Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/45/01. The contractual start date was in July 2013. The draft report began editorial review in November 2013 and was accepted for publication in July 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Campbell et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
There is a considerable body of evidence demonstrating the benefits of physical activity, in terms of both treating and preventing diseases including coronary heart disease (CHD), stroke, type 2 diabetes mellitus, chronic back pain, osteoporosis, cancers, depression and dementia. 1,2 Current recommendations from the Department of Health1 suggest that adults should undertake at least 150 minutes of moderate-intensity activity each week (in the form of at least 30 minutes of activity on at least 5 days a week, which can be split into three 10 minutes bouts in the same day); however, according to the 2008 Health Survey for England, only 39% of men and 29% of women achieved these levels. 3
Interventions to promote increased levels of physical activity require a wide variety of approaches, with each facilitating small increments in behaviour change. 4 These may include interventions targeted at the population level, such as changes in the environment, as well as interventions targeted at the individual level, such as brief advice delivered in primary care. Over the past 10 years or so, there has been a shift in focus from promoting vigorous exercise to promoting moderate exercise, with more emphasis on lifestyle activity, because of the expanding body of evidence suggesting that there may be greater population gains through the least active becoming more active rather than moderately active people engaging in more vigorous forms of activity. 4
Description of technology under assessment
Primary care has been recognised as a potentially valuable setting for the promotion of physical activity in those who might benefit most. 5 One commonly used method to increase physical activity is the use of exercise referral schemes (ERSs). ERSs have seen considerable growth and are now the most common form of physical activity intervention in primary care. 6
Exercise referral is the practice of referring a person from primary care to a qualified exercise professional who uses relevant medical information about the person to develop a tailored programme of physical activity usually lasting from 10 to 12 weeks. In so doing, opportunities for exercise are provided and there is an expectation that levels of physical activity will increase, leading to positive changes in health behaviours over the long term. These types of schemes usually rely on a partnership between the local authority, primary care trust and private leisure service providers.
Chapter 2 Definition of the decision problem
Since the early 1990s there has been a considerable growth in the number of ERSs in the UK. 5 By 2005, 89% of primary care organisations in England ran an ERS, making it one of the most common forms of physical activity intervention in primary care. 6
Five previous systematic reviews7–11 have been undertaken in this area exploring the effectiveness of ERSs. There was a lack of consistency in the included studies in each of these reviews, revealing a different understanding and interpretation of ERSs between authors. Despite these varying definitions, these previous systematic reviews conclude that ERSs have a small effect in increasing physical activity in the short term, with little or no evidence of long-term sustainability (i.e. 12 months or longer). There was also evidence of a reduced level of depression for participants given exercise referral compared with usual care. 11 However, owing to the considerable uncertainty surrounding the clinical effectiveness and cost-effectiveness of ERS, in 2006, the National Institute for Health and Care Excellence (NICE) Public Health Intervention programme determined that there was insufficient evidence to recommend the use of ERSs as an intervention, other than as part of research studies in which their effectiveness could be evaluated.
The NICE guidance Four commonly used methods to increase physical activity: brief interventions in primary care, exercise referral schemes, pedometers and community-based walking and cycling,9 which included guidance for ERSs, drew on a review of evidence which included four randomised controlled trials (RCTs). 12–15 An additional four studies have been included in a more recent review11 and its update,33 three of which have been published since 2006. 16–18
Physical activity can be promoted in primary care in different ways, including through delivery of advice, provision of written materials and referral to an exercise programme. The UK has seen an expansion in ERSs since 1990,5 but there are concerns that this might not produce sustained change in physical activity beyond the typical programme length of 12 weeks. 19 In 2006, NICE20 advised that there was insufficient evidence to recommend the use of ERSs to promote physical activity other than as part of research studies where their effectiveness can be evaluated. Despite this recommendation, the schemes are still widely used.
A model-based economic evaluation of ERSs concluded that the cost-effectiveness of an ERS is highly sensitive to small changes in the effectiveness and cost of an ERS and is subject to significant uncertainty, mainly as a result of limitations in the clinical effectiveness evidence base. 21
Given the considerable public health benefits of increasing levels of physical activity, it is important that any initiatives for its promotion are kept under consideration and review. Within this short report, newly available effectiveness evidence will be used to update the existing knowledge base and inform NICE guidance for ERSs referred from primary care. The report will address the question ‘what is the clinical effectiveness and cost-effectiveness of ERS to promote physical activity?’ Key factors that will be addressed will include an analysis of effects for those referred for particular clinical conditions, and an exploration of subgroups for whom intervention effectiveness might have a greater effect than for others, including differences between sexes and age groups. We shall also explore where there may be differences in outcomes that relate to key elements of the intervention, such as frequency of contact with the exercise service. The economic evaluation will also build on previous work to explore whether or not the cost-effectiveness of ERSs differs for those referred for particular clinical conditions (hypertension, obesity and depression).
Report methods for synthesis of evidence of clinical effectiveness
This report will be an update of the Pavey et al. 11 systematic review of the evidence; updated searches will be carried out in order to identify new evidence. Any new evidence that is identified will be reviewed systematically and the findings integrated with those of the existing review. The scope of the review will be more limited than in Pavey et al. ,11 owing to the time and resource constraints of this project. We will only include RCTs and systematic reviews of RCTs to analyse effectiveness. We will use only the included RCTs to explore issues of adherence and uptake further. We will do this in two ways: (1) we shall explore adherence and uptake in the trials and (2) we shall examine explanations given within the papers by the authors. This will be done by qualitatively analysing the discussion and conclusion sections of the included trials as well as by extracting data on the numbers of participants who were included in the trials and the drop-out rates. In addition, using the included RCTs, we shall identify qualitative studies undertaken as part of a mixed-methods analysis of exercise referral.
Overall aims and objectives of assessment
-
To identify any new research evidence that has become available since 2009 to inform the review of effectiveness of ERSs.
-
To update the Pavey et al. 11 review with any additional evidence.
-
To qualitatively analyse the discussion and conclusion sections of the included studies to identify potential barriers and facilitators to the implementation, uptake and adherence to ERSs.
-
To explore, where data allow, any characteristics of the intervention or the population that might influence the effectiveness of the intervention.
-
To update the cost-effectiveness evaluation with any new evidence that has become available.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
Identification of studies
The search strategy comprised the following main elements:
-
searching of electronic databases
-
contact with experts in the field
-
scrutiny of bibliographies of retrieved papers.
The search strategies used in the Pavey et al. 11 systematic review of the evidence were used in this review. These consisted of two search strategies (stage 1 and stage 2), details of which are included in Appendix 1 of this report. The stage 1 search was a focused phrase search, with stage 2 being a more sensitive search combining the terms for exercise referral with study type and setting terms (primary care). Searches were limited by English language and a publication date of October 2009 to current (8 May 2013 for stage 1 and 17 June 2014 for stage 2). SPORTDiscus was not available to the research team; therefore, Scopus via Elsevier was used, and the stage 1 search was conducted in this data source. Key sports and exercise science journals such as Medicine and Science in Sports and Exercise and International Journal of Sports Psychology have been covered, as they are indexed in one or more of the databases listed below. The Journal of Aging and Physical Activity was found not to be indexed in the databases searched, hence this journal was hand-searched by scanning the electronic table of contents available at http://journals.humankinetics.com/japa-contents (2009–current and in-press articles as of September 2013).
Sibling studies
In order to identify any sibling studies (qualitative studies conducted as part of a mixed-method evaluation of the intervention), two searches were undertaken. First, the names of authors and project names of the included trials papers were searched for in Google Scholar (Google, Mountain View, CA, USA). To augment this search, citation searches of the included trials were undertaken in the Science Citation Index and proceedings and Social Science Citation Index and proceedings [via Web of Science Thomson Institute for Scientific Information (ISI)].
The following electronic databases were searched: MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid); EMBASE (via OvidSP); PsycINFO (via OvidSP); Scopus (via Elsevier); The Cochrane Library, including the Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), NHS Health Technology Assessment (HTA) Database, NHS Economic Evaluation Database (NHS EED), Database of Abstracts of Reviews of Effects (DARE); Science Citation Index and proceedings and Social Science Citation Index and proceedings (via Web of Science Thomson ISI), UK Clinical Research Network Study Portfolio (UKCRN) portfolio database; Current Controlled Trials; and ClinicalTrials.gov.
An example of the stage 1 and 2 search strategies is shown in Appendix 1.
Inclusion/exclusion criteria
Population
The population included any adult (aged 18 years or over) with or without a medical diagnosis and deemed appropriate for ERSs.
Interventions
The ERS exercise/physical activity programme is required to be more intensive than simple advice and needs to include one or a combination of: counselling (face to face or via telephone), written materials and supervised exercise training. Programmes or systems of exercise referral initiated in secondary or tertiary care, such as conventional comprehensive cardiac or pulmonary rehabilitation programmes, were excluded. We will exclude trials of exercise programmes for which individuals will be recruited from primary care, but there was no clear statement of referral by a member of the primary care team.
Comparators
Comparators included any control, for example usual (brief) physical activity advice, no intervention, attention control or alternative forms of ERSs.
Outcomes
Outcomes included physical activity (self-reported or objectively monitored), physical fitness [e.g. maximal oxygen uptake (VO2max), health outcomes (e.g. blood pressure), adverse events (e.g. musculoskeletal injury)] and uptake and adherence to ERSs. We will also explore how patient characteristics, (age, sex and diagnosis) and programme factors (e.g. length and intensity of the exercise programme) might influence the outcome of ERSs.
Study design
We included any new RCT evidence, identified in searches of electronic databases published from October 2009 to May/June 2013 (see Appendix 1). Data were extracted and the data extraction tool was modelled on that used in the Pavey et al. 11 review. We also searched for any systematic reviews of ERSs published from 2009 to May/June 2013. Their lists of included studies were hand-searched to identify any further relevant studies.
For any new RCTs that we identified, any qualitative data that have been reported as part of a mixed-methods evaluation an ERS intervention were also included.
Any ongoing studies that we identify will also be reported. These would offer the most relevant insights into the particular factors influencing the adherence and uptake of that particular ERS intervention.
Titles and abstracts were examined for inclusion by two reviewers independently. Disagreement was resolved by consensus.
Exclusion criteria
-
Animal models.
-
Pre-clinical and biological studies.
-
Narrative reviews, editorials, opinions.
-
Non-English-language papers.
-
Reports published as meeting abstracts only, in which insufficient methodological details are reported to allow critical appraisal of study quality.
Quality assessment strategy
The Cochrane risk-of-bias tool was used to assess study quality. 19 Consideration of study quality included assessment of the following trials characteristics:
-
method of randomisation
-
allocation concealment
-
blinding
-
numbers of participants randomised, excluded and lost to follow-up.
-
whether or not intention-to-treat analysis has been performed
-
methods for handling missing data
-
baseline comparability between groups.
Methods of analysis/synthesis
Data from new studies published since 2009 were tabulated and discussed in a narrative review. The data from studies already identified and analysed by Pavey et al. 11 were used as published and data from new studies were integrated with them.
Meta-analyses were used to estimate a summary measure of effect on relevant outcomes based on intention-to-treat (ITT) analyses. These meta-analyses used data published in the Pavey et al. 11 review and new data were added.
Meta-analysis was carried out using fixed- and random-effects models, using Review Manager 12 software (Thomson Reuters, Toronto, ON, Canada). Heterogeneity will be explored through consideration of the study populations, methods and interventions, by visualisation of results and, in statistical terms, by the chi-squared test for homogeneity and the I2 statistic.
In order to extend our understanding of the factors that predict uptake and adherence, we undertook a qualitative thematic analysis of the discussion and conclusion sections of the included RCTs. This yielded insights into the factors identified by the triallists that influenced variations in uptake or adherence. The results will be described in a narrative, and a logic model used to explore and explain associations between multiple and varied barriers and facilitators to uptake and adherence of ERSs.
Results
Quantity and quality of research available
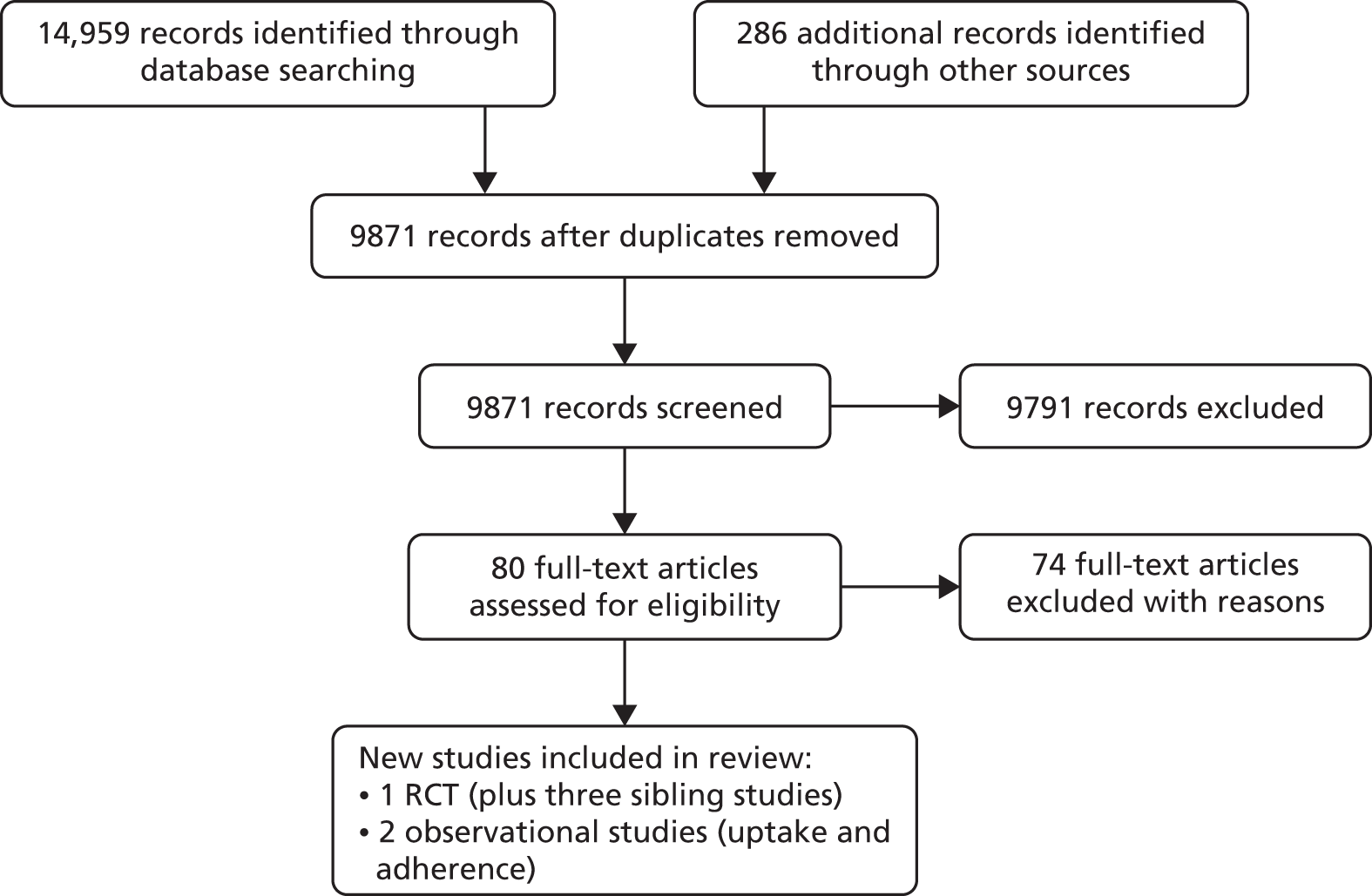
Our search of electronic databases and relevant journals yielded 9627 titles, of which one primary study was judged to meet the inclusion criteria. Figure 1 summarises the process of identifying the inclusion and exclusion process. In two studies,22,23 additional data were supplied by the authors. One study,24 identified in bibliographic searching, could not be retrieved. The main reasons for excluding studies included non-RCT design (n = 44), participants recruited from primary care for inclusion in an exercise programme but without referral from a health-care professional (n = 20), the intervention was a prescription to undertake exercise but not a referral to a third-party exercise provider (n = 5), the population was not appropriate (n = 1), participants were already part of an intervention prior to randomisation (n = 1) or randomisation occurred prior to the baseline assessment (n = 1). Appendix 2 provides the full list of excluded studies. The included studies are summarised in Table 1, which includes the studies incorporated in the Pavey et al. review. 11 The new evidence is highlighted in bold type within the tables.
FIGURE 1.
Flow diagram demonstrating the process of identifying new studies for inclusion in the review.
| Study | Country | Number of GP practices | Date study conducted | RCT design | Overall (n) | Randomised (n) | Follow-up periods |
|---|---|---|---|---|---|---|---|
| aTaylor et al.12,25 | UK | 3 | January to December 1994 | Individual | 142 | 97 ERS/45 control | 8, 16, 26 and 37 weeks |
| Stevens et al.26 | UK | 1 | Not stated | Individual | 714 | 363 ERS/351 control | 8 months |
| Harrison et al.15 | UK | 46 | March 2000 to December 2001 | Individual | 545 | 275 ERS/270 control | 6, 9 and 12 months |
| Isaacs et al.18 | UK | 88 | October 1998 to April 2002 | Individual | 943 | 317 leisure centre/315 walking/311 control | 10 weeks, 6 and 12 months |
| aSorensen et al.27 | Denmark | 14 | 2005 to 2006 | Individual | 52 | 28 ERS/24 control | 4 and 10 months |
| Gusi et al.28 | Spain | 4 | Not stated | Cluster | 287 | 127 ERS/160 control | 6 months |
| Duda et al.17 | UK | Not reported (13 leisure-centre sites) | November 2007 to July 2008 | Cluster | 347 | 184 ERS/163 control | 3 and 6 months |
| Murphy et al. 23 | UK | 12 local health boards | Not stated. National ERS rolled out in 2007 | Individual | 2160 | 1080 ERS/1080 control | 6 and 12 months |
Characteristics of included studies
The characteristics of the included ERS studies are summarised in Table 2. All of the included studies were RCTs. The Pavey et al. 11 review included seven trials. 12,15,18,26–28,31 The data from these studies are included in the tables of this review, with new data emboldened. One additional study was identified (four publications). 23,25,26,32 This study was a mixed-methods evaluation, incorporating RCT and qualitative evidence, undertaken in Wales. It was larger than previous studies, with 2160 participants. The earlier studies ranged in size from 52 to 943 participants. The total number of participants in all eight studies was 5190.
| Study | Country | Age range of patients (years) | Inclusion criteria | Exclusion criteria | Inclusion/exclusion criteria determined/evaluated by | Number of participants excluded |
|---|---|---|---|---|---|---|
| Taylor et al.12 | UK | 40–70 | Smokers, hypertension (140/90 mmHg), overweight (BMI > 25 kg/m2) | SBP > 200 mmHg, history of MI or angina pectoris, diabetes mellitus, musculoskeletal condition preventing PA, previous ERS referral | Research team and GP determined and evaluated | 44 |
| Stevens et al.26 | UK | > 18 | Sedentary – < 20 × 30 minutes of moderate-intensity PA or < 12 × 20 vigorous-intensity PA in the past 4 weeks | Medical reasons for exclusion (e.g. registered disabled, diagnosis of heart disease) | Research team determined and evaluated | 113 |
| Harrison et al.15 | UK | > 18 | Sedentary, participating in < 90 minutes of moderate/vigorous PA a week, additional CHD risk factors; obesity, previous MI, on practice CHD risk management register, diabetes mellitus | GP identified contradiction to PA, SBP > 200 mmHg, not sedentary, only one family member (to avoid contamination – research team criterion) | GP evaluation using the trial’s ERS-determined criteria | 285 |
| Isaacs et al.18 | UK | 40–74 | Not active (no definition reported), raised cholesterol, controlled mild/moderate hypertension, obesity, smoking, diabetes mellitus, family history of MI at early age | Pre-existing overt CVD, uncontrolled hypertension, uncontrolled insulin-dependent diabetes, psychiatric or physical conditions preventing PA, conditions requiring specialist programme | GP evaluation using criteria determined by an existing ERS | Not reported |
| Sorensen et al.27 | Denmark | > 18 | Patients must meet all criteria:
|
Not meeting the inclusion criteria | GP evaluation using the trial’s ERS-determined criteria | Not reported |
| Gusi et al.28 | Spain | > 60 | Moderately depressed (6–9 points on the Geriatric Depression Scale), overweight (BMI 25–39.9 kg/m2), capable of walking for more than 25 minutes | Severe obesity, major depression, debilitating medical condition, known unstable cardiac condition, attention or comprehension problems | Research team determined, GP evaluation | 32 |
| Duda et al.17 | UK | > 18 | Two or more risk factors for CHD; people with chronic medical conditions, such as asthma, bronchitis, diabetes mellitus, mild anxiety or depression; people for whom regular activity might delay the onset of osteoporosis, people with borderline hypertension and those perceived by the GP or practice nurse to possess motivation to change People suffering from well-controlled chronic medical conditions: mild or controlled asthma, chronic bronchitis, controlled diabetes mellitus, mild to moderate depression and/or anxiety, people for whom the onset of osteoporosis may be delayed through regular exercise (i.e. post-menopausal women, borderline hypertensive patients with a blood pressure no higher than 160/102 mmHg prior to medication, people exhibiting motivation to change) |
Angina pectoris, moderate-to-high (or unstable) hypertension ≥ 160/102 mmHg Poorly controlled insulin-dependent diabetes mellitus, history of MI within the last 6 months – unless the patient has completed stage III cardiac rehabilitation, established cerebrovascular disease, severe chronic obstructive airways disease, uncontrolled asthma |
GP evaluation using the trial’s ERS-determined criteria | Not reported |
| Murphy et al. 23 | UK | > 16 | The patient must be sedentary (defined as not moderately active for > 3 times per week or deconditioned through age or inactivity) and have at least one of the following medical conditions: CHD risk factors (raised BP, BMI > 28 kg/m 2 , controlled diabetes mellitus, impaired glucose tolerance, raised cholesterol, family history of heart disease or diabetes mellitus, referral from cardiac rehab schemes); mental health (mild anxiety, depression or stress); musculoskeletal (at risk of osteoporosis, arthritis, poor mobility, musculoskeletal pain); respiratory/pulmonary (chronic obstructive pulmonary disease, mild/well-controlled asthma, bronchitis, emphysema); neurological conditions (multiple sclerosis); other (smoking, chronic fatigue) | Aged ≤ 16 years, unstable angina, blood pressure uncontrolled or above 180/100 mmHg, cardiomyopathy, uncontrolled tachycardia, cardiac arrhythmia, valvular heart disease, congenital heart disease, unexplained dizzy spells, excessive or unexplained breathlessness on exertion, uncontrolled diabetes mellitus, uncontrolled epilepsy, history of falls or dizzy spells in previous 12 months, uncontrolled asthma, first 12 weeks of pregnancy, awaiting medical investigation, aneurysms, history of cerebrovascular disease, unstable or newly diagnosed angina, established CHD, any other uncontrolled condition | Clinicians in normal practice referred to evaluation team | 1493 |
The studies of Duda et al. 17 and Gusi et al. 28 used cluster allocation, with the other studies using individual-level randomisation. Follow-up duration ranged from 2 to 12 months. The general practitioner (GP) was the main referrer, usually using a bespoke referral form to a fitness or exercise instructor/officer. The Murphy et al. 23 study included referrals by health professionals working in a range of health-care settings.
Five studies12,15,26,23,28 compared ERSs with a usual-care group, which consisted of no exercise intervention or simple advice on physical activity. Sorensen et al. 27 compared ERSs with motivational counselling aimed at increasing daily physical activity. The Isaacs et al. study18 also included an instructor-led walking programme. The Duda et al. study17 compared two forms of ERSs, that is, standard ERS versus a combined ERS plus self-determination theory (SDT)-based intervention.
Characteristics of participants
A total of 5190 participants were included in the eight trials. 12,15,17,18,23,26–28 A summary of the characteristics of participants is presented in Table 3. All of the studies recruited participants who were sedentary or who were believed by their GP to be able to improve health by an increased physical activity level. They all also excluded individuals who had poorly controlled hypertension, diabetes mellitus or heart disease. Gusi et al. 28 also excluded those with severe obesity or major depression. Sorensen et al. 27 included participants who were willing to pay 750 Danish krone for the intervention.
| Study | Country | Mean age (years) | Sex (% male) | Ethnicity (%) | Reported diagnosed conditions for risk factors (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||
| Taylor et al.12 | UK | 54.1 | 54.4 | 37 | 38 | Not reported | Not reported | Smokers: 43 | Smokers: 40 |
| Overweight: 77 | Overweight: 71 | ||||||||
| Hypertensive: 46 | Hypertensive: 58 | ||||||||
| Stevens et al.26 | UK | 59.1 | 59.2 | 40 | 44 | White: 87 | White: 83 | BMI > 25 kg/m2: 46 | BMI > 25 kg/m2: 42 |
| Black: 5 | Black: 4 | Smoker: 18 | Smoker: 17 | ||||||
| Asian: 4 | Asian: 6 | ||||||||
| Other: 4 | Other: 5 | ||||||||
| Harrison et al.15 | UK | 18–44 (n = 111) | 18–44 (n = 107) | 33 | 34 | White: 71.9 | White: 74.1 | Smoker: 24.4 | Smoker: 20.7 |
| 45–59 (n = 101) | 45–59 (n = 98) | ||||||||
| > 60 (n = 63) | > 60 (n = 65) | At least one CHD risk factor: 75.3 | At least one CHD risk factor: 75.2 | ||||||
| Isaacs et al.18 | UK | 57.1 | Usual care: 57 | ERS: 35 | Control: 32 | White: 75.7 | White (control/walking): 76.5/75.9 | Raised cholesterol (exercise/walking): 24.0 | Raised cholesterol (control/walking): 17.1/21.5 |
| Hypertension (exercise/walking): 44.5 | Hypertension (control/walking): 43.5/46.3 | ||||||||
| Obesity (exercise/walking): 65.9 | Obesity (control/walking): 63.5/58.5 | ||||||||
| Smoking (exercise/walking): 10.4 | Smoking (control/walking): 8.3/12.2 | ||||||||
| Type 2 diabetes mellitus (exercise/walking): 12.3/11.3 | Diabetes mellitus (control/walking): 15.6/11.3 | ||||||||
| Walk: 56.9 | Walk: 31 | Asian: 16.7 | Asian (control/walking): 14/12.2 | Family history of MI (exercise/walking): 13.9 | Family history of MI (control/walking): 16.2/12.9 | ||||
| Sorensen et al.27 | Denmark | 53.9 | 52.9 | 43 | 37 | Not reported | Not reported | Metabolic syndrome: 36 | Metabolic syndrome: 25 |
| Type 2 diabetes mellitus: 18 | Type 2 diabetes mellitus: 21 | ||||||||
| CVD: 32 | Heart disease: 42 | ||||||||
| Other diseases: 14 | Other diseases: 13 | ||||||||
| Gusi et al.28 | Spain | 71 | 74 | 0 | 0 | Not reported | Not reported | Overweight (BMI > 25 kg/m2): 80 | Overweight: 86 |
| Type 2 diabetes mellitus: 39 | Type 2 diabetes mellitus: 37 | ||||||||
| Moderate depression: 34 | Moderate depression: 38 | ||||||||
| Duda et al.17 | UK | < 30 (n = 19) | < 30 (n = 11) | 24 | 30 | White: 74.9 | White: 67.5 | Smoker: 22.1 | Smoker: 23.1 |
| Hypertension: 38 | Hypertensive: 37.5 | ||||||||
| Overweight (BMI > 25 kg/m2): 25.3 | Overweight: 26.3 | ||||||||
| Obese (BMI > 30 kg/m2): 52.3 | Obese: 51.9 | ||||||||
| 30–49 (n = 76) | 30–49 (n = 77) | Black: 10.6 | Black: 14.9 | Morbidly obese (BMI > 40 kg/m2): 12.1 | Morbidly obese: 13.5 | ||||
| 50–64 (n = 64) | 50–64 (n = 50) | Asian: 9.5 | Asian: 14.9 | Probable anxiety: 34.2 | Probable anxiety: 31.9 | ||||
| > 65 (n = 25) | > 65 (n = 25) | Other: 5 | Other: 2.6 | Probable depression: 21.9 | Probable depression: 15.3 | ||||
| Murphy et al. 23 | UK | 52 (SD 14.7) | – | 44% | – | White: 96 | – | CHD risk factors: 72 | – |
| Mental health issues: 24 | |||||||||
The participants included in the Murphy et al. study23 shared a similar profile to those already included in earlier studies. Most of those recruited were middle-aged white adults, with at least one medical condition, who might benefit from an increased level of physical activity (Table 4). In the Murphy et al. study,23 72% of participants had CHD risk factors and 24% had mental health problems.
