Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/06/07. The contractual start date was in May 2014. The draft report began editorial review in April 2015 and was accepted for publication in July 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Guthrie et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
The Health Technology Assessment (HTA) programme is a research funding programme that supports research that is tailored to the needs of UK NHS decision-makers, patients and clinicians. The programme is part of the wider National Institute for Health Research (NIHR) and funds UK researchers to conduct a mix of primary research and evidence syntheses that address the needs of the NHS.
Hanney et al. (2007)1 described how the first formal NHS research and development (R&D) strategy, launched in 1991, led directly to the establishment of the HTA programme in 1993. 2 The R&D strategy Assessing the Effects of Health Technologies2 highlighted the importance of health technology assessment. In this section of the report, we outline the policy developments and changes that have affected the programme over the last 10 years, describe the programme’s current structure and approach, provide an overview of previous work reviewing the impact of the programme and set out the aims of this study.
Policy developments affecting the Health Technology Assessment programme from 2003 to 2013
One of the key developments over the last 10 years was the publication of the 2006 national health research strategy Best Research for Best Health. 3 The strategy changed the way that health research is funded in the UK and led, among other things, to the creation of the NIHR. The Best Research for Best Health strategy3 explicitly refers to the HTA programme, which was to be included within the remit of the newly created NIHR, stating that the HTA programme’s purpose is to ‘ensure that high-quality research information on the costs, effectiveness and broader impact of health technologies is produced in the most effective way for those who use, manage and provide care in the NHS’ (p. 22). The report also reaffirms the HTA programme’s links to the National Institute for Health and Care Excellence (NICE) and suggests that alongside addressing questions of importance to the NHS and its users, it should provide ‘dedicated support for the work of the National Institute for Health and Clinical Excellence (NICE) by commissioning both primary research and Technology Assessment Reviews’ (p. 22). A key outcome of the Best Research for Best Health strategy3 was the introduction for a major new programme of pragmatic clinical trials. These pragmatic trials were intended to address topics of direct relevance to the NHS and to operate largely in response mode. By this point, the HTA programme had already started to work with the UK Clinical Research Network to identify and support relevant trials that were thought to have value for the NHS, but the formation of a new programme for pragmatic clinical trials formalised the shift in focus of the HTA programme to fund a wider range of clinical trials. The HTA programme also continued to support clinical trials and evidence syntheses (initially the primary focus of the programme) through its established commissioning routes, based on the research priorities identified through engagement with specialist groups, the NHS and researchers.
Over the period from 2003 to 2013 the size of the HTA programme grew significantly, as did the profile of its research. According to the Best Research for Best Health HTA implementation report,4 by 2009, 54 project grants had been awarded through the new clinical trials funding stream, which had been renamed the HTA Clinical Evaluation and Trials. The new health strategy also led to an increase in funding for the programme, with its annual budget planned to grow by ‘a further £29m as a result of the Best Research for Best Health Research Strategy; and a further £48m following the Comprehensive Spending Review and the increase in the Joint Health Research Fund, both by 2012/13 compared to 2005/06 levels’ (p. 2). 4 The overall budget of £88M by 2012–13 was intended to fund more HTA trials through all commissioning routes. The Best Research for Best Health implementation report4 also referenced the quality of HTA research, noting that the roughly 50 monographs were published each year in the ‘internationally acclaimed’ Health Technology Assessment (HTA) journal, which had an impact factor (a measure of the level of citation of articles within the journal) of 3.87 in 2007, ranking it among the top 10% of health and medical-related journal titles.
Prior to the publication of the Best Research for Best Health report,4 there had already been some changes to the HTA programme in response to the public health White Paper Choosing Health: Making Healthy Choices Easier5 and the 2004 Wanless Report Securing Good Health for the Whole Population. 6 A new HTA panel on public health was established in 2005 and supported by £9.2M in funding. According to the Best Research for Best Health implementation plan,4 the panel was ‘developing close and complementary links with the recently established NIHR Public Health Research programme, which evaluates non-NHS public health interventions’ (p. 2) by 2009.
The Cooksey Review,7 A Review of UK Health Research Funding, also had implications for the strategy and focus of the HTA programme. A key recommendation of the Cooksey Review7 was the establishment of the Office for Strategic Coordination of Health Research to determine the government’s health research strategy, set the budget for health research, and distribute the budget for health research between the NIHR and Medical Research Council (MRC). The Cooksey Review7 also recommended the establishment of NIHR as a real, rather than a virtual, institute, and clarification of the roles of the NIHR and MRC, which had implications for the HTA programme. HTA research had previously been funded by both the MRC and the NIHR, but health technology assessment, along with health services research and applied public health research, was brought exclusively within the remit of the NIHR in response to these recommendations. The Cooksey Review7 explicitly stated that the HTA programme should benefit from these new arrangements by receiving a greater proportion of the financial support for this type of research within the overall UK funding portfolio. The report also praised the HTA programme as ‘very successful in its role of Knowledge Production, by providing NHS decision-makers with a high-quality evidence base, in meeting needs created by “R&D market failure” and for its innovation and flexibility’ (p. 85) and as ‘a global leader in this area’ (p. 99). The review recommended that the HTA programme be expanded to meet the increasing information needs of the NHS. Specific areas recommended for expansion included strengthening the commissioned workstreams for primary research, clinical trials and themed calls, following up on research recommendations from NICE and funding research of HTA methodologies. It is interesting to note that the Cooksey Review7 recommended that a set of metrics should be developed to evaluate the impact of the expansion of the HTA programme to inform future spending decisions.
More recent changes in the NHS, outlined in the 2011 document Equity and Excellence: Liberating the NHS,8 have been less closely focused on research and have had a less substantial impact on the HTA programme. Although the Equity and Excellence document8 does not refer to the HTA programme specifically, it reinforces the core role of research in the NHS. The document states that ‘the Government is committed to the promotion and conduct of research as a core NHS role’ (paragraph 3.16). The Equity and Excellence document8 also highlights the importance of the NIHR and clinical research, describing how the NHS has ‘an increasingly strong focus on evidence-based medicine, supported by internationally respected clinical researchers with funding from the National Institute for Health Research’ (paragraph 1.6). It also notes the importance of patient involvement in research, and states that to support the development of quality standards NICE will advise the NIHR (including the HTA programme) on research priorities.
Current structure and approach of the Health Technology Assessment programme
Aims
Recent NIHR briefing documents state the aim of the HTA programme as to ‘research information about the effectiveness, costs and broader impact of health-care treatments and tests for those who plan, provide or receive care in the NHS’ (p. 2),9,10 demonstrating that although the specific strategy of the programme has changed over time, the overarching aims of the programme have remained consistent.
Research funding
The HTA programme commissions and funds research via four routes:
-
Commissioned workstream This stream funds research on specific topics identified by a range of stakeholders, from patients to professional bodies, and prioritised by the six advisory panels (described below). Typically, the HTA programme advertises three calls per year, which consist of a set of specified research questions that are described in commissioning briefs. Responses from applicants to each commissioning brief are reviewed based on their scientific merit, feasibility and value for money by the HTA Commissioning Board.
-
Researcher-led workstream This stream funds research questions put forward by researchers. The Clinical Evaluation and Trials Board assesses proposals based on their relevance to clinical practice in the NHS and the importance of the outcomes to patients.
-
Themed calls Themed calls aim to increase the evidence base for key health priorities through funding a number of projects across the NIHR in a particular area. They are evaluated separately by independent review boards set up for that purpose. Previous calls include obesity, medicines for children, diagnostic tests, dementia and primary care.
-
Technology Assessment Reports (TARs) These provide evidence to support NICE’s technology appraisal and diagnostic assessment programmes (TARs are described in detail below).
Six advisory panels and the prioritisation group support the review boards across all of these programmes. The prioritisation group balances the relative priority of research topics for the commissioned research workstream and applications received from the researcher-led funding stream. The six advisory panels are as follows: Diagnostic Technologies & Screening Programmes; Elective and Emergency Specialist Care; Interventional Procedures; Maternal, Neonatal and Child Health; Mental, Psychological and Occupational Health; Primary Care, Community and Preventive Interventions. The role and remit of each of these advisory panels is described in more detail below (see Priority setting).
The programme supports both evidence syntheses and primary research across all six panels and across all the funding streams. Over the last 10 years, there has been a relative increase in both response mode-funded research and primary research, which is in line with the recommendations set out in Best Research for Best Health3 and the Cooksey review7 (see Policy developments affecting the Health Technology Assessment programme from 2003 to 2013, above). In 2001, the HTA programme had published only five clinical trials,11 whereas by April 2014 the programme had supported 530 primary research studies, 260 of them completed and published in the HTA journal. An overview of the HTA programme’s portfolio, by type of study and status of the project, is provided in Table 1.
| Type of study | Complete | Waiting to publish | In progress | Waiting to start | Total |
|---|---|---|---|---|---|
| NICE TARs | 157 | 9 | 5 | 0 | 171 |
| NICE diagnostic assessments | 12 | 6 | 3 | 0 | 21 |
| NICE ERG report | 152 | 0 | 34 | 0 | 186 |
| HTA TARs | 130 | 11 | 6 | 0 | 147 |
| Methodology reports | 121 | 1 | 0 | 0 | 122 |
| Evidence synthesis | 205 | 29 | 14 | 4 | 252 |
| Primary research | 260 | 65 | 198 | 7 | 530 |
Relationship with policy-making organisations
An important part of the HTA programme’s strategy is the way it engages and influences NICE, the National Screening Committee (NSC) and other policy-makers. The HTA programme has direct links with NICE through its commissioning of TARs to inform NICE guidance. However, HTA programme-funded research also feeds in to other aspects of NICE’s work. For example, HTA programme-funded research is often cited in NICE guidelines, but the links between the HTA programme and the guideline-producing parts of NICE are less strong than the links between the TAR programme and NICE. The HTA programme also has direct links with the NSC, which are less formalised than the programme’s links with NICE. In addition to its well-established links with NICE and the NSC, the HTA programme also has informal links with other policy-makers (e.g. NHS England). The remainder of this subsection discusses the links between the HTA programme and each of those organisations.
The HTA programme commissions independent TARs to meet the urgent needs of NICE and other users of TARs. 12 Nine TAR centres, based in universities and academic centres across the country and contracted by the Department of Health (DH), conduct all of the TARs for NICE. TARs provide evidence to support NICE’s technology appraisal and diagnostic assessment programmes. There are three types of TARs:
-
Single Technology Appraisal Reports (STAs) aim to ‘assess the strength and quality of the research evidence submitted by manufacturers to NICE as part of the evaluation of a single new drug or device close to when they are first licensed’ (p. 3)12 and are produced within 8 weeks.
-
Multiple Technology Appraisal Reports (MTAs) aim to ‘identify, assess and synthesise the research evidence (including data submissions) from across a number of interventions in a given healthcare area’ (p. 3). 12 MTAs typically provide estimates of the relative effectiveness and cost-effectiveness of different interventions. They are larger research reviews than the STAs and take 26–28 weeks to produce.
-
Diagnostic Assessment Reports aim to ‘identify, assess clinical outcomes and synthesise the research evidence for single or multiple diagnostic technologies in a given pathway’ (p. 3). 12 Diagnostic Assessment Reports typically provide estimates of the relative effectiveness and cost-effectiveness of different diagnostic technologies. Diagnostic Assessment Reports are also relatively large reviews and take 24 weeks to produce.
Recent users of TARs also include the Chief Medical Officer, the National Specialised Commissioning Team, and the Policy Research Programme. TARs produced for other policy-makers may take different forms, and their content is tailored to meet the needs of the particular policy-making organisation. However, they typically include a systematic review of evidence in a particular area and economic modelling.
The NICE Guidelines Programme also uses HTA programme-funded research, but the link between the policy impact and the HTA programme is less direct. The HTA programme also has direct links with the NSC, which relies heavily on HTA programme-funded research for formulating evidence-based advice for government. According to interviewees, the HTA programme sends research related to screening directly to the NSC. The HTA programme and the NSC also have regular meetings to discuss both ongoing and recently published HTA programme-funded research related to screening. The NSC also submits research topics directly to the HTA programme. However, the links between the HTA programme and the NSC seem to rely primarily on relationships between individuals rather than formal links between the two organisations.
Research budget
‘The research budget of the HTA programme has now grown considerably from the initial £1M in 1993/4 to £8M in 2003/4 and £60M in 2012/13’ [NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC), 3 April 2014, personal communication, reproduced with permission]. Adjusting for inflation, that growth is equivalent to an increase of more than 400%, in real terms, over the last 10 years. This funding increase reflects the growth in scope of the programme over that time period, particularly the expansion of the clinical trials element of the programme because clinical trials are typically much more costly than evidence syntheses.
Priority setting
The HTA programme identifies potential research topics from a number of sources. It engages key stakeholders in the NHS and the NIHR, in part through the NIHR horizon scanning centre. The programme also sources research topics from the James Lind Alliance (JLA) Priority Setting Partnerships and from research recommendations in published research, particularly systematic reviews, and guidelines. The programme also elicits research suggestions from policy-makers (notably NICE and the NSC) and other health-care-related organisations (e.g. the Royal Colleges and patient groups). Research topics can also be submitted by members of the public through the HTA website.
The initial prioritisation process consists of a number of steps. First, potential research topics are reviewed to assess whether or not they fall within the remit of the programme and to determine whether or not the research questions are sufficiently different from existing or ongoing research. Research topics that do not fall within the remit of the HTA programme are passed on to other NIHR research programmes.
The remaining research topics are then reviewed by one of the six Topic Identification, Development and Evaluation (TIDE) panels. These panels comprise a range of professional, public and patient members who act to advise the HTA programme on the importance of the research topic to patients and the NHS. There are six such panels in the following areas:
-
Primary Care, Community and Preventive Interventions Covers interventions that are delivered in primary care or the community.
-
Elective and Emergency Specialist Care Focuses on interventions delivered in hospitals or by specialists.
-
Maternal, Neonatal and Child Health Examines interventions related to obstetrics, paediatrics and specific maternal health issues.
-
Interventional Procedures Covers all surgical interventions, drugs used for interventional procedures and interventional radiology.
-
Mental, Psychological and Occupational Health Covers rehabilitation, learning difficulties, mental health, cognitive deficits and occupational health.
-
Diagnostic Technologies & Screening Programmes Covers all tests used to diagnose, monitor or select patients for treatment; or to monitor a disease or the effect of its treatment.
The members of each panel consist primarily of clinicians and health-care professionals working in the front-line NHS but also include public and patient representatives. At panel meetings, the members discuss and refine research suggestions. The members of the panel then vote on the relative importance of the topics to the NHS. The most important topics are then developed into vignettes that guide ongoing work on the selected topics. The vignettes are discussed at the panel’s Methods Group, which provides advice on the proposed research design and methodology. After the panel finalises the vignettes, they send them to the HTA Prioritisation Group.
For researcher-led work, the TIDE panel reviews anonymised extracts from proposals against the ‘criteria to guide the setting of HTA Priorities’. They evaluate the importance of the research question and produce a ranked list of proposals for the HTA Prioritisation Group.
After the six advisory panels have completed the vignettes and the TIDE panel has reviewed researcher-led proposals, the HTA Prioritisation Group then determines which research should be funded. The HTA Prioritisation Group consists of the Programme Director of the NIHR HTA programme, the chairs from each of the six HTA panels and the two funding boards, and senior representatives from NETSCC. The role of the Prioritisation Group is to develop a portfolio of research that reflects the needs of the NHS, fits within the available programme budget and provides good value for money. The group reviews and prioritises topics from the panels based on the vignettes prepared, and decides which should be developed into commissioning briefs. The HTA Prioritisation Group also reviews researcher-led proposals and prioritises them for consideration at the Clinical Evaluations and Trials Board. Finally, the Group reviews funding recommendations from the HTA commissioning boards and prepares a final list for approval by the DH.
The Health Technology Assessment journal
The HTA programme has its own journal, Health Technology Assessment, which is part of the wider NIHR journals library. The HTA programme endeavours to publish all HTA programme-funded research in the HTA journal as a monograph, and includes a full description of the methods, results and conclusions of the research. The monographs include an abstract, scientific summary and plain English summary. Although the programme aims to publish all HTA programme-funded research in the HTA journal, the research must be of sufficiently high scientific quality, as assessed by external peer reviewers and the journal’s editors, to be published in the journal. The purpose of the journal is to ensure that the full results of all studies are publicly available. However, the HTA programme also encourages the publication of results in other peer-reviewed journals. Prior publication of HTA programme-funded research results in other journals is not a limitation to the publication of a monograph in the HTA journal. Typically, the HTA journal article is one of the final outputs of any project.
However, not all HTA programme-funded research is published in the HTA journal. For example, STAs are typically not published in the HTA journal (with a limited number of exceptions) because they are usually based on commercial data that were provided in confidence, and they primarily review manufacturers’ evidence rather than present original research. Despite the growth in the size of the HTA programme, there is evidence that the programme has maintained its academic quality, with the HTA journal’s 5-year impact factor of 5.595 ranking it fourth among all journals in the Health Care Sciences and Services category. 13
Adding value in research
The HTA programme has also been influenced by the work of Chalmers and Glasziou (2009)14 on research waste and, more recently, by the same authors and others in a series of publications on research waste in The Lancet in January 2014. The five papers15–19 published in The Lancet each expand on one of the key themes identified in the earlier paper by Chalmers and Glasziou (2009):14
-
decisions about which research to fund based on issues relevant to users of research15
-
appropriate research design, methods and analysis16
-
efficient research regulation and management17
-
fully accessible research information18
-
unbiased and usable research reports. 19
The HTA programme and the NIHR have used these themes to develop the Adding Value in Research framework. 20 The aim of the framework is to ensure that NIHR-funded research ‘answers questions relevant to clinicians, patients and the public; uses appropriate design and methods; is delivered efficiently; results in accessible full publication; and produces unbiased and usable reports’ (p. 1). 21 In fact, the HTA programme has been considered an exemplar of good practice in many of these areas. For example, Chalmers and Glasziou (2009)14 note:
Some elements of these recommendations reflect policies already implemented by some research funders in some countries. For example, the NIHR’s Health Technology Assessment Programme routinely requires or commissions systematic reviews before funding primary studies, publishes all research as web-accessible monographs, and, since 2006, has made all new protocols freely available.
p. 8814
The HTA programme’s perceived success in adding value in research contrasts with the evidence that shows that researchers more widely are not making sufficient use of existing evidence in the design and execution of their research. For example, the study by Clark et al. (2013)22 showed that of 446 trials submitted to research ethics committees in the UK, only 4% used meta-analyses of data from relevant previous studies to plan target sample sizes. Similarly, an analysis of clinical trials by Robinson et al. (2011)23 found that less than one-quarter of previous trials were cited in reports. This evidence suggests that not all research funders adhere to the UK policy on research governance in the biomedical research sector. According to the DH’s advice on research governance ‘all existing sources of evidence, especially systematic review, must be considered carefully before undertaking research. Research which duplicates other work unnecessarily, or which is not of sufficient quality to contribute something useful to exiting knowledge, is unethical’. 24
The Adding Value in Research framework is used by the HTA programme and the NIHR more widely for self-assessment and ongoing improvement. Through the Research on Research programme that was established in 2007, the NIHR funds studies on how they manage their research programmes and the projects they fund. Adding Value in Research is one of three core strategic areas of the Research on Research programme. Recent work funded through the programme has shown that the HTA programme is performing well against several of the elements of the framework. Wright et al. (2014)25 provided evidence that the programme is supporting research of clinical relevance. Turner et al. (2013)26 found that 95.7% of all HTA studies either have published, or will publish, a monograph in the HTA journal, and that that percentage increases to 98% for studies commissioned after 2002. Chinnery et al. (2013)27 showed that these publications were available promptly. They also found that the median time to publication for a HTA monograph was 9 months shorter than an external journal article.
However, published evidence suggests that the HTA programme could improve the value that it adds. Work by Douet et al. (2014)28 showed that not all the reports published in the HTA journal provide sufficient information to replicate the work, with components missing in 69.4% of the 98 reports analysed. In particular, only 58.2% of reports had complete patient information. Jones et al. (2013)29 looked at the use of systematic reviews in planning a sample of randomised trials and found that although the majority of HTA programme-funded research referenced the systematic review in their application, only half used the systematic review to inform the design of the study. Turner et al. (2010)30 found that, in one particular research area, eight similar HTA studies had been funded by different international HTA organisations. Although the studies looked at the issue within different contexts, the similarity between studies suggests that potential duplication of effort and research waste may have taken place. The authors suggest that such resource wastage could be minimised by the use of a toolkit designed to help adapt HTA reports between different contexts.
Complementary to the Adding Value in Research framework is the ‘needs-led, science-added’ approach, which is a set of principles used across the NIHR, including the HTA programme, which aims to maximise the utility of research for decision-makers. The underpinning principle is that research should be ‘needs led’ in that it should reflect the key information needs of decision-makers, while also being of high quality. To this end, the NIHR undertakes a range of activities, such as involving stakeholders – including policy-makers, patients and the public – in topic identification and prioritisation (as described above); conducting peer review of proposals and making the funding decisions via expert panels; ongoing monitoring and contact with projects in process; and comprehensive publication of findings in the NIHR journals library.
Patient and public involvement in research
Both the Adding Value in Research framework and the ‘needs-led, science-added’ approach advocate the involvement of patients and the public in the design and conduct of research. The NIHR defines involvement of patients and the public as:
. . . an active partnership between the public and researchers,
research done with or by members of the public, not to or about them,
the public getting involved in the research process itself.
p. 231
Health Technology Assessment programme-funded studies are expected to demonstrate that they will involve patients and the public, and this forms part of the assessment of proposals submitted for funding. The NIHR provides guidance to researchers on what that involvement could consist of, suggesting that patients and the public could be involved in:
-
designing questionnaires and patient information sheets
-
helping to find participants and designing the best way to approach them
-
participating in advisory or steering groups
-
undertaking aspects of the research
-
contributing to or commenting on the final report.
One of the main support mechanisms that the NIHR provides support for researchers is the Research Design Service, which can give researchers access to relevant patients or members of the public that they have recruited into their patient and public involvement (PPI) panels. In a study of a sample of HTA programme-funded projects, 85% of proposals described PPI representation in their application. However, only 41% of reviewer comments across the trials commented on the PPI plan, which suggests that more guidance could be provided for reviewers on this process. 32
Previous work assessing the impact of the Health Technology Assessment programme
Several previous studies have been conducted to assess the impact of the HTA programme. Hanney et al. (2007)1 undertook an assessment of the impact of the first 10 years of the HTA programme, which was commissioned by the NIHR. Using payback case studies, Hanney et al. (2007)1 identified a range of impacts results from the HTA programme and the mechanism through which those impacts arose. Hanney et al. (2007)1 found that the HTA programme primarily has an impact on knowledge generation, but that it also has a perceived impact on health policy and, to some extent, clinical practice. Hanney et al. (2007)1 suggested that the policy relevance of the funded studies contributed to the observed high impact of the programme. Hanney et al. (2007)1 also reported that the methodological rigour and strict peer review facilitated the publication of HTA programme-funded research in high-quality, peer-reviewed journals.
A second study examining the impact of the HTA programme was carried out by RAND Europe on the impact of a small sample of clinical trials funded by the HTA programme. 33 The study33 attempted to monetise the potential benefits to the NHS and patients of the findings of a small sample of HTA studies and compared these benefits to the cost of the HTA programme. The study33 provides quantitative evidence of the impact of the HTA programme in a limited number of cases, and demonstrates that a small subset of the work of the HTA programme has potential returns that would be greater than the total costs of the HTA programme if the findings of those studies were fully implemented and delivered the expected long-run returns. However, the approach has several limitations. First, only a limited range of types of benefit were captured. As well as providing evidence that new treatments could be effective and cost-effective (potentially influencing health policy, clinical practice, health outcomes and the economy), the programme also identifies new technologies that are no better than the existing standard of care, which prevents new, potentially less effective or more expensive technologies, being adopted by the health service. Similarly, the approach does not capture wider impacts of the HTA programme, such as its impact on the research system. In addition, the approach focused only on clinical trials, whereas part of the value of the HTA programme comes from its systematic reviews and evidence syntheses.
A recent paper by Raftery and Powell (2013)11 looked at the impact of the HTA programme over the last 20 years. They describe examples of when HTA programme-funded research has had an impact on health policy and clinical practice, and the impact of the programme at a wider level. Raftery and Powell (2013)11 see the programme as a provider of evidence to NICE and the NSC, and as an exemplar of good practice in the promotion of full, open access publication of all results, the registration of trial protocols at the outset of research and the insistence on systematic review before funding primary research. The study11 concludes by identifying key challenges for the HTA programme, which include ensuring that funding and publication of research is timely, addressing the methodological challenges around the research that it funds, ensuring that trials are incorporated into updated meta-analyses, and maintaining their independence from government. Another key challenge that Raftery and Powell (2013)11 identified is maintaining funding for the HTA programme from the NHS in the current economic climate. Raftery and Powell (2013)11 suggest that the HTA programme needs to demonstrate that it is cost-effective through the effect that it has on health service resources and public health. The authors recommended that the HTA programme funds more ‘research on research’, including work looking at the HTA programme. As well as considering other approaches, including the issues of adding value in research and the contribution of trials to subsequent systematic review, Raftery and Powell (2013)11 recommended the application of the payback framework to look at the second decade of the programme.
The present study is intended to be complementary to the previous studies on the HTA programme and, in particular, to provide an assessment of the impact of the HTA programme over the last 10 years, as recommended by Raftery and Powell (2013). 11
Aim of this study
The aim of this study is to review the impact of the NIHR HTA programme from 2003 to 2013. This study considers a broad range of potential impacts, spanning academic, health policy, clinical practice, and health and economic outcomes. Although the study’s approach was largely retrospective, reviewing impact from 2003 to 2013, it also included a forward-looking component, which considered how the HTA could increase its impact in the future, based on the evidence collected for the retrospective analysis.
Considering the objectives of the programme, and taking into account the different ways in which the HTA programme can have an impact, through the HTA programme-funded research and the programme itself, we identify three key research questions:
-
What has been the impact of HTA programme-funded research on the NHS, patients, clinicians, health policy, academia, the research system, industry and the economy from 2003 to 2013?
-
What has been the impact of the HTA programme on the NHS, patients, clinicians, health policy, academia, the research system, industry and the economy from 2003 to 2013?
-
What actions can the HTA programme take to increase its impact on the NHS, patients, clinicians, health policy, academia, the research system, industry and the economy?
Chapter 2 Methodology
We have taken a broad approach to assessing the impact of the HTA programme, aiming to explore the full range of impacts resulting from HTA research across the programme, including the impacts resulting from the programme itself, not just as a body of individual projects. Therefore, the work presented in this report consists of two elements:
-
Analysis of impact across the HTA programme To do this, we have:
-
conducted 20 interviews with key stakeholders, spanning a range of viewpoints, to understand the impact of the HTA programme in different contexts
-
conducted a bibliometric analyses of the HTA programme
-
analysed all available survey data from Researchfish® (Cambridge, UK; www.researchfish.com) for HTA programme-funded research over the period. Researchfish is an online system that is designed to capture research outcomes for researchers and funding organisations through questions on a series of types of outcomes and impacts.
-
-
Analysis of the impact of a sample of individual HTA projects To do this, we have conducted 12 detailed payback case studies.
These tasks are mapped against the key study questions in Table 2.
| Question | Source |
|---|---|
| What broad impacts on the NHS and patients, policy, academia, the research system, industry and the economy have resulted from the HTA at a programme level over the period 2003–13? | Interviews with stakeholders Bibliometrics Survey data |
| What is the impact on the NHS and patients, policy, academia, the research system, industry and the economy of the HTA programme at a project level over the period 2003–13? | Interviews with stakeholders Bibliometrics Survey data Payback case studies |
| What actions can the HTA programme take to help increase its impact on the NHS and patients, policy, academia, the research system, industry and the economy in the future? | Cross-cutting analysis across all tasks |
Our methodology is based on the payback framework developed by Buxton and Hanney (1996). 34 This approach was specified by the commissioning brief and was selected because it is a useful way to collect information on the impact of research systematically and, comparably, allowing useful comparisons to be drawn across data sources to generate wider insights. In addition, it is well established and has been widely used as a method to investigate and catalogue the impacts of health research,35–38 and was used previously to assess the impact of the first 10 years of the HTA programme, following an assessment of potential approaches that could be used. 1 Using this approach allows us to make direct comparisons of our findings to those of the previous assessment.
The payback framework consists of two elements: a classification system to capture and categorise the outputs and outcomes of research, and a logic model which helps to break down the research and translation process. As such, the payback framework helps not only to evaluate the range and nature of outputs from research, but also to conceptualise the process through which these outputs are generated. Although the logic model is linear in format, which is a simplification of the research process, it also explicitly includes feedback between different stages of the process.
The payback framework has five categories of impact to capture the range of impacts resulting from research. We used these to structure our data collection – primarily for the case studies (described in Case studies) but also for our other streams of evidence.
-
Knowledge production This category covers the knowledge produced as a result of the research conducted, and this knowledge is in general captured in publications. Peer-reviewed articles are generally the most common measure, but editorials, meeting abstracts, reviews and patent filings are other examples of knowledge production. Citation analysis is one approach to understanding and measuring the output in this category.
-
Research targeting and capacity building This category captures benefits for future research created by the research conducted both in terms of the direction of research and research priorities, and the building of research capacity in terms of infrastructure, skills and staff development.
-
Informing policy and product development This category captures the impact of research on health policy (illustrated by such things as citation on clinical guidelines) and on product development as findings are taken up by the private sector for commercialisation (possible measures are licensing intellectual property, contract research work and public–private joint ventures, along with new start-ups).
-
Health and health sector benefit This category covers health benefits and other benefits for the health sector (such as improved efficiency or cost savings) resulting from the findings of the research being put into practice. This typically occurs via the uptake of the policy, products or processes outlined in the previous category.
-
Broader economic benefit This final category covers the wider socioeconomic benefits resulting from the research. They might be the outcome of the increased productivity of a healthier workforce resulting from the health benefits described, or might result from increased employment or the development of new markets, stemming from the development of new products or processes. This can be very challenging to measure owing to the challenges of attributing such change to a particular piece of research.
Although we used the payback approach to structure our data collection, in our analysis of the impacts of the programme we categorised the impacts slightly differently to better reflect the aims and priorities of the HTA programme, particularly its focus on the UK, and the NHS and patients. Although our categorisation does not explicitly mention every potential impact of the programme (and indeed, it is not possible to identify and capture every possible impact), we felt that it was appropriate to focus primarily on assessing the impact of the programme against its own aims, while remaining open to other types of impact that may also be identified. The categories used, as defined below, are intended to be broad enough to capture the full range of impact identified, but also focused primarily on the key aims of programme. We used the following categories:
-
Impact on the NHS and patients This captures impacts on health and the health sector, and those socioeconomic benefits that relate to the improved health of patients.
-
Impact on UK policy Impact on health policy in the UK only. Part of what is captured under improving policy and product development.
-
Academic impact The equivalent of ‘Knowledge production’.
-
Impact on the research system The equivalent of ‘Research targeting and capacity building’.
-
Impact on industry and the economy This covers parts of what is captured under improving policy and product development, focusing on the product development side, but also takes in economic impacts when they do not relate to the NHS or patient health.
-
International impact This captures impacts across all categories outside the UK, with the exception of academic impact, which is hard to separate in this way. In particular, this category covers impacts on policy and practice outside the UK, and influences on wider research systems and structures.
These categories were used to structure the analysis of the results across the various data collection methods used, combining the broad findings across the programme from the interviews, bibliometrics and survey data with the in-depth examples provided by the case studies. The qualitative data from the interviews and case studies were coded and analysed by impact type and stakeholder group. Within the impact categories, we conducted a thematic analysis of the data coded in each impact grouping to identify key messages. The same categories were used to analyse the quantitative data from the bibliometric analyses and the Researchfish survey. The rationale for using the same impact categories to analyse all of the different data sources was to allow the identification of key messages and themes that were supported by the data.
In the following sections, we describe each of the methods used in more detail.
Interviews
The purpose of the interviews was to understand the broad impact of the HTA programme on the medical research funding, practice and policy landscape. Potential interview candidates were identified from the following sources:
-
analysis of key policy documents to identify important perspectives and, where possible, relevant individuals
-
suggestions of the advisory board
-
when a relevant perspective was identified, but not an individual, we looked at the structure of relevant organisation(s) to identify the appropriate contact point
-
snowballing based on suggestions from previous interviews.
Through the sample of interviewees chosen, we aimed to cover a wide range of perspectives, but the final sample of interviewees was ultimately pragmatic, based on our ability to identify appropriate, informed informants who were available and willing to participate in an interview over the relevant time frame. A total of 20 interviews were conducted with informants, covering a range of perspectives. A full list of the interviews conducted and the perspectives covered is provided in Table 3.
| Interview | Academic | Patient and public | NICE | NSC | SIGN | Cochrane | NHS | DH/political | International | HTA expert | MRC/other funder | Internal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (I1) | ✗ | ✗ | ✗ | |||||||||
| 2 (I2) | ✗ | |||||||||||
| 3 (I3) | ✗ | ✗ | ✗ | |||||||||
| 4 (I4) | ✗ | ✗ | ✗ | |||||||||
| 5 (I5) | ✗ | ✗ | ||||||||||
| 6 (I6) | ✗ | ✗ | ||||||||||
| 7 (I7) | ✗ | |||||||||||
| 8 (I8) | ✗ | ✗ | ✗ | |||||||||
| 9 (I9) | ✗ | ✗ | ||||||||||
| 10 (I10) | ✗ | ✗ | ||||||||||
| 11 (I11) | ✗ | |||||||||||
| 12 (I12) | ✗ | |||||||||||
| 13 (I13) | ✗ | |||||||||||
| 14 (I14) | ✗ | ✗ | ||||||||||
| 15 (I15) | ✗ | |||||||||||
| 16 (I16) | ✗ | |||||||||||
| 17 (I17) | ✗ | |||||||||||
| 18 (I18) | ✗ | ✗ | ||||||||||
| 19 (I19) | ✗ | |||||||||||
| 20 (I20) | ✗ | |||||||||||
| Total | 4 | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 5 | 4 | 2 | 8 |
An important limitation to note is the potential inherent bias in using key informant interviews to collect information on the HTA programme. For interviews to be productive, it is necessary that the individuals interviewed are informed about the programme. However, informed individuals are typically (although not exclusively) in some way involved with the programme and, as such, will potentially be biased, with the most likely risk being that the perspectives offered are likely to be supportive of the programme. However, those that were previously involved in the programme (and are no longer) may be critical of the programme, and indeed critical from an informed perspective. In either case, it is important to note that those who are informed enough to provide information on the programme are likely to have had some direct engagement with the programme and, as such, are likely to bring their own personal perspective to the information provided. As such, analysis of the data provided need to be conducted in the context of those personal views and experiences.
Interviews were conducted by one of two researchers on the project team by telephone and took between 30 minutes and 1 hour. For the first few interviews, both interviewers joined the interview so that they could ensure that they were taking a similar approach and had a shared understanding of the protocol and how it should be applied. The remaining interviews were undertaken by one interviewer only. Interviews were recorded, but the recordings were kept for internal use only and were destroyed after completion of the analysis.
The interview questions were open ended and the protocol was flexible to allow the interviewer to focus on the most relevant questions for that particular stakeholder. A semistructured approach ensured that interviewers covered a consistent range of issues in each interview, but also allowed the particular context and circumstances relevant to the different groups to be discussed. The protocol consisted of three main sections. The first section was of a set of introductory questions to explore the informants’ backgrounds and engagement with the HTA programme. This information, together with our prior knowledge and research on the key informants, allowed us to select the relevant subsets of questions to ask in the second section, which was a set of focused questions relevant to particular perspectives (e.g. questions on policy impact, international impact or academic impact). The final section covered some overview questions about the impact of the programme at a high level, which could be used as required to pick up any issues not covered in the second section. The full protocol, including the detailed questions for all three sections, is provided in Appendix 1.
Interview notes were written up by the team member who carried out the interviews. These notes were intended to be comprehensive in capturing the points made by the interviewee, and were compiled with reference to the interview recording but were not word-for-word transcriptions. However, when the interviewers identified particular quotes of interest, the interviewers transcribed those quotes verbatim from the interview recordings. These notes were coded in NVivo version 10 (QSR International, Warrington, UK), a qualitative analysis software package, against a common codebook. Initially interviews were double coded by both interviewers to ensure consistency in coding and a common understanding of the codebook. Subsequently, the interviews were coded by one interviewer (the person who had conducted the interview). The initial codebook was generated based on previous experience and a review of the study aims and questions, but was developed iteratively over the coding process, with new codes added as required, and regular meetings taking place between the two coders to ensure that all interviews were coded consistently and that any new codes added were applied to all interviews, and that there was a common understanding of the meaning of the codes used. The same codebook was also used for the analysis of the case studies to allow cross-comparisons across both data sets to be made. A full version of the final codebook is provided in Appendix 2.
Bibliometrics
Bibliometrics is the study of scientific publications and their dissemination and use in the scientific community. It offers a powerful set of methods and measures for studying the structure and process of scholarly communication, and has become a standard tool of science policy and research management. Bibliometrics utilises quantitative methods to analyse patterns of scientific publications and their citation, based on the reference lists of scientific journal articles. Citation analysis, a component of bibliometrics, is used extensively to measure the impact and quality of scientific work, as well as the intellectual influence of scientists and scholars. Primarily, it is based on the assumption that new papers will cite other articles that are perceived as useful for informing new research. In this regard, a citation is viewed as a measure of the ‘utility’ and ‘visibility’ of a piece of research. If a researcher or a piece of research has more utility as shown by a larger number of citations, it is assumed that the research is of higher quality. Therefore, a citation is perceived as a proxy for research quality and a measure for research achievement and excellence.
Benefits, drawbacks and common pitfalls of bibliometrics
The advantages of bibliometric methods are straightforward: they provide a quasi-objective and quantitative method of evaluating the performance of research, researchers, institutions and research systems. However, caution is needed when interpreting bibliometric results, as a number of caveats and drawbacks exist.
First, and foremost, citations are not a complete representation of research quality and are only ever a proxy. Bibliometric analysis provides a quantitative reflection of research performance and does not take into account the nuance that can be achieved with a more in-depth, qualitative analysis. Although it is generally accepted that there is a strong correlation between citations and scientific quality at the article, individual researcher and national levels, it does not necessarily follow that an article with a low number of citations is of low scientific value. Furthermore, ‘impact’ as demonstrated by a high level of citation should be considered in a narrow sense as impact within academia, and does not necessarily imply that the research will go on to impact on policy, practice or society more widely. 39 In order to build a more complete picture of research quality and impact, bibliometric indicators should be considered alongside other research evaluation methods, as we have done in this study. It is also important to use a variety of bibliometric indicators, as each has its own particular strengths and weaknesses, for example in relation to sensitivity to skewed distributions or small sample sizes. The specific indicators used in this study are explained further below.
Another issue to be aware of is that different research fields exhibit different citation patterns, with some fields attracting a larger number of citations than others. This can be attributed to a number of factors, including the size of the field; the number of journals and the number of times per year these journals are published; the number of journals indexed in Web of Science (WoS); and the publication norms of the field (e.g. the publication of research as book chapters rather than journal articles is more common in the humanities than the fields of natural and social sciences). These factors contribute to research in some fields having a higher probability of being published and/or cited. Additionally, assessment can be distorted by ‘fashions’ in particular fields; areas seen as particularly topical may attract large amounts of funding, more researchers and more citations. It is possible to control for these differences between fields, to some extent, and further detail on how this was achieved in this study is provided below.
Citations can also sometimes be manipulated by researchers and research organisations to unfairly represent the value of their scientific output. Citation counts can be enhanced by researchers citing their own work (self-citations) as well as researchers publishing flawed or controversial work that is likely to gain a number of citations in other articles that criticise the original research. These types of ‘negative citations’ are not easily recognised using bibliometric analysis, as all citations are assumed to be equal. Identification of negative citations requires a more in-depth, qualitative analysis, which is resource intensive and outside the scope of the current study.
Finally, bibliometric analysis tends to under-represent the research output of non-English speaking countries. The majority of journals indexed in citation databases, such as WoS, are English-language journals and the small number of non-English-language journals indexed may unfairly represent the value of research published in other languages. This issue should not prove particularly problematic in evaluating the HTA programme’s research, as it can reasonably be assumed that all project outputs are published in English.
Bibliometrics in this study
Bibliometric analysis was used in this study to provide a quantitative analysis of the academic output and impact of HTA programme-funded projects. It was carried out at two levels: an overall assessment of the entire HTA portfolio and specific project-level profiles of the studies selected as case studies. Although many of the indicators were the same for these two purposes, there were differences in the methods used, particularly in compiling the relevant data. Each of these is described below, in turn (see Identifying the Outputs of Health Technology Assessment programme-funded research).
Bibliometric data source
The Centre for Science and Technology Studies (CWTS) was contracted by RAND Europe to provide the bibliometric analysis for this project. CWTS is an interdisciplinary research institute housed within the Faculty of Social Sciences at Leiden University in the Netherlands, which specialises in advanced quantitative analysis of scientific research and its connections to technology, innovation and society.
The CWTS maintains a bibliometric database of scientific publications for the period 1980 to the present, generated from the Thomson Reuters WoS database. WoS is a bibliographic database that covers the publications of about 12,000 journals in the natural sciences, the social sciences, and the arts and humanities. Each journal in WoS is assigned to one or more subject categories representing different fields of research. The CWTS in-house database makes a number of improvements to the original WoS data, most importantly by using a more advanced citation-matching algorithm and an extensive system for address unification. The database is based on the journals and serials of the Science Citation Index and associated citation indices: the Science Citation Index, the Social Science Citation Index, and the Arts & Humanities Citation Index, extended with six so-called specialty Citation Indices (Chemistry, Compumath, Materials Science, Biotechnology, Biochemistry & Biophysics, and Neuroscience).
Identifying the outputs of Health Technology Assessment programme-funded research
Programme level
The programme-level analysis required two different types of publication to be identified: papers published in the HTA journal and outputs from HTA programme-funded projects published in other WoS indexed journals. Three different data sources were used to compile the complete publication list:
-
All articles and reviews published in the HTA journal during the period 2004–12. This list comprised 512 papers.
-
Papers listed on all HTA project pages of the NETSCC website (data provided by NETSCC). More than 80% of these papers were matched to the CWTS database, resulting in a list of 474 papers to be included in the analysis.
-
Papers reported by researchers in Researchfish. Matching this list to the CWTS database resulted in 331 unique papers (excluding those published in the HTA journal).
Papers that were not matched to WoS in lists (2) and (3) tended either to have been published too recently to have been indexed in the database or be editorial material (our analysis included only articles and reviews, as other document types do not usually contribute substantially to scientific knowledge). Once duplicates were removed from lists (2) and (3), the final data set comprised 1087 papers.
Although we are confident that this data set covers the majority, and likely the most visible/cited portion, of HTA programme-funded outputs, there will inevitably be publications that are not included. A number of other methods of verifying our data set and identifying missing papers were tested to explore how comprehensive our list was:
-
A review of the NETSCC and Researchfish data revealed that although the NETSCC data appeared to be more complete, Researchfish often provided additional publications for currently active projects, suggesting that it may be particularly useful in identifying more recent publications and that both sources were valuable in building a robust data set for this study.
-
An alternative approach to identifying HTA programme-funded publications would have been to build the data set up from individual papers that acknowledge the HTA programme as their source of funding. Since 2008, WoS has systematically recorded this information, when available, from papers. A brief search revealed more than 20 valid variants of the HTA programme’s name (e.g. UK HTA, HTA programme, NIHR HTA), and these variants retrieved around two-thirds of the number of papers in our existing data files for the covered time period, suggesting that many publications do not acknowledge the HTA programme in an easily recognisable form. Given this variation, and the fact that the acknowledgement data in WoS are available from only mid-2008 onwards, this approach was not considered a reliable and efficient method for contributing to the set of publications for this study.
-
For a small sample of projects we searched WoS for the associated grant number, for which there is also a specific field. However, grant numbers are not always provided in a consistent format in publications and this approach also proved unreliable.
Project level
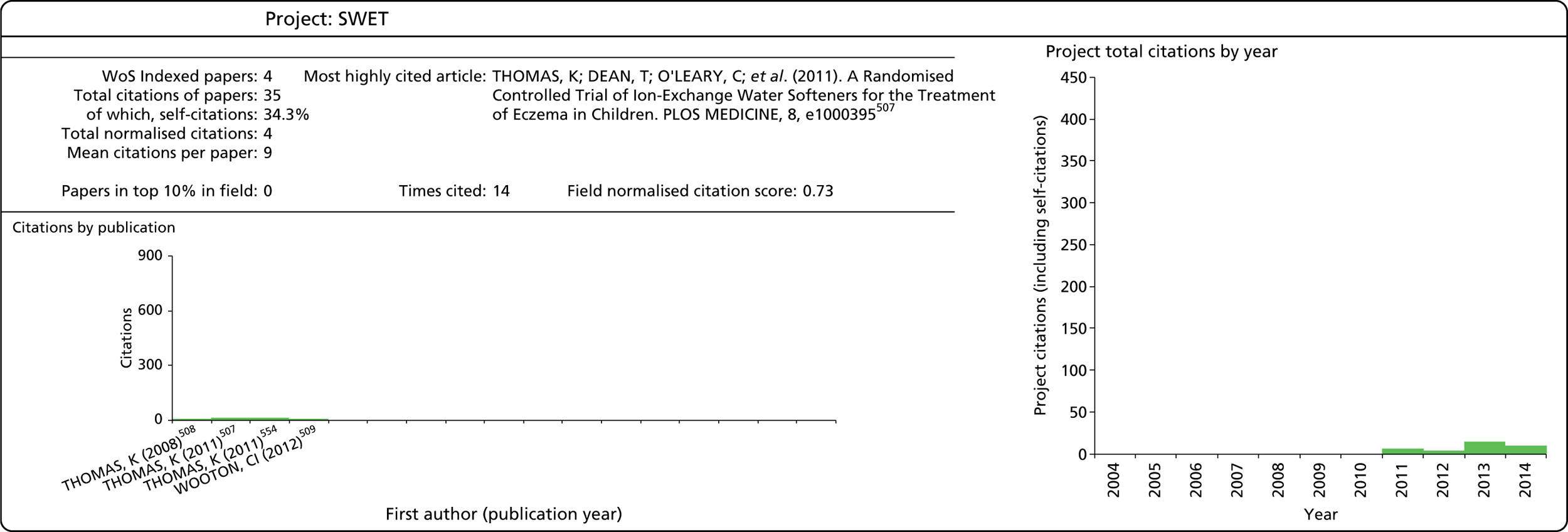
Publication lists for individual case study projects were initially compiled from the sources used at the programme level and a review of the CI’s publication record in WoS. The resulting list was then shared with the CI for verification.
Indicators
As noted above, it is useful to use a variety of bibliometric indicators to reflect different aspects of the HTA programme’s research output. A summary of these is provided in Table 4. A more in-depth discussion of the meaning and use of these indicators follows below.
| Indicator | Description |
|---|---|
| Number of publications | Number of individual publications produced |
| Field of publication | The field of the journal in which a paper appears, based on WoS subject categories; journals can be categorised to more than one field |
| Number of citations | Total number of citations |
| Field of citation | The field of the journal of a citing paper, based on WoS subject categories; journals can be categorised to more than one field |
| MNCS | Average number of citations, normalised according to each paper’s field and year of publication, relative to the world average |
| MNJS | The average number of citations received by articles in a journal in a field, relative to the world average |
| Papers in top 10% in field | Percentage (or number) of publications that belong to the most cited 10% of papers in their field |
| Collaboration | Articles including authors from more than one institution (based on all author addresses) |
| International collaboration | Articles with at least one author with an address outside the UK |
Number of publications
This indicator is calculated simply by counting the total number of publications attributable to the HTA programme or to a particular project. Only publications classified as an article or review in WoS are taken into account. Publications of other document types usually do not make a significant scientific contribution and are commonly excluded from bibliometric analysis.
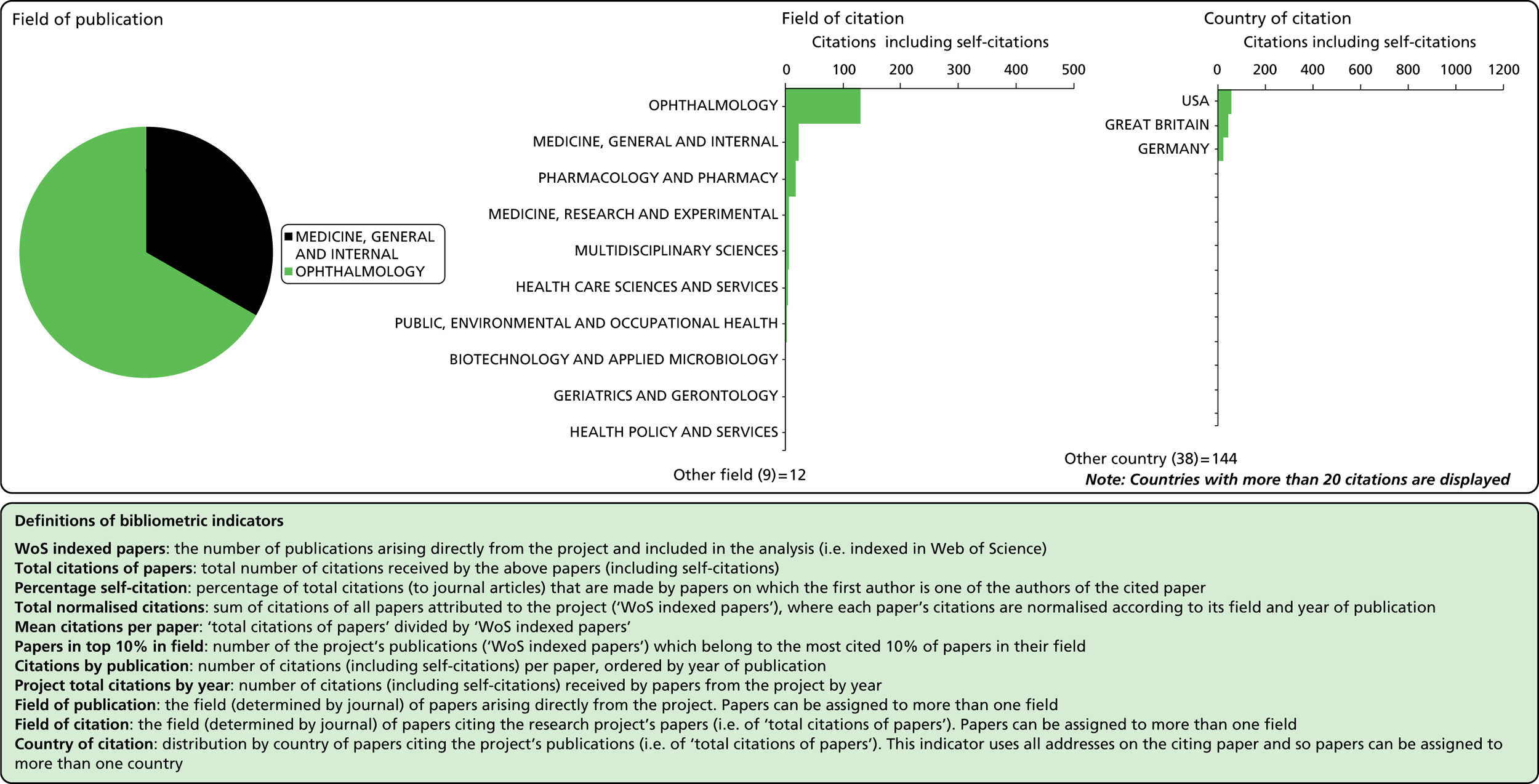
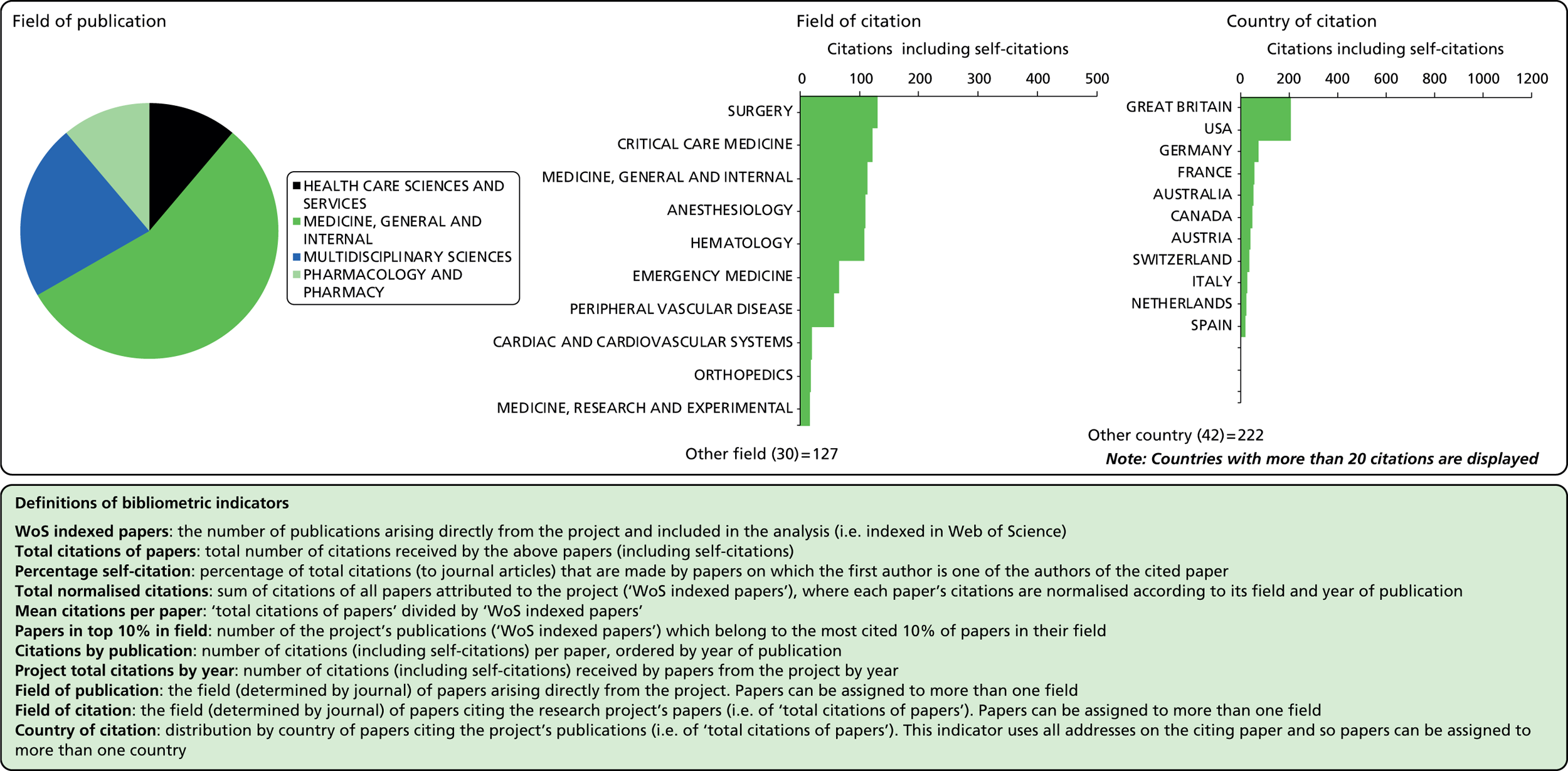
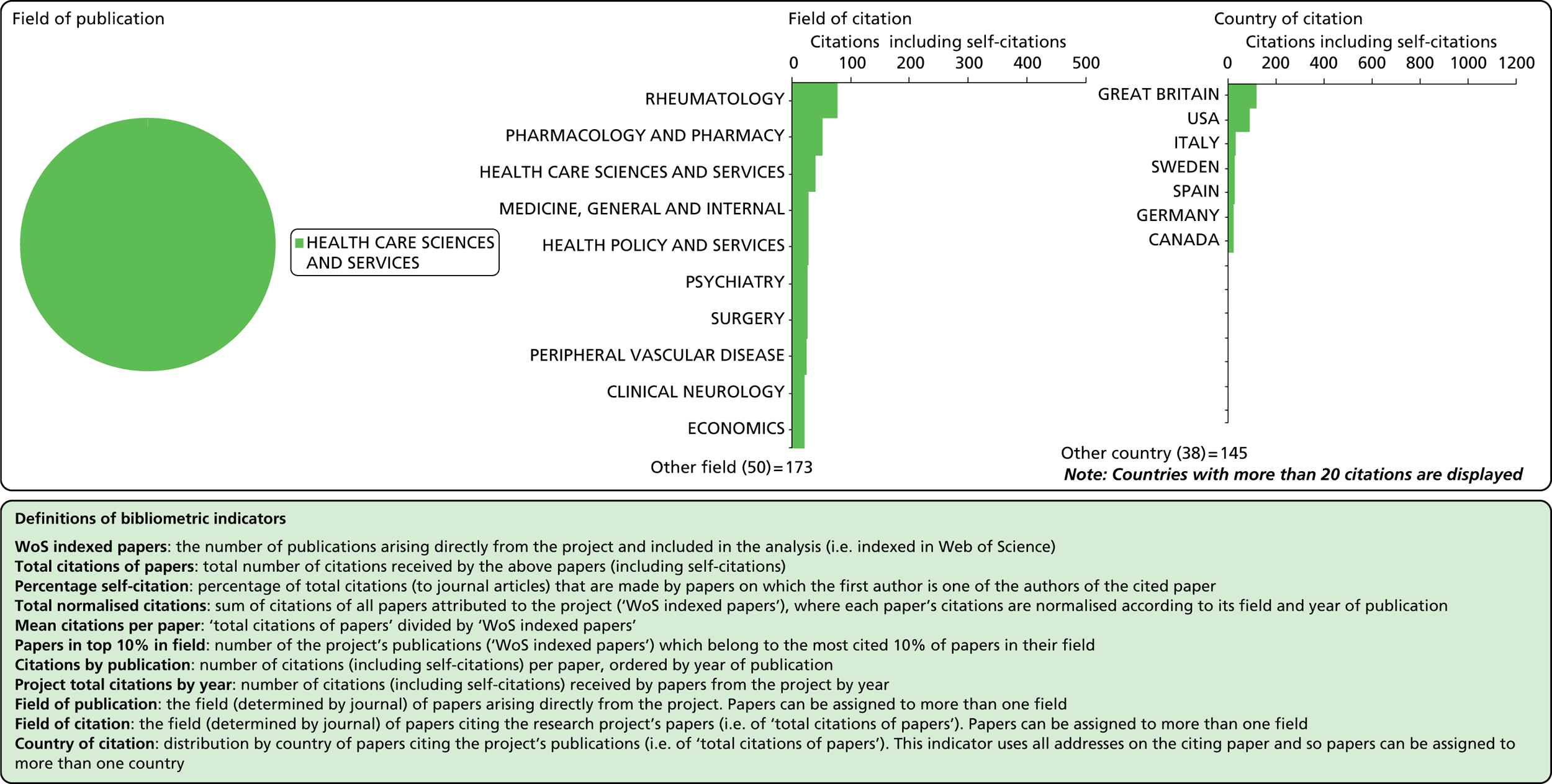
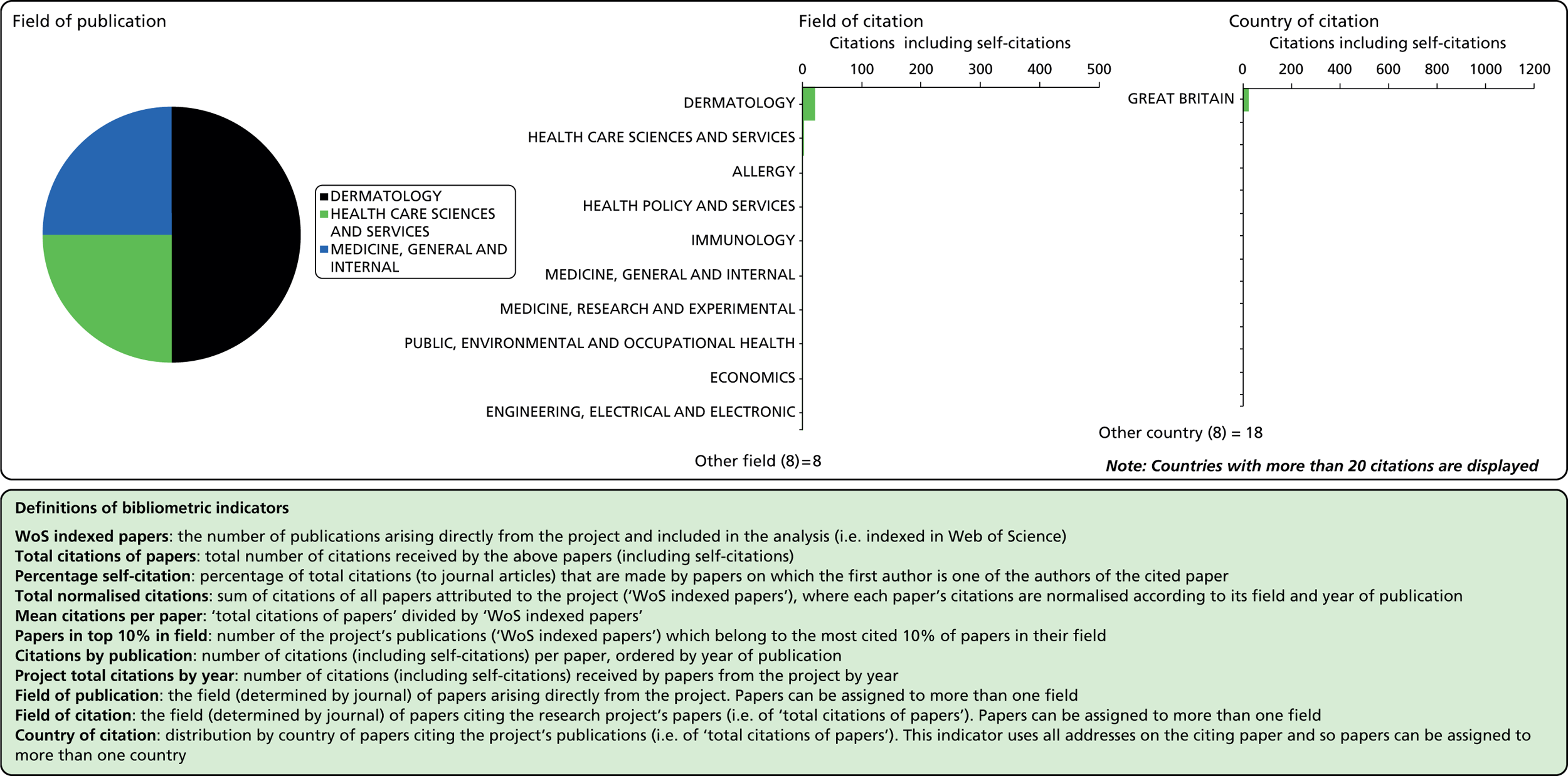
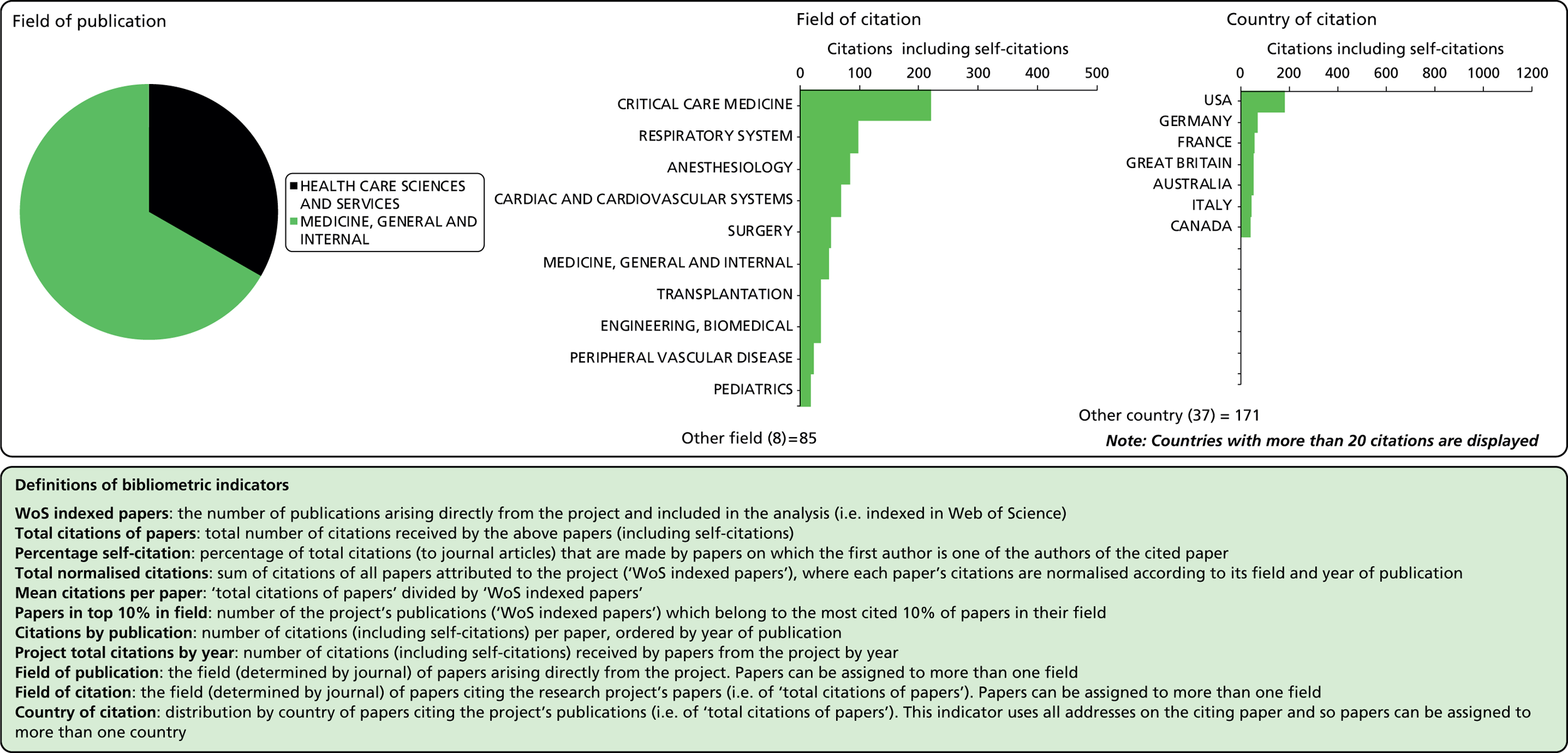
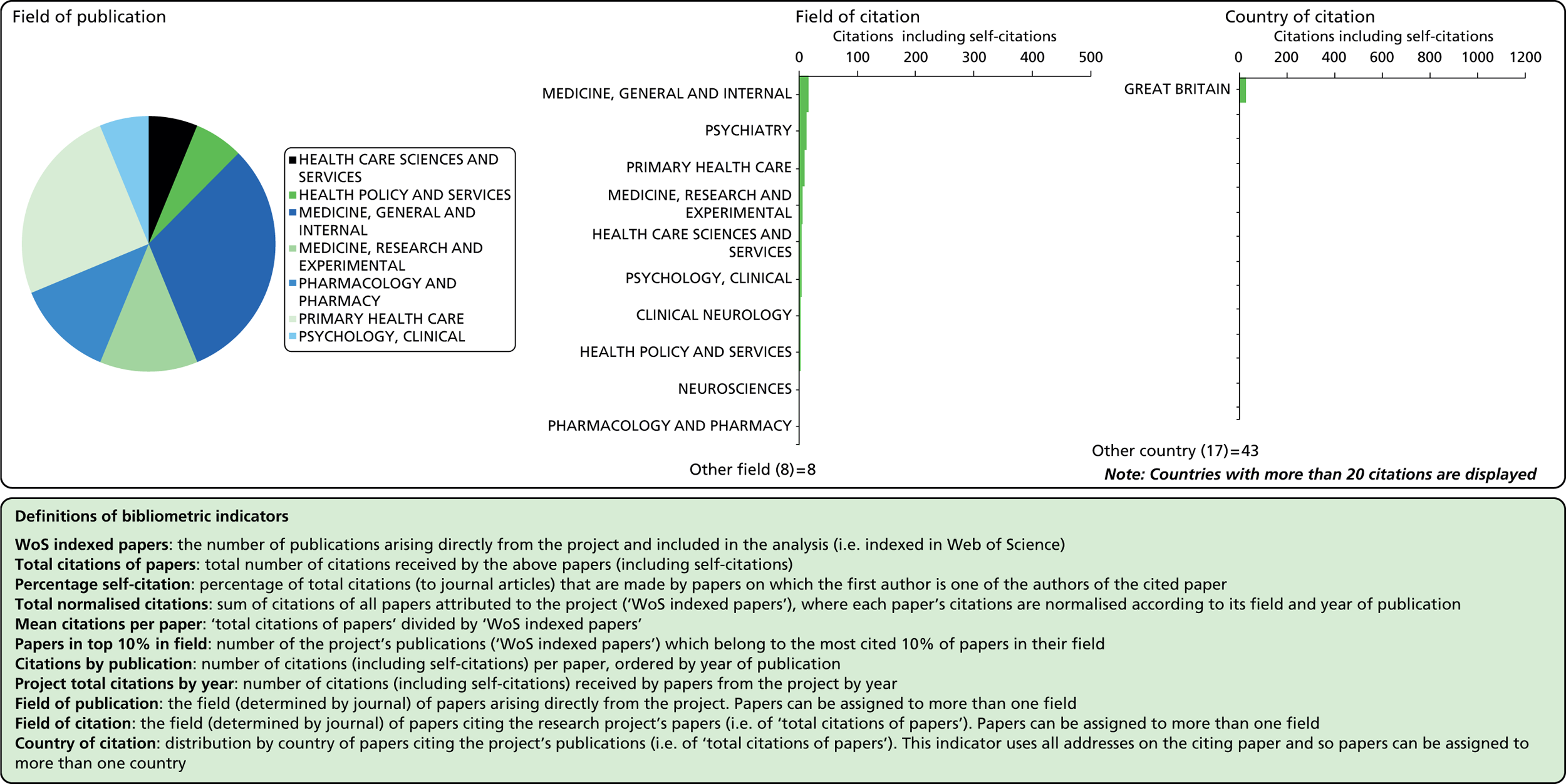
Field of publication
Each journal in WoS is assigned to one or more subject categories, of which there are approximately 250. These subject categories can be interpreted as scientific fields, and thus the field to which a paper belongs is determined by the journal in which it is published. Publications in multidisciplinary journals such as Nature, Proceedings of the National Academy of Sciences, and Science are individually allocated, if possible, to subject fields on the basis of their references.
Number of citations
This refers to the total number of citations that an article has received during the period 2004–13. In this analysis, a variable citation window has been used, i.e. the time period over which citations are counted varies according to the year of publication (papers published nearer the start of our study period have had more time to accumulate citations). Given this, it is important to also consider a normalised indicator that takes account of the variable citation window, as described below.
Field of citation
Considering the fields in which a particular paper has been cited can provide some indication of the diffusion of knowledge to different scientific fields. As noted above, the field of a paper is determined by the WoS subject category (or categories) to which the journal in which it appears has been assigned.
Mean Normalised Citation Score
Normalisation is applied to correct for differences in citation characteristics between publications from different scientific fields and between publications of different ages (in the case of a variable-length citation window). The normalised citation score of a publication is the ratio of the actual and the expected number of citations of the publication, for which the expected number of citations is defined as the average number of citations of all publications in WoS belonging to the same field and having the same publication year. The Mean Normalised Citation Score (MNCS) indicator is then obtained by taking the average of the normalised scores of all papers produced by a programme, researcher, institution, or other unit of analysis. If the MNCS has a value of ‘1’, it means that, on average, those publications have been cited as frequently as the world average for papers in their field and of similar age. Similarly, a score of ‘2’ would indicate that, on average, the papers are cited twice as often as would be expected for that field and publication year. A score of ‘< 1’ indicates a citation level that is below the world average.
Mean Normalised Journal Score
This indicator, closely related to the MNCS, is a measure of the visibility of the journals in which the papers of a researcher, institution, programme or other unit are published. The difference is that rather than using the actual number of citations of a paper, as in the MNCS, it uses the average number of citations of all articles published in a journal in a specified year. The interpretation of the Mean Normalised Journal Score (MNJS) indicator is analogous to the interpretation of the MNCS indicator. If a unit has a MNJS indicator of ‘1’, this means that, on average, papers appear in journals that are cited as frequently as would be expected based on the field to which they belong.
Papers in top 10% of field
Alongside the MNCS, we also look at this indicator as a measure of citation impact. It is the percentage of publications (of a programme, researcher, etc.) that belong to the most cited 10% of papers in their field published in the same year. For example, a research programme with 20% of its papers in the top 10% for its field would be doing twice as well as an average programme. This indicator is complementary to the MNCS, in that although both are measures of utility or impact, they are sensitive to different aspects of the distribution of the sample of papers. One of the weaknesses of the MNCS indicator is that it is sensitive to extreme outliers in the data; one very highly cited paper can skew the mean dramatically. However, a paper belongs to the top 10% if it is cited more frequently than 90% of similar papers, regardless of the actual number of citations. The weakness of this indicator is that there is a somewhat false dichotomy created between the top 10% and other papers, whereas at the boundary the actual difference in citation level can be very small. Considering it alongside the MNCS provides a more complete picture of the distribution of publications.
Indicators of collaboration
Indicators of scientific collaboration are based on an analysis of addresses listed in the publications produced by the research unit. Collaboration exists when authors are from more than one institution, and international collaboration exits when at least one of these institutions is outside the UK.
Analysis of Researchfish data
All HTA awards that were active from June 2003 onwards were taken as a sample for the portfolio of work funded by the HTA scheme. TARs are excluded from Researchfish, because the nature of the TARs, for which researchers often move therapeutic area with each piece of work, means that it would be very difficult for researchers to track the impact of any one piece of work. In addition, researchers will have involvement with many TARs, so it would be particularly burdensome to report on. We were able, however, to collect evidence about the impact of TARs through a case study.
Researchfish is an online questionnaire that enables research funders and research institutions to track the impacts of their investments, and researchers to log the outputs, outcomes and impacts of their work (www.researchfish.com). It is currently used by more than 90 funders in the UK and internationally to gather information from researchers about the outcomes from their work. The question set descends from the Arthritis Research UK’s RAND/ARC Impact Scoring System and MRC’s e-Val. 39
The project team used Researchfish, rather than a bespoke survey, because the NIHR already held data on the impact of HTA awards since 2009. This allowed us to reduce the burden on the HTA researchers by reducing the duplication of information requests. Similarly to other questionnaire-based data collection, Researchfish data have various limitations – which is why it was used in concert with other forms of evidence. The data are self-reported and therefore could be biased towards the categories of impact that the researcher deems most valuable. For researchers who are unfamiliar with the Researchfish interface, limited time may mean that they put in only a selection of their impacts. The project team were not aware of any research on the accuracy and completeness of Researchfish data.
The request for data went out to a total of 619 awards. The submission period in Researchfish was open for 5 weeks, between 6 August and 10 September 2014. The chief investigator (CI) for each award was sent a request to complete the survey, and two reminders while the survey was live. Overall, 109 awards responded to the request for data. Data – submitted for the annual NIHR submission in November 2013 – were already held on an additional 269 awards, and, subsequently, these were combined with data collected for the annual NIHR submission in November 2014, providing data on an additional 44 awards. Therefore, combining the sources, we had data on 68% (422/619 awards). A consequence of this data collection method, which builds on a precollected data set, is that our sample is likely to be biased towards more recent grants because they are required to report through Researchfish on an ongoing basis.
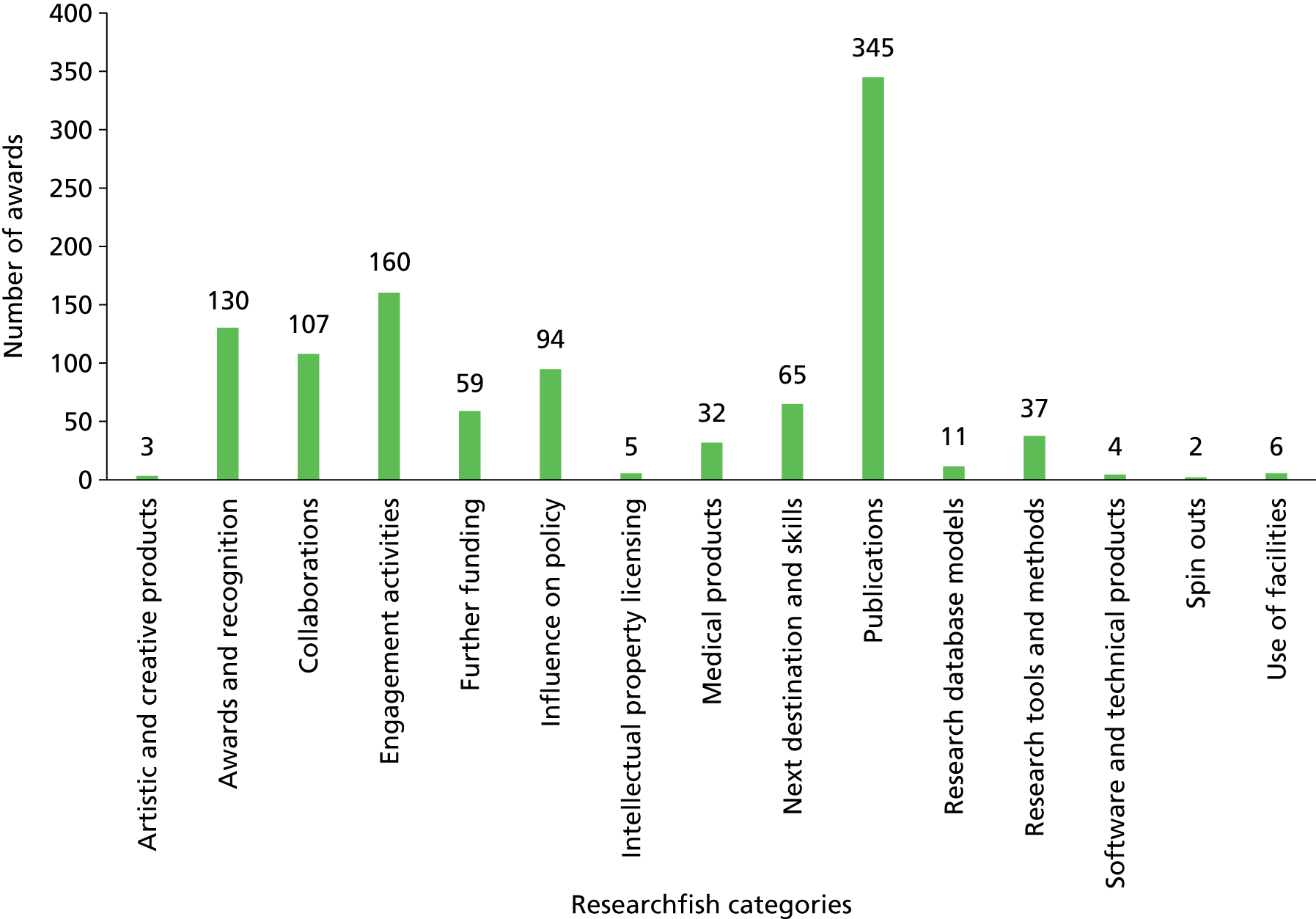
Researchfish is structured to allow researchers to provide data on a wide range of impacts (across all of the payback categories); however, there is no requirement for researchers to indicate ‘none’ in categories for which they have not had an impact. This means that it is not possible to calculate response rates for individual categories or questions. Overall, 2099 pieces of data were captured across the 13 categories of impact, as defined within Researchfish (Figures 1 and 2). We used Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA) to analyse the data on the impact of the portfolio. We also used the bibliometric information as a factor in the selection of case studies.
FIGURE 1.
Number of instances of each category of impact in Researchfish recorded for the NIHR HTA programme.

FIGURE 2.
Number of HTA awards reporting impacts in the Researchfish impact categories.
Case studies
Case study selection
We conducted 12 case studies with individual HTA projects as the unit of analysis for the case studies. As described at the start of the chapter, the case studies were conducted using a payback case study approach. Following the discussion with the advisory board, we decided to select a purposive sample of case studies of projects that we expected to have had high impacts across a range of different areas. The intention was that this would allow us to explore the range of impacts emerging from the HTA programme and the routes by which that impact occurred. One disadvantage of this selection approach was that it tells us less about cases in which research did not have an impact – to partially address this we examined the barriers to impact that our case studies had overcome. The selection approach also means that we cannot generalise from the case studies to extrapolate the overall impact of the HTA programme. In addition, case studies were chosen to reflect the diversity of the research funded through the programme. Overall, case study selection was based on four main criteria:
-
high impact
-
mix of primary research, evidence synthesis and TARs
-
mix of research areas
-
timing of the publication of final results.
Each of these is described in more detail below.
High impact
In order to identify relevant studies for inclusion, we compiled a long list of potential studies for inclusion from the following sources:
-
high-impact projects listed on the HTA website
-
projects highlighted at the NIHR HTA Conference 201340
-
projects highlighted by Raftery and Powell11
-
suggestions from our project advisory board
-
suggestions and examples from the key informant interviews
-
highly cited publications identified through the bibliometric analysis (among the top 15 most highly cited publications in the set of publications analysed, normalised for field)
-
projects with a range of impacts according to the Researchfish survey data.
We selected all of the case studies based on evidence that they have already had, or would likely have, a high level of impact in one or more of the following categories: knowledge production, capacity building, and policy and practice.
Mix of primary research, evidence synthesis and Technology Assessment Reports
Among the studies selected for in-depth analysis41–52 through case studies were two case studies focused on TARs,45,52 two focused on evidence synthesis42,47 and eight focused on primary research. 41,43,44,46,48–51 This distribution of case studies among the different research types funded by the HTA programme was chosen to reflect the focus of the programme over the last 10 years.
Mix of research areas
The case studies were also selected to cover the main research areas of the HTA programme: screening and diagnostics, surgery, medical devices, pharmaceuticals and mental health interventions. We included at least one, and no more than three, case studies from each of these groupings.
Timing of the publication of final results
An additional selection criterion was the timing of the studies. We required that all projects selected for inclusion had published their full study results, ideally as a final HTA journal article, at the time we started to conduct the case studies (September 2014). Initially, we considered focusing on older studies (e.g. studies that had their final HTA journal article published in 2010 or earlier) because of the time needed for research to be translated into impact. However, we decided to loosen this selection criterion for a number of reasons. First, from our work in the earlier stages of the project looking at the impact of the programme overall, and through our discussions with the advisory board, it became apparent that, in some instances, HTA research can have an impact shortly after completion, as it is typically very close to practice. In addition, the findings of HTA programme-funded research are often published in other journals well before the monograph is published in the HTA journal. Second, it became apparent that selecting an earlier publication deadline as an inclusion criterion would significantly limit our sample and would prevent us from including more recent larger studies, the size of which were previously unprecedented in the HTA programme’s history. These more recent, large studies were also longer in duration because of their scale, and hence have published their findings only recently. Even this looser criterion – that the key study findings should have been published – meant that we had to exclude some high-profile and potentially high-impact studies, such as the ProtecT Trial (Prostate testing for cancer and Treatment), which had been recommended to us by a number of sources. However, we felt that it was important that the full results of the trial were at least publicly available to conduct the case studies effectively.
The final sample of case studies selected is set out in Table 5, demonstrating the range of projects covered. More description of the individual projects selected is available in Chapter 3 (see Table 6).
| Case study | Project number | Primary | Evidence synthesis | MTA/STA | Screening/diagnostics | Pharmaceuticals | Surgery | Devices | Mental health | Other | Knowledge production | Capacity building | Policy/practice | Value of award (£) | Dates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
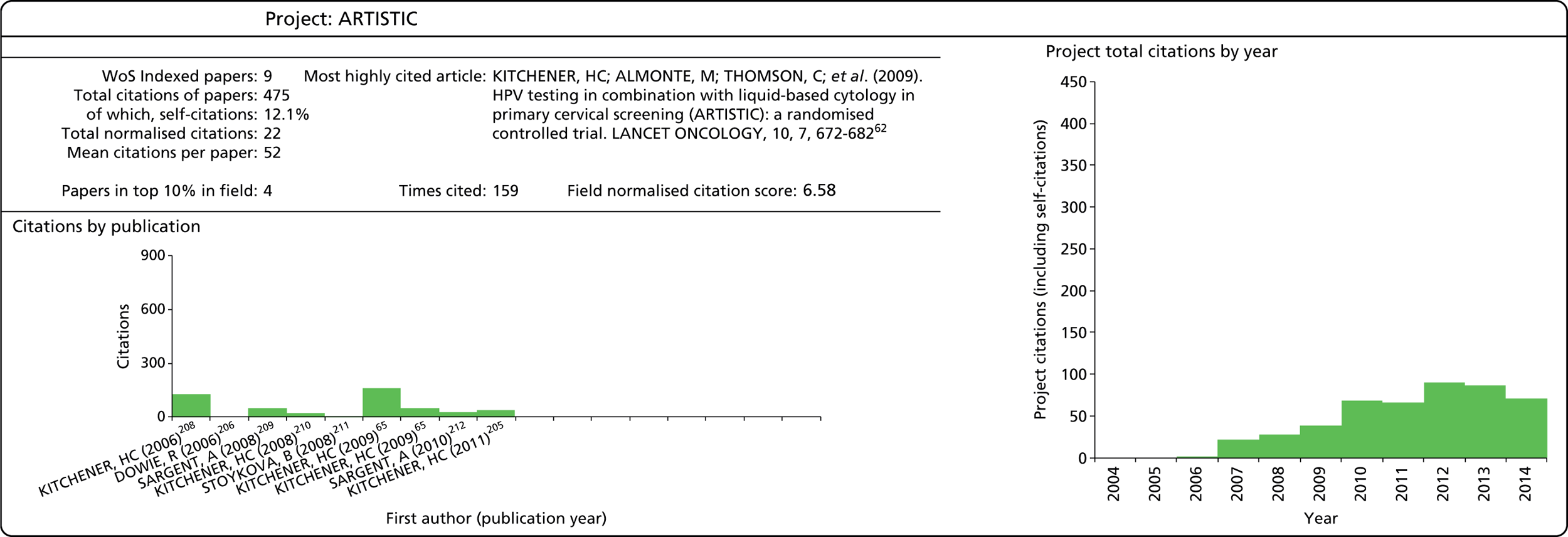
| ARTISTIC41 | 98/04/64 | ✗ | ✗ | ✗ | 1,187,000 | 2001–9 | |||||||||
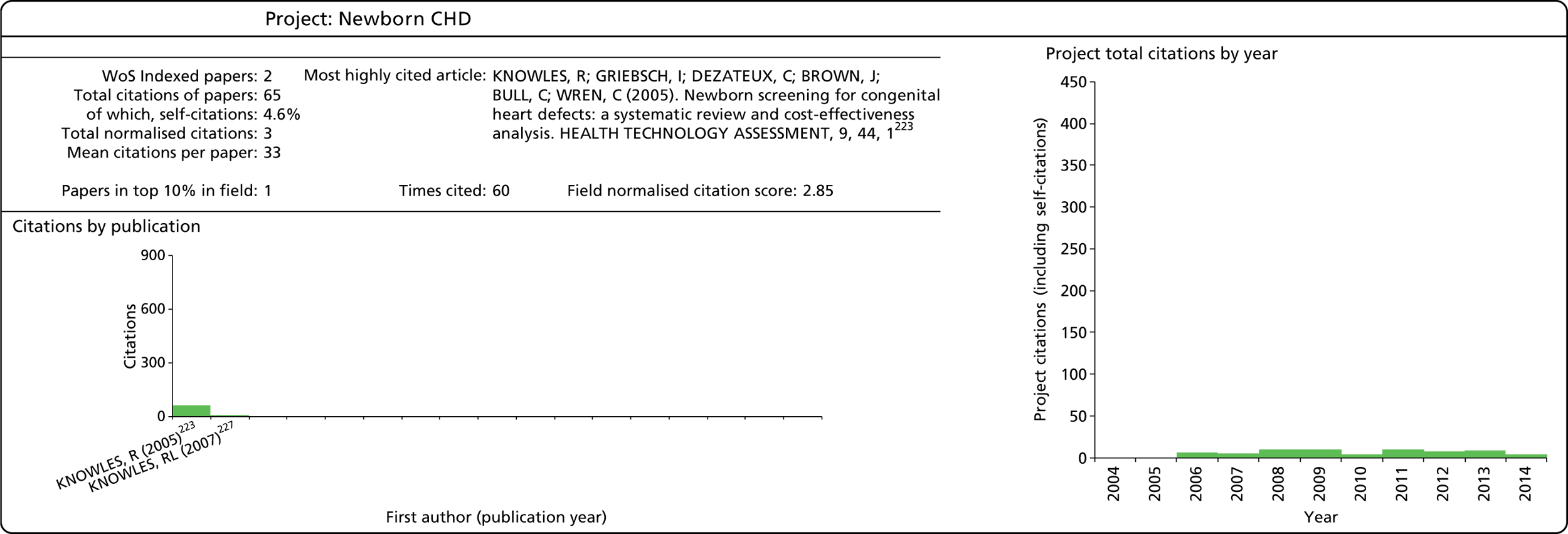
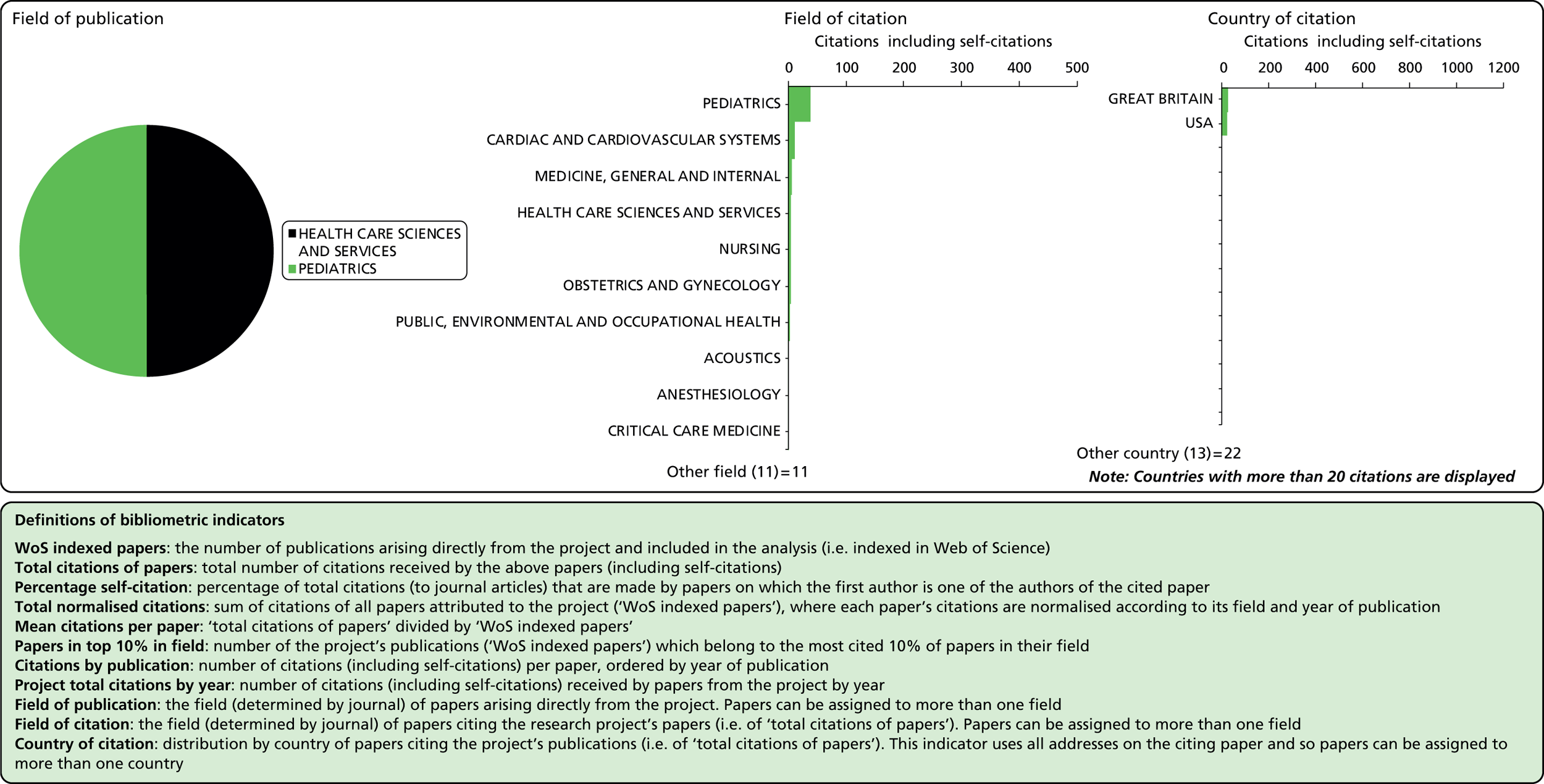
| Newborn CHD42 | 99/45/01 | ✗ | ✗ | ✗ | 60,000 | 2001–5 | |||||||||
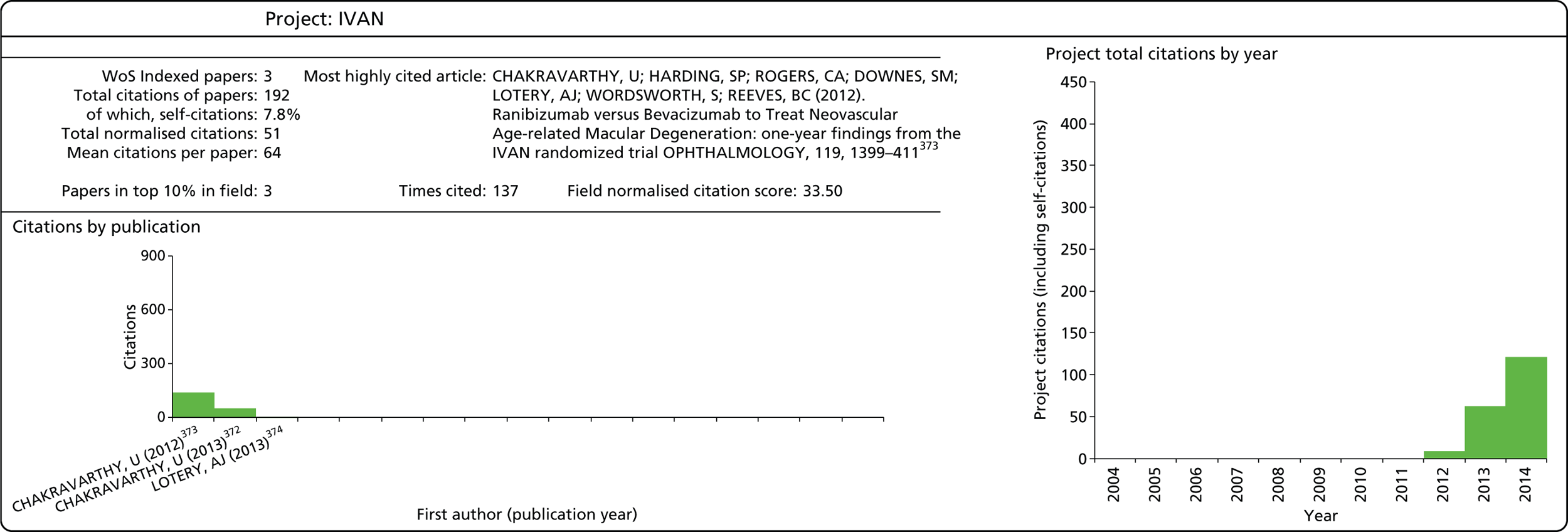
| IVAN43 | 07/36/01 | ✗ | ✗ | ✗ | ✗ | 3,346,000 | 2007–14 | ||||||||
| CRASH-244 | 06/303/20 | ✗ | ✗ | ✗ | ✗ | 2,546,000 | 2007–13 | ||||||||
| RA45 | 04/26/01 | ✗ | ✗ | ✗ | Unknowna | 2005–6 | |||||||||
| EVAR46 | 95/02/99 | ✗ | ✗ | ✗ | ✗ | 901,000 | 2005–12 | ||||||||
| Carotid Stenosis47 | 01/37/03 | ✗ | ✗ | ✗ | 111,000 | 2003–6 | |||||||||
| SWET48 | 05/16/01 | ✗ | ✗ | ✗ | 912,000 | 2006–11 | |||||||||
| CESAR49 | 99/01/01 | ✗ | ✗ | ✗ | ✗ | 1,425,000 | 2000–10 | ||||||||
| CoBalT50 | 06/404/02 | ✗ | ✗ | ✗ | 1,541,000 | 2008–14 | |||||||||
| CUtLASS51 | 96/19/06 | ✗ | ✗ | ✗ | 1,297,000 | 1999–2006 | |||||||||
| STAs at SHTAC/ScHARR-TAG52 | ✗ |
Conducting the case studies
A case study lead was identified for each case study, and this researcher led all data collection for that case study. In addition, a second person was assigned to provide support on each case study, to act as a sounding board for discussion and also ensure consistency of approach across all of the case studies. A total of five researchers conducted all of the case studies, with overlaps between researchers to ensure that best practice and learning was shared among the team.
We gathered data from two main sources: document review and interviews. When possible, we conducted interviews with the CIs for each study in the early stages of the data collection process to establish the key impacts of the study, the publications resulting from the study and relevant stakeholders for further interviews. If the CI was not available at an early stage, another key member of the study team (e.g. the clinical lead) was interviewed initially, and the CI was interviewed at a later stage if possible. Interview data were then supplemented by analysis of relevant documents, including published papers and reports, guidelines, policy documents, systematic reviews, meta-analyses, project records (such as end of grant reports) and, where available, original proposal documentation and curricula vitae (CVs) of project team members. As well as verifying and expanding upon information from interviews, the document analysis was used to identify key informants for further interviews.
Interviews were then conducted with members of the project team, policy-makers, clinicians and patient representatives. Interviews were semistructured, based on a protocol tailored to the payback framework, but adapted for the types of research conducted by the HTA programme (provided in Appendix 3). The full protocol was used only with the CI (or in the initial interview with a key member of the project team when the CI was not able to be contacted), but relevant sections were selected and supplemented as required for use with other informants, based on their expertise and the gaps in our knowledge. Interviews were conducted by telephone and were recorded with permission from the key informants. Recordings were confidential and for the use of the research team only, and were destroyed upon completion of the study. We conducted between two and seven interviews per case study, each lasting approximately 1 hour. The interviews, in turn, supported the identification of further relevant documentation for analysis. All interview participants were given the opportunity to review and comment on the final case study, and request that any quotations attributed to them in the case study were anonymised or removed.
In each of the case studies, we analysed the initial and long-term outputs and outcomes of HTA research, including translation of the research findings into the knowledge base or clinical practice. Through key informant interviews, we explored possible attribution problems by asking the studies’ CIs, and other key stakeholders, what would have happened in the absence of the HTA research. In particular, the case studies focused on the outcomes of the research for the NHS and patients.
