Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/116/06. The contractual start date was in July 2010. The draft report began editorial review in October 2014 and was accepted for publication in April 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dr Aimee Spector reports personal fees from NHS trusts, outside the submitted work. Professor Alistair Burns reports personal fees from the International Journal of Geriatric Psychiatry, personal fees from NHS England, non-financial support from King’s College London and non-financial support from the Driver and Vehicle Licensing Agency, outside the submitted work. Professor Robert Woods reports royalties for group cognitive stimulation therapy manuals paid to Dementia Services Development Centre Wales, Bangor University (Hawker Publication and Freiberg Press, USA) outside the submitted work. Professor Ian Russell reports grants from University College London, both for the submitted work and outside the submitted work. Lauren Yates, Professor Martin Orrell, Phuong Leung, Dr Aimee Spector, Professor Robert Woods and Dr Vasiliki Orgeta have a patent on the individual cognitive stimulation therapy manual.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Orgeta et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction to the individual Cognitive Stimulation Therapy trial
Background
Cognitive stimulation therapy for dementia
Developing and evaluating psychosocial interventions for people with dementia and their family carers is becoming increasingly important both in the UK and internationally. In fact, recent evidence indicates that psychosocial interventions are useful and may therefore be utilised either as standalone treatments or in addition to pharmacological treatments for dementia. Although a large number of non-pharmacological interventions are available, there is now consistent evidence of at least moderate quality that those interventions incorporating psychosocial1,2 or psychological approaches3 can benefit people with dementia.
Given the growing development and application of cognitive-based interventions in dementia care, it has been emphasised that each of the different approaches should be clearly defined, in order to avoid confusion in relation to the various interventions used. 4 For example, cognitive stimulation approaches allow opportunities for general enhancement of cognitive and social functioning through a range of activities, usually in a group setting, that differ from cognitive training and cognitive rehabilitation techniques. 2
Cognitive stimulation approaches originate from reality orientation (RO),5 a theoretical tradition postulating that provision of intellectual and social stimulation for people with dementia can positively affect their well-being. Cognitive stimulation interventions are, in fact, those that have been researched most frequently and have been associated with benefits for people with dementia. A recent Cochrane review, for example, has concluded that cognitive stimulation programmes can benefit cognition in people with mild to moderate dementia and may have a potential beneficial effect for self-reported quality of life. 2
Among cognitive stimulation approaches used, cognitive stimulation therapy (CST)6 is an evidence-based psychosocial intervention for people with mild to moderate dementia, consisting of structured sessions of stimulating activities delivered in a group setting. Being one of the few manualised approaches and designed to be facilitated by health and social care professionals, the original CST programme was evaluated in a single-blind, multicentre, randomised controlled trial (RCT), which reported benefits in cognition and quality of life for people with dementia living in residential care. 6 CST compares favourably with trials of acetylcholinesterase inhibitors, and economic analysis has demonstrated that CST is cost-effective. 7
Participation in cognitive stimulation approaches for people with dementia has been recommended by the National Institute for Health and Care Excellence (NICE)8 and the 2011 World Alzheimer’s Report,9 which concluded that CST interventions are among the few associated with evidence of efficacy in comparison to other psychosocial interventions currently available and should be made available to people with mild to moderate dementia. The recent Cochrane review2 suggested that, although cognitive stimulation interventions can benefit cognition, further randomised controlled studies are needed to evaluate effects of modality of delivery, such as carer-led, home-based approaches. The Cochrane review also suggested that there is currently minimal evidence in relation to in-depth qualitative studies of these approaches.
Individual cognitive stimulation approaches are likely to increase access to this intervention, specifically for those people with dementia who are unable to participate in groups owing to local service constraints, personal preference, or health or mobility issues. The justification of evaluating individual carer-led programmes stems from promising findings from studies that have applied home-based cognitive interventions. A carer-led, home-based programme of active training in memory management including cognitive stimulation, orientation and family carer counselling10 reported long-term benefits for cognition for people with dementia, reduced care home admissions and improved carer well-being. Home-based cognitive stimulation has also been associated with improvements in problem solving and memory, and a reduction in carers’ depressive symptoms,11 whereas the first RCT evaluating a home-based RO/cognitive stimulation intervention demonstrated improvements in cognition for the person with dementia. 12
Costs of dementia and use of cognitive stimulation interventions
Dementia is a health and social care priority for many countries worldwide. It is currently estimated that population ageing is likely to exert an enormous impact on the dementia epidemic, with rapid increases in the number of people affected in many countries. 13 Over 700,000 older people currently living in the UK have dementia, leading to high costs of treatment, care and support, with the current overall annual cost of dementia exceeding £26B. 14 Given that a high proportion of this overall economic impact is the value of unpaid care and support by family and other carers,14 it is important to find interventions that make good use of society’s limited resources, including the substantial inputs from carers. Cognitive stimulation approaches have been shown to be promising in this regard, being among the few post-diagnostic support interventions demonstrated to be cost-effective. 7 Moreover, given the high proportion of cost carried by unpaid carers, it is important that interventions targeting well-being in people with dementia also accommodate any associated effects on family carers and support them in their caring roles.
Aims and objectives
This report presents data on the development and evaluation of a home-based individual cognitive stimulation therapy (iCST) programme for people with dementia and their family carers. The intervention was evaluated using a pragmatic, single-blind, RCT design comparing the effectiveness of iCST to treatment as usual (TAU). The objectives of the study were the following:
-
to develop an individual, home-based programme of CST for people with dementia and their family carers
-
to assess the effectiveness of iCST in improving cognition and quality of life for people with dementia, mental and physical health in carers, and other outcomes in conjunction with TAU
-
to assess the cost-effectiveness of iCST in comparison with TAU.
Chapter 2 Intervention development
Development of individual cognitive stimulation therapy: overview and framework of development studies
The development of the iCST programme involved three separate components that informed the key characteristics of the intervention and its delivery in the main trial. We followed guidelines of the Medical Research Council15 for the development and evaluation of complex interventions characterised by multiple interacting components. 16 Following these guidelines, we used the best available evidence on group CST,2,6 appropriate theories5,17 and a series of development studies in order to develop the individual CST programme. The key objectives of the development phase were: (1) to ensure that the therapeutic materials were easy to use, clear and appropriately tailored to the needs of people with dementia and their carers; (2) to collect professional expertise data on the development of iCST; and (3) to gather data in relation to feasibility, appropriateness of materials and factors influencing fidelity. 18
Development study 1: service users’ views about the intervention
This component of the development phase involved assessing the appropriateness and acceptability of the intervention, by consulting people with dementia and their family carers using individual interviews and focus groups. During initial consultations of manual development, two family carers and two health and social care professionals were consulted. Carers’ and professionals’ feedback was sought in relation to the adaptation of group CST approaches6,19 and the key characteristics of previous individual cognitive-based interventions involving carers. 10,12 These consultations concluded that a carer-led manual should adapt similar layouts to those used in previous literature (i.e. group CST) but overall length should be reduced, academic terms should be simplified and simple instructions should be incorporated. At this stage, carers and professionals also identified the need to emphasise the dyadic nature of the intervention and to ensure that the manual is engaging. Data gathered from these first consultations resulted in the first draft of the iCST manual (and associated workbook). People with dementia and family carers taking part in the individual interviews and focus groups were recruited from local NHS and voluntary organisations in the North East London Foundation Trust. First drafts of the iCST manual and activity workbook were presented to service users for appraisal in a series of 10 individual interviews and six focus groups. Demographic characteristics of the sample taking part in Development study 1 can be seen in Appendix 1.
Ten caregiving dyads were recruited to participate in the individual interviews. People with dementia and their carers were interviewed separately, using a discussion guide that was applied to direct the content of the interviews, with each interview typically lasting 30–45 minutes. The interview with the person with dementia started with an appreciation of the concept of mental stimulation and involved completing a sample of iCST activities and collecting feedback about participants’ enjoyment and the level of difficulty. A general discussion about perceptions of, and needs for, a home-based programme of mentally stimulating activities followed. The aims of the carer interviews were similar, focusing primarily on collecting data about the quality and appropriateness of iCST, and identifying any practical issues that might affect the delivery of the programme and areas of support for carers.
Six focus groups were conducted with people with dementia and family carers. Thirty-two people participated in the groups, which aimed to identify key characteristics of mentally stimulating activities, to assess the feasibility of a home-based activity programme and to obtain feedback about the quality of materials presented. Two groups were held with carers, three groups were held with people with dementia and one group was held with both carers and people with dementia. Group discussions were conducted in a semistructured style guided by a series of pre-determined focus points. Details of the discussion points of the individual interviews and focus groups are presented in Appendix 1.
All individual interviews and focus groups were transcribed. Inductive thematic analysis techniques were employed in the coding and analysis of data,20 with meaningful excerpts of text extracted and used for categories emerging (i.e. ‘perceived barriers’). Two researchers independently reviewed all excerpts and additionally examined whether or not any could be coded to more than one category, reaching consensus over their category placement. Data from all focus groups were collated, then examined further by source (carers and people with dementia) to identify any variations in views. Individual interview data were also grouped by source and compared with data gathered from the focus groups.
The response to the first draft of the iCST manual and activity workbook was positive overall, with carers commenting that materials were clearly laid out and written in a way that was easy for people with dementia and carers to understand. Recommended changes to the manual included editorial changes to improve the clarity of instructions provided and alterations to the size of text and images. ‘Monitoring progress’ forms of overall enjoyment of activities underwent significant adjustments at this stage in response to feedback from carers that appraisal sessions should be informal in order to avoid the person with dementia feeling that their performance on the activities is being scrutinised. Appendix 1 provides a summary of the qualitative results of both the individual interview and focus group data.
Development study 2: expert feedback
The main purpose of Development study 2 was to gather ‘expert data’ on the suitability of the iCST manual and associated workbook. We used consensus methods guidelines to collect ‘expert data’ from dementia care professionals. 21 Additional objectives were to address and identify key components of the intervention in order to inform its final development. This study comprised an online survey evaluating the draft of the iCST manual and associated workbook, followed by a consensus conference. Health-care professionals, academics, service users, and private and voluntary sector professionals were invited to participate in an online survey and were asked to provide their opinions on the suitability of the iCST manual and workbook. We also sought input from European experts in dementia care.
Experts evaluated the iCST manual and activity workbook on the following domains: overall quality and layout; language used; font size; amount of information presented; clarity and variety of activities; and level of engagement. Statements were evaluated on a 5-point scale as follows: ‘strongly disagree’ (1); ‘disagree’ (2); ‘neutral’ (3); ‘agree’ (4); and ‘strongly agree’ (5). Very high agreement was defined as a median of 5, an interquartile range of 0 and ≥ 80% of participants scoring 4 or 5. High agreement was defined by a median of 5, an interquartile range of ≤ 1 and ≥ 80% scoring a 4 or 5. Moderate agreement was defined by a median value of 4–5, an interquartile range of ≤ 2 and ≥ 60% of participants scoring a 4 or 5. 22 For overall quality a 4-point scale was used: ‘poor’ (1); ‘fair’ (2); ‘good’ (3); ‘excellent’ (4).
A total of 25 dementia experts took part in the online consensus survey, of which two were family carers (8%), 11 (44%) were working in the NHS or social services, three (12%) were working in the private sector, seven (28%) were academics in dementia care and two (8%) were professionals working in the voluntary sector. Of these experts, 16 (64%) attended the consensus conference and additional workshops. Results of the online survey for the iCST manual and associated workbook appear in Appendix 2.
Additional qualitative comments from the online survey data were subjected to a thematic analysis. Analyses of these data emerged in the following key domains:
-
Person-centred focus: the activities and key principles of iCST should be more person-centred; there should be an emphasis on positive emotions and pleasurable experiences for both the person with dementia and family carer, and the manual should emphasise the dyadic nature of the intervention.
-
iCST activities: for each activity the level of difficulty of the session should be clearly separated and additional activities for people with mobility issues or compromised hearing should be accommodated. It was emphasised that activities should be accessible to all demographics and that all iCST sessions should be ‘collaborative’, highlighting that people with dementia and carers ‘work together’.
-
Layout and clarity of intervention: the manual should provide key information about the intervention only; it should incorporate a smaller and more manageable number of key principles and should emphasise that discussion is one of the key purposes of iCST by including open-ended questions.
Consensus workshops
An additional component of Development study 2 was consensus workshops inviting experts to comment on specific components of the development of the intervention, with a total of 16 experts taking part. Themes discussed were: Getting started with iCST, iCST toolkit of resources, Overview of iCST manual and associated workbook, Support for carers and Home-based training in iCST. A thematic analysis was used to analyse data in each of the four workshops. In the Getting started with iCST theme, results indicated that this aspect of the intervention would need to be tailored to individual needs and that carers should be encouraged to identify their own style, with the use of warm-up activities that incorporate person-centred interests. In relation to the iCST toolkit of resources theme, experts commented that physical games should be adapted for indoors use and have an increased cognitive component, and that maps of counties will be a useful relevant resource. In the workshop discussing the Overview of iCST manual and associated workbook theme, it was discussed that principles of iCST should avoid replication and should be shortened to a more manageable key list.
In the remaining two consensus expert workshops, issues of support for carers and set-up of home-based training for iCST were discussed. Analyses indicated emphasis by experts on the importance of empowering carers by providing extra information and help if requested, encouraging the involvement of other family members and inclusion of a Carers Diary providing easy access and navigation to sessions completed. Experts advised that a digital versatile disc (DVD) could be used for problem solving but that it should not become a barrier for carers adopting their own style.
Development study 3: field testing for final refinement prior to the main trial
The main aim of the third development study was to assess further key elements and components of adaptation of the intervention through field testing. Twenty-six people with dementia and their carers took part in this study. The majority of carers were family members of the person with dementia and were recruited either from the voluntary sector or from memory services in the North East London Foundation Trust. A small proportion of carers taking part were paid carers (n = 6), who were recruited from a private home care agency. People with dementia were screened for eligibility using the inclusion criteria of the main trial. Family carers were usually approached about the research study first by dementia care professionals (consultant old age psychiatrist) and then by the research team. A senior member of staff at the home care agency contacted potential carers working with people with mild to moderate dementia. If the dyad consented to participate, a set-up visit was arranged by local researchers. Written consent was provided by both the carer and the person with dementia at the beginning of the set-up visit. Demographic characteristics of the sample can be seen in Appendix 3.
For the purposes of the field testing, the 75-session programme was divided into six draft manuals and accompanying workbooks. Manuals 1–5 comprised a total of 60 sessions and manual 6 contained the remaining 15 sessions. Carers were asked to deliver up to 3 sessions per week. Participants were asked to complete a total of 24 sessions on average.
Training and support
Family carers were trained by a member of the research team in their own home. Paid carers were trained as a group, with training sessions lasting 1–1.5 hours. Carers were provided with materials including the iCST manual and associated workbook and additional toolkit items (such as dominoes and playing cards). The first part of the session focused on describing the programme, familiarising carers with the iCST materials and explaining key principles of the intervention. A clip of a DVD developed in the recent maintenance group CST trial19 was shown in order to demonstrate principles and examples of activities. The second part of the session focused on practising an iCST session with support from the researcher. In all set-up visits, carers were invited to participate in a session with their relative with dementia. At the group training session for paid carers, staff were divided into pairs in order to practise iCST. The guided session aimed to help carers understand the key principles of iCST and how these can be implemented in the delivery of the sessions.
Carers completed a short evaluation questionnaire at the end of the training session rating knowledge and confidence in delivering iCST, alongside information about the anticipated amount of support needed. An evaluation questionnaire was also completed by the researcher in order to assess the success of the carer’s training session and to measure additional fidelity-related components. Researchers contacted carers weekly to provide support and to gather feedback about the dyads’ experiences with the iCST programme. Carers provided additional feedback about each activity on ‘monitoring progress’ forms, rating the person with dementia’s interest, communication, enjoyment and level of difficulty of each session using a 5-point Likert scale.
Final visit
Debriefing visits were arranged with dyads who completed their allocated sessions. The purpose of the visit was to collect written feedback about the sessions and to interview the dyad about their experience in using iCST. Carers completed a modified version of the training visit evaluation questionnaire, assessing knowledge and confidence in delivering iCST, the quality of support received and their perceived success in engaging with the person with dementia. Similar data were also completed by the researcher at the end of the visit.
Appendix 3 shows number of sessions completed for dyads taking part in the field-testing phase. The average number of sessions completed was 12 out of 24, indicating that most dyads were able to complete approximately half of the sessions, as opposed to 3 sessions per week. For a total of nine dyads, additional information for each of the sessions was collected in the areas of enjoyment, communication and interest by the person with dementia. Information related to the difficulty level for each of the sessions was also collected, as well as fidelity parameters (see Appendix 3). Pre–post evaluations of the field-testing phase showed that carers’ knowledge of [before: mean = 2.78, standard deviation (SD) = 0.97; after: mean = 3.11, SD = 0.93] and confidence in iCST (before: mean = 3.78, SD = 0.83; after: mean = 4.11, SD = 0.78) increased.
Information from the field-testing phase was incorporated into the main trial, by revising sessions that were rated either low on items of interest, communication and enjoyment for the person with dementia, or rated high in levels of difficulty. Qualitative data were also collected during Development study 3 via standard telephone interviews. Carers delivering iCST were asked to comment on parameters such as barriers to being able to complete the sessions, and on the content of the manual and activity workbook. An overview of these findings appears in Appendix 3. The most common barrier in iCST was lack of time and availability for delivering the sessions followed by issues around the health of the person with dementia or the carer. An additional barrier noted by both family and paid carers was experiencing difficulties in motivating and engaging people with dementia in the sessions. When discussing potential gains, a high percentage of carers mentioned that iCST provided opportunities to spend more time with their relative and assisted in improving the caregiving relationship.
Patient and public involvement
We held initial meetings with two members of the public as part of our public and patient involvement for the iCST study. Both were family carers of people with dementia and were asked to read all previous group CST manuals and comment on their content and suitability for carer use. During the development phase of iCST and the main trial, we invited two additional family carers to be consultants on the project to comment on carer training issues and support around iCST. They both took part in meetings held to provide peer supervision to unblinded researchers supporting carers and people with dementia with iCST. Their feedback emphasised the need for paying attention to the needs of both people with dementia and family carers as well as the importance of focusing on positive aspects of the intervention during carer support.
Chapter 3 Final intervention tested in the main trial
The iCST intervention was developed primarily as a home-based programme of structured iCST for people with dementia to be delivered by carers. Dyads completed up to 3 30-minute sessions per week over 25 weeks. The programme consisted of a total of 75 themed activity sessions, including being creative, number games and art discussion (Box 1), which were intended to provide opportunities for general cognitive stimulation via a choice of specific activities. In order to accommodate personal interests, dyads were encouraged to adapt the materials provided and to take a flexible approach in relation to choosing sessions, such as omitting any activities not suited to their interests, or revisiting activities that were particularly enjoyable. Each iCST session followed a consistent structure, where the first few minutes involved engaging in discussions of orientation information prompted by family carers (i.e. day, date, weather, time, location), followed by discussion of current events (i.e. a news story, a community event or family occasion) and the main iCST activity (15–20 minutes).
-
My life.
-
Current affairs.
-
Food.
-
Being creative.
-
Number games.
-
Quizzes.
-
Sounds.
-
Physical games.
-
Categorising objects.
-
Household treasures.
-
Useful tips.
-
Thinking cards.
-
Visual clips discussion.
-
Art discussion.
-
Faces/scenes.
-
Word games.
-
Slogans.
-
Associated words.
-
Orientation.
-
Childhood.
We used recent guidelines23 aimed at improving the description of interventions evaluated in RCTs consistent with the Consolidated Standards of Reporting Trials (CONSORT) 2010 and Standard Protocol Items: Recommendations for Interventional Trials statements, which enables replication of the specific intervention tested. An overview of the iCST intervention using the Template for Intervention Description and Replication checklist and guide is presented in Table 1.
| Item | Description |
|---|---|
| Name | iCST for people with dementia |
| Why | iCST is based on theoretical principles of RO and uses the same structure and principles of the group CST approach. Carer-led iCST adapts a person-centred approach, in which the person is engaging in home-based cognitive stimulation activities. iCST activities focus more on opportunities to express opinions and less on discussing factual information, and place an emphasis on the person with dementia and their family carer spending enjoyable time together, using a specific framework of discussion |
| What | Materials |
| iCST manual (guidance for carers) | |
| iCST activity workbook (activities materials) | |
| iCST carer diary (reporting and evaluating activities) | |
| iCST toolkit (boules, playing cards, dominoes, magnifying card, sound activity compact discs, coloured pencils, and world and UK map) | |
| iCST training pack (iCST role-play exercises) | |
| Procedure | |
| Carers trained at their home, using a standardised training package aimed at demonstrating key principles of iCST. Additional support provided over the phone and additional home support visits | |
| Provider | iCST was provided by family carers. Carer training and support was provided by the research team of unblinded researchers who were either mental health nurses, clinical psychologists, occupational therapists or research assistants. All unblinded researchers received standardised training in supporting family carers in iCST |
| How | iCST was delivered by carers. iCST carer training was delivered by unblinded researchers. Unblinded researchers received training in a group. Support on iCST was provided as an additional home visit and over the phone by unblinded researchers |
| Where | iCST was delivered at the dyad’s home |
| When and how much | iCST consisted of 75 sessions delivered by the family carer for 30 minutes, three times a week, over 25 weeks |
| Tailoring | Tailoring included additional home visits or telephone support, or provision of additional resources related to the intervention (i.e. books, DVD player) |
| Modifications | No modifications occurred during the intervention |
| How well | Planned: intervention compliance was assessed by self-reported questionnaires completed by carers and researchers |
| Actual: mean compliance was 31.6 (SD = 26.8) sessions, where 22% completed 0 sessions, 13% completed 1–10 sessions and 51% completed more than 30 sessions |
Contents of individual cognitive stimulation therapy
Dyads were provided with the iCST manual, the iCST activity workbook, two carer diaries and the iCST toolkit. The iCST manual provides guidance on how to run the iCST sessions, the key principles of iCST (Box 2) and guidance on activities. This manual was disseminated at the ninth UK Dementia Congress and has been published. 24 The iCST activity workbook contains paper-based resources for activities suggested in the manual, such as word puzzles and images to stimulate discussion.
iCST should:
-
be ‘person-centred’
-
offer people with dementia choice in a range of home-based CST activities
-
focus on opinions rather than facts
-
use reminiscence
-
always use a focus such as senses, stimuli or objects
-
aim at maximising potential
-
recognise that enjoyment and fun are key for both the person with dementia and their family carer
-
provide opportunities to stimulate language
-
provide opportunities to strengthen the caregiving relationship.
Carer training
Carers were trained in their home by an unblinded researcher. A standardised training package was developed with interactive features, including a role-play exercise and the opportunity to see clips of group CST activities. The first part of the training session introduces the dyad to the iCST materials (manual, activity workbook, toolkit and carer diaries) and explains the session structure and key principles. Carers were encouraged to take part in a role-play exercise with the researcher, developed to demonstrate ‘good’ and ‘bad’ practice in iCST. In the final part of iCST training, family carers were invited to deliver their first iCST session with support from the unblinded researcher, who provided feedback afterwards. Where multiple family carers were involved in delivering the programme, the researcher invited them to be trained with the main carer.
Individual cognitive stimulation therapy support for carers
An unblinded researcher provided dyads with telephone support throughout their participation, providing weekly, fortnightly or monthly telephone support depending on carer needs and preference. Additional monitoring visits took place at 12 weeks and 25 weeks, which were aimed at collecting the iCST carer diaries, providing further support if necessary and completing measures of compliance. There were a total of 21 unblinded researchers supporting carers, of whom 81% were qualified professionals working in the local NHS trusts. Among qualified professionals, the majority were nurses (n = 13) or clinical psychologists (n = 3), with one member of staff being a qualified occupational therapist. The remaining staff were clinical studies officers (n = 2) or research assistants (n = 2). All unblinded researchers supporting carers received training in iCST.
Individual cognitive stimulation therapy fidelity
To ensure that psychosocial interventions can be replicated, and to ensure that the treatment delivered was indeed the treatment intended (known as ‘treatment integrity’),25 iCST components were described in detail in a treatment protocol. This protocol formed the basis of training of all unblinded researchers supporting and training family carers in delivering iCST. iCST diaries provided a method of tracking compliance to the programme, whereby in each theme, carers were required to record whether or not the session had been completed, the date of completion and their relative’s interest, enjoyment and communication. Space was provided for dyads to provide any additional comments related to the sessions. The progress of dyads and compliance to the programme was also measured during support activities via treatment questionnaires during both the telephone support and visits. A telephone support questionnaire was used to gather data on the average number of sessions completed per week, average duration of sessions, average time spent preparing and any difficulties encountered. Carers completed a self-report measure of confidence and knowledge in the delivery of iCST, level of engagement of the person with dementia, use of iCST principles and satisfaction with the support provided in treatment visits.
Chapter 4 Trial phase methods
Design
A multicentre, pragmatic, single-blind, two-treatment arm (iCST vs. TAU), randomised, controlled, clinical trial was conducted over 26 weeks. Participants were randomised to the two groups using a dynamic adaptive randomisation stratified for site and whether or not the person with dementia was taking acetylcholinesterase inhibitors at baseline. Data collection was at baseline, 13 weeks and 26 weeks after completion of the intervention. The primary outcomes were assessed at both time points, with the primary hypothesis examining outcomes at 26 weeks.
Ethics approval
A protocol was submitted for ethical review to the East London 3 Research Ethics Committee (REC) (reference number 10/H0701/71) in January 2010, with provisional approval being granted in July 2010. The following information was identified by the Committee as needing further clarification and amendment:
-
Participant Information Sheet to be modified to cover video-recordings and information about the control/usual care group and minor editing changes (i.e. language use)
-
consideration of a cross-over or add-on scheme and whether or not participants will be given a copy of a DVD and manual
-
provision of additional information about the interviews in both consent and participant forms and further clarification over whether or not recruitment posters and leaflets would be used.
Final approval was granted in September 2010. Participating centres obtained approval from the appropriate local REC and relevant NHS research and development departments.
Intervention and control conditions
Participants randomised to the intervention group received iCST at their own home. The control condition was TAU, with participants in this group receiving no additional intervention. The services and interventions available to people with dementia and family carers randomised to receive TAU varied between and within the iCST centres and may have changed over time. We recorded the use of drugs and services across the two groups and any changes that occurred. In general, services offered to the TAU group were also available to those in the active treatment group condition.
It is very unlikely that any comparable (or even any other) individual cognitive stimulation intervention for the person with dementia would have been available, as these types of therapies are generally unavailable in the UK. We followed standard best-practice methods around pragmatic trials involving an intervention group compared with usual care. Outside the iCST intervention, both groups, in general, would have access to the same kinds of mentally stimulating activities. It is possible that some participants in the TAU group may have engaged in some form of mentally stimulating activities in day-centres; however, this is unlikely to have been as structured as iCST. We asked sites to note instances in which the person with dementia may have been engaged in cognitive stimulation groups by their local services. Those participants who have engaged in such activities during the 3 months prior to recruitment were considered to be ineligible.
Study population
Eight centres in England and Wales were involved in the study: London, Bangor, Hull, Manchester, Norfolk and Suffolk, Dorset, Lincolnshire and Devon (covering Devon North and Devon South), which comprised 12 recruitment sites in total. Researchers in three centres (London, Bangor and Manchester) were based in universities, whereas those in Hull, Norfolk and Suffolk, Dorset, Lincolnshire and Devon were based in NHS mental health services (Table 2). Recruitment commenced in April 2012 and was completed in July 2013.
| iCST centre | iCST recruitment site |
|---|---|
| London | North East London NHS Foundation Trust |
| Barnet Enfield and Haringey Mental Health NHS Trust | |
| Bangor | Betsi Cadwaladr University Health Board |
| Hull | Hull Humber NHS Foundation Trust |
| Manchester | Manchester Mental Health and Social Care Trust |
| Lancashire Care NHS Foundation Trust Site A | |
| Lancashire Care NHS Foundation Trust Site B | |
| Dorset | Dorset Healthcare University NHS Foundation Trust |
| Lincolnshire | Lincolnshire Partnership NHS Foundation Trust |
| Norfolk and Suffolk | Norfolk and Suffolk NHS Foundation Trust |
| Devon | Devon Partnership NHS Trust |
| Northern Devon Healthcare NHS Trust |
Eligibility criteria
Inclusion
All participants were people with dementia who:
-
met the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition criteria for dementia of any type (Alzheimer’s, vascular, Lewy body type and mixed)
-
scored 10 or above on the Mini Mental State Examination (MMSE)
-
had some ability to communicate and understand communication, indicated by a score of 1 or 0 on the relevant items of the Clifton Assessment Procedures for the Elderly – Behaviour Rating Scale
-
could see/hear well enough to participate in the programme activities
-
had no major physical illness or disability affecting their participation
-
lived in the community at baseline, had regular contact with a relative or other unpaid carer who could act as an informant and could participate in the intervention.
