Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/71/01. The contractual start date was in June 2010. The draft report began editorial review in November 2014 and was accepted for publication in April 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor John Norrie is a member of the National Institute for Health Research Health Technology Assessment Commissioning Board and a member of the National Institute for Health Research Efficacy and Mechanism Evaluation and Health Technology Assessment Editorial Boards.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Pickard et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
In 2008, the UK Government Department of Health’s National Institute for Health Research (NIHR) Health Technology Assessment programme called for a randomised controlled trial (RCT) to answer the following clinical care question: ‘What is the effectiveness and cost-effectiveness of the use of calcium channel blockers and α-blockers to facilitate urinary stone passage in people with ureteric colic?’ This report describes the research [the SUSPEND (Spontaneous Urinary Stone Passage ENabled by Drugs) trial] that was subsequently commissioned.
The SUSPEND trial was a large, pragmatic, UK-based, multicentre RCT. It aimed to establish whether or not the use of either alpha-blockers or calcium channel blockers was clinically effective as medical expulsive therapy (MET) to facilitate spontaneous stone passage for people requiring emergency care for ureteric colic in comparison with placebo, and whether or not their use was cost-effective from the perspective of the UK NHS.
Background
Target population for trial
Ureteric colic describes the pain felt when a stone formed in the urine collection part of the kidney (usually resulting from the aggregation of calcium-based crystals) passes down the ureter, the urine drainage tube connecting the kidney to the bladder (Figure 1). The pain is typically severe and recurrent; each episode usually lasts for an hour or two and is interspersed with periods without pain. Female sufferers consider it to be more intense than the pain experienced during childbirth. 1 Repeated episodes have a detrimental influence on perception of quality of life. 2 Pain is usually felt in the abdomen but can go down into the groin and scrotum in males, and labia in females. Some people also get increased frequency and urgency of urination. The pain is likely to relate to direct contact of the stone with the epithelial cells lining the ureter and sustained contraction (spasm) of the smooth muscle surrounding the ureter in response to the obstruction of urine flow. 3 The severity of the pain leads to secondary symptoms such as nausea, vomiting and fever. Pain episodes generally continue until the stone passes into the bladder from where it can be freely voided by urination. Kidney stone disease is highly prevalent in most countries, where it affects 5% to 10% of the population,4 and, as there is no effective disease-modifying treatment, sufferers may have repeated episodes following the first bout of colic, with an approximate lifetime risk of recurrence of 50%. 5,6 The cause of kidney stone formation is multifactorial with genetic, environmental and dietary influences all playing a part. In epidemiological terms it is more common in people aged 15–60 years, in men and in those of Caucasian race, and there is a higher incidence during the summer months. 7
FIGURE 1.
Anatomy of urinary tract showing definition of ureteric segments. Reproduced, with permission, from Medindia. URL: www.medindia.net/news/michigan-hospitals-lead-the-way-in-preventing-common-and-costly-urinary-tract-infections-116433-1.htm (accessed July 2015).

Ureteric colic is one of the most common reasons for people to seek emergency health care, with an annual incidence of around 30 out of 100,000 in high-resource parts of the world. 8 In the UK, 83,000 people required emergency care for ureteric colic in 2009,9 and NHS England health episode statistics data for 2012–13 showed 31,000 hospital admissions with a 1-day median stay and a NHS tariff cost of £19.3M. 10 In the USA, there were 600,000 emergency room visits in 2000 at an estimated health-care cost of US$490M. 7 In both countries, the incidence of ureteric colic increased by over 50% during the previous decade. 7,9
Clinical assessment
The diagnosis of ureteric colic is usually straightforward from the characteristic history of pain, the lack of abdominal tenderness and, to some extent, the finding of non-visible haematuria on reagent strip testing of urine. 8 At this point the patient is given effective pain relief in the form of opiates or non-steroidal anti-inflammatory drugs (NSAIDs), alone or in combination. 11,12 Urine is then checked for infection by microscopy and culture, and renal function is estimated by measurement of serum creatinine. Once the patient is comfortable, the presence of a stone in the ureter can be reliably confirmed by imaging using computerised tomography scanning of the kidneys, ureters and bladder (CT KUB) without the use of intravascular contrast agents. Further analysis of the CT KUB images will localise the stone to the upper, mid or lower ureter (see Figure 1) and will estimate stone size defined by the maximum linear dimension in millimetres. Less commonly, patients may have evidence of severe urinary and bloodstream infection (urosepsis) as a result of the stone obstructing drainage from the affected kidney; they may have stones in both ureters; or they may have a single kidney, all of which cause marked impairment of renal function. Each of these situations will require resuscitation and urgent intervention and, therefore, such patients are not the focus of this trial.
Interventions for ureteric colic
Guidance on patient management options with summarised relative benefits and harms has been formulated under a joint initiative between the European Association of Urology and the American Urological Association, and form the basis of clinical management for people with kidney and ureteric stones, particularly across Europe and North America. 13 The options for treatment fall under three headings – symptom management, MET and active treatment – and these are discussed below.
Symptom management
For the majority of patients (approximately 75%) without a reason for prompt intervention to drain the affected kidney, management consists of pain relief, antiemetics and encouragement of adequate oral fluid intake. Once the pain is controlled, care can continue at home with oral analgesics but with the facility for rapid readmission if there is deterioration, together with a planned reassessment at approximately 4 weeks to assess whether or not spontaneous stone passage has occurred. Reasons for changing to active management would include poor pain control, the onset of systemic infection or concerns regarding deterioration in kidney function.
Medical expulsive therapy
Patients with ureteric stones face the uncertainty of when spontaneous stone passage and associated episodic pain will occur. Simple adjunctive treatments that lessen stone symptoms, hasten stone passage and increase the likelihood of eventual passage, thus reducing the risk of requiring active treatment, would reduce this burden. After many years of different agents being trialled, recent meta-analyses of multiple RCTs have encouraged clinicians to prescribe MET. An alpha-blocker drug [typically tamsulosin hydrochloride (Petyme, TEVA UK Ltd) 400 µg once daily] or a calcium channel blocker [typically nifedipine (Coracten®, UCB Pharma Ltd) 30 mg once daily] is prescribed to patients who, following assessment and diagnosis, can be treated by symptom management while awaiting spontaneous stone passage. 14,15 The results of most individual trials, and the summarised effects in the meta-analyses, suggest that these two drug classes have efficacy for the three desired outcomes of reduced pain, quicker stone passage and higher rate of eventual stone passage. However, the small sample size and the single-centre nature of the individual trials included limits, certainty and generalisability of the treatment effect, hence the need for a large, more pragmatic, trial such as SUSPEND. Current status of the evidence basis for use of MET is discussed later in this introduction.
Active treatment
Definitive removal of the stone can be achieved in two ways. Extracorporeal shockwave lithotripsy (ESWL) is an ambulatory treatment whereby acoustic waves that can be generated by a number of different energy sources are focused on the stone from outside the body. The physical characteristics of the stone allow it to be fragmented without significant damage to surrounding tissue. The treatment does require continued imaging of the stone by radiography or ultrasound, however, and the patient has to pass the fragments in the urine. Alternatively, stones can be directly visualised by passing a fine endoscope (ureteroscope) up from the bladder. The stone can then be extracted whole or fragmented in situ with a variety of energy sources and removed in pieces. This ureteroscopic technique gives greater certainty of stone removal but does involve a hospital admission and general anaesthetic. 16 Emergency drainage of an obstructed infected kidney can be achieved either by retrograde passage of a drainage tube (ureteric stent) up the ureter to the kidney from below or by direct insertion from above of a tube (nephrostomy) through the skin of the back into the urine collection part of the kidney.
Outcomes of interest
Cohort studies and observations from placebo or standard therapy groups of RCTs suggest that about 50–85% of people with ureteric colic will pass their stone unaided (spontaneous stone passage) within 4 weeks of diagnosis. Speedier passage of the stone would tend to reduce overall pain burden and thereby lessen the impact of any pain on the patient’s lifestyle, in terms of time off work and interference in day-to-day activities. From a clinical perspective, confirming stone passage is difficult. Patients are sometimes encouraged to sieve their urine (most stones are the size of a match head), but adherence is doubtful and small fragments can easily be missed. Simple imaging by plain single abdominal radiography or by ultrasound to confirm the absence of a stone has low diagnostic accuracy,17 whereas further definitive imaging by repeated CT KUB gives levels of radiation exposure that are generally considered to be unacceptable for this predominantly young patient group. 18 Clinical definition of stone passage is, therefore, the absence of pain or other relevant symptoms or signs. For the 30% of patients who continue to experience symptoms, further intervention (sometimes with MET if not previously used, but more usually with active stone removal by ESWL or ureteroscopy) will be required following repeat imaging. The urgency of any active intervention will depend on individual patient circumstances and resource availability in the particular health-care setting. There is an increased likelihood that active treatment will be required with larger stones (conventionally described as > 5 mm) and with stones located in the mid or upper ureter at the time of first presentation (Table 1). The need for further treatment is an important and routinely measurable outcome for both patients and providers of health care, as active intervention is associated with harm to patients and increased health-care costs. 13
Current evidence base for use of medical expulsive therapy
Background
Given that care for most patients with ureteric stones is delivered with the expectation of spontaneous stone passage, several strategies aimed at reducing pain, hastening stone passage and increasing the rate of stone passage have been proposed and trialled. Such strategies are termed MET. Agents that initially appeared to be useful but then failed to show efficacy in more robustly designed studies included diuretics and administration of high fluid load to increase urinary hydrostatic pressure above the stone;20 steroidal anti-inflammatory drugs and NSAIDs to reduce ureteric oedema and inflammation around the stone;21,22 and antimuscarinic drugs to inhibit ureteric muscular contraction. 23 The two drug classes that appear to show efficacy in repeated small-scale RCTs and subsequent meta-analysis are calcium channel antagonists and alpha-adrenoreceptor antagonists. 24
Experiments using animal and human tissue models demonstrate that ureteric smooth muscle contraction can be stimulated by activation of adrenergic receptors, particularly the alpha-1D subtype. 25–28 Blockade of alpha-1 receptors by specific pharmacological antagonists (alpha-blockers) such as doxazosin,29 terazosin,30 alfuzosin31 and, most typically, tamsulosin32,33 results in ureteric smooth muscle relaxation. It was hypothesised that this would translate into clinical benefit for people with ureteric colic. Smooth muscle contraction is stimulated in part by the influx of calcium ions into the smooth muscle cell through specific channels in the cell membrane, which are opened and closed by changes in the degree of electrical polarisation. These channels can be blocked by specific pharmacological antagonists, such as nifedipine, resulting in less calcium influx and reduced smooth muscle contraction. Experimental work demonstrating this effect in vitro encouraged translation to the clinical care of people with ureteric colic. 34–38
Pharmacological characteristics of putative agents
Tamsulosin is a readily available alpha-blocker which is licensed by the European Medicines Agency and the US Food and Drug Administration at a dose of 400 µg in the form of a modified-release (MR) oral tablet to be taken daily for relief of lower urinary tract symptoms in men. 39,40 It is generally well tolerated but is associated with common (0.1–1%) risks of dizziness and retrograde ejaculation in men. The calcium channel blocker nifedipine is available in the form of MR tablets or capsules in varying doses, ranging from 20 mg to 60 mg, with 30 mg being most often used. 40 The drug is licensed for the treatment of hypertension, Raynaud’s phenomenon and prophylaxis of angina, although it has been largely superseded for these indications by more specific and effective calcium channel antagonists. The common side effects (0.1–1%) include headache, dizziness, flushing, constipation and oedema of the lower limbs.
Evidence review for efficacy of tamsulosin and nifedipine as medical expulsive therapy
Multiple RCTs have been carried out testing the efficacy of both alpha-blockers (typically tamsulosin 400 µg) and calcium channel blockers (typically nifedipine 30 mg) compared with placebo, standard care, which may include other interventions, and each other. The medications are prescribed for use either up until the time of spontaneous stone passage or for up to one per month without stone passage. The conduct, quality and results of these trials have been examined by a number of systematic reviews and the results combined in meta-analyses, the findings of which concerning the main outcomes of interest will now be summarised.
Stone clearance
Comparison of the rate of spontaneous stone passage between MET and control is the primary outcome for the great majority of RCTs included in the meta-analyses. The absolute rates of stone passage are likely to vary according to trial eligibility criteria, such as type of diagnostic imaging used, stone location, stone size, the time point at which the outcome is censored and the protocol used to decide whether or not the stone has passed. Variation in these trial features is illustrated from 22 studies included in a Cochrane review14 that compared tamsulosin 400 µg with control (Table 2).
| Trial features | Option 1 (n) | Option 2 (n) | Option 3 (n) |
|---|---|---|---|
| Diagnostic imaging | CT KUB (4) | Any imaging (15) | Unclear (3) |
| Stone size | ≤ 10 mm (10) | 4–10 mm (4) | Other (8) |
| Stone location | Distal (20) | Proximal (1) | Any (1) |
| Follow-up duration | < 4 weeks (11) | 4 weeks (10) | > 4 weeks (1) |
| Follow-up assessment | CT KUB (3) | Imaging (10) | Unclear (9) |
| Primary outcome | Rate of stone passage (20) | Time to stone passage (2) |
Despite these inconsistencies in trial design, the available meta-analyses all demonstrate an apparent beneficial effect of both tamsulosin and nifedipine as agents to increase the proportion of patients with ureteric colic who spontaneously pass their stone within a reasonable time frame (Table 3).
| Review | Comparators | Stone clearance | |||
|---|---|---|---|---|---|
| Stone free | RR (95% CI) | Number of studies | Number of participants | ||
| Hollingsworth et al., 200624 | Tamsulosin vs. controla | 72% vs. 47% | 1.5 (1.2 to 1.9) | 4 | 222 |
| EAU/AUA, 200713 | Tamsulosin vs. control | NA | 29% (20% to 37%)b | 5 | 280 |
| Singh et al., 200741 | Alpha-blocker vs. control | 80% vs. 52% | 1.6 (1.4 to 1.8) | 16c | 1235 |
| Seitz et al., 200915 | Tamsulosin vs. control | 82% vs. 59% | 1.4 (1.3 to 1.6) | 10 | 816 |
| Campschroer et al., 201414 | Tamsulosin vs. placebo | 80% vs. 65% | 1.5 (1.3 to 1.7) | 6d | 629 |
| Lu et al., 201242 | Tamsulosin vs. standard caree | 75% vs. 50% | 1.4 (1.1 to 1.7) | 9 | 661 |
| Fan et al., 201343 | Tamsulosin vs. control | 80% vs. 52% | 1.5 (1.4 to 1.7) | 15 | 1593 |
| Campschroer et al., 201414 | Alpha-blocker vs. control | 78% vs. 49% | 1.6 (1.3 to 1.9) | 21f | 1565 |
| Campschroer et al., 201414 | Tamsulosin vs. standard | 77% vs. 52% | 1.5 (1.3 to 1.7) | 24 | 1875 |
| EAU/AUA, 200713 | Tamsulosin vs. nifedipine | NA | 20% (–7% to 45%)b | 2 | Not stated |
| Lu et al., 201242 | Tamsulosin vs. nifedipine | 84% vs. 73% | 1.2 (1.0 to 1.3) | 6 | 597 |
| Campschroer et al., 201414 | Tamsulosin vs. nifedipine | 94% vs. 73% | 1.2 (1.0 to 1.4) | 4 | 3486 |
| Hollingsworth et al., 200624 | Nifedipine vs. control | 71% vs. 47% | 1.5 (1.1 to 1.9) | 2 | 135 |
| EAU/AUA, 200713 | Nifedipine vs. control | NA | 9% (–7% to 25%)b | 4 | 160 |
| Singh et al., 200741 | Nifedipine vs. control | 78% vs. 52% | 1.5 (1.3 to 1.7) | 9 | 686 |
| Seitz et al., 200915 | Nifedipine vs. control | 79% vs. 53% | 1.5 (1.3 to 1.7) | 9 | 686 |
Regarding the effect of stone size, the meta-analysis by Seitz et al. 15 reported a relative risk (RR) in favour of tamsulosin versus control of 1.25 [95% confidence interval (CI) 1.12 to 1.40] for stones < 5 mm, and 1.62 (95% CI 1.50 to 1.74) for stones ≥ 5 mm. For nifedipine against control, the RR for stones < 5 mm was 1.49 (95% CI 1.17 to 1.88) and for stones ≥ 5 mm was 1.49 (95% CI 1.31 to 1.69). Similarly, Campschroer et al. 14 reported absolute rates of stone clearance for alpha-blocker against control for stones ≤ 5 mm of 83% versus 56%, with a RR of 1.4 (95% CI 1.2 to 1.7), and for stones > 5 mm of 67% versus 39%, with a RR of 1.7 (95% CI 1.3 to 2.1). Summarised results for the effect of stone location on clearance rates for tamsulosin against standard therapy were reported by Fan et al. ,43 with a RR of 1.55 for lower ureteral stones (95% CI 1.43 to 1.68) and a RR of 1.28 for upper ureteral stones (95% CI 1.04 to 1.57). Similarly, Campschroer et al. 14 reported absolute rates of stone clearance for alpha-blockers against control for stones in the lower (distal) ureter of 79% versus 55% (RR 1.4, 95% CI 1.2 to 1.6) and for stones in the mid or upper ureter of 39% versus 27% (RR 1.5, 95% CI 0.9 to 2.4). The results of these meta-analyses should be interpreted with caution given the uncertainties associated with estimating effect size in subgroups of the overall trial population. Overall, it does appear that these drugs demonstrate efficacy for passage of larger stones and for stones in the upper section of the ureter that are considered to be less likely to pass without active intervention. 8
Time to stone passage
Shorter duration of stone episode is likely to be associated with less pain and less social inconvenience, such as time off work, which may be of benefit to patients. Most RCTs examined time to stone passage as a secondary outcome, although the degree to which this was reported varied, thus making meta-analysis difficult. However, the direction of effect was consistent in favouring tamsulosin or nifedipine (Table 4). It should also be noted that censoring of exact time of passage is reliant on precise patient report or timing of follow-up imaging leading to reporting inaccuracy.
| Review | Comparators | Time to stone passage (days) | |||
|---|---|---|---|---|---|
| Absolute number of days | RR (95% CI) | Number of studies | Number of participants | ||
| Singh et al., 200741 | Tamsulosin vs. controla | 5 vs. 8 | – | 8 | 584 |
| Lu et al., 201242 | Tamsulosin vs. controla | – | –3 (–5 to –2) | 6 | 454 |
| Fan et al., 201343 | Tamsulosin vs. control | – | –3 (–5 to –3) | 7 | 555 |
| Campschroer et al., 201414 | Alpha-blocker vs. placebo | – | –2 (–4 to 1) | 4b | 293 |
| Campschroer et al., 201414 | Alpha-blocker vs. standardc | – | –3 (–4 to –1) | 18d | 1388 |
| Singh et al., 200741 | Nifedipine vs. controla | 8 vs. 13 | – | 9 | 686 |
Pain episodes/use of analgesia
Pain is likely to be the main reason for continued ill health in people with ureteric stones and it drives both transient quality-of-life detriment and the need for further intervention. However, it is difficult to measure in an ambulatory setting, particularly for conditions such as ureteric colic which are characterised by episodic pain of varying severity and frequency. The RCTs and subsequent meta-analyses reported this outcome, but the differences in definition make measurement of comparative effect uncertain (Table 5).
| Review | Comparators | Number of pain episodes | |||
|---|---|---|---|---|---|
| Absolute rate | RR (95% CI) | Number of studies | Number of participants | ||
| Lu et al., 201242 | Tamsulosin vs. controla | – | –0.4 (–0.7 to –0.1) | 8 | 633 |
| Fan et al., 201343 | Tamsulosin vs. controla | 24% vs. 40% | – | 4 | 326 |
| Campschroer et al., 201414 | Tamsulosin vs. placebo | – | –0.7 (–1.3 to –0.1) | 1 | 96 |
| Campschroer et al., 201414 | Tamsulosin vs. standardb | – | –0.5 (–1.0 to –0.0) | 6 | 555 |
Need for hospitalisation
The final main outcome of interest is the proportion of patients that require further active management, which will mainly consist of stone removal using ESWL or ureteroscopy. Within the RCTs and subsequent meta-analyses this is mainly reported as the rate of further hospital admissions for treatment of a ureteric stone (Table 6).
| Review | Comparators | Need for active intervention | |||
|---|---|---|---|---|---|
| Absolute rate | RR (95% CI) | Number of studies | Number of participants | ||
| Seitz et al., 200915 | Tamsulosin vs. controla | 5% vs. 25% | – | 5 | 480 |
| Fan et al., 201343 | Tamsulosin vs. controla | 12% vs. 34% | 0.4 (0.2 to 0.6) | 5 | 325 |
| Campschroer et al., 201414 | Alpha-blocker vs. standardb | 14% vs. 37% | 0.4 (0.1 to 1.0) | 4c | 313 |
| Seitz et al., 200915 | Nifedipine vs. controla | 20% vs. 34% | – | 1 | 140 |
Cost-effectiveness
A cost-minimisation study has been performed which assessed the potential health economic benefit of MET compared with observation alone. 44 A decision analytical model was used to predict comparative costs and resource use for a MET strategy using tamsulosin based on cost data obtained from the USA and four European countries, and efficacy data from existing meta-analyses. The costs of adverse events and other treatment-related complications were not included and a cost–utility analysis was not performed. The study found that use of tamsulosin for MET might lead to a saving of US$1132 over observation, with this conclusion being unchanged by sensitivity analyses. The cost-effectiveness of MET for different health-care systems remains unknown.
Summary
All seven meta-analyses using different selection criteria and reporting protocols appear to suggest that treatment with either tamsulosin or nifedipine at the time of presentation with acute ureteric colic increases the likelihood of eventual spontaneous stone passage, hastens the time to stone passage and reduces the risk of unwanted consequences such as pain and the need for an intervention to remove the stone. This leads the authors of these reviews to conclude that the balance of evidence supports routine use of these therapies, although with caveats regarding individual trial quality and trial size, with all but one trial having fewer than 100 participants in each group (Table 7). One large trial compared the use of tamsulosin (400 µg) against nifedipine (30 mg) as MET for stones sized 4–7 mm located in the very distal ureter (ureter course within the bladder wall) across 10 centres in China. 45 The trial randomised 3189 patients, and, at 4 weeks, the stone expulsion rate was 96% for tamsulosin and 74% for nifedipine with no further details given, although the Cochrane review14 gave a RR of 1.3 (95% CI 1.2 to 1.4) and found the trial to have low risk of bias. However, limited details given in the trial report makes quality assessment difficult; there was no sample size calculation to justify the large sample and diagnosis was by any imaging method, although follow-up and primary outcome assessment was by weekly CT KUB until stone passage or up to 4 weeks. Mean time to stone passage was given as 78.35 hours in the tamsulosin group and 137.93 hours in the nifedipine group. This duration of stone episode appears much shorter than that recorded in previous trials and may relate to the inclusion criteria and recruitment policies for this particular study. Additionally, the Jadad quality score was low (one) and the trial was supported by a manufacturer of tamsulosin.
| Review | Blinding | Overall quality | Ascertainment bias | Publication bias | Research recommendation | Recommendation for practice |
|---|---|---|---|---|---|---|
| Hollingsworth et al., 200624 | Majority unblinded | Not assessed | Mediterranean setting mainly | No | High-quality RCT | Viable option |
| Fan et al., 201343 | 27% low risk | Variable | Good | Unlikely | None | None |
| Lu et al., 201242 | Varied: potential source of bias | Varied | Varied | Yes | Large RCT | Tamsulosin recommended for distal stones of < 10 mm |
| Singh et al., 200741 | Majority unblinded | Poor | NA | No risk for nifedipine; small risk for tamsulosin | Large RCT | Promising but await large trial |
| Seitz et al., 200915 | Majority not blinded | NA | Varied imaging | Yes | Large study | MET for < 10 mm |
| Campschroer et al., 201414 | Noted lesser effect size in placebo studies | Variable | NA | Small risk | Large, multicentre RCT with CT for diagnosis | Alpha-blocker for distal < 10 mm |
The possibility that the conclusion from published meta-analyses regarding the benefits of MET is incorrect has to be borne in mind, as up to one-third of meta-analyses that show positive outcomes of a therapy are later altered by the inclusion of results from single, large, multicentre, robust, well-designed RCTs. 46 In the case of MET trials it is noted that use of less diagnostically accurate methods of imaging for participant inclusion prior to the widespread availability of CT KUB may lead to selection bias, because either smaller or radiolucent stones are missed, or people without a stone are included. Similarly, older, less accurate methods of imaging were widely used to decide if the stone had passed. This, together with lack of blinding in non-placebo-controlled studies, could lead to ascertainment bias in favour of the intervention group.
