Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/37/16. The contractual start date was in September 2009. The draft report began editorial review in April 2014 and was accepted for publication in November 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Barbara Gregson and A David Mendelow received a grant from the Medical Research Council and Efficacy and Mechanism Evaluation Programme to undertake Surgical Trial In spontaneous lobar intraCerebral Haemorrhage (STICH II). A David Mendelow also reports grants from Newcastle Neurosurgery Foundation Ltd for sponsorship of meetings outwith this study and personal fees from Stryker Ltd for consultancy work. Elaine McColl is a member of the National Institute for Health Research Journals Library Editorial Group, but had no role in editorial decisions concerning this publication. Newcastle University is reimbursed for the time devoted to editorial work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Gregson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Much of this text is reproduced from Gregson BA, Rowan EN, Mitchell PM, Unterberg A, McColl EM, Chambers IR, et al. Surgical trial in traumatic intracerebral hemorrhage (STITCH(Trauma)): study protocol for a randomized controlled trial. Trials 2012;13:193. © 2012 Gregson et al. ; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
In the UK there are 1.4 million presentations of head injury at emergency departments each year. 1 The mortality rate for severe isolated traumatic brain injury varies between 16% and 40%. 2 More than 150,000 of those who present to emergency departments with head injury are admitted to hospital each year. Of these, about 20,000 injuries are serious. One year after a serious head injury, 35% of patients are dead or severely disabled. Intracranial haemorrhage occurs in more than 60% of serious head injuries in one or more of three types: extradural haematomas (EDH), subdural haematomas (SDH) and intracerebral haemorrhage (ICH). Prompt surgical removal of significant SDH and EDH is of established and widely accepted value. Intraparenchymal haemorrhage is more common than both the other types put together and is found in more than 40% of severe head injuries. It is clearly associated with a worse outcome, but the role of surgical removal of the clot remains undefined. Several terms are used to describe the condition, including traumatic intracerebral haemorrhage (TICH), traumatic intraparenchymal haemorrhage and contusion. Prospectively collected data in over 7000 head-injured patients in Newcastle upon Tyne showed that contusions are more common in older head-injured patients and occurred in patients with less severe injury.
Surgical practice in the treatment of TICH differs widely. Several issues inform the debate:
-
Contused brain does not recover but appears as encephalomalacic brain tissue loss on convalescent phase imaging. This suggests that removing TICH does not increase tissue loss.
-
Extravasated blood is believed to be neurotoxic, leading to secondary injury that may be avoided by surgical removal.
-
Larger TICHs may be associated with an ischaemic penumbra of brain tissue that could be salvaged.
-
Some TICHs expand to the point at which they cause mass effect with high intracranial pressure (ICP), resulting in secondary brain injury.
The aim of early surgical TICH removal is to prevent secondary brain injury from these mechanisms. A detailed description of the pathophysiology of ICH was published in 1993. 3 This information translated into the initial hypothesis that led to the Surgical Trial In spontaneous intraCerebral Haemorrhage (STICH), which began in 1993. 4 This was the justification for a trial of surgical intervention. However, use of the operation varies around the world. It is more frequently done in the Far East than in Europe or the USA.
Traditional neurosurgical care of patients with severe head injury is frequently based on ICP measurement. Patients with high ICP (> 30 mmHg) and TICH would undergo craniotomy and those with low ICP (< 20 mmHg) would be managed conservatively. Patients with ICP between 20 and 30 mmHg would be observed with ongoing ICP monitoring and undergo craniotomy if the ICP rises. 5 This ICP-based approach has been recommended by the Brain Trauma Foundation6 but its authority has recently been challenged in the Trial of Intracranial Pressure Monitoring in Traumatic Brain Injury (BEST TRIP) published by Chesnut et al. 7 from Latin America. In the light of this controversy, the early management of patients with TICH needs evaluation to determine if Early Surgery should become part of the standard of care in the same way it is for significant EDH8 and SDH. 9
There have been trials of surgery for spontaneous ICH (SICH),4,10 but none so far of surgery for TICH. The Cochrane Review (2nd edition) has shown benefit from surgical evacuation for SICH. 11 There are differences in the pathogenesis, clinical behaviour and outcome of the two conditions (SICH and TICH). 12 Patients suffering a TICH tend to be younger, by about 15 years on average, than patients suffering SICH and, therefore, the level of disability may have a larger effect on their ability to return to work and their economic output. TICHs are more likely to be lobar, to be superficial and to have a medium-sized volume (25–65 cc). These differences between the conditions mean that we cannot derive the role of surgery for TICH from results of the 15 published trials of surgery for spontaneous ICH; however, the STICH trials4,10 showed a trend towards better outcome with surgery for the group of spontaneous supratentorial ICH that are most like TICH: superficial haematomas with no intraventricular bleed.
We already know that surgery is effective in patients with traumatic EDH and SDH and that Early Surgery produces more favourable outcomes than delayed surgery. This is not known for TICH. If Early Surgery is of benefit to these patients, then implementation of early referral and diagnosis with immediate treatment may reduce death and disability in this specific group of head-injured patients.
Several authors13–15 have compared surgery with conservative treatment in single-centre retrospective series and recommended surgery for larger TICHs even if patients were in an apparently good clinical state initially. Matheisen et al. 13 found that patients with an admission Glasgow Coma Score (GCS) of at least 6 and a lesion volume of at least 20 ml who had surgery without previous neurological deterioration had significantly better outcomes than those who did not have surgery or had surgery after deterioration. None of the patients who had surgery before any deterioration died or was vegetative, as opposed to 39% of those who had surgery after deterioration and 50% of those who did not have surgery. Choksey et al. 14 found that 38% of patients with a low GCS and a volume of the TICH > 16 ml who had surgery had a poor outcome, compared with 56% of those who did not have surgery. Zumkeller et al. 15 found that the poor-outcome rate in the operated patients was 29%, compared with 59% in the non-operated group.
Boto et al. 16 evaluated the characteristics of severely head-injured patients with basal ganglia TICHs and found that the TICH tended to enlarge in the acute post-traumatic period. They found that patients with a TICH of > 25 ml and those in whom TICH enlargement or raised ICP had occurred had the worst outcomes. They suggested that these patients might benefit from more aggressive surgical treatment.
D’Avella et al. 17 published a series and suggested that non-comatose patients with smaller TICHs may be treated conservatively but that surgery is indicated for patients with larger TICHs. Most of their comatose patients who were severely injured had a poor outcome whatever treatment was used.
None of these studies involved randomisation into surgical and non-surgical groups. They also differed in the characteristics of the parenchymal blood. Such uncontrolled observational studies may be potentially misleading and a randomised controlled trial was needed.
Guidelines for the surgical management of traumatic brain injury were published in 2006. 18 These confirmed that studies in this area have been observational and that there is a lack of evidence from well-designed randomised controlled trials. Those studies that attempt to compare outcome between surgical and non-surgical groups cannot adequately control for known prognostic variables.
The National Institute for Health and Care Excellence (NICE) recommended in the Clinical Guidelines CG56 Head Injury: Triage, Assessment, Investigation and Early Management of Head Injury in Infants, Children and Adults19 that research is needed to develop a consensus on criteria for lesions not currently considered to be surgically significant, namely TICH. This trial, Surgical Trial in Traumatic intraCerebral Haemorrhage [STITCH(TRAUMA)], was recommended by that NICE Head Injury Guideline Development Group.
Chapter 2 Methods
Much of this text is reproduced from Gregson BA, Rowan EN, Mitchell PM, Unterberg A, McColl EM, Chambers IR, et al. Surgical trial in traumatic intracerebral hemorrhage (STITCH(Trauma)): study protocol for a randomized controlled trial. Trials 2012;13:193. © 2012 Gregson et al. ; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Trial objectives
The principal aim of the trial was to determine whether or not a policy of Early Surgery in patients with TICH improves outcome compared with a policy of Initial Conservative Treatment.
In addition, we wanted to assess the relative costs and consequences of Early Surgery versus Initial Conservative Treatment in UK patients and those in a subgroup of countries covering the likely highest-recruiting centres. (Following the trial being halted by the funding agency this objective was changed and reference to a UK-specific analysis removed.)
Finally, we hoped to confirm appropriate thresholds for ICP and cerebral perfusion pressure (CPP) for clinical management in the subgroup of head-injured patients with TICH who undergo such monitoring. (Following the trial being halted by the funding agency this objective was changed to: to investigate the use of ICP monitoring for clinical management of head-injured patients with TICH and its effect on treatment decisions.)
Trial design
STITCH(TRAUMA) was an international, multicentre, pragmatic, patient-randomised, parallel-group trial comparing early surgical evacuation of TICH with Initial Conservative Treatment. Only patients for whom the treating neurosurgeon was in equipoise about the benefits of early surgical evacuation compared with Initial Conservative Treatment were eligible for the trial. An independent 24-hour telephone randomisation service based in the Aberdeen Clinical Trials Unit was used and backed by 24-hour availability of trial investigators who could advise on patient eligibility. Minimisation with a random component was used to ensure that the two groups were balanced within country, age and severity. Outcome was measured at 6 and 12 months via a postal questionnaire using the Extended Glasgow Outcome Scale (GOSE).
Additional data were collected in those centres that practised invasive brain monitoring to see if there was evidence that such monitoring techniques added value to clinical decision-making. The aim was to obtain an unbiased assessment of the effect of clot removal or not on ICP/CPP and to evaluate if monitoring ICP/CPP provided additional information that informed improved clinical management (the third objective). Such monitoring was not mandatory for a patient to be enrolled in the trial.
It was planned that relevant health-care costs be assessed in the UK, including length of hospital stay and the costs associated with surgical treatment (theatre time, consumables, overheads); health-care resource use outside hospital (e.g. district nurse, physiotherapy) together with productivity costs arising from absence from work; and additional costs for family members through extra caring responsibilities. Consequences were to be measured by combining data on quality of life, measured using the European Quality of Life-5 Dimensions (EQ-5D), with survival to generate quality-adjusted life-years (QALYs). Existing questionnaires were adapted for use with TICH patients where appropriate, and they formed an additional 3-month postal questionnaire and part of the 6-month and 12-month postal questionnaires for patients.
The trial protocol was published in Trials. 20
Pilot study
An internal pilot phase21 was conducted with criteria for stopping the trial early if the recruitment of centres and/or patients was slower than projected or if unexpected difficulties arose in signing up collaborating centres.
The target was not fewer than 12 centres signed up at the end of year 1. If this target was met, then the pilot was to continue until a second point when the trial had a total of 12 recruiting centre-years. If at this point the average recruitment rate was less than 2 per centre-year, the trial was to be terminated.
Screening logs
Screening logs were to be maintained by each centre to record the patients admitted to the neurosurgical unit with any traumatic ICH; whether or not they were eligible for the trial and whether or not they were recruited (and, if not, why not, if the reason could be ascertained). These were to be used to provide a context for the study, to monitor recruitment rates and as the basis for constructing the Consolidated Standards of Reporting Trials (CONSORT) diagram for reporting the trial.
Centre recruitment
Suitable centres were recruited from those already collaborating successfully with the team in other studies [STICH, Surgical Trial In spontaneous lobar intraCerebral Haemorrhage (STICH II), RESCUEicp] plus those identified by the various networks: TARN (Trauma Audit and Research Network), EBIC (European Brain Injury Consortium) and EMN (Euroacademia Multidisciplinaria Neurotraumatologica), BrainIT, EANS (European Association of Neurosurgical Societies), GNAMED (Glasgow, Newcastle, Aberdeen, Middlesbrough, Edinburgh, Dundee; Scottish and Newcastle Neurosurgery Research Group), SBNS (Society of British Neurological Surgeons), BNRG (British Neurosurgery Research Group), ICRAN (International Conference on Recent Advances in Neurotraumatology) and WFNS (World Federation of Neurosurgical Societies) Neurotrauma Committee.
Centres were required to demonstrate effective trial experience and previous adherence to trial guidelines with high follow-up rates. In order to be eligible, centres should have been able to recruit a minimum of one patient per year. They had to be able to communicate with the research team. (At least one member of the local team had to be proficient in English and provide contact details where they could be reached easily to support the local centre and respond to the trial management team in Newcastle.) They had to be able to provide computed tomography (CT) images of sufficient quality to the study centre in Newcastle and be able to arrange follow-up for patients with limited literacy.
Each centre was required to obtain ethics approval and other permissions as needed to conform with local and national legislation and governance frameworks and to provide documentary evidence to the trial management team that these permissions were in place, prior to site registration and initiation. Each site was also required to sign an agreement with the sponsor (Newcastle upon Tyne Hospitals NHS Foundation Trust) and the contractor (Newcastle University). Applications by the lead collaborator in each centre for ethical approval (or Site-Specific Assessment in the UK) were supported by the trial manager and the clinical lead for the centre and country in which the centre was located. Also within the UK, research and development approval was sought in respect of all participating centres and the study was open to audit (‘for cause’ or as part of the routine 10% check) by the appropriate research governance teams in the participating Trusts. A member of the STITCH(TRAUMA) study team visited centres with high volume recruitment or where there were concerns about patient eligibility (identified by central monitoring) to confirm patient existence and monitor adherence to the trial protocol.
Multicentre Research Ethics Committee approval for the study was obtained from Southampton Multicentre Research Ethics Committee (Reference: 09/H0502/68) on 15 June 2009 and the trial was registered (ISRCTN19321911).
Patient recruitment
To be considered for STITCH(TRAUMA), patients had to have had CT to confirm the diagnosis and the size and location of the haematoma. Any clotting or coagulation problems had to be corrected prior to randomisation in line with standard local clinical practice.
Inclusion criteria
-
Adults aged 14 years or over.
-
Evidence of a TICH on CT with a confluent volume of attenuation significantly raised above that of the background white and grey matter that has a total volume greater than 10 ml calculated by (length × width × height)/2 in cm. 22
-
Within 24 hours of head injury. This criterion was later increased to 48 hours following discussion at an investigators’ meeting in order to increase the number of patients eligible, allowing more time for patients to reach the neurosurgery department and for the TICH to develop.
-
Clinical equipoise. Only patients for whom the responsible neurosurgeon was uncertain about the benefits of either treatment were eligible.
Exclusion criteria
-
A significant surface haematoma (EDH or SDH) requiring surgery. (The indications for intervention for these patients were already very well defined.)
-
Three or more separate haematomas fulfilling the inclusion criteria.
-
If surgery could not be performed within 12 hours of randomisation.
-
Severe pre-existing physical or mental disability or severe comorbidity which would lead to a poor outcome even if the patient made a full recovery from the head injury. (Examples would be a high level of dependence before the injury or severe irreversible associated injury such as complete spinal cord injury.)
-
Permanent residence outside a study country preventing follow-up.
-
Patient and/or relative having a strong preference for one treatment modality.
There was no specified upper age limit. The need for clinical equipoise and explicit exclusion of patients with severe pre-existing physical or mental disability or severe comorbidity which might lead to a poor outcome even if the patient made a good recovery from the head injury excluded the less able older patient while allowing a fit older person to be included. Haematoma rates were known to be more common in the older head-injured patient.
Recruitment was encouraged by quarterly glossy newsletters sent to each centre expressing interest, monthly e-mail newsletters to the site co-ordinators and investigators, regular attendance and presentations at national and international meetings, having stands at national and international meetings, sending an e-mail to the investigators and co-ordinators whenever a patient was recruited and awarding certificates to every surgeon who recruited a patient so that they would be able to demonstrate their involvement in clinical trials.
Consent procedure
Written witnessed informed consent of the patient or relative was obtained by trained neurosurgical staff prior to randomisation. The member of neurosurgical staff provided a written information sheet and allowed as much time as possible to discuss the options. One copy of the consent form was given to the patient, one was filed in the patient notes and one was filed with the trial documentation. If the patient was unable to give consent him- or herself because of the nature of the haemorrhage, a personal representative was approached to give consent on behalf of the patient. The personal representative was described as someone with a close personal relationship with the patient who was capable and willing to consent on behalf of the patient. (If the patient was unable to consent and the closest relative was not available the patient could not be included in the study. This study did not permit a professional legal representative to give consent because of the need to establish a relationship with potential carers to facilitate complete follow-up by postal questionnaire.)
Randomisation (treatment allocation)
Randomisation was achieved via the independent 24-hour randomisation service based in the Aberdeen Clinical Trials Unit either by telephone or by using the web-based version. The randomisation information was entered and at the end of the phone call or the web entry the neurosurgeon was informed of the patient identifier number for the trial and the treatment group the patient was allocated to. The neurosurgeon recorded this information on the randomisation form and then faxed the form to the STITCH(TRAUMA) office.
The data manager checked the form against the information received from the randomisation centre and entered the data into an anonymised password-protected database.
The 24-hour randomisation service was backed by 24-hour availability of the project team, who could advise on patient eligibility. If the site had problems contacting the randomisation service a member of the project team undertook the randomisation for them.
Allocation was stratified by geographic region, with a minimisation algorithm based on age group (< 50 years, 50 years to < 70 years, ≥ 70 years) and severity (as measured by whether the pupils are equal and reacting or not) and with a random component (i.e. with probability of 80%).
Trial interventions
The two trial interventions were early evacuation of the haematoma by a method of the surgeon’s choice (within 12 hours of randomisation), combined with appropriate best medical treatment versus best medical treatment combined with delayed (more than 12 hours after randomisation) evacuation if it became appropriate later. Both groups were monitored according to standard local neurosurgical practice.
Best medical treatment included (depending on the practices within the centre) monitoring of ICP or other modalities and management of metabolism, sodium osmotic pressure, temperature and blood gases.
All patients also had an additional CT at 5 days (± 2 days) to assess changes in the haematoma size with and without surgery.
Compliance
Patients or their relatives could withdraw consent for an operation, or, conversely, request an operation, after randomisation, thereby leading to crossover between the arms. In surgical trials it is common for the patient’s condition to change over time and a patient randomised to Initial Conservative Treatment might deteriorate and require surgery later. Such crossovers and the reasons for them were documented.
Information was collected about the status (GCS and focal signs) of patients through the first 5 days of their trial progress and ICP/CPP measures in invasively monitored patients in order to be able to describe the change in status that might lead to a change in equipoise for the treating neurosurgeon, and subsequent surgery in patients initially randomised to conservative treatment.
Compliance with treatment allocation was monitored by the data manager. In surgical trials, patients allocated to the non-surgical arm of the trial may later deteriorate and surgeons may intervene. This was the case in the Medical Research Council (MRC)-funded STICH4 and STICH II,10 in trials of cardiac surgery compared with angioplasty, in the MRC-funded back pain trial23 and in the Spine Patient Outcomes Research Trial (SPORT). 24 These crossover rates to surgery were 26%, 21%, 28%, 28% and 30% respectively. While surgical trials will always have such crossovers when surgeons perceive that there is value in operating on patients who deteriorate after initial randomisation into the conservative limb of the trial, it is important to understand, monitor and report the rates of such crossovers. During the recruitment of centres and at investigator meetings the importance of clinical equipoise and minimising crossovers was emphasised and all crossovers were investigated. Centres exhibiting high crossover rates could be withdrawn from the study.
Data collection
To preserve confidentiality all patients were allocated a unique study identifier during the randomisation process, which was used on all data collection forms and questionnaires. Only a limited number of members of the research team were able to link this identifier to patient identifiable details; this was necessary in order to carry out centralised follow-up.
Baseline
Before randomisation, a one-page baseline form was completed by the responsible neurosurgeon recording demographic (age, gender) and clot characteristics [site, side, length (A), width (B) and height (C) measures to define volume] and status at randomisation (GCS, pupils equal and reacting or not). This information was required in order to randomise the patient.