The final results of each case study are presented in a standard template using the payback framework to enable comparability across all of the case studies. The full text of all of the case studies is provided in Appendix 4. We conducted a cross-cutting qualitative analysis of all of the case studies, using the impact categories described at the start of this chapter. The aim of the qualitative analysis of the case studies was to identify the key impact mechanisms associated with HTA research, as well as success factors, i.e. things that support the successful translation and implementation of the findings of HTA research. To conduct this analysis, we coded all of the case studies in NVivo using the codebook utilised for the analysis of interviews (to allow comparison with those findings). Each case study was coded by the lead researcher involved in conducting each case study, plus one other researcher from the study team. One researcher coded all case studies to ensure comparability and consistency in the use of the codebook. Coding was conducted iteratively, with potential new codes identified, discussed as a team, added where appropriate and coded across all case studies to ensure consistency. Before the coding began, the team met to discuss all of the codes to ensure a shared understanding of the coding approach. Coding was then compared between both researchers who were coding each case study in order to analyse the level of consistency and resolve any disagreements in coding. The final coded case studies were then examined, code by code, with two key topics in mind. First, the nature, range and extent of impact observed across the case study set. Second, the barriers and facilitators of that impact, with the intention of making observations around actions that the HTA programme might be able to take to increase those impacts in the future. The codes were examined iteratively through a process of separate analysis and team workshops to identify emerging themes and observations. The analysis was conducted in the context of the findings of the earlier stages of the project. All of the case study authors were involved in the workshops and the analysis process to ensure that the tacit knowledge obtained through conducting the case studies, which may not have been fully captured in the case study text, was included in the wider analysis.
Cross-cutting analysis
To develop our conclusions we integrated the evidence emerging from the interviews, bibliometrics, Researchfish and case studies. We did this by starting with the coded interview data for each stakeholder and type of impact. We then put the case study material coded against the same stakeholders and type of impacts alongside it, and looked for themes and parallels between the data sources. We took these emerging themes and checked them against what was emerging from closely related stakeholders and impacts. Finally, we added in relevant quantitative data from the bibliometrics and Researchfish data to identify themes that were supported across the different data sets.
Advisory board
The project was supported by an advisory board consisting of four members: Professor Ruairidh Milne, Professorial Fellow in Public Health and Consultant Adviser at the NETSCC; Professor Kieran Walshe, Professor of Health Policy and Management at Manchester Business School, and Associate Director of the NIHR Health Services and Delivery Research programme; Professor Stephen Hanney, Professorial Research Fellow at the Health Economics Research Group, Brunel University London, and one of the developers of the payback framework; and Lester Firkins, OBE, adviser in PPI in research.
The advisory board was consulted at three crucial stages of the project: at the start of the project, to finalise methods and approach; at the selection of case studies, after the wider project level data had been collected; and when data collection was completed to discuss the study findings and conclusions. The process of consultation consisted of half-day workshops at which ideas were presented and discussed among the whole study team, along with the four advisory board members.
The advisory board was the main route through which the views of patients and the public were taken into account. As noted, Lester Firkins was selected specifically to provide a public and patient perspective input to the work, and he was consulted through the three meetings and at other times on an ad hoc basis to support the development of study methods, the process of conducting the research, and the interpretation and presentation of the study findings. In particular, he provided significant input around our assessment of the use of PPI in the HTA programme, and advised on what would be considered good practice in terms of PPI. Lester Firkins has played a range of roles in the representation of patients and the public, including Co-chairperson of PPI governance for NETSCC, and Chairperson of Strategy Development for the JLA.
Chapter 3 The impact of the Health Technology Assessment projects
The primary route to impact for the HTA programme is through the projects that it supports. This chapter presents our findings on the project-based impacts of the HTA programme, categorised into the following impact areas: the NHS and patients, UK policy, academic, the research system, industry and the economy, and international.
To collect data that gave us an indication of the impact of all HTA programme-funded research, we used the interviews, bibliometric analysis and Researchfish data. To supplement this overview with a more detailed understanding of how that impact developed, we carried out 12 case studies of high-impact research projects, which we selected to illustrate the variety and range of impacts arising from HTA projects. Because of this selection framework, the results from the case studies are not generalisable to the entire portfolio of HTA research, but rather provide examples of the range and nature of the different impacts of the programme. The 12 case studies are briefly summarised in Table 6 and the full text of all of the case studies can be found in Appendix 4. Table 7 provides an overview of the impacts observed for the 12 case studies. The impacts resulting from HTA programme-funded research are discussed in more detail in the remainder of this chapter.
| Case study | Type of research | Field | Value of award (£) | Dates | Summary |
|---|---|---|---|---|---|
| ARTISTIC41 | Primary | Screening/diagnostics | 1,187,000 | 2001–9 | RCT into the effectiveness and cost-effectiveness of HPV testing in primary screening as either an adjunct to cytology or as a stand-alone test, compared with the current national screening programme, which relies on cytology alone |
| Newborn CHD42 | Evidence synthesis | Screening/diagnostics | 60,000 | 2001–5 | Systematic review and cost-effectiveness analysis of newborn screening for CHDs |
| IVAN43 | Primary | Pharmaceuticals | 3,346,000 | 2007–14 | RCT comparing two drugs for the treatment of wet AMD, a chronic and progressive condition that is the leading cause of sight loss in older people |
| CRASH-244 | Primary | Pharmaceuticals | 2,546,000 | 2007–13 | RCT investigating whether or not TXA could be used to treat trauma victims shortly after their injury and reduce their chance of dying |
| RA45 | TAR | Pharmaceuticals | Unknown | 2005–6 | MTA consisting of a systematic review and economic analysis of three drugs for the treatment of rheumatoid arthritis in adults |
| EVAR46 | Primary | Surgery | 901,000 | 2005–12 | RCT comparing the use of endovascular repair with existing treatments for the correction of AAA |
| Carotid Stenosis47 | Evidence synthesis | Screening/diagnostics | 111,000 | 2003–6 | Systematic review and modelling to determine whether or not novel non-invasive treatments were as effective as the traditional (invasive) therapy in diagnosing carotid stenosis with the aim of reducing the risk of stroke |
| SWET48 | Primary | Devices | 912,000 | 2006–11 | RCT to determine whether or not ion-exchange water softeners improve atopic eczema in children with moderate to severe eczema and the likely cost and cost-effectiveness of such an intervention |
| CESAR49 | Primary | Devices | 1,425,000 | 2000–10 | RCT of ECMO for severe adult respiratory failure, compared with standard care |
| CoBalT50 | Primary | Mental health | 1,541,000 | 2008–14 | RCT into use of CBT as an adjunct to usual care (including drug treatment) for TRD after initial treatment has failed |
| CUtLASS51 | Primary | Mental health | 1,297,000 | 1999–2006 | RCT comparing atypical antipsychotics to older, typical drugs for the treatment of schizophrenia |
| STAs52 | TAR | Overview of STAs and their impact (particular focus on Southampton and Sheffield TAR centres) |
| Case study | NHS and patients | UK policy | Academic/research system | Industry and the economy | International |
|---|---|---|---|---|---|
| ARTISTIC41 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Newborn CHD42 | ✗ | ✗ | ✗ | ✗ | ✗ |
| IVAN43 | ✗ | ✗ | ✗ | ||
| CRASH-244 | ✗ | ✗ | ✗ | ✗ | ✗ |
| RA45 | ✗ | ✗ | ✗ | ✗ | ✗ |
| EVAR46 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Carotid Stenosis47 | ✗ | ✗ | ✗ | ✗ | ✗ |
| SWET48 | ✗ | ✗ | ✗ | ✗ | |
| CESAR49 | ✗ | ✗ | ✗ | ✗ | ✗ |
| CoBalT50 | ✗ | ✗ | |||
| CUtLASS51 | ✗ | ✗ | ✗ | ✗ | |
| STAs52 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Total | 7 (4) | 10 | 12 | 7 (4) | 9 |
Impact on the NHS and patients
The primary route to impact of Health Technology Assessment programme-funded research is through guidelines, particularly National Institute for Health and Care Excellence and National Screening Committee guidelines
Interviewees reported that the primary route to impact of HTA programme-funded research on patients is through its impact on clinical guidelines, which, in turn, affects clinical care. If the HTA programme funds high-quality research that is then incorporated into clinical guidelines which clinicians follow; patients receiving that care can be said to have benefited from the initial research that led to the improvement in care. One interviewee thought that the impact of HTA programme-funded research on patient care was widespread (I19):
Somebody who is receiving treatment as a result of a NICE appraisal, based on an HTA assessment by the HTA programme, will be affected by the HTA programme but will have no idea of that.
I19
The fact that the HTA programme primarily has an impact on patients through clinical guidelines complicated the assessment of the impact of HTA programme-funded research because it is not possible to attribute that impact directly to the HTA programme, as both the underlying research and the resulting guidelines contributed to the observed impact. Two interviewees highlighted the challenges of attributing the impact of improved patient care as a result of better guidelines to the HTA programme. The interviewees reported that, when looking at the impact of HTA research on clinical practice, it is legitimate to look at the joint impact of NICE and the HTA programme (I4, I16). One interviewee described this joint impact in more detail:
They have helped each other considerably and probably have brought about a bigger impact than if they were working separately . . . They could exist without each other, but if NICE didn’t have the HTA then it would have to rely on what individual assessors have sent to drug companies, which is what happens in some other countries, and if NICE didn’t exist then the HTA programme would be producing reports that wouldn’t have the same traction.
I16
Another challenge in attributing the impact of HTA programme-funded research on patients to the HTA programme is that many users of HTA programme-funded research may not be aware that the HTA programme was the funder of the research. For example, users of scientific evidence do not always note the funder of research before reading and using the findings of research. Interviewees highlighted that many clinicians have heard of the HTA programme but that it is likely that their clinical practice has been influenced by the research funded by the HTA programme (I8, I19, I9). For example, one interviewee noted:
I think the impact is through guidelines. I don’t think the average clinician had the slightest idea what the HTA was, had never heard of it, would never look it up, directly. Physicians got HTA research results indirectly. They are following many of the things that HTA might have suggested.
I8
One interviewee felt that the attribution challenges resulting from the lack of awareness of the HTA programme among users of HTA programme-funded research may be more widespread, extending beyond clinicians to all users of HTA programme-funded research. The interviewee reported that the HTA programme directly or indirectly influences everyone who has an interest in the clinical effectiveness, cost-effectiveness and wider impacts of health technologies, whether or not those users of HTA research are aware of the HTA programme (I19).
Individual pieces of Health Technology Assessment programme-funded research can have an impact on the NHS and patients
More than half of the case studies (7 out of 12) provided some evidence that HTA programme-funded research has an impact on the NHS and patients, which is consistent with the findings reported by interviewees. In addition to the seven case studies41,42,44–46,49,52 that had an impact on the NHS and patients, four studies47,48,50,51 included limited evidence of changes in clinical practice.
The observed impacts included health benefits for patients; an increase in patient choice; and potential cost savings. Two case studies [ARTISTIC (A Randomised Trial of HPV testing in primary cervical screening)41 and Newborn CHD42 (Newborn Screening for congenital heart defects)] will probably have a significant impact on patients and the NHS in future, as they have both led to screening pilots that may eventually be implemented nationally. We also identified two cases where HTA programme-funded research confirmed the appropriateness of existing treatment [CoBalT (Cognitive Behavioural Therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care trial)50 and SWET (Softened Water Eczema Trial)48]. However, identifying the impact of studies that provide the evidence for existing practice is challenging because changes in clinical practice are unlikely to occur. Finally, two studies [IVAN (A randomised controlled trial of alternative treatments to Inhibit VEGF in patients with Age-related choroidal Neovascularisation)43 and CUtLASS (Cost Utility of the Latest Antipsychotics in Severe Schizophrenia trial)51] demonstrated potential for impact on the NHS and patients but barriers to that impact exist within the health-care system (see External factors can influence the implementation of HTA-funded research, below). The impacts of the case studies on the NHS and patients are summarised in Table 8 and described in more detail in the following sections.
| Name of case study | Evidence of impact | Summary of impact on the NHS and patients |
|---|---|---|
| ARTISTIC41 | ✗ | Limited use in small pilot, but likely health benefits for those screened |
| Newborn CHD42 | ✗ | Some use in the NHS (around 20%), but likely health benefits for those screened |
| IVAN43 | ✗ | Possible slight cost reduction due to renegotiation of Lucentis (ranibizumab; Roche and Novartis) price; Avastin (manufactured by Roche) not generally available in the NHS |
| CRASH-244 | ✗ | TXA now used widely in trauma, reasonable to assume health benefits and link to this research is strong |
| RA45 | ✗ | Increased patient choice since more drug options available |
| EVAR46 | ✗ | Increased patient choice; endovascular aneurysm repair preferred by patients and now widely available |
| Carotid Stenosis47 | ✗ | Reduced incidence of stroke, benefit to patients, but evidence around implementation levels weak |
| SWET48 | ✗ | Possible cost savings for patients |
| CESAR49 | ✗ | Health benefits from the use of ECMO for patients with respiratory failure in specialised ECMO centres |
| CoBalT50 | ✗ | May be limited, use of longer CBT treatment suggested |
| CUtLASS51 | ✗ | Reasonable to assume benefits to patients from improved dosing and reduced polypharmacy, but link to this study may be weak |
| STAs52 | ✗ | Level of implementation of guidance changes will differ but there are likely to be some changes in NHS practice impacting on patient choice |
Three of the case studies demonstrated evidence of health benefits for patients through implementation in the NHS
Three of the case studies44,47,49 found evidence that use in the NHS had led to health benefits for patients. For example, the drug tranexamic acid (TXA), which was investigated in the CRASH-2 trial44 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage), is now widely used in trauma. Based on the trial results, it is reasonable to assume that use of TXA in trauma has led to survival benefits for patients. The link between the CRASH-2 study44 and changes in clinical practice is strong because it is the only major trial44 that has investigated the use of TXA for trauma. The link is also plausible because the CI has been actively involved in the dissemination and implementation of the findings. Similarly, it is reasonable to assume that the CESAR study49 (Conventional ventilator support vs. Extracorporeal membrane oxygenation for Severe Adult Respiratory failure trial) has also led to survival benefits for patients with respiratory failure treated in ECMO centres in the UK. The link between the CESAR trial49 and the survival benefits is also strong because it was that trial, together with a subsequent study of extracorporeal membrane oxygenation (ECMO) during the H1N1 epidemic (a worldwide pandemic in 2009, caused by the H1N1 flu virus), which led to the establishment of the five adult ECMO centres in the UK. The Carotid Stenosis study47 maybe also have contributed to a reduced incidence of stroke, but the level of implementation of the findings from that study is less clear.
In addition, there is evidence that the Newborn CHD42 study, together with the PulseOx trial (see Appendix 4.2), has led to changes in newborn screening for CHD. Results from a survey found that the pulse oximetry screening is already being used in 20% of paediatric units. However, the attribution of the observed changes in screening practices in the paediatric units cannot be exclusively attributed to the Newborn CHD42 systematic review, as that study then resulted in the PulseOx trial (see Appendix 4.2), such that it is possible to say that only these two HTA programme-funded studies jointly contributed to the observed changes in screening practice.
The ARTISTIC trial41 has had a limited impact on the NHS to date. The study41 played an important role in the development of a pilot on the use of human papillomavirus (HPV) screening by the NSC, and where used, the evidence from the study41 and a pooled analysis of a wider data set suggest that there are likely to be health benefits from the use of HPV screening. However, the use of HPV screening is still in the pilot phase and the level of implementation is confined to individuals in the pilot.
The evidence on the impact of the CoBalT50 and CUtLASS51 case studies is also less clear. The CoBalT study50 may have influenced the practice of a limited number of clinicians who are familiar with the study,50 and this is likely to have been only a subtle adjustment in the way in which practice is implemented [using 18 sessions of cognitive–behavioural therapy (CBT) as an adjunct to primary treatment, rather than 12 sessions]. There is some suggestion that the CUtLASS study51 led to more careful prescribing of antipsychotics, for example with reduced dosing levels and reduced use of multiple drugs. If so, this may have improved patient health by reducing the side effects of these drugs. However, the evidence linking this to the study51 is not strong.
Three case studies described an impact on patient choice
There are also a number of studies that have had an impact through increased patient choice, which has a positive impact on quality of care, as treatments may have different side effects for different patients or may fit better with other aspects of a patient’s lifestyle. For example, the EVAR study46 (EndoVascular Aneurysm Repair) provided evidence that endovascular aneurysm repair treatment was safe to be used instead of open repair for the treatment of abdominal aortic aneurysm (AAA). It was one of several studies looking at this issue but there is evidence from the case study46 that this particular study was important in changing practice. Endovascular aneurysm repair is generally preferred by patients, and this study46 was important in allowing them to access this treatment option. The Rheumatoid Arthritis (RA) study45 also broadened patient choice, in terms of the drugs available for the treatment of rheumatoid arthritis. This study45 was a MTA TAR that fed directly into NICE decision-making and resulted in the inclusion of additional drugs in the NICE guidance, making them available on the NHS. Typically, STAs are likely to increase patient choice, as they are reviews of manufacturers’ submissions regarding new drugs that they would like to introduce to the UK market. Based on the STA evidence, and other inputs, NICE will determine whether or not the drug is effective and cost-effective, and hence whether or not it can be made available in the NHS. Because STAs do not compare drugs, it is most likely that the result will be the introduction (or not) of a new drug, rather than the replacement of an existing treatment, so the impact is most likely to be an increase in patient choice.
Two case studies indicated some limited evidence of potential cost savings
The IVAN study43 is likely to have played a role in saving the NHS money by contributing to a reduction in the cost of Lucentis (ranibizumab; Roche and Novartis) to the NHS through renegotiations, although this is nowhere near as significant as the potential cost savings that could result from the findings of this study43 as described below.
The SWET48 did not find any evidence that water softeners are effective for the treatment of atopic eczema in children, and there is some suggestion that this could potentially save patients money, as they can avoid spending money on treatments that do not work. However, evidence is not available around the level of sales before and after the study,48 so the extent to which this impact has been realised is unclear.
Two case studies demonstrated that existing NHS practice is appropriate and hence it is particularly difficult to identify their impact
In both of the SWET48 and CoBalT50 studies, the results largely confirmed that existing NHS practice and corresponding guidance is correct and, as such, it is difficult to see the impact that they have had on practice. In the case of CoBalT,50 there is some scope for minor adjustment of the details of implementation of CBT in terms of the number of sessions offered, but in SWET48 the results demonstrated that water softeners that were not widely used in the treatment of eczema, and certainly not funded by the NHS for the treatment of eczema, are not effective. However, this does not mean that the two studies48,50 do not have value: they provided evidence that confirmed that existing practice is appropriate. However, it is not possible or appropriate for either study48,50 to have a significant visible impact on changing NHS practice.
There is some evidence that Health Technology Assessment programme-funded research can have a direct impact on patient care
In some cases, clinicians may change clinical practice as a direct result of HTA programme-funded research if the effect demonstrated in the study is sufficiently large and they are aware of the study findings. One interviewee reported that widespread dissemination of the study results, or publicity of the results, may lead to adoption of the new technology before guidelines have been updated. Another potential direct impact that HTA programme-funded research may have on patient care is by improving the skills of clinicians in the NHS through their participation in research. One interviewee suggested that if clinical trials increase the skills of clinicians then that increase in skill is likely to have a positive impact on patient care.
The case studies found evidence that implementation of HTA programme-funded research sometimes occurs outside the conventional pathways (not via NICE or the NSC). For example, TXA in trauma is being used in the NHS as a result of the CRASH-2 study44 findings, even although TXA for trauma has not yet been included in NICE guidelines (as described in more detail below). The inclusion of TXA for trauma in the Joint Royal Colleges Ambulance Liaison Committee guidelines, and clinicians’ use of TXA off-label, has facilitated adoption in the NHS. However, the research team has also been actively involved in the widespread dissemination of the research findings, as described in the next section. A number of interviewees noted that the HTA programme’s framework for dissemination is not designed to support widespread dissemination (I14, I8, I9, I13) – one commented that it is ‘essentially an academic one’ (I9) – assuming that impacts on practice will occur through guideline bodies.
Data from Researchfish show that > 70% of dissemination activities recorded consisted of a talk or presentation, or attending a scientific meeting (Figure 3). However, although academics were a key audience for dissemination (n = 128), the largest group targeted by dissemination activities listed in Researchfish were health professionals and practitioners (n = 463), suggesting that HTA researchers are more active in direct dissemination than our interviewees might have been aware (Figure 4).
FIGURE 3.
Dissemination activities recorded in Researchfish.
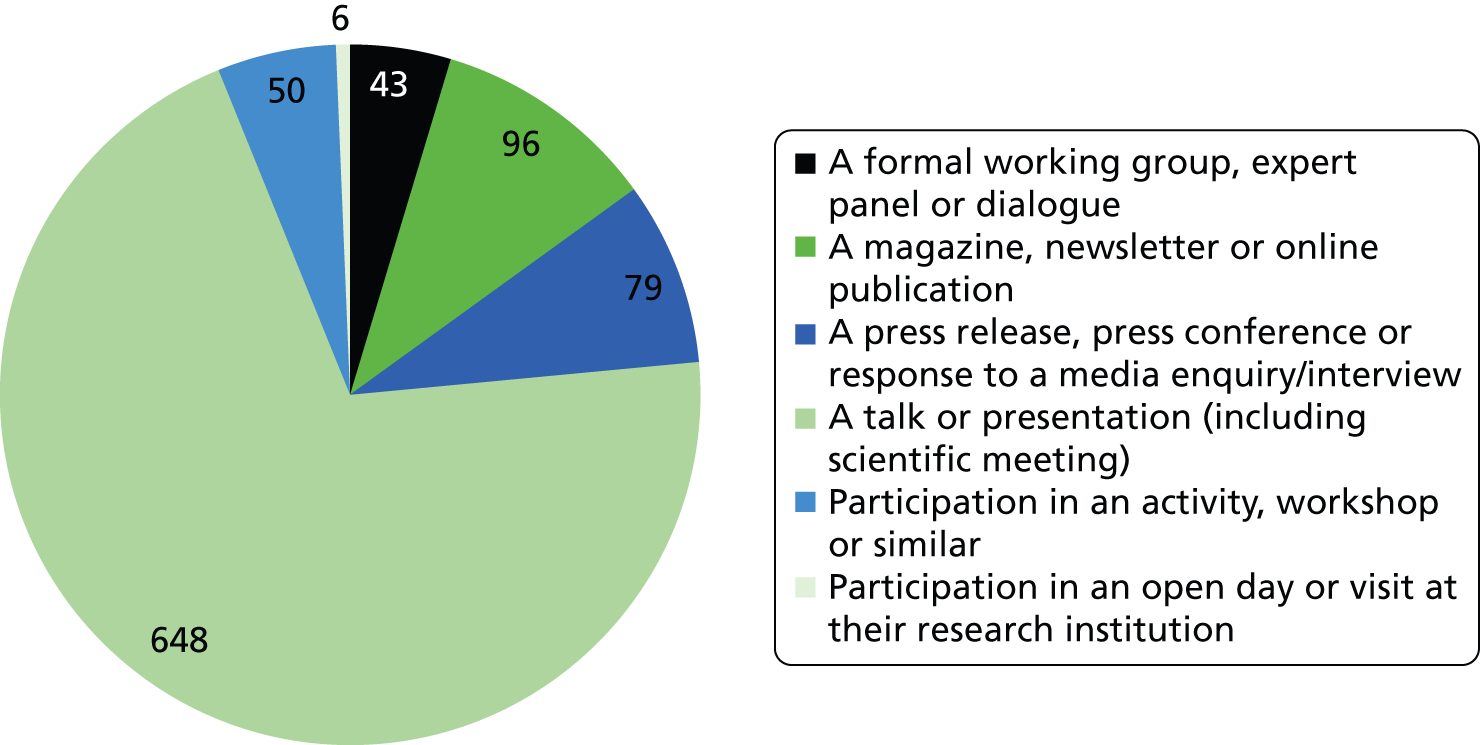
FIGURE 4.
Audiences for dissemination activities recorded in Researchfish.
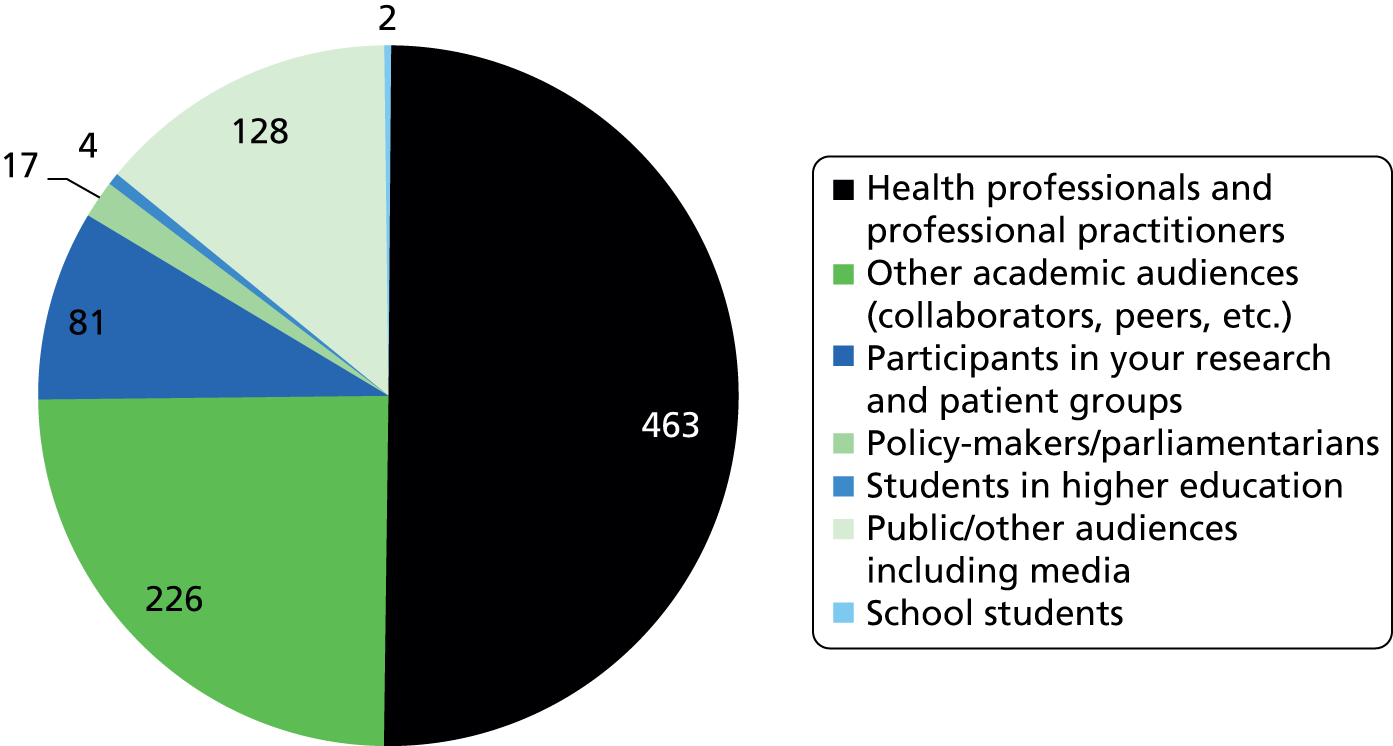
The HTA programme is not involved in the direct implementation of HTA research in clinical practice, but rather funds research that is intended to provide evidence for clinical practice in the NHS. Although the HTA programme is implementation focused in terms of the research topics it funds, the active implementation of research is currently outside its remit as a research programme. Some interviewees viewed the division of labour between the HTA programme as a funder of research and the NHS as an implementer as desirable, although one interviewee disagreed with the view that the HTA programme receives funding to do research and that the NHS receives money to implement new technologies:
Unless the HTA programme is thinking about implementation while it’s doing its work and developing implementation strategies, it’s less likely that things will get picked up.
I8
In order for HTA programme-funded research to be implemented in the NHS, the research must be implementation focused, such that the findings are useful to those who manage and provide care in the NHS. However, the challenge of translating research into clinical practice is not unique to the HTA programme. Interviewees stressed that implementation of research (from any source) in the NHS is challenging and takes time (I2, I12, I19).
However, it is possible for the HTA programme and researchers to influence the ease with which HTA research is adopted in the NHS, particularly through dissemination. One interviewee noted that the HTA programme relies too much on the NIHR’s existing communication channels and that it does not make adequate use of other communication channels, such as the clinical and professional networks of HTA programme-funded researchers (I12). Some interviewees reported that the challenges the HTA programme faces in terms of dissemination apply to the NIHR as a whole, rather than to just the HTA programme (I15, I13), and that the new NIHR dissemination centre may address some of the dissemination challenges that the programme faces (I6).
However, one interviewee noted that the primary objective of the HTA programme, as part of the NIHR, is to inform decisions that related to practice within the NHS, whereas the primary objective of the Policy Research Programme is to inform wider policy-related decisions (e.g. how best to commission services) (I19). The results of this divisions between the two research funders is that the main audience of HTA research is patients, clinicians, managers and organisations at the meta-level, such as NICE, which makes decisions on their behalf. That interviewee suggested that the HTA programme’s dissemination strategy should target clinicians and patients directly:
The HTA programme is designed to inform decision-making by clinicians, managers and patients in the NHS. So, the way that it does that through the policy-makers is by influencing NICE who influences decision-makers, but the whole idea is to go straight to the decision-makers so that clinicians are aware of the latest evidence in their field . . . It is the professionals that will know that and, increasingly, the patients that they treat, which is why [the HTA programme] is very keen on plain English summaries of research.
I19
Funding for the dissemination of research was identified as a challenge in many of the case studies. In some of the case studies, dissemination of the research findings seems to have been impeded by a lack of incentive to implement the research findings in the NHS or, in some cases, the opposition to their implementation by some stakeholders. We discuss this issue in detail in the next section.
There are particular issues associated with the dissemination of the research findings from TARs, which are unique to the TAR programme. For example, members of the RA45 team were not clear whether or not it was appropriate to actively disseminate their research findings or whether or not the research was intended solely to inform NICE decisions. In the TAR centre case study, the TAR team’s desire to disseminate their work was also constrained by the continual change in focus and research topics within the centre and a lack of time for dissemination of older studies while carrying out new ones.
It is plausible that HTA programme-funded research could lead to improved care in the NHS by improving the skills and knowledge of clinicians. Interviewees also suggested that HTA research could lead to direct improvements in health through participation in research, which one interviewee suggested could have an impact on the overall quality of care provided in the NHS because of the volume of research funded in the NHS. However, another interviewee noted that there is insufficient evidence on whether or not participation in research leads to improved skills among clinicians and whether or not it has a significant effect on patient care (I14).
External factors can influence the implementation of Health Technology Assessment programme-funded research
Wider system factors and the priorities of other stakeholders can have a substantial influence on the impact of HTA research. Looking across our case studies, we identified five ways in which this can happen: findings that challenge commercial interests; findings that are in stark contrast with what is expected; changes in the commissioning structure of the NHS; changes or uncertainties around the cost of implementation; and factors completely outside the health system.
Findings that challenge commercial interests
Two of our case studies43,44 had findings that challenged commercial interests – this meant there was no obvious industry stakeholder to champion the dissemination and adoption of the new approach.
The IVAN study43 compared two drugs for the treatment of wet age-related macular degeneration (AMD), a chronic and progressive condition that is the leading cause of sight loss in older people. The study43 found that the two drugs, Lucentis and Avastin (manufactured by Roche), were equally effective, but that Avastin, which is much less expensive, was more cost-effective. However, Lucentis was already approved for use in the UK (and several other countries), and, as both drugs were marketed by the same company, there was no industry incentive to pursue a marketing authorisation, meaning that NICE has not reviewed Avastin and it is not widely used in the NHS. This is also partly because there have been threats of legal action from industry when Avastin has been used in the UK and internationally. So, although the study43 has the potential to result in significant cost savings for the NHS, these have largely yet to be realised.
The CRASH-244 study provides a contrasting example. Here, despite a lack of incentives for industry to act, there has been widespread uptake of the findings. This can largely be attributed to members of the study44 team being extremely active in the dissemination and implementation of the findings, largely without additional support (they received a small dissemination grant from the HTA programme but indicated that it was not sufficient to cover the costs of their efforts). It was noted specifically by one member of CRASH-2 study44 team that, compared with delivering the trial, the dissemination and implementation stage, which is largely unfunded, has been particularly time-consuming; they contrasted this with the approach for new products launched by the pharmaceutical industry.
Findings in stark contrast with expectations
Another study51 produced results that were in stark contrast with the expectations of the researchers, industry, clinicians and patient groups. The results of the CUtLASS study51 showed that atypical antipsychotic drugs offered no clear advantage over the older, typical drugs that had been used for the treatment of psychosis. Although NICE recommendations were changed as a result, just prior to and during the early stages of the study,51 there had been a dramatic increase in the use of atypical antipsychotics, amidst a general belief in the field that they would be superior in both effectiveness and reducing side effects. Our case study51 interviewees suggested that the study results, although supported by a similar study in the USA, have probably had little impact on reversing this trend and have had little effect on the choice of drug that clinicians make. The study51 may have had some impact on prescribing practices around dosing and use of multiple drugs, but interviewees told us it had not led, and is unlikely to lead, to a shift back to the use of the older typical drugs.
The converse example is the EVAR study,46 in which the uptake of findings was helped by the fact that they aligned with the preferences expressed by patients and clinicians along with other stakeholders.
Particularly in cases in which the findings run counter to expectations, the extent to which other studies are available, which support the findings of the work, can also affect the credibility and subsequent impact of those studies. This may be particularly important when findings are unexpected or run counter to the wishes of stakeholders. For example, the findings of the CUtLASS51 and IVAN43 studies were both controversial with some stakeholder groups, so the fact that other large studies in the USA showed similar findings lent important credibility to their outcomes.
Changes in the commissioning structure of the NHS
Two case studies46,49 showed the contrasting ways in which changes in the commissioning and administrative structure of the NHS can affect the impact of the study. In the case of the CESAR study,49 which studied ECMO for severe adult respiratory failure, the devolution of clinical commissioning to regional areas complicated the provision of ECMO in the UK. This devolution occurred as part of the Health and Social Care Act. Prior to the devolution, ECMO services were commissioned at a national level. However, after the devolution, payment for patients transferred between hospital regions or ECMO became more complicated. In contrast, for the EVAR study,46 uptake in the UK has been influenced by reconfiguration of vascular surgery services over the last few years. Inequitable provision led to a government decision to focus services into a limited number of high-volume centres. Combined with a parallel quality improvement programme for aortic surgery, which included the recommendation that centres that are unable to offer endovascular aneurysm repair as a treatment option should be able to show that they could refer patients to another centre for that treatment, this has resulted in all these larger centres all now offering endovascular aneurysm repair as a treatment option (as centres typically do not want to send work away).
Changes or uncertainties around the cost of implementation
The CUtLASS case study51 also illustrates how changes in the status, and hence price, of medicines can render some parts of the analysis, notably the cost-effectiveness analysis, out of date. In this case many atypicals are now off patent, so the cost difference between atypicals and typical antipsychotics is not as dramatic as it once was and, as such, parts of the pharmacoeconomic analysis may no longer apply.
This was also seen in the EVAR study,46 where the cost-effectiveness calculations were called into question at least in part because the calculations are based on follow-up using computerised tomography scanning, which is expensive. Now a significant proportion of patient follow-up is conducted using ultrasound, which is much less expensive.
Another issue around cost, which could have implications for implementation, is the way in which HTA studies are typically conducted. Although studies include a cost-effective analysis typically, this does not always cover all of the upfront costs of implementation. For example, in the EVAR study46 it was noted that there would probably be some training costs involved in introducing the new treatment, and that these are not accounted for in the study46 analysis. This was also the case for the ARTISTIC study,41 where the shift to HPV screening would likely be complex and time-consuming, as infrastructure for the existing screening approach is already in place, and could also have labour market implications.
Factors beyond the health system
Finally, wider events outside the health and research systems can also affect the uptake of findings. For example, in the case of the CESAR study,49 the H1N1 epidemic probably increased the rate of introduction of ECMO centres in the UK. In the case of the CRASH-2 studies,44 there is some suggestion that the war in Afghanistan may have played a role in the study being funded, as well as its uptake, particularly among the UK and US armed forces who were relatively early and influential adopters.
Study design can influence whether or not research is implemented in the NHS
In several cases, the study design played an important role in facilitating the uptake of the findings subsequently. Many of the studies were clearly tailored to use in the NHS, reflecting the wider priorities of the HTA programme. It is important to note that there is typically a tension between this type of pragmatic design and the design of a robust clinical trial that is appropriate for inclusion in wider meta-analyses. Indeed, because of such design, several of the studies received methodological criticisms, particularly outside the UK. However, nonetheless, reflecting the priorities of the programme, many of the studies took this approach, which then allowed the findings to be used to assess the relative effectiveness and cost-effectiveness of the approaches considered in the specific context of the UK health system.
For example, the trial design for the CESAR study49 was considered controversial because only the patients randomised to the intervention were moved to the ECMO centre. In some previous trials, everyone who met the criteria for ECMO was transferred to an ECMO centre, and half received standard care whereas the other half received ECMO. The research team made the decision that transferring patients to an ECMO centre to receive standard care was not appropriate for a UK context because, in the UK, patients who were not going to receive ECMO would never be transferred but, rather, would be treated locally. This focus on designing the trial to reflect the UK context meant that results were less comparable to other studies in the field.
Other elements of the study design were also seen to contribute to the usefulness of the findings in several case studies. The EVAR study,46 for example, was the only one among four international trials that, in addition to comparing endovascular aneurysm repair to open repair, also looked at the use of endovascular aneurysm repair among patients who were unfit to undergo open repair to see if the less serious surgery could be an option for this group. Furthermore, it has the longest-term follow-up out of all of the studies internationally, offering additional information about the long-term safety and outcomes of endovascular aneurysm repair devices. In the CRASH-2 study,44 the choice of TXA as the drug to be investigated (among a choice of several potential options) was important for the subsequent uptake of findings, as it is inexpensive and already familiar to clinicians because it is used in other types of surgery. In SWET,48 the careful consideration of the outcome measure was very important to the objectivity of findings, as it was not possible to blind participants to their treatment allocation.
Impact on policy
The Health Technology Assessment programme funds high-quality scientific evidence
The HTA programme has a number of measures in place to ensure that the research that it funds produces robust scientific evidence. For example, all research applications and HTA monographs are peer reviewed. Many interviewees praised the quality of HTA programme-funded research (I2, I3, I19) and viewed the evidence that HTA programme-funded research produces on the effectiveness and cost-effectiveness of health-care interventions as invaluable to policy-makers (I2, I3, I6–I19). For example, one interviewee reported that the most important achievement of the HTA programme has been its provision of evidence to the government about the effectiveness of health technologies:
It’s really important to clock that and the importance of the decisions that are informed by it.
I19
An important aspect of HTA programme-funded research is the health-economic evaluation that is a component of many HTA programme-funded research projects. Some interviewees report that the cost-effectiveness analysis is particularly important for NICE decision-making. One interviewee reports that it is ‘exactly what [NICE] would do’ and prevents NICE from having to conduct the analysis separately (I18).
As noted above (see The primary route to impact of Health Technology Assessment programme-funded research is through guidelines, particularly National Institute for Health and Care Excellence and National Screening Committee guidelines), the primary route to impact of HTA programme-funded research on patients is through clinical guidelines. Similarly, the primary impact of HTA programme-funded research on health policy is on clinical guidelines. Many interviewees cited the inclusion of HTA research in guidelines as evidence that the HTA programme informs decisions about the delivery of health-care services (I3, I6–I9, I14–I18). One interviewee noted that:
The HTA programme is the vodka in the cocktail. It’s the crucial ingredient without many things people know and love like clinical guidelines cannot work.
I3
However, the importance of HTA programme-funded research to clinical guidelines has changed over time because of the shift in attitudes towards evidence-based medicine. One interviewee noted that:
When HTA started, there wasn’t really guidance. So, the idea was that the programme was aimed at the practitioners. But, with the development of NICE particularly of course, but many other guidance producers, it has turned out, I don’t think intentionally, that guidance producers are the primary consumers of quality pragmatic primary research that the HTA produces.
I9
Understanding the remit of the HTA programme is essential to interpreting the impact of the HTA programme on health policy. For example, the HTA programme does not produce guidelines itself, but rather is a funder of research that results in scientific evidence, which may then be included in clinical guidelines. Two interviewees stressed that the HTA programme does not make policy decisions or produce policy advice, but, rather, stops very clearly at the point of funding research, analysing and presenting the findings and discussing their implications (I3, I19).
Health Technology Assessment research has had wide range of different types of impacts on policy
Data from the 68% (422 out of 619) of awards captured in Researchfish indicated that the HTA programme has had a range of impacts on policy. Of the 422 projects with data, 22% reported having an impact on policy (15% of the overall portfolio). More detail on the sample that responded and the specifics of Researchfish response rates is given in Chapter 2. The Researchfish data identified a range of different types of impacts across the portfolio of HTA funding, with some projects mentioning more than one impact within influence on policy. The types of impact include: influenced training of practitioners or researchers; membership of a guidance committee; participation in an advisory committee; citation in clinical reviews; citation in other policy documents; citation in systematic reviews; citation in clinical guidelines; implementation circular, rapid advice or letter; and gave evidence to a government review as shown in Figure 5.
FIGURE 5.
Impacts on policy recorded in Researchfish.
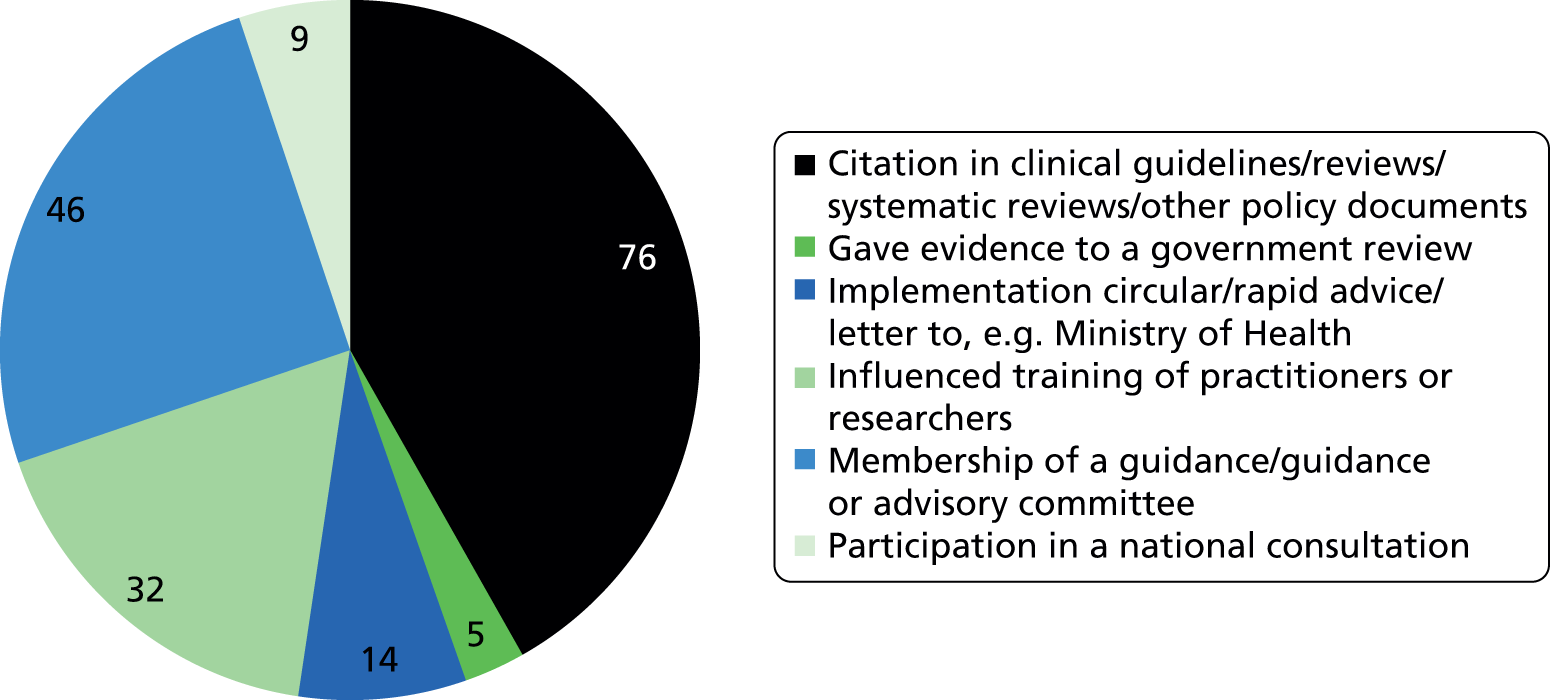
According to the Researchfish data, these impacts on policy occurred mainly in the UK, as shown in Figure 6 . Out of the projects that reported an impact on policy, nearly 60% were within the UK. However, 30% also reported an impact on policy and practice internationally. The kinds of international impacts reported include citation of the research in other countries’ guidelines, and participation and membership in international working groups and committees.
FIGURE 6.
Reach of impacts on policy recorded in Researchfish.

The case studies also illustrated the wide range of impacts on UK policy. Of the 12 case studies,41–52 10 studies41,42,44–49,51,52 indicated evidence of some impact on UK policy. The main routes to policy impact were through citation on guidance, typically although not exclusively NICE guidance, and through a NSC pilot. These are outlined in Table 9 and described in the following sections.
National Institute for Health and Care Excellence and the National Screening Committee are key users of Health Technology Assessment research
Both NICE and the NSC use HTA programme-funded research in their guidelines. For example, Raftery and Powell (2013)11 found that up to July 2013 the HTA programme has funded an academic assessment of all of the 293 topics appraised by the NICE Technology Appraisal Programme. The authors also found that HTA programme-funded research underpins many of the NICE and NSC guidelines. For example, they found that HTA programme-funded research was the main supporting evidence referenced for 9 out of the 20 disease-specific screening programmes in England. They also found that 55 out of the 106 screening topics considered by the NSC since 1986 were informed by HTA programme-funded research.
Most interviewees identified NICE and the NSC as important users of HTA research (I2, I3, I7–I9, I15, I16, I19). However, interviewees also noted that the way policy-makers use HTA research will vary depending on the type of HTA programme-funded research (e.g. systematic review or primary research). For example, the HTA programme produces bespoke evidence syntheses for both NICE and the NSC, which have a direct impact on policy (I2–I8, I15, I16, I19). Similarly, all of the HTA research that is funded to support NICE technology appraisals has a direct, nearly one-to-one, impact on NICE technology appraisals (I7, I15, I16, I18, I19). One interviewee noted that in both these instances, it would be exceptional if the HTA research did not have an impact on policy (I15). HTA research also feeds into NICE clinical and public health guidelines (I15, I16, I18), which, as one interviewee (I15) explained, happens in three ways: the guidelines may reference a HTA programme-funded trial, take advantage of some early finding or subsume previous NICE guidelines that included HTA research. Lastly, the HTA also funds non-bespoke evidence syntheses for the NSC when they have identified a gap in the existing evidence. However, for those non-bespoke evidence syntheses, the research is more likely to have a longer-term impact.
The importance of NICE and NSC as routes for policy impact are also illustrated by the case studies. Seven45–49,51,52 of the case studies had an impact on policy by influencing NICE guidance and a further two41,42 had an impact on NSC policy by leading to a pilot – we elaborate on the details of those impacts below.
Seven case studies influenced National Institute for Health and Care Excellence guidance
Two of the case studies discussed NICE TARs (STAs52 and RA45). As such, these studies45,52 were conducted with the expressed aim of informing NICE decision-making. Both studies45,52 were provided to, and briefed to, the NICE guidance committee and will have, alongside other inputs, directly informed the decision taken around those particular NICE technology appraisals. The guidance does not typically directly cite evidence from TARs, but there is strong reason to suppose that they have a significant impact on the process.
Both the CUtLASS51 and EVAR46 studies led to straightforward changes in NICE guidance, suggesting a wider choice of treatment options. For example, as a result of the CUtLASS51 findings, NICE changed its recommendation from using an atypical antipsychotic as first-line treatment to discussing with the patient which drug should be used, with no mention of whether or not that should be typical or atypical. However, as described above, this may have had little effect on practice. Similarly, the NICE Interventional Procedure Guidance states that ‘current evidence on the efficacy and short-term safety of stent–graft placement in AAA appears adequate to support the use of this procedure’ based largely on the EVAR46 data, supplemented by a systematic review, and a NICE technology appraisal also cites the study. However, in this case the guidance has been implemented and endovascular aneurysm repair is widely used.
The CUtLASS study51 is an interesting case because the impact it has had on policy is quite different from that it has had on practice. As described above, the impact on policy is to broaden the range of treatment options available to patients and to encourage discussion between the clinician and patient about benefits and side effects in determining the most appropriate medication. However, in practice it is likely that very few patients are receiving the older typical antipsychotics because of changes in practice that had already taken place while the study51 was being conducted (and prior to it to some extent), meaning that very few clinicians are able (or possibly willing) to prescribe those drugs. However, there is some suggestion that the findings have resulted in more careful consideration of dosage and the effects of using of multiple drugs in practice, potentially reducing side effects. This change in practice does not correspond to the codified policy change laid out in the guidance.
The evidence of SWET48 is listed in the reviewed sources on the relevant NICE guidance, but there is no direct citation or change in policy, which is appropriate, as the study48 found that existing policy and practice in the UK should not be changed. As such, although the findings are not directly cited, this is as significant an impact on policy as could be expected given the nature of the study48 findings.
The Carotid Stenosis study47 is cited in the NICE guidance for stroke, developed by the Royal College of Physicians in 2008 as the evidence for the use of imaging techniques for the assessment of risk of stroke. This work also been cited in Scottish Intercollegiate Guidelines Network (SIGN) guidance.
The two screening case studies led to a National Screening Committee screening pilot
Both the ARTISTIC41 and the Newborn CHD42 studies have led to NSC pilots which, if successful, will result in roll-out at the national level. The process in this context is somewhat different from those studies that feed in to NICE guidance. The process of policy change can be slower but in some senses is more direct, as, if the pilots are successful, the change in practice will be rolled out and implemented nationally. By contrast, uptake of guidance may be patchier. 53–56
In both cases, attribution of this policy change is clear, with the decision to pilot pulse oximetry based on the Newborn CHD study42 and one other study, and the decision to pilot HPV primary screening based primarily on the ARTISTIC trial,41 which is directly cited in the minutes of the NSC meeting where that decision was taken.
Other policy-makers also use Health Technology Assessment programme-funded research
A number of other policy-makers also use HTA programme-funded research. A number of interviewees recognised other guideline producers, such as SIGN, and individuals involved in the production of systematic reviews or experts in a particular field in which the HTA programme funds research as other important users of HTA research (I5, I14, I15, I17).
One interviewee noted that the wider policy teams within the DH would not usually have direct engagement with the HTA programme, but rather that the DH R&D directorate brokers that on their behalf. The interviewee noted that a particular policy team’s awareness of the programme would depend on how interested that team is in the source of the research:
Whether they would just note that this evidence was produced by a programme and that it was robust evidence and therefore they use it or whether they would know the HTA programme by name . . . would depend on their personal level of interest. But that’s the same as whether they would know that the British Heart Foundation funds heart research or the MRC funds medical research.
I19
Although there are other users of HTA research, the primary users of HTA programme-funded research seem to be NICE and the NSC. One interviewee noted that a possible criticism of the HTA programme is that it is too NICE centric, commenting that:
It is extremely important that [the HTA programme] continues to serve a wide range of masters, not just NICE. NICE should be the primary customer. But [the programme] should continue to consider needs of other decision makers.
I3
The potential limitations of an entirely NICE-focused policy dissemination approach are illustrated by two examples from the case studies,43,44 for which despite strong evidence that a change in policy may be warranted, the NICE guidance has not been updated for regulatory reasons. In the case of both the CRASH-244 and the IVAN study,43 impact on NICE guidance has been hampered by the lack of any incentive for industry to seek a marketing authorisation for the drug in question.
In the case of the CRASH-2 study,44 NICE have not reviewed TXA as part of their standard guideline review process. This is partly because there was no direct push to do so, as the drug manufacturers have not applied for a UK marketing authorisation for use in the trauma setting, and this is unlikely to change, as it is now a generic drug and there is therefore little incentive for pharmaceutical companies to pursue this. Following some lobbying by members of the study44 team, NICE developed an evidence review based on the results of the CRASH-2 trials,44 describing its use in this context. According to the NICE website, TXA may be included within the scope of the NICE clinical guideline on the assessment and management of major trauma, which is estimated to be published in February 2016 (www.nice.org.uk/guidance/indevelopment/gid-cgwave0642). The CI is continuing to press for the drug to be authorised in the UK, as that will allow the drug to be marketed properly. The findings have also been included in the 2012 Joint Royal Colleges Ambulance Liaison Committee guidelines, which recommend pre-hospital TXA for all patients triaged to a trauma centre.
In the case of the IVAN study,43 the situation is more extreme. The drug Avastin, which was found to be as effective but much more cost-effective than Lucentis for neovascular AMD, has not been assessed, and the existing NICE guidance still recommends the use of Lucentis. To assess Avastin would require the DH to commission NICE to carry it out, but this is made more difficult by the fact that the drug’s manufacturer does not intend to apply for a licence to market Avastin for a new indication of neovascular AMD. There is no commercial incentive for the pharmaceutical industry to carry out the relevant safety trials and apply for a licence, as both patents are held by the same company. However, in December 2014 NICE was asked by the DH to begin developing a guideline for the diagnosis and management of macular degeneration. The scoping workshop for this guideline was to be held in April 2015 and it was expected that the guideline would be published in August 2017. It was unclear whether or not this guidance would cover Avastin.
Although in both of these cases industry interests have hampered the inclusion of the relevant drugs in NICE guidance, the impact on practice differed significantly as described above. TXA is widely used off-label for the treatment of trauma, but Avastin is not commonly used in the NHS, as industry has threatened such use with legal action. This may reflect a difference in circumstances: TXA is a generic drug and no company has any interest in pursuing market authorisation; however, in the case of Avastin the pharmaceutical companies involved have an interest in preserving the use of Avastin solely in the cancer indications for which a licence was granted, while marketing Lucentis for its licensed indication of AMD.
Impacts on policy may be delayed by the time taken to publish and the increased focus on primary research in the Health Technology Assessment programme
Although the HTA programme has an important impact on health policy, there are some factors that may delay the programme’s impact. Interviewees highlighted two factors that can delay the impact of HTA research: the increased focus on primary research and the time to publication for research.
Relative increase in funding for primary research
Two interviewees noted that there has been a shift in the focus of HTA funding over the last 10 years, with a relative increase in funding for primary research compared with secondary research (I7, I15). The reason for the shift was explained by one of the interviewees as follows:
Cochrane has now done many more systematic reviews so there are fewer that need to be done by the HTA programme. The reason for large trials is that some areas are relatively evidence free so there is a need for primary evidence and partly because improvements in healthcare are very much incremental so in order to identify small but important benefits one does need some very large trials.
I7
The increased focus on systematic reviews can delay the impact of HTA research on patient care and clinical practice because the results of trials are less likely to have an immediate effect on policy than systematic review. One interviewee described how the programme’s increased focus on systematic reviews has probably changed the impact of HTA programme-funded research:
By changing funding [towards] primary research, you’re shifting the eventual impact slightly upstream, away from a relative focus on decision-makers, commissioners, NICE, international organisations, slightly towards the people who would find the results of large trials of interest, who would tend to be, perhaps more specialist clinicians, people doing research, people who are compiling systematic reviews, etc.
I15
Although primary research is often included in later systematic reviews, which then influence policy and practice, the HTA programme’s more recent focus on primary research potentially decreases the immediate usefulness of HTA research to policy-makers and delays the impact of HTA programme-funded research on health policy.
Time to publication of Health Technology Assessment programme-funded research
Another barrier to the impact of HTA programme-funded research is the time to publication of the research findings. One interviewee highlighted the delay between the academic publication and the monograph as a particular challenge for policy-makers because the academic publication does not include all of the results, such that policy-makers may be wary of making decisions without the full HTA report (I18). More generally, several interviewees noted that HTA programme-funded research is slow to complete and publish (I7–I9, I18). However, interviewees also recognised that the time taken to publish results is a problem with clinical research in general because it takes time to do it thoroughly (I7–I9, I15). One interviewee noted that while the timeliness of research remains ’a big issue’, it is improving (I7).
Academic impact
The HTA programme has made a substantial contribution to health research through the publication of the monographs in the HTA journal as well as by encouraging independent publication in peer-reviewed journals
Through the HTA journal and wider publications, the HTA programme has funded a large volume of research which is now available to the wider academic community. Figure 7 shows the total number of publications from HTA programme-supported projects by year. The data suggest an increase in the number of papers being published in journals other than the HTA journal since 2010, as well as a general increase in output over time.
FIGURE 7.
Total number of publications from HTA programme-funded projects by year. Note: this is based on a sample of publications identified through three sources: all articles and reviews published in the HTA journal during the period 2004–12; papers listed on all HTA project pages of the NETSCC website; and papers reported by researchers in Researchfish. As such, this may not be a complete record of all publications related to HTA programme-funded research.

A clear policy of the HTA programme, which is supported by the HTA journal in particular, is that the vast majority of research funded is published (and in an open access format). The monographs in the HTA journal are intended to provide a full and detailed account of the work conducted, and 96% of projects are published in the HTA journal at least (excluding TARs). 26 This is a very high rate of publication compared with other funding streams nationally and internationally,57 and ensures that HTA research is made widely available. Indeed, a Cochrane review58 in 2007 concluded that ‘less than half of all studies, and about 60% of randomized or controlled clinical trials, initially presented as summaries or abstracts at professional meetings are subsequently published as peer-reviewed journal articles’. Interviewees commented favourably on this high level of publication as ‘exemplary’ (I1) and ‘absolutely unique in the world’ (I6). One interviewee thought this was the programme’s biggest achievement: ‘making sure that the dissemination gets published in a form that is usable by academics, ensuring that it is all there in full format’ (I7); another suggested that this was ‘a very valuable resource for researchers’ (I9).
In addition to publication in the HTA journal, the programme encourages publication in other journals. This allows the programme to ensure that the research is comprehensively written up and published, while also ensuring that it is presented in the journals that are likely to be read by those in each particular field. This is felt to be important for dissemination of the work, and for improving its credibility in the academic community (I15). It was also considered to be ‘good for the scientific endeavour and careers of researchers’ (I14). One interviewee also commented that wider publication was important for the international visibility of the research (I7).
Bibliometric data provides one way to illustrate the wide range of fields covered by HTA programme-funded research. Table 10 shows the range of fields in which HTA papers have been published. The classification is based on WoS subject categories, which are applied at the journal level. As such, papers published in the HTA journal are not included in this table (since they are all in the same category). The table indicates the range of journals publishing HTA programme-supported research.
| Field | Papers |
|---|---|
| Medicine, general and internal | 137.83 |
| Surgery | 28.58 |
| Public, environmental and occupational health | 27.70 |
| Medicine, research and experimental | 27.20 |
| Health-care sciences and services | 26.33 |
| Cardiac and cardiovascular systems | 21.00 |
| Oncology | 20.50 |
| Obstetrics and gynaecology | 19.33 |
| Radiology, nuclear medicine and medical imaging | 19.33 |
| Paediatrics | 15.83 |
| Psychiatry | 15.50 |
| Infectious diseases | 13.83 |
| Peripheral vascular disease | 12.83 |
| Multidisciplinary sciences | 12.00 |
| Rheumatology | 12.00 |
The Health Technology Assessment programme produces high-quality, rigorous academic research
There was a clear consensus from the interviews conducted that the research conducted through the HTA programme is of excellent academic quality (I2–I5, I8, I12, I15, I18, I19). This is reflected in the bibliometric data shown in Figure 8. This sets out MNCS per year, both for all publications, as well as separately for papers in the HTA journal. In both cases, the average value that would be expected (normalised for field and year of publication) is one, so on average work funded by the HTA programme is cited more than twice as frequently as would be expected on average, with little difference in the citation rates between the HTA journal and external publications.
FIGURE 8.
Mean normalised citation score per year, for all publications from HTA programme-funded research and for papers in the HTA journal only. Note: this is based on a sample of publications identified through three sources: all articles and reviews published in the HTA journal during the period 2004–12; papers listed on all HTA project pages of the NETSCC website; and papers reported by researchers in Researchfish. As such, this may not be a complete record of all publications related to HTA programme-funded research.
Although MNCS can be skewed by very highly cited articles, the proportion of publications in the top 10% of papers in their field shows that this does not seem to be the case for HTA-supported research (Figure 9). Across the time period of the study, 31% of papers in the HTA journal, and 29% of papers published in other journals, featured in the top 10%.
FIGURE 9.
Proportion of publications in the top 10% of papers in their field, for all publications from HTA programme-funded research and for papers in the HTA journal only. Note: this is based on a sample of publications identified through three sources: all articles and reviews published in the HTA journal during the period 2004–12; papers listed on all HTA project pages of the NETSCC website; and papers reported by researchers in Researchfish. As such, this may not be a complete record of all publications related to HTA programme-funded research.
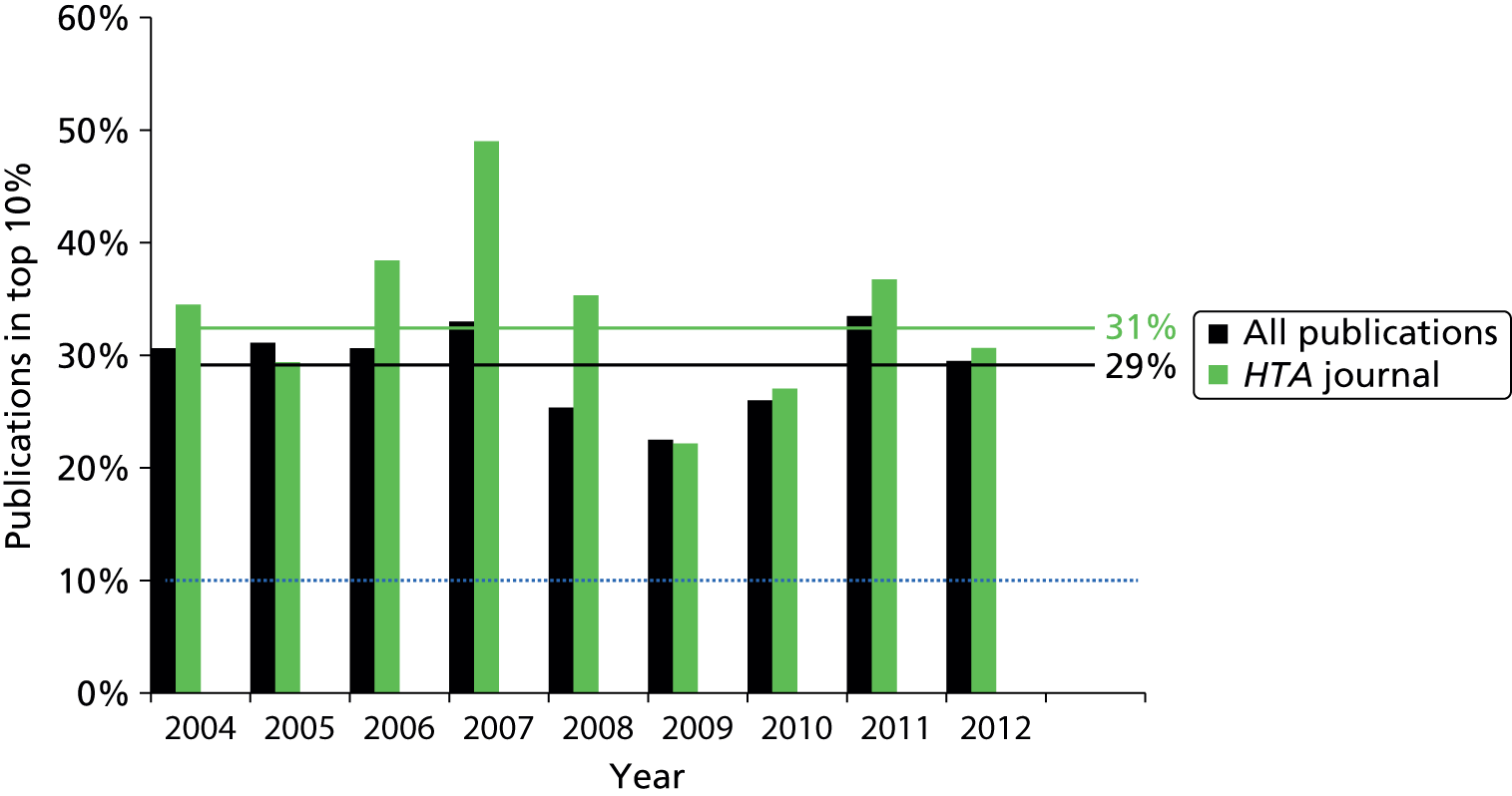
The fact that HTA journal articles and articles published in other journals performed similarly in terms of MNCS, proportion of papers in the top 10% and proportion of papers uncited (7% of publications in the HTA journal, 6% of wider publications) suggests that the two are fairly similar in terms of citation distribution, and that publication in addition the HTA journal is the norm.
It is worth noting that interviewees also commented on the ‘unique’ combination of this high academic quality with application focused research (I2, I3, I18). For example, one interviewee commented that ‘. . . [HTA research] is academically rigorous and high quality, but nonetheless clearly and deliberately linked to policy and practical decisions’ (I3). Some comments were also made around the way that the HTA’s internal processes supported this. One interviewee commented that the HTA is ‘. . . rigorous about the methods in uses. For example, the HTA will insist that there is a systematic review before there is a randomised trial. They set high standards’. (I5) Another commented on the prioritisation process, stating that ‘prioritisation in HTA ensures really important questions that need really good quality research are addressed rigorously and be made publicly available’ (I18).
Research quality is also illustrated by the case studies, most of which produced further highly cited articles in addition to the Health Technology Assessment journal publication
Of the 12 case studies,41–52 1041–44,46–51 refer to additional publications outside the HTA journal article. Based on a bibliometric analysis, the quality of these publications as indicated by the number of citations received is high (Table 11). With one exception,48 all of the studies had more than one-quarter of their publications in the top 10% of the field, and this was very high, ≥ 50%, for seven42–47,49 of the case studies. Several of the studies also produced a large number of publications. Although normalised citations take account of the fact that more recent papers have had less time to accumulate citations, it is worth noting that some of the case study projects have not yet published all of their outputs, and we cannot anticipate the impact of future publications in the analysis. For example, there are at least five further publications planned for the IVAN43 project.
| Case study | Total indexed publications | Total citations | Total normalised citations | Papers in top 10% of field |
|---|---|---|---|---|
| ARTISTIC41 | 10 | 475 | 22 | 4 |
| CESAR49 | 3 | 526 | 30 | 2 |
| CRASH-244 | 9 | 645 | 57 | 5 |
| CUtLASS51 | 7 | 710 | 31 | 2 |
| Carotid Stenosis47 | 2 | 165 | 6 | 2 |
| CoBalT50 | 7 | 39 | 12 | 2 |
| EVAR46 | 17 | 2842 | 123 | 11 |
| IVAN43 | 3 | 192 | 51 | 3 |
| Newborn CHD42 | 2 | 65 | 3 | 1 |
| RA45 | 1 | 349 | 20 | 1 |
| SWET48 | 4 | 35 | 4 | 0 |
Although we did not explicitly explore HTA publication policy in the case studies, one researcher (ARTISTIC41) explicitly praised the policy of allowing publication in other journals before the HTA monograph was produced, allowing study results to be released sooner and made available in highly visible journals.
The exception is the Technology Assessment Report programme, particularly the Single Technology Appraisals, for which academic publication can be challenging
For the RA45 study, the only publication was the HTA journal article. The case study suggests that the results did have the potential for wider publication but that this did not take place because of the time constraints involved in the conducting TARs (see Appendix 4). Researchers immediately move to a new topic and have to produce work on a tight timescale. This means that they typically do not follow up on a particular study and do not continue to work in a particular field, limiting opportunities for publication. It was also noted that it can be difficult to publish TARs because a lot of the data were provided by pharmaceutical companies, as commercial-in-confidence reports, for which the primary sources could not be cited.
Many of the same concerns also apply to STAs. 52 However, in this case, the main output of the work is a report that is used by the NICE Appraisal Committee in their deliberations but is not typically published in the HTA journal (there are a limited number of exceptions among the earlier STAs, but publication as a HTA monograph is no longer carried out). The case study suggests that in addition to the concerns described above for MTAs, the STAs are not perceived to be of the same scientific rigour as MTAs, which further limits publication opportunities (see Appendix 4). However, at ScHARR-TAG (School of Health and Related Research Technology Assessment Groups) they have found a route of publications for many of their TARs in the Pharmacoeconomics journal series.
It should also be noted that, although the HTA journal article is the only output from the RA45 study, it is extremely highly cited, receiving 20 times the expected level of citation for the field, and being among the top 15 most highly cited publications across the entire HTA portfolio analysed at the programme level. However, it is noted in the case study that, despite the high level of citation in this case, solely publishing in the HTA journal could be considered to be limiting in that there is a perception that it is not often read by clinicians (see Appendix 4).
Impact on the research system
The Health Technology Assessment programme is viewed as an important funder of clinical research, which has had a positive impact on the careers of Health Technology Assessment researchers and research capacity in the UK
The HTA programme has led to the investment of a large amount of money in clinical research, and in HTA research in particular, and the many interviewees felt that this had played an important role in building capacity in the field in the UK (I4, I6, I9, I14, I15, I19). One interviewee felt that this sizeable investment in the field was the key achievement of the programme, stating that:
The most important achievement of the HTA programme is itself. That is to say, it is the commitment of a very large sum of research money on improving practice. If you wanted to celebrate what it does, that’s what you should celebrate, rather than any individual achievement or accomplishment.
I9
This is reflected, according to one interviewee, in the ability of the UK to retain capacity in this field, commenting that:
There are few people working in HTA that go somewhere else. You tend to find in other fields people get sucked to other countries. So, I think it shows that people feel that there is quite a critical mass of researchers in this field in the UK. There hasn’t been large-scale flight of people to other countries. You can’t say that about other fields of research.
I4
This is supported by evidence in the Researchfish database, which provide data from some researchers on the ‘next destination’ of staff who had moved on subsequent to undertaking the research. Positions are generally within the UK, although instances of relocation to China, Qatar and Sweden are mentioned.
There is limited evidence from Researchfish that HTA funding can advance researchers’ careers in other ways. For example, there are 85 instances of individuals gaining skills through HTA programme funding. However, only a very small number of qualifications were reported [three Master of Science/Master of Art; one Doctor of Medicine; eight Doctor of Philosophy (PhDs); five undergraduates].
There is also evidence that further funding was secured based on existing HTA awards recorded in Researchfish from 40 awards. Some of these awards reported more than one instance of follow-on funding, and, across the 89 examples described and set out in Table 12, the median value was £189,029. Over one-quarter of this funding is follow-on funding from NIHR, DH or NHS.
| Source | Number of instances | Total value of leveraged funding from this source (£) |
|---|---|---|
| Charity | 6 | 1,505,782 |
| Industry | 4 | 876,725 |
| Foundation | 5 | 3,494,650 |
| Government | 22 | 54,360,651 |
| DH | 14 | 44,536,853 |
| Other | 8 | 9,823,798 |
| HEI | 3 | 1,933,260 |
| Society | 2 | 71,720 |
| Total | 42 | 62,242,788 |
However, one person made a specific comment around the career challenges resulting from working on TARs, particularly STAs that have been introduced relatively recently (I4). These offer fewer opportunities for publications, as they are typically not published in the HTA journal because they are commentary on work that others have done as part of the manufacturer’s submission rather than a lot of original work (in most cases). This has potential career implications in terms of their publication record for the researchers involved.
Overall, the impression from interviewees is that ‘the HTA is prominent in clinical research in the UK’ (I15) and that it ‘support the academic community in this country’ (I19). There is also some suggestion by one of the interviewees that clinical research had previously been in decline in the UK, and that the HTA programme, and NIHR more generally, had played an important role in building back up that research base, although it had taken some time (I6).
Half of the studies were extended or had additional elements added to the initially planned work
In several cases, when the study was considered to be successful because it demonstrated significant impact on the primary outcome, additional follow-up time points were added to the study to allow the effects of interventions to be understood over a longer time period. This took place for the EVAR,46 CoBalT,50 IVAN43 and ARTISTIC41 studies. In some cases, this was coordinated with other international studies on the topic in question. For example, the IVAN43 study has been extended to allow the team to bring back participants for 4- and 5-year follow-ups to look at the longer-term effects of the treatments, matching the 5-year outcomes data captured by the similar CATT (Comparison of AMD Treatments Trials) study in the USA (see Appendix 4.3).
In addition, five case studies (CRASH-2,44 IVAN,43 SWET,48 ARTISTIC41 and EVAR46) described additional ‘nested’ studies, which were made possible by the initial HTA study, using the data produced through the study in a different way, or extending a particular aspect of the study. For example, two ‘nested’ studies, also funded by the HTA programme, stemmed from the CRASH-244 trial. One focused on developing and validating a model to predict death in patients with traumatic bleeding, as well as using that model to then evaluate the effect of TXA on mortality levels. The other looked at the data available in the CRASH-244 data set on intracranial bleeding and the effect of TXA in traumatic brain injury. The IVAN study43 also provides interesting examples of additional ‘bolt-on’ funding, such as creating a serum and deoxyribonucleic acid (DNA) biobank to analyse genetic interactions with the drugs, and exploring the mechanistic aspects of vascular endothelial growth factor (VEGF) metabolism. Although these were of little direct relevance to the aims of the HTA programme-funded project, the samples collected were then able to be used in subsequent analyses funded from other sources.
Most of the studies shaped future research in the field, either in terms of topic or methods
In several cases, members of the study team went on to conduct further trials building on the expertise developed in the HTA study. Some of these were also HTA funded. In other cases, members of the study team advised others on how to set up their trials. For example, members of the SWET48 study team shared their learning about the development of objective outcome measures, how to deal with open trails and patient recruitment. Sometimes studies shaped the development of other trials through publications rather than direct communication, such as the case of the Newborn CHD42 study, which was cited in the methods for two other trials internationally.
In two cases (RA45 and Newborn CHD42), the models developed in the studies42,45 were used by others in further work. For example, the Birmingham Rheumatoid Arthritis Model version 2 (BRAM2) model, which was refined in the RA45 study, has been used in other cost-effectiveness assessments of treatments for rheumatoid arthritis, both by members of the study45 team and others internationally.
Two of the studies41,46 pooled results with other similar studies internationally. In the case of the ARTISTIC study,41 members of the research team were involved in conducting the pooled analysis to the pooled analysis, which included the data from the ARTISTIC study,41 but, in the case of the EVAR study,46 the team led the synthesis, which was funded by the HTA programme and is scheduled for publication in the next year. This raises an interesting point, as with these studies there is sometimes a trade-off between the extent to which the study is tailored for applications in the context of the NHS, and comparability of the study with international results, allowing for this type of pooled analysis, as described previously (see Study design can influence whether or not research is implemented in the NHS, above).
Three studies contributed to stimulating their research field more widely
The publication of the CRASH-244 study findings in 2010 seems to have contributed to wider interest in TXA in the research system, as reflected by an increased level of publication around the topic in PubMed since that point. The IVAN study43 was one of the first of its kind in the UK, so also contributed to shaping the work that has followed in the field. Similarly, at the time the EVAR46 study was conducted, trials were not commonplace in the vascular surgery community at the time, and the EVAR46 study team was one of the early groups to start to conduct trials in this field. As such, the trial46 and the team became a source of knowledge and advice for others in vascular surgery who were looking to conduct trials.
Although no further studies have been conducted on the use of water softeners for the treatment of eczema, SWET contributed to increased interest in the relationship between water hardness and eczema. For example, work looking at the association between hard water and the development of eczema is ongoing, and preparing a national follow-up trial looking at whether or not water softeners might help prevent eczema from birth, rather than treat established eczema. SWET48 also influenced the field more widely through the Harmonising Outcome Measures for Eczema initiative, which was established after the trial (www.nottingham.ac.uk/homeforeczema/index.aspx). Members of the team contributed their expertise in developing objective outcome measures for eczema research and this should contribute to the improvement of eczema research internationally.
All of the studies had some capacity for building impacts for the individuals directly involved in the study, although scope for this was often limited because the researchers were already well established
There was evidence that the studies benefited the careers of those involved, from overall reputational benefit of being involved (ARTISTIC,41 RA,45 STAs,52 CoBalT50) to the award of PhD or other qualifications to members of the team (ARTISTIC,41 Carotid Stenosis,47 EVAR,46 CoBalT50); the winning of awards, honours or prizes (ARTISTIC,41 EVAR,46 SWET48); and through team members securing advisory roles, for example on guidelines committees (STAs,52 Newborn CHD,42 ARTISTIC41).
In 841,42,44,46,48–51 of the 12 case studies, however, it is indicated that the researchers involved in conducting the study already had established careers, so the scope for this particular study to contribute to their career progression was limited, or at least the study was only one of many important studies that contributed to their reputation and any other indicators of esteem. So although you can often see an upwards career trajectory for researchers involved, it is hard to attribute it to this study in particular. This also, perhaps, suggests that the HTA typically funds more established researchers, although this may be an artefact of the case studies selected.
Many studies made important contributions to building capacity more widely, outside the immediate study team
In several cases, the studies contributed to the building of capacity outside the immediate study team. In some cases, this refers to building of wider research capacity within the institution. For example, the IVAN43 study allowed the Clinical Trials Unit (CTU) at Bristol to apply for UK Clinical Research Collaboration (UKCRC) registration and expand rapidly in both number of staff and expertise, whereas the EVAR46 study provided wider capacity-building opportunities for others working at Imperial College London, in terms of learning how to set up and run trials in the area through mentoring and observation with members of the study46 team, who, having previously run the UK Small Aneurysm Trial, were quite experienced. Three studies also mentioned the development of enhanced networks (CoBalT,50 IVAN,43 SWET48).
Five studies43–46,49 mentioned wider research capacity-building activities outside the institution. Work conducted through the RA45 study contributed to the development of HTA internationally, as members of the team briefed the work in other countries as an example illustrating how the HTA process works in the UK. Members of the CRASH-244 study proactively undertook a range of capacity-building activities as part of this work, including travelling to a number of the key collaborating sites overseas (e.g. in Nigeria and Pakistan) to support them in terms of developing relevant capacity in the management of research, around issues such as monitoring of trials and ethics processes. Three cases43,46,49 also suggested that the studies were important in building up research capacity in a new or emerging area, for example by bringing more researchers into the field (CESAR,49 EVAR,46 IVAN43). For example, the IVAN43 case study suggests that the work, as the first large-scale randomised controlled trial (RCT) in ophthalmology in the country, served as a flagship trial, and established models and structures that have benefited subsequent studies, advancing ophthalmology research in the UK overall.
Finally, a number of case studies mentioned that the work had an impact on the teaching and training of clinicians (Carotid Stenosis,47 CESAR,49 EVAR,46 Newborn CHD,42 CUtLASS,51 CRASH-244). In addition, for the IVAN43 study it was suggested that the work led to an increased awareness of research among clinicians, and increased enthusiasm for, and interest in, initiating and participating in studies subsequently. This reflects, perhaps, the fact that the study43 was both large and a flagship trial in the field, as described above.
Impact on industry and the economy
Interviewees suggest that there is little overlap between the majority of the research funded by the Health Technology Assessment programme and industry, as the programme intentionally does not fund research that industry would support
Typically, HTA programme-funded research is in areas for which there is little or no commercial incentive to carry out research, such as when a drug is off patent. As such, that means that typically industry involvement in research is limited in most cases (I6, I14). However, there are notable exceptions to this, and, in some cases, the research priorities of the HTA and the pharmaceutical industry conflict directly (I7, I14, I19). One example noted by an interviewee is the IVAN43 trial, in which a HTA study compared two drugs owned by the same company, one of which was not licensed for that indication. The IVAN43 trial is described in more detail as one of the case studies in Appendix 4. As another interviewee noted, the research that the programme funds will sometimes come into conflict with industry because it ‘challenges some of the assertions that it makes about the effectiveness and cost-effectiveness of its products’ (I7). STAs are an example of this, when TAR teams critique manufacturers’ submissions to NICE. This inevitably will lead to conflict with industry in some cases.
Given the focus of HTA programme-funded research, it is perhaps not surprising that the production of new intellectual property or the discovery of new molecules is relatively unusual, as suggested by one interviewee (I7). This is supported by the data available in Researchfish, which indicates that there are five instances of Interventional Procedures reported (in five separate awards), which focus around the production of resource materials and manuals that have been copyright protected.
More than half of case studies demonstrated impacts on industry and product development
Of the 12 case studies,41–52 seven41,42,45,46,49,51,52 indicated clear impacts on industry or product development, as set out in Table 13. Most of these impacts on product development were through the refinement of products or markets, rather than the development of new products. Several studies also suggest that there may have been an impact on industry through increased sales, which, although plausible, is hard to evidence. In addition, the TARs had an impact on industry via NICE, which is discussed in the next section.
| Name of case study | Evidence of impact | Summary of impact on industry and product development |
|---|---|---|
| ARTISTIC41 | ✗ | Informed the optimal threshold for the Hybrid Capture 2 Test for HPV detection and so improved its relative sensitivity Development of new primary HPV screening tests and a decrease in the price of tests |
| Newborn CHD42 | ✗ | May have contributed to improved design of pulse oximetry devices to make suitable for children |
| IVAN43 | ||
| CRASH-244 | ||
| RA45 | ✗ | Anecdotal evidence that model developed has been used by some pharmaceutical companies in their applications for approval of similar therapies by NICE Inclusion of drug into guidance, so reimbursement on UK market |
| EVAR46 | ✗ | Contributed to growth of stent market and to changes in design of products |
| Carotid Stenosis47 | ||
| SWET48 | ✗ | Water softening industry may have benefited from the publicity associated with being part of a high profile trial, and many trial participants purchased the unit despite findings showing no benefit from it |
| CESAR49 | ✗ | Limited evidence that study influenced the more recent developments in ECMO technology in three companies by demonstrating potential market |
| CoBalT50 | ||
| CUtLASS51 | ✗ | Potential negative impact – may have reduced industry research in schizophrenia drugs |
| STAs52 | ✗ | Inform NICE decisions and hence the drugs purchased by the NHS |
Impact through the refinement of products or markets
Three case studies41,42,46 indicated a potential impact on products, either through the refinement of existing products or changes to the market for those devices (ARTISTIC,41 Newborn CHD,42 EVAR46). For example, the Newborn CHD42 study may have contributed to improved design of pulse oximetry devices to make them suitable for children. Only one study41 noted an impact through the development of a new product: work conducted through the ARTISTIC41 study contributed to the development of new primary HPV screening tests and also a decrease in the price of those tests.
It should be noted that there is one case for which it could be argued that the study had the effect of reducing R&D. The CUtLASS case study51 suggests that the findings showing that atypical antipsychotics were not superior to the older drugs may have contributed to a reduction in research in the pharmaceutical industry into developing new drugs for schizophrenia. Although it is not straightforward to attribute it to the study,51 it is clear that several companies have withdrawn completely from the field.
Potential impact through increased sales
Several studies that demonstrated that a particular technology is effective and hence increased its use could plausibly be considered to have had an impact, through increased sales, on those companies that make those technologies. This applies to the Newborn CHD42 study, EVAR,46 Carotid Stenosis47 and CESAR. 49 However, in all cases we do not have clear evidence on sales levels before and after the study. Such data are not routinely collected or tracked, perhaps reflecting the priorities of both the HTA programme and those conducting research within it. The aim of the work is to impact on policy and NHS practice, and, as such, those involved are often aware of such impact. Benefits to industry are not a key aim of the work and, as such, are not tracked in the same way.
The Health Technology Assessment programme also has an indirect impact on industry through the National Institute for Health and Care Excellence
Although the research funded through the HTA programme does not typically overlap with industry interests (with some exceptions), the programme does have an impact on industry through NICE (I3, I4, I8, I14). As one interviewee explains, it is difficult to disentangle the impact of the HTA programme from the impact of NICE here:
One of major outputs of HTA programme is the work for NICE, which impacts the pharmaceutical industry. Imagine the HTA programme without NICE . . . the pharmaceutical industry would not be as impacted upon. But people have been doing HTA for ages. In countries that don’t have an equivalent of NICE, people can take HTA reports or leave them . . . they just get diffused into the general literature.
I4
One of the ways that the impact of the HTA programme specifically can be observed is through its methodological impact on the pharmaceutical industry (I4, I8, I9, I14). Because of the work that NICE commissions through the HTA programme, the methods that NICE uses, particularly for the economic analysis, are those developed and used through the HTA programme. One interviewee explains this as follows:
Pharmaceutical companies are trying to get products licensed and the regulatory framework for research to achieve product licensing is out by different regulatory bodies. That is independent of the HTA, but the important connection with the HTA is through the work that the HTA programme commissions for NICE, which includes a lot of cost-effectiveness analyses in primary and secondary research, because the pharmaceutical industry is particularly concerned about NICE decisions, which means that they have to use the methods that are used for NICE to make its decisions. Those methods are the methods used by the HTA programme.
I9
The RA45 case study provides a useful illustration of these two ways in which HTA research, particularly TAR studies, can impact on industry, largely because of the influence they have on NICE. First, the study45 led to the inclusion of new drugs into NICE guidance, so the relevant companies will now be able to receive reimbursement on the UK market for those drugs. In addition, there is anecdotal evidence that the cost-effectiveness model developed and refined through this study45 has been used by some pharmaceutical companies in their applications for approval of similar therapies by NICE. This illustrates how the TAR studies can have methodological impacts on industry.
Similarly, the STAs52 are likely to have an impact on industry insofar as they influence NICE decisions and hence the drugs purchased by the NHS. There may also be individual examples of methodological impacts, although none is noted in the case study,52 and perhaps the scope for methodological advancement in the short turnaround of a STA is limited.
The cost-effectiveness analysis conducted in Health Technology Assessment studies can affect industry but also the economy more widely through more efficient allocation of NHS resources
The HTA programme may sometimes have a positive impact on industry when a particular drug, device or other treatment is shown to be cost-effective, but it may also have the opposite effect when it shows that a particular drug is not cost-effective (I7, I19).
However, this cost-effectiveness analysis has wider implications. The HTA programme has an indirect impact on the economy by providing evidence on the cost-effectiveness of different treatments, which policy-makers can use to allocate resources more efficiently (I3, I7, I19). This ultimately may have even further impacts on the health and hence productivity of the population as set out by one interviewee:
[The HTA programme] results in more effective use of healthcare resources, which both frees up some of the NHS’ money and has led to a healthier, potentially more productive, population.
I7
As described previously, two of the case studies43,48 provide some limited evidence of potential cost savings for the NHS or patients. The IVAN43 study is likely to have played a role in saving the NHS money by contributing to a reduction in the cost of Lucentis to the NHS through renegotiations, and SWET48 showed that water softeners are not effective for the treatment of atopic eczema in children, potentially saving patients money, as they can avoid spending money on treatments that do not work, although the evidence around this is unclear.
In addition, three of the case studies44,47,49 suggest use in the NHS of new effective (and cost-effective) treatments has led to health benefits for patients (CRASH-2,44 CESAR49 and Carotid Stenosis47). It may be possible to extrapolate this to economic benefits, through the improved health and productivity of the population, but to do so in any concrete way is extremely challenging. The CRASH-244 case study does allude to this. The study44 authors, in the HTA journal article, reflect on the groups most affected by trauma. It is noted that the poor are disproportionately affected by trauma, with the risk higher for more disadvantaged groups in the UK or internationally. It is also noted that the group that is predominantly affected by trauma is young men – the average age of participants in the CRASH-244 trial was 35 years old, and 85% of participants were male. They suggest that this means that the social and economic benefits of reducing death by trauma could be very significant. However, providing clear evidence of these type of benefits, or quantifying them, has not been possible within the scope of any of the case studies.
At a higher level, there is evidence from a previous study looking at a sample of 10 HTA programme-funded studies, that if 12% of the potential net benefit of implementing the findings of that sample of 10 studies for 1 year was realised, it would cover the cost of the HTA programme from 1993 to 2012. 33
Impact internationally
Health Technology Assessment research is used outside the UK, particularly by other Health Technology Assessment organisations and those conducting systematic reviews
Research funded by the NIHR HTA programme is viewed as a reference resource by people internationally (I3, I16), and a lot of HTA organisations around the world rely heavily on NIHR HTA research (I3). This is because, in contrast with HTA programmes in most other countries, the programme does original research rather than relying on existing studies and analysing them (I8). One interviewee also described how ‘people in other countries that are in the HTA or systematic review business are often aware that there is an important HTA trial under way and are keen to see its results’ (I14). Several interviewees mentioned in particular a high number of web hits and downloads from the HTA website, including internationally (I7, I14, I16). Data provided by NETSCC show that the HTA website received 228,777 (unique) hits between March 2014 and February 2015, and that over the same period there were 111,102 documents downloaded, of which 93,879 were of full HTA monographs. It is not clear, however, how many of these were within or external to the UK.
It was generally felt that the programme is well known internationally, particularly among other HTA organisations (I3, I4, I7, I8, I10, I14, I16), with one interviewee describing the programme as having ‘a very strong brand’ (I19). However, by contrast, some interviewees (I3, I4, I9, I12, I18) expressed concern that there was confusion regarding the identity of the programme, both internationally and in the UK. In particular, there is confusion between the HTA programme and NICE, perhaps reflecting the fact that these roles are often combined in one organisation in other countries. There is also some confusion between the HTA programme and NIHR more widely, part of which may come from the fact that funding from the different NIHR streams is not always clearly delineated (I5).
One interviewee also noted that HTA research is often cited in guidelines outside the UK as ‘international HTA programmes turn to the HTA programme’s work to inform their own guidelines’ (I8).
Most case studies had some international impact, across academia, policy and practice
Of the 12 case studies,41–52 nine studies41–47,49,52 found evidence of international impact. As illustrated in Table 14, these spanned academia, policy and practice in particular. Each of these areas is explored in more detail in the following sections.
| Case study | Overall | Academic/research system | Policy | Practice | Industry/product development |
|---|---|---|---|---|---|
| ARTISTIC41 | ✗ | ✗ | ✗ | ||
| Newborn CHD42 | ✗ | ✗ | ✗ | ✗ | ✗ |
| IVAN43 | ✗ | ✗ | ✗ | ✗ | |
| CRASH-244 | ✗ | ✗ | ✗ | ✗ | |
| RA45 | ✗ | ✗ | ✗ | ||
| EVAR46 | ✗ | ✗ | ✗ | ✗ | |
| Carotid Stenosis47 | ✗ | ✗ | ✗ | ||
| SWET48 | |||||
| CESAR49 | ✗ | ✗ | ✗ | ✗ | |
| CoBalT50 | |||||
| CUtLASS51 | |||||
| STAs52 | ✗ | ✗ |
Most of the studies have had an impact on policy and practice internationally, and only in the case of the Technology Assessment Reports was this mediated through National Institute for Health and Care Excellence
More than half of the studies have been cited on international policy statements or guidance, either for individual countries outside the UK or wider international guidance. For example, as a result of the CRASH-244 study, TXA is listed by the World Health Organization (WHO) as an essential medicine, and five studies42,44,46,47,49 are cited on European level guidance. Through this tangible impact on international guidance, many studies indicate that the studies have influenced practice internationally, although there is more concrete evidence of this in only a few cases. In the majority of cases, this impact has occurred directly as a result of the study itself, which was important in the field and hence is directly referred to by international sources. In the case of the TARs, this effect is mediated through NICE, where NICE guidance, drawing on HTA evidence, influences international policy and practice. One case (ARTISTIC41) indicates that the international impact occurred through the study41 contributing to pooled results, the analysis of which has influenced policy and practice internationally.
Impacts on research internationally stemmed from the sharing of results, tools and practices, and developing the field both through capacity building and the targeting of future research
As described previously, several studies pooled results with other studies internationally, or shared research tools and practices with other research groups internationally. Contributions were also made to the capacity building of overseas collaborators through the work as described for CRASH-244 in Chapter 4 (see Funding research that can make a difference, below). Two case studies43,49 referred to methods being used in the development of other studies internationally. For example, the IVAN43 study influenced a number of RCTs in other countries, which began during the course of IVAN through the sharing of protocols. By contrast, an international study looking at ECMO has designed its approach to address the one of the perceived methodological limitations that was criticised in the case of the CESAR49 study. Several studies referred to related studies being conducted subsequently in other countries.
Clear evidence of international impact on industry and product development is more difficult to demonstrate and is typically inferred
Where research has had an impact on product development, those products are typically developed by global manufacturers and, as such, the impacts are international. However, the details of how and where within industry those impacts have occurred has not been investigated in detail. In the case of the STAs,52 it is also noted that they will probably have an international impact just because NICE guidance is so influential internationally. For example, it is suggested that both NICE guidance is likely to impact on wider international marketing decisions that would have international implications for industry.
The Health Technology Assessment programme has an impact internationally through the international reputation of the National Institute for Health and Care Excellence
In addition to the influence of the HTA programme independently, it also has an impact internationally because of the reputation and influence of NICE internationally, influence which is amplified by NICE’s policy of providing free access to its publications. As described above (see The Health Technology Assessment programme also has an indirect impact on industry through NICE), part of the reason that NICE is so important to industry is because of its international influence (I3, I4, I8). One interviewee described how NICE had an impact on policy and practice internationally:
NICE’s influence is both national and international: other countries are setting up their own version of NICE or taking NICE’s views into consideration when designing their own policies. NICE made HTA programme influential.
I8
This is seen in the two case studies focusing on TARs,45,52 as described above, where any international impact was mediated by NICE rather being a direct impact of the HTA research itself. However, as described above, many of the studies directly impacted policy internationally. This is partly because of the sample of case studies selected. Many of them are important internationally known studies in their fields, which show significant results. As such, they are likely to impact on international policy directly. Impact via NICE as an important decision-making body with international recognition may be more significant for other less well-known studies.
Chapter 4 The impact of the Health Technology Assessment programme
In addition to the impact of projects in the HTA programme, discussed in the last chapter, the programme itself has influenced thinking in the health system and the attitudes of researchers, policy-makers and clinicians, both within and beyond the UK. This chapter summarises the programme-level impacts of the HTA programme, those impacts above and beyond the collection of impacts of the individual projects. These observations are based primarily on the interviews, but also draw on the other data sources and fall into the following categories: changing attitudes; supporting research effectively; and funding important research.
Changing attitudes
The Health Technology Assessment programme has contributed to the cultural change in attitudes towards medical research, which has involved a paradigmatic shift towards evidence-based and, more recently, economic evidence-based medicine
Many interviewees commented on the role that the HTA programme has played in the shift towards evidence-based medicine (I6, I8, I9, I14, I16, I19). This includes not just its role in providing an important part of the underpinning evidence required to support an evidence-based NHS, but also in changing attitudes within the research community towards conducting this type of research, and its importance and validity. For example, one interviewee stated that:
The research establishment has completely changed its view about the validity and important of the HTA’s kind of work. The HTA isn’t the only driver of this change but it exemplifies the change and was at the centre of that change. The HTA did not cause the changed but it played a role in the general paradigmatic change in what is meant by good health research in the last 20 years.
I8
Another commented that:
[the HTA programme] has had a fundamental role to play in increasing the focus on clinical and applied research . . . it has also been important in changing perceptions about the importance of applied and practice based research, clinical and applied research. It has changed the views of the funding bodies and the universities about that.
I19
In addition, one interviewee commented:
In the 1990s and early 2000s, the HTA programme was one of the drivers behind the change in the attitude towards research in that research should be applicable, needs-led and impactful work.
I8
Interviewees also commented on the importance of the HTA programme in changing attitudes towards research in the NHS (I14, I19). For example, one interviewee stated that:
Initially, for the first 50 years of the NHS’s existence, it did not fund research. Now, it puts 1% of its budget into research. The HTA programme is only a small part of that but it’s the part that provides the justification for spending money on research.
I14
Another commented that:
for the people in the NHS, people feel that some research is very abstract and obtuse and a long way from practice, and the HTA programme answers questions that are deeply practical questions to people in the NHS and the patients that they serve.
I19
One interviewee noted that the HTA programme has also had an important impact on policy-makers, beyond its impact on policy, through helping to change the perception of policy-makers from thinking of research as very academic and esoteric to something that is practice and answers relevant questions (I19). This is, however, difficult to observe and evidence.
Comments were also made about the role the programme has played, alongside NICE, in the increasing focus not just on effectiveness, but also cost-effectiveness in medicine (I3, I8, I9, I16, I19). For example, one interviewee commented that:
Over the last 30 years or so, the whole way of thinking of medicine has changed to an evidence-based mode and, in the last ten years or so, to an economics of medicine evidence-based mode and the HTA programme is the principal vehicle.
I16
This is summarised in what one interviewee felt was the most important achievement of the HTA programme:
Changing perceptions of HTA research, resulting in a shift in esteem with which health service research was held, and making sure people take account of population effectiveness and cost-effectiveness.
I8
The Health Technology Assessment programme has had an impact on the wider Health Technology Assessment movement internationally
Interviewees suggested that the HTA programme is viewed favourably compared with other HTA programmes internationally (I8, I16), although many respondents note that the HTA programme is very different from other countries’ programmes because most other HTA programmes focus on only secondary research/systematic reviews (I3, I14). The HTA programme’s connection to policy-makers, particularly NICE, was seen as a particular strength of the programme compared with other countries (I16). Another important difference is the separation of functions between the HTA programme in NICE. In many countries, the appraisal and assessment functions are combined in one body (I6).
Broadly, the programme is considered to be a thought leader in the HTA space internationally (I10, I15, I19). For example, one interviewee described the programme as a ‘pioneer in this area’ (I10), and another set out that ‘a lot of its international standing is based on its academic strength’ (I19). One interviewee described how the UK HTA programme had played an important role in a wider international movement, increasingly recognising the importance of this type of research:
There’s a big international movement in health technology assessment and the HTA programme has played a big and leading role in that internationally . . . in helping governments to see the benefits of assessing health technologies and appraising them.
I15
Supporting research effectively
Oversight from the Health Technology Assessment programme is generally considered proportional and supportive, and in several cases can be seen to directly contribute to the success of the work
The general sense across the case studies was that the level of oversight provided on the work was largely appropriate. For example, one interviewee from the ARTISTIC41 case study suggests that ‘they maintained some oversight, but it was very light touch’. Similarly, according to the CRASH-244 case study, the CI did not consider the monitoring to be disproportionate to the scale of the funding. However, one study did comment on increasing bureaucracy (EVAR46), referring in particular to delays in the contracting process for the follow-on work delaying progress.
Although the overall picture was similar, interim reporting requirements seemed to differ between studies. For example, for the ARTISTIC study,41 this consisted of submission of interim reports, meeting minutes from the Trial Steering Committee (TSC) and the independent Data Monitoring Committee. The CESAR49 and SWET48 studies suggested that the project had to submit annual reports, whereas for CoBalT50 these were every 6 months, and SWET48 was subject to a monitoring visit towards the end of the recruitment period. This may reflect changes in the process over time, or differences depending on the scale and nature of the research.
Many case studies provide evidence that the HTA was supportive. This could be through their intermediary role in the case of the TARs, which was highlighted as important to the TAR team in the case of the RA45 study by one of the researchers, as they were involved in negotiating with NICE when timelines were perceived to be too short. They also provided support for follow-on work for several studies. As an example, the IVAN case study notes that regular updates were provided, and the HTA programme remained aware of progress throughout, which meant that there were no concerns about granting a 3-month extension to the study43 when requested by the team (see Appendix 4). More generally, several studies include comments on the positive attitude of the HTA programme overall and commented favourably on the interaction with the programme. For example, one interviewee from the IVAN43 case study commented that ‘of all the funders I’ve ever dealt with, I’ve always found the HTA to be the most reasonable and interested in making the research happen. It was and continues to be an extraordinarily refreshing process compared to previous funders’.