| Study | Country | Referrer | Format of referral | Referred to where | Participant cost | Referred to who |
|---|---|---|---|---|---|---|
| Taylor et al.12 | UK | GP | Signed prescription card | Leisure centre | Half-price admission | Fitness instructor |
| Stevens et al.26 | UK | GP | Letter | Leisure centre | Not reported | Exercise development officer |
| Harrison et al.15 | UK | GP | Faxed referral form | Leisure centre | ‘Subsidised‘ | Exercise officer |
| Isaacs et al.18 | UK | GP or practice nurse | Specially prepared ‘prescription pad’ – referral form | Leisure centre | Free | Fitness instructor |
| Sorensen et al.27 | Denmark | GP | Not reported | Clinic | Pay €100 | Physiotherapist |
| Gusi et al.28 | Spain | GP | Not reported | Supervised walks in a public park or forest tracks | Not reported | Qualified exercise leaders |
| Duda et al.17 | UK | Member of the primary-care team | Not reported | Leisure centre | Not reported | Health and fitness adviser |
| Murphy et al. 23 | UK | Clinician | Form | Evaluation team | Access to one-to-one exercise instruction and/or group exercise classes. Discounted rate for exercise activities, £1 per session | Evaluation team |
Characteristics of interventions
The characteristics of the interventions are summarised in Table 5. Interventions ran for between 10 weeks14,21,33 and 6 months28 and included instructor-led exercise classes or walks,28 some form of consultation aimed at increasing activity22,33 or a combination of the two. 14,17,21,23,34 The intervention by Sorensen et al. 27 was based on the transtheoretical model of behaviour change, which categorises people into stages of readiness for change and postulates 10 experiential and behavioural processes of change, pro and con beliefs and self-efficacy beliefs (beliefs in personal ability to carry out the behaviour in question). 22 The Duda et al. intervention17 was based on SDT, which emphasises the determinants and consequences of different reasons for behavioural engagement, which vary in their degree of self-determination. 23 The intervention aimed to promote autonomy support, which is assumed to foster participants’ feelings of competence, autonomy and relatedness and, as a result, enhance autonomous motivation for physical activity and associated mental health outcomes. 17 The only study added since the Pavey HTA,11 by Murphy et al. ,23 was also the only study to explicitly use motivational interviewing. However, fidelity to this intervention method during consultations was considered to be low. Motivational interviewing is ‘a collaborative, person-centred form of guiding to elicit and strengthen motivation for change’. 35 In those studies that held supervised exercise sessions or walks, the number of sessions per week varied between one32 and three,28 with the most common being two sessions per week,14,17,21 although one study27 held two sessions per week for the first 2 months and then one session per week for the following 2 months. Supervised exercise sessions or walks lasted between 30–40 minutes12 and 1 hour. 17,34 Two interventions were group interventions28,34 and four were at group and/or individual levels. 14,17,22,23
| Study | Country | Initial screen/assessment | Scheme duration | Provider | Exercise sessions per week | Exercise session intensity | Group or individual | Duration of follow-up |
|---|---|---|---|---|---|---|---|---|
| Taylor et al.12 | UK | Yes | 10 weeks | Leisure centre | 2 × 30–40 minutes | Moderate intensity | Group and/or individual | 37 weeks |
| Stevens et al.26 | UK | Yes | 10 weeks | Leisure centre | Not reported | Not reported | Not reported | 8 months |
| Harrison et al.15 | UK | Yes | 12 weeks | Leisure centre | 2 × 1 hour | Individually based | Group and/or individual | 6 months |
| Isaacs et al.18 | UK | Yes | 10 weeks | Leisure centre | 2 × 45 minutes | Not reported | Group and/or individual | 6 months |
| Sorensen et al.27 | Denmark | Yes (and motivational counselling) | 4 months | Clinic | First 2 months: 2 sessions × 1 hour; second 2 months: 1 session × 1 hour | More than 50% of heart rate reserve for a minimum of 20 minutes | Group | 10 months |
| Gusi et al.28 | Spain | Not reported | 6 months | Walking scheme | 3 × 50 minutes | Not reported | Group | 6 months |
| Duda et al.17 | UK | Yes | 12 weeks | Leisure centre | Individually based | Individually based | Group and/or individual | 6 months |
| Murphy et al. 23 | UK | With exercise professional on entry: lifestyle questionnaire, health check (resting heart rate, blood pressure, BMI and waist circumference), introduction to leisure-centre facilities, motivation interview and goal-setting | 16 weeks | Exercise professionals | Access to one-to-one exercise instruction and/or group exercise classes |
4-week telephone contact with exercise professional: review of goals, motivational interview, relapse prevention
16-week consultation with exercise professional: review of goals, motivation interview, health check, lifestyle questionnaire, service evaluation questionnaire and advice on continuing with exercise after the programme 8-month telephone contact by exercise professional to ask about their exercise behaviour and relapse prevention |
Individual | 12 months review including repeat of health check carried out at entry and Chester fitness step test |
Risk of bias
Table 6 summarises the risk of bias for each of the included studies. Most included a power calculation and allocated participants using an appropriately generated random number sequence. However, the reporting of concealment of trial group allocation was poor, although there was good evidence of participant characteristics of intervention and control groups at baseline. Although blinding of participants and intervention providers in these studies was not feasible, blinding of outcome assessment was possible. Outcome blinding is particularly important in preventing assessment bias in the case of outcomes that require observer judgement or involvement (e.g. blood pressure measurement or exercise testing). Two studies17,23 reported outcome blinding. Recall Questionnaire was assessed via telephone to maintain blinding. The reporting and handling of missing data were detailed for most studies, and all studies, except one,12 reported the use of ITT analysis. The level of missing data at follow-up ranged across studies from 16.5% to 50%. Most studies used imputation methods (last observation carried forward or complete case average values) to replace missing data values at follow-up. Overall, three studies12,15,26 were judged to be at moderate overall risk of bias and five17,18,23,27,28 to be at low overall risk of bias.
| Risk-of-bias criterion | Study and country | |||||||
|---|---|---|---|---|---|---|---|---|
| Taylor et al.,12 UK | Stevens et al.,26 UK | Harrison et al.,15 UK | Isaacs et al.,18 UK | Sorensen et al.,27 Denmark | Gusi et al.,28 Spain | Duda et al.,17 UK | Murphy et al.,23 UK | |
| Power calculation reported? | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Method of random sequence generation described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | Yes |
| Method of allocation concealment described? | Yesa | Unclear | Unclear | Unclear | Yes | Yes | Unclear | Yes |
| Method of outcome (assessment) blinding described? | Unclear | Unclear | Unclear | No | Unclear | Unclear | Yes | Yes |
| Were groups similar at baseline? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was ITT analysis used? | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was there any statistical handling of missing data? | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Yes |
| Were missing data (dropout and loss to follow-up) reported? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Exercise referral scheme eligibility, uptake and adherence
There was a considerable range in the proportion of individuals randomised compared with those deemed eligible (Table 7). In both the Sorensen et al. 27 and Duda et al. 17 studies, of those deemed eligible for ERSs, a substantial number refused participation in the trial. In the Sorensen et al. 27 study this low number may be reflective of the 750 Danish krone payment by patients as part of a standard Danish Exercise on Prescription. In the Duda et al. 17 study, this may be related to the workload and training needs of the health and fitness advisors at the time of recruitment.
| Study | Country | Number deemed eligible (n) | Total n randomised (%) | ERS (n) | Control (n) | ERS uptake, n (%) |
|---|---|---|---|---|---|---|
| Taylor et al.12 | UK | 345 | 142 (41) | 97 | 45 | 85 (88) |
| Stevens et al.26 | UK | 827 | 714 (86) | 363 | 351 | 126 (35) |
| Harrison et al.15 | UK | 830 | 545 (66) | 275 | 270 | 232 (84) |
| Isaacs et al.18 | UK | 1305 | 949 (73) | 317 leisure centre, 315 walking | 311 | 293 (92) |
| Sorensen et al.27 | Denmark | 327 | 52 (16) | 28 | 24 | 28 (100) |
| Gusi et al.28 | Spain | 160 | 127 (79) | 64 | 63 | Not reported |
| Duda et al.17 | UK | 1683 | 347 (21) | 184 | 163 | Not reported |
| Murphy et al. 23 | UK | 3286 | 2160 (66) | 1080 | 1080 | n = 919 (85) |
The terms ‘adherence’ and ‘uptake’ can be used variably within the literature. ‘Uptake’ refers to initial attendance, take-up or enrolment. ‘Adherence’ describes the level and duration of participation, and the threshold for determining adherence may vary in different studies. 34
Rates of uptake varied in the included studies. Taylor et al. ,12 Isaacs et al. ,18 Sorensen et al. 27 and Murphy et al. 23 reported uptake rates in excess of 85%, and in the Stevens et al. study26 only 126 (35%) of the 363 randomised to ERSs attended the first consultation. Stevens et al. 26 discussed how the low uptake they experienced may have been reflective of the nature of the invitation letter sent to participants and the point of randomisation (pre-invitation letter). Furthermore, they hypothesise that a change in the format of the letter (e.g. including a specific appointment date for the first ERS appointment) would have improved participation. Uptake was not reported by Duda et al. 17 or Gusi et al. 28
Adherence was assessed differently between the trials, and levels of adherence also varied. Stevens et al. 26 and Gusi et al. 28 reported ERS programme completion rates of 25% and 86%, respectively. However, these rates do not reflect the number of sessions attended, only those who attended a second consultation26 or follow-up assessment. 28
Sorensen et al. 27 reported that an average of 18 out of a total of 24 ERS exercise sessions were attended and 68% and 75% of participants attended the counselling sessions at 4 and 10 months, respectively. Both Taylor et al. 12 and Isaacs et al. 18 provide a detailed description of ERS programme adherence. Taylor et al. 12 reported 13% attending no exercise sessions and 28% attending 75–100% of exercise sessions, with an average of 9.1 out of 20 prescribed exercise sessions attended. Isaacs et al. 18 reported 7.6% attending no exercise sessions and 42% attending 75–100% of exercise sessions in the leisure centre group. In the walking group, 23.5% attended no exercise sessions, with 21.5% attending 75–100% of exercise sessions. As shown in Table 8, there was no consistent difference in attendance rates between those in at-risk groups and the overall study population in the studies of Taylor et al. 12 and Isaacs et al. 18 In the Isaacs et al. study,18 the 60–69 years age group had the highest adherence in both the ERS (53.3%) and the walking (24.2%) groups. There were no significant differences in attendance rate based on employment status, educational level, socioeconomic status, ethnicity or relationship status. However, Murphy et al. 23 did find differences in uptake and adherence between participants from deprived and less-deprived areas. Adherence was lower for those without access to private transport in both the ERS and walking groups. Harrison et al. 15 and Duda et al. 17 did not provide information on participants’ adherence to the ERS intervention.
| Study | Country | Smoking (%) | Obesity (%) | Hypertension (%) | Overall (%) |
|---|---|---|---|---|---|
| Taylor et al.12 | UK | 12 | 28 | 23 | 28 |
| Isaacs et al.,18 ERS group | UK | 45.5 | 38.8 | 46.1 | 42 |
| Isaacs et al.,18 control walking group | UK | 26.3 | 18.7 | 22.9 | 21.5 |
Murphy et al. 23 found that participants already active at baseline were the most likely to enter the ERS, but that they were also most likely to partially attend a programme. Men and younger participants were slightly less likely to enter the scheme, and women were less likely to complete the programme. Participants in the least-deprived areas were more likely to take up the scheme, although the differences in adherence between the most- and least-deprived areas were smaller. Non-car owners were less likely to take up the scheme or to adhere to it if they joined. Those referred for mental health reasons were more likely not to enter the ERS, and only one in three mental health patients completed the programme.
In a univariate regression analysis of predictors of uptake and adherence, the only significant correlates of uptake in the Murphy et al. study23 were car ownership and deprivation. Those in moderately deprived areas were less likely to enter the ERS than those in the least-deprived areas. Car owners were significantly more likely to enter the ERS than non-car owners. Older participants, those referred for non-weight-related CHD risk factors, non-mental health patients and those already moderately active at baseline were most likely to complete the programme.
In a multivariable regression analysis, the significant difference between low and medium deprivation areas remains and car ownership remains predictive of uptake. Associations of CHD risk factors with adherence become non-significant and associations of age and mental health status remain significant. Associations of baseline activity with adherence are strengthened in the multivariable analysis, with the contrast between inactive and moderately inactive participants becoming more significant (see Barriers and facilitators of referral, uptake and adherence to exercise referral schemes for further discussion of factors influencing uptake and adherence).
Assessment of effectiveness
Only Isaacs et al. 18 reported all outcome domains applicable to this systematic review (Table 9). New data added to the Pavey et al. 11 review are emboldened within the tables.
| Study | PA | PA measure | Physical fitness | Clinical outcomes | Psychological well-being | HRQoL | Patient satisfaction | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Taylor et al.,12 UK | Yes | Self-report, 7 day-PAR | Yes, submax. HR | Yes, BP, BMI, BF%, waist to hip | Yes, PSW | No | No | No |
| Stevens et al.,26 UK | Yes | Self-report, 7 day-PAR | No | No | No | No | No | No |
| Harrison et al.,15 UK | Yes | Self-report, 7 day-PAR | No | No | No | No | Yes | No |
| Isaacs et al.,18 UK | Yes | Self-report, Minnesota LTPAQ | Yes, submax. bike test, submax. walking test | Yes, BP, cholesterol, lipoproteins, triglycerides, weight, BMI, BF%, waist-to-hip ratio, FEV, PEF | Yes, anxiety, depression | Yes, SF-36 mental | Yes | Yes, GP records |
| Sorensen et al.,27 Denmark | Yes | Self-report, unspecified | Yes, submax. bike test | Yes, weight, BMI | No | Yes, SF-12 mental, physical | No | No |
| Gusi et al.,28 Spain | Not reported | N/A | No | Yes, BMI | Yes, anxiety, depression | Yes, EQ-5D | No | No |
| Duda et al.,17 UK | Yes | Self-report, 7 day-PAR | No | Yes, BMI | Yes, anxiety, depression | Yes, Dartmouth QoL | No | No |
| Murphy et al.,23 UK | Yes | Self-report, 7 day-PAR | No | Measured at baseline | Yes, HADs | Yes, EQ-5D | Yes, Client Service Receipt Inventory | No |
Physical activity
All studies, with the exception of Gusi et al. ,28 provided a measure of self-reported physical activity. Self-reported measures included the validated 7-Day Physical Activity Recall (7-Day PAR) questionnaire12,23,26,27 and the validated Minnesota Leisure Time Activity questionnaire. 18 None of the studies reported methods of measuring physical activity using an objective method of measurement, and all relied on self-report tools.
Exercise referral schemes versus usual care/advice only
The most consistently reported physical activity outcome across studies was the proportion of individuals achieving 90–150 minutes of at least moderate-intensity activity per week. Data for this outcome in the Murphy et al. study23 were supplied by the author (Professor Simon Murphy, Cardiff University, 2013, personal communication). When pooled across studies the relative risk (RR) was 1.12 [95% confidence interval (CI) 1.04 to 1.20] of achieving this outcome with ERS compared with usual care at 6–12 months’ follow-up (Figure 2). There was no evidence of heterogeneity in this analysis (I2 0%). This analysis draws on data published by Pavey et al. 11 Three studies12,15,23 reported this outcome based on the number of individuals who were available at follow-up. These results show a decrease in the RR found by Pavey et al. 11 (RR 1.16, 95% CI 1.03 to 1.30). In order to assess the potential (attrition) bias in using completers, the denominators of these three studies were adjusted to all individuals randomised in order to perform an ITT analysis (Figure 3). It was assumed that all missing cases did not meet the physical activity threshold. In the pooled ITT analyses, the proportion achieving the physical activity threshold in the ERS group compared with usual care was RR 1.08 (95% CI 1.00 to 1.17). There was no evidence of heterogeneity in this analysis (I2 0%). This is also a reduction on the RR found by Pavey et al. 11 (RR 1.11, 95% CI 0.99 to 1.25).
FIGURE 2.
Number achieving 90–150 minutes physical activity/week (updated meta-analysis). df, degrees of freedom; M–H, Mantel–Haenszel; PA, physical activity.
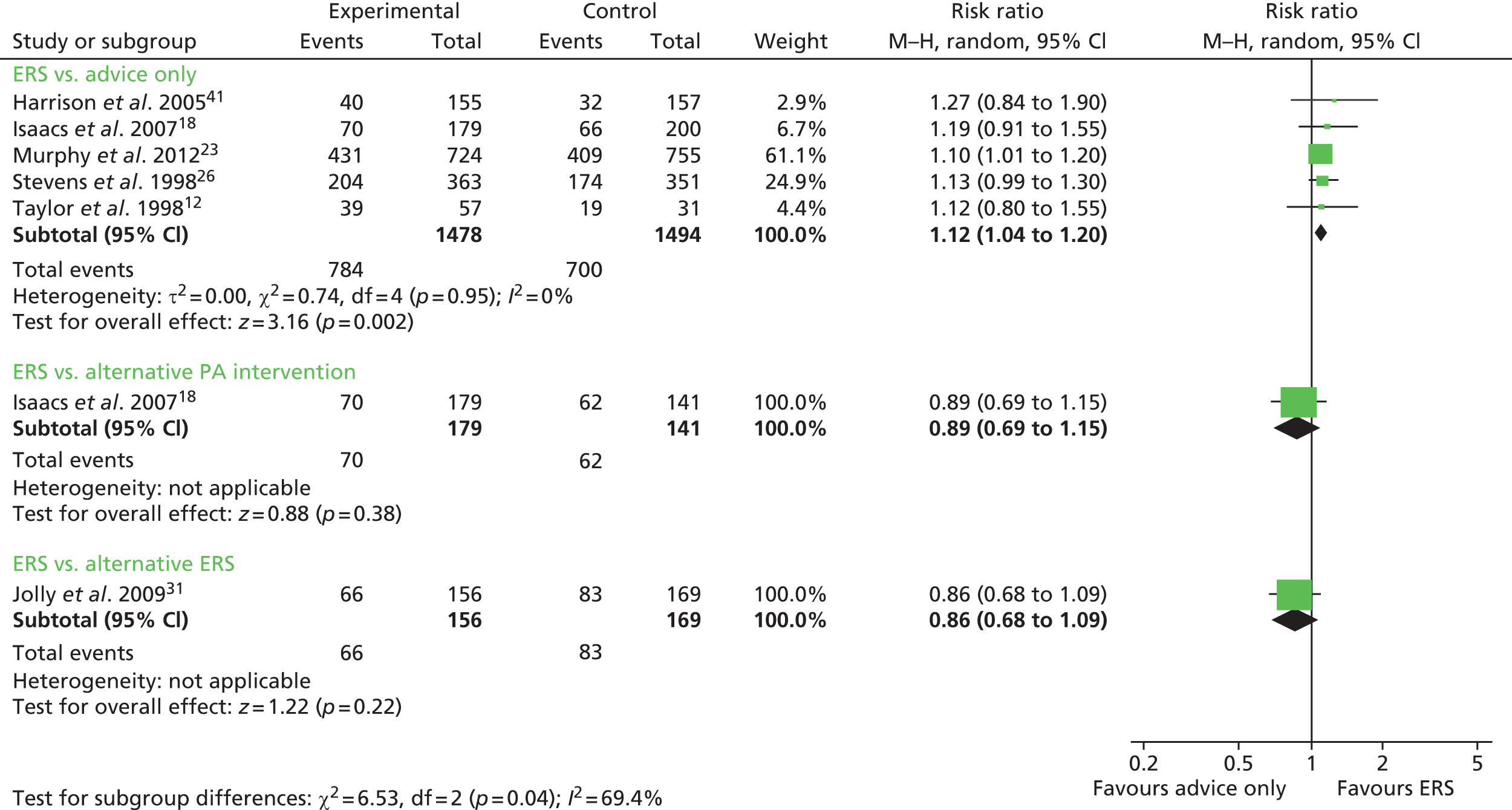
FIGURE 3.
Number achieving 90–150 minutes physical activity/week (ITT analysis) (updated meta-analysis). df, degrees of freedom; M–H, Mantel–Haenszel; PA, physical activity.

Total minutes of physical activity were reported by Isaacs et al. 18 and Murphy et al. 23 When these data were pooled, there was a significant increase in the number of minutes of physical activity per week in the ERS group; mean difference 55.10 minutes (95% CI 18.47 to 91.73 minutes) (Figures 4–6).
FIGURE 4.
Minutes spent in at least moderate-intensity physical activity per week at 6–12 months’ follow-up. 11 df, degrees of freedom; IV, inverse variance; PA, physical activity; SD, standard deviation.
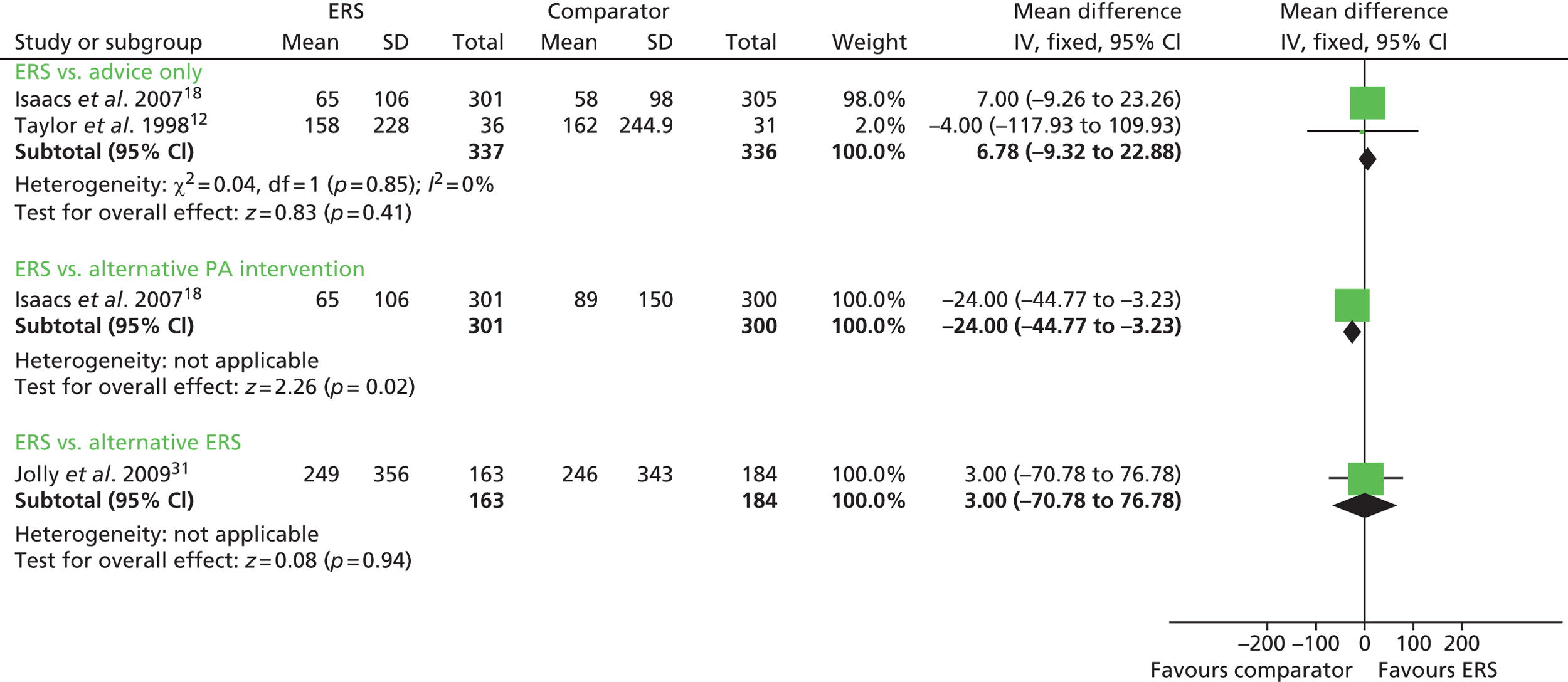
FIGURE 5.
Minutes of total physical activity/week at 6–12 months’ follow-up ERS vs. advice only (updated meta-analysis). df, degrees of freedom; IV, inverse variance; SD, standard deviation.

FIGURE 6.
Minutes of total physical activity/week at 6–12 months’ follow-up ERS vs. alternative physical activity. 11 df, degrees of freedom; IV, inverse variance; PA, physical activity; SD, standard deviation.

Exercise referral schemes versus alternative physical activity intervention
Sorensen et al. 27 reported a higher level of energy expenditure with ERS than with physical activity counselling. In contrast, the study by Isaacs et al. 18 observed a higher level of physical activity (minutes of total and moderate-intensity activity, energy expenditure) in those in the walking programme than in the ERS group. When pooled across studies, there was no significant difference in the total amount of physical or energy expenditure between ERSs and alternative physical activity interventions (Figures 7 and 8).
FIGURE 7.
Energy expenditure (kcal/kg/day) ERSs vs. usual care at 6–12 months’ follow-up. 11 df, degrees of freedom; IV, inverse variance; SD, standard deviation.
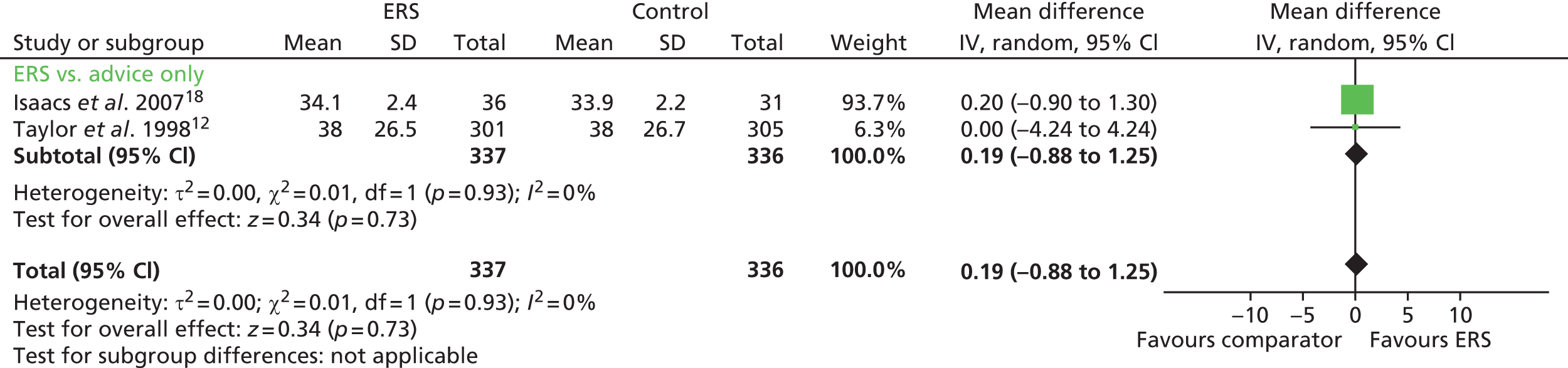
FIGURE 8.
Energy expenditure ERSs vs. alternative physical activity intervention at 5–12 months’ follow-up. 11 df, degrees of freedom; IV, inverse variance; SD, standard deviation.