Two week/discharge
At 2 weeks after randomisation or at discharge or death (whichever occurred first) the discharge/2-week form was completed. This form recorded the date, the event that triggered the form and the patient’s status at that time, whether or not the patient had had surgery and when (and why if randomised to Initial Conservative Treatment or why not if randomised to Early Surgery), the patient’s GCS and localising features for the 5 days following randomisation, the occurrence of any adverse events (including death, pulmonary embolism, deep-vein thrombosis, surgical site infection) following randomisation, past medical history and status prior to the head injury. This form, together with copies of the randomisation CT image and the 5-day post-randomisation CT image, was sent to the STITCH office within 2 weeks. The data manager entered the data into the anonymised password-protected database.
Computed tomography
Copies of two CT images were required: the diagnostic CT image prior to randomisation and a 5-day image. All patients had undergone a diagnostic CT imaging as standard practice. The 5-day image was due between 3 and 7 days after randomisation. Many patients received this as part of standard treatment and the study accepted and used any CT taken for clinical purposes during this period. Only patients who did not receive such a CT during this period required additional imaging.
The preferred scanning technique was CT with volume acquisition 32 × 0.5 mm (or equivalent), 120 kV, 400 mA (or equivalent) and 220 mm field of view. The preferred angle was parallel with the anterior cranial fossa, coverage from base of skull to vertex, reconstruct 5 mm whole head, soft tissue filter.
The preferred method of sending CT images was in DICOM (Digital Imaging and Communications in Medicine)-compatible format, on separate CDs for the two time points, anonymised by use of a patient identifier. They were checked by the data manager initially on receipt at the STITCH(TRAUMA) office to ensure that the haematoma characteristics at randomisation conformed to the required inclusion criteria. Where protocol deviations were suspected, the data manager arranged for the image to be viewed by a trained reader to check whether or not the centre needed to be contacted immediately to prevent repetitions.
The data manager loaded the images into a specialised password-protected image management program. They were allocated a separate randomly created identifier by the data manager, so that it would not be possible for the reader to identify the before-and-after images of the same patient. A separate list identifying patient identifier and scan identifier was kept by the data manager.
The CT images were to be analysed subsequently by trained readers using the image management program and a defined protocol. Their passwords gave access only to scans blinded to treatment group and patient identity.
Follow-up
Postal questionnaires had previously been designed for the STICH4 and STICH II10 studies and were translated into most of the languages required. Where new countries with different languages were recruited, the National Investigator was asked to arrange translation and another principal investigator from the country was asked to check the translation. Postal follow-up was due at 6 months and 12 months. The patient’s general practitioner (in the UK) or consultant (outside the UK) was contacted at 4.5 months and 10 months to check that the patient was alive and to confirm his/her place of residence. At that time the clinician was also requested to complete a major adverse events form to include death, pulmonary embolism, deep-vein thrombosis, stroke or any other serious adverse event. The 6-month outcome questionnaire was mailed to the patient at 5 months for completion by the patient or relative if the patient was unable to complete it him- or herself. If necessary, a reminder was sent at 6 months and telephone follow-up was carried out by ‘blinded’ clerical or nursing staff at 7 months to enhance response rates. Similarly, the 12-month questionnaire was mailed at 11 months with reminders at 12 and 13 months. In countries where the postal system was poor, patients were requested to attend a follow-up clinic at which the questionnaire was distributed and collected. In countries where literacy or language/dialect was a problem, a ‘blinded’ interviewer administered the questionnaire. This methodology had been developed for use and applied to good effect in STICH and STICH II.
Costs
The costs associated with surgical treatment (theatre time, consumables, overheads) were collected from published resources and local cost surveys undertaken by the study health economist. Length of stay, health-care resource use outside hospital, together with productivity costs arising from absence from work, and additional costs for family members through extra caring responsibilities were collected using the additional 3-month postal questionnaire and extended 6-month and 12-month postal questionnaires in the UK. Data were to be combined on quality of life with survival to generate QALYs. This included measurement of health-care costs, quality of life [EQ-5D-3 level (EQ-5D-3L)] work absence [World Health Organization (WHO) Health and Performance Questionnaire – Clinical Trial Version] and carer activities (measured by the discrete choice experiment developed by the Health Economics Research Unit). EQ-5D-3L and survival were collected for all patients by the postal outcome questionnaires in order to generate QALYS for the whole study and for a UK-only analysis.
Serious adverse events
Serious adverse events were recorded on the Major Adverse Events form. Serious adverse events were defined as an adverse experience that resulted in any of the following outcomes:
-
death
-
life-threatening
-
inpatient hospitalisation or prolongation of existing hospitalisation
-
persistent or significant disability or incapacity.
All serious adverse events were to be reported to the STITCH Office within 7 days of the local investigator becoming aware of the event and to the local ethics committee or other regulatory bodies as required.
Outcome measures
-
Primary: unfavourable outcome was defined as death or severe disability on the Glasgow Outcome Scale (GOS), which was recorded using a self-completed structured questionnaire based on the 8-point GOS. 25
-
Secondary: Rankin, EQ-5D-3L, Mortality, Survival, Major Adverse Events (death, pulmonary embolism or deep-vein thrombosis, infection, rebleeding), QALYs, total health-care costs, social costs.
Structured postal questionnaires were used to collect follow-up data. They contained the GOSE, Rankin Scale and EQ-5D-3L and were translated into the necessary languages. GOSE and Rankin Scale had been translated by a national investigator for a previous study and were back-translated to check them. EQ-5D-3L was obtained in the necessary validated translated versions.
The GOS is the specific measure for head injury and the 8-point scale provides more sensitivity than the 5-point scale. Initially the plan had been to use a prognosis-based26,27 outcome measure such that for patients with a very poor prognosis an outcome of good recovery, moderate disability or upper severe disability would be regarded as a favourable outcome, while for patients with a better prognosis a favourable outcome would be good recovery or moderate disability. Following the withdrawal of funding, the analysis plan was altered to a simple dichotomised split for all patients. The Rankin Scale is widely used as a functional outcome measure in stroke and allows comparison of results between this study of patients with traumatic ICH and studies of patients with spontaneous ICH. EQ-5D-3L is the standard measure of quality of life incorporating a utility value and has been developed in many languages.
Sample size
Previous studies had suggested a favourable outcome in the non-operated group of about 40% and a favourable outcome in the surgical group of about 60–70%. However, this was in observational studies. Given that many randomised controlled trials observe a more favourable outcome than seen in observational data it was assumed that the favourable outcome (good recovery or moderate disability on the GOS) in the conservative treatment group would be 50% in order to calculate sample size. A total sample size of 776 would therefore be required to show a 10% benefit (i.e. 50% vs. 60%) from surgery (2p < 0.05) with 80% power. A safety margin of 9.5% was built in to allow for loss to follow-up, making a total sample size of 840 to be recruited and randomised (420 per arm).
In order to achieve this sample size in a reasonable time span and to provide robust evidence, it was necessary to recruit patients from outside the UK. In England and Wales there are only 30 neurosurgical units, and only one-third of these participate in randomised controlled trials. Experience with interested neurosurgical centres in previous studies had shown that about 25% of recruited centres fail to recruit any patients and a further 25% only recruit one or two patients. The best recruiting centres will recruit about 10 patients per year; therefore, to complete patient recruitment within the time scale, we estimated that we would need to approach at least 150 centres.
Loss to follow-up was reduced as much as possible. In the previous STICH study, the loss was about 5%. In STITCH(TRAUMA), the patient population was younger and likely to be more mobile; however, we implemented procedures that we had developed to minimise loss to follow-up. Methods of follow-up were adapted to those most likely to be successful within each country and centre according to local population and care characteristics. Residence in any study country was an eligibility criterion so patients who suffered a head injury while on holiday would be able to be followed up.
Following the decision by the funding agency to halt the trial, the power of the trial was recalculated. Using the uncorrected chi-squared test and assuming equal sample sizes in the two groups, and given an average favourable outcome of 60% (as observed after the recruitment and follow-up of 96 patients), there would be 26.4% power to detect a 10% difference. The achieved sample size of 170 gave 80% power to detect a 21% difference.
Blinding
It was not possible to blind either patients or treating surgeons to whether or not the patient had surgery or when. To minimise possible sources of bias, randomisation was undertaken centrally, thus ensuring concealment of allocation from the enrolling clinician, patient and relatives. The multidisciplinary team in the co-ordinating centre and the principal investigators were blinded to the results until after the data set was locked following receipt of the final outcome questionnaire. Only the data manager and Data Monitoring Committee (DMC) had access to unblinded data.
Statistical analysis
Initially the primary analysis was planned to be a simple categorical frequency comparison using the uncorrected chi-squared test for prognosis-based26,27 favourable and unfavourable outcomes at 6 months. Patients with a good prognosis would be categorised as having a favourable outcome if they achieved good recovery or moderate disability on the GOS. Patients with a poor prognosis would be categorised as having a favourable outcome if they achieved good recovery, moderate disability or upper severe disability on the GOSE. Secondary outcomes were also to be analysed using the prognosis-based method as specified in STICH. 28
However, STITCH(TRAUMA) was halted early by the funding agency for failure to recruit in the UK. The achieved sample size, at the point when recruitment was halted on instruction from the funding agency, was 170 patients, with 4% from the UK. The analysis plan was, therefore, adapted from the original analysis statement published in the protocol20 in the light of this much reduced sample size. The new plan was developed and published on the study website without access to treatment allocation prior to unblinding the data.
Primary outcome
Analysis was on an intention-to-treat basis. The primary analysis was a simple categorical frequency comparison using the uncorrected chi-squared test for favourable and unfavourable outcomes at 6 months. Patients were categorised as having a favourable outcome if they achieved good recovery or moderate disability on the GOS. Patients were categorised as having an unfavourable outcome if they had severe disability on the GOS, were vegetative or had died. A sensitivity analysis using logistic regression was undertaken to adjust for age, volume of haematoma and GCS.
Secondary outcomes
Secondary outcome analyses included the proportional odds model analysis of GOS, GOSE and Rankin Scale scores at 6 months, Kaplan–Meier survival curve with log-rank test, mortality at 6 months and dichotomised Rankin Scale score (0–2, 3–6) at 6 months (comparing patients able to look after their own affairs with patients who need help). For dichotomised outcomes, absolute differences and 95% confidence intervals (CIs) were reported. Secondary outcomes were also collected at 12 months but were not required by the funding agency.
Subgroup analysis
Minimal subgroup analysis was undertaken and regarded as exploratory. Odds ratios (ORs) and 95% CIs were reported for the following subgroups: age (two bands using randomisation strata: < 50 years and ≥ 50 years – there were very few patients aged over 70 years so the two upper age bands were combined), volume of haematoma (using median split: ≤ 23 ml, > 23 ml), GCS (using standard classification of head injury severe, moderate, minor: 3–8, 9–12, 13–15), time from ictus to randomisation (using median split < 21 hours, ≥ 21 hours) and geographical region (four bands: Europe, India, China, other). Interaction tests (chi-squared test for heterogeneity) were undertaken and relevant p-values reported.
Protocol violations
There were two cases of major protocol violation in that the treatment decision was taken prior to randomisation. One patient randomised to surgery had already had a decision made for no surgery to take place and one case randomised to Initial Conservative Treatment had already had surgery. These two cases will be excluded from all analyses.
Health economics analysis
A cost–utility analysis was conducted from a health service perspective and based on an intention-to-treat principle.
Health-care resource use data were sourced from individual case report forms and participant questionnaires and supplemented using additional questionnaires administered to a sample of trial recruiting centres. Standard unit cost estimates were applied to resource use data for intervention delivery, hospital length of stay and rehospitalisation. Where standard unit costs were not available, supplemental information was collected from the additional questionnaire (e.g. cost of an hour of a surgeon’s time) and WHO choice data for the cost per night in hospital. Intervention and follow-up costs were summed to generate total costs to the health services for each individual within the trial. Costing followed recommended procedures for international studies29,30 and costs were transformed into 2013 international dollars. 31 Standard general linear regression methods were used to estimate the impact of treatment group (conservative or Early Surgery) on costs. Estimates were calculated for all countries as well as country subgroups classified according World Bank classifications32 based on gross national income (GNI) per capita as follows: low income (GNI ≤ Int$1005, e.g. Nepal), lower middle income (GNI Int$1006–3975, e.g. India), upper middle income (GNI Int$3976–12,275, e.g. China), and high income (GNI ≥ Int$12,276, e.g. Western Europe, USA). Owing to small sample sizes, regression analyses were not undertaken.
Reporting statistics
For categorical variables, the number and percentage in each group are reported. Percentages are reported to no decimal places. For continuous variables, the median, quartiles, maximum and minimum are reported. Where p-values are reported, these are given to three decimal places or to one significant figure if four decimal places are required and then < 0.0001 if smaller still. The absence of data is reported. Outcomes are reported as ORs with 95% CIs and to two decimal places and p-values to three decimal places. Absolute benefits with 95% CIs to one decimal place are also reported.
Research governance
To conform with the Research Governance Framework for Health and Social Care, the role of sponsor for this study was taken on by the Newcastle upon Tyne Hospitals Foundation NHS Trust. All study-attached staff were appropriately qualified and trained in Good Clinical Practice appropriate to their role in the study.
Trial Steering Committee
Independent oversight of the study was provided by a Trial Steering Committee (TSC). The TSC met at 6-monthly intervals during the study. It provided overall supervision of the trial on behalf of the Health Technology Assessment (HTA) programme. It considered progress of the trial (in particular, success in site and patient recruitment), adherence to the protocol, patient safety and consideration of new information. The trial was conducted according to the standards set out in the MRC Guidelines for Good Clinical Practice.
Data Monitoring Committee
In order to monitor accumulating data on patient safety and treatment benefit, a DMC was established. The DMC considered data from interim analyses after 50, 100 and 150 patients had been recruited. It reported to the TSC. Interim analyses were strictly confidential and the committee would recommend stopping the trial early only if one or other treatment showed an advantage at a very high significance level or if the recruitment rates were unexpectedly low. The DMC recommended at each review that the trial should continue.
Management committee
This group met weekly to monitor progress and compliance. On a quarterly basis, funnel plots showing the proportion of patients who had died by the number recruited per site and the proportion of crossovers were reported to the group.
National Investigators
In countries with multiple centres, one centre investigator was required to fulfil the role of National Investigator and be responsible for obtaining national ethics approval and other permissions as required, for ensuring that documentation was translated from English as required, for identifying suitable centres within their country, for encouraging recruitment and for acting as a liaison person between the management team and the centres if required.
Centre investigators
Each centre had a centre investigator responsible for the conduct of the trial in that centre.
Patient and public involvement
There are two main charities involved with head injury research in the UK: Headway and UK Acquired Brain Injury Forum. Both organisations were invited to propose representatives to sit on the TSC. These representatives supported the study, providing advice on all aspects of the trial including design of the questionnaires, changes in the protocol and recruitment to the study.
Chapter 3 Results
Timelines
Funding was activated on 1 September 2009 and the first site opened to recruitment on 1 October 2009. The first patient was recruited in December 2009. In March 2012, the HTA programme announced that it would be withdrawing funding because of the low UK recruitment. It felt that the study was not viable in the UK and that there was no realistic chance of that changing in the near future. It did agree to a managed process of withdrawal, particularly for non-UK centres, to enable the project team to investigate the possibility of support from another funder in order to continue the trial and to fulfil the ethical responsibility for follow-up of current study participants and maximise data available for future meta-analyses. Despite considerable efforts to identify further funding both in the UK and abroad, the project team were unsuccessful in achieving this in the short time available and the final patient was recruited by 30 September 2012.
Centre recruitment
Interest in the study was expressed by 139 centres, and 59 of these completed all the regulatory requirements to become registered centres. The registered centres were located in 20 countries: the UK (n = 9), Armenia (n = 1), Bulgaria (n = 1), Canada (n = 1), the People’s Republic of China (n = 3), the Czech Republic (n = 1), Egypt (n = 2), Germany (n = 6), Hungary (n = 1), India (n = 14), Italy (n = 1), Latvia (n = 1), Lithuania (n = 2), Malaysia (n = 2), Nepal (n = 1), Pakistan (n = 1), Romania (n = 3), Russia (n = 1), Spain (n = 2) and the USA (n = 6). A further 13 centres had obtained ethical approval but were unable to complete the regulatory processes prior to the study being halted. Figure 1 shows the accumulation of centres through the trial. Regulatory processes are difficult and time-consuming and the difficulties in recruiting centres to surgical trials have been reported previously. 33 The times to recruitment for individual centres are shown in Figure 2 and the variability within countries in Figure 3.
FIGURE 1.
Recruitment of centres over time.

FIGURE 2.
Time to achieve regulatory approval.

FIGURE 3.
Time to approval by country.

Screening logs
Screening logs were returned by 32 centres from 15 countries, covering 251 centre-months out of a potential 1028 centre-months. The logs reported 1735 patients screened, of whom 1542 were reported to be ineligible, 134 eligible but not included and 59 recruited. The main reasons for ineligibility were that the ICH was too small (49%) or that the patient had an EDH or SDH that required surgery (20%). Reasons for not recruiting eligible patients included no consent (77%) and failure to randomise (23%). Further descriptions of these data are given in Francis et al. 34
Patient recruitment
Between December 2009 and September 2012, 170 patients were recruited from 31 centres in 13 countries and randomly assigned to treatment groups: 83 to Early Surgery and 87 to Initial Conservative Treatment (Figure 4). After an initial slow recruitment phase, when the number of centres recruiting was low, the rate of recruitment picked up and in fact matched the recruitment seen in a previous SICH study. However, announcement of the plan to withdraw funding resulted in a slowdown in recruitment even though it continued for a further 6 months. This was probably a result of trial fatigue and a decision by the trial team not to encourage further centre recruitment until attempts to obtain further funding had been exhausted.
FIGURE 4.
Patient recruitment over time.
