Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 13/48/01. The protocol was agreed in November 2013. The assessment report began editorial review in July 2014 and was accepted for publication in October 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Edwards et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Description of health problem
Bradycardia
Bradycardia is defined as a resting heart rate below 60 beats per minute (b.p.m.). A slow heart rate can occur naturally under various circumstances and is not necessarily associated with a medical condition. For example, some highly trained athletes have bradycardia. However, there is also pathological bradycardia, which is caused by conditions that affect the electrical conduction system of the heart, including sick sinus syndrome (SSS) and/or atrioventricular (AV) block. 1 Bradycardia does not necessarily require treatment unless it causes symptoms. People suffering from symptomatic bradycardia can present with dizziness, confusion, palpitations, breathlessness, exercise intolerance and syncope (blackout or fainting). However, bradycardia, and symptoms related to it, may be intermittent or may be non-specific, particularly in the elderly.
Sick sinus syndrome
Sick sinus syndrome is caused by dysfunction of the sinus node, the heart’s natural pacemaker. The sinus node consists of a cluster of cells that is situated in the upper part of the right atrium (the right upper chamber of the heart). The sinus node generates the electrical impulses that are conducted through the heart and stimulate it to contract. SSS covers a spectrum of arrhythmias with different underlying mechanisms, manifested as bradycardia, tachycardia (fast heart rate) or a mix of the two, but also as chronotropic incompetence (the inability of the heart to increase its rate appropriately with increased activity, leading to exercise intolerance). SSS manifesting as bradyarrhythmias includes sinus bradycardia, sinus arrest, sinoatrial (SA) exit block and alternating bradyarrhythmias and tachyarrhythmias such as bradycardia–tachycardia syndrome (BTS). 1,2
In sinus arrest or sinus pause, the sinus node transiently ceases to generate electrical impulses. 3 The pause can last from a couple of seconds to several minutes. The sinus pause usually allows escape beats or rhythms to occur, when other pacemakers in the heart initiate contraction of the ventricles. In SA exit block, the sinus node depolarises normally, but the signal is blocked before it leaves the sinus node, leading to intermittent delay (first-degree SA block) or failure (second-degree SA block) of atrial depolarisation.
Atrioventricular block
Atrioventricular block can occur independently from SSS, and so patients suffering from symptomatic bradycardia due to SSS may also have or develop AV block. In AV block, the electrical impulses from the sinus node in the right atrium to the ventricular chambers are slowed or blocked at the AV node or within the His–Purkinje system, which conducts electrical impulses between the atria and ventricular chambers. Although heart block can be present at birth (congenital), people are more likely to develop the condition, with the risk increasing with age along with the incidence of heart disease. As in SA block, there are several degrees of AV block. 4 First-degree AV block is usually asymptomatic and occurs when the electrical impulses slow as they pass through the AV node, but all impulses reach the ventricles. In second-degree AV block, some of the electrical impulses from the sinus node are unable to reach the ventricles, a condition that is more likely to present with symptoms such as syncope. In third-degree AV block (complete heart block), there are no electrical impulses between the atrial and ventricular chambers. In the absence of any electrical impulses from the atria, the ventricles produce escape beats, which are usually slow.
Aetiology and pathology
The resting heart rate in healthy people does not change with increasing age;5 however, bradycardia due to SSS becomes more common in older people because of idiopathic degeneration or development of scarring of the sinus node, both of which occur with ageing. 2 However, SSS can also be caused by extrinsic factors that can mimic or exacerbate SSS, such as some types of medication (e.g. calcium channel blockers and beta-blockers), electrolyte disturbances, hypothyroidism, hypothermia and toxins. SSS has also been linked with diseases and conditions that cause scarring or damage to the heart’s electrical system, such as atrial fibrillation (AF) and heart failure (HF). 1,2
Atrioventricular block can also be either congenital or acquired. Acquired AV block is associated with coronary heart disease, myocardial infarction, cardiomyopathy, heart surgery and with the use of many antiarrhythmic agents.
Incidence and prevalence
Sick sinus syndrome usually occurs in older adults, but it can affect persons of all ages, and it affects men and women equally. 2 The incidence of AV conduction abnormalities also increases with advancing age. 6 However, the prevalence of bradyarrhythmias due to SSS requiring permanent pacemaker implant is unknown,7 as is the breakdown of the prevalence of SSS with and without a concurrent AV block. Hospital Episode Statistics data from October 2012 to September 2013 included 2490 patients with a primary diagnosis of SSS in NHS hospitals in England.
Diagnosis
Diagnosis of SSS is made by considering a patient’s medical history and symptoms and through the use of electrocardiography (ECG). Diagnosis sometimes proves difficult because symptoms and electrocardiographic abnormalities are intermittent. When 12-lead ECG does not yield a diagnosis, prolonged ECG monitoring, such as Holter monitoring (ECG monitoring for 24–48 hours) or longer-duration cardiac monitoring either with event ECG recorders for weeks at a time or with an implantable loop recorder for months at a time, may help accurate diagnosis. 2,8 SSS manifested as chronotropic incompetence is usually assessed through various exhaustive and symptom-limited exercise tests; however, there are no well-validated standards for diagnosing SSS in this setting. 5
Atrioventricular conduction is also assessed by ECG. Adequate AV conduction, that is, absence of AV block, has been defined as presence of 1 : 1 conduction at rates of 140 b.p.m. 9
Prognosis and impact of health problem
The prognosis of bradycardia due to SSS depends on the aetiology. If the underlying cause is, for example, medication, hypothyroidism or electrolyte imbalance, then the bradycardia may resolve if the triggering cause is treated or removed. However, for most people, SSS is idiopathic and progressive, with a highly variable development of the disease. People with asymptomatic SSS do not require therapy. The only effective treatment for patients suffering from symptoms is implantation of a permanent pacemaker. 2 However, pacemaker implantation does not cure or affect the prognosis of SSS, and pacemakers are implanted with the aim of alleviating symptoms and improving the patient’s quality of life (QoL). Pacemaker implantation is associated with considerable risk for the patient, and therefore careful consideration must be given to the balance between potential benefits and adverse effects of treatment. Although pacemaker implantation has been shown to improve QoL for patients with bradycardia and sinus node dysfunction,10,11 it has been noted that women and older adults may achieve lower levels of improvement in QoL than other groups. 12 Additionally, research suggests that there may be differences between the sexes at pacemaker implantation, with less favourable outcomes for women in terms of complications. 13
Patients with SSS are at risk of developing a complete AV block, with considerable variation in the estimates of risk of AV block (from < 1% up to 4.5% per year). 4,14 A patient with SSS who develops AV block will require ventricular pacing (VP) and, consequently, an upgrade to a dual-chamber pacemaker if they already have a single-chamber atrial pacemaker. People with SSS may also develop BTS with AF as the tachyarrhythmia, which in turn leads to an increased risk of stroke. 2
Measurements of disease
Symptomatic bradycardia, and implantation of permanent pacemakers to relieve the symptoms, can have a significant impact on a patient’s QoL. 4 QoL has been measured using many different generic and disease/treatment-specific measures in pacemaker trials. Recommended generic measures include the Short Form Questionnaire-36 items (SF-36), a health questionnaire with 36 questions, which looks at functional health, general well-being and physical and mental health. 15
The Karolinska Questionnaire,16 which has been validated in patients paced for bradyarrhythmia, contains 16 questions on cardiovascular symptoms relevant to pacemaker patients. The Specific Activity Scale (SAS)17 is another disease-specific questionnaire for the functional classification of patients with cardiovascular disease (CVD). Based on physical capacity, patients are divided into class I (unlimited exercise capacity) to class IV (very low exercise tolerance). Many pacemaker trials also use the New York Heart Association (NYHA) functional scale, which is used to classify patients’ cardiac disease according to the severity of their symptoms. Similar to the SAS, patients can fall into four categories based on the limitations on physical activity, from class I (no limitation of physical activity) to class IV (symptoms of HF at rest and inability to carry out any physical activity without discomfort).
Current service provision
Current guidelines
The National Institute for Health and Care Excellence (NICE)’s technology appraisal (TA) number 88,18 which was published in 2005, recommends dual-chamber pacemakers for patients with symptomatic bradycardia that is due to SSS, AV block or a combination of the two. 18 However, in a few exceptional cases single-chamber atrial or ventricular pacemakers are preferred:
-
single-chamber atrial pacemakers for patients with SSS in whom, after full evaluation, there is no evidence of impaired AV conduction
-
single-chamber ventricular pacemakers for patients with AV block with continuous AF
-
single-chamber ventricular pacemakers for patients with AV block alone, or in combination with SSS, when patient-specific factors, such as frailty or the presence of comorbidities, influence the balance of risks and benefits in favour of single-chamber VP.
Similarly, guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA), published in 2008, recommend dual-chamber pacemakers for AV block and for SSS if there is a suspected abnormality of AV conduction or increased risk of future AV block. 4 Single-chamber ventricular pacemakers are recommended for patients with AV block and chronic AF or other atrial tachyarrhythmias, and single-chamber atrial pacemakers are recommended for patients with SSS with no suspected abnormality of AV conduction and who are not considered to be at increased risk of future AV block.
In 2013, the European Society of Cardiology (ESC) published its guidelines on cardiac pacing and cardiac resynchronisation therapy. 7 ESC recommends dual-chamber pacemakers as a first choice for patients with SSS and/or AV block, with the exception of patients with a persistent AV block and continuous AF, for whom a single-chamber ventricular pacemaker is recommended.
The differences in recommendations between the more recent ESC guidelines and those of NICE and the ACC/AHA are linked to the completion and publication of the DANPACE trial,19 which has provided new evidence on the comparison of single-chamber atrial pacing with dual-chamber pacing in SSS with no evidence of AV block. The objectives for this multiple technology appraisal (MTA) were to evaluate formally the data from DANPACE1 study and to identify any other evidence in this area.
Current pacemaker usage in the NHS
During 2012–13 in England, more than 20,000 people had a single- or a dual-chamber pacemaker implanted and just over 8000 people had an implanted pacemaker renewed. 20 The median length of hospital stay was 2 days for implantation of both single- and dual-chamber pacemaker systems, resulting in 82,000 bed-days in the UK in 2012–13. Of the newly implanted single and dual pacemakers, SSS was the fourth most prevalent primary diagnosis (9.5%), after AF and flutter (22.5%), complete AV block (18.8%) and second-degree AV block (10.6%). 8 Among patients with a primary diagnosis of SSS (2490 patients), 67.5% had a dual-chamber pacemaker implanted, 14.8% had a single-chamber pacemaker and 2.2% had a reoperation on an existing implanted pacemaker. 8
The target for the implantation rate of new pacemakers in England and Wales is 700 pacemakers per million people. In 2012, the total implant rate in England and Wales fell short of this target, reaching 559 per million of the population. 21 In England, implantation rates varied between 379 and 638 new pacemaker implants per million people in different parts of the country, although a decrease in variability was noticed across the country from 2010 to 2012. 21
Description of technology under assessment
Pacemakers
Pacemakers are small battery-driven devices which regulate abnormal heart rhythms. A pacemaker consists of a generator and one or more leads, which are connected to the heart. The leads will sense the heart’s electrical activity and, when it becomes too slow, an electrical impulse from the generator will initiate contraction of the heart.
Single-chamber pacemakers have one lead which is attached to either the atrium (atrial pacing) or the ventricle (VP). Dual-chamber pacemakers have two leads: one lead is attached to the atrium and the second to the ventricle.
The North American Society of Pacing and Electrophysiology (NASPE) and the British Pacing and Electrophysiology Group (BPEG) have established nomenclature to describe the different pacing modes of pacemakers, which is a four-letter combination (Table 1). 22 The first letter indicates which chamber or chambers are paced, and the second letter specifies which chamber(s) are sensed. Letters I and II are usually, but not necessarily, the same. The third letter describes the mode of response to sensing. The pacemaker can be inhibited (I), if it senses a spontaneous depolarisation; triggered (T), if it senses that no depolarisation has occurred (uncommon); or both inhibited and triggered (D).
| Position | I | II | III | IV |
|---|---|---|---|---|
| Category | Chamber paced | Chamber sensed | Response to sensing | Rate modulation |
| Codes |
|
|
|
|
In an AAI or VVI pacemaker, the pacemaker senses an atrial or ventricular event and withholds its signal. DDI pacemakers will inhibit the output of the device in either chamber where it senses a signal. The most common example of the letter D in the third position is in DDD pacemakers, which have dual functionality. On sensing an atrial signal, the DDD pacemaker initially inhibits the atrial output, which triggers a timer that, after a set time interval (AV delay), initiates a ventricular output. If the DDD device senses a ventricular signal during the triggering interval, the pacemaker also inhibits the ventricular output. The fourth letter specifies whether or not the pacemaker is programmed to sense and increase the heart rate in response to physical, mental or emotional activity. This is termed rate response.
Modern pacemakers have numerous programmable features that can be altered to optimise pacemaker function. Programming is a complex and rapidly evolving technical area, and a detailed description of pacemaker programming is beyond the scope of this report; thus, a few key parameters are summarised:
-
Rate responsiveness As mentioned previously, some pacemakers can be programmed to vary the pacing rate in response to the patient’s activity level. Rate-responsive pacemakers control heart rate by sensing body movement or breathing or by closed-loop stimulation. Closed-loop stimulation determines the appropriate heart rate based on intracardiac impedance measurements, which reflect information from the autonomic nervous system.
-
AV delay The AV delay is the time interval between an atrial paced or sensed event and the delivery of a VP stimulus in dual-chamber pacemakers. If intrinsic conduction is more rapid than the duration of the programmed AV delay, the intrinsic signal will inhibit VP.
-
Mode switching Dual-chamber pacemakers may have an additional feature called mode switching. 23 Mode-switch algorithms track tachyarrhythmias, such as AF, and when these occur trigger a non-tracking mode, or VP to avoid tachycardia. Atrial arrhythmias would otherwise cause sustained high ventricular rates. When the atrial rate falls below the rate programmed for mode switch, the pacemaker changes back to a tracking mode. 23
Implant procedure and follow-up
Pacemakers are usually implanted under local anaesthetic. An incision is made below the collarbone to facilitate lead implantation and a pocket is created under the skin to hold the pacemaker device. The pacing lead is inserted into the heart through a major vein. One end of the lead is securely lodged in the tissue of the heart and the other end is connected to the pacemaker. The position of the lead is checked using radiographic imaging. Testing and programming of the pacemaker may sometimes be done wirelessly and can be changed at any time. The hospital stay is usually brief and the implant procedure could be carried out as day surgery or might require a single overnight stay in hospital. Implantation of a dual-chamber pacemaker may take longer than implantation of a single-chamber pacemaker, because dual-chamber pacemakers require the insertion and placement of two leads. The requirement for an additional lead in dual- versus single-chamber pacemakers might result in an associated increased risk of complications, such as lead displacement. 24
People with permanently implanted pacemakers require regular follow-up to check the function of the pacemaker leads, the frequency of utilisation and the battery life of the pacemaker, and for abnormal heart rhythm. 25 The battery life of a pacemaker is about 5–8 years; after this time, replacement of the pacemaker will be required. Replacement of the pacemaker involves making an incision over the previous site of insertion, removing the old pacemaker generator, checking the lead(s), and, if satisfactory, attaching a new generator to the existing lead(s). Problems with pacemaker leads, such as loss of contact between the lead and the heart, require reoperation. Where repair of a fault with a lead is necessary, the old lead may be left in place but disconnected from the pacemaker and a new lead implanted. Removal of old leads can be complicated by the formation of scar tissue connecting the lead to the vein and/or the heart.
Complications
Most complications occur during or soon after implantation of a pacemaker. Some of the more common complications are lead displacement (1.4–2.1%) and puncture of the lung when placing the leads, which can lead to a pneumothorax (1.9%) or haemothorax. 26,27 One of the most serious, but rarer, complications that can arise during the implant procedure is cardiac perforation. There is also the risk of infection of the pacemaker pocket or the leads. 27,28 Complications occurring at a later date mainly involve dysfunction of the pacemaker or of the leads, that is, failure to pace or sense appropriately. Other late complications include infection or erosion of the pacemaker site or its leads. 28
Reoperation may be required as a result of a complication, such as lead displacement, infection or pacemaker erosion, but it can also be because of a need for pacemaker upgrade (single to dual) or pacemaker replacement as a result of changed clinical needs or end of battery life. 24 The complication rate associated with a reoperation is substantially higher than that associated with initial implantation. 29
Costs associated with intervention
The cost of pacemaker implantation comprises several elements:
-
price of the generator and leads
-
implant procedure (setting and personnel)
-
personnel involved prior to and following implantation
-
regular routine follow-up
-
management of perioperative complications
-
management of late complications
-
replacement or upgrade at the end of the life of the pacemaker or in response to changing clinical need.
Further details on the costs associated with pacemaker implantation are given in Chapter 4, Costs.
Chapter 2 Definition of the decision problem
Decision problem
Population
The population of interest to this review is people with symptomatic bradycardia due to SSS without AV block, that is, with intact AV conduction, and who required permanent pacemaker implantation.
Intervention and comparator
The review considered permanent implantable dual-chamber pacemakers programmed to dual-chamber pacing compared with permanent implantable pacemakers (single or dual) programmed to atrial pacing.
All programmable features, such as rate responsiveness, mode switch and VP-minimising features, were allowed.
Outcomes
The outcomes of interest considered for this review included:
-
mortality (all-cause)
-
HF
-
AF
-
stroke
-
exercise capacity
-
cognitive function
-
requirement for further surgery
-
adverse effects of pacemaker implantation (including peri- and post-operative complications, AF and device replacement)
-
health-related quality of life (HRQoL).
Overall aims and objectives of assessment
The aims of this MTA were to appraise the clinical effectiveness and cost-effectiveness of dual-chamber pacemakers for treating symptomatic bradycardia in people with SSS in whom there is no evidence of impaired AV conduction and to update the recommendations of NICE’s TA8818 in relation to this indication.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
The clinical effectiveness of single-chamber atrial and dual-chamber pacemakers for the treatment of symptomatic bradycardia due to SSS without AV block was assessed by conducting a systematic review of published research evidence. The review was undertaken following the general principles published by the Centre for Reviews and Dissemination (CRD) and the Cochrane Collaboration. 30,31 The protocol for the systematic review was registered on PROSPERO (registration number CRD42013006708).
Identification of studies
To identify relevant randomised controlled trials (RCTs), multiple electronic databases were searched, including MEDLINE, EMBASE and The Cochrane Library [including the Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects and Health Technology Assessment (HTA) database]. Bibliographies of retrieved studies identified as relevant were manually reviewed for potentially eligible studies. In addition, experts in the field were contacted with a request for details of published and unpublished studies of which they may have knowledge. Furthermore, submissions submitted to NICE were assessed for unpublished data.
The search terms included medical subject heading (MeSH) and text terms for the interventions: artificial pacemakers and pacing; dual-chamber pacemakers/pacing; and single-chamber atrial pacemakers/pacing. As the scoping search using this search strategy identified all relevant trials known from the previous MTA, search terms for the condition (i.e. bradycardia and SSS) were not used. To keep in line with the original MTA, which focused on RCT evidence, the search strategy included a RCT filter developed and validated by the Scottish Intercollegiate Guidelines Network. 32 No language or date restriction was applied to the searches. Electronic databases were initially searched on 7 January 2014 and results uploaded into Reference Manager Version 11.0 (Thomson ResearchSoft, San Francisco, CA, USA) and deduplicated. An update search was carried out on 12 May 2014. Full details of the terms used in the searches are presented in Appendix 1, Literature search strategies.
Two reviewers independently screened all titles and abstracts according to the inclusion criteria (see Table 2). Full paper manuscripts of any titles/abstracts of potential relevance were obtained and assessed independently by two reviewers. If a study was only reported as a meeting abstract or if full-paper manuscripts could not be obtained, the study authors were contacted to gain further details. Studies for which insufficient methodological details were available to allow critical appraisal of study quality were excluded. Discrepancies between the two reviewers were resolved by consensus, with involvement of a third reviewer when necessary.
Inclusion and exclusion criteria
Inclusion criteria and exclusion criteria for the review of effectiveness were based on the decision problem outlined in Table 2. The review included RCTs of parallel and crossover design. Systematic reviews and non-randomised studies were excluded.
| Domain | Inclusion criterion |
|---|---|
| Study design | RCTs of parallel or crossover design |
| Intervention | Permanent implantable dual-chamber pacemakers |
| Population | People with symptomatic bradyarrhythmias due to SSS without AV block |
| Comparator | Permanent implantable single-chamber atrial pacemakers |
| Outcomes | Mortality (all-cause) |
| HF | |
| AF | |
| Stroke | |
| Exercise capacity | |
| Cognitive function | |
| Requirement for further surgery | |
| Adverse effects of pacemaker implantation (including peri- and post-operative complications, AF and device replacement) | |
| HRQoL |
The intervention was permanent implantable dual-chamber pacemakers compared with single-chamber atrial pacemakers or dual-chamber pacemakers programmed primarily to atrial pacing. Studies were not excluded based on programming of the pacemakers; both rate-responsive and non-rate-responsive programming were included. The review also allowed other programmable features, such as prolonging or eliminating the AV interval in order to minimise VP.
Randomised controlled trials were included if the relevant pacing modes were compared in a population with symptomatic bradycardia, documented SSS, BTS and normal AV conduction. Studies were excluded if none of the outcomes of interest was reported.
Data abstraction strategy
Data were extracted independently by two reviewers using a standardised data extraction form. Information extracted included details of the study’s design and methodology, baseline characteristics of participants and results, including clinical outcome efficacy and any adverse events reported. Where there was incomplete information, the study authors were contacted with a request for further details. Discrepancies were resolved by discussion, with involvement of a third reviewer if necessary. Data extraction forms for the included studies are provided in Appendix 2, Data abstraction.
Critical appraisal strategy
The quality of the clinical effectiveness studies was assessed independently by two reviewers. Any disagreements were resolved by consensus and if necessary a third reviewer was consulted. The study quality was assessed according to recommendations by the CRD30 and the Cochrane Handbook for Systematic Reviews of Interventions31 and recorded using the Cochrane risk of bias tool. 31
Methods of data synthesis
Details of results on clinical effectiveness and quality assessment for each included study are presented in structured tables and as a narrative summary. The possible effects of study quality on the effectiveness data and review findings are discussed. Standard pairwise meta-analysis was performed to evaluate the clinical effectiveness for several outcomes based on intention-to-treat analysis. Intention-to-treat analysis was defined as analysis of patients in the trial arm to which they were allocated at randomisation regardless of whether or not they changed pacing mode, withdrew or were lost to follow-up.
Dichotomous outcomes data were meta-analysed using Mantel–Haenszel odds ratio (OR) with 95% confidence intervals (CIs) and a random-effects model. Individual trial data were analysed and presented in the same way as meta-analysed data for comparison where appropriate. In addition, if hazard ratios (HRs) were presented in the original publication of a trial, these have been reproduced in this report for comparison. Missing data were imputed and analysed as treatment failures.
For the dichotomous outcomes reported in this review (mortality, HF, AF, stroke, further surgery and adverse events), only RCTs with a parallel-group design have been considered, excluding RCTs with a crossover design. RCTs with a crossover design are most appropriate for symptomatic treatment of chronic or relatively stable conditions, such as symptomatic bradycardia treated by artificial pacing with a permanently implanted pacemaker. 33 However, crossover trials are appropriate only when looking at treatment effects that are likely to be reversible and short-lived, and inappropriate when studying outcomes where an outcome event may alter the baseline risk, that is, on entry to the second phase the patients systematically differ from their initial state. 33
Data for the continuous outcomes exercise capacity, cognitive functioning and QoL were primarily reported in included crossover trials. Data from parallel and crossover RCTs have been reported separately. It was planned a priori to analyse continuous outcome data from crossover studies using the mean difference (or the difference between the means) of dual-chamber and single-chamber atrial pacing and the standard deviation (SD) or standard error (SE) for the within-person differences. However, the included crossover trials reported means and SDs for treatment-specific outcomes but did not report paired results. One crossover trial provided individual patient data (IPD) for exercise capacity34 and one for QoL35 from which the mean difference and SE for within-person difference could be obtained. However, because there was a lack of reporting of relevant data across the included crossover trials, a meta-analysis of data was not performed for these trials.
A meta-analysis was carried out for the parallel-group trials using Review Manager Version 11 (Thomson ResearchSoft, San Francisco, CA, USA), with the use of a random-effects model. Statistical heterogeneity between included studies was assessed using the I2 test. In the presence of heterogeneity (I2 > 30%), possible sources were investigated, including differences between individual studies’ populations, methods or interventions. The possibility of publication bias and/or small study effects was not investigated because of the low number of included studies.
Stakeholders’ submissions
A joint manufacturers’ submission from the Association of British Healthcare Industries (ABHI) was expected for this MTA; however, the only submission to NICE in relation to this MTA was from the British Cardiovascular Society. As such, this report does not contain confidential information from stakeholders. No data additional to the studies identified in the systematic review were presented in the submission.
Results
Quantity and quality of research available
Database searches retrieved 492 records (post deduplication). One additional reference was identified through hand searching, giving a total of 493 references that were screened for inclusion (Figure 1). Full references were sought for 34 of these, which were potentially eligible for inclusion. Of the records identified as potentially relevant, only one reference was unobtainable. 36 However, this reference was identified in the original NICE MTA TA8818 and excluded because it was a pre-clinical study. Of the remaining 33 records, nine references describing six studies were included in the review. Characteristics of the studies included in the review are given in Table 3. A list of excluded references (with reason for exclusion) is presented in Appendix 4, Table of excluded studies.
FIGURE 1.
Preferred Reporing Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the clinical effectiveness review.

| Study | Population | Intervention | Comparator 1 | Comparator 2 | Randomisation | Country | Number of patients | Follow-up | Supplementary publications |
|---|---|---|---|---|---|---|---|---|---|
| Parallel-group RCTs | |||||||||
| Albertsen et al.37 | Sinus arrest/SA block, BTS, sinus bradycardia | DDD(R) | AAI(R) | N/A | Device | Denmark | 50 | 12 months | None identified |
| DANPACE19 | SA block/sinus arrest, sinus bradycardia, BTS | DDDR | AAIR | N/A | Device | Denmark, UK, Canada | 1415 | Mean 5.4 years (SD 2.6 years) | Andersen et al.;38 Nielsen et al.;39 Riahi et al.40 |
| Nielsen et al.41 | Sinus bradycardia, SA block, BTS | DDDR-s | AAIR | DDDR-l | Device | Denmark | 177 | Mean 2.9 years (SD 1.1 years) | Kristensen et al.42 |
| Crossover RCTs | |||||||||
| Gallik et al.34 | Sinus node disease | DDDR | AAIR | N/A | Programming | NR | 12 | < 1 day | None identified |
| Lau et al.35 | SSS | DDDR | AAIR | N/A | Programming | NR | 15 | 4 weeks per treatment | None identified |
| Schwaab et al.43 | Sinus bradycardia | DDDR | AAIR | N/A | Programming | Germany | 21 | 3 months per treatment | None identified |
No additional studies were retrieved from submissions made to NICE as part of the appraisal of this technology.
Randomised controlled trial characteristics
A summary of study characteristics (populations, interventions, comparator and follow-up) is shown in Table 3.
Six RCTs described and reported in nine publications were included in the review. The review included one trial34 that was identified but excluded from the original MTA, NICE’s TA88. 18 In NICE’s TA88,18 studies of fewer than 48 hours’ duration, such as Gallik et al. ,34 were excluded, whereas no time limitation was specified for the purposes of this review. This review also includes two trials that have been completed and published since NICE’s TA88: Albertsen et al. 37 and DANPACE. 19
Information about and results from DANPACE19 have been published in three publications included in this review: the protocol, the primary publication and one publication focusing on subgroup analyses of HF data. 19,38,40 One other included trial was reported in a main publication41 and an additional paper focusing on AF and thromboembolism analyses. 42
Study design
Three RCTs with a parallel-group design19,37,41 and three crossover RCTs34,35,43 were identified as relevant and were included in this review.
The follow-up period varied greatly among the included studies. Of the parallel-group RCTs, Albertsen et al. had a set follow-up of 12 months,37 the DANPACE trial had a follow-up of up to 10 years with an average of 5.4 years (SD 2.6 years),19 and, in Nielsen et al. the follow-up ranged from 6 days to 5.3 years (mean 2.9 years, SD 1.1 years). 41
The follow-up in the crossover trials was shorter than in the parallel studies. In Lau et al. 35 and Schwaab et al. ,43 patients spent 4 weeks and 3 months, respectively, before crossing over to the other pacing mode. Gallik et al. 34 studied the immediate effects of pacing mode during exercise; haemodynamic effects were measured during bicycle exercise first in one pacing mode and after 0.5–1 hour’s rest, the exercise was repeated in the other pacing mode.
Intervention and comparator
The three parallel RCTs randomised patients to receive single- or dual-chamber pacemakers. 19,37,41 In the crossover trials, all patients were implanted with a dual-chamber pacemaker and then randomised to a pacing programme of dual-chamber or single-chamber atrial pacing, followed by the alternative pacing mode. 34,35,43
Most trials randomised patients before pacemaker implantation, including the trials randomising patients by device (parallel RCTs),19,37,41 and two of the studies randomising by pacing programme. 35,43 The remaining trial, by Gallik et al. ,34 randomised patients who had recently had a dual pacemaker implanted.
The single and dual pacemakers used in the included trials were from several different manufacturers including Medtronic (Minneapolis, MN, USA), ELA Medical Inc. (Arvada, CO, USA), Boston Scientific (Marlborough, MA, USA); St Jude Medical (St Paul, MN, USA), Guidant (Indianapolis, IN, USA), Pacesetter (St Jude Medical; St Paul, MN, USA); Cardiac Pacemakers Inc. (St Paul, MN, USA), Telectronics Pacing Systems (Englewood, CO, USA) and Intermedics Inc. (Angleton, TX, USA).
The included trials compared DDD(R) with AAI(R) pacing. However, Nielsen et al. 41 included two DDDR trial arms with different programmed AV delay: DDDR-s with a short AV delay (< 150 milliseconds) and DDDR-l with a fixed long AV delay (300 milliseconds). Data for these two study arms have been combined in analyses in this review. However, for each outcome, either the impact of combining the study arms has been explored in a sensitivity analysis or data from each study arm have been presented separately.
The DANPACE19 study was the only trial that specifically stated that programmable features prolonging or eliminating the AV interval, in order to minimise VP, were not permitted in the trial.
In all the included studies, all or the majority of patients within each study received pacemakers programmed with the rate-adaptive function activated. The rate-adaptive function was activated in all patients in Albertsen et al. ,37 DANPACE,19 Gallik et al. ,34 Lau et al. 35 and Schwaab et al. 43 In Nielsen et al. ,41 all but two patients had the rate-adaptive function active.
The programmed AV delay in the dual-chamber pacing mode differed greatly across the studies and between study arms, as shown in Table 4. The studies had, for each study arm with dual-chamber pacing, an AV delay that was set at a specific value,34,41 in a range19,35,37 or optimised according to a programmed algorithm. 43 Gallik et al. ,34 Lau et al. 35 and the DDDR-s arm in Nielsen et al. 41 employed relatively short AV delays, up to 150 milliseconds. By contrast, the DDDR-l arm in Nielsen et al. 41 had an AV delay of 300 milliseconds. The AV delay in Albertsen et al. 37 and the DANPACE trial19 was around 220 milliseconds.
| Study | Intervention | Rate adaptive | AV delay | Mode switch |
|---|---|---|---|---|
| Parallel-group RCTs | ||||
| Albertsen et al.37 | DDDR | On | Maximum 220–225 milliseconds | On |
| DANPACE19 | DDDR | On in all but two patients | 140–220 milliseconds | On |
| Nielsen et al.41 | DDDR-s | On | 150 milliseconds | On |
| DDDR-l | On | 300 milliseconds | On | |
| Crossover RCTs | ||||
| Gallik et al.34 | DDDR | On | 100 milliseconds | NR |
| Lau et al.35 | DDDR | On | 96 milliseconds (SD 7 milliseconds) to 140 milliseconds (SD 5 milliseconds) | NR |
| Schwaab et al.43 | DDDR | On | AV delay was optimised based on the maximum time velocity integral of the aortic flow | On, but not in all patients |
The mode switch function was active in all three parallel-group RCTs. 19,37,41 In Schwaab et al. ,43 mode switch was activated in some patients, but the number of patients was not specified. Gallik et al. 34 and Lau et al. 35 did not report mode switch settings; however, mode switching may not have been available at the time of these trials.
Population
Most of the parallel and crossover RCTs included patients with symptomatic bradycardia or SSS in combination with certain ECG criteria, for example indicating normal AV conduction.
Schwaab et al. 43 had slightly different inclusion criteria: patients had to have chronotropic incompetence, have experienced at least two documented episodes of atrial tachyarrhythmia, be on antiarrhythmic medication for prevention of atrial flutter or AF, as well as being eligible for a dual-chamber pacemaker for symptomatic bradycardia.
The parallel RCTs19,37,41 had similar exclusion criteria, excluding patients if they had chronic AF, AV block, carotid sinus syndrome, vasovagal syncope, bundle branch block, surgery, a short life expectancy, dementia or cancer. Lau et al. 35 did not report specific exclusion criteria, and Gallik et al. 34 excluded patients with evidence of AV node disease or who were unable to exercise.
Summaries of the characteristics of the study populations in the included RCTs are presented in Table 5 (parallel RCTs) and Table 6 (crossover RCTs). More detailed baseline characteristics can be found in Appendix 2, Data abstraction.
| Patient characteristics | Albertsen et al.37 | DANPACE19 | Nielsen et al.41 | ||||
|---|---|---|---|---|---|---|---|
| DDDR, n (%) | AAIR, n (%) | DDDR, n (%) | AAIR, n (%) | DDDR-s, n (%) | DDDR-l, n (%) | AAIR, n (%) | |
| Number of participants | 26 | 24 | 708 | 707 | 60 | 63 | 54 |
| Age (years), mean (SD) | 73 (13) | 72 (10) | 72.4 (11.4) | 73.5 (11.2) | 74 (9) | 74 (9) | 74 (9) |
| Sex (male) | 8 (31) | 10 (42) | 267 (37.7) | 235 (33.2) | 23 (43) | 26 (43) | 24 (38) |
| Sinus arrest/sinoatrial block | 16 | 14 | NR | NR | 17 | 16 | 19 |
| BTS | 12 | 11 | NR | NR | 38 | 36 | 27 |
| Sinus bradycardia | 8 | 4 | NR | NR | 5 | 11 | 8 |
| Previous history of AF | NR | NR | 318 | 303 | NR | NR | NR |
| Previous stroke | 1 | 5 | 53 | 61 | NR | NR | NR |
| NYHA class, n | |||||||
| I | 18 | 19 | 522 | 503 | 38 | 46 | 32 |
| II | 8 | 3 | 158 | 172 | 22 | 14 | 18 |
| III | 0 | 2 | 24 | 29 | 0 | 3 | 2 |
| IV | 0 | 0 | 2 | 0 | 0 | 0 | 1 |
| Anticoagulant drugs | NR | NR | 89 | 108 | 5 | 11 | 5 |
| Beta-blockers | 11 | 6 | 132 | 159 | 5 | 7 | 4 |
| Diuretics | 11 | 14 | 263 | 304 | NR | NR | NR |
| Calcium channel blockers | 5 | 5 | 142 | 137 | 7 | 11 | 14 |
| ACE inhibitors | 10 | 11 | 170 | 160 | NR | NR | NR |
| Cardiac glycoside | NR | NR | 62 | 73 | 9 | 11 | 11 |
| Sotalol | NR | NR | 44 | 43 | 8 | 10 | 7 |
| Amiodarone | NR | NR | 24 | 25 | NR | NR | NR |
| Aspirin | 14 | 20 | 361 | 369 | 40 | 36 | 35 |
| Class I antiarrhythmics | NR | NR | 20 | 14 | NR | NR | NR |
| Patient characteristics | Gallik et al.34 | Lau et al.35 | Schwaab et al.43 |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Number of participants | 12 | 15 | 21 |
| Age (years), mean (SD) | 61 (SE 4) | 62 (2) | 70 (7) |
| Sex (male) | 8 (67) | 5 (42) | 11 (58) |
| Previous history of AF | NR | Some of the patients | NR |
| Previous stroke | NR | NR | NR |
| NYHA class | NR | NR | NR |
| Beta-blockers | 4 | 1 | NR |
| Class I antiarrhythmics | NR | NR | 2 |
| Calcium channel blockers | 4 | 2 | NR |
| ACE inhibitors | NR | 1 | NR |
| Cardiac glycoside | 3 | 3 | NR |
| Potassium channel blockers | NR | 1 | 18 |
| Aspirin | NR | 1 | NR |
| Nitrates | NR | 2 | NR |
The parallel RCTs19,37,41 varied in size from 50 to 1415 randomised patients. The crossover studies34,35,43 were smaller, with between 12 and 21 participants. The RCTs all included patients with SSS or sinus node dysfunction (SND). The parallel RCTs, Albertsen et al. 37 and Nielsen et al. ,41 reported the breakdown of pacing indication of the participants for sinus arrest/SA block BTS and sinus bradycardia, with some imbalances between the trial arms; most notably, there were more people with BTS in the two dual-chamber pacing arms than in the AAIR arm in Nielsen et al. 41
Mean age was similar across the three parallel RCTs,19,37,41 and between study arms (72–74 years). The participants in the crossover trials34,35,43 had a slightly lower mean age, of 61–70 years. Only the DANPACE trial1 reported previous history of AF, with around 44% of the participants having a history of AF in each trial arm. 3 Previous stroke was captured in Albertsen et al. and the DANPACE trial. In the smaller study by Albertsen et al. ,37 the number of patients with prior stroke was low but with a notable difference between groups in the proportion of people with prior stroke (5 out of 24 patients in the AAIR arm and only 1 out of 26 in the DDDR arm). In the DANPACE trial, there was no statistically significant difference between the trial arms in the percentage of patients having experienced a stroke at trial entry (7.5% and 8.6%, respectively). 19 In the parallel RCTs that reported NYHA class at baseline19,37,41 the majority of participants were NYHA class I or II (96%) with no or mild symptoms of HF.
Outcomes
The outcomes of interest to this review that were reported in the included studies are listed in Table 7. For several of the outcomes, the trials had used different scales or measurements, which have been reported separately. These include HF, exercise capacity and HRQoL.
| Outcome | Parallel RCTs | Crossover RCTs | ||||
|---|---|---|---|---|---|---|
| Albertsen et al.37 | DANPACE19 | Nielsen et al.41 | Gallik et al.34 | Lau et al.35 | Schwaab et al.43 | |
| All-cause mortality | ✗ | ✓ | ✓ | N/A | N/A | N/A |
| CV mortality | ✗ | ✗ | ✓ | N/A | N/A | N/A |
| HF | ✓ | ✓ | ✓ | N/A | N/A | N/A |
| AF | ✗ | ✓ | ✓ | N/A | N/A | N/A |
| Stroke | ✗ | ✓ | ✓ | N/A | N/A | N/A |
| Exercise capacity | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ |
| Cognitive functioning | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
| Further surgery | ✗ | ✓ | ✗ | N/A | N/A | N/A |
| Adverse events | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ |
| HRQoL | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ |
Randomised controlled trial quality
This section describes the trial designs and methodology employed in the trials, which may give rise to an increased risk of bias in terms of selection, detection, performance, and attrition bias. Additionally, other potential sources of bias, such as statistical methods used, are also assessed. Tables 8 and 9 summarise the results of critical appraisal of the included parallel and crossover RCTs, respectively. A more detailed description of the quality assessment of the trials can be found in Appendix 3, Quality assessment.
| Outcome | Potential source of bias | Albertsen et al.37 | DANPACE19 | Nielsen et al.41 |
|---|---|---|---|---|
| All | Random sequence generation | ?a | ? | ? |
| Allocation concealment | ? | ✓ | ? | |
| Selective reporting | ✓ | ✗ | ✓ | |
| Mortality | Blinding of participants and personnel | N/A | ✓ | ✓ |
| Blinding of outcome assessment | N/A | ✓ | ✓ | |
| Incomplete outcome data | N/A | ? | ✓ | |
| Stroke | Blinding of participants and personnel | N/A | ✓ | ✓ |
| Blinding of outcome assessment | N/A | ✓ | ✓ | |
| Incomplete outcome data | N/A | ? | ✓ | |
| AF | Blinding of participants and personnel | N/A | ? | ? |
| Blinding of outcome assessment | N/A | ? | ? | |
| Incomplete outcome data | N/A | ? | ✓ | |
| HF | Blinding of participants and personnel | ? | ? | ? |
| Blinding of outcome assessment | ✗ | ? | ? | |
| Incomplete outcome data | ✓ | ? | ✓ | |
| Requirement for further surgery | Blinding of participants and personnel | N/A | ? | N/A |
| Blinding of outcome assessment | N/A | ? | N/A | |
| Incomplete outcome data | N/A | ✓ | N/A | |
| Exercise capacity | Blinding of participants and personnel | ? | N/A | N/A |
| Blinding of outcome assessment | ✗ | N/A | N/A | |
| Incomplete outcome data | ✓ | N/A | N/A | |
| Adverse events | Blinding of participants and personnel | ? | N/Ab | N/A |
| Blinding of outcome assessment | ? | N/Ab | N/A | |
| Incomplete outcome data | ✓ | N/Ab | N/A |
| Outcome | Potential source of bias | Gallik et al.34 | Lau et al.35 | Schwaab et al.43 |
|---|---|---|---|---|
| All | Random sequence generation | ?a | ? | ? |
| Allocation concealment | ? | ? | ✓ | |
| Selective reporting | ✓ | ? | ✗ | |
| Exercise capacity | Blinding of participants and personnel | ? | N/A | ✓ |
| Blinding of outcome assessment | ? | N/A | ✓ | |
| Incomplete outcome data | ✓ | N/A | ✓ | |
| Cognitive function | Blinding of participants and personnel | N/A | N/A | ✓ |
| Blinding of outcome assessment | N/A | N/A | ✓ | |
| Incomplete outcome data | N/A | N/A | ✓ | |
| HRQoL | Blinding of participants and personnel | N/A | ✓ | ✓ |
| Blinding of outcome assessment | N/A | ✓ | ✓ | |
| Incomplete outcome data | N/A | ✓ | ✓ |
Selection bias
None of the full publications of the included trials described how the randomisation sequence had been generated. However, based on correspondence with the researchers for Albertsen et al. ,37 randomisation was performed in a 1 : 1 ratio. 37 Each patient was asked to draw one envelope, containing the allocation, from a batch of 10. Albertsen et al. ,37 the DANPACE trial19 and Schwaab et al. 43 gave some details about how the allocation sequence had been concealed from staff involved in the enrolment and assignment of participants. In these studies, the allocation was performed using sealed envelopes before pacemaker implantation19,37 or programming of the first pacing mode. 43 Nielsen et al. ,41 Gallik et al. 34 and Lau et al. 35 did not describe allocation concealment.
Performance and detections bias
Participants and investigators were blinded to the pacing mode in Lau et al. 35 and Schwaab et al. 43 Albertsen et al. ,37 Nielsen et al. 41 and Gallik et al. 34 did not describe the trial design regarding blinding. Based on correspondence with the researchers of the DANPACE trial,1 it was confirmed that this study was an open-label trial with participants, researchers and outcome assessors aware of the type of pacemaker and pacing mode in each patient. 19
In Nielsen et al. ,41 physical examinations and echocardiography were carried out unblinded, in contrast to the study by Albertsen et al. ,37 in which echocardiographic analyses were carried out blinded to the pacing mode. 37 However, blinding of echocardiography would have had only limited impact on the outcomes of interest captured in Albertsen et al. :37 HF, exercise capacity and adverse events. 37 In the DANPACE trial,19 a committee adjudicated stroke and thromboembolic events unaware of the assigned pacing mode. Gallik et al. 34 did not specify the blinding status of any outcome assessors.
Attrition bias
As mentioned previously, the DANPACE trial19 and Nielsen et al. 41 study participants were followed up for a variable length of time. In both studies, patients were followed up from enrolment to death or end of study, with no loss to follow-up. In Albertsen et al. ,37 one patient randomised to single-chamber atrial pacing was lost to follow-up, which has been accounted for as a treatment failure in the Technology Assessment Group (TAG)’s analyses.
Despite the small number of patients lost to follow-up, the number of patients changing pacemaker or pacing mode from the one to which they were randomised was relatively high and uneven between the trial arms in all three parallel RCTs. 19,37,41 In all three trials, the number of patients in the single-chamber atrial pacing arm who switched to (predominantly) DDDR pacing was higher than the number of patients in the dual-chamber pacing arm who switched to another pacing mode.
Among the crossover trials, three patients in Lau et al. 35 and two patients in Schwaab et al. 43 were excluded from the trials. The reasons for exclusion in Lau et al. 35 were pacemaker failure (n = 2) and patient non-compliance (n = 1), and, in Schwaab et al. ,43 development of chronic AF (n = 1) and death (n = 1). As expected, the crossover trials had to exclude participants who did not complete both intervention periods.
Reporting bias
In an early publication of the DANPACE trial results,19 outlying the protocol for the study,38 one of the secondary end points listed was a QoL evaluation, comprising elements from the general health questionnaire SF-36. However, no result of this outcome was published in either of the identified references linked to this study. 1,40
All three crossover trials34,35,43 reported results for each pacing mode separately, with mean and SE or SD. Exact p-values were not provided for the within-patient difference for any of the outcomes: the p-value was not reported, was described as non-significant or was reported to be less than a certain value. Lau et al. 35 reported IPD for general well-being [as measured by a visual analogue scale (VAS)] and Gallik et al. 34 reported IPD for exercise time, which were used to calculate the within-patient difference for these outcomes. The lack of reporting of p-value for the paired t-test for other outcome data in the crossover trials rendered the data unsuitable for meta-analysis.
Statistical analysis
Both the DANPACE trial19 and Nielsen et al. 41 were suspended before reaching the target number of participants and are consequently underpowered to show a statistically significant difference in the primary outcome: all-cause mortality in the DANPACE trial19 and changes in left atrium size and left ventricle (LV) size and function in Nielsen et al. A total of 450 patients were to be included in Nielsen et al. ,41 but recruitment was stopped after randomisation of 177 patients because recruitment for the DANPACE trial19 had started. However, recruitment for the DANPACE trial19 was also stopped early, at 1415 randomised patients, compared with the target of 1900 patients. 1 This was as a result of the increasing use of dual-chamber pacemakers with features that prolong or eliminate the AV interval to minimise VP in patients with SSS, which were not permitted in the trial and which therefore led to a decrease in the recruitment rate. In addition, a planned interim analysis showed that no statistically significant difference could be reached with respect to the primary outcome of all-cause mortality even with the planned 1900 patients.
Overall trial quality
Overall trial design and methodology were appropriate in the included trials; however, detailed descriptions of randomisation and allocation concealment were sparse. The parallel RCTs were either open label or it was unclear if and how patients, trial personnel and outcome assessors were blinded to the pacing modes. Blinding is likely to have a limited effect on the result of objective outcomes such as mortality, stroke and adverse events; however, for more subjective outcomes, including patient-reported outcomes such as QoL and HF questionnaires and exercise capacity, there is an increased risk of introducing bias into the results. The two crossover RCTs reporting results on QoL were both described as double blind. The risk of attrition bias was generally low as few patients were lost to follow-up in the parallel RCTs and the crossover trials excluded a small number of patients from the analyses. However, the number of patients in the parallel RCTs who changed pacing mode during the follow-up period was uneven between the pacing modes, which may lead to a conservative estimate of the effect of pacing mode.
Assessment of effectiveness
Change in pacing mode
Several patients in the parallel RCTs19,37,41 changed pacing mode during the study from the one to which they were randomised. Among the 857 patients randomised to DDDR and 785 to AAIR in the three trials, significantly more people with single-chamber atrial pacing than with dual-chamber pacing changed pacemaker and pacing mode (OR 0.50, 95% CI 0.37 to 0.67; Figure 2). There was no statistical heterogeneity in the meta-analysis of the three trials and only modest uncertainty; however, the result was mainly driven by the largest and longest trial, DANPACE.
FIGURE 2.
Results from analysis of change in pacing mode.

Most patients who changed from AAI(R) changed to DDD(R). The primary reasons for the implantation of a dual-chamber pacemaker in patients with a single-chamber atrial pacemaker were development of a high-degree AV block, or Wenckebach block during implantation. However, there were also a small number of patients who switched from AAIR to VVI. Patients randomised to DDD(R) who changed pacing mode during implantation or during follow-up primarily changed to VVI pacing because of development of persistent AF. One patient was lost to follow-up in the AAIR arm of Albertsen et al. ,37 and has been included in this analysis as changing pacing mode.
Per cent atrial and ventricular pacing
The rate of atrial pacing and VP (%) varied greatly among the studies, study arms and pacing modes (Table 10). Differences between studies in the rate of paced atrial or ventricular beats may be associated with differences in other outcome measures. VP has been associated with an increased incidence of AF. 44
| Study | Pacing mode | AV delay | % VP | % atrial pacing |
|---|---|---|---|---|
| Parallel RCTs | ||||
| Albertsen et al.37 | DDDR | Paced AV delay maximum 220–225 milliseconds | 66 | 62 |
| AAIR | N/A | Two patients, 3% and 99%, respectivelya | 53 | |
| DANPACE19 | DDDR | Mean maximum paced AV delay 225 (SD 39 milliseconds) | 65 (SD 33) | 59 (SD 31) |
| AAIR | N/A | 103/122 patients, 53 (SD 35)a | 58 (SD 29) | |
| Nielsen et al.41 | DDDR-s | 150 milliseconds | 90 | 57 |
| DDDR-l | 300 milliseconds | 17 | 67 | |
| AAIR | N/A | NRa | 69 | |
| Crossover RCTs | ||||
| Gallik et al.34 | DDDR | 100 milliseconds | NR | NR |
| AAIR | N/A | N/A | NR | |
| Lau et al.35 | DDDR | 96 milliseconds (SD 7 milliseconds) to 140 milliseconds (SD 5 milliseconds) | 64 (SD 11) | NR |
| AAIR | N/A | N/A | NR | |
| Schwaab et al.43 | DDDR | AV delay was optimised based on the maximum time velocity integral of the aortic flow | 99 (SD 2) | 95 (SD 5) |
| AAIR | N/A | N/A | 96 (SD 5) | |
In the DANPACE trial19 and Nielsen et al. ,41 the percentages of atrial pacing and VP were calculated using the mean of the number of paced beats at each follow-up, which was captured by the pacemaker event counters. Schwaab et al. 43 used stored pacemaker histograms to capture the percentage of paced beats in the atrium and ventricle. Albertsen et al. 37 and Lau et al. 35 did not describe how the percentages of atrial pacing and VP were captured, and Gallik et al. 34 did not report data on the percentages of atrial pacing or VP.
Schwaab et al. 43 had the highest rate of atrial pacing and VP, with patients being paced in both the atrium and ventricle for almost every beat. The amount of atrial pacing was balanced between the trial arms in Schwaab et al. 43 (95–96%) and in the DANPACE trial19 (58–59%). By contrast, in Albertsen et al. 37 the percentage of atrial pacing was higher in the DDDR (62%) than in the AAIR group (53%), and in Nielsen et al. 41 there were similar amounts of atrial pacing in the AAIR (69%) and DDDR-l (67%) group but less in DDDR-s (57%). Lau et al. 35 did not report the percentage of atrial pacing.
The variation between the trials in percentage of VP was even greater than for atrial pacing. The VP in the dual-chamber pacing arm was 64–66% in Albertsen et al. ,37 the DANPACE trial1 and Lau et al. 35 However, the dual-chamber pacing arm with the long AV delay in Nielsen et al. 41 (DDDR-l) had only 17% VP compared with a VP percentage of above 90% in the dual-chamber pacing arm with short AV delay (DDDR-s) in the same trial. VP was also above 90% in Schwaab et al. 43 The programmed AV delay varied between the included studies, which may explain some of the variation in the percentage of VP.
All-cause mortality
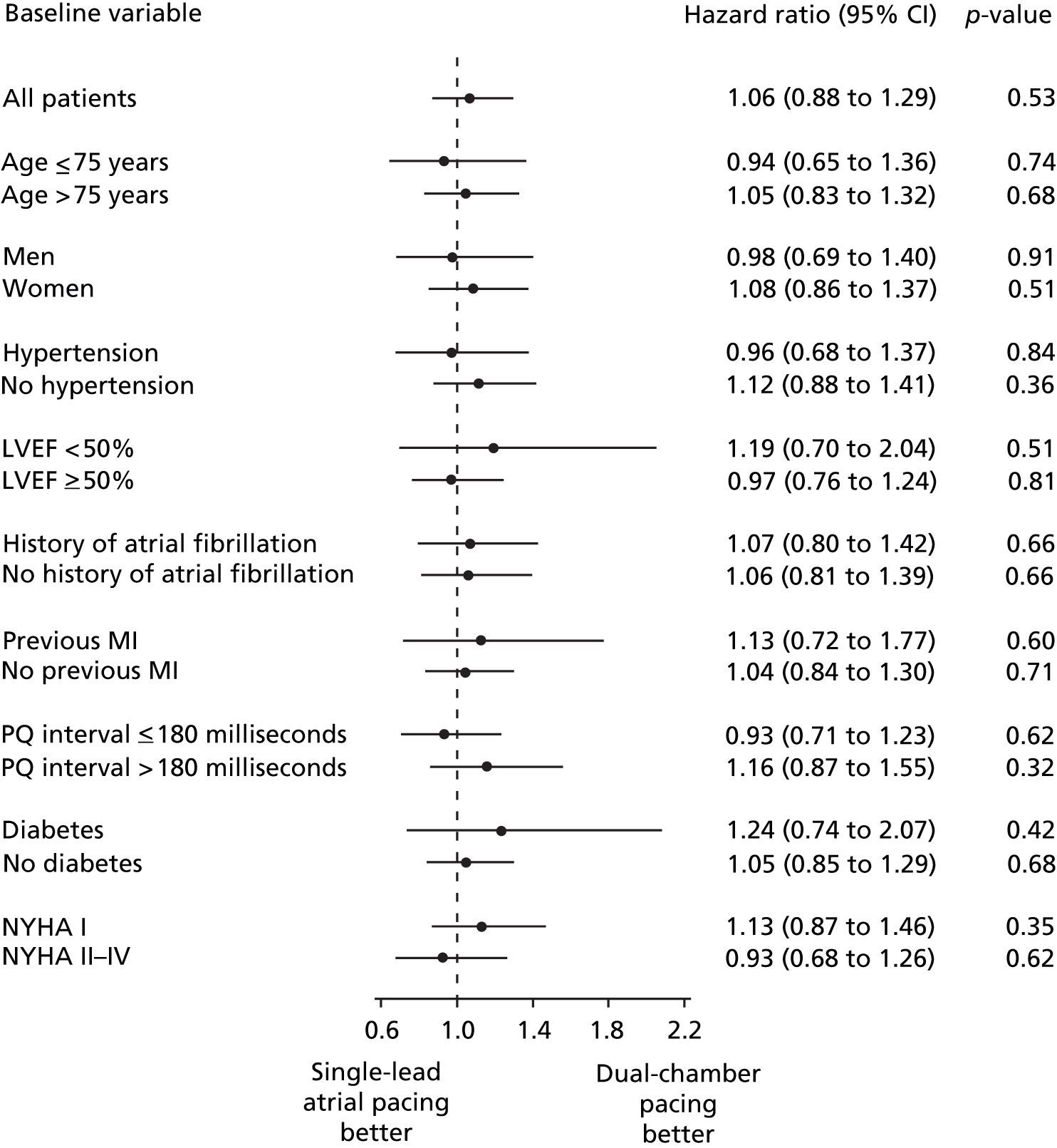
The DANPACE trial19 and Nielsen et al. 41 reported all-cause mortality. With 831 people randomised to DDDR pacing and 761 patients to AAIR pacing in total, there were fewer deaths among patients with dual-chamber pacing than single-chamber atrial pacing, but the difference was not statistically significant (OR 0.97, 95% CI 0.67 to 1.41; Figure 3).
FIGURE 3.
Results from analysis of all-cause mortality.

