Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 12/69/01. The protocol was agreed in September 2013. The assessment report began editorial review in April 2014 and was accepted for publication in September 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Picot et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of underlying health problem
Breast cancer is the most common cancer in women in England, with 41,523 new diagnoses in 2011. 1 It accounts for about one-third of all cancers in women2 but is rare in men, accounting for < 0.25% of cancers in 2011 (303 new diagnoses in England in 2011). 1 Consequently, the primary focus of this report is breast cancer in women and, when data are presented for men, this is clearly indicated.
Breast cancer aetiology
Breast cancer, in common with all other cancers, is caused by deoxyribonucleic acid (DNA) mutations that disrupt the normal maintenance of cellular identity, growth and differentiation. 3 The majority of breast and other cancers develop from somatic mutations3,4 resulting from errors in processes such as DNA replication, DNA modification or DNA repair,4,5 which in turn may be influenced by environmental and/or dietary factors. 6 A small proportion of cancer types arise from inheritable single-gene disorders,3 for example BRCA1 (breast cancer 1) and BRCA2 (breast cancer 2) are genes associated with inheritable breast cancer. 4,7–9
There are two main forms of breast cancer: non-invasive, in which the cancer cells have not spread; and invasive, in which the breast cancer cells can potentially spread to the surrounding breast tissue or beyond. Approximately 10% of newly diagnosed breast cancer cases are non-invasive, the majority (approximately 90%) being ductal carcinoma in situ (DCIS). 10 In DCIS, cancer cells have developed inside milk ducts but have not yet developed the ability to spread beyond the ducts. DCIS is usually identified by mammography as it rarely presents as a lump. The remaining 90% of newly diagnosed breast cancer cases are various types of invasive breast cancer.
When breast cancer is diagnosed, information is gathered to describe and classify it according to a variety of characteristics. Much of the information required can be obtained only from samples taken during surgical removal of the primary tumour. Key aspects include:11
-
histological type (e.g. invasive ductal carcinoma, invasive lobular carcinoma)
-
histological grade, ranging from low (generally slow growing) to high (generally fast growing)
-
stage, based on the tumour node metastasis (TNM) classification (Tables 1 and 2)
-
oestrogen receptor (ER) alpha status
-
human epidermal growth factor receptor-2 (HER-2) status
-
DNA profile.
| STAGE | TNM (see Table 2) |
|---|---|
| Stage 0 | Tisa N0 M0 |
| Stage I | T1 N0 M0 |
| Stage IIa | T1 N1 M0 or T2 N0 M0 |
| Stage IIb | T2 N1 M0 or T3 N0 M0 |
| Stage IIIa | T2 N2 M0 or T3 N1 M0 or T3 N2 M0 |
| Stage IIIb | T4 N0 M0 or T4 N1 M0 or T4 N2 M0 |
| Stage IIIc | Any T N3 M0 |
| Stage IV | Any T any N M1 |
| Tumour stage | Nodal stage | Distant metastasis | |||
|---|---|---|---|---|---|
| Tisa | Tumour in situ | N0 | No regional lymph node metastasis | M0 | No distant metastasis |
| T1 | Tumour < 2 cm in diameter | N1 | Mobile regional lymph node metastasis | M1 | Distant metastasis |
| T2 | Tumour 2–5 cm in diameter | N2 | Fixed regional lymph node metastasis | ||
| T3 | Tumour > 5 cm in diameter | N3 | Supraclavicular lymph node metastasis | ||
| T4 | Tumour fixed to skin/chest wall or inflammatory cancer | ||||
This information is essential for deciding what local and systemic treatments may be required and provides information about prognosis. The focus of this assessment is the treatment of early breast cancer; however, it should be noted that there is no internationally agreed single definition of early breast cancer (e.g. in terms of TNM stage). Typically, however, early breast cancer would be classified as TNM stage I or II (either IIa or IIb), with potentially some stage III tumours (those for which treatment could be curative).
The aim of treatment for early breast cancer is to provide a cure. As already stated, there are two major categories of early breast cancer: non-invasive (in situ) disease (predominantly in the form of DCIS) and invasive cancer. 11 For invasive cancer to be categorised as early breast cancer, the tumour should not have spread beyond the breast or the lymph nodes (which remain mobile) in the armpit ipsilateral to (on the same side as) the affected breast. 13 Once an invasive cancer has spread to distant sites (which may occur after initial treatment with curative intent), it is no longer curable, but can be treated to control symptoms.
Breast cancer epidemiology
In England, in 2011, the age-standardised rates of breast cancer incidence per 100,000 of the population were 124.8 for women and 0.9 for men. 1 For the period 2008–10 the age-standardised rate for women in England was 125.7 [95% confidence interval (CI) 125.0 to 126.4]. 14 The strongest risk factor for breast cancer is increasing age and, consequently, over 80% of new diagnoses of breast cancer in England are in women aged > 50 years1 and in men aged > 60 years. 1 Other important risk factors include obesity, alcohol consumption and lack of physical activity, which are estimated to be linked to about 18.5% of UK female breast cancer cases. 15
There were 9702 deaths of women and 64 deaths of men from breast cancer in England in 2011. 16 The UK age-standardised mortality rate from breast cancer per 100,000 women in 2008–10 was 25.3 (95% CI 25.0 to 25.6 per 100,000 women). 14 For women diagnosed with breast cancer during 2004–6 and followed up to 2011, the age-standardised 1-year survival rate for all breast cancers was 94.7% and the 5-year survival was 83.3%. 17 Between 2002 and 2006, a statistically significant annual increase in 1-year survival of 0.3% and in 5-year survival of 0.9% was observed. 17 The rise in survival estimates has been due to earlier detection and improved treatment of breast cancer in women. 2 An analysis of survival by stage at diagnosis for women in the UK diagnosed with invasive breast cancer (DCIS was excluded) during 2000–718 reported 1-year and 3-year net survival as shown in Table 3.
| TNM stage | 1-year net survival (%) (95% CI) | 3-year net survival (%) (95% CI) |
|---|---|---|
| TNM stage 1 | 100 (100 to 100) | 99.3 (99.2 to 99.4) |
| TNM stage 2 | 99.2 (99.2 to 99.3) | 92.4 (92.2 to 92.7) |
| TNM stage 3 | 90.9 (90.5 to 91.4) | 70.7 (69.9 to 71.5) |
| TNM stage 4 | 53.0 (52.0 to 54.0) | 27.9 (26.9 to 28.9) |
Breast cancer diagnosis
In England, the main routes to diagnosis for the majority of breast cancer cases are via the NHS Breast Cancer Screening Programme or urgent (2-week wait) referrals from a general practitioner (GP) due to a suspicion of cancer. The Breast Cancer Screening Programme targets women aged 50–69 years (with extension from 47 years to 73 years ongoing, and expected to be completed after 2016). In 2006–8, just over 50% of breast cancer cases in the 50–69 years age group were diagnosed through screening, whereas, in other age groups (< 50 years and ≥ 70 years), over 50% of cases were diagnosed through the urgent GP referral route. 19 Breast cancer screening aims to detect cancers at an early stage when they are too small to cause changes to the breast that can be observed or felt. In England in 2011–12, 40.7% (6403) of all the breast cancers detected by screening were invasive but small (< 15 mm in diameter). 20 In the case of breast cancers detected via routes other than screening, there are no regularly published data on stage of breast cancer at diagnosis;21 however, evidence suggests that the majority (at least 80%) of women are diagnosed with early disease (stage I or stage II) whatever their route to diagnosis. 22
The 2009 National Institute for Health and Care Excellence (NICE) guideline Early and Locally Advanced Breast Cancer: Diagnosis and Treatment11 provides recommendations for breast cancer diagnosis. Diagnosis is made after triple assessment consisting of a clinical assessment, mammography and/or ultrasound imaging, and core biopsy and/or fine-needle aspiration cytology. 11 A multidisciplinary team should review and discuss the test results and, if a cancer diagnosis is pathologically confirmed, suggest a treatment plan.
Breast cancer natural history and prognosis
The natural history of breast cancer is variable and incompletely understood. 23 If left untreated, a typical invasive breast cancer might progress in the following manner. Initially, the breast cancer cells multiply, thereby increasing the size of the tumour;24 as the tumour proliferates, the risk that metastatic cells will be generated increases. 25 A key route for metastatic spread of breast cancer cells is via the lymphatic system. If a breast cancer spreads, the first place it spreads to is often the first lymph node (or nodes) receiving direct lymphatic drainage from the tumour;24,25 this lymph node is called the sentinel lymph node. 26 The tumour can also spread to more distant lymph nodes and to systemic sites via the bloodstream (e.g. bone, lung, liver, brain). It is also possible for tumour cells to metastasise via the vascular system directly to systemic sites;25 however, not all breast cancers metastasise. Evidence from screening studies suggests that some screen-detected breast cancers may regress spontaneously27 and natural history may vary according to a variety of factors, for example genotype,28 hormone receptor status29 and race. 30
The heterogeneous nature of breast cancer natural history has an impact when trying to provide a prognosis and tools have been developed which aim to predict invasive breast cancer outcome. For example, the Nottingham Prognostic Index (NPI)31 (Table 4) is a tool that combines information on the size of the tumour, the number of lymph nodes involved and the histological grade to produce an overall score, with a higher score indicating a worse prognosis. Other models have been developed which aim to more accurately predict outcome by including alternative indicators and/or more explanatory factors, for example Predict33 and the Galway Index of Survival. 34 The program Adjuvant! enables prognostic estimates of outcome either with or without therapy to be produced based on estimates of individual patient prognosis and data on the efficacy of a range of adjuvant therapy options and is available online (www.adjuvantonline.com/index.jsp). 35
| NPI = (T × 0.2) + L + G | ||
|---|---|---|
| Score | Prognostic group | 10-year survivala |
| 2.08–2.4 | Excellent | 96% |
| 2.42 to ≤ 3.4 | Good | 93% |
| 3.42 to ≤ 4.4 | Moderate I | 81% |
| 4.42 to ≤ 5.4 | Moderate II | 74% |
| 5.42 to ≤ 6.4 | Poor | 50% |
| 6.5 to 6.8 | Very poor | 38% |
Impact of breast cancer
Psychological distress, chiefly in the form of anxiety, may be experienced by women from the initial diagnostic procedures for a suspected breast cancer36 through all stages of treatment and beyond. 37,38 In addition to psychological aspects, women may experience a range of physical problems, for example arm and breast symptoms and/or lymphoedema39,40 and fatigue. 40
An analysis of patients’ free-text comments from the Cancer Patient-Reported Outcome Measures (PROMs) Survey in England41 identified a range of issues that may affect patients diagnosed with breast cancer. These included poor body image following breast surgery, ongoing problems following surgery such as pain and lymphoedema and problems associated with other non-surgical treatments, for example hot flushes related to hormone treatments, burns following radiotherapy and neuropathy during and following chemotherapy. In addition, some patients found that existing comorbidities such as arthritis and osteoporosis were exacerbated by their treatment. Some survey respondents highlighted that, during and/or following treatment, a lack of energy affected their everyday life, and some found that they had cognitive problems and memory loss. Both during and after treatment some patients suffered from feelings of depression, loneliness and isolation. A continuing fear of recurrence was also an issue for some. Other problems highlighted by the survey were social and financial issues, for example relating to employment and obtaining insurance.
The impact of breast cancer for the NHS is likely to increase across all facets of the breast cancer care pathway in the future. This is because the population of England is growing in both size and age, which will lead to increasing rates of breast cancer given that the strongest risk factor for breast cancer is age.
Current service provision
Surgery is usually the first treatment option for early breast cancer (DCIS and invasive breast cancer). Pre-operative assessment of the breast and axilla determines the size of the primary tumour relevant to the volume of breast and this information is used to decide whether or not wide local excision (WLE) of the tumour (‘lumpectomy’) is possible, allowing breast-conserving surgery (BCS) instead of mastectomy (removal of the breast). Patients who have a mastectomy can have immediate breast reconstruction (carried out at the same time as the mastectomy) or delayed breast reconstruction.
Pre-operative assessment of the axilla includes ultrasound to determine whether or not morphologically abnormal lymph nodes are present. If abnormal lymph nodes are identified, ultrasound-guided needle biopsy is offered to obtain a tissue sample for testing. If there is no evidence of lymph node involvement on ultrasound, or the ultrasound-guided needle biopsy outcome is negative, lymph node clearance is not performed during BCS. The NICE guideline Early and Locally Advanced Breast Cancer: Diagnosis and Treatment11 recommends, instead, sentinel lymph node biopsy (SLNB) as the preferred technique (SLNB was undertaken for 84% of invasive breast cancers identified during breast cancer screening between April 2011 and March 201242). The tissue from SLNB has typically been analysed using post-operative histopathology with a 5–15-day wait for results. If macrometastases (tumour deposits with at least one dimension over 2 mm) are identified, a second operation takes place to remove the remaining axillary lymph nodes (axillary lymph node dissection). 43 In August 2013, NICE recommended whole lymph node analysis using the RD-100i one-step nucleic acid amplification (OSNA) system as an option for detecting sentinel lymph node metastases. This analysis is carried out during breast surgery, takes approximately 30 to 45 minutes and means that, if the result is positive for metastases (cytokeratin-19 gene expression identified which is a marker associated with breast cancer), axillary lymph node dissection can be completed during the initial surgery, removing the need for a second operation. 43 The advisory group for this assessment indicated that there are 22 RD-100i OSNA systems currently in use in the UK and use is increasing.
After surgical removal of the primary tumour (and axillary lymph nodes if indicated), the information on prognostic and predictive factors obtained by histological examination, the outcome of tests for ER and HER-2 status, and other patient and tumour characteristics are used by the breast cancer multidisciplinary team to consider options for adjuvant therapy for all patients with early breast cancer. Decisions regarding adjuvant therapy are made following discussion with the patient. 44 Adjuvant chemotherapy or radiotherapy should start as soon as clinically possibly and within 31 days of being ‘fit to treat’ after surgery. 45,46
Data from the NHS Breast Screening Programme Audit 2011–1242 indicate that, in practice, some trusts are struggling to meet this 31-day standard for radiotherapy. Overall, 57% of women received radiotherapy within 60 days and 92% within 90 days of their final surgery. 42 Advice from the advisory group for this assessment suggested that the figures for symptomatic cancer (i.e. not screen detected) were likely to be similar and that meeting the 31-day goal for adjuvant chemotherapy may also be difficult.
The range of recommended breast cancer treatment options described by the 2009 NICE guideline Early and Locally Advanced Breast Cancer: Diagnosis and Treatment11 are summarised in Table 5.
| Adjuvant treatment | Treatment options | Comments |
|---|---|---|
| Radiotherapy | Whole-breast radiotherapy following BCS | |
| Post-mastectomy radiotherapy to chest wall | For example, if at high risk of local recurrence | |
| Boost to tumour bed following BCS | For example, if at high risk of local recurrence | |
| Radiotherapy to nodal areas | For example, if four or more involved axillary lymph nodes | |
| Systemic therapy for metastatic disease | Endocrine therapy | For example, tamoxifen or aromatase inhibitor for ER-positive tumours only |
| Chemotherapy | For example, anthracycline-containing regimens, docetaxel | |
| Biological therapy | For example, trastuzumab (Herceptin®, Roche) | |
| May need assessment and treatment for bone loss | ||
| Primary systemic therapy | ||
| Chemotherapy | Before surgery, e.g. to shrink tumour before surgery, to observe response in the primary tumour before its surgical removal | |
| Endocrine therapy | ||
After BCS, whole-breast external beam radiotherapy (WB-EBRT) substantially reduces the risk of recurrence (15.7% absolute reduction in 10-year risk of any first recurrence) and moderately reduces the risk of breast cancer death (3.8% absolute reduction in 15-year risk of breast cancer death) for patients with early invasive breast cancer. 47 Therefore, post-operative WB-EBRT is the standard of care for all patients with early invasive breast cancer after breast-conserving therapy (as per the 2009 NICE guideline11). WB-EBRT works by directing a beam, or multiple beams, of radiation through the skin directly at the tumour and surrounding cancer cells to destroy them. The radiation beam is generated by an instrument, known as a linear accelerator, which is capable of producing high-energy X-rays or electrons. The most common types of external radiotherapy use photon beams (as X-rays). 48 From the patient’s perspective, external radiotherapy is similar to having an X-ray, only the radiation is more intense. In the UK, a hypofractionated regimen is standard practice, with NICE guidelines recommending that patients with early invasive breast cancer who have undergone BCS receive 40 Gy in 15 fractions. 11 The 15 fractions are typically delivered to patients by hospital radiotherapy departments at short (10–15-minute) treatment sessions each day, Monday to Friday, with a rest at the weekends. The course is usually given for 3 weeks, but may last longer. This course of radiotherapy can be followed by a ‘boost’ dose (e.g. 12 Gy in four fractions, 10 Gy in five fractions or 16 Gy in eight fractions) to the tumour bed over a further 1–2 weeks in patients considered to be at a higher risk of local recurrence (e.g. aged < 40 years, grade 3 disease and lymph node positive). 11 In many other parts of the world standard practice for whole-breast radiotherapy is 50 Gy in 25 fractions given daily (Monday to Friday) over 5 weeks. 49 For patients with apparently localised DCIS treated with BCS, there is a 25% risk of a local recurrence over 10 years if there is no further therapy and half of the recurrences will be of invasive cancer. 11 Unfortunately, there is no reliable way to identify the patients who will not be at risk of local recurrence. 50 Therefore, adjuvant radiotherapy should be offered to all patients with DCIS following BCS alongside a discussion of the potential benefits and risks. 11
The treatment schedule described above can be difficult for some women to undertake (e.g. if they live a long way from their nearest treatment centre, if they have caring responsibilities, if they are elderly and/or disabled). Whole-breast radiotherapy may also be associated with short-term adverse effects (e.g. skin soreness/redness, tiredness, nausea) as well as long-term adverse effects (e.g. changes to breast size and texture/feel, lung or heart problems), and can be impossible to deliver effectively in patients who are unable to lie flat or in those unable to raise the shoulder on the side receiving treatment.
When chemotherapy is indicated, WB-EBRT is nearly always given when chemotherapy has been completed and after a gap of 2–3 weeks that minimises overlapping and/or enhancing toxicities. For patients who require biological therapy or endocrine therapy, this is typically administered concurrently with WB-EBRT.
Radiotherapy is viewed as a cost-effective treatment. The total spend on radiotherapy (not limited to breast cancer) has been estimated to constitute just 5% of the estimated total NHS spend on cancer care. 45
Description of technology under assessment
The INTRABEAM® Photon Radiotherapy System (Carl Zeiss, Oberkochen, Germany) has a miniature, electronic, high-dose-rate, and low-energy X-ray source (XRS) which is used to deposit high-dose radiation directly to a tumour or tumour bed. 51 In the USA, INTRABEAM gained US Food and Drug Administration approval in 1997, and in Europe it was awarded Conformité Européenne (CE) certification in 1999. 52 As INTRABEAM uses a low-energy XRS, the system does not have to be contained within the kind of specially designed room that is required for high-energy radiation sources (e.g. linear accelerators). 51 This means that INTRABEAM can be used to deliver intraoperative radiation therapy (IORT) in an ordinary operating theatre at the same time as surgery. In addition, the system is mobile so it can be moved with care between different operating theatres.
The XRS component of the device has a 10-cm-long probe51 and one of a variety of applicators of different shapes and sizes can be attached to this depending on the anatomical site being treated. For breast cancer, a set of eight reusable spherical applicators is available with diameters from 1.5 to 5.0 cm. 52 An applicator is chosen for irradiating the tumour bed after lumpectomy depending on the size of the resection cavity. The INTRABEAM technical specifications state that the dose is usually entered by one person (usually a physicist) and must be checked by a doctor, who verifies the dose planning and confirms it by entering a password. 52 The tissue adjacent to the resection cavity is then irradiated by the INTRABEAM device for typically 20–30 minutes. 51 A characteristic of the low-energy X-rays produced by the INTRABEAM device is that the maximum dose of radiotherapy is delivered to the tissues at the surface of the cavity, but, because the dose attenuates steeply as tissue depth increases, peripheral healthy tissue is spared. 53 As a result, the surface of the tumour bed typically receives 20 Gy in this single-fraction treatment. 53 After this treatment the incision is closed. The design of the INTRABEAM equipment ensures that the tissue most at risk of developing a local recurrence, that is, comprising the wall of the resection cavity adjacent to the resected tumour, receives the largest dose of irradiation.
The INTRABEAM device has been used in patients with early breast cancer to deliver IORT to the cavity wall resulting from lumpectomy for treatment of the primary tumour. Patients at low risk of recurrence do not receive any further local treatment. Patients with a higher risk of recurrence (e.g. histopathology showing invasive lobular carcinoma, extensive intraductal component, grade 3, node involvement, close margins) may go on to receive an additional course of WB-EBRT to the whole breast but without a tumour bed boost because the INTRABEAM device has already delivered therapy directly to the tumour bed. Other adjuvant treatments, for example endocrine therapy, chemotherapy, biological therapy, will also be given if indicated.
Six centres in the UK (four in London, one in Winchester and one in Dundee) are known to have used the INTRABEAM device to treat breast cancer but in the absence of NICE guidance, the equipment has not entered into routine use. In addition to these six centres, information received from the advisory group for this assessment suggests that Liverpool and Harlow have purchased the equipment for neurosurgical and breast use, respectively. Ten other NHS trusts have expressed an interest in purchasing the device and private providers may also have or be intending to purchase the INTRABEAM device.
The device manufacturer has indicated that the cost of the INTRABEAM device in the UK is £435,000. This cost includes a set of spherical applicators, each of which would need to be replaced, at a cost of £3170 per applicator, after 100 treatments. A fully inclusive service contract for maintenance of the device would cost £35,000 annually. Additionally, there are associated consumable costs, for example radiation protection shields (pack of 10 costs £1041, sufficient for five treatments), and sterile plastic drapes (pack of five £95.00, sufficient for five treatments).
Chapter 2 Definition of the decision problem
Decision problem
In line with the scope54 of the NICE appraisal, this assessment will consider the intraoperative use of the INTRABEAM Radiotherapy System as an alternative to post-operative WB-EBRT to the whole breast, and as a boost during BCS before WB-EBRT is provided. Its use for local recurrence will not be considered.
The comparator for this review is WB-EBRT delivered by linear accelerator. As already noted, post-operative WB-EBRT is the standard of care for all patients with early invasive breast cancer after breast-conserving therapy (as per the 2009 NICE guideline11).
The population of patients included within this assessment is people with early operable breast cancer who are eligible for WLE of the tumour followed by whole-breast radiotherapy. If the cancer has spread to the regional lymph nodes, the metastasis remains mobile (not fixed to other structures). Although there is no single definition of early breast cancer, a common definition is disease that is confined to the breast and draining nodes for which treatment could be curative. The majority of people with early breast cancer are, therefore, likely to have tumours classified as TNM stage I or II (either IIa or IIb) but some with stage III tumours could also be considered to have early breast cancer using this definition. People with a local recurrence are excluded from the assessment. The NICE scope that underpins this assessment did not identify any relevant subgroups for consideration.
As specified in the NICE scope,54 the following outcome measures are included in the decision problem:
-
overall survival
-
disease-free survival
-
ipsilateral local recurrence
-
adverse effects of treatment
-
health-related quality of life (HRQoL).
Overall aims and objectives of assessment
The aim of this assessment is to assess the clinical effectiveness and cost-effectiveness of the INTRABEAM Photon Radiotherapy System for the adjuvant treatment of early breast cancer during surgical removal of the tumour.
Other intraoperative techniques were not included as comparators in the NICE scope because they are not currently in use in clinical practice. These techniques were also not included as interventions alongside the INTRABEAM Photon Radiotherapy System because their use was not considered sufficiently comparable.
Chapter 3 Methods
The a priori methods for systematically reviewing the evidence of clinical effectiveness and cost-effectiveness are described in the research protocol, which was sent to our expert advisory group for comment. None of the comments we received identified specific problems with the methods of the review which has been undertaken following the general principles outlined in Systematic Reviews: CRD’s Guidance For Undertaking Reviews In Health Care. 55 The methods outlined in the protocol are briefly summarised below.
Identification of studies
The search strategies were developed and tested by an experienced information scientist. The strategies were designed to identify all relevant clinical effectiveness studies of the INTRABEAM Photon Radiotherapy System for people with early operable breast cancer. Separate searches were conducted for the economic evaluation to identify studies of cost-effectiveness and HRQoL.
The following databases were searched for published studies and ongoing research from inception to March 2014: The Cochrane Library [including the Cochrane Database of Systematic Reviews (CDSR), the Cochrane Central Register of Controlled Trials, Centre for Reviews and Dissemination (CRD) (University of York) Database of Abstracts of Reviews of Effectiveness (DARE), the NHS Economic Evaluation Database (NHS EED), the Health Technology Assessment (HTA) database], MEDLINE (via Ovid), EMBASE (via Ovid), MEDLINE In-Process & Other Non-Indexed Citations (via Ovid), Web of Science with Conference Proceedings, Science Citation Index Expanded (SCIE) and Conference Proceedings Citation Index (CPCI) – Science (ISI Web of Knowledge), Bioscience Information Service (BIOSIS) Previews (ISI Web of Knowledge), Zetoc (Mimas), National Institute for Health Research (NIHR) – Clinical Research Network Portfolio, ClinicalTrials.gov, Current Controlled Trials, and World Health Organization International Clinical Trials Registry Platform (WHO ICTRP). Searches were limited to randomised controlled trials (RCTs) and controlled clinical trials (CCTs) for the assessment of clinical effectiveness. Although searches were not restricted by language, only full texts of English-language articles were retrieved during the study selection process.
Bibliographies of included articles, systematic reviews and clinical guidelines were also searched. The manufacturer’s submission (MS) to NICE was searched for any additional studies that met the inclusion criteria. Members of our advisory group were asked to identify additional published and unpublished evidence. Further details including search dates for each database and an example search strategy can be found in Appendix 1.
Inclusion and exclusion criteria
The inclusion and exclusion criteria were derived from the final scope54 issued by NICE.
Study design
-
For the systematic review of clinical effectiveness, RCTs were eligible for inclusion. If the data from available RCTs were incomplete (e.g. absence of data on outcomes of interest), evidence from good-quality CCTs was eligible for consideration.
-
For the systematic review of cost-effectiveness, full economic evaluations (cost-effectiveness, cost–utility or cost–benefit analyses) reporting on measures of both costs and consequences were eligible for inclusion.
-
For the systematic review of HRQoL, primary research studies based in the UK, Europe, North America and Australasia were eligible for inclusion.
-
Abstracts or conference presentations of studies were eligible for inclusion only if sufficient details were presented to allow an appraisal of the methodology and the assessment of results to be undertaken.
-
Case series, case studies, narrative reviews, editorials and opinions were excluded, as were non-English-language studies. Systematic reviews and clinical guidelines were used only as a source of references.
Intervention(s)
-
INTRABEAM Photon Radiotherapy System with or without post-operative WB-EBRT.
Comparator(s)
-
External beam radiotherapy delivered by a linear accelerator.
Population
-
For the systematic review of clinical effectiveness, people with early operable breast cancer (as defined by the trials).
-
For the systematic review of HRQoL, people with breast cancer (not limited to early-stage breast cancer).
-
People with a local recurrence were excluded.
Outcomes
Studies were included if they reported on one or more of the following outcomes:
-
overall survival
-
disease-free survival
-
ipsilateral local recurrence
-
adverse effects of treatment
-
HRQoL
-
cost-effectiveness [expressed in natural units such as life-years gained (cost-effectiveness analysis), quality-adjusted life-years (QALYs) (cost–utility analysis), or in monetary units (cost–benefit analysis)].
Inclusion screening process
Studies were selected for inclusion through a two-stage process. Literature search results (titles and, if present, abstracts) identified by the search strategy were screened independently by two reviewers to identify all citations that potentially met the inclusion/exclusion criteria detailed above. Full manuscripts of selected citations that appeared potentially relevant were obtained. These were assessed by one reviewer against the inclusion/exclusion criteria using a flow chart and checked independently by a second reviewer before a final decision regarding inclusion was agreed. At each stage any disagreements were resolved by discussion, with the involvement of a third reviewer when necessary.
Data extraction process
Data were extracted by one reviewer using a standardised data extraction form and each data extraction was checked for accuracy by a second reviewer. Discrepancies in the extracted data were resolved by discussion, with involvement of a third reviewer when necessary.
Critical appraisal strategy
The risk of bias of the included clinical effectiveness studies was assessed using criteria devised by the Cochrane Collaboration. 56 Criteria were applied by one reviewer and checked by a second reviewer with any disagreements resolved by consensus and involvement of a third reviewer where necessary. The methodological quality of included cost-effectiveness studies was assessed using criteria adapted by the review authors from checklists for appraising economic evaluations by Drummond et al. 57 The economic evaluation included in the MS [Multiple Technology Appraisal (MTA) INTRABEAM Photon Radiosurgery System for the Adjuvant Treatment of Early Breast Cancer. Carl Zeiss, UK. 2014] to NICE was assessed using criteria adapted by the review authors from checklists for appraising economic evaluations by Drummond et al. ,57 supplemented with additional criteria for critical appraisal of model-based evaluations by Philips et al. 58 For the systematic review of HRQoL, the included studies were assessed against a critical appraisal checklist adapted by the review authors from common themes found in other published assessment forms for HRQoL studies. 59–62
Method of data synthesis
Clinical effectiveness, cost-effectiveness and HRQoL data were synthesised through narrative reviews that included critical appraisal of study methods, critical assessment of data used in any economic models and tabulation of the results of included studies.
Chapter 4 Clinical effectiveness
Results
Quantity and quality of research available
Titles and, where available, abstracts of a total of 655 citations were screened and full copies of 44 references were obtained. Of these, 38 were excluded after inspection of the full article (see Appendix 2). The most common primary reason for exclusion was that the reference was an abstract containing insufficient details to allow appraisal of methodology and/or results (n = 25); a further eight records were excluded chiefly because the outcome was not relevant to the review, three records were excluded chiefly because of an incorrect intervention, one record was excluded on the basis of study design and one record was excluded because it related to an ongoing study (see Chapter 4, Ongoing studies). One RCT, the TARGeted Intraoperative radioTherapy Alone (TARGIT-A) trial, met the inclusion criteria for the review (Figure 1). The primary and secondary outcomes for the whole trial population were described by two full papers and three linked abstracts. Five substudies of the TARGIT-A trial which report outcome data from participants at just one or two centres were identified. Four of these substudies were excluded from this systematic review on the grounds of outcome (see Appendix 2). One substudy has been included which reports data on HRQoL from patients at one TARGIT-A trial centre. 63 Table 6 provides a summary description of the TARGIT-A study publications included in the clinical effectiveness systematic review.
| Author | Study | Details |
|---|---|---|
| Vaidya et al., 201064 | TARGIT-A trial | Initial results of local recurrence and complications, n = 2232 |
| Vaidya et al., 201465 | TARGIT-A trial | Updated longer-term results of local recurrence, complications and survival, n = 3451 |
| Welzel et al., 201363 | TARGIT-A trial substudy, one centre (Germany) | QoL outcome, n = 88 |
Overview of the TARGIT-A trial
The key characteristics of the TARGIT-A trial64,65 are shown in Table 7 with further details in the data extraction form (see Appendix 3). The TARGIT-A trial is the pivotal trial evaluating the concept of delivering a single dose of targeted IORT at the time of surgery using the mobile INTRABEAM Photon Radiotherapy System.
| Study | Methods | Key inclusion/exclusion criteria | Key participant characteristicsa | Outcomes |
|---|---|---|---|---|
| Vaidya et al., 201064 and 201465 TARGIT-A trial Number of centres: 33 (six in UK) Countries: 11 (Europe, USA, Canada and Australia) |
Design: international, multicentre, non-inferiority RCT Intervention: TARGIT (INTRABEAM device) Dose: typically 20 Gy to surface of tumour bed attenuating to 5–7 Gy at a depth of 1 cm Comparator: WB-EBRT |
Inclusion criteria:
|
Reported in updated 2014 paper (n = 3451):65 Age (years):
|
Primary outcome:Secondary outcomes:
|
| Sponsor: academic and government bodies | Dose: typically 40–56 Gy ± boost of 10–16 Gy Other interventions used: adjuvant systemic treatment as appropriate. Participants in the INTRABEAM group with unfavourable pathological features found subsequently (e.g. lobular carcinoma) received WB-EBRT in addition after INTRABEAM Number of participants: n = 3451:65
Follow-up: median 2 years and 5 months (IQR 12–52 months) |
Nodes involved:
|
||
PgR status:
|
||||
Additional characteristics reported only in 2010 paper (n = 2232):64
|
||||
DCIS:
|
||||
Adjuvant therapy:
|
Design
The TARGIT-A trial is an international, multicentre, non-inferiority RCT that recruited participants in 33 centres in 11 countries including the UK (six centres), Europe (17 centres in six countries), the USA (seven centres), Canada (one centre) and Australia (two centres). The trial evaluated IORT using the INTRABEAM device compared with conventional WB-EBRT. The planned follow-up for trial participants was at least 10 years. 69 Median follow-up achieved for the most recent 2014 publication65 is 2 years 5 months.
As a non-inferiority trial, the RCT sought to determine whether or not INTRABEAM treatment was no worse than WB-EBRT. The pre-stated non-inferiority margin was an absolute difference of 2.5% in the primary end point (local recurrence) between groups. The 2.5% non-inferiority margin was chosen at the trial outset because it seemed clinically acceptable to both clinicians and patients. 64 However, it should be noted that, when the non-inferiority margin was chosen, the estimated local recurrence rate (LRR) (based on the literature available in 1999)70,71 was 6%, and since then recurrence rates have fallen. Two patient preference studies72,73 suggest that patients would be willing to accept an increase in the risk of local recurrence for the convenience of INTRABEAM treatment but it should be noted that these studies were conducted in countries in which WB-EBRT is typically delivered over 5–6 weeks and it is not known whether or not patient preference would be similar in England where WB-EBRT is typically delivered over 3 weeks.
The trial randomised participants in three strata: pre pathology, post pathology and contralateral breast cancer. In the initial 2010 publication,64 pre-pathology entry accounted for two-thirds of patients, post pathology approximately 30% and contralateral breast cancer patients < 4%. It is not clear if these proportions were maintained in the additional patient numbers reported in the updated 2014 publication. 65 The baseline stratification data show differences between centres in the number of patients entering the trial according to the three timings of delivery strata, particularly pre and post pathology (see Appendix 3 for further details). Patients who entered the trial in the pre-pathology stratum were randomised to either INTRABEAM or WB-EBRT prior to WLE of the primary tumour (Figure 2a). The trial was pragmatic in that if participants randomised to INTRABEAM were subsequently found to have unfavourable pathological features (unexpected lobular carcinoma, extensive intraductal component, positive margins at first excision), and hence were at high risk of recurrence elsewhere in the breast, they received WB-EBRT in addition (i.e. INTRABEAM + WB-EBRT, approximately 15% of INTRABEAM patients). The protocol also allowed for post-pathology entry of patients whereby patients underwent initial surgery and then, providing no unfavourable pathological features were identified, were randomised in a second stratum to receive INTRABEAM delivered as a second procedure or WB-EBRT (Figure 2b). Post-pathology entrants to the trial were randomised within 30 days after lumpectomy and the median time between initial lumpectomy and post-pathology INTRABEAM treatment was 37 days. The timing of INTRABEAM delivery was not specified in the intervention description within the NICE scope and, therefore, the post-pathology participants are included in this systematic review. Additionally, patients with a history of previous contralateral breast cancer were also included and randomised in a third stratum. Treatment for breast cancer in the contralateral breast is not an exclusion criterion for this review and, therefore, these participants are also judged to meet the criteria for inclusion.
FIGURE 2.
Flow diagram for the two main trial strata. (a) Pre pathology; and (b) post pathology.


Participants
The TARGIT-A trial was a moderately large trial, recruiting 3451 women with early breast cancer eligible for BCS (2298 to the pre-pathology stratum, 1153 to the post-pathology stratum, as noted above final proportion of contralateral breast cancer patients not reported). 65 Participants had to be ≥ 45 years of age and have invasive ductal carcinoma that was unifocal on conventional examination and imaging. The trial protocol specifically defined early invasive breast cancer as T1 and small T2, N0–1, M0. 69 The initial trial publication64 stipulated the pre-operative diagnosis of lobular carcinoma as a single exclusion criterion, although the trial protocol specified additional exclusion criteria. 69 Furthermore, because the trial was pragmatic, each participating centre had the option to predefine more restrictive entry criteria than in the core protocol (e.g. age, tumour size, grade, node) and to stipulate local policy for the delivery of WB-EBRT.
The majority of women (77%) were aged between 51 and 70 years. Approximately one-third of participants had a grade 1 tumour and around half had grade 2 tumour, while only 15% had a grade 3 tumour. The publications64,65 did not specify the grading system used, but it is likely to have been the standard Bloom–Richardson system74 or the Nottingham system,75 which is modification of the Bloom–Richardson system. In the majority of women, tumour sizes were small (87% < 2 cm) and were associated with a good prognosis – nodes were uninvolved (84%) and ER status and progesterone receptor (PgR) status were positive (93% and 82%, respectively). 65 Two-thirds of women were receiving hormone therapy as adjuvant systemic treatment, while around 12% were receiving chemotherapy. 64
Intervention
The INTRABEAM patients received a typical dose of 20 Gy to the surface of the tumour bed (attenuating to 5–7 Gy at a 1 cm depth).
Comparator
External beam radiotherapy patients received a typical dose of 40–56 Gy with/without an additional boost to the tumour bed of 10–16 Gy. Trial centres were allowed to stipulate local policy for the delivery of WB-EBRT and, therefore, there would have been some differences between WB-EBRT delivered at different centres. It is presumed that, in UK centres, 40 Gy in 15 fractions would have been the likely treatment schedule, whereas in some other centres local policy was an alternative schedule, for example 56 Gy in 28 fractions. 63
Outcomes
The primary outcome of the trial was pathologically confirmed local recurrence in the conserved breast. In the initial 2010 paper,64 survival free of recurrence (i.e. disease-free survival) was reported, but, in the 201465 paper, the data on recurrence are not presented in that format. Secondary outcomes were rates of local toxicity or morbidity, which were assessed using a complications form that contained a pre-specified checklist. The timing of the data collection for complications was unclear in the trial publications, being described as ‘early’ in the 2010 paper64 and ‘arising 6 months after randomisation’ in the 2014 paper. 65 Complications recorded on the pre-specified checklist were haematoma, seroma, wound infection, skin breakdown, delayed wound healing and Radiation Therapy Oncology Group (RTOG) toxicity grade 3 or 4 (for dermatitis, telangiectasia, pain in irradiated field, or other). Overall survival was reported as a secondary outcome measure in the 2014 updated publication. 65 No data on HRQoL have been published for the whole trial population; however, one small substudy63 is included in this systematic review which reports on HRQoL for 88 participants enrolled at one centre in Mannheim, Germany. HRQoL was assessed by two validated questionnaires of the European Organisation for Research and Treatment of Cancer (EORTC), the quality of life (QoL) questionnaire – C30 (QLQ-C30, version 3; European Organization for Research and Treatment of Cancer, Brussels, Belgium) and the QoL questionnaire – Breast Cancer Module (QLQ-BR23). Data presented in the initial TARGIT-A trial publication64 suggest that all the participants enrolled at this centre were randomised to the pre-pathology stratum.
For most outcomes, analyses were by intention to treat (ITT), one exception being local recurrence in the conserved breast which, because of the nature of the outcome, could not include women who had undergone a mastectomy (approximately 2%). For a superiority trial, the Consolidated Standards of Reporting Trials (CONSORT) statement76 states that analysis should be by ITT. However, the TARGIT-A trial is a non-inferiority trial. An extension to the CONSORT statement77 for non-inferiority trials indicates that non-ITT analyses might be desirable and that there would be greater confidence in the results if these were consistent between ITT and non-ITT analyses. Therefore, an analysis by treatment received in addition to the ITT analyses presented for the TARGIT-A trial would have been welcome. Outcomes of local recurrence and overall survival were reported for the whole trial population and separately for the pre- and post-pathology strata. Data from participants who received INTRABEAM only and from those who received INTRABEAM with WB-EBRT in addition were analysed together for most outcomes. Median length of follow-up for participants in the initial 2010 publication was not reported, although it was stated that maximum follow-up was 10 years. 64 The more recent 2014 publication65 reported an overall median follow-up of 2 years 5 months, with 2020 (59%) participants reaching a median 4 years and 1222 (35%) reaching a median 5 years.
Quality assessment of TARGIT-A trial
Overall, the methodological quality of the TARGIT-A trial was judged to be good with a low risk of bias. Table 8 shows the judgements of risk of bias in the various domains. For the HRQoL substudy, the assessment of selection bias and reporting bias for the main trial was judged to apply. For the remaining criteria it was judged that the HRQoL substudy could potentially differ from the main trial and, therefore, separate assessments were conducted (see Table 8). Overall, the substudy was judged to be at a high risk of bias owing to the lack of blinding and it is not clear how representative the results are for the total trial population because the substudy represents only about 2.5% of the overall trial population. Therefore, the substudy results should be interpreted with caution.
| Cochrane criteria for assessment of risk of bias in RCTs56 | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Low risk | Computer-generated randomisation schedules |
| Allocation concealment | Low risk | Central allocation |
| Performance bias | ||
| Blinding of participants and personnel in the TARGIT-A trial | Low risk | Neither patients nor investigators were blinded. However, outcomes of mortality and recurrence were unlikely to be influenced by lack of blinding |
| Blinding of participants and personnel in the HRQoL substudy | High risk | As part of the TARGIT-A trial neither patients nor investigators were blinded and the outcome could potentially be influenced by the lack of blinding |
| Detection bias | ||
| Blinding of outcome assessment in the TARGIT-A trial | Low risk | Some investigators and teams were not blinded and it is not clear whether or not all the analyses were performed unblinded. However, outcomes of mortality and recurrence are objective measures and hence unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment in the HRQoL substudy | Unclear risk | No information reported for this substudy |
| Attrition bias | ||
| Incomplete outcome data addressed in the TARGIT-A trial | Low risk | Low proportion of withdrawals and participants not receiving allocated treatment (reasons similar between groups). Analyses by ITT |
| HRQoL sub-study | Low risk | Reason for loss of one participant given |
| Reporting bias | ||
| Selective reporting | Low risk | The protocol is available online (www.nets.nihr.ac.uk/__data/assets/pdf_file/0007/51892/PRO-07-60-49.pdf)69 and specifies all outcomes including relapse-free survival and overall survival (as secondary outcomes) |
| Other bias | ||
| Other sources of bias in the TARGIT-A trial | Low risk | None evident |
| Other sources of bias in the HRQoL substudy | Unclear risk | Retrospective questionnaire with no baseline QoL measurement |
Randomisation schedules that were generated by computer and held securely in two centres, with requests for randomisation made by telephone or fax, meant that the risk of selection bias was low.
Owing to the nature of the interventions, it was not feasible to blind the patients or investigators in the trial, which could potentially introduce performance bias. However, given that the main trial outcomes (recurrence and survival) were objective measures, it was deemed unlikely that patients or investigators were influenced by the lack of blinding and thus performance bias was judged to be low. Similarly, for the main trial, although not all outcome assessors were blinded, the risk of detection bias was judged to be low because the main trial outcomes (recurrence and survival) were objective measures. For the substudy,63 the lack of patient and investigator blinding led to a judgement of a high risk of performance bias, and detection bias was judged as unclear owing to a lack of information.
The risk of attrition bias (differences between groups in withdrawals from the study) was deemed to be low in the TARGIT-A trial. There was a low proportion of withdrawals, and the rate appeared similar between treatment groups (0.5% INTRABEAM, 1.6% WB-EBRT). 65 Similar numbers of patients in the two treatment groups received their allocated treatment (91% INTRABEAM, 92% WB-EBRT)65 and all randomised patients were included in an ITT analysis for most outcomes. However, as noted above (see Overview of the TARGIT-A trial, Outcomes), an additional analysis by treatment received would have been desirable. The substudy63 was deemed to be at low risk of attrition bias because only one patient was reported as lost to follow-up.
The risk of bias due to selective reporting was deemed low as all outcomes specified in the trial protocol69 were reported in either the original or updated publication. 64,65 No other sources of bias in the total trial population were identified. The substudy63 used a retrospective questionnaire without reporting baseline measurements and was therefore deemed to be at unclear risk of other sources of bias.
Assessment of clinical effectiveness
The majority of the results presented in the following section are the most recent data for the TARGIT-A trial reported in the updated publication by Vaidya et al. 65 Results are presented for ipsilateral local recurrence, overall survival, and morbidity and toxicity. The main trial outcome data are supplemented with some morbidity data from the initial trial publication (see Vaidya et al. 64). The TARGIT-A trial presented outcomes of recurrence and survival for the whole trial population, and separately for the pre- and post-pathology strata. The separate analysis of these two strata was pre-specified. No data were presented from the third stratum (participants with a history of previous contralateral breast cancer) and no data on HRQoL have been published for the whole trial population. However, limited data on the secondary outcome of QoL are provided by a substudy at one trial centre. 63
Ipsilateral local recurrence
Local recurrence in the conserved breast was the primary outcome in the TARGIT-A trial. Recurrence was defined as a recurrent tumour in the ipsilateral breast and was confirmed pathologically by clinical examination and cytology or biopsy. 69 The most recent data from the 201465 publication are shown, which were not expressed in terms of disease-free survival. Results are presented in Tables 9 and 10 and show data for the whole cohort and for the two pre-specified randomisation strata (pre pathology and post pathology) representing the different timings in delivery of INTRABEAM therapy. The trial authors also report results separately for the mature cohort (participants previously reported in the initial publication in 201064) and the earliest cohort (which excludes participants enrolled in the last 4 years of the study) in order to ‘assess stability over time’65 (see Table 10). However, there has been criticism of this approach78 because all patients included in the earliest cohort are also included in, and account for, just over half of the mature cohort and are included again in the whole cohort representing approximately one-third of this. The assessment team and the advisory group for this assessment also have concerns about the approach taken. For the INTRABEAM arm, data from participants who received INTRABEAM only and from those who received INTRABEAM and WB-EBRT were analysed together.
| Local recurrence | INTRABEAM events/n; 5-year cumulative risk (%) (95% CI)65 | WB-EBRT events/n; 5-year cumulative risk (%) (95% CI)65 | Absolute difference in Kaplan–Meier estimate at 5 years; p-value |
|---|---|---|---|
| Whole group (n = 3375)a | 23/1679; 3.3 (2.1 to 5.1) | 11/1696; 1.3 (0.7 to 2.5) | 12 (2.0%); p = 0.042 |
| Pre-pathology stratum (n = 2234)a | 10/1107; 2.1 (1.1 to 4.2) | 6/1127; 1.1 (0.5 to 2.5) | 4 (1.0%); p = 0.31 |
| Post-pathology stratum (n = 1141)a | 13/572; 5.4 (3.0 to 9.7) | 5/569; 1.7 (0.6 to 4.9) | 8 (3.7%); p = 0.069 |
| Local recurrence65 | Median follow-up | Events, n | Absolute difference (%) (90% CI) in the binomial proportionsa of ipsilateral local recurrence (INTRABEAM minus WB-EBRT) | z-value | p non-inferiority |
|---|---|---|---|---|---|
| Whole trial | |||||
| All patients | 2 years 5 months | 34 | 0.72 (0.2 to 1.3) | –5.168 | < 0.0001 |
| Mature cohortb | 3 years 7 months | 32 | 1.13 (0.3 to 2.0) | –2.652 | 0.0040 |
| Earliest cohortc | 5 years | 23 | 1.14 (–0.1 to 2.4) | –1.750 | 0.0400 |
| Pre pathology | |||||
| All patients | 2 years 4 months | 16 | 0.37 (–0.2 to 1.0) | –5.954 | < 0.0001 |
| Mature cohortb | 3 years 8 months | 14 | 0.60 (–0.3 to 1.5) | –3.552 | 0.0002 |
| Earliest cohortc | 5 years | 9 | 0.76 (–0.4 to 2.0) | –2.360 | 0.0091 |
| Post pathology | |||||
| All patients | 2 years 4 months | 18 | 1.39 (0.2 to 2.6) | –1.503 | 0.0664 |
| Mature cohortb | 3 years 7 months | 18 | 2.04 (0.3 to 3.8) | –0.429 | 0.3339 |
| Earliest cohortc | 5 years | 14 | 1.80 (–1.2 to 4.8) | –0.382 | 0.3511 |
By nature of the outcome, the recurrence data do not include women who underwent mastectomy (n = 76). Statistical significance levels were set at p < 0.01 for recurrence. The rationale for setting p < 0.01 for recurrence but p < 0.05 for survival (see Overall survival) is not provided.
As can be seen in Table 9, the 5-year risk of local recurrence in the conserved breast in the whole cohort of patients was higher in patients receiving INTRABEAM than in those treated with WB-EBRT, but the absolute difference did not exceed the pre-stated non-inferiority margin of 2.5% (3.3% vs. 1.3%, respectively; absolute difference 2.0%; p = 0.042). With the statistical significance level set at p < 0.01 for recurrence, the difference between groups was not statistically significant. Similarly, in the pre-pathology stratum (INTRABEAM delivered at the time of BCS), the absolute difference in recurrence did not exceed the 2.5% non-inferiority margin (2.1% INTRABEAM vs. 1.1% WB-EBRT, absolute difference 1.0%; p = 0.31) and the difference between groups was not statistically significant. However, in the post-pathology stratum (INTRABEAM delivered after BCS as a secondary procedure), although the difference between groups was not statistically significant (and the analysis may not have been powered to detect a difference), the 5-year local recurrence was higher in INTRABEAM patients, with the difference being larger than the pre-defined non-inferiority margin of 2.5% (5.4% INTRABEAM vs. 1.7% WB-EBRT, absolute difference 3.7%; p = 0.069). Therefore, INTRABEAM has been shown to be non-inferior to WB-EBRT for the whole group and for the pre-pathology stratum but not for participants in the post-pathology stratum (based on a non-inferiority margin of 2.5%).
The data on recurrence were used to generate a non-inferiority statistic (pnon-inferiority) for the absolute difference in the binomial proportions of ipsilateral local recurrence (see Table 10). INTRABEAM was shown to be non-inferior to WB-EBRT for the whole cohort (absolute difference in binomial proportions 0.72%, 90% CI 0.2% to 1.3%; pnon-inferiority < 0.0001) and for all pre-pathology patients (absolute difference in binomial proportions 0.37%, 90% CI –0.2% to 1.0%; pnon-inferiority < 0.0001). However, non-inferiority was not established for the post-pathology patients (absolute difference in binomial proportions 1.39%, 90% CI 0.0% to 2.8%; pnon-inferiority = 0.0664).
The non-inferiority statistic was also reported separately for two cohorts of participants within the trial that had longer follow-up. As already noted, the stated aim of these analyses was to ‘assess stability over time’,65 but participants in the earliest cohort are also included in the mature cohort and whole trial population and there are concerns about this approach; therefore, the results should be interpreted cautiously. For the mature cohort, which comprised participants previously reported on in 2010,64 and the earliest cohort, which had a median follow-up of 5 years, results reflect those of the ‘all-patients’ analyses. It is worth noting that the number of local recurrence events in the earliest cohort (median follow-up 5 years) was 23 events for the whole trial, just nine of which occurred in pre-pathology participants.