Several studies also provide evidence that interaction with HTA programme management supported the successful completion of work or its impact. In the CoBalT50 study, this took the form of advice on how to conduct the study. The HTA group suggested ways to improve recruitment rates, which had been problematic, and this advice proved to be very helpful. In the case of the ARTISTIC41 study, this took the form of flexibility around the reporting of the study. One member of the team noted that being able to publish results in other journals ahead of the monograph being released allowed work to have a quicker impact. In the case of the IVAN43 study, there was some urgency in getting the project started because of the changing policy environment, and the case study43 notes that the HTA programme did expedite the process as much as possible to ensure that the study43 was able to take place.
The Health Technology Assessment journal is generally considered to make an important contribution to the academic impact of Health Technology Assessment programme-funded research
As described above, the HTA journal provides a way to ensure the research funded through the programme is published in full as standard. However, several interviewees (I3, I4, I6, I7, I16) commented on the quality of the journal in addition, noting its high levels of citation and the fact that it may be more widely picked up (and potentially by different audiences) compared with publication in a standard academic journal. One interviewee commented that ‘something that is published through the HTA programme is probably more read and more cited than a more standard paper in an academic journal’ (I4).
This is supported by the bibliometric evidence in Figure 10, which shows the MNJS for the publications analysed. This measure indicates the level of citation of journals in which HTA research is published normalised for research field. The average value is one, so values of ‘< 1’ indicate that research is cited more frequently than would be expected for that field. The MNJS indicates that the HTA journal is cited 2.31 times more frequently than other journals in the same field. The other journals that HTA-supported research is published in tend to be slightly less visible, with an average MNJS of 2.14 over time, but this is still more than twice the average for their fields of publication. The MNJS has remained fairly stable over time, with no obvious trend.
FIGURE 10.
Mean normalised journal score per year, for all publications from HTA programme-funded research and for the HTA journal only. Note: this is based on a sample of publications identified through three sources: all articles and reviews published in the HTA journal during the period 2004–12; papers listed on all HTA project pages of the NETSCC website; and papers reported by researchers in Researchfish. As such, this may not be a complete record of all publications related to HTA programme-funded research.
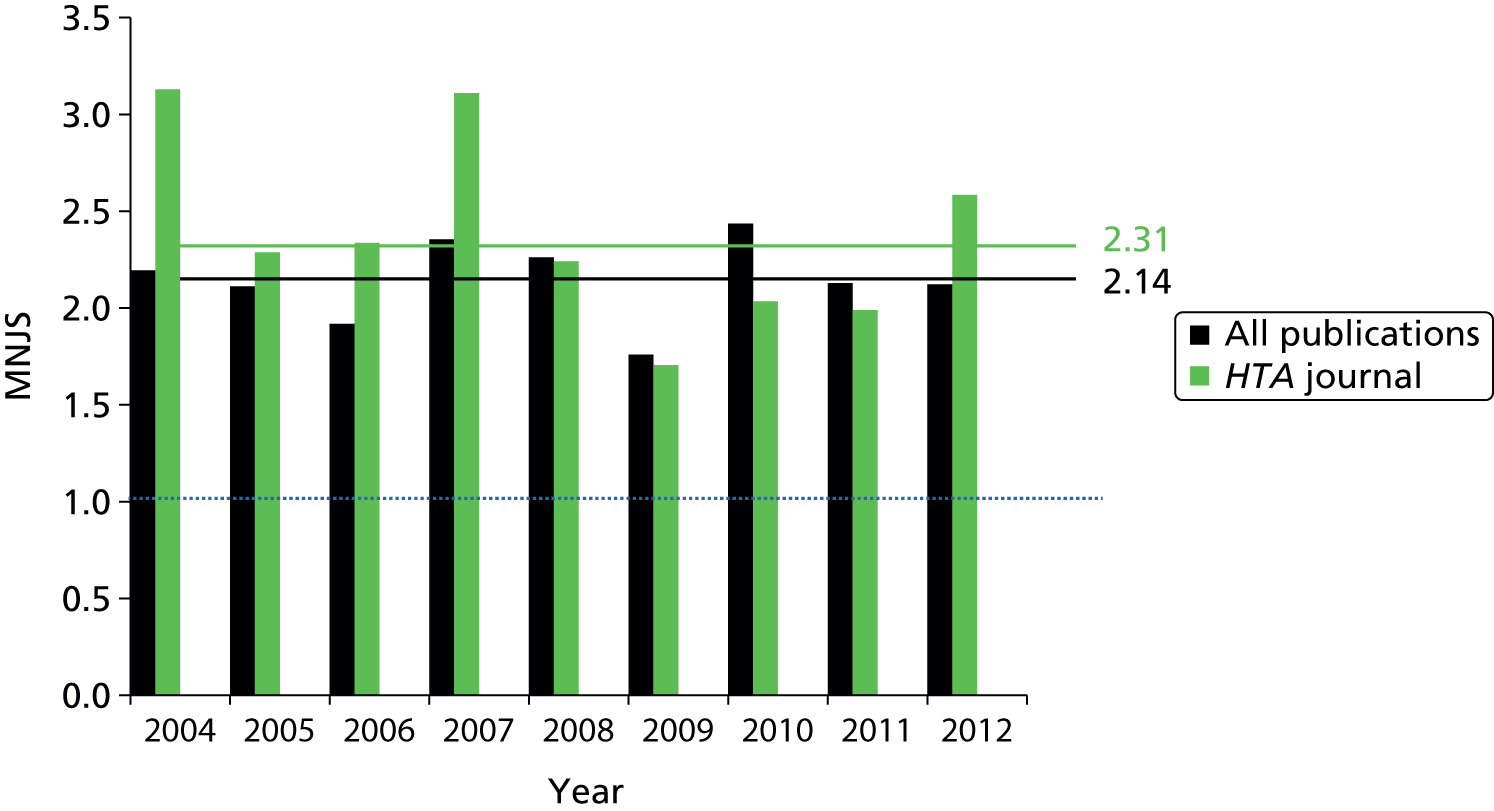
Also noted in interviews was the high number of downloads of the HTA journal (I3, I6, I16), including from outside the UK. Data provided by NETSCC indicate that over the year 1 March 2014 to 28 February 2015 there was a total of 111,102 PDF downloads, of which 93,879 were for the full HTA monograph.
However, some respondents questioned the added value of the HTA monograph to the independent publications resulting from the same research (I7, I14). For example, one interviewee suggested that ‘the monographs simply reproduce the academic publication or don’t go sufficiently beyond it to justify the production of the monograph’ (I14), and another commented that it is ‘hard to know impact of monographs over and above the journal articles’ (I7). One interviewee suggested that this should be monitored to ensure that the monograph does provide a lot more detail than other published outputs to avoid research waste (I14). However, one interviewee from the ARTISTIC41 case study stressed the importance of the HTA monographs. The interviewee noted that the monographs are invaluable to policy-makers because they provide much more detail than the academic publications. This should be contrasted, however, with the role the HTA journal plays in ensuring that virtually all HTA research is published as outlined above.
The Health Technology Assessment programme tries to ensure that its research delivers added value, although some respondents provided specific examples of duplication of research effort
The HTA programme, and the NIHR more widely, have adopted the ‘Adding Value in Research’ framework20 based on the work of Chalmers and Glasziou (2009). 14 As described above, one element of this in which the HTA programme is considered ‘exemplary’ (I1) is in the publication of the vast majority of its research. This is an area in which there is also suggestion from the interviewees that other research funders are trying to emulate the achievements of the HTA programme (I1, I10). Another area receiving praise was the use of systematic reviews in the determination of which primary research to fund (I1). One interviewee stated that they ‘don’t know of any research funding agency in the world that has done those two things systematically as the HTA programme’ (I1). Another interviewee pointed to flexibility with the HTA in their funding processes allowing them to save money, pointing to a specific example where they were open to alternative approaches which might offer the same information more cheaply than a clinical trial (I5).
Avoiding duplications of research is also important and an area where the picture is more mixed. Some interviewees pointed to the importance of some individuals in the HTA programme in ensuring that this does not take place (I1, I15), but others could identify specific examples where such duplication between funders had occurred (I1, I14).
One researcher also commented on the process of applying for HTA funding as being bureaucratic and not very user friendly, suggesting that they could benefit from ‘a user group to advise them on the experience’ (I14). This suggests that there may also be potential to prevent wasted time by streamlining this process, balancing, of course, the need for appropriate data collection and oversight.
The Health Technology Assessment programme is recognised as being one of the first public funders to require patient and public involvement and has continued to be viewed as a leader in this area; however, a number of respondents noted that the impact of that involvement is unclear and not well monitored
Several interviewees felt that the HTA programme had played a role in the development of the use of PPI in the UK and that it was an early actor in this area (I3, I5, I7, I8, I13, I19). One interviewee commented that ‘the HTA programme was the first national, publicly funded programme to insist on PPI’ (I8), another that ‘it’s probably one of the programmes with the longest standing relationship with involvement of patients and the public’ (I13).
In particular, the programme was praised for involving PPI throughout the research process, and several commented that it is a leader in this field in demonstrating that this is feasible. Two interviewees singled this out as the most important achievement of the programme: ‘giving PPI credibility’ (I8) and ‘demonstrating that a research funder can involve PPI throughout the research process’ (I13). One of the interviewees described how this process works:
The HTA programme has a lot of engagement: within its own processes has public and patients involved in all stages of the research, prioritising and funding process. Public/patients are on all of the boards and committees and conduct peer reviews. There is a patient and public steering group.
I7
However, despite this positive picture overall, there was some questions among some respondents around the impact of that involvement. In particular, at present it is hard to see how effective that PPI is, and what impact it has on the way research is funded and conducted. A lack of monitoring was mentioned by two interviewees, one commenting that:
What I can’t put my hand on my heart and say is: the impact of the PPI involvement is this . . . I can’t say that they always have an enormous impact. My gut-feeling is that, they do, but I can’t prove it.
I7
This lack of evidence around the effectiveness of PPI is not unique to the HTA programme. 59 However, the observation remains that although the HTA programme has been a leader in terms of its processes for PPI, the impact of its investment in PPI is less clear and does not appear to be well monitored.
Evidence from the case studies supports the notion that the HTA programme actively encourages the use of PPI in its studies, but it is again difficult to conclude anything about the impact of that PPI. There was much less formal PPI at the time many of these studies started, and the change in attitudes to PPI over the time period is noted in five45–47,50,51 of the case studies. Where it is mentioned, there is generally a positive attitude towards PPI, but it is hard to conclude anything quantitative from our sample about the value of PPI.
What we can say, however, is that nearly all of the studies involved some PPI. This tended to be focused on patients and their families, rather than the public. Six case studies showed significant involvement of patients for example through focus groups, protocol development, determination of outcome measures, dissemination strategies and steering committees (Newborn CHD,42 SWET,48 CESAR,49 CoBalT,50 IVAN,43 CRASH-244). For example, SWET48 involved the National Eczema Society and a local group of carers in the work, with a patient representative involved during all stages of project from design to write up and dissemination, and the CRASH-244 study involved patients in defining outcome measures and the development of protocol at start of study. Four of the studies stated that inspiration from patients fed into the studies through the clinical work of members of the team (CUtLASS,51 EVAR,46 CoBalT,50 Carotid Stenosis47). For example, in the CUtLASS51 case study it is noted that there was significant insight into patient need as result of the lead researchers being clinicians directly treating patients and in contact with families. In the case of the two TARs,45,52 PPI input was via NICE, with patient representatives contributing to TAR selection and sitting on the NICE Appraisal Committee. In particular, one study (Newborn CHD42) was remarked on as being at the forefront of PPI for the time.
Most PPI was through patient groups or appointed representatives. Of the six case studies42–44,48–50 with significant independent PPI, four studies42,43,48,49 engaged with patient and family groups and three studies48–50 used appointed patient representatives. Other forms of input, each used in one study, were practitioner input, the use of learning from patients in a previous project (around appropriate outcome measures), and use of learning from patients in pilot work.
The structures and relationships that the Health Technology Assessment programme has developed, linking its research to the National Institute for Health and Care Excellence and the National Screening Committee, are important in facilitating the impact of its work
As described in Chapter 3 (see The primary route to impact of Health Technology Assessment-funded research is through guidelines, particularly National Institute for Health and Care Excellence and National Screening Committee guidelines and National Institute for Health and Care Excellence and the National Screening Committee are key users of Health Technology Assessment research), the relationships that the HTA, at a programme level, has with NICE and the NSC are important in facilitating much of the impact of the work that it funds. This was identified in the previous study into the impact of the first 10 years of the programme by Hanney et al. (2007)1 and remains the case. In particular, the structures around priority setting and the TAR programme are important in ensuring that research addresses questions of relevance to these key users and that the findings impact on policy and, ultimately, practice.
The STAs are an interesting example of this, which have emerged over the last 10 years. As described in the case study, this stream of research was introduced with the aim of producing independent evidence more quickly to support NICE decision-making, and, through speeding up that NICE decision-making process, they facilitate faster impacts on practice, with potential benefits for both patients and industry.
Although the specific impacts come through the individual projects, by having these structures and relationships in place, the HTA programme enables its work to have a more direct impact than might otherwise be possible.
Funding research that can make a difference
The Health Technology Assessment programme answers questions that would not be answered by other funders and fills important research gaps
The HTA programme’s stated aim is to ‘research information about the effectiveness, costs and broader impact of health-care treatments and tests for those who plan, provide or receive care in the NHS’. Interviewees felt that the programme was not only successful in doing this, but also that it focuses its resources on topics that would not be supported through other means (I3, I6, I8, I16, I18). As one interviewee describes it:
[The HTA programme] answers questions that aren’t going to be answered by other means of research, which are the basic research imperatives that fund the universities and pharmaceutical company research, which doesn’t usually answer quite the questions we want to know.
I16
Another interviewee stated that the programme ‘plugs a really important gap in research we need to make recommendations’ (I18), whereas one commented that the HTA programme is ‘funding things industry won’t fund’ (I3).
The HTA’s prioritisation process plays an important role in this and was praised by one interviewee as ‘[ensuring] really important questions that need really good quality research are addressed rigorously and made publicly available’. Another commented that the ‘philosophy of the programme has been consistent: NHS relevance’ (I6).
However, some interviewees criticised a lack of transparency in the priority-setting process (I13, I14). For example, one interviewee noted that although the HTA programme has a good relationship with the NSC, it does not keep a good record of the work that it does for the NSC (I14), and, similarly, noted that looking at HTA programme-funded studies, it is ‘very hard to identify the source of the topic and particularly to identify how many of them came from the public’ (I14).
An important development over the last 10 years has been the growth of researcher-led research, particularly alongside the growth of the primary research stream. Given the clear priority-setting agenda in the HTA programme, initially the programme had been entirely commissioned-mode funding. However, this caused some challenges, as set out by one interviewee:
Researchers had good ideas that they might not want to share with [the HTA programme] because they might not get the contract. A lot of researchers were not giving [the HTA programme] their best topics. By creating the responsive mode, they can keep ownership of topic themselves.
I6
However, the researcher-led arm of the portfolio is still subject to the same prioritisation process. The topic is reviewed in the same way that a topic under the commissioned arm would be and the researcher still has to make the case around why it is important to the NHS. As one interviewee commented:
The research questions come from the practice community rather than the academic or research community. That remains the core of the HTA programme, even though it has now added on the researcher-led stream.
I9
The Health Technology Assessment programme has a track record of commissioning work that may be controversial
Evidence from the case studies suggests that the HTA programme is not afraid to commission controversial work or studies in areas for which many stakeholders already hold strong assumptions about the outcomes, which, when contradicted, can lead to controversy. Many of the case studies are noted as being in some way controversial. In particular, two of the studies41,43 were commissioned in directly controversial environments (ARTISTIC,41 IVAN43). The IVAN43 study in particular is noted as being politically high profile from the start, with a number of discussions taking place in the House of Commons about the difference in cost between Avastin and Lucentis, and the availability of both on the NHS.
Several other studies were commissioned in situations for which stakeholders such as industry, patients, and clinicians had existing expectations about the likely outcomes of the study. When these assumptions were confirmed, such as in the case of the EVAR46 study, this facilitated uptake of the findings. However, where the assumptions are contradicted, there seems to be reluctance to change practice (e.g. CESAR,49 CUtLASS51). Although specific groups with vested interests in the study results can lead to an unpleasant environment for researchers – for example, one of the researchers involved in the CUtLASS study suggested that it took around 2 years for the findings to be accepted in the field, during which time he described himself as being ‘kind of ostracised’ by the pharmaceutical industry – these studies also have a significant potential for impact on practice when implementation can occur, as they are challenging existing assumptions and practices.
In addition to controversy around study findings, several case studies comment on controversy around the methods used in the studies. These tend to fall into to two groups. First, there are cases when there is also controversy because the results are unexpected or not as desired (EVAR,46 RA,45 CUtLASS51). In other cases, there is some controversy in another country (typically the USA) because the methods do not correspond to existing practice there. This reflects the NHS focus of the work and study designs, as described in Chapter 3 (see Study design can influence whether or not research is implemented in the NHS).
Chapter 5 Discussion
In this chapter, we return to the key study questions: what has been the impact of HTA programme-funded research and the HTA programme from 2003 to 2013, and how can it be increased in the future? The first section provides an overview of the impact of the HTA programme from 2003 to 2013, looking at evidence both at the programme level and from the individual projects as described in the previous two chapters. In the second section, we explore what lessons can be learned from the last 10 years of the HTA programme, and ways that the HTA programme can increase its impact in the future. Finally, we include a discussion of the limitations of the study approach and findings, and provide some overall conclusions and recommendations for future research.
What is the impact on policy, practice, health, the economy, society more widely and research of the Health Technology Assessment programme over the period 2003–13?
The Health Technology Assessment programme has had important impacts on patients through health policy and practice, and impact on practice can be direct as well as via the National Institute for Health and Care Excellence and the National Screening Committee
Across the programme as a whole, the HTA’s impact on patient care is primarily an indirect impact, mediated through guidelines. The case studies provide evidence of the types of impact that the HTA’s research can have on patients and the NHS, including changes in NHS practice, health benefits for patients and increased patient choice. However, it is sometimes difficult to determine the impact of HTA programme-funded research on clinical practice because HTA studies are typically commissioned when the evidence is unclear, such that many studies show that existing practice is appropriate, which we observed in two of the case studies. 48,50
Given the close links between the HTA programme and NICE through, for example, the TAR programme, it is legitimate to look at the joint impact of NICE and the HTA programme on clinical practice, and it is often not possible to attribute any observed impact to either organisation, as both contributed. However, the case studies illustrated a number of examples for which NICE was not the most effective route to impact for HTA research. For example, because of the interests of other groups, NICE has not yet been an effective route of implementation for the treatments assessed in the CRASH-244 and IVAN43 studies because of the conflicting interest of other groups (notably industry). Despite this, the results of the CRASH-244 study have been widely implemented.
For screening studies, the relationship with the NSC provides another important route to impact on clinical practice. Where evidence from the HTA programme suggests that screening for a particular condition is both effective and cost-effective, the national screening programme often then undertakes a screening pilot programme. Two examples of this are ARTISTIC41 and Newborn CHD42 case studies. The NSC pilot itself has an impact on the patients involved in the study, and any resulting national screening programmes then have an impact on all of the patients in the target population.
There are also other ways through which the HTA programme can have impact. As the major funder of clinical research in the UK, the programme may have an impact on the quality of care provided in the NHS through supporting a high volume of research in the NHS by increasing the skills of clinicians. However, the impact of research on clinical skill has not yet been well researched. There is also some evidence that patients benefit from participation in clinical trials. However, the primary impact of HTA programme-funded research on patients is through producing high-quality scientific evidence that results in improved guidance for clinicians which, if implemented, should improve patient care.
Interviewees and the case studies suggested that impact on practice beyond the NICE and NSC routes might be limited by the HTA programme’s dissemination strategy. It was recognised that this challenge of dissemination and adoption is not unique to the HTA programme. However, the overall dissemination strategy was considered limited and too academically focused. This challenge in dissemination was reflected in the case studies, where several note a lack of support for dissemination and implementation, which may have limited the impact of HTA programme-funded research on NHS practice. The case studies illustrated that this challenge was particularly pronounced where other stakeholders have interests or expectations that run counter to project findings. However, the case studies also illustrated that some of these obstacles can be overcome by active and enthusiastic project teams.
The Health Technology Assessment programme has an impact on UK policy, primarily through its close links with the National Institute for Health and Care Excellence and the National Screening Committee
The HTA programme funds robust scientific research that is clearly and deliberately linked to policy-makers. Many interviewees viewed the evidence that the HTA programme produces on the effectiveness and cost-effectiveness of treatments as invaluable to policy-makers. Of the 12 case studies,41–52 10 studies41,42,44–49,51,52 indicated evidence of some impact on UK policy. The main routes to policy impact were through citation in clinical guidelines, typically NICE guidance and NSC pilots, which supports the finding that NICE and the NSC are key users of HTA research. The programme has close relationships with both NICE, particularly through the TAR programme, and the NSC. These findings regarding the impact of HTA programme-funded research on health policy are consistent with the findings of Hanney et al. (2007)1 on the impact of the HTA programme from 1993 to 2003.
However, we found that there are also other users of HTA research, including other guideline producers such as SIGN. The shift in focus towards primary research may also have an impact on the appropriate audience for HTA research, shifting it upstream from policy-makers towards specialist clinicians, other researchers and systematic reviewers. The case studies also provide examples where there are other routes to impacts on policy and practice. The impact of both the CRASH-244 and IVAN43 studies on NICE guidance has been hindered by the lack of incentive for industry to seek a marketing authorisation for the drug in question, but, in the CRASH-244 case at least, alternative routes to impact on NHS policy and practice have been found.
Health Technology Assessment research has an impact on policy and practice
Health Technology Assessment research is used outside the UK, particularly by other HTA organisations and those conducting systematic reviews. This is reflected in the case studies, with most of the studies having had an impact on policy and practice internationally. In the majority of cases, this impact has occurred directly as a result of the study itself, which was important in the field and hence is directly referred to by international sources.
The programme also has an international influence through its impact on HTA practice. The NIHR HTA programme is considered to be a thought leader in this area, and plays an important role in a wider international movement that increasingly recognises the importance of this type of research. This international influence is reflected in the case studies, with impacts on research internationally stemming from the sharing of results, tools and practices, and developing the field both through capacity building and the targeting of future research. In addition to the influence of the HTA programme independently, it also has an international impact through the reputation and influence of NICE internationally.
The work of the Health Technology Assessment programme is highly cited and considered academically rigorous, but the academic impact of the Technology Assessment Reports may be limited
The HTA programme has made a substantial contribution to health research through the publication of the monographs in the HTA journal, as well as by encouraging independent publication in other peer-reviewed journals. The work produced by the HTA programme is academically rigorous research, which is reflected in the bibliometric data both at the project level and across the programme as a whole, with citation levels more than double the expected level for the field. This finding regarding the academic quality of the research is also consistent with Hanney et al. ’s (2007)1 assessment of the impact of the first 10 years of the HTA programme from 1993 to 2003.
The HTA journal makes an important contribution to the academic impact of HTA programme-funded research, ensuring that all research is published in full, making much more detail available than would typically be present in academic publications, without restricting the ability to publish findings elsewhere. These observations about the HTA journal were reflected in the case studies, where most of the studies published academic articles outside the HTA journal publication, and both those articles and the HTA journal articles were typically highly cited.
One notable exception is the TAR stream of HTA research, for which publication opportunities are limited as a result of the nature of the research, in terms of the content, much of which is provided by industry in confidence, and in terms of the tight timelines for delivery of the work and the changing focus of researchers moving from one topic to another. STAs are not typically included in the HTA journal and finding other routes to publication can be challenging. However, these concerns should be balanced with the benefits that this stream of work offers, particularly in delivering timely evidence with a direct link into the policy-making process, which allows changes in practice to happen quickly.
Capacity building is not limited to members of the research teams, reflecting the importance of the programme as a funder of UK clinical research
The HTA programme is viewed as an important funder of clinical research, which has had a positive impact on both the careers of HTA researchers and on research capacity to carry out high-quality HTA research in the UK. The HTA programme has made a substantial investment in clinical research, and in HTA research in particular, which has played an important role in building and retaining HTA research capacity in the UK. This finding is consistent with the findings of Hanney et al. (2007),1 who described the programme as ‘an important and independent funding source’ (p. 73) and suggested that in several cases researchers felt that finding funding from other sources for particular trials would have been difficult.
Looking at the evidence from the case studies, all of the studies had some capacity-building impacts for the individuals directly involved in the study, although scope for this was sometimes limited because the researchers already had well-established research careers. However, the studies provided important examples of wider capacity building outside the study team by sharing methods and expertise in the conduct of clinical trials, the development of networks, and building up research capacity in a new or emerging area (e.g. by bringing more researchers into the field). Most of the case studies shaped future research in the field, either in terms of research priority setting or the development of new research methods.
The Health Technology Assessment programme has had broader impacts on the research system
As well as the direct academic and capacity-building aspects of the programme, the HTA programme has had an impact on the health research system more widely, through changing attitudes towards research, particularly HTA research, and through funding work addressing the needs of the NHS that would not have been supported by other funders.
The HTA programme has contributed to the cultural change in attitudes towards medical research, which has involved a paradigmatic shift towards evidence-based and, more recently, economic evidence-based medicine. This includes not just its role in providing an important part of the underpinning evidence required to support an evidence-based NHS, but also in changing attitudes within the research community towards conducting this type of research and its importance and validity. Interviewees also commented on the importance of the HTA programme in changing attitudes towards research in the NHS, largely because of the way that it addresses questions that are of NHS relevance. Interviewees commented on the role that the programme has played, alongside NICE, in the increasing focus not just on effectiveness, but also cost-effectiveness in medicine.
The significance of the HTA programme compared with other programmes in some of these changing attitudes is perhaps reflected in the nature of the research that it funds, which would not likely be supported by other funders. The research is not just NHS focused, but aims to focus resources on topics that, although valuable to patients and the NHS, would not be supported through other means, including topics that are not of interest to industry or academia.
What actions can the Health Technology Assessment programme take to increase its impact on policy, practice, health, the economy, society more widely and research in the future?
Provide targeted support for dissemination
Findings from the interviews and case studies suggest that the dissemination of HTA programme-funded research could be improved. The case study evidence in particular suggests that a targeted approach to dissemination resources could be the most effective approach. Based on evidence from the case studies, an approach that allocates fundings after the completion of the research may be the most effective, as it would allow support for dissemination to be focused on projects for which it would provide most value. For example, if the results indicate that existing practice is appropriate, there is no need for the provision of dissemination resources to ensure that the findings are implemented in practice.
The resources provided for dissemination should also take into account the context of the study and the extent to which other stakeholders are likely to champion (or oppose) the study findings. The case study evidence suggests that when there is no champion for dissemination, a role often played by industry, research is more difficult to implement. Also, when results are counter to the expectations and/or desires of stakeholders, including patients, clinicians and industry, uptake may be slower.
By taking a targeted approach at the end of studies to allow the team to remain engaged in the active dissemination of the work, the HTA programme is likely to increase the impact of its work on health policy and clinical practice. However, there are some limitations to this approach. The inclination and skill of researchers in dissemination activities will vary and there may be legal and regulatory obstacles that researchers cannot overcome on their own. As such, the support needed from the HTA programme management may include their influence and input as well as their financial support.
Maintain close relationships with the National Institute for Health and Care Excellence and the National Screening Committee, but also consider working more closely with other policy-making organisations
The close relationships that the HTA programme has with NICE and the NSC contribute to the programme’s impact on health policy and clinical practice. However, the relationships operate quite differently in each case. For the NSC, the links seem more informal but nonetheless direct and effective in both the communication of research needs and the sharing and implementation of findings. For NICE, the relationship consists of many elements, including formal links to the TAR programme, for which the HTA commissions work on behalf of NICE to support its technology assessment process, but also more informal links with other parts of NICE, which inform wider HTA priority setting and commissioning, as well as parts of NICE involved in the preparation of clinical guidelines. These relationships are crucial to the impact that the HTA programme has on policy and practice, and should be maintained. However, it is worth considering whether or not the input of these two important policy-making organisations (and indeed other stakeholders, including members of the public) is sufficiently transparent, particularly with regard to priority setting. Better record keeping and disclosure might allow the programme to better demonstrate how and why it is generating its research priorities. This is discussed further below (see Consider funding research on the implementation of Health Technology Assessment-funded research).
It should also be noted, however, that NICE and the NSC are not the only routes through which the HTA programme can have an impact on policy and practice, as illustrated in several of the case studies. Being aware of other potential audiences – including clinicians, systematic reviewers and guideline producers – will allow the programme to ensure that it is also meeting the needs of these wider groups and thus increasing its potential impact.
Maintain the academic quality of the work and focus on NHS need
The combination of research that is both academically rigorous and of relevance to the NHS was noted as an important feature of the HTA programme. The work is academically rigorous and this is reflected in its bibliometric analysis, but it is also tailored to the needs of policy-makers and the NHS. This balance has been a feature of the HTA programme, as its inception and maintaining it is important in maintaining the impact of HTA programme-funded research. Maintaining the practicality of the work without the rigour would raise concerns regarding the use of the evidence in decision-making and would also lower the academic esteem for, and engagement with, the programme and potentially this type of research. Maintaining the quality without the practicality would significantly affect the programme’s ability to have an impact on health policy and clinical practice. Maintaining both the quality and relevance of the HTA programme’s work is therefore crucial to the future success of the programme and its impact.
Maintain good relationships with researchers and flexibility in the way the programme supports research
As well as being an important funder for academics in clinical research, and HTA research in particular, researchers consulted in the case studies were positive about the contribution of the HTA programme and praised the level of oversight, supportiveness and positive interactions with programme management. Some of the case studies also provided specific examples of how the interaction with programme management and oversight directly contributed to the success and impact of the research. Another important element for academics was the level of flexibility and academic freedom that the programme offers. However, one interviewee reported that the programme’s flexibility has decreased over time. Maintaining these good relationships and positive interactions with researchers is likely to be beneficial in terms of ensuring that communication is maintained and the programme is best able to facilitate the impact of the work it funds.
Particular consideration needs to be given to the TAR programme, which although beneficial in terms of providing a direct link to NICE and a clear route to impact on policy, has proved challenging for academics. Although working with the HTA and NICE is beneficial to academic institutions in terms of the prestige that it offers, interviewees expressed concerns about the fast turnaround of work, the range of topics and the limited opportunities for academic publication. Different TAR centres operate in quite different ways and it may be that there is potential for learning between them about how best to manage the demands of conducting this particular type of work alongside pursuing other academic research. For example, one of the centres described how they had found a route for publication for STAs, which perhaps others could emulate. Similarly, different centres had different numbers of staff involved in conducting TARs and thought could be given to the best balance there to allow staff to pursue other interests while also making sure that teams conducting TARs have the availability and skills to do so. The HTA programme could help facilitate this learning, and think about how the TAR programme could be better supported to allow academics to contribute sustainably to this important source of evidence for policy-makers.
Consider funding research on the implementation of Health Technology Assessment programme-funded research
It was noted in several case studies that even although an intervention had been shown to be cost-effective, there were still cost barriers to implementation. This is because the HTA studies do not usually consider practical issues around implementation in the NHS – such as training and infrastructure – and the economic analyses consider the operation of the approach as standard practice, not the upfront costs of implementation. This can be important, particularly where HTA research is serving as an important input to NICE decision-making. The programme may wish to consider whether or not, and how, the programme could more fully address these issues to make the findings of its work more complete from an implementation perspective. One way to do this would be by providing funding to expand studies in which the evidence suggests that there may be a need for a change in practice, or where these costs seem significant enough to affect policy and practice decisions. This would allow the programme to provide better information to decision-makers and better facilitate the uptake of findings where appropriate. Selection of the relevant studies for this type of extension could be on the basis of the importance of such an update for NHS policy and practice, using the same prioritisation approach as used throughout the programme, based on the suggestions of relevant stakeholders.
Improve the transparency of the priority-setting process and monitoring the impacts of patient and public involvement
The HTA programme is recognised as being one of the first public research funders to require PPI in research and has continued to be viewed as a leader in this area. Attitudes and approaches to PPI have changed over the last 10 years, but almost all of the case studies investigated had some PPI. However, what is not clear, either at the programme or the project level, is what the impact of that PPI has been. Views regarding the level of PPI and impact of PPI were generally positive, and although record keeping around what was done in terms of PPI is in place for more recent studies, there is little information about what changed as a result of PPI and, as a result, the evidence of the impact of that change. Increased transparency, monitoring and measurement of the effectiveness of PPI would not only allow the programme to better demonstrate its commitment to PPI, but also would allow programme management to improve and develop its PPI strategy to increase its impact on the effectiveness of the research that it funds.
A similar argument applies to the priority-setting process for the HTA programme. There is overall information provided at the programme level about what the priority-setting process consists of, and that input is taken from policy bodies, clinicians, researchers, patients and members of the public. However, it is difficult to trace the origins of a particular piece of commissioned research, and, indeed, little information is available about the relative importance of the different sources of project ideas. For example, it is not clear how often, if ever, suggestions from the public reach the point of being commissioned as a study. Increased transparency and monitoring here would, again, not only allow the programme to better demonstrate the quality of its priority-setting process, but also would give programme management the ability to better understand the effectiveness of that process and how it could be improved.
This recommendation reflects the findings of previous work. For example, Raftery and Powell (2013)11 note that given the programme’s needs-led research focus, it has always sought the opinions of clinicians, policy-makers and patients, and that the methods of doing this should be assessed and reviewed on an ongoing basis. Hanney et al. (2007),1 in the previous assessment of the programme, also suggested that there was a need to maintain more detailed records of the process for prioritisation and tendering of research topics, noting that the records kept were not sufficient for monitoring and evaluation of the successes and failures of the programme.
Consider ways to protect the future of the programme through improved recognition and planning for change
Looking forward, the HTA programme faces a range of potential challenges. In times when the NHS is facing increasing budgetary challenges, its budgetary pressures are likely to increase for any elements of the health system that are not delivering front-line care. Being able to provide evidence on the effectiveness and impact of the programme will be important as the programme looks to secure funding, as noted previously by Raftery and Powell (2013). 11 The need to clarify the role of the programme relative to other bodies, such as NICE and NIHR as a whole, will be important. As discussed in Chapter 3 (see Health Technology Assessment research is used outside the UK, particularly by other Health Technology Assessment organisations and those conducting systematic reviews), there is some evidence that the brand of the HTA programme is not always well known and understood outside those working closely with the programme, which may be a risk to its future status. There is also a need to consider succession planning. Several key individuals have played important roles in the success of the programme. If succession planning is not already under way, thought needs to be given to how the departure of key individuals will be managed. It will also be important to continue to change and adapt to the needs of a changing NHS to ensure that the research the HTA funds is timely and relevant to the needs of clinicians, policy-makers and patients. The HTA programme seems to have been successful at adapting to the changing needs of the NHS over the last 20 years, and the programme will need to maintain this adaptability to ensure that it meets the future needs of the NHS.
Limitations
In considering the conclusions of the project it is important to bear in mind the limitations of this study, which are discussed in detail in Chapter 2. In summary, it proved to be difficult to identify interviewees who were both knowledgeable about, and generally critical of, the HTA programme. Although this suggests that the HTA programme is generally viewed positively, it is possible that we missed more critical view points. We also used citation level, normalised by field, as one source of evidence for academic quality. Although general correlation between citation and quality is widely accepted, there is much debate about the exact meaning of citation. Researchfish data are similar to survey data, and they cover a wide range of types of impact, but they are self reported and biased towards more recent projects because reporting the impact of research in Researchfish has become mandatory only recently. We selected the case studies to include higher-impact projects that would reveal more about the pathways through which HTA research has impact, but the consequence of this selection process is that the observed levels of impact cannot be generalised across the entire HTA portfolio. However, this multi-method study was designed to address these limitations by triangulating findings from all four sources of evidence. One area in which this approach has been harder is attempting to quantify the impact on industry and the economy, for which it has not been possible to obtain financial and sales information.
Conclusions and recommendations for future research
The HTA programme has had an impact on health policy, clinical practice, academia and the research system in the UK. These impacts stem from the quality of the research, the focus on NHS priorities, good governance and close relationships with key policy stakeholders. To maintain and grow this impact the programme could consider funding for dissemination and additional cost analysis when required. The programme should maintain its focus on needs-led research and preserve the flexibility in its support of researchers. The HTA has been a leader in terms of its transparency, the comprehensiveness of its publications and its PPI activities. This openness could be extended to its priority-setting process and an examination of the impact, benefits and scope for improvement of its PPI activities. In an ever-changing NHS, the programme needs to maintain its ability to change and adapt, while still delivering its mission to ‘ensure that high-quality research information on the costs, effectiveness and broader impact of health technologies is produced in the most effective way for those who use, manage and provide care in the NHS’.
Future research topics could include the following:
-
Study into the effects and impact of PPI on HTA programme-funded research There is a lack of information on the impacts of the PPI process on HTA programme-funded research. A study investigating the impact that PPI has on HTA research and its ultimate effects on health policy, clinical practice and patients would allow the programme to better demonstrate the importance of PPI, and allow it to determine whether or not there is scope for improvement in the PPI process. The work would provide the most effective support for the programme if it also provided a framework for ongoing monitoring and evaluation of PPI within the programme to improve transparency, and to allow for continued development and improvement of the process in the future. Ongoing work by Professor Gamble and colleagues, scheduled for publication later this year (HS&DR project 10/2001/29) (Gamble C, Dudley L, Allam A, Bell P, Buck D, Goodare H, et al. An evidence base to optimise methods for involving patient and public contributors in clinical trials. Health Serv Deliv Res 2015; in press), may partly address this research need.
-
Continued collection of case studies A rolling programme of case studies collected on an ongoing basis would benefit the programme by providing a detailed and evolving understanding of the routes to impact of HTA research. It would also allow the programme to build up a portfolio of standardised and comparable examples of impact, which could be used to demonstrate the impact of the programme and provide information on where and how improvements could be made to maintain its impact. The payback framework would probably be the most effective template for such case studies, particularly as the programme has an existing portfolio of payback case studies from this study and the previous work by Hanney et al. (2007). 1
-
Study measuring the impact of studies that do not recommend a change in practice Two of the case studies48,50 provided evidence that supported NHS practice, such that no changes in clinical practice were observed. It is likely that much of the value that the programme provides is through such recommendations to continue existing practice. However, in contrast with cases, for example, in the recommendations show that a change in practice could bring health benefits or cost savings, it is difficult to measure the value of these studies to the UK health system. It would be useful, therefore, to conduct a study to look at how to value studies that recommend that standard practice should continue. A value-of-information approach might be appropriate here.
Acknowledgements
Many thanks to our advisory board for their helpful and constructive input. Thanks, in addition, to all of those who participated in interviews.
The bibliometric data were provided by CWTS, Leiden University.
Contribution of authors
Dr Susan Guthrie (Senior Analyst, Policy Research) conducted a number of interviews, two case studies, and led the analysis and reporting.
Teresa Bienkowska-Gibbs (Analyst, Policy Research) conducted a number of interviews, four case studies, conducted much of the analysis of the interview data and contributed to reporting.
Dr Catriona Manville (Senior Analyst, Policy Research) conducted the analysis of Researchfish data, three case studies and contributed to reporting.
Alexandra Pollitt (Senior Analyst, Policy Research) conducted the bibliometric analysis, two case studies and contributed to reporting.
Dr Anne Kirtley (RAND Europe) prepered the CoBalT case study.
Dr Steven Wooding (Senior Research Leader, Policy Research) provided oversight to the whole project and contributed to reporting.
All the study team were involved in the analysis, interpretation and drafting of the article.
Data sharing statement
If you wish to make use of data collected for this study, please contact the corresponding author. Interview data were collected under guarantees of anonymity and so cannot be made openly available. Bibliometric data were obtained via a third party from Thomson Reuters’ WoS database. The licence agreement with Thomson Reuters does not allow us to make the data freely available. Readers can contact Thomson Reuters to obtain the data (http://thomsonreuters.com/thomson-reuters-web-of-science/). ResearchFish data were provided from the NIHR under an agreement that does not allow us to make it freely available. Readers can contact the NIHR to request the data.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Hanney S, Buxton M, Green C, Coulson D, Raftery J. An assessment of the impact of the NHS Health Technology Assessment Programme. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11530.
- Assessing the Effects of Health Technologies: Principles, Practice, Proposals. London: DH; 1992.
- Department of Health . Best Research for Best Health 2006. www.gov.uk/government/publications/best-research-for-best-health-a-new-national-health-research-strategy (accessed April 2015).
- National Institute for Health Research . Implementation Plan 6.3a. NIHR Health Technology Assessment (HTA) Programme Version 5 2009.
- Choosing Health: Making Healthy Choices Easier. London: HMSO; 2004.
- Wanless D. Securing Good Health for the Whole Population: Final Report. London: HMSO; 2004.
- Cooksey D. A Review of UK Health Research Funding. London: HMSO; 2006.
- Department of Health . Equity and Excellence: Liberating the NHS 2010. www.gov.uk/government/uploads/system/uploads/attachment_data/file/213823/dh_117794.pdf (accessed April 2015).
- National Institute for Health Research . Briefing Document: 3.1 Commissioned Research Programmes, Version 7 2014. www.nihr.ac.uk/files/pdfs/Briefing%20documents/3.1%20Commissioned%20research%20programmes.pdf (accessed April 2015).
- National Institute for Health Research . Briefing Document: 3.2 Response Mode Research Programmes, Version 7 2014. www.nihr.ac.uk/documents/about-NIHR/Briefing-Documents/3.2-Response-mode-research-programmes.pdf (accessed April 2015).
- Raftery J, Powell J. Health Technology Assessment in the UK. Lancet 2013;382:1278-85. http://dx.doi.org/10.1016/S0140-6736(13)61724-9.
- National Institute for Health Research . Health Technology Assessment (HTA) Programme: Working With NICE and Other Policy Customers 2013. www.nets.nihr.ac.uk/__data/assets/pdf_file/0005/66875/policy-customers-leaflet.pdf (accessed April 2015).
- National Institute for Health Research . Health Technology Assessment Journal Sees Rise in Impact Factor n.d. www.nets.nihr.ac.uk/news/all/2012/health-technology-assessment-journal-sees-rise-in-impact-factor (accessed April 2015).
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet 2009;374:86-9. http://dx.doi.org/10.1016/S0140-6736(09)60329-9.
- Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gülmezoglu AM, et al. How to increase value and reduce waste when research priorities are set. Lancet 2014;383:156-65. http://dx.doi.org/10.1016/S0140-6736(13)62229-1.
- Ioannidis JPA, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014;3838:166-75. http://dx.doi.org/10.1016/S0140-6736(13)62227-8.
- Salman RA-S, Beller E, Kagan J, Hemminki E, Phillips RS, Savulescu J, et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014;3838:176-85. http://dx.doi.org/10.1016/S0140-6736(13)62297-7.
- Chan A-W, Song F, Vickers A, Jefferson T, Dickersin K, Gøtzsche PC, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet 2014;383:257-66. http://dx.doi.org/10.1016/S0140-6736(13)62296-5.
- Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014;3838:267-76. http://dx.doi.org/10.1016/S0140-6736(13)62228-X.
- National Institute for Health Research . Adding Value in Research Framework 2013. www.nets.nihr.ac.uk/about/adding-value-in-research (accessed April 2015).
- National Institute for Health Research . What Is Adding Value in Research? 2013. www.nets.nihr.ac.uk/__data/assets/pdf_file/0005/80159/NIHR-Adding-Value-in-Research-A5-Flyer_Sept2013.pdf (accessed April 2015).
- Clark T, Berger U, Mansmann U. Sample size determinations in original research protocols for randomised clinical trials submitted to UK research ethics committees: review. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f1135.
- Robinson KA, Goodman SN. A systematic examination of the citation of prior research in reports of randomized, controlled trials. Ann Intern Med 2011;154:50-5. http://dx.doi.org/10.7326/0003-4819-154-1-201101040-00007.
- Department of Health . The Research Governance Framework for Health and Social Care 2005. www.gov.uk/government/uploads/system/uploads/attachment_data/file/139565/dh_4122427.pdf (accessed April 2015).
- Wright D, Young A, Iserman E, Maeso R, Turner S, Haynes RB, et al. The clinical relevance and newsworthiness of NIHR HTA-funded research: a cohort study. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-004556.
- Turner S, Wright D, Maeso R, Cook A, Milne R. Publication rate for funded studies from a major UK health research funder: a cohort study. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002521.
- Chinnery F, Young A, Goodman J, Ashton-Key M, Milne R. Time to publication for NIHR HTA programme-funded research: a cohort study. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-004121.
- Douet L, Milne R, Anstee S, Habens F, Young A, Wright D. The completeness of intervention descriptions in published National Institute of Health Research HTA-funded trials: a cross-sectional study. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-003713.
- Jones AP, Conroy E, Williamson PR, Clarke M, Gamble C. The use of systematic reviews in the planning, design and conduct of randomised trials: a retrospective cohort of NIHR HTA funded trials. BMC Med Res Methodol 2013;13. http://dx.doi.org/10.1186/1471-2288-13-50.
- Turner S, Adams N, Cook A, Price A, Milne R. Potential benefits of using a toolkit developed to aid in the adaptation of HTA reports: a case study considering positron emission tomography (PET) and Hodgkin’s disease. Health Res Policy Syst 2010;8. http://dx.doi.org/10.1186/1478-4505-8-16.
- National Institute for Health Research . Public and Patient Involvement: Information for Researchers 2013. www.nets.nihr.ac.uk/programmes/hta/application-process/PPI-leaflet-Researchers.pdf (accessed April 2015).
- Gamble C, Dudley L, Newman J. Evidence base for patient and public involvement in clinical trials (EPIC). Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-S1-O34.
- Guthrie S, Hafner M, Bienkowska-Gibbs T, Wooding S. Returns on Research Funded Under the NIHR Health Technology Assessment (HTA) Programme: Economic Analysis and Case Studies (RR-666-DH). Cambridge: RAND Europe; 2015.
- Buxton M, Hanney S. How can payback from health services research be assessed?. J Health Serv Res Policy 1996;1:35-43.
- Hanney SR, Home PD, Frame I, Grant J, Green P, Buxton MJ. Identifying the impact of diabetes research. Diabet Med 2006;23:176-84. http://dx.doi.org/10.1111/j.1464-5491.2005.01753.x.
- Wooding S, Hanney S, Buxton M, Grant J. Payback arising from research funding: an evaluation of the Arthritis Research Campaign. Rheumatology 2005;44:1145-56. http://dx.doi.org/10.1093/rheumatology/keh708.
- Levitt R, Celia C, Diepeveen S, Ni Chonaill S, Rabinovich L, Tiessen J. Assessing the Impact of Arts and Humanities Research at the University of Cambridge (TR-816-CUAHRC). Cambridge: RAND Europe; 2010.
- Making an Impact: A Preferred Framework and Indicators to Measure Returns on Investment in Health Research. Ottawa, Canada: Canadian Academy of Health Sciences; 2009.
- Wooding S, Hanney S, Pollitt A, Buxton M, Grant J. Project Retrosight. Understanding the Returns from Cardiovascular and Stroke Research. Cambridge: RAND Europe; 2011.
- National Institute for Health Research . NIHR HTA Conference 2013 n.d. www.profbriefings.co.uk/nihrhta2013/ (accessed June 2015).
- National Institute for Health Research . HTA – 98 04 64: ARTISTIC: A Randomised Trial of Human Papillomavirus (HPV) Testing in Primary Cervical Screening 2015. www.nets.nihr.ac.uk/projects/hta/980464 (accessed June 2015).
- National Institute for Health Research . HTA – 99 45 01: Newborn Screening for Congenital Heart Defects: A Systematic Review and Cost-Effectiveness Analysis 2015. www.nets.nihr.ac.uk/projects/hta/994501 (accessed June 2015).
- National Institute for Health Research . HTA – 07 36 01: A Randomised Controlled Trial (RCT) of Alternative Treatments to Inhibit VEGF in Patients With Age-Related Choroidal Neovascularisation (IVAN) n.d. www.nets.nihr.ac.uk/projects/hta/073601 (accessed June 2015).
- National Institute for Health Research . HTA – 06 303 20: The CRASH-2 Trial: A Randomised Controlled Trial and Economic Evaluation of the Effects of Tranexamic Acid on Death, Vascular Occlusive Events and Transfusion Requirement in Bleeding Trauma Patients n.d. www.nets.nihr.ac.uk/projects/hta/0630320 (accessed June 2015).
- National Institute for Health Research . HTA – 04 26 01: A Systematic Review of the Effectiveness of Adalimumab, Etanercept and Infliximab for the Treatment of Rheumatoid Arthritis in Adults and an Economic Evaluation of Their Cost-Effectiveness n.d. www.nets.nihr.ac.uk/projects/hta/042601 (accessed June 2015).
- National Institute for Health Research . HTA – 95 02 99: The UK EndoVascular Aneurysm Repair (EVAR) Trials: Randomised Trials of EVAR Versus Standard Therapy n.d. www.nets.nihr.ac.uk/projects/hta/950299 (accessed June 2015).
- National Institute for Health Research . HTA – 01 37 03: Accurate, Practical and Cost-Effective Assessment of Carotid Stenosis in the UK n.d. www.nets.nihr.ac.uk/projects/hta/013703 (accessed June 2015).
- National Institute for Health Research . HTA – 05 16 01: A Multicentre Randomised Controlled Trial and Economic Evaluation of Ion-Exchange Water Softeners for the Treatment of Eczema in Children: The Softened Water Eczema Trial (SWET) n.d. www.nets.nihr.ac.uk/projects/hta/051601 (accessed June 2015).
- National Institute for Health Research . HTA – 99 01 01: Randomised Controlled Trial and Parallel Economic Evaluation of Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) n.d. www.nets.nihr.ac.uk/projects/hta/013703 (accessed June 2015).
- National Institute for Health Research . HTA – 06 404 02: Clinical Effectiveness and Cost-Effectiveness of Cognitive Behavioural Therapy As an Adjunct to Pharmacotherapy for Treatment-Resistant Depression in Primary Care: The CoBalT Randomised Controlled Trial n.d. www.nets.nihr.ac.uk/projects/hta/0640402 (accessed June 2015).
- National Institute for Health Research . HTA – 96 19 06: Randomised Controlled Trials of Conventional Antipsychotic Versus New Atypical Drugs, and New Atypical Drugs Versus Clozapine, in People With Schizophrenia Responding Poorly To, or Intolerant Of, Current Drug Treatment n.d. www.nets.nihr.ac.uk/projects/hta/961906 (accessed June 2015).
- National Institute for Health Research . Working With Policy Customers n.d. www.nets.nihr.ac.uk/programmes/hta/policy-customers (accessed June 2015).
- Audit Commission . Managing the Financial Implications of NICE Guidance 2005. archive.audit-commission.gov.uk/auditcommission/SiteCollectionDocuments/AuditCommissionReports/NationalStudies/ManagingTheFinancialImplicationsOfNiceGuidance08Sep05REP.pdf (accessed April 2015).
- Pettit L, Leonard S, Brooks-Rooney C, Hamerslag L, Kusel J. Assessing the Implementation of NICE Guidance: Is There a Correlation Between Recommendations and Uptake in Clinical Practice n.d. www.costellomedical.com/wp-content/uploads/2014/03/Poster_PHP1041.pdf (accessed April 2015).
- Grol R. Successes and failures in the Implementation of evidence-based guidelines for clinical practice. Med Care 2001;39. http://dx.doi.org/10.1097/00005650-200108002-00003.
- Garrido MV, Kristensen FB, Nielsen CP, Busse R. Health Technology Assessment and Health Policy-Making in Europe: Current Status, Challenges and Potential. Observatory Studies Series No. 14. Copenhagen: EUnetHTA and European Observatory; 2008.
- Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14080.
- Scherer RW, Langenberg P, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev 2007;2. http://dx.doi.org/10.1002/14651858.mr000005.pub3.
- Mockford C, Staniszewska S, Griffiths F, Herron-Marx S. The impact of patient and public involvement on UK NHS health care: a systematic review. Int J Qual Health Care 2001;24:28-3. http://dx.doi.org/10.1093/intqhc/mzr066.
- Public Health England . NHS Cervical Screening Programme n.d. www.cancerscreening.nhs.uk/cervical/ (accessed April 2015).
- Public Health England . NHS Cervical Screening Programme: About Cervical Screening n.d. www.cancerscreening.nhs.uk/cervical/about-cervical-screening.html (accessed April 2015).
- Kitchener HC, Almonte M, Thomson C, Wheeler P, Sargent A, Stoykova B, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009;10:672-82. http://dx.doi.org/10.1016/S1470-2045(09)70156-1.
- Schiffman MH, Bauer HM, Hoover RN, Glass AG, Cadell DM, Rush BB, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst 1993;85:958-64. http://dx.doi.org/10.1093/jnci/85.12.958.
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:1-9. http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F.
- Kitchener HC, Almonte M, Gilham C, Dowie R, Stoykova B, Sargent A, et al. ARTISTIC: a randomised trial of-human-papillomavirus (HPV) testing in primary cervical screening. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13510.
- The University of Manchester . Professor Henry Kitchener (MD FRCOG FMedScie) – Personal Details 2015. www.manchester.ac.uk/research/henry.c.kitchener/ (accessed April 2015).
- The University of Manchester . Institute of Cancer Sciences: Professor Henry Kitchener 2015. www.cancer.manchester.ac.uk/staff/HenryKitchener (accessed April 2015).
- European Commission . European Cervical Cancer Screening Network 2015. www.cancer-network.de/cervical/ (accessed April 2015).
- National Cancer Institute . International Cancer Screening Network 2015. http://appliedresearch.cancer.gov/icsn/ (accessed April 2015).
- London School of Hygiene and Tropical Medicine . Julian Peto 2015. www.lshtm.ac.uk/aboutus/people/peto.julian (accessed April 2015).
- Stern PL, Brown M, Stacey SN, Kitchener HC, Hampson I, Abdel-Hady ES, et al. Natural HPV immunity and vaccination strategies. J Clin Virol 2000;19:57-66. http://dx.doi.org/10.1016/S1386-6532(00)00128-1.
- Martin-Hirsch P, Jarvis G, Kitchener H, Lilford R. Collection devices for obtaining cervical cytology samples. Cochrane Database Syst Rev 2000;3. http://dx.doi.org/10.1002/14651858.cd001036.
- Paraskevaidis E, Lolis ED, Koliopoulos G, Alamanos Y, Fotiou S, Kitchener HC. Cervical intraepithelial neoplasia outcomes after large loop excision with clear margins. Obstet Gynecol 2000;95:828-31. http://dx.doi.org/10.1016/S0029-7844(00)00791-2.
- Martin-Hirsch PL, Kitchener H. Interventions for preventing blood loss during the treatment of cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2000;2.
- Martin-Hirsch PL, Paraskevaidis E, Kitchener H. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2000;2.
- Martin-Hirsch PL, Jarvis G, Kitchener H, Lilford R. Progestagens for endometrial cancer. Cochrane Database Syst Rev 2000;2.
- Sengupta PS, Shanks JH, Buckley CH, Ryder WD, Davies J, Reynolds K, et al. Requirement for expert histopathological assessment of ovarian cancer and borderline tumors. Br J Cancer 2000;82:760-2. http://dx.doi.org/10.1054/bjoc.1999.0994.
- Martin-Hirsch P, Jarvis G, Kitchener H, Lilford R. Collection devices for obtaining cervical cytology samples. Cochrane Database Syst Rev 2000;2. http://dx.doi.org/10.1002/14651858.cd001036.
- Chew GK, Jandial L, Paraskevaidis E, Kitchener HC. Pattern of CIN recurrence following laser ablation treatment: long-term follow-up. Int J Gynecol Cancer 1999;9:487-90. http://dx.doi.org/10.1046/j.1525-1438.1999.99066.x.
- Onon TS, Kitchener HC. The use of vaccines in treating cervical cancer: present status and future prospects. Int J Gynecol Cancer 1999;9:265-78. http://dx.doi.org/10.1046/j.1525-1438.1999.99022.x.
- Melville A, Eastwood A, Kleijnen J, Kitchener H, Martin-Hirsch P, Nelson L. Management of gynaecological cancers. Qual Health Care 1999;8:270-9. http://dx.doi.org/10.1136/qshc.8.4.270.
- Paraskevaidis E, Kalantaridou SN, Georgiou I, Koliopoulos G, Pappa L, Malamou-Mitsi V, et al. Spontaneous evolution of human papillomavirus infection in the uterine cervix. Anticancer Res 1999;19:3473-8.
- Martin-Hirsch P, Lilford R, Jarvis G, Kitchener HC. Efficacy of cervical-smear collection devices: a systematic review and meta-analysis. Lancet 1999;354:1763-70. http://dx.doi.org/10.1016/S0140-6736(99)02353-3.
- Cruickshank ME, Buchan S, Melvin WT, Kitchener HC. Human papillomavirus type 16 and 18 detection in the management of mild dyskaryosis. Br J Obstet Gynaecol 1999;106:969-76. http://dx.doi.org/10.1111/j.1471-0528.1999.tb08439.x.
- Kitchener HC. Gynaecological cancer. Postgrad Med J 1999;75:332-8. http://dx.doi.org/10.1136/pgmj.75.884.332.
- Papadimitriou DS, Martin-Hirsch P, Kitchener HC, Lolis DE, Dalkalitsis N, Paraskevaidis E. Recurrent borderline ovarian tumours after conservative management in women wishing to retain their fertility. Eur J Gynaecol Oncol 1999;20:94-7.
- Milner BJ, Brown I, Gabra H, Kitchener HC, Parkin DE, Haites NE. A protective role for common p21WAF1/Cip1 polymorphisms in human ovarian cancer. Int J Oncol 1999;15:117-19. http://dx.doi.org/10.3892/ijo.15.1.117.
- Kitchener HC, Symonds P. Detection of cervical intraepithelial neoplasia in developing countries. Lancet 1999;353:856-7. http://dx.doi.org/10.1016/S0140-6736(98)00417-6.
- Razzaq R, Carrington BM, Hulse PA, Kitchener HC. Abdominopelvic CT scan findings after surgery for ovarian cancer. Clin Radiol 1998;53:820-4. http://dx.doi.org/10.1016/S0009-9260(98)80193-1.
- Martin-Hirsch PL, Whitehurst C, Buckley CH, Moore JV, Kitchener HC. Photodynamic treatment for lower genital tract intraepithelial neoplasia. Lancet 1998;351. http://dx.doi.org/10.1016/S0140-6736(98)24019-0.
- Kitchener HC. Gynaecological cancer services: the way forward. Br J Hosp Med 1997;58:364-6.
- Flannelly G, Langhan H, Jandial L, Mana E, Campbell M, Kitchener H. A study of treatment failures following large loop excision of the transformation zone for the treatment of cervical intraepithelial neoplasia. Br J Obstet Gynaecol 1997;104:718-22. http://dx.doi.org/10.1111/j.1471-0528.1997.tb11983.x.
- Cruickshank ME, Angus V, Kelly M, McPhee S, Kitchener HC. The case for stopping cervical screening at age 50. Br J Obstet Gynaecol 1997;104:586-9. http://dx.doi.org/10.1111/j.1471-0528.1997.tb11537.x.
- Soutter WP, de Barros Lopes A, Fletcher A, Monaghan JM, Duncan ID, Paraskevaidis E, et al. Invasive cervical cancer after conservative therapy for cervical intraepithelial neoplasia. Lancet 1997;349:978-80. http://dx.doi.org/10.1016/S0140-6736(96)08295-5.
- Kitchener HC. Gynaecological cancer services: time for change. Br J Obstet Gynaecol 1997;104:123-6. http://dx.doi.org/10.1111/j.1471-0528.1997.tb11030.x.
- Du Bois A, McKenna CJ, Andersson H, Lahousen M, Kitchener H, Pinter T, et al. A randomised, double-blind, parallel-group study to compare the efficacy and safety of ondansetron (GR38032F) plus dexamethasone with metoclopramide plus dexamethasone in the prophylaxis of nausea and emesis induced by carboplatin chemotherapy. Oncology 1997;54:7-14. http://dx.doi.org/10.1159/000227652.
- Bhattacharya S, Mollison J, Pinion S, Parkin DE, Abramovich DR, Terry P, et al. A comparison of bladder and ovarian function two years following hysterectomy or endometrial ablation. Br J Obstet Gynaecol 1996;103:898-903. http://dx.doi.org/10.1111/j.1471-0528.1996.tb09909.x.
- Kaye SB, Paul J, Cassidy J, Lewis CR, Duncan ID, Gordon HK, et al. Mature results of a randomized trial of two doses of cisplatin for the treatment of ovarian cancer. Scottish Gynecology Cancer Trials Group. J Clin Oncol 1996;14:2113-19.
- Buchan S, Jiang G, Cruickshank ME, Melvin WT, Kitchener HC. Can HPV16 detection rationalise the management of women with mild or moderate dyskaryosis?. Biochem Soc Trans 1996;24.
- Bell S, Porter M, Kitchener H, Fraser C, Fisher P, Mann E. Psychological response to cervical screening. Prev Med 1995;24:610-16. http://dx.doi.org/10.1006/pmed.1995.1096.
- Flannelly G, Kitchener H. Every woman with an abnormal cervical smear should be referred for treatment: debate. Clin Obstet Gynecol 1995;38:585-91. http://dx.doi.org/10.1097/00003081-199509000-00018.
- Flannelly G, Jiang G, Anderson D, Melvin W, Mann E, Kitchener H. Serial quantitation of HPV-16 in the smears of women with mild and moderate dyskaryosis. J Med Virol 1995;47:6-9. http://dx.doi.org/10.1002/jmv.1890470103.
- Evans MJ, Langlois NE, Kitchener HC, Miller ID. Is there an association between long-term tamoxifen treatment and the development of carcinosarcoma (malignant mixed Mullerian tumor) of the uterus?. Int J Gynecol Cancer 1995;5:310-13. http://dx.doi.org/10.1046/j.1525-1438.1995.05040310.x.
- Cruickshank ME, Flannelly G, Campbell DM, Kitchener HC. Fertility and pregnancy outcome following large loop excision of the cervical transformation zone. Br J Obstet Gynaecol 1995;102:467-70. http://dx.doi.org/10.1111/j.1471-0528.1995.tb11319.x.
- Cruickshank ME, Kitchener HC. Towards optimal management of the mildly abnormal smear. Curr Opin Obstet Gynecol 1995;7:20-3. http://dx.doi.org/10.1097/00001703-199507010-00005.
- Penney GC, Kitchener HC, Templeton A. The management of carcinoma of the vulva: current opinion and current practice among consultant gynaecologists in Scotland. Health Bull 1995;53:47-54.
- Cooper KG, Kitchener HC, Parkin DE. Cerebral metastases from epithelial ovarian carcinoma treated with carboplatin. Gynecol Oncol 1994;55:318-23. http://dx.doi.org/10.1006/gyno.1994.1297.
- Paraskevaidis E, Kitchener HC, Malamou-Mitsi V, Agnanti N, Lolis D. Thermal tissue damage following laser and large loop conization of the cervix. Obstet Gynecol 1994;84:752-4.
- Macleod A, Kitchener HC, Parkin DE, Sarkar T, Miller ID, Mann E, et al. Cervical carcinoma in the Grampian region (1980–1991): a population-based study of survival and cervical cytology history. Br J Obstet Gynaecol 1994;101:797-803. http://dx.doi.org/10.1111/j.1471-0528.1994.tb11949.x.
- Flannelly G, Anderson D, Kitchener HC, Mann EM, Campbell M, Fisher P, et al. Management of women with mild and moderate cervical dyskaryosis. BMJ 1994;308:1399-403. http://dx.doi.org/10.1136/bmj.308.6941.1399.
- Paraskevaidis E, Kitchener H, Adonakis G, Parkin D, Lolis D. Incomplete excision of CIN in conization: further excision or conservative management?. Eur J Obstet Gynecol Reprod Biol 1994;53:45-7. http://dx.doi.org/10.1016/0028-2243(94)90136-8.
- Kitchener HC. The role of human papillomavirus in the genesis of cervical cancer. Cancer Treat Res 1994;70:29-41. http://dx.doi.org/10.1007/978-1-4615-2598-1_3.
- Milner BJ, Allan LA, Eccles DM, Kitchener HC, Leonard RC, Kelly KF, et al. p53 mutation is a common genetic event in ovarian carcinoma. Cancer Res 1993;53:2128-32.
- Milner BJ, Allan LA, Kelly KF, Cruickshank D, Hall M, Johnston A, et al. Linkage studies with 17q and 18q markers in a breast/ovarian cancer family. Am J Hum Genet 1993;52:761-6.
- Kitchener HC, Neilson L, Macnab JC. Oestrogen receptor message in premalignant and normal cervical cells: a methodological study. Eur J Cancer 1993;29:252-5. http://dx.doi.org/10.1016/0959-8049(93)90186-J.
- Eccles DM, Russell SE, Haites NE, Atkinson R, Bell DW, Gruber L, et al. Early loss of heterozygosity on 17q in ovarian cancer. The Abe Ovarian Cancer Genetics Group. Oncogene 1992;7:2069-72.
- Kaye SB, Lewis CR, Paul J, Duncan ID, Gordon HK, Kitchener HC, et al. Randomised study of two doses of cisplatin with cyclophosphamide in epithelial ovarian cancer. Lancet 1992;340:329-33. http://dx.doi.org/10.1016/0140-6736(92)91404-V.
- Anderson DJ, Flannelly GM, Kitchener HC, Fisher PM, Mann EM, Campbell MK, et al. Mild and moderate dyskaryosis: can women be selected for colposcopy on the basis of social criteria?. BMJ 1992;305:84-7. http://dx.doi.org/10.1136/bmj.305.6845.84.
- Seynaeve C, Schuller J, Buser K, Porteder H, Van Belle S, Sevelda P, et al. Comparison of the anti-emetic efficacy of different doses of ondansetron, given as either a continuous infusion or a single intravenous dose, in acute cisplatin-induced emesis. A multicentre, double-blind, randomised, parallel group study. Ondansetron Study Group. Br J Cancer 1992;66:192-7. http://dx.doi.org/10.1038/bjc.1992.241.
- Paraskevaidis E, Kitchener HC, Miller ID, Mann E, Jandial L, Fisher PM. A population-based study of microinvasive disease of the cervix: a colposcopic and cytologic analysis. Gynecol Oncol 1992;45:9-12. http://dx.doi.org/10.1016/0090-8258(92)90483-Y.
- Kitchener HC. United Kingdom colposcopy survey, British Society for Colposcopy and Cervical Pathology. Br J Obstet Gynaecol 1991;98:1112-16. http://dx.doi.org/10.1111/j.1471-0528.1991.tb15363.x.
- Kitchener HC, Neilson L, Burnett RA, Young L, Macnab JC. Prospective serial study of viral change in the cervix and correlation with human papillomavirus genome status. Br J Obstet Gynaecol 1991;98:1042-8. http://dx.doi.org/10.1111/j.1471-0528.1991.tb15344.x.
- Kernohan NM, Best PV, Jandial V, Kitchener HC. Palisading granuloma of the ovary. Histopathology 1991;19:279-80. http://dx.doi.org/10.1111/j.1365-2559.1991.tb00036.x.
- Paraskevaidis E, Jandial L, Mann EM, Fisher PM, Kitchener HC. Pattern of treatment failure following laser for cervical intraepithelial neoplasia: implications for follow-up protocol. Obstet Gynecol 1991;78:80-3.
- Cruickshank D, Kitchener H. Screening for uterine neoplasms. Lancet 1990;336:562-3. http://dx.doi.org/10.1016/0140-6736(90)92115-X.
- Macnab JC, Kitchener HC. Sexually transmitted cancers and viruses. Biomed Pharmacother 1989;43:167-72. http://dx.doi.org/10.1016/0753-3322(89)90210-2.
- Kitchener HC. Does HPV cause cervical cancer?. Br J Obstet Gynaecol 1988;95:1089-91. http://dx.doi.org/10.1111/j.1471-0528.1988.tb06783.x.
- Kitchener HC. Genital virus infection and cervical neoplasia. Br J Obstet Gynaecol 1988;95:182-91. http://dx.doi.org/10.1111/j.1471-0528.1988.tb06849.x.
- Sutherland IH, Arulkumaran S, Kitchener HC. Atypical condylomas of the cervix uteri in Singapore women: a histopathological and immunohistochemical study. Aust N Z J Obstet Gynaecol 1986;26:151-4. http://dx.doi.org/10.1111/j.1479-828X.1986.tb01554.x.
- Eglin RP, Kitchener HC, MacLean AB, Denholm RB, Cordiner JW, Sharp F. The presence of RNA complementary to HSV-2 (herpes simplex virus) DNA in cervical intraepithelial neoplasia after laser therapy. Br J Obstet Gynaecol 1984;91:265-9. http://dx.doi.org/10.1111/j.1471-0528.1984.tb04765.x.
- Park M, Kitchener HC, Macnab JC. Detection of herpes simplex virus type-2 DNA restriction fragments in human cervical carcinoma tissue. EMBO J 1983;2:1029-34.
- Peto J, Mack TM. High constant incidence in twins and other relatives of women with breast cancer. Nat Genet 2000;26:411-14. http://dx.doi.org/10.1038/82533.
- Deacon JM, Evans CD, Yule R, Desai M, Binns W, Taylor C, et al. Sexual behaviour and smoking as determinants of cervical HPV infection and of CIN3 among those infected: a case–control study nested within the Manchester cohort. Br J Cancer 2000;83:1565-72. http://dx.doi.org/10.1054/bjoc.2000.1523.
- Easton D, Peto J. Re: Presenting statistical uncertainty in trends and dose–response relations. Am J Epidemiol 2000;152:393-4. http://dx.doi.org/10.1093/aje/152.4.393.
- La Vecchia C, Decarli A, Peto J, Levi F, Tomei F, Negri E. An age, period and cohort analysis of pleural cancer mortality in Europe. Eur J Cancer Prev 2000;9:179-84. http://dx.doi.org/10.1097/00008469-200006000-00005.
- Lakhani SR, Gusterson BA, Jacquemier J, Sloane JP, Anderson TJ, van de Viver MJ, et al. The pathology of familial breast cancer: histological features of cancers in families not attributable to mutations in BRCA1 or BRCA2. Clin Cancer Res 2000;6:782-9.
- Popat S, Stone J, Coleman G, Marshall G, Peto J, Frayling I, et al. Prevalence of the APC E1317Q variant in colorectal cancer patients. Cancer Lett 2000;149:203-6. http://dx.doi.org/10.1016/S0304-3835(99)00360-2.
- Bullard J, Coleman MP, Robinson D, Lutz JM, Bell J, Peto J. Completeness of cancer registration: a new method for routine use. Br J Cancer 2000;82:1111-16. http://dx.doi.org/10.1054/bjoc.1999.1048.
- Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 1999;91:943-9. http://dx.doi.org/10.1093/jnci/91.11.943.
- Stone J, Bevan S, Cunningham D, Hill A, Rahman N, Peto J, et al. Low frequency of germline E-cadherin mutations in familial and nonfamilial gastric cancer. Br J Cancer 1999;79:1935-7. http://dx.doi.org/10.1038/sj.bjc.6690308.
- Peto J, Decarli A, La Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br J Cancer 1999;79:666-72. http://dx.doi.org/10.1038/sj.bjc.6690105.
- Osin P, Gusterson BA, Philp E, Waller J, Bartek J, Peto J, et al. Predicted anti-oestrogen resistance in BRCA-associated familial breast cancers. Eur J Cancer 1998;34:1683-6. http://dx.doi.org/10.1016/S0959-8049(98)00248-2.
- Crook T, Brooks LA, Crossland S, Osin P, Barker KT, Waller J, et al. p53 mutation with frequent novel condons but not a mutator phenotype in BRCA1- and BRCA2-associated breast tumours. Oncogene 1998;17:1681-9. http://dx.doi.org/10.1038/sj.onc.1202106.
- Lakhani SR, Jacquemier J, Sloane JP, Gusterson BA, Anderson TJ, van de Viver MJ, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst 1998;90:1138-45. http://dx.doi.org/10.1093/jnci/90.15.1138.
- Osin P, Crook T, Powles T, Peto J, Gusterson B. Hormone status of in-situ cancer in BRCA1 and BRCA2 mutation carriers. Lancet 1998;351. http://dx.doi.org/10.1016/S0140-6736(98)24020-7.
- Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998;62:676-89. http://dx.doi.org/10.1086/301749.
- Yamada T, Manos MM, Peto J, Greer CE, Munoz N, Bosch FX, et al. Human papillomavirus type 16 sequence variation in cervical cancers: a worldwide perspective. J Virol 1997;71:2463-72.
- Dunning AM, Chiano M, Smith NR, Dearden J, Gore M, Oakes S, et al. Common BRCA1 variants and susceptibility to breast and ovarian cancer in the general population. Hum Mol Genet 1997;6:285-9. http://dx.doi.org/10.1093/hmg/6.2.285.
- Stewart AC, Eriksson AM, Manos MM, Muñoz N, Bosch FX, Peto J, et al. Intratype variation in 12 human papillomavirus types: a worldwide perspective. J Virol 1996;70:3127-36.
- Easton DF, Matthews FE, Ford D, Swerdlow AJ, Peto J. Cancer mortality in relatives of women with ovarian cancer: the OPCS Study. Office of Population Censuses and Surveys. Int J Cancer 1996;65:284-94. http://dx.doi.org/10.1002/(SICI)1097-0215(19960126)65:3<284::AID-IJC2>3.0.CO;2-W.
- Peto J, Easton DF, Matthews FE, Ford D, Swerdlow AJ. Cancer mortality in relatives of women with breast cancer: the OPCS Study. Office of Population Censuses and Surveys. Int J Cancer 1996;65:275-83. http://dx.doi.org/10.1002/(SICI)1097-0215(19960126)65:3<275::AID-IJC1>3.0.CO;2-X.
- Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet 1995;57:1457-62.
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst 1995;87:796-802. http://dx.doi.org/10.1093/jnci/87.11.796.
- Peto J, Hodgson JT, Matthews FE, Jones JR. Continuing increase in mesothelioma mortality in Britain. Lancet 1995;345:535-9. http://dx.doi.org/10.1016/S0140-6736(95)90462-X.
- Peyton CL, Jansen AM, Wheeler CM, Stewart AC, Peto J, Bosch FX, et al. A novel human papillomavirus sequence from an international cervical cancer study. J Infect Dis 1994;170:1093-5. http://dx.doi.org/10.1093/infdis/170.5.1093.
- Peto J, Darby S. Lung cancer. Radon risk reassessed. Nature 1994;368:97-8. http://dx.doi.org/10.1038/368097a0.
- Wooster R, Ford D, Mangion J, Ponder BA, Peto J, Easton DF, et al. Absence of linkage to the ataxia telangiectasia locus in familial breast cancer. Hum Genet 1993;92:91-4. http://dx.doi.org/10.1007/BF00216153.
- Jha PK, Beral V, Peto J, Hack S, Hermon C, Deacon J, et al. Antibodies to human papillomavirus and to other genital infectious agents and invasive cervical cancer risk. Lancet 1993;341:1116-18. http://dx.doi.org/10.1016/0140-6736(93)93128-N.
- Smith SA, Easton DF, Ford D, Peto J, Anderson K, Averill D, et al. Genetic heterogeneity and localization of a familial breast-ovarian cancer gene on chromosome 17q12–q21. Am J Hum Genet 1993;52:767-76.
- Easton D, Ford D, Peto J. Inherited susceptibility to breast cancer. Cancer Surv 1993;18:95-113.
- Anderson KE, Easton DF, Matthews FE, Peto J. Cancer mortality in the first degree relatives of young breast cancer patients. Br J Cancer 1992;66:599-602. http://dx.doi.org/10.1038/bjc.1992.321.
- Peto J. Meta-analysis of epidemiological studies of carcinogenesis. IARC Sci Publ 1992;116:571-7.
- Eden OB, Lilleyman JS, Richards S, Shaw MP, Peto J. Results of Medical Research Council Childhood Leukaemia Trial UKALL VIII (report to the Medical Research Council on behalf of the Working Party on Leukaemia in Childhood). Br J Haematol 1991;78:187-96. http://dx.doi.org/10.1111/j.1365-2141.1991.tb04415.x.
- Piolatto G, Negri E, La Vecchia C, Pira E, Decarli A, Peto J. Bladder cancer mortality of workers exposed to aromatic amines: an updated analysis. Br J Cancer 1991;63:457-9. http://dx.doi.org/10.1038/bjc.1991.106.
- Piolatto G, Negri E, La Vecchia C, Pira E, Decarli A, Peto J. An update of cancer mortality among chrysotile asbestos miners in Balangero, northern Italy. Br J Ind Med 1990;47:810-14. http://dx.doi.org/10.1136/oem.47.12.810.
- Rawson NS, Peto J. An overview of prognostic factors in small cell lung cancer. A report from the Subcommittee for the Management of Lung Cancer of the United Kingdom Coordinating Committee on Cancer Research. Br J Cancer 1990;61:597-604. http://dx.doi.org/10.1038/bjc.1990.133.
- Easton D, Peto J. The contribution of inherited predisposition to cancer incidence. Cancer Surv 1990;9:395-416.
- Peto J. Oral contraceptives and breast cancer: is the CASH study really negative?. Lancet 1989;1. http://dx.doi.org/10.1016/S0140-6736(89)90088-3.
- Tidy JA, Parry GC, Ward P, Coleman DV, Peto J, Malcolm AD, et al. High rate of human papillomavirus type 16 infection in cytologically normal cervices. Lancet 1989;1. http://dx.doi.org/10.1016/S0140-6736(89)90023-8.
- Peto J, Easton D. Cancer treatment trials: past failures, current progress and future prospects. Cancer Surv 1989;8:511-33.
- Peto J. Fibre carcinogenesis and environmental hazards. IARC Sci Publ 1989;90:457-70.
- Easton DF, Peto J, Doll R. Cancers of the respiratory tract in mustard gas workers. Br J Ind Med 1988;45:652-9. http://dx.doi.org/10.1136/oem.45.10.652.
- Eden OB, Lilleyman J, Shaw MP, Richards S, Peto J. Medical Research Council Childhood Leukaemia Trial VIII compared with trials II-VII: lessons for future management. Haematol Blood Transfus 1987;30:448-55. http://dx.doi.org/10.1007/978-3-642-71213-5_79.
- Peto J, Doll R. Passive smoking. Br J Cancer 1986;54:381-3. http://dx.doi.org/10.1038/bjc.1986.187.
- Chilvers C, Peto J. Stress and cancer surveys. Lancet 1986;1. http://dx.doi.org/10.1016/S0140-6736(86)91592-8.
- Decarli A, Peto J, Piolatto G, La Vecchia C. Bladder cancer mortality of workers exposed to aromatic amines: analysis of models of carcinogenesis. Br J Cancer 1985;51:707-12. http://dx.doi.org/10.1038/bjc.1985.106.
- Kinlen LJ, Webster AD, Bird AG, Haile R, Peto J, Soothill JF, et al. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet 1985;1:263-6. http://dx.doi.org/10.1016/S0140-6736(85)91037-2.
- Peto J. Early- and late-stage carcinogenesis in mouse skin and in man. IARC Sci Publ 1984;56:359-71.
- Peto J, Cuckle H, Doll R, Hermon C, Morgan LG. Respiratory cancer mortality of Welsh nickel refinery workers. IARC Sci Publ 1984;53:37-46.
- Clifford P, O’Connor AD, Carter RL, Dalley VM, Darby AJ, Deutsch G, et al. Combination treatment in advanced head and neck cancer. Lancet 1982;2:708-9. http://dx.doi.org/10.1016/S0140-6736(82)90726-7.
- Peto J, Seidman H, Selikoff IJ. Mesothelioma mortality in asbestos workers: implications for models of carcinogenesis and risk assessment. Br J Cancer 1982;45:124-35. http://dx.doi.org/10.1038/bjc.1982.15.
- Peto J. CNS relapse in childhood leukaemia. Lancet 1981;2:753-4. http://dx.doi.org/10.1016/S0140-6736(81)91083-7.
- Greaves MF, Janossy G, Peto J, Kay H. Immunologically defined subclasses of acute lymphoblastic leukaemia in children: their relationship to presentation features and prognosis. Br J Haematol 1981;48:179-97. http://dx.doi.org/10.1111/j.1365-2141.1981.tb02704.x.
- Hardisty RM, Till MM, Peto J. Acute lymphoblastic leukaemia: four-year survivals old and new. J Clin Pathol 1981;34:249-53. http://dx.doi.org/10.1136/jcp.34.3.249.
- Kinlen LJ, Peto J, Doll R, Sheil AG. Cancer in patients treated with immunosuppressive drugs. BMJ (Clin Res Edn) 1981;282. http://dx.doi.org/10.1136/bmj.282.6262.474-b.
- Peto J. Lung cancer mortality in relation to measured dust levels in an asbestos textile factory. IARC Sci Publ 1980;30:829-36.
- Peto J. The incidence of pleural mesothelioma in chrysotile asbestos textile workers. IARC Sci Publ 1980;30:703-11.
- Peto R, Pike MC, Day NE, Gray RG, Lee PN, Parish S, et al. Guidelines for simple, sensitive significance tests for carcinogenic effects in long-term animal experiments. IARC Monogr Eval Carcinog Risk Chem Hum Suppl 1980;2:311-426.
- Kinlen LJ, Sheil AG, Peto J, Doll R. Collaborative United Kingdom-Australasian study of cancer in patients treated with immunosuppressive drugs. BMJ 1979;2:1461-6. http://dx.doi.org/10.1136/bmj.2.6203.1461.
- Sutcliffe SB, Wrigley PF, Peto J, Lister TA, Stansfeld AG, Whitehouse JM, et al. MVPP chemotherapy regimen for advanced Hodgkin’s disease. BMJ 1978;1:679-83. http://dx.doi.org/10.1136/bmj.1.6114.679.
- MacLennan IC, Peto J, Kay HE. Analysis of treatment of childhood leukaemia. V. Advantage of reduced chemotherapy during and immediately after cranial irradiation. Br J Cancer 1977;36:625-33. http://dx.doi.org/10.1038/bjc.1977.240.
- Berry RJ, Laing AH, Newman CR, Peto J. The role of radiotherapy in treatment of inoperable lung cancer. Int J Radiat Oncol Biol Phys 1977;2:433-9. http://dx.doi.org/10.1016/0360-3016(77)90154-7.
- Villa LL, Perez G, Kjaer SK, . FUTURE II Study Group . Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356:1915-27. http://dx.doi.org/10.1056/NEJMoa061741.
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007;369:2161-70. http://dx.doi.org/10.1016/S0140-6736(07)60946-5.
- National Institute for Health Research . HTA – 98 04 01: A Systematic Review of the Role of Human Papillomavirus Testing Within a Cervical Screening Programme 2015. www.nets.nihr.ac.uk/projects/hta/980401 (accessed June 2015).
- Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al. A systematic review of the role of human papillomavirus testing within a cervical screening programme. Health Technol Assess 1998;3.
- Naucler P, Ryd W, Törnberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007;357:1589-97. http://dx.doi.org/10.1056/NEJMoa073204.
- Bulkmans N, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007;370:1764-72. http://dx.doi.org/10.1016/S0140-6736(07)61450-0.
- National Institute for Health Research . HTA – 98 04 99: The Clinical Effectiveness and Cost-Effectiveness of Primary Human Papillomavirus Cervical Screening in England: Extended Follow-up of the ARTISTIC Randomised Trial Cohort Through the Three Screening Rounds 2015. www.nets.nihr.ac.uk/projects/hta/980499 (accessed June 2015).
- National Institute for Health Research . HTA – 09 66 01: A Modelled Analysis Using Data from the ARTISTIC Trial of the Potential Effectiveness and Cost-Effectiveness of HPV Testing in Cervical Cancer Screening 2015. www.nets.nihr.ac.uk/projects/hta/096601 (accessed June 2015).
- Kitchener H, Canfell K, Gilham C, Sargent A, Roberts C, Desai M, et al. The clinical effectiveness and cost-effectiveness of primary human papillomavirus cervical screening in England: extended follow-up of the ARTISTIC randomised trial cohort through three screening rounds. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18230.
- National Institute for Health and Care Excellence . Guidance on the Use of Liquid-Based Cytology for Cervical Screening 2003. www.nice.org.uk/guidance/ta69 (accessed April 2015).
- Public Health England . Liquid Based Cytology (LBC): NHS Cervical Screening Programme n.d. www.cancerscreening.nhs.uk/cervical/lbc.html (accessed April 2015).
- Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014;383:524-32. http://dx.doi.org/10.1016/S0140-6736(13)62218-7.
- Kitchener HC, Gilham C, Sargent A, Bailey A, Albrow R, Roberts C, et al. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening: extended follow up in the ARTISTIC trial. Eur J Cancer 2011;47:864-71. http://dx.doi.org/10.1016/j.ejca.2011.01.008.
- Dowie R, Stoykova B, Crawford D, Desai M, Mather J, Morgan K, et al. Liquid-based cytology can improve efficiency of cervical smear readers: evidence from timing surveys in two NHS cytology laboratories. Cytopathology 2006;17:65-72. http://dx.doi.org/10.1111/j.1365-2303.2006.00304.x.
- Dowie R, Stoykova B, Desai M. Assessing the wellbeing of cytoscreeners: experience in two NHS cytology laboratories. Cytopathology 2006;17:366-73. http://dx.doi.org/10.1111/j.1365-2303.2006.00383.x.
- Kitchener H, Almonte M, Wheeler P, Desai M, Gilham C, Bailey A, et al. HPV testing in routine cervical screening: cross sectional data from the ARTISTIC trial. Br J Cancer 2006;95:56-61. http://dx.doi.org/10.1038/sj.bjc.6603210.
- Sargent A, Bailey A, Almonte M, Turner A, Thomson C, Peto J, et al. Prevalence of type-specific HPV infection by age and grade of cervical cytology: data from the ARTISTIC trial. Br J Cancer 2008;98:1704-9. http://dx.doi.org/10.1038/sj.bjc.6604324.
- Kitchener HC, Fletcher I, Roberts C, Wheeler P, Almonte M, Maguire P. The psychosocial impact of human papillomavirus testing in primary cervical screening: a study within a randomized trial. Int J Gynecol Cancer 2008;18:743-48. http://dx.doi.org/10.1111/j.1525-1438.2007.01113.x.
- Stoykova B, Kuzmanov G, Dowie R. Putting National Institute for Health and Clinical Excellence guidance into practice: a cost minimization model of a national roll-out of liquid based cytology in England. Int J Technol Assess 2008;24:391-8. http://dx.doi.org/10.1017/S0266462308080513.
- Sargent A, Bailey A, Turner A, Almonte M, Gilham C, Baysson H, et al. Optimal threshold for a positive hybrid capture 2 test for detection of human papillomavirus: data from the ARTISTIC trial. J Clin Microbiol 2010;48:554-8. http://dx.doi.org/10.1128/JCM.00896-09.
- National Institute for Health Research . HTA – 03 04 02: MAVARIC – A Comparison of Automation-Assisted and Manual Cervical Screening: A Randomised Controlled Trial 2015. www.nets.nihr.ac.uk/projects/hta/030402 (accessed June 2015).
- National Institute for Health Research . HTA – 05 41 02: A Study of Cellular Counting to Determine Minimum Thresholds for Adequacy for Liquid-Based Cervical Cytology Using a Survey and Counting Protocol n.d. www.nets.nihr.ac.uk/projects/hta/054102 (accessed August 2015).
- National Institute for Health Research . HTA – 09 164 01: Strategies to Increase Cervical Screening Uptake at First Invitation (STRATEGIC) 2015. www.nets.nihr.ac.uk/projects/hta/0916401 (accessed June 2015).
- Periodic Report Summary – PREHDICT (Health-economic Modelling of Prevention Strategies for HPV-related Disease in European Countries). Brussels: European Commission; 2013.
- Kitchener HC, Blanks R, Cubie H, Desai M, Dunn G, Legood R, et al. MAVARIC: a comparison of automation-assisted and manual cervical screening: a randomised controlled trial. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15030.
- Kitchener H, Walker P, Nelson L, Hadwin R, Patnick J, Anthony GB, et al. HPV testing as an adjunct to cytology in the follow up of women treated for cervical intraepithelial neoplasia. BJOG 2008;115:1001-7. http://dx.doi.org/10.1111/j.1471-0528.2008.01748.x.
- UK National Screening Committee . Note of the Meeting Held on 25 April 2012 at Royal Free Hampstead NHS Trust n.d. www.screening.nhs.uk/getdata.php?id=13050 (accessed April 2015).
- Ewer A, Furmston A, Middleton L, Deeks JJ, Daniels JP, Pattison HM, et al. Pulse oximetry as a screening test for congenital heart defects in newborn infants: a test accuracy study with evaluation of acceptability and cost-effectiveness. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16020.
- European Surveillance of Congenital Anomalies . Prevalence Tables 2015. www.eurocat-network.eu/accessprevalencedata/prevalencetables (accessed April 2015).
- Bernier PL, Stefanescu A, Samoukovic G, Tchervenkov CI. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2010;13:26-34. http://dx.doi.org/10.1053/j.pcsu.2010.02.005.
- Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C. Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9440.
- UCL Institutional Research Information Services . Prof Carol Dezateux 2015. http://iris.ucl.ac.uk/iris/browse/profile?upi=CDEZA65 (accessed April 2015).
- UCL Institutional Research Information Services . Dr Rachel Knowles 2015. http://iris.ucl.ac.uk/iris/browse/profile?upi=RKNOW69 (accessed April 2015).
- Griebsch I, Knowles RL, Brown J, Bull C, Wren C, Dezateux CA. Comparing the clinical and economic effects of clinical examination, pulse oximetry, and echocardiography in newborn screening for congenital heart defects: a probabilistic cost-effectiveness model and value of information analysis. Int J Technol Assess Health Care 2007;23:192-204. http://dx.doi.org/10.1017/S0266462307070304.
- Knowles RL, Griebsch I, Bull C, Brown J, Wren C, Dezateux C. Quality of life and congenital heart defects: comparing parent and professional values. Arch Dis Child 2007;92:388-93. http://dx.doi.org/10.1136/adc.2005.075606.
- Hall DM, Elliman D. Health for all Children. Oxford: Oxford University Press; 2006.
- Wren C, Richmond S, Donaldson L. Presentation of congenital heart disease in infancy: implications for routine examination. Arch Dis Child Fetal Neonatal Ed 1999;80:F49-53. http://dx.doi.org/10.1136/fn.80.1.F49.
- Ainsworth S, Wyllie JP, Wren C. Prevalence and clinical significance of cardiac murmurs in neonates. Arch Dis Child Fetal Neonatal Ed 1999;80:F43-5. http://dx.doi.org/10.1136/fn.80.1.F43.
- Abu-Harb M, Hey E, Wren C. Death in infancy from unrecognised congenital heart disease. Arch Dis Child 1994;71:3-7. http://dx.doi.org/10.1136/adc.71.1.3.
- Ewer AK, Middleton LJ, Furmston AT, Bhoyar A, Daniels JP, Thangaratinam S, et al. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet 2011;378:785-94. http://dx.doi.org/10.1016/S0140-6736(11)60753-8.
- Knowles R, Hunter R. Screening for Congenital Heart Defects: External Review Against Programme Appraisal Criteria for the UK NSC 2013. www.screening.nhs.uk/policydb_download.php?doc=337 (accessed April 2015).
- Jackson E, Stocks J, Pilgrim L, Dundas I, Dezateux C. A critical assessment of uncalibrated respiratory inductance plethysmography (Respitrace) for the measurement of tidal breathing parameters in newborns and infants. Pediatr Pulmonol 1995;20:119-24. http://dx.doi.org/10.1002/ppul.1950200212.
- Godward S, Dezateux C. Validation of the reporting bases of the orthopaedic and paediatric surveillance schemes. Arch Dis Child 1996;75:232-6. http://dx.doi.org/10.1136/adc.75.3.232.
- Stocks J, Henschen M, Hoo AF, Costeloe K, Dezateux C. Influence of ethnicity and gender on airway function in preterm infants. Am J Respir Crit Care Med 1997;156:1855-62. http://dx.doi.org/10.1164/ajrccm.156.6.9607056.
- Rahi JS, Dezateux C. The future of preschool vision screening services in Britain. BMJ 1997;315:1247-8. http://dx.doi.org/10.1136/bmj.315.7118.1247.
- Dezateux C. Evaluating newborn screening programmes based on dried blood spots: future challenges. Br Med Bull 1998;54:877-90. http://dx.doi.org/10.1093/oxfordjournals.bmb.a011735.
- Peckham CS, Dezateux C. Issues underlying the evaluation of screening programmes. Br Med Bull 1998;54:767-78. http://dx.doi.org/10.1093/oxfordjournals.bmb.a011728.
- Godward S, Dezateux C. Surgery for congenital dislocation of the hip in the UK as a measure of outcome of screening. MRC Working Party on Congenital Dislocation of the Hip. Medical Research Council. Lancet 1998;351:1149-52. http://dx.doi.org/10.1016/S0140-6736(97)10466-4.
- Elbourne D, Dezateux C. Screening toddlers for iron deficiency anaemia in general practice. Effect of delaying timing of clamping of cord is being studied. BMJ 1998;316:145-6. http://dx.doi.org/10.1136/bmj.316.7125.145.
- Rahi JS, Dezateux C. National cross sectional study of detection of congenital and infantile cataract in the United Kingdom: role of childhood screening and surveillance. The British Congenital Cataract Interest Group. BMJ 1999;318:362-5. http://dx.doi.org/10.1136/bmj.318.7180.362.
- Rahi JS, Dezateux C. Capture-recapture analysis of ascertainment by active surveillance in the British Congenital Cataract Study. Invest Ophthalmol Vis Sci 1999;40:236-9.
- Rahi JS, Dezateux C. Congenital and infantile cataract in the United Kingdom: underlying or associated factors. British Congenital Cataract Interest Group. Invest Ophthalmol Vis Sci 2000;41:2108-14.
- Lees CM, Davies S, Dezateux C. Neonatal screening for sickle cell disease. Cochrane Database Syst Rev 2000;2. http://dx.doi.org/10.1002/14651858.cd001913.
- Merelle ME, Lees CM, Nagelkerke AF, Dezateux C. Newborn screening for cystic fibrosis. Cochrane Database Syst Rev 2000;2.
- Merelle ME, Nagelkerke AF, Lees CM, Dezateux C. Newborn screening for cystic fibrosis. Cochrane Database Syst Rev 2001;3. http://dx.doi.org/10.1002/14651858.cd001402.
- Dezateux C. Screening newborn infants for cystic fibrosis. J Med Screening 2001;8:57-8. http://dx.doi.org/10.1136/jms.8.2.57.
- Rahi JS, Dezateux C. Measuring and interpreting the incidence of congenital ocular anomalies: lessons from a national study of congenital cataract in the UK. Invest Ophthalmol Vis Sci 2001;42:1444-8.
- Zeuner D, Ades A, Karnon J, Brown J, Dezateux C, Anionwu E. Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis. Health Technol Assess 1999;3.
- Hall D. A Program for Child Health Surveillance. Oxford: Oxford University Press; 1996.
- Bove E, Bull C, Stark J, De Leval M, Macartney F, Taylor J. Congenital heart disease in the neonate: results of surgical treatment. Arch Dis Child 1983;58:137-41. http://dx.doi.org/10.1136/adc.58.2.137.
- Spiegelhalter DJ, Harris NL, Bull K, Franklin RC. Empirical evaluation of prior beliefs about frequencies: methodology and a case study in congenital heart disease. J Am Stat Assoc 1994;89:435-43. http://dx.doi.org/10.1080/01621459.1994.10476765.
- Bull C. Current and potential impact of fetal diagnosis on prevalence and spectrum of serious congenital heart disease at term in the UK. Lancet 1999;354:1242-47. http://dx.doi.org/10.1016/S0140-6736(99)01167-8.
- Bull C, Yates R, Sarkar D, Deanfield J, De Leval M. Scientific, ethical, and logistical considerations in introducing a new operation: a retrospective cohort study from paediatric cardiac surgery. BMJ 2000;320:1168-73. http://dx.doi.org/10.1136/bmj.320.7243.1168.
- Bull C. Key Issues in Retrospective Evaluation of Morbidity Outcomes Following Paediatric Cardiac Surgery 2001. http://webarchive.nationalarchives.gov.uk/20090811150351/http:/www.bristol-inquiry.org.uk/final_report/annex_b/images/otherpdfs/replacements/Bull_morbidity_R.pdf (accessed April 2015).
- Wren C, Oslizlok P, Bull C. Natural history of supravalvular aortic stenosis and pulmonary artery stenosis. J Am Coll Cardiol 1990;15:1625-30. http://dx.doi.org/10.1016/0735-1097(90)92837-R.
- De Leval M, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg 1988;96:682-95.
- Rubay J, de Leval M, Bull C. To switch or not to switch? The Senning alternative. Circulation 1988;78:III1-4.
- Bull C. Interventional catheterisation in infants and children. Br Heart J 1986;56. http://dx.doi.org/10.1136/hrt.56.3.197.
- Haworth SG, Bull C. Physiology of congenital heart disease. Arch Dis Child 1993;68:707-11. http://dx.doi.org/10.1136/adc.68.5.707.
- Magnay A, Bull C. Life-threatening pulmonary hypertensive crises occurring preoperatively. Int J Cardiol 1992;36:227-9. http://dx.doi.org/10.1016/0167-5273(92)90011-Q.
- Knowles R, Blackburn M, Zahir M, Russell M, Carrier A, Nevrkla E. The implementation of a new parallel child health record. Child Care Health Dev 1999;25:253-66. http://dx.doi.org/10.1046/j.1365-2214.1999.00097.x.
- Davies C, Brown J. UK cervical cancer screening programme: setting a research agenda. J Med Screening 1994;1:249-54.
- Bryan S, Brown J. Extrapolation of cost-effectiveness information to local settings. J Health Serv Res Policy 1998;3:108-12.
- Johnston K, Gerard K, Brown J. Generalizing costs from trials: analyzing center selection bias in a breast screening trial. Int J Technol Assess Health Care 1998;14:494-50. http://dx.doi.org/10.1017/S0266462300011478.
- Brown J. Economic evaluation of cancer treatments: a review of the methods. Clin Oncol (R Coll Radiol) 1999;11:78-83. http://dx.doi.org/10.1053/clon.1999.9018.
- Karnon J, Brown J. Selecting a decision model for economic evaluation: a case study and review. Health Care Manag Sci 1998;1:133-40. http://dx.doi.org/10.1023/A:1019090401655.
- Wren C. Subcostal echocardiography in ventricular tachycardia. Am Heart J 1984;108:1579-82. http://dx.doi.org/10.1016/0002-8703(84)90720-8.
- Wren C. Broad-complex tachycardias. Lancet 1986;2. http://dx.doi.org/10.1016/S0140-6736(86)90619-7.
- Wren C. Arrhythmias in children: the influence of exercise and the role of exercise testing. Eur Heart J 1987;8:25-8. http://dx.doi.org/10.1093/eurheartj/8.suppl_D.25.
- Hunter S, Wren C. Balloon dilatation in congenital heart disease. Arch Dis Child 1987;62:768-70. http://dx.doi.org/10.1136/adc.62.8.768.
- Wren C. Hyperkalaemia, cardiac arrhythmias, and cerebral lesions in high risk neonates. Arch Dis Child 1988;63:681-2. http://dx.doi.org/10.1136/adc.63.6.681-a.