The large DANPACE trial,19 which stopped recruitment before reaching the planned 1900 patients, was not powered to detect a difference in mortality between the two pacing modes. However, from a planned interim analysis of the DANPACE trial results,19 it was calculated that no statistically significant difference in all-cause mortality would have been observed even if all 1900 patients had been recruited. As the meta-analysis of the DANPACE trial19 and Nielsen et al. considers only 1592 patients, it is unlikely to have sufficient power to identify a statistically significant difference. The breakdown of the number of deaths in the two DDD trial arms in Nielsen et al. is shown in Table 11. 41
| Outcome | Dual-chamber pacing | Atrial pacing | p-value | ||||
|---|---|---|---|---|---|---|---|
| DDDR-s | DDDR-l | AAIR | |||||
| n | N | n | N | n | N | ||
| Mortality | 14 | 60 | 14 | 63 | 9 | 54 | 0.51 |
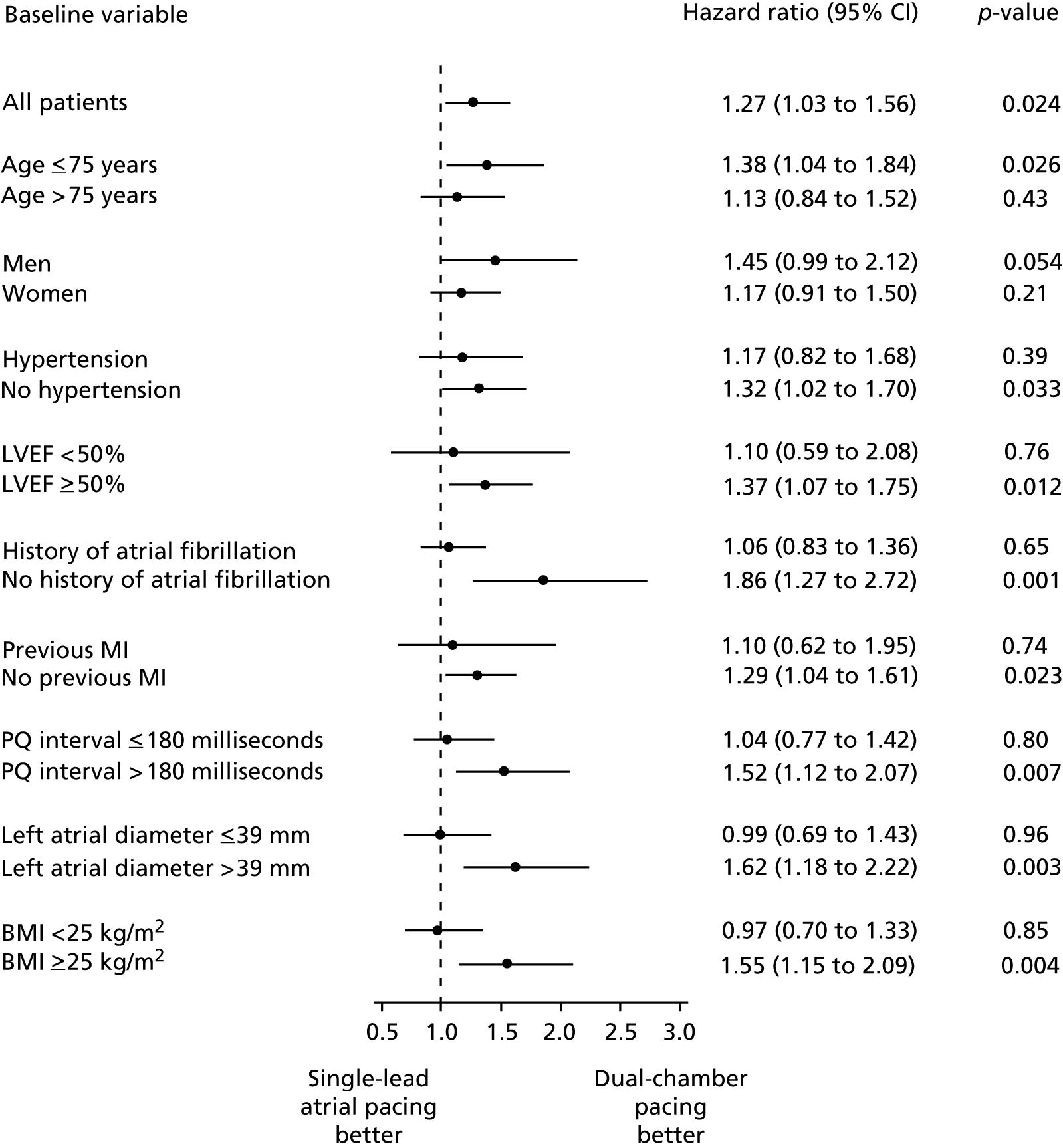
The DANPACE trial,19 in which the primary outcome was all-cause mortality, presented this outcome as a HR. The HR presented in the full publication was in line with the meta-analysis of mortality OR of the two included trials. The unadjusted HR for AAIR pacing compared with DDDR pacing was 1.06 (95% CI 0.88 to 1.29; p = 0.53). The HR after adjustment for baseline variables [age, sex, prior history of AF, prior myocardial infarction, left ventricular ejection fraction (LVEF) < 50%, and hypertension] was 0.94 (95% CI 0.77 to 1.14; p = 0.52) for AAIR pacing versus DDDR pacing. The all-cause mortality incidence was similar in all predefined subgroups (age > or ≤ 75 years; sex; hypertension; LVEF < or ≥ 50%; history of AF; previous myocardial infarction; PQ-interval > or ≤ 180 milliseconds; diabetes; NYHA class I or II–IV), with the smallest p-value for interaction of 0.45 (Figure 4).
FIGURE 4.
Subgroup analyses of all-cause mortality in the DANPACE trial. 1 MI, myocardial infarction.
In Albertsen et al. ,37 which did not report mortality as an outcome, one patient was lost to follow-up in the AAIR group, and may have died within the follow-up period.
Heart failure
Heart failure was reported in all three parallel RCTs. 19,37,41 However, the outcome measures varied between the studies (Table 12). In the three trials, HF was captured as NYHA class at the end of follow-up; number of patients taking diuretics; HF leading to hospitalisation; number of cases of new HF (defined as new NYHA class IV or new NYHA class III with the presence of oedema and/or dyspnoea); number of patients with an increase in consumption of diuretics; and number of patients with an increase of at least one NYHA class.
| Study | Time point | Dual-chamber pacing | Atrial pacing | Estimate of effect | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | N | n | N | n | N | ||||
| Albertsen et al.37 | DDDR | AAIR | |||||||
| NYHA class | |||||||||
| I | Baseline | N/A | N/A | 18 | 26 | 19 | 24 | NR | NR |
| II | Baseline | N/A | N/A | 8 | 26 | 3 | 24 | NR | NR |
| III | Baseline | N/A | N/A | 0 | 26 | 2 | 24 | NR | NR |
| IV | Baseline | N/A | N/A | 0 | 26 | 0 | 24 | NR | NR |
| I | End of follow-up | N/A | N/A | 14 | 26 | 18 | 23a | NR | NR |
| II | End of follow-up | N/A | N/A | 10 | 26 | 5 | 23a | NR | NR |
| III | End of follow-up | N/A | N/A | 1 | 26 | 0 | 23a | NR | NR |
| IV | End of follow-up | N/A | N/A | 1 | 26 | 0 | 23a | NR | NR |
| DANPACE19 | DDDR | AAIR | AAIR vs. DDDR | ||||||
| NYHA class | |||||||||
| I | Baseline | N/A | N/A | 522 | 708 | 503 | 707 | NR | NR |
| II | Baseline | N/A | N/A | 158 | 708 | 172 | 707 | NR | NR |
| III | Baseline | N/A | N/A | 24 | 708 | 29 | 707 | NR | NR |
| IV | Baseline | N/A | N/A | 2 | 708 | 0 | 707 | NR | NR |
| NYHA class | |||||||||
| I | End of follow-up | N/A | N/A | 341 | 666 | 364 | 666 | NR | 0.43 |
| II | End of follow-up | N/A | N/A | 260 | 666 | 231 | 666 | NR | |
| III | End of follow-up | N/A | N/A | 61 | 666 | 67 | 666 | NR | |
| IV | End of follow-up | N/A | N/A | 4 | 666 | 4 | 666 | NR | |
| Patients on diuretics | End of follow-up | N/A | N/A | 328 | 708 | 324 | 707 | NR | 0.89 |
| HF (leading to hospitalisation) | End of follow-up | N/A | N/A | 28 | 708 | 27 | 707 | HR 1.06 | 0.84 |
| New HF (new NYHA IV or III + symptoms) | End of follow-up | N/A | N/A | 169 | 708 | 170 | 707 | Unadjusted | |
| HR 1.00 | 0.87 | ||||||||
| Adjusted | |||||||||
| HR 1.09 | 0.44 | ||||||||
| Nielsen et al.41 | DDDR-s | DDDR-l | AAIR | ||||||
| Patients with increased consumption of diuretics | End of follow-up | 19 | 60 | 13 | 63 | 15 | 54 | NR | 0.34 |
| Patients with at least one NYHA class increase | End of follow-up | 18 | 60 | 29 | 63 | 17 | 54 | NR | 0.17 |
All the outcome measures for HF with a reported measure of uncertainty consistently showed no statistically significant difference between dual-chamber and single-chamber atrial pacing (see Table 12). However, because of low event rates or relatively small sample sizes the uncertainty was large around the HF outcome measures reported by Nielsen et al. 41 (patients with increased consumption of diuretics, patients with an increase of at least one NYHA class) and HF leading to hospitalisation reported by the DANPACE trial. 19
Pre-defined subgroup analyses in the DANPACE trial19 showed a statistically significant difference between single-chamber atrial pacing and dual-chamber pacing for patients aged ≤ 75 years and patients aged > 75 years in the number of patients developing new HF. In the younger subgroup (≤ 75 years), patients with AAIR were at a lower risk than those with DDDR of developing HF (HR 0.72, 95% CI 0.53 to 1.00), and in the older subgroup (> 75 years) patients were at a higher risk when on AAIR (HR 1.34, 95% CI 1.00 to 1.80). All other subgroup analyses were non-significant (sex; hypertension; LVEF < or ≥ 50%; previous myocardial infarction; PQ interval > or ≤ 180 milliseconds; NYHA class I or II–IV; diuretics, p > 0.31).
Atrial fibrillation
The DANPACE trial19 and Nielsen et al. 41 reported results on the incidence of AF. In both studies, DANPACE19 and Nielsen et al. ,41 AF was diagnosed by standard 12-lead ECG at planned follow-up visits. In the DANPACE trial,19 AF was defined as either paroxysmal (the first diagnosis of AF detected on ECG and verified by the pacemaker telemetry at a planned follow-up visit) or chronic (AF at two consecutive follow-up visits and at all subsequent follow-up visit). The results of paroxysmal and chronic AF have been combined to simplify the comparison with the results from Nielsen et al. (Table 13), but they are also reported separately (Table 14).
| Study | Outcome | Dual-chamber pacing | Atrial pacing | Effect estimate DDDR vs. AAIRa | |||
|---|---|---|---|---|---|---|---|
| n | N | n | N | OR | 95% CI | ||
| Nielsen et al.41 | AF | 25 | 123 | 4 | 54 | 3.19 | 1.05 to 9.67 |
| Sensitivity analysis | Subgroup | n | N | n | N | OR | 95% CI |
| Nielsen et al.41 | DDDR-l | 11 | 63 | 2 | 27 | 2.64 | 0.54 to 12.84 |
| DDDR-s | 14 | 60 | 2 | 27 | 3.80 | 0.80 to 18.10 | |
| Study | Outcome | Dual-chamber pacing | Atrial pacing | Effect estimate DDDR vs. AAIRa | |||
|---|---|---|---|---|---|---|---|
| n | N | n | N | OR | 95% CI | ||
| DANPACE19 | Paroxysmal AF | 163 | 708 | 201 | 707 | 0.75 | 0.59 to 0.96 |
| Chronic AF | 76 | 708 | 79 | 707 | 0.96 | 0.68 to 1.33 | |
Nielsen et al. found that the risk of developing AF with dual-chamber pacing was significantly higher than with single-chamber atrial pacing (OR 3.19, 95% CI 1.05 to 9.67; see Table 13). 41 A sensitivity analysis of the DDDR-l and DDDR-s trial arms analysed separately similarly shows a larger proportion of patients developing AF in either dual-chamber pacing arms than in the trial arm paced with a single-chamber atrial pacemaker (see Table 13). In the sensitivity analysis, the single-chamber atrial pacing group has been split in two, so as to avoid double counting of patients.
In contrast to the results in Nielsen et al. ,41 in the DANPACE trial,19 the risk of developing paroxysmal AF was significantly lower with dual-chamber pacing than with single-chamber atrial pacing (OR 0.75, 95% CI 0.59 to 0.96; see Table 14). By contrast, no statistically significant difference between dual-chamber and single-chamber atrial pacing was identified when focusing on the number of patients who developed chronic AF (OR 0.96, 95% CI 0.68 to 1.33; see Table 14); substantial uncertainty was identified in this analysis.
The HRs for paroxysmal and chronic AF reported in the DANPACE trial19 (unadjusted and adjusted for age, sex, prior history of AF, prior myocardial infarction, LVEF < 50% and hypertension), comparing single-chamber atrial pacing with dual-chamber pacing, support the analyses (Table 15).
| Study | Outcome | Dual-chamber pacing | Atrial pacing | Unadjusted effect estimate AAIR vs. DDDR | Adjusted effect estimate AAIR vs. DDDR | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | N | n | N | HR | 95% CI | HR | 95% CI | ||
| DANPACE19 | Paroxysmal AF | 163 | 708 | 201 | 707 | 1.27 | 1.03 to 1.56 | 1.24 | 1.01 to 1.52 |
| Chronic AF | 76 | 708 | 79 | 707 | 1.02 | 0.74 to 1.39 | 1.01 | 0.74 to 1.39 | |
There are several possible reasons for the disparity in the result of AF between the DANPACE trial19 and Nielsen et al. ,41 including differences in baseline characteristics of patients enrolled in the studies and differences in pacemaker programming. Various hypotheses have been put forward around factors that may have an effect on the incidence of AF:
-
Previous history of AF: in the DANPACE trial19 the strongest predictor of paroxysmal AF was prior history of AF (HR 3.23, 95% CI 2.59 to 4.03; p = 0.001). However, the DDDR and AAIR pacing arms were well balanced for previous AF at baseline. Nielsen et al. 41 did not report previous history of AF; however, they did report a breakdown of the underlying pacing indications including BTS, in which the tachyarrhythmia often is AF. In Nielsen et al. 41 there was an imbalance in the number of patients with BTS, with a larger proportion among patients in the two dual-chamber pacing arms than in the single-chamber pacing arm. Nielsen et al. 41 found a correlation between BTS at implantation and an increased risk of AF (relative risk 3.3, 95% CI 1.3 to 8.1; p = 0.01).
-
PQ-interval: the result of a subgroup analysis of 650 patients in the DDDR group in the DANPACE trial19 indicates that a longer baseline PQ-interval (> 180 milliseconds) is associated with an increased risk of AF (p < 0.001). There was a slight difference in PQ interval at baseline between the studies; however, the PQ interval was well balanced between the different trial arms within each study (Table 16). 1,41
-
Programmed AV interval and percentage VP: both DDDR and AAIR preserve AV synchrony. However, in AAIR normal ventricular activation pattern is preserved, whereas DDDR causes some degree of unnecessary VP with changes to the ventricular activation and contraction pattern, which has been associated with an increased risk of AF. 44,45 The programmed AV delay is closely linked to the resulting VP percentage; the DDDR-l arm in Nielsen et al. 41 had a programmed AV delay of 300 milliseconds and just 17% VP, whereas the DDDR-s arm had an AV delay of 150 milliseconds and 90% VP. In the DANPACE trial19 the patients in the DDDR arm had an AV delay and VP percentage in the middle of the range observed in Nielsen et al. 41 [225 milliseconds (SD 39 milliseconds) and 65% (SD 33%), respectively; see Table 10]. However, in Nielsen et al. ,41 there were significantly more patients with AF in both the DDDR-l and the DDDR-s arms than in the AAIR group, despite having low and high VP percentages, respectively. A subgroup analysis of 650 patients with a DDDR pacemaker in the DANPACE trial19 showed no statistically significant association between percentage VP or the length of the AV delay and risk of AF. 39
| Baseline characteristic | Nielsen et al.41 | DANPACE | |||
|---|---|---|---|---|---|
| DDDR-s | DDDR-l | AAIR | DDDR | AAIR | |
| PQ baseline (millisecond), mean (SD) | 183 (28) | 184 (27) | 186 (27) | 179 (30) | 179 (29) |
Both studies, DANPACE19 and Nielsen et al. ,41 seem to be of good quality, although there are some differences in the methods (e.g. programmed AV interval) and in the baseline characteristics of the patients in the two studies. However, the DANPACE trial19 is almost 10 times the size of Nielsen et al. 41 and it has a longer mean follow-up [5.4 years (SD 2.6 years) compared with 2.9 years (SD 1.1 years), respectively]; thus, it is reasonable to have more confidence in the results from the DANPACE trial1 than from the Nielsen et al. study.
Subgroup analyses of paroxysmal AF in the DANPACE trial19 showed a statistically significant difference between the subgroups of patients with and without a prior history of AF, body mass index (BMI) ≥ or < 25 kg/m2, and a left atrial diameter > or ≤ 39 mm at baseline (Figure 5). 1 In these three subgroups the incidence of paroxysmal AF was lower with DDDR than with AAIR pacing in patients without a prior history of AF, a higher BMI and a dilated left atrium at baseline (p < 0.05). The subgroup analysis of patients with different PQ-interval > or ≤ 180 milliseconds indicated a lower risk of paroxysmal AF with DDDR than AAIR pacing in patients with a longer PQ-interval (p = 0.084). The p-values for all other interaction were > 0.34.
Stroke
The studies, DANPACE19 and Nielsen et al. ,41 captured the number of patients suffering a stroke as an outcome. In Nielsen et al. ,41 the diagnosis of stroke was given when neurological symptoms of presumably cerebral ischaemic origin persisted for more than 24 hours, or if patients died within 24 hours from an acute cerebrovascular event. The definition of stroke in the DANPACE trial19 was similar: the sudden development of focal neurological symptoms lasting more than 24 hours. As for several other outcomes, the number of events was low, the uncertainty considerable and no statistically significant difference was shown (OR 0.93, 95% CI 0.60 to 1.45; Figure 6).
FIGURE 6.
Results from analysis of stroke.

The DANPACE trial19 reported an unadjusted HR for stroke of 1.13 (95% CI 0.72 to 1.80; p = 0.59) for patients with single-chamber atrial pacing compared with dual-chamber pacing. The HR when adjusted for age, sex, prior history of AF, hypertension and prior stroke was similar (HR 1.11, 95% CI 0.70 to 1.77; p = 0.65).
The breakdown of the number of patients suffering a stroke in the trial arms in Nielsen et al. 41 is shown in Table 17.
| Outcome | Dual-chamber pacing | Atrial pacing | p-value | ||||
|---|---|---|---|---|---|---|---|
| DDDR-s | DDDR-l | AAIR | |||||
| n | N | n | N | n | N | ||
| Stroke | 7 | 60 | 4 | 63 | 3 | 54 | 0.32 |
Exercise capacity
Exercise capacity was measured in the parallel RCT Albertsen et al. 37 and the crossover trials Gallik et al. 34 and Schwaab et al. 43 Albertsen et al. 37 used the 6-minute walking test (6MWT) to test exercise tolerance/capacity. 37 The 6MWT measures the distance an individual is able to walk over a total of 6 minutes on a hard, flat surface. In Gallik et al. ,34 exercise capacity was tested through an upright bicycle exercise. The initial workload was 200 kilopond metres (kpm), which was increased incrementally by 200 kpm every 3 minutes. The aim was to achieve a peak heart rate ≥ 85% predicted by age, and the outcome measure was exercise time. Schwaab et al. 43 used bicycle ergometry by incremental exercise test to exhaustion, using workload increments of 15 W/minute. 43 Outcome measures included total exercise duration and maximum workload.
Gallik et al. 34 presented IPD for exercise duration, whereas Schwaab et al. 43 presented only data for the individual treatment periods, but the results of paired t-tests of the within-patient difference for both studies were only reported as significant or not, without the numerical details of p-values.
In Albertsen et al. ,37 there was no statistically significant difference between the trial arms in the 6MWT at baseline, but, at 12 months’ follow-up, patients with a single-chamber atrial pacemaker walked significantly further than patients with a dual-chamber pacemaker (Table 18). 37 Although the result was statistically significant and the mean difference just reached the minimal clinically important difference of 54–80 m,46 there was substantial uncertainty around this value. One patient in the single-chamber atrial pacing group was lost to follow-up during the study, which may have had a small impact on the overall result.
| Parallel RCT | Time frame | Dual-chamber pacing | Atrial pacing | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | ||||
| Albertsen et al.37 | |||||||||
| 6MWT (m) | Baseline | 415 | 76 | 26 | 444 | 105 | 24 | NS | |
| 12 months | 446 | 96 | 26 | 500 | 89 | 23 | < 0.05 | ||
| Crossover RCT | N | Dual-chamber pacing | Atrial pacing | Within-patient difference | |||||
| Mean | SD | Mean | SD | Mean | p-value | ||||
| Gallik et al.34 | |||||||||
| Exercise durationa (seconds) | 12 | 416 | 140 | 411 | 122 | 6 | 0.74 | ||
| Schwaab et al.43 | |||||||||
| Exercise duration (seconds) | 19 | 402 | 102 | 423 | 127 | –21 | < 0.05 | ||
| Maximum workload (W) | 19 | 96 | 27 | 103 | 31 | –7 | < 0.05 | ||
Schwaab et al. 43 also showed a significantly better exercise capacity with single-chamber atrial pacing than with dual-chamber pacing for bicycle exercise duration and workload. However, Gallik et al. 34 did not detect a statistically significant difference between the pacing modes for a similar bicycle test. It is noteworthy that Gallik et al. 34 evaluated pacemakers over a markedly shorter test period, with both pacing modes tested on the same day with 0.5 to 1 hour’s rest between modes, which may partly explain the difference in result between Gallik et al. 34 and Schwaab et al. 43 However, as with the 6MWT in Albertsen et al. , there was substantial uncertainty around the result of the exercise testing in both Schwaab et al. 43 and Gallik et al. 34
Further surgery
One of the outcomes in the DANPACE trial19 was pacemaker reoperation during follow-up. The need for pacemaker reoperation was decided by the physician in charge of follow-up. Significantly fewer participants in the DDDR arm than in the AAIR arm needed a reoperation (OR 0.48, 95% CI 0.36 to 0.63; Table 19) during the relatively long-term follow-up in the DANPACE trial [5.4 years (SD 2.6 years)]. 19 The difference in reoperations between the pacing modes was statistically significant with only modest uncertainty around the result. Need for surgical change of pacing mode was the only reason for reoperation for which the difference between the pacing modes was statistically significant (Table 20). The reported unadjusted and adjusted HRs for AAIR compared with DDDR pacing are also listed in Table 19.
| Dual-chamber pacing | Atrial pacing | Effect estimate DDDR vs. AAIRa | Unadjusted effect estimate AAIR vs. DDDR | Adjusted effect estimate AAIR vs. DDDR | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | N | n | N | OR | 95% CI | HR | 95% CI | HR | 95% CI |
| 84 | 708 | 156 | 707 | 0.48 | 0.36 to 0.63 | 1.99 | 1.53 to 2.59 | 2.00 | 1.54 to 2.61 |
| Indication | Dual-chamber pacing | Single-chamber atrial pacing | p-value | ||
|---|---|---|---|---|---|
| n | N | n | N | ||
| Battery depletion | 42 | 708 | 59 | 707 | 0.09 |
| Need for surgical change of mode of pacing | 4 | 708 | 66 | 707 | < 0.001 |
| Lead complications | 30 | 708 | 37 | 707 | 0.42 |
| Surgical or mechanical complications | 7 | 708 | 10 | 707 | 0.52 |
| Infection | 3 | 708 | 3 | 707 | 0.98 |
| Skin erosion | 3 | 708 | 1 | 707 | 0.31 |
| Device failure | 2 | 708 | 2 | 707 | 0.99 |
Adverse effects of pacemaker implantation
Although all three parallel-group RCTs randomised patients by device, only two of the trials reported data on adverse effects linked to pacemaker implantation: Albertsen et al. 37 and DANPACE. 19 Albertsen et al. looked at complications around implantation; in the 50 randomised participants there were no lead displacements, infections or haematomas at pacemaker implantation (Table 21). 37 No other peri- or post-operative adverse effects of pacemaker implantation were reported. One patient in the single-chamber atrial pacing group was lost to follow-up during the study and could have had an adverse event after withdrawing from the trial.
| Complication | Dual-chamber pacing | Atrial pacing | ||
|---|---|---|---|---|
| n | N | n | N | |
| Lead displacements | 0 | 26 | 0 | 24 |
| Infections | 0 | 26 | 0 | 24 |
| Haematomas | 0 | 26 | 0 | 24 |
The DANPACE trial19 did not report adverse effects at implantation at randomisation; however, the indications for reoperations during follow-up were detailed. Of 1415 patients, 240 underwent one or more reoperations during the follow-up period (see Table 19). The more frequent indications for reoperation were battery depletion, lead complications and need for change of pacing mode (see Table 20). Less common adverse effects leading to reoperation were surgical or mechanical complications, infection, skin erosion or device failure. The only indication that was significantly different between the dual-chamber and single-chamber atrial pacemaker arm was surgical change in pacing mode.
Health-related quality of life and symptoms
Quality of life was studied in the crossover trials of Lau et al. 35 and Schwaab et al. 43 Both studies used different instruments to measure symptoms, QoL and functional status. Lau et al. 35 used VAS for general well-being; the SAS functional questionnaire for physical capacity (described in Chapter 2, Stakeholder’s submissions); the 12-item General Health Questionnaire (12-GHQ), symptom questionnaire; and the somatic symptoms inventory (SSI) adapted for local use from the Bradford Somatic Inventory. The 12-GHQ is a measure of current mental health. Each item is rated on a four-point scale (less than usual, no more than usual, rather more than usual or much more than usual). The symptom questionnaire assessed the incidence and frequency of dyspnoea, palpitations, dizziness, chest pain, sleep disturbance and neck pulsations, rated between 1 (all the time) and 5 (never). The SSI measures adequacy of daily life activities, emotional adjustment, social interactions (frequency, range and quality), work adjustment, sleep, fatigue and appetite.
Schwaab et al. 43 used three different self-administered questionnaires relevant to this review: VAS for general well-being, physical, emotional and cognitive functioning; the VAS Karolinska questionnaire including 16 questions on cardiovascular symptoms relevant to pacemaker patients; and the SAS functional questionnaire.
Lau et al. 35 presented IPD for general well-being; however, for all other outcomes of interest, Lau et al. 35 and Schwaab et al. 43 presented data for only the individual treatment periods. The results of paired t-tests of the within-patient difference were reported only as significant or not, without the numerical details of p-values.
General well-being and functional status
General well-being was similar across the studies of Lau et al. 35 and Schwaab et al. 43 There was no statistically significant difference between the pacing mode in either trial (Table 22). For functional status, the results were also similar between the trials with no statistically significant difference between the pacing modes. Although both trials were relatively small and with limited follow-up, there was a substantial amount of uncertainty around these results.
| Outcome | Dual-chamber pacing | Atrial pacing | Within-patient difference | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | p-value | |
| General well-being | ||||||
| aLau et al.35 | 7.1 | 1.2 | 6.8 | 1.3 | 0.25 | 0.32 |
| Schwaab et al.43 | 67 | 20 | 67 | 23 | 0 | NS |
| SAS | ||||||
| Lau et al.35 | 1.5 | 0.2 | 1.4 | 0.2 | 0.1 | NS |
| Schwaab et al.43 | 1.6 | 0.74 | 1.6 | 0.67 | 0 | NS |
Multidimensional measures
In the Schwaab et al. study,43 one of the multidimensional QoL questionnaires (self-perceived health status) included a section on cognitive function, an outcome specified in the scope of this review. Schwaab et al. 43 was the only included study to capture this outcome. There was no statistically significant difference between dual-chamber and single-chamber atrial pacing mode for cognitive functioning or the other elements of the self-perceived health status questionnaire in Schwaab et al. 43 (Table 23). Similarly, there was no statistically significant difference between the pacing modes for tests of mental well-being (12-GHQ, SSI), or for most symptoms in either Lau et al. 35 or Schwaab et al. 43 (see Table 23). Schwaab et al. 43 did report that patients experienced less dizziness with single-chamber atrial pacing than with dual-chamber pacing (p < 0.05), where Lau et al. 35 did not find a difference for the same symptom.
| Outcome | Dual-chamber pacing | Single-chamber atrial pacing | Within-patient difference | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | p-value | |
| Lau et al.35 | ||||||
| 12-GHQ | 14.3 | SE 2.2 | 15.2 | SE 2.1 | –0.9 | NS |
| SSI | 71.5 | SE 3.3 | 70.2 | SE 3.5 | 1.3 | NS |
| Activities of daily living | 31.2 | 2.0 | 32.8 | 2.1 | –1.6 | NS |
| Emotional adjustment | 24.2 | 1.7 | 23.2 | 1.8 | 1.0 | NS |
| Social interactions | ||||||
| Frequency | 11.3 | 1.1 | 11.8 | 1.2 | –0.5 | NS |
| Range | 2.1 | 0.2 | 2.2 | 0.3 | –0.1 | NS |
| Quality | 21.5 | 1.2 | 22.4 | 1.1 | –0.9 | NS |
| Work adjustment | 0.4 | 0.1 | 0.3 | 0.1 | 0.1 | NS |
| Sleep | 0.3 | 0.1 | 0.3 | 0.1 | 0.0 | NS |
| Fatigue | 0.6 | 0.1 | 0.6 | 0.1 | 0.0 | NS |
| Appetite | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | NS |
| Symptoms (1–5) | ||||||
| Dyspnoea | 3.4 | 0.45 | 3.95 | 0.25 | –0.55 | NS |
| Palpitations | 4.25 | 0.25 | 3.95 | 0.3 | 0.3 | NS |
| Dizziness | 4.25 | 0.25 | 3.95 | 0.3 | 0.3 | NS |
| Chest pain | 4.55 | 0.25 | 4.6 | 0.25 | –0.05 | NS |
| Sleep disturbance | 4.2 | 0.25 | 4.6 | 0.2 | –0.4 | NS |
| Neck pulsations | 4.95 | 0.1 | 4.95 | 0.1 | 0 | NS |
| Schwaab et al.43 | ||||||
| Self-perceived health status (%) | ||||||
| General well-being | 67 | 20 | 67 | 23 | 0 | NS |
| Physical functioning | 59 | 25 | 56 | 25 | 3 | NS |
| Emotional functioning | 63 | 27 | 63 | 27 | 0 | NS |
| Cognitive functioning | 56 | 23 | 51 | 27 | 5 | NS |
| Karolinska questionnaire (%) | ||||||
| Chest pain | 73 | 20 | 76 | 19 | –3 | NS |
| Palpitations | 78 | 17 | 79 | 20 | –1 | NS |
| Dizziness | 71 | 16 | 82 | 11 | –11 | < 0.05 |
| Dyspnoea | 67 | 24 | 71 | 20 | –4 | NS |
The results of the multidimensional QoL measures are limited by the same factors as the results for general well-being and functional status: both trials were relatively small and had limited follow-up, and there was a substantial amount of uncertainty around the results.
Discussion
Summary of quantity and quality of research available
The systematic review of clinical effectiveness identified six RCTs of relevance to this MTA. Three of these were of a parallel-group design and three were crossover trials. The trials all evaluated the efficacy of dual-chamber pacing compared with single-chamber atrial pacing in people with symptomatic bradycardia due to SSS, with no evidence of impaired AV conduction.
Both parallel-group and crossover trials are appropriate designs for the evaluation of pacing modes. In crossover trials, it is easy to switch between pacing modes with implantation of dual-chamber pacemakers and there is negligible concern for carryover effects or the need for a wash-out period between pacing modes. Crossover trials have an advantage over parallel-group trials as a result of higher power to detect a difference between interventions for the same number of participants. However, the crossover trials included in this review had small patient numbers (12–21 patients) and short durations (up to 3 months), which limited the outcomes that could reasonably be captured and the power to detect any differences between the pacing modes. The crossover trials provided data on exercise capacity, symptoms and QoL measures.
The parallel-group RCTs were larger (50–1415 patients) with longer follow-up (mean follow-up from 1 year to 5.4 years) than the crossover trials. The parallel RCTs captured mortality, HF, AF, stroke, need for reoperation, exercise capacity and adverse events of pacemaker implantation. No QoL measures were reported in any of the parallel RCTs. The parallel RCTs were trials of device, whereas the crossover trials were trials of pacing mode programming.
The quality of the trials was generally high, with appropriate trial design and methodology. The trials appeared to be appropriately randomised with a small number of participants excluded or lost to follow up. The DANPACE study19 was an open-label trial, whereas the blinding in the other two parallel RCTs was unclear. However, blinding is likely to have a limited effect on the result of objective outcomes such as mortality, stroke, AF and adverse events captured in these trials. For more subjective outcomes, including patient-reported outcomes such as QoL and HF questionnaires, and exercise capacity, there is an increased risk of introducing bias in the results. However, the two crossover RCTs reporting results on QoL were both described as double blind. The baseline characteristics were similar between the trial arms and across the parallel and crossover RCTs. However, in Nielsen et al. ,41 there were more people with BTS in the dual-chamber pacing groups than in the single-chamber atrial pacing group. The DANPACE study19 was the only one of the included trials reporting previous history of AF, which was balanced between the trial arms. The number of patients in the parallel RCTs who changed pacing mode during the follow-up period was uneven between the trial arms, which may lead to a conservative estimate of the effect of pacing mode for certain outcomes.
Summary of assessment of clinical effectiveness
-
Dual-chamber pacing was associated with a statistically non-significant improvement in mortality in the Nielsen et al. 41 and DANPACE studies. 19 The meta-analysis strengthens this conclusion. However, the meta-analysis is unlikely to have sufficient power to identify a statistically significant difference.
-
In the three parallel RCTs,19,37,41 the incidence of HF was captured using a wide range of measures, which limited the possibility to meta-analyse data for HF. Dual-chamber pacing was not associated with a statistically significant difference in HF compared with single-atrial pacing for any of the outcome measures. In a subgroup analysis, the DANPACE trial1 showed that younger patients (≤ 75 years) with AAIR were at a lower risk of developing HF than those with DDDR (HR 0.72, 95% CI 0.53 to 1.00) and that older patients (> 75 years) were at a higher risk when on AAIR (HR 1.34, 95% CI 1.00 to 1.80).
-
There were conflicting results for AF from the DANPACE19 and Nielsen et al. studies. 41 Dual-chamber pacing was associated with a statistically significant increase in AF in Nielsen et al. (OR 3.19, 95% CI 1.05 to 9.67), whereas in the DANPACE trial19 dual-chamber pacing was associated with a statistically significant decrease in paroxysmal AF (OR 0.75, 95% CI 0.59 to 0.96), but no statistically significant improvement in chronic AF. The disparity in the results between the DANPACE trial19 and Nielsen et al. 41 may have many causes, including differences in baseline characteristics such as pacing indication, prior history of AF and PQ interval. Other factors may include differences in intervention, that is, programming of AV delay leading to difference in VP percentages. However, the DANPACE trial1 is by far the largest study with the longest follow-up and balanced baseline characteristics; thus, it is reasonable to have more confidence in the results from the DANPACE trial19 than from Nielsen et al. 41
-
In a meta-analysis of data from the two studies, DANPACE19 and Nielsen et al. ,41 dual-chamber pacing was not associated with a statistically significant improvement in the risk of stroke.
-
There were limited data (relatively small number of patients with limited follow-up) on exercise capacity showing a small, but statistically significant, improvement with single-chamber atrial pacing compared with dual-chamber pacing in one parallel37 and one crossover trial. 43 One additional short-term crossover trial showed no statistically significant difference for this outcome.
-
In the three parallel RCTs,19,37,41 pacing mode was changed in significantly more patients with single-chamber atrial pacing than with dual-chamber pacing (OR 0.50, 95% CI 0.37 to 0.67). For people implanted with a single-chamber atrial pacemaker, the need to change pacing mode was predominantly a result of the development of AV block requiring upgrade to a dual-chamber pacemaker.
-
The DANPACE study19 was the only trial which specifically looked at reoperations, and found a statistically significant difference in the need for reoperations with the number of participants with dual-chamber pacing needing a reoperation being significantly lower than the number of patients with single-chamber atrial pacing (OR 0.48, 95% CI 0.36 to 0.63). In line with the results of change in pacing mode, the difference in reoperations was primarily driven by a surgical need for change of pacing mode in patients with single-chamber atrial pacemakers.
-
Adverse effects of pacemaker implantation were poorly reported. Albertsen et al. 37 reported no complications at implantation. The DANPACE trial19 reported indications for reoperations, of which the more frequent indications were battery depletion, lead complications and need for surgical change of pacing mode. The last indicator was significantly less common in dual-chamber pacing than in single-chamber atrial pacing.
-
Health-related quality of life and symptoms were assessed in two small crossover trials with limited follow-up using a wide range of measures. No statistically significant difference was shown between dual-chamber and single-chamber atrial pacing for general well-being, functional status or multidimensional QoL measures including for cognitive functioning.
Generalisability of results
The DANPACE study19 is a relatively large trial of good quality and good follow-up, which gives a reasonable evidence base for dual-chamber pacing compared with single-chamber atrial pacing for people with SSS without evidence of impaired AV conductance. The additional studies identified in this review had small sample sizes and short follow-up in comparison, giving them little weight to inform the question of dual-chamber pacing versus single-chamber atrial pacing. Although the time horizon in the DANPACE trial1 was reasonable, the results for patients needing a change in pacing mode and reoperation were probably conservative, as the proportion of these resulting from the development of a high-grade AV block would be expected to increase steadily over time. In addition, the DANPACE trial1 did not allow pacemaker algorithms designed to minimise VP in patients with intact AV conduction, which are now more common since the beginning of this trial. Although the DDDR pacemakers in the DANPACE trial1 were programmed in a way intended to reduce unnecessary VP, VP was still 65% (SD 33%), which may offset some of the benefit of implanting a dual-chamber pacemaker.
Conclusions
This review has shown dual-chamber pacing to be associated with a lower risk of paroxysmal AF and fewer reoperations than single-chamber atrial pacing. No statistically significant difference was shown between the pacing modes for mortality, stroke, QoL, chronic AF or HF. However, for patients younger than 75 years of age, the risk of HF seems to be higher with a dual-chamber pacemaker than a single-chamber atrial pacemaker, and for patients older than 75 years the risk seems to be lower with dual-chamber pacing than with single-chamber atrial pacing.
Hence, there are arguments in favour of both dual-chamber pacing and single-chamber atrial pacing in patients with SSS without evidence of impaired AV conduction.
With single-chamber atrial pacing:
-
Patients who do not go on to develop AV block have been paced appropriately and avoid any unnecessary VP, which may have adverse consequences for cardiac function.
-
The risk of HF may be lower than for dual-chamber pacing if the patient is younger than 75 years of age.
-
The implantation procedure is generally shorter than for dual-chamber pacemakers.
-
The follow-up takes less time than for dual-chamber pacemakers.
-
The risks of complications associated with pacemaker implantation may be lower than for dual-chamber pacemakers, as only one lead is inserted.
With dual-chamber pacing:
-
Patients who do go on to develop AV block will be protected by the presence of a ventricular lead and will not need a further operation to upgrade the pacemaker and insert a second lead, which is associated with higher risk of complications than for first time implant.
-
The risk of developing paroxysmal AF is lower than with single-chamber atrial pacemaker.
-
The risk of HF may be lower than for single-chamber atrial pacing if the patient is older than 75 years of age.
In conclusion, in patients with SSS without evidence of impaired AV conduction, the risk of developing a complete AV block and the lack of tools to identify patients at high risk of developing the condition argues for the implantation of a dual-chamber pacemaker programmed to minimise unnecessary VP. However, considerations have to be made around the risk of developing HF, which may depend on age and device.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
This section describes the TAG’s review of the existing cost-effectiveness evidence for pacing in the management of bradycardia. The sections Narrative summary of included UK economic evaluations, Narrative summary of included non-UK economic evaluations and Narrative summary of included costing studies provide narrative summaries of UK-specific economic evaluations, non-UK specific economic evaluations and costing studies, respectively, identified in the review. A joint manufacturers’ submission was expected from the ABHI but it was not submitted for consideration as part of this MTA. The section Summary and conclusions of available cost-effectiveness evidence summarises the available evidence and draws conclusions about the published assessments of cost-effectiveness.
A systematic review of MEDLINE (via Ovid), EMBASE (via Ovid), the HTA database (HTA; via The Cochrane Library) and the NHS Economic Evaluations Database (NHS EED; via The Cochrane Library) was carried out in December 2013. The review aimed to identify published economic evaluations or costing studies of relevance to the decision problem that is the focus of this MTA.
To facilitate the identification of all potentially relevant information, the MEDLINE and EMBASE search strategies combined terms capturing population (pacing), interventions (dual-chamber pacemakers) and economic evaluations/costing studies, with terms designed to capture a broader range of comparators (e.g. single-chamber ventricular pacemakers) than those specified in the scope; economic evaluations or costing studies in patients receiving single-chamber VP were considered likely to be informative in the development of a de novo economic evaluation.
The search strategy for HTA and NHS EED combined terms for the target condition (AV block, SSS) with terms for the intervention (pacemaker). All databases were searched from inception; full details of the search terms are presented in Appendix 1, Literature search strategies.
In addition to searches of the above databases, additional sources of potentially relevant publications were explored:
-
Experts in the field were contacted with a request for details of relevant published and unpublished studies of which they may have knowledge.
-
The NICE website was searched for any recently published TAs in pacing that had not already been identified via the database searches.
-
Reference lists of key identified studies were reviewed for any potentially relevant studies.
No restrictions on language or setting were applied to any of the searches. The titles and abstracts of papers identified through the searches were independently assessed for inclusion by two health economists using the criteria outlined in Table 24.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
The systematic review was updated in June 2014. The search strategy remained the same as outlined above; however, results were limited from 16 December 2013 to 6 June 2014 to identify additional relevant studies.
A total of 228 papers were identified from the December 2013 search (Figure 7). Of these papers, 112 were excluded on the basis of title and abstract and 90 were duplicates. Therefore, a total of 26 papers were identified as potentially relevant and were reviewed in full. Of the 26 papers, 15 were excluded after review of the full paper. For a description of the reasons for exclusion of the ordered papers, see Appendix 4, Table of excluded studies. Eleven papers from the December 2013 search were identified as being relevant to the review of the economic literature. A further nine papers were identified from the update search in June 2014. Of these, seven were excluded on the basis of title and abstract, one was a duplicate and one paper was identified as potentially relevant and was reviewed in full. Overall, one additional paper was identified in the update search as being relevant to the review of the economic literature.
FIGURE 7.
Identified economic evaluation and costing studies.

Of the 12 studies identified from the searches, 11 were economic evaluations and one was a UK-specific costing study (Box 1 shows a summary of studies, full extraction tables are provided in Appendix 2, Data abstraction).
Mahoney 1994. 47
Sutton et al. 1996. 48
Reporting the National Institute for Health and Care Excellence’s technology appraisal number 8818Castelnuovo et al. 2005. 49
Caro et al. 2006. 50
Additional studiesClarke et al. 1998. 51
Deniz et al. 2008. 52
O’Brien et al. 2005. 53
Oddershede et al. 2014. 54
Osman et al. 2010. 55
Rinfret et al. 2005. 56
Ray et al. 1992. 57
Wiegand et al. 2001. 58
Of the 11 economic evaluations identified, five were UK-based studies,48–50 and two were carried out in the USA,47,56 with the remaining four studies carried out in Denmark,54 Italy,52 Canada53 and Germany. 58
With the exception of studies carried out by Caro et al. 50 and Castelnuovo et al. ,49 the identified UK-specific economic evaluations were simple comparisons of costs and benefits. That is, differences in costs were not analysed in relation to differences in benefits. Furthermore, single-centre costs were predominantly used and estimates of benefit informed by either retrospective analysis of patient records51,55 or unadjusted pooling of incidence data identified in a literature review. 48
The analyses carried out by Caro et al. 50 and Castelnuovo et al. 49 were evaluations of cost–utility. Caro et al. focused on the cost–utility of dual-chamber (DDD/DDDR) versus single-chamber ventricular pacemakers (VVI/VVIR) in people with bradycardia resulting from SND or AV block. The study reported by Castelnuovo et al. 49 relates to NICE’s TA88,18 of which this MTA is in part a review and update, and therefore considers, among others, the question that is the scope of this MTA. However, for the cost–utility of dual-chamber versus single-chamber atrial pacemakers in people with SSS and no AV block, Castelnuovo et al. 49 found that atrial pacing dominates (i.e. is less expensive and more effective than) dual-chamber pacing over a 10-year time horizon. A narrative review of all UK-specific economic evaluations is presented in Narrative summary of included UK economic evaluations, with quality assessment against the NICE reference case and the Philips checklist59 presented in Appendix 3, Quality assessment.
The six non-UK economic evaluations identified were of varying quality and relevance. 47–58 One study54 considered the cost-effectiveness of dual-chamber versus single-chamber atrial pacing, making it the most relevant non-UK economic evaluation. The remaining four studies considered the cost-effectiveness of dual-chamber versus single-chamber VP. 47,56,52,53 Of these, two studies compared DDD or AAI devices with VVI devices. 47,53 A narrative review of these studies is presented in the section Narrative summary of included non-UK economic evaluations, with quality assessment against the NICE reference case and Philips checklist59 presented in Appendix 3, Quality assessment.
The single UK-specific costing study57 identified in the TAG’s systematic review of the economic literature provides information on the cost of devices incurred by a single centre in 1991 and is therefore of limited use to inform an up-to-date economic evaluation.
Narrative summary of included UK economic evaluations
Caro et al.50
Caro et al. 50 estimated the cost–utility of dual-chamber pacemakers compared with single-chamber ventricular pacemakers in people with bradycardia as a result of SND or AV block. The analysis was carried out from a UK NHS perspective, using costs from 2003 discounted at 6% per annum over a 5-year time horizon; benefits, namely quality-adjusted life-years (QALYs), were discounted at a rate of 1.5% per annum.
Model structure and assumptions
A discrete event simulation approach was used to estimate the incremental cost-effectiveness ratio (ICER) of the dual-chamber and single-chamber pacemakers under consideration. The model simulated two hypothetical populations of patients: population A and population B. With the exception of age (sampled from 2002 UK pacemaker implantation population statistics60) and systolic blood pressure (sampled from the Framingham Heart Study for patients with AF61), the characteristics of each (n = 1000) hypothetical patient in population A were sampled from distributions derived from the baseline characteristics of people enrolled into the Canadian Trial of Physiological Pacing (CTOPP). Each patient in population A was ‘cloned’ to produce population B. Both populations entered the simulation at the point of pacemaker implantation; population A received a dual-chamber (DDD or DDDR) pacemaker and population B received a single-chamber ventricular (VVI or VVIR) pacemaker.
Thereafter, simulated patients were exposed to the risk of one of four events: onset of AF, an implantation-related complication, pacemaker syndrome or death. The time to each possible event was estimated through samples of the corresponding failure time distribution; each event was assumed to be independent of other simulated events. The simulation selected and processed the consequences of each event in the order in which they were estimated to occur, with death resulting in no future events and the onset of AF resulting in exposure to the risk of stroke.
Outcome data
The onset of AF with respect to device type was estimated from data collected in the CTOPP. 62,63 Analysis indicated that dual-chamber pacing was associated with an 18% and 27% reduction in the onset of AF (lasting more than 15 minutes) and chronic AF (permanent AF), respectively. Data from the MOde Selection Trial (MOST)64 were used to inform the rate of post-operative complications associated with dual-chamber devices, whereas complication rates associated with single-chamber ventricular devices were derived from application of the HR (0.42) versus dual-chamber pacing observed in CTOPP. 62 The incidence and severity (i.e. whether or not symptoms were severe enough to warrant pacemaker upgrade) of pacemaker syndrome in patients implanted with single-chamber ventricular devices were estimated from data reported in the MOST and CTOPP. Mortality observed in the CTOPP was used to inform simulated life expectancy. After the onset of AF, the risk of stroke was estimated using the Framingham risk equation;61 patients receiving anticoagulation treatment (assumed to be 65% of the patients with chronic AF, based on data from the CTOPP) incurred a relative risk reduction of 0.55, based on a study by Hart et al. 65 QoL utility weights are reportedly derived from ‘data collected using the time trade-off approach during MOST’,50 but details of utility value derivation are not provided or cited.
Resource use and cost data
Direct medical costs incurred by the UK NHS were included in the analysis and encompassed costs of device, initial implantation, device replacement, anticoagulation and stroke. With the exception of device costs, standard NHS cost resources were used (NHS Reference Costs 2002,66 summary of product characteristics); device costs in Caro et al. 50 were obtained from a personal communication from the Consortium of Pacemaker Manufacturers. The cost of anticoagulation included warfarin therapy. The costs of monitoring and laboratory tests in Caro et al. 50 were obtained from a personal communication with the Department of Coagulation, Sheffield Haemophilia and Thrombosis Centre, Royal Hallamshire Hospital. The cost of stroke, initial implantation and replacement implantations were derived from relevant Healthcare Resource Group (HRG) codes.
Summary of results
The average (based on 100 simulations) additional cost associated with a dual-chamber pacemaker compared with a single-chamber ventricular pacemaker was estimated to be £43 per patient, over 5 years. This additional cost was estimated to be associated with an average gain in QALYs of 0.09 per person, resulting in an average ICER of £477 per QALY gained. Univariate sensitivity analysis revealed that the cost-effectiveness results are sensitive to assumptions regarding the proportion of patients requiring pacemaker replacement as a result of pacemaker syndrome. Multivariate sensitivity analysis indicated robust cost-effectiveness estimates, with 29% of simulations resulting in the dominance of dual-chamber pacemakers over single-chamber ventricular pacemakers and 31% indicating an ICER of < £1000 per QALY. No simulations estimated an ICER of more than £10,000 per QALY.
Critique
The study by Caro et al. 50 provides a useful example of a simulation approach in a disease area similar to that specified in the scope of this MTA. The model structure used represents a reasonable approximation of the health condition under evaluation, but is limited by the exclusion of HF and the use of a time horizon shorter than the expected lifetime of the interventions considered. With the exception of QoL utility weights, the data that form the basis of the economic evaluation are generally well described; however, it is unclear how data sources were identified. Assessment of parameter uncertainty has been carried out to sufficient depth to understand the potential impact of model parameters on the cost-effectiveness results; however, assessment of structural or methodological uncertainty is missing.
Castlenuovo et al.49
The review and economic evaluation carried out by Castelnuovo et al. 49 informed NICE’s TA88, an MTA of which this review is in part an update. Consequently, the scope of the review reported by Castelnuovo et al. 49 was broader than the decision problem that is the focus of this MTA. Castelnuovo et al. 49 considered the clinical effectiveness and cost-effectiveness of dual-chamber versus single-chamber pacemakers for the management of bradycardia as a result of SSS and/or AV block. The analysis was carried out over 5- and 10-year time horizons from the perspective of the UK NHS. Costs (from 2003) were discounted at a rate of 6% per annum and benefits (QALYs) were discounted at a rate of 1.5% per annum, according to the NICE reference case of the time.
Model structure and assumptions
A series of Microsoft Excel-based (Microsoft Corporation, Redmond, WA, USA) Markov models were used to assess the cost-effectiveness of three different treatment choices:
-
dual-chamber pacemakers versus single-chamber ventricular pacemakers in people with AV block
-
dual-chamber pacemakers versus single-chamber ventricular pacemakers in people with SSS
-
dual-chamber pacemakers versus single-chamber atrial pacemakers in the SSS population.
The patient population of each analysis were assumed to be homogeneous, that is, either all patients had SSS or all had AV block. A simplified outline of the structure of the Markov models is displayed in Figure 8. Health states were designed to reflect disease course and potential outcomes following pacemaker implantation. People receiving a dual-chamber pacemaker were initially exposed to the risk of perioperative and subsequent complications and over the longer term to the risk of AF, HF or stroke. People who developed AF were exposed to a higher risk of HF or stroke than people without AF. In addition, people with a dual-chamber device (either initially or following upgrade) who went on to develop AF had their device reprogrammed to act as a single-chamber ventricular device. Mortality was also accounted for within the model, with people exposed to the risk of death from any cause, perioperative mortality, death from stroke or death from HF. The subsequent disease pathways for people experiencing HF or stroke were not explicitly modelled; broad assumptions regarding the ongoing cost and utility associated with these health states were made.
People receiving a single-chamber pacemaker were exposed to the same risks as people receiving a dual-chamber pacemaker. However, those receiving a single-chamber ventricular pacemaker were also at risk of developing pacemaker syndrome, which could result in upgrade to a dual-chamber pacemaker. Similarly, people receiving a single-chamber atrial pacemaker were at risk of developing AV block, necessitating upgrade to a dual-chamber pacemaker.