Need for a further trial and implications for design
The change of practice recommendations in some of the published meta-analyses has led to the adoption of MET for people with expectantly managed ureteric colic. The most widely used care guidance document gives a ‘Grade A’ recommendation for use of an alpha-blocker with follow-up within 14 days. 47 The extent of use of MET is hard to measure, but a survey of urologists in the USA suggests that 25% would recommend its use for stones in the mid and upper ureter and 32% would recommend its use for stones in the distal ureter. 48 The routine use of MET appears to be increasing, at least in the USA, with rates of 14% in 200949 rising to 64% in 2012. 50 In the UK, anecdotal discussion at the British Association of Urological Surgeons (BAUS), Section of Endourology, meetings suggests that use is also widespread in response to the EAU guideline recommendation. Despite this practice recommendation and widespread adoption of MET, the uncertainties expressed by most meta-analyses and associated suggestions of the need for a large RCT should be borne in mind. This is of particular importance as the use of ineffective treatment is both wasteful and potentially harmful.
Conclusions
Ureteric stone disease is a significant health problem in the UK and worldwide in terms of its impact on patients and the use of resources. A large proportion of patients with a ureteric stone will ultimately experience spontaneous stone passage. However, any drug treatment that facilitates and increases the chance of this (i.e. MET) will bring added benefit in terms of reduced pain, a reduced need for active intervention and a quicker return to normal activity. Provided the drug is effective and safe, the benefits of use will probably lead to cost savings for the NHS and society as a whole. The available evidence from meta-analyses of a high number of predominantly small, underpowered studies with a high degree of clinical and statistical heterogeneity suggest that MET with either tamsulosin or nifedipine may have some of those advantages over standard therapy of observation and supportive therapy, which has led to increasing adoption as part of routine care. However, as a result of significant uncertainties and knowledge gaps within the evidence base, it was clear that a large, multicentre, well-designed trial was required. From an effectiveness perspective, any trial would also need to measure the impact on pain burden, quality of life and cost-effectiveness from the perspective of the NHS.
Trial objectives
The aim of the SUSPEND trial was to determine the clinical effectiveness and cost-effectiveness of the use of tamsulosin and nifedipine in the management of people with symptomatic ureteric stones. The following question was addressed:
In patients with a symptomatic ureteric stone of ≤ 10 mm in maximum dimension, what is the clinical benefit and cost-effectiveness of using either tamsulosin 400 µg or nifedipine 30 mg once a day for up to 4 weeks over placebo?
In the context of a trial group receiving placebo, two pragmatic comparisons of equal importance were made in the evaluation of MET for facilitating ureteric stone passage:
-
tamsulosin 400 µg or nifedipine 30 mg once daily versus placebo
-
tamsulosin 400 µg once daily versus nifedipine 30 mg once daily.
The hypotheses being tested were:
-
The use of tamsulosin or nifedipine will result in an absolute increase in the spontaneous stone passage rate of at least 25% compared with placebo (from 50% to 75%).
-
The use of tamsulosin will result in an absolute increase of 10% in the spontaneous stone passage rate compared with nifedipine (from 75% to 85%).
Chapter 2 Trial design
The SUSPEND trial was a multicentre, randomised, placebo-controlled trial evaluating the benefit of two drugs as MET, the alpha-blocker tamsulosin and the calcium channel blocker nifedipine to increase stone clearance rate for UK NHS patients with symptomatic ureteric stones.
The trial protocol has been published by McClinton et al. 51
Participants
Potential participants were adults presenting as an emergency with a diagnosis of ureteric colic at UK NHS hospitals and identified according to the inclusion and exclusion criteria specified below.
Inclusion criteria
-
Patients presenting acutely with ureteric colic.
-
Patients aged ≥ 18 years to ≤ 65 years.
-
Presence of stone confirmed by non-contrast CT KUB.
-
Stone within any segment of the ureter.
-
Unilateral ureteric stone.
-
Largest dimension of the stone ≤ 10 mm.
-
Female participants who were willing to use two of the listed methods of contraception prior to taking any trial medication until at least 28 days after receiving the last dose of trial medication, who were post menopausal (defined as 12 months with no menses and without an alternative medical cause) or who had undergone permanent sterilisation. Acceptable forms of contraception for trial purposes included:
-
Established use of hormonal methods of contraception.
-
Placement of an intrauterine device or intrauterine system.
-
Barrier methods of contraception: condom or occlusive cap (diaphragm or cervical/vault caps) plus a spermicidal foam/gel/film/cream/suppository.
-
Male partner was sterile (with the appropriate post-vasectomy documentation of the absence of sperm in the ejaculate) prior to a woman partner starting the trial and was the sole partner of the female participant.
-
True abstinence: when this was in line with the preferred and usual lifestyle of the person willing to take part in the trial. Periodic abstinence (e.g. calendar, ovulation, symptothermal, post-ovulation methods) and withdrawal were not acceptable methods of contraception for trial purposes.
-
-
Capable of giving written informed consent, which includes compliance with the requirements of the trial.
Exclusion criteria
-
Women who have a known or suspected pregnancy (confirmed by a pregnancy test).
-
Women who are breastfeeding.
-
Women intending to become pregnant during anticipated period of participation in trial.
-
Asymptomatic incidentally found ureteric stone.
-
Stone not previously confirmed by CT KUB.
-
Stone with any one dimension of > 10 mm on CT KUB.
-
Kidney stone without the presence of ureteric stone.
-
Multiple (i.e. ≥ 2) stones within one ureter.
-
Bilateral ureteric stones.
-
Stone in a ureter draining an either anatomically or functionally solitary kidney.
-
Patients with abnormal upper urinary tract anatomy (such as a duplex system, horseshoe kidney or ileal conduit).
-
Presence of urinary sepsis.
-
Chronic kidney disease stage 4 or worse (estimated glomerular filtration rate of < 30 ml/minute).
-
Patients currently taking an alpha-blocker.
-
Patients currently taking a calcium channel blocker.
-
Patients currently taking a phosphodiesterase type 5 inhibitor.
-
Contraindication or allergy to tamsulosin or nifedipine.
-
Patients who are unable to understand or complete trial documentation.
Participants were randomised to one of the three trial groups on a 1 : 1 : 1 basis. The randomisation algorithm used trial centre (site), stone size (≤ 5 mm, > 5 mm) and stone location (upper, middle or lower ureter) as minimisation covariates. A remote telephone interactive voice response randomisation application hosted by the Centre for Healthcare Randomised Trials (CHaRT), Health Services Research Unit (HSRU) at the University of Aberdeen, UK, was used to perform randomisation.
The main criterion for selection of UK NHS hospital sites where participant recruitment could take place was a high rate (> 15 per month) of patient emergency admissions owing to ureteric colic. Information on admission rates was obtained from a national audit of ureteric stone management undertaken by the BAUS Section of Endourology in 2007 (see www.BAUS.org.uk). A total of 24 UK NHS sites took part in the trial (Figure 2). These were Aberdeen Royal Infirmary, Aberdeen; Addenbrooke’s Hospital, Cambridge; Bristol Royal Infirmary, Bristol; Broadgreen Hospital, Liverpool; Cheltenham General Hospital, Cheltenham; Derriford Hospital, Plymouth; Freeman Hospital, Newcastle upon Tyne; Guy’s Hospital, London; Manchester Royal Infirmary, Manchester; Morriston Hospital, Swansea; Norfolk and Norwich University Hospital, Norwich; Pinderfields Hospital, Wakefield; Queen Elizabeth Hospital, Birmingham; Raigmore Hospital, Inverness; Royal Hallamshire Hospital, Sheffield; Southampton General Hospital, Southampton; Southmead Hospital, Bristol; St George’s Hospital, London; St James’s University Hospital, Leeds; Sunderland Royal Hospital, Sunderland; The James Cook University Hospital, Middlesbrough; Torbay Hospital, Torquay; University Hospital of South Manchester, Manchester; and the Western General Hospital, Edinburgh.
FIGURE 2.
The UK location of the 24 SUSPEND trial sites.

Trial interventions
Two active treatments were being investigated and compared with a placebo group and each other:
-
tamsulosin hydrochloride 400 µg MR once daily up to a maximum of 28 days
-
nifedipine 30 mg MR once daily up to a maximum of 28 days
-
placebo [lactose-filled capsule (Tayside Pharmaceuticals)] once daily up to a maximum of 28 days.
Medication was overencapsulated to ensure that both participants and trial staff remained blinded to the identity of allocated medication.
Apart from allocated trial medication, all participants received the standard care for expectant management of people with ureteric colic. This included other medications to relieve symptoms, such as analgesics and antiemetics, advice on general measures, such as adequate fluid intake and resumption of normal activity, and appropriate review arrangements to detect stone passage, progressive symptoms or complications such as sepsis. No other adjunctive medications thought to promote stone passage, such as corticosteroids, are approved for use in the UK and clinical staff were asked to avoid use of such agents at site initiation visits.
Duration of interventions
Participants took one capsule of trial medication per day until stone passage occurred or further intervention was decided upon, up to a maximum of 28 days after randomisation.
Comparisons
Two pragmatic comparisons were made evaluating MET for facilitating ureteric stone passage:
-
tamsulosin (alpha-blocker) or nifedipine (calcium channel blocker) versus placebo
-
tamsulosin versus nifedipine.
Outcome measures
Primary outcome measures
Clinical effectiveness
The primary outcome was spontaneous passage of ureteric stones at 4 weeks after randomisation. This was defined as no further intervention planned or carried out to facilitate stone passage at up to 4 weeks. A further intervention was classified as any clinical record entry detailing actual interventions reported to have been carried out within 4 weeks or any further planned intervention. This information was reported on the 4- and 12-week case report form (CRF). Patients’ returned questionnaires at 4 weeks and 12 weeks were also assessed for additional interventions that may not have been captured by the CRF.
Health economic
The health economic outcome was incremental costs per quality-adjusted life-years (QALYs) gained at 12 weeks. QALYs were calculated from participant responses to the European Quality of Life-5 Dimensions questionnaire 3 level response (EQ-5D-3L™)52 completed at baseline, 4 weeks and 12 weeks post randomisation, and costs from use of primary and secondary NHS health-care services from the responses to the 12-week participant questionnaire and the 4- and 12-week CRFs.
Secondary outcome measures
Patient reported
Patient-reported secondary outcomes were:
-
severity of pain
-
health-related quality of life (HRQoL)
-
self-reported use of analgesics
-
time to stone passage
-
self-reported discontinuation of trial medication (and reasons).
Severity of pain related to the pain on the day of completion of the 4-week questionnaire as measured by the numeric rating scale (NRS) from 0 (no pain) to 10 (worst pain imaginable). 53,54 Analgesic use was measured using the self-reported number of days that pain medication was used up to the time of completion of the 4-week participant questionnaire. HRQoL was measured using the generic health profile measure Short Form questionnaire-36 items (SF-36),55 and the generic health status measure European Quality of Life-5 Dimensions (EQ-5D™),56 at baseline, 4 weeks and 12 weeks. Time to stone passage was derived from the 4-week CRF where there was passage of stone confirmed by imaging. Self-reported stone passage was not used to assess time to stone passage. Discontinuation of trial medication up to 4 weeks after randomisation was measured in the 4-week participant questionnaire.
Chapter 3 Methods
Research ethics and regulatory approvals
The SUSPEND trial was a clinical trial involving investigational medicinal products (CTIMPs). It was conducted under the European Union Clinical Trials Directive and was reviewed and approved by the UK Medicines and Healthcare products Regulatory Agency and allocated the EudraCT number 2010–019469–26. The trial was also given a favourable opinion prior to commencement by the East of Scotland Research Ethics Service Research Ethics Committee 2 (reference 10/S0501/31). It was approved by the sponsors (NHS Grampian and University of Aberdeen) and by the research and development departments of the NHS organisations at each participating site prior to trial commencement at each site. The trial was conducted in accordance with the principles of good clinical practice and was registered on the UK Clinical Research Network Portfolio (UKCRN Study ID 9184) and assigned an International Standard Randomised Clinical Trial number (ISRCTN69423238). Prior to starting recruitment at each site, a site initiation visit took place where central trial staff detailed and explained trial procedures to the local principal investigator and clinical research team, and provided a trial-specific site file.
Participants
Trial flow
The flow of participants through the trial is detailed in Figure 3.
FIGURE 3.
Flow of participants through the trial. GP, general practitioner.

Identification of patients (screening)
Patients were identified by clinicians working in the urology or accident and emergency departments of participating sites, who were supported by local clinical research teams. Approved trial publicity material in the form of posters was used to help alert staff that the trial was taking place at specific sites.
Recruitment process
Clinicians assessed patients presenting with suspected ureteric calculi in accordance with standard practice. A screening log was completed and included all patients assessed at participating sites to document the reasons for inclusion or non-inclusion in the trial. Following adequate pain relief and confirmation of a single ureteric stone by CT KUB, identified eligible patients were given a patient information leaflet (PIL; see Appendix 1) to inform them of the purpose and need for the trial as well as the uncertainties around the clinical usefulness of MET. The PIL was developed in conjunction with the BAUS Section of Endourology Patient Group. Following receipt of the PIL, a member of the local research team asked the patient if they were interested in the trial and ensured any questions that the patients had were answered appropriately. Further checking against eligibility criteria, particularly around the use of tamsulosin and nifedipine as MET, was performed by local research staff. When a patient was eligible and happy to take part in the trial they were asked to sign a trial consent form (see Appendix 2).
Randomisation and intervention allocation
Eligible and consenting participants were allocated using minimisation to one of the two intervention groups or the placebo group on a 1 : 1 : 1 basis using the telephone interactive voice response randomisation application hosted by the CHaRT, HSRU, at the University of Aberdeen. The minimisation algorithm used the trial centre (site), stone size (≤ 5 mm, > 5 mm) and stone location (upper, middle, lower ureter) as covariates.
Blinding
At randomisation, the participant was allocated a unique participant study number and assigned a numbered participant pack. The packs were provided by an independent supplier containing the overencapsulated trial medication to ensure that the participant, local investigator and trial personnel remained blinded to treatment allocation.
Unblinding
The treatment code was broken only in the case of a serious adverse event (SAE), when it was necessary for the principal investigator at site or treating health-care professional to know which intervention the participant was receiving to determine a management plan.
Each participant was given a card to carry with details of a contact telephone number at the site to be used in the event that unblinding was necessary. Contact information was also available in the participant’s hospital records. If unblinding was necessary, a member of the research team or a member of clinical staff at the local recruiting site telephoned the dedicated randomisation service at the CHaRT in Aberdeen on the number provided using the trial centre identification and the participant study number. In the unlikely event of the randomisation service not being able to field the query, the on-call pharmacist at Aberdeen Royal Infirmary was contacted and the same procedure followed.
Following any unblinding via the telephone randomisation service, automatic e-mails were sent to the chief investigator, trial manager and members of the CHaRT management team. If an on-call pharmacist performed the unblinding they would e-mail the same list of people. These e-mails did not contain the treatment code, and the trial team remained blinded as far as was practicable. The chief investigator then ascertained why unblinding had taken place. If the patient was unblinded because of a SAE this was then reported.
Interventions
The trial interventions were:
-
tamsulosin hydrochloride in the form of 400-µg MR capsules
-
nifedipine in the form of 30-mg MR capsules
-
placebo (lactose-filled capsules).
A summary of product characteristics for each of the investigational medicinal products (tamsulosin hydrochloride and nifedipine) used in the trial is included in Appendix 3.
All the medicinal products were overencapsulated to maintain the blinding of the trial. Trial medication was presented as capsules in amber plastic containers with a childproof closure and labelled in accordance with Annex 13 of Volume 4 of The Rules Governing Medicinal Products in the European Union: Good Manufacturing Practices. 57 All disguised drug packs were stored at site pharmacies under temperature-controlled conditions until dispensed to participants. The medicinal products and the placebo were overencapsulated, packaged and labelled by Tayside Pharmaceuticals, Ninewells Hospital, Dundee, UK, in accordance with Good Manufacturing Practice.
Participants were instructed to store the medication in accordance with the manufacturer’s instructions. Unused medication and/or empty packaging were returned to the site by the participant at the 4-week follow-up visit or returned directly to the pharmacy; alternatively, if participants did not attend the 4-week visit, they were instructed to dispose of surplus trial medication appropriately.
Data collection
Questionnaires were designed to obtain information on stone passage or further intervention, pain, HRQoL and resource use, including NHS and personal costs. Participants were asked to complete trial questionnaires at baseline, 4 weeks post randomisation and 12 weeks post randomisation. The baseline questionnaire was completed in hospital before randomisation. Further questionnaires were sent to each participant by post from the trial office (CHaRT, Aberdeen) with pre-paid envelopes at 4 weeks and 12 weeks post randomisation (see Appendix 4). If a participant did not return the questionnaire a reminder letter was sent out approximately 2 weeks later with a short form of the questionnaire containing the EQ-5D only (see Appendix 4).
In addition, CRFs were completed by the research team at the recruiting site at baseline and at the follow-up visit 4 weeks after randomisation (see Appendix 5). If the participant did not attend the follow-up visit, the CRF was completed from the participant’s health-care records. If participants indicated on their 12-week questionnaire that they had received further intervention for their stone since their 4-week questionnaire, or if they completed only a short form of the questionnaire, or if no 12-week questionnaire was returned, a further CRF was completed 12 weeks post randomisation from their health-care records (see Appendix 5). The outcome measures collected and their timings of measurement are described in Table 8.
| Outcome measure | Source | Timing | ||
|---|---|---|---|---|
| Recruitment | 4 weeks post randomisation | 12 weeks post randomisation | ||
| Further intervention planned | CRF | ✓ | ✓ | |
| Pain (NRS) | PQ | ✓ | ✓ | |
| Use of analgesics | PQ | ✓ | ||
| Further interventions received | PQ and/or CRF | ✓ | ✓ | |
| Health status: SF-36 and EQ-5D | PQ | ✓ | ✓ | ✓ |
| Adverse events | PQ | ✓ | ✓ | |
| Time to passage of stone | PQ and CRF | ✓ | ✓ | |
| NHS primary and secondary health-care use | PQ and CRF | ✓ | ✓ | |
| Participant personal costs | PQ | ✓ | ||
Safety reporting
Non-serious adverse events were not collected or reported. Planned hospital visits for conditions other than those associated with the ureteric stone were not collected or reported. Hospital admissions (planned or unplanned) associated with the treatment of the ureteric stone diagnosed at the time of entry to the trial were expected to occur for a proportion of participants. These were recorded as an outcome measure, but were not recorded or reported as SAEs.
All suspected SAEs were assessed in respect of severity, potential relationship to trial medication and whether they were expected or unexpected. Confirmed SAEs were reported to the Trial Office and then to the chief investigator and sponsor, who subsequently provided their assessment and action plan.
Participants who had left hospital were advised to contact their general practitioner (GP) should they experience an adverse event. This is current standard clinical practice for participants receiving tamsulosin or nifedipine within the NHS. As part of their notification that one of their patients was participating in the trial, GPs were asked to inform the trial office of any SAEs or reactions. This provided a robust system for the notification of any serious adverse reactions or SAEs occurring outside hospital research sites.
Change of status/withdrawal
The trial status of some participants changed during the trial for a number of reasons. These included post-randomisation exclusion, participant withdrawal and medical withdrawal. Participants were free to withdraw from the trial at any time without giving a reason. If a participant withdrew from receiving the trial questionnaires, permission was sought for the research team to continue to collect outcome data from their hospital records. In the event that a participant was told to stop taking trial medication by clinical or trial staff for any reason, the participant continued in the trial and was asked to complete the trial documents unless he or she did not wish to do so.
Data management
Clinical data were entered into the electronic SUSPEND database through the trial web portal (https://viis.abdn.ac.uk/HSRU/suspend/), together with data from participant questionnaires, by the research team working at each hospital site. Questionnaires returned by post to the trial office were entered into the database by the central research team. Staff in the trial office worked closely with the local research teams to ensure that the data were complete and accurate. All trial staff and the statistician responsible for analysing the data remained blinded to allocation until completion of the trial and locking of the database.
Data collected during the course of the research were kept strictly confidential and accessed only by members of the trial team. Participants’ details were stored on a secure database under the guidelines of the Data Protection Act 1998, including encryption of any identifiable data. 58 Participants were allocated an individual specific trial number and all data, other than personal data, were identified only by this unique study number.
A random 10% sample of all trial data was generated by the database for re-entry by the trial office to validate correct data entry input. Any discrepancies between original data entry and the re-entered data were reviewed against the original paper copy and incorrect entries corrected accordingly. An initial data entry error rate of > 5% would have triggered a requirement to re-enter the entire data set from that questionnaire. This was not found to be necessary.
Trial oversight committees
The trial Data Monitoring Committee (DMC) comprised three independent individuals who met initially at the beginning of the trial when terms of reference and other committee procedures were agreed. The DMC then met a subsequent four times during the course of the trial to monitor unblinded trial baseline and outcome data provided by the trial statistician and details of SAEs. The DMC reported any recommendations to the chairperson of the Trial Steering Committee (TSC).
The TSC was chaired by a clinician independent from the trial and consisted of two other independent members as well as the grant holders. The TSC met five times over the duration of the trial.
Patient and public involvement
A patient representative was involved in the study design and conduct, with input into production of the PIL and other trial documentation, and membership of both the trial management group and the TSC. The patient representative contributed to, and reviewed, the trial protocol and final report. Additionally, the PIL for the trial (see Appendix 1) was developed in conjunction with the BAUS Section of Endourology Patient Group.
Important changes to methods after trial commencement
Serious adverse events
During the initial stages of the trial, a number of SAEs were reported and recorded which, on investigation, were found to be a result of readmissions for continuing treatment of the participant’s ureteric stone (i.e. the primary outcome). These were, therefore, being recorded as a SAE as well as an outcome. To ensure that such events were recorded only as an outcome, the wording regarding the collection of these events was clarified to state:
Hospital admissions (planned or unplanned) associated with the treatment of the ureteric stone diagnosed at the time of entry to the trial are expected. These will be recorded as an outcome measure, but will not be recorded or reported as serious adverse events.
Strategies to improve questionnaire return rate
A number of strategies were implemented during the trial to improve participant questionnaire return rate. A substudy investigating the use of text message notification to participants to inform them that their questionnaire would arrive shortly, combined with e-mail delivery of questionnaires with a link to complete an online version, did not affect response rate. A short version of the 4- and 12-week questionnaires designed to collect the information needed for the primary outcomes of the trial was sent instead of the full questionnaire as a reminder to encourage completion. 59 This did not have any effect on response rate.
A Cochrane review on strategies to improve retention in RCTs found monetary incentives to be one of the few approaches to be effective in increasing response rates to participant questionnaires. 60 Part-way through the trial, a £5 high-street voucher was sent out with the 12-week questionnaire to encourage response. This appeared effective, in that response rate increased from 46% to 57%, but influence from other confounders cannot be ruled out.
Statistical methods and trial analysis
Sample size and power calculation
We combined the data from two meta-analyses,24,41 which suggested a RR of approximately 1.50 comparing MET (either alpha-blocker or calcium channel blocker) against ‘standard care’ as the primary outcome. These reviews indicated that the percentage of spontaneous stone passage was approximately 50% in control groups of included RCTs. Only three of the included RCTs directly compared a calcium channel blocker and an alpha-blocker, and these suggested that alpha-blockers were potentially superior to calcium channel blockers. From an analysis of data from Singh et al. 41 and Hollingsworth et al. ,24 we estimated that proportions of stone passage in the alpha-blocker and calcium channel blocker groups were approximately 85% and 75%, respectively. The most conservative sample size was required to detect superiority between the two active treatments, and the trial was powered on this basis. To detect an increase of 10% in the primary outcome (spontaneous stone passage) from 75% in the calcium channel blocker group to 85% in the alpha-blocker group, with type I error rates of 5% and 90% power, required 354 participants per group (1062 in total); adjusting for 10% loss to follow-up inflated this to 400 per group. We aimed to recruit 1200 participants (randomising 400 to each of the three treatment groups: alpha-blocker, calcium channel blocker and placebo) to provide sufficient power (> 90%) for all other comparisons of interest, and allowing for an anticipated 10% loss to follow-up.
