Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/01/27. The contractual start date was in September 2011. The draft report began editorial review in September 2014 and was accepted for publication in April 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
James Raftery is a member of the National Institute for Health Research Editorial Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Williamson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Definition
The term otitis media with effusion (OME) is often used synonymously with ‘glue ear’. 1 The descriptive definition is based on the intraoperative findings of sticky mucinous secretions behind the eardrum that can hamper the free movement of the ossicles in the middle ear. The viscosity of the middle ear fluid has been found to vary and the fluid is sometimes described as serous or secretory. 1,2 Effusions are associated with notional conductive hearing losses of about 15–45 dB owing to damping effects on sound transmission to the inner ear. OME occurs usually in one ear, but frequently in both ears. 3 Probably the earliest and most relevant description of the condition from a primary care perspective was made by Dr John Fry, who wrote about the ‘catarrhal child’: a syndrome of coughs, colds, catarrh and subhealth, in which OME and frequent or persistent upper respiratory infections are the predominant features, and which presents most commonly in young children of early school age. 4
Natural history and scale of the problem
As many as 80% of children of all ages develop OME. 3 Most such episodes are anticipated to resolve naturally, with an average duration of 6–10 weeks, and with just 10% of episodes lasting ≥ 1 year. 5,6 The problem with such a common condition is that it is often regarded as normal. The prevalence rises to 46% (a secondary peak) in the early school years,6 when recurrent ear-related symptoms and broader developmental concerns most often bring the condition to light,7,8 and not infrequently results in surgical referral for grommets. 1,9,10 Time to resolution of effusions remains clinically unpredictable; many last ≥ 3 months and 30% are recurrent. 3,11 In the UK, about 200,000 children are seen every year with this problem in primary and community care. 1,12 The full scale of the total health burden is only partly reflected in high international rates for grommet surgery,13–15 but with falling rates observed in the UK. 16 In the USA, in 2004, as many as 2.2 million people were diagnosed, at an estimated cost of US$4B. 17
The impact of this very common condition on child physical health, hearing, speech, behaviour, development and mapped quality of life (QoL) has been found to be just as great in a primary care sample as in hospital samples. 9,18–21
Clinical management
Diagnostic evaluation in primary care
Initial temporising management in primary care is often pragmatic, ad hoc and influenced mainly by parental concerns. 1,4 Research about diagnosis of OME suggests that more could be done in this setting to improve diagnostic accuracy. 22–25
Although the history alone has moderate specificity, it is not particularly sensitive for how a child is likely to function, for example in a noisy learning environment. 26,27 OME has been described as a chronic ‘invisible’ illness that can be relapsing and frustrating for both the parent and child. It has been clinically noted that uncertainties are often expressed by families regarding whether the root cause is a behavioural issue or a genuine hearing problem. Concerns about school achievement have also been suggested as an important driver for surgical intervention. 28 Case ascertainment for treatment poses several dilemmas, caused not just by (a) the current lack of available ‘proportionate and cost-effective’ treatments (see The evidence for non-surgical interventions), but also by (b) lack of agreement within the profession about what one is treating: whether a disease or condition, a disorder, a disability or a global qualitative impact, as one moves from the more biomedical to a patient-centred model of the condition. 19,29 In general practice, evaluating this multidimensional aspect of OME appears to approximate to a simple formula or rule of thumb as: history of persistence (reported duration) × perceived severity (number of related surgery attendances and/or level of concern about salient markers in different domains, e.g. hearing-/speech-/ear-related physical health/behaviour and development). Symptoms and concerns are reasonable but variable predictors of the state of the child’s ears in terms of current effusion status,30 reinforcing the case for improving clinical assessment in primary care both at the point of treatment and to improve accuracy of referrals.
A careful clinical history is of central importance for case recognition and appears reasonably good for purpose; UK ear, nose and throat (ENT) referrals, although variable, are fairly conservative: about 15% referred in the General practice Nasal steroid trial of Otitis Media with Effusion (GNOME) trial, and with ≈50% of children nationally who are referred on, but who are subsequently found not to meet the strict criteria for grommets (after a period of waiting conducted in hospital, which will necessarily include natural resolution effects). 9,10,30,31 The majority of all affected children will initially undergo observation in primary care or in the community. 1 The more frequently that parents report ear-related episodes in their child in the previous 12 months, the greater the predictive values for the finding of an effusion on screening tympanometry [two or more episodes have an odds ratio (OR) of 2.9, and five or more have an OR of 4.3]. 32 However, it is probable that children with OME-related impacts remain under-recognised. 33 The intermittent history is problematic, and the spectrum of need is wide. 1,34 In this context, the current markedly limited range of evidence-based treatments both on offer and capable of informing policy requires strengthening.
A detailed history, for example finding evidence of reported hearing difficulty with other typical symptoms/concerns associated with OME, may be supplemented by good-quality otoscopy (using a halogen light) or, better still, by using tympanometry. 1 There are no studies of symptom predictors of effusions from primary care, but the sensitivity of history is thought to be around 70%. 1 Both otoscopy and tympanometry are more objective measures in pinpointing the current status of effusion(s) than parent report alone, and have some potential to improve case recognition and clarify those requiring treatment. Tuning fork tests are unfortunately unreliable for the vast majority of children when age at presentation is considered. Relevant audiological tests that improve precision include free-field voice testing done by specialists, and this is probably the most reliable evaluation of hearing for the presenting age group. 1 Accurate pure-tone audiography is not really feasible and is seldom valid in primary care settings.
However, all currently used tests and assessments, irrespective of setting, remain only weakly predictive of the QoL experienced by children and their families. 21 Patient-reported outcome measures (PROMs) can capture such information and are relatively new for the condition of OME. They are moving from the research to clinical audit stage in secondary care, but remain at the research stage in primary care. 9,18,29 Such outcomes are intrinsically holistic and both child and family centred, and thus well suited to primary care use in their shortest available pragmatic form(s). They are endorsed as an important part of the battery of assessments by Cochrane, and are also seen to be of high priority by other experts. 35
Prognosis
Prognostic factors for likely persistence have been extensively reviewed, and nearly all have ORs below 2, curtailing their usefulness somewhat in clinical settings. 31,32,36 Age appears to be the best available predictor of population outcome, with fewer children aged > 6–7 years ‘troubled’ by OME. There is also a clear effect of season, with fewer cases found in the summer months (some of these may be allergy rather than infection related). 3,31,32
Birthweight and skull size have been mooted to have prognostic relevance as have genetic factors,8,31 but such variables have not found any clinical application. There appears to be little, if any, effect of sex at the level of disease/condition, although it is known that boys are slower to read than girls, and so sex may be a confounding factor in presentation and by contributing to outcome severity. This illustrates the importance of cofactors that may either heighten (or reduce) the impact of the OME, such as poor communicating styles at home or school, or reduced ability to lip read because of uncorrected poor visual acuities. 37
Sharing age-related natural history-based prognosis with families is very important, but generally it is difficult on the individual level to predict which children will persist with effusions for 3 months, thus partly justifying a watch-and-wait period.
The evidence for non-surgical interventions (available for use in primary care)
Published systematic reviews have evaluated many studies across a wide selection of non-surgical interventions that are currently used in the treatment of OME. The selected interventions considered here have been made with multidisciplinary input from (a) the current British Medical Journal Clinical Evidence team;34 (b) ENT colleagues editing the Scott-Brown Otolaryngology series;31 and (c) the National Institute for Health and Care Excellence (NICE)’s 2009 grommets review. 1 The original (trial protocol) searches were based on a MEDLINE search from 1966 to March 2010, EMBASE from 1980 to March 2010, and the Cochrane Database of Systematic Reviews from inception to 2010. All searches have since been updated using MEDLINE and EMBASE for systematic reviews to include individual randomised controlled trials (RCTs), and all publications using the key terms ‘otitis media’ and/or ‘OME’ that have been published between January 2006 up to August 2014. The main interventions of interest and summarised below are antibiotics, steroids, antihistamines/decongestants and autoinflation. Cochrane has underlined the importance of OME as a condition of considerable importance, with nine current published reviews on the topic and one protocol on its website (www.cochrane.org), with several of these reviews updated over the study period.
The evidence for antibiotics
The most recent updated Cochrane review suggests that antibiotics are unlikely to be beneficial. The research was extensive and included 23 trials and 3027 subjects. 38 The author’s main conclusion was that there is no statistically significant evidence that antibiotics produce resolution of OME in the short term. Six studies39–44 (n = 738 children) were combined and show slight benefit of long-term antibiotics at 6 months, but none of these was from primary care. Unwanted effects of antibiotics reported in the literature include rashes, nausea, vomiting, diarrhoea and anaphylaxis. Unnecessary use of antibiotics also promotes the development of antibiotic resistance and the medicalisation of minor illness. 45–50 A few uncertainties remain for targeted, well-considered and selective use of antibiotics. 46 Speculatively, this may include secondary care subgroups as an alternative to surgery, or for any antibiotic-sensitive biofilm infection, or where recurrent acute otitis media (AOM) rather than OME is deemed to be the predominant underlying pathology. 51
To conclude, with inadequate evidence for routine use of antibiotics from primary care, a number needed to treat (NNT) was estimated to be over 20,52 and with the escalating level of threat from antibiotic resistance, antibiotics should not be recommended for routine use.
The evidence for steroids
A Cochrane systematic review,53 search date August 2010, which included 12 studies and 945 patients, found some evidence for improved short-term resolution of OME in those treated in secondary care with oral steroids, either alone or combined with antibiotics. However, there is insufficient evidence to date to determine their effect on resolution of OME-related symptoms or on longer-term outcomes. The systematic review also included several trials, and a UK primary care study of topical intranasal corticosteroids, and concluded that there was sufficient evidence to make the statement that there was ‘no benefit from topical intranasal steroids’. 18,53 Oral steroids have, however, been mooted as a simple, cheap treatment with the advantage that they could be used for a wide age range of selected affected children. However, better evidence of their effectiveness in clearing effusions, in improving patient-centred outcomes in the short and longer term, evidence for their cost-effectiveness and, importantly, a comprehensive evaluation of any associated harms is still required. There are concerns from both parents and professionals about the appropriateness and safety of courses of oral steroids for very young children in this clinical context, that is, a chronic intermittent condition of variable severity that eventually self-limits. These important issues and desired outcomes are being addressed in an ongoing trial funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (project number 11/01/26), with selected patients being recruited from hospital settings.
The evidence for autoinflation
Autoinflation is a promising technique, with some preliminary efficacy and effectiveness demonstrated in several small hospital-based trials reported in two meta-analyses. 35,54 In total, seven clinical trials were found suitable to be included in Cochrane. 55–61 Subsequently, a literature search (search date November 2013), which is an update of the original Cochrane systematic review, was performed using the standard Cochrane search method, as described in full elswehere. 35 Two additional references to clinical trials were found, one of which was a completed RCT carried out on a total sample of 40 children. 62 Different methods of autoinflation using Valsalva manoeuvre techniques, party blowers and face masks, as well as purpose-manufactured devices, were considered in both meta-analyses. The nasal balloon technique using Otovent (ABIGO Medical, Askim, Sweden), which was developed by Professor Stangerup (see Chapter 3, Trial intervention for a full description), and essentially consists in inflating a purpose-manufactured balloon through the nose, and the EarPopper® (Micromedics, St Paul, MN, USA) (a device operating to give a steady flow of air to the nose, which needs to be co-ordinated with the act of swallowing, which opens up the Eustachian tubes) are the only two purpose-manufactured standardised delivery devices, and appear also to yield the most promising results so far in terms of beneficial ORs. 35 Four small studies54–56,62 (one unpublished except in a review) that used the balloon intervention (Otovent) as the method for autoinflation were the basis of our power calculation. Combining these trials gives a potentially homogeneous total of 336 children and is completely dominated by secondary care studies. For the tympanometric outcomes at 1 month, the OR based on all four relevant studies was 2.4, but was not significant. The Cochrane authors, although finding a large aggregate effect size for the autoinflation method with a relative risk (RR) of improvement of 2.47 for tympanometric outcomes, reported wide confidence intervals (CIs) going through 1 (95% CI 0.93 to 6.8). The authors recognised that evidence to recommend widespread use of autoinflation in general practice was missing (a view echoed in subsequent NICE guidance),1 and highlighted the need for a large-scale pragmatic trial in primary care with a longer follow-up term of > 1 month. 35
The most recent updated MEDLINE search in August 2014 revealed one further autoinflation study. 63 This small pilot study involved a modified face mask with an external counter-pressure system intended for use in very young children. One must conclude that the clinical efficacy and effectiveness of autoinflation in a primary care population remains completely untested and requires full evaluation before wide-scale use in the NHS. Primary care is the best setting to evaluate effectiveness of autoinflation, because most children with OME are seen in primary care and in the community, and it is increasingly clear that there are, as yet, no evidence-based treatments that work in this setting. 1,31,34,35,52,53 Lack of a good non-surgical intervention is arguably a major factor fuelling the substantial rates of inappropriate early referral for consideration of surgery, which is thus far the only proven effective treatment. 16,37
There are no known or reported harms associated with nasal autoinflation to date, with higher respiratory tract infection (RTI) rates (including AOM) noted in the control groups in two studies, making it unlikely that the increased pressure in the nose during autoinflation can spread infections, or that it acts as an object that produces cross-infection. 55,56 Patent details outline advantages of controlled air flow and non-damaging pressures inside the nose (the latest patent was filed in September 2008, patent reference US 20100071707A1).
Perhaps compliance is the major potential weakness with this technique,31 and it can probably reliably be performed only in school-aged children (4–5 years and older). Nasal balloon autoinflation using Otovent (≈£5) has the advantage of being considerably less costly than using the EarPopper (≈US$200). Otovent also has better preliminary evidence than the single manufacturer randomised study of just 94 children for the EarPopper,57 thus making the nasal balloon method the intervention of choice for further evaluation.
In summary, the current best, but very limited, evidence from three small homogeneous moderate-quality studies combined at 1 month in the Cochrane review35 suggests there may be a higher rate of short-term tympanometric resolution in children using a purpose-manufactured balloon device (Otovent) than in control subjects. 55,56,61 All the meta-analysed studies, however, failed to provide a definitive answer; many lacked intermediate follow-up and all lacked any long-term follow-up. The Cochrane 3-month meta-analysis, although reporting statistically significant results, used combined (audiometric and tympanometric) outcomes. Furthermore, no relevant important patient-centred outcomes were included in the review, and all identified studies were conducted entirely in highly selected secondary care/specialist populations.
The evidence for other interventions
A British Medical Journal clinical evidence review34 found that mucolytics are unlikely to be beneficial, and that antihistamines and/or oral decongestants are likely to be ineffective and have unwanted side effects. 64 Hearing aids have not as yet been properly evaluated, with no good comparator studies available. 1
The evidence for surgery
For the sake of completeness, and context, a brief synopsis of the evidence for surgery is included here to help bring out some of the issues with current management. Surgery is demonstrably and clearly effective for a carefully selected minority of children, that is those with more severe histories and/or intractable presentations. 1,37 OME/glue ear remains consistently one of the commonest reasons for childhood surgery (inserting grommets/removing adenoids). 15–17,65
However, surgery is known to have a number of significant disadvantages, ranging from high costs and child-and-family preference for a non-surgical option to risks from anaesthetic (with post-operative adverse events that include otorrhoea,66 perforation, tympanosclerosis, residual hearing loss1,14,31 and significant re-insertion rates8). But arguably the most significant limitation of surgery is that, although effective, it is a treatment that is selectively applied post hoc, allowing many children to remain disadvantaged by their hearing loss and other clinically and socially significant OME impacts over a wait of approximately 9 months, rather than in a more timely fashion. This observation has been labelled the treatment paradox.
Conclusion
Temporising medical management is frequently given in general practice, and often includes prescribing antibiotics, decongestants and antihistamines, all of which have been shown to be clinically ineffective, and, worse still, have significant harms. 1,12,34,38,64 Furthermore, these interventions are associated with substantial NHS costs. Antibiotic prescription in primary care is rising progressively again, and has now exceeded the peak in the late 1990s, further driving the development of antibiotic resistance, which may lead to serious infections becoming untreatable. 47,50 The high prevalence of OME, and the fact that it is estimated that one-third of all cases of otitis media are primarily OME related,2,12 means that estimated rates of 80% antibiotic prescribing for all types of otitis media episodes in primary care are potentially reducible by a further 20–30%. 12 Finding an appropriate, feasible and low-cost management option for primary care for the majority of affected children must therefore be seen as an urgent priority, with the status quo of ‘watch and wait’ sometimes interpreted by parents as ‘doing nothing’ or as a form of demand suppression. 67
Autoinflation has been identified by a systematic search through the evidence as the best potential option for primary care. If found to be the case from research, a low-cost, safe and clinically effective treatment might resolve effusions and related symptoms, concerns and global impact on the child’s life and development. There is thus potential to improve child and parent QoL, increase satisfaction and adherence to a recommended watch-and-wait strategy, and also to reduce the harms of overprescribing antibiotics and other presently misapplied treatments.
A simple autoinflation method (Otovent) used for 1–3 months is proposed here in a pragmatic, open, randomised two-arm controlled trial in UK primary care, in which both arms receive usual (routine or standard) management, in order, primarily, to evaluate both clinical effectiveness and cost-effectiveness of the intervention. Health economic outcomes are proposed to be collected up to 12 months post randomisation.
Primary aim and objectives
The primary aim of the study is to evaluate the clinical effectiveness of autoinflation in resolving OME at 1 and 3 months by assessing the proportions of children showing rigorously defined improvement as accepted by Cochrane, that is, tympanometric resolution in at least one ear per child of a type B tympanogram (fluid) to normal, type A/C1, tympanograms. 35
Secondary aims and objectives
-
Tympanometric: assessment of the proportions of ears showing resolution from B to A/C1 types at 1 and 3 months. 35
-
Clinical outcomes: evaluation of the clinical effectiveness of the intervention on total symptoms (e.g. hearing difficulty, earache and difficulty concentrating) using a total diary score. We also used a total ear problem (mapped) QoL measure using a PROM [a 14-point questionnaire on the impact of OME (OMQ-14), which is a subset of the Medical Research Council-developed 30-point questionnaire on the impact of OME (OM8-30)].