Outcome data
Randomised controlled trial data were used to inform the incidence of perioperative and subsequent complications, incidence and severity of pacemaker syndrome, progression to AV block (in people with SSS) and the onset of AF. Operative complication rates were taken from the CTOPP62 and Pacemaker Selection in the Elderly (PASE) study,67 with complication rates from an upgrade procedure assumed to be twice that of an initial procedure. The incidence and severity of pacemaker syndrome in people implanted with a single-chamber ventricular device were estimated based on data from the MOST and CTOPP. 62,68 Progression to AV block (1.9% per annum) in people with SSS was sourced from Nielsen et al. 41 The likelihood of developing AF was taken from data presented in the MOST, UKPACE (commercial in confidence) and the Nielsen et al. study. 41
The incidence of stroke and HF in people without AF and receiving single-chamber ventricular or dual-chamber pacemakers was synthesised in a meta-analysis from evidence identified in the clinical effectiveness review that formed part of the work undertaken for NICE’s TA88. 18 The incidence of HF and stroke for people without AF receiving single-chamber atrial pacing was taken from the study reported by Nielsen et al. 41 In people with AF, the likelihood of experiencing stroke or HF was estimated from data presented in Chugh et al. 69 and Wang et al. ,70 respectively. Table 25 provides a summary of the outcome data used to inform the Markov models that formed the basis of the economic evaluation considered in NICE’s TA88. 18
| Outcome | Single-chamber VP | Single-chamber atrial pacing | Dual-chamber pacing | |||
|---|---|---|---|---|---|---|
| Input | Source | Input | Source | Input | Source | |
| Incidence of perioperative complicationsa | 3.3% | CTOPP62 | 3.3% | CTOPP62 | 6.6% | CTOPP62 |
| Perioperative mortalityb | 0.25% | PASE67 | 0.25% | PASE67 | 0.25% | PASE67 |
| Subsequent complications | 0.1% | Assumption | 0.1% | Assumption | 0.1% | Assumption |
| Subsequent complications mortality | 0.5% | Assumption | 0.5% | Assumption | 0.5% | Assumption |
| Incidence of mild pacemaker syndrome | 44% (1 month); 77% (6 months); 23% (thereafter) | PASE71 | N/A | N/A | N/A | N/A |
| Severe pacemaker syndrome | 16.5% of pacemaker syndrome cases | CTOPP62 | N/A | N/A | N/A | N/A |
| Progression to AV block | N/A | N/A | 1.9% per annum | Nielsen et al.41 | N/A | N/A |
| AF onset | SSS: 12% (6 months); 27% (thereafter) | MOST68 | SSS: 12% (6 months); 27% (thereafter) | MOST68 | SSS: 12% (6 months); 27% (thereafter) | MOST68 |
| AV block: CiC from UKPACE | AV block: CiC from UKPACE | AV block: CiC from UKPACE | ||||
| Progression to stroke (no AF) | 1.25% per annum | Castelnuovo et al.49 | RR vs. dual-chamber 0.62 | Nielsen et al.41 | 1.07% per annum | Castelnuovo et al.49 |
| Progression to stroke (with AF) | 3.2% per annum | Chugh et al.69 | 3.2% per annum | Chugh et al.69 | 3.2% per annum | Chugh et al.69 |
| Stroke mortality | 33% per annum | Appelros et al.72 | 33% per annum | Appelros et al.72 | 33% per annum | Appelros et al.72 |
| HF (no AF) | 2.6% per annum | Castelnuovo et al.49 | RR vs. dual-chamber 1.07 | Nielsen et al.41 | 2.5% per annum | Castelnuovo et al.49 |
| HF (with AF) | 3.3% per annum | Wang et al.70 | 3.3% per annum | Wang et al.70 | 3.3% per annum | Wang et al.70 |
| HF mortality | 20.8% per annum | MacIntyre et al.73 | 20.8% per annum | MacIntyre et al.73 | 20.8% per annum | MacIntyre et al.73 |
Resource use and cost data
Costs associated with the intervention, procedure (including complications), device reprogramming, management of pacemaker syndrome, AV block, AF, stroke and HF were included in the economic evaluation carried out by Castelnuovo et al. (Table 26). 49 Intervention costs were sourced from an economic evaluation carried out alongside the, at the time unpublished, UKPACE trial. 74 Procedure (including complications) costs were calculated from HRG codes reported in the resource cost initiative database (NHS Executive75). The cost of device reprogramming was assumed to include a cardiological consultation, pacing check and electrocardiography. Costs associated with device upgrade, severe pacemaker syndrome and AV block were excluded, as they were assumed to involve the same type of resource use and, therefore, cost as device reprogramming. Costs associated with mild pacemaker syndrome were assumed to be equivalent to those associated with a routine follow-up visit.
| Cost | Single-chamber pacing | Dual-chamber pacing |
|---|---|---|
| Device | VVI: £690 | DDD: £1365 |
| VVIR: £1099 | DDDR: £2107 | |
| Atrial lead: £175 | Atrial lead: £175 | |
| Ventricular lead: £172 | Ventricular lead: £172 | |
| Device implantation | £4025 | £4925 |
| Perioperative complications | £816 | £894 |
| Subsequent complications | £816 | £894 |
| Upgrade to dual chamber | £4925 | N/A |
| Reprogramming dual chamber to act as single-chamber ventricular | N/A | £176 |
| Cost (per cycle) | ||
| Follow-up | £40 | £40 |
| Mild pacemaker syndrome | £40 (VP only) | N/A |
| Severe pacemaker syndrome | £176 (VP only) | N/A |
| AV block prior to upgrade | £176 | N/A |
| AF | £41 | £41 |
| Stroke | £820 | £820 |
| HF | £152 | £152 |
Broad assumptions were made regarding the resource use and cost associated with the management of AF, stroke and HF. Estimates of treatment allocation for people with AF were taken from two studies, with costs for antithrombotics taken from a cross-sectional community study carried out in 199876 and costs for digoxin, beta-blockers and calcium channel blockers based on the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial. 77–79 In addition, people with chronic AF were assumed to make eight visits per year to their general practitioner (GP). Those on warfarin were assumed to require a further two specialist outpatient visits and a further eight anticoagulation clinic visits. Resources used after a stroke were derived from a UK study of resource use in people living with stroke80 and combined with 2003 community care and NHS reference costs. The resource use and cost assumed to be associated with HF is stated as being ‘based on assumptions regarding hospital admission and drug use’. 49
Health-related quality of life
Quality of life was incorporated into the model with the use of weights (utilities) associated with time spent in each of the model’s health states, adjusting the value of that time with respect to the severity of the health state. The majority of these weights were identified from a study by Lopez-Jimenez et al. ,10 which reported health values elicited and valued by a subset of patients enrolled in the PASE trial. 71 Table 27 summarises the utility weights used for each health state in the NICE’s TA8818 models.
| Health state | Utility | Source |
|---|---|---|
| Pacemaker implant | 0.76 | Lopez-Jimenez et al.10 |
| Perioperative complications | 0.75 | Assumption based on Lopez-Jimenez et al.,10 0.01 less than pacemaker implant |
| Subsequent complications | ||
| Well with pacemaker | 0.925 | Lopez-Jimenez et al.10 |
| Mild pacemaker syndrome | 0.80 | Equivalent to people with NYHA class I or II HF, Lopez-Jimenez et al.10 |
| Severe pacemaker syndrome | 0.62 | Equivalent to people with NYHA class III or IV HF, Lopez-Jimenez et al.10 |
| AV block prior to upgrade | 0.76 | Lopez-Jimenez et al.10 |
| Upgrade to dual-chamber pacemaker | 0.915 | Lopez-Jimenez et al.10 |
| Perioperative complications during upgrade | ||
| AF | 0.875 | Hogenhuis et al.81 |
| Reprogramming dual chamber to single-chamber ventricular following onset of AF | 0.875 | Assumed equal to AF |
| Stroke | 0.64 | Tengs et al.82 |
| HF | 0.39 | Lopez-Jimenez et al.10 |
Summary of results
For each of the three treatment choices considered in NICE’s TA88,18 the ICERs estimated deterministically are as follows:
-
dual- versus single-chamber ventricular pacemakers in people with AV block: £8458 per QALY over 5 years, £5483 per QALY over 10 years
-
dual- versus single-chamber ventricular pacemakers in people with SSS: £9552 per QALY over 5 years, £5732 per QALY over 10 years
-
dual- versus single-chamber atrial pacemakers in people with SSS: dual-chamber pacemakers are dominated by (i.e. are more costly and less effective than) single-chamber atrial pacemakers over 5 and 10 years.
Univariate sensitivity analysis identified the cost of implantation and the incidence, duration and utility associated with mild pacemaker syndrome as key drivers of the deterministic cost-effectiveness results. In addition, mortality and the incidence of AF were noted as having a moderate effect on the ICERs associated with each treatment choice. Probabilistic sensitivity analysis highlighted a high degree of uncertainty in the models, with results spread across the four quadrants of the cost-effectiveness plane.
Critique
Overall, the work carried out by Castelnuovo et al. 49 was of high quality. The model structure used coherently maps the clinical pathway of the health condition under consideration and all model assumptions have been clearly stated and justified. The use of shorter (5 years) and longer (10 years) time horizons is useful for understanding the potential impact of the broad assumptions made regarding the sequelae of AF, HF and stroke. The health states considered are generally appropriate. However, no rationale is provided for the exclusion of subsequent complications following upgrade to a dual-chamber pacemaker. Data on which the models are based were predominantly identified systematically, with quality assessment of source data carried out and choices between sources justified. However, the identification of some data sources, for example Chugh et al. 69 for the progression to stroke after AF, has not been explained. Treatment effects have been appropriately synthesised using the best techniques and data available at the time. Extrapolation has been described and justified and the potential impact explored in a sensitivity analysis. All costs and QoL weights included in the model have been clearly justified and calculated. However, the utility associated with an upgrade procedure [0.915, after application of a disutility of 0.01 to the utility associated with being well with pacemaker (0.925)] seems high and may overestimate the benefit of single-chamber pacemakers. In general, results have been sufficiently explained and contextualised by the existing literature and areas of remaining uncertainty, for example conflicting trial results, have been highlighted.
Clarke et al.51
Clarke et al. 51 carried out a retrospective follow-up of patients implanted with single-chamber atrial pacemakers in a single centre between 1992 and 1996. The aim of the study was to ascertain the rate of development of AV block and estimate potential cost-saving from use of single-chamber atrial pacing instead of dual-chamber pacing in people with SND and no AV block. A retrospective analysis of case notes identified 81 patients implanted with a single-chamber atrial pacemaker between 1992 and 1996. Of these, eight patients died during the analysis period and case notes were unobtainable for five patients. Fifteen (22%) of the 68 patients for whom case notes were available required a revision procedure. Of these, 10 patients (67%) required revision as a result of complications or manufacturer recall and four (5.8%) patients required revision following the development of AV block. Based on these data and on the cost of implantation (£2885 and £3844 for single-chamber and dual-chamber pacemakers, respectively), Clarke et al. estimated cost-savings of £103,000 a year associated with the use of single-chamber atrial pacemakers instead of dual-chamber pacemakers in people with SND and no AV block.
Critique
The cost savings estimated by Clarke et al. seem to be based on the assumption that an additional cost (i.e. the cost of upgrade) would be accrued only by patients developing AV block. The additional cost associated with revision procedures after experiencing complications does not seem to have been taken into account. In addition, the sample size (n = 68) is small and based on the experience of a single health-care centre; therefore, these data cannot be assumed to reliably inform the rate of development of AV block in people with SND.
Osman et al.55
The aim of the study reported by Osman et al. 55 was to assess the safety and potential cost savings associated with same-day procedures (as opposed to procedures followed by an overnight stay) for implantation of new pacemaker devices (i.e. first pacemaker implants). The study used retrospective safety and cost data from a single centre to assess the level of complications associated with a same-day procedure in patients scheduled for a new pacemaker implant.
Summary of results
Records from 780 patients intended for same-day implantation of a new pacemaker were identified for the period of April 2001 to December 2006. Box 2 summarises the immediate (occurring < 24 hours after implantation) and early (occurring > 24 hours and < 6 weeks after implantation) complications recorded and the reason for any unplanned overnight stay.
-
Haematoma (12 patients).
-
Pneumothorax (three patients).
-
Observation at physician’s request (13 patients).
-
Social reasons (seven patients).
-
The development of angina (three patients).
-
AF (one patient).
-
Warfarin with INR > 2.0 (two patients).
-
Displaced atrial leads (two patients).
-
Elevated ventricular threshold (one patient).
-
Sensing problems on the atrial lead (two patients).
-
Haematoma (one patient).
-
Lead displacements (five patients).
-
High pacing thresholds (six patients).
-
Wound infection (three patients).
-
Sensing problems (two patients).
-
Subclavian vein thrombosis (one patient).
INR, international normalised ratio.
The cost associated with an overnight stay (£203.60) was obtained from the centre’s finance department. Based on this and on retrospective assessment of procedures carried out from November 2005 to November 2006 (109 procedures, of which two required an unplanned overnight stay), the authors concluded that savings of £21,785 [(109 × £203.60) – (2 × £203.60)] were made for the year November 2005 to November 2006.
Critique
The study by Osman et al. 55 provides a useful insight into the potential complications associated with pacemaker implantation procedures in the UK. Although the cost information provided is limited to the cost of an overnight stay, details are given regarding, for example, the use of pre- and peri-implantation antibiotics, which may be useful to inform a de novo economic evaluation.
Sutton et al.48
Sutton et al. 48 carried out a comparison of costs and benefits associated with atrial (AAI/DDD) versus ventricular (VVI) pacing in people with SSS or AV block. The analysis was carried out over a 10-year time horizon; however, no discounting was applied to costs or benefits. A generic unit of currency, based on 1991 UK prices, was used to inform all estimates of cost. Furthermore, the perspective of the analysis was not explicitly stated.
Model structure and assumptions
The authors state that a ‘computer model’48 was developed to estimate the incidence and prevalence of complications considered within the analysis, namely AF, AV block, stroke and any resulting disability, HF, pacemaker upgrade and mortality. The number of surviving patients with AF, stroke, disability as a result of stroke or HF was calculated and recorded annually for:
-
patients with SSS initially implanted with a VVI device
-
patients with SSS upgraded to a DDD device
-
patients with SSS initially implanted with a DDD device
-
patients with AV block initially implanted with a VVI device
-
patients with AV block upgraded to a DDD device
-
patients with AV block initially implanted with a DDD device.
In addition, the following assumptions were made in relation to the analysis:
-
Stroke occurs in 30% of AF cases.
-
Thirty per cent of strokes result in long-term disability.
-
Mortality is equivalent for patients with and without HF or AF.
-
No generator change or lead replacement is required within the 10-year time horizon.
Outcome data
A literature review was carried out to inform estimates of the incidence of AF, AV block, HF, stroke and mortality. Outcome data used in the analysis carried out by Sutton et al. 48 are summarised in Table 28. It is unclear how estimates of mortality have been derived, that is, the authors report an average mortality (based on 13 studies) of 6.4% and 3.6% per annum in SSS patients paced with VVI and DDD devices, respectively. However, the estimates of mortality used to inform the analysis are 6% and 3% for SSS patients paced with VVI and DDD devices, respectively. Furthermore, the source of mortality estimates for AV block patients paced with VVI (7% per annum) and DDD (5% per annum) devices is not stated. In addition to the lack of clarity regarding estimates of mortality, the percentage of patients assumed to experience stroke is 30%, whereas evidence from the literature review suggests a 39% stroke-to-AF ratio. Finally, the source(s) used to estimate the incidence of pacemaker syndrome is (are) not provided.
| Outcome | SSS | AV block | ||
|---|---|---|---|---|
| VVI (%) | DDD (%) | VVI (%) | DDD (%) | |
| Year 1 | ||||
| AF | 10 | 2 | 5 | 1 |
| Stroke | 3 | 0.6 | 1.5 | 0.3 |
| Disability | 0.9 | 0.2 | 0.45 | 0.09 |
| HF | 6 | 2 | 6 | 25 |
| Pacemaker syndrome | 2 | 0 | 2 | 0 |
| Mortality | 6 | 3 | 7 | 5 |
| Year 2 onwards | ||||
| AF | 7 | 1.5 | 3 | 0.5 |
| Stroke | 2.1 | 0.45 | 0.9 | 0.15 |
| Disability | 0.63 | 0.14 | 0.27 | 0.045 |
| HF | 6 | 2 | 6 | 2 |
| Pacemaker syndrome | 2 | 0 | 2 | 0 |
| Mortality | 6 | 3 | 7 | 5 |
Resource use and cost data
As noted above, a generic unit of currency is used to inform all estimates of cost. The reference cost for this currency is the 1991 UK price of a VVI device, which is set as 100 currency units. 48 Table 29 summarises the costs used in the analysis by Sutton et al. 48
| Cost component | VVI | DDD | Source |
|---|---|---|---|
| Pulse generator | 100 | 166 | Six manufacturers active on the UK market |
| Leads | 13 | 26 | |
| Implantationa | 117 | 148 | Single-centre costs |
| Follow-up | 4 | 8 | |
| Upgradeb | 340 | – | |
| AF treatment | 10 | 10 | |
| Strokec | 100 | 100 | |
| Disability | 1733 | 1733 | Local area costs of long-term care |
| HFd | 243 | 243 | UK drug prices, single-centre care costs |
Summary of results
Based on the inputs listed above, the analysis carried out by Sutton et al. 48 estimated that survival is increased with atrial pacing. By contrast, twofold and fivefold reductions are estimated in HF and disability from stroke, respectively. Assessment of costs associated with atrial pacing and VP suggests that there are equal cost implications to both pacing modes 3 years after implantation. However, in SSS patients, the 10-year cumulative cost of VVI pacing is 12 times that of DDD pacing. Furthermore, the 10-year cumulative cost of VVI pacing in patients with AV block is eight times that of DDD pacing in the same indication.
Univariate sensitivity analysis was carried out to assess the impact of AF incidence, mortality, stroke incidence (as a proportion of AF incidence), disability costs and the incidence of HF on the modelled cost estimates. In SSS patients, the cost of DDD pacing increases with increasing disability costs and increasing stroke incidence, but at a faster rate than the cost of VVI pacing. Conversely, DDD costs increase at a lower rate than VVI costs with increasing AF and HF incidence. However, it is important to note that sensitivity analysis around the incidence of AF assumes no difference between VVI and DDD pacing, an assumption that is unlikely to be reflected in clinical practice.
In AV block patients, the cost of DDD pacing increases more rapidly than the cost of VVI pacing with increasing AF and HF incidence. Conversely, costs of DDD pacing increase more slowly than the cost of VVI pacing with increasing disability costs and stroke incidence.
Critique
The study by Sutton et al. ,48 although based predominantly on a review of the literature, is limited in that estimates of incidence obtained from the literature are simply pooled (i.e. an average taken) without adjustment for patient characteristics. This is likely to introduce potentially considerable bias into estimates of ongoing incidence and prevalence of common pacemaker sequelae. In addition, the use of a generic currency unit based on unpublished costs does not facilitate uprating of costs to current prices. Therefore, while providing a potentially useful source of health states and structural assumptions that may be used to inform a de novo economic evaluation, the analysis carried out by Sutton et al. 48 is not informative because of methodological limitations.
Narrative summary of included non-UK economic evaluations
Oddershede et al.54
Oddershede et al. 54 considered the cost–utility, from a Danish health-care system perspective, of dual-chamber versus single-chamber atrial pacemakers in people with SSS and preserved AV conduction. Costs and benefits (QALYs) were discounted at a rate of 3.5% per annum over a period of 60 years to estimate lifetime costs and effects. A Markov model comprising four health states (well, first stroke, second stroke and dead) was used to analyse cost-effectiveness. Patients without a history of stroke entered the model in the health state well, whereas those with a history of stroke entered the model in the health state first stroke. The model had monthly cycles and allowed patients to develop up to seven strokes.
The authors estimated cost-effectiveness using three approaches: an adjusted and an unadjusted approach using data from the DANPACE trial19 and an adjusted pooled approach using data from the DANPACE trial19 pooled with two other Danish clinical trials. 41,45,83 Patients were divided according to predicted survival probability into three groups to account for heterogeneity, with group 1 categorised as the group at highest risk of death and group 3 at the lowest risk of death. A Cox proportional hazards model was used to estimate the characteristics of the three groups. Survival probability was reported to change with:
-
age
-
sex
-
previous myocardial infarction
-
history of AF
-
proportion of patients entering the model at the health state first stroke.
The cost-effectiveness of DDDR was assessed by calculating net monetary benefit, which combines lifetime costs and QALYs. Therefore, a net monetary benefit greater than zero indicated that DDDR was cost-effective. A probabilistic sensitivity analysis was carried out to test the robustness of the results.
Outcome data
Stroke occurrence and death were the outcomes of interest. The authors justified not including HF based on the findings reported by Riahi et al. ,40 who found no statistically significant difference in occurrence of HF by pacing mode. Patient-level data on clinical effectiveness from the DANPACE trial19 were pooled with data from two other Danish RCTs reported by Andersen et al. 45,83 and Nielsen et al. 41
Resource use and cost data
Resource use with initial pacemaker implantation was collected from the DANPACE trial,19 with data collected on surgery, complications and duration of initial hospitalisation. The model assumed that patients had outpatient follow-up visits at 3 months, 2 years, 4 years and every subsequent year, as per routine practice at Aalborg University Hospital, Denmark. Costs were calculated in Danish kroner (2012 prices) and then converted to pounds sterling at a rate of £1 = 8.73 DKK. The cost of an outpatients clinic was £101 (SE £10), with stroke and death costing £13,348 (SE £1335) and £1314 (SE £131), respectively.
Summary of results
Cost-effectiveness was reported based on the different approaches used and disaggregated according to risk groups for the adjusted analysis. Probability of cost-effectiveness was calculated across 10,000 simulations at willingness-to-pay (WTP) thresholds of £20,000 and £30,000. Table 30 shows a summary of the cost-effectiveness results.
| Population | Incremental cost (£) | Incremental benefit (QALYs) | Net monetary benefit (£) at a WTP of £20,000 per QALY | Net monetary benefit (£) at a WTP of £30,000 per QALY | Probability DDDR is cost-effective at a WTP of £20,000 per QALY (%) | Probability DDDR is cost-effective at a WTP of £30,000 per QALY (%) |
|---|---|---|---|---|---|---|
| Adjusted approach | ||||||
| Risk group 1 | –3336 | –0.022 | 2918 | 2694 | 77 | 69 |
| Risk group 2 | –2570 | –0.029 | 1996 | 1709 | 60 | 55 |
| Risk group 3 | –5045 | –0.041 | 4220 | 3442 | 64 | 59 |
| Adjusted pooled approach | ||||||
| Risk group 1 | –4170 | –0.103 | 2103 | 1069 | 71 | 58 |
| Risk group 2 | –3856 | –0.170 | 460 | –1238 | 51 | 42 |
| Risk group 3 | –7521 | –0.218 | 3160 | 980 | 62 | 51 |
| Unadjusted approach | ||||||
| All patients | –2310 | 0.277 | 7847 | 10,615 | 88 | 86 |
Critique
The analysis carried out by Oddershede et al. 54 was clearly reported, with baseline characteristics of patients, utility values and resource cost from the DANPACE trial19 all presented in the paper. The model seemed reasonable and accounted for clinical heterogeneity in patient populations, which makes the results more robust. A weakness of the pooled analysis is the fact that the combined clinical effectiveness data included a study that compared single atrial pacing with single VP. 45 The authors reported that single VP was excluded from the analysis, which implies that data from a randomised trial were pooled without a comparator arm. This would mean breaking the benefits of randomisation and turning the data set into observational data. In addition, a breakdown of the stroke and death costs would have been useful, particularly with death reported to cost the equivalent of £1314.
Deniz et al.52
Deniz et al. 52 considered the cost–utility, from an Italian government perspective, of dual-chamber versus single-chamber ventricular pacemakers in people with bradycardia as a result of SND or AV block. Costs and benefits (QALYs) were discounted at a rate of 3% per annum over a 5-year time horizon. The authors adapted a discrete event simulation that was originally developed to assess the cost–utility of dual-chamber pacemakers for the management of bradycardia as a result of SND or AV block in the UK50 to consider an Italian government perspective. The model structure used in the analysis reported by Deniz et al. 52 is identical to that described by Caro et al. 50 Similarly, outcome data used in the economic analysis reported by Deniz et al. 52 are identical to those used to inform the economic analysis reported by Caro et al. 50
Resource use and cost data
The analysis reported by Deniz et al. 52 included the costs associated with devices, initial implantation, device replacement, anticoagulation and stroke. Device costs were obtained in Deniz et al. 52 from a personal communication from the medical devices company Medtronic Europe. All other included costs were obtained from ‘Regional information published for specific diagnosis-related groups in Italy’ via the Italian National Institute of Statistics website. 84 The cost associated with stroke was assumed to be equivalent to that associated with a stroke-related hospitalisation. The cost of anticoagulation included warfarin at a dose of 5 mg per day and a physician visit.
Summary of results
Based on 100 replications of 1000 simulated patients, the ICER estimated for dual-chamber versus single-chamber VP was €260 per QALY [equivalent to £215 per QALY (converted on 20 January 2014 by http://markets.ft.com/research/Markets/Currencies)]. Univariate sensitivity analysis revealed that the result was sensitive to assumptions regarding the percentage of patients assumed to upgrade to a dual-chamber device following the onset of pacemaker syndrome. In other words, under the assumption that 5% of patients (rather than 16.7% as in the base case) experiencing severe pacemaker syndrome upgrade to a dual-chamber device, the ICER rises to €14,233 per QALY; however, this does not take account of any reduction in HRQoL for patients with severe pacemaker syndrome. Multivariate sensitivity analysis based on 1000 replications, of 1000 simulated patients, in which parameter uncertainty is included, estimated that dual-chamber devices provided more benefit at a lower cost in 45% of replications.
Critique
Akin to the analysis carried out by Caro et al. ,50 the analysis reported by Deniz et al. 52 is based on a reasonable model of bradycardia in people with SND or AV block; however, consideration of HF as a potential sequelae would have provided greater face validity to the analysis. Similarly, a longer time horizon would have enabled a full comparison of costs and benefits accrued over the lifetime of the devices considered. Further detail on the derivation of stroke costs and QoL weights would have been useful to the critical appraisal of this analysis. Assessment of structural and methodological uncertainty would also have contributed to the robustness of the analysis.
Mahoney47
The study reported by Mahoney47 compares (without the use of modelling) costs and outcomes associated with single-chamber ventricular (VVI) pacing versus single-chamber atrial (AAI) pacing and dual-chamber (DDD) pacing; the patient population is not specified. The study purports to assess the long-term costs of care for patients receiving each type of pacing considered. However, the time horizon of the analysis is not stated and no discounting is applied to costs or benefits.
Outcome data
The outcomes considered in the comparison are AV block, AF, congestive heart failure (CHF), pacemaker syndrome, stroke, thromboembolism and mortality. A meta-analysis of ‘35 published studies comparing dual and single chamber [pacing] modes’ is reported (no reference supplied) as the source of data on the considered outcomes. Based on the meta-analysis, Mahoney states that, compared with VVI pacing, DDD pacing significantly reduces the incidence of AF, pacemaker syndrome, thromboembolism, stroke and mortality. When compared with VVI pacing, AAI pacing is reported to reduce significantly the incidence of AF, thromboembolism, stroke, CHF and mortality. However, the probability of development of AV block is reported as being greater in people implanted with AAI than in those with VVI pacemakers.
Resource use and cost data
The ‘long-term’ costs of care for people receiving each considered pacing mode include device costs (source not stated) and the cost of treating outcomes associated with pacing, for example AF. The ‘national average urban Diagnostic Related Group payment (e.g. Minneapolis, MN)’47 is used to inform the costs associated with treatment of outcomes.
Summary of results
The overall cost of VVI is reported to be 24–27% higher than DDD and 34–35% higher than AAI. In addition, the cost of treating patients for AF, CHF, stroke and pacemaker syndrome is higher in VVI than in DDD pacing and higher still in VVI than in AAI pacing.
Critique
The study carried out by Mahoney47 is of poor quality, with an absence of references. Although the outcomes included are reasonable, the lack of referencing prevents validation of the comparative treatment effects. In addition, it is unclear which elements are included in the cost of treating outcomes and the time period over which these costs are considered.
O’Brien et al.53
O’Brien et al. 53 carried out an economic evaluation alongside the CTOPP. The CTOPP considered the effects of physiological (dual-chamber or single-chamber atrial) pacing compared with VP in people without chronic AF indicated for initial pacemaker implantation for the management of symptomatic bradycardia. 62 Resource use and cost data were collected from a subset (n = 1058) of patients enrolled in CTOPP (n = 2568) and adjusted for censoring using methods described by Lin et al. 85 Life expectancy and the number of AF episodes by type of pacing (physiological vs. ventricular) were estimated from the full trial population of CTOPP.
Summary of results
Cost-effectiveness was assessed per life-year gained and per AF episode avoided, with results further disaggregated into subgroups by intrinsic (unpaced) heart rate (IHR; IHR ≤ 60 b.p.m. or IHR > 60 b.p.m.). Table 31 summarises the cost-effectiveness results reported by O’Brien et al. 53
| Patient group | Incremental cost, C$ | Incremental benefit, C$ | ICER, C$ (£) |
|---|---|---|---|
| Per LYGa | |||
| All patients | 2976 | 0.01 | 297,600 (164,611) |
| IHR ≤ 60 b.p.m. | 4091 | 0.25 | 16,004 (9040) |
| IHR > 60 b.p.m. | 2020 | –0.11 | Physiological pacing dominated by VP |
| Per AF episode avoideda | |||
| All patients | 2976 | –0.04 | 74,000 (40,931) |
| IHR ≤ 60 b.p.m. | 4091 | –0.04 | 102,275 (56,571) |
| IHR > 60 b.p.m. | 2020 | –0.04 | 40,400 (22,346) |
Critique
Although of limited relevance to the decision problem that is the focus of this MTA, the economic evaluation carried out by O’Brien et al. 53 is robust with respect to methods of analysis and data used. Furthermore, this economic evaluation may provide a useful external validation of resource use, AF incidence and life expectancy in people implanted with physiological pacemakers.
Rinfret et al.56
Rinfret et al. 56 assessed the cost–utility of dual-chamber (DDDR) versus single-chamber ventricular (VVIR) pacemakers in people paced for SSS. Analyses was carried out from a US societal perspective across a within-trial time horizon (4 years) and a lifetime time horizon, with costs and benefits discounted at 3% per annum.
Model structure and assumptions
Within-trial analysis used Kaplan–Meier survival data to adjust yearly estimates of cost and utility obtained from the MOST. 44,68,86 These data were extrapolated over a lifetime time horizon using a Markov model calibrated to trial data. Within the Markov model, individuals were classified according to their current mode of pacing and their history of AF, HF or stroke. State-specific costs and utilities were estimated using multiple linear regression models that incorporated the following independent (or predictor) variables:
-
initial pacing mode
-
year of trial (year 1 vs. years 2 to 5)
-
crossover in previous year
-
crossover during current year
-
non-fatal event (AF, HF or stroke) during current year
-
one prior non-fatal event
-
two or more prior non-fatal events
-
death in current year.
Outcome data
Data on the incidence of crossover from VVIR to DDDR pacing as a result of pacemaker syndrome, AF, HF, stroke and event-specific mortality were collected from the MOST. 68 These data were supplemented with age- and sex-adjusted data from US life tables and expert opinion on the requirement for generator replacement. Utility data were elicited directly from patients enrolled in the MOST using ‘a standard [time trade-off] instrument’. 56
Resource use and cost data
Detailed data on resource use gathered as part of the MOST were used to inform the analyses carried out by Rinfret et al. 56 Table 32 displays the majority of costs considered in the analyses of Rinfret et al. 56 In addition to the costs summarised in Table 32, medication costs for each class of prescription drugs reported in the MOST were obtained from the 2001 Redbook,85 and were based on doses considered to be clinically average.
| Cost component | DDDR (US$) | VVIR (US$) | Source |
|---|---|---|---|
| Initial pacemaker implantation | |||
| Device cost | 7720 | 5277 | IMS Hospital Supply Index™ |
| Procedure costs | 1894 | 1732 | Single-centre costs |
| Post-procedure hospitalisation | 901 | 877 | Single-centre costs |
| Physician fees | 688 | 679 | Medicare physician fee schedule |
| Follow-up (per annum) | |||
| Year 1 | 4387 | 3825 | MOST68 |
| Subsequent years | 3328 | 2766 | MOST68 |
| Events (one-off cost) | |||
| Crossover to DDDR | – | 14,451 | Single-centre costs |
| First non-fatal event occurring in current year | 4529 | 4529 | MOST68 |
| Second non-fatal event occurring in current year | 11,261 | 11,261 | MOST68 |
| Death occurring in current year | 6878 | 6878 | MOST68 |
| Generator change | 7100 | 5737 | IMS Hospital Supply Index |
Summary of results
A within-trial cost–utility analysis estimated an ICER of US$52,814 per QALY for DDDR versus VVIR pacing over 4 years. A lifetime cost–utility analysis estimated an ICER of US$6800 per QALY for DDDR versus VVIR pacing. Bootstrap analysis (1000 samples with replacement) estimated that, at a WTP threshold of US$50,000 per QALY, DDDR pacing was cost-effective in 91.9% of all samples.
Univariate sensitivity analysis revealed that the model was highly sensitive to the cost associated with implantation of a dual-chamber pacemaker and to assumptions regarding generator lifespan. Cost-effectiveness results were also moderately sensitive to follow-up costs and QoL by pacing mode.
Critique
The analyses carried out by Rinfret et al. 56 are clearly described and are underpinned by high-quality evidence. The use of calibration to ensure consistency between modelled and observed outcomes is a key strength of the Markov-based analysis. However, the use of single-centre costs somewhat inhibits the generalisability of the results.
Wiegand et al.58
Wiegand et al. 58 considered the costs and benefits of single-lead VDD pacemakers compared with DDD pacing in patients with AV block and normal sinus node function. The analysis was carried out based on clinical data gathered in a single-centre prospective study, over an average time horizon of 42 months. No discounting was used and HRQoL was not considered.
Outcome data
Kaplan–Meier data were used to assess the maintenance of AV synchrony and event-free survival in patients paced with a VDD versus a DDD pacemaker, and data were compared with the log-rank test.
Resource use and cost data
Resource use was categorised as primary or secondary. Primary resource use was assumed to be any resource associated with initial pacemaker implantation, and included two nights of hospital stay, three doses of the antibiotic cefacolin (Elzogram®, Eli Lilly, Basingstoke, UK), one routine pacemaker interrogation, one 24-hour Holter electrocardiogram and one chest radiograph.
Resources used in the ongoing management of pacemaker patients were categorised as secondary and included prolonged stay or readmission of patients; laboratory examinations; antibiotic therapy; additional chest radiography, Holter recordings and pacemaker interrogations; operative revision, device explantation and reimplantation; and the treatment of atrial arrhythmias.
The cost associated with devices, leads, single-use operation material and sterilisation were estimated from the average cost of each incurred by a single centre. Fees for implanting physicians, nurses and medical technicians were sourced from German standard implantation charges.
Summary of results
No significant differences in the occurrence of AF, cardiac disease or pacemaker-related complications were identified between patients paced with VDD compared with DDD devices. Similarly, no significant differences in the event-free survival of the two patient groups were observed. However, cumulative costs of DDD pacing were significantly higher than for VDD pacing. The authors concluded that this was likely to be a consequence of the higher hardware and initial implantation costs associated with DDD devices, which is further compounded by higher follow-up costs.
Critique
The study by Wiegand et al. 58 is thorough and transparent, with assumptions and potential limitations clearly stated. However, the time horizon considered is unlikely to be sufficient to capture the full cost–benefit of VDD versus DDD devices. With respect to transferability of the study findings to different health-care systems, the authors state that the assumptions and standardisations carried out to calculate costs can be reliably transferred. Although application of the assumptions made to different settings may not be entirely feasible given the variation in care across health-care centres and countries, the transparency of the study as described by Wiegand et al. 58 may facilitate the comparison of resource use assumptions.
Narrative summary of included costing studies
Ray et al.57
The aims of the study carried out by Ray et al. 57 were twofold: first, to assess the impact of the 1990 BPEG guidelines on clinical practice and, second, to assess the impact of full guideline adherence on cost. An audit of patients undergoing first pacemaker implant for the period of March 1990 to August 1991 was carried out and these data were used to assess changes in clinical practice and average device costs.
Summary of results
The 1990 BPEG guideline recommendations by pacing indication are summarised in Table 33. Also presented are the percentage of patients implanted with recommended devices during the study period, the average cost of recommended devices as estimated by Ray et al. 57 and the estimated cost of full guideline adherence. Based on these data, the authors estimated that full adherence to BPEG recommendations would increase the annual budget for pacing hardware by 94% or 61% with respect to optimal or alternative pacing recommendations, respectively.
| Pacing indication | BPEG recommended pacing mode | Percentage of recommended pacing mode used in study period (%) | Average cost of recommended pacing mode (£) | Cost of full guideline adherence (£)a | ||||
|---|---|---|---|---|---|---|---|---|
| Optimal | Alternative | Optimal | Alternative | Optimal | Alternative | Optimal | Alternative | |
| SND (148 patients) | AAIR | AAI | 5.4 | 27.0 | 1642 | 927 | 243,016 | 137,196 |
| AV block (329 patients) | DDD | VDD | 15.8 | 0 | 1811 | Unknown | 595,819 | Unknown |
| SND and AV block (6 patients) | DDDR | DDD | 16.7 | 33.3 | 1992 | 1811 | 11,952 | 10,866 |
| AV block and AF (52 patients) | VVIR | VVI | 13.5 | 86.5 | 1773 | 631 | 92,196 | 32,812 |
| CSS/MVVS (15 patients) | DDI | – | 53.3 | – | 1845 | – | 27,675 | – |
Critique
The study by Ray et al. 57 provides information on the cost of devices incurred by a single centre in 1991 and is, therefore, of limited use to inform an up-to-date economic evaluation. However, the study itself seems to have been well conducted, if poorly reported.
Summary and conclusions of available cost-effectiveness evidence
Aside from the work carried out to inform NICE’s TA88,18 no economic evaluations considering the cost-effectiveness of dual-chamber versus single-chamber atrial pacemakers in a UK health-care setting were identified by the TAG’s systematic review. Castelnuovo et al. 49 considered the impact of complications, upgrade (as a result of AV block), AF, HF, stroke and death on the cost–utility of dual-chamber versus single-chamber atrial pacemakers. Based on the clinical evidence available at the time of NICE’s TA88,18 single-chamber atrial pacing was estimated to dominate dual-chamber pacing for patients with SSS and no AV block. However, new clinical evidence of the relative effectiveness of dual-chamber versus single-chamber atrial pacing has emerged.
In addition to the cost–utility of dual-chamber versus single-chamber atrial pacing, Castelnuovo et al. 49 considered the cost–utility of dual-chamber versus single-chamber VP. Similar to the analysis of dual-chamber versus single-chamber atrial pacing, these analyses considered the impact of complications, upgrade (as a result of pacemaker syndrome), AF, HF, stroke and death on estimates of cost–utility.
Subsequent to the publication of NICE’s TA88,18 four evaluations of the cost-effectiveness of dual-chamber (or physiological) pacing versus single-chamber VP have been published. 50,56,52,53 Of these, two were based on an evidence submission from the ABHI (carried out by Caro Research) that was submitted as part of NICE’s TA88. 50,52 These evaluations employed a discrete event simulation considering the impact of implantation-related complications, pacemaker syndrome (potentially resulting in an upgrade procedure), AF, stroke and death on the cost–utility of dual-chamber versus single-chamber ventricular pacemakers. Of the two remaining cost-effectiveness analyses, one was carried out alongside the CTOPP53 and considered costs in relation to the relative extension of life or prevention of AF in patients paced with dual-chamber versus single-chamber ventricular pacemakers. The remaining study assessed the impact of complications, upgrade as a result of pacemaker syndrome, AF, HF, stroke and death on the cost–utility of dual-chamber versus single-chamber ventricular pacemakers. 56
In the updated search conducted by the TAG, a cost-effectiveness analysis that included the DANPACE trial19 was identified. 54 The main strength of this evaluation was that it was informed by individual patient-level data. However, it was conducted from the perspective of the Danish health-care system and principally focused on the occurrence of stroke or death as the clinical outcomes of interest in the model.
Therefore, based on review of the current economic literature, the TAG considered there to be a need for a de novo economic analysis of dual-chamber versus single-chamber atrial pacing in people with bradycardia as a result of SSS and with no AV block. Furthermore, review of the economic literature around pacing revealed common consideration of the following pacemaker sequelae:
-
peri- and post-operative complications
-
the potential for upgrade requirements in people paced with single-chamber devices
-
onset of AF
-
HF
-
stroke
-
cardiovascular mortality
-
all-cause mortality.
Consequently, following consultation with clinical experts, these outcomes were incorporated into the economic evaluation developed by the TAG (see Chapter 3, Results).
Independent economic assessment
Overview
The TAG constructed a de novo economic model in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) to estimate the cost-effectiveness of dual-chamber versus single-chamber atrial pacemakers in a population of patients with bradycardia as a result of SSS without AV block. A Markov model was utilised with monthly cycle length to carry out the analysis. The perspective used in the economic model is that of the NHS and Personal Social Services (PSS). Costs and benefits are discounted at 3.5% per annum and the model uses a monthly cycle length. Full details of the population modelled, model structure used, inputs, outputs and sensitivity analyses are presented in the sections that follow.
Comparison to scope
The final scope issued by NICE for this MTA is summarised in Table 34, alongside a commentary detailing to what extent the TAG’s economic analysis adheres to the scope.
| NICE’s scope | TAG’s de novo analysis | |
|---|---|---|
| Intervention | Permanent implantable dual-chamber pacemakers | Yes |
| Population(s) | People with symptomatic bradyarrhythmias due to SSS without AV block | Yes |
| Comparator | Single-chamber atrial pacemakers | Yes |
| Outcomes | The outcome measures to be considered include:
|
Partially. Clinical, but not economic, assessment of the relative difference in the outcomes of exercise capacity and cognitive function were carried out |
| Economic analysis | The reference case stipulates that the cost-effectiveness of treatments should be expressed in terms of incremental cost per QALY. The reference case stipulates that the time horizon for estimating clinical effectiveness and cost-effectiveness should be sufficiently long to reflect any differences in costs or outcomes between the technologies being compared. Costs will be considered from a NHS and PSS perspective | Yes, incremental cost per QALY assessed, time horizon is 10 years (to capture expected lifetime of devices), only costs relevant to a NHS and PSS perspective are included |
| Other considerations | Guidance will only be issued in accordance with the CE marking | NICE has formally requested information on CE marking from manufacturers, but it was not made available in time for inclusion in this MTA |
Population
The population that is the focus of this MTA is people with symptomatic bradyarrhythmias due to SSS with, after full evaluation, no evidence of impaired AV conduction. As discussed in Chapter 3, Assessment of effectiveness, from the trials identified in the TAG’s review of the clinical effectiveness literature, pooled estimates of clinical outcomes were available for:
Although the review of the clinical effectiveness literature identified six relevant studies, disparity across the trials in definition of and reporting of clinical outcomes precluded meta-analysis for several outcomes. Thus, estimates of the relative effect of dual-chamber versus single-chamber atrial pacemakers are not available for all clinical outcomes considered in the economic model. Furthermore, the data from which pooled estimates of stroke and change in pacing mode are derived differ by outcome. Based on this, and on clinical expert opinion of the reliability of the DANPACE trial,19 the TAG used data from the trial, rather than pooled estimates, to inform the base-case economic model. The impact of incorporating pooled estimates on the cost-effectiveness of dual-chamber pacemakers versus single-chamber atrial pacemakers, where possible, is explored in sensitivity analysis (see Approach to uncertainty).
The age and sex percentages of patients considered in the TAG’s de novo economic evaluation mirror the baseline age (73 years) and sex percentages (35% male) of patients enrolled in the DANPACE trial. 19 However, the percentages of patients with a history of AF (DANPACE: 44%), stroke (DANPACE: 8%) or HF (DANPACE: 12%) are assumed to be zero on entry into the economic model. This is a simplifying assumption (to avoid the need for multiple health states within the economic model) based on the use of measures of treatment effect (HRs) that are adjusted for potentially confounding factors, such as a history of AF, stroke or HF (see Treatment effectiveness).
Interventions and comparators
The interventions and comparators of interest in this MTA are dual-chamber pacemakers versus single-chamber atrial pacemakers. As discussed in Chapter 1, Description of technology under assessment, pacemakers may or may not be rate responsive, that is, having the functionality to sense and increase the heart rate in response to physical, mental or emotional activity. A variety of pacing modes are available in dual-chamber and single-chamber atrial pacemakers, for example:
-
DDDR-s, dual-chamber pacing with short AV delay (< 150 milliseconds)
-
DDDR-l, dual-chamber pacing with a fixed long AV delay (300 milliseconds).
The DANPACE19 trial, and therefore the TAG’s economic evaluation, considers DDDR (dual-chamber pacing with rate control) versus AAIR (single-chamber atrial pacing with rate control) pacemakers.
Model structure
The TAG economic model is a Markov cohort model consistent with that used in NICE’s TA88,18 of which this MTA is, in part, an update. Furthermore, to facilitate a comparison of the cost-effectiveness of dual-chamber pacemakers versus single-chamber atrial pacemakers, the model structure employed by the TAG is derived from that used in NICE’s TA88 to assess the cost-effectiveness of these interventions in people with SSS and no AV block (Figure 9). The cycle length of the model is 1 month, as, according to clinical experts, 1 month is sufficient for patients to feel the benefit of pacemaker implantation.
FIGURE 9.
Overview of TAG’s economic model structure. AVB, atrioventricular block.
Patients enter the model requiring a pacemaker and are assigned to receive either a dual-chamber pacemaker (implant dual-chamber pacemaker; n = 1000) or a single-chamber atrial pacemaker (implant single-chamber atrial pacemaker; n = 1000). After implantation of the pacing devices, patients transition into the ‘with pacemaker’ health states: the ‘with dual-chamber pacemaker’ and ‘with single-chamber atrial pacemaker’ health states.
The risk of reoperation is only possible for patients initially implanted with a single-chamber device (see Treatment effectiveness). Based on a subgroup analysis of reoperation data from the DANPACE trial,19 all patients requiring reoperation are assumed to receive a dual-chamber device. An analysis of reasons for reoperation in the DANPACE trial19 indicated that a statistically significantly larger percentage of people who initially received a single-chamber pacemaker required reoperation to change pacing mode compared with those who received a dual-chamber pacemaker (Table 35); all other reasons for reoperation were found to be not statistically significant.
| Reason for reoperation | Treatment arm | p-value | |
|---|---|---|---|
| AAIR, n (%) | DDDR, n (%) | ||
| Battery depletion | 59 (8.3) | 42 (5.9) | 0.09 |
| Change of mode of pacing | 66 (9.3) | 4 (0.6) | < 0.001 |
| Lead complications | 37 (5.2) | 30 (4.2) | 0.42 |
| Surgical or mechanical complications | 10 (1.4) | 7 (1.0) | 0.52 |
| Infection | 3 (0.4) | 3 (0.4) | 0.98 |
| Skin erosion | 1 (0.1) | 3 (0.4) | 0.31 |
| Device failure | 2 (0.3) | 2 (0.3) | 0.99 |
For people implanted with single-chamber atrial devices, the need to change pacing mode is predominantly a result of the development of AV block requiring upgrade to a dual-chamber device. Therefore, to capture this statistically significant difference in the need for reoperation between the two pacemaker types, the cost and QoL of patients requiring a reoperation was based on the difference in event rates between the two arms and was applied in the model solely to patients receiving a single-chamber atrial device. This simplification in the model was tested in a structural sensitivity analysis (see Approach to uncertainty).
Furthermore, to maintain the focus of the model on the statistically significant difference between the device arms attributed solely to reoperation to change pacing mode, only one instance of reoperation was permitted within the model time horizon.
Patients residing in the with pacemaker health states are at risk of developing the sequelae of AF, stroke or HF, and patients who develop HF or stroke remain at risk of reoperation; however, in the event of reoperation, patients do not transition from the HF or the stroke health states, but instead simply incur the cost of reoperation. Patients who develop AF are at risk of the further sequelae of HF or stroke. However, once patients develop AF they are at no further risk of reoperation, as, after consultation with clinical experts, it has been assumed that, on development of AF, pacing will either cease or patients will be given a VP device.
All patients are at risk of death, regardless of health state (see Mortality).
Overview of model parameters, sources and assumptions
A summary of parameters and the accompanying distributions used to inform the TAG’s economic model is provided in Table 36.
| Parameter | Mean value | Variance | Source | Section in this report |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 73 | Estimated 95% CI 50.85 to 95.15 | Assumption | Population |
| Probabilities dual-chamber pacemakers | ||||
| Paroxysmal AF | 0.23 | 95% CI 0.16 to 0.35a | Nielsen et al. 201119 (163 events, 708 patients) | Treatment effectiveness |
| Chronic AF | 0.11 | 95% CI 0.08 to 0.7a | Nielsen et al. 201119 (76 events, 708 patients) | Treatment effectiveness |
| HF | 0.24 | 95% CI 0.17 to 0.36a | Nielsen et al. 201119 (169 events, 708 patients) | Treatment effectiveness |
| Stroke | 0.05 | 95% CI 0.03 to 0.07a | Nielsen et al. 201119 (34 events, 708 patients) | Treatment effectiveness |
| HR: clinical sequelae for single-chamber atrial pacemakers compared with dual-chamber pacemakers | ||||
| Paroxysmal AF | HR 1.24 | 95% CI 1.01 to 1.52 | Nielsen et al. 201119 | Treatment effectiveness |
| Chronic AF | HR 1.01 | 95% CI 0.74 to 1.39 | Nielsen et al. 201119 | Treatment effectiveness |
| HF | HR 1.09 | 95% CI 0.88 to 1.35 | Riahi et al. 201240 | Treatment effectiveness |
| Stroke | HR 1.11 | 95% CI 0.70 to 1.77 | Nielsen et al. 201119 | Treatment effectiveness |
| Mortality | ||||
| Implantation health states | Age-specific rate of all-cause mortality from UK general population; weighted by the proportion of male and female patients modelled | N/A | ONS88 | Mortality |
| With pacemaker health states | ||||
| HR: mortality | ||||
| AF (vs. general population) | 2.08 | 95% CI 2.01 to 2.16 | Miyasaka et al. 200789 | Mortality |
| Stroke: males (vs. general population) | 3.59 | 95% CI 2.38 to 5.42b | Carter et al. 200790 | Mortality |
| Stroke: females (vs. general population) | 3.14 | 95% CI 2.26 to 4.38 | Carter et al. 200790 | Mortality |
| HF (vs. general population) | 1.32 | Estimated 95% CI 1.17 to 1.48 | Pocock et al. 200691 | Mortality |
| AF and stroke (vs. stroke population) | 1.33 | 95% CI 1.01 to 1.76 | Carter et al. 200790 | Mortality |
| AF and HF (vs. HF population) | 1.11 | 95% CI 1.00 to 1.23 | Pocock et al. 200691 | Mortality |
| Health state utility values | ||||
| Implant pacemaker | 0.73 | – | Fleischmann et al. 200692 | Health-related quality-of-life data |
| With pacemaker | 0.83 | – | Fleischmann et al. 200692 | Health-related quality-of-life data |
| Stroke | Month 1, 0.64 | 95% CI 0.15 to 1.00 | Luengo-Fernandez et al. 201393 | Health-related quality-of-life data |
| After 1 month, 0.70 | 95% CI 0.28 to 1.00 | |||
| Change from with pacemaker to AF | 0.02 | 95% CI 0.01 to 0.03 | Fleischmann et al. 200994 | Health-related quality-of-life data |
| AF and stroke | Month 1, 0.64 | 95% CI 0.15 to 1.00 | Assumption that values are the same as stroke without AF93 | Health-related quality-of-life data |
| After 1 month, 0.70 | 95% CI 0.28 to 1.00 | |||
| HF | 0.64 | 95% CI 0.44 to 0.91 | Lopez-Jimenez et al. 200210 | Health-related quality-of-life data |
| AF and HF | 0.64 | 95% CI 0.44 to 0.91 | That values are the same as HF without AF10 | Health-related quality-of-life data |
| Death | 0 | N/A | Assumption | Health-related quality-of-life data |
| Costs | ||||
| Unit costs | ||||
| Single-chamber atrial pacing | £1875 | Estimated 95% CI £1191 to £2366 | Weighted average calculated from NHS Reference Costs 2012–201395 | Costs |
| Dual-chamber pacing | £2438 | Estimated 95% CI: £1642, £3040 | Weighted average calculated from NHS Reference Costs 2012–201395 | Costs |
| HF episode | £1228 | Estimated 95% CI £1004 to £1541 | Weighted average calculated from NHS Reference Costs 2012–201395 | Costs |
| Stroke episode | £1427 | Estimated 95% CI £988 to £1616 | Weighted average cost calculated from NHS Reference Costs 2012–201395 | Costs |
| Cardiologist non-admitted non-face- to-face attendance, follow-up | £86 | Estimated 95% CI £40 to £107 | NHS Reference Costs 2012–2013 95 | Costs |
| Total UK direct health-care cost of CVD | £8,680,892,000 | 95% CI £6,267,529,000 to £13,422,948,000a | Townsend et al. 201296 | Costs |
| Average annual post-stroke hospitalisation cost | £1444 | 95% CI £1043 to £2,234a | Calculated from average of post-first year hospitalisation costs [2009 cost year US dollars converted to UK pounds sterling according to conversion rate reported in study (US$1 = £0.64)], Luengo-Fernandez et al. 201297 | Costs |
| Total annual UK stroke medication costs | £86,172 | 95% CI £62,215 to £133,245a | Townsend et al. 201296 | Costs |
| Total UK stroke primary care costs | £40,034 | 95% CI £28,904 to £61,903a | Townsend et al. 201296 | Costs |
| Episode cost of stroke in people with AF | £10,413 | 95% CI £215 to £53,539 | Luengo-Fernandez et al. 201398 | Costs |
| Average annual post-stroke hospitalisation cost in people with AF | £3370 | 95% CI £0.85 to £24,371 | Annual costs for people surviving past the 90-day acute period, Luengo-Fernandez et al. 201398 | Costs |
| Cost of GP referrals for AF | £49,800 | 95% CI £35,955 to £77,004a | Stewart et al. 200499 | Costs |
| Cost of hospital outpatient referrals for AF | £36,400 | 95% CI £26,280 to £56,284a | Stewart et al. 200499 | Costs |
| Cost of hospital admissions with principal diagnosis of AF | £271,600,000 | 95% CI £196,093,000 to £419,965,000a | Stewart et al. 200499 | Costs |
| Cost of post-discharge outpatient visits | £31,700 | 95% CI £22,887 to £49,017a | Stewart et al. 200499 | Costs |
| Cost of anticoagulation in AF patients | ||||
| Apixaban | £1.10 (daily) | N/A | BNF 67100 | Costs |
| Dabigatran | £1.10 (daily) | N/A | BNF 67100 | Costs |
| Rivaroxaban | £2.20 (daily) | N/A | BNF 67100 | Costs |
| Warfarin | £6.08 (monthly) | N/A | eMIT101 | Costs |
Treatment effectiveness
The effect of dual-chamber versus single-chamber atrial device implantation on the clinical outcomes considered in the TAG’s economic model were predominantly informed by the results reported from the DANPACE trial. 19 In particular, the risk of reoperation because of change of mode of pacing was estimated from summary statistics reported by Nielsen et al. 19 Similarly, the risks of developing AF or stroke were based on summary statistics reported by Nielsen et al. and the risks of HF were based on summary statistics reported by Riahi et al. 19,40 Targeted literature searches were carried out in Google Scholar (Google, Mountain View, CA, USA) to identify up-to-date published sources of the risks of stroke and HF in people with AF.
Probabilities of reoperation
Nielsen et al. 19 reports the number of patients requiring reoperation for various indications, including battery depletion, need for surgical change of mode of pacing, lead complications, surgical or mechanical complications, infection, skin erosion and device failure. Overall, reoperation is statistically significantly (single-chamber atrial vs. dual-chamber pacing, adjusted HR 2.00, 95% CI 1.54 to 2.61; p < 0.001)19 different between treatment arms, with a higher rate of reoperation in people receiving a single-chamber atrial pacemaker (see Model structure).
As discussed in Model structure, of conditions requiring reoperation, a statistically significant difference between pacemaker types was identified only for surgical change of mode of pacing, with a significantly larger percentage of people in the AAIR treatment arm requiring reoperation compared with the DDDR treatment arm (9.3% AAIR vs. 0.6% DDDR; p < 0.001) over an average follow-up period of 5.4 years.
For people implanted with single-chamber atrial devices, the need to change pacing mode is predominantly a result of the development of AV block, which requires an upgrade to a dual-chamber device. For the model, the difference in event rates for reoperation was used to estimate the risk of patients with a single-chamber atrial device developing AV block per patient per month, and this was applied as a constant risk for the time horizon covered by the model.
To derive the probabilities required for the economic model, the difference in monthly event rates in the single-chamber and dual-chambers arms was calculated using:
where r = event rate, ns = number of events in the single-chamber atrial pacemaker arm, Ns = number of patients receiving a single-chamber atrial pacemaker, nd = number of events in the dual-chamber pacemaker arm and Nd = number of patients receiving a dual-chamber pacemaker.
This monthly rate was then converted into a monthly probability, using a standard formula:
where p = monthly probability, r = event rate and t = time (months).
This resulted in a monthly rate of 0.142% and a monthly probability of 0.142%.
The decision to use this approach was based on the need to capture the uncertainty associated with reoperation for one-way sensitivity analysis (OWSA) and probabilistic sensitivity analysis (see Approach to uncertainty). However, as the Kaplan–Meier plot presented by Nielsen et al. 19 suggests a non-linear decline in reoperation, as opposed to a constant rate, an alternative approach using reoperation as a time-dependent parameter was explored as a structural sensitivity analysis (see Approach to uncertainty).
At 96 months post implantation, based on an 8-year battery life, all patients who had not yet experienced reoperation or developed AF were assumed to receive a replacement dual-chamber device.
Probabilities of atrial fibrillation, heart failure and stroke
Nielsen et al. 19 and Riahi et al. 40 report the number of cases of AF, stroke and HF observed per arm, together with HRs of single-chamber atrial pacemakers versus dual-chamber pacemakers over an average follow-up period of 5.4 years. Therefore, to derive the probabilities required for the economic model, the event rates in the dual-chamber arm were calculated using:
where r = event rate, n = number of events and N = number of patients receiving a dual-chamber pacemaker.
The event rates in the single-chamber atrial pacemaker arm were calculated by applying the event specific HR to the event rate in the dual-chamber arm.
These rates were then converted into monthly probabilities, using a standard formula:
where p = monthly probability, r = event rate and t = time (months).
Table 37 summarises the monthly probabilities used to estimate the number of people implanted with a single-chamber atrial or dual-chamber device who go on to experience AF, HF or stroke.
| Outcome | Single-chamber atrial pacemaker arm | Dual-chamber pacemaker arm | |||
|---|---|---|---|---|---|
| HR | Monthly rate (%) | Monthly probability (%) | Monthly rate (%) | Monthly probability (%) | |
| Paroxysmal AF | 1.24 | 0.50 | 0.68 | 0.40 | 0.58 |
| Chronic AF | 1.01 | 0.18 | 0.18 | ||
| HF | 1.09 | 0.46 | 0.46 | 0.42 | 0.42 |
| Stroke | 1.11 | 0.08 | 0.08 | 0.08 | 0.08 |
Probabilities of heart failure and stroke in people with atrial fibrillation
As discussed in Approach to uncertainty, patients who develop AF are at risk of the further sequelae of HF or stroke. HF and AF are often comorbid conditions and AF is a well-known risk factor for stroke, in particular ischaemic stroke. 69 Therefore, targeted searches of the literature were carried out in Google Scholar to identify recent publications estimating the risk of HF and stroke in people with AF. No studies were identified in which the risk of HF in people with AF was estimated; therefore, it was assumed within the economic analysis that the risk of HF was the same in people with and without AF.