Exercise referral schemes versus a self-determination theory informed delivery of exercise on referral
In the Duda et al. study,17 the proportion of patients achieving at least 150 minutes of moderate physical activity per week increased in the standard ERS group from 27% at baseline to 63% at 3 months and 46% at 6 months. There were no significant differences in these proportions between the standard ERS and a SDT-informed delivery of ERSs (Table 10).
| Study and time of follow-up | Patients achieving PA guidance (90–150 minutes/at least moderate intensity per week) | At least moderate intensity PA (minutes per week) | Total PA (minutes per week) | Energy expenditure (kcal/kg/day)a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ERS, n/N | Control, n/N | ERS, mean (SD) | Control, mean (SD) | ERS, mean (SD) | Control, mean (SD) | ERS, mean (SD) | Control, mean (SD) | |||
| Moderate | Vigorous | Moderate | Vigorous | |||||||
| ERS vs. usual care | ||||||||||
| Taylor et al.12 | ||||||||||
| 8 weeksb | 51/63 | 20/31c,d | 247 (174) | 49 (60) | 145 (178)e | 21 (61)e | Not reported | Not reported | 34.6 (1.2) | 33.7 (1.7)e |
| 16 weeksb | 51/57 | 18/31d,e | 226 (252) | 59 (72) | 160 (262)c | 21 (72)e | Not reported | Not reported | 34.6 (1.2) | 33.9 (1.7)e |
| 26 weeksb | 39/47 | 18/31d,e | 183 (234) | 56 (108) | 206 (251)c | 34 (111)c | Not reported | Not reported | 34.4 (1.8) | 34.3 (1.2)c |
| 37 weeksb | 39/57 | 19/31c,d | 158 (228) | 42 (96) | 162 (245)c | 23 (106)c | Not reported | Not reported | 34.1 (2.4) | 33.9 (2.2)c |
| Stevens et al.26 | ||||||||||
| 8 monthsf | 204/363 | 174/351c,d | Not reported | – | Not reported | – | Not reported | Not reported | Not reported | Not reported |
| Harrison et al.15 | ||||||||||
| 6 monthsb | 38/168 | 22/162d,e | Not reported | – | Not reported | – | Not reported | Not reported | Not reported | Not reported |
| 9 monthsb | 36/149 | 31/140c | Not reported | – | Not reported | – | Not reported | Not reported | Not reported | Not reported |
| 12 monthsb | 40/155 | 32/157c | Not reported | – | Not reported | – | Not reported | Not reported | Not reported | Not reported |
| Isaacs et al.18 | ||||||||||
| 10 weeksg | 48/164 | 29/157d,e | 93 (115) | – | 79 (114)c | – | 584 (479) | 668 (555)c | 34 (26) | 36 (32)c |
| 6 monthsg | 70/179 | 66/200c,d | 65 (106) | – | 58 (98)c | – | 692 (496) | 647 (463)c | 38 (27) | 35 (27)c |
| Gusi et al.28 | ||||||||||
| 6 months | Not reported | Not reported | Not reported | – | Not reported | – | Not reported | Not reported | Not reported | Not reported |
| Murphy et al. 23 | ||||||||||
| 12 months | 431/724 | 409/755 | – | – | – | – | 335.53 (442.47) | 277.42 (371.70) | – | – |
| ERS vs. alternative PA intervention | ||||||||||
| hSorensen et al.27 | ||||||||||
| 4 monthsb | Not reported | Not reported | Not reported | – | Not reported | – | 63 (114) | 23 (107)c | 43 (2.4) | 41 (4.8) |
| 10 monthsb | Not reported | Not reported | Not reported | – | Not reported | – | 20 (124) | 20 (152)c | 41 (2.1) | 40 (5) |
| Isaacs et al.18 | ||||||||||
| 10 weeksg | 48/164 | 53/92d,e | 93 (115) | – | 113 (291)c | – | 584 (479) | 863 (1026)e | 34 (26) | 43 (38)e |
| 6 monthsg | 70/179 | 62/141c,d | 65 (106) | – | 89 (150)e | – | 692 (496) | 759 (539)c | 38 (27) | 42 (27)c |
| ERS vs. SDT-informed ERS | ||||||||||
| Duda et al.17 | ||||||||||
| 3 monthsg | Not reported | Not reported | 319 (338)c | – | 331 (336)c | – | Not reported | Not reported | Not reported | Not reported |
| 6 monthsg | 66/156 | 83/169c,d | 249 (356)c | – | 246 (343)c | – | Not reported | Not reported | Not reported | Not reported |
Subgroup analysis exploring the impact of patient variables on effects of exercise referral schemes on levels of physical activity
Pavey et al. 11 reported the following analysis of subgroups within seven studies: Harrison et al. 15 reported no statistical significant interaction effects between the ERS effect and pre-specified baseline variables (i.e. CHD risk factors, sex and age). Comparing high adherers (> 75% attendance at ERS) with low adherers (< 75% attendance at ERS) in the Isaacs et al. 18 study, 32 high adherers and 16 low adherers were achieving > 150 minutes of moderate physical activity per week at 10 weeks. At 6 months, 41 high adherers and 29 low adherers were achieving > 150 minutes of moderate physical activity per week. However, these proportions were not significantly different. In the Duda et al. study,17 age, sex, deprivation (Index of Multiple Deprivation score), ethnicity, depression at baseline and level of physical activity at baseline were assessed by regression methods as predictors of physical activity at 6 months. Only physical activity at baseline was associated with physical activity at 6 months’ follow-up (p < 0.001). Murphy et al. 23 also found that effectiveness was highly dependent on adherence, with significantly greater differences in all outcomes among those who completed the 16-week programme compared with those who attended only partially or not at all.
Murphy et al. 23 reported that referral and participation in ERSs increased physical activity significantly for those referred for CHD risk factors [odds ratio (OR) 1.29, 95% CI 1.04 to 1.60]. However, among those referred for mental health reasons, either solely or in combination with CHD, there was no difference in physical activity between the ERSs and normal-care participants at 12 months’ follow-up. The effect of being in the ERS group on all referrals was an increase in levels of physical activity at 12 months, but this finding was of borderline statistical significance (OR 1.19, 95% CI 0.99 to 1.43).
Physical fitness
No additional data were available for this outcome; the following results are taken from Pavey et al. 11 The studies by Taylor et al. ,12 Isaacs et al. 18 and Sorensen et al. 27 reported physical fitness outcomes (Table 11).
| Study and time of follow-up | Mean predicted heart rate at a workload of 150 W | VO2max (ml/kg/minute) | Submaximal bike ergometer (minutes) | Submaximal shuttle walk (m) | Isometric knee strength (N) | Leg extension power (W) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERS, mean (SD) | Control, mean (SD) | ERS, mean (SD) | Control, mean (SD) | ERS, mean (SD) | Control, mean (SD) | ERS, mean (SD) | Control, mean (SD) | ERS, mean (SD) | Control, mean (SD) | ERS, mean (SD) | Control, mean (SD) | |
| ERS vs. usual care | ||||||||||||
| Taylor et al.12 | ||||||||||||
| 16 weeksa | 138.6 (23.0) | 147.2 (29.7)b | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| 26 weeksa | 136.3 (22.6) | 142.3 (28.5)b | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| 37 weeksa | 134.2 (19.0) | 146.0 (24.2)c | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Isaacs et al.18 | ||||||||||||
| 10 weeksa | Not reported | Not reported | Not reported | Not reported | 9.65 (1.5) | 8.87 (1.5)c | 456 (102) | 434 (104)b | 277 (54) | 265 (56)b | 174 (31) | 165 (31)b |
| 6 monthsa | Not reported | Not reported | Not reported | Not reported | 8.86 (1.7) | 9.08 (1.7)b | 445 (96) | 434 (97)b | 265 (58) | 267 (66)b | 173 (66) | 167 (68)b |
| ERS vs. alternative PA intervention | ||||||||||||
| Sorensen et al.27 | ||||||||||||
| 4 monthsa | Not reported | Not reported | 23.8 (7.1) | 21.7 (11.0)b | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| 10 monthsa | Not reported | Not reported | 23.0 (8.2) | 22.4 (12.7)b | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Isaacs et al.18 | ||||||||||||
| 10 weeksa | Not reported | Not reported | Not reported | Not reported | 9.65 (1.5) | 8.92 (1.7)b | 456 (102) | 437 (100)b | 277 (54) | 275 (58)b | 174 (31) | 166 (32)b |
| 6 monthsa | Not reported | Not reported | Not reported | Not reported | 8.86 (1.7) | 8.92 (1.8)b | 445 (96) | 448 (95)b | 265 (58) | 264 (66)b | 173 (66) | 164 (68)b |
Exercise referral schemes versus usual care
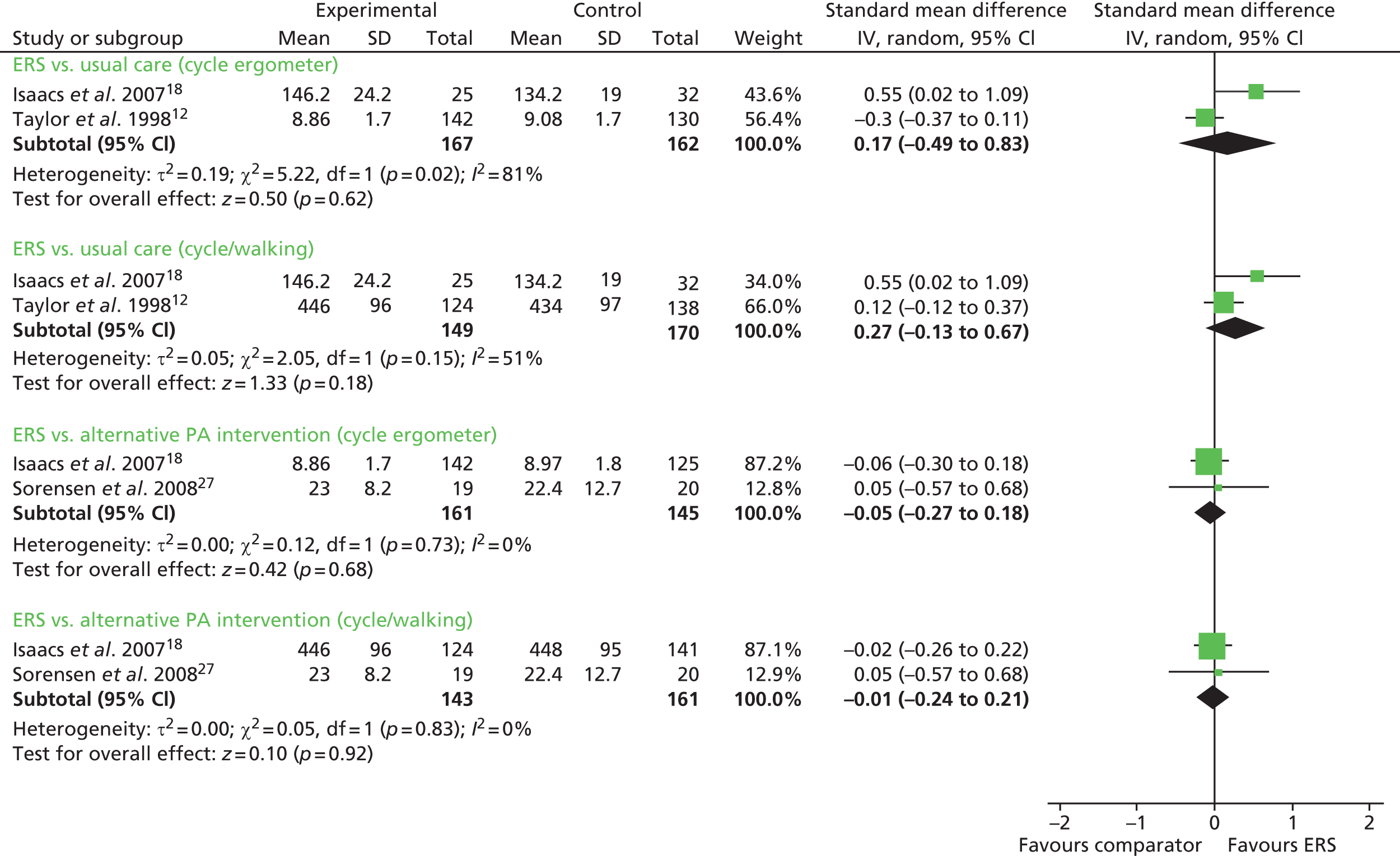
Taylor et al. 12 reported a lower (more favourable) submaximal heart rate (at 150 W) for ERS compared with usual care. Isaacs et al. 18 reported no significant differences in any of the physical fitness measures (submaximal bike and shuttle walk, isometric knee strength, leg extension power) between the ERS and usual-care groups at follow-up, except at 10 weeks for the submaximal bike ergometer test. Pooling of the cardiorespiratory measures (mode: cycle ergometer or cycle/walking) showed no difference between ERSs and usual care (Figure 9). There was considerable evidence of statistical heterogeneity.
FIGURE 9.
Physical fitness at 6–12 months’ follow-up. df, degrees of freedom; IV, inverse variance; PA, physical activity; SD, standard deviation.
Exercise referral schemes versus alternative physical activity intervention
Isaacs et al. 18 and Sorensen et al. 27 reported no significant differences in any of the physical fitness measures between the ERS and the alternative physical activity intervention groups at follow-up (see Figure 9).
Exercise referral schemes versus exercise referral scheme plus self-determination theory
The study of Duda et al. 17 did not assess physical fitness.
Clinical factors
Five studies provided information on clinical outcomes, that is, CHD risk factors (Table 12), weight and obesity measures (Table 13) and respiratory function (Table 14).
| Study and time of follow-up | SBP (mmHg) | DBP (mmHg) | Cholesterol (mmol/l) | High-density lipoproteins (mmol/l) | Low-density lipoproteins (mmol/l) | Triglycerides (mmol/l) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | |
| ERS vs. usual care | ||||||||||||
| Taylor et al.12 | ||||||||||||
| 16 weeksa | 130 (14.5) | 130 (14)b | 84 (8) | 84 (8)b | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| 26 weeksa | 130 (14) | 131 (14)b | 84 (8) | 84 (8)b | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| 37 weeksa | 130 (17) | 131 (18)b | 85 (9) | 83 (9)b | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Isaacs et al.18 | ||||||||||||
| 10 weeksa | 133 (10) | 132 (10)b | 82 (6) | 83 (6)b | 5.68 (0.53) | 5.71 (0.42)b | 1.35 (0.18) | 1.35 (0.18)b | 3.41 (0.46) | 3.44 (0.47)b | 2.12 (0.71) | 2.14 (0.71)b |
| 6 monthsa | 133 (12) | 133 (12)b | 82 (6) | 82 (7)b | 5.65 (0.50) | 5.60 (0.50)b | 1.37 (0.25) | 1.38 (0.17)b | 3.40 (0.48) | 3.37 (0.50)b | 2.04 (0.74) | 2.00 (0.84)b |
| ERS vs. alternative PA intervention | ||||||||||||
| Isaacs et al.18 | ||||||||||||
| 10 weeksa | 133 (10) | 134 (10)b | 82 (6) | 84 (6)b | 5.68 (0.53) | 5.69 (0.53)b | 1.35 (0.18) | 1.33 (0.17)b | 3.41 (0.46) | 3.45 (0.46)b | 2.12 (0.71) | 2.05 (0.76)b |
| 6 monthsa | 133 (12) | 134 (12)b | 82 (6) | 83 (6)b | 5.65 (0.50) | 5.56 (0.57)b | 1.37 (0.25) | 1.37 (0.16)b | 3.40 (0.48) | 3.36 (0.48)b | 2.04 (0.74) | 1.95 (0.74)b |
| ERS vs. ERS plus SDT | ||||||||||||
| Duda et al.17 | ||||||||||||
| 6 monthsa | 130 (17) | 127 (16)b | 82 (11) | 79 (11)b | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Study and time of follow-up | Weight (kg) | BMI (kg/m2) | Body fat (%) | Waist-to-hip ratio (cm) | ||||
|---|---|---|---|---|---|---|---|---|
| ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | |
| ERS vs. usual care | ||||||||
| Taylor et al.12 | ||||||||
| 16 weeksa | Not reported | Not reported | 27.5 (0.6) | 27.6 (0.6)b | 70 (8) | 76 (8)c | 0.87 (0.08) | 0.83 (0.09)b |
| 26 weeksa | Not reported | Not reported | 27.3 (1.3) | 27.5 (1.1)b | 70 (11) | 75 (11)c | 0.87 (0.08) | 0.83 (0.09)b |
| 37 weeksa | Not reported | Not reported | 27.5 (1.3) | 27.6 (1.1)b | 71 (13) | 76 (13)c | 0.87 (0.08) | 0.84 (0.09)b |
| Isaacs et al.18 | ||||||||
| 10 weeksd | 81 (3) | 81 (3)b | 30.2 (0.8) | 30.1 (1.5)b | 37.4 (1.9) | 37.5 (1.9)b | 0.88 (0.06) | 0.89 (0)b |
| 6 monthsd | 82 (3) | 82 (3)b | 30.5 (1.1) | 30.4 (1.1)b | 37.8 (2.4) | 37.8 (2.4)b | 0.88 (0) | 0.88 (0)b |
| Gusi et al.28 | ||||||||
| 6 monthsa | Not reported | Not reported | 29.7 (4.2) | 30.6 (4.3)c | Not reported | Not reported | Not reported | Not reported |
| ERS vs. alternative PA intervention | ||||||||
| fSorensen et al.27 | ||||||||
| 4 monthse | –1.1 (4) | –1.1 (4)b | –0.3 (1.3) | –0.04 (1.6)b | Not reported | Not reported | Not reported | Not reported |
| 10 monthse | –0.3 (4.4) | –0.3 (4.4)b | –0.1 (1.9) | –0.6 (2.8)b | Not reported | Not reported | Not reported | Not reported |
| Isaacs et al.18 | ||||||||
| 10 weekse | 81 (3) | 81 (3)b | 30.2 (0.8) | 30.2 (1.6)b | 37.4 (1.9) | 37.1 (1.9)b | 0.88 (0.06) | 0.88 (0.06)b |
| 6 monthse | 82 (3) | 82 (3)b | 30.5 (1.1) | 30.5 (1.1)b | 37.8 (2.4) | 37.8 (1.1)b | 0.88 (0) | 0.88 (0)b |
| ERS vs. ERS plus SDT | ||||||||
| Duda et al.17 | ||||||||
| 6 monthse | Not reported | Not reported | 32.8 (6.9) | 32.8 (6.4)b | Not reported | Not reported | Not reported | Not reported |
| Study and time of follow-up | FEV : FVC ratio | PEF | ||
|---|---|---|---|---|
| ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | |
| ERS vs. usual care | ||||
| Isaacs et al.18 | ||||
| 10 weeksa | 0.86 (0.0) | 0.86 (0.06)b | 417 (58) | 409 (58)b |
| 6 monthsa | 0.86 (0.09) | 0.86 (0.09)b | 407 (115) | 411 (117)b |
| ERS vs. alternative PA intervention | ||||
| Isaacs et al.18 | ||||
| 10 weeksa | 0.86 (0.0) | 0.85 (0.06)b | 417 (58) | 407 (61)b |
| 6 monthsa | 0.86 (0.09) | 0.85 (0.09)b | 407 (115) | 416 (117)b |
Exercise referral schemes versus usual care
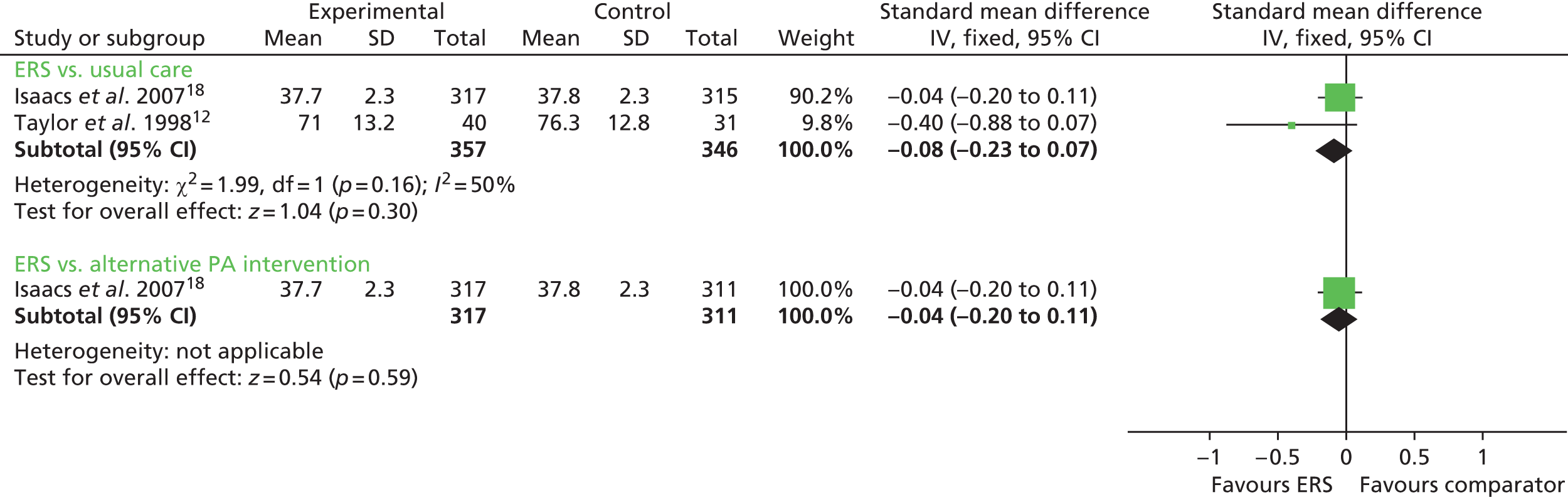
Taylor et al. 12 reported percentage of body fat in ERS participants compared with usual care at follow-up and found no statistically significant difference between the groups. Gusi et al. 28 reported a lower body mass index (BMI), with no other between-group differences in weight and body fat outcomes for the other measured clinical factors (Figures 10 and 11). There was no significant difference in resting blood pressure, serum lipids or respiratory function between ERSs and usual care at follow-up (Figures 12 and 13).
FIGURE 10.
Systolic blood pressure at 6–12 months’ follow-up. 11 df, degrees of freedom; IV, inverse variance; PA, physical activity; SD, standard deviation.

FIGURE 11.
Diastolic blood pressure at 6–12 months’ follow-up. 11 df, degrees of freedom; IV, inverse variance; PA, physical activity; SD, standard deviation.

FIGURE 12.
Body mass index at 6–12 months’ follow-up. 11 df, degrees of freedom; IV, inverse variance; PA, physical activity; SD, standard deviation.
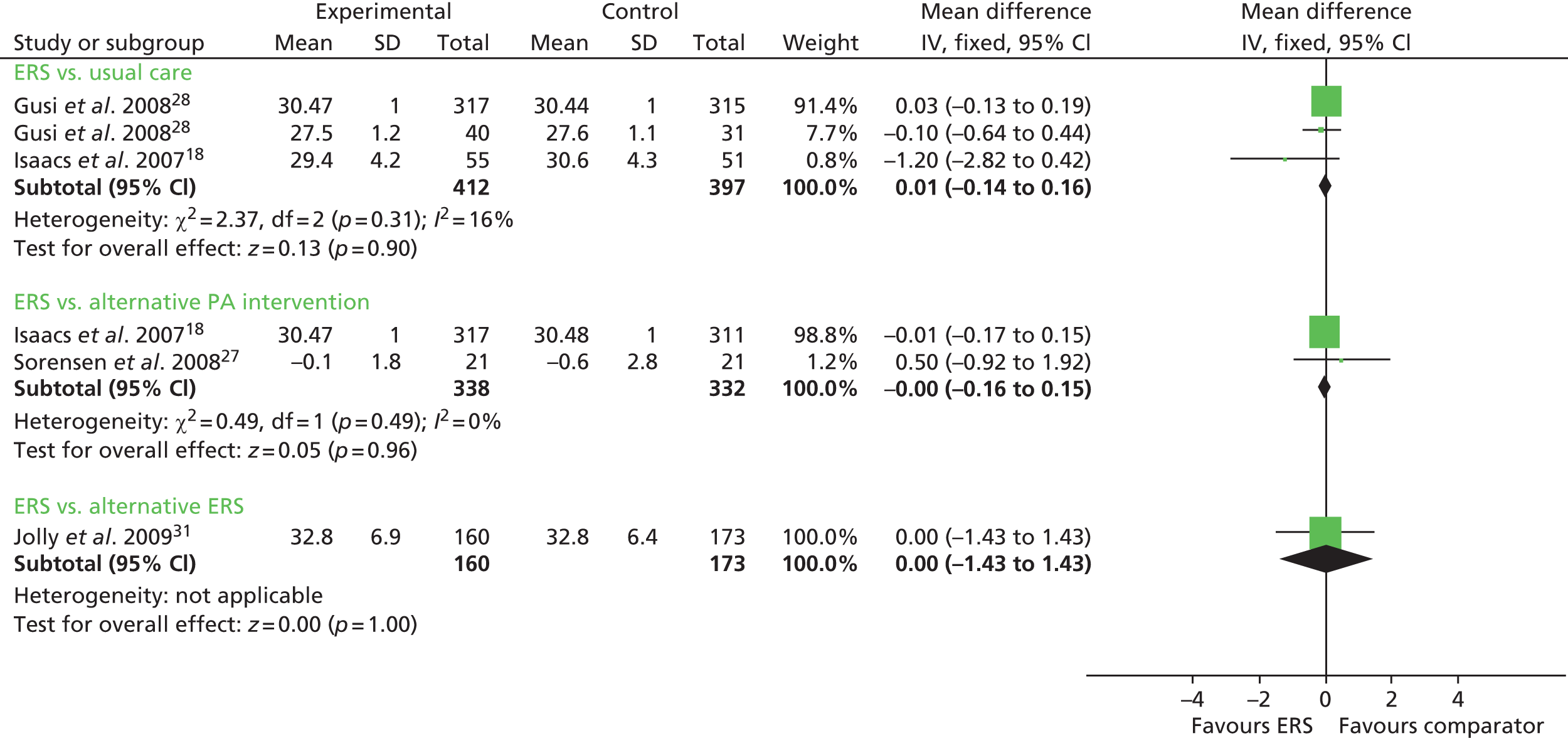
FIGURE 13.
Body fat at 6–12 months’ follow-up. 11 df, degrees of freedom; IV, inverse variance; PA, physical activity; SD, standard deviation.
Exercise referral schemes versus alternative physical activity intervention
In both the studies by Isaacs et al. 18 and Sorensen et al. 27 there were no significant between-group differences at follow-up in resting blood pressure (see Figures 10 and 11), BMI (see Figure 12), body fat outcomes, serum lipids and respiratory function. The Sorensen et al. trial27 reported reduced levels of glycosylated haemoglobin (HbA1c) in both the ERS group (mean –0.26%, 95% CI –0.79% to 0.27%) and the physical activity counselling group (mean –0.23%, 95% CI –0.47% to 0.02%) at 4 months’ follow-up, although there was no difference between groups.
Exercise referral schemes versus exercise referral scheme plus self-determination theory
Duda et al. 17 reported no significant difference between standard ERS and ERS plus SDT in BMI or resting blood pressure.
Psychological well-being
Four studies17,18,23,25,28 reported psychological well-being outcomes and the results are summarised in Table 15.
| Study | Psychological well-being | Anxiety | Depression | Anxiety/depression | ||||
|---|---|---|---|---|---|---|---|---|
| ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | |
| ERS vs. usual care | ||||||||
| aTaylor and Fox25 | ||||||||
| 16 weeksb | 2.31 (0.79) | 2.31 (0.67)c | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| 37 weeksb | 2.41 (0.79) | 2.42 (0.54)c | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| dIsaacs et al.18 | ||||||||
| 6 monthse | Not reported | Not reported | 6.9 | 7.1f | 4.8 | 4.9f | Not reported | Not reported |
| Gusi et al.28 | ||||||||
| 6 monthse | Not reported | Not reported | 14.1 (9) | 22.2 (9.8)c | 1.8 (2.3) | 2.9 (2.5)c | 1.2 (0.4) | 1.5 (0.7)c |
| Murphy et al.23 | ||||||||
| 12 months | Not reported | Not reported | HADS 7.82 (95% CI 7.39 to 8.25) (n = 472) | HADS 8.35 (95% CI 7.92 to 8.77) (n = 502) | HADS 6.14 (95% CI 5.73 to 6.54) (n = 471) | HADS 6.93 (95% CI 6.53 to 7.32) (n = 506) | ||
| ERS vs. alternative PA intervention | ||||||||
| dIsaacs et al.18 | ||||||||
| 6 monthse | Not reported | Not reported | 6.9 | 7.5f | 4.8 | 5.1f | Not reported | Not reported |
| ERS vs. ERS plus SDT | ||||||||
| Duda et al.17 | ||||||||
| 3 monthsb | Not reported | Not reported | 7.7 (4.4)f | 8.89 (4.3) | 5.9 (4.2)f | 6.68 (4.1) | Not reported | Not reported |
| 6 monthsb | Not reported | Not reported | 7.9 (4.8)f | 8.86 (4.7) | 6.1 (4.4)f | 6.65 (4.3) | Not reported | Not reported |
Exercise referral schemes versus usual care
Taylor and Fox25 reported physical self-perceptions measures, with improvements shown in physical self-worth, and perceptions of physical condition and physical health-collected physical self-perceptions data, and reported significant differences favouring the ERS group compared with usual-care group at 16 and 37 weeks. Isaacs et al. 18 reported no differences between the ERS and usual-care groups in the anxiety and depression scores using the Hospital Anxiety Depression Scale (HADS) at 6 months. In the Gusi et al. study,28 all measures [Geriatric Depression Scale, State Trait Anxiety Inventory and the anxiety/depression subscale of the European Quality of Life-5 Dimensions (EQ-5D)] at 6 months were found to favour ERS participants compared with those receiving the usual care. In the Murphy et al. study,23 in participants who were referred for mental health reason or in combination with CHD, there were significantly lower levels of anxiety (OR –1.56, 95% CI –2.75 to –0.38) and depression (OR –1.39, 95% CI –2.60 to –0.18) but no effect on physical activity. Murphy et al. 23 also found significant interactions with sex for both mental health outcomes, with the beneficial effect of the intervention apparent only among women. There was a suggestion that the intervention was more effective on mental health outcomes among the youngest age group (18–44 years), although this was not statistically significant. Effects did not vary significantly by deprivation status.