Exclusion
People with dementia were excluded if:
-
they were not living in the community (i.e. living in a care home)
-
they had no available family carer to deliver the sessions and act as an informant.
Sample size
The main analysis was based on intention to treat for the primary outcome of cognition [Alzheimer’s Disease Assessment Scale – Cognitive Subscale (ADAS-Cog)]. Our group CST study6 had an effect size of 0.32. Our Cochrane review of RO26 found a standardised mean difference (SMD) of 0.58, whereas the individual RO/CST study12 found a SMD of 0.41. Taking a conservative approach, we estimated the SMD relative to TAU to be 0.35. A sample size of 260 will have to yield 80% power to detect a SMD of 0.35 using a two-group t-test with a 0.05 (two-sided) significance level comparing the iCST and the TAU groups. Assuming 15% attrition, we originally proposed to recruit 306 people with dementia. During the course of the trial the observed attrition rate was nearer to 25% than the 15% accounted for in the sample size calculation and, therefore, the target recruitment was revised to account for this. The final recruited sample size was 356.
Recruitment procedures
In each iCST centre, people with dementia and their family carers were recruited through mental health services for older people, such as Memory Clinics and Community Mental Health Teams, through dementia care professionals, including psychiatrists, and through local voluntary sector organisations, such as the Alzheimer’s Society (see www.alzheimers.org.uk). The centres in London, Bangor and Manchester were supported by clinical studies officers accessed through the National Institute for Social Care and Health Research Clinical Research Centre in Wales and the Dementias and Neurodegenerative Disease Research Network (DeNDRoN) in England. In Hull and the East Riding of Yorkshire, all patients and carers referred with dementia (and their general practitioners who currently have additional DeNDRoN support to assist with recruitment to dementia trials) were automatically provided with ‘opt-in information’ on current NHS portfolio studies in dementia care, via a centralised clinical academic unit, The Hull Memory Clinical Resource Centre.
The aim of the project was briefly described to potential participants by members of the research and clinical team, and permission for them to be contacted by local researchers was obtained prior to further contact. Research assistants discussed the project and provided full details to participants, answered any questions related to the project and, if participants agreed, undertook informed consent.
Informed consent
Participants enrolled to the study only after providing informed consent in line with guidelines set by the Mental Capacity Act 2005. 27 Participants were in the mild to moderate stages of dementia and were therefore expected to be competent to give informed consent for participation, provided that appropriate care was taken in explaining the research and sufficient time was allowed for them to reach a decision. It was helpful for a family member to be involved, and we aimed to ensure that this was done wherever possible. Both people with dementia and family carers were informed that no disadvantage would accrue if they chose not to participate, and all participants were provided with at least 24 hours to review information about the study prior to making a decision. In seeking consent, we followed current guidance from the British Psychological Society28 on the evaluation of capacity. In this context, consent is regarded as a continual process rather than a one-off decision, and willingness to continue participating was continually checked through discussion with participants during the assessments. If, at any point, the person with dementia or family carer became uncomfortable with the assessments, these were discontinued.
Ethical arrangements
The study was approved through the appropriate REC. All researchers received training in Good Clinical Practice guidelines. 29 There appear to be no documented harmful side effects from participating in CST interventions or other types of cognitive-based interventions. Regular monitoring by, and support from, the key local unblinded researchers in each centre was undertaken during the intervention to ensure that people with dementia participating in the iCST sessions did not feel deskilled or undervalued.
Prospective participants were fully informed of the potential risks and benefits of the project. A reporting procedure was put in place to ensure that serious adverse events (SAEs) were reported to the Chief Investigator (see Appendix 8). On becoming aware of an adverse event involving people with dementia or their carers, a member of the research team assessed whether or not it was ‘serious’. A SAE was defined as any untoward occurrence experienced by either a person with dementia or carer that:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
was otherwise considered medically significant by the investigator
-
came within the scope of the Protection of Vulnerable Adults protocol,30 which was in place to ensure that suspected cases of abuse or neglect were followed up in an appropriate manner.
A reporting form was submitted to the Chief Investigator who assessed whether or not the SAE reported was:
-
related to the conduct of the trial
-
unexpected.
Serious adverse events that were judged to be related and unexpected were to be reported to the REC and the trial Data Monitoring and Ethics Committee (DMEC) within 15 days of occurrence.
Randomisation
Remote randomisation of participant allocation treatment was undertaken via a web-based randomisation service managed by the North Wales Organisation for Randomised Trials in Health (NWORTH) clinical trials unit, after baseline assessment and informed consent. Randomisation was completed using a dynamic adapative allocation method,31 with an overall allocation ratio of 1 : 1. Random allocation was stratified by site and receipt of acetylcholinesterase inhibitors (AChEIs). For each participant randomised, the likelihood of their allocation to each treatment group is recalculated based on the participants already recruited and allocated. This recalculation is done at the overall allocation level, within stratification variables and within stratum level (the relevant combination of stratification levels). By undertaking this recalculation, the algorithm ensures that balance is maintained within acceptable limits of the assigned allocation ratio while maintaining unpredictability.
Allocation concealment
The randomisation database was held at NWORTH, and the analysts involved in the trial did not have access to the database. The dynamic adaptive algorithm is tuned using weighting parameters. These parameters are chosen by simulation modelling to ensure that the balance is maintained at an acceptable level, while ensuring that the sequence of allocations does not become predictable. Strong parameters would make the randomisation algorithm behave in a deterministic way, thus making allocation concealment difficult. Unblinded researchers were the only staff who were informed at each of the iCST centres of participants’ allocation.
Implementation
A web-based randomisation system was set up at NWORTH. Unblinded researchers could log into the system, enter participants’ details and receive randomisation results on screen and by confirmation e-mail. The system ensured that each entry had a unique trial identification number.
Blinding
As with all psychosocial interventions, participants cannot be blind to the allocation they receive. Within each iCST centre, there were nominated blinded and unblinded researchers; both were able to conduct baseline assessments and request randomisation. However, once participants were randomised, follow-up data were collected by the team of blinded researchers only, whereas the training and carer support in delivering iCST was run by unblinded researchers. Given that participants may occasionally and inadvertently inform researchers of the treatment they are receiving, we aimed to reduce this bias by use of self-report measures wherever feasible and brief reminders to participants. We asked all blinded researchers to record their impression of the group to which each participant was allocated and their confidence in that prediction. Statisticians remained blind to allocation for the main analysis, whereas compliance analysis incorporating compliance to the intervention was conducted after the main analyses only.
Data collection
Primary and secondary measures were completed at baseline, 13 weeks after baseline (week 13) and 26 weeks after baseline (week 26). Researchers were instructed to conduct all week-13 assessments within the 13-week period, but no later than 2 months from the scheduled first follow-up appointment (starting at date of baseline assessment), and to conduct all week-26 assessments by 26 weeks, but no later than 2 months from the scheduled second follow-up (starting at date of baseline assessment).
Most interviews were conducted in dyads’ homes. All questionnaire instruments were arranged in the form of booklets, with additional show cards of responses supporting the person with dementia during the assessment. If, at any point, the person with dementia felt uncomfortable with the assessment this was discontinued and was only rescheduled to take place during a second visit where appropriate.
Measures
Primary outcome measures for person with dementia
-
Cognition for the person with dementia, assessed by ADAS-Cog,32 measuring the severity of the most important cognitive symptoms of Alzheimer’s disease (AD). ADAS-Cog is the most popular cognitive testing instrument used in clinical trials of drug treatments for dementia consisting of 11 tasks assessing disturbances of memory, language, praxis, attention and other cognitive abilities, often referred to as the core symptoms of AD. This widely used test has good reliability and validity,33 and is scored from 0 to 70, with higher scores indicative of greater cognitive impairment.
-
Quality of life of the person with dementia, measured using the Quality of Life in Alzheimer’s Disease Scale (QoL-AD). 34 QoL-AD is a widely used, brief, self-report questionnaire, covering 13 domains of quality of life. The QoL-AD has good validity and reliability. 35 Both self and carer ratings were collected, in which higher scores indicate better quality of life.
Secondary outcome measures
-
Quality of life, assessed using the Dementia Quality of Life (DEMQOL) measure,36 covering five domains of quality of life, including daily activities, health and well-being, cognitive functioning, social relationships and self-concept. The scale uses self-rated reports of quality of life administered to the person with dementia. The measure was also administered to the family carer in order to collect DEMQOL-proxy ratings.
-
Neuropsychiatric symptoms, measured by the Neuropsychiatric Inventory. 37 The Neuropsychiatric Inventory assesses 10 behavioural disturbances occurring in people with dementia, using a screening strategy to minimise administration time by examining and scoring only those behavioural domains with positive responses to screening questions. Both frequency and severity of each behaviour are determined, with both validity and reliability for the measure established. 37
-
Functional ability for the person with dementia, measured by the Bristol Activities of Daily Living Scale (BADLS),38 a carer-rated instrument consisting of 20 daily-living abilities. The BADLS shows sensitivity to change in people with AD receiving anticholinesterase medication and significantly correlates with changes in the MMSE and the ADAS-Cog. 39
-
Depression, measured using the Geriatric Depression Scale (GDS)-15,40 one of the most commonly used self-rating depression scales in geriatric populations. The shorter version of the scale comprises easy-to-use items, designed to exclude somatic symptoms of depression that are also seen in non-depressed elderly people. The GDS-15 has acceptable sensitivity and specificity when used with people with mild to moderate dementia. 41
-
Quality of the relationship, measured by the Quality of Caregiver–Patient Relationship (QCPR),42 applicable to both spousal and adult child carers, completed by both the person with dementia and family carer. The QCPR has good internal consistency and concurrent validity with other measures of relationship quality and carer distress. 42
-
Use of health and social care services provided by public or non-public bodies, as measured on the Client Service Receipt Inventory (CSRI),43 adapted for use in this study. The CSRI was used to collect information on the identified carer’s costs and the participant’s use of health and social care services. Additional data collected included medications for mental health, the carer’s provision of unpaid care and employment status, and out-of-pocket costs to both participant and carer (travel expenses to health and social care appointments, payment for equipment and adaptations).
Primary outcome measures for carers
-
Mental and physical health, measured by the Short Form questionnaire-12 items (SF-12). 44 The SF-12 measures health by scoring standardised responses, which are expressed in terms of two meta-scores: the physical component summary (PCS) and the mental component summary (MCS).
Secondary outcome measures
-
Depression, measured by the Hospital Anxiety and Depression Scale (HADS),45 a self-completed measure, generating scores for generalised anxiety and depression, used widely to identify caseness for clinically significant depression and anxiety. 46
-
Health-related quality of life, measured using the three-level response version of the European Quality of Life-5 Dimensions (EQ-5D™) (hereafter EQ-5D-3L),47 a standardised instrument for use as a measure of health outcome. Applicable to a wide range of health conditions, the EQ-5D-3L provides a simple descriptive profile and a single index value for health status.
-
Resilience, measured by the Resilience Scale-14 items,48 in which responses are summed and higher scores indicate stronger resilience. The measure demonstrates high construct validity. 49
Data checking
A full data-management plan was written, encompassing data storage and processing, data filing, data sharing, data freezing and data archiving. Data were collected in questionnaire packs and entered into a data-management system (MACRO version 4.1.2.3750, InferMed, London), which was audited for data entry accuracy, before being exported to Statistical Package for the Social Sciences (SPSS) files. SPSS Predicative Analytics SoftWare version 20 (IBM Corporation, Armonk, NY, USA) was used for all further data manipulations and analysis. In all SPSS files, cleaning processes were undertaken, including checks for consistency and out-of-range data. If applicable, questionnaire data were cross-checked with the SPSS data to explore any issues of inconsistency. CSRI data were cleaned and analysed in Stata 13 (StataCorp LP, College Station, TX, USA), again checking for consistency. Adherence data were entered by unblinded researchers into the MACRO system and used in both outcomes and economic analyses.
Data analysis
Missing data for clinical effectiveness
Data were not imputed for participants who did not provide any information at a particular time point. However, standard statistical tests were employed to ensure that there were no significant differences in demographics or baseline outcome scores between those who completed at a time point and those who did not. There were two types of missing data: missing items within measures and missing measures at time points.
Missing items within measures: pro-rating
For items missing within measures, the rules for completing missing data for the relevant measure were applied. The missing data rules implemented for each measure are considered to be part of the validated tool and were therefore used as designed in line with the original validation.
Pro-rating within participant measures were undertaken at the 20% missing level (i.e. if there was one item missing for a 5-item score, this was completed with the mean of the other items).
Missing measures at time points: regression model using multiple imputation
A regression within the treatment group was applied to impute total scores in line with the trend seen in the group, as multiple imputations, allowing an assessment of the sensitivity of the data. The multiple imputation model included demographic variables such as sex, age, ethnicity, type of relationship and site. It also included the completed scores for the other outcome measures at each time point. At both follow-up time points, the model included the allocated treatment group. Scores at baseline were used to predict scores at week 13. Scores at baseline and week 13 were used to predict scores at week 26.
Baseline characteristics
No statistical tests were conducted for significant differences in baseline characteristics between the two treatment arms. 50
Interim analyses
No interim analyses were planned for the data. No additional analyses were requested or identified by the DMEC.
Primary effectiveness analyses
We used an analysis of covariance (ANCOVA) model to assess the differences between the two groups in the ADAS-Cog and the QoL-AD as the primary outcome measures for people with dementia. The dependent variable in the model was the outcome at week 26, with covariates being the baseline measurement, age of participants with dementia and relationship with the carer. The fitted fixed factors considered were sex, marital status and receipt of acetylcholinesterase inhibitors. Site was added as a random factor. Both stratification variables were included in the model (site and acetylcholinesterase inhibitors).
A similar ANCOVA model was fitted for the carer primary outcome. The dependent variable in the model was the outcome at week 26, with the covariates being the baseline measurement, age of carer and relationship with the person with dementia. The fitted fixed factors considered were sex and marital status. Site was fitted as a random factor.
Secondary effectiveness analyses
The ANCOVA model described above was used to assess the differences between the two groups on all secondary outcomes for people with dementia. A similar ANCOVA model was fitted for all carer secondary outcomes.
Additional analyses
A basic adherence analysis was undertaken. The number of iCST sessions completed was held as a continuous variable and added to the model of the main analysis. This would allow an insight into whether or not the number of sessions completed was important to the outcome.
Economic analyses
The economic evaluation was a cost-effectiveness analysis, conducted first from a health and social care perspective and, second, from a societal perspective. The primary outcome measures for the person with dementia were the incremental cost of achieving:
-
one SMD (taken to be 2.4 points on the scale) in the ADAS-Cog
-
one SMD (taken to be 1.7 points on the scale) in the QoL-AD.
The primary outcome measure for the carer was the incremental cost per quality-adjusted life-year (QALY) (derived using the EQ-5D-3L with societal weights).
In the analysis plan, the secondary economic measures for the person with dementia were set out as: QALYs derived from the DEMQOL-U and DEMQOL-Proxy-U, the MMSE, BADLS, GDS-15 and QCPR. Secondary economic measures for the carer were the HADS, MCS-12, PCS-12 and QCPR.
Valuation strategy for outcomes
Carers’ utility scores were calculated from the EQ-5D-3L, applying published societal weights. 51 We also derived utility scores for people with dementia based on self-ratings and carer proxy-ratings (the DEMQOL-U and DEMQOL-Proxy-U indexes, respectively) from the DEMQOL and DEMQOL-Proxy instruments, using published societal weights. 52 All QALYs were calculated using the area-under-the-curve method with linear interpolation between the three assessment points and the last value carried forward from the final assessment to 12 months post-baseline.
The ADAS-Cog scores were reversed so that an increased score can be interpreted as a positive change for the purposes of deriving net benefit in order to plot cost-effectiveness acceptability curves (CEACs). For the QCPR, an estimate of the points equivalent of the SMD was obtained by taking the baseline SD for the QCPR and multiplying by the effect size53 of 0.35 set for the study. This gave a difference of 3.1.
Costs
Perspective
Costs from the health and social care perspective covered services used by the person with dementia, including care/nursing home care, hospital care (inpatient, day, outpatient and accident and emergency services), primary and community health and social care. Costs from a societal perspective covered the aforesaid services, plus the costs of care and support provided by unpaid carers.
Time horizon
The economic analysis used the mean end point (week 26) outcome measure (as the primary outcome for people with dementia and secondary outcomes) and costs over the 26-week period. In the case of the ICER for the primary outcome for caregivers (cost per QALY), we calculated QALYs over the year from the baseline assessment; we likewise assumed that mean costs remained unchanged since the end of the intervention period and calculated annual equivalent costs by doubling the costs estimated for the 26-week period.
Cost data collections
Costs were calculated based on several collections:
-
data on services used by the person with dementia, as observed and reported by carers using the CSRI. 43 Service use items were collected and aggregated into cost categories
-
data on time spent by professionals (unblinded researchers) in supporting carers to deliver the training package, using one of a suite of ‘treatment adherence’ forms
-
data on professionals’ labour costs, using a pro-forma distributed to the unblinded researchers
-
data on time spent by carers to deliver the training package:
-
average time spent in preparing and in delivering the session: these data were collected by unblinded researchers during their telephone contacts, using one of a suite of ‘treatment adherence’ forms
-
number of sessions completed: this information was collected from carers who were asked to complete a workbook feedback form after every session. A manual count of sessions completed was conducted by unblinded researchers at each follow-up monitoring visit (one per follow-up period)
-
-
data on carer time spent on care and support activities, and lost employment and out-of-pocket costs, using the CSRI
-
costs of training materials (excluding costs of the initial development and testing of the package), supplied by the project management team.
Unit costs/valuation of health and social services and unpaid care
Unit costs were applied to units of resources in the estimation of per-participant costs. The base year for unit costs was 2012–13. Unit costs employed are summarised in Table 3 (unit costs and sources are given in further detail in Appendix 4). Costs of health and social care were calculated from service-use data by applying relevant, nationally generalisable unit costs [e.g. NHS reference costs54 and the Personal Social Services Research Unit (PSSRU) costs compendium55]. Costs of carers’ inputs were calculated using two methods: replacement costs and opportunity costs. 56–58 The primary analysis used opportunity costs, attaching a value equal to the national minimum wage to each hour of unpaid carer time spent on care and the cost of lost production; a replacement costs approach was employed in the sensitivity analyses (valuing time spent on care at the unit cost of a home care worker) (see Table 3). Certain out-of-pocket payments were also considered to be a cost to the dyad rather than to health and social care: the travel costs of accompanying the person with dementia to dementia-related appointments by car, public transport or taxi, and the private purchasing of equipment and adaptations.
| Service use item | Unit cost (£), 2012–13 |
|---|---|
| Inpatient bed-day, per specialty (range) | 344–1495 |
| Inpatient bed-day, weighted average across adult specialties | 577 |
| Day attendances, per specialty (range) | 540–817 |
| Day case, weighted average across specialties | 693 |
| Outpatient attendances (range) | 27–468 |
| A&E attendances, admitted and non-admitted (range) | 115–160 |
| Outpatient, weighted average of follow-up attendances across adult specialties | 98 |
| Primary, community and community mental health services, per contact (range) | 36–115 |
| Primary and community health services, per minute (range) | 0.5–3.6 |
| Residential care, per day (range) | 76–143 |
| Nursing home care, per day | 107 |
| Community-based social care, per minute (range) | 0.4–2.7 |
| Day services, per session/day (range) | 5–38 |
| Medications, standard quantity units (range) | 0.1–6.0 |
| Equipment and adaptations, cost over 3 months (range) | 0.1–104.0 |
| Carer hour, valued at replacement cost: home care worker, per hour | 19 |
| Carer hour, valued at opportunity cost: minimum wage, per hour | 6 |
Valuation strategy for intervention costs
The iCST intervention was produced from both professional and carer inputs. Unblinded researchers from the nursing and psychology disciplines worked to set up and train carers to deliver the sessions and then provided ongoing face-to-face and telephone support to carers throughout the study period.
In order to value the costs of professional time taken to deliver the intervention, we collated information on each researcher’s Agenda for Change band,59 on costs and full-time equivalents on the project. We estimated workers’ indirect and direct overheads using PSSRU unit costing methods;56 in estimating capital costs we assumed that workers were based in premises with a shared treatment space. A weighted hourly cost of professional support was then calculated based on the full-time equivalent contribution per Agenda for Change band. In addition, we calculated site-level average travel costs per visit, including professionals’ travel time and costs of mileage (unblinded researchers were asked to estimate their average travel time and miles driven in visiting participants on their iCST case load in each site). The costs of iCST training, including professional time and travel expenses and venue costs, were provided by the University College London project team. The project team also provided an estimate of the cost of the iCST training manual and materials (excluding the costs of developing the manual). We calculated an average training cost per participant and the average cost of manual materials per participant. The unit costs of professional support time and travel, training and materials costs of the intervention are summarised in Table 4. These unit costs were used to calculate a total cost of the package of materials and professional support in the cost-effectiveness analysis. We attached the weighted hourly cost of professional time to the reported telephone and face-to-face contact time for each participant, and a site average cost of mileage and travel time to reported face-to-face visits. We spread the per-participant manual and training costs, and the costs of professionals’ time spent in providing the set-up visit, across the two follow-up periods, allocating half the cost to each period.
| Costs of professional support (intervention) | Unit cost (£), 2012–13 |
|---|---|
| Total iCST training costs | 17,288 |
| Per participant (total divided by 180 intervention participants) | 96 |
| iCST manual and materials, per participant | 94 |
| Professional time, per hour | 49 |
| Mileage costs per one-way journey (per site) (range) | 4–32 |
Carers provided data on the number of iCST sessions completed over each follow-up period (see also Cost data collections); they were asked by the unblinded researchers to estimate the time spent in preparing for and delivering the sessions during scheduled telephone support calls. The average time spent in preparing and delivering sessions in each follow-up period was calculated and this estimate was used in turn to calculate the total hours spent in these activities in each period. This time was valued at the national minimum wage in the primary analysis and at the unit cost of a home care worker in the sensitivity analysis.
Missing data for cost-effectiveness
For missing service-use data (collected from the CSRI), the following rules were applied: when service use was indicated but frequency was missing, a suitable nationally applicable unit cost was used if available (e.g. cost per visit). If no suitable unit cost was available, the cost was calculated as follows: (1) establish the mean duration of use of those with frequency information; (2) assign that mean value to cases with missing duration data; (3) estimate the average cost by multiplying frequency by duration by unit cost and estimate the mean cost of those where any use of the service has been indicated; (4) assign the mean cost to cases where both frequency and duration of use information are missing. For each case, items in each cost category (see Trial results) were summed to give a total cost per category. Category-level costs were summed to give a total overall cost per case. If all costs in the category were missing, the category total (per case) was also calculated as missing; if some items were missing, these were treated as zeros and the case was assigned the cost of the sum of available costs in the category. Missing outcomes and costs data were multiply imputed separately for the person with dementia and the carer. We used the MI impute chained command in Stata 1360 to build a regression model, including demographic variables (site, whether or not acetylcholinesterase inhibitors were being taken, sex, relationship with the other member of the dyad, ethnicity, who the carer/person lived with, level of education), treatment allocation, cost categories and scale-level non-missing primary and secondary outcome measure variables as predictors. For the adherence data entered by unblinded researchers, the same procedure was followed; professional support costs were imputed within the model for imputing carers’ costs. The cost of carers’ time in delivering the intervention was imputed in cases where unblinded researchers had not recorded the time taken to prepare and deliver sessions within the adherence forms (so that the cost of the session time could not be estimated). Again, these costs were imputed within the model for imputing carers’ costs. The multiple imputation procedure was used to generate five complete data sets, to be combined according to Rubin’s rules. 60,61 Service use and intervention contact counts were not multiply imputed, only the costs. If the CSRI had not been completed (e.g. no questions or just one or two initial questions had been answered), these cases were considered wholly missing and were not included in the cost-effectiveness analyses.
Cost-effectiveness analyses
The iCST intervention was to be defined as cost-effective compared with TAU if it was:
-
less costly and more effective
-
more costly and more effective, and society is willing to pay the additional cost in order to achieve the gain in outcome
-
less costly and less effective, and society is willing to sacrifice some of the outcome difference in order to make a saving.
The iCST intervention was to be defined as not cost-effective if it was both significantly more costly and less effective compared with TAU.
The criteria for this decision was based on the following rule:
where ΔC represents the additional cost, ΔE represents the gain in outcome associated with the treatment and λ represents the willingness to pay (WTP) for that outcome gain. 62 The incremental cost-effectiveness ratio (ICER) (ΔC : ΔE) must be below λ to be considered cost-effective.
The ICER was defined as the difference in the mean costs of the iCST and TAU groups over the period of follow-up, divided by the difference in the mean end point outcome measure (primary outcome for people with dementia and secondary outcomes) between groups. In the case of the ratio of incremental costs and QALY, the denominator was the difference in the mean QALY over the year from the baseline assessment. The numerator was the difference between annualised costs, calculated by doubling the costs estimated over the full follow-up period. The decision rule can be rearranged to be expressed in terms of the net monetary benefit as λ × ΔE – ΔC > 0,62 the monetary value of gains in outcome associated with the treatment at a given WTP, net of the additional cost of the treatment. 62 CEACs were produced to represent graphically the uncertainty around the point estimate of the ICER.
Health economic modelling methods
Incremental costs and outcomes and their ratio (ΔC : ΔE) were estimated by seemingly unrelated regressions (SURs), with bootstrapped standard errors (SEs). This system of equations was used to obtain the cost/outcome difference between groups by estimating the coefficients on the intervention term in each (cost/outcome) equation. The SUR approach is useful for obtaining an estimator for the ICER and for net benefit for a given WTP, while also allowing for adjustment for a set of baseline covariates (which can differ between cost and outcome equations). 63 The analyses were performed on 300 bootstrapped replications from each complete data set (generated by the multiple imputation process), using the Stata command gsem, and the results combined. The estimates of costs and outcomes for the person with dementia were adjusted for the covariates: site, whether or not the person with dementia was taking AChEIs, who the person lived with, and baseline costs (cost equation only) or baseline outcome (outcome equation only). The estimates of costs and outcomes for the carer were adjusted for the covariates: site, whether or not the person with dementia was taking AChEls, baseline costs and who the person with dementia lived with (cost equation only), who the carer lived with, carer sex, carer age (outcome equation only) and baseline outcome (outcome equation only). This approach was used to calculate the net monetary benefit over a range of WTP values for incremental benefits (SMDs in the primary and secondary outcome measures, QALY gains), including the £20,000–30,000 NICE threshold range. 64
Summary of changes to the protocol
Approval was obtained from the REC for one substantial amendment to the protocol during the trial. This was related to the inclusion of additional questionnaires for both the person with dementia and their family carer. There was one non-substantial amendment notified to the study’s sponsor (University College London) representative, which was related to minor changes to the study protocol and inclusion of new sites and investigators. There was one protocol violation (this is described in detail in Appendix 9).