General methods
Treatment groups were described at baseline and follow-up using means [with standard deviations (SDs)], medians (with interquartile ranges) and numbers (with percentages) where relevant. Primary and secondary outcomes were compared using generalised linear models. Treatment effects were estimated from unadjusted and adjusted models. Adjusted models included the trial centre (random effect), stone size (≤ 5 mm, > 5 mm) and stone location (upper, middle or lower ureter). All estimates of treatment effect are presented with 95% CIs. The analysis strategy was by allocated group (intention to treat). Two a priori comparisons were considered for the primary trial analysis:
-
MET [an alpha-blocker (tamsulosin) or a calcium channel blocker (nifedipine)] versus placebo
-
an alpha-blocker (tamsulosin) versus a calcium channel blocker (nifedipine).
We also made two post-hoc comparisons between tamsulosin and placebo, and nifedipine versus placebo. All analyses were carried out using Stata® 13 (StataCorp LP, College Station, TX, USA).
Primary outcome
The primary outcome was analysed using logistic regression. We summarised treatment effects as odds ratios (ORs) and absolute percentage differences, from both adjusted and unadjusted models and presented with 95% CIs. Subgroup analyses (appropriately analysed by testing treatment by subgroup interaction) explored the possible effect modification of stone size (≤ 5 mm or > 5 mm to 10 mm), location in ureter, (upper, mid or lower) and sex, all using stricter levels of statistical significance (99% CIs; p-value < 0.01). 61 Subgroup analyses were also summarised visually using forest plots. 62 There was no correction for multiple testing. 63 During the planning of the SUSPEND trial it was anticipated that there would be few or no missing primary outcome data (owing to the algorithm specifying the primary outcome) and the primary outcome was analysed using complete-case analysis. The pragmatic nature of the trial made assessing the adherence unreliable, and no attempt was made to incorporate any analysis of treatment received.
Secondary outcomes
The secondary outcomes were analysed in a similar manner to the primary outcome using the appropriate link functions. Quality-of-life data were analysed using a mixed model that allowed treatment effects to vary at each time point.
Timings and frequency of analysis
The DMC considered interim inspection of the data on four occasions during the trial. The committee met to review and consider data on outcome measures and SAEs after randomisation of 300, 600 and 900 participants had occurred. Having seen and considered these data, the DMC did not make any recommendations to alter the progression of the trial on any of the occasions on which they met.
Algorithm for primary outcome
The primary clinical outcome is spontaneous passage of ureteric stones at 4 weeks (defined as no further intervention required to facilitate stone passage at up to 4 weeks). The algorithm to create this outcome can be found in Appendix 6.
Missing data
Baseline data were collected prior to randomisation. Where baseline data were missing, no imputation was undertaken for the reporting of the baseline covariates of the trial cohort. If there were missing data for covariates that were used in the analyses of the trial outcomes, single imputation was performed using the guidelines set out in White and Thompson64 (i.e. centre-specific means for continuous variables and an indicator for categorical variables). It was anticipated that the nature of the clinical condition and the algorithm to generate the final outcome would result in few cases of missing primary outcome data and, as such, no plan was made to impute missing primary outcome data. Participants with missing primary outcome data were excluded from analysis of the primary outcome. For other outcomes, participants were included where they provided data under a missing-at-random assumption. Sensitivity analyses were planned to follow guidelines laid out by White et al. 65 to assess the impact of any missing outcome data on quality-of-life data from patient questionnaires. The analysis of quality-of-life outcomes was repeated using multiple imputation models with predictions based on all baseline covariates collected. Results were combined across 10 imputed data sets. The robustness of the results was then tested using pattern mixture models, which imputed missing data across a range of potential values from minus half of the observed SD to plus half of the SD of the outcome being analysed.
Economic methods
Introduction
Given that the condition under study was anticipated to be a short-term resolving problem for patients and the NHS, the main planned economic analysis was a ‘within-trial’ economic evaluation using data collected during the 12 weeks of individual participation. The question addressed was: ‘What is the cost-effectiveness of medical expulsive therapy using either tamsulosin or nifedipine compared to no treatment (placebo)?’ The trial was set within the perspective of the NHS, although it included both the NHS costs as well as those health-care costs falling on the participants.
Measurement of resource utilisation
Resource use and costs were estimated for each participant. Resource data collected included the costs of the intervention drugs and simultaneous and consequent use of primary and secondary NHS services by participants. Personal health-care costs, such as purchase of medication, were also estimated.
At recruitment, data were collected on the intervention that the participants received, the diagnostic tests conducted and the medications prescribed at the admission. At 12 weeks post randomisation, participants were asked to provide information by questionnaire of their primary and secondary health-care service use. They were asked for details of medications purchased, the cost of these and whether or not they had any visits to non-NHS health-care providers.
The consequential use of health services was recorded prospectively for each participant in the trial. Resource utilisation data were based on responses to the participant questionnaires and the CRFs completed by the local research teams. The CRFs recorded information on non-protocol visits (protocol visits are those scheduled for the purposes of data collection), outpatient visits and readmissions relating to the use and consequences of drug treatment. Use of primary care services, such as prescription medications, and contacts with primary care practitioners (e.g. GPs and practice nurses) were collected via the health-care utilisation questions administered in the participant 12-week questionnaire. Details of the sources used to estimate resource utilisation are included in Table 9.
| Resource | Relevant variable | Source | Reported outcome |
|---|---|---|---|
| Intervention | Drug (e.g. tamsulosin) | CRFa | Number |
| Diagnostic tests | CRFa | Number | |
| Analgesic/antibiotics | CRFa | Number | |
| Primary care visits | GP doctor visits | PQ | Number |
| GP nurse visits | PQ | Number | |
| Secondary care | Outpatient visit | CRFa and PQ | Number |
| Active further intervention (e.g. insertion of stent) | CRFa and PQ | Number | |
| Admissions days | CRFa and PQ | Number |
Identification of unit costs
Unit costs were obtained from published sources such as the British National Formulary (BNF)40 and NHS reference costs. 66 The unit cost data source year was 2012–13 and the currency was British pounds.
The cost of the trial intervention included the cost of the drug to which the participants were allocated, the costs of diagnostic tests performed to confirm ureteric stone and the cost of the medications or antibiotics prescribed at diagnosis. The unit costs of medications were obtained from the BNF40 and the diagnostic tests costs were obtained from NHS reference costs. 66 The unit costs of medicines given on admission were assumed to be those of the most commonly used drugs. The unit cost of NSAIDs was that of diclofenac (50 mg) given as a tablet, and the cost of opiates was based on the cost of morphine (10-mg injection). Antibiotic costs were based on the unit cost of a 3-day course of ciprofloxacin (500 mg). The initial secondary care attendances prior to and at recruitment were not included as costs because they were considered to be the same across all trial groups. The unit cost for the diagnostic test received was based on the average NHS reference cost for a computerised tomography (CT) scan ordered by the urology department.
The costs of subsequent resource use comprised costs to both the primary (GP appointments) and secondary (outpatient appointments and admissions) NHS care services. Unit costs for GP visits were obtained from the Personal Social Services Research Unit costs of primary care. 67 Outpatient visit unit cost was based on the average NHS tariff for a urology department consultant-led outpatient appointment obtained from the reference costs. 66 A summary of the unit costs of the resources used is provided in Table 10.
| Resource | Unit cost | Source | Notes |
|---|---|---|---|
| Drug | |||
| Alpha-blocker (tamsulosin) | £4.76 | BNF40 | The 28-day cost of the non-proprietary tamsulosin hydrochloride, daily cost of £0.17 |
| Calcium channel blocker (nifedipine) | £6.95 | BNF40 | The 28-day cost of the non-proprietary nifedipine, daily cost of £0.25 |
| Diagnostic test | £60.00 | Reference costs66 | RA08A CT scan, one area, no contrast, 19 years and over, diagnostic imaging: direct accessa |
| Analgesia | |||
| NSAID | £2.49 | BNF40 | Based on cost of diclofenac (Voltarol®, Novartis) |
| Opiate | £2.60 | BNF40 | Based on cost of morphine injection |
| Other | £2.55 | Average of above 2 | |
| Antibiotic used | £0.80 | BNF40 | Based on most frequently administered antibiotic (ciprofloxacin, non-proprietary) |
| Cost additional day in hospital | £264.00 | Reference costs66 | LB05G intermediate percutaneous kidney or ureter procedures, 19 years and over, with CC score of 0 [non-elective inpatient (long-stay) excess bed-days] |
| Percutaneous insertion of nephrostomy tube | £1207.00 | Reference costs66 | LB09D intermediate endoscopic ureter procedures, 19 years and over [non-elective inpatients (short stay)] |
| £691.00 | Day case | ||
| Antegrade insertion of stent into ureter | £647.00 | Reference costs66 | LB05G intermediate percutaneous kidney or ureter procedures, 19 years and over, with CC score of 0–2 [non-elective inpatients (short stay)] |
| £566.00 | Day case | ||
| Therapeutic ureteroscopic operations | £1458.00 | Reference costs66 | LB65E major endoscopic kidney or ureter procedures, 19 years and over with CC score 0–2 [non-elective inpatients (short stay)] |
| £1434.00 | Day case | ||
| Endoscopic insertion/removal of stent into ureter | £524.00 | Reference costs66 | LB72A diagnostic flexible cystoscopy, 19 years and over [non-elective inpatients (short stay)] |
| £402.00 | Day case | ||
| ESWL of calculus in ureter | £775.00 | Reference costs66 | LB36Z ESWL [non-elective inpatients (short stay)] |
| £504.00 | Day case | ||
| Hospital admission without procedure | £470.00 | Reference costs66 | LB40G urinary tract stone disease without interventions, with CC score of 0–2 [non-elective inpatients (short stay)] |
| £456.00 | Day case | ||
| More than one intervention | £1719.00 | Reference costs66 | LB40D urinary tract stone disease with interventions, with CC score of 0–2 [non-elective inpatients (short stay)] |
| Outpatient visit | £101.00 | Reference costs66 | Based on the average unit cost of outpatient attendances (both consultant- and non-consultant-led) to urology department |
| Practice nurse visit | £15.50 | Based on cost per consultation | |
| GP visit | £44.46 | Per surgery consultation lasting 11.7 minutes | |
| Cost of visit to other health-care professionals | As indicated by participant | Based on information on PQ | |
| Medications | As indicated by participant | Based on information on PQ | |
| Visits to non-NHS providers | As indicated by participant | Based on information on PQ | |
Unit costs of further active intervention for the ureteric stone were derived from costs associated with different urology Healthcare Resource Group codes as detailed in Table 10. For occasions when a participant received two interventions on the same day, unit cost use was defined as the average cost of treatment of urinary tract stone disease with intervention. For those that had an admission with no intervention, the cost of urinary stone disease without intervention was used. As the median stay in the urology department was 1 day, any extra admissions days were costed using the long-stay excess days tariff. 66
The participant resource use data and unit cost were combined for each of the primary and secondary NHS care services to give an estimate of the total health-care cost per participant, as well as the average cost for each identified resource and the average total cost for each group of the trial.
Participant costs
Participant costs comprised self-purchased health care, such as prescription costs (for participants who pay prescription charges), over-the-counter medications and visits to non-NHS health-care providers. Information about participant resource use was collected using the 12-week health-care utilisation questionnaire (see Appendix 4).
Health status
Health-related quality-of-life measures were collected at baseline, 4 weeks and 12 weeks by participant completion of the EQ-5D and the SF-36 questionnaires. The EQ-5D divides health status into five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Each of these dimensions has three levels, so 243 possible health states exist. Responses on the participants’ EQ-5D questionnaires were transformed using a standard algorithm to produce a health-state utility at each time point for each participant. The utility scores obtained at baseline, 4 weeks and 12 weeks were used to estimate the mean QALY score for each group52 over the 12-week (approximately 0.25 years) period of observation.
Responses from the SF-36 questionnaire were also used as the basis of QALYs as a sensitivity analysis to validate the EQ-5D scores. They were mapped onto the existing Short Form questionnare-6 Dimensions (SF-6D) measure using a standard algorithm68 to allow utility values to be estimated for each time point. These utility scores were transformed to QALYs using the methods described above to provide an alternative measure of QALYs for each participant.
Data analysis
Resource use, cost and QALY data were summarised and analysed using Stata 13. As data were collected over a short (12-week) period, discounting was not carried out. The main cost-effectiveness analysis reports the results of participants with complete data. All the difference estimates are presented with 95% CIs. Data reported as mean costs for both active treatment groups and the placebo group were derived for each item of resource use and then compared using unpaired Student’s t-test and linear regression adjusted for baseline values. The mean incremental costs were estimated using general linear models, with adjustment for minimisation variables [centre at which participant was recruited, stone size (≤ 5 mm, > 5 mm), stone location at diagnosis (lower, mid or upper ureter) and sex]. The general linear model allowed for heteroscedasticity by specifying a distributional family which reflects the relationship between mean and variance. 69 A modified Park’s test was conducted to identify the appropriate family, which identified a gamma family. This allows for the skewness of cost data and assumes that variance is proportional to the square of the means as appropriate. A link function needs to be identified for the general linear model to specify the relationship between the set of regressors and the conditional mean. The link test recognised the identity link as the appropriate link function. The identity link leaves the interpretation of the coefficients unchanged from that of the ordinary least squares, as the covariates act additively to the mean. The mean incremental QALYs were estimated using ordinary least squares and were adjusted for minimisation factors, as well as for the baseline EQ-5D score.
Incremental cost-effectiveness
Cost-effectiveness of the trial interventions from the perspective of the NHS during the period of observation was measured in terms of the number of participants needing further treatment within 12 weeks, and in terms of QALYs accrued by participants in each group at 12 weeks. The results are presented as point estimates of mean incremental costs, number of further treatments needed, QALYs, incremental cost per further treatment needed and incremental cost per QALY. Measures of variance for these outcomes required bootstrapping of the point estimates. Incremental cost-effectiveness data are presented by cost-effectiveness acceptability curves (CEACs). Forms of uncertainty (e.g. concerning the unit cost of a resource from the different centres) are addressed using deterministic sensitivity analysis. As the data were not normally distributed, non-parametric bootstrapping was used to generate 1000 estimates of mean costs and QALYs for each treatment group. CEACs were generated using these 1000 estimates, using the net monetary benefit (NMB) approach. The NMB associated with a given treatment option is given by the formula:
where effects are measured in QALYs and Rc is the ceiling ratio of willingness to pay (WTP) per QALY. Using this formula, the strategy with greatest NMB is identified for each of the 1000 bootstrapped replicates of the analysis, for different ceiling ratios of WTP per QALY. Plotting the proportion of bootstrap iterations favouring each treatment option (in terms of the NMB) against increasing WTP per QALY produces the CEAC for each treatment option. These curves graphically present the probability of each treatment strategy being considered optimal at different levels of WTP per QALY gained.
The degree of missing data for the variables used in the derivation of costs was very low, and the data that were missing were considered to be missing completely at random. However, the number of participants with completely missing data for EQ-5D scores at 4 weeks and 12 weeks used for the derivation of QALYs was high (available data: tamsulosin group = 164/383; nifedipine group = 165/383; and placebo group = 157/384). Several methods of imputation were used as described in the sensitivity analysis.
Sensitivity analysis
There are elements of uncertainty owing to the lack of available information; therefore, various sensitivity analyses were conducted to explore the importance of such uncertainties. One-way sensitivity analyses using extreme values were performed around the QALY estimates. As the base-case analyses were performed using participants with complete cases, multiple imputation was carried out using chained equations in Stata 13 to replace missing cost and EQ-5D variables with a plausible value in 20 imputed data sets.
There was uncertainty around the QALY estimates as they were derived using the EQ-5D. There was some uncertainty over whether or not the dimensions in the EQ-5D are sensitive enough to capture the loss in quality of life, particularly in reference to acute pain. Therefore, SF-36 responses were mapped on the SF-6D measure using the algorithm by Brazier et al. 68 to validate the estimate of utility value for each time point derived from the EQ-5D. These scores were used in the same way as the EQ-5D to provide an alternative measure of QALYs for each participant.
A modelling exercise had been planned to extrapolate the estimates of the cost–utility analysis to a longer time horizon than that considered by the trial. However, the decision was taken not to perform any further analysis as the trial data suggested that there were very few cases that had not resolved by the end of the 12-week trial period and there was no chance of recurrence of the same stone.
Chapter 4 Participant baseline characteristics
Trial recruitment
In total, 1167 patients presenting to 24 UK NHS hospitals for emergency treatment of ureteric colic were randomised during the 35 months between January 2011 and December 2013, and followed up to March 2014. The trajectory of recruitment from all sites during the course of the trial is shown in Figure 4, and Table 11 lists the recruiting sites and individual recruitment duration, total recruitment and average recruitment rate per month.
FIGURE 4.
SUSPEND recruitment over time.
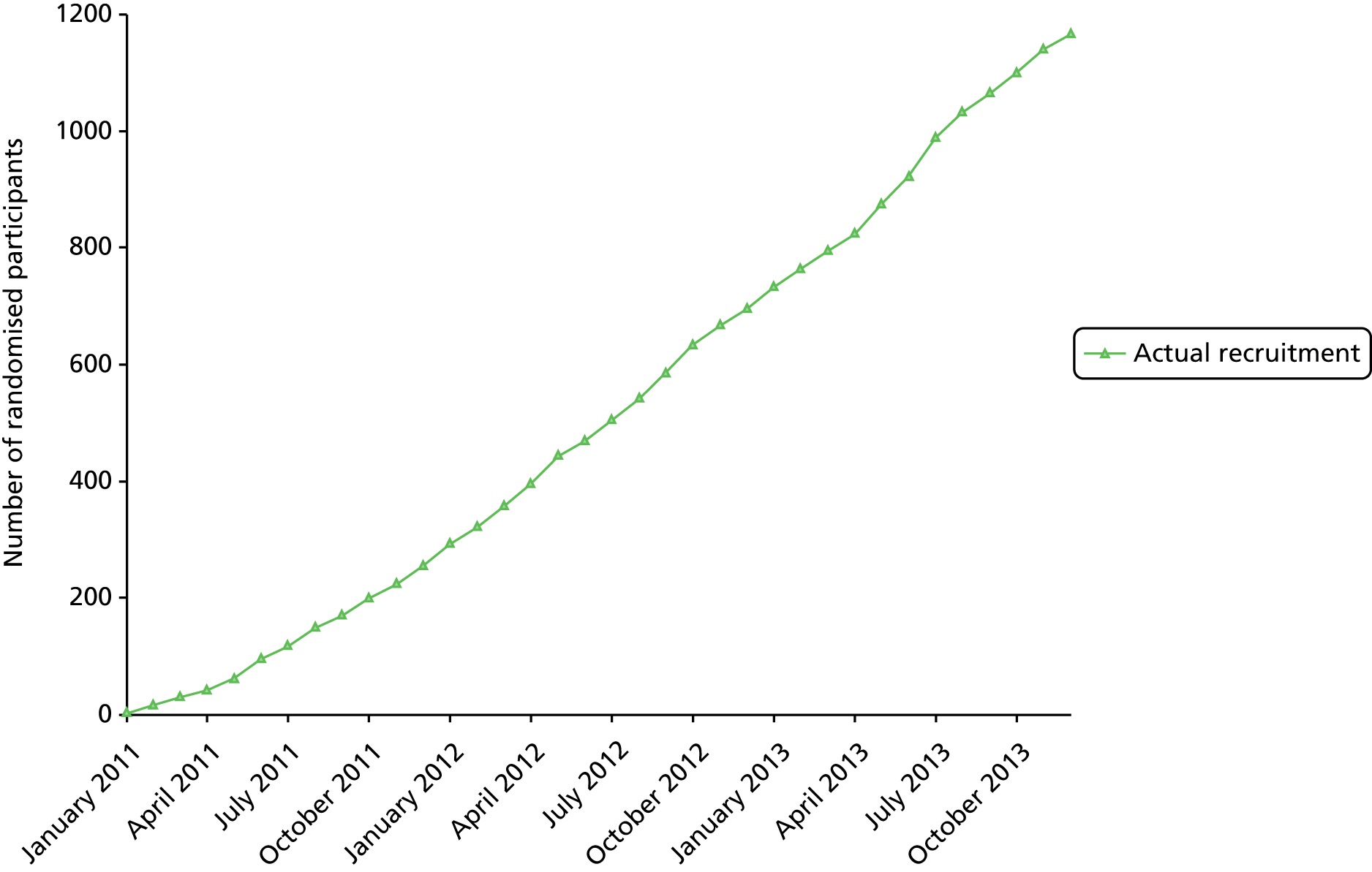
| Recruiting site | Number randomised | Percentage of total recruitment (n = 1167) | Number of recruiting months | Average number recruited per month |
|---|---|---|---|---|
| Freeman Hospital, Newcastle | 211 | 18 | 36 | 5.86 |
| Pinderfields General Hospital, Wakefield | 154 | 13 | 32 | 4.81 |
| Southmead Hospital, Bristol | 109 | 9 | 24 | 4.54 |
| Norfolk and Norwich University Hospital, Norwich | 86 | 7 | 32 | 2.69 |
| Cheltenham General Hospital, Cheltenham | 24 | 2 | 9 | 2.67 |
| Morriston Hospital, Swansea | 75 | 6 | 33 | 2.27 |
| Wythenshawe Hospital, Manchester | 36 | 3 | 17 | 2.12 |
| Aberdeen Royal Infirmary, Aberdeen | 75 | 6 | 36 | 2.08 |
| St George’s Hospital, London | 56 | 5 | 31 | 1.81 |
| St James’ University Hospital, Leeds | 25 | 2 | 14 | 1.79 |
| Addenbrooks Hospital, Cambridge | 35 | 3 | 21 | 1.67 |
| Royal Hallamshire Hospital, Sheffield | 41 | 4 | 25 | 1.64 |
| The James Cook University Hospital, Middlesbrough | 46 | 4 | 29 | 1.59 |
| Derriford Hospital, Plymouth | 53 | 5 | 34 | 1.56 |
| Southampton General Hospital, Southampton | 19 | 2 | 13 | 1.46 |
| Sunderland Royal Infirmary, Sunderland | 34 | 3 | 25 | 1.36 |
| Bristol Royal Infirmary, Bristol | 7 | 1 | 6 | 1.17 |
| Raigmore Hospital, Inverness | 29 | 2 | 26 | 1.12 |
| Queen Elizabeth Hospital, Birmingham | 2 | 0 | 2 | 1.00 |
| Torbay Hospital, Torquay | 20 | 2 | 27 | 0.74 |
| Guy’s Hospital, London | 8 | 1 | 11 | 0.73 |
| Broadgreen Hospital, Liverpool | 18 | 2 | 27 | 0.67 |
| Manchester Royal Infirmary, Manchester | 3 | 0 | 16 | 0.19 |
| Western General Hospital, Edinburgh | 1 | 0 | 13 | 0.08 |
Participant flow
The progress of participants through the trial from screening to final outcome measurement at 12 weeks after randomisation is shown in Figure 5, which complies with current recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statement. 70 A total of 17 participants were withdrawn following randomisation and have not been included in the main analysis, for the reasons stated in Table 12. Fourteen participants did not have any primary outcome data recorded, which left 1136 (97%) randomised participants included in the primary outcome analysis.
FIGURE 5.
The SUSPEND trial CONSORT diagram.