Pavey et al. 33 in a review updating his earlier review,11 pooled HADS score data from Murphy et al. 23 and Gusi et al. 28 (Figure 14). This showed a significant reduction in depression (standardised mean difference –0.82, 95% CI –1.28 to –0.35) but not in anxiety (standardised mean difference –4.12, 95% CI –11.52 to 3.28) for ERSs compared with usual care.
FIGURE 14.
Meta-analysis of depression and anxiety in patients, at 6–12 months’ follow-up: fixed-effects model used. (Pavey et al. 33 reproduced with permission, ©2011 BMJ). df, degrees of freedom; IV, inverse variance; SE, standard error.

Exercise referral schemes versus alternative physical activity intervention
Isaacs et al. 18 reported no differences between the ERS and walking programme in anxiety or depression outcomes at the 6-month follow-up.
Exercise referral schemes versus exercise referral scheme plus self-determination theory
Duda et al. 17 reported no difference between groups in anxiety or depression outcomes at either the 3- or 6-month follow-up.
Health-related quality of life
Five studies17,18,23,27,28 reported health-related quality of life (HRQoL), as summarised in Table 16.
| Study and time of follow-up | SF-36 mental | SF-12 mental | SF-12 physical | EQ-5D | Dartmouth QoL (overall QoL scale) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | |
| ERS vs. usual care | ||||||||||
| aIsaacs et al.18 | ||||||||||
| 6 monthsb | 54.2 | 54.3c | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Gusi et al.28 | ||||||||||
| 6 monthsc | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 0.89 (0.18) | 0.51 (0.2)d | Not reported | Not reported |
| Murphy et al. 23 | ||||||||||
| 12 months | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 0.64 (0.32) ( n = 395) | 0.61 (0.32) ( n = 391) | Not reported | Not reported |
| ERS vs. alternative PA intervention | ||||||||||
| Sorensen et al.27 | ||||||||||
| 4 monthsb | Not reported | Not reported | 40 (10.7) | 37 (11.9)e | 49 (1017.6) | 46 (13.1)e | Not reported | Not reported | Not reported | Not reported |
| 10 monthsb | Not reported | Not reported | 41 (10.8) | 39 (10.9)e | 51 (11.6) | 45 (15.4)e | Not reported | Not reported | Not reported | Not reported |
| aIsaacs et al.18 | ||||||||||
| 6 monthsb | 54.3 | 53c | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| ERS vs. ERS plus SDT | ||||||||||
| Duda et al.17 | ||||||||||
| 3 months | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 3.16 (0.8)e | 3.25 (0.7)c |
| 6 months | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 3.15 (0.8)e | 3.24 (0.8)c |
Exercise referral schemes versus usual care
Isaacs et al. 18 reported no differences between the ERS and usual-care groups at follow-up on the Short Form questionnaire-36 items (SF-36) mental health scale. Gusi et al. 28 observed higher EQ-5D scores in the ERS group than in the usual-care group at 6 months. Murphy et al. 23 also observed higher EQ-5D scores in the ERS group than in the usual-care group at 12 months, but it was not statistically significant.
Exercise referral schemes versus alternative physical activity intervention
Isaacs et al. 18 reported no differences between the ERS and walking groups at follow-up on the SF-36 mental health scale score. Similarly, Sorensen et al. 27 found no differences in scores between the groups at follow-up on the Short Form questionnaire-12 items (SF-12) items mental and physical scales.
Exercise referral schemes versus exercise referral scheme plus self-determination theory
Duda et al. 17 reported no difference between groups in overall Dartmouth CO-OP (Primary Care Cooperative Information Project) chart score, although there was a difference for the feelings subscale at 6 months in favour of the alternative ERS group (not tabularised).
Patient satisfaction
Two studies15,18 reported patient satisfaction and results are summarised in Table 17.
| Study | Satisfied with received information (%) | Needed further information (%) | ||
|---|---|---|---|---|
| ERS, mean (SD) | Usual care, mean (SD) | ERS, mean (SD) | Usual care, mean (SD) | |
| ERS vs. usual care | ||||
| Harrison et al.15 | ||||
| 3 months | 92 | 69a | 43 | 54a |
| ERS vs. alternative PA intervention | ||||
| Isaacs et al.18 | ||||
| 10 weeks | 97 | 96b | 15 | 17b |
Exercise referral schemes versus usual care
The Harrison et al. study15 reported that the ERS group participants were significantly more satisfied with the information they received and felt they needed less information about physical activity compared with the usual-care group. In the Taylor et al. study,12 comments about the concept of ERSs (measured at 8 weeks) identified that 50% of patients were positive, 35% had mixed feelings and 15% had only negative comments. Negative comments included a long waiting time before introductory session, lack of staff support, crowded facilities and inconvenient facility times.
Exercise referral schemes versus alternative physical activity intervention
In the Isaacs et al. 18 study, there was no between-group difference in participant satisfaction with received information or the need for additional information. In the ERS group, 97.8% felt better for taking part and enjoyed the programme compared with the walking group, in which 93.8% felt better for taking part and 95.2% enjoyed the programme.
Exercise referral schemes versus exercise referral scheme plus self-determination theory
Duda et al. 17 did not assess participant satisfaction.
Adverse events
Although participation in ERSs has the potential to lead to negative events (e.g. an increase in exercise-related musculoskeletal injuries or exercise-related cardiac complications), only the Isaacs et al. 18 study assessed such events. Using GP records, the authors assessed the change in consultations before and after ERSs. There was evidence of a small increase in GP visits for falls and fractures in the ERSs and walking groups compared with usual-care control after the start of the study (Table 18).
| Adverse events | Leisure centre | Walking control | Advice-only control |
|---|---|---|---|
| Visits for chest pain | |||
| 12–6 months before start of study | 1 (%) | 3 | 7 |
| 6 months before start of study | 3 (%) | 4 | 7 |
| Start of study to 6 months | 2 (%) | 9 | 7 |
| 6–12 months after start of study | 10 (%) | 4 | – |
| Visits for aches/pains | |||
| 12–6 months before start of study | 64 | 48 | 56 |
| 6 months before start of study | 62 | 53 | 55 |
| Start of study to 6 months | 52 | 42 | 44 |
| 6–12 months after start of study | 63 | 44 | – |
| Visits for sprains | |||
| 12–6 months before start of study | 2 | 2 | 7 |
| 6 months before start of study | 3 | 6 | 2 |
| Start of study to 6 months | 1 | 4 | 6 |
| 6–12 months after start of study | 2 | 0 | – |
| Visits for falls | |||
| 12–6 months before start of study | 1 | 1 | 0 |
| 6 months before start of study | 1 | 1 | 2 |
| Start of study to 6 months | 9 | 2 | 0 |
| 6–12 months after start of study | 3 | 6 | – |
| Visits for fractures | |||
| 12–6 months before start of study | 0 | 1 | 1 |
| 6 months before start of study | 0 | 0 | 0 |
| Start of study to 6 months | 1 | 0 | 0 |
| 6–12 months after start of study | 0 | 4 | – |
Health-care utilisation
No studies reported hospitalisations, primary care visits or use of medication.
Intervention characteristics relating to effectiveness and adherence outcomes
Since the impact of ERSs on physical activity outcomes was not significant relative to control in most studies, it is difficult to tease out the characteristics of effective interventions. The only study to find a significant (albeit small) benefit of an ERS over and above brief advice on some physical activity outcomes utilised a counselling intervention (three sessions) based on motivational interviewing plus access to subsidised exercise classes for 16 weeks, compared with normal care and brief written information. 23 The duration of the scheme was slightly longer than most other interventions, and the components of the scheme were similar to the other interventions, apart from the use of motivational interviewing as a tool in exercise counselling (although fidelity was found to be poor). The comparison condition may have been less intensive than comparison conditions in some other studies, and, interestingly, levels of adherence were low. See Table 19 for a summary of the included studies intervention and control characteristics relative to effectiveness and adherence. In addition, see Table 20 for further details of the components of the ERS interventions.
| Study | Scheme duration | Number of sessions per week | Duration of exercise sessions | Group or individual | Components | Theoretical basis | Control condition | Follow-up | Effectiveness | Adherence |
|---|---|---|---|---|---|---|---|---|---|---|
| Taylor et al. 199812/200525 | 10 weeks | 2 | 30–40 minutes | Group and/or individual | Exercise prescription; subsidised exercise sessions | Not reported | HEA leaflets on preventing CHD (but no specific PA advice) | 8, 16, 26 and 37 weeks | RR 1.12 (95% CI 0.80 to 1.55);a RR 0.95 (95% CI 0.63 to 1.45)b | 88% |
| Stevens et al. 199826 | 10 weeks | Not reported | NA | Not reported | Counselling | Not reported | Information on PA and health, and local facilities | 8 months | RR: 1.13 (95% CI 0.99 to 1.30);a RR 1.13 (95% CI 0.99 to 1.30)b | 35% |
| Harrison et al. 200415 | 12 weeks | 2 | 1 hour | Group and/or individual | Counselling; subsidised exercise sessions | Not reported | Written information only | 6 months, 9 months and 12 months | RR 1.27 (95% CI 0.84 to 1.90);a RR 1.23 (95% CI 0.80 to 1.89)b | 84% |
| Sorensen et al. 200627 | 4 months | 2 for first 2 months, 1 for second 2 months | 1 hour | Group | Counselling; supervised exercise sessions | Transtheoretical model | Low-intensive intervention (PA counselling) | 4 months and 10 months | 4 months: 48 minutes per week (95% CI 31 to 128 minutes per week); 10 months: 10 48 minutes per week (95% CI 74 to 9448 minutes per week)c | 92% |
| Isaacs et al. 200718 | 10 weeks | 2 | 45 minutes | Group and/or individual | Supervised exercise sessions | Not reported | Advice only (tailored PA advice, information on local facilities) | 6 months and 1 year | Mean difference 45.00 (95% CI –31.42 to 121.42);d RR 1.19 (95% CI 0.91 to 1.55);a RR 1.05 (95% CI 0.78 to 1.42)b | 100% |
| Gusi et al. 200828 | 6 months | 3 | 50 minutes | Group | Supervised exercise sessions | Not reported | Best care in general practice (incorporating brief advice) | 6 months | Not reported | 86% |
| Duda et al. 201417 | 12 weeks | Not reported | NA | Group and/or individual | Counselling; self-management booklet | SDT | Standard ERS provision | 3 months and 6 months | Not reported | 55.6% |
| Murphy et al. 2012 23 | 16 weeks | 1 | Not reported | Group and/or individual | Counselling; subsidised exercise classes | Motivational interviewing | Normal care and brief written information | 12 months | Mean difference: 58.11 [16.38, 99.84];d RR: 1.10 [1.01, 1.20];a RR: 1.05 [0.95, 1.17]b | 43.8% |
| Study | Description |
|---|---|
| Taylor et al. 199812/200525 | Exercise prescription and 10-week programme of reduced-price sessions at a leisure centre (group and/or individual) |
| Stevens et al. 199826 | Two consultations centred around becoming more active with a focus on what participants already did, options for becoming more active and keeping a physical activity diary (no formal exercise sessions) (group and/or individual) |
| Harrison et al. 200415 | Consultation at a leisure centre followed by subsidised 12-week borough-wide leisure pass; participants were encouraged to attend at least two centre-based sessions a week (group and/or individual) |
| Sorensen et al. 200627 | Health profiles (lifestyle and health questions) and motivational counselling (based on the transtheoretical model of behaviour change), followed by 4-month group-based training involving aerobic conditioning (e.g. Nordic walking and aerobics), light strength conditioning, stretching and games, with additional health profiles and motivational counselling after 2 and 7 months (group) |
| Isaacs et al. 200718 | Instructor-led exercise classes in a leisure-centre setting (types of class available were aerobics, body conditioning, aqua-aerobics, gymnasium and an optional swimming class) and instructor-led walks (graded 1–5 on difficulty, participants could choose) (group and/or individual) |
| Gusi et al. 200828 | Supervised walks that consisted of walking alternating with specific exercises (joint mobility, brisk walking, strengthening, stretching and brisk walking with footsteps and hand claps) (group) |
| Duda et al. 201417 | Consultations based on SDT: initial 1-hour one-to-one person-centred interview, including optional fitness appraisal and exercise promotion booklet, then 15–20 minute face-to-face or telephone consultation at 1 month, a brief 5-minute telephone call at 2 months, followed by a 20- to 30-minute face-to-face or telephone consultation at 3 months, including a self-management booklet centred on maintaining physical activity (group and/or individual) |
| Murphy et al. 2012 23 | Motivational interviewing – initial consultation with an exercise professional (including introduction to leisure-centre facilities and goal-setting), 4-week telephone contact, 16-week consultation and then an 8-month telephone contact and 12-month review, plus discounted access to one-to-one and/or group exercise classes (group and/or individual) |
Summary
-
An exhaustive search of electronic databases, hand-searching journals, bibliographic searches and citation searching, identified 9871 titles published since 2009. Eighty full-text articles were retrieved.
-
This review is an update of an earlier systematic review by Pavey et al. 11 This update also included the study by Murphy et al. 23 This review has incorporated additional data supplied by the study authors. Further additional data have also been incorporated exploring issues of uptake and adherence.
-
One additional RCT,23 by Murphy et al. , was identified since publication of the Pavey et al. 11 HTA. The study author supplied additional data, allowing an update of the meta-analyses exploring the impact of referral to and ERS minutes of physical activity as measured using a 7-day physical activity recall, and the number of participants achieving 90–150 minutes of physical activity per week.
-
When the effectiveness of an ERS is compared with usual-care group that is receiving advice only, the RR of achieving 90–150 minutes of physical activity per week at 6–12 months’ follow-up is 1.12 (95% CI 1.04 to 1.20). This is lower than the RR reported earlier by Pavey et al. 11 (RR 1.16, 95% CI 1.03 to 1.30).
-
The meta-analysis of minutes of total physical activity/per week at 6–12 months’ follow-up also showed an increase of 55.10 minutes (95% CI 18.47 to 91.73 minutes) for those in the ERS groups compared with those receiving advice only in the control groups.
-
Murphy et al. 23 is the largest RCT published to date and was considered to be at low risk of bias. It was undertaken in Wales, and its findings are highly relevant to the UK context.
-
Murphy et al. 23 reported that referral and participation in ERS increased physical activity significantly for those referred for CHD risk factors (OR 1.29, 95% CI 1.04 to 1.60). However, among those referred for mental health reasons, either solely or in combination with CHD, there was no difference in physical activity between the ERS and normal-care participants at the 12-month follow-up. The effect of being in the ERS group on all referrals was an increase in levels of physical activity at 12 months, but this finding was of borderline statistical significance (OR 1.19, 95% CI 0.99 to 1.43).
-
No additional new evidence was available for the impact of ERSs on physical fitness, patient satisfaction, clinical factors or adverse effects.
-
The only significant improvements that have been measured for people referred to ERSs are in self-reported measures of physical activity. These types of measures may be vulnerable to self-report bias, particularly in the absence of blinding at outcome assessment. Only two studies attempted to blind those collecting outcome data to the participants’ allocated groups. There was no evidence that the interventions lead to positive changes in body fat, BMI or blood pressure.
-
Subgroup analyses offer some insights into which groups of people are most like to take up and adhere to ERS. The evidence is not consistent across the trials. Those who adhere to the scheme are most likely to increase levels of physical activity. Those with higher levels of physical activity at baseline are also more likely to increase levels of physical activity. There is also some evidence that those referred for CHD risk factors are more likely to increase their levels of physical activity.
-
Despite exploring the differences between the ERSs, it was not possible to identify particular features of the interventions that promoted changes in levels of physical activity.
-
The cut-off point for ‘sedentary’ seems quite high and in most studies related to not meeting physical activity guidelines, which may be problematic as it would capture those with a broad range of activity levels. Around one-half of the studies did not specify that it was necessary to be sedentary for referral to the scheme, which again has implications for the interpretation of the findings.
-
Interventions with a theoretical basis did not provide much explicit detail on how the theory drove the intervention.
Barriers and facilitators of referral, uptake and adherence to exercise referral schemes
Quantitative results of randomised controlled trial and observational studies
Quantitative analyses of the predictors of uptake and adherence to ERSs have been explored by Pavey et al. 11 It is beyond the scope of this short report to update this element of the review. However, as new data have become available we have added these to the data from the existing Pavey et al. 11 review.
Pavey et al. 11 identified five RCTs and 14 observational studies for inclusion in their review. One additional trial23 and two observational studies34,36 were identified for inclusion in this update, although our search for new observational data was not exhaustive. See Table 21 for a summary of uptake and adherence to ERSs across studies.
| Study | Uptake | Adherence | |
|---|---|---|---|
| % (n/N) | % (n/N) of patients who took up ERSs | % (n/N) of patients who were referred to ERSs | |
| RCTs | |||
| Taylor et al.,12 UK | 88 (85/97) | 28 (24/85) | 25 (24/97) |
| Stevens et al.,26 UK | 35 (126/363) | Not reported | Not reported |
| Harrison et al.,41 UK | 84 (232/275) | Not reported | Not reported |
| Isaacs et al.,18 UK | 92 (293/317) | 45 (133/293) | 42 (133/317) |
| Sorensen et al.,27 UK | 100 (28/28) | Not reported | Not reported |
| Murphy et al.,23 UK | 85 (919/1080) | 51 (473/919) | Completed 16 weeks: 43.8 (473/1080) |
| Partial attendance 41.3 (446/1080) | |||
| Observational studies | |||
| Damush et al.,42 USA | 28 (113/404) | Not reported | Not reported |
| Dinan et al.,43 UK | 89 (216/242) | 82 (178/216) | 74 (178/242) |
| aDugdill et al.,4 UK | B: 68 (1825/2696) | A: 34 (336/958); B: 46 (849/1829) | B: 32 (849/2698) |
| Edmunds et al.,44 UK | Not reported | 51 (25/49) | Not reported |
| Harrison et al.,41 UK | 79 (5225/6610) | Not reported | Not reported |
| Jackson et al.,45 UK | Not reported | 70 (466/686) | Not reported |
| Jones et al.,46 UK | 78 (119/152) | 65 (77/119) | 51 (77/152) |
| Lord and Green,47 UK | 60 (252/419) | 31 (77/252) | 18 (77/419) |
| Martin and Woolf-May,48 UK | Not reported | 12 (60/490) | Not reported |
| Morton et al.,36 UK | Not reported | 40 (12/30) | Not reported |
| Roessler and Ibsen,37 Denmark | Not reported | 70 (811/1156) | Not reported |
| Sowden et al.,38 UK | 58 (3565/6101) | 39 (1404/3565) | 23 (1404/6101) |
| James et al.,49 UK | Not reported | 57 (750/1315) | Not reported |
| bGidlow et al.,39 Crone et al.,40 James et al.,49 UK | 66 (1930/2908) | 48 (931/1930) | 32 (931/2908) |
| Tobi et al., UK24 | Not reported | 58 at 13 weeks (407/701) | Not reported |
| 45 at 20–26 weeks (315/701) | |||
| Hanson et al.,50 UK | 81(1811/2233) | 53.5 at 12 weeks (968/1811) | 43.3 (968/2233) at 12 weeks |
| 42.9 (777/1811) at 24 weeks | 34.8 (777/2233) at 24 weeks | ||
Sample sizes ranged across studies from 30 to 6610 participants in the observational studies and from 97 to 2068 in the RCTs. Mean age ranged from 44.9 to 51.9 years across the observational studies and from 53.0 to 59.1 years for the RCTs. Uptake and adherence were described differently in the included studies. Uptake was defined in one of two ways: attendance at the initial consultation with the ‘exercise professional’ or attendance at least one exercise session.
Most studies provided a definition of adherence – completion of a set number of exercise sessions, either numerically (e.g. completed 20 sessions) or a percentage (e.g. > 80% attendance). For four studies,37–40 attendance at a post-ERS consultation was also required to meet the definition of adherence.
The uptake of ERSs across the RCTs ranged from 35% to 100%. Murphy et al. 23 had an uptake of 85%, which was slightly above the pooled result (80%, 95% CI 61% to 98%) reported in the Pavey et al. 11 review. Adherence to the scheme throughout compares favourably with the pooled rate of 37% (95% CI 20% to 54%) across schemes assessed by trials in the review.
Levels of uptake to ERSs ranged from 28% to 81% in the observational studies and adherence ranged from 12% to 70%. Two additional studies36,48 reported adherence levels of 58% and 54%.
Demographic factors
Four studies23,38,47,49 found that women were more likely to take up ERSs than men, but two studies41,44 found no association between sex and uptake. The data for adherence, however, are less consistent, with one study49 reporting that men are more likely to adhere to ERSs than women and another that women are more likely to adhere to ERSs. 51 Increasing age was a factor that strongly predicted for both uptake of ERSs and adherence to the scheme in six studies. 6,23,24,38,49,51 Three studies found no such association. 18,42,47
Three studies23,39,49 found that those most deprived were less likely to take up ERSs and two studies23,49 found that deprivation was a predictor for not adhering to ERSs. Two studies23,49 found no such association with take up. Car ownership23 was also a predictor of both uptake and adherence to ERSs. Living in a rural location was also found to be associated with a lower likelihood of uptake. 39 Two studies24,42 found no association between ethnicity and uptake, and one found no association with adherence. 24 Those most active at baseline were also most likely to take up and to adhere to ERSs. 51
Tables 22–24 describe the factors that have been associated with uptake and adherence in the included studies.
| Factor | Harrison et al.41 (n = 6610) | Sowden et al.38 (n = 6160) | Gidlow et al.39 (n = 2958) | Damush et al.42 (n = 404) | Murphy et al.23 (n = 2068) | Tobi et al.24 (n = 701) | Hanson et al.49 (n = 2233) | Lord and Green et al.47 (n = 419) | Dugdill et al.4 (n = 2696) | Isaacs et al.18 (n = 317) | Number of studies on this factor | + | – | No significant association |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 0 | 0 | ||||||||||||
| Female | 0 | + | 0 | + | + | + | 6 | 4 | 2 | |||||
| Increasing age | + | + | 0 | + | + | 0 | + | 0 | 8 | 5 | 3 | |||
| Deprivation | 0 | 0 | – | – | – | 5 | 3 | 2 | ||||||
| Car ownership | + | 1 | 1 | |||||||||||
| Rural living | – | 1 | 1 | |||||||||||
| Ethnicity | 0 | 0 | 2 | 2 | ||||||||||
| CHD risk | + | + | ||||||||||||
| Mental health illness | + | – | – | |||||||||||
| Overweight/obesity | – | 0 | 0 | 3 | 1 | 2 | ||||||||
| Leisure site | 0 | + | 2 | 1 | 1 | |||||||||
| Clinic location | 0 | |||||||||||||
| Referral by cardiac rehabilitation nurse | + | 1 | 1 | |||||||||||
| Referred by GP | – | 1 | 1 |
| Factor | Harrison et al.41 (n = 6610) | Sowden et al.38 (n = 6160) | Gidlow et al.39 (n = 2958) | Damush et al.42 (n = 404) | Murphy et al.23 (n = 2068) | Tobi et al.24 (n = 701) | Hanson et al.49 (n = 2233) | Leijon et al.51 (n = 1358) | Lord and Green et al.47 (n = 419) | Dugdill et al.4 (n = 2696) | Isaacs et al.18 (n = 317) | Number of studies on this factor | + | – | No significant association |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | – | + | 2 | 1 | 1 | ||||||||||
| Female | + | 0 | 2 | 1 | 1 | ||||||||||
| Increasing age | + | + | + | + | 4 | 4 | |||||||||
| Deprivation | – | – | 2 | 2 | |||||||||||
| Car ownership | + | 1 | 1 | ||||||||||||
| Moderately active | + | 1 | 1 | ||||||||||||
| Inactive at baseline | – | 1 | 1 | ||||||||||||
| Rural living | |||||||||||||||
| Ethnicity | 0 | 1 | 1 | ||||||||||||
| CHD risk | + | – | 2 | 1 | 1 | ||||||||||
| Mental health illness | – | 1 | 1 | ||||||||||||
| Overweight/obesity | + | – | 2 | 1 | 1 | ||||||||||
| Leisure site | + | 1 | 1 | ||||||||||||
| Home-based activities | + | 1 | 1 | ||||||||||||
| Referral by cardiac rehabilitation nurse | + | 1 | 1 |
| Psychosocial factors | Edmunds et al.44 | Jones et al.46 | Morton et al.36 | Number of studies on this factor | + | – | No significant association |
|---|---|---|---|---|---|---|---|
| Stage of change | 0 | 1 | 1 | ||||
| Self-efficacy | 0 | 1 | 1 | ||||
| Expectations of change (health and fitness) | 0 | 1 | 1 | ||||
| Expectations of change (personal development) | + | 1 | 1 | ||||
| Psychological well-being | 0 | 1 | 1 | ||||
| Need satisfaction | 0 | 1 | 1 | ||||
| Perceived autonomy | 0 | 1 | 1 | ||||
| Support | 0 | 1 | 1 | ||||
| Self-determination | + | 1 | 1 |
Medical diagnosis
The evidence for the role of medical conditions at baseline in influencing both uptake and adherence is not consistent across the studies.
Harrison et al. 41 found that those with mental health problems were more likely to take up ERSs than those with no specified referral reason (OR 1.70, 95% CI 1.24 to 2.39). Gidlow et al. 39 found that those with mental health problems were less likely to take up ERSs than those referred with cardiovascular disease (OR 0.33, 95% CI 0.27 to 0.57). Gidlow et al. 39 and Moore et al. 52 both found that those with mental health conditions were less likely to adhere than those referred for other reasons (Gidlow et al. 39 22% vs. 34%; Moore et al. 52 OR 0.57, 95% CI 0.43 to 0.75).
Harrison et al. 41 found that the response to referral for respiratory problems was associated with deprivation, with those most deprived less likely to take up ERSs than those least deprived (OR 1.45, 95% CI 1.06 to 1.99).
Participants referred because of overweight or obesity were found to be less likely to take up the intervention39 (OR 0.63, 95% CI 0.50 to 0.81; p < 0.01) or to adhere50 to the intervention than those without these problems. However, this factor was not significant in two other studies14,23
Those referred with musculoskeletal or orthopaedic problems were also less likely to take up ERSs39 (OR 0.75, 95% CI 0.58 to 0.99). Tobi et al. 24 also found that they were less likely than participants with metabolic conditions to adhere to ERSs (OR 0.25, 95% CI 0.07 to 0.94).
Participants referred with CHD risk factors were also found to have lower odds of adhering than those with metabolic conditions24 (OR 0.18, 95% CI 0.05 to 0.70).
Sowden et al. 38 reported that patients with diabetes mellitus were less likely to adhere to an ERS (OR 0.76, 95% CI 0.63 to 0.93) than those with cardiovascular disease (CVD) (OR 1.22, 95% CI 1.03 to 1.45) when compared with those without either condition.
Psychosocial
Pavey et al. 11 reports the results of three studies36,44,46 that assess the psychosocial predictors of adherence. Morton et al. 36 found participant self-determination to positively predict ERS adherence, whereas Edmunds et al. 44 found no such association. An expectation for change in personal development was also found to be positively predictive of ERS adherence.
