Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/167/02. The contractual start date was in December 2011. The draft report began editorial review in May 2014 and was accepted for publication in August 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none.
Disclaimer
this report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Bruce et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Otitis media with effusion in children with cleft palate
Cleft lip and palate are among the most common congenital malformations, with an overall incidence of around 1 in 700 individuals. 1,2 Approximately 90% of children with cleft palate (CP) have a history of non-trivial otitis media with effusion (OME). 1–3 OME (also known as ‘glue ear’) is the accumulation within the middle-ear space of a mucoid or serous fluid. Although the exact mechanism for the development of OME is not fully understood, dysfunction of the Eustachian tube connecting the middle-ear space to the postnasal space is thought to be of fundamental importance. The function of the Eustachian tube is to equalise pressure either side of the tympanic membrane, avoiding the development of negative pressure in the middle ear. In children, the Eustachian tube does not work as efficiently, with the resultant tendency towards the development of negative middle-ear pressure and the accumulation of fluid within the middle-ear space (OME). This tendency towards Eustachian tube dysfunction is further increased in children with CP as a result of dysfunction of the muscles originating from the palate which act to open the Eustachian tube orifice. 4,5
A prospective longitudinal study following children between the ages of 1 and 5 years demonstrated that the overall prevalence of OME was 75% in children with cleft lip and palate compared with 19% in children without clefts. 2 This difference in prevalence of OME between children with and without clefts was also significant at individual time points throughout the study period. As well as being more common in children with CP, OME is likely to persist longer in children with CP. A retrospective longitudinal study of adolescents with various types of CP has demonstrated a decrease in prevalence of abnormal middle ears over time, with the decline in OME in patients with isolated CP occurring between 13 and 16 years of age. 6 Other studies have shown a similar decline in the prevalence of OME in late adolescence. 7,8 A questionnaire-based study of the natural history and outcome of middle-ear disease in children with CP reported that ear problems (ear infections and/or hearing loss) were most prevalent in the age range 4–6 years, only settling in adolescence, with 26% of the 13- to 15-years age group reported to have experienced ear problems in the preceding year. 9 However, 24% were still reported to have below-normal hearing when reaching early adulthood (16 years and above). Therefore, the prevalence of OME from early childhood into adolescence is an important factor when considering the optimum treatment strategy for OME in children with CP.
Otitis media with effusion commonly presents with hearing loss, but may also cause language delay, poor educational progress, recurrent ear infections, otalgia, behavioural deterioration, imbalance, tinnitus and hyperacusis. OME may also have a negative impact on quality of life (QoL) in affected children, with hearing being considered important at key stages in the development of language and behavioural and social relationships. 10
Management options for otitis media with effusion in children with cleft palate
The diagnosis of OME requires a focused history, including information on the clinical features of OME and the general health and developmental status of the child. Clinical examination should include otoscopy, examination of the upper respiratory tract, tympanometry and an age-appropriate hearing test.
There are several possible approaches to the management of persistent OME in children with CP, which can be broadly divided into surgical, non-surgical and combination treatment. 4 The surgical treatment of persistent OME consists of the insertion of ventilation tubes (VTs, also known as grommets) into the tympanic membrane, which, while patent and in situ, prevent the development of the differential pressure between the surrounding environment and the middle-ear space, thought to be an important factor in the pathogenesis of OME. VTs have recognised complications, which include persistent tympanic membrane perforation, ear infections and early extrusion. 1 Adjuvant adenoidectomy is not recommended in children with CP owing to the risk of velopharyngeal competence. Hearing aids (HAs) provide an alternative non-surgical treatment option for OME, with the aim of amplifying the sound delivered to the middle ear, compensating for the ‘dampening’ of the sound signal as it crosses the middle-ear space to reach the cochlea. HAs may also lead to ear infections and may not be considered cosmetically acceptable by a proportion of children and parents. Compliance with HAs in children with CP with or without cleft lip and OME has been reported to be only 52% (16/31 patients). 4 However, the same study reported otological complications in 5% (2/44) of children managed non-surgically and 38% of those treated with VTs, with the authors subsequently advocating VTs only in children not compliant with HAs or those who develop recurrent ear infections. 4 Combination treatment describes the scenario in which the chosen treatment strategy changes between surgical and non-surgical (or vice versa) owing to persistence or recurrence of symptoms.
In a systematic review directed at the early routine insertion of VTs for the management of OME in children with CP, the authors identified 18 eligible studies (case series, retrospective cohorts, prospective cohorts and randomised studies), but only one of these was a randomised clinical trial. This randomised trial had several significant methodological limitations which critically limited interpretation. The authors concluded that the majority of studies were small or of poor quality, with many having no formal sample size calculation, with the resultant risk of being underpowered to demonstrate a clinically important effect of treatment. 1
When we consider outcomes, we see that studies have used diverse measures, mostly selected from clinicians’ point of view and with limited consistency between studies. As OME can impair hearing at stages thought to be important in the development of language and behavioural and social relationships before the start of school, it could be suggested that outcomes relevant to these issues should be used in future studies. It is clear, therefore, that if further research into this treatment is to be commissioned, those outcomes relevant to parents and patients should be considered.
Guidelines for the management of otitis media with effusion in children with cleft palate
In 2008, the National Institute for Health and Care Excellence (NICE) published clinical guideline 60 (CG60), entitled Surgical Management of Otitis Media With Effusion in Children, which included a section specific to children with CP. 11 The guideline highlighted the particular problems posed by OME in children with CP, which included early onset, prolonged clinical course and higher rate of recurrence. For children in general, the guideline recommends that
Children with persistent bilateral OME documented over a period of 3 months with a hearing level in the better ear of 25–30 dBHL or worse averaged at 0.5, 1, 2 and 4 kHz (or equivalent dBA where dBHL not available) should be considered for surgical intervention.
The recommendations for children with OME and CP were:
1.8.1 The care of children with cleft palate who are suspected of having OME should be undertaken by the local otological and audiological services with expertise in assessing and treating these children in liaison with the regional multidisciplinary cleft lip and palate team.
1.8.2 Insertion of ventilation tubes at primary closure of the cleft palate should be performed only after careful otological and audiological assessment.
1.8.3 Insertion of ventilation tubes should be offered as an alternative to hearing aids in children with cleft palate who have OME and persistent hearing loss.
The guideline also concluded that the evidence for a benefit of VT insertion in CP was lacking and that the optimal treatment for OME in children with CP had not been determined. In the absence of strong evidence, clinicians were recommended to base the management of OME in children with CP on the needs of the individual. Although the needs of each patient should be central to the decision-making process, there clearly remains a need to determine which treatment strategy is the most appropriate for these children.
Commissioning brief and objectives
The management of Otitis Media with Effusion in children with cleft palate (mOMEnt) study has been funded through a Health Technology Assessment (HTA) programme-commissioned call (project number 09/167/02) to address the uncertainty in the treatment of OME and to address the question ‘What is the most appropriate way to manage OME in children with CP?’ by completing a feasibility study.
Randomised controlled trials (RCTs) provide the highest level of evidence in the evaluation of health care. However, trials are expensive and require considerable additional effort from health-care staff and patients, which may create particularly high barriers to recruitment and successful completion of a study. From evaluating previous surgical trials, it appears that there are several challenges for a potential trial of care of OME in children with CP. 12 These concern feasibility, choice of comparator treatment, selection of relevant outcomes, and surgical compliance and skill. Furthermore, from the patient’s point of view there may be difficulties with equipoise as surgical and non-surgical treatments have different risks.
The aim of our research was to provide information on the feasibility of carrying out a RCT or strong prospective cohort studies of the management of OME in children with CP. The project involved a set of studies and a value of information (VOI) study with the aim of identifying the optimum study design to add to knowledge of the treatment of OME in children with CP.
The study had the following components:
-
Study Advisory Group (SAG) We formed a SAG comprising clinical and methodological experts with nominations from the Craniofacial Society of Great Britain and Ireland. This included audiologists, speech and language therapists, and ear, nose and throat (ENT) surgeons. The SAG had the following specific roles: (i) to regularly provide advice for the study; (ii) to have an input into the design of the clinical survey and Delphi study; (iii) to advise on key parameters to explore the VOI analysis; and (iv) to have full input into the final exercise on feasibility.
-
Clinician surveys Surveys of clinicians were carried out to (i) identify the current UK practice for the treatment of OME in children with CP; and (ii) evaluate the feasibility of performing a RCT, or other relevant type of study, of VTs in comparison with ‘usual methods’ for the treatment of OME in children with CP.
-
A qualitative project The qualitative research project was designed to capture patient and parent opinions on willingness to take part in the trial, and to identify their needs for the content and form of information required to provide or withhold informed consent. Opinions on outcomes were also explored and data collected contributed to the core outcome set (COS) development.
-
The development of a COS A COS for a potential trial was developed. This would reflect the values of both providers and consumers of care.
-
VOI analysis This component was a VOI analysis that provided information on whether or not the extent of existing decision uncertainty about OME care for children with CP justifies the costs of the proposed research.
-
Evaluation The final part of the project was an evaluation of the data collected in the above components, in order to make recommendations on the feasibility of a potential study design.
Chapter 2 Clinician survey
Aim and objectives
The aim of the clinician survey was to collect data on the current clinical practice in cleft lip and palate centres in the UK, using a survey.
The main objective of the clinician survey was to collect information that would enable a decision to be taken on the feasibility of carrying out a trial or cohort investigation. As a result, within this report we are only including information on the following:
-
clinical provision and practice
-
method of delivery of care (centralised or ‘hub and spoke’)
-
caseload of the centres of children with non-syndromic CP.
A copy of the survey form is provided in Appendix 1.
Methods
We developed a survey form with the input of the SAG. This involved the preparation of drafts and a face-to-face discussion with the group, followed by development of the final form by e-mail ‘discussion’. The form was piloted in two cleft lip and palate units and further changes were made. The survey form is included in Appendix 1.
We then approached the clinical directors of each of the cleft lip and palate networks and asked them to identify the lead ENT/audiology clinicians who could complete the forms. They were sent the survey electronically. We utilised several methods to obtain a high response rate to the survey. These included:
-
encouraging the clinical directors to discuss the study with the lead clinicians
-
contacting the clinicians by e-mail several times
-
telephone contact with the clinicians to discuss the form and the study.
When centres were unable to provide the full data set requested, we subsequently asked them to provide information on the three most important questions that were relevant to the decision regarding the potential study design (Table 1).
| Question number in survey | Question text |
|---|---|
| 2.5 | What tests do you routinely use to diagnose and guide the subsequent management of OME? |
| 2.13 | What is your view on the optimum age for inserting VTs? |
| 3.1 | Do children attend cleft clinics outside your trust? |
Information on the caseload of the centres of children with non-syndromic CP was collected from two sources. Firstly, the cleft network co-ordinators were approached and asked to provide data for their centre; secondly, the Craniofacial Anomalies Network (CRANE) database, a national database that includes data on the caseload and treatment outcomes of cleft centres in England and Wales, was consulted.
Results
The response rate for the survey is provided in Table 2.
| Number of centres invited to complete the survey | 16 |
| Number of full responses received | 10 |
| Number of partial responses to an abbreviated questionnaire | 14 |
The full data are included in Appendix 2.
The key responses received from each centre are outlined below for the associated survey questions.
Clinical provision and practice
2.1 Does your cleft service have dedicated audiology input based at your centre?
The majority of centres (9/10) completing this question indicated that they had access to a named health-care professional who undertook age-appropriate hearing testing in children with CP.
2.4 How often do children with cleft palate receive routine audiological assessment at your cleft centre? If assessment varies by age please give frequency of routine audiological assessment and age ranges
Details received would suggest that although testing regimes vary, most children undergo at least four hearing assessments by the age of 5 years (in addition to Universal Newborn Hearing Screening). Children may be seen more regularly, depending on clinical need.
2.6 At primary cleft palate repair, how is the decision made to insert ventilation tubes or not, and who is involved in the decision-making process?
The responses received indicate that it is not standard practice in the centres surveyed to sanction the insertion of VTs at primary cleft repair.
2.8 After what period of time would a conductive hearing loss (> 25–30 dBHL) trigger ‘active’ intervention (referral for/decision to insert ventilation tubes or prescribe hearing aids) at your centre?
Most centres would need evidence of persistence of OME over at least a 3-month period to recommend HAs or VTs. The response to this question would suggest adherence to the recommendations contained in NICE CG6011 regarding a 3-month period of ‘watchful waiting’ prior to making a decision to recommend an intervention for OME.
2.9 Please describe the decision-making process to provide hearing aids or to insert/refer to ear, nose and throat for consideration of ventilation tubes as the first-line treatment for persistent otitis media with effusion. Please include any involvement of parents and/or the child
The responses indicated that patient choice is an important factor in the decision-making process, again adhering to NICE CG60,11 which recommends that ‘treatment and care should take into account children’s needs and preferences together with those of their parents or carers’.
2.5 What tests do you routinely use to diagnose and guide the subsequent management of otitis media with effusion?
All centres had access to a comprehensive set of audiological tests that would enable accurate assessment of frequency-specific hearing thresholds from approximately 6 months of age (visual reinforced audiometry 6 months to 3 years) through to adulthood (play audiometry 3–5 years, pure tone audiometry 4–5 years+). Tests were also available in certain centres that would enable threshold assessment in children < 6 months of age (auditory brainstem response) and assessment of other aspects of hearing, including the perception of speech.
This would indicate that all of the centres replying to the clinician survey were in a position to participate in a subsequent study to determine the most effective treatment for OME in children with CP.
2.13 What is your view on the optimum age for inserting ventilation tubes?
There was a spread of ages given in the responses received, with several centres indicating that clinical need was the more important determinant (Table 3).
| Age | Number of centres (n = 13) |
|---|---|
| Under 1 year (at palatal repair) | 2/13 (15%) |
| 1–4 years | 4/13 (31%) |
| 5 years and above | 1/13 (8%) |
| No optimum age/when clinical need dictates | 6/13 (46%) |
The results for this question should be interpreted cautiously, as the wording of some answers suggested that the question asked for a minimum age for inserting VTs, as opposed to an optimum age. Although there was variance in practice, the majority of centres considered that the decision to insert VTs was either influenced by clinical need and not age (6/13 centres), or 1–4 years (4/13 centres). Only 3 out of 13 centres stated that the optimum age to insert VTs was under 1 year or over 5 years. It is likely that a subsequent study would include children (> 1 year old) of nursery, pre-school and school age. Therefore, with respect to age at VT insertion, the majority of centres would not be required to agree to a significant change in clinical practice.
Method of delivery of care: centralised or ‘hub and spoke’?
Most centres (12/13) have a ‘hub-and-spoke’ clinics infrastructure, with the number of ‘spoke’ clinics ranging from 2 to 14. It should be noted that the one centre that stated it did not have any spoke sites indicated in response to a later question that it makes recommendations to clinics outside its cleft service.
The response to this question regarding structure of service was particularly relevant to the design and management of a future study, indicating that the majority of centres (12/13) had a hub-and-spoke infrastructure model. The number of spoke/outreach clinics varied and would have implications for any study design, especially randomisation, as well as standardisation of hearing testing and the need to obtain trust research and development (R&D) approval for multiple sites.
Caseload of the centres of children with non-syndromic cleft palate
Centres were unable to provide specific data on the number of non-syndromic patients only, and instead provided information on the total new referrals. This, unfortunately, appeared inaccurate according to the SAG. As a result, we have based our figures on yearly caseload on the data derived from the CRANE database. The advice from the CRANE database co-ordinator was that the caseload of non-syndromic CP/cleft lip and palate is approximately 75–80% of all registered cases. In generating these data, they assumed that this proportion was uniform across centres (Table 4).
| Centre | Number of new referrals (number of children with CP with or without cleft lip) | Estimate of numbers who would be recruited into a trial |
|---|---|---|
| Newcastle | 65 (49) | 6 |
| Leeds | 65 (49) | 6 |
| Liverpool | 64 (48) | 6 |
| Manchester | 69 (52) | 7 |
| Nottingham | 93 (70) | 8 |
| Birmingham | 121 (91) | 10 |
| Cambridge | 87 (65) | 8 |
| North Thames | 173 (130) | 14 |
| Oxford | 45 (34) | 4 |
| Salisbury | 53 (40) | 5 |
| Swansea | 51 (38) | 5 |
| Bristol | 65 (49) | 6 |
| South Thames | 145 (109) | 12 |
| Belfast | 31 (23) | 3 |
| Edinburgh | 29 (22) | 3 |
| Glasgow | 46 (35) | 4 |
| Total | 1202 (902) | 107 |
CRANE does not collect information for Scottish centres and so we have used the data directly from the centres, as this seemed logical to the SAG. We have calculated an estimation of the number of patients per year who would enter a trial based on the number of patients who are likely to have OME (90%), then taking a conservative estimate of those who would meet trial eligibility criteria (50%), and finally factoring in the predicted consent rate, estimated from the qualitative research described in Chapter 3 (25%).
Discussion
The survey has provided useful information for the potential study design. However, the response rate was disappointing in that only 10 out of the 16 centres provided us with a full response. We made multiple efforts to engage with the clinicians; this included liaising with the clinical director of each network/centre, multiple e-mail contacts and reminder telephone calls. In spite of these efforts, our data set is not complete for all networks/centres.
The low response rate may be due to variation between centres in the method of delivery of care and structure of the service. For example, although 90% of those responding had access to an audiologist within their cleft team, this was not the case for all centres. Importantly, in those centres where there is no dedicated audiologist/ENT surgeon, the patients are referred to a general paediatric clinic. This made it difficult to identify the appropriate clinician to respond to the survey. We did make efforts to identify if these issues were relevant to centres that did not respond completely, but we found that information was very limited.
It is therefore clear that when designing a future study, the structure of the clinical team at each centre/network and the engagement with the current study are important considerations when identifying sites to participate. One option would be to only approach those sites that provided a good response to the present study.
The clinician survey has highlighted several key factors for the design and delivery of a subsequent study, and has suggested that UK cleft centres are in a position to participate in a study to determine the most effective treatment for OME in children with CP. The results indicate that centres would be able to nominate a lead ENT/audiologist for a study to act as local primary investigator and that the centres are able to perform age-appropriate hearing tests from 1 year of age through to adolescence. The survey also suggested that centres adhered to the 3-month ‘watchful waiting’ period prior to considering an intervention for OME, as recommended by NICE CG60,11 and children were seen regularly for audiological assessment up to the age of 5 years. The majority of centres considered either the period from 1 to 4 years of age or any age based on clinical need to be the appropriate time for insertion of VTs. Therefore, a subsequent study is likely to be more readily acceptable to centres if it uses the criteria for intervention as recommended by NICE CG60, recruits patients within the first 5 years of life and concentrates testing within the same period to minimise additional clinic visits. The importance of parental opinion in the decision-making process regarding OME management was emphasised, and this has implications for the information provision contained in any study design.
The method of delivery of care was important for potential study design. It is clear that most of the cleft networks operated a hub-and-spoke infrastructure for clinics, in that the patients were seen at the centres, but their audiological/ENT care was provided in local clinics and hospitals. This has several important implications. Firstly, it would be difficult to engage peripheral clinicians with the random allocation of care as they may not be in equipoise, and the probability of protocol deviations would be high. Furthermore, obtaining trust R&D approval for multiple sites with potentially low caseloads would be problematic and inefficient. Finally, there will be the additional problem of standardising both audiological assessment and treatment away from the hub clinic. The potential numbers of eligible patients for recruitment were provided in Table 4, with the recruitment rate and required recruitment period being influenced by the study design and sample size.
Chapter 3 Qualitative interviews with parents and children with cleft palate
Background
There has been very little qualitative research on treatment or living with CP from the perspective of either parents13 or children,14 and none related to OME.
Aims
The aims of the qualitative interviews were to explore in depth (a) parents’ views about their willingness for a child to take part in a potential trial comparing VTs and HAs, and (b) outcomes of the management of OME considered important by parents and children.
Methods
A qualitative methodology was adopted to enable individuals to recount experiences in their own words, highlighting what is important to them. 15 We focused initially on descriptions provided by participants, but, as the study progressed, took a more interpretive approach to data, in line with the principles of framework analysis. 16
Participants
Parents were recruited from two cleft centres in northern England. They were eligible to take part if they had a non-syndromic child with CP (including cleft lip and palate) between 0 and 11 years of age, who had a current or past diagnosis of OME. This age range was selected because it is the common time period for children to experience OME, as reflected by the NICE17 guideline on management of this condition, which is specific to the care of those aged under 12 years. Families with particularly difficult social circumstances (e.g. domestic violence, recent bereavement) were not approached. Children aged 6–11 years were interviewed, if they were happy to talk to the researcher. We felt that children younger than this would have difficulty expressing their thoughts on the research topic. Participants had to be able to converse in English. The interviewer was a researcher who did not have a clinical background and was not involved in participants’ care.
A purposive approach to sampling was taken to ensure variation in terms of children’s age, treatment experiences for OME and gender. A sampling matrix was developed for this purpose18 to guide recruitment as it progressed. We intended to recruit parents of approximately 30 children with a range of treatment experiences, including VTs only, HAs only, both VTs and HAs, and neither VTs nor HAs (the watchful waiting group). Initially, any parent meeting the inclusion criteria was invited to take part. As recruitment progressed, practitioners were asked to identify specific individuals to ensure variation in the sample. Data collection continued until the sample was diverse in terms of children’s age, gender and treatment experiences, and it was judged that data saturation had been reached.
Procedures
In unit A, a designated member of the cleft team screened clinic lists and medical notes on a regular basis for potential participants due to attend. Children who had a CP and a clinical history of OME but no concurrent syndrome were identified. The researcher was informed in advance when eligible participants had an appointment, and visited the unit on these dates. A member of the cleft team talked to the identified parent and asked if he or she was happy to meet the researcher. If he or she agreed, the researcher introduced herself and gave the parent a copy of the participant information sheet. She also took a telephone number and called a day or two later to see if the parent was willing to be interviewed. As recruitment progressed, the clinic lists were not screened; rather, the researcher would attend on days when she might capture individuals missing from quota matrices (e.g. clinics for 10-year-olds).
In unit B, a designated member of the team screened clinic lists and patient notes to see whether or not eligible parents were due for an appointment, using the same criteria as for unit A. These individuals were sent a participant information sheet in the post. The researcher would visit clinic on dates when people identified as possible participants were attending. A member of the cleft team checked that parents were happy to talk to the researcher. If this was the case, she introduced herself and asked if they had received information in the post. When parents stated that they had and were happy to take part, a time and date were arranged for the interview. Sometimes parents said that they had not received information through the post or had not had time to read it. The researcher would give these parents a copy of the information sheet, taking a telephone number so that she could call them a day or two later to see if they were willing to be interviewed. As recruitment progressed, the practitioner screening clinic lists was advised to identify individuals who contributed to cells of the quota matrix that were lacking in numbers. For example, over time patients who had received HAs only were targeted.
Modified versions of information sheets, with simpler language and less text, were developed for children aged 6–7 years and a slightly more detailed version for 8- to 11-year-olds. These were given out at clinic in unit A or sent in the post for unit B along with study invitations to parents. Information sheets were piloted with families attending unit A in advance of data collection, and revised in light of their comments.
Data collection
In line with the qualitative methodology, semistructured interviews were conducted to gather data on views and experiences. Interviews took place at a time and place convenient to participants (mostly in their home, see Results) between March and August 2012. They were recorded with parents’ consent and transcribed verbatim for analysis, with identifying features removed during this process (including names of health-care professionals).
A topic guide was developed for parents, based on relevant literature and discussion among the research team in relation to the project’s aims (see Appendix 3). Interviews took the form of a conversation in which parents initially told the story of their child’s OME, prompted by questions including:
-
When did you first notice a problem with your child’s ears? What alerted you?
-
What information did you receive about different treatments for glue ear?
-
What made you choose [treatment] for your child?
-
How satisfied were you with treatment your child received?
-
What would you advise other parents about treatment for glue ear?
About midway through an interview, parents were invited to reflect on important results (outcomes) of treatment for OME, which were recorded on electronic ‘sticky notes’ on a tablet computer. Parents were able to move these around to demonstrate their importance; they were encouraged to elaborate on reasons for items they had listed and the order they placed them in. Towards the end of an interview, parents were introduced to the concept of a RCT comparing treatments for OME and asked for their thoughts on whether or not they would allow their child to be part of such a study.
The topic guide was revised as data collection progressed to incorporate additional topics raised during interviews. For example, parent 1 talked about the difficulties she found with obtaining a regular supply of batteries for her child’s HA; hence, subsequent parents of children who had HAs were asked specifically about battery supplies. Likewise, parent 2 mentioned struggling to understand feedback she received from audiology after her child’s hearing tests; this was added to the topic guide as an area for exploration in other interviews.
The first child to be interviewed was given the option of whether he wanted to talk to the researcher before or after his parent. He opted to go first. However, after interviewing his mother, the researcher had a better understanding of the child’s condition and a greater awareness of his character, interests and likes. Therefore, subsequent interviews with children tended to be carried out after data collection with their parent(s). This approach had specific advantages; as well as allowing more details to be gathered about the condition’s history, it enabled the child to (a) see their parent(s) interacting with the researcher, (b) become familiar with the researcher’s presence and (c) observe the conversational tone and format of the interview.
Children were interviewed separately from parents to avoid the difficulty of disentangling individual perceptions in joint interviews and the potential for children to sense that they should agree with parents. 19 Parents were in the same room or an adjacent one when a child’s interview was being conducted. Overall, children responded well to questions posed, but sometimes parents added comments to statements made or elaborated when a son or daughter struggled to verbalise his or her thoughts; this is something that others have noted to be helpful when gathering qualitative data from children. 20
Interviewing parents and children separately was necessary because a different approach to data collection was used with the latter. There is a wealth of literature on how to conduct investigations with children that aims to offer an in-depth understanding of their experiences or views. Within such work, a recurring theme is the need for participatory techniques, including songs, drawings and stories, because children are said to communicate better through such media,21 and the need for creativity in how data are collected. 22 We prepared a range of activities to engage children and to maintain interest among those with limited concentration. 23 Most were carried out on a tablet computer. For example, interviewees were shown a picture of a child and informed that this individual had just been told that he/she had glue ear. This indirect approach reduced the need for personal disclosure from the child straight away. They were then asked questions about how the child in the picture might feel about different treatments and to complete speech bubbles on the tablet computer to show what this child might be thinking. Activities were used as a starting point for discussion on areas relevant to the study’s aims. They were piloted with a group of children without clefts on the topic of healthy eating, to see which appeared best at facilitating conversation with the researcher. Interviewees enjoyed playing on the tablet computer, which made data collection a fun event. All the children could use this device, even if they had not seen one before. Questions asked when carrying out activities included:
-
Can you tell me about any problems you’ve had with your ears?
-
How do you feel when you have to go and see the doctor about your ears?
-
What’s the good thing about having grommets/HAs?
-
What’s not so good about having grommets/HAs?
Analysis
Framework analysis was applied to interview data. 24 This allows for the sharing of information within a team, by summarising data into charts. It is suited to applied qualitative research that has specific questions and objectives,25 and provides a clear record of how ideas moved from participants’ words to final findings. 26 Framework analysis is divided into five stages: (1) familiarisation with the data (becoming immersed in material collected); (2) development of a thematic framework (identifying key issues in the transcripts), which involved constantly comparing emerging codes and categories with original data, across all cases; (3) indexing data (labelling key issues that emerge across cases); (4) devising a series of thematic charts (allowing the full pattern across cases to be explored and reviewed); and (5) mapping and interpreting data (looking for associations, providing explanations, highlighting key characteristics and ideas). It facilitates either theme-based or case-based analysis, or a combination of the two, through the development of charts that can be read across rows (cases) or down columns (categories). It also allowed us to explore data based on specific interviewee characteristics, such as the child’s treatment experience or age.
Three researchers and three clinicians (surgeon, consultant in audiovestibular medicine, orthodontist) formed the analysis team. PC and ST led the process, meeting approximately once a week during data collection to discuss what participants were saying, consider areas to follow up in later interviews and debate emerging ideas. The analysis team came together halfway through data collection. Before this meeting, each member was given four to six interview transcripts to review and identify potential codes, which were discussed as a team. ST used ideas from this meeting, and her knowledge of the entire data set, to develop a thematic framework in consultation with PC. This was shared with the team for their comments via e-mail before being used to index all interview data within the qualitative computer package NVivo 9 (QSR International, Warrington, UK). At the descriptive stage of analysis, separate thematic frameworks were developed for parents and children, to ensure that children’s views were not lost among those of parents, whose more articulate expression could have dominated a single thematic framework for the entire data set.
Once all transcripts had been indexed using the thematic frameworks, ST charted data, again in NVivo 9. This involved summarising what participants had said in relevant cells of a chart (Table 5). PC checked 10% of transcripts and agreed how ST had indexed data overall; any disagreements were resolved through discussion between ST and PC. Charted data were sent to the analysis team in advance of a second meeting to talk about these summaries and to start interpreting data, a process that ST and PC continued in follow-up analysis sessions. To illustrate aspects of the analysis, we have included direct quotations from participants in this chapter. We have not used names, to avoid identification. Numbers are employed when referring to the sample, reflecting the order of data collection, with ‘C’ denoting a child interviewee, ‘P’ a parent, (m) a mother and (f) a father.
| Sequence of care | Glue ear vs. other aspects of cleft care | Hearing tests | GP’s role in treating glue ear | |
|---|---|---|---|---|
| P25 4-year-old child Male Unilateral CLP VTs and HA Centre A |
Tests when born suggested hearing was fine. Then at 18 months diagnosed with glue ear. Told from early on hearing could be problem but hard to take everything in then – focus on feeding. Happy when first tests came back OK – felt one less thing to worry about. Did not realise hearing could become a problem later on. Felt ‘devastated’ when told son had hearing problems. With HAs can hear as long as are in. So in bed and in morning cannot hear until put in | Had to make decision whether to have second set VTs or HAs. With cleft no decision to make – just go with what doctors advise | Just went for routine hearing test – did not think there was a problem but told there was. ‘Devastated’ with the news because thought would interfere with his speech | |
| P12 4-year-old child Male Unilateral CLP Watchful waiting Centre B |
Aware a number of problems associated with cleft, including hearing, even before the birth. Told at time about VTs that it was just a little operation that could help with this | Child went through so much with palate operations – at start did not really see ears as major, especially since everyone gets ear infections. But now is having ear infections all time and been through all major palate operations, mum’s concerned with ears and how these might affect child at school | Not had a hearing test for about 1 year. At last test, clinicians were impressed with child’s hearing and could not see a problem but since then he has had a number of ear infections. Coped well with hearing tests because some play involved | Goes to doctors as soon as child seems to have ear infection. GP gives antibiotics. Tends to clear up but then returns. Feels nothing else has been offered to stop infections recurring. Going to speak to GP next time child has infection to see if VTs would help with this as it has affected child’s sleep. But not had an infection for about 4 months |
Ethical considerations
Ethical approval for the qualitative interviews with parents and children was obtained from the National Research Ethics Service North East Committee – Greater Manchester East (reference 11/NW/0586). Approval was also obtained to further contact participants with an invitation to take part in the final consensus meeting described in Chapter 4.
All parents gave informed written consent to their participation and the use of quotations from their interviews for dissemination purposes. They also consented to the involvement of children aged ≥ 6 years. Assent was obtained from children, who were made aware from the outset that there were no wrong or right answers, that data collection was a confidential process and that the researcher was not coming to provide treatment, but just to ask about their views and experiences. They were given the option of saying ‘no’ to taking part, even if their parents had consented to their involvement. To put children at ease, at the start of the interview the researcher showed them the digital recorder. She gave them the chance to take on the role of interviewer, inviting them to ask her any question they wanted or to choose a question from a selection she had prepared. This gave them the opportunity to understand how the recorder worked and introduced them to the conversational tone and form of the interview. Participants (parents and children) had the opportunity to ask questions prior to starting data collection and at the end of the interview. When the interview finished, children were asked to indicate how they felt by selecting one of a range of cartoon faces showing different emotions (e.g. happy, sad, angry, confused). There was a general sense of happiness at being listened to and being able to possibly help other children.
Rigour
Based on guidelines for producing good-quality qualitative research, we employed the following strategies. 27,28
-
Reflexive notes were made during data collection by ST. In these, she recorded contextual information relating to where interviews took place and emerging ideas relating to analysis.
-
Data were sought from a diverse group of individuals, in terms of factors thought to be pertinent to experiences of OME (e.g. age, type of treatment).
-
More than one person was involved in the analysis; the analysis team comprised members with different experiences in terms of the care of children with CP.
-
We looked for disconfirming data while developing themes to deepen the analysis and to ensure all aspects of transcripts were considered.
Results
Interviews were conducted with 37 families (five from minority ethnic groups). Twenty-eight were recruited from unit A and nine from unit B. This represented a 71% response rate among those invited to participate, as shown in the flowchart in Figure 1. After comparing narratives from those in units A and B, no obvious differences were identified. Therefore, data from both sets of interviewees were combined within the analysis.
FIGURE 1.
Recruitment to the study.
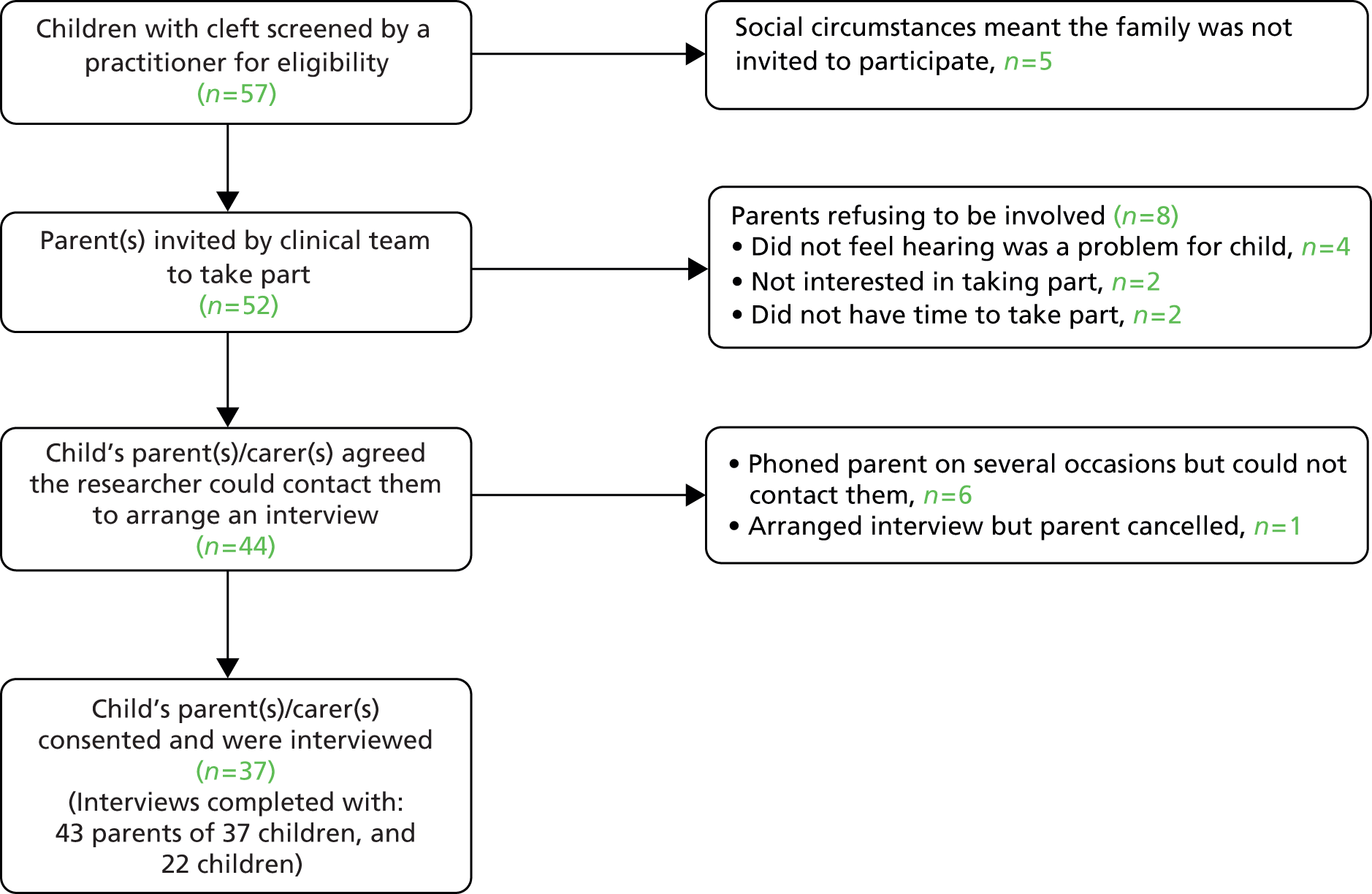
Twelve parents were interviewed as couples, while one father and 30 mothers were interviewed on their own. The mean age of parents was 34.9 years [standard deviation (SD) 6.7 years]. Data were collected from 22 children, comprising 13 boys and 9 girls; two children aged 6–11 years did not want to take part, so only their mothers were interviewed. The mean age of children interviewed was 8.8 years (SD 1.3 years). The type of treatment and cleft experienced by children is illustrated in Tables 6 and 7. The most difficult group to identify was children with experience of HAs only, especially those in the younger age group. It appeared that VTs had often been inserted at a young age during an anaesthetic for another cleft procedure. In addition, children who had received HAs only were often syndromic and, therefore, not eligible to participate.
| Interview number | Parent(s) interviewed | Cleft type | Treatment experience | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mother | Father | UCLP | BCLP | CP | VTs | HAs | Both | WW | |
| 2 | ✓ | ✓ | ✓ | ||||||
| 4 | ✓ | ✓ | ✓ | ||||||
| 6 | ✓ | ✓ | ✓ | ✓ | |||||
| 8 | ✓ | ✓ | ✓ | ||||||
| 11 | ✓ | ✓ | ✓ | ||||||
| 12 | ✓ | ✓ | ✓ | ||||||
| 13 | ✓ | ✓ | ✓ | ||||||
| 15 | ✓ | ✓ | ✓ | ✓ | |||||
| 19 | ✓ | ✓ | ✓ | ✓ | |||||
| 20 | ✓ | ✓ | ✓ | ||||||
| 21 | ✓ | ✓ | ✓ | ||||||
| 23 | ✓ | ✓ | ✓ | ||||||
| 25 | ✓ | ✓ | ✓ | ||||||
| Child number | Parent(s) interviewed | Cleft type | Treatment experience | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mother | Father | UCLP | BCLP | CP | VTs | HAs | Both | WW | |
| 1 | ✓ | ✓ | ✓ | ||||||
| 3 | ✓ | ✓ | ✓ | ||||||
| 5 | ✓ | ✓ | ✓ | ||||||
| 7 | ✓ | ✓ | ✓ | ||||||
| 9 | ✓ | ✓ | ✓ | ||||||
| 10 | ✓ | ✓ | ✓ | ||||||
| 14 | ✓ | ✓ | ✓ | ||||||
| 16 | ✓ | ✓ | ✓ | ||||||
| 17 | ✓ | ✓ | ✓ | ✓ | |||||
| 18 | ✓ | ✓ | ✓ | ||||||
| 22 | ✓ | ✓ | ✓ | ✓ | |||||
| 24 | ✓ | ✓ | ✓ | ||||||
| 26 | ✓ | ✓ | ✓ | ||||||
| 27 | ✓ | ✓ | ✓ | ||||||
| 28 | ✓ | ✓ | ✓ | ✓ | |||||
| 29 | ✓ | ✓ | ✓ | ||||||
| 30 | ✓ | ✓ | ✓ | ||||||
| 31 | ✓ | ✓ | ✓ | ||||||
| 32 | ✓ | ✓ | ✓ | ||||||
| 33 | ✓ | ✓ | ✓ | ||||||
| 34 | ✓ | ✓ | ✓ | ||||||
| 35 | ✓ | ✓ | ✓ | ||||||
| 36 | ✓ | ✓ | ✓ | ||||||
| 37 | ✓ | ✓ | ✓ | ||||||
Interviews with parents lasted between 20 and 65 minutes (average 40 minutes). Those with children ranged from 10 to 40 minutes (average 20 minutes). Most were conducted at a participant’s home but five interviews with parents and three with children were conducted in clinic, at the parents’ request. All but one interview was digitally recorded and transcribed verbatim; one mother requested that her interview was not recorded but was happy for the researcher to take written notes.
For parent interviews, the team identified 139 initial codes through the familiarisation stage of analysis. These were clustered into 10 categories (service provision, family and social life, VTs, HAs, ear infections, communication, child’s education, parents’ involvement, outcomes from treatment, views of being part of a trial), each of which had subcategories. For child interviews, 54 initial codes were collapsed into six categories (everyday life and hearing, VTs, HAs, ear infections, clinical encounters, school); again, each of these had subcategories. Table 8 shows how data from initial codes relating to parents’ views of HAs were combined to create four subcategories. Categories and subcategories formed the indexing scheme that structured the summarising of data into charts. These charts were then used to describe and interpret interviewees’ words.
| Initial codes | Subcategories | Examples from transcripts |
|---|---|---|
|
Impact on hearing (to include impact on speech) | . . . when she put them in his ears for the first time and turned them on his face he was like that [pulls a face] and it was only about a year ago I think, maybe 18 months ago that he heard a microwave ping for the very first time ’cause obviously it’s all trial and error with hearing aids, getting the right frequencies . . .P1 (m)As soon as we got that [HA] it did, it did improve his speech. It has improved his speech since we got it ’cause he was hardly talking. He’s still not, I wouldn’t say he’s at the age of a 3-year-old speech-wise cause his little friends in school are all talking a lot better and are a lot more clearer as well.P15 (m) |
|
Visibility | I know a lot of parents who wouldn’t have hearing aids because of social reasons, people pick up on oh he’s deaf or whatever, whereas grommets they’re not seen, nobody has a clue.P2 (m)She was the only one with hearing aids so everybody loved them and we sent books in to school so she could read them with her friends to show them that this is what I’m going to get and everybody was dead excited and all of her friends keep saying ‘I want hearing aids’.P13 (m) |
|
Parents’ beliefs about HAs | . . . it [HA] looks like she’s relying on something, yeah rather than trying hard like for herself but obviously if it does affect her hearing during the class and she does need one then as I said you can’t see it from outside, I think it’s just for us as her parents, we were worried that it may not be comfortable for her, that’s all.P11 (m). . . if you put a grommet in it’s opening the tube so the tube, the fluid can drain, whereas these [HAs] are just, well it’s just making the hearing a little bit better. It’s not, so it’s still bunged up. It’s still, the eardrum is still as flat as anything. It’s not solving anything . . . it’s just assisting.P27 (m) |
|
Getting child to wear | It was just whether he would tolerate them, you know, for a long time because he’s a wee bit of a fussy wee boy [laughs]. He doesn’t like anything really that interferes with him and I just was worried that perhaps maybe he wouldn’t wear them or maybe he would just wear them for a week and then decide that these weren’t for him.P4 (m). . . he only wears the one, he only wears it at school. He doesn’t wear it at home or anything, he doesn’t really need it at home, he can manage watching the telly or listening to us. So I’ve never made him wear it at home.P33 (m) |
|
Supplies and maintenance | I soak them twice a week in warm water and . . . we have to make sure there’s not water in the tubes, so it’s using a puffer and puffing the water out. It’s not too bad.P3 (m)He doesn’t bother about the hearing aids ’cause they’re snazzy aren’t they. At the minute he’s got like red and yellow in and last time he had stickers in them and so he can do what he wants with them really . . . The batteries, through no fault of anyone, you just forget and you’d think oh I need to get batteries. They’re so tight. You think well, you go in and say can I have some batteries, for instance, [child] has school, here and his dad’s. So we leave them at all places but they’ll give you like one packet and you’re like oh great [sarcastic tone], you know, but no getting them is fine.P37 (m) |
As mentioned above, two main areas were explored during the interviews: willingness to enter a child into a RCT and outcomes of importance for children and parents following management of OME. Given that these are distinct topics, results and a discussion of what was found relating to involvement in a trial is followed by results and a discussion of what was found about perceptions of outcomes.
Analysis strand 1: parents’ views about their child’s participation in a potential trial
This section describes parents’ comments about whether or not they would allow their child to be part of a trial comparing VTs and HAs, and factors influencing their decision. It covers views of randomisation and explores possible barriers to recruitment. We focus on the opinions of mothers and fathers rather than those of children because they would make the ultimate decision of whether or not to participate. In addition, it was felt that children would struggle to understand the concepts of randomisation and equipoise. Parents were asked to state whether or not they would allow their child to take part in a trial to test the best method of treating OME. However, one mother (P9) was not asked because she expressed negative views about VTs with such emotion that it was inappropriate to explore if she would enter her son into a study where there was a 50% chance of receiving this treatment. An outline of how the topic was approached within the interview is shown in Box 1.
At the moment it’s not clear what treatment is best for glue ear. We would like to do a trial comparing two different treatments. The best way to do a fair test between two types of treatments is for there to be an equal chance of children receiving treatment A or treatment B. This could be done by a computer programme or by rolling a dice – for example, if an even number comes up the child receives treatment A and if an odd number comes up they receive treatment B. If a parent agreed to let their child be part of this type of trial it wouldn’t be a doctor who decided what treatment they received or the parent, and the child would have an equal chance of receiving treatment A or B. What they did receive would be down to chance. What are your views of letting your child be part of such a trial if [child’s name] got either treatment A or treatment B by chance?
(Invariably parents would ask which treatments at this point, so the researcher mentioned VTs vs. HAs.)
Follow-up questions: (a) What made you say [yes, no, unsure]?; (b) Is there anything that would change your view?; (c) What would you want to know before you decided?
Parents’ willingness to enter their child into a trial comparing ventilation tubes and hearing aids
In nine parent interviews, participants stated that they would allow their child to be part of the trial, whereas in 19 the answer was negative, and in eight, participants were unsure what they would do. Table 9 groups interviewees based on their response to taking part in a trial (‘no’, ‘unsure’, ‘yes’) and summarises key factors influencing their decision-making as recorded in interview transcripts. Patterns which emerged on how individuals responded are shown in Table 10.
| Reason for response | Parents saying ‘yes’ | Parents saying ‘no’ | Parents who were ‘unsure’ | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interviewee identifier | ||||||||||||||||||||||||||||||||||||
| 1 | 12 | 18 | 19 | 22 | 26 | 27 | 28 | 31 | 4 | 6 | 7 | 8 | 10 | 11 | 14 | 15 | 16 | 17 | 20 | 21 | 23 | 30 | 32 | 33 | 34 | 36 | 37 | 2 | 3 | 5 | 13 | 24 | 25 | 29 | 35 | |
| Trust in medical professionals | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||||||||||||
| Benefits to child of being in trial | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||||||||||||||
| Altruism/advance knowledge | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||||||
| Want control/choice/optionsa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||||
| Child not getting best treatment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||||||||||
| Preference stated for VTs | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||||||||||||||
| Preference stated for HAs | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||||||||||||
| Drawbacks related to VTs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||||
| Drawbacks related to HAsb | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||||||||||||||
| Those saying ‘no’ | Those who were ‘unsure’ | Those saying ‘yes’ |
|---|---|---|
|
|
|
Most interviewees were reticent about entering their child into the proposed trial, although they recognised the need to advance scientific knowledge. Some of their reluctance stemmed from concerns about not being able to choose treatment and a risk of their child not being allocated to the most effective arm. Furthermore, several parents had pre-existing views about the benefits or drawbacks of VTs or HAs, as suggested in the following interview extracts:
Urm, possibly not, just because if she then fell in the grommets group, she would have to have an operation and I wouldn’t want her to go under general again just for grommets. So probably not.
P7 (m)
I’ve had the results and I’ve witnessed it and he was a changed child. He could hear perfectly well. I mean he went for his hearing test after his grommets and he was passing them with flying colours . . . So no I wouldn’t be happy with that and I wouldn’t want a hearing aid because it’s there, it’s on view, children will poke at it and say ‘what’s that in your ear?’ and it’s the sheer embarrassment for a child, I would say no, absolutely no way.
P8 (m)
Hence, interviewees saying ‘no’ or ‘unsure’ did not see the two treatments as sufficiently equivalent to accept randomisation and did not appear to feel that risks associated with one intervention were warranted. Some parents expressed fears that there could be social consequences of HAs, including the potential for bullying:
Just the stigma, the stigma with hearing aids isn’t it. It’s, you don’t want anything that anyone can say to, to the chances of your child being bullied, being picked on and you know yourself it’s like kids and it’s just one more that they can, he’s not a very confident child, you know you worry that he, that’s why we were concerned whether his speech, would that cause bullying and stuff like that. It’s just a stigma isn’t it really, it’s like anything.
P16 (m)
Others had strong views about physical risks that they associated with VTs (e.g. causing damage to the ear or infections):
I think she was about 1 when she had her first set of grommets that they said would help sort out the glue ear and that unfortunately made things worse . . . I think the hearing aids have actually helped more than the grommets ever did because we haven’t had an ear infection for over a year . . . They [VTs] never worked. She’d be ill, she’d be off school. We’d get phone calls saying she wasn’t in but it wasn’t our fault.
P24 (m)
Participants were divided in terms of their favoured treatment, including parents of children in the watchful waiting group (Table 11). Hence, even when interviewees had no personal experience of VTs or HAs, they could still hold strong opinions about these approaches, informed by conversations with friends or relatives, media outlets or social norms. Parents with children who had received VTs only tended to prefer this approach. Just one person in this group expressed a preference for HAs; P29 did not think it was fair to put her child under anaesthetic when the VTs kept falling out. Most parents with a child who had experienced HAs only described being encouraged to try them by a health-care professional. They expressed a preference for these devices in part because they eliminated the need for anaesthetic, which they worried could be required on several occasions if VTs fell out. These parents talked about their own struggles witnessing their son or daughter being anaesthetised. They also mentioned their child’s difficulties with surgery:
. . . he had a bad reaction . . . he was being sick . . . it was just horrible . . . I didn’t want to put him through another operation no matter how big or small.
P33 (m)
. . . we’ve had a lot of battles with her going for surgery . . . she goes to the play specialist a couple of months before surgery . . . cannulas . . . they are the major issue with her.
P34 (m)
| Interviewee | Prefer VTs | Prefer HAs | No preference | Not clear |
|---|---|---|---|---|
| Child had VTs | ||||
| P4 | ✓ | |||
| P6 | ✓ | |||
| P8 | ✓ | |||
| P14 | ✓ | |||
| P17 | ✓ | |||
| P21 | ✓ | |||
| P22 | ✓ | |||
| P26 | ✓ | |||
| P28 | ✓ | |||
| P29 | ✓ | |||
| P31 | ✓ | |||
| P32 | ✓ | |||
| P36 | ✓ | |||
| Child had HAs | ||||
| P27 | ✓ | |||
| P30 | ✓ | |||
| P33 | ✓ | |||
| P34 | ✓ | |||
| P37 | ✓ | |||
| Child had VTs and HAs | ||||
| P1 | ✓ | |||
| P3 | ✓ | |||
| P5 | ✓ | |||
| P7 | ✓ | |||
| P9 | ✓ | |||
| P10 | ✓ | |||
| P13 | ✓ | |||
| P15 | ✓ | |||
| P18 | ✓ | |||
| P24 | ✓ | |||
| P25 | ✓ | |||
| Child in watchful waiting | ||||
| P2 | ✓ | |||
| P11 | ✓ | |||
| P12 | ✓ | |||
| P16 | ✓ | |||
| P19 | ✓ | |||
| P20 | ✓ | |||
| P23 | ✓ | |||
| P35 | ✓ | |||
It was notable that though interviewees with children who wore HAs said they had been worried about teasing, these fears had not been realised; most stated that their child coped better wearing HAs than they had expected. As for those whose children had tried both treatments, HAs tended to be preferred as a result of poor previous experiences with VTs (e.g. falling out, repeated insertions, ear infections that parents attributed to VTs). Of those receiving both, most children had received VTs followed by HAs; just C13 and C18 had HAs first. Only P25 in this group of parents preferred VTs because, unlike HAs, they allowed for a constant improvement in hearing while in place:
So sort of when you go to bed they’re not in and when you get up on a morning they’re not in so obviously you’ve got that lull of whereas it’s like putting contact lenses in, you can constantly see rather than put your glasses on you know that type of thing, that was the only thing in my head to compare it to . . . the grommets give you a more rounded hearing cause it’s always there as opposed to just when they’re in.
P25 (m)
Views on the presentation of information about trial participation
Interviewees stated that prior to deciding whether or not to allow their child to be part of the proposed trial, they would like to talk to a researcher about it and wanted written information which they could take home and reflect on with family members. They also suggested that information should be provided to children if they were old enough. Participants stated that the information they would like to help make a decision included:
-
General That neither treatment would make the child’s situation worse, potential benefits and drawbacks of each treatment and what might happen if either did not work.
-
HAs What this would involve for parents and how often they would have to take their child to get new moulds fitted.
-
VTs What might go wrong, how many sets would be inserted, the chances of them falling out and any after effects.
Some parents were clear that how the idea of randomisation was presented could affect their willingness to contemplate their child’s involvement:
I don’t know if kind of like, I know it’s, to me kind of like roll of a dice sounds a bit like a board game . . . you’re thinking about kind of like your child’s kind of like welfare and to think of a dice, you’re thinking I don’t know whether or not I like the idea of that, whereas if you’ve got kind of like a computer generated list . . . then that’s fine.
P5 (m)
This is consistent with previous research, which has noted that explaining randomisation in terms of pulling names out of a hat or coin tossing may influence willingness to be part of a trial, leaving individuals feeling as if the approach is haphazard. 29
Conditions for agreeing to participation
This section moves on from the description of beliefs, experiences and knowledge to consider how individuals could be clustered based on factors influencing their willingness to allow their child to be part of a trial comparing VTs and HAs. By reflecting on participants’ responses, we grouped them according to key factors shaping their decision-making:
-
‘protecting’: not wanting to put their child at undue risk of harm (physical or psychosocial)
-
‘fixing’: believing that one treatment was more appropriate and/or convenient
-
‘following’: being persuaded by the views of professionals
-
‘helping’: wanting to advance knowledge and assist patients in the future.
The response of those characterised as ‘protecting’ was influenced mainly by previous experience, either direct or vicarious. These individuals were concerned about perceived physical or social risks associated with either VTs or HAs. Some had witnessed their child having several sets of VTs and some believed that these had caused permanent damage inside the ear. Others were reluctant to agree to the possibility of HAs, seeing these devices as an additional burden on top of scars from surgery and speech difficulties:
Just because urm I think I had so many other things, so many other problems as well then to sort of . . . be picked out for grommets or hearing aids and think it’s going back to the hearing aids issue for us, I think we would say no.
P36 (m)
Whereas parents classed as ‘protecting’ rejected either HAs or VTs, those defined as ‘fixing’ articulated a preference for one of these treatments and a sense of knowing what was best for their son or daughter. Some valued the opportunity to capitalise on their child undergoing an anaesthetic for a palatal closure to have VTs inserted at the same time. Others felt that HAs were preferable because they could avoid the need for repeated VT insertions.
Data from parents whose response was shaped predominantly by a wish to protect or fix implied that accepting uncertainty about which option is most effective (clinical equipoise) was a necessary but not sufficient condition. Agreement to trial participation could also require what we refer to as ‘parental equipoise’. This relates to the wider impact a treatment may have on everyday life. For example, those defined as ‘fixing’ felt that one approach to managing OME was more suitable in terms of its bearing on their child’s psychosocial well-being. This could be expressed as a preference for the expedient solution of inserting VTs while the child was anaesthetised for another procedure. Alternatively, HAs could be preferred as an acceptable solution to hearing loss that avoided the need for surgery. Those classed as ‘protecting’ were not in parental equipoise because they had significant beliefs about potential adverse consequences of either VTs or HAs.
If parents expressed concerns or preferences for VTs or HAs, they were unable to agree to participation. When these were not overriding factors influencing decision-making, some individuals agreed to their child participating in a trial because they trusted practitioners. We have described this as ‘following’. Alternatively, those we designated as ‘helping’ talked about being motivated mainly by a wish to progress knowledge and assist others in a similar situation. These interviewees did not voice strong views about VTs or HAs and, in that sense, appeared to accept there was sufficient clinical and parental equipoise to allow their child to take part in a trial. In their narratives they reflected on the widespread benefits of participation for future generations of patients.
Figure 2 summarises conditions associated with agreeing to be in the proposed trial, which were derived from interview data. It highlights that those saying ‘no’ or ‘unsure’ expressed views associated with ‘protecting’ or ‘fixing’ which were not articulated by those saying ‘yes’, who were willing to follow a practitioner’s suggestion or to help advance knowledge. It also underlines differing moral drivers shaping people’s decisions. Those characterised as ‘protecting’ exhibited the socially expected role of safeguarding their child. Likewise, interviewees described as ‘fixing’ demonstrated parental authority by suggesting a need to improve their child’s circumstances in what they felt was the most efficient way possible. ‘Following’ could result from a sense of loyalty to staff involved in patient care, if they are the people asking parents to take part. ‘Helping’ suggested a wish to assist others in a similar position in the future.
FIGURE 2.
Conditions associated with agreeing to be part of a trial.
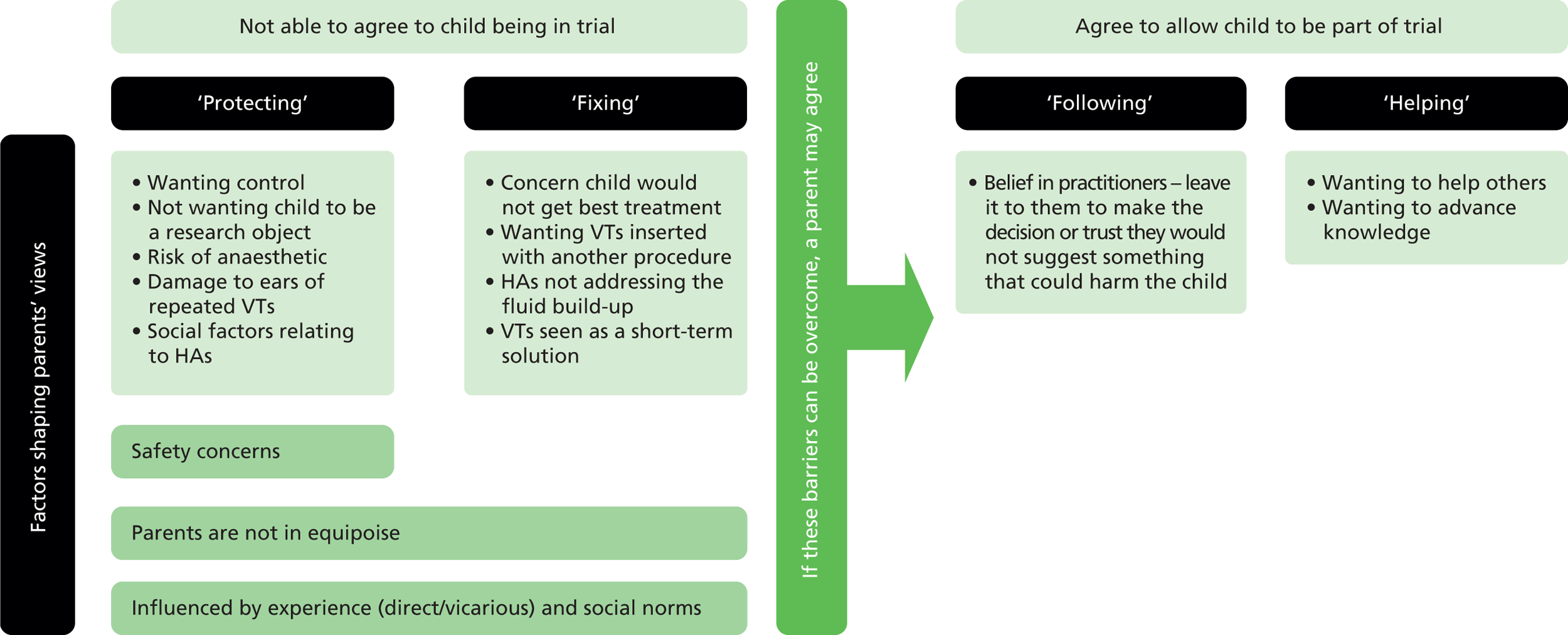
These different conditions associated with decision-making (‘protecting’, ‘fixing’, ‘following’, ‘helping’) highlight possible barriers to and enablers of recruitment. They suggest that allaying concerns around a perceived need to protect children from risks, and/or addressing beliefs that one treatment has particular benefits making it more suitable for the child, might be essential prior to recruitment. If parents can be reassured about the safety of interventions proposed, and that there is no definite advantage to either, they may be more likely to move on to ‘following’ or ‘helping’ and, therefore, saying ‘yes’ to randomisation. Further research could test how these drivers of decision-making relate to recruitment, which could allow for individualised approaches to trial presentation based on whether a parent’s view, at recruitment, was most reflective of ‘protecting’, ‘fixing’, ‘following’ or ‘helping’.
Nature of the two interventions being compared
We have described how most interviewees were resistant to the idea of entering their child into the proposed trial because they did not regard it as a fair comparison. As noted by P20:
I don’t think I’d be too happy . . . because they’re two extremes, you’ve got the grommets, which is an operation, or the hearing aid, which isn’t an operation and to me it’s, I’d be, you’d be scared to be put in the grommets group . . .
P20 (m)
To test the influence this perceived lack of equivalence had on parents’ response to our question about trial participation, after 10 interviews we explored participants’ views of their child being involved in a study of a hypothetical medication for OME. As shown in Table 12, they appeared much more receptive to this treatment route. Those whose initial response was characterised by a drive to protect agreed if convinced that the medication was safe, as did parents classed as ‘fixing’, if they saw it as an attractive solution to the child’s hearing problem. In general, participants described medication as a treatment that was easier to accept than surgery or HAs. They talked about taking medication being a ‘normal’ activity compared with the possibility of social stigma associated with HAs or the invasive nature of VTs. In addition, this option was seen as being more in parents’ control, with any side effects rectified by stopping the medication:
. . . you have to try new things because we’re never gonna learn are we and we’re never gonna progress, we’ve got to try new things and it’s a medicine. If he was ill from it, it would either come out of his bottom or through his mouth and I’d stop giving it him.
P37 (m)
| Willingness to consent to trial of surgical or medical intervention | Parent identifiera | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 26 | 27 | 28 | 31 | 32 | 33 | 34 | 37 | |
| Yes to trial comparing VTs and HAs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Yes to a study of medication for OME | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
The main hurdle that parents saw with this type of treatment was how the medication tasted, which might put the child off taking it.
Only one person agreeing to a child’s participation in a trial comparing VTs and HAs would not allow her son to be part of a study examining medication for OME. Her rationale related to a concern about potential side effects, and in that sense ‘protecting’ came to dominate her decision-making:
. . . the grommets will go in his ear but they come out after so long and . . . they’re not gonna damage him, you know, there’s nothing major that that’s gonna do to his body but obviously him taking a medication, I don’t know whether he could be allergic to something that’s in the medication . . . I wouldn’t really want to put him through that if there was no real, ’cause obviously they couldn’t promise me that something wasn’t gonna happen to [child].
P12 (m)
Two parents who said ‘no’ to the trial of VTs and HAs also said ‘no’ to a study of a hypothetical medication for OME. Their reasons related to a concern about side effects and not wanting to try a new treatment route for this condition.
Discussion: strand 1
Interviewees diverged in their opinions of VTs and HAs, with some favouring the former while others preferred the latter. They often held strong opinions about treatment, but also reflected the uncertainty that surrounds best practice in managing OME in those with CP. Our data show that recruitment could be difficult in research comparing VTs and HAs, a common obstacle with trials30 and a key reason for their failure. 31 Only one-quarter of those interviewed said that they would enter their child into such a study, although parents’ explanations for their responses are potentially more useful than this figure because they identify concerns that, if addressed, might facilitate a higher rate of recruitment.
Other authors have talked about ‘individual’ or ‘patient’ as opposed to ‘collective’ equipoise. 32 We used the term ‘parental equipoise’ to highlight that interviewees thought more broadly than a treatment’s clinical effectiveness, considering its impact on a child’s and the family’s psychosocial well-being. Most participants were not in parental equipoise, a condition that is necessary, we suggest, before agreeing to enter a child into a trial comparing VTs and HAs. Individuals were distributed across a continuum that included phases of decision-making shaped primarily by a need to protect, fix, follow or help. Data suggested that a lack of parental equipoise would need to be addressed if it were not to be a significant hurdle to trial recruitment.
Parents whose responses we have described as ‘protecting’ were not in equipoise because of the risks they associated with treatments. Some were particularly concerned to avoid their child having general anaesthetics. Negative views about VTs could also be based on personal or vicarious experience of multiple insertions, ear infections and/or concerns about long-term damage inside the ear. Therefore, HAs were preferred in some cases because they were non-invasive. Alternatively, parents might worry about their child’s or others’ responses to HAs, owing to social norms with regard to visible difference and disability. The response of interviewees indicates how a range of parental concerns may need to be addressed in order to recruit to a trial, including social experiences as well as clinical benefits and risks.
‘Protecting’ could be seen as focusing on perceived negative implications of a treatment, whereas ‘fixing’ suggested that individuals were making a positive choice of one way of managing OME over another. Parents described as ‘fixing’ believed that a particular treatment would be of greater benefit to a child and/or was more convenient than the alternative. Some considered the risks associated with VTs to be minimal, particularly if the insertion took place while a child was anaesthetised for another procedure. In contrast, HAs may be seen as requiring a longer commitment from parents, ensuring that children wore them, that batteries were replaced and that the HAs were not lost or broken. However, if VTs had not been an effective treatment, for example because they fell out soon after insertion, or if parents had seen the child flourish with HAs, they may believe this to be the most appropriate intervention for their son or daughter.
Parents who did not have strong beliefs about the benefits or risks of either VTs or HAs could be prepared to enter their children into a trial. In the case of those whom we characterised as ‘following’, experience again played an important role in their decisions; if they had a positive rapport with practitioners, whom they had trusted to guide them through previous treatments for CP, they may be happy to accept the request to be involved in a trial. This is not to say that those described as ‘protecting’ or ‘fixing’ mistrusted clinicians, but what was clear from their narratives were strong beliefs about treatments based on experience (whether personal or vicarious) and possibly limited knowledge to make an informed decision. If such beliefs could be addressed, there may be potential for these individuals to be more willing to agree to participation.
Altruism was an important motivation for interviewees agreeing to the potential trial, as reported in other studies. 33,34 However, the fact that parents decide about participation for a child leads to a bioethical debate about whether or not one can act altruistically on behalf of another. 19 Parents may be concerned about making the ‘right decision’, seeing the need to protect their child from harm as fundamental. 35 As a consequence, although some studies have found that parents are motivated to consent to a trial for altruistic reasons,36,37 it can be conditional on reassurances about concerns for a child’s safety and well-being. 35 This reflects findings that ‘protecting’ or ‘fixing’ were potential barriers to trial involvement.
Randomisation was a concept that many interviewees struggled to comprehend. The term itself may imply a haphazard approach to treatment allocation,29 with some people believing it is an unethical way of deciding how a condition will be managed. 38 Parents we interviewed talked about their dislike of the term ‘rolling a dice’, feeling that it devalued treatment choices and decision-making. Misunderstanding of randomisation shows that clear information is required when asking mothers and fathers to involve their child in a trial. Shilling et al. 19 reported that parents they interviewed liked being able to take away information on which to reflect and to share with family members. Likewise, in our study, parents wanted verbal details about the study to be accompanied by written material which they could discuss with others. However, previous research underlines that not everyone will read this information. 39 In addition, parents vary in terms of how much information they want about a trial. 40,41 A wish for more information has been associated with increased anxiety and less sense of control,42 but if parents feel inadequately informed it can dissuade them from participating. 43 Anxiety may be moderated by trust in medical research and the relationship parents have with practitioners. 35 Hence, confidence in a clinical team may be a factor influencing agreement to trial involvement. 19,44 A higher proportion of our interviewees may have said ‘yes’ to their child’s participation in a potential study comparing VTs and HAs if asked by a member of the cleft team, rather than a researcher they had only met for this qualitative study. Conversely, they may have felt more able to communicate their reservations to the researcher, without any fear of upsetting or letting down someone involved in the long-term care of their son or daughter.
Conclusion to strand 1
The diversity of views expressed in interviews indicates that parents did not share a consistent preference for either VTs or HAs. Data suggested that because many parents were not in equipoise when talking about VTs and HAs, recruitment to a trial comparing the two could be problematic. In order to perceive these two management approaches as equivalent, parents require information about how they work and potential drawbacks. Parental equipoise is a term we used to reflect the wider range of considerations, over and above clinical effectiveness, which might influence views on entering a child into a trial. The continuum of parental equipoise presented above (‘protecting’, ‘fixing’, ‘following’, ‘helping’) highlights potential factors driving decision-making around participation, and implies that specific issues may need to be considered when planning a trial comparing VTs and HAs:
-
stressing the safety procedures within the trial set up
-
especially if parents are characterised as ‘protecting’
-
-
emphasising and clearly explaining equipoise (recognising that parental equipoise is wider than clinical equipoise)
-
especially if parents are characterised as ‘protecting’ or ‘fixing’
-
-
exploring people’s previous experience/understanding of the two treatments
-
especially if parents are characterised as ‘protecting’ or ‘fixing’
-
-
being careful about who introduces the study to mothers and fathers
-
especially if parents are characterised as ‘following’
-
-
highlighting how results will advance knowledge for future generations
-
especially if parents are characterised as ‘helping’.
-
As well as equipoise, parents’ understanding of randomisation, and how this process of allocation is described (e.g. throwing a dice, computer generated), could influence recruitment. Parents may believe that practitioners will select the treatment that is best for their child, which could make the concept of uncertainty and random allocation difficult to accept. Prior to recruitment, time may need to be set aside to talk about how randomisation is performed and reasons for its use in this type of research.
Analysis strand 2: important outcomes for parents and children
This section presents parents’ and children’s views of important outcomes in the management of OME. As mentioned above, there was a specific section during interviews when they were invited to focus on this topic. However, overall narratives were also explored and interpreted for data relating to outcomes that researchers could assess in clinical trials.
Defining outcomes
The Oxford English Dictionary45 suggests the noun ‘outcome’ refers to the way a thing turns out; a consequence. It is a term familiar to researchers when defining the end point measured in a study to examine whether or not a treatment is effective but is less common in everyday conversation in relation to health. This makes it a potentially difficult topic to explore with service users. Nevertheless, research has shown that collecting people’s perspectives on outcomes can be a fruitful endeavour. For example, in the field of arthritis care, feeling less fatigue was found to be more important to those with the condition than traditional areas measured, such as joint tenderness and stiffness. 46
Parents’ views when talking specifically about outcomes following treatment for otitis media with effusion
Towards the middle of an interview, parents were asked what results or improvements they would look for following treatment of OME. If they had difficulty responding, the researcher posed the question ‘What do you think grommets or hearing aids should do for a child with glue ear?’ In our research, two parents found it difficult to distinguish processes from outcomes, listing ‘good aftercare’ as an important result of treatment (see Table 13). However, eventually most individuals were able to say something about important outcomes (only P35 could not, stating it was too difficult because his daughter had not experienced VTs or HAs). Figure 3 shows the responses on electronic ‘sticky notes’ to this part of the interview for P15 and P16. The left-hand picture from the interview with P15 shows that this mother and father could not identify a key outcome, rating hearing and speech as being equally important (which was also the case for P26: see Table 13).
FIGURE 3.
An example of sticky notes from P15 and P16 (left-hand sticky notes relate to important outcomes; those on the right relate to a question about drawbacks of treatment received by their child).

Table 13 presents outcomes referred to by parents when they were asked to focus on this topic within their interview. Green squares indicate the outcome(s) they defined as most important. This tended to be hearing but not for everyone; some people rated communication or seeing their child less frustrated as key, although both were dependent on improved hearing. Parents whose son or daughter had not received treatment (the watchful waiting group) referred to a narrower range of outcomes compared with other interviewees (see Table 13). Hearing, in particular, was listed as key by this group, with only P12 rating something else as more important; she explained that her son had experienced repeated ear infections, so a primary result of treatment should be to address this problem. Similarly, P9 proposed this to be her key outcome, after her son had endured recurring ear infections, which she attributed to repeated VTs. This mother formed part of a group whose child had received VTs and HAs. Reducing pain was listed frequently as an outcome by these interviewees (see Table 13), because a son or daughter had often had HAs following a bad experience with VTs falling out on several occasions and believing they caused damage to the ear.
| Interviewee identifier | Child had VTs | Child had HAs | Child had VTs and HAs | Child had no treatment | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 14 | 17 | 21 | 22 | 26 | 28 | 29 | 31 | 32 | 36 | 27 | 30 | 33 | 34 | 37 | 1 | 3 | 5 | 7 | 9 | 10 | 13 | 15 | 18 | 24 | 25 | 2 | 11 | 12 | 16 | 19 | 20 | 23 | |
| Improvement in hearing | ||||||||||||||||||||||||||||||||||||
| Speech/communication | ||||||||||||||||||||||||||||||||||||
| Child is less frustrated | ||||||||||||||||||||||||||||||||||||
| Make the child more alert | ||||||||||||||||||||||||||||||||||||
| Glue ear not to return | ||||||||||||||||||||||||||||||||||||
| Reduce pain/not to cause pain | ||||||||||||||||||||||||||||||||||||
| Reduced number of ear infections | ||||||||||||||||||||||||||||||||||||
| Remove fluid from the ears | ||||||||||||||||||||||||||||||||||||
| Improvement in school work | ||||||||||||||||||||||||||||||||||||
| Improved social interactions | ||||||||||||||||||||||||||||||||||||
| To have good aftercare | ||||||||||||||||||||||||||||||||||||
| To be discreet (in relation to HAs) | ||||||||||||||||||||||||||||||||||||
Those with a child who had received only HAs were similar to the no treatment group in their focus on hearing and communication as outcomes. In contrast, the VTs-only group listed a variety of potential outcomes (see Table 13). Members of this group talked about HAs not addressing what they believed to be the underlying problem of fluid build-up. They were the only individuals to refer to ‘OME not returning’ as an outcome, which suggests they viewed VTs as a cure for the condition. This could cause disappointment when OME recurred if VTs fell out soon after insertion:
Yeah it was a bit of a let down really that they’d, they’d said that it would be better if she had them [VTs] and then it came out more or less straight away . . .
P6 (m)
Education was mentioned by just a handful of parents in response to specific questions asking about outcomes (see Table 13). They listed ‘improved concentration’ and ‘being able to follow a teacher’s instructions’ on their virtual ‘sticky notes’. However, at other moments within the interview participants referred to their joy and relief at seeing a son or daughter advance at school once hearing loss had been addressed. Participants did not mention absence from school as an important outcome, yet during interviews they did raise the issue of time lost from school due to ear infections and for HA appointments:
. . . I’ve just had a right ticking off by the school because of his hearing. He has a green are good, amber is mediocre and red is warning and he’s just got a warning because of his attendance and that is just because of the time spent in hospital and that’s all because of his ENT appointments.
P5 (m)
Improved social interaction was a wish expressed by several parents for their child following treatment for OME. They wanted their son or daughter to be understood by others and for the child to understand others so they could communicate in social settings, with peers and at school. When unable to engage in this manner, children could remove themselves from socialising:
In the playground they would be playing and chasing each other and she wouldn’t be able to hear and she would have to try and look at faces so she would withdraw herself, she withdrawed herself for years.
P24 (m)
Age of children and outcomes
Given the wide age range represented in the study (0–11 years), potential differences in outcomes mentioned by parents for younger and older children were explored. Looking across ages, responses broadly followed the pattern outlined in Table 14, which suggests some differences based on age.
| Parents of preschool children (age 0–4 years) | Parents of young primary school children (age 5–7 years) | Parents of older primary school children (age 8–11 years) |
|---|---|---|
| Focused on hearing, pain and ear infections | Focused on hearing, pain and ear infections | Focused on hearing, pain and ear infections |
| Focused also on speech and language | Focused on social interaction more than just speech and language | Focused on social interaction and educational performance |
| Talked about a wish for glue ear to be removed |
It is understandable that parents of preschool children tended to mention speech and language because at this age these skills are developing. There was some concern expressed about children being competent in speech and language prior to starting school so they could flourish in this setting. At school, children become less dependent on parents and begin developing their own social network. Hence, an ability to interact is important during this life stage. Parents of older children tended to worry about educational performance, perhaps because difficulties in this area were more evident at this age and concerns about coping at secondary school arose. Several of these parents had experienced OME in their child for several years and thought that hearing would have been ‘normal’ by this age (≥ 8 years). Hence, there was some anxiety among parents of older children that their son or daughter would have on-going difficulties with hearing.
Parental stress
Interview transcripts were replete with indications of parental anxiety and strain. For example, seeing a child in pain, usually due to ear infections or damage, was upsetting for parents, who felt helpless to relieve this discomfort. Parental stress could also stem from decision-making around whether to have VTs or HAs and whether or not to try VTs more than once. Some parents talked about the need to maintain HAs (e.g. cleaning them, ordering batteries), although this was depicted as a hassle rather than a major problem. What appeared to be more stressful was when a child lost a HA; parents then had a child who was unable to hear and they had the embarrassment of contacting an audiology service to request a new HA. Frustration exhibited by children when unable to hear caused parental disquiet in case others thought their son or daughter was ‘just being naughty’ (P22 m). It could also lead to disharmony at home, as children were moody with their siblings and parents disagreed about how to manage such behaviour:
. . . it [child’s behaviour] was causing a lot of stress in our house . . . parents could be falling out with each other, saying ‘well why are you not being firmer’ . . .
P18 (m)
They [school] even made an appointment for us to see somebody . . . about her behaviour. So that made me quite anxious and worried and thought that we were, you know, she was really naughty and going off the rails but when we sort of realised that it was more cause of . . . her ears then I was angry at the school more after that because I’d, not taken it out on her but . . . I’d been a bit upset and taking it out on myself more than anything, thinking it was something that we were doing or that I was doing personally.
P22 (m)
Physical and psychosocial outcomes
Parental interviews contained data on a mixture of physical and psychosocial outcomes (Table 15). Interview data suggest that when considering outcomes, parents saw the health problem (OME) and associated symptoms more broadly within the context of the individual child’s life. For example, the physical problem of not hearing appeared to contribute to difficulties in the child’s psychological functioning (e.g. frustration, fear, sadness) and social life (e.g. learning and communication, being unable to take part in activities like swimming). Physical outcomes were related, primarily, to VTs (e.g. infections and perforations). Some interviewees felt that a resolution of hearing loss should not be sought if it was going to cause further significant discomfort for the child:
. . . she had two major ear infections with them [VTs] and they are now blocked so she has to go back and get them looked at. So she’s had nothing but trouble with them . . . The infections and the pain and I don’t think you know her being in pain outweighs her hearing, if that makes sense.
P13 (m)
| Broad area | Specific outcomes | Reality of parents’ experiences | Quotations from interviews |
|---|---|---|---|
| Social | Education | Once hearing had been addressed parents felt children blossomed educationally and were more able to concentrate | . . . to be at school and not feel that you weren’t hearing everything and being able to . . . take part fully in school . . .P18 (m) |
| Interaction | Speech and language improved after treatment for OME – parents felt once hearing was rectified, children were more able to be part of their social world and were aware of what was happening around them | . . . she was very closed off from people at school . . . She didn’t really talk to anybody and I didn’t understand how difficult that was until we found out how much of a hearing loss she had. But since having the hearing aids she’s, she’d made a few friends.P13 (m) | |
| Psychological | Child feeling less frustrated | Children became less frustrated when able to hear what others were saying | Socially it [treatment] helps them and it helps them with any frustrations because it’s annoying when you can’t hear things properly all the time.P31 (m) |
| Child being more confident | Children seemed to become more self-confident in social and school settings – parents saw their child’s personality come out when they could hear better | . . . everyone was like ‘wow he’s come out of his shell hasn’t he’.’ Wow, where’s his quiet side gone’. I said ‘it’s them bloody hearing aids and it were’.P37 (m) | |
| Physical | Rectify hearing loss (once and for all if possible) | VTs helped some children’s hearing, if they did not fall out, as did HAs for others – some parents suggested treatment should mean the hearing problem would not return when discussing VTs | The first thing that I wanted was for him to hear. Better hearing.P5 (m) |
| Reduce ear infections and blocked ears | Some parents felt VTs reduced ear infection frequency, others believed they caused this problem; with HAs there could be a difficulty of not being able to wear them with an ear infection and it was felt that HAs did not address the underlying fluid build-up | I’m not expecting it [VTs] to stop him having ear infections completely but to kind of control it so you know it wasn’t as often as he’s getting them cause I mean I don’t know how often kids get ear infections but to me it seemed like a lot to be having.P12 (m) | |
| Pain-free | Parents suggested that HAs should be comfortable and that VTs should not cause pain, which some parents attributed to their insertion (e.g. earache) | She wouldn’t be ill for a start. She wouldn’t have leaky ears and pain. It’s just [with VTs], this is all it’s been is pain, blood, sick. We’ve not had a result, there’s never been a result.P24 (m) |
Parents described the change in their child once hearing loss had been addressed in very emotive terms, emphasising, once again, that OME could affect numerous areas of a child’s social functioning and psychological well-being:
He just was like in this bubble and it was just, I just, to me I can just picture the ears just opening up [when VTs were inserted] and everything getting in . . . So really it was just participating in more things perhaps, being involved a little bit more in everything that was going on around him, being able to give him the chance to do it all . . . He was even more enthusiastic and just a completely different boy to me really [laughs].
P4 (m)
Their narratives often centred on ‘normality’. They wanted their son or daughter to have the same opportunities in life as their peers and to reach their potential at school, noting that OME could prevent this from occurring. It was implied that successful treatment would help the child become confident and content by allowing them to hear what was taking place around them, so they did not feel like an outsider. When OME was present, parents suggested it could mean their son or daughter was disconnected from the social world, resulting in the child becoming agitated:
Oh, he was not a very nice toddler [laughs]. He was quite difficult as a toddler, like we’d go to like places, he was alright at pre-school, he was better for other people but when it was like, especially when he was really little . . . when other kids are all starting to speak and he couldn’t communicate properly with other children. So he’d get, he’d like lash out, you know, he’d try and talk to them but obviously he wasn’t making the right sounds.
P33 (m)
A desire for the child to have the same life chances as their peers and not to miss out educationally relates to a concept underpinning parental interviews around achieving developmentally appropriate independence. For example, parents worried if their child was reliant on siblings for friends and social activities, and encouraged them to pursue their own interests. Interviewees appeared to value a progression towards autonomy and talked about seeing the child’s personality emerging as a positive. Box 2 provides some exemplar quotations from parents on this topic.
. . . without his hearing aids he would be very limited. There’s no way I would let him, I mean at the minute if he wants to play out and he’ll play out just in front of the house. Without hearing aids it wouldn’t happen because he wouldn’t hear the cars coming down . . .
P3 (m)
. . . she was more clingy than any of the other children would have been. In lots of situations she didn’t like being left . . . when she first started [school] she was very quiet, she wasn’t even answering the register . . . so there was a tiny bit of concern at one stage . . .
P18 (m)
The teacher would have to come and say ‘you can do it, you know how to do it’, and go through it with her, ‘no, no I can’t do it’, rather than trying herself, she’d rather the teacher come and be with her and try and help her . . . But at the minute they say she’s trying so much better and she’ll give it a go rather than saying straight away ‘I can’t do it’. She’ll give it a go and quite often she’ll get it right, she knows what she’s doing.
P22 (m)
Children’s views of outcomes
The term ‘outcomes’ could have been difficult for children to understand so they were invited to say what they thought was ‘good’ and ‘not so good’ about VTs or HAs. Responses to this question are listed in Table 16, which shows that children varied in their views, with some struggling to provide an answer because they had received treatment (usually VTs) several years previously or were in the watchful waiting group.
| Identifier | Treatment | What’s good about VTs or HAs? | What’s not so good about VTs and HAs? |
|---|---|---|---|
| 1 | VTs and HAs | Nothing | People touch it (HAs) It’s irritating (HA) Sometimes my ear hurts (HAs aggravates) Tickles when getting moulds made (HAs) |
| 3 | VTs and HAs | Helps me listen (HAs) Miss school (for appointments) (HAs) Likes playing with putty when getting HAs made |
Small children (noisy in restaurants) (HAs) Annoying when batteries go (HAs) |
| 5 | VTs and HAs | Nothing | Felt tight inside the ear (VTs) |
| 7 | VTs and HAs | Excellent because they are pretty (HAs) | Annoying because they fall out (HAs) |
| 9 | VTs and HAs | Hearing is louder when in (HAs) | You have to be put to sleep and don’t know what they [doctors] are doing (VTs) They [doctors] force you to have it (VTs) |
| 10 | VTs and HAs | They make you hear properly (HAs) | I hate wearing hearing aids They are annoying cause they itch (HAs) Can’t change the levels on HAs |
| 14 | VTs | Hearing improves (VTs) Confidence (when had VTs knew there was nothing to worry about the operation) |
Irritating (could feel the VTs were there) |
| 16 | Watchful waiting | Not asked because had not had treatment | |
| 17 | VTs | Not asked because child did not have much to say on the topic | |
| 18 | VTs and HAs | Hearing is better (HAs) | Not always comfortable, especially when get a new mould (HAs) Have to take them in and out (HAs) Batteries – make a noise when running out (HAs) |
| 22 | VTs | It helps you to hear (VTs) | Painful ear just after surgery (VTs) |
| 24 | VTs and HAs | They are useful and they help you to understand people (HAs) | Putting them in was painful (needle) and they cause pain (VTs) (had recurrent ear infections) |
| 26 | VTs | Can hear better (said this about HAs but had not had them) | Annoying cause you have to wear them (HAs – even though he did not have personal experience of these devices) |
| 27 | HAs | They help you hear and do more (HAs) | You have to keep changing batteries (HAs) Can hurt when getting the mould made (HAs) |
| 28 | VTs | Hear better (VTs) | Nothing |
| 31 | VTs | Rest of the interview suggested child would struggle with the task | |
| 32 | VTs | Child struggled with giving opinions | |
| 33 | HAs | Rest of the interview suggested child would struggle with the task | |
| 34 | HAs | Hear better (HAs) | Tickles when getting moulds made (HAs) |
| 35 | Watchful waiting | Child struggled because had no experience of treatment | |
| 36 | VTs | Child struggled because had no experience of HAs and had VTs a number of years ago | |
| 37 | HAs | Hear better (HAs) You get to pick your own colours (HAs) |
Running out of batteries (HAs) Tickles when getting moulds made (HAs) |
Hearing was referred to by children as a primary result of treatment for OME because it allowed them to understand others and to join in activities. However, one 10-year-old girl said an improvement in hearing had to be balanced against problems associated with interventions such as HAs:
They [HAs] make you hear properly. But I’d rather no hearing. I’d rather, I’d rather not hear properly than wear those things. I hate them so much.
What makes you hate them so much?
Everything about them. Sometimes they can get way too loud and I can’t really turn them down … and then sometimes they’re really, really low when you turn them on and then they stay like that . . . I’d be sitting there [in the classroom] with the hearing in my ear blocking all the sounds from my right ear cause, I’d be like, ‘what, what, what did you say sir?’
For participants who wore HAs, being involved in their design was a positive factor associated with these devices. Conversely, when discussing negative aspects of HAs, children mentioned their visibility; that they could fall out when playing; having to change batteries; an inability to alter the volume setting; and they could be uncomfortable in their ear. The main issues they associated with VTs included having to undergo an operation; a belief that they could cause infections; and feeling their presence inside the ear. In Box 3, ideas relating to children’s expectations of treatment for OME have been summarised into four main areas, alongside illustrative quotations. More views were expressed (positive and negative) about HAs compared with VTs, possibly because children had experienced them recently or currently wore these devices, whereas VTs may have been inserted several years prior to interview.
-
Help with hearing
C3C14
It’s a bit hard to explain but they [HAs] help by letting me hear people more and I can urm, if they wasn’t invented I’d just be completely deaf like that and I wouldn’t even hear anything.
I could tell [hearing had improved] because urm I could like mum didn’t have to repeat things over and over again [after he had VTs] like she did sometimes because I just wasn’t, I was concentrating on something else but urm before urm she had to do it quite a lot . . .
-
To be aesthetically pleasing
C7
Every time I have new hearing aids my friends say ‘oh they look nice’ [laughs] . . . I think they’re a bit pretty.
-
Not to cause discomfort, pain or irritation
C5C27
Urm, when it [VTs] goes in it feels too tight . . . Inside my ear . . . Soon as I’d had the operation.
I find it OK but if the, one of the wires goes too far in I find it, it urm hurts a bit . . . Well they put the mould in [for the HA], then they put this wire in and they put like a mic over it or something.
-
Not to interfere with activities
C3C18
. . . when the batteries go . . . I feel like tearing it [HA] out because when you’re doing it, it’s always there, just in the back of your mind, when you’re playing a game and then it just pops into the back of your head you go hooray, I think it might be charged up again, put it back on and it goes on and then a few moments later goes off.
. . . the bad thing is it [HA] does fall out sometimes at school . . . That was a surprise that was cause I didn’t know it was gonna fall out.
Combining children’s and parents’ views
Children and parents placed an importance on hearing as an outcome. Yet, as mentioned above, it was not referred to in isolation and interviewees did not focus on hearing as simply a physical improvement that could be measured. Instead, they associated it with psychosocial functioning, such as:
-
independence (freedom for the child to go and do things on their own)
-
performance at school (more attentive, able to read because they could hear words)
-
able to interact and connect with the social world (being understood and being able to understand what others were saying)
-
less frustration because of better communication, resulting in improved behaviour and self-confidence
-
engagement in everyday activities (e.g. swimming, cinema)
-
impact on home life (less shouting, TV less loud).
Views of outcomes from children and parents are merged in Figures 4 and 5. Figure 4 depicts physical outcomes mentioned within interviews, whereas Figure 5 centres on psychosocial ones. Figures 4 and 5 illustrate a pathway from hearing to physical and psychosocial outcomes, respectively, based on data from interviewees. White rectangles in these figures indicate outcomes mentioned by parents only, whereas shaded ones represent those referred to by children and parents. Children’s views were not inconsistent with those of parents, but mothers and fathers did have more to say about outcomes that could be used to assess the effectiveness of interventions for OME.
FIGURE 4.
Physical outcomes described in interviews (shaded boxes = mentioned by parents and children; white boxes = mentioned by parents only).

FIGURE 5.
Psychosocial outcomes described in interviews (shaded boxes = mentioned by parents and children; white boxes = mentioned by parents only).

Treatment-specific outcomes
Certain aspects of managing OME were specifically connected to a treatment. These are highlighted in Figure 6, which shows that VTs, in particular, were associated with physical outcomes. For example, parents often referred to VTs as bringing additional benefits compared with HAs by addressing fluid build-up inside the ear. In some interviews, parents believed that VTs reduced the frequency of ear infections experienced by their child. Conversely, others blamed VTs for constant pain:
I think him having the grommets has caused more agony and ear infections and sleepless nights . . . He’s had very many sleepless nights to the point where you give paracetamol but the child still says ‘I can’t sleep, it’s painful’ and you as a parent don’t have anything to do other than be there for your child, console them . . .
P9 (m)
Ventilation tubes were also associated with procedural issues and, to a lesser degree, social consequences. In contrast, HAs were related mainly to social consequences (see Figure 6). Although parents expressed concerns that HA would result in their child being bullied, in reality this fear did not appear to be realised; children who wore HAs were said to adapt very well to these devices and did not report the social stigma that parents anticipated. However, as children got older they appeared to become more self-conscious about having a HA. One boy, aged 8 years, talked about his frustration when classmates tried to touch his HAs:
They just talk to me all the time about them and everyone touches them.
Everybody touches them, at school . . . How do you feel about that?
I feel weird.
Why do you feel weird?
Because everyone keeps touching them.
FIGURE 6.
Outcomes associated with treatment described by parents and children (green rectangle = mentioned by parents and children; green oval = mentioned by parents only; white rectangle = mentioned by children only).