| Reason for exclusion | Intervention | ||
|---|---|---|---|
| Tamsulosin (n = 8) | Nifedipine (n = 4) | Placebo (n = 5) | |
| Age out of range | 5 | 2 | 5 |
| Given tamsulosin after randomisation | 3 | ||
| Found to have multiple stones | 1 | ||
| Stone not within ureter | 1 | ||
Baseline characteristics
Our randomisation algorithm, including minimisation by the variables of stone size and location, ensured that the three trial groups were well balanced across all relevant and measured covariates (Table 13). Participants were drawn from the expected age range within the limits imposed by the nature of the trial as a CTIMP set at 18–65 years. Those over 65 years old were not included as there is a requirement to titrate the dose of nifedipine in this age group (see Appendix 3) which was not possible. Women accounted for 19% of the trial population. The groups were well balanced for opiate and NSAID use prior to randomisation, in line with a similar duration of pain symptoms prior to randomisation. The overall proportion of participants that fell into the pre-specified subgroups related to stone size and location was similar to previous cohorts and they were well balanced between the three groups. One-third of participants had suffered a previous stone episode at some point in the past.
| Baseline characteristics | Intervention | ||
|---|---|---|---|
| Tamsulosin (N = 383) | Nifedipine (N = 383) | Placebo (N = 384) | |
| Age (years), mean (SD) | 43.1 (11.5) | 42.3 (11.0) | 42.8 (12.3) |
| Female, n (%) | 68 (17.8) | 66 (17.2) | 85 (22.1) |
| Stone size (mm), mean (SD) | 4.6 (1.6) | 4.5 (1.6) | 4.5 (1.7) |
| ≤ 5 mm, n (%) | 287 (74.9) | 286 (74.7) | 286 (74.5) |
| > 5 mm, n (%) | 96 (25.1) | 97 (25.3) | 98 (25.5) |
| Stone location | |||
| Upper ureter, n (%) | 94 (24.5) | 89 (23.2) | 93 (24.2) |
| Mid ureter, n (%) | 40 (10.4) | 43 (11.2) | 44 (11.5) |
| Lower ureter, n (%) | 249 (65.0) | 251 (65.5) | 247 (64.3) |
| History of previous stone episode, n (%) | 130 (33.9) | 118 (30.8) | 137 (35.7) |
| Duration of pain (days), mean (SD) | 3.0 (5.1) | 2.6 (3.3) | 3.2 (5.5) |
| Pain visual analogue score, mean (SD) | 4.0 (3.4) | 3.9 (3.4) | 3.6 (3.2) |
| Analgesic medication pre-admission, n (%) | |||
| NSAID | 132 (34.5) | 110 (28.7) | 117 (30.5) |
| Opiate | 63 (16.4) | 67 (17.5) | 81 (21.1) |
| Other | 79 (20.6) | 86 (22.5) | 79 (20.6) |
| Analgesic medication on admission, n (%) | |||
| NSAID | 279 (72.8) | 289 (75.5) | 278 (72.4) |
| Opiate | 224 (58.5) | 230 (60.1) | 230 (59.9) |
| Other | 127 (33.2) | 141 (36.8) | 133 (34.6) |
| Antibiotic medication on admission, n (%) | 38 (9.9) | 46 (12.0) | 41 (10.7) |
| SF-36 physical component summary, mean (SD) | 47.0 (9.0) | 46.5 (9.2) | 46.1 (9.7) |
| SF-36 mental component summary, mean (SD) | 50.2 (10.8) | 50.6 (10.8) | 49.6 (11.6) |
| EQ-5D, mean (SD) | 0.677 (0.311) | 0.674 (0.332) | 0.701 (0.306) |
Site staff and participant response rates
Completion rates for the 4- and 12-week CRFs from site staff, and participant response rates for the 4- and 12-week questionnaires are detailed in Table 14. Average response rates were 63% for the 4-week participant questionnaire and 49% for the 12-week participant questionnaire with no differences between trial groups. Denominators in the results sections are the number of participants that were included in that specific analysis and reflect the number of participants with available data.
| Type of response | Intervention | ||
|---|---|---|---|
| Tamsulosin (N = 383) | Nifedipine (N = 383) | Placebo (N = 384) | |
| 4-week questionnaire, n (%) | 247 (64.5) | 241 (62.9) | 231 (60.2) |
| 4-week CRF, n (%) | 378 (98.7) | 379 (99.0) | 379 (98.7) |
| 12-week questionnaire, n (%) | 187 (48.8) | 194 (50.7) | 183 (47.7) |
| 12-week CRF, n (%) | 357 (93.2) | 356 (93.0) | 358 (93.2) |
Chapter 5 Outcomes and results
Primary outcome
A primary outcome was attributed to 1136 of the 1150 included participants (99%), with 14 participants (1%) completely lost to follow-up. The occurrence of the primary outcome at any time up to 4 weeks after randomisation (number of participants not requiring further intervention for the symptomatic ureteric stone) was 307 out of 378 (81.2%) in the tamsulosin group and 304 out of 379 (80.2%) in the nifedipine group, compared with 303 out of 379 (79.9%) for those randomised to placebo. These primary results are described in more detail in Table 15 using both raw and adjusted analyses. The full logistic regression model is detailed in Appendix 7.
| Analyses type | Intervention | ||
|---|---|---|---|
| Tamsulosin (N = 378) | Nifedipine (N = 379) | Placebo (N = 379) | |
| No further intervention, n (%) | 307 (81.2) | 304 (80.2) | 303 (79.9) |
| OR, 95% CI; p-value | Risk difference, 95% CI | ||
| MET vs. placebo | |||
| Unadjusted | 1.04, 0.77 to 1.43; 0.76 | 0.8%, –4.1% to 5.7% | |
| Adjusted | 1.06, 0.70 to 1.60; 0.78 | 0.9%, –5.1% to 6.8% | |
| Tamsulosin vs. nifedipine | |||
| Unadjusted | 1.07, 0.74 to 1.53; 0.73 | 1.0%, –4.6% to 6.6% | |
| Adjusted | 1.06, 0.73 to 1.53; 0.77 | 0.8%, –4.5% to 6.1% | |
| Tamsulosin vs. placebo | |||
| Unadjusted | 1.08, 0.76 to 1.56; 0.76 | 1.2%, –4.4% to 6.9% | |
| Adjusted | 1.09, 0.67 to 1.78; 0.73 | 1.3%, –5.7% to 8.3% | |
| Nifedipine vs. placebo | |||
| Unadjusted | 1.02, 0.71 to 1.45; 0.93 | 0.2%, –5.4% to 5.9% | |
| Adjusted | 1.03, 0.68 to 1.56; 0.88 | 0.5%, –5.6% to 6.5% | |
We also recorded the number of participants having an intervention planned between 4 weeks and 12 weeks on the 12-week CRF. A further 27 (7.1%) participants in the tamsulosin group, 25 (6.4%) in the nifedipine group and 28 (7.4%) in the placebo group were recorded as having had an intervention between these time points.
Secondary outcomes
Estimated time to stone passage
The outcome for time to passage of stone as measured by clinical report, and confirmed by imaging, was available for 237 (21%) participants and showed no difference between groups (Table 16).
| Analyses type | Intervention | ||
|---|---|---|---|
| Tamsulosin (n = 79) | Nifedipine (n = 74) | Placebo (n = 84) | |
| Time to stone passage (days) | |||
| Mean (SD) | 16.5 (12.6) | 16.2 (14.5) | 15.9 (11.3) |
| Median (25th, 75th centile) | 14 (5, 27) | 13 (4, 26) | 14 (5, 25) |
| MET vs. placebo (difference, 95% CI; p-value) | |||
| Unadjusted | 0.5, –2.9 to 3.9; 0.78 | ||
| Adjusted | 0.6, –2.6 to 4.0; 0.71 | ||
| Tamsulosin vs. nifedipine (difference, 95% CI; p-value) | |||
| Unadjusted | 0.4, –3.7 to 4.4; 0.86 | ||
| Adjusted | 0.6, –2.5 to 3.7; 0.72 | ||
Pain
Participants scored the severity of pain during the day of completion of the baseline and 4-week questionnaire using the NRS. 53,54 The duration of pain and use of analgesic medication was recorded as the number of days pain medication was used on the 4-week participant questionnaire. The results are shown in Table 17.
| Outcome | Intervention | ||
|---|---|---|---|
| Tamsulosin (N = 247) | Nifedipine (N = 239) | Placebo (N = 231) | |
| Any self-reported pain medication during first four weeks, n/N (%) | 139/245 (56.7) | 133/239 (55.6) | 136/231 (58.9) |
| Number of days of pain medication usea | |||
| Mean (SD) | 11.6 (8.7) | 10.7 (9.0) | 10.5 (8.2) |
| Median (25th, 75th centile) | 10 (4, 17) | 7 (4, 14) | 7 (4, 14) |
| MET vs. placebo (difference, 95% CI; p-value) | 0.6, –1.6 to 2.8; 0.45 | ||
| Tamsulosin vs. nifedipine (difference, 95% CI; p-value) | 0.8, –1.6 to 3.2; 0.50 | ||
| EQ-5D pain domain at 4 weeks | (N = 244) | (N = 239) | (N = 229) |
| No pain or discomfort, n (%) | 170 (69.7) | 159 (66.5) | 154 (67.2) |
| Moderate pain or discomfort, n (%) | 66 (27.0) | 71 (29.7) | 65 (28.4) |
| Extreme pain or discomfort, n (%) | 8 (3.3) | 9 (3.8) | 10 (4.4) |
| MET vs. placebo (OR, 95% CI; p-value) | 0.94, 0.73 to 1.21; 0.66 | ||
| Tamsulosin vs. nifedipine (OR, 95% CI; p-value) | 0.82, 0.62 to 1.09; 0.17 | ||
| VAS pain scale at 4 weeksa | (N = 233) | (N = 231) | (N = 216) |
| Mean (SD) | 1.0 (2.0) | 1.3 (2.2) | 1.2 (2.2) |
| MET vs. placebo (difference, 95% CI; p-value) | 0.0, –0.4 to 0.4; 0.96 | ||
| Tamsulosin vs. nifedipine (difference, 95% CI; p-value) | –0.3, –0.7 to 0.1; 0.095 | ||
| EQ-5D pain domain at 12 weeks | (N = 183) | (N = 188) | (N = 177) |
| No pain or discomfort, n (%) | 126 (68.9) | 136 (72.3) | 133 (75.1) |
| Moderate pain or discomfort, n (%) | 50 (27.3) | 46 (24.5) | 41 (23.2) |
| Extreme pain or discomfort, n (%) | 7 (3.8) | 6 (3.2) | 3 (1.7) |
| MET vs. placebo (OR, 95% CI; p-value) | 1.26, 0.87 to 1.82; 0.21 | ||
| Tamsulosin vs. nifedipine (OR, 95% CI; p-value) | 1.14, 0.84 to 1.56; 0.39 | ||
Health status
Generic health profile as measured by the SF-36 and EQ-5D at baseline and 4 weeks and 12 weeks after randomisation is shown in Table 18 and Figure 6.
| Quality-of-life measures | Intervention | ||
|---|---|---|---|
| Tamsulosin (N = 383) | Nifedipine (N = 383) | Placebo (N = 384) | |
| SF-36 physical component summary, n [mean] (SD) | |||
| Baseline | 369 [46.5] (9.2) | 372 [47.0] (9.0) | 369 [46.1] (9.7) |
| 4 weeks | 229 [48.0] (9.4) | 228 [47.9] (9.7) | 213 [47.9] (8.8) |
| 12 weeks | 177 [51.2] (9.7) | 177 [51.4] (9.2) | 167 [51.6] (9.0) |
| Effect estimates, 95% CI; p-value | |||
| MET vs. placebo | Tamsulosin vs. nifedipine | ||
| 4 weeks | –0.2, –1.4 to 1.0; 0.83 | 0.4, –1.0 to 1.8; 0.61 | |
| 12 weeks | 0.0, –1.4 to 1.3; 0.96 | 0.8, –0.7 to 2.3; 0.30 | |
| SF-36 mental component summary, n [mean] (SD) | |||
| Baseline | 369 [50.6] (10.8) | 372 [50.2] (10.8) | 369 [49.6] (11.6) |
| 4 weeks | 229 [47.7] (11.9) | 228 [47.7] (11.9) | 213 [46.5] (11.8) |
| 12 weeks | 177 [49.3] (11.7) | 177 [50.4] (10.3) | 167 [51.3] (9.9) |
| Effect estimates, 95% CI; p-value | |||
| MET vs. placebo | Tamsulosin vs. nifedipine | ||
| 4 weeks | 0.5, –1.0 to 2.0; 0.53 | –0.3, –2.1 to 1.4; 0.70 | |
| 12 weeks | –1.5, –3.1 to 0.2; 0.09 | –1.5, –3.4 to 0.4; 0.11 | |
| EQ-5D, n [mean] (SD) | |||
| Baseline | 373 [0.674] (0.332) | 369 [0.677] (0.311) | 373 [0.701] (0.306) |
| 4 weeks | 243 [0.837] (0.271) | 238 [0.853] (0.241) | 226 [0.846] (0.242) |
| 12 weeks | 182 [0.859] (0.242) | 187 [0.868] (0.240) | 175 [0.898] (0.184) |
| Effect estimates, 95% CI; p-value | |||
| MET vs. placebo | Tamsulosin vs. nifedipine | ||
| 4 weeks | 0.001, –0.035 to 0.037; 0.96 | –0.003, –0.045 to 0.038; 0.87 | |
| 12 weeks | –0.028, –0.068 to 0.011; 0.16 | 0.002, –0.043 to 0.048; 0.91 | |
FIGURE 6.
Quality-of-life scores measured at baseline, 4 weeks and 12 weeks. Graphs summarise mean and SD for SF-36 physical component summary (PCS) and mental component summary (MCS), median and 25th to 75th centiles for the EQ-5D.


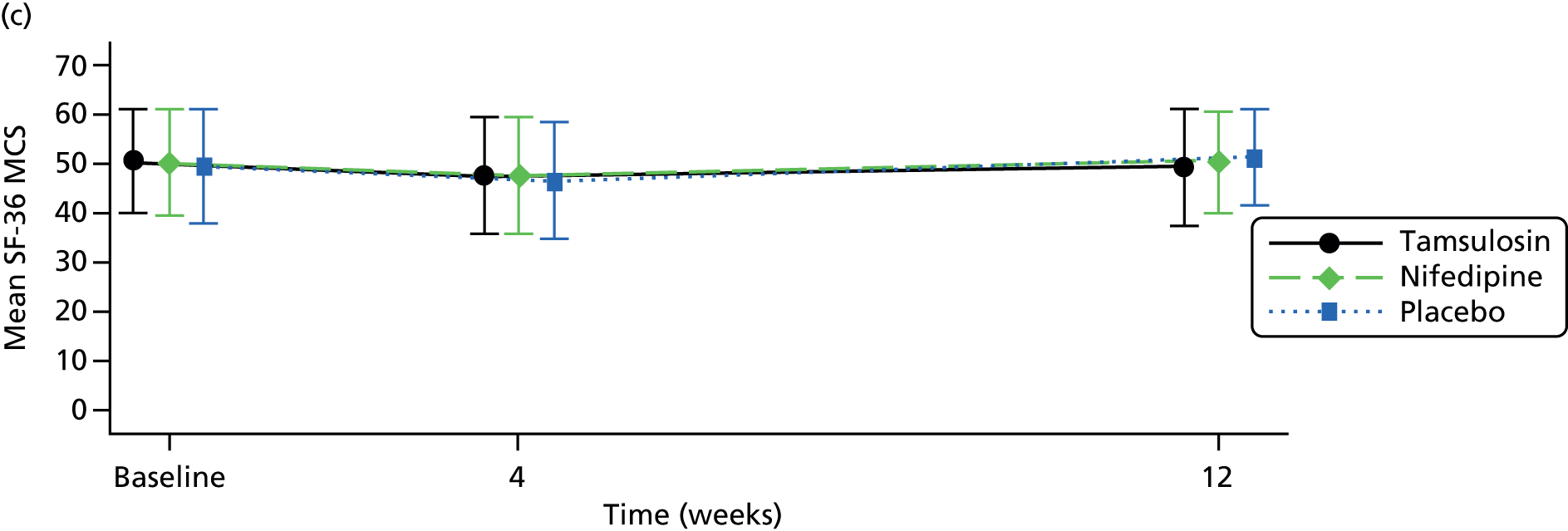
On both the EQ-5D and the SF-36 physical component summary, participants had impaired HRQoL at baseline which returned to population average (SF-36) or full health (EQ-5D) levels by 12 weeks. There was no evidence of a difference between any groups when comparing MET to placebo or tamsulosin to nifedipine at either of the time points. Owing to the high proportion of missing data, the robustness of these results was tested using multiple imputation and pattern mixture models. Younger participants and those with higher baseline SF-36 physical and mental component summary scores were less likely to respond at 4 weeks and 12 weeks. Multiple imputation models gave practically identical treatment effect estimates but with slightly tighter CIs for all treatment effect estimates in Table 18. The results were also robust when varying the pattern of missing data for all but implausible scenarios; for example, missing data in MET group were no different from observed data, but in the placebo group the missing data were over one-third of a SD lower (i.e. 3 points on the SF-36 physical component summary) than observed data. This was the case for all treatment effects summarised in Table 18.
Duration of hospitalisation
The number of participants with further hospital admissions was low and similar in all the groups (see Table 21). The average stay of additional hospital admissions up to 12 weeks after randomisation was 0.17 days (SD 0.64 days) in the tamsulosin group, 0.23 days (SD 1.06 days) in the nifedipine group and 0.25 days (SD 1.13 days) in the placebo group.
Significant clinical events
Participant discontinuation of trial medication was reported from responses to a single question on the 4-week participant questionnaire. Discontinuation rates solely as a result of side effects were 10% (25/247) for tamsulosin, 17% (40/241) for nifedipine and 6% (15/231) for placebo (Table 19). Serious adverse reactions (defined as SAEs that were thought to be possibly or definitely related to trial medication) were recorded by sites using a SAE form. The event rates are shown in Table 19 with further details of the reactions reported in Table 20.
| Quality-of-life measures | Intervention | ||
|---|---|---|---|
| Tamsulosin (N = 247) | Nifedipine (N = 241) | Placebo (N = 231) | |
| Participant discontinuation of medication as a result of side effects, n (%) | 25 (10) | 40 (17) | 15 (6) |
| Serious adverse reactions, n | 0 | 3 | 1 |
| Description of serious adverse reaction | Intervention | ||
|---|---|---|---|
| Tamsulosin (n = 0) | Nifedipine (n = 3) | Placebo (n = 1) | |
| Right loin pain, diarrhoea, vomitinga | 0 | 1 | 0 |
| Headache, dizziness, light-headedness, chronic abdominal pain | 0 | 0 | 1 |
| Malaise, headache, chest paina | 0 | 1 | 0 |
| Severe chest pain, difficulty breathing, left arm pain | 0 | 1 | 0 |
Subgroup analysis
We explored the potential moderating effect of several factors previously reported in the literature by analysing the following subgroups:
-
sex
-
stone size (≤ 5 mm, > 5 to 10 mm)
-
stone location (upper, mid, lower ureter).
Use of analgesia was considered as a subgroup analysis. However, as 98% of participants had used analgesia prior to randomisation it was therefore felt that any subgroup analysis would be uninformative.
Results of the subgroup analysis are summarised graphically using forest plots in Figures 7 and 8 for MET versus placebo and tamsulosin versus nifedipine, respectively. The forest plots present ORs and 99% CIs. There was no evidence of any subgroup by treatment interaction. The p-values for the interaction terms were 0.59 for sex, 0.23 for stone size, and 0.12 for upper and 0.04 for mid ureter (with lower ureter as the reference location) for stone location comparing MET versus placebo. For tamsulosin versus nifedipine, these p-values were 0.39 for sex, 0.13 for stone size, 0.54 for upper ureter and 0.70 for mid ureter. The full breakdown of primary outcome by subgroup is summarised in Appendix 8.
FIGURE 7.
Subgroup analysis: stone size and stone location on stone passage (MET vs. placebo).

FIGURE 8.
Subgroup analysis: stone size and stone location on stone passage (tamsulosin vs. nifedipine).
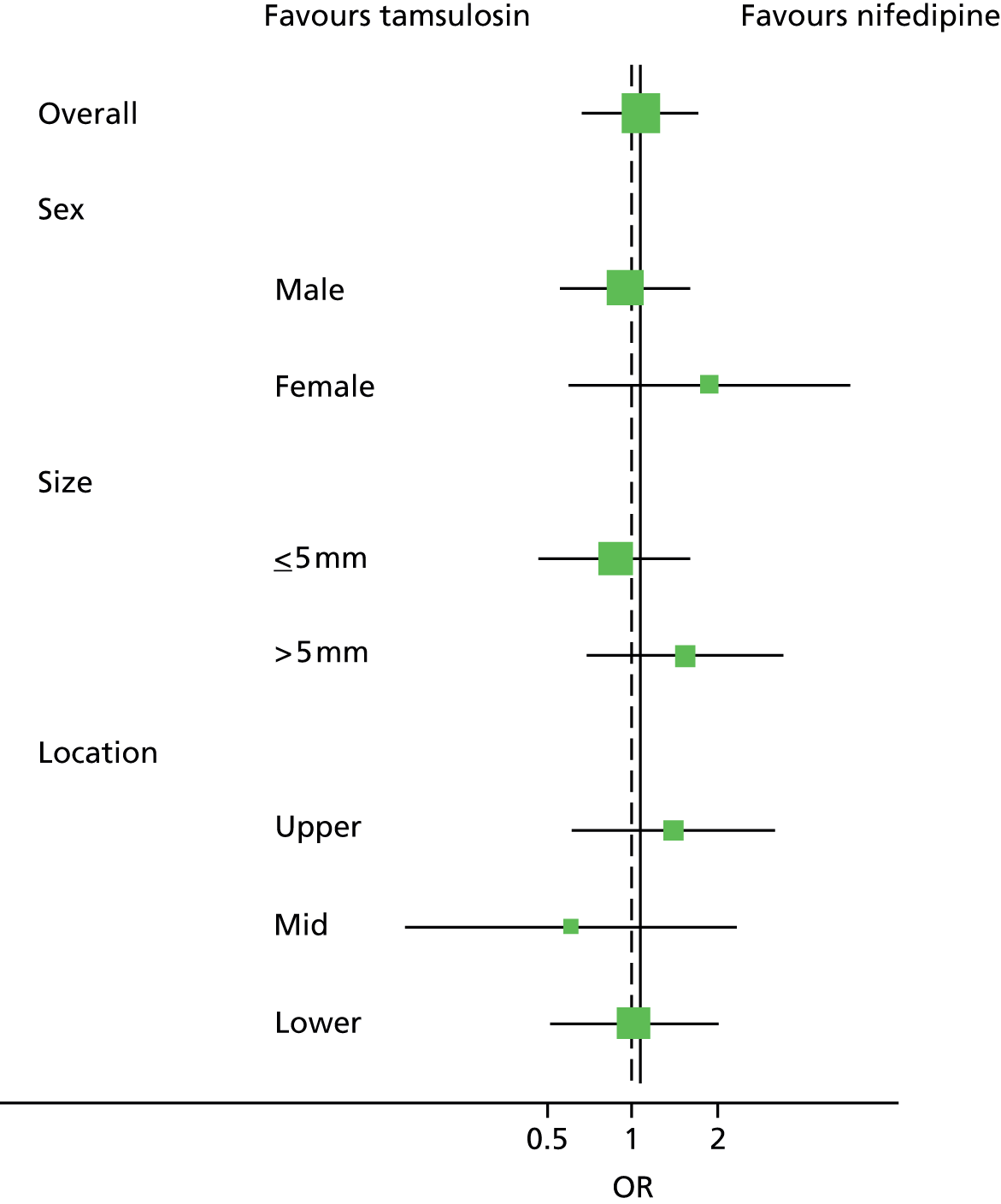
Chapter 6 Resource use, costs and cost-effectiveness
The average total resource use for the interventions and the subsequent use of health services over the 12 weeks are detailed in Table 21. The pattern of resource use was similar across all three groups and there were no statistically significant differences between the three groups. Very few participants used resources, as indicated by the zero median value, and resource use was skewed, with most participants having little or no resource use and a few having high resource use.