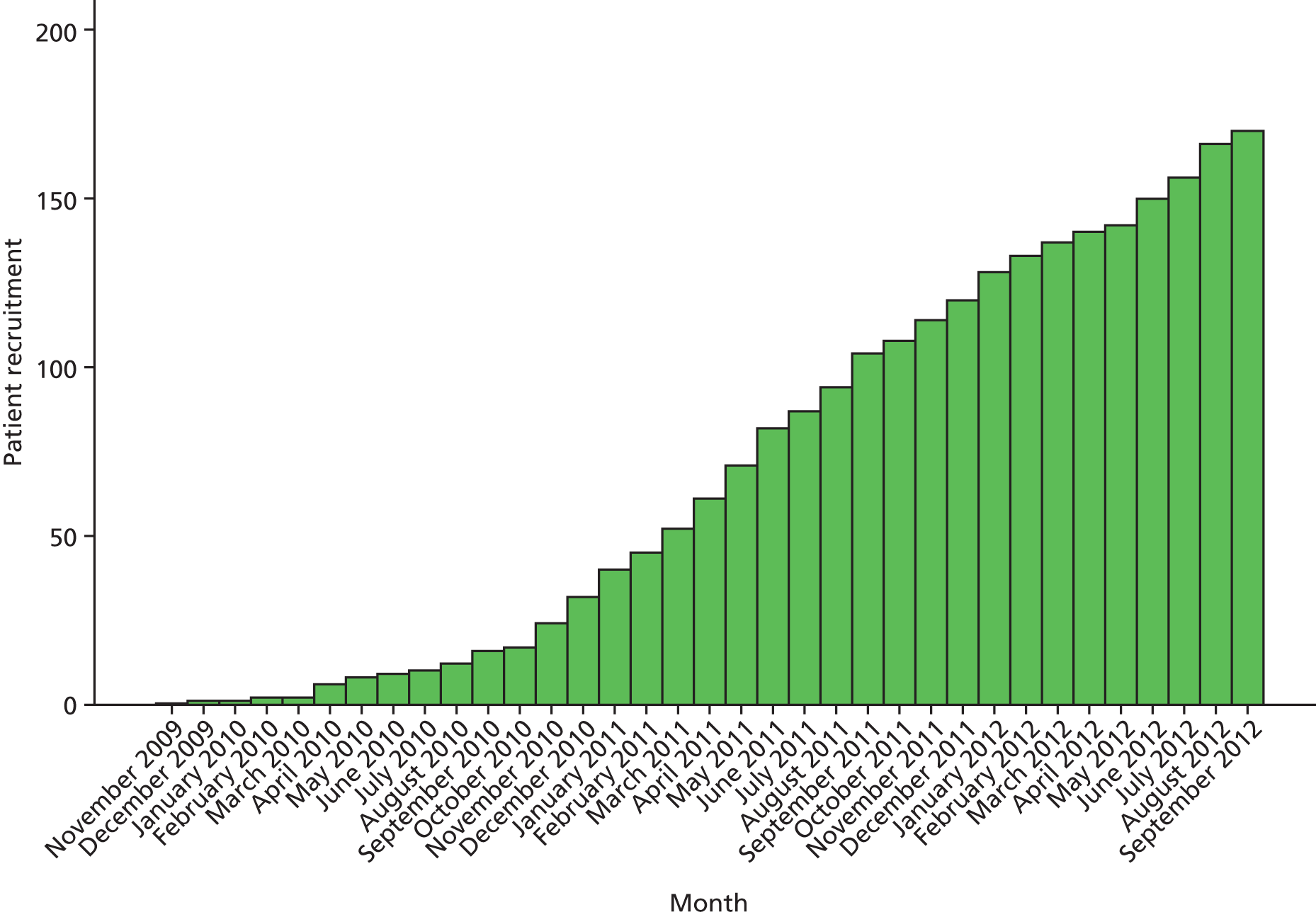
Figure 5 shows recruitment by centre and country. India was the highest-recruiting country, with a total of 74 patients, followed by China with 43. There were 27 patients recruited in Europe, including six in the UK, and 26 in the other countries (Pakistan, Malaysia, Nepal and Egypt).
FIGURE 5.
Recruitment by centre and country. (a) Centre; and (b) country. NIMHANS, National Institute of Mental Health and Neuro Sciences.


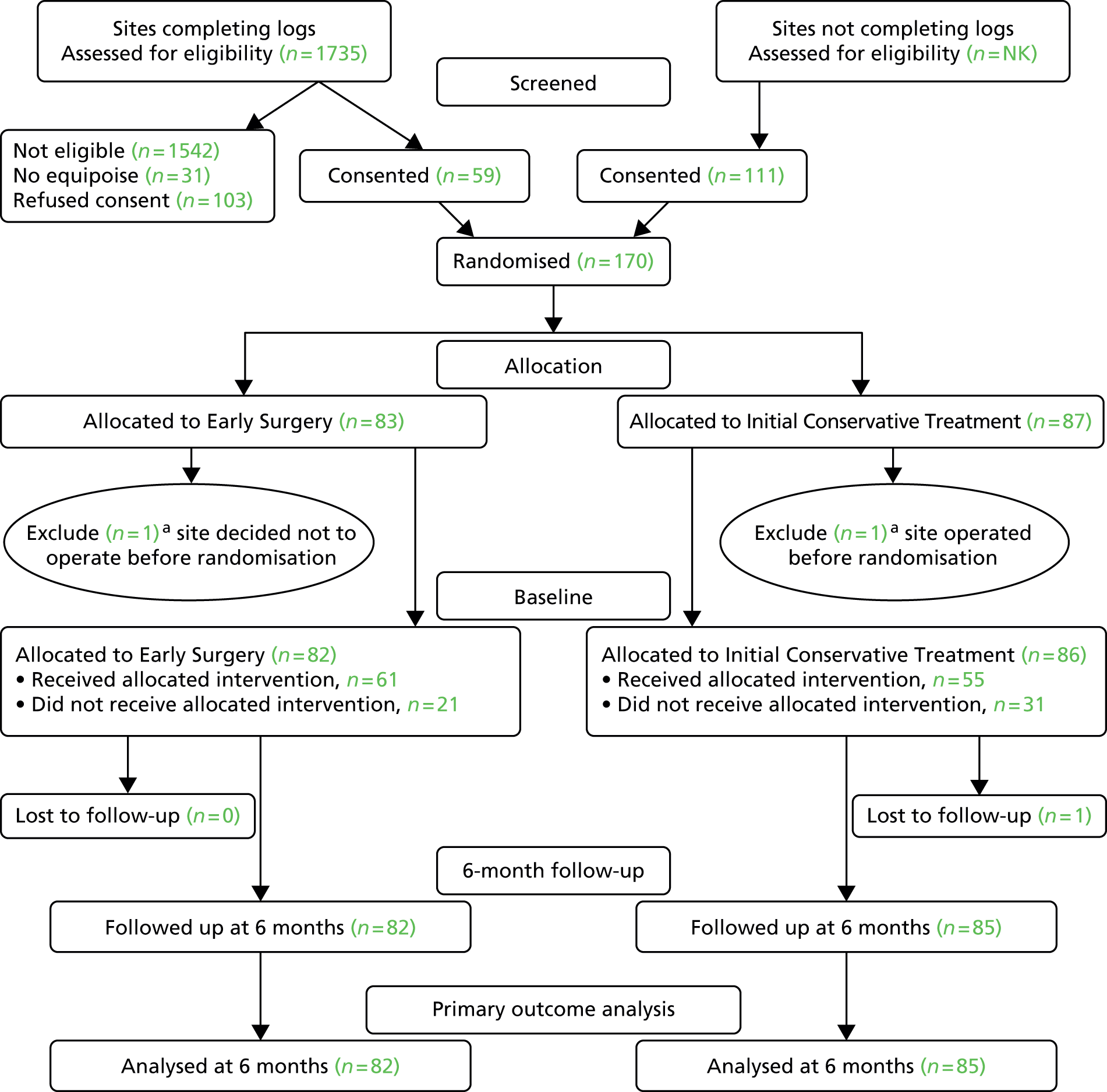
Figure 6 shows the CONSORT diagram for the trial. Two patients were excluded because the treatment decision was made prior to randomisation: in one case the patient was operated on prior to randomisation and in the other a decision was made not to operate prior to randomisation. These were serious protocol violations. All other patients were included in the analysis. Only one patient was lost to 6-month follow-up. This report therefore reports baseline measures for 168 patients and results for 167 patients, 82 patients assigned to Early Surgery and 85 patients assigned to Initial Conservative Treatment.
FIGURE 6.
Consolidated Standards of Reporting Trials flow chart for STITCH(TRAUMA) patients. NK, not known. a, One site recruited one patient but had undertaken surgery prior to randomisation; the patient was allocated to Initial Conservative Treatment. Another site recruited one patient for whom a treatment decision not to operate was made before the patient was randomised; this patient was allocated to Early Surgery. Because of the severe breach of protocol, these patients were excluded. Reproduced from Mendelow et al. 35 © A. David Mendelow, Barbara A. Gregson, Elise N. Rowan, Richard Francis, Elaine McColl, Paul McNamee, Iain R. Chambers, Andreas Unterberg, Dwayne Boyers, and Patrick M. Mitchell 2015; Published by Mary Ann Liebert, Inc. This Open Access article is distributed under the terms of the Creative Commons License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
Baseline measures
Tables 1–6 show the distribution of baseline variables between the two treatment groups. Patients ranged in age from 16 to 83 years, with a median age of 50 years, and 122 (73%) were male (Table 1). Despite 13% of patients being over the age of 70 years, only 7% were on any anticoagulant or antiplatelet medication. Prior to the head injury, 164 patients (98%) scored 0 or 1 on the Rankin Scale, and 22 (13%) had a medical history of cardiovascular disease (Table 2). The main causes of the head injury were road traffic accidents (113, 67%) and falls (47, 28%) (Table 3). Most of those who were in a road traffic accidents were motorbike riders (n = 45, 40%) or pedestrians (n = 29, 26%). Patients from Europe were most likely to have suffered a fall (74%) while patients from Asia were most likely to have had a road traffic accident (76%) either on a motorbike or as a pedestrian.
| Variable | Early Surgery (N = 82) | Initial Conservative Treatment (N = 86) |
|---|---|---|
| Age (years) | ||
| Median (IQR) | 51 (32–63) | 50 (33–61) |
| Range | 18–83 | 16–77 |
| Mean (SD) | 48 (17.7) | 48 (16.9) |
| Age band (years), n (%) | ||
| < 50 | 37 (45) | 42 (49) |
| 50–69 | 34 (42) | 33 (38) |
| ≥ 70 | 11 (13) | 11 (13) |
| Sex, n (%) | ||
| Male | 57 (70) | 65 (76) |
| Female | 25 (30) | 21 (24) |
| Pre-ICH Rankin Scale score, n (%) | ||
| 0 | 74 (90) | 78 (91) |
| 1 | 6 (7) | 6 (7) |
| 2 | 2 (2) | 0 (0) |
| 3 | 0 (0) | 1 (1) |
| 4 | 0 (0) | 0 (0) |
| 5 | 0 (0) | 1 (1) |
| Pre-ICH mobility, n (%) | ||
| Able to walk 200 m | 80 (98) | 82 (95) |
| Able to walk indoors | 1 (1) | 3 (3) |
| Unable to walk | 1 (1) | 1 (1) |
| Handedness,a n (%) | ||
| Right | 80 (98) | 84 (99) |
| Left | 2 (2) | 1 (1) |
| Variable | Early Surgery, n (%) (N = 82) | Initial Conservative Treatment, n (%) (N = 86) |
|---|---|---|
| Significant medical history | ||
| Cardiovascular | 10 (12) | 12 (14) |
| Gastrointestinal | 4 (5) | 3 (3) |
| Musculoskeletal | 3 (4) | 1 (1) |
| Oncological | 1 (1) | 1 (1) |
| Renal | 1 (1) | 0 (0) |
| Epilepsy | 2 (2) | 2 (2) |
| Endocrine | 4 (5) | 5 (6) |
| Haematological | 2 (2) | 0 (0) |
| Neurological | 0 (0) | 3 (3) |
| Pulmonary | 1 (1) | 1 (1) |
| Social history | 3 (4) | 3 (3) |
| Antiepileptics | 1 (1) | 2 (2) |
| ENT | 1 (1) | 2 (2) |
| Hepatic | 2 (2) | 2 (2) |
| Previous TBI | 1 (1) | 2 (2) |
| Psychiatric | 0 (0) | 0 (0) |
| Developmental | 0 (0) | 0 (0) |
| Prior to ICH anticoagulant | 2 (2) | 0 (0) |
| Prior to ICH antiplatelet | 3 (4) | 7 (8) |
| Prior to ICH thrombolytic | 0 (0) | 0 (0) |
| Variable | Early Surgery, n (%) (N = 82) | Initial Conservative Treatment, n (%) (N = 86) |
|---|---|---|
| Cause of injury | ||
| Road traffic accident | 54 (66) | 59 (69) |
| Fall domestic | 15 (18) | 15 (17) |
| Fall outside home | 8 (10) | 9 (10) |
| Work | 0 (0) | 1 (1) |
| Violence/assault | 3 (4) | 2 (2) |
| Animal attack | 2 (2) | 0 (0) |
| Mechanism of injurya | ||
| Acceleration/deceleration | 13 (16) | 23 (28) |
| Direct impact | 38 (47) | 25 (30) |
| Crush | 2 (2) | 1 (1) |
| Fall from ground | 18 (22) | 26 (31) |
| Fall from height | 8 (10) | 8 (10) |
| Fall (details unknown) | 2 (2) | 0 (0) |
Sixty-eight (40%) patients were admitted to another hospital prior to their transfer to the neurosurgical unit, and the time from injury to randomisation varied between 3 and 48 hours with a median of 22 hours (Table 4). Two-thirds (n = 111; 66%) had an initial loss of consciousness, 25 (15%) pretraumatic amnesia and 41 (24%) post-traumatic amnesia.
| Variable | Early Surgery (N = 82) | Initial Conservative Treatment (N = 86) |
|---|---|---|
| Time to randomisation (hours) | ||
| Median (IQR), range | 21 (13–31), 3–48 | 22 (14–28), 4–48 |
| Mean (SD) | 22 (11.7) | 22 (10.6) |
| Emergency services provided for the airway, n (%) | ||
| None | 28 (34) | 21 (24) |
| Oxygen | 45 (55) | 56 (65) |
| Intubation | 6 (7) | 8 (9) |
| Oxygen and intubation | 3 (4) | 1 (1) |
| Site of additional injuries, n (%) | ||
| Skin | 35 (43) | 46 (53) |
| Head and neck | 63 (77) | 67 (78) |
| Face | 34 (41) | 37 (43) |
| Chest | 11 (13) | 11 (13) |
| Abdomen | 7 (9) | 1 (1) |
| Extremities | 17 (21) | 11 (13) |
| Spine | 3 (4) | 2 (2) |
| Initial loss of consciousness, n (%) | ||
| Yes | 57 (70) | 54 (63) |
| No | 15 (18) | 20 (23) |
| Unknown | 10 (12) | 12 (14) |
| Pre-traumatic amnesia, n (%) | ||
| Yes | 16 (20) | 9 (10) |
| No | 33 (40) | 40 (47) |
| Unknown | 33 (40) | 37 (43) |
| Post-traumatic amnesia, n (%) | ||
| Yes | 22 (27) | 19 (22) |
| No | 27 (33) | 34 (40) |
| Unknown | 33 (40) | 33 (38) |
| Secondary insults, n (%) | ||
| Hypoxic | 3 (4) | 3 (3) |
| Hypotensive | 2 (2) | 2 (2) |
| Hypothermic | 0 (0) | 0 (0) |
| Cardiac arrest | 0 (0) | 0 (0) |
| Referral details, n (%) | ||
| Primary admission | 45 (55) | 55 (64) |
| Secondary admission | 37 (45) | 31 (36) |
The volume of the largest haematoma varied between 10 ml and 97 ml, with a median of 23 ml, and 61 (36%) patients had a second haematoma between 0 ml and 26 ml, with a median of 3 ml (Table 5). All of the reported haematomas were located in the lobar regions of the brain, particularly in the frontal or temporal areas. The distribution of baseline variables between the two groups was very similar. At the time of randomisation, 70 (42%) patients had a GCS of 13–15, 78 (46%) a GCS of 8–12 and 20 (12%) a GCS of < 8 (Table 6). More than 85% of patients had a motor score on the GCS of 5 or more.
| Variable | Early Surgery (N = 82) | Initial Conservative Treatment (N = 86) |
|---|---|---|
| Volume of largest haematoma (ml) | ||
| Median (IQR), range | 25 (18–37), 11–96 | 23 (15–32), 10–97 |
| Mean (SD) | 31 (18.0) | 27 (16.8) |
| Location of largest haemorrhage, n (%) | ||
| Frontal | 36 (44) | 43 (50) |
| Temporal | 39 (48) | 37 (43) |
| Parietal | 4 (5) | 5 (6) |
| Occipital | 3 (4) | 1 (1) |
| Second haematoma present, n (%) | ||
| Yes | 28 (34) | 33 (38) |
| No | 54 (66) | 53 (62) |
| Volume of second haematoma if applicable (ml) | ||
| Median (IQR), range | 3 (1–10), 0–20 | 4 (2–8), 0–26 |
| Mean (SD) | 6 (6.3) | 6 (6.6) |
| Location of second haemorrhage, n (%) | ||
| Frontal | 20 (24) | 15 (17) |
| Temporal | 6 (7) | 12 (14) |
| Parietal | 1 (1) | 6 (7) |
| Occipital | 1 (1) | 0 (0) |
| No second haemorrhage | 54 (66) | 53 (62) |
| Variable | Early Surgery, n (%) (N = 82) | Initial Conservative Treatment, n (%) (N = 86) |
|---|---|---|
| GCS: eye | ||
| 1 | 7 (9) | 12 (14) |
| 2 | 21 (26) | 17 (20) |
| 3 | 23 (28) | 25 (29) |
| 4 | 31 (38) | 32 (37) |
| GCS: verbal | ||
| 1 | 12 (15) | 15 (17) |
| 2 | 21 (26) | 19 (22) |
| 3 | 8 (10) | 14 (16) |
| 4 | 23 (28) | 20 (23) |
| 5 | 18 (22) | 18 (21) |
| GCS: motor | ||
| 1 | 0 (0) | 1 (1) |
| 2 | 2 (2) | 1 (1) |
| 3 | 4 (5) | 5 (6) |
| 4 | 6 (7) | 4 (5) |
| 5 | 32 (39) | 33 (38) |
| 6 | 38 (46) | 42 (49) |
| GCS: total | ||
| 3 | 0 (0) | 1 (1) |
| 4 | 0 (0) | 0 (0) |
| 5 | 1 (1) | 2 (2) |
| 6 | 6 (7) | 3 (3) |
| 7 | 4 (5) | 3 (3) |
| 8 | 1 (1) | 6 (7) |
| 9 | 11 (13) | 8 (9) |
| 10 | 11 (13) | 14 (16) |
| 11 | 6 (7) | 8 (9) |
| 12 | 6 (7) | 7 (8) |
| 13 | 10 (12) | 8 (9) |
| 14 | 14 (17) | 13 (15) |
| 15 | 12 (15) | 13 (15) |
| Pupils | ||
| Both reactive | 77 (94) | 79 (92) |
| One reactive | 3 (4) | 3 (3) |
| Both unreactive | 2 (2) | 4 (5) |
Surgery
Of the 82 patients in the Early Surgery group, only 61 (74%) had surgery, 57 (93%) of these within 12 hours of randomisation (Table 7). The reasons for not having surgery were patient or relative refusal (n = 15), improvement (n = 1), deterioration (n = 2), seizures (n = 1), anaesthetic risk (n = 1) and change of history suggesting spontaneous rather than traumatic ICH (n = 1). Although informed consent was obtained prior to randomisation, patients often had more than one relative and further discussion could lead to a change of opinion.
| Variable | Early Surgery: surgical cases only, N = 61 (74%) | Initial Conservative Treatment: surgical cases only, N = 31 (36%) |
|---|---|---|
| Method, n (%) | ||
| Craniotomy | 59 (97) | 25 (81) |
| Other | 2 (3) | 6 (19) |
| Other surgical details | ||
| Bone flap replaced | 47 (77) | 13 (42) |
| Other cranial surgery | 1 (2) | 3 (10) |
| Paralysed and sedated | 17 (28) | 12 (39) |
| Any non-cranial surgery | 1 (2) | 2 (7) |
| Pre-operative GCS: eye, n (%) | ||
| 1 | 5 (8) | 15 (48) |
| 2 | 18 (30) | 8 (26) |
| 3 | 19 (31) | 5 (16) |
| 4 | 19 (31) | 3 (10) |
| Pre-operative GCS: verbal, n (%) | ||
| 1 | 13 (21) | 16 (52) |
| 2 | 15 (25) | 7 (23) |
| 3 | 6 (10) | 5 (16) |
| 4 | 18 (30) | 0 (0) |
| 5 | 9 (15) | 3 (10) |
| Pre-operative GCS: motor, n (%) | ||
| 1 | 0 (0) | 4 (13) |
| 2 | 2 (3) | 1 (3) |
| 3 | 6 (10) | 3 (10) |
| 4 | 4 (7) | 6 (19) |
| 5 | 26 (43) | 14 (45) |
| 6 | 23 (38) | 3 (10) |
| Time randomisation to surgery (hours) | ||
| Median (IQR), range | 3 (1–6), < 1–24 | 25 (6–79), < 1–318 |
| Mean (SD) | 4 (4.5) | 58 (75.6) |
| Surgery within 12 hours of randomisation, n (%) | 57 (93) | 10 (32) |
| Time injury to surgery (hours) | ||
| Median (IQR), range | 23 (16–36), 4–69 | 45 (26–99), 9–332 |
| Mean (SD) | 26 (13.8) | 78 (79.0) |
| Surgery within 12 hours of injury, n (%) | 9 (15) | 3 (10) |
Of the 86 patients randomised to Initial Conservative Treatment, 31 (36%) had surgery within 14 days of randomisation, 10 (32%) of these within 12 hours. The reasons for having surgery were neurological deterioration (n = 29), no shrinkage in haematoma size (n = 1) and rise in ICP (n = 1). Neurological deterioration was identified by a drop in GCS, enlargement of the haematoma or increase in midline shift, increase in weakness or change in pupil size or reactivity.