Discussion: strand 2
Interviews started with participants talking about experiences of OME and then midway through they were invited to focus discussion on the topic of outcomes. Therefore, most parents and children identified outcomes directly by listing them on a tablet computer and indirectly through describing how this condition and its management affected their lives; outcomes were explored within the context of this broader reflection on experiences, meaning that although reference to outcomes was sometimes explicit, it also involved drawing out from participants what were important consequences of OME based on their overall narratives about living with this condition.
The key outcome emerging in participants’ narratives was hearing. This was referred to by children and parents, who saw it as more than a restoration of physical functioning. Instead, hearing was described as important largely because of the psychosocial consequences associated with its loss, such as relationships with others and ability to progress in life. This could be summarised as the child’s ‘connection with their social world’. Figure 5, in particular, delineates how psychosocial variables were linked to hearing. However, some parents and children implied that hearing should not be sought at any cost; comfort and absence of pain were felt to be more important in some cases than being able to hear everything that was occurring. Hence, a degree of hearing that allowed children to function in the social world without excessive discomfort was a happy medium that certain interviewees depicted as acceptable.
Data suggest that particular outcomes may become increasingly important over time, with parents of older children being more concerned about social interaction and education; whereas earlier, focus on speech and language development may be a priority. Others have noted that hearing loss can leave individuals behind educationally. 47 Education was only mentioned by parents; children were more concerned with a wish to fit in with friends and not missing out on valued activities (e.g. swimming and playing). Parents identified consequences for children’s self-esteem and confidence as important outcomes, which could be related to a notion of experiencing a ‘normal’ childhood and reaching their potential. This highlights that outcomes discussed during interviews did not necessarily relate to a discrete endpoint, implying that a distinction may be required between immediate and longer-term consequences of treatment for OME.
Conclusion to strand 2
Hearing was a key outcome referred to by parents and children. This was probably to be expected, given the nature of the problem and aims of treatment. Yet although hearing was central to interviewees’ concerns, this was largely because of its psychosocial consequences. Hence, rather than absolute hearing at one moment in time, participants talked about the impact of not hearing on social development and psychological well-being; interviewees were looking for a health gain in terms of hearing to engender improvements in the child’s functioning in their social world and to bring them psychological comfort, including self-confidence, a move towards independence and the opportunity to develop their personality. Interviewees talked about significant psychosocial difficulties that stemmed from hearing loss, including poor social interaction and educational performance. Furthermore, both physical and psychosocial outcomes contributed to parental stress, with hearing difficulties adding to anxiety and pressures already associated with caring for a child who had a cleft.
Strengths and limitations of the study
Findings were strengthened by interviewing a range of participants in terms of the child’s age, gender and treatment received for OME. We involved fathers as well as mothers and recruited from more than one site. We talked with children, allowing their voice to be heard on important outcomes. Interviews addressed an event that may have happened several years previously (i.e. VTs insertion), which could result in recall bias. In addition, parents were asked to comment hypothetically on entering their child into a potential trial. We cannot be certain that their response would be the same if they were asked by a member of their team to be part of a real investigation. Parents we talked to who drew on personal experiences of treatment may not be representative of those invited to enter their children into a future trial at the first intervention for OME. However, interviewees included those whose children had only experienced watchful waiting, which indicates that even if someone has no direct experience, they may still have views about one intervention or another through listening to others.
Parents taking part had agreed to be interviewed. It could be argued that they might be predisposed to say ‘yes’ to trial participation. However, we spoke to people with a range of views about allowing their child to enter a trial comparing VTs and HAs. We interviewed 71% of those invited to take part, a high response rate for this type of study. It is not clear whether or not those refusing to be interviewed were put off by the more involved nature of data collection compared with other studies (e.g. completing a questionnaire), although one mother did refuse because she only wanted to be interviewed by telephone due to her busy family life. Two children declined to take part because they did not like talking to strangers but their mothers were interviewed. To encourage children to talk, we used a tablet computer to perform activities. We explored what they thought it was like for other children to undergo an operation or to wear a HA and asked them to comment on what might be good and not so good about each treatment. Children said at the end of their interview that they had enjoyed being listened to by a researcher and doing activities.
We took a systematic approach to the analysis, involving a mixed team of researchers and clinicians who cared for children with CP. This helped to add to the depth of the analysis and allowed for the checking and challenging of data interpretation. Using the computer package NVivo 9 meant a clear trail of how the analysis progressed was kept, from initial coding through to categorising and development of key ideas reported above.
Chapter 4 Development of a core outcome set for use in clinical trials of treatment for otitis media with effusion in children with cleft palate
Background
Clinical trials should have defined primary and secondary outcomes that answer questions generated by the main hypotheses. However, when we consider outcomes that may be used in studies of the treatment of OME in children with CP, it appears that these are numerous and diverse, and include outcomes such as chronic otitis media, OME, hearing loss, eustachian tube function, behaviour, receptive language and side effects of treatment to name a few. Furthermore, some of these outcomes may be influenced by other factors associated with clefting, for example the effect of the palatal cleft on speech.
Heterogeneity of outcomes across trials has been illustrated by a recent review which aimed to identify COSs for trials of treatment of childhood conditions. The authors categorised outcomes measured in clinical trials for a variety of paediatric conditions into six broad domains: disease activity, physical consequence of disease, functional status, social outcome and QoL, side effects of therapy and health resource utilisation. 48 Importantly, in this review, they did not retrieve any studies relating to OME.
In summary it appears that the domains in which the most tangible benefits from the users and providers of care for children with CP and OME are unknown and traditionally researchers have used diverse outcomes. This situation could lead to the following potential problems.
Heterogeneity between studies
This may be illustrated by considering the findings of a recently published systematic review directed at the early routine insertion of VTs for management of OME in children with CP. In this review the authors evaluated the literature up to 2006 (including RCTs, controlled clinical trials, case series and historical cohort studies). 1 Eighteen studies satisfied the inclusion criteria. When the outcomes were evaluated the studies were shown to have used varied primary outcome measures including hearing loss, tympanosclerosis, parental satisfaction with treatment, speech and language, and OME. Furthermore, there was inconsistency in the method of assessment for some outcomes; in particular speech and language which was assessed by undefined speech and language therapist assessment, a study specific scale or using the Reynell Developmental Language Scale. This limited consistency between studies, leading to marked heterogeneity, may result in difficult interpretation and comparison of findings and hinder potential meta-analysis. 48,49
Outcome reporting bias
Another relevant factor is outcome reporting bias. This occurs when only a selection of results for measured outcomes are reported in a study, and the choice of which to report is based on the results. For example, a tendency to report only significant or positive findings results in a biased representation of the results of a trial. There is overwhelming empirical evidence that this phenomenon occurs. 50
Core outcome sets
One strategy that has been suggested to overcome these issues is the development of a COS which should be measured and reported in all RCTs of a specific condition. 48,49,51–54 As a result, the risk of outcome reporting bias and heterogeneity is reduced for those outcomes in the set, and the potential for carrying out a meta-analysis for key outcomes is increased.
The outcome measures that could potentially be used to evaluate OME treatment are numerous and diverse, and may also be affected by specific factors in clefting. There is currently no COS available for clinical trials of the management of OME in children with CP.
Aim and objectives
Aim
The aim of this part of the project was to contribute to the development of a COS, relevant to studies of the treatment of OME in children with CP.
Objectives
Specific objectives were:
-
to systematically review the literature to identify the outcomes that had been previously reported in studies of the treatment of OME
-
to prioritise outcomes from the clinician perspective
-
to prioritise outcomes from the perspective of patients who can express their views, and parents
-
to compare outcomes of importance to health professionals and parents/patients
-
to integrate patient/parent and health professional outcomes into a combined COS.
Systematic review and development of a list of outcomes previously reported
Aim
To identify the outcomes that had been used in previous research evaluating the management of OME in children with CP.
Methods
The systematic review was carried out using two sources of publications:
-
Studies of the early placement of VTs for children. This was done by updating the search from a previous systematic review. 1
-
Studies of other surgical interventions for OME in children with and without cleft obtained from relevant Cochrane reviews.
Population
Children, with and without CP, aged < 18 years with OME.
Intervention
Any surgical intervention used to manage OME.
Comparison
Untreated control or other surgical interventions.
Outcome
Any outcome that was reported.
Criteria for considering studies for updating the Bristol review1 and Cochrane reviews identified through Cochrane Central Register of Controlled Trials search
We included the following types of studies:
-
systematic reviews with/without meta analyses
-
RCTs
-
controlled clinical trials
-
case series
-
prospective cohorts
-
retrospective cohorts.
Search methods for identification of studies
An identical search strategy to Ponduri et al. 1 was used and applied to the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, MEDLINE, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) (January 2006–April 2011).
The detailed search strategies applied are given in Appendix 4.
Multiple databases were utilised to maximise the sensitivity of a search. CENTRAL comprises only studies which are deemed to be controlled trials by a team of reviewers; EMBASE, MEDLINE and CINAHL include published research of various study designs. The advantages conferred by using CENTRAL in addition to the other databases is that trials from other sources of research (e.g. journals not indexed in MEDLINE and conference proceedings) are hand-searched, and controlled trials from these are included. This improves the chances of identifying all relevant studies.
Eligibility of studies
Two researchers (MOS and KOB) independently assessed the abstracts of studies resulting from the searches using a screening proforma. Full copies of all potentially relevant studies and those appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, were then obtained.
The full-text papers were assessed independently by two review authors (MOS and KOB) and any disagreement on the eligibility of included studies resolved through discussion. Where resolution was not possible, a third review author (IAB) was consulted.
For the purpose of this study there was no synthesis of outcome data from the RCTs, and hence a critique of the methodological quality of the studies was not necessary.
Data extraction
Two reviewers (NH and IAB) independently extracted the data. NH and IAB then reviewed the extracted data together to assess consensus and to ensure that all outcomes have been identified. Disagreement was resolved through discussion, where resolution was not possible, a third review author (KO) was consulted. The following data was extracted from each study:
-
study type
-
author details
-
year and journal of publication
-
intervention(s) under investigation
-
whether the study population was exclusively paediatric and CP or mixed (children and adults, with or without CP)
-
age and number of children included in the study population
-
inclusion and exclusion criteria.
Outcomes:
-
the outcomes which were measured, including the method of measurement
-
the time points at which they were measured
-
if stated, the designated primary outcome
-
designated secondary outcome(s).
Data analysis and presentation
For analysis purposes the data was initially tabulated so that for each study outcomes were listed.
The outcome domains were then determined following a review of the extracted outcomes by the authors (IAB and NH). The outcomes were then grouped under these domains. Finally, the outcome domains and included outcomes were reviewed by the SAG to assess suitability of domain name and grouping of outcomes.
Systematic review: results
Figure 7 illustrates the generation of the eligible studies. The search retrieved 85 potentially eligible studies, after screening titles and abstracts, all but nine studies were deemed to be irrelevant. After further analysis of the full texts, one further study was excluded as it was undertaken to determine the frequency that children with CP pass their newborn hearing test. 55 In addition, full texts of the 18 studies identified in the previous review1 were retrieved. Six Cochrane systematic reviews relating to OME were also retrieved to examine outcomes measured in studies of other interventions for OME in children with and without cleft. 56–61 This yielded another 24 studies.
FIGURE 7.
Retrieved studies flow chart.

Description of included studies
The characteristics of included studies together with outcomes measured are given in Table 17. Ponduri et al. 1 reported 18 studies of children with CP and 17 have been included in this review. 62,63,65–78 One paper of the original 18 identified110 was excluded from the present study as the results included are reported in a later paper by the same author.
| Study number | Author (year) | Study type | Duration of follow up | Sample size | Participants | Interventions | Outcomes measured | Method of measurement | Comments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Møller (1982)62 | Retrospective cohort | 12 months–4 years | 70 | Children with CP aged 12–156 months at study entry | CP with bilateral VT, unilateral VT, anterior quadrant VT | Incidence of otorrhoea | Incidence of otorrhoea within 1 year of VT | Primary outcome not specified in paper |
| Necessity to remove VT | Necessity to remove VT | ||||||||
| Middle ear ventilation | Position of tympanic membrane (measure of middle ear ventilation) | ||||||||
| Tympanosclerosis | Tympanosclerosis | ||||||||
| Middle ear ventilation | Retraction | ||||||||
| Necessity for new VT | Necessity for new VT | ||||||||
| OMEa | Otoscopic findings (OME) | ||||||||
| 2 | Potsic et al. (1979)63 | Retrospective cohort | 5 years | 69 | Children with CP | No treatment vs. VT when needed vs. VT early insertion | Hearing impairmenta | Pure tone audiometry | Primary outcome not specified in paper Hearing impairment included as an outcome with two methods of assessment |
| Hearing impairmenta | Speech audiometry | ||||||||
| OME | Otoscopic findings | ||||||||
| 3 | Paradise and Bluestone (1974)64 | Case series | 32 months | 138 | Children with CP aged < 24 months. Pittsburgh USA | n/a | Incidence of otorrhoea | Otorrhoea – 6 months post operation | Primary outcome not specified in paper OME included as an outcome with two methods of assessment |
| Duration of otorrhoea | |||||||||
| OMEa | Pneumatic otoscopy | ||||||||
| OMEa | Microscopy | ||||||||
| Perforation | Perforation | ||||||||
| Tympanic membrane on otoscopy | Fullness or bulging of the tympanic membrane | ||||||||
| Tympanic membrane on otoscopy | Erythema or colour of the tympanic membrane | ||||||||
| 4 | Møller (1981)65 | Prospective cohort | 36 months | 261 | Children with CP aged 1 month–20 years (mean 7 years). Western and Northern Norway | 28 bilateral VT, 40 unilateral VT | Hearing impairmenta | Pure tone audiometry | Primary outcome not specified in paper Disagreement between reviewers on primary outcome requiring further discussion. Consensus reached that hearing impairment was considered the primary outcome |
| Middle ear pressure | tympanometry | ||||||||
| OME | Otoscopic findings | ||||||||
| Scarring of the tympanic membrane | Otoscopic findings | ||||||||
| Atelectasis of the tympanic membrane | Otoscopic findings | ||||||||
| Tympanosclerosis of the tympanic membrane | Otoscopic findings | ||||||||
| AOM | Otoscopic findings | ||||||||
| Chronic perforation | Otoscopic findings | ||||||||
| Stapedial reflex | Tympanometry | ||||||||
| 5 | Smith et al. (1994)66 | Case series | 65 months | 81 | Children with CP, age not stated. North Carolina, USA | – | Eustachian tube dysfunctiona | Tympanometry | Primary outcome not specified in paper |
| Number of VTs until normal tympanometry | Tympanometry/note review | ||||||||
| Average time to extrusion of VT | Note review | ||||||||
| Hearing | Pure tone audiometry | ||||||||
| Perforation of the tympanic membrane | Otoscopy | ||||||||
| 6 | Hormann and Roehrs (1991)67 | Prospective cohort | Ages 8 and 16 years | 184 | Children with CP. Hamburg, Germany (n = 126) and Ioqa, USA (n = 58) | Grommets vs. early grommets | Risk of otorrhoea | Risk of otorrhoea | Article in German. Abstract and Ponduri et al. 20091 review used |
| Middle ear status | Unknown | ||||||||
| 7 | Broen et al. (1996)68 | Prospective cohort | 3-monthly follow-up for 9–30 months | 28 CP; 29 non-CP | Children with and without CP. Minnesota, USA | Cleft vs. non cleft | Hearing impairmenta | Sound field audiometry | Primary outcome not specified in paper Disagreement between reviewers on primary outcome requiring further discussion. Consensus reached that hearing impairment was considered to be the primary outcome Hearing impairment included as an outcome with three methods of assessment |
| Hearing impairmenta | Visual reinforcement audiometry | ||||||||
| Hearing impairmenta | Pure tone audiometry | ||||||||
| Middle ear function | Tympanometry | ||||||||
| Hearing screening failures | |||||||||
| Number of VTs | Audiometry number of VTs | ||||||||
| 8 | Frable et al. (1985)69 | Case series | 6-monthly follow-up for 10 years | 36 | Children with CP | CP children with bilateral VTs placed | Number of VTs | Note review | Primary outcome not specified in paper |
| Tympanic membrane atresiaa | Otoscopic findings | ||||||||
| COMa | Otoscopic findings | ||||||||
| Incidence of AOM | Parent report | ||||||||
| OME | Otoscopic findings | ||||||||
| Hearing loss | Audiometry | ||||||||
| 9 | Sheahan et al. (2002)70 | Case series | Mean follow-up 6 years and 11 months | 104 | Children with CP. Dublin, Ireland | CP children treated for OME | COMa | Otoscopic findings | Primary outcome not specified in paper Cholesteatoma, retraction and perforation have been grouped in this paper and are considered to be signs of COM |
| Cholesteatoma | Otoscopic findings | ||||||||
| Retraction | Otoscopic findings | ||||||||
| Perforation | Otoscopic findings | ||||||||
| OME | Otoscopic findings | ||||||||
| Hearing impairment | Pure tone audiometry | ||||||||
| 10 | Hubbard et al. (1985)71 | Retrospective study | 60–132 months | 48 | Children with CP aged 3 months at study entry. Pittsburgh, USA | VT at 3 months vs. VT 30 months | Scarring of the tympanic membranea | Otoscopy | Primary outcome not specified in paper |
| Hearing loss | Pure tone audiometry | ||||||||
| Hearing loss | Speech reception thresholds | ||||||||
| Middle ear pressure | Pneumatic otoscopy | ||||||||
| Middle ear pressure | Tympanometry | ||||||||
| OME | Otoscopic findings | ||||||||
| Hypernasality of speech | 5-point scale completed by speech and language therapist | ||||||||
| Consonant articulation | Test of articulation | ||||||||
| Social maturity | Vineland Social Maturity Scale | ||||||||
| Social maturity | Wechsler Intelligence Scale for Children | ||||||||
| Self-esteem | Coopersmith Self-Esteem Inventory | ||||||||
| Behaviour | Child Behaviour Checklist | ||||||||
| Nasal resonance | Assessment by speech and language therapist | ||||||||
| Perforation | Otoscopic findings | ||||||||
| 11 | Gordon et al. (1988)72 | Retrospective study | Follow-up 10–16 years post treatment | 50 | Children with CP. New Zealand | VTs vs. no treatment | Hearing impairment | Pure tone audiometry | Primary outcome not specified in paper |
| Middle ear pressure | Tympanometry | ||||||||
| Tympanosclerosisa | Otoscopy | ||||||||
| Scarring of the tympanic membranea | Otoscopy | ||||||||
| Retraction | Otoscopy | ||||||||
| OMEa | Otoscopy | ||||||||
| Otorrhoeaa | Otoscopy | ||||||||
| Cholesteatomaa | Otoscopy | ||||||||
| 12 | Robson et al. (1992)73 | Retrospective | 30–60 months’ follow-up | 74 | Children with CP. Bristol, UK | VTs vs. no treatment | Otorrhoea | Otoscopy | Primary outcome not specified in paper Communication disorder (verbal comprehension and expression) as an outcome with four methods of assessment |
| Perforations of the tympanic membrane | Otoscopy | ||||||||
| Tympanosclerosis | Otoscopy | ||||||||
| Episodes of otalgia | |||||||||
| Middle ear pressure | Tympanometry | ||||||||
| Atelectasis of the tympanic membrane | Otoscopy | ||||||||
| Retraction of the tympanic membrane | Otoscopy | ||||||||
| Velopharyngeal insufficiency | SLT assessment | ||||||||
| OME | Otoscopy | ||||||||
| Hearing impairment | Pure tone audiogram | ||||||||
| Educational performance | Parent questionnaire (is child above/below average) | ||||||||
| Level of speech therapy support required | Level of speech therapy support required | ||||||||
| Behaviour | Parent questionnaire (is child above/below average) | ||||||||
| Communication disorder (verbal comprehension and expression)a | Cleft-related articulation at SLT assessment | ||||||||
| Communication disorder (verbal comprehension and expression)a | Phonological problem at SLT assessment | ||||||||
| Communication disorder (verbal comprehension and expression)a | Language difficulties at SLT assessment | ||||||||
| Communication disorder (verbal comprehension and expression)a | Learning difficulties at SLT assessment | ||||||||
| 13 | Greig et al. (1999)74 | Case series | 60 months | 36 | Children with CP. London, England | CP children with bilateral VT tubes | Hearing improvement | Parental questionnaire | |
| Change in otorrhoea | Parental questionnaire | ||||||||
| Parental satisfaction with VT treatmenta | Parental questionnaire | ||||||||
| Speech improvement | Parental questionnaire | ||||||||
| Receptive language | SLT assessment | ||||||||
| Expressive language | SLT assessment | ||||||||
| Speech development | SLT assessment | ||||||||
| Global development | Unclear | ||||||||
| Hearing impairment | Audiometry (not details given) | ||||||||
| Nasal escape | SLT assessment | ||||||||
| Cleft speech | SLT assessment | ||||||||
| Perforation | Presume otoscopy | ||||||||
| 14 | Shaw et al. (2003)75 | Retrospective study | 10 years | 72 | Children with CP. Liverpool, UK | Children grouped into LAHSAL cleft classification | Speech – resonancea | SLT assessment | Primary outcome not specified in paper |
| Speech – articulationa | SLT assessment | ||||||||
| Number of grommets | Number of grommets | ||||||||
| 15 | Freeland and Evans (1981)76 | Retrospective study | Followed 6-monthly for 48 months | 68 | Children with CP. Oxford, UK | Recruited at birth with regular follow-up | OME | Otoscopic findings | |
| Retraction | Otoscopic findings | ||||||||
| Perforation | Otoscopic findings | ||||||||
| Tympanosclerosis of the tympanic membrane | Otoscopic findings | ||||||||
| Language developmenta | Reynell Developmental Language Scale | ||||||||
| 16 | Zheng et al. (2003)77 | RCT | 6-month postoperative (VTs), 20-month postoperative (control) | 62 | Children with CP. China | Palatoplasty (n = 24) vs. palatoplasty + VT (n = 38) | Presence of OMEa | Unknown | Article in Chinese. Abstract and Ponduri et al. 20091 review used |
| Hearing levels | Unknown | ||||||||
| 17 | Liu et al. (2004)78 | Case series | 2 weeks–18 months postoperatively | 19 | Children with CP. China | Unilateral VT vs. other ear as control with no VT | Complications | Unknown | Article in Chinese. Abstract and Ponduri et al. 20091 review used |
| Hearing lossa | Audiometry | ||||||||
| Middle ear status | Unknown | ||||||||
| 18 | Tanpowpong et al. (2007)79 | Cohort | 10 months | 23 | 6 children with CP and 17 without CP. Bangkok | Myringotomy and VT insertion | Hearing impairmenta | Pure tone audiometry | Primary outcome not specified in paper |
| Middle ear pressure | Tympanometry | ||||||||
| Otorrhoea | Otoscopy | ||||||||
| Time to extrusion of VTs | Note review | ||||||||
| 19 | Civelek et al. (2007)5 | Retrospective | 72-month follow-up | 41 | 56 children with CP, 15 children without CP and history of VT insertion. Turkey | CP vs. non-CP with VTs | Perforationa | Otoscopic findings | Primary outcome not specified in paper |
| Tympanosclerosisa | Otoscopic findings | ||||||||
| Cholesteatomaa | Otoscopic findings | ||||||||
| Retractiona | Otoscopic findings | ||||||||
| Hearing impairmenta | Pure tone audiometry | ||||||||
| Middle ear pressure | Tympanometry | ||||||||
| Velopharyngeal insufficiency | Speech assessment (method not specified) | ||||||||
| 20 | Phua et al. (2009)80 | Retrospective | Minimum 2 years, maximum 15 years | 234 | Children with CP. Auckland, New Zealand | VT (n = 45) vs. no treatment (n = 189) | Hearing lossa | Pure tone audiogram | Primary outcome not specified in paper |
| Recurrent AOM | Note review | ||||||||
| Persistent OME | Otoscopic findings | ||||||||
| Retraction | Otoscopic findings | ||||||||
| Perforation | Otoscopic findings | ||||||||
| Cholesteatoma | Otoscopic findings | ||||||||
| Subjective hearing loss Number of VTs |
Note review | ||||||||
| 21 | Reiter et al. (2009)81 | Retrospective | 6 years | 116 | Children with CP. Germany | Divided age and type of cleft then ± VTs | Cholesteatomaa | Otoscopic findings | Primary outcome not specified in paper |
| Hearing loss | Pure tone audiogram | ||||||||
| Middle ear pressure | Tympanometry | ||||||||
| Atelectasis of the tympanic membranea | Otoscopic findings | ||||||||
| Perforations of the tympanic membranea | Otoscopic findings | ||||||||
| Retraction of the tympanic membranea | Otoscopic findings | ||||||||
| OME | Otoscopic findings | ||||||||
| 22 | Szabo et al. (2010)82 | Retrospective | 5 years | 86 | Children with CP. Connecticut, USA | All cases VT | Hearinga | Newborn hearing screening | Primary outcome not specified in paper |
| Number of surgeries | Note review | ||||||||
| Atelectasis of the tympanic membrane | Otoscopic findings | ||||||||
| Perforations of the tympanic membranea | Otoscopic findings | ||||||||
| Retraction of the tympanic membrane | Otoscopic findings | ||||||||
| Tympanosclerosis | Otoscopic findings | ||||||||
| Scarring of the tympanic membrane | Otoscopic findings | ||||||||
| 23 | Hornigold et al. (2008)83 | Long-term follow-up data of RCT | 20 years post-VT insertion in original RCT | 7 | Children with CP who had participated in previous RCT. UK | VT insertion vs. control | Hearing loss | Pure tone audiometry | Primary outcome not specified in paper In this study primary outcomes were grouped in the paper as symptomatology |
| Middle ear function | Tympanometry | ||||||||
| Mucosal COM | Otoscopic findings | ||||||||
| Cholesteatoma (squamous COM) | Otoscopic findings | ||||||||
| Otorrhoeaa | Patient interview | ||||||||
| Subjective hearing lossa | Patient interview | ||||||||
| Otalgiaa | Patient interview | ||||||||
| Vertigoa | Patient interview | ||||||||
| Tinnitusa | Patient interview | ||||||||
| Need for further surgerya | Patient interview | ||||||||
| 24 | Merrick et al. (2007)84 | Cohort study | Mean 8 years of age. Follow-up post-palatoplasty | 100 | 50 children with CP, 50 children without CP. UK | VT (n = 50) vs. control (n = 50) | OME | Otoscopy | Primary outcome not specified in paper |
| Speech intelligibilitya | CP speech assessment audit form | ||||||||
| Speech nasalitya | Assessment audit form | ||||||||
| Nasal air flowa | Assessment audit form | ||||||||
| Consonant productiona | Assessment audit form | ||||||||
| Cleft type characteristicsa | Assessment audit form | ||||||||
| 25 | Curtin et al. (2009)85 | Prospective cohort | 6-months post-palate repair | 33 | Children with CP. Stanford, USA | VT at 9 months with a 6-month follow-up | Incidence of otorrhoeaa | Parent report | Hearing impairment as an outcome with three methods of assessment |
| VT patency | Tympanometry | ||||||||
| Hearing impairment | Behavioural audiometry | ||||||||
| Hearing impairment | Sound field audiometry | ||||||||
| Hearing impairment | Newborn infant hearing screen | ||||||||
| 26 | Mandel et al. (2002)86 | RCT | 4 weeks | 111 | Children without CP. Pittsburgh, USA | Bilateral VT vs. bilateral myringotomy vs. no surgery | Middle ear effusiona | Pneumatic otoscopy | Primary outcome not specified in paper |
| Middle ear pressure | Tympanometry | ||||||||
| Recurrence of OME following resolution | Note review | ||||||||
| Incidence of AOM | Note review | ||||||||
| Hearing impairment | Age-appropriate hearing test (procedures varied according to age) | ||||||||
| Adverse events | Incidence of hyperactivity, increased appetite, vomiting, irritability, diarrhoea, abdominal discomfort, rash | ||||||||
| 27 | Casselbrant et al. (2009)87 | RCT | Up to 36 months | 98 | Children without CP. Pittsburgh, USA. | Myringotomy + VT (n = 32), adenoids with myringotomy + VT (n = 32), adenoidectomy with myringotomy (n = 34) | Percentage of time with OMEa | Pneumatic otoscopy | Percentage of time with OME as an outcome with three methods of assessment |
| Percentage of time with OMEa | Tympanometry | ||||||||
| Percentage of time with OMEa | Otoscopy | ||||||||
| Requirement for further surgery | Note review | ||||||||
| Incidence of AOM | Outpatient assessment | ||||||||
| Incidence of otorrhoea | Outpatient assessment | ||||||||
| VT functional status | Tympanometry | ||||||||
| Perforation of the tympanic membrane | Otoscopy | ||||||||
| 28 | Koivunen et al. (2004)88 | RCT | 2 years | 180 | Children without CP. Oulu, Finland | Adenoidectomy (n = 60), chemoprohpylaxis (n = 60), placebo (n = 60) | Intervention failurea | Two acute episodes in 2 months or three in 6 months based on symptom diaries; or middle ear effusion for at least 2 months as assessed by study otolaryngologist using pneumatic otoscope | Necessity to visit doctor – definition of doctor not specified in paper, would assume that the outcome describes unplanned visits to the GP and not planned study visits to the consultant |
| Incidence of AOM | Symptom diary | ||||||||
| Necessity to visit doctor | Symptom diary | ||||||||
| Requirement for antibiotics | Symptom diary | ||||||||
| Days with rhinitis | Symptom diary | ||||||||
| Days with earache | Symptom diary | ||||||||
| Days with fever | Symptom diary | ||||||||
| Incidence of adverse events | Symptom diary | ||||||||
| 29 | Matilla et al. (2003)89 | RCT | Follow-up until age 2 years, mean follow-up 7 months | 137 | Children without CP. Helsinki, Finland | Bilateral VTs (n = 63) vs. bilateral VTs + adenoidectomy (n = 74) | Rate of AOMa | Otoscopy | Rate of AOM as an outcome with two methods of assessment |
| Rate of AOMa | Symptomology | ||||||||
| Rate of otitis media episodes caused by S. pnemoniae | Culture | ||||||||
| Rate of otitis media episodes caused by H. influenzae | Culture | ||||||||
| Rate of otitis media episodes caused by M. catarrhali | Culture | ||||||||
| Middle ear pressure | Tympanometry | ||||||||
| Number of days with otorrhoea | Follow-up card | ||||||||
| 30 | Paradise et al. (1990)90 | RCT and cohort | 3 years | 99 in RCT and 114 in cohort | Children without CP. Pittsburgh, USA | Adenoidectomy (n = 99) vs. control (n = 114) | Proportion of time with otitis mediaa | Interpolation of visit data. Pneumatic otoscopy and biweekly enquiries by nurse | Proportion of time with otitis media |
| Number of VT insertions | Case note review | ||||||||
| Number of days when experienced otalgia | Biweekly enquiries by nurse | ||||||||
| Number of days receiving antibiotics | Biweekly enquiries by nurse | ||||||||
| 31 | Paradise et al. (1999)91 | RCT | Up to 3 years | 461 (304 in three-way trial and 157 in two-way trial) | Children without CP. Pittsburgh, USA | Adenoidectomy (n = 100) vs. adenotonsillectomy (n = 180) vs. control (n = 181) | Number of episodes of AOMa | Not specified | |
| Proportion of time with otitis media | Interpolation of visit data. Pneumatic otoscopy and biweekly enquiries by nurse | ||||||||
| Number of VT insertions | Case note review | ||||||||
| Number of days when experienced otalgia | Biweekly enquiries by nurse | ||||||||
| Number of myringotomies | Not specified | ||||||||
| Number of days receiving antibiotics | Biweekly enquiries by nurse | ||||||||
| 32 | Rynneldagoo et al. (1978)92 | Prospective controlled study | 24 months | 76 | Children without CP. Sweden | Adenoidectomy (n = 37), control (n = 39) | Change in frequency of common cold | Unknown | Primary outcome unknown |
| Change in frequency of purulent otitis media | Unknown | ||||||||
| Change in frequency of serious otitis media | Unknown | ||||||||
| Change in frequency of nasal obstruction | Unknown | ||||||||
| 33 | Gates et al. (1987)93 | RCT | 24 months | 491 | Children without CP. Texas, USA | Bilateral myringotomy (n = 107) vs. bilateral VTs (n = 129) vs. adenoidectomy (n = 130) vs. adenoidectomy + bilateral VTs (n = 125) | Time with hearing loss | Unknown | Primary outcome unknown |
| Time with OME | Unknown | ||||||||
| Time to recurrence of OME | Unknown | ||||||||
| Requirement for further VT insertion | Unknown | ||||||||
| 34 | Hammaren-Malmi et al. (2005)94 | RCT | 12 months | 217 | Children without CP. Helsinki, Finland | Adenoidectomy + bilateral VTs (n = 109) vs. bilateral VTs only (n = 108) | Number of AOM episodes in 12 monthsa | Patient diary and review by GP (primary care doctor) | IAB and NH agreed only one outcome in paper. This was noted under outcome measures in the paper but not explicitly as a primary outcome |
| 35 | Roydhouse (1980)95 | RCT | 36 months | 169 | Children without CP. Auckland, New Zealand | Bilateral VT + adenoids (n = 50), bilateral VT no adenoids (n = 50), control (n = 69) | Presence of OME | Unknown | Primary outcome unknown |
| Requirement for repeated grommets | Unknown | ||||||||
| Number of relapses | Unknown | ||||||||
| 36 | Black et al. (1990)96 | RCT | 2 years | 149 | Children without CP. Oxford, UK | Adenoidectomy, myringotomy and VTs (n = 37) vs. adenoidectomy and VTs (n = 38) vs. myringotomy and VTs (n = 37) vs. VTs (n = 37) | Hearing impairmenta | Pure tone audiometry | Main outcomes stated as hearing loss, results of impedance tympanometry and parental views on child progress. Primary outcome not specifically stated |
| Developmental progress | Parental opinion of child’s progress | ||||||||
| Presence of an abnormal tympanogram | Impedance tympanometry | ||||||||
| Adverse side effects of treatment | Parental opinion (favourable, uncertain or unfavourable) | ||||||||
| Requirement for further surgery | Note review | ||||||||
| Parents opinion on treatment | 3-point scale | ||||||||
| 37 | Dempster et al. (1993)97 | RCT | 12 months | 78 | Children without CP. Glasgow, UK | Adenoidectomy + VTs (n = 37) vs. VTs only (n = 35) | Presence of OMEa | Otoscopy | Presence of OME likely to be primary outcome but paper states two primary outcomes, presence of OME and hearing |
| Presence of OMEa | Tympanometry | ||||||||
| Hearinga | Pure tone audiometry | ||||||||
| Tympanosclerosis | Otoscopy | ||||||||
| Perforation | Otoscopy | ||||||||
| Retraction | Otoscopy | ||||||||
| 38 | Maw et al. (1999)98 | RCT | 18 months | 182 | Children without CP. Bristol, UK | Adenoidectomy (n = 47) vs. adenoidectomy and tonsilectomy (n = 47) vs. control (n = 56) | Hearing loss | Age-appropriate hearing test | Expressive language, verbal comprehension (grouped as language development) |
| Expressive languagea | Reynell Developmental Language Scale scores at 9 and 18 months | ||||||||
| Verbal comprehensiona | Reynell Developmental Language Scale scores at 9 and 18 months | ||||||||
| Mental development | Griffiths Mental Development Scales | ||||||||
| Middle ear pressure | Tympanometry | ||||||||
| Presence of OME | Otoscopy | ||||||||
| Requirement for further VTs | Note review | ||||||||
| 39 | Rach et al. (1991)99 | RCT | 6 months | 43 | Children without CP. Netherlands | VTs vs. no surgery | Language development – verbal expressiona | Reynell Developmental Language Scale | Primary outcome not specified in paper |
| Language development – verbal comprehensiona | Reynell Developmental Language Scale | ||||||||
| Middle ear pressure | Tympanometry | ||||||||
| Duration of VT tube in situ | Tympanometry | ||||||||
| Presence of OME | Tympanometry | ||||||||
| 40 | Rovers et al. (2000)100 | RCT | 12 months | 187 | Children without CP. Netherlands | VT (n = 93) vs. no surgery (n = 94) | Expressive languagea | Schlichting Test for Expressive Language | Primary outcome not specified in paper but study designed to detect a difference in language development |
| Expressive languagea | Lexi test | ||||||||
| Comprehensive languagea | Reynell Developmental Language Scale | ||||||||
| Hearing loss | Visual reinforcement audiometry | ||||||||
| IQ | Bayley Scale of Infant Development | ||||||||
| OME | Otoscopy | ||||||||
| OME | Tympanometry | ||||||||
| 41 | Johnston et al. (2004)101 | Screening followed by RCT | Follow-up to age 3 years | 429 | Children without CP. Pittsburgh, USA | VT (n = 216) vs. delayed VT (n = 213) | Tympanoscerosisa | Otoscopy | Primary outcome not specified in paper Outcome, tympanic membrane abnormalities which includes tympanosclerosis, fibrosis, atrophy, retraction pocket, perforation and cholesteatoma |
| Fibrosisa | Otoscopy | ||||||||
| Atrophya | Otoscopy | ||||||||
| Retraction pocketa | Otoscopy | ||||||||
| Perforationa | Otoscopy | ||||||||
| Hearing impairment | Pure tone audiometry | ||||||||
| Cholesteatomaa | Otoscopy | ||||||||
| 42 | Paradise et al. (2001)102 | RCT | Follow-up to age 3 years | 429 | Children without CP. Pittsburgh, USA | VT (n = 216) vs. delayed VT (n = 213) | Cognitiona | General cognitive index of McCarthy Scales of Children’s Abilities | Primary outcome not specified in paper |
| Hearing lossa | Age-appropriate hearing tests | ||||||||
| Behavioura | Child Behaviour Checklist | ||||||||
| Receptive languagea | Peabody-revised picture vocabulary test | ||||||||
| Expressive languagea | Number of different words | ||||||||
| Expressive languagea | Percentage of consonants correct – revised | ||||||||
| Expressive languagea | Mean length of utterance in morphemes | ||||||||
| Parental distressa | Parental stress index, short form | ||||||||
| Duration of OME | Pneumatic otoscopy | ||||||||
| Duration of OME | Tympanometry | ||||||||
| 43 | Maw (1983)103 | RCT | 12 months | 222 | Children without CP. Bristol, UK | Adenoidectomy (n = 36) vs. no surgery (n = 33) vs. adenotonsillectomy (n = 34) | Presence OMEa | Pneumatic otoscopy | Primary outcome not specified in paper Although other methodologies listed these were to confirm OME as part of patient screening and were not used as an outcome |
| 44 | Zielhus et al. (1989)104 | Screening followed by RCT | Up to age 4 years | 43 | Children without CP. Netherlands | VT (n = 22) vs. no treatment (n = 21) | Presence OME | Tympanometry | Primary outcome not specified in paper Verbal comprehension and verbal expression grouped as language development |
| OME | Otoscopy | ||||||||
| Verbal comprehensiona | Reynell Developmental Language Scale | ||||||||
| Verbal expressiona | Reynell Developmental Language Scale | ||||||||
| 45 | Fiellau-Nikolajsen et al. (1980)105 | RCT | 6 months post operative | 42 | Children without CP. Aarhus, Denmark | Myringtomy with adenoids (n = 20) vs. myringotomy only (n = 22) | Middle ear pressurea | Tympanometry | Primary outcome not specified in paper but middle ear pressure considered to be the primary outcome by the reviewers |
| Presence of OME | Otoscopy | ||||||||
| Duration of OME | Otoscopy (repeated over time) | ||||||||
| Hearing impairment | Pure tone audiometry | ||||||||
| Presence of middle ear reflexes | Impedance audiometry | ||||||||
| Middle ear pressurea | Tympanometry | ||||||||
| 46 | Nguyen et al. (2004)106 | RCT | Minimum 12 months | 63 | Children without CP. Quebec, Canada | VTs and adenoidectomy (n = 23) vs. VTs only (n = 40) | Recurrence AOMa | Patient questionnaire | Primary outcome not specified in paper Outcome in paper considered to be the primary outcome was ‘treatment failure’ as defined by the three outcomes listed and marked with ‘a’ |
| Recurrence of OME over 3 monthsa | Patient questionnaire | ||||||||
| Reinsertion of VTsa | Patient questionnaire | ||||||||
| 47 | Paradise et al. (2007)107 | RCT | Follow-up at ages 9–11 years | 429 in original study. 391 in follow-up | Children without CP | VTs (n = 216) vs. delayed treatment (n = 213) | Literacy | Woodcock Reading Mastery tests | Primary outcome not specified in paper Multiple methods of assessment for outcomes listed in paper Follow up of 2003 and 2005 paper |
| Literacy | Oral fluency – the number of words in a grade-level passage read correctly | ||||||||
| Literacy | Spelling and writing samples subtests of the Woodcock–Johnson III Tests of Achievement | ||||||||
| Phonological awareness | Ellison and Rapid Letter Naming subtests of the Comprehensive Test of Phonological Processing | ||||||||
| Auditory processing ability | Children’s version of the Hearing in Noise Test | ||||||||
| Attention, impulsivity and psychological functioning | Disruptive Behavior Disorders Rating Scale | ||||||||
| Attention, impulsivity and psychological functioning | Child Behaviour Checklist | ||||||||
| Attention, impulsivity and psychological functioning | Impairment Rating Scale | ||||||||
| Attention, impulsivity and psychological functioning | Social Skills Scale of the Social Skills Rating System | ||||||||
| Intelligence and academic achievement | Wechsler Abbreviated Scale of Intelligence | ||||||||
| Intelligence and academic achievement | Calculation subtest of the Woodcock–Johnson III Tests of Achievement | ||||||||
| Attention, impulsivity and psychological functioning | Continuous Performance Test – visual | ||||||||
| Attention, impulsivity and psychological functioning | Continuous Performance Test – auditory | ||||||||
| 48 | Paradise et al. (2005)108 | RCT | Follow-up at age 6 years | 429 | Children without CP. Pittsburgh, USA | VT (n = 216) vs. delayed treatment (n = 213) | Intelligence and academic achievement | Wechsler Abbreviated Scale of Intelligence | Primary outcome not specified in paper Follow-up of 2003 paper109 |
| Receptive vocabulary | Peabody Picture Vocabulary Test | ||||||||
| Behaviour | Parent-reported inventories – Child Behaviour Checklist | ||||||||
| Phonologic memory | Non-word repetition task | ||||||||
| Auditory processing and language | SCAN test, a measure of central auditory processing | ||||||||
| Vocabulary diversity | Conversational sample – number of different words | ||||||||
| Sentence length and grammatical complexity | Conversational sample – length of utterances in morphemes | ||||||||
| Speech sound production | Conversational sample – percentage of consonants correct | ||||||||
| Parent–child stress | Parent–reported inventories – Parental Stress Index | ||||||||
| 49 | Paradise et al. (2003)109 | RCT | Follow up at age 4 years | 429 | Children without CP. Pittsburgh, USA | VT (n = 216) vs. delayed treatment (n = 213) | Cognition | McCarthy Scales of Children’s Abilities | Primary outcome not noted as primary but main outcome. However, this included more than one outcome |
| Receptive language | Peabody Picture Vocabulary Test – Revised | ||||||||
| Phonological memory | Non-word repetition test | ||||||||
| Expressive language | Word diversity (number of difference words) | ||||||||
| Expressive language | Sentence length and grammatical complexity (mean length of utterance in morphemes) | ||||||||
| Expressive language | Speech sound production (Percentage of Consonants Correct – Revised) | ||||||||
| Parent–child stress | Parental Stress Index | ||||||||
| Behaviour | Child Behaviour Checklist |
A further eight studies of children with CP have been included. 5,79–85 In addition, the search was expanded to studies of children without CP or where the presence or absence of a cleft was not specified, this yielded a further 24 studies. 86–89,91–109
Studies did not always clearly state the primary outcome and when this was the case the primary outcome was inferred from the sample size calculation, the study title and/or the results presented first in the results section. Papers which did not explicitly identify a primary outcome are detailed in Table 17. The primary outcome was determined independently by NH and IAB, and reviewed with any disagreement discussed further, there was disagreement for only two papers with further discussion required to agree the primary outcome. Papers with disagreement are noted in Table 17.
Each outcome measured was listed by study, only individual outcomes were included (e.g. where an outcome was measured using different methods this was counted as one outcome but the methods of measurement noted).
Generation of an outcome list for use in the Delphi survey of health professionals
The number of outcomes measured in an individual study varied with a median of 6 outcomes (range 1–14 outcomes) per paper. All outcomes measured were reviewed by NH and IAB, and grouped into possible outcome and outcome domain headings. After the initial review a total of 43 outcomes were listed under 13 domain headings (Table 18). Outcomes related to resource use were considered to be outside of the scope of the COS and would instead be considered as part of the VOI analysis (see Chapter 5). Consequently, the outcomes ‘necessity to visit doctor’ and ‘level of speech therapy support required’ were not considered in the initial list of 45 outcomes.
| Outcome domain | Outcome | Outcome identified in individual study | Study number of papers retrieved from systematic review (see Table 17) |
|---|---|---|---|
| Outcomes related to COM | COM | COMa | 8 |
| COMa | 9 | ||
| Mucosal COM | 23 | ||
| Fibrosis | 41 | ||
| Cholesteatoma | Cholesteatoma | 9 | |
| Cholesteatomaa | 11 | ||
| Cholesteatomaa | 19 | ||
| Cholesteatoma | 20 | ||
| Cholesteatomaa | 21 | ||
| Cholesteatoma (squamous COM) | 23 | ||
| Cholesteatoma | 41 | ||
| Tympanosclerosis | Tympanosclerosis | 1 | |
| Scarring of the tympanic membrane | 4 | ||
| Tympanosclerosis of the tympanic membrane | 4 | ||
| Scarring of the tympanic membranea | 10 | ||
| Scarring of the tympanic membranea | 11 | ||
| Tympanosclerosisa | 11 | ||
| Tympanosclerosis | 12 | ||
| Tympanosclerosis of the tympanic membrane | 15 | ||
| Tympanosclerosisa | 19 | ||
| Scarring of the tympanic membrane | 22 | ||
| Tympanosclerosis | 22 | ||
| Tympanosclerosis | 37 | ||
| Tympanosclerosis | 41 | ||
| Atelectasis | Atelectasis of the tympanic membrane | 4 | |
| Tympanic membrane atresiaa | 8 | ||
| Atelectasis of the tympanic membrane | 12 | ||
| Atelectasis of the tympanic membranea | 21 | ||
| Atelectasis of the tympanic membrane | 22 | ||
| Atrophy | 41 | ||
| Persistent tympanic membrane perforation | Perforation | 3 | |
| Perforation of the tympanic membrane | 5 | ||
| Perforation | 9 | ||
| Perforation | 10 | ||
| Perforations of the tympanic membrane | 12 | ||
| Perforation | 13 | ||
| Perforationa | 15 | ||
| Perforationa | 19 | ||
| Perforation | 20 | ||
| Perforations of the tympanic membranea | 21 | ||
| Perforations of the tympanic membranea | 22 | ||
| Perforation of the tympanic membrane | 27 | ||
| Perforation | 37 | ||
| Perforation | 41 | ||
| Chronic perforation | 4 | ||
| Tympanic membrane | 3 | ||
| Tympanic membrane on otoscopy | 3 | ||
| Persistent tympanic membrane retraction | Retraction | 9 | |
| Retraction | 11 | ||
| Retraction of the tympanic membrane | 12 | ||
| Retractiona | 15 | ||
| Retractiona | 19 | ||
| Retraction | 20 | ||
| Retraction of the tympanic membranea | 21 | ||
| Retraction of the tympanic membrane | 22 | ||
| Retraction | 37 | ||
| Retraction pocket | 41 | ||
| Outcomes related to behaviour | Behaviour | Behaviour | 10 |
| Behaviour | 10 | ||
| Behaviour | 12 | ||
| Behaviour | 12 | ||
| Behavioura | 42 | ||
| Behavioura | 42 | ||
| Attention, impulsivity and psychological functioning | 47 | ||
| Behaviour | 48 | ||
| Behaviour | 49 | ||
| Outcomes related to ear symptoms | Otalgia | Episodes of otalgia | 12 |
| Otalgiaa | 23 | ||
| Days with earache | 28 | ||
| Number of days on which ear pain occurred | 30 | ||
| Number of days when experienced otalgia | 31 | ||
| Otorrhoea | Incidence of otorrhoea | 1 | |
| Incidence of otorrhoea | 3 | ||
| Duration of otorrhoea | 3 | ||
| Risk of otorrhoea | 6 | ||
| Otorrhoea | 11 | ||
| Otorrhoea | 12 | ||
| Change in otorrhoea | 13 | ||
| Otorrhoea | 18 | ||
| Otorrhoeaa | 23 | ||
| Incidence of otorrhoeaa | 25 | ||
| Incidence of otorrhoea | 27 | ||
| Number of days with otorrhoea | 29 | ||
| Number of episodes of secondary otorrhoea | 30 | ||
| Vertigo | Vertigoa | 23 | |
| Hearing impairmenta | 2 | ||
| Hearing impairmenta | 4 | ||
| Hearing | 5 | ||
| Hearing impairmenta | 7 | ||
| Hearing screening failures | 7 | ||
| Hearing loss | 8 | ||
| Hearing impairment | 9 | ||
| Hearing loss | 10 | ||
| Hearing loss | 10 | ||
| Hearing impairment | 11 | ||
| Hearing impairment | 12 | ||
| Hearing impairment | 13 | ||
| Hearing improvement | 13 | ||
| Hearing levels | 16 | ||
| Hearing loss | 17 | ||
| Hearing impairmenta | 18 | ||
| Hearing impairmenta | 19 | ||
| Hearing lossa | 20 | ||
| Subjective hearing loss | 20 | ||
| Hearing loss | 21 | ||
| Hearinga | 22 | ||
| Hearing loss | 23 | ||
| Subjective hearing lossa | 23 | ||
| Hearing impairment | 25 | ||
| Hearing impairment | 26 | ||
| Time with hearing loss | 33 | ||
| Hearing impairmenta | 36 | ||
| Hearinga | 37 | ||
| Hearing loss | 38 | ||
| Hearing loss | 40 | ||
| Hearing impairment | 41 | ||
| Hearing lossa | 42 | ||
| Hearing impairment | 45 | ||
| Tinnitus | Tinnitusa | 23 | |
| Outcomes related to nasal symptoms | Nasal obstruction | Nasal air flowa | 24 |
| Change in frequency of nasal obstruction | 34 | ||
| Rhinitis | Days with rhinitis | 28 | |
| Outcomes related to development | Developmental progress | Global development | 13 |
| Developmental progress | 36 | ||
| Mental development | 38 | ||
| Intelligence and academic achievement | Educational performance | 12 | |
| IQ | 40 | ||
| Intelligence and academic achievement | 47 | ||
| Intelligence and academic achievement | 48 | ||
| Psychosocial development | Self-esteem | 10 | |
| Social maturity | 10 | ||
| Psychosocial development | 47 | ||
| Literacy | 47 | ||
| Phonological memory | Phonologic memory | 48 | |
| Phonological memory | 49 | ||
| Cognitive development | Cognitiona | 42 | |
| Cognitive development | 47 | ||
| Cognition | 49 | ||
| Outcomes related to middle ear status | Eustachian tube function | Middle ear ventilation | 1 |
| Middle ear ventilation | 1 | ||
| Middle ear pressure | 4 | ||
| Eustachian tube dysfunctiona | 5 | ||
| Middle ear status | 6 | ||
| Middle ear function | 7 | ||
| Middle ear pressure | 10 | ||
| Middle ear pressure | 11 | ||
| Middle ear pressure | 12 | ||
| Middle ear status | 17 | ||
| Middle ear pressure | 18 | ||
| Middle ear pressure | 19 | ||
| Middle ear pressure | 21 | ||
| Middle ear function | 23 | ||
| Middle ear pressure | 26 | ||
| Middle ear pressure | 29 | ||
| Presence of an abnormal tympanogram | 36 | ||
| Middle ear pressure | 38 | ||
| Middle ear pressure | 39 | ||
| Middle ear pressurea | 45 | ||
| Stapedial reflex | Presence of middle ear reflexes | 45 | |
| Stapedial reflex | 4 | ||
| Outcomes related to speech and language | Speech | Speech sound production | 48 |
| Speech intelligibility | Speech intelligibilitya | 24 | |
| Receptive language skills | Receptive language | 13 | |
| Receptive languagea | 42 | ||
| Receptive vocabulary | 48 | ||
| Receptive language | 49 | ||
| Auditory processing ability | 47 | ||
| Auditory processing and language | 48 | ||
| Expressive language skills | Expressive language | 13 | |
| Expressive languagea | 38 | ||
| Language development – verbal expression | 39 | ||
| Expressive language | 40 | ||
| Expressive languagea | 42 | ||
| Expressive languagea | 42 | ||
| Verbal expressiona | 44 | ||
| Vocabulary diversity | 48 | ||
| Expressive language | 49 | ||
| Expressive language | 45 | ||
| Speech and language development | Speech development | 13 | |
| Speech development | 47 | ||
| Phonological awareness | 47 | ||
| Parents perspective of speech | Speech improvement (parent reported) | 13 | |
| Language skills – comprehension and expression | Communication disorder (verbal comprehension and expression)a | 12 | |
| Language development | 47 | ||
| Language development – verbal | 39 | ||
| Sentence length and grammatical complexity | 48 | ||
| Language development | 15 | ||
| Verbal comprehensiona | 38 | ||
| Verbal comprehensiona | 44 | ||
| Comprehensive language | 40 | ||
| Consonant production – cleft vs. non-cleft speech difficulties | Consonant articulation | 10 | |
| Consonant productiona | 24 | ||
| Speech – articulationa | 14 | ||
| Cleft-related speech patterns | Cleft speech | 13 | |
| Cleft type characteristicsa | 24 | ||
| Speech signs of velopharyngeal insufficiency | Velopharyngeal insufficiency | 12 | |
| Velopharyngeal insufficiency | 19 | ||
| Speech – resonancea | 14 | ||
| Speech nasalitya | 24 | ||
| Hypernasality of speech | 10 | ||
| Nasal resonance | 10 | ||
| Nasal escape | 13 | ||
| Speech – resonancea | 14 | ||
| Speech nasalitya | 24 | ||
| Outcomes related to otitis media | AOM | AOM | 4 |
| Incidence of AOM | 8 | ||
| Recurrent AOM | 20 | ||
| Incidence of AOM | 26 | ||
| Incidence of AOM | 27 | ||
| Incidence of AOM | 28 | ||
| Rate of AOMa | 29 | ||
| Rate of otitis media episodes caused by H. influenzae | 29 | ||
| Rate of otitis media episodes caused by M. catarrhalis | 29 | ||
| Rate of otitis media episodes caused by S. pnemoniae | 29 | ||
| Episodes of suppurative otitis mediaa | 30 | ||
| Number of days on which antimicrobial treatment was received | 30 | ||
| Incidence of AOMa | 31 | ||
| Number of days receiving antibiotics | 31 | ||
| Number of AOM episodes in 12 months | 34 | ||
| Change in frequency of purulent otitis media | 34 | ||
| Recurrence AOMa | 46 | ||
| Requirement for antibiotics | 28 | ||
| Days with fever | 28 | ||
| OME | OMEa | 1 | |
| OME | 2 | ||
| OMEa | 3 | ||
| OME | 4 | ||
| OME | 8 | ||
| OME | 9 | ||
| OME | 10 | ||
| OMEa | 11 | ||
| OME | 12 | ||
| OME | 15 | ||
| Presence of OME | 16 | ||
| Persistent OME | 20 | ||
| OME | 21 | ||
| OME | 24 | ||
| Middle ear effusiona | 26 | ||
| Recurrence of OME following resolution | 26 | ||
| Percentage of time with OME | 27 | ||
| Percentage of time with OMEa | 27 | ||
| Percentage of time with OMEa | 27 | ||
| Proportion of time with OMEa | 30 | ||
| Proportion of time with otitis media | 31 | ||
| Time to recurrence of OME | 33 | ||
| Time with OME | 33 | ||
| Change in frequency of serious otitis media | 34 | ||
| Number of relapses | 35 | ||
| Presence of OME | 35 | ||
| Presence of OMEa | 37 | ||
| Presence of OMEa | 37 | ||
| Presence of OME | 38 | ||
| Presence of OME | 39 | ||
| OME | 40 | ||
| Duration of OME | 42 | ||
| Presence of OME | 43 | ||
| Presence of OME | 44 | ||
| Presence of OME | 45 | ||
| Duration of OME | 45 | ||
| Recurrence of OME over 3 monthsa | 46 | ||
| Intervention failurea | 28 | ||
| Temporary tympanic membrane perforation | This outcome was identified from outcomes listed as part of persistent tympanic membrane perforation | ||
| Outcomes related to VTs (grommets) | Requirement for repeated VTs | Necessity for new VT | 1 |
| Number of VTs until normal tympanometry | 5 | ||
| Number of VTs | 7 | ||
| Number of VTs | 8 | ||
| Number of grommets | 14 | ||
| Number of VTs | 20 | ||
| Number of surgeries | 22 | ||
| Need for further surgerya | 23 | ||
| Requirement for further surgery | 27 | ||
| Number of tympanostomy tube procedures | 30 | ||
| Number of VT insertions | 31 | ||
| Requirement for further VT insertion | 33 | ||
| Requirement for repeated grommets | 35 | ||
| Requirement for further surgery | 36 | ||
| Requirement for further VTs | 38 | ||
| Reinsertion of VTsa | 46 | ||
| Necessity to remove VTs | Necessity to remove VT | 1 | |
| Early extrusion or blockage of VTs | Average time to extrusion of VT | 5 | |
| Time to extrusion of VTs | 18 | ||
| VT patency | 25 | ||
| VT functional status | 27 | ||
| Duration of VT tube in situ | 39 | ||
| Side effects of treatment | Side effects of treatment | Complications | 17 |
| Adverse events | 26 | ||
| Incidence of adverse events | 28 | ||
| Adverse side effects of treatment | 36 | ||
| Upper respiratory tract infections | Upper respiratory tract infection | Change in frequency of common cold | 34 |
| Parent and child stress | Parental stress | Parental distressa | 42 |
| Child stress | Parent–child stress | 49 | |
| Parent–child stress | 48 | ||
| Parental satisfaction with treatment | Parental satisfaction with treatment | Parental satisfaction with VT treatmenta | 13 |
| Parents opinion on treatment | 36 | ||
The draft list of outcomes was then circulated to the mOMEnt SAG. The SAG comprised cleft clinicians representing speech and language therapists (n = 2), cleft surgeons (n = 1), ENT surgeons (n = 2), audiologists (n = 2) and clinical psychologists (n = 1). Members of the SAG reviewed outcomes relevant to their clinical field. The SAG also commented on the suitability of the overall domain under which outcomes are grouped.
Following review by the SAG and further review by the Study Management Group (SMG) the following actions were taken (Figure 8):
-
The SAG were asked if ‘temporary membrane retraction’ should also be included as an outcome as temporary retraction was included. All agreed that it was not necessary to include this in the list of outcomes.
-
The outcome ‘language and comprehension’ was removed as this is duplicated by the individual outcomes of ‘receptive language’ and ‘expressive language’.
-
Speech’ was grouped with ‘speech and language development’.
-
Speech intelligibility was listed as a separate outcome.
-
Outcomes related to behaviour were split into ‘externalising behaviour’ and ‘internalising behaviour’ based on the domains used in the Child Behaviour Checklist.
-
‘Intelligence and academic achievement’ was split into two individual outcomes.
FIGURE 8.
Development of final list of outcomes to be used in the Delphi survey of health professionals. SSC, Study Steering Committee.
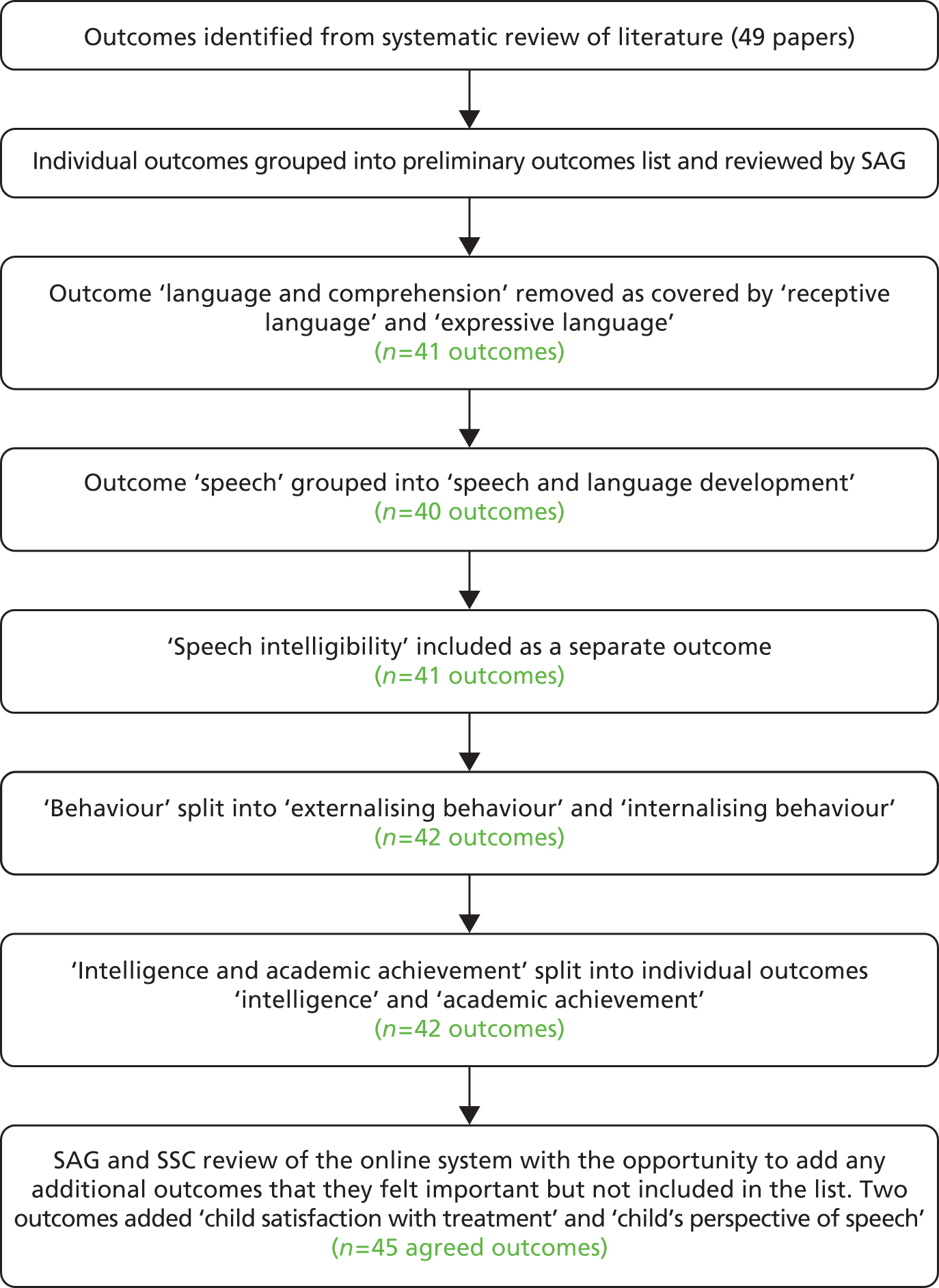
Both the SAG and the Study Steering Committee (SSC) tested the online system with an opportunity to score each outcome and to add any additional outcomes that they felt were important. Two members of the SAG added outcomes related to HAs; however, these were outside of the scope of the COS, surgical management of OME, and were not included in the outcome list. The SSC noted two additional outcomes ‘child’s satisfaction with treatment’ and ‘child’s perspective of speech’ which were taken forward to the list of outcomes to be scored by clinicians.
The final list of outcomes used in round 1 of the clinician Delphi comprised 45 individual outcomes (43 identified from the systematic review of the literature and two by the SSC) grouped under 14 domains (Table 19). To aid all participants completing the Delphi an outcome tip was written for each outcome and suitability confirmed with the SAG.
| Domain | Outcome | Tip (where there is no tip the outcome was considered to be understandable by all clinical roles) |
|---|---|---|
| Outcomes related to behaviour | Externalising behaviour | Externalising behaviours are directed outwards (e.g. having a tantrum) |
| Internalising behaviour | Internalising behaviours are directed inwards (e.g. being withdrawn or lonely) | |
| Outcomes related to COM | Atelectasis | Retraction of the thin tympanic membrane with loss of the normal middle ear space |
| Cholesteatoma | Structure made of keratin not usually found in the middle ear. Has tendency to enlarge and cause recurrent ear discharge and hearing loss | |
| COM | Fluid in the middle ear persisting for over 3 months | |
| Persistent tympanic membrane perforation | Hole in the tympanic membrane | |
| Persistent tympanic membrane retraction | Tympanic membrane pulled backwards due to negative pressure in the middle ear | |
| Tympanosclerosis | Damage to the tympanic membrane with resultant deposition of calcium within tympanic membrane | |
| Outcomes related to development | Academic achievement | |
| Cognitive development | ||
| Developmental progress | Progress in relation to developmental milestones | |
| Intelligence | ||
| Literacy | Reading | |
| Phonological memory | Ability to remember a sequence of unfamiliar sounds | |
| Psychosocial development | ||
| Outcomes related to ear symptoms | Hearing loss | Hearing ability below the normal range for the population |
| Otalgia | Earache | |
| Otorrhoea | Ear discharge | |
| Tinnitus | The perception of noises without an accompanying external signal | |
| Vertigo | Hallucination of movement | |
| Outcomes related to middle ear status | Eustachian tube function | The eustachian tube is responsible for equalising the pressure in the middle ear with the outside world. In eustachian tube dysfunction this does not happen appropriately leading to negative pressure in the middle ear |
| Stapedial reflex | This reflex occurs in response to loud noise and is thought to play a protective role, limiting the potential for noise-induced hearing loss. When a noise of certain loudness is heard the stapedial muscle contracts making the ossicular chain of bones in the middle ear stiffer and less energy is carried to the cochlea | |
| Outcomes related to nasal symptoms | Nasal obstruction | Blocked nose |
| Rhinitis | Inflammation of the lining of the nose. Sometimes as a result of infection or allergy | |
| Outcomes related to otitis media | AOM | Infection involving the middle ear |
| OME | The presence of fluid within the middle ear | |
| Temporary tympanic membrane perforation | Hole in the tympanic membrane | |
| Outcomes related to speech and language | Consonant production | How clearly consonants are pronounced |
| Consonant production – cleft related speech patterns | Unusual consonant patterns that speech and language therapists attribute to CP/velopharyngeal dysfunction | |
| Expressive language skills | The child’s ability to produce language, including vocabulary, grammar, use of language, and sentence length and structure (syntax) | |
| Parent’s perspective of speech | Parents’ views of their child’s speech | |
| Receptive language skills | The child’s ability to understand spoken language | |
| Speech development | The predictable pattern of speech sound development leading to the production of words | |
| Speech intelligibility | How easy it is to understand a child’s speech | |
| Speech signs of velopharyngeal insufficiency | E.g. nasal tone of voice, nasal airflow accompanying speech, visible nasal grimace during speech, prevalence of consonants (‘m’ ‘n’ ‘ng’) and absent ‘ “f” “ssh” “t” “d” ‘ | |
| Outcomes related to VTs (grommets) | Early extrusion or blockage of VTs | Extrusion means the VT falls out of the tympanic membrane |
| Necessity to remove VTs | ||
| Requirement for repeated VTs | ||
| Parent and child stress | Child stress | |
| Parental stress | ||
| Parent/child satisfaction with treatment | Parental satisfaction with treatment | |
| Side effects of treatment | Side effects of treatment | |
| Upper respiratory tract infections | Upper respiratory tract infection | Infection involving the ears, nose or throat |
| Additional outcomes from SAG | Child’s satisfaction with treatment | |
| Child’s perspective of speech | Ability to hear speech noises |
Identification of outcomes of importance to parents and children with cleft palate
Introduction
The opinions of parents and children about the treatment of OME for children with CP are important because this group will experience both the benefits and adverse effects of treatments, and be involved in the decision-making about which treatment to have, and should therefore have opportunities to contribute to identification of the most appropriate outcomes for use in future trials of OME.
Outcomes of importance to parents and children with CP were identified from two sources:
-
qualitative interviews with a purposive sample of parents and children
-
an online survey of children with CP and their parents.
The qualitative interviews with parents and resulting important outcomes are described in Chapter 3 and Table 13. The results of the interviews, based on responses to specific questions about outcomes, were cross-checked against the outcomes list generated from systematic review of the literature and, as the qualitative interviews were in parallel to the first round of the health professionals Delphi, any additional outcomes from round 1 to assess if any new outcomes were identified. IAB and NH mapped each outcome from the interviews against the list of outcomes after round 1 with no new outcomes identified. (Table 20). Outcomes mentioned in the interviews that were related to HAs or service delivery were not considered. Although parental stress was not specifically referenced by parents when asked about outcomes, it was a topic that was talked about repeatedly in interviews and this represented an outcome already identified from review of the literature – ‘parental stress’. 111
| Outcome mentioned in interviews | Outcome domain from outcomes list used in health professionals survey round 1 | Associated outcome/s from outcomes list used in health professionals survey round 1 | Number of parents interviewed | Number of parents who mentioned as an outcome | Percentage of parents who mentioned outcome | Number of parents who mentioned as the most important outcome | Percentage of parents who mentioned as the most important outcome |
|---|---|---|---|---|---|---|---|
| Improvement in hearing | Outcomes related to ear symptoms | Hearing | 36 | 30 | 83 | 21 | 58 |
| Speech/communication | Outcomes related to speech and language | Consonant production Consonant production – cleft-related speech patterns Expressive language skills Parent’s perspective of speech Receptive language skills Speech development Speech intelligibility Speech signs of velopharyngeal insufficiency Parent’s perspective of speech Child’s perspective of speech |
36 | 14 | 39 | 4 | 11 |
| Child is less frustrated | Outcomes related to behaviour | Externalising behaviour | 36 | 2 | 6 | 2 | 6 |
| Make the child more alert | Additional outcomes identified in round 1 | Listening skills | 36 | 2 | 6 | 2 | 6 |
| Glue ear not to return. Remove fluid from the ears | Outcomes related to otitis media | OME | 36 | 8 | 22 | 1 | 3 |
| Reduce pain/not to cause pain | Outcomes related to ear symptoms | Otalgia | 36 | 7 | 19 | 2 | 6 |
| Reduced number of ear infections | Outcomes related to otitis media | AOM | 36 | 9 | 25 | 1 | 3 |
| Improvement in school work | Outcomes related to development | Academic achievement Cognitive development Developmental progress Intelligence Literacy |
36 | 6 | 17 | 0 | 0 |
| Improved social interactions | Outcomes related to development Additional outcomes identified in round 1 |
Psychosocial development Psychosocial well-being |
36 | 10 | 28 | 2 | 6 |
Although the qualitative interviews completed with parents and children gave rich in-depth information on outcomes of importance, the sample (recruited from two cleft centres), was relatively small in comparison with the UK population of children with a CP. To gain a broader view of important outcomes an online survey similar to that completed by health professionals was developed. This was suggested by the Cleft Lip & Palate Association (CLAPA) Children and Young Persons Council (CYPC) after discussions about the mOMEnt study. The CYPC suggested ways in which the Delphi survey could be adapted for children based on their experiences and these suggestions were taken on board.
Methods
Children aged 7–16 years and their parents were invited to take part in an online survey. In addition, after advice from the CLAPA Adult Voices Group, the survey was also made available to adults with a CP who had experience of OME.
The survey included questions to ensure eligibility, namely confirmation of a CP or a child with CP and experience of OME. For the survey of parents and children the term ‘glue ear’ was used instead as this was considered to be a more appropriate terminology.
Advice was sought from the National Research Ethics Service who did not consider that ethical approval was required for an online survey of parental and child opinion.
Survey content
Parents and children were asked to score a list of outcomes which was based on the outcomes list generated from the systematic review. Each outcome was reviewed by NH and IAB, and a plain-language alternative suggested that was appropriate for a child aged 7–10 years. This description was tested for readability using the National Institute of Adult Continuing Education Simplified Measure of Gobbledygook calculator111 and was further checked for understanding with the CLAPA CYPC and the local Happy Faces Group. Some outcomes which related to specific clinical observations were combined so that parents and children scored a total of 36 outcomes representing the 45 outcomes identified from the systematic review and SAG/SSC contribution.
The same outcome wording was used for all participants with the exception of minor changes such as ‘your’/’your child’s’ to ensure appropriate context (Table 21).
| Original outcome | Outcome domain | Age 7–10 years | Age 11–16 years | Parents | Adults |
|---|---|---|---|---|---|
| Internalising behaviour | Things about behaviour/things about your child’s behaviour/things about behaviour | How lonely you feel, feeling like an outsider | How lonely you feel, feeling like an outsider | How lonely your child feels, feeling like an outsider | How lonely you felt, feeling like an outsider |
| Externalising behaviour | Things about behaviour/things about your child’s behaviour/things about behaviour | How angry you are towards others | How angry you are towards others | How angry your child is towards others | How angry you were towards others |
| Atelectasis | Things about having problems with your ears for a long time/things about your child having problems with their ears for a long time | Not having problems inside your ear caused by having lots of ear infections over a long time (more than 3 months) | Not having problems inside your ear caused by having lots of ear infections over a long time (more than 3 months) | Your child not having problems inside their ear caused by having lots of ear infections over a long time (more than 3 months) | Not having problems inside your ear caused by having lots of ear infections over a long time (more than 3 months) |
| Persistent tympanic membrane retraction | |||||
| Tympanosclerosis | |||||
| Cholesteatoma | Things about having problems with your ears for a long time/things about your child having problems with their ears for a long time | Not having problems inside your ear caused by bad skin growing behind your ear drum | Not having problems inside your ear caused by bad skin growing behind your ear drum | Your child not having problems inside their ear caused by bad skin growing behind your ear drum | Not having problems inside your ear caused by bad skin growing behind your ear drum |
| COM | Things about having problems with your ears for a long time/things about your child having problems with their ears for a long time | Not having problems inside your ear caused by having glue ear for a long time (more than 3 months) | Not having problems inside your ear caused by having glue ear for a long time (more than 3 months) | Your child not having problems inside their ear caused by having glue ear for a long time (more than 3 months) | Not having problems inside your ear caused by having glue ear for a long time (more than 3 months) |
| Original outcome | Outcome domain children aged 7–16 years and adults/parents | Age 7–10 years | Age 11–16 years | Parents | Adults |
| Persistent tympanic membrane perforation | Things about having problems with your ears for a long time/things about your child having problems with their ears for a long time | Not having problems inside your ear caused by having a hole in your ear drum for a long time (more than 3 months) | Not having problems inside your ear caused by having a hole in your ear drum for a long time (more than 3 months) | Your child not having problems inside their ear caused by having a hole in your ear drum for a long time (more than 3 months) | Not having problems inside your ear caused by having a hole in your ear drum for a long time (more than 3 months) |
| Academic achievement Cognitive development Developmental progress Intelligence Literacy Phonological memory |
Things about school and making friends | How well you are doing at school | How well you are doing at school or college | How well your child is doing at school or college | How well you did at school or college |
| Psychosocial development | Things about school and making friends | How well you are learning to make friends and speak to new people | How well you are learning to make friends and speak to new people | How well your child is learning to make friends and speak to new people | How well you learnt to make friends and speak to new people |
| Hearing | Things about how your ear feels and works/things about how your child’s ear feels and works | How well you can hear | How well you can hear | How well your child can hear | How well you could hear |
| Otalgia | Things about how your ear feels and works/things about how your child’s ear feels and works | How painful your ear is | How painful your ear is | How painful your child’s ear is | How painful your ear was |
| Otorrhoea | Things about how your ear feels and works/things about how your child’s ear feels and works | Not having infected liquid leaking out of your ear | Not having pus (infected liquid) leaking out of your ear | Your child not having pus (infected liquid) leaking out of their ear | Not having pus (infected liquid) leaking out of your ear |
| Tinnitus | Things about how your ear feels and works/things about how your child’s ear feels and works | How much you hear buzzing or ringing noises | How much you hear buzzing or ringing noises | How much your child hears buzzing or ringing noises | How much you heard buzzing or ringing noises |
| Vertigo | Things about how your ear feels and works/things about how your child’s ear feels and works | How dizzy you feel | How dizzy you feel | How dizzy your child feels | How dizzy you felt |
| Eustachian tube function | Things about how the middle part of your ear works/things about how the middle part of your child’s ear works | How well a special tube in your ear works. If this tube does not work properly you might hear popping and crackling noises | How well a special tube in your ear works. If this tube does not work properly you might hear popping and crackling noises | How well a special tube in your child’s ear works. If this tube does not work properly your might hear popping and crackling noises | How well a special tube in your ear worked. If this tube did not work properly you might have heard popping and crackling noises |
| Stapedial reflex | Things about how the middle part of your ear works/things about how the middle part of your child’s ear works | How well your ear works when it hears a loud noise | How well your ear works when it hears a loud noise | How well your child’s ear works when it hears a loud noise | How well your ear worked when it heard a loud noise |
| Nasal obstruction | Things about how your nose feels/things about how your child’s nose feels | How well you can breathe through your nose | How well you can breathe through your nose | How well your child can breathe through their nose | How well you could breathe through your nose |
| Rhinitis | Things about how your nose feels/things about how your child’s nose feels | How much your nose feels runny or stuffy | How much your nose feels runny or stuffy | How much your child’s nose feels runny or stuffy | How much your nose felt runny or stuffy |
| AOM | Things about glue ear and ear infections | Not having ear infections | Not having ear infections | Your child not having ear infections | Not having ear infections |
| OME | Things about glue ear and ear infections | Not having glue ear and being able to hear better | Not having glue ear and being able to hear better | Your child not having glue ear | Not having glue ear and being able to hear better |
| Temporary tympanic membrane perforation | Things about glue ear and ear infections | Not having a hole in your eardrum that only lasts for a few weeks | Not having a hole in your eardrum that lasts for a few weeks | Your child not having a hole in their eardrum that lasts for a few weeks | Not having a hole in your eardrum that lasts for a few weeks |
| Consonant production Consonant production – cleft-related speech patterns Expressive language skills |
Things about talking | Being able to say all your words clearly and grown-ups and children understanding what you say | Being able to say all your words clearly and grown-ups and children understanding what you say | Your child being able to say all their words clearly so that adults and other children can understand what they said | Being able to say all your words clearly so that adults and other children could understand what you said |
| Parent’s perspective of speech | Things about talking | How much you talk like someone without a CP | How much you talk like someone without a CP | How much your child talks like someone without a CP | How much you talked like someone without a CP |
| Receptive language skills | Things about talking | How well your parents think you are speaking | How well your parents think you are speaking | How well you think your child is speaking | How well your parents thought you were speaking |
| Speech development | Things about talking | Being able to listen and understand what other people say | Being able to listen and understand what other people say | Your child being able to listen and understand what other people say | Being able to listen and understand what other people say |
| Speech intelligibility | Things about talking | Speaking as well as other children the same age as you | Speaking as well as other children the same age as you | Your child speaking as well as other children who are the same age | Speaking as well as other children who were the same age as you |
| Speech signs of velopharyngeal insufficiency | Things about talking | Your speech not sounding different to other children | Your speech not sounding different to other children | Your child’s speech not sounding different to other children | Your speech not sounding different to other children |
| Early extrusion or blockage of VTs | Things about grommets | How often your grommets/VTs fall out or do not work | How often your grommets/VTs fall out or do not work | How often your child’s grommets/VTs fall out or do not work | How often your grommets/VTs fell out or did not work |
| Necessity to remove VTs | Things about grommets | Not needing another operation to take grommets/VTs out | Not needing another operation to take grommets/VTs out | Your child not needing another operation to take grommets/VTs out | Not needing another operation to take grommets/VTs out |
| Requirement for repeated VTs | Things about grommets | Not needing another operation to have new grommets/VTs because the old ones fell out | Not needing another operation to have new grommets/VTs because the old ones fell out | Your child not needing another operation to have new grommets/VTs because the old ones fell out | Not needing another operation to have new grommets/VTs because the old ones fell out |
| Child stress | Things about how you or your parents feel/things about how you or your child feels | How often you feel upset or angry | How often you feel upset or angry | How often your child feels tense or upset | How often you felt upset or angry |
| Parental stress | Things about how you or your parents feel/things about how you or your child feels | How often your parents feel upset or angry | How often your parents feel upset or angry | How often you feel tense or upset | How often your parents felt upset or angry |
| Parental satisfaction with treatment | Things about how well your child’s treatment has worked | How well your parents think that HAs or grommets have improved your hearing | How well your parents think that HAs or grommets have improved your hearing | How well you think that HAs or grommets have improved your child’s hearing | How well your parents thought that HAs or grommets improved your hearing |
| Side effects of treatment | Things about problems caused by treatment/things about problems caused by your child’s treatment | Not having problems, that can sometimes happen, that are caused by a treatment you have for glue ear | Not having problems, that can sometimes happen, that are caused by a treatment you have for glue ear | Your child not having problems, that can sometimes happen, that are caused by a treatment they have for glue ear | Not having problems, that can sometimes happen, that are caused by a treatment you had for glue ear |
| Upper respiratory tract infection | Things about infections in the ear, nose or mouth | Not having infections in your ear, nose or mouth | Not having infections in your ear, nose or mouth | Your child not having infections in their ear, nose or throat | Not having infections in your ear, nose or mouth |
| Child’s satisfaction with treatment | Other things | How much you think treatment has made you better | How much you think treatment has made you better | How much your child thinks that treatment has made them better | How much you think treatment made you better |
| Child’s perspective of speech | Other things | How normal you think you sound when you are talking | How normal you think you sound when you are talking | How normal your child thinks they sound when they are talking | How normal you thought you sounded when you were talking |
Parents and adults were asked to consider the appropriate question described in Table 22 and to then score each of the outcomes listed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) scale of 1–9. In the online survey the scale was presented in the format 1–9 with 1–3 labelled as ‘not that important’, 4–6 labelled as ‘important’ and 7–9 labelled as ‘really important’112 (Figure 9). Parents were also provided with an option to add anything else that they considered relevant in a free-text box.
| Group | Initial question |
|---|---|
| Parents | Think about when your child has had glue ear and how you might decide if their treatment for glue ear has worked. We would like you to look at the list below and tell us how important each thing on this list is in deciding if treatment has worked |
| Adults | Think about when you have had glue ear, as a child, and how you might decide if your treatment for glue ear worked. We would like you to look at the list below and tell us how important each thing on this list is in deciding if treatment has worked |
| Children aged 7–16 years | Think about when you have had glue ear and how you might decide if your treatment for glue ear has worked. We would like you to look at the list below and tell us how important each thing on this list is in deciding if treatment has worked. You can ask a grown-up to help you if you get stuck |
| Health professionals | What outcomes influence your management of children with CP, with, or at high risk of, OME? |
FIGURE 9.
Screen shots of online survey for children aged 7–16 years and parents.