| Resource | Intervention {n, mean [median] (SD)} | ||
|---|---|---|---|
| Tamsulosin | Nifedipine | Placebo | |
| Analgesics and antibiotics | 383, 1.84 [2] (0.87) | 383, 1.74 [2] (0.88) | 384, 1.78 [2] (0.86) |
| Diagnostic testsa | 383, 1.60 [2] (0.66) | 383, 1.56 [2] (0.60) | 384, 1.61 [2] (0.69) |
| Doctor visits | 329, 0.20 [0] (0.68) | 331, 0.18 [0] (0.68) | 325, 0.14 [0] (0.54) |
| Nurse visits | 329, 0.04 [0] (0.31) | 330, 0.02 [0] (0.15) | 325, 0.07 [0] (0.90) |
| Outpatient visitsb | 377, 0.72 [1] (0.73) | 378, 0.63 [1] (0.67) | 379, 0.67 [1] (0.69) |
| Percutaneous insertion of nephrostomy tubec | 378 [0] | 379 [0] | 379 [0] |
| Antegrade insertion of stent | 378, 0.01 [0] (0.09) | 379, 0.01 [0] (0.10) | 379, 0.03 [0] (0.16) |
| Ureteroscopic operations | 378, 0.10 [0] (0.30) | 379, 0.10 [0] (0.30) | 379, 0.11 [0] (0.31) |
| Endoscopic insertion of stent | 378, 0.06 [0] (0.24) | 379, 0.07 [0] (0.25) | 379, 0.08 [0] (0.27) |
| ESWL | 378, 0.06 [0] (0.24) | 379, 0.07 [0] (0.27) | 379, 0.08 [0] (0.29) |
| Other | 378, 0.12 [0] (0.34) | 379, 0.12 [0] (0.35) | 379, 0.10 [0] (0.33) |
| All interventionsd | 378, 0.29 [0] (0.65) | 379, 0.30 [0] (0.71) | 379, 0.31 [0] (0.70) |
| Excess admissions dayse | 375, 0.17 [0] (0.64) | 377, 0.23 [0] (1.06) | 375, 0.25 [0] (1.13) |
Costs
In terms of the costs incurred after the intervention was delivered, the mean total cost per participant in the tamsulosin group was £326 (SD £594), in the nifedipine group was £335 (SD £557) and in the placebo group was £367 (SD £619) (Table 22). On average, costs in the placebo group were higher than in either intervention group, which were mainly driven by the further interventions received and inpatient admissions. There was, however, no evidence of a statistically significant difference in the subsequent services used. Similar to the resource use, the cost data were skewed, as indicated by the many zero values in the summary statistics.
| Resource | Intervention {n, mean [median] (SD)} | ||
|---|---|---|---|
| Tamsulosin | Nifedipine | Placebo | |
| Intervention | 383, £4.96 | 383, £6.95 | 384, £0 |
| Analgesics and antibiotics | 383, £4 [5] (2) | 383, £4 [5] (2) | 384, £4 [5] (2) |
| Diagnostic testsa | 383, £96 [120] (40) | 383, £94 [120] (36) | 383, £98 [120] (41) |
| Doctor visits | 329, £9 [0] (30) | 331, £8 [0] (30) | 325, £6 [0] (24) |
| Nurse visits | 329, £0.57 [0] (5) | 330, £0.28 [0] (2) | 325, £1.14 [0] (14) |
| Outpatient visitsb | 377, £73 [101] (74) | 378, £64 [101] (67) | 379, £67 [101] (70) |
| All interventionsc | 378, £250 [0] (581) | 379, £267 [0] (608) | 379, £291 [0] (632) |
| Excess admissions daysd | 375, £44 [0] (169) | 377, £62 [0] (279) | 375, £65 [0] (298) |
| Total costse | 325, £326 [228] (494) | 329, £335 [227] (557) | 323, £367 [223] (619) |
Quality-adjusted life-years
The EQ-5D scores for each group of the trial at baseline, 4 weeks and 12 weeks are shown in Table 23. EQ-5D data were complete for just over 40% of trial participants in each group. The estimated mean QALY gained over the 12 weeks of trial participation was 0.19 (SD 0.05) for the tamsulosin group, 0.20 (SD 0.04) for the nifedipine group and 0.20 (SD 0.04) for the placebo group.
| EQ-5D score at | Intervention {n, mean [median] (SD)} | ||
|---|---|---|---|
| Tamsulosin | Nifedipine | Placebo | |
| Baseline | 373, 0.70 [0.80] (0.31) | 369, 0.70 [0.80] (0.29) | 373, 0.72 [0.80] (0.29) |
| 4 weeks | 243, 0.85 [1.00] (0.25) | 238, 0.86 [1.00] (0.22) | 226, 0.86 [1.00] (0.22) |
| 12 weeks | 185, 0.87 [1.00] (0.23) | 187, 0.87 [1.00] (0.23) | 175, 0.91 [1.00] (0.17) |
| QALY | 165, 0.19 [0.21] (0.05) | 164, 0.20 [0.21] (0.04) | 157, 0.20 [0.21] (0.04) |
Medical expulsive therapy versus placebo
The results of the analysis undertaken to compare MET against placebo are reported in Tables 24 and 25. The pattern of resource use was similar across both groups without any statistically significant differences. Resource use was low, as indicated by the zero median value, and skewed, with most participants having little or no resource use.
| Resource | Intervention {n, mean [median] (SD)} | |
|---|---|---|
| MET | Placebo | |
| Analgesics and antibiotics | 766, 1.80 [2] (0.87) | 384, 1.78 [2] (0.86) |
| Diagnostic testsa | 766, 1.58 [2] (0.63) | 384, 1.61 [2] (0.69) |
| Doctor visits | 660, 0.20 [0] (0.68) | 325, 0.14 [0] (0.54) |
| Nurse visits | 659, 0.04 [0] (0.25) | 325, 0.07 [0] (0.90) |
| Outpatient visitsb | 754, 0.68 [1] (0.70) | 379, 0.67 [1] (0.69) |
| Percutaneous insertion of nephrostomy tubec | 757, 0.00 [0] | 379, 0.00 [0] |
| Antegrade insertion of stent | 757, 0.01 [0] (0.10) | 379, 0.03 [0] (0.16) |
| Ureteroscopic operations | 757, 0.10 [0] (0.30) | 379, 0.11 [0] (0.31) |
| Endoscopic insertion of stent | 757, 0.07 [0] (0.25) | 379, 0.08 [0] (0.27) |
| ESWL | 757, 0.07 [0] (0.26) | 379, 0.08 [0] (0.29) |
| Other | 757, 0.12 [0] (0.34) | 379, 0.10 [0] (0.33) |
| All interventionsd | 757, 0.30 [0] (0.69) | 379, 0.31 [0] (0.70) |
| Excess admissions dayse | 752, 0.20 [0] (0.87) | 375, 0.25 [0] (1.13) |
| Resource | Intervention {n, mean [median] (SD)} | |
|---|---|---|
| MET | Placebo | |
| Intervention | 766, £4.96 | 384, £0 |
| Analgesics and antibiotics | 766, £4 [5] (2) | 384, £4 [5] (2) |
| Diagnostic testsa | 766, £95 [120] (38) | 383, £97 [120] (41) |
| Doctor visits | 660, £8 [0] (30) | 325, £6 [0] (24) |
| Nurse visits | 659, £0.42 [0] (4) | 325, £1.14 [0] (14) |
| Outpatient visitsb | 754, £68 [101] (71) | 379, £67 [101] (70) |
| All interventionsc | 757, £258 [0] (594) | 379, 291 [0] (632) |
| Excess admissions daysd | 752, £53 [0] (230) | 375, £65 [0] (298) |
| Total costse | 654, £330 [228] (526) | 323, £367 [223] (619) |
| Unadjusted mean difference (95% CI) | –£35 (–£39 to £110) | |
| Adjusted mean difference (95% CI) | £3 (–£67 to £70) | |
Costs
In terms of costs incurred after the intervention was delivered, the mean total cost per participant in the MET group was £330 (SD £526) and in the placebo group was £367 (SD £619) (see Table 25). On average, the MET group had lower costs than the placebo group, which were mainly driven by the further interventions received and inpatient admissions. The data were skewed, as indicated by the many zero values in the summary statistics. The unadjusted mean difference was –£35 (95% CI –£39 to £110) and favoured the MET group. The adjusted mean difference estimated using a generalised linear model fitted and adjusting for the minimisation factors and clustering for the centres between MET and placebo was £3 (95% CI –£67 to £70) and favoured the placebo group. There was, however, no evidence of a statically significant difference in the costs.
The EQ-5D results in Table 26 followed a similar pattern to those reported in Table 23 as, on average, the MET group had slightly lower QALY scores of 0.19 (SD 0.05), compared with 0.20 (SD 0.04) for placebo. The unadjusted mean difference was –0.003 (96% CI –0.006 to 0.011) and the adjusted mean difference was –0.001 (95% CI –0.007 to 0.006), but these differences were not statistically significant.
| EQ-5D score at | Intervention {n, mean [median] (SD)} | |
|---|---|---|
| MET | Placebo | |
| Baseline | 742, 0.70 [0.80] (0.30) | 373, 0.72 [0.80] (0.29) |
| 4 weeks | 481, 0.86 [1.00] (0.24) | 226, 0.86 [1.00] (0.22) |
| 12 weeks | 369, 0.87 [1.00] (0.23) | 175, 0.91 [1.00] (0.17) |
| QALY | 329, 0.19 [0.21] (0.05) | 157, 0.20 [0.21] (0.04) |
| Unadjusted QALY difference, 95% CI | –0.003 (–0.006 to 0.011) | |
| Adjusted QALY difference, 95% CI | –0.001 (–0.007 to 0.006) | |
The incremental cost difference based on the complete cases (participants with both QALY and cost data) was –£42 (95% CI –£188 to £104) and the incremental QALY difference was –0.001 (95% CI –0.008 to 0.006) (Table 27). These values are based on a smaller sample than the raw cost and QALY data in Tables 25 and 26. Thus, on average, costs in the MET group were lower but MET was also less effective than placebo. The probabilities that MET would be considered cost-effective at various thresholds of WTP are shown in Table 27.
| Cost effectiveness results | |
|---|---|
| Difference in costs, mean (95% CI) | −£42 (−£188 to £104)a |
| Difference in QALYs, mean (95% CI) | −0.001 (−0.008 to 0.006) |
| ICER | £4355b |
| Probability active is cost-effective when threshold is £0 per QALY | 71% |
| Probability active is cost-effective when threshold is £20,000 per QALY | 56% |
| Probability active is cost-effective when threshold is £30,000 per QALY | 51% |
| Probability active is cost-effective when threshold is £50,000 per QALY | 46% |
The empirical estimates of the joint distribution of mean costs and QALYs obtained using the results of the bootstrap replicates are shown in Figure 9. The figure shows that in most cases costs were lower in the MET group than in the placebo group, but QALYs gained were also lower.
FIGURE 9.
Representation of the uncertainty in differential mean costs and QALYs based on EQ-5D responses (MET vs. placebo). Strategy costs are costs incurred for each of the two treatment arms, MET and placebo.

The probability that the MET intervention group would be considered to be cost-effective at different thresholds of WTP was 56% at £20,000 and 51% at £30,000, as illustrated in Figure 10.
FIGURE 10.
Cost-effectiveness acceptability curve using QALYs based on EQ-5D responses (MET vs. placebo).

Tamsulosin versus nifedipine
Resource use
The average total resource use in terms of the interventions and the subsequent use of health services over the 12 weeks of the trial are detailed in Table 28. The pattern of resource use was similar across both groups and there were no statistically significant differences between the groups. Very few participants used resources, as indicated by the zero median value; resource use was skewed, with most participants having little or no resource use and a few of them having high resource use.
| Resource | Intervention {n, mean [median] (SD)} | |
|---|---|---|
| Tamsulosin | Nifedipine | |
| Analgesics and antibiotics | 383, 1.84 [2] (0.87) | 383, 1.74 [2] (0.88) |
| Diagnostic testsa | 383, 1.60 [2] (0.66) | 383, 1.56 [2] (0.60) |
| Doctor visits | 329, 0.20 [0] (0.68) | 331, 0.18 [0] (0.68) |
| Nurse visits | 329, 0.04 [0] (0.31) | 330, 0.02 [0] (0.15) |
| Outpatient visitsb | 377, 0.72 [1] (0.73) | 378, 0.63 [1] (0.67) |
| Percutaneous insertion of nephrostomy tubec | 378 [0] | 379 [0] |
| Antegrade insertion of stent | 378, 0.01 [0] (0.09) | 379, 0.01 [0] (0.10) |
| Ureteroscopic operations | 378, 0.10 [0] (0.30) | 379, 0.10 [0] (0.30) |
| Endoscopic insertion of stent | 378, 0.06 [0] (0.24) | 379, 0.07 [0] (0.25) |
| ESWL | 378, 0.06 [0] (0.24) | 379, 0.07 [0] (0.27) |
| Other | 378, 0.12 [0] (0.34) | 379, 0.12 [0] (0.35) |
| All interventionsd | 378, 0.29 [0] (0.65) | 379, 0.30 [0] (0.71) |
| Excess admissions dayse | 375, 0.17 [0] (0.64) | 377, 0.23 [0] (1.06) |
Costs
In terms of the costs incurred after the intervention was delivered, the mean total cost per participant in the tamsulosin group was £326 (SD £594) and in the nifedipine group was £335 (SD £557) (Table 29). On average, the nifedipine group had higher costs than the tamsulosin group. These costs were mainly driven by the further interventions received and inpatient admissions. There was, however, no evidence of a statistically significant difference in the subsequent use of services. Cost data were skewed, as indicated by the many zero values in the summary statistics. The adjusted mean difference was –£25 (95% CI –£84 to £34) favouring tamsulosin.
| Resource | Intervention {n, mean [median] (SD)} | |
|---|---|---|
| Tamsulosin | Nifedipine | |
| Intervention | 383, £4.96 | 383 £6.95 |
| Analgesics and antibiotics | 383, £4 [5] (2) | 383, £4 [5] (2) |
| Diagnostic testsa | 383, £96 [120] (40) | 383, £94 [120] (36) |
| Doctor visits | 329, £9 [0] (30) | 331, £8 [0] (30) |
| Nurse visits | 329, £0.57 [0] (5) | 330, £0.28 [0] (2) |
| Outpatient visitsb | 377, £73 [101] (74) | 378, £64 [101] (67) |
| All interventionsc | 378, £250 [0] (581) | 379, £267 [0] (608) |
| Excess admissions daysd | 375, £44 [0] (169) | 377, £62 [0] (279) |
| Total costse | 325, £326 [228] (494) | 329, £335 [227] (557) |
| Unadjusted mean difference (95% CI) | −£9 (−£90 to £72) | |
| Adjusted mean difference (95% CI) | −£25 (−£84 to £34) | |
Quality-adjusted life-years
Table 30 shows the EQ-5D scores for each group of the trial at baseline, 4 weeks and 12 weeks. The proportion of total trial participants with complete EQ-5D data at each time point was slightly greater than 40% for each group. The estimated mean QALYs were 0.19 (SD 0.05) for the tamsulosin group and 0.20 (SD 0.04) for the nifedipine group. The mean QALY difference (–0.003) between tamsulosin and nifedipine after adjusting for minimisation factors and baseline EQ-5D favoured the nifedipine group, but this was not statistically significant.
| EQ-5D measure at | Intervention {n, mean [median] (SD)} | |
|---|---|---|
| Tamsulosin | Nifedipine | |
| Baseline | 373, 0.70 [0.80] (0.31) | 369, 0.70 [0.80] (0.29) |
| 4 weeks | 243, 0.85 [1.00] (0.25) | 238, 0.86 [1.00] (0.22) |
| 12 weeks | 185, 0.87 [1.00] (0.23) | 187, 0.87 [1.00] (0.23) |
| QALY | 165, 0.19 [0.21] (0.05) | 164, 0.20 [0.21] (0.04) |
| Unadjusted QALY difference (95% CI) | −0.006 (−0.016 to 0.004) | |
| Adjusted QALY difference (95% CI) | −0.003 (−0.014 to 0.009) | |
Estimation of cost-effectiveness
For the initial base-case analysis, a generalised linear model adjusting for baseline EQ-5D and minimisation factors for complete case (respondents that had both cost and QALY data) was employed. An ordinary least squares model adjusting for baseline EQ-5D and the minimisation factors was used to estimate the QALY differences. As noted in Table 31, these values differ from those reported in Tables 29 and 30 as they are based on a smaller sample, as a result of the missing EQ-5D data. The incremental analysis in Table 31 reflects that, on average, the tamsulosin group was less costly than the nifedipine group but the tamsulosin group had lower QALYs than the nifedipine group.
| Cost-effectiveness results | |
|---|---|
| Difference in costs, mean (95% CI) | −£87 (−£200 to £26)a |
| Difference in QALYs, mean (95% CI) | −0.002 (−0.013 to 0.010) |
| ICER | £43,500b |
| Probability that tamsulosin is cost-effective when threshold is £0 per QALY | 85% |
| Probability that tamsulosin is cost-effective when threshold is £20,000 per QALY | 61% |
| Probability that tamsulosin is cost-effective when threshold is £30,000 per QALY | 55% |
| Probability tamsulosin is cost-effective when threshold is £50,000 per QALY | 48% |
The empirical estimates of the joint distribution of mean costs and QALYs obtained using the results of the bootstrap replicates are shown in Figure 11.
FIGURE 11.
Representation of the uncertainty in differential mean costs and QALYs based on EQ-5D responses (tamsulosin vs. nifedipine). Strategy costs are costs incurred for each of the two treatment arms, tamsulosin and nifedipine.

The probability that the tamsulosin intervention group would be considered to be cost-effective at different thresholds of WTP was 61% at £20,000 and 55% at £30,000 (Figure 12).
FIGURE 12.
Cost-effectiveness acceptability curve using QALYs based on EQ-5D responses (tamsulosin vs. nifedipine).

Sensitivity analysis using quality-adjusted life-years generated using Short Form questionnaire-36 items
Medical expulsive therapy versus placebo
The results of the analysis using the utility scores (Table 32) from the SF-6D were similar to the base-case analysis, although there were fewer respondents with complete data.
| SF-6D measure at | Intervention {n, mean [median] (SD)} | |
|---|---|---|
| MET | Placebo | |
| Baseline | 729, 0.72 [0.71] (0.15) | 367, 0.71 [0.69] (0.15) |
| 4 weeks | 441, 0.72 [0.74] (0.15) | 213, 0.72 [0.74] (0.15) |
| 12 weeks | 348, 0.79 [0.85] (0.15) | 165, 0.80 [0.85] (0.14) |
| QALY | 292, 0.17 [0.18] (0.03) | 141, 0.17 [0.18] (0.03) |
| Unadjusted difference in QALYs (95% CI) | −0.001 (−0.005 to 0.007) | |
| Adjusted difference in QALYs (95% CI) | −0.003 (−0.008 to 0.002) | |
The cost-effectiveness results indicate that, on average, the MET group was £37 less costly than the placebo group, but it was also 0.003 QALYs less effective than placebo (Table 33). The empirical estimates of the joint distribution of mean costs and QALYs obtained using the results of the bootstrap replicates are shown in Figure 13.
| Cost effectiveness results | |
|---|---|
| Difference in costs, mean (95% CI) | −£37 (−£151 to £77) |
| Difference in QALYs, mean (95% CI) | −0.003 (−0.008 to 0.002) |
| ICER | £12,333a |
| Probability intervention is cost-effective when threshold is £0 per QALY | 75% |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 41% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 32% |
| Probability intervention is cost-effective when threshold is £50,000 per QALY | 22% |
FIGURE 13.
Representation of the uncertainty in differential mean costs and QALYs based on SF-6D responses (MET vs. placebo). Strategy costs are costs incurred for each of the two treatment arms, MET and placebo.

The empirical estimates of the joint distribution of mean costs and QALYs obtained using the results of the bootstrap replicates are shown in Figure 11.
The probability that the MET intervention group would be considered cost-effective at different thresholds of WTP was 41% at £20,000 and 32% at £30,000, as shown in Figure 14.
FIGURE 14.
Cost-effectiveness acceptability curve using QALYs based on SF-6D responses (MET vs. placebo).
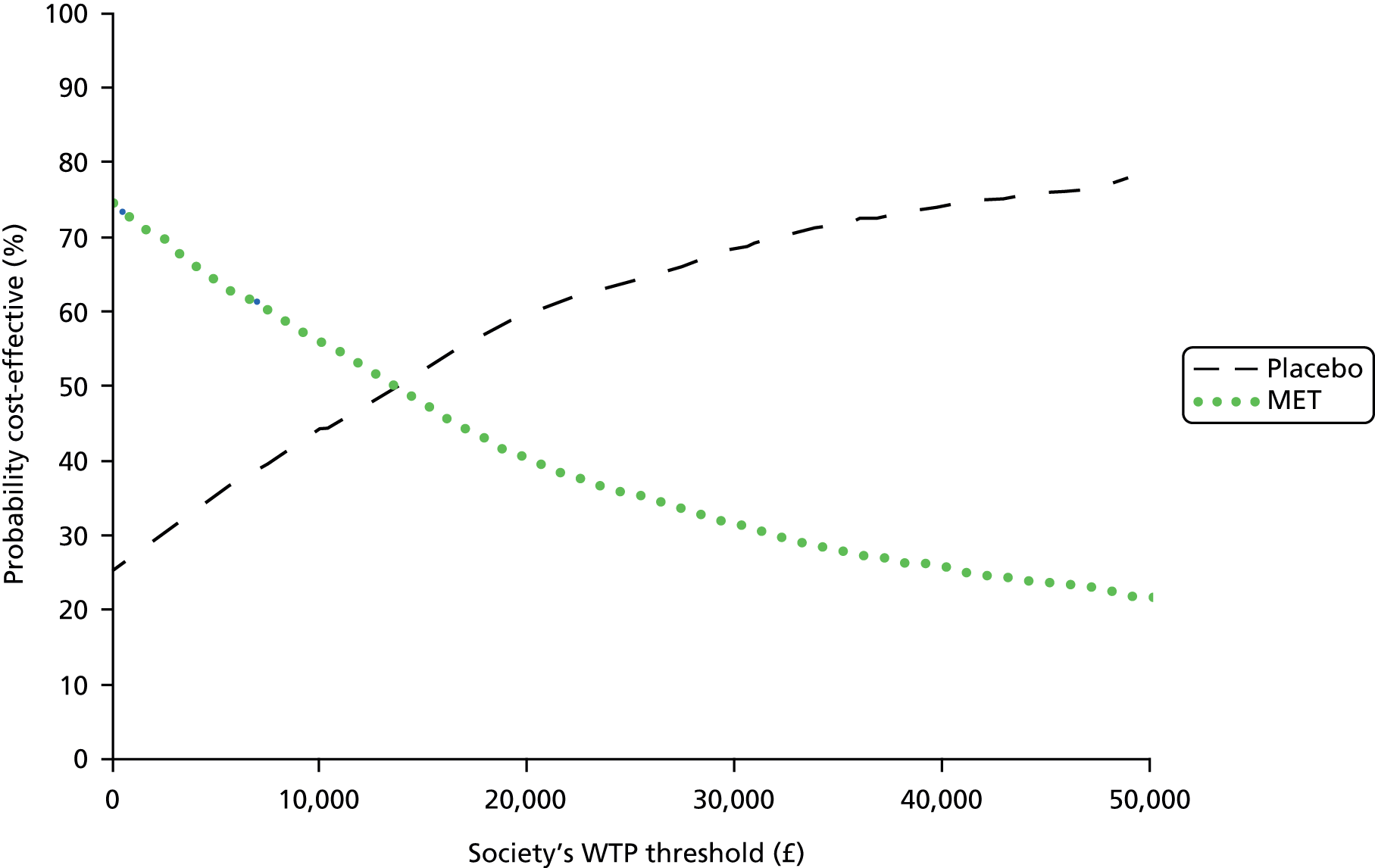
Tamsulosin versus nifedipine
The results of the analysis using the utility scores (Table 34) from the SF-6D were similar to the base-case analysis, although there were fewer respondents with complete data.
| SF-6D measure at | Intervention {n, mean [median] (SD)} | |
|---|---|---|
| Tamsulosin | Nifedipine | |
| Baseline | 363, 0.72 [0.70] (0.15) | 366, 0.720 [0.73] (0.15) |
| 4 weeks | 220, 0.82 [0.73] (0.15) | 221, 0.73 [0.74] (0.15) |
| 12 weeks | 174, 0.78 [0.85] (0.16) | 174, 0.79 [0.83] (0.14) |
| QALY | 147, 0.17 [0.17] (0.03) | 145, 0.17 [0.18] (0.03) |
| Difference in QALYs (95% CI) | −0.001 (−0.006 to 0.008) | |
The results of the incremental analysis are reported in Table 35. On average, the tamsulosin group had lower costs and lower QALYs than the nifedipine group. None of the differences was statistically significant.
| Cost-effectiveness results | |
|---|---|
| Difference in costs, mean (95% CI) | −£14 (−£146 to £117) |
| Difference in QALYs, mean (95% CI) | −0.0006 (−0.0050 to 0.0040) |
| ICER | £23,000a |
| Probability intervention is cost-effective when threshold is £0 per QALY | 63% |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 57% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 54% |
| Probability intervention is cost-effective when threshold is £50,000 per QALY | 51% |
The empirical estimates of the joint distribution of mean costs and QALYs obtained using the results of the bootstrap replicates are shown in Figure 15.