Surgical patients in the Early Surgery group were more likely to undergo craniotomy than surgical patients in the Initial Conservative Treatment group (97% vs. 81%; Fisher’s exact test, p = 0.016). One patient in the Initial Conservative Treatment group underwent burrhole surgery, but all other patients who did not have craniotomy underwent craniectomy. The bone flap was more likely to be replaced in the surgical patients in the Early Surgery group (77%) than in the Initial Conservative Treatment group (42%) (Fisher’s exact test, p = 0.001). As Table 3 demonstrates, surgical patients in the Early Surgery group had significantly higher pre-operative GCS values on all the subscales than those requiring surgery in the Initial Conservative Treatment group. Comparison of the baseline characteristics of patients in the Initial Conservative Treatment group who had surgery with those who did not showed that patients who deteriorated and went on to have surgery had larger haematomas initially (Mann–Whitney U-test, p = 0.010) and were more likely to have at least one pupil unreactive (Fisher’s exact test, p = 0.0005) but did not differ on age, GCS at the time of randomisation or presence of a second haematoma.
Hospital stay
At 2 weeks post randomisation, similar proportions of patients in the two groups were still on the neurosurgical ward: 29 (35%) of the Early Surgery patients and 32 (37%) of the Initial Conservative Treatment patients. The proportions that had been transferred to another ward or hospital were also similar: three (4%) and four (5%) respectively. However, 43 (52%) Early Surgery patients had been discharged, compared with 33 (38%) Initial Conservative Treatment patients. Furthermore, there was a significant difference in the percentage of patients who had died by 2 weeks: 7 (9%) Early Surgery patients compared with 17 (20%) Initial Conservative Treatment patients (Fisher’s exact test, p = 0.047).
At some point in the first 2 weeks, ICP was monitored in seven (9%) Early Surgery patients, compared with 16 (19%) Initial Conservative Treatment patients (p = 0.073), and this affected management decisions in one Early Surgery patient, compared with 10 Initial Conservative Treatment patients (p = 0.069) (Table 8). Patients were less likely to be monitored in India, where the rate was 4% (3 out of 74), compared with 21% elsewhere (20 out of 94).
| Variables | Early Surgery, n (%) (N = 82) | Initial Conservative Treatment, n (%) (N = 86) |
|---|---|---|
| ICP monitored | ||
| Yes | 7 (9) | 16 (19) |
| No | 75 (91) | 70 (81) |
| Monitor type (of ICP-monitored cases) | ||
| Intraventricular | 0 (0) | 1 (6) |
| Camino | 3 (43) | 5 (31) |
| Codman | 2 (29) | 7 (44) |
| Spiegelberg | 2 (29) | 3 (19) |
| Monitoring affected management (of ICP-monitored cases) | ||
| Yes | 1 (14) | 10 (63) |
| No | 6 (86) | 6 (37) |
Very few postrandomisation events were recorded during the first 2 weeks of the hospital stay (Table 9). The most frequently reported was pneumonia, with eight Early Surgery patients and eight Initial Conservative Treatment patients.
| Variable | Early Surgery, n (%) (N = 82) | Initial Conservative Treatment, n (%) (N = 86) |
|---|---|---|
| Death | 7 (9) | 17 (20) |
| Ischaemic stroke | 0 (0) | 1 (1) |
| Pulmonary embolism | 1 (1) | 2 (2) |
| Deep-vein thrombosis | 0 (0) | 0 (0) |
| Pneumonia | 8 (10) | 8 (9) |
| Postoperative EDHa | 0 (0) | 2 (2) |
| Septicaemia | 1 (1) | 0 (0) |
| Urinary tract infectiona | 1 (1) | 0 (0) |
| Epilepsy | 3 (4) | 0 (0) |
| Other | 5 (6) | 1 (1) |
Primary outcome
Six-month outcome was available for 82 Early Surgery patients and 85 Initial Conservative Treatment patients; one patient from the Initial Conservative Treatment group was lost to follow-up. Fifty-two (63%) Early Surgery patients had a favourable outcome on the dichotomised GOS, compared with 45 (53%) Initial Conservative Treatment patients (OR 0.65, 95% CI 0.35 to 1.21; p = 0.171): an absolute difference of 10.5% (95% CI –4.4% to 25.3%) (Table 10). Adjusting for age, volume and GCS gave an OR of 0.58 (95% CI 0.29 to 1.16; p = 0.122).
| Primary outcome (prognosis-based) | Early Surgery, n (%) (N = 82) | Initial Conservative Treatment, n (%) (N = 85) | Test and p-value, absolute difference (95% CI) |
|---|---|---|---|
| Unfavourable | 30 (37) | 40 (47) | Chi-squared test, p = 0.170, 10.5% (–4.4% to 25.3%) |
| Favourable | 52 (63) | 45 (53) |
Secondary outcomes
However, there was a highly significant difference in mortality at 6 months, with 12 (15%) Early Surgery patients dying compared with 28 (33%) Initial Conservative Treatment patients (OR 0.35, 95% CI 0.16 to 0.75, p = 0.007): absolute difference 18.3% (95% CI 5.7% to 30.9%) (Table 11). Figure 7 shows the Kaplan–Meier plot of survival for the two groups of patients, illustrating the significant advantage of Early Surgery compared with Initial Conservative Treatment (p = 0.008).
| Variable | Early Surgery, n (%) (N = 82) | Initial Conservative Treatment, n (%) (N = 85) | Test and p-value, absolute difference (95% CI) |
|---|---|---|---|
| Mortality at 6 months | |||
| Dead | 12 (15) | 28 (33) | Chi-squared test, p = 0.006,a 18.3 (5.7 to 30.9) |
| Alive | 70 (85) | 57 (67) | |
| GOS | |||
| Dead | 12 (15) | 28 (33) | Chi-squared test trend, p = 0.047,a POM, p = 0.153 |
| Vegetative | 0 (0) | 0 (0) | |
| Severely disabled | 18 (22) | 12 (14) | |
| Moderately disabled | 26 (32) | 18 (21) | |
| Good recovery | 26 (32) | 27 (32) | |
| GOSE | |||
| Dead | 12 (15) | 28 (33) | Chi-squared test trend, p = 0.052, POM, p = 0.127 |
| Vegetative | 0 (0) | 0 (0) | |
| Lower SD | 4 (5) | 8 (9) | |
| Upper SD | 14 (17) | 4 (5) | |
| Lower MD | 5 (6) | 3 (4) | |
| Upper MD | 21 (26) | 15 (18) | |
| Lower GR | 12 (15) | 12 (14) | |
| Upper GR | 14 (17) | 15 (18) | |
| Dichotomised Rankin Scale score | |||
| Unfavourable | 27 (33) | 37 (44) | Chi-squared test, p = 0.159, 10.6 (–4.0 to 25.3) |
| Favourable | 55 (67) | 48 (56) | |
| Rankin Scale score | |||
| 0 | 17 (21) | 18 (21) | Chi-squared test trend, p = 0.043,a POM, p = 0.147 |
| 1 | 27 (33) | 22 (26) | |
| 2 | 11 (13) | 8 (9) | |
| 3 | 8 (10) | 4 (5) | |
| 4 | 7 (9) | 3 (4) | |
| 5 | 0 (0) | 2 (2) | |
| Dead | 12 (15) | 28 (33) | |
| EQ-5D index | |||
| Median | 0.80 | 0.71 | Mann–Whitney U-test, p = 0.218 |
| IQR | 0.52–1.00 | 0.00–1.00 | |
| Range | –0.33 to 1.00 | –0.59 to 1.00 | |
| Limb movement | |||
| Worse-affected legb | |||
| Unaffected | 50 (72) | 47 (82) | Chi-squared test, p = 0.374 |
| Weak | 18 (26) | 9 (16) | |
| Paralysed | 1 (1) | 1 (2) | |
| Worse-affected armb | |||
| Unaffected | 48 (70) | 43 (75) | Chi-squared test, p = 0.464 |
| Weak | 21 (30) | 14 (25) | |
| Paralysed | 0 (0) | 0 (0) | |
FIGURE 7.
Kaplan–Meier: survival analysis. Log-rank test, p = 0.0081. Reproduced from Mendelow et al. 35 © A. David Mendelow, Barbara A. Gregson, Elise N. Rowan, Richard Francis, Elaine McColl, Paul McNamee, Iain R. Chambers, Andreas Unterberg, Dwayne Boyers, and Patrick M. Mitchell 2015; Published by Mary Ann Liebert, Inc. This Open Access article is distributed under the terms of the Creative Commons License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.

The main causes of death were the initial head injury (Early Surgery 5 vs. Initial Conservative Treatment 14) and pneumonia (Early Surgery 4 vs. Initial Conservative Treatment 2). Other causes of death in the Initial Conservative Treatment group included cachexia (n = 2), ischaemic stroke (n = 2), meningitis (n = 1), pulmonary embolism (n = 2), renal injury (n = 1), head injury and surgery (n = 1), seizure (n = 1) and unknown – sudden death in the community (n = 1). In the Early Surgery group, the other causes were hypovolaemic shock (n = 1), pulmonary embolism (n = 1), head injury and surgery (n = 1) and unknown in the community (n = 1). Only eight non-death-related major adverse events were recorded (in eight patients): seizure (n = 3), new/enlarged haematoma (n = 2), infection (n = 2) and other (n = 1).
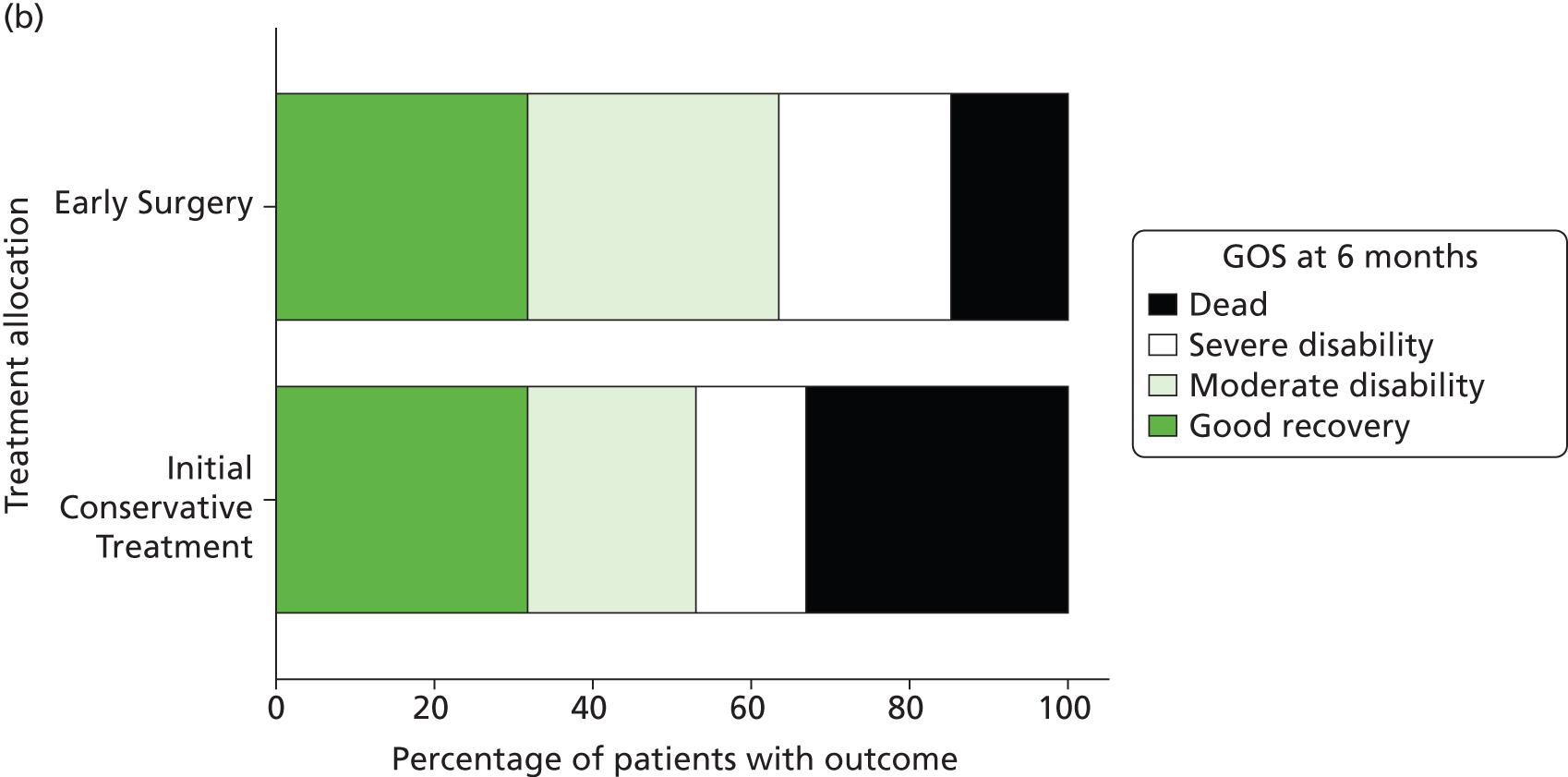
Table 11 and Figure 8 show the distribution of GOS, GOSE and Rankin Scale at 6 months by treatment group. For each of these secondary outcomes there is a significant trend towards better outcome in the Early Surgery group (p = 0.047, 0.052 and 0.043 respectively), although the proportional odds models did not reach statistical significance (OR 0.67, 95% CI 0.39 to 1.16, p = 0.153; OR 0.66, 95% CI 0.38 to 1.13, p = 0.127; OR 0.67, 95% CI 0.39 to 1.15, p = 0.147).
FIGURE 8.
(a) GOSE at 6 months; (b) GOS; and (c) Rankin Scale. Notes (a) proportional odds model, p = 0.127; chi-squared for trend, p = 0.052; (b) proportional odds model, p = 0.153; chi-squared for trend p = 0.047; and (c) proportional odds model, p = 0.147; chi-squared test for trend, p = 0.043. Reproduced from Mendelow et al. 35 © A. David Mendelow, Barbara A. Gregson, Elise N. Rowan, Richard Francis, Elaine McColl, Paul McNamee, Iain R. Chambers, Andreas Unterberg, Dwayne Boyers, and Patrick M. Mitchell 2015; Published by Mary Ann Liebert, Inc. This Open Access article is distributed under the terms of the Creative Commons License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.


There were also no significant differences in EQ-5D or limb movements between groups. Of 68 Early Surgery patients, 20 (29%) reported being employed at 6 months and, of 54 Initial Conservative Treatment patients, responding to the question 13 (24%) reported being employed.
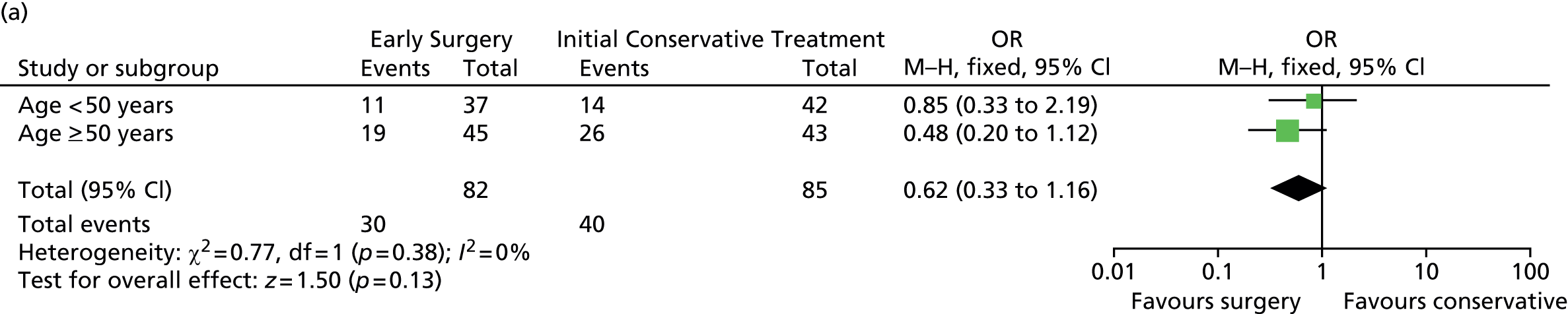
Subgroup analyses
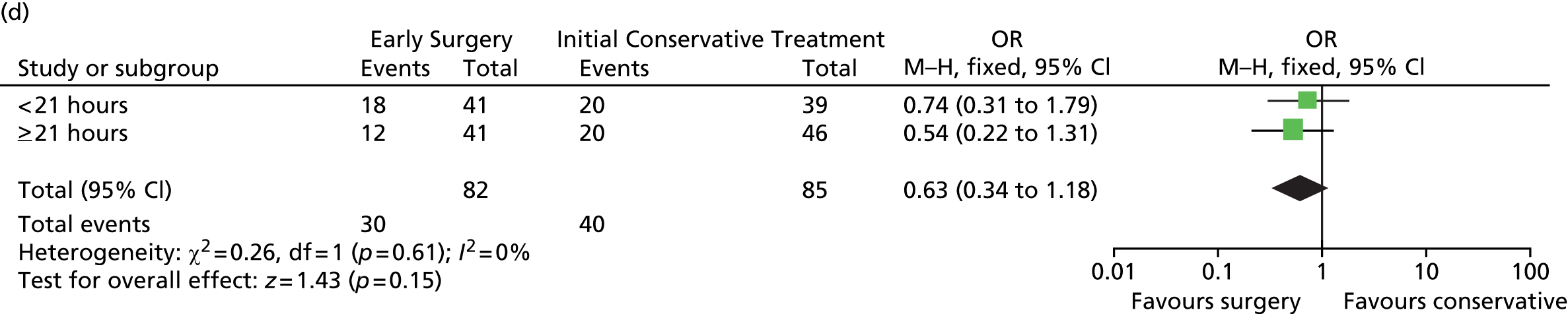
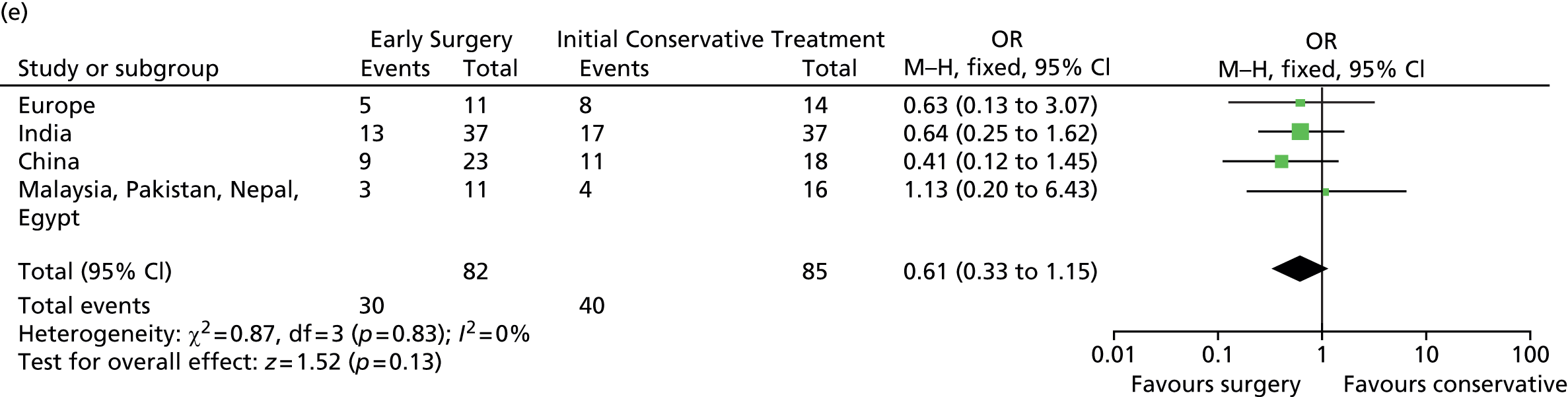
Prespecified subgroup analyses are shown in Figure 9. None of the subgroups display any significant heterogeneity of treatment response, although the patients with a GCS of 9–12 had the best response to Early Surgery.