Qualitative evaluation of the discussion sections of included studies
In order to explore a further source of data to improve understanding of the factors that will influence the uptake of and adherence to ERSs, we undertook a qualitative analysis of the discussion and conclusion sections of the included studies. Often authors will report their perceived reasons for success and failure of interventions. These may not be reported in the measurable outcomes described within the results sections of published papers and these views can give valuable additional insights that are particularly relevant to the studies included in the review.
As a result of this analysis we created a logic model to summarise and describe the complexity of factors that might impact on referral to ERSs, uptake, adherence and sustained change in levels of physical activity.
Factors that impact on the patterns of referral to exercise referral schemes
Crucial to the success of exercise referral programmes is ensuring that all those who might benefit are referred. This was identified in a number of the studies as an area that impacted on intervention effectiveness. Where researchers were involved in supporting the referral process, referral rates improved. Low referral rates were reported in studies in which only one GP was involved in making referrals and in pragmatic ‘real-world’ studies. 28,33,34
Factors that were perceived to reduce health professionals’ referral to ERSs were lack of enthusiasm for the project, poor knowledge of the scheme and poor interpersonal skills on behalf of the health professional. 12 Workload and competing demands and the extra time needed to make the referral were also considered barriers to referral. 26 There was also a view that certain characteristics of individuals might influence the likelihood of referral. Being younger appeared, in one study, to reduce the likelihood of being referred. 18
An invitation that was perceived by those referred to be judgemental in its tone was a barrier to the uptake of an ERS. Other aspects of the invitation that were more likely to lead to a positive response included issuing invitations with a specific appointment time. Where invitations required participants to make the appointment, there was poorer response to the referral. 26
An area where there is uncertainty about potential factors that might influence referral and response to referral is in the use of cold calling by health professionals as an alternative to the GP referring following a consultation. 12 There may also be a potential role for peers in the process of referral and uptake that is as yet unexplored. 28
Factors that influence uptake of exercise referral schemes
A number of characteristics of the intervention were described as being factors that might shape the uptake of ERSs and adherence to the programme. The attractiveness and appeal of the intervention was felt to be important, particularly in targeting these to the individuals most likely to benefit from the intervention. 18 Using an existing scheme was also felt to be advantageous. 12 The incorporation of motivational counselling, ongoing professional support and the quality of that counselling and support were considered important factors. Another element of motivational interviewing related to the fidelity of the intervention, with poor delivery compromising the effectiveness of the intervention. 52 Interventions that had tailored motivational strategies were seen as more likely to be taken up and adhered to than ones in which there was a lack of choice. Schemes where support was given in a non-judgemental way were considered important. 52 The speed of referral was considered important, with congestion in the system a barrier to uptake. 12
There were aspects of ERS design where further research was recommended to establish its value; this included the use of telephone calls to prompt attendance, follow-up sessions outside the scheme and the addition of motivational counselling. 14,21,33
A further barrier to uptake in trials of ERSs was that some participants did not want to be randomised. 27
Factors influencing adherence and sustained change in physical activity levels
A number of factors, relating particularly to the environment, were considered important in maintaining adherence to the programme and in maintaining levels of physical activity. These included perception of the environment and, linked to this, the presence of parks and green spaces in the urban environment. 18 An environment that is not, or is not perceived to be, conducive to changing levels of physical activity is a barrier to sustained behaviour change. Programmes that included strategies to make gradual small changes and move to levels of moderate activity, rather than interventions that promote more intense levels of physical activity, were felt to have greater appeal and to be more likely to promote adherence and sustained change. 26
A range of characteristics of participants were also reported to make a difference to their response to ERSs. Individuals who smoked were less likely to adhere to ERSs. 12 The evidence for people with obesity was more varied, with one study finding that those with obesity were more likely to take up the intervention and adhere to it than non-obese participants12 and another finding the opposite. 18 More research might identify what features of the intervention made it attractive or less attractive to people with obesity. Participants with greater pre-existing levels of physical activity were more likely to adhere to the intervention. 14,23 One paper raised the suggestion that those volunteering to participate may have already been motivated to become (more) active. 27 Along with reports that there were fewer referrals from more deprived wards,18 this also raises the possibility that, as an intervention, ERSs may serve to increase inequalities in health.
Additional questions
A number of areas for further investigations were identified in the included studies. These included gaining an understanding of the needs of particular groups; exploring why people withdraw from ERSs; and the potential value of additional home support. 17,21,23
The value of advice
Several studies explored the reasons why the intervention was not effective, when compared with advice-only groups (usual care). 17,21,34 One reason was that physical activity levels also increased in the control groups that were receiving advice about the benefits of physical activity but were not referred to an ERS. The reasons why advice works in the context of these trials, where individuals are deprived of the intervention, warrants further exploration.
Qualitative sibling studies
We searched for qualitative studies that were undertaken as part of a mixed-methods evaluation of an ERS included in this review.
One study23,25,26,32 undertook a mixed-method process evaluation, using structured observation, implementer interviews and routine data to assess the extent to which the ERS was implemented as intended. Semistructured patient interviews explored processes of change and the emergence of social patterning in responses to the scheme. 52
The features of the intervention were as follows: motivational interviewing – initial consultation with an exercise professional (including introduction to leisure-centre facilities and goal-setting), 4-week telephone contact, 16-week consultation and then an 8-month telephone contact and 12-month review, plus discounted access to one-to-one and/or group exercise classes (group and/or individual).
The intervention recruited participants who were sedentary and who had at least one CHD risk factor or suffered a mental health problem (mild anxiety, depression or stress).
The findings of the semistructured patient interviews are summarised.
Qualitative study component: patient experiences of the exercise referral schemes
Entering the scheme: routes into exercise referral schemes and motivations for attendance
Some patients described entering the ERS because their health professional had advised them to. However, others described initiating referral themselves through asking their doctor to refer them, having made an independent decision to become more active. In some centres, a majority of patients were referral seekers, with some commenting that health professionals had been unaware of the scheme until made aware of it by patients. Those who actively sought referral often had been previously active, but in many cases this had been interrupted. Joining the ERS became a way of overcoming barriers to becoming active once again.
Patients cited various motivations for attendance, including improved health outcomes such as reduced blood pressure and weight loss. Both referral seekers and those advised to attend commonly also linked behaviour change to personal values such as having a proactive role in treating and preventing illness. Older patients emphasised maintenance of autonomy and ability to perform everyday activities as principal motivators for attendance. For younger patients, however, primary motivations centred around maintaining occupational functioning or returning to work, with many having attended because of injuries or illness which prevented, or threatened to prevent, them from working.
Experiences of exercise referral schemes
Those who attended the ERS for several weeks were beginning to perceive progress towards their goals. Some mental health patients highlighted valued improvements in mood and increased social contact. Others highlighted medical improvements, such as reduced breathlessness and blood pressure, increased mobility or reduced pain. Lack of weight loss was a disappointment for several participants.
The expertise of the professionals and the support they offered was clearly an aspect of the interventions that was valued, particularly in helping participants as they became familiar with using exercise equipment and in knowing the extent to which they should exert themselves. The professional’s role in monitoring progress and promoting further progression was also valued.
The lack of variety in the forms of exercise enabling progression was a negative element of the intervention for some who were at the later stages of the programme. The limited number of centres where the scheme was offered was potentially a barrier to participation, particularly if this meant that participation was dependent on access to a car. Access was also more problematic for those who were working and were more constrained by time.
Peer support and the value of shared experiences with other patients was a positive element of the intervention, meaning that individuals felt that they would be viewed with empathy. Mixing with others who were at later stages in the programme provided positive role models. This made the exercise environment less intimidating and more supportive, which encouraged participants.
Leaving exercise referral scheme: the transition to independent activity
Some patients identified clear plans for how they would maintain increases in physical activity, sometimes having identified exit route classes they planned to enter or focusing upon a desire to return to former hobbies. Such patients were most commonly those with a prior history of activity, some of whom had entered ERSs as a means of overcoming the refusal of gyms to accept them as members.
Others, perhaps more dependent on the programme to provide ideas for long-term maintenance or activity, were unsure how they might maintain change. In some cases, formation of action plans appeared to have been hampered by a lack of information about available options after the programme. Some expressed concerns that, without a commitment to an agreed time and place, other aspects of daily life would crowd out time for physical activity, with the ERSs providing justification for taking time out from other commitments, but with this time becoming harder to protect after programme completion.
There were some differences in views between those at the early stages of the programme and those nearing the end of the programme. For those at the early stage, they expressed concerns regarding their ability to maintain increases without ongoing motivational and informational support from the professional. For those near the end of the programme there were perceived challenges including the loss of social support they gained while participating in the programme. The support element of having others to exercise with was an important factor in maintaining levels of physical activity. For some, there were concerns about moving into mainstream exercise settings as well as concerns about the cost of remaining active once the programme discount was withdrawn.
Summary
-
Figure 15 is a logic model, seeking to represent the many factors that can impact the pathway from sedentary levels of physical activity, to successful uptake and adherence to an ERS and to maintained healthy levels of physical activity.
-
Exercise referral schemes are ‘complex interventions’; therefore, what works in one setting may not mean the intervention will necessarily work in another. It is, therefore, important to understand what works, for whom and in what circumstances.
-
When considering uptake and adherence, issues relating to who is referred and also to maintenance of physical activity at the end of the intervention also need to be considered.
-
A range of factors can influence who is referred to an ERS, including the support for referral, buy-in by GPs and preconceived perceptions about patients by health professionals.
-
A range of characteristics can shape how participants respond to referral. These include age, pre-existing levels of physical activity and obesity. The nature of the invitation to an ERS can also shape response from potential participants.
-
Exercise referral schemes vary in many ways, including professional running schemes, group-based or individual sessions and the incorporation of motivational counselling. They will also vary in duration of contact and length of time in scheme. There is uncertainty over which elements of these schemes may have benefits for which groups. There is some suggestion that group-based work can be beneficial in promoting peer support. This in turn can promote sustainable change. It can make some individuals, particularly men and older people and overweight individuals, feel more uncomfortable in group settings.
-
Perceptions of the environment and the presence of green space supports sustained change.
Chapter 4 Assessment of cost-effectiveness
Background to independent economic assessment
In 2011, Anokye et al. 21 published the results of a cost-effectiveness model of ERSs based on data from a systematic review of the effectiveness of ERSs by Pavey et al. 11 They concluded that ERSs are associated with a modest increase in lifetime costs and benefits and that the cost-effectiveness of ERSs is highly sensitive to small changes in the effectiveness and cost of ERSs and is subject to some significant uncertainty, mainly as a result of limitations in the clinical effectiveness evidence base.
This model was later amended to inform the NICE guidance on brief advice in primary care to promote physical activity (PH44). 53 This later model differed from that used by Pavey et al. 11 in terms of both the model structure and its data inputs (see Chapter 4, Comparison of the Anokye adapted to exercise referral scheme model and the Pavey exercise referral scheme model for a comparison of these two models).
The scope for the economic analysis of ERSs for this brief report was to update the Anokye et al. brief advice model with evidence from an updated systematic review on the effectiveness of ERSs and to update the costs. 53
The economic analysis has, therefore, focused on updating only three groups of parameters and all other parameter values have remained unchanged. First, estimates of the relative clinical effectiveness of ERSs versus no ERSs have been updated using the results from the updated systematic review given that the review identified new evidence (see Chapter 3, Results). Second, costs were inflated to 2013 values using Personal Social Services (PSS) Research Unit inflation indices. 54 Third, the starting age has been set at 50 years, which is the mean age from the studies from which the effectiveness data have been taken. No other model parameter values were updated and the model structure remains unchanged.
There are several benefits to using an existing cost-effectiveness model to assess the cost-effectiveness of ERSs. Firstly, it builds on the existing evidence base already incorporated within the model, allowing resources to be focused on identifying and incorporating any new effectiveness evidence. Secondly, the repeated use of the same model across multiple NICE Public Health Appraisals is likely to increase internal consistency within the NICE process. Conversely, the lack of a de novo approach does limit our ability to explore alternative model structures and evidence sources. In this case, the use of an existing model was determined to be the best option given the resources available, and the model to be used was specified a priori in our protocol.
Methods
Population, intervention and comparator
The population, intervention and comparator are the same as those defined in Chapter 4, Comparison of the Anokye adapted to exercise referral scheme model and the Pavey exercise referral scheme model.
Horizon and perspective
The analysis was conducted for a hypothetical cohort of 100,000 patients with a mean age of 50 years receiving either exercise referral or usual care. A lifetime horizon was adopted in order to capture the potential long-term benefits and cost savings associated with physical activity. Mean life expectancy was based on UK interim life tables55 and the analysis did not consider males and females separately. The economic perspective of the model is the NHS and PSS in the UK. Costs and health benefits were discounted at an annual rate of 1.5% as recommended by the Methods for the Development of NICE Public Health Guidance. 56
Model structure
The model structure is described in full elsewhere but we provide a brief description here. 53 The model has a Markov structure and considers a cohort of individuals aged 50 years who present in a physically inactive state and are given a referral to a service designed to increase physical activity that includes a physical activity or exercise programme compared with a control group with no referral to an exercise service. The age of the population was selected to reflect the populations enrolled in the studies providing evidence on the effectiveness of ERS. 11,23 The model estimates the likelihood of becoming physically active and the consequent risk reduction this has on CHD, stroke and type 2 diabetes mellitus.
In the first year of the model individuals enter either an ‘inactive, healthy state’ or an ‘active, healthy’ state; this is considered a ‘run-in’ period where individuals reach a stable level of activity. From year 2 onwards, a proportion of individuals transit from the initial state into one of the following states: event free, CHD, stroke and type 2 diabetes mellitus. Stroke and CHD are then subdivided into non-fatal and fatal. Patients can also die from other causes. Figure 16 shows the model structure.
FIGURE 16.
Model structure from year 2 onwards.

Model parameters
Effectiveness of exercise referral schemes versus usual care
The evidence for the effectiveness of ERSs versus usual care is based on the results of the systematic review described in Chapter 3 of this report. The review identified one new study by Murphy et al. 23 This study conducted a large RCT, with 2160 participants randomised to receive ERS or usual care. A full description of the study can be found in Chapter 3 of this report. This study was combined in a meta-analysis with the studies identified by Pavey et al. 11 The RR of the number of individuals becoming active versus being inactive owing to ERSs was 1.12 (95% CI 1.04 to 1.20). This RR was calculated using the number of participants with study data at 12 months. A sensitivity analysis was also conducted using the RR estimated when taking an ITT approach (RR 1.08, 95% CI 1.00 to 1.17).
The effect of physical activity on disease outcomes
Evidence of the effect of physical activity on the development of CHD, stroke and type 2 diabetes mellitus was derived from a reference search of papers included in five national and international guideline reports that set out the science-based guidance on physical activity, fitness, and health for the UK, USA and Canada (see appendix 6 of Anokye et al. ). 53
The RR estimates for developing CHD (non-fatal and fatal), stroke (non-fatal and fatal) and diabetes mellitus were selected from Hu et al. ,57–59 respectively. These RR estimates were based on cohort follow-up periods of 19 years for CHD and stroke and 12 years for diabetes mellitus. The model applies these RR estimates for the initial 10-year period only on the assumption that it would be unrealistic for them to be applied after the follow-up period (Table 25).
| Disease conditions | RR (95% CI) | Source |
|---|---|---|
| CHD | 0.90 (0.83 to 0.99) | Hu et al.56 |
| Stroke | 0.86 (0.79 to 0.93) | Hu et al.57 |
| Diabetes mellitus | 0.67 (0.53 to 0.84) | Hu et al.58 |
Physical activity habits can be quite changeable. The impact of changing habits is incorporated in the cohort RR estimates for the disease conditions. The studies used followed up the same people (who were either active or inactive at baseline) for a number of years, during which time some of the inactive people might have become active or vice versa, diluting the observed relationships between activity and outcomes. 60–62 Hence, the protective effect of activity is assumed to last for the period in which these people were followed, that is 10 years.
The physical activity levels and study population used to measure the effect of activity on disease outcomes were similar to those of the effectiveness estimate.
Baseline risk of developing the disease conditions
The baseline risks for developing CHD and stroke were based on age-specific UK annual incidence rates used in a NICE technology appraisal of statins63 and the model developed as part of the update of the NICE guideline on hypertension. 64,65 In these models, data were obtained from the Bromley Coronary Heart Disease Register and the Oxfordshire Community Stroke project. The baseline risk for diabetes mellitus was taken from age-specific UK incidence rates for type 1 and type 2 diabetes mellitus from 1996 to 2005 estimated in Gonzalez et al. 66 Table 26 shows the baseline risks for the disease conditions in the general population.
| Age (years) | CHD | Stroke | Diabetes mellitus | Source(s) |
|---|---|---|---|---|
| 33–34 | 0.000035 | 0.00008 | – | Ward et al.,63,68 NICE69 |
| (33–39) | – | – | 0.00009 | Gonzalez et al.66 |
| 35–44 | 0.000465 | 0.00023 | – | Ward et al.,63,68 NICE69 |
| (40–49) | – | – | 0.00028 | Gonzalez et al.66 |
| 45–54 | 0.002095 | 0.00057 | – | Ward et al.,63,68 NICE69 |
| (50–59) | – | – | 0.000632 | Gonzalez et al.66 |
| 55–64 | 0.00631 | 0.00291 | – | Ward et al.,63,68 NICE69 |
| (60–69) | – | – | 0.001005 | Gonzalez et al.66 |
| 65–74 | 0.0097 | 0.0069 | – | Ward et al.,63,68 NICE69 |
| (70–79) | – | – | 0.001116 | Gonzalez et al.66 |
| 75–81 | 0.0097 | 0.01434 | – | Ward et al.,63,68 NICE69 |
| (80–81) | – | – | 0.001116 | Gonzalez et al.66 |
The derivation of the probabilities for developing CHD, stroke and diabetes mellitus used in the model involved a number of steps. First, the probability of developing these conditions among inactive people was derived by adjusting the general population age-specific incidence rates using the attributable risk fraction. 67 Second, the estimates were adjusted using RR estimates of the probability of developing the health states among active individuals reported in Hu et al. 57–59
The probability of a primary stroke or CHD event being fatal was based on incidence data from the Bromley Coronary Heart Disease Register and the Oxfordshire Community Stroke project. 63 This should be acknowledged as a simplification of the model, as in reality these probabilities might depend on level of physical activity. Lack of data, however, precluded accounting for such a possibility.
Mortality risks
The probabilities for CVD- (CHD and stroke) and non-CVD-related mortality for healthy people were derived from age-specific UK interim life tables prepared by the Government Actuary’s Department that were adjusted by age-specific UK annual incidence of mortality prepared by the Office for National Statistics (see Appendix 4). Although it is recognised that these estimates relate to the general population and, hence, include people with CHD, stroke and diabetes mellitus, the percentages of those disease groups are relatively small (< 8%) and, hence, we assume that these estimates are applicable to the healthy population. RR estimates for CHD-, stroke- and non-CVD-related mortality among people with CHD, stroke and diabetes mellitus were used to adjust the probabilities for the healthy people to derive probabilities of CHD-, stroke- and non-CVD-related mortality (see Table 27). The RR estimates for diabetic patients were based on a cohort of the Framingham Heart Study (aged 45–74 years) that was followed for up to 25 years in the Preis et al. study. 60 For stroke patients, data were obtained from Brønnum-Hansen et al. 61 who followed a Danish cohort of > 25-year-olds for 10 years after their first non-fatal stroke. As no equivalent data were found for CHD patients the model assumes CHD risk is the same as stroke risk. The relative risks for mortality applied in the model are summarised in Table 27.
| Mortality outcome | After non-fatal CHD | After non-fatal stroke | After diabetes |
|---|---|---|---|
| Non-CVD mortality | 1.71 | 1.71 | 1.49 |
| CVD mortality | 3.89 | 3.89 | 2.61 |
Utility values
Health state utility values were taken from Ward et al. ,68 who undertook a wide search for available evidence on utility estimates associated with CVD and diabetes mellitus health states (Table 28).
| Condition | Utility |
|---|---|
| Healthy | 1 |
| CHD initial event | 0.8 |
| Post-CHD event | 0.92 |
| Stroke initial event | 0.63 |
| Post-stroke event | 0.65 |
| Diabetes mellitus | 0.9 |
To account for the fact that HRQoL in the general population falls with age, the disease-specific utilities were weighted using age-specific utility scores for the general population. The age-specific utility scores were estimated using data from the Health Survey for England62 (see Table 29).
| Age (years) | Mean | SD |
|---|---|---|
| 33–44 | 0.90 | 0.184 |
| 45–54 | 0.86 | 0.229 |
| 55–64 | 0.82 | 0.264 |
| 65–74 | 0.78 | 0.266 |
| >75 | 0.72 | 0.275 |
Process utility
It is acknowledged that individuals benefit psychologically from physical activity, resulting in a so-called process utility, and it has been estimated that there is an average increase in utility values of 0.072. 11 Using a conservative approach, this utility gain is assumed to last for only the first year, as the evidence in a study by Campbell et al. 70 indicates that this is the period when individuals stayed active. Sensitivity analysis considers the impact of this quality of life (QoL) gain by setting it to zero in the univariate sensitivity analysis.
Intervention costs
An estimate of the cost of ERS was taken from Pavey et al. 11 and is based on a HTA by Isaacs et al.,18 which conducted a detailed micro-level costing exercise for a leisure centre-based ERS. Pavey et al. 11 updated this cost to 2010 prices (£222) and we have inflated this to a 2011/12 cost of £229 using inflation indices from the PSS Research Unit. 54 The cost to the participants is not included in the ERS model.
Treatment costs
Table 30 shows the annual costs per person attributed to the health states in the model. These costs were taken from a National Clinical Guidelines Centre report that provided an updated review of costs for various health states. 69
| Conditions | Annual cost per person (2011/12 prices, £) |
|---|---|
| Healthy | 0 |
| CHD initial event | 4198 |
| CHD annual treatment | 480 |
| Stroke initial event | 10,839 |
| Stroke annual treatment | 2380 |
| Diabetes mellitus annual treatment | 968 |
Individuals incur a one-off initial event cost when entering a CHD or stroke disease state. Furthermore, for all three disease states (CHD, stroke and diabetes mellitus) individuals incur an ongoing treatment cost for each year they remain in that disease state.
Injuries and adverse events
No new data relating to injuries and adverse events were identified in the systematic review and, therefore, adverse events are not included in this analysis.
Subgroup analysis
Subgroup analysis was carried out for individuals with a diagnosed condition known to benefit from physical activity. The three conditions included in the Pavey et al. HTA11 were obesity, hypertension and depression. Table 31 shows the probabilities of being active and inactive associated with these conditions taken from Pavey et al. and the calculated RRs that were used in these subgroup analyses. For events for which data were not available, that is diabetes mellitus for the hypertensive cohort, base case values have been assumed.
| Risks of adverse health outcomes by comorbidity | Relative risks | Condition | Calculated RRs |
|---|---|---|---|
| Obese | |||
| Probability of experiencing CHD when active | 0.0259 | CHD | 0.6888 |
| Probability of experiencing CHD when inactive | 0.0376 | ||
| Probability of experiencing stroke when active | 0.0259 | Stroke | 0.6888 |
| Probability of experiencing stroke when inactive | 0.0376 | ||
| Probability of experiencing type 2 diabetes mellitus when active | 0.0756 | Diabetes mellitus | 0.7667 |
| Probability of experiencing type 2 diabetes mellitus when inactive | 0.0986 | ||
| Hypertensive | |||
| Probability of experiencing CHD when active | 0.060 | CHD | 0.8108 |
| Probability of experiencing CHD when inactive | 0.074 | ||
| Probability of experiencing stroke when active | 0.060 | Stroke | 0.8108 |
| Probability of experiencing stroke when inactive | 0.074 | ||
| Depressive | |||
| Probability of experiencing CHD when active | 0.0336 | Depressive | 0.4195 |
| Probability of experiencing CHD when inactive | 0.0801 | ||
For the depressive subgroup analysis, data from the Murphy et al. study23 were provided to us (Professor Simon Murphy, personal communication) for which enabled us to estimate the RR of achieving recommended levels of physical activity for ERSs in a subgroup of individuals with a mental health referral (RR 1.02, 95% CI 0.83 to 1.24). As these data were more directly applicable to the population with depression, we have applied this as the base case efficacy parameter in this subgroup analysis. For the obese and hypertensive subgroups, for which subgroup-specific effectiveness data were available, we have applied the meta-analysed effectiveness data from all studies included in the clinical effectiveness review, which are not specific to patients with these conditions. The results for the depressive cohort are also provided using these RRs for comparison. All other parameter values are the same as the base case analysis.
Univariate sensitivity analysis
Univariate sensitivity analyses were undertaken to examine the impact of changes to a number of parameter values on the cost-effectiveness results (Table 32). Following completion of the report, further additional analyses were requested by the NICE Public Health Appraisal Committee (PHAC). These additional analyses are summarised in Appendices 6 and 7.
| Purpose (impact of) | Parameter | Changes in parameter estimates |
|---|---|---|
| Changes in people who become physically active (at 1 year) after exposure to ERSs | Effectiveness estimate (via RR) |
|
| Changes in persistence of protective effects (adjusted for decay rates) of physical activity | RR for developing disease conditions | Base case = protective effects persists up to 10 years. Changes:
|
| Changes in discount rate | Discount rate | Change discount rate for costs and QALYs from 1.5% to 3.5% |
| Assuming no psychological QoL gain from physical activity | Process utility | Change process utility from 0.072 to 0.000 |
| Process utility lasts longer | Process utility | Threshold analysis. |
| Risk of developing disease conditions change | RR of developing disease | RRs from Pavey et al.11 |
| Lower intervention cost | Intervention cost | Threshold analysis |
| Change in QoL utility values | QoL utility | QoL utilities from Pavey et al.11 |
Probabilistic analysis
Uncertainties around all parameters in the model (except baseline mortality) are addressed simultaneously using probabilistic sensitivity analyses (PSAs). Baseline mortality data were excluded from the PSA because mortality data come from census data and national databases where the uncertainty in the mean estimates is expected to be small.
The choice of distributions and their respective alpha and beta calculations draws on Briggs et al. 64 In cases where there are no data on standard errors they were subjectively assigned at 10% of the mean value. We have checked that the model results are stable and this information was used to determine the number of runs (10,000).
Results
Deterministic results
Table 33 shows the cost-effectiveness results per individual person based on point estimates of parameter values. The deterministic analysis indicates that ERSs are expected to produce a small health gain [0.003 quality-adjusted life-years (QALYs)] at an additional cost of £225 compared against usual care. This resulted in an incremental cost-effectiveness ratio (ICER) of £76,059 per QALY gained.
| Intervention | Mean cost (£) | Mean QALY | Incremental cost (£) | Incremental QALY | ICER (£) |
|---|---|---|---|---|---|
| ERS | 4572 | 18.136 | 225.4 | 0.003 | 76,059 |
| Usual care | 4346 | 18.133 | – | – | – |
Probabilistic results
Table 34 shows the PSA results per individual person. The PSA results are very similar to the deterministic results, with an ICER of £76,276.
| Intervention | Mean cost (£; 95% CI) | Mean QALY (95% CI) | Incremental cost (£) | Incremental QALY | ICER (£) |
|---|---|---|---|---|---|
| ERS | 4570 (4568 to 4571) | 18.1284 (18.006 to 18.161) | 226 | 0.0030 | 76,276 |
| Usual care | 4344 (4342 to 4346) | 18.1254 (18.093 to 18.158) | – | – | – |
Figure 17 shows a cost-effectiveness plane of the probabilistic results. Although all of the PSA estimates indicate a positive health gain and increase in costs, there is uncertainty in the magnitude of that cost and QALY gain. However, as can be seen from the cost-effectiveness acceptability curve in Figure 18, the probability that ERSs are cost-effective at a willingness-to-pay threshold of £30,000 per QALY gained is only 0.004.
FIGURE 17.
Cost-effectiveness plane.

FIGURE 18.
Cost-effectiveness acceptability curve.