FIGURE 15.
Representation of the uncertainty in differential mean costs and QALYs based on SF-6D responses (tamsulosin vs. nifedipine). Strategy costs are costs incurred for each of the two treatment arms, tamsulosin and nifedipine.
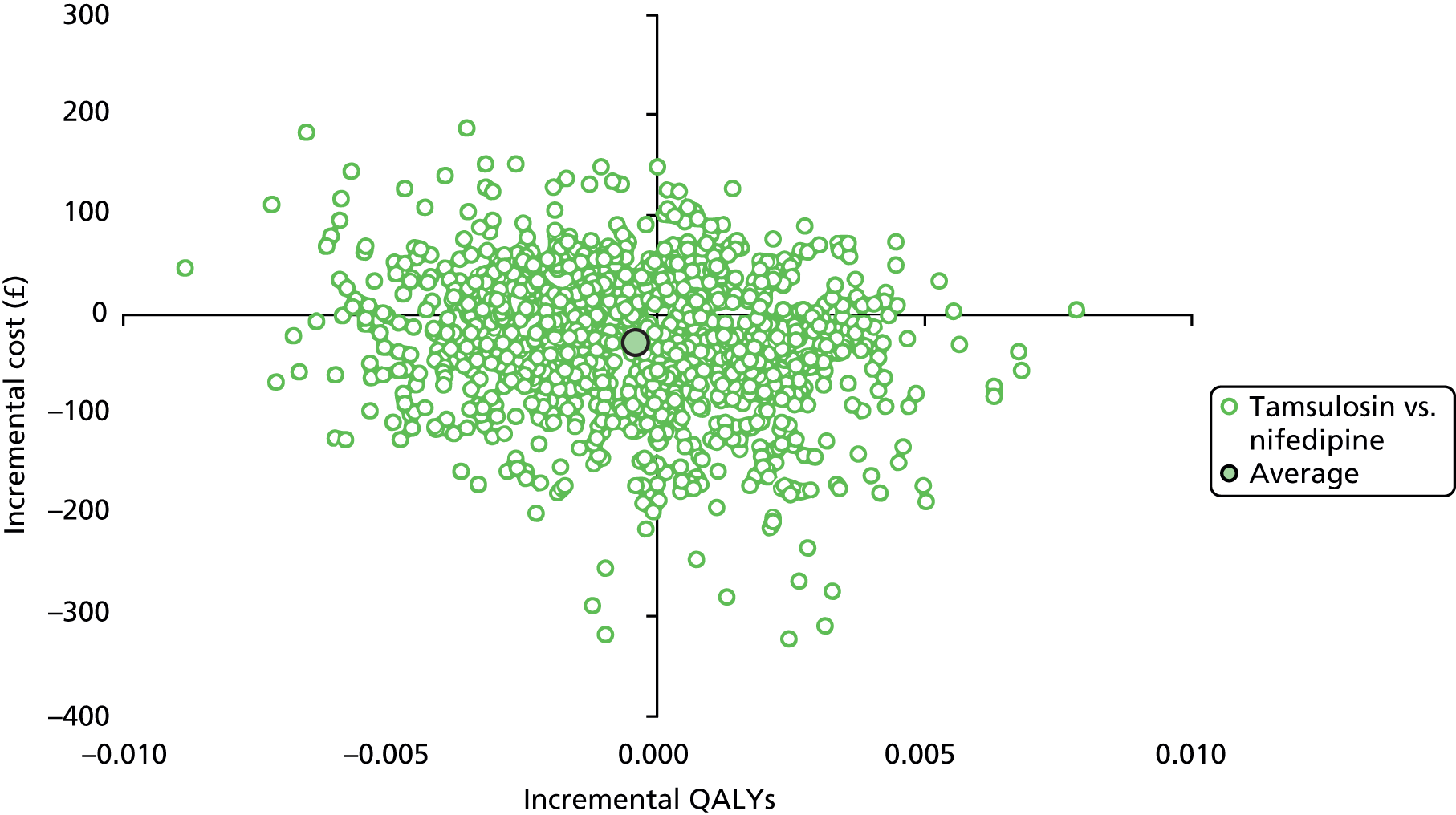
The probability that tamsulosin would be considered cost-effective at different thresholds of WTP was 57% at £20,000 and 54% at £30,000, as illustrated in Figure 16.
FIGURE 16.
Cost-effectiveness acceptability curve using QALYs based on the SF-6D responses (tamsulosin vs. nifedipine).

Sensitivity analysis using extreme European Quality of Life-5 Dimensions scores
The first one-way sensitivity analysis replaced all the missing QALY data with the highest EQ-5D score for the specific group at that particular time point. As illustrated by Tables 36 and 37, this did not change the results.
| EQ-5D measure at | Intervention {mean [median] (SD)} | ||
|---|---|---|---|
| Tamsulosin (n = 383) | Nifedipine (n = 383) | Placebo (n = 384) | |
| Baseline EQ-5D | 0.70 [0.80] (0.31) | 0.71 [0.80] (0.29) | 0.73 [0.80] (0.29) |
| 4 weeks | 0.91 [1.00] (0.21) | 0.92 [1.00] (0.19) | 0.92 [1.00] (0.18) |
| 12 weeks | 0.94 [1.00] (0.17) | 0.94 [1.00] (0.17) | 0.96 [1.00] (0.12) |
| QALY | 0.20 [0.2] (0.04) | 0.21 [0.21] (0.03) | 0.21 [0.22] (0.03) |
| EQ-5D measure at | Intervention {mean [median] (SD)} | ||
|---|---|---|---|
| Tamsulosin (n = 383) | Nifedipine (n = 383) | Placebo (n = 384) | |
| Baseline EQ-5D | 0.66 [0.80] (0.37) | 0.65 [0.80] (0.37) | 0.69 [0.80] (0.36) |
| 4 weeks | 0.45 [0.80] (0.56) | 0.45 [0.80] (0.56) | 0.40 [0.70] (0.57) |
| 12 weeks | 0.29 [−0.24] (0.58) | 0.30 [−0.24] (0.58) | 0.28 [−0.24] (0.58) |
| QALY | 0.10 [0.11] (0.10) | 0.10 [0.12] (0.10) | 0.10 [0.11] (0.10) |
Sensitivity analyses using multiple imputation
Medical expulsive therapy versus placebo
The results of the imputation are presented in Table 38. The cost and QALY differences between the two groups are similar to those of the complete case analysis; on average, MET is less costly and less effective than placebo. The cost difference reduced from –£42 (95% CI –£188 to £104) (see Table 27) to –£6 (96% CI –£106 to £92) (see Table 38). The QALY difference remained the same with a very small change in the 95% CI. However, none of these differences was statistically significant.
| Cost-effectiveness results | |
|---|---|
| Difference in costs, mean (95% CI) | −£6 (−£106 to £92) |
| Difference in QALYs, mean (95% CI) | −0.001 (−0.007 to 0.004) |
| ICER | £6000a |
| Probability intervention is cost-effective when threshold is £0 per QALY | 53% |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 33% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 29% |
| Probability intervention is cost-effective when threshold is £50,000 per QALY | 26% |
The probability that MET will be cost-effective compared with placebo at a given WTP per QALY gained threshold reduced from 56% (see Table 27) to 33% for the £20,000 threshold, and from 51% (see Table 27) to 29% for the £30,000 threshold (Figures 17 and 18).
FIGURE 17.
Representation of the uncertainty in differential mean costs and QALYs based on EQ-5D imputation results (MET vs. placebo). Strategy costs are costs incurred for each of the two treatment arms, MET and placebo.
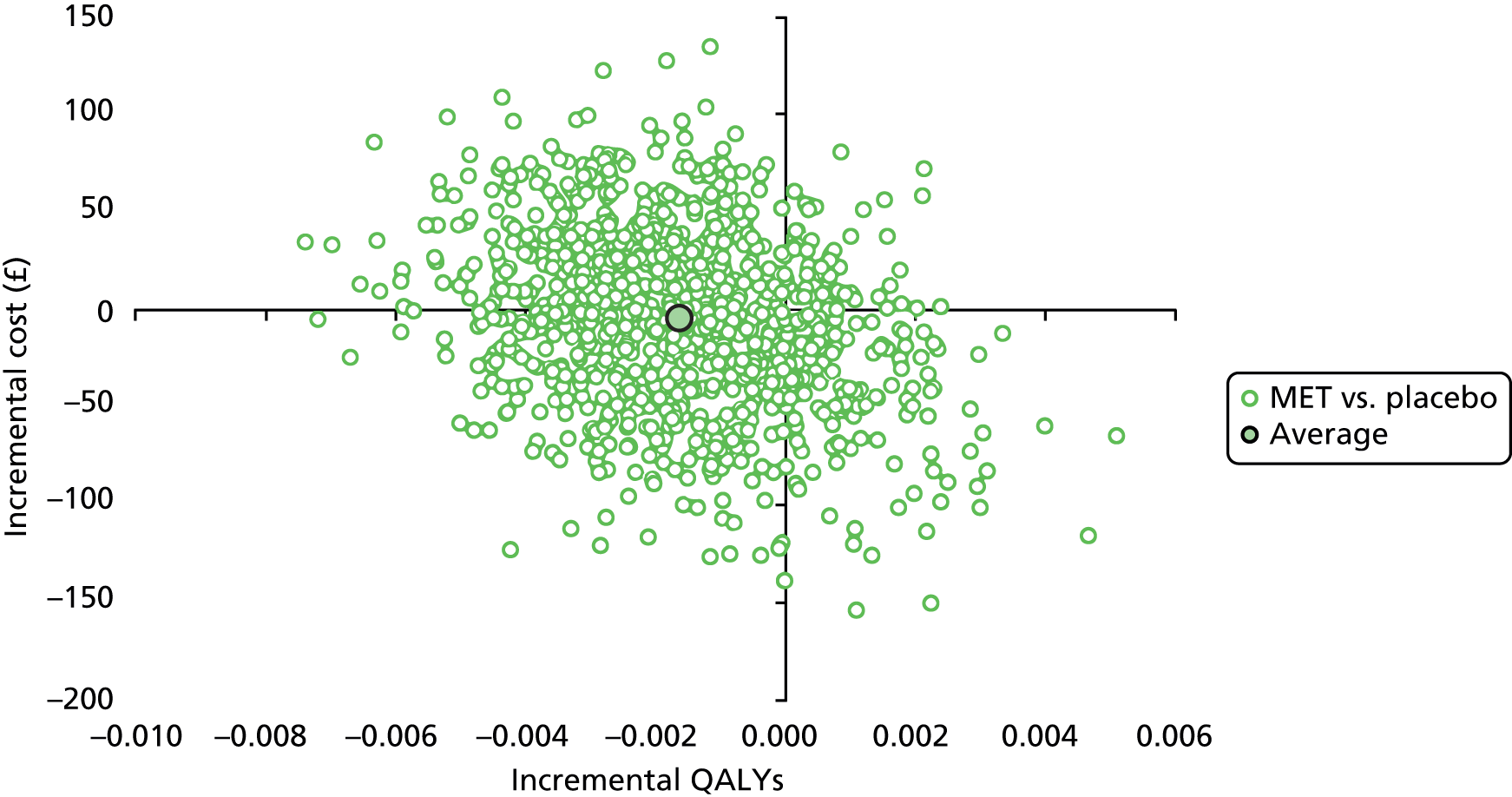
FIGURE 18.
Cost-effectiveness acceptability curve using QALYs based on EQ-5D imputation results (MET vs. placebo).
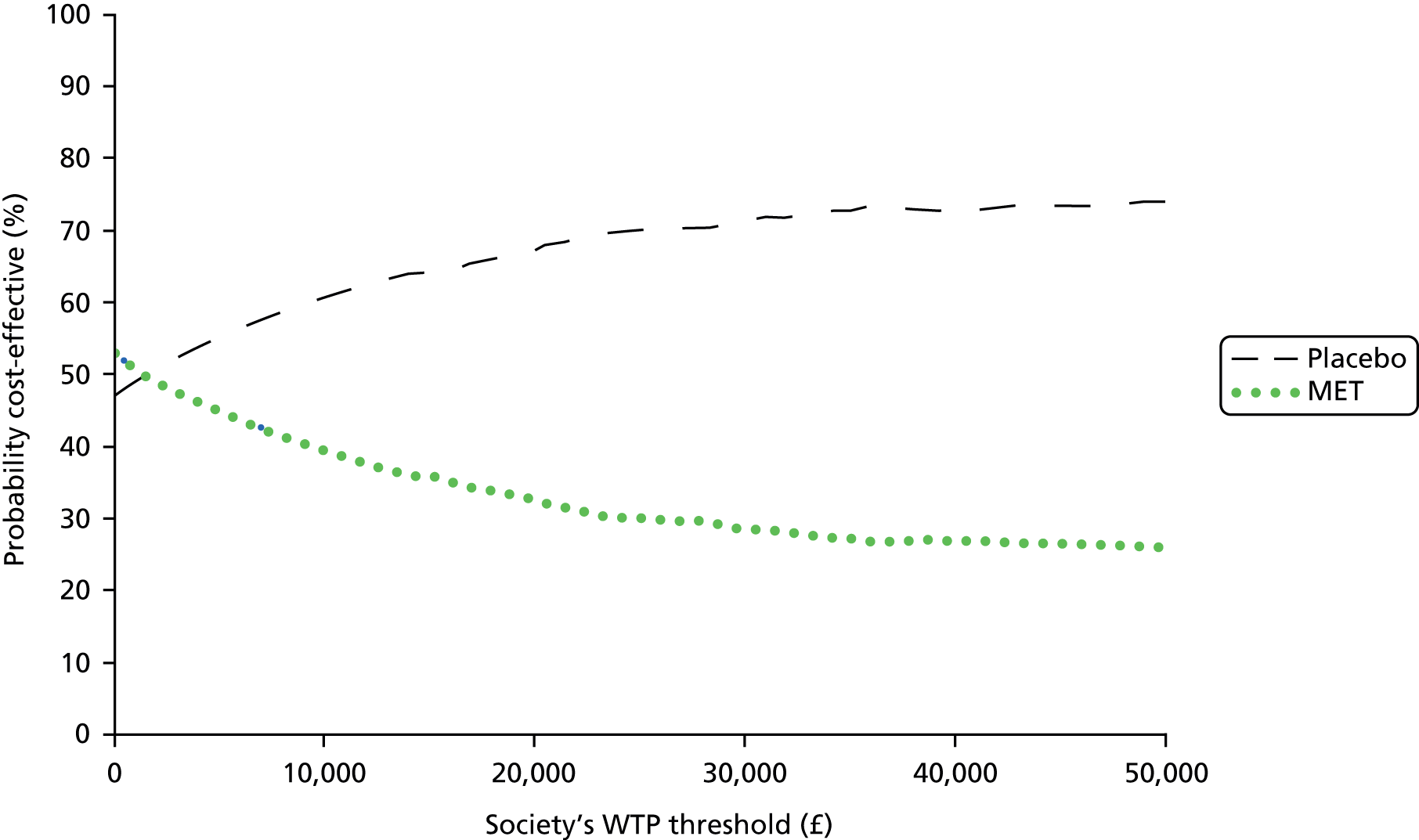
Tamsulosin versus nifedipine
The results of the imputation for the tamsulosin and nifedipine comparison are presented in Table 39. None of these differences was statistically significant. The direction of the difference in costs and QALYs changed from negative to positive compared with the complete case analysis using EQ-5D (Table 31), with tamsulosin costing more than nifedipine but also being more effective.
| Cost-effectiveness results | |
|---|---|
| Difference in costs, mean (95% CI) | £11 (−£57 to £80) |
| Difference in QALYs, mean (95% CI) | 0.0004 (−0.0070 to 0.0040) |
| ICER | £24,677 |
| Probability intervention is cost-effective when threshold is £0 per QALY | 43% |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 50% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 53% |
| Probability intervention is cost-effective when threshold is £50,000 per QALY | 54% |
The shape of the CEAC also changed, with the chance that tamsulosin would be considered to be cost-effective increasing over different thresholds and that of the nifedipine group decreasing compared with the complete case analysis using the EQ-5D reported in Table 31 (Figures 19 and 20).
FIGURE 19.
Representation of the uncertainty in differential mean costs and QALYs based on EQ-5D imputation results (tamsulosin vs. nifedipine). Strategy costs are costs incurred for each of the two treatment arms, MET and placebo.

FIGURE 20.
Cost-effectiveness acceptability curve using QALYs based on EQ-5D imputation results (tamsulosin vs. nifedipine).
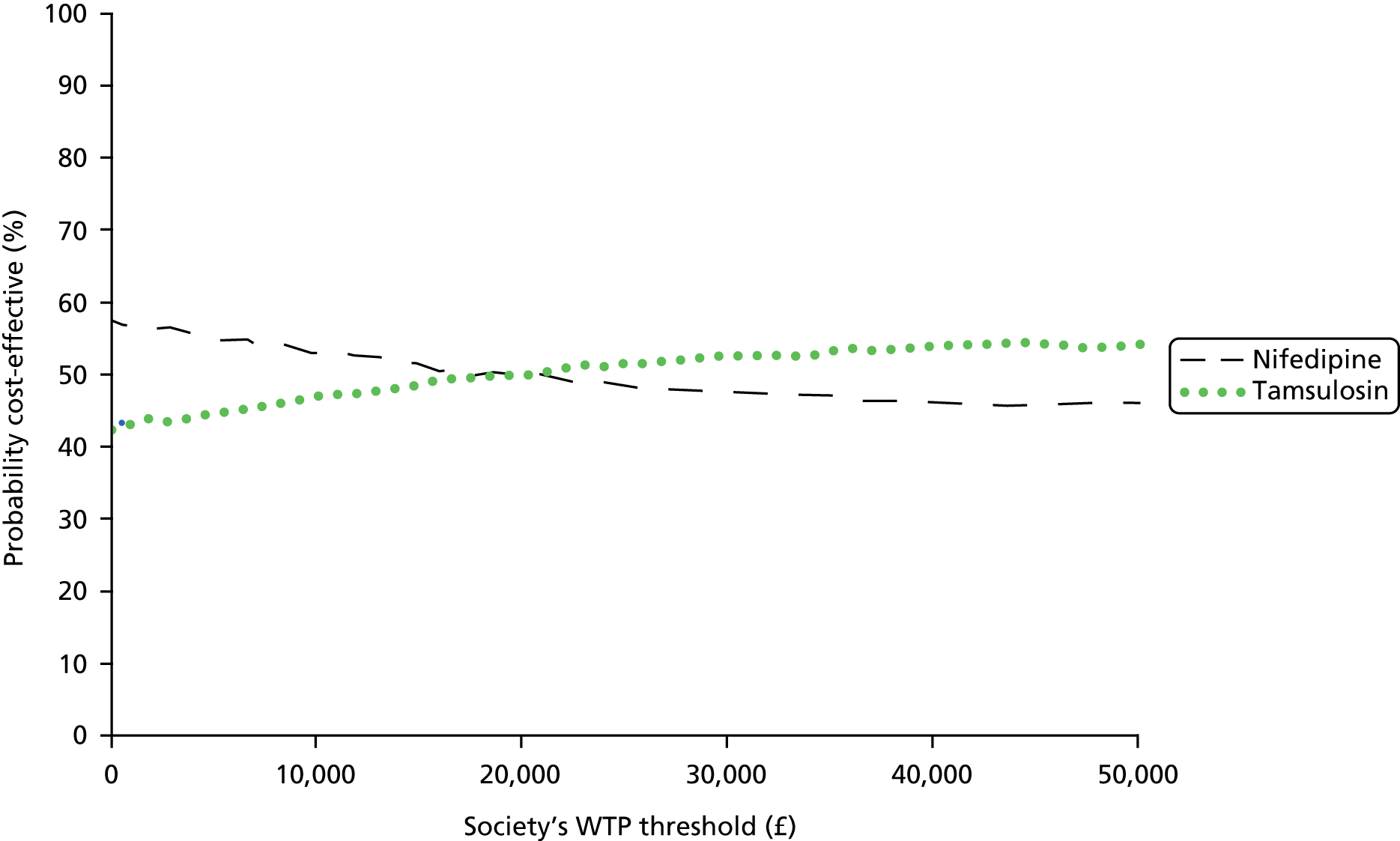
Interpretation of results
The results were not sensitive to the different assumptions applied and concur with the clinical results that reflected that there were no statistically significant differences in the clinical outcome. The utility scores indicated a general improvement in quality of life from baseline to 12 weeks; however, there was no statistically significant difference between the groups. The sensitivity analyses using data imputation to determine the possible effects of missing data did not lead to any change in the overall conclusions. The imputation analysis comparing MET and placebo resulted in a smaller cost difference, that was not statistically significant, and a reduction in the probability of MET being considered to be cost-effective at the £20,000 and £30,000 WTP thresholds. The complete case analysis tended to favour tamsulosin over nifedipine in cost, but the imputed analysis favoured tamsulosin in QALYs; however, neither of these results was statistically significant. The chance of tamsulosin being considered to be cost-effective in the imputed analysis with nifedipine, at the £20,000 and £30,000 WTP thresholds also reduced. The incremental cost-effectiveness ratios (apart from those in the imputation analysis reported in Table 39) all reflected the saving per QALY lost. For the MET to be considered cost-effective, these values would have to be greater than £30,000. These results have to be interpreted taking into account the uncertainty surrounding the estimates. The results emphasise that there are no significant differences in both the costs and QALYs, and if the National Institute for Health and Care Excellence-recommended thresholds of £20,000 to £30,000 are considered, none of the active treatments has much higher than a 50% chance of being considered cost-effective, meaning that none has any cost-effectiveness advantage over placebo.
Chapter 7 Discussion
Statement of principal findings
Primary outcome
The SUSPEND trial was designed, firstly, to determine whether or not the possible benefit of MET for expectantly managed ureteric colic suggested by meta-analyses of a number of small previous RCTs was borne out in a large, controlled, effectiveness trial carried out in a routine care setting. Secondly, the trial investigated which of the two candidate classes of agent was superior in facilitating stone passage. The results showed no benefit for either tamsulosin (an alpha-blocker) or nifedipine (a calcium channel blocker) over 4 weeks in altering the rate of stone passage as measured by the lack of need for further intervention. The finding of no effect is robust in that the trial recruited to the planned sample size with near-complete collection of the primary outcome, delivering the necessary power to detect what was established as the minimum clinically important difference of a 25% (50–75%) increase in spontaneous stone passage. Indeed, the trial results have sufficient precision to rule out a 10% absolute benefit between both active drugs and placebo at the 95% confidence level. These results were unchanged by pre-planned sensitivity analyses examining interaction with stone size, stone location, sex of participant and place of treatment.
Secondary outcomes
Pain is a significant burden for this patient population and has an impact on state of health. Improved pain control would therefore be of benefit to sufferers of ureteric colic and would also relieve burden on the health-care system in terms of contact with health-care professionals for advice and to obtain analgesia. For the 61% of participants who completed and returned the 4-week questionnaire, there was no benefit of either drug compared with placebo for the outcomes of degree of pain at 4 weeks, measured on a Likert-type pain score, and the recollected number of days of analgesia use over 4 weeks. Concerning quality of life, baseline scores for health status taken just prior to randomisation showed the expected detriment caused by an acute episode of ureteric colic across all five domains. Subsequently, there was progressive improvement over the 12 weeks of the trial back to the level of the general population. 52 For the outcome of recovery of general health, there were no statistically significant differences in any of the time points between the groups, thus indicating no benefit from the drugs tested.