The absolute differences in the 5-year Kaplan–Meier estimates of percentage with local recurrence in the conserved breast were calculated and presented as a figure in the trial publication65 for the pre-pathology stratum only. Data were estimated from the figure using Engauge digitising software (version 4.1, © Mark Mitchell) (see Appendix 3). The Kaplan–Meier estimates were consistent across the three cohorts with increasing median follow-up, with absolute differences in percentage with local recurrence in the conserved breast of 1.1 (whole cohort), 1.1 (mature cohort) and 1.0 (earliest cohort).
Overall survival
Overall survival was a secondary outcome in the TARGIT-A trial and was reported in the more recent 2014 publication. 65 Overall survival was defined as the time interval between randomisation and death69 and included breast cancer deaths and non-breast cancer deaths. Statistical significance levels were set at p < 0.05 for survival. As already noted, the rationale for setting p < 0.05 for survival but p < 0.01 for recurrence was not provided.
There were no statistically significant differences in overall mortality between women who received INTRABEAM compared with those who received WB-EBRT (3.9% vs. 5.3%, respectively; difference –1.4%; p = 0.099) (Table 11). When mortality was split into breast cancer and non-breast cancer deaths, rates of breast cancer death were similar between the two treatments (2.6% vs. 1.9%; p = 0.56), but there were significantly fewer non-breast cancer deaths in the INTRABEAM group than in the WB-EBRT group (1.4% vs. 3.5%, respectively; p = 0.0086).
| Mortality65 | INTRABEAM events/n; 5-year cumulative risk (%) (95% CI)65 | WB-EBRT events/n; 5-year cumulative risk (%) (95% CI)65 | Absolute difference; p-value |
|---|---|---|---|
| Overall mortality | |||
| All patients (n = 3451) | 37/1721; 3.9 (2.7 to 5.8) | 51/1730; 5.3 (3.9 to 7.3) | –14 (–1.4%); p = 0.099 |
| Pre-pathology stratum (n = 2298) | 29/1140; 4.6 (1.8 to 6.0) | 42/1158; 6.9 (4.3 to 9.6) | –13 (–2.3%); p = NR |
| Post-pathology stratum (n = 1153) | 8/581; 2.8 (1.3 to 5.9) | 9/572; 2.3 (1.0 to 5.2) | –1 (0.5%); p = NR |
| Breast cancer mortality | |||
| All patients (n = 3451) | 20/1721; 2.6 (1.5 to 4.3) | 16/1730; 1.9 (1.1 to 3.2) | p = 0.56 |
| Pre-pathology stratum (n = 2298) | 17/1140; 3.3 (1.9 to 5.8) | 15/1158; 2.7 (1.5 to 4.6) | p = 0.72 |
| Post-pathology stratum (n = 1153) | 3/581; 1.2 (0.4 to 4.2) | 1/572; 0.5 (0.1 to 3.5) | p = 0.35 |
| Non-breast cancer mortality | |||
| All patients (n = 3451) | 17/1721; 1.4 (0.8 to 2.5) | 35/1730; 3.5 (2.3 to 5.2) | p = 0.0086 |
| Pre-pathology stratum (n = 2298) | 12/1140; 1.3 (0.7 to 2.8) | 27/1158; 4.4 (2.8 to 6.9) | p = 0.016 |
| Post-pathology stratum (n = 1153) | 5/581; 1.58 (0.62 to 3.97) | 8/572; 1.76 (0.7 to 4.4) | p = 0.32 |
In the pre-pathology stratum (INTRABEAM delivered at the time of BCS), overall mortality was slightly lower in the INTRABEAM group (4.6% vs. 6.9% for INTRABEAM and WB-EBRT, respectively; difference –2.3%; no p-value reported). When split into causes of death, the same pattern was observed as for the whole cohort for which deaths attributable to breast cancer were similar between the two treatments (3.3% vs. 2.7% for INTRABEAM and WB-EBRT, respectively; p = 0.72), but there were significantly fewer non-breast cancer deaths in the INTRABEAM group (1.3%) than in the WB-EBRT group (4.4%; p = 0.016). When INTRABEAM was delivered after BCS as a delayed procedure (post-pathology stratum), rates of overall mortality, breast cancer and non-breast cancer mortality were similar between treatment groups (see Table 11).
For non-breast cancer mortality, which was statistically significantly different between the INTRABEAM and WB-EBRT groups, Vaidya et al. 65 detailed the causes of death. These included other types of cancer, cardiovascular causes and other causes. Details can be found in the data extraction form in Appendix 3.
The absolute differences in the 5-year Kaplan–Meier estimates of percentage overall mortality were calculated and presented in the published paper65 for the pre-pathology stratum only (as with local recurrence, see Ipsilateral local recurrence) for the three cohorts with increasing median follow-up. As noted in section Ipsilateral local recurrence, there are concerns about the approach taken and, therefore, the results should be interpreted cautiously. The Kaplan–Meier estimates were similar across the three cohorts, with absolute differences in percentage mortality of –2.3 (whole cohort), –2.6 (mature cohort) and –2.2 (earliest cohort) (the data extracted from the published figure are available in Appendix 3). These data and the absolute differences in the 5-year Kaplan–Meier estimates of percentage with local recurrence in the conserved breast (see Ipsilateral local recurrence) were presented together in the 2014 trial publication65 to demonstrate the relationship between local recurrence and mortality whereby women receiving INTRABEAM experience more local recurrences but fewer deaths than those receiving WB-EBRT.
Morbidity and toxicity
Complications, in the form of local toxicity and morbidity, were reported as secondary outcomes. The initial publication by Vaidya et al. 201064 reported early complications but did not specifically define ‘early’, although the trial protocol69 stipulated that the period of serious adverse event observation extended from the time of registration onto the trial until 90 days after the completion of randomised treatment. The more recent TARGIT-A publication65 reported complications arising 6 months after randomisation.
As can be seen in Table 12, the incidence of any early complication was similar in the two treatment groups. Clinically significant complications were also similar between groups with the exception of two. Wound seroma requiring more than three aspirations occurred more frequently in women receiving INTRABEAM than in those receiving WB-EBRT (2.1% vs. 0.8%, respectively; p = 0.012), while, conversely, a RTOG toxicity score of grade 3 or 4 was less frequent in the INTRABEAM group than in the WB-EBRT group (0.5% vs. 2.1%; p = 0.002). 64 Separate data were not reported for the categories of dermatitis, telangiectasia, pain in irradiated field, or other that contributed to the RTOG toxicity grade 3 or 4 outcome. A member of the advisory group for this assessment indicated that the clinical impact for patients with grade 3 or 4 toxicity is much greater than for those with a seroma requiring several aspirations.
| Earlya complications | INTRABEAM (n = 1113) | WB-EBRT (n = 1119) | p-value |
|---|---|---|---|
| Number of complications per patient64 | |||
| 0 | 917/1113 (82.4%) | 946/1119 (84.5%) | NR |
| 1 | 151/1113 (13.6%) | 139/1119 (12.4%) | NR |
| 2 | 29/1113 (2.6%) | 27/1119 (2.4%) | NR |
| 3 | 11/1113 (1.0%) | 5/1119 (0.4%) | NR |
| 4 | 3/1113 (0.3%) | 0/1119 | NR |
| 5 | 2/1113 (0.2%) | 0/1119 | NR |
| 6 | 0/1113 | 3/1119 (0.3%) | NR |
| Any complicationa | 196/1113 (17.6%) | 174/1119 (15.5%) | χ2 1.74; p = 0.19b |
| aClinically significant complications64 | |||
| Haematoma needing surgical evacuation | 11/1113 (1.0%) | 7/1119 (0.6%) | 0.338 |
| Seroma needing > 3 aspirations | 23/1113 (2.1%) | 9/1119 (0.8%) | 0.012 |
| Infection needing i.v. antibiotics or surgical intervention | 20/1113 (1.8%) | 14/1119 (1.3%) | 0.292 |
| Skin breakdown or delayed wound healingc | 31/1113 (2.8%) | 21/1119 (1.9%) | 0.155 |
| RTOG toxicity grade 3 or 4d | 6/1113 (0.5%) | 23/1119 (2.1%) | 0.002 |
| Major toxicitye | 37/1113 (3.3%) | 44/1119 (3.9%) | 0.443 |
| Wound-related complications arising 6 months after randomisation65 | INTRABEAM (n = 1721) | WB-EBRT (n = 1730) | p-value |
| Haematoma/seroma needing > 3 aspirations | 4/1721 (0.2%)f | 2/1730 (0.1%)f | NR |
| Infection needing i.v. antibiotics or surgery | 12/1721 (0.7%)f | 9/1730 (0.5%)f | NR |
| Skin breakdown or delayed wound healing | 3/1721 (0.2%)f | 5/1730 (0.3%)f | NR |
| Total | 19/1721 (1.1%) | 16/1730 (0.9%) | 0.599 |
| Radiotherapy-related complications65 | |||
| RTOG toxicity grade 3 or 4 | 4/1721 (0.2%) | 13/1730 (0.8%) | 0.029 |
The incidence of complications arising 6 months after randomisation (reported by the 2014 publication65) was lower in both treatment groups, although it is not clear whether or not these complications occurred in any of the same patients who were reported in the 2010 publication64 as having clinically significant complications. There appeared to be no differences between treatment groups in any single defined wound-related complication (see Table 12) (p-values not reported), or in total complications (1.1% INTRABEAM vs. 0.9% WB-EBRT; p = 0.599). The incidence of radiotherapy-related complications (RTOG toxicity score of grade 3 or 4) remained higher in women receiving WB-EBRT (0.8%) than in those receiving INTRABEAM (0.2%), but the difference between the groups was no longer statistically significant (p = 0.29).
Substudy reporting quality of life for participants at one trial centre
No data on HRQoL have been published for the whole trial population; however, Welzel et al. 63 have assessed QoL retrospectively in one small substudy of 88 participants enrolled at one centre in Mannheim, Germany. The initial TARGIT-A trial publication64 indicates that all the participants enrolled at this centre were randomised to the pre-pathology stratum. QoL was assessed by using two validated questionnaires of the EORTC: the QLQ-C30 (version 3) and the QLQ-BR23. Participants (n = 88) were asked to report on their situation in the last week and these participants represent 2.5% of the total TARGIT-A trial population. The results of both an ITT analysis and an as-treated analysis (with a threshold for significance of p < 0.01 in both cases) are presented in Table 13. The as-treated analysis removes five participants from the INTRABEAM group and moves four of them to the WB-EBRT group because this was the treatment received, with the fifth (who refused WB-EBRT) not contributing data. The ITT analysis did not identify any statistically significant differences in QoL measures (global health status, restrictions in daily activities, general pain, breast or arm symptoms) reported by the INTRABEAM arm in comparison with the WB-EBRT arm. The as-treated analyses were not presented in the same way as the ITT analysis. For the as-treated analyses, the results for the INTRABEAM arm were reported separately for those who received INTRABEAM therapy only and those who received INTRABEAM + WB-EBRT with statistical comparisons of INTRABEAM only versus WB-EBRT, INTRAEAM only versus INTRABEAM + WB-EBRT, and WB-EBRT versus INTRABEAM + WB-EBRT being reported. Thus, a statistical comparison between the original randomised groups is not reported. For the comparison of the INTRABEAM-only group with the WB-EBRT-treated group the as-treated analyses showed a statistically significant benefit of INTRABEAM for restrictions in daily activities, general pain, breast symptoms and arm symptoms, but there was no statistically significant difference in the global health status subscale. When comparing the INTRABEAM-only group with the INTRABEAM + WB-EBRT group, the only statistically significant difference in the reported QoL measures was for breast symptoms. No statistically significant differences were reported for comparisons of QoL measures between the INTRABEAM + WB-EBRT and the WB-EBRT groups. These data should be interpreted cautiously owing to their non-randomised nature and the small numbers involved. The breast and arm symptoms most commonly reported by participants were moderate or severe pain in the arm or shoulder, difficulty in raising/moving arm sideways and pain in area of affected breast. No statistically significant differences between groups were reported for the as-treated analysis of frequency of symptoms.
| ITT analysis, QoL outcome, mean (SD) | INTRABEAM (N = 46; IORT n = 30, INTRABEAM + WB-EBRT n = 16) | WB-EBRT (n = 42) | p-valuea | |
|---|---|---|---|---|
| Global health statusb | 61.6 (21.7), n = 46 | 54.8 (19.9), n = 40 | 0.183 | |
| Restrictions in daily activitiesb | 72.8 (32.3), n = 46 | 61.8 (29.2), n = 41 | 0.055 | |
| General painc | 29.3 (32.8), n = 46 | 42.5 (33.0), n = 42 | 0.048 | |
| Breast symptomsc | 17.0 (20.8), n = 45 | 18.1 (20.2), n = 42 | 0.629 | |
| Arm symptomsc | 24.4 (26.7), n = 45 | 31.1 (27.9), n = 40 | 0.279 | |
| As-treated analysis, QoL outcome, mean (SD) | INTRABEAM (n = 25) | INTRABEAM + WB-EBRT (n = 16) | WB-EBRT (n = 46) | p-value |
| Global health statusb | 63.6 (24.2) | 60.9 (19.9) | 52.4 (22.1) | > 0.01 |
| Restrictions in daily activitiesb | 78.7 (35.2) | NR | 60.5 (29.5) | 0.007d |
| General painc,e | 21.3 (95% CI NRf to 54.4) | 43.7 (95% CI 11.6 to 75.9) | 40.9 (95% CI 8.6 to 73.2) | 0.007d 0.018g |
| Breast symptomsc,e | 7.2 (95% CI NRf to 20.9) | 29.7 (95% CI 6.8 to 52.5) | 19.0 (95% CI NRf to 39.2) | 0.001d < 0.001g 0.021h |
| Arm symptomsc,e | 15.2 (95% CI NRf to 37.2) | 32.6 (95% CI 6.8 to 58.4) | 32.8 (95% CI 4.2 to 61.5) | 0.009d 0.011f |
| As-treated analysis, frequency breast/arm symptomsi | INTRABEAM (n = 25), % moderate/severe | INTRABEAM + WB-EBRT (n = 16), % moderate/severe | WB-EBRT (n = 46), % moderate/severe | p-value |
| Pain in area of affected breast | 4/0 | 25/13 | 11/4 | > 0.01 |
| Swelling in area of affected breast | 0/0 | 7/7 | 4/2 | |
| Oversensitivity in area of affected breast | 4/0 | 20/7 | 9/7 | |
| Skin problems on or in area of affected breast | 4/4 | 13/6 | 9/4 | |
| Pain in arm or shoulder | 8/8 | 33/20 | 18/23 | > 0.01 |
| Swelling in arm or hand | 8/4 | 6/6 | 9/7 | |
| Difficulty in raising or moving arm sideways | 20/0 | 13/7 | 24/12 | > 0.01 |
Summary of clinical effectiveness
-
One RCT64,65 met the inclusion criteria for the systematic review. It evaluated IORT using the INTRABEAM device compared with conventional WB-EBRT. In addition to the main trial,64,65 one substudy reported on participants from an individual trial centre for the outcome of QoL. 63 Other publications from TARGIT-A were not included.
-
The RCT was a non-inferiority trial that sought to determine whether or not INTRABEAM treatment was no worse than WB-EBRT. The pre-stated non-inferiority margin was an absolute difference of 2.5% in the primary end point (local recurrence) between groups. However, the choice of non-inferiority margin was based on an estimated 5-year LRR of 6%, but since then trial recurrence rates have reduced.
-
The RCT had two randomisation strata. Participants in the pre-pathology stratum were randomised to INTRABEAM or WB-EBRT prior to surgery to remove the tumour. Any participants in the INTRABEAM arm who were subsequently found to have unfavourable pathological features received WB-EBRT in addition (i.e. INTRABEAM + WB-EBRT). Participants in the post-pathology stratum received surgery to remove the tumour and were entered into the trial providing initial histopathology showed no adverse criteria. Participants in the INTRABEAM arm found to have unfavourable pathological features on final histopathology received INTRABEAM + WB-EBRT.
-
The quality of the RCT was judged to be good with a low risk of bias.
-
Local recurrence in the conserved breast was the primary outcome of the RCT, with the pre-stated non-inferiority margin being an absolute difference of 2.5% between groups. Overall survival was a secondary outcome. The median follow-up was 2 years 5 months, with 2020 (59%) of the total study population reaching a median follow-up of 4 years and 1222 (35%) reaching a median follow-up of 5 years. Results were presented for the whole trial population, the pre-pathology stratum and the post-pathology stratum.
Whole trial population
-
Local recurrence for the whole trial population was higher in the INTRABEAM group, but the absolute difference in 5-year risk of local recurrence did not exceed the 2.5% non-inferiority margin. Analysis of the non-inferiority statistic for local recurrence indicated that INTRABEAM was non-inferior to WB-EBRT.
-
The difference in overall survival for the whole trial population between women who received INTRABEAM and those who received WB-EBRT was not statistically significant. Analysis of breast cancer and non-breast cancer deaths showed that rates of breast cancer death were similar between the two treatments but there were significantly fewer non-breast cancer deaths in the INTRABEAM group than in the WB-EBRT group.
-
When considering these results for differences in 5-year risks it should be remembered that median follow-up was just under 2.5 years and 1222 participants had completed 5 years of follow-up. The initial sample size calculation required 2232 participants be enrolled; however, this was based on a background 5-year recurrence rate of 6% whereas the observed recurrence rate in the trial to date is lower than 6% so a smaller sample size could achieve the same statistical power.
Pre-pathology stratum
-
Local recurrence for the pre-pathology stratum was higher in the INTRABEAM group but the absolute difference in 5-year risk of local recurrence did not exceed the 2.5% non-inferiority margin. Analysis of the non-inferiority statistic for local recurrence indicated that INTRABEAM was non-inferior to WB-EBRT.
-
Overall, mortality was slightly lower in the INTRABEAM group because, although breast cancer deaths were similar between the two treatments, there were significantly fewer non-breast cancer deaths in the INTRABEAM group.
-
Participants in the pre-pathology stratum treated with INTRABEAM experienced a 1% increase in local recurrence but this was counterbalanced with a potential 2.3% decrease in overall mortality.
-
When considering these results, the same issues regarding median length of follow-up apply to the pre-pathology stratum as have already been noted for the whole trial population. It should also be remembered that 2298 participants were randomised to the pre-pathology stratum.
Post-pathology stratum
-
Local recurrence in the post-pathology stratum was higher in the INTRABEAM arm and the absolute difference in the 5-year local recurrence exceeded the pre-defined non-inferiority margin of 2.5%. Analysis of the non-inferiority statistic indicated that non-inferiority was not established for the post-pathology patients.
-
Overall mortality, breast cancer mortality and non-breast cancer mortality were similar between treatment groups.
-
When considering these results, the same issues regarding median length of follow-up apply as noted for the whole trial population. In addition, it should be remembered that 1153 participants were randomised to the post-pathology stratum
-
-
Numbers of early complications reported were similar in the two treatment groups. Clinically significant complications were also similar, except for wound seroma requiring more than three aspirations, which occurred more frequently in the INTRABEAM group, whereas an RTOG toxicity score of grade 3 or 4 was less frequent in the INTRABEAM group. Complications arising 6 months after randomisation appeared similar between the groups and, although RTOG toxicity of grade 3 or 4 remained more common among WB-EBRT arm participants, the difference between groups was no longer statistically significant.
-
One substudy reported QoL for participants at one trial centre:
-
The outcomes from this substudy should be treated with some caution because of the risks of bias identified and the small proportion of the overall trial population involved.
-
ITT analysis did not identify any statistically significant differences in QoL measures between the study arms.
-
The Southampton Health Technology Assessments Centre’s review of clinical effectiveness in the manufacturer’s submission to the National Institute for Health and Care Excellence
Carl Zeiss, UK, (INTRABEAM manufacturer) submitted a report and economic model to NICE. The clinical effectiveness evidence has been briefly appraised (see Appendix 4) and a review of the economic model and cost-effectiveness results included in the MS can be found in Chapter 5 (see Review of evidence submission from Carl Zeiss, UK, to National Institute for Health and Care Excellence).
The manufacturer did not conduct a formal systematic review of the clinical effectiveness evidence. Although the databases searched and the dates of searches were specified, no information is provided to indicate how the results of this search were screened to identify relevant studies, no detailed inclusion or exclusion criteria were presented and there is no quality assessment of the included studies. The manufacturer did not report searching for any ongoing studies but information is included from conference proceedings.
The MS contains a narrative summary of the single key RCT, the TARGIT-A trial, which is also included in the Southampton Health Technology Assessments Centre (SHTAC)’s systematic review. However, there are two differences in the evidence presented. First, the MS excludes evidence from the initial TARGIT-A trial publication from 2010,64 reasoning that the 2010 results are expected to be included in the more recent (201465) publication but, in contrast, the SHTAC’s systematic review includes evidence on early complications from the 2010 TARGIT-A trial publication64 as these are not reported by the more recent 2014 trial paper. 65 The second difference in the TARGIT-A trial evidence presented is that the MS includes a cohort study79 reporting on post-operative complications within the first week following surgery at the TARGIT-A trial centre in Mannheim, Germany. This cohort study is excluded from SHTAC’s systematic review because it is likely that the data reported are either partially or wholly contained within the early complications reported by the initial TARGIT-A trial publication64 and, furthermore, Tuschy et al. 79 report no comparable data for the WB-EBRT group.
In addition to evidence from the TARGIT-A RCT, the MS also provides a narrative summary of evidence from a further 22 studies72,79,80–99 (six reported as conference abstracts) that did not meet the inclusion criteria of the SHTAC’s review, chiefly on the grounds of study design.
The MS Interpretation of clinical evidence subsections a, b, and c (MS pp. 42–46) focuses on the TARGIT-A trial data and, consequently, with just one included trial there is no discrepancy for the key outcomes of recurrence and overall survival between the MS and the SHTAC’s systematic review.
Ongoing studies
The clinical effectiveness search and the search for ongoing studies identified one ongoing RCT (TARGIT-B),100,101 one prospective single-arm study (TARGIT-E)102 and three registry database studies (TARGIT-R,103 TARGIT-BQR104 and TARGIT-US). 105 A brief description of each study is provided in Table 14.
| Title, database identifier(s) | Study type (country), estimated enrolment | Summary description of study aims | Start date | End date (primary end date) | Funding and/or sponsor |
|---|---|---|---|---|---|
| TARGIT-B,100,101 NCT01792726, HTA 10\104\07 | RCT multicentre, multinational, n = 1796 | To evaluate whether or not a tumour bed boost in the form of a single fraction of radiotherapy given intraoperatively and targeted to the tissues at the highest risk of local recurrence is superior (in terms of local tumour control) to standard post-operative WB-EBRT boost after BCS in women undergoing breast-conserving therapy who have a higher risk of local recurrence | March 2013 | April 2022 (January 2022) | HTA |
| TARGIT-E,102 NCT01299987 | Prospective multicentre single-arm, Phase II, n = 265 | To investigate the efficacy of a single intraoperative radiotherapy treatment (based on the protocol of TARGIT-A) within elderly low-risk patients which is followed by WB-EBRT only when risk factors are present. In presence of risk factors, post-operative WB-EBRT will be added according to international guidelines | January 2011 | November 2025 (November 2015) | Sponsor: University Hospital Mannheim |
| TARGIT-R,103 ISRCTN91179875 | Registry database multicentre, multinational, n not provided | To monitor the long-term effectiveness and safety of TARGIT treatment in women who receive TARGIT outside of a clinical trial. Recruitment start mid-2013 continuing to at least mid-2015 | July 2013 | July 2023 | Royal Free Charity (UK) |
| TARGIT-BQR,104 NCT01440010 | Registry database (Germany), n = 1000 | A quality control registry collecting data on LRR, toxicity and overall survival. For women with breast cancer receiving TARGIT with the INTRABEAM system as an advanced boost followed by shortened WB-EBRT | September 2011 | Not provided | Sponsor: University Hospital Mannheim |
| TARGIT-US,105 NCT01570998 | Registry trial (USA), n = 755 | A pragmatic registry trial (modelled on TARGIT-A) to continue the use of intraoperative radiotherapy for a select population of women, and to follow outcomes of local and regional control, toxicity and morbidity | May 2012 | Not provided (January 2015) | Sponsor: University of California, San Francisco |
Chapter 5 Economic analysis
The aim of this section is to assess the cost-effectiveness of the INTRABEAM Photon Radiotherapy System for the adjuvant treatment of early operable breast cancer.
The economic analysis comprises:
-
a systematic review of the literature on the cost-effectiveness of the INTRABEAM Photon Radiotherapy System for the adjuvant treatment of early operable breast cancer
-
a systematic review of studies of the HRQoL of patients with breast cancer
-
a review of the INTRABEAM MS to NICE
-
an independent economic model and cost-effectiveness evaluation (the SHTAC’s model).
Systematic review of existing cost-effectiveness evidence
The methods and inclusion criteria considered for this review of economic evaluations are presented in Chapter 2, Decision problem, and details of the search strategy are documented in Appendix 1.
A total of 184 citations were identified through the systematic searches. Following examination of titles and abstracts, 10 potentially relevant papers were retrieved for a more detailed inspection. Of these, seven papers were excluded, some for more than one reason. The primary reasons for exclusion were as folows: full economic evaluation was not conducted (four studies), publications were abstracts with insufficient details to allow an appraisal of the methodology and results (two studies ) and the intervention was not INTRABEAM (one study) (for details, see list of excluded studies in Appendix 5). A summary of the selection process and the reasons for exclusion is presented in Figure 3.
FIGURE 3.
Flow chart of identification of studies for inclusion in the review of cost-effectiveness.

Three publications were eligible for inclusion, two of which reported the same economic model: Alvarado et al. 106 reported a full economic evaluation based on the trial results of TARGIT-A; and Esserman et al. 107 assessed the level of confidence of the TARGIT-A trial results and the impact of early and late adoption of the trial results. The remaining study by Shah et al. 108 conducted an economic evaluation based on TARGIT-A and the Electron Intraoperative Radiotherapy (ELIOT) trial; however, the analysis based on the ELIOT trial is not relevant to this systematic review. Characteristics of the included studies106–108 are shown in Table 15 and discussed in more detail subsequently. The full data extraction forms are shown in Appendix 6.
| Characteristic | Alvarado et al.106,107 | Shah et al.108 |
|---|---|---|
| Publication year | 2013, 2014 | 2014 |
| Country | USA | USA |
| Funding source | Not stated | Not stated |
| Study type | Cost–utility analysis | Cost–utility analysis; cost minimisation analysis |
| Perspective | Societal | Societal |
| Study population | Women with early breast cancer included in TARGIT-A trial | Women with early breast cancer as included in TARGIT-A trial |
| Intervention(s) | INTRABEAM | INTRABEAM |
| Comparator(s) | 6-week WB-EBRT with a standard 33 fractions | Whole-breast irradiation (WB-EBRT) |
| Intervention effect | Kaplan–Meier estimate of local recurrence in the conserved breast at 4 years: 1.2% (95% CI 0.53 to 2.71) for INTRABEAM and 0.95% (95% CI 0.39 to 2.31) for WB-EBRT (TARGIT-A trial) | LRRs 3.3% for INTRABEAM and 1.3% for WB-EBRT (TARGIT-A trial) |
| Currency base | US$ 2011 | US$ (price year not stated) |
| Model type, health states | A Markov decision-analytic model with six health states based on the TARGIT-A trial | Not reported explicitly, analyses were based on reimbursement models |
| Time horizon | 10 years | Not clearly stated, assumed to be 10 years |
| Baseline cohort | Women aged ≥ 55 years with early breast cancer defined as stage I-IIA ER+ | TARGIT-A trial: women with early-stage ductal breast cancer who were ≥ 45 years |
| Base-case results | Costs: INTRABEAM $28,879; 6-week WB-EBRT $34,070 | Reimbursement costs ranges:a INTRABEAM $3094 to $10,179; WB-EBRT $11,726 to $13,743 |
| LY: INTRABEAM 8.38240; 6-week WB-EBRT 8.38257 | QALY: INTRABEAM 9.04; WB-EBRT 9.08 | |
| QALY: INTRABEAM 7.66020; 6-week WB-EBRT 7.65994 | ICERs for local recurrence: range $1782 to $2172 for WB-EBRT based on difference in whole-breast irradiation rates (15–21%) | |
| ICER: 6-week WB-EBRT dominated | Costs per QALY for WB-EBRT compared with INTRABEAM: range $89,234/QALY to $108,735/QALY depending on the difference in whole-breast irradiation rates |
Critical appraisal of the economic evaluations
The included cost-effectiveness studies were assessed against a critical appraisal checklist (Table 16), which appraised the quality of the studies and their generalisability to the UK. Any concerns identified by the assessment group (AG) are described below.
| Item | Alvarado et al.106,107 | Shah et al.108 |
|---|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Yes | Yes |
| 2. Is the setting comparable to the UK? | No | No |
| 3. Is the analytical and modelling methodology appropriate? | Yes | Yes |
| 4. Are all the relevant costs and consequences for each alternative identified? | Yes | Yes |
| 5. Are the data inputs for the model described and justified? | Yes | Yes |
| 6. Are health outcomes measured in QALYs? | Yes | Yes |
| 7. Is the time horizon considered appropriate? | No | ? |
| 8. Are costs and outcomes discounted? | Yes | No |
| 9. Is an incremental analysis performed? | Yes | No |
| 10. Is uncertainty assessed? | Yes | No |
Both studies clearly defined the decision problem and used the relevant intervention and comparator for the purpose of this review, although the number of fractions used in the comparator arm of WB-EBRT was not relevant to UK practice (a standard of 33 fractions was used by Alvarado et al. ,106 whereas standard UK practice is 15 fractions over 3 weeks; the number of fractions was not reported by Shah et al. 108). The patient groups of interest as well as the perspective of the studies (societal) were stated; however, as the studies were based in the USA, they are not generalisable to the UK NHS setting. It is to be noted that the TARGIT-A trial, on which both the economic evaluations were based, included pre- and post-pathology patients. The study type and modelling methodology adopted by Alvarado et al. 106 are appropriate for the decision problem in this review. Shah et al. ,108 on the other hand, do not describe the methodology but do state that the methodologies are described elsewhere.
The study by Alvarado et al. 106,107 was transparent with respect to the information on model inputs and the assumptions used. Health state-specific costs109–118 and utilities119 were populated from published literature, although it was unclear if systematic reviews were conducted to inform these parameters. Both direct and indirect costs were reported. 106,107 The utilities associated with the health states in the base-case model were obtained via standard gamble technique in the source study119 and health outcomes were reported in terms of QALYs and life-years gained. A 10-year time horizon was used; this is considered inappropriate as risk of local recurrence continues over a lifetime. A series of one-way and two-way sensitivity analyses were conducted to assess uncertainty. In addition, scenario analysis of the 3-week accelerated WB-EBRT schedule of 16 fractions was performed. Although the results of the one-way sensitivity analyses favoured INTRABEAM over WB-EBRT in the treatment of patients with early-stage breast cancer, the robustness of the results still remains questionable as a probabilistic sensitivity analysis (PSA) was not conducted. The external validity of the economic model was assessed by comparing the findings with the published results of TARGIT-A, as well as against an online tool for adjuvant therapy and published cost-effectiveness evidence in the disease area using WB-EBRT as one of the comparator arms. The results of the base-case model were comparable with these sources.
Shah et al. 108 reported that all assumptions and methodology adopted in the analyses were based on, and consistent with, previously published articles, references of which were obtained and examined by the AG. The methodologies adopted to estimate reimbursement costs as well as the assumptions used in cost estimations were adequately described in the references provided. The study reported health outcomes in terms of QALYs. The time horizon for the analysis was not clearly stated but based on the estimation of mean utility by reimbursement technique it was assumed to be 10 years. No sensitivity or validation checks were reported, thus raising questions about the robustness of the results presented.
Description and results of the published economic evaluations
Alvarado et al.106
Modelling approach
Alvarado et al. 106 developed a Markov decision-analytic model in TreeAge Pro 11 software (TreeAge Software, Inc., Williamstown, MA, USA) to assess the cost-effectiveness of INTRABEAM compared with WB-EBRT, based on the results of the TARGIT-A trial. The analysis was conducted over a 10-year time horizon with annual model cycles. Patients’ transition through the model was clearly stated. The six health states were:
-
disease-free status post BCS
-
disease free following local recurrence + salvage mastectomy
-
disease free following local recurrence + salvage lumpectomy
-
metastases
-
death due to other causes
-
death due to metastatic breast cancer.
All patients entering the model were assumed to be in a healthy state without evidence of the disease, having initially undergone BCS and allocated radiation treatment. Patients with local recurrence who initially received WB-EBRT were treated with salvage mastectomy followed by immediate reconstruction; however, patients with local recurrence who had initially received INTRABEAM also had the option of salvage lumpectomy followed by WB-EBRT. Patients could die from any other causes at any time in the model, although death resulting from breast cancer was possible only for those women who had metastatic breast cancer. A total of 14.1% of women with INTRABEAM received an additional 5 weeks (28 fractions) of WB-EBRT. Costs and benefits were discounted at 3% per annum. Costs were expressed in US$ and the price year was 2011.
Assumptions
Alvarado et al. 106 incorporated the following assumptions to inform the cost–utility model:
-
It was assumed that LRRs progressed linearly over 10 years. This is a strong assumption and should be treated with caution.
-
For women treated with INTRABEAM followed by WB-EBRT, it was assumed that they incurred the same LRRs as those who had INTRABEAM alone.
Estimation of effectiveness
Alvarado et al. 106 sourced inputs for rates and probabilities from published literature. 64,109,120–122 Data for the 4-year LRRs from the TARGIT-A trial64 were converted to annual transitional probabilities and projected over a 10-year period. The Kaplan–Meier estimate of local recurrence in the conserved breast at 4 years was estimated to be 1.20% (95% CI 0.53 to 2.71) for the INTRABEAM arm and 0.95% (95% CI 0.39 to 2.31) in the WB-EBRT arm.
Estimation of quality-adjusted life-years
Alvarado et al. 106 stated that, where possible, health state utilities were obtained via standard gamble preferences. These were sourced from a 1998 publication which evaluated HRQoL in breast cancer patients treated with lumpectomy and radiotherapy. 119 The utilities for INTRABEAM, 6-week WB-EBRT and INTRABEAM followed by 5-week WB-EBRT were assumed to be the same, at 0.92. The utility associated with salvage mastectomy was valued at 0.82 and that associated with salvage mastectomy followed by WB-EBRT at 0.87. Patients with metastatic breast cancer were assigned a value of 0.70.
Estimation of costs
A societal perspective was adopted for the analyses, including both direct and indirect costs (resource use was not reported). Direct costs included by Alvarado et al. 106 were costs of the physician, facility fees for various surgical and radiotherapy therapy treatments and costs of the metastatic health state. Surgical and treatment costs were estimated using Medicare reimbursements and the costs associated with the metastatic states were sourced from published literature. The intervention costs were reported as follows: INTRABEAM, US$5547; 6-week WB-EBRT, US$10,464; INTRABEAM followed by 5-week WB-EBRT, US$13,640; and 3-week WB-EBRT, US$6,640.
Indirect costs were derived from published data and were estimated as follows: INTRABEAM followed by 5-week WB-EBRT, US$1244; 6-week WB-EBRT, US$1467; and 3-week WB-EBRT, US$667.
Cost-effectiveness results
For the base-case analysis, Alvarado et al. 106 found that INTRABEAM resulted in a QALY gain of 0.00026 and cost US$5191 less than 6-week WB-EBRT. Therefore, the incremental cost-effectiveness ratio (ICER) of INTRABEAM dominated 6-week WB-EBRT as it was cheaper and more effective. One-way and two-way sensitivity analyses, conducted to check uncertainty in the base-case model prediction, further supported the base-case results. External validity of the model was assessed and the predicted 4-year recurrence rate of INTRABEAM in the model was similar to that in TARGIT-A trial as well as the predicted 10-year overall survival in the model compared with the results of an online tool of an adjuvant therapy and a published cost-effectiveness model.
Summary of key issues
-
The study Alvarado et al. 106 was based on the US health-care system; hence it is not generalisable to the UK setting. Further, a societal perspective was adopted which differed from the UK NHS and Personal Social Services (PSS) perspectives.
-
The model included results from both pre-pathology and post-pathology patients.
-
The number of fractions of WB-EBRT was not relevant to UK practice. The study used the assumption of using WB-EBRT with a standard 33 fractions whereas the current standard UK practice is 15 fractions. (The impact of variations in WB-EBRT fractions is explored in the AG’s Independent Economic Evaluation with results presented in Results of independent economic analysis and Table 39.)
-
Uncertainty around the base-case results was not fully explored, a very limited number of one-way and two-way sensitivity analyses were conducted, and PSA was not performed.
-
The economic analysis was conducted for a time horizon of 10 years, which is inappropriate given that risk of local recurrence continues over a lifetime.
Shah et al.108
Modelling approach
Shah et al. 108 analysed the cost-effectiveness of IORT compared with WB-EBRT and accelerated partial breast irradiation (APBI) through reimbursement models based on the results of two trials, TARGIT-A and ELIOT. The results based on the ELIOT trial were not extracted as the intervention was not eligible for inclusion in this systematic review. The study estimated reimbursement models in four ways:
-
reimbursement only (professional and facility)
-
reimbursement incorporating additional medical costs (e.g. increased operative time with IORT, fraction of IORT patients requiring additional radiation)
-
reimbursement requiring non-medical costs
-
reimbursement incorporating costs associated with recurrences.
A cost minimisation analysis was also conducted based on the absolute difference in reimbursements by techniques. The ICER analysis provided the increased reimbursement required to use WB-EBRT or APBI compared with IORT per percentage point of improvement in local recurrence. The study, in general, did not adhere to the prescribed modelling techniques advocated by NICE. Price year and discount rates were not reported.
Estimation of effectiveness
Shah et al. 108 obtained LRRs for both the INTRABEAM and WB-EBRT arms (3.3% for INTRABEAM vs. 1.3% for WB-EBRT) from the TARGIT-A trial.
Estimation of quality-adjusted life-years
The utility values used by Shah et al. 108 were obtained from the same source119 as Alvarado et al. ,106 as outlined above. A utility of 0.92 was assigned to the ‘no recurrence’ health state, 0.779 to ‘local recurrence’, and 0.685 to the ‘other recurrence’ health state.
Estimation of costs
A societal perspective was adopted for the analyses, including both direct and indirect costs. Details of the costs (direct and indirect) used in the analysis by Shah et al. 108 are described elsewhere. 80,123–125 A detailed overview of the methods to estimate non-medical costs, follow-up costs and costs of local recurrence or other recurrence (including salvage mastectomy) was presented. Reimbursement costs for INTRABEAM and WB-EBRT were reported as outlined in Table 17. Non-medical costs were reported as US$44.96 and US$89.92 per day for once-daily and twice-daily treatment schedules, respectively. Non-medical costs were estimated as follows (Shah et al.,108 p. 143):
-
Average round trip travel was 40 miles to the radiation centre (36 cents per mile).
-
The time involved was 2 hours per treatment, including travel, of which 30 minutes was spent receiving treatment (US$14.78 per hour).
-
Patients receiving twice daily treatment returned to work during the interfraction period.
| Reimbursement type | INTRABEAM | WB-EBRT |
|---|---|---|
| Total reimbursement | US$3094 | US$11,726 |
| Reimbursement including additional medical costsa | US$8003–8706 | US$11,726 |
| Reimbursement including medical and non-medical costsa | US$8192–8971 | US$12,985 |
| Reimbursement including medical, non-medical and recurrence costs (TARGIT)a | US$9399–10,179 | US$13,743 |
Cost-effectiveness results
Based on the TARGIT-A trial results, Shah et al. 108 reported that the ICERs for local recurrence ranged from US$1782 to US$2172 for WB-EBRT, based on the difference in whole-breast irradiation rates (15–21%), when all associated costs were incorporated. The costs per QALY for WB-EBRT compared with INTRABEAM ranged from US$89,234/QALY to US$108,735/QALY depending on the difference in whole-breast irradiation rates. Results from the cost minimisation analysis indicated that the use of INTRABEAM was associated with cost savings of US$3.6–4.3M when compared with WB-EBRT.
Summary of key issues
Shah et al. 108 reported the results of cost-effectiveness analysis based on reimbursement models. This study also had a number of limitations:
-
The study was based in the USA and adopted a societal perspective, which is not generalisable to the UK NHS and PSS setting.
-
Limited information was reported on the model approach and assumptions in the included paper; however, details on model structure and assumptions were reported elsewhere.
-
The time horizon for the analysis was not clearly stated.
-
Although the techniques adopted to estimate costs associated with non-medical, follow-up, local recurrence or other recurrence (including salvage mastectomy) were mentioned, the costs were not reported, except for non-medical costs.
-
Sensitivity analysis was not conducted as part of the analysis, thereby raising questions on the robustness of the model predictions.
Summary of cost-effectiveness studies
-
Two cost-effectiveness studies, reported in three publications,106–108 were identified.
-
Both studies were based on the findings of the TARGIT-A trial.
-
Cost–utility analyses were performed to evaluate QALYs, costs and ICERs of INTRABEAM compared with WB-EBRT.
-
The analyses were conducted for a time horizon of 10 years in one study;106,107 for the other study108 it is assumed that a similar time horizon was adopted, although this was not clearly stated.
-
The quality of utility data used in both the studies is questionable. The source study by Hayman et al. 119 was an old publication and more recent data would have been appropriate, such as those identified in Southampton Health Technology Assessments Centre’s systematic review of health-related quality-of-life studies. It was also not clear whether or not a systematic approach was adopted to identify this source.
-
The perspectives, settings and comparators of both studies were not generalisable to the UK setting.
-
Alvarado et al. 106 found INTRABEAM to be a more valuable strategy with less cost and greater QALYs than WB-EBRT. Shah et al. 108 concluded that although INTRABEAM represented a potential cost-saving alternative compared with WB-EBRT, the latter represented a cost-effective modality compared with INTRABEAM when additional medical and non-medical costs were factored in.
Southampton Health Technology Assessments Centre’s systematic review of health-related quality-of-life studies
A systematic review of HRQoL was undertaken, which aimed to identify utility data to populate the planned independent economic model of INTRABEAM for breast cancer discussed in Independent economic evaluation.
The methods used to identify studies are described in Chapter 3, Methods, although the selection criteria were modified slightly. First, as stated in Chapter 3, Inclusion and exclusion criteria, inclusion was not limited to women with early breast cancer. After considering previous research, such as the TARGIT-A trial (discussed in Chapter 4, Quantity and quality of research available) and other cost-effectiveness studies (discussed Systematic review of existing cost-effectiveness evidence), it was anticipated that the following health states would be of potential relevance for developing an economic model. These health states were then specified a priori as eligibility criteria for the systematic review of HRQoL:
-
disease free after WLE
-
WLE + INTRABEAM
-
WLE + WB-EBRT
-
WLE + INTRABEAM + WB-EBRT
-
mastectomy and reconstruction
-
disease free after local recurrence
-
distant recurrence/metastases.
Second, although the initial intention was to include studies that reported either preference-based measures of health such as European Quality of Life-5 Dimensions (EQ-5D), Short Form questionnaire-6 Dimensions, Health Utilities Index Mark 3, disease-specific measures such as EORTC QLQ-BR23, EORTC QLQ-C30; or Short Form questionnaire-36 items, this resulted in a large number of HRQoL studies of potential relevance. Therefore, the selection criteria were narrowed to only those studies that reported patients’ QoL using the EQ-5D measure. The EQ-5D consists of five dimensions of health: mobility, self-care, ability to undertake usual activities, pain and discomfort, and anxiety and depression. It is the preferred measure of HRQoL by NICE, as it permits comparison of cost-effectiveness (e.g. in terms of QALYs) with other health-care interventions to inform decisions about recommended treatments. In addition, it has been widely used and validated in many different patient populations.
The eligibility criteria for the systematic review of QoL are summarised below.
-
Participants
-
Women with breast cancer and meeting any of the health states defined above.
-
-
Intervention/comparator
-
Radiotherapy, endocrine/hormonal therapy, chemotherapy.
-
-
Outcomes
-
EQ-5D index [EQ-5D visual analogue scale (VAS) was excluded].
-
-
Design
-
Primary research studies [mapping studies (which seek to create a mathematical link between two different QoL instruments) were excluded].
-
Studies based in the UK, Europe, North America and Australasia.
-
Studies published as abstracts or conference presentations were included only if sufficient details were provided to allow an appraisal of the methodology and assessment of the results.
-
Non-English-language studies were excluded.
-
A total of 939 potentially relevant studies were identified through the systematic searches, the majority of which (874 studies) were excluded based on titles and abstracts. Full papers of the remaining 65 studies were retrieved for further inspection. These studies were first screened to check they met all of the following five selection criteria:
-
breast cancer patients (including metastases)
-
primary research
-
EQ-5D
-
published in the English language
-
full paper or abstract with sufficient information available.
Any study that did not meet any of the above five criteria was excluded. If studies met all five criteria, they were further screened to check:
-
if EQ-5D data were reported for any of the seven health states of interest
-
if the geographical origin of the participants was the UK, Europe, North America or Australasia. The geographical locations were limited to these regions owing to similar racial compositions.
Studies were included in this review if they met all of the above criteria.
Nine studies met the inclusion criteria. Some studies were excluded for more than one reason and the main reasons for exclusion of the remaining 55 studies were: not primary research (n = 3), abstracts with insufficient details (n = 19), inappropriate participants (n = 9), studies not reporting EQ-5D data (n = 11) and no utility data on any of the seven health states of interest for the purpose of this review (n = 13). A summary of the selection process and the reasons for exclusion are presented in Figure 4 and Appendix 7, respectively.
FIGURE 4.
Flow chart of identification of studies for inclusion in the review of QoL.
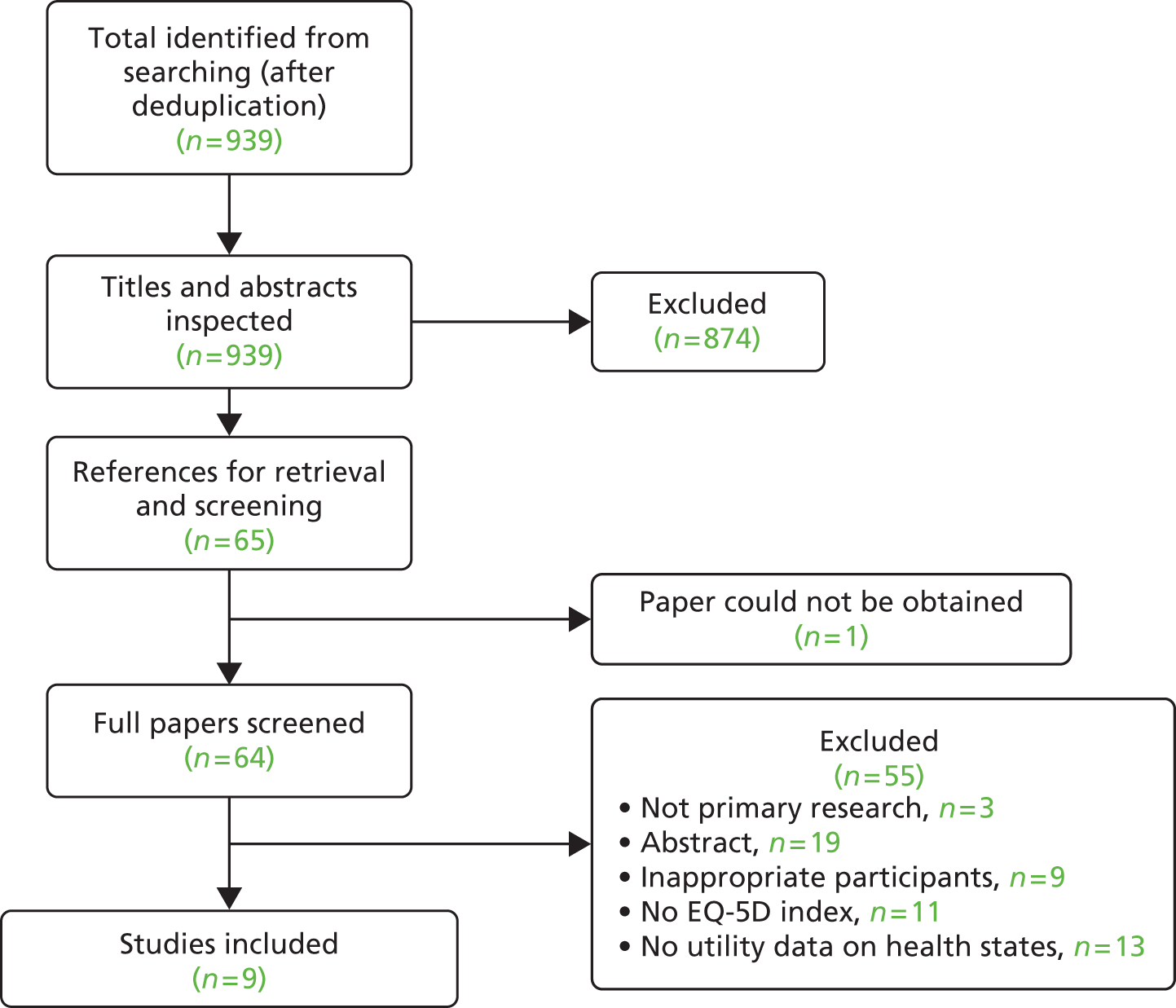
The characteristics of the nine included studies are presented (Table 18) and discussed according to the health states outlined earlier. The studies were diverse in terms of their aims, comparisons made, patient characteristics and locations. Full data extraction of all the included studies is shown in Appendix 8. The nine studies provided data for five out of the seven health states potentially relevant for the independent model: disease free after WLE (one study),126 WLE + WB-EBRT (three studies),127–129 disease free after local recurrence (one study),132 mastectomy and reconstruction (two studies),130,131 and distant recurrence/metastatic breast cancer (three studies). 132,133,134 No EQ-5D data were identified for the health states WLE + INTRABEAM or WLE + INTRABEAM + WB-EBRT. Out of the nine studies, two studies each were based in the UK,126,128 the USA,127,129 and Sweden,131,132 one study each was based in Canada130 and Germany,134 and the remaining study was based on a RCT conducted across the UK and USA. 133
| Author | Turnbull et al.126 | Freedman et al.127 | Prescott et al.128 | Serra et al.129 | Conner-Spady et al.130 | Robertson et al.131 | Lidgren et al.132 | Sherrill et al.133 | Hildebrandt et al.134 |
|---|---|---|---|---|---|---|---|---|---|
| Publication year | 2010 | 2010 | 2007 | 2012 | 2005 | 2012 | 2007 | 2008 | 2014 |
| Country | UK | USA | UK | USA | Canada | Sweden | Sweden | UK and USA | Germany |
| Study type | RCT | Single cohort study | RCT and a non-randomised cohort | Single cohort study | 2-year longitudinal study | Retrospective descriptive study | Cross-sectional observational study | RCT Q-TWiST analysis | Cross-sectional survey |
| Health state relevant to the SHTAC’s model | Disease free after WLE | WLE + WB-EBRT | WLE + WB-EBRT | WLE + WB-EBRT | Mastectomy and immediate reconstruction | Mastectomy and immediate reconstruction | Disease free after local recurrence, distant metastases | Distant metastases | Distant recurrence/metastases |
| Study population | 1625 women with biopsy-proven primary breast cancer | 1050 women with early-stage breast cancer treated with BCS and radiation with or without systemic therapy | 253 women with ‘low-risk’ axillary node-negative breast cancer undergoing BCS + endocrine therapy | 66 women undergoing radiation therapy for breast cancer | 52 women with stages II and III breast cancer at high risk of relapse | 223 IBR patients with implants | 345 women with a previous diagnosis of breast cancer | 399 women with advanced or metastatic HER-2+ breast cancer who had progressive disease following prior therapy including an anthracycline, a taxane and trastuzumab (Herceptin®, Roche) | 592 patients with breast (n = 497), cervical, endometrium, ovarian or other gynaecological cancer |
| Study population age | MRI scan: 56.38 years (SD 9.67 years); no MRI scan: 56.59 years (SD 10.09 years) | 18–44 years: 13%; 45–64 years: 68%; > 64 years: 30% | Radiotherapy: 72.3 years (SD 5.0 years); no radiotherapy: 72.8 years (SD 5.2 years) | 57 years (range 28–77 years) | 44.7 years (SD 8.5 years) | Mean age at IBR: 50 years | 57 years (range 28–93 years); < 50 years: 26%; 50–64 years: 52%; ≥ 65: 22% | Not reported | All patients: 59.07 years (range 20.12–83.33 years) |
| Comparator population | No MRI scan | No comparator | No radiotherapy | No comparator | No comparator | No comparator | No comparator | Capecitabine (Xeloda®, Roche) | No comparator |
| Interventions | MRI scan | BCS and radiation | Radiotherapy | Guided imagery (a stress reduction technique) | HDC treatment with autologous blood stem cell transplantation | Immediate breast reconstruction | No intervention | Lapatinib (Tyverb®, GSK) combined with capecitabine | No intervention |
| QoL instrument used | EQ-5D | EQ-5D | EQ-5D | EQ-5D | EQ-5D | EQ-5D | EQ-5D | EQ-5D | EQ-5D |
| Time period where HRQoL instruments administered | Baseline, 8 weeks post randomisation, 6 and 12 months post initial surgery | 5 years, 10 years, 15 years | Baseline, 3.5 months, 9 months, 15 months | Prior to start of guided imagery treatment; end of radiation therapy | Pre-induction; day 1 third cycle of FAC chemotherapy; 3 week post HDC; 6 months; 12 months; 18 months; 24 months | Median 4 years post operatively | Administered once | HRQoL data specific to the different time points of the study were not reported; the study reported only average utility values | Administered once |
Critical appraisal of the included studies
A summary of the critical appraisal of the included studies is presented in Appendix 9.