Children aged 7–16 years were shown the same list of outcomes as parents and were asked the question shown in Table 22. However, under the recommendation of the CYPC, scoring was completed using a traffic light system where scores of 1–3 were represented by a red box labelled as ‘not that important’, scores of 4–6 as an amber box labelled as ‘important’ and scores of 7–9 as a green box labelled ‘really important’ (see Figure 9). A free-text box was also provided so that participants could add anything else they considered relevant.
The study management team discussed whether or not parents, children and adults should see a free-text box first, before the list of outcomes, as there were concerns that participants might not feel that they had a voice once the list was shown. However, feedback received from parents and children was that it would be better to have the list first as that would help them to think about things and whether or not anything was missing.
Participants
Participants of the survey for parents and children were identified using the CLAPA mailing list and social media pages with a potential reach of 4710 and 9564 respectively. There is likely to be substantial overlap with membership of multiple Facebook pages (Facebook, Inc., Cambride, MA, USA) and groups, but it was not possible to assess this. An individual e-mail was sent to all those on the CLAPA mailing list together with a reminder in their e-newsletter. A link was posted on the Facebook page which included the researcher’s name (NH) and a link together with a photograph. Examples of the newsletter and social media post are provided in Appendix 5.
Results
Although the survey was sent to a large number it was only accessed 293 times. Of this, 252 answered the initial question regarding eligibility and of the 235 eligible only 22% completed the survey. The 51 responses received comprised 35 parents, eight adults and eight children. Of the eight children four were in the 7- to 10-years age group and four were aged 11–16 years (Figure 10).
FIGURE 10.
Response rate to parent and child survey.
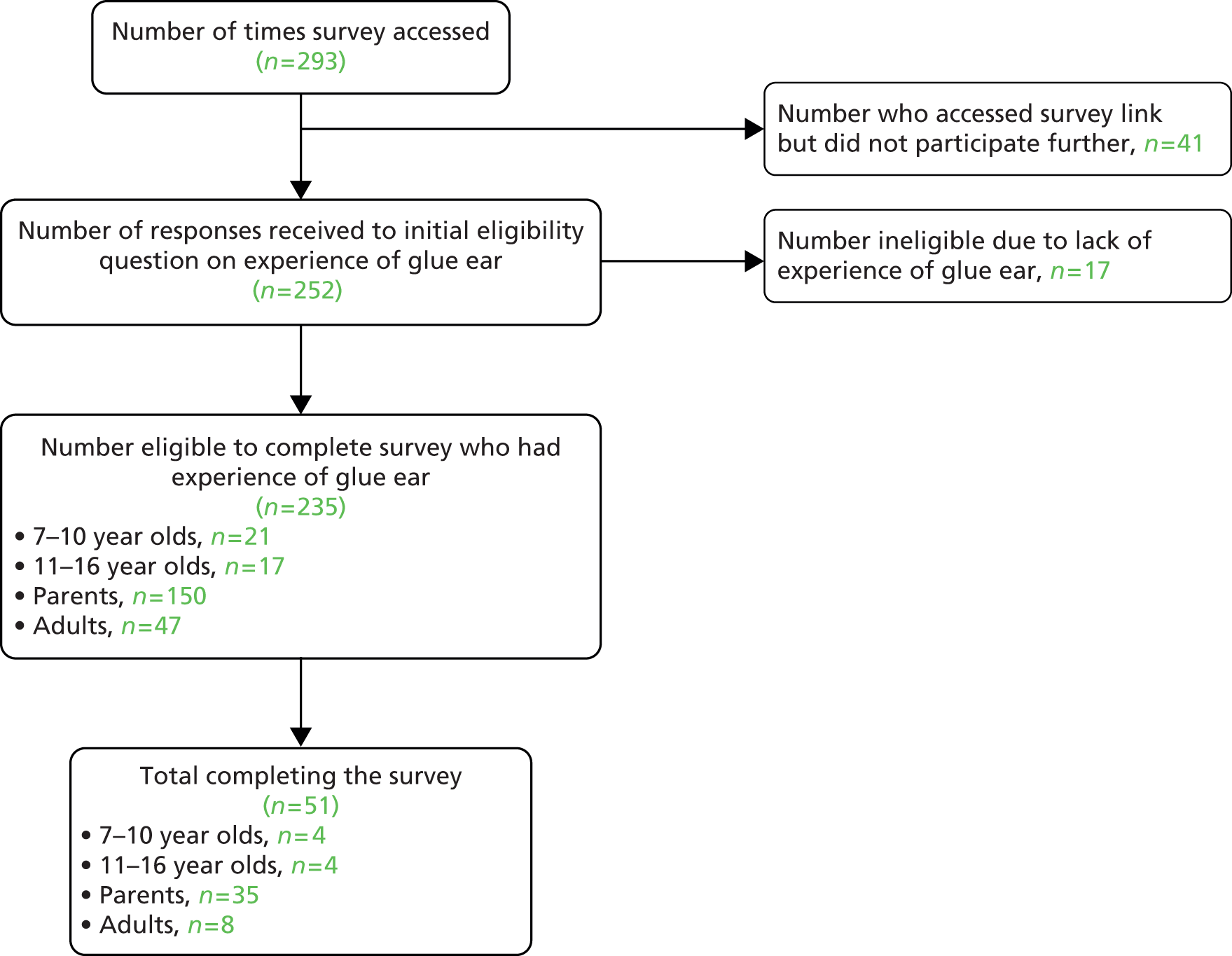
Twenty-two free-text responses were received in addition to participant scores. Four responses were from adults who had completed the survey. The adult responses suggested that they might not have scored based on reflection of having OME as a child and instead based on current issues (Table 23). This, together with the small numbers of adults participating meant that adult scores were not considered further for the COS development.
| Verbatim free-text response to ‘Is there anything else you would like to tell us that is important to you?’ | Outcomes mentioned | Notes |
|---|---|---|
| I had grommets because of my glue ear but it is only years later that I have found out that I have scars from them that I now have side effects and still hard of hearing and will need a hearing aid in the near future. | Tympanosclerosis; side effects of treatment; hearing | No new outcomes represented in free-text response |
| I’m not sure this makes sense do u want to know how important these things were to me or how much having glue ear effected these things? I would like to take part I’m 30 and still get clue ear. It effects my every day life especially though the winter. I’m miserable when I can’t hear.I’m deaf now and don’t want to go see my friends because I hate them having to repeat themselves constantly. It makes me so angry I want to punch someone or sometimes it makes me so sad I don’t want to be around anymore.It’s the worst part of my life. When I get to many colds and I can feel my ears going it’s my worst feeling ever. I think at least deaf people have a constant deafness and can get used to poor hearing . . .The only treatment I got when I was younger were grommets. They worked well but I loved the sea and couldn’t swim. Later in childhood I those swimming over grommets and ear infections. The joke was ull go surfing all weekend then not again for a few months whilst I got ear infections and my ears recovered.Now I have hearing aids when my ears are bad but they r rubbish because my hearing fluctuates and the sounds I hear are muffled still. Hearing aids just make my hearing hat louder muffled noises. | Externalising behaviour; internalising behaviour; AOM; upper respiratory tract infections; OME; chronic OME; psychosocial well-being | No new outcomes represented in free-text response. Free-text response suggests that this comment is based on current experience rather than when a child |
| More research is need in this area and your test scale is very poor in terms of its wording a Likert scale would have been more appropriate for your results analysis.I also feel that lots of people have been denied the appropriate care and attention when it comes to grommets/glue ear etc and not enough has been done to amend this. | No new outcomes represented in free-text response | |
| This is the first time I have heard of this term today. I had tubes inserted as a child and removed. Now as a 50 year old adult I have major hearing loss. | No new outcomes represented in free-text response. Free-text response suggests that this comment is based on current experience rather than when a child |
Free-text responses from children aged 7–10 years and 11–16 years did not mention any new outcomes but did reiterate some outcomes already in the outcomes list (Table 24). There were 15 free-text responses provided by parents and one of these represented a new outcome that was not included in the original outcomes list (Table 25). The new outcome ‘hyperacusis’, sensitivity to loud noises, was included in round 3 of the Delphi survey for health professionals.
| Child’s age (years) | Verbatim free-text response to ‘Is there anything else you would like to tell us that important to you?’ | Outcomes mentioned | Notes |
|---|---|---|---|
| 7–10 | If I keep getting ear infections or if I need to get another operation. | AOM; requirement for repeated VT (could also be necessity to remove VTs as both require another operation); cholesteatoma | Cholesteatoma would also require operation therefore added. No new outcomes represented in free-text response |
| 11–16 | Think that it should be know straight away whether an operation is needed for glue ear instead of having loads of operations, the grommets need to last longer and make sure they work so you don’t need an operation every 6 months . . . | Necessity to remove VTs; requirement for repeated VTs; early extrusion | No new outcomes represented in free-text response |
| 11–16 | Unable to complete questionnaire as my glue ear of which i still suffer has been has been controlled with a hearing aid and is reliant on myself and my mum to decide if it needs to be worn and is also reliant on my mum chasing appointments which are few and far between. My mum says it is the one part of the cleft palate service that is lacking. | No new outcomes represented in free-text response |
| Verbatim free-text response to ‘Is there anything else you would like to tell us that important to you?’ | Outcomes mentioned | Notes |
|---|---|---|
| Glue ear significantly affects the development of a child at crucial ages. School teachers do not understand intermittent hearing loss and are usually not sympathetic or helpful to a child with glue ear (due to lack of understanding of the nature of the condition) and often label a child as ‘lazy’ when they are inattentive. Glue ear significantly affcts a child’s behaviour. | Internalising behaviour; externalising behaviour; listening skills; developmental progress | No new outcomes represented in free-text response |
| Grommets being put in during the Cleft surgery meant the decision was a no brainer. It is hard to gauge how well they worked, but not hard to remember how many ear infections he had, whilst they were in. Now they are out, and he still has glue ear, our choices have been given on the basis of his hearing, not any other issues glue ear may cause (no dizziness, and his speech is very good) – as the decision is hearing versus ear infections, short term hearing aids would SEEM to be the best bet for us (it’s about how he hears and how that affects his making friends/learning, not what that looks like. What I worry about, having completed this survey, is that we don’t know the true COST of not having grommets (do they improve the condition or just the effects?) | AOM; hearing; psychosocial well-being; academic achievement; cognitive development; intelligence; literacy; phonologial memory; psychosocial development; OME | No new outcomes represented in free-text response |
| Having the treatment explained verbally AND visually to the child and parents in a way in which they will understand. Depending on the age of the child, have a folder available for them to take home with the details and/or photo’s of key members of staff, the ward/medical setting where they will be staying, including pictures of the theatre and anaesthetic rooms where the ops take place, before they are admitted to hospital. And the chance to visit the ward during the pre-op assessment for those children who need it. | No outcomes noted | Information needs only. No new outcomes represented in free-text response |
| I have had swim plugs made for my daughter who had long term grommets fitted after short term grommets fell out. If only we’d been made more aware of how crucial it is to prevent water from entering the ear canal after this kind of treatment we could have spared her countless years of ear infections! She uses them in the shower too. I had to go to a private hospital to have them made as she wasn’t offered them routinely on the NHS. I forgot the plugs for one swimming lesson and she got a horrible bleeding infection that lasted 6 weeks which proved how essential the plugs are. | AOM; early extrusion of VTs | No new outcomes represented in free-text response |
| I have put everything as being very important, because to me nothing is more important than the health and happiness of my child, but perhaps the survey would gather better data by asking How Successful treatment has been and how often issues and arrise and the severity of these issues. My child has constant glue ear but in consultation with her doctors we decided not to have further grommit surgery as the first surgery was unsuccessful and caused my child to become extremely unwell due to breathing problems and affects of anaesthetics. There is no mention of bone conductor or other hearing aids. My daughter uses this attached to glasses and we are happy that it is having a clear beneficial affect. | COM; OME; requirement for repeated VTs; hearing; side effects of treatment | No new outcomes represented in free-text response |
| I think early treatment for glue ear is better my daughter did not have grommets fitted until she was 5. After having the grommets put in there was a very noticeable change to her hearing and speech. | Hearing; consonant production – cleft-related speech patterns; expressive language skills; receptive language skills; speech development; speech intelligibility; speech signs of velopharyngeal insufficiency; parent’s perspective of speech; child’s perspective of speech | No new outcomes represented in free-text response |
| I wish that he had been screened before he started school. His hearing probs not identified until he had started school and by that time he had started to drift off in lessons because he could not hear properly, also had constant colds and stuffy noses. | Listening skills; hearing; upper respiratory tract infection; rhinitis | No new outcomes represented in free-text response |
| last time my son had glue ear, the volume, and consequently tension levels in our house rose considerably. My son struggles to sleep through the night when he has glue ear and feels emotional as he is unable to fully participate in many social activities. We opted for a 4th set of grommets and it was literally like flicking a switch – the atmosphere in our home changed almost immediately. We appreciate that should the glue ear re-occur, we will have to treat the subsequent hearing loss with hearing aids but appreciated as parents being given the option of either hearing aids or grommets last time around as this seems to help all aspects of the glue ear rather than just the hearing issue. | Parental stress; child stress; psychosocial well-being; internalising/externalising behaviour | No new outcomes represented in free-text response |
| My child is 5 years old and started school last September. He has a cleft lip and pallet. Since having Grommits put in he has more confidence and has come out off he’s shell. In fact he never stop’s talking. We are pleased with results. Thank you | Psychosocial well-being | No new outcomes represented in free-text response |
| My daughter has had hearing aids (short time) and gromits. I am astounded at the differance gromits have made to her. Her behaviour has totally changed, gone is my clingy girl. Now she runs off in playgroup by herself to play with others. And her speech has really come on. I am angry therefore that I had to really fight to get her gromits. Hearing aids were strongly pushed. I have seen for myself how great gromits have been for her so will gladly do battle again on her behalf to get more when the current ones come out. However my daughter has many medical issues and this is something that I would really prefer not to have to worry about. | Psychosocial well-being; internalising and externalising behaviour; consonant production – cleft-related speech patterns; expressive language skills; receptive language skills; speech development; speech intelligibility; speech signs of velopharyngeal insufficiency; parent’s perspective of speech; child’s perspective of speech | No new outcomes represented in free-text response |
| My son had grommets fitted and by the time we went to a cleft clinic just over a week later one was out and sitting on the ear canal – the grommets made no discernible difference. We then decided to have hearing aids and they have transformed our lives. I didn’t realise how much my son could not hear until he had hearing aids and I used to get very angry at his seemingly bad behaviour – as a family we are happier and more content. The audiologist and consultant had a ‘wait and see’ approach to my son’s hearing, to see if the glue ear righted itself. In hindsight I think that condemned my son to 3 lost years where he couldn’t hear properly when it was easily fixable. When we did ask the consultant for hearing aids we were left in no doubt that he was not happy at this choice, which I find strange. | Early extrusion or blockage of VTs; externalising behaviour; parental stress; child stress; hearing | No new outcomes represented in free-text response |
| Professionals need to be aware that repeated grommet insertion is not always the best thing for a cleft child. The cause of the glue ear can be different than in a non-cleft child. My daughter was under the care of a normal ENT Surgeon and had one set of grommets, which fell out. She was all set to have another set inserted, but was then moved to an ENT surgeon who specialised in cleft children. He was reluctant to carry out repeated grommet operations as each operation carries the risks associated with a general anaesthetic/surgery, and the risk of the ear drum perforating. His preference is to fit the child with hearing aids – they are non-invasive and should the child grow out of glue ear you simply stop using the hearing aid. | Early extrusion of VTs; persistent/temporary tympanic membrane perforation; side effects of treatment. | No new outcomes represented in free-text response |
| The biggest measure of success for us as a family, was my daughter asking what the funny noise was in the supermarket, it was the ‘beeping’ of the checkout scanning items, she had never heard it before her grommets were inserted – clear indication of a great improvement in hearing. | Hearing | No new outcomes represented in free-text response |
| The length of time it has taken to get to the stage where the fitting of grommets was deemed required was 12 months which I find unacceptable. Then the surgeon hadn’t even seen the last test results on the day of the fitting and refused to do one ear absolute joke, frustrating for myself and partner, distressing for my son who had already had a pre op at this point, some of the care we have received has been brilliant but on others has bordered on the negligent | No new outcomes represented in free-text response | |
| There is also the problem of the variation in hearing levels that children with glue ear can have – often on a daily basis, which means that hearing aids aren’t always at the right level. Another issue is that children with glue ear are treated as deaf children rather than ones with fluctuating hearing and with other issues associated with their hearing, such as earache, sensitivity to loud noise and subject to hearing popping sounds. Also once hearing levels improve (if they do) to an extent where hearing aids are not needed, then no further treatment is considered, even though they still experience the pain, popping sounds, problems with loud noise and variation in hearing levels related to glue ear. There is no firm opinion as to when/if to have grommets fitted and yet once hearing rises just above the 20 dB level grommets are no longer an option even though glue ear is still causing a problem. | AOM; eustachian tube function; hearing; otalgia | New outcome identified. Hyperacusis – sensitivity to loud noises |
Consensus matrix: parents and children
The results of the one-off survey of parents and children were reviewed against the definition of consensus agreed prior to the start of the study (see Table 28). 10 Using this definition parents and children had reached consensus in 26 and 9 outcomes respectively (Table 26).
| Original outcome | Outcome scored by parents/children | Parents | Children |
|---|---|---|---|
| Internalising behaviour | How lonely you feel, feeling like an outsider | ||
| Externalising behaviour | How angry you are towards others | ||
| Atelectasis | Not having problems inside your ear caused by having lots of ear infections over a long time (more than 3 months) | ✓ | ✓ |
| Persistent tympanic membrane retraction | |||
| Tympanosclerosis | |||
| Cholesteatoma | Not having problems inside your ear caused by bad skin growing behind your ear drum | ||
| COM | Not having problems inside your ear caused by having glue ear for a long time (more than 3 months) | ✓ | ✓ |
| Persistent tympanic membrane perforation | Not having problems inside your ear caused by having a hole in your ear drum for a long time (more than 3 months) | ✓ | |
| Academic achievement | How well you are doing at school | ✓ | |
| Cognitive development | |||
| Developmental progress | |||
| Intelligence | |||
| Literacy | |||
| Phonological memory | |||
| Psychosocial development | How well you are learning to make friends and speak to new people | ✓ | ✓ |
| Hearing | How well you can hear | ✓ | ✓ |
| Otalgia | How painful your ear is | ✓ | |
| Otorrhoea | Not having infected liquid leaking out of your ear | ||
| Tinnitus | How much you hear buzzing or ringing noises | ✓ | |
| Vertigo | How dizzy you feel | ✓ | |
| Eustachian tube function | How well a special tube in your ear works. If this tube does not work properly you might hear popping and crackling noises | ✓ | |
| Stapedial reflex | How well your ear works when it hears a loud noise | ✓ | |
| Nasal obstruction | How well you can breathe through your nose | ||
| Rhinitis | How much your nose feels runny or stuffy | ||
| AOM | Not having ear infections | ✓ | |
| OME | Not having glue ear and being able to hear better | ✓ | ✓ |
| Temporary tympanic membrane perforation | Not having a hole in your eardrum that only lasts for a few weeks | ||
| Consonant production | Being able to say all your words clearly and grownups and children understanding what you say | ✓ | |
| Consonant production – cleft-related speech patterns | |||
| Expressive language skills | |||
| Parent’s perspective of speech | How much you talk like someone without a CP | ✓ | |
| Receptive language skills | How well your parents think you are speaking | ✓ | ✓ |
| Speech development | Being able to listen and understand what other people say | ✓ | |
| Speech intelligibility | Speaking as well as other children the same age as you | ||
| Speech signs of velopharyngeal insufficiency | Your speech not sounding different to other children | ✓ | |
| Early extrusion or blockage of VTs | How often your grommets/VTs fall out or do not work | ✓ | |
| Necessity to remove VTs | Not needing another operation to take grommets/VTs out | ✓ | ✓ |
| Requirement for repeated VTs | Not needing another operation to have new grommets/VTs because the old ones fell out | ✓ | ✓ |
| Child stress | How often you feel upset or angry | ✓ | |
| Parental stress | How often your parents feel upset or angry | ||
| Parental satisfaction with treatment | How well your parents think that HAs or grommets have improved your hearing | ✓ | |
| Side effects of treatment | Not having problems, that can sometimes happen, that are caused by a treatment you have for glue ear | ✓ | |
| Upper respiratory tract infection | Not having infections in your ear, nose or mouth | ✓ | |
| Child’s satisfaction with treatment | How much you think treatment has made you better | ✓ | |
| Child’s perspective of speech | How normal you think you sound when you are talking | ✓ |
Identification of outcomes of importance to health professionals
Methods
To investigate outcomes of importance to clinicians a Delphi approach was adopted so that the anonymous opinions of the participant can be obtained in a way that gives equal influence to all who participate, and avoids an individual participant being overtly influenced by the opinions of any other participant. 113,114 An overview of the Delphi exercise is given in Figure 11. The method was prespecified at the start of the study and the protocol published. 10
FIGURE 11.
Overview of Delphi process. ID, identification.
Participants: health professionals
The Delphi study was conducted with the clinical teams from all UK cleft centres. Health professionals were selected from only UK centres due to time and cost constraints. The clinical lead at each of the UK cleft centres was asked to provide the names and roles of all members of the cleft team. The clinical roles considered key for completion of the Delphi were audiologists, ENT surgeons, speech and language therapists and specialist nurses. Other health professionals (e.g. paediatricians, clinical psychologists, clinical geneticists and cleft surgeons) were identified after consultation with the clinical director or cleft service co-ordinator at each cleft centre and were invited accordingly.
All health professionals, identified through correspondence with the clinical lead at UK sites, were contacted directly by e-mail with information about the mOMEnt study and instructions on how they could participate (see Appendix 6). A summary of invited health professionals is given in Table 27. All participants were asked to confirm their clinical role and cleft centre on registration for the mOMEnt Delphi survey.
| Cleft centre number | Cleft surgeon, n | ENT surgeon, n | Geneticist, n | Specialist cleft nurse, n | Speech and language therapist, n | Paediatrician, n | Psychologist, n | Audiologist, n | Total at site, N |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 3 | 1 | 4 | 4 | 2 | 3 | 2 | 23 |
| 2 | 3 | 1 | 0 | 1 | 6 | 1 | 1 | 0 | 13 |
| 3 | 1 | 0 | 0 | 2 | 10 | 0 | 0 | 1 | 14 |
| 4 | 1 | 2 | 1 | 4 | 6 | 0 | 3 | 0 | 17 |
| 5 | 1 | 1 | 2 | 0 | 2 | 0 | 1 | 1 | 8 |
| 6 | 2 | 0 | 2 | 4 | 5 | 0 | 3 | 3 | 19 |
| 7 | 2 | 0 | 2 | 3 | 4 | 2 | 4 | 2 | 19 |
| 8 | 1 | 0 | 1 | 2 | 2 | 0 | 0 | 1 | 7 |
| 9 | 2 | 2 | 1 | 3 | 2 | 0 | 1 | 1 | 12 |
| 10 | 5 | 0 | 0 | 6 | 6 | 0 | 2 | 0 | 19 |
| 11 | 2 | 1 | 1 | 3 | 6 | 2 | 2 | 0 | 17 |
| 12 | 2 | 0 | 1 | 2 | 3 | 1 | 2 | 1 | 12 |
| 13 | 4 | 2 | 1 | 5 | 10 | 1 | 4 | 1 | 28 |
| 15 | 2 | 1 | 0 | 5 | 3 | 0 | 0 | 1 | 12 |
| 16 | 3 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 8 |
| Total, n | 35 | 13 | 14 | 46 | 69 | 9 | 27 | 15 | 228 |
| Percentage representation | 15 | 6 | 6 | 20 | 30 | 4 | 12 | 7 | 100 |
At the beginning of the exercise participants were reminded of the importance of completing the entire Delphi process. Reminder e-mails were sent to aid completion of each round together with follow-up telephone calls to encourage completion. Participants who took part were given a unique identifier to allow tracking of attrition at each round. All data was stored against the unique identifier and participants were not able to identify other participants or individual responses.
Delphi survey: round 1
In the first round of the online questionnaire participants were asked to provide their name and e-mail address together with their clinical role and cleft centre. This information was stored in a separate database to the scores and used to provide the respondent with a unique identifier. The unique identifier was used to allow identification of individuals completing all rounds of the Delphi exercise and to track attrition between rounds.
In round 1 participants scored the list of outcomes generated from the systematic review of the literature and through SAG and SSC contributions (see Table 19). Outcomes were ordered alphabetically, to avoid potential weighting of outcomes caused by the order in which they are displayed, and this was stated in the introductory text. Participants were asked to consider the question ‘What outcomes influence your management of children with CP, with, or at high risk of, OME?’ and to then score each of the outcomes listed using the GRADE scale of 1–9. In the Delphi exercise the scale was presented in the format 1–9 with 1–3 labelled as ‘not important’, 4–6 labelled as ‘important but not critical’ and 7–9 labelled as ‘critical’. Participants were also provided with an option to add additional outcomes that they considered relevant together with a score for each outcome added (Figure 12).
FIGURE 12.
Example of online system for participants to score each outcome and to add additional outcomes they consider relevant.
Analysis of round 1
Additional outcomes listed by participants were reviewed and coded by two members of the study team (NH and IAB) to ensure they represent new outcomes. If there was uncertainty the SMG were consulted and the SAG if appropriate. For each outcome, the number of participants who scored the outcome and the distribution of scores (as percentage that have scored each outcome) were summarised by the stakeholder group. All outcomes were carried forward to round 2.
Response rate in round 1
The number of participants in each stakeholder group who responded to round 1 were assessed following round 1 closure. Results will be presented as:
-
total number of registrations
-
breakdown of respondents who have completed the survey and their inclusion in the initial e-mail invitation
-
total number of respondents who completed the round
-
total number of respondents in each stakeholder group
-
percentage of respondents compared with potential respondents as identified from the information provided by clinical leads
-
percentage of respondents from other sources (not included in original e-mail invitation).
Continuation to round 2 was considered based on the response to round 1. If a low number of responders (< 10) was observed for one or more stakeholder groups, the Delphi protocol for future rounds was reviewed and revised. Where there is only one stakeholder group with a small number of respondents (potentially due to the sample available from clinical teams) then consideration was given to grouping with another stakeholder group. This was actioned in consultation with the SAG to ensure appropriateness of grouping.
Delphi survey: round 2
In round 2, each participant was presented with the number of respondents and distribution of scores for each outcome for their particular stakeholder group. Participants were shown their score from round 1, asked to consider responses from the other members of their stakeholder group and asked to rescore the outcome.
Any changes to scores in light of the stakeholder group or overall response were documented. Those who did not take part in round 1 and did not provide a score were not invited to participate in round 2 or participate further in the Delphi.
Analysis of round 2
The total number of participants invited to take part in round 2 was recorded. For each outcome, the number of participants who have scored the outcome and the distribution of scores was summarised by stakeholder group. All outcomes were carried forward to round 3.
Delphi round 3
In round 3, participants were shown the distribution of scores, for each outcome, for all stakeholder groups separately as well as a summary of the results from the survey of parents and children. Participants were asked to rescore all outcomes.
Analysis round 3
The total number of participants invited to take part in round 3 was recorded. For each outcome, the number of participants who scored the outcome and the distribution of scores was summarised together with the number of participants who scored the outcome in all rounds. Results of the stakeholder group response were compared with the whole group response and the percentage agreement used to determine the structure and focus of the final consensus meeting. Each outcome was classified as ‘consensus in’, ‘consensus out’ or ‘no consensus’ according to the classifications in Table 28.
| Consensus classification | Description | Definition |
|---|---|---|
| Consensus in | Consensus that outcome should be included in the COS | ≥ 70% participants scoring as 7–9 and < 15% participants scoring as 1–3 |
| Consensus out | Consensus that outcome should not be included in the COS | ≥ 70% participants scoring as 1–3 and < 15% of participants scoring as 7–9 |
| No consensus | Uncertainty about importance of outcome | Anything else |
Consensus meeting
The final phase of the study was a face-to-face consensus meeting with participants who had completed either the health professional Delphi, parent and child survey or the qualitative study and members of the SAG. Additional ethical approval was required to invite parents who participated in interviews to attend the consensus meeting. This was obtained from the Greater Manchester East Research Ethics Committee (reference 11/NW/0586).
The responses from round 3 of the health professionals Delphi and the parent and child survey were used to inform the structure and content of the consensus meeting.
Definition of consensus
The classification described in Table 28 was used to determine if consensus was reached.
For consensus to have been reached that an outcome should be in the COS, agreement by the vast majority regarding the critical importance of the outcome, with only a small minority considering it to be not important at all, is required. Conversely, for consensus to have been reached that an outcome should not be in the COS, agreement by the vast majority regarding the lack of importance of the outcome, with only a small minority considering it to be critically important, is required.
Although the choice of thresholds is inevitably somewhat subjective, the specification of the definition of consensus upfront aimed to reduce the chance of consensus being defined post hoc in such a way as to bias the results towards the beliefs of the research team.
Statistical considerations
Sample size
There is currently no standard method for sample size calculation in Delphi processes thus a pragmatic approach was taken. The number of participants in the present study is limited by the composition and number of UK cleft centres. Efforts were made to maximise the response rate across centres and stakeholder groups.
Results
Participation rates
The rate of completion was carefully monitored in each round against the number invited/eligible to participate. Regular reminder e-mails were sent which included information on the round together with the reason why a response was needed (see Appendix 6). In the third round a non-monetary incentive was used, e-mails were sent by a female member of staff and an image was included with the e-mail (as these methods have been previously reported to improve response rate). 115 In rounds 2 and 3 telephone calls were also made to participants and personalised e-mails were sent by the study co-ordinator in an effort to increase the response rate. The overall response rate per round is given in Table 29, the response rates by stakeholder group are discussed in the results of each round.
| Round | Date open | Date closed | Number of days round available (weekdays) | Number of participants eligible to complete | Number of participants completing round (% of total eligible) |
|---|---|---|---|---|---|
| 1 | 22 November 2012 | 29 April 2013 | 158 (113) | 228 | 104 (46) |
| 2 | 13 June 2013 | 15 November 2013 | 155 (112) | 99 | 85 (86) |
| 3 | 9 December 2013 | 13 February 2014 | 66 (49) | 81 | 73 (81) |
Round 1: health professionals
Of the 228 participants invited to round 1 a total of 104 completed the round with four of these participants providing partial scores only. Those who provided partial scores but scored > 50% of outcomes were invited to take part in round 2. The breakdown of participants by stakeholder group is shown in Table 30. After round 1 the free-text responses, from two of the three geneticists who had completed the round (Table 31), indicated that clinical geneticists were not directly involved in the care of children with OME and so were not invited to further rounds. In addition, of the three paediatricians who took part, all had a speciality in audiology, consequently for future rounds their scores were combined with audiologists into a new group ‘audiologists and audiological physicians’ as agreed by the SAG.
| Stakeholder group | Cleft surgeon | ENT surgeon | Geneticist | Specialist cleft nurse | Speech and language therapist | Paediatrician | Psychologist | Audiologist | Total |
|---|---|---|---|---|---|---|---|---|---|
| Number invited to participate | 35 | 13 | 14 | 46 | 69 | 9 | 27 | 15 | 228 |
| Number of respondents | 15a | 9b | 3 | 18c | 36d | 3 | 13 | 7 | 104 |
| Percentage completing round 1 | 43 | 69 | 21 | 39 | 52 | 33 | 48 | 46 | 46 |
| Verbatim comment | Score | Stakeholder group |
|---|---|---|
| I do not think many of these outcomes are particularly relevant to a clinical geneticist | 0 | Clinical geneticist |
| I am fairly new to the role so still have so much to learn therefore my answers are based on my previous experience and what I have learnt so far therefore am not sure my responses will be reflective of specialist nurses as a whole. | 0 | Specialist cleft nurse |
| As a Clinical Psychologist, this study is really hard to respond to! | 0 | Psychologist |
| As a clinical geneticist my involvement is more in the diagnostic proccess rather than the decisions regarding surgery so I have been unable to comment on many of the survey questions. | 0 | Clinical geneticist |
| I found it difficult to complete this survey as it referred to ‘your’ management of children wth otitis media. There were many factors I gueesed were probably important but as an SLT it would not be my role to manage these. So I put unable to score for those | 0 | Speech and language therapist |
| Duplication of otological findings. Some of the questions used are too broad | 0 | ENT surgeon |
| found it difficult to fill in – unclear how to rate it. | 0 | Speech and language therapist |
| This is just a comment regarding signs of velopharyngeal incompetence/insufficiency – I do not think that this is an outcome that is indicative of the management of OME, but it may be correlated with and possibly contributory to the development of OME and therefore should be measured. | 8 | Cleft surgeon |
Free-text responses provided in round 1
Eighteen free-text responses were provided by health professionals in round 1, all of which were reviewed by the SMG and SAG for classification. Eight responses represented a comment only with no associated score (see Table 31), two related to the use of HAs and were considered outside of the scope of the study (Table 32), the remaining eight described outcomes (Table 33). Of the eight potential outcomes two related to attention and behaviour, these outcomes are included in the broad domains of internalising and externalising behaviour and so were not carried forward. One participant listed both attention and listening, listening skills was considered to be a separate outcome and was taken forward to round 2. Two participants listed outcomes related to psychosocial well-being and this also represented a new outcome taken forward to round 2. Two responses were received about parental involvement and patient willingness to participate in speech activities, these were not considered to represent an outcome of treatment.
| Verbatim comment | Score | Stakeholder group | Resulting actions/decision |
|---|---|---|---|
| Use of hearing aid | 6 | Audiologist | SMG and SAG agreed that this was outside of the scope of the current study which was for surgical interventions |
| Outcomes related to hearing aids | 9 | Cleft surgeon | SMG and SAG agreed that this was outside of the scope of the current study which was for surgical interventions |
| Verbatim comment | Score | Stakeholder group | Resulting actions/decision |
|---|---|---|---|
| Attention/hyperactivity levels | 8 | Psychologist | Attention problems and ADHD are covered under the Child Behaviour Checklist as one of the domains categorised into higher domain of internalising/externalising activity |
| Attention and listening behaviour/skills | 8 | Speech and language therapist | Attention problems and ADHD are covered under the Child Behaviour Checklist as one of the domains categorised into higher domain of internalising/externalising activity Listening skills considered to represent a new outcome and added to the outcomes list for scoring in round 2 |
| Self-esteem/self-concept/social confidence | 9 | Psychologist | After discussion with the SAG it was agreed to include an additional outcome in round 2 ‘psychosocial well-being’ |
| Functional measure of how the patient is doing, e.g. making friends, chatting to peers, speaking on the phone etc. | 9 | Speech and language therapist | After discussion with the SAG it was agreed to include an additional outcome in round 2 ‘psychosocial well-being’ |
| Prevention of otis media – affects of breast feeding/early palate surgery/nasal regurgitation when eating and drinking | 7 | Specialist cleft nurse | After discussion with the SAG this was not considered to represent an outcome related to treatment of OME |
| Auditory responsiveness to speech stimuli in classroom/at home/in speech therapy | 9 | Speech and language therapist | Auditory responsiveness was considered to be part of ‘listening skills’ which is considered to represent a new outcome and was added to the outcomes list for scoring in round 2 |
| Willingness to participate in speech activities | 7 | Speech and language therapist | After discussion with the SAG this was not considered to represent an outcome related to treatment of OME |
| Parental involvement (doing activities/exercises at home) | 8 | Speech and language therapist | After discussion with the SAG this was not considered to represent an outcome related to treatment of OME |
Following completion of round 1 the scores were compared with the definition of consensus to determine which stakeholder groups had reached the definition of ‘consensus in’. The number of outcomes for which ‘consensus in’ was reached varied across stakeholder groups. ENT surgeons and audiologists/audiological physicians had reached consensus for the fewest outcomes (n = 6), whereas specialist cleft nurses considered 33 outcomes for inclusion in the COS.
The outcome with the most stakeholder groups (five out of six) reaching ‘consensus in’ after round 1 was ‘hearing’.
Details of the stakeholder groups reaching consensus in for each outcome are shown in Table 34.
| Outcome | Cleft surgeon | ENT surgeon | Specialist cleft nurse | Speech and language therapist | Psychologist | Audiologist/audiological physician |
|---|---|---|---|---|---|---|
| Internalising behaviour | ✓ | ✓ | ||||
| Externalising behaviour | ✓ | ✓ | ||||
| Atelectasis | ||||||
| Cholesteatoma | ✓ | ✓ | ✓ | |||
| COM | ✓ | ✓ | ✓ | |||
| Persistent tympanic membrane perforation | ✓ | ✓ | ||||
| Persistent tympanic membrane retraction | ✓ | ✓ | ||||
| Tympanosclerosis | ✓ | ✓ | ||||
| Academic achievement | ✓ | |||||
| Cognitive development | ✓ | |||||
| Developmental progress | ✓ | ✓ | ✓ | |||
| Intelligence | ||||||
| Literacy | ✓ | |||||
| Phonological memory | ||||||
| Psychosocial development | ✓ | |||||
| Hearing | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Otalgia | ||||||
| Otorrhoea | ✓ | |||||
| Tinnitus | ||||||
| Vertigo | ✓ | |||||
| Eustachian tube function | ✓ | ✓ | ||||
| Stapedial reflex | ✓ | |||||
| Nasal obstruction | ||||||
| Rhinitis | ||||||
| AOM | ✓ | ✓ | ✓ | |||
| OME | ✓ | ✓ | ✓ | ✓ | ||
| Temporary tympanic membrane perforation | ✓ | ✓ | ||||
| Consonant production | ✓ | ✓ | ||||
| Consonant production – cleft-related speech patterns | ✓ | ✓ | ||||
| Expressive language skills | ✓ | ✓ | ||||
| Parent’s perspective of speech | ✓ | ✓ | ||||
| Receptive language skills | ✓ | ✓ | ||||
| Speech development | ✓ | ✓ | ✓ | |||
| Speech intelligibility | ✓ | ✓ | ✓ | |||
| Speech signs of velopharyngeal insufficiency | ✓ | ✓ | ||||
| Early extrusion or blockage of VTs | ✓ | ✓ | ||||
| Necessity to remove VTs | ✓ | ✓ | ||||
| Requirement for repeated VTs | ✓ | ✓ | ✓ | |||
| Child stress | ✓ | ✓ | ||||
| Parental stress | ✓ | ✓ | ||||
| Parental satisfaction with treatment | ✓ | ✓ | ||||
| Side effects of treatment | ✓ | ✓ | ✓ | |||
| Upper respiratory tract infection | ||||||
| Child’s satisfaction with treatment | ✓ | ✓ | ||||
| Child’s perspective of speech | ✓ | ✓ | ✓ |
Round 2: health professionals
In round 2 a total of 47 outcomes, representing the original list of 45 plus the addition of two outcomes identified by health professionals’ free-text responses in round 1, were scored by participants. Participants were shown their own score in round 1 together with the percentage of participants giving each score for their stakeholder group (Figure 13). A total of 85 responses were received in round 2 (86% of those completing round 1). The breakdown of participants by stakeholder group is shown in Table 35. After round 1 two participants, both speech and language therapists, went on maternity leave and so did not take part in future rounds of the study.
FIGURE 13.
Example of presentation of round 1 stakeholder group and individual scores.

| Stakeholder group | Cleft surgeon | ENT surgeon | Geneticist | Specialist cleft nurse | Speech and language therapist | Paediatrician | Psychologist | Audiologist/audiological physician | Total |
|---|---|---|---|---|---|---|---|---|---|
| Number invited to participate | 15 | 9 | 0a | 17b | 34c | 0d | 13 | 10 | 98 |
| Number of respondents | 13e | 9 | n/a | 14 | 28f | n/a | 12 | 9g | 85 |
| Percentage completing round 1 | 87 | 100 | n/a | 82 | 82 | n/a | 92 | 90 | 86 |
Three of the 85 participants completing the round provided partial scores only. The percentage of outcomes scored ranged from 21% to 62%. In this round all participants who provided scores were carried forward to round 3 irrespective of the percentage of outcomes scored.
Attrition bias in round 2
To identify whether or not attrition in round 2 would introduce bias, the scores from round 1 were compared with those completing both rounds (n = 85) versus those completing round 1 only (n = 14). Figure 14 shows the distribution of the average score across all 47 outcomes by stakeholder group. The results of those who did not complete round 2 did not represent extreme views suggesting that bias had not been introduced through attrition between round 1 and 2.
FIGURE 14.
Round 1 average scores across all outcomes by stakeholder group. Shaded bars represent those who provided scores in round 1 only, open bars represent those scoring in both rounds 1 and 2. (a) Cleft surgeon; (b) ENT surgeon; (c) specialist care nurse; (d) speech and language therapist; (e) psychologist; and (f) audiologist.


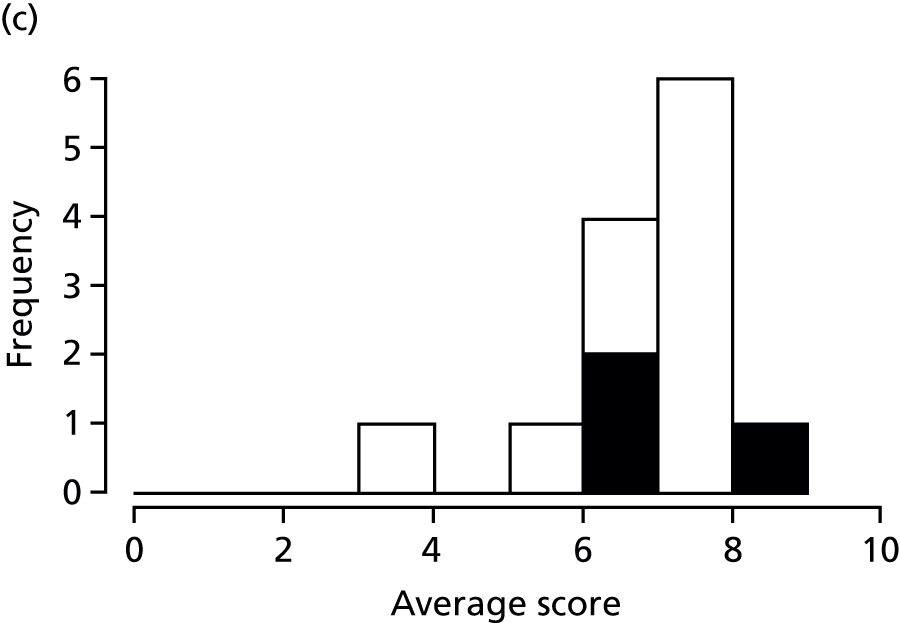
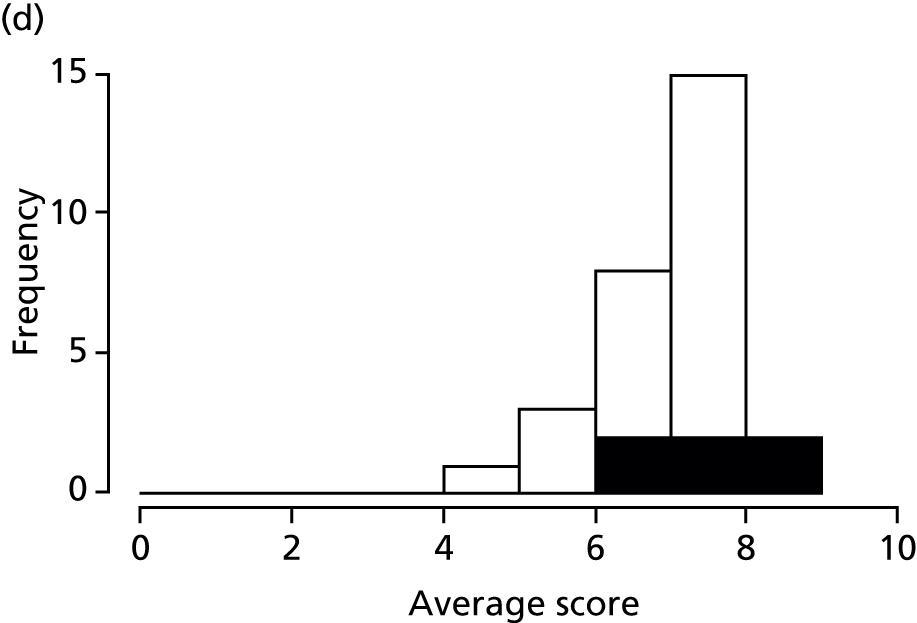
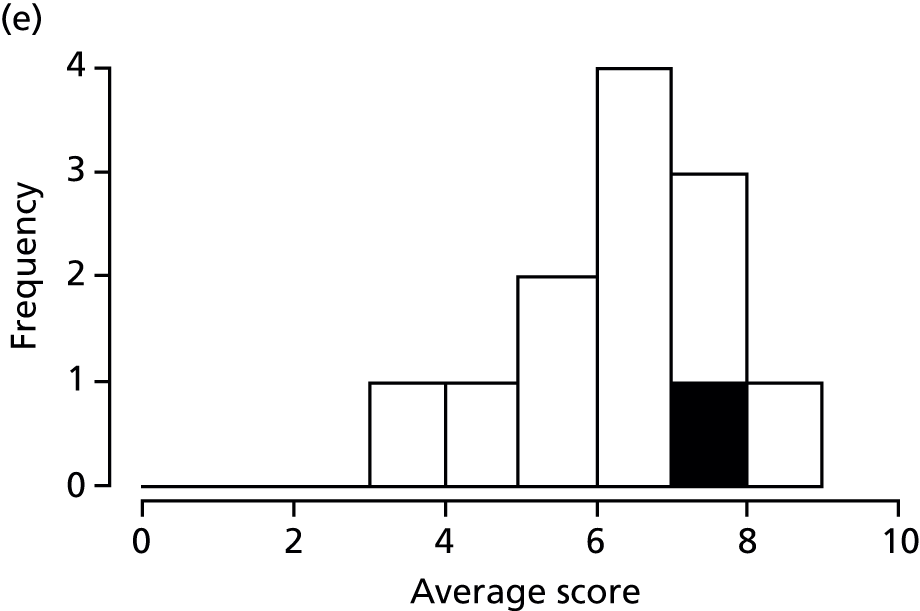
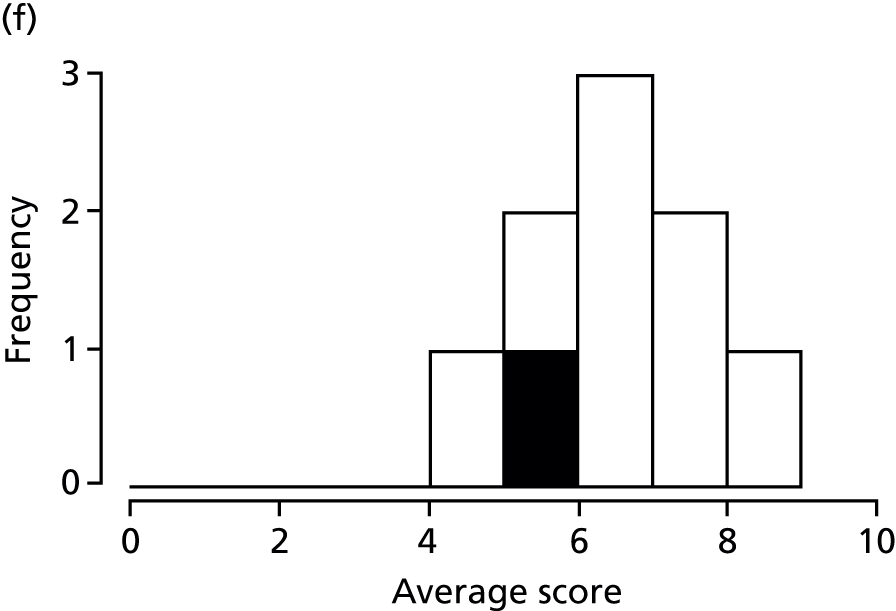
Changes in score between rounds 1 and 2
In round 2 participants were shown the results for their stakeholder group and asked to rescore each outcome. Participants were informed that they could change their score or keep it the same as their score in round 1. Six participants (7%) did not change any scores between rounds 1 and 2, whereas two participants (2%) changed between 80% and 100% of their scores (Figure 15).
FIGURE 15.
Percentage of scores changed between rounds 1 and 2 after viewing the results by stakeholder group.

Consensus matrix
The scores in round 2 were again compared against the definition of consensus to determine which stakeholder groups had reached the definition of ‘consensus in’ (Table 36).
| Outcome | Cleft surgeon | ENT surgeon | Specialist cleft nurse | Speech and language therapist | Psychologist | Audiologist/audiological physician |
|---|---|---|---|---|---|---|
| Internalising behaviour | ✓ | ✓ | ||||
| Externalising behaviour | ✓ | ✓ | ||||
| Atelectasis | ✓ | ✓ | ||||
| Cholesteatoma | ✓ | ✓ | ✓ | ✓ | ✓ | |
| COM | ✓ | ✓ | ✓ | ✓ | ||
| Persistent tympanic membrane perforation | ✓ | ✓ | ✓ | ✓ | ||
| Persistent tympanic membrane retraction | ✓ | ✓ | ||||
| Tympanosclerosis | ✓ | ✓ | ||||
| Academic achievement | ✓ | |||||
| Cognitive development | ✓ | ✓ | ✓ | ✓ | ||
| Developmental progress | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Intelligence | ||||||
| Literacy | ✓ | ✓ | ||||
| Phonological memory | ✓ | |||||
| Psychosocial development | ✓ | ✓ | ✓ | |||
| Hearing | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Otalgia | ✓ | |||||
| Otorrhoea | ✓ | ✓ | ||||
| Tinnitus | ✓ | |||||
| Vertigo | ✓ | ✓ | ||||
| Eustachian tube function | ✓ | ✓ | ||||
| Stapedial reflex | ✓ | ✓ | ||||
| Nasal obstruction | ||||||
| Rhinitis | ||||||
| AOM | ✓ | ✓ | ✓ | |||
| OME | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Temporary tympanic membrane perforation | ✓ | |||||
| Consonant production | ✓ | ✓ | ✓ | |||
| Consonant production – cleft-related speech patterns | ✓ | ✓ | ✓ | |||
| Expressive language skills | ✓ | ✓ | ||||
| Parent’s perspective of speech | ✓ | |||||
| Receptive language skills | ✓ | ✓ | ✓ | ✓ | ||
| Speech development | ✓ | ✓ | ✓ | ✓ | ||
| Speech intelligibility | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Speech signs of velopharyngeal insufficiency | ✓ | ✓ | ✓ | ✓ | ||
| Early extrusion or blockage of VTs | ✓ | ✓ | ||||
| Necessity to remove VTs | ✓ | ✓ | ||||
| Requirement for repeated VTs | ✓ | ✓ | ✓ | |||
| Child stress | ✓ | ✓ | ||||
| Parental stress | ✓ | ✓ | ||||
| Parental satisfaction with treatment | ✓ | ✓ | ✓ | |||
| Side effects of treatment | ✓ | ✓ | ✓ | |||
| Upper respiratory tract infection | ||||||
| Child’s satisfaction with treatment | ✓ | ✓ | ✓ | ✓ | ||
| Child’s perspective of speech | ✓ | ✓ | ✓ | ✓ | ||
| Psychological well-being | ✓ | ✓ | ✓ | |||
| Listening skills | ✓ | ✓ | ✓ | ✓ |
The percentage of outcomes scored as consensus in was again lowest in the ENT surgeon group and highest in the specialist cleft nurse group. However, between rounds the most important outcomes had changed. For example, in round 1 ENT surgeons had reached ‘consensus in’ for the outcomes ‘acute otitis media’ (AOM) and ‘side effects of treatment’, but in round 2 these were no longer included and had been replaced by ‘speech intelligibility’ and ‘speech signs of velopharyngeal insufficiency’. In the audiologist/audiological physicians group the number of outcomes where ‘consensus in’ had been reached increased from 6 to 19.
As in round 1 five of the six stakeholder groups had reached ‘consensus in’ for the outcome ‘hearing’. However, in round 2 five of the six groups also reached consensus that the outcomes ‘OME’, ‘psychosocial development’ and ‘speech intelligibility’ should be included in the COS.
Round 3: health professionals
Forty-nine outcomes were scored in round 2. These represented the 47 outcomes scored in round 2 together with the outcome ‘hyperacusis’ identified from the free-text response to the parent/child survey and ‘psychosocial well-being’. An error in the entry of outcomes onto the online system in round 2 meant that ‘psychosocial well-being’ was listed as ‘psychological well-being’ which is considered to be a different outcome. Therefore in round 3 this was clarified and participants asked to score ‘psychosocial well-being’ as well as rescore ‘psychological well-being’. Participants were shown their own score in round 2 together with the scores for each of the stakeholder groups including parents and children. A total of 73 responses were received in round 2 (86% of those completing round 1). The breakdown of participants by stakeholder group is shown in Table 37. After round 2, four participants left the cleft service and so were no longer eligible to participate in round 3.
| Stakeholder group | Cleft surgeon | ENT surgeon | Geneticist | Specialist cleft nurse | Speech and language therapist | Paediatrician | Psychologist | Audiologist/audiological physician | Total |
|---|---|---|---|---|---|---|---|---|---|
| Number invited to participate | 12a | 8b | 0c | 13d | 27e | 0f | 12 | 9 | 81 |
| Number of respondents | 11 | 7g | n/a | 13h | 24i | n/a | 11 | 7 | 73 |
| Percentage completing round 1 | 92 | 88 | n/a | 100 | 89 | n/a | 92 | 92 | 90 |
Attrition bias in round 3
To identify whether or not attrition in round 3 would introduce bias, the scores from round 1 were compared for those completing both rounds (n = 73) with those completing round 1 only (n = 8). Figure 16 shows the distribution of the average score across all 47 outcomes by stakeholder group. The results of those who did not complete round 3 did not represent extreme views suggesting that bias had not been introduced through attrition between rounds 2 and 3.
FIGURE 16.
Average scores in round 2 across all outcomes by stakeholder group. Shaded bars represent those who provided scores in round 2 only, open bars represent those scoring in both round 2 and 3. (a) Cleft surgeon; (b) ENT surgeon; (c) specialist care nurse; (d) speech and language therapist; (e) psychologist; and (f) audiologist.

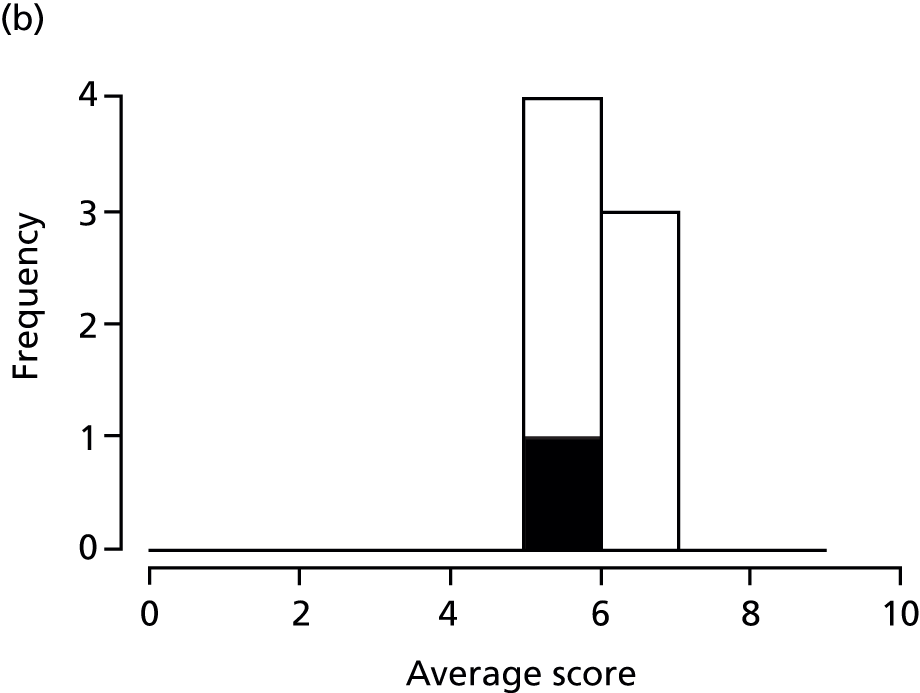
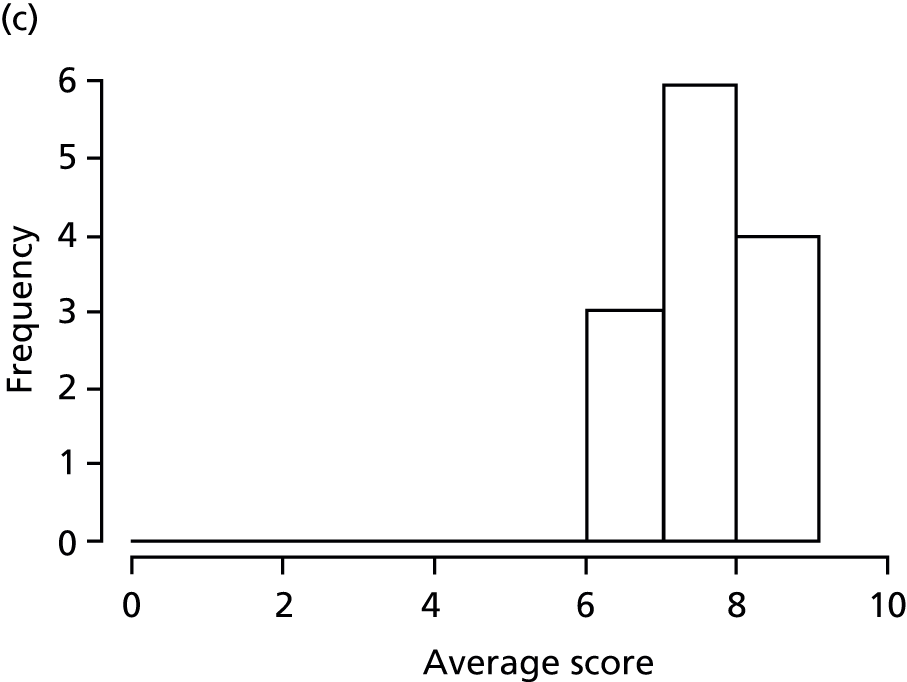
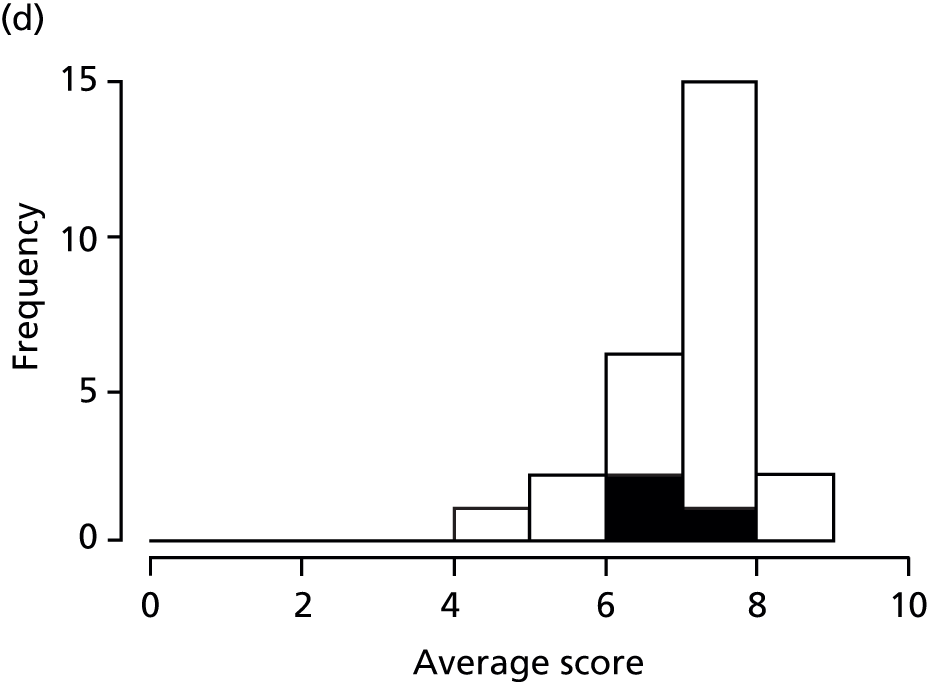
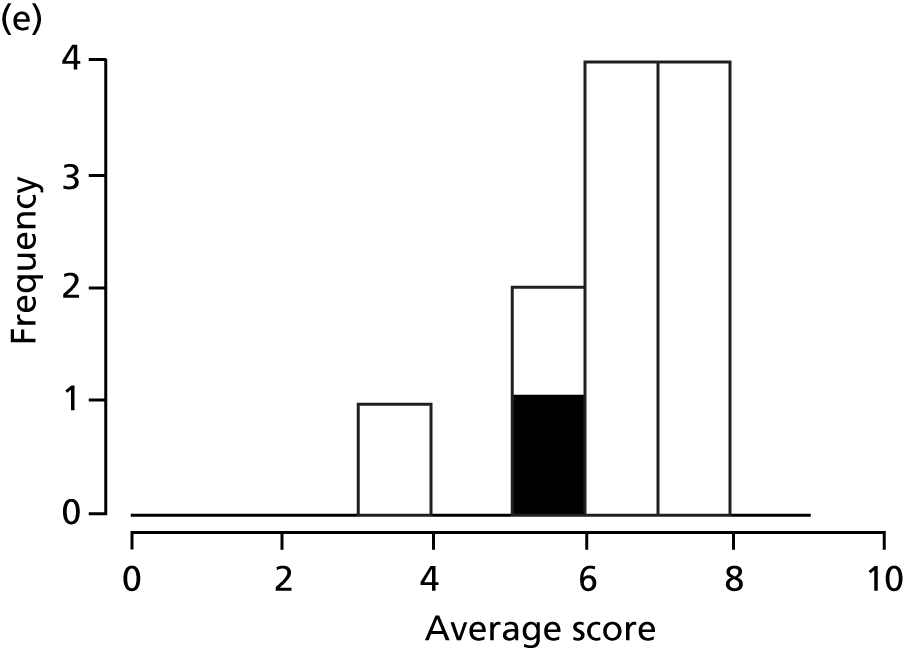

Changes in score between rounds 2 and 3
In round 3 participants were shown the results for all stakeholder groups, which also included the results of the parent and child survey, and were asked to rescore each outcome. Participants were informed that they could change their score or keep it the same as their score in round 2. Three participants (4%) did not change any scores between rounds 2 and 3. A larger proportion of participants changed between 20% and 60% of their scores in round 3 (46%) compared with round 2 (38%) (Figure 17).
FIGURE 17.
Percentage of score changed between rounds 2 and 3 after viewing the results by stakeholder group including the results of the survey of parents and children.

Consensus matrix
The scores in round 3 were again compared against the definition of consensus to determine which stakeholder groups had reached the definition of ‘consensus in’.
After round 3 all eight of the stakeholder groups (health professionals plus parents and children) had reached ‘consensus in’ for the outcome ‘hearing’. Seven of the stakeholder groups had reached consensus in for the outcomes ‘OME’ and ‘COM’. Details of the stakeholder groups reaching consensus in for each outcome are shown in Table 38.
| Outcome | Cleft surgeon | ENT surgeon | Specialist cleft nurse | Speech and language therapist | Psychologist | Audiologist/audiological physician |
|---|---|---|---|---|---|---|
| Internalising behaviour | ✓ | ✓ | ||||
| Externalising behaviour | ✓ | ✓ | ||||
| Atelectasis | ✓ | ✓ | ✓ | |||
| Cholesteatoma | ✓ | ✓ | ✓ | ✓ | ✓ | |
| COM | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Persistent tympanic membrane perforation | ✓ | ✓ | ||||
| Persistent tympanic membrane retraction | ✓ | ✓ | ||||
| Tympanosclerosis | ✓ | |||||
| Academic achievement | ✓ | |||||
| Cognitive development | ✓ | ✓ | ✓ | |||
| Developmental progress | ✓ | ✓ | ✓ | |||
| Intelligence | ||||||
| Literacy | ✓ | |||||
| Phonological memory | ✓ | |||||
| Psychosocial development | ✓ | ✓ | ✓ | |||
| Hearing | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Otalgia | ✓ | ✓ | ||||
| Otorrhoea | ✓ | ✓ | * | |||
| Tinnitus | ✓ | |||||
| Vertigo | ✓ | |||||
| Eustachian tube function | ✓ | ✓ | ||||
| Stapedial reflex | ✓ | |||||
| Nasal obstruction | ||||||
| Rhinitis | ||||||
| AOM | ✓ | ✓ | ✓ | ✓ | ||
| OME | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Temporary tympanic membrane perforation | ✓ | |||||
| Consonant production | ✓ | ✓ | ✓ | ✓ | ||
| Consonant production – cleft-related speech patterns | ✓ | ✓ | ✓ | |||
| Expressive language skills | ✓ | ✓ | ||||
| Parent’s perspective of speech | ✓ | |||||
| Receptive language skills | ✓ | ✓ | ✓ | ✓ | ||
| Speech development | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Speech intelligibility | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Speech signs of velopharyngeal insufficiency | ✓ | ✓ | ||||
| Early extrusion or blockage of VTs | ✓ | ✓ | ||||
| Necessity to remove VTs | ✓ | ✓ | ✓ | |||
| Requirement for repeated VTs | ✓ | ✓ | ✓ | |||
| Child stress | ✓ | ✓ | ||||
| Parental stress | ✓ | ✓ | ||||
| Parental satisfaction with treatment | ✓ | ✓ | ✓ | ✓ | ||
| Side effects of treatment | ✓ | ✓ | ✓ | |||
| Upper respiratory tract infection | ||||||
| Child’s satisfaction with treatment | ✓ | ✓ | ✓ | ✓ | ||
| Child’s perspective of speech | ✓ | ✓ | ✓ | ✓ | ||
| Psychological well-being | ✓ | ✓ | ✓ | |||
| Listening skills | ✓ | ✓ | ✓ | ✓ | ||
| Psychosocial well-being | ✓ | ✓ | ✓ | ✓ | ||
| Hyperacusis |
Variability in outcomes achieving consensus between rounds
Between rounds there was variability in the number of outcomes achieving consensus. Fewer outcomes achieved consensus in all rounds compared with those achieving consensus in round 3 only (Table 39). This was consistent across all stakeholder groups.
| Health professional stakeholder group | Number of outcomes reaching consensus in all 3 rounds | Number reaching consensus in round 2 and staying in consensus in round 3 | Number only reaching consensus in round 3 | Additional outcomes achieving consensus in round 3 compared with round 2 |
|---|---|---|---|---|
| Cleft surgeon | 14 | 18 | 20 | 2 |
| ENT surgeon | 4 | 5 | 9 | 4 |
| Specialist cleft nurse | 32 | 36 | 41 | 5 |
| Speech and language therapist | 13 | 21 | 22 | 1 |
| Psychologist | 7 | 11 | 13 | 2 |
| Audiologist | 6 | 12 | 19 | 7 |
In round 3 participants were shown the results of all stakeholder groups, including parents and children with CP. In round 3 more outcomes were considered important, by the individual health professional stakeholder groups, and achieved consensus compared with round 2 (suggesting that the availability of the scores from all stakeholder groups influenced responses). The individual outcomes reaching consensus across all three rounds for each health professional stakeholder group are shown in Tables 40–45.
| Outcome | R1 consensus | R2 consensus | R3 consensus |
|---|---|---|---|
| Atelectasis | ✓ | ✓ | |
| Cholesteatoma | ✓ | ✓ | ✓ |
| COM | ✓ | ✓ | ✓ |
| Persistent tympanic membrane perforation | ✓ | ✓ | ✓ |
| Persistent tympanic membrane retraction | ✓ | ✓ | ✓ |
| Tympanosclerosis | ✓ | ✓ | |
| Cognitive development | ✓ | ||
| Developmental progress | ✓ | ||
| Hearing | ✓ | ✓ | ✓ |
| Otalgia | ✓ | ✓ | |
| Otorrhoea | ✓ | ✓ | ✓ |
| Tinnitus | ✓ | ||
| Vertigo | ✓ | ||
| Eustachian tube function | ✓ | ✓ | ✓ |
| Stapedial reflex | ✓ | ||
| AOM | ✓ | ✓ | ✓ |
| OME | ✓ | ✓ | ✓ |
| Consonant production | ✓ | ||
| Temporary tympanic membrane perforation | ✓ | ||
| Consonant production – cleft-related speech patterns | ✓ | ✓ | ✓ |
| Receptive language skills | ✓ | ✓ | |
| Speech development | ✓ | ✓ | ✓ |
| Speech intelligibility | ✓ | ✓ | ✓ |
| Speech signs of velopharyngeal insufficiency | ✓ | ||
| Necessity to remove VTs | ✓ | ✓ | ✓ |
| Requirement for repeated VTs | ✓ | ✓ | ✓ |
| Side effects of treatment | ✓ | ||
| Listening skills | ✓ | ✓ | |
| Psychosocial well-being | ✓ |
| Outcome | R1 consensus | R2 consensus | R3 consensus |
|---|---|---|---|
| Cholesteatoma | ✓ | ✓ | ✓ |
| COM | ✓ | ||
| Hearing | ✓ | ✓ | ✓ |
| OM | ✓ | ||
| Otorrhoea | ✓ | ||
| OME | ✓ | ✓ | ✓ |
| Speech development | ✓ | ||
| Speech intelligibility | ✓ | ✓ | |
| Speech signs of velopharyngeal insufficiency | ✓ | ||
| Parental satisfaction with treatment | ✓ | ✓ | ✓ |
| Side effects of treatment | ✓ | ✓ |
| Outcome | R1 consensus | R2 consensus | R3 consensus |
|---|---|---|---|
| Internalising behaviour | ✓ | ✓ | ✓ |
| Externalising behaviour | ✓ | ✓ | ✓ |
| Atelectasis | ✓ | ✓ | |
| Cholesteatoma | ✓ | ✓ | |
| COM | ✓ | ✓ | ✓ |
| Persistent tympanic membrane perforation | ✓ | ✓ | ✓ |
| Persistent tympanic membrane retraction | ✓ | ✓ | ✓ |
| Tympanosclerosis | ✓ | ✓ | ✓ |
| Academic achievement | ✓ | ✓ | ✓ |
| Cognitive development | ✓ | ✓ | ✓ |
| Developmental progress | ✓ | ✓ | ✓ |
| Literacy | ✓ | ✓ | ✓ |
| Psychosocial development | ✓ | ✓ | ✓ |
| Hearing | ✓ | ✓ | ✓ |
| Otalgia | ✓ | ||
| Tinnitus | ✓ | ||
| Vertigo | ✓ | ✓ | ✓ |
| Eustachian tube function | ✓ | ✓ | ✓ |
| Stapedial reflex | ✓ | ✓ | ✓ |
| AOM | ✓ | ✓ | ✓ |
| OME | ✓ | ✓ | ✓ |
| Temporary tympanic membrane perforation | ✓ | ✓ | ✓ |
| Consonant production | ✓ | ✓ | ✓ |
| Consonant production – cleft-related speech patterns | ✓ | ||
| Expressive language skills | ✓ | ✓ | ✓ |
| Parent’s perspective of speech | ✓ | ||
| Receptive language skills | ✓ | ✓ | ✓ |
| Speech development | ✓ | ✓ | ✓ |
| Speech intelligibility | ✓ | ✓ | ✓ |
| Speech signs of velopharyngeal insufficiency | ✓ | ✓ | ✓ |
| Early extrusion or blockage of VTs | ✓ | ✓ | ✓ |
| Necessity to remove VTs | ✓ | ||
| Requirement for repeated VTs | ✓ | ✓ | ✓ |
| Child stress | ✓ | ✓ | ✓ |
| Parental stress | ✓ | ✓ | ✓ |
| Parental satisfaction with treatment | ✓ | ✓ | ✓ |
| Side effects of treatment | ✓ | ✓ | ✓ |
| Child’s satisfaction with treatment | ✓ | ✓ | ✓ |
| Child’s perspective of speech | ✓ | ✓ | ✓ |
| Psychological well-being | ✓ | ✓ | |
| Listening skills | ✓ | ✓ | |
| Psychosocial well-being | ✓ |
| Outcome | R1 consensus | R2 consensus | R3 consensus |
|---|---|---|---|
| Cholesteatoma | ✓ | ✓ | |
| COM | ✓ | ✓ | ✓ |
| Persistent tympanic membrane perforation | ✓ | ||
| Cognitive development | ✓ | ✓ | |
| Developmental progress | ✓ | ✓ | ✓ |
| Literacy | ✓ | ||
| Phonological memory | ✓ | ✓ | |
| Psychosocial development | ✓ | ✓ | |
| Hearing | ✓ | ✓ | ✓ |
| AOM | ✓ | ✓ | |
| OME | ✓ | ✓ | ✓ |
| Consonant production | ✓ | ✓ | ✓ |
| Consonant production – cleft-related speech patterns | ✓ | ✓ | ✓ |
| Expressive language skills | ✓ | ✓ | ✓ |
| Parent’s perspective of speech | ✓ | ✓ | ✓ |
| Receptive language skills | ✓ | ✓ | ✓ |
| Speech development | ✓ | ✓ | ✓ |
| Speech intelligibility | ✓ | ✓ | ✓ |
| Speech signs of velopharyngeal insufficiency | ✓ | ✓ | ✓ |
| Child’s satisfaction with treatment | ✓ | ✓ | |
| Child’s perspective of speech | ✓ | ✓ | ✓ |
| Psychological well-being | ✓ | ✓ | |
| Listening skills | ✓ | ✓ | |
| Psychosocial well-being | ✓ |
| Outcome | R1 consensus | R2 consensus | R3 consensus |
|---|---|---|---|
| Internalising behaviour | ✓ | ✓ | ✓ |
| Externalising behaviour | ✓ | ✓ | ✓ |
| Cognitive development | ✓ | ✓ | |
| Developmental progress | ✓ | ✓ | ✓ |
| Psychosocial development | ✓ | ✓ | |
| Hearing | ✓ | ||
| Child stress | ✓ | ✓ | ✓ |
| Parental stress | ✓ | ✓ | ✓ |
| Parental satisfaction with treatment | ✓ | ✓ | |
| Child’s satisfaction with treatment | ✓ | ✓ | ✓ |
| Child’s perspective of speech | ✓ | ✓ | ✓ |
| Psychological well-being | ✓ | ✓ | |
| Psychosocial well-being | ✓ |
| Outcome | R1 consensus | R2 consensus | R3 consensus |
|---|---|---|---|
| Atelectasis | ✓ | ||
| Cholesteatoma | ✓ | ✓ | ✓ |
| COM | ✓ | ✓ | |
| Persistent tympanic membrane perforation | ✓ | ||
| Developmental progress | ✓ | ||
| Hearing | ✓ | ✓ | ✓ |
| Otorrhoea | ✓ | ✓ | |
| AOM | ✓ | ||
| OME | ✓ | ✓ | |
| Consonant production | ✓ | ✓ | |
| Consonant production – cleft-related speech patterns | ✓ | ||
| Receptive language skills | ✓ | ✓ | |
| Speech development | ✓ | ✓ | |
| Speech intelligibility | ✓ | ✓ | |
| Early extrusion or blockage of VTs | ✓ | ✓ | ✓ |
| Necessity to remove VTs | ✓ | ✓ | ✓ |
| Requirement for repeated VTs | ✓ | ✓ | ✓ |
| Parental satisfaction with treatment | ✓ | ||
| Side effects of treatment | ✓ | ✓ | ✓ |
| Child’s satisfaction with treatment | ✓ | ✓ | |
| Child’s perspective of speech | ✓ | ✓ | |
| Listening skills | ✓ | ✓ |
Consensus meeting
A meeting was held on the 6 March 2014 to review the results of the mOMEnt study COS development. The meeting was attended by health professionals, parents, parent representatives and observers. Each outcome from the list scored by parents and health professionals, in the online survey and Delphi survey, respectively, was reviewed at the meeting with some discussed further and rescored for importance.
The meeting resulted in a preliminary COS together with a set of outcomes that would need further discussion with parents. In addition, a set of outcomes related to speech that possibly represent ‘how’ a particular outcome would be measured were discussed with the SAG.
Pre meeting
An invitation to attend the COS consensus meeting was sent to:
-
health professionals who had completed all rounds of the online Delphi survey and expressed an interest in attending future meetings
-
all parents who had completed an online survey, expressed an interest and provided contact details to be informed about future meetings
-
parents who had taken part in a qualitative interview whose contact details were still valid
-
CLAPA members in the north west based on CLAPA mailing list.
All those who confirmed attendance received an e-mail with information on what they should expect at the meeting and also three documents related to the meeting content: the Core Outcome Measures in Effectiveness Trials (COMET) plain language summary, the meeting agenda and a meeting overview document (see Appendix 7).
A separate session was scheduled immediately before the main consensus meeting for 30 minutes to allow HB and ST to meet with parents. This meeting allowed parents to meet one another and for any questions to be answered about the structure of the day, expectations and for additional information to be given on COS development.
Twenty-five participants attended the consensus meeting of whom 14 were eligible to vote (Table 46). All stakeholder groups with the exception of clinical psychologists were represented (Table 47). Two parents were in attendance (another two were due to attend, but shortly before the meeting notified NH that they were unable to do so due to child illness for one and jury duty for the other). On the day, three health professionals (one audiologist, one cleft surgeon and one speech and language therapist) were unable to attend due to illness or the need to cover a colleague’s clinic.
| Initials | Meeting role | Stakeholder group | Membership |
|---|---|---|---|
| PW | Meeting facilitator | n/a | SMG |
| IAB | Presenter – introduction to mOMEnt | ENT surgeona | SMG |
| NH | Presenter – methods | n/a | SMG |
| ST | Presenter – qualitative results | n/a | SMG |
| PC | Presenter – qualitative results | n/a | SMG |
| HB | COMET PPI co-ordinator | n/a | COMET |
| AHB | Participant | Speech and language therapista | SAG |
| RC | Participant | Audiologist/audiological physiciana | SAG |
| PH | Participant | Cleft surgeona | SAG |
| AH | Participant | Audiologist/audiological physiciana | Health professionals Delphi |
| SD | Participant | ENT surgeona | Health professionals Delphi |
| NHu | Participant | Cleft nurse specialista | Health professionals Delphi |
| FJ | Participant | Speech and language therapista | Health professionals Delphi |
| TB | Participant | Cleft nurse specialista | Health professionals Delphi |
| CH | Participant | Speech and language therapista | Health professionals Delphi |
| AC | Participant | Speech and language therapista | Health professionals Delphi |
| JH | Participant | Parenta | Parent online survey |
| LH | Participant | Parenta | Parent interviews |
| RP | Participant | Chief executive of CLAPAa | CLAPA |
| CB | Meeting organiser | n/a | SMG |
| KOB | Observer | n/a | SMG |
| BS | Observer | n/a | SMG |
| KW | Observer | n/a | University of Liverpool |
| AW | Observer | n/a | University of Liverpool |
| BE | Observer | n/a | The Healing Foundation |
| JT | Observer | n/a | The Healing Foundation |
| Stakeholder group | Number of voting members attending consensus meeting | Percentage representation at consensus meeting |
|---|---|---|
| ENT surgeon | 2 | 14 |
| Cleft nurse specialist | 2 | 14 |
| Speech and language therapist | 4 | 29 |
| Audiologist/audiological physician | 2 | 14 |
| Cleft surgeon | 1 | 7 |
| Clinical psychologist | 0 | 0 |
| Parent/parent representative | 3 | 21 |
Meeting agenda
The meeting was structured according to the moMEnt consensus meeting agenda version 1.0 (see Appendix 7) with the exception of the meeting summary which, due to the time taken for discussion of each outcome, was sent to participants after the meeting.
The day began with an informal session where participants were asked to sit next to someone they did not know and to find out a little bit about them including one of their favourite things or places. This encouraged good interaction and all participants were energetic and engaged. PW stressed at the beginning of the meeting that all should feel free to ask questions and that no question was trivial.
After the introduction IAB gave an overview of the mOMEnt study and of OME. This included some technical terms.
PW then went on to describe what was meant by an outcome and what a COS represents. After that the plan for the day was summarised reiterating that everyone should feel free to ask questions and to share their opinions or experiences.
NH presented the methods used in mOMEnt for both health professionals and parents and children. TB asked how we had anticipated people would respond when they saw other health professionals’ scores, as when she received them in round 2 of the survey she was unclear on how to react and spent time thinking about this. PW responded that this was exactly what we wanted participants to do; we wanted them to think about their own score and how it fitted with the scores of others. Those at the meeting were reminded that health professionals in the Delphi were advised that they could change their score or keep it the same.
CH said that in round 3 it was helpful to have the terminology used for parents to help understand each outcome.
JH asked how the health professionals were identified and why there were smaller numbers of audiologists and ENT surgeons. NH responded to say that clinical leads at each of the UK cleft centres were contacted and asked to provide the names, clinical roles and contact details of their teams. Not all cleft centres in the UK have a dedicated ENT surgeon or audiologist which is reflected in the numbers invited and has also been supported by responses to the clinician survey. There was general agreement from health professionals in the room to this response.
NH went on to describe the survey for parents and children including the number of parents and children who had completed. NH also presented the definition of consensus that was agreed at the start of this study and published in the trial protocol. NH concluded by summarising that the meeting would aim to bring the information from all of the sources together but that we would really like to do more work to get further input from parents and children.
ST and PC then presented the results of the qualitative interviews. PC gave a clear and lay explanation of what qualitative research is and how the data was analysed. ST summarised what children and parents said about physical and psychological outcomes and how the research team perceived they were interconnected.
PW presented the results of the Delphi survey, in terms of the numbers responding, attrition bias and the effect of each round on changes to individual’s scores. The responses by stakeholder group are provided in Appendix 8.
The summary of round 3 results based on which of the eight stakeholder groups (cleft surgeons, ENT surgeons, specialist cleft nurses, speech and language therapists, psychologists, audiologists, parents and children) had reached the definition of consensus for each outcome were tabled (Table 48).
| Outcome | Round 3 and survey of parents and children with CP | |||||||
|---|---|---|---|---|---|---|---|---|
| Cleft surgeon | ENT surgeon | Specialist cleft nurse | Speech and language therapist | Psychologist | Audiologist | Parent | Child | |
| Internalising behaviour | ✓ | ✓ | ||||||
| Externalising behaviour | ✓ | ✓ | ||||||
| Atelectasis | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Cholesteatoma | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| COM | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Persistent tympanic membrane perforation | ✓ | ✓ | ✓ | |||||
| Persistent tympanic membrane retraction | ✓ | ✓ | ✓ | ✓ | ||||
| Tympanosclerosis | ✓ | ✓ | ✓ | |||||
| Academic achievement | ✓ | ✓ | ||||||
| Cognitive development | ✓ | ✓ | ✓ | ✓ | ||||
| Developmental progress | ✓ | ✓ | ✓ | ✓ | ||||
| Intelligence | ✓ | |||||||
| Literacy | ✓ | ✓ | ||||||
| Phonological memory | ✓ | ✓ | ||||||
| Psychosocial development | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Hearing | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Otalgia | ✓ | ✓ | ✓ | |||||
| Otorrhoea | ✓ | ✓ | ✓ | |||||
| Tinnitus | ✓ | ✓ | ||||||
| Vertigo | ✓ | ✓ | ||||||
| Eustachian tube function | ✓ | ✓ | ✓ | |||||
| Stapedial reflex | ✓ | ✓ | ||||||
| Nasal obstruction | ||||||||
| Rhinitis | ||||||||
| AOM | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| OME | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Temporary tympanic membrane perforation | ✓ | |||||||
| Consonant production | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Consonant production – cleft-related speech patterns | ✓ | ✓ | ✓ | ✓ | ||||
| Expressive language skills | ✓ | ✓ | ✓ | |||||
| Parent’s perspective of speech | ✓ | ✓ | ||||||
| Receptive language skills | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Speech development | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Speech intelligibility | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Speech signs of velopharyngeal insufficiency | ✓ | ✓ | ✓ | |||||
| Early extrusion or blockage of VTs | ✓ | ✓ | ✓ | |||||
| Necessity to remove VTs | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Requirement for repeated VTs | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Child stress | ✓ | ✓ | ✓ | |||||
| Parental stress | ✓ | ✓ | ||||||
| Parental satisfaction with treatment | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Side effects of treatment | ✓ | ✓ | ✓ | ✓ | ||||
| Upper respiratory tract infection | ✓ | |||||||
| Child’s satisfaction with treatment | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Child’s perspective of speech | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Psychological well-being | ✓ | ✓ | ✓ | |||||
| Listening skills | ✓ | ✓ | ✓ | ✓ | ||||
| Psychosocial well-being | ✓ | ✓ | ✓ | ✓ | ||||
| Hyperacusis | ||||||||
After lunch PW continued to present the results. There was an initial test of the voting buttons. Participants who were eligible to vote are detailed in Table 46.
Results of voting and review of outcomes
Each outcome was considered in turn. Those presented first represented those on which the most stakeholder groups had reached consensus. For each outcome, participants decided if they wanted to discuss it, to simply revote or to not discuss it further. A summary of the result for each outcome is given in Table 49. Detailed notes of the discussion and a breakdown of scores are given in Appendix 9.
Each outcome has been categorised based on the following:
-
discussed and voted
-
discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured
-
discussed and agreed that further discussion with parents is needed
-
agreed not to discuss further or vote – not in the COS.
| Outcome | Number of the eight stakeholder groups achieving consensus prior to meeting | Percentage of meeting participants scoring 7–9 | Percentage of meeting participants scoring 1–3 | Category of meeting conclusion | Description of category of meeting conclusion |
|---|---|---|---|---|---|
| Hearing | 8 | 100 | 0 | 1 | Discussed and voted |
| COM | 7 | 100 | 0 | 1 | Discussed and voted |
| OME | 7 | 93 | 7 | 1 | Discussed and voted |
| Speech intelligibility | 6 | 85 | 0 | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Also agreed by SAG post meeting to include as ‘how’ of speech development |
| Receptive language skills | 6 | 100 | 0 | 1 | Discussed and voted |
| Speech development | 6 | 93 | 7 | 1 | Discussed and voted |
| Atelectasis | 5 | 46 | 9 | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Atelectasis to be combined with ‘COM’ |
| Cholesteatoma | 5 | 84 | 0 | 3 | Discussed and agreed that further discussion with parents is needed |
| Psychosocial development | 5 | 71 | 7 | 1 | Discussed and voted |
| AOM | 5 | 78 | 7 | 1 | Discussed and voted |
| Consonant production | 5 | 76 | 8 | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Also agreed by SAG post meeting to include as ‘how’ of speech development |
| Necessity to remove VTs | 5 | 0 | 67 | 4 | Agreed not to discuss further or vote – not in the COS as this relates to a specific treatment |
| Requirement for repeated VTs | 5 | 44 | 27 | 4 | Agreed not to discuss further or vote – not in the COS as this relates to a specific treatment |
| Parental satisfaction with treatment | 5 | 69 | 8 | 1 | Discussed and voted |
| Child’s satisfaction with treatment | 5 | 61 | 0 | 1 | |
| Child’s perspective of speech | 5 | 69 | 0 | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Also agreed by SAG post meeting to include as ‘how’ of speech development |
| Persistent tympanic membrane retraction | 4 | n/a | n/a | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Persistent tympanic membrane retraction to be combined with ‘COM’ |
| Cognitive development | 4 | n/a | n/a | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured To be combined with ‘how well you are doing at school’ |
| Developmental progress | 4 | n/a | n/a | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Developmental progress to be combined with ‘how well you are doing at school’ |
| Consonant production – cleft-related speech patterns | 4 | n/a | n/a | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Consonant production cleft-related speech patterns to be combined with ‘consonant production’ Also agreed by SAG post meeting to include as ‘how’ of speech development |
| Side effects of treatment | 4 | 100 | 0 | 1 | Discussed and voted |
| Listening skillsa | 4 | 84 | 0 | 1 | Discussed and voted |
| Psychosocial well-beinga | 4 | 69 | 0 | 1 | Discussed and voted |
| Tympanosclerosis | 3 | n/a | n/a | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Tympanosclerosis to be combined with ‘COM’ |
| Persistent tympanic membrane perforation | 3 | n/a | n/a | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Persistent tympanic membrane perforation to be combined with ‘COM’ |
| Otalgia | 3 | 67 | 24 | 1 | Discussed and voted |
| Otorrhoea | 3 | 50 | 8 | 1 | Discussed and voted |
| Eustachian tube function | 3 | 27 | 0 | 3 | Discussed and agreed that further discussion with parents is needed |
| Expressive language skills | 3 | n/a | n/a | 3 | At the consensus meeting this was considered as part of the ‘how’ speech development is measured and it was agreed not to vote. However, post-meeting discussion with the SAG identified that the grouping of this outcome for parents might not have been appropriate and so this outcome should be discussed further |
| Speech signs of velopharyngeal insufficiency | 3 | n/a | n/a | 3 | Wording of lay description should be revisited and discussed with parents |
| Early extrusion or blockage of VTs | 3 | n/a | n/a | 4 | Agreed not to discuss further or vote – not in the COS as this relates to a specific treatment |
| Child stress | 3 | 51 | 26 | 1 | Discussed and voted |
| Psychological well-beinga | 3 | n/a | n/a | 3 | Discussed and agreed that further discussion with parents is needed |
| Internalising behaviour | 2 | n/a | n/a | 4 | Agreed not to discuss further or vote – not in the COS |
| Externalising behaviour | 2 | n/a | n/a | 4 | Agreed not to discuss further or vote – not in the COS |
| Academic achievement | 2 | 66 | 8 | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Academic achievement to be combined with ‘how well you are doing at school’ |
| Literacy | 2 | n/a | n/a | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Literacy to be combined with ‘how well you are doing at school’ |
| Phonological memory | 2 | n/a | n/a | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured To be combined with ‘how well you are doing at school’ |
| Tinnitus | 2 | 25 | 50 | 3 | Discussed and agreed that further discussion with parents is needed |
| Vertigo | 2 | 67 | 0 | 1 | Discussed and voted |
| Stapedial reflex | 2 | 0 | 50 | 1 | Discussed and voted |
| Parent’s perspective of speech | 2 | n/a | n/a | 2 | Discussed and agreed to be considered as part of the ‘how’ an outcome is measured relating to ‘speech development’. Also agreed by SAG post meeting to include as ‘how’ of speech development |
| Parental stress | 2 | 43 | 14 | 1 | Discussed and voted |
| Intelligence | 1 | n/a | n/a | 2 | Discussed and agreed to combine with another outcome and to be considered as part of the ‘how’ an outcome is measured Intelligence to be combined with ‘how well you are doing at school’ |
| Temporary tympanic membrane perforation | 1 | n/a | n/a | 4 | Agreed not to discuss further or vote – not in the COS |
| Upper respiratory tract infection | 1 | 0 | 43 | 3 | Discussed and agreed that further discussion with parents is needed |
| Nasal obstruction | 0 | n/a | n/a | 4 | Agreed not to discuss further or vote – not in the COS |
| Rhinitis | 0 | n/a | n/a | 4 | Agreed not to discuss further or vote – not in the COS |
| Hyperacusisa | 0 | n/a | n/a | 3 | Discussed and agreed that further discussion with parents is needed |
The results of the health professionals Delphi, the parent and child Delphi and discussion at the consensus meeting contributed to a preliminary COS (Table 50). This was agreed at a follow-up meeting with the SAG.
| Outcome | Number of stakeholder groups scoring as ‘consensus in’ | Percentage scoring 7–9 at meeting | Percentage scoring 1–3 at meeting |
|---|---|---|---|
| Hearing | 8 | 100 | 0 |
| COM | 7 | 100 | 0 |
| OME | 7 | 93 | 7 |
| Receptive language skills | 6 | 100 | 0 |
| Speech development | 6 | 93 | 7 |
| Psychosocial development | 5 | 71 | 7 |
| AOM | 5 | 78 | 7 |
| Side effects of treatment | 4 | 100 | 0 |
| Listening skills | 4 | 84 | 0 |
The consensus meeting also identified outcomes where ambiguity may have been introduced by the wording used and which all agreed would benefit from further discussion with parents. After a post-meeting discussion with the SAG about the outcomes which represented the ‘how’ of speech development, a further outcome, ‘expressive language’, was identified as needing further exploration with parents as the grouping with other outcomes for the parent survey might have been misleading. The outcomes requiring further discussion with parents due to potential issues around the wording used to describe the outcome are described in Table 51.
| Outcome | Number of stakeholder groups scoring as ‘consensus in’ | Percentage scoring 7–9 at meeting | Percentage scoring 1–3 at meeting | Notes |
|---|---|---|---|---|
| Speech signs of velopharyngeal insufficiency | 3 | n/a | n/a | Further discussion with parents and further consideration of wording used to describe the outcome needed |
| Upper respiratory tract infection | 1 | 0 | 43 | Further discussion with parents and further consideration of wording used to describe the outcome needed |
| Eustachian tube function | 3 | 27 | 0 | Further discussion with parents and further consideration of wording used to describe the outcome needed |
For some outcomes which were seen less frequently among children with CP and OME concerns were raised that the sample of respondents and those present at the meeting may not have had experience of this outcome which, in turn, might have affected its relative importance. All agreed that these outcomes, described in Table 52, should be discussed further with a larger group of parent.
| Outcome | Number of stakeholder groups scoring as ‘consensus in’ | Percentage scoring 7–9 at meeting | Percentage scoring 1–3 at meeting | Notes |
|---|---|---|---|---|
| Cholesteatoma | 5 | 84 | 0 | Further discussion with parents |
| Tinnitus | 2 | 25 | 50 | Further discussion with parents |
| Hyperacusis | 0 | n/a | n/a | Further discussion with parents |
Three of the outcomes discussed at the meeting were scored as ‘consensus in’ by parents and also scored highly at the meeting yet did not meet the definition of consensus in. In addition, participants of the meeting felt that one outcome ‘psychological well-being’ needed development of a description that could then be explored further with parents as this was not included in the parent survey. The four outcomes that will be discussed further with parents are detailed in Table 53.
| Outcome | Number of stakeholder groups scoring as ‘consensus in’ | Percentage scoring 7–9 at meeting | Percentage scoring 1–3 at meeting | Notes |
|---|---|---|---|---|
| Psychological well-being | 3 | n/a | n/a | Further discussion with parents needed with input from clinical psychologists. Clinical psychology was not represented at the consensus meeting to provide support to the interpretation of this outcome |
| Academic achievement grouped into ‘how well your child is doing at school’ | 2 | 66 | 8 | Parents in the online survey and three health professional groups in Delphi reached consensus on outcomes included in parent outcome ‘how well your child is doing at school’. No consensus at face-to-face meeting and so this will be discussed further with parents |
| Otalgia | 3 | 67 | 24 | Parents in the online survey and two health professional groups in Delphi reached consensus. No consensus at face-to-face meeting and so this will be discussed further with parents |
| Child stress | 3 | 51 | 26 | Parents in the online survey and two health professional groups in Delphi reached consensus. No consensus at face-to-face meeting and so this will be discussed further with parents |
Meeting feedback
The results of the meeting were summarised in a report which was circulated to all meeting participants. This report described outcomes that had been identified for inclusion in the COS together with outcomes that need further discussion. The resulting preliminary COS was confirmed by the SAG. The outcomes detailed in Tables 51–53 needed to be discussed further with parents to determine inclusion in a COS representing outcomes that should be measured in all future studies of OME in children with CP. If consensus was reached, the outcome was added to the COS.
The engagement of parents in the next stages of the mOMEnt study will be essential and the study team will work with CLAPA to identify how best to engage their members.
Discussion
There is currently no published COS for effectiveness trials of interventions for OME in children with CP, and indeed the outcomes measured in previous studies are variable. The development of a COS in this clinical area aims to improve the interpretation and comparison of future studies, and reduce the risk of ORB and heterogeneity across studies.
The preliminary COS includes nine outcomes related to the management of OME in children with CP (see Table 50). The next steps will involve consideration of how each of these outcomes should be defined and how each outcome should be measured. The definition of each outcome will need to take into consideration the meaning of terms to both health professionals and parents as each may have a different understanding of the outcome and potential definitions. Each outcome will also be assessed for potential outcome measurement instruments, whether or not a validated tool already exists and what methods have been used to measure this outcome in previous studies as described in the systematic review.
For the outcomes included in the preliminary COS this will include:
-
Consideration of methods of assessing hearing that might be influenced by the intervention (e.g. differing methods depending on VT or HA use).
-
Agreeing a definition of COM and methods of measurement.
-
Determining which aspects of speech development should be measured and identifying if methods of measurement are already available.
-
Reviewing methods for assessment of receptive language, psychosocial development, AOM and listening skills.
-
Establishing the most appropriate way to measure side effects of treatment. The question asked in the Delphi survey of health professionals and the survey of parents and children did not specify outcomes in relation to surgical management of OME and so on further reflection, and discussion at the consensus meeting, outcomes related to VT tubes were excluded from the COS in the same way as outcomes specific to HAs. The outcome ‘side effects of treatment’ included in the COS may be dependent on the interventions/treatments that are being compared. There should also be consideration of potential crossover of AOM as both an individual outcome and a potential side effect of VT insertion.
Guidelines on the selection on outcome measurement instruments to be included in a COS developed by the Core Outcome Measurement Instrument Selection project will be consulted when available. Furthermore, the UK Cleft Audit means that for some outcomes there are potentially methods of measurement that have already been agreed by health professionals providing cleft care in the UK. For example, measures of psychosocial outcomes are included in the audit process at 5, 10, 15 and 20 years. These include both generic measures (to facilitate comparisons with population norms) and questions tailored to those affected by cleft. Speech is also measured using a validated tool which has been tested for its reliability, validity and applicability. 116 Additionally, outcome measures which include multiple domains related to OME, such as the OM8-30 and OMQ-14, warrant further investigation as a method of assessing outcomes.
Internationally, the need for a harmonised approach to outcome assessment in the general management of CP has been identified with recommendations made for standardised data sets. 117,118
The mOMEnt study has involved multiple key stakeholder groups to ensure that a COS is suitable and well accepted in future research. However, although a preliminary COS has been developed, further engagement is needed with parents to ensure that all outcomes relevant to this group have been adequately considered and that the wording of each outcome was interpreted as the study team had intended. The number of parents attending the consensus meeting resulted in low representation of parents particularly in comparisons with the number of health professionals present (of the voting participants two parents and 12 health professionals). The number of health professionals in attendance was also lower than expected based on the response rate to the health professional Delphi, with clinical psychologists not being represented at all. In the mOMEnt study the consensus meeting was held for both health professionals and parents to allow integration of views.
Previous studies included in the systematic review of the literature identified papers from a range of countries. The mOMEnt COS has been developed with input from health professionals, parents and children with CP who are based in the UK, but to promote good uptake of the COS into future studies international consensus is needed. Cleft organisations exist in both Europe (the European Cleft Organisation) and the USA (American Cleft Palate-Craniofacial Association), and may represent an opportunity to engage international health professionals through their membership.
Although OME is prevalent in children with CP, affecting around 75%, it is also a common childhood condition for children without cleft, with estimates that approximately 19% of non-cleft children are affected by the condition. 2 The preliminary COS described in the current study includes outcomes that have been identified from previous studies in both cleft and non-cleft populations suggesting that they may also be of relevance to studies of OME in children without CP. Future work will involve contacting a wider group, such as the British Academy of Audiology, so that the relevance of the COS to non-cleft children can be assessed.
Lessons learnt
There are aspects of the mOMEnt study which have identified lessons learnt or future considerations for COS development.
The mOMEnt study aimed to bring together the views of health professionals and parents in a final face-to-face meeting. However, the number of participants in each stakeholder group who were able to attend was lower than expected based on the numbers invited. Health professionals and parents may each have different preferences for the timing of meeting attendance or specific needs, such as child care, which should be taken into consideration for future studies. In the present study both child care and travel costs were reimbursed and travel booked on behalf of participants, but child care was organised by participants themselves which might be challenging if parents do not regularly use child care services. Further discussion with parents will explore the optimum day/timing of a similar type of meeting to inform future COS development of relevance to children with CP.
The low response rate of parents completing the online survey might reflect the transient nature of OME or that this is in fact a lower priority compared with the other needs of a child with CP only, unless perhaps their child is experiencing pain/discomfort or noted issues with hearing. The online system used was not optimised for use with a smartphone or tablet which may too have contributed to the small numbers responding, particularly in the 11–16 years age group where it is estimated that nearly half of UK teenagers own a smartphone. 119 The health professional Delphi survey benefited from SAG input into the design of the online system. For the survey of parents and children with CP all 36 outcomes were shown on one page which might have been potentially off-putting, warranting a future approach which involves system testing with parents and children with CP to explore accessibility and preferences.
The COS development for the mOMEnt study has involved multiple stakeholders with different clinical backgrounds. For health professionals this means that the role each stakeholder group plays in the management of OME and the knowledge of the condition across stakeholder groups is variable. At the consensus meeting a health professional noted that the parent/child descriptors of each outcome were helpful, illustrating the need to use plain language to ensure that the process of COS development is accessible to all. The varying roles of stakeholders may have also influenced the level of engagement with a COS for OME in children with CP. This could, in part, be alleviated by carefully considering the initial invitation, ensuring that the e-mail subject is relevant to all and giving consideration to providing a list of outcomes to be scored with the initial invite so that assumptions about relevance are not made. Substantially longer was taken to respond to the online survey than had been originally anticipated and multiple reminder e-mails and telephone calls were needed to improve the response rate. Clinical workload is also likely to have contributed to the time taken to respond.
The impact of the COS cannot be assessed in the present study but details will be included in the COMET database so that the COS is readily accessible to future studies of OME in children with CP allowing a future comparison of uptake.
Chapter 5 Economic analysis
Introduction
A key objective of the mOMEnt feasibility study was to perform a decision-analytic model-based economic evaluation to assess the potential impact of the surgical insertion of VTs for the management of persistent bilateral OME in children with CP. The primary aim of the economic analysis was to provide information on the potential key drivers of cost-effectiveness given the current evidence base for each of the potential strategies for managing children with CP and OME. The objectives of the economic analysis involved:
-
conducting a systematic review and critical appraisal of published economic models that aimed to evaluate various treatment options for the management of persistent bilateral OME in children with CP
-
structuring and populating a de novo economic model to estimate the incremental costs and quality-adjusted life-years (QALYs) of four potential strategies for managing children with CP and OME
-
performing VOI analyses to quantify the potential value of future research.
Methods
Systematic review of existing model-based economic evidence
A systematic search of the literature was conducted to identify published decision-analytic model-based economic evaluations of treatment options for the management of OME in children with CP. The search strategy (see Appendix 11) was designed to retrieve relevant studies from the following databases: MEDLINE, EMBASE and the American Economic Association’s electronic bibliography (EconLit). These databases were searched from the date of their inception to January 2014. The systematic search did not identify any published economic studies. One published study120 was found which gave a qualitative summary of guidelines on the surgical management of children with OME (CG60) produced by NICE.
A systematic review completed to inform CG6011 identified three economic evaluations121–123 that explored the cost-effectiveness of treatment options for the management of OME, but these were not model-based evaluations. A de novo economic model was developed to inform the recommendations for treatment options as part of CG60. 11 A decision tree was constructed that compared the deterministic incremental costs and benefits of four strategies [HAs; VTs; VTs plus adenoidectomy; and do nothing (DN)] for the management of children with persistent bilateral OME. The decision problem addressed in CG60 was not directly relevant to the management of OME in children with CP. Furthermore, the analysis made additional assumptions that limited its generalisability: (i) the time horizon for the analysis was 12 months; (ii) three surgical insertions of VTs were permitted to take place within a 12-month time frame to a proportion of children that is unlikely to reflect actual clinical practice; (iii) no gain in QALYs were assumed for children in the HAs and DN strategies; (iv) no probabilistic sensitivity analysis (PSA) was conducted. Neither the recommendations from the NICE CG60,11 nor the results of the previously published economic evaluations, can be generalised to a population of children with CP. Therefore a de novo economic model was developed to evaluate the incremental cost-effectiveness of relevant treatment options for the management of OME in children with CP.
Economic model
A decision-analytic model was structured to estimate the costs and QALYs associated with four strategies for the management of persistent bilateral OME in children with CP: (i) VTs; (ii) HAs; (iii) HAs plus VTs; and (iv) DN.
The model structure
The model followed a hypothetical cohort of 10,000 children with CP and persistent OME under the age of 12 years. This age range for the relevant study population was used as there is evidence to indicate OME-related ear problems settle in children with CP after the age of 12 years. 124 Furthermore, the incidence65 and consequences125 of OME decline as a child grows older. The model structure was informed by a previously published model that had been used to develop national guidance (CG60) on the management of children with OME. 11
The model used the UK NHS perspective for costs. Hearing gain was the primary clinical outcome which was transformed, using published utility values, to generate an estimate of the impact on health-related quality of life (HRQoL). A decision tree was used to represent care pathways for a 24-month time horizon. The time frame for the analysis was based on the advice from clinical experts (n = 3) in the mOMEnt SMG and the literature which indicated two reasons: (i) a period of 24 months is a reasonable follow-up period to detect key outcomes following an intervention for OME;11,78,80 and (ii) the total number of potential multiple VTs insertions and retractions could reasonably be performed within a 24-month period. All costs and benefits incurred beyond 12 months were discounted at an annual rate of 3.5%, as recommended by the NICE reference guide for the methods of technology appraisal. 126
The schematic for the decision tree and care pathways represented is shown in Figure 18. Following the criteria for selecting an appropriate modelling approach set out by Barton et al. ,127 a decision tree structure was considered to be the most appropriate modelling approach for this decision problem. As the children within the model are assumed to be independent of each other, the care pathways outlined following consultation with clinical experts can adequately be represented by a probability tree and a short follow-up time horizon was considered. The model was built in Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA) and programmed using Visual Basic for Applications® to estimate the expected costs and benefits for each strategy.
FIGURE 18.
The decision tree diagram.
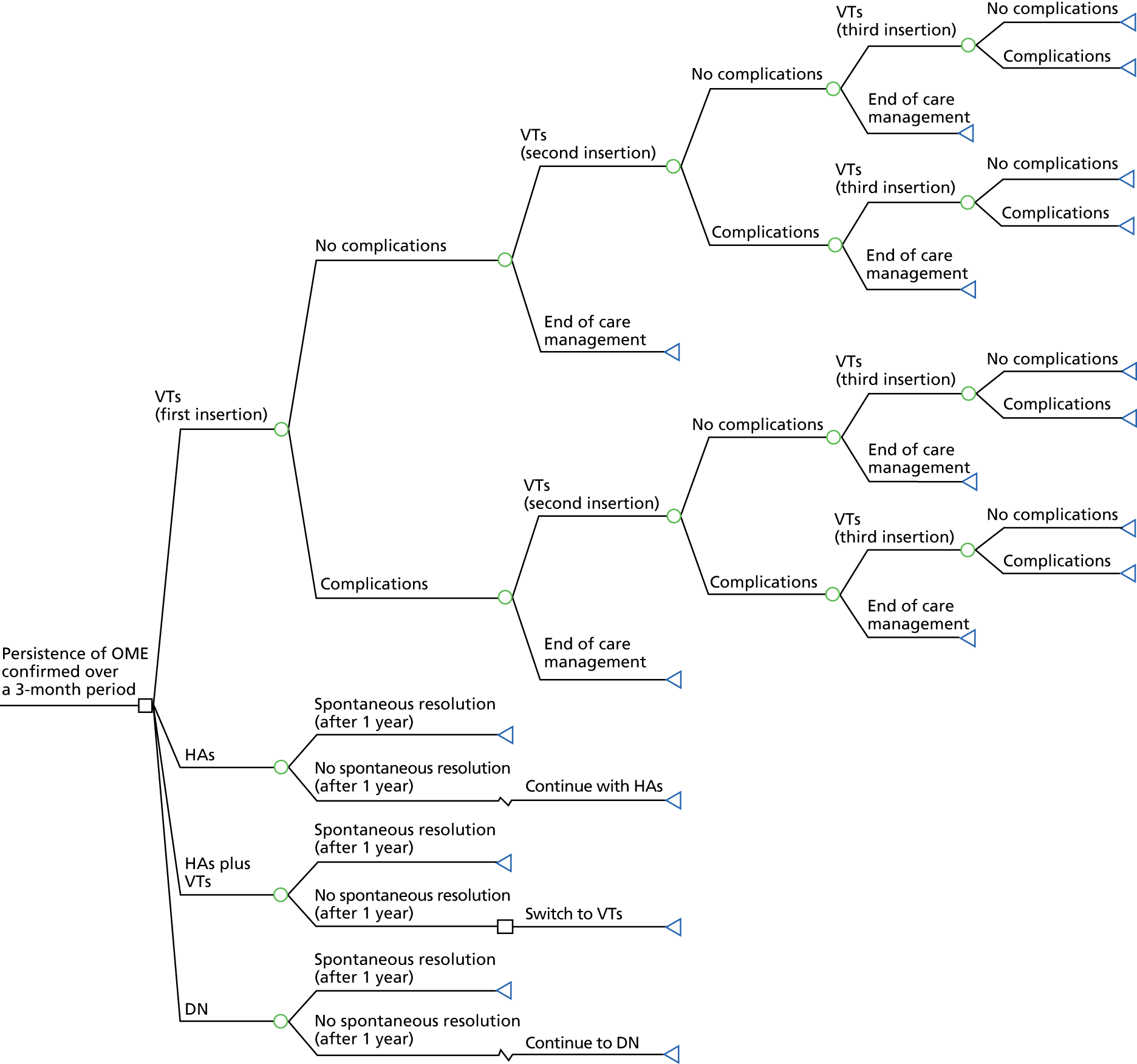
The treatment strategies
The four strategies included in the economic analysis and the key assumptions associated with these strategies are now described.
Ventilation tubes strategy
The strategy involving the surgical insertion of VTs assumes that children with CP are recommended for the intervention once the persistence of OME has been confirmed over a 3-month period. The model starts at the point when the first surgical insertion of VTs takes place. A proportion of children are assumed to have a second insertion of VTs and a smaller proportion of children will require a third insertion because of persistent or relapsing OME,11,124 or early extrusion of the VTs from the eardrum. 1,11 To avoid the high frequency of insertion in a single year, the second surgical insertion of VTs is assumed to take place within the first year of management follow-up, while the third surgical insertion is assumed to take place within the second year of management follow-up. Based on the literature,11,124,128 the maximum number of VT insertions per child was limited to three within the assumed 24-month time horizon because children who undergo several VT insertions increase the risk of conductive hearing loss in the long run70 and also to prevent scarring of the eardrum from repeated operations. The end point of the VT strategy was defined as ‘end of care management’ to represent when children do not require a subsequent insertion of VTs within the assumed 24-month time horizon for the care pathway.
The surgical insertion of VTs is commonly accepted as a safe operation, but there are some potential minor postoperative complications. None of the potential complications related to the VTs insertion operation are life-threatening. Following each surgical insertion, there is a probability of complications such as otorrhoea, granulation tissue formation and eardrum perforation. These complications were assumed to occur as reflected in the estimates extracted from the identified published literature (see Table 54). The risk of eardrum perforation is usually higher after repeated VTs insertion;80,129 thus, a higher risk was expected for eardrum perforations in subsequent surgeries. Cholesteatoma (the abnormal collection of skin cells) formation was not included as a complication of OME because previous investigators among many others suggested that VT insertion can avert sequelae of OME such as cholesteatoma formation. 11,128,130,131 Although there may be a chance of calcium deposition within the eardrum with subsequent increased eardrum rigidity (tympanosclerosis) that could be either due to AOM,132 OME itself133 or VT insertion. 134 Therefore no incidence of tympanosclerosis formation was predicted as part of any of the strategies included in this analysis given that the actual cause of tympanosclerosis is not fully understood. 135
No serious injury or surgical death was assumed to occur in this strategy since it is extremely unlikely for children to suffer serious injury or death from an insertion of VTs under modern anaesthesia. 136,137 Occasionally there may be a need to have the VTs removed,11,80 and the impact of this has been explored in the model. It was estimated that children will have their first ENT review within 6 weeks of an operation and subsequent ENT reviews every 26 weeks thereafter until the mean ‘extrusion time’ (i.e. the time which the VTs should naturally fall out by) of 39 weeks. 11 It was also estimated that children will require one or two audiological review(s) after each surgery based on the advice published in current guidelines (CG60)11 that hearing levels of children who underwent the insertion of VTs for OME should be reassessed postoperatively. The model assumed that a proportion of children who suffer from otorrhoea and/or granulation tissue formation will need a visit to a general practitioner (GP) for a course of antibiotics or eardrops.
Hearing aids strategy
The HAs strategy assumed that children with CP are offered the intervention once the persistence of OME has been confirmed over a 3-month period. It was estimated that in some children OME will resolve spontaneously by the end of 12 months. 4,138,139 However, the model assumed that children in whom OME had not naturally resolved by 12 months will continue with using their HAs in the hope of spontaneous resolution without surgery. The initial costs of this strategy include the HAs, batteries for HAs, ear moulds (to help fit the HAs into a child’s ear and enable the amplified sound to enter the ear canal), HAs care kit and HAs fitting in an audiology department. Batteries for HAs are estimated to need replacing every 4 weeks. 11 In addition, ear moulds are estimated to need replacing every 13 weeks11 because ear moulds repeatedly turn yellow and inflexible with time and, hence, require replacement on a regular basis. For a proportion of children some of these costs are expected to be incurred again due to breakage or loss of HAs. 11
Acute otitis media is a common sequelae in children who suffer from OME, which if left untreated will generally lead to episodes of AOM that require active intervention with a course of appropriate antibiotics. 139–141 AOM is the most common reason for children to take antibiotics. 139 The antibiotic, amoxicillin, is the treatment of choice to cure the AOM infection. 141,142 The HAs strategy included the use of antibiotics, and based on the meta-analysis conducted by Rosenfeld and Kay,140 an average 2.8 episodes [95% confidence interval (CI) 2.2 to 3.4 episodes] of AOM were predicted to occur each year. AOM is one of the foremost causes of doctors’ consultations139 and prescribing antibiotics for AOM is known to encourage GP visits for subsequent episodes. 141 Thus, the children with untreated OME in this strategy were assumed to make 2.8 GP visits (on average) every year due to AOM episodes. 139–141 It was estimated that children will make one or two ENT visit(s) every year. 138,142 Furthermore, it was assumed that children will have their first audiological review after 13 weeks and subsequent audiological reviews every 26 weeks thereafter. 11 Previous work has suggested that adherence to wearing HAs is a problem,4,138 because children frequently take the HAs device out. To reflect the impact of acceptability of wearing the HAs, the model also included an estimate of the level of adherence to wearing the HAs and associated impact on QALYs.
Hearing aids plus ventilation tubes strategy
The combined strategy of HAs plus VTs assumed that children with persistent OME confirmed over a 3-month period will initially be fitted with HAs. Children who do not experience spontaneous resolution of OME by the end of the first 12 months were then assumed to switch to the VTs strategy for the remainder of the follow-up period. Therefore, in effect, the pathways of this strategy resembled that of the first 12 months of the HAs strategy followed by the first 12 months of the VTs strategy.
Do nothing strategy
Do nothing is defined as an ‘extended period of watchful waiting’. The DN strategy in the model therefore reflected extending the initial watchful waiting period of 3 months by a further 24-month period. This strategy assumed that children with CP have no planned intervention,4,98,138 but they will be offered an appropriate course of antibiotics to treat any emerging instances of AOM. 139–141 Similar to the HAs strategy, children in this strategy were expected to experience 2.8 episodes (95% CI 2.2 to 3.4 episodes) of AOM every year. 140 As such, every year, the children with untreated OME were assumed to make 2.8 annual visits to the GP (on average) due to AOM episodes. 139–141 Furthermore, the children were assumed to require ongoing contact with health-care services including one or two audiological review(s)4,138,142 and one or two ENT visit(s)138,142 every year. The model assumed that, apart from the direct costs related to HAs devices and the need for any subsequent audiological reviews, the resource consequences of this strategy will effectively be similar to the HAs strategy.
Model input parameters
The data used to populate the model were derived from a variety of sources including systematic reviews of clinical effectiveness and existing economic evaluation literature, and rapid reviews of resource use and utility literature. Pondhuri et al. 1 also conducted a systematic review to identify all studies that reported on the association between early insertion of VTs and subsequent outcome in children with CP. Most of the studies identified from their systematic review of the relevant clinical literature were judged to be of low quality. The main challenges in terms of study quality were that identified studies were small, without sample size calculations and generally had poor reporting of data. The results of the systematic review of clinical effectiveness literature (see Chapter 4) confirmed that it was not possible to run a meta-analysis to estimate an overall measure of clinical effect because of study heterogeneity; thus, it was necessary to purposively select the papers deemed to have most direct relevance to the study population of interest. The model inputs in terms of probabilities, clinical effectiveness, utility values, resource use and unit costs are now described.
Probabilities
The probabilities identified for each aspect of the care pathway associated with the VTs strategy (and sources of data) are shown in Table 54 (for VT-related complications) and in Table 55 (for VT insertion).
| Complication | Probability | Distribution for PSA | Source | Notes |
|---|---|---|---|---|
| Otorrhoea | 0.25 | Beta∼(3,9) | Maheshwar et al.4 | In view of incidence of otorrhoea, a more conservative value was used. The value of 0.25 reported (for cleft population) by Maheshwar et al.4 is in line with the meta-analysed value of 0.26 reported (for non-cleft population) by Kay et al.129 Maheshwar et al.4 conducted their retrospective study in the UK. Russell et al.143 claimed a slightly lower risk of otorrhoea for the cleft population than the non-cleft population |
| Granulation tissue formation | 0.042 | Beta∼(37,850) | Kay et al.129 | Granulation tissue formation is a post-operative complication cited by Kay et al.129 and CG6011 among others |
| Eardrum perforation (first procedure) | 0.024 | Beta∼(2,81) | Phua et al.80 | A retrospective New Zealand-based study. Only one study was identified relevant to children with CP from a published systematic review that reported a subsequent higher risk of perforations of the eardrum due to repeated VTs insertion |
| Eardrum perforation two or more procedures) | 0.098 | Beta∼(4,37) | Phua et al.80 |
| Parameter | Probability | Distribution for PSA | Source | Notes |
|---|---|---|---|---|
| Removal of VTs (first procedure) | 0.072 | Beta∼(6,77) | Phua et al.80 | This was the only identified study of children with CP from a published systematic review that reported different risk of retraction following subsequent VTs insertion procedures |
| Removal of VTs (two or more procedures) | 0.171 | Beta∼(7,34) | Phua et al.80 | |
| Reinsertion of second VT | 0.38 | Beta∼(68,110) | Sheahan et al.124 | This was a questionnaire-based study of cleft children. This was the only identified study that reported a reinsertion rate for two or more VTs. A previous model used a reinsertion rate of 0.25 for general children11 |
| Reinsertion of third VT | 0.38 | Beta∼(68,110) | Sheahan et al.124 | |
| Time to extrusion | VTs fall out by 39 weeks | Normal∼(39,2.93)a | CG6011 | The ‘time to extrusion’ is defined as the time by when the VTs naturally should fall out |
For the HAs strategy, a probability for breakage or loss over a 12-month period of 16.44% was used to populate the model. This value was calculated from estimates that 25% of children break or lose their HAs over a period of 21 months, presented by NICE,11 under the assumption of a constant hazard data. Rosenfeld and Kay140 reported a meta-analysis that generated the value for spontaneous resolution of chronic OME documented for 3 months or longer, and in line with this, the model predicted a spontaneous resolution rate of 30.8% by the end of the first 12 months for children in the non-surgical strategies. The resolution of OME was assumed to have a constant rate over 12 months, following advice from clinical experts and the evidence base. In calculating the QALY gain associated with the HAs strategy, the model assumed that 90.9% of the cohort of children will adhere to wearing their HAs, based on a published non-adherence rate of 9.1%. 138 The 9.1% of children who do not adhere to wearing their HAs were then assumed to have gain in QALY equivalent to that used in the DN strategy.
Health gain and utilities
Hearing level measured in decibels (dBHL) over two pre-defined time periods of 12 and 24 months was used to value the impact of each strategy on children with CP and OME. The study conducted by Maw and Bawden144 was identified as the primary source to provide estimates for the quantity of hearing gain associated with each strategy, and in the baseline analysis the assumed hearing gains were (i) 13.06 dBHL after 12 months and 12.24 dBHL after 24 months for the VTs strategy; and (ii) 4.88 dBHL after 12 months and 7.57 dBHL after 24 months for the non-surgical strategies (HAs and DN). QALYs were then calculated to value the ‘quality’ of the observed gain in hearing, as a change in HRQoL, which was assumed to be a linear function of potential improvement in hearing. To calculate the QALYs, a utility value per unit increase in hearing gain was attached to the identified dBHLs for each strategy.
A systematic search strategy (see Appendix 11) was designed to identify relevant utility data suitable for informing estimates of QALYs for the economic model. The search was carried out in MEDLINE, EMBASE and EconLit. These databases were searched from the date of their inception to January 2014. Studies were considered as being eligible for inclusion if the studies (i) were published in peer-reviewed journals as full papers; (ii) reported HRQoL data based on utility values for ‘hearing’ of OME-affected children with no other comorbidity; and (iii) reported utilities that are appropriate for estimating QALYs. Eighteen references were identified from the electronic search strategy; none of which met the inclusion criteria.
Published expert opinion was used to apply a value for the assumed utility gain associated with per unit increase in dBHL. This estimate of a utility gain per unit increase in dBHL of 0.00874 (95% CI 0.00500 to 0.01200)11 was based on the interpretation of an unpublished study by Kubba (2004)145 that collected individual-patient data on the Health Utility Index Mark III for children with a median age of 5 years. The use of VTs can improve the level of a child’s hearing by approximately 50.5% (95% CI 47.0% to 54.5%) when compared with no intervention. 140 To estimate the impact of HRQoL the utility gain per unit increase in dBHL of 0.00874 was reduced by 50.5% for the children in the DN strategy.
Resource use and costs
Table 56 summarises the point estimates of resource use and unit costs used for each strategy and pathway for the model together with the assumed ranges and distributions used in the PSA. All prices are presented in UK£ for the year 2010–11.
| Parameter | Resource use | Cost | Source | Notes | ||
|---|---|---|---|---|---|---|
| Mean description (per child) | Distribution for PSA | Mean unit cost (£) | Distribution for PSA | |||
| Insertion of VTs | One procedure | Fixed | 891 | Gamma∼(130.45,6.83) | NSRC1146 2010–11 (HRG146 code CZ08T; day case) | Minor ear procedures for children aged ≤ 18 years through tympanic membrane. One procedure is equivalent to inserting two VTs for each child due to bilateral OME |
| Tympanoplasty | One procedure | Fixed | 1831 | Gamma∼(100.11,18.29) | NSRC1146 2010–11 (HRG146 code CZ10U; day case) | Major ear procedures for children aged ≤ 18 years due to perforation of eardrum |
| Removal of VTs | One procedure | Fixed | 891 | Gamma∼(130.45,6.83) | NSRC1146 2010–11 (HRG146 code CZ08T; day case) | Minor ear procedures for children aged ≤ 18 years through tympanic membrane. One procedure is equivalent to removing one or two VT(s) for each child due to bilateral OME |
| HA | Two units | Fixed | 80 | Fixed | GDG11 estimate has been inflated based on the HCHS147 index 2010–11 (PSSRU147) | Two HAs are required for each child due to bilateral OME |
| Ear mould | Eight units per year | Fixed | 17 | Fixed | GDG11 estimate has been inflated based on the HCHS147 index 2010–11 (PSSRU147) | Two ear moulds are required for each child every 13 weeks11 |
| HA care kit | One unit | Fixed | 20.88 | Fixed | Connevans148 | This cost incurred once due to the maintenance of the HA(s) |
| HA battery | Twenty-six units per year | Fixed | 0.49 | Fixed | HAB149 | Batteries are required to be replaced every 4 weeks11 |
| HA fitting | One procedure | Fixed | 76 | Gamma∼(71.03,1.07) | NHS reference costs (2005–6)11 (service code AS1FA) has been inflated based on the HCHS147 index 2010–11 (PSSRU147) | HAs fitting in an audiology department. One procedure is equivalent to fit two HAs for each child due to bilateral OME |
| GP visit/antibiotic medication | VTs: 0.25 (otorrhoea) + 0.042 (granulation) time | See Table 54 | GP visit: 41 Antibiotic medication: 11 |
Fixed | GP visit: PSSRU147 (unit costs of health and social care 2010–11) Antibiotic medication: GDG11 estimate has been inflated based on the HCHS147 index 2010–11 (PSSRU147) |
GP visit and cost for a course of antibiotics or eardrops due to otorrhoea, granulation and/or AOM |
| HAs: 2.8 times | Normal∼(2.8,0.3) | |||||
| DN: 2.8 times | Normal∼(2.8,0.3) | |||||
| Audiological review | VTs: 1.5 visits following each surgery | Uniform∼(1,2) | 48 | Gamma∼(64,0.75) | NHS reference costs (2005–6)11 (service code AS1FU) has been inflated based on the HCHS147 index 2010–11 (PSSRU147) | One or two visit(s) following each surgery for VTs.11 One visit within 13 weeks and subsequent visits every 26 weeks thereafter for HAs.11 One or two visit(s) every year for DN4,11,138,142 |
| HAs: 1 visit initially + 1.5 visits per year thereafter | Fixed | |||||
| DN: 1.5 visits per year | Uniform∼(1,2) | |||||
| ENT specialist visit | VTs: 2.5 visits following each surgery | Fixed | 91.72 | Gamma∼(84.14,1.09) | NSRC1146 2010–11 (service code 120) | One visit within 6 weeks of each surgery and subsequent visits every 26 weeks thereafter until the time of extrusion.11 One or two visit(s) every year138,142 for both HAs and DN |
| HAs: 1.5 visits per year | Uniform∼(1,2) | |||||
| DN: 1.5 visits per year | Uniform∼(1,2) | |||||
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was used to quantify the joint uncertainty in the model by assigning a range and specific distribution to each of the input parameters. The PSA was run using 10,000 iterations. Gamma distributions were used to represent the uncertainty in the cost parameters (see Table 56), because these values are constrained to be zero or positive. The gamma distribution is parameterised by two parameters (shape and scale), which are expressed as functions of the expectation and variance of the distribution. Beta distributions were used to represent the uncertainty in the probability parameters (see Tables 54 and 55) as these values are defined on the interval with a minimum (0) and maximum (1) value. The beta distribution is parameterised by two parameters (alpha and beta); alpha corresponds to the ‘number of events’ observed and beta corresponds to the ‘number of non-events’ observed. Normal distributions were used to represent the uncertainty in the hearing gain parameters to reflect the likelihood of an increase or decrease unit in dBHL during the recovery period: Normal∼(13.06,9.49) after 12 months and Normal∼(12.24,9.1) after 24 months for the VTs strategy; and Normal∼(4.88,11.11) after 12 months and Normal∼(7.57,12.76) after 24 months for the non-surgical strategies. 144 The utility gain per unit increase in dBHL was parameterised by a Gamma distribution [Gamma∼(24.38, 0.0004)] with the shape (24.38) and scale (0.0004) determined from the mean and variance reported by Kubba. 145
The probability of spontaneous resolution was sampled from the distribution labelled as Beta∼(61,137). 140 The probability of breakage or loss of HAs was re-estimated within the PSA based on uncertainty surrounding the original 21-month data that was represented by a Beta∼(6,18). 138 Adherence to HAs was sampled from Beta∼(20,2),138 whereas expected episodes of AOM were sampled from Normal∼(2.8,0.3). 140 Based on the statement described earlier that VTs can improve a child’s quality of hearing by approximately 50.5% (95% CI 47.0% to 54.5%)150 when compared with DN, the QALY gain associated with the DN strategy was adjusted according to a normal distribution [Normal∼(0.505,0.02)].
Value of information analysis
Using the decision tree structure and subsequent PSA, an expected value of perfect information (EVPI) analysis was conducted to estimate the potential value of future research. Equation 1 shows that EVPI estimates the difference between the expected value of a decision made with perfect information and the expected value of a decision made on the basis of the current evidence base:
where EθmaxjNB(j,θ) represents the expected value of the decision with perfect information and maxjEθNB(j,θ) represents the expected value without perfect information. Using Equation 2, the net benefit (NB) associated with each treatment strategy was calculated by combining the respective health gain and expected cost consequences:
where λ represents the willingness-to-pay (WTP) threshold, E represents the QALY gained and C represents the expected cost.
In its simplest form, EVPI represents the maximum amount that a decision-maker would be willing to pay to gain access to perfect information. However, the societal value of research should ideally be estimated across the population of future patients for whom the decision is pertinent since the information provided by research is a public good. Equation 3 shows the calculation of the population-level expected value of perfect information (pEVPI):
where T = effective lifetime of a technology; It = incidence of the condition relevant to the health technology in period T; and r = discount rate. pEVPI represents an upper bound of the expected benefit of conducting further research. If pEVPI is greater than the expected cost for conducting further research, then it should potentially be considered worthwhile to conduct the further research. Here the estimate of the population was based on an assumption that every year 720 children will be eligible for VTs in the UK. Data from the CRANE database showed there were 800 children born with CP in England, Wales and Northern Ireland in 2012. 151 Of which, 720 (90% of 800113,124,152,153) were assumed to suffer from OME. The lifetime for the technology was assumed to reflect that the decision would be relevant for 10 years (T). Armstrong154 first described the use of VTs in 1954, and, since then, use of VTs to restore hearing to normal has been increased. Given the historical longevity of the technology revealed in the literature, it seemed reasonable to assume that use of VTs will last for at least another 10 years before a new technology comes along and replaces it. A discount rate of 3.5% (r) was used to be in line with the recommendations in the NICE reference guide for methods of technology appraisal. 155
The basic method for estimating EVPI was then extended to identify the type of evidence which will be most important by identifying the parameter(s) for which more precise estimates would be most valuable. 156,157 The expected value of partial perfect information (EVPPI) can be estimated using Equation 4:
Here, ϑ represents parameter(s) of interest, φ represents other uncertainties, θ represents all parameters, EϑmaxjEφ/ϑNB(j,φ,ϑ) corresponds to the expected value with perfect information for parameter ϑ, and maxjEθNB(j,θ) corresponds to the expected value of current information for all parameters θ. The EVPPI analysis was conducted on four parameters including (1) unit cost of surgical procedure (in isolation); (2) dBHL (in isolation); (3) unit measurement of dBHL (in isolation); and (4) dBHL plus utility gain per unit increase in dBHL (in group). These parameters were identified a priori by clinical members of the mOMEnt study team (n = 3) as parameters most likely to impact on the relative expected costs and QALY gains of each of the four management strategies. Following the recommendations made in by Brennan et al. ,156 a total of 100,000 simulations (100 simulations in the outer loop and 1000 simulations in the inner loop) were used to estimate the maximum possible EVPPI values.
Results
The results of the baseline (deterministic) and probabilistic analyses are shown in Table 57. All results were initially generated for a hypothetical cohort of 10,000 children but the final results are presented in terms of costs and QALYs gained per child. The expected values from the PSA showed that the use of HAs plus VTs strategy was the most expensive option at £2663 per child. The insertion of VTs was the second most costly strategy with a cost of £2086 per child compared with £1237 per child for the HAs strategy and £593 per child for the DN strategy. The associated gains in QALY were 0.218 for the VTs strategy; 0.136 for HAs plus VTs; 0.102 for HAs; and 0.053 for DN.
| Strategy | Deterministic | Probabilistic | ICER per QALY gained | |||
|---|---|---|---|---|---|---|
| Cost (£) | QALY | Cost (£) | QALY | Deterministic | Probabilistic | |
| DN | 592 | 0.0528 | 593 | 0.0529 | – | – |
| HAs | 1235 | 0.1017 | 1237 | 0.1019 | Extended dominated by VTs | Extended dominated by VTs |
| VTs | 2083 | 0.2175 | 2086 | 0.2176 | £9053 (VTs vs. DN) | £9065 (VTs vs. DN) |
| HAs plus VTs | 2661 | 0.1357 | 2663 | 0.1358 | Dominated by VTs | Dominated by VTs |
The incremental cost-effectiveness ratios (ICERs) per QALY gained based on the expected values from the PSA were (i) £13,143 per QALY gained for the HAs strategy compared with the DN strategy; and (ii) £7338 per QALY gained for the VTs strategy compared with the HAs strategy. The HAs plus VTs strategy was dominated by the VTs strategy because it was shown to be less effective and more costly. Applying the weak dominance principle shows that the HAs strategy was extended dominated by the VTs strategy because HAs compared with DN has an ICER (£13,143 per QALY gained) greater than that of VTs compared with HAs (£7338 per QALY gained). This means that the VTs strategy should be compared with the DN strategy, giving an ICER of £9065 per QALY gained. All four strategies were included in the subsequent PSA and VOI analyses as there could be some possible realisations of the uncertainty where the order of costs and QALYs gained would change, which would affect the relevant comparators for the incremental analyses.
Figure 19 shows the scatter plot of expected incremental costs and effects (gain in QALYs) for each of the 10,000 iterations run in the PSA. The DN strategy was assumed to be the status quo and hence has been anchored at the origin. Comparing the values for positive expected incremental costs and gain in QALYs, some 77% of the simulated values for HAs versus DN, 90% for VTs versus DN and 85% for HAs plus VTs versus DN, fell in the north-east quadrant of the cost-effectiveness plane. The north-east quadrant represents values in which an intervention would be more costly and more effective compared with its comparators, and it then becomes necessary to make a decision about the threshold value for WTP for an additional QALY. The PSA revealed that the HAs strategy was extended dominated by the VTs strategy for some 61% of the simulated realisations. Figure 19 indicates that some of the expected costs and gain in QALYs would result in negative ICERs. For this reason, it was not appropriate to calculate pseudo-CIs around the mean estimates of ICERs from the PSA.
FIGURE 19.
Scatterplot of incremental expected costs and QALYs obtained from PSA. The circles represent the expected value from the PSA. The DN strategy was anchored at the origin.
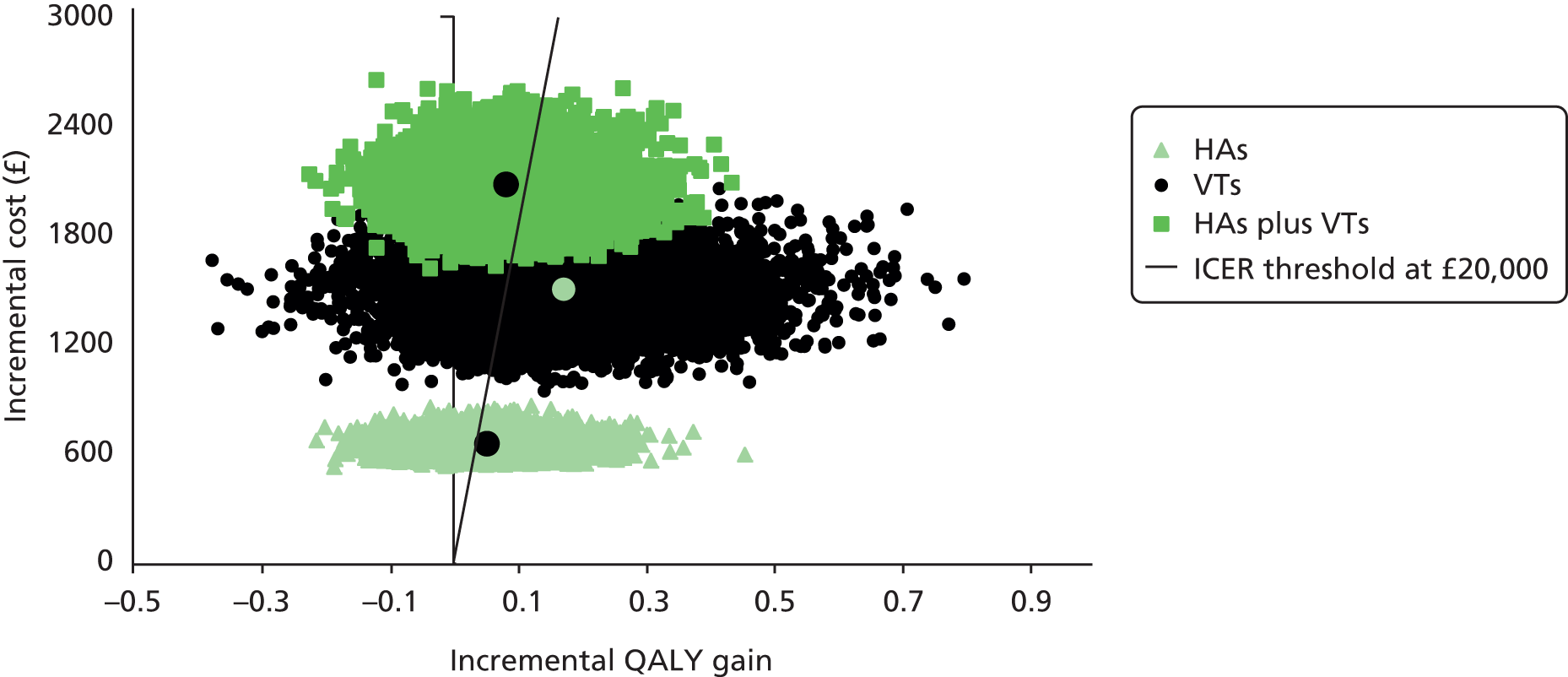
Figure 20 presents cost-effectiveness acceptability curves for each of the strategies based on the results of the PSA. The probability that the VTs strategy was cost-effective is 0.49 at the WTP threshold of £10,000 per QALY and 0.63 at the WTP threshold of £20,000 per QALY.
FIGURE 20.
Cost-effectiveness acceptability curve for four strategies.
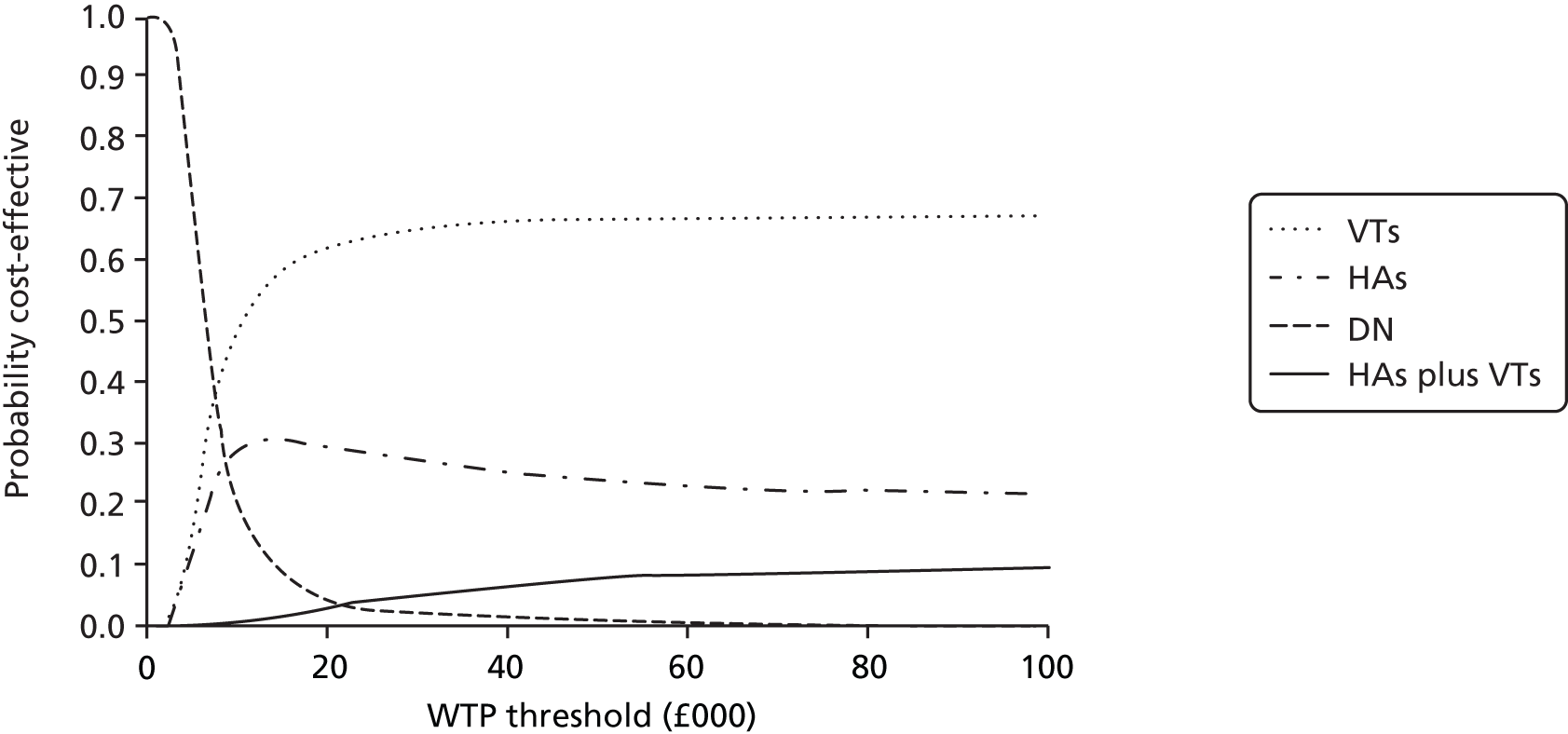
The EVPI values at both individual and population levels for a range of different values of the WTP threshold are presented in Table 58. At the population level, the maximum potential value of approximately £5.24M at an assumed WTP threshold of £20,000 suggests that further research work in assessing the impact of the surgical insertion of VTs for the management of persistent bilateral OME in children with CP could potentially be worthwhile, provided that the total cost of undertaking the further research remains under this estimated EVPI value.
| WTP threshold (£) | Individual EVPI (£) | pEVPI over a 10-year decision horizon (£) | pEVPI over a 5-year decision horizon (£) |
|---|---|---|---|
| 5000 | 102 | 632,148 | 343,191 |
| 10,000 | 641 | 3,972,619 | 2,156,720 |
| 15,000 | 721 | 4,468,422 | 2,425,889 |
| 20,000 | 845 | 5,236,916 | 2,843,101 |
| 25,000 | 988 | 6,123,164 | 3,324,242 |
| 30,000 | 1136 | 7,040,399 | 3,822,205 |
Figure 21 shows the relationship between the pEVPI values and different values of WTP per QALY gained. Figure 21 indicates that the value of further research exceeds £4M for all values of the WTP threshold beyond £10,000 per QALY gained. This value is likely to exceed the total cost of future research. At a WTP threshold of £30,000 per QALY gained, the value of further research exceeds £7M. However, should the WTP threshold be < £1500, the pEVPI is zero indicating that there is no value in additional information from future research.
FIGURE 21.
Expected population value of perfect information at various WTP thresholds.