Time to stone passage was determined from the date of stone passage recorded on the CRFs, which were completed by local research staff. This question also established whether or not stone passage was confirmed by repeat imaging. Imaging during follow-up was not required as part of the trial protocol and this was performed only if directed by the local clinical team on the basis of local practice or clinical need. There was no clear record of stone passage for 79% of participants, which is to be expected in the routine care setting, given that once the stone is in the bladder it then passes during micturition without further discomfort. Therefore, although the best estimate of time to stone passage showed no evidence of any difference between groups, it is acknowledged that there are uncertainties concerning these data owing to the limited sample.
In line with the clinical findings, there was no evidence that the drugs tested offered any advantage in terms of cost-effectiveness, because QALY gain was equivalent across the three groups. In addition, the costs were broadly in line with the low drug costs for tamsulosin and nifedipine, and with the similar rates of further costly intervention to remove a stone between the groups.
The results of this large, pragmatic, UK-based, multicentre RCT set within routine care provide no support for the continued use of MET. Neither tamsulosin (400 µg) nor nifedipine (30 mg) reduced the need for further intervention over 4 weeks compared with placebo as part of the expectant phase of management for people presenting with a symptomatic ureteric stone.
Strengths and weakness of trial
Strengths
The SUSPEND trial was commissioned by the UK NHS to define whether or not drugs to increase the rate of ureteric stone passage, and thereby benefit patients and reduce health-care costs, were clinically useful. The SUSPEND trial was designed to fulfil this brief and, in particular, sought to embed the trial within the current standard NHS care pathway using the primary outcome measure of ‘need for further intervention’. This outcome was reliable and valid in terms of recording, attribution and relevance to patients and the NHS. The trial design and outcome measure used allows immediate implementation of the findings into routine NHS care.
During protocol development, the need to include only those people presenting for emergency treatment of a single ureteric stone was clear, because this was the group in which MET would potentially be used in the UK NHS. To achieve this, mandatory identification of a single ureteric stone by CT KUB, which has 98% diagnostic accuracy,71 was incorporated as the main inclusion criterion. During the recruitment period a number of sites were in the later stages of transition between previous imaging modalities and CT KUB recommended by the relevant guidelines. 47 Overall, this resulted in 17% of patients screened being ineligible for the trial because CT KUB had not been performed. Ineligibility as a result of a lack of CT KUB was high in the first 6 months of recruitment, but fell markedly during the recruitment period (Figure 21) as the slower adopting centres successfully implemented CT KUB as a mandatory part of their loin pain emergency care pathway during the course of the SUSPEND trial. This supports our strategy of requiring CT KUB for trial entry, which anticipated this key pathway change (see Figure 21).
FIGURE 21.
Percentage of screened participants found to be ineligible owing to lack of CT KUB during the trial.
As expected for a large trial, baseline characteristics that might influence stone passage rates were similar between groups, with no differences in age and sex distribution, or in stone size and stone location. In addition, the characteristics of the SUSPEND trial population were similar to those recently published in a cohort of people presenting with ureteric colic. 72 The proportion of participants requiring further intervention by 4 weeks in the SUSPEND trial (20%) was similar to previous RCTs that used this as a measured outcome (20%)14 and from a recent case series (14%). 72 In this trial, only 345 of the 4483 (7.6%) screened patients had a contraindication to the trial medication, suggesting that MET would be widely applicable as a therapeutic option. However, subsequent treatment discontinuation rates owing to perceived drug adverse effects were significantly higher for tamsulosin (10%) and nifedipine (17%) than for placebo (6%), which would limit routine application. Stone characteristics were also similar to the most recently reported case series, with 75% being sized at 5 mm or less and 65% being found in the lower ureter, compared with 88% being sized at 5 mm or less and 66% being found in the lower ureter in the recent case series from the Republic of Korea. 72 The design used for the SUSPEND trial ensured inclusion of only people with a single ureteric stone that could be managed expectantly as well as recruitment of a population with similar key characteristics to case series of people with ureteric colic. 72 The equivalence of the SUSPEND trial population to the total population of people who might be considered for MET means that the finding of no effect is generalisable across the target population for MET both within the UK and worldwide.
A key aspect of the trial design was to ensure that allocation of participants to trial group was concealed from participants, clinical staff, and local and central trial staff. Selection bias in terms of clinical subgroups was not present, as emphasised by the equivalence of baseline characteristics. The well-established safety profile of the medications used meant that cases of unblinding were few (six in total; 0.5%) and that blinding was maintained until trial completion and database locking, thereby minimising the risk of ascertainment bias. Given the variation in spontaneous stone passage rates observed in control groups of previous RCTs, a placebo control was chosen despite the presumed likely efficacy of the active drugs. The continued need for the placebo group was monitored throughout the trial and supported by the DMC.
By using established clinical research networks within the UK and promotion by relevant professional organisations, it was ensured that the trial recruited effectively to the planned sample size. Over the 35-month recruitment window, 1167 participants were randomised and 1136 (97%) of these had the primary outcome recorded against the targets of 1200 and 1080 set out at the start of the trial, ensuring that the findings were robust.
It was anticipated that patient-driven outcomes, such as health status and pain, would be difficult to collect and that it would not be possible to estimate time to stone passage reliably without non-routine repeated imaging for all participants. The primary outcome, need for further intervention, was chosen because it aligned with the evidence needs of patients, clinicians and health-care providers. The avoidance of further intervention is a key outcome for patients as this involves invasive procedures each with a benefit and harm profile, together with the social and economic inconvenience of further hospital attendance and recovery time. For clinicians working in managed health-care systems such as the UK NHS, reduction in demand from emergency presentations makes planned service delivery more straightforward and avoids disruption to more efficient elective care delivery. For providers of health care, any reduction in interventions will reduce requirement for non-elective activity and cost and, hence, increase efficiency. A further advantage, considering the target population of a predominantly younger working age group with stable domicile, was that the recording of the primary outcome was achievable in a high percentage and could be validated locally through routinely collected NHS data. The success of this strategy is shown by attribution of primary outcome to 98% of the trial population, allowing an unbiased precise estimate of treatment effect.
Weaknesses
The conversion rate from screening to randomisation was 26%, which may have resulted in a degree of inclusion bias, although this was not evident from the similarity of baseline characteristics between our trial population and the previous unselected case series from the Republic of Korea. 72 The proportion of women in the trial population (19%) was lower than that recorded in other cohorts such as Hospital Episode Statistics data for 2012–13 [Office of Population Censuses and Surveys Classification of Surgical Operations and Procedures (fourth revision) (OPSC) code N23; 39.5%] and the recent Korean case series (32%). 72 However, the proportion of women was balanced across the groups of the trial and there was no evidence of interaction between sex and treatment for the primary outcome. Women were more likely to be excluded from the screened population, mainly as a result of not having a diagnostic CT KUB and having a stone located within the kidney rather than ureter. An additional small number were excluded because of the need to comply with the regulatory requirements regarding contraceptive use, as tamsulosin is not licensed for use in women. 73 Despite being unable to include patients older than 65 years, owing to restrictions in nifedipine use, the overall age range was identical with previous series as was the proportion of previous stone formers.
During the trial design, a number of methods of measurement of the event of stone passage were considered before deciding on the need for further active intervention. Most previous trials in this area have recorded stone passage rates up to a suitable cut-off time, usually at 4 weeks. These trials used various methods of measuring stone passage, which were predominantly based on the absence of visible stone on repeat imaging. In the routine NHS care pathway, repeat imaging following an episode of ureteric colic is not used for asymptomatic patients, regardless of whether or not they feel they have passed the stone. Those with continued pain or signs of infection are reimaged either by ultrasound with a full bladder and plain abdominal film of kidneys, ureters and bladder or, sometimes, by repeat CT KUB. For the purposes of detecting stone passage, a combination of ultrasound and plain radiography has the advantage of low radiation dose and, despite lower diagnostic accuracy (approximately 60%) compared with CT KUB, is recommended in current guidance. 17 Repeat CT KUB has been used in a number of trials and will give a high degree of certainty regarding the presence of a stone but exposes the patient to a significant radiation dose, equivalent to about 2.5 years of background. 18 A recent report from the UK Government18 has emphasised the need for clinicians to justify every CT scan requested because of the risks of increased radiation exposure. For a single CT carried out in a 44-year-old person, this would amount to a lifetime additional cancer risk of 240 in 1 million. For this reason, repeat imaging at 4 weeks was not required to censor stone passage in our trial protocol. Rather, the active outcome of need for further intervention was measured, which has immediate consequences for the patient, clinician and health-care provider, and we were successful in documenting this for 98% of our trial population.
Reducing time to stone passage has been used as an important marker for the efficacy of MET; however, it is less appropriate for an effectiveness trial such as SUSPEND set within routine care. To measure this outcome accurately requires regular and frequent imaging, which was not logistically, ethically or economically feasible within our research setting. A strict set of criteria for inclusion of participants for this outcome was used, including (1) being reported by trial staff on the 4-week CRF; (2) being confirmed by imaging that a stone had passed; and (3) having a credible date of stone passage entered by the local research team on the 4-week CRF. Using these criteria, time to stone passage was confirmed for only 237 (21%) participants, with no difference between groups. Given that stone passage is often unnoticed following presentation with ureteric colic (except by absence of continuing pain) and considering repeat imaging was not mandatory, our results for time to stone passage have limited reliability and validity. Nevertheless, the lack of any discernible benefit from the tested drugs regarding time to stone passage from available data is consistent with the findings regarding the primary and other secondary outcomes.
The primary outcome was censored at 4 weeks, using the reasoning that most stone episodes would be completed by this time and that this end point aligned with previous trials. It could be thought, however, that a 4-week period of treatment with MET might reduce symptoms without stone passage, with symptoms returning once treatment had been completed. Data were collected up to 12 weeks and showed that a further 27 (7.1%) participants in the tamsulosin group, 25 (6.4%) in the nifedipine group and 28 (7.4%) in the placebo group had an intervention between 4 weeks and 12 weeks that was not planned at 4 weeks post randomisation. Inclusion of these data as a sensitivity analysis to the main results did not alter the finding of no difference.
The other possible benefit of MET is that it may reduce the amount of pain suffered by people as the stone descends down the ureter. The episodic nature of the pain and the community setting of stone episode follow-up presented challenges to reliable measurement of pain severity and duration during the 4-week observation period of the SUSPEND trial. Pain symptoms were recorded by responders to the 4-week participant questionnaire as the number of days that pain medication was taken for in the 4 weeks post-randomisation, a NRS for pain severity at the 4-week time point and the level of pain over the past 4 weeks on the EQ-5D. Although none of these measures showed any differences between treatment groups and the responses were consistent across the three measurements, recorded outcomes were available for only 63% of participants at 4 weeks and only 50% of participants at 12 weeks. There was no difference in response rates between trial groups, and the only baseline characteristic associated with failure to return a questionnaire was younger age, which is in accordance with findings from a previous community-based trial. 74 A number of interventions aimed at increasing questionnaire response rates were implemented throughout the trial, including pre-notification of questionnaire delivery by short message service (SMS) text message; e-mail delivery of reminder questionnaires; sending out a shortened questionnaire format; and including a monetary incentive as recognition of the burden required for questionnaire completion and appreciation for trial involvement. A £5 high-street voucher sent out with the 12-week questionnaire successfully increased response rate from 46% to 57%. There was a small, non-significant, increase in response rate to the 4-week (but not the 12-week) questionnaire following a SMS text pre-notification on questionnaire response rate (response rate was increased from 52% to 57%). The remaining interventions did not have any impact on response rate.
Results in context
Previous efficacy studies
In general, previous studies have sought to demonstrate efficacy for MET using a primary outcome of stone passage rates directly measured as no stone seen on repeat imaging. Rates of further intervention are infrequently recorded in published work, with only three trials, involving 248 participants, of tamsulosin versus control (standard therapy), reporting a mean rate of 18% compared with 31% (RR 0.58, 95% CI 0.38 to 0.90),14 and one trial, involving 140 participants, comparing nifedipine with an antispasmodic, phloroglucinol, reporting a rate of 20% versus 34% (p-value = 0.8). 15 The overall rate of further intervention in the SUPEND trial is in line with these previous reports, but in our large sample no difference was found across trial groups. Adding the SUSPEND trial results for tamsulosin into the Cochrane meta-analysis14 using a random-effects model for this outcome gave a RR of 0.76 (95% CI 0.55 to 1.05; p-value = 0.1) (Figure 22).
FIGURE 22.
Forest plot showing inclusion of the SUSPEND trial result for comparison of tamsulosin vs. control for the outcome of hospitalisation for further intervention within a previous meta-analysis conducted as part of the Cochrane review. 14 The SUSPEND study refers to the current report. df, degrees of freedom; M–H, Mantel–Haenszel.
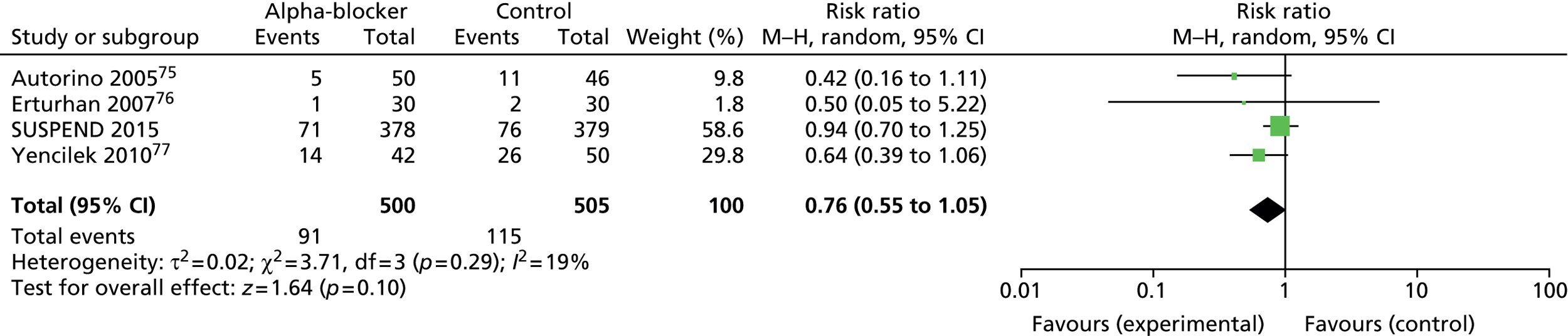
It is difficult to place these results in the context of the previous studies and associated meta-analyses because of marked differences in design, size, conduct and outcome measures. Specifically, this trial was planned to provide a ‘stand-alone’ definitive answer to whether or not MET is a clinically useful intervention in the appropriate health-care setting. This was achieved by using definitive diagnostic confirmation of the target condition by CT KUB, using broad inclusion criteria, particularly in relation to stone size and location, and recruiting to a sample size sufficient to detect an effect size that may be considered to be the minimum required to change practice. To guard against allocation and ascertainment bias, robust methods of randomisation, allocation concealment and protection of blinding until trial completion were used. A placebo control group was used in case of a no-effect result, which indeed transpired. The primary outcome was chosen to reflect the chief concern of patients, clinicians and health-care providers, while being straightforward to collect and minimising waste of trial resources. These elements set this trial apart, and it is not felt that addition of these data to previous meta-analyses is valid. Instead, it is more appropriate for seekers of evidence concerning the advisability of use of MET to consider the positive results seen from meta-analysis of a series of small efficacy-focused RCTs against the no-effect results of this large effectiveness trial.
Meaning of trial
Ureteric colic continues to be a common reason for younger people of working age to seek emergency health care, with over 30,000 episodes resulting in hospital admission recorded for NHS England in 2012–13. 66 Simple, safe therapies to reduce the need for invasive interventions and alleviate pain associated with upper urinary tract stones would probably be welcomed by patients suffering ureteric colic as well as by the clinicians treating them and providers of health care. Unfortunately, the promise shown for tamsulosin, an alpha-blocker, and nifedipine, a calcium channel blocker, in meta-analysis of smaller trials has not been borne out by this large, pragmatic, multicentre RCT set within the routine care setting in the UK NHS. Patients with ureteric colic, their clinicians and clinical guidance authorities need to consider our results in conjunction with other evidence and decide whether or not to use MET for people presenting with ureteric colic. This does require urgent consideration as the prevalence of use of MET appears to be increasing, at least in the USA, from 14% in 200949 to 64% in 2012. 50 In our view, the results of the SUSPEND trial are clear and show that MET is not effective using the agents, dose and duration tested, suggesting that this trend of increased use should be reversed.
Chapter 8 Recommendations and further research
Medical expulsive therapy had no effect in this large, pragmatic, UK-based, adequately powered, high-quality RCT with a low risk of bias. This should be considered by interested clinicians, guideline writers and health-care policy-makers, especially against the positive findings from previous meta-analyses of a number of predominantly small, low-quality trials. In particular, the key design aspects of the SUSPEND trial that directly relate to the current care pathway for people with ureteric colic in the UK NHS (including accurate diagnosis by CT KUB, expectant management at home after a short hospital stay and the outcome of need for further intervention) should be given due weight. The routine use of MET is currently recommended by relevant clinical guidance bodies, and recent cohort studies suggest that the majority of patients with ureteric colic are prescribed one of the agents, predominantly tamsulosin, although this remains an unlicensed use of the drug. The SUSPEND trial results should reverse this trend, and guideline writers will need to reconsider clinical practice recommendations and their strength in the light of our reliable and precise estimates of effect size. This is especially in light of the higher rate of participants in both the tamsulosin and nifedipine groups who discontinued trial medication owing to adverse effects compared with placebo. It is of particular importance for women (especially those of child-bearing age) prescribed tamsulosin, as this medication is not licensed for use in this patient group and, therefore, the necessary safety profile has not been established.
Our baseline measurements reinforce previous understanding that ureteric colic is associated with considerable pain and disturbance to health state, with the consequent increased use of health services and disruption of social and economic activity. The degree of ill health does appear to resolve for most sufferers of ureteric colic within 4 weeks, but there remains a minority (20% in this trial) of patients who fail to pass their stone spontaneously and in whom further intervention is required to remove it. This intervention inevitably leads to a further period of disability and ongoing use of health-care resources. Therefore, despite the null results concerning the clinical effectiveness of tamsulosin and nifedipine, there remains a need for simple treatments that can reduce the need for intervention, help relieve pain and hasten stone passage, and a number of agents are in the early phases of development. We consider research priorities to be:
-
continued early-phase work to identify putative drugs or simple devices that show efficacy to hasten or increase likelihood of stone passage
-
promising agents should be tested in multicentre studies adequately powered to demonstrate a useful treatment effect and designed to minimise important biases
-
further work is required to investigate the phenomenon of large, high-quality trials showing smaller effect size than meta-analysis of several small, lower-quality studies. In particular, uncertainty regarding the results of these meta-analyses should be better communicated to the seeker of evidence both statistically and in the written conclusions of published papers
-
work should be done to ensure that guideline-producing bodies and their writers are well-informed regarding the need for careful consideration and interpretation of findings from published meta-analyses and grade their practice recommendations cautiously.
Acknowledgements
We thank the NIHR Health Technology Assessment (HTA) programme for funding the SUSPEND trial and the staff of the NIHR HTA for their helpful administrative support. Special thanks must go to all of the SUSPEND trial participants and staff at each of our recruiting centres for taking part in this trial.
The SUSPEND trial would not have been possible without the dedication of the SUSPEND trial study office staff (Sarah Cameron, Kirsty Shearer, Karen McIntosh, Lindsay Grant, Jess Wood, Karen Innes, Kathryn Starr, Ruth Thomas and Alison McDonald) based in the CHaRT within the HSRU, University of Aberdeen. The trial received invaluable statistical support from Charles Boachie and Graeme MacLennan. Gladys McPherson and her team provided excellent information technology (IT) and database support. Fiona Stewart and Cynthia Fraser provided expert information management support. We gratefully acknowledge this support and the assistance of all in the HSRU based at the University of Aberdeen. The HSRU is core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates; however, the opinions expressed in this publication are those of the authors and may not be shared by the Chief Scientist Office.
We thank the Section of Endourology, BAUS, for carrying out audits that informed trial design and for supporting the trial throughout its design and recruitment phases.
We also thank the independent members of the TSC [Professor Mike Bishop (chairperson), Mr Frank Keely, Dr Miles Witham (in part) and Professor Sean Treweek (in part)] who provided invaluable advice and guidance during the course of the trial, and the DMC [Professor Elaine McColl (chairperson), Mr Ken Hastie, Dr Lee Shepstone and Professor John Norrie (in part)] whose voluntary support and advice was essential to the success of the SUSPEND trial.
Sincere thanks are due to the staff of the East of Scotland Research Ethics Service Research Ethics Committee 2 (formerly Fife and Forth Valley Research Ethics Committee) and the UK Medicines and Healthcare Products Regulatory Agency for considering our application and amendments during the course of the trial. The SUSPEND study team acknowledges the support of NIHR, through the Comprehensive Local Research Networks in England, Wales and Scotland.
Contributions of authors
Professor Robert Pickard (Professor of Urology and co-investigator) contributed his clinical expertise to the design of the study, recruitment, interpretation of the trial findings, and to the writing and delivery of the final report.
Ms Kathryn Starr (Trial Manager) was responsible for the day-to-day management of the study, delivery of the trial, and the writing and delivery of the final report.
Mr Graeme MacLennan (Senior Statistician and co-investigator) contributed to the statistical analysis of the study, and writing of the results and discussion chapters.
Ms Mary Kilonzo (Research Fellow, Health Economics and co-investigator) contributed to the analysis of the health economics component of the study and also to the writing of the health economics chapters.
Mr Thomas Lam (Senior Clinical Lecturer in Urology and co-investigator) contributed his clinical expertise to the design of the study, the day-to-day clinical support of the trial and the final report writing.
Dr Ruth Thomas (Research Manager and co-investigator) contributed her expertise to the design of the study, the day-to-day support of the trial and the final report writing.
Dr Jennifer Burr (ex-Director of CHaRT and co-investigator) contributed to the design of the study, the delivery of the trial and to the writing of the final report.
Professor John Norrie (Director of CHaRT) contributed to the design of the study, the delivery of the trial and the writing of the final report.
Dr Gladys McPherson (Senior IT Manager) contributed to the delivery of the trial and the writing of the final report.
Mrs Alison McDonald (Senior Trial Manager) contributed to the delivery of the trial and the writing of the final report.
Dr Kirsty Shearer (Trial Manager) contributed to the delivery of the trial and the writing of the final report.
Dr Katie Gillies (Research Fellow Health Services Research and co-investigator) contributed to the delivery of the trial and the writing of the final report.
Mr Kenneth Anson (Consultant Urological Surgeon and reader in urology) contributed to the design of the study, the delivery of the trial and the writing of the final report.
Mr Charles Boachie (Statistician) contributed to the analysis of the trial and the writing of the final report.
Professor James N’Dow (Professor of Urology and co-investigator) contributed to the design of the study and the writing of the final report.
Mr Neil Burgess (Consultant Urologist and co-investigator) contributed to the design of the study, delivery of the trial and the writing of the final report.
Mr Terry Clark (Member Stone Patient Advisory Group and co-investigator) contributed to the design of the study, the delivery of the trial and the writing of the final report.
Ms Sarah Cameron (Trial Manager) contributed to the delivery of the trial and the writing of the final report.
Professor Samuel McClinton (Professor of Urology) was the chief investigator of the study; he had complete involvement and oversight of the study design, execution and data collection, and was responsible for the final report.
Grant holders
Professor Samuel McClinton, Professor James N’Dow, Mr Thomas Lam, Mr Neil Burgess, Mr Kenneth Anson, Professor Glen Preminger, Professor Robert Pickard, Dr Uday Patel, Mrs Kathryn McMullen, Dr Jennifer Burr, Mr Graeme MacLennan, Mr Charles Boachie, Ms Mary Kilonzo, Dr Katie Gillies, Dr Patrick Wright, Dr Ruth Thomas and Mr Terry Clark.
Trial Steering Committee
Professor Mike Bishop (chairperson), Mr Frank Keely, Dr Miles Witham (in part) and Dr Sean Treweek (in part).