Chapter 5 Trial results
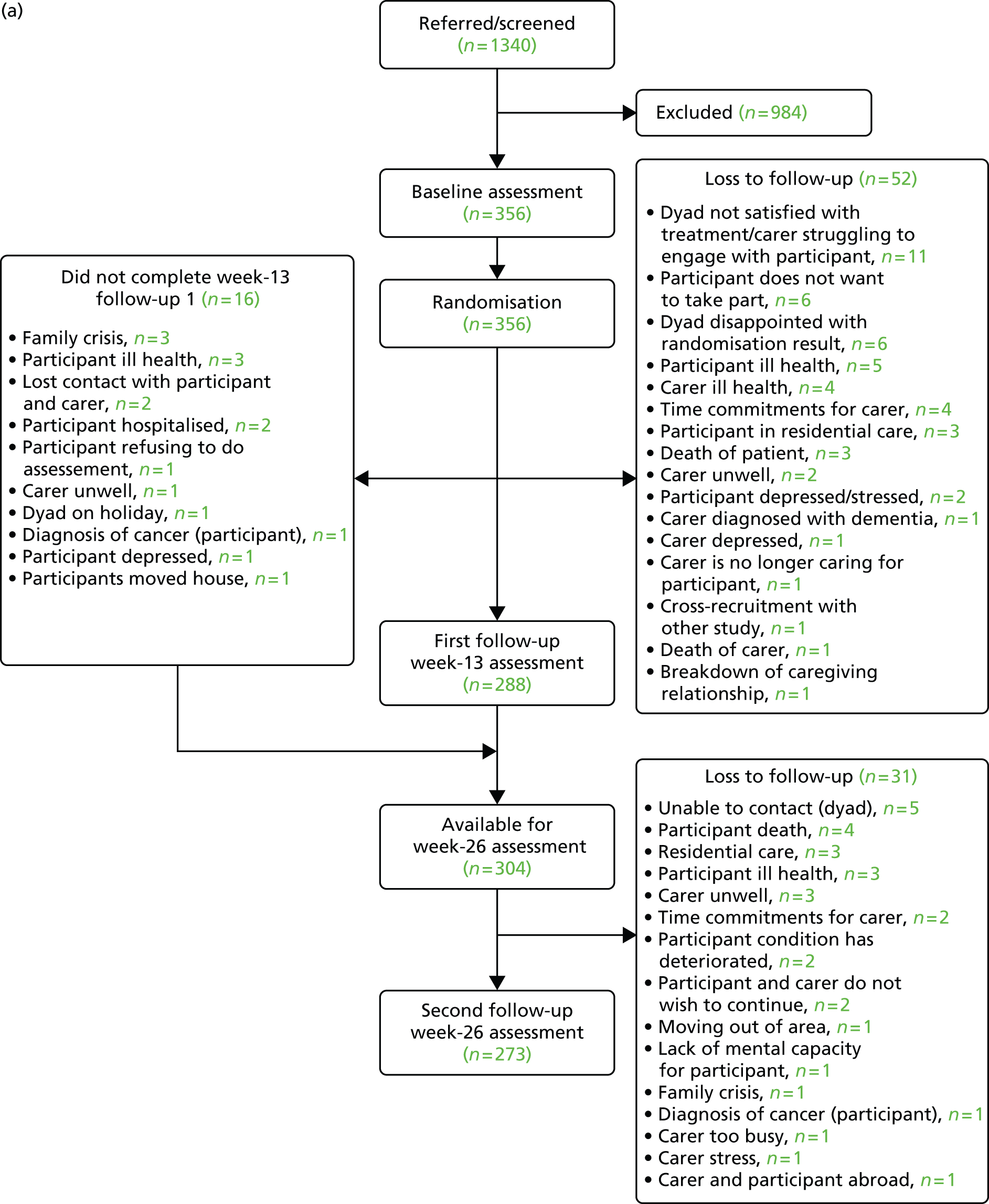
Figure 1 presents the details of the flow of participants through the trial. In total, 1340 people were considered for recruitment to the study. From these, 356 were randomised and together constituted the final sample for the study. The most common reason for loss between referral and randomisation was participants not wishing to take part in the study. Losses in 22% of cases were attributable to people with dementia not meeting the clinical criteria, indicating that this factor was, to some extent, a barrier to study recruitment (Table 5). Table 6 shows sources of referrals to the project, of which 45% came from Memory Clinics. Conversion of referrals to randomisation for each of the centres can be seen in Table 7. Variation between centres may be attributable to, in part, differences in recruitment methods (e.g. note screening vs. personal invitation by clinician).
FIGURE 1.
(a) Participant flow through trial; and (b) participant flow through trial indicating treatment allocation. DNC, did not complete.

| Reason | Total, n (%) |
|---|---|
| Does not wish to take part | 320 (24) |
| iCST exclusion criteria apply | 295 (22) |
| Dyad has not responded | 215 (16) |
| Could not make contact/reason not known | 53 (4) |
| Not available owing to holiday/family/work commitments | 33 (2) |
| Health problems for dyad | 21 (2) |
| Subtotals | 937 (70) |
| Other | |
| Prefers group activities/does activities at home/considers intervention not suitable | 18 (1) |
| Already participating in similar study | 16 (1) |
| Distressed during interview | 4 (< 1) |
| Family not discussing diagnosis | 3 (< 1) |
| Moved out the area | 3 (< 1) |
| Person with dementia has died | 3 (< 1) |
| Subtotals | 47 (3) |
| Total lost between referral/screening and randomisation | 984 (73) |
| Total number randomised | 356 (27) |
| Total referred or screened | 1340 |
| Source | Total, n (%) |
|---|---|
| Memory Clinic | 602 (45) |
| Consultant psychiatrist referral | 315 (23) |
| CMHT | 119 (9) |
| DeNDRoN clinical studies officer | 67 (5) |
| Consultant psychologist referral | 57 (4) |
| Alzheimer’s Society | 52 (4) |
| Primary care dementia practitioner | 41 (3) |
| Previous studies | 25 (2) |
| Local Voluntary Organisation | 20 (1) |
| Carers Support Services/Association | 19 (1) |
| Age Concern | 10 (< 1) |
| Newspaper article/media release | 7 (< 1) |
| Local day centre | 4 (< 1) |
| Admiral nurse | 2 (< 1) |
| Total | 1340 |
| Centre | Total referrals, n | Total randomisations, n (%) |
|---|---|---|
| London | 255 | 127 (50) |
| Bangor | 296 | 35 (12) |
| Hull | 111 | 45 (40) |
| Manchester | 482 | 53 (11) |
| Dorset | 29 | 20 (69) |
| Lincolnshire | 36 | 20 (55) |
| Norfolk and Suffolk | 83 | 28 (34) |
| Devon | 48 | 28 (58) |
| Total | 1340 | 356 |
Randomised allocation
The 356 dyads gave informed consent to the study and were randomised after completion of the baseline assessment between April 2012 and July 2013. A total of 180 dyads were randomised to iCST and 176 to TAU. Table 7 provides rates of randomisation for each of the iCST centres.
Follow-up retention rates
Retention rates at week 13
Between randomisation and week 13 there were a total of 68 losses (Table 8), of which four were deaths. Sixteen of the dyads were not available to complete the week-13 assessment but indicated availability to complete the week-26 assessment. There were no differential rates of retention between sites at week 13 [χ2 = 11.9; degrees of freedom = 11; p-value = 0.37].
| Centre | Baseline | Completed week 13 (retention rate), n (%) | Completed week 26(retention rate), n (%) |
|---|---|---|---|
| London | 127 | 101 (79) | 96 (76) |
| Bangor | 35 | 30 (86) | 31 (89) |
| Hull | 45 | 34 (75) | 32 (71) |
| Manchester | 53 | 39 (74) | 37 (70) |
| Dorset | 20 | 18 (90) | 18 (90) |
| Lincolnshire | 20 | 16 (80) | 14 (70) |
| Norfolk and Suffolk | 28 | 26 (93) | 23 (82) |
| Devon | 28 | 24 (86) | 22 (79) |
| Total | 356 | 288 (81) | 273 (77) |
Retention rates at week 26
At week 26 (see Table 8), a further 31 dyads were lost to follow-up, which included a further four deaths, equating to a total of 83 losses (including eight deaths) and a retention rate of approximately 77%, which was the predicted rate used in the updated sample size calculations. There were no differential retention rates between sites at week 26 (χ2 = 12.5; degrees of freedom = 11; p-value = 0.33). In terms of losses to follow-up from the study (i.e. excluding deaths), the attrition rate was 21%.
Retention rates by allocated group
Table 8 indicates that there was a total of 83 losses to follow-up. There were 46 (25%) losses in the intervention group and 37 (21%) in the TAU group. Analyses comparing the baseline characteristics of those who dropped out in the intervention group versus those in the TAU group did not indicate any significant differences. Baseline characteristics for the test included sex, ethnicity, marital status, relationship with the person with dementia, living with the person with dementia or not, living with other, living alone, highest level of education and age.
Ratings of perception of allocation of dyads by blinded researchers
A total of 264 perception ratings were completed during week 13. These perception ratings asked the blinded researcher to assess, after a follow-up visit, which treatment the dyad had been allocated to using a 5-point Likert-type scale (‘definitely in iCST group’, ‘more likely to be in iCST group’, ‘equally likely to be in iCST or TAU’, ‘more likely to be in TAU group’, ‘definitely in TAU group’). Table 9 provides the results of these perception ratings in terms of the proportion that were correct, neutral and incorrect for treatment allocation at week 13. A total of 60% of blinded researchers rated dyads as being equally likely to receive iCST or TAU, with 23% making a correct judgement, of which 7% of these ratings were a definite judgement, and 17% making an incorrect judgement, of which only 5% were definite.
| Researcher rating | Actual treatment allocation (N = 264) | ||
|---|---|---|---|
| iCST, n (%) | TAU, n (%) | Total, n (%) | |
| Correct ‘definite’ judgement | 13 (12) | 6 (4) | 19 (7) |
| Correct ‘more likely’ judgement | 14 (13) | 28 (18) | 42 (16) |
| Equally likely to be in iCST or TAU | 68 (65) | 92 (58) | 160 (60) |
| Incorrect ‘more likely’ judgement | 11 (10) | 20 (13) | 31 (12) |
| Incorrect ‘definite’ judgement | 0 | 12 (7) | 12 (5) |
| Total | 106 | 158 | 264 |
A total of 255 perception ratings were completed during week 26. Table 10 provides the results of these perception ratings in terms of the proportion that were correct, neutral and incorrect for treatment allocation at week 26. In a similar manner to the judgements for week 13, 57% of researchers rated treatment allocation equally to iCST and TAU, with a total of 23% making a correct judgement, of which 10% were definite judgements. A similar percentage to week 13 (20%) also made an incorrect judgement about dyad allocation.
| Researcher rating | Actual treatment allocation (N = 255) | ||
|---|---|---|---|
| iCST, n (%) | TAU, n (%) | Total, n (%) | |
| Correct ‘definite’ judgement | 22 (19) | 4 (3) | 26 (10) |
| Correct ‘more likely’ judgement | 17 (15) | 17 (12) | 34 (13) |
| Equally likely to be in iCST or TAU | 65 (57) | 80 (57) | 145 (57) |
| Incorrect ‘more likely’ judgement | 10 (9) | 31 (22) | 41 (16) |
| Incorrect ‘definite’ judgement | 0 | 9 (6) | 9 (4) |
| Total | 114 | 141 | 255 |
Analysis
Baseline characteristics by treatment allocation
Demographic information
Demographics information for people with dementia and their family carers appears in Tables 11 and 12, respectively. Table 13 provides the means and SDs for age for people with dementia and their carers. There were 226 spousal and 130 non-spousal caregiving dyads. For the 130 non-spousal dyads there were 113 carers (113/356, 31.7%) who were the son or daughter, son-in-law or daughter-in-law, or brother or sister of the person with dementia. The remaining carers were described as having other relationships with the person with dementia (9/356, 2.5%) or as being another relative (8/356, 2.2%). At baseline, 270 people with dementia were taking AChEIs. In practice, it would be expected that a relatively small proportion would be stopping/starting AChEIs and randomisation should mean that this would be consistent across groups.
| Characteristic | Total, n/N (%) | iCST, n/N (%) | TAU, n/N (%) |
|---|---|---|---|
| Sex | |||
| Female | 165/356 (46) | 83/180 (50) | 82/176 (50) |
| Ethnicity | |||
| White | 331/356 (93) | 164/180 (50) | 167/176 (50) |
| Marital status | |||
| Married/cohabiting/civil partnership | 252/356 (71) | 125/180 (50) | 127/176 (50) |
| Living situation | |||
| Living with spouse/partner | 225/356 (63) | 113/180 (50) | 112/176 (50) |
| Highest level of education | |||
| School leaver (14–16 years) | 213/356 (60) | 113/180 (53) | 100/179 (47) |
| Taking acetylcholinesterase inhibitors | |||
| Yes | 270/356 (76) | 136/180 (76) | 134/176 (76) |
| Characteristic | Total, n/N (%) | iCST, n/N (%) | TAU, n/N (%) |
|---|---|---|---|
| Sex | |||
| Female | 261/356 (73) | 135/180 (52) | 126/176 (48) |
| Ethnicity | |||
| White | 329/356 (92) | 164/180 (50) | 166/176 (50) |
| Marital status | |||
| Married/cohabiting/civil partnership | 297/356 (84) | 149/180 (50) | 148/176 (50) |
| Living situation | |||
| Living with spouse/partner | 236/356 (66) | 119/180 (50) | 117/176 (50) |
| Highest level of education | |||
| School leaver (14–16 years) | 156/356 (45) | 79/180 (50) | 80/179 (50) |
| Dyad participant | iCST | TAU | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Person with dementia | 180 | 78.40 | 7.30 | 176 | 78.00 | 7.70 | 356 | 78.20 | 7.49 |
| Carer | 179a | 66.01 | 12.76 | 174 | 65.49 | 13.11 | 353a | 65.73 | 12.92 |
| Spousal carer | 112 | 73.11 | 7.57 | 111 | 72.50 | 8.22 | 223a | 72.80 | 7.89 |
| Non-spousal carer | 67 | 54.13 | 10.67 | 63 | 53.16 | 11.00 | 130 | 53.66 | 10.80 |
Table 14 details the sex of carers/participants in the caregiving dyads. Details of dementia diagnosis for the sample are provided in Table 15. A total of 68% of participants had a diagnosis of AD alone, 13% had a diagnosis of vascular dementia and 8% had a diagnosis of AD in combination with vascular dementia.
| Sex of carer and participant in caregiving dyads | Sex of person with dementia | |
|---|---|---|
| Female | Male | |
| Sex of carer | 165 | 191 |
| Female | 82 | 179 |
| Male | 83 | 12 |
| Total | 165 | 191 |
| Diagnosis | Total, n/N (%) | iCST, n/N (%) | TAU, n/N (%) |
|---|---|---|---|
| AD | 227/355 (64) | 108/179 (60) | 119/176 (68) |
| Vascular dementia | 40/355 (11) | 18/179 (10) | 22/176 (13) |
| Lewy body dementia | 11/355 (3) | 5/179 (3) | 6/176 (3) |
| Mixed AD and vascular dementia | 36/355 (10) | 22/179 (12) | 14/176 (8) |
| Not known | 41/355 (12) | 26/179 (15) | 15/176 (8) |
Severity of dementia was measured by the Clinical Dementia Rating Scale (CDR),65 and general cognition by the MMSE. 66 A total of 70% of the sample had a CDR score of 1, 18% had a CDR score of 0.5, 12% had a CDR score of 2, and one person with dementia received a score of 0. The total mean MMSE score for the sample was 21.23 (SD = 4.30), with those allocated to iCST scoring a mean of 21.12 (SD = 4.48) and those allocated to TAU scoring a total of 21.33 (SD = 4.11).
Primary analyses of outcomes
The mean values for the iCST group and TAU group at each of the time points are given in Table 16. ANCOVA models were fitted for each of the measures. The primary model fitted was ANCOVA using the week-26 time point as the dependent variable and site as the random factor. Fixed factors were marital status, living status, sex of participant, whether or not the person with dementia was currently on AChEIs and treatment allocation. Fitted covariates were age, baseline outcome score and relationship with the carer. Models have also been fitted using the shorter-term week-13 follow-up as an outcome.
| Outcome measure | Baseline | Week 13 | Week 26 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mis., n | iCST (n = 180), mean (SD) | Mis., n | TAU (n = 176), mean (SD) | Mis., n | iCST (n = 142), mean (SD) | Mis., n | TAU (n = 146), mean (SD) | Mis., n | iCST (n = 134), mean (SD) | Mis., n | TAU (n = 139), mean (SD) | |
| Person with dementia | ||||||||||||
| ADAS-Cog | 1 | 21.47 (9.22) | 1 | 19.79 (8.03) | 4 | 20.86 (9.73) | 6 | 19.50 (8.97) | 6 | 20.69 (9.39) | 5 | 20.39 (9.91) |
| QoL-AD | 38.01 (5.44) | 37.96 (6.04) | 2 | 37.90 (5.52) | 2 | 38.09 (5.63) | 5 | 37.86 (5.13) | 1 | 37.71 (5.91) | ||
| DEMQOL | 3 | 93.85 (11.76) | 3 | 92.18 (13.55) | 7 | 94.08 (10.92) | 4 | 94.05 (11.80) | 6 | 95.46 (11.17) | 3 | 95.12 (11.11) |
| NPI total | 11.21 (13.96) | 10.99 (11.98) | 2 | 10.67 (13.30) | 12.07 (12.61) | 1 | 11.57 (13.72) | 1 | 11.59 (12.80) | |||
| GDS-15 | 3 | 3.14 (2.64) | 3 | 3.16 (3.15) | 9 | 2.98 (2.56) | 3 | 3.03 (2.86) | 8 | 2.90 (2.55) | 3 | 2.85 (2.67) |
| QCPR total | 6 | 55.17 (8.89) | 2 | 56.72 (8.73) | 4 | 56.30 (8.98) | 3 | 55.82 (9.06) | 3 | 56.88 (8.59) | 1 | 55.55 (10.25) |
| QCPR (warmth) | 1 | 33.19 (5.08) | 1 | 33.98 (5.20) | 1 | 33.32 (5.49) | 33.25 (5.38) | 1 | 33.78 (4.97) | 33.07 (5.92) | ||
| QCPR (criticism and conflict) | 1 | 22.07 (4.78) | 1 | 22.69 (4.66) | 1 | 22.80 (4.46) | 22.54 (4.75) | 22.95 (4.59) | 22.46 (5.15) | |||
| MMSE | 21.12 (4.48) | 21.33 (4.11) | 1 | 20.59 (5.02) | 2 | 20.89 (4.83) | 4 | 20.68 (4.76) | 1 | 21.19 (5.21) | ||
| BADLS [P] | 5.16 (5.45) | 4.49 (4.09) | 14.53 (10.34) | 1 | 13.55 (8.20) | 3 | 15.39 (10.78) | 1 | 14.56 (8.86) | |||
| QoL-AD [P] | 0 | 32.88 (6.83) | 33.09 (6.22) | 1 | 32.64 (6.25) | 2 | 31.93 (5.84) | 1 | 32.46 (6.20) | 31.99 (6.30) | ||
| DEMQOL [P] | 1 | 97.99 (13.17) | 2 | 98.59 (12.76) | 2 | 99.26 (12.38) | 1 | 98.75 (11.96) | 1 | 99.42 (12.41) | 1 | 98.18 (12.80) |
| Carer | ||||||||||||
| SF-12 PCS | 1 | 51.46 (10.25) | 1 | 50.20 (10.32) | 51.13 (9.73) | 49.97 (10.34) | 50.06 (10.53) | 1 | 48.63 (10.87) | |||
| SF-12 MCS | 1 | 49.42 (8.10) | 1 | 48.14 (9.42) | 47.98 (9.90) | 2 | 47.93 (9.96) | 48.88 (9.41) | 47.88 (10.13) | |||
| HADS (total) | 3 | 9.63 (6.11) | 10.02 (6.67) | 1 | 10.37 (6.98) | 10.41 (6.89) | 1 | 10.06 (6.92) | 11.16 (7.59) | |||
| HADS (anxiety) | 3 | 5.84 (3.58) | 6.03 (3.88) | 1 | 6.33 (4.35) | 6.06 (4.00) | 1 | 6.03 (4.32) | 6.36 (4.44) | |||
| HADS (depression) | 3 | 3.79 (3.30) | 3.99 (3.40) | 1 | 4.03 (3.30) | 4.36 (3.48) | 1 | 4.03 (3.29) | 4.80 (3.81) | |||
| EQ-5D-3L health state today | 3 | 78.37 (16.63) | 76.24 (19.28) | 1 | 78.13 (16.84) | 76.45 (16.93) | 78.63 (17.09) | 76.58 (16.90) | ||||
| EQ-5D-3L calculated utility value | 1 | 0.83 (0.20) | 0.81 (0.21) | 1 | 0.82 (0.20) | 0.79 (0.22) | 2 | 0.82 (0.21) | 0.75 (0.25) | |||
| RS-14 | 2 | 83.39 (10.98) | 1 | 83.63 (10.43) | 83.27 (11.14) | 83.49 (10.47) | 1 | 83.44 (10.86) | 81.83 (12.90) | |||
| NPI (carer distress) | 3.15 (2.67) | 3.23 (2.59) | 2 | 3.13 (2.48) | 3.18 (2.39) | 1 | 3.11 (2.65) | 1 | 3.25 (2.41) | |||
| QCPR total | 3 | 59.21 (6.67) | 3 | 58.21 (6.63) | 8 | 60.12 (6.33) | 4 | 59.73 (6.67) | 9 | 60.14 (6.54) | 4 | 59.76 (6.77) |
| QCPR (warmth) | 3 | 34.94 (3.77) | 3 | 34.59 (3.64) | 8 | 35.20 (3.66) | 4 | 35.69 (3.45) | 8 | 35.25 (3.57) | 4 | 34.95 (3.51) |
| QCPR (criticism and conflict) | 3 | 24.26 (3.63) | 2 | 23.63 (3.81) | 8 | 24.92 (3.57) | 4 | 24.04 (4.41) | 9 | 24.90 (3.66) | 4 | 24.81 (4.01) |
The number of cases with missing data, ANCOVA group means, mean differences, 95% confidence intervals (CIs) of mean differences and p-values for the original data to compare the iCST and TAU groups for the dementia patient outcome measures at week 26 and week 13, after adjusting for the baseline outcome measures and covariates, are given in Tables 17 and 18. There was little difference between the imputed value data and complete data; therefore, for ease of reading, complete case data have been presented. The carer outcome measures at week 26 and week 13 after adjusting for the baseline outcome measures are given in Tables 19 and 20.
| Outcome measure | Missing | iCST (n = 134) | TAU (n = 139) | MD | 95% CI of MD | p-value |
|---|---|---|---|---|---|---|
| ADAS-Cog | 11 | 20.03 | 20.58 | –0.55 | –2.00 to 0.90 | 0.45 |
| QoL-AD | 6 | 37.90 | 37.92 | –0.02 | –1.04 to 1.00 | 0.97 |
| DEMQOL | 9 | 94.45 | 94.14 | 0.31 | –1.62 to 2.22 | 0.79 |
| NPI [P] | 2 | 8.10 | 8.42 | –0.32 | –2.78 to 2.12 | 0.79 |
| GDS-15 | 11 | 3.29 | 3.31 | –0.02 | –0.51 to 0.47 | 0.94 |
| QCPR (total)a | 4 | 57.42 | 55.65 | 1.77 | 0.26 to 3.28 | 0.02 |
| QCPR (warmth) | 1 | 33.74 | 32.93 | 0.81 | –0.11 to 1.73 | 0.09 |
| QCPR (criticism and conflict) | 23.51 | 22.65 | 0.86 | –1.74 to 0.02 | 0.06 | |
| MMSE | 5 | 19.63 | 20.10 | –0.47 | –1.26 to 0.30 | 0.23 |
| BADLS [P] | 4 | 11.91 | 12.57 | –0.66 | –2.07 to 0.75 | 0.36 |
| QoL-AD [P] | 1 | 32.45 | 32.00 | 0.45 | –0.71 to 1.60 | 0.45 |
| DEMQOL [P] | 2 | 99.67 | 97.94 | 1.73 | –0.61 to 4.07 | 0.15 |
| Outcome measure | Missing | iCST (n = 142) | TAU (n = 146) | MD | 95% CI of MD | p-value |
|---|---|---|---|---|---|---|
| ADAS-Cog | 10 | 22.00 | 21.71 | 0.29 | –1.10 to 1.68 | 0.68 |
| QoL-AD | 4 | 38.40 | 38.54 | –0.14 | –1.12 to 0.84 | 0.78 |
| DEMQOL | 11 | 91.72 | 92.05 | –0.33 | –2.31 to 1.65 | 0.74 |
| NPI [P] | 2 | 12.27 | 13.72 | –1.46 | –3.68 to 0.76 | 0.20 |
| GDS-15 | 12 | 3.27 | 3.36 | –0.09 | –0.56 to 0.38 | 0.71 |
| QCPR (total) | 7 | 56.62 | 55.52 | 1.10 | –0.15 to 2.35 | 0.09 |
| QCPR (warmth) | 1 | 34.04 | 33.65 | 0.39 | –0.43 to 1.21 | 0.36 |
| QCPR (criticism and conflict) | 1 | 22.49 | 21.85 | 0.63 | –0.10 to 1.36 | 0.09 |
| MMSE | 3 | 20.32 | 20.16 | 0.16 | –0.60 to 0.92 | 0.69 |
| BADLS [P] | 1 | 12.73 | 12.93 | –0.20 | –1.44 to 1.04 | 0.75 |
| QoL-AD [P] | 3 | 32.66 | 31.91 | 0.75 | –0.27 to 1.77 | 0.15 |
| DEMQOL [P] | 3 | 99.28 | 98.73 | 0.55 | –1.70 to 2.80 | 0.64 |
| Outcome measure | Missing | iCST (n = 134) | TAU (n = 139) | MD | 95% CI of MD | p-value |
|---|---|---|---|---|---|---|
| SF-12 PCS | 1 | 49.57 | 49.11 | 0.46 | –1.21 to 2.13 | 0.59 |
| SF-12 MCS | 0 | 48.44 | 48.31 | 0.13 | –1.65 to 1.91 | 0.89 |
| HADS total | 1 | 10.27 | 10.96 | –0.70 | –1.85 to 0.46 | 0.24 |
| HADS (anxiety) | 1 | 6.09 | 6.30 | –0.21 | –0.94 to 0.52 | 0.57 |
| HADS (depression) | 1 | 4.16 | 4.67 | –0.51 | –1.09 to 0.08 | 0.09 |
| EQ-5D-3L health state today | 0 | 78.20 | 76.99 | 1.21 | –2.14 to 4.57 | 0.48 |
| EQ-5D-3L calculated utility valuea | 2 | 0.82 | 0.76 | 0.06 | 0.01 to 0.10 | 0.01 |
| RS-14 | 1 | 83.42 | 81.85 | 1.58 | –0.37 to 3.52 | 0.11 |
| NPI (carer distress) | 2 | 3.13 | 3.22 | –0.09 | –0.55 to 0.37 | 0.70 |
| QCPR total | 13 | 59.65 | 60.21 | –0.56 | –1.93 to 0.82 | 0.43 |
| QCPR (warmth) | 12 | 35.05 | 35.13 | –0.08 | –0.84 to 0.68 | 0.83 |
| QCPR (criticism and conflict) | 13 | 24.65 | 25.05 | –0.40 | –1.19 to 0.39 | 0.32 |
| Outcome measure | Missing | iCST (n = 134) | TAU (n = 139) | MD | 95% CI of MD | p-value |
|---|---|---|---|---|---|---|
| SF-12 PCS | 0 | 50.51 | 50.57 | –0.06 | –1.45 to 1.33 | 0.93 |
| SF-12 MCS | 0 | 47.59 | 48.30 | –0.71 | –2.34 to 0.92 | 0.39 |
| HADS total | 1 | 10.47 | 10.31 | 0.16 | –0.81 to 1.15 | 0.74 |
| HADS (anxiety) | 1 | 6.34 | 6.05 | 0.29 | –0.35 to 0.91 | 0.37 |
| HADS (depression) | 1 | 4.13 | 4.27 | –0.14 | –0.67 to 0.39 | 0.60 |
| EQ-5D health state today | 1 | 77.55 | 77.00 | 0.55 | –2.59 to 3.69 | 0.73 |
| EQ-5D calculated utility value | 1 | 0.81 | 0.79 | 0.02 | –0.02 to 0.06 | 0.19 |
| RS-14 | 0 | 83.35 | 83.41 | –0.06 | –1.63 to 1.51 | 0.94 |
| NPI (carer distress) | 2 | 3.16 | 3.15 | 0.01 | –0.43 to 0.43 | 0.99 |
| QCPR total | 12 | 59.90 | 59.94 | –0.04 | –1.45 to 1.37 | 0.95 |
| QCPR (warmth) | 12 | 35.10 | 35.78 | –0.68 | –1.44 to 0.08 | 0.09 |
| QCPR (criticism and conflict) | 12 | 24.80 | 24.16 | 0.64 | –0.23 to 1.53 | 0.15 |
A further analysis, in which imputation of the entire data set (356 participants) using the regression model is detailed, can be found in Appendix 6. The tables presented there indicate the results for the data if the multiple imputation model had been used to impute the data for all 356 participants at each time point. Various methodologies for different types of missing data (e.g. death vs. ill health) have not been taken into account as the number of each of these circumstances was minimal and would have limited impact on the results of any imputation method. Ten imputation sets were created to establish a robust adjustment to the data missing from each time point. There was no substantial difference between any of the results presented.