- Cooper SG, Sullivan ID, Wren C. Treatment of recoarctation: balloon dilation angioplasty. J Am Coll Cardiol 1989;14:413-19. http://dx.doi.org/10.1016/0735-1097(89)90195-2.
- Sreeram N, Hunter S, Wren C. Acute myocardial infarction in infancy: unmasking of anomalous origin of the left coronary artery from the pulmonary artery by ligation of an arterial duct. Br Heart J 1989;61:307-8. http://dx.doi.org/10.1136/hrt.61.3.307.
- Sreeram N, Wren C. Supraventricular tachycardia in infants: response to initial treatment. Arch Dis Child 1990;65:127-9. http://dx.doi.org/10.1136/adc.65.1.127.
- Stuart G, Wren C. Endocarditis with acute mitral regurgitation caused by Fusobacterium necrophorum. Pediatr Cardiol 1992;13:230-2. http://dx.doi.org/10.1007/BF00838782.
- O’Sullivan J, Smith R, Coulthard M, Hamilton L, Wren C. Haemophilus influenzae type B endocarditis and meningitis. Pediatr Infect Dis J 1992;11:776-7. http://dx.doi.org/10.1097/00006454-199209000-00025.
- Skinner JR, Bexton R, Wren C. Aortic coarctation endarteritis and aneurysm: diagnosis by transoesophageal echocardiography. Int J Cardiol 1992;34:216-18. http://dx.doi.org/10.1016/0167-5273(92)90160-5.
- Till J, Wren C. Atrial flutter in the fetus and young infant: an association with accessory connections. Br Heart J 1992;67:80-3. http://dx.doi.org/10.1136/hrt.67.1.80.
- Nicholson WR, Matthews JN, O’Sullivan JJ, Wren C. Ambulatory blood pressure monitoring. Arch Dis Child 1993;69:681-4. http://dx.doi.org/10.1136/adc.69.6.681.
- O’Sullivan J, Cottrell AJ, Wren C. Ondine’s curse and neurally mediated syncope: a new and important association. Eur Heart J 1993;14:1289-91. http://dx.doi.org/10.1093/eurheartj/14.9.1289.
- O’Sullivan JJ, Jameson S, Gold RG, Wren C. Endocardial pacemakers in children: lead length and allowance for growth. Pacing Clin Electrophysiol 1993;16:267-71. http://dx.doi.org/10.1111/j.1540-8159.1993.tb01575.x.
- Abu-Harb M, Wyllie J, Hey E, Richmond S, Wren C. Presentation of obstructive left heart malformations in infancy. Arch Dis Child Fetal Neonatal Ed 1994;71:F179-83. http://dx.doi.org/10.1136/fn.71.3.F179.
- O’Sullivan J, Bain H, Hunter S, Wren C. End-on aortogram: improved identification of important coronary artery anomalies in tetralogy of Fallot. Br Heart J 1994;71:102-6. http://dx.doi.org/10.1136/hrt.71.1.102.
- O’Sullivan JJ, Gardiner HM, Wren C. Digoxin or flecainide for prophylaxis of supraventricular tachycardia in infants?. J Am Coll Cardiol 1995;26:991-4. http://dx.doi.org/10.1016/0735-1097(95)00291-9.
- Abu-Harb M, Wyllie J, Hey E, Richmond S, Wren C. Antenatal diagnosis of congenital heart disease and Down’s syndrome: the potential effect on the practice of paediatric cardiology. Br Heart J 1995;74:192-8. http://dx.doi.org/10.1136/hrt.74.2.192.
- O’Sullivan JJ, McCarthy PT, Wren C. Differences in amiodarone, digoxin, flecainide and sotalol concentrations between antemortem serum and femoral postmortem blood. Hum Exp Toxicol 1995;14:605-8. http://dx.doi.org/10.1177/096032719501400709.
- Wren C. Mechanisms of fetal tachycardia. Heart 1998;79:536-7. http://dx.doi.org/10.1136/hrt.79.6.536.
- Estlin EJ, Bennett MK, Skinner JR, Milligan DW, Wren C. Atrial fibrillation with neonatal pulmonary lymphangiectasia. Acta Paediatr 1998;87:1304-6. http://dx.doi.org/10.1111/j.1651-2227.1998.tb00957.x.
- Goodship J, Cross I, LiLing J, Wren C. A population study of chromosome 22q11 deletions in infancy. Arch Dis Child 1998;79:348-51. http://dx.doi.org/10.1136/adc.79.4.348.
- Wren C. Incessant tachycardias. Eur Heart J 1998;19:E32-6.
- O’Sullivan JJ, Derrick G, Griggs PE, Wren C. Validation of the Takeda 2421 ambulatory blood pressure monitor in children. J Med Eng Technol 1998;22:101-5. http://dx.doi.org/10.3109/03091909809062474.
- Wren C. Treatment of tachycardia in infants and children. Indian Pediatr 1999;36:1091-6.
- Wren C. Prolonged QTc interval as an important factor in sudden infant death syndrome. Arch Dis Child 1999;81. http://dx.doi.org/10.1136/adc.81.3.278k.
- Wren C. Catheter ablation in paediatric arrhythmias. Arch Dis Child 1999;81:102-4. http://dx.doi.org/10.1136/adc.81.2.102.
- Wren C. Cardiac causes for syncope or sudden death in childhood. Arch Dis Child 1999;81:289-91. http://dx.doi.org/10.1136/adc.81.4.289.
- O’Sullivan JJ, Derrick G, Griggs P, Foxall R, Aitkin M, Wren C. Ambulatory blood pressure in schoolchildren. Arch Dis Child 1999;80:529-32. http://dx.doi.org/10.1136/adc.80.6.529.
- Gregory J, Emslie A, Wyllie J, Wren C. Examination for cardiac malformations at six weeks of age. Arch Dis Child Fetal Neonatal Ed 1999;80:F46-8. http://dx.doi.org/10.1136/fn.80.1.F46.
- Wren C, Richmond S, Donaldson L. Temporal variability in birth prevalence of cardiovascular malformations. Heart 2000;83:414-19. http://dx.doi.org/10.1136/heart.83.4.414.
- Leonard H, Derrick G, O’Sullivan J, Wren C. Natural and unnatural history of pulmonary atresia. Heart 2000;84:499-503. http://dx.doi.org/10.1136/heart.84.5.499.
- Wren C. Radiofrequency ablation in children. Cardiol Young 2000;10:303-6. http://dx.doi.org/10.1017/S1047951100009604.
- Wren C. Ventricular pre-excitation producing resolution of complete atrioventricular block. Heart 2000;83. http://dx.doi.org/10.1136/heart.83.1.102.
- Leonard H, Barrett AM, Scott JE, Wren C. The influence of congenital heart disease on survival of infants with oesophageal atresia. Arch Dis Child Fetal Neonatal Ed 2001;85:F204-6. http://dx.doi.org/10.1136/fn.85.3.F204.
- Culyer AJ, Newhouse JP. Handbook of Health Economics. Amsterdam: Elsevier; 2000.
- Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P. The Role of Modelling in Prioritising and Planning Clinical Trials. Tunbridge Wells: Gray Publishing; 2003.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64. http://dx.doi.org/10.1016/S0167-6296(98)00039-3.
- Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute for Clinical Excellence (NICE). Lancet 2002;360:711-15. http://dx.doi.org/10.1016/S0140-6736(02)09832-X.
- Knowles RL, Smith A, Lynn R, Rahi JS. Using multiple sources to improve and measure case ascertainment in surveillance studies: 20 years of the British Paediatric Surveillance Unit. J Public Health (Oxf) 2006;28:157-65. http://dx.doi.org/10.1093/pubmed/fdl005.
- Lynn R, Pebody R, Knowles R. Twenty years of active paediatric surveillance in the UK and Republic of Ireland. Euro Surveill 2006;11.
- Knowles R, Bull C, Wren C, Dezateux C. Factors influencing height and weight at 10–14 years in UK children with congenital heart defects. J Epidemiol Commun H 2008;62. http://dx.doi.org/10.1371/journal.pone.0106806.
- Knowles RL, Bull C, Wren C, Dezateux C. Ethics, governance and consent in the UK: implications for research into the longer-term outcomes of congenital heart defects. Arch Dis Child 2011;96:14-20. http://dx.doi.org/10.1136/adc.2008.152975.
- Knowles RL, Friend H, Lynn R, Mitchell S, Michie C, Ihekweazu C. Surveillance of rare diseases: a public health evaluation of the British Paediatric Surveillance Unit. J Public Health (Oxf) 2012;34:279-86. http://dx.doi.org/10.1093/pubmed/fdr058.
- Tadic V, Knowles R, Rahi J. Patient-Reported Outcome and Experience Measures (PROMS and PREMS) in Routine Clinical Practice 2012. http://discovery.ucl.ac.uk/1397058/ (accessed April 2015).
- Tadic V, Knowles R, Hogan A, Rahi J. Paediatric Patient-Reported Outcome and Experience Measures (PROMS and PREMS) in Routine Clinical Practice 2012. http://discovery.ucl.ac.uk/1382617/ (accessed April 2015).
- Khalid JM, Oerton JM, Dezateux C, Hindmarsh PC, Kelnar CJ, Knowles RL. Incidence and clinical features of congenital adrenal hyperplasia in Great Britain. Arch Dis Child 2012;97:101-6. http://dx.doi.org/10.1136/archdischild-2011-300234.
- Knowles R, Veldtman G, Hickey EJ, Bradley T, Gengsakul A, Webb GD, et al. Functional health status of adults with tetralogy of Fallot: matched comparison with healthy siblings. Ann Thorac Surg 2012;94:124-32. http://dx.doi.org/10.1016/j.athoracsur.2011.09.056.
- Tadić V, Hogan A, Sobti N, Knowles RL, Rahi JS. Patient-reported outcome measures (PROMs) in paediatric ophthalmology: a systematic review. Br J Ophthalmology 2013;97:1369-81. http://dx.doi.org/10.1136/bjophthalmol-2013-303350.
- Knowles RL, Bull C. Longer-term survival and health outcomes for children living with congenital heart defects. Paediatr Child Health 2013;23:73-7. http://dx.doi.org/10.1016/j.paed.2012.09.005.
- Dezateux C, Brocklehurst P, Burgess S, Burton P, Carey A, Colson D, et al. Life Study: a UK-wide birth cohort study of environment, development, health, and wellbeing. Lancet 2013;382. http://dx.doi.org/10.1016/S0140-6736(13)62456-3.
- Knowles RL, Olafsdottir F. Initial Clinical Referral Standards After Newborn Screening for Congenital Hypothyroidism: Final Report of the UK Newborn Screening Programme Centre Expert Working Group and Systematic Evidence Review 2010–2011 2013. http://discovery.ucl.ac.uk/1385570/1/FINAL%20REPORT_ICR_CHT_REVIEW_Jan%202013.pdf.
- Knowles RL, Bull C, Wren C, Wade A, Goldstein H, Dezateux C, et al. Modelling survival and mortality risk to 15 years of age for a national cohort of children with serious congenital heart defects diagnosed in infancy. PLOS One 2014;9. http://dx.doi.org/10.1371/journal.pone.0106806.
- Knowles RL, Day T, Wade A, Bull C, Wren C, Dezateux C. Patient-reported quality of life outcomes for children with serious congenital heart defects. Arch Dis Child 2014;99:413-19. http://dx.doi.org/10.1136/archdischild-2013-305130.
- Knowles RL, Khalid JM, Oerton JM, Hindmarsh PC, Kelnar CJ, Dezateux C. Late clinical presentation of congenital adrenal hyperplasia in older children: findings from national paediatric surveillance. Arch Dis Child 2014;99:30-4. http://dx.doi.org/10.1136/archdischild-2012-303070.
- Tregay J, Wray J, Bull C, Franklin RC, Daubeney P, Barron DJ, et al. Unexpected deaths and unplanned re-admissions in infants discharged home after cardiac surgery: a systematic review of potential risk factors. Cardiol Young 2014;25:1-14. http://dx.doi.org/10.1017/S1047951114002492.
- Knowles R, Elbahy L, Dezateux C. The Life Study Journey. London, UK: Life Study (UCL); 2014.
- Public Health England . Newborn Pulse Oximetry Screening Pilot 2015. http://newbornphysical.screening.nhs.uk/cms.php?folder=2858 (accessed April 2015).
- National Institute for Health and Care Excellence . Antenatal Care. Appendix A: The Guideline Development Group 2008. www.nice.org.uk/guidance/CG62/chapter/Appendix-A-The-Guideline-Development-Group (accessed April 2015).
- Griebsch I, Brown J, Boggis C, Dixon A, Dixon M, Easton D, et al. Cost-effectiveness of screening with contrast enhanced magnetic resonance imaging vs X-ray mammography of women at a high familial risk of breast cancer. Br J Cancer 2006;95:801-10. http://dx.doi.org/10.1038/sj.bjc.6603356.
- Brown B, Diamantopoulos A, Bernier J, Schöffski P, Hieke K, Mantovani L, et al. An economic evaluation of cetuximab combined with radiotherapy for patients with locally advanced head and neck cancer in Belgium, France, Italy, Switzerland, and the United Kingdom. Value Health 2008;11:791-9. http://dx.doi.org/10.1111/j.1524-4733.2007.00302.x.
- Asseburg C, Frank M, Kohne CH, Hartmann JT, Griebsch I, Mohr A, et al. Cost-effectiveness of targeted therapy with cetuximab in patients with K-ras wild-type colorectal cancer presenting with initially unresectable metastases limited to the liver in a German setting. Clin Ther 2011;33:482-97. http://dx.doi.org/10.1016/j.clinthera.2011.04.010.
- Lang I, Kohne CH, Folprecht G, Rougier P, Curran D, Hitre E, et al. Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur J Cancer 2013;49:439-48. http://dx.doi.org/10.1016/j.ejca.2012.08.023.
- Novello S, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Analysis of patient-reported outcomes from the LUME-Lung 1 trial: a randomised, double-blind, placebo-controlled, Phase III study of second-line nintedanib in patients with advanced non-small cell lung cancer. Eur J Cancer 2015;51:317-26. http://dx.doi.org/10.1016/j.ejca.2014.11.015.
- Popat S, Mellemgaard A, Fahrbach K, Martin A, Rizzo M, Kaiser R, et al. Nintedanib plus docetaxel as second-line therapy in patients with non-small-cell lung cancer: a network meta-analysis. Future Oncol 2014;11:409-20. http://dx.doi.org/10.2217/fon.14.290.
- Griebsch I, Palmer M, Fayers PM, Ellis S. Is progression-free survival associated with a better health-related quality of life in patients with lung cancer? Evidence from two randomised trials with afatinib. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005762.
- Wissinger E, Griebsch I, Lungershausen J, Byrnes M, Travers K, Pashos CL. The humanistic burden of head and neck cancer: a systematic literature review. PharmacoEconomics 2014;32:1213-29. http://dx.doi.org/10.1007/s40273-014-0199-x.
- Marsh K, Xu P, Orfanos P, Gordon J, Griebsch I. Model-based cost-effectiveness analyses for the treatment of chronic lymphocytic leukaemia: a review of methods to model disease outcomes and estimate utility. PharmacoEconomics 2014;32:981-93. http://dx.doi.org/10.1007/s40273-014-0187-1.
- Clark MJ, Harris N, Griebsch I, Kaschinski D, Copley-Merriman C. Patient-reported outcome labeling claims and measurement approach for metastatic castration-resistant prostate cancer treatments in the United States and European Union. Health Qual Life Outcomes 2014;12. http://dx.doi.org/10.1186/s12955-014-0104-5.
- Popat S, Mok T, Yang JC, Wu YL, Lungershausen J, Stammberger U, et al. Afatinib in the treatment of EGFR mutation-positive NSCLC: a network meta-analysis. Lung Cancer 2014;85:230-8. http://dx.doi.org/10.1016/j.lungcan.2014.05.007.
- Marsh K, Xu P, Orfanos P, Benedict A, Desai K, Griebsch I. Model-based cost-effectiveness analyses for the treatment of chronic myeloid leukaemia: a review and summary of challenges. PharmacoEconomics 2014;32:853-64. http://dx.doi.org/10.1007/s40273-014-0177-3.
- Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. PharmacoEconomics 2014;32:865-82. http://dx.doi.org/10.1007/s40273-014-0169-3.
- Griebsch I. Immunosuppressive treatment following kidney transplantation. Pharm Unserer Zeit 2005;34:322-30. http://dx.doi.org/10.1002/pauz.200500132.
- Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 2005;365:1769-78. http://dx.doi.org/10.1016/S0140-6736(05)66481-1.
- Griebsch I, Coast J, Brown J. Quality-adjusted life-years lack quality in pediatric care: a critical review of published cost-utility studies in child health. Pediatrics 2005;115:e600-14. http://dx.doi.org/10.1542/peds.2004-2127.
- Beswick AD, Rees K, West RR, Taylor FC, Burke M, Griebsch I, et al. Improving uptake and adherence in cardiac rehabilitation: literature review. J Adv Nurs 2005;49:538-55. http://dx.doi.org/10.1111/j.1365-2648.2004.03327.x.
- Rees K, Victory J, Beswick AD, Turner SC, Griebsch I, Taylor FC, et al. Cardiac rehabilitation in the UK: uptake among under-represented groups. Heart 2005;91:375-6. http://dx.doi.org/10.1136/hrt.2003.032946.
- Roberts T, Barton P, Auguste P, Middleton L, Furmston A, Ewer A. Pulse oximetry as a screening test for congenital heart defects in newborn infants: a cost-effectiveness analysis. Arch Dis Child 2012;97:221-6. http://dx.doi.org/10.1136/archdischild-2011-300564.
- Thangaratinam S, Daniels J, Ewer AK, Zamora J, Khan KS. Accuracy of pulse oximetry in screening for congenital heart disease in asymptomatic newborns: a systematic review. Arch Dis Child Fetal Neonatal Ed 2007;92:F176-80. http://dx.doi.org/10.1136/adc.2006.107656.
- Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet 2012;379:2459-64. http://dx.doi.org/10.1016/S0140-6736(12)60107-X.
- De-Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganäs L, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39 821 newborns. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.a3037.
- Wennerholm U-B, Daxberg E-L, Fassoulas A, Hafström O, Liljegren A, Samuelsson O, et al. Pulse Oximetry (POX) Screening for Congenital Heart Defects in Newborns 2011. www2.sahlgrenska.se/upload/SU/HTA-centrum/HTA-rapporter/HTA-report%20POX%20%202011-06-30%20till%20publicering%202.pdf (accessed April 2015).
- Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation 2009;120:447-58. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.192576.
- Sendelbach DM, Jackson GL, Lai SS, Fixler DE, Stehel EK, Engle WD. Pulse oximetry screening at 4 hours of age to detect critical congenital heart defects. Pediatrics 2008;122:e815-20. http://dx.doi.org/10.1542/peds.2008-0781.
- Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine: results from a prospective multicenter study. Eur J Pediatr 2010;169:975-81. http://dx.doi.org/10.1007/s00431-010-1160-4.
- Aamir T, Kruse L, Ezeakudo O. Delayed diagnosis of critical congenital cardiovascular malformations (CCVM) and pulse oximetry screening of newborns. Acta Paediatr 2007;96:1146-9. http://dx.doi.org/10.1111/j.1651-2227.2007.00389.x.
- Hines AJ. A nurse-driven algorithm to screen for congenital heart defects in asymptomatic newborns. Adv Neonatal Care 2012;12:151-7. http://dx.doi.org/10.1097/ANC.0b013e3182569983.
- UK National Screening Committee . Screening for Congenital Heart Defects: Pulse Oximetry. Policy Review Summary [13 334] 2014. http://legacy.screening.nhs.uk/policydb_download.php?doc=391 (accessed August 2015).
- Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, . CATT Research Group . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-908. http://dx.doi.org/10.1056/NEJMoa1102673.
- Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng C-Y, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106-16. http://dx.doi.org/10.1016/S2214-109X(13)70145-1.
- Reeves B, Harding S, Langham J, Grieve R, Tomlin K, Walker J, et al. Verteporfin photodynamic therapy for neovascular age-related macular degeneration: cohort study for the UK. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16060.
- Tufail A, Patel PJ, Egan C, Hykin P, da Cruz L, Gregor Z, et al. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c2459.
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432-44. http://dx.doi.org/10.1056/NEJMoa062655.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-31. http://dx.doi.org/10.1056/NEJMoa054481.
- Martin DF, Maguire MG, Fine SL, Ying G-S, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388-98. http://dx.doi.org/10.1016/j.ophtha.2012.03.053.
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2005;36:331-5.
- Rosenfeld PJ. Intravitreal Avastin: the low cost alternative to Lucentis?. Am J Ophthalmol 2006;142:141-3. http://dx.doi.org/10.1016/j.ajo.2006.03.036.
- Raftery J, Clegg A, Jones J, Tan SC, Lotery A. Ranibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmology 2007;91:1244-6. http://dx.doi.org/10.1136/bjo.2007.116616.
- Martin DF, Maguire MG, Fine SL. Identifying and eliminating the roadblocks to comparative-effectiveness research. N Engl J Med 2010;363:105-7. http://dx.doi.org/10.1056/NEJMp1001201.
- Kodjikian L, Souied EH, Mimoun G, Mauget-Faÿsse M, Behar-Cohen F, Decullier E, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL noninferiority randomized trial. Ophthalmology 2013;120:2300-9. http://dx.doi.org/10.1016/j.ophtha.2013.06.020.
- Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 122:146-52. http://dx.doi.org/10.1016/j.ophtha.2014.07.041.
- Krebs I, Schmetterer L, Boltz A, Told R, Vécsei-Marlovits V, Egger S, et al. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Opthalmology 2013;97:266-71. http://dx.doi.org/10.1136/bjophthalmol-2012-302391.
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;382:1258-67. http://dx.doi.org/10.1016/S0140-6736(13)61501-9.
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119:1399-411. http://dx.doi.org/10.1016/j.ophtha.2012.04.015.
- Lotery AJ, Gibson J, Cree AJ, Downes SM, Harding SP, Rogers CA, et al. Pharmacogenetic associations with vascular endothelial growth factor inhibition in participants with neovascular age-related macular degeneration in the IVAN Study. Ophthalmology 2013;120:2637-43. http://dx.doi.org/10.1016/j.ophtha.2013.07.046.
- Dakin HA, Wordsworth S, Rogers CA, Abangma G, Raftery J, Harding SP, et al. Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005094.
- Moja L, Lucenteforte E, Kwag Koren H, Bertele V, Campomori A, Chakravarthy U, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2014;9. http://dx.doi.org/10.1002/14651858.cd011230.
- Reeves BC, Underwood W, Cappel-Porter H, Baos S, Rogers CA, Arnott M, et al. Documentation of adverse events in non-commercial trials of intravitreal injection of anti-VEGF drugs to treat wet age-related macular degeneration (AMD). Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-S1-A13.
- Kodjikian L, Decullier E, Souied EH, Girmens J-F, Durand EE, Chapuis FR, et al. Bevacizumab and ranibizumab for neovascular age-related macular degeneration: an updated meta-analysis of randomised clinical trials. Graefes Arch Clin Exp Ophthalmol 2014;252:1529-37. http://dx.doi.org/10.1007/s00417-014-2764-6.
- Lotery A, MacEwen C. What is stopping the NHS from using bevacizumab for macular degeneration and other retinal disorders?. BMJ 2014;349. http://dx.doi.org/10.1136/bmj.g6887.
- Lock D. Avastin and Lucentis – It’s Time for NHS Commissioners to Act Rationally by Limiting the Choices for Wet AMD Patients. BMJ Blogs 2014. http://blogs.bmj.com/bmj/2014/09/26/david-lock-avastin-and-lucentis-its-time-for-nhs-commissioners-to-act-rationally/ (accessed April 2015).
- National Institute for Health and Care Excellence (NICE) . Macular Degeneration n.d. www.nice.org.uk/guidance/indevelopment/gid-cgwave0658 (accessed April 2015).
- Royal College of Ophthalmologists . Age-Related Macular Degeneration: Guidelines for Management 2013. www.rcophth.ac.uk/wp-content/uploads/2014/12/2013-SCI-318-RCOphth-AMD-Guidelines-Sept-2013-FINAL-2.pdf (accessed April 2015).
- Annual Report 2011. London: RCO; 2011.
- Royal College of Ophthalmologists . Review Concludes That Avastin and Lucentis Are Equally Effective in Treating Wet AMD 2011. http://rcophth.nemisys2.uk.com/news.asp?itemid=647&itemTitle=RCOphth+Review+concludes+that+Avastin+and+Lucentis+are+equally+effective+in+treating+wet+AMD&section=24&sectionTitle=News (accessed April 2015).
- Jack A. Patients Urged to Sue for Best Types of Drug . Financial Times 2012. www.ft.com/cms/s/0/e8890da0-3a0e-11e1-8707-00144feabdc0.html#axzz3PJX1sKi5 (accessed February 2015).
- Cohen D. Why have UK doctors been deterred from prescribing Avastin?. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h1654.
- Lock D. Avastin and Lucentis: a guide through the legal maze. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h1377.
- Adams B. Novartis to Drop Legal Case Against NHS Body. PharmaTimes 2012. www.pharmatimes.com/Article/12-10-03/Novartis_to_drop_legal_case_against_NHS_body.aspx (accessed April 2015).
- Grogan K. Pharma Files Complaint to Stop Italy Off-Label Law. PharmaTimes 2015. www.pharmatimes.com/Article/15-02-09/Pharma_files_complaint_to_stop_Italy_off-label_law.aspx (accessed April 2015).
- Jolly D. Italy Fines Novartis and Roche in Collusion Case. New York Times 2014. www.nytimes.com/2014/03/06/business/international/italy-fines-novartis-and-roche-in-collusion-case.html?_r=2 (accessed April 2015).
- EUR-lex . Judgment of the Court (Third Chamber) of 29 March 2012. European Commission V. Republic of Poland 2012. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:62010CJ0185 (accessed April 2015).
- Killick J, Kritikos M. on behalf of White & Case . The European Court Affirms That Public Safety Comes First: Financial Considerations Cannot Justify the Placing on the Market of Unlicensed Medicines 2012. www.whitecase.com/files/Publication/5d54d7d1–3722–4f08-a89bf413f762d106/Presentation/PublicationAttachment/e0e85eae-3087–4f7c-97d8-fab2fed3d7f3/Article-European-Court-affirms-public-safety-comes-first-unlicensed-medicines.pdf (accessed April 2015).
- Calkin S. CCGs Poised to Defy Pharma by Switching to Cheaper, Unlicensed Drug. Health Service Journal 2014. www.hsj.co.uk/news/commissioning/ccgs-poised-to-defy-pharma-by-switching-to-cheaper-unlicensed-drug/5077111.article#.VOHq-fmsV8E (accessed April 2015).
- Generics and Biosimilars Initiative . Biosimilars of Bevacizumab n.d. http://gabionline.net/Biosimilars/General/Biosimilars-of-bevacizumab (accessed April 2015).
- Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17100.
- Shakur H, Roberts I, Bautista R, Caballero J, Coats T, . CRASH-2 trial collaborators . Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23-32. http://dx.doi.org/10.1016/S0140-6736(10)60835-5.
- Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, . CRASH-2 collaborators . The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011;377:1096-101. http://dx.doi.org/10.1016/S0140-6736(11)60278-X.
- Guerriero C, Cairns J, Perel P, Shakur H, Roberts I, . CRASH 2 trial collaborators . Cost-effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH-2 trial. PLOS One 2011;6. http://dx.doi.org/10.1371/journal.pone.0018987.
- Edwards P, Shakur H, Barnetson L, Prieto D, Evans S, Roberts I. Central and statistical data monitoring in the clinical randomisation of an antifibrinolytic in significant haemorrhage (CRASH-2) trial. Clin Trials 2014;11:336-43. http://dx.doi.org/10.1177/1740774513514145.
- Alvarado JC, Dewan Y, Elsayed HF, Gogichaishvili T, Gupta S, Hunt BJ, et al. Improving trauma care worldwide. Tranexamic acid in trauma: we need stronger global health policy. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4593.
- Roberts I. The CRASH-2 trial was not about global scientific supremacy. Lancet 2013;382:207-8. http://dx.doi.org/10.1016/S0140-6736(13)61596-2.
- Shakur H, Roberts I, Piot P, Horton R, Krug E, Mersch J. A promise to save 100,000 trauma patients. Lancet 2012;380:2062-3. http://dx.doi.org/10.1016/S0140-6736(12)62037-6.
- Pealing L, Perel P, Preito-Merino D, Roberts I. CRASH-2 Trial Collaborators . Risk factors for vascular occlusive events and death due to bleeding in trauma patients; an analysis of the CRASH-2 cohort. PLOS One 2012;7. http://dx.doi.org/10.1371/journal.pone.0050603.
- Roberts I, Perel P, Prieto-Merino D, Shakur H, Coats T, Hunt BJ, et al. Effect of tranexamic acid on mortality in patients with traumatic bleeding: prespecified analysis of data from randomised controlled trial. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5839.
- Roberts I. Tranexamic acid: a recipe for saving lives in traumatic bleeding. Int J Epidemiol 2011;40. http://dx.doi.org/10.1093/ije/dyr141.
- CRASH-2 Trial Collaborators . Effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study). BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d3795.
- Roberts I. CRASH-2: Antifibrinolytic treatment in traumatic brain injury. J Neurotrauma 2011;28:A33-4.
- Roberts I. The CRASH-2 trial of an antifibrinolytic agent in traumatic haemorrhage: an international collaboration. Indian J Med Res 2007;125:5-7.
- CRASH-2 Trial Collaborators . Improving the evidence base for trauma care: progress in the International CRASH-2 trial. PLOS Clin Trials 2006;1. http://dx.doi.org/10.1371/journal.pctr.0010030.
- Perel P, Prieto-Merino D, Shakur H, Roberts I. Development and validation of a prognostic model to predict death in patients with traumatic bleeding, and evaluation of the effect of tranexamic acid on mortality according to baseline risk: a secondary analysis of a randomised controlled trial. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17240.
- Perel P, Al-Shahi Salman R, Kawahara T, Morris Z, Prieto-Merino D, Roberts I, et al. CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) intracranial bleeding study: the effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo-controlled trial. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16130.
- Shakur H, Elbourne D, Gülmezoglu M, Alfirevic Z, Ronsmans C, Allen E, et al. The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-40.
- Roberts I, Shakur H, Ker K, Coats T. CRASH-2 Trial collaborators . Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev 2012;12. http://dx.doi.org/10.1002/14651858.cd004896.pub3.
- London School of Hygiene and Tropical Medicine Clinical Trials Unit . TRANMAN – A Clot Stabiliser Can Save the Lives of Trauma Victims 2012. http://youtu.be/pIoYJUf1uls (accessed April 2015).
- University of Leicester . TXA Implementation Pages – How To Do It n.d. www2.le.ac.uk/departments/cardiovascular-sciences/research/cardiovascular-physiology-and-pathophysiology/emergency-medicine-group/research/injury/txa-implementation-pages-how-to-do-it (accessed April 2015).
- Fentiman P. Tranexamic Acid Manga Offers Comic Relief for Medics. The Chariot 2012. http://blogs.lshtm.ac.uk/news/2012/09/11/tranexamic-acid-manga-offers-comic-relief-for-medics/ (accessed April 2015).
- EMCrit Blog . Podcast 67 – Tranexamic Acid (TXA), Crash 2, &Amp; Pragmatism With Tim Coats 2012. http://emcrit.org/podcasts/tranexamic-acid-trauma/ (accessed April 2015).
- Morrison J, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg 2012;147:113-19. http://dx.doi.org/10.1001/archsurg.2011.287.
- Napolitano LM, Cohen MJ, Cotton BA, Schreiber MA, Moore EE. Tranexamic acid in trauma: how should we use it?. J Trauma Acute Care Surg 2012;74:1575-86. http://dx.doi.org/10.1097/TA.0b013e318292cc54.
- Macroeconomics and Health: Investing in Health for Economic Development. Geneva: World Health Organization; 2001.
- Edwards P, Roberts I, Green J, Lutchmun S. Deaths from injury in children and employment status in family: analysis of trends in class specific death rates. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38875.757488.4F.
- Nantulya VM, Reich MR. Equity dimensions of road traffic injuries in low- and middle-income countries. Inj Control Saf Promot 2003;10:13-20. http://dx.doi.org/10.1076/icsp.10.1.13.14116.
- Aeron-Thomas A, Jacobs GD, Sexton B, Gururaj G, Rahman F. The Involvement and Impact of Road Crashes on the Poor: Bangladesh and India Case Studies 2004. www.dfid.gov.uk/r4d/Output/5429/Default.aspx (accessed April 2015).
- National Institute for Health Research . HTA – 01 62 01: The Clinical and Cost-Effectiveness of Anakinra for the Treatment of Rheumatoid Arthritis in Adults: A Systematic Review and Economic Analysis n.d. www.nets.nihr.ac.uk/projects/hta/016201 (accessed April 2015).
- Clark W, Jobanputra P, Barton P, Burls A. The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8180.
- Jobanputra P, Barton P, Bryan S, Burls A. The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess 2002;6. http://dx.doi.org/10.3310/hta6210.
- Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8110.
- Barton P. Development of the Birmingham Rheumatoid Arthritis Model: past, present and future plans. Rheumatology 2011;50:iv32-8. http://dx.doi.org/10.1093/rheumatology/ker244.
- Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10420.
- Jobanputra P, Maggs F, Deeming A, Carruthers D, Rankin E, Jordan AC, et al. A randomised efficacy and discontinuation study of etanercept versus adalimumab (RED SEA) for rheumatoid arthritis: a pragmatic, unblinded, non-inferiority study of first TNF inhibitor use: outcomes over 2 years. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2012-001395.
- Ruiz Garcia V, Jobanputra P, Burls A, Cabello JB, Gálvez Muñoz JG, Saiz Cuenca ESC, et al. Certolizumab pegol (CDP870) for rheumatoid arthritis in adults. Cochrane Database Syst Rev n.d.;2.
- National Institute for Health and Care Excellence Decision Support Unit . Sequential Use of TNF-α Inhibitors for the Treatment of Rheumatoid Arthritis 2006. www.nicedsu.org.uk/PDFs%20of%20reports/RA%202006%20final%20report.pdf (accessed April 2015).
- Adalimumab, Etanercept, Infliximab, Rituximab and Abatacept for the Treatment of Rheumatoid Arthritis after the Failure of a TNF Inhibitor. London: NICE; 2010.
- Abatacept for the Treatment of Rheumatoid Arthritis. London: NICE; 2008.
- Hill S, Garattini S, van Loenhout J, O’Brien BJ, de Joncheere K. Technology Appraisal Programme of the National Institute for Clinical Excellence: A Review by the WHO. London: World Health Organization; 2003.
- Ingoldby CJ, Wujanto R, Mitchell JE. Impact of vascular surgery on community mortality from ruptured aortic aneurysms. Br J Surg 1986;73:551-3. http://dx.doi.org/10.1002/bjs.1800730711.
- Collin J, Araujo L, Walton J, Lindsell D. Oxford screening programme for abdominal aortic aneurysm in men aged 65 to 74 years. Lancet 1988;2:613-15. http://dx.doi.org/10.1016/S0140-6736(88)90649-6.
- Scott RA, Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA. The long-term benefits of a single scan for abdominal aortic aneurysm (AAA) at age 65. Eur J Vasc Endovasc Surg 2001;21:535-40. http://dx.doi.org/10.1053/ejvs.2001.1368.
- Lederle FA, Johnson GR, Wilson SE, Gordon IL, Chute EP, Littooy FN, et al. Relationship of age, gender, race, and body size to infrarenal aortic diameter. The Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Investigators. J Vasc Surg 1997;26:595-601. http://dx.doi.org/10.1016/S0741-5214(97)70057-0.
- Lederle FA, Johnson GR, Wilson SE. Abdominal aortic aneurysm in women. J Vasc Surg 2001;34:122-6. http://dx.doi.org/10.1067/mva.2001.115275.
- Cornuz J, Sidoti PC, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health 2004;14:343-9. http://dx.doi.org/10.1093/eurpub/14.4.343.
- Guessous I, Periard D, Lorenzetti D, Cornuz J, Ghali WA. The efficacy of pharmacotherapy for decreasing the expansion rate of abdominal aortic aneurysms: a systematic review and meta-analysis. PLOS One 2008;3. http://dx.doi.org/10.1371/journal.pone.0001895.
- The UK . Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998;352:1649-55. http://dx.doi.org/10.1016/S0140-6736(98)10137-X.
- Volodos NL, Karpovich IP, Troyan VI, Kalashnikova Y, Shekhanin VE, Ternyuk NE, et al. Clinical experience of the use of self-fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction. Vasa Suppl 1991;33:93-5.
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991;5:491-9. http://dx.doi.org/10.1007/BF02015271.
- Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16090.
- Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. EVAR trial participants . Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004;364:843-8. http://dx.doi.org/10.1016/S0140-6736(04)16979-1.
- Yusuf SW, Whitaker SC, Chuter TA, Wenham PW, Hopkinson BR. Emergency endovascular repair of leaking aortic aneurysm. Lancet 1994;344. http://dx.doi.org/10.1016/S0140-6736(94)90443-X.
- Brown LC, Thompson SG, Greenhalgh RM, Powell JT. Endovascular Aneurysm Repair trial participants . Incidence of cardiovascular events and death after open or endovascular repair of abdominal aortic aneurysm in the randomized EVAR trial 1. Br J Surg 2011;98:935-42. http://dx.doi.org/10.1002/bjs.7485.
- Brown LC, Greenhalgh RM, Powell JT, Thompson SG. EVAR Trial Participants . Use of baseline factors to predict serious complications and re-interventions after endovascular aneurysm repair (EVAR) in patients with a large abdominal aortic aneurysm: results from the UK EVAR trials. Br J Surg 2010;97:1207-17. http://dx.doi.org/10.1002/bjs.7104.
- Wyss TR, Brown LC, Powell JT, Greenhalgh RM. Rate and predictability of graft rupture after endovascular and open abdominal aortic aneurysm repair: data from the EVAR trials. Br J Surg 2010;98. http://dx.doi.org/10.1097/sla.0b013e3181fcb44a.
- Wyss TR, Brown LC, Powell JT, Greenhalgh RM. Rate and predictability of graft rupture after endovascular and open abdominal aortic aneurysm repair data from the EVAR trials. Ann Surg 2010;252:805-12. http://dx.doi.org/10.1097/SLA.0b013e3181fcb44a.
- Brown LC, Greenhalgh RM, Powell JT, Thompson SG. EVAR Trial Participants . Use of baseline factors to predict complications and reinterventions after endovascular repair of abdominal aortic aneurysm. Br J Surg 2010;97:1207-17. http://dx.doi.org/10.1002/bjs.7104.
- Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, . United Kingdom EVAR Trial Investigators . Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863-71. http://dx.doi.org/10.1056/NEJMoa0909305.
- Brown LC, Brown EA, Greenhalgh RM, Powell JT, Thompson SG. UK EVAR Trial Participants . Renal Function and Abdominal Aortic Aneurysm (AAA). The Impact of Different Management Strategies on Long-Term Renal Function in the UK EndoVascular Aneurysm Repair (EVAR) Trials. Ann Surg 2010;251:966-75. http://dx.doi.org/10.1097/SLA.0b013e3181d9767c.
- Brown LC, Greenhalgh RM, Thompson SG, Powell JT. EVAR Trial Participants . Does EVAR alter the rate of cardiovascular events in patients with abdominal aortic aneurysm considered unfit for open repair? Results from the Randomised EVAR Trial 2. Eur J Vasc Endovasc Surg 2010;39:396-402. http://dx.doi.org/10.1016/j.ejvs.2010.01.002.
- Epstein DM, Sculpher MJ, Manca A, Michaels J, Thompson SG, Brown LC, et al. Modelling the long-term cost-effectiveness of endovascular or open repair for abdominal aortic aneurysm. Br J Surg 2008;95:183-90. http://dx.doi.org/10.1002/bjs.5911.
- Powell JT, Brown LC, Greenhalgh RM, Thompson SG. The rupture rate of large abdominal aortic aneurysms: Is this modified by anatomical suitability for endovascular repair?. Ann Surg 2008;247:173-9. http://dx.doi.org/10.1097/SLA.0b013e3181557d2a.
- Greenhalgh RM. EVAR Trial Participants . Secondary interventions and mortality following endovascular aortic aneurysm repair: device-specific results from the UK EVAR trials. Eur J Vasc Endovasc Surg 2007;34:281-90. http://dx.doi.org/10.1016/j.ejvs.2007.03.021.
- Brown LC, Greenhalgh RM, Howell S, Powell JT, Thompson SG. Patient fitness and survival after abdominal aortic aneurysm repair in patients from the UK EVAR trials. Br J Surg 2007;94:709-16. http://dx.doi.org/10.1002/bjs.5776.
- Greenhalgh RM. EVAR Trial Participants . Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365:2179-86. http://dx.doi.org/10.1016/S0140-6736(05)66627-5.
- Greenhalgh RM. EVAR Trial Participants . Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet 2005;365:2187-92. http://dx.doi.org/10.1016/S0140-6736(05)66628-7.
- Brown LC, Epstein D, Manca A, Beard JD, Powell JT, Greenhalgh RM. The UK endovascular aneurysm repair (EVAR) trials: design, methodology and progress. Eur J Vasc Endovasc Surg 2004;27:372-81. http://dx.doi.org/10.1016/j.ejvs.2003.12.019.
- Greenhalgh RM, Powell JT. Endovascular repair of abdominal aortic aneurysm. N Engl J Med 2008;358:494-501. http://dx.doi.org/10.1056/NEJMct0707524.
- Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D. The UK EVAR Trial Investigators . Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med 2010;362:1872-80. http://dx.doi.org/10.1056/NEJMoa0911056.
- Waton S, Johal A, Groene O, Cromwell D, Mitchell D, Loftus I. National Vascular Registry 2013 Report on Surgical Outcomes: Consultant-level Statistics. London: The Royal College of Surgeons of England; 2013.
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004;351:1607-18. http://dx.doi.org/10.1056/NEJMoa042002.
- Blankensteijn JD, de Jong SE, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SM, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med 2005;352:2398-405. http://dx.doi.org/10.1056/NEJMoa051255.
- De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1881-9. http://dx.doi.org/10.1056/NEJMoa0909499.
- Becquemin JP, Pillet JC, Lescalie F, Sapoval M, Goueffic Y, Lermusiaux P, et al. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low- to moderate-risk patients. J Vasc Surg 2011;53:1167-73. http://dx.doi.org/10.1016/j.jvs.2010.10.124.
- Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA 2009;302:1535-42. http://dx.doi.org/10.1001/jama.2009.1426.
- Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Matsumura JS, Padberg FT, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med 2012;367:1988-97. http://dx.doi.org/10.1056/NEJMoa1207481.
- Powell JT, Sweeting MJ, Thompson MM, Ashleigh R, Bell R, . IMPROVE trial investigators . Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.f7661.
- Drury D, Michaels J, Jones L, Ayiku L. Interventional Procedures Programme NICE Review Body Report. Sheffield: The University of Sheffield School of Health and Related Research; 2005.
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms. Clinical Practice Guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg 2011;41:S1-58. http://dx.doi.org/10.1016/j.ejvs.2010.09.011.
- Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: The Society for Vascular Surgery practice guidelines. J Vasc Surg 2009;50:S2-49. http://dx.doi.org/10.1016/j.jvs.2009.07.002.
- Stather PW, Sidloff D, Dattani N, Choke E, Bown MJ, Sayers RD, et al. Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. Br J Surg 2013;100:863-72. http://dx.doi.org/10.1002/bjs.9101.
- Rayt HS, Sutton AJ, London NJ, Sayers RD, Bown MJ. A systematic review and meta-analysis of endovascular repair (EVAR) for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2008;36:536-44. http://dx.doi.org/10.1016/j.ejvs.2008.08.008.
- Chambers D, Epstein D, Walker S, Fayter D, Paton F, Wright K, et al. Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13480.
- Lombard Medical . Lombard Medical Technologies PLC: Pre-Close Trading and Strategic Update 2014. www.lombardmedical.com/files/pre-close_trading_and_strategic_update.pdf (accessed April 2015).
- Waton S, Johal A, Groene O, Cromwell D, Mitchell D, Loftus I. Outcomes After Elective Repair of Infra-renal Abdominal Aortic Aneurysm. London: The Royal College of Surgeons of England; 2013.
- The National Vascular Database Report 2009. London: The Royal College of Surgeons of England; 2009.
- 2013/14 NHS Standard Contract for Specialised Vascular Services (Adults). Redditch: NHS England; 2012.
- The Vascular Society . Framework for Improving the Results of Elective AAA Repair 2011 2011. www.vascularsociety.org.uk/wp-content/uploads/2012/11/VSGBI-AAA-QIF-2011-v4.pdf (accessed April 2015).
- Schanzer A, Greenberg RK, Hevelone N, Robinson WP, Eslami MH, Goldberg RJ, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011;123:2848-55. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.014902.
- National Institute for Health Research . HTA – 09 22 169: An Assessment of the Cost-Effectiveness of Magnetic Resonance Including Diffusion-Weighted Brain Imaging in Patients With Transient Ischaemic Attack and Minor Stroke n.d. www.nets.nihr.ac.uk/projects/hta/0922169 (accessed April 2015).
- Wardlaw JM, Chappell F, Stevenson M, De Nigris E, Thomas S, Gillard J, et al. Accurate, practical and cost-effective assessment of carotid stenosis in the UK. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10300.
- Wardlaw JM, Chappell FM, Best JJK, Wartolowska K, Berry E. on behalf of the NHS R&D Health Technology Assessment Carotid Stenosis Imaging Group . Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta-analysis. Lancet 2006;367:1503-12. http://dx.doi.org/10.1016/S0140-6736(06)68650-9.
- Chappell FM, Raab GM, Wardlaw JM. When are summary ROC curves appropriate for diagnostic meta-analyses?. Stat Med 2009;28:2653-68. http://dx.doi.org/10.1002/sim.3631.
- Wardlaw JM, Stevenson MD, Chappell F, Rothwell PM, Gillard J, Young G, et al. Carotid artery imaging for secondary stroke prevention: both imaging modality and rapid access to imaging are important. Stroke 2009;40:3511-17. http://dx.doi.org/10.1161/STROKEAHA.109.557017.
- Wardlaw JM, Chappell FM, Best JJ, Wartolowska K, Berry E. Database of Abstracts of Reviews of Effects (DARE). York: Centre for Reviews and Dissemination (CRD), University of York; 2006.
- Chappell FM, Wardlaw JM, Young GR, Gillard JH, Roditi GH, Yip B, et al. Carotid artery stenosis: accuracy of noninvasive test: individual patient data meta-analysis. Radiology 2009;251:493-502. http://dx.doi.org/10.1148/radiol.2512080284.
- National Institute for Health Research . HTA – 96 08 01: What Is the Best Imaging Strategy for Acute Stroke? n.d. www.nets.nihr.ac.uk/projects/hta/960801 (accessed April 2015).
- Wardlaw J, Brazzelli M, Miranda H, Chappell F, McNamee P, Scotland G, et al. An assessment of the cost-effectiveness of magnetic resonance, including diffusion-weighted imaging, in patients with transient ischaemic attack and minor stroke: a systematic review, meta-analysis and economic evaluation. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18270.
- National Institute for Health and Care Excellence . Stroke: National Clinical Guideline for Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA) 2008. www.nice.org.uk/nicemedia/live/12018/41363/41363.pdf (accessed April 2015).
- Scottish Intercollegiate Guidelines Network . Management of Patients With Stroke or TIA: Assessment, Investigation, Immediate Management and Secondary Prevention 2008. http://sign.ac.uk/pdf/sign108.pdf (accessed April 2015).
- European Stroke Organization (ESO) Executive . Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008;25:457-50. http://dx.doi.org/10.1159/000131083.
- Canadian Best Practice Recommendations for Stroke Care . Hyperacute Stroke Care 2013. http://strokebestpractices.ca/wp-content/uploads/2013/05/Ch3_SBP2013_Hyper-Acute-_23MAY13_EN_-FINAL4.pdf (accessed April 2015).
- The Australian National Stroke Foundation . Clinical Guidelines for Stroke Management 2010 n.d. http://strokefoundation.com.au/site/media/Clinical_Guidelines_Acute_Management_Recommendations_2010.pdf (accessed April 2015).
- Latchaw R, Alberts MJ, Lev MH, Connors JJ, Harbaugh RE, Higashida RT, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke 2009;40:3646-78. http://dx.doi.org/10.1161/STROKEAHA.108.192616.
- Warlow C, van Gijn J, Dennis M, Wardlaw J, Bamford J, Hankey G, et al. Stroke: Practical Management. Oxford: Blackwell Scientific Ltd; 2008.
- Kane I, Whiteley W, Sandercock P, Wardlaw J. Availability of CT and MR for assessing patients with acute stroke. Cerebrovasc Dis 2008;25:375-7. http://dx.doi.org/10.1159/000120688.
- Brazzelli M, Shuler K, Quayyum Z, Hadley D, Muir K, McNamee P, et al. Clinical and imaging services for TIA and minor stroke: results of two surveys of practice across the UK. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-003359.
- McNally N, Williams H, Phillips D, Smallman-Raynor M, Lewis S, Venn A, et al. Atopic eczema and domestic water hardness. Lancet 1998;352:527-31. http://dx.doi.org/10.1016/S0140-6736(98)01402-0.
- Miyake Y, Yokoyama T, Yura A, Iki M, Shimizu T. Ecological association of water hardness with prevalence of childhood atopic dermatitis in a Japanese urban area. Environ Res 2004;94:33-7. http://dx.doi.org/10.1016/S0013-9351(03)00068-9.
- Thomas K, Koller K, Dean T, Gilbert C, Sach T, Frost A. A multicentre randomised controlled trial and economic evaluation of ion-exchange water softeners for the treatment of eczema in children: the Softened Water Eczema Trial (SWET). Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15080.
- Thomas K, Dean T, O’Leary C, Sach TH, Koller K, Frost A, et al. A randomised controlled trial of ion-exchange water softeners for the treatment of eczema in children. PLOS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1000395.
- Thomas K, Sach T. A multicentre randomized controlled trial of ion-exchange water softeners for the treatment of eczema in children: protocol for the Softened Water Eczema Trial (SWET) (ISRCTN: 71423189). Br J Dermatol 2008;159:561-6. http://dx.doi.org/10.1111/j.1365-2133.2008.08704.x.
- Wootton C, Koller K, Lawton S, O’Leary C, Thomas K. Are accelerometers a useful tool for measuring disease activity in children with eczema? Validity, responsiveness to change, and acceptability of use in a clinical trial setting. Br J Dermatol 2012;167:1131-7. http://dx.doi.org/10.1111/j.1365-2133.2012.11184.x.
- National Institute for Health Research . NIHR Researchfish 2014. www.nihr.ac.uk/about/nihr-researchfish.htm (accessed April 2015).
- Williams H. Atopic eczema. BMJ 1995;311. http://dx.doi.org/10.1136/bmj.311.7015.1241.
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess 2000;4.
- Arnedo-Pena A, Bellido-Blasco J, Puig-Barbera J, Artero-Civera A, Campos-Cruañes JB, Pac-Sa MR, et al. Domestic water hardness and prevalence of atopic eczema in Castellon (Spain) schoolchildren. Salud Publica Mex 2007;49:295-301. http://dx.doi.org/10.1590/S0036-36342007000400009.
- Williams H. Is the prevalence of atopic dermatitis increasing?. Clin Exp Dermatol 1992;17:385-91. http://dx.doi.org/10.1111/j.1365-2230.1992.tb00244.x.
- Williams H. Giving early solids to infants. Beware of misclassifying eczema. BMJ 1993;307. http://dx.doi.org/10.1136/bmj.307.6901.444-c.
- Williams H. Atopic dermatitis: new information from epidemiological studies. Br J Hosp Med 1994;52:409-12.
- Williams H. On the definition and epidemiology of atopic dermatitis. Dermatol Clin 1995;13:649-57.
- Williams H. Diagnostic criteria for atopic dermatitis. Lancet 1996;348:1391-2. http://dx.doi.org/10.1016/S0140-6736(05)65466-9.
- McNally N, Phillips D, Williams H. The problem of atopic eczema: aetiological clues from the environment and lifestyles. Soc Sci Med 1998;46:729-41. http://dx.doi.org/10.1016/S0277-9536(97)00174-3.
- Williams H. Do topical steroids reduce relapses in adults with atopic dermatitis?. Arch Dermatol 1999;135:1530-1. http://dx.doi.org/10.1001/archderm.135.12.1530.
- Williams H. Diagnostic criteria for atopic dermatitis: where do we go from here?. Arch Dermatol 1999;135:583-6. http://dx.doi.org/10.1001/archderm.135.5.583.
- Williams H. Epidemiology of atopic dermatitis: recent advances and future predictions. Curr Probl Dermatol 1999;28:9-17. http://dx.doi.org/10.1159/000060595.
- Charman C, Venn A, Williams H. Measurement of body surface area involvement in atopic eczema: an impossible task?. Br J Dermatol 1999;140:109-11. http://dx.doi.org/10.1046/j.1365-2133.1999.02617.x.
- Williams H. Epidemiology of atopic dermatitis. Clin Exp Dermatol 2000;25:522-9. http://dx.doi.org/10.1046/j.1365-2230.2000.00698.x.
- Charman C, Morris A, Williams H. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol 2000;142:931-6. http://dx.doi.org/10.1046/j.1365-2133.2000.03473.x.
- Emerson R, Charman C, Williams H. The Nottingham eczema severity score: preliminary refinement of the Rajka and Langeland grading. Br J Dermatol 2000;142:288-97. http://dx.doi.org/10.1046/j.1365-2133.2000.03300.x.
- Charman C, Williams H. Outcome measures of disease severity in atopic eczema. Arch Dermatol 2000;136:763-9. http://dx.doi.org/10.1001/archderm.136.6.763.
- Ravenscroft JC, Thomas KS, Williams HC. Current management of atopic eczema. Practitioner 2002;246:690-5.
- Williams H. New treatments for atopic dermatitis. BMJ 2002;324:1533-34. http://dx.doi.org/10.1136/bmj.324.7353.1533.
- Williams H. Prevention of atopic eczema: a dream not so far away?. Arch Dermatol 2002;138:391-2. http://dx.doi.org/10.1001/archderm.138.3.391.
- Thomas K, Armstrong S, Avery A, Po AL, O’Neill C, Young S, et al. Randomised controlled trial of short bursts of a potent topical corticosteroid versus prolonged use of a mild preparation for children with mild or moderate atopic eczema. BMJ 2002;324. http://dx.doi.org/10.1136/bmj.324.7340.768.
- Charman C, Venn A, Williams H. Reliability testing of the six area, six sign atopic dermatitis severity score. Br J Dermatol 2002;146:1057-60. http://dx.doi.org/10.1046/j.1365-2133.2002.04644.x.
- Gradwell C, Thomas K, English J, Williams H. A randomized controlled trial of nurse follow-up clinics: do they help patients and do they free up consultants’ time?. Br J Dermatol 2002;147:513-17. http://dx.doi.org/10.1046/j.1365-2133.2002.04901.x.
- Williams H. Evening primrose oil for atopic dermatitis. BMJ 2003;327:1358-9. http://dx.doi.org/10.1136/bmj.327.7428.1358.
- Williams H. Objective measures of atopic dermatitis severity: in search of the Holy Grail. Arch Dermatol 2003;139:1490-2. http://dx.doi.org/10.1001/archderm.139.11.1490.
- Charman C, Chambers C, Williams H. Measuring atopic dermatitis severity in randomized controlled clinical trials: what exactly are we measuring?. J Invest Dermatol 2003;120:932-41. http://dx.doi.org/10.1046/j.1523-1747.2003.12251.x.
- Charman C, Williams H. The use of corticosteroids and corticosteroid phobia in atopic dermatitis. Clin Dermatol 2003;21:193-200. http://dx.doi.org/10.1016/S0738-081X(02)00368-1.
- Charman CR, Venn AJ, Williams H. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004;140:1513-19. http://dx.doi.org/10.1001/archderm.140.12.1513.
- Williams H. Twice-weekly topical corticosteroid therapy may reduce atopic dermatitis relapses. Arch Dermatol 2004;140:1151-2. http://dx.doi.org/10.1001/archderm.140.9.1151.
- Flohr C, Johansson S, Wahlgren C-F, Williams H. How atopic is atopic dermatitis?. J Allergy Clinl Immunol 2004;114:150-8. http://dx.doi.org/10.1016/j.jaci.2004.04.027.
- Perkin M, Strachan D, Williams H, Kennedy C, Golding J. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol 2004;15:221-9. http://dx.doi.org/10.1111/j.1399-3038.2004.00160.x.
- Williams J, Burr M, Williams H. Factors influencing atopic dermatitis: a questionnaire survey of schoolchildren’s perceptions. Br J Dermatol 2004;150:1154-61. http://dx.doi.org/10.1111/j.1365-2133.2004.05869.x.
- Van Coevorden A, Coenraads P, Svensson A, Bavinck JN, Diepgen TL, Naldi L, et al. Overview of studies of treatments for hand eczema: the EDEN hand eczema survey. Br J Dermatol 2004;151:446-51. http://dx.doi.org/10.1111/j.1365-2133.2004.06040.x.
- Charman CR, Venn AJ, Williams H, Bigby M. Measuring atopic eczema severity visually: which variables are most important to patients?. Arch Dermatol 2005;141:1146-51. http://dx.doi.org/10.1001/archderm.141.9.1146.
- Johansson S, Flohr C, Wahlgren C-F, Williams H. Role of immunoglobulin E sensitization in eczema, previously referred to as atopic dermatitis. Expert Rev Clin Immunol 2005;1:257-62. http://dx.doi.org/10.1586/1744666X.1.2.257.
- Williams H. Atopic dermatitis. N Engl J Med 2005;352:2314-24. http://dx.doi.org/10.1056/NEJMcp042803.
- Silcocks P, Williams H. A scientific look at seasonality of symptom severity in atopic dermatitis. J Invest Dermatol 2005;124:xviii-xix. http://dx.doi.org/10.1111/j.0022-202X.2004.23638.x.
- Ashcroft DM, Dimmock P, Garside R, Stein K, Williams H. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38376.439653.D3.
- Haileamlak A, Dagoye D, Williams H, Venn AJ, Hubbard R, Britton J, et al. Early life risk factors for atopic dermatitis in Ethiopian children. J Allergy Clin Immunol 2005;115:370-6. http://dx.doi.org/10.1016/j.jaci.2004.10.024.
- Haileamlak A, Lewis S, Britton J, Venn AJ, Woldemariam D, Hubbard R, et al. Validation of the International Study of Asthma and Allergies in Children (ISAAC) and UK criteria for atopic eczema in Ethiopian children. Br J Dermatol 2005;152:735-41. http://dx.doi.org/10.1111/j.1365-2133.2005.06511.x.
- Flohr C, Pascoe D, Williams H. Atopic dermatitis and the ‘hygiene hypothesis’: too clean to be true?. Br J Dermatol 2005;152:202-16. http://dx.doi.org/10.1111/j.1365-2133.2004.06436.x.
- Williams H. Educational programmes for young people with eczema. BMJ 2006;332:923-4. http://dx.doi.org/10.1136/bmj.332.7547.923.
- Langan S, Thomas K, Williams H. What constitutes a flare of atopic eczema?. J Invest Dermatol 2005;125.
- Thomas K, Koller K, Foster K, Perdue J, Charlesworth L, Chalmers JR. The UK clinical research network-has it been a success for dermatology clinical trials?. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-153.
- British Broadcasting Corporation . South Today. Features: Soft Water Eczema Trial 2009. www.bbc.co.uk/southtoday/content/articles/2009/01/09/eczema_water_feature.shtml (accessed April 2015).
- British Broadcasting Corporation . Radio 4. Case Notes Programme on Eczema 2009. www.bbc.co.uk/programmes/b00lv28n (accessed April 2015).
- Nankervis H, Thomas K, Delamere F, Barbarot S, Williams H. Systematic review of treatments for eczema. NIHR Journal 2015.
- Thomas K, Langan S, Stuart B, Schmidtt J, Williams H. Validation of treatment escalation as a definition of atopic eczema flares. PLOS One 2015;10. http://dx.doi.org/10.1371/journal.pone.0124770.
- Devonshire J, Koller K, Davies-Jones S. A trial into whether water softeners can help eczema. Dermatol Nurs 2008;7:20-51.
- Davies-Jones S, Grundy J, Simmonds R. Can childhood eczema be improved by the installation of a water softener at home?. Dermatol Nurs 2011;10:44-9.
- The University of Nottingham . People Look-Up: Hywel Williams 2015. www.nottingham.ac.uk/medicine/people/hywel.williams (accessed April 2015).
- National Institute for Health Research . HTA – 11 65 01: Randomised Controlled Trial of Silk Therapeutic Clothing for the Long-Term Management of Eczema in Children (CLOTHES Trial: CLOTHing for the Relief of Eczema Symptoms) 2015. www.nets.nihr.ac.uk/projects/hta/116501 (accessed April 2015).
- Eleftheriadou V, Thomas K, Ravenscroft J, Whitton M, Batchelor J, Williams H. Feasibility, double-blind, randomised, placebo-controlled, multi-centre trial of hand-held NB-UVB phototherapy for the treatment of vitiligo at home (HI-Light trial: Home Intervention of Light therapy). Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-51.
- National Institute for Health Research . HTA – 11 153 01: BATHE (Bath Emollients for Treatment of Childhood Eczema) 2015. www.nets.nihr.ac.uk/projects/hta/1115301 (accessed April 2015).
- National Institute for Health Research . HTA – 12 67 12: A Randomised Controlled Trial to Determine Whether Skin Barrier Enhancement With Emollients Can Prevent Eczema in High Risk Children 2015. www.nets.nihr.ac.uk/projects/hta/126712 (accessed April 2015).
- The University of Nottingham . HOME – Harmonising Outcome Measures for Eczema 2015. http://nottingham.ac.uk/homeforeczema/index.aspx (accessed April 2015).
- Jancin B. Hard Water Linked to Infant Eczema 2014. www.edermatologynews.com/home/article/hard-water-linked-to-infant-eczema/12e6a599f0d1f468bfb1123dde425964.html (accessed April 2015).
- British Broadcasting Corporation News . Eczema Study into Hard Water Link 2007. http://news.bbc.co.uk/2/hi/uk_news/england/7115304.stm (accessed April 2015).
- British Broadcasting Corporation News . Water Softener Eczema Relief Hope 2009. http://news.bbc.co.uk/2/hi/health/7820081.stm (accessed April 2015).
- The University of Nottingham . Water Softeners Not Found to Improve Childhood Eczema 2011. www.nottingham.ac.uk/news/pressreleases/2011/february/watersoftenerseczema.aspx (accessed April 2015).
- The University of Nottingham . Video of Professor Hywel Williams. Centre of Evidence Based Dermatology: Softened Water Eczema Trial 2015. www.nottingham.ac.uk/research/groups/cebd/projects/swet.aspx (accessed April 2015).
- Nottingham Post . City’s University Study Proves Water Softeners Do Not Help Eczema 2011. www.nottinghampost.com/City-s-university-study-proves-water-softeners-help-eczema/story-12245607-detail/story.html (accessed April 2015).
- Nottingham Post . Eczema Theory Disproved 2011. www.nottinghampost.com/Eczema-theory-disproved/story-12246140-detail/story.html (accessed April 2015).
- Stein J. Water Softeners May Not Drastically Ease Eczema Symptoms for Children, a Study Finds 2011. http://articles.latimes.com/2011/feb/15/news/la-heb-eczema-20110215 (accessed April 2015).
- U.S. News & World Report . Softening Water Does Not Seem to Ease Eczema 2011. http://health.usnews.com/health-news/family-health/infectious-diseases/articles/2011/02/22/softening-water-does-not-seem-to-ease-eczema (accessed April 2015).
- United Press International . Eczema Not Helped by Water Softener 2011. www.upi.com/Health_News/2011/02/17/Eczema-not-helped-by-water-softener/93861297973641/ (accessed April 2015).
- Medical News Today . Water Softeners Not Found To Improve Childhood Eczema 2011. www.medicalnewstoday.com/releases/216699.php (accessed April 2015).
- Annual Report 2009–2010. n.d.
- EvidenceUpdates from BMJ . SWET HTA Report 2011.
- F1000Prime . A Randomised Controlled Trial of Ion-Exchange Water Softeners for the Treatment of Eczema in Children 2011. http://f1000.com/prime/13200035 (accessed April 2015).
- The University of Nottingham . CLAHRC BITE: A Bite-Sized Summary of Research from the Centre of Evidence Based Dermatology (CEBD) 2012. http://ctsu.nottingham.ac.uk/ts0504b/docs/CLAHRC_BITE9.pdf (accessed April 2015).
- Gamble R, Dellavalle R. Ion-exchange water softener use and eczema. Arch Dermatol 2011;147:1208-10. http://dx.doi.org/10.1001/archdermatol.2011.249.
- Ewence A, Rumsby P, Rockett L, Davey A, Williams H, Danby S, et al. A Review of Skin Irritation and Tap Water Quality. Report for the Drinking Water Inspectorate 2011. http://dwi.defra.gov.uk/research/completed-research/reports/dwi70-2-257.pdf (accessed April 2015).
- Welsh S, Wilson N. Report by Sue Welsh (Wirral) and Niall Wilson (Liverpool) n.d. www.bad.org.uk/events/archive/annual-meeting-2011 (accessed April 2015).
- Atopic Eczema in Children: Management of Atopic Eczema in Children from Birth up to the Age of 12 Years. London: NICE; 2007.
- Atopic Eczema in Children. Management of Atopic Eczema in Children from Birth up to the Age of 12 Years. London: NICE; 2014.
- Atopic Eczema in Children. London: NICE; 2011.
- Scottish Intercollegiate Guidelines Network . Management of Atopic Eczema in Primary Care 2011. www.sign.ac.uk/guidelines/fulltext/125/index.html (accessed April 2015).
- Bartlett RH. Clinical research in acute fatal illness: lessons from extracorporeal membrane oxygenation [published online ahead of print Sep 15 2014]. J Intensive Care Med 2014. http://dx.doi.org/10.1177/0885066614550278.
- Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study. JAMA 1979;242:2193-96. http://dx.doi.org/10.1001/jama.1979.03300200023016.
- Morris A, Wallace C, Menlove R, Clemmer TP, Orme JF, Weaver LK, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;149:295-30. http://dx.doi.org/10.1164/ajrccm.149.2.8306022.
- Green TP, Timmons OD, Fackler JC, Moler FW, Thompson AE, Sweeney MF. The impact of extracorporeal membrane oxygenation on survival in pediatric patients with acute respiratory failure. Crit Care Med 1996;24:323-29. http://dx.doi.org/10.1097/00003246-199602000-00023.
- Schumacher RE, Roloff DW, Chapman R, Snedecor S, Bartlett RH. Extracorporeal membrane oxygenation in term newborns: a prospective cost-benefit analysis. ASAIO J 1993;39:873-9. http://dx.doi.org/10.1097/00002480-199339040-00010.
- O’Rourke PP, Crone RK, Vacanti JP, Ware JH, Lillehei CW, Parad RB, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics 1989;84:957-63.
- Geven W. A Comparative Trial of ECMO for Neonatal Respiratory Failure in the Netherlands. Dearborn, MI: Extracorporeal Life Support Organization; 1995.
- Bartlett RH, Roloff DW, Cornell RG, Andrews AF, Dillon PW, Zwischenberger JB. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics 1985;76:479-87.
- UK Collaborative ECMO Trial Group . UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet 1996;348:75-82. http://dx.doi.org/10.1016/S0140-6736(96)04100-1.
- Bartlett RH, Combes A, Peek GJ. Extracorporeal life support. Ann Am Thorac Soc 2014;11. http://dx.doi.org/10.1513/AnnalsATS.201404-182LE.
- Peek GJ, Clemens F, Elbourne D, Firmin R, Hardy P, Hibbert C, et al. CESAR: conventional ventilatory support vs. extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res 2006;6. http://dx.doi.org/10.1186/1472-6963-6-163.
- Peek GJ, Elbourne D, Mugford M, Tiruvoipati R, Wilson A, Allen E, et al. Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR). Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14350.
- University of London . Professor Diana Elbourne 2014. www.londoninternational.ac.uk/people/lshtm/professor-diana-elbourne (accessed June 2015).
- Extracorporeal Life Support Organization (EuroELSO) . EuroELSO 2015. www.elso.org/Members/Chapters/EuroELSO.aspx (accessed June 2015).
- Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A (H1N1). JAMA 2011;306:1659-68. http://dx.doi.org/10.1001/jama.2011.1471.
- Thalanany MM, Mugford M, Hibbert C, Cooper NJ, Truesdale A, Robinson S, et al. Methods of data collection and analysis for the economic evaluation alongside a national, multi-centre trial in the UK: conventional ventilation or ECMO for Severe Adult Respiratory Failure (CESAR). BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-94.
- Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. http://dx.doi.org/10.1016/S0140-6736(09)61069-2.
- Thalanany MM, Mugford M, Mitchell-Inwang C. Visiting adult patients in intensive care: the importance of relatives’ travel and time costs. Intensive Crit Care Nurs 2006;22:40-8. http://dx.doi.org/10.1016/j.iccn.2005.10.004.
- Elbourne D. The UK collaborative ECMO trial. Midwives Chron 1994;107:312-16.
- Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Failure in Adults. London: NICE; 2011.
- Extracorporeal Life Support Organization (ELSO) . Guidelines for Adult Respiratory Failure V 1.3 2013. www.elso.org/Portals/0/IGD/Archive/FileManager/989d4d4d14cusersshyerdocumentselsoguidelinesforadultrespiratoryfailure1.3.pdf (accessed April 2015).
- Elbourne D. Subjects’ views about participation in a randomized controlled trial. J Reprod Infant Psychol 1987;5:3-8. http://dx.doi.org/10.1080/02646838708403468.
- Altman DG, Elbourne D. Combining results from several clinical trials. Br J Obstet Gynaecol 1988;95:1-2. http://dx.doi.org/10.1111/j.1471-0528.1988.tb06474.x.
- Mitchell GG, Elbourne DR. The Salford Third Stage Trial. Oxytocin plus ergometrine versus oxytocin alone in the active management of the third stage of labor. Online J Curr Clin Trials 1993;83.
- Johnson A, Wincott E, Grant A, Elbourne D. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation: results at 30 months. Arch Dis Child 1994;71. http://dx.doi.org/10.1136/fn.71.2.F147.
- Snowdon C, Garcia J, Elbourne D. Making sense of randomization; responses of parents of critically ill babies to random allocation of treatment in a clinical trial. Soc Sci Med 1997;45:1337-55. http://dx.doi.org/10.1016/S0277-9536(97)00063-4.
- Van Asten H, Scanlan S, Truesdale A, Macfarlane A, Elbourne D. A placebo-controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstet Gynecol Scand 1997;76:608-9. http://dx.doi.org/10.3109/00016349709024596.
- Fooks J, Mutch L, Yudkin P, Johnson A, Elbourne D. Comparing two methods of follow up in a multicentre randomised trial. Arch Dis Child 1997;76:369-76. http://dx.doi.org/10.1136/adc.76.4.369.
- Snowdon C, Garcia J, Elbourne D. Reactions of participants to the results of a randomised controlled trial: exploratory study. BMJ 1998;317:21-6. http://dx.doi.org/10.1136/bmj.317.7150.21.
- Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. Lancet 1998;351:693-9. http://dx.doi.org/10.1016/S0140-6736(97)09409-9.
- Fairlie FM, Marshall L, Walker JJ, Elbourne D. Intramuscular opioids for maternal pain relief in labour: a randomised controlled trial comparing pethidine with diamorphine. Br J Obstet Gynaecol 1999;106:1181-7. http://dx.doi.org/10.1111/j.1471-0528.1999.tb08145.x.
- Albers L, Garcia J, Renfrew M, McCandlish R, Elbourne D. Distribution of genital tract trauma in childbirth and related postnatal pain. Birth 1999;26:11-7. http://dx.doi.org/10.1046/j.1523-536x.1999.00011.x.
- Arsiwala S, Peek G, Davies M, Sosnoski A, Firmin R. Hypereosinophilic syndrome: cause of prosthetic valve obstruction. J Thorac Cardiovasc Surg 1995;110:545-6. http://dx.doi.org/10.1016/S0022-5223(95)70253-9.
- Peek GJ, Firmin RK, Arsiwala S. Chest tube insertion in the ventilated patient. Injury 1995;26:425-6. http://dx.doi.org/10.1016/0020-1383(95)00055-E.
- Peek GJ, Firmin RK. Isolated sternal fracture: an audit of 10 years’ experience. Injury 1995;26:385-8. http://dx.doi.org/10.1016/0020-1383(95)00051-A.
- Firmin RK, Peek GJ, Sosnowski AW. Role of extracorporeal membrane oxygenation. Lancet 1996;348. http://dx.doi.org/10.1016/S0140-6736(05)65241-5.
- Peek GJ, Firmin RK, Moore HM, Sosnowski AW. Cannulation of neonates for venovenous extracorporeal life support. Ann Thorac Surg 1996;61:1851-2. http://dx.doi.org/10.1016/0003-4975(96)00173-7.
- Peek GJ, Firmin RK, Sosnowski AW. Limb perfusion during cardiopulmonary support. Ann Thorac Surg 1996;61:1291-2. http://dx.doi.org/10.1016/S0003-4975(96)80911-8.
- Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for adult respiratory failure. Chest 1997;112:759-64. http://dx.doi.org/10.1378/chest.112.3.759.
- Kolvekar SK, Peek GJ, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenator for pulmonary embolism. Ann Thorac Surg 1997;64:883-4.
- Peek GJ, Firmin RK. Reducing morbidity from insertion of chest drains. Patients must be disconnected from positive airways pressure before insertion of drains. BMJ 1997;315.
- Peek GJ, Arsiwala SS, Chan KC, Hickey MS. Absorbable pulmonary artery band. Ann Thorac Surg 1997;64:539-41. http://dx.doi.org/10.1016/S0003-4975(97)00546-8.
- Peek GJ, Firmin RK. Extracorporeal membrane oxygenation for cardiac support. Coron Artery Dis 1997;8:371-88. http://dx.doi.org/10.1097/00019501-199706000-00006.
- Peek GJ, Firmin RK. Extracorporeal membrane oxygenation, a favourable outcome?. Br J Anaesth 1997;78:235-7. http://dx.doi.org/10.1093/bja/78.3.235.
- Peek GJ, Sosnowski AW. Extra-corporeal membrane oxygenation for paediatric respiratory failure. Br Med Bull 1997;53:745-56. http://dx.doi.org/10.1093/oxfordjournals.bmb.a011645.
- O’Toole G, Peek G, Jaffe W, Ward D, Henderson H, Firmin RK. Extracorporeal membrane oxygenation in the treatment of inhalation injuries. Burns 1998;24:562-5. http://dx.doi.org/10.1016/S0305-4179(98)00061-8.
- Peek GJ, Killer HM, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation: potential for adults and children?. Hosp Med 1998;59:304-8.
- Peek GJ, White S, Scott AD, Hall AW, Moore HM, Sosnowski AW, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg 1998;227:572-4. http://dx.doi.org/10.1097/00000658-199804000-00020.
- Peek GJ, Wong K, Morrison C, Killer HM, Firmin RK. Tubing failure during prolonged roller pump use: a laboratory study. Perfusion 1999;14:443-52. http://dx.doi.org/10.1177/026765919901400607.
- Peek GJ, Scott R, Killer HM, Jarvis MA, Kolvekar S, Forbes D, et al. A porcine model of prolonged closed chest venovenous extracorporeal membrane oxygenation. ASAIO J 1999;45:488-95. http://dx.doi.org/10.1097/00002480-199909000-00023.
- Peek GJ, Firmin RK. The inflammatory and coagulative response to prolonged extracorporeal membrane oxygenation. ASAIO J 1999;45:250-63. http://dx.doi.org/10.1097/00002480-199907000-00003.
- Ichiba S, Jenkins DR, Peek GJ, Brennan KJ, Killer HM, Sosnowski A, et al. Severe acute respiratory failure due to legionella pneumonia treated with extracorporeal membrane oxygenation. Clin Infect Dis 1999;28:686-7. http://dx.doi.org/10.1086/517219.
- Firmin R, Lowes A, Hickson B. A case of rhabdomyosarcoma of the right ventricle. Br Heart J 1978;40:1426-8. http://dx.doi.org/10.1136/hrt.40.12.1426.
- Salem R, Devitt P, Johnson J, Firmin R. Emergency geriatric surgical admissions. BMJ 1978;2:416-17. http://dx.doi.org/10.1136/bmj.2.6134.416.
- Naunton Morgan TC, Firmin R, Mason B, Monks V, Caro D. Prophylactic povidone iodine in minor wounds. Injury 1980;12:104-6. http://dx.doi.org/10.1016/0020-1383(80)90132-1.
- Firmin RK, Fragomeni LS, Lennox SC. Complete cleft sternum. Thorax 1980;35:303-6. http://dx.doi.org/10.1136/thx.35.4.303.
- Firmin RK, Tolhurst-Cleaver C. Safe intrapleural drainage. Anaesthesia 1980;35:79-80. http://dx.doi.org/10.1111/j.1365-2044.1980.tb03751.x.
- Fragomeni LS, Firmin RK, Lennox SC. Limited axillary thoracotomy in the management of recurrent spontaneous pneumothorax. AMB Rev Assoc Med Bras 1982;28:69-70.
- Heard BE, Dewar A, Firmin RK, Lennox SC. One very rare and one new tracheal tumour found by electron microscopy: glomus tumour and acinic cell tumour resembling carcinoid tumours by light microscopy. Thorax 1982;37:97-103. http://dx.doi.org/10.1136/thx.37.2.97.
- Roizen MF, Hunt TK, Beaupre PN, Kremer P, Firmin R, Chang CN, et al. The effect of alpha-adrenergic blockade on cardiac performance and tissue oxygen delivery during excision of pheochromocytoma. Surgery 1983;94:941-5.
- Firmin RK, Lima R, Anderson RH, Yen Ho S, Lincoln C. Anatomic problems associated with arterial switch procedures for double outlet right ventricle with subpulmonary ventricular septal defect. Thorac Cardiovasc Surg 1983;31:365-8. http://dx.doi.org/10.1055/s-2007-1022020.
- Gottrup F, Firmin R, Chang N, Goodson WH, Hunt TK. Continuous direct tissue oxygen tension measurement by a new method using an implantable silastic tonometer and oxygen polarography. Am J Surg 1983;146:399-403. http://dx.doi.org/10.1016/0002-9610(83)90427-0.
- Firmin RK, Azariades M, Lennox SC, Lincoln JC, Paneth M. Sleeve lobectomy (lobectomy and bronchoplasty) for bronchial carcinoma. Ann Thorac Surg 1983;35:442-9. http://dx.doi.org/10.1016/S0003-4975(10)61599-8.
- Gottrup F, Firmin R, Hunt TK, Mathes SJ. The dynamic properties of tissue oxygen in healing flaps. Surgery 1984;95:527-36.
- Campalani G, Firmin RK, Vaughan M, Ross DN. Surgical repair of coarctation of the aorta using the internal mammary artery as a free autogenous graft. J Thorac Cardiovasc Surg 1985;90:928-31.
- Firmin RK, Bouloux P, Allen P, Lima RC, Lincoln JC. Sympathoadrenal function during cardiac operations in infants with the technique of surface cooling, limited cardiopulmonary bypass, and circulatory arrest. J Thorac Cardiovasc Surg 1985;90:729-35.
- Lima RC, Firmin RK, Pillai R, Lennox SC, Cleland WP, Paneth M, et al. Surgical management of adults with cyanotic congenital heart disease. Arq Bras Cardiol 1985;45:175-80.
- Firmin RK, Turley K, Jacobs S, Ebert PA. Changes in the respiratory gas tensions of a pneumonectomy space and their application to the diagnosis of bronchopleural fistula. Thorac Cardiovasc Surg 1985;33:173-5. http://dx.doi.org/10.1055/s-2007-1014110.
- Betts TE, Firmin R, Guerreiro D. The use of a flexible film isolator to maintain specified concentrations of oxygen. Lab Anim 1985;19:109-10. http://dx.doi.org/10.1258/002367785780942660.
- Kanter K, Anderson R, Lincoln C, Firmin R, Rigby M. Anatomic correction of double-outlet right ventricle with subpulmonary ventricular septal defect (the ‘Taussig-Bing’ anomaly). Ann Thorac Surg 1986;41:287-92. http://dx.doi.org/10.1016/S0003-4975(10)62771-3.
- Gottrup F, Firmin R, Rabkin J, Halliday BJ, Hunt TK. Directly measured tissue oxygen tension and arterial oxygen tension assess tissue perfusion. Crit Care Med 1987;15:1030-6. http://dx.doi.org/10.1097/00003246-198711000-00008.
- Hosie KB, Dunning JJ, Bailey JS, Firmin RK. Glove perforation during sternotomy closure. Lancet 1988;2. http://dx.doi.org/10.1016/S0140-6736(88)90987-7.
- Hunt TM, Firmin RK, Johnstone MJ. Management of a patient with Wilms’s tumour extending into the right heart chambers: a case report and a review of other published reports. Br Heart J 1988;60:165-8. http://dx.doi.org/10.1136/hrt.60.2.165.
- Maurus KE, Firmin RK, Leanage R. A rare anomaly of the aortic arch complex. Int J Cardiol 1988;20:149-51. http://dx.doi.org/10.1016/0167-5273(88)90328-2.
- Azariades M, Firmin RK, Lincoln C, Lennox SC. Cerebral function analysis during deep hypothermia and total circulatory arrest in infant lambs. Thorac Cardiovasc Surg 1988;36:133-6. http://dx.doi.org/10.1055/s-2007-1020060.
- Sosnowski AW, Bonser SJ, Field DJ, Graham TR, Firmin RK. Extracorporeal membrane oxygenation. BMJ 1990;301:303-4. http://dx.doi.org/10.1136/bmj.301.6747.303.
- Azariades M, Firmin R, Lincoln C, Lennox S. The effect of propranolol on the cerebral electrical response to deep hypothermia and total circulatory arrest in lambs. J Thorac Cardiovasc Surg 1990;99:1030-6.
- Sosnowski AW, Graham TR, Firmin RK. Extracorporeal membrane oxygenation. Lancet 1990;335:971-2. http://dx.doi.org/10.1016/0140-6736(90)91034-8.
- Shawkat S, Sarangi PP, Firmin RK. A technique for replacing a prosthetic aortic valve after total aortic root replacement. Br Heart J 1990;63:260-1. http://dx.doi.org/10.1136/hrt.63.4.260.
- Arnold IR, Hubner PJ, Firmin RK. Blood filled cyst of the papillary muscle of the mitral valve producing severe left ventricular outflow tract obstruction. Br Heart J 1990;63:132-3. http://dx.doi.org/10.1136/hrt.63.2.132.
- Thomson AH, Beardsmore CS, Firmin R, Leanage R, Simpson H. Airway function in infants with vascular rings: preoperative and postoperative assessment. Arch Dis Child 1990;65:171-4. http://dx.doi.org/10.1136/adc.65.2.171.
- Morgan WE, Salama FD, Beggs FD, Firmin RK, Rowles JM. Thoracic injuries sustained by the survivors of the M1 (Kegworth) aircraft accident. The Nottingham, Leicester, Derby, Belfast Study Group. Eur J Cardiothorac Surg 1990;4:417-20. http://dx.doi.org/10.1016/1010-7940(90)90070-G.
- Field DJ, Firmin RK, Sosnowski A. Neonatal extracorporeal membrane oxygenation. Lancet 1991;337:1344-5. http://dx.doi.org/10.1016/0140-6736(91)93015-2.
- Brack MJ, Hubner PJ, Firmin RK. Successful operation on a coronary arteriovenous fistula in a 74-year-old woman. Br Heart J 1991;65:107-8. http://dx.doi.org/10.1136/hrt.65.2.107.
- Field DJ, Firmin RK. Extracorporeal membrane oxygenation. Lancet 1991;337:51-2. http://dx.doi.org/10.1016/0140-6736(91)93369-K.
- Firmin RK. Extracorporeal membrane oxygenation in the 1990s: a personal view. Perfusion 1991;6:161-6. http://dx.doi.org/10.1177/026765919100600302.
- Weerasena N, Firmin R, Bailey J, Leannage R, Elliott E, Jagger C. Paediatric cardiology in a sub-regional cardiothoracic unit. Health Trends 1991;23:144-6.
- Underwood MJ, Weeresena N, Graham TR, Bailey JS, Firmin RK. Attitudes of cardiothoracic surgeons in the UK to human immunodeficiency virus. Br J Surg 1992;79. http://dx.doi.org/10.1002/bjs.1800791044.
- Pearson GA, Field DJ, Firmin RK, Sosnowski AS. UK experience in neonatal extracorporeal membrane oxygenation. Arch Dis Child 1992;67:822-5. http://dx.doi.org/10.1136/adc.67.7_Spec_No.822.
- Pearson GA, Firmin RK, Sosnowski A, Field D. Neonatal extracorporeal membrane oxygenation. Br J Hosp Med 1992;47:646-53.
- Prentice MB, Flower AJ, Morgan GM, Nicholson KG, Rana B, Firmin RK, et al. Infection with hepatitis B virus after open heart surgery. BMJ 1992;304:761-4. http://dx.doi.org/10.1136/bmj.304.6829.761.
- Pearson GA, Firmin RK, Sosnowski A, Reeves R. Support when gas exchange fails. Clin Intensive Care 1992;3.
- Sayers RD, Thompson MM, Underwood MJ, Graham T, Hartshorne T, Spyt TJ, et al. Early results of combined carotid endarterectomy and coronary artery bypass grafting in patients with severe coronary and carotid artery disease. J R Coll Surg Edinb 1993;38:340-3.
- Underwood MJ, Firmin RK, Graham TR. Current concepts in the use of intra-aortic balloon counterpulsation. Br J Hosp Med 1993;50:391-7.
- Underwood MJ, More R, Weeresena N, Firmin RK, De Bono DP. The effect of surgical preparation and in-vitro distension on the intrinsic fibrinolytic activity of human saphenous vein. Eur J Vasc Surg 1993;7:518-22. http://dx.doi.org/10.1016/S0950-821X(05)80363-9.
- Underwood MJ, Weerasena N, Graham TR, Hosie K, Dunning J, Bailey JS, et al. Prevalence and prevention of glove perforation during cardiac operations. J Thorac Cardiovasc Surg 1993;106:375-7.
- Sayers RD, Naylor AR, Parry AD, Firmin RK, Bailey J, Bell PR. The management of cardiac trauma by general surgeons in non-cardiothoracic units. J R Coll Surg Edinb 1993;38:142-4.
- Underwood MJ, Firmin RK, Jehu D. Aspects of psychological and social morbidity in patients awaiting coronary artery bypass grafting. Br Heart J 1993;69:382-4. http://dx.doi.org/10.1136/hrt.69.5.382.
- Thompson MM, Graham TR, Bolia AA, Firmin RK, Bell PR. Intrahepatic leiomyosarcoma of the inferior vena cava with extension into the right atrium. Eur J Vasc Surg 1993;7:204-7. http://dx.doi.org/10.1016/S0950-821X(05)80764-9.
- Thompson MM, Firmin RK, Bolia AA, Graham TR, Bell PR. Primary venous leiomyosarcoma: a rare but lethal disease. J Vasc Surg 1993;17:447-8. http://dx.doi.org/10.1016/0741-5214(93)90431-K.
- Underwood MJ, Sosnowski A, Graham TR, Firmin RK. Operative management of the calcified aorta: a practical method of aortic occlusion. J Thorac Cardiovasc Surg 1993;105:377-8.
- Pearson GA, Grant J, Field D, Sosnowski A, Firmin RK. Extracorporeal life support in paediatrics. Arch Dis Child 1993;68:94-6. http://dx.doi.org/10.1136/adc.68.1.94.
- Pearson GA, Sosnowski A, Chan KC, Firmin RK. Salvage of postoperative pulmonary hypertensive crisis using ECMO via cervical cannulation in a case of truncus arteriosus. Eur J Cardiothorac Surg 1993;7:390-1. http://dx.doi.org/10.1016/1010-7940(93)90073-K.
- Evans MJ, McKeever PA, Pearson GA, Field D, Firmin RK. Pathological complications of non-survivors of newborn extracorporeal membrane oxygenation. Arch Dis Child 1994;71:F88-92. http://dx.doi.org/10.1136/fn.71.2.F88.
- Hurrion EM, Pearson GA, Firmin RK. Childhood pulmonary alveolar proteinosis. Extracorporeal membrane oxygenation with total cardiopulmonary support during bronchopulmonary lavage. Chest 1994;106:638-40. http://dx.doi.org/10.1378/chest.106.2.638.
- Fenton AC, Foweraker JE, Pearson GA, Firmin RK. Bronchopulmonary infection with Moraxella catarrhalis in infants requiring extracorporeal membrane oxygenation. Pediatr Pulmonol 1994;17:393-5. http://dx.doi.org/10.1002/ppul.1950170610.
- Sadiq M, Fenton AC, Firmin RK. False aneurysm of the right ventricular outflow tract after total correction of tetralogy of Fallot: diagnosis by echocardiography and successful repair by neck cannulation for cardiopulmonary bypass. Br Heart J 1994;71:566-8. http://dx.doi.org/10.1136/hrt.71.6.566.
- Claydon AH, Nicholson KG, Wiselka MJ, Firmin RK. Varicella pneumonitis: a role for extra-corporeal membrane oxygenation?. J Infect 1994;28:65-7. http://dx.doi.org/10.1016/S0163-4453(94)94192-0.
- Kockelbergh RC, Harris AM, John RM, Bailey JS, Firmin RK. Prolonged suction drainage prevents serous wound discharge after cardiac surgery. Ann R Coll Surg Engl 1994;76:30-2.
- Davies MJ, Arsiwala SS, Moore HM, Kerr S, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for the treatment of massive pulmonary embolism. Ann Thorac Surg 1995;60:1801-3. http://dx.doi.org/10.1016/0003-4975(95)00622-2.
- Underwood MJ, Pearson JA, Waggoner J, Lunec J, Firmin RK, Elliot MJ. Changes in ‘inflammatory’ mediators and total body water during extra-corporeal membrane oxygenation (ECMO). A preliminary study. Int J Artif Organs 1995;18:627-32.
- Firmin RK, Khan Y, Sosnowski A. Extracorporeal membrane oxygenation (ECMO) for cardiorespiratory failure in children: the Leicester experience. Int J Artif Organs 1995;18:603-6.
- Khan JY, Kerr SJ, Tometzki A, Tyszczuk L, West J, Sosnowski A, et al. Role of ECMO in the treatment of respiratory syncytial virus bronchiolitis: a collaborative report. Arch Dis Child Fetal Neonatal Ed 1995;73:F91-4. http://dx.doi.org/10.1136/fn.73.2.F91.
- Dhillon R, Pearson GA, Firmin RK, Chan KC, Leanage R. Extracorporeal membrane oxygenation and the treatment of critical pulmonary hypertension in congenital heart disease. Eur J Cardiothorac Surg 1995;9:553-6. http://dx.doi.org/10.1016/S1010-7940(05)80004-1.
- Davies MJ, Dyamenahalli U, Leanage RR, Firmin RK. Total one-stage repair of aortopulmonary window and interrupted aortic arch in a neonate. Pediatr Cardiol 1996;17:122-4. http://dx.doi.org/10.1007/BF02505097.
- Goldman AP, Kerr SJ, Butt W, Marsh MJ, Murdoch IA, Paul T, et al. Extracorporeal support for intractable cardiorespiratory failure due to meningococcal disease. Lancet 1997;349:466-9. http://dx.doi.org/10.1016/S0140-6736(96)12106-1.
- Hamilton DL, Firmin RK, Millar-Craig MW. Images in cardiology. Pseudoaneurysm of the thoracic aorta presenting with angina: a late complication of aortic valve replacement. Heart 1998;79. http://dx.doi.org/10.1136/hrt.79.3.310.
- Firmin RK, Killer HM. Extracorporeal membrane oxygenation. Perfusion 1999;14:291-7. http://dx.doi.org/10.1177/026765919901400409.
- Kovac JD, Spyt TJ, Firmin RK, Verma PK, de Bono DP. Chronic haemoptysis as delayed complication of ventricular aneurysmectomy. Int J Cardiol 1999;69:299-303. http://dx.doi.org/10.1016/S0167-5273(98)00360-X.
- Mugford M. A comparison of reported differences in definitions of vital events and statistics. World Health Stat Q 1983;36:201-12.
- Macfarlane A, Mugford M. The outcome of pregnancy: what can we learn from official statistics?. Nurs Times 1984;80:42-4.
- Mugford M, Drummond MF, Henderson J, Mooney G. Chorionic villus sampling. Lancet 1985;2:384-5. http://dx.doi.org/10.1016/S0140-6736(85)92514-0.
- Mugford M, Mutch L, Elbourne D. Standard perinatal data: suggestions for regular review of facilities for perinatal care within a regional health authority. Community Med 1985;7:157-68.
- Mugford M, Somchiwong M, Waterhouse IL. Treatment of umbilical cords: a randomised trial to assess the effect of treatment methods on the work of midwives. Midwifery 1986;2:177-86. http://dx.doi.org/10.1016/S0266-6138(86)80043-2.
- Szczepura A, Mugford M, Stilwell JA. Information for managers in hospitals: representing maternity unit statistics graphically. BMJ (Clin Res Ed) 1987;294:875-80. http://dx.doi.org/10.1136/bmj.294.6576.875.
- Mugford M. A review of the economics of care for sick newborn infants. Community Med 1988;10.
- Mugford M, Szczepura A, Lodwick A, Stilwell J. Factors affecting the outcome of maternity care. II. Neonatal outcomes and resources beyond the hospital of birth. J Epidemiol Community Health 1988;42:170-6. http://dx.doi.org/10.1136/jech.42.2.170.
- Stilwell J, Szczepura A, Mugford M. Factors affecting the outcome of maternity care. I. Relationship between staffing and perinatal deaths at the hospital of birth. J Epidemiol Community Health 1988;42:157-69. http://dx.doi.org/10.1136/jech.42.2.157.
- Mugford M, Kingston J, Chalmers I. Reducing the incidence of infection after caesarean section: implications of prophylaxis with antibiotics for hospital resources. BMJ 1989;299:1003-6. http://dx.doi.org/10.1136/bmj.299.6706.1003.
- Mugford M. The role of health economics. BMJ 1989;299. http://dx.doi.org/10.1136/bmj.299.6701.741.
- Mugford M, Banfield P, O’Hanlon M. Effects of feedback of information on clinical practice: a review. BMJ 1991;303:398-402. http://dx.doi.org/10.1136/bmj.303.6799.398.
- Mugford M, Piercy J, Chalmers I. Cost implications of different approaches to the prevention of respiratory distress syndrome. Arch Dis Child 1991;66:757-64. http://dx.doi.org/10.1136/adc.66.7_Spec_No.757.
- Clark L, Mugford M, Paterson C. How does the mode of delivery affect the cost of maternity care?. Br J Obstet Gynaecol 1991;98:519-23. http://dx.doi.org/10.1111/j.1471-0528.1991.tb10362.x.
- Mugford M, Chapple J. How have recent changes in the NHS affected perinatal audit?. Arch Dis Child 1993;69:322-6. http://dx.doi.org/10.1136/adc.69.3_Spec_No.322.
- Mugford M, Howard S. Cost effectiveness of surfactant replacement in preterm babies. PharmacoEconomics 1993;3:362-73. http://dx.doi.org/10.2165/00019053-199303050-00004.
- Jefferson T, Mugford M, Demicheli V. QALY league tables. Health Econ 1994;3. http://dx.doi.org/10.1002/hec.4730030309.
- Howard S, Normand C, Mugford M, Elbourne D, Field D, Johnson A, et al. Costing neonatal care alongside the Collaborative ECMO trial: how much primary research is required?. Health Econ 1995;4:265-71. http://dx.doi.org/10.1002/hec.4730040403.
- Mugford M. Publishing economically. Lancet 1995;345:1127-8. http://dx.doi.org/10.1016/S0140-6736(95)90971-0.
- Mugford M. The cost of neonatal care: reviewing the evidence. Soz Praventivmed 1995;40:361-8. http://dx.doi.org/10.1007/BF01325418.
- Jefferson T, Mugford M, Gray A, Demicheli V. An exercise on the feasibility of carrying out secondary economic analyses. Health Econ 1996;5:155-65. http://dx.doi.org/10.1002/(SICI)1099-1050(199603)5:2<155::AID-HEC194>3.0.CO;2-O.
- Howard S, Mugford M, Normand C, Elbourne D, Grant A, Field D, et al. A cost-effectiveness analysis of neonatal ECMO using existing evidence. Int J Technol Assess Health Care 1996;12:80-92. http://dx.doi.org/10.1017/S0266462300009417.
- Mugford M, Hutton G, Fox-Rushby J. Methods for economic evaluation alongside a multicentre trial in developing countries: a case study from the WHO Antenatal Care Randomised Controlled Trial. Paediatr Perinat Epidemiol 1998;12:75-97. http://dx.doi.org/10.1046/j.1365-3016.1998.00008.x.
- Roberts T, Mugford M, Piercy J. Choosing options for ultrasound screening in pregnancy and comparing cost effectiveness: a decision analysis approach. Br J Obstet Gynaecol 1998;105:960-70. http://dx.doi.org/10.1111/j.1471-0528.1998.tb10258.x.
- Hopayian K, Mugford M. Conflicting conclusions from two systematic reviews of epidural steroid injections for sciatica: which evidence should general practitioners heed?. Br J Gen Pract 1999;49:57-61.
- University Hospitals of Leicester NHS Trust . Extra Corporeal Membrane Oxygenation (ECMO) 2015. www.leicestershospitals.nhs.uk/aboutus/departments-services/heart-services/ecmo/ (accessed June 2015).
- Peek GJ, Elbourne D, Mugford M. Ventilatory support versus ECMO for severe adult respiratory failure. Reply. Lancet 2010;375. http://dx.doi.org/10.1016/S0140-6736(10)60224-3.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Zwischenberger JB, Lynch JE. Will CESAR answer the adult ECMO debate?. Lancet 2009;374:1307-8. http://dx.doi.org/10.1016/S0140-6736(09)61630-5.
- Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A (H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013;187:276-85. http://dx.doi.org/10.1164/rccm.201205-0815OC.
- Combes A, Bacchetta M, Brodie D, Müller T, Pellegrino V. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care 2012;18:99-104. http://dx.doi.org/10.1097/MCC.0b013e32834ef412.
- Crucean A, Peek G. CESAR Study: Justification of Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure. IRBM 2010;31:S46-51. http://dx.doi.org/10.1016/S1959-0318(10)70009-9.
- Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 2014;190:488-96. http://dx.doi.org/10.1164/rccm.201404-0630CP.
- ECLS Registry Report. Ann Arbor, MI; 2015.
- Thomas LJ, Kessler D, Campbell J, Morrison J, Peters TJ, Williams C, et al. Prevalence of treatment-resistant depression in primary care: cross-sectional data. Br J Gen Pract 2013;63:e852-8. http://dx.doi.org/10.3399/bjgp13X675430.
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D Report. Am J Psychiatry 2006;163:1905-17. http://dx.doi.org/10.1176/appi.ajp.163.11.1905.
- Souery D, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J Clin Psychiatry 2007;68:1062-70. http://dx.doi.org/10.4088/JCP.v68n0713.
- Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol 2007;17:696-707. http://dx.doi.org/10.1016/j.euroneuro.2007.03.009.