The designs of the included studies varied: three were RCTs,126,128,133 two were single-cohort studies,127,129 one was a longitudinal study,130 one was a retrospective descriptive study131 and two were cross-sectional studies. 132,134
All nine included studies defined the study question and explained the treatment strategies. Across the studies, the study designs as well as the methods of recruiting participants were clearly outlined. The studies were transparent with regard to the information provided for the methodologies applied. One study did not include patients aged < 65 years,128 another excluded those aged > 65 years130 and three studies did not state clearly if any individuals relevant to this review were excluded. 129,131,134 One study131 did not describe participant characteristics. With respect to the sample size, only two studies126,129 provided an appropriate justification for the study sample size. The response rates to EQ-5D were not reported in two studies128,133,134 thereby raising questions on the validity of the reported results as a lower response rate could possibly result in biased outcomes. Similarly, loss to follow-up was not reported by four studies127,129,131,134 and loss to follow-up would also impact on the validity of the results.
The included studies were assessed on the basis of their relevance to the NICE reference case. Of the nine included studies, only three128,126,132 met all of the criteria (see Appendix 8). Five studies did not meet one of the criteria, as valuations of HRQoL were not undertaken from the general UK population. 127,129,131,133,134 The population characteristics in the remaining study did not match those described in the decision problem as they included women with a poor prognosis (stage II/III). 130
Of the included studies, only one study reported utility value for disease free after WLE. 126 This study was UK based and included patients aged ≥ 18 years. Three studies reported utility values for the WLE + WB-EBRT health state, of which one was based in the UK128 and two were US based. 127,129 Patients in the study by Freedman et al. 127 were > 18 years of age and those in the study by Serra et al. 129 ranged from 28 years to 77 years of age. The UK-based study by Prescott et al. 128 excluded women aged < 65 years and the mean age of the baseline cohort was 72 years. It was observed that the baseline patient characteristics with respect to age differed across the three studies. Freedman et al. 127 included women with early-stage breast cancer for their analysis, which was similar to the population of interest for the independent model. In addition, they reported outcomes at a longer follow-up of up to 15 years.
The utility values for the health state of mastectomy and immediate reconstruction were reported by two studies. 130,131 Robertson et al. 131 conducted a retrospective study based on Swedish breast cancer patients who had undergone immediate breast reconstruction with implants. Conner-Spady et al. ,130 on the other hand, conducted a longitudinal study in Canadian women with stage II or III breast cancer and at high risk of relapse. The study by Robertson et al. 131 had advantages over Conner-Spady et al. 130 with respect to larger sample size, recent publication date and longer follow-up period. Furthermore, women aged > 65 years were not included in the Canadian study. 130
Three studies reported utility associated with distant metastases,132,133,134 one of which also reported utility associated with disease-free status after local recurrence. 132 Sample size ranged from 345132 to 497. 134 In two of these studies, the median age of population was reported and was 57 years132 and 59 years;134 no information related to age was provided in the other study. 133 Lidgren et al. 132 included women with a previous diagnosis of breast cancer, while Sherrill et al. 133 focused on those with advanced or metastatic HER-2-positive (HER-2+) breast cancer who had progressive disease. Hildebrandt et al. 134 included both male and female patients affected by breast, cervical, endometrium, ovarian and other gynaecological cancer, and reported data separately for each disease.
Results
The utility values for the potentially relevant health states extracted from the nine included studies are tabulated in Table 19.
| Study (country) | Health state | EQ-5D estimates | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Turnbull et al.126 (UK) | Disease free after WLE | MRI scanNo MRI scanBaseline0.85670.86018 weeks post randomisation0.77910.77286 months post initial surgery0.80400.793512 months post initial surgery0.81010.8112 | MRI scan | No MRI scan | Baseline | 0.8567 | 0.8601 | 8 weeks post randomisation | 0.7791 | 0.7728 | 6 months post initial surgery | 0.8040 | 0.7935 | 12 months post initial surgery | 0.8101 | 0.8112 | |
| MRI scan | No MRI scan | ||||||||||||||||
| Baseline | 0.8567 | 0.8601 | |||||||||||||||
| 8 weeks post randomisation | 0.7791 | 0.7728 | |||||||||||||||
| 6 months post initial surgery | 0.8040 | 0.7935 | |||||||||||||||
| 12 months post initial surgery | 0.8101 | 0.8112 | |||||||||||||||
| Freedman et al.127 (USA) | WLE + WB-EBRT | 0.89 (95% CI: 0.87 to 0.91) at 5 years | |||||||||||||||
| 0.90 (95% CI: 0.86 to 0.94) at 10 years | |||||||||||||||||
| 0.90 (95% CI: 0.83 to 1.00) at 15 years | |||||||||||||||||
| Prescott et al.128 (UK) | WLE + WB-EBRT | RadiotherapyNo radiotherapyBaseline0.770.743.5 months0.780.769 months0.760.7215 months0.740.73 | Radiotherapy | No radiotherapy | Baseline | 0.77 | 0.74 | 3.5 months | 0.78 | 0.76 | 9 months | 0.76 | 0.72 | 15 months | 0.74 | 0.73 | |
| Radiotherapy | No radiotherapy | ||||||||||||||||
| Baseline | 0.77 | 0.74 | |||||||||||||||
| 3.5 months | 0.78 | 0.76 | |||||||||||||||
| 9 months | 0.76 | 0.72 | |||||||||||||||
| 15 months | 0.74 | 0.73 | |||||||||||||||
| Serra et al.129 (USA) | WLE + WB-EBRT | 0.88 prior to the start of guided imagery therapy, 0.86 at the end of therapy | |||||||||||||||
| Conner-Spady et al.130 (Canada) | Mastectomy and immediate reconstruction | Pre induction: 0.78 | |||||||||||||||
| Day 1 third cycle of FAC chemotherapy: 0.75 | |||||||||||||||||
| 3 week post HDC: 0.61 | |||||||||||||||||
| 6 months or 8 weeks post HDC: 0.79 | |||||||||||||||||
| 12 months: 0.84 | |||||||||||||||||
| 18 months: 0.84 | |||||||||||||||||
| 24 months: 0.89 | |||||||||||||||||
| Robertson et al.131 (Sweden) | Mastectomy and immediate reconstruction | 0.83 | |||||||||||||||
| Lidgren et al.132 (Sweden) | Disease free after local recurrence, distant metastases | Patients in their first year after a primary breast cancer: 0.696 (95% CI 0.634 to 0.747) | |||||||||||||||
| Patients in first year after a recurrence: 0.779 (95% CI 0.700 to 0.849) | |||||||||||||||||
| Patients in their second and following years after primary breast cancer/recurrence: 0.779 (95% CI 0.745 to 0.811) | |||||||||||||||||
| Patients with metastatic disease: 0.685 (95% CI 0.620 to 0.735) | |||||||||||||||||
| Sherrill et al.133 (UK and the USA) | Distant metastases | Lapatinib (Tyverb®, GSK) + capecitabine (Xeloda®, Roche)CapecitabineToxicity grade (3/4)0.600.59TWiST0.660.66Relapse0.410.44 | Lapatinib (Tyverb®, GSK) + capecitabine (Xeloda®, Roche) | Capecitabine | Toxicity grade (3/4) | 0.60 | 0.59 | TWiST | 0.66 | 0.66 | Relapse | 0.41 | 0.44 | ||||
| Lapatinib (Tyverb®, GSK) + capecitabine (Xeloda®, Roche) | Capecitabine | ||||||||||||||||
| Toxicity grade (3/4) | 0.60 | 0.59 | |||||||||||||||
| TWiST | 0.66 | 0.66 | |||||||||||||||
| Relapse | 0.41 | 0.44 | |||||||||||||||
| Hildebrandt et al.134 (Germany) | Distant recurrence/metastases | Breast cancerMedianOverall0.8870Primary disease0.8870Metastatic disease0.8870Recurrent disease0.8870Both0.8870 | Breast cancer | Median | Overall | 0.8870 | Primary disease | 0.8870 | Metastatic disease | 0.8870 | Recurrent disease | 0.8870 | Both | 0.8870 | |||
| Breast cancer | Median | ||||||||||||||||
| Overall | 0.8870 | ||||||||||||||||
| Primary disease | 0.8870 | ||||||||||||||||
| Metastatic disease | 0.8870 | ||||||||||||||||
| Recurrent disease | 0.8870 | ||||||||||||||||
| Both | 0.8870 |
Disease free after wide local excision
Turnbull et al. 126 reported EQ-5D estimates for women with biopsy-proven primary breast cancer who were scheduled for WLE. The utility estimate for women randomised to the group undergoing magnetic resonance imaging (MRI) was 0.86 at baseline, 0.78 at 8 weeks post randomisation, and 0.80 and 0.81 at 6 and 12 months post initial surgery, respectively. Those randomised to receive no MRI scan had similar utility estimates to those receiving a MRI scan at baseline and 12 months post initial surgery, but slightly lower values of 0.77 and 0.79 at 8 weeks post randomisation and 6 months post initial surgery, respectively.
Wide local excision plus whole-breast external beam radiotherapy
Freedman et al. 127 reported EQ-5D estimates for women in early-stage breast cancer treated by BCS and radiotherapy with or without systemic therapy as 0.89, 0.90 and 0.90 at 5 years, 10 years and 15 years, respectively.
Prescott et al. 128 included breast cancer patients who had undergone BCS and endocrine therapy to assess the QoL and cost-effectiveness of omission of post-operative radiotherapy in women with ‘low-risk’ axillary node-negative breast cancer (T0–2). For the radiotherapy arm, reported EQ-5D estimates varied between 0.77 at baseline and 0.74 at 15 months and utility estimates varied between 0.74 at baseline and 0.73 at 15 months for the no radiotherapy arm. This study did not include patients aged < 65 years.
Serra et al. 129 assessed EQ-5D estimates on people undergoing radiotherapy for breast cancer to evaluate the impact of guided imagery (a stress reduction technique). The utility values prior to the start of radiotherapy plus guided imagery therapy and at the end of radiation therapy were reported as 0.88 and 0.86, respectively. One of the disadvantages of this study was that it reported very limited details on the inclusion/exclusion criteria; hence, it was not transparent whether or not any relevant individuals were excluded from the analysis.
Mastectomy and immediate reconstruction
Conner-Spady et al. 130 evaluated EQ-5D estimates in Canadian patients with stage II/III breast cancer who were at high risk of relapse and were receiving high-dose chemotherapy (HDC) treatment with autologous blood stem cell transplantation. There was a decrease in HRQoL from pre-induction (0.78) to 3 weeks post HDC (0.61) and return to baseline levels at 8 weeks post HDC (0.79). The EQ-5D estimate at 2 years was 0.89. In the short term, there was a negative impact on HRQoL by treatment, but this quickly rebounded and no data were available for the long term. EQ-5D estimates specific to different types of surgery (modified radical mastectomy, total mastectomy and segmental surgery) were not reported. Patients aged > 65 years were excluded.
Robertson et al. 131 presented an audit of all immediate breast reconstructions (IBRs) during the period 2005–8 performed by breast surgeons and investigated post-operative HRQoL in a Swedish setting. The EQ-5D estimate was reported as 0.83. The study did not state clearly if any relevant individuals were excluded; therefore, generalisability of the results is unclear.
Disease free after local recurrence, distant metastases
In a cross-sectional observational study, Lidgren et al. 132 estimated HRQoL for different breast cancer disease states in Swedish women with a previous diagnosis of breast cancer. This study reported EQ-5D estimates for two health states: disease free after local recurrence and distant metastases. Patients in the first year after a primary breast cancer had a EQ-5D estimate of 0.696. EQ-5D estimates in the first year after local recurrence and in the second and following years after both primary breast cancer and local recurrence were same at 0.779, and patients in metastatic disease had a EQ-5D estimate of 0.685.
Sherrill et al. 133 conducted a quality-adjusted time without symptoms of disease or toxicity of treatment (Q-TWiST) analysis in patients with advanced or metastatic HER-2+ breast cancer who had progressive disease following prior therapy including an anthracycline, a taxane and trastuzumab (Herceptin®, Roche). The study compared health states in patients receiving combination therapy of lapatinib (Tyverb®, GlaxoSmithKline) plus capecitabine (Xeloda®, Roche) and those receiving capecitabine alone. The EQ-5D estimate associated with the relapse health state was reported as 0.41 for the lapatinib plus capecitabine arm compared with 0.44 for capecitabine monotherapy arm. However, this trial was stopped early before attaining the sample size.
In a cross-sectional survey, Hildebrandt et al. 134 investigated health utilities as cardinal values of individuals’ preferences for specific health-related outcomes in women treated in Germany in the fields of gynaecological oncology and mastology to provide local German data. The study found that patients with breast cancer who had primary disease had the highest estimates of QoL as measured by EQ-5D VAS and these declined if the disease was already advanced. However, this difference was not evident from the EQ-5D health index in patients with primary disease, metastatic disease, recurrent breast cancer, or both recurrence and metastatic disease, which had a consistent median value of 0.8870.
When comparing the EQ-5D estimates across the potentially relevant health states in breast cancer patients reported in the studies included in this review, it is observed that there are variations in EQ-5D estimates for similar health states. These differences could be explained by the differences in patient characteristics, country settings, nature of the intervention(s) and comparators(s) used in the treatment of breast cancer patients across different countries, and length of follow-up.
Summary and conclusions of the health-related quality-of-life review
The key findings of this systematic review are summarised below.
-
Nine studies met the inclusion criteria of the HRQoL systematic review.
-
Two studies were UK based and the remaining studies were based in Europe and North America.
-
The included studies were diverse with respect to their aims, population of interest, geographical locations, interventions, comparators, study designs and methodologies adopted.
-
The review identified utilities that could be used to inform the independent cost-effectiveness model for five out of seven potentially relevant health states: disease free after WLE; WLE + WB-EBRT; disease free after local recurrence; mastectomy and immediate reconstruction; and distant recurrence.
-
The review did not identify any relevant study to populate the utilities for two potentially relevant health states: WLE + INTRABEAM or WLE + INTRABEAM + WB-EBRT.
Review of the evidence submission from Carl Zeiss, UK, to the National Institute for Health and Care Excellence
A structured data extraction form was used to guide the review of the submission by Carl Zeiss, UK, to NICE (see Appendix 4). The MS evaluated the cost-effectiveness of INTRABEAM in early breast cancer patients when compared with radiotherapy, which is usually given in the UK over 3–6 weeks as WB-EBRT. The total costs, QALYs gained and cost-effectiveness associated with the intervention and comparator under consideration in the appraisal were reported in the MS. The perspective adopted in the submission was that of the NHS, capturing direct costs and benefits only. A systematic review of any relevant cost-effectiveness models was not conducted. Very limited information on the model was presented in the main submission document and, although further details were contained within the Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) model, these too were limited.
Modelling approach
A multistate Markov model, developed in Microsoft Excel, was used in the submission. The model used a cohort of breast cancer patients aged ≥ 55 years who were disease free after WLE. The economic model was based on the results of the pre-pathology stratum of the TARGIT-A trial65 with 2298 patients. This was because results were less favourable in post-pathology stratum (see Chapter 4, Assessment of effectiveness) and the submission recommended that INTRABEAM be used in pre-pathology patients only (MS, pp. 3–4).
It was not reported whether the model was constructed de novo or adapted from another previously existing model. The model consisted of four health states:
-
disease free
-
local recurrence treated by mastectomy/lumpectomy
-
non-breast cancer death
-
breast cancer death.
Patients in the disease-free state could remain in that state or transition to either local recurrence or non-breast cancer death. Those in the local recurrence state could remain in that state or die from either non-breast cancer or breast cancer-related deaths. The two death states were the absorbing states. The analysis was conducted for a time period of 20 years with an annual cycle length.
Assumptions
The manufacturer’s model made the following assumptions:
-
After local recurrence, INTRABEAM patients would undergo salvage lumpectomy.
-
After local recurrence, WB-EBRT patients would undergo salvage mastectomy. There is also an undocumented assumption that all patients undergoing mastectomy have reconstruction, which is reflected in the high cost of mastectomy.
-
The death rate in disease-free patients was equal to that in the general population.
-
An average of 23 fractions of WB-EBRT per patient were delivered, based on 15–30 fractions in the clinical practice.
-
All patients were given INTRABEAM concurrent with initial lumpectomy (pre-pathology stratum of TARGIT-A trial).
A few of the model assumptions are not relevant to UK practice. The model assumed that INTRABEAM patients would undergo salvage lumpectomy after local recurrence; however, clinical experts advised that in the UK most patients would undergo mastectomy after local recurrence instead. Furthermore, the undocumented assumption that all mastectomy patients would undergo reconstruction is not in line with UK practice, as only around 31% of the patients undergoing mastectomy will have reconstructions, as shown in the independent model discussed in Methods for economic analysis. In addition, the assumption of using an average of 23 fractions of WB-EBRT per patient was not appropriate as the current standard UK practice is 15 fractions.
Critical appraisal of model
The manufacturer’s economic evaluation was appraised for methodological quality and generalisability to the UK NHS using a checklist adapted from the NICE reference case requirements and the Philips et al. 58 checklist (Table 20). The evaluation met half of the requirements for methodological quality and generalisability, and the remaining criteria were either not met or unclear; therefore, the evaluation did not fully meet the NICE reference case. A brief description is presented below.
| Item number | Item | Carl Zeiss |
|---|---|---|
| 1 | Is there a clear statement of the decision problem? | Yes |
| 2 | Is the comparator routinely used in UK NHS? | ?a |
| 3 | Is the patient group in the study similar to those of interest in UK NHS? | ?b |
| 4 | Is the health care system comparable to that in the UK? | Yes |
| 5 | Is the setting comparable to the UK? | Yes |
| 6 | Is the perspective of the model clearly stated? | Yes |
| 7 | Is the study type appropriate? | Yes |
| 8 | Is the modelling methodology appropriate? | ? |
| 9 | Is the model structure described and does it reflect the disease process? | Yes |
| 10 | Are assumptions about model structure listed and justified? | No |
| 11 | Are the data inputs for the model described and justified? | No |
| 12 | Is the effectiveness of the intervention established based on a systematic review? | No |
| 13 | Are health benefits measured in QALYs gained? | Yes |
| 14 | Are health benefits measured using a standardised and validated generic instrument? | Yes |
| 15 | Are the resource costs described and justified? | No |
| 16 | Have the costs and outcomes been discounted? | Yes |
| 17 | Has uncertainty been assessed? | ? |
| 18 | Has the model been validated? | No |
The manufacturer’s evaluation provided a clear statement of the decision problem to be addressed, which appeared to follow the scope for the appraisal issued by NICE. Although the comparator included WB-EBRT, which is routinely used within the NHS, its appropriateness is questionable as the number of WB-EBRT fractions used in the UK practice is 15 compared with 23 fractions used in the model.
Six out of 33 centres in the TARGIT-A trial were based in the UK and centres were allowed to follow local policy for WB-EBRT delivery. The MS reported 23 fractions as the average of the range between 15 and 30 fractions being used in all the countries in the trial, but it was not clear if this was a weighted average of fractions used in the trial or a midpoint. The perspective adopted in the model was appropriate and, although the MS reported that the analysis was UK based, limited details were provided on the baseline characteristics of the patient population. A Markov modelling methodology was used, which seemed appropriate given the clinical nature of breast cancer; however, the AG considered that the reported model was a simplified structure with only four health states and that an additional health state for progressed disease would have been appropriate. Another limitation was that a lifetime horizon was not adopted.
Patients entering the model were aged 55 years (on average) and were followed for 20 years. This time span might not reflect the entire follow-up period of the disease. Patients transitioned through the health states in annual cycles, which is an appropriate assumption. The model structure was presented diagrammatically but no justification of the key assumptions and description of the data inputs used was provided. Measures of clinical effectiveness were obtained from a single study;65 however, no other relevant trials were identified by the SHTAC’s systematic review. Benefits for the model were measured in QALYs using standard gamble for measuring utility, although the source study was dated 1997. 135 It was not clear if a systematic review was conducted to identify the study. The model extrapolated local recurrence and survival data beyond 5 years by tacitly assuming an exponential fit to time to local recurrence; however, the AG considers that a log-normal distribution would be the best fit based on comparison with external data (see Data sources). All benefits and costs were discounted at 3.5% as outlined in NICE guidance. Uncertainty was assessed through PSA and no one-way or scenario analyses were conducted. Finally, no details around model validation were provided.
Estimation of effectiveness
Data on effectiveness for both the intervention (INTRABEAM) and the comparator (WB-EBRT) were derived from a single RCT (TARGIT-A) by Vaidya et al. 65 and 5-year cumulative risks reported in the source study were converted to annual probabilities and populated in the model. It was not reported whether or not a systematic review was conducted to identify the source study; however, no other relevant trials were identified by the SHTAC’s systematic review (see Chapter 4, Quantity and quality of research available). No adverse events were included in the analysis, which was considered appropriate by the AG.
Estimation of quality-adjusted life-years
Health-related quality-of-life utility values were assigned to patients in the disease-free state, those undergoing salvage lumpectomy and those undergoing salvage mastectomy. A utility value of 0.92 was assigned to patients in the disease-free state, a value of 0.87 to patients undergoing salvage lumpectomy and a value of 0.82 to those undergoing salvage mastectomy. The MS obtained these values from a single study by Hayman et al. published in 1997. 135 No details were provided of the method of deriving these values or the rationale used. The source study135 used a standard gamble approach to estimate utility values, which were not obtained from the general population. This is a limitation as it was shown in the systematic review of HRQoL (see Southampton Health Technology Assessments Centre’s systematic review of health-related quality-of-life studies) that there were several other more recent and relevant HRQoL studies that used the EQ-5D measure.
Estimation of costs
Treatment unit costs were obtained from the following sources: expert opinion, reference costs 2012–13,136 payments by results tariff 2013–14,137 and the study by Wolowacz et al. 138 As with clinical effectiveness and utilities, the methods of deriving the costs were not adequately described. The costs associated with travel/parking/accommodation were appropriately not included within the WB-EBRT arm (it was stated that these expenses might range from £50 to £100 per patient per fraction delivered).
The validity of the costs estimates is questionable. The cost of INTRABEAM per patient was obtained from expert opinion and although the manufacturer provided the cost compositions of INTRABEAM, it was not transparent in explaining the assumed cost per patient. In addition, cost of WB-EBRT was obtained from an inappropriate Healthcare Resource Group (HRG) code, the code used in the model for WB-EBRT was for ‘other radiotherapy treatment’, whereas the AG considers that the HRG code description required for the purpose of this analysis is ‘deliver a fraction of radiotherapy on a megavoltage machine’, which includes WB-EBRT delivered by linear accelerator, as per the NICE scope. The AG considers that HRG codes SC22Z and SC23Z are required for treatment delivery, and SC45Z, SC46Z, SC47Z and SC48Z are required for WB-EBRT (see Data sources and Table 31). Costs were only varied by ± 10% in PSA. There were also inconsistencies in the sources used to populate the reported costs; for instance, the costs of treating post-INTRABEAM local recurrence (salvage lumpectomy) and that of treating post-WB-EBRT local recurrence (salvage mastectomy) were obtained from payments by results tariff 2013–14, whereas the cost of WB-EBRT was obtained from the reference costs 2012–13. 137 The use of reference costs is preferable and would be considered standard practice.
Cost-effectiveness results
The base-case results from the submission are shown in Table 21 and indicate that INTRABEAM is associated with higher QALYs and lower costs. The submission states that the incremental analysis shows dominance of INTRABEAM over WB-EBRT.
| Intervention | Mean QALYs | Mean cost (£) | ICER vs. WB-EBRT (cost/QALY) |
|---|---|---|---|
| INTRABEAM | 13.230 | 14,461 | Dominates |
| WB-EBRT | 13.223 | 20,926 | |
| Incremental | 0.012 | –6465 |
One-way sensitivity analyses and scenario analyses were not conducted. A PSA was undertaken using Monte Carlo simulation with 1000 iterations. The cost parameters in the model were assigned to beta-project evaluation and review technique (PERT) distributions and beta distributions were assigned to utilities. For the cost parameters, the AG considers that gamma distribution would have been a more standard choice. It is not usual practice to assign beta-PERT distribution; however, it is expected that this would have little impact. For the PSA, at the £20,000 and £30,000 willingness-to-pay (WTP) thresholds, INTRABEAM has the highest probability of being cost-effective, at 100% for both thresholds.
Critique of the manufacturer’s submission
-
The MS provides very limited information on model structure, baseline characteristics of the patient population and setting.
-
Limited information is provided with respect to input parameters such as costs and utilities. The MS is not transparent in providing the methodology adopted to inform the input parameters.
-
The method to derive costs of INTRABEAM is not clear.
-
No rationale is provided for using the specific distributions assigned to the parameters.
-
The method of extrapolation of local recurrence and survival data is not justified.
-
The number of fractions for the WB-EBRT arm used in the model (23 fractions) is higher than UK practice; this will lead to an overestimation of WB-EBRT costs.
-
The manufacturer’s model assesses health benefit in terms of QALYs, which is a valid measure of health in the UK NHS setting. The source study135 used standard gamble from a 1997 publication to estimate utilities. No details were provided as to whether or not a systematic search was conducted to identify utilities for the model.
-
Model validation was not conducted; hence, the generalisability of model results remains questionable.
-
Probabilistic sensitivity analysis was conducted for only 1000 simulations and no one-way or scenario analyses were conducted. Limited sensitivity analyses conducted around the base-case model results raise questions on the robustness of the model predictions.
-
In summary, results of the MS model should be viewed with caution owing to the methodological and reporting limitations outlined above.
Independent economic evaluation
Overview
We developed a new model to estimate the costs, benefits and cost-effectiveness of the INTRABEAM Photon Radiotherapy System compared with WB-EBRT for early operable breast cancer.
The effects of the intervention on the clinical course of the disease are obtained from the TARGIT-A trial included in the systematic review of clinical effectiveness (see Chapter 4). The patient population included in the economic model reflects the patient population in the pre-pathology stratum of this trial. This is because the TARGIT-A study recommends INTRABEAM concurrent with lumpectomy as an alternative to post-operative WB-EBRT65 but does not recommend the use of post-operative INTRABEAM as an alternative to WB-EBRT (as non-inferiority was not established in this stratum). Furthermore, use of the pre-pathology stratum provides consistency with the manufacturer’s economic model, which is also based on the results of the pre-pathology stratum.
The analysis takes the perspective of the NHS and PSS in the UK. The model adopts a lifetime (40-year) horizon to estimate costs and benefits from each treatment. Future costs and benefits are discounted at 3.5% per annum as recommended by the UK Treasury. 139 The outcome of the economic evaluation is reported as the cost saved per QALY lost.
Methods for economic analysis
The model uses transition probabilities obtained from the clinical literature to simulate the progression of early operable breast cancer in a cohort of patients and to estimate the cost-effectiveness of the radiotherapy treatments under consideration. The model was constructed using the TreeAge Pro 2014 software (TreeAge Software, Inc., Williamstown, MA, USA). The model structure was informed by a review of other published models of early breast cancer106,109,120,140–142 and the evidence available to inform disease progression, which is drawn from the only existing RCT, the TARGIT-A trial65 (see Chapter 4).
The model structure follows the disease pathway for early-stage breast cancer. It is slightly modified from an economic model structure used in a previous HTA report to NICE140 in order to reflect the clinical evidence available. The structure is also similar to the model structure adopted by Alvarado et al. 106 in their cost-effectiveness analysis of IORT. The SHTAC’s model uses six distinct health states: recurrence free, local recurrence, disease free after local recurrence, any other recurrence, death from breast cancer, and death from other causes (Figure 5). The local recurrence, disease free after local recurrence and any other recurrence health states were chosen pragmatically in order to match the definitions and data supplied by the TARGIT-A trial publication. 65
FIGURE 5.
Influence diagram for the SHTAC’s breast cancer cost-effectiveness model.

Local recurrence is defined in the TARGIT-A trial as recurrence in the conserved breast while any other recurrence incorporates regional recurrence (axilla plus supraclavicular), contralateral breast recurrence and distant recurrence. 65 The AG notes that regional recurrence, contralateral recurrence and distant recurrence have very different prognoses and costs but they are not modelled separately as no data were available to inform possible transitions to or from these health states.
Non-death health states are associated with a HRQoL utility and a cost estimate.
All patients start the model in the recurrence-free state and may then either stay in the recurrence-free state, have a local recurrence and move to the local recurrence state, have another type of recurrence and move to the any other recurrence state, or die from non-breast cancer causes. From the local recurrence state, a patient may move to the disease free after local recurrence state, suffer any other recurrence or die from other causes. A patient in the disease free after local recurrence state may remain either in this state, suffer any other recurrence or die from other causes. From the any other recurrence state, it is possible to die from breast cancer, die from other causes or stay in the state. The local recurrence state is temporary and it is only possible to remain here for one cycle.
Model cycle length is 1 year and a lifetime horizon of 40 years was adopted in the base case, which is sufficiently long to capture all clinically and economically important events. A half-cycle correction was applied.
The baseline disease progression parameters used in the model were obtained from the TARGIT-A trial (see Chapter 4). 65 These inform the annual probabilities of local recurrence, any other recurrence while recurrence free, and death from breast cancer. Data from de Bock et al. 143 were used to inform the probability of any other recurrence given local recurrence at the suggestion of the advisory group. Data from the Office for National Statistics (ONS) were used to inform the probability of all-cause mortality by age. 144 Parametric curves were fitted to Kaplan–Meier data in order to provide the probabilities of local recurrence in both treatment arms.
The costs included in the model are those for initial radiation treatment and repeat lumpectomy and mastectomy and reconstruction, with or without radiation treatment, at local recurrence. Full details of the costs used in the model are given in Data sources.
The model includes the following assumptions:
-
All patients enter the model in the recurrence-free state after initial BCS and radiation therapy.
-
It is not possible to die from breast cancer while in the local recurrence state or the disease free after local recurrence state. It is only possible to die from breast cancer while in the any other recurrence state.
-
Only one local recurrence is allowed; repeat local recurrence is not modelled.
-
Death rates for non-breast cancer causes are based on mortality statistics for England and are applied across all health states.
-
The survival of patients with recurrence of any sort is assumed to be independent of the time of recurrence.
A further simplification is that, owing to data limitations, the costs of post-progression therapies are not included in the base case.
In each cycle, the total costs and QALYs are calculated by multiplying the individual costs and HRQoL of each model state by the proportion of the model cohort in that state, for each of the radiotherapy types. The total discounted lifetime costs and QALYs are calculated by aggregating the costs and QALYs for all cycles. The ICER is calculated as:
where convention therapy A is the current standard of care and therapy B is a new therapy. The associated incremental net monetary benefit (NMB) of a specific treatment compared with a comparator may be calculated as:
when the incremental QALYs and incremental costs are simply the denominator and numerator, respectively, of equation (1) and WTP is the maximum amount a decision-maker is prepared to pay per QALY gained. 57 As long as the incremental NMB is more than zero, then a treatment is cost-effective and larger NMBs represent greater cost-effectiveness than smaller NMBs.
Model validation
The model was validated by checking the model structure, calculations and data inputs for correctness. The structure was reviewed by clinical experts to establish that it was appropriate for the disease and its treatment. Internal consistency was examined by varying input values and verification that any change to the input values produced changes in the model outputs of the expected direction and magnitude. A second modeller reproduced the model in Microsoft Excel and checked that the outputs were the same as the TreeAge Pro implementation. To establish its external consistency, the model results were compared with published outcomes of survival in early breast cancer.
Evaluation of uncertainty
The evaluation of the cost-effectiveness of radiotherapy treatment options for early operable breast cancer is based on uncertain information that includes uncertainty about the clinical effects of treatment, HRQoL while in the various health states, and resource use. Such uncertainty is examined using deterministic and probabilistic sensitivity analyses.
One-way deterministic sensitivity analyses were conducted to test the robustness of the cost-effectiveness results to variations in parameter input values when altered one at a time (see Results of independent economic analysis).
Joint variation and potential correlation in multiple parameters was addressed using PSA (see Results of independent economic analysis). In the PSA, probability distributions were assigned to the parameter point estimates used in the base-case analysis. The model was then run for 10,000 iterations with parameter values sampled at random from these distributions. The uncertainty surrounding the cost-effectiveness of the treatments is represented on a cost-effectiveness acceptability curve (CEAC), which plots the probability that an intervention will be cost-effective at a particular WTP threshold.
Scenario analysis was used to investigate the effect of uncertainty in model assumptions and structure.
Data sources
Recurrence-free state: probability of local recurrence
The baseline risk of local recurrence in the economic model is taken from the pre-pathology subgroup of the TARGIT-A trial. 65 The TARGIT-A trial was the only trial included in the review of clinical effectiveness (see Chapter 4) and as such is the main source of evidence of the clinical efficacy of INTRABEAM.
Local recurrence probabilities in the pre-pathology substratum for INTRABEAM and WB-EBRT were extracted from a Kaplan–Meier plot in the trial publication65 using the digitising software PlotDigitizer (© 2000–14 Joseph A Huwaldt) and the method of survival curve reconstruction described in Guyot et al. 145 Parametric survival models were then fitted to the observed data using Stata software version 11.0 (StataCorp LP, College Station, TX, USA) in order to extrapolate local recurrence beyond the 5 years reported. 65 In line with the recommendation of Latimer146 all of the ‘standard’ parametric models were considered (exponential, Weibull, Gompertz, log-logistic, log-normal).
Akaike information criterion (AIC) values obtained for each distribution are given in Table 22, which shows that the log-normal, log-logistic and Weibull distributions provide the best fit to the data based on this criterion. The Gompertz and exponential distributions fit the data less well. The log-normal and Weibull fits are compared graphically with the 5 years of trial data in Figure 6. The log-logistic fit is similar to the log-normal and is not considered further. Figure 6 demonstrates that the log-normal and Weibull fits are similar over this time period. Figure 7 shows the behaviour of the log-normal and Weibull fits over the model time horizon of 40 years and it can be seen that local recurrences continue to occur throughout the time horizon with both models, but that the proportion with local recurrence after 40 years is much higher under the Weibull model than under the log-normal model. Previous economic evaluations to NICE have assumed that patients who have experienced an episode of early-stage breast cancer but are in remission after 15 years will have the same risk of progression as the general population. 140 However, clinical advice to the AG is that the risk of local recurrence continues throughout life and is relatively linear over time. Data on local recurrence at 9 years from the ELIOT trial,147 and the study of Kreike et al. ,148 which follows up BCS + radiotherapy patients for 15 years, also suggest that risk of local recurrence does not decrease over time.
| Model | AIC |
|---|---|
| Log-normal | 213.0 |
| Log-logistic | 214.2 |
| Weibull | 214.2 |
| Gompertz | 217.6 |
| Exponential | 219.2 |
FIGURE 6.
Kaplan–Meier plot of local recurrence in the pre-pathology subgroup of the TARGIT-A trial65 compared with fitted log-normal and Weibull local recurrence curves.

FIGURE 7.
Kaplan–Meier plot of local recurrence in the pre-pathology subgroup of the TARGIT-A trial65 compared with fitted log-normal and Weibull local recurrence curves over 40 year time horizon.
The model adopts the log-normal curve in the base case. Not only is this a better fit by the AIC criterion, but the rate of local recurrence does not increase as steeply over time as in Weibull model (see Figure 7; see also Figure 6 for more detail of the first 5 years). This behaviour means that median survival is longer under this model and, thus, it provides a better fit to other published data on survival after breast cancer (see Model validation). Coefficients of the fitted log-normal regression model are given in Table 23.
Recurrence-free and local recurrence states: probability of any other recurrence
The baseline risk of any other recurrence while in the recurrence-free state is taken from the pre-pathology subgroup of the TARGIT-A trial. 65 The 5-year probability of any other recurrence in the WB-EBRT pre-pathology subgroup is given in the trial publication as 4.7%. The corresponding 5-year probability for INTRABEAM is 4.8%. 65 These probabilities are converted to 1-year probabilities for use in the economic model to inform the transition from the recurrence free health state to the any other recurrence health state (Table 23).
| Variable | Values | Transition probability per one year model cycle | Source |
|---|---|---|---|
| Log-normal model of time to local recurrence WB-EBRT | Constant = 4.97, sigma = 0.436 | Varies through time | Model fitted to KM data in Vaidya 201465 |
| β-coefficient for INTRABEAM in log-normal model of time to local recurrence | –0.256 | NA | Model fitted to KM data in Vaidya 201465 |
| Probability of any other recurrence WB-EBRT while recurrence free | 0.047 (5 years) | 0.0096 | Vaidya 201465 |
| Probability of any other recurrence INTRABEAM while recurrence free | 0.048 (5 years) | 0.0098 | Vaidya 201465 |
| Probability of any other recurrence given local recurrence | 0.416 (10.2 years) | 0.0514 | de Bock et al.144 |
| Probability of breast cancer death WB-EBRT | 0.027 (5 years) | 0.0055 | Vaidya 201465 |
| Probability of breast cancer death INTRABEAM | 0.033 (5 years) | 0.0067 | Vaidya 201465 |
| Probability of breast cancer death given other recurrence WB-EBRT | – | 0.5698 | Calculation |
| Probability of breast cancer death given other recurrence INTRABEAM | – | 0.6832 | Calculation |
| Probability of non-breast cancer death | Age dependent | Varies through time | ONS mortality tables145 |
The probability of any other recurrence is higher for those who have already experienced a local recurrence than for those who have not, but these more detailed data are not available from the TARGIT-A trial and would not be robust owing to the low numbers in TARGIT-A with local recurrence. 65 A previous HTA submission to NICE140 uses the study of Kamby and Sengelov149 to inform a model transition from locoregional relapse to metastatic disease. In this study, the proportion with distant disease was 72% at 10 years after locoregional relapse, giving a 1-year probability of distant disease of 0.1195 (see Table 23). In an analysis of 3601 women enrolled in randomised trials and treated for early-stage breast cancer, de Bock et al. 143 report that, of 310 women who experience locoregional recurrence, 129 experienced distant metastases after locoregional recurrence, at a median follow-up of 10.2 years. This broadly equates to a 1-year probability of distant disease given local recurrence of 0.0514. This probability is based on a much bigger sample and is more recent than the study of Kamby and Sengelov. 149 Consequently, the probability of 0.0514 derived from de Bock et al. 143 data is adopted for use in the economic model to inform the transitions from the local recurrence and disease free after local recurrence health states to the any other recurrence health state (see Table 23).
Probability of breast cancer death
In common with other economic models of early breast cancer, the SHTAC’s model assumes that all breast cancer deaths occur from the ‘any other recurrence’ state, which includes metastatic cancer. 120,140,142 Thus, in the model a breast cancer death is conditional on having had any other recurrence beforehand (see Figure 5). The TARGIT-A trial ascribed a death to breast cancer if breast cancer was present at the time of death. 65 Consequently, it is possible that a small proportion of the breast cancer deaths observed in the TARGIT-A trial occurred while a patient was experiencing local recurrence, before repeat surgery. However, given the small numbers of likely deaths from the local recurrence state, which patients only pass through for one model cycle, this is felt to be an acceptable modelling simplification.
The model requires the probability that a patient in the ‘any other recurrence’ state dies from breast cancer in a given cycle. The TARGIT-A trial publication reports both the probability of death from breast cancer and the probability of any other recurrence, by treatment arm. 65 Thus, with the model assumption that all breast cancer deaths occur after ‘any other recurrence’, the 5-year probability of death from breast cancer, given any other recurrence, can be calculated. For the WB-EBRT pre-pathology subgroup, this probability is approximately given by 0.0055/0.0096 (= 0.5698, with no input data rounding), while for the INTRABEAM pre-pathology subgroup the corresponding probability is approximately 0.0067/0.0098 (= 0.6832, with no input data rounding) (see Table 23). Assuming that time to death after any other recurrence is exponentially distributed, these probabilities correspond to a mean survival after any other recurrence of around 21 months for WB-EBRT and 17.5 months for INTRABEAM.
Probability of non-breast cancer death
The general underlying risk of mortality was modelled using a cohort life table generated from the 2010–12 female interim life tables for England. 144 The age-related mortality for each year in the model was determined from these data using the demographic characteristics of breast cancer patients in England. Specifically, in the base case, patients enter the model at an age of 62 years. This is the median age at which breast cancer is diagnosed in females in England. 150
In the model base case, the same probabilities of non-breast cancer death by age are used for both treatment arms; however, the TARGIT-A trial publication notes a statistically significant difference in non-breast cancer deaths between treatment arms, with fewer deaths in the INTRABEAM arm. 65 These data are based on a small number of events (12 non-breast cancer deaths on the INTRABEAM arm and 27 on the WB-EBRT arm). The TARGIT-A trial publication shows that the higher number of deaths on the WB-EBRT arm is due to cardiovascular causes and other cancers and states that it is improbable that there was a substantial imbalance in baseline comorbidities between the two randomised groups. 65 The AG notes, however, that patients on the WB-EBRT arm were slightly older at baseline. 64 A mean age is not supplied but the AG calculates a mean age of 62.5 years for the WB-EBRT arm and of 62 years for the INTRABEAM arm, for all patients. (Ages at baseline for the pre-pathology stratum alone are not supplied.) The AG has also compared the annual probabilities of death on the WB-EBRT arm with annual all-cause mortality probabilities obtained from ONS data144 and found that they are similar. Therefore, the AG does not consider that there is an excess of deaths on the WB-EBRT arm, but rather a shortfall of deaths on the INTRABEAM arm, which is likely to have arisen owing to chance and/or the slightly younger mean age of patients in this arm of the trial.
Therefore, the model does not adopt trial-observed non-breast cancer mortality data for use in the base case, but they are examined in scenario analysis reported in Results of independent economic analysis.
Health-related quality of life
The systematic review of HRQoL identified nine studies that met the inclusion criteria (see Table 18). Six of the included studies provide EQ-5D values for the ‘recurrence-free’ state in the economic model (see Table 19). 126–128,132,134 Two of these studies are US based,127,129 one is Swedish,132 one is German134 and two are UK based. 126,128 Breast cancer treatment in other countries can differ from the UK and so a UK-based study is preferable. However, one of the UK-based studies128 has a mean participant age of approximately 72 years. This is 10 years older than the population under consideration here. Consequently, the other UK study, the COMICE (comparative effectiveness of MRI in breast cancer) trial of Turnbull et al. ,126 was selected as it provides EQ-5D values for younger patients after WLE. 126 The COMICE trial was a reasonably large RCT (1623 participants in two arms) of women with biopsy-proven primary breast cancer scheduled for WLE and reports EQ-5D values at four time points. Participants had a mean age at randomisation of 57 years. The time points of ‘8 weeks post randomisation’ and ‘12 months post initial surgery’ were chosen from the no intervention arm of the trial for use in the recurrence-free state in the model. These reflect utility in the first year after WLE and utility thereafter (Table 24).
| Model health state | EQ-5D (SE) | Source |
|---|---|---|
| Recurrence free in first year | 0.7728 (0.0079) | Turnbull et al.,126 no MRI arm at the 8-week post randomisation time point |
| Recurrence free after first year | 0.8112 (0.0072) | Turnbull et al.,126 no MRI arm at the 12 months post initial surgery time point |
| Local recurrence | 0.8112 (0.0072) | Turnbull et al.,126 no MRI arm at the 12 months post initial surgery time point |
| Disease free after local recurrence | 0.8112 (0.0072) | Turnbull et al.,126 no MRI arm at the 12 months post initial surgery time point |
| Any other recurrence | 0.685 (0.0293) | Lidgren et al.132 |
The Swedish study by Lidgren et al. 132 identified in the systematic review of QoL provides EQ-5D estimates for four states of breast cancer and uses the UK EQ-5D index tariff (see Table 19). A total of 52% of participants in this study were aged 50–64 years and 22% were aged ≥ 65 years and, as such, it conforms reasonably well to the population age in the SHTAC’s model. The study indicates that utilities in the first year after local recurrence and in the second and following years, after both primary breast cancer and local recurrence, are the same. 132 Accordingly, the SHTAC’s model uses the same utility value from the COMICE trial of 0.8112 for these three health states, as shown in Table 24.
The similarity of EQ-5D values across breast cancer health states is also reflected in the recent study in the German population by Hildebrandt et al. 134 which found the same median EQ-5D scores for primary disease, metastatic disease and recurrent disease (see Table 19). A previous HTA report to NICE uses utilities valued by either patients or clinical experts using time trade-off (TTO). 140 This set of utilities is examined in scenario analysis described in Results of independent economic analysis. It is not adopted in the base case as the utilities were not valued by the general population and were not obtained via the EQ-5D.
It is assumed that utility while in the ‘any other recurrence’ health state is equivalent to utility for metastatic disease. The Lidgren et al. 132 study gives a utility of 0.685 for metastatic disease (see Table 19). 132 This was adopted in the economic model as no utility for metastatic disease is given in the COMICE trial publication. 126 A utility for metastatic disease is given in Sherrill et al. ,133 but this is based on an international multicentre study of relatively young participants (median in pooled population approximately 52 years)151 and, therefore, does not appear to be as relevant to the model; however, the EQ-5D value of 0.66 is similar to the value of 0.685 given in Lidgren et al. 132 for this state (see Table 24). Alternative values are examined in scenario analysis (see Results of independent economic analysis).
The systematic review of QoL identified two studies that give EQ-5D values for mastectomy and immediate reconstruction. 130,131 Conner-Spady et al. 130 do not report the EQ-5D for mastectomy patients specifically. Robertson et al. 131 report a EQ-5D value of 0.83 for mastectomy and reconstruction at a median of 4 years’ follow-up, but an immediate post-operative value is not reported. The value of 0.83 is higher than the utility given in the COMICE trial at the 12-month time point after WLE. 126 This may reflect the lower mean age of 50 years131 but, on the basis of this study, mastectomy and reconstruction does not appear to be associated with disutility compared with WLE utility observed in the COMICE trial. Consequently, a mastectomy disutility is not included in the base case, but is examined in scenario analysis described in section Results of independent economic analysis.
In common with the manufacturer’s economic model and the IORT economic evaluation of Alvarado et al. ,106 the SHTAC’s model does not reflect any utility benefit associated with initial INTRABEAM treatment. Given that the duration of WB-EBRT in England is 3 weeks, any utility benefit associated with the one-off INTRABEAM delivery is likely to be very small when considered within the annual model cycle length. Any impact of treatment on HRQoL is assumed to occur because of its effect on disease progression and this is already accounted for in the model.
A summary of the health state utility values used in the economic model base case is given in Table 24.
Resource use and costs
This section considers the resource use and costs associated with the clinical pathway of the modelled population.
The proportion of INTRABEAM patients who also receive WB-EBRT is taken from the TARGIT-A trial, in which 15.2% of INTRABEAM patients also received WB-EBRT (Table 25). 65 The model assumes that 15 WB-EBRT deliveries are required to complete a course of treatment, as recommended in NICE Clinical Guideline 80. 11 Alternatives to this value are examined in deterministic sensitivity analysis (DSA) described in Results of independent economic analysis.
| Parameter | Units | Value | Source |
|---|---|---|---|
| Proportion of INTRABEAM patients who also receive WB-EBRT | Proportion | 0.152 | Vaidya 201465 |
| Number of WB-EBRT deliveries required to complete a course of treatment | Deliveries | 15 | NICE Clinical Guideline 8011 |
| Proportion of INTRABEAM patients having mastectomy at local recurrence | Proportion | 0.8 | Clinical advice |
| Proportion of mastectomy patients who have reconstruction | Proportion | 0.31 | National Mastectomy and Breast Reconstruction Audit 2011152 |
In contrast to the manufacturer’s model, in which it is assumed that all INTRABEAM patients will undergo repeat lumpectomy in the event of local recurrence, the SHTAC’s model assumes that only a minority of INTRABEAM patients will undergo repeat lumpectomy on local recurrence. Clinical advice to the AG is that the most common and evidence-based approach in the UK is to offer mastectomy for local recurrence and that approximately 70–80% of patients opt for this. The SHTAC’s model assumes 80% in the base case (see Table 25). All WB-EBRT patients are assumed to undergo mastectomy for local recurrence based on clinical opinion from the advisory group and evidence-based clinical practice.
Clinical advice to the AG also indicates that well under 50% of patients who undergo mastectomy will opt for reconstruction. This is borne out by figures obtained from the National Mastectomy and Breast Reconstruction Audit,152 which shows that only around 31% of those undergoing mastectomy choose to have a reconstruction (see Table 25).
The working lifetime of an INTRABEAM device is assumed to be 10 years in the base case (Table 26). This value is informed by the manufacturer and radiotherapy expert opinion; an alternative value of 5 years is examined in DSA described in Results of independent economic analysis.
| Parameter | Units | Value | Source |
|---|---|---|---|
| Lifetime of INTRABEAM device | Years | 10 | Carl Zeiss, UK |
| Proportion of INTRABEAM patients requiring radiation shield | Proportion | 1 | AG assumption |
Use of INTRABEAM requires appropriate shielding from radiation. The manufacturer observes that radiation protection shields are not required in all hospitals in England (J Richardson, NICE, 2014, personal communication); however, the proportion of hospitals that would not need shields is unclear. The SHTAC’s model base case therefore assumes that radiation shields are required in all cases (see Table 26) and examines alternative values for this proportion in DSA.