Univariate sensitivity analysis
Univariate sensitivity analyses were undertaken to examine the impact of assumptions about the effectiveness of ERSs, the duration of the protective effect of physical activity and the discount rate on the cost-effectiveness of ERSs compared with usual care. Table 35 lists the effect of these changes.
| Parameter | Baseline | Sensitivity analysis | ICER (£) (baseline £76,000) |
|---|---|---|---|
| RR of effectiveness of ERSs on physical activity uptake | 1.12 | 1.31 | < 30,000 |
| 1.47 | < 20,000 | ||
| 1.08 (ITT) | 113,931 | ||
| Length of protective effect of physical activity | 10 years | 1 year | 124,193 |
| Lifetime | 33,056 | ||
| Discount rate | 1.5% | 3.5% | 88,943 |
| Process utility | 0.072 | 0 | 188,834 |
| Process utility threshold analysis: how long does the effect need to last for the ICER to fall below £30,000 per QALY? | First year only | 4 years | 27,893 |
| RR of developing disease (active vs. inactive) | CHD 0.9 | 0.52 | 37,676 |
| Stroke 0.86 | 0.73 | ||
| Diabetes mellitus 0.67 | 0.5 | ||
| Intervention cost threshold analysis: reduction required to achieve an ICER < £30,000 per QALY | £229 | 60% reduction (£92) | 29,746 |
| Utility values for disease states changed to those used by Pavey et al.11 | CHD initial event 0.8 | 0.55 | 62,343 |
| Ongoing CHD 0.92 | |||
| Stroke initial event 0.63 | 0.52 | ||
| Ongoing stroke 0.65 | 0.70 | ||
| Diabetes mellitus 0.90 |
The model is very sensitive to increases of the RR of ERSs on physical activity uptake, with a small increase from 1.12 to 1.31 leading to the ICER falling below £30,000 per QALY gained. Using an ITT approach to estimate the efficacy of ERSs compared with usual care reduced the RR to 1.08 and substantially increased the ICER to over £100,000 per QALY. Increasing the protective effect of physical activity over the duration of an individual’s lifetime leads to an ICER that is around £33,000 per QALY gained. However, as expected, excluding the process utility raises the ICER considerably, whereas assuming that this utility lasts for 4 years reduces the ICER to around £28,000 per QALY gained. Using the Pavey et al. 11 RRs of developing physical activity-related diseases leads to an ICER of around £38,000. A 60% reduction in the cost of the intervention is required before the ICER falls below £30,000. Using the Pavey et al. 11 utilities results in an ICER of around £62,000.
Results for the additional analyses requested by PHAC are summarised in Appendices 6 and 7.
Results of the subgroup analysis
Tables 36–39 show the results of the subgroup analysis. In all subgroups, the ICER is above £37,000.
| Intervention | Mean cost (£) | Mean QALY | Incremental cost (£) | Incremental QALY | ICER (£) |
|---|---|---|---|---|---|
| ERS | 4764 | 18.063 | 221 | 0.005 | 45,905 |
| Usual care | 4543 | 18.058 | – | – | – |
| Intervention | Mean cost (£) | Mean QALY | Incremental cost (£) | Incremental QALY | ICER (£) |
|---|---|---|---|---|---|
| ERS | 4632 | 18.111 | 224 | 0.004 | 61,602 |
| Usual care | 4408 | 18.107 | – | – | – |
| Intervention | Mean cost (£) | Mean QALY | Incremental cost (£) | Incremental QALY | ICER (£) |
|---|---|---|---|---|---|
| ERS | 4695 | 18.035 | 228 | 0.001 | 227,948 |
| Usual care | 4467 | 18.034 | – | – | – |
| Intervention | Mean cost (£) | Mean QALY | Incremental cost (£) | Incremental QALY | ICER (£) |
|---|---|---|---|---|---|
| ERS | 4688 | 18.040 | 221 | 0.006 | 37,488 |
| Usual care | 4467 | 18.034 | – | – | – |
Comparison of the Anokye adapted to exercise referral scheme model and the Pavey exercise referral scheme model
The aim of this short report was to adapt the Anokye et al. 21 brief advice model into a model comparing ERSs with usual care. The parameters we changed were replacing the RR of brief advice versus usual care with the same RR of ERSs versus usual care used by Pavey et al. ,11 changing the starting age from 33 years to 50 years to reflect the Pavey et al. data and inflating all costs to 2011–12 values. The adapted model appears to generate results that are less favourable to ERSs, even though the effectiveness estimate applied in the updated model is slightly more favourable. We will attempt here to explain why the model results differ in a manner not explained solely by the update of efficacy evidence. We do not have access to the Pavey et al. 11 model and so our explanation of these discrepancies is based on the Pavey et al. health technology report.
Comparison of model structure
The aim of the Pavey et al. 11 model is the same as the adapted model, that is, to assess the impact of ERSs on CHD, stroke and diabetes mellitus through the beneficial effects of increased physical activity. The intervention (and effectiveness of the intervention), comparator, perspective and time horizons are the same in both models. The two main differences are:
-
Pavey used a decision tree approach whereas the adapted model has a Markov structure
-
the benefits of physical activity were assumed to be constant over lifetime in Pavey et al. ,11 whereas in the adapted model, the benefits of physical activity are limited to 10 years
-
many of the input parameters differ as detailed in Comparison of input parameters.
Comparison of input parameters
Relative risks
In Pavey et al. ,11 the RR for developing a disease for active versus inactive individuals was 0.52, 0.73 and 0.5 for CHD, stroke and diabetes mellitus, respectively. In the adapted model the RR was 0.9, 0.86 and 0.67, respectively. The effect of using Pavey et al. ’s11 RRs is explored in our sensitivity analysis. Table 40 shows the parameters used and describes the sensitivity analysis conducted to explore their impact on cost-effectiveness.
| Change in the RRs associated with active vs. inactive people developing a disease to those used by Pavey et al.11 | ||
|---|---|---|
| RRs | Current | Pavey et al. |
| CHD | 0.9 | 0.52 |
| Stroke | 0.86 | 0.73 |
| Diabetes mellitus | 0.67 | 0.50 |
| Change in the QoL utility values for disease states to those used by Pavey et al.11 | ||
| Health state utility values | Current | Pavey et al. |
| CHD initial event | 0.80 | 0.55 |
| Ongoing CHD | 0.92 | 0.55 |
| Stroke initial event | 0.63 | 0.52 |
| Ongoing stroke | 0.65 | 0.52 |
| Diabetes mellitus | 0.90 | 0.70 |
Quality-of-life utility values
Table 40 also shows the health state utility values in the two models. The values used by Pavey et al. 11 are markedly lower than those in the updated model.
Costs
Pavey et al. 11 report the discounted (3.5%) lifetime costs of CHD, stroke and diabetes mellitus used in their model (shown in Table 41, inflated to 2011/12 prices). They also assume an average length of life-years remaining of 18.41, 5.12 and 28.13 for CHD, stroke and diabetes mellitus respectively. In order to compare the lifetime costs of Pavey et al. 11 with the updated model we used the following method to estimate lifetime costs in our model:
-
apply discounting to the estimate of life expectancy for each health condition to estimate the discounted life-years gained in each health state
-
multiply the discounted life-years by the ongoing cost of the condition used in the updated model
-
add on the cost of the initial event for the condition (zero for diabetes mellitus) used in the updated model.
| Parameter | Pavey model lifetime costs (£) | Updated model lifetime costs (£) |
|---|---|---|
| CHD | 18,857 | 8953 |
| Stroke | 2090 | 21,101 |
| Diabetes mellitus | 53,512 | 10,392 |
Table 41 shows the discounted lifetime costs used in the Pavey et al. 11 model and the estimates from the updated model. The costs vary widely between the two models.
Comparison of results
Table 42 shows the baseline results from Pavey et al. 11 and the deterministic results from the adapted model with the same RR used by Pavey et al. 11 The Pavey et al. 11 model has a smaller incremental cost but gains 2.7 times the number of QALYs, resulting in an ICER of around £21,000.
| Updated ERS model | Mean cost (£) | Mean QALY | Incremental cost (£) | Incremental QALY | ICER (£) |
|---|---|---|---|---|---|
| ERS | 4572 | 18.137 | 226 | 0.003 | 88,742 |
| Usual care | 4346 | 18.134 | – | – | – |
| Pavey et al.11 model | – | – | – | – | – |
| ERS | 2492 | 16.743 | 169 | 0.008 | 20,876 |
| Usual care | 2322 | 16.735 | – | – | – |
Number of events avoided and quality-adjusted life-years gained
The number of events avoided and the QALYs gained reported by Pavey et al. and from the updated model are shown in Table 43. Exercise referral avoids far more CHD, stroke and diabetes mellitus cases in the Pavey et al. 11 model than in the updated model, thus resulting in much a higher incremental QALY gain in the Pavey et al. model. The difference in event rates is related to the differences in the duration of the protective effect of physical activity and the strength of this protective effect.
| Event | Pavey et al.11 | Updated model |
|---|---|---|
| CHD | 51 | 12.6 |
| Stroke | 16 | 6.1 |
| Diabetes mellitus | 86 | 9.6 |
| QALYs gained | 800 | 476 |
Discussion
There are several limitations to the analysis based on the updated model. The model only estimates the impact of physical activity on selected morbidities and the cost-effectiveness of ERSs may therefore be underestimated. It is possible that there is a benefit from physical exercise on other morbidities, but previous modellers have been unable to find the necessary data. 11 The updated model does not include the impact of adverse events or injuries; however, previous authors have commented that they are rare events and are unlikely to affect the results. 11
A limitation in assessing subgroups (obesity, hypertension and depression) is that, with the exception of the depression subgroup, the efficacy of ERSs is assumed to be the same as the whole inactive population. Further data would be needed on the efficacy of ERSs in these subgroups to test this assumption. The model also assumes that the starting utility for these subgroups is the same as the general population. If the starting utility were found to be lower in any of these subgroups, this may have the effect of lowering the incremental QALY gains, resulting in a higher ICER than that generated within our sensitivity analysis.
We were unable to assess whether or not less-intensive ERSs could be effective at a lower cost and therefore be cost-effective. The sensitivity analysis indicated that schemes would need a 60% reduction in costs to achieve an ICER below £30,000 per QALY gained. However, less-intensive schemes may be less effective and so data on effectiveness and costs would be required to assess cost-effectiveness. (This issue is explored in more detail in Appendix 7.)
The very small incremental QALYs gained by ERSs mean that the results are very sensitive to small changes in some of the model parameters. A relatively small increase in the efficacy of ERSs or a 3-year increase in the length of the process utility gain both lead to ICERs that are below £30,000 per QALY gained. In contrast, removing the process utility attributed to ERSs results in an ICER in excess of £180,000 per QALY gained, and using efficacy data from the ITT analysis, which provides a more conservative estimate of effectiveness (RR 1.08, 95% CI 1.00 to 1.17), resulted in an ICER of around £114,000.
The ICER estimated by Pavey et al. 11 was much lower than that estimated in the present analysis. However, we found that several parameter changes reduced the ICER, and it was particularly sensitive to changes in the duration over which the process utility was applied. It may be the case that minor changes to a combination of parameter values (i.e. process utility, length of protective effect, RR of developing the disease and the utility values) would bring the ICER down to a value more consistent with that estimated by Pavey et al. 11 (This supposition is explored in more detail in Appendices 6 and 7.)
Owing to time and data constraints, we were unable to model separately individuals with a pre-existing condition and also those considered to be healthy. The model therefore assumes that patients do not have CHD, stroke or type 2 diabetes mellitus at the start of the model. Although the population of interest for the report as a whole includes those with a pre-existing medical condition, the economic analysis focuses on a cohort without any of these three specific conditions. We have conducted subgroup analyses for individuals who have hypertension, obesity or depression at the start of the model, as these subgroups were already specified within the existing model. However, subgroup-specific effectiveness data were only available for the depression subgroup.
The model oversimplifies the clinical situation because it does not recognise that more than one of the three conditions can be present in the same individual and also that the presence of one comorbidity may impact the likelihood of experiencing another. Again, we are constrained here to using an existing economic model in which type 2 diabetes mellitus, CHD and stroke are treated as mutually exclusive conditions. Also, the model does not account for the fact that stroke patients are at a higher risk of having recurrent strokes and thus the utility loss and additional costs associated with this are not taken into account. The impact of these limitations on the cost-effectiveness of ERSs is difficult to estimate.
Given that the effectiveness estimates applied in the model are based on data from a 1-year follow-up, good-quality data on the sustainability of activity levels would lessen the uncertainty in the model that comes from extrapolating from short- to long-term outcomes.
Conclusion
Our analysis indicates that the ICER for ERSs compared with usual care is around £76,000 per QALY, although the cost-effectiveness of ERSs is subject to considerable uncertainty and is particularly sensitive to the assumptions made regarding the effectiveness of ERSs in increasing physical activity and the size and duration of process utility gains.
Chapter 5 Discussion
Statement of principal findings
Systematic review of exercise referral schemes
This is an update of an existing review,11 and, therefore, this review uses the search results, data extraction tools, data and findings of that review in this update. One additional RCT,31 additional qualitative data49 and two additional observational studies36,48 have been included in this update.
A total of eight RCTs14,17,21,22,28,32–34 met the inclusion criteria and were included in the review. Six RCTs14,17,21,28,32,33 compared ERSs with usual care, which in all cases included some sort of advice regarding physical activity. Two RCTs21,34 compared an alternative physical activity-promoting intervention and one RCT31 compared an alternative form of ERS (i.e. ERS plus SDT intervention) with usual care.
There was considerable heterogeneity in the nature of the exercise/physical activity intervention across studies. Studies recruited predominantly sedentary middle-aged adults who had evidence of at least one lifestyle risk factor, and five of the studies also included individuals with a medical diagnosis (e.g. hypertension, depression). ERSs usually took place at a leisure centre and involved 10–12 weeks of exercise intervention and where there was follow-up it took place at 6 and/or 12 months post randomisation. Studies were judged to have a moderate or low overall risk of bias.
The most commonly reported outcome was self-reported physical activity. When compared with usual care, there was weak evidence of an increase in the number of ERS participants who achieved 90–150 minutes of at least moderate-intensity physical activity per week at 6–12 months’ follow-up (pooled RR 1.12, 95% CI 1.04 to 1.20). There was no difference in physical activity between ERSs versus alternative physical activity promotion interventions or ERSs versus ERSs plus SDT at 6–12 months’ follow-up. We found no evidence to support differences across subgroups (e.g. age, sex) in terms of the impact of ERSs on physical activity. Murphy et al. 32 found that the intervention was effective in increasing levels of physical activity in those referred for a CHD risk factor. There was no consistent evidence for a difference between ERS and any of the comparator groups in the duration of moderate/vigorous intensity and total physical activity, physical fitness, blood pressure, serum lipids, glycaemic control, obesity indices (body weight, BMI, percentage fat), respiratory function, psychological well-being (perception of self-worth, or symptoms of depression or anxiety) or HRQoL.
We found considerable variation across studies in the level of uptake (i.e. attendance at the first induction visit) and adherence to ERSs (i.e. completion of the programme) across the 19 included studies (16 observational studies and six RCTs). Uptake levels were higher, on average, in RCTs than in observational studies, although there was no clear difference in adherence between the two. In bivariate and multivariate analyses, women and older people were more likely to take up ERS. In addition, although older people were also more likely to adhere, women were less likely to adhere than men. Very few studies reported associations between ERS uptake or adherence and participant psychosocial factors or programme-level predictors.
A consistent finding in the quantitative studies reporting adherence and uptake is the association between increasing age and likelihood of uptake and adherence to ERS. This reverses the trend seen in the general population, which is a decrease in physical activity with advancing age. A number of factors have been proposed to explain this including that older people are less time-constrained, are more likely to value the social interaction offered by the group-based approaches and may find it easier to incorporate the scheme exercise activities (such as walking, swimming and cycling) into their everyday life. The only group to show a statistically significant increase in physical activity in the Murphy et al. 23 study were those referred for CHD risk. This group would also have been likely to have been older. Increasing age or CHD risk factors may be confounding factors. It may be that these factors work in a complementary manner, with physical activity perceived to be a means of reducing CHD risk and CHD risk a perceived greater threat to those of advancing age.
One issue that may impact on trial results, as suggested in the qualitative review of discussion sections of included trials, is the possibility that those volunteering to participate in the trial in the first place may have been more motivated to become active or increase their physical activity. This may have contributed to the overall finding that physical activity levels increased in both intervention and comparison groups. It is therefore possible that trial participants (and perhaps also ERS participants in general) may be more intrinsically motivated initially to become physically active and thus may differ from the population as a whole in terms of their response to such a programme; according to SDT,71 intrinsic motivation is more likely to lead to behaviour change that is maintained over the longer term. Therefore, ERSs may be inadvertently creating inequality in service delivery and failing to reach those most in need of intervention, through the element of self-selection that could creep in in terms of uptake and adherence to the scheme.
In two recent systematic reviews (Orrow et al. 72 and Campbell et al. 70) exploring the effectiveness of advice or counselling delivered by health professionals within primary care to promote physical activity, positive benefits were seen with significant increases in self-reported physical activity at 12 months. In Campbell et al. where interventions were limited to brief advice the RR was 1.30 (CI 95% 1.12 to 1.50), favouring brief advice over usual care. Neither review found any evidence to support ERS over advice or counselling interventions.
This review did find benefit of ERSs over simply giving advice to promote physical activity. In all of the control groups, advice was given on the value of increasing levels of physical activity. This may have had the effect of reducing the apparent effectiveness of ERSs if compared with usual care which might mean no advice to promote levels of physical activity. The quality of advice may not have been as high as that delivered in the brief intervention studies, hence a difference was found between the effectiveness of ERS schemes and advice alone. The presence of bias may also influence the findings in these studies, of which only two22,32 attempted to blind at outcome assessment.
Strengths and limitations of the assessment
Clinical effectiveness
The strength of this update review is that it was able to use the robust findings of a previous systematic review. 11 The additional data included in this update supported the existing findings.
A rigorous search was carried out to identify any additional RCTs and qualitative studies done alongside the RCTs (sibling studies). The narrow focus of this review in terms of its definition of ERSs meant that similar interventions which may have yielded valuable insights were excluded from the review.
Measuring physical activity by methods of self-report has an obvious risk of self-report bias. There is no gold standard self-reporting measure of adherence to physical activity or physical activity levels. Many traditional instruments have shortcomings from a clinical perspective. Physical activity levels are often scored on scales that are not easily converted into a counselling message. It can also be difficult to assess small but clinically significant changes in physical activity levels in a practice situation. Problems with these instruments underscore the challenge of translating research findings into clinical practice. Self-report levels of physical activity are the only outcomes that show a significant effect. The reliance on outcomes that are subject to recall bias is a weakness of this review.
Cost-effectiveness
The scope for the economic analysis of ERSs for this brief report was to update the Anokye et al. 53 brief advice model, with evidence from the updated systematic review on the effectiveness of ERS, and to update the costs. As such, we did not conduct a systematic review of the existing evidence on the cost-effectiveness of ERSs, which limits our ability to place these results into a broader context. However, we have conducted a detailed comparison with the model used by Pavey et al. 11 to explore why the ICERs differ so much from those previously reported. Several of the assumptions made in our economic evaluation are more conservative than the assumptions made by Pavey et al. 11 In particular, the restriction of benefits to 10 years, which has had a substantial impact on the ICERs, may be considered to be a more reasonable assumption than the lifetime benefits assumed by Pavey et al. 11 (This issue is explored in more detail in Appendix 6.)
The estimate of process utility gain associated with physical activity, which is a particularly important driver of cost-effectiveness within the model, was based on cross-sectional data. 11 It was included in our base case analysis, as this was the approach taken in the economic evaluation of brief interventions to increase physical activity,53 but was actually excluded from the base case analysis in the economic evaluation of ERSs by Pavey et al. 11 In our clinical effectiveness review, some but not all of the RCTs that reported HRQoL found a statistically significant difference between ERSs and usual care, suggesting that this short-term benefit is still relatively uncertain. (This issue is explored in more detail in Appendix 7.)
One of the main limitations of the economic evaluation is that it does not fully explore the potential for cost-effectiveness to vary according to the exact nature of ERSs or the characteristics of the population to whom it is offered. The cost of an ERS is likely to be highly dependent on the exact nature of that scheme. The incremental cost is also dependent on the provision made to those who received usual care, which we assumed to have zero cost. There was substantial variation in both of these factors across the included studies, although our threshold analysis suggested that large changes in the incremental cost of ERS compared with usual care would be needed to bring the ICER under £30,000. (This issue is explored in more detail in Appendix 7.) Although we have provided some estimates of the cost-effectiveness of ERSs for patients who might be referred for ERSs because of a pre-existing health condition, this has been achieved by assuming that some data from the general population, such as the relative risks of being physically active, are transferrable to those patients with pre-existing conditions.
Another key limitation of the model is that it only captures the impact of physical activity on three health conditions and it does not allow for individuals to have multiple conditions. This fails to capture the many aspects of health that may be influenced by physical activity and the complex relationships that exist between different exercise-related conditions, such as the impact of type 2 diabetes mellitus on cardiovascular risk. In addition, the model does not account for the fact that stroke patients are at a higher risk of having recurrent strokes and, thus, the utility loss and additional costs associated with this are not taken into account. The impact of these limitations on the cost-effectiveness of ERSs is difficult to estimate.
Uncertainties
Clinical effectiveness of exercise referral schemes
A number of uncertainties remain regarding the clinical effectiveness of ERSs:
-
the potential value of different components of the ERS programme on promoting physical activity
-
the long-term changes in physical activity behaviour as a result of participating in these schemes
-
the extent to which people in the advice-only groups changed their levels of physical activity
-
whether or not the small increases in self-reported physical activity are clinically significant or lead to clinical significant differences.
Cost-effectiveness of exercise referral schemes
Good-quality data on the sustainability of activity levels, and the magnitude and sustainability of any associated process utility gains, would lessen the uncertainty in the model.
Chapter 6 Conclusions
Implications for service provision
In 2006, NICE commented that there is insufficient evidence for ERS and recommended that the NHS should make ERS available only as part of a controlled trial. Pavey et al. 11 updated the evidence available with the inclusion of four additional trials and also concluded that there remains very limited support for the potential role of ERSs in positively improving levels of physical activity. There was little evidence that interventions incorporated strategies that enabled participants to achieve a sustainable active lifestyle, and very little reference to the development of theoretically based interventions that draw on successful behaviour-change techniques. This update supports and reinforces these findings. The additional data from a large well-designed trial, conducted in the UK, which incorporated motivational interviewing, found that ERSs improved levels of physical activity, but this was of borderline statistical significance.
Suggested research priorities
-
The findings from both the quantitative and qualitative data suggest that referral for CHD risk and increasing age are stronger predictors both for uptake and adherence to ERSs. Interventions targeting older patients, at greater risk of reduced levels of physical activity and with CHD risk, may show greater benefit of ERS. This should be further tested in mixed-method evaluation, incorporating both RCT evidence and qualitative data, which will enable exploration of the elements of the intervention that are most effective.
-
Further research is needed to identify the mediating factors that influence uptake and adherence, with a greater understanding of who benefits and in which circumstances. Fewer men take up ERSs. Studies that explore the barriers and facilitators that men face in increasing levels of physical activity need to be explored.
-
Longitudinal studies that examine the relationship between increased levels of physical activity and impact on health outcomes should be developed.
-
Further research is needed to explore the effect of interventions with a theoretical basis and the fidelity of the intervention with that theory.
-
An understanding of the impact of advice in the control interventions which may have reduced the impact of ERSs in studies in which it is compared with an advice-only group would be beneficial.
-
Trials should be developed with a comparator group which addresses a different health behaviour or an intervention for something unrelated (e.g. singing in choirs) that may also have a social benefit to compare with ERS. Consider funding of three-arm trials or multiple comparison design trials so that the impact of advice only can be controlled for.
-
Adherence to ERSs, as measured by attendance, is only a proxy marker of exercise adherence, particularly as participants are unlikely to adhere to the recommended exercise level by attending one session per week. Further research is required to establish better methods of assessing exercise adherence.
-
Expected value of sample information techniques should be used to estimate whether or not the benefits of conducting further research into the areas identified above, to reduce decision uncertainty and potentially make different recommendations regarding the use of ERSs, would outweigh the cost of conducting that additional research. The existing model could be used as a starting point for that analysis, although it would need to be adapted to ensure that the structural uncertainties identified in this report are reflected within the expected value of sample information in addition to the parameter uncertainty currently included in the PSA.
Acknowledgements
Many thanks to our clinical advisors for providing support and advice for this review.
-
Dr Alistair Bradley, General Practitioner, Tramways Medical Centre, Hillsborough, Sheffield, UK.
-
Aimee Rogers, Personal Trainer, Revitalize Fitness, Sheffield, UK.
-
Sean McQuade, Belfast Health and Social Care Trust.
-
Andrew Power, Strategic Manager (Physical Activity), County Durham Sport.
Thank you to Andrea Shippam for providing administrative support and preparing and formatting the report.
This report was commissioned by the National Institute for Health Research HTA programme. The views expressed in this report are those of the authors and not necessarily those of the NHS research and development HTA programme. The final report and any errors remain the responsibility of the University of Sheffield. Eva Kaltenthaler and Matt Stevenson are guarantors.
Contributions of authors
Fiona Campbell and Emma Everson-Hock designed the protocol for, and carried out, the systematic review of clinical effectiveness. This included searching for and including new data in the updated analyses, writing the report and responding to amendments.
Mike Holmes, Sarah Davis and Nana Anokye designed and carried out the cost-effectiveness analysis.
Sarah Davis was responsible for further amendments and analysis of the cost-effectiveness data.
Helen Buckley Woods carried out the searches.
Paul Tappenden and Eva Kaltenthaler helped design the project and protocol and commented on draft versions of the report.
About the School of Health and Related Research
The School of Health and Related Research (ScHARR) is one of the nine departments that constitute the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research and the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the NIHR HTA Programme on behalf of a range of policy makers, including NICE. ScHARR-TAG is part of a wider collaboration of a number of units from other regions including Health Economics Research Unit and Health Services Research Unit, University of Aberdeen; Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Liverpool Reviews and Implementation Group (LRiG), University of Liverpool; Peninsular Technology Assessment Group (PenTAG), University of Exeter; the NHS Centre for Reviews and Dissemination, University of York; Warwick Evidence, University of Warwick; the BMJ Technology Assessment Group (BMJ-TAG), BMJ Evidence Centre and Kleijnen Systematic Reviews Ltd.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- At Least Five a Week. Evidence on the Impact of Physical Activity and its Relationship to Health. A Report from the Chief Medical Officer. London: Department of Health; 2004.
- Physical Activity Guidelines for Americans. Washington, DC: US Department of Health and Human Services; 2008.
- Craig Rachel, Mindell Jennifer, Hirani Basant. Health Survey for England 2008. Physical Activity and Fitness. Summary of Key Findings. Leeds: Health and Social Care Information Centre; 2009.
- Dugdill L, Graham RC, McNair F. Exercise referral: the public health panacea for physical activity promotion? A critical perspective of exercise referral schemes; their development and evaluation. Ergonomics 2005;48:1390-410. http://dx.doi.org/10.1080/00140130500101544.
- Exercise on Prescription: A Report for the Chartered Society of Physiotherapists. London: Labour Research Department; 2004.
- Sowden SL, Raine R. Running along parallel lines: how political reality impedes the evaluation of public health interventions. A case study of exercise referral schemes in England. J Epidemiol Community Health 2008;62:835-41. http://dx.doi.org/10.1136/jech.2007.069781.
- Morgan O. Approaches to increase physical activity: reviewing the evidence for exercise-referral schemes. Public Health 2005;119:361-70. http://dx.doi.org/10.1016/j.puhe.2004.06.008.
- Sorensen JB, Skovgaard T, Puggaard L. Exercise on prescription in general practice: a systematic review. Scand J Prim Health Care 2006;24:69-74. http://dx.doi.org/10.1080/02813430600700027.
- A Rapid Review of the Effectiveness of ERS to Promote Physical Activity in Adults. London: NICE; 2006.
- Williams N, Hendry M, France B, Lewis R, Wilkinson C. Effectiveness of exercise referral schemes to promote physical activity in adults: a systematic review. Br J Gen Pract 2007;57:979-86. http://dx.doi.org/10.3399/096016407782604866.
- Pavey TG, Anokye N, Taylor AH, Trueman P, Moxham T, Fox KR, et al. The clinical effectiveness and cost-effectiveness of exercise referral schemes: a systematic review and economic evaluation. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15440.
- Taylor AH, Doust J, Webborn N. Randomised controlled trial to examine the effects of a GP exercise referral programme in Hailsham, East Sussex, on modifiable coronary heart disease risk factors. J Epidemiol Community Health 1998;52:595-601. http://dx.doi.org/10.1136/jech.52.9.595.
- Halbert J, Silagy C, Finucane P, Withers R, Hamdorf P. Physical activity and cardiovascular risk factors: effect of advice from an exercise specialist in Australian general practice. Med J Aust 2000;173:84-7.