- Stimpson N, Agrawal N, Lewis G. Randomised controlled trials investigating pharmacological and psychological interventions for treatment-refractory depression. Systematic Review. Br J Psychiatry 2002;181:284-94. http://dx.doi.org/10.1192/bjp.181.4.284.
- Management of Depression in Primary and Secondary Care. London: NICE; 2004.
- Wiles NJ, Symmons DP, Harrison B, Barrett E, Barrett JH, Scott DG, et al. Estimating the incidence of rheumatoid arthritis: trying to hit a moving target?. Arthritis Rheum 1999;42:1339-46. http://dx.doi.org/10.1002/1529-0131(199907)42:7<1339::AID-ANR6>3.0.CO;2-Y.
- Wiles NJ, Peters TJ, Leon DA, Lewis G. Birth weight and psychological distress at age 45–51 years: Results from the Aberdeen Children of the 1950s Cohort Study. Br J Psychiatry 2005;187:21-8. http://dx.doi.org/10.1192/bjp.187.1.21.
- Wiles NJ, Hollinghurst S, Mason V, Musa M, Burt V, Hyde J, et al. A randomized controlled trial of cognitive behavioural therapy as an adjunct to pharmacotherapy in primary care based patients with treatment resistant depression: a pilot study. Behav Cogn Psychother 2007;36:21-33.
- National Institute for Health and Clinical Excellence . Depression in Adults 2009. www.nice.org.uk/guidance/cg90/resources (accessed April 2015).
- Wiles NJ, Thomas L, Abel A, Barnes M, Carroll F, Ridgway N, et al. Clinical effectiveness and cost-effectiveness of cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: the CoBalT randomised controlled trial. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18310.
- Turner N, Campbell J, Peters TJ, Wiles NJ, Hollinghurst S. A comparison of four different approaches to measuring health utility in depressed patients. Health Qual Life Outcomes 2013;11. http://dx.doi.org/10.1186/1477-7525-11-81.
- Wiles NJ, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 2013;381:375-84. http://dx.doi.org/10.1016/S0140-6736(12)61552-9.
- Hollinghurst S, Carroll FE, Abel A, Campbell J, Garland A, Jerrom B, et al. Cost-effectiveness of cognitive-behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: economic evaluation of the CoBalT trial. Br J Psychiatry 2014;204:69-76. http://dx.doi.org/10.1192/bjp.bp.112.125286.
- Barnes M, Sherlock S, Thomas L, Kessler D, Kuyken W, Owen-Smith A, et al. No pain, no gain: depressed clients’ experiences of cognitive behavioural therapy. Br J Clin Psychol 2013;52:347-64. http://dx.doi.org/10.1111/bjc.12021.
- Simmonds BAJ, Turner NL, Thomas LJ, Campbell J, Lewis GH, Wiles NJ, et al. Patients’ experiences of participating in a large scale cognitive behavioural therapy depression trial. Fam Pract 2013;30:705-11. http://dx.doi.org/10.1093/fampra/cmt028.
- Barnes M, Wiles N, Morrison J, Kessler D, Williams C, Kuyken W, et al. Exploring patients’ reasons for declining contact in a cognitive behavioural therapy randomised controlled trial in primary care. Br J Gen Pract 2012;62:e371-7. http://dx.doi.org/10.3399/bjgp12X641492.
- Thomas LJ, Abel A, Ridgway N, Peters T, Kessler D, Hollinghurst S, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment resistant depression in primary care: the CoBalT randomised controlled trial protocol. Contemp Clin Trials 2012;33:312-19. http://dx.doi.org/10.1016/j.cct.2011.10.016.
- Jenkinson CE, Winder RE, Sugg HVR, Roberts MJ, Ridgway N, Kuyken W, et al. Why do GPs exclude patients from participating in research? An exploration of adherence to and divergence from trial criteria. Family Pract 2014;31:364-70. http://dx.doi.org/10.1093/fampra/cmu005.
- Button KS, Turner N, Campbell J, Kessler D, Kuyken W, Lewis G, et al. Moderators of response to cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care. J Affect Disord 2014;174:272-80. http://dx.doi.org/10.1016/j.jad.2014.11.057.
- Campbell JL, Fletcher E, Britten N, Green C, Holt TA, Lattimer V. Telephone triage for management of same-day consultation requests in general practice (The ESTEEM trial): a cluster-randomised controlled trial and cost-consequence analysis. Lancet 2014;384:1859-68. http://dx.doi.org/10.1016/S0140-6736(14)61058-8.
- Andrew A, Knapp M, McCrone PR, Parsonage M, Trachtenberg M. Effective Interventions in Schizophrenia: The Economic Case. London, UK: Personal Social Services Research Unit, London School of Economics and Political Science; 2012.
- Psychosis and Schizophrenia in Adults: Treatment and Management. London: NICE; 2014.
- Lewis S, Davies L, Jones P, Barnes T, Murray R, Kerwin R, et al. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10170.
- Jones PB, Barnes TE, Davies L, Dunn G, Lloyd H, Hayhurst KP, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: cost utility of the latest antipsychotic drugs in schizophrenia study (cutlass 1). Arch Gen Psychiatry 2006;63:1079-87. http://dx.doi.org/10.1001/archpsyc.63.10.1079.
- Lewis S, Lieberman J. CATIE and CUtLASS: can we handle the truth?. Br J Psychiatry 2008;192:161-3. http://dx.doi.org/10.1192/bjp.bp.107.037218.
- Geddes J, Freemantle N, Harrison P, Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ 2000;321:1371-6. http://dx.doi.org/10.1136/bmj.321.7273.1371.
- Bagnall A-M, Jones L, Ginnelly L, Lewis R, Glanville J, Gilbody S, et al. A systematic review of atypical antipsychotic drugs in schizophrenia. Health Technol Assess 2003;7. http://dx.doi.org/10.3310/hta7130.
- Lloyd HM, Markwick AJ, Page ER, Lewis S, Barnes TR. Attitudes to atypical and conventional antipsychotic drug treatment in clinicians participating in the CUtLASS study. Int J Psychiatry Clin Pract 2005;9:35-40. http://dx.doi.org/10.1080/13651500510014738.
- Lewis SW, Barnes TRE, Davies L, Murray RM, Dunn G, Hayhurst KP, et al. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull 2006;32:715-23. http://dx.doi.org/10.1093/schbul/sbj067.
- Davies L, Lewis S, Jones P, Barnes T, Gaughran F, Hayhurst K, et al. Cost-effectiveness of first-v. second-generation antipsychotic drugs: results from a randomised controlled trial in schizophrenia responding poorly to previous therapy. Br J Psychiatry 2007;191:14-22. http://dx.doi.org/10.1192/bjp.bp.106.028654.
- Jones PB, Barnes TRE, Elton P, Davies L, Dunn G, Lloyd H, et al. First- vs second-generation antipsychotic drugs in schizophrenia. In reply. Arch Gen Psychiatry 2007;64:979-80. http://dx.doi.org/10.1001/archpsyc.64.8.979.
- Peluso MJ, Lewis SW, Barnes TR, Jones PB. Non-neurological and metabolic side effects in the Cost Utility of the Latest Antipsychotics in Schizophrenia Randomised Controlled Trial (CUtLASS-1). Schizophrenia Res 2013;144:80-6. http://dx.doi.org/10.1016/j.schres.2012.12.008.
- Barnes TR, Drake RJ, Dunn G, Hayhurst KP, Jones PB, Lewis SW. Effect of prior treatment with antipsychotic long-acting injection on randomised clinical trial treatment outcomes. Br J Psychiatry 2013;203:215-20. http://dx.doi.org/10.1192/bjp.bp.113.125807.
- Taylor D, Hayhurst K, Kerwin R. A controlled, mirror-image study of second-generation antipsychotics in the treatment of schizophrenia. Int Clin Psychopharmacol 2007;22:133-6. http://dx.doi.org/10.1097/YIC.0b013e3280148219.
- Hayhurst K, Drake R, Massie J, Dunn G, Barnes T, Jones P, et al. Improved quality of life over one year is associated with improved adherence in patients with schizophrenia. Eur Psychiatry 2014;29:191-6. http://dx.doi.org/10.1016/j.eurpsy.2013.03.002.
- Boter H, Peuskens J, Libiger J, Fleischhacker WW, Davidson M, Galderisi S, et al. Effectiveness of antipsychotics in first-episode schizophrenia and schizophreniform disorder on response and remission: an open randomized clinical trial (EUFEST). Schizophrenia Res 2009;115:97-103. http://dx.doi.org/10.1016/j.schres.2009.09.019.
- Clark NG. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabet Care 2004;27.
- Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects. CNS Drugs 2005;19:1-93.
- Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Ritz L, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) Study. Am J Psychiatry 2008;165:1420-31. http://dx.doi.org/10.1176/appi.ajp.2008.08050756.
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009;373:31-4. http://dx.doi.org/10.1016/S0140-6736(08)61764-X.
Appendix 1 Interview protocol
The NIHR has commissioned RAND Europe to conduct and assessment of the impact of the NIHR HTA programme. RAND Europe is an independent not-for-profit public policy research institute.
This study seeks to:
-
identify the impacts of the NIHR HTA programme over the period 2003–2013 across a broad range of areas, including: health policy, clinical practice, patient health, the economy and research
-
look at the impacts of the programme as a whole as well as specific case study examples
-
produce recommendations for how the HTA can maximise the impact of its research in the future.
To this aim, we have scheduled interviews with experts from a range of stakeholder groups.
Taking part in the interview is entirely voluntary. All information collected in the interview will be kept strictly confidential. Any quotes included in the assessment will be anonymised.
With your permission, we would like to record this interview for the purpose of writing up notes and conducting the analysis.
A. Details about their organisation
-
Could you please tell us a bit about your background and your involvement with the NIHR HTA programme?
B. Specific questions depending on perspective of interviewee (ask all that apply)
PPI questions
-
How effectively does the HTA programme engage and involve patients and the public?
-
At what stages are the public involved (prompts if needed: project design, execution, dissemination)?
-
Does the HTA address the right topics that matter to patients? What does it miss?
-
How has the HTA’s approach to PPI changed over time?
-
How does it differ from other research funders?
-
What does the HTA programme do well in terms of PPI?
-
What could it do to improve PPI?
‘Customer’ questions (just ask about their organisation – select as appropriate)
-
How does NICE/NSC/SIGN use HTA research?
-
How important is HTA research to NICE/NSC/SIGN?
-
How has this changed over time?
-
Is NIHR HTA evidence widely used in guideline development (e.g. for NICE – outside of TARs)?
-
What could be done to make it more useful?
-
What do you think the relationship between NICE/NSC/SIGN and the HTA programme should look like?
-
Optional: How widely are guidelines used in practice?
-
Is NIHR HTA research used in practice through other means (e.g. directly implemented on a local level)?
-
What does the HTA programme do well in terms of dissemination and communication of its research?
-
What challenges are there in the communication and uptake of HTA research?
NHS/health system questions
-
What impact does the HTA programme have on practice in the NHS?
-
Does the HTA programme address NHS priorities effectively?
-
Are HTA studies directly taken up in practice?
-
If so, how does this happen? What barriers are there? What enablers?
-
-
How widely are guidelines and other NICE/NSC products (e.g. TARs) used in practice? What influences this?
-
What could the HTA programme do better to achieve its mission of providing information on effectiveness, costs and broader impact of healthcare treatments and tests to those who plan, provide or receive care in the NHS?
-
What does the HTA programme do well?
-
What challenges are there in the communication and uptake of HTA research?
Industry questions
-
What impact does the HTA programme have on the pharmaceutical industry (e.g. drug development, research priorities, etc. )?
-
What impact does the work of the HTA programme have on the research conducted by the pharmaceutical industry?
-
Does the work of the HTA programme inform your industry’s research agenda?
-
How well does the HTA programme interact with research programmes in industry? What are the challenges?
International questions (only for those with international Health Technology Assessment expertise)
-
How does the HTA programme compare to similar organisations in other countries?
-
What does it do well compared to other countries?
-
What could it learn from others?
-
To what extent do you think the NIHR HTA programme has had an impact outside of the UK, if at all?
-
Does the HTA programme have an influence on international practice in this field? (Examples, which could be used as prompts if necessary and relevant: Do you require systematic reviews before conducting primary research? Are your protocols openly published? What proportion of your research leads to a publication, including negative results?)
-
Is UK HTA research taken up elsewhere internationally?
-
Any other impacts?
-
Internal/strategy questions
-
What is the intended impact of the HTA programme? How was that determined and developed?
-
What strategy does the programme take to achieve that impact?
-
How has this changed over the last ten years?
-
How far do you think the intended impact has been achieved?
-
What particular challenges does the HTA programme face? How have these changed over the last ten years?
-
What are the HTA’s priorities looking forward? How do you/does the programme plan to achieve them?
Academic questions
-
Have you ever applied for HTA funding? If so:
-
When and what for?
-
Can you describe the process?
-
Was the process satisfactory?
-
How did it differ from applications to other funders?
-
-
Have you ever been awarded HTA funding?
-
What requirements were placed on that funding?
-
What was your interaction with programme management like?
-
How did it differ from awards from other funders?
-
-
Would you consider applying for HTA funding (again)? Why/why not?
Political questions
-
What are the perceptions around the HTA programme in the Department of Health and in government more widely? (Possible follow ups: Is it considered to offer good value for money? What are the intended aims of the programme from a government perspective? Has it made the case for its continued funding?)
-
What influence does HTA research, or the programme more widely, have on policy, if any?
-
If it does have an influence, how does this happen? (Possible follow ups if needed: How is the research communicated? To whom? How is it used?)
Other funder questions
-
What overlap is there between your work and the work of the HTA programme?
-
Do you communicate regularly with HTA programme management?
-
What influence, if any, does the HTA programme have on your research strategy and portfolio?
-
What influence, if any, does the HTA programme have on your research processes? (Examples, which could be used as prompts if necessary and relevant: Do you require systematic reviews before conducting primary research? Are your protocols openly published? What proportion of your research leads to a publication, including negative results?)
-
What could the HTA programme learn from you?
C. Impact of the National Institute for Health Research Health Technology Assessment programme (all respondents, possibly with some questions excluded depending on questions already asked)
-
Could you please describe what groups or types of organisations the NIHR HTA programme has an impact on?
-
Could you please describe any impact of NIHR HTA programme on (prompt for nature of impact and specific examples):
-
Health policy (e.g. guidance, government policy, etc.)
-
The NHS (e.g. efficiency gains, cost savings, improved quality of care, etc.)
-
Clinical practice (e.g. improved quality of care, more effective treatment, etc.)
-
Patients (e.g. improve quality of care, increased life expectancy, etc.)
-
The economy (e.g. efficiency gains, cost savings, etc.)
-
Research (e.g. research agenda, best practice, etc.)
-
Other countries (e.g. health system, health policy, research practice, etc.).
-
-
If the HTA programme has not had an impact on any of the above, could you please describe any barriers to it having an impact (health policy, the NHS, clinical practice, patients, the economy, research, and other countries)?
-
Are you aware of any other research organisations that have built on the work of the HTA programme or used the methods developed by the HTA programme for their work? (Examples, which could be used as prompts if necessary and relevant: Do you require systematic reviews before conducting primary research? Are your protocols openly published? What proportion of your research leads to a publication, including negative results?)
-
Has the HTA programme had an impact on the research agenda (in the UK or internationally)? If so, could you please describe this impact?
-
Do you have any views on the extent to which HTA research is disseminated (in the UK or internationally)?
D. Views on the Health Technology Assessment programme (all respondents)
-
Do you have any views on the overall effectiveness of the NIHR HTA programme (e.g. overall, how successful do you think the HTA programme has been)?
-
Do you have any views of the research priorities of the NIHR HTA programme (e.g. are there some areas of research that are particularly well-funded or areas that are notably underfunded)?
-
Could you please describe what you would consider to be the most important benefits/achievements of the HTA programme (e.g. efficiency gains, improved quality of care, publications, etc. )?
-
Could you please describe what you would consider to be the most important shortcomings of the HTA programme?
-
Do you have any views on whether the NIHR HTA programme adds value to medical research in the UK and internationally?
-
Could you please describe what impact you foresee the HTA programme having in the short term and longer term?
Appendix 2 Codebook
| Code | Description | Unit of analysis | Categories (where applicable) |
|---|---|---|---|
| Researcher characteristics | |||
| Interest | Wider interests of researchers working on project | Researcher | Interests in other field(s) or areas (e.g. policy); Research is focused on single topic; Other |
| Motivation | Researcher motivation | Researcher | Curiosity focused/knowledge driven; Inspired by patients; Patient oriented, Evidence of strategic thinking |
| Network | Networks | Researcher | Evidence of strong research links within an institution; Evidence of research links across institutions; Evidence of links with industry; Evidence of links with policy-makers; Evidence of links with patients and families; Evidence of other networks |
| Skills | Particular skills that the researcher contributes | Researcher | N/A |
| Funding characteristics | |||
| Design | Comments on design of research, particularly external input | Project/programme | N/A |
| Field | Research field | Project | Screening and diagnostics; Pharmaceuticals; Surgery; Devices; Mental health; Methodology; Other |
| HTA budget | Comments on overall HTA funding and resources | Programme | N/A |
| Leverage | Suggestions that initial funding leveraged subsequent funding | Project | N/A |
| Multisite | Part of a multisite study | Project | N/A |
| Priorities | Comment on HTA research priorities or priority setting | Programme | N/A |
| Resources | Comment on level of resourcing of specific research projects | Project | N/A |
| Review | Review of research by funder | Project | Review comments helpful; Review comments unhelpful; Review comments neutral; Initial rejection; Other |
| Type | Type of funding | Project | Response mode; Commissioned; TAR; Other |
| Research characteristics | |||
| Application | Suggestions of application of new method in field | Project | N/A |
| Champion | Senior champion | Project/programme | Research; Implementation; Other |
| Changed | Suggestion that research plans changed from original ideas | Project | N/A |
| Collaboration | Collaboration | Project/programme | International; National; Local; Materials; Other (please note that categories are not mutually exclusive) |
| Controversy | Suggestions about the nature of research/scientific hypothesis | Project | Safe; Fashionable; Controversial; Contrary to established view; High risk; Other (please note that categories are not mutually exclusive) |
| Dissemination | Comments on the dissemination of research findings | Project/programme | N/A |
| Involve | Involvement in research process of wider stakeholders | Project/programme | N/A |
| Source | Source of research idea | Project/programme | NICE; NSC; PPI; Researcher; Other |
| Unplanned | Suggestion that research did not work out as planned – for better or worse | Project | Better; Worse; Other |
| System characteristics | |||
| Governance | Research governance by the HTA programme (and others) | Project/programme | N/A |
| Internal | Internal strategy at the HTA | Programme | N/A |
| Political | Influence of the political environment on research or research uptake | Project/programme | N/A |
| PPI | Influence of PPI on HTA programme and HTA research | Project/programme | N/A |
| Socioeconomic | Influence of wider social/economic environment on research/uptake | Project/programme | N/A |
| System | Influence of health system on research or research uptake | Project/programme | N/A |
| Research customers/stakeholders | |||
| Clinicians or practitioners | Any direct reference to that group | Project/programme | N/A |
| Relationship | Any direct reference to relationship with HTA | Project/programme | N/A |
| Government | Any direct reference to that group | Project/programme | N/A |
| Industry | Any direct reference to that group | Project/programme | N/A |
| JLA | Any direct reference to that group | Project/programme | N/A |
| Media | Any direct reference to that group | Project/programme | N/A |
| NETSCC | Any direct reference to that group | Project/programme | N/A |
| NHS | Any direct reference to that group | Project/programme | N/A |
| NICE | Any direct reference to that group | Project/programme | N/A |
| NIHR | Any direct reference to that group | Project/programme | N/A |
| NSC | Any direct reference to that group | Project/programme | N/A |
| Other customers | Any direct reference to other customers not included in above groups | Project/programme | N/A |
| Patients | Any direct reference to that group | Project/programme | N/A |
| Public | Any direct reference to that group | Project/programme | N/A |
| Scientists | Any direct reference to that group | Project/programme | N/A |
| SIGN | Any direct reference to that group | Project/programme | N/A |
| Other funders/research bodies | |||
| Cochrane | Cochrane reviews mentioned | Project/programme | N/A |
| Comparison | Comparison to other funders | Project/programme | HTA better; Other funder better; Other |
| Funder | Any direct reference to any other research funder or research body | Project/programme | N/A |
| Influencing other programmes | Any impact of the HTA on how other funders, HTA programmes, customers, etc. operate | Project/programme | N/A |
| International | Other HTA funders internationally | Programme | N/A |
| MRC | Any direct reference to that group | Project/programme | N/A |
| Overlap | Comments on overlap or duplication between funding body and HTA | Programme | N/A |
| Impacts | |||
| Academic | Academic quality, high citation, impacts on knowledge creation | Project/programme | N/A |
| Achievement | ‘The most important achievement of the HTA programme is . . .’ | Programme | N/A |
| Barrier | Barriers to impact | Project/programme | N/A |
| Capacity | Impact on capacity building | Project/programme | N/A |
| Economic | Economic impact | Project/programme | N/A |
| Facilitator | Facilitators of impact | Project/programme | N/A |
| Health | Impact on health and health sector | Project/programme | N/A |
| Industry | Impact on industry | Project/programme | N/A |
| International | Impact internationally/outside the UK | Project/programme | N/A |
| Limitations or areas for improvement | Negative aspects or challenges of the HTA programme | Programme | N/A |
| Patients | Impact on patients | Project/programme | N/A |
| Policy | Impact on policy | Project/programme | N/A |
| Practice | Impact on practice in the NHS or wider health-care systems | Project/programme | N/A |
| Product | Impacts on product development | Project/programme | N/A |
| Strengths | Positive aspects of HTA programme that don’t fall under ‘Achievement’ | Programme | N/A |
| Targeting | Impacts on research targeting | Project/programme | N/A |
| Other | |||
| Change | Changes that have occurred (particularly over the last 10 years) | Project/programme | N/A |
| Interesting | Interesting and notable | Any | N/A |
| Potential case studies | HTA work or specific projects mentioned by interviewees | Any | N/A |
| Potential interviewees | Experts mentioned or recommended interviewees | Any | N/A |
| Potential reference | References that may be of use in reporting stage | Project/programme | N/A |
| Quotes | All direct quotes in case studies | Any | N/A |
Appendix 3 Case study interview protocol
A. Introduction
RAND Europe has been commissioned by the HTA programme to assess its impact over the last 10 years.
RAND Europe is a non-profit policy research organisation, with an interest in research policy.
As part of this project, we are building a series of case studies around research funded by the HTA programme to identify the nature and range of impacts the programme has had, and how they came about. In a separate stream of work we are also looking across the programme as a whole for the impacts it has had using interviews, bibliometrics and a survey.
Our previous work in this area includes: payback studies of research impact on Mental Health research, Cardiovascular research, Arthritis research and Social science research. We have also carried out two studies for government and charitable research funders estimating the economic benefit of investing in medical research.
Drawing on a range of sources, we identified a long list of potential case studies and selected a stratified sample which included your study into XXXX published in the HTA journal article XXXX. We’d like to talk to you about how that research came about how it fitted into the other research you were doing at the time and how it developed.
For this project, we are looking at both how the findings of the research were developed and translated; and also, how the research undertaken developed the careers of the researchers involved.
We would like to record this interview. You will be given the opportunity to review the draft case study before it is published and request that any direct quotations used are removed or anonymised.
You should also emphasize that not all the questions will be relevant to their research project, and indeed we wouldn’t expect them all to be.
You shouldn’t stick to the protocol as written – it just provides guidance of the areas you should aim to cover. During your desk research you will have identified additional questions that you will want to ask and it’s probably best to add these to the protocol.
B. Introductory questions
To begin, talk briefly about their current work and how it relates to what they were doing at the time.
-
Can you tell us a bit about what you were doing at the time?
-
Where you were in your career?
-
Can you give us some background to this project?
-
Why do you think this project was seen as important?
C. Stage 0: Opportunity identification/research needs assessment
-
Was this project commissioned or was it a response mode grant?
-
If commissioned, what was the source of the idea for the research?
-
If response mode, what was the original impetus for the work? (Solely scientific curiosity? The desire to fill certain gaps in knowledge? Targeting of a particular disease state? Your own clinical experience?)
-
-
Was there a clear intended impact on policy or practice from the outset?
-
What other ideas were you pursuing at the time, how did they relate to this work?
-
Who influenced your decision to work in this area?
-
Was it a continuation of previous work?
-
How far was your identification of the research topic influenced by:
-
Research you had done before? Funded by whom?
-
The research of others? If so how did you hear about this research?
-
For primary research, an existing systematic review?
-
-
How much interaction was involved in determining your choice of research topic?
-
With representatives of patient or practitioner groups?
-
With funders?
-
With peers internationally in a specific research community?
-
-
Did institutional conditions such as lab space, equipment, or availability of researchers affect the research proposal?
D. Stage 1: Inputs to research and project specification and selection
-
How much funding did you receive from the HTA?
-
Were there other sources of funding which supported this work?
-
If so:
-
What were the different forms of support and why was each important?
-
Was there soft or core funding (e.g. funding the needs to be applied for vs. guaranteed funding)?
-
-
Did you make any unsuccessful applications for funding? Did you make any resubmissions?
-
Did any of the peer review or applications processes affect the design or direction of the work?
-
Did you have to compete for funding?
-
Did you consult with patients, the public or practitioners in developing the research design? What role did their input play?
-
What was the institutional setting (hospital, university, research institute) for the research?
-
Who were the main researchers involved in the project?
-
What was their level of research experience and seniority at that time?
-
Had they previously worked in this research area?
-
For primary research: did any existing systematic review play a role in your research design (e.g. in determining necessary sample sizes)?
-
Which of the following inputs were important?
-
Knowledge/expertise
-
Techniques
-
Samples/study recruits
-
Consumables
-
Space
-
Time
-
Money
-
Collaborators
-
Reputation.
-
E. Stage 2: Processes
-
Did the methods proposed prove to be appropriate? Which avenues of research were successful and which weren’t?
-
Was there any interaction with potential users of the research during the research processes?
-
How much freedom did you or the research group have to pursue different lines of enquiry/deviate from the original proposal? How important was this flexibility in achieving the final results?
-
Did you publish the research protocol at the start of the study?
-
Did the research require new techniques/new expertise/new approaches to the subject?
-
How would you describe your role in the research process?
-
What was the role of collaborators in the research process (both academic and industrial)?
-
Who else was working in the area?
-
What interaction did you have with HTA programme staff during the research process? How useful was this interaction?
F. Stage 3: Primary outputs
-
Which publications do you think were most important from this research and why?
-
Did this work have any impact on the agenda for your subsequent research?
-
Did this research make any impact on the career of any of the research team? For example: contribute to research training in terms of research degrees or the gaining of additional skills
-
enable them to establish themselves in the field?
-
help the lead researcher to build a team of researchers?
-
-
Are you aware of any other researchers who have built on this work or used the methods you developed? What is the role of collaborators in this?
-
Did the research spawn a new area of investigation or make a major impact on the approach used in subsequent research?
-
If the research was clinical, were any basic researchers also involved? If so did this influence their attitude to clinical research?
-
Were any health practitioners involved in assisting with the research, and if so did it have any impact on their attitude towards implementing research findings in general?
-
For primary research: has the research been included in any subsequent systematic reviews or meta-analyses?
-
For evidence synthesis: has any primary research been conducted based on the findings of your work?
-
Have you had any impact outside the field of research you are working in?
-
Were any findings of the research not published (e.g. dead ends, negative findings)?
G. Interface B: Dissemination
-
Apart from publications, what attempt did you make to disseminate the findings
-
to academic audiences?
-
to wider audiences? Did you work with funders or stakeholders to do this?
-
-
Did you use specially designed dissemination approaches to particular audiences, for example policy briefs for policy-makers? What were the most effective mechanisms for this?
-
What was the role of your networks in dissemination?
-
Did you receive support from funders/employers for dissemination? What form did this take?
H. Stage 4: Secondary outputs
-
Has the research been cited directly in any clinical guideline, audit criteria or similar document from a professional body or public policy-making body at national or local level?
-
Do you know how far the research directly influenced the formulation of any policy, or the realisation that a policy was needed?
-
Has any subsequent research by you or others that built on this project been cited in any clinical guideline, audit criteria or similar document from a professional body or public policy-making body at national or local level? Do you think this might happen in future?
-
Did the research from your project lead to any patents/licences? Was it taken up by industry? Has it contributed to any commercial products?
-
If the research has made some impact, what are the key reasons for this? If it has failed to have an impact what are the reasons for this?
-
What barriers were there to the research having an impact/being translated?
-
What factors facilitated the research having an impact/being translated?
-
Has your research had an impact on teaching for clinicians?
-
Has any advisory role to government, hospitals, industry led to an impact from your research? How did this come about?
I. Stage 5: Applications
-
Have the findings from the research influenced practitioners directly through them reading the articles or hearing a presentation about the research?
-
What were the impacts on practice through clinical guidelines or policies based either specifically on the research or on other research that built on your research?
-
Can you comment on the extent of implementation? How widely have those policies or guidelines been taken up?
-
Have the findings been disseminated through other routes such as networks or existing relationships with practitioners?
-
Has any impact been local, regional, national or international?
-
If the research has been taken up by industry, do you know what level of sales has been achieved by any product to which it contributed?
-
Do you expect any greater take-up of the findings in the future? Where and how?
-
Has there been an impact on practice through your own clinical work (if you have any)? What has been the knock-on effect of that on other clinicians?
J. Stage 6: Public engagement
-
Depending on answers to previous questions about involvement of the public in shaping the research agenda, ask how far there has been any interaction with patients, patient groups or the wider public about the findings and their implication. Has this led to any improvement in the way patients manage their own care or interact with therapy? Or had any impact on public attitudes to medical research? Please describe these.
-
Did engagement with the public/patient groups lead to changes in the researchers’ perceptions of these groups? Please describe.
K. Stage 7: Final outcomes
-
If the research has made impact on policy or practice, or on the behaviour of the public, is there any way of assessing the benefits in terms of: patient health gain? Qualitative improvements in the way the service is delivered that increase patient and/or practitioner satisfaction? Cost savings?
-
If it is possible to assess the potential benefit for one patient, approximately how many patients might be able to benefit from the improved therapy or organisation of the service?
-
If the improved therapy based on the research has resulted in a health gain, will this also result in fewer days lost from work/decreased benefits payments/decreased visits to secondary health care?
-
If the research has resulted in commercial development is anything known about the amount of employment generated, the level of import substitution, or the revenue generated for the company by the product?
L. Other general questions
-
Who else should we speak to about your research?
-
Are there other questions we should have asked or things that you want to talk about?
-
Are you happy for us to contact you to follow up on details arising from the case study research?
Appendix 4 Case studies
This appendix contains the full text of the 12 case studies41–52 conducted as part of this study. The order in which the case studies are presented, along with a brief summary of each, is set out in Table 15.
| Case study | Type of research | Field | Summary |
|---|---|---|---|
| ARTISTIC41 | Primary | Screening/diagnostics | RCT into effectiveness and cost-effectiveness of HPV testing in primary screening as either an adjunct to cytology or as a stand-alone test compared with the current screening programme, which relies on cytology alone |
| Newborn CHD42 | Evidence synthesis | Screening/diagnostics | Systematic review and cost-effectiveness analysis of newborn screening for CHDs |
| IVAN43 | Primary | Pharmaceuticals | Comparing two drugs for the treatment of wet AMD, a chronic and progressive condition that is the leading cause of sight loss in older people |
| CRASH-244 | Primary | Pharmaceuticals | RCT investigating whether or not TXA could be used to treat trauma victims shortly after their injury and reduce their chance of dying |
| RA45 | TAR | Pharmaceuticals | MTA consisting of a systematic review and economic analysis of three drugs for the treatment of rheumatoid arthritis in adults |
| EVAR46 | Primary | Surgery | RCT comparing the use of endovascular repair with existing treatments for the correction of AAA |
| CS47 | Evidence synthesis | Screening/diagnostics | Systematic review and modelling to determine whether or not novel non-invasive treatments were as effective as the traditional (invasive) therapy in diagnosing carotid stenosis with the aim of reducing the risk of stroke |
| SWET48 | Primary | Devices | Study to determine whether or not ion-exchange water softeners could improve atopic eczema in children with moderate to severe eczema, and likely cost and cost-effectiveness of such an intervention |
| CESAR49 | Primary | Devices | RCT of ECMO for severe adult respiratory failure, compared with standard care |
| CoBalT50 | Primary | Mental health | RCT into use of CBT as an adjunct to usual care (including drug treatment) as a ‘next-step’ treatment after initial treatment has failed |
| CUtLASS51 | Primary | Mental health | RCT comparing atypical antipsychotics to older, typical drugs for the treatment of schizophrenia |
| STAs52 | TAR | Overview of STAs and their impact (particular focus on Southampton and Sheffield TAR centres) |
Appendix 4.1: ARTISTIC trial
Summary
The ARTISTIC trial41 evaluated the effectiveness and cost-effectiveness of HPV testing in primary screening, as either an adjunct to cytology or as a stand-alone test, compared with the current screening programme, which relies on cytology alone. The results from the first two screening rounds found that HPV testing did not significantly improve the effectiveness of liquid-based cytology (LBC) and that it would not be cost-effective to screen with both HPV testing and cytology, but the trial also found that HPV testing is highly effective as a primary screening strategy. The follow-up study, which included data from three screening rounds, concluded that HPV testing, as a primary screening test, was significantly more protective over three screening rounds than current practice (cytology), and that the use of HPV testing in primary screening could safely allow the screening interval to be lengthened. The cost-effectiveness analysis found that primary HPV screening would probably be cost-saving in both vaccinated and unvaccinated cohorts.
The ARTISTIC41 trial resulted in the publication of two HTA monographs and eight peer-reviewed publications. It also had a positive impact on the career of the Principal Investigator (PI) and a number of other individuals involved in the study,41 which can be viewed as a proxy measure for an increase in skills and capacity building as a result of taking part in the HTA programme-funded trial. The ARTISTIC41 trial has had an important academic impact, particularly through its contribution to the pooled analysis of the four other European HPV screening trials, which showed that HPV testing as primary screening for cervical cancer results in a decrease in the incidence of cervical cancer. The ARTISTIC41 trial also resulted in the piloting of HPV testing as primary screening for cervical cancer, which may, in turn, result in national roll-out of HPV testing. Finally, the ARTISTIC41 trial, together with the other European trials, has had an impact on the development of new HPV tests internationally.
Introduction to case study
Background
Scientific background
The current NHS Cervical Screening Programme aims to reduce cervical cancer incidence and mortality. 60 In England, all women aged 25–64 years are invited to be screened. Women aged 25–49 years are invited every 3 years whereas women aged 50–64 years are invited every 5 years. 61 Current primary cervical screening uses cervical cytology to detect pre-invasive cancer lesions from a sample of cervical cells. 62 These pre-invasive lesions, known as Cervical Intraepithelial Neoplasia (CIN), usually precede the development of invasive cancer by many years, such that their detection offers the opportunity to intervene prior to the development of cancer.
However, during the 1990s, a number of studies established that cervical cancer was caused by HPV. 63,64 Walboomers et al. (1999)64 found that the prevalence of high-risk HPV in cervical carcinomas was 99.7% and concluded that ‘the extreme rarity of HPV-negative cancers reinforces the rationale for HPV testing in addition to, or even instead of, cervical cytology in routine cervical screening’. The purpose of the ARTISTIC trial41 was to determine whether or not HPV testing in primary screening, either as an adjunct to cytology or as a stand-alone test with cytology reserved for HPV positive women, would be more effective and cost-effective than the existing cervical screening programme. 65
Chief investigators’ background details
Henry Kitchener, Professor of Gynaecological Oncology at the University of Manchester and Honorary Consultant at St Mary’s Hospital in Manchester, was one of the CIs of the ARTISTIC41 study, as well as clinical PI. 66 Kitchener is a Fellow of the Academy of Medical Sciences, the Chairperson of the Department of Health Advisory Committee on Cervical Screening, Chairperson of Target Ovarian Cancer’s Scientific Advisory Boards, and a Trustee of the British Society of Colposcopy and Cervical Screening. 67 From 2012 to 2014, Kitchener was interim Director of the Institute of Cancer Sciences at the University of Manchester. He is also Associate Director of Governance at Central Manchester and Manchester Children’s University Hospitals NHS Trust and is involved in screening policy internationally as a member of the European Cervical Cancer Screening Network68 and the International Cancer Screening Network. 69
Julian Peto, Professor of Epidemiology at the London School of Hygiene and Tropical Medicine, was the other CI of the ARTISTIC41 study and the lead for statistics and epidemiology. 70 Peto’s Chair of Epidemiology at the London School of Hygiene and Tropical Medicine (LSHTM) is supported by Cancer Research UK.
The case study approach
The data collection process for this case study involved a series of interviews and a review of the primary and secondary data sources relating to the ARTISTIC41 trial. As shown in Table 16, six individuals were interviewed: both CIs, one of the trial statisticians, the first author of the pooled analysis of the four randomised trials of HPV-based screening for cervical cancer, the Director of the NHS Cancer Screening Programmes and the Director of the NSC.
| Interviewee | Reason for interview |
|---|---|
| Henry Kitchener | Co-CI |
| Julian Peto | Co-CI |
| Clare Gilham | Trial statistician |
| Guglielmo Ronco | First author of pooled analysis of four HPV screening trials |
| Julietta Patnick | Director of the NHS Cancer Screening Programmes |
| Anne Mackie | Director of the NSC |
Stage 0: topic/issue identification
A number of key factors influenced the research team’s decision to work in this area, as detailed in Box 1 and described below.
-
CIs’ clinical and epidemiology expertise.
-
Recently established link between HPV and cervical cancer.
-
Identified research need from a previous systematic review.
-
Uncertainty regarding the relative effectiveness of HPV testing, cytology and HPV/cytology co-testing.
-
HTA-commissioned call.
Principal Investigators’ clinical and epidemiology expertise
Prior to undertaking the ARTISTIC41 trial, Henry Kitchener and Julian Peto both had extensive research expertise. 67,70 Kitchener, the Clinical CI, had substantial clinical and research expertise in gynaecological cancers and clinical trials, and had published extensively on the diagnosis and treatment of such cancers. 71–131 Peto had extensive expertise in epidemiology, particularly the epidemiology of cancers, as evidenced by his numerous publications. 64,132–192
Recently established link between human papillomavirus and cervical cancer
In the 1990s, the link between cervical cancer and HPV became well established. 63,64 According to Kitchener et al. (2009),65 this link suggested two important clinical applications: primary prevention through HPV vaccination and HPV as a screening test for cervical cancer or clinical management. The former was addressed by two clinical trials,193,194 which resulted in the introduction of a national vaccination programme in 2008. The latter was addressed by the ARTISTIC41 trial.
Identified research need from a previous systematic review
In 1998, the NIHR R&D HTA programme, now the NIHR HTA programme, funded a systematic review on the role of HPV testing within the cervical screening programme. 195 The review found that HPV testing has higher sensitivity, but lower specificity, than cytology for high-grade CIN lesions. The authors concluded that HPV testing could not be recommended for implementation within the national screening programme. 196
The authors of the systematic review made the following recommendations for future research:
Full evaluation of HPV testing should provide information on the length of protection after a negative result, and consideration should be given to a very large trial with a reduction in cancer incidence as the end-point. Further studies and modelling simulations are needed to evaluate the range of potential roles and most cost-effective use of HPV testing, and how it should be implemented and integrated with other testing methodologies.
p. 1513196
Following the publication of the systematic review, the HTA programme commissioned primary research into the role of HPV testing in the cervical screening programme, which resulted in the funding of the ARTISTIC41 trial.
Uncertainty regarding the relative effectiveness and cost-effectiveness of human papillomavirus testing, cytology and human papillomavirus/cytology co-testing
According to one interviewee, at the time of the ARTISTIC trial, there was considerable debate regarding whether or not women should be screened with both cytology and HPV testing or HPV testing alone. The ARTISTIC41 trial thus set out to determine the effectiveness and cost-effectiveness of HPV testing as either an adjunct to cytology or as a stand-alone test, compared with the current practice of cytology alone.
Concurrent trials in other European countries
Shortly before the ARTISTIC41 trial was commissioned, two other European randomised trials were evaluating HPV testing in primary screening: the Swedescreen trial in Sweden197 and the POBASCAM trial in the Netherlands. 198 [Two other European trials commenced shortly after ARTISTIC: the Finnish Public Health Trial (http://onlinelibrary.wiley.com/doi/10.1002/ijc.10839/full) and the Italian New Technologies for Cervical Cancer Screening trial (www.ncbi.nlm.nih.gov/pubmed/22088483).] However, the Swedescreen197 and POBASCAM198 trials were looking at the effectiveness of HPV testing as a primary screen in the context of the Swedish and Dutch health-care systems, which, one interviewee noted, had limited generalisability to the NHS.
Health Technology Assessment programme-commissioned call
Following the publication of an earlier HTA programme-funded systematic review on the role of HPV testing in the cervical screening programme, the HTA programme commissioned primary research into the effectiveness and cost-effectiveness of HPV testing. According to one interviewee:
The topic was a no-brainer in the sense that it had to be done. It was clearly something that . . . there had been an accumulation of evidence to suggest that HPV primary screening could be the way to go. This didn’t come out of a clear blue sky. This was something that had been on the horizon.
In the commissioning brief, the HTA programme specified both the research question and the study design. 65 The brief specified the following research question: ‘What would be the performance (specificity and sensitivity), costs, effectiveness and impact on the cervical screening programme of HPV testing use along, or in conjunction with the cervical smear test?’ The commissioning brief also identified the following outcomes measures for inclusion: incidence of pre-cancer and cancer of the cervix, the diagnostic performance of HPV tests, quality of life and patient satisfaction issues, and duration of follow-up. The commissioning brief clearly stated that the primary research should assess the performance and properties of both HPV testing alone and in conjunction with conventional cytology.
Interface A: project specification and selection
Henry Kitchener and Julian Peto initially submitted separate applications to conduct primary research into the role of HPV testing in primary screening for cervical cancer. However, according to one interviewee, in the second round of the application, the HTA programme asked the two research groups to put in a joint application, which resulted in the funding of the ARTISTIC41 trial. According to one of the interviewees, Kitchener’s team had substantial clinical expertise, whereas Peto’s team had substantial epidemiological and statistical expertise. Henry Kitchener, Julian Peto, Stephen Moss, Robin Dowie, Gerald Corbitt, Mina Desai, Peter Maguire and Chris Roberts were all involved in the design of the study protocol. 65 It has not been possible to determine how many other applications were made to the HTA programme; however, as the HTA put out an open call to tender, it is likely that the application process was competitive. One interviewee noted that asking the two research teams to submit a joint application ultimately proved to be beneficial:
The success of the HTA, through combining [Julia Peto’s] bid, with Henry Kitchener’s, is perhaps underplayed by the HTA.
The ARTISTIC41 trial was a randomised trial that compared cervical cytology with cytology as an adjunct to HPV testing, over three screening rounds, which were 3 years apart. The first round of screening was intended to detect prevalent disease (the proportion of the population with HPV), whereas the second round was intended to detect a combination of incident disease (the proportion of the population that acquired HPV over the screening interval) and undetected prevalent disease from the first round (the proportion of the population with HPV in the first screening round that remained undetected until the second screening round). The third round looked at the effectiveness of HPV testing over a longer time period. The trial used LBC instead of conventional cytology, as LBC was set to be introduced into the NHS shortly after the start of the ARTISTIC41 trial. One interviewee noted that the NHS Cancer Screening Programmes advocated the use of LBC in the trial so that the results would be relevant to what was expected to be current practice in the NHS by the time of publication.
The research team did not have any interaction with patients or policy-makers in the design of the research. However, the ARTISTIC41 trial was designed to inform cervical cancer screening policy from the outset and the trial was embedded within the existing NHS Cervical Screening Programme.
Stage 1: inputs to research
Financial
The initial ARTISTIC41 trial received £1,186,678.76 in funding from the NIHR HTA programme41 and additional service support funding of £10 per recruited woman from the DH. 65 For the follow-up study, the research team received £276,915 from the HTA programme for the third screening round, and co-funding from the NHS Cervical Screening Programme,199 together with an additional £72,656 for a modelled analysis of the potential effectiveness and cost-effectiveness of HPV testing in cervical cancer screening after three screening rounds. 200 Alexandra Sargent received financial support from Roche to travel to project meetings in the follow-up study. 201
Prior to the ARTISTIC41 study, the model used in the economic evaluation was funded by the National Health and Medical Research Council Australia, the Medical Services Advisory Committee Australia, the National Screening Unit in New Zealand, the NHS Cervical Screening Programme in England and the Cancer Council in Australia. 201
Knowledge and expertise
The research team had considerable topic and methodological expertise. As noted above, Henry Kitchener and Julian Peto both had substantial expertise in gynaecological cancer research, with Kitchener’s earlier work having focused more on the clinical aspects of the research, and Peto’s on the epidemiological aspects. During the trial, Kitchener was the clinical lead, and Peto was the lead for statistics and epidemiology. Kitchener and Peto also worked with an experienced team with expertise in a number of areas: Clare Gilham had earlier expertise in statistics and epidemiology, Maribel Almonte and Christopher Roberts had expertise in statistics, Andrew Bailey and Alexandra Sargent had expertise in virology, and Robin Dowie had expertise in health economics.
Stage 2: research process
The ARTISTIC41 trial set out to evaluate the effectiveness and cost-effectiveness of HPV testing in primary screening as either an adjunct to cytology or as a stand-alone test, compared with the current screening programme, which relies on cytology alone. 65 HPV testing was undertaken in both arms of the trial,41 but concealed from those randomised to standard care with cytology only to allow the evaluation of HPV testing as a stand-alone test. The cohort data were then use to model the outcomes of three different screening strategies: cytology alone, HPV testing alone and cytology combined with HPV testing. The study41 concluded that HPV testing did not significantly improve the effectiveness of LBC and that it would not be cost-effective to screen with both HPV testing and cytology, but that HPV testing is highly effective as a primary screening strategy. According to one interviewee, a better trial design would have involved all trial participants being screened with both a HPV test and cytology.
The ARTISTIC41 trial used LBC instead of conventional cytology, whereas the four other European trials used conventional cytology. In LBC, cervical cell samples are collected in the same way as conventional cytology, but a brush-like device is used instead of a spatula. 202 The sample is then placed into a vial of preservative fluid so that most of the cervical cells are retained. The samples are then mixed in a laboratory to disperse the cells. The cellular debris (e.g. blood and mucus) is then removed and a thin layer of cervical cells is placed on a microscope slide, which is then stained. According to Kitchener et al. (2009),65 ‘the principal advantages of LBC are a major reduction in inadequate samples for reading and more rapid throughput of samples in laboratories’. At the time of the ARTISTIC41 trial, the NHS Cancer Screening Programme was piloting LBC for cervical screening. According to one interviewee, the ARTISTIC41 trial was run on LBC so that the results would be relevant to what was expected to be current practice in the UK by the time the trial would be published. National conversion from conventional cytology to LBC was completed in 2008. 203
According to one interviewee, the use of LBC complicated the interpretation of the trial results. As LBC had not yet been introduced in the UK at the time of the ARTISTIC41 trial, the cytologists involved in the trial had to be trained to use the new technology, which meant that they were over-cautious and found 16% of smears to be abnormal. The same interviewee also noted that the sensitivity of cytology to detect CIN3 (Grade 3) improved after the switch from conventional to LBC, which probably resulted in previously missed lesions being detected in the ARTISTIC41 trial. The other European trials that did not use LBC observed a larger difference between HPV testing and cytology. 204
Although the research team did not have any interaction with patients while conducting the study,41 they did have some interaction with policy-makers and the HTA programme. Henry Kitchener was a member of the Advisory Committee on Cervical Screening during the trial, and updated the Committee on the trial’s progress. According to one interviewee, policy-makers awaited the results of the trial and viewed the results as important. The research team also had some interaction with the HTA programme during the study,41 which involved the submission of interim reports, and meeting minutes from the TSC and the independent Data Monitoring Committee. According to one interviewee, ‘the HTA maintained some oversight, but it was light touch’.
The research team undertook a follow-up study, which started in 2008, and analysed the results of the extended follow-up of the trial cohort after a third screening round. 201 The objective of the follow-up study was to provide further insight into the duration of protection of a negative HPV test, through the use of long-term data, to determine whether or not it would be possible to lengthen the screening interval. In the extended follow-up study, all women underwent cytology, HPV testing and genotyping. According to one interviewee, the genotyping of all of the HPV-positive samples in the trial was also a unique feature of the ARTISTIC41 trial. The follow-up study concluded that HPV testing as a primary screening test was significantly more protective over three screening rounds than current practice (cytology), and that the use of HPV testing in primary screening could safely allow the screening interval to be lengthened. 201 The cost-effectiveness analysis found that primary HPV screening would be cost-saving in a number of different screening strategies for both vaccinated and unvaccinated cohorts.
Stage 3: primary outputs from research
Knowledge
The ARTISTIC41 trial resulted in numerous publications, including: two HTA monographs;65,201 a paper reporting on the results of the first two screening rounds;62 a paper reporting on the results of the third round follow-up;205 a paper on the efficiency of cervical smear readers;206 a paper on the well-being of cytoscreeners;207 an epidemiological study on the prevalence of HPV;208 an epidemiological study on the prevalence of different high-risk strains of HPV;209 and a paper on the psychosocial impact of HPV testing on women. 210 Figure 11 presents the results of the bibliometric analyses on the publications resulting from the ARTISTIC41 trial.
The Kitchener et al. (2009)62 paper and the first HTA monograph65 report on the results of the first two screening rounds of the ARTISTIC trial. Those results indicated that LBC combined with HPV testing resulted in a significantly lower detection rate of CIN3+ lesions in the second round of screening than with LBC alone, but that the effect size was small. Over the first two screening rounds, LBC combined with HPV testing did not detect a higher rate of either CIN3+ or CIN2+ lesions than LBC alone. The economic analysis found that it would not be cost-effective to screen with both LBC and HPV testing, but that HPV testing as either a triage tool or an initial test triaged by cytology would be cheaper than cytology alone.
The Kitchener et al. (2011) paper205 and the second HTA monograph201 report the results of the third screening round. It was found that the additional sensitivity of HPV testing compared with cytology could permit the lengthening of cervical screening intervals because a negative HPV test would provide protection over a longer period of time than cytology. 205 The study205 concluded that a negative HPV test was significantly more protective that LBC over the three screening rounds of the trial and that, as a result, the primary screening interval could be increased from 3 years to 6 years if HPV testing replaced cytology. The economic analysis found that HPV testing would be more effective than cytology and save costs. 201
The Dowie et al. (2006)206 study on the efficiency of cervical smear readers using LBC found that LBC can improve laboratory efficiency. However, the study206 also noted that decision-makers should consider the costs and benefits of introducing LBC into screening programmes, including the upfront cost of capital investment and the workforce implications for cytoscreeners.
Dowie et al. (2006)207 looked at the well-being of cytoscreeners in the NHS. The study207 found that there was a strong negative correlation between cynicism, or an indifference to work, and overall job satisfaction. Cynicism among cytoscreeners increased over the period in which the Greater Manchester laboratories partially converted to LBC.
The Kitchener et al. (2006)208 paper reports on the prevalence of high-risk HPV in relation to age, cytology and histology upon entry into the trial. Of the 24,510 women screened, aged 20–64 years, the cytology results were 87% normal, 11% borderline or mild, 1.1% moderate and 0.6% severe dyskaryosis or worse. 208 Kitchener et al. (2006)208 also found that the prevalence of HPV decreased sharply with age, but increased with cytological grade. Lastly, the study208 found that the majority of young women in the Greater Manchester area had been infected with a high-risk strain of HPV by the age of 30 years, such that the introduction of HPV testing as a routine screening test in women aged < 30 years would result in a substantial increase in retesting and referral rates.
Sargent et al. (2008)209 report on the prevalence of type-specific HPV infection by age and grade of cervical cytology. Among the 24,510 women screened, aged 20–64 years, the most common strains of HPV were HPV16, HPV18, HPV31, HPV51 and HPV52. Those strains accounted for 60% of all HPV cases detected. Although the prevalence of HPV declined with age, the proportion of cases caused by each strain did not vary with age. Multiple infections were common in those aged ≤ 30 years. Lastly, the study209 found that catch-up vaccination would probably reduce the number of women with moderate or worse cytology, but that it would not substantially reduce borderline to mild cytology.
The Kitchener et al. (2008)210 paper reports on the psychosocial impact of HPV testing on women in primary cervical screening. The study210 found that a revealed high-risk HPV test result did not have a significant impact on women with a revealed HPV-positive test result compared with those women with a concealed HPV-positive test result. The study210 concluded that HPV testing does not add significant psychological distress to cytology in routine primary cervical screening.
Finally, Sargent et al. (2010)212 report on the optimal threshold for a positive Hybrid Capture 2 test, used in the trial for the detection of high-risk HPV. The study212 found that a relative light unit/cut-off ratio of ≥ 2 achieved an increased specificity in the detection of CIN2+ lesions compared with the manufacturer’s recommended ratio of 1, which was achieved without a clinically significant loss of sensitivity.
Benefits to future research and research use
Capacity building and career development
As noted above, Henry Kitchener and Julian Peto both had well-established research careers prior to the ARTISTIC41 trial. However, according to one interviewee, the ARTISTIC41 trial probably had a positive reputational benefit on Kitchener’s career, as he was one of the CIs, and the first author on the two HTA monographs and a number of the other trial publications. After commencing the ARTISTIC41 trial, Kitchener received a number of large grants from the HTA programme for the MAVARIC213 trial on automation-assisted cervical screening compared to manual screening, a multi-stranded study on the minimum cellularity required for the reliable assessment of liquid-based cervical cytology samples,214 the extension of the ARTISTIC41 trial,199 a modelled analysis of the effectiveness and cost-effectiveness of HPV testing in cervical cancer screening (using data from the ARTISTIC41 trial)200 and a study on strategies to increase cervical screening uptake at first invitation (STRATEGIC215).
According to one interviewee, the ARTISTIC41 study also had a positive impact on the careers of a number of the other members of the research team. For example, an individual who conducted much of the viral testing subsequently went on to do a PhD based on the ARTISTIC41 trial, and, according to the same interviewee, that individual’s career has since prospered.
Targeting of future research
Ronco et al. (2013)204 included the ARTISTIC41 trial in a pooled analysis of the four European primary HPV testing trials, which was part of the wider European PREHDICT216 study (Health-economic modelling of prevention strategies for HPV-related disease in European countries). Three members of the research team contributed to the pooled analysis: Henry Kitchener, Clare Gilham and Julian Peto. It found that there was a reduced incidence of cancer among women who had been screened with a HPV test. According to one interviewee, the pooled analysis was particularly important because it was not possible to look at final outcomes in the individual trials (as invasive cancers are very rare in women who have been screened). The pooled analysis of the four studies provided sufficient power to detect the effect of HPV primary screening on cancer incidence.
The pooled analysis also highlighted some important differences between the four European trials. The ARTISTIC41 trial was the only trial that did not show a clear increase in sensitivity of HPV testing compared with cytology, which one interviewee suggested was probably attributable to the ARTISTIC41 trial’s use of LBC. The interviewee suggested that the heterogeneity of the ARTISTIC41 trial may have somewhat limited its international impact compared with the other European trials, but that ARTISTIC41 trial was, nevertheless, an important study. Lastly, the interviewee noted that the heterogeneity between the European trials was also useful because it allowed for comparison of the effect of the different protocols used.
After commencing the ARTISTIC41 trial, but prior to completing it, Henry Kitchener and others also undertook the related MAVARIC217 trial on automated assisted reading of cervical cytology slides. Although MAVARIC217 did not address any questions related to the effectiveness of HPV testing as a primary screening strategy, it informed policy-makers of another aspect related to cytology screening: the effectiveness of automated assisted reading of slides. The study217 found that automated reading was inferior to manual reading because it was 8% less sensitive.
Similarly, Kitchener et al. (2008)218 evaluated the use of HPV testing to determine cure after treatment for CIN. The study found that the cumulative incidence of failed treatment in women – who were cytology negative, but HPV positive, 6 months after treatment – was low, which indicated that women could be returned to regular 3-yearly recall instead of having annual cervical cytology for 10 years. According to one interviewee, this system was adopted by the NHS Cervical Screening Programme in 2012.
On a related topic, Kitchener is also undertaking the STRATEGIC215 study, funded by the HTA programme, which is looking at mechanisms for increasing the uptake of cervical screening by young women. Phase 1 of the study uses a pre-invitation leaflet to explain why individuals are being invited for screening, whereas Phase 2 focuses on those who failed to attend after receiving their invitation. In Phase 2, women are offered the opportunity to speak to a nurse, a HPV self-sampling test kit, a timed appointment or a choice between the nurse and the self-sampling kit. Although this study215 does not report directly on the effectiveness of HPV testing, it is a related study, as it will report on methods to increase the uptake of primary screening for cervical cancer.
Interface B: dissemination
According to the interviewees, the research team primarily disseminated the findings from the ARTISTIC41 trial through academic publications (described above) and conference presentations. The team presented their research at academic conferences, including the European Research Organisation on Genital Infection and Neoplasia (EUROGIN), and the International Papilloma Virus Society Conference. The research team also presented the results to the Advisory Committee on Cervical Screening to ensure that the Committee was aware of the study’s41 findings. However, one interviewee stressed that ‘by far, the most prominent method of dissemination was the publications in good journals’.
Stage 4: secondary outputs
According to one interviewee, the Sargent et al. (2010)212 paper on the optimal threshold for the Hybrid Capture 2 Test for HPV detection had a direct impact on policy. The paper showed that changing the cut-off from the manufacturers’ recommendations would increase the relative sensitivity of the test so that it would be more clinically useful. This finding was then translated into practice in the NHS. However, the interviewee noted that the Hybrid Capture 2 Test has now been superseded by other diagnostic tests but that, nevertheless, at the time, the paper had an impact on NHS practice.
The ARTISTIC41 trial also led to the undertaking of a national pilot of HPV primary screening in the UK, which began in 2013. The trial41 was cited in the NSC meeting minutes in April 2012. 219 The minutes state that ‘Members also asked about the cost-effectiveness of HPV TaPS [HPV Testing as Primary Screening]. Professor Patnick said the ARTISTIC trial had looked at both clinical and cost-effectiveness but further modelling would be needed as part of the feasibility study’. Additionally:
The UK NSC agreed that there is enough evidence to suggest that HPV TaPS would be cost and clinically effective. It was agreed that the UK NSC should consult on a recommendation to approve HPV as a primary screen for cervical cancer and that the feasibility study should explore implementation issues including length of time before a re-screen following a HPV negative result.
Henry Kitchener chairs the Steering Group for the HPV Primary Screening Pilot. According to one interviewee, the ARTISTIC41 trial has been essential for designing the national pilot. The interviewee noted that policy-makers have found the main paper and the monograph to be most valuable:
[The Cancer Screening Programmes] have been able to crawl all over the monograph to get details and answers that aren’t in the publication. The publication alone would not have been enough. [The Cancer Screening Programmes] do need the detail that’s in the monograph. If the HTA ever think about stopping doing that, I would vote against.
The results of the pilot will then inform any future changes in the NHS Cervical Screening Programme. However, according to one interviewee, the bureaucratic process may pose a barrier to the adoption of HPV testing in primary screening because the changeover from cytology to HPV testing will be expensive in the short run, even although it may be cost-effective in the long run.
One interviewee outlined the uncertainties that the HPV pilot is trying to address:
In terms of HPV, it is unfeasibly complicated. The acknowledgement that HPV as a primary screening is better than LBC is almost the easy bit. What [the National Screening Committee] has then got to do, in the context of HPV vaccination, is work out how on earth to do it. You change the screening intervals, you change the call intervals, you up the colposcopies, looking at the cervix in the short term, but reduce it in the long-term, and you hopefully reduce the length of time between call and recall for women that are HPV negative. That will have a huge effect on the labs, because you suddenly have to do millions of HPV tests and has the possibility of completely getting rid of cytology for all intents and purposes, reducing the cytology workforce to next to naught. There will need to be some, but not in the volumes that we’ve got. And of course that changes year on year when you do HPV vaccination, because hopefully this will more or less go out of style completely. And then of course, there is the question of whether women fancy being screened for a sexually transmitted disease, what effect that will have on it. Women will come back at varying intervals – whether the system is capable at managing varying bespoke intervals or a much more complicated set of call and recall.
Stage 5: adoption by practice and the public
The ARTISTIC41 trial is unlikely to have had a major impact on clinical practice to date as HPV testing for primary screening is being piloted in the NHS only now. However, as the pilot covers approximately 10% of women screened, it is likely that, through its impact on the pilot, the ARTISTIC41 trial has had a substantial impact on practice in the areas where the pilot is being conducted and on the health outcomes of women in the pilot regions. As the pilot is ongoing, and its results have not yet been published, it is not yet possible to say precisely what the impact has been on clinical practice (e.g. the costs of implementation) or the precise impact on patient health outcomes.
According to one interviewee, the ARTISTIC41 trial has probably also had an impact on the teaching of clinicians:
I’m sure it has. It depends what clinicians you are talking about. If you’re talking about gynaecologists and so on, probably it has. I’m sure it has become part of the canon of knowledge and evidence that people would be taught about.
One interviewee also stated the ARTISTIC41 trial has also had an impact outside the UK, primarily through its contribution to the Ronco et al. (2014)204 pooled analysis. The interviewee noted that:
Worldwide, we are all considering where we go next on HPV primary screening and how fast we can move and how safe it is to take each step. We’ve got well-established screening programmes. It’s not like a new programme where you’re just introducing a new test. You’re actually de-commissioning something that has been proven to work. It’s different to: shall we introduce lung-cancer screening, shall we introduce ovarian cancer screening, etc. We’re talking about getting rid of something that works well. The Pap test has saved millions of lives across the world and we’re now saying that we’re going to stop doing it.
Another interviewee noted that the impact of the European HPV trials may, collectively, be particularly important in developing countries that do not have the infrastructure for cervical cytology, as the results suggest that developing countries could safely introduce cervical cancer screening programmes that use HPV testing instead of cytology.
Stage 6: final outcomes
As noted above, HPV testing for primary cervical screening has not yet been adopted nationally in the NHS. However, if there is a national roll out of HPV testing for primary screening and the benefits of that screening are similar to those reported in the ARTISTIC41 trial, there could be substantial health gains. Although it would be difficult to attribute all of these health gains to the ARTISTIC41 trial, it undoubtedly made an important contribution to the evidence base that led to the pilot, which, in turn, may lead to national roll-out. However, when considering the potential health gains from national roll-out of HPV testing, one must also take into account the national HPV vaccination programme, which will decrease the overall benefit of HPV screening, as fewer women will acquire high-risk HPV strains. One interviewee noted that the most immediate impact of the ARTISTIC41 trial, should it result in changes to the Cervical Screening Programme, would be a reduction in cervical cancer among women who were beyond the age of the effect of the vaccine.
Implementation of HPV testing as a primary screening strategy would also have an important impact on the labour force. It would result in a substantial reduction in the need for cytoscreeners. According to one interviewee, some existing cytoscreeners would likely retire or be made redundant, whereas others would be re-trained to do viral testing instead of cytology. However, viral testing is much less labour intensive than cytology.
The ARTISTIC41 trial, together with the other European primary HPV testing trials, has likely had an impact on the development of new primary HPV screening tests. According to one interviewee:
It helped to provide substantial data to support HPV primary screening. Therefore, these big companies could see that if primary screening was coming down the line with HPV there would be a big market for the diagnostic kits.
According to another interviewee, the European trials have already had a big impact on the medical device industry. At the time that most of the trials started in the early 2000s, there were only two devices on the market, both of which were DNA diagnostic tests: Polymerase Chain Reaction and the Hybrid Capture Test. Now, according to the interviewee, there are eight or nine validated tests, and their development has caused a substantial decrease in the price of HPV tests.
Table of payback
Payback details for this case study are provided below in Table 17.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | Two HTA monographs and eight peer-reviewed publications Found that HPV testing is more protective than cytology over three screening rounds Found that the screening interval could be lengthened if HPV testing is used for primary screening for cervical cancer instead of cytology |
| Research Targeting and Capacity Building | Henry Kitchener obtained five project grants from the HTA programme after commencing the ARTISTIC41 trial One member of the research team obtained a PhD as a result of their involvement in the trial The ARTISTIC41 trial was included in a pooled analysis, which found that HPV testing decreases the incidence of cervical cancer The ARTISTIC41 trial data were used to model the effectiveness and cost-effectiveness of HPV vaccination |
| Informing Policy and Product Development | The ARTISTIC41 trial resulted in the piloting of HPV testing as primary screening in England |
| Health and Health Sector Benefits | The ARTISTIC41 trial has not yet had an impact outside those regions where HPV testing is being piloted, and the impact within the pilot regions has not yet been reported |
| Broader Social and Economic Benefits | Impact on the development of new HPV tests for primary screening |
Appendix 4.2: the Newborn screening for congenital heart defects study
Summary
The systematic review and cost-effectiveness analysis of the Newborn CHD study42 systematically reviewed the literature on outcomes of children with CHDs and compared the cost-effectiveness of different screening strategies. The study42 found that early detection of CHDs, through newborn screening, can improve the outcome of affected children, but that the current screening programme, which consists of clinical examination at birth and again at 6 weeks, is insufficiently accurate. The study42 concluded that pulse oximetry is a promising alternative screening strategy but that further research is required on its use for antenatal screening. The Newborn CHD study42 was the first to systematically review the evidence. It led directly to the HTA programme-funded PulseOx trial,220 which looked at the effectiveness and cost-effectiveness of using pulse oximetry to screen newborns for CHDs. The Newborn CHD42 study and the subsequent PulseOx trial,220 together with the increasing evidence on the effectiveness of pulse oximetry, resulted in the NSC undertaking a pilot project, the Newborn Pulse Oximetry Screening Pilot, which may result in a national roll-out of pulse oximetry screening for CHDs. Some neonatal units have already introduced pulse oximetry screening.
Introduction to case study
Background
Scientific background
Congenital heart disease is one of the most common causes of congenital anomalies. 221 In the UK, the incidence of CHDs is 6.4 per 1000 births. 222 Although many countries were using clinical examination to screen for CHDs at the time of the Newborn CHD study,42 no systematic review existed on the effectiveness of different newborn screening strategies. The systematic review and cost-effectiveness analysis of newborn screening for CHDs by Knowles et al. (2005)223 set out to (1) provide evidence that would inform policy decisions on newborn screening strategies for CHD and (2) identify priorities for future research in screening for CHD. The Newborn CHD study42 consisted of a systematic review of the published medical literature on outcomes of children with CHDs and a decision-analytic model to assess the cost-effectiveness of different screening strategies for CHDs in the UK. The alternative screening strategies considered included clinical examination at birth, and again at 6 weeks, with cardiac investigations for high-risk children and pulse oximetry or echocardiography, in addition to clinical examination. Pulse oximetry is a non-invasive test for measuring blood oxygen levels, which uses a portable oximeter that shines light from a probe attached to a newborn’s finger, toe or ear lobe to determine the percentage of haemoglobin saturated with oxygen. Echocardiography is a test that uses ultrasound to produce a moving, detailed image of the heart, which can provide information about the nature of a CHD.
Chief investigators’ background details
Carol Dezateux, Professor of Paediatric Epidemiology at the University College of London, was the CI of the systematic review on Newborn CHD. 223 Shortly after beginning the review, Dezateux became co-director of the UK Newborn Screening Programme Centre, a post that she held from 2002 to 2012. 224 While undertaking the review, Dezateux worked closely with Rachel Knowles at the Institute of Child Health, University College London. Shortly after beginning the Newborn CHD42 review, Knowles was awarded a MRC Clinical Research Training Fellowship in the Health of the Public, which she held from 2003 to 2007. 225
The case study approach
The data collection process for this case study involved a series of interviews and a review of the primary and secondary data sources relating to the Newborn CHD42 study. As shown in Table 18, four individuals were interviewed for this case study: the first author of the HTA report, the CI of the PulseOx trial,220 the Director of the NSC, and the Chief Executive of the Children’s Heart Federation.
| Interviewee | Reason for interview |
|---|---|
| Rachel Knowles | First author of HTA report and involved in all stages of the research |
| Andrew Ewer | CI of the PulseOx Trial220 |
| Anne Mackie | Director of the NSC |
| Anne Keatley-Clarke | Chief Executive of the Children’s Heart Federation |
The primary data sources for the case study included the interview data, the monograph in the NIHR HTA journal,223 the results of the economic analysis and value of information (VOI) analysis in the International Journal of Technology Assessment in Health Care,226 and a paper comparing parents and professional values on the quality of life of children with CHDs. 227 The background information reviewed for this study included current guidance of screening for CHDs,228 three studies on the effectiveness of clinical examination in detecting CHDs in the UK,229–231 the results of the PulseOx trial220,232 and the external review of screening for CHDs against the UK NSC’s programme appraisal criteria. 233
Stage 0: topic/issue identification
A number of key factors influenced the research team’s decision to work in this area, as detailed in Box 2 and described below. The Newborn CHD study42 was a commissioned call. Therefore, the key influencing factors outlined below include both the factors that motivated the initial commissioning of the study42 and the factors that motivated the research team to undertake the research.
-
CI’s longstanding interest in newborn and childhood screening interventions.
-
High prevalence of CHDs in the UK.
-
Poor performance of existing screening programmes in the UK.
-
No systematic evidence on the effectiveness and cost-effectiveness of different newborn screening strategies for CHDs.
-
Existing evidence suggested that echocardiography and pulse oximetry might be suitable for newborn screening for CHDs.
-
HTA-commissioned call.
Chief investigator’s longstanding interest in newborn and childhood screening interventions
Prior to the Newborn CHD42 study, Carol Dezateux had a history of interest in childhood and newborn screening interventions,234–249 which included both primary research and secondary research. Dezateux’s prior research into neonatal, newborn and childhood screening interventions ranged from antenatal and neonatal haemoglobinopathy screening250 to newborn screening for cystic fibrosis,246–248 newborn screening for sickle cell disease,245 childhood screening and surveillance for congenital and infantile cataract,242 surgery for congenital dislocation of the hip as a screening outcome measure,240 screening toddlers for iron deficiency anaemia241 and preschool vision screening. 237
High prevalence of congenital heart defects in the UK
It is likely that the high prevalence of CHDs in the UK contributed to the HTA’s decision to commission a review on the evidence on newborn screening for CHDs. At the time that the HTA commissioned the Newborn CHD42 study, a study by Wren et al. (1999)229 found that the prevalence of congenital anomalies was 7–8 per 1000 live-births. Christopher Wren, the lead author of that study229 and consultant paediatric cardiologist, was also involved in the Newborn CHD42 study.
According to the HTA commissioning brief:
Up to six in every 1000 live-born infants have a cardiovascular malformation. Most of these are asymptomatic at birth. Early recognition is important because clinical presentation and deterioration may be sudden and some treatable causes may cause death before diagnosis. Also, irreversible pulmonary vascular disease could be avoided by earlier ascertainment, and complications such as endocarditis reduced. Difficulties arise in the examination of the heart as the newborn period is a time of change for the cardiovascular system as adaptations continue to be made to extra uterine life. 223
Poor performance of existing screening programmes in the UK
It is also likely that the poor performance of existing screening programmes in the UK contributed to the HTA’s decision to commission the review. Current guidance on newborn screening for CHDs,230 which was put in place prior to the study by Knowles et al. (2005),223 recommends that the cardiovascular system of newborns be examined at birth and at 6 weeks. However, a previous population-based study by Wren et al. (1999)229 also found that more than half of all babies with undiagnosed CHDs were missed by existing neonatal examination and more than one-third were missed at the second clinical examination at 6 weeks. Similarly, another study published in the same year by Ainsworth et al. (1999)230 found that newborn examination detects only 44% of cardiac defects. In addition, an earlier study by Abu-Harb et al. (1994)231 found that only 31% of newborn infants with a life-threatening CHDs were identified at the newborn examination. Knowles et al. (2005)223 thus concluded that:
Current policy is associated with a low detection rates, especially for life-threatening defects. The newborn examination appears particularly crucial for such infants, most of whom will have presented with symptoms, collapse or death by the time of the second recommended screening examination at weeks of age.
No systematic evidence on the effectiveness and cost-effectiveness of different newborn screening strategies for congenital heart defects
The clear gap in the evidence on the effectiveness and cost-effectiveness of different newborn screening strategies for CHDs probably contributed to the HTA’s decision to commission the review. According to one of the interviewees, prior to the Newborn CHD42 review, there was no systematic evidence on the effectiveness and cost-effectiveness of different newborn screening strategies. According to the same interviewee, neonatal examination for CHDs had existed for many years in the UK but it had been implemented with very little evidence of its effectiveness.
Existing evidence suggested that echocardiography and pulse oximetry might be suitable for newborn screening for congenital heart defects
Technological developments in two different potential screening technologies, echocardiography and pulse oximetry, meant that their application to newborn screening for CHDs, in a population-level screening programme, could be feasible. 251 However, there was little systematic evidence on the effectiveness of those two screening technologies at the time.
Health Technology Assessment programme-commissioned call
In the context of the high prevalence of CHDs and the weak evidence underlying the current screening programme, the HTA put out a commissioned call for a systematic review and economic analysis of different strategies for newborn screening for CHDs. Specifically, the HTA commissioning brief identified the following research question for the systematic review: ‘What is the cost-effectiveness of auscultation and echocardiography in the detection of congenital heart disease in the newborn period and up to 1 year of life?’223
Interface A: project specification and selection
Carol Dezateux developed the original project proposal with Catherine Bull and Rachel Knowles. 223 At the time, Dezateux had substantial experience in research into newborn and childhood screening programmes,234–250 as noted above. Bull was a Consultant at Great Ormond Street Hospital for Children NHS Trust, and had previous experience in research in cardiology and CHDs. 252–262 At the time of the Newborn CHD42 study, Knowles, a public health doctor by training, was on a secondment to the Institute of Child Health at the University College of London to do some public health training, which resulted in her involvement in the study. Knowles had a history of interest in child health. 263
The commissioning brief asked that the systematic review should include the natural history of different CHDs, the properties of different screening tests of CHDs, the clinical impact of the different tests, the effectiveness of different management options for children who test positive for a CHD and the psychosocial impact of the test and diagnosis on patients. 223 The commissioning brief also requested cost-effectiveness modelling with sensitivity analyses. The research team modified the research question after their initial literature review to include pulse oximetry, as the evidence supporting the use of pulse oximetry for newborn screening for CHDs was published after the commissioning brief.
The Newborn CHD42 project had five aims. 223 The first was to conduct a systematic review of the epidemiology, natural history, treatment and outcomes of CHDs and the effects and costs of existing newborn screening strategies. The second was to classify different types of CHDs for newborn screening. The third was to assess the effects, costs and cost-effectiveness of different newborn screening strategies. The fourth was to assess the quality of life of children with a CHD, from the perspective of both parents and health professionals. The fifth was to explore parents’ experiences of newborn screening for, and diagnosis of, CHDs. The methods used in the study42 included a systematic review, a cost-effectiveness analysis and a VOI analysis.
According to one of the interviewees, the intention of the research team from the outset was to involve patients, clinicians and policy-makers in the later research stages, even although patients, clinicians (beyond the two cardiologists on the research team) and policy-makers were not heavily involved in the development of the initial research protocol. According to the same interviewee, Carol Dezateux had previously done a lot of work with the NSC and was aware of the issues of interest to the NSC. Similarly, the two cardiologists on the research team, Catherine Bull and Christopher Wren, had a long history of interest in population health and, according to the same interviewee, knew from past experience that it would be necessary to engage with clinicians early on to understand the implications of screening for clinical practice. Lastly, from the outset, the research team planned to involve the parents of children with congenital heart disease in focus groups to understand parents’ experiences of screening for, and the diagnosis of, CHDs.
According to an interviewee, the research team received feedback from the HTA on their initial application, which was used to refine the final application. However, it has not been possible to determine how the research protocol changed as a result of the feedback.
Stage 1: inputs to research
Financial
The research team received £59,749 in funding from the HTA. 42 No other funding sources for the study42 were identified in either the interviews or the document review.
Knowledge and expertise
The research team had considerable topic and methodological expertise prior to undertaking the Newborn CHD42 study. Carol Dezateux was the CI and, as noted above, had previously undertaken numerous studies on different neonatal and childhood screening programmes. 234–250 Jacqueline Brown was a Senior Scientist in the MRC Health Services Research Collaboration at the University of Bristol, with expertise in health economics. 264–268 Catherine Bull, as noted above, was a Consultant at Great Ormond Street Hospital for Children NHS Trust, and had substantial clinical and research experience in cardiology and CHDs. 252–262 Christopher Wren was a Consultant Paediatric Cardiologist at Freeman Hospital, and also had substantial clinical and research experience in cardiology and CHDs. 229–231,257,269–304 Rachel Knowles and Ingolf Griebsch had trained as a public health doctor and health economist, respectively, although both were close to the start of their research careers at that time.
Techniques
The Newborn CHD42 study used some relatively novel methods for the economic analysis. The research team developed a decision-analytic model, and conducted both deterministic and probabilistic sensitivity analyses, as well as an expected VOI analysis. Although these practices are relatively commonplace now, according to the Handbook of Health Economics ‘the theoretical application of [value of information analysis] VOI to clinical trial design and research prioritization is a relatively recent phenomenon (Claxton & Posnett, 1996; Meltzer, 2001)’. 305 In the Newborn CHD42 report, the authors note that the expected VOI approach had been highlighted as a potential tool for setting research priorities in health technology assessment,306–308 but that the methods had been applied to few health technology assessment studies. 223
Data sets
The study42 heavily relied on existing studies and data sets. Its purpose was to systematically review existing evidence and to extract data to inform the parameters of the decision analysis model. Therefore, the results from the review relied exclusively on published literature and data. Christopher Wren provided access to unpublished paediatric cardiology and epidemiological data sets from the Northern Region, also used to inform model parameters. The research team did also collect data on subjective probabilities for the economic analysis from experts for which no published data were available, while the remaining inputs into the economic model derived from the published, academic and grey literature.
Stage 2: research process
The study by Knowles et al. (2005)223 consisted of (1) a systematic review of the published medical literature on the epidemiology, natural history, treatment and outcomes of CHDs; (2) a cost-effectiveness analysis of alternative newborn screening strategies, which used a decision-analytic model and both deterministic and probabilistic sensitivity analyses, and a VOI analysis to explore the value of further research to policy-makers; (3) an empirical study on parental and clinician valuation of the quality of life of children with congenital heart disease, which used a visual analogue scale similar to that utilised by the European Quality of Life-5 Dimensions; and (4) an empirical study on parents’ perceptions of newborn screening for CHDs, which consisted of a systematic review of the literature and focus groups. 221
As noted above, the VOI analysis that was conducted for this study42 was a relatively novel method in health economics at the time. VOI analysis is used to estimate the potential benefit of collecting more information through increased research before making a decision. In the Newborn CHD42 study, the VOI analysis set out to identify the model parameters for which additional research would provide the most value. 223,226
The research team carried out focus groups in conjunction with Heartline (www.heartline.org.uk), which is a charity that supports the families of, and children with, heart conditions. 223 The focus groups were designed to include parents of children with CHDs, including both parents whose child had been diagnosed through an antenatal ultrasound scan, pulse oximetry, echocardiography and clinical examination, and parents whose child had become ill before receiving a diagnosis, as well as some parents whose child did not have a CHD. However, the final group predominantly included parents who had young children with more severe CHDs. In the focus groups, parents discussed their experience of having had their child screened for CHDs. Heartline allowed the research team to access the views of parents of affected infants. In the acknowledgement section of the report by Knowles et al. (2005),223 the authors note that ‘the authors would like to thank the national support group for families of children with congenital heart disease, Heartline, who provided us with invaluable assistance in recruiting and publicising the focus group’. According to one of the interviewees, this level of patient engagement was relatively novel at the time as it predated the current emphasis on the patient and public being involved in research, and the creation of organisations such as INVOLVE (www.invo.org.uk/).
A number of different individuals were involved in carrying out the research. Carol Dezateux, the CI, contributed to the development of the original project proposal, the review of the epidemiology of CHDs and the data extraction for economic model. 223 Rachel Knowles contributed to the review of the classification and coding systems for CHDs, the systematic review of outcomes of children with a CHD, the systematic review of parental views, the development and validation of the CHD classification for screening, the development of health states for the quality-of-life study, the organisation of the parent focus group, the compilation of the report on parents’ views, the systematic review for the model parameters, and the data extraction for the model parameters. Ingolf Griebsch conducted the review of cost parameters and contributed to the development of the decision analysis model and the expected VOI analysis. Jacqueline Brown contributed to the development of the decision analysis model and the expected VOI analysis. Catherine Bull contributed to the construction and validation of the CHD classification, the development of health states for the quality-of-life study, the organisation of the focus group, the development of the subjective probabilities for the model and the data extraction for the model parameters. Christopher Wren provided and analysed data from the Northern Region data set and contributed to the review of CHDs, the validation of the CHD classification and the development of the subjective probabilities. All members of the research team also participated in regular discussions and planning meetings, the administration of the questionnaire for the quality-of-life study, the development of the economic model, and the writing, proofreading and editing of the final report.
The research team did not have any specific international involvement in the study42 beyond the inclusion of research that originated from outside in the systematic review.
Adding value in research
Although the study by Knowles et al. (2005)223 was undertaken prior to the Chalmers and Glasziou (2009)14 publication on avoidable research waste and the NIHR’s introduction of the Adding Value in Research Framework,20 it is nevertheless feasible to assess to what extent the Newborn CHD42 study met all of the adding value in research criteria.
First, the research team set out to answer a question that was highly relevant to the users of research. The research question was prespecified by the HTA in a commissioning brief. However, the research team later altered the proposed research to include pulse oximetry so that the results would be of increased relevance to policy-makers. From the outset, the research team set out to address a high-priority question, with important outcomes, and involved clinicians in the research process. The research team did not, however, involve patients in setting the research agenda, but they did involve them in the research process.
Second, the research team used appropriate methods in the design, conduct and analysis of the study42 to address the question posed in the commissioning brief. The study42 did not identify an existing systematic review, but rather filled an important gap in the literature by conducting the first systematic review on the epidemiology, natural history, treatment and outcomes of newborns with CHDs.
Third, the research team completed the study42 within the timeline agreed with the HTA programme, received ethics approval from the Local Research Ethics Committee of the Institute of Child Health at Great Ormond Street Hospital Trust, and re-used existing data for all possible components of the study42 as well, to go above and beyond the commissioning brief by publishing a detailed paper on the economic analysis,226 and parents’ and clinicians’ valuation of the quality of life of children with congenital heart disease. 227
Fourth, the research team published the research findings in full in a HTA monograph and also used the same data to publish two additional studies,226,227 as noted above.
Lastly, the research team seems to have made every effort to produce an unbiased and usable report. The publications arising from the study42 clearly describe the research methods, the planned study outcomes and the interpretation of the findings in the context of the existing literature.
Stage 3: primary outputs from research
Knowledge
The Newborn CHD study42 resulted in the publication of two peer-reviewed papers and a HTA monograph. The publications cover the study results,223 the results of the cost-effectiveness model and VOI analysis,226 and the results of parents’ and professionals’ valuations of the quality of life of children with CHDs. 227 Figure 12 presents the results of the bibliometric analyses on the publications resulting from the Newborn CHD42 study.
The main results of the systematic review found that the prevalence of CHDs is 7–8 per 1000 live births, which accounts for 3% of infant deaths. 223 The study42 also identified a number of long-term conditions that result from CHDs, which included arrhythmias, infective endocarditis and pulmonary vascular obstructive disease. However, the review also identified important gaps in the evidence on long-term outcomes related to physical disability, the capacity to participate in normal childhood activities and neurodevelopmental, cognitive and psychosocial outcomes. The study42 also developed a classification for CHDs, which included three main types: life-threatening, clinically significant and non-clinically significant. Lastly, the review identified two alternative newborn screening strategies: pulse oximetry and echocardiography, in addition to clinical examination.
The economic analysis compared the cost-effectiveness of clinical examination to clinical examination with either pulse oximetry or echocardiography and found that the cost per additional timely diagnosis of life-threatening CHDs ranged from £4894 for pulse oximetry to £4,496,666 for echochardiography. 223,226 With the inclusion of clinically significant CHDs, the cost per additional diagnosis decreased to £1489 for pulse oximetry and £36,013 for echocardiography. The VOI analysis found that the maximum monetary value of additional research, at a cost-effectiveness threshold of £50,000 per timely diagnosis of a CHD, was £744,000 for the primary outcome, timely diagnosis of a life-threatening CHD, and £14,450,000 for the secondary outcome, diagnosis of a clinically significant CHD. The authors concluded that adding pulse oximetry to clinical examination is likely to be a cost-effective screening strategy for newborns, but that the VOI analysis supports targeting future research at reducing the uncertainty on the detection rate and false-positive rates for pulse oximetry.
The quality-of-life study compared the preferences of parents of children with CHD to those of health professionals for the longer-term health outcomes of children with CHDs, using a self-administered anonymous questionnaire with a visual analogue scale. 227 The study found that both parents and health professionals place similar values on the quality-of-life outcomes of children with a CHD, and agreed on the order of the ranking of different health states. Parents and health professionals both gave the lowest scores to health states with severe neurological disability.
The targeted review and focus groups found that parents support newborn screening for CHDs, prefer simple and accurate screening methods, experience negative psychosocial effects from poor management of the screening process and false test results, may experience short-lived anxiety in the event of a false-positive result until receipt of negative diagnostic test, and agree on the need for both universal screening standards and capable health professionals to discuss the outcomes of CHD screening. 223
Benefits to future research and research use
Capacity building and career development
Carol Dezateux had a well-established track record in childhood and antenatal research prior to the Newborn CHD42 study, and thus it is unlikely that the Newborn CHD42 study would have had a substantial impact on her career as it is one among her many past research projects. However, shortly after completing the Newborn CHD42 review, Dezateux was made a Fellow of the Academy of Medical Sciences in 2006, became the director of the University College London MRC Centre of Epidemiology for Child Health in 2007, a post that she held until 2012, and was awarded a Commander of the Order of the British Empire (CBE) for services to science in 2009. Dezateux is currently Director of the Life Study (www.lifestudy.ac.uk) and has ongoing research activities in CHDs, childhood and newborn screening, and paediatric epidemiology. 224
According to one interviewee, the Newborn CHD study42 probably had a positive impact on the careers of the two junior members of the team: Rachel Knowles and Ingolf Griebsch. Knowles has remained involved in CHD research, and, together with Dezateux, Catherine Bull and Christopher Wren, went on to set up a cohort study to monitor a group of children with CHDs – the UK Collaborative Study of Congenital Heart Defects. That cohort study, as well as Knowles’ other research since the Newborn CHD42 study, has resulted in a number of peer-reviewed publications. 309–326 Knowles also sits on the Newborn Pulse Oximetry Screening Pilot Project Board327 and sat on the NICE Guideline Development Group for Antenatal Care. 328 She also received a MRC training fellowship and funding from a British Heart Foundation grant. Griebsch went on to do additional work in health economics, which has resulted in a number of peer-reviewed academic publications. 226,329–346 Although it is not possible to attribute this later work by Knowles and Griebsch to their involvement in the Newborn CHD42 study, it was an important piece of academic work, to which they both made a substantial contribution, and it seems likely that it would have contributed to their subsequent academic careers.
Targeting of future research
The Newborn CHD study42 made three recommendations for further research. 223 The first was for research that would refine the detection rate of CHDs and provide further information on other aspects of pulse oximetry. The second was for more direct evaluation of antenatal screening strategies. The third was for investigation into the psychosocial effects of newborn screening for CHDs.
After the publication of the Newborn CHD42 study, the HTA commissioned primary research with the aim to reduce the uncertainties regarding the use of pulse oximetry as a population screening strategy. That commissioning brief resulted in the PulseOx trial,220 which was led by Andrew Ewer. According to one interviewee, the Newborn CHD42 study research team collaborated with the HTA programme in the drafting of the commissioning brief for primary research on pulse oximetry by advising the HTA on what the parameters of the study should be.
The PulseOx trial220 built on the Newborn CHD42 study by collecting primary data on the accuracy and associated costs of pulse oximetry in a UK setting. Ewer et al. (2012)220 developed a new decision-analytic model, described in detail by Roberts et al. (2012),347 based on the initial model developed in the Newborn CHD42 study by Knowles et al. (2005),223 which used the primary data on test accuracy and associated costs from the PulseOx trial. 220 Knowles et al. (2005)223 provided Ewer et al. (2012)220 with access to the initial decision-analytic model and the secondary data from the initial economic evaluation. According to one interviewee, the Newborn CHD42 research team designed the initial economic model so that it could be easily adapted, and had always intended to make it publicly available because of the research team’s understanding that the model was based on secondary data, but could likely be improved with the inclusion of primary data. Another interviewee noted that:
There’s very little trial detail in [the Knowles et al. ] study, so it didn’t influence the way [Ewer et al. ] undertook the protocol or developed the trial, but what it did was established fairly comprehensively the need for [the PulseOx] trial.
The Knowles et al. (2005)223 study was subsequently included in three reviews: a systematic review by Thangaratinam et al. (2007),348 a systematic review and meta-analysis by Thangaratinam et al. (2012),349 and a review of the current published literature by Knowles and Hunter (2013). 233 The Thangaratinam et al. (2007)348 review evaluated the accuracy of pulse oximetry as a screening tool for CHDs in asymptomatic newborns online, whereas the review by Knowles and Hunter (2013)233 formed part of the external review of the evidence for screening for CHDs against the programme appraisal criteria for the NSC, which includes an update of the cost-effectiveness model by Knowles et al. (2005)223 that incorporates evidence published since 2005. The most recent of these reviews, Knowles and Hunter (2013),233 concludes that routine pulse oximetry screening, in addition to clinical examination, is likely the most promising strategy. Knowles and Hunter (2013)233 also note that recent evidence reviews show that pulse oximetry has high specificity, moderate sensitivity and an acceptable false-positive rate, and that the addition of pulse oximetry to clinical examination would probably reduce the number of infants discharged from hospital before a CHD is recognised.
This view is reflected in the published literature that cites two of the publications from the Newborn CHD42 study: Knowles et al. (2005)223 and Griebsch et al. (2007). 226 For example, the Granelli et al. (2009)350 study on the impact of pulse oximetry on the detection of duct-dependent congenital heart disease in Sweden references the Griebsch et al. (2007) paper226 as the impetus for their trial in Sweden, which sought to reduce the number of false-positive results from pulse oximetry screening. A Swedish HTA report by Wennerholm et al. (2011)351 also references Knowles et al. (2005)223 in its review of the accuracy pulse oximetry screening, as well as Griebsch et al. (2007)226 in their review of the cost-effectiveness evidence. The Scientific Statement from the American Heart Association and American Academy of Pediatrics on the role of pulse oximetry in screening newborns for CHDs352 references both the Knowles et al. (2005)223 and Griebsch et al. (2007) papers226 in their systematic review. The Sendelbach et al. (2008)353 study references Knowles et al. (2005)223 as part of its rationale for conducting a prospective study on the feasibility and reliability of pulse oximetry screening in Texas.
A German study by Riede et al. (2010)354 on the effectiveness of newborn screening for congenital heart disease cites Griebsch et al. (2007)226 as part of their rationale for not looking at the costs of implementing pulse oximetry. Riede et al. (2010)354 note ‘in our study, we did not address the issue of costs which has been extensively examined by Griebsch et al. who came to the conclusion that POS [pulse oximetry screening] is at least cost-neutral in the short term’. Similarly, the study by Aamir et al. (2007)355 on the use of pulse oximetry to detect CHDs in New Jersey cites Knowles et al. (2005)223 as evidence that the cost of pulse oximetry is low. Hines et al. (2012)356 cite both Knowles et al. (2005)223 and Griebsch et al. (2007)226 in the background section of their evaluation of a pulse oximetry screening algorithm in New York as evidence that pulse oximetry is simple, cost-effective and useful as a complementary tool to prenatal ultrasound and postnatal clinical examination.
Interface B: dissemination
According to one of the interviewees, the research team kept Heartline informed of progress, and towards the end of the study42 held a workshop with NSC members and cardiologists to talk about the emerging findings. The research team also presented the results of their work at a Health Technology Assessment international (HTAi) Conference and the Royal College of Paediatrics and Child Health Conference.
Stage 4: secondary outputs
The study by Knowles et al. (2005)223 and the subsequent study by Ewer et al. (2012),220 together with the increasing evidence on the effectiveness of pulse oximetry, resulted in the NSC undertaking a pilot project – the Newborn Pulse Oximetry Screening Pilot. According to one of the interviewees, Knowles et al. (2005)223 led to Rachel Knowles’s role on the Newborn Pulse Oximetry Screening Pilot Project Board, as well as the NICE Guideline Development Group for Antenatal Care. The Newborn Pulse Oximetry Screening Pilot Project Board is responsible for the NSC pulse oximetry pilot work. The NICE Guideline Development Group for Antenatal Care assesses the evidence on topics relating to antenatal care to make recommendations for clinical practice.
The piloting of pulse oximetry will commence this year, in 2015. It will initially be piloted in six sites that are not yet using pulse oximetry in order to estimate the additional costs to the NHS of its implementation, because uncertainty remains regarding the likely extra burden on trusts from the follow-up of positive screening results, particularly in areas where there is not a cardiology centre close to the neonatal unit. In addition, the pilot will collect more information on the health services and cost implications of the pulse oximetry test identifying babies with low oxygen, but without a cardiac problem. The pilot project may prove to be a precursor to a national roll-out of pulse oximetry for newborn screening for CHDs, depending on its results. According to one of the interviewees:
What [the Newborn Pulse Oximetry Screening Pilot Board is] trying to do now is work out the feasibility of doing it on a national basis, what’s the right way to do it, who should do it, etc. So, it’s more the nuts and bolts of how it should happen rather than if or why it should happen.
Another interviewee succinctly summed up the current gaps in the evidence base regarding the likely impact of the national roll-out of pulse oximetry screening in the UK:
There are obviously a lot of little studies that have come out from different places in Europe about what you measure, how often you measure, what you do when you find something, etc. So actually there is not a consistency across those in terms of what you would expect if you were rolling out a national screening programme. You’d have everyone working to the same protocol.
The Knowles et al. (2005)223 review and related publications have also had an impact outside the UK. According to one of the interviewees, there is now a European initiative to start pulse oximetry screening in some other countries, such as France, Germany, Spain and Italy. The same interviewee also noted that the Sri Lankan government has approved the use of pulse oximetry for newborn screening for CHDs. The interviewee noted that:
I think it’s important to emphasize that this is not just a UK impact. There are many threads that lead to decisions, but I think the data that [Knowles et al. ] produced and [Ewer et al. ] produced have been inextricably linked into all of the national policies that are being or have been developed. There’s a lot going on.
In addition to the impact on policy, through the piloting of pulse oximetry, the Knowles et al. (2005) study,223 together with the subsequent work in the same area, has likely had an impact on the training of clinicians. According to one of the interviewees, trainees are very much aware of pulse oximetry now, which the interviewee noted is partly attributable to the Knowles et al. (2005) study223 and partly attributable to the Ewer et al. (2012)220 study.
According to another interviewee, since the publication of the Knowles et al. (2005) study,223 a number of new pulse oximetry devices have come on to the market. The interviewee noted that:
What the oximeter companies have done, obviously seeing the potential for every baby having this test, there’s a lot of oximeter machines to be sold as a result of that, they’ve focused on the particular difficulties of doing the test in babies.
According to the same interviewee, the software within the oximeters has been modified so that it is motion tolerant – so that if the patient moves, it is still possible to get a reading – and it has also been modified to work better in low perfusion states, i.e. to take into account that babies often have low perfusion at birth. It is not clear to what extent the Knowles et al. (2005) study223 contributed to these modifications in pulse oximetry devices but, as noted above, it is likely that, together with the more recent studies published on pulse oximetry, the study has had an impact.
Stage 5: adoption by practice and the public
A survey of 181 paediatric units, to which 77 sites responded, found that 21% (n = 21) of the units were already using pulse oximetry, 21% (n = 16) of those units not using pulse oximetry have plans to introduce it, 5% (n = 4) had no plans to introduce pulse oximetry, 21% (n = 16) of units were waiting for a UK NSC decision on whether or not to introduce pulse oximetry screening and 26% (n = 20) did not respond to the question regarding use or plans to use pulse oximetry. 357 It is not clear to what extent this uptake of pulse oximetry is attributable to the Knowles et al. (2005) study223 or to subsequent publications. However, as the Knowles et al. (2005)223 paper was among the first to highlight the potential benefits of using pulse oximetry for newborn screening for CHDs, it is likely that that paper has contributed to the subsequent uptake of pulse oximetry in the UK. Similarly, one of the interviewees noted:
It’s difficult to separate the impact of [the Ewer et al. ] study from the Knowles study, because they are linked. Without the Knowles’ study, [the Ewer et al. ] study would never have happened.
According to one of the interviewees, cardiologists and others differ in their views on whether or not they think using pulse oximetry to screen newborns for CHDs is beneficial or not. If neonatal units have a neonatologist within the unit who can produce echocardiograms then they might view pulse oximetry as beneficial because they can immediately address any issues if a baby has low oxygen saturation. The interviewee noted that the views of clinicians are likely to be influenced by whether or not they think pulse oximetry can identify children with a CHD and whether or not they can cope with the subsequent investigation of any children with low oxygen saturation screening results.
Stage 6: final outcomes
It is difficult to assess the health impacts of the findings of the study,42 as there has yet to be a national roll-out of pulse oximetry for newborn screening for CHDs. If pulse oximetry screening is introduced in the NHS, it is likely that a higher proportion of babies with CHDs will be diagnosed and treated earlier. However, it is also likely that a substantial number of babies with low oxygen, but no CHDs, will also be identified. The likely burden of follow-up examination of babies with low oxygen is not yet clear.
Some neonatal units have started using pulse oximetry, as noted above; however, it is not clear in what way they are using it, and what the resulting impact on health outcomes has been. In addition, because of the volume of work undertaken in pulse oximetry since the Knowles et al. (2005) study,223 it would be difficult to attribute any improved health outcomes to it and, for the same reason, it is not possible to determine the impact of the study on the sale of new pulse oximetry devices. However, as noted above, if the UK population is accruing health gains as a result of pulse oximetry, it is plausible that the Knowles et al. (2005) paper223 has contributed to those health gains, as it was among the first studies to highlight the potential benefits of its use for newborn screening for CHDs.
One interviewee elegantly summed up the challenge of attribution and the time lag in the benefits of the Newborn CHD42 study as follows:
I would say that in this area, in some ways, it’s quite a slow burn. Only now is the pilot being considered and embarked upon for pulse oximetry. I think sometimes looking at impacts too early means that you miss that these impacts can come at quite a long period of cumulative research and review and revising. It’s quite important to look at it long-term.
The Newborn CHD42 study was completed in 2005, but the final outcomes have yet to be determined. If a national screening programme for CHDs is implemented after completion of the pilot then the primary ‘final outcome’ of the study42 will have been achieved > 10 years after initial publication.