The EVPI analysis was important in deciding whether or not the value from undertaking further research could be worthwhile. The EVPPI analysis extended this analysis to provide the breakdown values of further research for key parameters. The EVPPI analysis, using an assumed WTP threshold of £20,000 per QALY, suggested that further research on dBHL with or without utility values could be potentially worthwhile. Figure 22 shows the maximum possible population EVPPI values associated with a number of key uncertain parameters at the WTP threshold of £20,000. For instance, improving the estimate of dBHL parameters would accrue the maximum possible return of approximately £3.5M at an assumed WTP threshold of £20,000.
FIGURE 22.
Population EVPPI values for key uncertain parameters at the WTP threshold of £20,000.

Discussion
The association between children with CP and hearing loss that results from OME is well documented. 64,158 The surgical insertion of VTs is one of the most common surgical procedures in childhood today. 134,136 Although the disagreement regarding the relative benefits and risks for the insertion of VTs in children with CP is unresolved within the surgical community, affected children still desire to function better and parents want their children to be in a position to participate fully in education. Despite a large body of evidence on incidence and prevalence rates of OME, there is still a paucity of research on the potential impact of the surgical insertion of VTs in children with CP. Therefore it was essential to assess whether or not the surgical insertion of VTs can have a positive impact on expected health benefits. A systematic search of the published literature was conducted to identify decision-analytic model-based economic evaluations of surgical insertion of VTs in the management of persistent bilateral OME in children with CP. No economic evaluations were identified that were relevant to children who are born with CP. Hence a de novo model-based economic analysis was carried out to assess the impact of surgical insertion of VTs compared with three alternatives in the management of bilateral OME persisting after the watchful waiting for 3 months in children with CP.
The insertion of VTs strategy was found to be the optimal strategy with the expected value of the ICER from the PSA of £9065 per QALY gained compared with the DN strategy. The HAs and VTs strategy was dominated by the VTs strategy. The HAs strategy was extended dominated by the VTs strategy. The ICER for VTs was well below the WTP threshold of £20,000 per QALY, which is commonly taken by NICE to be a reasonable threshold for WTP for an additional QALY. 159 The gain in QALY resulting from improvement in dBHL associated with the VTs strategy to manage OME came at a higher cost, which was mainly driven by the resource use attributable to the surgical process. The results of the PSA indicated the existence of considerable uncertainty surrounding the existence and extent of the incremental QALY gain associated with the VTs strategy compared with the DN strategy. In addition, there was some uncertainty surrounding the extent of the incremental cost associated with the VTs strategy compared with the DN strategy. This observed uncertainty perhaps explains why there remains disparity in the medical community regarding the use of VTs in individual children with CP for OME.
The results from this early economic analysis should not be used to inform any current changes in clinical practice; it was carried out to understand whether or not there is a need for further research regarding the utilisation of VTs in children with CP and persistent OME. A key strength of this analysis was that it extended the analysis beyond PSA and calculated the EVPI and EVPPI values to provide a measurable insight of whether or not further research in this area is potentially worthwhile. The feasibility and implications of using the EVPI and EVPPI methods for informing the future research prioritisation process have previously been well described and their use is recommended in the context of commissioned HTAs. 160 The maximum potential EVPI values of approximately £845 for every child and £5.24M for a population of children in England, Wales and Northern Ireland, assuming the WTP threshold of £20,000 per QALY and a decision horizon of 10 years, suggest that further research work in this area is potentially worthwhile.
This model was an early economic evaluation that used all available data from multiple sources. However, there were some limitations in terms of the availability and relevance of the data used for parameter inputs, which should be revised and reassessed once more relevant clinical effectiveness, resource use and utility data become available. The calculation of QALYs was driven by a combination of utility values and effectiveness data, specified in terms of dBHL. The key uncertainty in the model inputs indicated the need for further research to obtain more reliable and relevant values for the dBHL parameters. This analysis used a utility value taken from a published source and the same value has been used previously in an appraisal completed by the Guideline Development Group. 11 A further area of research is to explore whether or not gain in QALYs, that focus on measuring improvements in health status, are the only relevant outcome to assess an intervention aimed at young children. Other non-health gains such as improvements in educational attainment and ability to play with their peers may also be important outcomes for children and their parents.
The eligible patient population was also an area of uncertainty suggested to be important by clinical experts. This economic evaluation focused on a cleft population of children under the age of 12 years. This focus was necessary because of a paucity of epidemiological data for other age groups. This age group was selected for this analysis because clinical experts considered this group to represent children in which the condition is most prevalent. However, further work is needed, informed by robust epidemiological data, to understand the relative cost-effectiveness of the insertion of VTs in different age groups and also the most appropriate age for the surgical procedure in a child with CP. This analysis used the only available source for estimates of health-care resource use. These data were not directly relevant to a population of cleft children, but provided the best evidence in absence of directly relevant data. Using these data was likely to be a conservative approach as OME is relatively more common in cleft children compared with non-cleft children and has an extended recovery period. 124
This analysis posed two technical challenges. Interpretation of this analysis should be undertaken with caution as, with no definitive guidelines identified for the treatment of OME in children, the clinical pathway used to structure the economic evaluation was developed using assumptions based on available published evidence. Therefore the clinical pathways used to structure the economic model were developed using assumptions based on an existing economic model, used in a previous appraisal conducted by NICE,11 and adapted for a population of children with CP using advice from clinical experts. Furthermore, the limited number of studies meant that it was difficult to generate ranges based on empirical data around some parameters included in the PSA.
This is the first model-based economic evaluation to identify and quantify the costs and benefits of different management options of persistent bilateral OME in children with CP. The model has demonstrated the potential for resources to be released from other health-care interventions when VTs insertion is applied for managing OME. The total cost of the VTs strategy is relatively high, but this intervention appears to provide good value for money, based on the current evidence base, if used after the initial 3-month period of watchful waiting as a means to correct significant hearing impairment and prevent complications of untreated OME. The early management of OME-related complications should generate expected NBs that might compensate the additional expenditure incurred because of repeated clinic visits141 and prompt rapid hearing gain that is also important for childhood speech development and associated educational attainment. 161,162 Schönweiler et al. 163 showed that language development depends more on hearing ability than severity or surgical repair of CP. Paradise and Bluestone,64 some 40 years ago, advocated a policy of early VTs and replacements when necessary in order to decrease the long-term otological complications and minimise the effects on speech and language development. Furthermore, the use of VTs could release clinicians from the pressure to prescribe antibiotics to manage multiple instances of infections, which could impact on antibiotic resistance. 114,164 However, this analysis has shown limitations in the current evidence base and identified that it is potentially worthwhile undertaking further research in this area. Examples of the additional evidence that is needed include the link between hearing gain and utility gain; the actual use of health-care resources; and clinical effectiveness data to inform the appropriate age for the insertion of VTs in children with OME. Furthermore, another issue that could not be identified by using EVPPI, but is clearly relevant given the nature of the intervention and target patient population, is whether or not using hearing gain alone as an outcome is appropriate.
Conclusion
Based on the assumptions used in this analysis, the surgical insertion of VTs for the management of persistent bilateral OME in children with CP is most likely to be a cost-effective strategy, but the need for acquiring further information from future study is evident to inform this treatment choice. The probability that the VTs strategy is likely to be more cost-effective than its comparators was about 63% at the WTP threshold of £20,000 per QALY. The EVPI analysis has shown that undertaking further research in this area is potentially worthwhile. The EVPPI analysis indicated that the main uncertainty centres around the estimate of dBHL parameters. Consequently, if future research is to be undertaken it should then aim to improve the estimate of dBHL parameters using a RCT design. Future research may also focus on improving the estimate of utility values to the observed change in dBHL from a cohort study or substudy within a trial. The results presented here should not be considered as an option for all age groups; thus, further research is required to identify subgroups of cleft children likely to benefit most from the surgical insertion of VTs.
Chapter 6 Summary discussion and conclusions
This section of the report is concerned with a discussion of our findings that were presented to the SAG in order for them to make recommendations on potential study designs. It then concludes with our final recommendations on further studies.
Clinician survey
This part of the study suggested that some of the UK cleft centres were in a position to take part in a future study. These were centres that could nominate a lead ENT/audiologist to act as a local principal investigator and they were able to perform age-appropriate hearing testing from 1 year old to adolescence. These centres also exhibited adherence to the NICE Guideline Development Group guidelines and prescribed both HAs and VTs.
One of the most relevant findings of the survey was concerned with the method of delivery of care. It was clear that most of the networks operated a ‘hub and spoke’ infrastructure with the centre operating a monitoring service and recommending that care be provided locally to the patient in hospitals or smaller clinics. This has implications for potential study design. For example, it would be difficult to engage peripheral clinicians with the random allocation of care, as they may not be in equipoise and the probability of protocol deviations would be high. Furthermore, obtaining trust R&D for multiple sites with potentially low caseloads would be problematic and inefficient. Finally, there will be the additional problem of standardising both audiological assessment and treatment away from the ‘hub’ clinic. As a result, it is important that if a trial were to be carried out consideration should be given to an evaluation of whether or not the patients would need to receive their treatment in the hub or centre. This will result in a reduction in eligibility of patients in that they will have to either live in the catchment area of the centre or be willing to travel to the centre for their treatment.
Another important finding was that despite our best efforts in engaging the clinicians and encouraging them to participate in the survey, the co-operation level was not high. In effect, the clinicians were either unwilling or unable to provide us with all the information that we required, although the condensed information was good. This suggests that there is a lack of engagement of the wider clinical community with this study and this should be considered in identifying the design of a further study.
Finally, the yearly caseload figures of children with non-syndromic CP for each of the centres showed some uniformity in caseload with most centres seeing between 35 and 60 new referrals per year, three centres received between 90 and 130 referrals per year, while four had < 35 referrals per year. Although these figures need to be interpreted with a degree of caution as the CRANE database tends to slightly under-record patients (because not all parents consent for their child’s information to be included), it is generally felt to provide a good approximation of annual caseload. It is clear that for the effective running of a potential study that the centres with the higher caseloads are included. Table 59 includes data on yearly caseload and an estimate of numbers who would be recruited into a trial. This estimate is based on the number of patients who are likely to have OME (90%) and then taking a conservative estimate of those who would meet trial eligibility criteria (50%), and finally factoring in the predicted consent rate estimated from the qualitative research (25%).
| Centre | Number of new referrals (non-syndromic) | Estimate of potential numbers recruited into a trial |
|---|---|---|
| Newcastle | 65 (49) | 6 |
| Leeds | 65 (49) | 6 |
| Liverpool | 64 (48) | 6 |
| Manchester | 69 (52) | 7 |
| Nottingham | 93 (70) | 8 |
| Birmingham | 121 (91) | 10 |
| Cambridge | 87 (65) | 8 |
| North Thames | 173 (130) | 14 |
| Oxford | 45 (34) | 4 |
| Salisbury | 53 (40) | 5 |
| Swansea | 51 (38) | 5 |
| Bristol | 65 (49) | 6 |
| South Thames | 145 (109) | 12 |
| Belfast | 31 (23) | 3 |
| Edinburgh | 29 (22) | 3 |
| Glasgow | 46 (35) | 4 |
Qualitative component
This part of the study provided us with useful information on outcomes that were important to parents and patients, and their willingness to take part in a potential study. All the aims of this component were achieved.
It was clear that parents and children held strong opinions about treatment and participation in a future trial. Importantly, only 25% of those in our sample would be happy to enter their children into a trial. This reflected the fact that most parents were not in equipoise. We also felt that parents required comprehensive and detailed information about HAs and VTs. In addition to information on safety procedures in a trial, based on interview data, the following appear to be important: a clear explanation of clinical equipoise; a need for the investigators to understand patients/parents previous experience of treatment (bearing in mind that the burden of care for a child with a cleft is very high); ensuring that the study is introduced by clinicians with whom the parent and child are familiar and trust; and finally emphasising how the study will enhance knowledge and help others in the future. Addressing these issues may optimise trial recruitment.
When we evaluated outcomes that were important to interviewees, they stressed the significance of speech and language development, educational outcomes and establishing social networks. It was also clear that some of their concerns were not solely related to hearing difficulties but were associated with having a cleft. As a result, although hearing was the key outcome, this was largely because of its consequences on social and educational development and psychological well-being. The outcomes from this part of the project fed into the component of the study concerned with the development of a COS.
Core outcome set
This was the first time that a COS had been developed for research in children with cleft lip and palate. It is important to point out that we have only identified ‘what’ should be measured; we have not made any recommendations on ‘how’ the outcomes should be measured. This requires further development that is outside of the scope of this feasibility study. Although the current COS has had input from clinical stakeholders, parents and children with CP, the proportion of parents and children contributing was lower than we would have liked. We will build on our current work to ensure that the opinion of parents included in the current COS is representative.
Economic analysis
This work represents the first model-based economic evaluation study to identify and quantify the costs and benefits of different management options for children with clefts. The objective of this part of the feasibility study was to perform a decision-analytic model-based economic analysis to assess the potential impact of the surgical insertion of grommets for the management of persistent bilateral OME in children with CP.
The aim of this model was to provide information on the potential key drivers of cost-effectiveness given the current evidence base for each of the potential strategies for managing children with CP and OME. The focus of the health economics work involved:
-
completing a systematic review and critical appraisal of published models that aim to evaluate the incremental costs and benefits of the insertion of grommets in children
-
structuring and populating a decision-analytic model to determine the ICERs of ‘VTs’ strategy compared with ‘HAs’, ‘HAs plus VTs’ and ‘DN’ for the management of persistent bilateral OME in children with CP
-
performing the VOI analyses to demonstrate the value for money from the surgical insertion of VTs.
This provided highly relevant and useful information to the study. First, it emphasised the limitations of the current evidence base for the management of OME in children with CP. It also revealed that the surgical insertion of VTs is likely to be the most cost-effective option. Nevertheless, the need for additional information from a future study is required to inform this treatment choice. Importantly, the EVPI was approximately £5.24M for a population of children with CP in England, Wales and Northern Ireland, assuming the WTP of £20,000 per QALY and a decision horizon of 10 years, this suggests that further research work in this area is potentially worthwhile.
The EVPPI analysis also illustrates that if future research is to be commissioned, it should then prioritise improving the estimates of parameters (‘utility’ and ‘hearing gain in decibels’) used to calculate the QALY gain associated with each strategy because further information on these parameters could have a significant impact on decision uncertainty. However, interpretation of this analysis should be undertaken with caution, as with no definitive guidelines identified for the treatment of OME in children, the clinical pathway used to structure the economic evaluation was developed using assumptions based on available published evidence.
The effect of other research projects on potential co-operation of the centres
There are currently two major studies involving the cleft centres. These are a study into the timing of primary surgery (Timing of Primary Surgery for Cleft Palate; TOPS) and the Bristol Gene Bank and Birth Cohort. Although both these studies are involving younger children, it is important that we consider that there is risk of the staff in the cleft centres becoming ‘research fatigued’ and this will influence potential co-operation capability of some centres.
Meeting of the Study Advisory Group to decide on potential study design
The SAG met with members of the study team and evaluated the information that was derived from the components of the study. They made the following recommendations:
-
The primary outcome of a potential trial would be hearing. They generally supported the concept that a difference in hearing loss of 15 dB between two interventions would be a worthwhile clinical difference to detect.
-
The SAG suggested that we should explore the use of the OMQ-14 questionnaire to collect some information on hearing and other outcomes that may be included in the COS.
-
They suggested that the ‘ideal’ study design could be a cohort with a nested trial. All children aged 2 years old with OME, diagnosed by otoscopy and tympanometry, and CP would be entered into the cohort, with the study information including description of randomisation in the event that an intervention was needed for OME. Prior to commencement of the study, the audiological assessment protocol for centres enrolled in the study should, where necessary, be adapted and standardised. This would ensure conformity of assessment throughout the study and minimise impact on existing practice at individual centres. Additional information about the impact of OME could be collected between audiological assessment appointments using an appropriate validated questionnaire. At, or following, recruitment should a child meet the criteria for active treatment of OME (NICE CG6011) they would be asked to participate in the randomised trial of treatment involving HAs or VTs. All the children would be followed until they were aged 5 years. This is a standard data collection point for all children with clefts in the centres. 165
Nevertheless, they felt that this would be a highly ambitious study and we should make this recommendation with caution because of difficulties associated with the ‘hub and spoke’ model of care, the potentially low participation rate of patients and concerns with the engagement of clinicians.
Recommendations on future research
Before we make our final recommendations, it is useful to consider the HTA commissioning brief. This stated that the study should be directed at
What is the most appropriate way to manage OME in children with CP.
and
To carry out a feasibility and VOI study to assess the possibility of a randomised controlled trial or multicenter prospective cohort study. The primary outcome of the feasibility study is to identify the feasibility of a definitive study.
With this in mind, we have not attempted to design a trial but have aimed to provide information to the HTA programme which will help it to consider whether or not to commission further research and decide on options for the potential design of that research.
If we address the first point on whether or not a multicentre cohort is currently feasible, we are clear that a cohort study should not be explored for the following reasons:
-
The model of delivery of care would lead to major difficulties with monitoring the care provided and the uniformity of measurement of outcomes such as hearing, unless the cohort receives treatment in the centre and not in the spokes of the network.
-
There is a high risk of non-engagement of the clinical community.
-
There will be major issues with minimising bias.
-
If the cost of a cohort study would be similar to that of a trial, the trial would be the preferred option because the VOI, in terms of providing information that would reduce uncertainty, would be much greater with a trial.
Potential future trial
We decided that the primary outcome for a potential study would be hearing loss, based on the information from the economic analysis, the qualitative research, the COS and the opinion of the SAG. We asked the SAG to define a clinically meaningful difference for this between two interventions and they recommended that this was 10 dB. We could not find data of direct relevance to this study for children with CP, as all the trials identified have excluded children with CP. Nevertheless, we identified a trial of VTs versus ‘no treatment’ in children aged 19 months without CP, with a follow-up of 12 months. This is similar to the study we propose (ignoring the absence of CP patients) with our HA group not receiving a medical or surgical treatment which could effect the presence of OME (i.e. they will receive ‘no treatment’). 166
A sample size calculation based on data from this study shows that a study with a two-sided significance of 5% and a power of 90% would require 40 patients per intervention group to detect a difference of 10 dB with a standard deviation of 13.8. If we factor in potential loss to follow up of 10% we should aim for a total sample of 90 patients. If we then assume a 2-month run in for each recruiting site, the recruitment of 90 children in eight centres would take 20 months.
However, this sample size is likely to be an underestimate because the limited length of follow-up in the Rovers et al. study166 is likely to underestimate the variability in hearing loss.
Taking these factors into account, the issues that need to be considered in moving forward to a trial are:
-
there is limited data available for an accurate sample size calculation
-
the recruitment rate for any study may be low.
We suggest that additional data, which might strengthen the sample size calculation, could be obtained from a note review of hospital records to extract information for hearing levels in CP children at 2 and 5 years of age.
Concerns about the recruitment rate could be addressed by having an internal pilot in the trial, in order to identify likely recruitment rates and, through a qualitative research component, to identify barriers to recruitment and optimise methods of recruitment. For example, the qualitative component of our study suggested that parents were concerned about safety of their child, they were not in equipoise and they were not clear on the potential harms and benefits of the interventions that might be used. A decision on progression to the main trial could be taken 6 months after recruitment to the pilot has started.
Reflections on this project
We conclude with some reflections on this project. We had originally aimed to complete this project within 12 months and it was costed at a modest level because we underpinned some of the salary costs with resources from the Healing Foundation Cleft and Craniofacial Clinical Research Centre.
Unfortunately, the study over-ran by 16 months. Although the components directed at the qualitative research and health economics were completed inside the proposed time scale, there were major problems with the engagement from the clinical community which was needed to complete both the clinician survey and the COS project (although some centres were fully engaged and provided the information we required in a short time scale). In effect, we underestimated the time required to gain sufficient input from the clinical community. Despite all our efforts to facilitate this, the engagement was still slow and co-operation was not always forthcoming. We recommend that careful consideration be given to this in future studies that require extensive engagement with the cleft clinical community given how diverse it is.
Conclusion
There is need for further study of the management of OME in children with CP. This research should be a randomised trial based in eight of the UK cleft centres that compares the effectiveness of VTs with HAs. Children will enter the trial when they are 2 years old and followed for 3 years. An initial calculation suggests that the trial should enrol a sample of at least 90 children. The outcomes should be based on the COS that has been developed, with a primary outcome of hearing. However, there is uncertainty about the required sample size and likely recruitment rate for a trial.
As a result, we recommend that additional data should be obtained from a note review of hospital records to inform the sample size calculation.
Concerns about the recruitment rate could be addressed by having an internal pilot in the trial, in order to identify likely recruitment rates and, through a qualitative research component, to identify barriers to recruitment and optimise methods of recruitment. For example, the qualitative component of our study suggested that parents were concerned about the safety of their child, they were not in equipoise and they were not clear on the potential harms and benefits of the interventions that might be used. A decision on progression to the main trial could be taken 6 months after recruitment to the pilot has started.
Acknowledgements
This project was carried out by the Healing Foundation Cleft and Craniofacial Clinical Research Centre at the University of Manchester. The centre is funded by The Healing Foundation supported by Vocational Training Charitable Trust (VTCT).
This study was part-funded by the Healing Foundation supported by VTCT who funded trial staff including the study co-ordinator, information systems developer, study statistician, administrator and supervisory staff.
We acknowledge both the time and the efforts from:
Joyce Russell, Jeanette Mooney and Haydn Bellardie for their support and contribution to the qualitative protocol.
Study Advisory Group members: Debbie Sell (Senior Speech and Language Therapist, Great Ormond Street Hospital, London, UK), Anne Harding-Bell (Senior Speech and Language Therapist, Addenbrookes Hospital, Cambridge, UK), Raouf Chorbachi (Consultant Audiologist, Great Ormond Street Hospital for Children, London, UK), Per Hall (Lead Surgeon and Clinical Director, Plastic Surgeon, Addenbrookes Hospital, Cambridge, UK), Ravi Sharma (Consultant ENT surgeon, Alder Hey Children’s Hospital Trust, Liverpool, UK), Nichola Rumsey (Professor of Appearance Research, University of West of England, Bristol, UK) and Alex Bennett (Consultant ENT Surgeon, Royal Hospital for Sick Children, Edinburgh, Scotland).
Study Steering Committee members: Mike Clarke (Professor/Director of Medical Research Council Network of Hubs for Trials Methodology Research), Professor Anne Schilder (National Institute for Health Research Professor Director), Professor Andrea Manca (Professor of Health Economics, Centre for Health Economics) and Rosanna Preston (Chief Executive of the Cleft Lip and Palate Association).
Duncan Appelbe and Melanie Harper-Jones (Clinical Trials Research Centre, University of Liverpool) for their contribution to the design of the online system for the Delphi survey of health professionals and online survey of parents and children with CP, and Patricia Barnby (School of Dentistry/Healing Foundation Cleft and Craniofacial Clinical Research Centre, University of Manchester) for her contribution to the design of the online system for the Delphi survey of health professionals. Joyce Russell, Jeanette Mooney and Haydn Bellardie for their support and contribution to the qualitative protocol. Tri Tat (School of Dentistry/Healing Foundation Cleft and Craniofacial Clinical Research Centre, University of Manchester) for comments on an earlier version of the Delphi survey for health professional’s protocol. Claire Bennett (School of Dentistry/Healing Foundation Cleft and Craniofacial Clinical Research Centre, University of Manchester) for administrative support provided to the study and assisting with the formatting of the final report.
Contributions of authors
Iain Bruce contributed to the design of the study, acquisition, analysis and interpretation of the data (clinician survey, COS, health economics, final recommendations), drafted and revised the manuscript.
Nicola Harman contributed to the design of the study, acquisition, analysis and interpretation of the data (clinician survey, qualitative study, COS, final recommendations), drafted and revised the manuscript.
Paula Williamson contributed to the design of the study, acquisition, analysis and interpretation of the data (clinician survey, COS, final recommendations), drafted and revised the manuscript.
Stephanie Tierney contributed to the design of the study, acquisition, analysis and interpretation of the data (qualitative study, COS), drafted and revised the manuscript.
Peter Callery contributed to the design of the study, acquisition, analysis and interpretation of the data (qualitative study, COS, final recommendations), drafted and revised the manuscript.
Syed Mohuiddin contributed to the design of the study, acquisition, analysis and interpretation of the data (health economics), drafted and revised the manuscript.
Katherine Payne contributed to the design of the study, acquisition, analysis and interpretation of the data (health economics), drafted and revised the manuscript.
Elisabeth Fenwick contributed to the design of the health economics study, acquisition, analysis and interpretation of the data, and drafted and revised the health economics section of the manuscript.
Jamie Kirkham had input into the design and data analysis of the Delphi.
Kevin O’Brien contributed to the design of the study, acquisition, analysis and interpretation of the data (clinician survey, qualitative study, COS, final recommendations), drafted and revised the manuscript. He is the guarantor.
Data sharing statement
All data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Ponduri S, Bradley R, Ellis PE, Brookes ST, Sandy JR, Ness AR. The management of otitis media with early routine insertion of grommets in children with cleft palate – a systematic review. Cleft Palate Craniofac J 2009;46:30-8. http://dx.doi.org/10.1597/07-219.1.
- Flynn T, Möller C, Jönsson R, Lohmander A. The high prevalence of otitis media with effusion in children with cleft lip and palate as compared to children without clefts. Int J Pediatr Otorhinolaryngol 2009;73:1441-6. http://dx.doi.org/10.1016/j.ijporl.2009.07.015.
- Goldman JL, Martinez SA, Ganzel TM. Eustachian tube dysfunction and its sequelae in patients with cleft palate. South Med J 1993;86:1236-7. http://dx.doi.org/10.1097/00007611-199311000-00010.
- Maheshwar AA, Milling MAP, Kumar M, Clayton MI, Thomas A. Use of hearing aids in the management of children with cleft palate. Int J Pediatr Otorhinolaryngol 2002;66:55-62. http://dx.doi.org/10.1016/S0165-5876(02)00206-9.
- Civelek B, Celebioglu S, Sagit M, Akin I. Ventilation tubes in secretory otitis media associated with cleft palate: a retrospective analysis. Turk J Med Sci 2007;37:223-6.
- Flynn T, Moller C, Lohmander A, Magnusson L. Hearing and otitis media with effusion in young adults with cleft lip and palate. Acta Otolaryngol 2012;132:959-66. http://dx.doi.org/10.3109/00016489.2012.669497.
- Handzic-Cuk J, Cuk V, Gluhinic M, Risavi R, Stajner-Katusic S. Tympanometric findings in cleft palate patients: influence of age and cleft type. J Laryngol Otol 2001;115:91-6. http://dx.doi.org/10.1258/0022215011907668.
- Moller P. Long-term otologic features of cleft palate patients. Arch Otolaryngol 1975;101:605-7. http://dx.doi.org/10.1001/archotol.1975.00780390019005.
- Sheahan P, Blayney AW. Cleft palate and otitis media with effusion: a review. Rev Laryngol Otol Rhinol (Bord) 2003;124:171-7.
- Harman NL, Bruce IA, Callery P, Tierney S, Sharif MO, O’Brien K, et al. MOMENT – management of otitis media with effusion in cleft palate: protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-70.
- Surgical Management of Otitis Media With Effusion in Children. London: NICE; 2008.
- McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324:1448-51. http://dx.doi.org/10.1136/bmj.324.7351.1448.
- Nelson P, Glenny AM, Kirk S, Caress AL. Parents’ experiences of caring for a child with a cleft lip and/or palate: a review of the literature. Child Care Health Dev 2012;38:6-20. http://dx.doi.org/10.1111/j.1365-2214.2011.01244.x.
- Sharif MO, Callery P, Tierney S. The perspectives of children and young people living with cleft lip and palate: a review of qualitative literature. Cleft Palate Craniofac J 2013;50:297-304. http://dx.doi.org/10.1597/12-054.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research in health technology assessment: a review of the literature. Health Technol Assess 1998;2.
- Spencer L, Ritchie J, O’Connor W, Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. Thousand Oaks, CA: Sage Publications; 2003.
- Surgical Management of Otitis Media with Effusion in Children. London: National Collaborating Centre for Women’s and Children’s Health; 2008.
- Ritchie J, Lewis J, Elam G, Ritchie J, Lewis J. Qualitative Research Practice. London: Sage Publications; 2003.
- Shilling V, Williamson P, Hickey H, Sowden E, Smyth R, Young B. Processes in recruitment to randomised controlled trials of medicines for children (RECRUIT): a qualitative study. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15150.
- Irwin LG, Johnson J. Interviewing young children: explicating our practices and dilemmas. Qual Health Res 2005;15:821-31. http://dx.doi.org/10.1177/1049732304273862.
- O’Kane C, Christensen PM, James A. Research with Children: Perspectives and Practices. London: RoutledgeFalmer; 2000.
- Darbyshire P, Macdougall C, Schiller W. Multiple methods in qualitative research with children: more insight or just more?. Qual Res 2005;5:417-36. http://dx.doi.org/10.1177/1468794105056921.
- Hill M. Participatory research with children. Child Fam Soc Work 1997;2:171-83. http://dx.doi.org/10.1046/j.1365-2206.1997.00056.x.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. Thousand Oaks, CA: Sage Publications; 2003.
- Srivastava A, Thomson SB. Framework analysis: a qualitative methodology for applied policy research. J Admin Govern 2009;4:72-9.
- Ward DJ, Furber C, Tierney S, Swallow V. Using framework analysis in nursing research: a worked example. J Adv Nurs 2013;69:2423-31. http://dx.doi.org/10.1111/jan.12127.
- Elliott R, Fischer CT, Rennie DL. Evolving guidelines for publication of qualitative research studies in psychology and related fields. Br J Clin Psychol 1999;38:215-29. http://dx.doi.org/10.1348/014466599162782.
- Long T, Johnson M. Rigour, reliability and validity in qualitative research . . . including commentary by Burnard P with author response. Clin Eff Nurs 2000;4:30-7. http://dx.doi.org/10.1054/cein.2000.0106.
- Corbett F, Oldham J, Lilford R. Offering patients entry in clinical trials: preliminary study of the views of prospective participants. J Med Ethics 1996;22:227-31. http://dx.doi.org/10.1136/jme.22.4.227.
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7. http://dx.doi.org/10.1186/1745-6215-7-9.
- Clinical Trials for Tomorrow. London: Medical Research Council; 2003.
- Edwards SJ, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J. Ethical issues in the design and conduct of randomised controlled trials. Health Technol Assess 1998;2.
- Featherstone K, Donovan JL. ‘Why don’t they just tell me straight, why allocate it?’ The struggle to make sense of participating in a randomised controlled trial. Soc Sci Med 2002;55:709-19. http://dx.doi.org/10.1016/S0277-9536(01)00197-6.
- Daugherty C, Ratain MJ, Grochowski E, Stocking C, Kodish E, Mick R, et al. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol 1995;13:1062-72.
- Shilling V, Young B. How do parents experience being asked to enter a child in a randomised controlled trial?. BMC Med Ethics 2009;10. http://dx.doi.org/10.1186/1472-6939-10-1.
- Zupancic JA, Gillie P, Streiner DL, Watts JL, Schmidt B. Determinants of parental authorization for involvement of newborn infants in clinical trials. Pediatrics 1997;99. http://dx.doi.org/10.1542/peds.99.1.e6.
- Sammons H, Atkinson M, Choonara I, Stephenson T. What motivates British parents to consent for research? A questionnaire study. BMC Pediatr 2007;7. http://dx.doi.org/10.1186/1471-2431-7-12.
- Glogowska M, Roulstone S, Enderby P, Peters T, Campbell R. Who’s afraid of the randomised controlled trial? Parents’ views of an SLT research study. Int J Lang Commun Disord 2001;36:499-504. http://dx.doi.org/10.3109/13682820109177936.
- Kass NE, Sugarman J, Faden R, Schoch-Spana M. Trust, the fragile foundation of contemporary biomedical research. Hastings Cent Rep 1996;26:25-9. http://dx.doi.org/10.2307/3528467.
- Eder ML, Yamokoski AD, Wittmann PW, Kodish ED. Improving informed consent: suggestions from parents of children with leukemia. Pediatrics 2007;119:e849-59. http://dx.doi.org/10.1542/peds.2006-2208.
- Postlethwaite RJ, Reynolds JM, Wood AJ, Evans JH, Lewis MA, Eminson DM. Recruiting patients to clinical trials: lessons from studies of growth hormone treatment in renal failure. Arch Dis Child 1995;73:30-5. http://dx.doi.org/10.1136/adc.73.1.30.
- Sweetow RW, Rosbe KW, Philliposian C, Miller MT. Considerations for cochlear implantation of children with sudden, fluctuating hearing loss. J Am Acad Audiol 2005;16:770-80. http://dx.doi.org/10.3766/jaaa.16.10.2.
- Paediatric European Network for Treatment of AIDS . Parents’ attitudes to their HIV-infected children being enrolled into a placebo-controlled trial: the PENTA 1 trial. HIV Med 1999;1:25-31. http://dx.doi.org/10.1046/j.1468-1293.1999.00005.x.
- Singhal N, Oberle K, Burgess E, Huber-Okrainec J. Parents’ perceptions of research with newborns. J Perinatol 2002;22:57-63. http://dx.doi.org/10.1038/sj.jp.7210608.
- Oxford English Dictionary n.d. www.oed.com (accessed April 2014).
- Wells GA. Patient-driven outcomes in rheumatoid arthritis. J Rheumatol Suppl 2009;82:33-8. http://dx.doi.org/10.3899/jrheum.090129.
- Fitzpatrick E, Graham ID, Durieux-Smith A, Angus D, Coyle D. Parents’ perspectives on the impact of the early diagnosis of childhood hearing loss. Int J Audiol 2007;46:97-106. http://dx.doi.org/10.1080/14992020600977770.
- Sinha I, Jones L, Smyth RL, Williamson PR. A systematic review of studies that aim to determine which outcomes to measure in clinical trials in children. PLOS Med 2008;5. http://dx.doi.org/10.1371/journal.pmed.0050096.
- Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-39.
- Cooney R, Warren B, Altman D, Abreu M, Travis S. Outcome measurement in clinical trials for ulcerative colitis: towards standardisation. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-17.
- Chan A-W, Altman DG. Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38356.424606.8F.
- Tugwell P, Boers M, Brooks P, Simon L, Strand V, Idzerda L. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-38.
- Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia 2004;45:1416-28. http://dx.doi.org/10.1111/j.0013-9580.2004.02404.x.
- Ruperto N, Ravelli A, Murray KJ, Lovell DJ, Andersson-Gare B, Feldman BM, et al. Preliminary core sets of measures for disease activity and damage assessment in juvenile systemic lupus erythematosus and juvenile dermatomyositis. Rheumatology 2003;42:1452-9. http://dx.doi.org/10.1093/rheumatology/keg403.
- Chen JL, Messner AH, Curtin G. Newborn hearing screening in infants with cleft palates. Otol Neurotol 2008;29:812-15. http://dx.doi.org/10.1097/MAO.0b013e318180a4e0.
- Simpson SA, Thomas CL, van der Linden MK, Macmillan H, van der Wouden JC, Butler C. Identification of children in the first four years of life for early treatment for otitis media with effusion. Cochrane Database Syst Rev 2007;1. http://dx.doi.org/10.1002/14651858.cd004163.pub2.
- Thomas CL, Simpson S, Butler CC, van der Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2006;3. http://dx.doi.org/10.1002/14651858.cd001935.pub2.
- van den Aardweg MT, Schilder AG, Herkert E, Boonacker CW, Rovers MM. Adenoidectomy for otitis media in children. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd007810.pub2.
- Griffin GH, Flynn C, Bailey RE, Schultz JK. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.cd003423.pub2.
- Browning GG, Rovers MM, Williamson I, Lous J, Burton MJ. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2010;10. http://dx.doi.org/10.1002/14651858.cd001801.pub3.
- Perera R, Haynes J, Glasziou P, Heneghan CJ. Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.cd006285.
- Møller P. Seletive use of ventilating tubes in the treatment of secretory otitis media and retractions of the ear drum. Acta Otolaryngol 1982;93:158-60. http://dx.doi.org/10.3109/00016488209108504.
- Potsic WP, Cohen M, Randall P, Winchester R. Retrospective study of hearing impairment in 3 groups of cleft-palate patients. Cleft Palate J 1979;16:56-8.
- Paradise JL, Bluestone CD. Early treatment of the universal otitis media of infants with cleft palate. Pediatrics 1974;53:48-54.
- Møller P. Hearing, middle-ear pressure and otopathology in a cleft-palate population. Acta Otolaryngol 1981;92:521-8. http://dx.doi.org/10.3109/00016488109133291.
- Smith TL, DiRuggiero DC, Jones KR. Recovery of eustachian tube function and hearing outcome in patients with cleft palate. Otolaryngol Head Neck Surg 1994;111:423-9.
- Hormann K, Roehrs M. Middle-ear findings in young cleft lip and palate children. Comparison of two treatment clinics. Dtsche Z Mund Kiefer Gesichtschir 1991;15:149-52.
- Broen PA, Moller KT, Carlstrom J, Doyle SS, Devers M, Keenan KM. Comparison of the hearing histories of children with and without cleft palate. Cleft Palate Craniofac J 1996;33:127-33. http://dx.doi.org/10.1597/1545-1569(1996)033<0127:COTHHO>2.3.CO;2.
- Frable MA, Brandon GT, Theogaraj SD. Velar closure and ear tubings as a primary procedure in the repair of cleft palates. Laryngoscope 1985;95:1044-6. http://dx.doi.org/10.1288/00005537-198509000-00004.
- Sheahan P, Blayney AW, Sheahan JN, Earley MJ. Sequelae of otitis media with effusion among children with cleft lip and/or cleft palate. Clin Otolaryngol 2002;27:494-500. http://dx.doi.org/10.1046/j.1365-2273.2002.00607.x.
- Hubbard TW, Paradise JL, McWilliams BJ, Elster BA, Taylor FH. Consequences of unremitting middle-ear disease in early life. Otologic, audiologic, and developmental findings in children with cleft palate. N Engl J Med 1985;312:1529-34. http://dx.doi.org/10.1056/NEJM198506133122401.
- Gordon ASD, Jeanlouis F, Morton RP. Late ear sequelae in cleft-palate patients. Int J Pediatr Otorhinolaryngol 1988;15:149-56. http://dx.doi.org/10.1016/0165-5876(88)90066-3.
- Robson AK, Blanshard JD, Jones K, Albery EH, Smith IM, Maw AR. A conservative approach to the management of otitis-media with effusion in cleft-palate children. J Laryngol Otol 1992;106:788-92. http://dx.doi.org/10.1017/S0022215100120894.
- Greig AV, Papesch ME, Rowsell AR. Parental perceptions of grommet insertion in children with cleft palate. J Laryngol Otol 1999;113:1068-71.
- Shaw R, Richardson D, McMahon S. Conservative management of otitis media in cleft palate. J Craniomaxillofac Surg 2003;31:316-20. http://dx.doi.org/10.1016/S1010-5182(03)00074-X.
- Freeland AP, Evans DM. Middle-ear disease in the cleft-palate infant – its effect on speech and language-development. Br J Plast Surg 1981;34:142-3. http://dx.doi.org/10.1016/S0007-1226(81)80081-1.
- Zheng Q, Xu H, He Y. Effects of tympanotomy and pressure equilibrium tube insertion during palatoplasty on prognoses of otitis media with effusion. Hua Xi Kou Qiang Yi Xue Za Zhi 2003;21:28-30.
- Liu L, Sun Y-G, Ma L, Zhao W, Wu R. Effect of ventilation tube insertion on otitis media with effusion in cleft palate children. Zhonghua Er Bi Yan Hou Ke Za Zhi 2004;39:216-18.
- Tanpowpong K, Saisukul I, Kittimanont H, Rattanasiri S. Outcome of myringotomy with ventilation tube for otitis media with effusion in Thai children: Ramathibodi experiences. J Med Assoc Thai 2007;90:1866-71.
- Phua YS, Salkeld LJ, de Chalain TM. Middle ear disease in children with cleft palate: protocols for management. Int J Pediatr Otorhinolaryngol 2009;73:307-13. http://dx.doi.org/10.1016/j.ijporl.2008.10.026.
- Reiter R, Haase S, Brosch S. Repaired cleft palate and ventilation tubes and their associations with cholesteatoma in children and adults. Cleft Palate Craniofac J 2009;46:598-602. http://dx.doi.org/10.1597/08-166.1.
- Szabo C, Langevin K, Schoem S, Mabry K. Treatment of persistent middle ear effusion in cleft palate patients. Int J Pediatr Otorhinolaryngol 2010;74:874-7. http://dx.doi.org/10.1016/j.ijporl.2010.04.016.
- Hornigold R, Morley A, Glore RJ, Boorman J, Sergeant R. The long-term effect of unilateral t-tube insertion in patients undergoing cleft palate repair: 20-year follow-up of a randomised controlled trial. Clin Otolaryngol 2008;33:265-8. http://dx.doi.org/10.1111/j.1749-4486.2008.01670.x.
- Merrick GD, Kunjur J, Watts R, Markus AF. The effect of early insertion of grommets on the development of speech in children with cleft palates. Br J Oral Maxillofac Surg 2007;45:527-33. http://dx.doi.org/10.1016/j.bjoms.2007.02.003.
- Curtin G, Messner AH, Chang KW. Otorrhea in infants with tympanostomy tubes before and after surgical repair of a cleft palate. Arch Otolaryngol Head Neck Surg 2009;135:748-51. http://dx.doi.org/10.1001/archoto.2009.106.
- Mandel EM, Casselbrant ML, Rockette HE, Fireman P, Kurs-Lasky M, Bluestone CD. Systemic steroid for chronic otitis media with effusion in children. Pediatrics 2002;110:1071-80. http://dx.doi.org/10.1542/peds.110.6.1071.
- Casselbrant ML, Mandel EM, Rockette HE, Kurs-Lasky M, Fall PA, Bluestone CD. Adenoidectomy for otitis media with effusion in 2–3-year-old children. Int J Pediatr Otorhinolaryngol 2009;73:1718-24. http://dx.doi.org/10.1016/j.ijporl.2009.09.007.
- Koivunen P, Uhari M, Luotonen J, Kristo A, Raski R, Pokka T, et al. Adenoidectomy versus chemoprophylaxis and placebo for recurrent acute otitis media in children aged under 2 years: randomised controlled trial. BMJ 2004;328:487-90. http://dx.doi.org/10.1136/bmj.37972.678345.0D.
- Mattila PS, Joki-Erkkila VP, Kilpi T, Jokinen J, Herva E, Puhakka H. Prevention of otitis media by adenoidectomy in children younger than 2 years. Arch Otolaryngol Head Neck Surg 2003;129:163-8. http://dx.doi.org/10.1001/archotol.129.2.163.
- Paradise JL, Bluestone CD, Rogers KD, Taylor FH, Colborn DK, Bachman RZ, et al. Efficacy of adenoidectomy for recurrent otitis-media in children previously treated with tympanostomy-tube placement – results of parallel randomized and nonrandomized trials. JAMA 1990;263:2066-73. http://dx.doi.org/10.1001/jama.1990.03440150074029.
- Paradise JL, Bluestone CD, Colborn DK, Bernard BS, Smith CG, Rockette HE, et al. Adenoidectomy and adenotonsillectomy for recurrent acute otitis media – parallel randomized clinical trials in children not previously treated with tympanostomy tubes. JAMA 1999;282:945-53. http://dx.doi.org/10.1001/jama.282.10.945.
- Rynneldagoo B, Ahlbom A, Schiratzki H. Effects of adenoidectomy – controlled 2-year follow-up. Ann Otol Rhinol Laryngol 1978;87:272-8. http://dx.doi.org/10.1177/000348947808700223.
- Gates GA, Avery CA, Cooper JC, Prihoda TJ. Effectiveness of adenoidectomy and tympanostomy tubes in the treatment of chronic otitis media with effusion. New Engl J Med 1987;317:1444-51. http://dx.doi.org/10.1056/NEJM198712033172305.
- Hammaren-Malmi S, Saxen H, Tarkkanen J, Mattila PS. Adenoidectomy does not significantly reduce the incidence of otitis media in conjunction with the insertion of tympanostomy tubes in children who are younger than 4 years: a randomized trial. Pediatrics 2005;116:185-9. http://dx.doi.org/10.1542/peds.2004-2253.
- Roydhouse N. Adenoidectomy for otitis-media with mucoid effusion. Ann Otol Rhinol Laryngol 1980;89:312-15.
- Black NA, Sanderson CFB, Freeland AP, Vessey MP. A randomized controlled trial of surgery for glue ear. BMJ 1990;300:1551-6. http://dx.doi.org/10.1136/bmj.300.6739.1551.
- Dempster JH, Browning GG, Gatehouse SG. A randomized study of the surgical-management of children with persistent otitis-media with effusion associated with a hearing impairment. J Laryngol Otol 1993;107:284-9. http://dx.doi.org/10.1017/S0022215100122844.
- Maw R, Wilks J, Harvey I, Peters TJ, Golding J. Early surgery compared with watchful waiting for glue ear and effect on language development in preschool children: a randomised trial. Lancet 1999;353:960-3. http://dx.doi.org/10.1016/S0140-6736(98)05295-7.
- Rach GH, Zielhuis GA, Vanbaarle PW, Vandenbroek P. The effect of treatment with ventilating tubes on language-development in preschool-children with otitis-media with effusion. Clin Otolaryngol 1991;16:128-32. http://dx.doi.org/10.1111/j.1365-2273.1991.tb01960.x.
- Rovers MM, Straatman H, Ingels K, van der Wilt GJ, van den Broek P, Zielhuis GA. The effect of ventilation tubes on language development in infants with otitis media with effusion: a randomized trial. Pediatrics 2000;106. http://dx.doi.org/10.1542/peds.106.3.e42.
- Johnston LC, Feldman HM, Paradise JL, Bernard BS, Colborn DK, Casselbrant ML, et al. Tympanic membrane abnormalities and hearing levels at the ages of 5 and 6 years in relation to persistent otitis media and tympanostomy tube insertion in the first 3 years of life: a prospective study incorporating a randomized clinical trial. Pediatrics 2004;114:E58-67. http://dx.doi.org/10.1542/peds.114.1.e58.
- Paradise JL, Feldman HM, Campbell TF, Dollaghan CA, Colborn DK, Bernard BS, et al. Effect of early or delayed insertion of tympanostomy tubes for persistent otitis media on developmental outcomes at the age of three years. N Engl J Med 2001;344:1179-87. http://dx.doi.org/10.1056/NEJM200104193441601.
- Maw AR. Chronic otitis-media with effusion (glue ear) and adenotonsillectomy – prospective randomized controlled-study. BMJ 1983;287:1586-8. http://dx.doi.org/10.1136/bmj.287.6405.1586.
- Zielhuis GA, Rach GH, Vandenbroek P. Screening for otitis-media with effusion in preschool-children. Lancet 1989;1:311-14. http://dx.doi.org/10.1016/S0140-6736(89)91317-2.
- Fiellau-Nikolajsen M, Falbe-Hansen J, Knudstrup P. Adenoidectomy for middle-ear disorders – a randomized controlled trial. Clin Otolaryngol Allied Sci 1980;5:323-7. http://dx.doi.org/10.1111/j.1365-2273.1980.tb00898.x.
- Nguyen LHP, Manoukian JJ, Yoskovitch A, Al-Sebeih KH. Adenoidectomy: selection criteria for surgical cases of otitis media. Laryngoscope 2004;114:863-6. http://dx.doi.org/10.1097/00005537-200405000-00014.
- Paradise JL, Feldman HM, Campbell TF, Dollaghan CA, Rockette HE, Pitcairn DL, et al. Tympanostomy tubes and developmental outcomes at 9 to 11 years of age. N Engl J Med 2007;356:248-61. http://dx.doi.org/10.1056/NEJMoa062980.
- Paradise JL, Campbell TF, Dollaghan CA, Feldman HM, Bernard BS, Colborn DK, et al. Developmental outcomes after early or delayed insertion of tympanostomy tubes. N Engl J Med 2005;353:576-86. http://dx.doi.org/10.1056/NEJMoa050406.
- Paradise JL, Dollaghan CA, Campbell TF, Feldman HM, Bernard BS, Colborn DK, et al. Otitis media and tympanostomy tube insertion during the first three years of life: developmental outcomes at the age of four years. Pediatrics 2003;112:265-77. http://dx.doi.org/10.1542/peds.112.2.265.
- Paradise JL. Mix of middle ear effusions in infants with cleft palate. Ann Otol Rhinol Laryngol Suppl 1976;85:285-8.
- National Institute of Adult Continuing Education . SMOG Calculator 2014. www.niace.org.uk/misc/SMOG-calculator/smogcalc.php (accessed 4 April 2014).
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395-400. http://dx.doi.org/10.1016/j.jclinepi.2010.09.012.
- Grant HR, Quiney RE, Mercer DM, Lodge S. Cleft-palate and glue ear. Arch Dis Child 1988;63:176-9. http://dx.doi.org/10.1136/adc.63.2.176.
- Britten N, Ukoumunne O. The influence of patients’ hopes of receiving a prescription on doctors’ perceptions and the decision to prescribe: a questionnaire survey. BMJ 1997;315:1506-10. http://dx.doi.org/10.1136/bmj.315.7121.1506.
- Edwards PJ, Roberts I, Clarke MJ, Diguiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev n.d.;3. http://dx.doi.org/10.1002/14651858.mr000008.pub4.
- John A, Sell D, Sweeney T, Harding-Bell A, Williams A. The cleft audit protocol for speech-augmented: a validated and reliable measure for auditing cleft speech. Cleft Palate Craniofac J 2006;43:272-88. http://dx.doi.org/10.1597/04-141R.1.
- Shaw WC, Semb G, Nelson P, Brattstrom V, Molsted K, Prahl-Andersen B, et al. The Eurocleft Project 1996–2000: overview. J Cranio-MaxilloFac Surg 2001;29:131-40. http://dx.doi.org/10.1054/jcms.2001.0217.
- Global Strategies Towards Reducing the Health-Care Burden of Craniofacial Anomalies. Geneva: WHO; 2002.
- Offcom . The Communications Market Report: United Kingdom 2011. http://stakeholders.ofcom.org.uk/market-data-research/market-data/communications-market-reports/cmr11/uk/ (accessed April 2014).
- Khanna R, Lakhanpaul M, Bull PD, Group GD. Surgical management of otitis media with effusion in children: summary of NICE guidance. Clin Otolaryngol 2008;33:600-5. http://dx.doi.org/10.1111/j.1749-4486.2008.01844.x.
- Berman S, Roark R, Luckey D. Theoretical cost effectiveness of management options for children with persisting middle ear effusions. Pediatrics 1994;93:353-63.
- Hartman M, Rovers MM, Ingels K, Zielhuis GA, Severens JL, van der Wilt GJ. Economic evaluation of ventilation tubes in otitis media with effusion. Arch Otolaryngol Head Neck Surg 2001;127:1471-6. http://dx.doi.org/10.1001/archotol.127.12.1471.
- Mui S, Rasgon BM, Hilsinger RL, Lewis B, Lactao G. Tympanostomy tubes for otitis media: quality-of-life improvement for children and parents. Ear Nose Throat J 2005;84.
- Sheahan P, Miller I, Sheahan JN, Earley MJ, Blayney AW. Incidence and outcome of middle ear disease in cleft lip and/or cleft palate. Int J Pediatr Otorhinolaryngol 2003;67:785-93. http://dx.doi.org/10.1016/S0165-5876(03)00098-3.
- Gould HJ. Hearing loss and cleft palate: the perspective of time. Cleft Palate J 1990;27:36-9. http://dx.doi.org/10.1597/1545-1569(1990)027<0036:HLACPT>2.3.CO;2.
- Guide to the Methods of Technology Appraisal. London: NICE; 2004.
- Barton P, Bryan S, Robinson S. Modelling in the economic evaluation of health care: selecting the appropriate approach. J Health Serv Res Policy 2004;9:110-18. http://dx.doi.org/10.1258/135581904322987535.
- Kwan WMY, Abdullah VJ, Liu K, van Hasselt CA, Tong MCF. Otitis media with effusion and hearing loss in chinese children with cleft lip and palate. Cleft Palate Craniofac J 2011;48:684-9. http://dx.doi.org/10.1597/10-006.
- Kay DJ, Nelson M, Rosenfeld RM. Meta-analysis of tympanostomy tube sequelae. Otolaryngol Head Neck Surg 2001;124:374-80. http://dx.doi.org/10.1067/mhn.2001.113941.
- Fior R, Veljak C. Late results and complications of tympanostomy tube insertion for prophylaxis of recurrent purulent otitis media in pediatric age. Int J Pediatr Otorhinolaryngol 1984;8:139-46. http://dx.doi.org/10.1016/S0165-5876(84)80062-2.
- Vlastarakos PV, Nikolopoulos TP, Korres S, Tavoulari E, Tzagaroulakis A, Ferekidis E. Grommets in otitis media with effusion: the most frequent operation in children. But is it associated with significant complications?. Eur J Pediatr 2007;166:385-91. http://dx.doi.org/10.1007/s00431-006-0367-x.
- Gibb AG. President’s address. Tympanosclerosis. Proc R Soc Med 1976;69:155-62.
- Williamson I. Otitis media with effusion in children. BMJ Clin Evid 2011;2011.
- Lous J, Burton MJ, Felding JU, Ovesen T, Rovers MM, Williamson I. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2005;1. http://dx.doi.org/10.1002/14651858.cd001801.pub2.
- NHS Choices . Complications of Glue Ear 2013. www.nhs.uk/Conditions/Glueear/Pages/Complications.aspx (accessed 10 July 2013).
- Hoffmann KK, Thompson GK, Burke BL, Derkay CS. Anesthetic complications of tympanostomy tube placement in children. Arch Otolaryngol Head Neck Surg 2002;128:1040-3. http://dx.doi.org/10.1001/archotol.128.9.1040.
- Oxford Radcliffe Hospitals NHS Trust . Grommets Surgery for Glue Ear – Information for Patients 2012. www.ouh.nhs.uk/patient-guide/leaflets/files%5C101018grommets.pdf (accessed 10 April 2013).
- Gani B, Kinshuck AJ, Sharma R. A review of hearing loss in cleft palate patients. Int J Otolaryngol 2012;2012. http://dx.doi.org/10.1155/2012/548698.
- Rovers MM, Schilder AGM, Zielhuis GA, Rosenfeld RM. Otitis media. Lancet 2004;363:465-73. http://dx.doi.org/10.1016/S0140-6736(04)15495-0.
- Rosenfeld RM, Kay D. Natural history of untreated otitis media. Laryngoscope 2003;113:1645-57. http://dx.doi.org/10.1097/00005537-200310000-00004.
- Rovers MM, Glasziou P, Appelman CL, Burke P, McCormick DP, Damoiseaux RA, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet 2006;368:1429-35. http://dx.doi.org/10.1016/S0140-6736(06)69606-2.
- Gunasekera H, O’Connor TE, Vijayasekaran S, Del Mar CB. Primary care management of otitis media among Australian children. Med J Aust 2009;191:S55-9.
- Russell C, Black O, Dutt D, Ray A, Devlin M, Wynne D. Are ventilation tubes (grommets) in cleft children truly associated with increased complication rates? Results of a nested case control study of cleft and non-cleft children. Br J Oral Maxillofac Surg 2012;50:S2-3. http://dx.doi.org/10.1016/j.bjoms.2012.04.151.
- Maw R, Bawden R. Spontaneous resolution of severe chronic glue ear in children and the effect of adenoidectomy, tonsillectomy, and insertion of ventilation tubes (grommets). BMJ 1993;306:756-60. http://dx.doi.org/10.1136/bmj.306.6880.756.
- Kubba H. Quality of Life Assessment in Paediatric Otolaryngology 2004.
- Department of Health . NHS Reference Costs 2010–11 n.d. www.gov.uk/government/publications/2010-11-reference-costs-publication (accessed March 2013).
- Personal Social Services Research Unit . Unit Costs of Health and Social Care 2011 n.d. www.pssru.ac.uk/pdf/uc/uc2011/uc2011.pdf (accessed March 2013).
- Connevans Limited n.d. www.connevans.co.uk (accessed 3 December 2012).
- HAB . Hearing Aid Batteries and Accessories n.d. www.hearing-aid-batteries.org.uk (accessed 3 December 2012).
- Rosenfeld RM, Bhaya MH, Bower CM, Brookhouser PE, Casselbrant ML, Chan KH, et al. Impact of tympanostomy tubes on child quality of life. Arch Otolaryngol Head Neck Surg 2000;126:585-92. http://dx.doi.org/10.1001/archotol.126.5.585.
- CRANE Database . Annual Report on Cleft Lip and Or Palate 2013 n.d. www.craniofacialsociety.org.uk/downloads/CRANE%20Annual%20Report%202013%20(Final).pdf (accessed March 2014).
- Doyle WJ, Cantekin EI, Bluestone CD. Eustachian-tube function in cleft-palate children. Ann Otol Rhinol Laryngol 1980;89:34-40.
- Stool SE, Randall P. Unexpected ear disease in infants with cleft palate. Cleft Palate J 1967;4:99-103.
- Armstrong BW. A new treatment for chronic secretory otitis media. AMA Arch Otolaryngol 1954;59:653-4. http://dx.doi.org/10.1001/archotol.1954.00710050665001.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Brennan A, Kharroubi S, O’hagan A, Chilcott J. Calculating partial expected value of perfect information via Monte Carlo sampling algorithms. Med Decis Making 2007;27:448-70. http://dx.doi.org/10.1177/0272989X07302555.
- Groot Koerkamp B, Myriam Hunink MG, Stijnen T, Weinstein MC. Identifying key parameters in cost-effectiveness analysis using value of information: a comparison of methods. Health Econ 2006;15:383-92. http://dx.doi.org/10.1002/hec.1064.
- Bluestone CD. Eustachian tube obstruction in infant with cleft palate. Ann Otol Rhinol Laryngol 1971;80:1-30.
- Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224-7. http://dx.doi.org/10.1136/bmj.329.7459.224.
- Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8310.
- Kalcioglu MT, Cokkeser Y, Kizilay A, Ozturan O. Follow-up of 366 ears after tympanostomy tube insertion: why is it draining?. Otolaryngol Head Neck Surg 2003;128:560-4. http://dx.doi.org/10.1016/S0194-5998(03)00120-7.
- Schönweiler R, Lisson JA, Schönweiler B, Eckardt A, Ptok M, Tränkmann J, et al. A retrospective study of hearing, speech and language function in children with clefts following palatoplasty and veloplasty procedures at 18–24 months of age. Int J Pediatr Otorhinolaryngol 1999;50:205-17. http://dx.doi.org/10.1016/S0165-5876(99)00243-8.
- Schönweiler R, Schönweiler B, Schmelzeisen R. Hearing capacity and speech production in 417 children with facial cleft abnormalities. HNO 1994;42:691-6.
- Macfarlane J, Holmes W, Macfarlane R, Britten N. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. BMJ 1997;315:1211-14. http://dx.doi.org/10.1136/bmj.315.7117.1211.
- NHS England . NHS Standard Contract for Cleft Lip and Or Palate Services Including Non-Cleft Velopharyngeal Dysfunction (VPD) (All Ages) n.d. www.england.nhs.uk/wp-content/uploads/2013/06/d07-cleft-lip.pdf (accessed April 2014).
- Rovers MM, Straatman H, Ingels K, van der Wilt GJ, van den Broek P, Zielhuis GA. The effect of short-term ventilation tubes versus watchful waiting on hearing in young children with persistent otitis media with effusion: a randomized trial. Ear Hear 2001;22:191-9. http://dx.doi.org/10.1097/00003446-200106000-00003.
Appendix 1 mOMEnt clinician survey V1.0 30 October 2013
Appendix 2 Full results of clinician survey
The survey closed end of working Monday 3 February 2014 and final data entry occurred on Tuesday 4 February 2014.
The statistical analysis was completed on 4 February 2014 and disseminated on the 5 February 2014.
Statistical summaries
Sections refer to sections of the questionnaire sent to cleft sites (see Appendix 1).
Section 2: clinical provision and practice
2.1: Does your cleft service have dedicated audiology input based at your centre?
| Centre ID | Audiologist: dedicated audiology input | Clinical scientist: dedicated audiology input | Paediatrician: dedicated audiology input | Audiovestibular physician: dedicated audiology input | Number of professions |
|---|---|---|---|---|---|
| 13 | Yes | Yes | No | No | 2 |
| 10 | Yes | No | No | No | 1 |
| 12 | No | No | Yes | No | 1 |
| 16 | Yes | Yes | No | Yes | 3 |
| 1 | No | Yes | No | No | 1 |
| 17 | Yes | No | No | No | 1 |
| 8 | Yes | No | Yes | Yes | 3 |
| 7 | Yes | No | No | No | 1 |
| 2 | Yes | Yes | Yes | Yes | 4 |
| 6 | |||||
| 11 | |||||
| 4 | No | No | No | No | 0 |
| 5 | |||||
| 15 | |||||
| 14 | |||||
| 9 | |||||
| Overall n/N (%) | 7/10 (70.0) | 4/10 (40.0) | 3/10 (30.0) | 3/10 (30.0) | None = 1/10 (10.0) One profession = 5/10 (50.0) Two professions = 1/10 (10.0) Three professions = 2/10 (20.0) Four professions = 1/10 (10.0) |
2.2: Does your cleft service have a dedicated ENT clinician?
2.3: Is there anyone else in your centre that is involved in the management of OME in children with cleft? For example, community paediatrician (audiology) or audiovestibular physician.
| Centre ID | Question 2.2 | Question 2.3 | ||
|---|---|---|---|---|
| ENT consultant: dedicated ENT clinician | Associate specialist/staff dedicated: ENT clinician | Other dedicated ENT clinician/text | Anyone else involved in management of OME/text | |
| 13 | Yes | No | No | Yes/all other ENT consultants, associate specialists, specialist trainees and audiologists in [location] |