-
Health economic (HE) assessment of the cost-effectiveness of autoinflation in terms of the cost per additional child achieving resolution of OME at 1 and 3 months, and also in terms of cost per quality-adjusted life-year (QALY). Evaluation by notes audit30 of the 12-month HE outcomes.
-
Qualitative: to describe the experience of using autoinflation, including nurse-observed competence and reported compliance, and develop an easy-to-use training package for everyday practice.
Chapter 2 Pilot study
Introduction
A pilot study was proposed in advance of a main trial of autoinflation to test the feasibility of recruitment rates, randomisation procedures, training of practice staff, acceptability to patients and compliance with the autoinflation device. The pilot also improved costing estimates for the main trial.
Methods
Aims and objectives
The principal aims of the pilot study were to test the feasibility of conducting a trial of autoinflation in a primary care setting and, in particular, to evaluate children’s compliance in using the device. Other benefits of piloting are as indicated above. 68
Setting
The pilot study was set in four general practitioner (GP) practices recruited through the Primary Care Research Network (PCRN) (in Hampshire, Wiltshire, Buckinghamshire and West Berkshire).
Ethics approval and research governance
Ethics approval for the pilot and main study was awarded by the National Research Ethics Service (NRES) on 10 August 2009, reference number 09/H0504/75 (see Appendix 1). Research governance approval was obtained from four primary care trusts (PCTs) (in Hampshire, Wiltshire, Buckinghamshire and West Berkshire).
Recruitment of practices and research nurses
Four practices participated in the study. All nurses had current good clinical practice (GCP) training and attended a structured study training day held at their practices and delivered by the research team. On-site training of the nurses included identifying and inviting potential participants, informed consent process, performing each assessment and use of the specific study equipment (otoscope, tympanometer and autoinflation device). In addition, a prototype study manual was given to each research nurse (RN) for reference purposes, and provided preliminary support.
Recruitment of children
School-aged children (aged 4–11 years) were identified for the study either by initial computer searches or by opportunistic case finding within the practices. Children were eligible for inclusion if they displayed symptoms typical of OME in the previous 3 months or their notes recorded a history of ear problems in the previous 12 months or a relevant presenting problem. Full details are presented in Chapter 3, Recruitment of children. The youngest children (aged 4–6 years) attending school were deemed to have highest base level of risk for OME, so were selected for screening provided they had recent symptoms irrespective of notes history.
Eligibility and informed consent
Children were assessed for eligibility according to the criteria in Box 1. Prior to tympanometry, all parents gave written, informed consent for screening and children were also invited to give written assent wherever deemed applicable by the RN.
-
Children aged 4–11 years and attending school.
-
At least one ear with a type B tympanogram (using the modified Jerger classification). 69–71
PLUS
-
(a) For children (aged 4–6 years) identified from the practice age/sex register, parental concern with report of at least one relevant symptom/concern associated with OME in the previous 3 months from the following symptom/concern checklist:1,2,8,30,72
-
a prolonged or bad cold, cough or chest infection
-
an earache
-
appears to mishearing what is said
-
hearing loss has been suspected by anyone
-
says ‘eh what?’ or ‘pardon’ a lot
-
needs the television turned up
-
may be irritable or withdrawn
-
appears to be lip reading
-
not doing so well at school as you or the teacher think, e.g. with reading
-
has noises in the ear or is dizzy
-
snores, blocked nose or poor sleep
-
speech seems behind other children’s
-
any suspected ear problem.
OR
-
(b) For children identified in the targeted attendance screen (aged 7–11 years), a history of recent and/or recurrent otitis media or OME in the previous 12 months recorded in the child’s medical records OR ear-related problems in the previous year including suspected hearing loss, snoring, concerns about child’s behaviour, speech or educational development. 30
OR
-
(c) For children newly presenting, relevant expressed clinical concern from the health team about OME as a cause. 30
-
Children with a grommet already in the eardrum, or who have been referred or listed for ear surgery.
-
Children with a latex allergy (owing to use of latex nasal balloons).
-
Children with uncommon conditions and syndromes at high risk of recurrent disease including cleft palate, Down syndrome, Kartagener syndrome, primary ciliary dyskinesia and immunodeficiency states for whom early referral is indicated.
Randomisation and concealment of allocation
Eligible children were individually randomised to autoinflation plus routine care or routine care alone via a telephone dial-in service to the Primary Care and Vaccines Collaborative Clinical Trials Unit (PCVC-CTU) at the University of Oxford.
The randomisation method used an algorithm with minimisation based on three previously found key variables: age, sex and baseline severity of OME. 18,30 Owing to the nature of the intervention use of placebo was not possible, and therefore nurses, children and families were unable to be masked to treatment allocation.
Intervention
The intervention in the pilot involved the autoinflation method using a nasal balloon (Otovent) three times per day through each nostril for 1–3 months plus routine care compared with routine care alone.
Assessments
The primary outcomes assessed were tympanometric, resolution of type B (effusions) at 1 and 3 months. Tympanometric outcomes were assessed blind to intervention group by the chief investigator.
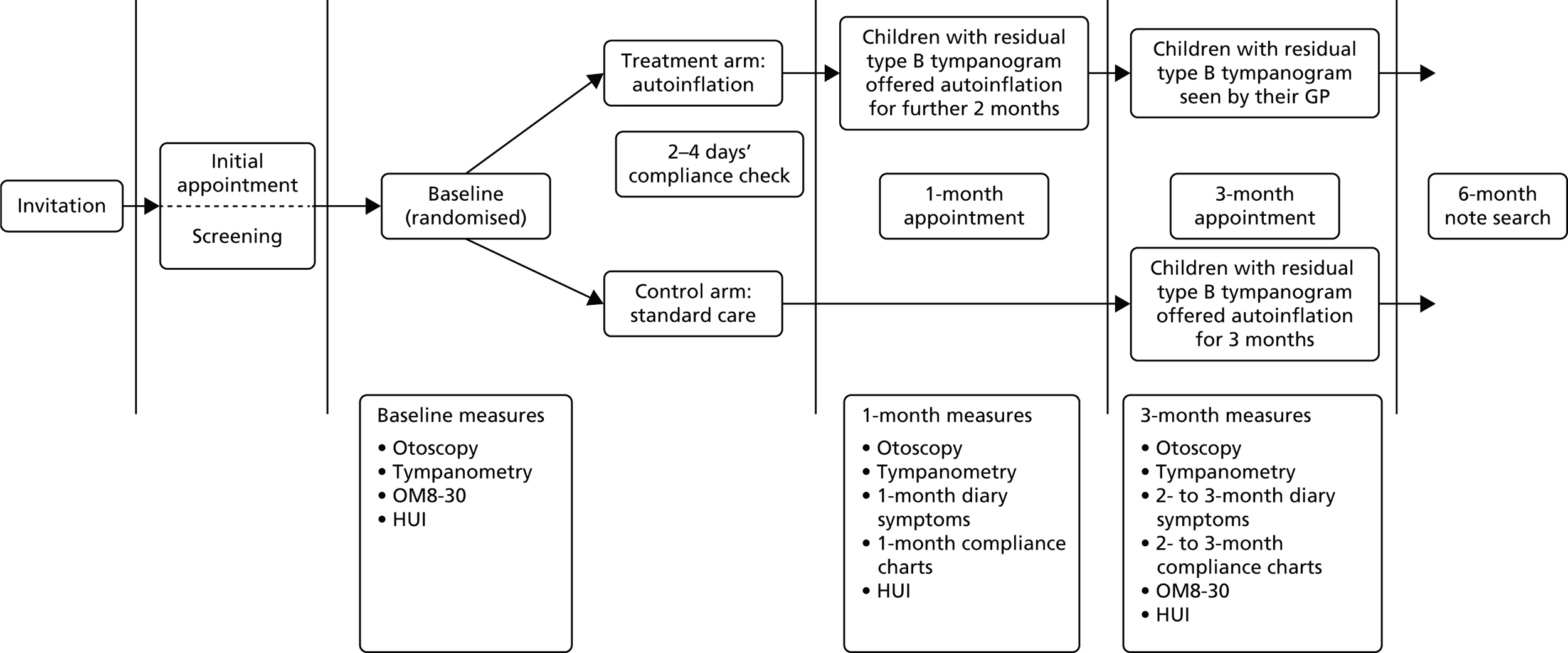
Compliance with the autoinflation device over the study period was recorded using a daily sticker reward chart completed by the child and was also parent reported (as use of autoinflation on a 4-point Likert scale and number of times per day). The ear-related QoL questionnaire (OM8-30) and Health Utilities Index (HUI) were also evaluated in the pilot study (Figure 1). 30
FIGURE 1.
Flow of participants through the pilot study.
Results
Patient recruitment
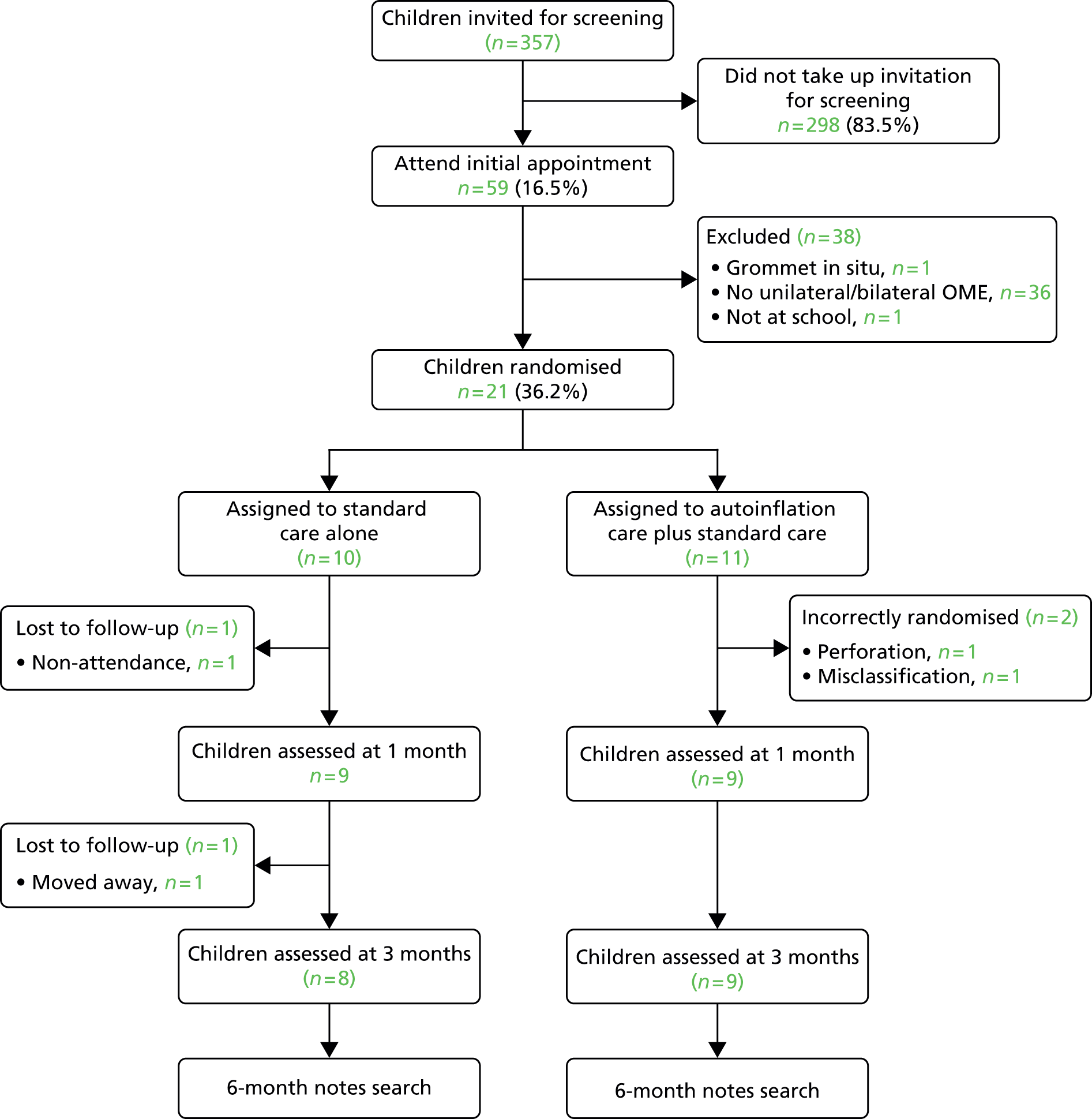
Practices commenced their computer searches in November 2009, with 357 children invited for screening from four practices between January 2010 and May 2010. Fifty-eight children were consequently screened for eligibility and 21 were randomised into the pilot.
The pilot Consolidated Standards of Reporting Trials (CONSORT) diagram, illustrated in Figure 2, details the numbers of children that progressed or otherwise through the study. Two children had to be excluded after randomisation, both in the autoinflation group, one because of an existing perforation of the eardrum and the second because of a tympanometric misclassification error, leaving 19 patients who were correctly randomised into the pilot.
FIGURE 2.
The pilot CONSORT diagram.
The baseline characteristics of children screened for the study are presented in Table 1 and all correctly randomised children are presented in Table 2.
| Variable | n (%) |
|---|---|
| Age at screening (years) | |
| 4–5 | 12 (21) |
| 5–6 | 32 (55) |
| 6–7 | 4 (7) |
| 7–11 | 10 (17) |
| Sex | |
| Male | 30 (52) |
| Female | 28 (48) |
| Variable | Autoinflation (n = 9), n (%) | Standard care (n = 10), n (%) |
|---|---|---|
| Sex | ||
| Female | 5 (56) | 5 (50) |
| Male | 4 (44) | 5 (50) |
| Age (years) | ||
| 4–5 | 3 (33) | 2 (20) |
| 5–6 | 4 (45) | 8 (80) |
| 6–7 | – | – |
| 7–11 | 2 (22) | – |
| Ethnicity | ||
| White | 8 (89) | 9 (90) |
| Oriental | – | – |
| Afro-Caribbean | – | – |
| Bangladeshi/Indian | – | 1 (10) |
| Mixed | – | – |
| Other | 1 (11) | – |
Outcome measures
Objective resolution was defined by ear as change from at least one type B (fluid) to A/C1 (clear) tympanogram. Intermediate negative-pressure C2 tympanograms were considered insufficient evidence of clearance of fluid.
The main effects of autoinflation were estimated using the difference in proportions of children with fluid resolved between the two treatment arms at both 1 and 3 months.
The estimated difference between groups at 1 month based on the by-child results from Table 3 is 11.1% (95% CI –36.0% to 58.2%), which is equivalent to an OR of 1.75 (95% CI 0.20 to 14.20).
| Severity | Resolution | 1 month | 3 months | ||
|---|---|---|---|---|---|
| Autoinflation | Standard care | Autoinflation | Standard care | ||
| Bilateral | Both ears resolved | 1 | 0 | 1 | 0 |
| One ear resolved | 2 | 1 | 2 | 1 | |
| Neither resolved | 3 | 3 | 3 | 3 | |
| Unilateral | Resolved | 0 | 1 | 1 | 3 |
| Not resolved | 3 | 4 | 1 | 2 | |
| % of children resolved (n/N) | 33.3% (3/9) | 22.2% (2/9) | 57.1% (4/7) | 44.4% (4/9) | |
The estimated difference at 3 months is 12.7% (95% CI –44.6% to 70.0%), which is equivalent to an OR of 1.7 (95% CI 0.2 to 12.2). This is comparable to the effect found in the meta-analysis from the Cochrane systematic review35 for full resolution (an OR of 2.4). The power calculation estimates for the main study were considered not to require revision by our statistician.
Compliance and use of the autoinflation device
Reported compliance was very high and consistent across both reward charts and parent-reported usage (Table 4). However, prospective use of a reward sticker chart was considered to be the most reliable method.
| Group | n | Reward chart (mean times per day) | Parent-reported frequency at 1 month | |
|---|---|---|---|---|
| Frequency | Mean number of times per day | |||
| Improved | 6 | 2.95 | All of the time, n = 5 | 3 |
| Most of the time, n = 1 | ||||
| Not improved | 3 | 2.70 | All of the time, n = 3 | 3 |
| Most of the time, n = 0 | ||||
Discussion
The pilot study under-recruited compared with the target set of 15 children per group. The main reasons for under-recruitment were twofold. First, delays in obtaining necessary ethics, governance and site permissions resulted in a late recruitment start date of January 2010, leaving only half the anticipated recruitment time frame (OME is a seasonal condition that substantially tails off around April). Second, the lowest recruiting of the four practices involved an enthusiastic GP who, because of time pressures, started recruitment extremely late.
A previous primary care trial estimated the need for 2940 children to be invited, in order to screen 1176 (40%), of whom 294 (25%) would be randomised. 30 Using the pilot CONSORT figures for a revision of the estimates, it was predicted that, of 5570 invitations for screening, 891 (16%) would respond and 294 (33%) would be randomised. This enabled a more precise estimate of practice recruitment and costs for the main study. More 4- to 6-year-olds than anticipated were identified, and approximately half of all those with symptoms who were screened were eligible and subsequently randomised. No parent/guardian of an eligible child refused consent for screening or to participate in the pilot, revealing very high acceptance rates for the study, and overall retention was good.
Web-based randomisation was the preferred option for site staff, and considered a more robust and inclusive system for randomisation, and was therefore implemented in the main study.
Children randomised to autoinflation were noted to be fully compliant with the method of autoinflation. No withdrawals occurred once children had started using the device. Feedback obtained from both parents and children about using the device was incorporated into subsequent training days for the main trial. Feedback included what to expect from the technique; the importance of prior stretching of new balloons; the need to involve the parents in demonstrating the method; and the need for persistence, especially over the first few days.