With respect to the risk of stroke in people with AF, a recent publication by Gallagher et al. 102 was identified in targeted searches. Gallagher et al. 102 report the results of a population-based cohort study of people with AF. Incidence rates of stroke, adjusted for covariates such as CHADS2 score, age and smoking status, were presented for:
-
all patients (2.3 per 100 person-years)
-
patients currently exposed to warfarin therapy (0.9 per 100 person-years)
-
patients recently exposed to warfarin therapy (2.2 per 100 person-years)
-
patients with a history of warfarin therapy (2.4 per 100 person-years)
-
patients with no history of warfarin therapy (3.4 per 100 person-years).
Current guidelines recommend effective anticoagulation therapy for stroke prevention in people with paroxysmal and persistent AF. 103 Therefore, the incidence rate of stroke in people currently exposed to warfarin therapy (0.9 per 100 person-years) was considered the most suitable to inform the risk of stroke in people with AF. The TAG notes that this is close to the monthly rate of stroke (0.08%) identified in the DANPACE trial. 19
Mortality
Within the TAG’s economic model, patients are at risk of death following entry into the model until transition into the absorbing state of death. Based on pooled estimates of all-cause and cardiovascular mortality identified in the clinical literature review, the risk of death is assumed to be consistent across treatment arms. However, the level of mortality risk to which patients are exposed varies with respect to age and the health state in which they reside. Table 38 summarises the risk of all-cause mortality, by health state, along with the sources of these data (identified in targeted searches of the literature).
| Health state | Rate of all-cause mortalitya | Source (country) |
|---|---|---|
| Implantation health states | Age-specific rate of all-cause mortality from UK general population; weighted by the proportion of male and female patients modelled | ONS 2012 (UK)88 |
| With pacemaker health states | ||
| AF | HR of 2.08 vs. the general population all-cause mortality rate | Miyasaka et al. 2007,89 a 21-year community-based study analysing the all-cause mortality risk of people with AF vs. an age- and sex-matched general population (USA) |
| Stroke | HRs of 3.59 and of 3.14 vs. the general population for males and females, respectively | Carter et al. 2007,90 a hospital-based cohort study to determine all-cause mortality with ischaemic stroke compared with an age-matched healthy cohort (UK) |
| AF and stroke | HR of 1.33 vs. people with stroke and no AF | |
| HF | HR of 1.32, calculated from a weighted average of HR for people with NYHA class III (1.30, n = 3985) and NYHA class IV (1.68, n = 197) | Pocock et al. 2006,91 an analysis of data from the CHARM programme to develop predictive models of all-cause mortality (international) |
| AF and HF | HR of 1.11 vs. people with HF and no AF |
As no evidence of inflated mortality risks for people requiring a pacemaker or people implanted with a pacemaker was identified, patients residing in the implantation and with pacemaker health states were assumed to be at the same risk of death as the age- and sex-matched UK general population.
Based on publications identified by Miyasaka et al. ,89 Carter et al. 90 and Pocock et al. ,91 people with AF, stroke or HF are assumed to be at increased risk of all-cause mortality versus the UK general population. Furthermore, people with AF and stroke and people with AF and HF are assumed to be at further risk of death as a result of the concomitant conditions.
In addition to all-cause mortality, patients experiencing events such as stroke and HF are at risk of death as a direct result of the event experienced. Case fatality as a result of stroke was calculated from data presented by Carter et al. 90 Carter et al. 90 report the number of people dying within 30 days of an acute stroke event (n = 32/545) and, of these, the number with concomitant AF (n = 14). Table 39 summarises the calculation of the probability of stroke case fatality in people with and without AF.
| AF (N) | Fatal stroke (n) | Probability of fatal stroke (%) |
|---|---|---|
| Yes (103) | 14 | 13.59 |
| No (442) | 18 | 4.07 |
Case fatality after development of HF is derived from information presented by Cowie et al. 104 and Mosterd et al. 105 for people without and with AF, respectively. Cowie et al. 104 report the results of a population-based observational study (west London, UK) of patients with a new diagnosis of HF; 81% of patients were reported as being alive 1 month after developing HF. Therefore, in the TAG’s economic model, 19% of new HF cases were assumed to be fatal. Mosterd et al. 105 report the results of prognostic analyses of a population-based cohort study (Rotterdam, the Netherlands) in patients with HF; cardiac death in people with AF was associated with a HR of 2.08, which was applied to the 19% risk of fatal HF in people to calculate the risk of fatal HF in people with AF. Table 40 summarises the probability of death following event sequelae used in the base-case economic analysis. All the probabilities used to inform mortality in the economic model are tested in one-way and probabilistic sensitivity analyses (see Approach to uncertainty).
Adverse events
Reviews of the clinical effectiveness and safety evidence for single-chamber atrial pacemakers versus dual-chamber pacemakers identified information on the following adverse events:
-
lead displacement
-
infection
-
haematoma
-
pacemaker-mediated tachycardia
-
oversensing
-
loss of pacing capture
-
pacing system explantation
-
atrial arrhythmia
-
ventricular arrhythmia
-
syncope
-
skin erosion
-
device failure.
No statistically significant differences in the rates of these adverse events were identified between treatment arms. Therefore, with the exception of adverse events leading to reoperation, which are captured within the reoperation data from the DANPACE trial19 (see Treatment effectiveness), no adverse events were included in the base-case economic model.
Health-related quality-of-life data
A systematic review was carried out in December 2013 to identify published HRQoL evidence relevant to the decision problem that is the focus of this MTA, that is, dual-chamber pacemakers versus single-chamber atrial pacemakers in patients with bradycardia as a result of SSS without AV block. The following databases were searched:
-
MEDLINE (via Ovid)
-
EMBASE (via Ovid)
-
HTA database (HTA, The Cochrane Library)
-
NHS EED (The Cochrane Library).
To facilitate the identification of all potentially relevant information, the MEDLINE and EMBASE search strategies used terms capturing population (pacing), interventions (dual-chamber pacemakers) and HRQoL studies combined with terms designed to capture a broader range of comparators than those specified in the final scope. HRQoL evidence in patients receiving single chamber VP was considered likely to be transferable to the patient population that is the focus of this MTA.
The search strategy for HTA and NHS EED combined terms for the target condition (AV block, SSS), terms for the intervention (pacemaker) and terms for HRQoL (quality of life, QoL and QALY). All databases were searched from inception; full details of the search terms are presented in Appendix 1, Literature search strategies. In addition to database searching, the reference lists of identified studies were reviewed for any potentially relevant studies.
No restrictions on language or setting were applied to any of the searches. The titles and abstracts of papers identified through the searches were independently assessed for inclusion by two health economists using the criteria outlined in Table 41.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
The systematic review was updated in June 2014. The search strategy remained the same as outlined above; however, results were limited from 17 December 2013 to 6 June 2014 in order to identify only additional relevant studies.
A total of 501 papers were identified from the December 2013 search (Figure 10). Of these papers, 425 were excluded following review of the title and abstract. Therefore, a total of 76 papers were identified as being potentially relevant. Of these papers, 13 were identified, from the abstract, as reporting condition-specific measures of HRQoL and 12 as reporting generic non-preference-based measures of HRQoL (mostly SF-36), with 51 papers identified as potentially reporting generic, preference-based measures of HRQoL (Q1, see Table 41). To be as inclusive as possible, studies for which it was unclear from the abstract which type of HRQoL measure was used were labelled as potentially reporting a generic, preference-based measure of HRQoL.
FIGURE 10.
Identified HRQoL studies, December 2013 and June 2014 searches.

Papers identified as reporting either condition-specific measures of HRQoL or generic non-preference-based measures of HRQoL during the December 2013 search were provisionally excluded. That is, these studies were reserved for inclusion, and the full text reviewed only if no suitable generic, preference-based measures of HRQoL were identified; this is because, as specified in the NICE reference case,106 generic preference-based measures of HRQoL, such as the European Quality of Life-5 Dimensions (EQ-5D), are preferred for the purposes of economic evaluation.
Review of the 51 papers potentially reporting generic preference-based QoL studies identified six studies as relevant, and these studies were listed for final inclusion. The remaining 45 studies were excluded (or provisionally excluded) for the following reasons (full details provided in Appendix 4, Table of excluded studies):
-
14 reported generic non-preference-based measures of QoL
-
10 did not report any QoL data
-
nine reported condition-specific measures of QoL
-
six were published in a language other than English
-
three were irretrievable
-
two were reviews
-
one study did not consider pacing.
As HRQoL data from the UK were considered to be the most relevant to the decision problem that is the focus of this MTA, papers published in languages other than English were provisionally excluded at this stage. Five papers were identified from the updated search in June 2014. However, after reviewing the titles and abstracts, none was identified as reporting HRQoL data relevant to the scope of this review.
Six studies were identified as reporting generic preference-based HRQoL data. Full details of the populations and health states considered and instruments and utility values reported in these studies are presented in Appendix 2, Data abstraction; a summary of these data is presented in Table 42.
| Included study | Country | Populationa | Health states | Instrument |
|---|---|---|---|---|
| Fleishmann et al. 200994 | USA | Patients enrolled in the MOST68 | No AF, developing PAF, CAF | TTO |
| Fleishmann et al. 200692 | USA | Patients enrolled in the MOST68 | DDDR pacing, VVIR pacing | TTO |
| Shukla et al. 2005108 | USA | Patients enrolled in the MOST68 | Pacemaker sensing mode (accelerometer, piezoelectric crystal or blended) | TTO |
| Link et al. 2004107 | USA | Patients enrolled in the MOST,68 who were randomised to VVIR pacing mode and went on to develop pacemaker syndrome | Baseline, pre crossover, post crossover | TTO |
| Lamas et al. 200268 | USA | Patients enrolled in the MOST68 | DDDR pacing, VVIR pacing | TTO |
| Lopez-Jimenez et al. 200210 | USA | Patients enrolled in the PASE trial10 | Baseline and 3 months, 9 months and 18 months after implantation | TTO |
All the HRQoL studies identified for inclusion, report time trade-off (TTO) utility data collected directly from patients (i.e. patient measurement and valuation). Of these, five68,92,94,107,108 report the results of QoL analyses carried out with patients enrolled in the MOST. 68 The remaining study10 reports utility data collected from patients enrolled in the PASE trial. 71 These studies are described in further detail in Appendix 2, Data abstraction.
Narrative summary of included health-related quality-of-life studies
Fleischmann et al.94
Fleischmann et al. 94 assess the impact of AF on the QoL and functional status of patients enrolled in the MOST. 68 Patients enrolled in the MOST had SSS and were randomised to either DDDR or VVIR pacing. The average age of patients was 73 years, 52% were male, approximately 45% had paroxysmal AF and 20% had some form of AV block. HRQoL was assessed using the SF-36 general health survey, and utilities were elicited using standard TTO methodology. Functional status was assessed using the SAS. Measurements were collected at baseline and 3 and 12 months post implantation in 1841 patients, who were subdivided into:
-
those without AF (n = 1737)
-
those who developed paroxysmal AF, but not chronic AF (n = 75)
-
those with chronic AF (n = 29).
The changes observed in each measure (SF-36, TTO and SAS) between baseline and 12 months and between 3 months and 12 months of follow-up were analysed, with adjustments for age, sex, history of AF, history of HF, treatment arm and baseline QoL score. In addition, to avoid confounding as a result of crossover, the last observed scores of patients who crossed over from VVIR to DDDR pacing were carried forward for the remainder of the analytical time frame. A summary of the results reported by Fleischmann et al. 94 is presented in Table 43; statistically significant differences in utility, as assessed using TTO methodology, were identified.
| Measure | No AF | Paroxysmal AF | Chronic AF | p-valuea |
|---|---|---|---|---|
| Baseline to 12 months after implantation | ||||
| PCS mean change in score (SEM) | 2.50 (0.44) | 0.90 (0.77) | –0.30 (1.62) | 0.04 |
| SAS mean change in score (SEM) | 0.03 (0.03) | 0.15 (0.06) | 0.21 (0.12) | 0.05 |
| TTO | 0.07 | 0.06 | 0.11 | > 0.05 |
| 3 months to 12 months after implantation | ||||
| SAS mean change in score (SEM) | 0.05 (0.03) | 0.12 (0.10) | 0.44 (0.14) | 0.02 |
| TTO | 0.00 | –0.02 | 0.03 | > 0.05 |
Based on the analyses conducted, the authors concluded that ‘AF was not a major determinant of most QoL measures’. 94 However, statistically significant changes in the physical component of the SF-36 score and in the SAS measure of functional capacity indicate that the presence of AF may impair the physical improvement associated with pacemaker implantation.
Fleischmann et al.92
In this analysis of serial QoL measures elicited from people enrolled in the MOST,68 Fleischmann et al. 92 consider the impact of pacemaker implantation and pacemaker mode (DDDR vs. VVIR) on QoL. SF-36, SAS and TTO measures were used to assess QoL at baseline, and after 3 months and 12 months of follow-up, followed by yearly estimates; last observations were carried forward in people who crossed over from VVIR to DDDR pacing. Table 44 displays the TTO utilities, adjusted for age and sex, by pacing mode as presented by Fleischman et al. 92
| Time point | DDDR | VVIR |
|---|---|---|
| Baseline (n = 1935) | 0.72 | 0.73 |
| 3 months (n = 1736) | 0.83 | 0.82 |
| 12 months (n = 1639) | 0.83 | 0.82 |
| 24 months (n = 1208) | 0.83 | 0.81 |
| 36 months (n = 748) | 0.86 | 0.83 |
| 48 months (n = 392) | 0.83 | 0.87 |
The authors conclude that, irrespective of sex, the presence of HF or level of comorbidity, pacemaker implantation was associated with statistically significant improvements in QoL. Furthermore, although a small but measurable effect of pacemaker mode was noted with the SF-36, no significant differences were observed in TTO utility estimates.
Shukla et al.108
Shukla et al. 108 assessed the impact of pacemaker sensor type (accelerometer, piezoelectric or blended) on the QoL of patients enrolled in MOST. 68 The SF-36, SAS, 0–100 scale and TTO measures were used to elicit QoL and, in the case of TTO, to value QoL. Measures were taken at baseline, 3 months post transplant and yearly thereafter; adjustments were made for age, sex, pacing mode (DDDR vs. VVIR), follow-up time and baseline QoL. Patients implanted with a blended sensor device reported statistically significantly worse physical function at (physical function, p = 0.009; physical summary score, p = 0.039; and physical role function, p = 0.08) than patients with accelerometer or piezoelectric sensors. However, no other statistically significant differences in QoL were identified.
The authors concluded that patients implanted with blended sensor devices had lower physical function and absolute QoL scores. However, the authors considered that these observations may be a result of ‘clinical selection of the most sophisticated sensor for the most ill patient’. 108
Link et al.107
A subset (18.3%) of patients enrolled in the MOST68 and randomised to VVIR pacing went on to develop pacemaker syndrome according to pre-specified criteria. That is, they developed ‘either congestive signs and symptoms associated with retrograde conduction during VVIR pacing or a ≥ 20 mmHg reduction of systolic blood pressure during VVIR pacing, associated with reproducible symptoms of weakness, light[-]headedness, or syncope’. 107 Link et al. report the QoL (SF-36, SAS, TTO and 0–100 score) measured in these patients at baseline, prior to crossover and after crossover. Significant decrements in QoL were observed in six of the 10 SF-36 scales from baseline (i.e. pre implantation) to pre crossover (Table 45). Utility was also lower than at baseline, but the difference was not statistically significant. After crossover, statistically significant improvements were seen in all measures of QoL (see Table 47).
| QoL measure | Baseline score (SD) (n = 153) |
Pre-crossover score (SD) (n = 80)a |
Post-crossover score (SD) (n = 136)b |
|---|---|---|---|
| SF-36 | |||
| Physical–composite | 35.8 (10.7) | 33.3 (10.1) | 38.0 (11.6) |
| Mental–composite | 51.5 (9.5) | 49.8 (10.9) | 52.7 (11.6) |
| Physical–function | 56.4 (27.6) | 39.8 (28.1) | 55.0 (29.7) |
| Role–physical | 28.4 (38.7) | 28.4 (39.3) | 50.6 (43.0) |
| Pain | 66.8 (29.1) | 70.9 (24.7) | 69.5 (26.3) |
| Health perception | 57.2 (21.1) | 52.4 (20.7) | 56.5 (21.5) |
| Energy | 39.6 (23.2) | 32.1 (21.4) | 49.9 (24.5) |
| Social–function | 67.7 (24.7) | 62.2 (26.5) | 71.1 (24.1) |
| Role–emotional | 80.4 (34.7) | 75.4 (39.2) | 83.6 (32.9) |
| Mental health | 77.4 (17.2) | 73.5 (19.6) | 77.5 (17.8) |
| SAS | |||
| Score | 2.09 (0.93) | 2.50 (0.91) | 2.07 (0.94) |
| TTO | |||
| Score | 0.75 (0.34) | 0.73 (0.35) | 0.82 (0.31) |
The authors concluded that ‘quality of life, as assessed by a variety of metrics, decreased at the time of diagnosis of pacemaker syndrome and improved after the pacemaker was reprogrammed to a physiologic mode. (However, a placebo effect cannot be truly ruled out, because neither patients nor physicians were blinded to the crossover status)’. 107
Lamas et al.68
Lamas et al. 68 report the results, including HRQoL, of the MOST. QoL was assessed with the SF-36, SAS and TTO measures. Table 46 presents the changes in QoL measures from baseline as reported by Lamas et al. 68
| QoL measure | Baseline score | 48-month score | Change from baseline | p-value (dual vs. ventricular) | ||
|---|---|---|---|---|---|---|
| Dual | Ventricular | Dual | Ventricular | |||
| SF-36 | ||||||
| Physical function | 58.8 | 58.9 | –3.2 | –0.1 | 1.9 | 0.04 |
| Physical role | 35.7 | 34.6 | 18 | 26.7 | 8.6 | < 0.01 |
| Social function | 63.5 | 62.6 | 6.4 | 9.8 | 2.5 | < 0.01 |
| Energy | 41.9 | 42.6 | 3.6 | 5.2 | 4.1 | < 0.01 |
| Mental health | 72 | 72 | 4.7 | 4.6 | 1.2 | 0.05 |
| Emotional role | 74 | 74 | 4.8 | 12.3 | 3.6 | < 0.01 |
| Pain | 67.5 | 67 | 6.9 | 5.1 | 0.5 | 0.57 |
| Health perception | 60 | 60.2 | –3.5 | –2.5 | 1.1 | 0.09 |
| Mental component summary | 48.4 | 48.4 | 2.4 | 3.5 | 1.1 | < 0.01 |
| Physical component summary | 38.5 | 38.4 | 1 | 2.2 | 1.2 | < 0.01 |
| SAS | ||||||
| Score | 2.01 | 1.97 | 0.16 | 0.13 | 0.002 | 0.94 |
| TTO | ||||||
| Utility (%) | 73 | 72 | 6 | 6 | 2 | 0.06 |
Similar to the conclusions of Fleischmann et al. ,92 the authors noted that, compared with VP, dual-chamber pacing provided significant improvements in six of the 10 SF-36 subscales, including the physical and mental component summary. However, no significant differences in utility, as assessed by TTO, were observed by pacing mode.
Lopez-Jimenez et al.10
Lopez-Jimenez et al. 10 report the results of the PASE study, which is a RCT with the primary endpoint of HRQoL. Patients enrolled in the PASE study were in sinus rhythm and indicated for permanent pacing as a result of bradycardia, and were randomised to VVIR or DDDR pacing mode. The average age of patients was 76 years, 59% were male, 57% had AV block and 28% had NYHA class III or IV HF. HRQoL was assessed using the TTO, SF-36 and 0–100 scoring systems. In addition to HRQoL, patients’ functional status was assessed with SAS. Measurements were collected at baseline and at 3, 9 and 18 months after enrolment. Table 47 summarises the estimates of HRQoL over time obtained by Lopez-Jimenez et al. 10
| QoL measure | Baseline (n = 398) | 3 monthsa (n = 284) | 9 monthsb (n = 291) | 18 months (n = 250) |
|---|---|---|---|---|
| TTO | 0.74 | 0.91 | 0.87 | 0.87 |
| SAS | 2.0 | 1.89 | 1.7 | – |
| 0–100 | 64.1 | 71.0 | 68.8 | – |
| SF-36 | ||||
| Physical function | 53.9 | 57.5 | 57.0 | – |
| Physical role | 34.7 | 62.4 | 57.0 | – |
| Social function | 63.0 | 76.7 | 70.2 | – |
| Energy | 43.3 | 55.3 | 52.2 | – |
| Mental health | 72.7 | 78.2 | 78.3 | – |
| Emotional role | 68.6 | 89.5 | 82.3 | – |
| Pain | 66.7 | 70.2 | 71.3 | – |
| Health perception | 60.5 | 62.6 | 59.9 | – |
In order to assess the validity of the TTO measure, the authors carried out ‘known group validity tests’ in patients with and without CHF and in patients with and without stable angina. As expected, people with a history of CHF had a significantly lower utility than people with no history of CHF (0.64 vs. 0.78; p < 0.001). Furthermore, people with NYHA class III or IV had a significantly lower utility than people with NYHA class I or II (0.62 vs. 0.80; p = 0.0001).
Utility estimates were not adjusted for potential covariates such as age or sex. However, subgroup analyses suggest that improvements in utility following pacemaker implantation were consistent regardless of implantation diagnosis, pacing mode, sex, age, employment status or history of angina.
The authors concluded that pacemaker implantation improves HRQoL to a mean level close to that of the general population.
Quality-of-life data selected for the economic model
The TAG’s economic model has the following health states, for which estimates of utility are required to facilitate the use of QALYs as the measure of benefit:
-
implant single-chamber atrial pacemaker
-
implant dual-chamber pacemaker
-
with single-chamber atrial pacemaker
-
with dual-chamber pacemaker
-
AF
-
stroke
-
HF
-
AF and stroke
-
AF and HF
-
death.
Table 48 summarises the utility values used, along with the source of these data and the rationale for selecting these data to inform the base case model. The analyses carried out by Fleishmann et al. 92,94 and Lopez-Jimenez et al. 10 were identified in the TAG’s systematic review of the HRQoL literature. A targeted search for utility associated with stroke (with or without AF) identified a study by Luengo-Fernandez et al. 93 that evaluated QoL after transient ischaemic attack and stroke. 93
| Health state | Utility | Source | Rationale for use in base case model |
|---|---|---|---|
| Implant single-chamber atrial pacemaker | 0.725a | Fleischmann et al. 200692 | Largest and most homogeneous HSUV study identified in people with SSS, based on age- and sex-adjusted analysis |
| Implant dual-chamber pacemaker | |||
| With single-chamber atrial pacemaker | 0.825a | ||
| With dual-chamber pacemaker | |||
| AF | 0.805 | Fleischmann et al. 200994 | Only HSUV study of the impact of AF in people paced for bradycardia |
| Stroke | 0.640 (month 1), 0.70 thereafter | Luengo-Fernandez et al. 201393 | Most recent and robust study identified by targeted search |
| HF | 0.640 | Lopez-Jimenez et al. 200210 | Only HSUV study identified which reporting utility data for bradycardia patients with HF |
| AF and stroke | 0.640 (month 1), 0.70 thereafter | Luengo-Fernandez et al. 201393 | Assumption |
| AF and HF | 0.640 | Lopez-Jimenez et al.10 | Assumption |
| Death | 0 | Assumption | |
Clinical expert opinion suggests that the QoL of patients suffering a stroke in the presence of AF is lower than that of people suffering a stroke in the absence of AF. However, no utility data for AF patients suffering a stroke were identified from the literature. Therefore, the base-case model assumes that the utility of stroke is the same regardless of the patient’s AF status. Death is assumed to be associated with a utility of 0.
Costs
The costs accounted for within the TAG’s economic model are categorised as follows:
-
device and implantation costs
-
monitoring costs
-
episode costs
-
long-term costs.
No currently relevant UK costing studies were identified in the TAG’s systematic review of the economic literature. Therefore, where possible, standard UK sources [NHS Reference Costs 2012–2013,95 NHS Generic Pharmaceuticals electronic market information tool101 or the British National Formulary (BNF)100] were used to inform the unit costs applied within the TAG’s economic model; these are described in more detail in the following sections. In addition, targeted searches for UK-specific resource use and costing studies of AF, HF and stroke were carried out in Google Scholar by trying different combinations of the terms ‘costs’, ‘NHS’ and ‘UK’ with the various conditions ‘atrial fibrillation’, ‘heart failure’, ‘stroke’ and ‘cardiovascular disease’. Of these, the following publications were selected to provide base-case model inputs:
-
Townsend et al. 96 Coronary Heart Disease Statistics. Birmingham: British Heart Foundation; 2012
-
Luengo-Fernandez et al. 109 Hospitalization resource use and costs before and after TIA and stroke: results from a population-based cohort study (OXVASC). Value in Health 2013;16:280–7
-
Luengo-Fernandez et al. 98 Population-based study of acute and long-term care costs after stroke in patients with AF. International Journal of Stroke 2013;8:308–14.
Device and implantation costs
The procedure costs (including hardware cost) associated with implantation of a single-chamber or dual-chamber device were obtained from a weighted average of episode costs associated with relevant HRG codes (NHS Reference Costs 2012–201395). Table 49 summarises the HRG codes used to inform each procedure cost used within the TAG’s economic model. Upgrade procedures were assumed to cost the same as an initial implantation of a dual-chamber device. Spell-level (rather than episode-level) data for each HRG code were used in sensitivity analysis (see Approach to uncertainty).
| Procedure | Unit costs (HRG code) | Activity (n times occurred) | Total costa |
|---|---|---|---|
| Implantation of a single-chamber device | £2937 (EA03A, pace 1: single-chamber or implantable diagnostic device, with CC score of 11+) | 2233 | £1875 |
| £2277 (EA03B, pace 1: single-chamber or implantable diagnostic device, with CC score of 8–10) | 2711 | ||
| £2085 (EA03C, pace 1: single-chamber or implantable diagnostic device, with CC score of 5–7) | 5291 | ||
| £2083 (EA03D, pace 1: single-chamber or implantable diagnostic device, with CC score of 2–4) | 11,768 | ||
| £1509 (EA03E, pace 1: single-chamber or implantable diagnostic device, with CC score of 0–1) | 19,218 | ||
| Implantation of a dual-chamber device | £3367 (EA05A, pace 2: dual chamber, with CC score of 9+) | 1904 | £2438 |
| £2630 (EA05B, pace 2: dual chamber, with CC score of 5–8) | 4504 | ||
| £2466 (EA05C, pace 2: dual chamber, with CC score of 2–4) | 9328 | ||
| £2146 (EA05D, pace 2: dual chamber, with CC score of 0–1) | 9898 |
Device and implantation costs are applied at two points in the model at first implantation and at upgrade (i.e. to patients in the implant single-chamber atrial pacemaker or implant dual-chamber pacemaker health states).
Monitoring costs
Following pacemaker implantation, patients receive follow-up checks from a cardiologist (WF01C, cardiologist non-admitted, non-face-to-face attendance, follow-up, £86). 94 Based on expert clinical opinion, initial follow-up is assumed to be 1 week after implant, with a second follow-up carried out at 2 months post implantation and subsequent annual visits. 110 Therefore, within the model, the cost of a follow-up visit is applied on entry into the implant single-chamber atrial pacemaker, implant dual-chamber pacemaker, with single-chamber atrial pacemaker and with dual-chamber pacemaker health states. The cost of a follow-up visit is also applied annually to all patients in the with single-chamber atrial pacemaker and with dual-chamber pacemaker health states.
Episode costs
Over the course of the TAG’s economic model, patients are exposed to the risk of HF and stroke, with or without the presence of AF. In the absence of AF, the occurrence of HF or stroke is associated with a cost that is based on a weighted average of episode-level costs (spell-level costs are used in sensitivity analysis, see Approach to uncertainty) associated with relevant HRG codes (Table 50). In the presence of AF, however, the episode cost of stroke is assumed to be £11,275 based on evidence from the OXford VASCular (OXVASC) population-based cohort study reported by Luengo-Fernandez et al. 98 No evidence was identified indicating that the episode cost of HF would differ in the presence of AF.
| Episode | Unit costs (HRG code) | Activity (n times occurred) | Total costa |
|---|---|---|---|
| HF | £2398 (EB03A, heart failure or shock with CC score of 14+) | 5832 | £1228 |
| £1919 (EB03B, heart failure or shock with CC score of 11–13) | 26,264 | ||
| £1389 (EB03C, heart failure or shock with CC score of 8–10) | 47,488 | ||
| £1034 (EB03D, heart failure or shock with CC score of 4–7) | 81,459 | ||
| £799 (EB03E, heart failure or shock with CC score of 0–3) | 23,133 | ||
| Stroke | £5497 (AA35A, stroke with CC score of 16+) | 3263 | £1427 |
| £4428 (AA35B, stroke with CC score of 13–15) | 9563 | ||
| £2433 (AA35C, stroke with CC score of 10–12) | 28,388 | ||
| £1575 (AA35D, stroke with CC score of 7–9) | 58,580 | ||
| £1060 (AA35E, stroke with CC score of 4–6) | 114,664 | ||
| £1023 (AA35F, stroke with CC score of 0–3) | 92,215 | ||
| Stroke following AFb | – | – | £11,275 |
The episode cost of HF is applied to patients entering the HF and the AF and HF health states. The episode cost of stroke is applied to patients entering the stroke health state and the episode cost of stroke following AF is applied to patients entering the AF and stroke health state.
In people with dual-chamber pacemakers, the onset of AF may be associated with a need to reprogramme the device to act as a ventricular pacemaker. In line with clinical expert opinion and assumptions made in NICE’s TA88,18 this cost comprises a cardiological consultation (WF01C, cardiologist non-admitted, non-face-to-face attendance, follow-up, £86)94 and electrocardiography (DIAGIMDA, simple echocardiography, 19 years and over, £41). 94 People with single-chamber atrial pacemakers who develop AF may also require VP, in which case the single-chamber atrial device will need to be replaced with a single-chamber ventricular device. The cost associated with device replacement is assumed to be equivalent to that of initial single-chamber implantation (£1875).
Based on expert clinical opinion, the cost of reprogramming and of device replacement is applied to one-third of people developing AF from the with dual-chamber pacemaker and with single-chamber atrial pacemaker health states. The impact of this assumption was tested in sensitivity analyses.
Long-term costs
Following the onset of HF, stroke or AF, patients are assumed to accrue costs over the long term, for example medication, hospitalisation and primary-care costs. For people with HF, these costs were determined from national prevalence and cost statistics reported in the British Heart Foundation’s Coronary Heart Disease Statistics 2012 publication. 96 The following data were extracted from the British Heart Foundation’s report:96
-
total UK direct health-care costs of CVD, 2009: £8,680,892,000
-
UK prevalence of HF, 2011: 0.90% in men and 0.70% in women, total 160,719 cases
-
UK prevalence of CVD, 2007–10 (Table 51).
| Year | Prevalence of CVD | |
|---|---|---|
| Men (%) | Women (%) | |
| 2007 | 10.9 | 9.7 |
| 2008 | 11.1 | 9.4 |
| 2009 | 11.4 | 9.5 |
| 2010 | 11.7 | 10.1 |
Based on the prevalence data presented in Table 51, the relative prevalence of HF as a percentage of CVD was calculated (7.53% for men and 6.39% for women; average 6.96%). Thereafter, the 2011 UK direct health-care costs of CVD (£8,680,892,000) and of HF (£632,586,584) were estimated, resulting in a per-person cost of HF of £3316 per annum (£276.34 per cycle) at 2013 prices. Full calculation details are available in Appendix 6, Calculation of long-term care costs associated with heart failure. The long-term costs associated with HF were applied monthly to people residing in the HF and the AF and HF health states.
For people experiencing stroke, the costs of hospitalisation estimated from the OXVASC population-based cohort study reported by Luengo-Fernandez et al. 98,109 were used. The costs of medication and primary care reported by Townsend et al. 96 were used to inform the base case. Table 52 summarises the unit costs used to inform long-term costs for people experiencing stroke.
| Cost component | Annual cost | Source |
|---|---|---|
| Post-stroke hospitalisation cost | £1564 | Average of post-first year hospitalisation costs [2009 cost year US$ converted to UK pounds according to conversion rate reported in study (US$1 = £0.64)], Luengo-Fernandez et al.97 inflated to 2013 prices |
| Post-stroke hospitalisation cost (in people with AF) | £3649 | Annual costs for people surviving past the 90-day acute period, Luengo-Fernandez et al.98 inflated to 2013 prices |
| Medication costs | £81 | Calculated from the 2009 stroke medication costs reported by Townsend et al.96 (inflated to 2010–11 costs) divided by the 2010–11 stroke prevalence also reported by Townsend et al.96 inflated to 2013 prices |
| Primary care costs | £38 | Calculated from the 2009 stroke primary care costs reported by Townsend et al.96 (inflated to 2010–11 costs) divided by the 2010–11 stroke prevalence also reported by Townsend et al.96 inflated to 2013 prices |
| Total cost per cycle stroke health state | £140 | |
| Total cost per cycle stroke and AF health state | £400 | |
The long-term costs associated with people with stroke and people with AF and stroke were applied monthly to people residing in the stroke and the AF and stroke health states, respectively.
In people with AF, long-term costs of primary care and hospitalisation were identified from a predictive study carried out by Stewart et al. ,99 who evaluated the UK health and social services cost of AF in 1995, and projected costs to 2000 based on epidemiological trends. Table 53 summarises the calculation of per-person primary care and hospitalisation costs, based on information presented by Stewart et al. 99
| Year | Information | Data |
|---|---|---|
| 2000 | Total number of AF cases | 601,149 |
| Cost of GP referrals | £49,800,000 | |
| Cost of hospital outpatient referrals | £36,400,000 | |
| Cost of hospital admissions with principal diagnosis of AF | £271,600,000 | |
| Cost of post-discharge outpatient visits | £31,700,000 | |
| Total non-medication and non-secondary hospital admission cost of AF | £390,101,149 | |
| Annual per person cost of AFa | £649 | |
| 2013 | Annual per person cost of AFb | £955 |
In addition to the costs of primary and hospital care, people with AF are assumed, in line with current clinical guidance,112 to receive effective anticoagulation therapy with apixaban, dabigatran etexilate (hereafter referred to as dabigatran), rivaroxaban or warfarin. Analysis of 2013 prescribing data indicates that, in primary care, the current market shares of apixaban, dabigatran, rivaroxaban and warfarin are 0.0004%, 0.47%, 0.15% and 99.38%, respectively. 113 However, data from the Hospital Prescribing Audit Index database indicate that, since 2012, the use of apixaban, dabigatran and rivaroxaban has changed by +179,873.2%, +132.4% and –21.5% , respectively. 114 Given that these therapies have recently been recommended for use in the prevention of stroke and systemic embolism in people with non-valvular AF, it is likely that market share will continue to change over the coming years. Therefore, although current market share estimates are used in the base case, the model is set up to have market share as a user input and different market share scenarios are assessed in sensitivity analyses (see Approach to uncertainty). Table 54 summarises the calculation of a per-person monthly cost of oral anticoagulation used in the TAG’s base-case model.
| Treatment | Market share (%) | Unit costs (£) | Daily dose |
|---|---|---|---|
| Apixaban | 0.0004 | 1.10a | Twice daily |
| Dabigatran | 0.47 | 1.10a | Twice daily |
| Rivaroxaban | 0.15 | 2.20a | Once daily |
| Warfarin | 99.38 | 6.08b | Once daily |
| Monthly cost of oral anticoagulationc | – | – | £6.45 |
The long-term cost associated with people with AF and stroke was applied monthly to people residing in the AF, the AF and stroke and the AF and HF health states.
Approach to uncertainty
Assessment of uncertainty associated with the TAG’s economic model is carried out probabilistically (with mean estimates of costs and QALYs used to calculate the base-case results), deterministically (OWSA) and through structural and scenario analyses.
Probabilistic
The TAG economic model has been constructed probabilistically, that is, to account simultaneously for the impact of parameter uncertainty on the cost-effectiveness results. Probability distributions were assigned to parameters used within the model, from which values have been simultaneously sampled 1000 times. Table 55 summarises the type of distribution, and rationale for selection of the distribution, used to inform each group of parameters; full details of distributional specifications are provided in Table 36.
| Parameter type | Parameter description | Distribution(s) used | Rationale |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deterministic
A series of OWSAs was carried out by using the lower and upper limits of the 95% CIs of the following parameters to assess their the impact on the ICER: age; efficacy values; utility values; costs; all-cause mortality; and HF hospitalisation. The estimates for the upper and lower 95% CIs are displayed in Table 36. Each key parameter was alternately assigned a low and high value and the deterministic cost-effectiveness results using this value were recorded.
Structural sensitivity analysis
The base-case analysis assumes a time horizon of 10 years, which requires an extrapolation of the data available from the pivotal RCT, DANPACE, beyond the typical duration that patients were followed up within the trial (5.4 years). 19 As a structural sensitivity analysis, the time horizon was reduced to 5 years to assess the degree of impact this extrapolation may have had on the base-case results.
In addition, as discussed in Treatment effectiveness, the risk of reoperation because of AV block was based on reoperation because of need for surgical change of mode of pacing, where it was found to be significantly higher in the AAIR treatment arm compared with the DDR treatment arm (9.3% AAIR vs. 0.6% DDDR; p < 0.001) over an average follow-up period of 5.4 years. However, the Kaplan–Meier plot (Figure 11) suggests a non-linear decline in reoperation, as opposed to a constant rate. In order to test the assumption of reoperation as a constant risk in the base case, a structural sensitivity analysis was undertaken based on the Kaplan–Meier data.
FIGURE 11.
Time-to-event curve for freedom from reoperation from the DANPACE trial. 19 Unadjusted p-value (log-rank test) shown.

In the structural sensitivity analysis, while residing in the with dual-chamber pacemaker and with single-chamber atrial pacemaker health states, patients are at risk of experiencing complications that require a reoperation, such as lead displacement or device failure. The risk of reoperation is time dependent and all patients requiring reoperation are assumed to be implanted with a dual-chamber device, regardless of the reason for reoperation or which pacemaker was originally implanted. The assumption that all patients requiring reoperation receive a dual-chamber device is based on reoperation data collected in the DANPACE trial. 19 As in the base case, only one instance of reoperation was permitted within the model time horizon.
The monthly probability of reoperation, by treatment arm, estimated from Kaplan–Meier data presented by Nielsen et al. 19 can be found in Appendix 7, Monthly probability of reoperation by treatment arm. These probabilities were estimated through digitisation of the Kaplan–Meier plot using the freely available online software WebPlotDigitizer (version 3.8, Ankit Rohatgi, Austin, TX, USA; http://arohatgi.info/WebPlotDigitizer/). The digitisation process provided monthly estimates of the ‘survival’ function S(t), from which the monthly probabilities of reoperation were calculated using the following formula:
where t = time (months) and p(re-operation)t = probability of re-operation at time t.
Scenario analysis
Various assumptions have been made in the construction of the TAG’s base-case model. Where possible, these have been tested in scenario analysis. Table 56 lists the scenario analyses carried out by the TAG, the parameters used to inform these scenarios and the rationale for each analysis.
| Scenario analysis | Parameter definition | Rationale |
|---|---|---|
| Cost scenarios | ||
| Cost of pacemaker implant/implantation | Spell costs of single-chamber pacemaker £3362.18 | To assess the impact of utilising an alternative source of cost of pacemaker implant/implantation |
| Spell costs of dual-chamber pacemaker £4142.11 | ||
| Cost per cycle for HF | £205.63 uplifted from NICE’s TA8818 | To assess the impact of utilising an alternative source for cost of HF |
| Cost per cycle for stroke | £1104 uplifted from NICE’s TA8818 | To assess the impact of utilising an alternative source for cost of stroke |
| Cost per cycle for stroke | £343 uplifted from Saka et al. 2009116 | To assess the impact of utilising an alternative source for cost of stroke |
| Cost of reprogramming and of device replacement in people developing AF | Applied to 0% and 100% of people developing AF | To test the impact of using extreme values for reprogramming/device replacement in people developing AF |
| Other | ||
| Alternative discount rates for costs and benefits | Discount rate for costs and benefits assumed to be 1% or 6% | As per NICE methods guides106 |
| Market share change for apixaban, dabigatran, rivaroxaban and warfarin | Assumed 15% receive each of apixaban, dabigatran and rivaroxaban, and 55% receive warfarin | To assess the potential impact of future increased uptake of apixaban, dabigatran, rivaroxaban |
Base case results
Incremental deterministic and probabilistic results are presented in Table 57. In the deterministic analysis, the mean cost associated with dual-chamber pacemakers was £9211.41, whereas single-chamber atrial pacemakers had a mean cost of £9480.47, resulting in an incremental cost of £269.06. The mean number of QALYs gained was 5.56 and 5.51 for dual-chamber pacemakers and single-chamber atrial pacemakers, respectively, with a resultant ICER of £6506 per QALY.
| Intervention | Total costs | Total QALYs | Incremental costs | Incremental QALYs | ICER (cost per QALY) |
|---|---|---|---|---|---|
| Deterministic results | |||||
| Single-chamber atrial pacemakers | £9211.41 | 5.51 | – | – | – |
| Dual-chamber pacemakers | £9480.47 | 5.56 | £269.06 | 0.04 | £6506 |
| Probabilistic results | |||||
| Single-chamber atrial pacemakers | £8828.23 | 5.25 | – | – | – |
| Dual-chamber pacemakers | £9104.81 | 5.30 | £276.59 | 0.05 | £6068 |
When accounting for uncertainty surrounding parameters, the mean cost associated with dual-chamber pacemakers was £9104.81 across 1000 simulations, whereas single-chamber atrial pacemakers accrued a mean cost of £8828.23, thus yielding an incremental cost of £276.59. The mean number of QALYs gained was 5.30 and 5.25 for dual-chamber pacemakers and single-chamber atrial pacemakers, respectively, with a resultant ICER of £6068 per QALY.
Results of the sensitivity analysis
Probabilistic sensitivity analysis
Using a time horizon of 10 years, the results of the probabilistic analysis are presented in Figures 12 and 13. Probabilistic sensitivity analysis revealed that, in the majority (66.00%) of cases, implanting patients with dual-chamber pacemakers resulted in greater costs and greater QALYs than implanting single-chamber atrial pacemakers. Furthermore, dual-chamber pacemakers produced more QALYs at a lower cost in 24.1% of cases and were dominated by single-chamber atrial pacemakers in 9% of cases. At a WTP threshold of £20,000, the probability of dual-chamber pacemakers being cost-effective is 72.9%, which increases to 78.7% at a WTP threshold of £30,000.
FIGURE 12.
Scatterplot of cost-effectiveness results for dual-chamber pacemakers vs. single-chamber atrial pacemakers using a time horizon of 10 years (dark-green line indicates threshold of £20,000 per additional QALY, light-green line indicates threshold of £30,000 per additional QALY). PSA, probabilistic sensitivity analysis.

FIGURE 13.
Cost-effectiveness acceptability curve for dual-chamber pacemakers vs. single-chamber atrial pacemakers using a time horizon of 10 years.
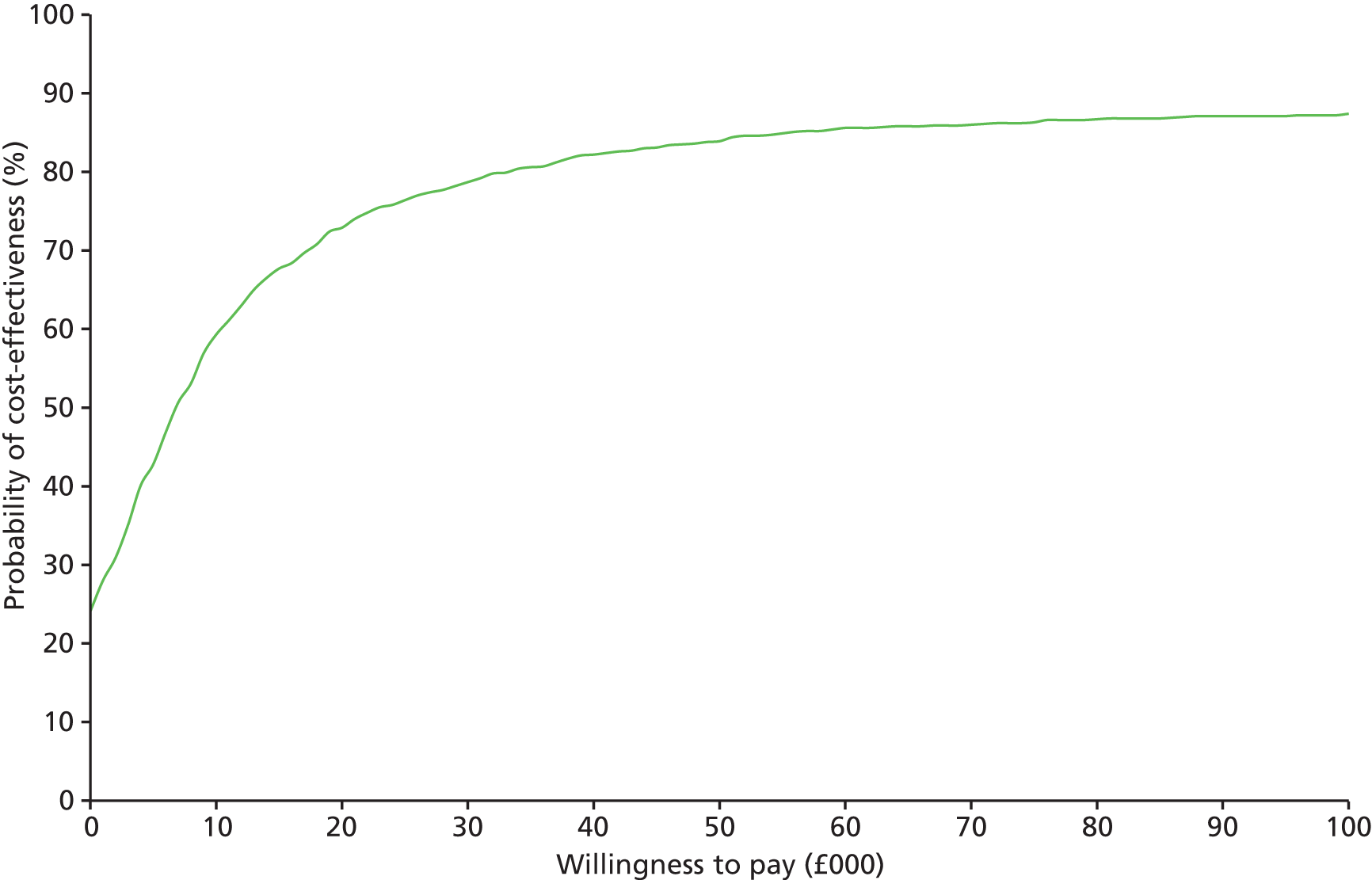
Structural sensitivity analysis
Table 58 presents the results of the structural sensitivity analysis in which the time horizon was reduced from 10 years to 5 years. The difference in costs in the deterministic results falls to £224.53, with a reduction in QALYs accrued to 0.02. This results in an increase in the deterministic ICER for the base case from £6506 to £14,261. Similarly, the probabilistic results demonstrate a fall in the incremental costs to £228.97 and a reduction in the incremental QALYs gained to 0.02, resulting in an increased ICER of £13,837. These results are perhaps to be expected; a halving of the time horizon results in roughly a halving of the difference in incremental QALYs and roughly a doubling of the resulting ICER.
| Intervention | Total costs | Total QALYs | Incremental costs | Incremental QALYs | ICER (cost per QALY) |
|---|---|---|---|---|---|
| Deterministic results | |||||
| Single-chamber atrial pacemakers | £4854.82 | 3.48 | – | – | – |
| Dual-chamber pacemakers | £5079.35 | 3.49 | £224.53 | 0.02 | £14,261 |
| Probabilistic results | |||||
| Single-chamber atrial pacemakers | £4718.12 | 3.35 | – | – | – |
| Dual-chamber pacemakers | £4947.09 | 3.37 | £228.97 | 0.02 | £13,837 |
The results of the probabilistic sensitivity analysis when the time horizon is reduced to 5 years are presented in Figures 14 and 15. At a WTP threshold of £20,000 the probability of dual-chamber pacemakers being cost-effective is 55.3%, which increases to 64.0% at a WTP threshold of £30,000.
FIGURE 14.
Scatterplot of cost-effectiveness results for dual-chamber pacemakers vs. single-chamber atrial pacemakers using a time horizon of 5 years (dark-green line indicates threshold of £20,000 per additional QALY, light-green line indicates threshold of £30,000 per additional QALY). PSA, probabilistic sensitivity analysis.
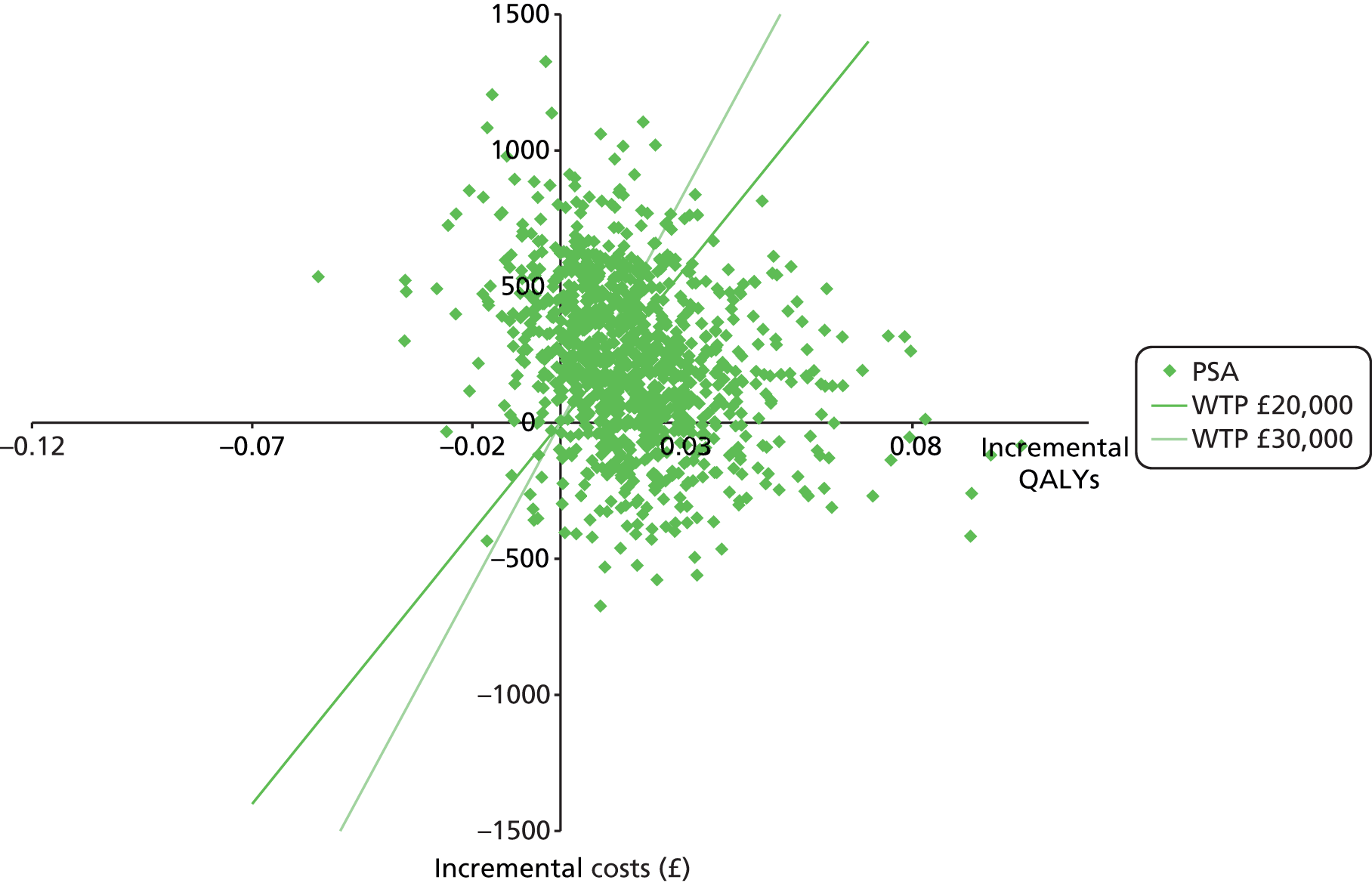
FIGURE 15.
Cost-effectiveness acceptability curve for dual-chamber pacemakers vs. single-chamber atrial pacemakers using a time horizon of 5 years.

Table 59 shows the results of the structural sensitivity analysis when risk of reoperation was taken from Kaplan–Meier data instead of using a constant risk. The incremental costs of dual-chamber pacemakers decreased to £136.43 compared with the deterministic base case whereas QALYs remained the same. This caused the ICER to decrease to £3425 per QALY.
| Intervention | Total costs | Total QALYs | Incremental costs | Incremental QALYs | ICER (cost per QALY) |
|---|---|---|---|---|---|
| Single-chamber atrial pacemakers | £9488.81 | 5.52 | – | – | – |
| Dual-chamber pacemakers | £9625.25 | 5.56 | £136.43 | 0.04 | £3425 |
One-way sensitivity analysis
As discussed in Approach to uncertainty, in addition to probabilistic sensitivity analysis, OWSA was carried out on the following parameters: age, clinical outcomes, health state utility values and all-cause mortality. The full results of these analyses are presented in Appendix 5, One-way sensitivity analysis. The TAG notes that many of the parameters tested in sensitivity analysis had minimal impact on the deterministic cost-effectiveness results and, therefore, the parameters that the ICER is most sensitive to are presented in Figure 16.
FIGURE 16.
Tornado diagram of parameters to which the cost-effectiveness of dual-chamber pacemakers vs. single-chamber atrial pacemakers is most sensitive. DC, dual chamber; SC, single chamber.
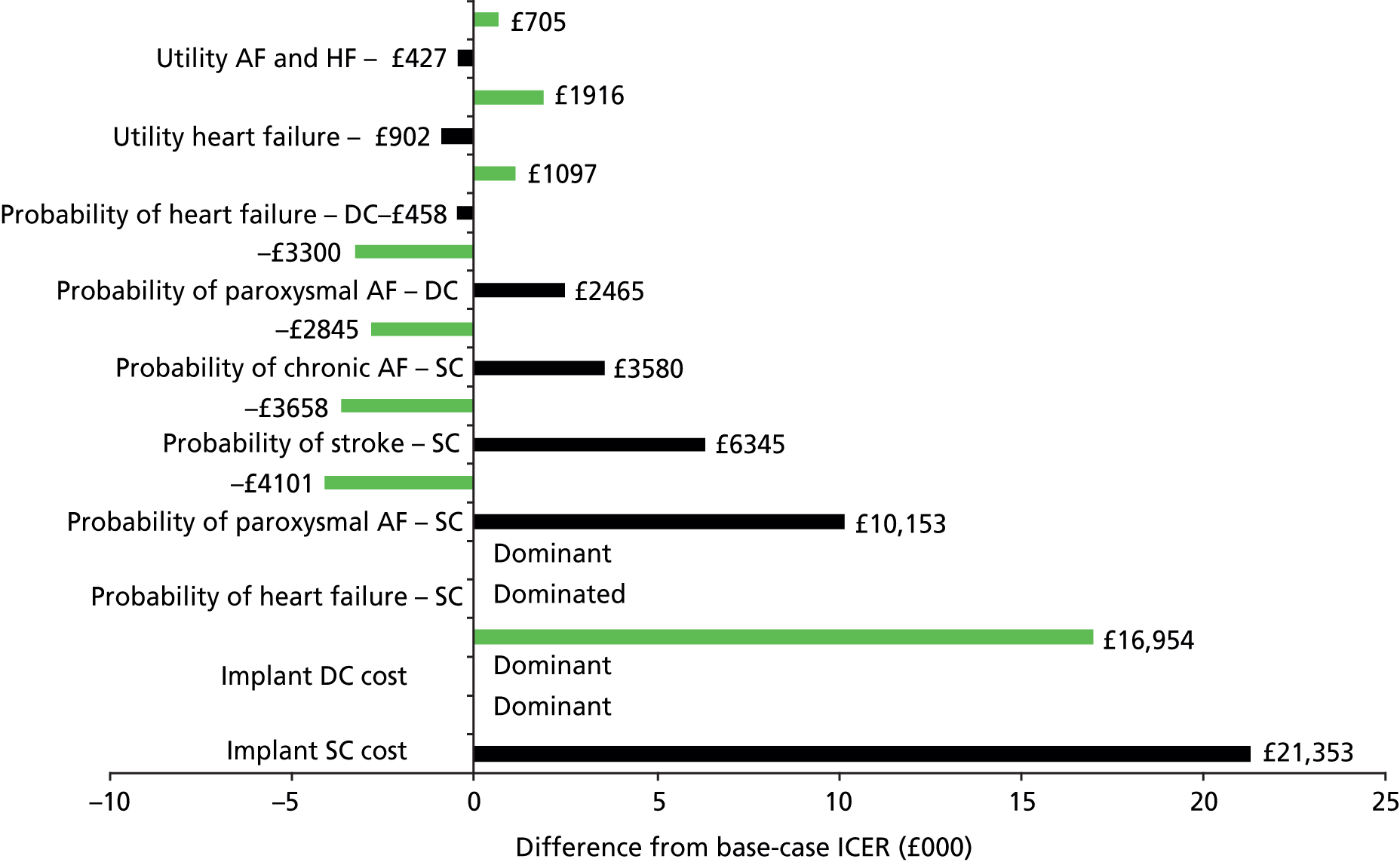
The ICER of dual-chamber pacemakers versus single-chamber atrial pacemakers was most sensitive to probability of HF in patients implanted with single-chamber atrial pacemakers. Dual-chamber pacemakers become dominated when the minimal probability is applied to single-chamber atrial pacemakers (i.e. when HF was less likely to occur in patients with single-chamber atrial pacemakers than in those with dual-chamber pacemakers). The ICER was also sensitive to implant/implantation costs for both dual-chamber and single-chamber atrial pacemakers.