Person with dementia outcomes
The ADAS-Cog primary outcome for people with dementia was not statistically significant between the iCST and TAU groups either at week 13 or at the primary time point of week 26. However, between week 13 and week 26, the estimated adjusted marginal means decreased more in the iCST group than in the TAU group, with a lower ADAS-Cog score being an improvement. For QoL-AD, there was no significant difference between the groups. Similarly, there were no differences between the groups for most secondary outcomes. There was a significant improvement for the iCST group relative to the TAU group for QCPR total score with a mean difference of 1.77 (95% CI 0.26 to 3.28; p-value = 0.02) at week 26. There was no evidence of significant differences for primary outcomes or secondary outcomes at week 13.
Carer outcome measures
There was no statistically significant difference in the primary outcome of SF-12 for carers between iCST and TAU at either week 26 or week 13. At week 26, the EQ-5D calculated utility value for the carer was significantly better for the iCST group with a mean difference of 0.06 (95% CI 0.01 to 0.10; p-value = 0.014). At week 26 there was an indication that the HADS depression subscale was lower in the iCST than in the TAU group, with decreases in depressive symptoms of –0.51 (95% CI –1.09 to 0.08; p-value = 0.090), but this did not reach significance.
At week 13 there were no significant differences between the two groups for any of the secondary outcome measures. The QCPR warmth subscale was close to significance between the iCST and TAU groups, with a mean difference of –0.68 (95% CI –1.44 to 0.08; p-value = 0.09) (see Table 19). In view of these results, and the fact that the QCPR measure for carers had the most missing data, we have also presented the results for the imputed analysis. Table 21 shows the imputation analyses, with the final two columns showing the range of F-values for the five imputed data sets. The suggestion from the observed data that the QCPR warmth subscale may have an effect was not substantiated by the imputed data sets with a pooled mean difference of –0.65 (F-range 2.35–3.66; p-range 0.06–0.13).
| QCPR outcome and subscales | iCST, n = 142; TAU, n = 146 | Mis., n | Pooled mean | Pooled SE | Pooled mean difference (iCST–TAU) | Pooled SE | Median F-value | Low F-value | High F-value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p-value | F | p-value | F | p-value | |||||||
| Carer | ||||||||||||
| QCPR total | iCST | 8 | 59.73 | 0.51 | –0.087 | 0.708 | 0.017 | 0.897 | 0.005 | 0.944 | 0.097 | 0.756 |
| TAU | 4 | 59.81 | 0.50 | |||||||||
| QCPR (warmth) | iCST | 8 | 35.05 | 0.28 | –0.650 | 0.392 | 2.900 | 0.090 | 2.353 | 0.126 | 3.660 | 0.057 |
| TAU | 4 | 35.70 | 0.27 | |||||||||
| QCPR (criticism and conflict) | iCST | 8 | 24.68 | 0.32 | 0.565 | 0.441 | 1.681 | 0.196 | 1.257 | 0.263 | 2.115 | 0.147 |
| TAU | 4 | 24.11 | 0.31 | |||||||||
Adherence analysis
The number of sessions completed by people with dementia and their carers ranged from 0 to 75 over the 26 weeks. Table 22 shows the number of sessions completed by the 180 participants randomised to receive iCST available for analysis. Overall, 22% of participants did not complete any sessions, whereas 51% completed more than 30 sessions.
| Number of sessions completed | 0 | 1–10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71–75 |
|---|---|---|---|---|---|---|---|---|---|
| n (%) | 39 (22) | 23 (13) | 10 (5) | 17 (9) | 19 (11) | 20 (11) | 14 (8) | 18 (10) | 20 (11) |
| Number of sessions completed, mean (SD) | 31.68 (26.81) | ||||||||
A number of options were explored in terms of the best way to consider analyses for adherence. The analysis methods for beginning to understand the ‘dose’ relationship were considered exploratory in nature. These options included whether or not an average of 1.5 or 2 sessions per week had been completed up to week 13 and week 26. The most efficient approach was considered to be to model the total number of sessions attended at each time point. Linear regression was used to assess the relationship between the follow-up outcome measures and the number of iCST sessions attended after adjusting for baseline outcome measures. Appendix 7 reports observed data with values of coefficients, SEs, F-values and p-values, and pooled data showing pooled coefficients, SEs, median F-values, with F-values and p-values for both the person with dementia and carer, respectively, at each time point.
Outcomes for person with dementia
There was no statistical relationship between the number of sessions completed by the person with dementia and the ADAS-Cog primary outcome at any time point (see Appendix 7). However, the total number of sessions completed from baseline to week 26 was significant, with an improvement for both the QCPR total (p-value = 0.003) and QCPR criticism subscale (p-value = 0.001) for the iCST group. This result was consistent for the QCPR total after regression analysis with imputed data. The imputation was not conducted for QCPR (criticism) at week 26 as no data were missing. At week 13, only QCPR (criticism) had a significant association with the number of sessions (p-value = 0.004). However, several measures were close to significance, suggesting a potential pattern of improvement for people with dementia undertaking iCST. This included the QCPR total (p-value = 0.06; imputed values: p-range 0.06–0.06), the MMSE (p-value = 0.10; imputed values: p-range 0.09–0.12) and the QoL-AD (p-value = 0.08: imputed values; p-range 0.08–0.10).
Outcomes for carer
There were no significant associations between the SF-12 primary outcome and the number of sessions completed by carers at either week 13 and week 26. At week 26, HADS (depression) showed a significant reduction in the iCST group (p-value = 0.02), suggesting that a higher number of iCST sessions was associated with a decrease in depression scores (see Appendix 7). This was supported by the imputation analysis (p-range 0.01–0.02). Imputation suggested that the HADS total (p-range 0.03–0.05) was also associated with numbers of sessions completed. The EQ-5D utility showed a trend in favour of iCST (p-value = 0.09; imputed values: p-range 0.08–0.10). At week 13, there were no significant associations between the secondary outcomes and the number of sessions completed; however, there was borderline significance for carer EQ-5D health state being associated with higher adherence (p-value 0.06; imputed values: p-range 0.05–0.06).
Adverse events
Individual cognitive stimulation therapy was not a Clinical Trial of an Investigational Medicinal Product, so there were no associated risks to participants by taking part. However, qualitative interviews indicated that for some people with dementia, taking part in iCST was a difficult process, which for a small number of participants resulted in feelings of frustration, especially when the activities were judged by the person with dementia to be very easy or not challenging enough.
Economic evaluation
Data on service use and unpaid care were collected using the CSRI at each assessment point. The rates of completion of the CSRI were high at each point. At baseline, one iCST participant/carer dyad did not complete the CSRI. At week 13, of 142 iCST dyads completing outcome assessments, 141 completed a CSRI; of 146 TAU dyads completing outcome assessments, 145 completed a CSRI. At week 26, of 134 iCST dyads completing outcome assessments, 133 completed a CSRI; of 139 TAU dyads completing outcome assessments, 138 completed a CSRI. Thirteen dyads in the TAU group and 16 dyads in the iCST group completed only one follow-up CSRI. Information was available at all three time points for 129 iCST dyads and for 135 TAU dyads. There was a difference of nine dyads in the complete case information available for the costs analyses relative to the 26-week outcomes analyses (264 vs. 273). Owing to a protocol violation (see Appendix 9), one participant who should have been assigned to the control group received the intervention. For the cost-effectiveness analyses, costs of all participants have been retained and the analysis carried out according to the intended allocation.
Care and support services and costs
Professional time spent in supporting the carers of participants receiving iCST is presented in Table 23. The amount of time (including travel) spent in delivering the set-up visit was about 2 hours. The time spent in monitoring visits and telephone calls was similar between week 13 and week 26. Carers spent 16.8 hours, on average, delivering the intervention at week 13, and 13.1 hours at week 26 (Table 24). Resources used by both groups are given in Tables 25–27. Use of health and social care services was very similar between groups during the period prior to baseline, and during the two follow-up periods (Tables 28–30).
| Type of contact | Intervention, mean (SE)a | Valid, n |
|---|---|---|
| Week 13 | ||
| Set-up visitb | 2 (0) | 126 |
| Monitoring visitsb | 1.3 (0) | 124 |
| Telephone calls | 0.6 (0) | 118 |
| Week 26 | ||
| Monitoring visitsb | 1.2 (0) | 121 |
| Telephone callsb | 0.4 (0) | 97 |
| Hours of carers’ time | Intervention, mean (SE)a | Valid, n |
|---|---|---|
| Week 13, hours | 16.8 (0.8) | 118 |
| Week 26, hours | 13.1 (1.2) | 91 |
| Baseline | Unit | iCST (SE) (n = 129) | TAU (SE) (n = 135) | Mean difference (95% CI)a |
|---|---|---|---|---|
| A&E department | Attendances | 0.0 (0.0) | 0.1 (0.0) | –0.1 (–0.2 to 0.0) |
| Inpatient services | Admissions | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0 to 0.1) |
| Inpatient services | Days | 0.2 (0.1) | 0.1 (0.1) | 0.1 (–0.1 to 0.3) |
| Day services | Days | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0 to 0.1) |
| Days in inpatient and day services | Days | 0.2 (0.1) | 0.1 (0.1) | 0.1 (–0.1 to 0.4) |
| Outpatient services | Visits | 0.7 (0.1) | 1.0 (0.1) | –0.3 (–0.7 to 0.1) |
| GP | Visits | 1.7 (0.2) | 1.6 (0.1) | 0.1 (–0.3 to 0.5) |
| Practice nurse | Visits | 0.9 (0.2) | 0.7 (0.1) | 0.2 (–0.3 to 0.7) |
| Community/district nurse | Visits | 0.3 (0.1) | 0.5 (0.3) | –0.2 (–0.8 to 0.5) |
| Physiotherapist | Visits | 0.2 (0.1) | 0.2 (0.1) | 0.0 (–0.2 to 0.2) |
| Occupational therapist | Visits | 0.1 (0.0) | 0.1 (0.0) | 0.0 (–0.1 to 0.1) |
| Dietician | Visits | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0 to 0.0) |
| Specialist nurse | Visits | 0.1 (0.0) | 0.1 (0.0) | –0.1 (–0.2 to 0.1) |
| Optician | Contacts | 0.2 (0.0) | 0.3 (0.0) | 0.0 (–0.1 to 0.1) |
| Chiropodist | Contacts | 0.4 (0.1) | 0.4 (0.1) | 0.0 (–0.2 to 0.2) |
| Dentist | Contacts | 0.5 (0.1) | 0.5 (0.1) | 0.0 (–0.2 to 0.2) |
| Mental health nurse | Contacts | 0.5 (0.2) | 0.5 (0.1) | –0.1 (–0.5 to 0.4) |
| Psychiatrist | Visits | 0.3 (0.1) | 0.4 (0.1) | –0.1 (–0.3 to 0.1) |
| Psychologist | Visits | 0.1 (0.0) | 0.1 (0.0) | 0.0 (–0.1 to 0.1) |
| Counsellor | Visits | 0.0 (0.0) | 0.1 (0.1) | –0.1 (–0.3 to 0.1) |
| Mental health team worker | Visits | 0.1 (0.0) | 0.3 (0.1) | –0.1 (–0.3 to 0.1) |
| Social worker/care manager | Visits | 0.1 (0.0) | 0.4 (0.2) | –0.3 (–0.7 to 0.1) |
| Home care/home help | Visits | 12.2 (4.3) | 9.3 (2.9) | 2.9 (–7.3 to 13.1) |
| Cleaner | Visits | 3.9 (0.9) | 2.8 (0.5) | 1.1 (–0.9 to 3.0) |
| Meals on wheels | Meals | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0 to 0.0) |
| Laundry service | Contacts | 0.8 (0.7) | 0.3 (0.2) | 0.5 (–0.8 to 1.8) |
| Sitting service (e.g. Crossroads) | Visits | 0.5 (0.3) | 0.9 (0.3) | –0.3 (–1.2 to 0.5) |
| Carer support worker | Visits | 0.1 (0.0) | 0.3 (0.3) | –0.3 (–1.0 to 0.4) |
| Daycentre | Attendances | 2.0 (0.6) | 4.5 (1.9) | –2.5 (–6.6 to 1.5) |
| Lunch club | Attendances | 1.0 (0.5) | 0.6 (0.3) | 0.5 (–0.6 to 1.6) |
| Patient education classes | Attendances | 0.2 (0.1) | 0.5 (0.2) | –0.2 (–0.7 to 0.2) |
| Items of equipment | Items | 1.1 (0.2) | 0.9 (0.2) | 0.2 (–0.3 to 0.8) |
| Medicationsb | Units | 0.9 (0.1) | 0.9 (0.1) | 0.0 (–0.2 to 0.2) |
| Unpaid care by primary carer | Hours | 646.3 (75.0) | 652.5 (68.1) | –6.2 (–205.3 to 192.9) |
| Unpaid care by other friends/relatives | Hours | 84.6 (23.0) | 40.3 (7.4) | 44.3 (–2.7 to 91.3) |
| Week 13 | Unit | iCST (SE) (n = 129) | TAU (SE) (n = 135) | Mean difference (95% CI)a |
|---|---|---|---|---|
| A&E department | Attendances | 0.1 (0.0) | 0.1 (0.0) | 0.0 (–0.1 to 0.1) |
| Inpatient services | Admissions | 0.0 (0.0) | 0.0 (0.0) | 0.0 (–0.1 to 0.0) |
| Inpatient services | Days | 0.1 (0.1) | 0.2 (0.1) | –0.1 (–0.4 to 0.2) |
| Day services | Days | 0.0 (0.0) | 0.0 (0.0) | 0.0 (–0.1 to 0.0) |
| Days in inpatient and day services | Days | 0.1 (0.1) | 0.3 (0.1) | –0.1 (–0.4 to 0.2) |
| Outpatient services | Visits | 0.8 (0.1) | 0.7 (0.1) | 0.1 (–0.2 to 0.4) |
| GP | Visits | 1.4 (0.2) | 1.6 (0.2) | –0.2 (–0.6 to 0.3) |
| Practice nurse | Visits | 0.8 (0.1) | 0.7 (0.1) | 0.2 (–0.1 to 0.5) |
| Community/district nurse | Visits | 0.2 (0.1) | 1.2 (0.9) | –1.0 (–2.8 to 0.8) |
| Physiotherapist | Visits | 0.1 (0.0) | 0.3 (0.1) | –0.2 (–0.4 to 0.1) |
| Occupational therapist | Visits | 0.1 (0.0) | 0.1 (0.0) | 0.0 (–0.1 to 0.1) |
| Dietician | Visits | 0.0 (0.0) | 0.0 (0.0) | 0.0 (–0.1 to 0.0) |
| Specialist nurse | Visits | 0.1 (0.1) | 0.1 (0.1) | –0.1 (–0.2 to 0.1) |
| Optician | Contacts | 0.2 (0.0) | 0.2 (0.0) | 0.0 (–0.2 to 0.1) |
| Chiropodist | Contacts | 0.4 (0.1) | 0.5 (0.1) | –0.2 (–0.3 to 0.0) |
| Dentist | Contacts | 0.4 (0.1) | 0.4 (0.1) | –0.1 (–0.2 to 0.1) |
| Mental health nurse | Contacts | 0.2 (0.1) | 0.3 (0.1) | –0.1 (–0.3 to 0.1) |
| Psychiatrist | Visits | 0.2 (0.0) | 0.3 (0.0) | –0.1 (–0.2 to 0.0) |
| Psychologist | Visits | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0 to 0.0) |
| Counsellor | Visits | 0.0 (0.0) | 0.2 (0.1) | –0.2 (–0.5 to 0.1) |
| Mental health team worker | Visits | 0.1 (0.1) | 0.3 (0.1) | –0.1 (–0.4 to 0.1) |
| Social worker/care manager | Visits | 0.2 (0.1) | 0.3 (0.2) | –0.1 (–0.6 to 0.3) |
| Home care/home help | Visits | 10.3 (3.8) | 11.6 (3.4) | –1.4 (–11.4 to 8.7) |
| Cleaner | Visits | 3.4 (0.6) | 2.8 (0.6) | 0.6 (–1.0 to 2.3) |
| Meals on wheels | Meals | 1.2 (0.8) | 0.0 (0.0) | 1.2 (–0.3 to 2.8) |
| Laundry service | Contacts | 0.3 (0.1) | 0.5 (0.3) | –0.2 (–0.9 to 0.5) |
| Sitting service (e.g. Crossroads) | Visits | 0.4 (0.2) | 0.3 (0.2) | 0.0 (–0.4 to 0.5) |
| Carer support worker | Visits | 0.1 (0.0) | 0.4 (0.2) | –0.3 (–0.8 to 0.1) |
| Daycentre | Attendances | 2.7 (0.7) | 3.1 (0.8) | –0.5 (–2.5 to 1.5) |
| Lunch club | Attendances | 1.2 (0.5) | 0.2 (0.1) | 1.0 (–0.1 to 2.0) |
| Patient education classes | Attendances | 0.4 (0.1) | 0.9 (0.3) | –0.4 (–1.0 to 0.2) |
| Items of equipment | Items | 1.1 (0.2) | 0.9 (0.2) | 0.3 (–0.2 to 0.8) |
| Medicationsb | Units | 0.8 (0.1) | 0.9 (0.1) | –0.1 (–0.2 to 0.1) |
| Unpaid care by primary carer | Hours | 705.6 (71.9) | 732.7 (71.8) | –27.1 (–227.3 to 173.0) |
| Unpaid care by other friends/relatives | Hours | 87.7 (22.9) | 40.9 (8.6) | 46.8 (–0.7 to 94.4) |
| Intervention: professional supportc | Contacts | 4.9 (0.2) | 0.04 (0.0) | 4.9 (4.5 to 52) |
| Week 26 | Unit | iCST (SE) (n = 129) | TAU (SE) (n = 135) | Mean difference (95% CI)a |
|---|---|---|---|---|
| A&E department | Attendances | 0.2 (0.1) | 0.1 (0.0) | 0.1 (–0.1 to 0.3) |
| Inpatient services | Admissions | 0.1 (0.0) | 0.1 (0.0) | 0.0 (–0.1 to 0.0) |
| Inpatient services | Days | 0.4 (0.2) | 0.6 (0.3) | –0.3 (–0.9 to 0.4) |
| Day services | Days | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0 to 0.0) |
| Days in inpatient and day services | Days | 0.4 (0.2) | 0.6 (0.3) | –0.3 (–0.9 to 0.4) |
| Outpatient services | Visits | 0.8 (0.2) | 0.5 (0.1) | 0.3 (–0.1 to 0.6) |
| GP | Visits | 1.2 (0.1) | 1.4 (0.1) | –0.2 (–0.5 to 0.2) |
| Practice nurse | Visits | 0.7 (0.1) | 0.6 (0.1) | 0.1 (–0.2 to 0.4) |
| Community/district nurse | Visits | 0.6 (0.3) | 0.4 (0.2) | 0.2 (–0.5 to 0.8) |
| Physiotherapist | Visits | 0.2 (0.1) | 0.2 (0.1) | 0.0 (–0.4 to 0.3) |
| Occupational therapist | Visits | 0.1 (0.0) | 0.3 (0.2) | –0.2 (–0.6 to 0.2) |
| Dietician | Visits | 0.0 (0.0) | 0.0 (0.0) | 0.0 (–0.1 to 0.0) |
| Specialist nurse | Visits | 0.1 (0.0) | 0.2 (0.1) | –0.1 (–0.2 to 0.1) |
| Optician | Contacts | 0.2 (0.0) | 0.2 (0.0) | 0.0 (–0.1 to 0.1) |
| Chiropodist | Contacts | 0.4 (0.1) | 0.5 (0.1) | –0.1 (–0.3 to 0.1) |
| Dentist | Contacts | 0.4 (0.1) | 0.4 (0.1) | 0.0 (–0.1 to 0.2) |
| Mental health nurse | Contacts | 0.2 (0.0) | 0.1 (0.0) | 0.1 (0.0 to 0.2) |
| Psychiatrist | Visits | 0.1 (0.0) | 0.2 (0.0) | 0.0 (–0.1 to 0.1) |
| Psychologist | Visits | 0.0 (0.0) | 0.0 (0.0) | 0.0 (–0.1 to 0.1) |
| Counsellor | Visits | 0.0 (0.0) | 0.1 (0.1) | –0.1 (–0.3 to 0.1) |
| Mental health team worker | Visits | 0.2 (0.0) | 0.2 (0.1) | 0.0 (–0.3 to 0.2) |
| Social worker/care manager | Visits | 0.1 (0.0) | 0.2 (0.1) | –0.1 (–0.3 to 0.0) |
| Home care/home help | Visits | 14.0 (4.9) | 14.5 (4.0) | –0.5 (–12.9 to 11.9) |
| Cleaner | Visits | 3.2 (0.6) | 4.3 (0.8) | –1.1 (–3.1 to 0.9) |
| Meals on wheels | Meals | 1.2 (0.8) | 0.0 (0.0) | 1.2 (–0.3 to 2.7) |
| Laundry service | Contacts | 0.1 (0.1) | 0.2 (0.1) | –0.1 (–0.4 to 0.2) |
| Sitting service (e.g. Crossroads) | Visits | 1.0 (0.4) | 0.9 (0.3) | 0.1 (–0.8 to 1.1) |
| Carer support worker | Visits | 0.6 (0.5) | 0.2 (0.2) | 0.4 (–0.6 to 1.4) |
| Daycentre | Attendances | 2.0 (0.6) | 3.4 (0.8) | –1.4 (–3.3 to 0.5) |
| Lunch club | Attendances | 1.4 (0.5) | 0.7 (0.3) | 0.7 (–0.5 to 1.9) |
| Patient education classes | Attendances | 0.7 (0.3) | 1.2 (0.4) | –0.5 (–1.4 to 0.3) |
| Items of equipment | Items | 1.0 (0.2) | 0.5 (0.1) | 0.5 (–0.0 to 0.9) |
| Medicationsb | Units | 0.8 (0.1) | 0.9 (0.1) | 0.0 (–0.2 to 0.1) |
| Unpaid care by primary carer | Hours | 720.5 (72.5) | 912.8 (79.0) | –192.4 (–403.9 to 19.1) |
| Unpaid care by other friends/relatives | Hours | 69.0 (15.4) | 51.9 (12.2) | 17.2 (–21.3 to 55.7) |
| Intervention: professional supportc | Contact | 2.6 (0.14) | 0.0 (0.0) | 2.5 (2.3 to 2.8) |
| Baseline | iCST, mean (SE) (n = 129) | TAU, mean (SE) (n = 135) | Mean difference (95% CI)a |
|---|---|---|---|
| Hospital | 233 (62) | 297 (69) | –64 (–248 to 121) |
| Primary and community health | 150 (13) | 144 (10) | 6 (–26 to 38) |
| Respite in residential/nursing home | 0 (0) | 13 (9) | –13 (–32 to 6) |
| Community-based social care | 901 (243) | 817 (175) | 84 (–502 to 670) |
| Community mental health | 126 (31) | 118 (18) | 8 (–62 to 78) |
| Daycare (any provider) | 685 (176) | 1012 (227) | –327 (–897 to 243) |
| Equipment and adaptations | 4 (1) | 3 (1) | 1 (–3 to 5) |
| Medicationsb | 172 (15) | 176 (14) | –4 (–45 to 36) |
| Health and social carec | 2271 (312) | 2581 (317) | –309 (–1186 to 567) |
| Unpaid care and supportd | 4535 (477) | 4282 (414) | 253 (–988 to 1493) |
| Week 13 | iCST, mean (SE) (n = 129) | TAU, mean (SE) (n = 135) | Mean difference (95% CI)a |
|---|---|---|---|
| Hospital | 253 (57) | 198 (46) | 55 (–89 to 199) |
| Primary and community health | 125 (13) | 142 (12) | –17 (–51 to 17) |
| Respite in residential/nursing home | 31 (22) | 0 (0) | 31 (–11 to 73) |
| Community-based social care | 845 (245) | 1072 (235) | –227 (–895 to 441) |
| Community mental health | 36 (7) | 76 (12) | –40 (–68 to –12) |
| Daycare (any provider) | 327 (69) | 439 (75) | –112 (–314 to 89) |
| Equipment and adaptations | 4 (2) | 2 (1) | 2 (–2 to 6) |
| Medicationsb | 188 (17) | 217 (15) | –29 (–72 to 15) |
| Health and social care excluding interventionc | 1809 (268) | 2146 (279) | –337 (–1099 to 426) |
| Intervention: professional supportd | 249 (4) | 1 (1) | 249 (240 to 257) |
| Health and social care including intervention (professional)e | 2058 (267) | 2147 (278) | –88 (–849 to 673) |
| Unpaid care and supportf | 4930 (448) | 4770 (444) | 159 (–1083 to 1401) |
| Intervention: carer deliveredg | 101 (5) | 1 (1) | 100 (90 to 110) |
| Intervention: professional support and carer deliveredh | 350 (8) | 2 (2) | 348 (333 to 363) |
| Societal excluding interventioni | 6739 (540) | 6916 (587) | –177 (–1751 to 1396) |
| Societal including intervention (professional and carer)j | 7089 (539) | 6918 (586) | 171 (–1402 to 1744) |
| Week 26 | iCST, mean (SE) (n = 129) | TAU, mean (SE) (n = 135) | Mean difference (95% CI)a |
|---|---|---|---|
| Hospital | 364 (106) | 377 (111) | –12 (–316 to 291) |
| Primary and community health | 122 (12) | 138 (14) | –16 (–53 to 21) |
| Respite in residential/nursing home | 34 (24) | 0 (0) | 34 (–12 to 81) |
| Community-based social care | 1010 (281) | 1142 (227) | –131 (–840 to 577) |
| Community mental health | 37 (8) | 40 (8) | –3 (–25 to 19) |
| Daycare (any provider) | 505 (109) | 783 (130) | –278 (–613 to 58) |
| Equipment and adaptations | 5 (2) | 1 (1) | 4 (0 to 8) |
| Medicationsb | 200 (16) | 207 (14) | –7 (–49 to 35) |
| Health and social care excluding interventionc | 2279 (360) | 2688 (344) | –409 (–1389 to 571) |
| Intervention: professional supportd | 231 (4) | 1 (1) | 231 (223 to 239) |
| Health and social care including intervention (professional)e | 2510 (360) | 2688 (344) | –178 (–1158 to 801) |
| Unpaid care and supportf | 4902 (448) | 5941 (481) | –1039 (–2336 to 258) |
| Intervention: carer deliveredg | 71 (6) | 1 (1) | 70 (58 to 82) |
| Intervention: professional support and carer deliveredh | 302 (8) | 1 (1) | 301 (285 to 317) |
| Societal excluding interventioni | 7181 (605) | 8629 (630) | –1448 (–3171 to 274) |
| Societal including intervention (professional and carer)j | 7483 (604) | 8630 (630) | –1147 (–2869 to 574) |
Carers were asked to report which types of care and support activities they typically engaged in (see Appendix 4). The proportions of carers reporting each type of activity did not vary significantly between groups. The majority reported assisting with supervision, helping the person with dementia with finances and medication, and taking them to appointments. At week 26, approximately 40% of carers across the groups were assisting the person with dementia with personal care.