One child did experience nosebleeds while using autoinflation. The parent reported that the child had suffered from previous recurrent nosebleeds, but chose to continue with the study anyway. Dr Stangerup (Otovent inventor) told us that there had been no previous reports of nosebleeds as a complication of nasal balloon inflation (Professor Sven Eric Stangerup, University of Copenhagen, 2011, personal communication). However, it was decided on review that it would be best to avoid any such prior histories based on a degree of hypothetical risk. A new exclusion criterion was therefore added (Box 2).
-
Change in method of randomisation: a continuously available web-based randomisation, instead of telephone randomisation, would be employed for the main study.
-
Additional exclusion criterion: an additional exclusion criterion would be added to the main study as follows: ‘A recent nosebleed in the previous 3 weeks, or more than one episode of nosebleeds in the preceding 6 months’.
-
Web-based hearing test [Two Alternative Auditory Disability and Speech Reception Test (TADAST)]:27 the TADAST forced-choice hearing disability test (previously evaluated in primary care) would be used as a baseline and 1-month measure. It was agreed that this would be optional in the main study and used in secondary analyses.
-
An improved, pragmatic version of the OM8-30 questionnaire (OMQ-14):20,21,30 the OMQ-14 became available courtesy of Professor Mark Haggard and was used in the main study. Database analyses showed that the OMQ-14 retained the validity, sensitivity and reliability for the most important symptoms and concerns of relevance for primary care, and was anticipated to have better completion rates that the longer OM8-30 and would be more amenable to primary care use.
The trial materials and operating manual were deemed satisfactory and were well accepted. The pilot study highlighted that not all nurses were confident with tympanometry, and three nurses would have liked additional training in use of the machine and interpretation (Box 3).
-
Trial management: the trial management group suggested revising the research management structure to allow for recruitment to be co-ordinated from Southampton with close supervision from the chief investigator once the trial design had been finalised and data collection tools and other trial materials had been finalised. The PCVC-CTU would continue to provide Clinical Trials Unit (CTU) supervision and expertise in data management and statistics.
-
Improved support for nurses: regional training days with an audiologist and the Otovent suppliers, and improved tympanometric support by the management team (fax and telephone) for screening and recruitment purposes were recommended for the main study.
-
Recruitment: more precise recruitment logistics and costing were made for the main study. It was agreed to use trained RNs rather than GPs (for whom time pressures were considered to be greater). Owing to the seasonal nature of OME, it was agreed to push for recruitment before the Christmas period to increase uptake.
Conclusion
This small-scale study, once under way, was successful in recruiting patients, compliance was excellent and no major protocol changes were needed for the main study and costing was improved. A great number of useful and practical points were learned from the study, in both a systematic and informal way, from parents and nurses. The overall performance of the pilot was encouraging and appeared sufficient in terms of recruitment and in answering what was felt to be the major unknown issue about whether or not children were able perform the technique and achieve sufficient compliance over 1 month in a primary care setting. 35
Chapter 3 Methods
Study design
We conducted an open, pragmatic RCT in primary care. We examined the difference in effectiveness between regular autoinflation with standard care and standard care alone.
Setting
The study was set in primary care in three regions of England: the South West, Thames Valley and Cheshire regions. The main study recruited children from 43 family practices from 17 UK PCTs, between January 2012 and February 2013.
Ethics approval and research governance
Ethics approval was awarded by the NRES on 10 August 2009, reference number 09/H0504/75 (see Appendix 1), and local research governance approval was obtained from all participating PCTs.
Recruitment and training of research nurses
Practices were recruited to the study by the PCRNs. At each practice a GP acting as principal investigator and a RN were assigned to the study. Practices were reimbursed for nurse time by the Department of Health service support costs.
Recruitment of practices
A total of 50 general practices were recruited to the study, of which 43 practices actively screened and randomised children during the study period (Tables 5 and 6). During winter 2011, 39 practices received training; seven did not continue to patient screening because of practice withdrawal (n = 3), slow start (n = 2), staff illness (n = 1) or research governance delay (n = 1). In order to maximise recruitment, an additional 11 practices were trained in autumn 2012 to replace nine low-/non-recruiting practices from the first season. Reasons for low recruitment included change in nursing staff (n = 3), practice withdrawal owing to other commitments (n = 2), recruitment problems (n = 1) and small practice list size that limited further recruitment (n = 1).
| PCRN/PCT | Number of practices (n = 43) |
|---|---|
| PCRN South West | 27 |
| Bath and North East Somerset | 4 |
| Bournemouth and Poole | 1 |
| Dorset | 1 |
| Gloucestershire | 2 |
| Hampshire | 9 |
| Somerset | 3 |
| Southampton City | 1 |
| Wiltshire (North, East and West) | 5 |
| Wiltshire (South) | 1 |
| PCRN Thames Valley | 13 |
| Berkshire East | 1 |
| Berkshire West | 3 |
| Milton Keynes | 1 |
| Oxfordshire | 8 |
| Cheshire CLRN | 3 |
| Central and Eastern Cheshire | 3 |
| Characteristics | Number of practices (n = 43) |
|---|---|
| Practice list size | |
| 1–4999 | 3 |
| 5000–14,999 | 32 |
| 15,000+ | 8 |
| Number of GP partners | |
| 0–5 | 15 |
| 6–10 | 21 |
| 11–15 | 6 |
| 16+ | 1 |
| Deprivation score | |
| High | 1 |
| Mid | 11 |
| Low | 31 |
| Main duties of participating research staff | |
| Practice nurse fitting in research around other duties | 24 |
| RN within GP practice | 13 |
| RN from outside GP practice | 3 |
| GP conducting research | 3 |
Training of research personnel
Five regional training days took place between November 2011 and January 2012 in Southampton (n = 2), Chippenham (n = 1), Oxford (n = 1) and Nantwich (n = 1). This provided convenient training locations for RNs and helped establish good and effective communication between RNs and the study team. One practice received on-site training and three practices requested additional pre-study visits from the study manager. One further training day took place in September 2012 (Oxford) for the 11 additional practices.
The training days involved the chief investigator, the trial co-ordinator, an audiologist, PCRN observers and a company representative. Study aims and methods were comprehensively covered. These included procedures for identifying and screening patients, taking consent, how to perform web-based randomisation, patient assessment and data management. A training manual was provided giving full details of the study methods and outcome assessments. Training in otoscopic examination and tympanometry was provided by the chief investigator and an audiologist from Starkey Laboratories Ltd, who gave detailed information and a practical demonstration of the technology and interpretation of the tympanometric results. This included classification of the four main types (A, C1, C2 and B) of tympanograms, recognition of obstructing wax, perforations, grommets and appropriate canal volumes for age, etc. Nurses had the opportunity to practise and refine their techniques during the training day. Brief training in the correct use of the autoinflation device (Otovent) was given by a representative from the suppliers (Kestrel Medical Ltd). All nurses or recruiting GPs had current training in GCP by the start of the trial.
As a means of additional support and training, nurses were invited to fax their initial screening tympanograms to the co-ordinating centre, where they were independently reviewed for categorisation based on all available parameters and observations. Any discrepancies in tympanometric classification were then fed back to nurses to help improve their precision of diagnosis in the field. Extra on-site training was offered by two experienced members of the study team where requested. Regional meetings were scheduled at the start of the second recruitment season to provide a study update and a further review of tympanometry and interpretation.
Recruitment of children
Participating practices were asked to invite 140 children as a target for screening. It was conservatively estimated from the pilot study that the proportion of invited children who would be recently symptomatic and/or whose parents would have concerns and who would, therefore, attend for screening would be 16%, and that one-third of these would be eligible for randomisation (140 children invited; 22 screened; and seven randomised in each participating practice, i.e. 1 in 20 children).
Children were identified by practice-based computer search or opportunistic case finding by practitioners, nurses and health visitors as follows.
Computer searches
-
High-risk children, that is those aged 4–6 years, were identified from practice age/sex registers and those with one or more OME-related symptoms or concerns in the previous 3 months were invited for screening.
-
An audit of the attendance records of 7- to 11-year-old children identified those with ear-related problems in the previous year.
Opportunistic case finding
-
General practitioners, nurses and health visitors identified children leading to an in-practice referral to the RN.
The parents of children identified for the study received an invitation letter and information sheet, and children received an age-appropriate information sheet (see Appendices 2 and 3). Parents gave written informed consent for screening and children were invited to give written assent if deemed appropriate by the RN (see Appendix 4). Table 7 shows features of study entry by practice and PCT.
| PCRN/PCTs | Participating practices | Number of children screened | Number of children randomised |
|---|---|---|---|
| PCRN South West | 27 | 843 | 212 |
| Bath and North East Somerset | 4 | 84 | 18 |
| Bournemouth and Poole | 1 | 5 | 0 |
| Dorset | 1 | 66 | 11 |
| Gloucestershire | 2 | 92 | 22 |
| Hampshire | 9 | 316 | 81 |
| Somerset | 3 | 126 | 34 |
| Southampton City | 1 | 24 | 5 |
| Wiltshire (North, East and West) | 5 | 116 | 37 |
| Wiltshire South | 1 | 14 | 4 |
| PCRN Thames Valley | 13 | 253 | 77 |
| Berkshire East | 1 | 11 | 1 |
| Berkshire West | 3 | 23 | 6 |
| Milton Keynes | 1 | 8 | 3 |
| Oxfordshire | 8 | 211 | 67 |
| Cheshire CLRN | 3 | 139 | 31 |
| Central and East Cheshire | 3 | 139 | 31 |
| Total | 43 | 1235 | 320 |
At the screening visit, the RN checked both ears for any obstructing wax, perforations or grommets using otoscopy. If the ear canal was occluded with wax, olive oil ear drops were recommended to soften and disperse the wax, and rescreening was rescheduled. Tympanometric screening was performed to assess full eligibility for the study, that is, every symptomatic child had to have one or two type B tympanograms confirming the presence of uni- or bilateral OME (Table 8 shows details of tympanometric classification used). This examination was requested to be repeated wherever the interpretation was unsatisfactory or inconclusive. The inclusion and exclusion criteria are shown in Box 1.
| Tympanometric classification | Middle ear pressure | Tympanogram | Positive predictive value for OME |
|---|---|---|---|
| Type A | +200 to –99 | Peak | Normal |
| Type C1 | –100 to –199 | Peak | Normal |
| Type C2 | –200 to –399 | Peak | 54% |
| Type B | –400 | Flat trace | 88% |
Randomisation and masking
Eligible children were individually randomised to autoinflation plus routine care or routine care alone within 1 week of screening. An independent external agency provided a centralised web-based randomisation system (www.sealedenvelope.com) for nurses to access while recruiting children to the study. The Oxford Primary Care CTU independently managed, co-ordinated, analysed and checked the data validity. The randomisation used an algorithm with minimisation based on three potential effect modifiers/confounders: age (< 6.5 years vs. > 6.5 years), sex and baseline severity of OME (one vs. two baseline type B tympanograms). 18 Owing to the nature of the intervention, use of placebo was not possible and therefore nurses, children and families were not masked to treatment allocation.
Trial intervention
The simple autoinflation treatment used in this trial involved inflating a purpose-manufactured balloon (Otovent), by blowing through each nostril into a connecting nozzle three times per day for 1–3 months (Figure 3). 55,56,60,61,73 Children in the treatment arm were instructed by watching the nurse and/or parent demonstrate the procedure, starting with stretching the balloon (by hand or mouth blowing). A website, which included a short instruction video, was also available as a back-up for parents and children (www.gluear.co.uk). A sticker book was provided to encourage the child’s ongoing participation. Children still recording a type B tympanogram in either ear at 1 month were advised to continue with nasal balloon autoinflation for a further 2 months. All study children (both arms) received their usual/routine clinical care as normal. At the end of the 3-month clinical study period, children in the standard care arm with tympanometric evidence of glue ear were offered a 1-month supply of nasal balloons.
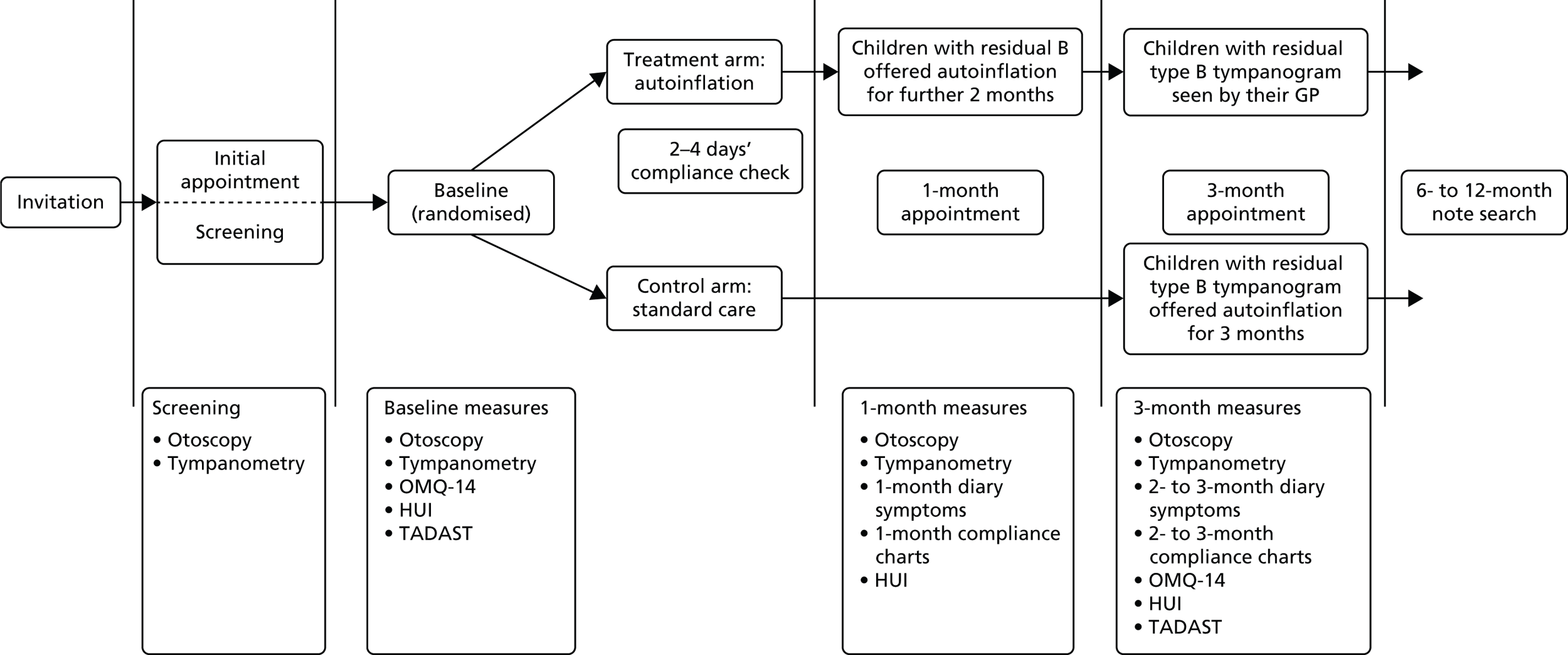
FIGURE 3.
Nasal balloon autoinflation (Otovent). Reproduced with permission from Kestrel Medical Ltd.

Assessments
Baseline assessment was conducted within 1 week of screening. Parents of children in the intervention group were routinely contacted by telephone after 3 days to provide additional support for autoinflation, if required. All children were followed up at 1 and 3 months (Figure 4).
FIGURE 4.
Flow of participants through the main study.
Advanced appointments were made for the next follow-up assessment at each visit and an appointment card given. A postcard reminder was sent 1 week before the next appointment to encourage attendance. In the case of non-attendance, the RN was asked to attempt contact twice by telephone and twice with a missed appointment card, after which the patient was considered lost to follow-up.
Withdrawals
In accordance with GCP, parents/guardians were free to withdraw their children from the study at any time without affecting their medical care. Children were withdrawn from the study in the event of incorrect diagnosis at the time of randomisation (no type B tympanogram confirmation).
Outcome measures
Children were assessed for the most important clinical outcomes at 1 and 3 months post randomisation over a recommended 3-month waiting or monitoring period, over which time natural resolution effects would be expected to occur in some children. 3,5,6 It was anticipated that, if the method was effective, it would most likely be at 1 month, when taking into account poor compliance reported in one secondary care study,58 and implied or suggested concerns for general use and durability of the method in primary care. 1,35,37,74 Health economic outcomes were collected for 1, 3 and 12 months. With the protocol indicating that routine care should not be affected in any way during the clinical phase (3 months) of the study, referrals in particular would be difficult to assess without longer-term follow-up (12 months). The flow diagram for the main study was essentially unchanged from the pilot except that (1) the OMQ-14 replaced the OM8-30 at baseline and 3 months; (2) the TADAST hearing performance test (see Two alternative auditory disability and speech reception tests hearing test) was added at baseline and 1 month; and (3) a notes audit for HE purposes was completed up to 12 months post randomisation.
Main outcomes
Primary outcomes in children at 1 and 3 months
-
The primary outcome was dichotomous: the difference in the proportion of children showing definite tympanometric resolution (from a type B tympanogram to a type A or C1 tympanogram, i.e. back to normal middle ear pressures) in at least one affected ear at 1 month. Intermediate negative-pressure C2 tympanograms were considered insufficient evidence of resolution of fluid, that is, not classified for analysis purposes as resolved (see Table 7). 69,71 Tympanometry has better test characteristics for the presence of effusion than history and/or simple otoscopy, and is thus a good choice for primary care studies, in which it has been shown to be a reliable diagnostic instrument. 1,18,24,31,36 It provides a reasonably objective outcome measure that can also be assessed blind to allocation arm. Two members of the trial team, trained in tympanometry, independently reviewed anonymised tympanometry printouts (for the main 1- and 3-month outcomes, see Tympanometric assessments). Cases of disagreement were settled by an independent audiologist. All MTP-10 micro-tympanometers (Interacoustics, Assens, Denmark), previously used in the GNOME study,18,30 were recalibrated by PC Werth Ltd prior to the start of the study and subsequently on an annual basis while in use.