- Lamb S, Bartlett H, Ashley A, Bird W. Can lay-led walking programmes increase physical activity in middle aged adults? A randomised controlled trial. J Epidemiol Community Health 2002;56:246-52. http://dx.doi.org/10.1136/jech.56.4.246.
- Harrison RA, Roberts C, Elton PJ. Does primary care referral to an exercise programme increase physical activity 1 year later? A randomized controlled trial. J Public Health 2004;27:25-32. http://dx.doi.org/10.1093/pubmed/fdh197.
- Murphy S, Raisanen L, Moore G, Edwards R, Linck P, Hounsome N, et al. The Evaluation of the National Exercise Referral Inwales 2010.
- Duda J, Williams G, Ntoumanis N, Daley A, Eves F, Mutrie N, et al. Effects of a standard provision versus an autonomy supportive exercise referral programme on physical activity, quality of life and well-being indicators: a cluster randomised controlled trial. Int J Behav Nutr Phys Act 2014;11. http://dx.doi.org/10.1186/1479-5868-11-10.
- Isaacs A, Critchley J, Tai S, Buckingham K, Westley D, Harridge S, et al. Exercise evaluation randomised trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11100.
- Higgins J, Altman D. Assessing Risk of Bias in Included Studies 2008. http://dx.doi.org/10.1002/9780470712184.ch8.
- Four Commonly Used Methods to Increase Physical Activity. NICE Public Health Guidance 2. London: NICE; 2006.
- Anokye NK, Trueman P, Green C, Pavey TG, Hillsdon M, Taylor RS, et al. The cost-effectiveness of exercise referral schemes. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-954.
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviours. Am Psychol 1992;47:1102-14. http://dx.doi.org/10.1037/0003-066X.47.9.1102.
- Murphy SM, Edwards RT, Williams N, Raisanen L, Moore G, Linck P, et al. An evaluation of the effectiveness and cost effectiveness of the National Exercise Referral Scheme in Wales, UK: a randomised controlled trial of a public health policy initiative. J Epidemiol Community Health 2012;66:745-53. http://dx.doi.org/10.1136/jech-2011-200689.
- Tobi P, Estacio E, Seesaghur A, Nabingi S, Cawley J. Evaluation of Healthwise Exercise Referral Scheme (Final Report). Prepared for Greenwich Teaching Primary Care Trust and Greenwich Leisure Limited. London: University of East London, Institute for Health and Human Development; 2009.
- Taylor AH, Fox KR. Effectiveness of a primary care exercise referral intervention for changing physical self-perceptions over 9 months. Health Psychol 2005;24:11-2. http://dx.doi.org/10.1037/0278-6133.24.1.11.
- Stevens W, Hillsdon M, Thorogood M, McArdle D. Cost-effectiveness of a primary care based physical activity intervention in 45–74 year old men and women: a randomised controlled trial. Br J Sports Med 1998;32:236-41. http://dx.doi.org/10.1136/bjsm.32.3.236.
- Sorensen JB, Kragstrup J, Skovgaard T, Puggaard L. Exercise on prescription: a randomized study on the effect of counselling vs counselling and supervised exercise. Scand J Med Sci Sports 2008;18:288-97. http://dx.doi.org/10.1111/j.1600-0838.2008.00811.x.
- Gusi N, Reyes MC, Gonzalez-Guerrero JL, Herrera E, Garcia JM. Cost–utility of a walking programme for moderately depressed, obese, or overweight elderly women in primary care: a randomised controlled trial. BMC Public Health 2008;8:1-10. http://dx.doi.org/10.1186/1471-2458-8-231.
- Taylor AH. Evaluating GP Exercise Referral Schemes. Findings From a Randomised Control Study. Brighton: University of Brighton; 1996.
- Sorensen JB, Kragstrup J, Kjaer K, Puggard L. Exercise on prescription: trial protocol and evaluation of outcomes. BMC Health Serv Res 2007;7. http://dx.doi.org/10.1186/1472-6963-7-36.
- Jolly K, Duda JL, Daley A, Ntoumanis N, Eves F, Rouse P. An Evaluation of the Birmingham Exercise on Prescription Service: Standard Provision and a Self-Determination Focused Arm. Final Report. Birmingham; 2009.
- Deci EL, Ryan RM. The ‘what’ and the ‘why’ of goal pursuits: human needs and the self-determination of behavior. Psychol Inq 2000;11:227-68. http://dx.doi.org/10.1207/S15327965PLI1104_01.
- Pavey TG, Taylor AH, Fox KR, Hillsdon M, Anokye N, Campbell JL, et al. Effect of exercise referral schemes in primary care on physical activity and improving health outcomes: systematic review and meta-analysis. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d6462.
- Tobi P, Estacio EV, Yu G, Renton A, Foster N. Who stays, who drops out? Biosocial predictors of longer-term adherence in participants attending an exercise referral scheme in the UK. BMC Public Health 2012;12. http://dx.doi.org/10.1186/1471-2458-12-347.
- Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychother 2009;37:129-40. http://dx.doi.org/10.1017/S1352465809005128.
- Morton KL, Biddle SJ, Beauchamp MR. Changes in self-determination during an exercise referral scheme. Public Health 2008;122:1257-60. http://dx.doi.org/10.1016/j.puhe.2007.11.006.
- Roessler KK, Ibsen B. Promoting exercise on prescription: recruitment, motivation, barriers and adherence in a Danish community intervention to reduce type 2 diabetes, dyslipidemia and hypertension. J Public Health 2009;17:187-93. http://dx.doi.org/10.1007/s10389-008-0235-4.
- Sowden SL, Breeze E, Barber J, Raine R. Do general practices provide equitable access to physical activity interventions?. Br J Gen Pract 2008;58:699-702. http://dx.doi.org/10.3399/bjgp08X342237.
- Gidlow C, Johnston LH, Crone D, Morris C, Smith A, Foster C, et al. Socio-demographic patterning of referral, uptake and attendance in Physical Activity Referral Schemes. J Public Health 2007;29:107-13. http://dx.doi.org/10.1093/pubmed/fdm002.
- Crone D, Johnston LH, Gidlow C, Henley C, James DVB. Uptake and participation in physical activity referral schemes in the UK: an investigation of patients referred with mental health problems. Issues Ment Health Nurs 2008;29:1088-97. http://dx.doi.org/10.1080/01612840802319837.
- Harrison RA, McNair F, Dugdill L. Access to exercise referral schemes – a population based analysis. J Public Health 2005;27:326-30. http://dx.doi.org/10.1093/pubmed/fdi048.
- Damush TM, Stump TE, Saporito A, Clark DO. Predictors of older primary care patients’ participation in a submaximal exercise test and a supervised, low-impact exercise class. Prev Med 2001;33:485-94. http://dx.doi.org/10.1006/pmed.2001.0919.
- Dinan S, Lenihan P, Tenn T, Iliffe S. Is the promotion of physical activity in vulnerable older people feasible and effective in general practice?. Br J Gen Pract 2006;56:791-3.
- Edmunds J, Ntoumanis N, Duda JL. Adherence and well-being in overweight and obese patients referred to an exercise on prescription scheme: a self-determination theory perspective. Psychol Sport Exerc 2007;8:722-40. http://dx.doi.org/10.1016/j.psychsport.2006.07.006.
- Jackson C, Bell F, Smith RA, Dixey R. Do adherers and non-adherers to a GP exercise referral scheme differ in their long-term physical activity levels?. J Sports Sci 1998;16.
- Jones F, Harris P, Waller H, Coggins A. Adherence to an exercise on prescription scheme: the role of expectations, self-efficacy, stage of change and psychological well-being. Br J Health Psychol 2005;10:359-78. http://dx.doi.org/10.1348/135910704X24798.
- Lord JC, Green F. Exercise on prescription: does it work?. Health Educ J 1995;54:453-64. http://dx.doi.org/10.1177/001789699505400408.
- Martin C, Woolf-May K. The retrospective evaluation of a general practitioner exercise prescription programme. J Human Nutr Diet 1999;12. http://dx.doi.org/10.1046/j.1365-277X.1999.00005.x.
- James DVB, Johnston LH, Crone D, Sidford AH, Gidlow C, Morris C, et al. Factors associated with physical activity referral uptake and participation. J Sports Sci 2008;26:217-24. http://dx.doi.org/10.1080/02640410701468863.
- Hanson CL, Allin LJ, Ellis JG, Dodd-Reynolds CJ. An evaluation of the efficacy of the exercise on referral scheme in Northumberland, UK: association with physical activity and predictors of engagement. A naturalistic observation study. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002849.
- Leijon ME, Faskunger J, Bendtsen P, Festin K, Nilsen P. Who is not adhering to physical activity referrals, and why?. Scand J Prim Health Care 2011;29:234-40. http://dx.doi.org/10.3109/02813432.2011.628238.
- Moore GF, Raisanen L, Moore L, Din N, Murphy S. Mixed-method process evaluation of the Welsh National Exercise Referral Scheme. Health Educ 2013;113. http://dx.doi.org/10.1108/HE-08-2012-0046.
- Anokye N, Lord J, Fox-Rushby J. National Institute for Health and Clinical Excellence Public Health Intervention Guidance on Physical Activity – Brief Advice for Adults in Primary Care: Economic Analysis. London: NICE; 2012.
- Curtis L. Unit Costs of Health and Social Care 2011 2011. www.pssru.ac.uk/project-pages/unit-costs/2011/index.php (accessed May 2014).
- Office for National Statistics (ONS) . United Kingdom Interim Life Tables 2010 2010. www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2010-2012/stbilt2012.html (accessed May 2010).
- Methods for the Development of NICE Public Health Guidance (Second Edition. London: NICE; 2009.
- Hu G, Jousilahti P, Borodulin K, Barengo N, Lakka T, Nissinen A, et al. Occupational, commuting and leisure-time physical activity in relation to coronary heart disease among middle-aged Finnish men and women. Atherosclerosis 2007;194:490-7. http://dx.doi.org/10.1016/j.atherosclerosis.2006.08.051.
- Hu G, Sarti C, Jousilahti P, Silventoinen K, Barengo N, Tuomilehto J. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke 2005;36:1994-9. http://dx.doi.org/10.1161/01.STR.0000177868.89946.0c.
- Hu G, Qiao Q, Silventoinen K, Eriksson J, Jousilahti P, Lindstrom J, et al. Occupational, commuting, and leisure-time physical activity in relation to risk for Type 2 diabetes in middle-aged Finnish men and women. Diabetologia 2003;46:322-9.
- Preis S, Hwang S, Coady S, Pencina M, D’Agostino RS, Savage P, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 2009;119:1728-35. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.829176.
- Brønnum-Hansen H, Davidsen M, Thorvaldsen P. Long-term survival and causes of death after stroke. Stroke 2001;32:2131-6. http://dx.doi.org/10.1161/hs0901.094253.
- Health Survey for England – 2008: Physical Activity and Fitness. Leeds: Health and Social Care Information Centre; 2009.
- Ward S, Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11140.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press Inc.; 2006.
- Anokye N, Jones T, Fox-Rushby J. National Institute for Health and Clinical Excellence Public Health Intervention Guidance Physical Activity: Brief Advice for Adults in Primary Care: Component 2 Economic Analysis. Review of Economic Evidence. London: NICE; 2012.
- Gonzalez E, Johansson S, Wallander M, Rodriguez L. Trends in the prevalence and incidence of diabetes in the UK: 1996–2005. J Epidemiol Community Health 2009;63:332-6. http://dx.doi.org/10.1136/jech.2008.080382.
- Jamrozik K. Estimate of deaths attributable to passive smoking among UK adults: database analysis. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38370.496632.8F.
- Ward S, Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. Statins for the Prevention of Coronary Events 2005.
- Hypertension: The Clinical Management of Primary Hypertension in Adults. Clinical Guideline: Methods, Evidence and Recommendations. London: NICE; 2011.
- Campbell F, Blank L, Messina J, Day M, Buckley Wood H, Payne N, et al. National Institute for Health and Clinical Excellence Public Health Intervention Guidance Physical Activity: Brief Advice for Adults in Primary Care. Review of Effectiveness Evidence. London: NICE; 2012.
- Curtis L. Unit Costs of Health and Social Care 2012 2012. www.pssru.ac.uk/project-pages/unit-costs/2012/ (accessed May 2014).
- Orrow G, Kinmonth A-L, Sanderson S, Sutton S. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e1389.
Appendix 1 Literature search strategies
Searches were limited by English language and publication date of October 2009 to present (8 May 2013 for stage 1 and 17 June 2013 for stage 2).
Stage 1 search
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to present>
Search strategy
-
physical activity referral*.ti,ab.
-
exercise on prescription.ti,ab.
-
exercise referral*.ti,ab.
-
supervised exercise.ti.
-
1 or 2 or 3 or 4
Stage 2 search
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to present>
Search strategy
-
“Referral and Consultation”/
-
(exercise* or physical*).ti,ab.
-
1 and 2 (2396)
-
((physical* or exercise*) adj2 (superv* or subsid* or prescrib*)).ti,ab.
-
(exercise* adj2 (fit* or train* or activit* or promot* or program* or intervention*)).ti,ab.
-
(physical* adj2 (fit* or train* or activit* or promot* or program* or intervention*)).ti,ab.
-
((physical* or exercise*) and referral*).ti,ab.
-
4 or 5 or 6 or 7
-
Randomized controlled trial.pt.
-
Randomized Controlled Trial/
-
(random$ or placebo$).ti,ab,sh.
-
((singl$ or double$ or triple$ or treble$) and (blind$ or mask$)).tw,sh.
-
9 or 10 or 11 or 12
-
controlled clinical trial.pt.
-
(retraction of publication or retracted publication).pt.
-
13 or 14 or 15
-
(family medicine$ or family practice$ or general practice$ or primary care or primary health care or primary health service$ or primary healthcare or primary medical care or family medical practice$ or family doctor$ or family physician$ or family practitioner$ or general medical practitioner$ or general practitioner$ or local doctor$).ti,ab.
-
Family Practice/
-
Primary Health Care/
-
Physicians, Family/
-
Community Health Centers/
-
(community healthcare or community health care).ti,ab.
-
(GP or GPs).ti,ab.
-
general practic*.ti,ab.
-
17 or 18 or 19 or 20 or 21 or 22 or 23 or 24
-
(referral* or promot* or program* or intervent*).ti,ab.
-
25 or 26
-
Exercise/
-
Exercise Therapy/
-
28 or 29
-
27 and 30
-
3 or 8 or 31
-
(child* or adolescent* or school* or pediatric* or paediatric*).ti.
-
32 not 33
-
16 and 34
-
(animals not humans).sh.
-
35 not 36
-
(“2009 October*” or “2009 November*” or “2009 December*” or “2010*” or “2011*” or “2012*” or “2013*”).dp.
-
37 and 38
-
limit 39 to english language
Appendix 2 Table of excluded studies with rationale
| Paper | Comment |
|---|---|
| Ackermann RT, Deyo RA, LoGerfo JP. Prompting primary providers to increase community exercise referrals for older adults: a randomized trial. J Am Geriatr Soc 2005;53:283–9 | Randomisation before baseline assessment |
| Anokye NK, Trueman P, Green C, Pavey TG, Hillsdon M, Taylor RS, et al. The cost-effectiveness of exercise referral schemes. BMC Public Health 2011;11:954 | Cost-effectiveness model |
| Baker M. Exercise Scheme Guidelines. Analysis. Practice Nurse 2001;21:14 | Not a RCT |
| Beynon CM, Luxton A, Whitaker R, Cable NT, Frith L, Taylor AH, et al. Exercise referral for drug users aged 40 and over: results of a pilot study in the UK. BMJ Open 2013;3:e002619 | Not a RCT |
| Bull FC, Milton KE. A process evaluation of a “physical activity pathway” in the primary care setting. BMC Public Health 2010;10:463 | Not a RCT |
| Casey D, De CM, Dasgupta K, Casey D, De Civita M, Dasgupta K. Understanding physical activity facilitators and barriers during and following a supervised exercise programme in Type 2 diabetes: a qualitative study. Diabet Med 2010;27:79–84 | Not a RCT |
| Chambers MJ. Exercise: a prescription for a good night’s sleep? Phys Sportsmed 1991;19:106–12 | Not a RCT |
| Cobiac LJ, Vos T, Barendregt JJ. Cost-effectiveness of interventions to promote physical activity: a modelling study. PLOS Med 2009;6:e1000110 | Cost-effectiveness model |
| Corbett C, Woodiwiss B. Exercise on prescription. Professional Nurse 1900;18:666–7 | Not a RCT |
| Davies T, Craig A. Developments & opportunities for exercise prescription . . . This article is the first in a series focussing on issues in exercise prescription. SportEX Medicine 1999;1:20–2 | Not a RCT |
| Dugdill L, Graham RC, McNair F. Exercise referral: the public health panacea for physical activity promotion? A critical perspective of exercise referral schemes; their development and evaluation. Ergonomics 2005;48:1390–410 | Not a RCT |
| Elley CR, Garrett S, Rose SB, O’Dea D, Lawton BA, Moyes SA, et al. Cost-effectiveness of exercise on prescription with telephone support among women in general practice over 2 years. BJSM Online 2011;45:1223–9 | No primary care referral |
| Elley CR, Kerse N, Arroll B, Robinson E. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. Br Med J 2003;326:793–6 | No third-party exercise provider |
| Gademan MG, Deutekom M, Hosper K, Stronks K, Gademan MGJ, Deutekom M, et al. The effect of exercise on prescription on physical activity and wellbeing in a multi-ethnic female population: a controlled trial. BMC Public Health 2012;12:758 | Not a RCT |
| Gidlow C, Johnston LH, Crone D, Morris C, Smith A, Foster C, et al. Socio-demographic patterning of referral, uptake and attendance in Physical Activity Referral Schemes. J Public Health (Oxf) 2007;29:107–13 | Not a RCT |
| Graham RC, Dugdill L, Cable NT. Health practitioner perspectives in exercise referral: implications for the referral process. J Sports Sci 2005;24:636–7 | Not a RCT |
| Grandes G, Sanchez A, Sanchez-Pinilla RO, Torcal J, Montoya I, Lizarraga K, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med 2009;169:694–701 | No primary care referral |
| Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns 2008;70:31–9 | No primary care referral |
| Harland J, White M, Drinkwater C, Chinn D, Farr L, Howel D. The Newcastle exercise project: a randomised controlled trial of methods, to promote physical activity in primary care. Br Med J 1999;319:828–32B | No primary care referral |
| Hellenius M-L. Prescribing exercise in clinical practice. Curr Cardiovasc Risk Rep 2011;5:331–9 | Not a RCT |
| Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485–92 | No third-party exercise provider |
| Johnston LH, Warwick J, De Ste CM, Crone D, Sidford A. The nature of all ‘inappropriate referrals’ made to a countywide physical activity referral scheme: implications for practice. Health Educ J 2005;64:58–69 | Not a RCT |
| Jolly K, Daley A, Adab P, Lewis A, Denley J, Beach J, et al. A randomised controlled trial to compare a range of commercial or primary care led weight reduction programmes with a minimal intervention control for weight loss in obesity: the Lighten Up trial. BMC Public Health 2010;10:439 | No third-party exercise provider |
| Josyula LK. Examination of physical activity for health promotion, and attitudes towards aging, among adults – cross-cultural comparisons; healthcare provider recommendations; toolkit evaluation. Dissertation Abstracts International: Section B: The Sciences and Engineering 1942;71(7-B) | Not a RCT |
| Karjalainen JJ, Kiviniemi AM, Hautala AJ, Niva J, Lepojarvi S, Makikallio TH, et al. Effects of exercise prescription on daily physical activity and maximal exercise capacity in coronary artery disease patients with and without type 2 diabetes. Clin Physiol Funct Imaging 2012;32:445–54 | No third-party exercise provider |
| Kolt GS, Schofield GM, Kerse N, Garrett N, Ashton T, Patel A, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med 2012;10:206–12 | Not appropriate population |
| Kolt GS, Rosenkranz RR, Savage TN, Maeder AJ, Vandelanotte C, Duncan MJ, et al. WALK 2.0 – Using Web 2.0 applications to promote health-related physical activity: a randomised controlled trial protocol. BMC Public Health 2013;13:436 | No primary care referral |
| Lawton BA, Rose SB, Elley CR, Dowell AC, Fenton A, Moyes SA. Exercise on prescription for women aged 40–74 recruited through primary care: two year randomised controlled trial. BMJ 2008;337:a2509 | No primary care referral |
| Lawton BA, Rose SB, Raina EC, Dowell AC, Fenton A, Moyes SA, et al. Exercise on prescription for women aged 40–74 recruited through primary care: two year randomised controlled trial. BJSM Online 2009;43:120–3 | No primary care referral |
| Lawton BA. Exercise on prescription for women aged 40–74 recruited through primary care: two year randomised controlled trial. BMJ 2009;339:a2509 | No primary care referral |
| Leung W, Ashton T, Kolt GS, Schofield GM, Garrett N, Kerse N, et al. Cost-effectiveness of pedometer-based versus time-based Green Prescriptions: the Healthy Steps Study. Aust J Prim Health 2012;18:204–11 | No primary care referral |
| Lord JC, Green F. Exercise on prescription: does it work? Health Educ J 1995;54:453–64 | Not a RCT |
| Markland D, Tobin VJ. Need support and behavioural regulations for exercise among exercise referral scheme clients: The mediating role of psychological need satisfaction. Psychol Sport Exerc 2010;11:91–9 | Not a RCT |
| Mckay J, Wright A, Lowry R, Steele K, Ryde G, Mutrie N. Walking on prescription: The utility of a pedometer pack for increasing physical activity in primary care. Patient Educ Couns 2009;76:71–6 | No primary care referral |
| Mills H, Crone D, James DV, Johnston LH, Mills H, Crone D, et al. Exploring the perceptions of success in an exercise referral scheme: a mixed method investigation. Eval Rev 2012;36:407–29 | Not a RCT |
| Murphy MH, McNeilly AM, Murtagh EM. Physical activity prescription for public health. Proc Nutr Soc 2010;69:178–84 | Not a RCT |
| Mutrie N, Doolin O, Fitzsimons CF, Grant PM, Granat M, Grealy M, et al. Increasing older adults’ walking through primary care: results of a pilot randomized controlled trial. Fam Pract 2012;29:633–42 | No primary care referral |
| Nicolai SPA, Kruidenier LM, Leffers P, Hardeman R, Hidding A, Teijink JAW, et al. Supervised exercise versus non-supervised exercise for reducing weight in obese adults. J Sports Med Phys Fitness 2009;49:85–90 | No primary care referral |
| Orrow G, Kinmonth AL, Sanderson S, Sutton S. Republished research: effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BJSM Online 2013;47:27 | Not a RCT |
| Phillips EM, Kennedy MA. The exercise prescription: a tool to improve physical activity. PM&R 2012;4:818–25 | Not a RCT |
| Pringle A, Gilson N, McKenna J, Cooke C. An evaluation of the Local Exercise Action Pilots and impact on moderate physical activity. Health Educ J 2009;68:179–85 | Not a RCT |
| Pugh D. Time to encourage patients to take more exercise. The Practitioner 2012;256:25–8 | Not a RCT |
| Richards J, Foster C, Thorogood M, Hillsdon M, Kaur A, Wickramasinghe KK, et al. Face-to-face interventions for promoting physical activity. Cochrane Database Syst Rev 2013;9:CD010392 | Not a RCT |
| Rimer J, Dwan K, Lawlor DA, Greig CA, McMurdo M, Morley W, et al. Exercise for depression. Cochrane Database Syst Rev 2012;7:CD004366 | Not a RCT |
| Rouse PC, Ntoumanis N, Duda JL, Jolly K, Williams GC, Rouse PC, et al. In the beginning: role of autonomy support on the motivation, mental health and intentions of participants entering an exercise referral scheme. Psychol Health 2011;26:729–49 | Not a RCT |
| Rome A, Persson U, Ekdahl C, Gard G. Physical activity on prescription (PAP): costs and consequences of a randomized, controlled trial in primary healthcare. [Provisional abstract]. Scand J Prim Health Care 2009;27:216–22 | Part of intervention prior to randomisation |
| Sabti Z, Handschin M, Joss MK, Allenspach EC, Nuscheler M, Grize L, et al. Evaluation of a physical activity promotion program in primary care. Fam Pract 2010;27:279–84 | Not a RCT |
| Santos C, Santos J, Morais L, Rodrigues F, Barbara C. Pulmonary rehabilitation in COPD: effects of two aerobic exercise intensity in patient-centered outcomes – a randomized study. [Abstract]. Chest 2011;140:853A | No primary care referral |
| Slade SC, Keating JL. Effects of preferred-exercise prescription compared to usual exercise prescription on outcomes for people with non-specific low back pain: a randomized controlled trial [ACTRN12608000524392]. BMC Musculoskelet Disord 2009;10:14 | No primary care referral |
| Slade SC, Keating JL. Exercise prescription: a case for standardised reporting. BJSM Online 2012;46:1110 | Not a RCT |
| Sorensen J, Sorensen JB, Skovgaard T, Bredahl T, Puggaard L, Sorensen J, et al. Exercise on prescription: changes in physical activity and health-related quality of life in five Danish programmes. Eur J Public Health 2011;21:56–62 | Not a RCT |
| Sukala WR, Page R, Cheema BS, Sukala WR, Page R, Cheema BS. Exercise training in high-risk ethnic populations with type 2 diabetes: a systematic review of clinical trials. Diabetes Res Clin Pract 2012;97:206–16 | Not a RCT |
| Taylor TR, Makambi K, Sween J, Roltsch M, Adams-Campbell LL, Taylor TR, et al. The effect of a supervised exercise trial on exercise adherence among African American Men: a pilot study. J Natl Med Assoc 2011;103:488–91 | Not a RCT |
| Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 2012;344:e2088 | Not a RCT |
| Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Duda W, Borowjack E, et al. Interventions to reduce or prevent obesity in pregnant women: A systematic review. Health Technol Assess 2012;16(31) | Not a RCT |
| van Midde Koop M, Rubinstein SM, Verhagen AP, Ostelo RW, Koes BW, van Tulder MW. Exercise therapy for chronic nonspecific low-back pain. Best Pract Res Clin Rheumatol 2010;24:193–204 | Not a RCT |
| van Hoecke AS, Delecluse C, Bogaerts A, Boen F. The long-term effectiveness of need-supportive physical activity counselling compared with a standard referral in sedentary older adults. J Aging Phys Activity 2015: in press | No primary care referral |
| Vermunt PW, Milder IE, Wielaard F, de Vries JH, Baan CA, van Oers JA, et al. A lifestyle intervention to reduce Type 2 diabetes risk in Dutch primary care: 2.5-year results of a randomized controlled trial. Diabet Med 2012;29:e223–31 | No primary care referral |
| Vermunt PW, Milder IE, Wielaard F, de Vries JH, van Oers HA, Westert GP, et al. Lifestyle counselling for type 2 diabetes risk reduction in Dutch primary care: results of the APHRODITE study after 0.5 and 1.5 years. Diabetes Care 2011;34:1919–25 | No primary care referral |
| Voet NB. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev 2010;1:CD003907 | Not a RCT |
| Voet NB, van der Kooi EL, Riphagen II, Lindeman E, van Engelen BG, Geurts AC, et al. Strength training and aerobic exercise training for muscle disease. [Update of Cochrane Database Syst Rev 2005;1:CD003907; PMID: 15674918]. Cochrane Database Syst Rev 2010;1:CD003907 | Not a RCT |
| Ward M, Phillips CJ, Farr A. Heartlinks – a real world approach to effective exercise referral. Int J Health Promo Educ 2010;48:20–7 | Not a RCT |
| Waryasz GR, McDermott AY. Exercise prescription and the patient with type 2 diabetes: A clinical approach to optimizing patient outcomes. J Am Acad Nurse Pract 2010;22:217–27 | Not a RCT |
| Wu YT, Hwang CL, Chen CN, Chuang LM, Wu YT, Hwang CL, et al. Home-based exercise for middle-aged Chinese at diabetic risk: a randomized controlled trial. Prev Med 2011;52:337–43 | No primary care referral |
| Wyatt-Williams J. Setting up a national exercise referral scheme (in Wales). J Aging Phys Activ 2012;20:S259 | Not a RCT |
| Yildirim Y, Soyunov S. Relationship between learning strategies of patients and proper perception of the home exercise program with non-specific low back pain. J Back Musculoskeletal Rehabil 2010;23:137–42 | No primary care referral |
| Yoshida H, Ishikawa T, Suto M, Kurosawa H, Hirowatari Y, Ito K, et al. Effects of supervised aerobic exercise training on serum adiponectin and parameters of lipid and glucose metabolism in subjects with moderate dyslipidemia. J Atheroscler Thromb 2010;17:1160–6 | Not a RCT |