The (imputed) costs of health and social care and unpaid carer inputs, including the costs of delivering the intervention, are given in Tables 28–30. The costs of delivering the intervention were estimated from the health and social care perspective, including the costs of paid/professional staff, training and materials, and also from the broader perspective of the time costs to carers (see Tables 29–30). The paid staff costs of delivering the intervention were slightly higher and the unpaid carer costs were approximately 40% higher at the week-13 follow-up compared with the week-26 follow-up period. The mean cost of the intervention, taking paid staff time into account, was £481 over the period of the study; the mean cost of delivering the intervention, if carer time was valued at the minimum wage, was £171. It cost £652 to deliver the intervention, considering both paid staff and unpaid carer inputs. Costs of other health and social care and carer costs were similar between the groups at baseline and, for the most part, did not significantly differ (at the 5% level) between the groups during the follow-up period. The total raw mean costs of health and social care and costs to carers at week 26 were somewhat, but not significantly, less for the intervention than the control group (7483 iCST vs. 8631 TAU; t = 1.312; p-value = 0.191).
Cost-effectiveness analyses
The primary outcomes and costs for the person with dementia and their carers are presented in Tables 31–34. Only primary outcomes (ADAS-Cog, QoL-AD, QALY) and secondary outcomes (QCPR, person with dementia) with significant between-group differences at the 5% level are presented here.
| Outcomes | iCST (n = 129) (95% CI) | TAU (n = 135) (95% CI) | Difference (n = 264) (95% CI)a |
|---|---|---|---|
| ADAS-Cog | 20.53 (19.19 to 21.87) | 21.19 (19.78 to 22.61) | –0.66 (–2.25 to 0.92) |
| QoL-AD | 37.96 (37.09 to 38.84) | 37.71 (36.86 to 38.57) | 0.25 (–0.81 to 1.3) |
| QCPR | 56.95 (55.63 to 58.27) | 55.24 (53.74 to 56.75) | 1.71 (0.15 to 3.27) |
| Costs | iCST (n = 129) (95% CI) | TAU (n = 135) (95% CI) | Difference (n = 264) (95% CI) |
|---|---|---|---|
| Health and social care (£)a,b | 4740 (3790 to 5700) | 4670 (3680 to 5660) | 70 (–1050 to 1190) |
| Societal (£)a,c | 9770 (8410 to 11140) | 10630 (9140 to 12120) | –860 (–2750 to 1040) |
| Outcomes | iCST (n = 129) (95% CI) | TAU (n = 135) (95% CI) | Difference (n = 264) (95% CI) |
|---|---|---|---|
| QALYa,b | 0.82 (0.78 to 0.85) | 0.77 (0.74 to 0.80) | 0.05 (0.01 to 0.09) |
| Costs | iCST (n = 129) (95% CI) | TAU (n = 135) (95% CI) | Difference (n = 264) (95% CI) |
|---|---|---|---|
| Annual equivalent: health and social care (£)a,b | 9480 (7370 to 11,600) | 9340 (7300 to 11,390) | 140 (–2360 to 2640) |
| Annual equivalent: societal (£)a,c | 19,550 (16,730 to 22,370) | 21270 (18,170 to 24,350) | –1710 (–5570 to 2150) |
Outcomes and costs for the person with dementia
The SUR analyses produced very similar estimates of between-group differences on these outcomes at week 26 to the ANCOVA approach used in the main analysis (see Primary analyses of outcomes). On the primary outcomes for the person with dementia (see Table 31), the groups’ mean outcome scores did not differ significantly. The mean (adjusted) difference in ADAS-Cog scores was –0.66 (95% CI –2.25 to 0.92). On the QoL-AD, the intervention group scores were marginally, but not significantly, higher than those of the intervention (difference of 0.25, 95% CI –0.81 to 1.3). On the secondary outcomes, the mean between-group difference in the QCPR was 1.71 (95% CI 0.15 to 3.27).
For the costs for the person with dementia (see Table 32), the groups’ (adjusted) mean health and social care costs did not differ significantly. The (adjusted) mean week-26 health and social care costs were slightly, but not significantly, higher in the intervention group, giving a between-group difference of £70 (95% CI –£1050 to £1190). In terms of societal costs, however, the control group’s (adjusted) mean week-26 costs were £860 (95% CI –£2750 to £1040) higher than those in the intervention group (but not significantly so).
Outcomes and costs for carers
Mean QALYs (see Table 33) for the carers in the intervention group were significantly higher than for carers in the control group [0.82 (95% CI 0.78 to 0.85) vs. 0.77 (95% CI 0.74 to 0.8), respectively, a difference of 0.05, 95% CI 0.01 to 0.09]. The annual-equivalent health and social costs in the intervention group were slightly higher than in the control group (see Table 34). From the broader societal perspective, the annual equivalent costs in the intervention group were somewhat lower than in the control, by £1710 (95% CI –£5570 to £2150).
Incremental cost-effectiveness ratios
The ICERs for people with dementia and for carers are presented in Table 35.
| Cost perspectivea | ADAS-Cog (95% CI)b | QoL-AD (95% CI)c | QCPR (95% CI)d | QALY (95% CI)e |
|---|---|---|---|---|
| Person with dementia, 6 months | ||||
| Health and social caref | 300 (unbounded to unbounded) | 600 (unbounded to unbounded) | 100 (–3200 to 5400) | NA |
| Societalg | –3300 (unbounded to unbounded) | –7100 (unbounded to unbounded) | –1600 (–13,300 to 2400) | NA |
| Carer, annual equivalent costs and outcomes | ||||
| Health and social caref | NA | NA | NA | 3100 (–71,000 to 84,200) |
| Societalg | NA | NA | NA | –38,400 (–236,000 to 47,300) |
Person with dementia
The incremental health and social care cost of a difference of 2.4 points in the ADAS-Cog score at week 26 was £300. The probability of cost-effectiveness on the ADAS-Cog from the health and social care perspective rises from 45% at a WTP of £0 to approximately 80% at a WTP of £20,000 (see Figure 2). As the control group’s mean (adjusted) 6-month societal costs were greater than those in the intervention group, which constitutes a cost-saving (not significant at the 5% level), the ICER produced is negative. The probability of cost-effectiveness by reference to ADAS-Cog (see Figure 2) from the broader societal perspective rises from 80% at a WTP of £0 and then falls to approximately the same level as given by the health and social care perspective. This is a pattern that can occur when the joint density of incremental outcomes and costs crosses all quadrants of the cost-effectiveness plane. 67
The incremental health and social care cost of a difference of 1.7 points in QoL-AD score at week 26 was £600. The ICER from the societal perspective was negative (–£7100). The probabilities of cost-effectiveness follow a fairly similar pattern as in the case of the ADAS-Cog measure (see Figure 3).
Going beyond the ICER point estimates and taking sampling uncertainty into account, on both ADAS-Cog and QoL-AD measures, and from either the health and social care or the societal perspective, the CEACs and the (absence of) confidence limits of the ICERs indicate that there is no positive WTP where we can be 95% confident that the two interventions differ in terms of cost-effectiveness. 68
On the secondary outcomes, the incremental cost to achieve a difference of 3.1 points in the QCPR was £100 from the health and social care perspective, whereas the ICER from the societal perspective was negative. Taking sampling uncertainty into account, we can be confident that the intervention is cost-saving at a WTP of over £5400 considering health and social care costs, or at a lower WTP of over £2400 considering societal costs. The CEAC (see Figure 4) indicates that the probability of cost-effectiveness exceeds 97.5% over these WTP thresholds.
Carers
The incremental cost per QALY gained was £3100 (95% CI –£71,000 to £84,200). Given the 95% CIs of the ICER, we cannot be certain that the iCST and TAU alternatives are different in terms of cost-effectiveness at a WTP below the upper limit of about £84,200, but we can be confident that the intervention is cost-effective above this upper limit. 68 The probability of iCST being cost-effective from this perspective was 72% at a WTP per QALY of £20,000 and 81% at a WTP per QALY of £30,000.
From the broader societal perspective, as costs were lower and QALYs gained were higher in the intervention group, the ICER point-estimate was negative. The probability of iCST being cost-effective from this perspective was 90% at a WTP per QALY of £20,000 and 93% at a WTP per QALY of £30,000 (see Figure 5). There is a > 97.5% probability of cost-effectiveness at WTP values over £47,300 per QALY, given the upper confidence limit of the ICER, and we can be confident that the intervention is cost-effective above this upper limit.
Sensitivity analyses
The estimates of societal costs were tested by varying the valuation of time spent by carers in providing assistance with care and delivering the intervention using a replacement cost approach (Tables 36 and 37). This approximately doubled ICERs for all outcomes (Table 38). As measured by achieving a difference of 3.1 points in the QCPR and seen from this cost perspective, we can be confident that the intervention is cost-saving at a WTP of over £11,700, in light of sampling uncertainty. The probability of achieving a QALY gain at a WTP of £30,000 was lower than in the main analysis, at 73% (see Figure 5). The upper confidence limit for the ICER is much higher than in the main estimates, at £244,500 (see Table 38).
| Costs | iCST (n = 129) (95% CI) | TAU (n = 135) (95% CI) | Difference (n = 264) (95% CI)a |
|---|---|---|---|
| Societal: replacement cost (£)b | 35,510 (30,900 to 40,120) | 37,250 (32,470 to 42,030) | –1740 (–7920 to 4430) |
| Costs | iCST (n = 129) (95% CI) | TAU (n = 135) (95% CI) | Difference (n = 264) (95% CI)a |
|---|---|---|---|
| Annual equivalent (societal: replacement cost) (£)b | 71,010 (61,420 to 80,600) | 74,520 (64,020 to 85,020) | –3510 (–16,170 to 9140) |
| Cost perspectivea | ADAS-Cog (95% CI)b | QoL-AD (95% CI)c | QCPR (95% CI)d | QALY (95% CI)e |
|---|---|---|---|---|
| Person with dementia, 6 months | ||||
| Societal: replacement costf | –6600 (unbounded to unbounded) | –14,500 (unbounded to unbounded) | –3200 (–35,600 to 11,700) | NA |
| Carer, annual equivalent costs and outcomes | ||||
| Societal: replacement costf | NA | NA | NA | –78,300 (–595,100 to 244,500) |
Chapter 6 Qualitative study
Background
Despite the increasing number of RCTs evaluating cognitive-based interventions for people with dementia, little is known about the experiences of people with dementia taking part. This is the first RCT to conduct an embedded qualitative study exploring experiences of people with dementia taking part in a cognitive stimulation intervention alongside their family carers. In this study we used qualitative methods to explore the views of people with dementia taking part in the iCST programme. Given that qualitative data from the development phase of the trial indicated that interpersonal aspects of the caregiving relationship may be particularly relevant within the context of a home-based, carer-led individual cognitive stimulation approach, we additionally explored whether or not taking part in iCST was associated with any changes in interpersonal aspects of quality of life such as the relationship between the person with dementia and their family carer.
Aims
To explore the experiences of people with dementia and family carers taking part in the iCST programme.
Methods
Sampling and recruitment to the qualitative study
This was a qualitative study using semistructured in-depth interviews, using a convenience sub-sample of participants allocated in the treatment group, regardless of how many sessions they completed. During the baseline assessments participants were informed about the iCST qualitative study and were invited to consent to the possibility of participating in the qualitative interview if they were assigned to the intervention group. Unblinded researchers made contact with the participants to discuss the qualitative component of the trial after they completed the intervention (at week 25). The contact was usually at monitoring visit 2, via telephone and, occasionally, via e-mail. The unblinded researcher asked participants if they would be willing to take part in an individual interview about their experiences of taking part in the iCST programme. If participants agreed to be interviewed, a confirmation letter and Participant Information Sheet were sent to them.
A subsample of 23 dyads of the group allocated to receive iCST took part, consisting of 22 people with dementia (one participant refused to participate) and 23 family carers. Demographic characteristics of the sample can be seen in Table 39. The mean age was 74.73 years for participants and 65.87 for family carers. People with dementia taking part in the qualitative study had a mean baseline MMSE score of 22.5, indicative of mild dementia. There were 17 spousal carers, 5 adult–child carers and 1 sibling carer. The minimum number of sessions completed was 18 and the maximum was 75, with 61% of the sample completing more than 38 sessions.
| Characteristics | People with dementia, n = 22 | Carers, n = 23 |
|---|---|---|
| Mean (%) | Mean (%) | |
| Age | 74.73 (6.00) | 65.87 (13.68) |
| Sex | ||
| Male | 16 (73) | 4 (17) |
| Female | 6 (27) | 19 (83) |
| Ethnicity | ||
| White British | 15 (68) | 20 (87) |
| Other white | 4 (18) | 2 (8) |
| Caribbean | 3 (14) | 1 (4) |
| Education | ||
| School leaver (14–16 years) | 12 (54) | 10 (43) |
| School leaver (18 years of age) | 4 (18) | 2 (8) |
| Higher education | 4 (18) | 6 (26) |
| Further education | 2 (9) | 4 (17) |
| Postgraduate | 0 | 1 (4) |
| MMSE | 22.5 (3.38) | |
| Number of iCST sessions completed | 49.41 (18.38) | |
Data collection
The researcher undertaking the individual interviews was not involved in recruiting or providing the intervention to participants. Semistructured, in-depth interviews took place at the participants’ homes, with each interview fully audio-recorded and transcribed. The interview questions were established by focusing mainly on non-directive questions, with overall interview duration ranging from 30 to 45 minutes. People with dementia and their carers were asked separately to describe their experiences in taking part in the iCST programme using initially open-ended questions, followed by questions focusing on specific domains, presented in Box 3. All interviews started with an informal conversation while the dyad welcomed the interviewer into their home, in order to build up optimal rapport and increase the reliability of the interview. 69 In the second part the interviewer went through the Participant Information Sheets with the person with dementia and their carer and explained topic areas of the questions.
How would you describe your experiences of taking part in the iCST programme?
How did you find the iCST programme?
How would you describe taking part in iCST to someone else?
Have you experienced any changes as a result of your participation in iCST?
Have you experienced any changes in your everyday life?
Have you experienced any changes in your relationship with your relative?
Data analysis
Interviews were analysed using Framework Analysis,70 which is considered to be a suitable and reliable method of qualitative analysis providing a systematic model for managing and mapping the data. 71 Framework Analysis consists of stages of familiarisation, identifying a thematic framework, indexing, charting, mapping and interpretation. The interviews were transcribed verbatim, checked for accuracy and read thoroughly by two independent researchers. After several readings of the interviews, a coding frame was developed using Nvivo 10 software (QSR International, Warrington, UK). Initial codes were refined and modified during the analytic process. A coding scheme was created to organise the data to interpretations. A spreadsheet was used to generate a matrix with data ‘charted’ into the matrix. Researchers compared and contrasted styles of summarising in the early stages of the analysis process to ensure consistency. 70 Quotations were tagged automatically by using Nvivo10.
Results
A total of 22 people with dementia and 23 carers were interviewed. Ten dyads were recruited from London, four dyads from Manchester, five dyads from Norfolk and Suffolk and four dyads from Dorset. The analyses identified five main themes in relation to participation in iCST which were common across transcripts for both people with dementia and their carers. These themes were the following: (1) iCST was described as providing opportunities for both general and specific intellectual stimulation; (2) iCST was of value and useful for both the person with dementia and carer; (3) iCST offered opportunities for enjoyment and allowed the person to take part in pleasant activities; (4) iCST promoted being active in everyday life; and (5) iCST brought the carer and person with dementia closer. An additional theme identified in the carer data was that iCST provided opportunities for carers to become more aware of the ‘needs’ of the person with dementia and the experience of ‘living with dementia’ from their perspective.
Opportunities for stimulation
A total of 73% of people with dementia and 65% of carers reported that iCST provided opportunities for general mental stimulation and non-specific memory improvement. Some participants described their experience of mental stimulation as being alert and noted that the intervention helped in terms of both raising general ‘awareness of what is happening’ and enabling them to ‘think better’. A number of carers noticed changes in their relative’s alertness, orientation and likelihood of engaging, and being active, in discussions. Non-specific memory changes were reported by both people with dementia and their carers.
It helped to try and get my memory back and look at different things.
Participant (Manchester)
The course has re-stimulated me to think.
Participant (London)
I found it interesting, looking at the different things and trying to remember some of the logos and different things from the past . . . It keeps the mind active and it is a good thing . . . how I would describe it . . . really it was good.
Participant (Dorset)
. . . It varied the awareness of what is happening I think, otherwise just carry on not thinking about things but then get aware of what it is all about and start thinking a little bit more about it.
Participant (Norfolk and Suffolk)
Made my relative more alert.
Carer (London)
He became a little more active, more willing to participate in discussions, activities that we did together outside of the activities in the book. He tended to snooze a lot before.
Carer (London)
It does sharpen up what you are doing.
Participant (Norfolk and Suffolk)
It does obviously get your mind focused on trying to think of the past as well, very interesting.
Participant (Dorset)
The activities motivate you to think better.
Participant (Norfolk and Suffolk)
However, 20% of the people with dementia did not find iCST stimulating or not stimulating enough as the activities were too easy. Although some participants and carers found that the activities were not challenging enough, they were aware of, and emphasised, the fact that the intervention was probably designed to meet the different ‘needs’ of a wide range of people. Carers commented that the effectiveness of the activities can vary and that this possibly depends on the topics, levels of interest and issues around level of ‘difficulty’ of cognitive impairment. Some participants were not quite sure about iCST when they first started the programme but later found the activities to be worthwhile.
I didn’t honestly find the material all that stimulating for me.
Participant (London)
I would say there was much of it I enjoyed but I didn’t gain much from it, because a lot of it wasn’t appropriate for my particular stage of difficulty . . . you must have so many people at different stages, with different needs.
Participant (London)
Sometimes it is difficult, depending on the topic on the subject, the reaction is quite different sometimes.
Carer (London)
Well I wasn’t quite sure, but now I have started to appreciate it.
Participant (Manchester)
Most of the things were mind stretching in the actual books but some of the things weren’t, but then of course you have got a whole range of people to actually hit the book with haven’t you and I thought it was worthwhile.
Participant (Norfolk and Suffolk)
Individual cognitive stimulation therapy was useful and of value for the person with dementia and carer in terms of communication
Most people with dementia and their carers found that the programme was very useful and important. Although most described iCST as a useful programme, one participant valued it as a ‘learning course’. Both people with dementia and carers found the design and structure of the programme easy to adjust to and remember overall, thus providing a focus. Carers mentioned that engaging their relative in conversation can be difficult and that doing the iCST activities not only helped to stimulate thinking but helped to frame a conversation.
It was useful for us and perhaps even for other people at some time . . . it was all worthwhile in my opinion, I found it extremely good.
Participant (Norfolk and Suffolk)
It is mostly about the course, about learning and recalling . . . it has re-stimulated me to think that maybe I should talk a bit more.
Participant (London)
It does give a structure and a focus on something that you can do together and just sharpen things up and I would guess if you are bit further along it would be even more important.
Carer (London)
I would just describe it as a pack with activities to help stimulate the mind of the person with dementia and help the carer find new ways of communicating and talking to that person.
Carer (Manchester)
Opportunities for enjoyment and allowing the person with dementia to increase pleasant activities
A total of 82% of people with dementia found iCST to be enjoyable. They described the activities as pleasurable, entertaining and interesting. Carers found the activities to be enjoyable for both their relative and for themselves. Some participants mentioned that they did not remember the activities, but they were able to reflect on the enjoyment related to taking part. For one participant, the feelings of enjoyment and achievement outweighed any experience of remembering the activities.
I enjoyed the whole programme . . . if I didn’t enjoy it, then there is something wrong with me and there is something wrong all together.
Participant (London)
I think we both really enjoyed it and I think some work better than others, but it was a good way of sitting down having some time dedicated to actually talking about our particular subject or something specific, and I think that was helpful and it was fun as well.
Carer (London)
I don’t remember the activities, but I enjoyed what we were doing.
Participant (London)
Yeah even though like things might not stay with me . . . but it’s brilliant.
Participant (Manchester)
I have felt I have done something when it is time to pack up, and put the things away . . . I enjoy doing them . . . feel you’ve accomplished something.
Participant (London)
Individual cognitive stimulation therapy promotes being active in everyday life
People with dementia and their carers reported that being involved in the iCST programme added value to their daily experience in terms of taking up new activities, feeling motivated and raising awareness of ‘things around’. Some participants applied iCST sessions to everyday life. One couple made a journey to their home town to compare the old town with new changes. For some participants, taking part in iCST was an ‘obligation’, which has helped them not only to ‘think better’ but also to engage in other activities. A few people with dementia found that some of the activities helped them to revisit activities/hobbies that they enjoyed in the past or to look for new activities and interests. Some carers experienced changes in their everyday life, such as having ‘more of a focus’ or looking for further information related to cognitive stimulation activities for their relative.
It makes you aware of what is going on . . . It just keeps you going otherwise you would slump away and sleep the day away.
Participant (Norfolk and Suffolk)
It gives you a point of reference to do something and not just sit.
Participant (London)
Makes me more inquisitive and enlightens me about things.
Participant (Norfolk and Suffolk)
We were going through the street of that town, trying to remember each shop.
Participant (Norfolk and Suffolk)
I think having the obligation as we did here, to sit down in a particular day of the week or whenever it was, to actually do that, and it does make you think and work what’s left of my brain, and things like that, and yes I think it did well, it did well.
Participant (Norfolk and Suffolk)
It’s made me start thinking about doing what I used to do which was painting . . . over there there’s about two or three paintings over there, that I’ve done on that table and I think I could do more painting and that might make me better, you know and I can get up and do things more easily.
Participant (London)
It gives you a break from everyday life because while you’re doing this, you’re concentrating on doing that, therefore your mind relaxes from other problems . . . with this it makes him think and I’ve noticed a difference. When he was doing that, you see, that helped him think of normal ordinary day living things.
Carer (Norfolk and Suffolk)
Individual cognitive stimulation therapy gets the carer and person with dementia ‘together’
Most people with dementia and carers reported that iCST activities brought them closer, kept their ‘relationship going’ and helped people with dementia to ‘build up confidence’. A carer emphasised that spending time doing iCST together with her relative gave her the opportunity to ‘listen’.
It brings you together in a very nice way and it is good . . . we ought to continue them because it does get you together.
Participant (Norfolk and Suffolk)
It has really brought us closer . . . through it . . . because we were doing things together instead of things apart . . . If he’s left alone to his own devices he loses confidence, but through doing these sort of things that helps to build them up.
Carer (Norfolk and Suffolk)
I think if it involves you in something, and particularly if you can get a laugh out of it, the barriers come down . . . We enjoyed that time together.
Participant (Norfolk and Suffolk)
. . . Just opening topics of conversation, maybe listening to her, encouraging her to express herself and talk about things rather than just getting on with the business of how are we going to sort out the mess in this flat, what shopping do we need or what bills need to be dealt with. All that kind of stuff that is quite tedious and I think it is nice to just have the time to sit down and spend half an hour just looking through some photos or looking at a book together.
Carer (Manchester)
Carers were made aware of the ‘needs’ of the person with dementia and ‘understanding dementia in everyday life’
The final theme emerging from the carer data only was that engaging in the iCST activities with the person with dementia allowed carers to have a better understanding of their needs and to become more aware of situations their relative is likely to encounter in everyday life.
On the whole the experience has been really good . . . It disciplined us . . . so I think it raised an awareness of the need.
Carer (London)
I did not really notice any drastic changes . . . but the main changes were in how I was probably relating to her and thinking about how she would understand things, and how that could be in everyday situations . . . The change is probably more about me that I noticed about her.
Carer (Manchester)
My understanding of my relative’s needs that have changed a lot.
Carer (London)
Discussion
The study aimed to explore experiences of people with dementia and their carers taking part in the iCST programme through semistructured qualitative interviews. Major themes emerging from the qualitative data were opportunities for stimulation, changes in everyday life, interpersonal issues, such as the caregiving relationship, and understanding dementia in everyday life. The study findings suggested that taking part in the iCST programme provided opportunities for both general and intellectual stimulation, helping people with dementia to ‘think better’ and increase their alertness and awareness. 72 Some participants perceived the design and structure of the iCST programme as a tool which helped them to open up conversations and provided a frame for communication. A large number of participants and carers found that iCST offered opportunities to be involved in enjoyable and pleasant activities, revisiting or focusing on new interests and hobbies. In line with previous qualitative findings, although a few people with dementia expressed that they could not remember all of the activities they have completed during the intervention, they were able to reflect on moments of enjoyment and their feelings. 73
Interestingly, reports were consistent with the observation that iCST provided opportunities for people with dementia and their carers to ‘come closer’ and further strengthen their relationship. This is in line with results of the secondary analyses of more positive perceptions of relationship quality by people with dementia. As most people with dementia are cared for at home by family carers, it will be important for future research to understand better the factors that are likely to influence the caregiving relationship within the context of a carer-led, cognitive-based intervention. A considerable number of carers emphasised that iCST provided a framework for gaining a better understanding of the needs of the person with dementia, possibly by making carers more aware of the nature of the difficulties encountered by people with dementia and the experience of dementia in everyday life from the perspective of the person with dementia.
There are several limitations in this qualitative study, especially in relation to the fact that this was a convenience subsample. Most participants interviewed have done well with the intervention and reported higher than average compliance, which may have biased findings towards reporting positive changes related to the intervention. We did not interview dyads that did not complete any sessions or reported poor compliance (i.e. 10 sessions or fewer). In addition, data could have been affected by social desirability bias and participants thinking that the intervention has worked. Most people with dementia and their carers found the iCST programme to be interesting, useful and of value; however, a few reported that the programme was too easy and did not help them in terms of stimulating their thinking, judging the programme overall as not suitable for them. Future research should investigate issues of suitability of cognitive-based interventions and the importance of matching activities to personal preferences and ‘level of stimulation’.
This study reflected the experiences of people with dementia and their carers taking part in iCST and provided further insight into the perspectives of people with dementia and their feelings relating to engaging in a home-based cognitive stimulation programme. Some participants did not remember details of the activities but they were able to reflect on feelings of enjoyment and expressed the importance and value of taking part, its impact on their everyday life and their relationship with their family carer. The results indicate that many carers and people with dementia found iCST to be of great value in promoting mental stimulation and communication, and enhancing their relationship. These findings indicate that it will be important for future studies to include qualitative data in the design and evaluation of cognitive-based interventions for people with dementia and their carers.
Chapter 7 Discussion
Main findings
The iCST trial was a pragmatic, multicentre, RCT of a complex, individual, carer-led cognitive stimulation intervention. The trial was designed to evaluate the effects of iCST on cognition and quality of life for people with dementia and their family carers. We recruited a total of 356 caregiving dyads, making this study the largest in the current literature on CST-based approaches.
Primary outcomes
For people with dementia, the primary outcomes did not indicate any specific benefit for those allocated to receive iCST, given that there was no clinically significant improvement in cognition or quality of life compared with people with dementia receiving usual care. Carers’ physical and mental health was not significantly different between the intervention and control groups.
Secondary outcomes
We found no evidence that iCST reduced behavioural and psychological symptoms or depressive symptoms for people with dementia. There was also no evidence of change in activities of daily living. Although no effects were observed on most of the secondary outcomes, analyses indicated that people with dementia allocated to receive the intervention reported improvements in relationship quality with their family carer. iCST did not improve secondary outcomes such as carers’ mood, resilience or relationship quality with the person with dementia. Despite no differences in most secondary outcomes, health-related quality of life ratings for family carers allocated to the intervention group improved at the primary end point. This, however, was in contrast to no evidence of improvement on carers’ SF-12 component scores. This discrepancy in findings between the two generic instruments (SF-12 and EQ-5D-3L) may reflect intrinsic differences between these two instruments or differences in terms of each instrument’s sensitivity to change.