| 10 | Yes | No | Noa | No |
| 12 | Yes | No | Noa/[name] as general ENT adviser but children more likely to be seen by local ENT services | Yesb/I and my team offer follow-up to all CLEFT children . . . the team comprises audiologists and community paediatricians |
| 16 | Yes | No | Yes/ENT fellow | No |
| 1 | No | No | Yes/associate specialist ENT | Yes/audiologists, paediatricians (audiology) and ENT at each of the spoke hospital centres |
| 17 | Yes | No | Yes/SPR/post-CCT ENT fellow | No |
| 8 | No | No | No | No |
| 7 | Yes | No | No | Yes |
| 2 | Yes | No | No | No |
| 6 | ||||
| 11 | ||||
| 4 | No | |||
| 5 | ||||
| 15 | ||||
| 14 | ||||
| 9 | ||||
| Overall n/N (%) | 7/9 (77.8) | 0/9 (0.0) | 3/9 (33.3) | 4/10 (40.0) |
2.4: How often do children with CP receive routine audiological assessment at your cleft centre. If assessment varies by age please give frequency of routine audiological assessment and age ranges.
Individual listing of answers.
| Centre ID | Text answer to ‘how often receive routine audiological assessment?’ |
|---|---|
| 13 | NBHS. 8–10 Months. 18 months. 3 yrs. School screen. 5, 10, 15 and 20 yrs @ audit clinics |
| 10 | Normal hearing – 6 mthly if preschool and annually if school age. If a hearing loss is identified these children will follow pathway for CDHL |
| 12 | Children seen at 8 months age and then as frequently as clinical needs directs |
| 16 | All children seen at 8 months/3 yrs/5 yrs/7 yrs/10 yrs. Seen as clinical need dictates between these times for management of any hearing issues |
| 1 | All receive audiological assessment one week prior to primary palate surgery (approx 6 mths – 12 mths depending of palate surgery timing). They receive behavioural assessment follow up before 12 months as per national NHSP protocol requirements. Between 1–4 yrs: they are assessed locally until normal hearing or a sensorineural hearing loss is established bilaterally without intervention (i.e. grommets). Follow up timing is determined on a case by case basis depending on hearing results, timings are likely to be 3 months, 6 months or annual review depending on the hearing results, hearing history, speech and language therapists feedback and parental views. Assessments are ‘open’ and can be rearranged as and when in response to parental/professional concerns, i.e. families can access services before the scheduled review date. All children with a palatal cleft are recalled for a 5 year audit assessment between 5 : 0 to 5 : 11 as per national cleft service requirements. The 5 yr assessment is reported nationally as part of the cleft service quality dash board. 5yr+ audiological assessment and management as required and/or indicated, in cases of persistent conductive (fluctuating temporary or permanent) or sensorineural hearing loss. There is a requirement for 10 year audit, in our experience this is difficult to achieve in families with no hearing concerns, families with hearing concerns are already in the system. Due to the ‘burden’ of appointments families rarely want to come at 10 years for a hearing assessment if there are not hearing concerns, particularly if the child is involved in orthodontic care, and are missing school on a regular basis for ortho appointments |
| 17 | Post Palate repair (approx 9m), 18M, 2y, 5y, 10y |
| 8 | Audiological assessment will take place when babies/children attend the cleft centre at [centre]. I.e. Post palate repair, 18 months, 3 years, 5 years. We do not attend the clinics for children older than 7 years |
| 7 | Under 2y every 6mths, over 2y every 12mths. (if hearing is normal). If hearing is down or glue ear, then can change to 3 or 6 mths depending on loss or uni or bil glue |
| 2 | 3m, 8m, 12m, 18m, 3yr, then as per CSAG |
| 6 | |
| 11 | |
| 4 | |
| 5 | |
| 15 | |
| 14 | |
| 9 |
Schematic summary: how often receive routine audiological assessment?
| Timings of routine audiological assessment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time point | 13 | 10 | 12 | 16 | 1 | 17 | 8 | 7 | 2 |
| Newborn | ✗ | Every 6 months | ✗ | Every 6 months | ✗ | ||||
| 9 months | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| 18 months | ✗ | As frequently as clinical needs directs | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| 3 years | ✗ | ✗ | ✗ | ✗ (at 2 years) | ✗ | Every 12 months | ✗ | ||
| 5 years | ✗ | Every 12 months | ✗ | ✗ | ✗ | ✗ | As per CSAG | ||
| 7 years | ✗ | ||||||||
| 10 years | ✗ | ✗ | ✗ | ✗ | |||||
| 15 years | ✗ | ||||||||
| 20 years | ✗ | ||||||||
2.5: What tests do you routinely use to diagnose and guide the subsequent management of OME? If you have a protocol please attach it to this questionnaire. Please tick all that apply.
| Centre ID | Otoscopy | Tympanometry | Age-appropriate hearing test | Breakdown of age-appropriate hearing test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Automated brainstem audiometry | Visual reinforced audiometry | Distraction testing | Performance/play audiometry | Speech testing | Pure tone audiogram | Other test/text | ||||
| 13 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 12 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 16 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes/OAEs |
| 1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes/age-appropriate speech assessments |
| 17 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| 8 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes/OAEs |
| 7 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes/OAEs |
| 2 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 6 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Noa |
| 11 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yesb |
| 4 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes/OAEs |
| 5 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes/OAE |
| 15 | ||||||||||
| 14 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yesc |
| 9 | ||||||||||
| Overall n/N (%) | 14/14 (100.0) | 14/14 (100.0) | 14/14 (100.0) | 13/14 (92.9) | 14/14 (100.0) | 13/14 (92.3) | 14/14 (100.0) | 13/14 (92.3) | 14/14 (100.0) | 8/14 (57.1) |
Whether or not protocol was provided?
| Centre ID | Protocol provided |
|---|---|
| 13 | No |
| 10 | No |
| 12 | No |
| 16 | No |
| 1 | Yes |
| 17 | No |
| 8 | No |
| 7 | No |
| 2 | No |
| 6 | No |
| 11 | No |
| 4 | No |
| 5 | No |
| 15 | |
| 14 | Yes |
| 9 | |
| Overall n/N (%) | 2/14 (14.3) |
2.6: At primary CP repair how is the decision made to insert VTs or not and who is involved in the decision-making process?
Individual listing of answers.
| Centre ID | Text answer to ‘how is decision made to insert VTs and who decides?’ |
|---|---|
| 13 | Not routinely used, as no evidence to support this. Each decision is made on an individual need basis but at this age it is likely that a soft band will be used and not grommet insertion. Decision to insert grommets would be made by ENT consultant, based on info provided by Speciaist [Specialist] audio team and following discussion with parents |
| 10 | ENT consultant decides |
| 12 | Unlikely unless primary repair very late |
| 16 | Dependent on clinical need as assessed by local or regional ENT |
| 1 | Audiological assessment and audiological history acts as a gateway for ENT to be present at the same time as palate surgery. ENT decision based on audiological report and clinical presentation |
| 17 | We don’t insert VT at primary cleft repair |
| 8 | We follow the NICE guidelines. Children will be seen in their local audiology departments twice (usually at 8 months and 11 months) and the clinician will recommend grommets or not at palate repair if this has not already happened |
| 7 | In the [region], the local audiologist does the hearing assessments (following the hearing assessment and the treatments are discussed by them. If parents show an inclination for grommets, then the audiologists lets me [name] and the cleft coordinator know. I would normally see the patient during their admission or if parents want, arrange to see them in my clinic. I would liaise with the Cleft surgeons and arrange for the Grommets to be inserted and send a letter back to the referring audiology department to pick up the hearing assessments following grommets. If the patient is found to have other pathology – airway/cholesteatoma/Retraction pockets, then I would arrange a follow up appointment in my clinic |
| 2 | Audiological Physicians and ENT |
| 6 | |
| 11 | |
| 4 | |
| 5 | |
| 15 | |
| 14 | |
| 9 |
Schematic summary: how is decision made to insert VTs and who decides?
| Centre ID | No VT at primary | ENT consultant | Audiological physician | Single or joint professional decision | ||
|---|---|---|---|---|---|---|
| ENT alone | Audio alone | Both ENT + audio | ||||
| 13 | ✗ | ✗ | ✗ | |||
| 10 | ✗ | ✗ | ||||
| 12 | ✗ | |||||
| 16 | ✗ | ✗ | ||||
| 1 | ✗ | ✗ | ✗ | |||
| 17 | ✗ | |||||
| 8 | ✗ | ✗ | ||||
| 7 | ✗ | ✗ | ✗ | |||
| 2 | ✗ | ✗ | ✗ | |||
| Overall n/N (%) | 2/9 (22.2) | 6/7 (85.7) | 5/7 (71.4) | 2/7 (28.6) | 1/7 (14.3) | 4/7 (57.1) |
2.7: What factors influence the decision to insert VTs at primary cleft repair?
Individual listing of answers.
| Centre ID | Text answer to ‘what factors influence the decision to insert VTs at primary cleft repair?’ |
|---|---|
| 13 | Individual clinics need e.g. recurrent OME, but rarely used at this age!! |
| 10 | ENT consultant decides |
| 12 | |
| 16 | Any hearing loss and impact on development |
| 1 | Newborn hearing screen results. Parental concerns. Otology history to date. Audiological assessment results (Otoscopy, tympanometry, behavioural audiometry assessment) |
| 17 | |
| 8 | Hearing levels raised (the actual level that is used as the threshold can differ from clinician to clinician and it may also depend on the general health of the child, eg always full of a cold) and presence of middle ear fluid |
| 7 | Nice guidelines with hearing assessments. Discussion between audiologist and parents. Parents choice. Child’s ear canal anatomy (Narrow ear canals in syndromic kids, making access difficult) |
| 2 | Hearing levels, parent informed choice, practical clinical considerations |
| 6 | |
| 11 | |
| 4 | |
| 5 | |
| 15 | |
| 14 | |
| 9 |
Summary: most common factor is hearing loss with some centres referencing to NICE (CG60). 11 Other contributing factors including parental choice and concerns, otoscopy history, anatomical abnormality restricting surgical access and practice of individual surgeons.
2.8: After what period of time would a conductive hearing loss > 25–30 dBHL trigger ‘active’ intervention (referral for/decision to insert VTs or prescribe HAs) at your centre?
Individual listing of answers.
| Centre ID | Text answer to ‘time period of conductive hearing loss > 25–30 dBHL that triggers “active” intervention’ |
|---|---|
| 13 | NO specific timeframe but if after > 3/12 documented HL [hearing loss] and history of symptoms/parental or professional concerns, then intervention would be discussed/considered |
| 10 | 2 consecutive hearing tests showing CD hearing loss |
| 12 | Dependent on needs of the child – is it delaying language, social interaction, is it associated with ear infections etc – intervention decision would be discussed with family? |
| 16 | Any child seen within Audiology service with a loss gets referral to ENT straight away. Management then depends on clinical need on an individual basis |
| 1 | When persistent hearing loss demonstrated by two tests 3 mths apart. Where specialist cleft speech and language therapists identify hearing related speech characteristics with audiological assessment demonstrating hearing loss. Multiple/repeated ear infections/tympanic membrane perforations over a short period of time – decision made by ENT – seek clarification from ENT lead |
| 17 | Minimum 3m observation and only if the clinical history correlates with the audiological data |
| 8 | 3 months |
| 7 | Persistent hearing loss beyond 3 months |
| 2 | Depends on additional clinical factors, speech and middle ear health |
| 6 | |
| 11 | |
| 4 | If the OME is persistent over approximately 2 month period. Following the referral to ENT (about 6 wks wait) evidence of 3 months of persistent OME will have been recorded and can inform the intervention decision as per Nice guidelines |
| 5 | |
| 15 | |
| 14 | |
| 9 |
Schematic summary: time period of conductive hearing loss > 25–30 dBHL that triggers ‘active’ intervention.
| Centre ID | One hearing test showing hearing loss | Two hearing tests showing hearing loss | Depends on factors (in addition to hearing loss) | |
|---|---|---|---|---|
| 3 months apart | Unknown months apart | |||
| 13 | ✗ | |||
| 10 | ✗ | |||
| 12 | ✗ | |||
| 16 | ✗ | |||
| 1 | ✗ | |||
| 17 | ✗ | |||
| 8 | ✗ | |||
| 7 | ✗ | |||
| 2 | ✗ | |||
| 4 | ✗ | |||
| Overall n/N (%) | 1/10 (10.0) | 6 (60.0) | 1/10 (10.0) | 2/10 (20.0) |
2.9: Please describe the decision-making process to provide HAs or to insert/refer to ENT for consideration of VTs as the first-line treatment for persistent OME. Please include any involvement of parents and/or the child.
Individual listing of answers.
| Centre ID | Text answer to ‘how decide to provide HAs or insert VTs as first-line treatment’ |
|---|---|
| 13 | At this age the patient is usually being seen by the Specialist team including Audiologists and community paediatrics +/– ENT consultant. The need for amplification based on neuralplasitcity is discussed. Usually parents are asked to try a softband before contemplating Grommet insertion, because of the expected need for treatment over a long period of time/limitation to number of grommets might arise |
| 10 | Parents always contribute to this decision, and to date they have decided VT for management |
| 12 | Monitor hearing loss. Assess receptive speech . . . and expressive speech. School concerns? History of ear infections? Otoscopy. Ask parents. Ask child if old enough |
| 16 | All children referred to ENT, all options discussed with family – who then make their choice |
| 1 | All hearing management options are explained to parents with the pros and cons of each approach by the audiologist. Often parents choose grommets as a first line approach |
| 17 | If child has persistent OME and there is both clinical and audiological evidence of hearing loss; the parents are given a choice of either trial BC hearing aid or if > 4y VT tube insertion. VT tube insertion for < 4y is limited to children who have failed a trial of BC/AC hearing aid, who have recurrent AOM or have evidence of Tympanic membrane (TM) damage |
| 8 | If hearing levels are raised (usually 45 dBHL or greater) on two occasions, 12 weeks apart, we will discuss the management options with parents and child if old enough |
| 7 | The first time a child is seen with glue ear and reduced hearing, results are explained, NDCS glue ear booklet given and a review appt booked. Its explained to parents that to start with we always follow WW and hopefully it will clear on its own. If at next appt things haven’t changed then we will discuss the different management options, these are outlined in the booklet given. At next appt [appointment], again results explained and if both h.aids [hearing aids] or grommets are an option, they are discussed and explained then its parents who decide which to go for. (or child if old enough) |
| 2 | Discussions with the parents about the impact of the hearing loss and the benefits, risks, and alternatives of treatment, between Grommets vs Hearing aids and Hearing tactics (only) |
| 6 | |
| 11 | |
| 4 | Presence of persistent OME (as described earlier) confirmed through age appropriate hearing assessment and tympanometry. ENT consultant will discuss surgical intervention vs mangment with hearing aids so parents can make an informed choice regarding further management |
| 5 | |
| 15 | |
| 14 |
Schematic summary: how decide to provide HAs or insert VTs as first-line treatment.
| Decision process | 13 | 10 | 12 | 16 | 1 | 17 | 8 | 7 | 2 | 4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Seen by specialist team (audiologist, community paediatrics, ENT consultant) Monitor hearing loss |
✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Discuss with family (parents/child if old enough) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Assess receptive speech and expressive speech | ✗ | |||||||||
| School concerns | ✗ | |||||||||
| History of ear infections | ✗ | |||||||||
| Tests (otoscopy, tympanometry, age-appropriate hearing tests) | ✗ | ✗ |
2.10: Please describe the decision-making process to provide HAs or VTs if the child has already received treatment and has persistent OME.
Individual listing of answers.
| Centre ID | Text answer to ‘how do you decide to provide HAs or insert VTs if child has already received treatment’ | |
|---|---|---|
| For patients who have received HAs as initial treatment | For patients who have received VTs as initial treatment | |
| 13 | See centre response for 2.9, for very young. Later on if hearing rehabilitation needs to be discussed both Has and vent tubes will be discussed. The pros and cons of each are discussed with parents, who can then consider the options based on their child and likelihood of utising the Has etc. | See centre response for 2.9, but the risks associated with repeated grommet insertion may need to be discussed in more details depending on clinical findings on examination |
| 10 | Evidence of ongoing OME over a period of time. | |
| 12 | Monitor hearing loss. Assess receptive speech . . . and expressive speech. School concerns? History of ear infections? Otoscopy. Ask parents. Ask child if old enough | And again . . . as above |
| 16 | Down to patient choice | Patient choice |
| 1 | VT would be discussed where the child is unable to consistently use the hearing aids, either due to non-compliance with hearing aid use, because the hearing loss is no longer manageable with hearing aids, the tympanic membranes have become ‘unsafe’ and require VTs to prevent damage or active infection as a result of severe retraction or because hearing aids can no longer be used due to multiple ear infections or external auditory canal inflammation (otitis externa) through allergy or contact dermatitis or because the amplification available via the aid is no longer adequate to manage the hearing loss. Clarify with lead ENT – Children with persistent OME and had received three grommet/VT insertion should ideally be given hearing aid unless parents are not happy for their child using HA or clinically not suitable for using hearing aid | Hearing aids would be discussed as per with the first line treatment/management discussions, hearing aids as with grommets are discussed as a treatment option with each episode of OME where the clinical decision it to actively manage the OME. Families may choose hearing aids for treatment after previously choosing VTs depending on their experience of VTs. Hearing aids may be the recommended management because of a contraindication to further VT insertion for example if the child suffered with multiple ear infections and required the grommets (VTs) to be removed previously or if they have already had 3 sets of VTs or if the VTs lasted only a very short period of time and were found to be a less effective management option for the individual |
| 17 | Continue with hearing aid until OME resolves or the child stops using the Aid. If OME persists then VT tubes are offered | Continue with VT tubes. If parents choose hearing aids then the child is observed closely for evidence of TM damage. IF TM damage then VT tubes are placed |
| 8 | If already using hearing aids, they will be under regular review by their local audiology department and any changes in their hearing thresholds will be discussed and hearing aids reprogrammed if necessary. If they have previously had hearing aids but these were removed because their hearing improved but has now got worse again, I would discuss management options with parents/child. It probably depends what their experience of hearing aids was like whether they decide to go for aids again or try grommets. Some parents are happier to consider grommets once their child is a little older | If they have previously had grommets, I would discuss the options emphasising the problems of repeated grommet insertion |
| 7 | Children are always reviewed and if the child is not getting on with the aids, different management options are discussed and again parents decide what to go for.(for example, referral to ENT, different type of h.aid [hearing aid]). [name] | If ventilation tubes have come out or are blocked and OME is back, we would again follow WW. If after 3 mth [months] it had not cleared, then we would discuss all options again (grommets, hearing aids). For some children a second set of grommets may not be appropriate but that would be the decision of ENT [name] |
| 2 | See centre response for 2.9, with more emphasis on the importance of follow up and engagement with hearing aid use, also to monitor middle ear health | See centre response for 2.9, but in addition will highlight the small increase in complications with repeated grommets |
| 6 | ||
| 11 | ||
| 4 | Presence of persistent OME (as described earlier) confirmed through age appropriate hearing assessment and tympanometry. ENT consultant will discuss surgical intervention vs management with hearing aids so parents can make an informed choice regarding further management | Presence of persistent OME (as described earlier) confirmed through age appropriate hearing assessment and tympanometry. ENT consultant will discuss surgical intervention vs management with hearing aids so parents can make an informed choice regarding further management. The increased risks of TM perforation with repeat sets of grommets will be discussed |
| 5 | ||
| 15 | ||
| 14 | ||
| 9 | ||
Schematic summary: how decide to provide HAs or insert VTs if child has already received HAs as initial treatment.
| Decision process | 13 | 10 | 12 | 16 | 1 | 17 | 8 | 7 | 2 | 4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Similar to decision process for first-line treatment using HAs or insert VTs | ✗ | ✗ | ✗ | ✗ | ||||||
| Pros and cons of HAs and VTs are discussed with parents | ✗ | |||||||||
| Patient choice | ✗ | ✗ | ✗ | ✗ | ||||||
| VTs discussed where non-compliance with HA use | ✗ | ✗ | ||||||||
| VTs discussed where the amplification available via the aid is no longer adequate to manage the hearing loss | ✗ | ✗ | ||||||||
| VTs discussed where tympanic membranes have become ‘unsafe’ and require VTs to prevent damage or active infection as a result of severe retraction | ✗ | ✗ | ✗ | |||||||
| VTs discussed where HAs can no longer be used due to multiple ear infections or external auditory canal inflammation (otitis externa) through allergy or contact dermatitis | ✗ | ✗ | ||||||||
| Regular review by their local audiology department and any changes in their hearing thresholds will be discussed and hearing aids reprogrammed if necessary | ✗ | |||||||||
| Referral to ENT | ✗ | |||||||||
| Different type of HA | ✗ | |||||||||
| Monitor middle ear health | ✗ |
Schematic summary: how to decide to provide HAs or insert VTs if child has already received VTs as initial treatment.
| Decision process | 13 | 10 | 12 | 16 | 1 | 17 | 8 | 7 | 2 | 4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Similar to decision process for first-line treatment using HAs or insert VTs | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Risks associated with repeated grommet insertion discussed with parents | ✗ | ✗ | ✗ | ✗ | ||||||
| Patient choice | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Children with persistent OME and had received three grommet/VT insertions should ideally be given HAs unless parents are not happy for their child using a HA or clinically not suitable for using a HA | ✗ | |||||||||
| HAs may be the recommended management because of a contraindication to further VT insertion for example if the child suffered with multiple ear infections and required the grommets (VTs) to be removed previously | ✗ | |||||||||
| Continue with VTs. If parents choose HAs then the child is observed closely for evidence of TM damage. IF TM damage then VTs are placed | ✗ | |||||||||
| If VTs have come out or are blocked and OME is back, we would again follow WW. If after 3 months it had not cleared, then we would discuss all options again (grommets, HAs). For some children a second set of grommets may not be appropriate but that would be the decision of ENT | ✗ |
2.11: Under what specific circumstances would you advise against inserting VTs to treat persistent OME in a child with OME?
Individual listing of answers.
| Centre ID | Text answer to ‘advise against inserting VTs’ |
|---|---|
| 13 | Very young – recommend trial of softband first. Very atelectatic TM, with possible increased risk of TM Perfoatation development, which might make HA use difficult at a later date |
| 10 | Early extruders, thinning of TM |
| 12 | If greater risk from GA.?syndromic child, particularly if swallowing problems. Small ear canals. History of previous discharging ears with grommets |
| 16 | Where there are anatomical restrictions or history of repeated infections with previous VTs |
| 1 | VTs leading to multiple ear infections. VT insertion contraindicated by tympanic membrane appearance/structure for example too thin to maintain the VTs. I agree with above statement – SH |
| 17 | If child is under < 4y and hasn’t been tried with hearing aid. Syndromic child who is immune compromised |
| 8 | If they have had grommet(s) in the past. If they have dead ear on one side |
| 7 | Ent decision, or if child can’t have GA. [name] |
| 2 | If the ear canal access is difficult or there is an underlying sensorineural hearing loss |
| 6 | |
| 11 | |
| 4 | |
| 5 | |
| 15 | |
| 14 | |
| 9 |
Schematic summary: advise against inserting VTs.
| Specific circumstance | 13 | 10 | 12 | 16 | 1 | 17 | 8 | 7 | 2 |
|---|---|---|---|---|---|---|---|---|---|
| Very young – recommend trial of softband first If child is aged < 4 years and has not been tried with a HA |
✗ | ✗ | |||||||
| Very atelectatic TM, with possible increased risk of TM perfoatation development, which might make HA use difficult at a later date Thinning of TM VT insertion contraindicated by tympanic membrane appearance/structure for example too thin to maintain the VTs |
✗ | ✗ | ✗ | ||||||
| Early extruders | ✗ | ||||||||
| Greater risk from GA If child cannot have GA |
✗ | ✗ | |||||||
| Syndromic child, particularly if swallowing problems Syndromic child who is immune compromised |
✗ | ✗ | |||||||
| Small ear canals Anatomical restrictions If the ear canal access is difficult |
✗ | ✗ | ✗ | ||||||
| History of previous discharging ears with grommets History of repeated infections with previous VTs VTs leading to multiple ear infections |
✗ | ✗ | ✗ | ||||||
| If they have had grommet(s) in the past | ✗ | ||||||||
| If they have dead ear on one side | ✗ | ||||||||
| Underlying sensorineural hearing loss | ✗ |
2.12: What would be the maximum sets of VTs that you would consider in a child before advising against further VT insertion? Please explain your answer.
Individual listing of answers.
| Centre ID | Maximum sets of VT | Explanation of answer |
|---|---|---|
| 13 | Three setsa | 3 usually, but this would depend on findings at examination and other influencing factors |
| 10 | ENT consultant decides | |
| 12 | Four sets | Depends on gain V [versus] risk benefits |
| 16 | No specific number depends on individual patients | |
| 1 | Three sets | I agree with the above response. Too many myringotomies increases the risk of tympanic membrane perforation, tympano sclerosis. Perforated tympanic membrane increases the risk of ME infection with discharge making the use of HA difficult. SH |
| 17 | No maximum. After 2 sets of short term tubes we would place long term tubes | |
| 8 | One set | I have seen children who have had grommet insertion repeated a number of times and it is likely to affect the function of the TM. Also increasing the risk of chronic ear infections |
| 7 | Three sets | Or if the patient develops thin atrophic drum following extrusion of the last set of grommest. Or patient/parent choice |
| 2 | There is no absolute number, it is a clinical decision, I have seen patients who had 5–6 grommets elsewhere but these are the minority | |
| 6 | ||
| 11 | ||
| 4 | ||
| 5 | ||
| 15 | ||
| 14 | ||
| 9 |
Schematic summary: maximum sets of VTs.
| Centre ID | Maximum | |||
|---|---|---|---|---|
| One set | Three sets | Four sets | No maximum | |
| 13 | ✗ | |||
| 10 | ✗ | |||
| 12 | ✗ | |||
| 16 | ✗ | |||
| 1 | ✗ | |||
| 17 | ✗ | |||
| 8 | ✗ | |||
| 7 | ✗ | |||
| 2 | ✗ | |||
| Overall n/N (%) | 1/9 (11.1) | 3/9 (33.3) | 1/9 (11.1) | 4/9 (44.4) |
2.13: What is your view on the optimum age for inserting VTs?
Individual listing of answers.
| Centre ID | Text answer to ‘optimum age for inserting VTs’ |
|---|---|
| 13 | No optimum age, but I find we recommend them most from 3–4 yrs on |
| 10 | Decision should be based on clinical findings. |
| 12 | VT are of benefit for a limited period of time . . . so it is a judgment as to when is the best time for an individual child |
| 16 | None – when clinical need dictates |
| 1 | There is no age limitation in children with cleft palate, usually we prefer to perform myringotomy and grommet insertion when they undergo their first cleft surgery which is around six months. SH |
| 17 | Around age 5yr [years] |
| 8 | I don’t like to see them done too early but this is weighed against the convenience of putting in grommets at the time of palate repair although in practice this does not happen often now because of the NICE guidelines meaning that the repair is often done before the second hearing assessment |
| 7 | For OME in cleft children – generally beyond 2 years, when speech is developing |
| 2 | This is a clinical decision, I have not seen in my practice a child having grommets younger than 3 months |
| 6 | Difficult procedure in small syndromic infants with narrow ear canals. Individual decision based on Otoscopy and hearing results and speech and language. No age limits |
| 11 | Depends on indication – e.g. conductive hearing loss secondary to OME or recurrent acute AOM. I would insert them as soon as clinically indicated (with parental consent) |
| 4 | –9 |
| 5 | If needed early then 18–24 months |
| 15 | |
| 14 | We perform ventilation tube insertion with that of primary cleft palate repair surgery (8 months – 1 year), if persistent glue ear is confirmed. Alternatively if persistent glue ear is confirmed at any ventilation tubes are considered an option age as per national guidelines |
| 9 |
Schematic summary: optimum age for inserting VTs.
| Centre ID | Age | |||
|---|---|---|---|---|
| < 1 year (palate repair) | 1–5 years | > 5 years | No optimum age | |
| 13 | ✗ (most from 3–4 years onwards) | |||
| 10 | ✗ (based on clinical findings) | |||
| 12 | ✗ (clinical decision) | |||
| 16 [locaton] | ✗ (when clinical need dictates) | |||
| 1 | ✗ (myringotomy and grommet insertion when they undergo their first cleft surgery which is around 6 months) | |||
| 17 | ✗ | |||
| 8 | ✗ | |||
| 7 | ✗ | |||
| 2 | ✗ (clinical decision) | |||
| 6 | ✗ (individual decision based on otoscopy and hearing results and speech and language) | |||
| 11 | ✗ (insert them as soon as clinically indicated) | |||
| 5 | ✗ (if needed early then 18–24 months) | |||
| 14 | ✗ [insertion with that of primary CP repair surgery (8 months–1 year)] | |||
| Overall n/N (%) | 2/13 (15.4) | 4/13 (30.8) | 1/13 (7.7) | 6/13 (46.2) |
2.14: In what circumstances would you consider offering long-term ventilation T-tubes?
Individual listing of answers.
| Centre ID | Text answer to ‘circumstances to consider offering long-term ventilation T-tubes’ |
|---|---|
| 13 | On third set of grommets (so usually about 7 years +) and patient did not get on with trial of HA in past. Parents understand and accept the associated risks |
| 10 | ENT consultant decides |
| 12 | If grommets/aids not alternatives |
| 16 | After at least one previous set of VTs dependent on child’s age |
| 1 | In children who had persistent OME and had standard grommet insertions previously and who is not an appropriate candidate for hearing aid use.SH |
| 17 | Persistent OME following 2 sets of short term tubes or if there is evidence of TM damage (grade > 2) |
| 8 | That would not be my decision. I might say to parents that ENT may consider it but would never recommend it |
| 7 | Not for OME. Consider for persistent retraction pockets after first set grommets |
| 2 | In our practice in has not been necessary, and we have not had any |
| 6 | |
| 11 | |
| 4 | |
| 5 | |
| 15 | |
| 14 | |
| 9 |
Schematic summary: circumstances to consider offering long-term ventilation T-tubes.
| Circumstance | 13 | 10 h | 12 | 16 | 1 | 17 | 8 | 7 | 2 |
|---|---|---|---|---|---|---|---|---|---|
| On third set of grommets (so usually about ≥ 7 years) and patient did not get on with trial of HA in past If grommets/aids not alternatives In children who had persistent OME and had standard grommet insertions previously and who is not an appropriate candidate for hearing aid use |
✗ | ✗ | ✗ | ||||||
| ENT consultant decides That would not be my decision. I might say to parents that ENT may consider it but would never recommend it |
✗ | ✗ | |||||||
| After at least one previous set of VTs dependent on child’s age | ✗ | ||||||||
| Persistent OME following two sets of short-term tubes or if there is evidence of TM damage (grade > 2) | ✗ | ||||||||
| Not for OME. Consider for persistent retraction pockets after first set grommets In our practice in has not been necessary, and we have not had any |
✗ | ✗ |
2.15: In the last 5 years what is the average yearly number of patients with CP who receive HAs as primary management of conductive hearing loss secondary to OME?
Individual listing of answers.
| Centre ID | Text answer to ‘average yearly number of patients with CP who receive HAs as primary management of conductive hearing loss secondary to OME’ |
|---|---|
| 13 | NO idea. This data is not available to me at present, as ENT care is delivered locally (as per NICE guidelines) |
| 10 | None |
| 12 | Sorry do not have the ability to answer this |
| 16 | Data not available |
| 1 | We do not have this data as children are seen across several hospitals |
| 17 | There were originally 23 but some do not require them now or have tried them and then had grommets fitted. Audiology’s hearing aid database currently reports 12 Cleft hearing aid patients |
| 8 | No idea as hearing aids fitted in local services |
| 7 | |
| 2 | About 5% |
| 6 | |
| 11 | |
| 4 | Unable to extract data |
| 5 | |
| 15 | |
| 14 | |
| 9 |
Schematic summary: average yearly number of patients with CP who receive HAs as primary management of conductive hearing loss secondary to OME.
| Centre ID | Average yearly number of patients |
|---|---|
| 13 | Unknown number of patients |
| 10 | 0 |
| 12 | Unknown number of patients |
| 16 | Unknown number of patients |
| 1 | Unknown number of patients |
| 17 | 12 |
| 8 | Unknown number of patients |
| 7 | Unknown number of patients |
| 2 | Unknown number of patients (but 5% of unknown total) |
| [Location] | Unknown number of patients |
2.16: Does your centre offer HA technology other than behind the ear HAs, for example, bone conduction HAs?
| Centre ID | Centre offers HA technology other than BTE HAs | Details |
|---|---|---|
| 13 | Yes | Considered on individual need basis |
| 10 | Yes | Spectacle, BC and CROS. Refer to other service for BAHA |
| 12 | Yes | |
| 16 | Yes | BAHA on softband |
| 1 | Yes | BTE, bone conduction hearing aids, and soft band hearing aid technology available depending on the degree of hearing loss and the child |
| 17 | Yes | BC on soft band/hard band, BAHA softband, or implant |
| 8 | Noa | Hearing aids are not fitted at the centre, all done at local audiology service |
| 7 | Yes | Contact mini soft/hard bands [name] |
| 2 | Yes | |
| 6 | ||
| 11 | ||
| 4 | Yes | Bone Conduction Aids |
| 5 | ||
| 15 | ||
| 14 | ||
| 9 | ||
| Overall n/N (%) | 9/10 (90.0) |
2.17: Please describe the management of children with CP and OME who are not offered VTs or Has.
Individual listing of answers.
| Centre ID | Text answer to ‘management of children with CP and OME who are not offered VTs or HAs’ |
|---|---|
| 13 | Some children with be monitored with watchful waiting if appropriate for their individual clinical needs |
| 10 | Regular monitoring |
| 12 | If mild . . . advice to parents and school re ‘hearing tactics’ and good acoustic environment |
| 16 | Treatment is offered if required – otherwise hearing tactics for home/school given when parents decline treatment |
| 1 | All families are given the NDCS glue ear leaflet and locally written leaflets on cleft palate and hearing loss and early listening skills at their primary palate preadmission assessment appointment. The lead audiologist for the network explains the mechanism and impact of OME at the appointment using the NDCS leaflet diagrams, this allows parents to take the annotated diagrams home for future reference. Where grommets are required at the same time as surgery parents are given a leaflet about grommet inserion [insertion] addressing commonly asked questions associated with this procedure. SLT and audiology discuss communciatoin [communication] tactics at the regular SLT and Audiology appointments throughout early childhood. Families are given communication tactics regarding mild and or fluctuating hearing loss tailored to the individuals hearing loss and hearing history as part of their audiological assessment debrief/individual management plan setting. [location] run a specialsist [specialist] speech and hearing clinic run by the lead audiologist and specialist speech and language therapists. This clinic is for children with hearing and speech concerns and children/families from across the network can be referred and assessed in this clinic. The main aim is to explore hearing impact on speech and to tailor future therapy, communication, listening and hearing management advice using an MDT approach building a joint speech and audiological individual management plan |
| 17 | Observation for those who have OME but no clinical history of hearing issues. Observation and support for those syndromic children who have OME and VT or hearing aids are not appropriate |
| 8 | Continue watchful waiting, give parents advice about talking to child in quiet, ie turn off TV, child should sit at front of class, teacher should be aware that there is a hearing problem, talk to them from front etc, we have a leaflet with this advice |
| 7 | If a child has persistent glue ear and reduced hearing they would always be offered h.aid or grommets assessment [name] |
| 2 | Hearing tactics and Listening strategies |
| 6 | |
| 11 | |
| 4 | |
| 5 | |
| 15 | |
| 14 | |
| 9 |
Schematic summary: management of children with CP and OME who are not offered VTs or HAs.
| Circumstance | 13 | 10 | 12 | 16 | 1 | 17 | 8 | 7 | 2 |
|---|---|---|---|---|---|---|---|---|---|
| Watchful waiting Regular monitoring Observation for those who have OME but no clinical history of hearing issues |
✗ | ✗ | ✗ | ✗ | |||||
| Observation and support for those syndromic children who have OME and VT or HAs are not appropriate | ✗ | ||||||||
| Hearing tactics and listening strategies | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
| Written leaflets | ✗ | ✗ | |||||||
| If a child has persistent glue ear and reduced hearing they would always be offered HA or grommets assessment | ✗ |
2.18: Up to what age (in years) does your cleft service actively monitor a child’s hearing thresholds irrespective of OME?
Individual listing of answers.
| Centre ID | Maximum age monitor hearing (years) | How regularly assessments take place | Does answer change depending on OME history | Description of change depending on OME history |
|---|---|---|---|---|
| 13 | Adulthood | See question above asking about ‘routine monitoring’ | Yes | Will be monitored into adulthood if clinical need e.g. persistent ear problems (including retraction pockets, OME etc) are present |
| 10 | 16 | 6 mthly for preschool and annually thereafter sooner if a hearing loss is identified | No | Always dependent on hearing thresholds obtained |
| 12 | Cleft audit clinic at 5 10 and 15 years | Yes | Active monitoring dictated by clinical need | |
| 16 | 10 | See question 1 | Yes | If OME at 10 – this is monitored/treated as required |
| 1 | 5 | Audiological assessments are available for all up to 18 yrs and beyond this via the GP. If a child continues to have OME at 5 yrs then they would be monitored until this has resolved and their hearing is stable. Active management for all regardless of hearing status is until the 5 year audit point. All children are seen until hearing is within the normal range and stable at that level. The regularity of appointments is individaully set as per answer in 2.4 | No | Children are seen until hearing is within the normal range and stable at that level |
| 17 | Standard – 2y, 5y, 10y. If issues up to 16y frequency depends on history and progress | As above | ||
| 8 | 7 | They take place at the times that the child comes to the cleft unit (see above) | No | No because this will be done locally and the frequency of assessments will vary depending on the hearing thresholds, the degree of difficulty the child is experiencing etc. |
| 7 | 18 | See 2.4 [name] | Yes | More frequent review depending on hearing loss and bil or uni OME [name] |
| 2 | Adulthood | Please see answer 2.4 | Yes | And other otological and Audiological presentation of the case |
| 6 | ||||
| 11 | ||||
| 4 | ||||
| 5 | ||||
| 15 | ||||
| 14 | ||||
| 9 |
Section 3: spoke audiological/ear, nose and throat services
3.1: Do children in the service attend cleft clinics at any other sites outside of your trust?
| Centre ID | Attend other sites outside of your trust? | How many other sites outside of your trust provide audiological/ENT services? | |
|---|---|---|---|
| Number of sites | Text response | ||
| 13 | Yes | 7 | These are different questions!! Attend cleft clinics: 1 other site. The team go to [location] to hold clinics there for patients living nearer that area of [location]. No of Sites providing ENT/Audiology services: this equates to the number of healthe boards across [location] (7 I think) as ENT and Audiology services are provided locally (as per NICE) |
| 10 | No | 0 | |
| 12 | Yes | 6 | [locations] |
| 16 | Yes | Missing | All spokes in [location]region have audiology |
| 1 | Yes | 14 | 14 hospital sites |
| 17 | Yes | 12 | Approx 12 sites |
| 8 | Yesa | Missing | Do you mean dedicated cleft clinics within audiology depts. [departments]? I do not think this happens anywhere, children are put into regular assessment clinics |
| 7 | Yes | Missing | Audiology is provided at local services. [name] |
| 2 | Yes | 2 | 2 |
| 6 | Yes | 3 | Probably [locatons], and [location] – 3 other sites outside our trust |
| 11 | Yes | 7 | At least 7 spoke sites in the [location]. We call them all back to [location]for audit clinics though |
| 4 | Yes | ||
| 5 | |||
| 15 | |||
| 14 | Yes | 9 | Approx. 8 – 10 sites |
| 9 | |||
| Overall n/N (%) | 12/13 (92.3) | N = 9 Median = 7 spokes Minimum = 0 spokes Maximum = 14 spokes |
|
3.2: What, if any, are your recommendations to local audiology and or ENT services regarding the frequency of hearing tests and management of OME in patients with CP outside visits to your cleft service?
| Centre ID | Recommendations to local audiology and/or ENT services | Receive a copy of the results? | Local hospital make decisions regarding VTs and/or HAs | ||
|---|---|---|---|---|---|
| Text answer | Spoke protocol: same as hub or spoke specific? | Spoke decides? | Details | ||
| 13 | See answers on monitoring schedule above. This has been agreed with local Audiology and ENT services | Same as hub | Yes | Yes | This is very repetitive. All answers given above apply here also |
| 10 | 6 mthly for preschool and annually for school age, sooner if a hearing loss is identified | Same as hub | No | Yes | Unable to specify |
| 12 | Dependent on need | Same as hub | Missing | Yes | |
| 16 | All spokes are advised to follow same protocol as described above | Same as hub | No | Yes | Request insertion of VTs at same time as palate surgery in hub or insert within own institution |
| 1 | See attached network protocol | Same as hub | Yes | Yes | They make decisions as per the individual cases, in line with NICE guidelines and according to locally available technology in the case of hearing aids |
| 17 | No direct recommendations. They usually continue with the management recommended by us but do support and manage patients independently if appropriate | Same as hub | Yes | Yes | Depends if local ENT are involved |
| 8 | Our regional policy states 6 monthly assessments if hearing is normal but this will change if hearing affected. I think this pathway needs to be revised. In practice services see children at 8 months initially if newborn screen normal | Same as hub | Yes | Yes | The majority of hearing assessments are carried out locally, usually 6 monthly until about 7 years of age by which time most services report that if the hearing is normal they have usually DNA’d by then |
| 7 | None, they have their own policies. [name] | Spoke specific | Yes | Yes | They are their patients so they make all decisions. [name] |
| 2 | As per protocol 2.4 | Same as hub | Yes | Yes | |
| 6 | |||||
| 11 | |||||
| 4 | |||||
| 5 | |||||
| 15 | |||||
| 14 | |||||
| 9 | |||||
| Overall n/N (%) | Protocol same as hub = 8/9 (88.8); spoke-specific protocol = 1/9 (11.1) | 6/8 (75.0) | 9/9 (100.0) | ||
Section 4: case load
4.1: What is the average number of new CP (cleft lip and palate, and palate only) patients referred to your service each year?
| Centre ID | 2012 | 2011 | 2010 | 2009 | 2008 | Annual average |
|---|---|---|---|---|---|---|
| 13 | 45 | 55 | 43 | 48 | 45 | 47.2 |
| 10 | 29 | 29 | 38 | 40 | 44 | 36 |
| 12a | 51 | 47 | 48 | 39 | 60 | 49 |
| 16 | ||||||
| 1b | 178 | 147 | 188 | 182 | 210 | 181 |
| 17 | 117 | 108 | 107 | 108 | 106 | 109.2 |
| 8 | CP = 28; BCLP = 11; UCLP = 19; all types = 58 | CP = 36; BCLP = 16; UCLP = 12; all types = 64 | CP = 38; BCLP = 15; UCLP = 22; all types = 75 | CP = 40; BCLP = 4; UCLP = 19; all types = 63 | CP = 37; BCLP = 9; UCLP = 12; all types = 58 | CP = 35.8; BCLP = 11.0; UCLP = 16.8; all types = 63.6 |
| 7 | CP = 37; BCLP = 8; UCLP = 13; all types = 58 | CP = 40; BCLP = 9; UCLP = 14; all types = 63 | CP = 43; BCLP = 10; UCLP = 21; all types = 74 | CP = 46; BCLP = 10; UCLP = 12; all types = 68 | CP = 41; BCLP = 7; UCLP = 26; all types = 74 | CP = 41.4; BCLP = 8.8; UCLP = 17.2; all types = 67.4 |
| 2c | 233 | 132 | 119 | 104 | 86 | 134.8 |
| 6 | 29 | 31 | 36 | 33 | 37 | 33.2 |
| 11 | 46 | 41 | 45 | 56 | 60 | 49.6 |
| 4d | ||||||
| 5e | 73 | 67 | 73 | 74 | 76 | 72.6 |
| 15 | 33 (including 1 SMCP) | 45 (including 4 SMCP) | 44 (including 4 SMCP) | 48 (including 9 SMCP) | 44 (including 12 SMCP) | 42.8 (including SMCP); 37.2 (excluding SMCP) |
| 14 | 43 | 40 (including 1 SMCP) | 47 (including 5 SMCP) | 35 (including 4 SMCP) | 46 (including 3 SMCP) | 42.2 (including SMCP); 39.6 (excluding SMCP) |
| 9 | 155 | 146 | 120 | 131 | 131 | 136.6 |
Section 5: information
5.1: What information do you issue to your families about OME and the impact of OME?
| Centre ID | Information to your families | Attached written documentation | |
|---|---|---|---|
| Text answer | Information booklet | ||
| 13 | Baby booklets provided to parents at birth. Info leaflets e.g. that provided by NDCS on HAs, OME, are provided where appropriate | Own leaflets; NDCS | No |
| 10 | NDCS documentation | NDCS | No |
| 12 | Our own leaflet – sorry don’t have access to it electronically | Own leaflets | No |
| 16 | Families given NDCS leaflets – please see NDCS website for copies | NDCS | No |
| 1 | NDCS glue ear/cleft palate leaflet. Early listening skills. Cleft palate and hearing loss. Grommet insertion at the same time as primary palate repair | NDCS | No |
| 17 | Missing | No | |
| 8 | There is a section on hearing, OME and treatment options in the information given to all parents of babies with cleft palate. We sometimes hand out the NDCS booklet on cleft palate but usually use our in house generic leaflet on glue ear | Own leaflets; NDCS | Yes |
| 7 | NDCS glue gear booklet. NDCS cleft palate booklet. [name] | NDCS | No |
| 2 | Local patient information leaflets, in additional to NDCS and CLAPA information | Own leaflets; NDCS; CLAPA | No |
| 6 | |||
| 11 | |||
| 4 | I cannot answer for patients attending ENT services of cleft appointments, however in audiology NDCS info on OME is available | No | |
| 5 | |||
| 15 | |||
| 14 | |||
| 9 | |||
| Overall n/N (%) | Own leaflets = 4/9 (44.4); NDCS = 8/9 (88.9); CLAPA = 1/9 (11.1) | 1/10 (10.0) | |
5.2: When is the risk of OME and signs of OME first discussed with your families?
Individual listing of answers.
| Centre ID | Text answer to ‘when is the risk of OME and signs of OME first discussed with your families?’ |
|---|---|
| 13 | Mentioned in Baby booklet. By Cleft Nursing staff at time of home visits. Advised baby clinic, e.g. 6/52 age, by cleft surgeon. By SLT at Babble therapy workshops at 6/12. S/B [seen by] Audioogists [audiologists] at 8–10 months and further advice given |
| 10 | As soon as OME is confirmed |
| 12 | At first visit hearing is discussed |
| 16 | At every routine audiology appointment |
| 1 | OME is discussed actively from the early contacts and repeatedly by team members through the early years. It is FIRST discussed by the cleft nurses in the early home based appointments, then in the combined cleft MDT clinic appointments when the cleft diagnosis is clarified and surgery first discussed with the consultant surgeon, then again by the SLTs at the babble appointment (3mths of age) and again in detail at the preadmission appointment by the lead audiologist (6mths) one week before palate surgery when the information documented above is issued |
| 17 | At first ENT appointment (post palate repair) or when they first present with OME |
| 8 | It will usually all be mentioned at the first meeting with parents when they come to the cleft unit soon after birth. Virtually all the children have OME by the time they come for their assessment at 8 months (at local clinic) so we talk about effects this can have on the hearing. Risks are always discussed when it becomes necessary to discuss treatment options for OME with associated hearing loss |
| 7 | At first appt. [name] |
| 2 | Antenatal visit and in the initial attendance at the MDT |
| 6 | |
| 11 | |
| 4 | |
| 5 | |
| 15 | |
| 14 | |
| 9 |
Schematic summary: when is the risk of OME and signs of OME first discussed with your families?
| Centre ID | Antenatal | Newborn | 6 weeks | 3 months | 6 months | 9 months |
|---|---|---|---|---|---|---|
| 13 | Mentioned in Baby booklet Cleft Nursing staff at time of home visits |
Advised baby clinic, e.g. 6/52 age, by cleft surgeon | SLT at Babble therapy workshops | Audioogists at 8–10 months | ||
| 10 | As soon as OME is confirmed | |||||
| 12 | At first visit hearing is discussed | |||||
| 16 | At every routine audiology appointment | |||||
| 1 | Cleft nurses in the early home based appointments | Combined cleft MDT clinic appointments when the cleft diagnosis is clarified and surgery first discussed with the consultant surgeon | SLTs at the babble appointment (3mths of age) | Preadmission appointment by the lead audiologist (6mths) one week before palate surgery | ||
| 17 | At first ENT appointment (post palate repair) or when they first present with OME | |||||
| 8 | First meeting with parents when they come to the cleft unit soon after birth | Virtually all the children have OME by the time they come for their assessment at 8 months (at local clinic) so we talk about effects this can have on the hearing | ||||
| 7 | At first appt. [appointment] | |||||
| 2 | Antenatal visit | Initial attendance at the MDT | ||||
5.3: Who in your network gives advice and discusses OME with families?
Individual listing of answers.
| Centre ID | Text answer to: Who in your network gives advice and discusses OME with families |
|---|---|
| 13 | All professionals in contact with patient |
| 10 | Depends on clinic attended, sometimes the audiologist other times the ENT consultant – whoever leads the clinic |
| 12 | Cleft surgeons at initial visit . . . . . SLT Audiology |
| 16 | All audiology/ENT services |
| 1 | The whole team as required: Nurses, SLTs, audiologist, psychologists, consultant plastic surgeon. The written information and discussion around this is given by the lead audiologist |
| 17 | ENT doctor or audiologist seeing child |
| 8 | All of us involved in the audiology assessments i.e. consultants and audiologists. The cleft nurses give advice as do SALTS and cleft surgeons to some extent |
| 7 | Audiology team, ENT and cleft team. [name] |
| 2 | Audiological Physician and ENT, and on follow visits Audiologists |
| 6 | |
| 11 | |
| 4 | Audiologist/ENT cons [consultants]/Cleft Team |
| 5 | |
| 15 | |
| 14 | |
| 9 |
Schematic summary: who in your network gives advice and discusses OME with families?
| Centre ID | Cleft nurse | Cleft surgeon | SLT | Audiologists | ENT consultant | Psychologists |
|---|---|---|---|---|---|---|
| 13 | ✗ | ✗ | ✗ | ✗ | ||
| 10 | ✗ | ✗ | ||||
| 12 | ✗ | ✗ | ✗ | |||
| 16 | ✗ | ✗ | ||||
| 1 | ✗ | ✗ | ✗ | ✗ | ✗ | |
| 17 | ✗ | ✗ | ||||
| 8 | ✗ | ✗ | ✗ | ✗ | ||
| 7 | ✗ | ✗ | ✗ | ✗ | ||
| 2 | ✗ | ✗ | ||||
| 4 | ✗ | ✗ | ✗ | ✗ |
Section 6: service delivery
6.1: Are there any barriers to the care that you would like to provide to patients with cleft and OME?
Individual listing of answers.
| Centre ID | Text answer to ‘barriers to the care’ | |
|---|---|---|
| Yes/no | Details | |
| 13 | Yes | Integrated records: each dept has it’s own records. This means I rely on professionals remembering to copy letters to me for all cleft patients seen. Financial/Time: I would like to review every cleft child in ENT clinics from an early age but there is not the resource to allow this at present. Currently they are seen by my only at the audit clinics 5, 10 yrs [years] etc. Currently there is co co-ordination between local departments and the cleft team however! |
| 10 | No | |
| 12 | No | |
| 16 | Yes | Funding issues with unilateral ventilation |
| 1 | Yes | Lack of knowledge of the best practise approach to managing OME. Working across spoke hospitals ensures family centred care delivered close to home but makes collection of assessments and application of a single protocol or policy difficult to apply consistently, particularly in the absence of a nationally agreed protocol |
| 17 | Yes | BC hearing technology is still not as advanced as BAHA softband, but we can’t provide BAHA soft bands to all our patients who have OME! |
| 8 | Missing | |
| 7 | No | |
| 2 | Yes | Competing staff, equipment and testing rooms/theatre time with other initiatives and services within the department and with other Units externally |
| 6 | ||
| 11 | ||
| 4 | ||
| 5 | ||
| 15 | ||
| 14 | ||
| 9 | ||
6.2: If there is anything else that you would like to tell us about the management of OME in your centre please enter it here.
Individual listing of answers.
| Centre ID | Text answer to ‘anything else about the management of OME in your centre’ | Attached written protocol |
|---|---|---|
| 13 | No | |
| 10 | No | |
| 12 | No | |
| 16 | No | |
| 1 | We believe that the active early intervention model with all members of the team actively educating families on hearing improves the ability to inform individualised patient care and tailor individual management plans including how best to and when to manage OME. Working closely with specialist SLTs is critical for exploring the impact of OME on speech and listening skills. A close working relationship between the audiologist and SLT helps inform both audiological management and speech therapy advise planning and delivery. Giving parents information on signs to look out for with OME and raising their awareness of the presentation and time course of OME help to empower the parents/families to ask for hearing assessments/or ask for advice about hearing/behavioural presentations as they have concerns rather than waiting for set appointments | Yes |
| 17 | No | |
| 8 | No | |
| 7 | No | |
| 2 | No | |
| 6 | ||
| 11 | ||
| 4 | ||
| 5 | ||
| 15 | ||
| 14 | ||
| 9 |
Appendix 3 Topic guides for qualitative interviews
Appendix 4 Core outcome set systematic review search strategies
Cochrane Central Register of Controlled Trials
URL: www.cochrane.org/editorial-and-publishing-policy-resource/cochrane-central-register-controlled-trials-central via Ovid search strategy https://ovidsp.ovid.com/
Date range searched: 2006 to 14 April 2011.
Date search performed: 14 April 2011.
Search strategy
#1 MeSH descriptor cleft palate explode all trees (92)
#2 MeSH descriptor cleft lip explode all trees (69)
#3 (cleft* in All Text near/6 palate* in All Text) (176)
#4 (cleft* in All Text near/6 lip* in All Text) (131)
#5 “hare lip*” in All Text (2)
#6 harelip* in All Text (2)
#7 Palatoschisis in All Text (1)
#8 (orofacial* in All Text near/6 cleft* in All Text) (6)
#9 (facial* in All Text near/6 cleft* in All Text) (13)
#10 (face* in All Text near/6 cleft* in All Text) (7)
#11 (#1 or#2 or#3 or#4 or#5 or#6 or#7 or#8 or#9 or#10) (204)
#12 MeSH descriptor middle ear ventilation explode all trees (200)
#13 MeSH descriptor otitis media explode all trees (820)
#14 grommet* in All Text (72)
#15 (ear in All Text near/6 ventilat* in All Text) (232)
#16 otitis next media in All Text (1527)
#17 (ventilat* in All Text near/6 tube* in All Text) (408)
#18 tympanostom* in All Text (135)
#19 (glue in All Text near/6 ear* in All Text) (65)
#20 (#12 or#13 or#14 or#15 or#16 or#17 or#18 or#19) (1908)
#21 (#11 and#20) (17)
Cumulative Index to Nursing and Allied Health Literature via Ovid
URL: www.ebscohost.com/nursing/products/cinahl-databases/cinahl-complete via Ovid search strategy – https://ovidsp.ovid.com/
Date range searched: 1982 to February week 2 2006.
Date search performed: September 2012.
Search strategy
-
Cleft Palate/ (686)
-
Cleft Lip/ (526)
-
(cleft$ adj3 lip$).tw. (495)
-
(cleft$ adj3 palat$).tw. (617)
-
hare lip$.tw. (1)
-
harelip$.tw. (0)
-
Palatoschisis.tw. (0)
-
(orofacial$ adj3 cleft$).tw. (29)
-
(facial adj3 cleft$).tw. (40)
-
(oral adj3 cleft$).tw. (49)
-
(craniofacial adj3 cleft$).tw. (47)
-
or/1-11 (817)
-
Middle Ear Ventilation/ (224)
-
exp Otitis Media/ (1259)
-
grommet$.tw. (21)
-
(ear$ adj3 ventilat$).tw. (80)
-
otitis media.tw. (898)
-
(ventilat$ adj3 tube$).tw. (99)
-
tympanostom$.tw. (90)
-
(glue adj3 ear$).tw. (32)
-
or/13-20 (1634)
-
21 and 12 (17)
-
from 22 keep 1-17 (17)
Cumulative Index to Nursing and Allied Health Literature via EBSCOhost
URL: www.ebscohost.com/nursing/products/cinahl-databases/cinahl-com via EBSCOhost search strategy – www.ebsco.com/
Date range searched: February week 2, 2006 to 13 April 2011.
Date search performed: September 2012.
Search strategy
| CINAHL via Ovid | CINAHL via EBSCOhost | |||||||
|---|---|---|---|---|---|---|---|---|
| Keyword | Field | Results | Keyword | Field | Results for 1982–14 April 2014 | Results for 1982–February 2006 | ||
| 1 | Cleft Palate | Thesaurus | 686 | Cleft Palate | Thesaurus | 1436 | 751 | |
| 2 | Cleft Lip | Thesaurus | 526 | Cleft Lip | Thesaurus | 1099 | 567 | |
| 3 | cleft$ adj3 lip$ | Text Word (.tw.) | 495 | Cleft* N3 lip* | TI, AB,IN | 995 | 530 | |
| 4 | cleft$ adj3 palat$ | Text Word (.tw.) | 617 | Cleft* N3 palat* | TI, AB,IN | 1232 | 663 | |
| 5 | hare lip$ | Text Word (.tw.) | 1 | hare lip* | TI, AB,IN | 1 | 1 | |
| 6 | harelip$ | Text Word (.tw.) | 0 | Harelip* | TI, AB,IN | 0 | 0 | |
| 7 | Palatoschisis | Text Word (.tw.) | 0 | Palatoschisis | TI, AB,IN | 1 | 1 | |
| 8 | orofacial$ adj3 cleft$ | Text Word (.tw.) | 29 | Orofacial* N3 cleft* | TI, AB,IN | 93 | 33 | |
| 9 | facial adj3 cleft$ | Text Word (.tw.) | 40 | facial N3 cleft* | TI, AB,IN | 69 | 40 | |
| 10 | oral adj3 cleft$ | Text Word (.tw.) | 49 | oral N3 cleft* | TI, AB,IN | 115 | 50 | |
| 11 | craniofacial adj3 cleft$ | Text Word (.tw.) | 47 | craniofacial N3 cleft* | TI, AB,IN | 74 | 45 | |
| 12 | or/1-11 | 817 | or/1-11 | 1741 | 889 | |||
| 13 | Middle Ear Ventilation | Thesaurus | 224 | Middle Ear Ventilation | Thesaurus | 410 | 264 | |
| 14 | exp Otitis Media | Thesaurus | 1259 | exp Otitis Media | Thesaurus | 2403 | 1467 | |
| 15 | grommet$ | Text Word (.tw.) | 21 | grommet* | TI, AB,IN | 49 | 28 | |
| 16 | ear$ adj3 ventilat$ | Text Word (.tw.) | 80 | ear* N3 ventilat* | TI, AB,IN | 158 | 75 | |
| 17 | otitis media | Text Word (.tw.) | 898 | otitis media | TI, AB,IN | 1646 | 1104 | |
| 18 | ventilat$ adj3 tube$ | Text Word (.tw.) | 99 | ventilat* N3 tube* | TI, AB,IN | 266 | 166 | |
| 19 | tympanostom$ | Text Word (.tw.) | 90 | tympanostom* | TI, AB,IN | 171 | 110 | |
| 20 | glue adj3 ear$ | Text Word (.tw.) | 32 | glue N3 ear* | TI, AB,IN | 42 | 33 | |
| 21 | or/13-20 | 1634 | or/13-20 | 3210 | ||||
| 22 | 21 and 12 | 17 | 21 and 12 | 36 | ||||
| 23 | from 22 keep 1-17 | 17 | from 22 keep 1-17 | |||||
EMBASE
URL: www.elsevier.com/online-tools/embase via Ovid search strategy – https://ovidsp.ovid.com/
Date range searched: 2006 to 14 April 2011.
Date search performed: 14 April 2011.
Search strategy
-
cleft lip/ (3181)
-
Cleft Palate/ (5513)
-
Cleft Lip Face Palate/ (257)
-
Cleft Lip Palate/ (931)
-
(cleft$ adj3 lip$).tw. (4006)
-
(cleft$ adj3 palat$).tw. (5885)
-
hare lip$.tw. (23)
-
harelip$.tw. (31)
-
Palatoschisis.tw. (25)
-
(orofacial$ adj3 cleft$).tw. (243)
-
(facial adj3 cleft$).tw. (675)
-
(oral adj3 cleft$).tw. (290)
-
(craniofacial adj3 cleft$).tw. (259)
-
or/1-13 (9179)
-
middle ear ventilation/ (238)
-
exp Otitis Media/ (13,078)
-
tympanostomy tube/ (1111)
-
grommet$.tw. (305)
-
(ear$ adj3 ventilat$).tw. (901)
-
otitis media.tw. (9424)
-
(ventilat$ adj3 tube$).tw. (1075)
-
tympanostom$.tw. (640)
-
(glue adj3 ear$).tw. (216)
-
or/15-23 (16372)
-
14 and 24 (206)
-
from 25 keep 1-206 (206)
MEDLINE
URL: www.nlm.nih.gov/bsd/pmresources.html via Ovid search strategy – https://ovidsp.ovid.com/
Date range searched: 2006 to 14 April 2011.
Date search performed: 14 April 2011.
Search strategy
-
Cleft Palate/ (11,628)
-
Cleft Lip/ (8131)
-
(cleft$ adj3 lip$).tw. (6156)
-
(cleft$ adj3 palat$).tw. (8901)
-
hare lip$.tw. (68)
-
harelip$.tw. (99)
-
Palatoschisis.tw. (69)
-
(orofacial$ adj3 cleft$).tw. (280)
-
(facial adj3 cleft$).tw. (759)
-
(oral adj3 cleft$).tw. (326)
-
(craniofacial adj3 cleft$).tw. (213)
-
or/1-11 (15,658)
-
Middle Ear Ventilation/ (1469)
-
exp Otitis Media/ (15,239)
-
grommet$.tw. (336)
-
(ear$ adj3 ventilat$).tw. (616)
-
otitis media.tw. (10,785)
-
(ventilat$ adj3 tube$).tw. (1163)
-
tympanostom$.tw. (651)
-
(glue adj3 ear$).tw. (273)
-
or/13-20 (18,849)
-
21 and 12 (297)
-
exp animals/ not human/ (2,948,249)
-
22 not 23 (286)
-
22 not 24 (11)
-
from 25 keep 1-11 (11)
Appendix 5 Examples of invitations sent to parents and children via the Cleft Lip & Palate Association newsletter and social media
Appendix 6 Examples of invitation and reminder e-mails sent to health professionals to take part in the Delphi survey
Appendix 7 Documents provided to participants of the consensus meeting for core outcome set development
E-mail content
Dear <name>,
Thank you for agreeing to take to part in the MOMENT study event for the development of a core outcome set on the 6th March in Manchester.
The event will take place at the MacDonald Manchester Hotel and Spa, details of how to get the hotel are available on the hotel website: http://www.macdonaldhotels.co.uk/our-hotels/macdonald-manchester-hotel-spa/useful-information/
I have attached three documents for you to have a look at before the meeting, these are:
-
A summary of the day outlining who is involved and what the aims of the day are
-
A summary from an organisation called COMET describing what core outcome measures are.
-
An agenda for the day.
In the agenda there are two slots, one before and one after lunch, where we will present back to you the results of the surveys of health professionals, parents and children. These results will show how important people thought each of the outcomes of treatment for glue ear were.
In these results sessions we will ask you to think about the results we present and if you agree with them. We will ask everyone at the meeting, including those who have already completed the surveys, to score each of the outcomes. For some of these outcomes there may be some discussion needed within the group. For example, if it appears that there are large differences in opinions of how important an outcome is.
<We would like to welcome you to our meeting at 10.30am to meet with the team so that we can give you an overview of core outcome measures, introduce you to the study team and answer any questions that you might have.> – provided to parents only
If you would like us to book your travel arrangements and/or overnight accommodation and we have yet to confirm the arrangements with you please contact Claire (email address) who will be able to help with the booking. It would also be helpful if you could let us know whether you have any dietary requirements and if you are bringing along a friend or relative so that we know final numbers.
The MOMENT study team would like to take this opportunity to thank you for your interest in the study and the core outcome set meeting. We greatly value the contribution of parents, patient representatives and health care professionals in this study and look forward to meeting you on the 6th March.
Best wishes
Nicola
On behalf of the MOMENT study team.
Documents provided with e-mail
COMET plain language summary, URL: www.comet-initiative.org/resources/PlainLanguageSummary (accessed 9 April 2014).
mOMEnt consensus meeting agenda V1.0 24 February 2014
mOMEnt consensus meeting summary V2.0 19 February 2014
Appendix 8 Full responses to round 3 of the health professional Delphi survey and survey of parents and children with cleft palate
FIGURE 23.
Outcome: tinnitus.

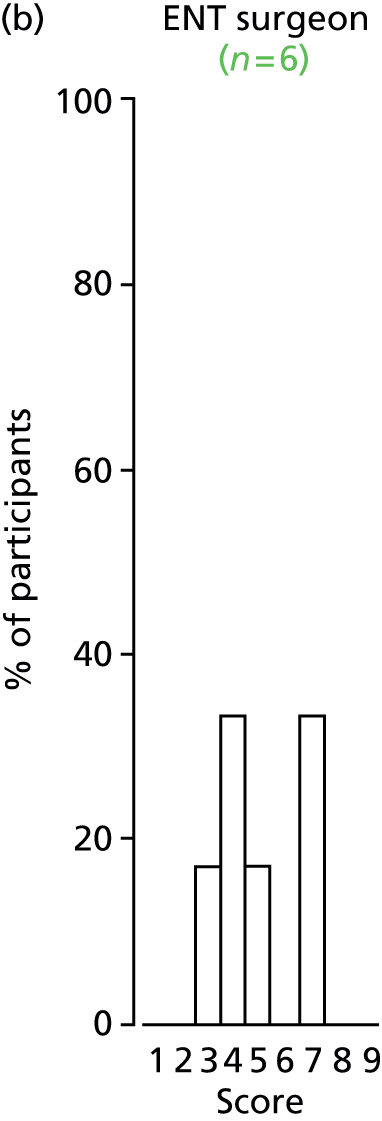
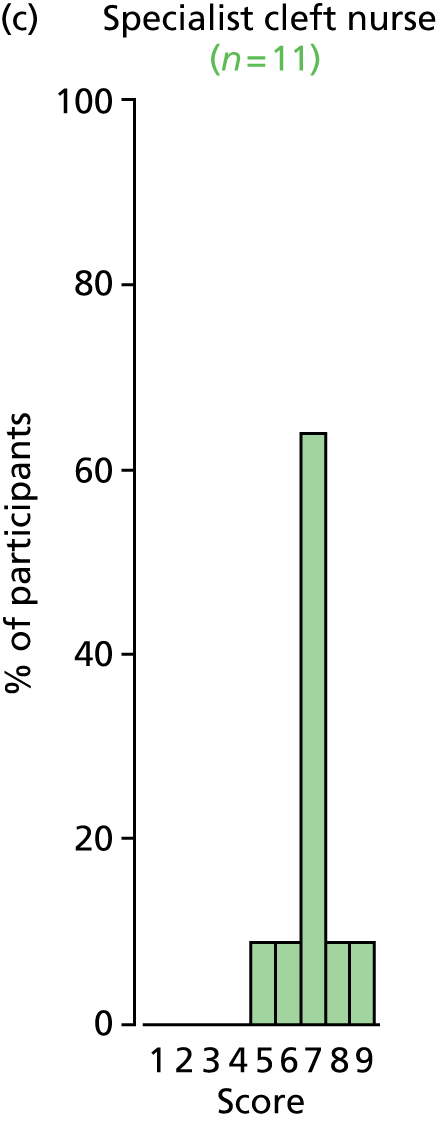

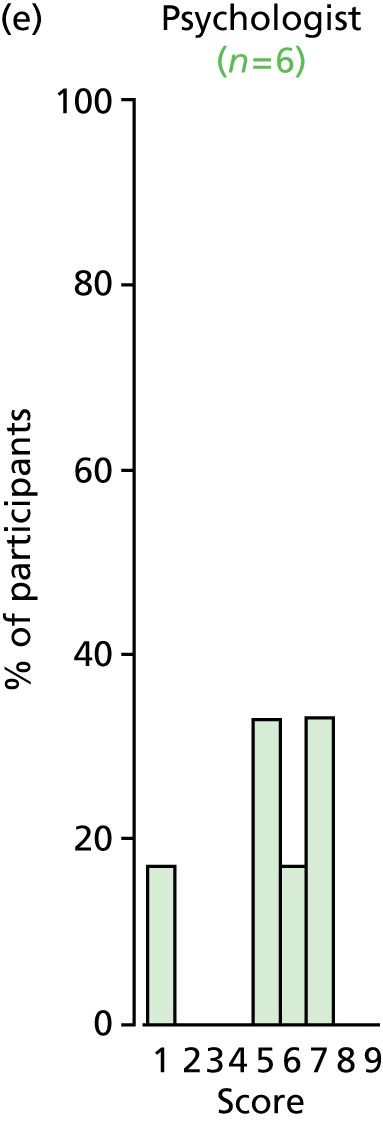
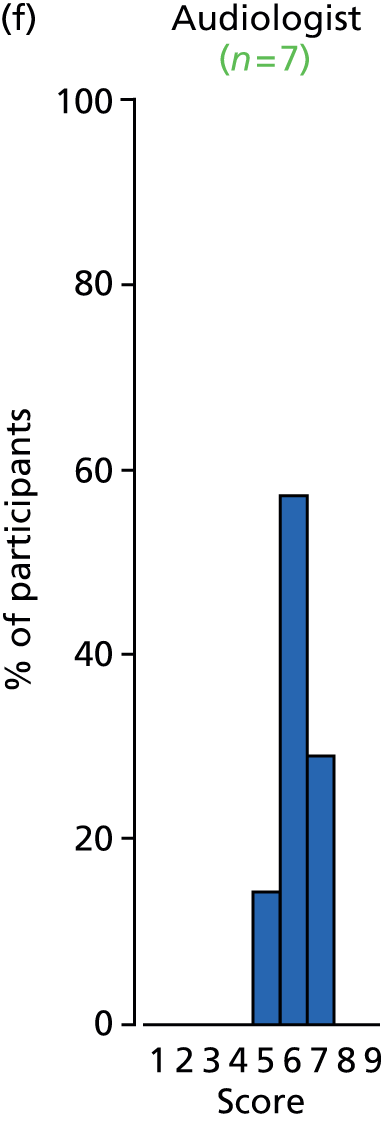


FIGURE 24.
Outcome: vertigo.
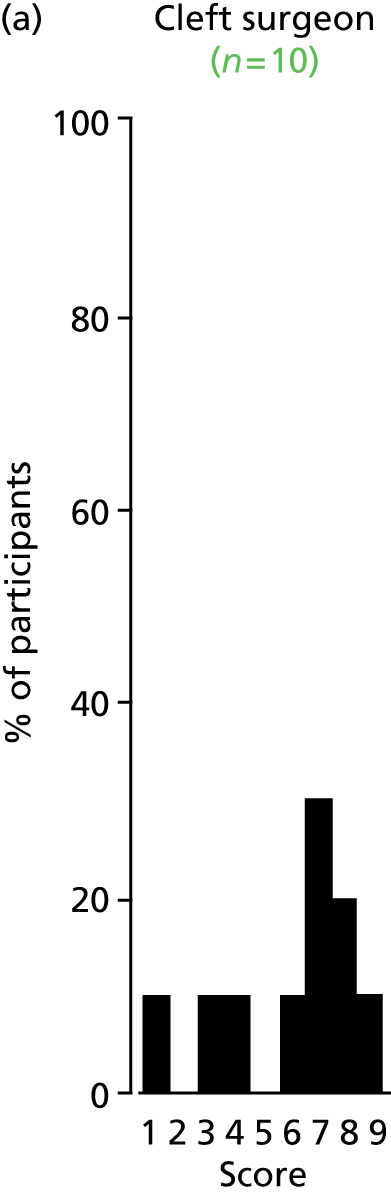
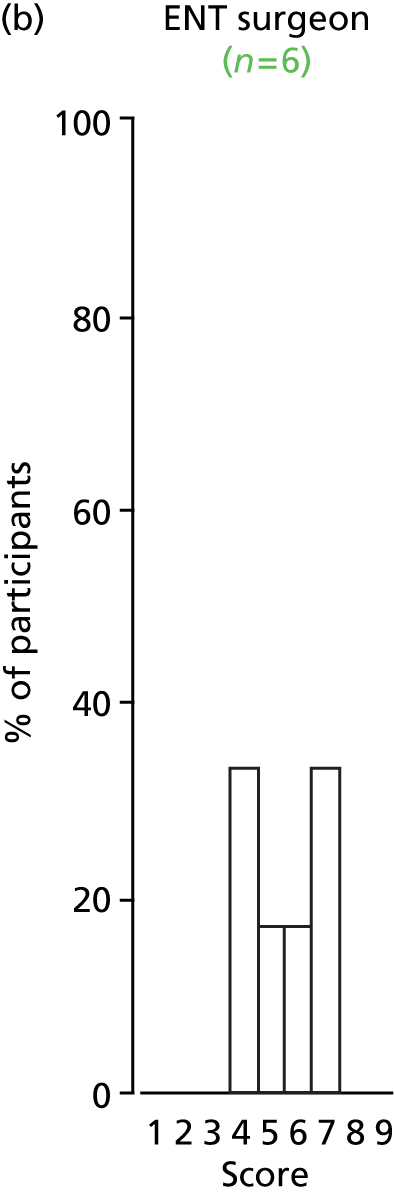


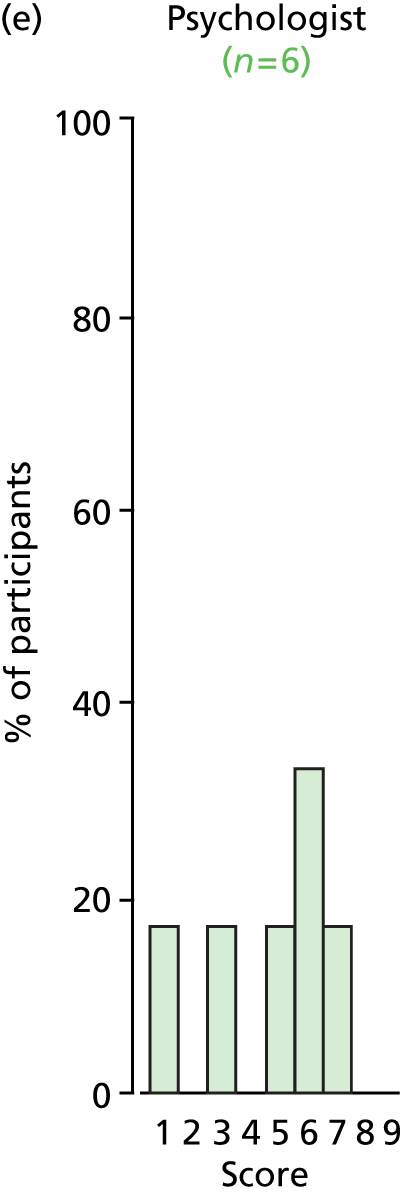
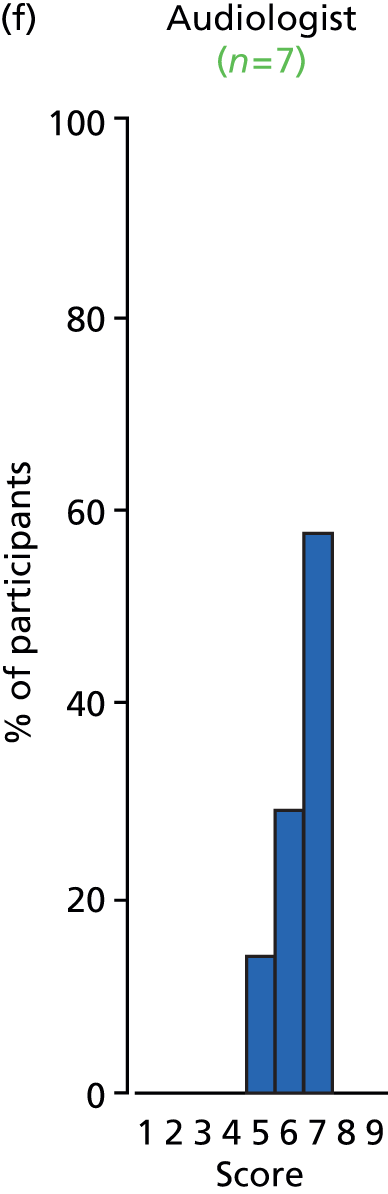


FIGURE 25.
Outcome: stapedial reflex.
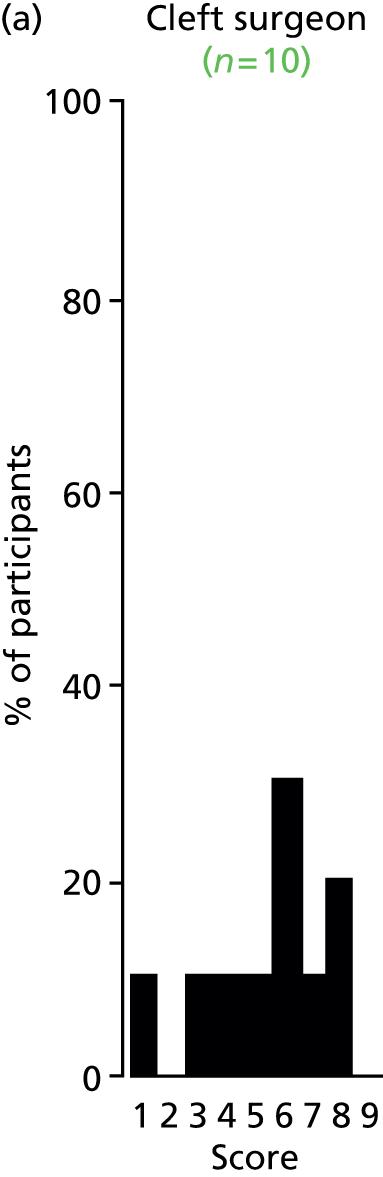

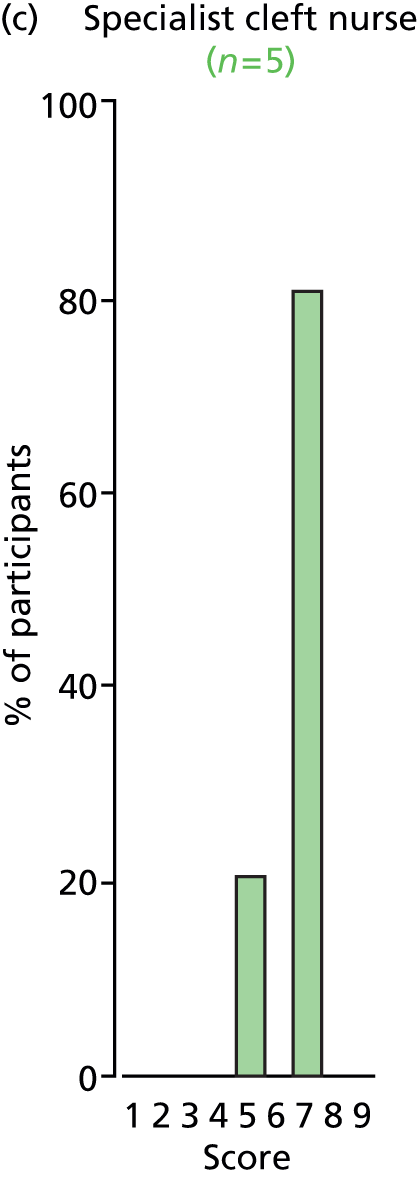
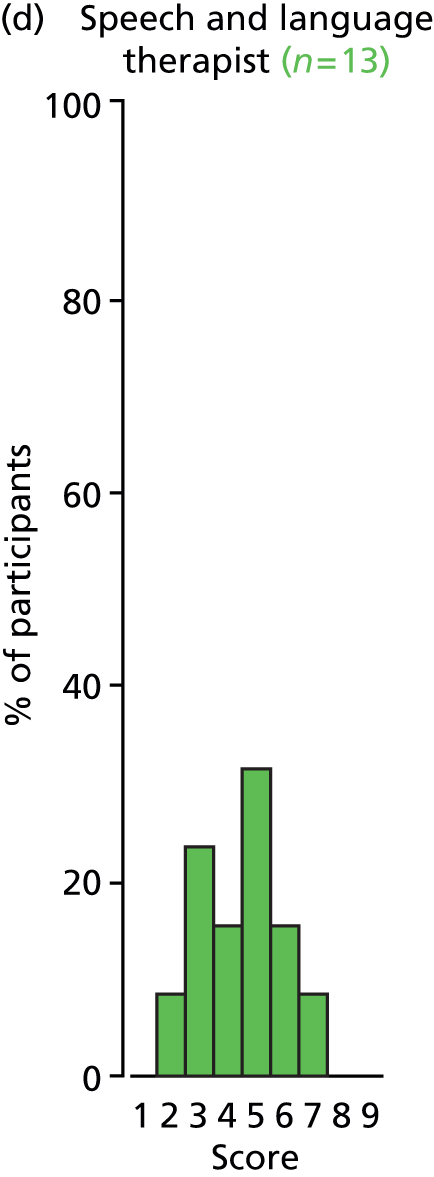
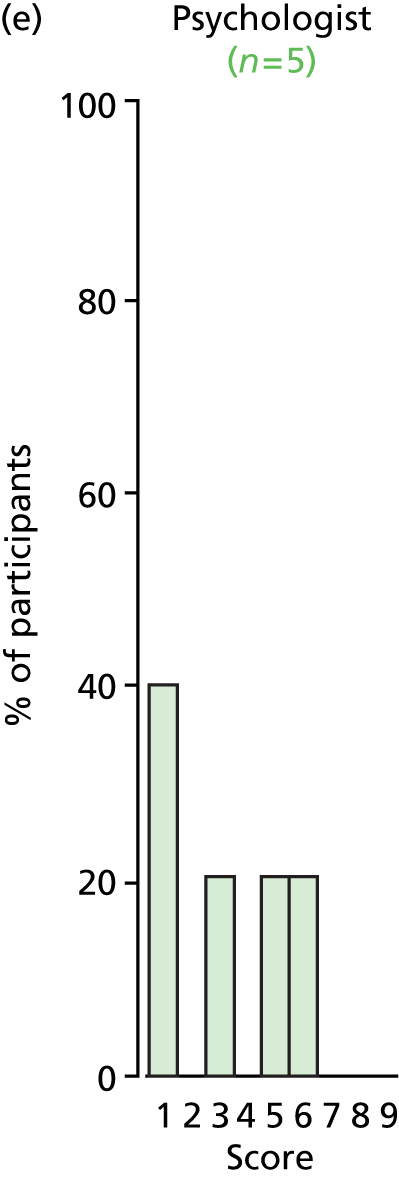

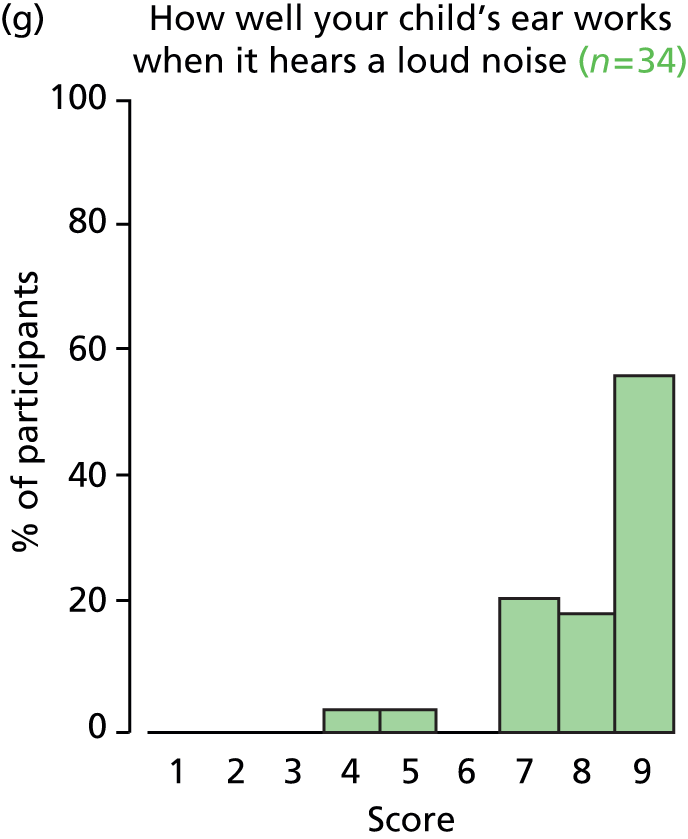
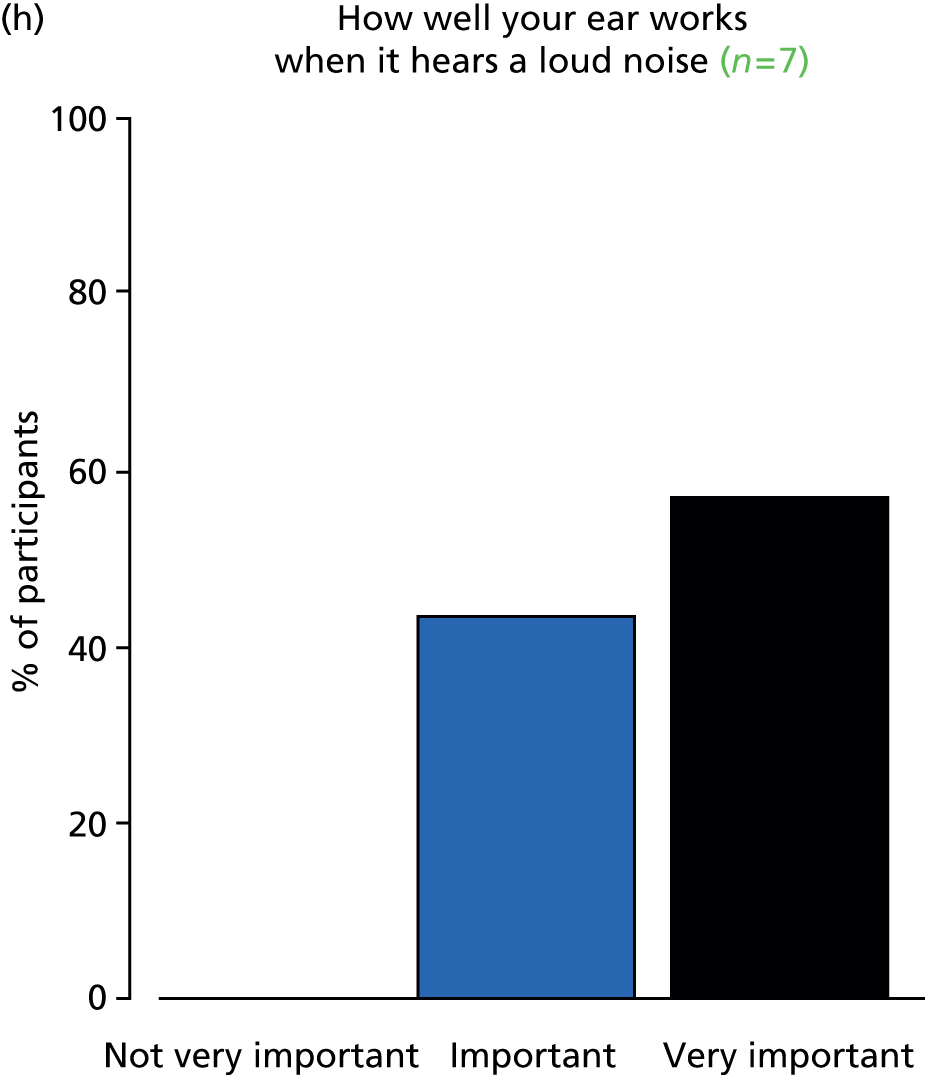
FIGURE 26.
Outcome: parent’s perspective of speech.