-
The difference in the proportion of children showing definite tympanometric resolution (of a type B tympanogram to a type A or C1, i.e. back to normal middle ear pressures) in at least one affected ear at 3 months was considered a second main outcome and is justified as above and in accordance with guideline suggestions for the monitoring period duration. 1,2
Secondary outcomes
Tympanometric resolution based on ears as the unit of analysis
Differences in the proportions of ears by group that show resolution (from type B to A/C1) at 1 and 3 months was a secondary outcome. This is justified as a means of demonstrating efficacy that provides additional power by using the data from both ears. Ears are not independent variables and previous trials that have not taken into account the correlation between ears in the analysis, resulting in overly precise CIs, have been justifiably criticised. Such outcomes are included in the main Cochrane meta-analyses, with post-hoc adjustments to account for this correlation. 35 Generalised estimating equations can be used to adjust the analysis for the non-independence of ears in a pre-specified manner. Thus, these robust outcomes are useful to demonstrate efficacy of the method in actually clearing children’s ears of effusions. However, such, and indeed all, tympanometric outcomes require additional clinical confirmation of effectiveness to better inform a child-centred management model of OME. 19,34,35,75
Non-tympanometric clinical outcomes
Ear-related QoL was measured at 3 months using the OMQ-14 (see OMQ-14 impact measures). Parents completed weekly diaries to record symptoms, adverse events and compliance, and also HUI version 3 (HUI3),76,77 with resource use questionnaires at baseline, 1 and 3 months to inform a HE analysis. Pure-tone audiometry was not conducted, as it cannot be done with adequate precision in non-specialist and noisy settings, and correlates only weakly with child and family QoL.
OMQ-14 impact measure
The OMQ-14 is a 14-item PROM developed by a process of extensive statistical refinement and iteration from two large primary and secondary care UK trials on OME [GNOME/TARGET (Trial of Alternative Regimens of Glue Ear Treatment)]9,18 and further evaluated in ongoing cohort/audit data sets from across Europe (Eurotitis 2) (Professor Mark Haggard, University of Cambridge, 2010, personal communication). 21 It is a functional health status measure that is reported by proxy. As a shortened form of the OM8-30,18,19,21,30,75,78 the 14 items selected have been demonstrated to efficiently optimise item mapping on to the HUI. 20 As a questionnaire it is simpler to administer and has better completion rates with fewer missing data than the longer OM8-30 (cf. HTA report for GNOME, where the missing outcome data was disappointing). 30 Its brevity also makes it more suitable for primary care use. It measures three domains found to map on to QoL in primary care: reported hearing difficulty and speech concerns; behavioural and developmental impact; and ear-related physical ill health. 21 It was decided a priori in the statistical plan to use the total instrument score (the OMQ-14 score used here is not the total integer ‘quick score’ intended for rapid field use, but the more precise decimal QoL-weighted sum of the three factor scores mentioned; the used version is slightly more precise) as the single most useful measure for family practice (and to avoid data dredging). The OMQ-14 total score refers to the 3-month period prior to completion, and in this study was completed at baseline and at 3 months (see Appendix 5). A standard deviation (SD) change of ≈0.3 in score is considered a clinically important effect for the child and family in terms of ear-related QoL.
Parent-reported symptom diary
Parents were asked to complete a weekly diary recording the number of days (0–7) of their child’s main symptoms of hearing loss, earache, difficulty concentrating, pain relief, disturbed sleep and absence from school.
In addition, a second diary of items was included to systematically record a number of other symptoms including nosebleeds, clumsiness/off-balance, systemic illness, nasal discharge and nasal congestion/snoring. Symptoms considered potentially adverse were collected on an adverse event form and some of these overlapped with the diary symptoms.
Two alternative auditory disability and speech reception tests hearing test
Hearing disability was evaluated at baseline and at 1 month for all children using the TADAST web-based test. TADAST is a forced-choice test, originally developed in primary care, that evaluates hearing disability associated with glue ear, and which has shown good test–retest repeatability in 4- to 11-year-old children. 27,79 Parents received an instruction card with details to log on to the new website at home after the baseline and 1-month assessments.
Compliance with the intervention
Compliance measures allow for the assessment of experiences of using the Otovent device (see Chapter 6), as concerns have been raised regarding what age autoinflation can be reliably performed. 80 All parents in the intervention group were contacted 2–4 days after the baseline visit primarily as a supportive measure but also to assess their compliance. If the parent reported problems with the autoinflation technique or adherence, a follow-up visit with the RN was offered so the parent/child could be given further specific tips and education about improving the technique, based directly on observation of use.
A sticker book diary was used to record compliance with the intervention, with children placing a sticker in the diary each time they inflate the nasal balloon. Parent-reported adherence was also recorded at the 1- and 3-month assessments (when use of the device was recorded as not at all, some of the time, most of the time and all of the time) and compared with the sticker book diary.
Parents of children in the standard care group were asked if Otovent had been used independently either because of self-purchase or because it was inadvertently prescribed during the study period.
Adverse events
The RN specifically asked parents about the occurrence of upper respiratory tract infections (URTIs) and nosebleeds at the 1- and 3-month assessments. In addition, the RN inspected the symptom diary for any further information. Serious adverse events were reported by fax to the co-ordinating centre within 24 hours of the practice being made aware of the event. The co-ordinating centre’s standard operating procedures were followed with respect to reporting to the sponsor, the Research Ethics Committee, the Data Monitoring and Ethics Committee (DMEC)/Trial Steering Committee and governance offices. Annual safety reports were submitted to the Research Ethics Committee.
Changes to the protocol
The majority of these were made as a result of the pilot study and are described in Chapter 2. During the main study a total of four substantial amendments were approved by the ethics committee and comprised minor changes to the study documentation, addition of a qualitative evaluation, a 12-month notes review (instead of 6 months post baseline) and a refinement to the study closure strategy, which allowed for a slight overshoot of recruitment. Full details are presented in Appendix 1.
Data management, cleaning and validation
All trial data were captured on paper case-report forms or participant-completed questionnaires. Trial data were tracked and managed using a clinical data management system [Open Clinica EnterpriseTM (OpenClinica LLC version 3.1, Waltham, MA, USA)]. Preliminary monitoring of received trial data was performed to assess completeness of forms, and compatibility and consistency of paperwork bundles in relation to participant identifiers. All trial data were double entered by two independent users. Self-evident modifications to captured data (correction of spelling errors, conversions, date formats, obvious updates based on supplementary data) were applied to reduce the number of data queries sent to the research sites. Responsible personnel at each research site reviewed and authorised a list of all prospective modifications. Validation of data was performed in three main ways:
-
On entry, programmed rules and range checks to highlight missing or inconsistent data would fire if predetermined conditions were met.
-
Listing checks were employed to identify potential discrepancies across participant visits/forms.
-
Review of the data set to identify discrepant, missing or outlying data was performed when participants completed their study schedule.
Requests for missing responses or clarification of inconsistent data (queries) were sent to the RNs at regular intervals to increase the likelihood of resolution.
Tympanometric assessments
Screening: finding cases
Tympanograms from 1104 of 1235 children screened (89%) were faxed to the co-ordinating centre soon after initial assessment as part of ongoing training in interpretation and to improve precision of diagnosis prior to randomisation. Only 55 of 2207 (2.5%) tympanograms (ears) were uninterpretable owing to poor technique or wax on expert review. All available data were used in the assessments, including otoscopic findings, shape of the curve, pressures, gradients and canal volume. The tympanogram classifications recorded in the database reflect the assessment made by the trial manager and/or chief investigator. Where these differed from the nurse classification, a data query was issued.
Outcome assessments
Tympanogram data captured from all follow-up assessments were retained as captured by the RN at the time of the patient assessment and a data query was issued only in the case of missing classifications. Tympanogram data collected during the follow-up assessments were reviewed in a separate fully anonymised process by the trial manager and chief investigator. For all tympanogram type classifications (A, C1, C2 and B), the expert inter-rater agreement was 89%. In all cases of disagreement, a blinded independent audiologist adjudicated. The reviewed outcome data were blinded to study identification number and treatment group. The final determined classifications for reviewed tympanogram data were entered into the clinical database using double data entry, consistent with all other data. These blinded agreed expert data assessments were the ones used in the final efficacy analyses at 1 and 3 months.
With regard to nurse interpretation of tympanometry and classification of all available ears, the summary level of agreement beyond chance between the nurse interpretation and the agreed blinded expert interpretation as the standard found substantial agreement that improved throughout the study (Table 9). 81
| Time point | n (ears) | Kappa (95% CI) |
|---|---|---|
| 1 month | 548 | 0.706 (0.689 to 0.771) |
| 3 months | 497 | 0.792 (0.782 to 0.823) |
Once all the follow-up assessment data were received, a 100% critical item review was performed on all tympanogram data and any inconsistencies rectified, particularly in the case where multiple tympanogram readings were taken during one assessment. The entire data set was then reviewed for inconsistencies and any further missing data points, before conducting a final quality control check on all data received for 19 patients randomly selected from all those recruited to the study. The final error rate was calculated to be 0.00% for critical items (tympanogram data) and 0.04% across all data points.
Statistical validation
Validation of all results presented in this report was conducted by Ly-Mee Yu, Oxford CTU. All results/major end points/primary end points were validated by independent programming using Stata version 13.0 (StataCorp LP, College Station, TX, USA).
Statistical methods
A detailed statistical analysis plan was developed at the start of the study.
Definition of populations used
Screened population
The screened population comprises all children who attended for the initial appointment and gave written informed consent.
Intention-to-treat population
The intention-to-treat (ITT) population comprises all children, out of those randomised, for whom tympanometric readings are available.
Per-protocol population
The per-protocol (PP) population comprises those randomised who satisfied the study eligibility criteria, received their allocated intervention, who did not used any autoinflation devices other than those provided for the study purposes and who used autoinflation at least twice per day for at least 70% of their treatment period during the first month. Children who presented more than 7 days before or after the scheduled 1-month visit date were considered not to have complied with the trial protocol and were excluded from the PP population.
Safety population
The safety population comprises all randomised children.
Primary analyses
The primary analysis was based on the ITT population. The proportion of children in each group with tympanometric resolution in at least one affected ear at 1 month (primary outcome) was compared using a generalised linear model with log-link function. 82 Results are presented as adjusted RRs with 95% CIs. The regression model83 adjusts for pre-specified baseline covariates: tympanometric baseline severity (one or two type B tympanograms), age, sex and PCT. Sensitivity analyses are performed on ITT and PP populations.
Multiple imputation of all missing data was performed using baseline variables as per the statistical plan: use of antibiotics, eczema, hay fever, asthma, age, sex, baseline severity, baseline OMQ-14 and follow-up OMQ-14 weighted scores. Multiple imputed data sets were created using Stata version 13 and the ‘ice’ and ‘mim’ functions. 84
For the 1-month ear-based analysis of tympanometric resolution, the non-independence (correlation) of the ears was adjusted for using generalised estimating equations with an independent working correlation structure. 85,86
Secondary analyses
Subgroup analyses
Four subgroups were compared using interaction tests on the primary outcome of resolution in at least one ‘B’ ear by 1 month. The subgroups considered were those described in the protocol, namely:
-
age: < 6.5 years or ≥ 6.5 years
-
severity: one or two B-type ears at baseline
-
OMQ-14 standardised total score: < 0 or ≥ 0
-
sex.
No interaction tests were significant; thus, results are not presented according to subgroups. The p-values ranged from 0.25 to 0.50.
Tympanometric resolution at 3 months
The analysis of the 3-month secondary end points by both child and ear were analysed in the same way as the primary end point using a generalised linear model that adjusts for a limited number of pre-specified baseline covariates.
Other tympanometric outcomes were analysed as per the statistical plan. Tympanometric deteriorations in normal ears at baseline were evaluated for potential confounding of results. However, as very few deteriorations occurred, no further analyses of this end point were undertaken.
Quality of life (OMQ-14)
The OMQ-14 standardised total scores at baseline and 3 months were calculated based on weightings provided by Professor Mark Haggard and Helen Spencer of the Eurotitis-2 Study Group (Cambridge University, 2011, personal communication). Standardised OMQ-14 change from baseline scores were analysed using a linear mixed-effects model with PCT as a random effect, and age, sex, baseline severity and OMQ-14 baseline score as fixed effects. Summary statistics for baseline and follow-up (3-month) standardised OMQ-14 scores are presented. Higher scores represent worse outcomes. The average change from baseline score is compared between groups. Questionnaires with more than four missing items were pre-specified as indicating separate analysis; however, this applied to only one person so no sensitivity analysis was carried out.
Diary card
Diary card symptom counts were quite skewed, with fewer children/parents reporting multiple diary symptoms. For this reason standard linear models could not be used to analyse these data and they were instead classified into categories based on the total number of weeks with symptoms (0, 1–7, 8–28, 29 + days). Category boundaries were decided on prior to data lock and were detailed in the amendment to the statistical analysis plan.
Data were analysed using an ordinal logistic regression model. 87 The model is an extension of the standard logistic regression model used for binary data, but allows for more than two categories for the outcome. The OR from an ordered logistic regression expresses the odds of being in a higher ordered category (i.e. more days with symptoms) when in the autoinflation group compared with the standard care group. Models were adjusted for age and sex as before.
Outputs from the analyses are displayed alongside a summary of the data for the 1- and 3-month diary cards.
Two Alternative Auditory Disability and Speech Reception Test
The TADAST score was a continuous variable out of a total of 36, with a chance score of 16 out of 36 to be compared between groups. However, because of problems with the website and late ethics permission, insufficient numbers of children completed the follow-up test (seven children at 1 month and two children at 3 months), so results are not presentable.
Sample size calculation
A 45% control resolution (improvement) rate at 1 month was anticipated in the calculations, as found in the previous GNOME trial. 18 The best estimates for the expected difference at 1 month were based on a meta-analysis of four small secondary care trials that used Otovent, included in the update for Cochrane. 35 Thus, for resolution at 1 month, the most conservative evidence-based estimate of effect size was an OR of 2.4. Given this effect size, 250 children were required (125 in each group) for a standard α = 5% and power = 90%. With 15% lost to follow-up, 295 were needed in total (for power = 80%, 226 were needed in total). The sample was also powered to detect a ≈0.3 SD effect on continuous variables such as the OMQ-14 total score at 3 months, which was deemed clinically significant.
Changes to the statistical analysis plan
-
Analyses were not completed using Stata version 11.2 (which is now an old version) but rather SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and Stata version 13 (StataCorp LP, College Station, TX, USA).
-
Twenty multiply imputed data sets were created for the sensitivity analysis of the primary end point rather than the five data sets specified, as more data sets result in more robust estimates.
Chapter 4 Results
Recruitment and trial flow profile
Screening commenced in December 2011. The first patient was randomised in January 2012 and the last child was randomised in February 2013. A total of 1235 children were screened, with 320 children (26%) randomised into the study over a period of 13 months. Table 10 displays the characteristics of screened children. The main reasons for ineligibility were no type B tympanogram, not currently at school or a recent nosebleed. Ineligible children reported fewer symptoms associated with OME in the preceding 3 months, and had fewer consultations for otitis media and OME in the previous 12 months.