FIGURE 9.
Subgroup analysis. (a) Age; (b) GCS; (c) volume of haematoma; (d) time from injury to randomisation; and (e) geographical region. df, degrees of freedom; M–H, Mantel–Haenszel. Reproduced from Mendelow et al. 35 © A. David Mendelow, Barbara A. Gregson, Elise N. Rowan, Richard Francis, Elaine McColl, Paul McNamee, Iain R. Chambers, Andreas Unterberg, Dwayne Boyers, and Patrick M. Mitchell 2015; Published by Mary Ann Liebert, Inc. This Open Access article is distributed under the terms of the Creative Commons License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.


Outcome by treatment allocation and treatment received
Looking at outcome by allocated and received treatment, 33% (20 out of 61) of patients who were allocated to Early Surgery and had surgery had died or were severely disabled at 6 months. However, 65% (20 out of 31) of patients who were allocated to Initial Conservative Treatment and had delayed surgery had died or were severely disabled at 6 months, compared with 37% (20 out of 54) of the conservative patients who did not have surgery.
Costs outcome
An unadjusted comparison of raw mean costs showed that Early Surgery was, on average, Int$476 more costly than Initial Conservative Treatment (Table 12). Generalised linear model (GLM) regression analysis, adjusting for patient characteristics, showed Early Surgery to be Int$1774 more costly (95% CI –Int$284 to Int$3831) than Initial Conservative Treatment. Sensitivity analyses showed that overall conclusions were robust to the choice of regression model for the analysis. Results from subgroup analyses (Table 13) were highly uncertain based on small sample sizes (and too small to conduct regression analysis) and should therefore be interpreted with caution.
| Cost (Int$) | Early Surgery (n = 82) | Initial Conservative Treatment (n = 86) | Difference of means | |||
|---|---|---|---|---|---|---|
| Resource use, mean (SD) | Costs, mean (SD) | Resource use, mean (SD) | Costs, mean (SD) | Raw difference | Adjusted difference | |
| All countries | ||||||
| Cost surgery | – | 981 (1678) | – | 515 (1206) | ||
| Cost ICU | 4.18 (4.20) | 2808 (5762) | 4.06 (4.61) | 2988 (6131) | ||
| Cost HDU | 1.72 (2.55) | 385 (1053) | 1.76 (3.01) | 461 (1445) | ||
| Cost ward | 11.88 (15.95) | 3595 (10,206) | 14.24 (29.43) | 3997 (13,789) | ||
| Cost readmission | 4.23 (14.43) | 1145 (5775) | 2.42 (9.63) | 421 (1720) | ||
| Total cost | – | 8812 (18,032)a | – | 8336 (18,685)a | 476 | GLM model 1774 (95% CI –284 to 3831) |
| Cost (Int$) | Early Surgery (n = 6) | Initial Conservative Treatment (n = 10) | Difference of means | ||
|---|---|---|---|---|---|
| Resource use, mean (SD) | Costs, mean (SD) | Resource use, mean (SD) | Costs, mean (SD) | ||
| Low-income countries | |||||
| Cost surgery | – | 142 (0) | – | 14 (45) | |
| Cost ICU | 0.83 (1.60) | 203 (391) | 1.20 (2.70) | 293 (659) | |
| Cost HDU | 3.83 (0.75) | 468 (92) | 3.50 (2.12) | 427 (259) | |
| Cost ward | 5.33 (1.03) | 325 (63) | 6.30 (6.43) | 384 (392) | |
| Cost readmission | 0 (0.00) | 0 (0) | 0 (0.00) | 0 (0) | |
| Total cost | – | 1139 (418) | – | 1118 (614) | 20 |
| Cost (Int$) | Early Surgery (n = 40) | Initial Conservative Treatment (n = 39) | Difference of means | ||
| Resource use, mean (SD) | Costs, mean (SD) | Resource use, mean (SD) | Costs, mean (SD) | ||
| Lower middle-income countries | |||||
| Cost surgery | – | 439 (511) | – | 176 (369) | |
| Cost ICU | 3.20 (3.78) | 580 (1449) | 2.38 (3.39) | 227 (314) | |
| Cost HDU | 1.93 (2.84) | 87 (128) | 1.97 (3.08) | 168 (513) | |
| Cost ward | 5.70 (5.84) | 64 (98) | 6.31 (5.29) | 125 (364) | |
| Cost readmission | 0.35 (2.21) | 3 (20) | 0.13 (0.59) | 1 (5) | |
| Total cost | – | 1174 (1583) | – | 697 (964) | 477 |
| Cost (Int$) | Early Surgery (n = 28) | Initial Conservative Treatment (n = 30) | Difference of means | ||
| Resource use, mean (SD) | Costs, mean (SD) | Resource use, mean (SD) | Costs, mean (SD) | ||
| Upper middle-income countries | |||||
| Cost surgery | – | 1089 (1174) | – | 822 (1031) | |
| Cost ICU | 5.43 (3.79) | 4272 (4134) | 6.93 (4.49) | 6010 (5588) | |
| Cost HDU | 0.93 (1.84) | 643 (1261) | 1.17 (3.26) | 821 (2295) | |
| Cost ward | 16.86 (16.30) | 3603 (4132) | 14.27 (26.05) | 3080 (6267) | |
| Cost readmission | 8.39 (20.55) | 997 (2986) | 6.23 (15.48) | 805 (1881) | |
| Total cost | – | 10,603 (7517) | – | 11,538 (10,149) | –936 |
| Cost (Int$) | Early Surgery (n = 8) | Initial Conservative Treatment (n = 7) | Difference of means | ||
| Resource use, mean (SD) | Costs, mean (SD) | Resource use, mean (SD) | Costs, mean (SD) | ||
| High-income countries | |||||
| Cost surgery | – | 4927 (3617) | – | 2020 (3542) | |
| Cost ICU | 7.25 (5.95) | 13,432 (14,847) | 5.14 (6.72) | 10,310 (15,989) | |
| Cost HDU | 1.88 (3.18) | 1089 (2668) | 0.57 (0.98) | 622 (964) | |
| Cost ward | 30.25 (31.45) | 23,671 (24,462) | 69.71 (68.18) | 34,662 (35,806) | |
| Cost readmission | 12.27 (22.53) | 8233 (16,895) | 2.29 (6.05) | 1719 (4547) | |
| Total cost | – | 46,489 (38,880)a | – | 47,483 (46,221)a | –994 |
Patients randomised to the Early Surgery group had an average gain of 0.019 QALYs over a 6-month period, 95% bootstrapped CI (–0.004 to 0.043), when compared with those randomised to the Initial Conservative Treatment. This is equivalent to an incremental QALY gain of 3.5 days over a 6-month period. The broad QALY gains are driven primarily by the increased chance of survival in the Early Surgery group. A detailed health economic evaluation comparing costs and QALYs has been conducted and is included as Appendix 4 to this report.
Chapter 4 Discussion
The question of whether or not it is beneficial to perform Early Surgery in patients with parenchymal ICH on CT image soon after trauma is one that should be addressed with high priority, as was identified by NICE in the second edition of its guidelines for head injury. 19 The STITCH(TRAUMA) trial was set up and funded in response to those guideline recommendations. Although the trial was stopped early by the UK National Institute for Health Research (NIHR) HTA programme, with an associated reduction in statistical power, there were some clinically significant results. These included a statistically significant survival advantage (85% vs. 67%) and a non-significant benefit on GOS, both associated with Early Surgery. This striking reduction in mortality was not accompanied by an increase in severe dependency and there were no vegetative survivors.
The early management of patients with TICH is not harmonised around the world. The timing of surgery in patients with parenchymal haematomas after head injury has not been standardised, and therefore no firm recommendations have been made. This contrasts with patients who develop EDH or acute SDH, because guidelines based on strong observational data8,9 have recommended early and expeditious scanning and surgery. Not all TICHs need to be removed, and nor do all the contusions associated with them. Generally, clinical deterioration and expansion of the haematomas and their associated oedema tend to trigger the need for surgery. If it were possible to foresee these changes, then secondary brain damage would be avoided. The objective of the STITCH(TRAUMA) trial was to discover if Early Surgery would prevent the secondary deterioration so often seen with conservative treatment for these lesions. While the primary outcome is not statistically significant, there is a strong signal that Early Surgery will indeed prevent such deterioration and save lives. This is seen in the highly significant reduction in mortality and the better outcomes in the ordinal analysis of the GOS and Rankin Scale scores. A larger trial is urgently needed to confirm or refute this signal, which is particularly strong in the patients with a randomisation GCS of 9–12. The groups of patients with GCS of 9–12 in the STICH and STICH II trials showed a similar trend, and a meta-analysis of these groups from all three trials shows a large beneficial effect, which is statistically significant and shows no heterogeneity (Figure 10).
FIGURE 10.
Meta-analysis of ICH subgroup of patients with randomisation GCS between 9 and 12 [prognosis-based outcome for STICH and STICH II and traditional outcome for STITCH(TRAUMA)]. df, degrees of freedom; M–H, Mantel–Haenszel.
Some units in some countries routinely measure ICP while others do not. The issue of Early Surgery for TICH is particularly important in those countries that do not measure ICP. This trial was stopped early by the funding authority because of poor recruitment in the UK, where ICP monitoring is ubiquitous. Therefore, the trial could not recruit to target and was expected to have a neutral conclusion given the reduced sample size. However, the evidence suggests that, if the trial had been allowed to continue to target, a significant difference would have been observed. This would probably be because 86% of patients were not monitored for ICP either because hospitals did not have the technology available or because they do not routinely use it for this patient group. 34 Unfortunately, despite considerable national and international efforts, the trial team was not able to secure alternative funding from other sources in order to continue the trial.
There were crossovers from Initial Conservative Treatment to Early Surgery and vice versa, as occurs in all surgical trials. This is because surgeons felt compelled to provide rescue surgery to those patients randomised to Initial Conservative Treatment who later deteriorated. On the other hand, some patients who were randomised to Early Surgery did not have surgery because their families withdrew consent. Despite these crossovers, which make the intention-to-treat analysis weaker, the effect of Early Surgery exceeded 10% and was statistically almost significant. If the total of 840 planned patients had been recruited, and if the same trend had transpired, this would have been a statistically significant result. In addition, the patients who had delayed surgery had deteriorated to a much poorer clinical state, and this was associated with a much poorer outcome (65% dead or severely disabled, compared with only 33% in those operated upon early).
Predicting which patients will deteriorate is complex and STICH II identified a small number of patients (GCS between 9 and 12) who may benefit from such anticipatory treatment. 10 In general, SICH patients with a good prognosis (GCS between 13 and 15) can be safely observed and require craniotomy only if they deteriorate. This is because there is enough time to perform a craniotomy before other secondary mechanisms such as brain oedema, mass effect with herniation and reduced CPP from elevated ICP cause harm. The RESCUEicp trial36,37 has set out to discover if decompressive craniectomy improves outcome in those patients who have already developed elevated ICP, and recruitment to that trial is now almost complete.
The economic analysis indicates that a strategy of Early Surgery is associated with a small non-significant increase in health-care costs. Furthermore, patients randomised to the Early Surgery group had an average gain of 0.019 QALYs over the initial 6-month period when compared with those randomised to the Initial Conservative Treatment. This is equivalent to an incremental QALY gain of 3.5 days over that period. The broad QALY gains are driven primarily by the increased chance of survival in the Early Surgery group. Based on the results of the study, and the WHO guidelines for cost-effectiveness,38 one could interpret the Early Surgery intervention as offering a high probability of cost-effectiveness in both high- and upper middle-income countries. There may also be a high probability of cost-effectiveness in lower middle-income countries; however, based on the cost-effectiveness acceptability curve (CEAC) analysis, this conclusion would be more sensitive to the threshold value of cost-effectiveness imposed by decision-makers.
Implications for health care
-
There is a strong case for operating on patients with TICH who have a GCS of 9–12. Those who are alert or just confused (GCS of 13–15) can probably be watched carefully for any deterioration because there is a safety margin, which diminishes the lower down the GCS the patient descends.
-
Once the GCS has dropped below 8, surgical intervention appears to be less effective.
-
Based on the results of the study, and the WHO guidelines for cost-effectiveness, the Early Surgery intervention could be interpreted as offering a high probability of cost-effectiveness in both high- and upper middle-income countries. There may also be a high probability of cost-effectiveness in lower middle-income countries; however, based on the CEAC analysis, this conclusion would be more sensitive to the threshold value of cost-effectiveness imposed by decision-makers.
Recommendations for research
This trial has given a very strong signal that Early Surgery is superior to Initial Conservative Treatment for patients with TICH. This signal was evident despite the sample size being only 20% of that originally planned. Given that there are 800,000 such injuries each year in the world (8000 per year in the UK because the UK accounts for 1% of the world’s 7 billion population) the 10.5% absolute improvement in favourable outcome represents 84,000 patients every year that could have a better outcome. If this is true, then the trial needs to be repeated with the utmost urgency to avoid this enormous annual excess death and disability rate that currently prevails for these patients in the UK and everywhere else in the world.
Acknowledgements
This project was funded by the NIHR HTA programme. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, central commisioning foundation, NIHR Evaluation, Trials and Studies Coordinating Centre, the HTA programme or the Department of Health.
Contributions of authors
Dr Barbara A Gregson (Trial Director, Principal Investigator and Statistician) and Professor A David Mendelow (Chief Investigator and Consultant Neurosurgeon) prepared this publication on behalf of all the STITCH(TRAUMA) investigators.
Dr Elise N Rowan (Trial Manager) and Dr Richard Francis (Data Manager) were responsible for supervising the acquisition of the data, checking and validation, analysis and interpretation.
Professor Paul McNamee (Professor of Health Economics) and Dr Dwayne Boyers (Research Associate in Health Economics) undertook the design of the economic study, the economic analysis and prepared Appendix 4.
Mr Patrick Mitchell (Consultant Neurosurgeon and Local Principal Investigator), Professor Elaine McColl (Professor of Health Services Research), Dr Iain R Chambers (Medical Physicist) and Professor Andreas Unterberg (Neurosurgeon and German National Principal Investigator) made a substantial contribution to the design of the study and the interpretation of the data.
Contribution of others
All members of the Steering Committee made a contribution to the design and overseeing of the study and to the interpretation of the work.
The members of the DMC made a contribution to the overseeing of the study and to the interpretation of the work.
The trial management team were responsible for the day-to-day running of the study.
The radiological committee were responsible for the coding and analysis of the CT scans.
The centre investigators were responsible for the acquisition of the data and interpreting the data.
STITCH(TRAUMA) investigators
Principal investigators
Professor A David Mendelow, MB BCh PhD FRCS.
Dr Barbara A Gregson, BSc PhD FSS.
Mr Patrick M Mitchell, BA MB BChir BSc FRCS PhD.
Professor Andy Unterberg, MD PhD.
Professor Elaine M McColl, BA MSc PhD.
Dr Iain R Chambers, BSc PhD CEng FIPEM.
Professor Paul McNamee, MA MSc PhD.
Steering committee
Mr J Steers (Independent Chairman).
Dr A Vail (Statistician).
Dr D Birchall (Neuroradiologist – Independent Member).
Mr J Timothy (Neurosurgeon – Independent Member).
Professor L Vale (Health Economist – Independent Member).
Mr A White (Lay Member – Headway).
Mr D O’Meara (Lay Member – UK Acquired Brain Forum).
Professor AD Mendelow.
Dr BA Gregson.
Mr PM Mitchell.
Dr A Unterberg.
Professor EM McColl.
Dr IR Chambers.
Dr P McNamee.
Dr E N Rowan.
Dr D Boyers.
Dr R Francis.
Data Monitoring Committee
Mr P Hutchinson (Chairman and Neurosurgeon).
Professor GD Murray (Independent Statistician).
Dr A Gholkar (Neuroradiologist).
Trial management team
Professor AD Mendelow (Chief Investigator).
Dr BA Gregson (Principal Research Associate and Trial Director).
Dr E Rowan (Senior Research Associate and Trial Manager).
Dr R Francis (Research Associate and Data Manager).
Dr D Boyers (Research Associate and Economist).
Miss H Atkinson (Trial Administrator 2009–2010).
Miss C Howe (Trial Administrator 2010–2013).
Mr P Mitchell (Neurosurgeon).
Radiological committee
Dr A Hassani.
Mr YK Yap.
Dr L Yap.
Dr A Gholkar.
Centre investigators
Below is a list of centre Investigators by country and centre together with the number of patients recruited. (In this list centres that recorded zero patients did return screening information.)
Canada
Toronto, St Michael’s Hospital (0) – RL Macdonald.
Bulgaria
Sofia, University Hospital Pirogov (2) – N Gabrovsky, N Velinov.
The Czech Republic
Brno, University Hospital Brno (1) – M Smrčka, G Hanoun.
Egypt
Alexandria, Alexandria University Hospitals (3) – OS Abdelaziz, I H Zidan.
Germany
Heidelberg, Heidelberg University Hospital (2) – AW Unterberg, C Beynon.
Munich, Bogenhausen Academic Teaching Hospital, Technical University of Munich (1) – CB Lumenta, DB Schul.
Ulm, University of Ulm School of Medicine (0) – M-E Halatsch, A Pala.
Hungary
Szeged, University of Szeged (0) – P Barzo, B Fulop.
India
Bangalore, BGS Global Hospital (1) – SAV Rao, NK Venkataramanaa.
Bangalore, National Institute of Mental Health and Neuro Sciences (NIMHANS) (5) – S Somanna, KVLN Rao, J lal Gangadharan.
Calcutta, Advanced Medical Research Institute (AMRI Hospitals) (0) – RN Bhattacharya.
Chennai, Fortis Malar Hospital (1) – K Sridhar, G Venkatprasanna.
Dehradun, Himalayan Institute of Medical Sciences (13) – KK Bansal, C Gupta, R Kumar.
Lucknow, King George’s Medical University [erstwhile Chhatrapati Shahuji Maharaj (CSM) Medical University] (29) – SK Singh, C Srivastava, BK Ojha, A Chandra.
Ludhiana, Christian Medical College & Hospital (3) – SS Grewal, B Gupta.
Maharashtra, Acharya Vinoba Bhave Rural Hospital (3) – A Agrawal.
Mullana (Ambala), Maharishi Markandeshwar (MM) Institute of Medical Sciences and Research (1) – A Agrawal.
Mysore, Mysore Clinisearch (2) – A Sangli.
New Delhi, All India Institute of Medical Sciences (8) – P Sarat Chandra, BS Sharma.