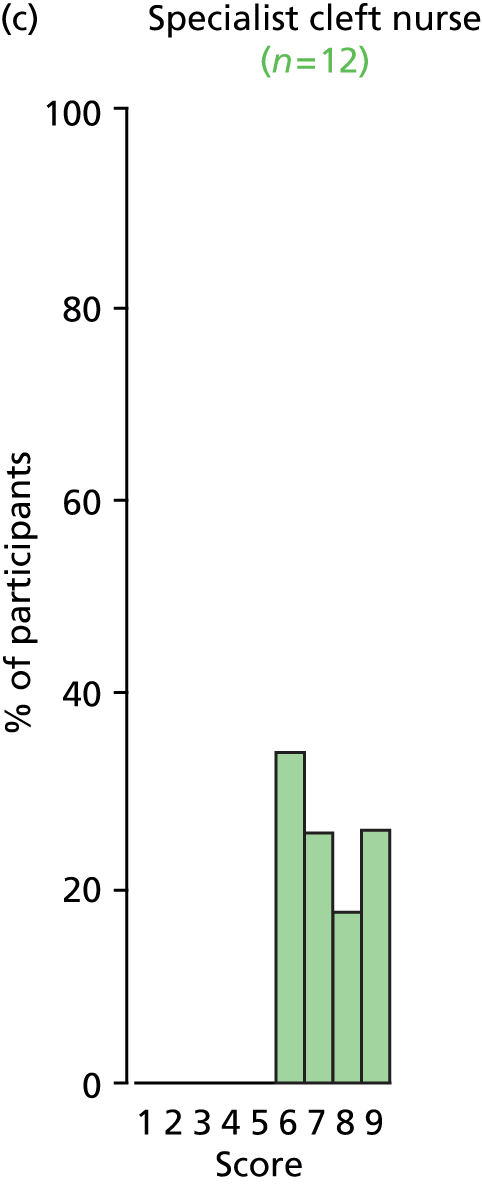
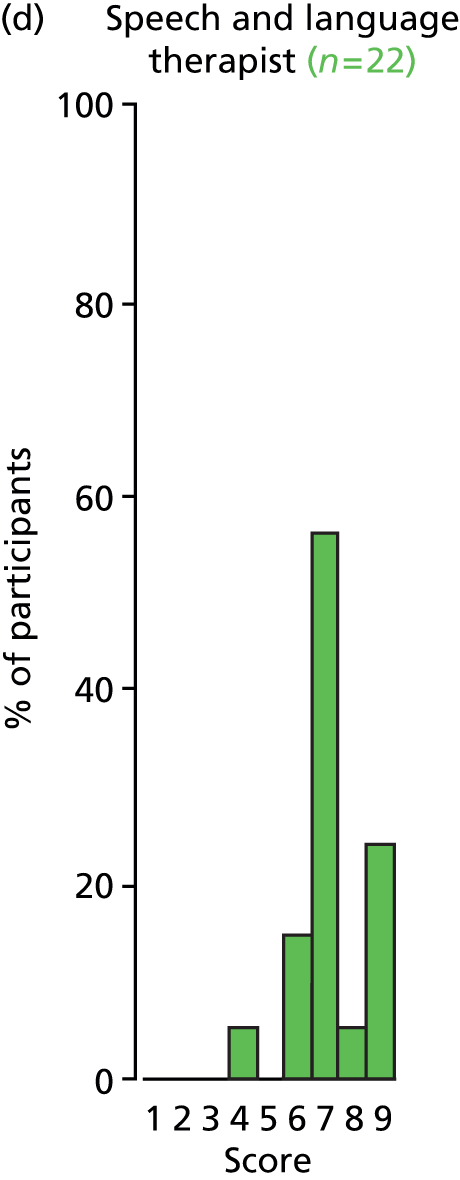

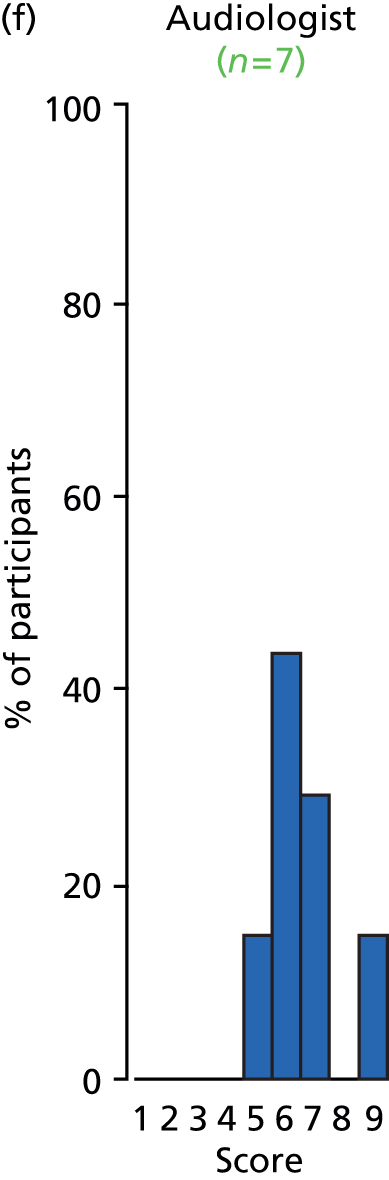
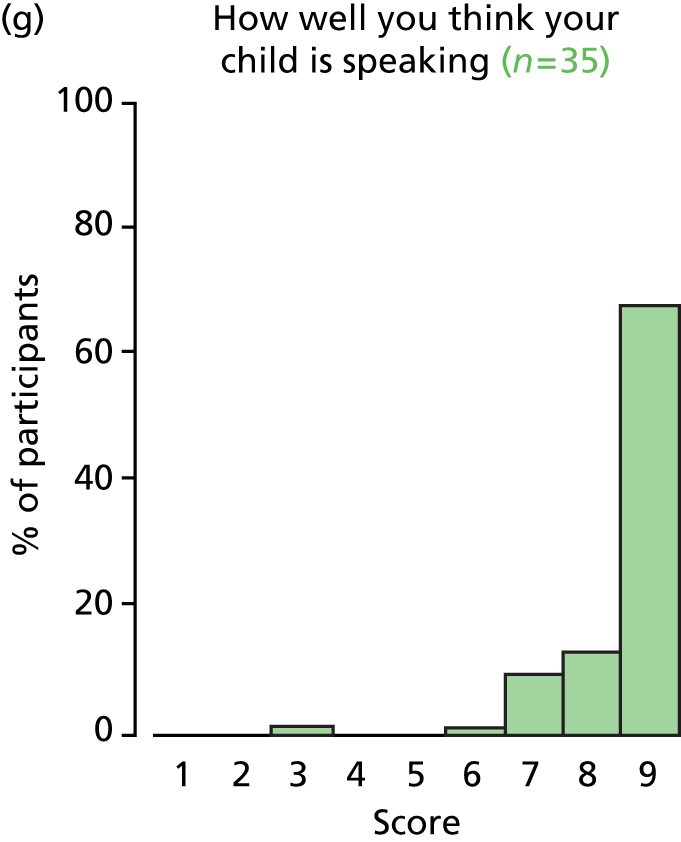

FIGURE 27.
Outcome: parental stress.
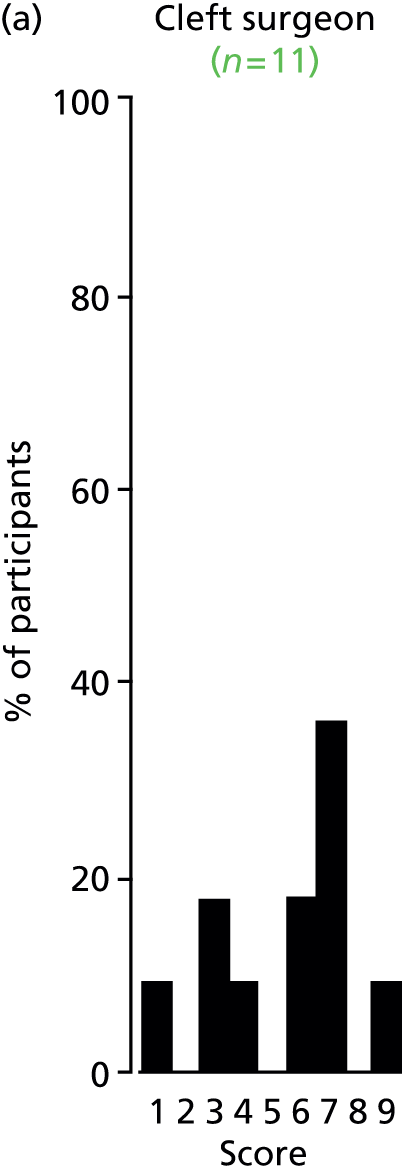





FIGURE 28.
Outcome: intelligence.
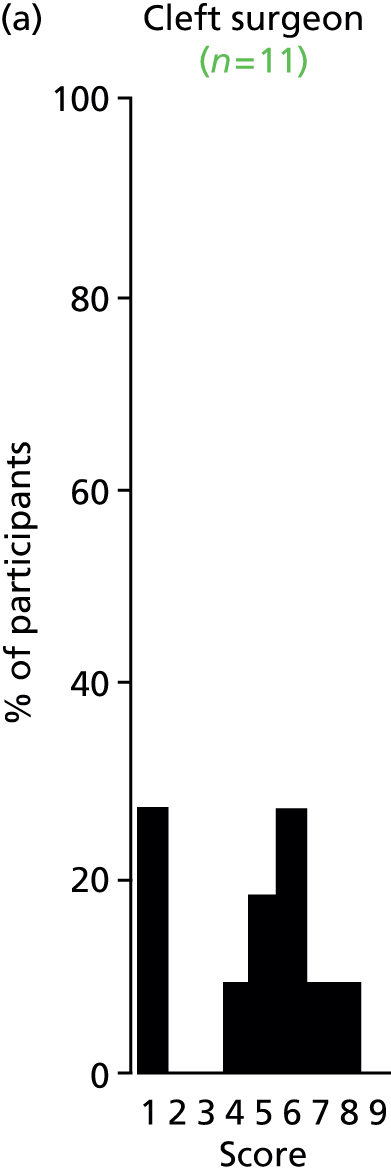
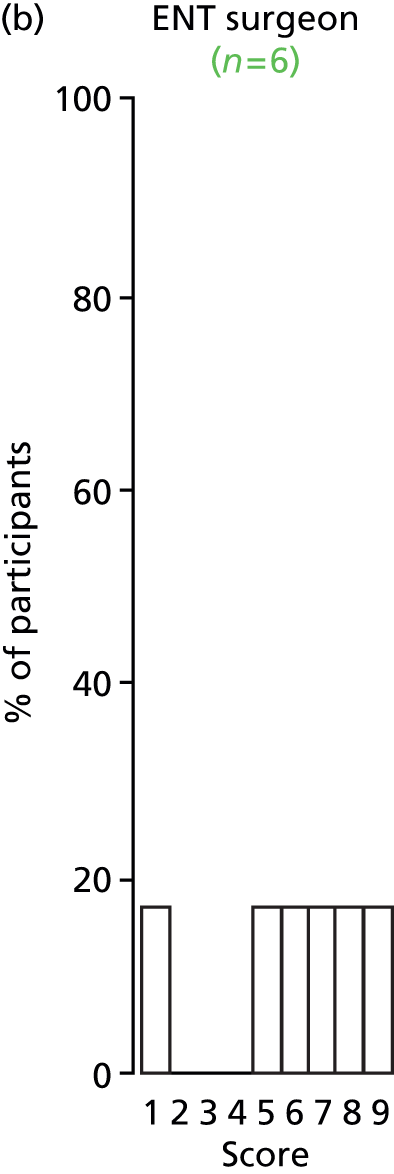

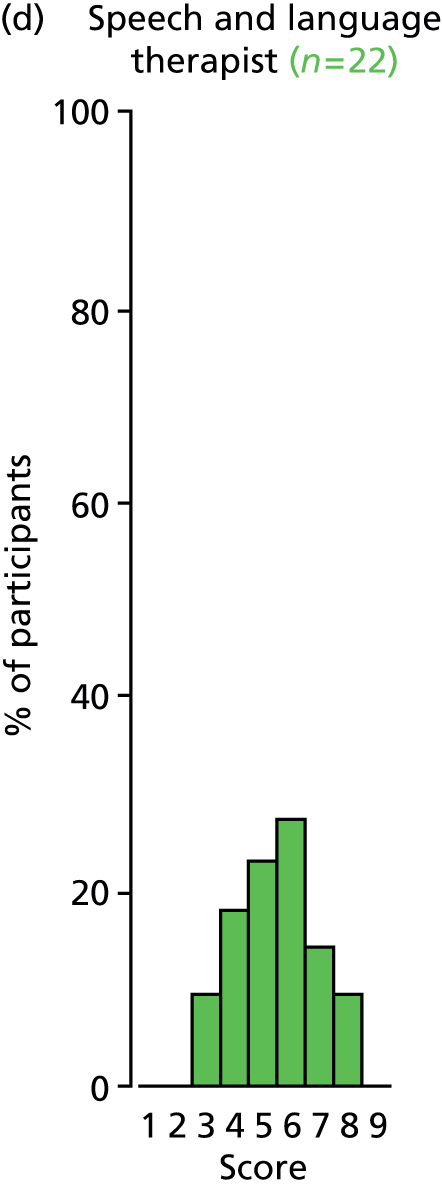
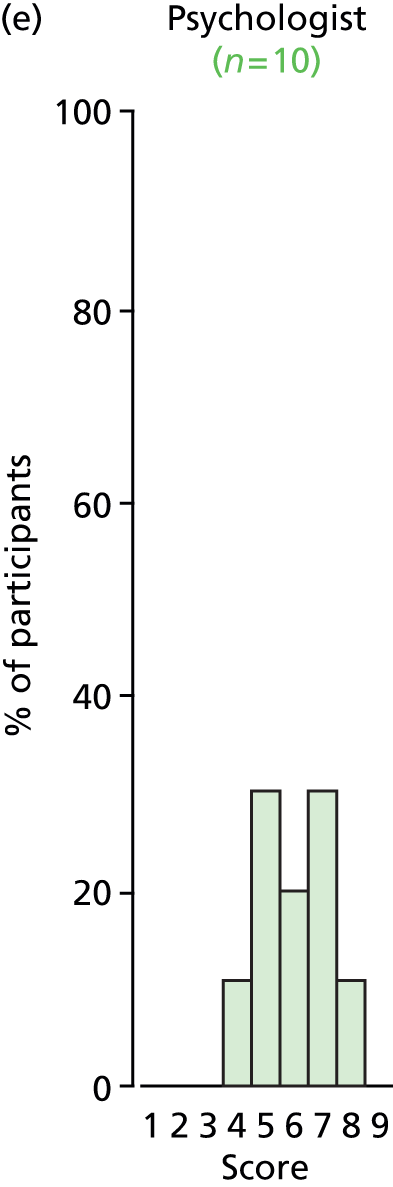

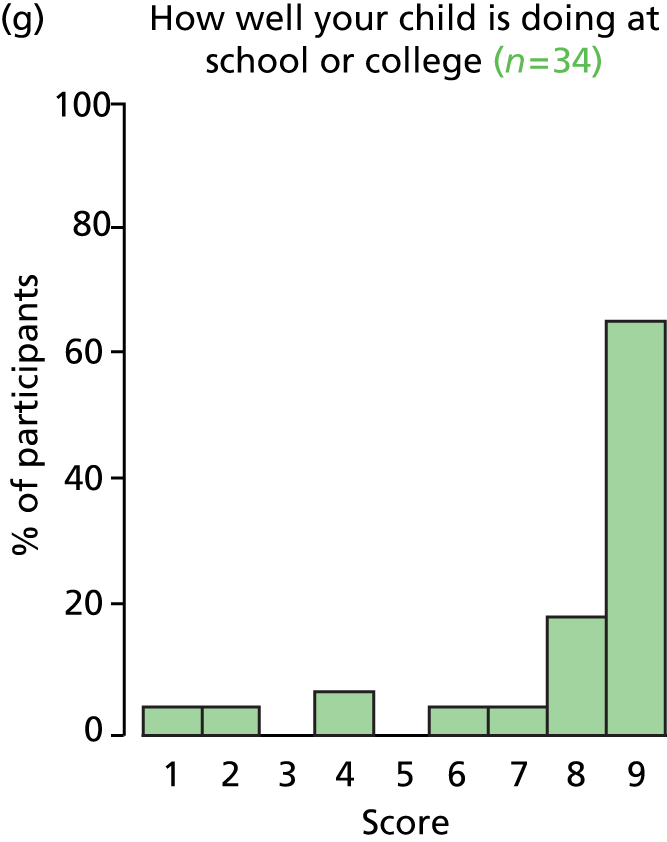

FIGURE 29.
Outcome: temporary tympanic membrane perforation.

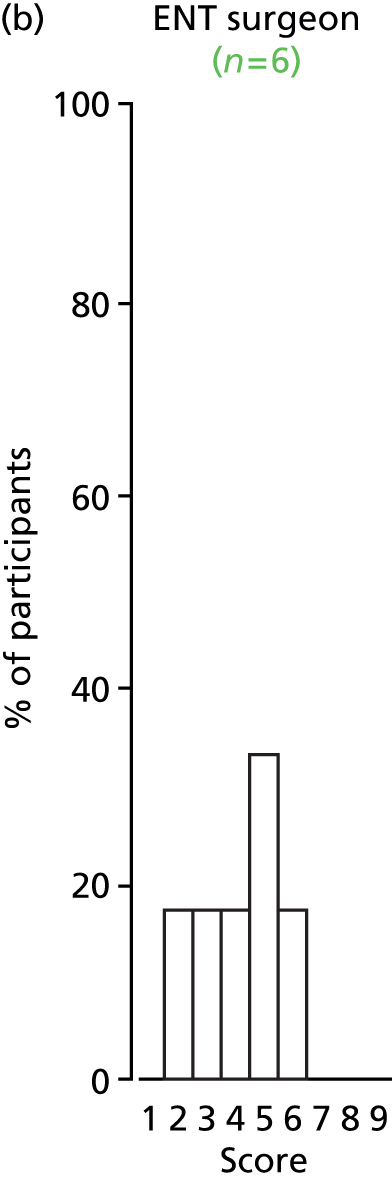

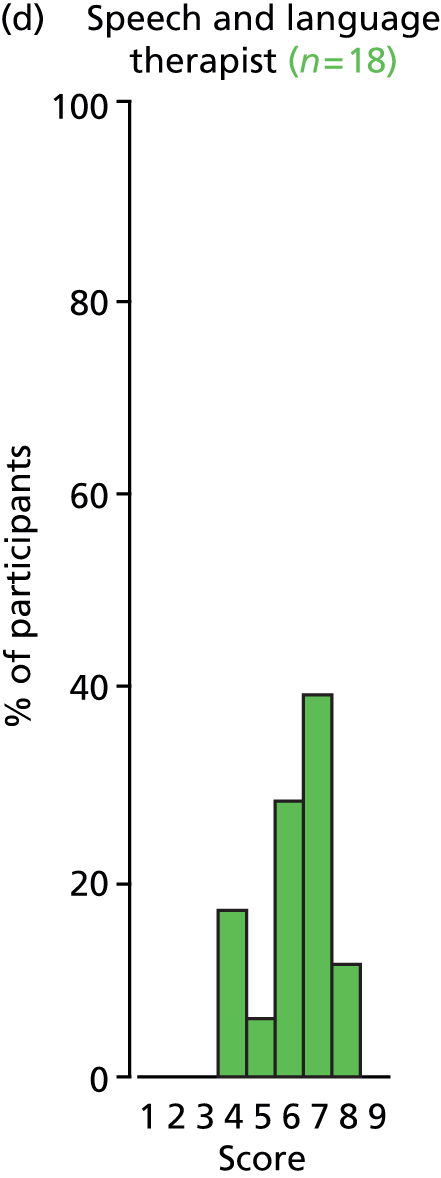


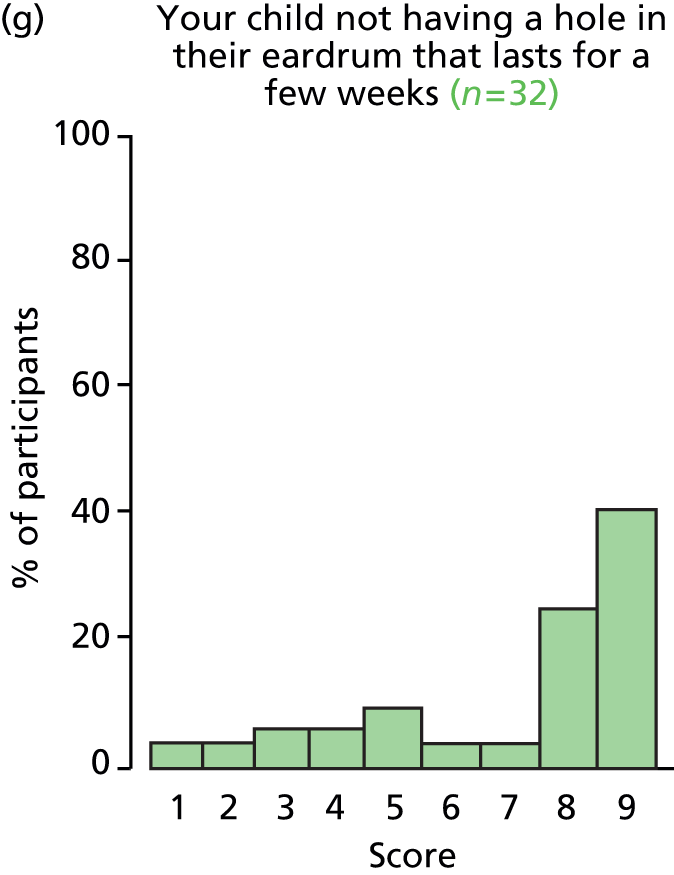
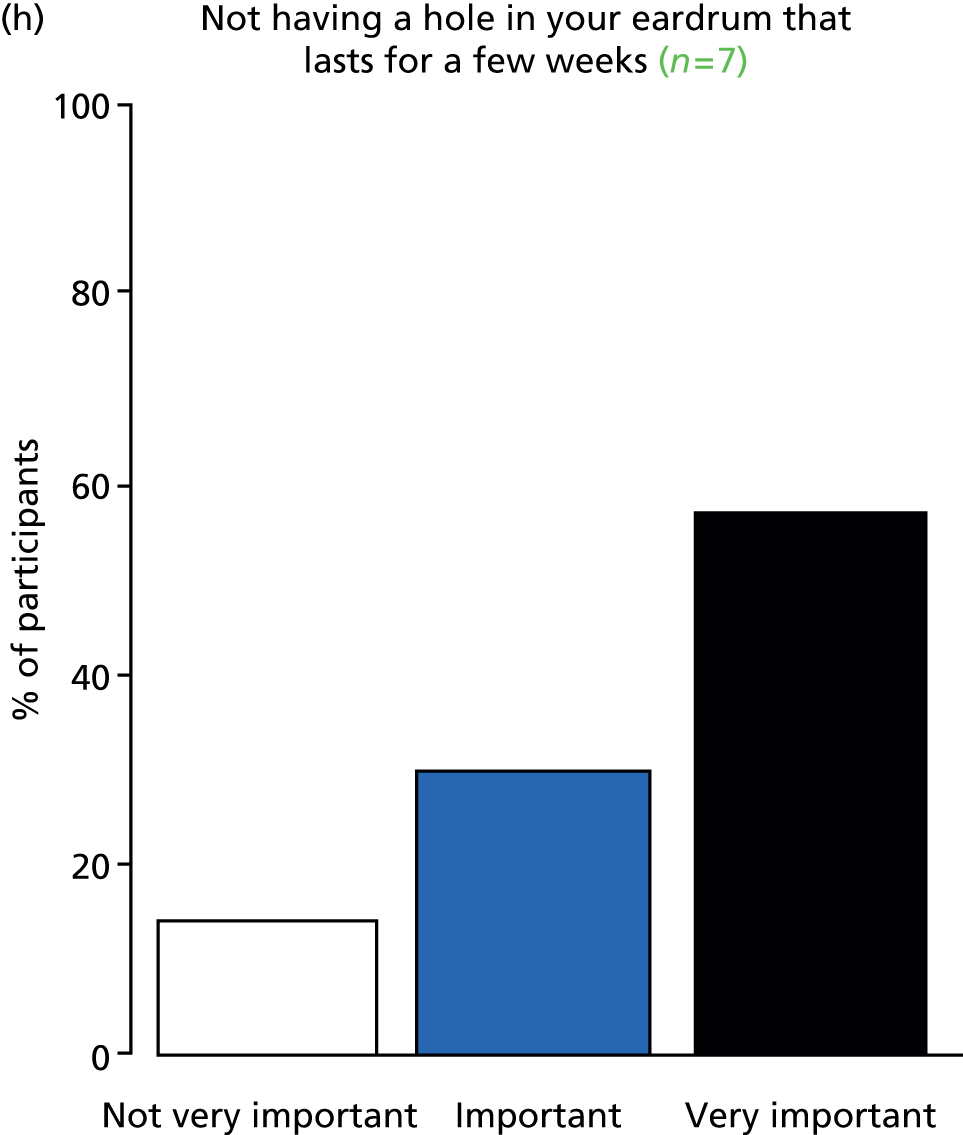
FIGURE 30.
Outcome: upper respiratory tract infection.




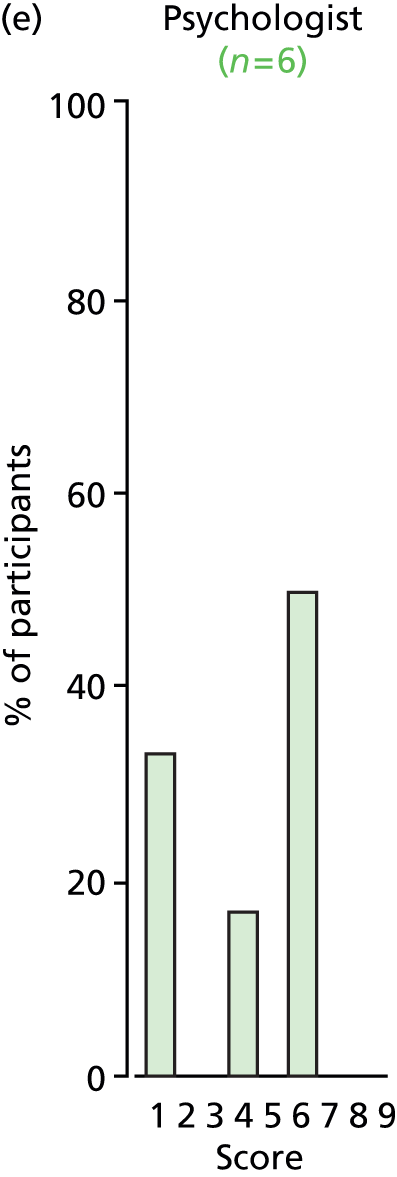

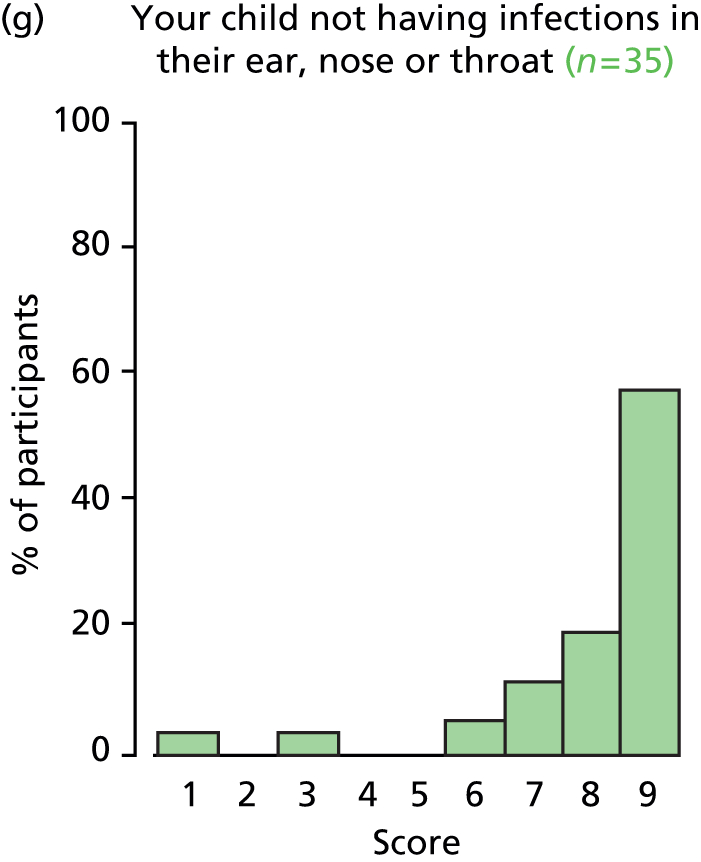

FIGURE 31.
Outcome: nasal obstruction.





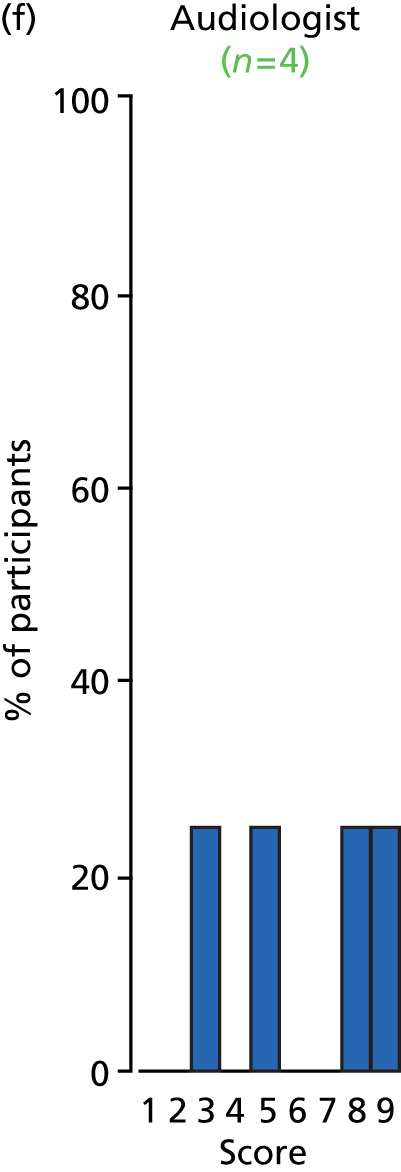


FIGURE 32.
Outcome: rhinitis.
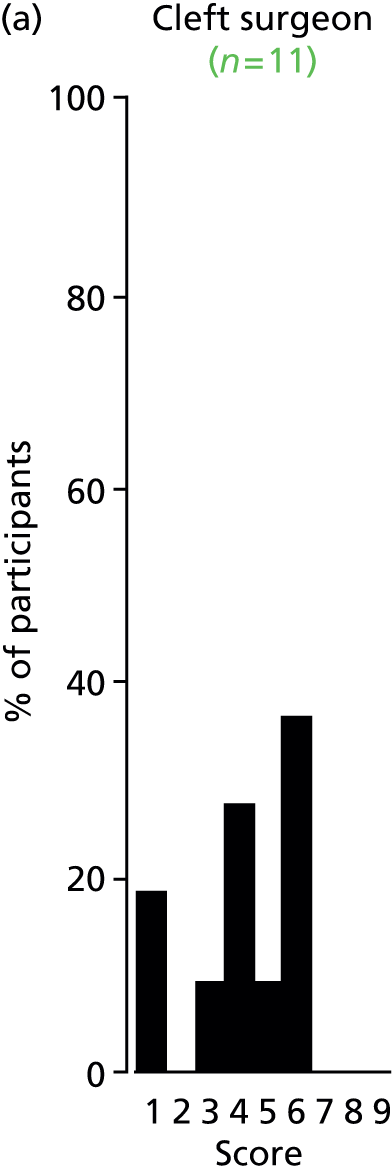



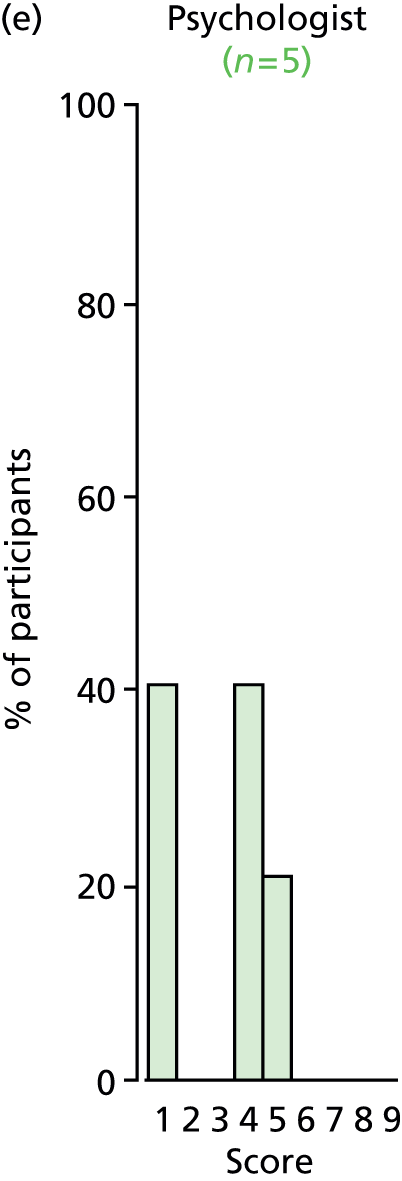
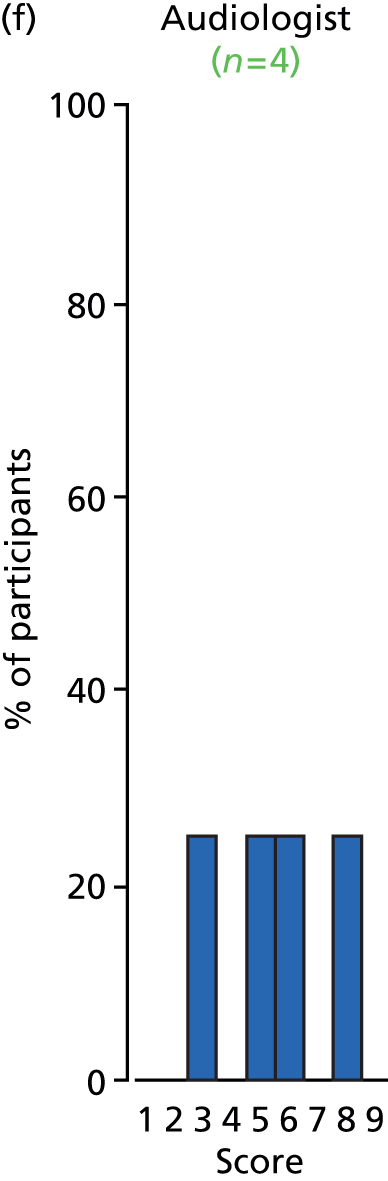

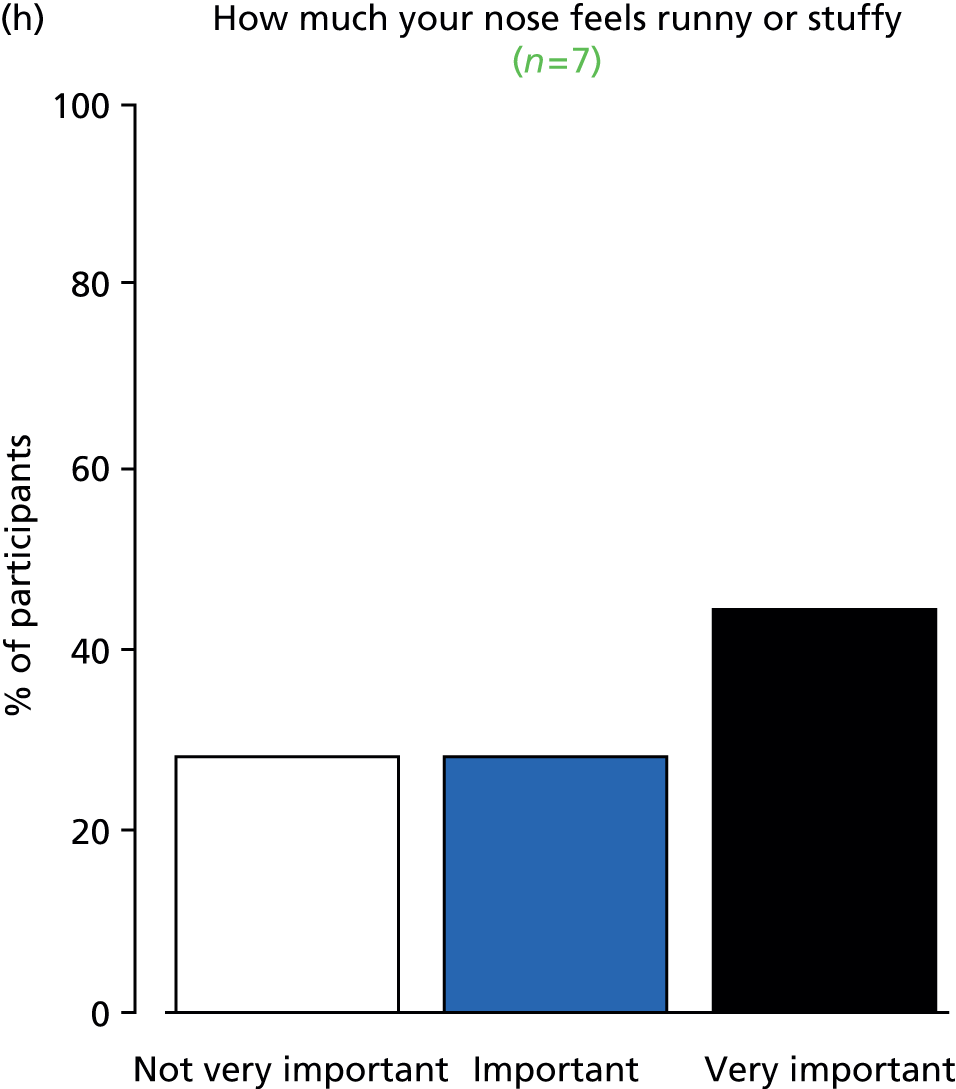
FIGURE 33.
Outcome: hyperacusis.

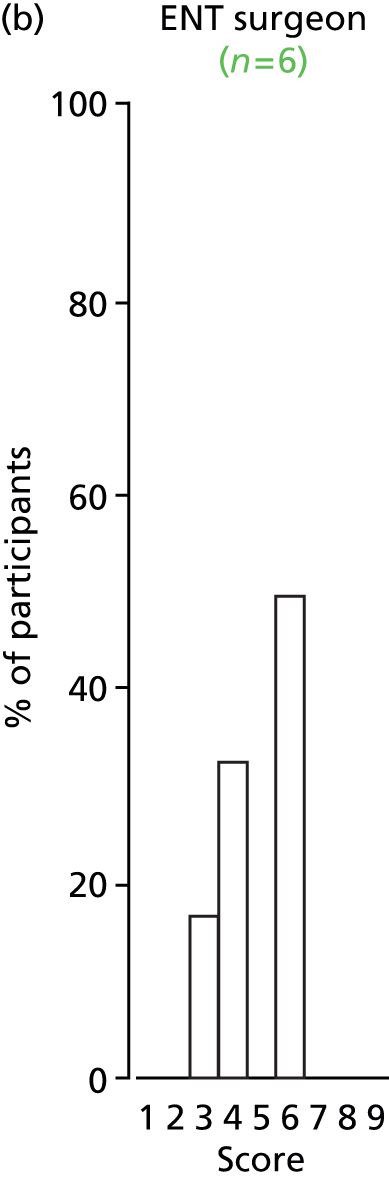
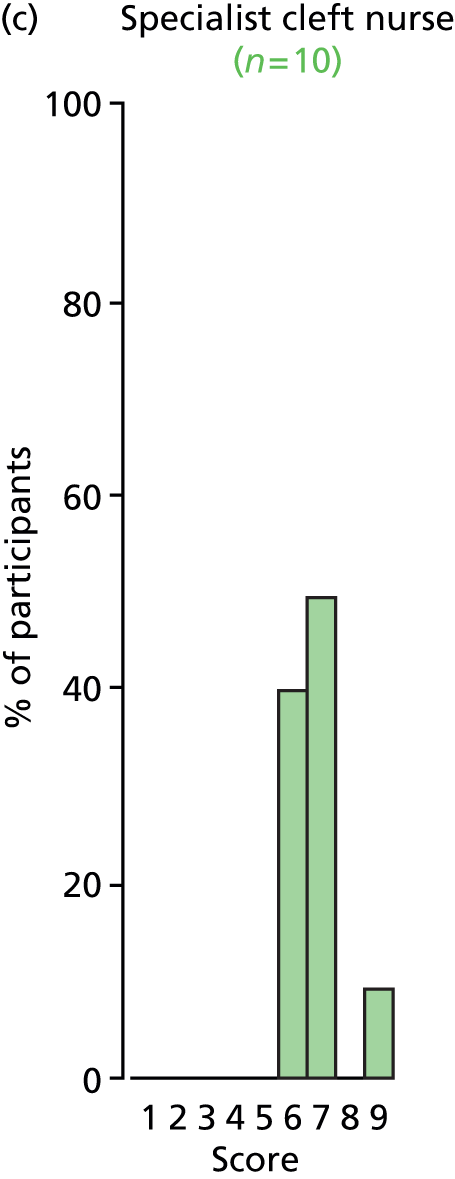



FIGURE 34.
Outcome: parental satisfaction with treatment.
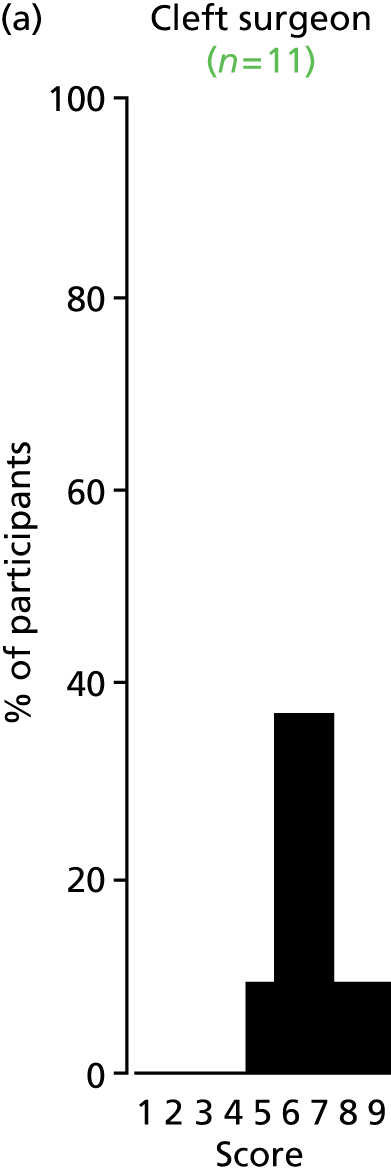
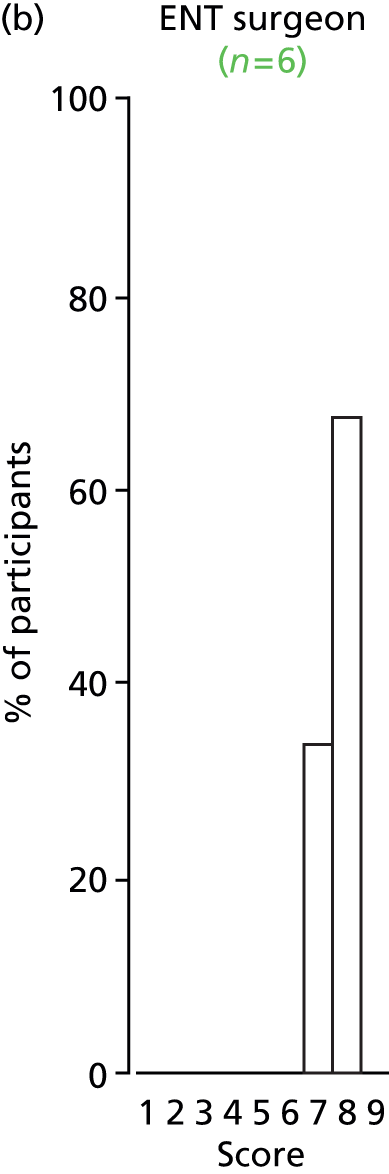


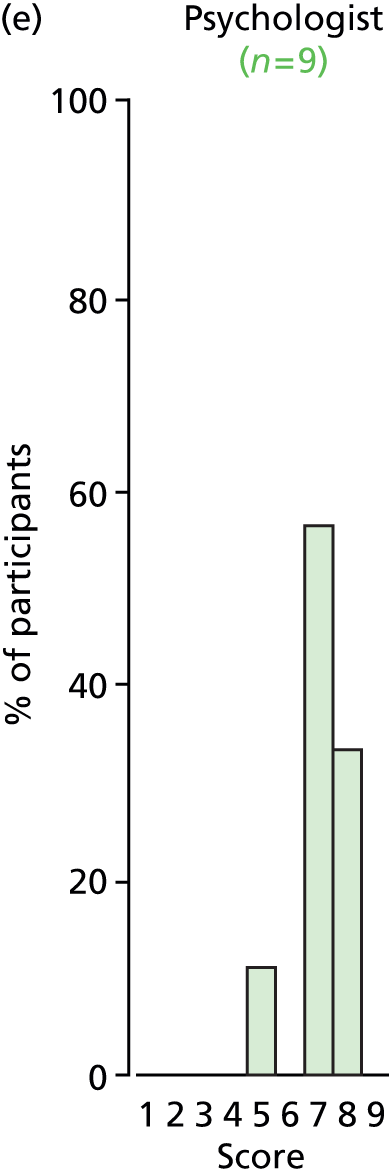
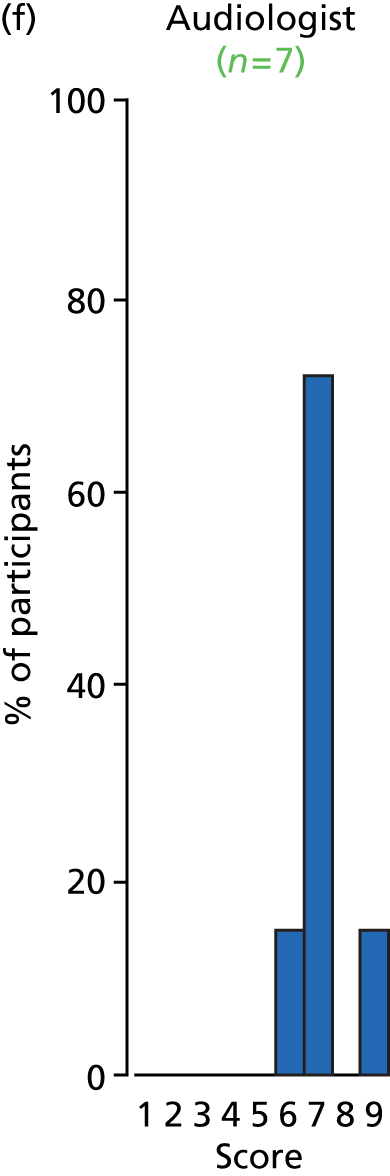


FIGURE 35.
Outcome: child’s satisfaction with treatment.


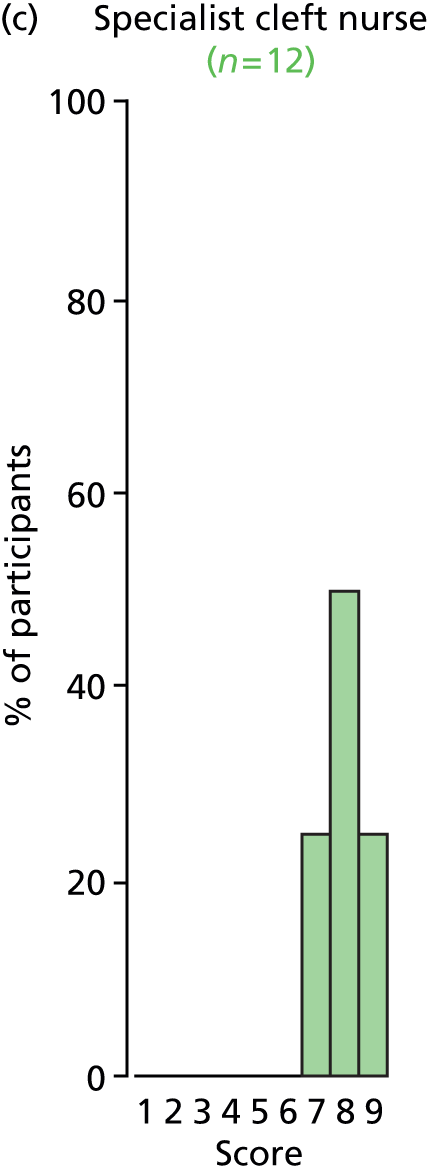
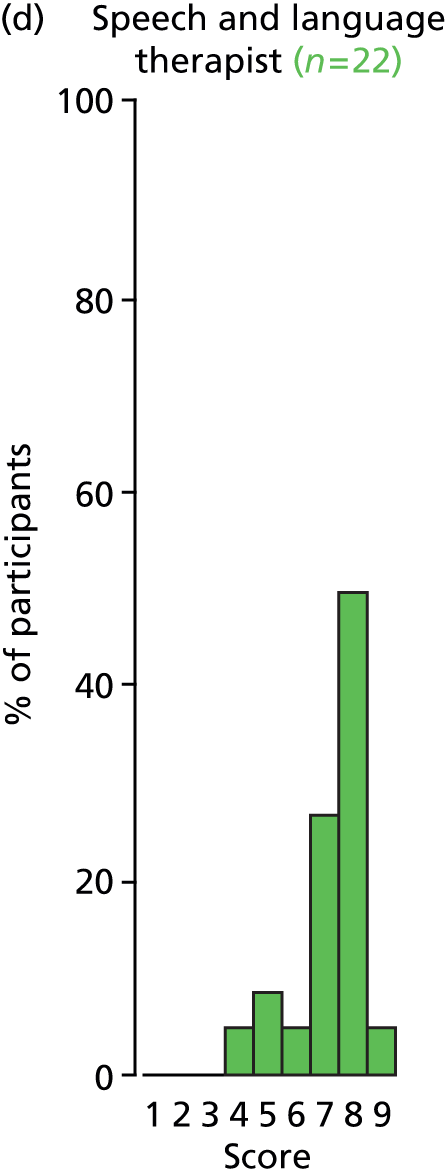




FIGURE 36.
Outcome: child’s perspective of speech.

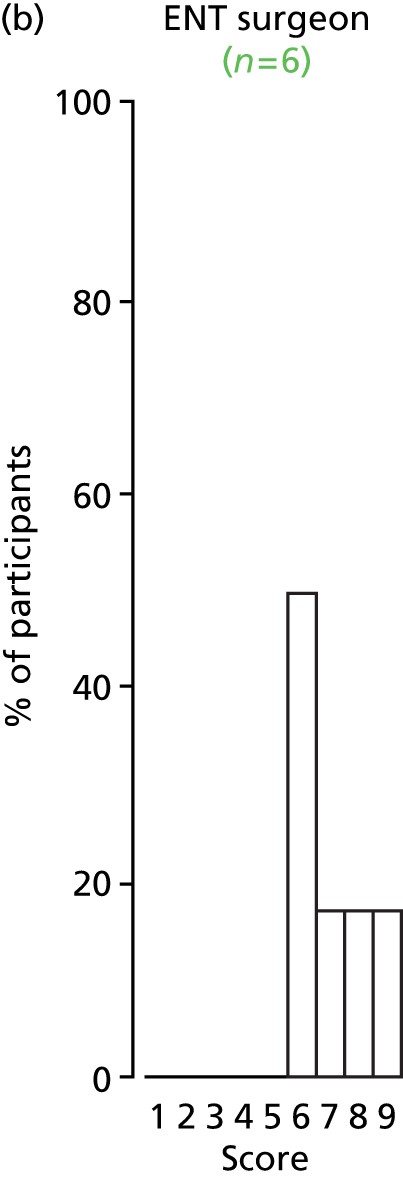
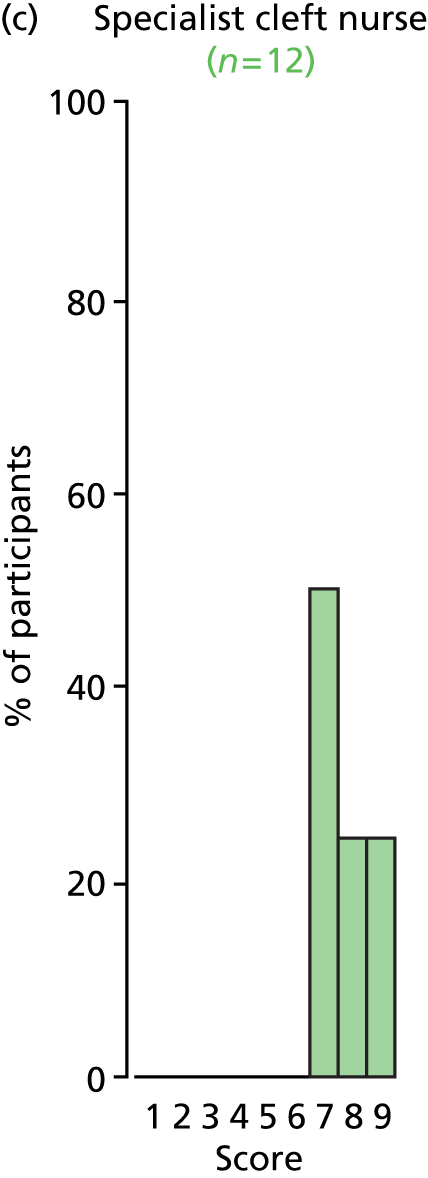

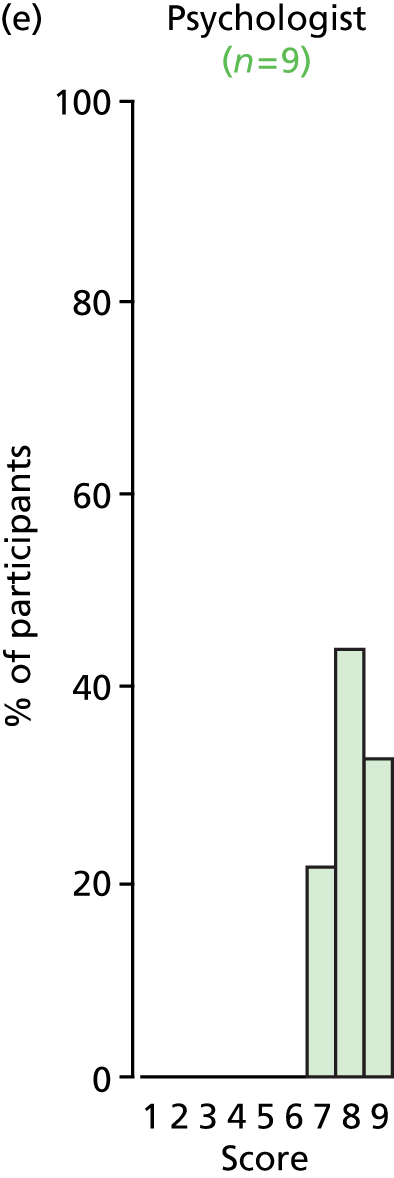
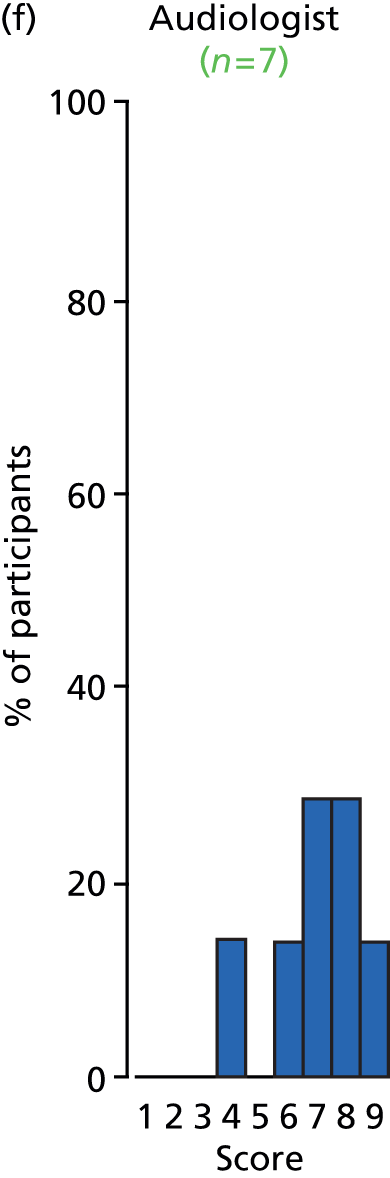
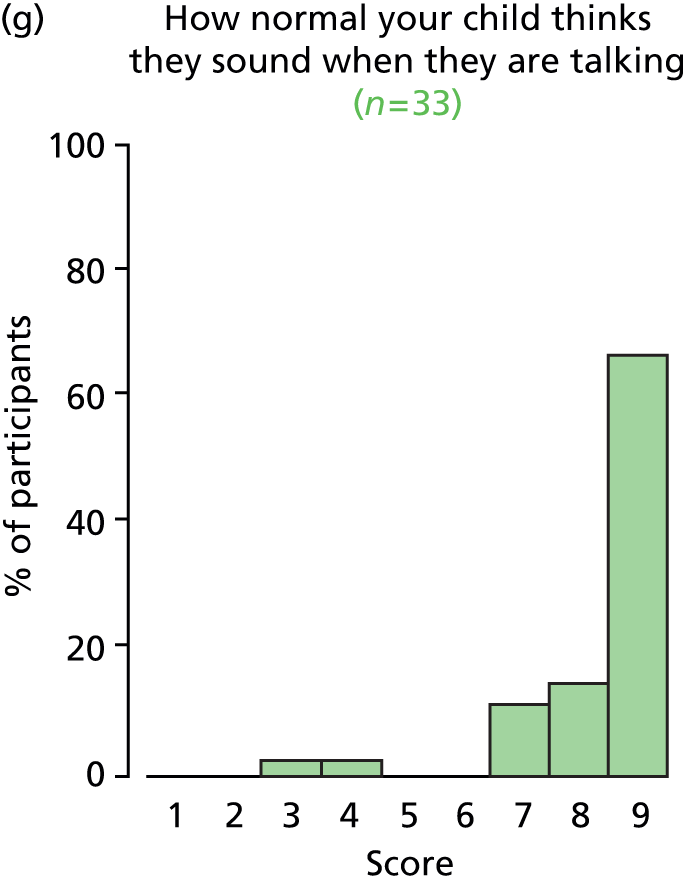

FIGURE 37.
Outcome: persistent tympanic membrane retraction.



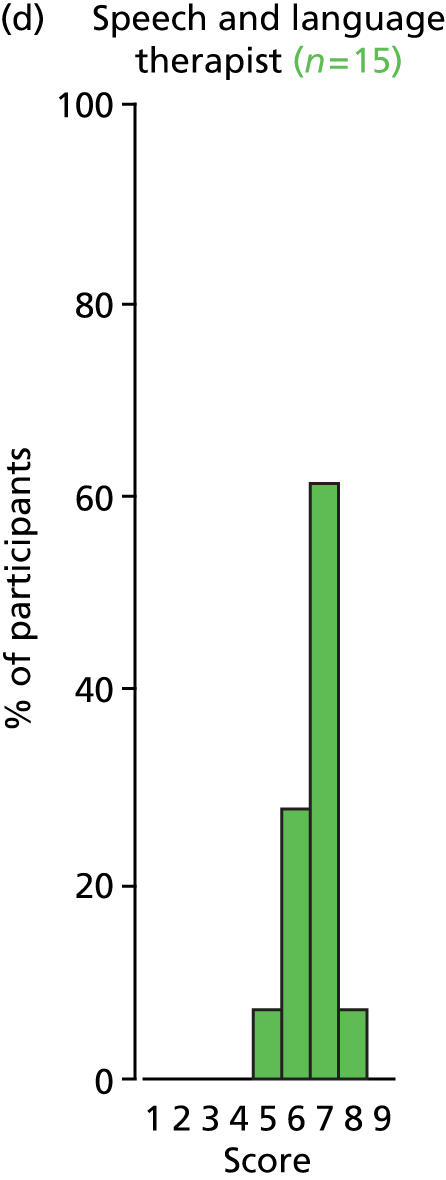
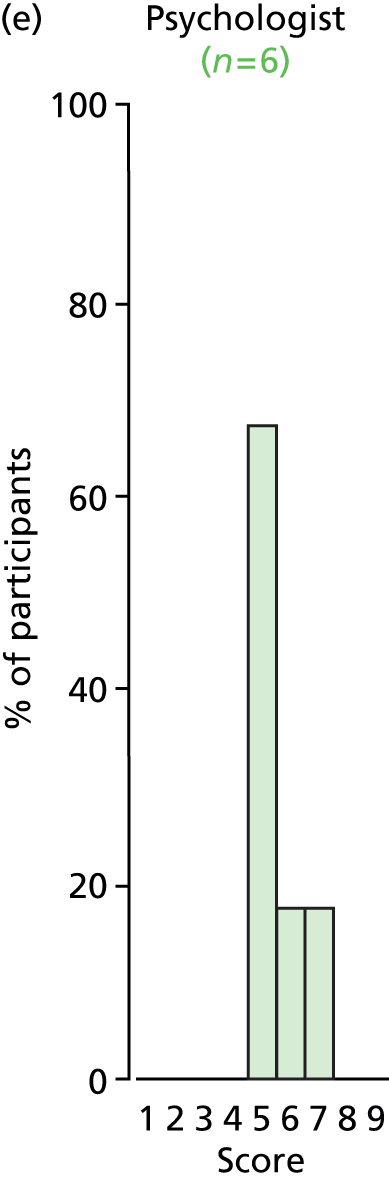
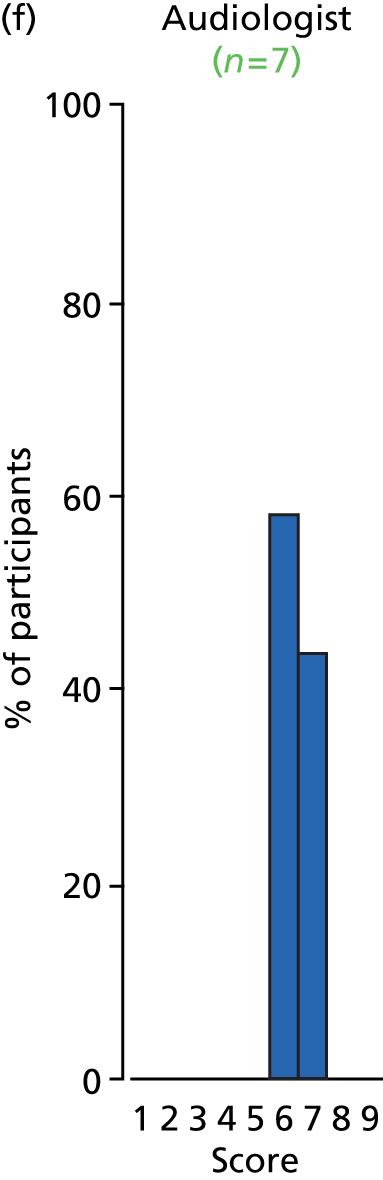
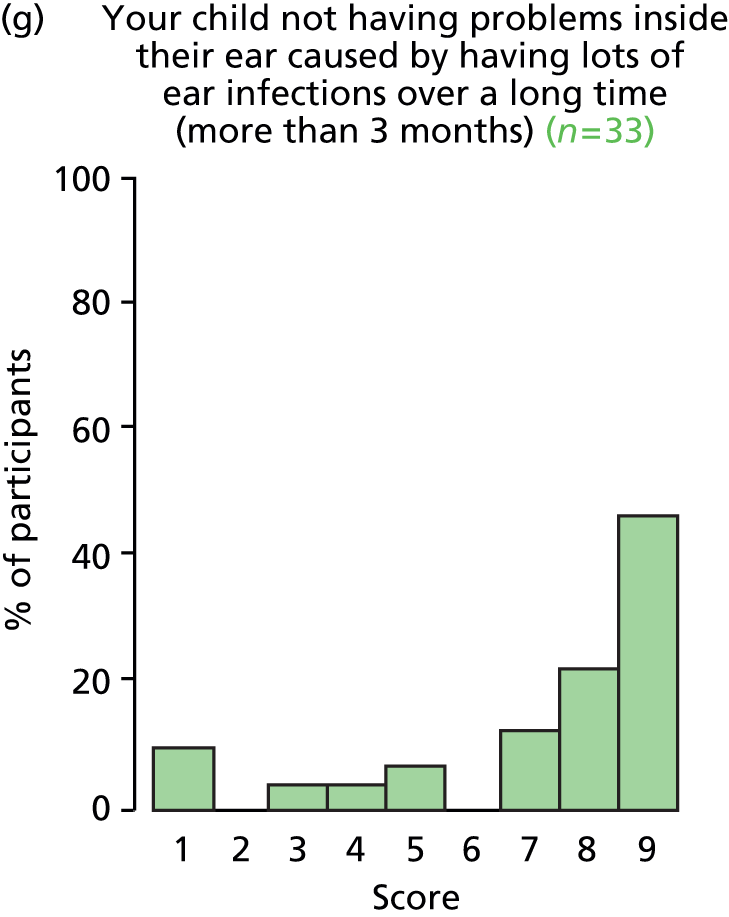
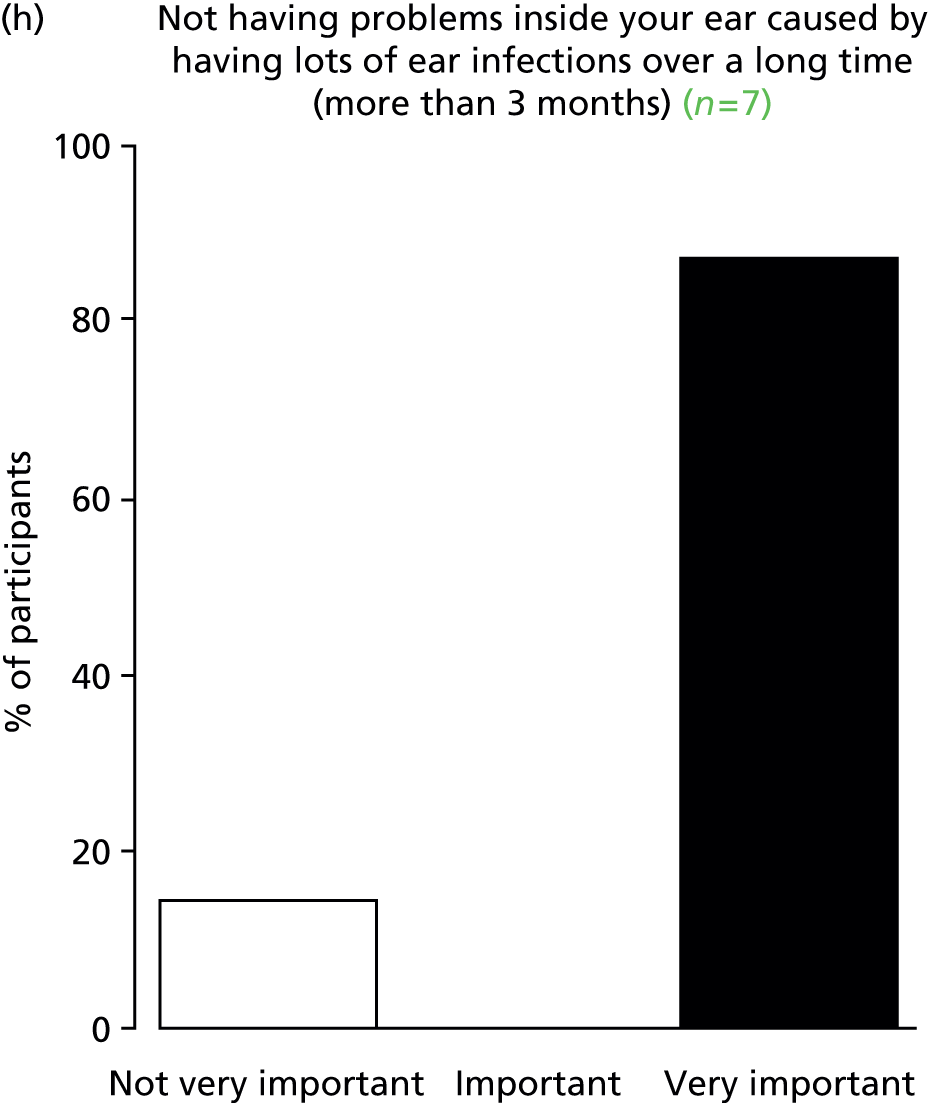
FIGURE 38.
Outcome: cognitive development.
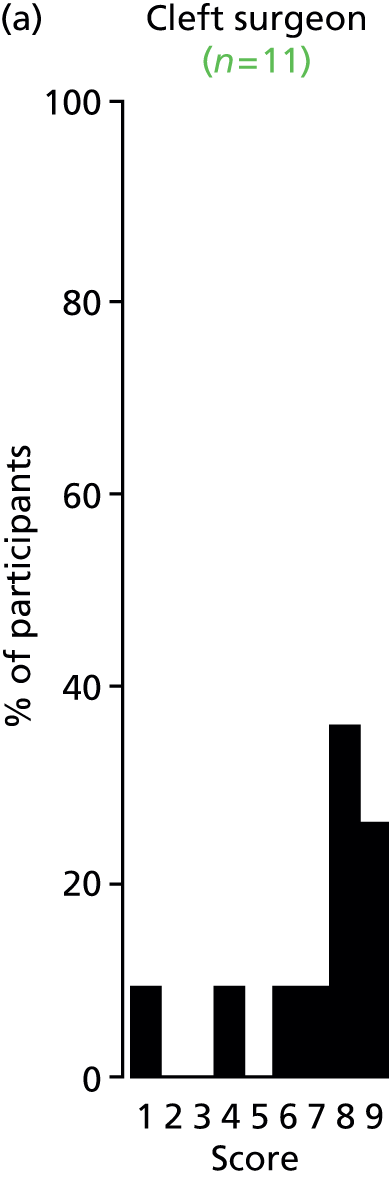
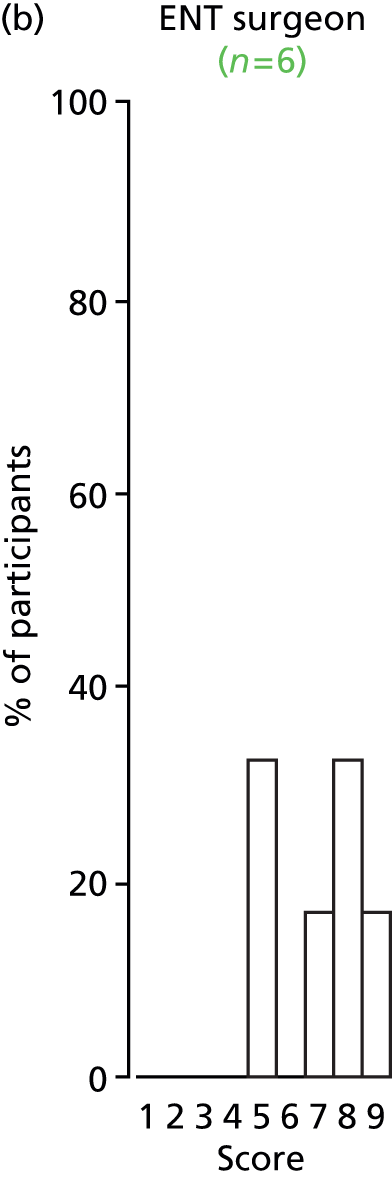
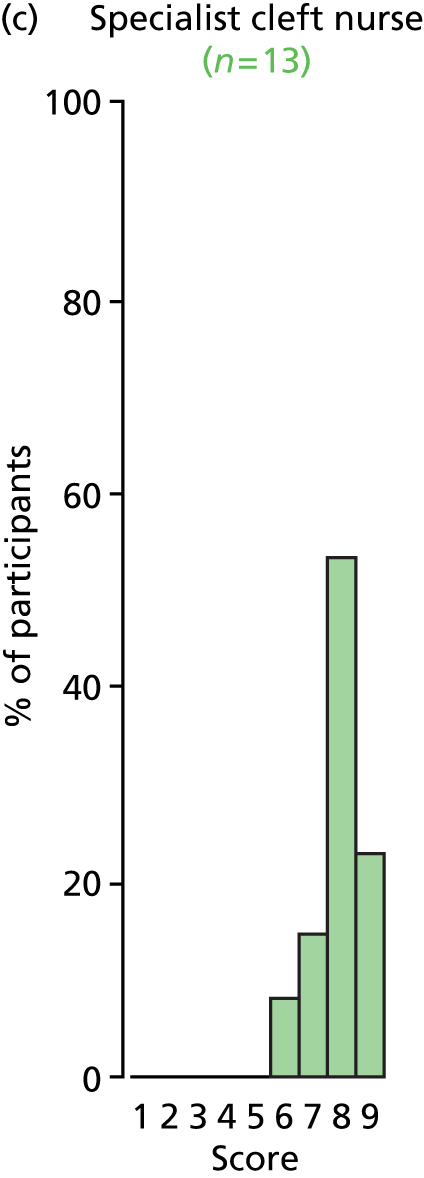


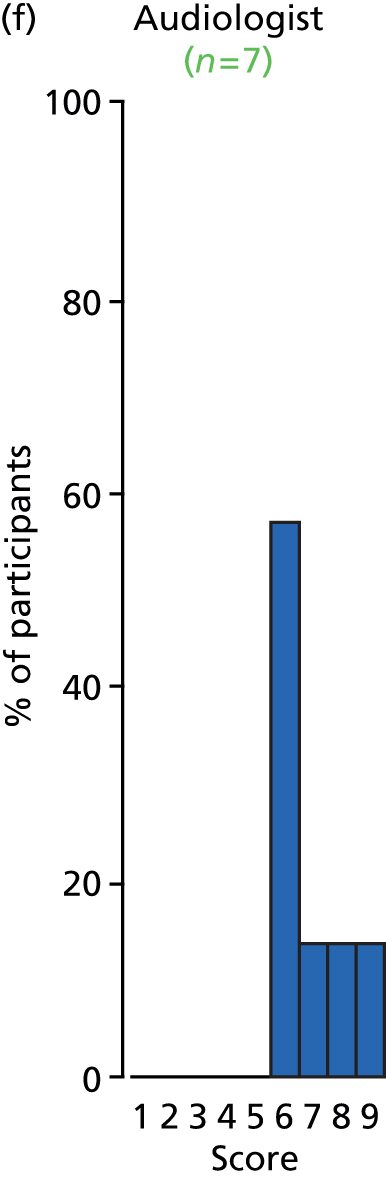

FIGURE 39.
Outcome: developmental progress.
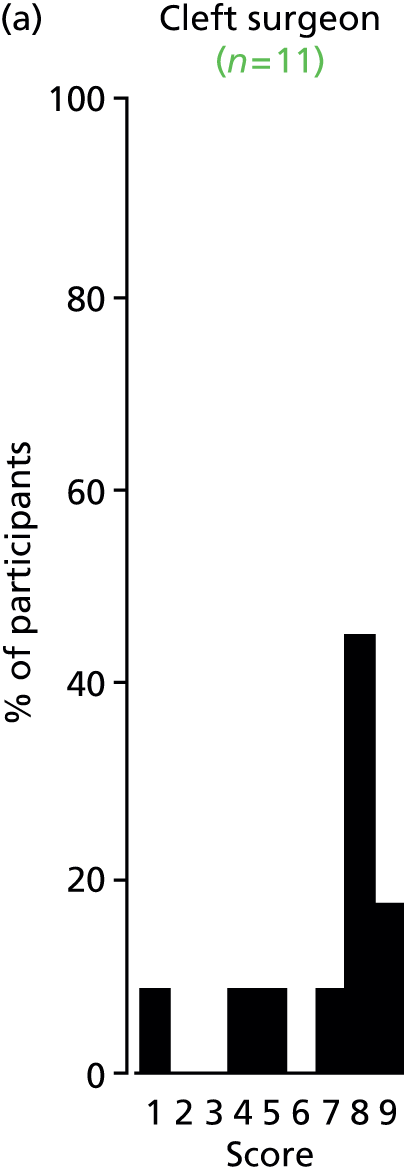


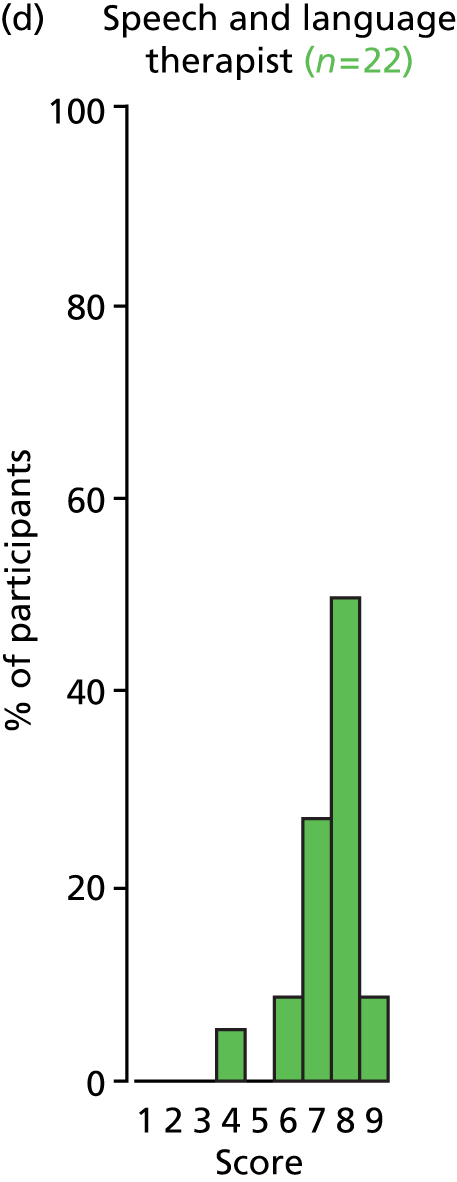
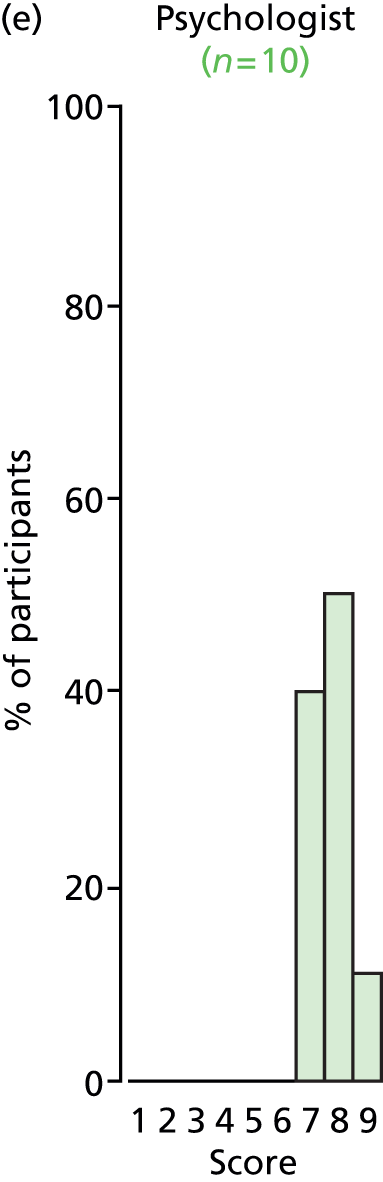
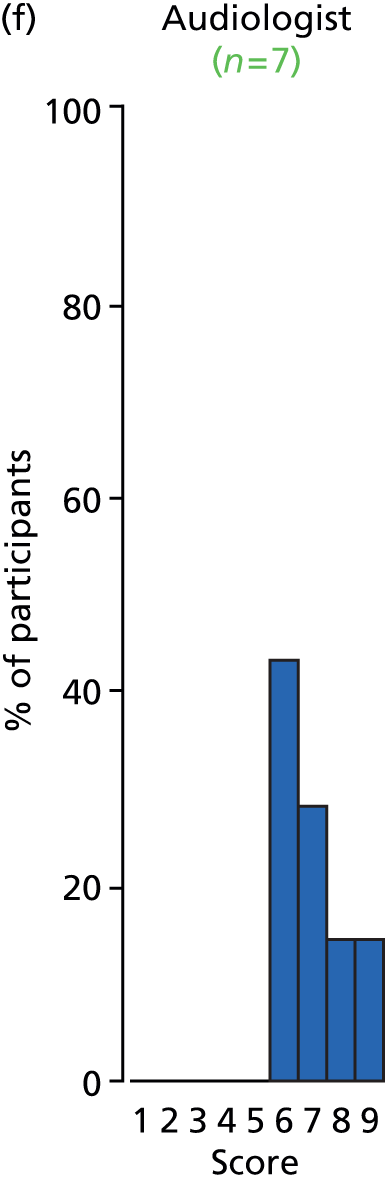
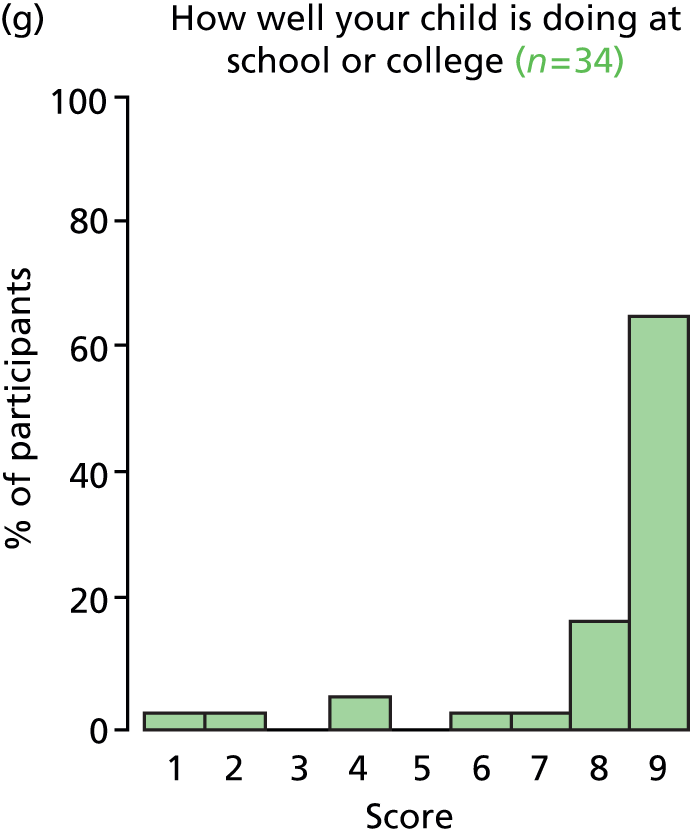

FIGURE 40.
Outcome: consonant production – cleft-related speech patterns.
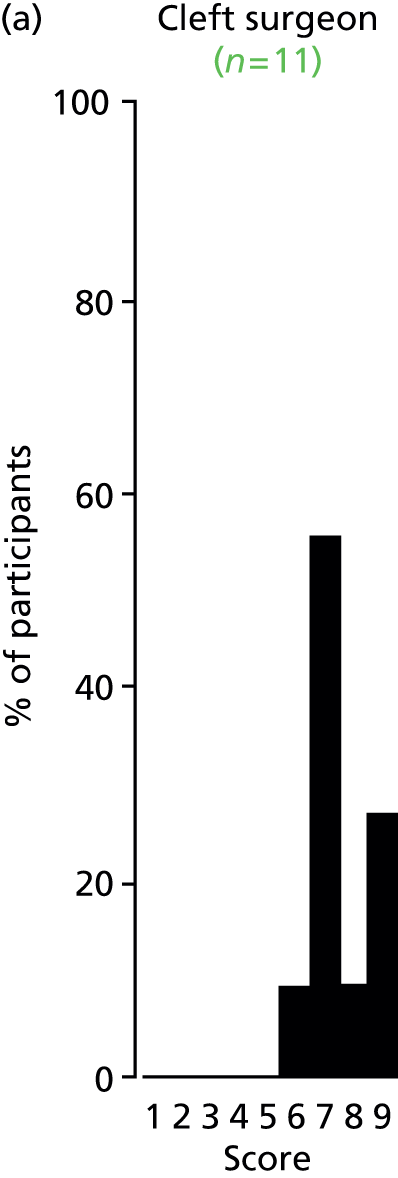
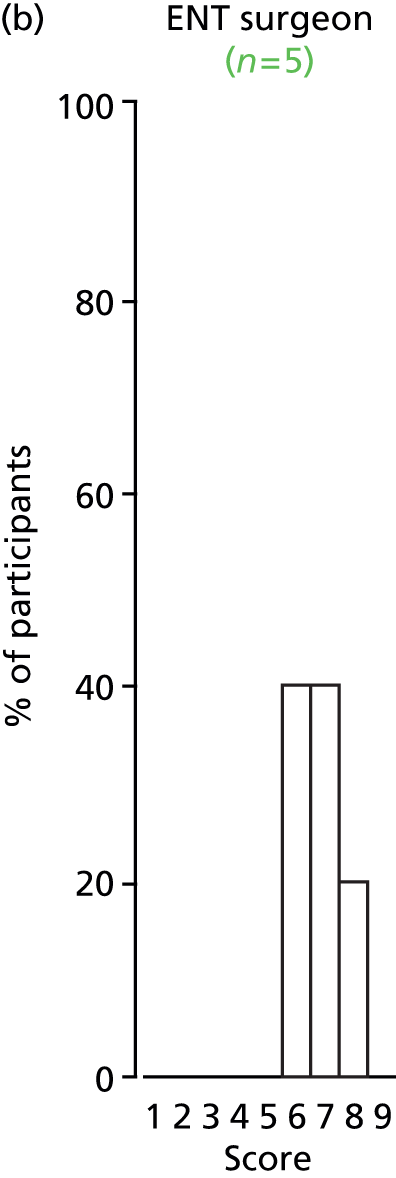
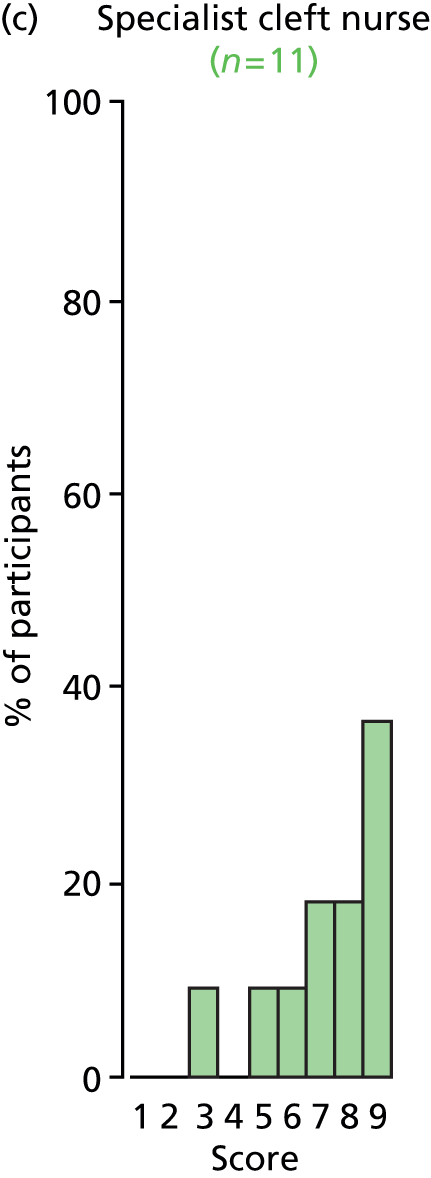


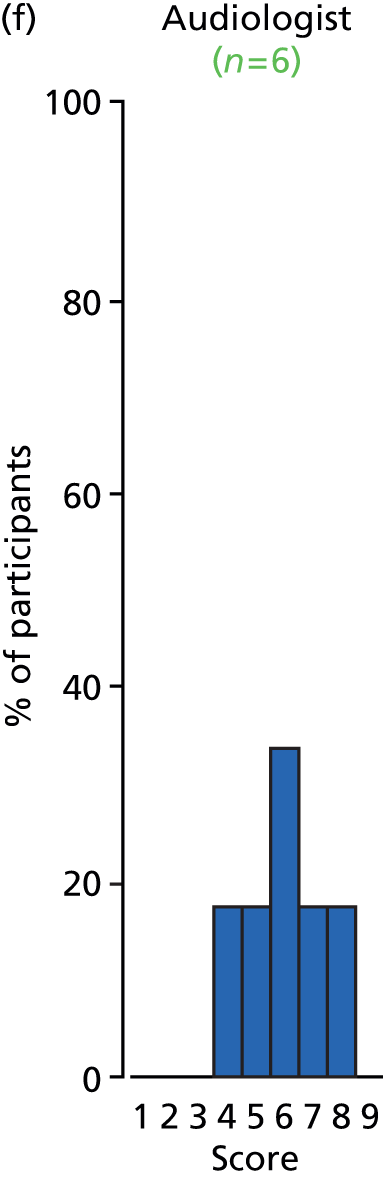

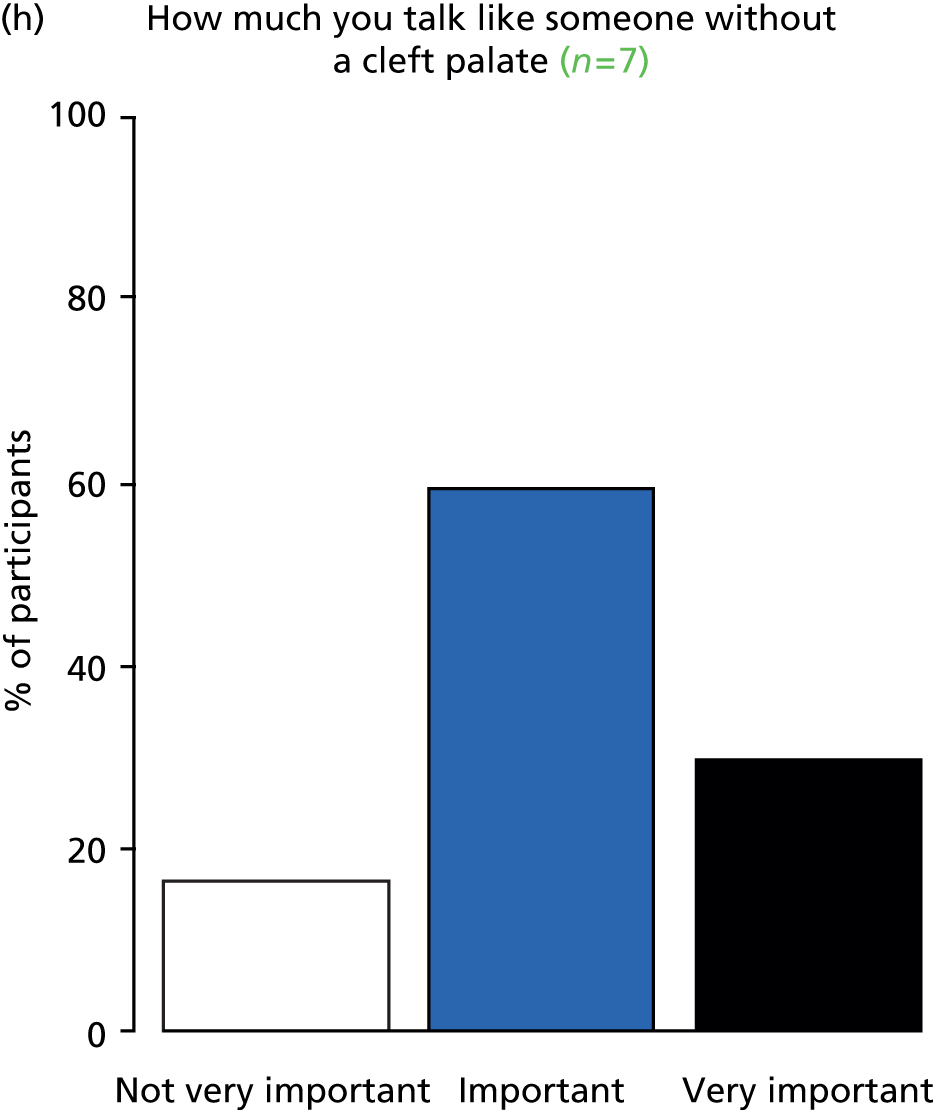
FIGURE 41.
Outcome: side effects of treatment.








FIGURE 42.
Outcome: listening skills.


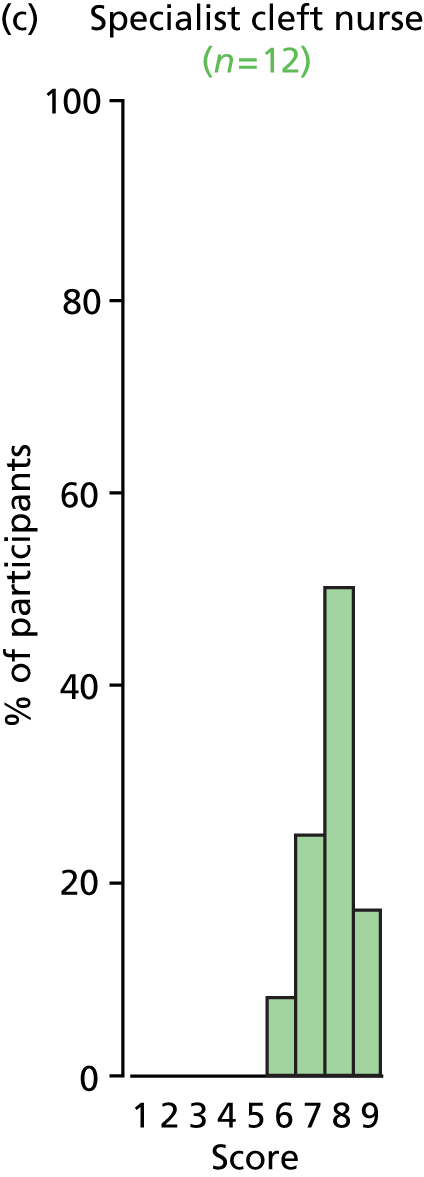



FIGURE 43.
Outcome: psychosocial well-being.