| Variable | Randomised, n (%) (N = 320) | Screened only, n (%) (N = 1235) |
|---|---|---|
| Sex | ||
| Female | 153 (47.8) | 429 (46.9) |
| Male | 167 (52.2) | 485 (53.0) |
| Missing | 0 (0.0) | 1 (0.1) |
| Age (years) | ||
| 4 | 58 (18.1) | 158 (17.3) |
| 5 | 147 (45.9) | 370 (40.4) |
| 6 | 77 (24.1) | 250 (27.3) |
| 7 | 21 (6.6) | 53 (5.8) |
| 8 | 8 (2.5) | 23 (2.5) |
| 9 | 6 (1.9) | 29 (3.2) |
| 10 | 3 (0.9) | 13 (1.4) |
| 11 | 0 (0.0) | 16 (1.7) |
| 12 | 0 (0.0) | 2 (0.2) |
| Missing | 0 (0.0) | 1 (0.1) |
| Was this child recruited from: | ||
| 4- to 6-year-old list | 265 (82.8) | 758 (82.8) |
| 7- to 11-year-old list | 21 (6.6) | 103 (11.3) |
| GP/nurse/health visitor referral | 34 (10.6) | 50 (5.5) |
| Missing | 0 (0.0) | 4 (0.4) |
| A prolonged or bad cold, cough or chest infection | ||
| No | 37 (11.6) | 178 (19.5) |
| Yes | 262 (81.9) | 662 (72.3) |
| Missing | 21 (6.6) | 75 (8.2) |
| Appears to be lip reading | ||
| No | 240 (75.0) | 756 (82.6) |
| Yes | 57 (17.8) | 84 (9.2) |
| Missing | 21 (6.6) | 75 (8.2) |
| An earache | ||
| No | 124 (38.8) | 504 (55.1) |
| Yes | 175 (54.7) | 336 (36.7) |
| Missing | 23 (7.2) | 75 (8.2) |
| Not doing as well at school as you or the teacher reasonably think | ||
| No | 219 (68.4) | 680 (74.3) |
| Yes | 79 (24.7) | 157 (17.2) |
| Missing | 22 (6.9) | 78 (8.5) |
| Often mishears what is said | ||
| No | 61 (19.1) | 323 (35.3) |
| Yes | 238 (74.4) | 516 (56.4) |
| Missing | 21 (6.6) | 76 (8.3) |
| Has noises in the ear or is dizzy | ||
| No | 225 (70.3) | 674 (73.7) |
| Yes | 73 (22.8) | 163 (17.8) |
| Missing | 22 (6.9) | 78 (8.5) |
| Hearing loss is suspected by anyone | ||
| No | 154 (48.1) | 606 (66.2) |
| Yes | 144 (45.0) | 232 (25.4) |
| Missing | 22 (6.9) | 77 (8.4) |
| Snores, blocked nose or poor sleep | ||
| No | 81 (25.3) | 318 (34.8) |
| Yes | 218 (68.1) | 522 (57.0) |
| Missing | 21 (6.6) | 75 (8.2) |
| Says ‘eh what?’ or ‘pardon’ a lot | ||
| No | 50 (15.6) | 252 (27.5) |
| Yes | 249 (77.8) | 587 (64.2) |
| Missing | 21 (6.6) | 76 (8.3) |
| Speech seems behind other children’s | ||
| No | 239 (74.7) | 689 (75.3) |
| Yes | 60 (18.8) | 152 (16.6) |
| Missing | 21 (6.6) | 74 (8.1) |
| Needs the television turned up | ||
| No | 119 (37.2) | 505 (55.2) |
| Yes | 180 (56.3) | 333 (36.4) |
| Missing | 21 (6.6) | 77 (8.4) |
| Any suspected ear problem | ||
| No | 177 (55.3) | 675 (73.8) |
| Yes | 122 (38.1) | 164 (17.9) |
| Missing | 21 (6.6) | 76 (8.3) |
| May be irritable or withdrawn | ||
| No | 207 (64.7) | 633 (69.2) |
| Yes | 92 (28.8) | 205 (22.4) |
| Missing | 21 (6.6) | 77 (8.4) |
| Observational register – was the child recruited from: | ||
| Computer records | 284 (88.8) | 859 (93.9) |
| Referral | 36 (11.3) | 53 (5.8) |
| Missing | 0 (0.0) | 3 (0.3) |
| How many episodes of OME have they had in the last 12 months? | ||
| 0 | 234 (73.1) | 787 (86.0) |
| 1 | 42 (13.1) | 69 (7.5) |
| 2 | 17 (5.3) | 14 (1.5) |
| 3 | 3 (0.9) | 10 (1.1) |
| 4 | 6 (1.9) | 2 (0.2) |
| 6 | 1 (0.3) | 0 (0.0) |
| 7 | 0 (0.0) | 1 (0.1) |
| Missing | 17 (5.3) | 32 (3.5) |
| How many episodes of OM have they had in the last 12 months? | ||
| 0 | 195 (60.9) | 678 (74.1) |
| 1 | 67 (20.9) | 154 (16.8) |
| 2 | 27 (8.4) | 34 (3.7) |
| 3 | 7 (2.2) | 15 (1.6) |
| 4 | 6 (1.9) | 3 (0.3) |
| 5 | 0 (0.0) | 1 (0.1) |
| 6 | 0 (0.0) | 1 (0.1) |
| 7 | 1 (0.3) | 0 (0.0) |
| Missing | 17 (5.3) | 29 (3.2) |
| Entries in their notes over the last 12 months for hearing loss | ||
| No | 243 (75.9) | 794 (86.8) |
| Yes | 61 (19.1) | 85 (9.3) |
| Missing | 16 (5.0) | 36 (3.9) |
| Entries in their notes over the last 12 months for snoring | ||
| No | 292 (91.3) | 848 (92.7) |
| Yes | 12 (3.8) | 30 (3.3) |
| Missing | 16 (5.0) | 37 (4.0) |
| Entries in their notes over the last 12 months for behaviour concerns | ||
| No | 298 (93.1) | 846 (92.5) |
| Yes | 6 (1.9) | 31 (3.4) |
| Missing | 16 (5.0) | 38 (4.2) |
| Entries in their notes over the last 12 months for speech concerns | ||
| No | 289 (90.3) | 834 (91.1) |
| Yes | 15 (4.7) | 43 (4.7) |
| Missing | 16 (5.0) | 38 (4.2) |
| Entries in their notes over the last 12 months for educational concerns | ||
| No | 295 (92.2) | 850 (92.9) |
| Yes | 9 (2.8) | 27 (3.0) |
| Missing | 16 (5.0) | 38 (4.2) |
The study population comprised 167 (52%) boys and 153 (48%) girls, with an age range of 4–10 years (mean 5.40 years; median 5.71 years); 181 (57%) children had unilateral OME and 135 (42%) had bilateral OME [on review, four children (1%) recruited were deemed not to have OME on tympanometry]. Two hundred and fourteen (67%) were recruited between October and March, while 106 (33%) were recruited between April and September.
The baseline characteristics of randomised children were well balanced between the two groups (Tables 11 and 12) with six being the median number of symptoms and concerns [interquartile range (IQR 4–8)] in the routine care arm and seven (IQR 5–9) in the intervention arm. The trial demographic data were comparable to national figures, but 33% of participating parents (vs. 27% nationally) were educated to degree level or higher. 88
| Variable | Standard care (n = 160) | Autoinflation (n = 160) | ||
|---|---|---|---|---|
| Age | ||||
| Years, mean (SD) | 5.4 (1.04) | 5.4 (1.24) | ||
| Sex | ||||
| Male | 83 (51.9) | 84 (52.5) | ||
| Severity of OME (number of type B tympanograms) | ||||
| No type B ears | 2 (1.3) | 2 (1.3) | ||
| One type B ear | 91 (56.9) | 90 (56.3) | ||
| Two type B ears | 67 (41.9) | 68 (42.5) | ||
| Month randomised | ||||
| October to March | 107 (66.9) | 107 (66.9) | ||
| April to September | 53 (33.1) | 53 (33.1) | ||
| Ethnicity | ||||
| White | 144 (90.0) | 152 (95.0) | ||
| Bangladeshi/Indian | 2 (1.3) | 2 (1.3) | ||
| Mixed race | 3 (1.9) | 1 (0.6) | ||
| Other group | 2 (1.3) | 2 (1.3) | ||
| No information | 9 (5.6) | 3 (1.9) | ||
| Education level of parent/carer | ||||
| School to 16 years, no qualifications | 11 (6.9) | 6 (3.8) | ||
| School to 16 years, GCSEs/O-level | 28 (17.5) | 33 (20.6) | ||
| Sixth form school or college, A-level | 19 (11.9) | 25 (15.6) | ||
| Highers, SCOTVEC or NVQ | 37 (23.1) | 38 (23.8) | ||
| University degree | 37 (23.1) | 31 (19.4) | ||
| Professional or postgraduate degree | 17 (10.6) | 22 (13.8) | ||
| No information | 30 (18.8) | 31 (19.4) | ||
| Parent-reported child characteristics | Missing | Missing | ||
| Asthma | 19 (11.9) | 9 (6) | 16 (10.0) | 4 (3) |
| Eczema | 15 (9.5) | 9 (6) | 20 (12.5) | 4 (3) |
| Hay fever | 40 (25) | 9 (6) | 42 (26.3) | 3 (2) |
| Antibiotics in previous month | 12 (7.5) | 9 (6) | 21 (13.1) | 3 (2) |
| Parent-reported symptoms in the previous 3 months (4- to 6-year-olds only) | (n = 135) | Missing, n (%) | (n = 130) | Missing, n (%) |
| A prolonged or bad cold, cough or chest infection | 113 (83.7) | 119 (91.5) | ||
| Appears to be lip reading | 27 (20.0) | 27 (20.8) | 1 (0.8) | |
| An earache | 74 (54.8) | 77 (59.2) | ||
| Not doing as well at school as expected | 32 (23.7) | 1 (0.7) | 39 (30.0) | |
| Often mishears what is said | 98 (72.6) | 112 (86.2) | ||
| Has noises in the ear or is dizzy | 29 (21.5) | 30 (23.1) | ||
| Hearing loss is suspected by anyone | 56 (41.5) | 1 (0.7) | 67 (51.5) | |
| Snores, blocked nose or poor sleep | 93 (68.9) | 101 (77.7) | ||
| Says ‘eh what?’ or ‘pardon’ a lot | 107 (79.3) | 114 (87.7) | ||
| Speech seems behind other children’s | 22 (16.3) | 31 (23.8) | ||
| Needs the television turned up | 78 (57.8) | 82 (63.1) | ||
| Any suspected ear problem | 48 (35.6) | 55 (42.3) | ||
| May be irritable or withdrawn | 43 (31.9) | 38 (29.2) | ||
| Median number of symptoms, n (IQR) | 6 (4–8) | 7 (5–9) | ||
| OMQ-14 | ||||
| Standardised score, SD (n) | –0.04, 0.95 (153) | 0.07, 1.00 (153) | ||
| Reason for visit | Standard care (n = 160) | Autoinflation (n = 160) |
|---|---|---|
| Episodes of OME, n (%) | ||
| 0 | 120 (75.0) | 114 (71.3) |
| 1 | 21 (13.1) | 21 (13.1) |
| ≥ 2 | 13 (8.1) | 14 (8.8) |
| No information | 6 (3.8) | 11 (6.9) |
| Episodes of otitis media, n (%) | ||
| 0 | 101 (63.1) | 94 (58.8) |
| 1 | 30 (18.8) | 37 (23.1) |
| ≥ 2 | 22 (13.8) | 19 (11.9) |
| No information | 7 (4.4) | 10 (6.3) |
| Hearing loss, n (%) | ||
| 0 | 124 (77.5) | 119 (74.4) |
| ≥ 1 | 30 (18.8) | 31 (19.4) |
| No information | 6 (3.8) | 10 (6.3) |
| Snoring, n (%) | ||
| 0 | 146 (91.3) | 146 (91.3) |
| ≥ 1 | 8 (5.0) | 4 (2.5) |
| No information | 6 (3.8) | 10 (6.3) |
| Behaviour concerns, (%) | ||
| 0 | 149 (93.1) | 149 (93.1) |
| ≥ 1 | 5 (3.1) | 1 (0.6) |
| No information | 6 (3.8) | 10 (6.3) |
| Speech concerns, (%) | ||
| 0 | 148 (92.5) | 141 (88.1) |
| ≥ 1 | 6 (3.8) | 9 (5.6) |
| No information | 6 (3.8) | 10 (6.3) |
| Educational concerns, (%) | ||
| 0 | 149 (93.1) | 146 (91.3) |
| ≥ 1 | 5 (3.1) | 4 (2.5) |
| No information | 6 (3.8) | 10 (6.3) |
Exclusions, withdrawals, loss to follow-up and missing outcomes
Details of all these with reasons are included in the CONSORT trial profile provided (Figure 5). Retention in the study was good, with 27 of 320 (8.4%) lost to follow-up at 1 month and 39 of 320 (12.2%) by 3 months. Uninterpretable tympanograms owing to poor technique (leakage or low canal volume) and clinical problems (wax or perforation) were similar in both groups, leaving 131 children in the autoinflation arm and 132 in the routine care arm in the ITT analysis for the primary outcome.
FIGURE 5.
Main study CONSORT diagram.
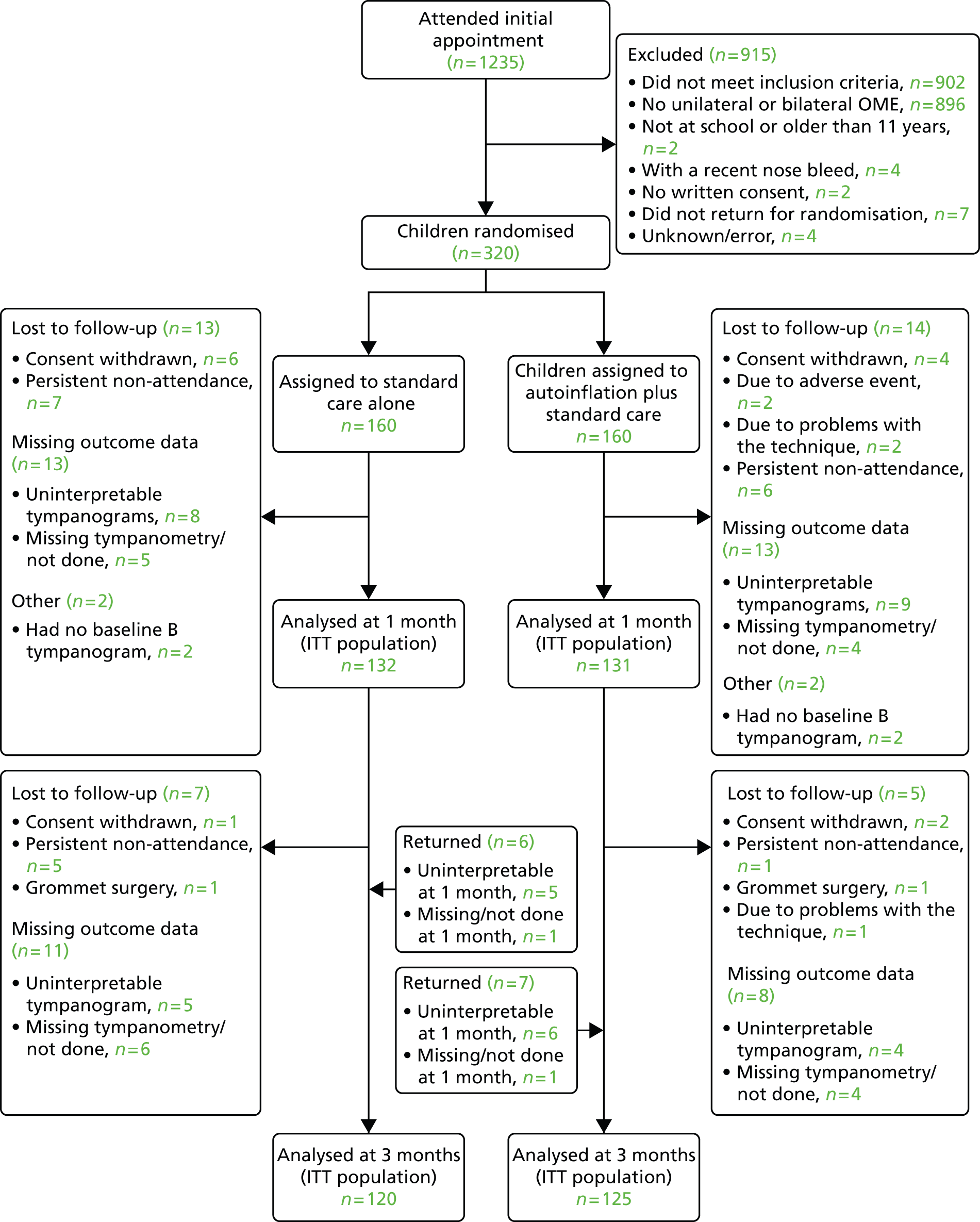
Time of follow-up assessments
Follow-up assessments were scheduled at 1 month (28 days) and 3 months (84 days) after randomisation. Table 13 shows the range the follow-up assessments timing. Parents were encouraged to return for assessment as close as possible to the scheduled date. Nine patients missed their 1-month assessment but returned for the 3-month assessment. In these cases, the 1-month data were considered as missing. Thirteen children with missing or uninterpretable tympanograms at 1 month returned at 3 months and were included in the analysis.
| Treatment group | Variable | n | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Standard care | 1-month visit | 141 | 32.4 | 8.3 | 19 | 77 |
| 3-month visit | 126 | 95.0 | 16.4 | 66 | 196 | |
| Autoinflation | 1-month visit | 140 | 32.4 | 7.6 | 21 | 82 |
| 3-month visit | 131 | 98.3 | 17.3 | 80 | 222 |
Main trial results
Primary outcome
Proportions of children showing clearance/resolution of at least one type B tympanogram (effusion) back to normal A/C1 pressures at 1 month
In the ITT population, 109 of 263 children experienced resolution of their B-type ears to A or C1 at 1 month: 62 of 132 (47%) children in the autoinflation group and 47 of 131 (36%) children in the standard care group. At 1 month, those in the autoinflation group were 36% more likely to have resolution of at least one B-type ear (RR 1.36, 95% CI 0.99 to 1.88; p = 0.0582). Sensitivity analyses using multiple imputations and a PP population analysis showed no significant difference between groups (Table 14).
| Analysis | n | Adjusted RR | 95% CI | p-value |
|---|---|---|---|---|
| ITT population | ||||
| Primary analysis (adjusteda) [Proc glimmix, dist = bin link = log] |
263 | 1.36 | 0.99 to 1.88 | 0.0582 |
| Sensitivity analysis (multiple imputationa,b) [Stata: mim: glm, fam(Poisson) link(log) vce(robust)] |
N = 320 M = 20 | 1.27 | 0.95 to 1.71 | 0.104 |
| PP population | ||||
| Sensitivity analysis: (PP populationa,c) [Proc glimmix, dist = bin link = log] |
195 | 1.14 | 0.80 to 1.63 | 0.4684 |
A further multiple imputation analysis was specified in the statistical plan, which proposed imputation for only those participants in whom data were missing for reasons that were not clinical (wax, perforation). This applied to only six participants, so this analysis was not performed.
Subgroup analyses
Pre-specified subgroups analyses of age (< 6.5 years vs. ≥ 6.5 years), severity (one vs. two B-type ears at baseline), OMQ-14 standardised total score (< 0 or ≥ 0) and sex were conducted on the primary outcome. In all cases no differences in treatment effects between subgroups were found. The p-values for the interaction term (treatment by subgroup) in the model ranged from 0.25 to 0.50. Although the interaction terms are not significant, they are of sufficient clinical interest to be considered further as additional (not pre-specified) secondary analyses (see Additional secondary analyses and Tables 24–29).