Table of payback
Payback details for this case study are provided below in Table 19.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | Three peer-reviewed publications First systematic review of the evidence on newborn screening for CHDs Found that pulse oximetry is a potential alternative screening strategy for CHDs and probably cost-effective Found that both parents and health professionals place similar values on the quality-of-life outcomes of children with a CHD Found that primary research into pulse oximetry would be of substantial value to decision-makers |
| Research Targeting and Capacity Building | The two junior members of the research team have experienced positive career progression since the publication of the study The study contributed to establishing a research network among its collaborators, with many of the authors continuing to work together after its completion Recommended funding primary research into pulse oximetry, which resulted in the PulseOx trial220 Included in three subsequent systematic reviews Cited in the international literature on pulse oximetry |
| Informing Policy and Product Development | Together with the PulseOx trial,220 resulted in the piloting of newborn pulse oximetry screening in the UK Impact on newborn screening policy outside the UK |
| Health and Health Sector Benefits | Newborn screening for CHDs in some neonatal units in the UK National roll-out of pulse oximetry would increase the early detection of newborn congenital heart disease but would also increase the detection of newborn babies with low oxygen but not a CHD |
| Broader Social and Economic Benefits | Development of new pulse oximetry technology, specifically for newborns |
Appendix 4.3: IVAN
Summary
The IVAN study43 was a multicentre, RCT comparing two drugs for the treatment of wet AMD, a chronic and progressive condition that is the leading cause of sight loss in older people. The study43 found that the two drugs, Lucentis (ranibizumab; Roche and Novartis) and Avastin (manufactured by Roche), were equally effective but that Avastin, which is much less expensive, was more cost-effective. However, by the time the trial reported, Lucentis was already approved for use in the UK (and several other countries), and as the patents for both drugs were ultimately held by the same company, there was no industry incentive to pursue a marketing authorisation for Avastin. Although there have been calls from the ophthalmology profession and at least one organisation representing people with wet AMD for NICE to review Avastin anyway, this has not yet happened and, consequently, it is not widely used in the NHS. There have also been threats of legal action from industry where Avastin has been used in the UK and internationally.
The IVAN study43 resulted in a number of peer-reviewed publications and had impacts on the career progression of the members of the team, helped establish a CTU at Bristol, and provided a ‘template’ for other trials in the field of ophthalmology in the UK and internationally. Alongside CATT358 (another trial of the two drugs in the USA), it has provided an evidence base that has supported the use of Avastin in the majority of cases of wet AMD in the USA, as well as in other countries, and is frequently cited as evidence to support an assessment of Avastin by NICE and the use of it in the NHS. The work may have contributed to a reduction in the cost of Lucentis to the NHS through renegotiations, and may have also influenced clinicians’ attitudes towards dosing. The work has the potential to save the NHS large amounts of money, but this has not yet been realised.
Introduction to case study
Background
Scientific background
Wet or neovascular AMD is a chronic and progressive condition that is the leading cause of sight loss in older people. 359 It is characterised by the abnormal growth of blood vessels in the macula and manifests as a blurring of the central visual field. In the mid-2000s the standard treatment for wet AMD was photodynamic therapy, but new drugs were beginning to emerge, which acted to block the action of VEGF, a protein that stimulates the growth of blood vessels (further details on treatments are provided in Emerging treatments for wet age-related macular degeneration, below).
Chief investigator’s background
The CI, Professor Usha Chakravarthy, is based at the Department of Ophthalmology at Queen’s University Belfast. After undertaking her medical training at the University of Madras, India, she moved to the UK and completed her PhD. Prior to the IVAN43 study, she worked on a number of other studies in AMD, including the verteporfin photodynamic therapy (VPDT) cohort study360 at Queen’s University Belfast in 1987, which preceded the IVAN study,43 and was also supported by the HTA programme.
The case study approach
The case study was based on a review of the relevant literature and four interviews, as listed in Table 20.
| Interviewee | Reason for interview |
|---|---|
| Usha Chakravarthy | CI |
| Barnaby Reeves | Co-investigator; Co-Director of the Clinical Trials and Evaluation Unit, University of Bristol |
| Dan Martin | CI of the CATT358 study |
| Deborah Cohen | Investigations Editor at the British Medical Journal |
Stage 0: topic/issue identification
A number of key factors influenced the research team’s decision to work in this area, as detailed in Box 3 and described below.
-
Emerging treatments for wet AMD.
-
Cost of Lucentis to the NHS.
-
The most effective way to use anti-VEGF treatments was unclear.
Emerging treatments for wet age-related macular degeneration
In the mid-2000s the standard treatment for neovascular AMD was VPDT. This was moderately effective at preventing further vision loss, but did not result in clinically significant recovery of visual acuity for most patients. 361 A previous HTA programme-funded study on VPDT, carried out by some members of the IVAN43 team (Chakravarthy, Reeves, Harding), had also found that many patients stopped the treatment. 360
Anti-VEGF treatment was developing fairly rapidly at the time (Chakravarthy interview) and one compound was already in use [Macugen (pegaptanib), Pfizer]; however, its effectiveness at improving visual acuity was similar to that of VPDT. 361 In 2005, evidence began to emerge on the effectiveness of another anti-VEGF agent, Lucentis (ranibizumab), which was shown to be more effective than photodynamic therapy (PDT) and Macugen in terms of visual acuity outcomes. 362,363
As it takes some time for a new drug to be assessed and licensed, some clinicians turned to Avastin (bevacizumab) in the meantime,364 another anti-VEGF compound already licensed for use in colorectal cancer. Bevacizumab is the parent molecule from which ranibizumab was derived, and initial studies suggested that Avastin might be superior to the existing licensed treatments: Macugen and PTD. 361,365 Within 6 months of these first case reports, use of Avastin off-label had spread across the world because of its apparent efficacy, short-term safety and low cost, despite it being licensed for use only in several forms of cancer. 366 It was estimated that within 6 months, more than 50,000 eyes had been treated using Avastin in the USA, with no RCT evidence, safety data or comparative data (Martin interview).
Lucentis was then licensed for use in the treatment of neovascular AMD in the USA in 2006, and in the European Union in January 2007. Avastin remains unlicensed for use in neovascular AMD. The patents for both Lucentis and Avastin are held by Genentech, a subsidiary of Roche, but Novartis holds the European marketing rights to Lucentis.
At the time of the commissioning of the IVAN study,43 Lucentis had not been recommended for use in the NHS by NICE and no direct, systematic comparison of Avastin and Lucentis had been conducted.
Cost of Lucentis to the NHS
Although Lucentis was effective in improving outcomes for neovascular AMD patients, as noted above, it was two or three times more expensive in comparison with the other treatments licensed at the time (Reeves). Importantly, it was also far more expensive than Avastin, a dose of which cost significantly less, as a result of splitting up doses licensed for cancer treatment into minute amounts. 367 The IVAN43 team had developed good relationships with the health authorities at the time and were able to discuss with them the emergence of new treatments and the potentially large costs that they might face if Lucentis became the standard treatment for neovascular AMD. The issue was politically high profile from the start, with a number of discussions taking place in the House of Commons about the difference in cost between Avastin and Lucentis, and the availability of both on the NHS (Cohen interview).
The most effective way to use anti-vascular endothelial growth factor treatments was unclear
Although part of the rationale of the study43 was the dramatic difference in the cost of Lucentis and Avastin, the team was also open to the possibility that Avastin might also be more effective (Reeves interview). As this was not specifically a hypothesis that the trial aimed to test, the use of anti-VEGF drugs was still relatively new at the time, and there remained a number of uncertainties about how they should best be used. There was some speculation that as Avastin was a larger molecule it might be retained in the eye for longer (Chakravarthy interview). In relation to this, an important aspect to explore was the frequency of treatment, and, in particular, whether or not treatment needed to be continuous or could be provided on an ‘as-needed’ basis.
Interface A: project specification and selection
A number of the researchers involved in the IVAN43 trial had been working together for several years prior to its development, in particular on the HTA programme-supported VPDT cohort study,360 looking at the use of verteporfin in photodynamic therapy (Usha Chakravarthy, Simon Harding, Barnaby Reeves). This study was initiated in 2004, and following the publication of key papers on the effectiveness of Lucentis in 2006 (the ANCHOR and MARINA studies362,363), the team began planning the IVAN43 study and proposed it to the HTA programme.
Other aspects of the VPDT cohort study360 were also important in the design of the IVAN43 study. The earlier study had established a large retinal research network of centres, which was cited in the IVAN43 study protocol as central to the new trial’s feasibility. The VPDT cohort study360 had also had representation on its steering committee from the Macular Society, with which the study team had established a longstanding relationship. The Macular Society was very supportive of the IVAN43 trial and was involved throughout the study, including on its steering committee.
The design and initiation of the CATT358 and IVAN43 trials proceeded in parallel over the period 2007–8, with the two research teams discussing their plans on several occasions to ensure that their data could be pooled when the trials completed.
The IVAN43 team also interacted with the HTA programme during the inception of the trial, as there was some urgency in beginning the work. Reeves commented that the study43 would have been much more difficult to conduct had NICE already recommended the use of Lucentis for neovascular AMD (this recommendation was issued in August 2008). Although this did not mean that the application process was altered or bypassed in any way, the HTA programme did expedite the process as much as possible. Reeves commented in interview:
Of all the funders I’ve ever dealt with, I’ve always found the HTA to be the most reasonable and interested in making the research happen. It was and continues to be an extraordinarily refreshing process compared to other funders I have worked with.
Stage 1: inputs to research
Financial
Funding for the study43 was provided by the HTA programme (£3,320,522) and the team did not approach any other funders prior to this. Reeves commented that the HTA programme was the obvious place to go for funding, with the only other realistic option at the time being the MRC. The HTA programme had also funded the group’s previous VPDT study.
In addition to the HTA’s funding of the research costs, NHS commissioning groups funded the treatment costs of the trial, which were substantial given the costs of the drugs involved (around £10M). Extensive collaboration and support was needed from commissioners, and there were several in particular who were helpful in negotiating with colleagues and facilitating the establishment of a central reservoir of funds to which different regions were willing to contribute. From this pooled funding, a pharmacy was commissioned to provide the drugs in the correct formulation.
Knowledge and expertise
The study43 was carried out by a multidisciplinary team, many of whom had worked together before, including on the PDT study. In addition to Chakravarthy, the team also included a number of other retinal experts: Simon Harding, Andrew Lotery, Susan Downes and James Talks. Reeves, whose research background was in ophthalmology (although it was not a primary focus of his work at the time), managed the trial in the Clinical Trials and Evaluation Unit at the University of Bristol, where Chris Rogers, the statistician on the study, and Lucy Culliford, the trial manager, were also based. This trials unit had also been involved in the latter stage of the VPDT Cohort Study,360 which Reeves brought with him from London when he moved to Bristol.
In addition to the ophthalmologists and the trials unit, the third important area of expertise was the independent Reading Centre, which received and graded the images generated during the study,43 and was masked to treatment allocation. Chakravarthy, Harding and Tunde Peto (Moorfields Eye Hospital) managed the UK Network of Ophthalmic Reading Centres at the time.
Drugs
A crucial input to the study43 was the drugs that were being tested, the supply of which proved challenging in the absence of support from the pharmaceutical companies that produced them. The first manufacturing pharmacy the team began working with subsequently discontinued negotiations, with representatives stating that collaborating with the IVAN43 trial was likely to jeopardise ongoing and future contracts with pharmaceutical clients (Reeves interview). Instead, the team began what has since become a long-term relationship with a NHS pharmacy in Liverpool. This pharmacy was able to produce prefilled syringes of Avastin, which they were then able to mask to prevent clinicians and participants from knowing which drug the participants were receiving. The pharmacy also developed the appropriate assays to quality assure the potency and stability of the drug in prefilled syringes, extending the expiry time after production from 2 weeks initially to 3 months by the end of the first year of the trial. This shelf-life is short compared with Lucentis (expiry date 2 years after manufacture) and caused some complexities with stock control of supplies of Avastin at sites. The trials unit and the pharmacy also collaborated to devise a way of maintaining a cold chain to deliver the drug around the UK (Reeves interview).
Stage 2: research process
The IVAN43 study was a multicentre, randomised controlled factorial trial. Participants were randomised to one of four arms:
-
Lucentis, continuous treatment
-
Lucentis, treatment only as needed after the first 3 months (‘discontinuous’ treatment)
-
Avastin, continuous treatment
-
Avastin, treatment only as needed after the first 3 months (‘discontinuous’ treatment).
Participants and clinicians were masked to the drug received, but not to the treatment frequency for both practical reasons and to allow the investigation of patients’ views on different treatment regimens.
Although the techniques used in the study43 were not new per se, the team was working in ‘uncharted territory’ in a number of ways. This was the first major non-commercial pharmacological trial that the UK had engaged with in ophthalmology, and of the 23 centres that participated, fewer than half had experience of recruiting patients to trials (Reeves interview). Similarly, the trials unit at Bristol had not conducted a trial on this scale prior to IVAN,43 and so although both Chakravarthy and Reeves commented that they would not make major changes to the study43 if conducting it again, there may be aspects of the process that could now be done more efficiently with the benefit of hindsight.
A number of related trials were undertaken in other countries around the time of IVAN,43 most notably the CATT study358 in the USA. In July 2005, the results from the first Phase III trial of Lucentis (MARINA) were presented. 363 This was followed shortly after by the ANCHOR study, which supported MARINA’s finding that Lucentis was superior to existing licensed treatments. 362 The CATT trial358 to compare Lucentis and Avastin was devised in 2005 and funded by the National Eyes Institute 3 weeks before Lucentis was approved by the Food and Drug Administration (Martin interview). This order of events meant that at the time of the conception and design of the trial the study team was unaware of the cost difference that there would be between the two drugs. Once this became clear there was a huge amount of interest in the trial.
Like the IVAN43 team, the CATT team358 experienced some challenges in conducting their study without industry support, both because of resistance from the pharmaceutical companies and difficulties in ensuring the supply of drugs for the trial. The nature of the problems was slightly different though. Whereas in the UK, the challenges related to producing, distributing and masking Avastin, in the USA it was the cost of Lucentis that proved problematic (Martin interview). The law at the time prevented Medicare from covering treatment costs for participants in clinical trials, even when these were the routine services that would have been received in the absence of the trial. This meant that the trial would need to fund US$25M of costs for Lucentis for the duration of the study. In 2007, the Medicare regulations were changed to cover 80% of the cost, but this left a substantial co-payment that one group of participants in the study would need to cover. This could have led to differential dropout in the two groups, and also raised major challenges for masking the drug being used (given that drugs are identified in billing documents and insurance company statements). In the end, the CATT team358 proposed an amendment to the Medicare Improvements for Patients and Providers Act of 2008, which allowed alternative payment mechanisms to be developed for National Institutes of Health-sponsored trials if they are needed to enhance the trial’s scientific integrity. 368
There was extensive interaction between the IVAN43 and CATT358 study teams while the studies were under way. Regular phone calls took place, and at each international ophthalmology meeting, for a number of years, the two teams scheduled a face-to-face meeting. The main aim of these was to ensure that any meta-analyses subsequently conducted could be of sufficient quality, and to enable the pooling of data from the two studies to create larger samples for exploring aspects such as safety. Although IVAN43 was a smaller study, it involved more intensive data collection, including on near-versus-distance vision, contrast sensitivity, reading speed, VEGF levels and other biochemical measures.
Other trials followed slightly behind IVAN43 and CATT358 – in France,369 Norway,370 Austria371 and Germany (VIBERA trial, status unknown) among others. There is a strong collaborative spirit in the field and the IVAN43 team had interactions with these research groups, sharing their protocol and advising on aspects such as dosing and parameters for efficacy (Chakravarthy interview).
The study43 team also had interactions with a number of other stakeholders throughout the study, and in particular before finalising the study’s protocol, including the Royal College of Ophthalmologists (RCO), commissioners and clinicians. The Macular Society was involved in the drafting of the information sheet for patients, ensuring that risks were clearly explained and that none of the details was confusing or misleading. Despite frequent claims from the pharmaceutical industry of safety issues, the Macular Society has continued to support the team’s work, including for the current long-term follow-up of the trial cohort (Reeves interview).
The team had frequent interactions with the HTA programme through the steering committee and data management committee. Regular updates were provided and the HTA programme remained aware of progress throughout. As a result, there were no concerns about granting a 3-month extension to the study43 when requested by the team (Chakravarthy interview).
The IVAN43 team did not interact with the pharmaceutical industry during the study, as the relevant companies were not supportive of the trial and raised a number of challenges during its course. As touched on above, the CATT team358 in the USA also encountered similar issues (Reeves and Martin interviews). Reeves commented that ‘everybody was terribly risk averse and the attention of the manufacturers to what we were doing was fairly intense’.
While in a trial of this kind there was little room for informal exploration or departures from the protocol, the team was able to build in to the design a number of bolt-ons to ensure that maximum use could be made of the study’s43 data. This included creating a serum and DNA biobank to analyse genetic interactions with the drugs, and an exploration of mechanistic aspects of VEGF metabolism – although this was of little direct relevance to the aims of the HTA programme, the samples collected were then able to be used in subsequent analyses funded from other sources.
Stage 3: primary outputs from research
Knowledge
The HTA monograph for the study43 will be published later in 2015, but a bibliometric summary of the main publications to date is provided in Figure 13. Papers were published reporting the 1- and 2-year findings of the study, both showing that Lucentis and Avastin had similar efficacy and safety. 372,373 This was in line with the findings from the CATT study,358 which reported results about 1 year ahead of the IVAN43 study, mainly because it recruited more quickly. 358,364
Both studies43,358 also looked at dosing frequency, which Martin considered to be the most interesting aspect of their findings. Both demonstrated very slightly better visual outcomes for patients in the continuous treatment arms, whereas the pooled safety data showed a slight increase in serious adverse events in the discontinuous arm. However, both studies also highlighted the need to consider the difference in cost between the two treatment regimens.
Martin commented that generating data on the provision of treatment only when needed has enabled a better understanding of the reasons why treatment is needed and an exploration of possible predictive factors. Work on this and other aspects of the original trials is still ongoing, including as a collaboration between the IVAN43 and CATT358 teams. Indeed, the IVAN43 team is still writing papers and plans to publish several more during 2015, whereas CATT358 researchers still have 19 related projects under way and continue to publish findings frequently.
Following the publication of the main findings, a pharmacogenetics paper was also published by the IVAN43 team, looking at genetic associations with patients’ responsiveness to anti-VEGF drugs. 374 This aimed to replicate three previously reported associations and to identify any novel associations from the trial. Although no associations were found, either novel or previously reported, Reeves commented that this was still an important paper to publish.
A cost-effectiveness assessment was also published following the main findings. 375 This estimated that the NHS could save at least £102M per year by switching from Lucentis to Avastin.
Given the concerns expressed about the relative safety of Lucentis and Avastin, an important output from the IVAN study43 and other trials taking place around the same time was a Cochrane review on safety. 376 As the IVAN43 and CATT358 findings had suggested, the review concluded that if there is a difference in the risk of adverse reactions between the two drugs, the difference is likely to be small, and noted that ‘health policies for the utilisation of ranibizumab instead of bevacizumab as a routine intervention for neovascular AMD for reasons of systemic safety are not sustained by evidence’.
As mentioned above, the IVAN43 team continue to publish results from the study and plan to submit a number of manuscripts in the first few months of 2015. Reeves commented that the only data generated from the study43 which are not (or will not be) in the public domain are some of the work on acquiring the evidence to show the safety, stability and potency of Avastin after its repackaging in prefilled syringes for the study. This information was considered to be ‘commercial in confidence’.
Benefits to future research and research use
Capacity building and career development
Although there was no direct training of students as part of the IVAN43 study (something that Reeves commented is difficult to include in RCTs of this nature), the study has been beneficial for the careers of those involved, a number of whom have received NIHR Senior Investigator awards on the basis of it (Chakravarthy interview). The group has also continued to work together, expanding their collaborations and initiating a number of new studies.
The IVAN43 study was particularly important for the trials unit at Bristol, which was fairly small at the time the study began. With another trial that was being conducted at the time, the IVAN43 study enabled the unit to apply for UKCRC registration and expand rapidly in both number of staff and expertise. Reeves commented that this would not have been possible without the IVAN43 study.
More broadly, Chakravarthy commented that the IVAN43 study has done a lot to advance ophthalmology research in the UK. As the first large-scale RCT in ophthalmology in the country, it served as a flagship trial and established models and structures that have benefited subsequent studies. For example, a large number of people in NHS trusts across the country participated, leading to an influx of resources into eye departments to support retina research. This has been sustained beyond the completion of the trial (Chakravarthy interview).
Through the study, the various centres involved also learned how to do research; training clinicians in these centres had been a vital part of the IVAN43 study, and, ultimately, may also have contributed to better care in these hospitals (Reeves interview). As Reeves commented:
I don’t have any doubt that doctors who take part in high quality clinical trials treat patients better. It’s intangible, it’s difficult to get evidence for, but that kind of research awareness really rubs off on how you run services and so on.
Interviewees commented that an increased awareness of research among clinicians has led to more enthusiasm for, and interest in, initiating and participating in studies following the IVAN43 study. The establishment of NIHR Clinical Research Networks, for which Chakravarthy is the national lead for ophthalmology, also helped IVAN hugely. Since IVAN,43 another dozen or so major studies have come into the retina network, and these were able to use the IVAN43 study as a template (Chakravarthy interview).
In terms of other study infrastructure, the Reading Centre enterprise had been established for the VPDT cohort study360 before its use in IVAN,43 but through the study it became more strongly established and was put on a firmer foundation (Reeves interview).
Targeting of future research
Although the original IVAN43 work finished in early 2014, an extension to the study has allowed the team to bring back participants for a final eye examination 4–5 years after recruitment to look at the longer-term effects of the treatments. The CATT study358 in the USA was funded to look at outcomes over 5 years, and Martin considers it very important that the IVAN43 study is also able to provide data over this time frame so that data can be pooled. In addition, as mentioned above, there are still other aspects of the study that members of the team are analysing and writing up for publication.
Reeves commented that everything that was done as part of the IVAN43 trial shaped the work that has followed, as the IVAN43 study was the first of its kind in the UK. As a result, the methods put in place for aspects such as the coding of adverse events have fundamentally changed data collection and coding for all of the studies that members of the team have since worked on. 377
As mentioned above, the IVAN43 study has helped shape the design of many subsequent trials, including a number of RCTs in other countries that began during the course of IVAN. These included the GEFAL (Groupe d’Evaluation Français Avastin vs Lucentis) study in France,369 the MANTA study in Austria,371 the LUCAS (Lucentis Compared to Avastin Study) study in Norway370 and the VIBERA [Prevention of Vision Loss in Patients With Age-Related Macular Degeneration (AMD) by Intravitreal Injection of Bevacizumab and Ranibizumab] study in Germany (status unknown). As noted above, the IVAN team shared their protocols with the studies that followed, and a recent meta-analysis which included several of these trials confirmed earlier meta-analyses that had demonstrated the non-inferiority of Avastin. 378
Interface B: dissemination
Findings were disseminated primarily through the major international ophthalmology meetings. A large number of abstracts were presented at the Association for Research in Vision and Ophthalmology annual conferences, as well as at the American Academy of Ophthalmology, EURETINA (European Society of Retina Specialists) and the International Congress for Ophthalmology. IVAN’s 1-year findings were presented at the same time as CATT’s 2-year findings,358 so to some extent CATT358 had prepared audiences for the results. Nevertheless, European audiences in particular were keen to see the UK results and the fact that the two studies produced consistent findings was very powerful. When the 1-year results of the two studies were combined, they showed very tight confidence intervals around the pooled beneficial effects. The pharmaceutical industry still raised concerns over the safety of Avastin, but these were largely addressed in the subsequent Cochrane review. 376
Reeves was also keen to disseminate the methodological lessons more widely, and so, in addition to ensuring that they were included in the HTA monograph, he also presented them at national trial methods meetings when possible.
Findings were distributed to a wider audience throughout the study. 43 As they emerged, data were made available through a regular newsletter to all stakeholder groups. Participants were informed of the findings from both IVAN and other studies that were ongoing, and the team presented in patient group meetings run by the Macular Society.
Stage 4: secondary outputs
The HTA monograph, which is currently in press, includes a meta-analysis of all of the outcomes for which this was possible, and the 2-year findings Lancet paper included a meta-analysis of visual acuity findings with CATT. 372 As noted above, the IVAN team also contributed to a Cochrane review on safety, which was published in July 2014. 376
Since 2008, NICE has recommended the use of Lucentis for neovascular AMD and no assessment of Avastin has been undertaken. Such an assessment would require the DH to commission NICE to carry it out, but this is made more difficult by the fact that the drug’s manufacturer has not applied for a licence to market Avastin for a new indication of neovascular AMD. 379 There is no commercial incentive for the pharmaceutical industry to carry out the relevant safety trials and apply for a licence, as both patents are held by the same company. 380 However, in December 2014 NICE was asked by the DH to begin developing a guideline for the diagnosis and management of macular degeneration. The scoping workshop for this guideline was held in April 2015, and it is expected that the guideline will be published in August 2017. 381
The guidance produced by the RCO does not rule out the use of Avastin, but states that if used:
It is extremely important to inform patients that it is unlicensed for this indication and that it has not undergone the usual rigorous clinical trials and independent evaluation by regulatory authorities. 382
The RCO has also encouraged an assessment of Avastin, as mentioned in, for example, its 2011 Annual Report:
While we continue to press the Department of Health (DH), the National Institute of Health and Clinical Excellence (NICE) the General Medical Council (GMC) and the NHS Executive to provide national guidance on the use of Avastin and Lucentis, off-label vs licensed drugs, we decided that we had to provide our own guidance that our members rightly demanded. This process culminated in the publication of the report of the working group and the College statement on the issue, both of which have been welcomed by the vast majority of members who prescribe these medications. 383
This report383 was accompanied by a statement citing the College’s working group’s findings that Lucentis and Avastin are equally effective and have a similar safety profile. The statement highlights the difference in cost to the NHS between the two (£740 per injection for Lucentis; £60 per injection for Avastin) and states:
The College believes that the NHS executive should urgently instruct NICE and the Medicines and Healthcare Products Regulatory Agency (MHRA) to evaluate the use of Avastin in the treatment of AMD and produce National Guidelines for the use of anti-VEGF agents in AMD. 384
The IVAN43 study’s findings have also been highlighted more broadly in the media, in particular because of the potential cost savings to the NHS. In January 2012, the Financial Times reported on the increasing pressure to assess Avastin for wet AMD:
Sir Michael [Rawlins] acknowledged there were intensifying pressures on the NHS to use the cancer drug Avastin beyond its regulatory authorisation to treat the eye condition age-related macular degeneration (AMD), rather than the more expensive approved treatment Lucentis. That would only intensify as fresh evidence emerged that Lucentis can treat the still more widespread eye condition of diabetic retinopathy, he said.
But in response to recent calls by the Royal College of Ophthalmologists for NICE to review the use of Avastin for AMD, he ruled out that any unilateral assessment should be launched by his agency. He said ministers should take the lead. ‘There is no way we would touch that without a request from the secretary of state [for health],’ he said.‘ ‘The costs are eye-watering. It is much better for [the government] to refer it to us. Their eyes are wide open. They know the issues’. 385
The General Medical Council (GMC) began a consultation on its guidance in 2011, which considered the possibility of inserting a clause allowing the use of off-label unlicensed drugs in cases in which the evidence shows that it is as safe and effective as the licensed alternative. However, following legal advice, the GMC reverted to its previous stance that an unlicensed drug should not be used on the basis of cost if a licensed alternative exists. 386,387
Stage 5: adoption by practice and the public
To date there have been no widespread changes in practice in the UK, because of the absence of clinical guidance and a lack of clarity around the legality of using Avastin: where there is a licensed alternative, that is the preferred choice. Reeves commented that many clinicians were very receptive to the idea of using Avastin, particularly given IVAN’s43 findings and the drug’s widespread use in the USA and elsewhere, but they were put off by a lack of clarity around whether or not it would be legal for them to prescribe it and an apparent lack of support from the GMC. 380 Certainly there is widespread awareness of the study’s findings among clinicians (Chakravarthy interview). Outside the NHS, Avastin is used to some extent in private sector treatment.
The Southampton, Hampshire, Isle of Wight and Portsmouth Primary Care Trust (PCT) cluster did, at one point, look to commission an Avastin-based service. Although NHS trusts must offer NICE-approved drugs, it was argued that there was nothing to prevent them from also offering other options and providing patients and clinicians with a choice. However, Novartis filed for a judicial review on the grounds that the cluster was using an unlicensed drug in order to save money when a licensed alternative was available. The case did not make it to court, in part because Novartis reduced the price of Lucentis through the provision of a patient access scheme, but also because the NHS was being restructured at the time and PCTs were being abolished (Reeves interview). The failure of this case to reach court as a test case means that there is still uncertainty over the legality of using Avastin in the NHS. Cohen argues that drug licensing relates to restrictions on only the indications for which a drug is marketed and not to the indications it can be used to treat. 386 Mike O’Brien Queen’s Counsel (QC), the former health minister, stated that:
The health secretary should condemn what Novartis is doing. Andrew Lansley should refuse to allow the NHS to be bullied by Novartis. The interests of the patient and the NHS must come first. 380
However, there was also support at senior levels for Novartis’s decision to push for the review, and at the time of the case being dropped, Sir Michael Rawlins, Chairman of NICE, suggested that more organisations should sue the NHS to ensure the uptake of NICE-approved drugs. 388 Cohen did comment, though, that other PCTs continued to offer a choice of drugs and have not been taken to judicial review. 386
In contrast with the UK’s very low level of use of Avastin, it is much more widely used in other countries. Reeves commented that at no time since Avastin was first used in the USA (which was before Lucentis was licensed) has the use of Lucentis exceeded the use of Avastin, despite Avastin never having been licensed for use in the eye. It is also widely used in developing countries where the cost of Lucentis is prohibitive.
In Europe, the Italian government initially approved Lucentis, but as the evidence grew for the effectiveness of Avastin, it attempted to introduce an Avastin-based service instead. The European Federation of Pharmaceutical Industries and Associations, the European Confederation of Pharmaceutical Entrepreneurs and the European association for bio-industries proceeded to file a complaint with the European Commission, arguing that the government was risking public health for purely budgetary reasons by promoting the off-label use of drugs. 389 The Italian government responded by fining Novartis and Roche €182.5M, accusing them of colluding to prevent the use of Avastin and overstating the dangers of its use. 390 Martin commented that this decision was largely related to the overstatement of Avastin-related risk by the pharmaceutical companies of the CATT study358 data. The claimed increased risk has not been confirmed in any other study (Martin interview).
In another instance, the European Commission brought a case against Poland, contending that a national law allowing off-label use of drugs was illegal when a licensed alternative was available on the national market. The European Court of Justice found that Poland had not fulfilled its obligations under European law and reiterated that public health must take priority over financial or economic considerations. 391 It has been suggested that this ruling provides some clarity to the debates around the use of Avastin in the UK and might suggest the likely outcome of future cases brought to court. 392 There still appears to be some conflation, though, between marketing a drug (for which a licence is required) and using a drug off-label, concepts which are governed by separate pieces of legislation. 386
Although there has not been a change in the use of Lucentis and Avastin in the NHS, the IVAN43 study may have contributed to a reduction in the price of Lucentis by way of the patient access scheme, and so generated savings for the NHS (Chakravarthy interview; Reeves interview). Even with the patient access scheme, though, the cost of Lucentis is still much higher than Avastin, and in 2013–14 the NHS spent £244M on Lucentis, the second largest spend on any drug. 393 Additionally, as anti-VEGF drugs are biologicals rather than chemically synthesised drugs, they cannot simply be produced as generics after their patent has expired. However, a number of biosimilars are reported to be in development. 394
There may also have been changes in the way that clinicians think about dosing, potentially contributing to improved care. This is due to the comparison in both the IVAN43 and CATT358 studies between continuous and discontinuous regimens providing a much richer knowledge of when and why treatment is needed (Martin interview).
Stage 6: final outcomes
Reeves commented that a frustration following the IVAN43 study is that, when taken at face value, a £12–15M investment (of which £3.5M was research costs) does not appear to have brought benefits for patients. He highlighted that there had been less tangible benefits through increasing skills in the ophthalmic community, initiating more research and developing teams that are able to provide services in a more efficient and coherent manner, but that through no failing of the study43 or the NIHR the potential impact of the findings in dramatically reducing costs had not been realised (Reeves interview).
Table of payback
Payback details for this case study are provided below in Table 21.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | Four peer-reviewed publications to date (indexed in WoS), plus contribution to a Cochrane review Further papers (at least five) in development currently Regular presentations at all international ophthalmology meetings |
| Research Targeting and Capacity Building | Valuable for career progression of members of the team (awarded NIHR Senior Investigator awards) Important in establishing the Clinical Trials and Evaluation Unit at Bristol (e.g. gaining UKCRC registration) Became a template for ophthalmology trials in the UK and sparked the expansion of the research field Trained NHS staff in trial centres in research methods Contributed to the Reading Centre enterprise becoming more strongly established Informed other trials in Europe through sharing protocols, etc., and influenced the study team’s future work |
| Informing Policy and Product Development | Included in several meta-analyses and a Cochrane review Cited in RCO guidelines on treatment of wet AMD Alongside CATT,358 frequently cited as evidence to support an assessment of Avastin by NICE and the use of it in the NHS |
| Health and Health Sector Benefits | With CATT,358 provided an evidence base which has supported the use of Avastin in the majority of cases of wet AMD in the USA, as well as in other countries May have contributed to a reduction in the cost of Lucentis to the NHS May have influenced clinicians’ attitudes towards dosing |
| Broader Social and Economic Benefits | Has the potential to save the NHS large amounts of money, but regulation means this has not yet been realised |
Appendix 4.4: CRASH-2
Summary
The aim of the CRASH-244 study was to investigate whether or not TXA could be used to treat trauma victims shortly after their injury and reduce their chance of dying. TXA, a low-cost generic drug, was already in use to reduce bleeding in patients undergoing surgery, but its use in trauma had not previously been studied through a clinical trial. The work found that early administration of TXA reduced death due to bleeding by 14%. The study44 team made significant efforts to disseminate the findings, including direct contact with guideline producers, and novel methods of dissemination such as videos, blogs and cartoons. TXA is now listed on the WHO list of essential medicines, and pre-hospital treatment with TXA is included in the 2012 Joint Royal Colleges Ambulance Liaison Committee guidelines. The European Trauma Bleeding Guideline has been changed to include early treatment with TXA, and NICE has developed an evidence review based on the results of the CRASH-244 trials describing its use in this context. The findings have also been incorporated into the British and American armies’ treatment protocols.
Introduction to case study
Background
Scientific background
Trauma is a significant cause of death worldwide, with three million people dying from trauma each year, of which one-third die because of blood loss. 395 TXA was already in use to reduce bleeding in patients undergoing surgery by encouraging clotting and preventing blood clots from breaking down. CRASH-244 study was therefore an attempt to see whether or not the same drug – a low-cost generic drug – could be used to treat trauma victims shortly after their injury and reduce their chance of dying. No previous trials had been conducted looking at the use of TXA in this context.
Chief investigator’s background
At the time the work was conducted, the CI, Ian Roberts, was Professor of Epidemiology and Public Health at LSHTM, and Director of the LSHTM CTU. In addition, he held the position of Coordinating Editor of the Cochrane Injuries Group.
Prior to this study,44 he had led the MRC-funded CRASH-1 trial, which was a large study of 10,000 patients with head injuries. The trial looked at the use of a group of drugs called corticosteroids in the case of severe head injury. They were already widely used in the treatment of severe head injury, but this study concluded that their use was harmful. Prior to that, he had not conducted previous trials in trauma, and his research had mostly been in injury prevention and public health.
The case study approach
The case study is based on interviews and document review. As shown in Table 22, four individuals were also interviewed, including the CI. Document review covered a range of materials including the key publications associated with the study44 and related studies (including systematic reviews and published critiques of the study), the HTA grant application, and relevant NICE and other guidance and protocols relating to TXA use.
| Interviewee | Reason for interview |
|---|---|
| Ian Roberts | CI |
| Tim Coats | Trial Management Group; Professor of Emergency Medicine at the University of Leicester and Chairperson of TARN |
| Haleema Shakur | Senior Lecturer in Clinical Trials and Co-Director of the LSHTM CTU |
| Andrew P Cap | Chief of Blood Research at the US Army Institute of Surgical Research, and Associate Professor of Medicine at the Uniformed Services University |
Stage 0: topic/issue identification
Two key factors influenced the research team’s decision to work in this area, as detailed in Box 4 and described below.
-
Existing network.
-
Cochrane review.
Existing network
Through the CRASH-1 study, Ian Roberts and colleagues had developed a wide network of researchers and trauma doctors. The process of building up that network had been extremely time-consuming and Roberts was keen to ensure that full use was made of the network. As the CRASH-1 study progressed, he was therefore starting to think about what the next important trial to use the network would be. Effectively, he had the network in place to conduct a trial and was looking for a good question.
Cochrane review
At the same time, Roberts was editor of the Cochrane Injuries Group, and through this he became aware of a Cochrane review of the effects of anti-fibrinolytic agents (including TXA) on haemorrhage. Anti-fibrinolytics are drugs that promote blood clotting by inhibiting fibrinolysis, the natural process that limits the growth of blood clots in the body. The review presented strong evidence that these agents reduced bleeding, and through his experience as a trauma doctor, he was aware that this could potentially have a use in trauma, as so many patients die because of blood loss. This idea was developed in discussion with Tim Coats following discussion at the Trauma Audit & Research Network (TARN) conference, and they worked together to conduct a Cochrane review of the existing evidence regarding the use of anti-fibrinolytics in trauma. This showed that the available evidence was limited and they decided to put together a trial application.
Interface A: project specification and selection
Prior to receiving full funding for the trial, a pilot phase was conducted with funding from the Molton Charitable Trust, the Bupa foundation and Novo Nordisk (a pharmaceutical company). The pilot was also supported by the WHO. Over 1 year, 2000 trauma patients were recruited into the pilot, and the trial procedures were tested and found to be effective. International interest and engagement with the trial was demonstrated through the number of centres engaged and patients recruited. The CI felt that this pilot phase was crucial in getting the wider funding for the trial, as it demonstrated that the study44 was feasible.
However, despite this, it took more than 1 year to get the study44 funded. Applications were made to the MRC, the Gates Foundation, the Formula 1 research foundation and the US Army, all of which were unsuccessful, before the work was ultimately funded by the HTA programme. The CI suggested at interview that this was not uncommon, and that a high degree of perseverance is required to get a large global trial funded. There were no major changes made to the approach between applications to different funders.
An important decision that was made at an early stage in specifying the project, before even the pilots started, was the decision to focus the study44 on TXA. The research team initially considered looking at aprotinin as the anti-fibrinolytic agent. However, the team decided to change this based on two main factors. First, they knew it would be an international trial, and aprotinin is an animal extract that might not be acceptable for use in some countries. Second, TXA was a generic drug and was much cheaper, which would facilitate its uptake if the trial findings were positive. Given that the evidence around TXA suggested that it was almost as effective an anti-fibrinolytic as aprotinin, they decided that it should be made the focus of the trial. TXA is also useful in terms of practical application because it is light and heat stable, meaning it is suitable for use in a wide variety of situations, including military applications.
The application for funding to the HTA programme was submitted by four co-applicants: Ian Roberts (the CI), Tim Coats, Beverly Hunt (from the Haematology and Rheumatology department at St Thomas’ Hospital) and Haleema Shakur (from the Department of Epidemiology & Population Health at LSHTM). The application process consisted of an initial outline application, and the team were then invited to submit a full application. Changes were made between the outline and full application, based on comments from the HTA funding board, as follows:
-
Clarification that a measure of morbidity would be collected from all trials participants at 28 days or at prior hospital discharge.
-
Justification of the lower age limit of 16 years – excluding children allows a fixed-dose treatment regimen rather than a body weight-related dosing regimen. In emergency situations, weighing patients and drawing up variable dosages is time-consuming and error prone. A fixed dosage simplifies the trial and enables large numbers of patients to be recruited.
-
The scope of the health-economic evaluation was clarified.
-
A meeting of the Data Monitoring and Ethics Committee (DMEC) was convened and a letter from the TSC chairperson in response to the DMEC report was included in the proposal.
-
The subgroup analyses were clarified.
-
The cost of the study were clearly defined, justified and explained in relation to the total funding available for CRASH-2. 44
Patient representatives and practitioners also made contributions to the proposal process. In terms of practitioners, the four applicants included a trauma doctor and a haematologist, so the links with clinical practice were clear. The CI has a long-standing relationship with the patient representative group RoadPeace, a trauma patient group representing the victims of road traffic accidents in the UK. This group was involved in the development of the research approach and the project as a whole. However, Haleema Shakur also noted that they were aware that there are particular challenges associated with PPI in the emergency medicine context, as people do not know in advance that they will be involved. Victim’s organisations typically do not reflect the full population of relevance, and what is desirable in terms of outcomes at the time of trauma – in terms of maximising survival – may not always correspond to the views of survivors, for example if they are disabled following the incident. Therefore, patient input has to be considered with the knowledge that the people that survive are only part of the population going into the research.
The CI suggests that the deaths of British soldiers in Afghanistan might have been a contributory factor to this work getting funded. The potential for application in the UK and other wider contexts is laid out in the application for grant funding, and the practical design of the study44 and pragmatic selection of the anti-fibrinolytic agent reflect the effort made by the project team to design the study44 in such a way as to facilitate uptake into clinical practice.
Stage 1: inputs to research
Funding
Although the study44 was predominantly funded by the HTA programme, there were a number of other funding inputs that were important in the success of the trial. The first of these was the pilot funding received from the Molton Charitable Trust and the Bupa Foundation. The CI suggested that this was important in providing the evidence that the trial could work, which was necessary to get the full funding for the study. 44 There was also some support from the pharmaceutical industry to cover the costs of the drug itself and a placebo.
An additional source of funding was the CTU at LSHTM. That unit receives a small amount of infrastructure support from the NIHR. So although most of the work conducted is still grant funded on a project-by-project basis, this provides a little bit of core funding. The CI suggests that this helps to ‘stop the “lumpiness” of trial funding. It helps you have continuity between trials so you can keep networks together’.
Existing networks
Maintaining existing networks was also important to this study. 44 The trial built on the existing network used in the CRASH-144 study, and indeed keeping that network which had been timely and difficult to bring together, was a major reason why the team decided to pursue a large-scale trial in the field of trauma. The CI suggested that there was probably about 40% overlap between the network of trauma doctors involved in CRASH-1 and CRASH-2. He emphasised the importance of that group, saying that ‘40% of people are really important because they get you going really quickly and they are often really good recruiting centres’. As described above, being able to access core funding through the CTU, and pilot funding to start the trial before full funding was obtained, was important in being able to maintain contact with that group and start the CRASH-2 trial almost immediately after CRASH-1.
Collaborators
The CRASH-244 study recruited 20,211 patients in 274 hospitals across 40 countries. Although there were only four people named on the HTA application, the group of key people involved in the study44 was actually very large. The CI emphasised the importance of this wider group.
The easy part of a clinical trial is writing the protocol and analysing the results. The hard bit is randomising thousands of patients. The doctors, who recruit the patients, they are the key . . . Having the idea is easy; it is the doing of it that is hard. In research, people tend to emphasise the ‘idea-havers’. The doers are the more important people: trial and data managers, and doctors and nurses.
Haleema Shakur also re-emphasised this point, suggesting that the network of global collaborators are often not sufficiently credited for the success of a trial, and that the role of a few core academics is typically over-emphasised.
To recruit 20,000 patients globally, it is almost impossible to fund a trial to cover its real cost. We are highly dependent on people contributing globally for nothing, or very little at all. They are the total unsung heroes . . . People have ideas all the time, but delivering on that idea is the most important thing.
Stage 2: research process
The research largely proceeded as planned and there was not anything exceptional about the methods used. The approach was largely quite similar to that used in CRASH-1, and as such was a tested approach. There were some improvements and developments made, for example the use of text messaging and social media to communicate with the doctors involved in the trial.
One notable thing about the trial was its scale. The study44 recruited 20,211 patients in 274 hospitals across 40 countries. The team were aware that the study44 would need to be very large, and in order to recruit the necessary number of patients a multi-country study would be required. The trial covered both developed and developing countries, and, as such, had to be designed with this in mind. However, it should be noted that in the developing world, the study44 was conducted through the larger teaching hospitals, which typically have standards similar to, or higher than, most European medical centres.
Patient groups were engaged in the research, recognising some of the challenges in doing this in emergency medicine as described above; however, their significant input was largely at the outset, contributing to the selection of outcome measures and development of the protocol. There was also ongoing interaction with the HTA programme, which monitored the progress of the project against recruitment targets and other such measures of progress. The CI did not consider this monitoring to be disproportionate to the scale of the funding.
Stage 3: primary outputs from research
Knowledge
The study44 showed that the use of TXA reduced 28-day all-cause mortality by 9%. 396 The risk of death due to bleeding specifically was also reduced, in this case by 14%. The time at which the TXA was administered was also found to be important: if administered within the first hour of injury, the risk of death due to bleeding was reduced by 31%, and if administered between 1 and 3 hours, the reduction in death due to bleeding was 21%. However, it should also be noted that if administered after 3 hours, the risk of death due to bleeding was increased. 397 This subgroup analysis, which was published after the initial publication of the initial findings, was very important in thinking how best to use the findings of the study44 in practice. Cost-effectiveness modelling suggested that administering TXA before 3 hours was highly cost-effective, with the incremental cost per life-year gained of administering TXA estimated to be US$64 (in the UK). 398 The key findings of the study44 were set out in 15 publications. 395–409 Bibliometric information on these publications is set out in Figure 14.
There are a number of reasons identified which could have contributed to the high profile and high citation rates of the CRASH-244 study. First, the result is clear with a simple outcome measure and a sufficiently large sample size to ensure the result is significant. Second, trauma is a huge public health problem worldwide for which there are few proven treatments. The study44 was also well publicised by the study team, as described below.
Benefits to future research and research use
Capacity building and career development
In terms of the four named researchers on the grant, they were all fairly senior at the time that the study44 was conducted and therefore it has not had a significant impact on their careers subsequently. The majority of their career development had already occurred prior to the start of this study. 44 However, there are some other participants who may have benefited from their involvement.
One group, which Haleema Shakur mentioned as important, and often overlooked in terms of career development, is the group of researchers such as trial managers, data managers and administrators, who need specialist skills but often do not have a clear career pathway. Typically, they are involved in a particular project but then leave and those skills are lost. At LSHTM, they hold a grant for staff development, which covers training costs for these groups who have the opportunity to study as part of their involvement for a master’s degree in clinical trials. This funding was not included in the HTA grant, and Shakur suggested that this funding could not usually be included in a research grant application, and so this type of staff development cannot usually be covered unless such separate funding is available. Several members of the team completed a master’s in clinical trials over the course of this research project.
Another group that may have benefited from this study44 in terms of career development are the international network of collaborators involved in the work. According to Shakur:
This is why people do collaborate. It is often their first opportunity to get involved in research, to see how to do research, see how a protocol is written, to see what is required to do a trial. Doctors need to have some research experience to get promotion, so it does help their careers, very much so . . . Many go on to do their own research.
The team proactively undertook a range of capacity-building activities as part of this work, including travelling to a number of the key collaborating sites to support them in terms of developing relevant capacity in the management of research, around issues such as monitoring of trials and ethics processes. Shakur in particular mentioned visits to Nigeria and Pakistan to support capacity building in the conduct of trials with the centres they had worked with in those countries. As well as benefiting the researchers at the participating centres in those countries, this also benefited members of the study44 team, in terms of sharing ideas, experience and issues. This reflects the wider attitude of the researchers that the project team for this type of research is not just the people in the office, but rather the wider global network.
Targeting of future research
The publication of the study44 findings in 2010 seems to have contributed to wider interest in TXA in the research sphere. This is illustrated by Figure 15, which shows the number of results in a search for ‘TXA’ in PubMed by year of publication.
FIGURE 15.
Number of results in PubMed for a search on the phrase ‘TXA’ by year of publication.

There are also a number of specific studies stemming from the CRASH-244 trial. Two ‘nested’ studies410,411 were funded by the HTA programme, led by Dr Pablo Perel, to make wider use of the CRASH-244 data set. One of these410 focused on developing and validating a model to predict death in patients with traumatic bleeding, as well as using that model to then evaluate the effect of TXA on mortality levels. The other study411 looked at the data available in the CRASH-244 data set on intracranial bleeding and the effect of TXA in traumatic brain injury. This study subsequently led on to the CRASH-3 trial (again led by Ian Roberts), which is ongoing and aims to look at the use of TXA in the treatment of significant traumatic brain injury through a large international RCT. At present, the study is in the pilot phase and has received some initial funding from the JP Moulton Charitable Trust, but full funding has yet to be confirmed.
Other studies have also taken forward the potential of TXA to reduce levels of death from bleeding in different contexts. The HALT-IT trial, again funded by the HTA programme (project number 11/01/04) is an ongoing piece of work looking at the effect of TXA on death and transfusion requirement in the case of acute gastrointestinal haemorrhage. The study is, again, led by Ian Roberts, and includes many of the same project team as the CRASH-2 study. 44 The TICH-2 study, also HTA funded (project number 11/129/109) is looking at TXA for hyperacute primary intracerebral haemorrhage. This piece of work is led by Nikola Sprigg at the University of Nottingham, and is largely conducted by a different team, although Roberts is involved on the trial steering group. The WOMAN trial412 is a study into the use of TXA for postpartum haemorrhage, and is being led by Roberts and involves some of the CRASH-244 project team. The work is currently in the pilot phase, supported by LSHTM and its trial coordinating centre.
The trial also led to a new Cochrane review that was initially conducted before the study, but was updated afterwards and now will be maintained by the team. 413 There was no additional funding provided for that by the HTA programme.
Interface B: dissemination
The project team considered the dissemination of the study44 findings to be very important and spent a lot of time both on developing their dissemination strategy and communicating their study44 findings in a range of different formats. The work was presented in the conventional way at conferences and through conversations with colleagues, but the team felt that this, although useful, reaches a limited audience.
At the time that the study44 was published in The Lancet, there was a press release and some media coverage of the trial’s finding, including national press, and the CI mentioned appearing on the Today programme on BBC Radio 4 to discuss it. They also used some more novel means of disseminating the findings, including a cartoon on YouTube,414 a song produced with trauma victims415 and a medical comic. 416 They also prepared specific publications aimed at spreading the message, such as an estimate of the number of lives that could be saved. Social media were used by the team, with the results presented in blogs and podcasts,417 and Coats described preparing free online medical education materials around TXA treatment. These materials were largely aimed at practising clinicians. According to one of the interviewees, there are several thousand people regularly listening to some of the podcasts, illustrating the wider audience that can be reached in this way.
The network of people involved in the trial was also important in dissemination. The CI suggested that those who took part in the clinical trial were more likely to then take up those findings and use the new intervention after the trial. Several team members also described how they became a resource for wider dissemination internationally. Press releases were produced in different languages, and trial participants were provided with packs to support dissemination among their networks, including material such as PowerPoint® (Microsoft Corporation, Redmond, WA, USA) presentations of the results, a list of frequently asked questions, and other relevant documentation. Many clinicians in the developing world are quite isolated and difficult to reach, so using these types of in-country networks was really valuable.
Some funding was received from the HTA programme to support dissemination. According to the study44 team they asked for half a million pounds, and received £30,000. It was noted by Tim Coats that, compared with delivering the trial, the dissemination and implementation stage, which is largely unfunded, has been particularly time-consuming. He noted, in particular, the contrast with the situation for new products uncovered by the pharmaceutical industry:
If this had been a drug discovered by a pharmaceutical company with such a positive result, there would have been a multi-million pound system of drug reps, publicity, advertisements, publicising to and informing doctors and reps would have been giving out pens to doctors. But because this was investigator led, there is an implementation gap. There isn’t all of that system.
Tranexamic acid was a cheap generic by the time this study44 was conducted, so according to the team the pharmaceutical industry had no interest in supporting any dissemination or implementation efforts.
Overall, the team felt that they did not get enough support for implementation and dissemination, and Shakur suggested that this is something that NIHR may need to consider more systematically.
[NIHR] need to think strategically about what they can do to support researchers when there is a positive result. They just seem to think it is just going to happen, but we have to think about strategies to get it into practice. And it takes money, and people.
Stage 4: secondary outputs
The findings of the study44 have influenced policy internationally. TXA is now listed on the WHO list of essential medicines following an application by members of the study44 team. The 2012 Joint Royal Colleges Ambulance Liaison Committee guidelines include pre-hospital TXA for all patients triaged to a trauma centre, and the European Trauma Bleeding Guideline has been changed to include early treatment with TXA.
In the UK, NICE were not able to review TXA as part of their standard guideline review process because the drug does not have a UK marketing authorisation for use in the trauma setting, and this is unlikely to change, as it is now a generic and there is therefore little incentive for pharmaceutical companies to pursue the matter. Following some lobbying by members of the study44 team, NICE developed an evidence review based on the results of the CRASH-244 trials describing its use in this context. According to the NICE website, TXA may be included within the scope of the NICE clinical guideline on the assessment and management of major trauma, which was to be published in June 2015 but has been pushed back until February 2016. Ian Roberts is continuing to press for the drug to be authorised in the UK, as that will allow the drug to be marketed properly, for example.
The findings have also been incorporated into the British and American Armies’ treatment protocols. The UK military were early adopters of the findings, which were timely as they coincided with the war in Afghanistan. The American military took a longer time to adopt the findings, but Coats suggests that in the worldwide context, the American military altering their protocols was influential in terms of changing practice internationally. The US military became interested in the study44 because of the difference in practice with the UK, and the MATTERS418 study was undertaken jointly comparing outcomes for those receiving TXA among US and British troops. The findings of this study coincided with the CRASH-244 results, and the protocols were subsequently revised in 2012.
Although the findings were picked up by the US military, wider adoption in the USA in the civilian sphere has been more limited. Interviewees suggested that this was largely because there were no US centres included in the CRASH-244 study. The reason for this was that the team could not afford the cost for indemnity insurance, which is required to conduct research in the USA, which would be covered in a grant from a US funder but is not provided by UK funders. The team suggested that it was because of this – and the wide spread of countries included in the study,44 including a number of developing countries – that practitioners in the USA were critical of the applicability of the findings of the study44 to their own practice.
The work was included in a Cochrane review of anti-fibrinolytic drugs most recently published in 2012. 413 The conclusions of the Cochrane review413 were similar to those of CRASH-2,44 and many of the study team members were also involved in conducting the systematic review.
Stage 5: adoption by practice and the public
The TARN collect data on all severely injured patients in the UK. According to Tim Coats, when the research came out, only around 3% of trauma patients received TXA, whereas now that proportion is around 75%, which would indicate that it took about 3 years from publication for the use of TXA to become very common practice. As described above, the drug does not have a UK marketing authorisation for trauma, which could have proved a barrier to uptake. However, according to Coats this is not typically an issue for clinicians, as they regularly use drugs off-label in particular contexts, but it was more of a problem for NICE, as the drug would not be reviewed through their standard processes (e.g. through a TAR), and this could potentially have inhibited communication and translation of the findings.
As described above, military use is widespread, in the US and UK armies and in some others. For example, the North Atlantic Treaty Organization (NATO) Blood Advisory Team has recommended use of TXA in combat trauma. Internationally, it is not clear how widely the findings have been implemented. Inclusion on the WHO list of essential medicines may have given support but it is not clear how quickly or widely these medicines are established. According to Shakur, use is likely patchy:
I was in Nigeria a year ago, and considering results came out 2010, and they were big part of trial, it is very still very limited in uptake. But I was in Malaysia last week and the uptake was amazing, it is available and doctors are aware. So it varies country to country, and this perhaps reflects the standard of care in those countries. If patients are not getting basic care, it is harder to get uptake on research findings.
As described above, civilian uptake in the USA has also been limited. This may have been at least partly because no US centres were involved in the trial as described above, and because of perceived difficulties in applying the results to their specific clinical context. Some of the key criticisms in relation to the CRASH-244 study from a US implementation perspective are set out by Napolitano et al. (2012). 419 They identify the following concerns about the CRASH-244 study in a table on p. 1578, which illustrates some of the concerns leading to limited uptake in the USA:
-
Approach to randomisation (‘Doctor is reasonably certain that anti-fibrinolytic agents are indicated or contraindicated – do not randomise’) created some concern regarding selection bias.
-
No data regarding injury severity of the patient cohort so unable to determine if cohorts are similar.
-
No data regarding shock in the patient cohort so unable to determine if cohorts are similar.
-
Small sample size of hypotensive patients (systolic blood pressure of < 90 mmHg), which is target population (only 31.5% of study patients).
-
Small sample size of tachycardic patients (heart rate of > 107 beats per minute), which is target population (only 48% of study patients).
-
No data regarding fibrinolysis on admission, no coagulation testing (rate of fibrinolysis at admission in North American trauma centres is < 5%).
-
Only 1063 deaths (35%) were caused by bleeding. The most common cause of death was traumatic brain injury.
-
TXA did not reduce blood transfusions.
-
No adverse events regarded as serious, unexpected or suspected to be related to the study treatment created a concern about possible inadequate reporting.
-
Patient follow-up reported as 100% considered difficult to believe.
-
Effect size small. Statistically significant but question whether or not it is a clinically meaningful finding.
-
Absolute increase in mortality if TXA given 3 hours after injury.
Members of the study44 team would probably refute many of these claims, or explain them as potential misunderstandings. For example, several of the concerns relate to the pragmatic nature of the trial design. Coats explains this approach is well established but can cause concern among some groups:
This was a pragmatic trial. Patients were included if the clinician felt that patient had or was likely to have significant bleeding. Some people don’t like that; they want a particular blood pressure or number to work with. We have to educate clinicians about this pragmatic approach. It is widely used and well established, but a lot of [people], particularly surgeons, like things very black and white. What is the number when I should give this? The answer is that there is not a specific number, but if you think there is significant bleeding. That’s a bit woolly for some people. But actually, in the trial, people know. Most clinicians know what a bleeding trauma patient looks like, but it’s hard to put into one number. Pragmatic methods ask you to just be a clinician, and if you are worried that the patient is bleeding then that’s the patient to give the treatment to.
However, it does serve to illustrate attitudes towards the study that have limited uptake of the findings in the USA. As a result of some of these concerns and the ongoing debate, uptake in the USA has been limited and patchy. 419
According to Andrew Cap, one key wider issue that emerged as part of the debate in the USA was the question around the measurement of fibrinolysis. As the drug was an antifibrinolytic, there were some questions around the mechanism of action, and whether or not measurement for fibrinolysis before use was desirable, which had not been directly addressed by the trial as described above. This created potential questions around the use of the drug – should it be given to bleeding trauma patients identified clinically as in the CRASH-244 trial, or only those manifesting clinically relevant fibrinolysis (which is hard to define and difficult to measure in an emergency situation)? This question is the focus of ongoing work in the USA, and practice in the USA with regard to this is not yet standardised.
A wider barrier to uptake described by Coats is the need to give the drug early in the management of the patient, which can often be a difficult time with many other important procedures taking place. Because it is not immediately lifesaving in the way that some other interventions at that point may be, it is easy for it to be overlooked. The drug affects odds of survival, but the effects are not necessarily immediately apparent. To help with this, the approach in the UK has been to include it in the pre-hospital 2012 Joint Royal Colleges Ambulance Liaison Committee guidelines. One of the key enablers of the uptake of the drug is that it is inexpensive. It is also familiar, as it is used in other contexts and is likely to be available and familiar to practitioners.
Stage 6: final outcomes
There has been an increase in the use of TXA in trauma – at least in the UK, and in the US and UK armed forces – and probably more widely. As the only major trial into the effects of TXA on trauma, and considering the considerable dissemination and implementation efforts made by the team, it seems reasonable to assume that the CRASH-244 trial played an important role in this change in practice. Based on the CRASH-244 data, early use of TXA in trauma reduces 28-day all-cause mortality by 15%. 396 Hence, the impact on patients in terms of survival rates could be significant. According to the HTA report:
If given promptly, the treatment reduces the risk of bleeding to death by about one-third. On the basis of these results, we estimate that giving TXA to bleeding trauma patients could save > 100,000 lives per year worldwide. 395
The treatment is also likely to be cost-effective. It is estimated in the study that early administration (within 3 hours) of TXA would cost US$48, US$66 and US$64 per life-year saved in Tanzania, India and the UK, respectively. 395 As outlined within the report, the WHO Commission on Macroeconomics suggests that health-care interventions costing less than gross domestic product per capita per disability-adjusted life-year (DALY) averted should be considered ‘very cost-effective’. 420 However, it should be noted that these estimates are purely life-years saved, not adjusted for quality of life [as required for the quality-adjusted life-year (QALY) measure used by NICE, or the DALY measure referred to here]. An additional caveat is that the numbers are extrapolated from 28-day outcomes to model survival over 12 months.
The study authors, in the HTA journal article, reflect on the groups most affected by trauma. It is noted that the poor are disproportionately affected by trauma with the risk higher for more disadvantaged groups in the UK or internationally. 421,422 It is also noted that the group predominantly affected by trauma is young men. The average age of participants in the CRASH-244 trial was 35 years old, and 85% of participants were male. 395 This means that the social and economic benefits of reducing death by trauma could be very significant. The authors illustrate this by reference to work in Bangladesh, which found that many urban households are made destitute by the death or injury of a family member in a road traffic crash. 423 These wider social and economic benefits are not easy to estimate but this does serve to illustrate the potential wider benefits for which the study findings have been implemented.
Table of payback
Payback details for this case study are provided below in Table 23.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | Fifteen peer-reviewed articles Only large-scale trial looking at the use of TXA in trauma Demonstrated that TXA was beneficial provided it was given within the first 3 hours Early TXA use reduces 28-day all-cause mortality by 15% |
| Research Targeting and Capacity Building | Capacity-building for international partners and building/maintenance of networks Some evidence of significant growth of papers on TXA following this study Several studies on TXA use in other contexts (mostly for the team involved in this work) |
| Informing Policy and Product Development | TXA listed on the WHO list of essential medicines Pre-hospital treatment with TXA included in the 2012 Joint Royal Colleges Ambulance Liaison Committee guidelines European Trauma Bleeding Guideline changed to include early treatment with TXA NICE evidence review based on the results of the CRASH-244 trials describing TXA use in trauma Findings incorporated into the British and American armies’ treatment protocols |
| Health and Health Sector Benefits | Some suggestion that level of adoption is around 75% in the UK; probably some adoption in other countries but level of adoption less clear Adopted by US and UK militaries Where adopted, study results suggest that it will increase trauma survival rates (by 9%, or higher if used up to only 3 hours after trauma as suggested) |
| Broader Social and Economic Benefits | Economic analysis included in the study suggests that the treatment is likely to be cost-effective in the UK and other countries Trauma typically affects young men (average age in study was 35 years) so potential social and economic impact of lives saved is high |
Appendix 4.5: a systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults, and an economic evaluation of their cost-effectiveness
Summary
An assessment report was conducted by the study team, commissioned through the HTA stream of the NIHR for use by NICE in their MTA of anti-tumour necrosis factor (TNF) inhibitors. The study team, comprising systematic reviewers, modellers and clinicians, conducted the research in approximately 6 months and wrote it up as a monograph to be published in the HTA journal. 45 Prior to publication it was used as a key piece of independent evidence in the NICE appraisal process by the committee, and informed its recommendations on the use of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in the NHS in England.
The Assessment Report is cited in NICE guidance issued in 2007, and the economic model used in the Assessment Report is also cited in a second piece of NICE guidance for treatments of rheumatoid arthritis published in 2010. The NICE guidance has led to increased choice in treatment available to patients and physicians. Overall, this has had a budgetary impact for health services, as the drugs involved have been deemed cost-effective for the patient, and overall drug approval has impacted on service delivery and is a factor for increased day-care use of rheumatology services.
Internationally, this project has been used as an example by members of the study team engaging with health technology assessors abroad to describe how the HTA and NICE conduct the process in the UK, thus informing practice overseas.
Introduction to case study
Background
Scientific background
Rheumatoid arthritis is a chronic systemic inflammatory disorder that primarily affects joints. Treatments include both medication and non-pharmacological measures – the goal being to control joint inflammation and prevent joint damage and disability. Within medication, there are both painkillers and anti-inflammatory drugs, including steroids, to suppress symptoms, but these do not stop the progression of the disorder. Alternatively, disease-modifying anti-rheumatic drugs (DMARDs), such as anti-TNF agents, have been shown to decrease the number of tender or swollen joints along with the pain and disability due to the disease. In this study three anti-TNF agents were compared.
In order to gain impartial evidence on the cost-effectiveness of potential therapies, Assessment Reports are commissioned through the HTA programme of NIHR, managed by NETSCC. These data are used to inform the appraisal of drugs and medical devices, which is undertaken by the technology appraisal programme of NICE, prior to availability from the NHS in England. As described below, this study was commissioned by NETSCC to fulfil a need from DH and NICE.
Chief investigator’s background
Professor Amanda Burls was based at the University of Birmingham at the time of the study as the Director of the West Midlands Health Technology Assessment Collaboration. At the same time, she was a Honorary Consultant in Public Health Medicine at South Birmingham PCT. She was the lead applicant for the collaborations call-off research contract for the TAR. The purpose was to undertake research synthesis, health technology assessments and methodological research to inform national-level NHS policy-makers, in particular NICE.
The case study approach
The case study was selected based on the high number of citations received by the HTA journal publication that came out of the study (see Figure 16). This is somewhat unusual for a TAR. Between 27 November and 14 January, six individuals with knowledge of the study and follow-on work were interviewed (Table 24). Individuals involved with the study were identified, and initially the project team spoke to Amanda Burls (CI) and Dr Yen-Fu Chen (first author on the HTA journal libraries output). From these interviews, other interviewees were suggested, who, in turn, recommended individuals to speak with, who could comment on other elements on the impact.
| Interviewee | Reason for interview |
|---|---|
| Amanda Burls | CI; Professor of Public Health, Health Services Research and Management Division, School of Health Sciences, City University London |
| Yen-Fu Chen | First author on HTA journal publication; Senior Research Fellow, Warwick Centre for Applied Health Research & Delivery, University of Warwick |
| Paresh Jobanputra | Clinical lead; Rheumatology Consultant and Clinical Service Lead at University Hospitals Birmingham NHS Foundation Trust |
| Zoe Garrett | Technical advisor, Centre for Health Technology Evaluation, NICE |
| Zoltan Kalo | Professor of Health Economics, Department of Health Policy and Economics, Eötvös Loránd University |
| Andrea Rita Horvath | FRCPA Clinical Director, SEALS North, Sydney, Australia |
In addition, a number of other people were contacted, but these people either declined to be interviewed or did not respond to our requests.
Bibliometric analysis was conducted on the outputs from the study. Web searches, using academic sources such as PubMed, the HTA website and wider online browsers such as Google, were conducted to search for further citations of the study’s outputs.
Stage 0: topic/issue identification
We have identified three key factors that influenced the selection of the topic and the research team’s ability to work in this area, as detailed in Box 5 and described below.
-
Introduction of new drugs to the market.
-
TAR contract and methodological expertise.
-
Previous work on rheumatoid arthritis.
Introduction of new drugs to the market
The request to conduct this piece of research came from the HTA programme contract, which is funded by NIHR and managed by NETSCC. The topic of research and the more specific questions are prioritised between NICE and DH. Ideas for topics to be investigated can be suggested from a broad range of stakeholders, including patients, the public, practitioners and industry. The topics for review are then chosen through a scoping phase, which includes a formal consultation with stakeholders on the scope of the appraisal (i.e. the document outlining the issues to be covered in the appraisal) and also a workshop. A range of stakeholders, including patient organisations and professional organisations, will be invited to the workshop to provide comments on the scope and the potential value of the appraisal. The decision that a technology appraisal is appropriate is based on factors such as the importance of the topic for health-related government policies; the potential health benefit of the technology; existing variation in the use of the technology; and the potential impact of the technology on NHS resource.
In this case, Chen told us that the team believed that the topic had been chosen because of the introduction of new and expensive drugs and the prevalence of rheumatoid arthritis, which resulted in a potential financial impact for NHS, which, without NICE guidance, could result in regional variation in use. In this instance, the treatments that were already available in the NHS (etanercept and infliximab) had received licensed extensions that could potentially increase their use, and a third drug (adalimumab) had come on to the market.
Technology Assessment Report contract and methodological expertise
As a TAR team, Burls and her group at the University of Birmingham worked on a broad range of disease areas conducting systematic reviews and cost-effectiveness studies. Their expertise was in the methodology of conducting assessments, including the cost-effectiveness element. They were selected for this expertise, not specific to the conduct of this study in terms of subject area, but more generally to the conduct of TARs in terms of wider methodological expertise. Disease-specific knowledge was obtained through the inclusion of clinicians in the project team.
Previous work on rheumatoid arthritis
Although the topics are set by NICE and the DH, the TAR teams see the list of proposed projects on offer, and can highlight those in which they are particularly interested. In this instance, because of the previous assessments of drugs in the same class (anti-TNF inhibitors) conducted for the HTA programme by this group, they had the expertise to be best placed to conduct this study as well. The previous related studies were entitled ‘The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis’424,425 and ‘The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation’. 426
The potential for impact from the research was not a motivating factor for the TAR team in conducting the study. Rather, Burls and Chen saw their role to systemically review the evidence, and that the NICE Appraisal Committee would use this evidence to inform their recommendations.
Interface A: project specification and selection
The project (award reference: 04/26/01) was assigned to the TAR team, West Midlands Health Technology Assessment Collaboration, based at the University of Birmingham and headed by Burls. The process of selection was not competitive; rather, as an academic centre, the group had bid to become an assessment group for NICE. Once this was successful, they were required to conduct a series of studies over a 3- to 4-year period (the length of the contract). As described above, the TAR teams had the opportunity to review the proposed studies in a list from NICE and then these were allocated, based on preferences stated, expertise, workload and availability. In this instance, the project team had conducted similar studies in the same area for the HTA, evaluating the clinical effectiveness and cost-effectiveness of anakinra, and infliximab and etanercept.
Once the protocol for the study is drafted, it is sent to NICE to comment on, and it cannot be finalised until it is agreed by both NICE and the TAR team. At this point in the process the protocol is sent to stakeholders, including representatives from manufacturers and clinicians, and stakeholders are invited to an information meeting at which the protocol is discussed.
Stage 1: inputs to research
Funding
Funding was provided to the TAR team in TAR units, which are allocated up front at the beginning of the year. This report was one TAR unit, which has an approximate value of £175,000. This was the only source of funding for the project. However, it is important to note that some members of the team, such as Paresh Jobanputra, were conducting this research in addition to his/her full-time position as a clinician.
As stated above, the core team’s expertise (lead by Amanda Burls, the CI) was in the methodology of conducting systematic reviews, rather than being disease specific. Chen was the main reviewer on the project. To complement their knowledge, the TAR team collaborated with modelling specialists and a consultant rheumatologist. The co-investigators on the project were:
-
Professor Stirling Bryan: health economist
-
Ms Anne Fry-Smith: information specialist
-
Dr Pelham Mervyn Barton: mathematical modeller
-
Dr Paresh Jobanputra: clinical rheumatologist
-
Dr Anke Mans: expert in rheumatoid arthritis biologics.
Techniques
Some members of the team had previously worked together, on the effectiveness of a specific treatment of rheumatoid arthritis,427 and during this work developed the BRAM. 428 During this study, the model was refined further. Jobanputra highlighted that the study was very collaborative and required the different inputs. For example, the modelling and systematic review could not happen without clinical input to provide context about how the rheumatoid arthritis pathway would be managed and the sequence of drugs that are chosen.
Engagement of industry
The main source of evidence used by the technology appraisal programme is often held within pharmaceutical companies, as a product of their research and industry-sponsored trials. These data may be available in published reports, but also may also be made available to the TAR team only through NICE, as commercial-in-confidence trial reports, and must be destroyed at the end of the project. In some instances, researchers stated that there is difficulty in gaining access to these reports, which are not routinely in the public domain, but that was not the case in this specific study. Over time, Chen believes that companies more readily provide trial reports. He felt that both the HTA programme and NICE have played a role in making it clear to industry that it is not acceptable to withhold relevant information.
Stage 2: research process
The aim of the study was to assess the clinical effectiveness and cost-effectiveness of adalimumab, etanercept and infliximab in adult patients with rheumatoid arthritis, by:
-
updating and undertaking a systematic review and meta-analysis of the clinical benefits and harms of adalimumab, etanercept and infliximab
-
reviewing published cost-effectiveness and cost–utility studies of these agents and economic evaluations included in manufacturer submissions
-
adapting the BRAM to evaluate the cost-effectiveness of these agents compared with other treatment options.
The research took approximately 6 months to complete and included a comparison of 29 RCTs. Different members of the team were responsible for different elements of the study:
-
Pelham Barton (Lecturer in Mathematical Modelling) constructed and analysed the new version of the BRAM; drafted the section of the report relating to the BRAM; responded to peer review; and read and edited the draft report.
-
Stirling Bryan (Professor in Health Economics) selected studies from the searches for published economic analyses; contributed to the economics review, review of submissions from industry, development of model structure and unit cost data collection; and edited the report.
-
Amanda Burls (Senior Clinical Lecturer in Public Health and Epidemiology) was senior reviewer on the report and provided project management and advice on all aspects of the report; participated in data extraction and analyses; drafted the results section, summary and discussion; compiled and edited the draft report; and takes final responsibility for the whole report.
-
Yen-Fu Chen (Systematic Reviewer) was main reviewer on the report and maintained day-to-day running of the review. He compiled the study protocol; carried out study selection and data extraction (mainly for etanercept and infliximab); and conducted meta-analyses. He also drafted the following sections: methods, narratives for included trials, and part of the results and discussion; and edited the report.
-
Wendy Clark (Information Pharmacist) applied the inclusion and exclusion criteria; was involved in data extraction principally for adalimumab; and commented on the draft report.
-
Anne Fry-Smith (Information Specialist) devised and implemented search strategies for bibliographic databases; drafted the searching methods section; and commented on the draft report.
-
Paresh Jobanputra (Consultant Rheumatologist) drafted the introduction; assisted with study selection; extracted data from some studies; contributed to the development of the economic model; identified data sources for parameters for the model; edited the report; and responded to peer-review comments.
-
Sue Jowett (Health Economist) wrote the review of existing economic evaluations.
Throughout the process the TAR team had regular contact with the HTA, which oversees the contract and ensure the project is delivered on time. Owing to the fact that this research feeds into the appraisal process, it is essential that deadlines are met to ensure that decision-making can occur with the best evidence available in a timely manner. The engagement with HTA ensures that the TAR team maintains an independent stance in terms of approach and the evidence it chooses to use, and also in terms of managing workload from a project management perspective. This intermediary function was highlighted as important to the TAR team – by one of the researchers – in negotiating with NICE, if the timelines are perceived to be too short.
There is also ongoing contact with NICE, mainly through the technical lead at NICE. Once the protocol for the study is drafted, it is sent to NICE for comment, and cannot be finalised until it is agreed by both NICE and the TAR team. The protocol is sent to stakeholders – including representation from manufacturers, clinicians and patient organisations – and they are invited to an information meeting at which the protocol is discussed. Once the research was commissioned, NICE comments on a draft version of the Assessment Report but there is no further engagement with other stakeholders until the report is sent for consultation before the first Appraisal Committee meeting.
Stage 3: primary outputs from research
Knowledge
The key findings of the study were:
-
Adalimumab, etanercept and infliximab are effective treatments compared with placebo for patients with rheumatoid arthritis who are not well controlled by conventional DMARDs, improving control of symptoms, improving physical function, and slowing radiographic changes in joints.
-
The combination of a TNF inhibitor with methotrexate was more effective than methotrexate alone in early rheumatoid arthritis, although the clinical relevance of this additional benefit is yet to be established, particularly in view of the well-established effectiveness of methotrexate alone.
-
An increased risk of serious infection cannot be ruled out for the combination of methotrexate with adalimumab or infliximab.
-
The results of the economic evaluation based on BRAM are consistent with the observations from the review of clinical effectiveness, including the ranking of treatments.
-
TNF inhibitors are most cost-effective when used as last active therapy. In this analysis, other things being equal, etanercept may be the TNF inhibitor of choice, although this may also depend on patient preference as to route of administration.
-
The next most cost-effective use of TNF inhibitors is third line, as recommended in the 2002 NICE guidance.
-
Direct comparative RCTs of TNF inhibitors against each other and against other DMARDs, and sequential use in patients who have failed a previous TNF inhibitor, are needed.
-
Longer-term studies of the quality of life in patients with rheumatoid arthritis and the impact of DMARDs on this are needed, as are longer studies that directly assess effects on joint replacement, other morbidity and mortality.
The sole publication from this study was the required output of a HTA journal article, omitting commercial-in-confidence material. 429 This was presented as evidence for the NICE Appraisal Committee and published online and alongside the guidance on the NICE website. Jobanputra noted that the research provided the opportunity for ‘major high profile journal publications . . . from the meta-analysis, the systematic review and the modelling but we didn’t have time [to write these up], as we were doing subsequent [HTA] assessments’. In addition, he noted that it could be difficult to publish from this type of project, as a lot of the data were provided by pharmaceutical companies as commercial-in-confidence reports, for which the primary sources could not be cited.
The HTA journal article was highly cited (349 times), with a normalised citation score of 19.70, and within the top 10% in field (Figure 16).
Benefits to future research and research use
Capacity building and career development
The study had an impact on the research career of Chen, as an early career researcher on the project, and he has subsequently stayed within the field of HTA research. However, as he pointed out, this is a ‘double-edged sword’. The benefits have included the reputation of HTA reports, and work for NICE has been helpful in his career development. He also highlighted the links between assessment groups that organise workshops to learn and share skills. However, the downside is that because of the short timelines of this type of work, all activity is focused on delivery and time between projects is limited: ‘Once it is finished you have to move straight on to another project’. Being based in a university environment, there are still other pressures on time, for example teaching. Therefore, outside HTA reports, Chen comments that his CV lacked other publications, which has had an impact on his career progression.
Jobanputra became involved in TARs after attending a course run on systematic reviewing taught by Burls and colleagues. This training enabled him to be involved with the multiple studies that the group conducted in the area of rheumatoid arthritis, as he was uniquely placed to understand the methodology as well as providing clinical expertise.
Targeting of future research
The effect of the study on the subsequent research agenda for the team was limited, as the choice of topic is driven by the requirements of NICE. However, owing to the expertise developed, the team subsequently was asked to do further assessments within the field of rheumatoid arthritis and the use of biologics. Burls stressed that the research had influenced the way in which the team promoted the use of appropriate trials for technology assessments. For example, as a result of this work and the expertise developed, Burls has been involved as an expert for the NICE Scientific Advice Programme and conducted some consultancy with the pharmaceutical sector to provide knowledge of appropriate comparators in trials.
The project team have gone on to use the model that was refined in this study (BRAM2) in other cost-effectiveness assessments of treatments for rheumatoid arthritis, for example on the use of further anti-TNFs and other biological treatments after the first TNF inhibitor has failed (which came out later) such as tocilizumab. Others have used the model, and international data from Spain and Poland has also been processed through the BRAM2 model, although it is not clear whether or not this has been used to inform their decisions on the availability and reimbursement of specific rheumatoid arthritis treatments.
One of the weaknesses identified in this study was that there is no direct comparative evidence between drugs in this field. Following this research, Jobanputra ran a head-to-head RCT to directly compare two anti-TNF inhibitors assessed in this report. 430 Jobanputra’s interest in conducting this research was that the agents were widely used in practice and yet it was not clear which drug a clinician should choose as a first option.
A further recommendation from the study was the need for long-term quality-of-life studies. These have subsequently been conducted – although Jobanputra questioned whether or not this was the direction the field was moving anyway and would have happened irrespective of their study.
As a result of the committee discussions and the interest in this area, registries were set up across Europe, in Spain and Germany, and data continued to be collected by the British Society of Rheumatologists in the UK to follow up on patients, as a way to gain long-term safety data. This was not directly an outcome of the research but an outcome of the process into which the research fed, although continued vigilance about potential harms was called for in the future research section of the report.
International
In the Spanish context, the individuals who Burls engaged with had not done a systematic review before. Subsequent to the interaction and training provided by Burls [through the Critical Appraisal Skills Programme (CASPe)], they have gone on to do further systematic reviews, to be on Cochrane review teams and write books about systematic reviewing. In addition, this research was picked up by the Cochrane collaboration, led by the group in Spain, who used this study’s data extraction in their process, and Burls was an author on the publication. 431
Interface B: dissemination
The Assessment Report was used was as evidence by the Appraisal Committee in the development of the guidance around the use of these drugs (see Stage 4: Secondary outputs/National Institute for Health and Care Excellence appraisal committee meeting and inclusion of the report in guidance, below, for further details of the committee process). Burls noted it was considered inappropriate for the project team to disseminate the research. She stated, ‘Our role was not to help people understand, it was to collect the evidence and then for NICE or the DH to disseminate’. Burls told us that this stance had ‘gradually changed over time as the project team are in the strongest position to understand the evidence’.
All three interviewees from the study team noted the lack of dissemination around this particular study. In one instance it was felt inappropriate, but predominantly it was a result of lack of time and the need to move on to the next topic. Linked to this, it is important to note that as the TAR teams’ expertise is in systematic reviewing or modelling and not the disease area, they do not follow the impact in a particular disease area. Burls, as CI, presented the study at a number of meetings, where interested parties in other countries wanted to understand the HTA process in the UK, and how to implement evidence-based policy. Chen noted that attempts to disseminate the research were limited, mainly because of time constraints on the project team. This was echoed by Jobanputra, who stated that owing to the collaborative nature of the project it was difficult to find time to publicise it, and the different individuals were being ‘driven in different directions’ depending on the setting within which they worked.
Stage 4: secondary outputs
National Institute for Health and Care Excellence appraisal committee meeting and inclusion of the report in guidance
The Assessment Report was submitted to the NICE Appraisal Committee to inform the guidance production. It is important to note that this is prior to publication in the HTA journal. The major difference between the two reports is that there is information supplied by industry that is included in the report to the NICE Committee but which is taken out before the final publication is made publicly available owing to confidentiality.
The Appraisal Committee is made up of individuals from a range of backgrounds including clinicians, lay experts, members of the NHS, statisticians, health economists and industry representatives. They would not all have specialist knowledge of the disease area, and therefore the committee is complemented by clinical specialists and patient representatives who attend the meetings to advise on clinical and patient matters. The project team were invited to attend the committee meetings, and usually the modeller, health economist, lead reviewer and the project leader are available to answer questions. In this instance, Jobanputra and Burls were there, and other members of the project team may have attended. Between the two committee meetings, the committee could also request clarification from the project team of any issues highlighted (which occurred in this instance), and public consultation is conducted around the draft NICE guidance.
The HTA TAR is the main – but not the only – source provided to inform the decision-making of the Committee. Other sources of evidence include submissions from related professional societies, manufacturers and patients. Chen highlighted that one of the important factors about the report is that ‘we carry out an independent assessment’. This includes general literature, and critique of all industry submissions. This was echoed by Garrett, stating that in a MTA ‘the independent report would usually be the main focus for the committee’ and confirming that it was in this instance.
Jobanputra highlighted the importance of this study and the outcome of the guidance for the rheumatology community. Therefore, there was great interest in NICE’s process. This created difficulties, especially for Jobanputra, who was a clinician in the field. The British Society for Rheumatology (BSR) also fed directly into NICE through a submission to the committee and provided recommendations about the use of the new agents.
In this instance there were more than the standard format of two meetings, as additional evidence was presented by the BSR. In addition, there was an appeal on the initial guidance from the manufacturers, BSR, Royal College of Nursing and patient groups, as the guidance about when the drugs could be used was more restrictive than they would have liked. In this instance the appeal was upheld and the appraisal was split so that guidance on the use of the first TNF inhibitor was published, and the consideration of the use of a second TNF inhibitor after the first has failed (outside the scope of the study) was returned to the committee for a further discussion of the evidence.
Changes to guidance
Recommendations, in the form of NICE guidance, were issued in 2007, which updated the 2002 guidance but was broadly similar. In response to the appeal outcome, the NICE decision support unit undertook a study, using BRAM, to research the sequential use of TNF inhibitors for treating rheumatoid arthritis before it went back to the Committee for further discussion. 432 The NICE guidance on the sequential use of TNF inhibitors resulting from the further work conducted by the decision support unit was also appealed. This second appeal was also upheld and a new Assessment Report focusing on the sequential use of TNF inhibitors was completed. This report also used the BRAM and was also undertaken by the West Midlands Health Technology Assessment Group.
The HTA journal article is cited as the independent economic analysis conducted using the BRAM in the current guidance for the treatment of rheumatoid arthritis (2010). 433 It was previously cited in the 2008 version. 434 Jobanputra commented that in his opinion, the guidance is adhered to broadly.
The NICE guidance published as a result of the new Assessment Report commissioned resulted in guidance for the sequential use of biological treatments for rheumatoid arthritis, with the inclusion of etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis. This guidance recommends adalimumab, etanercept and infliximab as possible treatments for people with rheumatoid arthritis who have:
-
already tried methotrexate and another DMARD, and
-
‘active’ rheumatoid arthritis, as assessed by a rheumatologist on two separate occasions; people who are treated with adalimumab, etanercept or infliximab should normally also be given methotrexate – if methotrexate does not suit them, they may be given adalimumab or etanercept on its own.
Garrett, who was the NICE technical adviser at the time, highlighted in interview that in a MTA ‘the independent report would usually be the main focus for the committee’. She stressed that this was the case in this appraisal and that ‘other [submissions] are supporting evidence’.
Citations in international guidance
As described above, this is a highly cited article. The reasons given by the project team for this included the common incidence of rheumatoid arthritis, the use of anti-TNF inhibitors as a new generation of drugs, and the potential financial implications. An important point about the project was the use of indirect or mixed-treatment comparison to compare therapies for which there are no direct head-to-head trials. This project helped explore to the need for this technique, which has now become a routine method.
On searching, we did not note that the HTA report is cited in international guidance and guidelines on the treatment of rheumatoid arthritis. However, anecdotally, Jobanputra recognised similar elements of guidance in Spain and Holland. It was highlighted that a barrier to tracking the work, and potentially to the project team achieving the recognition, is that if other countries based their guidance on the study undertaken for the HTA, or on the treatment pathway developed it in, it would reference the NICE guidance and not the original report, which affects citations.
Moving forwards, Jobanputra stressed that such work is of its time and would not have more impact, as the continual change as new drugs come on to the market, and others come off patent, means that there will be cheaper alternatives, which will drive practice and guidance. Therefore, much of the impact is in the model, which is still used to assess drugs today.
With the shift in the HTA programme to conducting STAs, there is a requirement for a manufacturer to submit their own model for critiquing by a TAR team. There is anecdotal evidence from Burls that the BRAM model has been used by some pharmaceutical companies in their NICE submissions for appraisals of similar therapies.
Stage 5: adoption by practice and the public
Impact on practice and patients
As a result of the NICE guidance, there was increased patient choice in the drugs available for treatment, and clinicians can prescribe them on the NHS. It provided evidence on which points in the treatment pathway certain drugs should be used. The availability of these drugs has a cost-effectiveness and budget impact for health services. However, in this instance it is important to note that this was a review of the initial appraisal. It increased the drug options available to patients, rather than providing a completely new mechanism of treatment.
Chen highlighted the impact of this approval beyond the UK market, noting that others look to what NICE determines owing to the transparency of the process and the speed at which they assess new medicines.
In general, the project team were not aware of the PPI involvement around the guidance, beyond the engagement of patients and patient groups at the Appraisal Committee. Chen noted that he received occasional e-mails from public asking his opinion on treatments, but referred them back to health professionals. As the majority of the project team are methodological specialists (systematic reviewers and modellers) rather than clinicians, once the project has ended they move on to a different topic and do not follow what has happened in one specific area.
However, Jobanputra stressed that the cost of the drugs and their availability has led to a change in the system. Prior to treatment, it is necessary to take a number of disease measurements, carried out in pre-treatment screening. This information is entered into a database and can be audited. The services have evolved to cope with this additional burden either through the development of specialist (biologic) nurses who triage the patients or when a consultant manages their own case load and is responsible for the measurement. Across the country, both of these systems are used, but it may create bottle necks to patient treatment, and could limit continuity of care when individuals are seen by multiple health-care professionals.
International interaction
Internationally, Burls has used this study as an example for training decision-makers and health economists in the set-up of HTA bodies and the use of evidence to support decision-making, building on her wider expertise in HTA. In particular, she has been involved in projects in Poland and Hungary, where there was a move to change their reimbursement policies for national health insurance funds to a more evidence-based approach. As a result of conducting studies, including this one, Burls has become an expert, able to advise on the HTA and NICE process for approval, and how other countries can conduct the necessary assessment in their own setting.
In Hungary, Zoltan and Horvath highlighted Burls’ methodological contribution as well the contribution to the policy around health technology appraisal and help in developing the system. However, they stressed that there were several actors in this space and could not disentangle the Burls’ contribution from others. Through Burls’ contribution, as well as others, Hungary was the first country in the Eastern block to introduce monetary cost-effectiveness to reimbursement of pharmaceuticals and medical devices. Now cost-effectiveness studies, in a Hungarian context, are used routinely in reimbursement as part of the evidence.
In the Spanish context, the individuals with whom Burls engaged had not done a systematic review before. Subsequent to the interaction and training provided by Burls (through the CASPe), they have gone on to do further systematic reviews, to be on Cochrane review teams and write books about systematic reviewing. In addition, this research was picked up by the Cochrane collaboration, led by the group in Spain, who used this study’s data extraction in their process, and Burls was an author on the publication. 431
In addition, the BRAM model was used to pilot the guidelines for clinical audit data, and data from Hungary was put through the model in Birmingham. However, at the time the drugs were not introduced, as Hungary could not afford the cost of the new drugs.
Chen has shared his experience of HTA assessments conducted for NICE with the HTA agency in Taiwan. He used this project as one of the examples to describe the general approach to evaluate evidence and how HTA and NICE use it to formulate guidance. He now has established regular contact, and has an ongoing relationship with the HTA agency in Taiwan.
Stage 6: final outcomes
Impact on service delivery
The NICE guidance resulting, in part, from the HTA report and the approval of these technologies, has led to increased day-case use in rheumatology services because of the approval of infliximab. Jobanputra stressed that this may have facilitated a change in practice such that many inpatient units shrank or were closed. As with many things, this was not the sole factor, but these technologies were a contributing factor to lesser use of inpatient care, which has made a positive contribution to cost saving. He also highlighted that any of the subsequent technologies have depended on day-case services to the extent that we had to develop community or home day-case infusion services (relatively few units in the country have performed home infusions).
Impact on industry
Recommendations in technology appraisals are important to manufacturers, as they provide guidance on the contexts within which drugs can be administered to patients. This therefore affects their market access, availability of treatments and financial reimbursement. In the field of rheumatoid arthritis, Burls stated that some subsequent appraised have used aspects of the BRAM model structure and inputs, whereas others have used alternative models.
Other impacts
After a review of the Institute by the House of Commons Health Select Committee in 2002, NICE commissioned a series of internal reviews, and requested the WHO Regional Office for Europe to carry out an external review on their methods and processes, as well as their scientific robustness. 435 This project was selected as a case study for the WHO’s external review – using this and three other TARs conducted in 2002, and extensive discussion with NICE staff, Appraisal Committee members, members of the Technical Assessment Groups and other stakeholders. Members of the project team were interviewed to input to the report. The report highlighted that NICE was ‘internationally a leading agency in technology assessment’, and provides recommendations and observations on how NICE could further develop the technology appraisal process, which was aimed at NICE and other countries with, or in the process of developing, a similar system.
Table of payback
Payback details for this case study are provided below in Table 25.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | One peer-reviewed report Adalimumab, etanercept and infliximab are effective treatments compared with placebo for patients with rheumatoid arthritis who are not well controlled by conventional DMARDs, improving control of symptoms, improving physical function and slowing radiographic changes in joints The combination of a TNF inhibitor with methotrexate was more effective than methotrexate alone in early rheumatoid arthritis, although the clinical relevance of this additional benefit is yet to be established, particularly in view of the well-established effectiveness of methotrexate alone |
| Research Targeting and Capacity Building | Methodology training for key team members As a result of producing the BRAM model (refined in the study), and development of expertise in the field of rheumatoid arthritis, the project team has undertaken subsequent assessments for the HTA of similar drugs Subsequent primary research, as head-to-head RCT, to directly compare two anti-TNF inhibitors assessed in this report. This was recommended as further research |
| Informing Policy and Product Development | The study was used as evidence by the NICE Technology Appraisal Committee for their deliberations regarding the use of biologics in the treatment of rheumatoid arthritis The report is cited as underpinning evidence in the NICE guidance on the use of anti-TNF inhibitors in the treatment of rheumatoid arthritis Internationally, this project has been used as an example by members of the study team engaging with HTAs abroad to describe how HTA and NICE conduct the process in the UK, thus informing practice overseas |
| Health and Health Sector Benefits | The technology has had an impact on increasing choice for patients; that has been facilitated by the guidelines, which created access to these drugs as to when they could be used and as a result has increased patient choice Drug approval has impacted on service delivery and is a factor for increased day-care use of rheumatology services |
| Broader Social and Economic Benefits | The availability of these drugs has a cost-effectiveness and budget impact for health services |
Appendix 4.6: endovascular repair for abdominal aortic aneurysm
Summary
Endovascular aneurysm repair is a new technique for the correction of AAA, which became available in the 1990s. Prior to that, the standard treatment was open surgical repair. Endovascular aneurysm repair is less invasive than open repair and can be performed under a local anaesthetic. Endovascular aneurysm repair can also be used in patients who are not in good enough health to undergo surgery under general anaesthetic as required for open repair, as the surgery is more minor. The aim of this study46 was to compare the efficacy of endovascular aneurysm repair with standard alternative management in patients with large AAA in two groups:
-
EVAR 1 Comparing endovascular aneurysm repair with open repair in patients deemed fit to undergo open repair.
-
EVAR 2 Comparing endovascular aneurysm repair with no intervention amongst patients fit to undergo endovascular aneurysm repair but not open repair.
The main study was undertaken between 1999 and 2010 and has also been funded recently to continue to follow the cohort out to 15 years post treatment. The study46 cost £901,457.65 and was led by Professor Roger Greenhalgh at Imperial College London. The trial46 included nearly 40 UK centres at which staff were trained to undertake the new treatment. Surgeons and radiologists worked together to conduct the procedure, and the work was supported by the Vascular Society and the British Society of Interventional Radiologists.
The EVAR 1 study found that endovascular aneurysm repair offered benefits in terms of 30-day operative mortality over open repair, but that over longer time periods (> 2 years) there was little difference in all-cause mortality between the two forms of treatment. In EVAR 2, endovascular aneurysm repair was not found to offer any benefits over no intervention. The HTA has now funded the study team to continue to monitor the EVAR 1 cohort out to 15 years.
The results have been cited in NICE Interventional Procedure Guidance and a NICE technology appraisal as well as in wider international guidance on the subject. Use of endovascular aneurysm repair in the UK and Europe has increased significantly over the last 10 years, and it is likely that this study made some contribution to these increases in uptake. This study in particular was influential for three main reasons. First, it is the only one of the four large-scale studies of endovascular aneurysm repair that has included long-term follow-up to measure the reliability of endovascular aneurysm repair over 10 years and more. Second, it is the only study that has looked at patients who were not fit for open repair, but who may be fit for endovascular aneurysm repair. Finally, the economic analysis that was included made the study particularly useful in considering the implementation of the treatment in the UK health system.
Introduction to case study
Background
Scientific background
Abdominal aortic aneurysm is a condition in which the aorta, which is the main artery that leaves the heart and travels down towards the legs, starts to bulge and expand at a section just below the diaphragm, level with the navel. In this region, the aorta normally measures about 1.5–2.5 cm in diameter but, with this condition, can grow much larger and in extreme cases can rupture, requiring an emergency operation and often resulting in death (approximately 80% mortality). 436 When the study started, the prevalence of AAA was around 5% in men aged > 65 years437 and it tends to increase with age,438 but is far less common in women. 439–441 There is no proven medical therapy to cure or slow the growth of the aneurysm and surgical correction remains the only course of treatment. 442 Previous work has shown that it is safe to delay surgical intervention until the aorta exceeds 5.5 cm in diameter, above which the probability of rupture increases markedly. 443
Around 1990, a new technique became available for the correction of AAA: endovascular aneurysm repair. 444,445 Prior to that, the standard treatment was open surgical repair, which had been used since the late 1950s. Endovascular aneurysm repair is less invasive than open repair and can be performed under a local anaesthetic. 446 This results in a shorter recovery time and a better chance of surviving within the first 30 days after the procedure. 447 However, problems following the operation are more likely and may require further small procedures to correct them. 446 Endovascular aneurysm repair can also be used with patients who are not in good enough health to undergo surgery under general anaesthetic as required for open repair, as the surgery is more minor. 446
The aim of the study was to compare, in two groups, the efficacy of endovascular aneurysm repair with standard alternative management in patients with large AAA:
-
EVAR 1 Comparing endovascular aneurysm repair with open repair in patients deemed fit to undergo open repair.
-
EVAR 2 Comparing endovascular aneurysm repair with no intervention amongst patients fit to undergo endovascular aneurysm repair but not open repair.
Chief investigator’s background
Professor Roger Greenhalgh was the CI for this project. At the time the research was conducted he was (and remains today) Emeritus Professor of Surgery and head of Imperial College Vascular Surgery Research Group. He was trained as a surgeon and still considers himself as such, saying that he was ‘attracted to surgery, to do difficult surgery’. Indeed, he was one of the pioneers of the endovascular aneurysm repair technique in the UK, conducting the first endovascular aneurysm repair in the UK, with an international expert, Parodi, in 1993. However, he also had good problem-solving skills and so developed an interest in research. According to Greenhalgh, because of his practical surgical experience, he was ‘very driven to only do research that would have a big impact on patient management’ and was ‘not interested in having a lot of papers’. Prior to the EVAR trial,46 Greenhalgh had been involved in the UK Small Aneurysm Trial (UKSAT),443 which was a MRC- and British Heart Foundation-funded study investigating whether or not the AAA diameter threshold of 5.5 cm was a safe size to consider surgical correction of the AAA. This trial443 found that it was safe to delay surgery until the AAA grew to 5.5 cm, and the findings, published in 1998, were important for AAA screening.
The case study approach
The case study was conducted based on interviews and document review. Four interviews were conducted, including with the CI, as detailed in Table 26. Documents reviewed included the key publications from the study, publications from other endovascular aneurysm repair trials, relevant systematic reviews and clinical guidelines, CVs of the project team members (where available) and the National Vascular Registry.
| Interviewee | Reason for interview |
|---|---|
| Roger Greenhalgh | CI |
| Jonathan Beard | Trial Management Committee; President of the Vascular Society, and Consultant Vascular Surgeon, at the Sheffield Vascular Institute |
| Louise Brown | Trial manager and statistician; Senior Statistician at the MRC CTU at University College London |
| Robert Sayers | Senior trainer at one of the trial centres; Professor of Vascular Surgery, University of Leicester, and Honorary Vascular/Endovascular Surgeon, University Hospitals of Leicester NHS Trust |
Stage 0: topic/issue identification
A number of key factors influenced the research team’s decision to work in this area, as detailed in Box 6 and described below.
-
Clinical use of the technique.
-
Registry data.
-
CI’s previous experience.
Clinical use of the technique
Endovascular aneurysm repair first came to attention in 1991 and was pioneered by Volodos et al. (1991)444 in the Ukraine and Parodi et al. (1991)445 in Argentina. The first report on the use of EVAR in an emergency situation was published in 1994. 448 According to Greenhalgh, between 1991 and 1996 there was increasing evidence that a commercial interest in this technique was emerging as various companies tried to make an appropriate commercial device. Professor Coats also explained that there was increasing use of the technique from the mid-1990s at some of the larger centres in the UK. In this context, it became clear to the project team that a trial would be necessary and the team proposed such a trial in 1996 and made plans to apply for funding, which was ultimately awarded by the HTA in 1999.