Scenario analysis
As discussed in Approach to uncertainty, a series of scenario analyses was conducted to test the robustness of the results to alternative sources for parameter estimates or testing broader assumptions (e.g. reprogramming/device replacement in patients developing AF) within the model. The results of the scenario analyses are depicted in Table 60.
| Analysis | Intervention | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (cost/QALY) (£) |
|---|---|---|---|---|---|---|
| Base case | Single chamber | 9211.41 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 269.06 | 0.04 | 6056 | |
| Efficacy | ||||||
| Assuming no impact on HF (i.e. HR set to 1) | Single chamber | 9025.43 | 5.54 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 455.05 | 0.02 | 22,213 | |
| Stroke used from meta-analysis (Chapter 3, Assessment of effectiveness) | Single chamber | 9205.51 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 274.96 | 0.04 | 6438 | |
| Cost scenarios | ||||||
| Spell-level costs of pacemaker implantation from NHS Reference Costs 2012–201395 | Single chamber | 11,416.24 | 5.51 | – | – | – |
| Dual chamber | 11,754.15 | 5.56 | £337.90 | 0.04 | 7605 | |
| Cycle cost for HF from NICE’s TA8818 | Single chamber | 8277.74 | 5.51 | – | – | – |
| Dual chamber | 8594.96 | 5.56 | 317.22 | 0.04 | 7140 | |
| Cycle cost for stroke from NICE’s TA8818 | Single chamber | 11,150.63 | 5.51 | – | – | – |
| Dual chamber | 11,305.08 | 5.56 | 154.44 | 0.04 | 3476 | |
| Cycle cost for stroke from Saka et al. 2009116 | Single chamber | 9619.42 | 5.51 | – | – | – |
| Dual chamber | 9864.37 | 5.56 | 244.95 | 0.04 | 5513 | |
| Reprogramming/device replacement for AF in 0% patients | Single chamber | 8997.42 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 483.05 | 0.04 | 10,872 | |
| Reprogramming/device replacement for AF in 100% patients | Single chamber | 9639.40 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 55.60–158.92 | 0.04 | 1251 dominant | |
| Other | ||||||
| Discount rate 0% | Single chamber | 10,664.51 | 6.27 | – | – | – |
| Dual chamber | 10,936.09 | 6.33 | 271.58 | 0.05 | 5045 | |
| Discount rate 6% | Single chamber | 8380.45 | 5.07 | – | – | – |
| Dual chamber | 8651.45 | 5.11 | 271.00 | 0.04 | 6938 | |
| Market share 55% warfarin 45% NOACa | Single chamber | 9683.98 | 5.51 | – | – | – |
| Dual chamber | 9903.55 | 5.56 | 219.57 | 0.04 | 4942 | |
Assuming no difference in the risk of developing HF with the two types of implant almost quadruples the ICER, increasing it to £22,213 from a base-case value of £6506.
The only outcome from the meta-analyses conducted in Chapter 3, Results that could be implemented in the economic model was stroke. However, utilising the risk of stroke from the TAG’s meta-analysis (see Chapter 3, Assessment of effectiveness) had only a modest impact on the ICER, increasing it to £6438.
Using spell-level costs for pacemaker implantation increased the costs of dual-chamber pacemaker implantation more than the costs of single-chamber atrial pacemaker implantation. This resulted in a modest increase in the incremental costs of dual-chamber pacemakers, resulting in a slightly higher ICER (£7605 compared with £6506). The alternative cost of HF was substantially higher than in the base case and increased the incremental difference in costs between interventions. This resulted in a slightly higher ICER compared with the base case (£7140 compared with £6506). The lower alternative cost of stroke from NICE’s TA8818 had a more modest effect on the incremental cost of interventions and a relatively modest change in ICER compared with the base case (£3476 compared with £6506). Similarly, the alternative cost per episode of stroke from Saka et al. 116 had little impact on the incremental cost or the resulting ICER compared with the base case (£5513 compared with £6506).
The proportion of patients experiencing AF resulting in either reprogramming or replacement of their pacemaker was estimated as one-third in the base case, based on advice from clinical experts. This was tested in two extreme scenario analyses in which it was assumed that reprogramming/replacement was required either in no one or in 100% of patients. These two scenarios had a pronounced impact on the resulting ICERs compared with the base case; the ICER increased to £10,872 when it was assumed that reprogramming/replacement was required in 0% of people, and dual-chamber pacemakers became dominant when this was number was set to 100% (i.e. less costly and more effective).
Varying the discount rate from 3.5% in the base case to either 0% or 6% had a modest impact on the ICER. Although costs and benefits increased overall at a discount rate of 0%, the ICER was reduced to £5045. Similarly, although increasing the discount rate to 6% decreased the cost and benefits overall, the impact on the ICER was an increase to £6938.
The final individual scenario analysis undertaken was to increase the proportion of prescribing of the novel oral anticoagulants (NOACs) to a more even level with warfarin. This was achieved by setting the market share for warfarin to 55% and the NOAC market share to 45% (evenly distributed in three blocks of 15% to apixaban, dabigatran and rivaroxaban). This resulted in an overall increase in costs but a reduction in the incremental cost between the two interventions and a modest reduction in the ICER to £4942 compared with £6506 in the base case.
The results of the individual scenario analyses suggest that the base-case ICER is robust to changes in costs of pacemaker implantation, stroke, discount rate and market share of NOACs. In addition, although varying the costs of HF and the proportion of patients whose pacemaker had to be reprogrammed or replaced because of AF to extreme values had a more pronounced impact on the resulting ICER; it was still well below the NICE threshold value of £20,000. In only one scenario, in which it was assumed that there is no difference in the development of HF, did the ICER exceed £20,000, and then only by £2213.
A cumulative worst-case scenario is depicted in Table 61, in which all efficacy and cost scenario analyses found to increase the ICER beyond the base case have been combined. This results in an ICER of £48,738 compared with the base-case ICER of £6506 at 10 years. However, if it is again assumed that the proportion of patients whose pacemaker has to be reprogrammed/replaced because of AF is one-third (as in the base case), the cumulative impact of the other adjustments result in an ICER of £27,918 at 10 years.
| Analysis | Intervention | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (cost/QALY) (£) |
|---|---|---|---|---|---|---|
| Base case | Single chamber | 9211.41 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 269.06 | 0.04 | 6056 | |
| Stroke used from meta-analysis (see Chapter 3, Assessment of effectiveness) | Single chamber | 9205.51 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 274.96 | 0.04 | 6438 | |
| Cycle cost for HF from NICE’s TA8818 | Single chamber | 8271.06 | 5.51 | – | – | – |
| Dual chamber | 8594.96 | 5.56 | 323.90 | 0.04 | 7584 | |
| Spell-level costs of pacemaker implantation from NHS Reference Costs 2012–201395 | Single chamber | 10,476.27 | 5.51 | – | – | – |
| Dual chamber | 10,868.64 | 5.56 | 392.37 | 0.04 | 9187 | |
| Reprogramming/device replacement for AF in 0% patients | Single chamber | 10,092.06 | 5.51 | – | – | – |
| Dual chamber | 10,868.64 | 5.56 | 776.58 | 0.04 | 18,183 | |
| Assuming no impact on HF (i.e. HR set to 1)a | Single chamber | 9956.17 | 5.54 | – | – | – |
| Dual chamber | 10,868.64 | 5.56 | 912.47 | 0.02 | 48,738 |
Overall, the ICER increases beyond £20,000 but remains below £30,000 if, in the cumulative worst-case scenario, it is assumed either that reprogramming/device replacement because of AF is required in 0% patients or that the pacemaker has no impact on HF. It exceeds £30,000 only when both scenarios are included in the cumulative worst-case scenario.
The overall adjusted HR for risk of developing HF used in the base case indicates a non-significant increase in risk with single-chamber atrial pacemakers compared with dual-chamber pacemakers (HR 1.09, 95% CI 0.88 to 1.35). 40 Based on feedback from our clinical experts, and as was assumed in Oddershede et al. ,54 we conducted a scenario analysis assuming that there was no difference in risk of HF based on implanted device (i.e. HR 1.00).
The results of the scenario analysis and the OWSA highlight how sensitive the results are to risk of HF, with dual-chamber pacemakers being considered cost-effective or dominated by single-chamber atrial pacemakers depending on the data used. These results warranted further investigation into HF, for which we assessed the subgroups analysed from the DANPACE trial. 40
The subgroups identified as statistically significant in an analysis of risk of HF from the DANPACE trial19 were a result of age (p = 0.05); all other subgroups assessed were found to be statistically non-significant (p > 0.31). 40 We explored the impact of using the HRs for the subgroups based on age (patients > 75 years or patients ≤ 75 years) as additional scenario analyses. The results are depicted in Table 62.
| Analysis | Intervention | HF, HRa | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (cost/QALY) (£) |
|---|---|---|---|---|---|---|---|
| Base case | Single chamber | 1.09 (95% CI 0.88 to 1.35)b | 9211.41 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 269.06 | 0.04 | 6056 | ||
| Assuming no impact on HF | Single chamber | 1.00 (N/A) | 9025.43 | 5.54 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 455.05 | 0.02 | 22,213 | ||
| Patients aged > 75 years40 | Single chamber | 1.34 (95% CI 1.00 to 1.80)c | 9706.42 | 5.45 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | –225.94 | 0.11 | Dominant | ||
| Patients aged ≤ 75 years40 | Single chamber | 0.72 (95% CI 0.53 to 1.00)c | 8418.67 | 5.61 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 1061.81 | -0.06 | Dominated |
The additional scenario analyses highlight the impact that risk of HF has on the results. When the risk of HF is adjusted by age, the ICER shows that, in patients aged > 75 years, dual-chamber pacemakers dominate single-chamber atrial pacemakers (i.e. are less expensive and more effective), whereas, in patients aged ≤ 75 years, dual-chamber pacemakers are dominated by single-chamber atrial pacemakers (i.e. they are more costly and less effective).
Summary of the Technology Assessment Group’s de novo economic evaluation
An overall summary of the results from the TAG’s economic model is presented in Table 63.
| Analysis | Intervention | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (cost/QALY) (£) |
|---|---|---|---|---|---|---|
| Base case | Single chamber | 9211.41 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 269.06 | 0.04 | 6056 | |
| Structural sensitivity analyses | ||||||
| Time horizon reduced to 5 years | Single chamber | 4854.82 | 3.48 | – | – | – |
| Dual chamber | 5079.35 | 3.49 | 224.53 | 0.02 | 14,261 | |
| Utilising Kaplan–Meier data as the basis for reoperation | Single chamber | 9488.81 | 5.52 | – | – | – |
| Dual chamber | 9625.25 | 5.56 | 136.43 | 0.04 | 3425 | |
| Probabilistic sensitivity analyses | ||||||
| Base case | Single chamber | 8828.23 | 5.25 | – | – | – |
| Dual chamber | 9104.81 | 5.30 | 276.59 | 0.05 | 6068 | |
| Time horizon reduced 5 years | Single chamber | 4718.12 | 3.35 | – | – | – |
| Dual chamber | 4947.09 | 3.37 | 228.97 | 0.02 | 13,837 | |
| Efficacy scenarios | ||||||
| Stroke used from meta-analysis (see Chapter 3, Assessment of effectiveness) | Single chamber | 9205.51 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 274.96 | 0.04 | 6438 | |
| Assuming no impact on HF (i.e. HR set to 1)a | Single | 9025.43 | 5.54 | – | – | – |
| Dual | 9480.47 | 5.56 | 455.05 | 0.02 | 22,213 | |
| Cost scenarios | ||||||
| Spell-level costs of pacemaker implantation from NHS Reference Costs 2012–201395 | Single chamber | 11,416.24 | 5.51 | – | – | – |
| Dual chamber | 11,754.15 | 5.56 | £337.90 | 0.04 | 7605 | |
| Cycle cost for HF from NICE’s TA8818 | Single chamber | 8277.74 | 5.51 | – | – | – |
| Dual chamber | 8594.96 | 5.56 | 317.22 | 0.04 | 7140 | |
| Cycle cost for stroke from NICE’s TA8818 | Single chamber | 11,150.63 | 5.51 | – | – | – |
| Dual chamber | 11,305.08 | 5.56 | 154.44 | 0.04 | 3476 | |
| Cycle cost for stroke from Saka et al.116 | Single chamber | 9619.42 | 5.51 | – | – | – |
| Dual chamber | 9864.37 | 5.56 | 244.95 | 0.04 | 5513 | |
| Reprogramming/device replacement for AF in 0% patients | Single chamber | 8997.42 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 483.05 | 0.04 | 10,872 | |
| Reprogramming/device replacement for AF in 100% patients | Single chamber | 9639.40 | 5.51 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | –158.92 | 0.04 | Dominant | |
| Other scenarios | ||||||
| Discount rate 0% | Single chamber | 10,664.51 | 6.27 | – | – | – |
| Dual chamber | 10,936.09 | 6.33 | 271.58 | 0.05 | 5045 | |
| Discount rate 6% | Single chamber | 8380.45 | 5.07 | – | – | – |
| Dual chamber | 8651.45 | 5.11 | 271.00 | 0.04 | 6938 | |
| Market share: 55% warfarin and 45% NOACb | Single chamber | 9683.98 | 5.51 | – | – | – |
| Dual chamber | 9903.55 | 5.56 | 219.57 | 0.04 | 4942 | |
| Worst-case scenario | ||||||
| All efficacy and cost scenarios where the ICER increases above the base case | Single chamber | 9956.17 | 5.54 | – | – | – |
| Dual chamber | 10,868.64 | 5.56 | 912.47 | 0.02 | 48,738 | |
| Additional scenarios for HF | ||||||
| Patients aged > 75 years (i.e. HR set to 1.34) from Riahi et al. 201240 | Single chamber | 9706.42 | 5.45 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | –225.94 | 0.11 | Dominant | |
| Patients aged ≤ 75 years (i.e. HR set to 0.72) from Riahi et al. 201240 | Single chamber | 8418.67 | 5.61 | – | – | – |
| Dual chamber | 9480.47 | 5.56 | 1061.81 | -0.06 | Dominated | |
Discussion of the Technology Assessment Group’s de novo economic evaluation
The economic evaluation conducted by the TAG of dual-chamber pacemakers for treating symptomatic bradycardia due to SSS without AV block is an update of NICE’s TA88. The previous assessment found dual-chamber pacemakers to be dominated by single-chamber atrial pacemakers (i.e. the former are more expensive and less effective than the latter). Our own evaluation is based on more up-to-date estimates employed within an economic model based on NICE’s TA88. The findings are quite different in that, although dual-chamber pacemakers are more expensive, they are more clinically effective. Analyses resulted in a deterministic ICER of £6506 at 10 years.
One-way sensitivity analysis was undertaken to identify the key drivers of cost-effectiveness in the economic model. Those drivers likely to increase the deterministic ICER over £20,000 were:
-
lowest risk of HF (dual-chamber pacemakers dominated by single-chamber atrial pacemakers)
-
highest cost of implant/procedure for dual-chamber pacemaker (ICER £23,010)
-
lowest cost of implant/procedure for single-chamber atrial pacemaker (ICER £27,409).
The result for the lowest risk of HF is driven by an increase in cost of £710 and a modest reduction in benefit (–0.01 QALYs) compared with dual-chamber pacemakers.
Using the extreme values for cost or implant/implantation increased the ICER substantially, with the lowest cost for single-chamber atrial pacemaker resulting in an ICER of £27,409.
One-way sensitivity analysis can be misleading in that it may under-represent the impact of parameter uncertainty in the results of the economic model. The results from the probabilistic sensitivity analysis capture the joint uncertainty across parameter estimates. The ICER from this analysis at 10 years is £6068, which is similar to the deterministic result (£6056). This is predominantly because of a reduction in the incremental costs rather than a change in the incremental QALYs.
Parameter uncertainty is not the only form of uncertainty found within an economic model. Structural uncertainty also needs to be accounted for. Two structural sensitivity analyses were undertaken in the current evaluation: the reduction of the time horizon from 10 to 5 years and the use of the risk of reoperation from Kaplan–Meier data presented in the DANPACE trial19 in contrast to implementing risk of reoperation as a constant risk.
Reducing the time horizon from 10 years to 5 years was undertaken to assess the impact of extrapolating the results from the DANPACE trial19 beyond the typical duration of a trial participant. The results are, perhaps as might be expected, halving of the time horizon results approximately halves the difference in incremental QALYs and approximately doubles the resulting ICER. The deterministic result changes from £6506 at 10 years to £14,261 at 5 years, whereas the result of the probabilistic sensitivity analysis changes from £6068 at 10 years to £13,837 at 5 years. Based on feedback from our clinical experts, a time horizon of 10 years would appear to be the most appropriate, as the development of AV block is expected to increase steadily over time.
In the structural sensitivity analysis, the risk of reoperation was implemented as a time-dependent parameter and all patients in whom reoperation was required were assumed to be implanted with a dual-chamber device, regardless of the reason for reoperation or which pacemaker was originally implanted. The impact of undertaking this more granular approach to reoperation within the model was modest. The deterministic ICER was reduced to £3425 at 10 years because of a small reduction in incremental costs. This is likely to be a result of the risk of being reoperated occurring slightly earlier than when a constant rate is assumed and indicates that the base case may be considered a conservative assumption.
A variety of scenario analyses were undertaken where an alternative source for a parameter estimate was used. Most had a minor impact on the resulting ICER with the exception of:
-
assuming no difference in risk of developing HF (ICER of £22,213)
-
reprogramming/device replacement for AF in 0% of patients (ICER of £10,872)
-
reprogramming/device replacement for AF in 100% of patients (dual-chamber pacemakers dominate).
In only one instance did a scenario analysis result in an ICER above £20,000, and even then it was by only £2213.
The results of the scenario analysis and the OWSA highlight how sensitive the results are to risk of HF. Subgroup analysis from the DANPACE trial19 identified a significant difference in HF as a result of age (p = 0.05); all other subgroups assessed were non-significant (p > 0.31). 40 When the risk of HF is adjusted by age, in patients aged > 75 years, dual-chamber pacemakers dominate single-chamber atrial pacemakers (i.e. the former are less expensive and more effective than the latter), whereas, in patients aged ≤ 75 years, dual-chamber pacemakers are dominated by single-chamber atrial pacemakers (i.e. the former are more costly and less effective than the latter).
Chapter 5 Assessment of factors relevant to the NHS and other parties
End-of-life criteria
Based on criteria outlined by NICE, the TAG considers that neither dual-chamber pacemakers nor single-chamber atrial pacemakers are eligible for consideration as end-of-life treatments.
Chapter 6 Discussion
This MTA sought to assess the available evidence to support the use of dual-chamber pacemakers to treat symptomatic bradycardia due to SSS without AV block in comparison with single-chamber atrial pacemakers. It is a partial update of NICE’s TA88,18 which had a wider remit investigating dual-chamber pacemakers for the treatment of symptomatic bradycardia due to SSS and/or AV block. With regard to the subset of patients of interest to this research, NICE’s TA88 recommends single-chamber atrial pacemakers for patients with SSS in whom, after full evaluation, there is no evidence of impaired AV conduction.
The TAG’s systematic review of the clinical effectiveness identified six RCTs in the population of interest. Three RCTs19,37,41 had a parallel-group design and three were crossover studies. 34,35,43 The crossover trials were generally small (12–21 patients) with limited follow-up (up to 3 months), which limited their opportunity to inform the outcomes of interest for this research. The parallel-group RCTs were relatively large (50–1415 patients), had longer follow-up than the crossover studies (1–5.4 years) and measured outcomes that were of direct interest to this research.
There was limited opportunity to combine the results using meta-analysis from the six RCTs identified from the published literature. When this was possible, the results were predominantly influenced by the largest trial, DANPACE,19 which accounted for over 80% of the weight in change in pacing mode, all-cause mortality and stroke. In no instance did the level of significance of an outcome from the DANPACE trial19 change as a result of its combination in a meta-analysis of that outcome [e.g. the OR for change in pacing mode was 0.52 (95% CI 0.38 to 0.71) from the DANPACE trial19 and 0.50 (95% CI 0.37 to 0.67) from the meta-analysis including the DANPACE trial,19 Albertsen et al. 37 and Nielsen et al. 41].
In this review, dual-chamber pacing was associated with a lower risk of AF and fewer reoperations than single-chamber atrial pacing. No statistically significant difference between the pacing modes was found for mortality, HF, stroke or QoL, and there were limited data on adverse effects of pacemaker implantation. However, in the case of patients younger than 75 years, the risk of HF seems to be higher with a dual-chamber pacemaker than with a single-chamber atrial pacemaker, and for patients older than 75 years the risk seems to be lower with dual-chamber pacing compared with single-chamber atrial pacing.
The DANPACE study19 is a relatively large trial of good quality with long follow-up, which gives a reasonable evidence base for dual-chamber pacing compared with single-chamber atrial pacing for people with SSS without evidence of impaired AV conductance. Although the time horizon in the DANPACE trial19 was reasonable, the increase in AV block increases the number of patients requiring a change in pacing mode which requires an operation for patients with a single-chamber pacemaker. Additionally, the DANPACE trial19 did not allow pacemaker algorithms designed to minimise VP in patients with intact AV conduction, which have become more common since the start of this trial. Although the DDDR pacemakers in the DANPACE trial19 were programmed in a way intended to reduce unnecessary VP, the incidence of VP was still 65% (SD 33%), which may offset some of the benefit of implanting a dual-chamber pacemaker.
Patients with single-chamber atrial pacing who do not go on to develop AV block will be paced appropriately and avoid any unnecessary VP, which may have adverse consequences for cardiac function. Implanting a single-chamber atrial pacemaker may also have additional benefits in terms of reducted time for the implantation procedure, a lower risk of complications associated with the implantation of a second lead and shorter (i.e. less time) follow-up appointments. However, patients who have a dual-chamber pacemaker implanted and who go on to develop AV block will be protected by the presence of a ventricular lead and will not need a further operation to upgrade the pacemaker and insert a second lead, which is likely to be associated with higher risk of complications than first-time implantation. Additionally, the DANPACE trial19 has shown that the risk of developing paroxysmal AF is lower with dual-chamber pacing than with single-chamber atrial pacing. In addition, subgroup analysis identified that, in patients younger than 75 years, the risk of HF may be higher with a dual-chamber pacemaker than with a single-chamber atrial pacemaker, whereas, in patients older than 75 years, the risk may be lower with dual-chamber pacing than with single-chamber atrial pacing.
The systematic review49 of existing cost-effectiveness analyses identified only one study that compared dual-chamber pacemakers and single-chamber atrial pacemakers for the treatment of symptomatic bradycardia due to SSS without AV block in a UK setting and which was based on the research carried out to inform NICE’s TA88. 18 In addition, in the update of the cost-effectiveness systematic review, a study by Oddershede et al. 54 was identified, which was based on the perspective of the Danish health-care system. This study is of particular interest, as it includes the DANPACE trial19 as well as two other Danish RCTs: the pilot study for the DANPACE trial (Nielsen et al. 41) and Andersen et al. 45
One of the strengths of the Oddershede et al. 54 approach is that it was based on IPD that allowed the researchers to account for baseline characteristics such as age, sex, previous myocardial infarction and history of AF. The researchers were also able to categorise patients as low risk or high risk (and, by deduction, the remainder of patients as at moderate risk) of a subsequent event. For each of the risk categories, and for an evaluation based on all patients, Oddershede et al. 54 found that the probability of dual-chamber pacemakers being cost-effective compared with single-chamber atrial pacemakers was > 50% at a WTP threshold of £20,000. This fell to > 40% at a WTP threshold of £30,000. This is likely to be a result of the incremental QALY decrement associated with dual-chamber pacemakers in their analysis. However, the model developed by Oddershede et al. 54 focused primarily on the occurrence of stroke and death, which may have restricted the comprehensiveness of the analysis to assess fully costs and benefits.
As no pre-existing economic evaluation adequately presents the cost-effectiveness of dual-chamber pacemakers in comparison with single-chamber atrial pacemakers for the treatment of symptomatic bradycardia due to SSS without AV block in a UK setting, the TAG developed a de novo economic model to help inform this important question.
As there were concerns around potential clinical heterogeneity as a result of different patient populations (e.g. prior history of AF) and different device programming used (e.g. different per cent VP) in the RCTs identified, the decision was made to base the model on the DANPACE trial. The base-case results of the TAG’s economic model demonstrate that dual-chamber pacemakers are more expensive but also more effective than single-chamber atrial pacemakers, resulting in an ICER of £6506. Probabilistic sensitivity analysis reduced this figure to £5989, principally because the incremental cost was lowered. This reduction in the difference in cost is likely to be because of the non-linearity of the minimum–maximum cost of implant/implantation of a single pacemaker compared with the minimum–maximum cost of implant/implantation of a dual pacemaker. The likelihood that dual-chamber pacemakers are cost-effective was found to be over 70% at a threshold of either £20,000 or £30,000.
As the deterministic results and the probabilistic results were so similar all subsequent analyses were based on the deterministic model.
A structural sensitivity analysis looking at a more granular approach to incorporating risk of reoperation using the available Kaplan–Meier data from the DANPACE trial19 reduced the ICER from £6506 to £3425. A second structural sensitivity analysis reducing the time horizon to 5 years more than doubled the base-case ICER to £14,261. In essence, halving the time horizon halved the incremental benefit.
One-way sensitivity analysis highlighted the key drivers of cost-effectiveness in the economic model. Those likely to increase the deterministic ICER to over £20,000 were:
-
lowest risk of HF (dual-chamber pacemakers dominated by single-chamber atrial pacemakers)
-
highest cost of implant/procedure for dual-chamber pacemakesr (ICER of £23,010)
-
lowest cost of implant/procedure for single-chamber atrial pacemakers (ICER of £27,409).
The result for the lowest risk of HF is being driven by an increase in cost of £710 and a modest reduction in benefit (–0.01 QALYs) compared with dual-chamber pacemakers.
A series of scenario analyses were undertaken to test the impact on the results when using alternative sources for parameter estimates or challenge assumptions in the model. The scenario analyses that raised the ICER above the base case were:
-
assuming no difference in HF (ICER of £22,213)
-
using the risk of stroke from the TAG’s meta-analysis (ICER of £6438)
-
using spell-level costs of pacemaker implantation (ICER of £7605)
-
using monthly cost of HF from NICE’s TA8818 (ICER of £7140)
-
using reprogramming/device replacement for AF of 0% (ICER of £10,897)
-
using a discount rate of 6% (ICER of £6938).
Only when we assume that the risk of developing HF is the same regardless of implanted device does the ICER increase beyond £20,000, albeit by a modest amount, to £22,213.
A cumulative worst-case scenario was also conducted that combined the monthly cost of HF from NICE’s TA88,18 the risk of stroke from the meta-analysis conducted by the TAG, the spell-level costs of implantation, reprogramming/device replacement for AF of 0% and the assumption of no difference in risk of developing HF between the two types of implant. This resulted in an ICER of £48,738.
The results of the scenario analysis and the OWSA highlight how sensitive the results are to risk of HF, with dual-chamber pacemakers being considered cost-effective or dominated by single-chamber atrial pacemakers depending on the data used. Subgroup analysis from the DANPACE trial19 identified a significant difference in HF owing to age (p = 0.05), but no significant differences were found in each subgroup (p > 0.31). 40 When the risk of HF is assessed by age, the ICER is reduced compared with the base case in patients aged > 75 years (£4918 vs. £6506, respectively), whereas dual-chamber pacemakers are dominated by single-chamber atrial pacemakers in patients aged ≤ 75 years (i.e. they are more costly and less effective).
Statement of principal findings
This MTA uses the best available evidence to explore the clinical and cost-effectiveness implications for using dual-chamber pacemakers rather than single-chamber atrial pacemakers to treat symptomatic bradycardia due to SSS without AV block. The DANPACE trial19 found that dual-chamber pacemakers significantly reduced the risk of reoperation to change mode of pacing compared with single-chamber atrial pacemakers (9.3% vs. 0.6%, p < 0.001). The difference is primarily because of the development of AV block requiring upgrade to a dual-chamber device. The DANPACE trial19 also demonstrated a reduced risk of paroxysmal AF with dual-chamber pacing compared with single-chamber atrial pacing (OR 0.75, 95% CI 0.59 to 0.96). No statistically significant difference between the pacing modes was found for mortality, HF, stroke or QoL. However, the risk of developing HF may vary with age and device.
The de novo economic model developed by the TAG shows that dual-chamber pacemakers are more expensive and more effective than single-chamber atrial devices resulting in a base case ICER of £6506. The ICER remains below £20,000 in probabilistic sensitivity analysis, structural sensitivity analysis and most scenario analyses and OWSA.
A potentially important finding of this MTA is the impact that HF may have on the decision to use dual-chamber pacemakers or single-chamber atrial pacemakers to treat symptomatic bradycardia due to SSS without AV block. The results of an analysis based on age (> 75 years or ≤ 75 years) and risk of HF indicate that dual-chamber pacemakers are the dominant treatment option in older patients (i.e. are less costly and more effective), whereas, in younger patients, dual-chamber pacemakers are dominated by single-chamber atrial pacemakers (i.e. the former are more expensive and less effective than the latter). However, these results are based on a subgroup analysis and should be treated with caution.
Strengths and limitations of the assessment
Strengths
-
The evidence used to inform the decision problem that is the focus of this MTA has been identified following the general principles published by the CRD. 30
-
Economic analyses have been carried out in accordance with the Guide to the Methods of Technology Appraisal 2013106 and the International Society For Pharmacoeconomics and Outcomes Research’s guidance for decision analytic models. 117
-
The economic model used to provide a framework for analysis is based primarily on the economic model constructed in NICE’s TA88. 18 In addition, parameter estimates have been informed by the best available evidence.
-
Expert clinical input has been sought and received throughout the project, in particular with respect to assumptions made in clinical and economic analyses and the face validity of final results and conclusions.
Weaknesses
-
The number of RCTs available to inform this decision question was limited, and those trials that were identified failed to report the results in a consistent manner.
-
The technologies under investigation are developing rapidly, such that the single-chamber atrial pacemakers or dual-chamber pacemakers used in current trials are likely to be superseded by newer implants (and/or pacing algorithms) prior to their completion.
-
A cohort approach using the adjusted trial level data from the DANPACE trial19 was used to populate the efficacy parameters within the economic model rather than a microsimulation informed by IPD.
-
The costs of the individual pacemakers under consideration were unavailable for use within the economic model and so the average costs reported within the appropriate HRG codes were used.
Uncertainties
The DANPACE study19 is the single largest RCT that has been conducted to compare single-chamber atrial pacemakers and dual-chamber pacemakers in patients with symptomatic bradycardia due to SSS and no evidence of AV block. However, it does not conclusively answer the clinically relevant questions concerning a difference in risk of HF, stroke and all-cause mortality. It seems unlikely that larger studies will be conducted to investigate these outcomes, and will not use the same pacemakers used in the DANPACE trial,19 as pacemaker design and pacing modes have rapidly changed over time and look likely to continue to change in the future.
Typically in a cost-effectiveness analysis, the acquisition costs of the interventions are known and the uncertainty in costs lies elsewhere. However, as the manufacturers declined the opportunity to make a submission and were unable to supply costs for devices in the time allowed, the costs for the individual pacemakers under consideration in this MTA were unavailable. We had to use the average costs reported within the appropriate HRG codes, which incorporate the cost of device plus the cost of implantation. There was considerable uncertainty in the economic evaluation as a result of implementing these costs. It was not possible to disentangle the uncertainty relating to cost of devices and cost of implantation.
Other relevant factors
Based on criteria outlined by NICE, the TAG considers that neither dual-chamber pacemakers nor single-chamber atrial pacemakers are eligible for consideration as end-of-life treatments.
Chapter 7 Conclusions
Implications for service provision
Feedback from our clinical experts indicates that many centres are generally implanting dual-chamber pacemakers rather than single-chamber atrial pacemakers in patients with symptomatic bradycardia due to SSS. Individual patient characteristics may dictate the use of single-chamber atrial pacemakers, for example concerns over potential ventricular remodelling over a prolonged period of time, but these would apply in specific circumstances only. As such, it appears that there would be minimal implications for service provision if dual-chamber pacemakers were to be favoured over single-chamber atrial pacemakers.
Suggested research priorities
Further RCTs investigating the impact of dual-chamber pacemakers compared with single-chamber atrial pacemakers and focusing on their impact on HF, stroke, and all-cause mortality would be desirable. However, the size of trials required to answer conclusively these important clinical questions may make them prohibitively expensive.
Assessment of the impact of treatments on patient QoL may be of interest to the wider clinical community, particularly in patients with and without AV block.
Further research into the cost of implantation and the adverse events associated with implanting a dual-chamber or single-chamber atrial pacemaker may also be warranted.
Acknowledgements
The Assessment Group would like to thank Dr Janet McComb (consultant cardiologist), Dr Alison Seed (consultant cardiologist) and Dr Derick Todd (consultant cardiologist) for providing clinical advice throughout the project. Thanks also to Dr Neil Sulke (consultant cardiologist) for providing comments on the technology assessment report. The TAG would also like to thank Dr Ifigeneia Mavranezouli (senior health economist) for providing feedback on the proposed economic analysis and for the economic sections of the report.
Contributions of authors
Steven J Edwards Project lead: supervised the production of the final report; contributed to the writing of the report; conducted critical appraisal of stakeholder submissions; conducted critical appraisal of the clinical evidence; and conducted critical appraisal of the economic evidence.
Charlotta Karner Devised and carried out the clinical literature searches; carried out study selection; carried out data extraction; contributed to the writing of the report; and conducted critical appraisal of the stakeholder submissions.
Nicola Trevor Devised and carried out the economic literature searches; carried out study selection; carried out data extraction; led the development of the economic model; contributed to the writing of the report; and conducted critical appraisal of stakeholder submissions.
Victoria Wakefield Devised and carried out the clinical literature searches; carried out study selection; carried out data extraction; contributed to the writing of the report; and conducted critical appraisal of the stakeholder submissions.
Fatima Salih Devised and carried out the economic literature searches; carried out study selection; carried out data extraction; led the development of the economic model; contributed to the writing of the report; and conducted critical appraisal of stakeholder submissions.
All authors read and commented on draft versions of the TAG’s report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation 2007;115:1921-32. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.616011.
- Semelka M, Gera J, Usman S. Sick sinus syndrome: a review. Am Fam Physician 2013;87:691-6.
- Ferrer MI. The sick sinus syndrome. Circulation 1973;47:635-41. http://dx.doi.org/10.1161/01.CIR.47.3.635.
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;51:e1-62. http://dx.doi.org/10.1016/j.jacc.2008.02.032.
- Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation 2011;123:1010-20. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.940577.
- Rose G, Baxter PJ, Reid DD, McCartney P. Prevalence and prognosis of electrocardiographic findings in middle-aged men. Br Heart J 1978;40:636-43. http://dx.doi.org/10.1136/hrt.40.6.636.
- Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281-329. http://dx.doi.org/10.1093/eurheartj/eht150.
- Adan V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician 2003;67:1725-32.
- British Pacing and Electrophysiology Group . Recommendations for pacemaker prescription for symptomatic bradycardia. Report of a working party of the British Pacing and Electrophysiology Group. Br Heart J 1991;66:185-91. http://dx.doi.org/10.1136/hrt.66.2.185.
- Lopez-Jimenez F, Goldman L, Orav EJ, Ellenbogen K, Stambler B, Marinchak R, et al. Health values before and after pacemaker implantation. Am Heart J 2002;144:687-92. http://dx.doi.org/10.1016/S0002-8703(02)00143-6.
- Mlynarski R, Wlodyka A, Kargul W. Changes in the mental and physical components of the quality of life for patients six months after pacemaker implantation. Cardiol J 2009;16:250-3.
- Barros RT, Carvalho SM, Silva MA, Borges JB. Evaluation of patients’ quality of life aspects after cardiac pacemaker implantation. Rev Bras Cir Cardiovasc 2014;29:37-44. http://dx.doi.org/10.5935/1678-9741.20140009.
- Nowak B, Misselwitz B, Erdogan A, Funck R, Irnich W, Israel CW, et al. Do gender differences exist in pacemaker implantation? Results of an obligatory external quality control program. Europace 2010;12:210-15. http://dx.doi.org/10.1093/europace/eup312.
- Rosenqvist M. Atrial pacing for sick sinus syndrome. Clin Cardiol 1990;13:43-7. http://dx.doi.org/10.1002/clc.4960130108.
- Stofmeel MA, Post MW, Kelder JC, Grobbee DE, van Hemel NM. Quality-of-life of pacemaker patients: a reappraisal of current instruments. Pacing Clin Electrophysiol 2000;23:946-52. http://dx.doi.org/10.1111/j.1540-8159.2000.tb00879.x.
- Gadler F, Linde C, Daubert C, McKenna W, Meisel E, Aliot E, et al. Significant improvement of quality of life following atrioventricular synchronous pacing in patients with hypertrophic obstructive cardiomyopathy. Data from 1 year of follow-up. PIC study group. Pacing In Cardiomyopathy. Eur Heart J 1999;20:1044-50. http://dx.doi.org/10.1053/euhj.1998.1331.
- Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation 1981;64:1227-34. http://dx.doi.org/10.1161/01.CIR.64.6.1227.
- Dual-Chamber Pacemakers for Symptomatic Bradycardia due to Sick Sinus Syndrome and/or Atrioventricular Block. London: NICE; 2005.
- Nielsen JC, Thomsen PE, Hojberg S, Moller M, Vesterlund T, Dalsgaard D, et al. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J 2011;32:686-96. http://dx.doi.org/10.1093/eurheartj/ehr022.
- Hospital Episode Statistics, Admitted Patient Care, England. 2012–13: Procedures and Interventions. Leeds: Health and Social Care Information Centre; 2013.
- Cunningham D, Charles R, Cunningham M, de Lange A. National Audit Of Cardiac Rhythm Management Devices. London: National Institute for Cardiovascular Outcomes Research; 2012.
- Bernstein AD, Camm AJ, Fletcher RD, Gold RD, Rickards AF, Smyth NP, et al. The NASPE/BPEG generic pacemaker code for antibradyarrhythmia and adaptive-rate pacing and antitachyarrhythmia devices. Pacing Clin Electrophysiol 1987;10:794-9. http://dx.doi.org/10.1111/j.1540-8159.1987.tb06035.x.
- Chow AW, Buxton AE. Implantable Cardiac Pacemakers and Defibrillators: All You Wanted to Know. Oxford: John Wiley & Sons; 2006.
- Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation 2010;122:1553-61. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.976076.
- van Eck JW, van Hemel NM, de Voogt WG, Meeder JG, Spierenburg HA, Crommentuyn H, et al. Routine follow-up after pacemaker implantation: frequency, pacemaker programming and professionals in charge. Europace 2008;10:832-7. http://dx.doi.org/10.1093/europace/eun093.
- Aggarwal RK, Connelly DT, Ray SG, Ball J, Charles RG. Early complications of permanent pacemaker implantation: no difference between dual and single chamber systems. Br Heart J 1995;73:571-5. http://dx.doi.org/10.1136/hrt.73.6.571.
- van Eck JW, van Hemel NM, Zuithof P, van Asseldonk JP, Voskuil TL, Grobbee DE, et al. Incidence and predictors of in-hospital events after first implantation of pacemakers. Europace 2007;9:884-9. http://dx.doi.org/10.1093/europace/eum113.
- Trappe HJ, Gummert J. Current pacemaker and defibrillator therapy. Dtsch Arztebl Int 2011;108:372-9. http://dx.doi.org/10.3238/arztebl.2011.0372.
- Harcombe AA, Newell SA, Ludman PF, Wistow TE, Sharples LD, Schofield PM, et al. Late complications following permanent pacemaker implantation or elective unit replacement. Heart 1998;80:240-4. http://dx.doi.org/10.1136/hrt.80.3.240.
- CRD’s Guidance for Undertaking Reviews in Healthcare. Centre for Reviews and Dissemination. York: CRD; 2011.
- Higgins J, Green SE. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration; 2011.
- Scottish Intercollegiate Guidelines Network (SIGN) . Search Filters 2013. www.sign.ac.uk/methodology/filters.html (accessed June 2014).
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31:140-9. http://dx.doi.org/10.1093/ije/31.1.140.
- Gallik DM, Guidry GW, Mahmarian JJ, Verani MS, Spencer WH, Gallik DM, et al. Comparison of ventricular function in atrial rate adaptive versus dual chamber rate adaptive pacing during exercise. Pacing Clin Electrophysiol 1994;17:179-85. http://dx.doi.org/10.1111/j.1540-8159.1994.tb01370.x.
- Lau CP, Tai YT, Leung WH, Wong CK, Lee P, Chung FL, et al. Rate adaptive pacing in sick sinus syndrome: effects of pacing modes and intrinsic conduction on physiological responses, arrhythmias, symptomatology and quality of life. Eur Heart J 1994;15:1445-55.
- Theodorakis G, Fitzpatrick A, Vardas P, Sutton R. Resting echo-Doppler estimation of cardiac output during AAI and DDD pacing, with varying AV delay, at different pacing rates. Eur J Cardiac Pacing Electrophysiol 1992;2:22-5.
- Albertsen AE, Nielsen JC, Poulsen SH, Mortensen PT, Pedersen AK, Hansen PS, et al. DDD(R)-pacing, but not AAI(R)-pacing induces left ventricular desynchronization in patients with sick sinus syndrome: tissue-Doppler and 3D echocardiographic evaluation in a randomized controlled comparison. Europace 2008;10:127-33. http://dx.doi.org/10.1093/europace/eum279.
- Andersen HR, Svendsen JH. The Danish multicenter randomized study on atrial inhibited versus dual-chamber pacing in sick sinus syndrome (the DANPACE study): purpose and design of the study. Heart Drug 2001;1:67-70. http://dx.doi.org/10.1159/000048939.
- Nielsen JC, Thomsen PE, Højberg S, Møller M, Riahi S, Dalsgaard D, et al. Atrial fibrillation in patients with sick sinus syndrome: the association with PQ-interval and percentage of ventricular pacing. Europace 2012;14:682-9. http://dx.doi.org/10.1093/europace/eur365.
- Riahi S, Nielsen JC, Hjortshøj S, Thomsen PE, Højberg S, Møller M, et al. Heart failure in patients with sick sinus syndrome treated with single lead atrial or dual-chamber pacing: no association with pacing mode or right ventricular pacing site. Europace 2012;14:1475-82. http://dx.doi.org/10.1093/europace/eus069.
- Nielsen JC, Kristensen L, Andersen HR, Mortensen PT, Pedersen OL, Pedersen AK, et al. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol 2003;42:614-23. http://dx.doi.org/10.1016/S0735-1097(03)00757-5.
- Kristensen L, Nielsen JC, Mortensen PT, Pedersen OL, Pedersen AK, Andersen HR, et al. Incidence of atrial fibrillation and thromboembolism in a randomised trial of atrial versus dual-chamber pacing in 177 patients with sick sinus syndrome. Heart 2004;90:661-6. http://dx.doi.org/10.1136/hrt.2003.016063.
- Schwaab B, Kindermann M, Schatzer-Klotz D, Berg M, Franow H, Frohlig G, et al. AAIR versus DDDR pacing in the bradycardia tachycardia syndrome: a prospective, randomized, double-blind, crossover trial. Pacing Clin Electrophysiol 2001;24:1585-95. http://dx.doi.org/10.1046/j.1460-9592.2001.01585.x.
- Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932-7. http://dx.doi.org/10.1161/01.CIR.0000072769.17295.B1.
- Andersen HR, Nielsen JC, Thomsen PEB, Thuesen L, Mortensen PT, Vesterlund T, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet 1997;350:1210-16. http://dx.doi.org/10.1016/S0140-6736(97)03425-9.
- Wise RA, Brown CD. Minimal clinically important differences in the six-minute walk test and the incremental shuttle walking test. COPD 2005;2:125-9. http://dx.doi.org/10.1081/COPD-200050527.
- Mahoney CB. Pacing modes and patient outcomes: the economic benefit of atrial-based pacing. J Cardiovasc Electrophysiol 1994;5:x-xi.
- Sutton R, Bourgeois I. Cost benefit analysis of single and dual-chamber pacing for sick sinus syndrome and atrioventricular block. An economic sensitivity analysis of the literature. Eur Heart J 1996;17:574-82. http://dx.doi.org/10.1093/oxfordjournals.eurheartj.a014911.
- Castelnuovo E, Stein K, Pitt M, Garside R, Payne E, Castelnuovo E, et al. The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation. Health Technol Assess 2001;9.
- Caro J, Ward A, Moller J. Modelling the health benefits and economic implications of implanting dual-chamber vs. single-chamber ventricular pacemakers in the UK. Europace 2006;8:449-55. http://dx.doi.org/10.1093/europace/eul042.
- Clarke KW, Connelly DT, Charles RG. Single chamber atrial pacing: an underused and cost-effective pacing modality in sinus node disease. Heart 1998;80:387-9. http://dx.doi.org/10.1136/hrt.80.4.387.
- Deniz HB, Caro JJ, Ward A, Moller J, Malik F, Deniz HB, et al. Economic and health consequences of managing bradycardia with dual-chamber compared to single-chamber ventricular pacemakers in Italy. J Cardiovasc Med 2008;9:43-50. http://dx.doi.org/10.2459/JCM.0b013e328013cd28.
- O’Brien BJ, Blackhouse G, Goeree R, Healey JS, Roberts RS, Gent M, et al. Cost-effectiveness of physiologic pacing: results of the Canadian Health Economic Assessment of Physiologic Pacing. Heart Rhythm 2005;2:270-5. http://dx.doi.org/10.1016/j.hrthm.2004.12.021.
- Oddershede L, Riahi S, Nielsen JC, Hjortshøj S, Andersen HR, Ehlers L, et al. Health economic evaluation of single-lead atrial pacing vs. dual-chamber pacing in sick sinus syndrome. Europace 2014;16:866-72. http://dx.doi.org/10.1093/europace/eut384.
- Osman F, Krishnamoorthy S, Nadir A, Mullin P, Morley-Davies A, Creamer J, et al. Safety and cost-effectiveness of same day permanent pacemaker implantation. Am J Cardiol 2010;106:383-5. http://dx.doi.org/10.1016/j.amjcard.2010.03.038.
- Rinfret S, Cohen DJ, Lamas GA, Fleischmann KE, Weinstein MC, Orav J, et al. Cost-effectiveness of dual-chamber pacing compared with ventricular pacing for sinus node dysfunction. Circulation 2005;111:165-72. http://dx.doi.org/10.1161/01.CIR.0000151810.69732.41.
- Ray SG, Griffith MJ, Jamieson S, Bexton RS, Gold RG. Impact of the recommendations of the British Pacing and Electrophysiology Group on pacemaker prescription and on the immediate costs of pacing in the Northern Region. Br Heart J 1992;68:531-4. http://dx.doi.org/10.1136/hrt.68.11.531.
- Wiegand UKH, Potratz J, Bode F, Schreiber R, Bonnemeier H, Peters W, et al. Cost-effectiveness of dual-chamber pacemaker therapy: does single lead VDD pacing reduce treatment costs of atrioventricular block?. Eur Heart J 2001;22:174-80. http://dx.doi.org/10.1053/euhj.2000.2126.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Central Cardiac Audit Database . National Pacemaker Database Annual Report 2002 2002. www.ccad.org.uk.
- Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D’Agostino RB, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. J Am Med Assoc 2003;290:1049-56. http://dx.doi.org/10.1001/jama.290.8.1049.
- Connolly SJ, Kerr CR, Gent M, Roberts RS, Yusuf S, Gillis AM, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med 2000;342:1385-91. http://dx.doi.org/10.1056/NEJM200005113421902.
- Skanes AC, Krahn AD, Yee R, Klein GJ, Connolly SJ, Kerr CR, et al. Progression to chronic atrial fibrillation after pacing: the Canadian Trial of Physiologic Pacing. CTOPP Investigators. J Am Coll Cardiol 2001;38:167-72. http://dx.doi.org/10.1016/S0735-1097(01)01326-2.
- Ellenbogen KA, Stambler BS, Orav EJ, Sgarbossa EB, Tullo NG, Love CA, et al. Clinical characteristics of patients intolerant to VVIR pacing. Am J Cardiol 2000;86:59-63. http://dx.doi.org/10.1016/S0002-9149(00)00828-6.
- Hart RG, Halperin JL, Pearce LA, Anderson DC, Kronmal RA, McBride R, et al. Lessons from the Stroke Prevention in Atrial Fibrillation trials. Ann Intern Med 2003;138:831-8. http://dx.doi.org/10.7326/0003-4819-138-10-200305200-00011.
- NHS Reference Costs 2002. London: DH; 2002.
- Link MS, Estes NA, Griffin JJ, Wang PJ, Maloney JD, Kirchhoffer JB, et al. Complications of dual chamber pacemaker implantation in the elderly. Pacemaker Selection in the Elderly (PASE) Investigators. J Interv Card Electrophysiol 1998;2:175-9. http://dx.doi.org/10.1023/A:1009707700412.
- Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002;346:1854-62. http://dx.doi.org/10.1056/NEJMoa013040.
- Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol 2001;37:371-8. http://dx.doi.org/10.1016/S0735-1097(00)01107-4.
- Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920-5. http://dx.doi.org/10.1161/01.CIR.0000072767.89944.6E.
- Lamas GA, Orav EJ, Stambler BS, Ellenbogen KA, Sgarbossa EB, Huang SK, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker Selection in the Elderly Investigators. N Engl J Med 1998;338:1097-104. http://dx.doi.org/10.1056/NEJM199804163381602.
- Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke 2003;34:122-6. http://dx.doi.org/10.1161/01.STR.0000047852.05842.3C.
- MacIntyre K, Capewell S, Stewart S, Chalmers JW, Boyd J, Finlayson A, et al. Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation 2000;102:1126-31. http://dx.doi.org/10.1161/01.CIR.102.10.1126.
- Jahangir A, Shen WK, Neubauer SA, Ballard DJ, Hammill SC, Hodge DO, et al. Relation between mode of pacing and long-term survival in the very elderly. J Am Coll Cardiol 1999;33:1208-16. http://dx.doi.org/10.1016/S0735-1097(99)00005-4.
- The New NHS – Reference Costs. Leeds: NHS Executive; 2002.
- Majeed A, Moser K, Carroll K. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994–1998: analysis of data from the general practice research database. Heart 2001;86:284-8. http://dx.doi.org/10.1136/heart.86.3.284.
- Cooper HA, Bloomfield DA, Bush DE, Katcher MS, Rawlins M, Sacco JD, et al. Relation between achieved heart rate and outcomes in patients with atrial fibrillation [from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Study]. Am J Cardiol 2004;93:1247-53. http://dx.doi.org/10.1016/j.amjcard.2004.01.069.
- Steinberg JS, Sadaniantz A, Kron J, Krahn A, Denny DM, Daubert J, et al. Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004;109:1973-80. http://dx.doi.org/10.1161/01.CIR.0000118472.77237.FA.
- Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825-33. http://dx.doi.org/10.1056/NEJMoa021328.
- Kavanagh S, Knapp M, Patel A. Costs and disability among stroke patients. J Public Health 1999;21:385-94. http://dx.doi.org/10.1093/pubmed/21.4.385.
- Hogenhuis W, Stevens SK, Wang P, Wong JB, Manolis AS, Estes NA, et al. Cost-effectiveness of radiofrequency ablation compared with other strategies in Wolff–Parkinson–White syndrome. Circulation 1993;88:II437-46.
- Tengs TO, Yu M, Luistro E. Health-related quality of life after stroke a comprehensive review. Stroke 2001;32:964-72. http://dx.doi.org/10.1161/01.STR.32.4.964.
- Andersen HR, Thuesen L, Bagger JP, Vesterlund T, Thomsen PEB. Prospective randomised trial of atrial versus ventricular pacing in sick-sinus syndrome. Lancet 1994;344:1523-8. http://dx.doi.org/10.1016/S0140-6736(94)90347-6.
- Italian Institute for Statistics (ISTAT) n.d. www.istat.it (accessed 27 February 2007).
- Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics 1997;53:419-34. http://dx.doi.org/10.2307/2533947.
- Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003;107:1614-19. http://dx.doi.org/10.1161/01.CIR.0000057981.70380.45.
- Cohen HE. Drug Topics Red Book. Montvale, NJ: Thomson Medical Economics; 2001.
- Office for National Statistics . Mortality Statistics: Deaths Registered in 2012 2013. www.ons.gov.uk/ons/rel/vsob1/mortality-statistics--deaths-registered-in-england-and-wales--series-dr-/2012/index.html (accessed June 2014).
- Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 2007;49:986-92. http://dx.doi.org/10.1016/j.jacc.2006.10.062.
- Carter AM, Catto AJ, Mansfield MW, Bamford JM, Grant PJ. Predictive variables for mortality after acute ischemic stroke. Stroke 2007;38:1873-80. http://dx.doi.org/10.1161/STROKEAHA.106.474569.
- Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 2006;27:65-7. http://dx.doi.org/10.1093/eurheartj/ehi555.
- Fleischmann KE, Orav EJ, Lamas GA, Mangione CM, Schron E, Lee KL, et al. Pacemaker implantation and quality of life in the Mode Selection Trial (MOST). Heart Rhythm 2006;3:653-9. http://dx.doi.org/10.1016/j.hrthm.2006.02.1031.
- Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology 2013;81:1588-95. http://dx.doi.org/10.1212/WNL.0b013e3182a9f45f.
- Fleischmann KE, Orav EJ, Lamas GA, Mangione CM, Schron EB, Lee KL, et al. Atrial fibrillation and quality of life after pacemaker implantation for sick sinus syndrome: data from the Mode Selection Trial (MOST). Am Heart J 2009;158:78-83. http://dx.doi.org/10.1016/j.ahj.2009.02.023.
- NHS Reference Costs 2012– 2013. London: DH; 2013.
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, et al. Coronary Heart Disease Statistics. Birmingham: British Heart Foundation; 2012.
- Luengo-Fernandez R, Gray AM, Rothwell PM. A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke 2012;43:3343-51. http://dx.doi.org/10.1161/STROKEAHA.112.667204.
- Luengo-Fernandez R, Yiin GS, Gray AM, Rothwell PM. Population-based study of acute- and long-term care costs after stroke in patients with AF. Int J Stroke 2013;8:308-14. http://dx.doi.org/10.1111/j.1747-4949.2012.00812.x.
- Stewart S, Murphy N, Walker A, McGuire A, McMurray JJV. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004;90:286-92. http://dx.doi.org/10.1136/hrt.2002.008748.
- British National Formulary (Online). London: BMJ and Pharmaceutical Press; 2014.
- Electronic Market Information Tool (eMit). London: DH; 2013.
- Gallagher AM, van Staa TP, Murray-Thomas T, Schoof N, Clemens A, Ackermann D, et al. Population-based cohort study of warfarin-treated patients with atrial fibrillation: incidence of cardiovascular and bleeding outcomes. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-003839.
- Camm AJ, Kirchhof P, Lip GYH, Schotten U, . European Association for Cardio-Thoracic Surgery (EACTS) . Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360-420. http://dx.doi.org/10.1093/europace/euq350.
- Cowie MR, Wood DA, Coats AJ, Thompson SG, Suresh V, Poole-Wilson PA, et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart 2000;83:505-10. http://dx.doi.org/10.1136/heart.83.5.505.
- Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A, et al. The prognosis of heart failure in the general population: The Rotterdam Study. Eur Heart J 2001;22:1318-27. http://dx.doi.org/10.1053/euhj.2000.2533.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Link MS, Hellkamp AS, Estes NA III, Orav EJ, Ellenbogen KA, Ibrahim B, et al. High incidence of pacemaker syndrome in patients with sinus node dysfunction treated with ventricular-based pacing in the Mode Selection Trial (MOST). J Am Coll Cardiol 2004;43:2066-71. http://dx.doi.org/10.1016/j.jacc.2003.10.072.
- Shukla HH, Flaker GC, Hellkamp AS, James EA, Lee KL, Goldman L, et al. Clinical and quality of life comparison of accelerometer, piezoelectric crystal, and blended sensors in DDDR-paced patients with sinus node dysfunction in the mode selection trial (MOST). Pacing Clin Electrophysiol 2005;28:762-70. http://dx.doi.org/10.1111/j.1540-8159.2005.00184.x.
- Luengo-Fernandez R, Silver LE, Gutnikov SA, Gray AM, Rothwell PM. Hospitalization resource use and costs before and after TIA and stroke: results from a population-based cohort study (OXVASC). Value Health 2013;16:280-7. http://dx.doi.org/10.1016/j.jval.2012.10.013.
- Olshansky B, Mazur A, Sandesara C, Li W, Gopinathannai R, Sullivan R. Bradycardia. BMJ Best Practice n.d. http://bestpractice.bmj.com/best-practice/monograph/832/highlights.html (accessed June 2014).
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47. http://dx.doi.org/10.1093/eurheartj/ehs253.
- Prescription Cost Analysis, England. Leeds: Health and Social Care Information Centre; 2012.
- Hospital Prescribing, England. Leeds: Health and Social Care Information Centre; 2012.
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 6: Embedding Evidence Synthesis In Probabilistic Cost-Effectiveness Analysis: Software Choices. London: NICE; 2011.
- Saka Ãm, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing 2009;38:27-32.
- Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices - Modeling Studies. Value Health 2003;6:9-17. http://dx.doi.org/10.1046/j.1524-4733.2003.00234.x.
- Newman D, Lau C, Tang AS, Irvine J, Paquette M, Woodend K, et al. Effect of pacing mode on health-related quality of life in the Canadian Trial of Physiologic Pacing. Am Heart J 2003;145:430-7. http://dx.doi.org/10.1067/mhj.2003.167.
- Tang AS, Roberts RS, Kerr C, Gillis AM, Green MS, Talajic M, et al. Relationship between pacemaker dependency and the effect of pacing mode on cardiovascular outcomes. Circulation 2001;103:3081-5. http://dx.doi.org/10.1161/01.CIR.103.25.3081.
- Office for National Statistics . Mortality Statistics: Cause (Series DH2) 2002 (Online Edition) 2002. www.statistics.gov.uk/STATBASE/Product.asp?vlnk=618&More=Y (accessed 16 December 2002).
Appendix 1 Literature search strategies
Clinical effectiveness studies
| # | Term |
|---|---|
| 1 | exp Pacemaker, Artificial/ |
| 2 | exp Cardiac Pacing, Artificial/ |
| 3 | (pacing or pacemaker$ or pace maker$ or paced or pacer$).ti,ab. |
| 4 | or/1-3 |
| 5 | ((dual or double) adj4 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 6 | (physiological$ adj2 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 7 | ((av or atrioventricular) adj2 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 8 | ((av or atrioventricular) adj2 (synchron$ or sequential) adj (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 9 | (dual adj2 chamber).mp. |
| 10 | (dual adj2 pac$).mp. |
| 11 | (double adj2 chamber).mp. |
| 12 | (physiologic$ adj2 pac$).mp. |
| 13 | (AV adj2 synchron$).mp. |
| 14 | (atrioventricular adj2 synchron$).mp. |
| 15 | (AV adj2 sequential).mp. |
| 16 | (atrioventricular adj2 sequential).mp. |
| 17 | DDD.mp. |
| 18 | DDDR.mp. |
| 19 | DDI.mp. |
| 20 | DDIR.mp. |
| 21 | VDD.mp. |
| 22 | VDDR.mp. |
| 23 | VDI.mp. |
| 24 | VDIR.mp. |
| 25 | or/5-24 |
| 26 | (single adj2 chamber).mp. |
| 27 | (single adj2 pac$).mp. |
| 28 | (atrial adj2 pac$).mp. |
| 29 | AAI.mp. |
| 30 | AAIR.mp. |
| 31 | or/26-30 |
| 32 | Randomized Controlled Trials as Topic/ |
| 33 | randomized controlled trial/ |
| 34 | Random Allocation/ |
| 35 | Double Blind Method/ |
| 36 | Single Blind Method/ |
| 37 | clinical trial/ |
| 38 | clinical trial, phase i.pt. |
| 39 | clinical trial, phase ii.pt. |
| 40 | clinical trial, phase iii.pt. |
| 41 | clinical trial, phase iv.pt. |
| 42 | controlled clinical trial.pt. |
| 43 | randomized controlled trial.pt. |
| 44 | multicenter study.pt. |
| 45 | clinical trial.pt. |
| 46 | exp Clinical Trials as topic/ |
| 47 | (clinical adj trial$).tw. |
| 48 | ((singl$ or doubl$ or treb$ or tripl$) adj (blind$3 or mask$3)).tw. |
| 49 | PLACEBOS/ |
| 50 | placebo$.tw. |
| 51 | randomly allocated.tw. |
| 52 | (allocated adj2 random$).tw. |
| 53 | or/32-52 |
| 54 | case report.tw. |
| 55 | letter/ |
| 56 | historical article/ |
| 57 | or/54-56 |
| 58 | 53 not 57 |
| 59 | 4 and 25 and 31 and 58 |
| # | Term |
|---|---|
| 1 | exp artificial heart pacemaker/ |
| 2 | heart pacing/ |
| 3 | (pacing or pacemaker$ or pace maker$ or paced or pacer$).ti,ab. |
| 4 | or/1-3 |
| 5 | ((dual or double) adj4 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 6 | (physiological$ adj2 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 7 | ((av or atrioventricular) adj2 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 8 | ((av or atrioventricular) adj2 (synchron$ or sequential) adj (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 9 | (dual adj2 chamber).mp. |
| 10 | (dual adj2 pac$).mp. |
| 11 | (double adj2 chamber).mp. |
| 12 | (physiologic$ adj2 pac$).mp. |
| 13 | (AV adj2 synchron$).mp. |
| 14 | (atrioventricular adj2 synchron$).mp. |
| 15 | (AV adj2 sequential).mp. |
| 16 | (atrioventricular adj2 sequential).mp. |
| 17 | DDD.mp. |
| 18 | DDDR.mp. |
| 19 | DDI.mp. |
| 20 | DDIR.mp. |
| 21 | VDD.mp. |
| 22 | VDDR.mp. |
| 23 | VDI.mp. |
| 24 | VDIR.mp. |
| 25 | or/5-24 |
| 26 | (single adj2 chamber).mp. |
| 27 | (single adj2 pac$).mp. |
| 28 | (atrial adj2 pac$).mp. |
| 29 | AAI.mp. |
| 30 | AAIR.mp. |
| 31 | or/26-30 |
| 32 | Clinical trial/ |
| 33 | Randomized controlled trial/ |
| 34 | Randomization/ |
| 35 | Single blind procedure/ |
| 36 | Double blind procedure/ |
| 37 | Crossover procedure/ |
| 38 | Placebo/ |
| 39 | Randomi?ed controlled trial$.tw. |
| 40 | Rct.tw. |
| 41 | Random allocation.tw. |
| 42 | Randomly allocated.tw. |
| 43 | Allocated randomly.tw. |
| 44 | (allocated adj2 random).tw. |
| 45 | Single blind$.tw. |
| 46 | Double blind$.tw. |
| 47 | ((treble or triple) adj blind$).tw. |
| 48 | Placebo$.tw. |
| 49 | Prospective study/ |
| 50 | or/32-49 |
| 51 | Case study/ |
| 52 | Case report.tw. |
| 53 | Abstract report/ or letter/ |
| 54 | or/51-53 |
| 55 | 50 not 54 |
| 56 | 4 and 25 and 31 and 55 |
| # | Term |
|---|---|
| 1 | MeSH descriptor: [Pacemaker, Artificial] explode all trees |
| 2 | MeSH descriptor: [Cardiac Pacing, Artificial] explode all trees |
| 3 | (pacing or pacemaker* or pace maker* or paced or pacer*):ti,ab. |
| 4 | or #1-#3 |
| 5 | ((dual or double) next/4 (pacing or pacemaker* or pace maker* or paced or pacer*)):ti,ab. |
| 6 | (physiological* next/2 (pacing or pacemaker* or pace maker* or paced or pacer*)):ti,ab. |
| 7 | ((av or atrioventricular) next/2 (pacing or pacemaker* or pace maker* or paced or pacer*)):ti,ab. |
| 8 | ((av or atrioventricular) next/2 (synchron* or sequential) next (pacing or pacemaker* or pace maker* or paced or pacer*)):ti,ab. |
| 9 | dual next/2 “chamber” |
| 10 | dual next/2 pac* |
| 11 | double next/2 “chamber” |
| 12 | physiologic* next/2 pac* |
| 13 | AV next/2 synchron* |
| 14 | atrioventricular next/2 synchron* |
| 15 | AV next/2 “sequential” |
| 16 | atrioventricular next/2 “sequential” |
| 17 | DDD |
| 18 | DDDR |
| 19 | DDI |
| 20 | DDIR |
| 21 | VDD |
| 22 | VDDR |
| 23 | VDI |
| 24 | VDIR |
| 25 | or #5-#24 |
| 26 | single next/2 “chamber” |
| 27 | single next/2 pac* |
| 28 | atrial next/2 pac* |
| 29 | AAI |
| 30 | AAIR |
| 31 | or #26-#30 |
| 32 | #4 and #25 and #31 |
Economic evaluations
| # | Terms |
|---|---|
| 1 | exp Pacemaker, Artificial/ |
| 2 | exp Cardiac Pacing, Artificial/ |
| 3 | (pacing or pacemaker$ or pace maker$ or paced or pacer$).ti,ab. |
| 4 | or/1-3 |
| 5 | ((dual or double) adj4 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 6 | (physiological$ adj2 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 7 | ((av or atrioventricular) adj2 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 8 | ((av or atrioventricular) adj2 (synchron$ or sequential) adj (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 9 | (dual adj2 chamber).mp. |
| 10 | (dual adj2 pac$).mp. |
| 11 | double adj2 chamber.mp. |
| 12 | physiologic$ adj2 pac$.mp. |
| 13 | (AV adj2 synchron$).mp. |
| 14 | (atrioventricular adj2 synchron$).mp. |
| 15 | (AV adj2 sequential).mp. |
| 16 | (atrioventricular adj2 sequential).mp. |
| 17 | DDD.mp. |
| 18 | DDDR.mp. |
| 19 | DDI.mp. |
| 20 | DDIR.mp. |
| 21 | VDD.mp. |
| 22 | VDDR.mp. |
| 23 | VDI.mp. |
| 24 | VDIR.mp. |
| 25 | or/5-24 |
| 26 | (single adj2 chamber).mp. |
| 27 | (single adj2 pac$).mp. |
| 28 | (atrial adj2 pac$).mp. |
| 29 | AAI.mp. |
| 30 | AAIR.mp. |
| 31 | (ventricular adj2 pac$).mp. |
| 32 | VVI.mp. |
| 33 | VVIR.mp. |
| 34 | or/26-33 |
| 35 | Health Economics.mp. |
| 36 | Economic evaluation.mp. |
| 37 | exp Costs and Cost Analysis/ |
| 38 | cost benefit analysis/ |
| 39 | exp models economic/ |
| 40 | exp fees/ |
| 41 | exp budgets/ |
| 42 | (economic adj2 burden).tw. |
| 43 | (expenditure* not energy).tw. |
| 44 | Cost Effectiveness Analysis.mp. |
| 45 | (unit cost or unit-cost or unit-costs or unit costs or drug cost or drug costs or hospital costs or health-care costs or health care cost or medical cost or medical costs).tw. |
| 46 | Cost Minimization Analysis.mp. |
| 47 | (cost adj2 (util$ or effective$ or efficac$ or benefit$ or consequence$ or analys$ or minimi$ or allocation$ or control$ or illness$ or affordable$ or fee$ or charge$)).tw. |
| 48 | (decision adj1 (tree* or analys* or model*)).tw. |
| 49 | (econom* or price* or pricing or financ*or fee* or pharmacoeconomic* or pharmaeconomic* or pharmaco-economic*).tw. |
| 50 | ((value or values or valuation) adj2 (money or monetary or life or lives or costs or cost)).tw. |
| 51 | Markov*.tw. |
| 52 | or/35-51 |
| 53 | 4 and 25 and 34 and 52 |
| # | Terms |
|---|---|
| 1 | exp Pacemaker, Artificial/ |
| 2 | exp Cardiac Pacing, Artificial/ |
| 3 | (pacing or pacemaker$ or pace maker$ or paced or pacer$).ti,ab. |
| 4 | or/1-3 |
| 5 | ((dual or double) adj4 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 6 | (physiological$ adj2 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 7 | ((av or atrioventricular) adj2 (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 8 | ((av or atrioventricular) adj2 (synchron$ or sequential) adj (pacing or pacemaker$ or pace maker$ or paced or pacer$)).ti,ab. |
| 9 | (dual adj2 chamber).mp. |
| 10 | (dual adj2 pac$).mp. |
| 11 | double adj2 chamber.mp. |
| 12 | physiologic$ adj2 pac$.mp. |
| 13 | (AV adj2 synchron$).mp. |
| 14 | (atrioventricular adj2 synchron$).mp. |
| 15 | (AV adj2 sequential).mp |
| 16 | (atrioventricular adj2 sequential).mp. |
| 17 | DDD.mp. |
| 18 | DDDR.mp. |
| 19 | DDI.mp. |
| 20 | DDIR.mp. |
| 21 | VDD.mp. |
| 22 | VDDR.mp. |
| 23 | VDI.mp. |
| 24 | VDIR.mp. |
| 25 | or/5-24 |
| 26 | (single adj2 chamber).mp. |
| 27 | (single adj2 pac$).mp. |
| 28 | (atrial adj2 pac$).mp. |
| 29 | AAI.mp. |
| 30 | AAIR.mp. |
| 31 | (ventricular adj2 pac$).mp. |
| 32 | VVI.mp. |
| 33 | VVIR.mp. |
| 34 | or/26-33 |
| 35 | Health Economics.mp. |
| 36 | Economic evaluation.mp. |
| 37 | exp Costs/ and Cost Analysis/ |
| 38 | cost benefit analysis/ |
| 39 | exp models economic/ |
| 40 | fees/ |
| 41 | exp budgets/ |
| 42 | (economic adj2 burden).tw. |
| 43 | (expenditure* not energy).tw. |
| 44 | Cost Effectiveness Analysis.mp. |
| 45 | (unit cost or unit-cost or unit-costs or unit costs or drug cost or drug costs or hospital costs or health-care costs or health care cost or medical cost or medical costs).tw. |
| 46 | Cost Minimization Analysis.mp. |
| 47 | (cost adj2 (util$ or effective$ or efficac$ or benefit$ or consequence$ or analys$ or minimi$ or allocation$ or control$ or illness$ or affordable$ or fee$ or charge$)).tw. |
| 48 | (decision adj1 (tree* or analys* or model*)).tw. |
| 49 | (econom* or price* or pricing or financ*or fee* or pharmacoeconomic* or pharmaeconomic* or pharmaco-economic*).tw. |
| 50 | ((value or values or valuation) adj2 (money or monetary or life or lives or costs or cost)).tw. |
| 51 | Markov*.tw. |
| 52 | or/35-51 |
| 53 | 4 and 25 and 34 and 52 |
Pacemakers (all fields).
Atrioventricular block (all fields).
Sick sinus syndrome (all fields).
Pacemakers (all fields).
Atrioventricular block (all fields).
Sick sinus syndrome (all fields).
Health-related quality of life
| # | Terms |
|---|---|
| 1 | exp Pacemaker, Artificial/ |
| 2 | exp Cardiac Pacing, Artificial/ |
| 3 | (pacing or pacemaker$or pace maker$or paced or pacer$).ti,ab. |
| 4 | or/1-3 |
| 5 | ((dual or double) adj4 (pacing or pacemaker$or pace maker$or paced or pacer$)).ti,ab. |
| 6 | (physiological$adj2 (pacing or pacemaker$or pace maker$or paced or pacer$)).ti,ab. |
| 7 | ((av or atrioventricular) adj2 (pacing or pacemaker$or pace maker$or paced or pacer$)).ti,ab. |
| 8 | ((av or atrioventricular) adj2 (synchron$or sequential) adj (pacing or pacemaker$or pace maker$or paced or pacer$)).ti,ab. |
| 9 | (dual adj2 chamber).mp. |
| 10 | (dual adj2 pac$).mp. |
| 11 | double adj2 chamber.mp. |
| 12 | physiologic$adj2 pac$.mp. |
| 13 | (AV adj2 synchron$).mp. |
| 14 | (atrioventricular adj2 synchron$).mp. |
| 15 | (AV adj2 sequential).mp. |
| 16 | (atrioventricular adj2 sequential).mp. |
| 17 | DDD.mp. |
| 18 | DDDR.mp. |
| 19 | DDI.mp. |
| 20 | DDIR.mp. |
| 21 | VDD.mp. |
| 22 | VDDR.mp. |
| 23 | VDI.mp. |
| 24 | VDIR.mp. |
| 25 | or/5-24 |
| 26 | (single adj2 chamber).mp. |
| 27 | (single adj2 pac$).mp. |
| 28 | (atrial adj2 pac$).mp. |
| 29 | AAI.mp. |
| 30 | AAIR.mp. |
| 31 | (ventricular adj2 pac$).mp. |
| 32 | VVI.mp. |
| 33 | VVIR.mp. |
| 34 | or/26–33 |
| 35 | Quality of Life/ |
| 36 | ((quality adj3 life) or life quality or QOL).ti,ab. |
| 37 | (HRQL or HRQOL or HRQoL).ti,ab. |
| 38 | (value adj2 life).ti,ab. or exp Value of Life/ |
| 39 | (life adj2 qualit$3).tw. |
| 40 | (quality-adjusted life year$1 or QALY or QALYs or quality adjusted life year$1).ti,ab. or exp Quality-Adjusted Life Years/ |
| 41 | daly.ti,ab. |
| 42 | (disabilit$3 adj2 life).ti,ab. |
| 43 | exp Health Status Indicators/ |
| 44 | (sf36 or sf-36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).tw. |
| 45 | (sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 46 | (sf6d or sf 6d or sf-6d or short form 6d or shortform 6d or sf six dimension$1 or short form six dimension$1).tw. |
| 47 | (sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).tw. |
| 48 | (sf16 or sf 16 or sf-16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 49 | (sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).tw. |
| 50 | (euroQoL or euro QoL or eq5d or eq 5d or eq-5d).tw. |
| 51 | (hye or hyes or health$year$equivalent$).tw. |
| 52 | hui$1.tw. |
| 53 | (willing$adj2 pay).tw. |
| 54 | (willing$adj2 accept).tw. |
| 55 | standard gamble$.tw. |
| 56 | (health adj3 (utilit$3 or value$2 or preference$2)).tw. |
| 57 | (visual analog$3 scale or VAS).tw. |
| 58 | patient preference$2.tw. |
| 59 | (person$trade-off or person$trade off or PTO).ti,ab. |
| 60 | (Contingent value or contingent valuation).ti,ab. |
| 61 | discrete choice.ti,ab. |
| 62 | health status.ti,ab. or exp Health Status/ |
| 63 | ((quality adj3 wellbeing index) or QWB).ti,ab. |
| 64 | (health utilities index or HUI).ti,ab. |
| 65 | (time trade off or time tradeoff or TTO or time trade-off).ti,ab. |
| 66 | (utility or utilities).ti,ab. |
| 67 | disutil$.ti,ab. |
| 68 | disability.tw. |
| 69 | (wellbeing or well-being or well being or qwb).ti,ab. |
| 70 | quality of well being.tw. |
| 71 | quality of wellbeing.tw. |
| 72 | or/35–71 |
| 73 | 4 and 25 and 34 and 72 |
| # | Terms |
|---|---|
| 1 | exp Pacemaker, Artificial/ |
| 2 | exp Cardiac Pacing, Artificial/ |
| 3 | (pacing or pacemaker$or pace maker$or paced or pacer$).ti,ab. |
| 4 | or/1–3 |
| 5 | ((dual or double) adj4 (pacing or pacemaker$or pace maker$or paced or pacer$)).ti,ab. |
| 6 | (physiological$adj2 (pacing or pacemaker$or pace maker$or paced or pacer$)).ti,ab. |
| 7 | ((av or atrioventricular) adj2 (pacing or pacemaker$or pace maker$or paced or pacer$)).ti,ab. |
| 8 | ((av or atrioventricular) adj2 (synchron$or sequential) adj (pacing or pacemaker$or pace maker$or paced or pacer$)).ti,ab. |
| 9 | (dual adj2 chamber).mp. |
| 10 | (dual adj2 pac$).mp. |
| 11 | double adj2 chamber.mp. |
| 12 | physiologic$adj2 pac$.mp. |
| 13 | (AV adj2 synchron$).mp. |
| 14 | (atrioventricular adj2 synchron$).mp. |
| 15 | (AV adj2 sequential).mp. |
| 16 | (atrioventricular adj2 sequential).mp. |
| 17 | DDD.mp. |
| 18 | DDDR.mp. |
| 19 | DDI.mp. |
| 20 | DDIR.mp. |
| 21 | VDD.mp. |
| 22 | VDDR.mp. |
| 23 | VDI.mp. |
| 24 | VDIR.mp. |
| 25 | or/5–24 |
| 26 | (single adj2 chamber).mp. |
| 27 | (single adj2 pac$).mp. |
| 28 | (atrial adj2 pac$).mp. |
| 29 | AAI.mp. |
| 30 | AAIR.mp. |
| 31 | (ventricular adj2 pac$).mp. |
| 32 | VVI.mp. |
| 33 | VVIR.mp. |
| 34 | or/26–33 |
| 35 | exp Quality of Life/ |
| 36 | ((quality adj3 life) or life quality or QOL).ti,ab. |
| 37 | (HRQL or HRQOL or HRQoL).ti,ab. |
| 38 | (value adj2 life).ti,ab. or exp Value of Life/ |
| 39 | (life adj2 qualit$3).tw. |
| 40 | (quality-adjusted life year$1 or QALY or QALYs or quality adjusted life year$1).ti,ab. or exp Quality-Adjusted Life Years/ |
| 41 | daly.ti,ab. |
| 42 | (disabilit$3 adj2 life).ti,ab. |
| 43 | exp Health Status Indicators/ |
| 44 | (sf36 or sf-36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).tw. |
| 45 | (sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 46 | (sf6d or sf 6d or sf-6d or short form 6d or shortform 6d or sf six dimension$1 or short form six dimension$1).tw. |
| 47 | (sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).tw. |
| 48 | (sf16 or sf 16 or sf-16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 49 | (sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).tw. |
| 50 | (euroQoL or euro QoL or eq5d or eq 5d or eq-5d).tw. |
| 51 | (hye or hyes or health$year$equivalent$).tw. |
| 52 | hui$1.tw. |
| 53 | (willing$adj2 pay).tw. |
| 54 | (willing$adj2 accept).tw. |
| 55 | standard gamble$.tw. |
| 56 | (health adj3 (utilit$3 or value$2 or preference$2)).tw. |
| 57 | (visual analog$3 scale or VAS).tw. |
| 58 | patient preference$2.tw. |
| 59 | (person$trade-off or person$trade off or PTO).ti,ab. |
| 60 | (Contingent value or contingent valuation).ti,ab. |
| 61 | discrete choice.ti,ab. |
| 62 | health status.ti,ab. or exp Health Status/ |
| 63 | ((quality adj3 wellbeing index) or QWB).ti,ab. |
| 64 | (health utilities index or HUI).ti,ab. |
| 65 | (time trade off or time tradeoff or TTO or time trade-off).ti,ab. |
| 66 | (utility or utilities).ti,ab. |
| 67 | disutil$.ti,ab. |
| 68 | disability.tw. |
| 69 | (wellbeing or well-being or well being or qwb).ti,ab. |
| 70 | quality of well being.tw. |
| 71 | quality of wellbeing.tw. |
| 72 | or/35–71 |
| 73 | 4 and 25 and 34 and 72 |
Pacemakers (all fields).
Atrioventricular block (all fields).
Sick sinus syndrome (all fields)
and
quality of life (all fields) or
QoL (all fields) or
QALY (all fields).
Pacemakers (all fields).
Atrioventricular block (all fields).
Sick sinus syndrome (all fields)
and
quality of life (all fields) or
QoL (all fields) or
QALY (all fields).
Appendix 2 Data abstraction
Clinical effectiveness studies
Parallel-group randomised controlled trials
| Study information | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study ID (author name, year or acronym) | Albertsen | |||||||
| Reference details for all references relating to the trial | Albertsen AE, Nielsen JC, Poulsen SH, Mortensen PT, Pedersen AK, Hansen PS, et al. DDD(R)-pacing, but not AAI(R)-pacing induces left ventricular desynchronization in patients with sick sinus syndrome: tissue-Doppler and 3D echocardiographic evaluation in a randomised controlled comparison 2. Europace 2008 Feb;10:127–3337 | |||||||
| Language of publication | English | |||||||
| Type of report | Full paper | |||||||
| Trial location and number of sites | One centre, Department of Cardiology, Aarhus University Hospital, Skejby, Denmark | |||||||
| Trial sponsor | The study was supported by grants from The Danish Heart Foundation | |||||||
| Conflicts of interest | None declared | |||||||
| Recruitment period | August 2003 to March 2005 | |||||||
| Patient enrolment | All patients referred to the Department of Cardiology, Aarhus University Hospital, Skejby, Denmark, for their first pacemaker implantation were screened for inclusion in the study | |||||||
| Trial design | Parallel-group RCT | |||||||
| Trial duration (including any run-in and follow-up period) | All data were collected at baseline within 12 hours before pacemaker implantation and again at 3 months’ and 12 months’ follow-up | |||||||
| Inclusion criteria | Patients with SSS (syncope, dizzy spells or HF) in combination with the electrocardiographic criteria (sinus arrest > 2 seconds, tachybrady syndrome with sinus pauses > 2 seconds or sinus bradycardia (< 40 b.p.m. in awake hour) | |||||||
| Exclusion criteria | AV/bundle branch block; chronic AF; AF at randomisation; carotid sinus syndrome; pacemaker implantation during surgery; impaired walking; no ECG documentation available; refusal; heart transplanted patients; vasovagal syncope; dementia or life expectancy < 1 year | |||||||
| Outcomes | Primary outcome: changes in LV dyssynchrony from baseline to 12 months’ follow-up recorded by tissue-Doppler echocardiography and LVEF measured with three-dimensional echocardiography | |||||||
| Secondary outcomes: NT-proBNP and 6MWT | ||||||||
| Subgroups | Not reported | |||||||
| Power calculation | Power calculation was done on the basis of LVEF. Calculation was performed before including patients in the study. The risk of type 1 error was set to 5% and the statistical power to 80%. On the basis of earlier studies from our laboratory the SD of the LVEF measured by means of three-dimensional echocardiography was assumed to be 6%. With a minimal relevant difference of 5% (absolute per cent) between LVEF in the AAI(R) and DDD(R) groups, a total of 44 patients were needed in the study. With an expected dropout rate of 10%, the total number of patients included was decided to be 50 | |||||||
| Intervention/comparator | Dual-chamber pacing DDD(R) | Atrial pacing AAI(R) | ||||||
| Pacemaker (type, brand, etc.) | Dual-chamber pacemakers from several different companies were used (Medtronic, St Jude Medical, Guidant, ELA) | Single-chamber pacemakers from several different companies were used (Medtronic, St Jude Medical, Guidant, ELA) | ||||||
| Implantation | Active fixation bipolar atrial leads were inserted transvenously in the right atrial appendage. An additional active fixation lead was inserted transvenously in the RV apex | All patients received active fixation bipolar atrial leads inserted transvenously in the right atrial appendage | ||||||
| Programming | All pacemakers were programmed with a basal rate of 60 b.p.m. and with rate modulation active to maximum 120–140 b.p.m. The paced AV delay was programmed to a maximum of 220–225 milliseconds and rate adaptive. The sensed AV delay was programmed 20 milliseconds shorter than the paced AV delay. Mode switch was active | All pacemakers were programmed with a basal rate of 60 b.p.m. and with rate modulation active to maximum 120–140 b.p.m. | ||||||
| Randomised, n | 26 | 24 | ||||||
| Withdrawals, n (change in pacing mode, loss to follow-up) | None | Switch to DDDR 2 (owing to Wenckebach block at atrial pacing 100 b.p.m. during the implantation procedure). Lost to follow-up: n = 1 | ||||||
| Atrial pacing, % | 62 | 53 | ||||||
| VP, % | 66 | The two patients who received right ventricular leads were paced in the ventricle 3% and 99% of the time, respectively | ||||||
| Follow-up | Total follow-up 12 months | |||||||
| Baseline patient characteristics | Dual-chamber pacing DDD(R), n (%) | Atrial pacing AAI(R), n (%) | p-value | |||||
| Age (years), mean (SD) | 73 (13) | 72 (10) | > 0.05 | |||||
| Male sex, n (%) | 8 (31) | 10 (42) | > 0.05 | |||||
| Previous history of AF, n (%) | NR | NR | – | |||||
| Previous stroke, n (%) | 1 | 5 | < 0.05 | |||||
| Cardiovascular medication, n | ||||||||
| Beta-blockers | 11 | 6 | < 0.05 | |||||
| Calcium channel blockers | 5 | 5 | > 0.05 | |||||
| ACE inhibitors/ARBs | 10 | 11 | > 0.05 | |||||
| Diuretics | 11 | 14 | > 0.05 | |||||
| Aspirin | 14 | 20 | > 0.05 | |||||
| Pacing indication, n | ||||||||
| Sinus arrest/sinoatrial block | 16 | 14 | NR | |||||
| BTS | 12 | 11 | ||||||
| Sinus bradycardia | 8 | 4 | ||||||
| NYHA class, n | ||||||||
| I | 18 | 19 | NR | |||||
| II | 8 | 3 | ||||||
| III | 0 | 2 | ||||||
| IV | 0 | 0 | ||||||
| Outcome | Definition | |||||||
| HF | NYHA classification at 12 months’ follow-up | |||||||
| Exercise capacity | 6MWT at 3 months’ and 12 months’ follow-up | |||||||
| Adverse effects | Complications following implantation: lead displacements, infections or haematomas | |||||||
| Dichotomous outcomes | Dual-chamber pacing DDD(R) | Atrial pacing AAI(R) | p-value | |||||
| n | N | n | N | |||||
| HF NYHA | ||||||||
| I | 14 | 26 | 18 | 23 | NR | |||
| II | 10 | 5 | ||||||
| III | 1 | 0 | ||||||
| IV | 1 | 0 | ||||||
| Adverse events | ||||||||
| Lead displacements | 0 | 26 | 0 | 24 | N/A | |||
| Infections | 0 | 26 | 0 | 24 | N/A | |||
| Haematomas | 0 | 26 | 0 | 24 | N/A | |||
| Continuous outcome | Time frame | Dual-chamber pacing | Atrial pacing | p-value | ||||
| Mean | SD | N | Mean | SD | N | |||
| Exercise capacity: 6MWT (minutes) | Baseline | 415 | 76 | 26 | 444 | 105 | 24 | > 0.05 |
| 12 months | 446 | 96 | 26 | 500 | 89 | 23 | < 0.05 | |
| Study information | ||||||
|---|---|---|---|---|---|---|
| Study ID (author name, year or acronym) | DANPACE19 (the Danish multicenter randomised trial on single-lead atrial pacing vs. dual-chamber pacing in SSS) | |||||
| Reference details for all references relating to the trial | Andersen HR, Svendsen JH. The Danish multicenter randomized study on atrial inhibited versus dual-chamber pacing in sick sinus syndrome (the DANPACE study): purpose and design of the study. Heart Drug 2001;1:67–7038 | |||||
| Nielsen JC, Thomsen PE, Højberg S, Møller M, Vesterlund T, Dalsgaard D, et al. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome 196. Eur Heart J 2011;32:686–9619 | ||||||
| Nielsen JC, Thomsen PE, Højberg S, Møller M, Riahi S, Dalsgaard D, et al. Atrial fibrillation in patients with sick sinus syndrome: the association with PQ-interval and percentage of ventricular pacing. Europace 2012;14:682–939 | ||||||
| Language of publication | English | |||||
| Type of report | Full papers | |||||
| Trial location and number of sites | Patients were enrolled from all Danish pacemaker centres and from selected centres in UK and Canada. Number of sites not reported | |||||
| Trial sponsor | Unrestricted grants from Medtronic, St Jude Medical, Boston Scientific, ELA Medical, Pfizer and The Danish Heart Foundation (10-04-R78-A2954-22779) | |||||
| Conflicts of interest | JCN and JHS received consultant honoraria and speakers fees from Medtronic, St Jude Medical and Biotronik. LSM is an employee of UNI-C and has been paid consultants fees for his participation in designing the study, taking care of data management and statistical analysis in the study, being a member of the study data monitoring board and reviewing the manuscript. WDT received a grant from Medtronic for follow-up of patients enrolled in a clinical trial of cardiac resynchronization therapy. JSH received a research grant from Boston for conduct of the Shockless IMPLant Evaluation (SIMPLE) trial – a 2500 patient study of implantable defibrillators; consulting fees and consultant honoraria from St Jude Medical; and speakers’ fees from Boston Scientific and St Jude Medical. The other authors report no conflicts | |||||
| Recruitment period | 10 March 1999 to 30 June 2008 | |||||
| Patient enrolment | Patients were enrolled from all Danish pacemaker centres and from selected centres in UK and Canada. All patients referred for first pacemaker implantation were evaluated for inclusion | |||||
| Trial design | Parallel-group RCT | |||||
| Trial duration | Follow-up took place after 3 months and again every year after implantation up to 10 years. Mean follow-up was 5.4 years (SD 2.6 years) | |||||
| Inclusion criteria | Symptomatic bradycardia; documented SA block or sinus-arrest with pauses > 2 seconds or sinus bradycardia < 40 b.p.m. for more than 1 minute while awake; PR interval ≤ 0.22 seconds if aged 18–70 years or PR interval ≤ 0.26 seconds if aged ≥ 70 years; and QRS width < 0.12 seconds; or bradycardia–tachycardia with QRS > 2 seconds (spontaneously or related to antiarrhythmic treatment; and age > 18 years at study enrolment; and able to attend outpatient study visits | |||||
| Exclusion criteria | AV block; bundle branch block; long-standing persistent AF (> 12 months); AF with ventricular rate < 40 b.p.m. for ≥ 1 minute or pauses > 3 seconds; a positive test for carotid sinus hypersensitivity; planned cardiac surgery; or a life expectancy shorter than 1 year; need for an ICD; cancer; severe psychiatric disease; severe dementia; planned major surgery in the near future. Documented paroxysmal AF was not an exclusion criterion | |||||
| Outcomes | Primary outcome: death from any cause | |||||
| Secondary outcomes: paroxysmal AF; chronic AF; first cardioversion for AF; stroke; peripheral embolism; HF (hospitalisation with HF as reported diagnosis and patients classified with new HF); % VP at each follow-up; mean % VP throughout the total follow-up period; % MS; cardiovascular mortality; need for pacemaker reoperation; and QoL | ||||||
| Subgroups | Age > or ≤ 75 years; sex; hypertension; LVEF < or ≥ 50%; history of AF; previous MI; PQ-interval > or ≤ 180 milliseconds; diabetes; NYHA class I or II–IV; left atrial diameter > or ≤ 39; BMI ≥ or < 25; diuretics | |||||
| Power calculation | It was assumed that the relative difference in mortality between AAIR pacing and DDDR pacing would be half of the difference observed between AAIR pacing and single-lead VP. Therefore, the study was planned to include 1900 patients followed for a mean of 5.5 years to identify a 6% absolute difference (32% vs. 26%) in death from any cause between treatment groups, with a power of 80% and an overall α = 0.05. Owing to the increasing use of dual-chamber pacemakers with new features prolonging or eliminating the AV interval in order to minimise VP in patients with SSS, which were not permitted in the trial, the recruitment rate decreased in several Danish centres from 2005. Fewer than the planned 1900 patients were included in the study. From a planned interim analysis, it could be foreseen that no significant difference could be reached with respect to the primary outcome even with the planned 1900 patients | |||||
| Intervention/comparator | Dual-chamber pacing DDD(R) | Atrial pacing AAI(R) | ||||
| Pacemaker (type, brand, etc.) | Contemporary DDDR pacemaker models (Boston Scientific, Medtronic and St Jude Medical) | NR | ||||
| Implantation | A bipolar lead was implanted in the right atrium and an additional lead was implanted in the right ventricle | A bipolar lead was implanted in the right atrium. An atrial pacing test was performed at 100 b.p.m. in all patients and 1 : 1 AV conduction was required for implantation of an AAIR pacemaker. In patients randomised to AAIR pacing demonstrating AV block when paced at 100 b.p.m., a ventricular lead and a DDDR pacemaker were implanted | ||||
| Programming | The rate-adaptive function was activated in all pacemakers and programmed with a lower rate of 60 b.p.m. and an upper rate of 130 b.p.m. The paced AV interval was programmed to 140–220 milliseconds according to a pre-specified algorithm; the paced AV interval was initially programmed to a value 10% longer than either the interval measured from the atrial pacing spike to start of the conducted QRS complex at 60 b.p.m. or the PR-interval if the sinus rate was faster than 60 b.p.m. If VP occurred with this programming, the paced AV interval was gradually increased in steps of 20 milliseconds until VP ceased or until a maximum of 220 milliseconds was reached. If VP still occurred at a programmed interval of 220 milliseconds, the paced AV interval was shortened to a length of 140–160 milliseconds, and the AV hysteresis function was activated to allow automatic search for intrinsic AV conduction with an AV interval of 220 milliseconds. The AV interval after sensed atrial beats was set 20–30 milliseconds shorter than the paced interval, and automatic shortening of the AV interval was allowed during rate increases. The maximum tracking rate was individualised and the mode switch function was activated. The mean programmed maximum paced AV delay in the dual-chamber group was 225 milliseconds (SD 39 milliseconds). New features prolonging or eliminating the AV interval in order to minimise VP in patients with SSS were not permitted in the trial | The rate-adaptive function was activated in all pacemakers and programmed with a lower rate of 60 b.p.m. and an upper rate of 130 b.p.m. The investigators were asked to only change the pacing mode from AAIR to DDDR pacing in cases of a high-grade AV block or a documented symptomatic AV block of the Wenckebach type. The incidental finding of a low Wenckebach block point at a follow-up visit was not an indication for change of pacing mode | ||||
| Randomised, n | 708 | 707 | ||||
| Withdrawals, n (%) (change in pacing mode, loss to follow-up) | First pacemaker implantation:
|
First pacemaker implantation:
|
||||
| Atrial pacing, % (SD) | 59 (31) | 58 (29) | ||||
| VP, % (SD) | 65 (33) | Pacemaker memory data were recorded in 103 of 122 patients who had a ventricular lead implanted at the first operation or at some point during follow-up. These 103 patients had a mean of 53% (SD 35%) VP | ||||
| Follow-up | Average follow-up 5.4 years (SD 2.6 years) | |||||
| Baseline patient characteristics | Dual-chamber pacing DDD(R), n (%) | Atrial pacing AAI(R), n (%) | p-value | |||
| Age (years), mean (SD) | 72.4 (11.4) | 73.5 (11.2) | 0.054 | |||
| Male sex, n (%) | 267 (37.7) | 235 (33.2) | 0.08 | |||
| Previous history of AF, n (%) | 318 (44.9) | 303 (42.9) | 0.44 | |||
| Previous stroke, n (%) | 53 (7.5) | 61 (8.6) | 0.43 | |||
| Medication, n (%) | ||||||
| Anticoagulation | 89 (12.6) | 108 (15.3) | 0.14 | |||
| Aspirin | 361 (51.1) | 369 (52.2) | 0.67 | |||
| Sotalol | 44 (6.2) | 43 (6.1) | 0.91 | |||
| Beta-blocker other than sotalol | 132 (18.7) | 159 (22.5) | 0.08 | |||
| Calcium channel blocker | 142 (20.1) | 137 (19.4) | 0.75 | |||
| Digoxin | 62 (8.8) | 73 (10.3) | 0.32 | |||
| Amiodarone | 24 (3.4) | 25 (3.5) | 0.88 | |||
| Class I antiarrhythmics | 20 (2.8) | 14 (2.0) | 0.30 | |||
| Angiotensin-converting enzyme inhibitors | 170 (24.0) | 160 (22.6) | 0.53 | |||
| Diuretics | 263 (37.2) | 304 (43.0) | 0.03 | |||
| Pacing indication, n | Not reported | |||||
| NYHA class, n | ||||||
| I | 522 (73.9) | 503 (71.4) | 0.33 | |||
| II | 158 (22.4) | 172 (24.4) | ||||
| III | 24 (3.4) | 29 (4.1) | ||||
| IV | 2 (0.3) | 0 | ||||
| Outcome | Definition | |||||
| Mortality | New deaths were identified by checking the study database against the Danish Civil Registration System and supplementary information regarding deceased patients was collected from hospitals and GPs | |||||
| AF | Paroxysmal AF, defined as the first diagnosis of AF detected in the 12-lead ECG and verified by the pacemaker telemetry at a planned follow-up visit and chronic AF was defined as AF at two consecutive follow-up visits and at all subsequent follow-up visits | |||||
| Stroke | Stroke was defined as the sudden development of focal neurological symptoms lasting more than 24 hours | |||||
| HF | NYHA functional class, use of diuretics, and hospitalisation for HF were used as indicators of HF. Patients were classified with new HF if (1) they presented in NYHA functional class IV or (2) if two or more of the following indicators were present: presence of oedema, presence of dyspnoea and NYHA functional class III | |||||
| Requirement of further surgery | Need for pacemaker reoperation was decided by the physician in charge of follow-up | |||||
| Adverse events | Not reported | |||||
| HRQoL | Measured using SF-36 | |||||
| Dichotomous outcomes | Dual-chamber pacing DDD(R) | Atrial pacing AAI(R) | Estimate of effect | 95% CI, p-value | ||
| n | N | n | N | AAIR vs. DDDR | ||
| Mortality | 193 | 708 | 209 | 707 | HR 1.06 (adjusted HR 0.94) | 0.88 to 1.29, 0.53 (adjusted 0.52) |
| HF (leading to hospitalisation) | 28 | 708 | 27 | 707 | HR 1.06 | 0.62 to 1.79, 0.84 |
| HF (new) | 169 | 708 | 170 | 707 | HR 1.00 | 0.79 to 1.22, 0.87 |
| HF NYHA class | ||||||
| I | 341 | 666 | 364 | 666 | NR | 0.43 |
| II | 260 | 231 | ||||
| III | 61 | 67 | ||||
| IV | 4 | 4 | ||||
| HF: diuretic use | 328 | 695 | 324 | 692 | NR | 0.89 |
| AF (paroxysmal) | 163 | 708 | 201 | 707 | HR 1.27 (adjusted HR 1.24) | 1.03 to 1.56, 0.024 (adjusted 0.042) |
| AF (chronic) | 76 | 708 | 79 | 707 | HR 1.02 (adjusted HR 1.01) | 0.74 to 1.39, 0.93 (adjusted 0.93) |
| Stroke | 34 | 708 | 39 | 707 | HR 1.13 (adjusted HR 1.11) | 0.72 to 1.80, 0.59 (adjusted 0.65) |
| Reoperation | 84 | 708 | 156 | 707 | HR 1.99 (adjusted HR 2.00) | 1.53 to 2.59, < 0.001 (adjusted < 0.001) |
| Battery depletion | 42 | 708 | 59 | 707 | – | 0.09 |
| Need for surgical change of mode of pacing | 4 | 708 | 66 | 707 | – | < 0.001 |
| Lead complications | 30 | 708 | 37 | 707 | – | 0.42 |
| Surgical or mechanical complications | 7 | 708 | 10 | 707 | – | 0.52 |
| Infection | 3 | 708 | 3 | 707 | – | 0.98 |
| Skin erosion | 3 | 708 | 1 | 707 | – | 0.31 |
| Device failure | 2 | 708 | 2 | 707 | – | 0.99 |
| Study information | ||||
|---|---|---|---|---|
| Study ID (author name, year or acronym) | Nielsen | |||
| Reference details for all references relating to the trial | Kristensen L, Nielsen JC, Mortensen PT, Pedersen OL, Pedersen AK, Andersen HR, et al. Incidence of atrial fibrillation and thromboembolism in a randomised trial of atrial versus dual-chamber pacing in 177 patients with sick sinus syndrome. Heart 2004;90:661–642 | |||
| Nielsen JC, Kristensen L, Andersen HR, Mortensen PT, Pedersen OL, Pedersen AK, et al. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol 2003 20;42:614–2341 | ||||
| Language of publication | English | |||
| Type of report | Full papers | |||
| Trial location and number of sites | Denmark, two sites, Skejby University Hospital, Aarhus, and Viborg County Hospital | |||
| Trial sponsor | Not reported | |||
| Conflicts of interest | Not reported | |||
| Recruitment period | Recruitment between December 1994 and March 1999 | |||
| Patient enrolment | The trial included consecutive patients referred to Skejby University Hospital, Aarhus, Denmark, for their first pacemaker implantation. In a 1-year period, patients were also enrolled at the neighbouring Viborg County Hospital | |||
| Trial design | Parallel-group RCT | |||
| Trial duration | Follow-up visits were after 3 months and 12 months and then once a year | |||
| Inclusion criteria | Patients with SSS, normal AV conduction, no bundle branch block, symptomatic bradycardia < 40 b.p.m. or symptomatic QRS pauses of more than 2 seconds | |||
| Exclusion criteria | Patients with AV block grade 1, 2 or 3; chronic AF; bundle branch block; AF > 50% of time; AF with QRS rate < 40 b.p.m.; cerebral disease including dementia; cardiac surgery planned; cancer; pacing for hypertrophic cardiomyopathy; age < 18 years; prior heart transplant; major surgery, non-cardiac; bradycardia and ventricular tachycardia; Wenckebach block < 100 b.p.m., known before implantation; carotid sinus syndrome; AF with RR intervals > 3 seconds | |||
| Outcomes | Primary outcome: changes in LA size and LV size and function during follow-up measured by M-mode echocardiography | |||
| Secondary outcomes: cardiographic end points – changes in LA volume and LV volume and LVEF measured by two-dimensional echocardiography; clinical end points – AF, thromboembolism, all-cause and cardiovascular mortality and congestive HF | ||||
| Subgroups | Not reported | |||
| Power calculation | Power calculations were based on M-mode echocardiographic data from an AAI vs. VVI study. With a statistical power of 80% and a 0.05 level of significance, a total of 450 patients were to be included in the study to detect a 10% difference between the AAIR group and the DDDR group in LA diameter. No differences between the DDDR-s and the DDDR-l groups were expected. However, inclusion was stopped after randomisation of 177 patients, because at that time a national multicentre trial of AAIR vs. DDDR pacing in patients with SSS was initiated and started in Denmark [the Randomised comparison of AAIR and DDDR pacing in 1900 patients with SSS (DANPACE) trial]. Patients included in the present study were not rolled over into the DANPACE study19 | |||
| Intervention/comparator | Dual-chamber pacing | Atrial pacing | ||
| DDDR-s | DDDR-l | AAIR | ||
| Pacemaker (type, brand, etc.) | Standard rate-adaptive dual-chamber pacemakers [Cardiac Pacemakers Inc. (St Paul, MN, USA), Pacesetter (St Paul, MN, USA), Medtronic (Minneapolis, MN, USA)] | Standard rate-adaptive single-chamber pacemakers [Cardiac Pacemakers Inc. (St Paul, MN, USA), Pacesetter (St Paul, MN, USA), Medtronic (Minneapolis, MN, USA)] | ||
| Implantation | All atrial leads were implanted in the upper parts of the right atrial wall. Among patients randomised to DDDR pacing, 37 patients had unipolar leads and 86 patients had bipolar leads in the right atrium. All patients randomised to DDDR pacing had unipolar leads with passive fixation implanted in the RV apex | All atrial leads were implanted in the upper parts of the right atrial wall. Among patients randomised to AAIR pacing, 19 patients had unipolar leads, and 35 patients had bipolar leads | ||
| AF at the time of pacemaker implantation was not a reason for implanting another pacemaker rather than according to the randomised mode. During implantation, an atrial pacing test at 100 b.p.m. was performed; 1 : 1 AV conduction was required for an atrial pacemaker to be implanted. If Wenckebach block occurred at a rate of 100 b.p.m., the patient received a DDDR pacemaker | ||||
| Programming | The rate response function was active in all but two patients | |||
| Lower and upper rates were programmed individually. Mode-switch function was active in all patients implanted with DDD pacemakers | ||||
| In patients randomised to DDDR-s pacing, the AV delay was 150 milliseconds and rate adaptive but even shorter if necessary to obtain VP with full capture | In patients randomised to DDDR-l pacing, the AV delay was fixed at 300 milliseconds. In four patients a shorter AV delay had to be programmed to avoid induction of endless loop tachycardia during initial pacemaker testing | |||
| Randomised, n | 60 | 63 | 54 | |
| Withdrawals, n (%) (change in pacing mode, loss to follow-up) |
|
|
|
|
| Atrial pacing, % | 57 | 67 | 69 | |
| VP, % | 90 | 17 | – | |
| Follow-up (years), mean (SD) | 2.8 (SD 1.5) | 2.8 (SD 1.4) | 3.1 (SD 1.3) | |
| Average follow-up was 2.9 years (SD 1.1 years; range 6 days to 5.3 years) | ||||
| Baseline patient characteristics | Dual-chamber pacing, n (%) | Atrial pacing, n (%) | p-value | |
| DDDR-s | DDDR-l | AAIR | ||
| Age, years (mean, SD) | 74 (9) | 74 (9) | 74 (9) | NR |
| Male sex, n (%) | 23 (43) | 26 (43) | 24 (38) | NR |
| Previous history of AF, n (%) | NR | |||
| Previous stroke, n (%) | NR | |||
| Medication, n | ||||
| Beta-blocker | 5 | 7 | 4 | NR |
| Calcium channel blocker | 7 | 11 | 14 | |
| Digoxin | 9 | 11 | 11 | |
| Sotalol | 8 | 10 | 7 | |
| Aspirin | 40 | 36 | 35 | |
| Warfarin | 5 | 11 | 5 | |
| Pacing indication, n | ||||
| Sinus bradycardia | 5 | 11 | 8 | NR |
| SA block | 17 | 16 | 19 | |
| BTS | 38 | 36 | 27 | |
| NYHA class, n | ||||
| I | 38 | 46 | 32 | NR |
| II | 22 | 14 | 18 | |
| III | 0 | 3 | 2 | |
| IV | 0 | 0 | 1 | |
| Outcome | Definition | |||
| Mortality | Cause of death was obtained by interviewing the doctors who had care of the patient and by review of hospital and necropsy reports | |||
| Cardiovascular mortality | Cause of death was obtained by interviewing the doctors who had care of the patient and by review of hospital and necropsy reports. Cardiovascular death included sudden death, death owing to congestive HF, arterial thromboembolism or a pulmonary embolus | |||
| AF | AF was diagnosed by standard 12-lead ECG at planned follow-up visits | |||
| Stroke | Stroke was diagnosed when neurological symptoms of presumably cerebral ischaemic origin persisted for more than 24 hours or if patients died within 24 hours from an acute cerebrovascular event | |||
| HF | HF was classified according to NYHA criteria and quantitated by the daily dose of diuretics | |||
| Dichotomous outcomes | Dual-chamber pacing | Atrial pacing | p-value | |
| DDDR-s (N = 60) | DDDR-l (N = 63) | AAIR (N = 54) | ||
| Mortality, n | 14 | 14 | 9 | 0.51 |
| Cardiovascular mortality, % | 11.7 | 14.3 | 7.4 | 0.43 |
| HF: increase in consumption of diuretics, % | 32 | 21 | 28 | 0.34 |
| HF: increase in at least one NYHA class, % | 30 | 46 | 31 | 0.17 |
| AF, n | 14 | 11 | 4 | 0.03 |
| Stroke, n | 7 | 4 | 3 | 0.32 |
Crossover randomised controlled trials
| Study information | ||||||
|---|---|---|---|---|---|---|
| Study ID (author name year, or acronym) | Gallik | |||||
| Reference details for all references relating to the trial | Gallik DM, Guidry GW, Mahmarian JJ, Verani MS, Spencer WH, III, Gallik DM, et al. Comparison of ventricular function in atrial rate adaptive versus dual chamber rate adaptive pacing during exercise. Pacing Clin Electrophysiol 1994;17:179–8534 | |||||
| Language of publication | English | |||||
| Type of report | Full paper | |||||
| Trial location and number of sites | Not reported | |||||
| Trial sponsor | Supported in part through a grant from TLL Temple Foundation, Lufkin, TX, USA. Computational assistance was provided by the CLINFO Project, funded by the Division of Research Resources of the National Institutes of Health, Bethesda, MD, USA, under grant RR-00350 | |||||
| Conflicts of interest | Not reported | |||||
| Recruitment period | Not reported | |||||
| Patient enrolment | Not reported | |||||
| Trial design | Two-period crossover RCT (AAIR, DDDR) | |||||
| Trial duration | Exercise tests for each patient were separated by a rest period of 0.5 to 1 hour to allow heart rate and blood pressure to return to baseline | |||||
| Inclusion criteria | Patients with sinus node disease, implanted dual-chamber, rate-adaptive, multiprogrammable (DDDR) pacemaker, no history of a second-degree AV block, no intraventricular conduction delay and demonstrated 1 : 1 AV conduction at an atrial pacing rate of 120 b.p.m. | |||||
| Exclusion criteria | Evidence of AV node disease, pregnancy, patients unable to exercise or those in whom exercise testing was contraindicated | |||||
| Outcomes | Exercise, haemodynamic parameters | |||||
| Subgroups | Not reported | |||||
| Power calculation | Not reported | |||||
| Intervention/comparator | ||||||
| Pacemaker (type, brand, etc.) | All patients had Synergyst II or Elite pacemakers (Medtronic, Inc., Minneapolis, MN, USA) | |||||
| Implantation | Patients recruited to the study had already had the pacemaker implanted | |||||
| Programming | DDDR with AV delay set at 100 milliseconds to maintain 100% ventricular capture and all other parameters left at the patient’s currently programmed settings, and AAIR. Rate response parameters were programmed to low threshold, rapid rate response (setting: 8) to try to achieve a maximal exercise test | |||||
| Randomised, n | 12 | |||||
| Withdrawals, n (%) (change in pacing mode, loss to follow-up) | Data were collected for all randomised patients | |||||
| Atrial pacing, % | Not reported | |||||
| VP, % | Not reported | |||||
| Follow-up | Both pacing modes were studied on the same day with 0.5–1.0 hour’s rest in between. No follow-up stated | |||||
| Baseline patient characteristics | ||||||
| Age (years), mean (SE) | 61 (4) | |||||
| Male sex, n (%) | 8 (67) | |||||
| Previous history of AF, n (%) | NR | |||||
| Previous stroke, n (%) | NR | |||||
| Medication, n |
|
|||||
| Pacing indication, n | NR | |||||
| NYHA class, n | NR | |||||
| Outcome | Definition | |||||
| Exercise capacity | Upright bicycle exercise was performed with an initial workload of 200 kpm, with increments in workload of 200 kpm every 3 minutes until peak heart rate ≥ 85% predicted based on age | |||||
| Continuous outcomes | N | Dual-chamber pacing | Atrial pacing | p-value | ||
| Mean | SD | Mean | SD | |||
| Exercise capacity | ||||||
| Exercise timea (seconds) | 12 | 416 | 140 | 411 | 122 | 0.74 |
| Study information | |||||||
|---|---|---|---|---|---|---|---|
| Study ID (author name, year or acronym) | Lau | ||||||
| Reference details for all references relating to the trial | Lau CP, Tai YT, Leung WH, Wong CK, Lee P, Chung FL, et al. Rate adaptive pacing in sick sinus syndrome: effects of pacing modes and intrinsic conduction on physiological responses, arrhythmias, symptomatology and quality of life. Eur Heart J 1994;15:1445–5535 | ||||||
| Language of publication | English | ||||||
| Type of report | Full paper | ||||||
| Trial location and number of sites | Not reported | ||||||
| Trial sponsor | UPCG research grant HKU37/91 (account code 338/041/0004) | ||||||
| Conflicts of interest | Not reported | ||||||
| Recruitment period | Not reported | ||||||
| Patient enrolment | Not reported | ||||||
| Trial design | Triple crossover RCT (AAIR, DDDR, VVIR) | ||||||
| Trial duration | Acute invasive testing phase, which was performed at admission of the patients into the study, and which was completed within a single clinical attendance. This was followed by a 12-week ambulatory phase in which the pacemaker was randomised to one of the three pacing modes for three 4-week periods | ||||||
| Inclusion criteria | SSS and intact AV conduction (1 : 1 conduction up to 100 b.p.m. and a pacing spike to R-interval ≤ 220 milliseconds) | ||||||
| Exclusion criteria | Not reported | ||||||
| Outcomes | Holter monitoring, ambulatory blood pressure monitoring, symptoms and QoL assessments | ||||||
| Subgroups | Not reported | ||||||
| Power calculation | Not reported | ||||||
| Intervention/comparator | |||||||
| Pacemaker (type, brand, etc.) | Dual-chamber rate-adaptive pacemaker with either activity of minute ventilation sensors for rate adaptation; minute ventilation pacemaker (META-DDDR, Model 1250, Telectronics Pacing Systems, Colorado); activity sensing pacemaker (Relay, Model 294–03, Intermedics Inc., Angleton, TX, USA) | ||||||
| Implantation | Not reported | ||||||
| Programming | Lower and upper rates of 60 and 150 b.p.m., respectively, were programmed, and the nominal rate-adaptive AV interval was used in all patients: 96 milliseconds (SD 7 milliseconds) to 140 milliseconds (SD 5 milliseconds) | ||||||
| Randomised, n | 15 | ||||||
| Withdrawals, n (%) (change in pacing mode, loss to follow-up) | Three withdrawals:
|
||||||
| Mode | DDDR | AAIR | |||||
| Atrial pacing, % (SD) | Not reported | Not reported | |||||
| VP, % (SD) | 64 (11) | N/A | |||||
| Follow-up | Total follow-up was 3 months for each pacing mode | ||||||
| Baseline patient characteristics | |||||||
| Age (years), mean (SD) | 62 (2) | ||||||
| Male sex, n (%) | 5 (42) | ||||||
| Previous history of AF, n (%) | Some of the patients | ||||||
| Previous stroke, n (%) | Not reported | ||||||
| Medication, n | |||||||
| Cardiac glycosides | 3 | ||||||
| Potassium channel blockers | 1 | ||||||
| Calcium channel blockers | 2 | ||||||
| Beta-blocker | 1 | ||||||
| Angiotensin-converting enzyme inhibitor | 1 | ||||||
| Acetylsalicylic acid | 1 | ||||||
| Nitrates | 2 | ||||||
| Pacing indication, n | Not reported | ||||||
| NYHA class, n | Not reported | ||||||
| Outcome | Definition | ||||||
| HRQoL | VAS for general well-being, 12-GHQ and the SSI adapted for local use from the Bradford Somatic Inventory | ||||||
| Continuous outcomes | N | Dual-chamber pacing | Atrial pacing | Mean difference | p-value | ||
| Mean | SD | Mean | SD | ||||
| General well-beinga (VAS) | 12 | 7.1 | 1.2 | 6.8 | 1.3 | 0.25 | 0.32 |
| 12-GHQ | 12 | 14.3 | SE 2.2 | 15.2 | SE 2.1 | NR | NS |
| The SSI adapted from the Bradford Somatic Inventory | 12 | 71.5 | SE 3.3 | 70.2 | SE 3.5 | NR | NS |
| Symptomsb | |||||||
| Dyspnoea | 12 | 3.4 | 0.45 | 3.95 | 0.25 | –0.55 | NS |
| Palpitations | 12 | 4.25 | 0.25 | 3.95 | 0.3 | 0.3 | NS |
| Dizziness | 12 | 4.25 | 0.25 | 3.95 | 0.3 | 0.3 | NS |
| Chest pain | 12 | 4.55 | 0.25 | 4.6 | 0.25 | –0.05 | NS |
| Sleep disturbance | 12 | 4.2 | 0.25 | 4.6 | 0.2 | –0.4 | NS |
| Neck pulsations | 12 | 4.95 | 0.1 | 4.95 | 0.1 | 0 | NS |
| Study information | |||||||
|---|---|---|---|---|---|---|---|
| Study ID (author name, year or acronym) | Schwaab | ||||||
| Reference details for all references relating to the trial | Schwaab B, Kindermann M, Schatzer-Klotz D, Berg M, Franow H, Frohlig G, et al. AAIR versus DDDR pacing in the bradycardia tachycardia syndrome: a prospective, randomized, double-blind, crossover trial 262. Pacing Clin Electrophysiol 2001;24:1585–9543 | ||||||
| Language of publication | English | ||||||
| Type of report | Full paper | ||||||
| Trial location and number of sites | Germany, number of sites not reported | ||||||
| Trial sponsor | All costs were paid for by the university clinic in Homburg/Saar | ||||||
| Conflicts of interest | Not reported | ||||||
| Recruitment period | Not reported | ||||||
| Patient enrolment | Not reported | ||||||
| Trial design | Two-period crossover RCT (AAIR, DDDR) | ||||||
| Trial duration | Four weeks after implantation, patients were randomised to either AAIR or DDDR mode. Three months after randomisation data were collected and the pacing mode switched to the other mode. After another 3 months data were collected again | ||||||
| Inclusion criteria | Patients had to have experienced at least two documented paroxysms of atrial tachyarrhythmia, be on antiarrhythmic medication for the prevention of atrial flutter or AF, and be eligible for a dual-chamber pacing system for spontaneous or medically induced symptomatic sinus bradycardia. Patients had to comply with each of the following definitions of chronotropic incompetence: peak exercise heart rate < 100 b.p.m., peak exercise heart rate < (220 – age) × 0.75 and heart rate at half the maximum workload < 60 + 2 b.p.m. per ml O2/kg/minute | ||||||
| Exclusion criteria | Complete bundle branch block, a bifascicular block, PQ interval > 240 milliseconds during sinus rhythm at rest, second or a third-degree AV block identified on preimplant 24-hour Holter electrocardiogram, significant valvular heart disease diagnosed by ECHO or Doppler echocardiography | ||||||
| Outcomes | QoL, LV outflow, aortic flow (peak flow velocity, time to peak flow velocity, the area under the systolic time–velocity curve, cardiac output), mitral flow (peak flow velocity of the early wave, time to peak flow velocity, deceleration of early diastolic flow, filling time, early diastolic closure rate of the anterior mitral valve leaflet), bicycle cardiopulmonary exercise testing (to assess exercise duration) development of AV block, oxygen consumption, carbon dioxide production, minute ventilation, breathing rate, respiratory rate exchange ratio, ventilatory equivalents for O2 and CO2, number of episodes and total duration of atrial tachyarrhythmia, incidence of AV block type I, II or III and maximum duration of the longest pause, percentage of paced atrial and ventricular beats | ||||||
| Subgroups | Not reported | ||||||
| Power calculation | Not reported | ||||||
| Intervention/comparator | |||||||
| Pacemaker (type, brand, etc.) | Any type of DDDR pacemaker | ||||||
| Implantation | Not reported | ||||||