The INTRABEAM device requires additional staff time both in support of the device and during its use. Staff time is costed in the SHTAC’s economic model using the NHS staff pay bands of surgical consultant and Agenda for Change bands 8b, 7 and 5. Hourly costs for each of these pay bands are taken from the Personal Social Services Research Unit (PSSRU)’s Unit Costs of Health and Social Care 2013153 and are given in Table 27.
| Staff band | Unit cost per hour (£) | Source |
|---|---|---|
| Surgical consultant | 100.00 | PSSRU’s Unit Costs of Health and Social Care 2013 (see table 15.6)153 |
| AfC band 8b | 73.00 | Mean annual basic pay from PSSRU’s Unit Costs of Health and Social Care 2013 (see table 17.3); overheads added as per other staff unit cost derivations in PSSRU 2013153 |
| AfC band 7 | 50.00 | PSSRU’s Unit Costs of Health and Social Care 2013 (see table 14.1)153 |
| AfC band 5 | 34.00 | PSSRU’s Unit Costs of Health and Social Care 2013 (see table 14.3)153 |
The staff time required in support of INTRABEAM at each pay band is detailed in Table 28 by activity. Radiotherapy and clinical expert opinion was used to identify these activities and estimate the staff time required at each band. Two experts were consulted and the cost of each activity shown in Table 28 is derived using the unit costs given in Table 27. It is assumed that operating procedure development and initial INTRABEAM training are one-off costs which are incurred only once within the lifetime of each device, that is, every 10 years in the base case. Technical commissioning and radiation protection refresher training costs are assumed to be required on an annual basis. Expert advice to the AG is that technical commissioning is required annually after annual maintenance by the manufacturer. All other costs are incurred on a per treatment basis (see Table 28). Variation in these costs is considered in DSA described in Results of independent economic analysis.
| Frequency of cost | Activity | Number of staff | Staff band | Time required | Cost (£) | Source |
|---|---|---|---|---|---|---|
| One off | INTRABEAM operating procedure development | 1 | 7 | 2 daysa | 757.00 | Expert opinion |
| One off | Initial INTRABEAM training | 4 | 7 | 2 daysa | 5227.00 | Expert opinion of time/assumption for number of staff and band |
| 2 | 8b | |||||
| Annual | Technical commissioning | 2 | 7 | 3 daysa | 2271.00 | Expert opinion |
| Annual | Technical commissioning sign off | 1 | 8b | 0.5 daysa | 275.00 | Expert opinion |
| Annual | Refresher training on radiation protection | 4 | 7 | 1 hour | 920.00 | Expert opinion of time/assumption for number of staff and band |
| 2 | 8b | |||||
| 5 | 5 | |||||
| 4 | Surgical consultantb | |||||
| Per treatment | Pre-treatment QC check | 1 | 7 | 30 minutes | 25.00 | Expert opinion |
| Per treatment | Planning INTRABEAM dose in operating theatre | 2 | Surgical consultantb | 6 minutes | 25.00 | Expert opinion/TARGIT-A trial |
| 1 | 7 | |||||
| Per treatment | Delivering INTRABEAM dose in operating theatre | 1 | Surgical consultantb | 33 minutes | 83.00 | Expert opinion/TARGIT-A trial |
| 1 | 7 | |||||
| Per treatment | Additional time required by medical physicist in support of INTRABEAM use | 1 | 7 | 1.5 hours | 76.00 | Expert opinion |
The costs of consumables required for INTRABEAM use, and the number of uses that each consumable supports, are given in Table 29. Other costs used in the model are shown in Table 30. These include the capital cost of each INTRABEAM device and its associated annual maintenance cost, provided by Carl Zeiss, UK. Based on a capital cost of £435,000, a device lifetime of 10 years and a discount rate of 3.5% the equivalent annual cost of INTRABEAM is £53,025 (see Table 30).
| Description | Cost per unit (£) | Number of treatments | Cost per treatment (£) | Source |
|---|---|---|---|---|
| Spherical applicator | 3170.00 | 100 | 31.70 | Carl Zeiss, UK |
| Radiation protection shields pack of 10 | 1041.00 | 5 | 208.20 | |
| Sterile plastic drapes pack of five | 96.00 | 5 | 19.20 |
| Description | Cost (£) | Source |
|---|---|---|
| INTRABEAM device capital cost | 435,000.00 | Carl Zeiss, UK |
| Annual maintenance INTRABEAM device | 35,000.00 | |
| INTRABEAM device equivalent annual cost of capital and initial costs | 53,025.00 | Calculation from capital cost and one-off costs (see Table 28) using device lifetime of 10 years and discount rate of 3.5% |
| Cost of 1 hour in operating theatrea | 569.00 | University Hospitals Southampton Finance Department January 2014 |
The INTRABEAM device use requires extra time in the operating theatre for both treatment planning and delivery. The cost of 1 hour in theatre at Southampton General Hospital is £569.00 (see Table 30). This cost includes nurse cost but does not include any medical staff or anaesthetist cost. Additional staff time in the operating theatre for INTRABEAM device use is costed separately and given in Table 28.
Costs for mastectomy with and without reconstruction, WLE, and planning and delivery of WB-EBRT were obtained as weighted averages from NHS Reference Costs 2012 to 2013136 and are given in Table 31 with HRG codes.
| HRG codes | Description | Weighted average unit cost (£) | Weighted average lower quartile (£) | Weighted average upper quartile (£) | Source |
|---|---|---|---|---|---|
| JA27Z, JA28Z | Mastectomy with reconstruction | 7822.00 | 6169.00 | 9241.00 | NHS Reference Costs 2012 to 2013 136 |
| JA24D, JA24E, JA24F | WLE | 1542.00 | 1185.00 | 1804.00 | NHS Reference Costs 2012 to 2013 136 |
| JA20D, JA20E, JA20F | Mastectomy | 2510.00 | 2041.00 | 2850.00 | NHS Reference Costs 2012 to 2013 136 |
| SC22Z, SC23Z | Deliver a fraction of radiotherapy on a megavoltage machine | 118.44 | 101.53 | 138.82 | NHS Reference Costs 2012 to 2013 136 |
| SC45Z, SC46Z, SC47Z, SC48Z | Preparation for simple radiotherapy | 323.65 | 198.08 | 413.75 | NHS Reference Costs 2012 to 2013 136 |
Only serious adverse events of common terminology criteria grades 3 and 4 which occur in > 5% of patients in any treatment arm are included in the economic model as these are considered to be those that incur additional NHS costs. Moreover, adverse events are included only if the adverse event incidence differs significantly between treatment arms, in line with the modelling guidelines of Philips et al. 58 The review of clinical effectiveness indicates that, although there are two statistically significant differences in adverse event incidence between treatment arms (see Table 12), these occur in < 3% of patients. Therefore, no costs for adverse events associated with INTRABEAM and WB-EBRT are included in the economic model. This is consistent with the manufacturer’s model and the model of Alvarado et al. 106
In order to avoid potentially confounding assumptions, the costs of post-progression therapies are not included in the model base case. These costs are also not included in the manufacturer’s model (which has no health state for any other recurrence) but are included in the IORT model of Alvarado et al. 106 The AG notes that, in order to accurately capture the costs of the ‘any other recurrence’ health state, it would be necessary to know the proportions in this state with regional recurrence, contralateral breast recurrence and distant recurrence as these types of recurrence are associated with very different costs. However, these proportions are not given in the trial publication for the pre-pathology stratum. 65 The advisory group notes that INTRABEAM is associated with higher mortality from breast cancer than WB-EBRT and that this may be because the proportions with each type of ‘any other recurrence’ differed between the treatment arms. Without information on the proportions with each type of recurrence the AG does not consider that it is appropriate to include post-progression costs in the base case. A scenario that does include post-progression costs is given in Results of independent economic analysis.
Demand for the INTRABEAM device
In the base case, the SHTAC’s model assumes that the INTRABEAM device is deployed in a large district hospital with a catchment population of 1,000,000. With approximately 41,523 incident breast cancer cases in England in 20111 and an English population in 2011 of approximately 53.1 million (Table 32), the expected number of breast cancer cases per year in a hospital catchment of this size is 782. Opinion obtained from two clinical expert members of the advisory group differed as to the proportion of these incident cases which might be suitable for treatment with INTRABEAM. One expert estimated 10–20% of cases, while a second expert suggested up to 50%. A study by Leonardi et al. 155 retrospectively applies the American Society for Radiation Oncology consensus statement guidelines for the application of APBI156 to participants in an intraoperative radiotherapy trial and finds that 16% of the patients would have been considered suitable using these guidelines. This figure corresponds with the lower estimate provided by the clinical experts and is adopted for use in the economic model base case. The alternative estimate of 50% is examined in DSA described in Results of independent economic analysis.
| Parameter | Units | Value | Source |
|---|---|---|---|
| Population served by one INTRABEAM device | People | 1,000,000 | Advisory group assumption |
| Incident breast cancer cases in England 2011 | People | 41,523 | ONS1 |
| Population of England 2011 | People | 53,107,200 | ONS154 |
| Proportion of incident breast cancer cases which are early breast cancer and suitable for INTRABEAM | Proportion | 0.16 | Expert opinion from one or more members of the advisory group and Leonardi et al.155 |
With a hospital catchment of 1,000,000 and 16% of incident cases of breast cancer suitable for INTRABEAM, 126 INTRABEAM procedures might be carried out per year. This is shown in Table 33.
| Population served by one device | Calculated number of INTRABEAM procedures per year | Calculated cost of INTRABEAM procedure by lifetime of device (£) | |
|---|---|---|---|
| 10-year lifetime | 5-year lifetime | ||
| 795,000 | 100 | 2069 | 2514 |
| 1,000,000 | 126 | 1882 | 2236 |
| 5,000,000 | 631 | 1302 | 1373 |
Table 33 also shows how variations to the base-case assumptions of hospital catchment size and INTRABEAM device lifetime affect the cost per INTRABEAM procedure. With a device lifetime of 10 years and a hospital catchment population of one million, the cost per INTRABEAM procedure is £1882. At 100 procedures per year, as assumed in the manufacturer’s economic model, the cost per procedure is £2069 (see Table 33). This is similar to the cost used in the manufacturer’s economic model of £2165 per procedure.
With a 5-year equipment lifetime, the cost per INTRABEAM procedure rises to £2236 with base-case assumptions (see Table 33). A 5-year device lifetime is examined in DSA described in Results of independent economic analysis.
Model validation
The overall survival predictions from the model base case are compared with the trial-observed Kaplan–Meier data for the pre-pathology subgroup in Figure 8. The model overall survival predictions in Figure 8 were obtained using TARGIT-A trial data to model non-breast cancer death for the first five model cycles and provide a good fit to the observed data. Data from the TARGIT-A trial show that overall survival in the INTRABEAM treatment arm is somewhat better than overall survival in the WB-EBRT arm at 5 years, and this is reflected in the model predictions (see Figure 8). The model thus appears to be performing satisfactorily in this respect.
FIGURE 8.
Kaplan–Meier plot of overall survival in the pre-pathology subgroup of the TARGIT-A trial65 compared with overall survival predicted by the SHTAC’s economic model using TARGIT-A trial data to model non-breast cancer death for first five cycles.

The model base case does not use trial-observed data for non-breast cancer death, for reasons given in Data sources. Figure 9 gives the model predictions for overall survival in each of the treatment arms in the pre-pathology subgroup when only ONS mortality data are used to model non-breast cancer death. Figure 9 shows that, when using these data, predicted overall survival in the INTRABEAM treatment arm is worse than observed in the trial, although the overall survival prediction for the WB-EBRT arm is still a good fit. This is to be expected because ONS age-specific all-cause mortality rates are higher than the non-breast cancer mortality rates seen on the INTRABEAM arm in the TARGIT-A trial. The model predictions change in reflection of these differences (compare Figure 8 with Figure 9) and so, again, the model appears to be working satisfactorily.
FIGURE 9.
Kaplan–Meier plot of overall survival in the pre-pathology subgroup of the TARGIT-A trial65 compared with overall survival predicted by the SHTAC’s economic model using ONS mortality data to model non-breast cancer death in all cycles.
It may be seen from Figures 8 and 9 that median overall survival predicted by the model base case for early operable breast cancer patients is approximately 21.5 years and that overall survival is approximately 56% at 20 years. Relative survival at 20 years is 82% and at 25 years is 77%. Relative survival compares the survival of people with the cancer to that of people without cancer in order to help correct for deaths from things other than breast cancer. Exact comparison with other data sources is difficult; however, the SEER (Surveillance, Epidemiology, and End Results) database of the US National Cancer Institute has 20-year relative survival of 64.7% in breast cancer patients aged ≥ 50 years diagnosed between 1985 and 1989. 157 Figures from Cancer Research UK for England and Wales indicate that relative survival from breast cancer at 20 years is 64.5%. 158 Thus, the relative survival of 82% at 20 years given by the model is somewhat higher than these estimates, but this is to be expected as treatment has improved in the 25 or so years since the patients on whom these estimates are based were diagnosed.
Relative survival compares the survival of people with the cancer to that of people without cancer in order to help correct for deaths from things other than breast cancer. Thus, it is reasonable that the overall survival of 56% in the model is lower than these published estimates of relative survival because it does reflect deaths from other causes.
Results of independent economic analysis
This section reports the cost-effectiveness of INTRABEAM compared with WB-EBRT in a cohort of early operable breast cancer patients. Base-case discounted cost-effectiveness summary results are given in Table 34 and are broken down by health state in Table 35. Results with no discounting of costs and outcomes are given in Table 36. INTRABEAM is less expensive but also less effective than WB-EBRT as it has lower total costs but also fewer total QALYs. Therefore, the ICERs given in Tables 34 and 36 represent the money saved per QALY lost that is associated with replacing WB-EBRT by INTRABEAM.
| Intervention | Total costs (£) | Total LYG | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£ saved/QALY lost) |
|---|---|---|---|---|---|---|
| WB-EBRT | 2368.00 | 20.72 | 11.329 | – | – | – |
| INTRABEAM | 2227.00 | 20.51 | 11.241 | –140.00 | –0.088 | 1596a |
| Health state | WB-EBRT | INTRABEAM | ||
|---|---|---|---|---|
| Total costs (£) | Total QALYs | Total costs (£) | Total QALYs | |
| Recurrence free | 2100 | 10.760 | 1882 | 10.551 |
| Local recurrence | 268 | 0.052 | 345 | 0.069 |
| Disease free after local recurrence | 0 | 0.348 | 0 | 0.469 |
| Any other recurrence | 0 | 0.169 | 0 | 0.152 |
| Dead background mortality | 0 | 0 | 0 | 0 |
| Dead breast cancer | 0 | 0 | 0 | 0 |
| Total | 2368 | 11.329 | 2227 | 11.241 |
| Intervention | Total costs (£) | Total LYG | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£ saved/QALY lost) |
|---|---|---|---|---|---|---|
| WB-EBRT | 2522 | 20.72 | 16.743 | – | – | – |
| INTRABEAM | 2346 | 20.51 | 16.576 | –177 | –0.167 | 1062a |
In situations in which a new intervention (INTRABEAM) is both less costly and less effective than the current standard of care (WB-EBRT), the ICER for INTRABEAM to replace WB-EBRT must lie above the usual NICE cost-effectiveness thresholds of £20,000 and £30,000 per QALY if INTRABEAM is to be considered a cost-effective alternative to WB-EBRT. However, the ICER value of £1596 saved per QALY lost, shown in Table 34, indicates that WB-EBRT is the cost-effective treatment option within the WTP threshold of £20,000 per QALY. Over the 40-year time horizon of the model, it is associated with more QALYs at broadly similar overall cost. WB-EBRT is also cost-effective in the undiscounted analysis in which incremental QALYs are nearly twice those seen in the discounted results and the ICER (£ saved/QALY lost) is smaller (see Table 36).
Sensitivity analysis
Deterministic and probabilistic sensitivity analyses were conducted in order to investigate the effect of uncertainty in model parameter values on the cost-effectiveness results. DSA was used to highlight the most influential parameters while the effect of uncertainty and interaction in multiple parameters was examined using PSA. Scenario analysis was used to investigate the effect of uncertainty in model assumptions and structure.
Each parameter was assumed to follow a probability distribution and these are given, with the distribution parameters, in Table 37. For beta distributions, the distribution parameters were fitted using either the method of moments or information on the sample size and number of events when available. Distribution parameters were fitted to the gamma distributions using the method of moments. In cases for which a standard error (SE) or standard deviation (SD) was not supplied in the source literature, the SE was calculated using an arbitrary ± 20% from the base-case value. Correlation between the parameters of the log-normal distribution used to inform time to local recurrence was incorporated by sampling from a multivariate normal distribution with covariance matrix as specified in Table 37.
| Parameter | Distribution | Distribution parameters | Mean/base case | 2.5th percentile for mean | 97.5th percentile for mean | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs | |||||||||||||||||
| INTRABEAM commissioninga | Gamma | α = 96.04; β = 26.51 | £2546 | £2062 | £3080 | ||||||||||||
| One WB-EBRT delivery | Gamma | α = 18.36; β = 6.45 | £118 | £71 | £178 | ||||||||||||
| WB-EBRT planning | Gamma | α = 4.10; β = 78.97 | £324 | £90 | £704 | ||||||||||||
| INTRABEAM setup costsa | Gamma | α = 96.04; β = 62.31 | £5984 | £4847 | £7239 | ||||||||||||
| Mastectomy and reconstruction | Gamma | α = 99.63; β = 78.51 | £7822 | £6362 | £9431 | ||||||||||||
| Mastectomy | Gamma | α = 147.71; β = 16.99 | £2510 | £2122 | £2931 | ||||||||||||
| One hour in operating theatrea | Gamma | α = 96.04; β = 5.92 | £569 | £461 | £688 | ||||||||||||
| Pre-treatment QC INTRABEAMa | Gamma | α = 96.04; β = 0.26 | £25 | £20 | £31 | ||||||||||||
| Staff time per hour in theatre during INTRABEAM deliverya | Gamma | α = 96.04; β = 1.57 | £150 | £122 | £182 | ||||||||||||
| Staff time per hour in theatre during INTRABEAM planninga | Gamma | α = 96.04; β = 2.61 | £250 | £203 | £303 | ||||||||||||
| Annual staff training in radiation protectiona | Gamma | α = 96.04; β = 9.58 | £920 | £745 | £1113 | ||||||||||||
| Staff time in support of INTRABEAM deliverya | Gamma | α = 96.04; β = 0.79 | £76 | £61 | £92 | ||||||||||||
| Repeat lumpectomy | Gamma | α = 95.55; β = 16.13 | £1542 | £1248 | £1866 | ||||||||||||
| Survival curve parameters | |||||||||||||||||
| Time to local recurrence | Multivariate normalb | ||||||||||||||||
| β(treatment arm) | Covariance matrix0.081–0.0770.531–0.0080.1310.035 | Covariance matrix | 0.081 | –0.077 | 0.531 | –0.008 | 0.131 | 0.035 | –0.256 | –0.815 | 0.307 | ||||||
| Covariance matrix | |||||||||||||||||
| 0.081 | |||||||||||||||||
| –0.077 | 0.531 | ||||||||||||||||
| –0.008 | 0.131 | 0.035 | |||||||||||||||
| Constant | 4.97 | 3.553 | 6.383 | ||||||||||||||
| Sigma | 0.436 | 0.072 | 0.797 | ||||||||||||||
| Probabilities | |||||||||||||||||
| Other recurrence INTRABEAM from recurrence free (5 years) | Beta | α = 19.1; β = 378 | 0.048 | 0.029 | 0.071 | ||||||||||||
| Other recurrence WB-EBRT from recurrence free (5 years) | Beta | α = 16.7; β = 337.9 | 0.047 | 0.028 | 0.071 | ||||||||||||
| Other recurrence after local recurrence (10.2 years) | Beta | α = 129; β = 181 | 0.416 | 0.362 | 0.471 | ||||||||||||
| INTRABEAM patient receives WB-EBRT | Beta | α = 239; β = 1332 | 0.152 | 0.135 | 0.170 | ||||||||||||
| Mastectomy patient has reconstruction | Beta | α = 5120; β = 11365 | 0.311 | 0.304 | 0.318 | ||||||||||||
| INTRABEAM patient has mastectomy at local recurrencea | Beta | α = 18.4; β = 4.6 | 0.800 | 0.618 | 0.933 | ||||||||||||
| INTRABEAM patient dies from breast cancer (5 years) | Beta | α = 10.6; β = 310.8 | 0.033 | 0.016 | 0.055 | ||||||||||||
| WB-EBRT patient dies from breast cancer (5 years) | Beta | α = 11.3; β = 407.8 | 0.027 | 0.014 | 0.045 | ||||||||||||
| Incident breast cancer patients suitable for INTRABEAMa | Beta | α = 294; β = 1528 | 0.161 | 0.145 | 0.179 | ||||||||||||
| Resource use | |||||||||||||||||
| INTRABEAM delivery timea | Normal | Mean = 33; SE = 3.37 | 33 | 26.40 | 39.60 | ||||||||||||
| INTRABEAM planning timea | Normal | Mean = 6; SE = 0.61 | 6 | 4.80 | 7.20 | ||||||||||||
| Utilities | |||||||||||||||||
| Recurrence free after the first year | Beta | α = 2400; β = 558.5 | 0.811 | 0.8 | 0.83 | ||||||||||||
| Recurrence free in the first year | Beta | α = 2161; β = 635.3 | 0.773 | 0.76 | 0.79 | ||||||||||||
| Other recurrence | Beta | α = 171; β = 78.7 | 0.685 | 0.63 | 0.74 | ||||||||||||
| Other | |||||||||||||||||
| Catchment population served by one INTRABEAM devicea | Normal | Mean = 1,000,000; SE = 102,041 | 1,000,000 | 800,004 | 1,199,996 | ||||||||||||
The model parameters were varied in DSA between the 2.5th and 97.5th percentiles of the assumed parameter distribution of the mean value and these are given in Table 37. Table 38 gives upper and lower bounds for parameters examined in DSA when these are different from the upper and lower bounds examined in PSA.
| Parameter | Base case | Lower value | Upper value |
|---|---|---|---|
| Proportion of incident breast cancer patients suitable for INTRABEAM | 0.16 | 0.1 | 0.5 |
| Fractions of WB-EBRT required to complete a course of treatment | 15 | 5 | 23 |
| Lifetime of INTRABEAM device (years) | 10 | 5 | 10 |
| Proportion of INTRABEAM patients requiring radiation shield | 1 | 0.25 | 1 |
| Age of cohort entering model (years) | 62 | 55 | 72 |
| Discount rate for costs (%) | 3.5 | 0.0 | 6.0 |
| Discount rate for health (%) | 3.5 | 0.0 | 6.0 |
Deterministic sensitivity analysis
Table 39 shows the results of the deterministic sensitivity analyses for INTRABEAM compared with WB-EBRT for the most influential parameters. A tornado diagram depicting the range in incremental NMB given in this table is given in Figure 10. A complete set of DSA results is given in Appendix 10.
| Variable description | Low value | High value | Low value incremental NMB (£) | High value incremental NMB (£) | Range (£) |
|---|---|---|---|---|---|
| 5-year probability of any other recurrence INTRABEAM | 0.029 | 0.071 | 5781 | –9171 | 14,952 |
| 5-year probability of any other recurrence WB-EBRT | 0.028 | 0.071 | –8760 | 5977 | 14,737 |
| Beta coefficient for INTRABEAM arm time to local recurrence | –0.815 | 0.307 | –4512 | 118 | 4630 |
| 5-year probability of death from breast cancer WB-EBRT | 0.014 | 0.045 | –4150 | –346 | 3804 |
| 5-year probability of death from breast cancer INTRABEAM | 0.016 | 0.055 | 1051 | –2518 | 3569 |
| Constant (time to local recurrence) | 3.553 | 6.383 | –3367 | –836 | 2531 |
| Discount rate for utilities (%) | 0 | 6 | –3192 | –1042 | 2150 |
| Number of WB-EBRT deliveries required in course of treatment | 5 | 23 | –2604 | –832 | 1772 |
| Starting age of model cohort (years) | 55 | 72 | –2273 | –757 | 1516 |
| Cost of delivering one fraction WB-EBRT (£) | 71 | 178 | –2211 | –877 | 1334 |
| Proportion of incident cases which are suitable for INTRABEAM | 0.1 | 0.5 | –2064 | –1128 | 936 |
| Sigma (time to local recurrence) | 0.072 | 0.797 | –1110 | –2018 | 908 |
FIGURE 10.
Tornado diagram showing key results of DSA for INTRABEAM vs. WB-EBRT. Bars indicate spread in incremental NMB between upper and lower parameter bounds (£). WTP set to £20,000 per QALY.

The incremental NMB rather than the ICER is used in Table 39 and Figure 10 as the ICER for INTRABEAM compared with WB-EBRT is sometimes negative (Figure 11) and incremental NMB has a more straightforward interpretation. A WTP of £20,000 and equation (2) (see Methods for economic analysis) were used to calculate the incremental NMB.
FIGURE 11.
Scatterplot of the costs and health benefits from PSA: INTRABEAM compared with WB-EBRT.
Table 39 and Figure 10 compare INTRABEAM incrementally with WB-EBRT in order to be consistent with the base case (see Table 34). Thus, a negative incremental NMB indicates that INTRABEAM is not cost-effective compared with WB-EBRT (or, conversely, that WB-EBRT is cost-effective compared with INTRABEAM). A positive incremental NMB indicates that INTRABEAM is cost-effective compared with WB-EBRT (or, conversely, that WB-EBRT is not cost-effective compared with INTRABEAM).
The results show that the incremental NMB is, above all, very sensitive to the probability of any other recurrence, which is assumed for both WB-EBRT and INTRABEAM as there is a very wide difference in the incremental NMB between the low and high values of these parameters. The differences lead to a switch in which treatment is considered cost-effective at a WTP threshold of £20,000 per QALY. At a low probability of any other recurrence on the INTRABEAM arm, INTRABEAM is cost-effective compared with WB-EBRT at a WTP of £20,000 (shown by positive incremental NMB in Table 39). At high probability of any other recurrence on the INTRABEAM arm, WB-EBRT is a cost-effective treatment option at the £20,000 per QALY WTP threshold (shown by negative incremental NMB in Table 39). With low probability of any other recurrence on the WB-EBRT arm, WB-EBRT is a cost-effective treatment option compared with INTRABEAM at the £20,000 per QALY WTP threshold, but this is reversed with high probability of any other recurrence on the WB-EBRT arm, that is, INTRABEAM becomes cost-effective at the £20,000 per QALY WTP threshold (see Table 39).
The model is also somewhat sensitive to the probability of death from breast cancer on the INTRABEAM arm and, again, this difference leads to a switch in which treatment is considered cost-effective at a WTP threshold of £20,000 per QALY. At low values for probability of death from breast cancer on the INTRABEAM arm, INTRABEAM is cost-effective at a WTP of £20,000 per QALY, but it is not cost-effective compared with WB-EBRT at high values for probability of death from breast cancer on the INTRABEAM arm (see Table 39).
Change in which treatment is considered cost-effective at a WTP threshold of £20,000 per QALY also occurs between the low and high parameter values considered for the beta coefficient for the INTRABEAM arm in the log-normal model of time to local recurrence (see Table 39). At low values of this coefficient, WB-EBRT is cost-effective compared with INTRABEAM but at the highest values considered, INTRABEAM becomes slightly more cost-effective than WB-EBRT.
In summary, the results of the DSA indicate that there is a degree of uncertainty surrounding the base-case results. In the case of four parameters, the difference between upper and lower values results in a switch in the treatment option, which is considered cost-effective at a WTP of £20,000 per QALY.
Probabilistic sensitivity analysis
A total of 10,000 PSA simulations were run, and the mean results for these simulations are presented in Table 40 and are similar to results for the base case given in Table 34. The scatterplot for cost and health outcomes is shown in Figure 11 and, similar to the DSA findings, indicates considerable uncertainty in the results. There are many points in the north-west quadrant of Figure 11, which demonstrates that in a large number of the PSA simulations INTRABEAM is less effective than WB-EBRT, as well as being more costly. Conversely, in many of the PSA simulations WB-EBRT is more effective and cheaper than INTRABEAM, shown by the large number of points in the south-east quadrant of Figure 11.
| Intervention | Total costs (£) | Total LYG | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£ saved/QALY lost) |
|---|---|---|---|---|---|---|
| WB-EBRT | 2398 | 20.73 | 11.327 | – | – | – |
| INTRABEAM | 2272 | 20.52 | 11.240 | –126 | –0.087 | 1447a |
The CEAC calculated from the PSA simulations is given in Figure 12 and indicates that at the £20,000 WTP threshold WB-EBRT has the highest probability (61.3%) of being cost-effective. WB-EBRT also has the highest probability of being cost-effective (61.4%) at a WTP of £30,000 per QALY. INTRABEAM has a higher probability of being cost-effective than WB-EBRT at WTP thresholds of around £5000 per QALY or less (see Figure 12).
FIGURE 12.
Cost-effectiveness acceptability curve from the PSA.
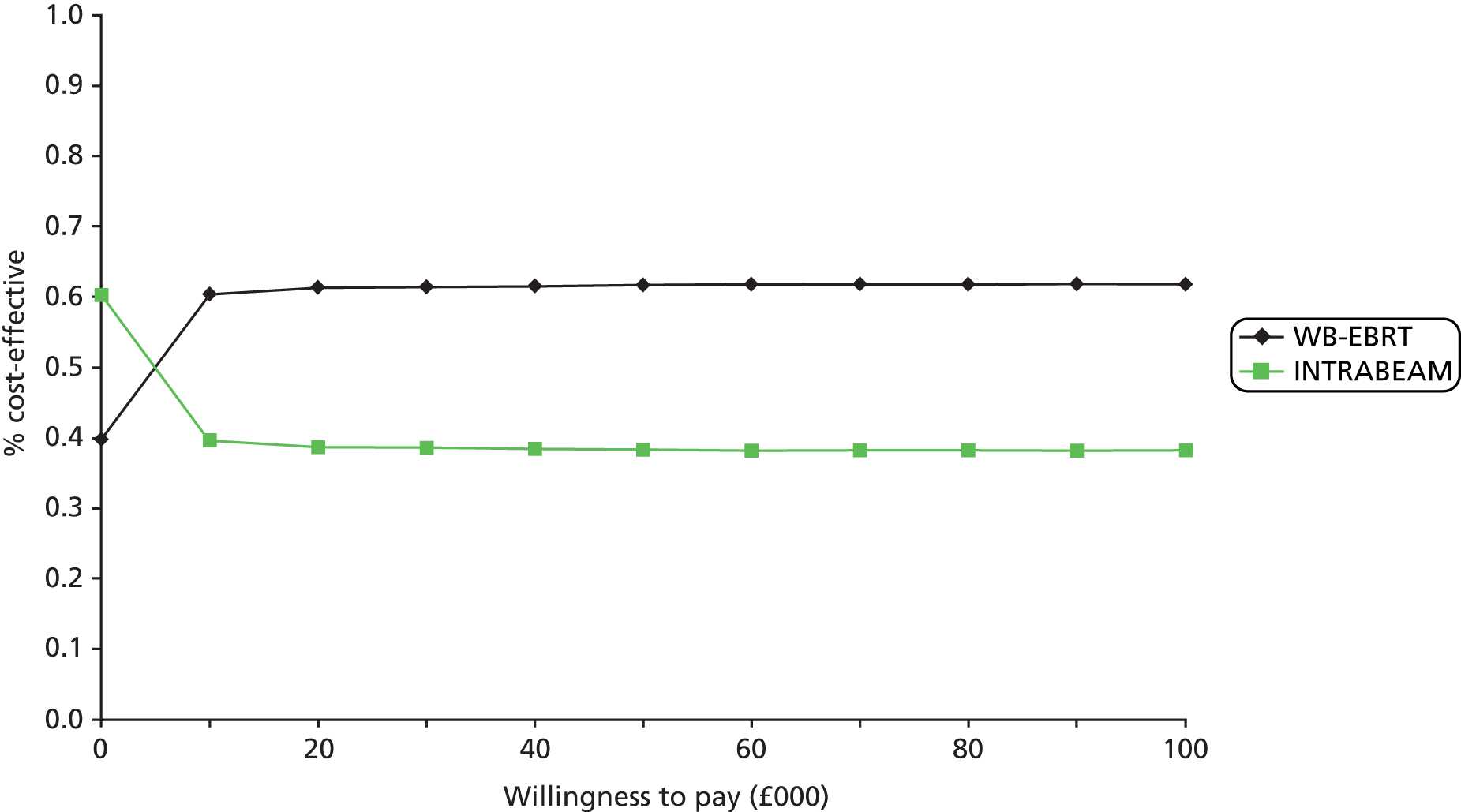
Scenario analysis
In addition to the sensitivity analyses, five scenarios were examined to investigate the uncertainty surrounding the structural assumptions made by the model.
The model base case uses ONS all-cause mortality tables to give the probability of non-breast cancer death. As an alternative to using ONS data in all model cycles, the use of non-breast cancer mortality data from the TARGIT-A trial was examined. A Weibull fit to TARGIT-A Kaplan–Meier data65 was used to obtain trial-observed non-breast cancer mortality probabilities for the first five model cycles. ONS mortality data were used thereafter. INTRABEAM dominates WB-EBRT in this scenario, as it is associated with lower total costs and greater total QALYs (Table 41).
| Intervention | Total costs (£) | Total LYG | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|---|
| WB-EBRT | 2366 | 20.58 | 11.259 | – | – | – |
| INTRABEAM | 2234 | 20.83 | 11.425 | –132 | 0.166 | Dominating |
The manufacturer’s model assumes that 100 patients are treated with INTRABEAM each year in a district general hospital (P Pinilla-Dominguez, NICE, 2014, personal communication). To replicate this assumption in the SHTAC’s model requires a corresponding assumption about the typical catchment population of a hospital offering INTRABEAM. In the base case, the SHTAC’s model assumes that the catchment population is one million, which implies 126 INTRABEAM procedures a year (see Table 33). A catchment population of 795,000 is required to give 100 INTRABEAM procedures a year. Results using this catchment population are given in Table 42. The table shows that INTRABEAM is now dominated by WB-EBRT as it is associated with slightly higher total cost, but fewer QALYs.
| Intervention | Total costs (£) | Total LYG | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|---|
| WB-EBRT | 2368 | 20.72 | 11.329 | – | – | – |
| INTRABEAM | 2414 | 20.51 | 11.241 | 47 | –0.088 | Dominated |
The manufacturer’s model uses a utility of 0.87 for lumpectomy at local recurrence and a utility of 0.82 for mastectomy. These figures imply a disutility for mastectomy of 0.05. The AG considers that it is unclear from the literature if mastectomy is associated with significant disutility to HRQoL as measured with EQ-5D. 160,161 A scenario analysis was conducted to examine the effect of a mastectomy disutility of 0.05 on model outcomes. In the SHTAC’s model it is assumed that this disutility is a weighted average of the disutilities associated with mastectomy and mastectomy and reconstruction.
The results are given in Tables 43 and 44. Table 43 shows results obtained when it is assumed that the mastectomy utility decrement applies to both the local recurrence and disease free after local recurrence health states; Table 44 shows the results obtained when it is assumed that the mastectomy utility decrement applies to the local recurrence health state only. Applying the utility decrement to both the local recurrence and disease free after local recurrence health states has more impact on final ICER than applying the decrement to the local recurrence state alone, but in neither case does the utility decrement make an appreciable difference to model outcome. The ICER decreases by less than £50 per QALY compared with the base case (see Table 34).
| Intervention | Total costs (£) | Total LYG | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£ saved/QALY lost) |
|---|---|---|---|---|---|---|
| WB-EBRT | 2368 | 20.72 | 11.304 | – | – | – |
| INTRABEAM | 2227 | 20.51 | 11.214 | –140 | –0.090 | 1563a |
| Intervention | Total costs (£) | Total LYG | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£ saved/QALY lost) |
|---|---|---|---|---|---|---|
| WB-EBRT | 2368 | 20.72 | 11.326 | – | – | – |
| INTRABEAM | 2227 | 20.51 | 11.238 | –47 | –0.088 | 1592a |
The decrease in ICER compared with the base case indicates that WB-EBRT becomes more cost-effective than INTRABEAM in this scenario. Although in the base case a smaller proportion of INTRABEAM patients undergo mastectomy for local recurrence (80% compared with 100% for WB-EBRT), more INTRABEAM patients experience a local recurrence. The net effect is that the total mastectomy utility decrement is greater on the INTRABEAM arm and, consequently, the incremental QALYs associated with WB-EBRT are slightly higher than in the base case.
The health state utilities used in the model base case are the same in the local recurrence health state and the recurrence-free health state after the first year (see Table 24). Although these utilities are based on the studies of Lidgren et al. 132 and Turnbull et al. ,126 it is arguably not appropriate that these two health states should have the same utility. Their identical values may arise because EQ-5D is not a particularly sensitive instrument to use when examining QoL in early breast cancer patients as found, for example, by Hildebrandt et al. 134 An alternative set of health state utility values used in a previous HTA report to NICE was examined. 140 These were valued by either patients or clinical experts using the TTO and are given in Table 45.
| Health state | Utility value | Source |
|---|---|---|
| Recurrence free | 0.78 | Hind et al.140 |
| Local recurrence | 0.61 | |
| Disease free after local recurrence | 0.71 | |
| Any other recurrence | 0.42 |
The results for the scenario are given in Table 46. These show that, although total QALYs decline in both treatment arms with use of the alternative utility set, the incremental QALYs do not change appreciably from the base case. Thus, the overall ICER is very similar to the base case: £1517 saved per QALY lost, compared with £1596 in the base case (see Table 34).
| Intervention | Total costs (£) | Total LYG | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£ saved/QALY lost) |
|---|---|---|---|---|---|---|
| WB-EBRT | 2368 | 20.72 | 10.812 | – | – | – |
| INTRABEAM | 2227 | 20.51 | 10.719 | –140 | –0.093 | 1517a |
The base case does not include costs of treatment after any other recurrence because of lack of information on the types of recurrence within this category. The trial publication reports the proportions with regional recurrence (1.1% INTRABEAM compared with 0.9% WB-EBRT) and distant recurrence (3.9% INTRABEAM compared with 3.2% WB-EBRT) for all patients, but does not give these data for the pre-pathology stratum. 65 However, the costs of treating these types of recurrence are quite different. 140 Using costs given in the HTA report of Hind et al. ,140 inflated to 2013 using the Hospital and Community Health Services prices index,153 the AG calculated the annual cost of metastatic disease (active treatment and supportive care) as £12,122 and the cost of end-of-life care for a breast cancer patient as £3669. In contrast, the costs of contralateral disease are more similar to those incurred at local recurrence. 140
For illustrative purposes, the AG has considered a scenario in which 60% of recurrences in the ‘any other recurrence’ health state are assumed to be distant recurrences and where mortality following any other recurrence is the same in both treatment arms (using the probability for WB-EBRT in the base case; see Table 23). This assumption is necessary because trial data show that mortality following any other recurrence is higher for INTRABEAM and, consequently, including costs for this state without such adjustment would simply result in additional incremental cost for WB-EBRT (as WB-EBRT patients live longer in this state). A figure of 60% with distant recurrence was estimated based on data given in the TARGIT-A publication for all patients and data in the literature. 140 The costs of distant recurrence are the major costs in the any other recurrence health state and as a simplification costs for the types of recurrence in this category were not considered. Using the costs given above for distant recurrence and end-of-life care, the results shown in Table 47 were obtained. Health state costs for this scenario are given in Table 48.
| Intervention | Total costs (£) | Total LYG | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£ saved/QALY lost) |
|---|---|---|---|---|---|---|
| WB-EBRT | 4652 | 20.72 | 11.329 | – | – | – |
| INTRABEAM | 4662 | 20.51 | 11.268 | –10 | –0.061 | 157a |
| Health state | WB-EBRT total costs (£) | INTRABEAM total costs (£) |
|---|---|---|
| Recurrence free | 2100 | 1882 |
| Local recurrence | 268 | 345 |
| Disease free after local recurrence | 0 | 0 |
| Any other recurrence | 1795 | 1897 |
| Dead background mortality | 0 | 0 |
| Dead breast cancer | 499 | 527 |
Table 47 shows that the base-case conclusion does not change when post-progression costs for distant disease and end-of-life care are considered, that is, INTRABEAM is not cost-effective compared with WB-EBRT at a WTP threshold of £20,000 per QALY. However, the cost saving associated with replacing WB-EBRT with INTRABEAM is much smaller as the ICER is reduced from £1596 saved per QALY lost in the base case to £157 saved per QALY lost in the scenario. INTRABEAM is only £10 less expensive than WB-EBRT per patient over the 40-year time horizon considered in the model.
Discussion
INTRABEAM is less expensive but also less effective than WB-EBRT as it is associated with lower total costs but fewer total QALYs. The base case ICER for replacing WB-EBRT with INTRABEAM is £1596 saved per QALY lost (this represents the money saved per QALY lost that is associated with replacing WB-EBRT by INTRABEAM). INTRABEAM is therefore not cost-effective compared with WB-EBRT at the WTP threshold of £20,000 per QALY as the cost saved per QALY lost is less than £20,000.
The CEAC calculated from PSA indicates that at the £20,000 WTP threshold WB-EBRT has a greater probability than INTRABEAM of being cost-effective, at 61.3%. WB-EBRT also has the highest probability of being cost-effective (61.4%) at a WTP of £30,000 per QALY.
The base-case result is subject to a degree of uncertainty as the disease progression parameters included in the model are largely drawn from the TARGIT-A trial. 65 As discussed in Chapter 4, Assessment of effectiveness, and Chapter 7, Statement of principal findings and Strengths and limitations of the assessment, this trial has relatively short follow-up. The numbers experiencing local recurrence in the pre-pathology stratum, which is used to inform the economic model, are also quite small. Results of DSA show that the base-case finding that INTRABEAM is not cost-effective at a WTP of £20,000 per QALY compared with WB-EBRT would be reversed if the probability of experiencing any other recurrence on the INTRABEAM arm was at the low end of its likely range, or if the probability of death from breast cancer on the INTRABEAM arm was at the low end of its likely range.
A strength of the economic model is that it is based on data identified from systematic searches for clinical effectiveness, cost-effectiveness and QoL evidence. Other strengths are that QoL/health state utility weights are taken from studies using the EQ-5D and valued using the UK general population tariff, and that a transparent approach was taken to costing the use of INTRABEAM per procedure by considering all elements of the cost base.
Possible weaknesses of the model are that the systematic review of QoL did not find EQ-5D values to populate all of the model health states and that the clinical effectiveness data used to inform disease progression in the model are drawn largely from one study which has a relatively short follow-up time. 64,65 This study also has a small number of events for the primary outcome in the pre-pathology stratum and the base-case results are therefore subject to some uncertainty. Owing to data limitations, the model does not include costs for the any other recurrence health state in the base case.
Comparison of the economic models
A key structural difference between the Carl Zeiss economic model and the SHTAC’s model is that the Zeiss model has four health states while the SHTAC’s model has six. The SHTAC’s model includes an additional (temporary) health state at local recurrence and also an ‘any other recurrence’ health state which includes metastatic disease. A further structural difference is that the Zeiss model uses an exponential assumption to extrapolate trial local recurrence data over the time horizon of the model, while the SHTAC’s model assumes a log-normal fit to these data. The Zeiss model is run over a 10-year time horizon rather than the 40-year horizon used in the SHTAC’s model.
Different cost and utility data were also used. The Zeiss model uses expert opinion to inform the cost of each INTRABEAM procedure while the SHTAC’s model uses a microcosting approach. The Zeiss model assumes that at local recurrence all INTRABEAM patients have salvage lumpectomy and that all WB-EBRT patients have salvage mastectomy. The cost of salvage mastectomy in the Zeiss model appears to include the cost of breast reconstruction for all patients. In contrast, the SHTAC’s model considers that most INTRABEAM patients will have mastectomy at local recurrence and that of patients having mastectomy, not all of them will have reconstruction.
Utilities used in the Zeiss model were obtained via standard gamble and were not obtained from the general population. Utilities used in the SHTAC’s model were obtained using the EQ-5D and valued with the UK tariff.
Chapter 6 Assessment of factors relevant to the NHS and other parties
The report ‘Radiotherapy Services in England 2012’161 states that there are currently 265 linear accelerators operating in the UK/England across 58 sites, with new sites planned. Breast cancer accounted for 28% of radiotherapy services activity for the year 2011/2012. To meet projected increases in the need for radiotherapy (owing to cancers in an ageing population) it has been estimated that 412 linear accelerators will be required by 2016. In contrast, as noted in Chapter 1, Description of technology under assessment, just eight INTRABEAM devices are known to have been purchased (four in London and one each in Winchester, Dundee, Liverpool and Harlow) for use in the NHS, with a further 10 NHS trusts expressing an interest in purchasing the device. Therefore, there would be a need for significant investment in INTRABEAM equipment if this technology were to be available across the NHS. Furthermore, in addition to the investment in equipment there would also need to be investment in staff training both for surgeons, physicists, oncologists and radiographers.
Advice from the advisory group for this assessment indicated that theatre capacity is also a consideration. The additional time needed in theatre to administer INTRABEAM therapy could add to pressure on breast clinics, especially if they already find it difficult to meet waiting time targets. However, in centres where lymph node analysis is already undertaken intraoperatively using the RD-100i OSNA system (Sysmex Europe GmbH, Norderstedt, Germany) (currently 22 in use in the UK; see Chapter 1, Current service provision), INTRABEAM therapy could be delivered and completed within this time and, therefore, would have less impact on theatre time.
As noted above, breast cancer currently accounts for about 28% of activity across radiotherapy centres. How much radiotherapy resource could be freed up by increased use of INTRABEAM therapy depends in part on the proportion of patients who would be eligible for INTRABEAM treatment. In the AG’s independent economic model (see Chapter 5, Data sources, Demand for INTRABEAM), the proportion of incident cases of early breast cancer suitable for INTRABEAM therapy is estimated at 16%. If this were the case, breast cancer would then account for about 24% of radiotherapy centre activity, a drop of 4%. However, it should be remembered that the actual drop would be likely to be lower than this for two reasons. First, after INTRABEAM treatment some patients may be found to have tumours with unfavourable features that put them at high risk of recurrence, in which case they would receive WB-EBRT in addition. Second, some patients will experience recurrence and, depending on their preference and extent of disease at recurrence, may opt for local excision and WB-EBRT.
In the future, radiotherapy resources may also be freed up if the current 3-week WB-EBRT treatment schedule can be shortened. For example, a clinical trial, the FAST-Forward non-inferiority RCT162 is currently testing a 1-week (5-fraction) course of WB-EBRT to see if it is as effective and as safe as the current UK 15-fraction standard. The estimated publication date for this HTA-funded trial is 2021. If this trial demonstrates that a 1-week course of WB-EBRT is as effective and safe in this patient group, then adoption of this shortened radiotherapy regimen would have a larger impact on radiotherapy resources than the introduction of INTRABEAM. The ability to identify a subset of women who could safely be treated without receiving WB-EBRT might also free up radiotherapy resources in the future.
From the patient perspective, INTRABEAM therapy may be viewed as an attractive option because the standard 15 fraction course of WB-EBRT would be avoided for the majority of those eligible for INTRABEAM treatment. The benefits of this include a reduction in the disruption to work and family life both in terms of time (for travel as well as for treatment) and costs (e.g. travel, parking, loss of earnings) which may be significant particularly for those who live farthest from a radiotherapy centre and for those at the lower end of the income spectrum.
Chapter 7 Discussion
Statement of principal findings
-
One international, multicentre, non-inferiority RCT64,65 was included in the systematic review of clinical effectiveness. It examined IORT using the INTRABEAM device compared with conventional WB-EBRT and was judged to be at a low risk of bias.
-
Participants could be randomised to INTRABEAM or WB-EBRT prior to surgery to remove the tumour (pre-pathology stratum) or could receive surgery to remove the tumour and be randomised into the trial after surgery providing initial histopathology showed no adverse criteria (post-pathology stratum). Participants in either stratum who were randomised to INTRABEAM and subsequently found to have unfavourable pathological features also received WB-EBRT (i.e. INTRABEAM + WB-EBRT).
-
The primary outcome of the RCT was local recurrence in the conserved breast. The pre-stated non-inferiority margin was an absolute difference of 2.5% between groups. Non-inferiority of INTRABEAM compared with WB-EBRT was demonstrated for the whole trial population and for the pre-pathology stratum. However, non-inferiority was not established for the post-pathology stratum for which the absolute difference in the 5-year local recurrence exceeded the pre-defined non-inferiority margin of 2.5%. In considering these results it should be remembered that the median follow-up of the total trial population was 2 years 5 months and 1222 (35%) had reached a median follow-up of 5 years.
-
Overall survival was a secondary outcome of the RCT. Differences between the groups in overall mortality and for breast cancer mortality were not statistically significant for the whole trial population, the pre-pathology stratum or the post-pathology stratum. In contrast, the analysis of non-breast cancer deaths showed that there were significantly fewer non-breast cancer deaths in the INTRABEAM group compared with the WB-EBRT group in the whole trial population and when the pre-pathology stratum was analysed separately. In the post-pathology stratum, there was no statistically significant difference in non-breast cancer mortality between the groups.
-
For participants in the pre-pathology stratum, treatment with INTRABEAM resulted in a 1% increase in local recurrence but this was counterbalanced with a potential 2.3% decrease in overall mortality.
-
Clinically significant complications reported to differ statistically significantly between the groups were wound seroma requiring more than three aspirations, which occurred more frequently in the INTRABEAM group, and RTOG toxicity score of grade 3 or 4, which was less frequent in the INTRABEAM group. Early complications and complications arising 6 months after randomisation appeared similar between the groups.
-
Limited information was available from one substudy undertaken by one trial centre on QoL. 63 Approximately 2.5% of the total trial population were involved in this study, which did not identify any statistically significant differences in QoL measures [EORTC QLQ-C30 (version 3) and the QLQ-BR23] between the study arms.
Cost-effectiveness
-
The systematic review identified two relevant economic evaluations,106,108 both of which were based on the TARGIT-A trial. Both studies were associated with a number of limitations.
-
Alvarado et al. 106 developed a Markov decision-analytic model with six health states. Costs and benefits were discounted at 3%, costs were expressed in US$ and the price year was 2011. INTRABEAM was found to be associated with less cost and greater QALYs than WB-EBRT.
-
Shah et al. 108 analysed cost-effectiveness through reimbursement models and conducted a cost-minimisation analysis. Methods and assumptions were based on previously published articles. The authors concluded that although INTRABEAM represented a potential cost-saving alternative, WB-EBRT represented a cost-effective modality compared with INTRABEAM based on cost per QALY analyses when additional medical costs and non-medical costs associated with INTRABEAM were factored in.
-
Both studies were based in the USA and adopted a societal perspective and are, therefore, not generalisable to the UK NHS.
-
The time horizon was 10 years in one study106 and not clearly stated in the other study108 (but assumed to be 10 years based on the estimation of mean utility), which is inappropriate as the risk of local recurrence continues over a lifetime.
-
Alvarado et al. 106 used a standard 33 fractions of WB-EBRT in their model, which is more than the current standard UK practice of 15 fractions and will lead to an overestimation of WB-EBRT costs. The number of fractions of WB-EBRT was not reported by Shah et al. 108
-
The quality of utility data used in both the studies is questionable. The source study119 was a publication dated 1998, and more recent data would have been appropriate, such as those identified in Chapter 5, Southampton Health Technology Assessments Centre’s systematic review of health-related quality-of-life studies.
Quality of life
-
The systematic review on HRQoL studies was conducted with an aim to identify utility data for the SHTAC’s independent model. Nine studies were identified, which were diverse with respect to their aims, interventions, comparators, study designs and methodologies. When assessing the studies on the basis of their relevance to the NICE reference case, only three met all of the criteria (details in Appendix 8). 126,128,132
-
The studies provide a source of EQ-5D data for five of the seven health states identified a priori as being potentially relevant for the SHTAC’s independent model. EQ-5D data were not identified for the health states WLE + INTRABEAM or WLE + INTABEAM + WB-EBRT.