This is the first economic analysis of an iCST intervention for people with dementia and their family carers. Although costs from either the health and social care or the societal perspective did not differ substantially between the groups at either follow-up time point, there was a consistent pattern of lower costs in the iCST group over the 26-week period. In terms of the primary outcomes for people with dementia, it appears that iCST is not more cost-effective than TAU from either cost perspective, when we take sampling uncertainty into account. There are no established societal WTP thresholds for improvements in ADAS-Cog, QoL-AD or QCPR. In terms of QALY gain for carers, iCST was more effective than TAU. Taking carers’ costs into account, costs in the iCST group were lower, but not significantly lower, than in the TAU group. Taking sampling uncertainty into account, and assuming no further change in utility or costs in either group for the following 6 months, the probability that iCST is cost-effective was 93% at a societal WTP per QALY of £30,000. Under the same assumptions, we can be confident that iCST is cost-effective at a societal WTP of approximately £47,300 to gain a QALY, and, excluding costs to carers from this calculation, we can be confident that iCST is cost-effective at approximately £84,200 per QALY. The intervention can be considered to be cost-effective in improving unpaid carers’ health-related quality of life only at a societal WTP well above the NICE threshold of £20,000–30,000. However, societal decision-makers may be willing to accept somewhat lower levels of certainty to achieve this outcome. Given the results of the sensitivity analysis, this conclusion is dependent on a relatively low valuation of carer time.
When considering the number of sessions received, as opposed to allocation, some improvements were observed. People with dementia completing more sessions were more likely to experience gains in terms of the caregiving relationship at 26 weeks. Reports of improvements in the caregiving relationship by people with dementia are consistent with previous studies indicating that meaningful activities conducted alongside family carers can preserve and enhance the caregiving relationship. 74 Improvements were observed for carers completing more sessions with their relative, expressed by a reduction in depressive symptoms. These findings are consistent with previous research, in which a home-based cognitive stimulation programme was associated with lower depressive affects in carers. 11
Overall, however, findings are in contrast to previous studies demonstrating that group short-term CST benefits cognition and quality of life for people with dementia,6 and maintains quality of life improvements when provided long term. 19 These results also contrast with previous RCTs reporting benefits in cognition for people with dementia associated with home-based individual RO/cognitive stimulation. 12 In relation to effects on cognition, not replicating results of group CST approaches may be attributable to the lack of a group setting when CST sessions are provided. In addition, although we tried to use similar activities to those provided in group CST, it is unlikely that the activities were the same, indicating that differences may relate to differences on group versus individual CST approaches. In relation to effects of quality of life, this is the first study to include a measure of quality of life for a home-based approach for people with dementia. Our results therefore indicate that the benefits in quality of life are more likely to be associated with interventions that combine or use CST approaches within a social setting. Our findings may be of importance in relation to updating the Cochrane review. This may indicate the need for separate analyses of group and individual approaches to cognitive stimulation.
Treatment implementation is an important parameter when evaluating psychosocial interventions. In order to ensure that iCST was ‘delivered as intended’, we used a treatment protocol that specified all components of the intervention in detail, in order to ensure that the intervention was delivered as planned. Each unblinded researcher received training in iCST, and there were frequent opportunities for researchers supporting carers in the delivery of iCST to receive supervision and feedback. Furthermore, there were a number of challenges associated with implementing iCST, particularly with regard to carers fitting iCST into a busy timetable, the lack of ‘stimulation’ for some participants and difficulties reported by carers in engaging in the intervention with their relative. Compliance will be, therefore, an important component to consider in order to optimise similar psychosocial interventions for people with dementia and their carers. At this stage, it is not clear if there are any specific characteristics that would identify the very low compliance group who completed no sessions at all, but we plan to look at further analyses on this topic in due course.
In line with our projections in relation to sample size, a total of 81% of the sample completed the 13-week assessments and 71% completed the 26-week assessments. The most common reason for not being available to complete follow-up appointments was reporting problems with engaging in iCST, indicating that, although the intervention may have a high uptake, it still may be difficult or not suitable for some carers and people with dementia. Although most carers were able to engage in iCST with their relative, there were frequent reports by carers of struggling to engage with their relative in the sessions and, often, this was a common reason of loss to follow-up. A total of 22% of dyads allocated to receive iCST did not complete any sessions and 13% completed fewer than 10. A threshold of completing over 38 sessions was set on the basis of number of sessions completed by the whole of the sample, as indicative of compliance to the intervention. We did not find any evidence of differential attrition, and analyses exploring differences between completers versus non-completers did not suggest any differences between the two groups in terms of baseline characteristics.
In terms of cognition and quality of life, our findings do not provide support for the use of home-based cognitive stimulation programmes for people with dementia, which is contrary to previous work on home-based memory rehabilitation10 and RO for people with dementia. 12 It is unclear which factors could account for the differential efficacy. It is likely that in some studies participants are highly selected, resulting in different patient groups recruited and, therefore, different levels of dementia severity and cognitive function. For example, in our study most of the sample had mild dementia in comparison to previous studies. Other factors could reflect differences between studies in terms of power or chance variation, given that previous studies are of varying quality with small samples overall. 2 Importantly, although treatment compliance is not reported in detail across studies, the ‘dose’ received in each of the studies may be an important determinant of efficacy.
Despite generally negative findings, people with dementia reported better relationship quality with their carer, indicating that individual cognitive stimulation interventions have the potential to improve inter-relationship outcomes for people with dementia. Relationship quality, rated by the person with dementia, has been shown to be an important contributor to quality of life for people with dementia and is the cornerstone of relationship-centred care. 75 We also found that health-related quality of life for family carers significantly increased, indicating that carer involvement in cognitive-based interventions may increase carer well-being. This may be related to the fact that, although iCST was developed largely as a home-based, carer-led, individual cognitive stimulation approach, the intervention incorporated additional psychoeducational elements such as communication, opportunities to increase pleasant events for both carers and people with dementia, which are components less likely to be incorporated in group CST-based approaches. This is consistent with the findings of the qualitative study where carers reported that iCST provided opportunities to understand dementia, its impact on communication and confidence for the person with dementia, and to increase pleasant activities both for themselves and their relative. Interventions that therefore target communication between people with dementia and their carers may reduce the strain on the caregiving relationship and may potentially improve general well-being outcomes for both.
A further variable of interest is unblinded researchers’ level of expertise and experience, which is an important factor to consider in the development of psychosocial interventions. Consultation groups with unblinded researchers indicated that they were generally well received by carers and people with dementia, although dyads differed widely in their level of engagement with the intervention. The level of expertise was judged as sufficient, and training and background in dementia care was considered to be important in supporting carers in delivering the sessions.
Implications for health care
It was expected that iCST may be beneficial for people with dementia in terms of cognition; however, this was based on smaller studies using both cognitive stimulation and RO techniques and using less well-defined methods. However, it is notable that participants in this study had better cognition (mean baseline MMSE score of 21) than those in the original group CST study (mean baseline MMSE score of 14), which was also limited to people with dementia scoring between 10 and 24 on the MMSE. This suggests that in the current study there was less scope for improvement, and some participants may have had cognitive function that was too high to benefit from iCST.
Most of the evidence on effects on cognition for cognitive stimulation is based on group approaches, so future research will need to focus on understanding the mechanisms that are more likely to be associated with the reported effects and differential effects of outcomes between group versus individual approaches. There was evidence in this trial that some people with dementia and their family carers will not be able to engage successfully in iCST, as 34% of the sample allocated to receive the intervention completed 0 or fewer than 10 sessions. Our qualitative findings indicated that, although most people with dementia and carers enjoyed the sessions, there were also a few comments that the iCST sessions were not challenging enough, indicating that the type of carer-led, cognitive-based intervention may be key in terms of producing a therapeutic effect. Despite overall negative findings, improvements on the carer–patient relationship and carers’ health-related quality of life suggest that iCST may have a key role in improving communication for people with dementia and their carers.
Limitations
Participants dropping out will have introduced bias if they had a different response to the intervention or TAU conditions compared with those that completed the trial. However, there was no evidence from demographic variables or baseline outcome scores that those who did complete the study were different from those who did not.
Although significant efforts were made to obtain outcome data, the trial may have been underpowered to detect significant differences for the primary outcome measure owing to the attrition rate and low levels of compliance in the overall number of sessions completed. Nevertheless, this is the largest RCT of a cognitive stimulation intervention in which carers lead the sessions and which shows no effect on cognition and quality of life for people with dementia compared with usual care.
An important limitation is that the observed differences of improvement in the caregiving relationship for people with dementia and health-related quality of life for carers may be attributable to incidental findings, driven by the multiple comparisons tested, and may, therefore, be attributable to chance. Despite people with dementia and carers expressing interest in the intervention, compliance was low overall, indicating that cognitive stimulation interventions delivered by carers may not be the ideal mode of delivery for many, thereby limiting wider applicability and generalisability of this approach, and indicating that better methods of monitoring and support for adherence are needed. Identifying subgroups of caregiving dyads that are more likely to benefit from this intervention is likely to be an important aim for future research.
Recommendations for future research
The null effect reported in this study leaves unanswered the question of whether or not carer-led cognitive stimulation interventions are effective. Despite the appeal of home-based programmes led by carers, cognitive stimulation approaches may be better provided on a group basis unless further evidence becomes available.
-
If carer-led interventions are to be further pursued, future research should identify which factors are more likely to make the intervention most successful and adaptable to the needs of people with dementia. As feedback from people with dementia in the qualitative interviews indicated that some sessions were not stimulating enough, future studies should consider that people earlier in the disease trajectory may have different cognitive stimulation needs from those with moderate dementia.
-
Given that less than half of the iCST group completed at least two sessions per week this reduced the power of the study to identify potential differences with the control group and indicates limitations in relation to the applicability of the intervention. Future work is needed to investigate the characteristics of caregiving dyads that are most likely to adhere to and benefit from carer-led individual cognitive stimulation interventions and methods to improve adherence. The involvement of paid domiciliary care workers or volunteer befrienders in delivering sessions should be explored further, as this may increase adherence by placing less responsibility on family carers.
-
Given that carer-led, cognitive-based approaches are relatively new treatments, research designs that address their efficacy are more relevant than designs addressing mechanisms at this point. Therefore, comparisons of group versus individual approaches are likely to be premature at this stage.
-
It is important that future research and different research groups evaluate further the effects of individual cognitive stimulation interventions. This work will help to ensure the reliability and robustness of the effects reported in this trial, in relation to benefits of relationship quality for people with dementia and health-related quality of life for carers.
Conclusions
The evidence from this trial suggests that taking part in iCST sessions does not result in improvements on cognition or quality of life for people with dementia. There was no evidence of improvements for carers’ mental and physical health. There was no evidence that iCST improved secondary outcomes for people with dementia, such as activities of daily living, mood or behavioural and psychological symptoms. Analyses indicated that iCST did not confer any benefit for carers’ mood, resilience or relationship quality with the person with dementia. Although people with dementia receiving iCST reported better relationship quality with their carer (in itself, an important component of quality of life for people with dementia) and carers reported improved health-related quality of life, these findings need to be interpreted with caution given limitations owing to multiple testing. Despite efforts to minimise loss to follow-up, the study may have still been underpowered to detect significant differences for the primary outcome. Given that iCST did not achieve the expected change, it is unlikely to lead to clinical benefit in improving cognition and quality of life for people with dementia.
Acknowledgements
This study was supported by the National Institute for Health Research Health and Technology Assessment (HTA) programme.
The authors would like to thank all the people with dementia and their family carers who took part in the iCST trial and development studies for their time and contribution.
We would like to thank the iCST Trial Steering Committee (TSC) and DMEC members. The TSC was chaired by Professor James Lindesay and included Dr Vincent Kirchner, Dr Jan Oyebode, Ms Rachel Thompson, Ms Catherine Crombie, Ms Alice Betts, Ms Elayne Dunn, Mr U Hla Htay, Professor Graham Stokes and Professor Robert Woods. The DMEC was chaired by Professor Jill Manthorpe and included Dr Jennifer Hellier (independent statistician), and Mr David Prothero (carer). We are grateful to both committees for their contribution and oversight.
We would like to thank all those who took part in the data collection, recruitment and set-up of iCST centres:
Bangor: Hannah Jelley, Julia Roberts, Dawn Jones, Claire Watkins and Kat Algar.
Devon North: Michael Van Buren, Arun Devasahayam, Mark Harrison, Paula Clark, Louise Wright, Sarah Bagwell, Amanda Skinner and Nathan Vernon.
Devon South: Leanne Timings, Joshua Williams, Gary Hodge, Natalie Portwine, Mike Visick, Wendy Colwell, Ruth Newman and San Sreenath.
Dorset: Caroline Coleman, Rebecca Weekes and Janet Craven.
Hull: Cathryn Hart, Saba Alam, Katie Gibson, Len Stevens, Gavin Dawson, Irene Carr, Rachel Whitehead and Lucy Webster.
Lincolnshire: Val Smith, Diane Brennan and Lizwi Nyathi.
London: Caroline Popham, Fara Hamidi, James Sinclair, Sharon Cooper, Tom Freeth, Linda Smith and Gillian Lasocki.
Manchester: Salman Karim, John Mulinga, Phillip Tinkler, Emma Oughton, Anita Davies, Kelly Birtwell, Lewis Harpin, Marianne Hare, Janice Birt, Kerry Ward, Rowen Callaghan, Jenny Maskell and Lesley Craven.
Norfolk and Suffolk: Kathryn Sams, Juniper West, Sarah Purdy, Bridget Veldhuis, Gemma Ridel, Ralph Woodcock, Angelica Schiza, Kim Clipsham, Moira Henderson, Wendy Dwornik and John Robinson.
Contributions of authors
Vasiliki Orgeta (Senior Research Associate) supervised and planned the development of iCST, contributed to the development of iCST materials, planned and conducted one of the development studies, was responsible for the day-to-day operation and management of the study in all centres, contributed to the study design and drafted most of the final report.
Phuong Leung (PhD researcher) contributed to the development of iCST materials, trial management and data collection, planned and conducted the qualitative study, and wrote sections of this report.
Lauren Yates (PhD researcher) contributed to the development of iCST materials, recruitment and data collection, planned and conducted two of the development studies, and wrote sections of this report.
Sujin Kang (Research Officer, Statistics) was a trial statistician, undertook the statistical analyses and drafted sections of the report.
Zoe Hoare (Research Fellow, Statistics) was a trial statistician, developed the statistical analysis plan, undertook the statistical analyses and drafted sections of the report.
Catherine Henderson (Research Fellow, Health Economics) advised on the supervision of data collection of health economics, undertook the health economic analyses and drafted the health economic section of the report.
Chris Whitaker (Senior Statistician) was trial statistician, advised on the statistical analysis plan, the statistical analyses and statistical input in sections of the report.
Alistair Burns (Professor of Old Age Psychiatry, Honorary Consultant Old Age Psychiatrist) was principal investigator of the Manchester site.
Martin Knapp (Professor of Health Economics) oversaw the health economics of the study.
Iracema Leroi (Clinical Senior Lecturer and Honorary Consultant in Psychiatry) was principal investigator of the Manchester site.
Esme D Moniz-Cook (Professor of Clinical Psychology and Ageing) was principal investigator of the Hull site.
Stephen Pearson (Consultant Psychiatrist in Old Age) was principal investigator of the Devon sites.
Stephen Simpson (Consultant Psychiatrist in Old Age) was principal investigator of the Dorset site.
Aimee Spector (Senior Lecturer in Dementia) contributed to the planning and design of the study and provided expertise in CST.
Steven Roberts (Head of Dementia Services) was principal investigator of the Lincolnshire site.
Ian T Russell (Professor of Clinical Trials) contributed to the planning and design of the study and oversaw the methodological aspects of the trial.
Hugo de Waal (Honorary Senior Lecturer, Consultant Psychiatrist in Old Age) was principal investigator of the Norfolk and Suffolk site.
Robert T Woods (Professor of Clinical Psychology of Older People) contributed to the planning and design of the study, provided expertise in CST and was principal investigator of the Bangor site.
Martin Orrell (Professor of Ageing and Mental Health) was the chief investigator, conceived, designed and planned the study, and supervised its conduct. He was the principal investigator for the London sites and contributed to all sections of this report.
All study authors contributed to the preparation and review of this report.
Publications
Orrell M, Yates LA, Burns A, Russell I, Woods RT, Hoare Z, et al. Individual Cognitive Stimulation Therapy for dementia (iCST): study protocol for a randomised controlled trial. Trials 2012;13:172.
Yates LA, Orrell M, Spector A, Orgeta V. Service users’ involvement in the development of individual Cognitive Stimulation Therapy (iCST) for dementia: a qualitative study. BMC Geriatr 2015;15:4.
Orrell M, Woods B, Spector A. Should we use individual cognitive stimulation therapy to improve cognitive function in people with dementia? BMJ 2012;344:e633.
Yates LA, Leung P, Orgeta V, Spector A, Orrell M. The development of individual Cognitive Stimulation Therapy (iCST) for dementia. Clin Interv Ageing 2014;10:95–104.
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG. Old Age Task Force of the World Federation of Biological Psychiatry . Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021. http://dx.doi.org/10.1176/appi.ajp.162.11.1996.
- Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev 2012;2. http://dx.doi.org/10.1002/14651858.cd005562.pub2.
- Orgeta V, Qazi A, Spector AE, Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst Rev 2014;1. http://dx.doi.org/10.1002/14651858.cd009125.pub2.
- Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev 2003;4. http://dx.doi.org/10.1002/14651858.cd003260.
- Taulbee LR, Folsom JC. Reality orientation for geriatric patients. Hosp Community Psych 1966;17:133-5. http://dx.doi.org/10.1176/ps.17.5.133.
- Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry 2003;183:248-54. http://dx.doi.org/10.1192/bjp.183.3.248.
- Knapp M, Thorgrimsen L, Patel A, Spector A, Hallam A, Woods B, et al. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. Br J Psychiatry 2006;188:574-80. http://dx.doi.org/10.1192/bjp.bp.105.010561.
- Dementia: Supporting People with Dementia and their Carers in Health and Social Care: Clinical Guideline. London: NICE–SCIE; 2006.
- Prince M, Bryce R, Ferri C. World Alzheimer Report: The Benefits of Early Diagnosis and Intervention. London: Alzheimer’s Disease International; 2011.
- Moniz-Cook E, Agar S, Gibson G, Win T, Wang M. A preliminary study of the effects of early intervention with people with dementia and their families in a memory clinic. Aging Ment Health 1989;2:199-211. http://dx.doi.org/10.1080/13607869856687.
- Quayhagen MP, Quayhagen M. Testing of a cognitive stimulation intervention for dementia caregiving dyads. Neuropsychol Rehabil 2001;11:319-32. http://dx.doi.org/10.1080/09602010042000024.
- Onder G, Zanetti O, Giacobini E, Frisoni GB, Bartorelli L, Carbone G, et al. Reality orientation therapy combined with cholinesterase inhibitors in Alzheimer’s disease: randomised controlled trial. Br J Psychiatry 2005;187:450-5. http://dx.doi.org/10.1192/bjp.187.5.450.
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63-75. http://dx.doi.org/10.1016/j.jalz.2012.11.007.
- Knapp M, Comas-Herrera A, Wittenberg R, Hu B, King D, Rehill A, et al. Scenarios of Dementia Care: What Are the Impacts on Cost and Quality of Life? 2014.
- Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. http://dx.doi.org/10.1136/bmj.321.7262.694.
- Woods RT, Britton PG. Psychological approaches to the treatment of the elderly. Age Ageing 1977;6:104-12. http://dx.doi.org/10.1093/ageing/6.2.104.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Orrell M, Aguirre E, Spector A, Hoare Z, Woods RT, Streater A, et al. Maintenance cognitive stimulation therapy for dementia: single-blind, multicentre, pragmatic randomised controlled trial. Br J Psychiatry 2014;204:454-61. http://dx.doi.org/10.1192/bjp.bp.113.137414.
- Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development. Thousand Oaks, CA: Sage; 1998.
- Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376-80. http://dx.doi.org/10.1136/bmj.311.7001.376.
- Junger S, Payne S, Brearley S, Ploenes V, Radbruch L. Consensus building in palliative care: a Europe-wide delphi study on common understandings and conceptual differences. J Pain Symptom Manage 2012;44:192-205. http://dx.doi.org/10.1016/j.jpainsymman.2011.09.009.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348.
- Yates L, Orrell M, Leung P, Spector AE, Woods B, Orgeta V. Making a Difference 3: Individualised CST–A Manual for Carers. London: Hawker Publications; 2014.
- Lichstein KI, Riedel BW, Grieve R. Fair tests of clinical trials: a treatment implementation model. Adv Behav Res Ther 1994;16:1-29. http://dx.doi.org/10.1016/0146-6402(94)90001-9.
- Spector A, Davies S, Woods B, Orrell M. Reality orientation for dementia: a systematic review of the evidence of effectiveness from randomized controlled trials. Gerontologist 2000;40:206-12. http://dx.doi.org/10.1093/geront/40.2.206.
- The National Archives . Mental Capacity Act 2005 n.d. www.legislation.gov.uk/ukpga/2005/9/contents (accessed 14 March 2011).
- Dobson C. Conducting Research with People Not Having the Capacity to Consent to Their Participation. Leicester: British Psychological Society; 2008.
- National Institute for Health Research Clinical Research Network . Good Clinical Practice Training n.d. www.crn.nihr.ac.uk/learning-development/good-clinical-practice/ (accessed 14 March 2011).
- Care Standards Act 2000: Elizabeth II. Part 7. London: The Stationery Office; 2000.
- Russell D, Hoare ZS, Whitaker R, Whitaker CJ, Russell IT. Generalized method for adaptive randomization in clinical trials. Stat Med 2011;30:922-34. http://dx.doi.org/10.1002/sim.4175.
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984;141:1356-64. http://dx.doi.org/10.1176/ajp.141.11.1356.
- Weyer G, Erzigkeit H, Kanowski S, Ihl R, Hadler D. Alzheimer’s Disease Assessment Scale: reliability and validity in a multicenter clinical trial. Int Psychogeriatr 1997;9:123-38. http://dx.doi.org/10.1017/S1041610297004298.
- Logsdon RG, Gibbons LE, McCurry SM. Quality of life in Alzheimer’s disease: patient and caregiver reports. J Ment Health Aging 1999;5:21-32.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med 2002;64:510-19. http://dx.doi.org/10.1097/00006842-200205000-00016.
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9100.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. http://dx.doi.org/10.1212/WNL.44.12.2308.
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing 1996;25:113-20. http://dx.doi.org/10.1093/ageing/25.2.113.
- Byrne LM, Wilson PM, Bucks RS, Hughes AO, Wilcock GK. The sensitivity to change over time of the Bristol Activities of Daily Living Scale in Alzheimer’s disease. Int J Geriatr Psychiatry 2000;15:656-61. http://dx.doi.org/10.1002/1099-1166(200007)15:7<656::AID-GPS163>3.0.CO;2-Q.
- Sheikh JI, Yesavage JA, Brink TL. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Haworth Press; 1986.
- Lach HW, Chang YP, Edwards D. Can older adults with dementia accurately report depression using brief forms? Reliability and validity of the Geriatric Depression Scale. J Gerontol Nurs 2010;36:30-7. http://dx.doi.org/10.3928/00989134-20100303-01.
- Spruytte N, Van Audenhove C, Lammertyn F, Storms G. The quality of the caregiving relationship in informal care for older adults with dementia and chronic psychiatric patients. Psychol Psychother 2002;75:295-311. http://dx.doi.org/10.1348/147608302320365208.
- Beecham J, Knapp M, Thronicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry 2001;179:540-4. http://dx.doi.org/10.1192/bjp.179.6.540.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Wagnild GM, Young HM. Development and psychometric evaluation of the Resilience Scale. J Nurs Meas 1993;1:165-78.
- Wagnild GM. The Resilience Scale User’s Guide for the US English version of the Resilience Scale and the 14-item Resilience Scale (RS-14). Montana: The Resilience Center; 2009.
- Matthews J. An Introduction to Clinical Trials. Arnold/Hodder Headline: London; 2000.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: University of York; 1995.
- Rowen D, Mulhern B, Banerjee S, Hout Bv, Young TA, Knapp M, . Estimating preference-based single index measures for dementia using DEMQOL and DEMQOL-Proxy. Value Health 2012;15:346-56. http://dx.doi.org/10.1016/j.jval.2011.10.016.
- Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics 1999;15:141-55. http://dx.doi.org/10.2165/00019053-199915020-00003.
- National Schedule of Reference Costs 2012–13. London: Department of Health; 2013.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Curtis L. Units Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Koopmanschap MA, van Exel JN, van den Berg B, Brouwer WB. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics 2008;26:269-80. http://dx.doi.org/10.2165/00019053-200826040-00001.
- Pritchard C, Sculpher M. Productivity Costs: Principles and Practice in Economic Evaluation. London: Office of Health Economics; 2000.
- NHS Employers . NHS Terms and Conditions of Service Handbook. Pay and Conditions Circular (AforC) 2 2014 2012.
- Stata Multiple-Imputation Reference Manual: Release 13.0. College Station, TX: StataCorp LP; 2013.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. http://dx.doi.org/10.1002/hec.843.
- Guide to the Methods of Technology Appraisal, 2008. London: National Institute for Health and Clinical Excellence; 2008.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566-72. http://dx.doi.org/10.1192/bjp.140.6.566.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. http://dx.doi.org/10.1002/hec.903.
- Glick H. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Nygard L. How can we get access to the experiences of people with dementia? Suggestions and reflections. Scand J Occup Ther 2006;12:101-12. http://dx.doi.org/10.1080/11038120600723190.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analyzing Qualitative Data. London: Routledge; 1994.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. http://dx.doi.org/10.1186/1471-2288-13-117.
- Spector A, Gardner C, Orrell M. The impact of Cognitive Stimulation Therapy groups on people with dementia: views from participants, their carers and group facilitators. Aging Ment Health 2011;15:945-9. http://dx.doi.org/10.1080/13607863.2011.586622.
- Sorensen LV, Waldorff FB, Waldemar G. Early counselling and support for patients with mild Alzheimer’s disease and their caregivers: a qualitative study on outcome. Aging Ment Health 2008;12:444-50. http://dx.doi.org/10.1080/13607860802224342.
- Hellstrom I, Nolan M, Lundh U. ‘We do things together’: a case study of ‘couplehood’; in dementia. Dementia 2005;4:7-22. http://dx.doi.org/10.1177/1471301205049188.
- Woods RT, Nelis SM, Martyr A, Roberts J, Whitaker CJ, Markova I, et al. What contributes to a good quality of life in early dementia? Awareness and the QoL-AD: a cross-sectional study. Health Qual Life Outcomes 2014;12. http://dx.doi.org/10.1186/1477-7525-12-94.
- NHS Staff Earnings Estimates April to June 2012. Leeds: Health and Social Care Information Centre; 2012.
- NHS Employers . New Mileage Arrangements for Agenda for Change Staff 2013. www.nhsemployers.org/PayAndContracts/AgendaForChange/mileage/Pages/Mileage-allowances.aspx (accessed 1 July 2013).
- UK Government . National Minimum Wage Rates 2013 n.d. www.gov.uk/national-minimum-wage-rates (accessed 17 February 2014).
- An OFT Market Study. London: Office of Fair Trading; 2012.
- NHS Sight Test Fee, Payments For Continuing Education and Training and Pre-Registration Supervisors Grant. London: Department of Health; 2012.
- Optics at a Glance 2012. London: Optical Confederation; 2012.
- Banerjee S, Hellier J, Romeo R, Dewey M, Knapp M, Ballard C, et al. Study of the use of antidepressants for depression in dementia: the HTA-SADD trial -- a multicentre, randomised, double-blind, placebo-controlled trial of the clinical effectiveness and cost-effectiveness of sertraline and mirtazapine. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17070.
- Prescription Cost Analysis, England 2012. Leeds: Health and Social Care Information Centre; 2013.