Visakhapatnam, Care Hospital (8) – PV Ramana, PM Jagannath.
Latvia
Riga, Pauls Stradins Clinical University Hospital (0) – E Valeinis.
Lithuania
Kaunas, Kaunas University of Health Sciences Hospital (2) – A Tamasauskas, R Vilcinis.
Klaipeda, Klaipeda University Hospital (0) – A Gvazdaitis.
Malaysia
Malaysia, Johor Bahru, Hospital Sultanah Aminah Johor Bahru (1) – NAA Rahman, A Ali.
Kota Bharu, Kelantan, Hospital Universiti Sains Malaysia (6) – JM Abdullah, TY Chin.
Nepal
Biratnagar, Neuro Hospital (16) – YB Roka, PR Puri.
Pakistan
Peshawar, Northwest General Hospital & Research Center Peshawar (2) – T Khan, F Filza.
People’s Republic of China
Beijing, Tiantan Hospital affiliated to Capital Medical University (30) – J Zhao, L Xu, J Li.
Shanghai, Huashan Hospital, Fudan University (9) – Y Sun, J Hu.
Tianjin, Tianjin Medical University General Hospital (4) – S Yang, R Jiang.
Romani
Romania, Cluj-Napoca, Cluj County Emergency Hospital (1) – I S Florian, M Rus.
Timisoara, Emergency County Hospital Timisoara (7) – H Ples, S M Hanas.
Spain
Santander, University Hospital Marqués de Valdecilla (2) – A Vázquez-Barquero.
Valladolid, Universitario Río Hortega (1) – R Sarabia, I Arrese.
United Kingdom
Cambridge, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust (0) – P J Kirkpatrick, A G Kolias.
Dundee, Ninewells Hospital and Medical School (2) – S Eljamel.
Haywards Heath, Hurstwood Park Neuroscience Centre (0) – G Critchley, J Norris.
Newcastle, Royal Victoria Infirmary (3) – P Bhattathiri, N Ross.
Southampton, Southampton General Hospital (1) – A Belli, D Bulters.
United States of America
Los Angeles, Los Angeles County & University of Southern California Medical Center (0) – JP Gruen.
Philadelphia, Temple University Hospital (0) – M W Weaver, F Sultan.
Portland, Legacy Emanuel Medical Center (0) – J W Chen, S Staat.
Publications
Kenyon GM, Mendelow AD, Gregson BA, Rowan E. Obtaining regulatory approval for multicentre randomised controlled trials: experiences in the STICH II trial. Br J Neurosurg 2011;25:352–6.
Francis R, Rowan E, Gregson BA, Mendelow AD. Traumatic intracerebral hemorrhage – to operate or not? World Neurosurg 2011;76:484–5.
Mendelow AD, Gregson BA, Rowan EN, Francis R, McColl E, McNamee P, et al. Early surgery versus initial conservative treatment in patients with traumatic intracerebral haemorrhage (STITCH[Trauma]): the first randomized trial. J Neurotrauma 2015;32:1312–23.
Francis R, Gregson BA, Mendelow AD. Attitudes to intracranial pressure monitoring of traumatic intracerebral haemorrhage. Br J Neurosurg 2014;28:663–5.
Gregson BA, Rowan EN, Mitchell PM, Unterberg A, McColl EM, Chambers IR, et al. Surgical trial in traumatic intracerebral hemorrhage (STITCH(Trauma)): study protocol for a randomized controlled trial. Trials 2012;13:193.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- NICE Clinical Guideline Sets Out Recommendations for NHS Care of People who have Suffered a Head Injury. London: NICE; 2003.
- Gabbe BJ, Biostat GD, Lecky FE, Bouamra O, Woodford M, Jenks T, et al. The effect of an organized trauma system on mortality in major trauma involving serious head injury: a comparison of the United Kingdom and Victoria, Australia. Ann Surg 2011;253:138-43. http://dx.doi.org/10.1097/SLA.0b013e3181f6685b.
- Mendelow AD. Mechanisms of ischemic brain damage with intracerebral hemorrhage. Stroke 1993;24:I115-17.
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005;365:387-97. http://dx.doi.org/10.1016/S0140-6736(05)17826-X.
- Gallbraith S, Teasdale G. Predicting the need for operation in the patient with an occult traumatic intracranial hematoma. J Neurosurg 1981;55:75-81. http://dx.doi.org/10.3171/jns.1981.55.1.0075.
- Bratton SL, . AANS/CNS Joint Section on Neurotrauma and Critical Care . Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma 2007;24:S55-8.
- Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med 2012;367:2471-81. http://dx.doi.org/10.1056/NEJMoa1207363.
- Mendelow AD, Karmi MZ, Paul KS, Fuller GA, Gillingham FJ. Extradural haematoma: effect of delayed treatment. Br Med J 1979;1:1240-2. http://dx.doi.org/10.1136/bmj.1.6173.1240.
- Seelig JM, Becker DP, Miller JD, Greenberg RP, Ward JD, Choi SC. Traumatic acute subdural hematoma: major mortality reduction in comatose patients treated within four hours. N Engl J Med 1981;304:1511-18. http://dx.doi.org/10.1056/NEJM198106183042503.
- Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013;382:397-408. http://dx.doi.org/10.1016/S0140-6736(13)60986-1.
- Prasad K, Mendelow AD, Gregson B. Surgery for primary supratentorial intracerebral haemorrhage. Cochrane Database Syst Rev 2008;4.
- Siddique MS, Gregson BA, Fernandes HM, Barnes J, Treadwell L, Wooldridge TD, et al. Comparative study of traumatic and spontaneous intracerebral hemorrhage. J Neurosurg 2002;96:86-9. http://dx.doi.org/10.3171/jns.2002.96.1.0086.
- Mathiesen T, Kakarieka A, Edner G. Traumatic intracerebral lesions without extracerebral haematoma in 218 patients. Acta Neurochir 1995;137:155-63. http://dx.doi.org/10.1007/BF02187188.
- Choksey M, Crockard HA, Sandilands M. Acute traumatic intracerebral haematomas: determinants of outcome in a retrospective series of 202 cases. Br J Neurosurg 1993;7:611-22. http://dx.doi.org/10.3109/02688699308995090.
- Zumkeller M, Hollerhage HG, Proschl M, Dietz H. The results of surgery for intracerebral hematomas. Neurosurg Rev 1992;15:33-6. http://dx.doi.org/10.1007/BF02352065.
- Boto GR, Lobato RD, Rivas JJ, Gomez PA, de la Lama A, Lagares A. Basal ganglia hematomas in severely head injured patients: clinicoradiological analysis of 37 cases. J Neurosurg 2001;94:224-32. http://dx.doi.org/10.3171/jns.2001.94.2.0224.
- D’Avella D, Cacciola F, Angileri FF, Cardali S, La Rosa G, Germano A, et al. Traumatic intracerebellar hemorrhagic contusions and hematomas. J Neurosurg Sci 2001;45:29-37.
- Bullock M, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell D, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery 2006;58:S25-46. http://dx.doi.org/10.1227/01.NEU.0000210365.36914.E3.
- Clinical Guidelines CG56 Head Injury: Triage, Assessment, Investigation and Early Management of Head Injury in Infants, Children and Adults. London: NICE; 2007.
- Gregson BA, Rowan EN, Mitchell PM, Unterberg A, McColl EM, Chambers IR, et al. Surgical trial in traumatic intracerebral hemorrhage (STITCH(Trauma)): study protocol for a randomized controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-193.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. http://dx.doi.org/10.1111/j. 2002.384.doc.x.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987-93. http://dx.doi.org/10.1161/01.STR.24.7.987.
- Fairbank J, Frost H, Wilson-MacDonald J, Yu L-M, Barker K, Collins R, et al. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38441.620417.8F.
- Weinstein JN, Tosteson TD, Lurie JD, Tosteson ANA, Hanscom B, Skinner JS, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial.[see comment]. JAMA 2006;296:2441-50. http://dx.doi.org/10.1001/jama.296.20.2441.
- Pettigrew LE, Wilson JT, Teasdale GM. Assessing disability after head injury: improved use of the Glasgow Outcome Scale. J Neurosurg 1998;89:939-43. http://dx.doi.org/10.3171/jns.1998.89.6.0939.
- Mendelow AD, Teasdale GM, Barer D, Fernandes HM, Murray GD, Gregson BA. Outcome assignment in the International Surgical Trial of Intracerebral Haemorrhage. Acta Neurochir 2003;145:679-81. http://dx.doi.org/10.1007/s00701-003-0063-9.
- Murray GD, Barer D, Choi S, Fernandes H, Gregson B, Lees KR, et al. Design and analysis of phase III trials with ordered outcome scales: the concept of the sliding dichotomy. J Neurotrauma 2005;22:511-17. http://dx.doi.org/10.1089/neu.2005.22.511.
- Briggs A, Gray A. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess 1999;3.
- Drummond M, Barbieri M, Cook J, Glick HA, Lis J, Malik F, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health 2009;12:409-18. http://dx.doi.org/10.1111/j.1524-4733.2008.00489.x.
- Manca A, Sculpher MJ, Goeree R. The analysis of multinational cost-effectiveness data for reimbursement decisions: a critical appraisal of recent methodological developments. Pharmacoeconomics 2010;28:1079-96. http://dx.doi.org/10.2165/11537760-000000000-00000.
- Campbell & Cochrane Economics Methods Group and the Evidence for Policy and Practice Information and Co-ordinating Centre (CCEMG – EPPI) . CCEMG – EPPI-Centre Cost Converter (v.1.2) n.d.
- World Bank . GNI Per Capita, How We Classify Countries n.d. http://data.worldbank.org/about/country-classifications (accessed 20 March 2014).
- Kenyon GM, Mendelow AD, Gregson BA, Rowan E. Obtaining regulatory approval for multicentre randomised controlled trials: experiences in the STICH II trial. Br J Neurosurg 2011;25:352-6. http://dx.doi.org/10.3109/02688697.2010.551675.
- Francis R, Gregson B, Mendelow A, Rowan E. Characteristics of Traumatic Intracerebral Haemorrhage: An Assessment of Screening Logs from the STITCH(Trauma) Trial 2014.
- Mendelow AD, Gregson BA, Rowan EN, Francis R, McColl E, McNamee P. Early surgery versus initial conservative treatment in patients with traumatic intracerebral haemorrhage (STITCH[Trauma]): the first randomized trial. J Neurotrauma 2015;32:1312-23.
- Hutchinson P. The RESCUEicp Study. RESCUEicp n.d. www.rescueicp.com/ (accessed 26 February 2014).
- Hutchinson PJ, Corteen E, Czosnyka M, Mendelow AD, Menon DK, Mitchell P, et al. Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEicp study. Acta Neurochir Suppl 2006;96:17-20. http://dx.doi.org/10.1007/3-211-30714-1_4.
- World Health Organization (WHO) . Cost Effectiveness and Strategic Planning (WHO CHOICE) n.d. www.who.int/choice/costs/CER_thresholds/en/ (accessed 27 March 2014).
- NHS Payment by Results 2013–14 National Tariff Information. London: DH; 2013.
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Briggs A, Wonderling D, Mooney C. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40. http://dx.doi.org/10.1002/(SICI)1099-1050(199707)6:4<327::AID-HEC282>3.0.CO;2-W.
- Simon J, Gray A, Duley L. Cost effectiveness of prophylactic magnesium sulphate for 9996 women pre-eclampsia from 33 countries: economic evaluation of the MAGPIE trial. BJOG 2006;113:144-51. http://dx.doi.org/10.1111/j.1471-0528.2005.00785.x.
- Kind P. The EuroQol Instrument: an Index of Health Related Quality of Life. Quality of life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincot-Raven; 1996.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: University of York; 1999.
Appendix 1 Consent
Patient information sheet_England_v2.1
Patient Consent Form_England_v2.1
Relative information sheet_England_v3.1
Relative consultation formV2
Retrospective patient information sheet_England_v1.1
Patient gaining capacity Consent Form _England v1
Appendix 2 Data collection forms
Randomisation form
Discharge/2-week follow-up form
Six-month postal follow-up for UK
Six-month postal follow-up for non-UK
Detailed ICP monitoring form
Major event form
Screening log
Appendix 3 Template letters
Initial standard general practitioner letter
6-month standard general practitioner letter
Template cover letter to accompany 6-month form
Appendix 4 Health economic analysis
Prepared by Dr Dwayne Boyers and Professor Paul McNamee
Introduction
The objective of the economic evaluation is to assess the cost-effectiveness of a strategy of Early Surgery compared with Initial Conservative Treatment in the management of traumatic ICH. This chapter reports the quality of life outcomes, resource use, costs and cost-effectiveness analyses performed alongside the STITCH(TRAUMA) randomised controlled trial, over a 6-month follow-up period.
It should be noted that the original intention of the economic component of the study was to conduct a cost–utility analysis from the UK NHS perspective, using cost and outcome data collected only on UK patients. We anticipated recruiting 150 UK patients into the trial to achieve this goal. However, UK recruitment achieved only six patients, which was not sufficient to produce a meaningful assessment of costs and outcomes for UK patients. An alternative analysis was therefore conducted, which used data from all participating centres in all countries recruiting into the trial. This approach has the advantage of being more relevant to a wider group of decision-makers. However, such an approach required the collection of additional cost data in non-UK sites, which proved challenging for some centres. Results are presented in terms of all patients recruited as a single analysis, as well as subgroup analyses based on World Bank country income group classifications (low-, lower middle-, upper middle- and high-income countries).
Methods
Resource use and costs
The main analysis focuses on results from an international perspective. However, given that the original objective of the cost-effectiveness analysis was to report from a UK perspective, we begin by reporting UK resource use for the six patients recruited into the trial.
Resource use and costs based on a UK analysis
Descriptive results are presented for the resource use and costs associated with UK participants for information only. Costs are assigned to resource use data based on 2013 health resource group (HRG) payment by results data. 1 We apply the non-elective cost associated with intracranial procedures for trauma with a diagnosis of intracranial injury (HRG code AA02). 39 Costs with (£6231 up to 43 days admission + £207 per day thereafter) and without (£4126 up to 18 days + £207 per day thereafter) complications are presented. As our data are presented in days in hospital, we calculate cost per day as the tariff value, divided through by the trim-point time. We then take the average tariff for those with and without complications. This means an overall cost per day applied to resource use in the analysis of £187 per day over the initial episode of care. Further, data from the patient questionnaire are used to estimate the number and length of hospital readmissions at 6 months’ follow-up. As the exact reason for readmission was not reported, we have assumed the same HRG codes would apply to these episodes of care also.
Resource use and costs based on international analysis
All data from the six UK trial participants are used in the costing analysis together with additional data collected from the other trial participating countries. Collection of this supplementary data required the development of a site-specific questionnaire, administered to all participating centres in the trial, to collect data on resource use and unit costs of care. The supplementary questionnaire used for the analysis is included as Appendix 5. Not all sites responded to this questionnaire which generated a substantial amount of missing data. Data were returned for 16 out of 31 sites (52%), representing 115 out of 168 (68%) patients recruited to the study. This required the imputation of cost and resource use data following some plausible assumptions, namely:
-
Where resource use and cost data were missing for some centres recruiting within a country, we have imputed weighted averages based on the number of patients recruited at centres where data are available.
-
Where resource use and cost data were missing for all centres within a country, we have pragmatically imputed weighted average data from all countries in the same income group, with country income subgroups determined according to World Bank classifications.
The impact of these data imputation methods is tested in sensitivity analyses, described in the sensitivity analyses section of this chapter.
The costing analysis is reported on the intention-to-treat principle and undertaken from an international health services perspective. The likely major drivers of costs (i.e. surgery, hospital stay and readmissions) are included. Surgery resource use (including staff time and overheads) and unit costs (e.g. surgeon’s salary and cost of theatre use) were collected using the site-specific questionnaires. Other health-care resource use data were collected, including days in intensive care, high-dependency units and general wards (all sourced from individual case report forms) and hospital readmissions (sourced from the participant 6-month questionnaire). The analysis follows recommendations from Drummond et al. 29 and Manca et al. ,30 reporting resource use and cost data at the country level. The costing of these hospital resource use data is undertaken in two stages. First, we apply country-specific unit costs for nights in hospital (intensive care unit, high-dependency unit, general ward) to resource use data to generate total costs. National average unit costs were not available for the majority of countries in the trial. Costs were therefore sourced directly from finance departments at specific sites and applied to resource use data to generate estimates of costs. Then, country-specific unit costs were transformed into international dollar costs (2013 values), using the Campbell & Cochrane Economics Methods Group purchasing power parity calculator. 31
To account for the highly skewed nature of cost data (i.e. a small proportion of patients incurring very high costs), we use GLM regression models, specifying a gamma family and identity link which best fits the distribution of the cost data. The choice of base-case model for the analysis was made on the basis of the lowest Akaike information criterion score, a method which is recommended as standard best practice. 30 Heteroskedastic robust standard errors were used for all analyses. The model estimates the impact of treatment group (Early Surgery compared with Initial Conservative Treatment) on costs adjusting for patient characteristics (age and sex). GLM models were bootstrapped using non-parametric bootstrapping techniques (n = 1000 repetitions) in Stata Version 13 (StataCorp LP, College Station, TX, USA) to generate data for developing CEACs. 31
Subgroup analyses of costs
Owing to the differences in organisation of care across countries and the value of money differences across jurisdictions, it is likely that the base-case analysis (a single analysis of all trial participants), while statistically more efficient, may have little relevance to informing decision-making at an individual country level. Results are, thus, also reported for groups of countries based on a measure of their development. For this purpose, all countries within the trial were ranked in ascending order of GNI per capita, according to World Bank classifications. The classification groups are low income, GNI per capita equal to Int$1005 or less (including Nepal); lower middle income, GNI per capita between Int$1006 and Int$3975 (including India); upper middle income, GNI per capita between Int$3976 and Int$12,275 (including China); and high income, GNI per capita of Int$12,276 or more (including most Western European countries). The logic is that such countries with similar GNI will deliver broadly comparable levels of care and reporting in this way improves the relevance of the costing for local policy-makers. It also provides an intuitive grouping of countries in the absence of enough data to conduct a country-specific analysis. This approach has been used successfully in a previous study. 40
Quality-adjusted life-years
The EQ-5D-3L generic quality of life instrument41 was administered to all trial participants at 6 months’ follow-up. Responses to the EQ-5D-3L questionnaire were valued using UK general population tariffs32 to generate a utility score for every patient within the trial. We assumed that all patients suitable for randomisation were in an unconscious state and would thus have a baseline utility of –0.402. 42 Given a lack of published tariff data across all trial recruiting countries, we have assumed that the UK tariffs offer a reasonable reflection of quality of life scores across all trial participants. This assumption is associated with limitations and assumes preferences for health states valued on the EQ-5D-3L are similar across countries. However, this approach offered the most pragmatic solution and has the advantage of applying tariffs derived from a standard method (i.e. time trade-off) to all quality of life data.
Quality-of-life data derived from the EQ-5D-3L are combined with mortality data from the trial, using the standard assumption that all participants who have died in the trial will have a utility value of 0. QALYs are then calculated on the basis of these assumptions using an area beneath the curve approach, assuming linear extrapolation of utility between time points [baseline (assumed –0.402) and 6 months’ follow-up]. Where the date of death was available, the QALY calculation has been modified to include this additional information. This introduces some asymmetries into the calculation between those who died and those who survived. The impact of assuming a linear extrapolation between time points for all patients is tested in the sensitivity analysis.