Manufacturer’s submission
-
The MS evaluated the cost-effectiveness of INTRABEAM in early breast cancer patients when compared with radiotherapy usually given in the UK over 3–6 weeks as WB-EBRT. The total costs, QALYs gained and cost-effectiveness associated with the intervention and comparator under consideration in the appraisal were reported. A multistate Markov model consisting of four health states was constructed. The analysis was conducted for a time period of 20 years with an annual cycle length. The perspective was that of the NHS and benefits and costs were discounted at 3.5%.
-
The base-case results indicate that INTABEAM is associated with greater QALYs and lower costs than WB-EBRT. One-way sensitivity analyses and scenario analyses were not conducted. PSA found that at the £20,000 and £30,000 WTP thresholds, INTRABEAM has the highest probability of being cost-effective, at 100% for both thresholds.
-
Limited information on the model structure and input parameters is provided in the MS and the AG has raised a number of concerns regarding the methods used; as a consequence the results of the MS model should be viewed with caution.
Southampton Health Technology Assessments Centre’s model
-
INTRABEAM is less expensive but less effective than WB-EBRT. The base-case ICER for replacing WB-EBRT with INTRABEAM is £1596 saved per QALY lost. INTRABEAM is, therefore, not cost-effective compared with WB-EBRT at the WTP threshold of £20,000 per QALY.
-
At the £20,000 WTP threshold WB-EBRT has a greater probability than INTRABEAM of being cost-effective, at 61.3%. WB-EBRT also has the highest probability of being cost-effective (61.4%) at a WTP of £30,000 per QALY.
-
The base-case result is subject to a degree of uncertainty. For four model parameters, the difference in their upper and lower values causes a switch in the treatment option, which is considered cost-effective at a WTP of £20,000 per QALY. Model outcomes are particularly sensitive to the probability of any other recurrence.
-
Alternative model health state utility values examined in scenario analysis do not substantively change the base-case findings. Other scenario analyses show that INTRABEAM is dominated by WB-EBRT if it is assumed to serve a smaller catchment population than the base case, and that INTRABEAM dominates WB-EBRT if trial-observed mortality data are used for the first five model cycles.
Strengths and limitations of the assessment
This assessment has the following strengths:
-
The systematic reviews and economic evaluation have been carried out independently of any vested interest and the results are presented in a consistent and transparent manner.
-
The systematic reviews have been undertaken following established methodology and principles for conducting a systematic review. The methods used were set out in a research protocol, which defined the research question in line with the NICE scope, and set out the inclusion and quality assessment criteria, data extraction process and the other methods to be employed during the evidence synthesis.
-
An advisory group has informed the review from its initiation. The research protocol was informed by comments received from the advisory group and the advisory group also commented on a draft of the final report.
-
A de novo economic model has been developed following recognised guidelines and the model structure and data inputs are clearly presented in this report. The main results have been summarised and presented. This should facilitate replication and testing of our model assumptions.
-
The economic model is based on data identified from systematic searches for clinical effectiveness, cost-effectiveness and QoL evidence.
-
The QoL/health state utility weights used in the economic model are taken from studies using the EQ-5D and valued using the UK general population tariff.
-
A transparent approach was taken to costing the use of INTRABEAM per procedure by considering all elements of the cost base.
-
The model is validated against external data.
In contrast, this assessment also has certain limitations:
-
Only one RCT has been published that met the inclusion criteria for the review.
-
The length of follow-up in the published reports of the included trial may be inadequate.
-
The economic model is based on estimates of efficacy from the included trial, which may have inadequate follow-up.
-
The systematic review of QoL did not find EQ-5D values to populate all of the model health states.
-
The economic model does not include any costs for the ‘any other recurrence’ health state in the base case owing to limitations in the evidence base.
Uncertainties
-
The TARGIT-A trial was a non-inferiority RCT with ITT results presented. An extension to the CONSORT statement77 for non-inferiority trials states that there would be greater confidence in the results of a non-inferiority trial if both ITT and non-ITT (per-protocol) results were presented and shown to be consistent with one another. As no per-protocol analysis was presented it is not known whether or not the results of such an analysis would confirm the findings of the ITT analysis.
-
In the WB-EBRT arm of the TARGIT-A trial, centres were allowed to stipulate local policy for the delivery of WB-EBRT and, therefore, there would have been some differences between WB-EBRT delivered at different centres, for example, in dose delivered or quality control. The impact of these differences is unknown however it seems unlikely that variations in WB-EBRT as delivered in non-UK TARGIT-A trial centres and the standard UK radiotherapy schedule (40 Gy in 15 fractions over 3 weeks11) would have an impact on results. Evidence from the UK-based START-B trial163 which was recruiting patients with operable early invasive breast cancer at a similar time to TARGIT-A compared a radiotherapy schedule of 50 Gy in 25 fractions over 5 weeks with 40 Gy in 15 fractions over 3 weeks. After a median follow-up of 6 years, START-B showed that 5-year local-regional relapse from a 40 Gy in a 15-fraction schedule (2.2%, 95% CI 1.3% to 3.1%) were as least as favourable as the 50 Gy in a 25-fraction schedule (3.3%, 95% CI 2.2% to 4.5%). A potentially more important consideration is the possibility of variable quality control of WB-EBRT between centres. The TARGIT-A trial protocol69 voiced the expectation that all trial investigators would be working to local or national standards conforming to international guidelines for quality assurance and thus no trial-specific quality control measures were put in place.
-
Some key estimates of clinical efficacy used in the economic model have wide CIs. Base-case results are therefore subject to a degree of uncertainty, which stems from uncertainty in the evidence base. For a few parameters [probability of any other recurrence assumed for WB-EBRT and INTRABEAM, the beta coefficient for the time to local recurrence (INTRABEAM) and the probability of death from breast cancer (INTRABEAM)] the cost-effectiveness findings are reversed when values at the upper and lower bounds of the appropriate CI are considered.
Chapter 8 Conclusions
Implications for service provision
There are only eight INTRABEAM devices currently available in the NHS. Therefore, there would be a need for significant investment in INTRABEAM equipment and in staff training for surgeons and physicists if this technology were to be available across the NHS. As indicated in Chapter 6, there is likely to be an impact on theatre capacity. If the use of INTRABEAM reduces the number of operations that can be completed in a given time, this could increase waiting list times, especially for centres that already find it difficult to meet waiting time targets.
Suggested research priorities
The evidence base for the use of INTRABEAM for the adjuvant treatment of early-stage breast cancer is limited to one RCT, the TARGIT-A trial, which has reported on outcomes after a median follow-up of 2 years and 5 months. The population enrolled in the trial has a low risk of local recurrence and of mortality and, therefore, there is scope for uncertainty about whether or not the results observed to date will hold over the longer term. To increase confidence in the results, longer-term follow-up data from the TARGIT-A trial are required. Future analyses should report the numbers experiencing each type of recurrence within the ‘any other recurrence’ category. ‘Any other recurrence’ included regional recurrence, contralateral breast recurrence and distance recurrence which have very different prognoses and contribute to the slightly higher breast cancer mortality associated with INTRABEAM. The economic model is very sensitive to this.
To address the effectiveness of INTRABEAM in a wider range of patients, analysis from other trials and analysis of registry data will be needed when sufficient data with an appropriate length of follow-up have been accrued [ongoing currently: one RCT (TARGIT-B),100,101 one prospective single-arm study (TARGIT-E)102 and three registry database studies,103–105 see Chapter 4, Ongoing studies].
Further HRQoL data are desirable. A very limited quantity has been published from the TARGIT-A trial and it is not clear whether or not HRQoL outcome data will be available for the whole trial population in the future.
Acknowledgements
We would like to thank members of our advisory group who provided expert advice and comments on the protocol and a draft of this report: Mr Dick Rainsbury, Consultant Surgeon, Hampshire Hospitals NHS Foundation Trust (Mr Rainsbury was involved in Winchester with the ‘TARGIT-A’ trial which used INTRABEAM equipment but had no financial interest in the trial); Professor John Yarnold, Professor of Clinical Oncology, The Institute of Cancer Research (chief investigator of the FAST-Forward trial); Dr Murray Brunt, Consultant Oncologist, University Hospital of North Staffordshire NHS Trust (no competing interests declared); Ms Sue Ward, Senior Operational Research Analyst, School of Health and Related Research, University of Sheffield; and Hilary Stobart, Independent Cancer Patients’ Voice (lay member of the National Cancer Research Institute (NCRI) clinical and translational working group and a member of a couple of radiotherapy trial patient advisory groups but none of these roles have involved discussions on INTRABEAM).
We would also like to thank Chris Brew-Graves at University College London (UCL) for providing cost information; Claire Birch, Head of the Radiotherapy Physics Service at University Hospital Southampton NHS Foundation Trust for providing estimates of staff time and staff grades for the use of INTRABEAM; the Finance Department at University Hospital Southampton for providing a cost estimate; Karen Welch, Information Scientist, SHTAC, University of Southampton, for generating and running the literature searches; and Emma Loveman, Senior Research Fellow, SHTAC, for reviewing a draft of the report.
Contribution of authors
Jo Picot (Senior Research Fellow) project managed the study, developed the research protocol, contributed to drafting the background section, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed the included study (clinical effectiveness), synthesised evidence, and drafted and edited the final report.
Vicky Copley (Senior Research Fellow) developed the research protocol, assessed studies for inclusion, synthesised evidence, developed the economic evaluation and drafted the report.
Jill L Colquitt (Senior Research Fellow) developed the research protocol, assessed studies for inclusion, extracted data from and quality assessed included studies (cost-effectiveness and QoL), synthesised evidence, drafted the report and acted as guarantor for the project.
Neelam Kalita (Research Fellow) assessed studies for inclusion, extracted data from and quality assessed included studies (cost-effectiveness and QoL), synthesised evidence, assisted with the economic evaluation and drafted the report.
Debbie Hartwell (Senior Research Fellow) developed the research protocol, contributed to drafting the background section, assessed studies for inclusion, extracted data from and quality assessed the included study (clinical effectiveness), synthesised evidence and drafted the final report.
Jackie Bryant (Principal Research Fellow) assisted with the development of the research protocol, assessed studies for inclusion, extracted data from and quality assessed included studies (cost-effectiveness and QoL) and drafted the report.
Data sharing statement
All available data relating to the systematic reviews is included in this report and its appendices. The economic model associated with this document is protected by intellectual property rights, which are owned by the University of Southampton. Anyone wishing to modify, adapt, translate, reverse engineer, decompile, dismantle or create derivative work based on the economic model must first seek the agreement of the property owners.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Cancer Registration Statistics, England, 2011. Newport: ONS; 2013.
- Office for National Statistics (ONS) . Breast Cancer: Incidence, Mortality and Survival, 2010 2012. www.ons.gov.uk/ons/rel/cancer-unit/breast-cancer-in-england/2010/sum-1.html (accessed 14 August 2013).
- Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer 2013;108:2419-25. http://dx.doi.org/10.1038/bjc.2013.233.
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. http://dx.doi.org/10.1038/nature12477.
- Alexandrov LB, Nik-Zainal S, Wedge DC, Campbell PJ, Stratton MR. Deciphering signatures of mutational processes operative in human cancer. Cell Rep 2013;3:246-59. http://dx.doi.org/10.1016/j.celrep.2012.12.008.
- Martin FL. Epigenetic influences in the aetiology of cancers arising from breast and prostate: a hypothesised transgenerational evolution in chromatin accessibility. ISRN Oncol 2013;2013. http://dx.doi.org/10.1155/2013/624794.
- Previati M, Manfrini M, Galasso M, Zerbinati C, Palatini J, Gasparini P, et al. Next generation analysis of breast cancer genomes for precision medicine. Cancer Lett 2013;339:1-7. http://dx.doi.org/10.1016/j.canlet.2013.07.018.
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994;266:66-71. http://dx.doi.org/10.1126/science.7545954.
- Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995;378:789-92. http://dx.doi.org/10.1038/378789a0.
- Lawrence G, Kearins O, Walton J, Lagord C, Cheung S. The Non-Invasive Breast Cancer Report. An Analysis of Non-Invasive Breast Cancers Diagnosed in England from 1 January 2006 to 31 December 2007. London: National Cancer Intelligence Network; 2011.
- Early and Locally Advanced Breast Cancer: Diagnosis and Treatment. London: NICE; 2009.
- Barrett SV. Breast Cancer. J R Coll Physicians Edinb 2010;40:335-9. http://dx.doi.org/10.4997/JRCPE.2010.418.
- Cancer Research UK . TNM Breast Cancer Staging n.d. www.cancerresearchuk.org/cancer-help/type/breast-cancer/treatment/tnm-breast-cancer-staging (accessed 14 August 2013).
- Statistical Bulletin: Cancer Incidence and Mortality in the United Kingdom, 2008–10. Newport: ONS; 2012.
- Parkin DM, Boyd L, Walker LC. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer 2011;105:S77-81. http://dx.doi.org/10.1038/bjc.2011.489.
- Cancer Research UK . Breast Cancer Mortality Statistics n.d. www.cancerresearchuk.org/cancer-info/cancerstats/types/breast/mortality/#country (accessed 3 April 2014).
- Statistical Bulletin: Geographic patterns of Cancer Survival in England: Patients followed up to 2011. Newport: ONS; 2013.
- Walters S, Maringe C, Butler J, Rachet B, Barrett-Lee P, Bergh J, et al. Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000–7: a population-based study. Br J Cancer 2013;108:1195-208. http://dx.doi.org/10.1038/bjc.2013.6.
- Routes to Diagnosis, 2006–8. NCIN Information Supplement. London: National Cancer Intelligence Network; 2012.
- Breast Screening Programme, England 2011–12. Leeds: Health and Social Care Information Centre; 2013.
- Office for National Statistics . Results of the ONS Cancer Incidence and Survival Statistics User Consultation 2013. www.ons.gov.uk/ons/about-ons/user-engagement/consultations-and-surveys/open-consultations/cancer-statistics-consultation/index.html (accessed 14 August 2013).
- Lyratzopoulos G, Abel GA, Barbiere JM, Brown CH, Rous BA, Greenberg DC. Variation in advanced stage at diagnosis of lung and female breast cancer in an English region 2006–9. Br J Cancer 2012;106:1068-75. http://dx.doi.org/10.1038/bjc.2012.30.
- Baum M. Modern concepts of the natural history of breast cancer: a guide to the design and publication of trials of the treatment of breast cancer. Eur J Cancer 2013;49:60-4. http://dx.doi.org/10.1016/j.ejca.2012.07.005.
- Ponten J. Natural history of breast cancer. Acta Oncol 1990;29:325-9. http://dx.doi.org/10.3109/02841869009090008.
- Leong SP. Paradigm shift of staging and treatment for early breast cancer in the sentinel lymph node era. Breast J 2006;12:S128-33. http://dx.doi.org/10.1111/j.1075-122X.2006.00326.x.
- Morton DL, Wen D, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992;127:392-9. http://dx.doi.org/10.1001/archsurg.1992.01420040034005.
- Zahl PH, Gotzsche PC, Maehlen J. Natural history of breast cancers detected in the Swedish mammography screening programme: a cohort study. Lancet Oncol 2011;12:1118-24. http://dx.doi.org/10.1016/S1470-2045(11)70250-9.
- Heijnsdijk EA, Warner E, Gilbert FJ, Tilanus-Linthorst MM, Evans G, Causer PA, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening-MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev 2012;21:1458-68. http://dx.doi.org/10.1158/1055-9965.EPI-11-1196.
- Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology 2012;26:688-94.
- Batina NG, Trentham-Dietz A, Gangnon RE, Sprague BL, Rosenberg MA, Stout NK, et al. Variation in tumor natural history contributes to racial disparities in breast cancer stage at diagnosis. Breast Cancer Res Treat 2013;138:519-28. http://dx.doi.org/10.1007/s10549-013-2435-z.
- Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 1992;22:207-19. http://dx.doi.org/10.1007/BF01840834.
- Blamey RW, Ellis IO, Pinder SE, Lee AH, Macmillan RD, Morgan DA, et al. Survival of invasive breast cancer according to the Nottingham Prognostic Index in cases diagnosed in 1990–9. Eur J Cancer 2007;43:1548-55. http://dx.doi.org/10.1016/j.ejca.2007.01.016.
- Wishart GC, Azzato EM, Greenberg DC, Rashbass J, Kearins O, Lawrence G, et al. PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res 2010;12. http://dx.doi.org/10.1186/bcr2464.
- Martin FT, O’Fearraigh C, Hanley C, Curran C, Sweeney KJ, Kerin MJ. The prognostic significance of nodal ratio on breast cancer recurrence and its potential for incorporation in a new prognostic index. Breast J 2013;19:388-93. http://dx.doi.org/10.1111/tbj.12122.
- Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 2001;19:980-91.
- Montgomery M, McCrone SH. Psychological distress associated with the diagnostic phase for suspected breast cancer: systematic review. J Adv Nurs 2010;66:2372-90. http://dx.doi.org/10.1111/j.1365-2648.2010.05439.x.
- Lim CC, Devi MK, Ang E. Anxiety in women with breast cancer undergoing treatment: a systematic review. Int J Evid Based Healthc 2011;9:215-35.
- Gilbert E, Ussher JM, Perz J. Sexuality after breast cancer: a review. Maturitas 2010;66:397-40. http://dx.doi.org/10.1016/j.maturitas.2010.03.027.
- Hayes SC, Johansson K, Stout NL, Prosnitz R, Armer JM, Gabram S, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer 2012;118:2237-49. http://dx.doi.org/10.1002/cncr.27467.
- Binkley JM, Harris SR, Levangie PK, Pearl M, Guglielmino J, Kraus V, et al. Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer 2012;118:2207-16. http://dx.doi.org/10.1002/cncr.27469.
- Corner J, Wagland R. National Cancer Survivorship Initiative: Text Analysis of Patients’ Free Text Comments: Final Report. Southampton: University of Southampton; 2012.
- An Audit of Screen Detected Breast Cancers for the Year of Screening April 2011 to March 2012. Birmingham: West Midlands NHS Breast Screening Quality Assurance Reference Centre; 2013.
- Intraoperative tests (RD-100i OSNA System and Metasin Test) for Detecting Sentinel Lymph Nodes metastases in Breast Cancer. London: NICE; 2013.
- National Institute for Health and Care Excellence (NICE) . NICE Pathways: Early and Locally Advanced Breast Cancer 2013. http://pathways.nice.org.uk/pathways/early-and-locally-advanced-breast-cancer (accessed 5 December 2013).
- Department of Health . Cancer Reform Strategy 2007. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_081007.pdf (accessed 10 February 2014).
- Improving Outcomes: A Strategy for Cancer. London: The Central Office of Information; 2011.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10801 women in 17 randomised trials. Lancet 2011;378:1707-16. http://dx.doi.org/10.1016/S0140-6736(11)61629-2.
- Cancer Research UK . External Radiotherapy n.d. www.cancerresearchuk.org/cancer-help/about-cancer/treatment/radiotherapy/external/about-external-radiotherapy#what (accessed 14 August 2013).
- Coles CE, Brunt AM, Wheatley D, Mukesh MB, Yarnold JR. Breast radiotherapy: less is more?. Clin Oncol (R Coll Radiol) 2013;25:127-34. http://dx.doi.org/10.1016/j.clon.2012.10.013.
- Moran MS, Bai HX, Harris EER, Arthur DW, Bailey L, Bellon JR, et al. ACR Appropriateness criteria ductal carcinoma in situ. Breast J 2011;18:8-15. http://dx.doi.org/10.1111/j.1524-4741.2011.01197.x.
- Radiotherapy System. Frequently Asked Questions. Jena: Carl Zeiss Meditec; 2013.
- Intrabeam Technical Specifications. Jena: Carl Zeiss Meditec; 2012.
- INTRABEAM Targeted Radiotherapy. Jena: Carl Zeiss Meditec; 2012.
- INTRABEAM Photon Radiotherapy System for the Adjuvant Treatment of Early Breast Cancer. Final Scope. London: NICE; 2013.
- Systematic Reviews: CRD’s Guidance For Undertaking Reviews In Health Care. York Publishing Services Ltd: Centre for Reviews and Dissemination; 2009.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011] 2011. www.cochrane-handbook.org (accessed 3 April 2014).
- Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Soh SE, Morris ME, McGinley JL. Determinants of health-related quality of life in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord 2011;17:1-76. http://dx.doi.org/10.1016/j.parkreldis.2010.08.012.
- Bartoli S, Aguzzi G, Tarricone R. Impact of quality of life of urinary incontinence and overactive bladder: a systematic literature review. Urology 2010;75:491-500. http://dx.doi.org/10.1016/j.urology.2009.07.1325.
- Efficace F, Bottomley A, Osoba D, Gotay C, Flechtner H, D’haese S, et al. Beyond the development of health-related quality of life (HRQoL) measures: A checklist for evaluating HRQoL outcomes in cancer clinical trials – does HRQoL evaluation in prostate cancer research inform clinical decision making?. J Clin Oncol 2003;21:3502-11. http://dx.doi.org/10.1200/JCO.2003.12.121.
- Papaioannou D, Brazier JE, Paisley S. The Identification, Review and Synthesis of Health State Utility Values from the Literature. Sheffield: University of Sheffield; 2011.
- Welzel G, Boch A, Sperk E, Hofmann F, Kraus-Tiefenbacher U, Gerhardt A, et al. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: results from the randomized phase III trial TARGIT-A. Radiat Oncol 2013;8. http://dx.doi.org/10.1186/1748-717X-8-9.
- Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. http://dx.doi.org/10.1016/S0140-6736(10)60837-9.
- Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. http://dx.doi.org/10.1016/S0140-6736(13)61950-9.
- Andersen KG, Gartner R, Kroman N, Flyger H, Kehlet H. Persistent pain after targeted intraoperative radiotherapy (TARGIT) or external breast radiotherapy for breast cancer: a randomized trial. Breast J 2012;21:46-9. http://dx.doi.org/10.1016/j.breast.2011.07.011.
- Sperk E, Welzel G, Keller A, Kraus-Tiefenbacher U, Gerhardt A, Sutterlin M, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat 2012;135:253-60. http://dx.doi.org/10.1007/s10549-012-2168-4.
- Keshtgar MR, Williams NR, Bulsara M, Saunders C, Flyger H, Cardoso JS, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat 2013;140:519-25. http://dx.doi.org/10.1007/s10549-013-2641-8.
- Vaidya JS. An international randomised controlled trial to compare targeted intra-operative radiotherapy (TARGIT) with conventional post-operative radiotherapy for women with early breast cancer (Project record). Health Technol Assess 2015.
- Clark RM, McCulloch PB, Levine MN, Lipa M, Wilkinson RH, Mahoney LJ, et al. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst 1992;84:683-9. http://dx.doi.org/10.1093/jnci/84.9.683.
- Early Breast Cancer Trialists’ Collaborative Group . Effects of radiotherapy and surgery in early breast cancer. An overview of the randomised trials. N Engl J Med 1995;333:1444-55. http://dx.doi.org/10.1056/NEJM199511303332202.
- Alvarado MD, Conolly J, Park C, Sakata T, Mohan AJ, Harrison BL, et al. Patient preferences regarding intraoperative versus external beam radiotherapy following breast-conserving surgery. Breast Cancer Res Treat 2014;143:135-40. http://dx.doi.org/10.1007/s10549-013-2782-9.
- Joseph D, Nowak A, Corica T, Saunders C, Herbert C, Bulsara M, et al. Patient preferences for adjuvant radiotherapy in early breast cancer – an Australian sub-study of the pilot TARGIT study. Eur J Surg Oncol 2006;32:S79-80. http://dx.doi.org/10.1016/S0748-7983(06)70699-0.
- Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 1957;11:359-77. http://dx.doi.org/10.1038/bjc.1957.43.
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403-10. http://dx.doi.org/10.1111/j.1365-2559.1991.tb00229.x.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28-55. http://dx.doi.org/10.1016/j.ijsu.2011.10.001.
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594-604. http://dx.doi.org/10.1001/jama.2012.87802.
- Association of Cancer Physicians (ACP) . Professional Organisation Statement on Behalf of the NCRI Breast CSG, RCP and ACP 2014.
- Tuschy B, Berlit S, Romero S, Sperk E, Wenz F, Kehl S, et al. Clinical aspects of intraoperative radiotherapy in early breast cancer: short-term complications after IORT in women treated with low energy X-rays. Radiat Oncol 2013;8. http://dx.doi.org/10.1186/1748-717X-8-95.
- Grobmyer SR, Lightsey JL, Bryant CM, Shaw C, Yeung A, Bhandare N, et al. Low-kilovoltage, single-dose intraoperative radiation therapy for breast cancer: results and impact on a multidisciplinary breast cancer program. J Am Coll Surg 2013;216:617-23. http://dx.doi.org/10.1016/j.jamcollsurg.2012.12.038.
- Deneve JL, Hoefer RA, Harris EE, Laronga C. Accelerated partial breast irradiation: a review and description of an early North American surgical experience with the intrabeam delivery system. Cancer Control 2012;19:295-308.
- Elliott R, DeLand M, Head JF, Elliott MC. Accelerated partial breast irradiation: initial experience with the Intrabeam system. Surg Oncol 2011;20:73-9. http://dx.doi.org/10.1016/j.suronc.2009.11.001.
- Merdad AA, Bahadur YA, Fawzy EE, Hassouna AH, Eltaher MM, Algaithy ZK, et al. Phase II study on the use of intraoperative radiotherapy in early breast cancer. Saudi Me J 2013;34:1133-8.
- Kolberg H, Stephanou M, Akplot-Basci L, Lövey G. Intraoperative Radiotherapy Using INTRABEAM® Device As Intraoperative Boost in Breast Conserving Therapy – A Single Institution Experience After First 200 Cases n.d. http://eccamsterdam2013.ecco-org.eu/Scientific-Programme/Abstract-search.aspx#.
- Jankiewicz M, Kurylcio A, Romanek J, Mielko L, Lewicka M, Cisel B, et al. Targeted Intraoperative Radiotherapy Using Low-Kilovoltage X-Rays for Early Breast Carcinoma: Single Centre Case Series With 5-Years Follow-up n.d. http://eccamsterdam2013.ecco-org.eu/Scientific-Programme/Abstract-search.aspx#.
- Steiner M, Leviov M, Biterman A, Shiloni E, Goldman J. Intraoperative Radiotherapy in Early Breast Cancer: 400 Consecutive Patients in One Institution n.d. http://meetinglibrary.asco.org/content/113394-132.
- Wenz F, Welzel G, Blank E, Hermann B, Steil V, Sütterlin M, et al. Intraoperative radiotherapy as a boost during breast conserving surgery using low-kilovoltage experience: the first 5 years of experience with a novel approach. Int J Radiation Oncology Biol Phys 2010;77:1309-14. http://dx.doi.org/10.1016/j.ijrobp.2009.06.085.
- Chua BH, Henderson MA, Milner AD. Intraoperative radiotherapy in women with early breast cancer treated by breast-conserving therapy. ANZ J Surg 2011;81:65-9. http://dx.doi.org/10.1111/j.1445-2197.2010.05431.x.
- Vaidya JS, Baum M, Tobias JS, Wenz F, Massarut S, Keshtgar M, et al. Long-term results of targeted intraoperative radiotherapy (Targit) boost during breast-conserving surgery. Int J Radiat Oncol Biol Phys 2011;81:1091-7. http://dx.doi.org/10.1016/j.ijrobp.2010.07.1996.
- Sperk E, Astor D, Welzel G, Gerhardt A, Sütterlin M, Wemz F. Intraoperative versus external beam boost for breast cancer: a matched pair analysis. J Clin Oncol 2013;31. http://meetinglibrary.asco.org/print/1153531.
- Vaidya JS, Bulsara M, Wenz F, Massarut S, Joseph D, Tobias JS, et al. Fewer Non-Breast Cancer Deaths in the TARGIT-A Trial Systematic Benefit of TARGIT or Lack of EBRT Toxicity? 2013. www.oncoletter.ch/index.tpl?rubrik=738.
- Malter W, Puppe J, Wuerstlein R, Semrau R, Bongartz R, Markiefka B, et al. Single center experience with intraoperative radiotherapy as a boost during oncoplastic breast-conserving surgery. Abstract EBBC 2012. Eur J Cancer 2012;48. www.ecco-org.eu/Events/Past-conferences/EBCC.aspx.
- Kraus-Tiefenbacher U, Bauer L, Scheda A, Schoeber C, Schaefer J, Steil V, et al. Intraoperative radiotherapy (IORT) is an option for patients with localized breast recurrences after previous external-beam radiotherapy. BMC Cancer 2007;7. http://dx.doi.org/10.1186/1471-2407-7-178.
- Kraus-Tiefenbacher U, Welzel G, Brade L, Hermann B, Siebenlist K, Wasser KS, et al. Postoperative seroma formation after intraoperative radiotherapy using low kilo- voltage X-ray given during breast conserving surgery. Int J Rad Oncol Biol Phys n.d.:1-6.
- Aziz MH, Schneider F, Clausen S, Blank E, Herskind C, Afza M, et al. Can the risk of secondary cancer induction after breast conserving therapy be reduced using intraoperative radiotherapy (IORT) with low-energy x-rays?. Radiat Oncol 2011;6. http://dx.doi.org/10.1186/1748-717X-6-174.
- Corica T, Nowak A, Saunders C, Bulsara M, Joseph D. Patient preferences for adjuvant radiotherapy in early breast cancer – an Australian sub-study of the INTRABEAM TARGIT trial. Abstract EBBC 2012. Eur J Cancer 2012;48. http://dx.doi.org/10.1016/S0959-8049(12)70547-6.
- Keshtgar MRS, Eaton DJ, Reynolds C, Pigott K, Davidson T, Gauter-Fleckenstein B, et al. Pacemaker and radiotherapy in breast cancer: is targeted intraoperative radiotherapy the answer in this setting?. Rad Oncol 2012;7. http://dx.doi.org/10.1186/1748-717X-7-128.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;386:987-98. http://dx.doi.org/10.1056/NEJMoa1209825.
- Keshtgar MRS, Tobias S, Wenz F, Joseph D, Stacey C, Metaxas MG, et al. Targeted intraoperative radiotherapy for breast cancer in patients in whom external beam radiation is not possible. Int J Rad Oncol Biol Phys 2011;80:31-8. http://dx.doi.org/10.1016/j.ijrobp.2010.01.045.
- TARGIT-B . A Comparison of Intra-Operative Radiotherapy Boost With External Beam Radiotherapy Boost in Early Breast Cancer. (TARGIT-B) n.d. https://clinicaltrials.gov/ct2/show/NCT01792726 (accessed 25 March 2014).
- TARGIT-B . An International Randomised Controlled Trial to Compare Targeted Intra-Operative Radiotherapy Boost With Conventional External Beam Radiotherapy Boost After Lumpectomy for Breast Cancer in Women With a High Risk of Local Recurrence n.d. www.nets.nihr.ac.uk/projects/hta/1010407 (accessed 25 March 2014).
- TARGIT-E . Prospective Phase II Study of Intraoperative Radiotherapy (IORT) in Elderly Patients With Small Breast Cancer (TARGIT-E) n.d. https://clinicaltrials.gov/ct2/show/NCT01299987 (accessed 25 March 2014).
- TARGIT-R . TARGeted Intraoperative RadioTherapy (TARGIT) Registry Database n.d. www.isrctn.com/ISRCTN91179875 (accessed 25 March 2014).
- TARGIT-BQR . TARGeted Intraoperative RadioTherapy With INTRABEAM As a Boost for Breast Cancer - A Quality Control Registry (TARGIT_BQR) n.d. https://clinicaltrials.gov/ct2/show/NCT01440010 (accessed 25 March 2014).
- TARGIT-US . Targeted Intraoperative Radiotherapy United States (TARGIT-US) Registry Trial n.d. https://clinicaltrials.gov/ct2/show/NCT01570998 (accessed 25 March 2014).
- Alvarado MD, Mohan AJ, Esserman LJ, Park CC, Harrison BL, Howe RJ, et al. Cost-effectiveness analysis of intraoperative radiation therapy for early-stage breast cancer. Ann Surg Oncol 2013;20:2873-80. http://dx.doi.org/10.1245/s10434-013-2997-3.
- Esserman LJ, Alvarado MD, Howe RJ, Mohan AJ, Harrison B, Park C, et al. Application of a decision analytic framework for adoption of clinical trial results: are the data regarding TARGIT-A IORT ready for prime time?. Breast Cancer Res Treat 2014;144:371-8. http://dx.doi.org/10.1007/s10549-014-2881-2.
- Shah C, Badiyan S, Khwaja S, Shah H, Chitalia A, Nanavati A, et al. Evaluating radiotherapy options in breast cancer: does intraoperative radiotherapy represent the most cost-efficacious option?. Clin Breast Cancer 2014;14:141-6. http://dx.doi.org/10.1016/j.clbc.2013.10.005.
- Suh WW, Hillner BE, Pierce LJ, Hayman JA. Cost-effectiveness of radiation therapy following conservative surgery for ductal carcinoma in situ of the breast. Int J Radiat Oncol Biol Phys 2005;61:1054-61. http://dx.doi.org/10.1016/j.ijrobp.2004.07.713.
- Medicare Physician Fee Schedule . US Department of Health and Human Services 2010. www.cms.gov/apps/physician-fee-schedule/overview.aspx.
- Outpatient Prospective Payment System . US Department of Health and Human Services 2010.
- Healthcare Common Procedure Coding System . US Department of Health and Human Services 2011.
- Highlights of Women’s Earnings in 2010 . Labor USDo, Editor, US Bureau of Labor Statistics 2011.
- CPI Inflation Calculator . US Bureau of Labor Statistics 2011. http://data.bls.gov/cgi-bin/cpicalc.pl.
- IRS announces 2011 standard mileage rates . Internal Revenue Service 2010.
- Gasoline and Diesel Fuel Update . US Energy Information Administration 2011. www.eia.gov/oog/info/gdu/gasdiesel.asp.
- Riley GF, Potosky AL, Lubitz JD, Kessler LG. Medicare payments from diagnosis to death for elderly cancer patients by stage at diagnosis. Med Care 1995;33:828-41. http://dx.doi.org/10.1097/00005650-199508000-00007.
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–20. J Natl Cancer Inst 2011;103:117-28. http://dx.doi.org/10.1093/jnci/djq495.
- Hayman JA, Hillner BE, Harris JR. Cost-effectiveness of routine radiation therapy following conservative surgery for early-stage breast cancer. J Clin Oncol 1998;16:1022-9.
- Sher DJ, Wittenberg E, Suh WW, Taghian AG, Punglia RS. Partial-breast irradiation versus whole-breast irradiation for early-stage breast cancer: a cost-effectiveness analysis. Int J Radiat Oncol Biol Phys 2009;74:440-6. http://dx.doi.org/10.1016/j.ijrobp.2008.08.015.
- Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513-20. http://dx.doi.org/10.1056/NEJMoa0906260.
- Salvadori B, Marubini E, Miceli R, Conti AR, Cusumano F, Andreola S, et al. Reoperation for locally recurrent breast cancer in patients previously treated with conservative surgery. Br J Surg 1999;86:84-7. http://dx.doi.org/10.1046/j.1365-2168.1999.00961.x.
- Suh WW, Pierce LJ, Vicini FA, Hayman JA. A cost comparison analysis of partial versus whole-breast irradiation after breast-conserving surgery for early-stage breast cancer. Int J Radiat Oncol Biol Phys 2005;62:790-6. http://dx.doi.org/10.1016/j.ijrobp.2004.10.039.
- Stokes ME, Thompson D, Montoya EL, Weinstein MC, Winer EP, Earle CC. Ten-year survival and cost following breast cancer recurrence: estimates from SEER-Medicare data. Value Health 2008;11. http://dx.doi.org/10.1111/j.1524-4733.2007.00226.x.
- Shah C, Lanni TB, Saini H, Nanavati A, Wilkinson JB, Badiyan S, et al. Cost-efficacy of acceleration partial-breast irradiation compared with whole-breast irradiation. Breast Cancer Res Treat 2013;138:127-35. http://dx.doi.org/10.1007/s10549-013-2412-6.
- Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, et al. Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE). Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14010.
- Freedman GM, Li T, Anderson PR, Nicolaou N, Konski A. Health states of women after conservative surgery and radiation for breast cancer. Breast Cancer Res Treat 2010;121:519-26. http://dx.doi.org/10.1007/s10549-009-0552-5.
- Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al. A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11310.
- Serra D, Parris CR, Carper E, Homel P, Fleishman SB, Harrison LB, et al. Outcomes of guided imagery in patients receiving radiation therapy for breast cancer. Clin J Oncol Nurs 2012;16:617-23. http://dx.doi.org/10.1188/12.CJON.617-623.
- Conner-Spady BL, Cumming C, Nabholtz JM, Jacobs P, Stewart D. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant 2005;36:251-9. http://dx.doi.org/10.1038/sj.bmt.1705032.
- Robertson S, Wengstrom Y, Eriksen C, Sandelin K. Breast surgeons performing immediate breast reconstruction with implants – assessment of resource-use and patient-reported outcome measures. Breast 2012;21:590-6. http://dx.doi.org/10.1016/j.breast.2012.01.003.
- Lidgren M, Wilking N, Jonsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res 2007;16:1073-81. http://dx.doi.org/10.1007/s11136-007-9202-8.
- Sherrill B, Amonkar MM, Stein S, Walker M, Geyer C, Cameron D. Q-TWiST analysis of lapatinib combined with capecitabine for the treatment of metastatic breast cancer. Br J Cancer 2008;99:711-15. http://dx.doi.org/10.1038/sj.bjc.6604501.
- Hildebrandt T, Thiel FC, Fasching PA, Graf C, Bani MR, Loehberg CR, et al. Health utilities in gynecological oncology and mastology in Germany. Anticancer Res 2014;34:829-35.
- Hayman JA, Fairclough DL, Harris JR, Weeks JC. Patient preferences concerning the trade-off between the risks and benefits of routine radiation therapy after conservative surgery for early-stage breast cancer. J Clin Oncol 1997;15:1252-60.
- Department of Health . NHS Reference Costs 2012 to 2013 n.d. www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013 (accessed 10 February 2014).
- Department of Health . Payments by Results in the NHS: Tariff for 2013 to 2014 n.d. www.gov.uk/government/publications/payment-by-results-pbr-operational-guidance-and-tariffs (accessed 10 February 2014).
- Wolowacz SE, Cameron D, Tate HC, Bagust A. Docetaxel in combination with Doxorubicin and cyclophosphamide as adjuvant treatment for early node-positive breast cancer: a cost-effectiveness and cost–utility analysis. J Clin Oncol 2008;26:925-33. http://dx.doi.org/10.1200/JCO.2006.10.4190.
- Green Book: appraisal and evaluation in central government. London: The Stationery Office; 2013.
- Hind D, Ward S, De NE, Simpson E, Carroll C, Wyld L. Hormonal therapies for early breast cancer: systematic review and economic evaluation. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11260.
- Bai Y, Ye M, Cao H, Ma X, Xu Y, Wu B. Economic evaluation of radiotherapy for early breast cancer after breast-conserving surgery in a health resource-limited setting. Breast Cancer Res Treat 2012;136:547-57. http://dx.doi.org/10.1007/s10549-012-2268-1.
- Gold HT, Hayes MK. Cost-effectiveness of new breast cancer radiotherapy technologies in diverse populations. Breast Cancer Res Treat 2012;136:221-9. http://dx.doi.org/10.1007/s10549-012-2242-y.
- de Bock GH, Putter H, Bonnema J, van der Hage JA, Bartelink H, van de Velde CJ. The impact of loco-regional recurrences on metastatic progression in early-stage breast cancer: a multistate model. Breast Cancer Res Treat 2009;117:401-8. http://dx.doi.org/10.1007/s10549-008-0300-2.
- England, Interim Life Tables, 1980–82 to 2010–12. Fareham: ONS; 2013.
- Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2012;12. http://dx.doi.org/10.1186/1471-2288-12-9.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making 2013;33:743-54. http://dx.doi.org/10.1177/0272989X12472398.
- Veronesi U, Orecchia R, Maisonneuve P, Viale G, Rotmensz N, Sangalli C, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. http://dx.doi.org/10.1016/S1470-2045(13)70497-2.
- Kreike B, Hart AAM, van de Velde T, Borger J, Peterse H, Rutgers E, et al. Continuing risk of ipsilateral breast relapse after breast-conserving therapy at long-term follow-up. Int J Radiat Oncol Biol Phys 2008;71:1014-21. http://dx.doi.org/10.1016/j.ijrobp.2007.11.029.
- Kamby C, Sengelov L. Pattern of dissemination and survival following isolated locoregional recurrence of breast cancer. A prospective study with more than 10 years of follow up. Breast Cancer Res Treat 1997;45:181-92. http://dx.doi.org/10.1023/A:1005845100512.
- The Second All Breast Cancer Report. London: National Cancer Intelligence Network; 2011.
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-43. http://dx.doi.org/10.1056/NEJMoa064320.
- NHS Information Centre for Health and Social Care . National Mastectomy and Breast Reconstruction Audit, Fourth Annual Report – 2011 n.d. www.ic.nhs.uk/catalogue/PUB02731/clin-audi-supp-prog-mast-brea-reco-2011-rep1.pdf (accessed 10 February 2014).
- Curtis L. Unit Costs of Health and Social Care 2013. Kent: PSSRU; 2013.
- Office for National Statistics . Population Estimates for England and Wales, Mid-2011 (2011 Census-Based) 2014. www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-england-and-wales/mid-2011--2011-census-based-/index.html (accessed 10 February 2014).
- Leonardi MC, Maisonneuve P, Mastropasqua MG, Morra A, Lazzari R, Rotmensz N, et al. How do the ASTRO consensus statement guidelines for the application of accelerated partial breast irradiation fit intraoperative radiotherapy? A retrospective analysis of patients treated at the European Institute of Oncology. Int J Radiat Oncol Biol Phys 2012;83:806-13. http://dx.doi.org/10.1016/j.ijrobp.2011.08.014.
- Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987-1001. http://dx.doi.org/10.1016/j.ijrobp.2009.02.031.
- SEER . SEER Cancer Statistics Review 1975–2009 2014. http://seer.cancer.gov/csr/1975_2009_pops09/browse_csr.php?sectionSEL=4&pageSEL=sect_04_table.17.html (accessed 10 February 2014).
- Cancer Research UK . Breast Cancer Survival Statistics 2014. www.cancerresearchuk.org/cancer-info/cancerstats/types/breast/survival/ (accessed 10 February 2014).
- Ahn SH, Park BW, Noh DY, Nam SJ, Lee ES, Lee MK, et al. Health-related quality of life in disease-free survivors of breast cancer with the general population. Ann Oncol 2007;18:173-82. http://dx.doi.org/10.1093/annonc/mdl333.
- Moro-Valdezate D, Buch-Villa E, Peiro S, Morales-Monsalve MD, Caballero-Garate A, Martinez-Agullo A, et al. Factors associated with health-related quality of life in a cohort of Spanish breast cancer patients. Breast Cancer 2014;21:442-52. http://dx.doi.org/10.1007/s12282-012-0402-x.
- Radiotherapy services in England 2012. London: Department of Health; 2012.
- Fast-Forward Protocol Development Group . Fast-Forward. Randomised Clinical Trial Testing a 1-Week Course of Curative Whole Breast Radiotherapy Against a Standard 3-Week Schedule in Terms of Local Cancer Control and Late Adverse Effects in Patients With Early Breast Cancer 2013. www.nets.nihr.ac.uk/__data/assets/pdf_file/0015/53115/PRO-09-01-47.pdf.
- Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371:1098-107. http://dx.doi.org/10.1016/S0140-6736(08)60348-7.
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008;112:533-43. http://dx.doi.org/10.1007/s10549-007-9885-0.
- Agency for Healthcare Research and Quality . Calculating the US Population-Based EQ-5D Index Score 2005. www.ahrq.gov/rice/EQ5Dscore.htm.
Appendix 1 Search dates and example MEDLINE search strategies for clinical effectiveness, cost-effectiveness and health-related quality of life
Databases searched for the systematic reviews of clinical effectiveness, cost-effectiveness and HRQoL are presented below. Searches were updated in March 2014.
| Database searched (host) | Clinical effectiveness searches | Cost effectiveness and QoL searches |
|---|---|---|
| Cochrane Central, Cochrane CDSR, Cochrane DARE, Cochrane HTA, and Cochrane Methods (The Cochrane Library) | All available years to 19 March 2014 | |
| Cochrane Central, Cochrane DARE, Cochrane Economic Evaluations, and Cochrane Methods (The Cochrane Library) | All available years to 18 March 2014 (QoL) and to 19 March 2014 (cost) | |
| CRD databases: DARE, HTA and NHS EED (CRD) | All available years to 19 March 2014 | All available years to 18 March 2014 (both) |
| CPCI – Science (Web of Science) | All available years to 19 March 2014 | All available years to 18 March 2014 (both) |
| Cost-effectiveness analysis registry (Tufts Medical Center) | Searched to 19 March 2014 (cost) | |
| EMBASE (via Ovid) | All available years to 19 March 2014 | All available years to 18 March 2014 (both) |
| MEDLINE(R) (via Ovid) | All available years to 19 March 2014 | All available years to 18 March 2014 (both) |
| MEDLINE(R) In-Process & Other Non-Indexed Citations (via Ovid) | Searched to 19 March 2014 | Searched to 18 March 2014 (both) |
| SCIE (Web of Science) | 1995 to 19 March 2014 | 1970 to 18 March 2014 (both) |
| ScienceDirect.com | Searched 19 March 2014 (cost) | |
| BIOSIS Previews (Web of Science) | 1995 to 19 March 2014 | All available years to 18 March 2014 (both) |
| Zetoc (via Mimas) | Searched to 19 March 2014 (cost) |
| Searched for ongoing trials (all searched on 25 March 2014) |
|---|
| NIHR Clinical Research Network (NIHR CRN Portfolio, formally UKCRN website) |
| Controlled-trials.com |
| ClinicalTrials.gov |
| WHO ICTRP |
| American Society of Clinical Oncology (ASCO) |
Example search strategies
Clinical effectiveness
-
exp Breast Neoplasms/
-
Carcinoma, Intraductal, Noninfiltrating/
-
(“ductal carcinoma* in situ” or DCIS).tw.
-
(breast* adj5 (neoplasm* or cancer* or tumo?r* or carcinoma* or adenocarcinoma* or sarcoma* or dcis or ductal* or infiltrat* or intraductal* or lobular or medullary or malignan*.tw.
-
(mammar* adj5 (neoplasm* or cancer* or tumo?r* or carcinoma* or adenocarcinoma* or sarcoma* or dcis or ductal* or infiltrat* or intraductal* or lobular or medullary or malignan*)).tw.
-
exp “Neoplasms, Ductal, Lobular, and Medullary”/
-
(breast or mammar*).tw.
-
6 and 7
-
or/1-5,8
-
intrabeam*.af.
-
Radiosurgery/ or radiosurg*.tw.
-
Radiotherapy, Adjuvant/
-
(radiother* or irradiat* or radiat* or xray or “x-ray”).tw.
-
or/12-13
-
“during surg*”.tw.
-
“radio* guided surg*”.tw.
-
(intraoperativ* or “intra operativ”).tw.
-
(“single dose” or “single fraction*”).tw.
-
or/15-18
-
14 and 19
-
IORT.tw.
-
(intraoperativ* adj5 radiotherap*).tw.
-
TARGIT*.tw.
-
“tumo?r bed”.tw.
-
(boost* or target*).tw.
-
13 and 24 and 25
-
9 and (10 or 11 or 20 or 21 or 22 or 23 or 26)
-
Randomized Controlled Trials as Topic/
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
Controlled Clinical Trial/
-
placebos/
-
random allocation/
-
Double-Blind Method/
-
Single-Blind Method/
-
(random* adj2 allocat*).tw.
-
placebo*.tw.
-
((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw.
-
crossover studies/
-
(crossover* or (cross adj over*)).tw.
-
Research Design/
-
((random* or control*) adj5 (trial* or stud*)).tw.
-
Clinical Trials as Topic/
-
random*.ab.
-
or/28-44
-
27 and 45
Cost-effectiveness
-
exp Breast Neoplasms/
-
Carcinoma, Intraductal, Noninfiltrating/
-
(“ductal carcinoma* in situ” or DCIS).tw.
-
(breast* adj5 (neoplasm* or cancer* or tumo?r* or carcinoma* or adenocarcinoma* or sarcoma* or dcis or ductal* or infiltrat* or intraductal* or lobular or medullary or malignan*)).tw.
-
(mammar* adj5 (neoplasm* or cancer* or tumo?r* or carcinoma* or adenocarcinoma* or sarcoma* or dcis or ductal* or infiltrat* or intraductal* or lobular or medullary or malignan*)).tw.
-
exp “Neoplasms, Ductal, Lobular, and Medullary”/
-
(breast or mammar*).tw.
-
6 and 7
-
or/1-5,8
-
intrabeam*.af.
-
Radiosurgery/ or radiosurg*.tw.
-
Radiotherapy, Adjuvant/
-
(radiother* or irradiat* or radiat* or xray or “x-ray”).tw.
-
or/12-13
-
“during surg*”.tw.
-
“radio* guided surg*”.tw.
-
(intraoperativ* or “intra operativ”).tw.
-
(“single dose” or “single fraction*”).tw.
-
or/15-18
-
14 and 19
-
IORT.tw.
-
(intraoperativ* adj5 radiotherap*).tw.
-
TARGIT*.tw.
-
“tumo?r bed”.tw.
-
(boost* or target*).tw.
-
13 and 24 and 25
-
9 and (10 or 11 or 20 or 21 or 22 or 23 or 26)
-
exp economics/
-
exp economics hospital/
-
exp economics pharmaceutical/
-
exp economics nursing/
-
exp economics medical/
-
exp “Costs and Cost Analysis”/
-
Cost Benefit Analysis/
-
exp models economic/
-
exp fees/ and charges/
-
exp budgets/
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic*).tw.
-
(value adj1 money).tw.
-
budget$.tw.
-
or/28-40
-
((energy or oxygen) adj cost).tw.
-
(metabolic adj cost).tw.
-
((energy or oxygen) adj expenditure).tw.
-
or/42-44
-
41 not 45
-
(letter or editorial or comment or historical article).pt.
-
46 not 47
-
27 and 48
Lines 50–54 added to strategy on 25/09/2013. Nothing extra found as a consequence.
-
accelerated partial breast irradiation.mp. 430
-
APBI.tw. 266
-
50 or 51
-
48 and 52
-
53 not 49
Health-related quality of life
-
exp Breast Neoplasms/
-
(breast* adj3 (neoplasm* or cancer* or tumo?r* or carcinoma* or adenocarcinoma* or sarcoma* or dcis or ductal* or infiltrat* or intraductal* or lobular or medullary or malignan*)).tw.
-
(mammar* adj3 (neoplasm* or cancer* or tumo?r* or carcinoma* or adenocarcinoma* or sarcoma* or dcis or ductal* or infiltrat* or intraductal* or lobular or medullary or malignan*)).tw.
-
or/1-3
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab.
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab.
-
(hui or hui1 or hui2 or hui3).ti,ab.
-
(health adj3 utilit$ ind$).mp.
-
“EORTC QLQ-BR23”.tw.
-
“FACT-B”.tw.
-
“Functional Assessment of Cancer Therapy Breast”.tw.
-
“BCQ”.tw.
-
“breast cancer chemotherapy questionnaire”.tw.