| Programming | DDDR or AAIR. Maximum pacing rate was set as (220 – age) × 0.9 and at least 10 b.p.m. below the Wenckebach point unless a lower rate was clinically indicated. AV delay was optimised based on the maximum time–velocity integral of the aortic flow. Rate adaptation was tailored individually during self-determined casual and brisk walks. Mode switch was not initiated in all patients | ||||||
| Randomised, n | 21 | ||||||
| Withdrawals, n (%) (change in pacing mode, loss to follow-up) | Two withdrawals:
|
||||||
| Mode | DDDR | AAIR | |||||
| Atrial pacing, % (SD) | 95 (SD 5) | 96 (SD 5) | |||||
| VP, % (SD) | 99 (SD 2) | N/A | |||||
| Follow up | Total follow-up was 3 months for each pacing mode | ||||||
| Baseline patient characteristics | |||||||
| Age (years), mean (SD) | 70 (7) | ||||||
| Male sex, n (%) | 11 (58) | ||||||
| Previous history of AF, n (%) | Not reported | ||||||
| Previous stroke, n (%) | Not reported | ||||||
| Medication, n | |||||||
| Sotalol | 13 | ||||||
| Flecainide | 2 | ||||||
| Amiodarone | 5 | ||||||
| Pacing indication, n | Not reported | ||||||
| NYHA class, n | Not reported | ||||||
| Outcome | Definition | ||||||
| Exercise capacity | Bicycle ergometry was performed under the same conditions and at the same time of day for each patient. An incremental exercise test to exhaustion was performed using workload increments of 15 W/minute in every patient. Exercise duration and maximal workload in watts were measured | ||||||
| Cognitive function | One dimension of first QoL questionnaire using VAS to assess cognitive functioning | ||||||
| HRQoL | Symptoms were assessed using four self-administered questionnaires: (1) VAS for general well-being, physical, emotional and cognitive functioning; (2) VAS Karolinska questionnaire including 16 questions on cardiovascular symptoms relevant to pacemaker patients; (3) SAS functional status questionnaire for physical capacity, grading patients from class I (unlimited exercise capacity) to class IV (very low exercise tolerance); (4) 5-point category scale to estimate the severity and prevalence of specific symptoms caused by pacemaker induced hemodynamic dysfunction as occurs in the pacemaker syndrome. 1 = severe and nearly persistent to 5 = free of symptoms | ||||||
| Continuous outcomes | Dual-chamber pacing | Atrial pacing | p-value | ||||
| Mean | SD | N | Mean | SD | N | ||
| Exercise capacity | |||||||
| Maximum exercise duration (second) | 402 | 102 | 19 | 423 | 127 | 19 | < 0.05 |
| Maximum workload (W) | 96 | 27 | 19 | 103 | 31 | 19 | < 0.05 |
| Self-perceived health status (%) | |||||||
| General well-being | 67 | 20 | 19 | 67 | 23 | 19 | NS |
| Physical functioning | 59 | 25 | 19 | 56 | 25 | 19 | NS |
| Emotional functioning | 63 | 27 | 19 | 63 | 27 | 19 | NS |
| Cognitive functioning | 56 | 23 | 19 | 51 | 27 | 19 | NS |
| Karolinska questionnaire (%) | |||||||
| Chest pain | 73 | 20 | 19 | 76 | 19 | 19 | NS |
| Palpitations | 78 | 17 | 19 | 79 | 20 | 19 | NS |
| Dizziness | 71 | 16 | 19 | 82 | 11 | 19 | < 0.05 |
| Dyspnoea | 67 | 24 | 19 | 71 | 20 | 19 | NS |
| SAS (1–4) | 1.6 | 0.74 | 19 | 1.6 | 0.67 | 19 | NS |
Economic evaluations
| Study ID (author name, year or acronym) | Caro, 2006, UK |
| Perspective; discounting; and cost year | UK NHS perspective; discounting: costs 6% and benefits 1.5%; cost year 2003 |
| Model type | Discrete event simulation, cost–utility, 5-year time horizon |
| Patient population | Bradycardia due to SND or AV block. Patient characteristics sourced from CTOPP. Age distribution based on 2002 UK pacemaker implantation population.58 Systolic blood pressure distribution |
| Intervention/comparator | Dual-chamber [DDD (52%) or DDDR (48%)] vs. single-chamber ventricular pacemakers [VVI (35%) or VVIR (65%)] |
| Costs (source) | Procedure costs (NHS Reference Costs 200266): initial procedure: outpatient (£1962), elective inpatient (£3177), non-elective inpatient (£3217) |
| Reoperation: day case (£1503), inpatient elective (£2395), inpatient non-elective (£2785) | |
| Pacemaker costs (Consortium of Pacemaker Manufacturers, personal communication50): DDD (£1260), DDDR (£1864), VVI (£673), VVIR (£9370) | |
| Anticoagulation (Summary of product characteristics and NHS Reference Costs 200266): cost of warfarin 5 mg per day (£0.06) and six physicians’ visits per year associated with monitoring | |
| Stroke (NHS Reference Costs 200266): £2157 per patient | |
| Outcomes (source) | Post-operative complications: |
| Results (including uncertainty | |
|---|---|
| Multivariate sensitivity analyses | Dual-chamber pacemakers dominant in 29% of replications, ICER < £1000/QALY in 31% of replications. ICER did not exceed £10,000/QALY in any analysis |
| Post-operative complications | Single-chamber (6.4%), dual-chamber (7.7%) |
| AF | Single-chamber (22%), dual-chamber (18%) |
| Death |
|
| Mean discounted cost over 5 years |
|
| Mean additional cost per patient | £43 |
| Mean cost–utility | £477 per discounted QALY |
| Univariate sensitivity analysis | Results are sensitive to the proportion of patients with a VVI(R) who would have a replacement device because of pacemaker syndrome |
| Study ID (author name, year or acronym) | Castelnuovo, 2005, UK |
| Perspective; discounting; and cost year | UK NHS perspective; discounting: costs 6% and benefits 1.5%; cost year 2003 |
| Model type | A series of Microsoft Excel-based Markov models, cost–utility, 5-year time horizon (10 years explored in sensitivity analysis |
| Patient population | Three homogeneous hypothetical cohorts of individuals with SSS or AV block |
| Intervention/comparator | The models compared three treatment options:
|
| Costs (source) | Hardware (unpublished at the time UKPACE): VVI (£690); VVIR (£1099); DDD (£1365); DDDR (£2107); atrial lead (£175); ventricular lead (£172) |
| Implantation procedure costs (resource cost initiative database): single-chamber pacemaker (£4025), dual-chamber pacemaker (£4925) | |
| Perioperative complications (NHS Resource Costs 200266): single-chamber ventricular pacemaker (£816); single-chamber atrial pacemaker (£894); dual-chamber pacemaker (£894) | |
| Pacemaker syndrome (NHS Reference Costs 200266), excludes upgrade costs: mild (£40); severe (£176) | |
| Development of AV block (NHS Reference Costs 200266), in SSS only patients; excludes upgrade costs: £176 | |
| AF (NHS Reference Costs 200266) £41 per month, including antithrombotic treatment, GP visits, INR monitoring and outpatient anticoagulation clinic visits | |
| HF: based on assumptions of hospital admission and drug use, £152 per month | |
| Stroke (Kavanagh et al.,80 NHS Reference Costs 200266): £816 per cycle | |
| Outcomes (source) | Proportion of patients receiving each device type is as reported in clinical trials |
| Incidence of perioperative complications (review of PASE and CTOPP),62,67 incidence rate doubled for upgrades | |
| Pacemaker syndrome, time-dependent incidence, proportion leading to upgrade (MOST and CTOPP)62,68 | |
| Development of AV block in SSS only patients, 1.9% per annum (Nielsen et al.)41 | |
Progression to AF:
|
|
| HF: | |
| Stroke: | |
| Reimplantation at the end of generator life: dual-chamber pacemaker, 0.7% in year 2, increasing to 25.5% in year 10 (The National Pacemaker Database60); single-chamber pacemaker, 0.6% in year 2, increasing to 18% in year 10 (The National Pacemaker Database60) | |
| Mortality: perioperative mortality, 2.5 per 1000 (PASE); perioperative mortality following upgrade operation, double initial operation perioperative mortality (assumption); all-cause mortality (ONS 2002120); stroke mortality, 33% (Appelros et al.)72 | |
| HF: 20.8% (MacIntyre et al.)73 | |
| Utility: pacemaker implant/AV block prior to upgrade, 0.76 (PASE); perioperative/subsequent complications, 0.75, assumption based on PASE); well with pacemaker, 0.925 (PASE); mild pacemaker syndrome, 0.80 (PASE); severe pacemaker syndrome, 0.62 (PASE); perioperative complications during upgrade, 0.915 (PASE); AF, 0.875 (Harvard database); HF, 0.64, (PASE); stroke, 0.39 (Tengs et al.)82 | |
| Results (including uncertainty) | Deterministic:
|
| Study ID (author name, year or acronym) | Clarke, 1998, UK |
| Perspective; discounting; and cost year | UK NHS; no discounting; cost year 1995/6 |
| Model type | Retrospective cost comparison/cost saving |
| Patient population | People with SSS and no AV block |
| Intervention/comparator | Single chamber atrial pacemaker vs. dual-chamber pacemaker |
| Costs (source) | Device (including implant) costs only, within institution cost (single-chamber atrial pacemaker: £2885; dual-chamber pacemaker: £3844 |
| Outcomes (source) | Development of AV block (retrospective analysis of within institution records from 1992–1996) |
| Results (including uncertainty) | Based on observed percentage of upgrades procedures, cost savings were estimated at £103,000 per year |
| Study ID (author name, year or acronym) | Deniz, 2008, Italy (based on model outlined in publication by Caro et al.) |
| Perspective, discounting, cost year | Italian government perspective, 3% discounting for costs and benefits, cost year 2004 |
| Model type | Cost–utility, DES, 5-year time horizon |
| Patient population | Individuals with SSS or AV block. Patient characteristics sourced from CTOPP.63 Age distribution based on 2002 UK pacemaker implantation population.60 Systolic blood pressure distribution from the Framingham Heart Study for patients with AF61 |
| Intervention/comparator | Dual-chamber [DDD (52%) or DDD(R)] vs. single-chamber ventricular pacemakers VVI(R) |
| Costs (source) | Procedure costs (Istat) initial procedure – outpatient (€5867), elective inpatient (€6934) Reoperation: day case (€2820), inpatient elective (€4302) Pacemaker costs (Medtronic Europe, Italy, personal communication):
Anticoagulation (Istat), includes warfarin 5 mg/day and a physician visit (€106 per year) |
| Outcomes (source) | Complications: baseline rate (MOST)64 HR for single-chamber device (CTOPP)62 |
| AF (CTOPP)62,63 6.6% of VVI(R) patients developed AF (documented episode lasting > 15 minutes) 18% risk reduction observed for ‘physiological’ pacing 3.84% of VVI(R) patients developed chronic AF (lasting at least 1 week) 27% risk reduction of chronic AF with ’physiological’ pacing |
|
| Stroke: in AF patients only (Framingham Heart Study)61 | |
| Clinically relevant pacemaker symptoms: CTOPP118 and MOST68 | |
| Death: assumed equivalent CTOPP119 | |
| QALYs: utility data (MOST)68 | |
| Results (including uncertainty) | Based on the mean of 100 replications of the simulation of 1000 patients ICER: €260 per QALY (£215 per QALY) Multivariate analysis based on 1000 replications of 1000 simulated patients accounting for parameter uncertainty indicated that dual-chamber devices were less costly and more effective in 45% of replications Univariate sensitivity analysis indicated that the cost-effectiveness results were sensitive to assumptions around device replication following the onset of pacemaker syndrome |
| Study ID (author name, year or acronym) | Mahoney, 1994, USA |
| Perspective, discounting, cost year | US-payer perspective, no discounting, cost year not stated |
| Model type | Comparison of benefits and costs, time horizon is stated as ‘long-term’ |
| Patient population | Patients receiving dual or single-chamber pacemakers |
| Intervention/comparator | DDD or AAI vs. VVI pacemakers |
| Costs (source) | Treatment (of outcomes associated with pacing, e.g. AF) costs (national average urban diagnostic-related group payment, Minneapolis), device costs (source not stated) |
| Outcomes (source) | AV block, AF, chronic heart failure, pacemaker syndrome, stroke, thromboembolism, mortality (meta-analysis of 35 published studies comparing dual-chamber to single-chamber pacing modes) |
| Results (including uncertainty) | Benefits:
|
Costs: the cost of treating patients for AF, chronic heart failure, stroke and pacemaker syndrome is higher in VVI pacing vs. DDD and vs. AAI pacing
|
|
| Including device cost, the overall cost of VVI vs. DDD is 24–27% higher and the overall cost of VVI vs. AAI is 34–35% higher | |
| Note: the diagrammatic representation of treatment costs seems to contradict the percentage cost increases reported. That is, in the figure, the treatment costs associated with AAI appear higher than those associated with DDD, and yet VVI is reported as costing proportionally more in comparison with AAI than with DDD pacing |
| Study ID (author name, year or acronym) | O’Brien, 2005, Canada |
| Perspective, discounting, cost year | Provincial government health-care payer (mostly Ontario) perspective, discounting 3% per annum, 2004 C$ |
| Model type | Economic evaluation alongside clinical trial, cost-effectiveness analysis, 5.2-year time horizon |
| Patient population | Patients without chronic AF who were scheduled for a first implantation of a pacemaker to treat symptomatic bradycardia |
| Intervention/comparator | Physiological pacing (dual-chamber or single-chamber atrial pacemakers) vs. single-chamber ventricular pacemakers |
| Costs (source) | Resource use and costs were collected from a subset of patients enrolled in CTOPP. Resource use included: initial pacemaker implantation, adjustment and replacement of pacemaker, length of (initial and subsequent) hospital stay, follow-up physician visits and consultations and antiarrhythmic drugs |
| Costs (adjusted for censoring using methods of Lin et al.85): hospital costs (Ontario Case Costing Project), device costs (Canadian market prices weighted by market share), physician’s services (Ontario Schedule of Benefits) and antiarrhythmic drugs (Ontario Drug Benefits schedule) | |
| Outcomes (source) | Life expectancy: Kaplan–Meier data (CTOPP – all patients) |
| AF: event data (CTOPP – all patients) | |
| Results (including uncertainty) | Cost-effectiveness:
|
Physiological pacing is dominated by single-chamber VP in people with IHR < 60
|
|
Sensitivity analysis:
|
| Study ID (author name, year or acronym) | Oddershede, 2014, Denmark | ||
| Perspective; discounting; and cost year | Danish health-care system; discounting: costs and benefits 3.5%; costs were converted from 2012 DKK to 2013 GBP | ||
| Model type | Markov model was used, cost–utility, life-time horizon | ||
| Patient population | Patients with SSS and preserved AV conduction model | ||
| Patients were divided into three groups with different levels of risk in terms of their predicted survival probability according to a Cox proportional hazard | |||
| Risk Group 1 was the group with the highest probability of deaths and Risk Group 3 was that with lowest probability of deaths | |||
| Intervention/comparator | DDDR vs. AAIR pacing | ||
| Costs (source) | Procedure costs:
|
||
Follow-up and complication costs (Danish diagnosis-related group and Danish ambulatory group costs 2012):
|
|||
| Outcomes (source)b | Stroke First stroke Second stroke Death QALYs |
||
| Results (including uncertainty) | Adjusted | Adjusted pooled | Unadjusted multistate |
Incremental costs (£)
|
Incremental costs (£):
|
Incremental costs (£): –2310 | |
Incremental effectiveness (QALYs):
|
Incremental effectiveness (QALYs):
|
Incremental effectiveness (QALYs): 0.277 | |
Net monetary benefit (£):
|
Net monetary benefit (£):
|
Net monetary benefit (£): 7847 (10,615) | |
| Probabilistic sensitivity analysis: at a WTP threshold of £20,000 DDDR pacing was cost-effective across all the scenarios. However, at a WTP threshold of £30,000 DDDR pacing was not cost-effective in Risk Group 2 | |||
| Study ID (author name, year or acronym) | Osman, 2010, UK |
| Perspective; discounting; and cost year | Single-centre perspective; no discounting; cost year 2005/6 |
| Model type | No model, safety and cost assessment of inpatient vs. same-pay elective pacemaker implant, 1-year time horizon for costs, 5.5-year time horizon for safety |
| Patient population | 780 patients scheduled for new pacemaker implant, included:
|
| Intervention/comparator | Same-day procedure vs. procedure followed by an overnight stay for new pacemaker implant |
| Costs (source) | Cost of an overnight stay, £203.60 (finance department of single-centre) |
| Outcomes (source) | Peri- and post-implant complications, hospital admissions after pacemaker implantation, mortality (single-centre pacing database) |
| Results (including uncertainty) | Unplanned overnight stay, 41 (5.3%) required an overnight stay as a result of:
|
Immediate complications, < 24 hours after implant, occurred in six patients:
|
|
Early complications, > 24 hours to 6 weeks after implantation, occurred in 17 patients:
|
|
| Cost savings, with respect to overnight stays avoided; of 109 patients undergoing elective same-day new pacemaker implantation between November 2005 and November 2006, two patients required an overnight stay as a result of lead displacement, resulting in cost-savings of £21,785 |
| Study ID (author name, year or acronym) | Ray, 1992, UK |
| Perspective; discounting; and cost year | Single-centre perspective; no discounting; cost year 1991 |
| Model type | No model, clinical practice and cost audit, 18-month time horizon |
| Patient population | Patients undergoing a first pacemaker implantation |
| Intervention/comparator | Any pacing device |
| Costs (source) | Average cost of pacemaker unit (single-centre cost records): VVI (£631) VVIR (£1773) AAI (£927) AAIR (£1642) DDD (£1811) DDDR (£1992) DDI (£1845) |
| Average cost of pacing type including the cost of any replacement lead or generator that had to be inserted within 1 month of the initial procedure (single-centre cost records) | |
| Outcomes (source) | N/A |
| Results (including uncertainty) | Change in practice: The percentage of SND patients receiving atrial (AAI/AAIR or DDD/DDDR) pacing as opposed to VP (VVI/VVIR) increased from 24% to 59% The proportion of AV block patients receiving a dual-chamber pacemaker increased from 12% to 19% The percentage of patients with AV block and AF receiving VVIR pacing increased from 10% to 22% |
| Cost impact of full guideline adherence Full guideline adherence was estimated as increasing the budget for pacing hardware by 94% (from 333,535 to 647,163) over the 18-month study period |
| Study ID (author name, year or acronym) | Rinfret, 2005, USA |
| Perspective; discounting; and cost year | Societal; 3% discounting; 2001 |
| Model type | In-trial cost-effectiveness analysis, extrapolated with a Markov model cost–utility analysis. In-trial time horizon 4 years and Markov extrapolation time horizon is lifetime |
| Patient population | Patients paced for SSS |
| Intervention/comparator | DDDR vs. VVIR |
| Costs (source) | Pacemaker implantation costs, including: Hardware (IMS Hospital Supply Index) Hospitalisation (single centre) Professional fees (Medicare physician fee schedule) |
| Follow-up outpatient costs, including emergency department visits, unscheduled outpatient visits and 50% of scheduled visits during the MOST (MOST68 data) | |
| Medication costs (2001 Redbook costs87) | |
| Rehospitalisation as a result of cardiovascular events (MOST68 data) | |
| Crossover – VVIR to DDDR – costs (single centre) | |
| Generator change (single centre) – resource use based on expert opinion of 8 and 11 years before replacement of DDDR and VVIR devices, respectively | |
| Outcomes (source) | AF (MOST68 data) Hospitalisation for HF (MOST68 data) Stroke (MOST68 data) Death (MOST68 data) Utility data (data from TTO instrument administered in MOST68) Age-specific background mortality (US life-tables) |
| Results (including uncertainty) | In-trial cost-effectiveness results: US$52,814 per QALY over 4 years Lifetime Markov model cost-effectiveness analysis result: US$6800 per QALY Sensitivity analysis, bootstrap analyses estimated that DDDR pacing would be cost-effective at a threshold of US$50,000 in 91.9% of samples |
| Study ID (author name, year or acronym) | Sutton, 1996, UK |
| Perspective; discounting; and cost year | Not stated, but includes costs relevant to UK NHS perspective; no discounting; cost year 1991 |
| Model type | ‘Computer model’, cost–benefit, 10-year time horizon |
| Patient population | Patients with SSS and/or AV block |
| Intervention/comparator | DDD pacing vs. VVI pacing |
| Costs (source) | Generic units of currency (based on UK prices) are used, with the cost of a VVI device equivalent to 100 cost units Device costs: a survey of six manufacturers active on the UK market Implantation costs: 45 minutes assumed for single-chamber, 60 minutes for dual-chamber plus two overnight stays for both (single-centre costs) Follow-up costs (single-centre costs) AF (single-centre costs) Stroke: assumed 7 days of inpatient care (single-centre costs) Disability from stroke (local area costs of long-term care) HF: includes the cost of therapy with ACE inhibitor and furosemide at average doses (standard UK prices) plus 1 week of inpatient care per year (single-centre costs) Upgrade: includes dual-chamber device costs, plus 60 minutes of operating time and one night inpatient stay, plus ‘waste of resources involved in disposing of the redundant generator’ |
| Outcomes (source) | Complications including AF, stroke, disability as a result of stroke, HF, pacemaker syndrome and mortality. In addition, AV block in SSS patients paced with an AAI device. Note: DDD and AAI pacing are considered equivalent (with the exception of AV blocks leading to upgrade) |
AF:
|
|
| Stroke: assumed to be 30% of AF rate | |
| HF: 6.5% in VVI paced patients, 2.1% in DDD paced patients (average incidence of four reports, including 414 patients) | |
Mortality:
|
|
| Results (including uncertainty) | Benefits: Survival: greater in patients initially implanted with a DDD, also 24/57 surviving SSS/VVI patients had upgraded to DDD. Similarly, 21/51 surviving AV block/VVI patients had upgraded to a DDD HF: incidence reduced by half with DDD pacing Disability from stroke: fivefold reduction in DDD patients as a result of reduction in AF Costs (not including generator replacement costs): approximately equal 3 years after implantation. Ten-year cumulative cost of VVI in SSS patients is 12 times that of DDD. The 10-year cumulative cost of VVI pacing in AV block is eight times that of DDD |
Sensitivity analysis:
|
| Study ID (author name, year or acronym) | Wiegand, 2001, Germany |
| Perspective, discounting, cost year | Not stated |
| Model type | No model was used. Cost–benefit analysis. Mean follow-up was 42 months (SD 15 months), ranging from 3 to 76 months |
| Patient population | Patients with AV block and normal sinus function admitted to the University Hospital of Luebeck between 1992 and 1997 |
| Intervention/comparator | Single-lead VDD vs. DDD devices |
| Costs (source) | Primary costs of pacemaker implantation included:
|
| Outcomes (source) | Kaplan–Meier curves for maintenance of AV synchrony and event-free survival of patients (single-centre prospective study) |
| Results (including uncertainty) | Costs: Average costs of VDD and DDD pacemaker devices did not differ Cost of entire pacemaker system was lower for VDD pacing: as a result of requiring one rather than two leads Implantation costs were lower for VDD pacing: as a result of shorter implantation time and fewer demands for lead introducers for subclavian vein puncture Cumulative costs of DDD pacing were significantly higher compared with VDD pacing during follow-up |
| Benefits: Mean post-operative hospitalisation was significantly prolonged in the DDD group No significant difference in AF, cardiac disease or pacemaker-related complications No significant difference between maintenance of AV synchrony and event-free survival |
Health-related quality of life
| Author, year, country | Population and methods | Health states | Instrument (valuation) | Utility results | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fleishmann, 2009,94 USA | The study analysed serial data from MOST,68 which randomised 2010 patients with SSS to VVIR or DDDR pacing. The average age of the study cohort was 73 years, and 52% of patients were male. The majority of patients were white and 22% had a history of diabetes. Prior myocardial infarction was reported in 26% and prior stroke in 11%. Prior HF was present on 18% of VVIR patients and 22% of DDDR patients. The QoL scores were measured at 3-month and 12-month visits and then yearly afterwards. After initial unadjusted analysis, change in QoL measures was stratified by age, sex, history of AF, history of HF, treatment arm and baseline QOL score for each measure. The last known QoL values were carried forward for patients who crossed over to dual-chamber pacing as a result of severe pacemaker syndrome |
|
TTO | Baseline utilities were not reported. Only change in utility values compared to values at baseline and 3 months were reported Change in QoL scores at 12 months compared with baselineScaleNo AFParoxysmal AFChronic AFSF-36Physical function+0.06–2.92–3.29Role physical+22.22+16.88+14.32Mental health+2.55+1.57+2.09Role emotional+6.85+4.10+12.39Vitality+7.68+7.04+4.46Pain+3.46+1.46–3.20Health perception–1.29–2.96–1.29Social function+6.80+4.98+6.61Physical summary+2.50+0.90–0.30Mental summary+2.50+2.32+2.87TTOScore+0.07+0.06+0.11SASScore+0.03+0.15+0.21 Change in QoL scores at 12 months compared with 3 monthsScaleNo AFParoxysmal AFChronic AFSF-36Physical function–2.21–4.36–3.45Role physical+2.44–1.80+0.83Mental health+0.25+0.50–3.31Role emotional+0.99–0.34+1.66Vitality–1.21–1.43–0.95Pain–0.92–3.13–7.09Health perception+3.28+2.27+0.79Social function–1.24+0.15–1.06Physical summary–0.60–2.49–0.50Mental summary0.11+0.80–0.74TTOScore–0.00–0.02+0.03SASScore+0.05+0.12+0.44 |
Change in QoL scores at 12 months compared with baseline | Scale | No AF | Paroxysmal AF | Chronic AF | SF-36 | Physical function | +0.06 | –2.92 | –3.29 | Role physical | +22.22 | +16.88 | +14.32 | Mental health | +2.55 | +1.57 | +2.09 | Role emotional | +6.85 | +4.10 | +12.39 | Vitality | +7.68 | +7.04 | +4.46 | Pain | +3.46 | +1.46 | –3.20 | Health perception | –1.29 | –2.96 | –1.29 | Social function | +6.80 | +4.98 | +6.61 | Physical summary | +2.50 | +0.90 | –0.30 | Mental summary | +2.50 | +2.32 | +2.87 | TTO | Score | +0.07 | +0.06 | +0.11 | SAS | Score | +0.03 | +0.15 | +0.21 | Change in QoL scores at 12 months compared with 3 months | Scale | No AF | Paroxysmal AF | Chronic AF | SF-36 | Physical function | –2.21 | –4.36 | –3.45 | Role physical | +2.44 | –1.80 | +0.83 | Mental health | +0.25 | +0.50 | –3.31 | Role emotional | +0.99 | –0.34 | +1.66 | Vitality | –1.21 | –1.43 | –0.95 | Pain | –0.92 | –3.13 | –7.09 | Health perception | +3.28 | +2.27 | +0.79 | Social function | –1.24 | +0.15 | –1.06 | Physical summary | –0.60 | –2.49 | –0.50 | Mental summary | 0.11 | +0.80 | –0.74 | TTO | Score | –0.00 | –0.02 | +0.03 | SAS | Score | +0.05 | +0.12 | +0.44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Change in QoL scores at 12 months compared with baseline | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Scale | No AF | Paroxysmal AF | Chronic AF | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical function | +0.06 | –2.92 | –3.29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role physical | +22.22 | +16.88 | +14.32 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental health | +2.55 | +1.57 | +2.09 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role emotional | +6.85 | +4.10 | +12.39 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitality | +7.68 | +7.04 | +4.46 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain | +3.46 | +1.46 | –3.20 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health perception | –1.29 | –2.96 | –1.29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social function | +6.80 | +4.98 | +6.61 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical summary | +2.50 | +0.90 | –0.30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental summary | +2.50 | +2.32 | +2.87 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TTO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Score | +0.07 | +0.06 | +0.11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Score | +0.03 | +0.15 | +0.21 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Change in QoL scores at 12 months compared with 3 months | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Scale | No AF | Paroxysmal AF | Chronic AF | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical function | –2.21 | –4.36 | –3.45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role physical | +2.44 | –1.80 | +0.83 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental health | +0.25 | +0.50 | –3.31 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role emotional | +0.99 | –0.34 | +1.66 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitality | –1.21 | –1.43 | –0.95 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain | –0.92 | –3.13 | –7.09 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health perception | +3.28 | +2.27 | +0.79 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social function | –1.24 | +0.15 | –1.06 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical summary | –0.60 | –2.49 | –0.50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental summary | 0.11 | +0.80 | –0.74 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TTO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Score | –0.00 | –0.02 | +0.03 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Score | +0.05 | +0.12 | +0.44 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAS (measure of cardiac function) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fleishmann, 2006,92 USA | Serial QoL data were collected and analysed as part of MOST. The paper refers to Lamas et al. 200268 for details of enrolment and data collection. A total of 2010 patients with SSS took part in MOST between 1995 and 1999. QoL was measured at 3-month and 12-month visits then yearly afterwards. In patients who crossed over because of severe pacemaker syndrome, a primary analysis was performed in which the last known QoL prior to crossover was carried forward. The baseline characteristics of the cohort are presented in the table below: CharacteristicVVIR (n = 996)DDDR (n = 1014)Age (year)73.1 (SD 11.0)72.9 (SD 11.1)Male, n (%)519 (52)536 (53)Non-white, n (%)144 (14)162 (16)Diabetes, n (%)204 (20)246 (24)Hypertension, n (%)608 (61)640 (63)Hypercholesterolaemia, n (%)340 (34)376 (37)Current smoker, n (%)85 (9)84 (8)Prior angina, n (%)280 (28)288 (28)Prior myocardial infarction, n (%)234 (24)279 (28)Prior HF, n (%)183 (18)221 (22)Prior stroke, n (%)108 (11)116 (11)Charlson Comorbidity Index1.46 (SD 1.65)1.54 (SD 1.67) |
Characteristic | VVIR (n = 996) | DDDR (n = 1014) | Age (year) | 73.1 (SD 11.0) | 72.9 (SD 11.1) | Male, n (%) | 519 (52) | 536 (53) | Non-white, n (%) | 144 (14) | 162 (16) | Diabetes, n (%) | 204 (20) | 246 (24) | Hypertension, n (%) | 608 (61) | 640 (63) | Hypercholesterolaemia, n (%) | 340 (34) | 376 (37) | Current smoker, n (%) | 85 (9) | 84 (8) | Prior angina, n (%) | 280 (28) | 288 (28) | Prior myocardial infarction, n (%) | 234 (24) | 279 (28) | Prior HF, n (%) | 183 (18) | 221 (22) | Prior stroke, n (%) | 108 (11) | 116 (11) | Charlson Comorbidity Index | 1.46 (SD 1.65) | 1.54 (SD 1.67) | Utility was measured at baseline and at different time points after implantation of single-chamber or dual-chamber pacemakers | TTO | Mean scores adjusted for age and sexPacemakerTTOSASBaseline (n = 1935)DDDR0.721.97VVIR0.732.003 months (n = 1736)DDDR0.831.92VVIR0.821.9412 months (n = 1639)DDDR0.831.99VVIR0.821.9724 months (n = 1208)DDDR0.831.99VVIR0.812.0136 months (n = 748)DDDR0.862.01VVIR0.831.9848 months (n = 392)DDDR0.832.01VVIR0.872.03 Mean scores adjusted for age and sexPacemakerBaseline1 year2 yearsPhysical functionDDDR58.961.058.6VVIR58.959.058.3Role physicalDDDR34.665.565.3VVIR35.756.259.9Mental healthDDDR72.176.778.7VVIR72.074.777.1Role emotionalDDDR74.085.989.1VVIR74.181.980.1VitalityDDDR42.651.552.1VVIR41.949.249.8PainDDDR42.671.173.0VVIR41.969.976.7Health perceptionDDDR67.058.458.6VVIR67.657.056.2Social functionDDDR60.371.473.8VVIR60.070.571.6 |
Mean scores adjusted for age and sex | Pacemaker | TTO | SAS | Baseline (n = 1935) | DDDR | 0.72 | 1.97 | VVIR | 0.73 | 2.00 | 3 months (n = 1736) | DDDR | 0.83 | 1.92 | VVIR | 0.82 | 1.94 | 12 months (n = 1639) | DDDR | 0.83 | 1.99 | VVIR | 0.82 | 1.97 | 24 months (n = 1208) | DDDR | 0.83 | 1.99 | VVIR | 0.81 | 2.01 | 36 months (n = 748) | DDDR | 0.86 | 2.01 | VVIR | 0.83 | 1.98 | 48 months (n = 392) | DDDR | 0.83 | 2.01 | VVIR | 0.87 | 2.03 | Mean scores adjusted for age and sex | Pacemaker | Baseline | 1 year | 2 years | Physical function | DDDR | 58.9 | 61.0 | 58.6 | VVIR | 58.9 | 59.0 | 58.3 | Role physical | DDDR | 34.6 | 65.5 | 65.3 | VVIR | 35.7 | 56.2 | 59.9 | Mental health | DDDR | 72.1 | 76.7 | 78.7 | VVIR | 72.0 | 74.7 | 77.1 | Role emotional | DDDR | 74.0 | 85.9 | 89.1 | VVIR | 74.1 | 81.9 | 80.1 | Vitality | DDDR | 42.6 | 51.5 | 52.1 | VVIR | 41.9 | 49.2 | 49.8 | Pain | DDDR | 42.6 | 71.1 | 73.0 | VVIR | 41.9 | 69.9 | 76.7 | Health perception | DDDR | 67.0 | 58.4 | 58.6 | VVIR | 67.6 | 57.0 | 56.2 | Social function | DDDR | 60.3 | 71.4 | 73.8 | VVIR | 60.0 | 70.5 | 71.6 | ||||||||||||||||||||||||||||||||||||||||||
| Characteristic | VVIR (n = 996) | DDDR (n = 1014) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (year) | 73.1 (SD 11.0) | 72.9 (SD 11.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Male, n (%) | 519 (52) | 536 (53) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-white, n (%) | 144 (14) | 162 (16) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diabetes, n (%) | 204 (20) | 246 (24) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hypertension, n (%) | 608 (61) | 640 (63) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hypercholesterolaemia, n (%) | 340 (34) | 376 (37) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Current smoker, n (%) | 85 (9) | 84 (8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prior angina, n (%) | 280 (28) | 288 (28) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prior myocardial infarction, n (%) | 234 (24) | 279 (28) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prior HF, n (%) | 183 (18) | 221 (22) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prior stroke, n (%) | 108 (11) | 116 (11) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charlson Comorbidity Index | 1.46 (SD 1.65) | 1.54 (SD 1.67) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean scores adjusted for age and sex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pacemaker | TTO | SAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline (n = 1935) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 0.72 | 1.97 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 0.73 | 2.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months (n = 1736) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 0.83 | 1.92 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 0.82 | 1.94 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 months (n = 1639) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 0.83 | 1.99 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 0.82 | 1.97 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24 months (n = 1208) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 0.83 | 1.99 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 0.81 | 2.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36 months (n = 748) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 0.86 | 2.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 0.83 | 1.98 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48 months (n = 392) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 0.83 | 2.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 0.87 | 2.03 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean scores adjusted for age and sex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pacemaker | Baseline | 1 year | 2 years | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical function | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 58.9 | 61.0 | 58.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 58.9 | 59.0 | 58.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role physical | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 34.6 | 65.5 | 65.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 35.7 | 56.2 | 59.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental health | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 72.1 | 76.7 | 78.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 72.0 | 74.7 | 77.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role emotional | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 74.0 | 85.9 | 89.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 74.1 | 81.9 | 80.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitality | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 42.6 | 51.5 | 52.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 41.9 | 49.2 | 49.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 42.6 | 71.1 | 73.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 41.9 | 69.9 | 76.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health perception | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 67.0 | 58.4 | 58.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 67.6 | 57.0 | 56.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social function | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 60.3 | 71.4 | 73.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 60.0 | 70.5 | 71.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAS (measure of cardiac function) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shukla, 2005,108 USA | The study reported a post-hoc analysis of elderly patients who were enrolled in MOST and received pacemakers that used accelerometer, piezoelectric crystal or blended sensors which were the most frequently used across a broad range of manufacturers. The QoL data of 1245 (613 DDDR and 632 VVIR) patients were analysed. Demographic, clinical and QoL data were collected at baseline, at 3 months and then annually in the trial | QoL values were compared according to type of sensor received | TTO | InstrumentUtilityp-valueTTOAccelerometer0.83 (0.015)0.45Blended0.80 (0.021)Piezoelectric crystal0.82 (0.012)SASAccelerometer2.38 (0.043)0.16Blended2.34 (0.56)Piezoelectric crystal2.30 (0.37)0–100 scaleAccelerometer70.9 (0.91)0.33Blended69.0 (1.3)Piezoelectric crystal70.9 (0.75) InstrumentUtilityp-valueSF-36Physical functionAccelerometer59.2 (1.2)0.009Blended53.7 (1.7)Piezoelectric crystal58.1 (1.0)Role, physical functionAccelerometer65.3 (1.9)0.08Blended59.8 (2.8)Piezoelectric crystal61.5 (1.5)PainAccelerometer72.1 (1.2)0.79Blended70.8 (1.7)Piezoelectric crystal71.8 (1.0)Health perceptionAccelerometer58.9 (0.9)0.11Blended56.0 (1.3)Piezoelectric crystal58.0 (0.8)EnergyAccelerometer51.9 (1.1)0.98Blended51.9 (1.5)Piezoelectric crystal51.8 (0.9)Social functionAccelerometer72.2 (1.1)0.42Blended70.1 (1.6)Piezoelectric crystal72.2 (0.9)Role, mental healthAccelerometer83.9 (1.4)0.79Blended84.1 (2.2)Piezoelectric crystal83.0 (1.2)Mental healthAccelerometer77.3 (0.8)0.51Blended76.3 (1.1)Piezoelectric crystal76.5 (0.6)Physical summaryAccelerometer41.0 (0.50)0.039Blended39.1 (0.70)Piezoelectric crystal40.4 (0.41)Emotional summaryAccelerometer52.1 (0.42)0.77Blended52.1 (0.61)Piezoelectric crystal51.8 (0.35) |
Instrument | Utility | p-value | TTO | Accelerometer | 0.83 (0.015) | 0.45 | Blended | 0.80 (0.021) | Piezoelectric crystal | 0.82 (0.012) | SAS | Accelerometer | 2.38 (0.043) | 0.16 | Blended | 2.34 (0.56) | Piezoelectric crystal | 2.30 (0.37) | 0–100 scale | Accelerometer | 70.9 (0.91) | 0.33 | Blended | 69.0 (1.3) | Piezoelectric crystal | 70.9 (0.75) | Instrument | Utility | p-value | SF-36 | Physical function | Accelerometer | 59.2 (1.2) | 0.009 | Blended | 53.7 (1.7) | Piezoelectric crystal | 58.1 (1.0) | Role, physical function | Accelerometer | 65.3 (1.9) | 0.08 | Blended | 59.8 (2.8) | Piezoelectric crystal | 61.5 (1.5) | Pain | Accelerometer | 72.1 (1.2) | 0.79 | Blended | 70.8 (1.7) | Piezoelectric crystal | 71.8 (1.0) | Health perception | Accelerometer | 58.9 (0.9) | 0.11 | Blended | 56.0 (1.3) | Piezoelectric crystal | 58.0 (0.8) | Energy | Accelerometer | 51.9 (1.1) | 0.98 | Blended | 51.9 (1.5) | Piezoelectric crystal | 51.8 (0.9) | Social function | Accelerometer | 72.2 (1.1) | 0.42 | Blended | 70.1 (1.6) | Piezoelectric crystal | 72.2 (0.9) | Role, mental health | Accelerometer | 83.9 (1.4) | 0.79 | Blended | 84.1 (2.2) | Piezoelectric crystal | 83.0 (1.2) | Mental health | Accelerometer | 77.3 (0.8) | 0.51 | Blended | 76.3 (1.1) | Piezoelectric crystal | 76.5 (0.6) | Physical summary | Accelerometer | 41.0 (0.50) | 0.039 | Blended | 39.1 (0.70) | Piezoelectric crystal | 40.4 (0.41) | Emotional summary | Accelerometer | 52.1 (0.42) | 0.77 | Blended | 52.1 (0.61) | Piezoelectric crystal | 51.8 (0.35) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Instrument | Utility | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TTO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 0.83 (0.015) | 0.45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 0.80 (0.021) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 0.82 (0.012) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 2.38 (0.043) | 0.16 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 2.34 (0.56) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 2.30 (0.37) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0–100 scale | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 70.9 (0.91) | 0.33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 69.0 (1.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 70.9 (0.75) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Instrument | Utility | p-value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical function | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 59.2 (1.2) | 0.009 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 53.7 (1.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 58.1 (1.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role, physical function | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 65.3 (1.9) | 0.08 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 59.8 (2.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 61.5 (1.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 72.1 (1.2) | 0.79 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 70.8 (1.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 71.8 (1.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health perception | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 58.9 (0.9) | 0.11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 56.0 (1.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 58.0 (0.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Energy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 51.9 (1.1) | 0.98 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 51.9 (1.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 51.8 (0.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social function | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 72.2 (1.1) | 0.42 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 70.1 (1.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 72.2 (0.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role, mental health | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 83.9 (1.4) | 0.79 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 84.1 (2.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 83.0 (1.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental health | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 77.3 (0.8) | 0.51 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 76.3 (1.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 76.5 (0.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical summary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 41.0 (0.50) | 0.039 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 39.1 (0.70) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 40.4 (0.41) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emotional summary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accelerometer | 52.1 (0.42) | 0.77 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Blended | 52.1 (0.61) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piezoelectric crystal | 51.8 (0.35) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0–100 scale | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Link, 2004,107 USA | The paper reports on the QoL of the subset of patients that took part in MOST who developed severe pacemaker syndrome according to the study protocol. A total of 182 patients assigned to VVIR pacing developed pacemaker syndrome. QoL was evaluated before and after crossover | Utility was measured at baseline and before and after crossover | TTO | InstrumentMean (SD) at baseline (n = 153)Mean (SD) at crossover (n = 80)Mean (SD) after crossover (n = 136)TTO0–1 TTO0.75 (0.34)0.73 (0.35)0.82 (0.31)SF-36Physical-composite35.8 (10.7)33.3 (10.1)38.0 (11.6)Mental-composite51.5 (9.5)49.8 (10.9)52.7 (11.6)Physical-function56.4 (27.6)39.8 (28.1)55.0 (29.7)Role-physical28.4 (38.7)28.4 (39.3)50.6 (43.0)Pain66.8 (29.1)70.9 (24.7)69.5 (26.3)Health perception57.2 (21.1)52.4 (20.7)56.5 (21.5)Energy39.6 (23.2)32.1 (21.4)49.9 (24.5)Social-function67.7 (24.7)62.2 (26.5)71.1 (24.1)Role-emotional80.4 (34.7)75.4 (39.2)83.6 (32.9)Mental health77.4 (17.2)73.5 (19.6)77.5 (17.8)SAS1–4 SAS2.09 (0.93)2.50 (0.91)2.07 (0.94)0–100 scaleScore65.8 (19.8)60.6 (20.0)70.8 (19.5) Change in QoL and p-values associated with the change are also reported in the paper |
Instrument | Mean (SD) at baseline (n = 153) | Mean (SD) at crossover (n = 80) | Mean (SD) after crossover (n = 136) | TTO | 0–1 TTO | 0.75 (0.34) | 0.73 (0.35) | 0.82 (0.31) | SF-36 | Physical-composite | 35.8 (10.7) | 33.3 (10.1) | 38.0 (11.6) | Mental-composite | 51.5 (9.5) | 49.8 (10.9) | 52.7 (11.6) | Physical-function | 56.4 (27.6) | 39.8 (28.1) | 55.0 (29.7) | Role-physical | 28.4 (38.7) | 28.4 (39.3) | 50.6 (43.0) | Pain | 66.8 (29.1) | 70.9 (24.7) | 69.5 (26.3) | Health perception | 57.2 (21.1) | 52.4 (20.7) | 56.5 (21.5) | Energy | 39.6 (23.2) | 32.1 (21.4) | 49.9 (24.5) | Social-function | 67.7 (24.7) | 62.2 (26.5) | 71.1 (24.1) | Role-emotional | 80.4 (34.7) | 75.4 (39.2) | 83.6 (32.9) | Mental health | 77.4 (17.2) | 73.5 (19.6) | 77.5 (17.8) | SAS | 1–4 SAS | 2.09 (0.93) | 2.50 (0.91) | 2.07 (0.94) | 0–100 scale | Score | 65.8 (19.8) | 60.6 (20.0) | 70.8 (19.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Instrument | Mean (SD) at baseline (n = 153) | Mean (SD) at crossover (n = 80) | Mean (SD) after crossover (n = 136) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TTO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0–1 TTO | 0.75 (0.34) | 0.73 (0.35) | 0.82 (0.31) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical-composite | 35.8 (10.7) | 33.3 (10.1) | 38.0 (11.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental-composite | 51.5 (9.5) | 49.8 (10.9) | 52.7 (11.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical-function | 56.4 (27.6) | 39.8 (28.1) | 55.0 (29.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role-physical | 28.4 (38.7) | 28.4 (39.3) | 50.6 (43.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain | 66.8 (29.1) | 70.9 (24.7) | 69.5 (26.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health perception | 57.2 (21.1) | 52.4 (20.7) | 56.5 (21.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Energy | 39.6 (23.2) | 32.1 (21.4) | 49.9 (24.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social-function | 67.7 (24.7) | 62.2 (26.5) | 71.1 (24.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role-emotional | 80.4 (34.7) | 75.4 (39.2) | 83.6 (32.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental health | 77.4 (17.2) | 73.5 (19.6) | 77.5 (17.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1–4 SAS | 2.09 (0.93) | 2.50 (0.91) | 2.07 (0.94) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0–100 scale | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Score | 65.8 (19.8) | 60.6 (20.0) | 70.8 (19.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0–100 scale | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Method of utility valuation was not stated | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lamas, 2002,68 USA | QoL data were collected by trained research co-ordinators as part of the MOST. A total of 2010 patients were enrolled from September 1995 to October 1999, at 91 clinical sites. Patients had to be at least 21 years old, undergoing initial implantation of a dual-chamber, rate modulated pacing system for SND and were in sinus rhythm when allocated to treatment. To be eligible for QoL analysis patients had to score 17 or higher on the Mini-Mental State Examination before implantation. Patients with serious concurrent illness as determined by the investigator at each site were excluded The baseline characteristics of the cohort are presented in the table below: CharacteristicVVIR (n = 996)DDDR (n = 1014)Median age (years)7474Female, n (%)477 (48)478 (47)Non-white, n (%)144 (14)162 (16)Diabetes, n (%)204 (20)246 (24)Hypertension, n (%)608 (61)640 (63)Hypercholesterolaemia, n (%)340 (34)376 (37)Current smoker, n (%)85 (9)84 (8)Prior angina, n (%)280 (28)288 (28)Prior myocardial infarction, n (%)234 (24)279 (28)Prior HF, n (%)183 (18)221 (22)Prior stroke, n (%)108 (11)116 (11)Charlson Comorbidity Index1.46 (SD 1.65)1.54 (SD 1.67) |
Characteristic | VVIR (n = 996) | DDDR (n = 1014) | Median age (years) | 74 | 74 | Female, n (%) | 477 (48) | 478 (47) | Non-white, n (%) | 144 (14) | 162 (16) | Diabetes, n (%) | 204 (20) | 246 (24) | Hypertension, n (%) | 608 (61) | 640 (63) | Hypercholesterolaemia, n (%) | 340 (34) | 376 (37) | Current smoker, n (%) | 85 (9) | 84 (8) | Prior angina, n (%) | 280 (28) | 288 (28) | Prior myocardial infarction, n (%) | 234 (24) | 279 (28) | Prior HF, n (%) | 183 (18) | 221 (22) | Prior stroke, n (%) | 108 (11) | 116 (11) | Charlson Comorbidity Index | 1.46 (SD 1.65) | 1.54 (SD 1.67) | Utility was measured at baseline and at different time points after implantation of single-chamber or dual-chamber pacemakers | TTO | QoL scaleVentricularDualTTOBaseline73723 months+7+812 months+5+824 months+4+736 months+4+848 months+6+6Change from baseline after 48 months+2, p = 0.06SASBaseline2.011.973 months–0.04–0.0612 months0.00+0.0224 months+0.03+0.0536 months+0.04+0.1148 months+0.16+0.13Change from baseline after 48 months+0.002, p = 0.94 SF-36 scaleBaseline48 monthsChange from baseline at 48 monthsPhysical functionVentricular58.8–3.2+1.9, p = 0.04Dual58.9–0.1Physical roleVentricular34.6+18.0+8.6, p < 0.01Dual35.7+26.7Social functionVentricular72.1+6.4+2.5, p < 0.01Dual72.0+9.8EnergyDDDR74.0+3.6+4.1, p < 0.01VVIR74.1+5.2Mental healthDDDR42.6+4.7+1.2, p = 0.05VVIR41.9+4.6Emotional roleDDDR42.6+4.8+3.6, p < 0.01VVIR41.9+12.3PainDDDR67.0+6.9+0.5, p = 0.57VVIR67.6+5.1Health perceptionDDDR60.3–3.5+1.1, p = 0.09VVIR60.0–2.5Mental summaryDDDR48.4+2.4+1.1, p < 0.01VVIR48.4+3.5Physical summaryDDDR38.5+1.0+1.2, p < 0.01VVIR38.4+2.2 Values of SF-36 components at 3 months, 12 months, 24 months and 36 are also reported in the paper |
QoL scale | Ventricular | Dual | TTO | Baseline | 73 | 72 | 3 months | +7 | +8 | 12 months | +5 | +8 | 24 months | +4 | +7 | 36 months | +4 | +8 | 48 months | +6 | +6 | Change from baseline after 48 months | +2, p = 0.06 | SAS | Baseline | 2.01 | 1.97 | 3 months | –0.04 | –0.06 | 12 months | 0.00 | +0.02 | 24 months | +0.03 | +0.05 | 36 months | +0.04 | +0.11 | 48 months | +0.16 | +0.13 | Change from baseline after 48 months | +0.002, p = 0.94 | SF-36 scale | Baseline | 48 months | Change from baseline at 48 months | Physical function | Ventricular | 58.8 | –3.2 | +1.9, p = 0.04 | Dual | 58.9 | –0.1 | Physical role | Ventricular | 34.6 | +18.0 | +8.6, p < 0.01 | Dual | 35.7 | +26.7 | Social function | Ventricular | 72.1 | +6.4 | +2.5, p < 0.01 | Dual | 72.0 | +9.8 | Energy | DDDR | 74.0 | +3.6 | +4.1, p < 0.01 | VVIR | 74.1 | +5.2 | Mental health | DDDR | 42.6 | +4.7 | +1.2, p = 0.05 | VVIR | 41.9 | +4.6 | Emotional role | DDDR | 42.6 | +4.8 | +3.6, p < 0.01 | VVIR | 41.9 | +12.3 | Pain | DDDR | 67.0 | +6.9 | +0.5, p = 0.57 | VVIR | 67.6 | +5.1 | Health perception | DDDR | 60.3 | –3.5 | +1.1, p = 0.09 | VVIR | 60.0 | –2.5 | Mental summary | DDDR | 48.4 | +2.4 | +1.1, p < 0.01 | VVIR | 48.4 | +3.5 | Physical summary | DDDR | 38.5 | +1.0 | +1.2, p < 0.01 | VVIR | 38.4 | +2.2 | ||||||||||||||||||||||||||||||||||||
| Characteristic | VVIR (n = 996) | DDDR (n = 1014) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median age (years) | 74 | 74 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Female, n (%) | 477 (48) | 478 (47) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-white, n (%) | 144 (14) | 162 (16) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diabetes, n (%) | 204 (20) | 246 (24) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hypertension, n (%) | 608 (61) | 640 (63) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hypercholesterolaemia, n (%) | 340 (34) | 376 (37) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Current smoker, n (%) | 85 (9) | 84 (8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prior angina, n (%) | 280 (28) | 288 (28) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prior myocardial infarction, n (%) | 234 (24) | 279 (28) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prior HF, n (%) | 183 (18) | 221 (22) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prior stroke, n (%) | 108 (11) | 116 (11) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charlson Comorbidity Index | 1.46 (SD 1.65) | 1.54 (SD 1.67) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| QoL scale | Ventricular | Dual | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TTO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 73 | 72 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months | +7 | +8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 months | +5 | +8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24 months | +4 | +7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36 months | +4 | +8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48 months | +6 | +6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Change from baseline after 48 months | +2, p = 0.06 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 2.01 | 1.97 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 months | –0.04 | –0.06 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 months | 0.00 | +0.02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24 months | +0.03 | +0.05 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36 months | +0.04 | +0.11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48 months | +0.16 | +0.13 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Change from baseline after 48 months | +0.002, p = 0.94 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 scale | Baseline | 48 months | Change from baseline at 48 months | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical function | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ventricular | 58.8 | –3.2 | +1.9, p = 0.04 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dual | 58.9 | –0.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical role | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ventricular | 34.6 | +18.0 | +8.6, p < 0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dual | 35.7 | +26.7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social function | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ventricular | 72.1 | +6.4 | +2.5, p < 0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dual | 72.0 | +9.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Energy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 74.0 | +3.6 | +4.1, p < 0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 74.1 | +5.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental health | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 42.6 | +4.7 | +1.2, p = 0.05 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 41.9 | +4.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emotional role | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 42.6 | +4.8 | +3.6, p < 0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 41.9 | +12.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 67.0 | +6.9 | +0.5, p = 0.57 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 67.6 | +5.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health perception | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 60.3 | –3.5 | +1.1, p = 0.09 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 60.0 | –2.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental summary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 48.4 | +2.4 | +1.1, p < 0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 48.4 | +3.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical summary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DDDR | 38.5 | +1.0 | +1.2, p < 0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VVIR | 38.4 | +2.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Appendix 3 Quality assessment