Secondary outcomes
Proportion of children showing clearance/resolution of at least one type B tympanogram (effusion) back to normal A/C1 pressures at 3 months
The resolution of at least one B-type ear at 3 months was analysed in the same way as the 1-month primary end point. There were 108 children with resolution of at least one B-type ear at 3 months. At 3 months, those in the autoinflation group were 37% more likely to have resolution of at least one B-type ear (RR 1.37, 95% CI 1.03 to 1.83; p = 0.0283) (Table 15).
| Tympanometric resolution in children of at least one affected type B ear | n | Standard care, n/N (%) resolved | Autoinflation, n/N (%) resolved | NNT | Adjusted RR (95% CI) | p-value |
|---|---|---|---|---|---|---|
| 1-month analysisa | 263 | 47/132 (35.6) | 62/131 (47.3) | 9 | 1.36 (0.99 to 1.88) | 0.06 |
| 3-month analysisb | 245 | 46/120 (38.3) | 62/125 (49.6) | 9 | 1.37 (1.03 to 1.83) | 0.03 |
Proportions of children’s ears showing resolution of a type B tympanogram back to A/C1 normal pressures at 1 and 3 months
An analysis of each ear separately was conducted, adjusting for the correlation between ears from the same child using generalised estimating equations (Table 16). Results were very similar to the main per-child analyses.
| Tympanometric resolution in B-type ears | n | Standard care, n/N (%) resolved | Autoinflation, n/N (%) resolved | Adjusted RR (95% CI) | p-value |
|---|---|---|---|---|---|
| 1-month analysisa,b | 375 B-type ears/263 children | 52/187 (28) | 73/188 (39) | 1.38 (1.01 to 1.87) | 0.0420 |
| 3-month analysisa,b | 348 B-type ears/245 children | 52/166 (31) | 74/182 (41) | 1.41 (1.5 to 1.88) | 0.0226 |
Tympanometric deterioration
There were 92 A- or C1-type ears at baseline for which valid tympanograms were also available at 1 month (Table 17). At 1-month follow-up, three A-type ears had deteriorated to B-type ears (one in the standard care group and two in the autoinflation group). Six of the C1-type ears had deteriorated to become B-type ears (four in the standard care group and two in the autoinflation group). Owing to the small number of deteriorations there was no further analysis on this end point.
| Group | Ears with no deterioration | Ears which deteriorated | Total |
|---|---|---|---|
| Standard care | 38 | 5 | 43 |
| Autoinflation | 45 | 4 | 49 |
| Total | 83 | 9 | 92 |
OMQ-14 impact measure
Table 18 displays summary statistics for baseline and follow-up (3-month) standardised OMQ-14 scores. Higher (more positive) scores represent worse outcomes. The average change from baseline in the standard care group was a decrease of 0.2 points, compared with a decrease of 0.7 points in the autoinflation group (p < 0.0001).
| Group | Time point | n | Number missing | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| Standard care | Baseline | 153 | 7 | –0.04 | 0.95 | –1.93 | 2.90 |
| 3 months | 121 | 39 | –0.37 | 1.06 | –2.15 | 3.06 | |
| Change from baseline | 121 | 39 | –0.21 | 0.90 | –3.11 | 1.90 | |
| Autoinflation | Baseline | 153 | 7 | –0.07 | 1.00 | –1.81 | 2.88 |
| 3 months | 127 | 33 | –0.70 | 1.01 | –2.12 | 3.11 | |
| Change from baseline | 126 | 34 | –0.69 | 0.84 | –2.58 | 1.44 |
A mean change in baseline score of –0.69 points (SD 0.84 points) at 3 months in the treated arm represents a large improvement.
At 3 months, the adjusted mean change from baseline in the standardised OMQ-14 total scores was greater in the autoinflation than in the routine care arm (Table 19). The difference between groups was –0.42 (95% CI –0.63 to –0.22) points. This score difference represents an adjusted effect size of 0.48 (of a SD; p < 0.0001) favouring the intervention (Figure 6).
| Group | n | Adjusted meana change from baseline | 95% CI | Mean difference | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Standard care | 121 | –0.22 | –0.40 to –0.04 | |||
| Autoinflation | 126 | –0.64 | –0.81 to –0.47 | –0.42 | –0.63 to –0.22 | < 0.001 |
FIGURE 6.
Standardised OMQ-14 scores at the 3-month follow-up. A lower/more negative score indicates a better outcome.
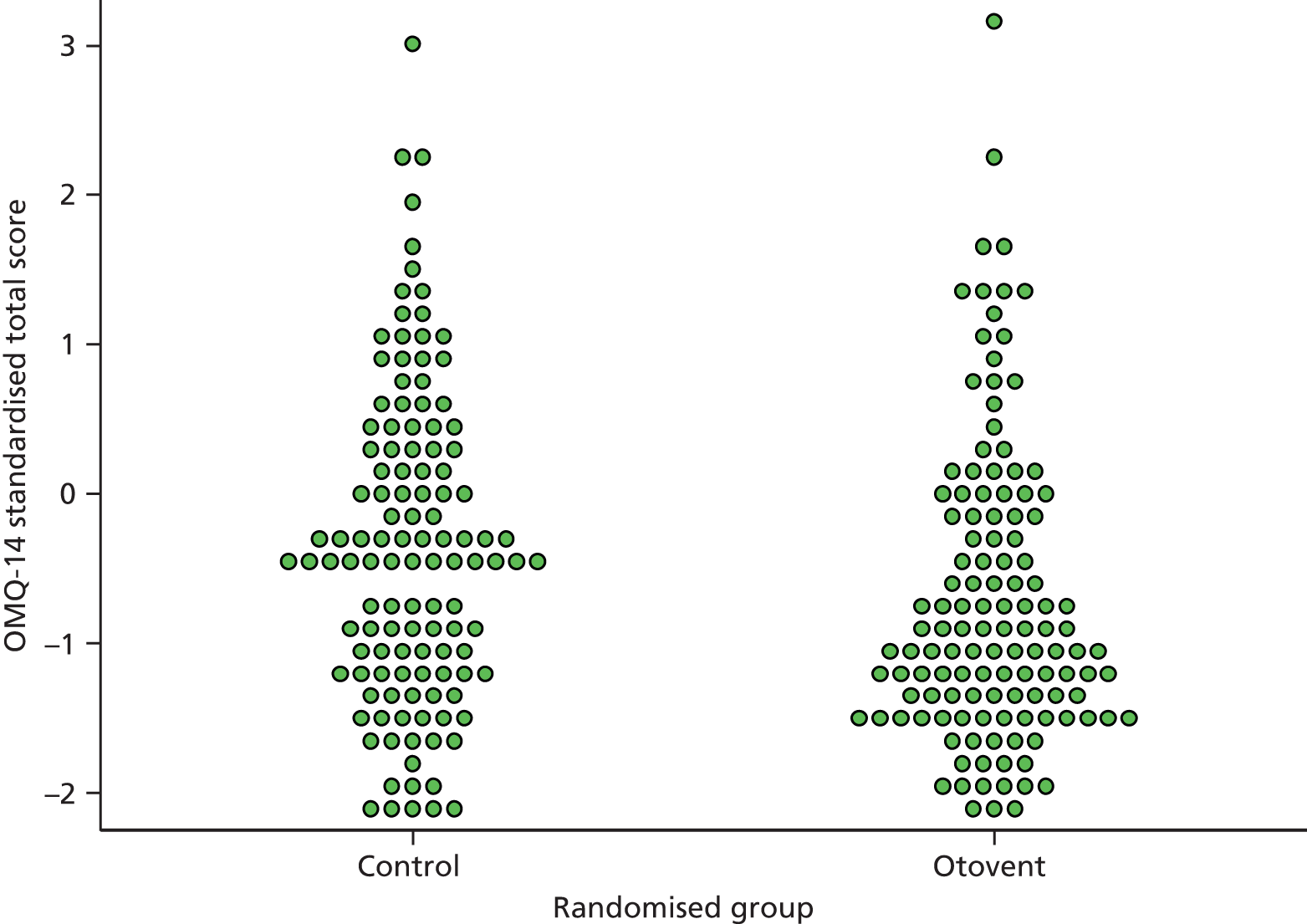
Main clinical diary symptoms
Days during which parents reported their child had hearing loss, earache, sleep disturbance or problems concentrating, and days requiring pain relief and days off school were summarised in accordance with the statistics plan (to avoid multiple outcomes) as days with any problem (Table 20). Overall, children in the autoinflation arm had fewer days/weeks with any symptom/problem at 1 month (OR 0.66, 95% CI 0.41 to 1.05; p = 0.08) and at 3 months (OR 0.58, 95% CI 0.37 to 0.90; p = 0.02).
| Days reporting one or more symptoms | Class | Standard care, n (%) | Autoinflation, n (%) | OR | 95% CI | p-valuea |
|---|---|---|---|---|---|---|
| 0–1 month: days reporting one or more symptomb | None | 9 (2.8) | 18 (5.6) | 0.658 | 0.413 to 1.048 | 0.0778 |
| 1–7 days | 47 (14.7) | 49 (15.3) | ||||
| ≥ 8 days | 82 (25.6) | 69 (21.6) | ||||
| Missing | 22 (6.9) | 24 (7.5) | ||||
| 1–3 months: days reporting one or more symptomb | None | 4 (1.3) | 9 (2.8) | 0.579 | 0.372 to 0.902 | 0.0157 |
| 1–7 days | 29 (9.1) | 30 (9.4) | ||||
| 8–28 days | 57 (17.8) | 73 (22.8) | ||||
| ≥ 29 days | 49 (15.3) | 27 (8.4) | ||||
| Missing | 21 (6.6) | 21 (6.6) |
Additional symptoms
Additional potentially adverse symptoms are reported in Table 21. See also Adverse events, which describes how the study collected symptoms from the reported adverse events sheets.
| OME diary question | 1-month totals | 3-month totals | ||||||
|---|---|---|---|---|---|---|---|---|
| Weeks with symptoms | Standard care, n (%) | Autoinflation, n (%) | p-valuea | Weeks with symptoms | Standard care, n (%) | Autoinflation, n (%) | p-valuea | |
| Has your child been clumsy/off-balance? | Missing | 22 (6.9) | 24 (7.5) | Missing | 21 (6.6) | 21 (6.6) | ||
| None | 81 (25.3) | 101 (31.6) | 0.0259 | None | 68 (21.3) | 92 (28.8) | 0.0073 | |
| 1 week | 14 (4.4) | 13 (4.1) | < 1 month | 31 (9.7) | 29 (9.1) | |||
| 2 weeks | 13 (4.1) | 10 (3.1) | 4–7 weeks | 23 (7.2) | 10 (3.1) | |||
| 3 weeks | 8 (2.5) | 5 (1.6) | ≥ 8 weeks | 17 (5.3) | 8 (2.5) | |||
| 4 weeks | 22 (6.9) | 7 (2.2) | ||||||
| Has your child been unwell/had a temperature? | Missing | 23 (7.2) | 24 (7.5) | Missing | 21 (6.6) | 21 (6.6) | ||
| None | 56 (17.5) | 61 (19.1) | 0.8345 | None | 33 (10.3) | 35 (10.9) | 0.6967 | |
| 1 week | 45 (14.1) | 45 (14.1) | < 1 month | 79 (24.7) | 78 (24.4) | |||
| 2 weeks | 20 (6.3) | 19 (5.9) | 4–7 weeks | 22 (6.9) | 24 (7.5) | |||
| 3 weeks | 9 (2.8) | 5 (1.6) | ≥ 8 weeks | 5 (1.6) | 2 (0.6) | |||
| 4 weeks | 7 (2.2) | 6 (1.9) | ||||||
| Has your child had a runny nose? | Missing | 23 (7.2) | 24 (7.5) | Missing | 21 (6.6) | 21 (6.6) | ||
| None | 43 (13.4) | 25 (7.8) | 0.0707 | None | 22 (6.9) | 10 (3.1) | 0.0201 | |
| 1 week | 25 (7.8) | 33 (10.3) | < 1 month | 60 (18.8) | 59 (18.4) | |||
| 2 weeks | 25 (7.8) | 24 (7.5) | 4–7 weeks | 30 (9.4) | 49 (15.3) | |||
| 3 weeks | 23 (7.2) | 21 (6.6) | ≥ 8 weeks | 27 (8.4) | 21 (6.6) | |||
| 4 weeks | 21 (6.6) | 33 (10.3) | ||||||
| Has your child had a blocked nose/been snoring? | Missing | 23 (7.2) | 24 (7.5) | Missing | 21 (6.6) | 21 (6.6) | ||
| None | 37 (11.6) | 31 (9.7) | 0.2211 | None | 21 (6.6) | 14 (4.4) | 0.1436 | |
| 1 week | 19 (5.9) | 17 (5.3) | < 1 month | 38 (11.9) | 43 (13.4) | |||
| 2 weeks | 9 (2.8) | 21 (6.6) | 4–7 weeks | 29 (9.1) | 42 (13.1) | |||
| 3 weeks | 20 (6.3) | 17 (5.3) | ≥ 8 weeks | 51 (15.9) | 40 (12.5) | |||
| 4 weeks | 52 (16.3) | 50 (15.6) | ||||||
| Has your child had any nosebleeds? | Missing | 23 (7.2) | 24 (7.5) | Missing | 21 (6.6) | 21 (6.6) | ||
| None | 124 (38.8) | 121 (37.8) | 0.3687 | None | 114 (35.6) | 114 (35.6) | 0.5546 | |
| 1 week | 10 (3.1) | 9 (2.8) | < 1 month | 24 (7.5) | 22 (6.9) | |||
| 2 weeks | 2 (0.6) | 3 (0.9) | 4–7 weeks | 0 | 2 (0.6) | |||
| 3 weeks | 0 | 3 (0.9) | ≥ 8 weeks | 1 (0.3) | 1 (0.3) | |||
| 4 weeks | 1 (0.3) | 0 | ||||||
Compliance
Of the 130 parents recorded, 116 (89%) reported the use of autoinflation as ‘most’ or ‘all of the time’ during the first month of treatment, consistent with the daily compliance charts. This level of compliance appears to be maintained in those continuing treatment up to 3 months (68/85, 80%) (Table 22). Figures 7 and 8 show median comparator estimates for the two methods used to assess compliance (diary sticker charts and parental report).
| Parent reported how often the child performed autoinflation | Diary compliant days at month 1a | Diary compliant days at months 2–3a | ||||
|---|---|---|---|---|---|---|
| n | Median | Range | n | Median | Range | |
| Not at all | 1 | 0 | 0–0 | 1 | 27 | 27–27 |
| Some of the time | 11 | 9 | 0–19 | 14 | 20.5 | 0.0–34.0 |
| Most of the time | 63 | 26 | 3–28 | 47 | 52 | 0–56 |
| All of the time | 53 | 28 | 0–28 | 21 | 56 | 33–56 |
| Missing | 2 | 13 | 5–21 | 2 | 37 | 18–56 |
| Overall | 130 | 26 | 0–28 | 85 | 51 | 0–56 |
FIGURE 7.
Compliance for months 0–1 (diary assessment vs. parental report of compliance).
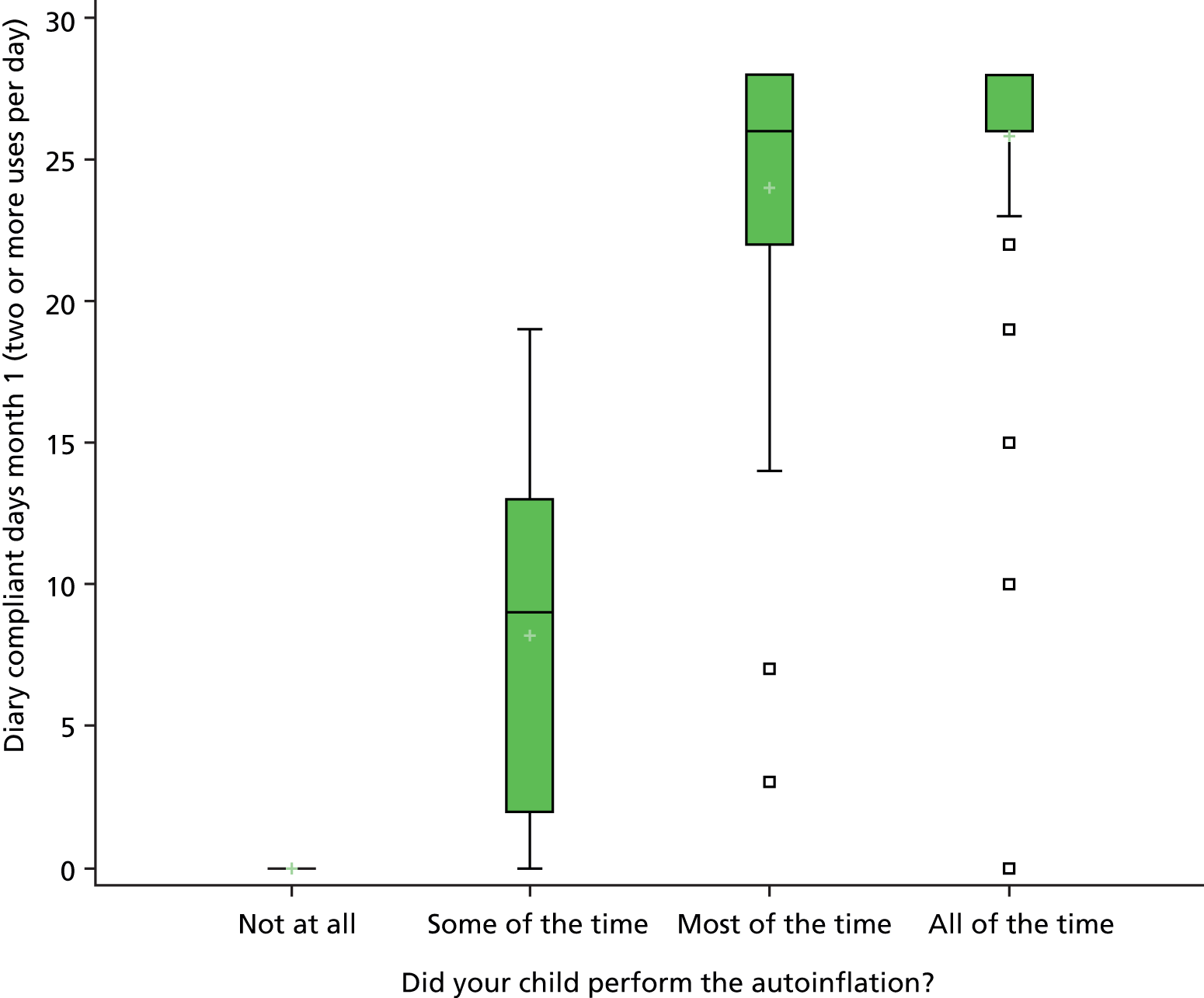
FIGURE 8.
Compliance for months 1–3 (diary assessment vs. parental report of compliance).

