011. Leeds: Lifestyle Statistics Team, Health and Social Care Information Centre; 2011.
- NHS Stop Smoking Services: Service and Monitoring Guidance 2010/11. London: Department of Health; 2009.
- Learning to Stay Stopped. West Yorkshire: West Yorkshire Smoking and Health; 2012.
- Armitage P, Berry G. Statistical Methods in Medical Research. Oxford: Blackwell Scientific Publications; 1994.
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 2003;5:13-25. http://dx.doi.org/10.1093/ntr/5.1.13.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. http://dx.doi.org/10.1111/j.1360-0443.2004.00995.x.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Schafer JL. Analysis of Incomplete Multivariate Data. New York, NY: Chapman & Hall; 1997.
- Mackinnon DP, Fairchild AJ. Current Directions in Mediation Analysis. Curr Dir Psychol 2009;18. http://dx.doi.org/10.1111/j.1467-8721.2009.01598.x.
- Hawe P, Shiell A, Riley T, Gold L. Methods for exploring implementation variation and local context within a cluster randomised community intervention trial. J Epidemiol Community Health 2004;58:788-93. http://dx.doi.org/10.1136/jech.2003.014415.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Goode J, Hewison J. Maximising the value of combining qualitative research and randomised controlled trials in health research: the QUAlitative Research in Trials (QUART) study–a mixed methods study. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18380.
- Grant A, Treweek S, Dreischulte T, Foy R, Guthrie B. Process evaluations for cluster-randomised trials of complex interventions: a proposed framework for design and reporting. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-15.
- Robson C. Real World Research. Chichester: John Wiley & Sons; 2011.
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London: Sage; 2006.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Dolan P, Roberts J. Modelling valuations for EQ-5D health states: an alternative model using differences in valuations. Med Care 2002;40:442-6. http://dx.doi.org/10.1097/00005650-200205000-00009.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Noble SM, Hollingworth W, Tilling K. Missing data in trial-based cost-effectiveness analysis: the current state of play. Health Econ 2012;21:187-200. http://dx.doi.org/10.1002/hec.1693.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;14:461-75. http://dx.doi.org/10.1002/hec.843.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:S68-S80. http://dx.doi.org/10.1177/0272989X9801800209.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401. http://dx.doi.org/10.1146/annurev.publhealth.23.100901.140534.
- Fenwick E, O’Brien BJ, Briggs AH. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. http://dx.doi.org/10.1002/hec.903.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2013.
- Health and Social Care Information Centre . Prescription Cost Analysis: England 2013 2014.
- National Schedule of Reference Costs 2012–13. London: DH; 2014.
- Borland R, Balmford J, Hunt D. The effectiveness of personally tailored computer-generated advice letters for smoking cessation. Addiction 2004;99:369-77. http://dx.doi.org/10.1111/j.1360-0443.2003.00623.x.
- Sheffer CE, Stitzer M, Brandon T, Bursac Z. Effectiveness of adding relapse prevention materials to telephone counseling. J Subst Abuse Treat 2010;39:71-7. http://dx.doi.org/10.1016/j.jsat.2010.03.013.
- Brandon TH, Simmons VN, Meade CD, Quinn GP, Lopez Khoury EN, Sutton SK, et al. Self-help booklets for preventing postpartum smoking relapse: a randomized trial. Am J Public Health 2012;102:2109-15. http://dx.doi.org/10.2105/AJPH.2012.300653.
- Bauld L. Longer Term Outcomes From Stop Smoking Services. 2014 UK National Smoking Cessation Conference. London: UK National Smoking Cessation Conference; 2014.
- Snuggs S, McRobbie H, Myers K, Schmocker F, Goddard J, Hajek P. Using text messaging to prevent relapse to smoking: intervention development, practicability and client reactions. Addiction 2012;107:39-44. http://dx.doi.org/10.1111/j.1360-0443.2012.04084.x.
- McNaughton B, Frohlich J, Graham A, Young QR. Extended interactive voice response telephony (IVR) for relapse prevention after smoking cessation using varenicline and IVR: a pilot study. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-824.
- McDermott MS, Beard E, Brose LS, West R, McEwen A. Factors associated with differences in quit rates between ‘specialist’ and ‘community’ stop-smoking practitioners in the english stop-smoking services. Nicotine Tob Res 2013;15:1239-47. http://dx.doi.org/10.1093/ntr/nts262.
- McEwen A, West R, McRobbie H. Effectiveness of specialist group treatment for smoking cessation vs. one-to-one treatment in primary care. Addict Behav 2006;31:1650-60. http://dx.doi.org/10.1016/j.addbeh.2005.12.014.
- Curry SJ, McBride CM. Relapse prevention for smoking cessation: review and evaluation of concepts and interventions. Annu Review Public Health 1994;15:345-66. http://dx.doi.org/10.1146/annurev.pu.15.050194.002021.
- O’Connell KA, Hosein VL, Schwartz JE. Thinking and/or doing as strategies for resisting smoking. Res Nurs Health 2006;29:533-42. http://dx.doi.org/10.1002/nur.20151.
List of abbreviations
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CO
- carbon monoxide
- CTU
- Clinical Trials Unit
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 Level
- GP
- general practitioner
- HTA
- Health Technology Assessment
- NIHR
- National Institute for Health Research
- NRT
- nicotine replacement therapy
- OR
- odds ratio
- p.p.m.
- parts per million
- QALY
- quality-adjusted life-year
- SHARPISH
- Self-Help And Relapse Prevention In Smoking for Health
- UEA
- University of East Anglia