-
or/5-13
-
4 and 14
Appendix 2 Excluded clinical effectiveness studies with rationale
| Excluded study | Primary reason for exclusion (comment) |
|---|---|
| Andersen KG, Gartner R, Kroman N, Flyger H, Kehlet H. Persistent pain after targeted intraoperative radiotherapy (TARGIT) or external breast radiotherapy for breast cancer: a randomized trial. Breast J 2012;21:46–9 | Outcome (substudy) |
| Andersen KG, Gartner R, Kroman N, Flyger H, Kehlet H. Persistent pain after targeted intraoperative radiotherapy (TARGIT) or external breast radiotherapy for breast cancer – a randomized trial. Eur J Cancer 2011;47:S388 | Abstracta |
| TARGIT-B: An international randomised controlled trial to compare targeted intra-operative radiotherapy boost with conventional external beam radiotherapy boost after lumpectomy for breast cancer in women with a high risk of local recurrence. URL: www.nets.nihr.ac.uk/projects/hta/1010407 (accessed 25 March 2014) | Ongoing (no data yet) |
| Baum M, Joseph DJ, Tobias JS, Wenz FK, Keshtgar MR, Alvarado M, et al. Safety and efficacy of targeted intraoperative radiotherapy (TARGIT) for early breast cancer: first report of a randomized controlled trial at 10-years maximum follow-up. J Clin Oncol 2010;28(Suppl. Abstract LBA517):18 | Abstracta |
| Baum M, Vaidya JS, Tobias JS, Keshtgar M, Williams NR, Wenz F, et al. Targit (targeted intra-operative radiotherapy for early stage breast cancer): Results from the targit a randomized controlled trial. Eur J Cancer Supplement 2010;8:19 | Abstracta |
| Drago S, Ciabattoni A, Piccirillo R, Bellotti A, Cresti R, Ciccone V, et al. Intraoperative radiation boost in early breast cancer: initial results of a randomized trial. Breast Cancer Res Treat 2004;88:S172 | Intervention (abstract) |
| Engel D, Schnitzer A, Brade J, Blank E, Wenz F, Suetterlin M, et al. Are mammographic changes in the tumor bed more pronounced after intraoperative radiotherapy for breast cancer? Subgroup analysis from a randomized trial (TARGIT-A). Breast J 2013;19:92–5 | Outcomesa |
| HAYES Inc. Intraoperative Radiation Therapy (IORT) for breast cancer. CRD Database Structured abstract. URL: www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32012000152&UserID=0 (accessed 25 September 2013) | Design |
| Holmes DR, Baum M, Joseph D. The TARGIT trial: targeted intraoperative radiation therapy versus conventional postoperative whole-breast radiotherapy after breast-conserving surgery for the management of early-stage invasive breast cancer (a trial update). Am J Surg 2007;194:507–10 | Abstracta |
| Joseph DJ. Targit. Radiother Oncol 2012;103:S4 | Abstracta |
| Keshtgar M, Vaidya J, Tobias J, Williams N, Baum M. TARGIT (Targeted intra-operative radiotherapy for early stage breast cancer): early results from the multi-centre randomized controlled trial. Eur J Surg Oncol 2010;36:1098 | Abstracta |
| Keshtgar M, Williams N, Corica T, Saunders C, Joseph D, Bulsara M. Cosmetic outcome after targit compared with external beam radiotherapy for early breast cancer. Radiother Oncol 2011;99:S251 | Abstracta |
| Keshtgar M, Williams N, Corica T, Saunders C, Joseph D, Bulsara M. Cosmetic outcome one, two, three and four years after intra-operative radiotherapy compared with external beam radiotherapy for early breast cancer: an objective assessment of patients from a randomised controlled trial. Breast 2011;20:S63 | Abstracta |
| Keshtgar M, Williams N, Corica T, Saunders C, Joseph D. Better cosmetic outcome after intraoperative radiotherapy compared with external beam radiotherapy for early breast cancer: objective assessment of patients from a randomized controlled trial. Ann Surg Oncol 2010;17:S178 | Abstracta |
| Keshtgar M, Williams N, Corica T, Saunders C, Joseph D. Cosmetic outcome one, two and three years after intra-operative radiotherapy compared with external beam radiotherapy for early breast cancer: An objective assessment of patients from a randomised controlled trial. Eur J Surg Oncol 2010;36:1105 | Abstracta |
| Keshtgar M, Williams N, Corica T, Saunders C, Joseph D. Significantly better cosmetic outcome after intraoperative radiotherapy compared with external beam radiotherapy for early breast cancer: objective assessment of patients from a randomized controlled trial. Ann Surg Oncol 2011;18:S171 | Abstracta |
| Keshtgar M, Williams NR, Corica T, Bulsara M, Saunders C, Flyger H, et al. An objective assessment of cosmetic outcome after intraoperative radiotherapy or external beam radiotherapy for early breast cancer in patients from a randomized controlled trial. Eur J Cancer 2013;49:S450 | Abstracta |
| Keshtgar M, Williams NR, Corica T, Hedges R, Saunders C, Joseph D. Early evidence of better cosmetic outcome after intra-operative radiotherapy compared with external beam radiotherapy for early breast cancer: Objective assessment of patients from a randomised controlled trial. Ann Surg Oncol 2010;17:S13 | Abstracta |
| Keshtgar M, Williams NR, Corica T, Saunders C, Bulsara M, Joseph D. Improved cosmetic outcome after TARGIT compared with external beam radiotherapy for early breast cancer. Eur J Cancer 2012;48:S186–7 | Abstracta |
| Keshtgar MR, Williams NR, Bulsara M, Saunders C, Flyger H, Cardoso JS, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat 2013;140:519–25 | Outcome (substudy)a |
| Keshtgar MR, Williams NR, Corica T, Bulsara M, Saunders C, Flyger H, et al. Cosmetic outcome after intraoperative radiotherapy or external beam radiotherapy for early breast cancer: an objective assessment of patients from a randomized controlled trial. J Clin Oncol 2013;31:(Suppl.; abstract 1110) | Abstracta |
| Keshtgar MR, Williams NR, Corica T, Bulsara M, Saunders C, Flyger H, et al. Cosmetic outcome after intraoperative radiotherapy or external beam radiotherapy for early breast cancer: an objective assessment of patients from a randomized controlled trial. J Clin Oncol 2013;15:1110 | Abstracta |
| Keshtgar MR, Williams NR, Corica T, Saunders C, Bulsara M, Joseph D. Cosmetic outcome one, two, three, and four years after intra-operative radiotherapy compared with external beam radiotherapy for treatment of early breast cancer: An objective assessment of patients from a randomized controlled trial. Int J Radiat Oncol Biol Physics 2011;81:S225 | Abstracta |
| Keshtgar MR, Williams NR, Corica T, Saunders C, Joseph DJ, Bulsara M. Cosmetic outcome 1, 2, 3, and 4 years after intraoperative radiotherapy or external beam radiotherapy for early breast cancer: an objective assessment of patients from a randomized controlled trial. J Clin Oncol 2011;29:94 | Abstracta |
| Keshtgar MR, Williams NR, Corica T, Saunders C, Joseph DJ. Cosmetic outcome two and three years after intraoperative radiotherapy compared with external beam radiotherapy for early breast cancer: an objective assessment of patients from a randomized controlled trial. J Clin Oncol 2010;28:570 | Abstracta |
| Sperk E, Welzel G, Keller A, Kraus-Tiefenbacher U, Gerhardt A, Sutterlin M, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Strahlenther Onkol 2012;188:62 | Abstract a |
| Sperk E, Welzel G, Keller A, Kraus-Tiefenbacher U, Gerhardt A, Sutterlin M, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat 2012;135:253–60 | Outcome (substudy)a |
| Sperk E, Welzel G, Keller A, Kraus-Tiefenbacher U, Gerhardt A, Sutterlin M, et al. Late Radiation Toxicity After Intraoperative Radiotherapy (IORT) for Breast Cancer: Results From the Randomized Phase III Trial TARGIT A. Eur J Cancer 2012;48:S187–8 | Abstracta |
| Vaidya JS, Baum M, Tobias JS, Houghton J, Keshtgar M, Sainsbury R, et al. Targeted intraoperative radiotherapy for breast cancer – a randomised trial. Breast Cancer Res Treat 2001;69:228 | Outcomesa (abstract) |
| Vaidya JS, Massarut S, Tobias JS, Wenz F, Bulsara M, Keshtgar M, et al. Targeted intra-operative radiotherapy boost-TARGIT-B trial: A randomized trial for young and high risk patients including those after post-neoadjuvant systemic therapy lumpectomy. Eur J Surg Oncol 2010;36:820 | Outcomes (abstract) |
| Vaidya JS, Tobias JS, Baum M, Houghton J, Keshtgar M, Sainsbury R. Targeted intra-operative radiotherapy (TARGIT) for breast cancer: a randomised trial. Radiology 2001;221:278 | Outcomesa (abstract) |
| Vaidya JS. An international randomised controlled trial to compare targeted intra-operative radiotherapy (TARGIT) with conventional post-operative radiotherapy for women with early breast cancer (Project record). Health Technol Assess 2015; in press | Outcomesa (trial protocol) |
| Valachis A, Mauri D, Polyzos NP, Mavroudis D, Georgoulias V, Casazza G. Partial breast irradiation or whole breast radiotherapy for early breast cancer: a meta-analysis of randomized controlled trials. Breast J 2010;16:245–51 | Intervention |
| Welzel G, Boch A, Blank E, Kraus-Tiefenbacher U, Keller A, Hermann B, et al. Radiation-related quality of life parameters after targeted intraoperative radiotherapy vs. whole breast radiotherapy in patients with breast cancer: results from the randomized phase III trial TARGIT-A. Int J Radiat Oncol Biol Physics 2011;81:S206–7 | Abstracta |
| Williams N, Keshtgar M, Corica T, Saunders C, Bulsara M, Joseph DJ. Cosmetic outcome after intra-operative radiotherapy for early breast cancer in women over 50 years. Radiother Oncol 2012;103:S128–9 | Abstracta |
| Williams NR, Keshtgar M, Corica T, Saunders C, Joseph D, Bulsara MK. Early breast cancer and cosmetic outcome one, two, three and four years after intra-operative radiotherapy compared with external beam radiotherapy: an objective assessment of patients from a randomised controlled trial (on behalf of the TARGIT trialists’ group). Eur J Cancer 2011;47:S365 | Abstracta |
| Williams NR, Keshtgar M, Corica T, Saunders C, Joseph D. Significantly better cosmetic outcome after intra-operative radiotherapy compared with external beam radiotherapy for early breast cancer: objective assessment of patients from a randomised controlled trial. Eur J Cancer Supplements 2010;8:129 | Abstracta |
| Zhou SF, Shi WF, Meng D, Sun CL, Jin JR, Zhao YT. Interoperative radiotherapy of seventy-two cases of early breast cancer patients during breast-conserving surgery. Asian Pac J Cancer Prev 2012;13:1131–5 | Intervention |
Appendix 3 Clinical effectiveness data extraction tables
| Reviewer 1: JP, date: 13 November 2013 | Reviewer 2: DH, date: 19 November 2013 | Version: 2 | |||
|---|---|---|---|---|---|
| Reference and design | Intervention and comparator | Participants | Outcome measures | ||
| Vaidya et al. 2014,65 2010.64 Linked substudies63,66–68 (separate data extractions). TARGIT-A trial Study design: international, multicentre, non-inferiority RCT Countries: UK, Europe, Australia, USA and Canada Number of centres: 33 centres in 11 countries65 [UK (6), Germany (7), Italy (3), Switzerland (2), Denmark (1), Poland (1), Norway (1), USA (7), Canada (1), Australia (2), France (2)]. For the mature cohort reported in 2010,64 28 centres in 10 countries [UK (5), Germany (6), Italy (2), Switzerland (2), Denmark (1), Poland (1), Norway (1), USA (7), Canada (1) and Australia (2)] Recruitment dates: 24 March 2000 to 25 June 2012 Funding: UCL Hospitals, UCL Comprehensive Biomedical Research Centre, UCL Hospital Charities, NIHR HTA programme (primary funder), Ninewells Cancer Campaign, National Health and Medical Research Council, German Federal Ministry of Education and Research. This was an academically driven trial and the funding bodies had no role in trial design, data analysis or interpretation, or writing the report |
Intervention: TARGITa (INTRABEAM device) Dose: typically 20 Gy to surface of tumour bed attenuating to 5–7 Gy at a depth of 1 cm Comparator: WB-EBRT Dose: typically 40–56 Gy ± boost of 10–16 Gy Other interventions used: adjuvant systemic treatment as appropriate – hormone therapy, chemotherapy or other (not specified). A risk-adapted approach in the TARGIT arm was pre-specified. Any participants in the TARGIT group with pre-specified unfavourable pathological features found subsequently received WB-EBRT after TARGIT. Three adverse features were defined in the core protocol (tumour-free margin < 1 mm; extensive in-situ component; and unexpected invasive lobular carcinoma) and centres could pre-specify additional features before starting recruitment |
Number of randomised participants: 2014 paper,65 n = 3451; TARGIT, n = 1721; WB-EBRT, n = 1730 (n = 2298 in pre-pathology stratum, n = 1153 in post pathology).65 2010 paper,64 n = 2232; TARGIT, n = 1113; WB-EBRT, n = 1119 (n = 1482 in pre-pathology stratum, n = 672 in post-pathology stratum, n = 78 in contralateral stratum)64 Inclusion criteria: women with early breast cancer, aged ≥ 45 years, suitable for WLE for invasive ductal carcinoma that was unifocal on conventional examination and imaging Exclusion criteria: pre-operative diagnosis of lobular carcinoma. (More detailed exclusion criteria are given in the protocol www.hta.ac.uk/project/1981.asp) |
Primary outcomes: local recurrence (in the conserved breast) Secondary outcomes: local toxicity or morbidity (complications pre-specified).64 Overall survival (breast cancer and non-breast cancer deaths).65 Specimen weight, margin status and reoperation for margins (analysed to compare the extent of local surgery)64 Method of assessing outcomes: described in the paper reporting initial results:64 assessments at entry, 3 and 6 months, then every 6 months for up to 5 years and every year for up to 10 years. Local recurrence was pathologically confirmed (no further details). Toxicity or morbidity assessed from data recorded on a complications form containing a pre-specified checklist (haematoma, seroma, wound infection, skin breakdown, delayed wound healing, RTOG toxicity grade 3 or 4 for dermatitis, telangiectasia, pain in irradiated field, or other). Skin breakdown or delayed wound healing or RTOG toxicity grade > 2 classified as major toxicity |
||
| Described in the 2014 paper:65 if breast cancer was present at the time of death, the death was presumed to be from breast cancer Length of follow-up: overall median 2 years and 5 months (IQR 12–52 months). A median follow-up of 4 years was reached by 2020 participants and of 5 years by 1222 participants. The mature cohort of 2232 participants (first reported on in 201064) had a median follow-up of 3 years and 7 months (IQR 30–61 months) in the 2014 paper.65 For the earlier 2010 paper, follow-up was up to 10 years (data lock 2 May 2010)64 |
|||||
| bBaseline characteristics65 | TARGIT (n = 1721) | WB-EBRT (n = 1730) | p-value | ||
| Age (years), n/N (%) | 0.274 | ||||
| ≤ 50 | 150/1721 (9) | 122/1730 (7) | |||
| 51–60 | 527/1721 (31) | 548/1730 (32) | |||
| 61–70 | 781/1721 (45) | 807/1730 (47) | |||
| > 70 | 263/1721 (15) | 253/1730 (15) | |||
| Pathological tumour size (cm), n/N (%) | 0.273 | ||||
| ≤ 1 | 611/1552 (39) | 597/1530 (39) | |||
| 1.1–2 | 751/1552 (48) | 726/1530 (48) | |||
| > 2 | 190/1552 (12) | 207/1530 (14) | |||
| Unknown | 169/1721 (10) | 200/1730 (12) | |||
| Grade,c n/N (%) | 0.394 | ||||
| 1 | 528/1517 (35) | 558/1505 (37) | |||
| 2 | 757/1517 (50) | 720/1505 (48) | |||
| 3 | 232/1517 (15) | 227/1505 (15) | |||
| Unknown | 194/1721 (11) | 225/1730 (13) | |||
| Lymphovascular invasion, n/N (%) | 0.224 | ||||
| Absent | 1348/1542(87) | 1343/1521 (88) | |||
| Present | 194/1542 (13) | 178/1521 (12) | |||
| Unknown | 179/1721 (10) | 209/1730 (12) | |||
| Nodes involved, n/N (%) | 0.091 | ||||
| 0 | 1307/1569 (83) | 1303/1543 (85) | |||
| 1–3 | 219/1569 (14) | 211/1543 (14) | |||
| > 3 | 43/1569 (3) | 29/1543 (2) | |||
| Unknown | 152/1721 (9) | 187/1721 (11) | |||
| ER status, n/N (%) | 0.090 | ||||
| ER+ | 1441/1561 (92) | 1433/1532 (94) | |||
| ER– | 120/1561 (8) | 99/1532 (7) | |||
| ER unknown | 160/1721 (9) | 198/1730 (12) | |||
| PgR status, n/N (%) | 0.179 | ||||
| PgR+ | 1232/1521 (81) | 1230/1495 (82) | |||
| PgR– | 289/1521 (19) | 265/1495 (18) | |||
| PgR unknown | 200/1721 (12) | 235/1730 (14) | |||
| HER-2, n/N (%) | 0.585 | ||||
| HER-2+ | 170/1499 (11) | 178/1487 (12) | |||
| HER-2– | 1329/1499 (89) | 1309/1487 (88) | |||
| Unknown | 222/1721 (13) | 243/1730 (14) | |||
| Additional baseline characteristics present only in the 2010 paper64 | TARGIT (n = 1113) | WB-EBRT (n = 1119) | Comments | ||
| Height (cm), median (IQR) | 164 (159–168) | 163 (159–168) | |||
| Weight (kg), median (IQR) | 70 (62–80) | 70 (62–80) | |||
| Tumour type, n/N (%) | |||||
| Invasive ductal carcinoma | 1012/1070 (95) | 1018/1079 (94) | |||
| Invasive lobular carcinoma | 47/1070 (4) | 45/1079 (4) | |||
| Mixed | 32/1070 (3) | 35/1079 (3) | |||
| Unknown | 43/1113 (4) | 40/1119 (4) | |||
| DCIS, n/N (%) | |||||
| Present | 529/1063 (50) | 547/1069 (51) | |||
| Absent | 534/1063 (50) | 522/1069 (49) | |||
| Unknown | 50/1113 (4) | 50/1119 (4) | |||
| Adjuvant therapy, n/N (%) | |||||
| Hormone therapy | 727/1113 (65) | 753/1119 (67) | |||
| Chemotherapy | 116/1113 (10) | 141/1119 (13) | |||
| Other | 48/1113 (4) | 41/1119 (4) | |||
| Unknown | 100/1113 (9) | 89/1119 (8) | |||
| Results | |||||
| Primary outcome:d events/N; 5-year cumulative risk (%) (95% CI)65 | TARGIT (n = 1721) | WB-EBRT (n = 1730) | Absolute difference; p-value | ||
| Local recurrence, all patientse | 23/1679; 3.3 (2.1 to 5.1) | 11/1696; 1.3 (0.7 to 2.5) | 12 (2.0%); p = 0.042 | ||
| Local recurrence, pre-pathology stratum | 10/1107; 2.1 (1.1 to 4.2) | 6/1127; 1.1 (0.5 to 2.5) | 4 (1.0%); p = 0.31 | ||
| Local recurrence, post-pathology stratum | 13/572; 5.4 (3.0 to 9.7) | 5/569; 1.7 (0.6 to 4.9) | 8 (3.7%); p = 0.069 | ||
| fLocal recurrence: calculation of pnon-inferiority65 | Median follow-up | Events, n/N | Absolute difference (90% CI) in the binomial proportionsg of ipsilateral local recurrence (TARGIT minus WB-EBRT) | z-value | pnon-inferiority |
| Whole trial: all patients | 2 years 5 months | 34/3451 | 0.72% (0.2% to 1.3%) | –5.168 | < 0.0001 |
| Whole trial: mature cohort | 3 years 7 months | 32/2232 | 1.13% (0.3% to 2.0%) | –2.652 | 0.0040 |
| Whole trial: earliest cohort | 5 years | 23/1222 | 1.14% (–0.1% to 2.4%) | –1.750 | 0.0400 |
| Pre-pathology: all patients | 2 years 4 months | 16/2298 | 0.37% (–0.2% to 1.0%) | –5.954 | < 0.0001 |
| Pre-pathology: mature cohort | 3 years 8 months | 14/1450 | 0.6% (–0.3% to 1.5%) | –3.552 | 0.0002 |
| Pre-pathology: earliest cohort | 5 years | 9/817 | 0.76% (–0.4% to 2.0%) | –2.360 | 0.0091 |
| Post pathology: all patients | 2 years 4 months | 18/1153 | 1.39% (0.2% to 2.6%) | –1.503 | 0.0664 |
| Post pathology: mature cohort | 3 years 7 months | 18/782 | 2.04% (0.3% to 3.8%) | –0.429 | 0.3339 |
| Post pathology: earliest cohort | 5 years | 14/405 | 1.8% (–1.2% to 4.8%) | –0.382 | 0.3511 |
| Local recurrence in conserved breast for pre-pathology stratumh | Absolute difference in 5-year Kaplan–Meier estimate (SE) | ||||
| Whole cohort (n = 2298), median follow-up 2 years 4 months | 1.1 (0.2 to 1.9) | ||||
| Mature cohort (n = 1450), median follow-up 3 years 8 months | 1.1 (0.2 to 1.9) | ||||
| Earliest cohort (n = 817), median follow-up 5 years | 1.0 (0.1 to 1.9) | ||||
| Secondary outcome: mortality, events n/N; 5-year cumulative risk (%) (95% CI)i | TARGIT | WB-EBRT | Absolute difference; p-value | ||
| Death, all patients | 37/1721; 3.9 (2.7 to 5.8) | 51/1730; 5.3 (3.9 to 7.3) | –14 (–1.4%); p = 0.099 | ||
| Death, pre-pathology stratum | 29/1140; 4.6 (1.8 to 6.0) | 42/1158; 6.9 (4.3 to 9.6) | –13 (–2.3%) | ||
| Death, post-pathology stratum | 8/581; 2.8 (1.3 to 5.9) | 9/572; 2.3 (1.0 to 5.2) | –1 (0.5%) | ||
| Breast cancer mortality, all patients | 20/1721; 2.6 (1.5 to 4.3) | 16/1730; 1.9 (1.1 to 3.2) | p = 0.56 | ||
| Breast cancer mortality, pre-pathology stratum | 17/1140; 3.3 (1.9 to 5.8) | 15/1158; 2.7 (1.5 to 4.6) | p = 0.72 | ||
| Breast cancer mortality, post-pathology stratum | 3/581; 1.2 (0.4 to 4.2) | 1/572; 0.5 (0.1 to 3.5) | p = 0.35 | ||
| Non-breast cancer mortality, all patients | 17/1721; 1.4 (0.8 to 2.5) | 35/1730; 3.5 (2.3 to 5.2) | p = 0.0086 | ||
| Non-breast cancer mortality, pre-pathology stratum | 12/1140; 1.3 (0.7 to 2.8) | 27/1158; 4.4 (2.8 to 6.9) | p = 0.016 | ||
| Non-breast cancer mortality, post-pathology stratum | 5/581; 1.58 (0.62 to 3.97) | 8/572; 1.76 (0.7 to 4.4) | p = 0.32 | ||
| Non-breast cancer mortality, causes of death, number of patients | TARGIT (n = 1721) | WB-EBRT (n = 1730) | |||
| Other cancers | 8 | 16 | |||
| Cardiovascular causes | |||||
| Cardiacj | 2 | 8 | |||
| Stroke | 0 | 2 | |||
| Ischaemic bowel | 0 | 1 | |||
| Otherk | 7 | 8 | |||
| Total | 17 | 35 | |||
| Overall mortality for pre-pathology stratuml | Absolute difference in 5-year Kaplan–Meier estimate (SE) | ||||
| Whole cohort (n = 2298), median follow-up 2 years 4 months | –2.3 (–0.7 to –3.9) | ||||
| Mature cohort (n = 1450), median follow-up 3 years 8 months | –2.6 (–1.0 to –4.2) | ||||
| Earliest cohort (n = 817), median follow-up 5 years | –2.2 (–0.3 to –4.1) | ||||
| Secondary outcome: earlym complications | TARGIT (n = 1113) | WB-EBRT (n = 1119) | |||
| Number of complications per patient:64 | |||||
| 0 | 917/1113 (82.4%) | 946/1119 (84.5%) | NR | ||
| 1 | 151/1113 (13.6%) | 139/1119 (12.4%) | NR | ||
| 2 | 29/1113 (2.6%) | 27/1119 (2.4%) | NR | ||
| 3 | 11/1113 (1.0%) | 5/1119 (0.4%) | NR | ||
| 4 | 3/1113 (0.3%) | 0/1119 (0%) | NR | ||
| 5 | 2/1113 (0.2%) | 0/1119 (0%) | NR | ||
| 6 | 0/1113 (0%) | 3/1119 (0.3%) | NR | ||
| Any complicationn | 196/1113 (17.6%) | 174/1119 (15.5%) | χ2 = 1.74; p = 0.19 | ||
| oClinically significant complications64 | |||||
| Haematoma needing surgical evacuation | 11/1113 (1.0%) | 7/1119 (0.6%) | 0.338 | ||
| Seroma needing more than three aspirations | 23/1113 (2.1%) | 9/1119 (0.8%) | 0.012 | ||
| Infection needing i.v. antibiotics or surgical intervention | 20/1113 (1.8%) | 14/1119 (1.3%) | 0.292 | ||
| Skin breakdown or delayed wound healingp | 31/1113 (2.8%) | 21/1119 (1.9%) | 0.155 | ||
| RTOG toxicity grade 3 or 4q | 6/1113 (0.5%) | 23/1119 (2.1%) | 0.002 | ||
| Major toxicityr | 37/1113 (3.3%) | 44/1119 (3.9%) | 0.443 | ||
| sComplications arising 6 months after randomisation65 events n/N (%) | TARGIT (n = 1721) | WB-EBRT (n = 1730) | p-value | ||
| Wound related: | |||||
| Haematoma/seroma needing > 3 aspirations | 4/1721 (0.2%)t | 2/1730 (0.1%)t | |||
| Infection needing i.v. antibiotics or surgery | 12/1721 (0.7%)t | 9/1730 (0.5%)t | |||
| Skin breakdown or delayed wound healing | 3/1721 (0.2%)t | 5/1730 (0.3%)t | |||
| Total | 19/1721 (1.1%) | 16/1730 (0.9%) | 0.599 | ||
| Radiotherapy related: RTOG grade 3 or 4 toxicity | 4/1721 (0.2%) | 13/1730 (0.8%) | 0.029 | ||
| Secondary outcome: extent of local surgery64 | TARGIT (n = 1113) | WB-EBRT (n = 1119) | |||
| Specimen weight (g)u | 46 (28–72) | 47 (29–76) | |||
| Margins at first excisionv | |||||
| Free | 970/1072 (90.5%) | 968/1073 (90.2%) | NR | ||
| DCIS only | 46/1072 (4.3%) | 43/1073 (4.0%) | NR | ||
| Invasive | 56/1072 (5.2%) | 62/1073 (5.8%) | NR | ||
| Unknown | 41/1113 (3.7%) | 46/1119 (4.1%) | NR | ||
| Re-excision for marginsw | |||||
| Pre-pathology stratum | 52/766 (6.8%) | 67/768 (8.72%) | NR | ||
| Post-pathology stratum | 27/347 (7.8%) | 36/351 (10.3%) | NR | ||
| Total | 79/1113 (7.1%) | 103/1119 (9.2%) | p = 0.07 | ||
| Exploratory outcomesx events n/N; 5-year cumulative risk (95% CI) | TARGIT | WB-EBRT | Absolute difference | ||
| Any other recurrence, all patients | 46/1679; 4.9% (3.5% to 6.9%) | 37/1696; 4.4% (3.0% to 6.4%) | 9 (0.5%) | ||
| Any other recurrence, pre-pathology stratum | 29/1107; 4.8% (3.1% to 7.3%) | 25/1127; 4.7% (3.0% to 7.4%) | 4 (0.1%) | ||
| Any other recurrence, post-pathology stratum | 17/572; 5.2% (3.0% to 8.8%) | 12/569; 3.7% (1.9% to 7.0%) | 5 (1.5%) | ||
| Regional recurrence (axillary and supraclavicular)y | 8/1679 | 6/1696 | Log-rank p = 0.609 | ||
Methodological comments
-
Allocation to treatment groups: described in detail in the paper reporting initial results. 64 Randomisation schedules were generated centrally by computer and kept securely in two centres (Perth for Australian centres, London for all other centres). Requests for randomisation were made (before lumpectomy65) via telephone or fax to one of the two centres where patient eligibility was checked. Treatment was allocated from a pre-printed randomisation schedule available to authorised staff only. Patients were randomly assigned in a 1 : 1 ratio with blocks stratified by centre and by timing of delivery of TARGeted Intraoperative radioTherapy (TARGIT) therapy. The 2010 paper reporting initial results64 states that timing of delivery of TARGIT therapy had three strata: pre-pathology entry, post-pathology entry/TARGIT as a second procedure, and history of previous contralateral breast cancer. The 2014 paper65 describes and reports results for only two strata: pre-pathology and post pathology and states that the post-pathology stratum was added via a protocol amendment in 2004. This was because the option to provide IORT as a second procedure (by reopening the wound) was requested by some centres planning to join the trial. The post-pathology stratum had a completely separate randomisation table. Post-pathology patients had to be randomised within 30 days of lumpectomy. 65
-
Blinding: no. The paper reporting initial results64 states that neither patients, investigators nor teams were masked to treatment (but given the nature of the treatments, this would not have been possible). Individual centres were not blinded to their own patients. States that confidential unblinded reports for the Data Monitoring Committee and blinded reports for the International Steering Committee (ISC) were produced by the trial statistician, but also states that unblinded analyses were performed according to a pre-specified statistical analysis plan. Hence, it is unclear whether or not the ISC reports were also unblinded. For ascertainment of cause of death, available data were reviewed by an independent senior clinician who was masked to randomisation. 65
-
Comparability of treatment groups: p-values are presented65 indicating no statistically significant differences in baseline characteristics between the groups. States that there was no significant difference between pre-pathology and post-pathology strata in the timing of delivery of WB-EBRT (p = 0.58). 65
-
Method of data analysis: all randomised patients were included in an ITT analysis. Patients who had undergone a mastectomy were not included in the analysis of local recurrence. 65 The separate analysis of the pre-pathology and post-pathology strata was planned. 65 A formal analysis for deaths from cardiovascular causes and deaths from other cancers was pre-specified. 65 Exploratory analyses (presumably not pre-specified) were conducted for regional recurrence, locoregional recurrence, distant recurrence, any other recurrence, and all recurrence. 65
-
In the 2010 paper reporting initial results:64 for the analysis of local recurrence, patients who underwent mastectomy as their definitive surgery and those who died or withdrew consent for further follow-up were censored on that date. All other recurrences in the conserved breast, but not axilla, were analysed and Kaplan–Meier curves were plotted to account for time to event and censoring of the data and included all patients. Analysis of the annual hazards of local recurrence was restricted to 4 years as < 20% patients had follow-up beyond this point. SAS System version 9.2 for Windows XP (SAS Institute Inc., Cary, NC, USA) and STATA version 11.0 were used for data compilation and analysis. Pearson chi-squared and log-rank tests were used to obtain p-values. Analysis was done in accordance with CONSORT guidelines.
-
In the 2014 paper:65 the non-inferiority statistic was analysed by calculating the difference in binomial proportions of local recurrences in the conserved breast between the two randomised groups (TARGIT vs. WB-EBRT). To assess stability over time, this statistic was also calculated for the mature cohort (n = 2232) reported in 201064 and for the earliest cohort (excluding the last 4 years of enrolment; n = 1222) who had a median follow-up of 5 years. Established methods were used to calculate the z-value and pnon-inferiority for the whole cohort and the two pre-specified strata (pre-pathology and post pathology). Overall mortality was also reported for the whole cohort, the mature cohort and the earliest cohort. If a patient had at least 5 years of follow-up, or if they were seen within the year before database lock, they were deemed to have adequate follow-up. Patients were censored when last seen or withdrawn from the trial. SAS System version 9.3 (SAS Institute Inc., Cary, NC, USA), Microsoft Excel 2011 (Microsoft Corporation, Redmond, WA, USA), STATA version 12.0 (StataCorp LP, College Station, TX, USA) and IBM SPSS version 20.0 (IBM Corporation, Armonk, NY, USA) were used for data compilation, validation and analysis. A log-rank test was used to compare the difference between survival function and to obtain p-values (significance levels set at p < 0.01 for local recurrence and p < 0.05 for survival).
-
-
Sample size/power calculation: described in detail in the paper reporting initial results. 64 The pre-defined non-inferiority margin was an absolute difference of 2.5% in the primary endpoint between groups. To test for non-inferiority with a background recurrence rate of 6% and an absolute non-inferiority margin of 2.5%, a total sample size of 2232 patients was calculated for 80% power at a 5% significance level. Randomisation continued after the initial analysis in 2010 to allow accrual in subprotocols and the trial was closed after the planned 1200 additional patients (1219 accrued) had been accrued. 65
-
Attrition/drop-out:
-
2010 paper64 TARGIT 17/1113 (1.5%) (4 withdrawn, 13 unknown); WB-EBRT 28/1119 (2.5%) (11 withdrawn, 17 unknown). Received allocated treatment:64 TARGIT 996/1113, WB-EBRT 1025/1119.
-
2014 paper:65 TARGIT 9/1721 withdrawn; 141 did not receive allocated treatment (78 received WB-EBRT, 42 had mastectomy, 21 received neither TARGIT nor WB-EBRT), 1571/1721 (91%) received allocated treatment [239/1571 (15.2%) received TARGIT + WB-EBRT; 1332/1571 (84.8%) received TARGIT alone). WB-EBRT 27/1730 withdrawn, 113 did not receive allocated treatment (12 received TARGIT, 14 received TARGIT + WB-EBRT, 34 had mastectomy, 53 received neither TARGIT nor WB-EBRT], 1590/1730 (92%) received allocated treatment.
-
States that 93.7% (3234/3451) of patients were seen in year before data lock or had at least 5 years of follow-up.
-
General comments
-
Generalisability: women with early breast cancer (although definition of ‘early’ is vague); international study with 6 out of 33 centres in the UK. Unsure whether or not population is typical of those with early breast cancer; also unclear how similar the WB-EBRT treatment is to standard WB-EBRT in the UK.
-
Outcome measures: outcomes reported are appropriate. Outcomes reported in linked publications, but are from only one or two participating centres, not for the whole trial population.
-
Intercentre variability: teams at each centre were trained and audited by a member of the trial ISC. 64 Observation of the baseline stratification data64 shows differences between centres in the number of patients entering the trial according to the three timings of delivery strata, particularly pre pathology and post pathology. Seven centres had patients in all three strata, 10 centres had patients in two strata (pre-pathology and post pathology, n = 3; pre pathology and contralateral, n = 6; post pathology and contralateral, n = 1), and 11 centres had patients in one stratum only (pre pathology, n = 8; post pathology, n = 3). 64 Centres were allowed to restrict the inclusion criteria beyond the core protocol (e.g. age, tumour size, grade, node) and to stipulate local policy for the delivery of WB-EBRT. Results are not presented by treatment centre nor any comment made in the text so intercentre variability in outcomes is unknown.
-
Conflict of interests: appear the same for both the 201064 and 201465 papers. Lead author received a research grant from Photoelectron Corp and Carl Zeiss and also honoraria; one author receives monthly consultancy fees from Carl Zeiss; one author has received a research grant and two authors have received honoraria from Carl Zeiss; Carl Zeiss sponsors most of the travel and accommodation costs for meetings/conferences relating to TARGIT. Only three authors’ travel/accommodation had not been sponsored by Carl Zeiss.
-
Other: pivotal trial for TARGIT (INTRABEAM). Registered with ClinicalTrials.gov number NCT00983684.
| Cochrane criteria for assessment of risk of bias in RCTs56 | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Low risk | Computer-generated randomisation schedules |
| Allocation concealment | Low risk | Central allocation |
| Performance bias | ||
| Blinding of participants and personnel | Low risk | Neither patients nor investigators were blinded. However, outcomes were unlikely to be influenced by lack of blinding |
| Detection bias | ||
| Blinding of outcome assessment | Low risk | Some investigators and teams were not blinded and it is not clear whether or not all the analyses were performed unblinded. However, most outcomes were objective measures and hence unlikely to be influenced by lack of blinding |
| Attrition bias | ||
| Incomplete outcome data addressed | Low risk | Low proportion of withdrawals and participants not receiving allocated treatment (reasons similar between groups). Analyses by ITT |
| Reporting bias | ||
| Selective reporting | Low risk | The protocol is available online (www.hta.ac.uk/project/1981.asp) and specifies all outcomes including relapse-free survival and overall survival (as a secondary outcome) |
| Other bias | ||
| Other sources of bias | Low risk | None evident |
| Reviewer 1: DH, date: 5 November 2013 | Reviewer 2: JP, date: 19 November 2013 | Version: 3, (Reviewer JC replaces DH 8 April 2014) | |||
|---|---|---|---|---|---|
| Linked study reference | Participants | Outcome measures | |||
| Substudy of TARGIT A trial:64,65 Welzel et al., 201363 Aim of substudy: to assess radiation-related QoL parameters in a sample of patients within the TARGIT RCT Number of centres contributing data: one Location of centres contributing data: Mannheim, Germany n = 15264 Other: cross-sectional analysis using retrospective QoL questionnaires Recruitment dates: June 2002 to February 2009 (consented during TARGIT trial). Questionnaires sent out 8 to 94 months following treatment |
Number of randomised participants: n = 123 eligible (aim was to assess the first 123 women accrued to TARGIT trial at this centre), n = 88 received questionnaires (ITT), n = 87 included in as-treated analysis TARGIT, n = 46 [further split into IORT (n = 30) and IORT with WB-EBRT boost (n = 16) original allocation]; ITT, (n = 41 as-treated); WB-EBRT, n = 42 ITT, (n = 46 as-treated) Doses: IORT: 20 Gy at applicator surface during surgery IORT-WB-EBRT: additional boost of 46 Gy in 23 fractions or 50 Gy in 25 fractions WB-EBRT: 56 Gy in 28 fractions (no additional boost) Additional inclusion criteria (beyond those of TARGIT): Patients had to be randomised in the TARGIT trial between 2002 and 2009 to qualify Additional exclusion criteria (beyond those of TARGIT): None reported |
Outcomes: radiation-related QoL measures Method of assessing outcomes: Two validated questionnaires of the EORTC: QLQ-C30, version 3, for global health status, role functioning and general pain; QLQ-BR23 for breast symptoms and arm symptoms. The time frame for these questions was the situation in the last week Length of follow-up: mean 32.1 months (median 25 months, range 9–94 months) |
|||
| Results | |||||
| QoL outcome, ITT analysis | TARGIT (N = 46; IORT n = 30, IORT + WB-EBRT n = 16) | WB-EBRT (n = 42) | |||
| N a | Mean (SD) | N a | Mean (SD) | p-value | |
| Global health statusb | 46 | 61.6 (21.7) | 40 | 54.8 (19.9) | 0.183 |
| Restrictions in daily activitiesb | 46 | 72.8 (32.3) | 41 | 61.8 (29.2) | 0.055 |
| General painc | 46 | 29.3 (32.8) | 42 | 42.5 (33.0) | 0.048 |
| Breast symptomsc | 45 | 17.0 (20.8) | 42 | 18.1 (20.2) | 0.629 |
| Arm symptomsc | 45 | 24.4 (26.7) | 40 | 31.1 (27.9) | 0.279 |
| QoL outcome, as-treated analysis, mean (SD) | IORT (n = 25) | IORT + WB-EBRT (n = 16) | WB-EBRT (n = 46) | p-value | |
| Global health statusb | 63.6 (24.2) | 60.9 (19.9) | 52.4 (22.1) | > 0.01 | |
| Restrictions in daily activitiesb | 78.7 (35.2) | NR | 60.5 (29.5) | 0.007e | |
| General painc,d | 21.3 (95% CI NRh to 54.4) | 43.7 (95% CI 11.6 to 75.9) | 40.9 (95% CI 8.6 to 73.2) | 0.007,e 0.018f | |
| Breast symptomsc,d | 7.2 (95% CI NRh to 20.9) | 29.7 (95% CI 6.8 to 52.5) | 19.0 (95% CI NRh to 39.2) | 0.001,e< 0.001,f 0.021g | |
| Arm symptomsc,d | 15.2 (95% CI NRh to 37.2) | 32.6 (95% CI 6.8 to 58.4) | 32.8 (95% CI 4.2 to 61.5) | 0.009,e 0.011f | |
| Frequency of moderate and severe breast/arm symptoms,i as-treated analysis, % moderate/% severe | IORT (n = 25) | IORT + WB-EBRT (n = 16) | WB-EBRT (n = 46) | p-value | |
| Pain in area of affected breast | 4%/0 | 25%/13% | 11%/4% | > 0.01 | |
| Swelling in area of affected breast | 0/0 | 7%/7% | 4%/2% | ||
| Oversensitivity in area of affected breast | 4%/0 | 20%/7% | 9%/7% | ||
| Skin problems on or in area of affected breast | 4%/4% | 13%/6% | 9%/4% | ||
| Pain in arm or shoulder | 8%/8% | 33%/20% | 18%/23% | > 0.01 | |
| Swelling in arm or hand | 8%/4% | 6%/6% | 9%/7% | ||
| Difficulty in raising or moving arm sideways | 20%/0 | 13%/7% | 24%/12% | > 0.01 | |
Methodological comments
-
Comparability of substudy population to main TARGIT-A trial population: narratively reports that, compared with patients in the whole TARGIT-A trial, patients in this substudy had largely similar demographic and clinical characteristics. On observation of the data, the reviewer would agree on the whole (but not all characteristics are presented in the substudy), although a lower proportion of the subsample had tumour size 0–1 cm and a greater proportion had tumour size 1–2 cm compared with the whole TARGIT-A population for both treatment arms.
-
Comparability of substudy treatment groups: demographic and clinical characteristics were similar between groups. p-values were reported and there were no statistical differences although presume this was for comparison of the three groups (i.e. IORT arm was split into IORT alone and IORT + WB-EBRT boost) and not IORT as a whole versus WB-EBRT.
-
Method of data analysis: reports all analyses were performed on an ITT and as-treated basis. The level of statistical significance was 0.01 (0.05/5) to reduce type-1 error in multiple comparisons. Chi-squared tests (or Fisher’s exact tests), Kruskal–Wallis one-way analysis of variance (ANOVA), and post hoc Mann–Whitney U-tests (or univariate ANOVA and post hoc Scheffe tests) were used to compare treatment groups. Independent effects of demographic and clinical factors on QoL were tested using univariate linear regression analysis. Variables with a p-value < 0.05 were further analysed with multiple linear regression analysis (stepwise forward method). The results from TARGIT-A patients were presented throughout as three groups with the IORT group split into IORT and IORT with WB-EBRT boost.
-
Attrition/drop-out: the main trial publication64 indicates that there were 152 participants at the Mannheim centre (for recruitment 24 March 2000 to 25 June 2012). This linked substudy aimed to assess the first 123 patients recruited from this centre (recruited June 2002 to February 2009), with 88 patients consenting (88/152 = 58%). Data are reported for the ITT (n = 88) and as-treated (n = 87) populations. Five patients did not receive IORT (four received WB-EBRT instead and one patient refused WB-EBRT). It is not possible to assess whether or not there are any other missing data as no ‘n’ is reported for tables or figures; however, none are apparent to the reviewer.
-
Other: the paper includes an additional two non-randomised control groups of WB-EBRT patients (from the same centre) treated with (1) IORT as a tumour bed boost + WB-EBRT (outside of TARGIT-A trial) or (2) WB-EBRT + WB-EBRT boost. These groups served as control groups for some analyses but are not reported on here.
General comments
-
Generalisability: this substudy reports on only 46 IORT and 42 WB-EBRT group participants from the TARGIT-A trial representing only about 2.5% of the total trial population of 3451 randomised participants (1721 TARGIT, 1730 WB-EBRT). 65 It is not clear how generalisable the results are to the remainder of the TARGIT-A trial population or to UK breast cancer patients.
-
Outcome measures: questionnaire response rate was 96–99%. The five functioning and symptom scales of the QLQ-C30 and QLQ-BR23 questionnaires were preselected during the design of the study based on a pilot study and relevance for radiation-related QoL in breast cancer. Other subscales and items of the questionnaires were not presented. In addition, states that four other QoL scales were used: the Hospital Anxiety and Depression Scale, the Functional Assessment of Cancer Therapy – Fatigue subscale, the Rosenberg Self-Esteem Scale and the Body Image Scale to control for differences that may inherently exist between treatment groups. Scores for each questionnaire were summed for each scale. However, the paper only narratively comments on differences between groups for these scales (no data).
-
On observation of the data, ITT and as-treated QoL outcomes seem similar for the WB-EBRT group, but are difficult to judge for the IORT group because of the way data are presented; for ITT results, IORT and IORT + WB-EBRT are presented as a single group whereas for as-treated results, IORT alone and IORT + WB-EBRT are reported separately.
Partial quality assessment
A complete risk of bias assessment has been conducted for the main TARGIT-A trial. 64 Only the criteria that could potentially differ in the substudy are reported here.
| Cochrane criteria for assessment of risk of bias in RCTs56 | Judgementa | Support for judgement |
|---|---|---|
| Performance bias | ||
| Blinding of participants and personnel in the HRQoL substudy | High risk | As part of the TARGIT-A trial, neither patients nor investigators were blinded and the outcome could potentially be influenced by the lack of blinding |
| Detection bias | ||
| Blinding of outcome assessment | Unclear risk | No information provided regarding blinding (or lack of) for the assessment of QoL measures |
| Attrition bias | ||
| Incomplete outcome data addressed | Low risk | Reason for loss of one patient given |
| Other bias | ||
| Other sources of bias | Unclear risk | Retrospective questionnaire with no baseline QoL measurement |
Appendix 4 Southampton Health Technology Assessments Centre’s critique of manufacturer’s submission
Southampton Health Technology Assessments Centre’s peer review of clinical effectiveness data presented in Carl Zeiss UK’s submission for the INTRABEAM Photon Radiotherapy System for early breast cancer Multiple Technology Appraisal
Comprehensiveness of ascertainment of published studies
Clinical effectiveness
The MS contains a narrative summary of the key RCT and other studies (non-randomised) with the results of each study presented separately. One table is presented in the executive summary detailing nine studies reporting on cosmesis and toxicity. Tables of patient and tumour characteristics are presented separately for each included study in Appendix 1. There is no formal systematic review of clinical effectiveness evidence although a systematic literature search is described.
-
Were databases and dates of searches specified?
-
Yes, pages 6 and 7 report that three databases were searched up to December 2013, with literature included only from 2007 onwards.
-
-
Were search strategies supplied?
-
Yes.
-
-
Was enough detail provided to be reproducible?
-
Yes.
-
-
Did they search/report on ongoing studies?
-
No searches for ongoing studies are reported.
-
-
Did they search for conference proceedings?
-
Unclear – conference proceedings may have been included in the three databases searched but this is not specifically stated. Information is included from some conference posters.
-
-
How many of the data are commercial in confidence/academic in confidence?
-
No data are commercial in confidence /academic in confidence.
-
Searches identified
-
Note the number of studies.
-
The MS does not state how many citations were identified by the search. The MS does not describe the processes or criteria (other than ‘related to the subject to be evaluated’) for selecting included studies. The MS does not state how many studies overall have been included in the submission. The reviewer has identified 26 studies, of which six are described as poster abstracts.
-
-
Note what study types.
-
The MS does not consistently identify the study types for the studies included in the review. Only one RCT is included, the majority of the remaining 25 citations appear to be cohort studies.
-
-
Did these meet our inclusion criteria?
-
The included RCT meets our inclusion criteria as do the studies reporting on subgroups of TARGIT-A participants. The remaining studies included in the MS did not meet our inclusion criteria, chiefly on the grounds of study design.
-
-
Were any studies identified that we have not included?
-
No.
-
-
Any key details/issues?
-
No.
-
Clinical analysis
-
Any major differences in evidence reported?
-
The MS discusses evidence from four articles that are all based on the key TARGIT-A trial and which are also included in the SHTAC’s systematic review. The MS has not included evidence from the initial TARGIT-A trial publication from 201064 stating that this is because more recent data are available and the 2010 results are expected to be included in the most recent (2014) publication. 65 The SHTAC’s systematic review does include evidence on early complications from the 2010 TARGIT-A trial publication as these are not reported by the more recent 2014 trial paper. The MS also does not include a study published by Sperk et al. 67 reporting on long-term toxicity following treatment either with the INTRABEAM (n = 54) device or WB-EBRT (n = 55) at one trial centre in Mannheim, Germany. The MS does, however, include a cohort study79 that reports on post-operative complications within the first week following surgery among 208 patients treated with INTRABEAM at a centre in Mannheim, Germany, who were participating in the TARGIT-A trial. Tuschy et al. 79 is excluded from the SHTAC’s systematic review because it is likely that the data reported are either partially or wholly contained within the early complications reported by the initial TARGIT-A trial publication64 and, in addition, Tuschy et al. 79 report no comparable data for the WB-EBRT group.
-
The MS also discusses evidence from n = 22 studies (six only reported as conference abstracts) that did not meet the inclusion criteria of the SHTAC’s review.
-
The MS provides a narrative summary for each individual study that has been included. Individual tables of baseline patient characteristics for 13 of the included studies are provided in an appendix. Aside from one table for eight of the nine studies listed in section 1.2, ‘Literature related to side effects and cosmetic outcome after IORT as a single treatment’, the MS does not provide summary tables for the included studies. There is no quality assessment of the included studies.
-
-
Are their conclusions similar to ours?
-
In the MS section ‘Interpretation of clinical evidence’ subsections a, b, and c, the focus is on the TARGIT-A trial data and, consequently, with only one included trial there is no evidence to draw together and interpret. Therefore, for the outcomes of recurrence and overall survival the MS and the SHTAC’s systematic review that report on the same data as published in the 2014 TARGIT-A trial publication. 65
-
In some of the remaining subsections of the MS ‘Interpretation of clinical evidence’, the MS discusses evidence for outcomes that are also included in the SHTAC’s systematic review (e.g. subsection d: Cosmetic outcome and toxicities, subsection f: Quality of life) drawing not only on evidence from the TARGIT-A trial but also on evidence from included cohort studies that support the data from the TARGIT-A trial. Where the SHTAC’s review reports a small amount of additional information on early complications reported by the initial TARGIT-A trial publication64 this does not impact on the overall conclusions. Other subsections of the MS ‘Interpretation of clinical evidence’ draw on cohort or other non-RCT studies to provide information to support other hypotheses that are not included within the SHTAC’s systematic review (e.g. subsection e: Side effects and impacts on critical organs are less in IORT than EBRT, subsection g: IORT can be administered to patients where EBRT is not advised, subsection i: Low risk of inducing secondary cancer).
-
-
Any indirect comparisons and if so, was this appropriate and what were key results?
-
There is no indirect comparison.
-
-
Any extra adverse event information?
-
None that meets the inclusion criteria for the SHTAC’s systematic review.
-
Interpretation
-
Does their interpretation of the clinical data match their analyses?
-
As already noted above, with only one included trial there is no evidence to draw together and interpret.
-
Questions
-
Any areas of uncertainty/discrepancy compared with the SHTAC’s review?
-
None related to the key TARGIT-A trial. Other evidence presented by the MS does not meet the inclusion criteria for the SHTAC’s systematic review.