Differences in QALY estimates across groups are analysed using ordinary least squares (OLSs) standard linear regression models. Bootstrapped regressions (1000 repetitions in Stata) are conducted to account for the non-normality of QALY data and regressions are adjusted for patient characteristics, namely age and sex, and to generate data for CEACs. 43 Heteroskedastic robust standard errors are applied to all models. Subgroup analyses are presented for the utility scores and QALYs for each country income group according to that outlined in the Subgroup analyses of costs section. This facilitates the production of incremental cost-effectiveness ratios (ICERs) for each country income group.
Cost–utility analysis
The health economic evaluation is a cost–utility analysis, reporting results as incremental cost per QALY gained for Early Surgery compared with Initial Conservative Treatment. The cost per QALY is presented using the ICER, calculated as the coefficient of treatment effect on costs divided by the coefficient of treatment effect on QALYs from the respective linear regression models. Estimates of the ICER should then be compared with the normal decision-making practice in individual countries or regions.
Costs and QALY differences are presented on the cost-effectiveness plane and CEACs are derived from the net benefit statistic to illustrate the probability of Early Surgery being the most cost-effective option. All statistical analyses were conducted using Stata and Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) was used for calculation of CEACs. Owing to the short period of follow-up of only 6 months, no discounting of costs or QALYs was necessary. No extrapolation to a longer-term time horizon was conducted. Owing to the acute nature of the clinical indication, and the likely recovery time after surgery, it is likely that all patients will either have recovered or died during the trial follow-up period, with no substantial additional costs or QALYs to be accrued over a lifetime horizon.
Sensitivity analyses and assumptions
Resource use and costs
A range of sensitivity analyses were undertaken in order to test the impact of assumptions and uncertainty surrounding resource use and cost data. Specifically:
-
We have tested the impact of variability of resource use and costs at different sites within a country. We conducted an assessment where costs for a given country were estimated on the basis of the highest and lowest cost sites within a country, with those costs applied to all sites in that country. This analysis helps to assess the variability in the organisation of care at different sites within a country and will to some extent address the impact of any differences in the private/public provision of care at different sites.
-
We have tested the impact of variability of resource use and costs at a regional level, where a region is defined by the World Bank country income classifications outlined above. The aim of this sensitivity analysis is to address the uncertainty across countries within an income group and present a plausible range of costs and ICERs for countries (including those not participating in the trial) at a regional level.
These and other assumptions used for the analysis of cost data are presented in Table 14. The table outlines the assumptions made together with justifications for each assumption and any sensitivity analyses conducted to assess the impact on costs and cost-effectiveness.
| Assumption no. | Issue arising | Assumption made | Justification for assumption | How uncertainty is incorporated | Implication for interpretation of results |
|---|---|---|---|---|---|
| C1 | Missing data from site-specific questionnaire regarding surgical costs and resource use | If only one centre in a country has reported data, then assume these data are applicable to all centres in that country. If more than one country has data available, impute the weighted average for missing data in that country | Provides the best possible assessment of average costs for each country, weighted for the largest recruiting centres in an individual country | Conduct sensitivity analysis imputing data from the highest and lowest resource use and cost centre data to all centres in that country | Sensitivity analysis will provide a within-country range of possible costs which can be presented to local decision-makers. This will account for differences in public/private mix of care which may impact on costs |
| C2 | Surgery and hospital stay costing. Payment methods to hospitals and reimbursement methods differ across countries | Take data reported in the questionnaires as being comparable | This assumption is made because the questionnaire was designed in a way to obtain similar information from all sites, although in practice some sites may have reported overheads while others may not | Conduct sensitivity analyses imputing the highest and lowest resource use and costs estimates from within a country income group to all countries in that income group | This assumption has implications for cross-country comparisons of cost-effectiveness. However, the questionnaire design provides a reasonably comparable and standardised method of asking resource use and costs across all centres. Sensitivity analyses assess the cross-country variation and its impact on cost-effectiveness |
| C3 | Missing data for all centres in a given country (e.g. Malaysia/Lithuania) | Assume that costs are equal to the average of all countries contributing data to that income group of countries (low, lower middle, upper middle, high) | The logic is that countries in the same income group will have broadly similar organisations of care and will incur similar values of costs | Sensitivity analysis conducted in C2 above deals with uncertainty in this assumption | Taken together, the base-case and sensitivity analyses will provide a plausible minimum and maximum range in which costs and incremental costs may fall, allowing the presentation of a range for the most likely ICER |
| C4 | HDU/ICU/ward unit cost data from the additional costing questionnaire. Missing data from the questionnaire | Take weighted averages as above wherever possible imputing for (a) site data to all sites in the country and (b) country data to all countries in an income group | As above | Sensitivity analyses conducted for C1 and C2 above address uncertainty in this assumption | Base-case and sensitivity analyses give a plausible range for the calculations |
Quality of life and quality-adjusted life-years
European Quality of Life-5 Dimensions-3 level follow-up responses and death data were fully recorded for all respondents who entered the trial and therefore QALY data were complete, with no missing data needing to be imputed. Owing to a large number of deaths within the trial, we have conducted sensitivity analyses exploring the impact of alternative methods of extrapolation of the EQ-5D-3L utility data for calculation of QALYs in the trial. The base-case analysis imputes a utility score of ‘0’ from the date of death to 6-month follow-up for those who died. Survivors’ QALYs are calculated on the basis of linear interpolation between time points. This reflects the fact that there may, in theory, be a QALY benefit to dying earlier in the trial, as opposed to remaining in a health state valued worse than death for a longer period of time. However, the use of these data in this way creates an asymmetry of information between those who survived and those who died over the trial follow-up, as we have more precise information for those who died. We, therefore, conducted an alternative analysis, in which all patients accrued QALY gains using the same linear interpolation, regardless of whether they survived or died. Although this method addresses the issues of asymmetry in the information available, it does not make use of all information available to us for QALY calculation. Assumptions surrounding QALY calculations, justifications and associated sensitivity analyses conducted where appropriate are outlined in Table 15.
| Assumption no. | Issue arising | Assumption made | Justification for assumption | How uncertainty is incorporated | Implication for interpretation of results |
|---|---|---|---|---|---|
| Q1 | No baseline utility data collected due to severity of initial injuries of patients in the trial | All patients enter in an unconscious state and thus have a baseline utility equivalent to that of the unconscious state, valued at –0.402 | This is reflective of the likely state of health, or close to the likely state of health of patients at the time of randomisation | Owing to the lack of alternative data, we have not conducted sensitivity analyses on this assumption. As all other clinical baseline estimates were similar at baseline, it is likely that our assumption will not bias outcomes | This assumption, assuming equality of baseline utility could in theory create a bias of for or against the Early Surgery. However, given similarity across groups of baseline clinical characteristics, it is unlikely that any biases would change cost-effectiveness results |
| Q2 | Most countries in the trial do not have published EQ-5D valuation tariffs | UK tariffs can be used for QALY calculations for all participants in the trial | A similar method is used and applied to all (i.e. the time trade-off). Using differing methods (e.g. visual analogue scale/standard gamble) could introduce greater biases | No sensitivity analyses undertaken, as alternative tariffs not available for participating countries | Results of the QALY analysis are important to all countries, but could be re-ran if new time trade-off tariffs for individual countries become available |
| Q3 | There were many deaths in the trial. QALYs may depend on how deaths are included in the calculations | A linear interpolation between time points was assumed | Standard method of analysis | Sensitivity analysis explores the impact of using date of death directly in the QALY calculations | Any differences between the methods of interpolating between time points for QALY calculation should be taken account of in interpretation of the results. The base-case and sensitivity analysis together will give a range for the ICER |
Analysis models of data and the impact of crossovers within the trial
In addition to the sensitivity analyses carried out on the resource use, unit cost and QALY calculations outlined in Tables 14 and 15, we conduct two further sensitivity analyses investigating the impact on results of (1) using an alternative non-parametric bootstrapped OLS regression model to account for the non-normal distribution of both cost and QALY data and (2) including interaction terms in the base-case analysis model to address the impact of crossovers on incremental costs, incremental QALYs and on cost-effectiveness.
Sampling uncertainty
Non-parametric bootstrapping techniques,44 based on 1000 repetitions of the GLM and OLS regressions were undertaken to determine the cost and QALY differences, respectively, between Early Surgery and Initial Conservative Treatment. Data from these bootstrapped regressions for cost and QALY differences were used to develop CEACs and to present scatterplots of cost and QALY differences on the cost-effectiveness plane. 44 CEACs are calculated using a net benefit approach and indicate the probability of an intervention being cost-effective at various threshold values of willingness to pay (WTP) for a QALY gain. They are especially useful when making decisions on cost-effectiveness on the balance of probabilities, and when incremental costs or effects fail to meet the traditional level of statistical significance (i.e. 95% confidence).
Results
Resource use and costs – site-specific costing questionnaire
Full hospital resource use data were available for all patients within the trial (n = 168). These data were supplemented by unit cost information and surgical resource use data collected from the additional site questionnaires. The questionnaires were sent to all 31 recruiting sites in 13 countries worldwide. Data were returned for 16 out of 31 sites (52%), representing 115 out of 168 (68%) patients recruited to the study. Completeness of data from the questionnaires is outlined in Figure 11.
FIGURE 11.
Data completeness (supplementary costing questionnaire) for surgical costs questionnaire, by patients recruited.

Data from the site-specific questionnaires returned were used to make plausible assumptions about resource use and unit costs in other countries where data were missing. These assumptions and sensitivity analyses undertaken have been outlined in Table 14.
The results of the surgery costing exercise show wide variation within countries, for example India (Figure 12) and across countries (Figure 13).
FIGURE 12.
Within country variation of cost of surgery (example of Indian centres). Note that only two sites in India reported complete surgery costing data.

FIGURE 13.
Across country variation in surgery costs (Int$).

Figure 12 shows substantial variation in costs across the two sites which reported data for India. This is because of different public/private mixes of care at these hospitals and illustrates the impact this variability can have on the cost estimates at a country level. The variation between sites in India is the most extreme of all the countries recruiting to the trial; however, it illustrates the need to conduct sensitivity analyses for the imputation of data at all the other centres who did not contribute data to the resource use and costing questionnaire. Figure 13 illustrates the variability in costing across countries.
There is wide variability across the different countries recruiting to the trial. As expected, countries in Europe have much greater treatment costs than those in lower-income countries. However, even within country income groups, there appears to be substantial variability. The most extreme variation in country groups is between the UK and the Czech Republic, which again illustrates the importance of sensitivity analyses to assess the impact of this variation on total cost estimates for the final cost-effectiveness calculations.
Resource use and costs: – UK analysis
The results of the resource use and costs associated with the six patients recruited from the UK into the study are presented in Table 16, using descriptive statistics and are presented for information only.
| Cost item | Early Surgery, mean (SD) resource use | Initial Conservative Treatment, mean (SD) resource use | Early Surgery, mean costs per patient (£)a | Initial Conservative Treatment, mean costs per patient (£)a | Mean cost difference (Early Surgery vs. Initial Conservative Treatment) (£) |
|---|---|---|---|---|---|
| n | 2 | 4 | 2 | 4 | – |
| Cost surgery | – | – | – | – | – |
| Cost ICU | 7 (9.9) | 3.5 (7) | – | – | – |
| Cost HDU | 3.5 (4.9) | 1 (1.5) | – | – | – |
| Cost ward | 28 (24) | 49.3 (61) | – | – | – |
| Cost of initial episode of care (without CC – with CC) | Mean total days = 38.5 | Mean total days = 53.8 | 7199.50 | 10,061 | –2861.50 |
| Cost hospital readmission (days) | 32.5 (46) | 4 (8) | 6077.50 | 748 | 5329.50 |
| Total costs | – | – | 13,277 | 10,809 | 2468 |
The remainder of this chapter refers to the costing and cost-effectiveness analysis from an international health-care provider perspective, using data from all participants recruited into the study.
Resource use and costs: international analysis
The results of the base-case costing analysis, showing mean [standard deviation (SD)] resource use and mean (SD) costs in international dollars, based on the intention-to-treat principle are reported in Table 17.
| Costs (Int$) | Early Surgery (n = 82) | Initial Conservative Treatment (n = 86) | Difference of means | |||
|---|---|---|---|---|---|---|
| Resource use; mean (SD) | Costs (Int$); mean (SD) | Resource use; mean (SD) | Costs (Int$); mean (SD) | Raw difference (Int$) | Adjusted difference (Int$) (95% CI) | |
| All countries | ||||||
| Cost surgery | – | 981 (1678) | – | 515 (1206) | 476 | 1774 (–132 to 3679) |
| Cost ICU | 4.18 (4.2) | 2808 (5762) | 4.06 (4.61) | 2988 (6131) | ||
| Cost HDU | 1.72 (2.55) | 385 (1053) | 1.76 (3.01) | 461 (1445) | ||
| Cost ward | 11.88 (15.95) | 3595 (10,206) | 14.24 (29.43) | 3997 (13,789) | ||
| Cost readmission | 4.23 (14.43) | 1145 (5775) | 2.42 (9.63) | 421 (1720) | ||
| Total cost | – | 8812 (18,032)a | – | 8336 (18,685)a | ||
For the whole sample, a comparison of raw mean costs shows that Early Surgery was, on average, Int$476 more costly than Initial Conservative Treatment. Using the general linear modelling estimates, with adjustment for patient characteristics of age and sex, Early Surgery is Int$1774 more costly (95% CI –Int$132 to Int$3679). The results are not significantly different between groups at the traditional 5% level of significance, but are significant at the 10% level. This suggests that there is weak evidence which suggests that Early Surgery is significantly more expensive than Initial Conservative Treatment. This perhaps indicates that patients, who are more likely to survive in the Early Surgery arm, are thus more likely to incur greater health-care costs also, through longer term treatment, and rehospitalisations as a result of their survival.
Costing subgroup analysis
Table 18 presents the cost breakdown of resource use and costs by income subgroups based on World Bank income classifications presented in the Methods section.
| Costs (Int$) | Early Surgery (n = 6) | Initial Conservative Treatment (n = 10) | Difference of means | |||
|---|---|---|---|---|---|---|
| Resource use, mean (SD) | Costs, mean (SD) | Resource use, mean (SD) | Costs, mean (SD) | Raw difference | Adjusted difference, (95% CI)a | |
| Low-income countries | ||||||
| Cost surgery | – | 142 (0) | – | 14 (45) | 20 | 205 (–48 to 459) |
| Cost ICU | 0.83 (1.60) | 203 (391) | 1.20 (2.70) | 293 (659) | ||
| Cost HDU | 3.83 (0.75) | 468 (92) | 3.5 (2.12) | 427 (259) | ||
| Cost ward | 5.33 (1.03) | 325 (63) | 6.30 (6.43) | 384 (392) | ||
| Cost readmission | 0.00 (0.00) | 0 (0) | 0.00 (0.00) | 0 (0) | ||
| Total cost | – | 1139 (418) | – | 1118 (614) | ||
| Costs (Int$) | Early Surgery (n = 40) | Initial Conservative Treatment (n = 39) | Difference of means | |||
| Resource use, mean (SD) | Costs, mean (SD) | Resource use, mean (SD) | Costs, mean (SD) | Raw difference | Adjusted difference, (95% CI) | |
| Lower middle-income countries | ||||||
| Cost surgery | – | 439 (511) | – | 176 (369) | 477 | 192 (–1010 to 395) |
| Cost ICU | 3.20 (3.78) | 580 (1449) | 2.38 (3.39) | 227 (314) | ||
| Cost HDU | 1.93 (2.84) | 87 (128) | 1.97 (3.08) | 168 (513) | ||
| Cost ward | 5.70 (5.84) | 64 (98) | 6.31 (5.29) | 125 (364) | ||
| Cost readmission | 0.35 (2.21) | 3 (20) | 0.13 (0.59) | 1 (5) | ||
| Total cost | – | 1174 (1583) | – | 697 (964) | ||
| Costs (Int$) | Early Surgery (n = 28) | Initial Conservative Treatment (n = 30) | Difference of means | |||
| Resource use, mean (SD) | Costs, mean (SD) | Resource Use, mean (SD) | Costs, mean (SD) | Raw difference | Adjusted difference, (95% CI) | |
| Upper middle-income countries | ||||||
| Cost surgery | – | 1089 (1174) | – | 822 (1031) | –936 | –1798 (–8378 to 781) |
| Cost ICU | 5.43 (3.79) | 4272 (4134) | 6.93 (4.49) | 6010 (5588) | ||
| Cost HDU | 0.93 (1.84) | 643 (1261) | 1.17 (3.26) | 821 (2295) | ||
| Cost ward | 16.86 (16.30) | 3603 (4132) | 14.27 (26.05) | 3080 (6267) | ||
| Cost readmission | 8.39 (20.55) | 997 (2986) | 6.23 (15.48) | 805 (1881) | ||
| Total cost | – | 10,603 (7517) | – | 11,538 (10,149) | ||
| Costs (Int$) | Early Surgery (n = 8) | Initial Conservative Treatment (n = 7) | Difference of means | |||
| Resource use, mean (SD) | Costs, mean (SD) | Resource use, mean (SD) | Costs, mean (SD) | Raw difference | Adjusted difference, (95% CI) | |
| High-income countries | ||||||
| Cost surgery | – | 4927 (3,617) | – | 2020 (3542) | –994 | –9679 (–41,613 to 22,256) |
| Cost ICU | 7.25 (5.95) | 13,432 (14,847) | 5.14 (6.72) | 10,310 (15,989) | ||
| Cost HDU | 1.88 (3.18) | 1089 (2668) | 0.57 (0.98) | 622 (964) | ||
| Cost ward | 30.25 (31.45) | 23,671 (24,462) | 69.71 (68.18) | 34,662 (35,806) | ||
| Cost readmission | 12.27 (22.53) | 8233 (16,895) | 2.29 (6.05) | 1719 (4547) | ||
| Total cost | 46,489 (38,880)b | 47,483 (46,221)b | ||||
Sample sizes were small for country subgroups and results should be interpreted with caution. This is particularly true for low- and high-income subgroups, which recruited 30 participants. Results should therefore be treated as exploratory. Owing to the small sample sizes, there is no evidence of significant differences in costs at the country income subgroup level.
Quality-adjusted life-years
For the purposes of this analysis, we assume that all patients in the trial started with an EQ-5D-3L health state of unconscious, corresponding to a baseline utility value of –0.402, applied to all patients in the trial. Figure 14 details the results of the responses to the individual EQ-5D-3L domains at 6 months’ follow-up. Data are presented on the basis of the percentage of respondents to the questionnaire who reported any problems on any of the EQ-5D-3L domains, broken down by randomised group.
FIGURE 14.
Responses to the EQ-5D-3L.

The results show that a greater proportion of respondents in the Early Surgery group experienced at least some problems in each of the five domains of the EQ-5D-3L, when compared with respondents in the Initial Conservative Treatment group. The most notable differences were in the Usual Activities, Pain/Discomfort and Anxiety/Depression domains, in which at least 20% more respondents in the Early Surgery group had at least some problems. In contrast, the proportion of patients who had died over the course of the 6-month follow-up period was twice as high in the Initial Conservative Treatment group as in the Early Surgery group (33% vs. 15% respectively). These data suggest that as more people survive in the Early Surgery group, they continue to have some problems in their quality of life at 6 months’ follow-up.