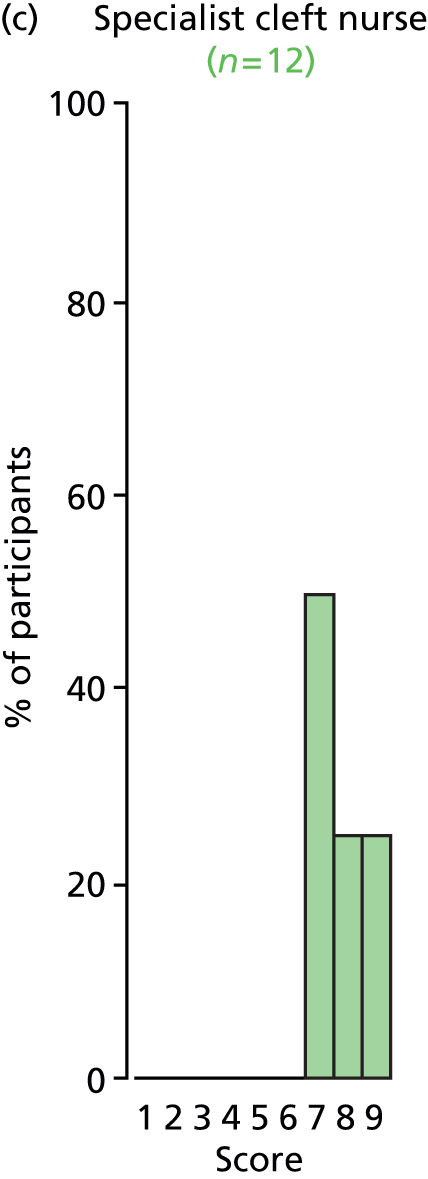
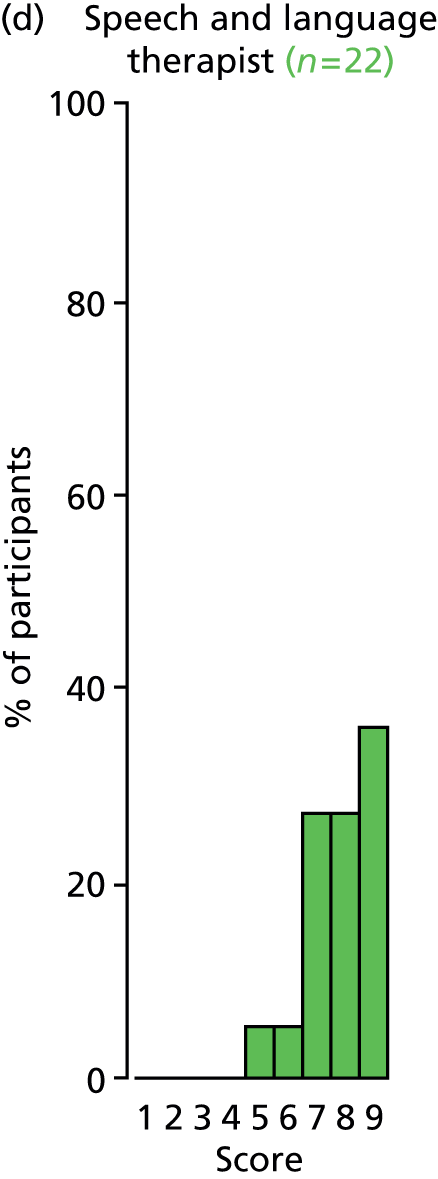


FIGURE 44.
Outcome: persistent tympanic membrane perforation.
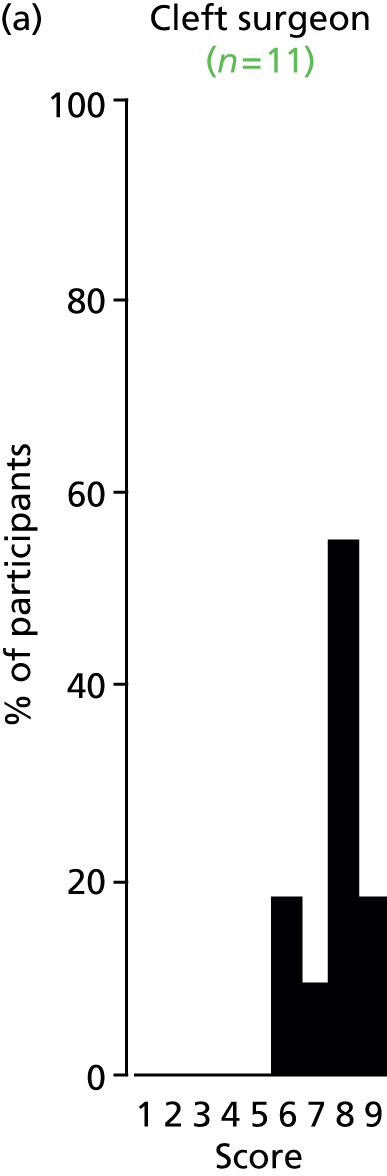


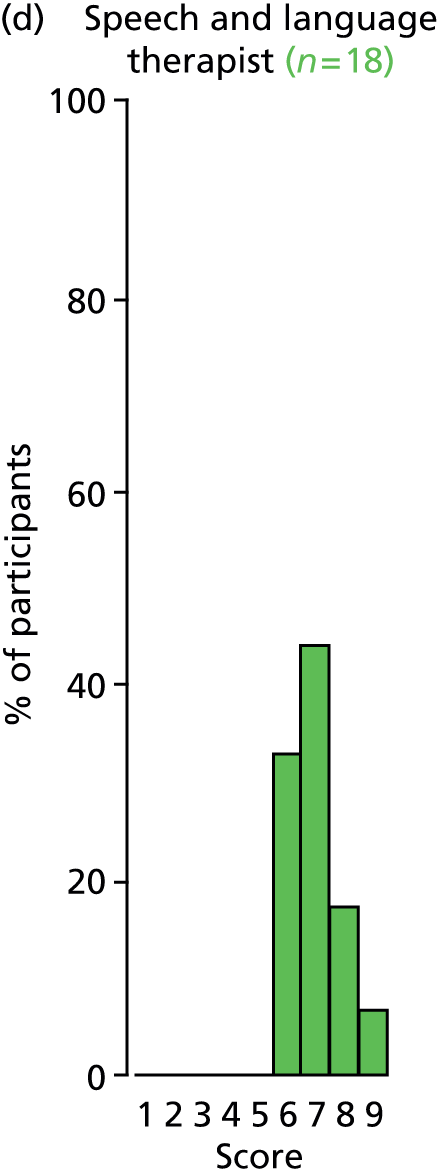
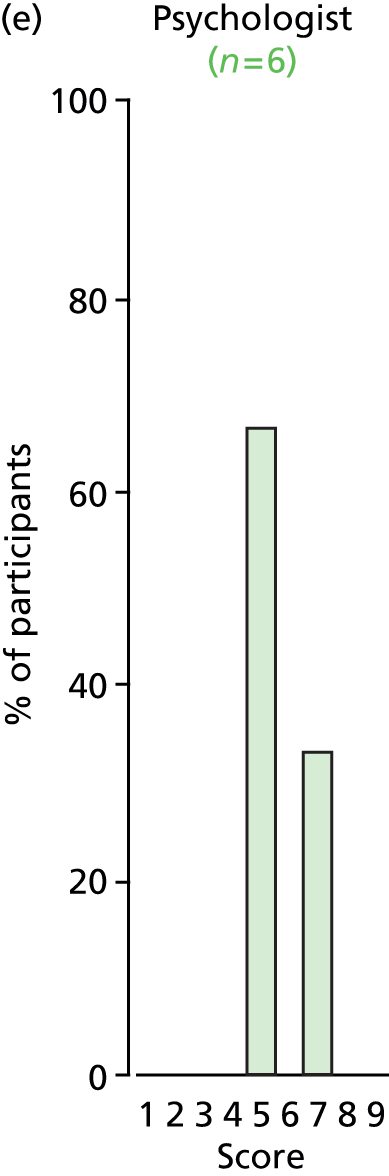
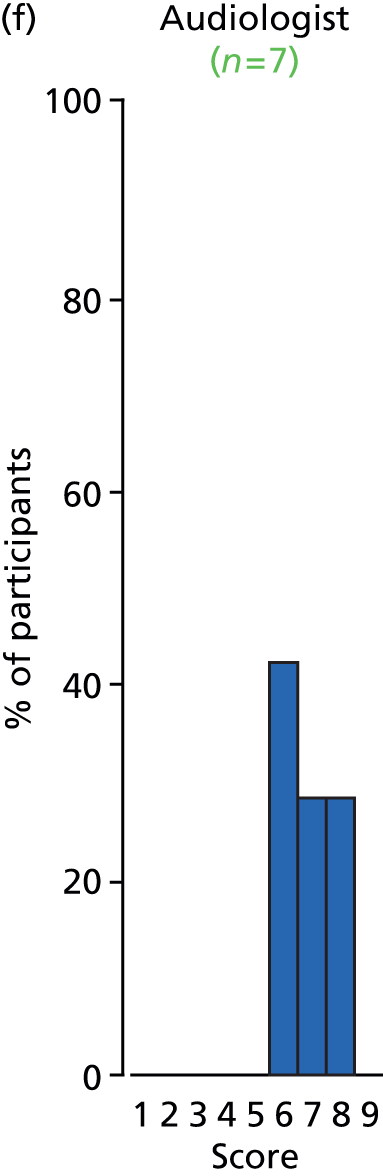
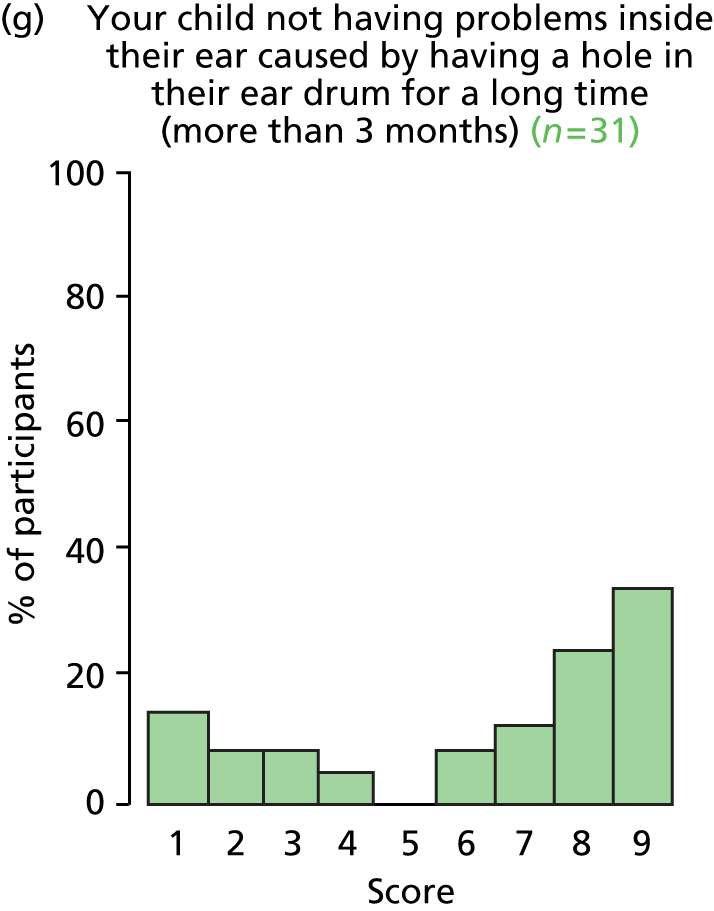

FIGURE 45.
Outcome: tympanosclerosis.
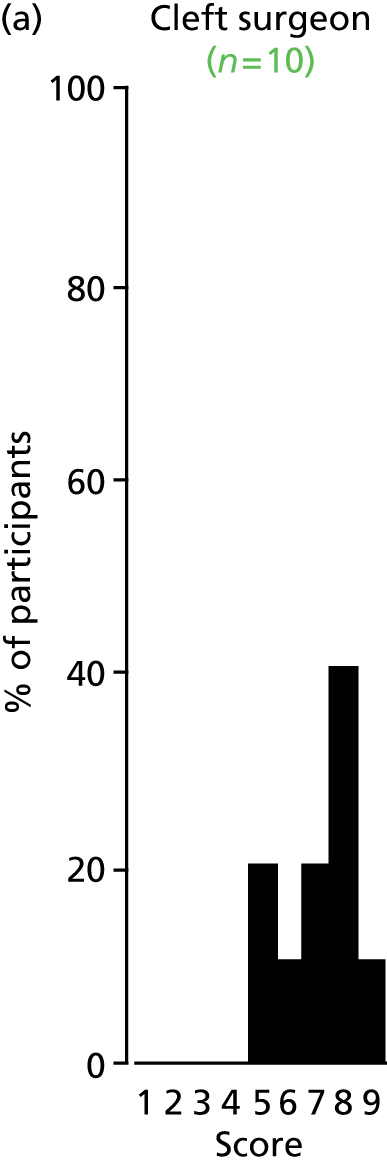
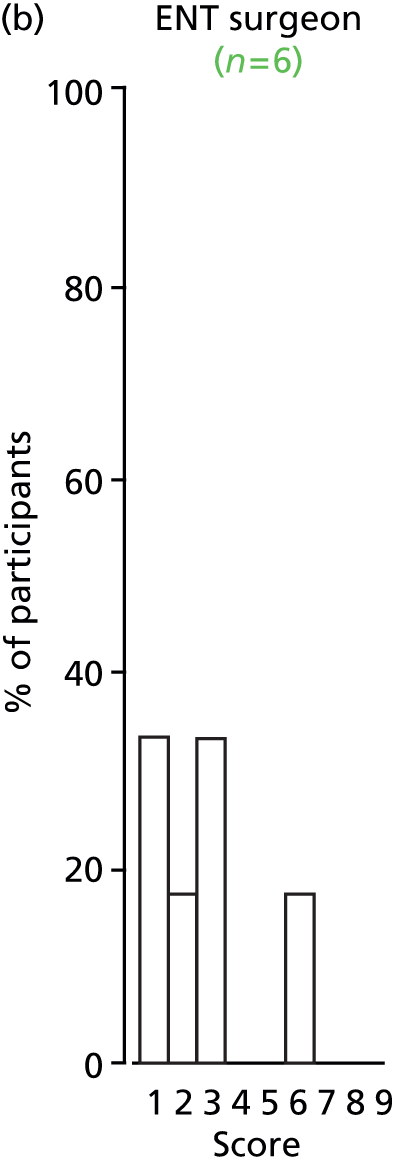
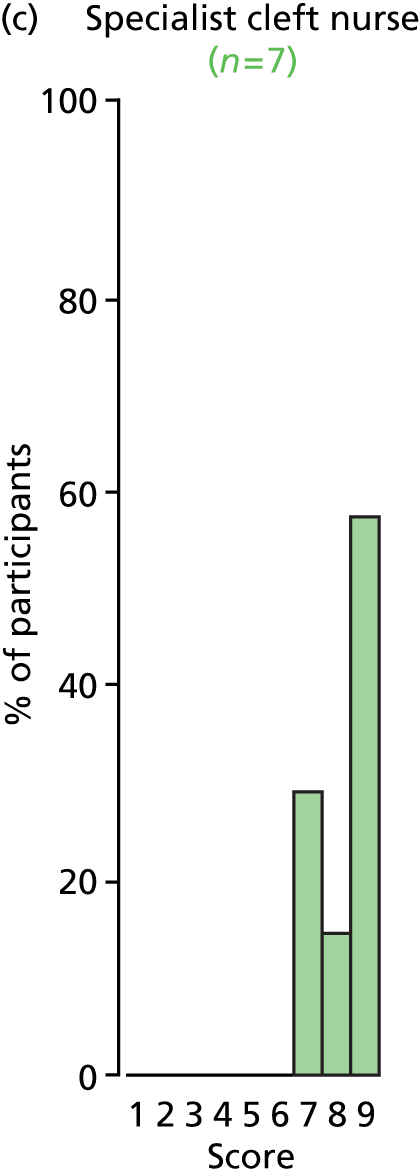

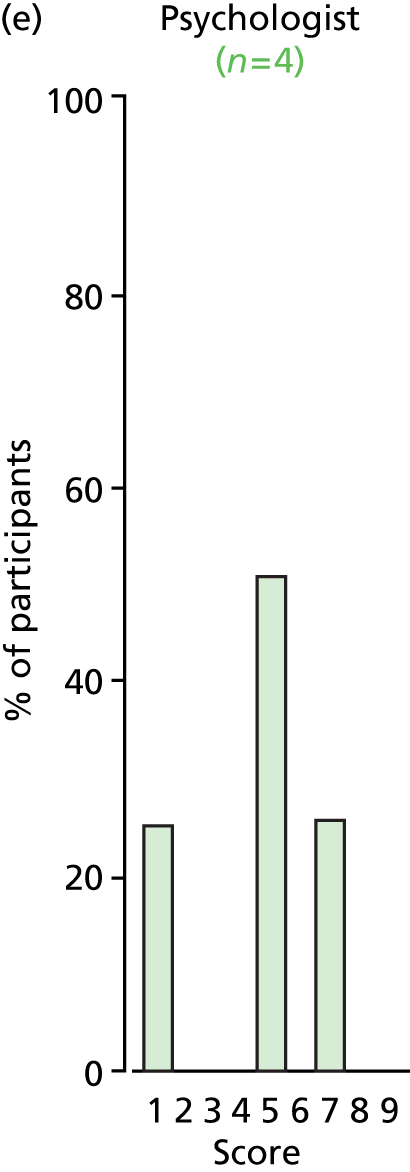
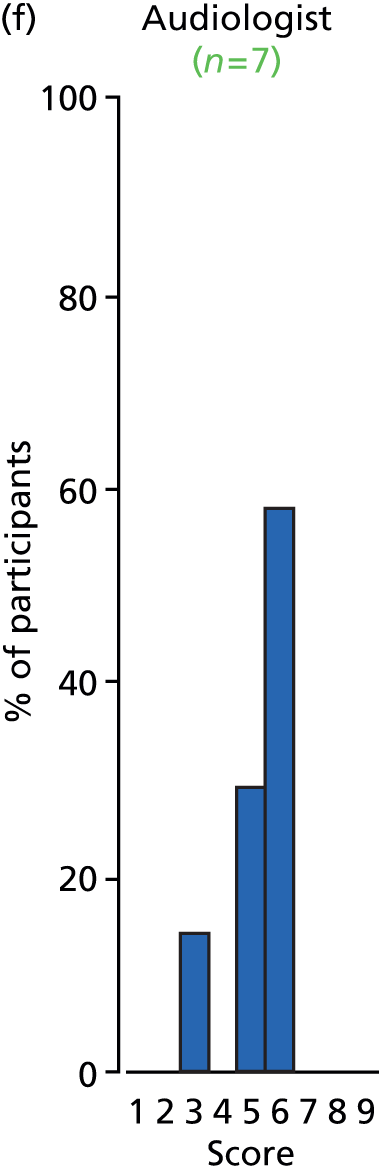

FIGURE 46.
Outcome: otalgia.
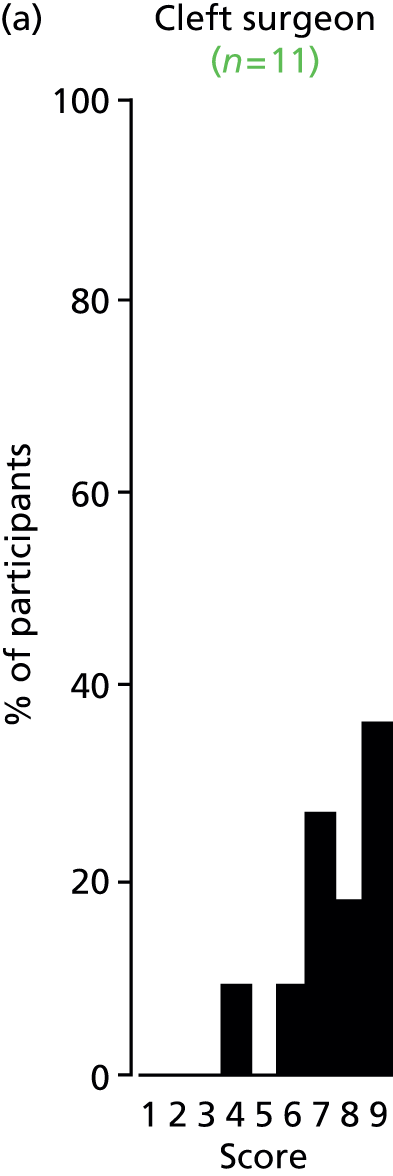
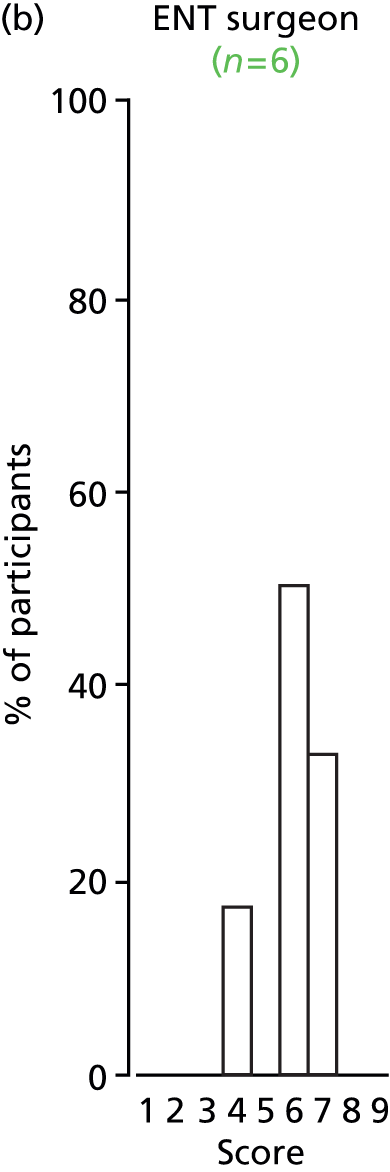
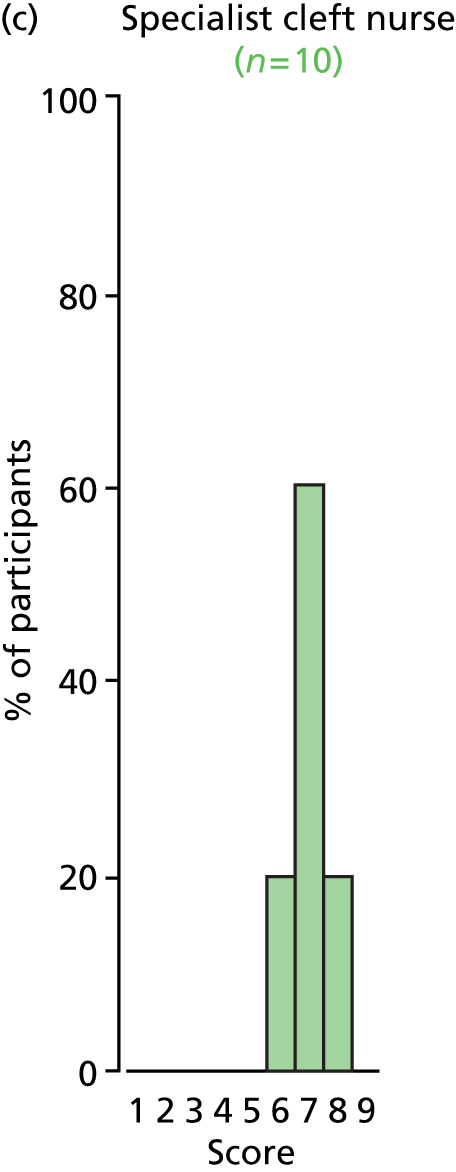





FIGURE 47.
Outcome: otorrhoea.

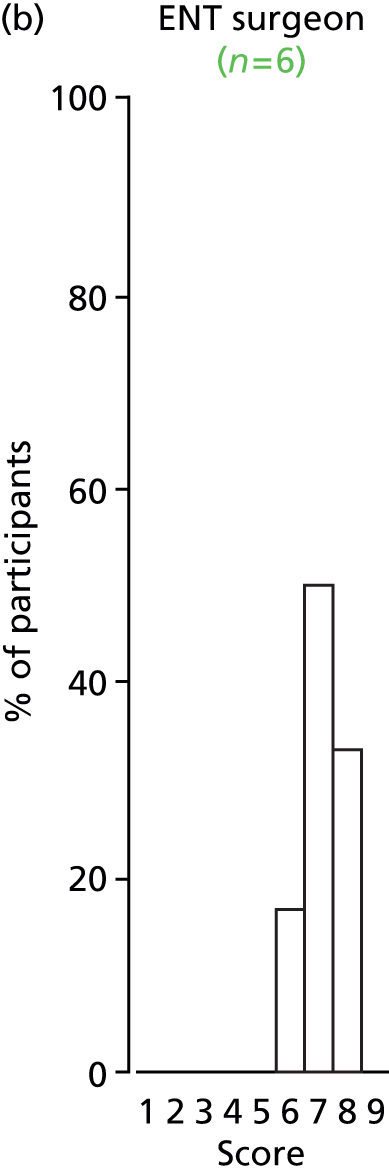

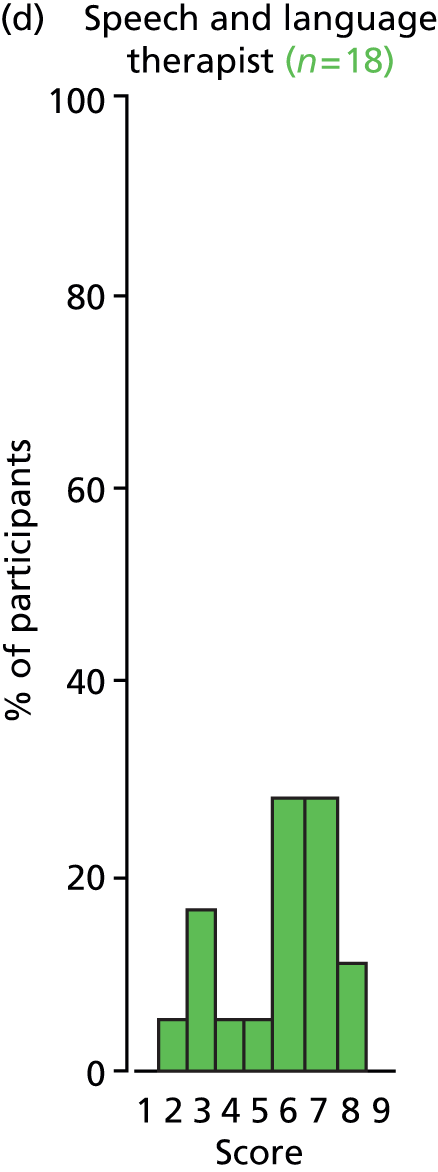



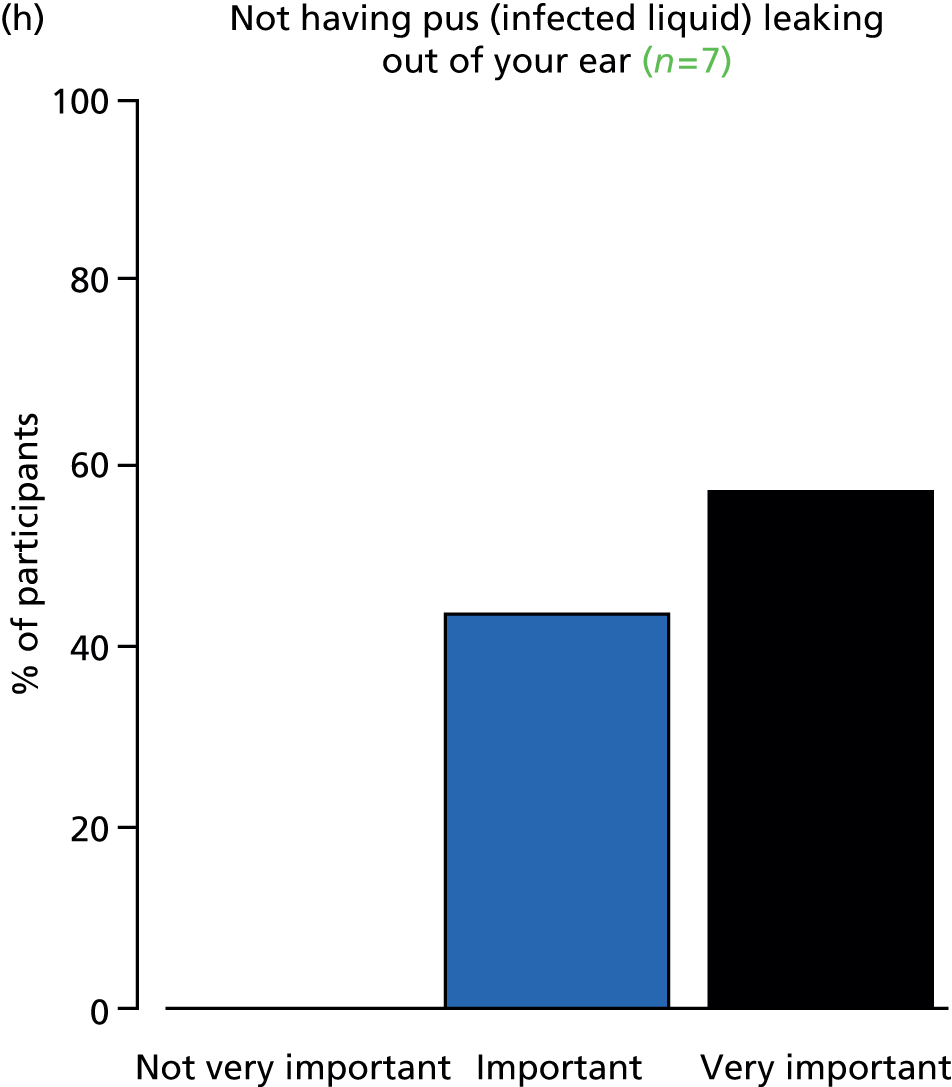
FIGURE 48.
Outcome: eustachian tube function.
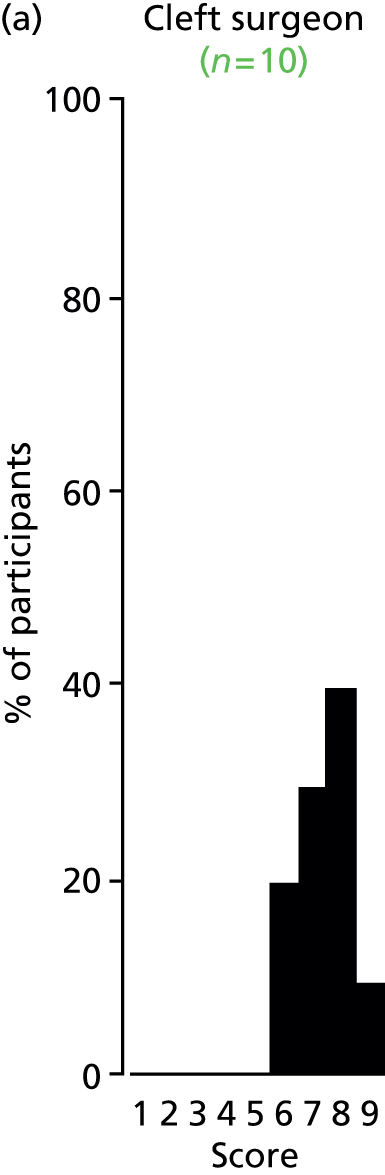
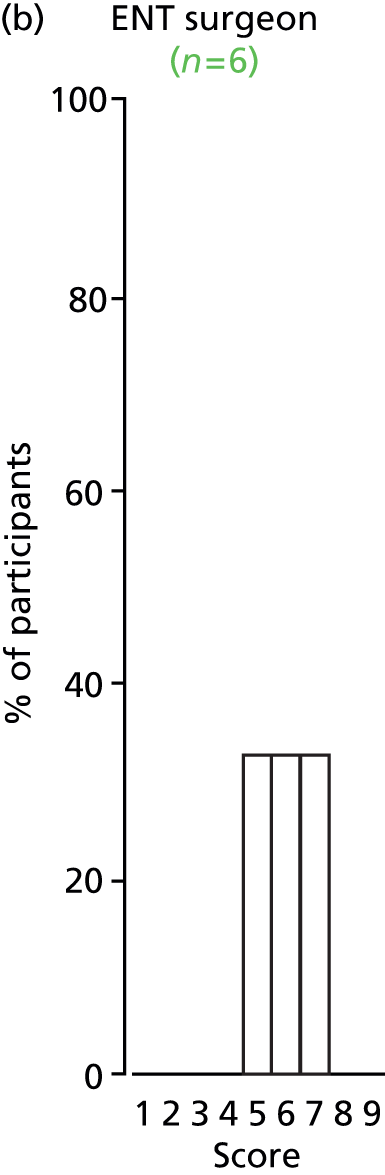

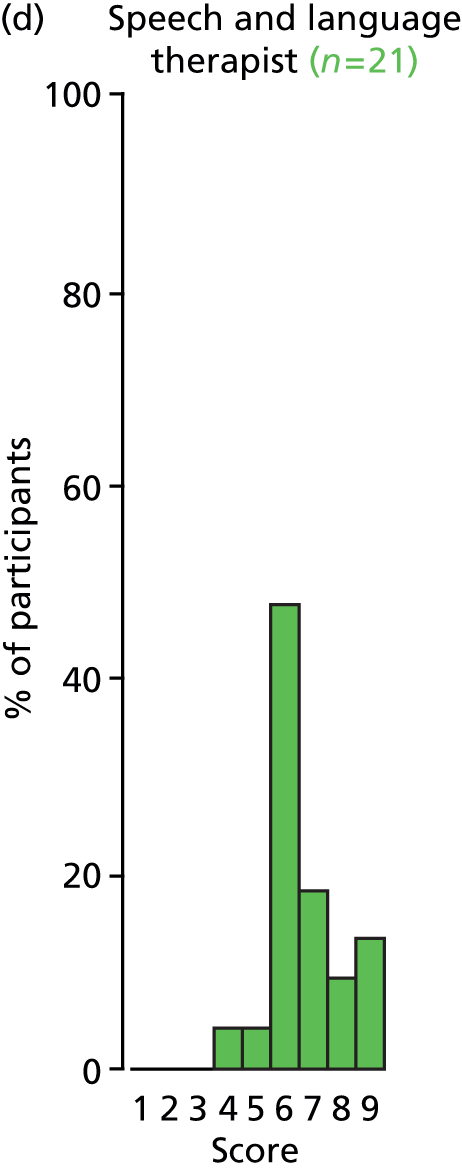
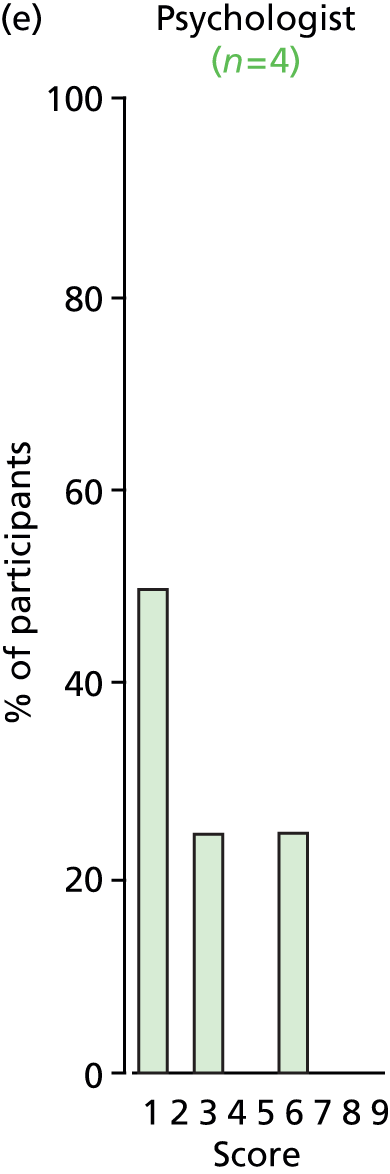
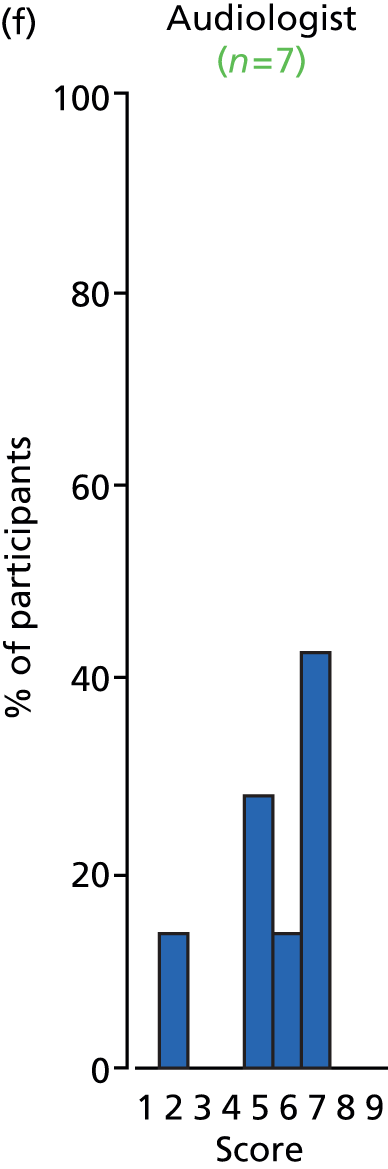


FIGURE 49.
Outcome: expressive language skills.

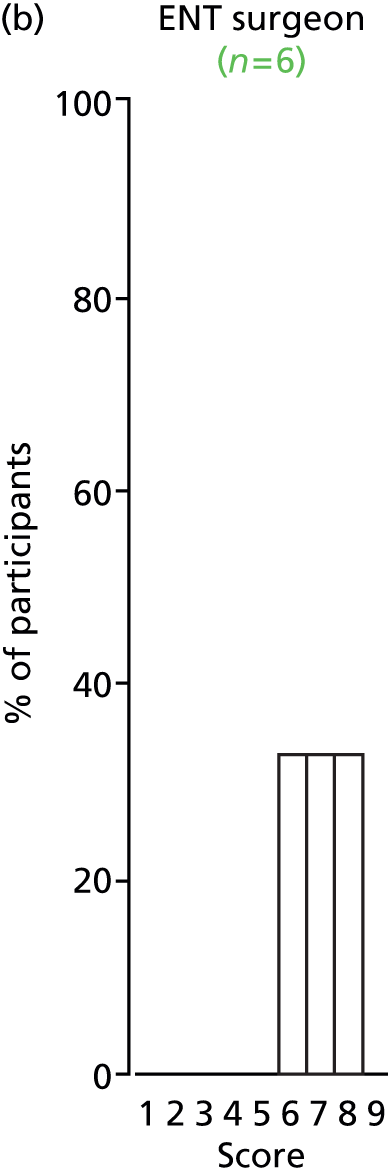






FIGURE 50.
Outcome: speech signs of velopharyngeal insufficiency.
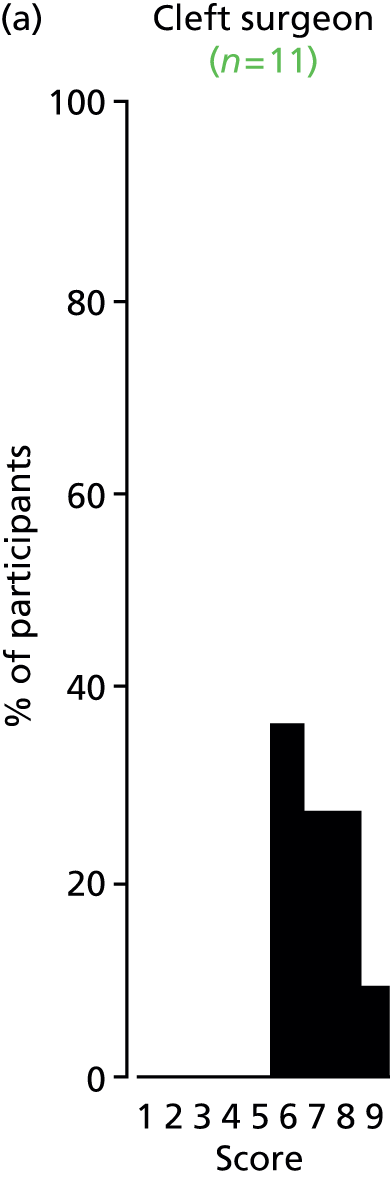
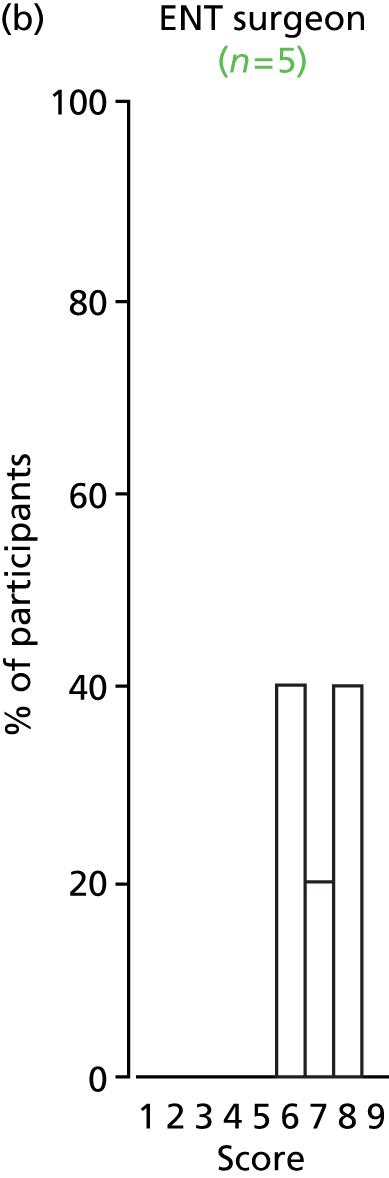

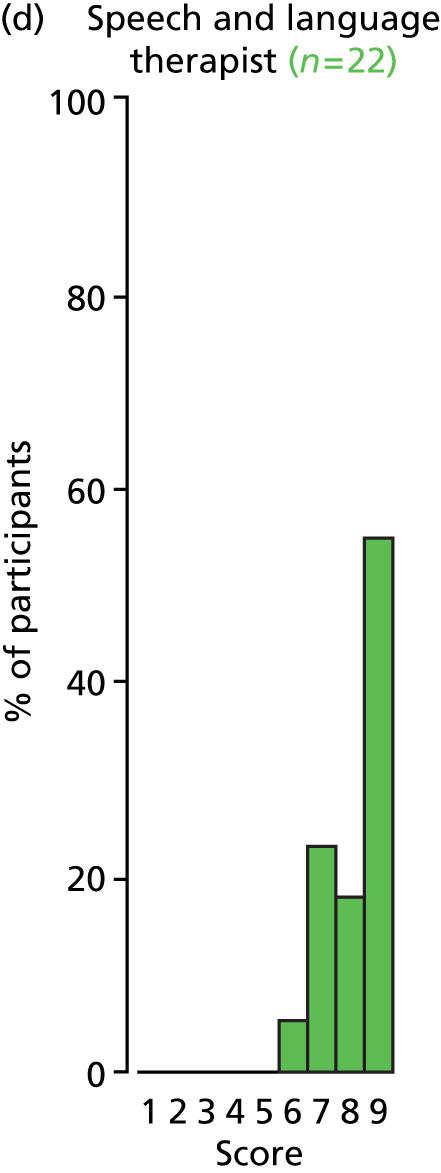


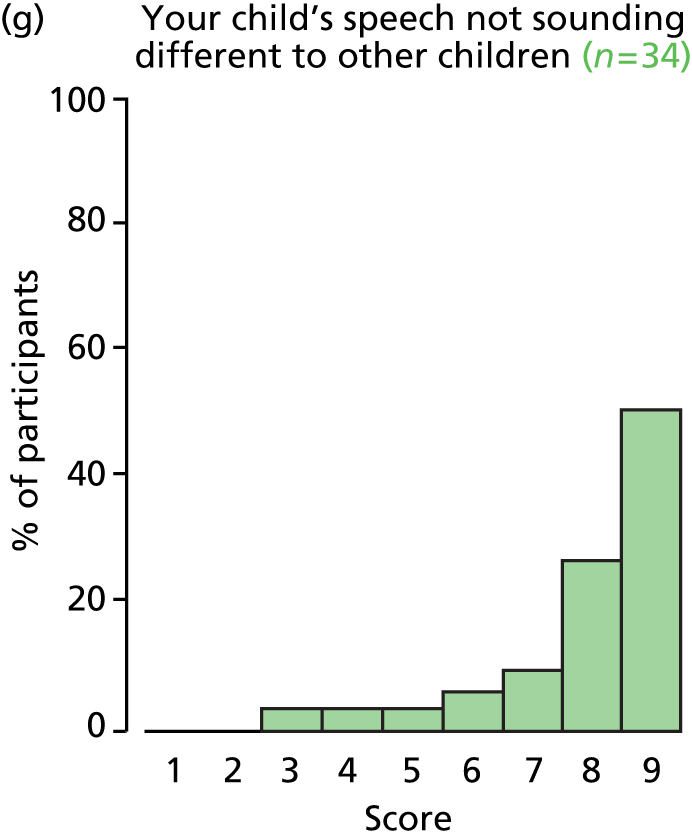

FIGURE 51.
Outcome: early extrusion or blockage of VTs.
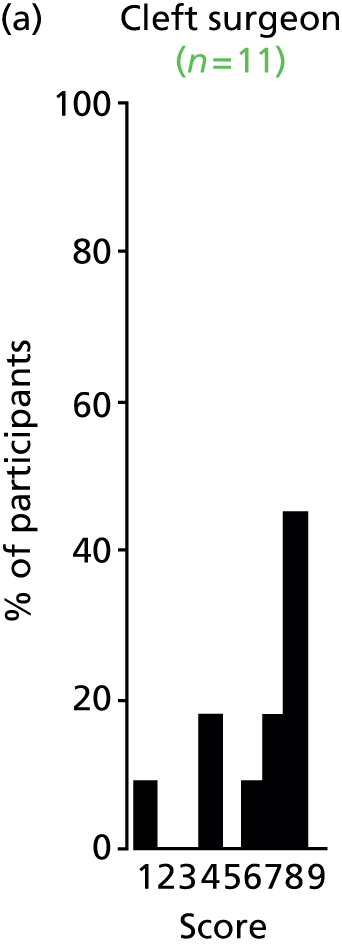
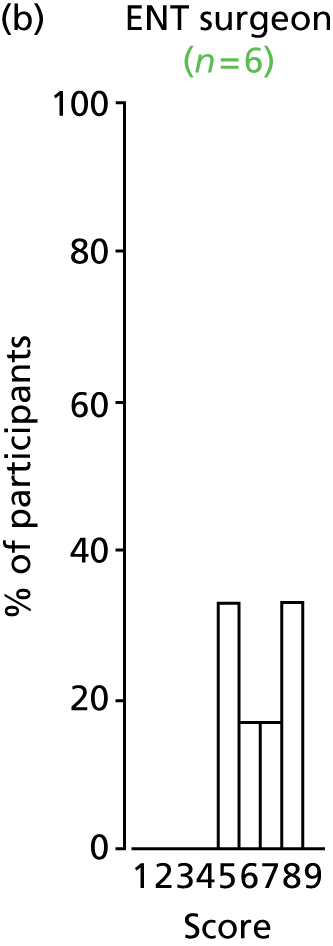






FIGURE 52.
Outcome: child stress.
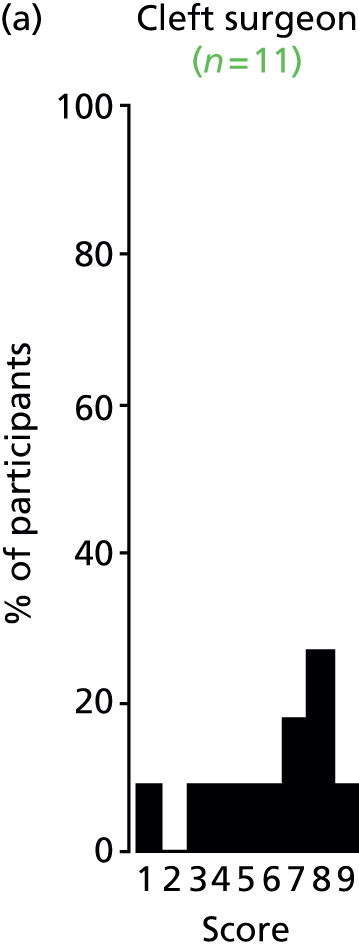
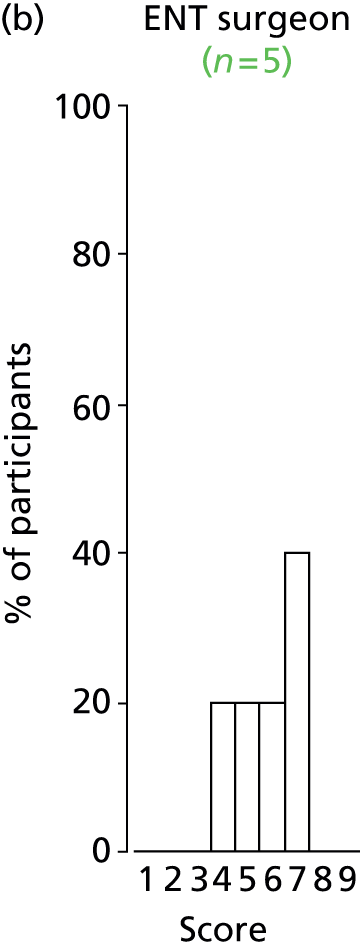
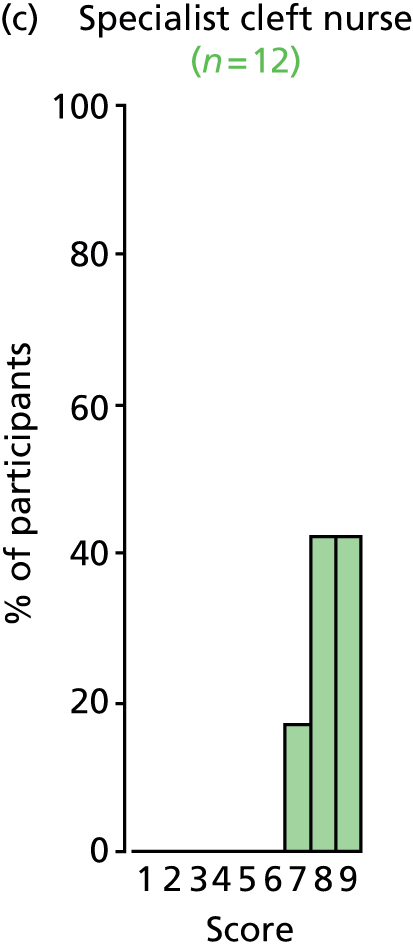
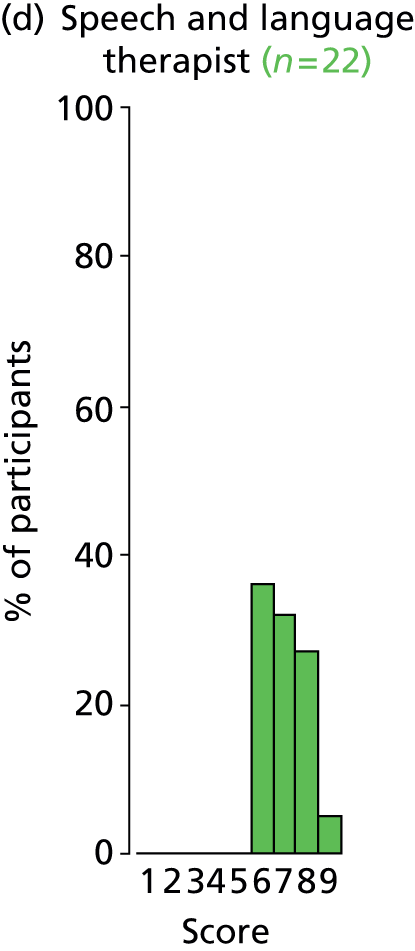




FIGURE 53.
Outcome: psychological well-being.

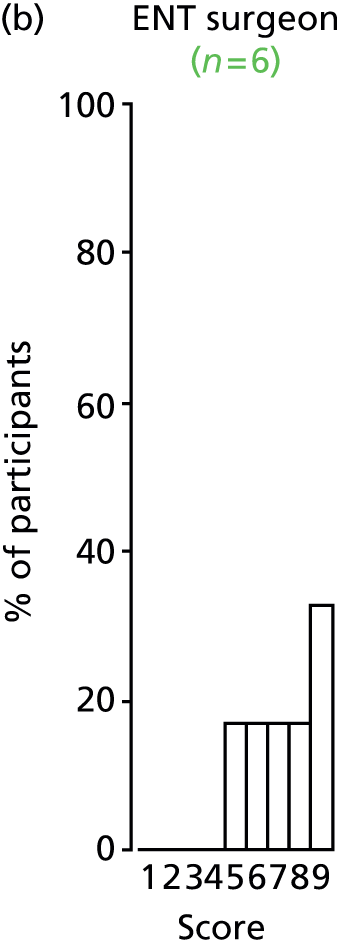
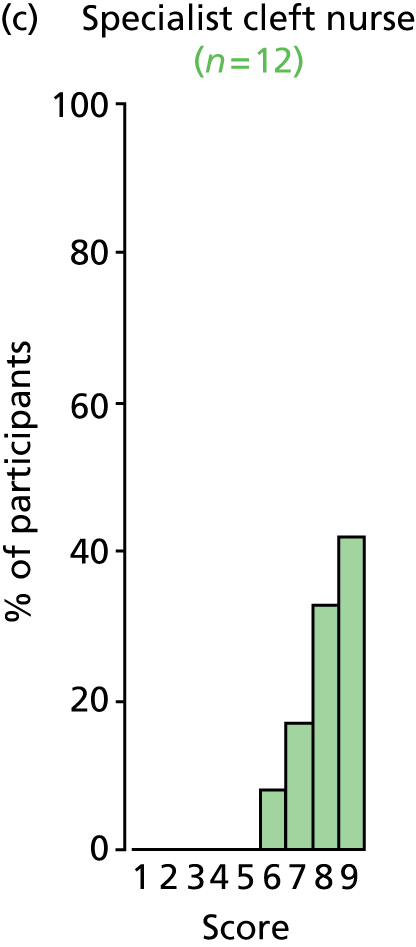
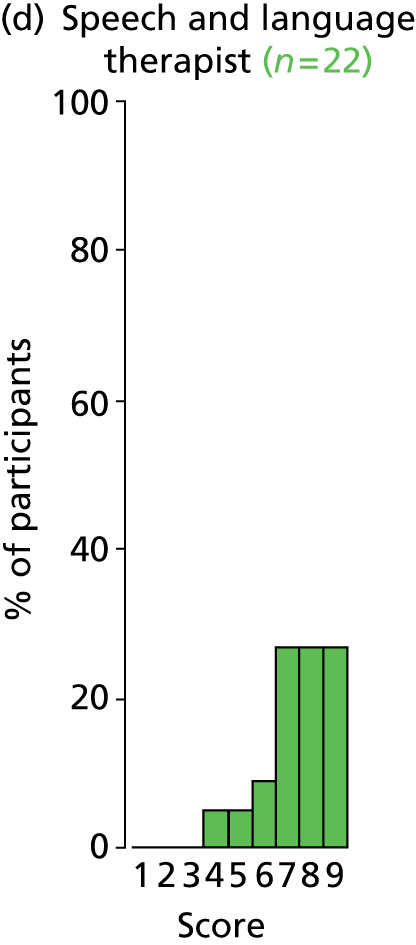

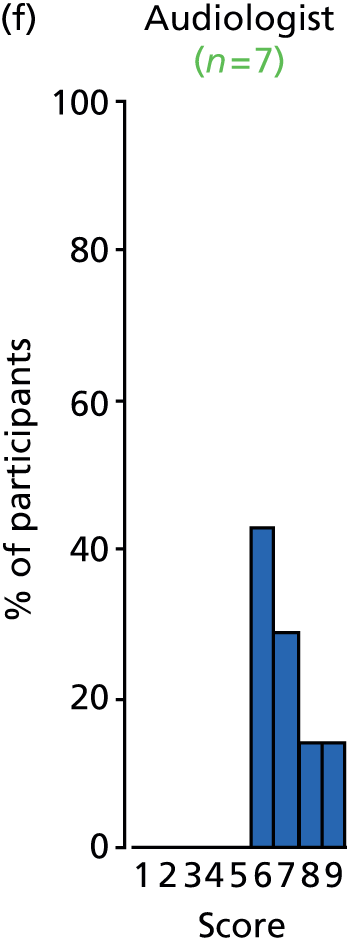
FIGURE 54.
Outcome: internalising behaviour.
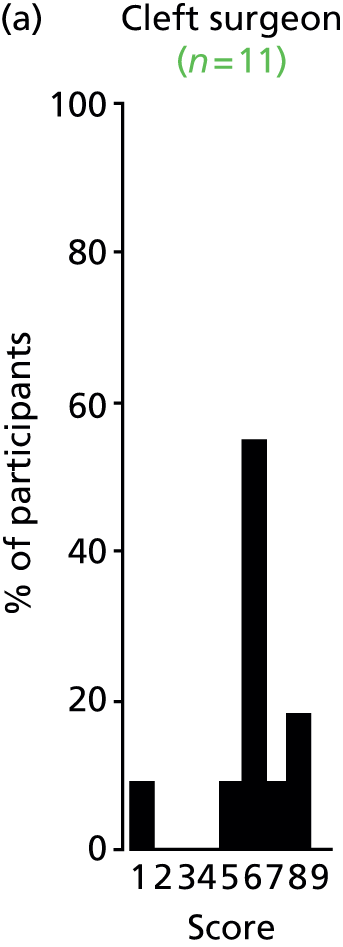
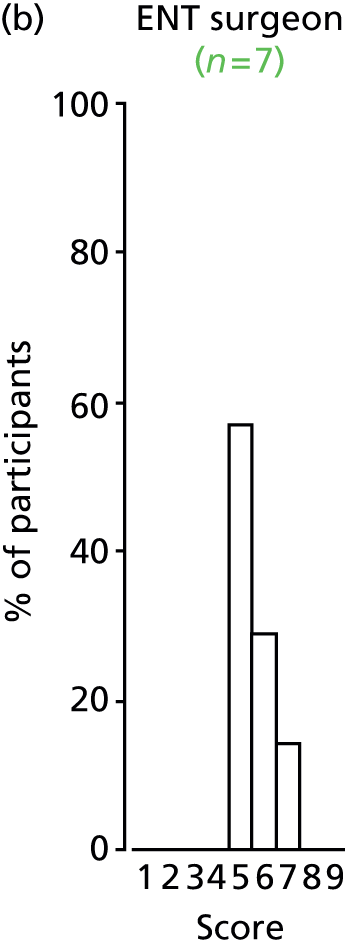
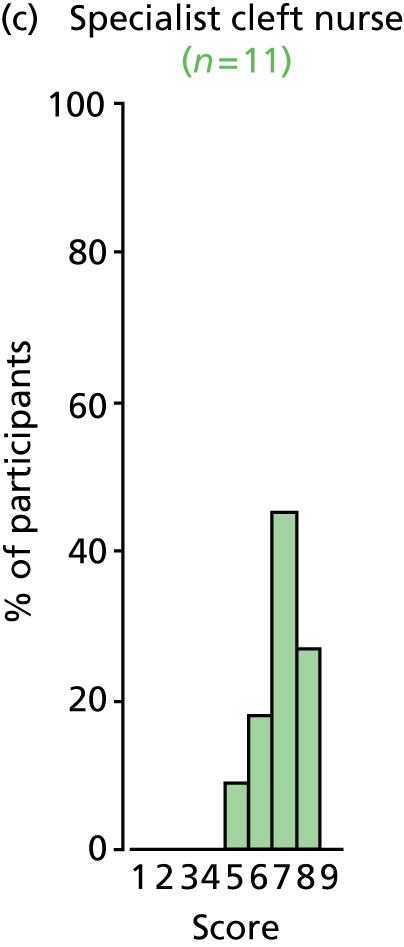
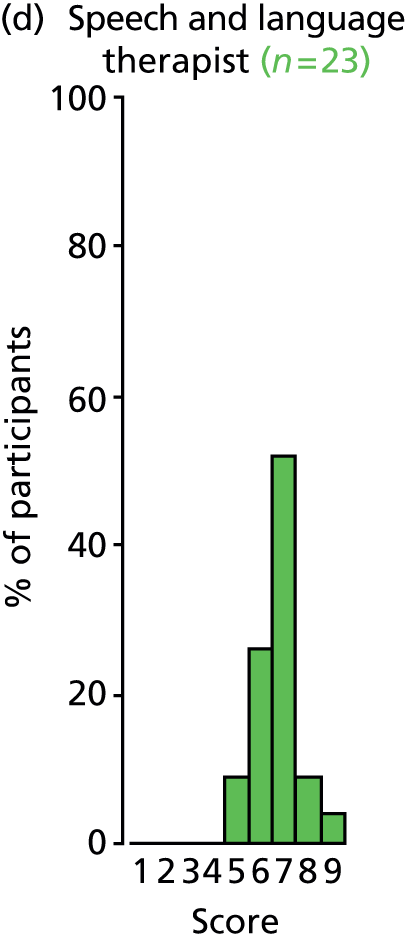
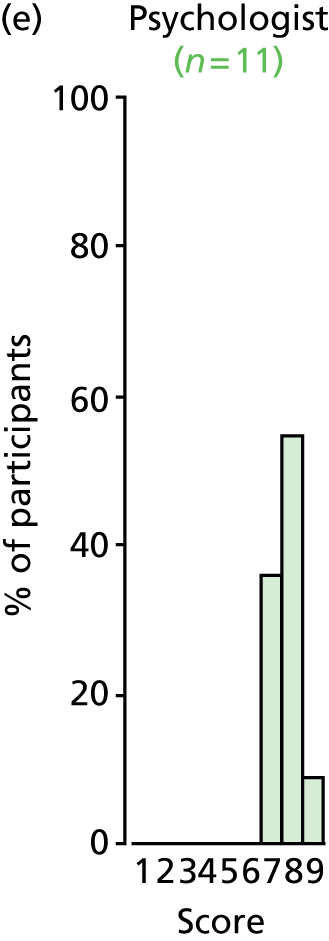
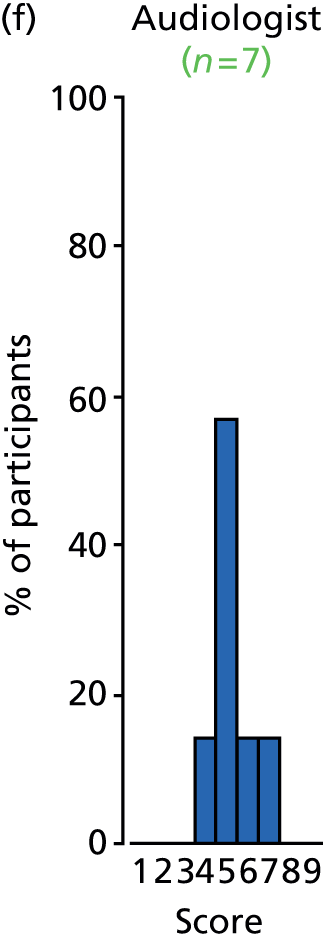

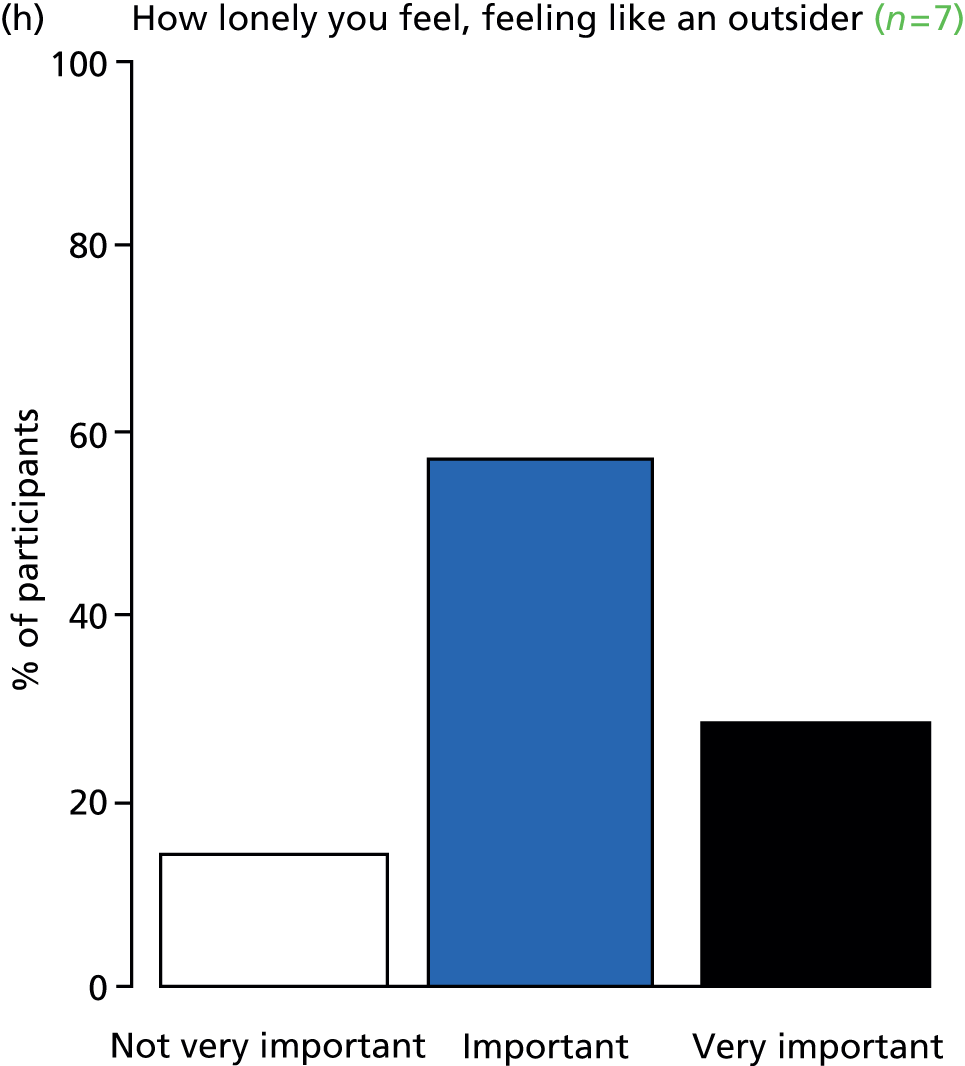
FIGURE 55.
Outcome: externalising behaviour.
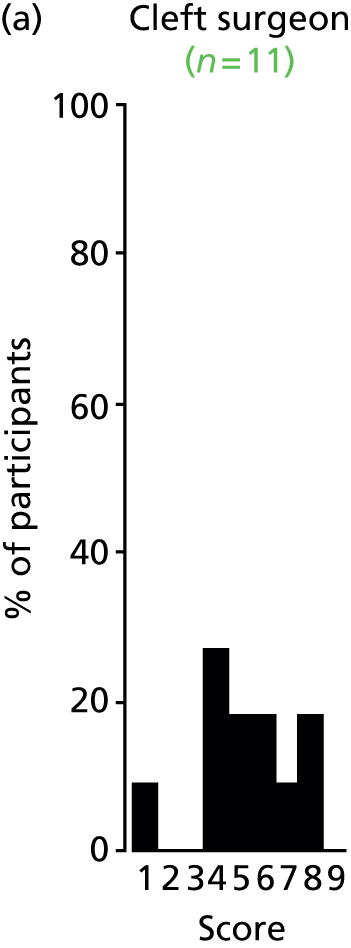
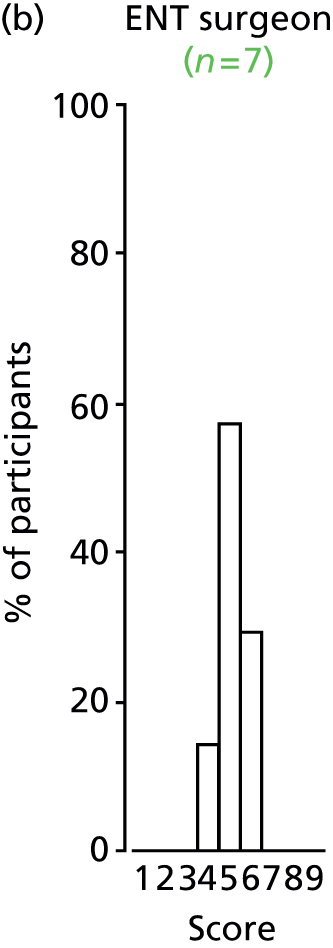

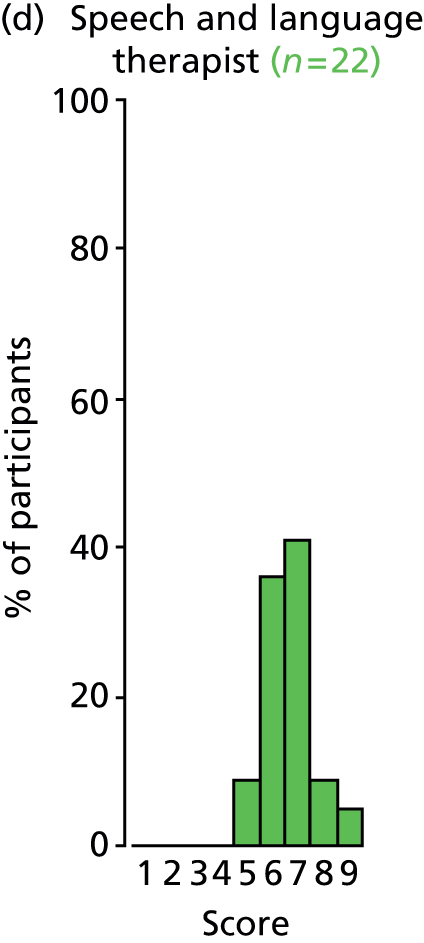
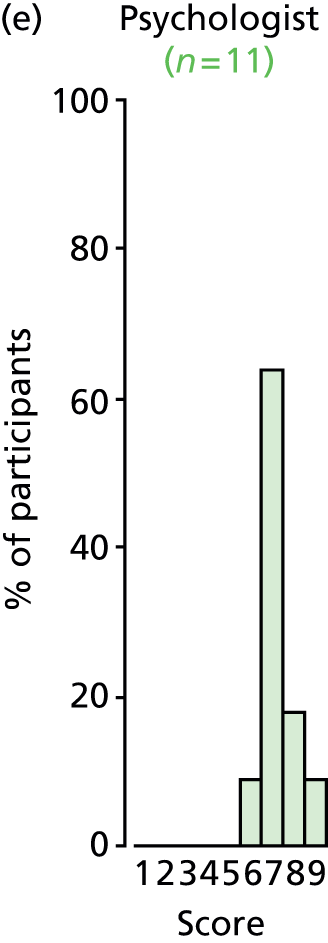


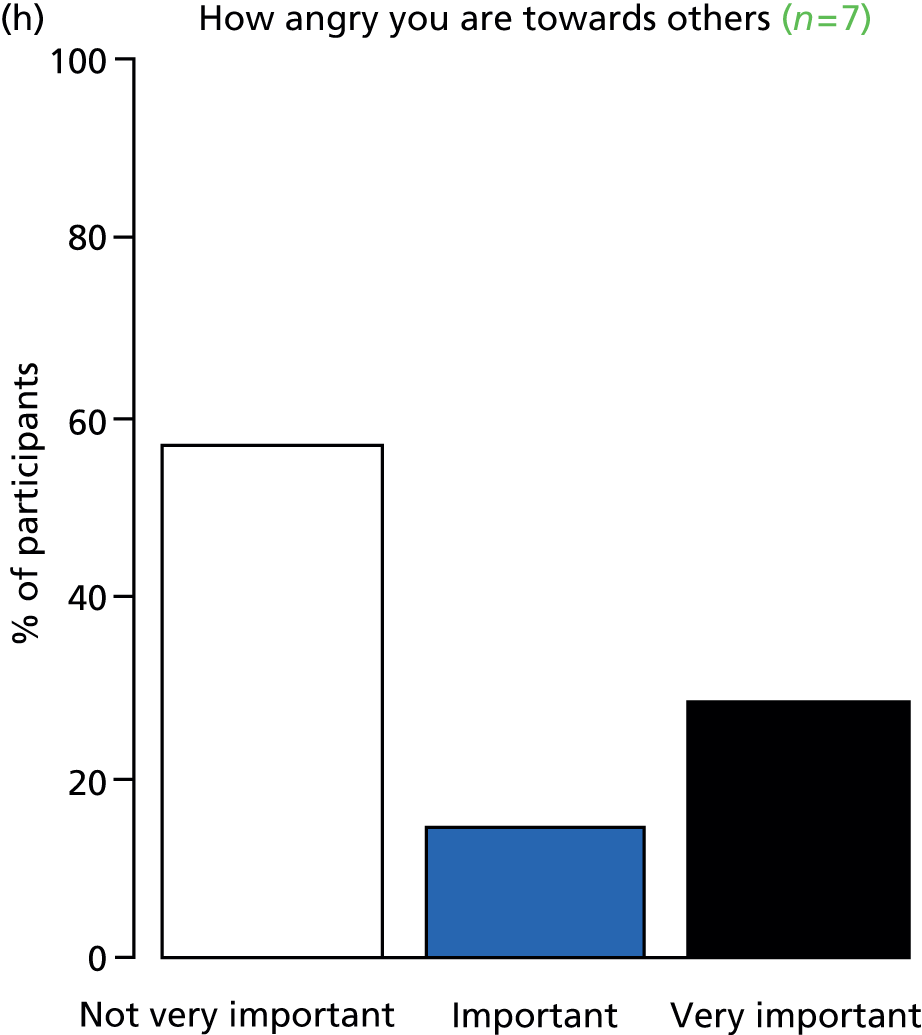
FIGURE 56.
Outcome: academic achievement.

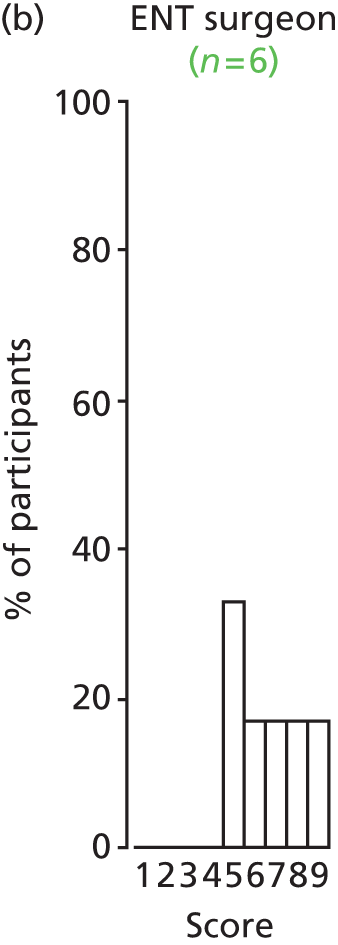
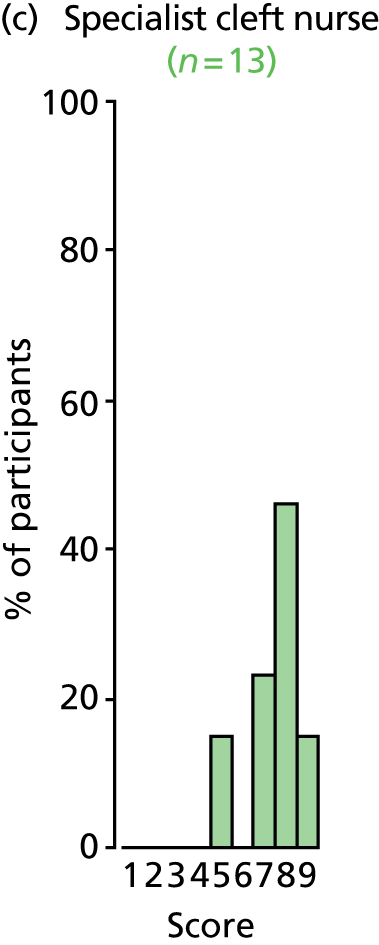
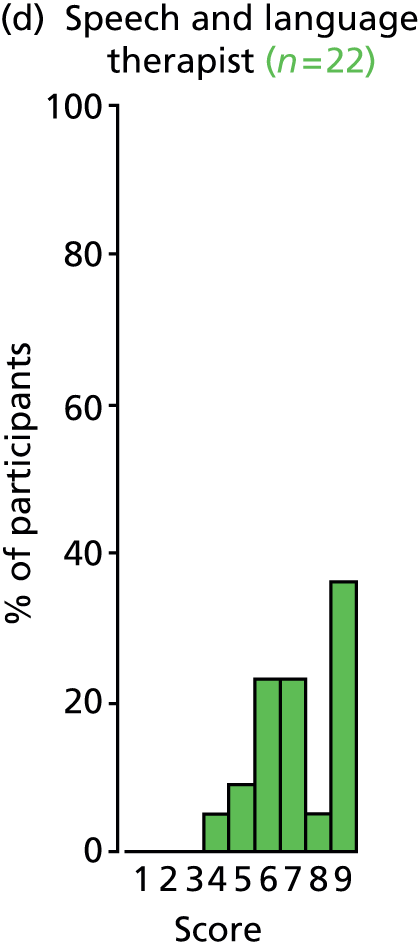

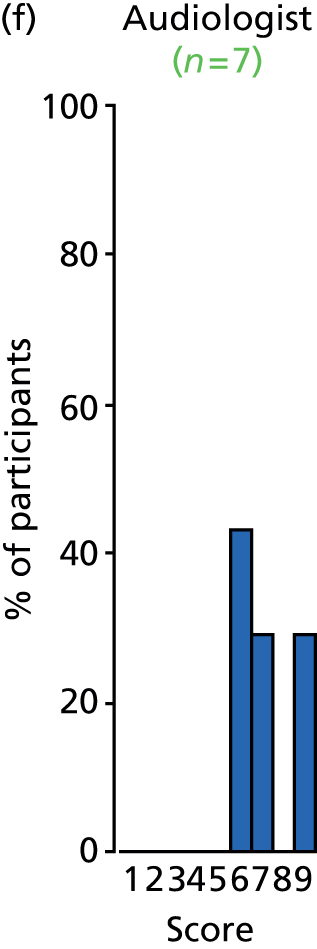
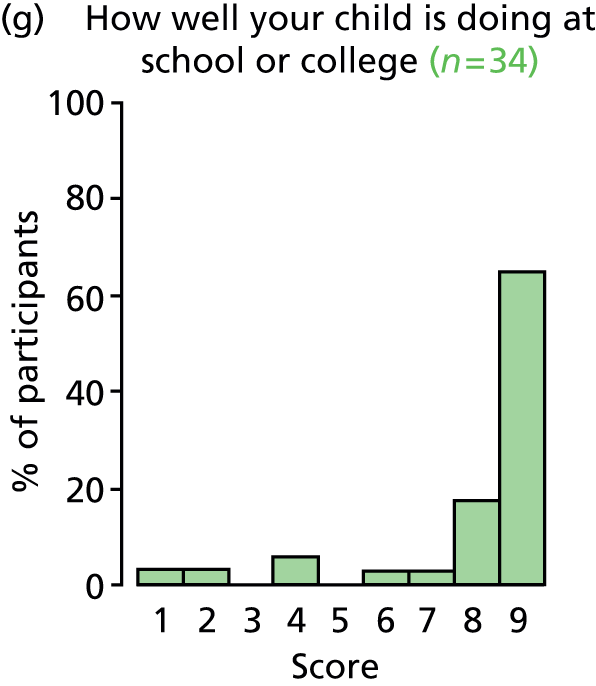
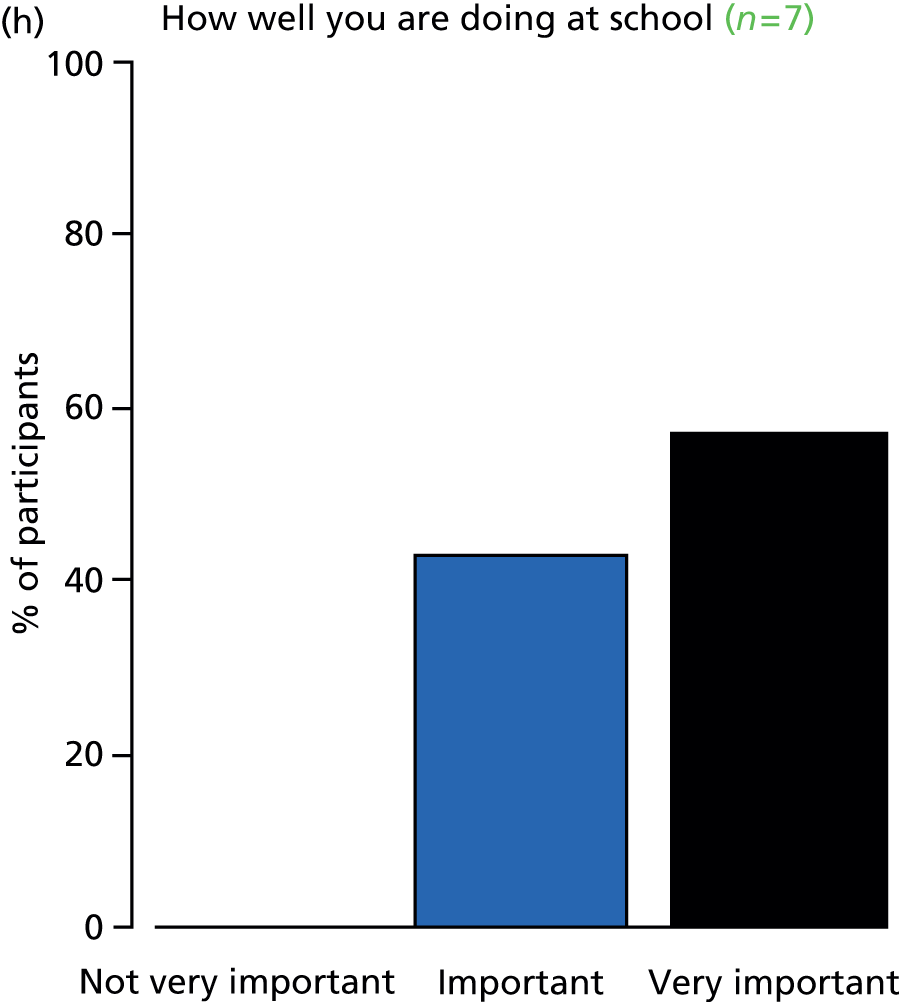
FIGURE 57.
Outcome: literacy.

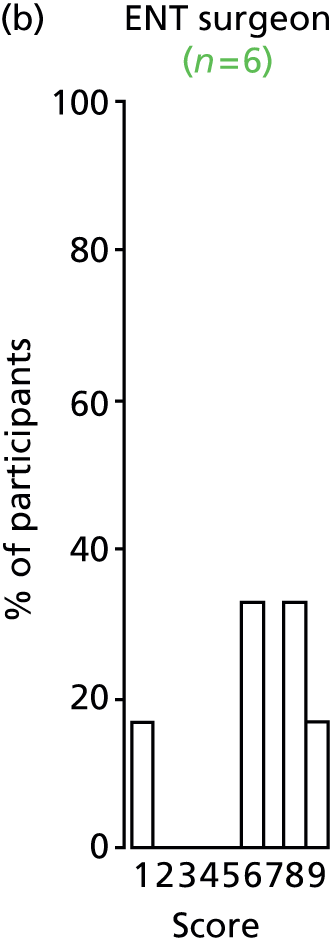
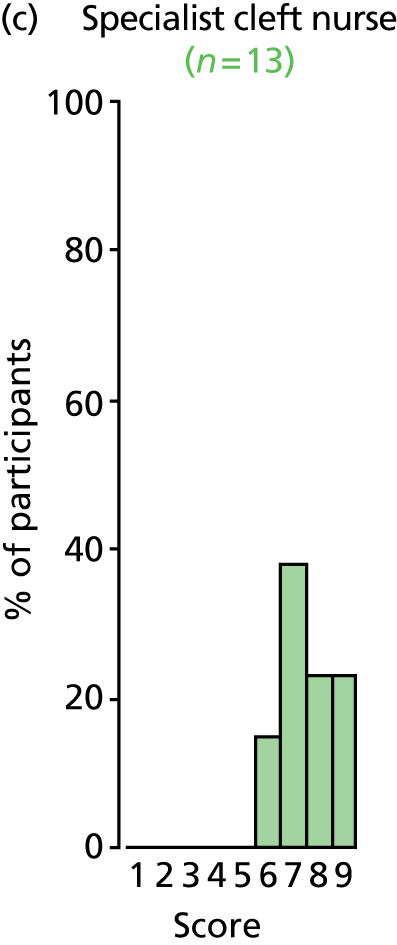



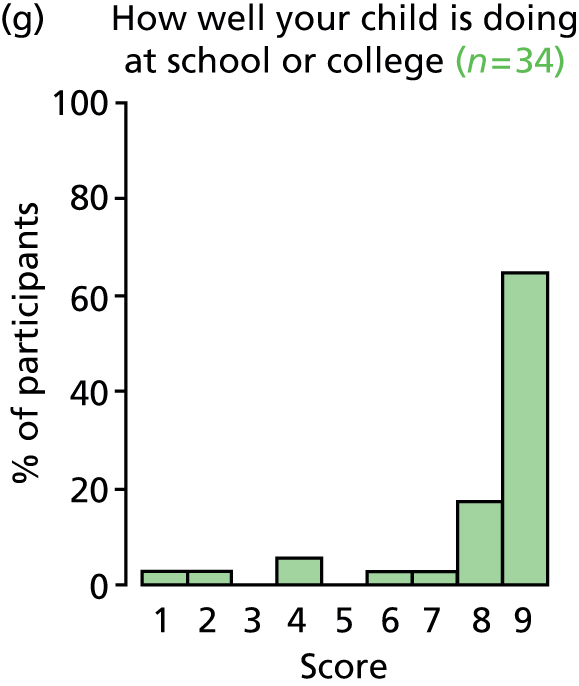
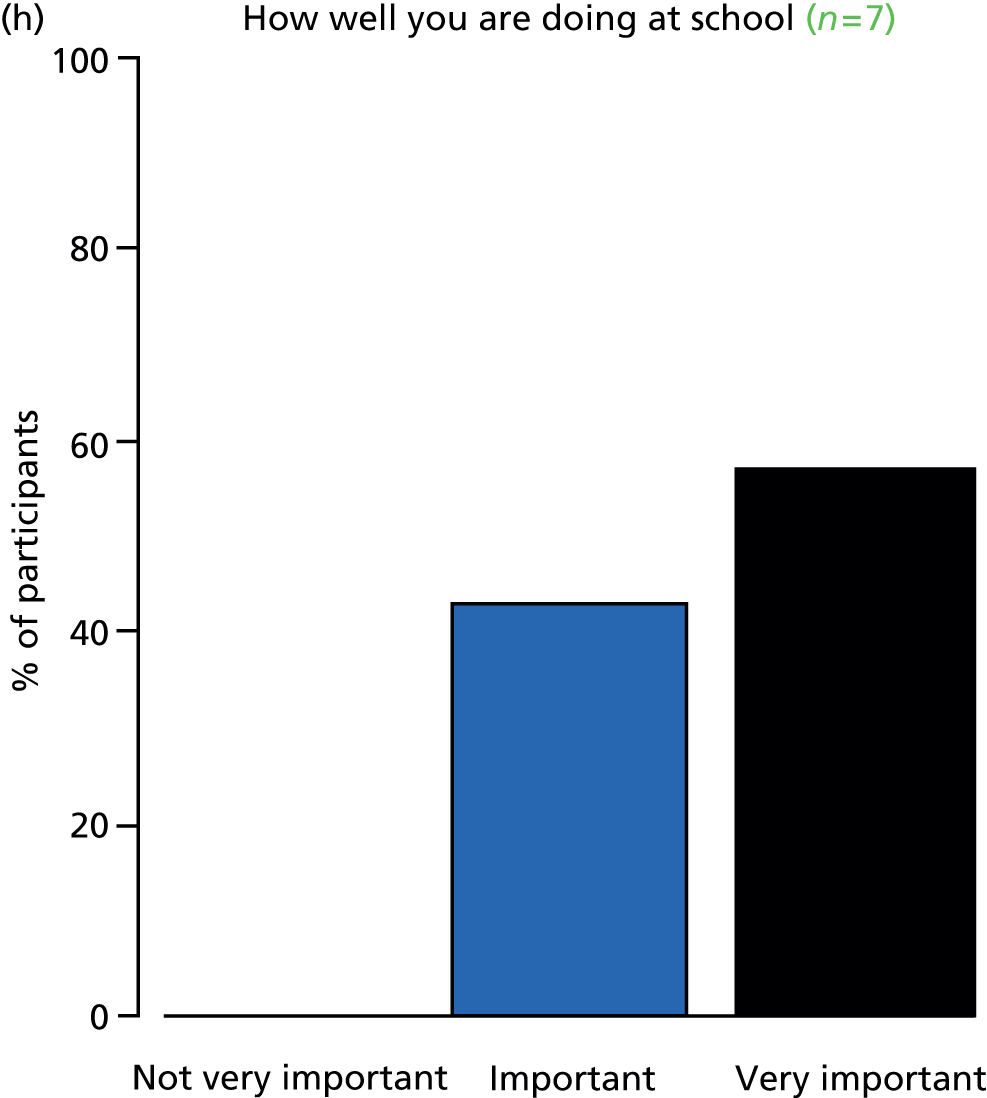
FIGURE 58.
Outcome: phonological memory.
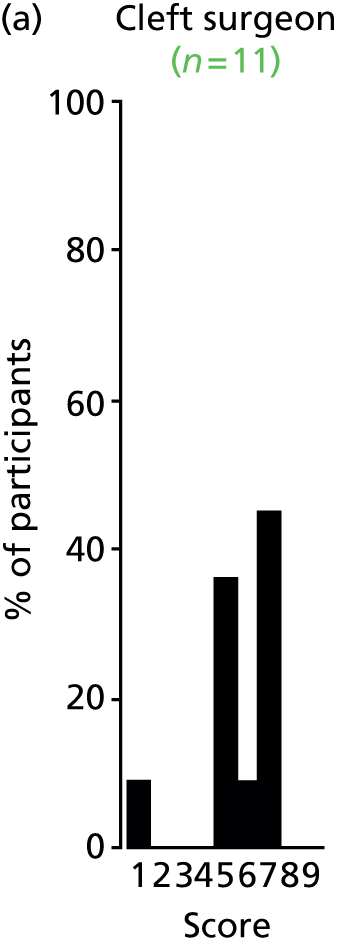

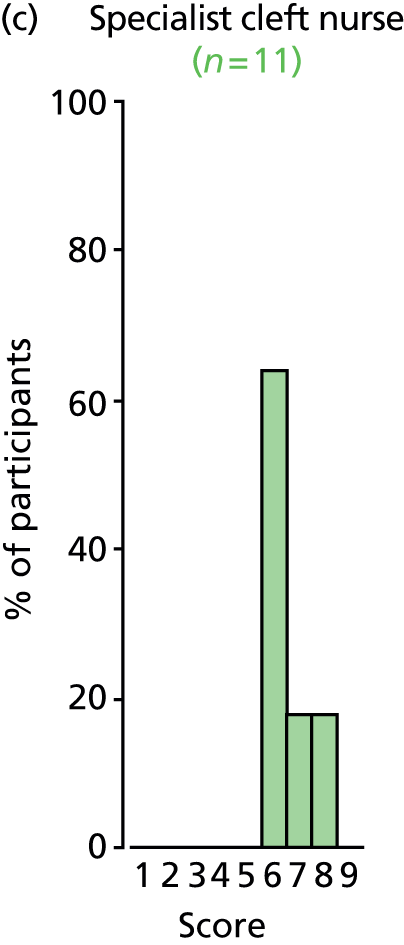
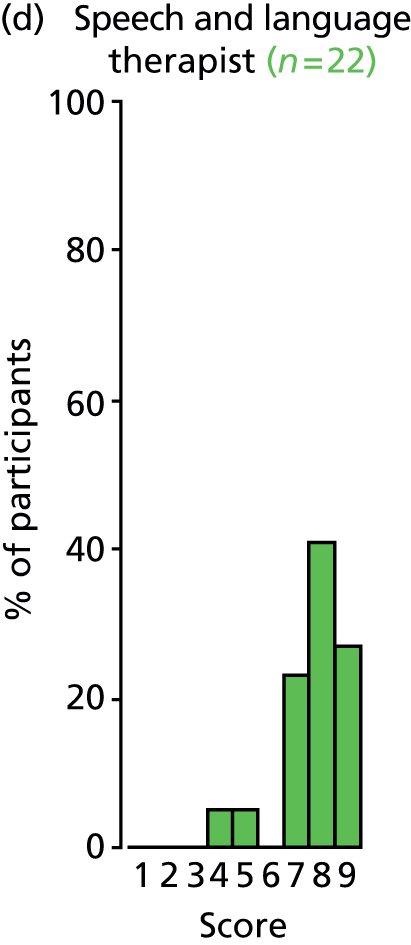
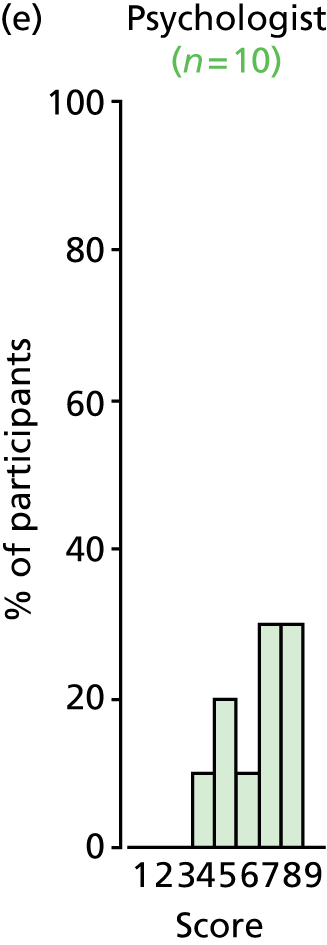


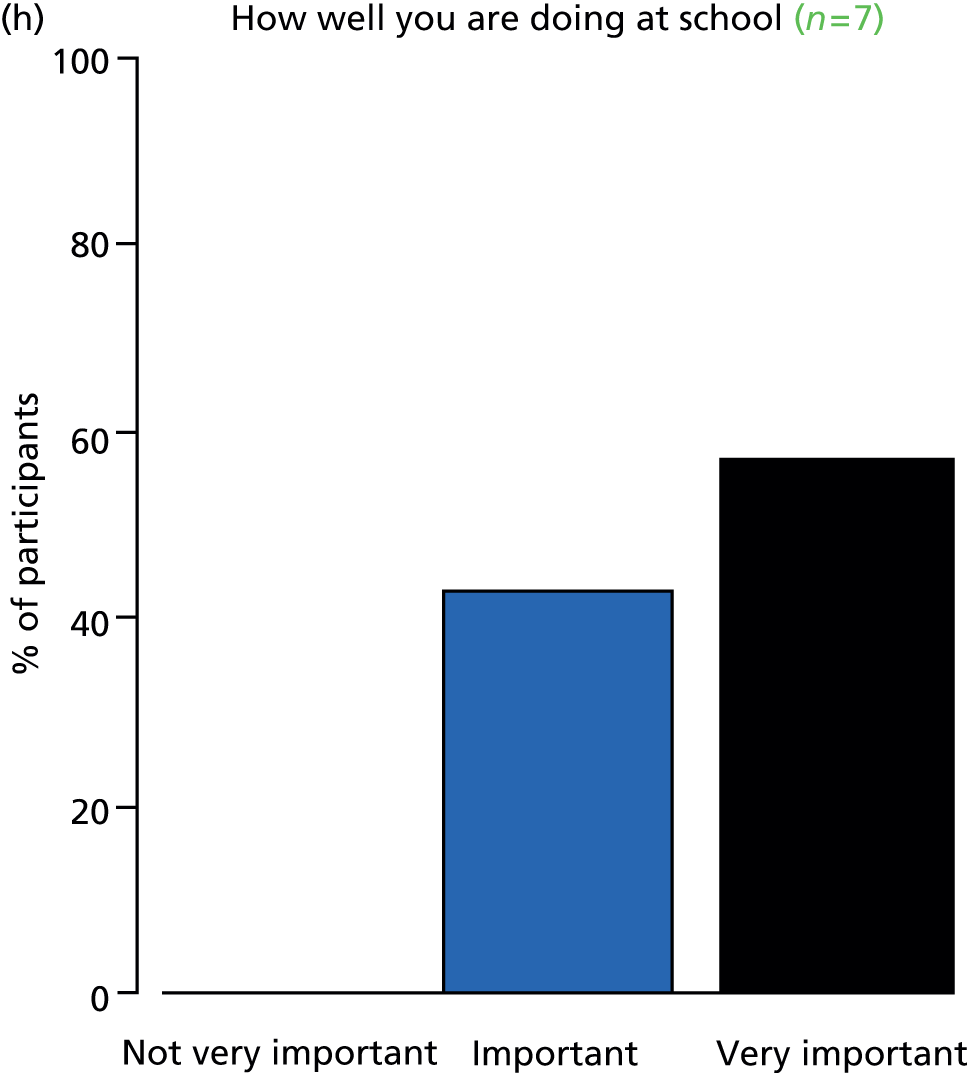
FIGURE 59.
Outcome: hearing.