-
Southampton Health Technology Assessments Centre’s critique of economic evaluation presented in Carl Zeiss UK’s submission for the INTRABEAM Photon Radiotherapy system for early breast cancer Multiple Technology Appraisal
Study characteristics
Reference
Carl Zeiss, UK, 2014.
Health technology
INTRABEAM Photon Radiotherapy System.
1.2 Interventions and comparators
What interventions/strategies were included?
INTRABEAM versus Whole-breast WB-EBRT (WB-EBRT).
Was a no treatment/supportive care strategy included?
No.
Describe interventions/strategies
New Innovative TARGeted Intra Operative Radio Therapy (IORT) using the INTRABEAM radiotherapy system.
Conventional therapy consisting of WB-EBRT.
1.3 Research question
What are the stated objectives of the evaluation?
To determine the cost-effectiveness of INTRABEAM in early breast cancer patients when compared with radiotherapy usually given in the UK over 3–6 weeks as WB-EBRT.
1.4 Study type Cost-effectiveness/cost–utility/cost–benefit analysis?
Cost–utility analysis.
1.5 Study population
What definition was used for [condition]? What are the characteristics of the baseline cohort for the evaluation?
The baseline cohort included patients aged 55 years who were disease free after WLE. The economic model was based on the results of the pre-pathology stratum of the trial with 2298 patients (this was because the outcome in patients in whom IORT was given only after the final pathology showed much less favourable results than in the patients who received IORT during lumpectomy).
1.6 Institutional setting: where is/are the intervention(s) being evaluated usually provided?
Not reported.
1.7 Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
UK; £. Price year for cost of INTRABEAM was unknown as based on expert opinion; price year of WB-EBRT was 2012–13; the price year of post IORT local recurrence and post WB-EBRT local recurrence was of 2013–14; and that of annual disease-free follow-up care was 2013. The cost was calculated to 2013 price using The Campbell and Cochrane Economics Methods Group – Evidence for Policy and Practice Information and Coordinating Centre Cost Converter.
1.8 Funding source
Carl Zeiss, UK.
1.9 Analytical perspective
What is the perspective adopted for the evaluation (health service, health and personal social services, third party payer, societal (i.e. including costs borne by individuals and lost productivity)]?
NHS health-care payer’s perspective
The MS notes that travel/parking/accommodation expenses for WB-EBRT patients were not included in the WB-EBRT costs (it was stated that these expenses might range from £50 to £100 per patient per fraction delivered).
Effectiveness
Were the effectiveness data derived from a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
Data for effectiveness were derived from a single study by Vaidya et al. 65 The source study reported 5-year cumulative risk which were converted to annual probabilities to populate the model by the manufacturer.
| Parameters | Probabilities |
|---|---|
| Local recurrence after IORT | 0.004 |
| Local recurrence after WB-EBRT | 0.002 |
| Breast cancer death after IORT | 0.007 |
| Non-breast cancer death after IORT | 0.003 |
| Breast cancer death after WB-EBRT | 0.005 |
| Non-breast cancer death after WB-EBRT | 0.009 |
Intervention costs
Were the cost data derived from a single (observational) study, a review/synthesis of previous studies expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used.
Cost data were obtained from the following sources: expert opinion, Reference Costs 2012–13,136 tariff information 2013–14,137 and the study by Wolowacz et al. 138 The methods of deriving costs were not adequately described.
The following costs were used in the model:
| Costs | Prices | Source |
|---|---|---|
| Costs of INTRABEAM | £2165 | Expert opinion |
| Costs of WB-EBRT | £7521 | HRG code SC29Z (Reference Cost 2012–13) |
| Cost of treating post IORT LR (salvage lumpectomy) | £1558 | HRG code JA09H (Tariff Information 2013–14) |
| Cost of treating post WB-EBRT LR (salvage mastectomy) | £6504 | HRG code JA16Z (Tariff Information 2013–14) |
| Annual disease-free follow-up care | £892 | Wolowacz et al.138 |
Indirect Costs (costs owing to lost productivity, unpaid inputs to patient care)
Were indirect costs included?
Not included.
Health state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from a single (observational) study, a review/synthesis of previous studies expert opinion. Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
The utility data were derived from a single study by Hayman et al. 135 The method of deriving these values was not reported.
List the utility values used in the evaluation?
| Health state | Utilities |
|---|---|
| Utility value in disease free patients | 0.92 |
| Utility value in salvage lumpectomy patients | 0.87 |
| Utility value in salvage mastectomy patients | 0.82 |
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported – list them if reported.
A multistate Markov model was developed, over a time-horizon of 20 years. It was not reported if the model was newly developed or adapted from a previously reported model.
The purpose of the model was to assess the cost-effectiveness of INTRABEAM compared with WB-EBRT. The model consisted of four health states as shown in the figure:
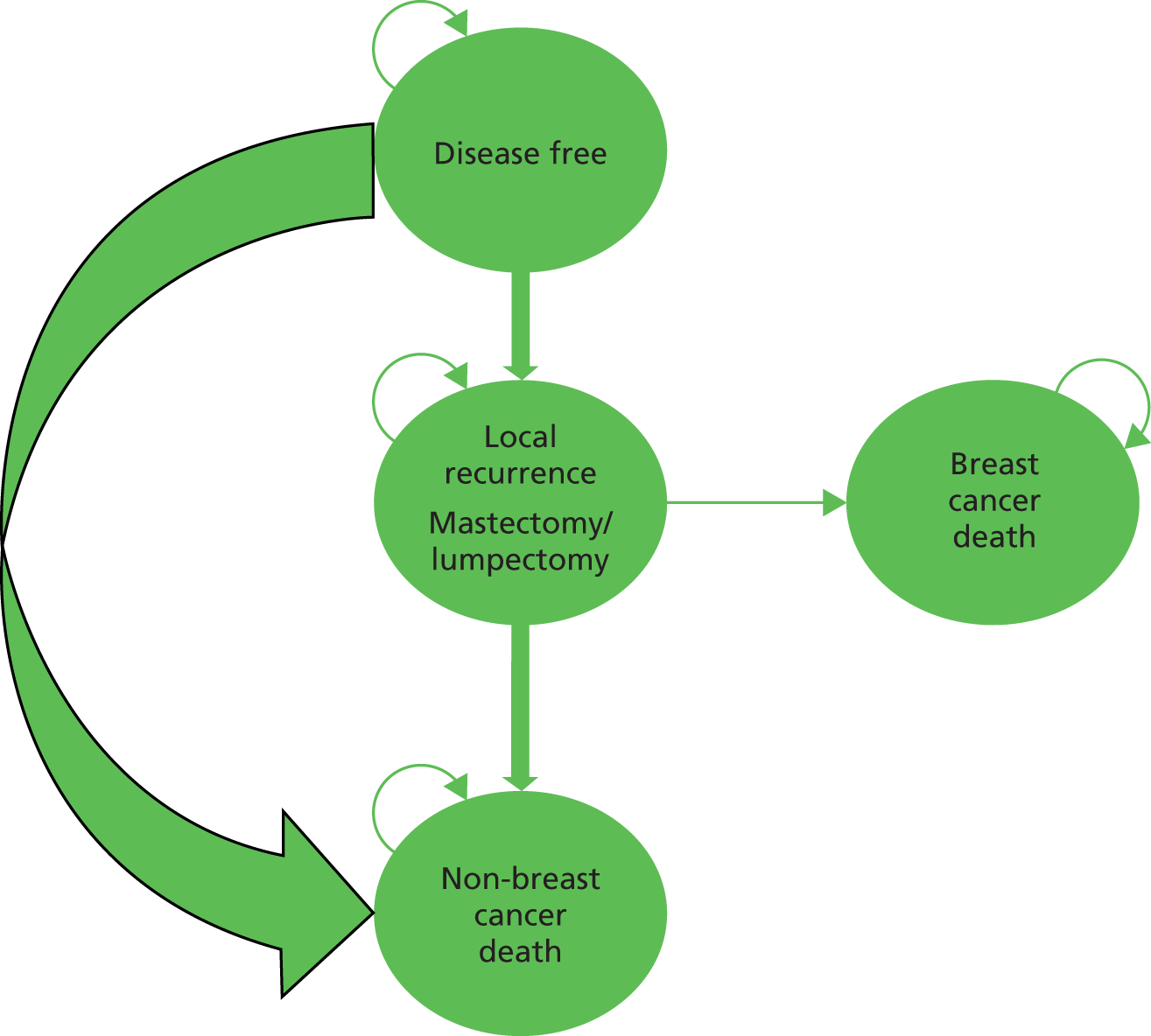
No description was provided on patient progression through the health states. The model assumptions were:
-
After local recurrence, IORT patients would have salvage lumpectomy.
-
After local recurrence, WB-EBRT patients would have salvage mastectomy.
-
Death rate in disease free patients was equal to general population.
-
Average 23 fractions of WB-EBRT per patient delivered based on 15–30 fractions in the clinical practice.
-
All patients were given IORT concurrent with initial lumpectomy (pre-pathology stratum of TARGIT-A trial).
Extract transition probabilities for [natural history/disease progression] model and show sources (or refer to table in text)
Data for transitional probabilities were extracted from Vaidya et al. 65
| Transitions | Annual probability | 95% CIa |
|---|---|---|
| Local recurrence after IORT | 0.0042 | 0.0022 to 0.0085 |
| Local recurrence after WB-EBRT | 0.0022 | 0.0010 to 0.0051 |
| Breast cancer death after IORT | 0.0067 | 0.0038 to 0.0119 |
| Non-breast cancer death after IORT | 0.0026 | 0.0014 to 0.0057 |
| Breast cancer death after WB-EBRT | 0.0055 | 0.0030 to 0.0094 |
| Non-breast cancer death after WB-EBRT | 0.0090 | 0.0057 to 0.0142 |
What is the model time horizon?
20 years.
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
Costs and outcomes were discounted at 3.5%.
Results/analysis
What measure(s) of benefit were reported in the evaluation?
Cost per QALY.
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation
| Strategies | Total QALYs (discounted) |
|---|---|
| IORT | 13.230 |
| WB-EBRT | 13.223 |
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
| Strategies | Total costs (discounted) |
|---|---|
| IORT | £14,461 |
| WB-EBRT | £20,926 |
Synthesis of costs and benefits: are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results
| vs. WB-EBRT | |||
|---|---|---|---|
| Incremental costs (discounted) | Incremental QALYs (discounted) | ICER | |
| IORT | –£6465 | 0.007 | Dominates |
Give results of any statistical analysis of the results of the evaluation
None.
Was any sensitivity analysis performed? If yes, what type(s) [i.e. deterministic (one-way, two-way etc.) or probabilistic]?
Probabilistic sensitivity analyses (ran for 1000 simulations).
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
No scenario analysis was conducted.
Give a summary of the results of the sensitivity analysis, did they differ substantially from the base-case analysis. If so, what were the suggested causes?
None; it was only reported that probabilistic results were similar to the base case results however no one-way sensitivity analysis was conducted.
Conclusions/Implications
Give a brief summary of the author’s conclusions from their analysis.
The authors concluded that INTRABEAM was a cost-effective strategy to treat early-stage breast cancer patients in the UK.
What are the implications of the evaluation for practice?
The MS stated that INTRABEAM could save valuable NHS resources in comparison with the current practice of WB-EBRT.
SHTAC’s commentary
Selection of comparators:
Number of fractions (23) for the WB-EBRT arm was not relevant to UK practice.
Validity of estimate of measure of benefit:
The manufacturer’s model assessed health benefit in terms of QALYs which was a valid measure of health in the UK NHS setting. Standard gamble was used to estimate utilities in the source study which was a 1997 publication;135 the reported values were not obtained from general population. In addition, no details were provided regarding whether or not a systematic search was conducted to identify utilities for the model.
Validity of estimate of costs:
The validity of the costs estimates remained questionable. The cost of INTRABEAM per patient was obtained from expert opinion. The manufacturer provided the cost compositions of INTRABEAM; however, it was not transparent in explaining the assumed cost per patient. In addition, cost of WB-EBRT was obtained from inappropriate HRG code: the code used in the model for WB-EBRT was for ‘other radiotherapy treatment’. On the contrary, the HRG code required for the purpose of this analysis was ‘external beam radiotherapy delivered by linear accelerator’ which required the weighted average of SC22Z and SC23Z (for delivery) and a weighed average SC45Z, SC46Z, SC47Z and SC48Z (for planning). Costs were only varied by ± 10% in PSA. There were also inconsistencies in the price years of the reported costs: cost of WB-EBRT was expressed in 2012–13; costs of treating post IORT local recurrence and post WB-EBRT local recurrence were in 2013–14; and cost of annual disease follow-up was in 2013.
| Item number | Item | MS |
|---|---|---|
| 1 | Is there a clear statement of the decision problem? | Yes |
| 2 | Is the comparator routinely used in UK NHS? | Uncleara |
| 3 | Is the patient group in the study similar to those of interest in UK NHS? | Unclearb |
| 4 | Is the health care system comparable to UK? | Yes |
| 5 | Is the setting comparable to the UK? | Yes |
| 6 | Is the perspective of the model clearly stated? | Yes |
| 7 | Is the study type appropriate? | Yes |
| 8 | Is the modelling methodology appropriate? | Unclearc |
| 9 | Is the model structure described and does it reflect the disease process? | Yesd |
| 10 | Are assumptions about model structure listed and justified? | No |
| 11 | Are the data inputs for the model described and justified? | No |
| 12 | Is the effectiveness of the intervention established based on a systematic review? | Noe |
| 13 | Are health benefits measured in QALYs? | Yes |
| 14 | Are health benefits measured using a standardised and validated generic instrument? | Yesf |
| 15 | Are the resource costs described and justified? | No |
| 16 | Have the costs and outcomes been discounted? | Yes |
| 17 | Has uncertainty been assessed? | Unclearg |
| 18 | Has the model been validated? | No |
Appendix 5 Excluded cost-effectiveness studies with rationale
| Excluded study | Reasons for exclusion |
|---|---|
| Xoft Axxent eBx electronic brachytherapy system (iCAD Inc.) for early-stage breast cancer. 2012 | Not full economic evaluation, inappropriate intervention and comparator |
| Alvarado M, Ozanne E, Mohan A, Esserman L. Cost-effectiveness of intraoperative radiation therapy for breast conservation. Journal of Clinical Oncology Conference: ASCO Annual Meeting 2011 Chicago, IL United States Conference Start: 20110603 Conference End: 20110607 Conference Publication 2011;29(Suppl. 1) | Abstract |
| BlueCross BlueShield Association. Accelerated partial breast irradiation as sole radiotherapy after breast-conserving surgery for early stage breast cancer. 2007 | Not full economic evaluation, inappropriate population of interest, intervention and comparator |
| BlueCross BlueShield Association. Accelerated radiotherapy after breast-conserving surgery for early stage breast cancer. 2012 | Not full economic evaluation |
| Santos M, Guerra JLL, Gordillo MJO, Fondevilla A, Calvo F, Samblas J, et al. Cost-effectiveness analysis of four validated techniques of accelerated partial breast irradiation for the treatment of early-stage breast cancer: Spanish public health system standard estimations. Value in Health 2012;15:A354 | Abstract, inappropriate intervention |
| Sher DJ, Wittenberg E, Suh WW, Taghian AG, Punglia RS. Partial-breast irradiation versus whole-breast irradiation for early-stage breast cancer: a cost-effectiveness analysis. Int J Radiat Oncol Biol Phys 2009;74:440–6 | Inappropriate intervention |
| Xie X, Dendukuri N, McGregor M. Single-dose intraoperative radiotherapy using Intrabeam® for early-stage breast cancer: a health technology assessment. 2012 | Not full economic evaluation |
Appendix 6 Cost-effectiveness data extraction tables
| Study | Alvarado, 2013;106 Esserman, 2014107 | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Research question | The study analysed, from a societal perspective, the cost-effectiveness of two radiation strategies for early-stage invasive breast cancer: single-dose IORT and the standard 6-week course of WB-EBRT | |||||||||||||||||||||||||||||||||||||||||||
| Country/setting | The model was based on the protocol of the international TARGIT-A trial; the economic evaluation is US based | |||||||||||||||||||||||||||||||||||||||||||
| Funding source | Not stated | |||||||||||||||||||||||||||||||||||||||||||
| Analysis type | Cost–utility analysis | |||||||||||||||||||||||||||||||||||||||||||
| Study type | A Markov decision-analytic model based on the TARGIT-A trial was developed consisting of six health states:
|
|||||||||||||||||||||||||||||||||||||||||||
| Perspective | Societal | |||||||||||||||||||||||||||||||||||||||||||
| Time horizon | 10-year period with annual cycle length | |||||||||||||||||||||||||||||||||||||||||||
| Model assumptions |
|
|||||||||||||||||||||||||||||||||||||||||||
| Discounting (rate) | Yes at 3% for both costs and effectiveness | |||||||||||||||||||||||||||||||||||||||||||
| Costing year, currency | 2011, US$ | |||||||||||||||||||||||||||||||||||||||||||
| Population | Trial name: TARGIT-A | |||||||||||||||||||||||||||||||||||||||||||
| Definition of condition: women with early breast cancer who were aged ≥ 55 years old | ||||||||||||||||||||||||||||||||||||||||||||
| Characteristics of baseline cohort/risk factors: early-stage was defined as stage I-IIA, ER-positive (ER+) breast cancer | ||||||||||||||||||||||||||||||||||||||||||||
| Intervention(s), comparator(s) | Intervention: single-dose IORT INTRABEAM | |||||||||||||||||||||||||||||||||||||||||||
| Comparator: 6-week course of WB-EBRT with a standard 33 fractions | ||||||||||||||||||||||||||||||||||||||||||||
| Intervention effect | Data for the 4-year LRRs from the TARGIT-A trial were transformed to annual transitional probabilities which were then estimated over a 10-year period. At 4 years, the Kaplan–Meier estimate of local recurrence in the conserved breast was estimated to be 1.2% (95% CI 0.53 to 2.71) in the INTRABEAM arm and 0.95% (95% CI 0.39 to 2.31) in the WB-EBRT arm | |||||||||||||||||||||||||||||||||||||||||||
| Health state utilities | Utility values associated with the health states were attained via standard gamble preferences, when feasible.119 Published literature was used to populate the remaining values (reference provided)119 Health state utilitiesBase-case valueRange valuesIORT0.920.87–0.973-week WB-EBRT0.920.87–0.976-week WB-EBRT0.920.87–0.97IORT followed by 5-week WB-EBRT0.920.87–0.97Salvage mastectomy0.820.77–0.87Salvage mastectomy and WB-EBRT0.870.82–0.92Metastatic breast cancer0.700.60–0.80Death0.00– Details on the measurement technique and valuation approach were not provided |
Health state utilities | Base-case value | Range values | IORT | 0.92 | 0.87–0.97 | 3-week WB-EBRT | 0.92 | 0.87–0.97 | 6-week WB-EBRT | 0.92 | 0.87–0.97 | IORT followed by 5-week WB-EBRT | 0.92 | 0.87–0.97 | Salvage mastectomy | 0.82 | 0.77–0.87 | Salvage mastectomy and WB-EBRT | 0.87 | 0.82–0.92 | Metastatic breast cancer | 0.70 | 0.60–0.80 | Death | 0.00 | – | ||||||||||||||||
| Health state utilities | Base-case value | Range values | ||||||||||||||||||||||||||||||||||||||||||
| IORT | 0.92 | 0.87–0.97 | ||||||||||||||||||||||||||||||||||||||||||
| 3-week WB-EBRT | 0.92 | 0.87–0.97 | ||||||||||||||||||||||||||||||||||||||||||
| 6-week WB-EBRT | 0.92 | 0.87–0.97 | ||||||||||||||||||||||||||||||||||||||||||
| IORT followed by 5-week WB-EBRT | 0.92 | 0.87–0.97 | ||||||||||||||||||||||||||||||||||||||||||
| Salvage mastectomy | 0.82 | 0.77–0.87 | ||||||||||||||||||||||||||||||||||||||||||
| Salvage mastectomy and WB-EBRT | 0.87 | 0.82–0.92 | ||||||||||||||||||||||||||||||||||||||||||
| Metastatic breast cancer | 0.70 | 0.60–0.80 | ||||||||||||||||||||||||||||||||||||||||||
| Death | 0.00 | – | ||||||||||||||||||||||||||||||||||||||||||
| Intervention cost |
|
|||||||||||||||||||||||||||||||||||||||||||
| Sources: | ||||||||||||||||||||||||||||||||||||||||||||
| Medicare Physician Fee Schedule. US Department of Health and Human Services; 2010. www.cms.gov/apps/physician-fee-schedule/overview.aspx | ||||||||||||||||||||||||||||||||||||||||||||
| Outpatient Prospective Payment System (OPPS). US Department of Health and Human Services; 2010 | ||||||||||||||||||||||||||||||||||||||||||||
| Indirect costs |
|
|||||||||||||||||||||||||||||||||||||||||||
The above figures were derived from the same sources:
|
||||||||||||||||||||||||||||||||||||||||||||
| Results Discounted/undiscountedIORT3-week WB-EBRT6-week WB-EBRTCosts$28,879$29,789$34,070Life-years8.382408.381528.38257QALY7.660207.646187.65994ICERDominatedDominatedCPI, consumer Price Index; IRS, Internal Revenue Service. |
Discounted/undiscounted | IORT | 3-week WB-EBRT | 6-week WB-EBRT | Costs | $28,879 | $29,789 | $34,070 | Life-years | 8.38240 | 8.38152 | 8.38257 | QALY | 7.66020 | 7.64618 | 7.65994 | ICER | Dominated | Dominated | CPI, consumer Price Index; IRS, Internal Revenue Service. | ||||||||||||||||||||||||
| Discounted/undiscounted | IORT | 3-week WB-EBRT | 6-week WB-EBRT | |||||||||||||||||||||||||||||||||||||||||
| Costs | $28,879 | $29,789 | $34,070 | |||||||||||||||||||||||||||||||||||||||||
| Life-years | 8.38240 | 8.38152 | 8.38257 | |||||||||||||||||||||||||||||||||||||||||
| QALY | 7.66020 | 7.64618 | 7.65994 | |||||||||||||||||||||||||||||||||||||||||
| ICER | Dominated | Dominated | ||||||||||||||||||||||||||||||||||||||||||
| CPI, consumer Price Index; IRS, Internal Revenue Service. | ||||||||||||||||||||||||||||||||||||||||||||
| Sensitivity analysis | ||||||||||||||||||||||||||||||||||||||||||||
| The model conducted a series of one-way and two-way sensitivity analyses. A scenario analysis of 3-week accelerated WB-EBRT schedule of 16 fractions was also conducted Parameter/scenarioValueICER ($/QALY)Utility of IORT0.97Dominated0.8712,820Utility of 6-week WB-EBRT0.9714,9650.87DominatedUtility of IORT followed by 5-week WB-EBRT0.97Dominated0.8791,517Utility of salvage lumpectomy after IORT0.92Dominated0.822,284,464LRR of IORT (10 year)6.0%746,1581.5%DominatedLRR of 6 week WB-EBRT (10 year)3.6%Dominated1.2%2.7 millionProportion of women who receive IORT followed by 5-week WB-EBRT28.2%267 million7.1%DominatedRate of MBC after salvage lumpectomy or mastectomy (10-year rates)40.0%21 million10%Dominated |
Parameter/scenario | Value | ICER ($/QALY) | Utility of IORT | 0.97 | Dominated | 0.87 | 12,820 | Utility of 6-week WB-EBRT | 0.97 | 14,965 | 0.87 | Dominated | Utility of IORT followed by 5-week WB-EBRT | 0.97 | Dominated | 0.87 | 91,517 | Utility of salvage lumpectomy after IORT | 0.92 | Dominated | 0.82 | 2,284,464 | LRR of IORT (10 year) | 6.0% | 746,158 | 1.5% | Dominated | LRR of 6 week WB-EBRT (10 year) | 3.6% | Dominated | 1.2% | 2.7 million | Proportion of women who receive IORT followed by 5-week WB-EBRT | 28.2% | 267 million | 7.1% | Dominated | Rate of MBC after salvage lumpectomy or mastectomy (10-year rates) | 40.0% | 21 million | 10% | Dominated | |
| Parameter/scenario | Value | ICER ($/QALY) | ||||||||||||||||||||||||||||||||||||||||||
| Utility of IORT | 0.97 | Dominated | ||||||||||||||||||||||||||||||||||||||||||
| 0.87 | 12,820 | |||||||||||||||||||||||||||||||||||||||||||
| Utility of 6-week WB-EBRT | 0.97 | 14,965 | ||||||||||||||||||||||||||||||||||||||||||
| 0.87 | Dominated | |||||||||||||||||||||||||||||||||||||||||||
| Utility of IORT followed by 5-week WB-EBRT | 0.97 | Dominated | ||||||||||||||||||||||||||||||||||||||||||
| 0.87 | 91,517 | |||||||||||||||||||||||||||||||||||||||||||
| Utility of salvage lumpectomy after IORT | 0.92 | Dominated | ||||||||||||||||||||||||||||||||||||||||||
| 0.82 | 2,284,464 | |||||||||||||||||||||||||||||||||||||||||||
| LRR of IORT (10 year) | 6.0% | 746,158 | ||||||||||||||||||||||||||||||||||||||||||
| 1.5% | Dominated | |||||||||||||||||||||||||||||||||||||||||||
| LRR of 6 week WB-EBRT (10 year) | 3.6% | Dominated | ||||||||||||||||||||||||||||||||||||||||||
| 1.2% | 2.7 million | |||||||||||||||||||||||||||||||||||||||||||
| Proportion of women who receive IORT followed by 5-week WB-EBRT | 28.2% | 267 million | ||||||||||||||||||||||||||||||||||||||||||
| 7.1% | Dominated | |||||||||||||||||||||||||||||||||||||||||||
| Rate of MBC after salvage lumpectomy or mastectomy (10-year rates) | 40.0% | 21 million | ||||||||||||||||||||||||||||||||||||||||||
| 10% | Dominated | |||||||||||||||||||||||||||||||||||||||||||
| Author’s conclusions | Alvarado et al.106 concluded that IORT was a better strategy as it was less costly and provided more QALYs compared with WB-ERT. Esserman et al.107 concluded that the result of TARGIT-A trial was not expected to change | |||||||||||||||||||||||||||||||||||||||||||
| Reviewer’s comments | Overall, the analysis was well conducted. The results of the analysis were in line with the study conclusions. However, the model did not incorporate any PSA. Further, only two sets of two-way sensitivity analyses were conducted. Hence, the robustness of the cost-effectiveness results remains questionable | |||||||||||||||||||||||||||||||||||||||||||
Quality assessment checklist for economic evaluations
| Item | Yes/no/unclear |
|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Yesa |
| 2. Is the setting comparable to the UK? | No |
| 3. Is the analytical and modelling methodology appropriate? | Yes |
| 4. Are all the relevant costs and consequences for each alternative identified? | Yes |
| 5. Are the data inputs for the model described and justified? | Yes |
| 6. Are health outcomes measured in QALYs? | Yes |
| 7. Is the time horizon considered appropriate? | Nob |
| 8. Are costs and outcomes discounted? | Yes |
| 9. Is an incremental analysis performed? | Yes |
| 10. Is uncertainty assessed? | Yesc |
Critical appraisal checklist for economic evaluations (based on Drummond et al.57)
| Study | Shah, 2014108 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Research question | The study analysed the cost-efficacy of IORT compared with WB-EBRT and APBI for early-stage breast cancer | ||||||||||||||||||
| Country/setting | The analysis was based on data from two phase III trials: TARGIT-A trial and the ELIOT trial; the economic evaluation was US based | ||||||||||||||||||
| Funding source | Not stated | ||||||||||||||||||
| Analysis type | Cost–utility analysis, cost minimisation analysis | ||||||||||||||||||
| Study type | The study used local recurrence data from two trials: TARGIT-A and ELIOT | ||||||||||||||||||
| For the cost-effectiveness analyses, reimbursement models were calculated in four ways: | |||||||||||||||||||
|
|||||||||||||||||||
| The ICER analysis provided the increased reimbursement required to use WB-EBRT or APBI compared with IORT per percentage point of improvement in local recurrence | |||||||||||||||||||
| Perspective | Societal | ||||||||||||||||||
| Time horizon | Not clearly stated; it is assumed that the time horizon was for 10 years based on the estimation of mean utility by technique | ||||||||||||||||||
| Model assumptions | The model assumptions based on Suh et al.123 were as follows:
|
||||||||||||||||||
| Discounting (rate) | Not stated | ||||||||||||||||||
| Costing year, currency | US $ (price year not stated) | ||||||||||||||||||
| Population | TARGIT-A trial: women with early-stage ductal breast cancer who were ≥ 45 years old | ||||||||||||||||||
| ELIOT trial: women with unicentric cancer less than 2.5 cm who were > 45 years old | |||||||||||||||||||
| Intervention(s), comparator(s) | Intervention: IORT (INTRABEAM in TARGIT-A trial) or electron intraoperative radiotherapy (in ELIOT trial). The latter is not eligible for inclusion in this review | ||||||||||||||||||
| Comparator(s): WB-EBRT 3D-CRT; APBI 3D-CRT; APBI IMRT; APBI SL; APBI ML; APBI interstitial | |||||||||||||||||||
| Intervention effect | LRRs for both the INTRABEAM and WB-EBRT arms (3.3% for IORT vs. 1.3% for WB-EBRT) were obtained from the TARGIT trial | ||||||||||||||||||
| Data from the ELIOT trial was not extracted as the intervention is not eligible | |||||||||||||||||||
| Health state utilities | The utility values for the outcome states (shown below) were based on the study by Hayman et al.119 Health state utilitiesBase-case valueNo recurrence0.92Local recurrence0.779Other recurrence0.685 |
Health state utilities | Base-case value | No recurrence | 0.92 | Local recurrence | 0.779 | Other recurrence | 0.685 | ||||||||||
| Health state utilities | Base-case value | ||||||||||||||||||
| No recurrence | 0.92 | ||||||||||||||||||
| Local recurrence | 0.779 | ||||||||||||||||||
| Other recurrence | 0.685 | ||||||||||||||||||
| Intervention cost | Reimbursement costs were reported Reimbursement typeIORTWB-EBRTTotal reimbursement$3094$11,726Reimbursement including additional medical costsa$8003–8706$11,726Reimbursement including medical and non-medical costsa$8192–8971$12,985Reimbursement including medical, non-medical, and recurrence costs (TARGIT)a$9399–10,179$13,743a Range based on differences in WB-EBRT rates (15–21%). |
Reimbursement type | IORT | WB-EBRT | Total reimbursement | $3094 | $11,726 | Reimbursement including additional medical costsa | $8003–8706 | $11,726 | Reimbursement including medical and non-medical costsa | $8192–8971 | $12,985 | Reimbursement including medical, non-medical, and recurrence costs (TARGIT)a | $9399–10,179 | $13,743 | a Range based on differences in WB-EBRT rates (15–21%). | ||
| Reimbursement type | IORT | WB-EBRT | |||||||||||||||||
| Total reimbursement | $3094 | $11,726 | |||||||||||||||||
| Reimbursement including additional medical costsa | $8003–8706 | $11,726 | |||||||||||||||||
| Reimbursement including medical and non-medical costsa | $8192–8971 | $12,985 | |||||||||||||||||
| Reimbursement including medical, non-medical, and recurrence costs (TARGIT)a | $9399–10,179 | $13,743 | |||||||||||||||||
| a Range based on differences in WB-EBRT rates (15–21%). | |||||||||||||||||||
| Data for APBI not extracted as it is not relevant for the purpose of this review | |||||||||||||||||||
| Indirect costs | Non-medical costs including travel costs were estimated to be $44.96 and $89.92 per day for once-daily and twice-daily schedules of treatment, respectively | ||||||||||||||||||
| Results | The results for QALY, ICER and costs per QALY are extracted based on the TARGIT-A trial as ELIOT trial was not relevant for the purpose of this review. These are: | ||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Sensitivity analysis | Not reported | ||||||||||||||||||
| Author’s conclusions | The authors concluded IORT to be a potential cost-effective strategy in the treatment of women with early-stage breast cancer. But, depending on cost per QALT analysis, the authors stated WBI and APBI to be more cost-effective strategies in delivering radiation therapy, despite IORT having reduced reimbursement rates | ||||||||||||||||||
| Reviewer’s comments | Limited information surrounding the model structure was presented in the study. Time horizon for the model was not clearly stated. Although the techniques adopted to estimate costs associated with non-medical, follow-up, local recurrence or other recurrence (including salvage mastectomy) were mentioned, the costs were not reported, except for non-medical costs. Sensitivity analysis was not conducted | ||||||||||||||||||
Quality assessment checklist for economic evaluations
| Item | Yes/no/unclear |
|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Yesa |
| 2. Is the setting comparable to the UK? | No |
| 3. Is the analytical and modelling methodology appropriate? | Yesb |
| 4. Are all the relevant costs and consequences for each alternative identified? | Yesc |
| 5. Are the data inputs for the model described and justified? | Yes |
| 6. Are health outcomes measured in QALYs? | Yes |
| 7. Is the time horizon considered appropriate? | Uncleard |
| 8. Are costs and outcomes discounted? | No |
| 9. Is an incremental analysis performed? | No |
| 10. Is uncertainty assessed? | No |
Appendix 7 Excluded quality-of-life studies with rationale
| Excluded study | Primary reason for exclusion |
|---|---|
| Bao T, Cai L, Snyder C, Betts K, Tarpinian K, Gould J, et al. Patient-reported outcomes in women with breast cancer enrolled in a dual-centre, double-blind, randomised controlled trial assessing the effect of acupuncture in reducing aromatase inhibitor-induced musculoskeletal symptoms. Cancer 2014;120:381–9 | Not EQ-5D |
| Bonnetain F, Conroy T, Velten M, Jolly D, Mercier M, Causeret S, et al. Impact of response shift in longitudinal postoperative quality of life (QoL) analysis among breast cancer (BC) patients: a randomised multicenter cohort study. J Clin Oncol 2010; Conference (var.pagings):15 | Abstract |
| Brown DS, Trogdon J, Ekwueme DU, Chamiec-Case L, Tangka FK, Guy GP et al. Preference-based estimates of the health utility impacts of breast cancer in women ages 18–44 in the United States. Value Health 2012; Conference(var.pagings):4 | Abstract |
| Chandwani KD, Thornton B, Perkins GH, Arun B, Raghuram NV, Nagendra HR et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integrative Oncol 2010;8:43–55 | Not EQ-5D |
| Chang J, Couture FA, Young SD, Lau CY, Lee MK. Weekly administration of epoetin alfa improves cognition and quality of life in patients with breast cancer receiving chemotherapy. Supportive Cancer Therapy 2004;2:52–8 | No relevant information on health states |
| Cheung YB, Lee CF, Luo N, Ng R, Wong NS, Yap YS, et al. Comparison of the measurement properties between the 5-level euroqol group’s 5-dimension (EQ-5D-5l) questionnaire and the functional assessment of cancer therapy-breast (FACT-B) in Asian breast cancer patients. Value Health 2012;15:A605 | Abstract |
| Cheville AL, Almoza M, Courmier JN, Basford JR. A prospective cohort study defining utilities using time trade-offs and the euroqol-5D to assess the impact of cancer-related lymphedema. Cancer 2010;116:3722–31 | Inappropriate participants |
| Conner-Spady B, Cumming C, Nabholtz JM, Jacobs P, Stewart D. Responsiveness of the EuroQol in breast cancer patients undergoing high dose chemotherapy. Qual Life Res 2001;10:479–86 | No relevant information on health states |
| Coyle D, Grunfeld E, Coyle K, Julian JA, Pond GR, Folkes A, et al. Cost-effectiveness of a survivorship care plan for breast cancer survivors. J Clin Oncol 2011; Conference(var.pagings):15 | Abstract |
| Crott R, Briggs A. Mapping the QLQ-C30 quality of life cancer questionnaire to EQ-5D patient preferences. European J Health Econ 2010;11:427–34 | Not primary research |
| Dabakuyo TS, Guillemin F, Conroy T, Velten M, Jolly D, Mercier M, et al. Response shift effects on measuring post-operative quality of life among breast cancer patients: a multicenter cohort study. Qual Life Res 2013;22:1–11 | Not EQ-5D |
| de KM, Dirksen CD, Kessels AG, van der Weijden T, van de Velde CJ, Roukema JA, et al. Cost-effectiveness of a short stay admission programme for breast cancer surgery. Acta Oncol 2010;49:338–46 | No relevant information on health states |
| Dolbeault S, Cayrou S, Bredart A, Viala AL, Desclaux B, Saltel P, et al. The effectiveness of a psycho-educational group after early-stage breast cancer treatment: results of a randomised French study. Psycho-Oncology 2009;18:647–56 | Not EQ-5D |
| Domeyer PJ, Sergentanis TN, Zagouri F, Zografos GC. Health-related quality of life in vacuum-assisted breast biopsy: short-term effects, long-term effects and predictors. Health Qual Life Outcomes 2010;8:11 | Inappropriate participants |
| Fang P, Tan KS, Troxel AB, Rengan R, Freedman G, Lin LL. High body mass index is associated with worse quality of life in breast cancer patients receiving radiotherapy. Breast Cancer Res Treat 2013;141:125–33 | Not EQ-5D |
| Fang P, Tan K, Troxel A, Rengan R, Freedman G, Lin L. High BMI associated with worse quality of life in breast cancer patients receiving radiation therapy. Int J Radiat Oncol Biol Physics 2013;87(Suppl. 1):S607 | No relevant information on health states |
| Farkkila N, Roine R, Jahkola T, Sintonen H, Hanninen J, Taari K, et al. Health state utilities in breast cancer. Value Health 2011; Conference (var.pagings):7 | Abstract |
| Haines TP, Sinnamon P, Wetzig NG, Lehman M, Walpole E, Pratt T, et al. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomised trial with economic evaluation. Breast Cancer Res Treat 2010;124:163–75 | No relevant information on health states |
| Hayran M, Cakir B, Cilingiroglu N, Erman M, Kilickap S, Ozisik YY, et al. Validation and clinical evaluation of different quality of life (QoL) scales in patients (pts) with breast cancer (BC) in Turkey. J Clin Oncol 2011; Conference (var.pagings):15 | Abstract |
| Jansen SJ, Otten W, van de Velde CJ, Nortier JW, Stiggelbout AM. The impact of the perception of treatment choice on satisfaction with treatment, experienced chemotherapy burden and current quality of life. Br J Cancer 2004;91:56–61 | No relevant information on health states |
| Jeruss JS, Hunt KK, Xing Y, Krishnamurthy S, Meric-Bernstam F, Cantor SB, et al. Is intraoperative touch imprint cytology of sentinel lymph nodes in patients with breast cancer cost-effective? Cancer 2006;107:2328–36 | Not primary research |
| Katharina WA, Schumacher A. Social connotations of breast cancer-work in progress. Psycho-Oncology 2013;22:222 | Abstract |
| Kimman ML, Dirksen CD, Falger P, Voogd A, Kessels A, Gijsen B, et al. Results of an RCT investigating the cost-effectiveness of four follow-up strategies after breast cancer. Eur J Cancer, Supplement 2009; Conference (var.pagings):2–3 | Abstract |
| Kimman ML, Dirksen CD, Lambin P, Boersma LJ. Responsiveness of the EQ-5D in primary breast cancer survivors. EJC Suppl 2008;6:73–4 | Abstract |
| Kimman ML, Dirksen CD, Voogd AC, Falger P, Gijsen BC, Thuring M, et al. Economic evaluation of four follow-up strategies after curative treatment for breast cancer: results of an RCT. Eur J Cancer 2011;47:1175–85 | Inappropriate participants |
| Lee CF, Luo N, Ng R, Wong NS, Yap YS, Lo SK, et al. Comparison of the measurement properties between a short and generic instrument, the 5-level EuroQoL Group’s 5-dimension (EQ-5D-5L) questionnaire, and a longer and disease-specific instrument, the Functional Assessment of Cancer Therapy-Breast (FACT-B), in Asian breast cancer patients. Qual Life Res 2013;22:1745–51 | Inappropriate participants |
| Lee CF, Ng R, Luo N, Wong NS, Yap YS, Lo SK, et al. The English and Chinese versions of the five-level EuroQoL Group’s five-dimension questionnaire (EQ-5D) were valid and reliable and provided comparable scores in Asian breast cancer patients. Supportive Care Cancer 2013;21:201–9 | Inappropriate participants |
| Lee J-A, Kim S-Y, Kim Y, Oh J, Kim H-J, Jo D-Y, et al. Comparison of health-related quality of life between cancer survivors treated in designated cancer centres and the general public in Korea. Japanese J Clin Oncol 2014;44:141–52 | No relevant information on health states |
| Lovrics PJ, Cornacchi SD, Barnabi F, Whelan T, Goldsmith CH. The feasibility and responsiveness of the health utilities index in patients with early-stage breast cancer: a prospective longitudinal study. Qual Life Res 2008;17:333–45 | Not EQ-5D |
| Matalqah LM, Radaideh KM, Yusoff ZM, Awaisu A. Health-related quality of life using EQ-5D among breast cancer survivors in comparison with age-matched peers from the general population in the state of Penang, Malaysia. J Public Health 2011;19:475–80 | Inappropriate participants |
| Milne RJ, Heaton-Brown KH, Hansen P, Thomas D, Harvey V, Cubitt A. Quality-of-life valuations of advanced breast cancer by New Zealand women. Pharmacoeconomics 2006;24:281–92 | Inappropriate participants |
| Moro-Valdezate D, Peiro S, Buch-Villa E, Caballero-Garate A, Morales-Monsalve MD, Martinez-Agullo A, et al. Evolution of Health-Related Quality of Life in Breast Cancer Patients during the First Year of Follow-Up. J Breast Cancer 2013;16:104–11 | No relevant information on health states |
| Ng R, Lee CF, Wong NS, Yap YS, Lo SK, Wong C, et al. Measurement properties and equivalence of the English and Chinese versions of the new 5-level EQ-5D in Asian breast cancer patients. Eur J Cancer 2011; Conference(var.pagings):S235 | Abstract |
| Oh S, Heflin L, Meyerowitz BE, Desmond KA, Rowland JH, Ganz PA. Quality of life of breast cancer survivors after a recurrence: a follow-up study. Breast Cancer Res Treat 2004;87:45–57 | Not EQ-5D |
| Peasgood T, Ward SE, Brazier J. Health state utility values in breast cancer: a review and metaanalysis. Value Health 2010; Conference(var.pagings):7 | Not primary research |
| Polsky D, Keating NL, Weeks JC, Schulman KA. Patient choice of breast cancer treatment: impact on health state preferences. Med Care 2002;40:1068–79 | Not EQ-5D |
| Polsky D, Mandelblatt JS, Weeks JC, Venditti L, Hwang YT, Glick HA, et al. Economic evaluation of breast cancer treatment: considering the value of patient choice. J Clin Oncol 2003;21:1139–46 | Not EQ-5D |
| Postma EL, Koffijberg H, Verkooijen HM, Witkamp AJ, van den Bosch MA, van HR. Cost-effectiveness of radioguided occult lesion localisation (ROLL) versus wire-guided localisation (WGL) in breast conserving surgery for nonpalpable breast cancer: results from a randomised controlled multicenter trial. Ann Surg Oncol 2013;20:2219–26 | No relevant information on health states |
| Rand KL, Otte JL, Flockhart D, Hayes D, Storniolo AM, Stearns V et al. Modelling hot flushes and quality of life in breast cancer survivors. Climacteric 2011;14:171–80 | No relevant information on health states |
| Shimozuma K, Shiroiwa T, Fukuda T, Mori M, Ohashi Y, Watanabe T. Comparison of Eq-5D Score Between Treatment with 4 Cycles of Anthracycline Followed by 4 Cycles of Taxane and 8 Cycles of Taxane for Node Positive Breast Cancer Patients After Surgery: N-Sas Bc 02 Trial. Value Health 2010;13:A274 | Abstract |
| Shiroiwa T, Fukuda T, Shimozuma K, Kuranami M, Suemasu K, Ohashi Y, et al. Comparison of EQ-5D scores among anthracycline-containing regimens followed by taxane and taxane-only regimens for node-positive breast cancer patients after surgery: the N-SAS BC 02 trial. Value Health 2011;14:746–751 | No relevant information on health states |
| Slovacek L, Slovackova B, Slanska I, Petera J, Priester P, Filip S, et al. Depression symptoms and health-related quality of life among patients with metastatic breast cancer in programme of palliative cancer care. Neoplasma 2009;56:467–72 | No relevant information on health states |
| Slovacek L, Slovackova B, Slanska I, Petera J, Priester P. Quality of life and depression among metastatic breast cancer patients. Med Oncol 2010;27:958–9 | Abstract |
| Sun Y, Kang E, Heo C, Kim D, Hwang Y, Yom C, et al. Comparison of Quality of Life According to the Surgical Techniques Among Breast Cancer Survivors. Breast 2013;22(Suppl. 1):S117–18 | Abstract |
| Sura K, Tan K, Freedman GM, Troxel AB, Lin LL. Factors affecting breast cancer patient quality of life in association with radiation. Int J Rad Oncol Biol Phys 2013; 87(Suppl. 1):S115–16 | Abstract |
| Takei H, Ohsumi S, Shimozuma K, Ohashi Y, Fujiki Y, Suemasu K, et al. Health-related quality-of-life and psychological distress of breast cancer patients after surgery during phase III randomised trial comparing tamoxifen, exemestane, and anastrozole: N-SAS BC 04. Breast Cancer Res Treat 2006;100(Suppl. 1):S189–90 | Not EQ-5D |
| Teckle P, Peacock S, McTaggart-Cowan H, van der Hoek K, Chia S, Melosky B, et al. The ability of cancer-specific and generic preference-based instruments to discriminate across clinical and self-reported measures of cancer severities. Health Qual Life Outcomes 2011;9:106 | Inappropriate participants |
| Velthuis MJ, May AM, Koppejan-Rensenbrink RA, Gijsen BC, van BE, de Wit GA, et al. Physical Activity during Cancer Treatment (PACT) Study: design of a randomised clinical trial. BMC Cancer 2010;10:272 | Not EQ-5D |
| Verkooijen HM, Buskens E, Peeters PH, Borel Rinkes IH, de Koning HJ, van Vroonhoven TJ, et al. Diagnosing non-palpable breast disease: short-term impact on quality of life of large-core needle biopsy versus open breast biopsy. Surg Oncol 2002;10:177–81 | Inappropriate participants |
| von Meyenfeldt MF, de KM, Kessels AGH, van der Weijden T, Bell AVRJ, Roukema JA, et al. Economic evaluation of a short stay admission programme for breast cancer surgery in four hospitals in the Netherlands. Eur J Cancer, Supplement 2010; Conference(var.pagings):3 | Abstract |
| Wilking N, Bernow M, Kossler I, Wilking U, Jonsson B. Health related quality of life (HRQoL) in Swedish relapse free breast cancer patients. A study of EQ5D and TTO in a patient advocacy population. Cancer Res 2009; 69:780S–1S | Abstract |
| Wu Y, Segreti A, Cella D, DiLeo A, Amonkar M, Koehler M, et al. Lapatinib plus paclitaxel versus paclitaxel alone for first line metastatic breast cancer (MBC) in ErbB(2+) patients – quality of Life (QOL) results. EJC Suppl 2008;6:171 | Abstract |
| Yaqata H, Iwase T, Ohtsu H, Komoike Y, Saji S, Takei H, et al. Baseline assessment of patient-reported outcomes (PROs) for breast cancer patients after 5-years of endocrine treatment in a randomised clinical trial: NSAS-BC 05. Breast 2011;20(Suppl. 1):S68 | Abstract |
| Zhou X, Cella D, Cameron D, Amonkar MM, Segreti A, Stein S, et al. Lapatinib plus capecitabine versus capecitabine alone for HER2+ (ErbB2+) metastatic breast cancer: quality-of-life assessment. Breast Cancer Res Treat 2009;117:577–89 | No relevant information on health states |
| Zhou X, Segreti A, Cella D, Cameron D, Geyer C, Amonkar M, et al. Lapatinib plus capecitabine versus capecitabine alone for ErbB2-positive metastatic breast cancer (MBC) – quality of Life (QOL) assessment. EJC Supplements 2008;6:216–17 | Abstract |
Appendix 8 Data extraction forms for health-related quality-of-life studies (presented in order of health states)
Reference
Turnbull, 2010. 126
Study characteristics
Research question
What are the stated objectives of the study?
To determine the potential benefits to the patient and to the NHS of the addition of MRI to the routine techniques employed for locoregional staging of primary breast cancer.
Describe the type of study and study design.
Randomised controlled trial.
Was the sample from i) the general population, ii) patients with the disease of interest, iii) individuals with knowledge of the disease, iv) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. > 80 years)?
Women with biopsy-proven primary breast cancer, who were scheduled for WLE following triple assessment (clinical, radiological and pathological).
Yes, the inclusion and exclusion criteria were clearly described; the study included patients aged 18 years or above.
What are the characteristics of the baseline cohort for the evaluation?
| Age | MRI scanNo MRI scanMean (years) (SD)56.38 (9.67)56.59 (10.09)Median (years) (range)57 (27–86)57 (58–85)NoteClinical details based on ITT population.Age (as randomised)MRI scanNo MRI scan< 50 years, n (%)187 (22.9)187 (23.2)≥ 50 years, n (%)629 (77.1)620 (76.8)NoteClinical details based on ITT population. | MRI scan | No MRI scan | Mean (years) (SD) | 56.38 (9.67) | 56.59 (10.09) | Median (years) (range) | 57 (27–86) | 57 (58–85) | NoteClinical details based on ITT population. | Age (as randomised) | MRI scan | No MRI scan | < 50 years, n (%) | 187 (22.9) | 187 (23.2) | ≥ 50 years, n (%) | 629 (77.1) | 620 (76.8) | NoteClinical details based on ITT population. | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRI scan | No MRI scan | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean (years) (SD) | 56.38 (9.67) | 56.59 (10.09) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median (years) (range) | 57 (27–86) | 57 (58–85) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NoteClinical details based on ITT population. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (as randomised) | MRI scan | No MRI scan | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 50 years, n (%) | 187 (22.9) | 187 (23.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≥ 50 years, n (%) | 629 (77.1) | 620 (76.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NoteClinical details based on ITT population. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Female 100% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Race (if appropriate) | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication/disease | Primary breast cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other characteristics (sample size) | n = 1625 (MRI scan: n = 817; no MRI scan: 808) VariablesaCategoryMRI scanNo MRI scanMenopausal status, n (%)Pre-menopausal232 (28.4)234 (29.0)Post menopausal574 (70.3)565 (70.0)Missing10 (1.2)8 (1.0)HRT use, n (%)Currently63 (7.7)46 (5.7)Previously232 (28.4)231 (28.6)Never514 (63.0)528 (65.4)Missing7 (0.9)2 (0.2)Pre-operative neoadjuvant therapy, n (%)Yes6 (0.7)11 (1.4)No808 (99.0)792 (98.1)Missing data2 (0.2)4 (0.5)In situ disease. Carcinoma in situ present, n (%)Yes586 (71.8)568 (70.4)No191 (23.4)193 (23.9)Missing data39 (4.8)46 (5.7)Grade, n (%)I177 (23.8)179 (24.8)II358 (48.2)331 (45.8)III200 (26.9)205 (28.4)Missing8 (1.1)8 (1.1)HRT, hormone replacement therapy.aInformation has been brought together from more than one place. n = 1625 is number randomised; however, the variables are for those analysed (n = 1623).NoteOther characteristics were reported but not data extracted. |
Variablesa | Category | MRI scan | No MRI scan | Menopausal status, n (%) | Pre-menopausal | 232 (28.4) | 234 (29.0) | Post menopausal | 574 (70.3) | 565 (70.0) | Missing | 10 (1.2) | 8 (1.0) | HRT use, n (%) | Currently | 63 (7.7) | 46 (5.7) | Previously | 232 (28.4) | 231 (28.6) | Never | 514 (63.0) | 528 (65.4) | Missing | 7 (0.9) | 2 (0.2) | Pre-operative neoadjuvant therapy, n (%) | Yes | 6 (0.7) | 11 (1.4) | No | 808 (99.0) | 792 (98.1) | Missing data | 2 (0.2) | 4 (0.5) | In situ disease. Carcinoma in situ present, n (%) | Yes | 586 (71.8) | 568 (70.4) | No | 191 (23.4) | 193 (23.9) | Missing data | 39 (4.8) | 46 (5.7) | Grade, n (%) | I | 177 (23.8) | 179 (24.8) | II | 358 (48.2) | 331 (45.8) | III | 200 (26.9) | 205 (28.4) | Missing | 8 (1.1) | 8 (1.1) | HRT, hormone replacement therapy.aInformation has been brought together from more than one place. n = 1625 is number randomised; however, the variables are for those analysed (n = 1623).NoteOther characteristics were reported but not data extracted. | |||
| Variablesa | Category | MRI scan | No MRI scan | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Menopausal status, n (%) | Pre-menopausal | 232 (28.4) | 234 (29.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Post menopausal | 574 (70.3) | 565 (70.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Missing | 10 (1.2) | 8 (1.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HRT use, n (%) | Currently | 63 (7.7) | 46 (5.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Previously | 232 (28.4) | 231 (28.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Never | 514 (63.0) | 528 (65.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Missing | 7 (0.9) | 2 (0.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pre-operative neoadjuvant therapy, n (%) | Yes | 6 (0.7) | 11 (1.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No | 808 (99.0) | 792 (98.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Missing data | 2 (0.2) | 4 (0.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| In situ disease. Carcinoma in situ present, n (%) | Yes | 586 (71.8) | 568 (70.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No | 191 (23.4) | 193 (23.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Missing data | 39 (4.8) | 46 (5.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Grade, n (%) | I | 177 (23.8) | 179 (24.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| II | 358 (48.2) | 331 (45.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| III | 200 (26.9) | 205 (28.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Missing | 8 (1.1) | 8 (1.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HRT, hormone replacement therapy.aInformation has been brought together from more than one place. n = 1625 is number randomised; however, the variables are for those analysed (n = 1623).NoteOther characteristics were reported but not data extracted. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| QoL instrument | EQ-5D | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Utility values, (yes/no) | Yes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment effect, if reported | Yes, reoperation rates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Country/setting
What is the country and setting for the evaluation?