Table 19 presents the descriptive statistics for the QALY analysis, based on the assumption of all patients commencing with a baseline utility score of –0.402, equivalent to being unconscious. Owing to the non-normal nature of the QALY data, means (SD) are presented together with median (intraquartile range) for information. Differences between groups are estimated using the bootstrapped regressions described in the Methods section.
| Utility | Early Surgery (n = 82) | Initial Conservative Treatment (n = 86) | Difference of means | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Raw difference | Adjusted differencea | |
| Baseline utilityb | –0.402 (0.000) | –0.402 (–0.402 to –0.402) | –0.402 (0.000) | –0.402 (–0.402 to –0.402) | ||
| 6-month utility | 0.663 (0.374) | 0.796 (0.516 to 1.000) | 0.530 (0.454) | 0.710 (0.000 to 1.000) | ||
| QALY (over 6 months’ follow-up) | 0.078 (0.074) | 0.0985 (0.0285 to 0.1495) | 0.06 (0.085) | 0.077 (–0.004 to 0.1495) | 0.018 | 0.019 (95% bootstrapped CI –0.004 to 0.043) |
Based on the regression analysis, incorporating date of death into the QALY calculation, and adjusting for patients’ characteristics of age and sex, we find that on average, patients randomised to the Early Surgery group had an average gain of 0.019 QALYs over a 6-month period, 95% bootstrapped CI (–0.004 to 0.043), when compared with those randomised to the Initial Conservative Treatment. This is equivalent to an incremental QALY gain of 3.5 days over a 6-month period. The broad QALY gains are driven primarily by the increased chance of survival in the Early Surgery group.
Subgroup analysis of quality-adjusted life-years data
Again applying UK-specific population weights to the quality of life scores from the EQ-5D-3L questionnaire, Table 20 presents the results by country income subgroup as classified by the World Bank country income subgroups described in the Methods section.
| Country income group | Early Surgery | Initial Conservative Treatment | QALY difference (95% bootstrap CI)a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | 6-month, mean (SD) | QALY, mean (SD) | n | Baseline, mean (SD) | 6-month, mean (SD) | QALY, mean (SD) | ||
| All countries | 82 | –0.402 (0) | 0.663 (0.374) | 0.078 (0.074) | 86 | –0.402 (0) | 0.53 (0.454) | 0.06 (0.085) | 0.019 (–0.004 to 0.043) |
| Low income | 6 | –0.402 (0) | 0.918 (0.094) | 0.129 (0.023) | 10 | –0.402 (0) | 0.876 (0.331) | 0.129 (0.051) | –0.018 (–0.055 to 0.019) |
| Lower middle income | 40 | –0.402 (0) | 0.658 (0.369) | 0.082 (0.062) | 39 | –0.402 (0) | 0.548 (0.453) | 0.069 (0.075) | 0.015 (–0.014 to 0.045) |
| Upper middle income | 28 | –0.402 (0) | 0.630 (0.369) | 0.07 (0.074) | 30 | –0.402 (0) | 0.391 (0.460) | 0.029 (0.094) | 0.037 (–0.006 to 0.08) |
| High income | 8 | –0.402 (0) | 0.606 (0.514) | 0.051 (0.129) | 7 | –0.402 (0) | 0.575 (0.378) | 0.052 (0.081) | 0.008 (–0.283 to 0.299) |
For all income subgroups, utility scores were, on average, higher in the Early Surgery group than in the Initial Conservative Treatment group at 6 months’ follow-up. There were negligible differences in raw mean QALYs for the low- and high-income countries, although sample sizes were very small and the regression outputs should be interpreted with caution. However, lower middle- and upper middle-income countries tended to show average QALY gains for those in the Early Surgery group. The results indicate that, although not reaching statistically significant QALY gains, patients in these groups are likely to experience QALY gains from Early Surgery. The results are promising for Early Surgery and indicate broad generalisability across the largest recruiting countries. Owing to the very small sample sizes recruited, it is not possible to draw conclusions on QALY outcomes for the low- and high-income countries.
Cost-effectiveness
The results of the base-case cost-effectiveness analysis are presented in Table 21.
| Base case result | Early Surgery, mean (SD) | Initial Conservative Treatment, mean (SD) | Difference of means | |
|---|---|---|---|---|
| Raw difference | Adjusted difference (95% CI)a | |||
| Costs (Int$) | 8812 (18,032) | 8336 (18,685) | 476 | 1774 (–132 to 3679) |
| QALYs | 0.078 (0.074) | 0.060 (0.085) | 0.018 | 0.019 (–0.004 to 0.043) |
| ICER (Int$) | – | – | 26,444 | 93,368 |
For the base-case analysis, the incremental costs for the whole group together were Int$1774, with average QALY gains of 0.019. Therefore, on this basis, the base-case ICER is Int$1774/0.019 = Int$93,368 per QALY gained for Early Surgery when compared with Initial Conservative Treatment.
However, the ICER in itself should be interpreted with caution, given the range of countries which contributed data to the costing process, and also relating to alternative thresholds of cost-effectiveness which may be used by decision-makers in different jurisdictions. This complicates interpretation of the ICER. It may thus be more informative to examine the incremental costs and QALYs for individual subgroups based on their country income classification. These data are presented in Table 22. Owing to uncertainty in incremental costs and effects, we have not presented ICERs in this table. Decision-makers are instead referred to the CEAC presented in Figure 17, which illustrates the uncertainty in cost-effectiveness at the subgroup level.
| Country group | Early Surgery costs (Int$) | Initial Conservative Treatment costs (Int$) | Incremental costsa (Int$) | Early Surgery QALYs (Int$) | Initial Conservative Treatment QALYs (Int$) | Incremental QALYsa (Int$) |
|---|---|---|---|---|---|---|
| Base-case analysis | 8812 | 8336 | 1774 | 0.078 | 0.06 | 0.019 |
| Low income | 1139 | 1118 | 205 | 0.129 | 0.129 | –0.018 |
| Lower middle income | 1174 | 697 | 192 | 0.082 | 0.069 | 0.015 |
| Upper middle income | 10,603 | 11,538 | –1798 | 0.070 | 0.029 | 0.037 |
| High income | 46,489 | 47,483 | –9679 | 0.051 | 0.052 | 0.008 |
As expected, there are substantial differences in costs across the subgroups. It appears that incremental costs of Early Surgery versus Initial Conservative Treatment could be decreasing for more developed countries. However, the results could equally be skewed by outliers in the data, which would have a large impact, given the small numbers. Despite substantial uncertainty in the presented results at a subgroup level, the data, on balance, suggest that favourable results could be achieved for Early Surgery in all subgroups with the exception of low-income countries.
Sensitivity analyses
A range of sensitivity analyses were undertaken focusing on the assumptions used to impute data from the site-specific questionnaire, QALY calculation methods and methods of analysis of the data. The impact of these analyses on cost-effectiveness results is outlined in Table 23. Sensitivity analyses on cost and QALY calculations are based on the assumptions outlined in the methods section and cross-referenced in the table below. Further analyses explore the impact of the model of analysis of cost data and the impact of crossovers within the study. Two ICERs are produced for each sensitivity analysis, the first based on a comparison of raw mean data across groups and the second based on the incremental costs and QALYs calculated using regression analyses described.
| Sensitivity analysis | Early Surgery costs (Int$) | Initial Conservative Treatment costs (Int$) | Incremental costs (Int$)a | Early Surgery QALYs | Initial Conservative Treatment QALYs | Incremental QALYsb | ICER (unadjusted data) (Int$/QALY) | ICER (modelled data) (Int$/QALY) |
|---|---|---|---|---|---|---|---|---|
| Base-case analysis | 8812 | 8336 | 1774 | 0.078 | 0.06 | 0.019 | 26,444 | 93,368 |
| Assumptions regarding missing data from centre-specific questionnaires (see Table 14) | ||||||||
| Assumption C1: Lowest resource use and cost data for individual centres applied to all centres in that country | 5940.00 | 5207.32 | 289.32 | 0.078 | 0.06 | 0.019 | 40,704 | 15,227 |
| Assumption C1: Highest resource use and cost data for individual centres applied to all centres in that country | 10,404.77 | 9256.37 | 2692.75 | 0.078 | 0.06 | 0.019 | 63,800 | 141,724 |
| Assumption C2: Lowest resource use and cost data for any country in a country income group, applied to all countries in that income group | 5362.30 | 3613.50 | 517.30 | 0.078 | 0.06 | 0.019 | 97,156 | 27,226 |
| Assumption C2: Highest resource use and cost data for any country in a country income group, applied to all countries in that income group | 16,291.51 | 14,211.96 | 1780.15 | 0.078 | 0.06 | 0.019 | 115,531 | 93,692 |
| Sensitivity analyses (see Table 15) | ||||||||
| Assumption Q3: Assume QALYs calculated with linear extrapolation from baseline directly to date of death, as opposed to current assumption of linear extrapolation to 6 months for those who died in the trial | 8812 | 8336 | 1774 | 0.065 | 0.032 | 0.0351 | 14,303 | 50,541 |
| Using a standard OLS regression method, with bootstrapped CI for costsc | 8812 | 8336 | 314c | 0.078 | 0.06 | 0.019 | 26,444 | 16,526 |
| Interaction terms in model to account for crossovers | 9316 | 5566 | 1021 | 0.087 | 0.078 | 0.011 | 416,667 | 92,818 |
Owing to the small sample size in two of the income subgroups, sensitivity analyses are performed only on the whole sample of patients recruited into the trial.
The results of the sensitivity analyses show some important impacts on cost-effectiveness calculations depending on the assumptions used in the analysis. For the costing assumptions, as expected, imputing higher cost estimates increased the ICERs, and lower cost estimates reduced the ICER. Based on the range of cost imputations tested in the sensitivity analyses, the ICER ranged from Int$15,227 to Int$141,724. This serves to illustrate the impact of within-country variation in reported unit costs and resource use from the centre-specific questionnaires, and the impact of assumptions around missing data on cost estimates used for the cost-effectiveness analysis. This variation is probably driven by the differing public/private mix of care in specific countries, and especially in relation to data provided for India. The results were less sensitive to cross-country variation, within World Bank country income groups. This adds some confidence to the reliability of cross-country comparisons within income subgroups and suggests results may to some extent be generalisable to other countries within income groups.
The sensitivity analysis with one of the greatest impacts on the ICER related to the method of QALY calculation. The base-case analysis makes use of all available information, imputing a utility score of ‘0’ for those who have died, from the date of death to the end of follow-up. We chose to use the date of death for QALY estimation because, given the initial negative utility, patients could in theory attain a QALY advantage from dying earlier in the follow-up period. However, the use of date of death will create an asymmetry of information on the time point at which utility is accrued in the trial between those who have died and those who survived, as we have no comparable information for survivors, other than at their 6-month follow up. Therefore, in order to illustrate the impact of this asymmetry, we have conducted an analysis assuming a linear interpolation between time points when calculating QALYs. This effectively ignores the date of death in the calculations but addresses the asymmetry of information. Take, for example, a patient who died at 10 days after surgery. In the base-case analysis, they would have [(–0.402 + 0)/2] × (10/365)] = –0.005507 QALY, over the first 10 days + 0 QALYs between death and 6 months. For the sensitivity analysis, the equivalent calculation would be –0.1005 QALYs [(–0.402 + 0)/2] × (182.5/365)]. These results show substantial differences in QALY calculation depending on whether or not date of death is accounted for.
In addition to the base-case GLM model, we conducted exploratory analysis on the impact of model of analysis on trial outcomes. For example, running a bootstrapped OLS regression model to account for the skewed distribution of the cost data shows Early Surgery being Int$314 more costly [95% bootstrapped CI (–Int$5139 to Int$5766)]. The resultant ICER falls to Int$16,526 per QALY gained. However, this analysis should only be interpreted as exploratory and closer examination of the data confirms that the GLM model with gamma distribution is the most appropriate fit to the data. However, the analysis serves to illustrate the potential uncertainty and impact of analysis model on the costing results.
Running an alternative model as a sensitivity analysis, which includes interaction terms to address crossovers in the GLM model, suggests on average higher costs and lower quality of life for groups which have crossed over in both directions, therefore indicating that crossovers have an important impact on results. Increases in costs and deterioration in QALYs were significant at the 10% and 5% levels respectively for crossovers from initial conservative management to surgery. These results would be expected, given that crossovers to surgery were likely a result of emergency circumstances. In the model which adjusts for crossovers, being randomised to Early Surgery, and receiving the randomised allocation suggests incremental costs of Int$1021 (95% CI –Int$367 to Int$2368) compared with being randomised to and receiving conservative management.
Sampling uncertainty
In order to address sampling uncertainty in our estimates, we present the bootstrapped iterations of incremental costs and incremental outcomes on a scatterplot of the cost-effectiveness plane. These are also presented in the form of a CEAC, outlining the probability of Early Surgery intervention being a cost-effective use of scarce health-care resource use. Figures 15 and 16 illustrate the probability of cost-effectiveness for the base-case analysis.
FIGURE 15.
Scatterplot of the cost-effectiveness plane: Early Surgery vs. Initial Conservative Treatment (all randomised patients – all countries). ES, Early Surgery; ICT, Initial Conservative Treatment.

FIGURE 16.
Cost-effectiveness acceptability curve: base-case analysis. ES, Early Surgery; ICT, Initial Conservative Treatment.

The graphical illustrations presented above indicate the probability of cost-effectiveness of Early Surgery compared with Initial Conservative Treatment and illustrate the sampling uncertainty surrounding the cost-effectiveness calculations. The scatterplot shows that there is a high probability of Early Surgery delivering improved QALYs; however, there is much uncertainty surrounding the incremental cost estimates. Figure 16 shows the probability of Early Surgery being cost-effective at certain threshold values of WTP for a QALY gain. The probability of cost-effectiveness increases as WTP increases, indicating approximately 50% probability of cost-effectiveness at a threshold value of WTP for a QALY gain of Int$50,000, increasing to approximately 65% when the threshold increases to Int$100,000. However, this graph should be interpreted with caution. Conclusions on cost-effectiveness would depend on a number of issues, including (1) how reflective these costs, which are based on all recruiting countries, are of individual country circumstances and (2) what amount of money decision-makers are willing to pay in international dollars for a QALY gain in their country. In the light of these two issues, Figure 17 presents the CEACs calculated for each of the individual income subgroups recruiting into the trial and may be more informative at a local decision-making level.
FIGURE 17.
Cost-effectiveness acceptability curves for base-case and country income subgroups. ES, Early Surgery; ICT, Initial Conservative Treatment.

The data broken down by subgroup and presented above indicate wide variation in the probability of cost-effectiveness depending on the country income subgroup considered. The probability of cost-effectiveness appears to be lowest for the low-income country group. However, data for both low- and high-income groups are based on very small numbers recruited and are as such unreliable to draw firm conclusions. Higher-income (e.g. UK, Germany), upper middle-income (e.g. China) and lower middle-income (e.g. India) countries have a probability of cost-effectiveness between 70% and 80% at a Int$50,000 threshold value of WTP for a QALY gain.
Interpretation of these country income subgroup CEACs is likely to depend on the wealth of individual countries and countries in income subgroups here should draw conclusions based on the data presented for their income subgroup but also in conjunction with normal threshold values of WTP for a QALY in their jurisdiction. The WHO Choosing Interventions that are Cost-Effective (CHOICE) project has issued guidance to assist with this process, suggesting that an ICER less than three times gross domestic product (GDP) per capita might be considered cost-effective and an ICER that is less than GDP per capita would be considered highly cost-effective.
Discussion
Our analysis is undertaken from an international health services perspective, with costs and QALYs presented by country income subgroup, according to World Bank classifications. Our analysis follows a similar approach to one previously used to report international data in major surgery trials. 28 Our results suggest that an improvement in average QALY outcomes may be achievable for additional costs of Early Surgery, with many of the QALY analyses falling just short of statistical significance at the 5% level. However, in one sensitivity analysis which varied the QALY calculation method, incremental QALYs were significant, highlighting the importance of how the date of death is treated within the calculations. In terms of costs, there were no significant differences between groups. The results are promising for the potential cost-effectiveness of Early Surgery and further studies are warranted to confirm these findings. These results indicate that, had the trial recruited to its original target, there is a high probability that we would see significant improvements in QALYs associated with Early Surgery.
The results of the analyses and the probability of cost-effectiveness are probably best interpreted on the income subgroup level. Although the subgroup analyses lack any statistical power, they are perhaps more relevant to decision-makers locally. The probability of cost-effectiveness at alternative threshold values of WTP for a QALY gain should be interpreted in the light of guidelines for cost-effectiveness in individual countries. Not all countries will have formal cost-effectiveness criteria for deciding on best practice guidelines. However, some studies7 have determined threshold values of WTP for a QALY gain. In addition, the WHO has suggested three levels of cost-effectiveness based on GDP per capita in that country. According to these guidelines, interventions could be considered cost-effective if their cost is < GDP per capita (very cost-effective); 1–3 times GDP per capita (cost-effective); > 3 times GDP per capita (not cost-effective). 38 The thresholds for 2013 could be calculated for any individual country in the trial, based on data from the World Bank, GDP per capita 2012 international dollars. 28 The threshold for each income country subgroup could be taken as the average of the GDP per capita in all of the trial participating countries in that country group. On this basis, the thresholds for low-income countries would be Int$1457 (very cost-effective) and Int$4371 (cost-effective); for lower middle-income countries Int$4389 (very cost-effective) and Int$13,167 (cost-effective); for upper middle-income countries Int$14,763 (very cost-effective) and Int$44,289 (cost-effective); and for high-income countries Int$32,363 (very cost-effective) and Int$97,089 (cost-effective).
This assessment of threshold values is designed to be a broad indicator of cost-effectiveness for individual countries and is based on the assumption that WTP for a QALY gain and WTP to avert a disability-adjusted life-year loss would be similar. This of course is an assumption which may not fall true in reality given the different make-up of the measures. However, while they are not identical, they have many similarities in their composition and could offer a broad estimate of the value placed on health in individual countries which may not have any formal measure of deciding on cost-effectiveness.
Based on the results of the study, and the WHO guidelines for cost-effectiveness,38 one could interpret the Early Surgery intervention as offering a high probability of cost-effectiveness in both high- and upper middle-income countries. There may also be a high probability of cost-effectiveness in lower middle-income countries also; however, based on the CEAC analysis, this conclusion would be more sensitive to the threshold value of cost-effectiveness imposed by decision-makers.
The results are based on a number of assumptions which have the potential to greatly influence the final cost-effectiveness results, as is evident from our sensitivity analyses. The data should therefore be interpreted as a preliminary indication of cost-effectiveness, based on currently available evidence. A larger trial population would provide more robust evidence.
Conclusions
The cost-effectiveness analysis indicates that Early Surgery may be associated with additional QALYs and increases in health-care expenditures. However, differences in costs and QALYs do not reach statistical significance. The results of our analyses, especially in relation to costs, should be interpreted with caution, in light of the assumptions outlined in this chapter. Further research is required to determine more conclusively whether Early Surgery is more cost-effective than Initial Conservative Treatment.
Appendix 5 Supplementary Costing Questionnaire
List of abbreviations
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPP
- cerebral perfusion pressure
- CT
- computed tomography
- DMC
- Data Monitoring Committee
- EDH
- extradural haematoma
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 level
- GCS
- Glasgow Coma Score
- GDP
- gross domestic product
- GLM
- generalised linear model
- GNI
- gross national income
- GOS
- Glasgow Outcome Scale
- GOSE
- Extended Glasgow Outcome Scale
- HRG
- health resource group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICH
- intracerebral haemorrhage
- ICP
- intracranial pressure
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OLS
- ordinary least square
- OR
- odds ratio
- QALY
- quality-adjusted life-year
- SD
- standard deviation
- SDH
- subdural haematoma
- SICH
- spontaneous intracerebral haemorrhage
- STICH
- Surgical Trial In spontaneous intraCerebral Haemorrhage
- STICH II
- Surgical Trial In spontaneous lobar intraCerebral Haemorrhage
- STITCH(TRAUMA)
- Surgical Trial In Traumatic intraCerebral Haemorrhage
- TICH
- traumatic intracerebral haemorrhage
- TSC
- Trial Steering Committee
- WHO
- World Health Organization
- WTP
- willingness to pay