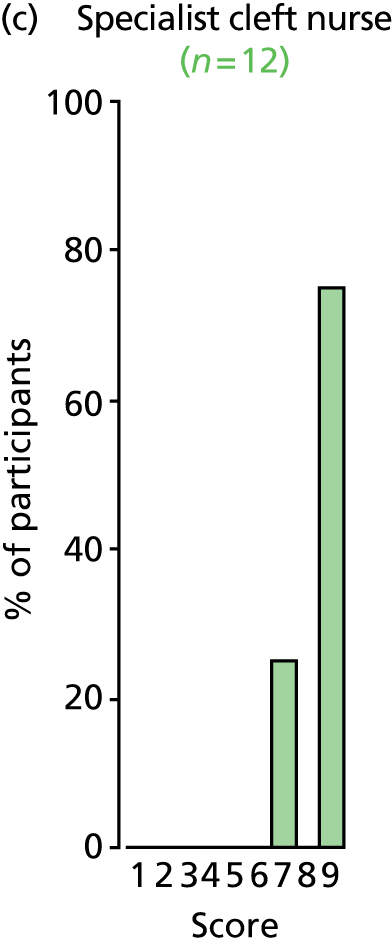



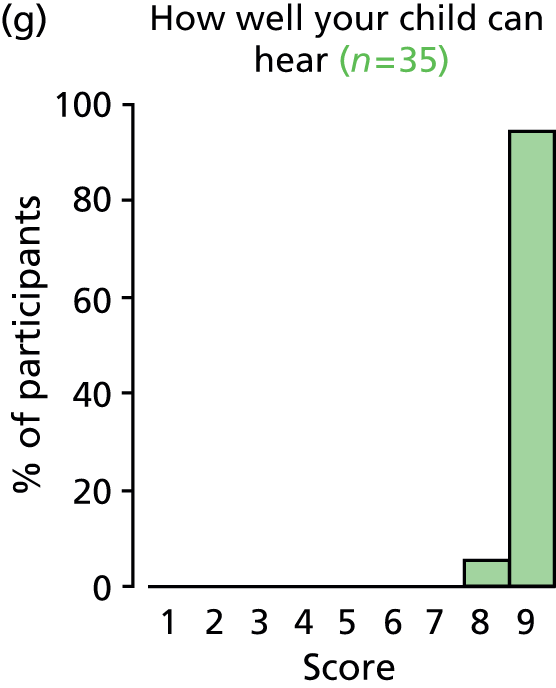

FIGURE 60.
Outcome: chronic otitis media.
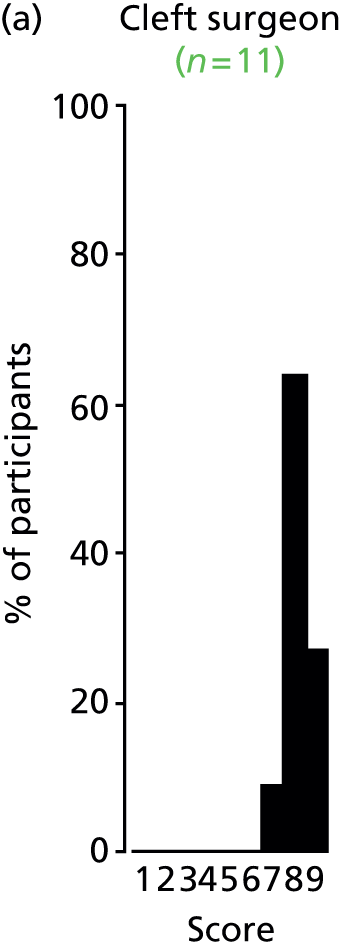
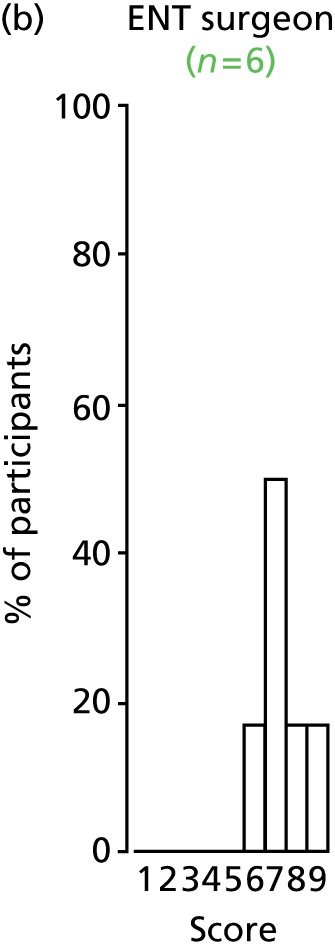
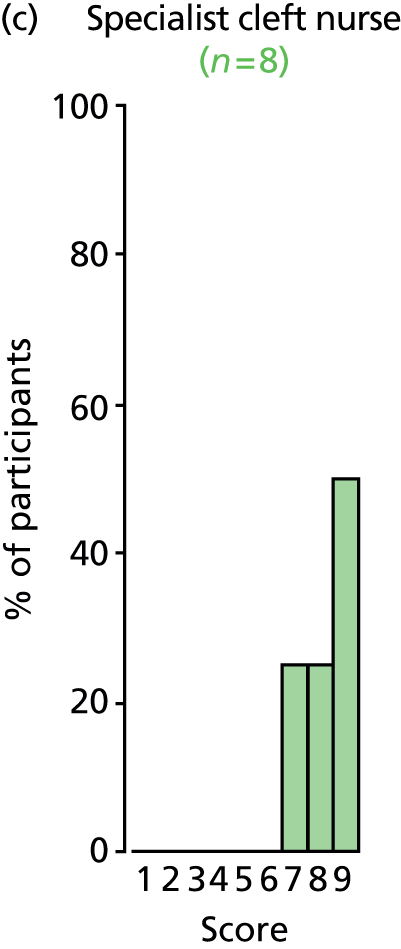



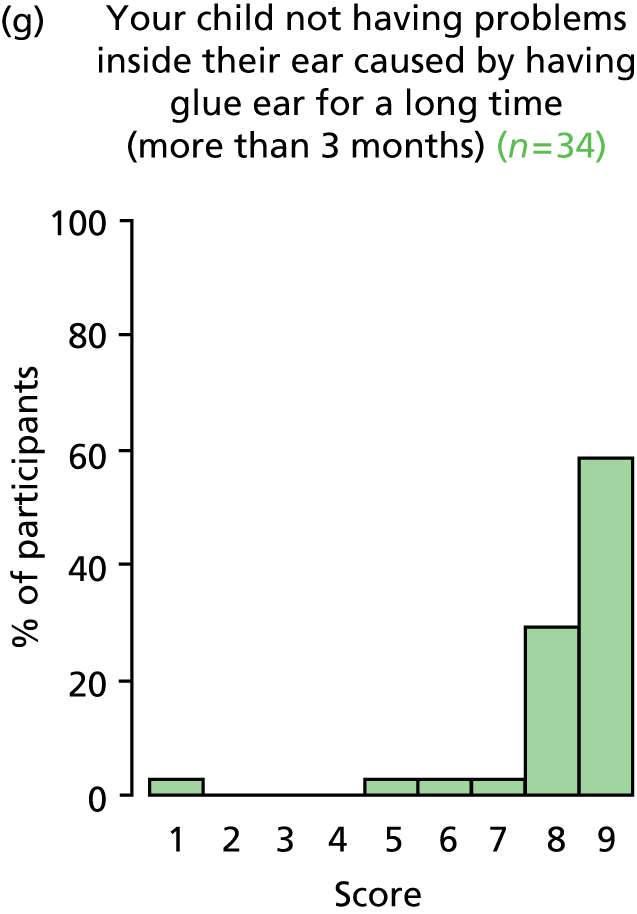

FIGURE 61.
Outcome: OME.
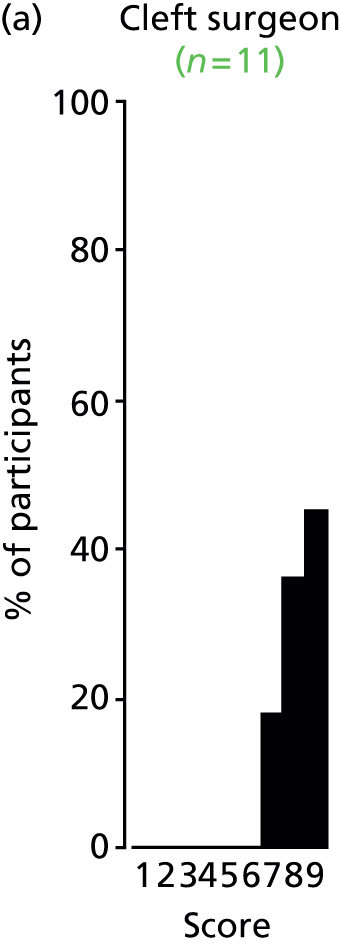




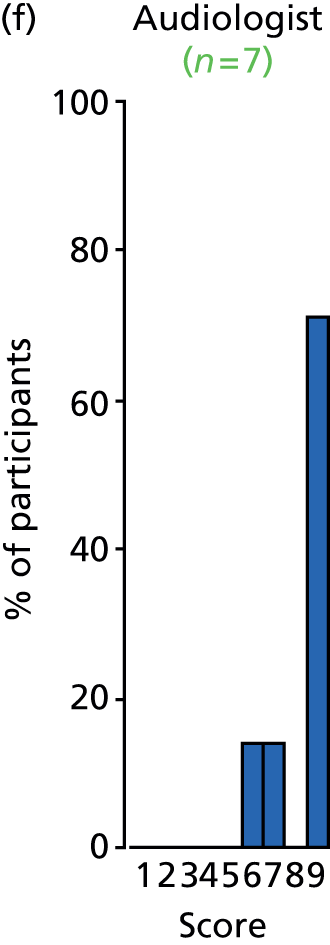
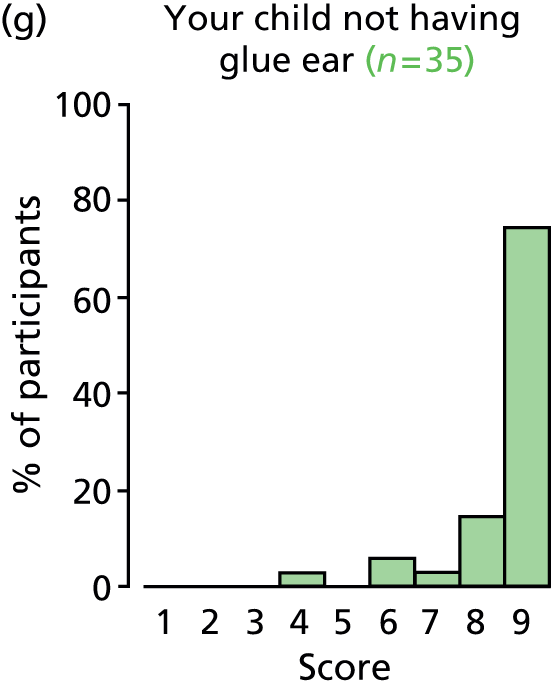

FIGURE 62.
Outcome: receptive language skills.

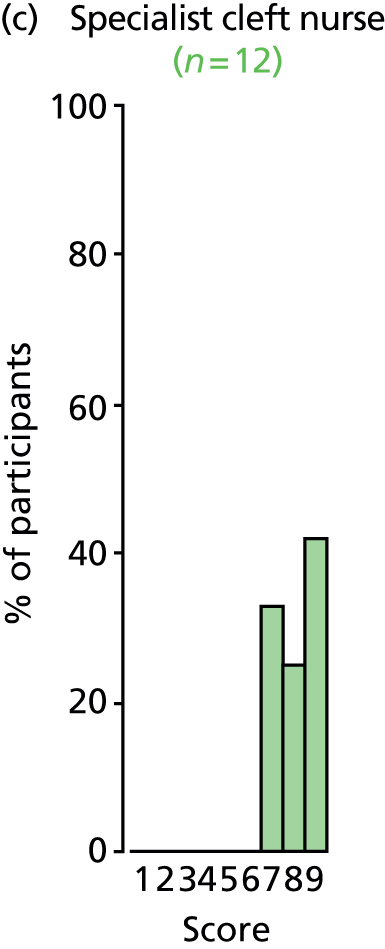
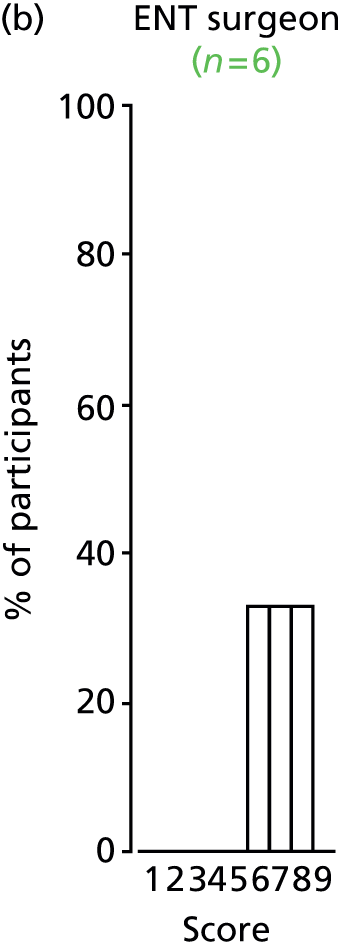

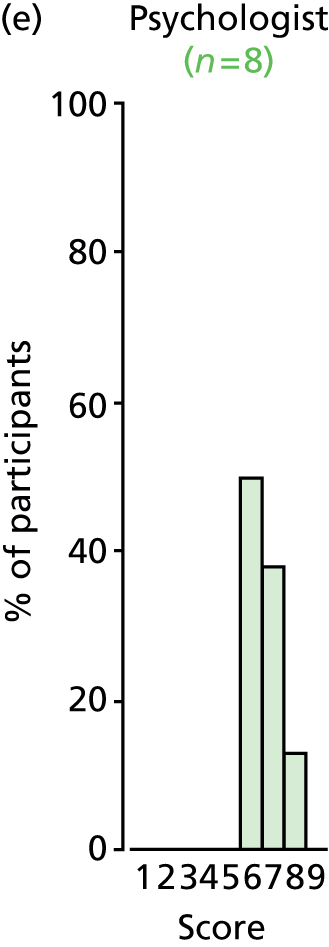
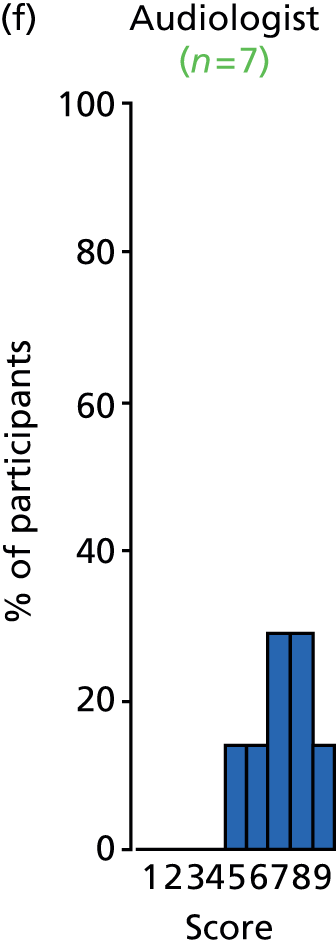
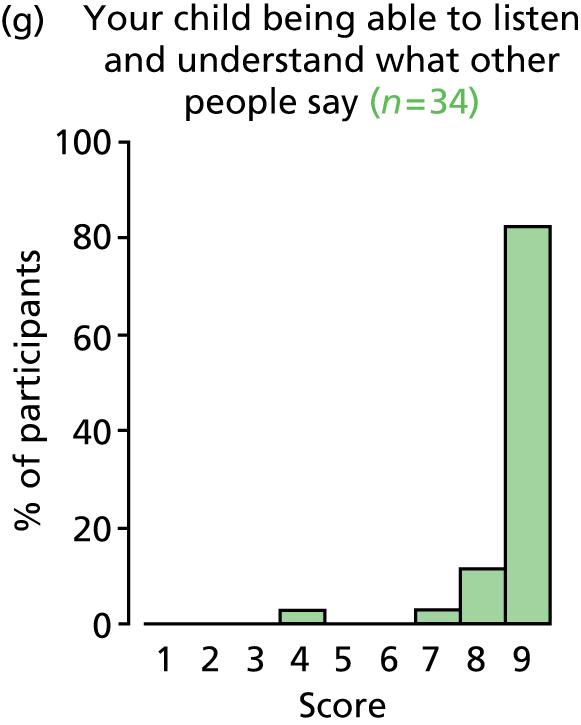
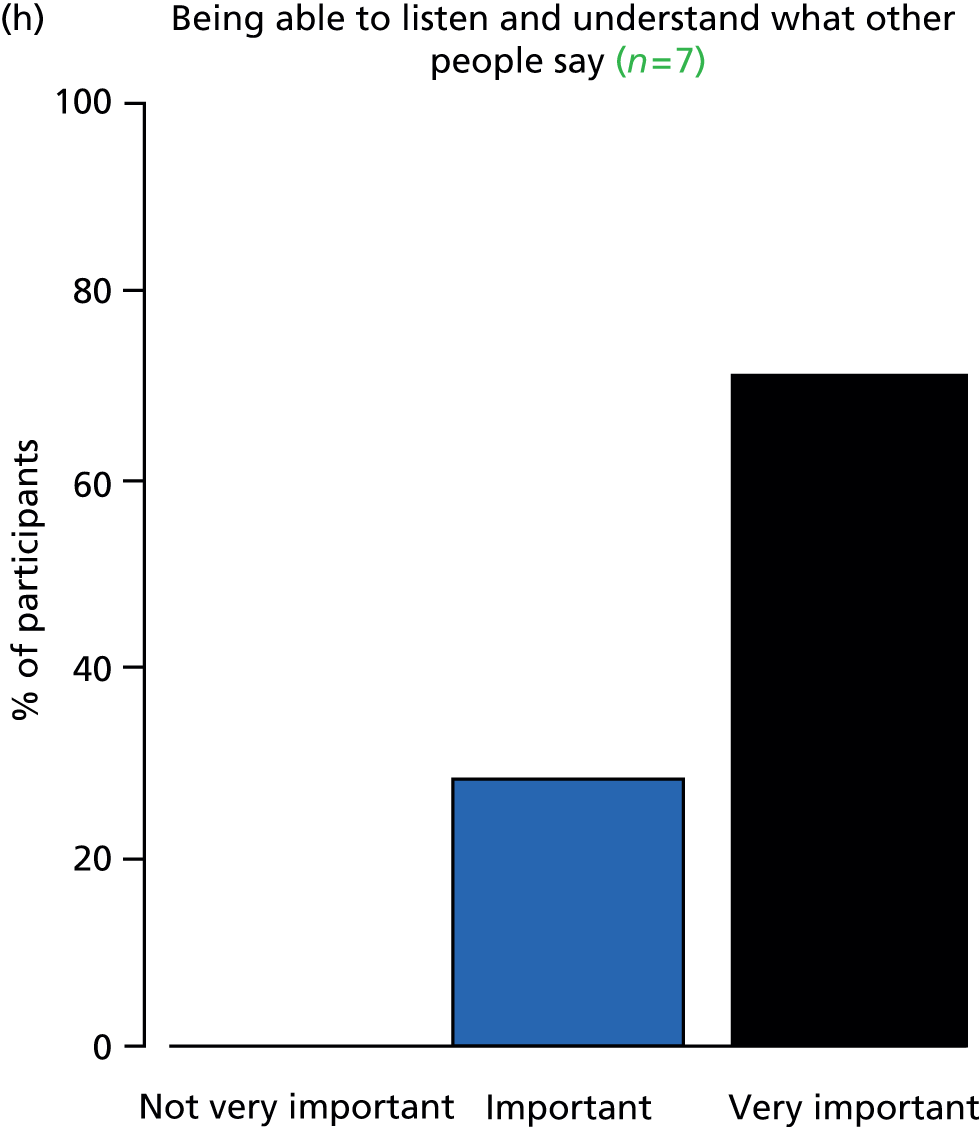
FIGURE 63.
Outcome: speech development.


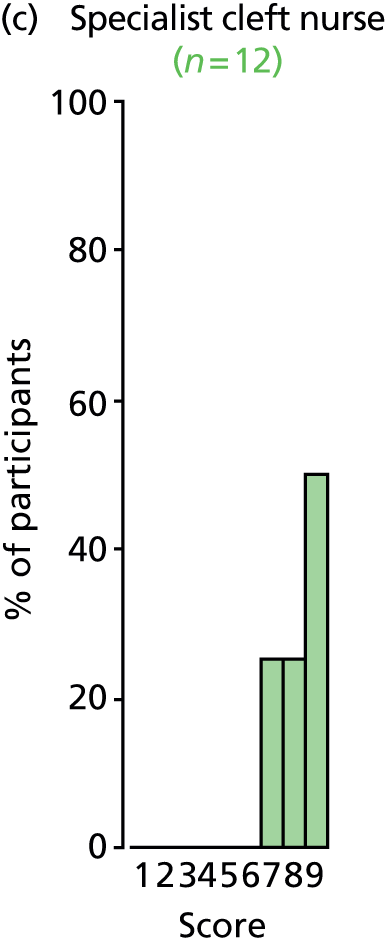



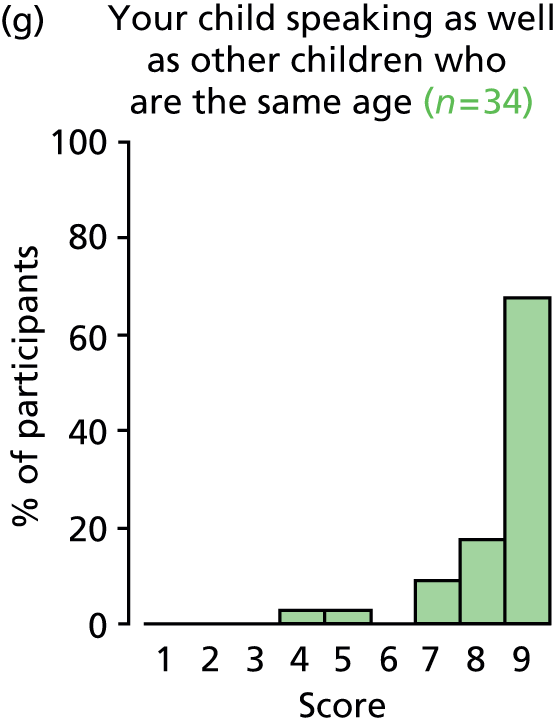

FIGURE 64.
Outcome: atelectasis.

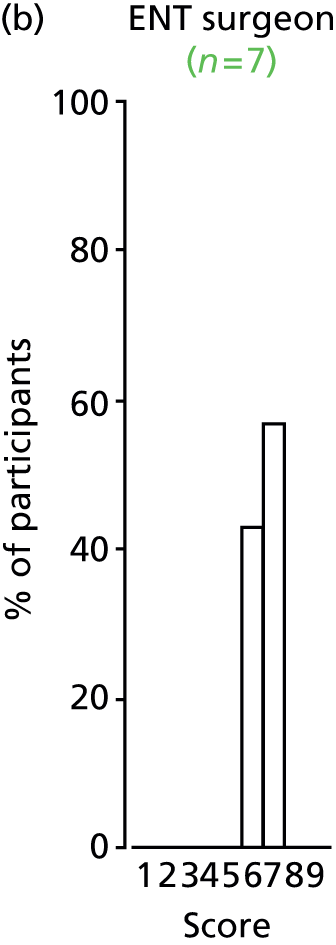



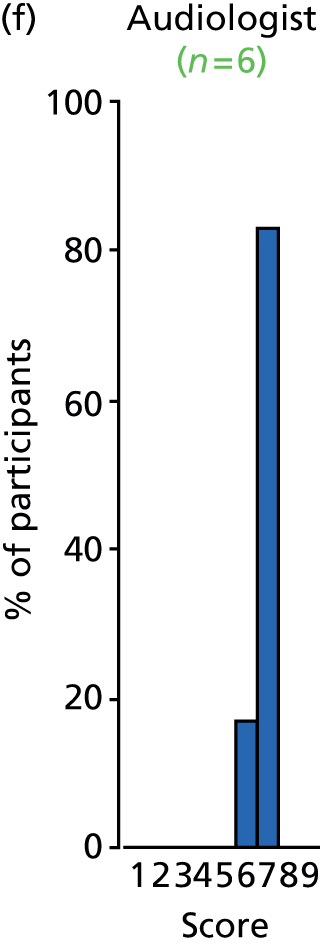


FIGURE 65.
Outcome: cholesteatoma.



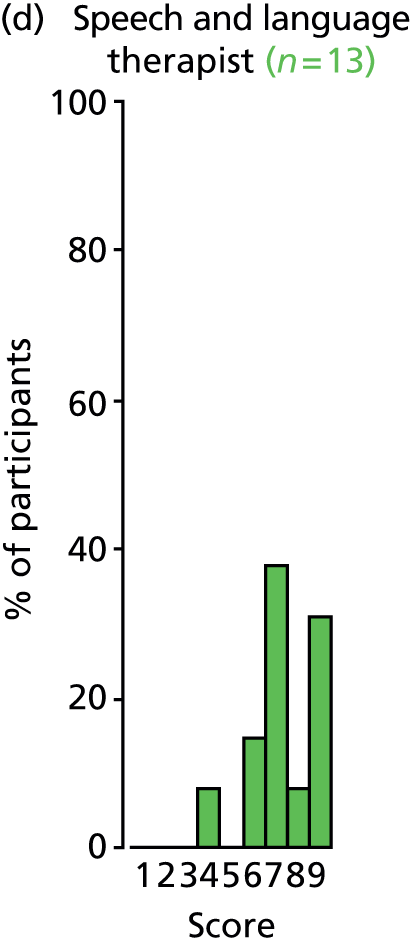
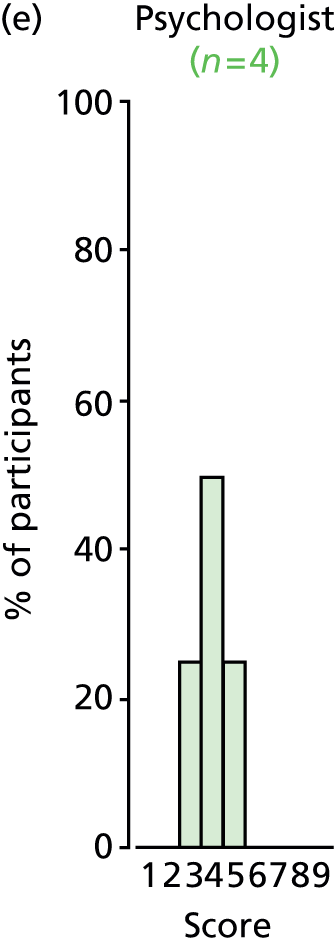
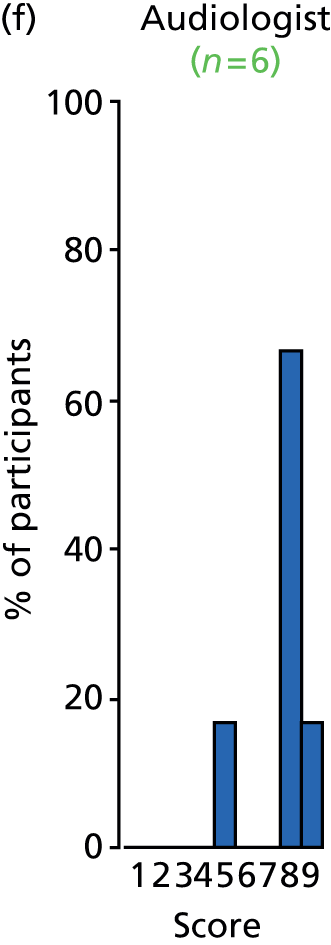


FIGURE 66.
Outcome: psychosocial development.
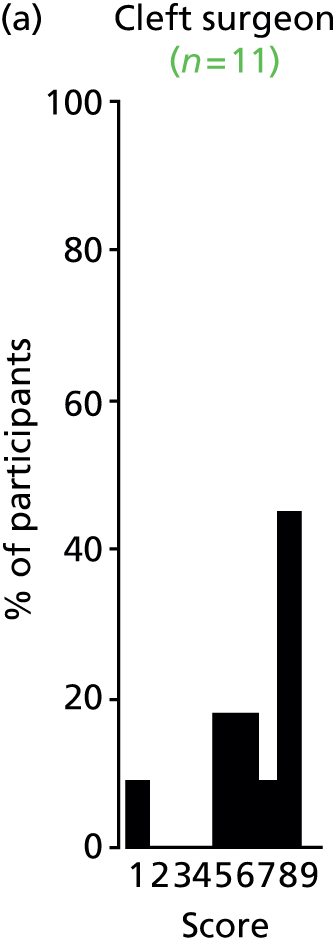
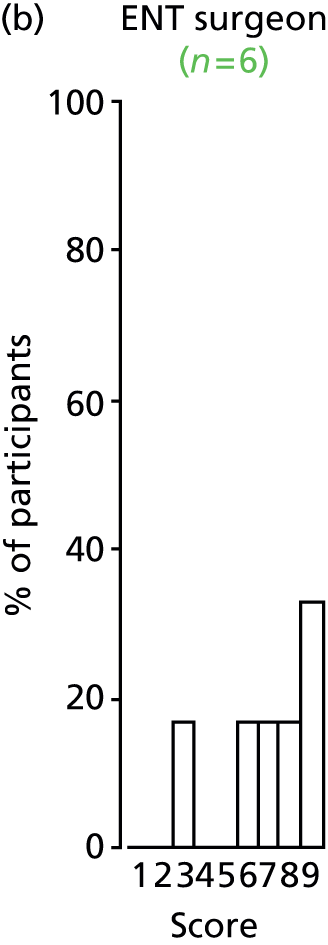


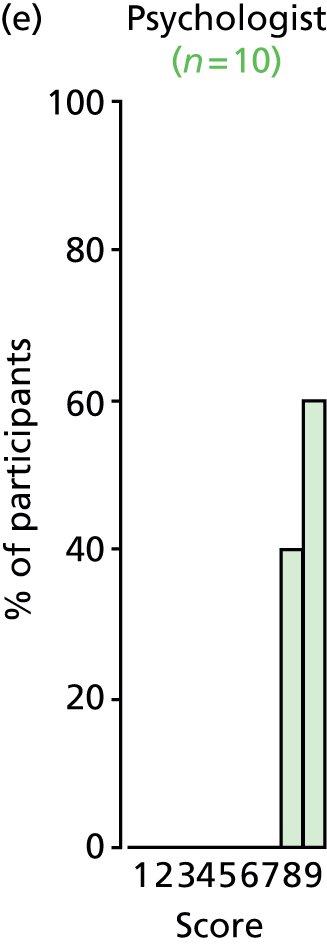
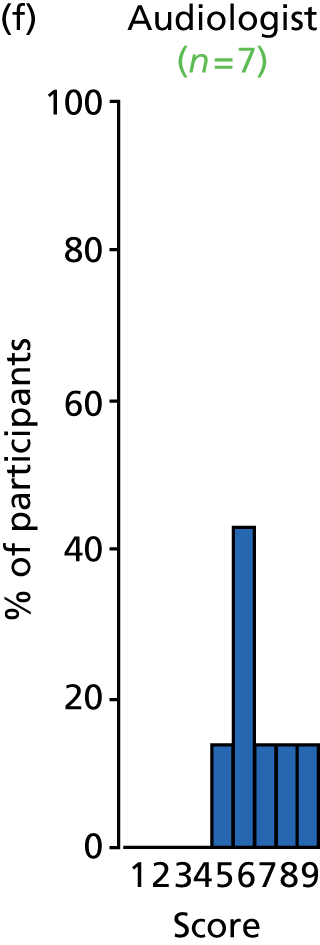

FIGURE 67.
Outcome: AOM.
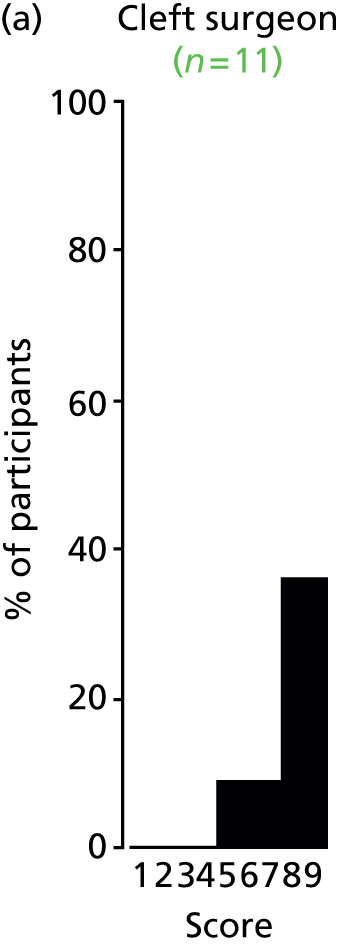
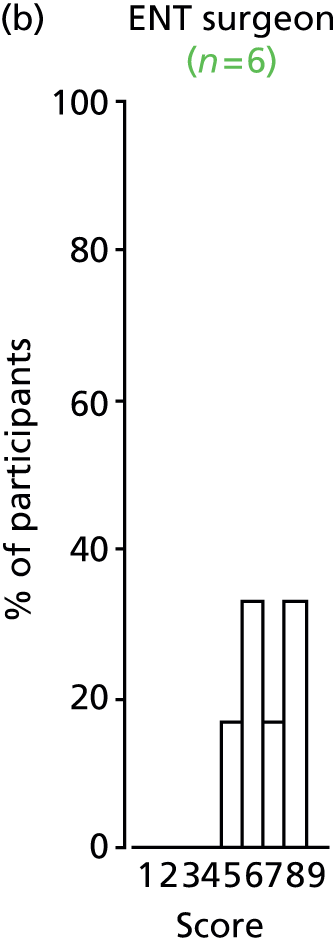

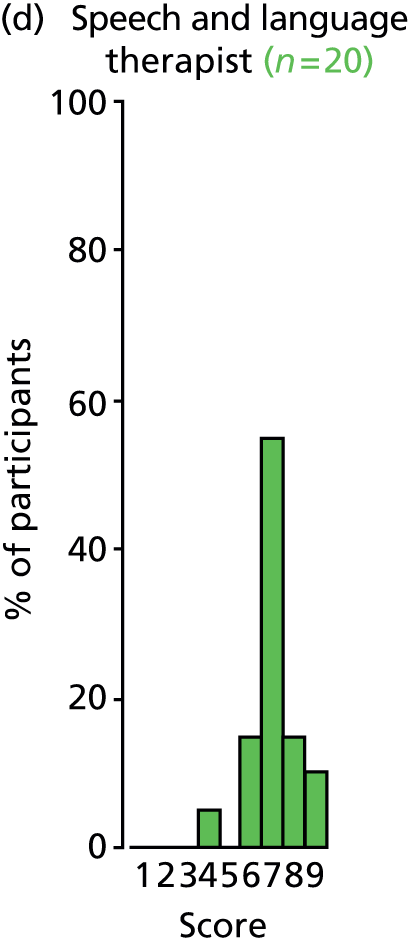




FIGURE 68.
Outcome: consonant production.
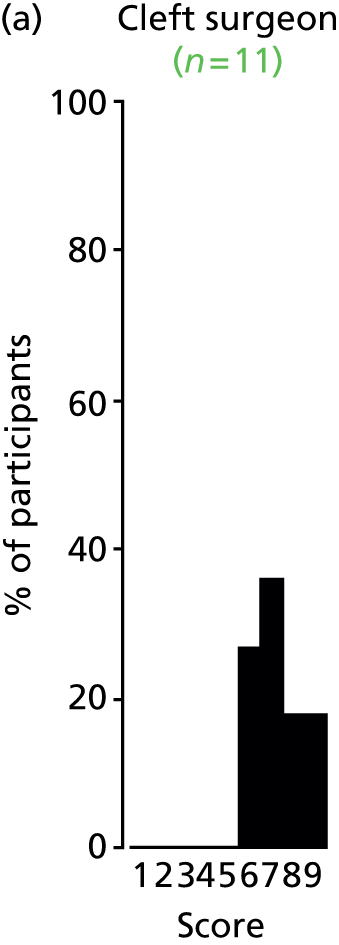
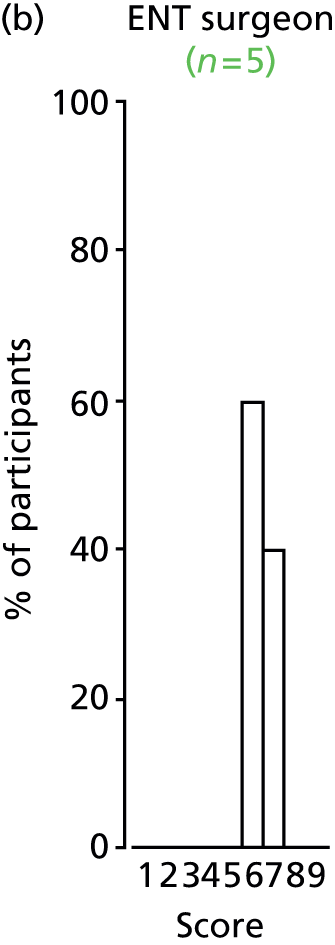
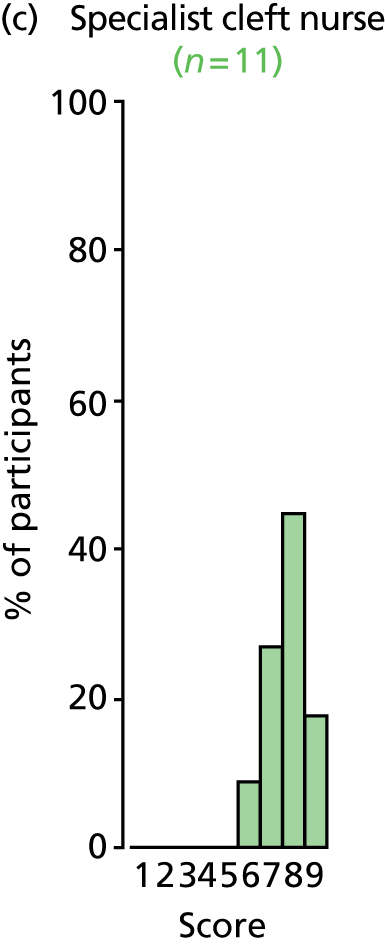

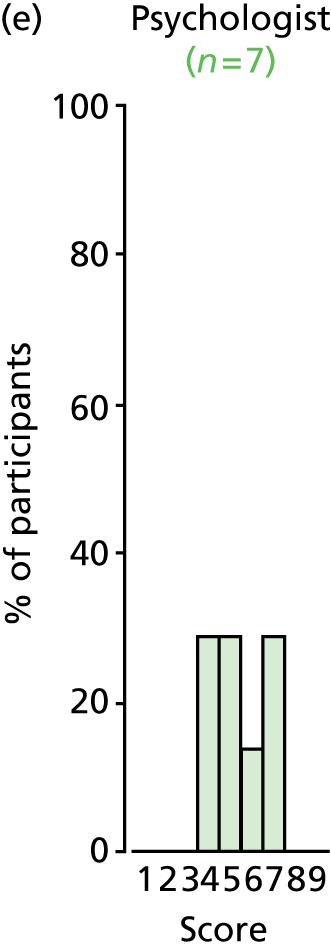

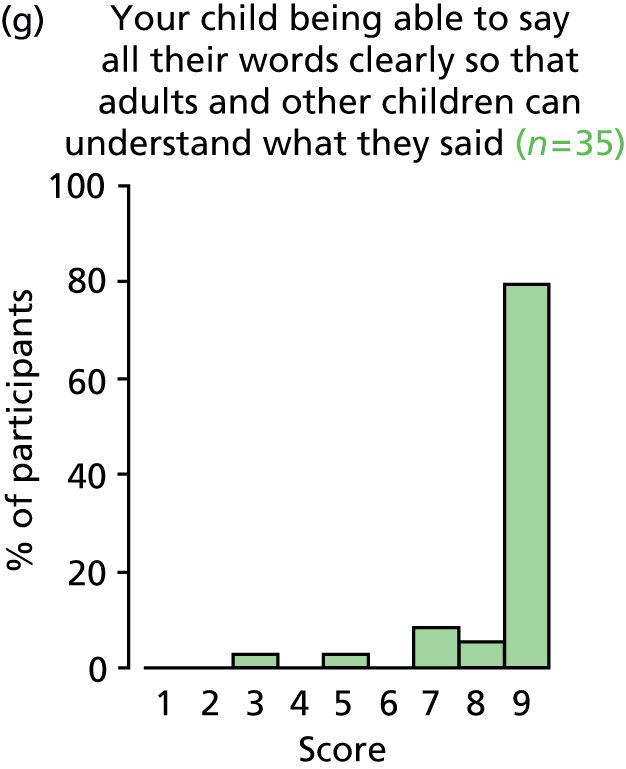
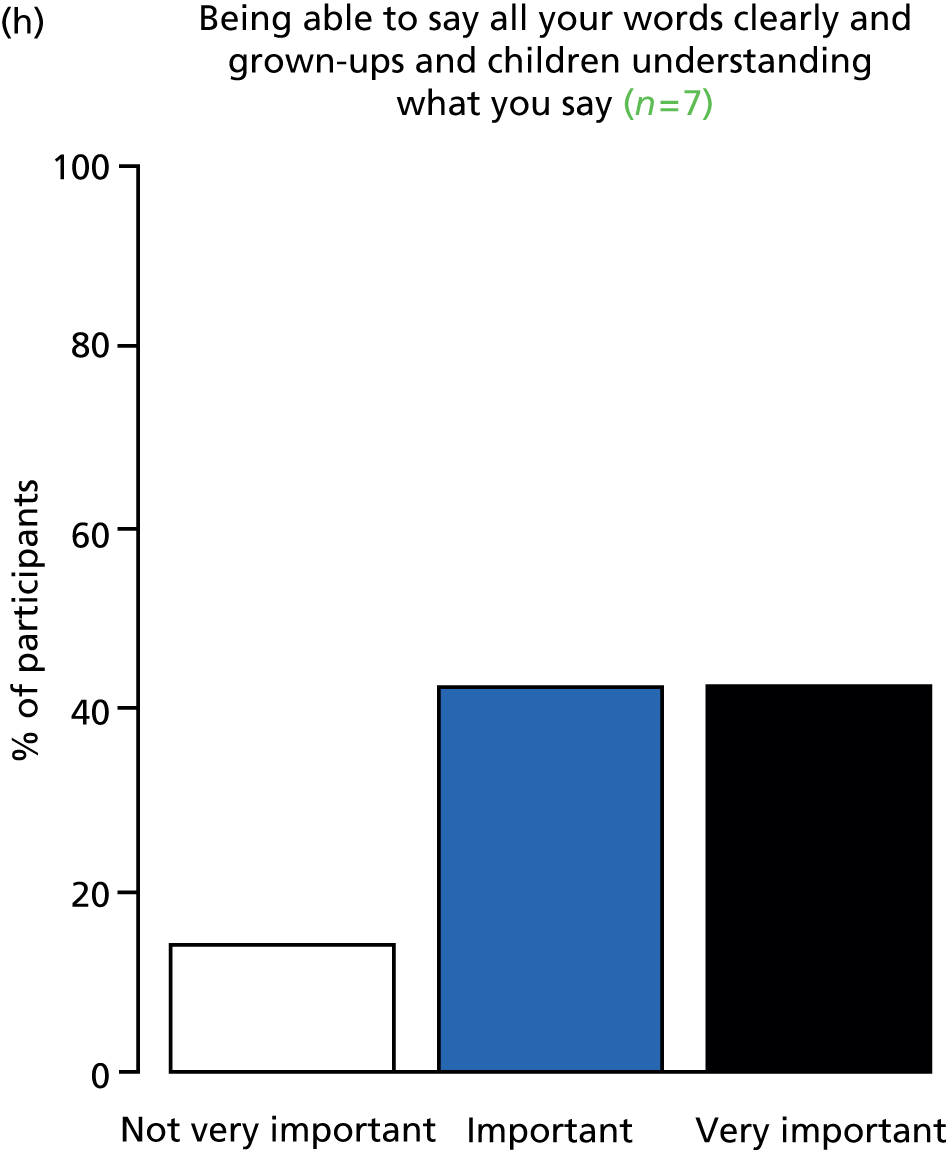
FIGURE 69.
Outcome: speech intelligibility.
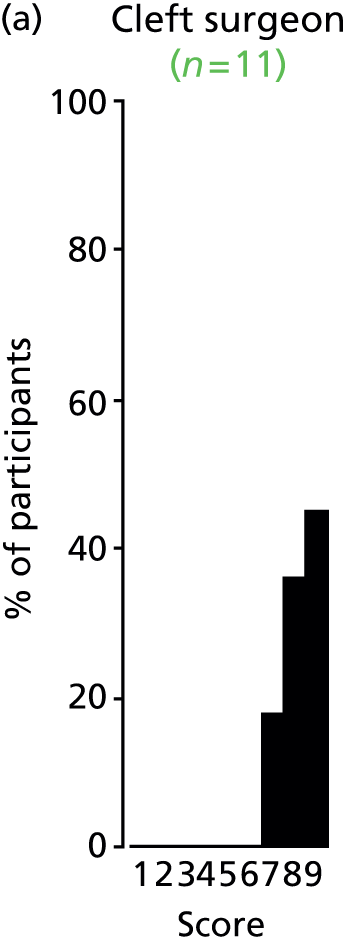
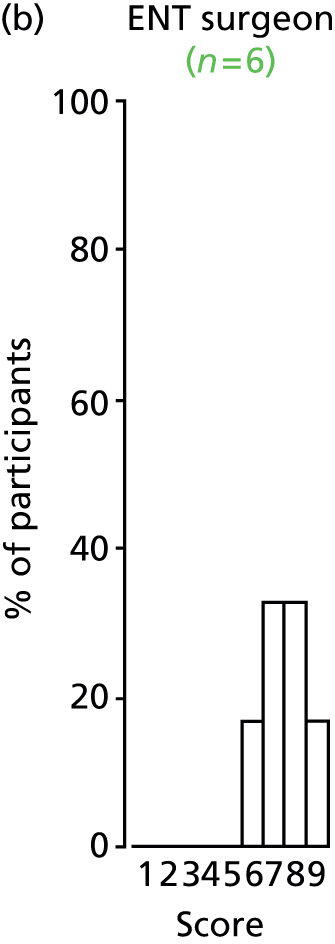

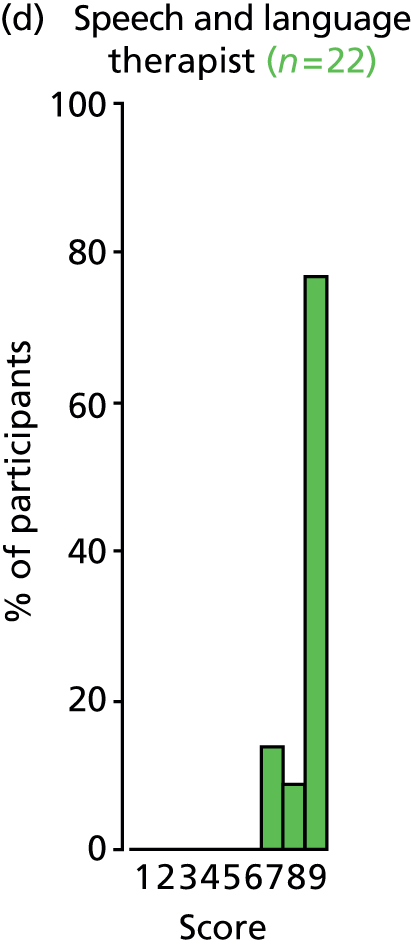

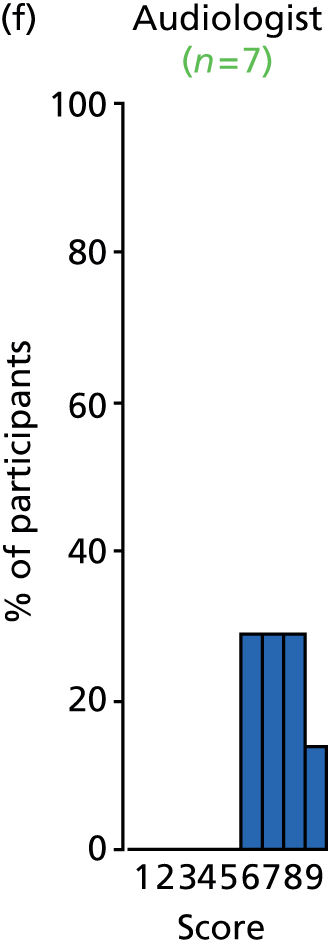
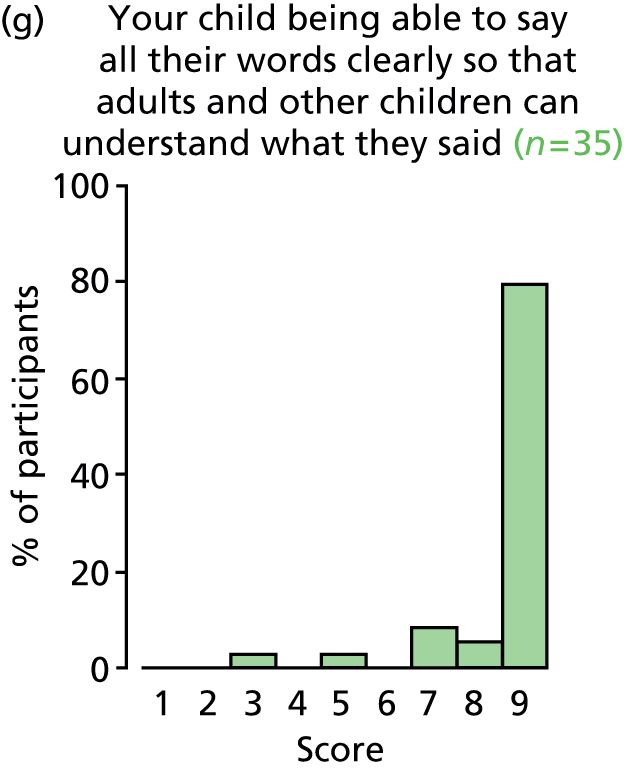

FIGURE 70.
Outcome: necessity to remove VTs.
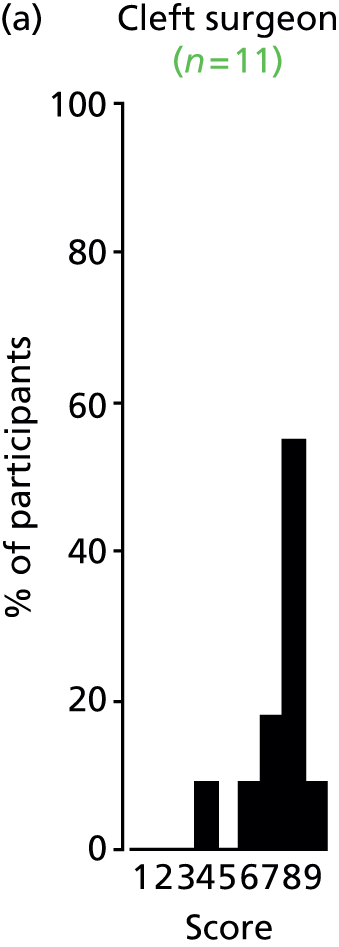

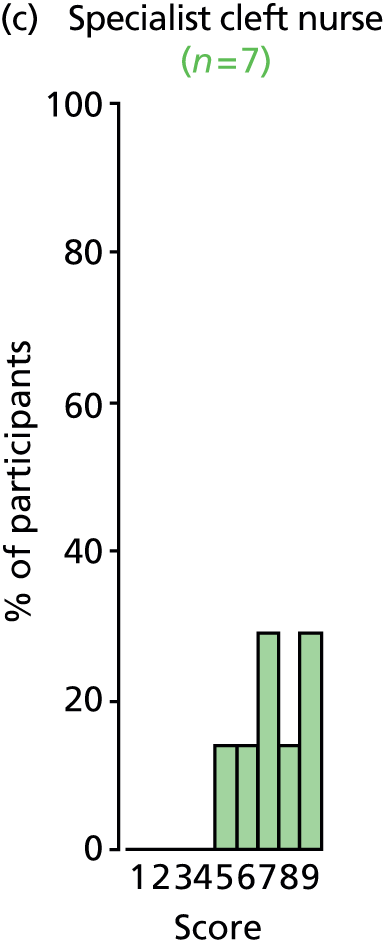
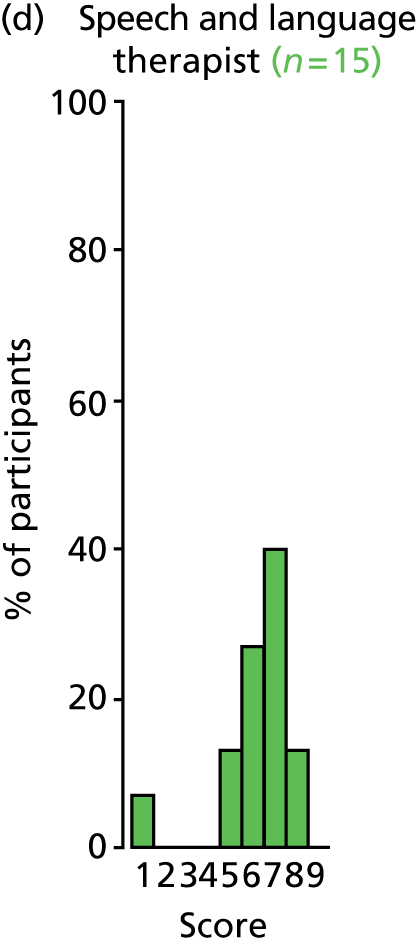
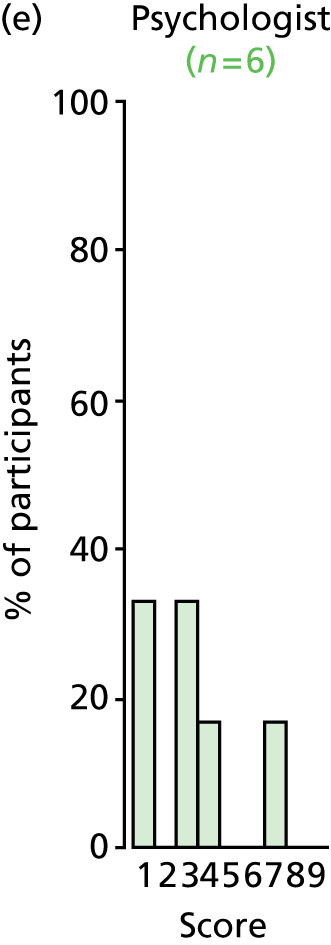



FIGURE 71.
Outcome: requirement for repeated VTs.
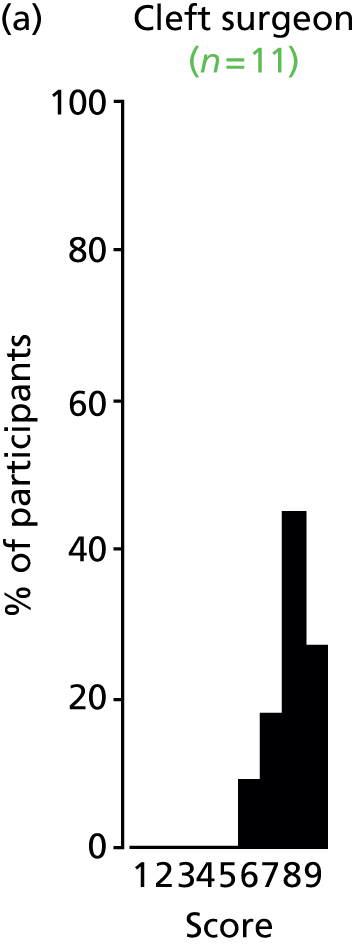






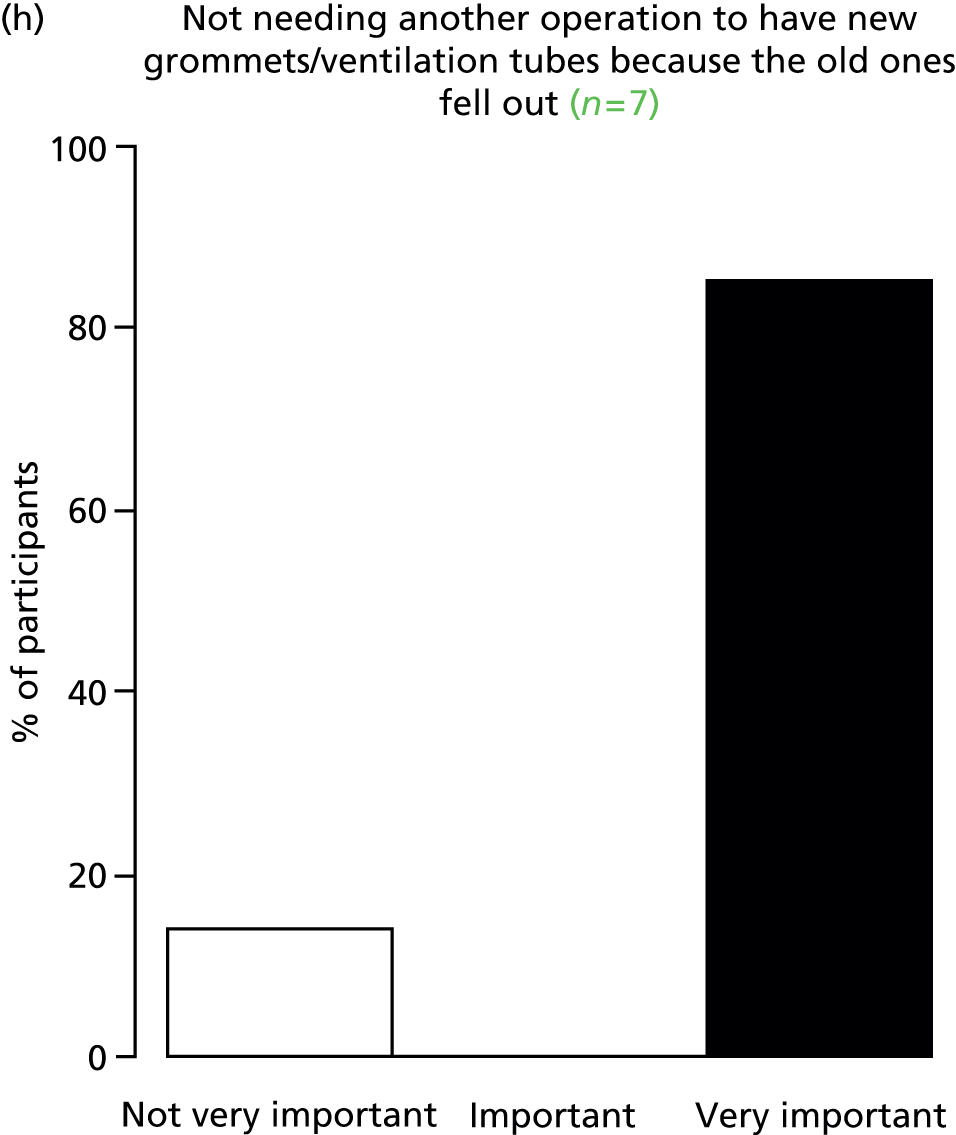
Appendix 9 Individual outcome discussions and results of scoring at consensus meeting
Note: the scores of participants scoring 1–9 only have been included. The figure for the number of participants scoring 1–9 has been given together with the number who answered ‘unable to score’. Some meeting participants left the meeting early and the outcomes affected by this are marked with an asterisk.
| Outcome number | 16 |
| Outcome name | Hearing |
| Number of participants scoring 1–9 | 14 |
| Number of participants unable to score | 0 |
FIGURE 72.
Results of scoring: hearing – how well your child can hear.
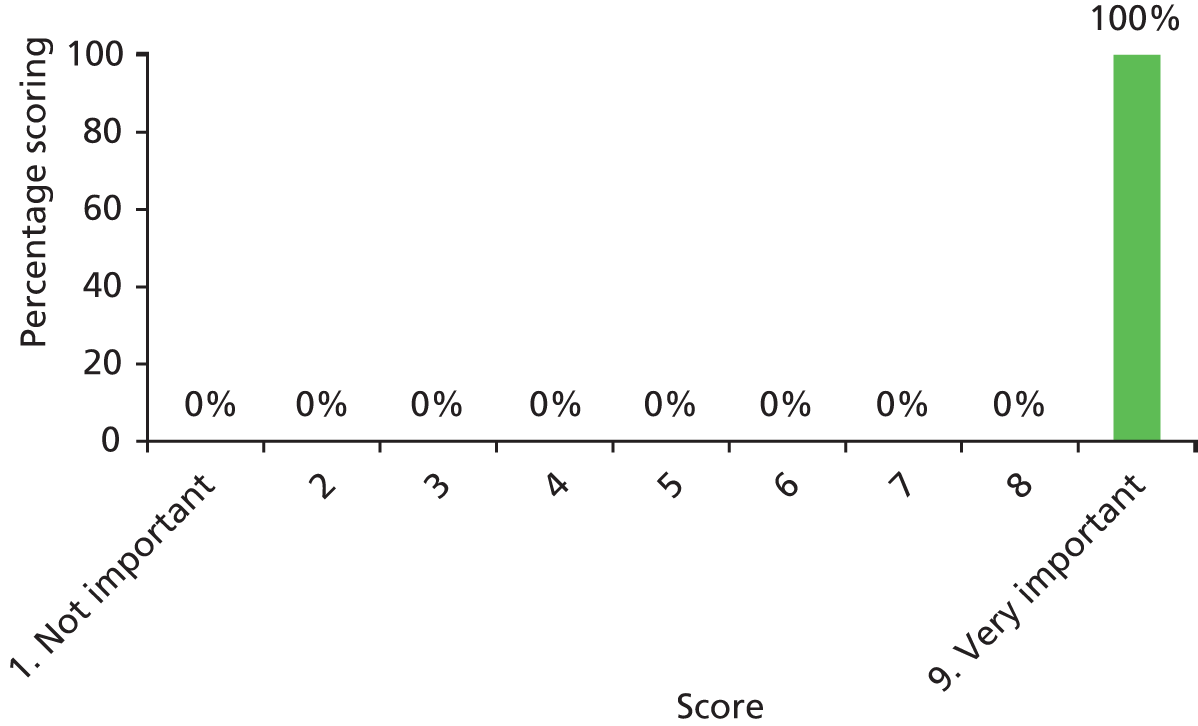
| Outcome number | 7 |
| Outcome name | COM |
| Number of participants scoring 1–9 | 14 |
| Number of participants unable to score | 0 |
FIGURE 73.
Results of scoring: chronic otitis media – your child not having problems inside their ear that can be caused by having glue ear for a long time (more than 3 months).
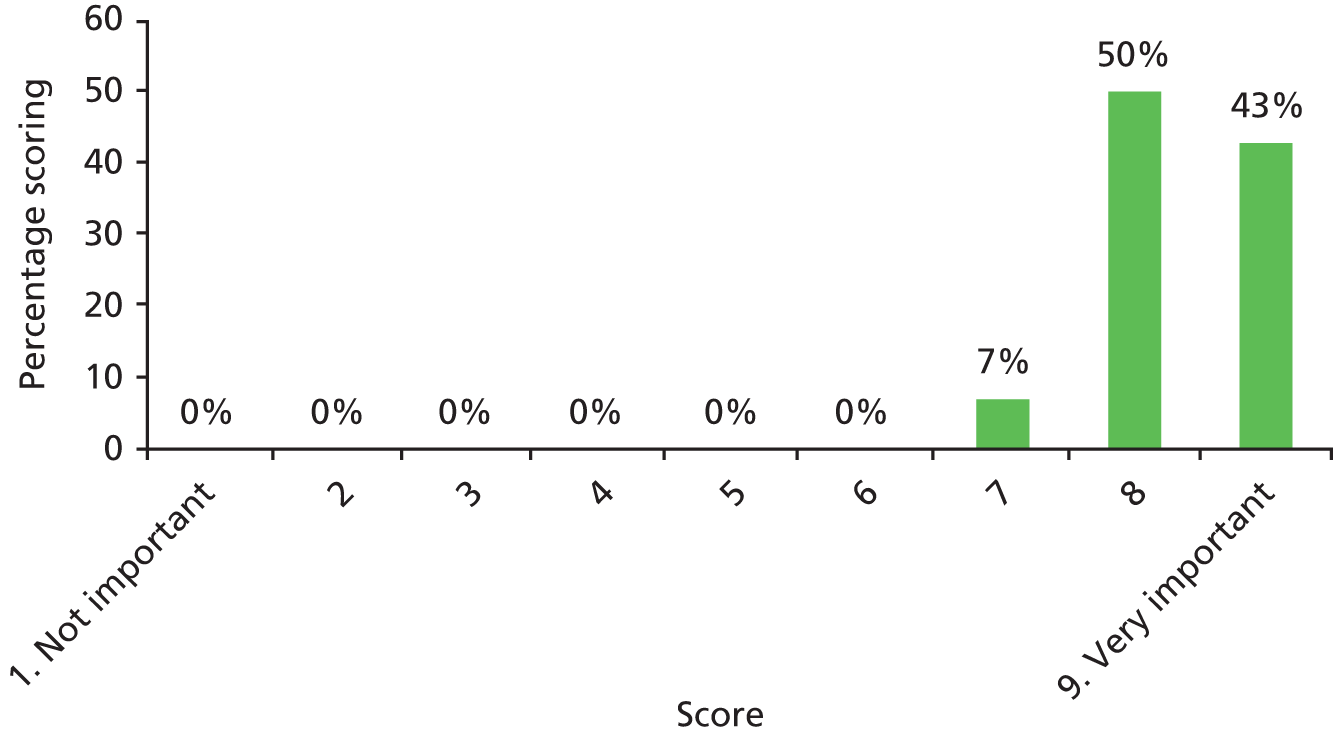
| Outcome number | 26 |
| Outcome name | OME |
| Number of participants scoring 1–9 | 14 |
| Number of participants unable to score | 0 |
FIGURE 74.
Results of scoring: OME – your child not having glue ear.

| Outcome number | 30 |
| Outcome name | Speech intelligibility |
| Number of participants scoring 1–9 | 13* |
| Number of participants unable to score | 0 |
FIGURE 75.
Results of scoring: speech intelligibility – your child being able to say all of their words clearly so that adults and other children can understand what they said.

| Outcome number | 33 |
| Outcome name | Receptive language skills |
| Number of participants scoring 1–9 | 14 |
| Number of participants unable to score | 0 |
FIGURE 76.
Results of scoring: receptive language skills – your child being able to listen and understand what other people say.
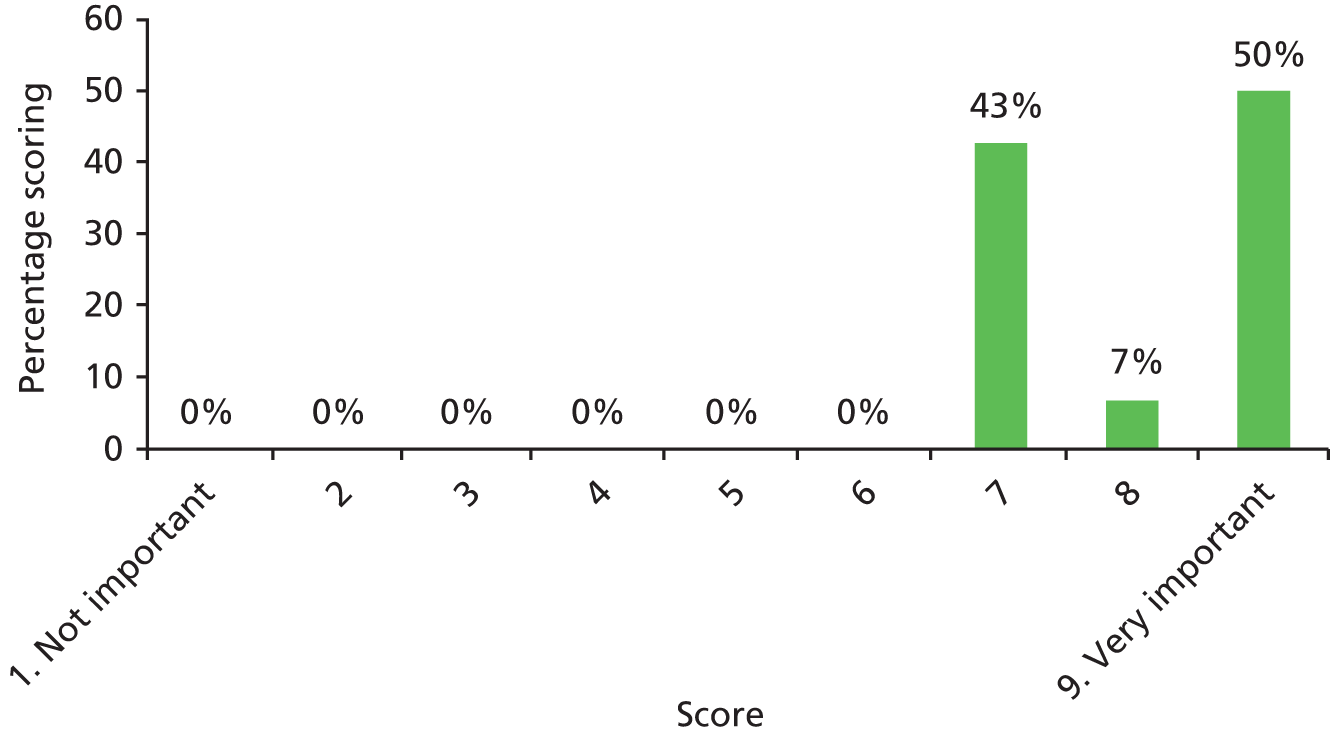
| Outcome number | 34 |
| Outcome name | Speech development |
| Number of stakeholder groups achieving consensus | 6 |
| Number of participants scoring 1–9 | 14 |
| Number of participants unable to score | 0 |
FIGURE 77.
Results of scoring: speech development – your child speaking as well as other children who are the same age.
| Outcome number | 3 |
| Outcome name | Atelectasis |
| Number of participants scoring 1–9 | 11 |
| Number of participants unable to score | 5 |
FIGURE 78.
Results of scoring: atelectasis – your child not having problems inside their ear caused by having lots of ear infections over a long time (more than 3 months).
| Outcome number | 6 |
| Outcome name | Cholesteatoma |
| Number of participants scoring 1–9 | 12 |
| Number of participants unable to score | 2 |
FIGURE 79.
Results of scoring: cholesteatoma – your child not having problems inside their ear caused by bad skin growing behind their ear drum.

| Outcome number | 15 |
| Outcome name | Psychosocial development |
| Number of participants scoring 1–9 | 14 |
| Number of participants unable to score | 0 |
FIGURE 80.
Results of scoring: psychosocial development – how well your child is learning to make friends and speak to new people.
| Outcome number | 25 |
| Outcome name | AOM |
| Number of participants scoring 1–9 | 14 |
| Number of participants unable to score | 0 |
FIGURE 81.
Results of scoring: AOM – your child not having ear infections.

| Outcome number | 28 |
| Outcome name | Consonant production |
| Number of participants scoring 1–9 | 13* |
| Number of participants unable to score | 0 |
FIGURE 82.
Results of scoring: consonant production – your child being able to say all of their words clearly so that adults and other children can understand what they say.

| Outcome number | 37 |
| Outcome name | Necessity to remove VTs |
| Number of participants scoring 1–9 | 12* |
| Number of participants unable to score | 1 |
FIGURE 83.
Results of scoring: necessity to remove VTs – your child not needing another operation to take grommets/VTs out.

| Outcome number | 38 |
| Outcome name | Requirement for repeated VTs |
| Number of participants scoring 1–9 | 11* |
| Number of participants unable to score | 2 |
FIGURE 84.
Results of scoring: requirement for repeated VTs – your child not needing another operation to take grommets/VTs because the old ones fell out.
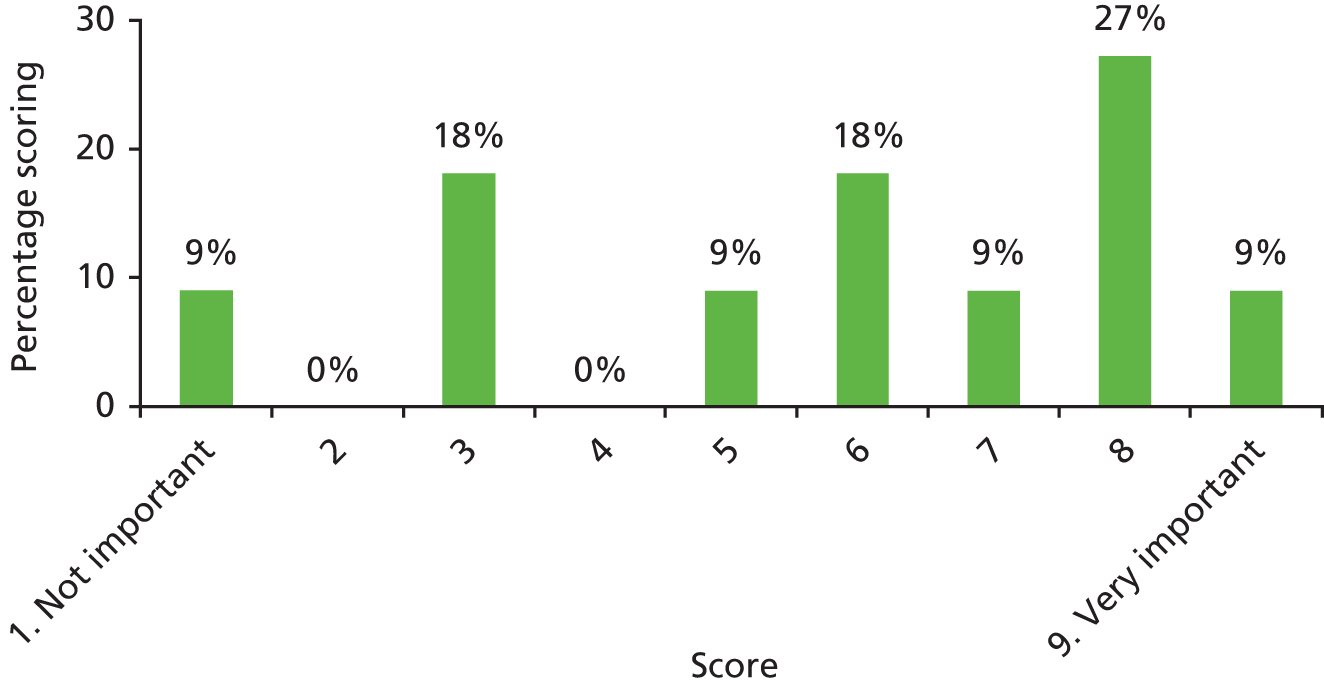
| Outcome number | 41 |
| Outcome name | Parental satisfaction with treatment |
| Number of participants scoring 1–9 | 13* |
| Number of participants unable to score | 0 |
FIGURE 85.
Results of scoring: parental satisfaction with treatment – how well you think that HAs or grommets have improved your child’s hearing.
| Outcome number | 44 |
| Outcome name | Child’s satisfaction with treatment |
| Number of participants scoring 1–9 | 13* |
| Number of participants unable to score | 0 |
FIGURE 86.
Results of scoring: child’s satisfactions with treatment – how much your child thinks that treatment has made them better.
| Outcome number | 45 |
| Outcome name | Child’s perspective of speech |
| Number of participants scoring 1–9 | 13* |
| Number of participants unable to score | 0 |
FIGURE 87.
Results of scoring: child’s perspective of speech – how normal your child thinks they sound when they are talking.
| Outcome number | 4 |
| Outcome name | Persistent tympanic membrane retraction |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 10 |
| Outcome name | Cognitive development |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 11 |
| Outcome name | Developmental progress |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 31 |
| Outcome name | Consonant production – cleft-related speech patterns |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 42 |
| Outcome name | Side effects of treatment |
| Number of participants scoring 1–9 (unable to score) | 7* |
| Number of participants unable to score | 1 |
FIGURE 88.
Results of scoring: side effects of treatment – your child not having problems, that sometimes happen, that are caused by a treatment they have for glue ear.

| Outcome number | 47 |
| Outcome name | Listening skills |
| Number of participants scoring 1–9 | 13* |
| Number of participants unable to score | 0 |
FIGURE 89.
Results of scoring: listening skills (not scored by parents or children).

| Outcome number | 48 |
| Outcome name | Psychosocial well-being |
| Number of participants scoring 1–9 | 13* |
| Number of participants unable to score | 0 |
FIGURE 90.
Results of scoring: psychosocial well-being (not scored by parents or children).
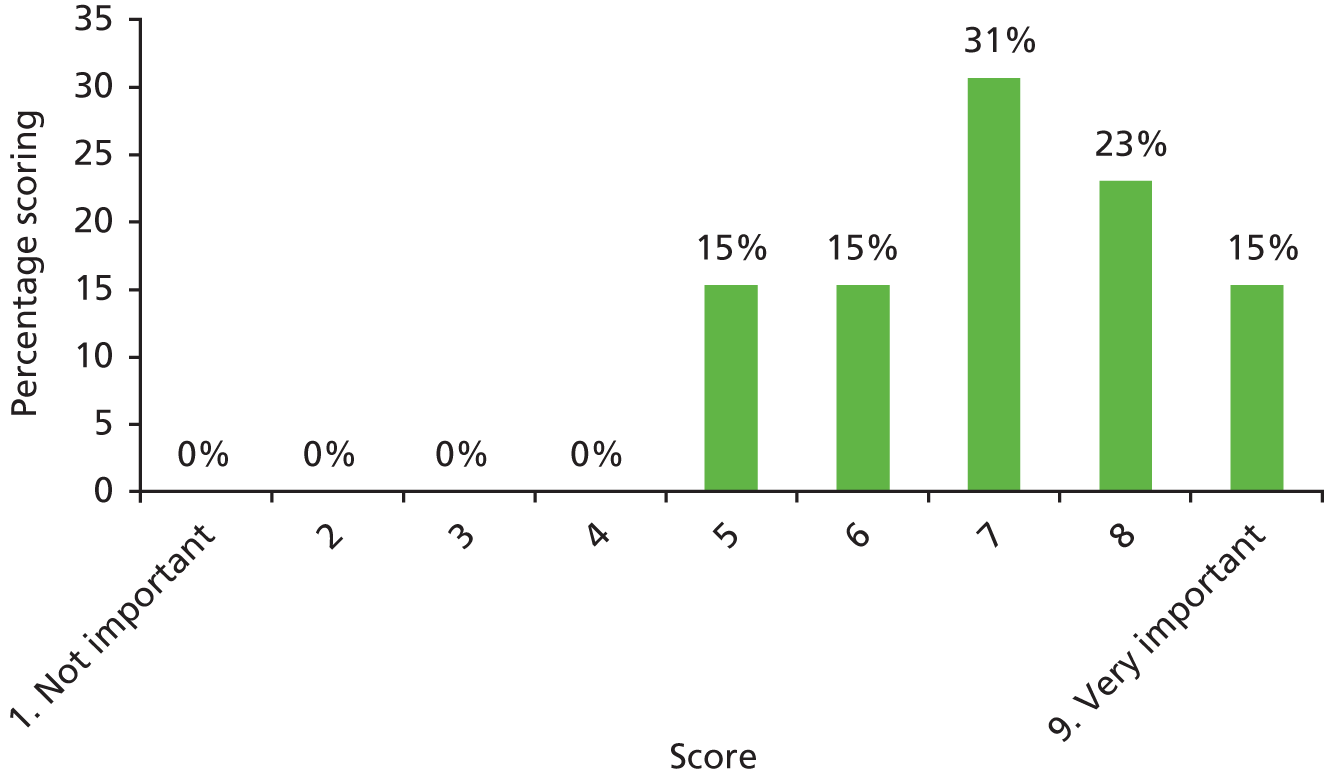
| Outcome number | 5 |
| Outcome name | Tympanosclerosis |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 8 |
| Outcome name | Persistent tympanic membrane perforation |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 17 |
| Outcome name | Otalgia |
| Number of participants scoring 1–9 | 12* |
| Number of participants unable to score | 0 |
FIGURE 91.
Results of scoring: otalgia – how painful your child’s ear is.
| Outcome number | 18 |
| Outcome name | Otorrhoea |
| Number of participants scoring 1–9 | 12* |
| Number of participants unable to score | 0 |
FIGURE 92.
Results of scoring: otorrhoea – your child not having infected liquid (pus) leaking out of their ear.

| Outcome number | 21 |
| Outcome name | Eustachian tube function |
| Number of participants scoring 1–9 | 11* |
| Number of participants unable to score | 1 |
FIGURE 93.
Results of scoring: eustachian tube function – how well a special tube in your child’s ear works, if this doesn’t work properly your child might hear popping and crackling noises.

| Outcome number | 29 |
| Outcome name | Expressive language skills |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 35 |
| Outcome name | Speech signs of velopharyngeal insufficiency |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 36 |
| Outcome name | Early extrusion or blockage of VTs |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 39 |
| Outcome name | Child stress |
| Number of participants scoring 1–9 | 8* |
| Number of participants unable to score | 0 |
FIGURE 94.
Results of scoring: child stress – how often your child feels tense or upset.
| Outcome number | 48 |
| Outcome name | Psychological well-being |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 1 |
| Outcome name | Internalising behaviour |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 2 |
| Outcome name | Externalising behaviour |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome numbers | 9–14 |
| Outcome name | Academic achievement |
| Number of participants scoring 1–9 | 12* |
| Number of participants unable to score | 0 |
FIGURE 95.
Results of scoring: academic achievement – how well your child is doing at school or college.

| Outcome number | 13 |
| Outcome name | Literacy |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 14 |
| Outcome name | Phonological memory |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 19 |
| Outcome name | Tinnitus |
| Number of participants scoring 1–9 | 12* |
| Number of participants unable to score | 0 |
FIGURE 96.
Results of scoring: tinnitus – how much your child hears ringing or buzzing noises.

| Outcome number | 20 |
| Outcome name | Vertigo |
| Number of participants scoring 1–9 | 12* |
| Number of participants unable to score | 0 |
FIGURE 97.
Results of scoring: vertigo – how dizzy your child feels.
| Outcome number | 22 |
| Outcome name | Stapedial reflex |
| Number of participants scoring 1–9 | 10* |
| Number of participants unable to score | 1 |
FIGURE 98.
Results of scoring: stepedial reflex – how well your child’s ear works when it hears a loud noise.

| Outcome number | 32 |
| Outcome name | Parent’s perspective of speech |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 40 |
| Outcome name | Parental stress |
| Number of participants scoring 1–9 | 7* |
| Number of participants unable to score | 0 |
FIGURE 99.
Results of scoring: parental stress – how often you feel tense or upset.
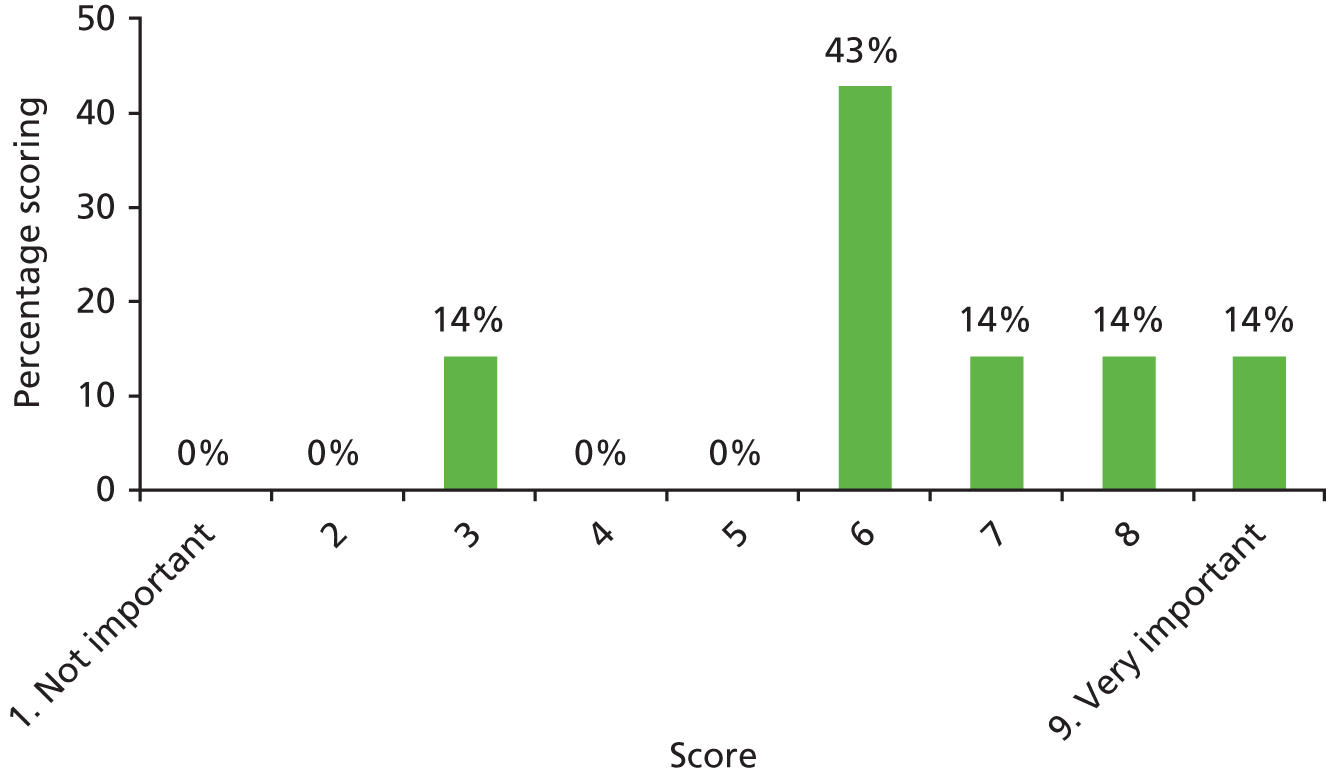
| Outcome number | 27 |
| Outcome name | Temporary tympanic membrane perforation |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Outcome number | 43 |
| Outcome name | Upper respiratory tract infection |
| Number of participants scoring 1–9 | 7* |
| Number of participants unable to score | 0 |
FIGURE 100.
Results of scoring: upper respiratory tract infection – your child not having infections in their ear, nose or throat.
| Outcome number | 24 |
| Outcome name | Rhinitis |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
| Results of discussion |
| Outcome number | 49 |
| Outcome name | Hyperacusis |
| Number of participants scoring 1–9 | – |
| Number of participants unable to score | – |
Appendix 10 Electronic search strategy to identify published model-based economic evaluations
MEDLINE, EMBASE and the American Economic Association’s electronic bibliography
URLs: MEDLINE, www.nlm.nih.gov/bsd/pmresources.html; EMBASE, www.elsevier.com/online-tools/embase; EconLit, www.aeaweb.org/econlit/; accessed via Ovid – https://ovidsp.ovid.com/
Date range searched: MEDLINE, from inception to 16 January 2014; EMBASE, from inception to 16 January 2014; EconLit, from inception to 16 January 2014.
Date search performed: January 2014.
Search strategy
-
otitis media.mp.
-
(glue ear or glue-ear).mp.
-
(middle ear effusion or effusion of the middle ear or middle ear infection or infection of the middle ear or middle ear inflammation or inflammation of the middle ear).mp.
-
or/1-3
-
(cleft$ or cheiloschisis or palatoschisis).mp.
-
4 and 5
-
(economic$ analys$ or economic$ evaluation$).mp.
-
(cost-effective$ or cost effective$).mp.
-
(cost-utility or cost utility).mp.
-
(cost-benefit or cost benefit).mp.
-
(cost-minimi$ or cost minimi$).mp.
-
(cost-consequence$ or cost consequence$).mp.
-
(value-of-information analys$ or value of information analys$).mp.
-
(decision-tree model$ or decision tree model$).mp.
-
(markov model$ or state-transition model$ or state transition model$).mp.
-
simulation model$.mp.
-
(individual-patient simulation or individual patient simulation).mp.
-
(individual patient-level model$ or individual patient level model$).mp.
-
(health-economic$ model$ or health economic$ model$).mp.
-
(decision-analytic$ model$ or decision analytic$ model$).mp.
-
(quality-adjusted life-year$ or quality-adjusted life year$ or QALY$).mp.
-
(disability-adjusted life-year$ or disability-adjusted life year$ or DALY$).mp.
-
or/7-22
-
6 and 23
Appendix 11 Electronic search strategy to identify estimates of impact on health-related quality of life and utility values
MEDLINE, EMBASE and the American Economic Association’s electronic bibliography
URLs: MEDLINE, www.nlm.nih.gov/bsd/pmresources.html; EMBASE, www.elsevier.com/online-tools/embase; EconLit, www.aeaweb.org/econlit/; accessed via Ovid – https://ovidsp.ovid.com/
Date range searched: MEDLINE, from inception to 16 January 2014; EMBASE, from inception to 16 January 2014; EconLit, from inception to 16 January 2014.
Date search performed: January 2014.
Search strategy
-
hearing.mp.
-
otitis media.mp.
-
(glue ear or glue-ear).mp.
-
(middle ear effusion or effusion of the middle ear or middle ear infection or infection of the middle ear or middle ear inflammation or inflammation of the middle ear).mp.
-
or/1-4
-
(utility index or utility-index).mp.
-
(utility score$ or utility-score$).mp.
-
(utility preference$ or utility-preference$).mp.
-
(utility value$ or utility-value$).mp.
-
(utility weight$ or utility-weight$).mp.
-
(health state utility or health-state utility or health-state-utility).mp.
-
(health state utilities or health-state utilities or health-state-utilities).mp.
-
(health utility index or health-utility index or health-utility-index).mp.
-
(health utility or health-utility).mp.
-
(utility measure$ or utility-measure$).mp.
-
(health related utility or health-related utility or health-related-utility).mp.
-
(health related utilities or health-related utilities or health-related-utilities).mp.
-
(time-trade-off or time trade-off or TTO).mp.
-
(standard gamble or standard-gamble or SG).mp.
-
(EQ-5D or EQ5D or EQ 5D or EuroQoL-5D or EuroQoL 5D or EuroQoL-five dimensions or EuroQoL five dimensions).mp.
-
(SF-6D or SF6D or SF 6D or short form-6D or short form 6D or short-form six-dimensions or short form six dimensions or short form-six dimensions).mp.
-
(quality of well being scale or quality of well-being scale or QWB).mp.
-
(quality of well being scale self administered or quality of well-being scale self-administered or QWB-SA).mp.
-
(health utilities index or HUI2 or HUI-2 or HUI-II or HUI3 or HUI-3 or HUI-III).mp.
-
(cost-utility or cost utility).mp.
-
(quality-adjusted life-year$ or quality adjusted life year$ or QALY$).mp.
-
or/6-26
-
5 and 27
-
grommet$.mp.
-
(ventilation tube$ or ventilation-tube$ or tympanostomy tube$ or tympanostomy-tube$).mp.
-
(hearing aid$ or hearing-aid$).mp.
-
(watchful waiting or watchful-waiting or do nothing or do-nothing).mp.
-
(adenoid$ or adenoidectomy).mp.
-
or/29-33
-
28 and 34
-
35 and (child or children).mp.
-
remove duplicates from 36
List of abbreviations
- AOM
- acute otitis media
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CG60
- clinical guideline 60
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CLAPA
- Cleft Lip & Palate Association
- COMET
- Core Outcome Measures in Effectiveness Trials
- COS
- core outcome set
- CP
- cleft palate
- CRANE
- Craniofacial Anomalies Network
- CYPC
- Children and Young Persons Council
- DN
- do nothing
- EconLit
- American Economic Association’s electronic bibliography
- ENT
- ear, nose and throat
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- GP
- general practitioner
- GRADE
- Grading of Recommendations, Assessment, Development and Evaluation
- HA
- hearing aid
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- mOMEnt
- management of Otitis Media with Effusion in children with cleft palate
- NB
- net benefit
- NICE
- National Institute for Health and Care Excellence
- OME
- otitis media with effusion
- pEVPI
- population-level expected value of perfect information
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- SAG
- Study Advisory Group
- SD
- standard deviation
- SMG
- Study Management Group
- SSC
- Study Steering Committee
- VOI
- value of information
- VT
- ventilation tube
- WTP
- willingness to pay