Data Monitoring Committee
Professor Elaine McColl (chairperson), Mr Ken Hastie, Dr Lee Shepstone (in part) and Professor John Norrie (in part before changing role to become CHaRT director).
Project Management Group
Professor Samuel McClinton, Ms Kathryn Starr, Professor James N’Dow, Mr Thomas Lam, Mr Neil Burgess, Mr Kenneth Anson, Professor Glen Preminger, Professor Robert Pickard, Dr Uday Patel, Mrs Kathryn McMullen, Dr Jennifer Burr, Mr Graeme MacLennan, Mr Charles Boachie, Ms Mary Kilonzo, Dr Katie Gillies, Dr Patrick Wright, Dr Ruth Thomas, Mr Terry Clark, Professor John Norrie, Mrs Alison McDonald and Dr Gladys McPherson.
Principal investigators
Professor Samuel McClinton, Mr Andrew Dickinson, Mr David Thomas, Mr Chandrasekharan Badrakumar, Mr Alaiyi West, Mr Ben Jenkins, Mr Alan Paul, Mr Anthony Timoney, Mr Stuart Irving, Mr Rob Calvert, Mr Matthew Bultitude, Mr Simon Phipps, Mr Kenneth Anson, Mr Paul Jones, Mr Tony Browning, Mr Robert Mason, Mr Jake Patterson, Mr James Hall, Mr Oliver Wiseman, Miss Kim Davenport, Mr Salah Albuhessi, Mr Kesavapilla Subramonian, Mr Richard Napier-Hemy, Mr Bhaskar Somani and Mr Graham Young.
Research nurses/fellows/clinical trial assistants
Gladys Makuta, Jane Sheran, Lyn Cogley, Wendy Robson, Bernadette Kilbride, Peter Murphy, Rashmi Bhardwaj, Nicola Brown, Kathleen MacLeod, Susan Walker, Eleanor Dungca, Charlotte Fox, Lorraine Lamb, Motaz El Mahdy, Wendy Wilmott, Jocelyn Keshett-Price, Tracey Stebbing, Nicola Hunt, David Tomlinson, Kee Wong, Katherine Lawrence, Marion McRury, Jane Watkins, Helen Heath, Michelle Lyall Rajab, Jordan Durrant, Andrew Symes, Chris Jones, Lisa Bastin, Bev Taylor, Jim Anderson, Jacqui Bartholemew, Kate Lindley, Andy Hall, Pauline Mercer, Barbara Finson, Gabrielle de Selincourt, Susannah Hulton, Anne Frost, Alison Hyde, Kelly Leonard, Sara Stearn, Annette Nilsson, Brian Parsons, Isi Routledge, Karen Bobruk, Katy Tucker, Richard Clark, Denise Whittaker, Julie Mitchell and Rebecca Ilyas.
Pharmacists
Kathryn McMullen, Patrician Cooper, Mike Marner, Maria Allen, Jack Oliver, Gordon Rushworth, Mary McKenzie, Jane Thompson, Rod Beard, Caroline Bedford, Simon Strange, Gail Healey, Ann Marron, Joanna Skwarski, Chi Kai Tam, Deirdre Wood, Sue Cromarty, Lisa Thomas, Jane Eastwood, Helen Kimber, Melody Cross, Helen Bowler, Naval Vyse, Bethan Willliams, Lindsay Ball, Amisha Desai, Carolyn Davies, Anna Song and Vivienne Benson.
NHS trusts and health boards
NHS Grampian, Plymouth Hospitals NHS Trust, Newcastle upon Tyne Hospitals NHS Foundation Trust, NHS Highland, South Tees Hospitals NHS Foundation Trust, City Hospitals Sunderland NHS Foundation Trust, The Leeds Teaching Hospitals NHS Trust, North Bristol NHS Trust, University Hospitals Bristol NHS Trust, Norfolk and Norwich University Hospitals NHS Foundation Trust, The Royal Liverpool and Broadgreen University Hospitals NHS Trust, NHS Lothian, Guy’s and St. Thomas’ NHS Foundation Trust, St George’s Healthcare NHS Trust, Abertawe Bro Morgannwg University Health Board, Mid Yorkshire Hospitals NHS Trust, South Devon Healthcare NHS Foundation Trust, Sheffield Teaching Hospitals NHS Foundation Trust, Cambridge University Hospitals NHS Foundation Trust, Gloucestershire Hospitals NHS Foundation Trust, University Hospitals Birmingham NHS Foundation Trust, Central Manchester University Hospitals NHS Foundation Trust, Southampton University Hospitals NHS Trust, and The University Hospital of South Manchester NHS Foundation Trust.
Publications
Pickard R, Starr K, MacLennan G, Lam T, Thomas R, Burr J, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial [published online ahead of print 19 May 2015]. Lancet 2015; in press.
Data sharing statement
All available data will be made available by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- American Urological Association . Kidney Stones 2012. www.auanet.org/education/kidney-stones.cfm (accessed November 2014).
- Diniz DH, Blay SL, Schor N. Quality of life of patients with nephrolithiasis and recurrent painful renal colic. Nephron Clin Pract 2007;106:c91-7. http://dx.doi.org/10.1159/000102995.
- Ross JA, Edmond P. The effects of calculi on ureteral function. Br J Surg 1972;59:45-9. http://dx.doi.org/10.1002/bjs.1800590112.
- Neisius A, Preminger GM. Stones in 2012: epidemiology, prevention and redefining therapeutic standards. Nature Rev Urol 2013;10:75-7. http://dx.doi.org/10.1038/nrurol.2012.253.
- Bihl G, Meyers A. Recurrent renal stone disease – advances in pathogenesis and clinical management. Lancet 2001;358:651-6. http://dx.doi.org/10.1016/S0140-6736(01)05782-8.
- Wilkinson H. Clinical investigation and management of patients with renal stones. Ann Clin Biochem 2001;38:3-7. http://dx.doi.org/10.1258/0004563011900623.
- Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project . Urologic diseases in America project: urolithiasis. J Urol 2005;173:848-57. http://dx.doi.org/10.1097/01.ju.0000152082.14384.d7.
- Bultitude M, Rees J. Management of renal colic. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5499.
- Turney BW, Reynard JM, Noble JG, Keoghane SR. Trends in urological stone disease. BJU Int 2012;109:1082-7. http://dx.doi.org/10.1111/j.1464-410X.2011.10495.x.
- Hospital Episode Statistics, Admitted Patient Care, England – 2012–13: Procedures and Interventions. Leeds: Health & Social Care Information Centre; 2013.
- Holdgate A, Pollock T. Systematic review of the relative efficacy of non-steroidal anti-inflammatory drugs and opioids in the treatment of acute renal colic. BMJ 2004;328:1401-24. http://dx.doi.org/10.1136/bmj.38119.581991.55.
- Safdar B, Degutis LC, Landry K, Vedere SR, Moscovitz HC, D’Onofrio G. Intravenous morphine plus ketorolac is superior to either drug alone for treatment of acute renal colic. Ann Emerg Med 2006;48:173-81. http://dx.doi.org/10.1016/j.annemergmed.2006.03.013.
- Preminger GM, Tiselius HG, Assimos DG, Alken P, Buck AC, Gallucci M, et al. 2007 Guideline for the management of ureteral calculi. Eur Urol 2007;52:1610-31. http://dx.doi.org/10.1016/j.eururo.2007.09.039.
- Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev 2014;4. http://dx.doi.org/10.1002/14651858.cd008509.pub2.
- Seitz C, Liatsikos E, Porpiglia F, Tiselius HG, Zwergel U. Medical therapy to facilitate the passage of stones: what is the evidence?. Eur Urol 2009;56:455-71. http://dx.doi.org/10.1016/j.eururo.2009.06.012.
- Bader MJ, Eisner B, Porpiglia F, Preminger GM, Tiselius HG. Contemporary management of ureteral stones. Eur Urol 2012;61:764-72. http://dx.doi.org/10.1016/j.eururo.2012.01.009.
- Fulgham PF, Assimos DG, Pearle MS, Preminger GM. Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: AUA technology assessment. J Urol 2013;189:1203-13. http://dx.doi.org/10.1016/j.juro.2012.10.031.
- Public Health England . Committee on Medical Aspects of Radiation in the Environment (COMARE) 16th Report: Patient Radiation Dose Issues Resulting from the Use of CT in the UK 2014. www.gov.uk/government/uploads/system/uploads/attachment_data/file/343836/COMARE_16th_Report.pdf (accessed November 2014).
- Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. Am J Roentgenol 2002;178:101-3. http://dx.doi.org/10.2214/ajr.178.1.1780101.
- Worster AS, Bhanich SW. Fluids and diuretics for acute ureteric colic. Cochrane Database Syst Rev 2012;2. http://dx.doi.org/10.1002/14651858.cd004926.pub3.
- Laerum E, Ommundsen OE, Gronseth JE, Christiansen A, Fagertun HE. Oral diclofenac in the prophylactic treatment of recurrent renal colic. A double-blind comparison with placebo. Eur Urol 1995;28:108-11.
- Phillips E, Kieley S, Johnson EB, Monga M. Emergency room management of ureteral calculi: current practices. J Endourol 2009;23:1021-4. http://dx.doi.org/10.1089/end.2008.0615.
- Holdgate A, Oh CM. Is there a role for antimuscarinics in renal colic? A randomized controlled trial. J Urol 2005;174:572-5. http://dx.doi.org/10.1097/01.ju.0000165337.37317.4c.
- Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet 2006;368:1171-9. http://dx.doi.org/10.1016/S0140-6736(06)69474-9.
- Davenport K, Timoney AG, Keeley FX. A comparative in vitro study to determine the beneficial effect of calcium-channel and alpha(1)-adrenoceptor antagonism on human ureteric activity. BJU Int 2006;98:651-5. http://dx.doi.org/10.1111/j.1464-410X.2006.06346.x.
- Hieble JP, Bondinell WE, Ruffolo RR. Alpha- and beta-adrenoceptors: from the gene to the clinic. 1. Molecular biology and adrenoceptor subclassification. J Med Chem 1995;38:3415-44. http://dx.doi.org/10.1021/jm00018a001.
- Sahin A, Erdemli I, Bakkaloglu M, Ergen A, Basar I, Remzi D. The effect of nifedipine and verapamil on rhythmic contractions of human isolated ureter. Arch Int Physiol Biochim Biophys 1993;101:245-7. http://dx.doi.org/10.3109/13813459309003918.
- Yamada S, Ito Y. α(1)-Adrenoceptors in the urinary tract. Handbook Exp Pharmacol 2011;202:283-306. http://dx.doi.org/10.1007/978-3-642-16499-6_14.
- Elliott HL, Meredith PA, Sumner DJ, McLean K, Reid JL. A pharmacodynamic and pharmacokinetic assessment of a new alpha-adrenoceptor antagonist, doxazosin (UK33274) in normotensive subjects. Br J Clin Pharmacol 1982;13:699-703. http://dx.doi.org/10.1111/j.1365-2125.1982.tb01439.x.
- Lepor H, Gup DI, Baumann M, Shapiro E. Laboratory assessment of terazosin and alpha-1 blockade in prostatic hyperplasia. Urology 1988;32:21-6.
- Lefevre-Borg F, O’Connor SE, Schoemaker H, Hicks PE, Lechaire J, Gautier E, et al. Alfuzosin, a selective alpha 1-adrenoceptor antagonist in the lower urinary tract. Br J Pharmacol 1993;109:1282-9. http://dx.doi.org/10.1111/j.1476-5381.1993.tb13762.x.
- Noble AJ, Chess-Williams R, Couldwell C, Furukawa K, Uchyiuma T, Korstanje C, et al. The effects of tamsulosin, a high affinity antagonist at functional alpha 1A- and alpha 1D-adrenoceptor subtypes. Br J Pharmacol 1997;120:231-8. http://dx.doi.org/10.1038/sj.bjp.0700907.
- Richardson CD, Donatucci CF, Page SO, Wilson KH, Schwinn DA. Pharmacology of tamsulosin: saturation-binding isotherms and competition analysis using cloned alpha 1-adrenergic receptor subtypes. Prostate 1997;33:55-9. http://dx.doi.org/10.1002/(SICI)1097-0045(19970915)33:1<55::AID-PROS9>3.0.CO;2-8.
- Forman A, Andersson KE, Henriksson L, Rud T, Ulmsten U. Effects of nifedipine on the smooth muscle of the human urinary tract in vitro and in vivo. Acta Pharmacol Toxicol (Copenh) 1978;43:111-18. http://dx.doi.org/10.1111/j.1600-0773.1978.tb02244.x.
- Golenhofen K, Lammel E. Selective suppression of some components of spontaneous activity in various types of smooth muscle by iproveratril (Verapamil). Pflugers Archiv 1972;331:233-43. http://dx.doi.org/10.1007/BF00589130.
- Hertle L, Nawrath H. Calcium channel blockade in smooth muscle of the human upper urinary tract. II. Effects on norepinephrine-induced activation. J Urol 1984;132:1270-4.
- Hertle L, Nawrath H. Calcium channel blockade in smooth muscle of the human upper urinary tract. I. Effects on depolarization-induced activation. J Urol 1984;132:1265-9.
- Salman S, Castilla C, Vela NR. Action of calcium antagonists on ureteral dynamics. Actas Urol Esp 1989;13:150-2.
- Center for Drug Evaluation & Research . Tamsulosin Hydrochloride (Flomax Capsules 0.4 Mg) 1997. www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/020579_s000.pdf (accessed November 2014).
- Joint Formulary Committee . British National Formulary October 2014 2014. www.medicinescomplete.com/mc/bnf/current/index.htm (accessed November 2014).
- Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med 2007;50:552-63. http://dx.doi.org/10.1016/j.annemergmed.2007.05.015.
- Lu Z, Dong Z, Ding H, Wang H, Ma B, Wang Z. Tamsulosin for ureteral stones: a systematic review and meta-analysis of a randomized controlled trial. Urol Int 2012;89:107-15. http://dx.doi.org/10.1159/000338909.
- Fan B, Yang D, Wang J, Che X, Li X, Wang L, et al. Can tamsulosin facilitate expulsion of ureteral stones? A meta-analysis of randomized controlled trials. Int J Urol 2013;20:818-30. http://dx.doi.org/10.1111/iju.12048.
- Bensalah K, Pearle M, Lotan Y. Cost-effectiveness of medical expulsive therapy using alpha-blockers for the treatment of distal ureteral stones. Eur Urol 2008;53:411-18. http://dx.doi.org/10.1016/j.eururo.2007.09.012.
- Ye Z, Yang H, Li H, Zhang X, Deng Y, Zeng G, et al. A multicentre, prospective, randomized trial: comparative efficacy of tamsulosin and nifedipine in medical expulsive therapy for distal ureteric stones with renal colic. BJU Int 2011;108:276-9. http://dx.doi.org/10.1111/j.1464-410X.2010.09801.x.
- LeLorier J, Gregoire G, Benhaddad A, Lapierre J, Derderian F. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med 1997;337:536-42. http://dx.doi.org/10.1056/NEJM199708213370806.
- Turk C, Knoll T, Petrik A, Sarica K, Skolarikos A, Straub M, et al. Guidelines on Urolithiasis 2014. www.uroweb.org/gls/pdf/22%20Urolithiasis_LR.pdf (accessed November 2014).
- Bandi G, Best SL, Nakada SY. Current practice patterns in the management of upper urinary tract calculi in the north central United States. J Endourol 2008;22:631-6. http://dx.doi.org/10.1089/end.2007.0186.
- Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int 2013;83:479-86. http://dx.doi.org/10.1038/ki.2012.419.
- Bagga H, Appa A, Wang R, Chi T, Miller J, Neilson J, et al. Medical expulsion therapy is underutilized in women presenting to an emergency department with acute urinary stone disease. J Urol 2013;189:e925-6. http://dx.doi.org/10.1016/j.juro.2013.02.2166.
- McClinton S, Starr K, Thomas R, McLennan G, McPherson G, McDonald A, et al. Use of drug therapy in the management of symptomatic ureteric stones in hospitalized adults (SUSPEND), a multicentre, placebo-controlled, randomized trial of a calcium-channel blocker (nifedipine) and an alpha-blocker (tamsulosin): study protocol for a randomized controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-238.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. Discussion Paper 172. York, University of York: Centre for Health Economics; 1999.
- Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis 1978;37:378-81. http://dx.doi.org/10.1136/ard.37.4.378.
- Keele KD. The pain chart. Lancet 1948;2:6-8. http://dx.doi.org/10.1016/S0140-6736(48)91787-5.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. http://dx.doi.org/10.1097/00005650-199206000-00002.
- EuroQol Group . EuroQol–a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- EudraLex: The Rules Governing Medicinal Products in the European Union. Volume 4 EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use. Annex 13 Investigational Medicinal Products. Brussels: European Commission; 2010.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Edwards PJ, Roberts I, Clarke MJ, Diguiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.mr000008.pub4.
- Brueton VC, Tierney J, Stenning S, Harding S, Meredith S, Nazareth I, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev 2013;12. http://dx.doi.org/10.1002/14651858.mr000032.pub2.
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000;355:1064-9. http://dx.doi.org/10.1016/S0140-6736(00)02039-0.
- Cuzick J. Forest plots and the interpretation of subgroups. Lancet 2005;365:1308-15. http://dx.doi.org/10.1016/S0140-6736(05)61026-4.
- Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet 2005;365:1591-5. http://dx.doi.org/10.1016/S0140-6736(05)66461-6.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. http://dx.doi.org/10.1002/sim.1981.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d40.
- NHS Reference Costs 2012–13. London: Department of Health; 2014.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. http://dx.doi.org/10.1016/S0167-6296(01)00130-8.
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. New York, NY: Oxford University Press; 2007.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c332.
- Worster A, Preyra I, Weaver B, Haines T. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med 2002;40:280-6. http://dx.doi.org/10.1067/mem.2002.126170.
- Tchey D-U, Ha YS, Kim WT, Yun SJ, Lee SC, Kim WJ. Expectant management of ureter stones: outcome and clinical factors of spontaneous passage in a single institution’s experience. Korean J Urol 2011;52:847-51. http://dx.doi.org/10.4111/kju.2011.52.12.847.
- Medicines and Healthcare Products Regulatory Agency . Flomax Relief MR (PL 00015 0280) 2009. www.mhra.gov.uk/home/groups/par/documents/websiteresources/con068200.pdf (accessed November 2014).
- Weissman J, Flint A, Meyers B, Ghosh S, Mulsant B, Rothschild A, et al. Factors associated with non-completion in a double-blind randomized controlled trial of olanzapine plus sertraline versus olanzapine plus placebo for psychotic depression. Psychiatry Res 2012;197:221-6. http://dx.doi.org/10.1016/j.psychres.2012.02.015.
- Autorino R, De Sio M, Damiano R, Di Lorenzo G, Perdona S, Russo A, et al. The use of tamsulosin in the medical treatment of ureteral calculi: where do we stand?. Urol Res 2005;33:460-4. http://dx.doi.org/10.1007/s00240-005-0508-0.
- Erturhan S, Erbagci A, Yagci F, Celik M, Solakhan M, Sarica K. Comparative evaluation of efficacy of use of tamsulosin and/or tolterodine for medical treatment of distal ureteral stones. Urology 2007;69:633-6. http://dx.doi.org/10.1016/j.urology.2007.01.009.
- Yencilek F, Erturhan S, Canguven O, Koyuncu H, Erol B, Sarica K. Does tamsulosin change the management of proximally located ureteral stones?. Urol Res 2010;38:195-9. http://dx.doi.org/10.1007/s00240-010-0257-6.
- Summary of Product Characteristics. Petyme. n.d.
- Summary of Product Characteristics. Coracten. n.d.
Appendix 1 Spontaneous Urinary Stone Passage ENabled by Drugs trial patient information leaflet
Appendix 2 Spontaneous Urinary Stone Passage ENabled by Drugs trial consent form
Appendix 3 Summary of product characteristics for the investigational medicinal products
Tamsulosin summary of product characteristics
Much of this information is reproduced from Medicines & Healthcare products Regulatory Agency78 © Crown Copyright 2015 under the Open Government Licence v3.0, https://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/.
Nifedipine summary of product characteristics
Much of this information is reproduced from Medicines & Healthcare products Regulatory Agency79 © Crown Copyright 2015 under the Open Government Licence v3.0, https://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/.
Appendix 4 Spontaneous Urinary Stone Passage ENabled by Drugs trial participant questionnaires
Appendix 5 Spontaneous Urinary Stone Passage ENabled by Drugs case report forms
Appendix 6 Algorithm to determine primary outcome
From the questions in the 4-week CRF, ‘Has the patient received any other ureteric stone treatment (excluding randomised medication)?’ and ‘Is further treatment/surgery planned for persistent ureteric stone?’, if any box was recorded as ‘yes’ then that participant has had further treatment for this episode of stone and has not spontaneously passed the stone within 4 weeks of randomisation in accordance with the definition of the primary outcome. In addition, if any box is ticked as ‘yes’ on the 12-week CRF in response to the question ‘Has the patient received any other ureteric stone treatment (excluding randomised medication)?’ and the date entered is prior to the 4-week endpoint for that individual participant, then that participant has had further treatment for this episode of stone and has not spontaneously passed the stone within 4 weeks in accordance with the definition of the primary outcome. All other participants are coded as having passed their stone spontaneously. A second validation check on this is provided by the question in the 4-week CRF: ‘Have you passed the stone?’.
Appendix 7 Full logistic regression models for the primary outcome
| Covariate | OR | 95% CI | p-value |
|---|---|---|---|
| MET | 1.06 | 0.70 to 1.60 | 0.780 |
| Stone > 5 mm | 0.34 | 0.25 to 0.45 | < 0.001 |
| Stone in upper ureter | 0.45 | 0.32 to 0.64 | < 0.001 |
| Stone in mid ureter | 0.66 | 0.41 to 1.04 | 0.070 |
| Covariate | OR | 95% CI | p-value |
|---|---|---|---|
| Nifedipine | 1.03 | 0.68 to 1.56 | 0.880 |
| Tamsulosin | 1.09 | 0.67 to 1.78 | 0.730 |
| Stone > 5 mm | 0.34 | 0.25 to 0.45 | < 0.001 |
| Stone in upper ureter | 0.45 | 0.32 to 0.64 | < 0.001 |
| Stone in mid ureter | 0.65 | 0.41 to 1.03 | 0.068 |
Appendix 8 Full breakdown of primary outcome subgroup summary
| Primary outcome subgroup summary | Intervention | ||
|---|---|---|---|
| Tamsulosin (N = 378) | Nifedipine (N = 379) | Placebo (N = 379) | |
| No further intervention, n (%) | 307 (81.2) | 304 (80.2) | 303 (79.9) |
| Subgroup, n/N (%) | |||
| Sex | |||
| Male | 252/313 (81) | 254/312 (81) | 239/297 (80) |
| Female | 55/65 (85) | 50/67 (75) | 64/82 (78) |
| Stone size, n/N (%) | |||
| ≤ 5 mm | 240/284 (85) | 246/285 (86) | 246/285 (86) |
| > 5 mm | 67/94 (71) | 58/94 (62) | 57/94 (61) |
| Stone location, n/N (%) | |||
| Upper ureter | 62/88 (70) | 58/92 (63) | 65/89 (73) |
| Mid ureter | 29/41 (71) | 32/40 (80) | 36/44 (82) |
| Lower ureter | 216/249 (87) | 214/247 (87) | 202/246 (82) |
List of abbreviations
- BAUS
- British Association of Urological Surgeons
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CT
- computerised tomography
- CTIMP
- clinical trial involving an investigational medicinal product
- CT KUB
- computerised tomography scanning of the kidneys, ureters and bladder
- DMC
- Data Monitoring Committee
- EQ-5D™
- European Quality of Life-5 Dimensions
- ESWL
- extracorporeal shockwave lithotripsy
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HSRU
- Health Services Research Unit
- IT
- information technology
- MET
- medical expulsive therapy
- MR
- modified release
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NRS
- numeric rating scale
- NSAID
- non-steroidal anti-inflammatory drug
- OR
- odds ratio
- PIL
- patient information leaflet
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnare-6 Dimensions
- SUSPEND
- Spontaneous Urinary Stone Passage ENabled by Drugs trial
- TSC
- Trial Steering Committee
- WTP
- willingness to pay