Registry data
An important factor in the identification of the topic and formulation of the approach for this work was the prospective voluntary Registry of Endovascular Treatment of Aneurysms (RETA), which was set up as a joint initiative by the Vascular Society and the British Society of Interventional Radiology in 1996. Guidance was issued (at a national level in the UK) that EVAR should be conducted only if data were then submitted to the RETA registry. Although it was not compulsory, the registry data were fairly complete. Based on sales data, the registry captured data on approximately 85% of procedures. The registry ran for 5 years, with the EVAR trial proposed in the third year, with the power calculations and design informed by the results of the RETA registry, which also provided evidence on the need for the study.
Chief investigator’s previous experience
As described above, the CI was one of the first clinicians to use the EVAR technique, and was interested in addressing topics of clinical significance. He also had previous experience conducting a trial in this field based on his work on the UKSAT. 443
Interface A: project specification and selection
The CI for this project was Professor Roger Greenhalgh; the co-investigators were Professor David Allison, Professor Sir Peter Bell (Leicester University), Professor Martin Buxton (Brunel University), Professor Peter Harris (Royal Liverpool & Broadgreen University Hospitals NHS Trust), Mr Brian Hopkinson (University of Nottingham), Professor Janet Powell (Imperial College London), Professor Ian Russell (Swansea University) and Professor Simon Thompson (University of Cambridge). The proposal was submitted to only the HTA programme (not to any other funders) but, nonetheless, some time elapsed between the proposal being submitted (1998) and the project starting (1999). The work was entirely funded by the HTA.
An important element of the project selection and specification process was a meeting between the HTA and a number of the members of the proposed project team, chaired by Professor Sir Miles Irving. A range of experts including clinicians, statisticians and health economists were in attendance. At this meeting, the approach to conducting the trial successfully at a national level was discussed. It was clear that the HTA was supportive of the idea from the outset, and the CI suggested that the interaction with the HTA in refining the project approach was very beneficial.
There was no patient interaction in the development of the project approach, which was not required at that time.
It is perhaps worth noting that although several other trials have been conducted internationally addressing the question investigated in the EVAR 1 trial – the comparison of EVAR to open repair – no other trials have been conducted echoing the EVAR 2 study, i.e. comparing EVAR to no intervention for those who are not fit to undergo open repair.
Stage 1: inputs to research
Financial
The initial study was undertaken between July 1999 and July 2005, with funding of £755,452.35. The study subsequently received a further £901,457.65 to extend the study to March 2012, and has also received another extension to follow the cohort for 15 years post treatment, with an additional £459,553.20 of funding. All of the funding was from the HTA programme.
Collaborators and supporters
The trial included nearly 40 UK centres at which staff were trained to undertake the new treatment. Surgeons and radiologists worked together to conduct the procedure, and the work was supported by the Vascular Society and the British Society of Interventional Radiologists. The vascular and radiology societies kept a registry of EVAR usage, which was useful in order to monitor the performance and learning curves of the participating centres.
Requirement to participate
An important input to the project was an idea developed by Sir Miles Irving of the HTA programme. He recognised the importance of linking funding of EVAR devices with participation in the trial. Thus any centre that wanted to use the EVAR technique had to be part of the EVAR trial, otherwise they would not receive governmental funding for the devices. The trial was also made easier to run by stating that the same funding would be received by participating centre whichever EVAR device they preferred to use. Because of this, recruitment into the trial ran ahead of schedule and the team were able to specify a training programme for participants.
Expertise of the team
The key members of staff involved in the study were Dr Louise Brown, the trial manager and statistician; Professor Roger Greenhalgh, the CI; Professor Janet Powell, a co-applicant who was also involved in leading the study; and Professor Simon Thompson, a co-applicant who oversaw the statistical analyses. However, there were many other researchers involved in the work, as well as a significant number of trial coordinators and staff at the recruiting centres.
Greenhalgh is an academic vascular surgeon with many years of relevant research experience that brought particular expertise to the clinical interpretation of the study and how to engage the clinical community to ensure a success. He was very much involved in promoting the trial and made significant academic input to its design.
Powell is a vascular biologist/pathologist who also has a lot of experience of vascular surgery and is well known in the vascular surgery community. She was key, along with Greenhalgh, in driving the structure of the studies. Her role was particularly in the day-to-day running of the study. She was also involved in the UKSAT,443 alongside Greenhalgh and Brown.
Brown was the trial manager and statistician for this work, and had initially worked as the trial manager for the UKSAT,443 but subsequently became more involved in the statistical analysis and completed her training in medical statistics during the completion of the EVAR trials. Brown moved off the project to her current position at the MRC CTU at the end of 2010.
Thompson was based in Cambridge and was less involved in the day-to-day running of the study, but was very much involved in quality review of the work and oversight of the statistical analyses, which were performed by Brown.
Stage 2: research process
Surgeons and radiologists needed to work together to conduct the EVAR procedure, and this requirement alongside other technical requirements (e.g. for equipment such as good X-ray and good computerised tomography scan facilities) meant that the number of centres involved was limited to just over 40 (compared with 98 for the UKSAT443).
The process of conducting the research had to be thought out in advance very carefully. One challenge that the team faced was that some centres joined the trial in order to get the government funding for EVAR, already thinking that EVAR was preferable to open repair. There was therefore some risk that they would therefore not always conform to the randomisation. This was particularly problematic for the EVAR 2 study, where some considered it ‘unethical’ not to offer EVAR to all patients.
The CI praised the interaction with the HTA over the course of the project as beneficial, with the HTA team being supportive and responsive throughout. In particular, the HTA’s willingness to fund extended follow-up out to 15 years – which was not done for the other three studies conducted internationally – is praised. However, it was noted that there has been a marked increase in bureaucracy over the duration of these studies. In particular, the contracting phase to start up the follow-on work (to cover follow-up out to 4 years) was delayed by 1 year due to contractual and bureaucratic obstacles. Changes in practice in the NHS were also noted by the CI. He suggested that there were some difficulties around the follow-up for patients in the later work, with some centres suggesting that research money should be available for general practitioners (GPs) to cover the follow-up costs but that money was not accounted for in the grant funding received. Because of NHS funding shortages, the team perceived that there is less willingness to engage in research than there was 5 years ago, so follow-up on longer timescales is becoming increasingly difficult. It proved necessary to apply for relevant Hospital Episode Statistics data to achieve optimal follow-up.
Stage 3: primary outputs from research
Knowledge
The findings were published as a HTA report in 2012446 and additionally through 18 other publications. 447,449–465 which were extremely highly cited as set out in the bibliometric information in Figure 17.
FIGURE 17.
Bibliometric information on the EVAR project.

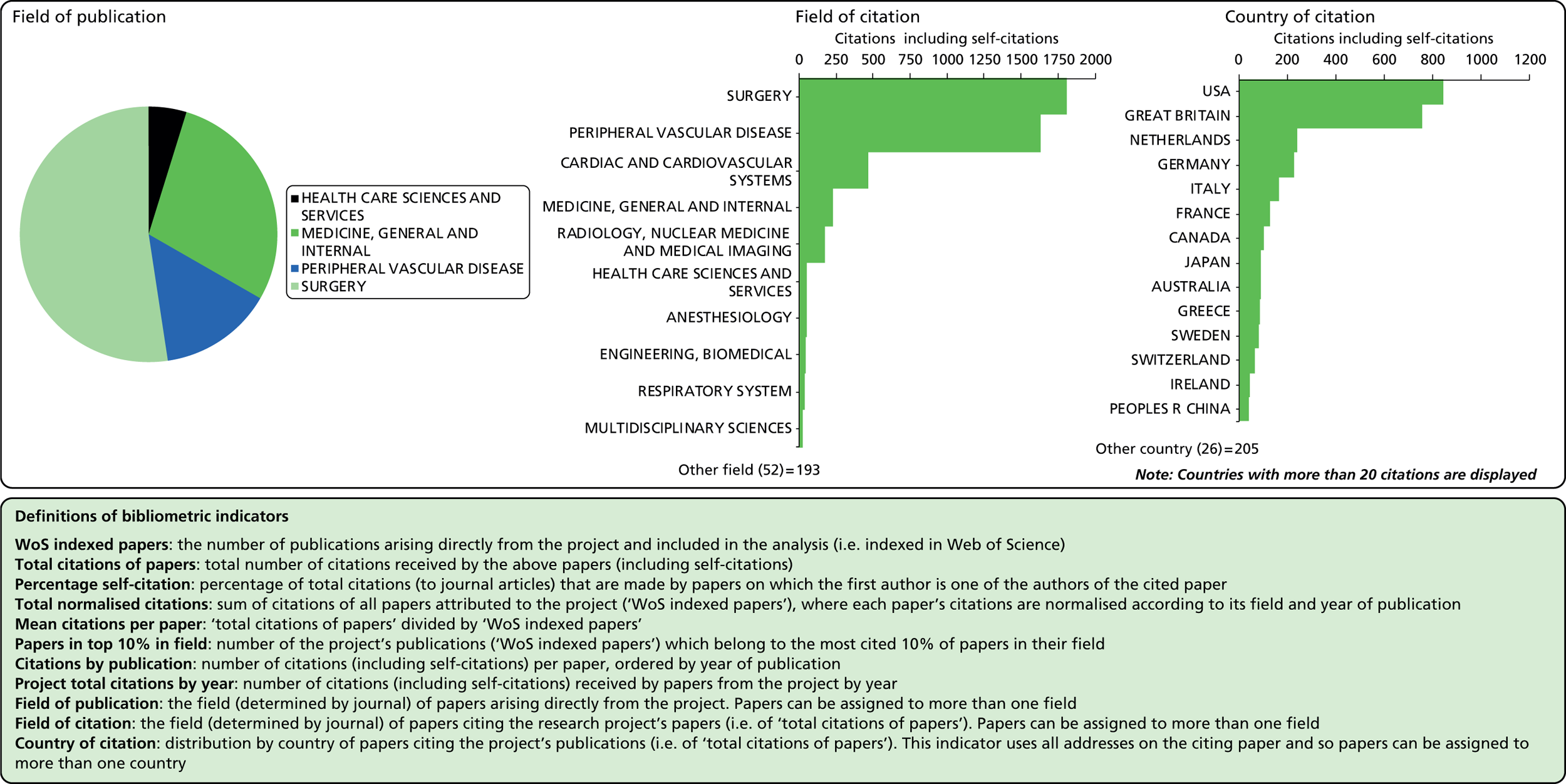
The primary outcome studied was mortality (operative, all-cause and AAA related). In 2004, the first results of the study were published covering 30-day mortality, in which EVAR performed better, with operative mortality rates of 1.8% compared with 4.3% for open repair. 447 However, this clear benefit was lost over longer timescales, as shown by results published in 2005, which showed no significant difference in all-cause mortality between the two groups over 2 years. 461 The EVAR procedure was more expensive than open repair (mean difference £1177) and given the lack of any difference in outcomes, was not found to be cost-effective, but the model was sensitive to specific assumptions. 446 The study also looked at outcomes over the longer term. Using a measured design to determine aneurysm-related (rather than all-cause) mortality, EVAR performed better than open repair over the first 5 years of follow-up, but, at around 6 years postoperatively the differences between the groups became insignificant. The study has so far followed up patients to 10 years and has found that aneurysm-related mortality is similar for both groups over that timescale. 454 In EVAR 2, the 30-day operative mortality was 7.3% in the EVAR group, but the EVAR group later demonstrated a significant advantage in terms of AAA-related mortality, but this became apparent only after 4 years. 462,465 However, this advantage did not result in any benefit in terms of all-cause mortality. Overall, as EVAR was more expensive than no intervention (mean difference £10,222), it was not found to be cost-effective. 446
According to members of the project team, there is some debate as to whether or not it is more appropriate to consider AAA-related mortality or all-cause mortality. Critics might suggest that EVAR is not beneficial, as it is not shown to reduce all-cause mortality. However, the alternative perspective is that it is not intended to treat all potential health risks for what, it should be noted, is a cohort of elderly men (of patients receiving either EVAR or open repair, 97% were aged > 60 years, 73% were aged > 70 years, and 87% were male). 466 Often they will die of unrelated conditions and it is debatable whether or not these should be considered as part of the outcomes for this intervention.
Several other trials have been conducted internationally, addressing the question investigated in the EVAR 1 trial: the comparison of EVAR to open repair. However, no other trials have been conducted echoing the EVAR 2 study: i.e. comparing EVAR to medical management for those unfit to undergo open repair. The other trials conducted, which are comparable to EVAR 1, are summarised below.
-
The Dutch Randomised Endovascular Aneurysm Management (DREAM) trial A trial using a similar protocol to EVAR 1 based in the Netherlands, based on 351 patients across 24 Dutch and four Belgian hospitals. Started soon after the EVAR trials, this study has published results on operative mortality and longer-term outcomes, with similar findings to the EVAR 1 trial. 467–469
-
The French Anévrisme de l’aorte abdominale, Chirurgie versus Endoprothèse trial According to Greenhalgh, this study was hampered by both bureaucratic delays to start-up, and the favourable 30-day mortality results from the EVAR 1 and DREAM trials in terms of recruitment. It began in 2003 but closed in 2008 after recruiting just over 300 patients. The results, in contrast with the other three trials, suggested no difference in operative mortality between the open and the endovascular repair arms, 0.6% versus 1.2%, respectively. 470
-
Open Versus Endovascular Repair trial A US trial recruiting across 43 centres through the Veterans Affairs program, this study looked at a slightly younger, fitter population than the EVAR trial, between 2002 and 2008, with operative mortality and 2-year outcomes published in 2009,471 and long-term results released in 2012. 472 The study found no significant difference in the primary outcome of long-term, all-cause mortality between the EVAR and open repair, with the short-term survival advantage seen for EVAR fading over the long term.
The HTA programme has now funded the study team to continue to monitor the cohort out to 15 years. None of the other three large trials conducted have followed up for this length of time so the study is important in terms of looking at the long-term outcomes following EVAR compared to alternative treatment approaches and potential long-term complications.
The key contribution of this study therefore is to show whether or not EVAR is safe over the long term. Although it is not found to be cost-effective in these studies when looking at mortality as a key outcome, it is still being used more widely. This is partly because, as described above, the use of all-cause mortality as an outcome for this group could be debated. However, it is also partly because EVAR is typically preferred by patients. This is because it is less painful and can be done under local anaesthetic – it is not major surgery in the same way as open repair. So given this preference and that EVAR is likely to be increasingly used for other reasons, such as commercial pressures, it is important to confirm whether or not it is stable and reliable over the long term. Training is required to conduct the procedure. As part of the trial, the research team looked at the number of operations needed as part of the training procedure and concluded that this number is around 30–40 operations. This means an investment in training is required for effective implementation. However, given that the operations are increasingly being carried out in a limited number of high-volume centres nationally, this is becoming less problematic.
As noted above, the EVAR 2 trial was the only trial comparing EVAR to no intervention for patients who were unfit to undergo open repair. However, there were some challenges with this trial. As described in the previous section, there was some crossover between randomised groups, i.e. individuals who were randomised not to have treatment then ended up having it anyway. Some suggested that there was also not a very clear definition of what is considered ‘unfit’, and this was particularly criticised in the USA. Finally, the trial design was based on American national history data on rupture by size of aneurysm. In the EVAR 2 trial, the rupture rate was not as high as expected, which meant that the trial might have been underpowered. Because of these issues, and potentially also because the findings of this study contradicted the assumptions or expectations of some vascular surgeons, uptake of these findings has been more problematic, particularly in the USA.
Benefits to future research and research use
Capacity building and career development
There is no clear evidence of impact of the study on the CI, Roger Greenhalgh, who was already a well-established researcher at that time. Indeed, Janet Powell, Louise Brown and Roger Greenhalgh all had similar roles in the prior UKSAT. 443 Since the study, Powell has received a lifetime achievement award from the Vascular Society (in 2012) but this is probably for her wider work, not just the EVAR study. Greenhalgh was made Honorary Fellow of the British Society of Interventional Radiology in 2006, and received a Honorary Fellowship from the Royal College of Surgeons Ireland in 2007, along with many other honours over the period 1999 to the present, although again the extent to which this can be attributed to this work is not clear. One of the other study researchers, Thomas Wyss, won the European surgical association’s first prize for a publication in 2010 in the Annals of Surgery based around these data. 452 During the completion of the EVAR Trials, Louise Brown moved more into statistical analysis and away from trial management, which is reflected in her subsequent career path and training as a qualified medical statistician. This was in part through some of the opportunities offered within the study, but also due to her part-time studies conducted alongside this work.
Brown outlined two other capacity-building contributions made by the study, which were not directly related to the careers of the key study members. The first was wider capacity-building opportunities for others working at Imperial College London in terms of learning how to set up and run trials in this area, through mentoring and observation with members of the study team who had previously run the UKSAT443 and were quite experienced. This reflects a wider issue that Brown raised, which was that large multicentre randomised trials were not commonplace in the vascular surgery community until the 1990s, and Greenhalgh and Powell, along with others, were one of the early groups to gain a lot of experience in conducting trials in this field. As such, they became a source of knowledge and advice for others in vascular surgery. Second, Brown also notes that several research fellows were involved in the work, looking, for example, at data on secondary outcomes, and that this research helped them to achieve their MD or MS qualifications. In particular, she notes that several fellows came from the University of Bern in Switzerland to work with them and this relationship developed such that they had a semi-regular sabbatical year arrangement with a group of researchers there, allowing fellows to come and work with the data they had available and learn from their trial design and management practices.
Targeting of future research
The HTA has now funded the study team to continue to monitor the cohort out to 15 years. None of the other three large trials conducted has been followed up for this length of time, so the study is important in terms of looking at the long-term outcomes following EVAR compared with alternative treatment approaches and potential long-term complications.
The team that conducted the EVAR study have now received the data from the other three trials that have been conducted and have been funded by the HTA programme to conduct an individual patient meta-analysis across all four trials. They hope that this larger data set will allow them to look in more detail at when and how complications occur to try and understand how the risk of uncommon but devastating complications can be minimised.
The HTA are also funding a further follow-on study stemming from the EVAR trial looking at whether or not EVAR should be performed in an emergency situation. This study, the IMPROVE473 trial (Immediate Management of Patients with Rupture: Open Versus Endovascular Repair), is a RCT looking at the use of EVAR compared with open repair for patients with a ruptured aneurysm, covering the influence of a range of factors including the time and manner of hospital presentation, fluid volume status, type of anaesthesia, type of endovascular repair and time to aneurysm repair. The primary outcome measure is 30-day mortality and the study is focused on patients with a proven diagnosis of ruptured or symptomatic AAA. This study is led by Janet Powell, who was involved in the EVAR study, and others, such as Greenhalgh, are also involved in the project. The 30-day results have already been published and show that EVAR offers no benefits over standard care. 473 Patients are now being followed up out to 12 months.
Interface B: dissemination
Dissemination efforts focused on academia and practitioners. The first route for dissemination was through publication in high-circulation journals. The work was published in journals such as the New England Journal of Medicine, The Lancet and Annals of Surgery. The work was also presented extensively at conferences and seminars, nationally and internationally, covering most of the major events in the field at the time. The work was presented at events in the USA and Australia in particular, but also in other countries. Examples include meetings of the American Society for Vascular Surgery, the Cardiovascular and Interventional Radiology Society of Europe and the European Society for Vascular and Endovascular Surgery. In the UK the relevant national society meetings (the Vascular Society and the British Society of Interventional Radiology) were important forums at which the work was presented. However, communication in the UK was thought to be in some sense less important, as most of the relevant members of the surgical world in the UK were involved directly in the study. There were no significant differences in terms of the findings between groups so most people were easily convinced by the findings. It could also be argued that in terms of EVAR 1, the findings were easy for practitioners to accept, as the trial confirmed their expectations that EVAR offered benefits over open repair. It was more challenging, perhaps, according to interviewees, to convince practitioners in the USA that the results, particularly with regard to the EVAR 2 study, were correct. Other dissemination routes described by the team include a video demonstrating the process of conducting an EVAR procedure, a front-page article on the paper Vascular News (of which Greenhalgh is Editor in Chief) and inclusion in relevant society newsletters. No extra funding was received from dissemination from the HTA programme or elsewhere. Typically, invited speaker costs were paid by the event organisers.
Stage 4: secondary outputs
The NICE Interventional Procedure Guidance (www.nice.org.uk/guidance/ipg163/documents/interventional-procedure-consultation-document-stentgraft-placement-in-abdominal-aortic-aneurysm) (issued 2006) states that ‘current evidence on the efficacy and short-term safety of stent–graft placement in abdominal aortic aneurysm appears adequate to support the use of this procedure’ based largely on the EVAR data, supplemented by a systematic review. 474 The guidance calls for more data on long-term outcomes. Similarly, a NICE technology appraisal (in 2009) (www.guidance.nice.org.uk/ta167) suggests that ‘Endovascular stent–grafts are recommended as a treatment option for patients with unruptured infrarenal AAAs, for whom surgical intervention (open surgical repair or EVAR) is considered appropriate’, echoing the findings of the EVAR study. Here, EVAR 1 was one of four trials used as evidence on EVAR compared with open repair; EVAR 2 provides the only evidence on a comparison with non-surgical management. EVAR was one of two main sources of economic data. This impact on UK guidance may have been partly facilitated by Greenhalgh’s involvement in the guidelines committee for the guidance produced in 2009. The work has also been cited in international guidelines including those of the European Society for Vascular and Endovascular Surgery475 and the American Society for Vascular Surgery,476 as well as a number of systematic reviews in the area,477,478 including a recent HTA programme-funded review. 479
This study in particular was influential for three main reasons. First, it is the only one of the four large-scale studies of EVAR that included long-term follow-up to measure reliability over 10 years and more. Second, it is the only study from that group that looked at patients who were not fit for open repair but who may be suitable for EVAR. Finally, the economic analysis that was included made the study particularly useful in considering the implementation of the treatment in the UK health system.
The work may have also had some impact on industry, which now produces the stents needed for the EVAR procedure. Devices are no longer home-made and are produced by four main suppliers (Cook, Medtronic, Gore and Vascutek) in what is estimated to be a global market for EVAR devices of approximately US$1.4B (according to a specialist EVAR device producer, Lombard Medical). 480 Depending on the extent to which uptake in the UK and other countries can be attributed (at least in part) to this work, part of that industry stems from these findings. According to interviewees, industry has modified the devices in response to some of the challenges demonstrated through research. For example, in response to evidence of device leakage, in some cases leading to high mortality levels, published in 2010, companies have since made adjustments to the devices to try and prevent this problem from occurring.
Stage 5: adoption by practice and the public
The usage of EVAR has increased dramatically, with evidence of a spike in use across Europe coincident with the publication of the key results from this study (in 2004, 2005 and 2010). In these years, a 14% or higher year-on-year increase in the use of EVAR was seen, with as high as 39% increase in 2004–5 (Roger Greenhalgh, 2014, personal communication).
In the UK, data from the National Vascular Registry show that EVAR was being used for 66.8% of cases for elective infra-renal AAA repair by 2012 (and 62.1% in 2010, 65.9% in 2011). Mortality rates before discharge were also favourable, at 0.8% for EVAR compared with 3.8% for open repair, although longer-range data are not available. The average length of stay was also shorter for EVAR patients, with a median of 4 days compared with 9 days. 481 Over a longer time range, between 2008 and 2012, 60% of patients undergoing elective treatment for AAA received EVAR in the UK, with mortality before discharge of 0.9% for the EVAR group compared with 4.1% for the open repair group. 466 The available data in the National Vascular Registry suggest that EVAR rates were much lower in the UK as recently as 2006–8, although the data over this period are less complete (EVAR accounts for 29% of procedures in 2006, and 44% in 2007 and 2008). 482
Uptake in the UK has been influenced by reconfiguration of vascular surgery services over the last few years. Owing to inequitable provision, there was a government-level decision to focus services into a limited number of high-volume centres reflecting, more widely, the observation that outcomes in such centres are typically better. 483 In parallel to that, the Vascular Society has introduced a quality improvement programme for aortic surgery since 2009, part of which is the recommendation that centres that are unable to offer EVAR as a treatment option should be able to show that they could refer patients to another centre for that treatment. 484 The overall result is that these larger centres all now need to be able to (and do) offer EVAR as a treatment option (as centres will not want to send work away). This has been a significant enabler in the uptake of the new technique. Because, as shown in the study, a reasonable level of training and experience is required to conduct EVAR, and it requires both a vascular surgeon and a radiologist to conduct, it may have been more difficult to introduce at smaller, lower-volume centres.
One potential barrier to uptake was cost. During the trial, the trial covered the costs of the devices. Once the trial finished, money had to be found from elsewhere to cover the device cost to use EVAR. This potentially would have deterred uptake if it had not been for the wider reconfiguration process as described above, which meant that if a centre could not offer EVAR, they had to send patients away, which would be even more costly. It was also suggested at interview that although the cost of EVAR devices are higher, there are cost savings in terms of bed occupancy so the overall financial picture is not clear.
Another factor suggested at interview as influencing the uptake of EVAR is the fact that vascular surgeons now have to publish their outcomes across the country. That makes it very desirable to reduce aortic mortality levels, and using EVAR is one way to do that.
Finally, the results of the EVAR 1 trial matched patient preferences, which made uptake much more straightforward. The procedure is much less invasive and less painful. As well as matching patient preferences, it was also what industry wanted to hear, and what the proponents of the technique among the surgical community want to hear. There was the suggestion at interview that if the study had produced clear findings to show that open repair was the better approach, the message might have been less readily accepted and adopted.
It should also be noted that despite all centres being required to offer EVAR, there are still significant variations in practice across the country. Although average levels of use are around 65%, there are some centres that are using EVAR in around 85% of cases. This raises questions of off-label use of the devices and adjunct procedures. As Beard noted:
It raises questions of whether [some centres] are implanting grafts outside instructions for use, off label. That’s a real issue because the EVAR trial had to be on IFU (instructions for use) but subsequent analysis showed a lot of cases in some centres outside IFU. That affects long-term outcomes in terms of reintervention rates.
There is evidence that off-label use has become a fairly significant issue in the USA, which means that there is likely to be the need for significant levels of reintervention in the future. 485
Stage 6: final outcomes
There are several benefits to patients having EVAR compared with open repair, where the treatment is appropriate. The first is that the procedure is less painful and can be done under local anaesthetic – it is not major surgery in the same way as open repair. Over the short to medium term at least, outcomes in terms of mortality are also better, as outlined above. There are some potential negatives, such as the need for surveillance scans, which can be inconvenient if that involves significant travel. Over the longer term, the benefits are less certain in terms of outcomes, which reinforces the importance of the ongoing work.
In terms of cost-effectiveness, the analysis in the study suggests that EVAR is not more cost-effective than open repair, as the improvement in outcomes and the increased cost effectively cancel each other out. However, these cost-effectiveness calculations were questioned by several interviewees for two main reasons. First, the calculations are based on follow-up using CT scanning, which is expensive and potentially dangerous. Now a significant proportion of patient follow-up is conducted using ultrasound, which is much less expensive. Second, although the devices are more expensive, the length of stay following treatment by EVAR is significantly shorter, and the cost of an intensive treatment unit bed is relatively high. Overall, this means that it is difficult to make any clear estimation of whether or not the increased use of EVAR has positive or negative impacts on the health system from an economic perspective.
There have been clear economic benefits of increased use of EVAR for those companies that produce the devices. There is some evidence, as stated in the previous section, that this is at least partly attributable to the EVAR trials, at least in Europe, so it is likely that the study made some economic contribution through this route.
Table of payback
Payback details for this case study are provided below in Table 27.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | Eighteen publications including many in high-profile journals Evidence on use of EVAR in patients unfit for open repair, which has not been replicated Long-term follow-up for patients undergoing EVAR in place of open repair, which is not replicated elsewhere Evidence that EVAR offers advantages over open repair in terms of short-term mortality as seen in other trials, but all-cause mortality advantages are not sustained in the long term |
| Research Targeting and Capacity Building | European surgical association’s first prize to Thomas Wyss Follow-on funding received for the study team to follow up to 15 years, and to conduct patient subgroup meta-analysis across all trials of EVAR vs. open repair A largely similar team is conducting the IMPROVE trial, a RCT looking at the use of EVAR compared with open repair for patients with a ruptured aneurysm |
| Informing Policy and Product Development | Provided some of the key evidence for NICE Interventional Procedure Guidance and a NICE technology appraisal advising that the technology can be used Cited on international guidelines including those of the European Society for Vascular Surgery and the American Society for Vascular Surgery |
| Health and Health Sector Benefits | Evidence of increase in use at the European level, which seems to align in terms of date with the release of key publications from this study Increased uptake in the UK, although this might have been supported by wider factors around reconfiguration of services |
| Broader Social and Economic Benefits | Increased use of EVAR brings benefits for EVAR device manufacturers, which can at least partly be attributed to this study EVAR is typically preferred by patients as less invasive and painful, although there are some down sides in terms of follow-up requirements |
Appendix 4.7: accurate, practical and cost-effective assessment of carotid stenosis in the UK
Summary
Through a response to a call from the HTA, Wardlaw and colleagues conducted a study to review different mechanisms for diagnosing blockages in blood vessels feeding the brain, with the aim of reducing the risk of stroke. They conducted a systematic review and modelling to determine whether or not novel non-invasive treatments were as effective as the traditional (invasive) therapy. Results showed that the non-invasive treatments were cost-effective, and highlighted that the treatment of choice depended on availability and familiarity with the procedure. The results have been included in guidance internationally including in the UK, Canada, USA and Australia. The reduction in strokes is estimated to have saved the NHS £30M annually through reduced admissions and care costs. Data from this analysis were also used in a further HTA study on the use of magnetic resonance brain imaging in stroke prevention, published in 2014. 486
Introduction to case study
Background
Scientific background
The carotid artery is the large vertical artery running from the aorta towards brain. A sticky deposit (plaque) often builds up where the artery divides at the base of the neck. This build-up narrows the artery and if pieces of the plaque break off and travel up the artery to the brain it can block circulation and cause death of brain tissue. The build-up can be prevented by treatment with drugs, but, in more severe conditions, surgical intervention can be required. This procedure can reduce the risk of stroke, in particular among those at risk after a transient ischaemic attack (TIA). This is a brief episode of neurological dysfunction resulting from an interruption in the blood supply to the brain or the eye, sometimes as a precursor to a stroke.
Accurate carotid imaging is used to assess the level of plaque and it is important to avoid operating on patients with less severe narrowing, i.e. in cases where the risks of surgery may outweigh the benefit.
Chief investigator’s background
Dr Joanna Wardlaw is a clinical academic, splitting her time between her role as a NHS consultant in neuroradiology and investigating the causes of stroke and brain vascular disease.
The case study approach
The case study was randomly selected to investigate an example of impact from a study funded as an evidence synthesis through the HTA. As shown in Table 28, two individuals were interviewed for this case study: the CI and the modeller on the project.
| Interviewee | Reason for interview |
|---|---|
| Joanna Wardlaw | CI |
| Matt Stevenson | Conducted modelling on the project |
Stage 0: topic/issue identification
The call for proposals for research on the topic of carotid stenosis was released by the HTA programme. It was an open competitive call. The following research question was identified by the Diagnostic Technologies and Screening panel: ‘What is the best method or combination of methods for assessing carotid artery stenosis?’.
We have identified two key factors that influenced the research team’s ability to work in this area and the need to commission this research, as detailed in Box 7 and described below.
-
Project teams’ disease and methodological expertise.
-
Change in availability of technology.
Project teams’ disease and methodological expertise
The authors constituted a panel of experts in stroke, imaging, vascular surgery, statistics and health-economic modelling. Individuals from different clinical settings were members of the team, to ensure that practice across settings was taken into account in the study.
Change in availability of technology
Carotid stenosis was originally measured using intra-arterial angiography (IAA), which is a risky invasive procedure. In the 1990s less-invasive imaging tests – ultrasound, magnetic resonance angiography (MRA), computed tomographic angiography and contrast-enhanced MRA (CE-MRA) – were becoming available. However, there was a lack of evidence on whether or not any of these procedures should be used over the established IAA procedure.
Interface A: project specification and selection
The application was submitted by Joanna Wardlaw, approved, and then the team commenced work. Wardlaw did not remember there being specific feedback on the application process in this instance, but noted that the HTA required an interim and final report during the course of the study.
Stage 1: inputs to research
Expertise
The authors were a panel of experts in stroke, imaging, vascular surgery, statistics and health-economic modelling. Individuals from different clinical settings were members of the team, to ensure that practice across settings was taken into account in the study. As Stevenson phrased it:
As Health Economists, certainly the way we work [for HTA], is that a lot of the time we come in, learn as much as we need to know about the disease to make the model have face validity and be clinically plausible, which is why there is always clinicians involved, we use our maths skills to make the model, turn the handle and write up the results.
He emphasised that the modellers’ role is to design and build models to a specific specification: ‘You need to learn new things [about disease areas], but you are an expert in building the models.’
Funding
Funding of £110,572 was provided by the HTA. Wardlaw felt that the project was underfunded, and with additional resources it could have been written up and published quicker. In particular, she linked the funding of the study to its impact, and felt that more generous funding would have been appropriate because of the monetary impact on changing practice (see Stage 5: Adoption by practice and the public). The funding was obtained in August 2003 and the report was published in 2006.
Techniques
Stevenson and the team, headed by Alan Brennan from the University of Sheffield, brought modelling expertise to the project. Stevenson had not worked on carotid artery stenosis previously (or since).
Stage 2: research process
The project aimed to determine the cost and benefits of the different techniques to enable comparison, and to understand the accuracy of the procedures. Using a combination of clinician knowledge, complemented by health economists and modellers, they developed a range of scenarios around the different machinery available in different hospitals. This was facilitated by the clinical members of the team who spanned a number of locations within the UK. They also reviewed scenarios, such as if the initial examination was conducted by a technician without medical content knowledge. In these instances, if you wanted a second opinion, which technique should you use?
As summarised in the HTA journal article, the aim of the study was to assess the use of less-invasive imaging techniques against the standard test through the following steps:
-
The accuracy of less-invasive carotid imaging was systematically reviewed and supplemented by individual patient data from primary research and audit studies in the UK.
-
A systematic review of the costs of less-invasive tests, outpatient clinics, endarterectomy (the gold standard invasive technique) and stroke was performed.
-
A model of the process of care following a TIA or minor stroke was developed, populated with data from stroke epidemiology studies in the UK, effects of medical and surgical interventions, outcomes, quality of life and costs.
-
A survey of UK stroke prevention clinics provided typical timings for imaging.
-
Some 22 different carotid imaging strategies were evaluated for short- and long-term outcomes, QALYS and net benefit.
The research took approximately 18 months to 2 years to complete. Funding was awarded in August 2003, and the monograph published in 2006. Different members of the team were responsible for different elements of the study:
-
Joanna Wardlaw (CI) responded to the HTA call, conceived the project, wrote the outline and full application, assembled the collaborative group, managed the project, coordinated the three full project meetings and had several other meetings with individual investigators, obtained data, supervised and conducted the systematic review, individual patient data analysis and UK survey of stroke prevention practice, obtained cost data, wrote the draft report and edited the revised report.
-
Francesca Chappell (Medical Statistician) contributed to the design of the study through discussions, performed the systematic literature review and the individual patient data analysis (including data transformation), attended three meetings to discuss progress and results including presenting data at meetings, kept meeting minutes, acted as information distributor for the study, performed the survey of UK stroke physicians, drafted two chapters and approved the final report.
-
Matt Stevenson (Senior Operational Researcher) contributed to the design of the study through discussions, designed and built the model in discussion with others, incorporated data provided by other parts of the project, ran and interpreted the model, drafted two chapters and approved the final report.
-
Enrico De Nigris (Research Assistant) performed the systematic literature review of costs and QALYs for stroke, obtained primary data on imaging tests in Sheffield, drafted one chapter and approved the final report.
-
Steven Thomas (Senior Lecturer and Consultant Vascular Radiologist) helped to assemble the collaborative group, edited the application, helped to develop and obtain data for the model, contributed to the design of the study, attended five meetings to discuss progress and consider results, helped to edit three chapters and approved the final report.
-
Jonathan Gillard (Reader and Honorary Consultant Neuroradiologist) provided individual patient data and data on costs of investigations from Cambridge, contributed to the design of the study through discussions, attended two meetings to discuss progress and consider results, helped to edit one chapter and approved the final report.
-
Elizabeth Berry (Senior Lecturer) provided data on MRA from a recent previous systematic literature review, helped to draft the application, contributed to the design of the study through discussions, attended three meetings to discuss progress and consider results, and helped to edit and approve the final report.
-
Gavin Young (Consultant Neurologist) contributed to the design of the study through discussions, attended three meetings to discuss progress and consider results, provided data for the individual patient data analysis, and helped to edit and approved the final report.
-
Peter Rothwell (Professor of Clinical Neurology) provided additional data from the carotid surgery trial data set and Oxfordshire Community Stroke Project and Oxford Vascular Study to help to populate the model, assisted in the design of the model and underlying assumptions, and approved the final report.
-
Giles Roditi (Consultant Radiologist) contributed to the design of the study through discussions, attended two meetings to discuss progress and consider results, provided data for the individual patient data analysis, and helped to edit and approve the final report.
-
Michael Gough (Consultant Vascular Surgeon) contributed to the design of the study through discussions, attended two meetings to discuss progress and consider results, suggested data sources for the individual patient data analysis, and helped to edit and approve the report.
-
Alan Brennan (Director of Health Economics and Decision Science) helped to write the full application, supervised Matt Stevenson and Enrico De Nigris, contributed to the design of the study through discussions, supervised the construction and running of the cost-effectiveness model, attended two meetings to discuss progress and consider results, and approved the final report.
-
John Bamford (Consultant Neurologist and Cerebrovascular Physician) contributed to the design of the study through discussions, provided data for the survey of UK stroke prevention clinics, attended a meeting to discuss progress and consider results, and approved the final report.
-
Jonathan Best (Professor of Medical Radiology) assisted with selection of papers and data extraction for the systematic review, attended one meeting to discuss progress and consider results, helped draft one chapter and approved the final report.
At the time that the study was carried out there was no routine patient involvement in studies. In more recent studies this has been included. As Wardlaw stated it has ‘become expected and essential to have participant engagement. It was always helpful, but wasn’t previously explicit’.
Wardlaw noted that communication with the HTA programme (through NETSCC) was only to monitor progress against the contract, and stated that there was no engagement with NICE, as the work fed into their process only subsequently (see Stage 4: Secondary outputs, Changes to guidance).
Stage 3: primary outputs from research
Knowledge
Overall, the study found that there were minor differences in accuracy between the three novel imaging techniques. The important factor was which could be accessed fastest, as delaying the scan could lead to a delay in diagnosis, during which time the patient could suffer a stroke.
The study showed that clinicians should be using the technique that was readily available and to conduct it regularly, so that the clinician was as accurate as possible, as a result of practice and familiarity with the procedure.
As presented in the HTA report, the key findings of the study were:
-
In the UK, less-invasive tests can be used in place of IAA if radiologists trained in carotid imaging are available. Imaging should be carefully audited.
-
Stroke prevention clinics should reduce waiting times at all stages to improve speed of access to endarterectomy.
-
In patients presenting late after TIA, test accuracy is very important.
-
More data are required to define the accuracy of the less-invasive tests, with improvements made in the data collection methods used and how data are presented. Consideration should also be given to the use of new technologies and randomised trials.
Wardlaw commented that the requirement to publish in a HTA monograph made it difficult to publish in high-impact journals, as some would not accept the study due to prior publication. This solution is either to conduct additional research, or to make the existing research sufficiently different. Wardlaw stressed that the HTA journal does not have ‘a bad impact factor, but in my field people haven’t found it and therefore you don’t see it cited’. Related to this, HTA articles are not as widely read and you have to ‘burrow to find the detail you want’. To avoid this it is necessary to publish in key peer-reviewed journals, but this doubles the time taken to publish. There were six publications in total resulting from the study. 487–492 Bibliometric analysis was conducted on a subset of two of these, as others were brought to our attention through interviews after the analysis had been conducted. Both publications were in the top 10% in their field (Figure 18).
FIGURE 18.
Bibliometric analysis for publications from the study entitled: accurate, practical and cost-effective assessment of carotid stenosis in the UK.
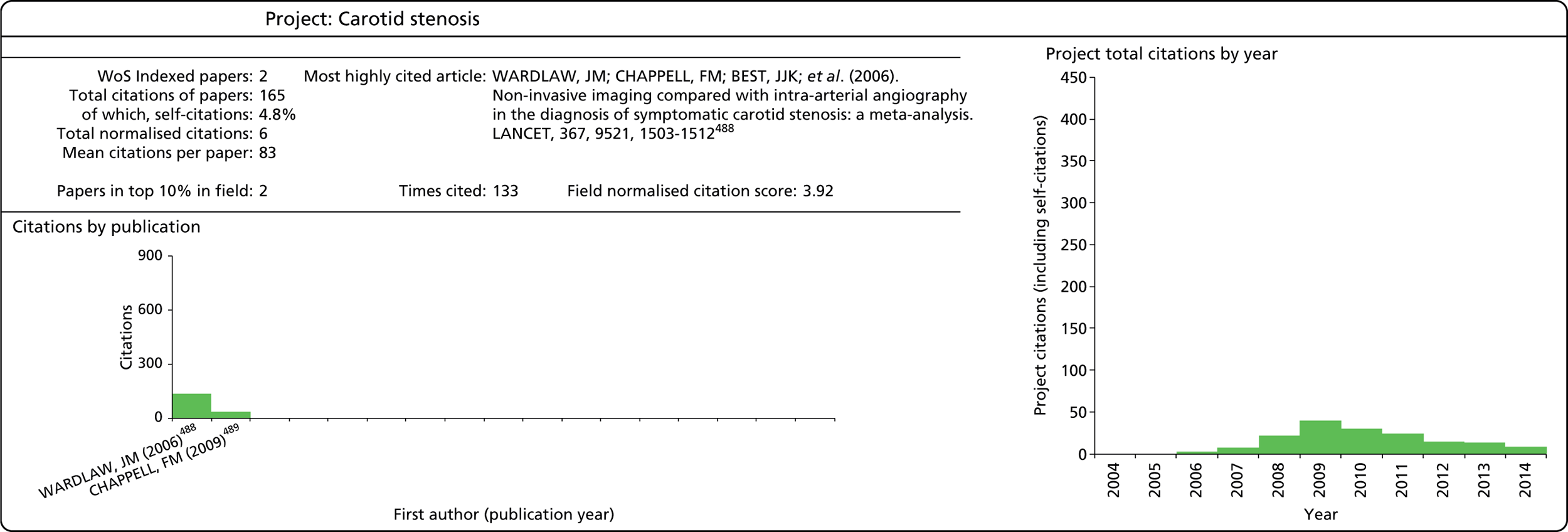

Benefits to future research and research use
Capacity building and career development
The study had an impact on the research career of Dr Chappell, who subsequently continued to research in this area, and received additional funding to complete a PhD building on data from this project (£114,598 from the Chief Scientist Office).
When pressed, Stevenson felt that this specific study did not have an impact on his career. However, taken as a body of work, the HTA projects he has worked on have allowed him to develop, through working with others, to becoming and independent researcher, and now playing a broader role in the oversight of others. In that vein, successful completion of this study, among others, had led to his promotions.
Wardlaw felt that the HTA projects were ‘hard work’ and took up a ‘substantial amount of time’, especially during reporting. There is an opportunity cost, as there were other avenues about the mechanism and treatment of stroke that she could not pursue while undertaking projects for the HTA.
Targeting of future research
This project is part of a body of work that Wardlaw has conducted for the HTA. Prior to this study, she led another assessment for the HTA on the topic of assessing cost-effectiveness of imaging for stroke diagnosis (entitled ‘What is the best imaging strategy for acute stroke?’493) and has since led a subsequent project on the use of brain imaging in TIA and stroke prevention. 486,494
The methodology has built across the three studies,486,494,495 from the use of a decision tree as to when to use imaging in diagnosis in the first study, to the inclusion of the element of time in the second study. The most recent study has combined these.
Stevenson stated that this project did not have an impact on his future research agenda, and that he has not worked on the subject of carotid stenosis since.
The project listed the following recommendations for further research:
-
More data are required to define the accuracy of less-invasive tests used at 50–69% stenoses, and in combination. Wardlaw’s colleague, Chappell, has been involved in updating the literature in this area.
-
The methodology for primary studies of the accuracy of less-invasive imaging tests needs to improve. The Cochrane Diagnostic Tests Methods group and the application of standards for reporting diagnostic test accuracy studies is better known about since this study, but in Wardlaw’s opinion there are still large loopholes.
-
Clearer presentation of data in reports of primary studies of diagnostic test accuracy would enable more key sensitivity analyses to be performed in future meta-analyses. As described above, reporting standards are in place but not enforced by many journals.
-
Methods of evaluating new technologies as they emerge are required.
-
Consideration should be given to new randomised trials to evaluate different less-invasive imaging strategies before endarterectomy.
-
Streamlined methods of collecting data to audit less-invasive tests when used in routine clinical practice are required to monitor test accuracy. Although Wardlaw and colleagues are collecting data she was not sure this was done more widely.
Interface B: dissemination
Wardlaw and Chappell presented the research at various European and UK conferences, around the topic of stroke, radiology and cost-effectiveness. Wardlaw noted that there was also a summary of the research on the HTA website, and she has been invited to give talks on the work.
Stevenson did not personally disseminate the report, as there was no funding in place to present at a conference. He stressed that ‘the papers [HTA article and journal article in Stroke] were good papers and so other than a conference presentation there were no other options’. 487,490
Wardlaw shared the research with her clinical network to ensure the evidence was known about by those reviewing and producing guidance.
There was no direct attempt at dissemination outside academia.
Stage 4: secondary outputs
The information below was provided by Joanna Wardlaw, who, as a clinician, is involved in the disease area on a day-to-day basis. When asked, Stevenson was unaware of the impacts of the research. He said, ‘as a modeller you only have “fleeting knowledge of a topic” and I have now moved on to look at different areas since. The only reason to look back is if subsequent projects come along’.
Changes to guidance
As the study was bid for, rather than being commissioned on behalf of NICE, the report did not automatically go to the NICE Appraisal Committee. Stevenson (part of the project team based at a TAR unit) saw this as a potential barrier to the uptake of this stream of research compared with Technology Appraisals. In such situations there was the risk that the research ‘could sit on the shelf’. He stressed that without the link to NICE, there is no direct funding earmarked to change practice and, therefore, even if the results are conclusive about a change in treatment, it may not be implemented as there is not provision for it within the health system.
However, Wardlaw was active in ensuring that the work was on the agenda thus informed subsequent guidance, through her clinical networks.
The NICE guidance for stroke, developed by the Royal College of Physicians in 2008, cites the study as evidence for the use of imaging techniques for the assessment of risk of stroke. It states:
All people with suspected non-disabling stroke or TIA who after specialist assessment (see section 5) are considered as candidates for carotid endarterectomy should have carotid imaging within 1 week of onset of symptoms. People who present more than 1 week after their last symptom of TIA has resolved should be managed using the lower-risk pathway. 495
This work also been cited in SIGN guidance,496 which states, referencing the HTA journal article: ‘A good quality meta-analysis showed that the most cost-effective diagnostic strategies for carotid stenosis are those that offer surgery to a larger proportion of patients quickly after the warning TIA/minor stroke’. As a result this paper and one other led to the following recommendations within the guidelines:
-
All patients with non-disabling acute stroke syndrome/TIA in the carotid territory who are potential candidates for carotid surgery should have carotid imaging.
-
Initial carotid imaging with duplex ultrasound or alternative should be performed rapidly once a diagnosis of ischaemic stroke or TIA in the carotid territory is made.
-
Initial carotid imaging should be performed within 48 hours of presentation.
-
Corroborative imaging is recommended to confirm and more accurately grade carotid disease if duplex carotid ultrasound is abnormal.
-
Non-invasive angiographic carotid imaging (CE-MRA) should be performed and interpreted by radiologists who are specifically trained and who have specialist interest in vascular imaging.
Duplex ultrasound criteria for grading of carotid disease should be standardised and regularly audited against another modalities and surgical findings.
Citations in international guidance
In addition to uptake in England, the study has been cited as a major source in guidance and guidelines abroad. 497–500 This guidance, in line with that from NICE, states that non-invasive imaging techniques should be used in a timely manner to detect risk of stroke.
Stage 5: adoption by practice and the public
Impact on teaching of clinicians
The research (and its impact on guidance) has had an impact on the training of clinicians who, according to Wardlaw, are now routinely taught these non-invasive techniques, and given the confidence to apply them. This confidence is important in ensuring that the techniques are regularly used to increase accuracy. The results of the study (and others) have been included in a textbook on the practical management of stroke, authored by Wardlaw and others, which is used by practitioners. 501
Impact on practice and patients
The study has changed practice, from the use of invasive to non-invasive methods and, accordingly to Wardlaw, it has given practitioners confidence to use the non-invasive methods. Further to the inclusion of the techniques of the prevention of stroke in the STROKE guidance in 2008, Wardlaw conducted a study to assess the impact of this change. A survey published in 2008502 showed that fewer patients were waiting > 24 hours for CT brain imaging compared with a survey in 2000–1. In 2012, Wardlaw and others503 commissioned a survey of UK practices to understand what happens in practice. In the mid-2000s, practitioners were still carrying out IAA; by 2010–11, Wardlaw stressed that this practice had vanished and been replaced by ultrasound, which uses ‘no needles and takes 10 minutes’. 494
Stage 6: final outcomes
Cost savings through the prevention of stroke
The introduction of the use of imaging techniques has led to cost savings for the NHS and wider society.
Performing immediate brain imaging in all patients with suspected acute stroke, compared with less-effective strategies, for the 120,000 patients who have a stroke each year in the UK is calculated to have resulted in 6000 more QALYs, and reduced the cost of stroke to the NHS by between £156M and £312M per year.
Based on the possible imaging combinations, Wardlaw estimates that use of these techniques has prevented 1760 strokes per year, making a cost saving of approximately £30M per year, through reduced length of stay in hospital and dependency of patients on services and carers. 490 She attributes these developments to this study and the work of her team on the previous HTA programme-funded grant.
International impact
Owing to inclusion in guidelines around the work, Wardlaw predicts that the cost-saving calculation of the impact in the UK could be multiplied to take into account the global impact.
Table of payback
Payback details for this case study are provided below in Table 29.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | Six peer-reviewed articles (five in addition to the required HTA journal article) In the UK, less-invasive tests can be used in place of IAA if radiologists who are trained in carotid imaging are available Stroke prevention clinics should reduce waiting times at all stages to improve speed of access to endarterectomy In patients presenting late after TIA, test accuracy is very important |
| Research Targeting and Capacity Building | One team member went on to use the data and develop the topic further in through PhD study, for which she was successful in obtaining funding |
| Informing Policy and Product Development | The recommendations from the study have been included in NICE and SIGN guidance, and the report cited Internationally the report has been cited in guidance and guidelines in Canada, the USA, Australia and at a Europe-wide level |
| Health and Health Sector Benefits | The results of the report, and their inclusion in guidance, have changed practice from the use of IAA to non-invasive practice, such as ultrasound As a result, these technologies can be used to support faster diagnosis and therefore prevent stroke Wardlaw estimates that use of these techniques has prevented 1760 strokes per year making a cost saving of approximately £30M per year, through reduced length of stay in hospital and dependency of patients on services and carers The results of the study (and others) have been included in a textbook on the practical management of stroke, which is used by practitioners |
| Broader Social and Economic Benefits | Cost savings through the prevention of stroke in the UK (as above) Cost savings internationally owing to the uptake of the research in guidance overseas, such as in the USA, Canada, Australia and Europe |
Appendix 4.8: Softened Water Eczema Trial
Summary
The SWET48 set out to determine whether or not ion-exchange water softeners could improve atopic eczema in children with moderate to severe eczema, as well as the likely cost and cost-effectiveness of such an intervention. SWET48 found no difference in any of the blinded outcomes, although it did find some small, but statistically significant, differences in some of the secondary non-blinded outcomes that parents observed. SWET48 addressed an important question, of relevance to patients, and contributed to the knowledge base on what is known about effective treatments for atopic eczema. The trial was also important in targeting future research on the connection between water hardness and eczema, and additional non-pharmacological interventions to treat eczema. The research team actively disseminated the research findings via peer-reviewed academic publications, the media and specific dissemination tools targeted towards patients and their carers. SWET48 received international media attention and seems to have had an impact in the UK and internationally. The study has had an impact on the National Eczema Society and has been taken into account by NICE. The main impact of the trial on clinical care is that clinicians can now give evidence-based advice on the use of water softeners for the treatment of eczema, and patients can avoid spending money on treatments that do not work.
Introduction to case study
Background
Scientific background
Eczema is a widespread problem in the UK, particularly in children. Previous studies have suggested that eczema is more common in hard water areas. 504,505 However, most existing treatments suppress only the symptoms of eczema and may have undesirable side effects. SWET48 set out to determine whether or not ion-exchange water softeners could improve atopic eczema in children with moderate to severe eczema, and the likely cost and cost-effectiveness of such an intervention. 506,507 The study was an observer-blind, parallel-group RCT. Participants had an ion-exchange water softener installed in their homes and used softened water for bathing and washing clothes, but continued to drink hard water. Research nurses were blinded to the intervention, but study participants were not. SWET48 found no difference in any of the blinded outcomes, although it did find some small, but statistically significant, differences in some of the secondary non-blinded outcomes that parents observed. The study concluded that it was likely that these small improvements in the non-blinded secondary outcomes were the result of response bias. Overall, the study did not find any evidence that water softeners provide additional benefit to usual care in children with moderate to severe eczema.
Chief investigator’s background
Hywel Williams, Professor of Dermato-epidemiology, was the CI of SWET. 48 He is Director of the Centre of Evidence Based Dermatology at the University of Nottingham, which includes the Cochrane Skin Group and the UK Dermatology Clinical Trials Network (DCTN), and was the founding director of the University of Nottingham CTU. In the early part of Williams’ career, he focused primarily on observational epidemiology, whereas more recently he has focused on systematic reviews and clinical trials, including SWET48 among others. Throughout Williams’ career, childhood eczema has remained a key research interest. Williams has worked closely with Kim Thomas, who was the lead investigator of SWET48 and an associate professor at the University of Nottingham at the time. SWET48 was coordinated from the Centre of Evidence Based Dermatology as one of a portfolio of trials supported through the UK DCTN.
The case study approach
The data collection process for this case study involved a series of interviews, a review of the primary and secondary data sources relating to SWET,48 and a targeted document review on the relationship between hard water and eczema. As shown in Table 30, five individuals were interviewed, including the chief and lead investigators of SWET,48 a consultant dermatologist, the former technical director/manager of the UK Water Trade Association (WTA) and a patient representative from the trial.
| Interviewee | Reason for interview |
|---|---|
| Hywel Williams | CI and Professor of Dermato-epidemiology |
| Kim Thomas | Lead Investigator, involved in all stages of the study, and Professor of Dermatology |
| Carsten Flohr | Consultant Dermatologist and Senior Lecturer |
| Anthony Frost | Former technical director/manager of the UK Water Trade Association |
| David Potter | Retired biochemist and patient representative |
The primary data sources for the case study were the interview data, the trial’s protocol,508 the write-up of the trial in PLOS Medicine,507 the full write-up of the trial in the HTA journal,506 a paper on the use of accelerometers for measuring disease activity in children509 and the NIHR Researchfish data510 on SWET,48 which included information on publications, awards and recognition, engagement activities and influence on policy. The background information reviewed for this study included an article on the links between eczema and the environment,511 a systematic review of treatments for eczema,512 and three ecological studies on the relationship between hard water and eczema. 504,505,513
Stage 0: topic/issue identification
A number of key factors influenced the research team’s decision to work in this area, as detailed in Box 8 and described below.
-
CI’s longstanding interest in childhood skin conditions and eczema research.
-
Ecological studies suggesting a relationship between water hardness and eczema.
-
Anecdotal evidence from patients that softened water improves eczema.
-
Identified research need from an earlier systematic review.
-
HTA-commissioned call.
The Chief investigator’s longstanding interest in childhood skin conditions and eczema research
Prior to SWET,48 Hywel Williams and Kim Thomas both had a history of interest in eczema research, as evidenced by Williams’ extensive publications on eczema and their joint publications on the same topic. 504,511,512,514–553 Shortly before undertaking SWET,48 Williams and Thomas had both been involved in a trial on the use of topical steroids for children with mild or moderate eczema. 531 SWET48 can be seen as a continuation of the Centre of Evidence Based Dermatology’s interest in eczema research.
Ecological studies suggesting a relationship between water hardness and eczema
In 1995, Hywel Williams published an editorial in the BMJ outlining the possible links between eczema and the environment. 511 Shortly thereafter, in 1998, Williams, together with medical geographers at the University of Nottingham, published an ecological study on the relationship between water hardness and the prevalence of eczema in children in Nottinghamshire. 504 That study found a positive relationship between the prevalence of eczema and water hardness, and concluded that domestic hard water may increase the risk of eczema in children. A subsequent ecological study in Japan505 also found a higher prevalence of eczema among children living in hard water areas (a third ecological study in Spain513 also found a higher prevalence of eczema in children living in hard water areas, but this study was published after the start date of SWET48 and thus would not likely have affected the identification of the research topic). These ecological studies provided the evidence base for the hypothesis that water softeners may be effective in the treatment of eczema. However, at that time, no interventional studies existed on the impact of water softeners for the treatment of eczema.
Anecdotal evidence from patients that softened water improves eczema
Anecdotal reports from patients with eczema also suggested that water softeners are an effective treatment for eczema. 508 Patients or their carers commonly reported having observed an improvement in their eczema when visiting a soft water area and would enquire whether or not the purchase of a water softener would improve their eczema.
Identified research need from an earlier systematic review
In 2000, a HTA programme-funded systematic review by Hoare et al. (2000)512 which was led by Hywel Williams, identified the use of water softeners for the treatment of eczema as a topic for future primary research. The UK DCTN was part of the affiliate scheme for submitting trial suggestions on behalf of the British Association of Dermatologists to the HTA programme, through the National Eczema Society, to look at the impact of softened water on eczema.
Health Technology Assessment programme-commissioned call
Shortly thereafter, the NIHR HTA programme put out a commissioned call to conduct primary research into the impact of water softeners on eczema. The commissioning brief specified the following research question: ‘What is the effectiveness and cost-effectiveness of water softeners for the treatment of atopic eczema?’ Hywel Williams and colleagues responded to the commissioning call and received funding from the HTA programme to conduct SWET48 and test the hypothesis that introducing a water softener in the home of children with moderate to severe eczema would improve their condition.
Interface A: project specification and selection
Prior to applying for funding from the HTA programme, the research team had submitted an application to do a similar trial to the MRC, which was initially rejected. None of the interviewees could recall a clear reason for their initial failure to receive funding from the MRC.
Subsequently, the research team spent a lot of time developing the trial design. They conducted an external pilot, funded by Kinetico UK Ltd, before undertaking SWET. 506 The pilot study had three main objectives: (1) to assess the appropriateness of the recruitment methods and trial procedures; (2) to inform calculations of the necessary sample size for the main trial; and (3) to determine whether or not it would be possible to blind participants to their treatment outcome (whether or not they had received a water softener). As a result of this piloting work, the research team realised that it would not be possible to blind participants to the observation because of the impact of ion-exchange water softeners on different observable characteristics of the water in the household (such as markedly increased suds with normal soap usage). This result informed the research team’s decision to conduct a single-blind trial for which the observers, the research nurses, were blinded from the treatment allocation. In addition, the research team noted that participants who had been assigned to the placebo were disappointed at not being able to try a water-softening unit, which might pose a challenge to recruitment. Therefore, the research team decided that a cross-over observational period at the end of the randomised evaluation period would be appropriate so that all participants would be exposed to a water softener in the course of the trial. The pilot probably played an important role in the research team receiving funding for the full trial because they were able to demonstrate the feasibility of conducting an unblinded trial, but with blinded objective outcome measures, to the HTA programme.
In the development of the initial design of the study,506 Williams and Thomas worked with the PIs for each of the sites in which the trial would be conducted. For example, they worked with Tara Dean, Professor of Health Sciences, who was the PI for Portsmouth and the Isle of Wight. Similarly, they worked with Nigel Burrows, a Consultant Dermatologist, who was the PI for Cambridgeshire, and Ian Pollock, a Consultant Paediatrician, who was the PI for Leicester. Andrew Nunn (Professor of Epidemiology), Sarah Meredith (Senior Epidemiologist) and Angela Crook (Senior Statistician) also contributed to the conceptualisation and design of the study. Tracey Sach, a Senior Lecturer in Health Economics, contributed to the design of the economic analysis. The research team also worked with Ian Pallett, a British Water Technical Consultant, and Anthony Frost, then Director of Aqua Focus Ltd, who later became the UK WTA representative. Lastly, the research team worked with David Potter, a retired biochemist, to ensure that patient views were taken into account in the study design.
The research team also consulted with the National Eczema Society, the main patient support group in the UK for people with eczema, and collaborated with the Nottingham Support Group for Carers of Children with Eczema, a local patient group in Nottinghamshire, during the development of the study. These two patient groups helped the research team establish that the issue of softened water for eczema was an important topic for families. For example, the National Eczema Society provided the research team with information on how many times they receive queries regarding the role of water softeners in the treatment of eczema.
The water-softening industry was also consulted. The research team met with representatives from British Water, a corporate membership association that covers different sectors of the water industry, and the water-softening industry. These meetings informed some of the logistics of the trial design, such as the design of a generic water softener that was encased in a cabinet which was designed specifically for the trial.
The research team did not have any interaction with the HTA programme during the development of the initial application, but did receive reviewer comments back from the HTA after they had submitted their initial bid. Overall, the research team viewed the feedback they received from the HTA as very helpful. Further changes (measuring medication use) were made after the first Trial Steering Group meeting.
Stage 1: inputs to research
Financial
The total funding awarded by the HTA programme for SWET48 was £912,257. The research team also received £180,000 matched funding from the water-softening industry for the water softeners and the salt for the water-softening units. However, it was not possible to ascertain the exact value of this matched funding. A consortium of representatives from the water industry, who were managed through two trade associations, provided the water-softening units and the salt, although the HTA funding paid for the installation of the units. The initial pilot of the study was funded by Kinetico UK Ltd.
Knowledge and expertise
The research team had considerable topic and methodological expertise. As noted above, Hywel Williams had a long history of research into eczema and had previously collaborated with Kim Thomas on a number of eczema-related research projects. The research team also included a number of other individuals with expertise in dermatology, including three dermatologists (Robin Graham-Brown, Edel O’Toole and Nigel Burrows), as well as a paediatrician with an interest in childhood skin conditions (Ian Pollock). In terms of methodological expertise, the research team also included a health economist (Tracey Sach), a trial statistician (Caroline O’Leary), an epidemiologist (Tara Dean), two senior statisticians (Andrew Nunn and Angela Crook), a senior trial methodologist (Sarah Meredith) and a trial manager (Karen Koller).
Collaborators
The research team worked closely with the water-softening industry through their trade associations. 506 Ian Pallett, the former Technical Director at British Water, was a co-applicant and was involved with both the pilot study and the main trial. The research team worked closely with Anthony Frost, the representative of the UK WTA, to deliver SWET. 48 Frost took over responsibility for subcontracting the water engineering work to the water-softening companies after the formation of the UK WTA. Representatives from 11 water-softening companies provided input into meetings during the trial development stages, as well as during the study. The water industry helped to inform the study design and assisted with installing the water-softening devices and monitoring samples, but it was not involved in data collection, analysis or interpretation.
The research team also collaborated with a patient representative, David Potter, who was responsible for liaising with the patient panel throughout the study to ensure that patients’ views were considered at every stage of the trial development and delivery. Potter was involved in the design of the study, trial management and oversight, data interpretation and the writing of the report.
Infrastructure
Early on in the study, the research team faced some challenges with recruitment due to unforeseen events (more houses than anticipated being unable to install a water-softening unit and changeover of nursing staff), which, among other strategies to boost recruitment, resulted in the research team approaching the NIHR Clinical Research Networks for support. 554 The research team worked with the Medicine for Children Research Network (MCRN), the Trent Comprehensive Local Research Network (CLRN), the Hampshire and Isle of Wight CLRN and the Primary Care Research Network (PCRN). The MCRN provided maternity cover for one of the study’s research nurses and allowed the research team to open two additional recruitment centres in Lincoln and South East London, while the Trent CLRN and the Hampshire and Isle of Wight CLRN provided nurse time in Nottingham and administrative trial support in the Isle of Wight, which allowed the research team to open two more recruitment centres in Leicester and Portsmouth. The PCRN assisted the research team with the identification of suitable patients from various GP databases. Lastly, the Trent CLRN provided invaluable administrative support following the appearance of Professor Tara Dean, the trial’s PI in Portsmouth, on BBC TV news,555 which resulted in a dramatic increase in calls to the coordinating centre. 554
Stage 2: research process
The trial design chosen was an observer-blind, parallel-group RCT of 12 weeks’ duration, followed by a 4-week observational period when participants in the usual care group were given a water softener to try for themselves. Research nurses, who were blinded to the intervention, assessed the eczema of the study participants at the baseline and 4, 12 and 16 weeks. The trial participants were recruited through secondary and primary care and through self-referral from the community. In total, 336 children with moderate to severe eczema who lived in a hard water area participated in the trial. Participants in group A had an ion-exchange water softener installed in their homes in addition to usual care for 12 weeks, whereas those in group B received usual care. During the observational period, water softeners were either switched off or removed from the homes of those in group A and installed in the homes of those in group B. Upon installation of a water softener, all water in the household was softened, with the exception of drinking water. The primary outcome for the trial was the mean change in disease severity between groups A and B compared with the baseline, at 12 weeks. The secondary outcomes of the trial included use of topical medications, night-time movement (assessed using accelerometers), patient-reported eczema severity and a number of quality-of-life measures.
The SWET48 was particularly interesting in a number of ways: its use of an objective outcome measure as the primary outcome in a trial where it was not possible to blind the study participants to the intervention, its collaboration with industry prior to, and during, the trial and the involvement of the UK Clinical Research Network during the trial. SWET48 also involved patients in the research, had ongoing interaction with the HTA programme throughout the trial, and complied with the different elements of the NIHR’s Adding Value in Research Framework. 20
The choice of an objective outcome measure proved to be a crucial decision in the trial design, as it was not possible to blind participants to their treatment allocation. 506 The research nurses involved in the trial were responsible for assessing the eczema of the patients in the trial and were blinded from the treatment allocation of the participants. All objective outcome measures (nurse-assessed severity, use of topical medications and night-time moving) showed no difference between the patients randomised to receive a water softener or not. However, the secondary non-blinded outcomes (participant-reported symptoms and quality of life), showed a small, statistically significant, positive benefit, but these effects were unlikely to be clinically relevant. It is therefore probable that the positive benefit observed in the non-blinded outcomes was due to response bias.
One of the interviewees succinctly summarised the importance of the use of an objective outcome measure in SWET:48
From that point of view, this study is a very good teaching example of why you have to use objective outcome measures for interventions that are effectively unblinded. If national policy was based on the use of subjective score scales, which were obviously influenced by the unblinded nature of the study, then water softeners could have been said to be effective.
The SWET48 also serves as an interesting example of working effectively with industry through relevant trade associations. 506 The research team worked closely with representatives from British Water and the UK WTA to deliver SWET. 48 Anthony Frost, the representative of the UK WTA, was responsible for subcontracting work to the water softener companies. The study involved more than 20 water engineers, who were responsible for the installation and removal of the water softeners, from 11 different water-softening companies. The UK WTA also addressed any issues regarding inappropriate (non-neutral) publicity of SWET48 on company websites. The research team struck an important balance between involving the water-softening industry in the study design and conduct, but not in data collection, analysis or interpretation. At the end of the study, all of the participants had the option of purchasing their water softener through the UK WTA.
There was ongoing patient involvement throughout the research process, including a patient representative, David Potter, who contributed to the conception and design of the study, trial management, trial oversight, interpretation of data, and writing of the final report. 506 The research team set up a patient panel as part of the trial, which helped to develop patient information leaflets and other resources during the set-up of the trial. Patients helped to advertise the trial and with the dissemination of the trial results, particularly through the Nottingham Eczema Support Group for Carers of Children with Eczema. A mother–child participant from the study, together with Williams, participated in a BBC Radio 4 programme on the trial results. 556 At the end of the trial, the research team also produced targeted dissemination material for patients, which included a newsletter and website information for the patient support groups. After SWET,48 one of the participants joined the UK DCTN’s Patient Panel (for all skin conditions) and is now an active research partner on a number of the Centre’s trials.
The research team had ongoing communication with the HTA programme throughout the conduct of the trial. This primarily involved sending annual progress reports and a monitoring visit towards the end of the recruitment period, which one interviewee noted was helpful in determining how to proceed with recruitment. The research team subsequently received a 6-month extension for the study and an additional £100,000 funding. 554 Members of the research team found the interaction with the HTA programme to be both helpful and supportive throughout the research process.
Adding value in research
From the development of the initial proposal through to the publication of the final outputs of the study, the research team adhered to the NIHR’s Adding Value in Research Framework. 20
First, the research team established that the issue of water softeners for the treatment of eczema was relevant to research users. The National Eczema Society also submitted a vignette to the HTA outlining the importance of this topic to patients. The research team worked with patient groups to establish the importance of the issue to patients, and involved both clinicians and patients in the study design.
Second, the research team invested a lot of time and effort in the design of the study to ensure appropriate research design, conduct, deliverability and analysis. Prior to undertaking SWET,48 Hywel Williams was the CI for the systematic review by Hoare et al. (2000),512 which identified the use of the water softeners for the treatment of eczema as in urgent need of primary research. In the development of the study design, conduct and analysis, the research team took active steps to reduce the potential for bias in the result. Wherever possible, the research team selected objective outcome measures, including for the trial’s primary outcome measure. In the conduct of the trial, the research nurses also collected digital images of participant’s eczema, which were scored by two independent dermatologists to assess the extent of observer bias. Trial participants were discouraged from discussing their treatment allocation with the research nurses, and the nurses kept records of all instances on which they thought that they may have become unblinded.
Third, although the research team needed a short extension of the study to account for unforeseen issues in recruitment (largely difficulties in installing the units in some older homes), the study otherwise met all of its targets and the research team delivered regular progress reports to the HTA programme during the study.
Fourth, the research results were published in full and served as a good example of the wide publication and dissemination of ‘negative’ research findings. The study demonstrated no benefit of water softeners for the management of established eczema. The results were clear, with narrow confidence intervals, which suggests that important differences between the two groups were unlikely to have been missed due to lack of study power. The research team published the research protocol in the British Journal of Dermatology at the start of the study,508 and published the final results in PLOS Medicine507 and the NIHR Health Technology Assessment journal (HTA journal). 506 The research team also used the trial data to conduct a number of substudies, including a paper in the British Journal of Dermatology on the usefulness of accelerometers for measuring disease activity in eczema patients,509 and another paper on the usefulness of the escalation of treatment as a measure of disease flare in eczema trials (submitted). The research team has widely publicised the findings of SWET48 and has been diligent in the re-use of the trial data to add to the knowledge base of what constitutes good practice in dermatology trials.
Lastly, the research team seems to have made every effort to produce unbiased and usable reports. The publications arising from SWET48 clearly describe the trial interventions, report on the planned study outcomes and interpret the results in the context of the existing evidence. In fact, the research team has since been involved in updating the initial systematic review by Hoare et al. (2000)512 on treatments for eczema, which will be published in the NIHR journal. 557
Stage 3: primary outputs from research
Knowledge
The main finding from SWET48 was that water softeners provide no additional benefit to usual care in children with moderate to severe eczema, which had not previously been assessed in a clinical trial. Although parents observed some small, but statistically significant, improvements in some of the secondary outcomes, the observed improvement likely resulted from response bias. The study48 was large enough not to have missed any small but clinically worthwhile benefits. Importantly, the trial demonstrated that overwhelming demand exists for non-pharmacological interventions for the treatment of eczema, which Thomas et al. (2011)506 conclude should be a priority for future research. Figure 19 presents the results of the bibliometric analyses on the publications resulting from SWET. 48
To date, SWET48 has resulted in the publication of four peer-reviewed papers and a fifth that has been submitted, but has not yet been published. These publications cover the trial protocol,508 the final results506,507 and two substudies on the usefulness of different outcome measures in eczema trials. 509,558 It also resulted in the publication of two short articles in the Dermatological Nursing journal. 559,560
Thomas and Sach (2008)508 published the trial protocol in the British Journal of Dermatology on behalf of SWET investigators at the start of the trial. The protocol summarises the background and rationale for the trial, and describes the methods and analysis plan in detail.
Thomas et al. (2011)506,507 published the trial results in both PLOS Medicine and the NIHR HTA journal. The PLOS Medicine publication507 reports the main trial findings, focusing on the results, analysis and conclusions, whereas the HTA publication506 reports all of the findings from the trial, including the initial pilot study, in great detail.
Wootton et al. (2012)509 and Thomas et al. (2015)558 published substudies on the usefulness of different outcome measures in eczema trials. Wootton et al. (2012)509 analysed the accelerometer data collected during SWET48 to assess the validity, responsiveness and acceptability of accelerometers for measuring disease activity in children with eczema. Importantly, the study concluded that the accelerometer data did not correlate well with disease severity or quality of life in SWET,48 and were not responsive to change over time, such that the authors concluded that further work is needed to establish superior methods for distinguishing between movements related to, and unrelated to, eczema. The study by Thomas et al. (2015)558 looked at treatment escalation as a measure of disease flare in eczema trials, which concluded that escalation of treatment may be a useful method for capturing eczema flares.
The SWET48 was also included as a case study in an article by Thomas et al. (2011)554 in Trials about the contribution of the UK clinical research network to dermatology clinical trials. The paper554 highlighted the importance of the NIHR Clinical Research Networks as a resource to researchers involved in clinical trials. In a case study on SWET,48 the paper outlines the contribution of the Clinical Research Networks to the trial’s recruitment. The CRNs provided maternity leave cover, nurse time, administrative time and identification of potential participants from GP databases.
Benefits to future research and research use
Capacity building and career development
Following the completion of SWET,48 a number of members of the research team experienced positive career developments in terms of promotions and recognitions. However, it is not possible to specifically attribute these developments directly to SWET,48 but rather it should be considered as one contributing factor.
Although SWET48 may have contributed to Hywel Williams’ career progression, it is only one among many studies to which he has contributed. For example, Williams has published over 400 peer-reviewed articles and, in the last 8 years, has received over £8M in funding for research on skin diseases. 561 After commencing SWET,48 from 2006 to 2009 Williams chaired the Research for Patient Benefit Programme for the East Midlands. In 2007, he was awarded a NHS Silver Merit award. In 2008, he was awarded a NIHR Senior Investigator award, which was subsequently renewed in 2012, and he was appointed national Chairperson of the NIHR CCRN Dermatology Specialty Group. In 2010, he was appointed Chairperson of the HTA Commissioning Board and Deputy Director of the HTA programme. In 2013, he was awarded a higher doctorate (DSc) for his work on eczema and a NHS Gold Distinction award. In 2014, he was nominated as a Fellow of the Academy of Medical Sciences.
At the time of SWET,48 Kim Thomas was an Associate Professor and Deputy Director of the Centre of Evidence Based Dermatology. SWET48 probably played a role in Thomas’ later career developments, as the study was a large, pragmatic trial that resulted in high-profile publications and media interest. In 2013, she was appointed Professor of Applied Dermatology Research at the University of Nottingham, and in 2015 became Co-Director of the Centre of Evidence Based Dermatology. However, as noted above in reference to Williams’ career progression, SWET48 is only one among several studies in which Thomas has been involved. She is currently CI for two NIHR HTA trials [CLOTHES562 (Clothing for the relief of Eczema Symptoms) and HI-LIGHT563 (Home Intervention of Light therapy: www.ncbi.nlm.nih.gov/pubmed/?term=HI-LIGHT)] and is co-applicant on a further two NIHR HTA programme-funded trials [BATHE564 (Bath Additivies for the Treatment of cHildhood Eczema) and BEEP565 (Barrier Enhancement for Eczema Prevention)]. She was also a co-applicant and programme manager for a recently completed NIHR Programme Grant for Applied Research, which included two work packages on eczema (one focusing on eczema prevention and the other eczema treatment). SWET48 prompted a research interest in outcome measures for dermatology trials, and both Williams and Thomas are members of the Executive Group for the HOME566 initiative. This international collaborative group is working to establish consensus as to core outcome measures for use in future eczema trials, and Thomas is lead for the long-term control working group. Thomas has published over 45 peer-reviewed publications since commencing SWET. 48
The SWET48 probably also contributed to the career progression of other members of the research team. For example, it was part of Tara Dean’s career pathway to become a professor. In addition, Joanne Chalmers, who helped to develop the study protocol, has since been promoted to senior research fellow. However, details regarding the contribution of SWET48 to the careers of the rest of the research team are less clear.
In addition, SWET48 contributed to the development of an informal research network. Many of the same researchers have worked together on subsequent studies. For example, Kim Thomas, Hywel Williams, Tara Dean, Ian Pollock, Nigel Burrows and Tracey Sach are all involved in the CLOTHES562 trial. Similarly, Kim Thomas, Hywel Williams, Joanne Chalmers and Tracey Sach are collaborating on the BEEP565 trial, and Kim Thomas is academic mentor for Tracy Sach on a NIHR fellowship to look at economic modelling to inform priorities for future eczema research.
Targeting of future research
Although no further studies have been conducted on the use of water softeners for the treatment of eczema, SWET48 contributed to increased interest in the relationship between water hardness and eczema. For example, Carsten Flohr is currently looking at the association between hard water and the development of eczema, and is preparing a national follow-up trial looking at whether water softeners might help prevent eczema from birth, rather than treat established eczema. 567
The SWET48 has also informed the design of subsequent trials, including the CLOTHES562 trial, the BATHE564 trial and the BEEP565 trial. SWET48 made an important contribution to the knowledge base for primary research in dermatology: how to recruit patients and how to measure clinical outcome and establishing an informal network for working with other researchers and patient groups. For example, the CLOTHES562 trial is looking at another non-pharmacological medical device intervention for the treatment of eczema, such that many of the same issues arose regarding the infeasibility of blinding participants to the intervention. In the BATHE564 and BEEP565 trials, the research team also faced many similar issues. In all three trials,562,564,565 the members of the research team were able to build on and share their experience from SWET,48 particularly in relation to the selection of objective outcome measures when it is not possible to blind participants to the intervention.
Following the completion of SWET,48 the HOME566 initiative was established. Hywel Williams and Kim Thomas are both members of the Executive Committee of the HOME566 initiative and have contributed their expertise in developing objective outcome measures for eczema research from SWET,48 among others. Through the HOME566 initiative, SWET48 will have important knock-on effects for dermatology research internationally, and should contribute to the improvement of eczema research internationally.
Interface B: dissemination
The research team has undertaken a number of engagement activities in relation to SWET. 48 In the early stages of the trial, members of the research team made several media appearances, which generated increased interest in participation in the trial from parents of children with eczema. For example, in 2007 the BBC News produced an item about the commencement of SWET,48 which included comments from Hywel Williams. 568 In 2009, Williams, together with a mother–child participant from the trial, was interviewed on the BBC Radio 4 Case Notes programme,556 and Tara Dean reported on SWET48 on the BBC South Today television news broadcast,555 which was subsequently picked up by the national BBC News. 569
After the completion of SWET,48 the University of Nottingham published a press release570 and a video of Hywel Williams commenting on the results,571 which generated widespread national and international media interest, as well as increased requests for further information from parents of children with eczema. In 2011, Williams was interviewed on BBC Radio Nottingham on the results of SWET,48 and the results were featured twice in the Nottingham Post. 572,573 Internationally, the results of SWET48 were featured in the Los Angeles Times,574 a U.S. News & World Report,575 an article by the United Press International,576 an article in Medical News Today,577 an article by MDLinx,578 an article in Health Orbit (webpage no longer available), and an article by Third Age online (web page no longer available). Collectively, this media coverage of the trial results generated increased enquiries into the results of the trial from parents of children with eczema.
In terms of clinical dissemination, the SWET HTA report48 was featured on the EvidenceUpdates from BMJ,579 and the PLOS Medicine article was included in an F1000Prime article. 580 The results of the trial were also reported in a CLAHRC BITE (Collaboration for Leadership in Applied Health Research and Care) from the Centre for Evidence-Based Dermatology,581 a research commentary in the Archives of Dermatology,582 and in a review on skin irritation and tap water by the Drinking Water Inspectorate. 583
In terms of academic dissemination, beyond the publications resulting from the study the research team also presented the findings of SWET48 at a number of conferences, including at the 91st Annual Meeting of the British Association of Dermatologists in 2011, at which SWET48 was awarded the CDA trophy for best academic paper. 584
Stage 4: secondary outputs
As a direct result of SWET,48 the National Eczema Society removed its factsheet called ‘Water softeners: do water softeners help eczema sufferers?’ from its website. The Chief Executive of the National Eczema Society confirmed, in writing, with the trial team that the decision to withdraw the factsheet was a direct result of the findings of SWET. 48
Although the NICE guidelines on atopic eczema in children585 and the most recent review of the guideline586 do not mention SWET,48 the previous review does cite SWET,587 but does not discuss the trial’s findings or make any specific policy recommendations. Similarly, the SIGN guidelines on the management of atopic eczema in primary care do not mention SWET. 588 It is likely that the NICE guidelines588 do not mention SWET48 because guidelines often do not list all of the treatments for particular conditions that should not be used, unless they were previously recommended.
Stage 5: adoption by practice and the public
The SWET48 found that water softeners provide no benefit to children with moderate to severe eczema, and the findings of the trial were widely disseminated to patients, clinicians and the public through the media and the various publications resulting from the trial. However, given that water softeners were shown to provide no benefit, it is difficult to establish the impact of the trial on clinical practice as water softeners for the treatment of eczema have never been funded by the NHS. The main impact of the trial on clinicians is that, for those aware of the findings, they can now give evidence-based advice to the patients and the carers of children with eczema. Conversely, the main impact of the trial on patients is that they can avoid spending money on water softeners in the hope that the treatment will improve their eczema. Given that there are approximately 400,000 children with moderate to severe eczema in the UK, and the cost of a water softener is approximately £750 per unit, this could represent important cost savings for families of children with eczema. However, as information on the proportion of families purchasing water softeners for the treatment of eczema before and after the trial is not available, it is not possible to draw final conclusions on the cost savings accruing to families in the UK from the results of this trial.
The main barriers to the research having an impact are the negative nature of the findings for those convinced by anecdotal evidence that water softeners improve eczema and the complexity of the trial results, which showed that, for all of the objective outcome measures, there was no benefit, whereas the more subjective outcome measures showed very small, statistically significant, but still clinically insignificant, differences. The negative findings may be a barrier to the research having an impact because some individuals strongly believe that softened water improves eczema and therefore are reluctant to accept the findings of SWET. 48 Similarly, the complex nature of the trial results may act as a barrier to the research having an impact because it may be difficult for some individuals to understand why the trial results were negative as the subjective outcome measures showed some benefit.
Stage 6: final outcomes
Since SWET48 showed that water softeners do not benefit patients with eczema, it is challenging to determine the impact of the uptake of the research findings in the absence of a plausible counterfactual (e.g. what would have happened if SWET48 had not been undertaken). Presumably, clinicians would have provided variable advice on the effectiveness of water softeners and eczema, and some patients would have continued to purchase water softeners in the hope that the devices would improve their eczema. The main impact of the study on society through the uptake of the findings will thus be cost savings for the carers of children with eczema.
It was not possible to determine the impact of the trial on the water-softening industry. However, it is likely that the industry benefited from the publicity that was associated with being part of a high-profile trial. It is also interesting to note that many families chose to purchase the unit at the end of the trial, despite the fact that the trial showed no treatment benefits.
Table of payback
Payback details for this case study are provided below in Table 31.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | Four peer-reviewed publications and a fifth submitted Only large trial looking at the use of water softeners for the treatment of eczema Found that water softeners provide no benefit for the treatment of moderate to severe eczema in children |
| Research Targeting and Capacity Building | Career development of members of research team Building and maintenance of networks Increase in dermatology trials for non-pharmacological treatments for eczema Increased interest in the connection between water hardness, eczema and the skin barrier in infants |
| Informing Policy and Product Development | The National Eczema Society withdrew its factsheet on water softeners and eczema NICE guidance on atopic eczema in children references SWET48 |
| Health and Health Sector Benefits | Clinicians are able to give evidence-based advice to patients that water softeners provide no benefit to children with moderate to severe eczema |
| Broader Social and Economic Benefits | Carers of children with eczema can avoid spending money on water softeners in the hope that they improve eczema |
Appendix 4.9: the Conventional ventilator support versus Extracorporeal membrane oxygenation for Severe Adult Respiratory failure trial
Summary
The CESAR49 study was a RCT of ECMO for severe adult respiratory failure compared with standard care. The trial compared the effectiveness and cost-effectiveness of ECMO with conventional treatment, 6 months after onset of acute respiratory distress syndrome (ARDS). The main results of the trial found a 16% ‘survival without severe disability benefit’ for patients referred to an ECMO centre compared with patients allocated to conventional treatment. Over a lifetime horizon, the cost per QALY for referral to ECMO was estimated to be £19,252. The authors concluded that transferring adult patients with severe, but potentially reversible, respiratory failure to a centre with an ECMO-based management protocol significantly improves patients’ ‘survival without severe disability’.
The CESAR49 trial was the first to show a ‘survival without disability benefit’ from ECMO in adults with respiratory failure. After commencing the CESAR49 trial, clinical lead Giles Peek was appointed as a Consultant Cardiothoracic Surgeon at Glenfield Hospital, University Hospitals of Leicester NHS Trust. A number of the research fellows involved in the trial49 went on to specialise in intensive care, whereas others decided not to pursue further training in that area. The CESAR49 trial, and more recent research into ECMO, brought additional researchers and clinicians into ECMO treatment and research. The CESAR49 trial also contributed to the targeting of future research, such as the later H1N1 flu virus trial and the Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial, which set out to address some of the uncertainties that remained after the CESAR49 trial. The CESAR49 trial led to a change in clinical practice in the UK, through the establishment of five adult ECMO centres. The CESAR49 trial, together with the H1N1 trial, resulted in an increase in the use of ECMO for adult ARDS. Finally, the CESAR49 trial likely contributed to the development of improved ECMO technology.
Introduction to case study
Background
Scientific background
Extracorporeal membrane oxygenation is a treatment for patients with respiratory failure, which uses cardiopulmonary bypass technology to provide temporary gas exchange. ECMO was first used in clinical practice in the 1970s for the treatment of neonatal respiratory failure, and began to be used for paediatric and adult respiratory failure in the mid-1990s. 589 Prior to the CESAR49 trial, there had been eight trials590–597 of ECMO in acute, potentially fatal, ARDS: two in adult respiratory failure,590,591 one in paediatric respiratory failure592 and five in neonatal respiratory failure. 593–597 The two prospective randomised trials of ECMO in adults showed no difference between ECMO and the control. According to Bartlett (2014),589 ‘these two early studies also demonstrate the negative effect of a premature study. Both studies are cited as demonstrating that ECMO is not effective in ARDS, essentially stopping research on the technique in adults for 30 years, except in a few centres.’ However, the five neonatal trials593–597 – one of which was conducted in the UK shortly before the CESAR49 trial – and the paediatric trial592 all showed survival benefits from ECMO, which resulted in the adoption of ECMO for neonatal respiratory failure in routine practice. Owing to the poor design of the earlier two adult trials,598 the results of which were at odds with the findings from the neonatal and paediatric trials, uncertainty within adult intensive care remained regarding the effectiveness of ECMO for the treatment of respiratory failure in adults, both in the UK and internationally. The CESAR49 trial set out to determine whether or not ECMO increases the ‘survival without disability’ of patients with potentially reversible ARDS and, if so, whether or not the procedure is cost-effective compared with conventional ventilator support. 599
Chief investigator’s background
Diana Elbourne, Professor of Healthcare Evaluation at the LSHTM, was the CI of the CESAR49 trial. 600 Prior to the CESAR49 study and taking up her post at LSHTM, Elbourne worked at the National Perinatal Epidemiology Unit in Oxford from 1981 to 1996. 601 While working in Oxford, Elbourne was involved in a neonatal ECMO trial in infants with respiratory failure,597 which led to her later involvement in the CESAR49 study. 600
During the CESAR49 trial, Elbourne worked closely with Giles Peek, a consultant cardiothoracic surgeon at Glenfield Hospital, University Hospitals of Leicester NHS Trust. Peek was the founding chairperson of the European Chapter of the Extracorporeal Life Support Organization (ELSO), in 2011. 602 Shortly after completing the CESAR49 trial, Peek conducted a matched-pairs trial to compare hospital mortality in patients with H1N1-related ARDS treated with ECMO versus conventional care. 603
The case study approach
The data collection process for this case study involved a series of interviews, a review of the primary and secondary data sources relating to the CESAR49 trial and a targeted review of the evidence on the effectiveness of ECMO for respiratory failure. As shown in Table 32, three individuals were interviewed for this case study: the CI, the clinical lead and an expert in ECMO.
| Interviewee | Reason for interview |
|---|---|
| Diana Elbourne | CI |
| Giles Peek | Clinical lead |
| Robert Bartlett | Expert in ECMO |
The primary data sources for the case study included the interview data, the research protocols,599,604 the monograph in the NIHR HTA journal,600 the final results of the trial published in The Lancet,605 and a paper on the importance of relatives’ travel and time costs for visiting adult patients in intensive care. 606 The background information reviewed for this study included a review of the clinical research on ECMO,589 the results of the earlier ECMO trials590–597,607 and both the NICE and ELSO guidance on ECMO for respiratory failure. 608,609
Stage 0: topic/issue identification
A number of key factors influenced the research team’s decision to work in this area, as detailed in Box 9 and described below.
-
Trial expertise of the PI and ECMO expertise of clinical lead.
-
Evidence from neonatal and paediatric trials that ECMO is an effective treatment for ARDS.
-
Uncertainty regarding the effectiveness of ECMO in adults.
-
Withdrawal of funding for adult ECMO in the UK.
-
Success of previous neonatal trials in the UK.
-
Fast-track commissioning of trial into ECMO for adults.
Trial expertise of the chief investigator and extracorporeal membrane oxygenation expertise of the clinical lead
Prior to undertaking the CESAR49 trial, Diana Elbourne had research expertise in clinical trials and clinical trial methodology research. 241,607,610–620 Elbourne was involved in the UK collaborative randomised trial of neonatal ECMO. 591 Giles Peek had published widely on extracorporeal life support and cardiothoracic surgery prior to the CESAR49 trial. 621–640
Evidence from neonatal and paediatric trials that extracorporeal membrane oxygenation is an effective treatment for acute respiratory distress syndrome
As noted above, there had been eight trials590–597 of ECMO in acute, potentially fatal, ARDS prior to the CESAR49 study: two in adult respiratory failure,590,591 one in paediatric respiratory failure592 and five in neonatal respiratory failure. 593–597 Although the two adult trials590,591 showed no survival benefit from ECMO in patients with severe acute respiratory failure, all of the neonatal and paediatric trials592–597 found a survival benefit from ECMO.
Uncertainty regarding the effectiveness of extracorporeal membrane oxygenation in adults
Owing to the poor design of the two adult trials,589 uncertainty remained regarding the effectiveness of ECMO for adults, even although ECMO had been shown to be effective in both newborns and children. According to Bartlett et al. (2014),598 ‘in retrospect, the 2 prospective randomised trials done in adult respiratory failure in the 1970s and 1980s were premature and poorly designed’. Bartlett et al. 598 highlight a number of flaws including inadequate characterisation of the prospective patient population in the study centres; poorly determined inclusion and exclusion criteria; lack of standardisation of the devices, technology and protocols; inadequate competence in the use of ECMO; and failure to report the flaws of the trial in publication.
Observational studies in adults suggested that there may be survival benefits from ECMO compared with conventional care. 599 Case series studies in the USA, UK and Germany found survival rates of up to 66% with ECMO compared with 44% for conventional care.
Withdrawal of funding for adult extracorporeal membrane oxygenation in the UK
According to one of the interviewees, prior to the CESAR49 study, funding for the use of clinical ECMO in the UK was withdrawn because of the reorganisation of the NHS that had occurred at that time. The Safety and Efficacy Register for New Interventional Procedures, which has now been replaced by NICE, assigned the ECMO procedure a ‘Cii categorisation’, which meant that it deemed the safety and/or efficacy of ECMO in adults to be not yet fully established and that the procedure required a fully controlled evaluation. 599
Success of previous neonatal extracorporeal membrane oxygenation trial in the UK
The earlier neonatal ECMO trial597 demonstrated the feasibility of conducting a trial in acute fatal illness in the UK, which set an important precedent for the CESAR49 trial. According to one interviewee, that trial demonstrated that the logistical problem of randomising patients to two different methods of care in the same intensive care unit (ICU) and the ethical problem of assigning patients to one strategy or the other – one of which ultimately will prove to have higher mortality – could be overcome. The interviewee noted that the UK neonatal ECMO trial597 addressed the logistical and ethical problems through a unique trial design in which many neonatal ICUs agreed to participate. With parental consent, patients who met the trial’s597 inclusion criteria (patients with pre-defined high mortality risk) were randomised to conventional treatment in the hospital’s ICU, the best available treatment at the time or to an ECMO centre for treatment.
Fast-track commissioning of trial into extracorporeal membrane oxygenation for adults
Sir Miles Irving and Professor Kent Woods brought the priority for undertaking a trial into ECMO for ARDS in adults directly to the HTA programme. As such, no commissioning brief was prepared, but rather applicants were invited to submit research proposals. This commissioning process resulted in the funding of the CESAR49 trial.
Interface A: project specification and selection
Diana Elbourne, Giles Peek, Richard Firmin and Miranda Mugford were involved in the design of the CESAR49 study. 600 According to one of the interviewees, once the neonatal ECMO trial was completed in the UK, a discussion regarding the possibility of conducting a similar trial in adults commenced. Three members of the research team for the neonatal trial were subsequently involved in the adult ECMO trial: Elbourne, Mugford and Firmin. The interviewee noted that the principles of both the neonatal and the adult ECMO trial were similar. Although the methods for the two trials were also similar, and some of the ECMO specialists involved in both trials were the same, the clinicians involved in the CESAR49 trial were primarily cardiothoracic surgeons, ECMO specialist nurses and perfusionists, rather than neonatologists. The ECMO specialist nurses were the most numerous. According to another interviewee, the initial planning of the trial took place in Ann Arbor, Michigan, in 1996 at the ELSO meeting, which convened experts in ECMO from around the world.
One interviewee noted that the research team worked closely with the DH during the design of the trial to determine the implications of covering the cost of the ECMO service during the trial and the immediate period after the trial, before the publication of the trial results. The DH also worked to support ECMO within the trial, while discouraging the use of ECMO outside the trial.
According to one of the interviewees, the CESAR49 study was designed to have an impact on policy and practice from the outset. Another interviewee noted that ‘it was designed to answer exactly that question: should Britain commission an adult ECMO service? So, it was completely pragmatic and it had a concurrent economic evaluation’.
Patients were not involved in the design of the initial research application, but, according to one interviewee, after the research team received confirmation of funding, patient representatives were included on the steering committee, and clinical stakeholders were involved in the drafting of the protocol and the research ethics application. The same interviewee also explained that relevant patient groups for ECMO are difficult to identify because there are only four, small, potentially relevant groups: people who had had ECMO in the past, the families of people who have had ECMO in the past and died, people who had had respiratory failure in the past, and the families of people who have had respiratory failure in the past and died. The research team involved patients who had previously had ECMO and a family member of someone with ARDS who had died in intensive care. However, according to one interviewee, patient involvement in the CESAR49 trial was relatively small because many of the issues of concern to patients had already been addressed in the design of the previous neonatal trial.
According to one interviewee, some members of the research team initially made one unsuccessful application for funding to the MRC before receiving funding from the HTA programme.
Stage 1: inputs to research
Financial
The research team received £1,424,795 in funding from the HTA programme. 49 The initial grant was for £933,237; however, the research team received extensions of £302,631 in October 2003 and £176,002 in May 2005. Although the costs of the trial were funded by the HTA programme, the clinical treatment costs for ECMO were funded by the NHS through the National Specialised Commissioning Advisory Group for England and Wales and through the Scottish Executive in Scotland. Conventional treatment was funded by the NHS under existing contracts. 599,600 According to one interviewee, the treatment and salary costs of the clinicians amounted to approximately £2,900,000 per year.
Knowledge and expertise
The research team had considerable methodological and clinical research expertise. At the time of the CESAR49 study, Diana Elbourne had substantial experience in clinical trial research, design and management,241,607,611–620 Giles Peek had substantial expertise in ECMO and cardiothoracic surgery,621–640 Richard Firmin had extensive clinical and research expertise in ECMO and cardiothoracic surgery,621–629,631,632,634–707 and Miranda Mugford had long-standing expertise in health economics. 708–732
Techniques
Extracorporeal membrane oxygenation, as a procedure, was an important input into the research. Robert Bartlett first developed the procedure in 1971, which Richard Firmin (a member of the research team) and Andrzej Sosnowski introduced to the UK in 1989 at Glenfield Hospital. 733 The technique requires substantial expertise and is delivered in only a limited number of centres globally. At the time of the CESAR49 study, Glenfield Hospital was the only adult ECMO centre in the UK, and it remains the only hospital in the UK to provide ECMO to both adults and newborns.
Stage 2: research process
The CESAR49 study was a RCT of ECMO for severe adult respiratory failure compared with standard care. 600 The trial compared the effectiveness and cost-effectiveness of ECMO with conventional treatment. Patients randomised to ECMO were treated in Leicester at the Glenfield Hospital, whereas patients randomised to conventional treatment were treated in hospitals throughout the UK. The primary outcome measure was death or severe disability at 6 months. The study49 found that fewer patients referred to the ECMO treatment centre died or had severe disability after 6 months (36.7% compared with 52.9% with conventional treatment), with a cost per QALY of £19,252.
According to one of the interviewees, the trial design proved to be controversial because only the patients randomised to the intervention were moved to the ECMO centre. In some previous trials, everyone who met the criteria for ECMO was transferred to an ECMO centre, and half the patients received standard care while the other half received ECMO. The research team made the decision that transferring patients to an ECMO centre to receive standard care was not appropriate for a UK context because, in the UK, patients who were not going to receive ECMO would never be transferred, but rather would be treated locally. Transferring patients to another treatment centre to receive standard care would have caused extra stress to critically ill patients. Another interviewee noted that ‘I think it was the right design. It was a controversial design, because it did not fit the US pattern, but it was a UK trial, and for doing the trial in the UK, it was the right design’.
The unique design of the CESAR49 trial also added complexity to the interpretation of the results, because it was not possible to compare the same type of care in both arms of the trial. In the control arm, conventional care, patients were treated in a different place, with a different team, but did not receive ECMO. Furthermore, a number of the patients in the ECMO arm did not receive ECMO because their condition improved at the ECMO centre, obviating the need to use it. However, patients with ARDS that were transferred to the ECMO treatment centre, regardless of whether they received ECMO or not, experienced lower mortality and lower rates of severe disability at 6 months than patients who received conventional care in their hospital’s ICU.
The research team had extensive interaction with other ICUs throughout the country when setting up the CESAR49 trial. According to one interviewee, the research team was in contact with > 100 ICUs before submitting the ethics application for the trial.