Clinical effectiveness studies
Parallel-group randomised controlled trials
| Outcome | Risk of bias | Risk assessmenta | Comments |
|---|---|---|---|
| All | Random sequence generation | ? | Not described |
| Allocation concealment | ? | Not described | |
| Selective reporting | ✓ | Results for all pre-specified outcomes of interest were reported | |
| HF | Blinding [who (participants, personnel) and method] | ? | Not described |
| Blinding of outcome assessment | ✗ | Knowledge of the pacing mode during collection of data at follow-up visits might have led to bias regarding NYHA classification | |
| Incomplete outcome data | ✓ | Only one patient was lost to follow-up in the AAIR group (4.2%) and none in the DDDR group. (Also note that two patients in AAIR group received DDDR) | |
| Exercise capacity | Blinding [who (participants, personnel) and method] | ? | Not described |
| Blinding of outcome assessment | ✗ | Knowledge of the pacing mode during collection of data at follow-up visits might have led to bias regarding 6MWT | |
| Incomplete outcome data | ✓ | Only one patient was lost to follow-up in the AAIR group (4.2%) and none in the DDDR group. (Also note that two patients in AAIR group received DDDR) | |
| Adverse effects of pacemaker implantation | Blinding [who (participants, personnel) and method] | ? | Not described |
| Blinding of outcome assessment | ? | Not described | |
| Incomplete outcome data | ✓ | Only one patient was lost to follow-up in the AAIR group (4.2%) and none in the DDDR group. (Also note that two patients in AAIR group received DDDR) |
| Outcome | Risk of bias | Risk assessmenta | Comments |
|---|---|---|---|
| All | Random sequence generation | ? | Not described |
| Allocation concealment | ✓ | ‘Randomisation by sealed envelope was performed before pacemaker implantation’19 | |
| Selective reporting | ✗ | A published protocol stated QoL as one of the outcomes to be captured in the trial. However, no results for this outcome were published in the primary or subsequent publications | |
| Mortality | Blinding [who (participants, personnel) and method] | ✓ | The trial was open label. However, the lack of blinding was deemed to have limited effect on the incidence of mortality |
| Blinding of outcome assessment | ✓ | The trial was open label. However, the lack of blinding was deemed to have limited effect on the incidence of mortality | |
| Incomplete outcome data | ? | No patients were lost to follow-up. However, the number of patients who switched pacing mode during follow-up was relatively uneven between the study arms (DDDR 9.7% and AAIR 17.4%) | |
| Stroke | Blinding [who (participants, personnel) and method] | ✓ | The trial was open label. However, the lack of blinding was deemed to have limited effect on the incidence of stroke |
| Blinding of outcome assessment | ✓ | A Clinical Event Committee, which was unaware of the assigned pacing mode adjudicated stroke and thromboembolic events | |
| Incomplete outcome data | ? | No patients were lost to follow-up. However, the number of patients who switched pacing mode during follow-up was relatively unequal between the study arms (DDDR 9.7% and AAIR 17.4%) | |
| AF | Blinding [who (participants, personnel) and method] | ? | The trial was open label |
| Blinding of outcome assessment | ? | The trial was open label | |
| Incomplete outcome data | ? | No patients were lost to follow-up. However, the number of patients who switched pacing mode during follow up was relatively unequal between the study arms (DDDR 9.7% and AAIR 17.4%) | |
| HF | Blinding [who (participants, personnel) and method] | ? | The trial was open label |
| Blinding of outcome assessment | ? | The trial was open label | |
| Incomplete outcome data | ? | No patients were lost to follow-up. However, the number of patients who switched pacing mode during follow up was relatively unequal between the study arms (DDDR 9.7% and AAIR 17.4%) | |
| Requirement of further surgery | Blinding [who (participants, personnel) and method] | ? | The trial was open label |
| Blinding of outcome assessment | ? | The trial was open label | |
| Incomplete outcome data | ✓ | No patients were lost to follow-up |
| Outcome | Risk of bias | Risk assessmenta | Comments |
|---|---|---|---|
| All | Random sequence generation | ? | Not described |
| Allocation concealment | ? | Not described | |
| Selective reporting | ✓ | Results for all pre-specified outcomes of interest were reported | |
| Mortality | Blinding [who (participants, personnel) and method] | ✓ | Not described |
| Blinding of outcome assessment | ✓ | Not described | |
| Incomplete outcome data | ✓ | The number of patients who switched pacing mode during follow-up was relatively low in all treatment arms, but also unequal (DDD-s 3.33%, DDDR-l 4.76% and AAIR 11.11%) | |
| Stroke | Blinding [who (participants, personnel) and method] | ✓ | Not described |
| Blinding of outcome assessment | ✓ | Not described | |
| Incomplete outcome data | ✓ | The number of patients who switched pacing mode during follow-up was relatively low in all treatment arms, but also unequal (DDD-s 3.33%, DDDR-l 4.76% and AAIR 11.11%) | |
| AF | Blinding [who (participants, personnel) and method] | ? | Not described |
| Blinding of outcome assessment | ? | Not described | |
| Incomplete outcome data | ✓ | The number of patients who switched pacing mode during follow-up was relatively low in all treatment arms, but also unequal (DDD-s 3.33%, DDDR-l 4.76% and AAIR 11.11%) | |
| HF | Blinding [who (participants, personnel) and method] | ? | Not described |
| Blinding of outcome assessment | ? | Not described | |
| Incomplete outcome data | ✓ | The number of patients who switched pacing mode during follow up was relatively low in all treatment arms, but also uneven (DDD-s 3.33%, DDDR-l 4.76% and AAIR 11.11%) |
Crossover randomised controlled trials
| Outcome | Risk of bias | Risk assessmenta | Comments |
|---|---|---|---|
| All | Random sequence generation | ? | Not described |
| Allocation concealment | ? | Not described | |
| Selective reporting | ✓ | IPD were reported for exercise duration | |
| Exercise capacity | Blinding [who (participants, personnel) and method] | ? | Not described |
| Blinding of outcome assessment | ? | Not described | |
| Incomplete outcome data | ✓ | Data were collected for all randomised patients |
| Outcome | Risk of bias | Risk assessmenta | Comments |
|---|---|---|---|
| All | Random sequence generation | ? | Not described |
| Allocation concealment | ? | Not described | |
| Selective reporting | ? | IPD were reported for general well-being. However, for other QoL measurements data for the individual treatment periods were reported but results of paired t-tests for each outcome were not | |
| HRQoL | Blinding [who (participants, personnel) and method] | ✓ | Double blind. Method of blinding not described |
| Blinding of outcome assessment | ✓ | Assessed by research nurse and a clinical psychologist who were blind to the pacemaker mode of the patient | |
| Incomplete outcome data | ✓ | Three patients were excluded from the trial |
| Outcome | Risk of bias | Risk assessmenta | Comments |
|---|---|---|---|
| All | Random sequence generation | ? | The randomisation sequence was generated by the principal investigator (Schwaab) in advance |
| Allocation concealment | ✓ | The randomisation sequence was hidden in envelopes that were closed. Thus, it was concealed from personnel at the time of recruitment. After written consent had been obtained by the patients, the envelope was opened and the first pacing mode was programmed | |
| Selective reporting | ✗ | Data for the individual treatment periods were reported. However, the results of paired t-tests for each outcome were not reported | |
| Exercise capacity | Blinding [who (participants, personnel) and method] | ✓ | Patients and all investigating physicians were blinded for the pacing mode |
| Blinding of outcome assessment | ✓ | Patients and all investigating physicians were blinded for the pacing mode | |
| Incomplete outcome data | ✓ | Two patients were excluded from the trial | |
| Cognitive function | Blinding [who (participants, personnel) and method] | ✓ | Patients and all investigating physicians were blinded for the pacing mode |
| Blinding of outcome assessment | ✓ | Patients and all investigating physicians were blinded for the pacing mode | |
| Incomplete outcome data | ✓ | Two patients were excluded from the trial | |
| HRQoL | Blinding [who (participants, personnel) and method] | ✓ | Patients and all investigating physicians were blinded for the pacing mode |
| Blinding of outcome assessment | ✓ | Patients and all investigating physicians were blinded for the pacing mode | |
| Incomplete outcome data | ✓ | Two patients were excluded from the trial |
Cost-effectiveness evidence
The National Institute for Health and Care Excellence’s reference case
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | No, patient population is SND or AV block, comparator is VP, whereas NICE scope specifies atrial pacing |
| Comparator(s) | As listed in the scope developed by NICE | No, comparator is VP |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | Yes |
| Perspective on costs | NHS and PSS | Yes |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | No, 5-year time horizon, devices may be expected to last beyond 5 years |
| Synthesis of evidence on health effects | Based on systematic review | No, utilities were obtained from head-to-head trial data (MOST) |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Partial, QoL weights are reported to be ‘based on the data collected using the [TTO] approach during MOST’;50 however, no further details are given as to the calculation of these weights and none are reported in the cited main trial report by Lamas et al.68 |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | Yes, patient response data were collected in MOST68 |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Unclear |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | Yes |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | Partial, 6% for costs and 1.5% for benefits is used in the base case, 3.5% for both costs and benefits is used in sensitivity analysis |
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | Yes; however, the scope of this review exceeds the scope of the current review |
| Comparator(s) | As listed in the scope developed by NICE | Yes, plus single-chamber VP |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | Yes |
| Perspective on costs | NHS and PSS | Yes |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes |
| Synthesis of evidence on health effects | Based on systematic review | Yes |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Yes, TTO |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | Yes |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No, patient valuation |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | Yes |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | Yes, but in sensitivity analysis only |
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | No, simple cost comparison |
| Comparator(s) | As listed in the scope developed by NICE | Yes |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | No |
| Perspective on costs | NHS and PSS | No, only device and implant costs accounted for |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | No, simple cost comparison |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | No, 4 years of follow-up considered |
| Synthesis of evidence on health effects | Based on systematic review | No, none other than development of AV block considered, within institution data used |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | No, none |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | No, none |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No, none |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | N/A |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | Only device and implant costs considered |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | No, none |
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | No, patient population is SND or AV block, comparator is VP, whereas NICE scope specifies atrial pacing |
| Comparator(s) | As listed in the scope developed by NICE | No, comparator is VP |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | Yes |
| Perspective on costs | NHS and PSS | No, Italian government perspective |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | No, 5-year time horizon, devices may be expected to last beyond 5 years |
| Synthesis of evidence on health effects | Based on systematic review | No, utilities were obtained from head-to-head trial data (MOST)68 |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Partial, QoL weights are reported to be ‘based on the data collected using the [TTO] approach during MOST’;52 however, no further details are given as to the calculation of these weights and none is reported in the cited main trial report by Lamas et al.68 |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | Yes, patient response data were collected in MOST |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Unclear |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | No |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | Partial, 3% for costs and benefits is used in the base case, 3.5% for both costs and benefits is used in sensitivity analysis |
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | Unclear, patient population is not stated |
| Comparator(s) | As listed in the scope developed by NICE | Indirectly, comparison is VVI vs. DDD and AAI |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | Yes |
| Perspective on costs | NHS and PSS | No, US payer perspective |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | No, non-simple comparison of benefits and costs |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Unclear, not stated |
| Synthesis of evidence on health effects | Based on systematic review | No, HRQoL was not considered |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | No, HRQoL was not considered |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | No, HRQoL was not considered |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No, HRQoL was not considered |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | No, HRQoL was not considered |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | No, US payer perspective |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | No, discounting was not used |
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | Partial, patient population (people with no AF and bradycardia) is broader than NICE scope |
| Comparator(s) | As listed in the scope developed by NICE | No, comparator is VP |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | Yes |
| Perspective on costs | NHS and PSS | No, provincial Canadian government health-care payer (mostly Ontario) |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | No, cost-effectiveness analysis |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | No, 5.2-year time horizon, devices may be expected to last beyond 5 years |
| Synthesis of evidence on health effects | Based on systematic review | No, HRQoL is not considered |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | No, HRQoL is not considered |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | No, HRQoL is not considered |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No, HRQoL is not considered |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | No, HRQoL is not considered |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | No, provincial Canadian government health-care payer (mostly Ontario) |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | No, 3% for costs and benefits |
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | No, mixed-patient population, procedure rather than intervention considered |
| Comparator(s) | As listed in the scope developed by NICE | No comparator considered |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | No, complications only |
| Perspective on costs | NHS and PSS | No, single-centre costs |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | No, safety and costs associated with same-day procedure |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | No, 5.5-year time horizon, devices may be expected to last beyond 5 years |
| Synthesis of evidence on health effects | Based on systematic review | No, complications only |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | No, HRQoL not considered |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | No, HRQoL not considered |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No, HRQoL not considered |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | No, HRQoL not considered |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | No, single-centre costs |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | No discounting |
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | No, audit study with a mixed patient population |
| Comparator(s) | As listed in the scope developed by NICE | No, audit study |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | No |
| Perspective on costs | NHS and PSS | No, single-centre costs |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | No, audit study |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | No, HRQoL not considered |
| Synthesis of evidence on health effects | Based on systematic review | No, HRQoL not considered |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | No, HRQoL not considered |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | No, HRQoL not considered |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No, HRQoL not considered |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | No, HRQoL not considered |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | No, single-centre costs |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | No discounting |
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | No, patient population is patients paced for SSS; however, comparator is VP |
| Comparator(s) | As listed in the scope developed by NICE | No, comparator is VP |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | Yes |
| Perspective on costs | NHS and PSS | No, US societal perspective |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes, 4-year in-trial cost-effectiveness analysis plus lifetime Markov model extrapolation |
| Synthesis of evidence on health effects | Based on systematic review | No, utilities were obtained from head-to-head trial data (MOST)68 |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Yes, TTO instrument used |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | Yes, patient response data were collected in MOST68 |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No, valuation carried out by patients |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | No, US societal perspective |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | No, 3% for costs and benefits |
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | No, mixed-patient population and, with the exception of developing AV block, AAI pacing is assumed equivalent to DDD |
| Comparator(s) | As listed in the scope developed by NICE | No, comparator is VVI |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | Yes |
| Perspective on costs | NHS and PSS | Unclear, perspective not stated, but appears to include all relevant NHS and PSS costs |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | No, cost–benefit |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes, 10-year time horizon |
| Synthesis of evidence on health effects | Based on systematic review | No, HRQoL not considered |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | No, HRQoL not considered |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | No, HRQoL not considered |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No, HRQoL not considered |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | No, HRQoL not considered |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | Partial, prices relevant to NHS used to inform cost estimates |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | No discounting |
| Element of HTA | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | No, patient population is AV block, comparator is single-lead VDD pacing |
| Comparator(s) | As listed in the scope developed by NICE | No, comparator is single-lead VDD pacing |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, for carers | No |
| Perspective on costs | NHS and PSS | No, costs from a single centre |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | No |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | No, follow-up was an average of 42 months |
| Synthesis of evidence on health effects | Based on systematic review | No, HRQoL not included |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | No, HRQoL not included |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | No, HRQoL not included |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | No, HRQoL not included |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | No, HRQoL not included |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | No, costs were from a single centre |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | No discounting reported |
The Philips checklist
| Attribute | Assessment | Comment |
|---|---|---|
| Structure | ||
| S1: statement of decision problem/objective | Yes | Stated |
| S2: statement of scope/perspective | Yes | Stated |
| S3: rationale for structure | Partial | Stated; however, the exclusion of HF compromises consistency with the disease area considered |
| S4: structural assumptions | Yes | Stated |
| S5: strategies/comparators | Yes | Stated |
| S6: model type | Yes | Stated, discrete event simulation |
| S7: time horizon | No | The 5-year time horizon may be insufficient to reflect all important differences between options, such as generator lifespan |
| S8: disease states/pathways | Partial | HF is excluded |
| S9: cycle length | – | N/A |
| Data | ||
| D1: data identification | Yes | Clearly stated and appropriate |
| D2: pre-model data analysis | Yes | Stated |
| D2a: baseline data | Yes | Stated |
| D2b: treatment effects | Yes | Stated |
| D2c: costs | Yes | Stated |
| D2d: QoL weights (utilities) | Yes | Stated |
| D3: data incorporation | Partial | Derivation of utility weights is not clearly reported |
| D4: assessment of uncertainty | Partial | Univariate and multivariate |
| D4a: methodological | No | Not reported |
| D4b: structural | No | Not reported |
| D4c: heterogeneity | No | Not reported |
| D4d: parameter | Yes | Assessed through deterministic and probabilistic analysis |
| Consistency | ||
| C1: internal consistency | No | Not reported |
| C2: external consistency | Yes | Stated |
| Attribute | Assessment | Comment |
|---|---|---|
| Structure | ||
| S1: statement of decision problem/objective | Yes | The objective of the model is specified and consistent with the stated decision problem |
| S2: statement of scope/perspective | Yes | The perspective of the model has been stated and the inputs used and outcomes considered are consistent with this perspective |
| S3: rationale for structure | Yes | The model structure represents a coherent theory of the health condition under consideration |
| S4: structural assumptions | Yes | Model assumptions have been clearly stated and justified |
| S5: strategies/comparators | Yes | All feasible and practical options have been evaluated |
| S6: model type | Yes | Markov model, appropriate for the decision problem |
| S7: time horizon | Yes | Both 5- and 10-year time horizons have been considered to facilitate understanding of the shorter-term cost-effectiveness of the interventions and cost-effectiveness over the clinically realistic lifetime of the technologies |
| S8: disease states/pathways | Partial | The health states considered are generally appropriate; however, no rationale is provided for the exclusion of subsequent complications following upgrade to a dual-chamber pacemaker |
| S9: cycle length | Partial | Cycle length is stated but not justified |
| Data | ||
| D1: data identification | Partial | Generally the data sources used were systematically identified and the quality assessment and choices between sources justified. However, the identification of some data sources, for example Chugh et al.69 for the progression to stroke after AF, have not been explained |
| D2: pre-model data analysis | Yes | Pre-model data analysis has been clearly stated |
| D2a: baseline data | Partial | Baseline data have been stated and justified. Half-cycle correction is not used and no explanation provided |
| D2b: treatment effects | Yes | Treatment effects have been appropriately synthesised. Extrapolation has been described and justified and the potential impact explored in sensitivity analysis |
| D2c: costs | Yes | Cost sources have been clearly described and included costs justified |
| D2d: QoL weights (utilities) | Yes | QoL weights have been clearly described. Patient valuation utilities have been used in the absence of valuations from the general population |
| D3: data incorporation | Yes | All data incorporated into the model have been described and referenced in sufficient detail |
| D4: assessment of uncertainty | Partial | Structural, heterogeneity and parameter uncertainty have been assessed |
| D4a: methodological | No | Not stated |
| D4b: structural | Yes | Structural uncertainty has been assessed through the use of different time horizons and assumption, for example pertaining to the time dependency of AF risk |
| D4c: heterogeneity | Yes | The patient population that is the scope of this review have been assessed as homogeneous subgroups |
| D4d: parameter | Yes | Univariate and multivariate sensitivity analysis has been carried out |
| Consistency | ||
| C1: internal consistency | No | Not stated |
| C2: external consistency | Yes | Results have been sufficiently explained and contextualised by the existing literature; areas of remaining uncertainty, for example conflicting trial results, have been highlighted |
| Attribute | Assessment | Comment |
|---|---|---|
| Structure | ||
| S1: statement of decision problem/objective | Yes | Stated |
| S2: statement of scope/perspective | Yes | Stated |
| S3: rationale for structure | Partial | Stated; however, the exclusion of HF compromises consistency with the disease area considered |
| S4: structural assumptions | Yes | Stated |
| S5: strategies/comparators | Yes | Stated |
| S6: model type | Yes | Stated, discrete event simulation |
| S7: time horizon | No | The 5-year time horizon may be insufficient to reflect all important differences between options, such as generator lifespan |
| S8: disease states/pathways | Partial | HF is excluded |
| S9: cycle length | – | N/A |
| Data | ||
| D1: data identification | Yes | Clearly stated and appropriate |
| D2: pre-model data analysis | Yes | Stated |
| D2a: baseline data | Yes | Stated |
| D2b: treatment effects | Yes | Stated |
| D2c: costs | Yes | Stated |
| D2d: QoL weights (utilities) | Yes | Stated |
| D3: data incorporation | Partial | Derivation of utility weights is not clearly reported |
| D4: assessment of uncertainty | Partial | Univariate and multivariate |
| D4a: methodological | No | Not reported |
| D4b: structural | No | Not reported |
| D4c: heterogeneity | No | Not reported |
| D4d: parameter | Yes | Assessed through univariate and multivariate sensitivity analysis |
| Consistency | ||
| C1: internal consistency | No | Not reported |
| C2: external consistency | Yes | Stated |
| Attribute | Assessment | Comment |
|---|---|---|
| Structure | ||
| S1: statement of decision problem/objective | Yes | Stated |
| S2: statement of scope/perspective | Yes | Stated |
| S3: rationale for structure | Yes | Stated |
| S4: structural assumptions | Yes | Stated |
| S5: strategies/comparators | Yes | Stated |
| S6: model type | Yes | Stated, calibrated Markov model used to extrapolate in-trial cost-effectiveness analysis |
| S7: time horizon | Yes | Lifetime |
| S8: disease states/pathways | Yes | Stated |
| S9: cycle length | Yes | Annual |
| Data | ||
| D1: data identification | Yes | Clearly stated and appropriate |
| D2: pre-model data analysis | Yes | Stated |
| D2a: baseline data | Yes | Stated |
| D2b: treatment effects | Yes | Stated |
| D2c: costs | Yes | Stated |
| D2d: QoL weights (utilities) | Yes | Stated |
| D3: Data incorporation | Yes | Clearly stated and appropriate |
| D4: assessment of uncertainty | Partial | Univariate, multivariate and structural |
| D4a: methodological | No | Not reported |
| D4b: structural | Yes | Modelled rather than actual data used to inform first 4 years of analysis |
| D4c: heterogeneity | No | Not reported |
| D4d: parameter | Yes | Assessed through univariate and bootstrap analysis |
| Consistency | ||
| C1: internal consistency | Yes | Stated |
| C2: external consistency | Yes | Stated |
| Attribute | Assessment | Comment |
|---|---|---|
| Structure | ||
| S1: statement of decision problem/objective | Yes | Stated |
| S2: statement of scope/perspective | Partial | Perspective is unclear |
| S3: rationale for structure | Partial | Limited information is provided on the model structure |
| S4: structural assumptions | Partial | Unclear whether all assumptions have been stated |
| S5: strategies/comparators | Yes | Stated |
| S6: model type | Yes | Stated |
| S7: time horizon | Yes | 10-year time horizon, long enough to capture device lifetime |
| S8: disease states/pathways | Partial | All relevant sequelae seem to have been incorporated but unclear which pathways are used |
| S9: cycle length | No | Not stated |
| Data | ||
| D1: data identification | Partial | Identification of cost sources is clearly stated; however, there is a lack of clarity regarding the source of estimates for the incidence of all considered complications |
| D2: pre-model data analysis | Partial | That average incidence is frequently used is stated; however, not all estimates are clearly explained |
| D2a: baseline data | No | Not stated |
| D2b: treatment effects | Partial | The derivation of incidence data is not clearly described for all outcomes |
| D2c: costs | Yes | Stated |
| D2d: QoL weights (utilities) | No | Not used |
| D3: data incorporation | Partial | Incorporation of data not consistently described |
| D4: assessment of uncertainty | Partial | Univariate analysis |
| D4a: methodological | No | Not reported |
| D4b: structural | No | Not reported |
| D4c: heterogeneity | No | Not assessed |
| D4d: parameter | Yes | Assessed through selected univariate sensitivity analysis |
| Consistency | ||
| C1: internal consistency | No | Not reported |
| C2: external consistency | Yes | Stated |
Appendix 4 Table of excluded studies
Clinical effectiveness review
| Full reference details | Reason for exclusion |
|---|---|
| Castelnuovo L, Stein K, Pitt M, Garside R, Payne E. The effectiveness and cost effectiveness of dual chamber pacing compared to single pacing for bradycardia – NICE Technology Assessment Report (Structured abstract). Health Technol Assess 2005;4(1) | Systematic review |
| Charalampopoulos AP. The effect of AAIR versus DDDR pacing mode in left ventricular ejection fraction, synchronization and NT-proBNP levels- A prospective study in sick sinus syndrome and normal ventricular function. European Society of Cardiology Congress 2010, Stockholm, Sweden, 28 August–1 September 2010. Eur Heart J 2010:31(Suppl. 1):P4828 | Pre-clinical study (left ventricular function, left atrial size, N-terminal prohormone of brain natriuretic peptide levels), no outcomes of interest |
| Clarke KW, Connelly DT, Charles RG. Single chamber atrial pacing: an underused and cost-effective pacing modality in sinus node disease. Heart 1998;80:387–9 | Non-randomised study |
| Davy JM, Hoffmann E, Frey A, Jocham K, Rossi S, Dupuis JM, et al. Near elimination of ventricular pacing in SafeR mode compared to DDD modes: a randomized study of 422 patients 1. Pacing Clin Electrophysiol 2012;35:392–402 | DDD(R) vs. dual-chamber pacing with VP minimising features |
| Delfaut P, Saksena S, Prakash A, Krol RB, Delfaut P, Saksena S, et al. Long-term outcome of patients with drug-refractory atrial flutter and fibrillation after single- and dual-site right atrial pacing for arrhythmia prevention. J Am Coll Cardiol 1998;32:1900–8 | Patients with drug-refractory symptomatic AF or flutter |
| Dretzke J, Lip G, Raftery J, Toff W, Fry-Smith A, Taylor R. Dual versus single chamber pacemaker therapy for atrioventricular block and sick sinus syndrome. Birmingham: West Midlands Health Technology Assessment Collaboration (WMHTAC). DPHE Report No. 32. 2002 | Systematic review |
| Fitts SM, Hill MR, Mehra R, Friedman P, Hammill S, Kay GN, et al. Design and implementation of the Dual Site Atrial Pacing to Prevent Atrial Fibrillation (DAPPAF) clinical trial. DAPPAF Phase 1 Investigators. J Interv Card Electrophysiol 1998;2:139–44 | Dual-chamber atrial pacing vs. single-chamber atrial pacing |
| French WJ, Haskell RJ, Wesley GW, Florio J. Physiological benefits of a pacemaker with dual chamber pacing at low heart rates and single chamber rate responsive pacing during exercise. Pacing Clin Electrophysiol 1988;11:1840–5 | Patients with complete heart block |
| Fukuoka S, Nakagawa S, Fukunaga T, Yamada H, Fukuoka S, Nakagawa S, et al. Effect of long-term atrial-demand ventricular pacing on cardiac sympathetic activity. Nucl Med Commun 2000;21:291–7 | Non-randomised study |
| Hildick-Smith DJW. Single-chamber versus dual-chamber pacemakers. N Eng J Med 1998;339:630–2 | Editorial correspondence |
| Jutzy RV, Florio J, Isaeff DM, Feenstra L, Briggs B, Levine PA. Limitations of testing methods for evaluation of dual chamber versus single chamber adaptive rate pacing. Am J Cardiol 1991;68:1715–17 | Dual vs. VP |
| Kuhne M, Schaer B, Kaufmann C, Moulay N, Cron T, Cueni T, et al. A randomized trial comparing two different approaches of pacemaker selection. Europace 2007;9:1185–90 | Standard (DDD for sinus rhythm) or tailored approach (AAI, VDD or DDD depending on AV conduction and chronotropic incompetence) |
| Maity AK, Ghosh SP, Dasbiswas A, Chatterjee SS, Chaudhury D, Das MK. Haemodynamic advantage with single chamber rate responsive pacemakers over dual chamber pacemakers during exercise in chronotropic incompetence. Indian Heart J 1992;44:231–4 | Patients with complete heart block |
| Miki Y, Ishikawa T, Inoue N, Yamakawa Y, Kobayashi T, Matsushita K, et al. Efficacy of consistent atrial pacing algorithm for suppression of atrial arrhythmias in patients with sick sinus syndrome and atrial fibrillation. Int Heart J 2008;49:273–80 | DDDR vs. atrial preference pacing (APP) algorithm |
| Mizutani N, Kobayashi T, Kato I. Optimal pacing mode for sick sinus syndrome. J Artif Organs 1997;26:369–74 | Non-randomised study |
| Nielsen JC, Bottcher M, Nielsen TT, Pedersen AK, Andersen HR, Nielsen JC, et al. Regional myocardial blood flow in patients with sick sinus syndrome randomized to long-term single chamber atrial or dual chamber pacing – effect of pacing mode and rate. J Am Coll Cardiol 2000;35:1453–61 | Pre-clinical study (myocardial blood flow), no outcomes of interest |
| Nielsen JC, Thomsen PE, Højberg S, Møller M, Riahi S, Dalsgaard D, et al. Atrial fibrillation in patients with sick sinus syndrome: the association with PQ-interval and percentage of ventricular pacing 195. Europace 2012;14:682–9 | Subgroup cohort |
| O’Brien BJ, Blackhouse G, Goeree R, Healey JS, Roberts RS, Gent M, et al. Cost-effectiveness of physiologic pacing: results of the Canadian Health Economic Assessment of Physiologic Pacing. Heart Rhythm 2005;2:270–5 | Dual vs. VP |
| Prakash AG. Impact of atrial and ventricular pacing on prevention of atrial fibrillation: insights from the preface study. 30th Annual Scientific Sessions, May 13-16, 2009, Boston, MA, USA. Heart Rhythm 2009;6:S1–536 | DDD vs. dual-chamber pacing with VP minimising features (anti-AA algorithms + SafeR, SafeR) |
| Psychari SN, Apostolou TS, Iliodromitis EK, Charalampopoulos A, Kremastinos DT. DDDR pacing results in left ventricular asynchrony with preservation of ejection fraction and NT-proBNP: a prospective study in sick sinus syndrome and normal ventricular function. Int J Cardiol 2010;144:310–12 | Pre-clinical study (left ventricular function, left atrial size, NT-proBNP levels), no outcomes of interest |
| Sami M. Are we ready for dual-site right atrial pacing? J Am Coll Cardiol 2002;40:1151–2 | Editorial correspondence |
| Theodorakis G, Fitzpatrick A, Vardas P, Sutton R. Resting echo-Doppler estimation of cardiac output during AAI and DDD pacing, with varying AV delay, at different pacing rates. EJCPE 1992;2:22–5 | Unable to obtain |
| Vardas PE, Simantirakis EN, Parthenakis FI, Chrysostomakis SI, Skalidis, EI. AAIR versus DDDR pacing in patients with impaired sinus node chronotropy: an echocardiographic and cardiopulmonary study. Pacing Clin Electrophysiol 1997;20:1762–8 | Non-randomised study |
| Xue-Jun R, Zhihong H, Ye W, Huifeng D, Jinrong Z, Fang C, et al. A clinical comparison between a new dual-chamber pacing mode-AAIsafeR and DDD mode. Am J Med Sci 2010;339:145–7 | DDDR vs. dual-chamber pacing with VP minimising features (AAISafeR) |
| Zhang YY, Wu DY, Fu NK, Lu FM, Xu J. Neuroendocrine and haemodynamic changes in single-lead atrial pacing and dual-chamber pacing modes. J Int Med Res 2013;41:1057–66 | Pre-clinical study (neuroendocrine and haemodynamic changes), no outcomes of interest |
Economic evaluations
| Bibliographic reference | Reasons for exclusion |
|---|---|
| Bauer A, Bauer J, Bauer M, Kelemen K, Voss F, Senges-Becker J, et al. [Efficiency potential in the pacemaker/implantable cardioverter defibrillator outpatient clinic]. Herzschrittmacherther Elektrophysiol 2006;17:26–34 | Non-UK costing study |
| Biffi M, Ziacchi M, Bertini M, Sangiorgi D, Corsini D, Martignani C, et al. Longevity of implantable cardioverter-defibrillators: implications for clinical practice and health care systems. Europace 2008;10:1288–95 | Not pacing |
| Goldberger Z, Elbel B, McPherson CA, Paltiel AD, Lampert R. Cost advantage of dual-chamber versus single-chamber cardioverter-defibrillator implantation. J Am Coll Cardiol 2005;46:850–7 | Not pacing |
| Roda JRB. Modeling of the clinical benefit and economic impact of pacemakers implantation with managed ventricular pacing. Pharmacoeconomics 2009;6:115–25 | Irretrievable |
| Dretzke J, Lip G, Raftery J, Toff W, Fry-Smith A, Taylor R, et al. Dual Versus Single Chamber Pacemaker Therapy for Atrioventricular Block and Sick Sinus Syndrome. 2002. URL: www.rep.bham.ac.uk/2002/pacemaker.pdf | Review |
| L’Agence Nationale d’Accreditation d’Evaluation en Sante (ANAES). Clinical and Economic Evaluation of Cardiac Pacemakers. Paris: ANAES. 1999. URL: www.has-sante.fr/ | Irretrievable |
| National Institute for Health and Care Excellence (NICE). Dual-Chamber Pacemakers for Symptomatic Bradycardia due to Sick Sinus Syndrome and/or Atrioventricular Block. London: NICE; 2005. URL: www.nice.org.uk/page.aspx?o=243281 | Duplicate of Castlenuovo49 |
| Emergency Care Research Institute (ECRI). Dual-chamber versus single-chamber pacemakers for sinus node dysfunction and atrioventricular block. Plymouth Meeting, PA: ECRI; 2005. p. 116. URL: www.ecri.org.uk/ | Irretrievable |
| Mundy L, Hiller JE. MRI Compatible Dual Chamber Pacemaker. Adelaide: Adelaide Health Technology Assessment (AHTA). Horizon Scanning Prioritising Summary 2010:29 | Not EE/cost |
| Marshall DA, Levy AR, Vidaillet H, Fenwick E, Slee A, Blackhouse G, et al. Cost-effectiveness of rhythm versus rate control in atrial fibrillation. Ann Int Med 2004;141:653–61 | Not pacing |
| Mitton CR, Rose S, Sheldon RS. A Cost–Utility Analysis of Pacemakers for the Treatment of Neurally Mediated Syncope. Working Paper; 98-5. Edmonton, AB: Institute of Pharmaco-Economics. 1998 | Not AV block or SSS |
| Namboodiri KK, Sharma YP, Bali HK, Grover A. Re-use of explanted DDD pacemakers as VDD-clinical utility and cost effectiveness. Indian Pacing and Electrophysiol J 2004;4:3–9 | Re-use of explanted pacemakers |
| Holt ND, Parry G, Tynan MM, Dark JH, McComb JM, et al. Permanent pacemaker implantation after cardiac transplantation: extra cost of a conservative policy. Heart 1996;76:439–41 | Not SSS or AV block |
| Kuhne M, Schaer B, Kaufmann C, Moulay N, Cron T, Cueni T, et al. A randomized trial comparing two different approaches of pacemaker selection. Europace 2007;9:1185–90 | Non-UK costing study |
| Yamamura KH, Kloosterman EM, Alba J, Garcia F, Williams PL, Mitran RD, et al. Analysis of charges and complications of permanent pacemaker implantation in the cardiac catheterization laboratory versus the operating room. Pacing Clin Electrophysiol 1999;22:1820–4 | Non-UK costing study |
Health-related quality of life
| Bibliographic reference | Reasons for exclusion |
|---|---|
| Alt E. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacing Clin Electrophysiol 1999;22:141–2 | Not QoL |
| Fitts SM, Hill MR, Mehra R, Friedman P, Hammill S, Kay GN, et al. Design and implementation of the Dual Site Atrial Pacing to Prevent Atrial Fibrillation (DAPPAF) clinical trial. DAPPAF Phase 1 Investigators. J Int Cardiac Electrophysiol 1998;2:139–44 | Not QoL |
| Funck RC, Adamec R, Lurje L, Capucci A, Ritter P, Shekan D, et al. Atrial overdriving is beneficial in patients with atrial arrhythmias: first results of the PROVE Study. [Erratum appears in Pacing Clin Electrophysiol 2001;24:viii]. Pacing Clin Electrophysiol 2000;23:1891–3 | Not QoL |
| Funck RC, Blanc JJ, Mueller HH, Schade-Brittinger C, Bailleul C, Maisch B, et al. Biventricular stimulation to prevent cardiac desynchronization: rationale, design, and endpoints of the ‘Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BioPace)’ study. Europace 2006;8:629–35 | Not QoL |
| Magovern GJ, Sr, Simpson KA. Clinical cardiomyoplasty: review of the ten-year United States experience. Ann Thoracic Surg 1996;61:413–19 | Not QoL |
| Mitsuoka T, Kenny RA, Yeung TA, Chan SL, Perrins JE, Sutton R, et al. Benefits of dual chamber pacing in sick sinus syndrome. Brit Heart J 1988;60:338–47 | Not QoL |
| Bortnik OE. Permanent parahisian pacing. Indian Pacing Electrophysiol J 2007;7:110–25 | Review paper |
| Pachon EIA. Ventricular endocardial right bifocal stimulation in the treatment of severe dilated cardiomyopathy heart failure with wide QRS. Pacing Clin Electrophysiol 2001;24:1369–76 | HF |
| Parsonnet V and Roelke M. Single-chamber versus dual-chamber pacemakers. N Eng J Med 1998;339:630–1 | Not QoL |
| Prech M, Grygier M, Mitkowski P, Stanek K, Skorupski W, Moszynska B, et al. Effect of restoration of AV synchrony on stroke volume, exercise capacity, and quality-of-life: can we predict the beneficial effect of a pacemaker upgrade? Pacing Clin Electrophysiol 2001;24:302–7 | Not QoL |
| Vassolo ML. Dual-chamber vs ventricular pacing in the elderly: quality of life and clinical outcomes. Eur Heart J 1999;20:1607–8 | Review |
| Wilkoff BL and the Dual Chamber and VVI Implantable Defibrillator trial investigators. The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial: rationale, design, results, clinical implications and lessons for future trials. Cardiac Electrophysiol Rev 2003;7:468–72 | Not QoL |
| Wilkoff BL, Kudenchuk PJ, Buxton AE, Sharma A, Cook JR, Bhandari AK, et al. The DAVID (Dual Chamber and VVI Implantable Defibrillator) II trial. J Am Coll Cardiol 2009;53:872–80 | Not pacing |
Appendix 5 One-way sensitivity analysis
| Parameter | Difference from ICER when using lower 95% CI | Difference from ICER when using upper 95% CI |
|---|---|---|
| Baseline age | £76 | £1584 |
| Probability of paroxysmal AF – DC | £2465 | –£3300 |
| Probability of chronic AF – DC | £174 | –£343 |
| Probability of HF – DC | –£458 | £1097 |
| Probability of stroke – DC | £574 | –£1034 |
| Probability of paroxysmal AF – SC | £10,153 | –£4101 |
| Probability of chronic AF – SC | £3580 | –£2845 |
| Probability of HF – SC | Dominated | Dominant |
| Probability of stroke – SC | £6345 | –£3658 |
| Proportion of new HF events leading to hospitalisation – DC | –£80 | £152 |
| Proportion of new HF events leading to hospitalisation – SC | £314 | –£597 |
| Probability of AV block | £567 | –£1095 |
| HR death AF (vs. general population) | £75 | –£82 |
| HR death stroke – males (vs. general population) | £48 | –£63 |
| HR death stroke – females (vs. general population) | £44 | –£55 |
| HR death HF (vs. general population) | £42 | –£43 |
| HR death AF and stroke (vs. stroke population) | –£6 | £7 |
| HR death AF and HF (vs. HF population) | –£2 | £3 |
| Implant SC cost | £21,353 | Dominant |
| Implant DC cost | Dominant | £16,954 |
| HF hospitalisation cost | –£1043 | £1137 |
| Stroke episode costs | £356 | –£153 |
| Monitoring cost | –£333 | £154 |
| Total UK direct health-care cost of CVD | £0 | £0 |
| Average annual post-stroke hospitalisation cost | £97 | –£191 |
| Total annual UK stroke medication costs | £7 | –£13 |
| Total UK stroke primary care costs | £3 | –£6 |
| Episode cost of stroke in people with AF | £309 | –£1305 |
| Average annual post-stroke hospitalisation cost in people with AF | £0 | £0 |
| Cost of GP referrals for AF | £120 | –£236 |
| Cost of hospital outpatient referrals for AF | £88 | –£173 |
| Cost of hospital admissions with principal diagnosis of AF | £656 | –£1289 |
| Cost of post-discharge outpatient visits | £77 | –£150 |
| Utility change from implant to HF | £306 | –£531 |
| Utility HF | –£902 | £1916 |
| Utility stroke (month 1) | £0 | £0 |
| Utility stroke (> month 1) | –£522 | £434 |
| Utility change from with pacemaker to AF | £106 | –£197 |
| Utility AF and stroke (month 1) | –£209 | £163 |
| Utility AF and stroke (> month 1) | £0 | £0 |
| Utility AF and HF | –£426 | £705 |
Appendix 6 Calculation of long-term care costs associated with heart failure
In 2011, the UK prevalence of HF was 0.90% in men and 0.70% in women. 96 No UK CVD prevalence data were identified for 2011; however, data are reported for 1988–2010 (Figure 17). By extrapolation of the four most recent data points, it is possible to estimate the UK CVD prevalence for 2011 (Figure 18). These extrapolations provide UK CVD prevalence estimates for 2011 of 11.95% for men and 10.95% for women, which give a relative prevalence of HF as a percentage of CVD of 7.53% for men and 6.39% for women: average 6.96%.
FIGURE 18.
Extrapolation of previous 4 years of CVD prevalence data.

The total UK direct health-care cost of CVD was £8,680,892,000 in 2009 and UK CVD prevalence increased by 1% between 2009 and 2011. Assuming that costs are directly proportional to prevalence, in 2009 prices, the 2011 total UK direct health-care cost of CVD can be estimated as £8,767,700,920 (£8,680,892,000 × 1.01), which when uplifted to 2011 prices is £9,086,227,882 (Table 116).
| Year | Pay and prices index (1987/8 = 100) | % increase |
|---|---|---|
| 2008/9 | 267 | – |
| 2009/10 | 268.6 | 0.60 |
| 2010/11 | 276.7 | 3.02 |
| 2011/12 | 282.5 | 2.10 |
| 2012/13 | 289.1 | 2.34 |
Based on the 2011 total direct health-care cost of CVD and the relative prevalence of HF as a percentage of CVD, the 2011 total direct health-care cost of HF can be estimated as £632,586,584 (£9,086,227,882 × 0.0696). There were 160,719 prevalent HF cases in the UK in 2011, which, combined with the estimated total direct health-care cost of HF, results in an estimated per person cost of HF of £3936 per annum (£4112 per annum at 2013 prices).
Appendix 7 Monthly probability of reoperation by treatment arm
| Time (months) | Single-chamber atrial pacemaker (%) | Dual-chamber pacemaker (%) |
|---|---|---|
| 0.00 | 0.00 | 0.00 |
| 1.00 | 2.82 | 2.51 |
| 2.00 | 1.29 | 0.32 |
| 3.00 | 0.98 | 0.32 |
| 4.00 | 0.00 | 0.65 |
| 5.00 | 0.66 | 0.00 |
| 6.00 | 0.66 | 0.00 |
| 7.00 | 0.33 | 0.00 |
| 8.00 | 0.34 | 0.33 |
| 9.00 | 0.67 | 0.00 |
| 10.00 | 0.00 | 0.33 |
| 11.00 | 0.00 | 0.00 |
| 12.00 | 0.68 | 0.00 |
| 13.00 | 0.34 | 0.33 |
| 14.00 | 0.00 | 0.00 |
| 15.00 | 0.00 | 0.00 |
| 16.00 | 0.34 | 0.00 |
| 17.00 | 0.34 | 0.00 |
| 18.00 | 0.00 | 0.00 |
| 19.00 | 0.00 | 0.00 |
| 20.00 | 0.00 | 0.00 |
| 21.00 | 0.34 | 0.33 |
| 22.00 | 0.69 | 0.00 |
| 23.00 | 0.00 | 0.33 |
| 24.00 | 0.70 | 0.00 |
| 25.00 | 0.35 | 0.00 |
| 26.00 | 0.00 | 0.00 |
| 27.00 | 0.35 | 0.00 |
| 28.00 | 0.71 | 0.00 |
| 29.00 | 0.00 | 0.00 |
| 30.00 | 0.36 | 0.33 |
| 31.00 | 0.00 | 0.00 |
| 32.00 | 0.00 | 0.33 |
| 33.00 | 0.36 | 0.33 |
| 34.00 | 0.00 | 0.00 |
| 35.00 | 0.00 | 0.00 |
| 36.00 | 0.36 | 0.33 |
| 37.00 | 0.00 | 0.00 |
| 38.00 | 0.72 | 0.00 |
| 39.00 | 0.36 | 0.00 |
| 40.00 | 0.36 | 0.00 |
| 41.00 | 0.00 | 0.34 |
| 42.00 | 0.00 | 0.00 |
| 43.00 | 0.00 | 0.00 |
| 44.00 | 0.73 | 0.00 |
| 45.00 | 0.00 | 0.00 |
| 46.00 | 0.00 | 0.00 |
| 47.00 | 0.37 | 0.00 |
| 48.00 | 0.00 | 0.00 |
| 49.00 | 0.00 | 0.34 |
| 50.00 | 0.00 | 0.00 |
| 51.00 | 0.00 | 0.00 |
| 52.00 | 0.37 | 0.00 |
| 53.00 | 0.00 | 0.00 |
| 54.00 | 0.00 | 0.00 |
| 55.00 | 0.00 | 0.00 |
| 56.00 | 1.11 | 0.34 |
| 57.00 | 0.00 | 0.00 |
| 58.00 | 0.00 | 0.00 |
| 59.00 | 1.12 | 0.00 |
| 60.00 | 0.00 | 0.00 |
| 61.00 | 0.38 | 0.00 |
| 62.00 | 0.00 | 0.00 |
| 63.00 | 0.00 | 1.02 |
| 64.00 | 0.00 | 0.00 |
| 65.00 | 0.00 | 0.00 |
| 66.00 | 0.00 | 0.00 |
| 67.00 | 0.76 | 0.34 |
| 68.00 | 0.38 | 0.34 |
| 69.00 | 0.00 | 0.34 |
| 70.00 | 0.00 | 0.00 |
| 71.00 | 0.00 | 0.00 |
| 72.00 | 0.77 | 1.38 |
| 73.00 | 0.78 | 0.70 |
| 74.00 | 2.34 | 0.35 |
| 75.00 | 2.00 | 1.06 |
| 76.00 | 2.86 | 1.43 |
| 77.00 | 1.68 | 1.09 |
| 78.00 | 1.71 | 0.37 |
| 79.00 | 1.30 | 0.37 |
| 80.00 | 1.32 | 0.74 |
| 81.00 | 1.79 | 1.12 |
| 82.00 | 0.91 | 0.38 |
| 83.00 | 1.83 | 2.27 |
| 84.00 | 1.87 | 0.78 |
| 85.00 | 1.90 | 0.00 |
| 86.00 | 1.94 | 1.56 |
| 87.00 | 3.47 | 3.57 |
| 88.00 | 6.15 | 2.47 |
| 89.00 | 5.46 | 2.11 |
| 90.00 | 6.94 | 3.02 |
| 91.00 | 1.24 | 3.11 |
| 92.00 | 0.00 | 0.46 |
| 93.00 | 2.52 | 0.46 |
| 94.00 | 0.00 | 1.39 |
| 95.00 | 0.00 | 1.41 |
| 96.00 | 1.94 | 0.48 |
Glossary
- Atrial fibrillation
- Atrial fibrillation/flutter is a heart rhythm disorder (arrhythmia). It usually involves a rapid heart rate, in which the upper heart chambers (atria) are stimulated to contract in a very disorganised and abnormal manner.
- Atrioventricular block
- Defective conduction at the atrioventricular node.
- Bradycardia
- Slow heart rate. Bradycardia may become pathological with decreased heart output. Symptoms of bradycardia may be specific (syncope) or chronic and non-specific (dizziness, fatigue and heart failure).
- Cost-effectiveness acceptability curve
- A graphical representation of the probability of an intervention being cost-effective over a range of monetary values for society’s willingness to pay for an additional unit of health gain.
- Incremental cost-effectiveness ratio
- An expression of the additional cost of health gain associated with an intervention relative to an appropriate comparator. Expressed as the difference in mean costs (relative to the comparator) divided by the difference in mean effects. Sometimes expressed with confidence intervals.
- International normalised ratio
- A measure of the degree of anticoagulation achieved using warfarin (intrinsic heart rate = 1.0 is equivalent to no anticoagulation).
- Kaplan–Meier curves
- Also called product limit method. A non-parametric method of compiling life or survival tables, developed by Kaplan and Meier in 1958. It combines calculated probabilities of survival and estimates to allow for censored observations, which are assumed to occur randomly. The intervals are defined as ending each time an event (e.g. death, withdrawal) occurs and are therefore unequal.
- New York Heart Association functional scale
- A scale used to classify patients’ cardiac disease according to the severity of their symptoms into four categories based on the limitations on physical activity, with class I patients having no limitation of physical activity and class IV patients having symptoms of heart failure at rest and inability to carry out any physical activity without discomfort.
- Physiological pacing
- Pacing mode that reproduces the natural sequence of atrioventricular contractions. This is achieved with the preservation of atrioventricular synchrony and rate response.
- Quality-adjusted life-year
- A term originally developed in cancer studies to balance poor quality of life (possibly with long life expectancy) with good quality of life (possibly with short life expectancy).
- Quality of life
- A concept incorporating all the factors that might impact on an individual’s life, including factors such as the absence of disease or infirmity as well as other factors which might affect physical, mental and social well-being.
- Rate modulation/rate responsiveness
- A feature of pacemakers in which the pacing rate varies according to the physical demands of the patient.
- Sick sinus syndrome
- This term covers a spectrum of arrhythmias with different underlying mechanisms, manifested as bradycardia, tachycardia (fast heart rate) or a mix of the two, but also as chronotropic incompetence (the inability of the heart to increase its rate appropriately with increased activity, leading to exercise intolerance).
- Tachyarrhythmia
- Abnormally fast heart rhythm.
- Tachycardia
- Increased heart rate.
List of abbreviations
- 6MWT
- 6-minute walking test
- 12-GHQ
- 12-Item General Health Questionnaire
- ABHI
- Association of British Healthcare Industries
- ACC
- American College of Cardiology
- AF
- atrial fibrillation
- AHA
- American Heart Association
- AV
- atrioventricular
- BMI
- body mass index
- BNF
- British National Formulary
- BPEG
- British Pacing and Electrophysiology Group
- b.p.m.
- beats per minute
- BTS
- bradycardia–tachycardia syndrome
- CHF
- congestive heart failure
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination
- CTOPP
- Canadian Trial of Physiological Pacing
- CVD
- cardiovascular disease
- ECG
- electrocardiography
- EED
- Economic Evaluations Database
- EQ-5D
- European Quality of Life-5 Dimensions
- ESC
- European Society of Cardiology
- GP
- general practitioner
- HF
- heart failure
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IHR
- intrinsic heart rate
- IPD
- individual patient data
- kpm
- kilopond metre
- LV
- left ventricle
- LVEF
- left ventricular ejection fraction
- MeSH
- medical subject heading
- MOST
- MOde Selection Trial
- MTA
- multiple technology appraisal
- NASPE
- The North American Society of Pacing and Electrophysiology
- NHS EED
- NHS Economic Evaluations Database
- NICE
- National Institute for Health and Care Excellence
- NOAC
- novel oral anticoagulant
- NYHA
- New York Heart Association
- OR
- odds ratio
- OWSA
- one-way sensitivity analysis
- OXVASC
- OXford VASCular study
- PASE
- Pacemaker Selection in the Elderly study
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SA
- sinoatrial
- SAS
- Specific Activity Scale
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form Questionnaire-36 items
- SND
- sinus node dysfunction
- SSI
- somatic symptoms inventory
- SSS
- sick sinus syndrome
- TA
- technology appraisal
- TAG
- Technology Assessment Group
- TTO
- time trade-off
- VAS
- visual analogue scale
- VP
- ventricular pacing
- WTP
- willingness to pay