UK, RCT.
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
QoL data were collected as part of the RCT.
Results
Summarise the results.
| EQ-5D scores | MRI scan: mean (SE), 95% CI | No MRI scan: mean (SE), 95% CI |
|---|---|---|
| Baseline | 0.8567 (0.0065), 0.8435 to 0.8699 | 0.8601 (0.0063), 0.8475 to 0.8728 |
| 8 weeks post randomisation | 0.7791 (0.0078), 0.7634 to 0.7948 | 0.7728 (0.0079), 0.7569 to 0.7887 |
| 6 months post initial surgery | 0.8040 (0.0094), 0.7844 to 0.8237 | 0.7935 (0.0078), 0.7781 to 0.8089 |
| 12 months post initial surgery | 0.8101 (0.0069), 0.7965 to 0.8236 | 0.8112 (0.0072), 0.7970 to 0.8253 |
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference-based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?)
Yes. EQ-5D was used to assess health states; the valuation of health states were from the UK population.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
Not applicable.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
-
The authors concluded that overall the two arms of the trial had similar QoL scores which decreased slightly between baseline and 8 weeks post randomisation but recovered between 6 and 12 months post initial surgery.
-
The authors reported that 12 months after initial surgery, there was no statistically significant difference in HRQoL as measured by EQ-5D between the two arms of the trial once baseline HRQoL and other covariates were controlled for. The nominal values of the point estimates of the mean changes between baseline and 12 months were also very similar.
What are the implications of the study for the model?
The utility values were derived from EQ-5D estimates based on UK population; therefore, the EQ-5D estimates reported for the no MRI arm could be used to inform the SHTAC’s model as this arm of the trial represented current UK treatment option for primary breast cancer. Specifically, the EQ-5D estimates in the baseline and 12 months post initial surgery for the cohort in no MRI arm could be used in the SHTAC’s model.
Criteria for assessment of study relevance to the National Institute for Health and Care Excellence’s reference case (adapted from Papaioannou et al.62)
| Relevance questions | Requirement for NICE |
|---|---|
| Do the population characteristics (e.g. age, sex, comorbidities, diagnosis, severity of disease) in the study match those described in the decision problem of the review and those modelled? | Yes |
| Was a generic preference-based instrument (preferably EQ-5D) used to describe the health states? | Yes |
| Was the change in HRQoL taken directly from the patient population? | Yes |
| Was the valuation of changes in patients’ HRQL undertaken from the general (UK) population? | Yes |
| Was the technique used to value the health states a choice-based method (such as TTO)? | Yes |
Reference
Freedman, 2010. 127
Study characteristics
Research question
What are the stated objectives of the study?
To use the EQ-5D instrument to evaluate the long-term health states of women with early-stage breast cancer treated by BCS and radiation.
Describe the type of study and study design.
Single cohort study.
Was the sample from i) the general population, ii) patients with the disease of interest, iii) individuals with knowledge of the disease, iv) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. > 80 years)?
Women with early breast cancer treated with BCS and radiation with or without systemic therapy.
Yes, the inclusion and exclusion criteria were clearly described and do not exclude any individuals that may be relevant (the study excluded male breast cancer, T3-T4 disease, stage IV disease, mastectomy, or patients treated without radiation).
What are the characteristics of the baseline cohort for the evaluation?
| Age (years) | 18–44: 13% | ||||||||||||||||||
| 45–64: 57% | |||||||||||||||||||
| > 64: 30% | |||||||||||||||||||
| Sex | Female, 100% | ||||||||||||||||||
| Race (if appropriate) | Not reported | ||||||||||||||||||
| Indication/disease | Early-stage breast cancer, American Joint Committee on Cancer stages 0, I, or II breast cancer | ||||||||||||||||||
| Other characteristics (sample size) | n = 1050Tumour stage, n (%)Tis192 (18%)T1714 (68%)T2141 (13%)Nodal stage, n (%)N0644 (61%)N1–3 positive174 (17%)N4+ positive38 (4%)NX194 (18%) | Tumour stage, n (%) | Tis | 192 (18%) | T1 | 714 (68%) | T2 | 141 (13%) | Nodal stage, n (%) | N0 | 644 (61%) | N1–3 positive | 174 (17%) | N4+ positive | 38 (4%) | NX | 194 (18%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumour stage, n (%) | |||||||||||||||||||
| Tis | 192 (18%) | ||||||||||||||||||
| T1 | 714 (68%) | ||||||||||||||||||
| T2 | 141 (13%) | ||||||||||||||||||
| Nodal stage, n (%) | |||||||||||||||||||
| N0 | 644 (61%) | ||||||||||||||||||
| N1–3 positive | 174 (17%) | ||||||||||||||||||
| N4+ positive | 38 (4%) | ||||||||||||||||||
| NX | 194 (18%) | ||||||||||||||||||
| QoL instrument | EQ-5D | ||||||||||||||||||
| Utility values, (yes/no) | Yes, presented in a figure over time and in text | ||||||||||||||||||
| Treatment effect, if reported | Not reported | ||||||||||||||||||
Country/setting
What is the country and setting for the evaluation?
USA, hospital outpatient clinic.
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
Single study.
Results
Summarise the results.
-
Mean descriptive index:
-
Mean scores by age:
-
No significant differences in health states between patients by age.
-
States no significant differences in mean index score by the use of adjuvant systemic therapy when compared with those treated by chemotherapy only, tamoxifen only, both or neither (p > 0.05); no data were reported.
-
States no apparent difference in mean score by use of IMRT versus conventional radiation although very few patients treated with IMRT had follow-up greater than 3 years. No data were reported.
-
States no significant differences between patients with and without a recurrence, although the number of questionnaires from patients with recurrence was small (n = 94) compared with those without recurrence (n = 2,386). No data were reported.
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference-based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?).
Yes. EQ-5D was used to assess health states. However, the valuation of health states were not from the UK general population – the study was US based.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
Not applicable.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
The authors concluded that patients reported high EQ-5D value, which was steady for up to 15 years following treatment with BCS and radiation. In addition, it was also observed that there was good level of statistical correlation between patient-reported outcomes by either descriptive system or VAS.
What are the implications of the study for the model?
The study is not UK based; therefore, the reported EQ-5D values could be used to inform the model for testing uncertainty or model validity. However, if no UK-based study is found, the mean EQ-5D score reported for WLE + WB-EBRT health state could be fed into the model. Data on mean index scores are reported for the entire cohort of patients (i.e. women treated with BCS and radiation) but report no significant difference between subgroups (e.g. the use of adjuvant systemic therapy, use of IMRT, recurrence, although the number of questionnaires from patients with recurrences was very small compared with those without recurrence).
Criteria for assessment of study relevance to the National Institute for Health and Care Excellence’s reference case (adapted from Papaioannou et al.62)
| Relevance questions | Requirement for NICE |
|---|---|
| Do the population characteristics (e.g. age, sex, comorbidities, diagnosis, severity of disease) in the study match those described in the decision problem of the review and those modelled? | Yes |
| Was a generic preference-based instrument (preferably EQ-5D) used to describe the health states? | Yes |
| Was the change in HRQoL taken directly from the patient population? | Yes |
| Was the valuation of changes in patients’ HRQL undertaken from the general (UK) population? | No |
| Was the technique used to value the health states a choice-based method (such as TTO)? | Yes |
Reference
Prescott, 2007. 128
Study characteristics
Research question
What are the stated objectives of the study?
To assess whether or not omission of post-operative radiotherapy in women with ‘low-risk’ axillary node-negative breast cancer (T0–2) treated by BCS and endocrine therapy improves QoL and is more cost-effective.
Describe the type of study and study design.
Randomised controlled trial. A non-randomised cohort was also recruited in order to complete a comprehensive cohort study.
Was the sample from i) the general population, ii) patients with the disease of interest, iii) individuals with knowledge of the disease, iv) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. > 80 years)?
Breast cancer patients undergoing BCS and endocrine therapy with complete excision on histological assessment
The inclusion and exclusion criteria were reported. The study did not include patients aged below 65 years.
What are the characteristics of the baseline cohort for the evaluation?
| Age | Randomised (n = 255)Radiotherapy (n = 127)No radiotherapy (n = 128)Mean age (years) at surgery (SD)72.3 (5.0)72.8 (5.2) | Randomised (n = 255) | Radiotherapy (n = 127) | No radiotherapy (n = 128) | Mean age (years) at surgery (SD) | 72.3 (5.0) | 72.8 (5.2) | ||
|---|---|---|---|---|---|---|---|---|---|
| Randomised (n = 255) | |||||||||
| Radiotherapy (n = 127) | No radiotherapy (n = 128) | ||||||||
| Mean age (years) at surgery (SD) | 72.3 (5.0) | 72.8 (5.2) | |||||||
| Sex | Female 100% | ||||||||
| Race (if appropriate) | Not reported | ||||||||
| Indication/disease | Breast cancer patients with ‘low risk’, axillary node negative | ||||||||
| Other characteristics (sample size) | n = 255 (randomised patients); 253 patients were evaluable; EQ-5D data were available for 203 patients | ||||||||
| QoL instrument | EQ-5D | ||||||||
| Utility values, (yes/no) | Yes | ||||||||
| Treatment effect, if reported | Not reported | ||||||||
Country/setting
What is the country and setting for the evaluation?
UK, RCT.
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
Yes, a RCT and a cohort study.
Results
Summarise the results.
| EQ-5D | Radiotherapy: n (n = 102), mean (95% CI) | No radiotherapy: n (n = 101), mean (95% CI) |
|---|---|---|
| Baseline | 0.77 (0.73 to 0.80) | 0.74 (0.70 to 0.77) |
| 3.5 months | 0.78 (0.74 to 0.81) | 0.76 (0.73 to 0.79) |
| 9 months | 0.76 (0.71 to 0.81) | 0.72 (0.68 to 0.76) |
| 15 months | 0.74 (0.70 to 0.78) | 0.73 (0.69 to 0.77) |
| Unadjusted QALYs | 0.95 (0.90 to 0.99) | 0.92 (0.88 to 0.95) |
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference-based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?)
Yes. EQ-5D was used to assess health status; the study was UK based.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
Not applicable.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
The authors concluded that patients in the radiotherapy arm had higher utility values at the baseline compared with those in the no radiotherapy arm. However, it was observed that the difference in adjusted QALYs for the two arms was too small to be statistically significant at the 5% level.
What are the implications of the study for the model?
As this is a UK-based study, the model inputs on utilities could be used to inform SHTAC’s cost-effectiveness model in development. In particular, this study could be used to populate the health state ‘Wide local excision followed by WB-EBRT’ with the value of 0.74 (95% CI 0.70 to 0.78).
Criteria for assessment of study relevance to the National Institute for Health and Care Excellence’s reference case (adapted from Papaioannou et al.62)
| Relevance questions | Requirement for NICE |
|---|---|
| Do the population characteristics (e.g. age, sex, co-morbidities, diagnosis, severity of disease) in the study match those described in the decision problem of the review and those modelled? | Yes |
| Was a generic preference-based instrument (preferably EQ-5D) used to describe the health states? | Yes |
| Was the change in HRQoL taken directly from the patient population? | Yes |
| Was the valuation of changes in patients’ HRQL undertaken from the general (UK) population? | Yes |
| Was the technique used to value the health states a choice-based method (such as TTO)? | Yes |
Reference
Serra, 2012. 129
Study characteristics
Research question
What are the stated objectives of the study?
To evaluate the impact of guided imagery (a stress reduction technique) on patients undergoing radiation therapy for breast cancer.
Describe the type of study and study design.
Single cohort study.
Was the sample from i) the general population, ii) patients with the disease of interest, iii) individuals with knowledge of the disease, iv) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. > 80 years)?
Women receiving radiation therapy for breast cancer.
Yes, inclusion/exclusion criteria were reported.
What are the characteristics of the baseline cohort for the evaluation?
| Age (years) | Mean age (range): 57 (28–77) | ||||||||||||||||||||||||
| Sex | Female 100% | ||||||||||||||||||||||||
| Race (if appropriate) | Not reported | ||||||||||||||||||||||||
| Indication/disease | Women undergoing radiation therapy for breast cancer | ||||||||||||||||||||||||
| Other characteristics (sample size) | N = 66 CharacteristicsnStage018I24II11III9Local recurrences4Adjuvant therapyChemotherapy and hormones13Chemotherapy only9Hormones only28None16 |
Characteristics | n | Stage | 0 | 18 | I | 24 | II | 11 | III | 9 | Local recurrences | 4 | Adjuvant therapy | Chemotherapy and hormones | 13 | Chemotherapy only | 9 | Hormones only | 28 | None | 16 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | n | ||||||||||||||||||||||||
| Stage | |||||||||||||||||||||||||
| 0 | 18 | ||||||||||||||||||||||||
| I | 24 | ||||||||||||||||||||||||
| II | 11 | ||||||||||||||||||||||||
| III | 9 | ||||||||||||||||||||||||
| Local recurrences | 4 | ||||||||||||||||||||||||
| Adjuvant therapy | |||||||||||||||||||||||||
| Chemotherapy and hormones | 13 | ||||||||||||||||||||||||
| Chemotherapy only | 9 | ||||||||||||||||||||||||
| Hormones only | 28 | ||||||||||||||||||||||||
| None | 16 | ||||||||||||||||||||||||
| QoL instrument | EQ-5D | ||||||||||||||||||||||||
| Utility values, (yes/no) | Yes | ||||||||||||||||||||||||
| Treatment effect, if reported | Not reported | ||||||||||||||||||||||||
Country/setting
What is the country and setting for the evaluation?
USA.
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
Single study.
Results
Summarise the results.
-
Health status was evaluated at two time points: prior to start of guided therapy (time 1) and at the end of radiation therapy (time 2).
-
EQ-5D index at time 1: 0.88 (n = 64), time 2 = 0.86 (n = 54).
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference-based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?)
Yes. EQ-5D questionnaire was used; the study was US based.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
-
Not applicable.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
The authors concluded that EQ-5D results indicated an increase in pain ratings attributed to the radiation-induced skin reactions which was also associated with a reduction in anxiety and depression. This reduction further reinforced the use of guided imagery.
What are the implications of the study for the model?
As the study was US based, the value of 0.86 (after radiation therapy) could be used to inform the health state of ‘wide local excision +WB-EBRT’ within the cost-effectiveness model, should there be no available UK-based data. However, patients also received guided imagery and there was no control arm in the study. It is therefore unclear what impact guided imagery had.
In other case, this value could be used in conducting sensitivity analysis.
Criteria for assessment of study relevance to the National Institute for Health and Care Excellence’s reference case (adapted from Papaioannou et al.62)
| Relevance questions | Requirement for NICE |
|---|---|
| Do the population characteristics (e.g. age, sex, comorbidities, diagnosis, severity of disease) in the study match those described in the decision problem of the review and those modelled? | Yes |
| Was a generic preference-based instrument (preferably EQ-5D) used to describe the health states? | Yes |
| Was the change in HRQoL taken directly from the patient population? | Yes |
| Was the valuation of changes in patients’ HRQL undertaken from the general (UK) population? | ? |
| Was the technique used to value the health states a choice-based method (such as TTO)? | Yes |
Reference
Conner-Spady, 2005. 130
Study Characteristics
Research question
What are the stated objectives of the study?
To examine changes in HRQoL in breast cancer patients with poor prognosis (stage II/III) receiving HDC treatment with autologous blood stem cell transplantation during long-term follow-up.
Describe the type of study and study design.
Prospective 2-year longitudinal study.
Was the sample from i) the general population, ii) patients with the disease of interest, iii) individuals with knowledge of the disease, iv) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. > 80 years)?
Patients with breast cancer with poor prognosis (stage II/III).
Yes. Inclusion/exclusion criteria were described clearly; consecutive patients aged between 18 and 65 years.
What are the characteristics of the baseline cohort for the evaluation?
| Age (years) | Mean age (range, SD): 44.7 (21–62, 8.5) Age distributionn%21–35 years611.536–50 years3261.551–62 years1426.9 |
Age distribution | n | % | 21–35 years | 6 | 11.5 | 36–50 years | 32 | 61.5 | 51–62 years | 14 | 26.9 | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age distribution | n | % | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21–35 years | 6 | 11.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36–50 years | 32 | 61.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 51–62 years | 14 | 26.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Not reported specifically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Race (if appropriate) | Not reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication/disease | Breast cancer patients with poor prognosis (stage II/III) who are at high risk of relapse | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other characteristics (sample size) | N = 52 VariablesCategorynPer centMarital statusSingle815.4Married/Partner4076.9Divorced23.8Widowed23.8Years of educationGrade 12 or less1835.3More than Grade 123364.7Stage of cancerII1834.6III3465.4Type of surgeryModified radical mastectomy2242.3Total mastectomy1936.5Segmental1121.2Nodal status10 or more3975.0TamoxifenYes510.0Menopausal statusPre3771.2Post1528.8 |
Variables | Category | n | Per cent | Marital status | Single | 8 | 15.4 | Married/Partner | 40 | 76.9 | Divorced | 2 | 3.8 | Widowed | 2 | 3.8 | Years of education | Grade 12 or less | 18 | 35.3 | More than Grade 12 | 33 | 64.7 | Stage of cancer | II | 18 | 34.6 | III | 34 | 65.4 | Type of surgery | Modified radical mastectomy | 22 | 42.3 | Total mastectomy | 19 | 36.5 | Segmental | 11 | 21.2 | Nodal status | 10 or more | 39 | 75.0 | Tamoxifen | Yes | 5 | 10.0 | Menopausal status | Pre | 37 | 71.2 | Post | 15 | 28.8 |
| Variables | Category | n | Per cent | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Marital status | Single | 8 | 15.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Married/Partner | 40 | 76.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Divorced | 2 | 3.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Widowed | 2 | 3.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Years of education | Grade 12 or less | 18 | 35.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| More than Grade 12 | 33 | 64.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stage of cancer | II | 18 | 34.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| III | 34 | 65.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of surgery | Modified radical mastectomy | 22 | 42.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total mastectomy | 19 | 36.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Segmental | 11 | 21.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nodal status | 10 or more | 39 | 75.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tamoxifen | Yes | 5 | 10.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Menopausal status | Pre | 37 | 71.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Post | 15 | 28.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| QoL instrument | EQ-5D | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Utility values, (yes/no) | Yes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment effect, if reported | Not reported |
Country/setting
What is the country and setting for the evaluation?
Canada; Phase II trial.
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
A prospective longitudinal study.
Results
Summarise the results.
-
Mean QoL scores across different time-points.
-
HRQoL decreased significantly from T1 to T3 but at T4, i.e. 8 weeks post HDC, it returned to baseline levels. Although in the short term there was a negative effect of treatment on HRQoL, it rebounded quickly.
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference-based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?)
Yes, EQ-5D questionnaire was used.
The valuation of health states was from a set of Canadian breast cancer patients group.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
Not applicable.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
The authors concluded that EQ 5D data indicated a decline in HRQoL following the administration of HDC but returned to baseline levels post HDC.
What are the implications of the study for the model?
The study did not report utility values for the health states that are relevant for the SHTAC’s cost-effectiveness model in development. However, as the patients included in the study had all undergone mastectomy/surgery, the utility value reported by EQ-5D at the end of 2 years (i.e. at time-point T7) valued at 0.89 could be used to represent the utility value for ‘mastectomy & reconstruction’ health state in the SHTAC’s cost-effectiveness model.
Criteria for assessment of study relevance to the National Institute for Health and Care Excellence’s reference case (adapted from Papaioannou et al.62)
| Relevance questions | Requirement for NICE |
|---|---|
| Do the population characteristics (e.g. age, sex, comorbidities, diagnosis, severity of disease) in the study match those described in the decision problem of the review and those modelled? | No |
| Was a generic preference-based instrument (preferably EQ-5D) used to describe the health states? | Yes |
| Was the change in HRQoL taken directly from the patient population? | Yes |
| Was the valuation of changes in patients’ HRQL undertaken from the general (UK) population? | Yesa |
| Was the technique used to value the health states a choice-based method (such as TTO)? | Yes |
Reference
Robertson, 2012. 131
Study characteristics
Research question
What are the stated objectives of the study?
To present an audit of all IBRs during the period 2005–8 performed by breast surgeons, including post-operative HRQoL.
Describe the type of study and study design.
Retrospective descriptive study.
Was the sample from i) the general population, ii) patients with the disease of interest, iii) individuals with knowledge of the disease, iv) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. > 80 years)?
Consecutive patients recruited between 2005 and 2008 who had undergone IBRs.
Inclusion and exclusion criteria were reported.
What are the characteristics of the baseline cohort for the evaluation?
| Age (years) | Mean age at IBR: 50 | |||||||||||||||||||
| Sex | Female 100% | |||||||||||||||||||
| Race (if appropriate) | Not reported | |||||||||||||||||||
| Indication/disease | IBR patients with implants | |||||||||||||||||||
| Other characteristics (sample size) | Sample size: 223 patients Indication for IBRMastectomy as first treatmentCompletion mastectomyIBTRTotal% (n)% (n)% (n)% (n)Patients62.8 (140)27.3 (61)9.9 (22)100 (223)IBTR, ipsilateral breast tumour recurrence. |
Indication for IBR | Mastectomy as first treatment | Completion mastectomy | IBTR | Total | % (n) | % (n) | % (n) | % (n) | Patients | 62.8 (140) | 27.3 (61) | 9.9 (22) | 100 (223) | IBTR, ipsilateral breast tumour recurrence. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication for IBR | Mastectomy as first treatment | Completion mastectomy | IBTR | Total | ||||||||||||||||
| % (n) | % (n) | % (n) | % (n) | |||||||||||||||||
| Patients | 62.8 (140) | 27.3 (61) | 9.9 (22) | 100 (223) | ||||||||||||||||
| IBTR, ipsilateral breast tumour recurrence. | ||||||||||||||||||||
| QoL instrument | EQ-5D | |||||||||||||||||||
| Utility values, (yes/no) | Yes | |||||||||||||||||||
| Treatment effect, if reported | Not reported | |||||||||||||||||||
Country/setting
What is the country and setting for the evaluation?
Sweden.
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
Single study.
Results
Summarise the results.
-
The calculated EQ-5D index for the patient population was 0.83.
-
EQ-5D questionnaire for patients’ current state of health at median of 4 years post operatively.
| Dimension | Severity level of problem | Missing | ||
|---|---|---|---|---|
| No problem | Moderate | Severe | ||
| % (n) | % (n) | % (n) | n | |
| Mobility | 86.6 (142) | 6.7 (11) | 0 (0) | 11 |
| Self-care | 92.7 (152) | 0.6 (1) | 0 (0) | 11 |
| Usual activities | 78 (128) | 13.4 (22) | 1.8 (3) | 11 |
| Pain/discomfort | 52.4 (86) | 37.8 (62) | 1.8 (3) | 13 |
| Anxiety/depression | 53.7 (88) | 37.8 (62) | 1.8 (3) | 11 |
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference-based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?)
Yes. EQ-5D was used to assess health status of the patients.
The valuation of health states was not from the UK general population; the study was based on Swedish population.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
Not applicable.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
The authors stated that although the rate of irradiated patients was high, patient-reported outcomes related to aesthetics of the breast reconstruction and items in day-to-day life were satisfactory. Furthermore, they observed that, compared with norm data, there was a high frequency of moderate problems associated with pain/discomfort and anxiety/depression at a median of 4 years following surgery, even though the general state of health was highly rated.
What are the implications of the study for the model?
The estimated EQ-5D score of 0.83 could be populated for the ‘mastectomy and reconstruction’ health state within the SHTAC’s cost-effectiveness model in development.
Criteria for assessment of study relevance to the National Institute for Health and Care Excellence’s reference case (adapted from Papaioannou et al.62)
| Relevance questions | Requirement for NICE |
|---|---|
| Do the population characteristics (e.g. age, sex, comorbidities, diagnosis, severity of disease) in the study match those described in the decision problem of the review and those modelled? | Yes |
| Was a generic preference-based instrument (preferably EQ-5D) used to describe the health states? | Yes |
| Was the change in HRQoL taken directly from the patient population? | Yes |
| Was the valuation of changes in patients’ HRQL undertaken from the general (UK) population? | No |
| Was the technique used to value the health states a choice-based method (such as TTO)? | Yes |
Reference
Lidgren, 2007. 132
Study characteristics
Research question
What are the stated objectives of the study?
To describe the HRQoL in different breast cancer disease states using preference-based measures.
Describe the type of study and study design.
Cross-sectional observational study.
Was the sample from i) the general population, ii) patients with the disease of interest, iii) individuals with knowledge of the disease, iv) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. > 80 years)?
Women with a previous diagnosis of breast cancer.
The inclusion criteria are reported, but exclusion criteria are not.
What are the characteristics of the baseline cohort for the evaluation?
| Age (years) | Mean age (range): 57 (28–93) Age distributionFrequencyPercentage< 50 years912650–64 years17852≥ 65 years7622Total345100 |
Age distribution | Frequency | Percentage | < 50 years | 91 | 26 | 50–64 years | 178 | 52 | ≥ 65 years | 76 | 22 | Total | 345 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age distribution | Frequency | Percentage | ||||||||||||||
| < 50 years | 91 | 26 | ||||||||||||||
| 50–64 years | 178 | 52 | ||||||||||||||
| ≥ 65 years | 76 | 22 | ||||||||||||||
| Total | 345 | 100 | ||||||||||||||
| Sex | Female, 100% | |||||||||||||||
| Race (if appropriate) | Not reported | |||||||||||||||
| Indication/disease | Women with a previous diagnosis of breast cancer | |||||||||||||||
| Other characteristics (sample size) | n = 361; n = 345 after exclusions | |||||||||||||||
| QoL instrument | EQ-5D | |||||||||||||||
| Utility values, (yes/no) | Yes | |||||||||||||||
| Treatment effect, if reported | Not reported |
Country/setting
What is the country and setting for the evaluation?
Sweden, breast cancer outpatient clinic.
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
A cross-sectional observational study.
Results
Summarise the results.
| State | n | % | Mean EQ-5D score | 95% CI |
|---|---|---|---|---|
| State P (patients in their first year after a primary breast cancer) | 72 | 21 | 0.696a | 0.634 to 0.747 |
| State R (patients in their first year after a recurrence) | 21 | 6 | 0.779 | 0.700 to 0.849 |
| State S (patients who had not had a primary breast cancer diagnosis or a recurrence during the previous year) | 177 | 53 | 0.779 | 0.745 to 0.811 |
| State M (patients with metastatic disease) | 65 | 19 | 0.685a | 0.620 to 0.735 |
The main driver behind the reduction in HRQoL was pain and discomfort as well as anxiety and depression.
EQ-5D dimensions (no problems, moderate problems and severe problems) were reported but no data were extracted.
| State | n | Mean EQ-5D score | 95% CI |
|---|---|---|---|
| Patients in state P receiving adjuvant chemotherapy | 23 | 0.620 | 0.509 to 0.697 |
| Patients in state P receiving hormone therapy | 17 | 0.744 | 0.573 to 0.841 |
| Patients in state R receiving adjuvant chemotherapy | 7 | 0.767 | 0.573 to 0.841 |
| Patients in state R receiving adjuvant hormone therapy | 4 | 0.816 | 0.729 to 0.963 |
| Patients in state S receiving adjuvant hormone therapy | 79 | 0.824 | 0.785 to 0.857 |
| Patients in state M receiving hormone therapy | 16 | 0.648 | 0.513 to 0.765 |
| Patients in state M receiving chemotherapy | 38 | 0.692 | 0.611 to 0.746 |
| Metastatic patients who had at least one new distant recurrences more than 1 month after their first distant recurrence | 10 | 0.661 | 0.454 to 0.812 |
| Metastatic patients who did not have a new distant recurrences more than 1 month after their first distant recurrence | 55 | 0.690 | 0.630 to 0.753 |
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference-based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?)
Yes. EQ-5D data were presented clearly. The valuation was based on Swedish patients.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
Not applicable.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
The authors found that there was an association between breast cancer and decline in HRQoL. This relationship was most evident in patients with metastatic disease.
What are the implications of the study for the model?
-
If UK-based data are not available: the utility value of 0.685 as derived for the patients with metastases could be used to inform the SHTAC’s cost-effectiveness model for the health state of distant recurrence, although the data are derived from Swedish patients. In addition, the value of 0.779 could be used to populate the utility value for health state ‘disease free after local recurrence’.
-
If UK-based data are available: the above values could be used for conducting sensitivity analysis.
Criteria for assessment of study relevance to the National Institute for Health and Care Excellence’s reference case (adapted from Papaioannou et al.62)
| Relevance questions | Requirement for NICE |
|---|---|
| Do the population characteristics (e.g. age, sex, comorbidities, diagnosis, severity of disease) in the study match those described in the decision problem of the review and those modelled? | Yes |
| Was a generic preference-based instrument (preferably EQ-5D) used to describe the health states? | Yes |
| Was the change in HRQoL taken directly from the patient population? | Yes |
| Was the valuation of changes in patients’ HRQL undertaken from the general (UK) population? | Yes; the study used UK EQ-5D index tariff |
| Was the technique used to value the health states a choice-based method (such as TTO)? | Yes |
Reference
Sherrill, 2008. 133
Study characteristics
Research question
What are the stated objectives of the study?
To examine whether or not patients receiving combination therapy of lapatinib + capecitabine would experience, on average, more time in a better health state compared with patients on capecitabine alone.
Describe the type of study and study design.
Randomised controlled trial; Q-TWiST analysis.
Was the sample from i) the general population, ii) patients with the disease of interest, iii) individuals with knowledge of the disease, iv) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. > 80 years)?
What are the characteristics of the baseline cohort for the evaluation?
| Age | Not reported | |||||||||||||||||||||||||
| Sex | Female, 100% | |||||||||||||||||||||||||
| Race (if appropriate) | Not reported | |||||||||||||||||||||||||
| Indication/disease | Advanced or metastatic HER-2+ breast cancer who had progressive disease following prior therapy | |||||||||||||||||||||||||
| Other characteristics (sample size) | N = 399Lapatinib + capecitabine armCapecitabine armn198201Patients characteristicsPrior therapyAnthracycline97%Taxane97%Trastuzumab97%Patients with metastatic disease96%Patients with visceral lesions78%Patients with visceral at three or more sites49% | Lapatinib + capecitabine arm | Capecitabine arm | n | 198 | 201 | Patients characteristics | Prior therapy | Anthracycline | 97% | Taxane | 97% | Trastuzumab | 97% | Patients with metastatic disease | 96% | Patients with visceral lesions | 78% | Patients with visceral at three or more sites | 49% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lapatinib + capecitabine arm | Capecitabine arm | |||||||||||||||||||||||||
| n | 198 | 201 | ||||||||||||||||||||||||
| Patients characteristics | ||||||||||||||||||||||||||
| Prior therapy | Anthracycline | 97% | ||||||||||||||||||||||||
| Taxane | 97% | |||||||||||||||||||||||||
| Trastuzumab | 97% | |||||||||||||||||||||||||
| Patients with metastatic disease | 96% | |||||||||||||||||||||||||
| Patients with visceral lesions | 78% | |||||||||||||||||||||||||
| Patients with visceral at three or more sites | 49% | |||||||||||||||||||||||||
| QoL instrument | EQ-5D | |||||||||||||||||||||||||
| Utility values, (yes/no) | Yes | |||||||||||||||||||||||||
| Treatment effect, if reported | Not reported | |||||||||||||||||||||||||
Country/setting
What is the country and setting for the evaluation?
UK and the USA; Phase III RCT.
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
Single study; patient-reported utility weights were derived from the EQ-5D using published algorithms. 165
Results
Summarise the results.
Average utility values by health state, based on EQ-5D scores.
| Health state ITT population | Lapatinib plus capecitabine | Capecitabine monotherapy |
|---|---|---|
| Toxicity:a Grade 3/4 | 0.60 (n = 27) | 0.59 (n = 17) |
| TWiST | 0.66 (n = 168) | 0.66 (n = 157) |
| Relapseb | 0.41 (n = 50) | 0.44 (n = 67) |
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference-based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?).
Yes, EQ-5D questionnaire was used.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
Not applicable.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
The authors concluded that Q-TWiST was greater in patients receiving the combination of lapatinib and capecitabine compared with those receiving capecitabine alone. Although the full impact of the combination therapy could not be assessed owing to the early closure to accrual and subsequent crossover, the authors envisaged that the average 7 weeks improvement underestimated the overall benefits.
What are the implications of the study for the model.
The utility value for the ‘relapse’ health state could be used to inform the ‘distant recurrence’ health state in the cost-effectiveness model.
Criteria for assessment of study relevance to the National Institute for Health and Care Excellence’s reference case (adapted from Papaioannou et al.62)
| Relevance questions | Requirement for NICE |
|---|---|
| Do the population characteristics (e.g. age, sex, comorbidities, diagnosis, severity of disease) in the study match those described in the decision problem of the review and those modelled? | Yes (for one of the health states of the model) |
| Was a generic preference-based instrument (preferably EQ-5D) used to describe the health states? | Yes |
| Was the change in HRQoL taken directly from the patient population? | Yes |
| Was the valuation of changes in patients’ HRQL undertaken from the general (UK) population? | Unclear |
| Was the technique used to value the health states a choice-based method (such as TTO)? | No |
Reference
Hildebrandt, 2014. 134
Study characteristics
Research question
What are the stated objectives of the study?
To investigate health utilities as cardinal values of the individual’s preferences for specific health-related outcomes in women treated in Germany in the fields of gynaecological oncology and mastology in order to provide local data from Germany.
Describe the type of study and study design.
Cross-sectional survey from May 2009 to December 2009.
Was the sample from i) the general population, ii) patients with the disease of interest, iii) individuals with knowledge of the disease , iv) other?
Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that may be relevant (e.g. aged > 80 years)?
The sample included patients (both men and women) who were affected by breast, cervical, endometrium, ovarian and other gynaecological cancer as well as healthy individuals.
Limited information was provided; relevant individuals do not appear to be excluded.
What are the characteristics of the baseline cohort for the evaluation?
| Age (years) | All patients with diseaseMedian age (years)59.07Range (years)20.12–83.33 | All patients with disease | Median age (years) | 59.07 | Range (years) | 20.12–83.33 | |
|---|---|---|---|---|---|---|---|
| All patients with disease | |||||||
| Median age (years) | 59.07 | ||||||
| Range (years) | 20.12–83.33 | ||||||
| Sex | Female, 99.4%; male, 0.6% | ||||||
| Race (if appropriate) | Not reported | ||||||
| Indication/disease | Patients with breast, ovarian, endometrial, cervical and other gynaecological cancer. | ||||||
| Other characteristics (sample size) | Number taking part in the survey: n = 655 (including 63 healthy controls) | ||||||
| Number with disease: n = 592 | |||||||
| Number of patients with breast cancer: n = 497 (including three men) | |||||||
| QoL instrument | EQ-5D | ||||||
| Utility values, (yes/no) | Yes | ||||||
| Treatment effect, if reported | Not reported |
Country/setting
What is the country and setting for the evaluation?
Germany; surgical and conservative oncological wards, specialist outpatient department for breast diseases and outpatient gynaecological oncology department.
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
Single study.
Results
Summarise the results
| Breast cancer | n | Minimum | Maximum | Median |
|---|---|---|---|---|
| Overall | 442 | 0.063 | 1.000 | 0.887 |
| Primary disease | 312 | 0.262 | 1.000 | 0.887 |
| Metastatic disease | 80 | 0.063 | 1.000 | 0.887 |
| Recurrent disease | 21 | 0.175 | 1.000 | 0.887 |
| Both | 29 | 0.788 | 1.000 | 0.887 |
Were the methods for deriving these data adequately described (give sources if using data from other published studies)? (Was a valid preference-based instrument used to describe health states, such as EQ-5D? Was the valuation of health states from the UK general population?).
EQ-5D valuation from German population.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
Not applicable.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
Patients with breast cancer who had primary disease had the highest estimates of QoL as measured by EQ-5D VAS and these declined in case the disease was already advanced. However, this difference was not evident from the EQ-5D health index in patients with primary, metastatic, recurrent, or both which had a consistent median value of 0.8870.
What are the implications of the study for the model?
The study could be used as a reference point for assuming similar utility values for ‘recurrence’ and ‘metastatic’ possible health states within the independent model.
Criteria for assessment of study relevance to the National Institute for Health and Care Excellence’s reference case (adapted from Papaioannou et al.62)
| Relevance questions | Requirement for NICE |
|---|---|
| Do the population characteristics (e.g. age, sex, comorbidities, diagnosis, severity of disease) in the study match those described in the decision problem of the review and those modelled? | Yes |
| Was a generic preference-based instrument (preferably EQ-5D) used to describe the health states? | Yes |
| Was the change in HRQoL taken directly from the patient population? | Yes |
| Was the valuation of changes in patients’ HRQL undertaken from the general (UK) population? | No |
| Was the technique used to value the health states a choice-based method (such as TTO)? | Yes |
Appendix 9 Critical appraisal checklist for health-related quality-of-life studies
| Criteria adapted from references59–62 | Issues to consider | Studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Turnbull et al.126 | Freedman et al.127 | Prescott et al.128 | Serra et al.129 | Conner-Spady et al.130 | Robertson et al.131 | Lidgren et al.132 | Sherrill et al.133 | Hildebrandt et al.134 | ||
| Conceptual | ||||||||||
| Study objectives | Were the objectives of the study clearly stated? HRQoL primary or secondary outcome? | Yes, secondary outcome | Yes, primary outcome | Yes, primary outcome | Yes, primary outcome | Yes, primary outcome | Yes, primary outcome | Yes, primary outcome | Yes, secondary outcome | Yes, primary outcome |
| HRQoL instrument | Was a reason provided to justify the HRQoL instrument selected? Was a validated tool used to assess QoL? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Methodology | ||||||||||
| Study design | Was the design of the study clearly described? (e.g. cohort, cross-sectional, survey) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes, RCT was described elsewhere | Yes |
| Respondent selection and recruitment | Was the sampling method for recruitment of participants adequately described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Inclusion/exclusion criteria | Are inclusion/exclusion criteria clearly described? Do these exclude any individuals that might be relevant (e.g. very elderly aged > 80 years old)? | Yes, eligibility criteria were described; no, relevant patient population was included | Yes, eligibility criteria were described; no, relevant patient population was included | Yes; the study did not include patients aged < 65 years | No, limited details were provided; it is unclear if the study excluded any individuals that might be relevant | Yes; the study did not include those aged > 65 years | No; it is unclear if the study excluded any relevant individuals | Yes; no, relevant patient population was included | Yes, reference provided; no, relevant patient population was included | No, limited information was provided but it could be assumed that no relevant groups were excluded |
| Participant characteristics | Were characteristics of participants clearly described (demographics and clinical variables)? | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes, reference provided | No |
| Sample size | Was the sample size used appropriately justified? | Yes | No, but the sample size was adequately large | Unclear. The sample size for the randomisation and that for the cost-effectiveness model were different | Yes | No | No | No | No, trial was stopped early before sample size reached | No |
| Instrument administration | Is it reported who and/or in which clinical setting the instrument was administered? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Timing of assessments | Is the timing of assessments reported? (e.g. baseline and/or at follow-up or after treatment) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Results | ||||||||||
| Response rates to instrument used | Are response rates reported and if so, are the rates likely to be a threat to validity? | Yes, response rates were reported; no, the rates are not likely to threaten the validity of results | Yes, response rates were reported; there was low response rates from women with recurrence compared with those without recurrence | Yes, response rates were reported; no, the rates are not likely to threaten the validity of results | Yes, the response rates were reported; no, the rates are not likely to threaten the validity of results | Yes, response rates were reported;no, the rates are not likely to threaten the validity of results | Yes, response rates were reported; no, the rates are not likely to threaten the validity of results | Yes, response rates were reported; no, the rates are not likely to threaten the validity of results | No, the response rates were not reported; unclear, possibly the rates could threaten the validity of the results | No, the response rates were not reported; NA |
| Loss to follow-up | Is the loss to follow-up reported and are reasons given? Are these likely to threaten the validity of results (e.g. characteristics of non-responders different to responders)? | Yes, loss to follow-up was reported; no, they are not likely to threaten validity of results | No, loss to follow-up was not reported; it is not clear if these were likely to threaten the validity of the results | Yes, loss to follow-up was reported; no, they are not likely to threaten validity of results | No, loss to follow-up was not reported; it is not clear if these were likely to threaten the validity of the results | Yes, loss to follow-up was reported; no, they are not likely to threaten validity of results | No, loss to follow-up was not reported; it is not clear if these were likely to threaten the validity of the results | NA; it is not clear | Yes, loss to follow-up was reported; no, they are not likely to threaten validity of results | No, loss to follow-up was not reported; it is not clear |
| Missing data | Are the levels of missing data reported? How are they dealt with? Could this threaten the validity of results? | Yes, missing data were reported; no, they are not likely to threaten the validity of the results | No, missing data were not reported; it is not clear if these were likely to threaten the validity of the results | Yes, missing data were reported; no, they are not likely to threaten the validity of the results | Mixed-model regression and generalised linear modelling allowed for the inclusion of patients with missing data over time on the assumption that the data were missing at random | Yes, missing data were reported; not clear; however, subset of 27 patients with complete data showed similar results | Yes, missing data were reported; it is not clear if these were likely to threaten the validity of the results | Yes, missing data were reported; it is not clear if these were likely to threaten the validity of the results | Yes, missing data were reported; it is not clear if these were likely to threaten the validity of the results | No, missing data were not reported; it is not clear if these were likely to threaten the validity of the results |
| Statistical analysis | Were appropriate statistical methods used? | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Only descriptive statistics was presented |
| Interpretation | ||||||||||
| Study findings | Were the key findings of the study clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Study limitations | Were limitations of the study clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Other | Relevance of location (e.g. patients not recruited in the UK) | Yes | This study was not UK based | Yes | Unclear, the study was based on US population | Unclear, the study was based on Canadian population | Unclear, this study was not UK based | Unclear, the study was based on Swedish population | Unclear, it was assumed centres were in the USA and the UK | Unclear, the study was based on German population |
Appendix 10 Complete set of results from deterministic sensitivity analysis, intraoperative radiation therapy compared with whole-breast external beam radiotherapy
Willingness to pay has been set to £20,000 per QALY.
| Variable description | Low value | High value | Low value incremental NMB (£) | High value incremental NMB (£) | Range (£) |
|---|---|---|---|---|---|
| 5-year probability of any other recurrence INTRABEAM | 0.029 | 0.071 | 5781 | –9171 | 14,952 |
| 5-year probability of any other recurrence WB-EBRT | 0.028 | 0.071 | –8760 | 5977 | 14,737 |
| Beta coefficient for INTRABEAM arm time to local recurrence (log-normal) | –0.815 | 0.307 | –4512 | 118 | 4630 |
| 5-year probability of death from breast cancer WB-EBRT | 0.014 | 0.045 | –4150 | –346 | 3804 |
| 5-year probability of death from breast cancer INTRABEAM | 0.016 | 0.055 | 1051 | –2518 | 3569 |
| Constant (time to local recurrence) (log-normal) | 3.553 | 6.383 | –3367 | –836 | 2531 |
| Discount rate for utilities (%) | 0 | 6 | –3192 | –1042 | 2150 |
| Number of WB-EBRT deliveries required to complete a course of treatment | 5 | 23 | –2604 | –832 | 1772 |
| Starting age of model cohort | 55 | 72 | –2273 | –757 | 1516 |
| Cost of delivering one-fraction WB-EBRT | 71 | 178 | –2211 | –877 | 1334 |
| Proportion of incident cases which are early breast cancer and suitable for INTRABEAM | 0.1 | 0.5 | –2064 | –1128 | 936 |
| Sigma (time to local recurrence) (log-normal) | 0.072 | 0.797 | –1110 | –2018 | 908 |
| WB-EBRT planning cost | 90 | 704 | –1813 | –1303 | 510 |
| Lifetime of INTRABEAM equipment (years) | 5 | 10 | –1973 | –1619 | 354 |
| Population served by one INTRABEAM device | 800,004 | 1,200,000 | –1800 | –1498 | 302 |
| Probability of any other recurrence given local recurrence | 0.362 | 0.471 | –1474 | –1764 | 290 |
| Proportion of patients requiring radiation shield | 0.25 | 1 | –1463 | –1619 | 156 |
| Cost of 1 hour in operating room | 461 | 688 | –1549 | –1696 | 147 |
| Utility recurrence-free subsequent years | 0.8 | 0.83 | –1658 | –1555 | 103 |
| Additional time required in theatre while delivering INTRABEAM | 26.4 | 33 | –1540 | –1619 | 79 |
| Discount rate for costs (%) | 0 | 6 | –1583 | –1658 | 75 |
| Prop of INTRABEAM who also received WB-EBRT | 0.135 | 0.17 | –1583 | –1657 | 74 |
| Utility associated with other recurrence state | 0.63 | 0.74 | –1592 | –1647 | 55 |
| Cost of staff time in theatre per hour of delivery time | 122 | 182 | –1603 | –1636 | 33 |
| Additional time required in theatre while planning INTRABEAM | 4.8 | 7.2 | –1603 | –1635 | 32 |
| Staff time required in supporting delivery of each INTRABEAM dose | 61 | 92 | –1604 | –1635 | 31 |
| Prop of INTRABEAM patients having mastectomy at local recurrence | 0.618 | 0.933 | –1611 | –1625 | 14 |
| Cost of staff time in theatre per hour of planning time | 203 | 303 | –1614 | –1624 | 10 |
| Cost of WLE | 1248 | 1866 | –1614 | –1624 | 10 |
| Cost of independent technical commissioning and calibration per year | 2062 | 3080 | –1615 | –1623 | 8 |
| Cost of mastectomy and reconstruction | 6362 | 9431 | –1617 | –1621 | 4 |
| Initial set up costs of INTRABEAM | 4847 | 7239 | –1618 | –1620 | 2 |
| Cost of mastectomy alone | 2122 | 2931 | –1619 | –1621 | 2 |
| Cost of annual radiation protection refresher training for theatre staff | 745 | 1113 | –1618 | –1620 | 2 |
| Cost of pre-treatment quality control checks | 20 | 31 | –1619 | –1619 | 0 |
| Proportion having reconstruction after mastectomy | 0.304 | 0.318 | –1620 | –1620 | 0 |
| Utility recurrence free first year after WLE + radiotherapy | 0.76 | 0.79 | –1619 | –1619 | 0 |
List of abbreviations
- AG
- assessment group
- AIC
- Akaike information criterion
- ANOVA
- analysis of variance
- APBI
- accelerated partial breast irradiation
- BCS
- breast-conserving surgery
- BIOSIS
- Bioscience Information Service
- CCT
- controlled clinical trial
- CDSR
- Cochrane Database of Systematic Reviews
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COMICE
- comparative effectiveness of MRI in breast cancer trial
- CONSORT
- Consolidated Standards of Reporting Trials
- CPCI
- Conference Proceedings Citation Index
- CRD
- Centre for Reviews and Dissemination
- DARE
- Database of Abstracts of Reviews of Effectiveness
- DCIS
- ductal carcinoma in situ
- DNA
- deoxyribonucleic acid
- DSA
- deterministic sensitivity analysis
- ELIOT
- Electron Intraoperative Radiotherapy trial
- EORTC
- European Organisation for Research and Treatment of Cancer
- EQ-5D
- European Quality of Life-5 Dimensions
- ER
- oestrogen receptor
- GP
- general practitioner
- HDC
- high-dose chemotherapy
- HER-2
- human epidermal growth factor receptor-2
- HER-2+
- human epidermal growth factor receptor-2 positive
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IBR
- immediate breast reconstruction
- ICER
- incremental cost-effectiveness ratio
- IORT
- intraoperative radiation therapy
- ISC
- International Steering Committee
- ITT
- intention to treat
- LRR
- local recurrence rate
- MRI
- magnetic resonance imaging
- MS
- manufacturer’s submission
- NCRI
- National Cancer Research Institute
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NPI
- Nottingham Prognostic Index
- ONS
- Office for National Statistics
- OSNA
- one-step nucleic acid amplification
- PERT
- project evaluation and review techniques
- PgR
- progesterone receptor
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- Q-TWiST
- quality-adjusted time without symptoms of disease or toxicity of treatment
- QALY
- quality-adjusted life-year
- QLQ-BR23
- QoL questionnaire – Breast Cancer Module
- QLQ-C30
- QoL questionnaire – C30
- QoL
- quality of life
- RCT
- randomised controlled trial
- RTOG
- Radiation Therapy Oncology Group
- SCIE
- Science Citation Index Expanded
- SD
- standard deviation
- SE
- standard error
- SHTAC
- Southampton Health Technology Assessments Centre
- SLNB
- sentinel lymph node biopsy
- TARGIT
- TARGeted Intraoperative radioTherapy trial
- TARGIT-A
- TARGeted Intraoperative radioTherapy Alone trial
- TNM
- tumour node metastasis
- TTO
- time trade-off
- UCL
- University College London
- VAS
- visual analogue scale
- WB-EBRT
- whole-breast external beam radiotherapy
- WHO ICTRP
- World Health Organization International Clinical Trials Registry Platform
- WLE
- wide local excision
- WTP
- willingness to pay
- XRS
- X-ray source