Adverse events
As there were no previously reported adverse events for autoinflation, apart from anecdotal otalgia, the events recorded here are otherwise biased towards overinclusivity, and some overlap with the additional symptoms reported in Table 21. There was very little difference between treatment arms in terms of numbers of children with a nosebleed (15% vs. 14%), but there were more reported RTIs in the treatment group (18% vs. 13% of children, 37 vs. 18 episodes). Most of the RTIs were classified, however, as mild afebrile rhinorrhoea. Eight children in the autoinflation arm (compared with two in routine care) reported otalgia (Table 23). One 8-year-old child in the autoinflation arm was hospitalised with mild/early mastoiditis on day 10 of treatment and made a full recovery after treatment with intravenous antibiotics.
| Adverse event | Standard care (n = 160) | Autoinflation plus standard care (n = 160) | ||
|---|---|---|---|---|
| Events, n | Affected children, n (%) | Events, n | Affected children, n (%) | |
| Nosebleed | 26 | 24 (15) | 26 | 22 (14) |
| URTI | 6 | 6 (4) | 20 | 13 (8) |
| Unspecified RTI | 4 | 4 (3) | 9 | 9 (6) |
| Lower RTI | 4 | 4 (3) | 2 | 2 (1) |
| AOM | 4 | 4 (3) | 6 | 5 (3) |
| Otalgia | 2 | 2 (1) | 8 | 7 (4) |
| Headache | – | – | 2 | 2 (1) |
| Hay fever | – | – | 1 | 1 |
| Serious adverse event | ||||
| Hospitalisationa | 1 | 1 (1) | 1 | 1 (1) |
No bacteriology was available to confirm the cause. Clinical details of relevance were obtained in full and revealed that the child had been investigated by a paediatrician for recurrent RTIs in the previous 12 months. The case was reviewed independently by the DMEC (see Appendix 7). It was noted that no previous cases of mastoiditis had been reported with the use of Otovent, a device available over the counter in the UK and abroad, and no cases had been reported in any of the previous trial literature (≈500 receiving autoinflation). The DMEC did a full risk assessment and advised that it was safe to continue with the study.
Additional secondary analyses
Tables 24–29 deal in more detail with specified subgroups and have been included because of clinical importance and interest to the general literature. The analyses are not sufficiently powered and show a number of non-significant p-values for each headed subgroup. Details of the overlapping CIs between the two study arms for resolution rates (primary outcome) are also shown.
| Randomised group | Bilateral | Unilateral | ||
|---|---|---|---|---|
| No resolution | Resolution | No resolution | Resolution | |
| Autoinflation, n (%) | 29 (48.33) | 31 (51.67) | 40 (56.34) | 31 (43.66) |
| Standard care, n (%) | 39 (65.00) | 21 (35.0) | 46 (63.89) | 26 (36.11) |
| RR (95% CI) | 1.48 (0.97 to 2.25) | 1.21 (0.81 to 1.81) | ||
| Randomised group | Male | Female | ||
|---|---|---|---|---|
| No resolution | Resolution | No resolution | Resolution | |
| Autoinflation, n (%) | 31 (46.97) | 35 (53.03) | 38 (58.46) | 27 (41.54) |
| Standard care, n (%) | 44 (65.67) | 23 (34.33) | 41 (63.08) | 24 (36.92) |
| RR (95% CI) | 1.54 (1.03 to 2.31) | 1.125 (0.730 to 1.720) | ||
| Randomised group | Age ≥ 6.5 years | Age < 6.5 years | ||
|---|---|---|---|---|
| No resolution | Resolution | No resolution | Resolution | |
| Autoinflation, n (%) | 13 (41.94) | 18 (58.06) | 54 (55.10) | 44 (44.90) |
| Standard care, n (%) | 18 (64.29) | 10 (35.71) | 65 (63.73) | 37 (36.27) |
| RR (95% CI) | 1.63 (0.91 to 2.90) | 1.24 (0.88 to 1.74) | ||
| Randomised group | OMQ-14 score of ≥ 0 | OMQ-14 score of < 0 | ||
|---|---|---|---|---|
| No resolution | Resolution | No resolution | Resolution | |
| Autoinflation, n (%) | 28 (49.12) | 29 (50.88) | 41 (56.94) | 31 (43.06) |
| Standard care, n (%) | 36 (67.92) | 17 (32.08) | 46 (61.33) | 29 (38.67) |
| RR (95% CI) | 1.59 (0.99 to 2.53) | 1.11 (0.75 to 1.64) | ||
| Randomised group | No GP visits | At least one GP visit | ||
|---|---|---|---|---|
| No Resolution | Resolution | No Resolution | Resolution | |
| Autoinflation, n (%) | 54 (55.10) | 44 (44.90) | 15 (45.45) | 18 (54.55) |
| Standard care, n (%) | 66 (68.75) | 30 (31.25) | 19 (52.78) | 17 (47.22) |
| RR (95% CI) | 1.44 (0.99 to 2.08) | 1.15 (0.73 to 1.84) | ||
| Randomised group | Spring: March–May | Summer: June–August | Autumn: September–November | Winter: December–February | ||||
|---|---|---|---|---|---|---|---|---|
| No resolution | Resolution | No resolution | Resolution | No resolution | Resolution | No resolution | Resolution | |
| Autoinflation, n (%) | 29 (47.54) | 32 (52.46) | 4 (57.14) | 3 (42.86) | 16 (69.57) | 7 (30.43) | 20 (50.00) | 20 (50.00) |
| Standard care, n (%) | 38 (61.29) | 24 (38.71) | 4 (50.00) | 4 (50.00) | 17 (73.91) | 6 (26.09) | 26 (66.67) | 13 (33.33) |
| RR (95% CI) | 1.36 (0.91 to 2.01) | 0.86 (0.29 to 2.58) | 1.167 (0.46 to 2.94) | 1.50 (0.87 to 2.58) | ||||
We have also, for reasons of clinical interest, added to this section the pre-baseline symptom (history) predictors of the finding of at least one type B tympanogram in the screened child. A univariate analysis was performed on all the parent-listed ear symptom/concerns items in the previous 3 months and those items that remained significant (Table 30) were then added in a stepwise multivariate analysis to derive a receiver operator characteristic (ROC) curve (Figure 9).
| Baseline-reported symptom | Univariate analysis (95% CI) | Multivariate OR (95% CI) |
|---|---|---|
| A prolonged or bad cold, cough or chest infection | 1.80 (1.23 to 2.64) | |
| Appears to be lip reading | 2.18 (1.51 to 3.15) | |
| An earache | 1.98 (1.51 to 3.15) | |
| Not doing as well at school as expected | 1.54 (1.13 to 2.59) | |
| Often mishears what is said | 2.55 (1.85 to 3.51) | 1.79 (1.26 to 2.56) |
| Hearing loss is suspected by anyone | 2.41 (1.83 to 3.17) | |
| Snores, blocked nose or poor sleep | 1.57 (1.17 to 2.10) | |
| Says ‘eh what?’ or ‘pardon’ a lot | 2.14 (1.52 to 3.02) | |
| Needs the television turned up | 2.32 (1.76 to 3.04) | 1.64 (1.22 to 2.23) |
| Any suspected ear problem | 2.74 (2.05 to 3.65) | 2.04 (1.48 to 2.79) |
| May be irritable or withdrawn | 1.28 (0.95 to 1.72) | |
| Speech behind other children | 1.14 (0.81 to 1.59) | |
| Noises in ear/dizzy | 1.32 (0.96 to 1.81) |
FIGURE 9.
Area under the ROC curve based on independent historical variables.
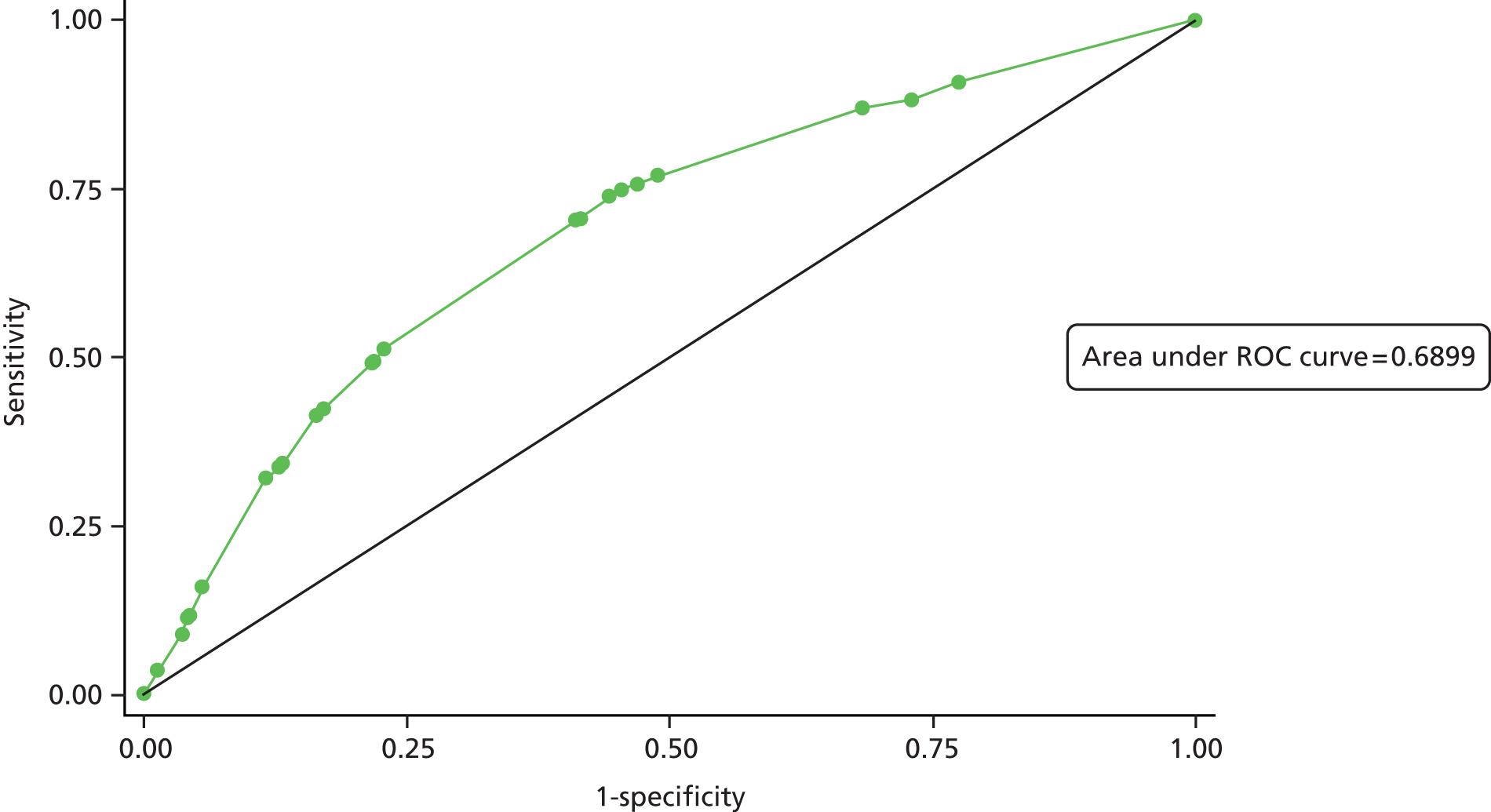
Meta-analysis for research in context
A meta-analysis with trials identified in the recent Cochrane review35 using similar outcomes at 1 month (ear-based analysis B to A/C1) favoured autoinflation (RR 1.61, 95% CI 1.26 to 2.06) (Figure 10). When the pilot study was combined with the main study as per the statistical plan, the primary care setting studies, when pooled, found a RR of 1.37 (95% CI 1.00 to 1.87) (Figure 11).
FIGURE 10.
Meta-analysis of 1-month outcomes (ear-based analysis). AIRS, AutoInflation Randomised Study; D + L, DerSimonian and Laird method; I-V, inverse variance method; SE, standard error.

FIGURE 11.
Meta-analysis primary care studies (per-child analysis) at 1 month. AIRS, AutoInflation Randomised Study; D + L, DerSimonian and Laird method; I-V, inverse variance method; SE, standard error.

Chapter 5 Health economic analysis
Introduction
The AutoInflation Randomised Study (AIRS) was designed to estimate the clinical effectiveness and cost-effectiveness of autoinflation alongside standard care compared with standard care alone in children with OME in primary care. An economic analysis was part of the design of the study. Data on resource use and health-related QoL using the HUI376,77,89 were collected during the trial. Outcomes were expressed both as cost per QALY gained and as cost per additional proportion of tympanic resolution of the intervention compared with standard care. This section reports that analysis.
Methods
The economic evaluation was taken from both the NHS and a Personal Social Services perspective. The baseline analysis was at 3 months. Resource usage beyond 3 months and the effects of including travel costs and those due to parents’ time off work were explored in scenario analysis.
Data collection
Resource usage data were extracted at 6 months after recruitment by study nurses through searching electronic records. A restricted set of resource use data were also extracted at 12 months focusing on hospital admissions related to otitis media. This was because grommet insertion, the most common specialist treatment, is often delayed by over 6 months after presentation. HUI3 forms were completed by patients at baseline, at 1 month and at 3 months. At 3 months after recruitment, parents completed an extra questionnaire on travel costs and time off work due to ear-related illness of their children.
Cost estimation
Resource use costs
Resource use included primary care consultations, prescribed medication, all otitis-related outpatient referrals, referrals for audiology, speech therapy or to community health-care professionals and any ear-related hospitalisations.
Medications
Medications recorded related to otitis media included antibiotics, decongestants and antihistamines, and analgesics. The names of medication, dosage and days of use were recorded. We used the pack price in costing all medications on the basis that this reflected the real costs of NHS resource use. The number of packs was estimated and costed based on actual duration. If no data were available on the duration of medications use was available, then one pack was assumed. The unit costs of medications were obtained from data published by the British National Formulary in September 2012 (Table 31). 90
| Name | Contents | Formulation | Pack volume | Pack/dose units | Price/pack |
|---|---|---|---|---|---|
| Amoxicillin (Amoxil®, GlaxoSmithKline) | 1 bottle | Suspension | 100 | ml | £1.13 |
| Cefalexin | 28 | Tablets | 250 | mg | £1.90 |
| Clarithromycin (Klaricid®, Abbott Healthcare) | 14 | Tablets | 250 | mg | £1.89 |
| Co-amoxiclav (Augmentin®, GlaxoSmithKline) | 1 bottle | Suspension | 100 | ml | £1.94 |
| Erythromycin | 28 | Tablets | 250 | mg | £23.43 |
| Ofloxacin (Exocin®, Allergan) | 1 | Eye drops | 5 | ml | £2.17 |
| Otomize spray (Cofradex®, Sanofi-aventis) | 1 | Ear spray | 5 | ml | £3.50 |
| Penicillin V Elixir | 28 | Tablets | 250 | mg | £1.40 |
| Ciprofloxacin (Ciloxan®, Alcon) | 1 | Eye ointment | 3.5 | g | £5.22 |
| Ciprofloxacin drops (Ciloxan®, Alcon) | 1 | Eye drops | 5 | ml | £4.70 |
| Betamethasone (Betnesol®, RPH) | 1 ampoule | Ampoule | 4 | mg | £1.22 |
| Trimethoprim | 14 | Tablets | 200 | mg | £0.98 |
Primary care consultations and secondary care costs
The unit costs of primary and secondary care consultations used were those published by Personal Social Services Research Unit at 2011–12 prices (see Table 25). 91 The Healthcare Resource Group costing applicable to study patients was based on their diagnoses, minor ear procedures, minor nose procedures and ENT outpatient costs (CZ12U).
Intervention costs
The intervention cost was based on the price charged by the company supplying Otovent packs to the NHS and the time required for training children in its use. The 2014 price to the NHS was £4.90 for one pack of Otovent (tube and five balloons). All patients in the intervention group were given one pack of the Otovent. However, if resolution was not achieved at 1 month, a second pack of Otovent was given to those patients. One consultation of 4 minutes with a practice nurse comprised training in the use of the Otovent. The dispensing cost was included (Table 32).
| Resource use item | Unit cost and source |
|---|---|
| Intervention cost (device and training on how to use) | Average 4 minutes per patient training with nurse (£52 per hour) = £3.47. Cost of device (4-week course) = £6.90 including dispensing costs |
| Practice nurse telephone call | Cost of standard nurse telephone call (6 minutes, at £40 per hour), including qualifications Source: PSSRU 2013 (table 10.6)91 = £4.00 |
| Practice nurse consultation in GP practice | Cost of standard face to face nurse consultation (£52 per hour), including qualifications Source: PSSRU 2013 (table 10.6)91 = £13.43 |
| GP consultation | Consultation lasting 11.7 minutes, including qualifications and direct care staff costs Source: PSSRU 2013 (table 10.8b)91 = £45 |
| GP home visit | Out-of-surgery visit, GP Source: PSSRU 2013 (table 10.8b)91 = £114 |
| Out-of-hours GP consultation | Out-of-hours benchmark, includes overheads = £61.14 Source: Primary Care Foundation, 201392 |
| ENT outpatient attendance | Paediatric ENT outpatient attendance, service code 215 = £95 |
| Ear-related inpatient cost, paediatrics | Minor ear procedures, elective inpatients, 18 years and under paediatric ENT CZ08T, service code 215 = £1295 Source: NHS Reference Costs 201393 |
| Adenoidectomy inpatient hospital cost, paediatrics | Minor nose procedures, elective inpatients, 18 years and under CZ12U, service code 215 = £1472 Source: NHS Reference Costs, 201393 |
| Non-ENT-related outpatient visit | Paediatric outpatient = £187, service code 420 Source: NHS Reference Costs, 201393 |
Quality of life
Self-completed questionnaires using the HUI3 were recorded at baseline, 1 month and 3 months during the trial. The data in eight categories were used to estimate utility scores for each individual child. The mapping algorithm and score functions were purchased from Health Utilities Inc. The algorithms map data from the 17-item interviewer-administered questionnaire to each of eight attributes (vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain) of the HUI3 classifications. Utility scores for each patient were calculated based on these algorithms.