Appendix 1 Development study 1: service users’ views about the intervention
| Characteristics | Focus groups | Individual interviews |
|---|---|---|
| People with dementia | n = 18 | n = 10 |
| Sex, n (%) | ||
| Male | 7 (39) | 5 (50) |
| Female | 11 (61) | 5 (50) |
| Mean age (years), n (SD) | 80.50 (5.80) | 84.44 (4.10) |
| Ethnicity, n (%) | ||
| White | 18 (100) | 10 (100) |
| Living status, n (%) | ||
| With spouse | 6 (33) | 6 (60) |
| With adult child | 2 (11) | 3 (30) |
| Alone | 4 (22) | 1 (10) |
| In care | 2 (12) | – |
| Unknown | 4 (22) | – |
| Family carers | n = 14 | n = 10 |
| Gender, n (%) | ||
| Male | 6 (43) | 3 (30) |
| Female | 8 (57) | 7 (70) |
| Mean age (years), n (SD) | 65.23 (9.65) | 67.67 (14.35) |
| Ethnicity, n (%) | ||
| White | 11 (79) | 10 (100) |
| Relationship, n (%) | ||
| Spouse | 7 (50) | 6 (60) |
| Adult child | 7 (50) | 4 (40) |
| Cohabiting, n (%) | ||
| Yes | 8 (57) | 9 (90) |
| Years caring, n (range, SD) | 5.61 (1–16, 4.03) | 2.89 (1.5–7, 1.78) |
| Themes | Discussion points | Focus group | Interview | |||
|---|---|---|---|---|---|---|
| Carer | Participant | Combined | Carer | Participant | ||
| Mental stimulation | Importance of mental stimulation | ✗ | ✗ | ✗ | ✗ | ✗ |
| Characteristics/types of mentally stimulating activities | ✗ | ✗ | ✗ | ✗ | ✗ | |
| iCST manual | ||||||
| Content | Additional information required | ✗ | ✗ | ✗ | ||
| Additional information required | ✗ | ✗ | ✗ | |||
| Layout | Size of text/images and quality | ✗ | ✗ | ✗ | ||
| Layout and format | ✗ | ✗ | ✗ | |||
| General | Positive/negative comments | ✗ | ✗ | ✗ | ||
| Ease of use | ✗ | ✗ | ✗ | |||
| iCST activity workbook | ||||||
| Content | Clarity of instructions | ✗ | ✗ | ✗ | ||
| Activities | ✗ | ✗ | ✗ | |||
| Layout | Layout and format | ✗ | ✗ | ✗ | ||
| Quality of images | ✗ | ✗ | ✗ | |||
| Activities | Level of difficulty | ✗ | ✗ | ✗ | ||
| Level of engagement/enjoyment | ✗ | ✗ | ✗ | |||
| Issues around improving activities | ✗ | ✗ | ✗ | |||
| Feasibility of iCST | Acceptability/iCST schedule (30 minutes/three times weekly) | ✗ | ✗ | ✗ | ✗ | ✗ |
| Anticipated difficulties | ✗ | ✗ | ✗ | |||
| Support needed | ✗ | ✗ | ✗ | |||
| Group- vs. home-based training | ✗ | ✗ | ✗ | |||
| Themes of qualitative analyses | Person with dementia | Family carer |
|---|---|---|
| Mental stimulation | ||
| Importance of mental stimulation | ‘Keep up to date’ and improve thoughts Keeps from ‘going backwards’ Keeps mind and body active |
Important for person with dementia Person is dependent on carer for stimulation Improves mood, alertness and quality of life |
| Mentally stimulating activities | Newspapers, watching TV, sports/exercise Puzzles/quizzes, household tasks |
Playing cards/quizzes, attending clubs/social events/trips, keeping fit, well-being activities |
| iCST manual/activity workbook | ||
| Content/language/terminology | Awakens thoughts/ideas, ‘opens the mind’ Include more images as ‘words are a problem’ |
Provides ideas to stimulate discussion Easy to understand, instructions clear |
| Layout/format/size of text/images | Ring-bound hard-copy material preferred Increase size of text Good, pleasing to the eye, bright and happy, putting you ‘in a good mood’ |
Increase size of text/images Layout should discourage feeling ‘being tested’ Ring-bound and hard-copy material preferred Outstanding, great colours, nicely done |
| iCST activities | Reduce difficulty level Include factual information |
Reduce difficulty level and avoid ‘deep’ questions Use activities that trigger memories |
| Feasibility | Limited time of family carers Important for carers to assist Household duties may be a barrier Person with dementia has plenty of time Carer will not need a lot of support |
Professionals delivering better suited Sessions feel ‘formal’ Useful for winter months Accommodate everyday events/personal needs Sessions in chunks would feel less like ‘therapy’ Mental/physical challenges of caring Training would be necessary |
| Resources/training | Resources forum/’iCST expert’ carers Phone support preferred over visits Group training encourages peer learning Individual training personal/easier to ask questions and not require arranging care |
|
Appendix 2 Development study 2: expert feedback
| Statement | Response options, n (%) | |||||
|---|---|---|---|---|---|---|
| Strongly disagree | Disagree | Neutral | Agree | Strongly agree | Conclusion | |
| Language is easy to understand | 0 | 2 (8) | 2 (8) | 6 (24) | 15 (60) | High agreement |
| Size of font is appropriate | 0 | 2 (8) | 1 (4) | 12 (48) | 10 (40) | Moderate agreement |
| Manual is stimulating/engaging | 0 | 1 (4) | 4 (16) | 16 (64) | 4 (16) | Moderate agreement |
| Amount of information is appropriate | 1 (4) | 1 (4) | 4 (16) | 14 (56) | 5 (20) | Moderate agreement |
| Activities are clearly presented | 0 | 0 | 2 (8) | 12 (48) | 11 (44) | Moderate agreement |
| Layout is appropriate | 0 | 0 | 2 (8) | 11 (44) | 12 (48) | Moderate agreement |
| Adequate variety in activities | 0 | 0 | 4 (16) | 15 (60) | 6 (24) | Moderate agreement |
| Participant/carer will enjoy the activities | 0 | 0 | 6 (24) | 16 (64) | 3 (12) | Moderate agreement |
| Statement | Response options, n (%) | |||||
|---|---|---|---|---|---|---|
| Strongly disagree | Disagree | Neutral | Agree | Strongly agree | Conclusion | |
| Language is easy to understand | 0 | 0 | 1 (4) | 11 (44) | 13 (52) | High agreement |
| Size of font is appropriate | 0 | 0 | 1 (4) | 14 (56) | 10 (40) | Moderate agreement |
| Workbook is stimulating/engaging | 0 | 0 | 3 (12) | 16 (64) | 6 (24) | Moderate agreement |
| Amount of information is appropriate | 0 | 0 | 1 (4) | 16 (64) | 8 (32) | Moderate agreement |
| Activities are clearly presented | 0 | 0 | 1 (4) | 13 (52) | 11 (44) | Moderate agreement |
| Layout is appropriate | 0 | 0 | 0 | 14 (56) | 11 (44) | Moderate agreement |
| Adequate variety in activities | 0 | 0 | 3 (12) | 15 (60) | 7 (28) | Moderate agreement |
| Participant/carer will enjoy the activities | 0 | 0 | 5 (20) | 14 (56) | 6 (24) | Moderate agreement |
Appendix 3 Development study 3: field testing of the intervention for final refinement prior to the main trial
| Demographic characteristics, N = 25 | |
|---|---|
| Family caregiving dyads, n = 19 | |
| People with dementia | |
| Age, mean (SD) | 80.60 (5.15) |
| Sex, % | |
| Male | 47.4 |
| Female | 52.6 |
| Ethnicity, % | |
| White | 78.9 |
| Black | 5.3 |
| Unknown | 15.8 |
| Living status, % | |
| Living with spouse | 42.1 |
| Living with adult child | 15.8 |
| Living alone | 26.3 |
| Unknown | 15.8 |
| Carers | |
| Age, mean (SD) | 65.00 (10.52) |
| Sex, % | |
| Male | 21.1 |
| Female | 78.9 |
| Ethnicity, % | |
| White | 84.2 |
| Mixed | 5.3 |
| Unknown | 10.5 |
| Relationship, % | |
| Spouse | 57.9 |
| Adult child | 42.1 |
| Years caring, mean (SD) | 4.32 (1.87) |
| Paid caregiving dyads, n = 6 | |
| People with dementia | |
| Age, mean (SD) | 83.25 (8.22) |
| Sex, % | |
| Male | 50.0 |
| Female | 50.0 |
| Ethnicity, % | |
| White | 83.3 |
| Unknown | 16.7 |
| Living status, % | |
| Living alone | 83.3 |
| Living with paid carer | 16.7 |
| Carers | |
| Age, mean (SD) | 42.6 (16.13) |
| Sex, % | |
| Male | 16.7 |
| Female | 83.3 |
| Ethnicity,% | |
| White | 33.3 |
| Black | 33.3 |
| Mixed | 33.3 |
| Years caring, mean (SD) | 1.75 (1.50) |
| Sessions completed (range 4–24) (N = 9) | |
|---|---|
| 0–6, n (%) | 1 (11) |
| 7–11, n (%) | 5 (56) |
| 12–16, n (%) | 2 (22) |
| 17–24, n (%) | 1 (11) |
| Mean number of sessions, n (SD) | 11.56 (5.59) |
| Evaluation ratings of iCST sessions, (n = 9) | |||||
|---|---|---|---|---|---|
| iCST theme | Frequency | ||||
| Interest | Communication | Enjoyment | Difficulty level | ||
| My life sessions (6 ratings) | Not at all (1) | 0 | 0 | 0 | 0 |
| A little (2) | 0 | 0 | 0 | 4 | |
| Moderately (3) | 6 | 1 | 4 | 2 | |
| Quite a lot (4) | 0 | 5 | 2 | 0 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 3.00 (0.00) | 3.83 (0.41) | 3.33 (0.52) | 2.33 (0.52) | |
| Current affairs (4 ratings) | Not at all (1) | 1 | 0 | 1 | 1 |
| A little (2) | 0 | 2 | 1 | 1 | |
| Moderately (3) | 1 | 0 | 2 | 2 | |
| Quite a lot (4) | 2 | 2 | 0 | 0 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 3.00 (1.41) | 3.00 (1.15) | 2.25 (0.95) | 2.25 (0.96) | |
| Food (6 ratings) | Not at all (1) | 1 | 0 | 2 | 1 |
| A little (2) | 3 | 2 | 2 | 0 | |
| Moderately (3) | 0 | 0 | 0 | 2 | |
| Quite a lot (4) | 2 | 4 | 2 | 1 | |
| Extremely (5) | 0 | 0 | 0 | 2 | |
| Mean ratings (SD) | 2.50 (1.22) | 3.33 (1.03) | 2.33 (1.37) | 3.50 (1.51) | |
| Being creative (5 ratings) | Not at all (1) | 2 | 2 | 2 | 4 |
| A little (2) | 0 | 1 | 0 | 1 | |
| Moderately (3) | 1 | 0 | 1 | 0 | |
| Quite a lot (4) | 2 | 2 | 2 | 0 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 2.50 (1.22) | 3.33 (1.03) | 2.33 (1.37) | 3.50 (1.51) | |
| Number games (4 ratings) | Not at all (1) | 0 | 0 | 0 | 2 |
| A little (2) | 1 | 0 | 2 | 1 | |
| Moderately (3) | 1 | 2 | 2 | 1 | |
| Quite a lot (4) | 2 | 2 | 0 | 0 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 3.25 (0.96) | 3.50 (0.58) | 2.50 (0.58) | 1.75 (0.96) | |
| Quiz games (2 ratings) | Not at all (1) | 0 | 0 | 0 | 2 |
| A little (2) | 0 | 0 | 0 | 0 | |
| Moderately (3) | 0 | 0 | 0 | 0 | |
| Quite a lot (4) | 2 | 1 | 1 | 0 | |
| Extremely (5) | 0 | 1 | 1 | 0 | |
| Mean ratings (SD) | 3.25 (0.96) | 3.50 (0.58) | 2.50 (0.58) | 1.75 (0.96) | |
| Sounds (3 ratings) | Not at all (1) | 0 | 0 | 2 | 0 |
| A little (2) | 0 | 0 | 0 | 1 | |
| Moderately (3) | 0 | 0 | 1 | 0 | |
| Quite a lot (4) | 3 | 3 | 0 | 2 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 4.00 (0.00) | 4.00 (0.00) | 1.67 (1.15) | 3.33 (1.15) | |
| Physical games (6 ratings) | Not at all (1) | 0 | 0 | 0 | 4 |
| A little (2) | 0 | 0 | 0 | 0 | |
| Moderately (3) | 2 | 2 | 2 | 2 | |
| Quite a lot (4) | 2 | 0 | 2 | 0 | |
| Extremely (5) | 2 | 4 | 2 | 0 | |
| Mean ratings (SD) | 4.00 (0.89) | 4.33 (1.03) | 4.00 (0.89) | 1.67 (1.03) | |
| Categorising objects (6 ratings) | Not at all (1) | 0 | 0 | 0 | 2 |
| A little (2) | 0 | 0 | 0 | 4 | |
| Moderately (3) | 4 | 4 | 2 | 0 | |
| Quite a lot (4) | 2 | 2 | 4 | 0 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 3.33 (0.52) | 3.33 (0.52) | 3.67 (0.52) | 1.67 (0.52) | |
| Household treasures (6 ratings) | Not at all (1) | 0 | 0 | 0 | 2 |
| A little (2) | 0 | 0 | 0 | 4 | |
| Moderately (3) | 4 | 2 | 6 | 0 | |
| Quite a lot (4) | 2 | 4 | 0 | 0 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 3.33 (0.52) | 3.67 (0.52) | 3.00 (0.00) | 1.67 (0.52) | |
| Useful tips (5 ratings) | Not at all (1) | 0 | 0 | 1 | 2 |
| A little (2) | 0 | 0 | 0 | 2 | |
| Moderately (3) | 3 | 1 | 2 | 1 | |
| Quite a lot (4) | 2 | 4 | 2 | 0 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 3.40 (0.55) | 3.80 (0.44) | 3.00 (1.22) | 1.80 (0.84) | |
| Thinking cards (2 ratings) | Not at all (1) | 0 | 0 | 0 | 0 |
| A little (2) | 0 | 0 | 0 | 2 | |
| Moderately (3) | 0 | 0 | 0 | 0 | |
| Quite a lot (4) | 2 | 2 | 2 | 0 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 4.00 (0.00) | 4.00 (0.00) | 4.00 (0.00) | 4.00 (0.00) | |
| Art discussion (4 ratings) | Not at all (1) | 0 | 0 | 0 | 4 |
| A little (2) | 0 | 0 | 0 | 0 | |
| Moderately (3) | 2 | 0 | 0 | 0 | |
| Quite a lot (4) | 0 | 2 | 2 | 0 | |
| Extremely (5) | 2 | 2 | 2 | 0 | |
| Mean ratings (SD) | 4.00 (1.15) | 4.50 (0.58) | 4.50 (0.58) | 1.00 (0.00) | |
| Faces/scenes (4 ratings) | Not at all (1) | 0 | 0 | 0 | 2 |
| A little (2) | 0 | 0 | 0 | 0 | |
| Moderately (3) | 0 | 0 | 2 | 2 | |
| Quite a lot (4) | 4 | 4 | 2 | 0 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 4.00 (0.00) | 4.00 (0.00) | 3.50 (0.58) | 2.00 (1.15) | |
| Word games (14 ratings) | Not at all (1) | 0 | 0 | 0 | 8 |
| A little (2) | 0 | 0 | 0 | 2 | |
| Moderately (3) | 0 | 2 | 2 | 4 | |
| Quite a lot (4) | 12 | 8 | 4 | 0 | |
| Extremely (5) | 2 | 4 | 8 | 0 | |
| Mean ratings (SD) | 4.14 (0.36) | 4.14 (0.66) | 4.43 (0.75) | 1.71 (0.91) | |
| Slogans and visual clips (4 ratings) | Not at all (1) | 0 | 0 | 2 | 2 |
| A little (2) | 0 | 0 | 0 | 0 | |
| Moderately (3) | 2 | 2 | 0 | 0 | |
| Quite a lot (4) | 2 | 2 | 2 | 2 | |
| Extremely (5) | 0 | 0 | 0 | 0 | |
| Mean ratings (SD) | 3.50 (0.58) | 3.50 (0.58) | 2.50 (1.73) | 2.50 (1.73) | |
| Orientation (7 ratings) | Not at all (1) | 0 | 0 | 2 | 2 |
| A little (2) | 2 | 2 | 2 | 2 | |
| Moderately (3) | 5 | 4 | 1 | 1 | |
| Quite a lot (4) | 0 | 1 | 2 | 0 | |
| Extremely (5) | 0 | 0 | 0 | 2 | |
| Mean ratings (SD) | 2.71 (0.48) | 2.86 (0.69) | 2.43 (1.27) | 2.71 (1.70) | |
| Using money (6 ratings) | Not at all (1) | 0 | 0 | 0 | 4 |
| A little (2) | 2 | 0 | 0 | 0 | |
| Moderately (3) | 2 | 4 | 4 | 2 | |
| Quite a lot (4) | 0 | 0 | 0 | 0 | |
| Extremely (5) | 2 | 2 | 2 | 0 | |
| Mean ratings (SD) | 3.33 (1.37) | 3.67 (1.03) | 3.67 (1.03) | 1.67 (1.03) | |
| Childhood (4 ratings) | Not at all (1) | 0 | 0 | 0 | 2 |
| A little (2) | 0 | 0 | 0 | 0 | |
| Moderately (3) | 0 | 0 | 0 | 2 | |
| Quite a lot (4) | 4 | 2 | 2 | 0 | |
| Extremely (5) | 0 | 2 | 2 | 0 | |
| Mean ratings (SD) | 4.00 (0.00) | 4.50 (0.58) | 4.50 (0.58) | 2.00 (1.54) | |
| Carer ratings | Researcher ratings | |||||
|---|---|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | |||
| iCST knowledge | 3.04 (1.00) | Success of set-up visit | 3.77 (0.75) | |||
| Poor (1) | 0 | Poor (1) | 0 | |||
| Fair (2) | 31.8 | Fair (2) | 0 | |||
| Good (3) | 45.5 | Good (3) | 41.2 | |||
| Very good (4) | 9.1 | Very good (4) | 41.2 | |||
| Excellent (5) | 13.6 | Excellent (5) | 17.6 | |||
| Confidence in iCST | 3.95 (0.78) | Successful engagement | 3.70 (0.47) | |||
| Very little (1) | 0 | |||||
| Some (2) | 0 | Not at all (1) | 0 | |||
| Fair (3) | 31.8 | A little (2) | 0 | |||
| Good (4) | 40.9 | Quite a bit (3) | 29.4 | |||
| Very confident (5) | 27.3 | A lot (4) | 70.6 | |||
| Support required | 2.10 (0.55) | Support required | 1.52 (0.51) | |||
| Not at all (1) | 5.0 | Not at all (1) | 47.1 | |||
| A little (2) | 85.0 | A little (2) | 52.9 | |||
| Quite a lot (3) | 5.0 | Quite a bit (3) | 0 | |||
| A lot (4) | 5.0 | A lot (4) | 0 | |||
| Training arrangement | ||||||
| Own home | 54.5 | |||||
| In groups | 22.7 | |||||
| Either | 22.7 | |||||
| Themes | Family carers | Paid carers |
|---|---|---|
| Barriers in iCST delivery | Time/availability Health of person with dementia/carer Job/family commitments Difficulties motivating/engaging person with dementia Life events |
Carer on annual leave Difficulties motivating/engaging person with dementia Life events Health of the person with dementia Visits suspended or person with dementia on holiday |
| Barriers specific to iCST activities/programme | Using opinion rather than facts Person with dementia finds activities difficult Locating resources to use |
Person with dementia demotivated if activity is too easy |
| iCST programme/structure | Doing sessions in ‘chunks’ Make sessions feel ‘informal’ Separate orientation with current affairs Skip orientation discussion Repeating sessions out of order helpful |
Make sessions feel ‘informal’ Completing sessions out of order helpful |
| Frequency/duration of iCST sessions | Difficult to complete three sessions weekly Sessions last longer than 30 minutes |
Feasible to complete three sessions weekly |
| iCST manual | Easy to use Promotes discussion/ideas Provides variation in difficulty of activities |
Easy to use Important to adjust ratings of difficulty Remove reference to ‘person with dementia’ |
| iCST resources | Feasible to use own resources when prompted | Feasible to use own resources when prompted |
| Response to the iCST activities from the person with dementia | Enjoying activities Asking questions can make person nervous Mood is lifted, sense of achievement Improvements in conversation |
Enjoying activities Mood is lifted Person interested/engaged in activities |
| Caregiving relationship | Gives carer a sense of purpose Brings dyad ‘closer together’ Activities help dyad to communicate Opportunities to laugh together Carer is giving person more of their time |
|
| Support in delivering iCST | None needed Friends and family help with sessions Family is often too busy to help |
None needed Family members help with sessions |
Appendix 4 Economic evaluation: unit costs and types of care and support tasks carried out by carers
| Service use item | Unit cost (£) | Sources |
|---|---|---|
| Costs of professional support (intervention) | ||
| iCST training costs | 17,288 | Project team |
| Per-participant (total divided by 180 intervention participants) | 96 | |
| iCST manual and materials, per participant | 94 | Project team |
| Weighted cost of professional support, per hour | 49 | Project data collection; Health and Social Care Information Centre 201276 |
| Mileage costs per one-way journey (per site) | Range: 4–32 | Project data collection; NHS Employers77 |
| Carer costs (value of time) | ||
| Replacement cost: average cost of independent sector, local authority-purchased home worker, per hour | 19 | Curtis 201355 |
| Opportunity cost: minimum wage, per hour | 6 | UK Government78 |
| Hospital costs | ||
| Inpatient bed-day, per specialty | Range: 344–1495 | Department of Health54 |
| Inpatient bed-day, weighted average across adult specialties | 577 | Department of Health54 |
| Day attendances | Range: 540–817 | Department of Health54 |
| Day case, weighted average across specialties | 693 | Department of Health54 |
| Outpatient attendances | Range: 27–468 | Department of Health54 |
| Outpatient, weighted average of follow-up attendances across adult specialties | 98 | Department of Health54 |
| Average cost of memory clinic contact | 465 | Curtis 201355 |
| A&E attendances, admitted and non-admitted | Range: 115–160 | Department of Health54 |
| Primary and community health services | ||
| District nursing time: average cost of direct contact, per contact | 38 | Department of Health54 |
| District nursing time: average cost of an hour of home visit, per minute | 1 | Curtis 201355 |
| District nursing visit: average cost of an hour of nurse time, per minute | 0.7 | Curtis 201355 |
| Practice nurse visit: average cost of an hour of direct contact time, per minute | 0.7 | Curtis 201355 |
| Practice nurse visit: per consultation, per consultation | 11 | Curtis 201355 |
| Specialist nursing; adult, face to face, per contact | Range: 45–90 | Department of Health54 |
| Weighted average of specialist nursing across adult specialities, per contact | 59 | Department of Health54 |
| NHS community occupational therapy, per minute | 0.5 | Curtis 201355 |
| NHS community occupational therapy, per visit | 73 | Curtis 201355 |
| NHS community physiotherapy, per visit | 50 | Department of Health54 |
| NHS community physiotherapy average cost of an hour of home visit, per minute | 0.5 | Curtis 201355 |
| GP time: average cost per minute of home visit, excluding direct staff and qualification, per minute | 4 | Curtis 201355 |
| GP time: per home visit lasting 23.4 minutes (including travel), per visit | 85 | Curtis 201355 |
| GP time: average cost per minute in clinic, excluding direct staff and qualification, per minute | 3 | Curtis 201355 |
| GP time: average cost of surgery visit of 11.7 minutes, excluding direct staff and qualification, per consultation | 34 | Curtis 201355 |
| Chiropodist (NHS): reference (average) cost of visit for community podiatry, episode/contact | 41 | Curtis 201355 |
| Dentist (NHS): reference (average) cost of visit for community dentistry, per contact | 115 | Department of Health54 |
| Dentist (private): average price of check-up, per contacta | 26 | Office of Fair Trading79 |
| NHS sight test: paid for sight test to optometrists, per test | 21 | Department of Health80 |
| Private sight test: average charge to patients for a private sight test, excluding discounts and special offers, per test | 22 | Optics at a glance81 |
| Social care | ||
| Private residential care, mean cost per day | 76 | Curtis 201355 |
| Local authority residential care, mean cost per day | 143 | Curtis 201355 |
| Private nursing home care, mean cost per day | 107 | Curtis 201355 |
| Social work: average cost per hour of face-to-face contact, per minute | 3 | Curtis 201355 |
| Independent home carer weekday face-to-face, per minute | 0.4 | Curtis 201355 |
| Meals on wheels: average cost per meal on wheels, per meal | 4 | Curtis 201355 |
| Community mental health services | Curtis 201355 | |
| Consultant psychiatrist: face-face contact, per minute | 4 | Curtis 201355 |
| Mental health nurse: face-to-face contact, per minute | 1 | Curtis 201355 |
| Counselling services in primary care: per surgery consultation | 58 | Curtis 201355 |
| Community psychologist: per hour of client contact per minute | 2 | Curtis 201355 |
| Day services | ||
| Day services: per session | 38 | Curtis 201355 |
| Day services: per day, weighted average over all services (stroke, elderly, other) | 155 | Department of Health54 |
| Lunch club: per session | 8 | Banerjee et al. 201382 |
| Social club: per session | 5 | Banerjee et al. 201382 |
| ‘Other’ | ||
| Medications: standard quantity units | Range: 0.1–6.0 | Health and Social Care Information Centre 201383 |
| Equipment and adaptations, cost over 3 monthsa | Range: 0.1–104.0 | Curtis 2012,56 Curtis 201355 |
| Type of care | iCST (SE) (n = 129) | TAU (SE) (n = 135) | Mean differencea |
|---|---|---|---|
| Baseline | |||
| Personal care | 43% | 36% | 6% |
| Helping with finances | 82% | 76% | 7% |
| Practical help | 78% | 77% | 1% |
| Taking the person to appointments | 91% | 86% | 6% |
| Medications | 78% | 79% | –1% |
| Keeping the person company | 89% | 91% | –2% |
| Making sure the person is safe (supervision) | 68% | 76% | –7% |
| Helping person to organise scheduleb | 2% | 2% | –1% |
| Helping person’s mental state: moraleb | 1% | 2% | –1% |
| Week 13 | |||
| Personal care | 40% | 30% | 11% |
| Helping with finances | 82% | 81% | 1% |
| Practical help | 81% | 80% | 1% |
| Taking the person to appointments | 87% | 89% | –2% |
| Medications | 81% | 80% | 1% |
| Keeping the person company | 91% | 91% | 0% |
| Making sure the person is safe (supervision) | 75% | 79% | –3% |
| Helping person to organise scheduleb | 2% | 1% | 0% |
| Helping person’s mental state: moraleb | 2% | 2% | –1% |
| Week 26 | |||
| Personal care | 43% | 40% | 3% |
| Helping with finances | 84% | 82% | 1% |
| Practical help | 84% | 85% | –1% |
| Taking the person to appointments | 89% | 90% | 0% |
| Medications | 82% | 81% | 1% |
| Keeping the person company | 92% | 96% | –3% |
| Making sure the person is safe (supervision) | 79% | 81% | –2% |
| Helping person to organise scheduleb | 2% | 4% | –3% |
| Helping person’s mental state: moraleb | 1% | 1% | 0% |
Appendix 5 Cost-effectiveness acceptability curves
FIGURE 2.
Cost-effectiveness acceptability curve: ADAS-Cog.