The research team also involved patients in the research process through the Trial Steering Group. One interviewee noted that patients’ views on the neonatal trial carried through to the design of the CESAR49 trial. For example, the neonatal trial had already consulted patients regarding an acceptable primary outcome, which should not just be death, but rather should include severe disability at 6 months to address quality-of-life concerns. Patients were also involved in the economic analysis and, according to one interviewee, were particularly helpful in determining whether or not patient diaries to document all services used from discharge to follow-up would be a useful way of collecting information on costs and resource use for the economic evaluation.
The research team also had ongoing interaction with the HTA programme throughout the research process. This interaction took the form of annual reports, which the research team submitted to the HTA programme, and two funding extensions over the life of the project. One interviewee noted that:
They were a good funder to work with. You work with them rather than adversarially. With some funders you feel like you have to justify your existence every five minutes . . . I think they were a very supportive funder, sort of critically supportive.
Adding value in research
Although the CESAR49 trial was undertaken prior to the Chalmers and Glasziou14 publication on avoidable research waste, and the NIHR’s introduction of the Adding Value in Research Framework,20 it is nevertheless feasible to assess the extent to which the CESAR49 trial met all of the adding value in research criteria of that framework.
First, the research team set out to answer a question that was relevant to the users of the research and designed the research to have an impact on policy and practice from the outset. The research team set out to address a high-priority question, with important outcomes, and involved clinicians throughout the research process. The research team did not, however, involve patients in setting the research agenda, but did involve patients in the research process.
Second, the research team used a trial design that was tailored to the UK context to address the issue of whether the ECMO for ARDS should be commissioned in the NHS. The study49 did not conduct a systematic review, as there had been only two previous trials in adult ECMO prior to the CESAR study, both of which have been deemed to be methodologically flawed. 589 However, the research team did review the existing evidence from those two trials, as well as the evidence from observational studies. 600
Third, although the research team needed two short extensions to account for unforeseen issues in recruitment, the study was otherwise delivered on time, and the research team delivered regular progress reports to the HTA programme during the study.
Fourth, the research team published their findings in full in a HTA monograph600 and in The Lancet,605 and also used the trial data to publish a substudy on the importance of relatives’ travel and time costs for visiting adult patients in intensive care. 606
Finally, the research team seems to have made every effort to produce an unbiased and usable report. The publications arising from the study clearly describe the research methods, the planned study outcomes and the interpretation of the findings.
Stage 3: primary outputs from research
Knowledge
The CESAR49 study resulted in the publication of two research protocols (one for the trial and one for the economic evaluation),599,604 two peer-reviewed publications,605,606 and a HTA monograph. 600 The research protocols describe the methods of the trial and economic evaluation in detail. The monograph and The Lancet paper605 report the results of the trial, and a substudy on the cost to families of visiting patients in intensive care presents the results of one component of the economic analysis. Figure 20 presents the results of the bibliometric analyses on the publications resulting from the CESAR49 trial.
The main results of the trial found a 16% ‘survival without severe disability’ benefit, post hospital admission, for patients referred to an ECMO centre compared with patients allocated to conventional treatment. According to one interviewee, all other reports of survival outcomes in patients with ARDS are based on 28 days post admission or hospital discharge. The 28-day survival outcome in the CESAR49 trial was 75% vs. 50%. 600,605 Over a lifetime horizon, the cost per QALY for referral to ECMO was estimated to be £19,252, which is below NICE’s cost-effectiveness threshold of £20,000–30,000 per QALY. 735 The authors concluded that transferring adult patients with severe, but potentially reversible, respiratory failure to a centre with an ECMO-based management protocol significantly improves patients’ ‘survival without severe disability’. The CESAR49 trial was the first to show a survival benefit from ECMO in adults with respiratory failure.
Commenting on the results, one interviewee noted:
That is a huge difference in acute clinical studies in fatal illness. Most clinicians would say if you could use some very expensive intervention and get a 10% improvement in survival then it’s worth it. Here it was twice that.
The substudy on the cost to relatives of visiting adult patients in intensive care investigated the costs to informal caregivers of visiting patients in ICUs. 606 The study606 found that the main cost to informal caregivers of visiting relatives in an ICU was related to travel.
However, there has also been some controversy regarding the results of the CESAR trial. 736 Although more patients referred to ECMO survived than patients randomised to conventional management, the survival benefit was not statistically significant (relative risk 0.73; 95% confidence interval 0.52 to 1.03; p = 0.07). The single referral centre was also viewed as a potential weakness of the study because potentially superior care at the trial ECMO centre might have led to the improved ‘survival without severe disability’ benefit observed in the trial. Another issue highlighted was the use of a local conventional management protocol rather than a standard critical care protocol. Zwischenberger and Lynch (2009)736 note that:
Most proponents of ECMO will conclude that patients treated in a hospital where ECMO was part of the algorithm fared better than patients in hospitals that did not offer ECMO. They will argue that the strengths of CESAR far outweigh the risks of transport and ECMO as well as any statistical critique . . . Detractors will argue that the intent-to-treat analysis and lack of protocol for ventilator and critical-care management in patients not randomised to receive ECMO weaken the conclusions . . . Critics will point out that the benefit is only directly applicable to the UK health-care system, and that the expense of training a team and maintaining equipment minimises any savings to be realised . . . This study will likely provide ammunition for both those in favour and those against the use of ECMO in the adult population.
The interviews picked up on some of this controversy. According to one interviewee, adult pulmonologists, mostly in the USA, were both surprised and sceptical. They criticised the study49 on the grounds that, of the 90 patients randomised to ECMO, 16 were transferred to the ECMO centre in Leicester but did not receive ECMO. These 16 patients improved with conventional care in the ECMO treatment centre, which differed from conventional care in the control arm of the study. The trial investigators acknowledged the lack of standardisation of conventional care between the ECMO centre and the ICUs as a major limitation of the study. That criticism highlights the importance of the interpretation of the trial: patients who were referred to the Leicester ECMO centre had a 16% ‘survival without disability’ advantage over patients managed in other ICUs in the UK. According to the same interviewee, the findings of the CESAR49 trial are now well understood and viewed positively within intensive care. The interviewee noted that:
If you ask any practising pulmonary intensivist ‘what should you do to improve survival in your severe ARDS patients?’ the answer is refer those patients to an ECMO centre where, number one, the care is better and, number two, they have ECMO available if needed.
Benefits to future research and research use
Capacity building and career development
After commencing the CESAR49 trial, in July 2004 Giles Peek was appointed as a Consultant Cardiothoracic Surgeon at Glenfield Hospital, University Hospitals of Leicester NHS Trust. In 2008, Peek became the Head of Service for the East Midlands Congenital Heart Centre and, in 2011, became Director of the Adult, Paediatric and Neonatal ECMO Programme. In 2015, he took up a visiting professorship in New York. Although it is not possible to determine to what extent Giles Peek’s career progression is attributable to his involvement in the CESAR49 trial, it is plausible that the trial made an important contribution, as his subsequent career developments have all occurred within the same field.
Prior to the CESAR49 trial, Diana Elbourne had a well-established track record in clinical trials and research into the methods of clinical trials, and thus it is unlikely that the CESAR49 study would have had a substantial impact on her career, as it is only one among many trials in which she has been involved.
According to one interviewee, the CESAR49 trial also had an important impact on a number of other individuals involved. Ravin Tiruvoipati, a Clinical Research Fellow at the time, was involved in the recruitment of both centres and patients, the clinical conduct of the research, project management, the interpretation of the results and writing of the final report; he decided to undergo intensivist training instead of surgical training after the CESAR49 trial. Nicky Jones, who was also a Clinical Research Fellow at the time, was involved in the recruitment of both centres and patients, the clinical conduct of the research and the project management group, and decided to move into general practice training instead of surgical training after the CESAR49 trial. According to the same interviewee, all of the ECMO clinicians, with the exception of Nikki Jones, confirmed their interests in the field and all of the researchers continued in their research pursuits.
One interviewee also maintained that the CESAR49 trial, together with more recent developments, brought additional researchers into ECMO. First, the CESAR49 trial led directly to the H1N1 trial, which looked at the same research question as the CESAR49 trial but in a relatively homogenous patient group that was restricted to patients with H1N1. Second, the flu pandemic independently increased the use of ECMO for adults with ARDS and research on ECMO in adults. The introduction of new, simpler, safer and more efficacious ECMO technology in 2008 also prompted increased interest in the use of ECMO for adults.
Targeting of future research
According to one interviewee, as the CESAR49 study progressed, an increasingly large number of people globally were performing ECMO. However, the ELSO registry is blinded to the source of cases entered and it is not possible to determine whether or not the CESAR49 study made any contribution to the global increase in ECMO use. According to the same interviewee, by the time that the CESAR49 study was published, interest in ECMO had increased, such that when the 2009 H1N1 flu epidemic occurred, there were quite a few ECMO centres that were able to treat patients with H1N1 using ECMO.
During the H1N1 epidemic, Giles Peek and others undertook another trial in ECMO which compared the hospital mortality of patients with H1N1-related ARDS referred to ECMO centres to matched patients who were not referred to ECMO. 603 The H1N1 study set out to address some of the controversy regarding the effectiveness of ECMO in adults that remained after the CESAR trial. It found that patients with H1N1-related ARDS that were referred to ECMO centres had lower hospital mortality than matched patients who did not receive ECMO: 27.5% of patients transferred to UK ECMO centres died before discharge compared with 52.5% for patients who were not referred for ECMO. However, a similar study in France found no difference in mortality between patients referred to ECMO centres and their matched pairs; however, only 50% of patients were matched in that study and the unmatched patients had lower mortality. 737
The international multicentre randomised EOLIA trial, which is led by Alain Combes in France, is looking at the early use of ECMO after diagnosis of ARDS: in a review article on research into adult ECMO to date, Combes et al. (2012)738 state ‘because the CESAR study was criticised for methodological limitations, new trials evaluating the impact of ECMO in severe respiratory failure are needed before widespread adoption of this technique’. The article738 notes that the EOLIA trial will address some of this uncertainty regarding the efficacy of early ECMO in ARDS by having close control over mechanical ventilation in the control group, initiation of ECMO prior to the transfer of patients to ECMO centres, and use of ECMO in all patients who are randomly assigned to receive ECMO.
Interface B: dissemination
Aside from the academic publications resulting from the CESAR49 study, Giles Peek has been active in the dissemination of the research findings. Through his role as Chairman of EuroELSO and his involvement in the broader ELSO organisation, Peek disseminated the findings of the trial through that network. He also presented the results of the trial on a number of occasions in different locations in the UK as well as internationally in Australia, Belgium, Brazil, Canada, China, France, Germany, Switzerland, the Netherlands, New Zealand and the USA. However, this dissemination activity was not covered by the project grant, but rather was supported by conference invitees or industry.
According to one of the interviewees, the research team also disseminated their findings to patients by sending summaries to all those who took part in the trial and by presenting the findings to patient groups. The research team also maintained a website throughout the project to provide updates and to disseminate the results at the end of the study (http://cesar.lshtm.ac.uk/).
Lastly, the research team translated the findings of the study into French,739 which made the research findings more readily available to the French-speaking academic community.
Stage 4: secondary outputs
According to one of the interviewees, the CESAR49 trial led directly to the creation of the five ECMO centres that currently exist in the UK, but that there were also a number of other events that contributed to the commissioning of the ECMO service. First, referrals to ECMO increased dramatically with the outbreak of the H1N1 flu epidemic, which coincided with the publication of the CESAR49 trial (at that time, the NHS had not yet commissioned ECMO services). The ECMO centre in Leicester collaborated with other centres to treat referred patients, which led to a doubling of ECMO capacity in the UK at that time. A similar experience was repeated the following year with the second outbreak of H1N1, which, according to the interviewee, then led to the commissioning of a more robust ECMO service, with five ECMO centres nationally.
The CESAR49 trial also had a direct impact on clinical guidelines in the UK. The NICE guidance on ECMO for severe acute respiratory failure in adults references the CESAR49 study as the primary evidence on ECMO for adults. 608 The guidance states that:
Evidence on the safety of extracorporeal membrane oxygenation (ECMO) for severe acute respiratory failure in adults is adequate but shows that there is a risk of serious side effects. Evidence on its efficacy is inadequate to draw firm conclusions: data from the recent CESAR . . . trial were difficult to interpret because different management strategies were applied among many different hospitals in the control group and a single centre was used for the ECMO. Therefore this procedure should only be used with special arrangements for clinical governance, consent and research.
The CESAR49 trial is also cited in the ELSO Guidelines for Adult Respiratory Failure, which is used by ECMO centres around the world. 609 In addition, the CESAR49 trial is cited in ECMONet’s position paper for ECMO programmes for acute respiratory failure in adult patients,740 as evidence that referral to an ECMO centre – where ECMO is part of a broader management protocol for acute respiratory failure – may improve patient outcomes.
According to one of the interviewees, the CESAR49 study has also had an impact on the teaching of clinicians in intensive care. The interviewee noted that although there is still a lot of scepticism and resistance, the results of the CESAR49 trial and ECMO are now something that students are required to know about. Lastly, the CESAR49 trial is also cited in ELSO’s Red Book (www.elso.org/Publications/RedBook4thEdition.aspx), which is a compendium of knowledge on ECMO.
Stage 5: adoption by practice and the public
According to one of the interviewees, ‘The neonatal trial changed the definition of standard practice around the world as soon as it was published. The CESAR49 trial did the same thing, from my point of view’. However, another interviewee noted that adoption of ECMO for ARDS in clinical practice has been relatively slow, but since the results of the CESAR trial it is a much more accepted treatment method. Since the publication of the CESAR study findings in 2009,605 the number of adult patients treated with ECMO globally has steadily increased from 3213 in 2009 to 5037 in 2014. 741 The same interviewee noted that clinicians are sometimes reluctant to change their clinical practice, despite evidence from primary research. The interviewee also pointed out that, in the past, there was a legitimate concern regarding the risk of transporting patients to ECMO centres, but that that risk has now largely been eliminated as a result of improvements in ECMO technology, because clinicians from ECMO centres can now cannulate patients prior to transport. Overall, the CESAR49 trial, together with the H1N1 ECMO study, seems to have led to an increase in the use of ECMO in the UK and internationally. However, it is not possible to apportion the increase in ECMO use to the two individual studies as they were published within 2 years of each other and both probably contributed to the observed increase.
One interviewee viewed the devolution of clinical commissioning to regional areas as an obstacle to the provision of ECMO in the UK. Prior to the recent reorganisation of the NHS, commissioning of ECMO services took place at a national level and ECMO centres worked collaboratively at the national level. However, because of changes in the commissioning of services, there is now a challenge regarding the costs of patients who are referred from a hospital in one region to an ECMO centre in another region.
One interviewee also noted that the CESAR49 trial has had an impact on practice internationally. They noted that ‘Every single health system is having to consider how they provide it, whether they’ll provide it, how they’ll fund it, who will do it. So I think it has had an enormous impact’.
According to the same interviewee, the number of clinicians performing adult ECMO has increased exponentially since the publication of the CESAR49 trial.
Stage 6: final outcomes
Peek et al. (2010)600 estimate that there may be up to 350 patients with severe, but potentially reversible, respiratory failure in the UK each year. The CESAR49 study and the subsequent H1N1 ECMO study showed that patients with ARDS that are referred to ECMO centres are much more likely to survive. One interviewee noted that although the number of patients referred to ECMO is small, the survival benefit for those patients is important. One interviewee also noted that, of the patients who survive a similar degree of illness (treated with either ECMO or conventional care), those on ECMO have much less ventilator-induced lung injury than those treated with conventional care, and have a much better quality of life.
One interviewee also noted that the CESAR49 study has influenced recent developments in ECMO technology because it demonstrated that there is a potential market for devices. Following the CESAR49 trial, three German companies developed improved ECMO machines. However, it is not possible to determine what level of sales were achieved for the companies and to what extent the development of the new technology can be attributed to the CESAR49 study.
The CESAR49 study has had a range of impacts, which one interviewee summarised succinctly:
It really has had an impact in several areas in the world, prompting industry to make devices and prompting hospitals to learn how to do the technology, and particularly getting them to realise that it’s not as simple as it seems, and in how to conduct research trials in acute fatal illness. It has certainly had an impact in all those areas.
Table of payback
Payback details for this case study are provided below in Table 33.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | First trial to show a survival without disability benefit of referral to an ECMO centre for adults with ARDS Two protocols, one monograph and two peer-reviewed publications |
| Research Targeting and Capacity Building | Giles Peek became a consultant cardiothoracic surgeon shortly after commencing the CESAR49 trial One research fellow went on to pursue training in intensive care, another went on to pursue training in general practice, and another continued on to work in clinical research Giles Peek was involved in the H1N1 ECMO trial immediately after the conclusion of the CESAR49 trial The EOLIA trial in France set out to address some of the remaining uncertainties regarding the use of ECMO for adult ARDS |
| Informing Policy and Product Development | Resulted in funding for adult ECMO in the NHS Resulted in the current five ECMO centres in the UK |
| Health and Health Sector Benefits | Global increase in use of ECMO for patients with ARDS |
| Broader Social and Economic Benefits | Survival without disability benefits patients with ARDS |
Appendix 4.10: CoBalT
Summary
In primary care, antidepressant drugs are often the first-line treatment for depression. Non-response to antidepressant treatment is a considerable problem. In the CoBalT50 study, the investigators conducted a large multi-site RCT to test the clinical effectiveness and cost-effectiveness of using CBT as an adjunct to pharmacotherapy for treatment-resistant depression (TRD) in primary care. The PI for this trial was Dr Nicola Wiles. The intervention was found to be clinically effective, significantly reducing depressive symptoms by 6 months. The response rate and the remission rate were found to be significantly greater in the intervention group than in the usual care group over the 12-month trial. Additionally, the improvement in quality of life over the 12 months was reported as being greater by those in the intervention group. The intervention was found to be cost-effective; if society is willing to pay £20,000 per QALY (the threshold used by NICE) the net monetary benefit per patient per year is £289, and the probability that the intervention is cost-effective is 0.74. The study also included a small qualitative study in which it was found that patients from the intervention group described CBT as challenging but that it had given them techniques to help them better manage their symptoms, for those that managed to complete the sessions. A follow-up study approximately 4 years post randomisation will report on these findings shortly. As the CoBalT50 findings were published only 2 years ago in February 2013, there has not yet been an opportunity for them to have a notable effect on society. However, there has been considerable impact on the careers of the more junior researchers involved in the study, and the research networks for the more senior researchers have been strengthened and increased both within and across different institutions. Many of the investigators involved in CoBalT50 have gone on to collaborate together in other research trials. There has not yet been an opportunity for the CoBalT50 findings to be cited by the NICE clinical guidelines, and so the impact on clinical practice has been limited. The next updates to the NICE guidelines for depression have an anticipated publication date of May 2017.
Introduction to case study
Background
Scientific background
In primary care, antidepressants are often the first-line treatment for depression. Non-response to antidepressant treatment is a considerable problem. 742 In a large US study more than half of the patients with depression recruited through primary care did not achieve remission after first-line antidepressant treatment,743 and in a European study half of the depressed patients did not respond to two consecutive courses of treatment with antidepressants. 744 There is no single accepted definition for ‘TRD’. 745 A systematic review published in 2002 found that no RCTs had been undertaken to examine psychological interventions for TRD. 746 In 2004, NICE published guidelines747 suggesting that combined antidepressant and CBT should be considered for treating TRD. The level of evidence for this recommendation was ‘grade B’, which is defined as evidence drawn from well-conducted clinical studies but no RCT on the topic of recommendation or extrapolated from a RCT. 747 CBT is a type of ‘talking therapy’ that has been shown to be effective as a treatment for some people with depression but thus far CBT alone has not been found to be efficacious for treating TRD (interviewee).
Principal Investigator background
Dr Nicola Wiles was the PI of the CoBalT50 study. To date she has had 68 publications. Her first paper was published in 1999 in the field of arthritis. 748 She moved from the University of Manchester to the University of Bristol in 2003, and, in doing so, moved from being an epidemiologist in rheumatology to an epidemiologist involved in mental health research (interviewee). Her first mental health research-related paper was published in 2005. 749 In 2008 Wiles published the results from a pilot trial for the CoBalT50 study, which provided proof that they would be able to recruit the appropriate subjects for the larger HTA programme-funded CoBalT50 RCT that followed (interviewee). 750
The case study approach
This case study was conducted by requesting interviews with the PI and other authors from the main HTA study report for this grant. Interviewees, listed in Table 34, included the PI and epidemiologist Nicola Wiles, a practising academic psychiatrist, and a GP/primary care health services researcher. This study was a multi-site RCT across three institutions. The majority of the authors as well as the PI were based at the University of Bristol, with other partners based at the University of Exeter and the University of Glasgow. At the time of the study, Glyn Lewis and John Campbell were based at the University of Bristol and the University of Exeter, respectively. In addition to interviews, further information was found through reading the publications produced as a result of this study and those that have cited these publications, and through looking at the study website. The NICE guidelines for depression and other journal article publications were reviewed to place the study in context.
| Interviewee | Reason for interview |
|---|---|
| Nicola Wiles | PI and epidemiologist at University of Bristol site |
| Glyn Lewis | Practising academic psychiatrist at University of Bristol site |
| John Campbell | GP and primary care services researcher at University of Exeter site |
Stage 0: topic/issue identification
A number of key factors influenced the research team’s decision to work in this area, as detailed in Box 10 and described below.
-
Professor Lewis was initially inspired by his experience with patients to think that CBT alongside pharmacotherapy might be beneficial.
-
Positive results from the pilot clinical trial suggested that this could be an effective intervention for TRD.
-
Despite inclusion in NICE guidelines in 2004 and 2009, evidence for the use of CBT alongside pharmacotherapy was weak.
-
Complemented other depression research in primary care settings being conducted within the department.
Professor Lewis was initially inspired by his experience with patients to think that cognitive–behavioural therapy alongside pharmacotherapy might be beneficial
The origin for this study goes back to the late 1990s when Professor Glyn Lewis, inspired by his clinical experience with his patients, conducted a small-scale trial, while he was at Cardiff University, to compare combined pharmacotherapy and CBT with pharmacotherapy alone in patients with TRD in secondary care. At the time, this field of research was very small in the UK. Approximately £10,000 was awarded by the Welsh government to support this trial (interviewee).
Positive results from the pilot clinical trial suggested that this could be an effective intervention for treatment-resistant depression
The initial small-scale trial at Cardiff was not very successful because Lewis was recruiting from secondary care and by this time the psychologists felt unable to randomise and also many of the patients had comorbid disorders, so they struggled to recruit enough people (interviewee). Lewis then moved to work at the University of Bristol, where he met an epidemiologist, Nicola Wiles, and together they designed a pilot trial experiment similar to Lewis’s previous trial but this time in a primary care population of individuals with TRD. A small grant from the Avon and Wiltshire Mental Health Partnership NHS Trust enabled them to conduct a pilot study. This study tested the robustness of their experimental design, proved that they could recruit the target population and yielded positive results. This study was very much the precursor to the application that they submitted to HTA (interviewees).
Despite inclusion in National Institute for Health and Care Excellence guidelines in 2004 and 2009, evidence for the use of cognitive–behavioural therapy alongside pharmacotherapy was weak
The NICE guidelines for depression,751 published in 2004 and 2009, both already include CBT as an adjunct to pharmacotherapy as the next step in treatment for TRD following pharmacotherapy alone. However, a systematic review published in 2002746 provided no evidence to support this ‘next step’ intervention for TRD. An update to this systematic review conducted by the Bristol team, first submitted for publication in 2006, found only three very small studies that provided evidence to support the effectiveness of providing patients in primary care with CBT treatment in addition to pharmacotherapy. One of these studies was their pilot study and the other two trials were conducted with very small sample sizes. 752 Although this lack of substantial change from the earlier systematic review meant that no-one was interested in publishing the Bristol team’s updated systematic review, it did help strengthen their application for the HTA grant (interviewee).
There was a large study being conducted in the USA called STAR*D, which was similar to the CoBalT50 study, but there was a critical difference between the two trials in relation to the suitability of the findings to test the intervention that was currently being recommended in the NICE guidelines. The STAR*D RCT did not test the effect of augmenting antidepressant medication with CBT as a ‘next-step’ treatment option, as there was there was no control group left on antidepressants while also having CBT (interviewee). 743 Additionally, the primary health-care systems in the UK and USA differ considerably, so it cannot be assumed that the findings from such trials will necessarily have the same effect in the UK (interviewee).
As the team were writing the CoBalT proposal in 2006, Improving Access to Psychological Therapies (IAPT) services, aimed at increasing access to CBT, started. There was debate within the team and with other people as to whether or not they still needed to do the study, but they felt that they should do it as evidence was still lacking and new initiatives lacking in evidence are vulnerable in a climate of funding cuts (interviewee).
Complemented other depression research in primary care settings being conducted within the department
The department at the University of Bristol that Nicola Wiles worked in had a strong background in research investigating interventions for depression in a primary care setting. At the time members of the department had either just finished or were conducting studies investigating the genetic indicators of treatment response for depression [MRC-funded GenPod study (GENetic and clinical Predictors Of treatment response in Depression)], the effectiveness of an online CBT chat room intervention for depression [the Bupa Foundation-funded IPCRESS study (Internet Psychotherapy for Depression)] and the effectiveness of exercise as an intervention for depression [HTA programme-funded TREAD study (Treatment for depression)]. In the CoBalT50 study they were keen to investigate a different intervention that they hoped might be more appealing to patients and practitioners (interviewees).
Interface A: project specification and selection
Nicola Wiles drafted the application. There was a large team working together for this study split between three teams based at the University of Bristol, University of Exeter and University of Glasgow. The CoBalT50 study was a response mode-funded grant (interviewees).
The team at the University of Bristol included the CI and epidemiologist Nicola Wiles, psychiatrist Glyn Lewis, GP David Kessler, statistician Tim Peters, health economist Sandra Hollinghurst, the head of psychology services from the Avon and Wiltshire Mental Health Partnership NHS Trust Bill Jerrom, qualitative researcher Katrina Turner and primary health care researcher Deborah Sharp. Sharp was involved in the submission of the proposal but did not take part in the study beyond that point. Many of the Bristol team had worked together previously on the IPCRESS study that investigated the effectiveness of CBT delivered by a therapist online, and this was useful in the process of putting together the team and proposal. The team at the University of Glasgow was led by psychiatrist and CBT expert Chris Williams and academic GP Jill Morrison, both of whom had been involved in similar trials before. The team at the University of Exeter was led by GP and primary care services researcher John Campbell from the Medical School, and clinical psychologist and CBT expert Willem Kuyken from the Mood Disorders Centre (interviewee).
The CoBalT50 study was a direct follow-on from a pilot trial conducted at the University of Bristol led by Wiles (interviewee). Wiles contacted John Campbell at the University of Exeter to ask if he was interested in joining the study, as he had a track record of recruiting to primary care studies. Campbell is a health services researcher and GP but although he had carried out quite a lot of mental health work he had not undertaken much in depression research. He facilitated bringing Willem Kukyen on board as a co-applicant of the trial, as he had very relevant expertise in depression and CBT (interviewee). Glyn Lewis suggested bringing Chris Williams from the University of Glasgow on to the team, given his expertise in CBT, and Williams then linked the team to Jill Morrison to provide primary care expertise in Glasgow (interviewee).
There were three main outcomes of the CoBalT study: the clinical effectiveness, the cost-effectiveness and the qualitative assessment of patient experience for individuals treated with CBT as an adjunct to pharmacotherapy.
A member of the study team explained that while there was no PPI in the format that is common nowadays, they did have some forms of user interaction for the design of the pilot study for the CoBalT, which then fed into the design of the CoBalT RCT. For the pilot study, in addition to the interactions between the academics and clinicians involved in the study, they drew in colleagues from the local Mental Health Trust, including the director of psychology services at that time, to collaborate on the project. This interaction facilitated the recruitment of therapists to deliver the therapy and contributed more generally to the development of the project. User feedback on how they felt about specific elements of a questionnaire used in the pilot trial was obtained by interacting with a larger trial being undertaken in the department at the same time, which was using questionnaires with similar elements (interviewee). When writing their proposal for their HTA application they actively recruited a patient user, Paul Lanham, to their steering group (interviewee).
None of the interviewees could remember if the peer review or application process affected the design of the study but they said that any comments would have been taken on board. However, they felt that any changes would have been only minor, as they had already established and tested the protocol in the pilot study. The CoBalT50 grant application was not submitted to any other funding body prior to being submitted to the HTA (interviewee).
Stage 1: inputs to research
The grant for the CoBalT50 study included a 6-month extension, and this, combined with the long-term follow-up study, came to just over £1.5M (HTA grant 06/404/02). Originally, the follow-up was administrated under the same code but now the codes have been switched (06/404/501) (interviewee). The CoBalT50 trial started in 2008. The HTA funded the research component of CoBalT50 but the NHS funded the treatment and therapist costs, which amounted to approximately £330,000 (interviewee).
The research in CoBalT50 did not particularly require new techniques, expertise or approaches to the subject. It was more about bringing together the correct multidisciplinary team to provide the breadth of experience and expertise needed to deliver a successful large-scale trial. A key characteristic that the researchers believed underlined the success of the CoBalT50 trial was the coming together and balance of an optimal team of experienced researchers from across all of the necessary fields of expertise with good reputations within the field (interviewee). It was claimed by two of the interviewees that the scale of the trial in primary care across multiple sites required exceptional communication and organisation skills that were ably orchestrated by Nicola Wiles (interviewee).
Although CoBalT50 did not involve developing new methodologies, the team did learn more about the methods they used, for example they published a paper on different approaches to measuring health utility. 753 They also worked out effective ways of conducting large-scale trials in primary care (interviewee).
The CoBalT50 trial was a multi-site clinical trial. Its success relied on productive collaboration between three universities, collaboration that took place between different departments within the universities, and between each university and their local primary care practices (interviewee). The University of Bristol was the lead institution, and the University of Exeter and University of Glasgow were collaborating institutions. As the study involved academic researchers, psychiatrists, psychologists and GPs, the teams involved in CoBalT50 at each university site were diverse and widespread. Each site had to contact local GP practices to recruit their own patients and then organise administration of the CBT treatment and trial follow-up appointments (interviewee). Across the three sites, 73 GP practices were involved in recruiting 469 patients for the trial. 752 This study was hugely collaborative and required excellent communication and organisation (interviewee). The actual team members at each site and their contributions to the study are listed above (see Interface A: Project specification and selection, above).
An example of within-university collaboration is from Exeter, where two researchers from different departments, the primary care services researcher John Campbell at the Medical School and the clinical psychologist CBT expert Willem Kuyken based at the Mood Disorders Centre located 2 miles apart, worked together for the first time. Therefore, much of the trial in Exeter was run from two separate centres. This was managed effectively by appointing a joint team and sharing the finances equitably between the two centres. Campbell started off as the lead in terms of delivering practices and recruiting patients, and then Kuyken took a lot of the lead for the rest of the study (interviewee).
Stage 2: research process
Recruitment was coordinated by the GPs at each of the three sites: David Kessler in Bristol, John Campbell in Exeter and Jill Morrison in Glasgow. For example, in Exeter, Campbell interacted directly with Nicola Wiles, Glyn Lewis and their research team in Bristol in order to agree on a financial model and staffing plan for recruitment in Exeter. In his team he had a local trial manager, two part-time researchers and an administrator working together to recruit practices and patients. Their approach to recruitment included sending a letter to GPs, which was signed by an academic research GP, a mood disorder specialist and the lead from the research network (Campbell, Kuyken and Philip Evans, respectively). They felt that it was important for them to be seen as working together in partnership for recruitment. In general, clinicians had no problems joining the programme and they were receptive to the idea of trying the treatments (interviewee). In total, 73 general practices agreed to take part in the study. 752
Each of the recruited practices searched their records for patients who had TRD. For this study TRD was defined as ‘those who have significant depressive symptoms following at least 6 weeks’ treatment with antidepressant medication at an adequate dose’. The recruitment criteria for this trial were that patients had to be between 18 and 75 years old, currently taking antidepressants and have done so for at least 6 weeks, have a Beck Depression Inventory-II (BDI-II) score of at least ‘14’, and fulfil the International Classification of Diseases, 10th edition, criteria for depression. Patients were not eligible for participation in the trial if they had bipolar disorder, psychosis or major alcohol/substance abuse, if they were unable to complete questionnaires, pregnant, currently receiving psychotherapy or had received CBT in the past 3 years, or were in secondary care for their depression. Some 469 eligible patients were recruited in total; 234 were randomly selected to receive CBT as an adjunct to their existing pharmacotherapy (intervention group), whereas the other 235 continued pharmacotherapy treatment as usual (usual care). The intervention group received a course of 12 sessions of individual face-to-face CBT with up to six more sessions if deemed appropriate by the therapist. Patients were followed up with face-to-face appointments with a researcher at 6 and 12 months, and over the telephone at 3 and 9 months. The primary outcome was ‘response’ defined as ‘at least 50% reduction in depressive symptoms using the BDI-II score at 6 months compared with baseline’. Secondary outcomes included the BDI-II as a continuous score, remission of symptoms, quality of life, anxiety and use of antidepressants at 6 and 12 months. Other data that were collected at 6 and 12 months included information on health and social care use, personal expenditure on private treatments, and time off work. 752
For the cost-effectiveness component of this trial, cost–consequence analyses were reported from the health and social care, patient and lost productivity perspectives. In addition to this a cost–utility analysis compared health and social care costs with QALYs. 752
For the qualitative component of this trial, 40 interviews were held face to face with patients 6 months after they started in the trial. A sampling strategy was used to ensure that patients from the intervention group, usual care group and those in the intervention group who did not complete the therapy, were interviewed. The information gathered in these interviews was analysed thematically to allow comparisons to be made within and across interviews, and to learn more about their experience of CBT and other specific issues. 752
Throughout the research process the team engaged their PPI representatives by telling the relevant people what they were planning to do and asking for comments. Key to this process was the patient user representative, Paul Lanham, on the TSC. The research team also consulted with the local Mental Health Research Network and used a service they provided in which you can submit materials for participants, such as participation leaflets, and the materials review service would provide feedback. This service was used prior to sending the patient information leaflets and consent forms, etc., to the ethics committee for review. They did also have one or two service user individuals who provided input on the design of the follow-up questionnaire during the course of the study. There was a consultation and feedback was taken into account for the final questionnaire. No commissioner users were involved in the CoBalT50 trial but the clinicians and therapist users were involved by default as they were part of the study. For example, Glyn Lewis works in the NHS 1 day per week (interviewee).
The most frequent interaction with the HTA staff was the progress reports that were requested by HTA every 6 months using a pro forma. The individuals on the CoBalT50 team who interacted with the HTA staff said that they found this interaction to be a helpful process. They appreciated being able to communicate with the funder and being able to update them on their progress. Throughout the duration of the trial they mainly had one contact; there was a change relatively early on.
They felt that they developed quite a good relationship with HTA through that contact and she was definitely very approachable (interviewee). In terms of interaction with HTA programme staff, they had a monitoring visit as part of their mid-term review, which included the then Deputy Chairperson for the HTA board Jenny Hewisson. One interviewee said that it was helpful going through what they were doing in these discussions, and that the CoBalT50 team acted on some helpful advice from the HTA group – that they should consider slimming down the screening process to try to improve their recruitment rate (interviewee). The team later requested a 6-month extension as a result of therapist capacity-related issues in two sites and a lower recruitment rate in the third site (interviewee).
Stage 3: primary outputs from research
Knowledge
Three key publications coming out of this research were published in 2013: a Lancet article reporting on clinical effectiveness,754 a companion article in the British Journal of Psychiatry on cost-effectiveness,755 and an article in the British Journal of Clinical Psychiatry reflecting the findings of the nested qualitative study (interviewee). 756 Figure 21 presents the results of the bibliometric analyses on the publications resulting from the CoBalT50 trial.
The intervention (CBT given as an adjunct to usual care, including pharmacotherapy) was found to be clinically effective. Some 46.1% in the intervention group (95 participants) met the criteria for response at 6 months compared with 21.6% in the usual care group (46 participants). The response rate and the remission rate were found to be significantly greater in the intervention group than in the usual care group over the 12-month trial. Additionally, the improvement in quality of life over the 12 months was reported as being greater by those in the intervention group. 752,754
The intervention was found to be cost-effective over 12 months. If society was willing to pay £20,000 per QALY then the net monetary benefit per patient per year would be £289, and the probability that the intervention is cost-effective would be 0.74. If society was willing to pay £30,000 per QALY then the net monetary benefit would be £859, and the probability that the intervention is cost-effective would be 0.91. NICE uses a threshold of £20,000 per QALY when investigating whether or not treatments are cost-effective. 752,755
The qualitative study found that interviewees from the intervention group reported that CBT had given them techniques to help them better manage their symptoms. Patients reported that they had struggled with components of the CBT, which, for some, resulted in them not completing therapy. However, those who did complete the therapy reported that they felt they had benefited it. The authors suggested that practitioners referring patients for CBT should discuss the potential challenges of the intervention with the patients to help them make an informed choice about referral for CBT. 752,756
There was not much freedom to pursue research that was not part of the original protocol as it was a RCT study and, by their very nature, these are focused studies. However, the team did publish several papers that used the data from the study. They reported on the prevalence of TRD in primary care. 742 They investigated patients’ experiences of participating in a large-scale depression trial, and found that the patients felt that they benefited from being in the trial because it enabled them to reflect on their feelings, and, for some, taking part increased their feelings of self-worth. 757 They explored patients’ reasons for declining to be contacted about a study of the effectiveness of CBT as a treatment for depression. The four main themes of reasons why patients declined were previous counselling experiences, negative feelings about the therapeutic encounter, perceived ineligibility and misunderstandings about the research. 758 They investigated why GPs exclude potentially eligible participants from a large-scale RCT and found that 67% were excluded because of trial criteria, 20% for other criteria (half of which were comorbid conditions) and 13% without reason. 759 They investigated potential moderators of response to the intervention for TRD but found that age was the only variable with evidence for effect modification. 760 They investigated the differences between four different approaches to measuring health utility in depressed patients, and found that there was a lack of agreement between utility scores generated by the different instruments. 753 They also published the protocol that they used for the trial. 761
Benefits to future research and research use
Capacity building and career development
Taking part in the CoBalT50 study enhanced the careers of all those that took part in it. It added to the already impressive profiles of the senior researchers and added to the body of work that will have contributed to the promotions of mid-career researchers. In general, for the more senior researchers the study maintained their continuity in the area and has opened up opportunities for new collaborations. Finally, some of the junior researchers were able to take the next step up in their career, and for two junior researchers the experience will have strengthened their applications for further qualifications for which they went on to train (interviewee).
In terms of Nicola Wiles’s career, it was the first large externally funded grant that she had led. It was a significant addition to her CV, and it will have been a contributing factor to her promotion to Reader at Bristol University. The CoBalT50 study also helped establish Wiles in the field of mental health and psychological treatments research, as well as helping her to become an expert on conducting large RCTs in a primary health-care setting (interviewee).
As a result of the CoBalT50 study, a NIHR-funded methodology fellow (post MSc but pre-doctoral) went on to be awarded a NIHR doctoral fellowship, and a local trials manager subsequently moved on to do clinical training for a DClinSci at the University of Exeter (interviewee). One individual improved his/her skill set and made a career progression from a researcher that conducted day-to-day data collection in the original study to a trial management role in a follow-up study (interviewee). For some of the junior researchers working on CoBalT50 the study certainly enhanced their profile through the publications that they co-authored. One of them, Caroline Jenkinson, worked very hard over the last year analysing some of the CoBalT data, and, as a result, she produced her own paper published in Family Practice in 2014;760 this was her first paper, which was important for her career. All of the junior researchers who worked for John Campbell on CoBalT50 have continued to work with him: Chris Wright, Rachel Winder and Caroline Jenkinson. Their hard work in CoBalT50 led him to continue to include them in subsequent work and to want to continue to support their career development (interviewee). There were 11 CBT therapists (health practitioners) involved in the CoBalT50 study, and it was reported that they found it to be a valuable experience and it helped inform the direction that they wanted to take with their own careers. Some of the therapists wrote a paper together (interviewee).
The output of CoBalT50 has had lots of effects, some of which are quite intangible. These include enhanced relationships, ongoing research submissions and collaborations. For example, Campbell’s professional partnerships and networks were enhanced through working on the CoBalT50 study. The success of TREAD and CoBalT,50 and the experience he gained from being involved in these studies, has enabled him to work effectively with a new Professor of Psychiatry at the Medical School at the University of Exeter, Chris Dickens. He brought Dickens on board for a new trial in which he was involved, looking at mirtazapine as an adjunct treatment for managing TRD called mirtazapine (MIR), which is contributing to Dickens’s career development (interviewee).
Targeting of future research
Campbell’s involvement in the CoBalT50 study added to his experience in conducting major clinical trials work, and, as such, strengthened his reputation as an established triallist. For example, he is now involved in another HTA programme-funded study with David Kessler, who was also a CoBalT50 co-applicant. Kessler saw that Campbell and his team were successful, committed and enterprising during CoBalT,50 and approached them to undertake the MIR trial with them, which is currently in progress. The commonality between CoBalT50 and MIR was TRD but with a different therapy. The MIR study was funded as a result of a HTA-commissioned rapid trial type of research call but the content of the trial was their idea. The HTA was looking to commission fast and easy to implement studies (interviewee).
In addition, having built a good track record, the University of Exeter team has gone on to secure their own HTA funding for the CADENCE (cardiac rehabilitation services for patients with new onset depression) study, which is looking at the management of depression in patients who have suffered acute cardiac events, and who are entering into cardiac rehabilitation. The CADENCE study is a feasibility and pilot trial for which the HTA awarded £430,000 for 2 years. The treatment being used in the CADENCE trial is behavioural activation, an enhanced psychological intervention. Campbell is the PI on the CADENCE trial. Although this study is led by the University of Exeter team, there is continued collaboration with the University of Bristol as Kessler is a co-applicant on the CADENCE grant. For this trial the relationship has reversed, as the University of Exeter team are leading, which shows that a dynamic relationship has been formed between the two teams in conducting these trials. In addition to the continued collaborative work that the University of Exeter team is conducting with the University of Bristol team, the former is also leading and developing their own mental health and psychological treatments research (interviewee).
The success of the CoBalT50 study has also enhanced Campbell’s career in the field of primary care services research. He recently completed a trial called ESTEEM, a study assessing the effectiveness and cost-effectiveness of telephone triage of patients requesting same-day consultations in general practice; which received £2.2M of HTA funding. ESTEEM is completely separate to CoBalT50 but his strong primary care services research track record with HTA through CoBalT and CADENCE will have helped his securing the ESTEEM grant. ESTEEM was a study about telephone triage in primary care involving 21,000 patients, 42 practices and four centres. The findings from ESTEEM were published in The Lancet at the end of 2014762 (interviewee).
In terms of the impact on the wider research field, the findings of the CoBalT50 study were published in February 2013, only 2 years ago, so the full impact has not yet been realised. The CoBalT50 study has been built on further by Nicola Wiles and the CoBalT50 team. They went on to test the long-term effectiveness of the CBT adjunct to pharmacotherapy treatment 4 years after randomisation. This was funded by means of an extension of the CoBalT50 grant. This study is now completed and the results have been submitted for publication (interviewee). Other than this study, none of the interviewees was aware of any other researchers who have built on their work from the CoBalT50 study. However, a prominent researcher in the USA who carries out CBT trials did approach one of the senior researchers for advice (interviewee).
Interface B: dissemination
Academic dissemination
As described above (see Stage 3: Primary outputs from research), there were three key publications coming out of this research. The team also published their trial protocol761 and five other publications. Between the team they did quite a lot of dissemination of the main study findings in terms of the academic environment both in the UK and internationally. At a British Association for Behavioural & Cognitive Psychotherapies (BABCP) conference, a primary CBT conference in the UK, they delivered a 1.5-hour symposium, during which they gave four presentations presenting different aspects of the studies to provide an overview of the CoBalT50 study. Four of the CoBalT50 team also presented in a symposium at a European CBT conference run by the European Association for Behavioural and Cognitive Therapies. The findings were also presented at the Society for Academic Primary Care (SAPC) annual meeting, at the American Behavioral and Cognitive Therapies conference, and the World CBT conference in Lima. Some of these conferences will have had clinicians and therapists in attendance in addition to academic researchers (interviewee).
Nicola Wiles’ experience of running the BABCP symposium was very different from the format in which she traditionally presented, such as the ‘10- to 15-minute talk plus 5 minutes for questions’ format at the SAPC conference. Attendees reported to her that they appreciated seeing the four talks – covering all aspects of the whole study – together, as it enabled them to have more in-depth understanding of the results. In addition to learning about the clinical effectiveness and cost-effectiveness of the intervention, the findings on participants’ experiences of the intervention, and learning about some of the barriers to completion were of particular interest to clinicians. The audience at these conferences was very clinically orientated, including CBT practitioners and people involved in psychological services, people running IAPT services, as well as the more traditional academic audience members (interviewee).
The feedback that Wiles received from her colleagues who presented at the international meetings was that the findings were well received. She did not know, but suspected, that the audiences at the international conferences would have been more restricted to academics and clinicians rather than people running services. Although this dissemination would feed into practice, it is important to be aware of the differences in provision of psychological services in other countries. For example, in the USA, access to psychological treatment is dependent on insurance and your ability to pay (interviewee).
Wider dissemination
The CoBalT50 team had funding from the HTA for some of the dissemination that they carried out. At the end of the study, they produced a newsletter, with the basic findings, for the GPs from all 73 practices as a ‘thank you’ for participating. They also sent similar newsletters to all participating patients as a ‘thank you’ for taking part. This newsletter was reviewed by the patient user representative before it went out to make sure that it was in plain English and written in a way that would be readily digestible for use (interviewee).
The findings of the CoBalT50 study were disseminated at a few events. One of these was a Mental Health Research Network South West conference, which brought together members of the academic community and service users. Some of those involved in commissioning services will have also learnt about the CoBalT50 findings at that meeting (interviewee). The University of Exeter team also disseminated the CoBalT50 findings at a local event at their Mood Disorders Centre, which was organised to bring together local GPs, people involved in the IAPT services and a few user representatives (interviewee). Campbell summarised some of the CoBalT50 findings at one of his local annual practice network events (interviewee).
Some of the members of the University of Bristol CoBalT50 team are involved in trying to create a health integration team in Bristol, which is looking to improve psychological services. One of their plans is to produce a couple of short videos, based on CoBalT50 findings, which they would then upload up on to a website such that they would be accessible to patients and clinicians. They particularly wanted to bring the qualitative findings about the patients’ experience of CBT to the clinicians’ attention, as they feel that these findings have the potential to inform the discussion between patient and clinicians regarding referral for therapy. They have received approximately £2000 from Bristol Health Partners to produce those videos (interviewee).
Although they did their best to disseminate their findings with the funding HTA provided, there were other forms of dissemination that they would have liked to have carried out. Campbell expressed that he would have liked to be able to organise more dissemination events for his practices, and he felt that when so much has been invested in a study such as CoBalT50 it really was worth spending the money to do more local dissemination, to get the practices together with the local psychiatrist, primary care people and the psychologists. He also thought it was important to target more dissemination to depression and mental health charities. From a practical point it was explained by Campbell that funding for dissemination needs to be available as soon as the results are obtained. If there is a delay of a couple of months then the investigators move on to their next projects and do not have time to get involved in broader dissemination activities and so a huge opportunity is missed (interviewee).
The CoBalT50 team have not received any HTA funding for dissemination of the follow-up study (interviewee). Campbell was not aware of any cases for which dissemination activities have been able to be worked into the follow-on grant. He suggested that potentially including such a section into their follow-on grant may have actually made them less likely to secure funding for the follow-on study. They could have built in £50,000 to hold a series of dissemination events but that would mean that the follow-on would be, for example, £200,000 as opposed to £150,000, and this would make the proposal less competitive and too high risk for the researcher (interviewee).
Stage 4: secondary outputs
So far there have been two secondary outputs from the CoBalT50 study. The first is the extension study that is following up the patients approximately 4 years post randomisation. These findings are currently being submitted for publication (interviewee). The other secondary output is the re-ignition of the systematic review. The team have published a Cochrane protocol on interventions – both pharmacological and psychological – for adults with TRD and they hope that the CoBalT50 study will feed into it and inform a wider research agenda in that area (interviewee).
The CoBalT50 findings are not yet cited by the NICE clinical guidelines. However, one of the interviewees explained that the NICE guidelines are not the sort of publications that you can actively approach to disseminate your findings; however, the CoBalT50 findings will be viewed as part of the background research used to develop the next NICE depression guidelines (interviewee). It is anticipated that these will be published in May 2017. In the 2009 NICE guidelines for depression it is stated in the section on sequencing treatments after initial inadequate response (Section 1.8) that ‘for a person whose depression has not responded to either pharmacological or psychological interventions, consider combining antidepressant medication with CBT’. 751 It is not mentioned in the guidelines how many sessions should be given. In the 2004 NICE guidelines they suggested that 12 sessions should be given. One of the interviewees said that this change was influenced by one of the clinical psychologists from the CoBalT50 study. Until the findings from the CoBalT50 study, there was no RCT evidence to support the use of CBT as an adjunct to pharmacotherapy for TRD (interviewee). The CoBalT50 findings have not been cited in any other clinical guidelines, audit criteria or similar document from a professional body or public policy-making body at a national or local level (interviewee).
Stage 5: adoption by practice and the public
The interviewees explained that it is very difficult to gauge whether or not there has been any impact on clinical practice by any individual clinicians, but there is some anecdotal evidence that the findings from the CoBalT50 study have more generally influenced some clinicians and practitioners. In the CoBalT50 study many of the patients received 18 sessions (the protocol was 12 sessions plus another six sessions if deemed necessary by the therapist). In the IAPT programme, the therapists are meant to deliver only four to six sessions. Now, if a therapist wants to deliver more sessions of CBT to their patients with TRD they can justify their request to their supervisor by referencing the CoBalT50 study. There is also potential for the CoBalT50 findings to influence IAPT in another way. One of the findings from the CoBalT50 qualitative study was that patients with TRD do not necessarily bring themselves to the health-care system therefore one action that the system might consider is to support an initiative to go out and find these patients (interviewee). The findings of CoBalT50 are not currently adopted into IAPT practice, but at least some IAPT commissioners are aware of the findings of the CoBalT50 study as they have attended conferences at which the CoBalT50 team have presented their findings (interviewee).
A potential barrier to the CoBalT50 findings being adopted into clinical practice and having an impact on patients is if the NHS does not have enough resources to implement the treatment as conducted in the study. There has already been a huge investment in psychological services but they think that CoBalT has shown that high-intensity therapies are required and it is variable in terms of how widely that is available across the UK (interviewee).
Stage 6: final outcomes
As the CoBalT50 findings were published only 2 years ago in February 2013, there has not yet been an opportunity for the CoBalT50 findings to have had a notable effect on society. As it stands, the NICE guidelines already recommend CBT as a ‘next step’ treatment for patients with TRD on pharmacotherapy, but this guidance was not based on any strong evidence and they do not recommend how many sessions should be included in the ‘next step’ treatment for TRD. The existence of the CoBalT50 findings now provide evidence to support this recommendation, and also provide evidence to suggest that there should be 12–18 sessions of CBT as part of this treatment. Anecdotally, there is a small number of clinicians who are aware of the CoBalT50 study and, as a result, are choosing to do 18 sessions of CBT as opposed to the more commonly administered 12 sessions. The CoBalT follow-up study has been completed and the results have been submitted for publication and will be disseminated shortly (interviewee).
Table of payback
Payback details for this case study are provided below in Table 35.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | Nine peer-reviewed articles Five academic meetings (two symposiums, one poster and two talks) |
| Research Targeting and Capacity Building | Two junior researchers moved on to doctoral courses PhDs obtained and techniques taught Career development of members of research team Targeting of further research by the original team, or by others building on the findings or refinement of methodology or techniques, etc. |
| Informing Policy and Product Development | As of yet, the findings from the CoBalT50 study have not informed policy but they expect that the findings will be incorporated in to the next NICE clinical guidelines for depression when they are published |
| Health and Health Sector Benefits | Within their network they may have affected the number of CBT sessions that clinicians are offering to their patients |
| Broader Social and Economic Benefits | Expanded the expertise for these large-scale primary care trials to more departments and researchers within the UK |
Appendix 4.11: CUtLASS
Summary
Schizophrenia is a chronic, severe and disabling disorder characterised by symptoms such as hallucinations, delusions, disordered thinking, movement disorders, social withdrawal and cognitive deficits. Pharmacological therapy has remained the mainstay of treatment since it was first introduced in the 1950s. Chlorpromazine, the first antipsychotic developed, was soon followed by a number of other similar drugs, and although these ‘typical’ (or first-generation) antipsychotic drugs had limited effectiveness and well-documented side effects, they remained the main options for treatment until the emergence of so-called ‘atypical’ (or second-generation) antipsychotics in the early 1990s. The main finding of the CUtLASS RCT was that atypical antipsychotics showed no advantage in terms of quality of life, symptoms or incidence of extrapyramidal side effects over the course of a year when compared with the older, typical drugs. There was also no clear preference among participants for one group or the other. These findings were surprising to patients and practitioners who had expected the atypical drugs to be superior.
As a result of the CUtLASS findings, NICE changed its recommendation from using an atypical antipsychotic as first-line treatment to discussing with the patient which drug should be used, with no mention of whether that should be typical or atypical. However, just prior to, and during the early stages of, the study there was a dramatic increase in the use of atypical antipsychotics, amid a general belief in the field that they would be superior in both effectiveness and reducing side effects. It is suggested that the study results, although supported by a similar study in the USA, have probably had little impact on reversing this trend and have had little effect on the choice of drug that clinicians make. However, the study may have led to clinicians making better use of the same drugs through being more aware of side effects, and reducing their practice of prescribing large doses of more than one drug (polypharmacy), which can increase safety risks and costs.
Introduction to case study
Background
Scientific background
Schizophrenia places a large burden on society, with a 2012 estimate suggesting that its cost to English society alone is £11.8B per year. 763
The National Institute for Health and Care Excellence recommends that schizophrenia should be treated by multidisciplinary teams, including community and home-based services, as well as a range of psychosocial interventions,764 but pharmacological therapy has remained the mainstay of treatment since it was first introduced in the 1950s. 765 Chlorpromazine, the first antipsychotic developed, was soon followed by a number of other similar drugs, and although these ‘typical’ (or first-generation) antipsychotics had limited effectiveness and well-documented side effects,766 they remained the main options for treatment until the emergence of so-called ‘atypical’ (or second-generation) antipsychotics in the early 1990s. The development of these new drugs was triggered by the re-introduction of clozapine to the market (Jones interview), it previously having been withdrawn because of safety concerns. These drugs were characterised by their lack of ‘extrapyramidal’ side effects (i.e. movement disorders such as tardive dyskinesia) and were subject to extensive promotional campaigns from the pharmaceutical companies involved. Jones described this period as a great example of ‘push and pull’ in the field of schizophrenia treatment: in addition to the ‘push’ from the industry side, the problems with the older drugs meant that clinicians, patients and family groups were also all in favour of the rapid adoption of atypicals in routine practice.
Chief investigator’s background
Shôn Lewis is Professor of Adult Psychiatry and Director of the Institute of Brain, Behaviour and Mental Health at the University of Manchester, where he has been based since 1994. He is also an honorary consultant psychiatrist in Manchester Mental Health and Social Care Trust and was R&D Director there from 2004 to 2008. His research focuses on risk factors and new interventions in schizophrenia and psychosis.
The case study approach
This case study was constructed based on a review of the HTA monograph and other literature relevant to the trial. As shown in Table 36, two of the principal clinicians involved in the study were also interviewed. Approaches to other members of the team and relevant individuals either did not receive a response or interviews were not possible within the study’s time frame.
| Interviewee | Reason for interview |
|---|---|
| Peter Jones | Co-investigator; Professor of Psychiatry at the University of Cambridge, and Director of the NIHR CLAHRC for the East of England |
| David Taylor | Co-investigator; Professor of Psychopharmacology at King’s College London |
Stage 0: topic/issue identification
A number of key factors influenced the research team’s decision to work in this area, as detailed in Box 11 and described below.
-
Emergence of new antipsychotics.
-
Increasing costs for psychiatric services.
Emergence of new antipsychotics
As described above, a new class of antipsychotic drugs had emerged during the early 1990s and there was optimism among clinicians that they would be both more effective and have fewer side effects than the typical antipsychotics available. Although used extensively already amid widespread belief that they were ‘better’ drugs, there was little systematic evidence on the relative effectiveness of the two generations of drugs and it was considered important to conduct an independent study to assess their value (Taylor interview). The HTA issued a commissioning brief for a study to this end in 1996.
Increasing costs for psychiatric services
Conducting such an assessment was made more urgent by increasing costs being incurred by the NHS. As the atypical drugs began to be more widely used, in part because of the apparent reduced extrapyramidal side effects,765 the cost to the NHS of providing treatment for schizophrenia increased rapidly (Jones interview). Prior to around 1996, drug costs had been only a small proportion of the total cost of psychiatric services, as the typical antipsychotics were all off-patent. The emergence of the new, far more expensive, atypical drugs led to prescribing becoming political, with a number of restrictions put in place regarding the new drugs (Jones interview). Jones noted that this was an unpopular situation among psychiatrists, as in some instances they were unable to make use of the first new drugs that had become available in a generation.
Interface A: project specification and selection
The HTA programme issued a commissioning brief in 1996 for a study looking at the clinical effectiveness and cost-effectiveness of first- and second-generation antipsychotics. Initially, three separates bids were in preparation by the researchers who ultimately undertook the study. As most of those involved knew each other, it was decided that by joining forces they would have a better chance of being awarded the grant and could benefit from economies of scale in terms of trial recruitment (as it would be possible for clinicians to refer patients to whichever part of the trial was most appropriate). This arrangement was organised by Shôn Lewis, who had previously worked at the Institute of Psychiatry and so knew well the team members based there.
The combination of the three groups resulted in a study in three distinct parts:
-
typical vs. atypical antipsychotics
-
clozapine vs. atypical antipsychotics
-
mirror image health economics study.
The study aimed to conform as closely as possible to routine clinical practice, so that outcomes could be measured in a ‘real world’ context, while also ensuring that it met the criteria for a robust randomised trial. The first part (‘band 1′) aimed to look at the overall clinical effectiveness and cost-effectiveness of atypicals compared with the older drugs. The second (‘band 2′) focused specifically on clozapine. As prescribing practice at the time meant that clozapine was used only when other first- and second-line drugs had not been effective, it was not considered appropriate to include clozapine in a three-way comparison with typicals and atypicals – it was unlikely that clinicians would be in a situation of choosing between all three options. The final part of the grant, the mirror image study, was carried out fairly independently of the other two, and used relapse and re-admission data to examine costs before and after switching from a typical to an atypical drug.
Although the study was initiated before formal public and patient involvement became commonplace, Jones commented that it was addressing a very real clinical problem, and that patient and family groups were aligned with other parties in supporting the study. At the time, all parties expected it to provide evidence to increase the availability of the new drugs (Jones interview).
There was little interaction with the pharmaceutical industry in the design of the study. Although pharmaceutical companies were not against the trial at the outset, they had less of a stake in it than in, for example, the CATIE (Clinical Antipsychotic Trials of Intervention Effectiveness)767 study in the USA – although in CATIE the participants were randomised to specific drugs, CUtLASS51 randomised only by class of drug, allowing clinicians to select the most appropriate drug within that for each patient. The aim of this design was to reflect the situation facing the NHS: although there were differences in pharmacology between the drugs within each class, the distinction reflected both the groupings used in clinical guidelines and the difference in acquisition costs for the NHS (as noted above, the off-patent typical drugs were far cheaper to prescribe than the new atypicals).
Stage 1: inputs to research
The HTA programme provided £1,297,444 of funding for the study. Other treatment costs were supported by regional R&D offices, although the precise mechanism for this varied by site.
It was also important for the team to have the buy-in of clinicians at each of the 14 NHS trusts that participated, as the study depended on their willingness to refer patients to the different bands of the study.
Shôn Lewis (University of Manchester), Peter Jones (University of Nottingham at the start of the study, then University of Cambridge), Thomas Barnes (Imperial College London) and Robin Murray (Institute of Psychiatry, London) were the main clinicians involved, but as the study was designed to be closely related to practice, conducting the trial was close to everyone’s day-to-day experience of seeing patients (Taylor interview). This meant that the study did not require substantial inputs beyond funding and the time of the researchers involved.
Stage 2: research process
The study took place across four sites initially (University of Manchester, University of Nottingham, Imperial College London and the Institute of Psychiatry, London). In the final year of recruitment, the University of Cambridge became a fifth site, following the move of Professor Jones from Nottingham. The study steering committee was chaired by Professor Lewis, and videoconferences were arranged to help collaboration. Professor Jones commented that this side of the trial worked very well.
The study faced some recruitment difficulties, in part because of clinicians’ opinions shifting in favour of atypicals during the late 1990s, which meant that some were unwilling to risk their patients being randomised to a typical drug (Taylor interview). However, the team subsequently found that the dropout rate was lower than anticipated and the outcome measures more sensitive to change than expected, with the result that the study was suitably powered (Jones interview).
A number of changes took place between the commissioning brief being issued in 1996 and the first participants being recruited in 1999, as well as during the course of the study. Most notably, there were changes in prescribing practices of atypical antipsychotics. Nationally, expenditure on atypicals increased from 38% of total antipsychotic spending in 1996 to 90% in 2002. 765 However, this increase was not uniform, and the team also noted that in Greater Manchester the prescribing rate of clozapine showed a more than 30-fold variation across the health providers involved in the same time period. 765
New antipsychotics were also licensed during the study: olanzapine and sertindole in 1996 (although sertindole was withdrawn on safety grounds in 1999), and quetiapine and amisulpride in 1997. In response to the newly available drugs, NICE issued drug treatment guidance in mid-2002 and the NICE schizophrenia clinical guideline later that year, both of which recommended atypicals as a first-line treatment and the wider use of clozapine in treatment-resistant schizophrenia.
Two drugs were explicitly added to the study during its course, as a result of their appearance in clinical guidelines of several of the trusts involved: amisulpride as an atypical, following its introduction in 1997, and sulpiride, which had been available since the mid-1960s. The inclusion of sulpiride as a typical drug drew some criticism following the study’s publication, as it is sometimes considered a ‘less typical’ typical antipsychotic due to claims that it has a reduced incidence of extrapyramidal side effects. 765 Its inclusion as a typical drug was supported by the fact that its cost was similar to the other drugs in that class, but Jones commented that it was being marketed as a new drug and so people often associated it with the atypicals (Jones interview). Taylor noted that after sulpiride’s addition to the study, the recruitment rate increased in band 1, with almost all of the newly referred patients being prescribed it, and suggested that this may be due to clinicians perceiving it as different from the other drugs in its class (Taylor interview).
A number of reviews and meta-analyses were also published during the study. Although some smaller reviews found small advantages for atypical drugs, a large systematic review by Geddes et al. (2000)768 concluded that there was insufficient evidence to conclude that atypicals were more effective, a finding supported by a subsequent systematic review by Bagnall et al. (2003). 769
At the same time as the CUtLASS51 trial in the UK, the CATIE767 trial was being carried out in the USA. It published slightly ahead of CUtLASS. 51 CATIE767 had broadly the same aim of comparing typical and atypical antipsychotics, but randomised participants to specific atypical drugs and compared against the typical antipsychotic perphenazine. The teams were aware of each other’s studies and there some informal discussions through personal contacts, but at the time the concept of meta-analysis was still in its infancy and was not a priority for the CUtLASS51 team (Jones interview).
The mirror image study was carried out fairly independently by Professors Taylor and Kerwin at the Institute of Psychiatry. It comprised a before-and-after comparison of patients who had switched from a typical antipsychotic to an atypical antipsychotic. Unlike many such studies, the team was able to include a control group who remained on the same medication throughout, a comparison that was possible owing to the level of funding provided by the HTA programme (Taylor interview).
Stage 3: primary outputs from research
Knowledge
The main finding from band 1 of the study was that atypical antipsychotics showed no advantage in terms of quality of life, symptoms or incidence of extrapyramidal side effects over the course of a year when compared with the older, typical drugs. There was also no clear preference among participants for one group or the other. 766
Professor Jones commented that these findings were a shock to everyone, given the widespread belief prior to the trial that atypical drugs were superior. Indeed an earlier paper published by the CUtLASS51 team that looked at the attitudes of clinicians found that 97% of respondents believed that atypicals had less severe side effects. 770 The paper reporting the study’s main results was refused by the British Medical Journal, despite strong scientific review, because they thought it was not of sufficient general interest (Jones interview). The paper was subsequently published in Archives of General Psychiatry,766 through which it has gone on to be a highly cited publication (Figure 22 provides a bibliometric summary of the project’s outputs).
Band 2 of CUtLASS51 concluded that clozapine was more effective at reducing symptoms in treatment-resistant schizophrenia than other atypicals, and also found that participants preferred it to the other drugs. 771
The mirror image study’s findings were published in 2007, and showed that participants switching to an atypical antipsychotic spent more days in hospital than those in the control group who had remained on a typical antipsychotic, regardless of whether or not participants had switched typical drugs within the control group. 776
The team also published a paper on cost-effectiveness, which showed that in comparison with atypical antipsychotics, typical antipsychotics may be cost saving and be associated with a QALY gain,772 as well as other papers on non-neurological and metabolic side effects,774 the use of long-acting injections versus oral preparations,775 and the determinants of changes in quality of life in schizophrenia patients. 777
Benefits to future research and research use
Capacity building and career development
Professor Taylor suggested that although the papers from the RCT component of CUtLASS51 were high profile, the study probably did not have a huge effect on the careers of the main researchers involved, who were already very well respected in the field. He also commented that the mirror image study has remained low profile, with its findings being one of his least cited publications.
Targeting of future research
The findings of both CUtLASS51 and CATIE767 were initially met with disbelief from clinicians, researchers and the pharmaceutical industry, and Professor Jones commented that it took around 2 years for the findings to be accepted in the field – a time during which he described himself as being ‘kind of ostracised’ by the pharmaceutical industry.
The next major trial to publish was EUFEST (European First Episode Schizophrenia Trial),778 a large multi-country study funded by industry, which compared the typical antipsychotic haloperidol with a range of atypical drugs. Although it showed better response and remission for most atypicals,778 Professor Jones commented that the use of ‘all cause discontinuation’ as the study’s outcome measure was, in retrospect, problematic. This measure is a pragmatic way of taking into account treatment discontinuation due to a drug’s (in)effectiveness and also its side effects, both important considerations for clinicians in prescribing an antipsychotic. Although this was also the measure used by CATIE,767 EUFEST778 used it in an open study (unlike in CATIE,767 the clinician was not blind to treatment group) and there is evidence that clinicians’ expectations that the new drugs were better resulted in them using a lower threshold for discontinuing the drugs they perceived as worse (Jones interview).
This was also around the time that other doubts were starting to arise about atypical drugs. In particular, evidence was beginning to accumulate on the metabolic side effects of some atypical drugs. 779,780 In 2002 the Committee on the Safety of Medicines and the Medicines Control Agency (now the MHRA) recommended blood glucose monitoring in patients with schizophrenia who were at risk of diabetes, and, subsequently, the TEOSS781 (Treatment of Early Onset Schizophrenia Spectrum disorders) study raised strong concerns about the use of atypical antipsychotics in young people.
Professor Taylor commented that the question of whether typical or atypical antipsychotics are superior as an entire class probably remains unanswered owing to methodological differences in the studies conducted. In the time since CUtLASS51 was published, no new drugs have been developed (Jones interview).
Interface B: dissemination
Professor Jones recounts that when the CUtLASS51 findings were first presented at an international conference in the USA, the room was full and the audience was somewhat sceptical. He commented that the initial correspondence they had around the findings and the citations they received was very negative, but after about 2 years the results became accepted in the field, and citation began to become more positive (Jones interview). The findings were published around the same time as the CATIE767 study, without which Professor Jones suggested the results may have been buried – CUtLASS’s51 consistency with a larger, US study has increased the profile of its findings and facilitated its impact.
Professor Jones commented that he considered it more important to maximise the impact of CUtLASS51 by disseminating its findings as widely as possible, rather than start another trial in the same area. Through British Association of Psychopharmacology masterclasses he teaches around 80 consultant psychiatrists twice per year, and so this provides a useful way of reaching large numbers of clinicians. He has also given talks at patient associations such as Rethink.
Stage 4: secondary outputs
As a result of the CUtLASS51 and CATIE767 findings, NICE changed its recommendation from using an atypical antipsychotic as first-line treatment to discussing with the patient which drug should be used, with no mention of whether that should be typical or atypical (Taylor interview). However, this may have had little effect on practice (Jones interview: see Stage 5: Adoption by practice and the public, below).
One of the potential downsides of CUtLASS51 and CATIE767 is that their findings may have contributed to a reduction in research in the pharmaceutical industry into developing new drugs for schizophrenia (Jones interview). Professor Jones commented that Eli Lilly has maintained development in the area, but that a recent promising compound had failed in larger trials, and that there had been a similar outcome for a compound that Roche had been developing for treating the negative symptoms of schizophrenia. Other companies have withdrawn completely from the field. Professor Taylor, though, suggested that pharmaceutical companies have recognised a lack of political will to develop new pharmacological treatments in mental health and have instead focused on areas that are higher on the political agenda (Taylor interview).
The choice of a quality-of-life scale as the main outcome measure was selected to reflect the main aim of clinicians in determining treatment (and so is appropriate in attempting to replicate a ‘real world’ setting as closely as possible). However, Professor Taylor commented that this, alongside the decision to randomise participants to classes of antipsychotic rather than to specific drugs (as was the case in CATIE767), has limited the extent to which the CUtLASS51 findings can be incorporated into meta-analyses.
Stage 5: adoption by practice and the public
As noted above, just prior to and during the early stages of the study there was a dramatic increase in the use of atypical antipsychotics, amid a general belief in the field that they were superior in both effectiveness and reducing side effects. Professor Jones suggested that the findings from CUtLASS51 have had little impact on reversing this trend and have had little effect on the choice of drug that clinicians make. He goes on to comment: ‘It’s very difficult to find a psychiatrist now under the age of 40 who knows how to prescribe the old drugs’ (Jones interview).
Similarly, Professor Taylor suggests that in the case of clozapine there has also been little effect on practice: although both CUtLASS51 and CATIE767 concluded that it was superior to other atypicals, this was largely already believed by clinicians to be the case (Taylor interview).
Although the particular drugs being prescribed may not have changed, it is likely that the CUtLASS51 findings have led to clinicians making better use of the same drugs (Jones interview). In particular, some of the subsidiary papers (e.g. on side effects and health economics) may have made clinicians more aware of side effects and adjusted their expectations around outcomes. Professor Jones suggested that, alongside this, one advantage of the rapid change in prescribing was that psychiatrists reduced their practice of prescribing large doses of more than one drug (polypharmacy), which can increase safety risks and costs, an issue touched on in a paper co-authored by the CUtLASS51 and CATIE CIs. 767 Among pharmaceutical companies there has also been a focus on determining the optimum dose when new atypicals have been introduced, in order to minimise side effects, as well as in using them as a single drug, allowing clinicians to observe their effects in isolation and gain a better understanding of how the patient responds (Jones interview).
Professor Taylor commented that the impact of the mirror image study was limited by its findings, in that if the study does not show that a marketable drug is superior to another treatment then the drug’s manufacturer has little incentive to publicise the findings.
The CUtLASS51 findings continue to be used as evidence that there is no advantage to using atypical antipsychotics over the older drugs, and serve to demonstrate that clinicians need to think carefully about choice of drug. There remains an issue around the definitions of typical and atypical antipsychotics, however (Taylor interview), and more recent reviews have questioned whether or not there are two distinct classes of antipsychotics, and whether or not all drugs within each can be considered equivalent. For example, Leucht et al. (2009)782 conclude that atypical antipsychotics are not homogeneous and suggest that more individualised treatment is needed. Professor Taylor also highlighted a potential risk in that the CUtLASS51 findings are sometimes used as evidence that prescribers should shift back to older drugs on the grounds of cost; as many atypicals are now off-patent, the cost difference is not as dramatic as it once was and parts of the pharmacoeconomic analysis may no longer apply (Taylor interview). In this regard, he considers the use of the study’s findings to promote the use of older drugs to be inappropriate.
Stage 6: final outcomes
Although the CUtLASS51 findings may have not had a substantial impact on the types of drugs being prescribed, they may have led to better prescribing, with more consideration of side effects and quality of life. However, the degree to which this can be attributed to CUtLASS51 rather than other studies or trends is very difficult to determine.
Table of payback
Payback details for this case study are provided below in Table 37.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | Ten peer-reviewed publications Presentation of findings at major international meetings Other knowledge production impacts, e.g. book chapters |
| Research Targeting and Capacity Building | Informed subsequent trials to some extent British Association of Psychopharmacology masterclasses to teach consultant psychiatrists about the findings |
| Informing Policy and Product Development | Limited citation in meta-analyses and systematic reviews Citation in NICE guideline Potential contribution to reduction of reduction of investment from the pharmaceutical industry in drug development |
| Health and Health Sector Benefits | May have contributed to better prescribing by clinicians in terms of dosing, polypharmacy and discussion of side effects with patients Potential risk of findings being used as an ‘excuse’ to prescribe older drugs when inappropriate |
| Broader Social and Economic Benefits | Possible benefit to patients’ quality of life if the study’s findings have led to better prescribing practice |
Appendix 4.12: an overview of Single Technology Appraisals and related processes conducted through the Technology Assessment Report programme 2003–13
Summary
A significant stream of the HTA funding is allocated to NICE STAs. In these, Evidence Review Groups (ERGs) (usually based at a university) review a manufacturer’s submission to NICE for recommendation for use of a medicine in the NHS in England. The independent assessment is reported to the Appraisal Committee but often not published beyond NICE’s website. This can have an impact on the careers of those undertaking the assessments, although it is recognised that work for the HTA and NICE carries some prestige. The work of the TAR team feeds directly into the appraisal process and informs the guidance that is developed. This timely assessment generally leads to the availability or otherwise of a medicine, which has an impact on patient lives, choices available and the profitability of the manufacturer. In addition, there is the potential for international impact, as other countries look to the NICE guidance and supporting evidence to inform their process.
Introduction to case study
Background
When a manufacturer produces a new drug, it provides an assessment of it to NICE as part of the process of requesting approval for its use in the NHS. To determine whether or not and how to approve the use of a new drug or technology, NICE requires a synthesis of the evidence and a cost-effectiveness model. This work is independently performed by centres, commissioned through, and funded by, the HTA programme.
There are different types of assessments that are conducted within the TAR contracts: STAs and MTAs, diagnostic assessments and highly specialised technologies. In a STA, a single technology is assessed. In this case, the manufacturer builds the economic model to evidence the value of the new technology and the TAR team critiques it. In the case of MTAs, multiple technologies for a single indication are reviewed simultaneously and compared. Here the academic group builds the model, utilising industry and independent data to inform it. Diagnostic assessments review diagnostic technologies, rather than drugs.
Over the past 5 years there has been a move towards STAs. The rationale is that these are quicker to complete and therefore decisions can be made faster, which is better for both patients and industry. They were introduced to ensure more timely guidance and to speed up patient access to medicines.
The case study approach
The case study was selected to provide an overview of STAs and their impact, as they are a stream of funding from the HTA’s programme. Owing to the nature of STAs (a rapid turnaround assessment of a manufacturers’ submission), researchers would not generally be expected to be aware of the impact, focusing instead on the methodological expertise. Without the ability to track a single piece of research, we decided not to focus on one example, rather taking the body of work and understanding the process and general outcomes of this stream of funding.
Between 12 January and 3 February, our team spoke to five individuals involved in various ways with the TAR contract and TAR teams (Table 38). Through the interviews, individuals suggested other contacts who might provide a perspective on the topic, and these individuals were subsequently contacted and interviewed. In addition, comments, where relevant, are included from interviewees for the other TAR case study conducted for this report, on the topic of rheumatoid arthritis. A number of researchers who had worked on several STAs were contacted but declined to be interviewed.
| Interviewee | Reason for interview |
|---|---|
| Meindert Boysen | Programme Director of Technology Appraisals, NICE |
| Paul Catchpole | Director of Value and Access, ABPI |
| Andrew Cook | Interim Director, SHTAC |
| Matt Stevenson | Professor of Health Technology Assessment, ScHARR-TAG, University of Sheffield |
| Pauline Swinburne | Senior Programme Manager, NIHR TAR programme, NETSCC |
Stage 0: topic/issue identification
The topics for review through the TAR programme, as a whole, are identified by broad engagement with a range of stakeholders. This includes industry, patients, clinicians and the public. For example, industry will highlight medicines, in its pipeline, which will require upcoming appraisal. This is a key source of ideas. The topics are selected on a 2-year rolling basis. The approval team is made up of representatives from the DH, NICE and NHS England. The topics are ultimately referred by a minister.
Approximately 40 topics are appraised on an annual basis, and Boysen estimates that currently 90% of these are STAs. The TAR teams do not have a say in the identification of topics.
Interface A: project specification and selection
Selection of Technology Assessment Report teams
The TAR teams bid for a contract on a 5-year basis. There are currently nine TAR centres in England and Scotland, which support the HTA by conducting the assessments required by NICE. When bidding for funding from NIHR, proposals incorporate full economic costing, including lighting, printing, heating, phone and building costs. In the two examples contacted, this cost is paid by the group to the institution within which they are based, in instalments over the lifetime of the contract.
Selection of specific projects
The topics for MTAs and STAs are identified as described above. Once this is finalised, the TAR team gets a list of upcoming studies (including STAs, MTAs, and non-NICE HTAs and scoping studies) on a monthly basis. Non-NICE studies are identified as topics by the HTA programme’s Prioritisation Group and the HTA programme Director. Allocation is managed by NETSCC on behalf of NIHR. The TAR team orders the prospective studies in ranking of preference to conduct them, with typically any for which they have disease-specific knowledge at the top (for continuity) and those with conflicts of interest ranked lowest. The HTA programme (managed by NETSCC) then allocates these across the centres, taking into account availability and size of contract.
Catchpole felt there was a lack of transparency on the criteria for allocation. This was also extended to the selection of clinicians by TAR teams, and how they are engaged in the process. This was important as he stated that the Association of the British Pharmaceutical Industry (ABPI) had previous mapped the STAs to centres, and there was variation in the number of recommendations from NICE depending on the different TAR teams that critiqued the evidence, potentially indicating to Catchpole some lack of consistency in the different groups’ take on interpreting the evidence for medicines. Counter arguments include that it may be more difficult to provide cost-effectiveness in some areas.
Stage 1: inputs to research
Expertise/techniques
Within ScHARR-TAG there is a pool of approximately 18–20 modellers and 10 systematic reviewers who can be used on any TAR assessment, with approximately 18 full-time equivalents employed by the ERG. This number is smaller in Southampton Health Technology Assessment Centre (SHTAC), which employs 12 full-time equivalents. Once expertise in a disease area has been developed, topics are allocated accordingly. However, it was acknowledged by one interviewee that there is the scope for medical knowledge to be lost within the TAR teams. On the other hand, the researcher recognised that they were not employed for this knowledge. NETSCC try to maintain skill in disease areas so that teams do not have to learn twice, but often they learn about a particular area and do not reuse that knowledge.
In general, the teams are made up of systematic reviewers, modellers and statisticians who use their methodologies to address questions regarding the cost-effectiveness of medicines. Clinical expertise is brought into the project team, as required, to articulate the pathway and ensure that the design of the model and parameter values are clinically correct. Catchpole stressed that there was a lack of transparency around this selection, which often appeared to be based on geographical proximity. Once relationships are formed, TAR teams will often work with the same clinician again on a specific disease area. This is facilitated by the fact they now understand the process that the reviewers and health economists are trying to undertake.
In addition, staff working on TARs get the opportunity – to a lesser or greater extent – to work on other grant-funded projects, although it is not clear how this impacts on their ability to conduct TARs and the overlap and transferability of knowledge and skills.
Resource
For any given project inside a team at SHTAC, there are two researchers, working on this study 50% of their time to conduct the clinical review, and two researchers conducting the economic assessment.
Funding
The contract is provided is in TAR units: 1 unit is worth approximately £175,000 and 1 MTA, and 1 STA is one-third of a unit. In general, this is not generous but it sufficient to conduct the research. If there is much additional research within a project, for example, then, as a result of committee comments, additional units or proportions of a unit, can be added. At SHTAC it is unusual to get more than 1 MTA per year. Cook stated that the contract has never been filled in his experience, and, on average, annually between 80% and 90% of it is used. This is the price for the flexibility that is required, and therefore according to Cook ‘the funder accepts a bit of inefficiency’. The TAR teams also conduct systematic reviews that would have otherwise had to be advertised by the HTA programme to make up the volume. These would require the same level of work as a STA, but are more likely to be published, which Cook stressed as being important for the team.
Role of industry
One interviewee (Chen) cited interaction with industry as a barrier to research, suggesting that in the past it has often been difficult to get access to the required commercial-in-confidence data from the manufacturers. As this is the input data, this can limit the applicability of the model developed. However, he stressed that this has become less of an issue over time, and now data are more readily available than they have been previously.
Patient perspective
Overall, the researchers with whom we spoke highlighted that patient perspective had gained importance and prevalence over the decade that we were reviewing. In 2015, patient–carer perspectives are required for grant applications to the HTA. However, one researcher suggested that this was not a requirement for TARs, perhaps reflecting the different process by which studies are allocated. Instead, patient involvement is gained through NICE and the HTA directly on these studies, for example through the presence of PPI representatives at guideline appraisal meetings at which STA/MTA evidence is used, or through the involvement of patients in the selection and prioritisation of topics for TARs (although their involvement is primarily around the selection of MTAs rather than STAs, which are driven by industry submissions). The perceived value of patient–carer engagement varied between individuals, with one CI stating that ‘other than being seen to be doing the right thing, I am not sure that patients/carers will have much input as they are not experts in the literature or health economics and they of course have vested interests’.
Stage 2: research process
The process for a STA is that within an 8-week time window a critique is undertaken of the manufacturer’s submission. Cook highlighted the impact of the short turnaround: ‘if you are on one you are generally working most evenings’. This level of intensity was echoed by other researchers.
The Programme Director of Technology Appraisals at NICE described the change of remit as moving from producing their own assessment to critiquing and helping companies make sure that they provide a reasonable evidence base. There were number of factors contributing to the introduction of STAs, in particular the sense from stakeholders (industry and clinicians) that NICE was taking too long to make recommendations about recent medicines. Owing to the urgency of STAs, MTAs can be delayed if there are STAs that require urgent attention.
Interaction with the National Institute for Health and Care Excellence
Each assessment has a technical lead who is a named person at NICE. There is a reasonable amount of communication with NICE regarding timetabling and scheduling issues, and requesting clarifications from manufacturers. All communication with industry is through NICE rather than directly from the study team. Communication is ensured through a number of teleconferences or e-mail correspondence between the team and NICE towards the beginning and end of each project. These typically cover the scope of the study and discussion between the team who conducted the assessment and NICE’s technical team who will present the information to the Appraisal Committee, to support them in their presentation and discussion of the findings.
The industry (represented by ABPI) has regular forums with NICE, at which they meet and exchange views on topics, and can provide feedback on the process. However, there is a more limited relationship with academic groups or engagement with NETSCC. Catchpole felt that greater engagement between ABPI and NETSCC would be beneficial, as the limited engagement so far had been positive. Catchpole also reflected on the lack of direct communication between the TAR teams and the manufacturers submitting information for review. He felt the process could be improved by more direct, straightforward, ‘mature’ communication. However, the current process whereby all communications are through NICE sometimes prevents this from taking place in an efficient manner. The lack of direct engagement with industry was also stressed by academics, who felt that this independence was required in order for assessments to be completed objectively. Any discussion, for example clarifications which the TAR team require, is handled through NICE. Academics suggested that this was helpful particularly when disputes or difficulties arose, as NICE could act as an intermediary.
Stage 3: primary outputs from research
Knowledge
The output of a STA is a report that is used by the NICE Appraisal Committee in their deliberations. These are published on the NICE website, but often not more widely published in academic journals. Reasons given by interviewees included the concern that they are not perceived to be of the same scientific rigour as MTAs, and that much of the data cannot be reviewed or published by peer reviewers as it is commercial-in-confidence data from industry. In addition, there is an argument that because of the short turnaround of these studies there is not time to write them up for wider publication. However, at ScHARR-TAG they publish them in the Pharmacoeconomics journal series. Stevenson highlighted that this is important as the teams work within an academic context and Higher Education Institution (HEI) employees are judged on publications for promotion.
Even solely publishing in the HTA journal was seen to be a limitation, as, although the studies have a high impact factor, there is a perception that the journal is not often read by clinicians. Once work has been published in the journal, it is not generally accepted by other peer-reviewed journals and can lead to a CV which is ‘fairly thin in terms of publication outside HTA reports’ or the requirement to do additional work to make the material ‘sufficiently different’ for wider publication (Burls and Chen). This can be difficult to balance in a university environment, especially alongside other commitments such as teaching.
Researchers commented on the delays in publication through the HTA journal, although how to reduce these delays was not clear to interviewees. For example, delays can generally be caused by the time taken to peer review the work, and the length of time it takes to make the required changes by the TAR team, who have often moved on to other work by this point.
Speaking on behalf of the industry, Catchpole recognised that independence is important, but argued that there is a disconnect between the requirement for academic outputs and NICE’s requirement of appropriate support for the appraisal process. For example, the manufacturer gets to comment on the report only at the same point as others have seen it. Although they can give feedback, Catchpole felt that there is no requirement for the TAR team to take it into account or address comments. The outcome of this can be the published result contradicting the eventual findings of NICE. Swinburne stressed the role of the ‘factual error check’ stage in the STA process, through which any errors identified are clearly referenced and an erratum included for the NICE committee.
Benefits to future research and research use
As individuals in TAR teams tend to be methodological specialists (health economists, modellers, statisticians and systematic reviewers), the impact of a particular piece of research on their future research agenda can be limited. However, as described above, the allocation process tries to align teams with topics with which they are familiar and therefore work in a specific disease area could lead to subsequent assessments.
With regard to use by others, STA reports are not typically published beyond the NICE website, and therefore there is little opportunity for others in academia to pick up on the research, or its findings, outside the team.
There is, however, in some instances the opportunity to use technology appraisal studies as examples when discussing the way the system works with others. For example, Burls advises teams internationally on the HTA and NICE processes in England, to support the methodological development of their own systems. In this instance she uses examples that they have worked on as case studies.
Capacity building and career development
The impact on career development was seen to be mixed. One researcher highlighted the prestige associated with conducting appraisals for HTA and NICE. However, several interviews noted on the other hand that the lack of publications causes difficulties within the academic environment.
Stevenson recognised that working on STAs full time could be demoralising, as it is not the normal academic model. He stressed, therefore, that this was the benefit of having a big team, and resource beyond those employed by the TAR contract, from which to pool resources, for which people can be phased in and out, spending time on other areas of interest in conjunction with TARs.
Cook highlighted that HTA projects can lead to advisory roles for more senior staff. This can be through sitting on NICE Appraisal Committees or advising the government.
Interface B: dissemination
The reports are delivered directly to NICE and disseminated by them through the Appraisal Committees. In addition, the team are available during the committee meetings to answer any specific questions and provide clarifications.
When pressed, researchers stated the lack of time and funding to disseminate made additional dissemination difficult to achieve. Overall dissemination is limited, with researchers interviewed often thinking that it is not part of their role (which is to provide the evidence to inform the decision process by the NHS and funders). For example, they felt that those on the NICE Appraisal Committee have the full report, and could ask questions as required, and questioned the value of reporting their findings directly to the public, whether in favour or against the use of medicines for a target population.
Stage 4: secondary outputs
When asked if specific projects had made a difference to practice on occasion, members of the original research team did not know. This could be because the researchers specialise in the methods and only had ‘fleeting knowledge of a topic’, and usually moved on to look at different areas, and that ‘the only reason to look back at a topic area is if subsequent projects come along’.
National Institute for Health and Care Excellence Appraisal Committee process
Once a STA is prepared, it is shared with the NICE Appraisal Committee in several ways. First, the full report is shared with the committee members. Second, the key findings are presented by a member of NICE staff (briefed by the study team) to the committee at the first meeting. Finally, members of the study team attend the committee meetings to answer any questions that arise.
Typically, when a STA is produced, there are at least two Appraisal Committee meetings (for a MTA there may be more). The STA approach allows the committee to make a decision on guidance in the first meeting. However, legally, after this, there is a consultation period with stakeholders and time for appeal. Therefore, in most cases, a preliminary decision is achieved and then the committee re-convene two or more months later to make the final decision. This allows the committee to identify missing data, analysis or evidence, which can be prepared between the two meetings.
Other evidence is also used alongside the manufacturer’s submission and the independent TAR. These sources include statements from clinical bodies, such as royal colleges, and patients. These aim to describe what is currently done, the perceived unmet needs, knowledge and expectations of new technology, what it is like to have the disease and consequences for carers. These stakeholders are also present at the meetings to present evidence. Researchers suggested that the TAR team will often receive criticism from the manufacturer if the findings go against the use of the medicine. The independent assessment by the academic team is ‘utterly necessary’ according to Andrew Cook. He felt that the committee required this ‘robust review of evidence and modelling’ to inform their decisions. The guidance is developed by the Appraisal Committee and submitted to the board of NICE who issue it. This view was different from Catchpole, who, on behalf of industry, felt that decisions of the NICE Appraisal Committee appeared over-reliant on the academics’ view and, in particular, the economic modelling, rather than taking into account and balancing a wider range of factors. Others felt that the independence of the ERG was important in providing the unbiased evidence.
Boysen highlighted that although the TAR report is a crucial part of the evidence provided to the Appraisal Committee, there was a need to consider other angles of interest to innovators and the health system, particularly in light of the areas of growth from the government and life science agenda.
Citations in international guidance
The NICE guidance is used internationally and interviewees felt that it has a large impact on guidance and practice in other markets due to the volume of information that is put into the public domain.
All interviewees felt that NICE guidance, and the HTA TAR reports that inform it, has an international impact. However, this is difficult to quantify. One statistic provided was that > 50% of the hits that the NICE website received are external to the UK. Recently, NICE have developed a Memorandum of Understanding with New Zealand, which is actively using NICE guidance
Cook stressed that there were many countries that will look at NICE guidance. In particular, this is due to the speed with which the HTA in England commissions STAs, and its transparency about publishing the related guidance, and, in the case of MTAs, the related evidence. Cook suggested that this was evidenced by the downloads of the HTA journal articles, which are accessed across every continent and by approximately two-thirds of countries in the world.
Boysen and Cook stated that other European countries will start their assessment from the NICE guidance and review. Through their own agencies they will then conduct economic reviews for their own health system. Outside Europe, Cook felt that countries often pick up NICE guidance and adopt it, especially where they do not have the resources to conduct the assessment themselves. This is particularly the case for MTAs, for which the report will look at a larger section of the treatment pathway. It is less the case for STAs, for which the economic component is key and less adaptable to different country contexts.
Stage 5: adoption by practice and the public
As a result of the committee deliberations and submission to the board of NICE, guidance will be issued. Depending on the outcome this may make new medicines available, changing practice or increasing choice.
Implementation of guidance
A division of NICE (currently called ‘implementation support’) is responsible for outreach and the adoption of products. When guidance is issued, it is advice to the NHS, providing options to the physician. It does not dictate which treatment must be prescribed. However, as a result of the guidance, commissioners cannot refuse treatment on the grounds of cost.
Access to medicines
The STA model helps to speed up timelines and has led to a drive towards faster access for patients to medicines. Boysen also highlighted that a drawback could be that two groups simultaneously could be assessing very similar drugs for the same disease and targeting the same population. These could even be presented at the same committee meeting, but the scope of STAs does not allow for comparison between these drugs. This is odd to health economists, and creates the concern that the process does not highlight what is preferential or most cost-effective, but rather provides the NHS with options of what to prescribe to a target population for a given condition.
Impact on patient health
The impact of the guidance from NICE and the HTA reports was described by Catchpole as ‘massive’. He suggested that it determines whether a patient is able to benefit from treatments that will extend or improve the quality of their lives. On the other hand, one could argue that it is important to prevent medicines that are not effective or cost-effective from being prescribed.
Catchpole argued that the process is time critical, in order to get up-to-date guidance into the NHS about whether (or not) a medicine is being supported within the UK. In reality, Catchpole felt that the process can often be delayed ‘by lengthy deliberations and arguments between health economists’. Stevenson felt that this deliberation was important in identifying the effect of favourable assumptions made by manufacturers in their submissions. Catchpole questioned whether or not there should be a more balanced view and a wider range of perspectives consulted in terms of access to ‘life-saving treatments’ for patients. Others felt that the introduction of STAs has led to more timely guidance.
The political nature of the work can make it difficult for the TAR team, especially when clinical members of the team are privy to information that is not in the public domain and which can affect prescribing behaviour. One clinician mentioned an occasion in which drugs were on the point of being withdrawn and the team was privy to confidential information about this, to conduct a meta-analysis. As clinicians, the time lag prior to removal of the drugs from market was uncomfortable.
Stage 6: final outcomes
Impact on industry
From an industry perspective, there is sometimes a tension over the role and scope of the academic group. Prior to appraisal and approval, companies have often spent 10 years or more conducting R&D in the area. Through the review, academic groups are privy to large amounts of new evidence and information that is at the leading edge of evidence for drugs and disease areas. This creates a tension between the work that they do in alignment with job specification for the appraisal and broader analysis of the data. Catchpole said that manufacturers sometimes felt that academic groups were adding things in to support their own research interests and intellectual curiosity, rather than the requirement of the assessment. On the other hand, academics felt that these additional questions were key to their ability to deliver the required report, highlighting the disconnect. In particular, Catchpole suggested that the requests for information from industry were often onerous and some of the information requested was often not necessary for the assessment. These requests are dealt with in multinational companies by global analytic groups and can be very demanding on the company, particularly because there is usually a short time window.
Catchpole highlighted that the decision of the NICE Appraisal Committee should take into account a range of factors, including the health-economic assessment. Therefore, there are instances when a journal article from the ERG is not aligned to the final position that is taken on a medicine as determined by the NICE Appraisal Committee, and ultimately the guidance issued.
Catchpole stressed the impact that such discrepancies can have for companies. As the publications are typically some of the earliest publicly available evidence for new medicines, there may be other reviews being conducted overseas simultaneously for licence and approval in other markets.
When there are contradictions within the evidence available in the public domain, it can impact on wider licensing decisions in other countries. Owing to the desire for the independence of the TAR teams, there is little interaction between them and industry. Catchpole suggested that the research could be more robust if there was better dialogue between the parties. For example, he stressed that the manufacturers are not given a chance to give input on or critique the HTA article to be published.
This illustrates the potential international impact of the decisions made by the NICE Appraisal Committees for industry, as Catchpole suggests that NICE decisions (and to a lesser extent and where published, HTA reports) are referred to by decision-makers internationally. However, looking at the impact within the UK alone, this can be significant for industry. The extent of the implementation of NICE guidance is not clear, but it is likely that guidelines do impact on practice in the UK and hence on sales of drugs in the UK market. Technology appraisals, in particular, are important in opening up access to drugs in the NHS, and so are likely to impact on the bottom line for companies. Of course, STAs are not the only contribution to the committee decision but it seems likely that – based on the processes outlined above – they are a very important input to that decision-making.
Other impacts
Cook and Stevenson stressed that it was difficult to identify impact from STAs beyond input to NICE guidance, and that the TAR unit does not collect information on impact systematically. In particular, this was as a result of the time lag for impact to occur, as they work on a variety of disease areas and move on to new topics very rapidly. Cook and Stevenson also suggested that there can be difficulties identifying and publicising impact when data are based on commercial-in-confidence data from manufacturers. This may become more of a problem going forward due to pressures from HEIs to have impact, as assessed through funding channels such as the Research Excellence Framework.
Table of payback
Payback details for this case study are provided below in Table 39.
| Payback category | Impacts from case study |
|---|---|
| Knowledge Production | Report made available to the NICE Appraisal Committee Occasionally (but not typically) there may be a HTA journal article It is often difficult to publish further owing to the turnaround time of projects and the availability of commercially sensitive industry data |
| Research Targeting and Capacity Building | Research targeting is limited as the teams’ expertise is in the methodologies of systematic reviewing, statistics and health economics rather than disease-specific areas However, over time, a group can build up a portfolio of work in a disease area Through the conduct of appraisals, researchers become experts in the system. Several advise internationally on how NICE and HTA work in the UK, and on elements which could be used overseas in other systems Working for NICE and the HTA is seen as prestigious, which is positive for career development. However, the potential lack of publications can have a negative impact on careers. In part it appears this depends on the size of the TAR centre and the possibility of moving people between positions |
| Informing Policy and Product Development | STA reports feed directly into the NICE appraisal process and inform the guidance that is developed |
| Health and Health Sector Benefits | As a result of NICE guidance, there can be increased access to medicines. This can increase available treatment, impacting on patient health or increasing choice. It also aims to prevent ineffective treatments being made available |
| Broader Social and Economic Benefits | The timely development of assessment and guidance has an impact on industry who have developed the medicines. In addition, this impact is broader than the UK market, as other countries look to the NICE guidance when conducting their own assessment There is an international impact on guidance issued around the world, with many countries looking to NICE guidance and supporting evidence to inform their process, either as a starting point or to adopt wholesale |
List of abbreviations
- AAA
- abdominal aortic aneurysm
- ARTISTIC
- A Randomised Trial of HPV testing in primary cervical screening
- BRAM2
- Birmingham Rheumatoid Arthritis Model, version 2
- CATT
- Comparison of AMD Treatments Trials
- CBT
- cognitive–behavioural therapy
- CESAR
- Conventional ventilator support vs. Extracorporeal membrane oxygenation for Severe Adult Respiratory failure trial
- CHD
- congenital heart defect
- CI
- chief investigator
- CoBalT
- Cognitive Behavioural Therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care trial
- CRASH-2
- Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage trial
- CTU
- Clinical Trials Unit
- CUtLASS
- Cost Utility of the Latest Antipsychotics in Severe Schizophrenia trial
- CV
- curriculum vitae
- CWTS
- Centre for Science and Technology Studies
- DH
- Department of Health
- DNA
- deoxyribonucleic acid
- ECMO
- extracorporeal membrane oxygenation
- EESC
- Elective and Emergency Specialist Care
- EVAR
- EndoVascular Aneurysm Repair trial
- HPV
- human papillomavirus
- HTA
- Health Technology Assessment
- IVAN
- A randomised controlled trial of alternative treatments to Inhibit VEGF in patients with Age-related choroidal Neovascularisation
- JLA
- James Lind Alliance
- MNCS
- Mean Normalised Citation Score
- MNJS
- Mean Normalised Journal Score
- MRC
- Medical Research Council
- MTA
- Multiple Technology Appraisal
- NETSCC
- NIHR Evaluation, Trials and Studies Coordinating Centre
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NSC
- National Screening Committee
- PhD
- doctor of philosophy
- PPI
- patient and public involvement
- R&D
- research and development
- RA
- Rheumatoid Arthritis (case study)
- RCT
- randomised controlled trial
- ScHARR-TAG
- School of Health and Related Research Technology Assessment Groups
- SIGN
- Scottish Intercollegiate Guidelines Network
- STA
- Single Technology Appraisal
- SWET
- The Softened Water Eczema Trial
- TAR
- Technology Assessment Report
- TIDE
- Topic Identification, Development and Evaluation
- TSC
- Trial Steering Committee
- TXA
- tranexamic acid
- UKCRC
- UK Clinical Research Collaboration
- VEGF
- vascular endothelial growth factor
- WHO
- World Health Organization
- WoS
- Web of Science