| Zanuso S, Jimenez A, Pugliese G, Corigliano G, Balducci S, Zanuso S, et al. Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol 2010;47:15–22 | Not a RCT |
| Zhang XD, Long BB, Wu H. Effects of aerobic exercise prescription intervention to female college students with simple obesity. Proceedings of the 21st Pan-Asian Congress of Sports and Physical Education, Application of Physiology and Psychology in Sports 2010;4:87–9 | No primary care referral |
| Zuazagoitia A, Grandes G, Torcal J, Lekuona I, Echevarria P, Gomez MA, et al. Rationale and design of a randomised controlled trial evaluating the effectiveness of an exercise program to improve the quality of life of patients with heart failure in primary care: The EFICAR study protocol. BMC Public Health 2010;10:33 | No third-party exercise provider |
Appendix 3 Inputs for probabilistic sensitivity analysis
| Parameter | Mean | Standard error | Distribution |
|---|---|---|---|
| Incidence rates for | |||
| CHD (available by age groups, years) | |||
| 33–34 | 0.000035 | 1.0881E-05 | Beta |
| 35–44 | 0.000465 | 3.9654E-05 | |
| 45–54 | 0.002095 | 8.41E-05 | |
| 55–64 | 0.00631 | 0.00014565 | |
| 65–74 | 0.0097 | 0.00018027 | |
| 75–81 | 0.0097 | 0.00018027 | |
| Stroke (available by age groups, years) | |||
| 33–34 | 0.00008 | 2.7602E-05 | Beta |
| 35–44 | 0.00023 | 4.6797E-05 | |
| 45–54 | 0.00057 | 7.3658E-05 | |
| 55–64 | 0.00291 | 0.00016623 | |
| 65–74 | 0.0069 | 0.00025546 | |
| 75–81 | 0.01434 | 0.0003669 | |
| Diabetes mellitus (available by age groups, years) | |||
| 33–39 | 9.00365E-05 | 6.9895E-06 | Beta |
| 40–49 | 0.000280353 | 1.2332E-05 | |
| 50–59 | 0.000631793 | 1.851E-05 | |
| 60–69 | 0.001004529 | 2.3336E-05 | |
| 70–79 | 0.001115584 | 2.459E-05 | |
| 80–81 | 0.001115584 | 2.459E-05 | |
| Probability | |||
| Fatality cases for CHD | |||
| 33–34 | 0.08773 | 0.008773 | Beta |
| 35–44 | 0.08773 | 0.008773 | |
| 45–54 | 0.08773 | 0.008773 | |
| 55–64 | 0.11553 | 0.011553 | |
| 65–74 | 0.21065 | 0.021065 | |
| 75–81 | 0.14763 | 0.014763 | |
| Fatality cases for stroke | |||
| 33–34 | 0.234636872 | 0.02346369 | Beta |
| 35–44 | 0.234636872 | 0.02346369 | |
| 45–54 | 0.234636872 | 0.02346369 | |
| 55–64 | 0.23279352 | 0.02327935 | |
| 65–74 | 0.23466258 | 0.02346626 | |
| 75–81 | 0.23420074 | 0.02342007 | |
| Relative risks for | |||
| Being active (at year 1) as a result of exercise referral | 1.1 | 0.06 | Log-normal |
| Developing disease conditions for active people | |||
|
0.90 | 0.04 | Log-normal |
|
0.86 | 0.04 | |
|
0.67 | 0.12 | |
| Non-CVD mortality after | |||
| Non-fatal CHD | 1.71 | 0.14 | Log-normal |
| Non-fatal stroke | 1.71 | 0.14 | |
| Diabetes mellitus | 1.49 | 0.13 | |
| CVD mortality after | |||
| Non-fatal CHD | 3.89 | 0.04 | Log-normal |
| Non-fatal stroke | 3.89 | 0.04 | |
| Diabetes mellitus | 2.61 | 0.14 | |
| Utility | |||
| Age (years)-specific QoL | |||
| 45–54 | 0.86 | 0.01 | Beta |
| 55–64 | 0.82 | 0.01 | |
| 65–74 | 0.78 | 0.01 | |
| 75+ | 0.72 | 0.01 | |
| Health state utility weight | |||
| Healthy | 1.00 | 0.10 | Gamma |
| CHD first event | 0.80 | 0.08 | |
| Ongoing CHD | 0.92 | 0.09 | |
| Stroke initial event | 0.63 | 0.06 | |
| Ongoing stroke | 0.65 | 0.07 | |
| Diabetes mellitus | 0.90 | 0.09 | |
| Mental health gain | 0.07 | 0.04 | Beta |
| Cost | |||
| Exercise referral | £229 | £23 | Normal |
| CHD first event | £4198 | £395 | |
| Ongoing CHD | £480 | £45 | |
| Stroke initial event | £10,839 | £1019 | |
| Ongoing stroke | £2380 | £224 | |
| Diabetes mellitus | £968 | £91 | |
Appendix 4 Mortality data
| Age (years) | All-cause mortality | CVD-cause mortality | Non-CVD-cause mortality |
|---|---|---|---|
| 50 | 0.002854 | 0.00054 | 0.00231 |
| 51 | 0.003087 | 0.00059 | 0.00250 |
| 52 | 0.003413 | 0.00065 | 0.00276 |
| 53 | 0.003693 | 0.00070 | 0.00299 |
| 54 | 0.004115 | 0.00078 | 0.00333 |
| 55 | 0.004513 | 0.00086 | 0.00366 |
| 56 | 0.004949 | 0.00094 | 0.00401 |
| 57 | 0.005334 | 0.00101 | 0.00432 |
| 58 | 0.005799 | 0.00110 | 0.00470 |
| 59 | 0.006403 | 0.00122 | 0.00519 |
| 60 | 0.006948 | 0.00132 | 0.00563 |
| 61 | 0.007478 | 0.00142 | 0.00606 |
| 62 | 0.008051 | 0.00153 | 0.00652 |
| 63 | 0.009034 | 0.00172 | 0.00732 |
| 64 | 0.010004 | 0.00190 | 0.00810 |
| 65 | 0.010801 | 0.00240 | 0.00840 |
| 66 | 0.011984 | 0.00266 | 0.00932 |
| 67 | 0.013043 | 0.00290 | 0.01015 |
| 68 | 0.014685 | 0.00326 | 0.01142 |
| 69 | 0.016104 | 0.00358 | 0.01253 |
| 70 | 0.017616 | 0.00391 | 0.01370 |
| 71 | 0.01932 | 0.00429 | 0.01503 |
| 72 | 0.021385 | 0.00475 | 0.01663 |
| 73 | 0.023881 | 0.00531 | 0.01858 |
| 74 | 0.026280 | 0.005838 | 0.020442 |
| 75 | 0.029173 | 0.007763 | 0.021410 |
| 76 | 0.032836 | 0.008737 | 0.024098 |
| 77 | 0.036376 | 0.009679 | 0.026696 |
| 78 | 0.040763 | 0.010847 | 0.029916 |
| 79 | 0.045782 | 0.012183 | 0.033600 |
| 80 | 0.051718 | 0.013762 | 0.037956 |
| 81 | 0.057861 | 0.015397 | 0.042465 |
| 82 | 0.064138 | 0.017067 | 0.047071 |
| 83 | 0.071322 | 0.018979 | 0.052343 |
| 84 | 0.080421 | 0.021400 | 0.059021 |
| 85 | 0.089901 | 0.023922 | 0.065978 |
| 86 | 0.099902 | 0.026584 | 0.073318 |
| 87 | 0.110316 | 0.029355 | 0.080961 |
| 88 | 0.122565 | 0.032614 | 0.089951 |
| 89 | 0.128800 | 0.034274 | 0.094527 |
| 90 | 0.143003 | 0.038053 | 0.104951 |
| 91 | 0.153263 | 0.040783 | 0.112480 |
| 92 | 0.175290 | 0.046644 | 0.128646 |
| 93 | 0.194175 | 0.051670 | 0.142505 |
| 94 | 0.214034 | 0.056954 | 0.157080 |
| 95 | 0.233037 | 0.062011 | 0.171026 |
| 96 | 0.251844 | 0.067015 | 0.184829 |
| 97 | 0.269651 | 0.071754 | 0.197898 |
| 98 | 0.290335 | 0.077258 | 0.213078 |
Appendix 5 Data extraction
Data extraction sheet for exercise referral scheme update
| Study details | Inclusion and exclusion criteria | Included population characteristics | Characteristics of referral, intervention and control | Outcomes | Quality Assessment |
|---|---|---|---|---|---|
| Murphy et al., 201223 Country: Wales, UK Study design: RCT Funding source: Welsh government |
Inclusion criteria Sedentary (identified as not moderately active for ≥ 3 times per week or deconditioned through age or inactivity) and had at least one of the following medical conditions: CVD risk factors
Mental health
|
Total number randomised:N = 2160 Intervention group:n = 1080 Control group:n = 1080 Age: 52 years (SD 14.7 years) Sex: 66% women Ethnicity: 96% white Disease status: CHD risk factors (72%), CHD and mental health issues (24%) |
Person making referral: clinician Reason for referral: identified opportunistically by clinicians in normal practice Format of referral: form Referred to who: evaluation team Referred to where: intervention delivered at leisure centres by exercise professionals in each local health board Consultations were based on motivation interview principles which facilitated the patient-centred achievable goals, including relapse prevention strategies at 4 and 16 weeks to review goals and encourage attendance Components of the intervention:
Total duration: 16 weeks Control group: given usual care and a leaflet highlighting the benefits of exercise, and were given the addresses of local facilities |
12 months follow-up Physical Activity (PA) 7-day physical activity recall administered by telephone ERS: median 200 (IQR 40–435), n = 724 Control: median 165 (IQR 50–370), n = 755 Psychological well-being HADS depression score ERS: mean 6.14 (95% CI 5.73 to 6.54), n = 471 Control: mean 6.93 (95% CI 6.53 to 7.32), n = 506 HADS anxiety score ERS: mean 7.82 (95% CI 7.39 to 8.25), n = 472 Control: mean 8.35 (95% CI 7.92 to 8.77), n = 502 |
Power calculation: yes, accounting for lost to follow-up Random allocation:yes, using random number generator Blinding at outcome assessment: yes Similar at baseline: yes ITT analysis: yes Statistical handling of missing data: yes Missing data reported: yes |
Appendix 6 Additional analyses conducted prior to the first committee meeting
Introduction
Prior to the first Committee meeting we were asked by the Centre for Public Health Practice to provide some additional analyses to inform the Committee’s discussions. In particular we were asked to provide additional analyses to support the following statement in Chapter 4, Discussion, ‘It may be the case that minor changes to a combination of parameter values . . . would bring the ICER down to a value more consistent with that estimated by Pavey et al. ’11
Based on the univariate sensitivity analyses already conducted, it can be seen that the model is sensitive to the duration of protective effect associated with physical activity for the conditions CHD, stroke and diabetes. In the base case analysis, the duration of protective effect was limited to 10 years. However, the RR estimates applied in the model were based on cohort studies with follow-up periods of 19 years for CHD57 and stroke58 and 12 years for diabetes mellitus. 59 We have, therefore, decided to explore the possibility of extending the protective effect to reflect the durations of these studies.
The model is also particularly sensitive to the process utility gain attributable to becoming physically active. In the base case analysis it is assumed that the process utility gain associated with physical activity is applied for 1 year only, as this is the duration of follow-up for the effectiveness studies. Without studies providing longer-term follow-up it is unclear how long people remain physically active. However, it is likely that some people who continue to be physically active at 1 year will carry on being physically active in the longer-term. To explore the impact of a gradual fall-off in the number remaining physically active, we have applied the process utility for 10 years, but assumed a linear decrease in the number who are physically active over those 10 years, such that none are receiving a process utility gain from being physically active after 10 years.
We also explored the effect on the ICER of combining these two less-conservative assumptions regarding the longer-term benefits of ERS.
Results
Presented are deterministic results for the whole cohort eligible to receive ERSs for several scenarios exploring less-conservative model assumptions.
Extending the duration of the protective effect associated with physical activity to 19 years for CHD and stroke and 12 years for diabetes mellitus, to reflect the follow-up periods in the studies used to estimate the RRs applied in the model, reduced the ICER to £50,634 as seen in Table 47.
| Intervention | Mean cost | Mean QALY | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| ERS | £4527 | 18.152 | £221 | 0.004 | £50,634 |
| Usual care | £4306 | 18.148 | – | – | – |
Applying the process utility for 10 years but assuming a linear decrease in the number who are physically active over those 10 years gave an ICER of £21,918, when all other assumptions were held constant, as shown in Table 48.
| Intervention | Mean cost | Mean QALY | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| ERS | £4572 | 18.218 | £225 | 0.010 | £21,918 |
| Usual care | £4346 | 18.208 | – | – | – |
Results when combining the extended duration of protective effect, with the gradual fall-off in the proportion remaining active are presented in Table 49, which shows an ICER of £18,935.
| Intervention | Mean cost | Mean QALY | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| ERS | £4527 | 18.234 | £221 | 0.012 | £18,935 |
| Usual care | £4306 | 18.222 | – | – | – |
The additional analyses conducted, which explore the effect on the ICER of applying some less-conservative model assumptions, demonstrate that ICERs as low as £19,000 per QALY can be achieved by combining a gradual fall-off in physical activity over 10 years and extending the duration of protective effect beyond 10 years. This supports the statement made in Chapter 4, Discussion that ‘It may be the case that minor changes to a combination of parameter values . . . would bring the ICER down to a value more consistent with that estimated by Pavey et al. ’11
Appendix 7 Additional analyses conducted prior to the second committee meeting
Introduction
Following the first PHAC on ERSs, NICE requested that ScHARR-TAG conduct additional analyses to inform the Committee’s discussion at the second PHAC. Incremental cost-effectiveness ratios were requested for a ‘combined scenario analysis’ incorporating:
-
costs for providing brief advice in the comparator arm
-
efficacy estimates from the ITT analysis
-
a 10-year linear fall-off in the process utility associated with being physical active, applied in combination with the original base case assumption that the protective effects of exercise are limited to 10 years.
It should be noted that the assumptions incorporated into this ‘combined scenario analysis’ requested by NICE do not necessarily reflect the authors’ preferred model assumptions.
In addition, several sensitivity analyses were also requested in which individual changes were made to the combined scenario analysis. First, a sensitivity analysis was requested exploring the impact of using EQ-5D data from the Murphy et al. 23 study as an alternative to the process utility gain estimated by Pavey et al. 11 and applied in the model used to inform the PH44. 65 Second, a sensitivity analysis was requested exploring the cost-effectiveness of less-intensive ERSs. Finally, a threshold analysis was also requested on the intervention cost for ERSs.
Further details on the methods used to conduct these analyses are provided in Methods, and the results are presented in Results.
Methods
Incorporating costs for brief advice
In the main report it was noted that in all of the studies comparing exercise referral to usual care, patients randomised to the usual-care arm received some form of advice regarding physical activity. In the model used by Pavey et al. 11 no cost was attributed to providing usual care in the comparator arm, which may have overestimated the incremental cost of ERS relative to usual care. In the appraisal of brief advice to promote physical activity, the cost of brief advice was estimated at £9.50 (2010/11 prices). 65 This was inflated to a 2011/12 cost of £9.81 using inflation indices from the PSS Research Unit71 and applied in the combined scenario analysis presented below.
Intention-to-treat analysis
In the main report efficacy results were meta-analysed using two different approaches. In Figure 2 the denominators used to calculate the RRs were the number of patients with data at follow-up for each study, whereas in Figure 3, in order to assess the potential (attrition) bias in using completers, the denominators of these three studies were adjusted to all individuals randomised in order to perform an ITT analysis. The cost-effectiveness results presented in the main report used the efficacy data based on individuals with follow-up (RR 1.12, 95% CI 1.04 to 1.20). In the combined scenario analysis presented below we use the efficacy data based on the ITT analysis (RR 1.08, 95% CI 1.00 to 1.17).
Duration of protective effect and process utility gain
In the model used to inform PH44,65 the protective effect of physical activity on stroke, type 2 diabetes mellitus and CHD was assumed to last for 10 years and a process utility associated with being physically active was assumed to apply for 1 year. In Appendix 6 we presented analyses exploring the impact of increasing the assumed duration of protective effect and the assumed duration over which the process utility is applied. In the combined scenario analysis presented, we assume that the duration of protective effect is maintained at 10 years but that there is a linear fall-off in the process utility gain over this 10-year period. This would be consistent with assuming that the number continuing to be physically active falls linearly over the 10 years after the intervention and that the full process utility gain is experienced until the individual stops being physically active.
Process utility gain
The process utility gain associated with being physically active reported by Pavey et al. ,11 and applied in the economic model used to inform PH44,65 is based on cross-sectional data and therefore may not represent a causal relationship between physical activity and health utility. However, Murphy et al. 23 report EQ-5D utility scores at 12 months for patients randomised to ERSs and usual care, allowing the utility gain attributable to ERSs to be calculated directly from a randomised comparison. Table 16 of the main report presents the mean EQ-5D scores from the two trial arms as 0.64 [standard deviation (SD) 0.32, n = 395] and 0.61 (SD 0.32, n = 391) for ERSs and usual care, respectively, giving a mean difference of 0.03 [standard error (SE) = 0.023]. It should be noted that this cannot be compared directly against the process utility of 0.072 given by Pavey et al. , as the value from Pavey et al. is an estimate of the difference between those who are physically active and those who are not physically active, whereas the value from Murphy et al. 23 is the difference between the randomised groups, each of which had a different proportion who became physically active.
In the additional sensitivity analysis reported below, we have removed the process utility gain of 0.072, which was applied in the base case analysis to all those who were physically active at 1 year, and added 0.03 to the utility values for all those receiving ERS. Nothing is added to the utility values of those receiving usual care, as this gain is measured relative to usual care. In this sensitivity analysis it is assumed that the utility gain measured by Murphy et al. 23 is experienced for only 1 year as this is the duration of study follow-up. Although it may be assumed that the process utility gain of being physically active may extend beyond the study duration, if people remain physically active, the utility gain associated with receiving ERS is the average gain across those who become active and those who do not and, therefore, it cannot be extrapolated in the same manner, as there is no rationale for a continued utility gain in those who received ERS but did not become physically active.
Less-intensive exercise referral schemes
In this sensitivity analysis we considered how the cost-effectiveness of a less-intensive form of ERS might differ from that assumed in the base case using evidence on a walking-based intervention (as opposed to a structured leisure centre-based intervention) from Isaacs et al. 18 This replicates a similar sensitivity analysis conducted by Pavey et al. 11 in which the intervention cost was reduced from £222 to £110. In our sensitivity analysis, the cost of the walking-based intervention was inflated to a cost of £114 (2011/12)31 to reflect current prices. We assumed that there was no change in efficacy associated with moving to a less-intensive ERSs.
Threshold analysis on the intervention cost for exercise referral schemes
A threshold analysis was conducted in which the intervention cost for ERSs was lowered until the ICER reached the £20,000 and £30,000 per QALY threshold boundaries, while holding constant all other data and assumptions applied in the combined scenario analysis.
Results
Main results for the cohort eligible to receive exercise referral schemes
The individual and combined effects of each of the changes to the model assumptions described in Methods are presented in Table 50. It can be seen that the addition of costs for providing some brief advice related to physical activity in the usual-care arm has little effect on the ICER. The application of the ITT efficacy data does have a small effect on the incremental costs but a greater proportionate effect on the incremental QALYs, resulting in a substantial increase in the ICER. Allowing for a 10-year linear reduction in the proportion remaining active, and therefore accruing a process utility gain, results in a substantial increase in incremental QALYs from 0.003 to 0.010 and results in the ICER falling to £21,918. The combined effect of all of three changes to the previous base case gives an ICER of £31,081 for the combined scenario analysis.
| Scenario | Description | ERS | Usual care | Incremental | ICER | |||
|---|---|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | Cost | QALYs | |||
| NA | Previous base case | £4572 | 18.136 | £4346 | 18.133 | £225 | 0.003 | £76,059 |
| 1 | Added cost of brief advice in the usual-care arm | £4562 | 18.136 | £4346 | 18.133 | £216 | 0.003 | £72,748 |
| 2 | ITT RR | £4573 | 18.135 | £4346 | 18.133 | £227 | 0.002 | £113,931 |
| 3 | 10-year fall-off in process utility | £4572 | 18.218 | £4346 | 18.208 | £225 | 0.010 | £21,918 |
| 1 + 2 + 3 | Combined scenario analysis | £4563 | 18.216 | £4346 | 18.209 | £217 | 0.007 | £31,081 |
Table 51 shows the PSA results per individual for the combined scenario analysis estimated from 10,000 samples. The PSA results are very similar to the deterministic results, with an ICER of £31,009.
| Intervention | Mean cost (95% CI) | Mean QALY (95% CI) | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| ERS | £4559 | 18.2154 | £217 | 0.0070 | £31,009 |
| Usual care | £4343 | 18.2084 | – | – | – |
Figure 19 shows a cost-effectiveness plane of the probabilistic results for the first 1000 samples. The cost-effectiveness acceptability curve in Figure 20 shows the probability that ERS is cost-effective at various willingness-to-pay thresholds (estimated from the full 10,000 samples). The probability that ERS is cost-effective compared with usual care is 0.41 for a willingness-to-pay threshold of £30,000 per QALY gained and 0.15 for a willingness-to-pay threshold of £20,000 per QALY.
FIGURE 19.
Cost-effectiveness plane for the combined scenario analysis.
FIGURE 20.
Cost-effectiveness acceptability curve for the combined scenario analysis.
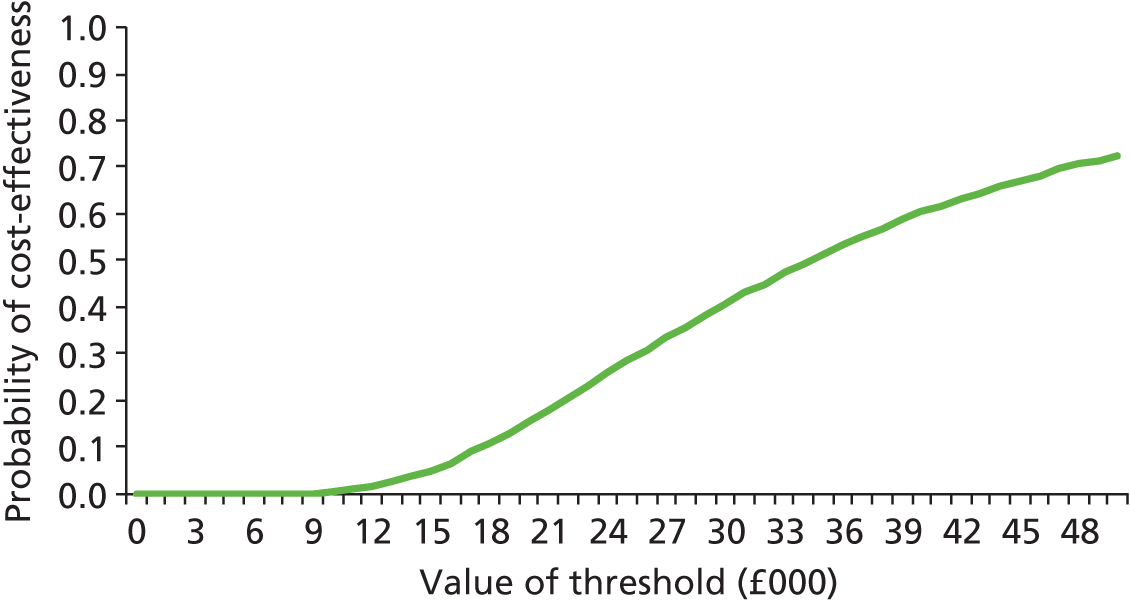
Subgroup analysis
The results for the subgroup analysis using the combined scenario analysis assumptions are presented in Tables 52–55. When assuming that the effectiveness of ERSs in each of these subgroups is similar to that in the eligible population as a whole, the ICERs are marginally more favourable than those of the whole population because of the greater risks of conditions associated with inactivity in these subgroups. However, applying the subgroup data for the depressed cohort from the Murphy et al. study resulted in a much higher ICER which reflects the estimate of low efficacy for this subgroup within the study reported by Murphy et al. 23
| Intervention | Mean cost | Mean QALY | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| ERS | £4757 | 18.142 | £213.8 | 0.008 | £26,015 |
| Usual care | £4543 | 18.134 | – | – | – |
| Intervention | Mean cost | Mean QALY | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| ERS | £4624 | 18.191 | £215.8 | 0.007 | £29,056 |
| Usual care | £4408 | 18.183 | – | – | – |
| Intervention | Mean cost | Mean QALY | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| ERS | £4685 | 18.112 | £218.0 | 0.002 | £96,462 |
| Usual care | £4467 | 18.110 | – | – | – |
| Intervention | Mean cost | Mean QALY | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| ERS | £4681 | 18.119 | £213.8 | 0.009 | £23,903 |
| Usual care | £4467 | 18.110 | – | – | – |
Additional sensitivity analyses
The sensitivity analysis in which the process utility gain for ERS versus usual care has been estimated directly from the Murphy et al. study23 is reported in Table 56. The QALY gain is similar to that reported by Murphy et al. (0.03),23 as it is largely driven by the utility gains derived during the 1-year trial period. Compared with the combined scenario analysis which uses the process utility gain attributable to being physically active, the QALY gain attributable to ERS is over three times greater (0.026 versus 0.007) when estimated using this directly measured EQ-5D resulting in an ICER of £8290.
| Intervention | Mean cost | Mean QALY | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| ERS | £4563 | 18.142 | £217 | 0.026 | £8290 |
| Usual care | £4346 | 18.115 | – | – | – |
The sensitivity analysis applying costs from a less-intensive intervention to increase physical activity (walking-based intervention) but assuming no change in efficacy from the combined scenario analysis is reported in Table 57. It can be seen that this reduced the ICER by approximately one-half, as the less-intensive walking-based intervention has approximately one-half of the cost of the structured leisure centre-based intervention which was used to estimate the cost of ERS in the combined scenario analysis.
| Intervention | Mean cost | Mean QALY | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| ERS | £4448 | 18.216 | £101.2 | 0.007 | £14,503 |
| Usual care | £4346 | 18.209 | – | – | – |
The results of the threshold analysis on the cost of ERS were as follows. A 4% reduction in the cost of ERSs from £229 to £220 gave an ICER just under £30,000 per QALY when holding all other conditions from the combined scenario analysis constant. A 36% reduction in the cost of ERSs from £229 to £147 gave an ICER just under £20,000 per QALY when holding all other conditions from the combined scenario analysis constant.
Conclusions
The ICER for the combined scenario analysis is £31,000 when using either the deterministic model or the PSA output. The application of the ITT data increased the ICER largely because of its impact on the absolute QALY gains, but this was more than counteracted by the assumption that process utility gain would be accrued for up to 10 years with a linear fall-off in those remaining active being assumed.
The sensitivity analysis exploring the application of the EQ-5D utility data from the study by Murphy et al. 23 shows that much larger QALY gains are estimated when applying the mean difference in utility between study arms rather than attributing a process utility gain only to those who become active. However, it should be noted that the EQ-5D difference measured by Murphy et al. 23 was not statistically significant and this uncertainty has not been captured in the results presented as there was insufficient time to run the PSA for this sensitivity analysis. Furthermore, in our clinical effectiveness review, some but not all of the studies that reported HRQoL data found a statistically significant difference between ERSs and usual care, suggesting that this short-term benefit is still relatively uncertain.
The threshold analysis on intervention cost and the sensitivity analysis applying a lower cost of ERSs based on the cost of a walking-based intervention, both demonstrate that the ICER is sensitive to the cost of ERSs. It should be noted that these analyses assume that there is no relationship between the cost of the scheme and its efficacy. It, therefore, cannot be concluded that lower-cost schemes are more cost-effective unless it can also be shown that they are equally efficacious.
List of abbreviations
- BMI
- body mass index
- CHD
- coronary heart disease
- CI
- confidence interval
- CVD
- cardiovascular disease
- EQ-5D
- European Quality of Life-5 Dimensions
- ERS
- exercise referral scheme
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PHAC
- Public Health Appraisal Committee
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SDT
- self-determination theory
- SF-36
- Short Form questionnaire-36