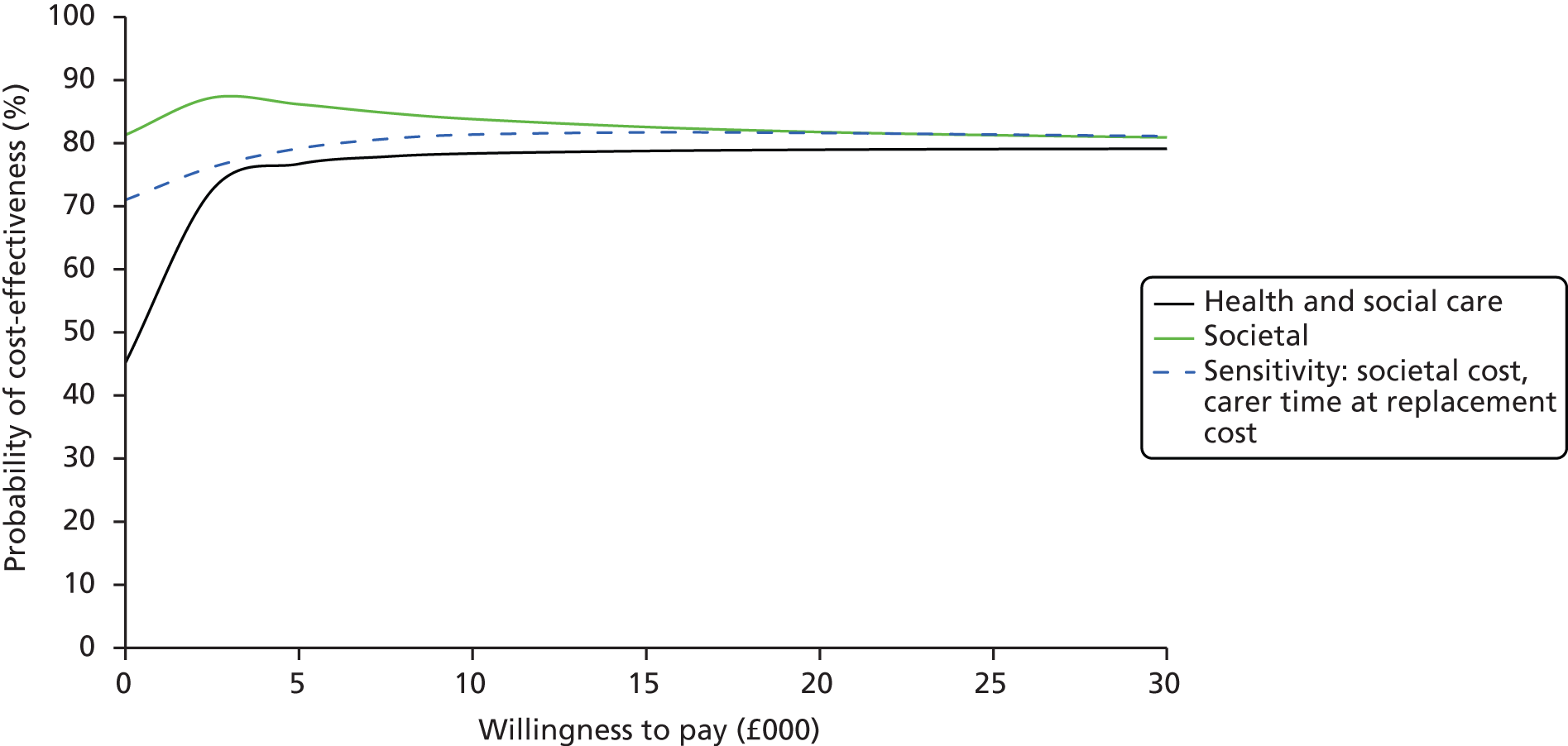
FIGURE 3.
Cost-effectiveness acceptability curve: QoL-AD.

FIGURE 4.
Cost-effectiveness acceptability curve: QCPR, person with dementia.

FIGURE 5.
Cost-effectiveness acceptability curve: QALY, carers.
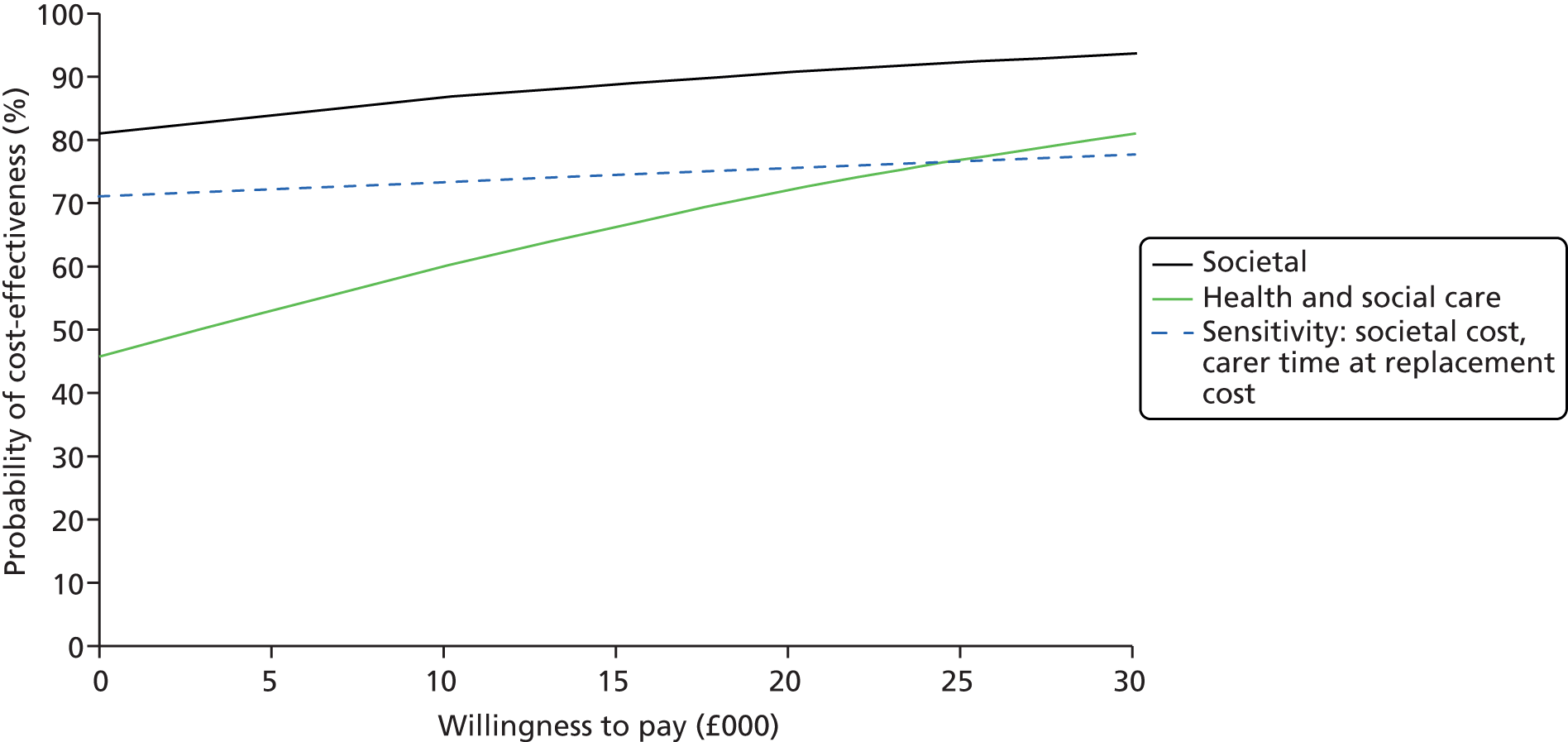
Appendix 6 Full imputation data set
The tables presented here indicate the results for the data if the multiple imputation model had been used to impute the data for all 356 participants at each time point. Various methodologies for different types of missing data (e.g. death vs. ill health) have not been taken into account as the number of each of these circumstances was minimal and would have limited impact on the results of any imputation method. Ten imputation sets were created to establish a robust adjustment to the data missing from each time point.
| Person with dementia outcome measure | iCST (N = 180) | TAU (N = 176) | Pooled MD | 95% CI of pooled MD | p-value |
|---|---|---|---|---|---|
| ADAS-Cog | 22.16 | 22.61 | –0.45 | –1.95 to 1.05 | 0.56 |
| QoL-AD | 37.74 | 37.71 | 0.03 | –1.94 to 2.00 | 0.98 |
| DEMQOL | 93.23 | 93.57 | –0.34 | –2.48 to 1.80 | 0.76 |
| NPI [P] | 10.52 | 9.80 | 0.72 | –1.85 to 3.29 | 0.58 |
| GDS-15 | 3.45 | 3.33 | 0.12 | –0.34 to 0.58 | 0.61 |
| QCPR total | 56.59 | 55.21 | 1.38 | –0.16 to 2.92 | 0.08 |
| QCPR (warmth) | 33.48 | 32.83 | 0.65 | –0.31 to 1.61 | 0.18 |
| QCPR (criticism and conflict) | 23.06 | 22.43 | 0.63 | –0.26 to 1.52 | 0.17 |
| MMSE | 18.98 | 19.68 | –0.7 | –1.44 to 0.04 | 0.07 |
| BADLS [P] | 13.38 | 13.47 | –0.09 | –1.40 to 1.22 | 0.89 |
| QoL-AD [P] | 31.27 | 31.03 | 0.26 | –0.91 to 1.38 | 0.69 |
| DEMQOL [P] | 99.05 | 97.94 | 1.11 | –1.16 to 3.38 | 0.34 |
| Carer outcome measure | iCST (N = 180) | TAU (N = 176) | Pooled MD | 95% CI of pooled MD | p-value |
|---|---|---|---|---|---|
| ADAS-Cog | 22.24 | 22.00 | 0.24 | –1.37 to 1.85 | 0.77 |
| QoL-AD | 38.28 | 38.20 | 0.08 | –0.85 to 1.01 | 0.86 |
| DEMQOL | 90.99 | 91.41 | –0.42 | –2.65 to 1.81 | 0.71 |
| NPI [P] | 13.41 | 14.52 | –1.11 | –3.24 to 1.02 | 0.31 |
| GDS-15 | 3.43 | 3.42 | 0.01 | –0.54 to 0.57 | 0.96 |
| QCPR total | 56.27 | 55.16 | 1.12 | –0.13 to 2.36 | 0.08 |
| QCPR (warmth) | 33.72 | 33.43 | 0.29 | –0.59 to 1.17 | 0.52 |
| QCPR (criticism and conflict) | 22.49 | 21.86 | 0.63 | –0.06 to 1.32 | 0.08 |
| MMSE | 20.06 | 20.01 | 0.05 | –0.70 to 0.80 | 0.89 |
| BADLS [P] | 12.96 | 13.18 | –0.22 | –1.45 to 1.01 | 0.73 |
| QoL-AD [P] | 32.56 | 31.76 | 0.80 | –0.31 to 1.92 | 0.16 |
| DEMQOL [P] | 101.57 | 100.58 | 0.99 | –1.22 to 3.2 | 0.38 |
| Carer outcome measure | iCST (N = 134) | TAU (N = 139) | MD | 95% CI of MD | p-value |
|---|---|---|---|---|---|
| SF-12 PCS | 47.80 | 46.99 | 0.81 | –1.18 to 2.80 | 0.43 |
| SF-12 MCS | 46.84 | 47.52 | –0.68 | –2.82 to 1.47 | 0.54 |
| HADS total | 10.56 | 10.67 | –0.12 | –1.39 to 1.16 | 0.86 |
| HADS (anxiety) | 6.04 | 5.97 | 0.07 | –0.74 to 0.88 | 0.87 |
| HADS (depression) | 4.63 | 4.83 | –0.20 | –0.83 to 0.43 | 0.53 |
| EQ-5D health state today | 75.33 | 74.90 | 0.42 | –3.03 to 3.88 | 0.81 |
| EQ-5D calculated utility valuea | 0.79 | 0.74 | 0.05 | 0.01 to 0.09 | 0.02 |
| RS-14 | 82.83 | 82.05 | 0.78 | –1.23 to 2.80 | 0.45 |
| NPI (carer distress) | 3.79 | 3.78 | 0.01 | –0.45 to 0.47 | 0.97 |
| QCPR total | 58.20 | 58.67 | –0.46 | –1.79 to 0.87 | 0.50 |
| QCPR (warmth) | 34.38 | 34.44 | –0.06 | –0.74 to 0.62 | 0.87 |
| QCPR (criticism and conflict) | 23.88 | 24.25 | –0.38 | –1.21 to 0.46 | 0.38 |
| Carer outcome measure | iCST (N = 180) | TAU (N = 176) | MD | 95% CI of MD | p-value |
|---|---|---|---|---|---|
| SF-12 PCS | 49.95 | 50.14 | –0.19 | –1.59 to 1.22 | 0.80 |
| SF-12 MCS | 46.28 | 47.08 | –0.80 | –2.48 to 0.88 | 0.35 |
| HADS total | 11.27 | 11.01 | 0.26 | –0.74 to 1.27 | 0.61 |
| HADS (anxiety) | 6.84 | 6.58 | 0.26 | –0.41 to 0.92 | 0.45 |
| HADS (depression) | 4.58 | 4.60 | –0.02 | –0.51 to 0.47 | 0.93 |
| EQ-5D health state today | 74.91 | 74.56 | 0.35 | –3.00 to 3.70 | 0.84 |
| EQ-5D calculated utility value | 0.80 | 0.78 | 0.02 | –0.01 to 0.06 | 0.21 |
| RS-14 | 81.37 | 81.75 | –0.38 | –2.00 to 1.25 | 0.65 |
| NPI (carer distress) | 3.14 | 3.04 | 0.10 | –0.32 to 0.52 | 0.64 |
| QCPR total | 58.05 | 58.21 | –0.16 | –1.66 to 1.34 | 0.84 |
| QCPR (warmth) | 34.76 | 35.54 | –0.77 | –1.61 to 0.06 | 0.07 |
| QCPR (criticism and conflict) | 23.30 | 22.67 | 0.64 | –0.26 to 1.53 | 0.16 |
Appendix 7 Compliance analysis
The model fitted uses linear regression to assess the relationship between outcome measure and number of iCST sessions attended at each outcome visit after adjusting for the baseline outcome measure.
| Person with dementia outcome measure | Observed data | Imputed data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 26 | Pooled coefficient | SE | Median F | Low F | High F | |||||||
| Participant with dementia | Coefficient | SE | F | p-value | F | p-value | F | p-value | F | p-value | ||
| ADAS-Cog | –0.013 | 0.014 | 0.837 | 0.361 | –0.015 | 0.015 | 1.156 | 0.283 | 0.633 | 0.427 | 1.488 | 0.224 |
| QoL-AD | 0.008 | 0.010 | 0.703 | 0.402 | 0.009 | 0.010 | 0.659 | 0.418 | 0.601 | 0.439 | 1.260 | 0.263 |
| DEMQOL | 0.007 | 0.019 | 0.158 | 0.691 | 0.008 | 0.019 | 0.210 | 0.647 | 0.068 | 0.794 | 0.352 | 0.553 |
| NPI total | –0.002 | 0.023 | 0.008 | 0.927 | –0.003 | 0.023 | 0.018 | 0.892 | 0.004 | 0.952 | 0.029 | 0.865 |
| GDS-15 | 0.001 | 0.005 | 0.055 | 0.815 | 0.002 | 0.005 | 0.091 | 0.763 | 0.019 | 0.889 | 0.270 | 0.604 |
| QCPR totala | 0.043 | 0.014 | 9.184 | 0.003 | 0.042 | 0.014 | 9.256 | 0.003 | 9.016 | 0.003 | 9.659 | 0.002 |
| QCPR (warmth) | 0.012 | 0.009 | 1.973 | 0.161 | 0.011 | 0.009 | 1.661 | 0.199 | 1.557 | 0.213 | 1.839 | 0.176 |
| QCPR (criticism and conflict)a,b | 0.029 | 0.008 | 12.633 | 0.001 | ||||||||
| MMSE | 0.006 | 0.008 | 0.559 | 0.455 | 0.006 | 0.008 | 0.447 | 0.504 | 0.383 | 0.536 | 0.846 | 0.359 |
| BADLS [P] | –0.015 | 0.013 | 1.254 | 0.264 | –0.015 | 0.013 | 1.272 | 0.260 | 1.230 | 0.268 | 1.413 | 0.236 |
| QoL-AD [P] | 0.012 | 0.011 | 1.225 | 0.269 | 0.012 | 0.011 | 1.225 | 0.269 | 1.225 | 0.269 | 1.225 | 0.269 |
| DEMQOL [P] | 0.013 | 0.023 | 0.344 | 0.558 | 0.013 | 0.023 | 0.326 | 0.569 | 0.312 | 0.577 | 0.421 | 0.517 |
| Person with dementia outcome measure | Observed data | Imputed data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 13 | Pooled coefficient | SE | Median F | Low F | High F | |||||||
| Person with dementia | Coefficient | SE | F | p-value | F | p-value | F | p-value | F | p-value | ||
| ADAS-Cog | 0.006 | 0.030 | 0.042 | 0.838 | 0.002 | 0.030 | 0.038 | 0.846 | 0.005 | 0.946 | 0.071 | 0.790 |
| QoL-AD | 0.019 | 0.021 | 0.866 | 0.353 | 0.019 | 0.021 | 0.824 | 0.365 | 0.685 | 0.409 | 1.015 | 0.315 |
| DEMQOL | –0.021 | 0.042 | 0.246 | 0.620 | –0.020 | 0.042 | 0.190 | 0.663 | 0.122 | 0.727 | 0.408 | 0.524 |
| NPI total | –0.045 | 0.048 | 0.880 | 0.349 | –0.046 | 0.048 | 0.903 | 0.343 | 0.877 | 0.350 | 0.916 | 0.339 |
| GDS-15 | –0.003 | 0.010 | 0.076 | 0.783 | –0.003 | 0.010 | 0.055 | 0.815 | 0.000 | 0.986 | 0.628 | 0.429 |
| QCPR total | 0.049 | 0.026 | 3.458 | 0.064 | 0.049 | 0.026 | 3.495 | 0.063 | 3.468 | 0.064 | 3.546 | 0.061 |
| QCPR (warmth) | 0.003 | 0.018 | 0.036 | 0.850 | 0.003 | 0.018 | 0.033 | 0.856 | 0.031 | 0.859 | 0.043 | 0.836 |
| QCPR (criticism and conflict)a | 0.043 | 0.015 | 8.268 | 0.004 | 0.043 | 0.015 | 8.383 | 0.004 | 8.377 | 0.004 | 8.386 | 0.004 |
| MMSE | 0.026 | 0.016 | 2.667 | 0.104 | 0.026 | 0.016 | 2.764 | 0.098 | 2.419 | 0.121 | 2.861 | 0.092 |
| BADLS [P]b | 0.024 | 0.029 | 0.671 | 0.413 | ||||||||
| QoL-AD [P] | 0.038 | 0.022 | 3.015 | 0.084 | 0.037 | 0.022 | 2.833 | 0.093 | 2.782 | 0.096 | 3.181 | 0.076 |
| DEMQOL [P] | 0.019 | 0.048 | 0.155 | 0.694 | 0.019 | 0.048 | 0.160 | 0.689 | 0.137 | 0.711 | 0.195 | 0.659 |
| Carer outcome measure | Observed data | Imputed data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 26 | Pooled coefficient | SE | Median F | Low F | High F | |||||||
| Carer | Coefficient | SE | F | p-value | F | p-value | F | p-value | F | p-value | ||
| SF-12 PCS | 0.018 | 0.016 | 1.196 | 0.275 | 0.017 | 0.016 | 1.052 | 0.306 | 1.171 | 0.280 | 1.253 | 0.264 |
| SF-12 MCSa | 0.017 | 0.017 | 0.921 | 0.338 | 0.017 | 0.017 | ||||||
| HADS totalb | –0.020 | 0.011 | 3.463 | 0.064 | –0.022 | 0.011 | 4.085 | 0.044 | 3.850 | 0.051 | 4.815 | 0.029 |
| HADS (anxiety) | –0.007 | 0.007 | 1.156 | 0.283 | –0.009 | 0.007 | 1.655 | 0.199 | 1.279 | 0.259 | 2.091 | 0.149 |
| HADS (depression)b | –0.013 | 0.006 | 5.684 | 0.018 | –0.014 | 0.006 | 6.275 | 0.013 | 5.549 | 0.019 | 6.667 | 0.010 |
| EQ-5D health state todaya | 0.020 | 0.032 | 0.406 | 0.525 | 0.020 | 0.032 | ||||||
| EQ-5D calculated utility value | 0.0007 | 0.0004 | 2.888 | 0.090 | 0.0007 | 0.0004 | 2.992 | 0.085 | 2.691 | 0.102 | 3.006 | 0.084 |
| RS-14 | 0.023 | 0.019 | 1.433 | 0.232 | 0.023 | 0.019 | 1.479 | 0.225 | 1.407 | 0.237 | 1.509 | 0.220 |
| NPI (carer distress) | –0.005 | 0.004 | 1.461 | 0.228 | –0.005 | 0.004 | 1.558 | 0.213 | 1.438 | 0.232 | 1.663 | 0.198 |
| QCPR | –0.006 | 0.013 | 0.179 | 0.673 | –0.004 | 0.013 | 0.065 | 0.798 | 0.010 | 0.919 | 0.184 | 0.669 |
| QCPR (warmth) | –0.0001 | 0.007 | 0.0002 | 0.988 | 0.001 | 0.007 | 0.019 | 0.890 | 0.001 | 0.982 | 0.044 | 0.833 |
| QCPR (criticism and conflict) | –0.004 | 0.008 | 0.273 | 0.602 | –0.003 | 0.008 | 0.229 | 0.633 | 0.060 | 0.806 | 0.326 | 0.568 |
| Carer outcome measure | Observed data | Imputed data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 13 | Pooled coefficient | SE | Median F | Low F | High F | |||||||
| Carer | Coefficient | SE | F | p-value | F | p-value | F | p-value | F | p-value | ||
| SF-12 PCSa | 0.015 | 0.030 | 0.270 | 0.604 | ||||||||
| SF-12 MCSa | 0.007 | 0.034 | 0.040 | 0.843 | ||||||||
| HADS total | –0.024 | 0.021 | 1.271 | 0.260 | –0.023 | 0.021 | 1.235 | 0.267 | 1.219 | 0.271 | 1.310 | 0.253 |
| HADS (anxiety) | –0.012 | 0.013 | 0.806 | 0.370 | –0.011 | 0.013 | 0.752 | 0.387 | 0.716 | 0.398 | 0.847 | 0.358 |
| HADS (depression) | –0.014 | 0.011 | 1.519 | 0.219 | –0.014 | 0.011 | 1.496 | 0.222 | 1.450 | 0.230 | 1.667 | 0.198 |
| EQ-5D health state today | 0.126 | 0.066 | 3.689 | 0.056 | 0.127 | 0.066 | 3.773 | 0.053 | 3.582 | 0.059 | 3.796 | 0.052 |
| EQ-5D calculated utility value | 0.001 | 0.001 | 2.573 | 0.110 | 0.001 | 0.001 | 2.566 | 0.110 | 2.558 | 0.111 | 2.607 | 0.108 |
| RS-14a | –0.031 | 0.033 | 0.891 | 0.346 | ||||||||
| NPI (carer distress) | –0.007 | 0.009 | 0.658 | 0.418 | –0.007 | 0.009 | 0.646 | 0.422 | 0.598 | 0.440 | 0.746 | 0.389 |
| QCPR total | –0.004 | 0.030 | 0.022 | 0.883 | –0.003 | 0.030 | 0.033 | 0.855 | 0.002 | 0.968 | 0.088 | 0.766 |
| QCPR (warmth) | –0.008 | 0.017 | 0.223 | 0.637 | –0.006 | 0.017 | 0.074 | 0.786 | 0.020 | 0.888 | 0.466 | 0.495 |
| QCPR (criticism and conflict) | 0.005 | 0.019 | 0.061 | 0.806 | 0.004 | 0.019 | 0.070 | 0.792 | 0.001 | 0.980 | 0.206 | 0.650 |
Appendix 8 Serious adverse events
Serious adverse events
In the iCST trial a SAE was defined as an untoward occurrence, experienced by the person with dementia or their carer, which:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
is otherwise considered medically significant by the principal investigator.
In addition, any cases in which action was taken in line with the protocol with regard to alleged or suspected abuse/neglect that required protection of vulnerable adults was considered to be a SAE.
All iCST centres were asked to report any of the above instances either for the person with dementia or their carer by categorising them as below:
-
death
-
life-threatening
-
hospitalisation or prolongation of existing hospitalisation
-
persistent or significant disability or incapacity
-
otherwise considered medically significant by the investigator
-
alleged/suspected abuse/neglect, as detailed in protection of vulnerable adults protocol.
There were a total of 51 SAEs reported during the trial, all of which were reported directly to the Chief Investigator. There were 25 SAEs reported in the iCST group and 26 events reported in the TAU group. There were 10 deaths reported, of which nine were deaths of the person with dementia and one was a carer death. There were only eight deaths noted on the CONSORT flowchart as in the remaining cases participants were lost to follow-up prior to their death being reported. A total of 44 of the SAEs related to the person with dementia and the remaining seven involved the carer. None of the SAEs reported was related to the trial. Details of these events broken down by treatment allocation are provided below (Table 60). Details of types of SAEs are presented below (Table 61). For three people with dementia there were two SAEs reported, which included hospitalisation, followed by death.
| SAE category | Total | iCST | TAU | Linked to the iCST trial |
|---|---|---|---|---|
| Death | 10 | 2 | 8 | 0 |
| Life-threatening | 5 | 3 | 2 | 0 |
| Hospitalisation | 32 | 16 | 16 | 0 |
| Disability | 0 | 0 | 0 | 0 |
| Medically significant | 4 | 4 | 0 | 0 |
| Protection of vulnerable adults | 0 | 0 | 0 | 0 |
| SAE category | Person with dementia | Carer |
|---|---|---|
| Angioplasty | 0 | 1 |
| Car accident | 0 | 1 |
| Chest infection | 2 | 0 |
| Diagnosis of cancer | 1 | 2 |
| Death | 9 | 1 |
| Fainting incident | 2 | 0 |
| Fall not requiring hospitalisation | 2 | 0 |
| Fall requiring hospitalisation | 3 | 0 |
| Fracture not requiring hospitalisation | 1 | 0 |
| Fracture of knee/arm/femoral shaft requiring hospitalisation | 3 | 1 |
| Heart attack | 1 | 0 |
| Heart operation | 0 | 1 |
| Irregular heart rhythm | 1 | 0 |
| Kidney stones/infection | 2 | 0 |
| Hospitalisation reason unknown | 1 | 0 |
| Oedema | 1 | 0 |
| Chronic obstructive pulmonary disease | 1 | 0 |
| Person with dementia missing/wandered away | 1 | 0 |
| Pneumonia | 3 | 0 |
| Psychiatric symptoms/confusion/delusions/suicidal thoughts/lethargy/loss of appetite | 5 | 0 |
| Seizure | 1 | 0 |
| Stroke | 1 | 0 |
| Urinary tract infection | 3 | 0 |
| Total | 44 | 7 |
Appendix 9 Protocol violations
Protocol violations
There was one protocol violation in the iCST trial, which was reported to the DMEC meeting and was attributable to an administrative error.
The protocol violation involved two dyads being accidently randomised with the wrong identification numbers. The data entry was performed remotely under the original identification numbers and questionnaire codes (not the ones that were actually used in randomisation). The solution proposed in consultations with the clinical trials unit was to change the numbers for the two participants in the database.
List of abbreviations
- AChEI
- acetylcholinesterase inhibitor
- AD
- Alzheimer’s disease
- ADAS-Cog
- Alzheimer’s Disease Assessment Scale – Cognitive Subscale
- ANCOVA
- analysis of covariance
- BADLS
- Bristol Activities of Daily Living Scale
- CDR
- Clinical Dementia Rating Scale
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CSRI
- Client Service Receipt Inventory
- CST
- cognitive stimulation therapy
- DEMQOL
- Dementia Quality of Life
- DeNDRoN
- Dementias and Neurodegenerative Disease Research Network
- DMEC
- Data Monitoring and Ethics Committee
- DVD
- digital versatile disc
- EQ-5D™
- European Quality of Life-5 Dimensions
- EQ-5D-3L™
- European Quality of Life-5 Dimensions-3 level response
- GDS
- Geriatric Depression Scale
- HADS
- Hospital Anxiety and Depression Scale
- ICER
- incremental cost-effectiveness ratio
- iCST
- individual cognitive stimulation therapy
- MCS
- mental component summary
- MMSE
- Mini Mental State Examination
- NICE
- National Institute for Health and Care Excellence
- NWORTH
- North Wales Organisation for Randomised Trials in Health
- PCS
- physical component summary
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QCPR
- Quality of Caregiver–Patient Relationship
- QoL-AD
- Quality of Life in Alzheimer’s Disease Scale
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RO
- reality orientation
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SMD
- standardised mean difference
- SPSS
- Statistical Package for the Social Sciences
- SUR
- seemingly unrelated regression
- TAU
- treatment as usual
- WTP
- willingness to pay