Analysis
The economic analysis was conducted using patient-specific resource use and QoL data. The time frame was 3 months for the primary outcome, but for 12 months a restricted data set was collected on resource use. The base case for the economic analysis (equivalent to the primary analysis of the main study) was at 3 months for both outcomes and costs. The data on resource use at 12 months were compared between arms with a focus on hospitalisations.
Accumulated total costs per patient were calculated by the sum of the products of resource use items and the associated unit costs, aggregated over the study period. QALYs for each patient were calculated according to the utility scores derived from HUI3 at baseline, 1 and 3 months using the area under the curve, and adjusted baseline difference in QoL scores, using the mean value between utility scores at baseline.
Missing data
Missing data in QoL scores derived from HUI3 were assumed to be equal to the mean for each treatment group by time point.
The mean cost per patient and QALYs associated with the intervention and standard care group were calculated and the differences between them evaluated in accordance with ITT principle. The bias-corrected bootstrap method was used to estimate mean costs, QALYs and the incremental cost-effectiveness ratio (ICER) with the associated 95% CI. Uncertainty around the costs and effectiveness estimates was illustrated using a scatterplot with a confidence ellipse. Cost-effectiveness acceptability curves were drawn to show the probability of the intervention being cost-effective given the level of willingness to pay per QALY gained.
Although the base-case analysis used ITT, a cost-effectiveness analysis was carried out on patients for whom clinical outcome data were available at 3 months (120 and 125 patients in the intervention and standard care arms respectively). The analyses were conducted in SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
The NHS costs at 3 months showed no statistically significant differences between arms (Tables 33 and 34). The mean cost per patient at 3 months was £17.61 (95% CI £10.42 to £24.81) for the routine care arm, compared with £31.94 (95% CI £23.69 to £40.19) for the Otovent plus routine care arm. The higher mean cost in the intervention arm was almost entirely caused by the cost of the intervention at £14.22. Only small differences were found between arms in use of the other resource headings.
| Intervention | n a | Mean | SD | 95% CIb |
|---|---|---|---|---|
| Standard care group | ||||
| Total cost | 132 | £17.613 | £41.771 | £10.42 to £24.805 |
| Primary care visits | 28 | £53.97 | £33.958 | £40.802 to £67.137 |
| Outpatient attendance | 8 | £95 | 0 | |
| Medication | 16 | £3.358 | £2.601 | £1.972 to £4.743 |
| Intervention | 0 | |||
| Autoinflation group | ||||
| Total cost | 131 | £31.941 | £47.733 | £23.69 to £40.192 |
| Primary care visits | 31 | £54.613 | £44.956 | £38.123 to £71.103 |
| Outpatient attendance | 5 | £114 | £42.485 | £61.248 to £166.752 |
| Medication | 19 | £3.046 | £2.295 | £1.94 to £4.152 |
| Intervention | 131 | £14.224 | £3.458 | £13.627 to £14.822 |
| Utility scores | Standard care (95% CI) (n = 132) | Autoinflation (95% CI) (n = 131) |
|---|---|---|
| Utility score at baseline | 0.781 (0.744 to 0.818) | 0.758 (0.717 to 0.798) |
| Utility score at month 1 | 0.787 (0.747 to 0.828) | 0.808 (0.768 to 0.847) |
| Utility score at month 3 | 0.843 (0.811 to 0.876) | 0.846 (0.808 to 0.885) |
| Utility score changed from baseline at 1 month | 0.006 (–0.03 to 0.042) | 0.050 (0.015 to 0.084) |
| Utility score changed from baseline at 3 months | 0.062 (0.025 to 0.1) | 0.089 (0.05 to 0.127) |
| QALYs (adjusted baseline difference) | 0.197 (0.188 to 0.205) | 0.200 (0.191 to 0.209) |
Quality of life
Utility scores at baseline, 1 month and 3 months and their changes from baseline at 1 month and 3 months are presented in Table 34. QALYs estimated from the HUI scores showed small differences between arms at baseline and at 1 and 3 months. The mean QALY gain was 0.197 (95% CI 0.188 to 0.205) in the control group and 0.200 (95% CI 0.191 to 0.209) in the intervention group. This small difference was not statistically significant.
Although the incremental difference in the primary outcome, tympanometric resolution of fluid, was statistically significant at 3 months, the difference in QALYs, although in the same direction, just missed statistical significance.
Mean incremental bootstrapped difference in QALYs, costs and ICERs (Table 35) put the ICER at £8463 (95% CI –£104,894 to £121,820). Although the QALY difference was not statistically significant, the bootstrapped cost difference was.
| Outcomes | Mean | 95% CI |
|---|---|---|
| QALY | ||
| Standard care group | 0.197 | 0.191 to 0.209 |
| Autoinflation group | 0.200 | 0.192 to 0.208 |
| Difference | 0.003 | –0.010 to 0.020 |
| Total cost | ||
| Standard care group | £17.5 | £10.5 to £24.9 |
| Autoinflation group | £31.8 | £24.2 to £39.9 |
| Difference | £14.3 | £3.5 to £25.2 |
| ICER | £8463 | –£104,894 to £121,820 |
The incremental cost per case resolved, based on those for whom data were available, was £132 (95% CI –£2315 to £2333) (Table 36).
| Outcomes | Mean | 95% CIa |
|---|---|---|
| Mean cost | ||
| Standard care group | £19.02 | £11.07 to £26.31 |
| Autoinflation group | £26.79 | £21.28 to £32.42 |
| Difference | £7.78 | –£1.29 to £17.61 |
| Tympanometric resolution | ||
| Standard care group | 0.39 | 0.29 to 0.47 |
| Autoinflation group | 0.50 | 0.41 to 0.58 |
| Difference | 0.11 | 0.01 to 0.24 |
| Incremental cost per case resolved | £132 | –£2315 to £2333 |
Sensitivity analysis
The cost-effectiveness acceptibility curve (probabilistic sensitivity analysis) for the cost per QALY put the probability of the intervention being cost-effective at 50.1% and 50.2% at a willingness-to-pay threshold of £20,000 and £30,000 per QALY respectively (see Figures 12 and 13).
FIGURE 12.
Scatterplot with 95% confidence ellipse. 95% confidence ellipse of difference in cost vs. QALY for Otovent vs. control.

FIGURE 13.
Cost-effectiveness acceptability curve.
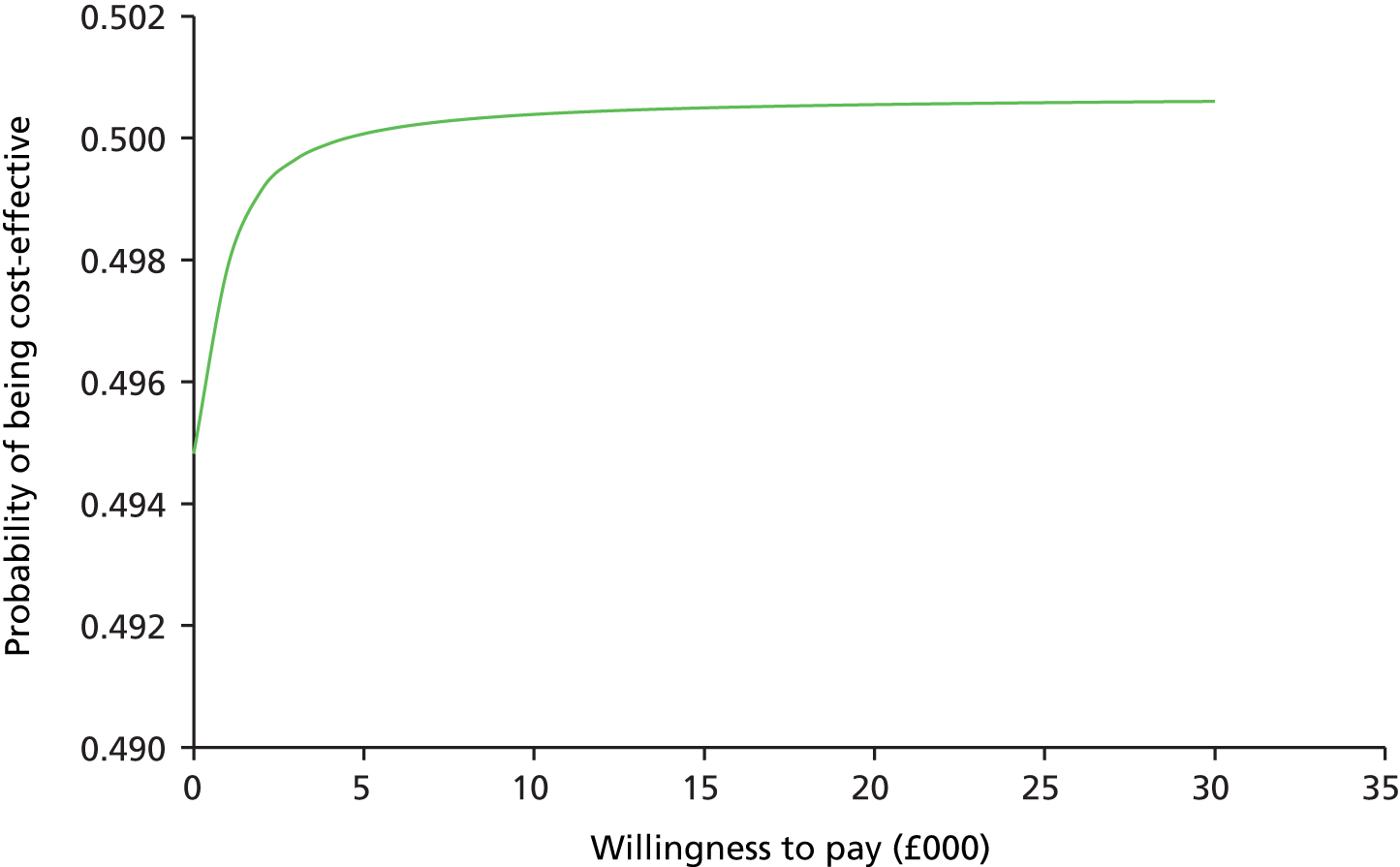
Scenario analyses
Separate analysis explored resource use and cost at 12 months. This showed few hospitalisations in either arm and reflected the cost differences reported above at 3 months.
Another analysis explored privately borne costs regarding travel and parents’ time off work. Only small differences were found in travel costs between arms. Similarly, few families reported time off work, with small differences between arms. Given the lack of difference in resource use between arms, this was as expected. Inclusion of these costs made minimal difference to the economic analyses reported above.
Full details of both above are available on request.
Discussion
The intervention improved outcomes at a low cost, leading to just over 50% probability of it being cost-effective at conventional cost per QALY thresholds. This analysis was based on ITT, a cost difference that was statistically significant when bootstrapped and a QALY difference that was not statistically significant.
The cost-effectiveness analysis was based on a smaller subset of patients for whom data were available. This showed a statistically significant difference in cases resolved at 3 months and put the cost per case resolved at £132.
The difference in mean cost was almost entirely caused by the cost of the intervention. The improved outcomes were not associated with any change in NHS service use, or with any privately borne costs. This may be because the improvement was insufficiently large to change patterns or service use, or it may be because of individual practitioners being reluctant to change their behaviours until evidence for an intervention is proven. Given that all unresolved children in the standard care arm were offered Otovent at 3 months as per the protocol (an ethical consideration), the effects are confounded beyond the end of the trial at 3 months.
Limitations of the CEA include:
-
The duration was short, with outcomes followed up only to 3 months and with selected resource use up to 12 months. Follow-up on service use to 12 months showed no difference in hospitalisations for related conditions, the main one being grommet surgery (± adenoidectomy).
-
HUI used US population weights. This was because of the greater experience of this instrument with children and its prior use in a similar study by the same team. 30 The QALY differences were reassuringly in the same direction as the primary outcome. Although US population weights were used, these are likely to be similar to those for the UK, as indicated by the similarity of US and UK weights for European Quality of Life-5 Dimensions. 94 The potentially beneficial effects of improvement in case resolution may over antibiotic prescribing have not been included.
-
The potentially beneficial effects of improvement in case resolution over antibiotic prescribing have not been included.
-
Although imputation was used in the clinical analysis of outcomes at 1 month, given that this made little difference to the results, a decision was made not to combine the methods of multiple imputation and bootstrapping.
Conclusions
The cost-effectiveness analysis based on the statistically significant difference in cases resolved puts the cost per case resolved at £132. Although the cost difference was not statistically significant, it was based almost entirely on the cost of the intervention.
The cost per QALY analysis showed the Otovent device to be just likely to be a cost-effective intervention. The uncertainty reflects the small and non-statistically significant difference in QALYs, a generic rather than condition-specific measure of outcome than the OMQ-14.
Chapter 6 Qualitative evaluation
Background
Autoinflation is a promising non-surgical treatment for OME, which has potential to improve natural resolution rates and QoL for children with OME-related concerns and symptoms, some of whom may be considered for ENT referral. The reliability of children inflating the nasal balloon and longer-term compliance with treatment has remained a concern regarding whether or not it could be a suitable treatment in primary care. 35 Although overall compliance has been assessed in the main trial, no previous qualitative work has been carried out with families or health-care professionals to explore facilitators and barriers to UK primary care management of OME, and the practicalities of use of autoinflation during this period.
This chapter reports a nested qualitative study, which is designed to inform the wider implementation of autoinflation in the primary care setting, including the monitoring process.
Objective
The qualitative study aims to explore the views and experiences of parents and practice nurses of both autoinflation and monitoring in primary care.
Methods
Participants and procedures
Participants were identified and recruited from general practices that participated in the main trial. A maximum variety sample95 of practice nurses were invited to participate, including nurses from high- and low-recruiting practices, career RNs and practice nurses who undertake research alongside their normal duties. A maximum variety sample of parent participants ensured a range of child characteristics including age, sex, baseline severity of OME and GP practice location. This sampling was carried out to select a wide variety of ‘information-rich cases’, to obtain in-depth information about the issues relevant to the study. 95
Interviews
Interviews were conducted either face to face or by telephone by a trained interviewer (JV), each lasting approximately 30 minutes. An interview guide was used to steer the interview while remaining sufficiently flexible to allow participants to raise issues that were important to them (see Appendix 8). Participants were asked about their views of screening and monitoring of glue ear in primary care, experiences of autoinflation including enablers and barriers to its use, and overall experiences of participating in AIRS. The interviews were digitally audio-recorded and transcribed verbatim, removing any identifiable data to ensure anonymity.
Analysis
Data were managed using NVivo 10 software and analysed using thematic analysis. 96 After initial familiarisation, the transcripts were systematically and comprehensively coded using open coding, a method of reducing the data while capturing the semantics and concepts of the data itself. The first three transcripts were coded by multiple coders and a coding framework agreed, improving the reliability of the study. Codes were refined into broad themes both inductively and guided by a priori knowledge of the topic area. Themes were then defined and described in relation to the research questions and existing literature.
Findings
Participants
A total of 33 participants took part in a research interview. Of these, 19 were practice nurses recruited from 18 GP practices across 10 former PCTs in the South West England, Thames Valley and Cheshire regions. Registered practice populations ranged from 3378 to 28,261, with the Index of Multiple Deprivation decile ranging from 6 to 10 (mid to low deprivation). Nurses variously described their employment status as practice nurses (n = 11), RNs (n = 7) and secondary care RNs (n = 1). The 14 parent participants were recruited from 10 practices in South West England and Thames Valley. All parents were the mothers, reflecting the usual carer who brought the child to the AIRS appointments.
Themes
Three key themes emerged from the analysis (Table 37). These themes are not an exhaustive account of the findings, but represent the major themes interpreted as relevant to the research question. Each theme is described in the following section and exemplar quotations are given to illustrate the subthemes.
| Theme | Subtheme |
|---|---|
| Rationalising | What parents knew about OME Rationalising treatment decisions |
| Primary care management | Screening for OME Practice nurse as OME case manager Referral expectations |
| Engaging with monitoring and treatment | Interactions between nurses and families Compliance with autoinflation |
Rationalising
This theme is defined as how parents seek information about OME and use their knowledge, experience and concerns to rationalise decisions about their child’s management.
What parents knew about otitis media with effusion
Parents used a range of information including tacit knowledge, personal experience and information gathered from friends, family and health professionals to make sense of glue ear and understand the implications for their child. There was a mixed knowledge base, with some parents having a good insight into the causes and natural history of the condition, while others had not heard of glue ear before. Referencing to normal childhood behaviours, including ignoring instructions and misbehaviour, often meant that hearing impairment was not always recognised.
I mean I thought sometimes it was sort of a bit like a, you know, a normal child at that age, they don’t want to answer you, sort of thing, they just ignore you anyway.
Parent participant 13
Parents gathered information from various sources including the internet, friends and family, charitable sources, ENT departments and their GP practice. Nurses signposted parents to online information, often to the website Patient (www.patient.co.uk), which was considered a useful source. However, many parents relied solely on the information provided by their GP surgery, finding the information on the internet somewhat overwhelming.
We were given a lot of websites to look at and sometimes you can go information overload on them can’t you?
Parent participant 8
Rationalising care decisions
Routine care [or active monitoring (AM)] was seen as a passive period of ‘wait and see’ rather than taking action, and this was unacceptable to some families. There was a general preference for non-surgical management of OME, although most parents would consider surgery if that was the only option or if the glue ear was considered to be particularly severe.
The grommets seem to be quite a good idea if . . . if, obviously, then if he had real bad problems.
Parent participant 14
Medical treatments such as antibiotics and steroids were not perceived by parents to be effective for OME, although there was some confusion with the diagnosis of AOM, for which antibiotics were seen as effective and acceptable. Autoinflation was described as a natural, holistic treatment that enables parents to feel that they are taking action, rather than waiting passively, as in the case with routine care.
Some parents don’t want to stick pills into their children; they don’t want to squirt stuff into their ear, they want to say, well, what else is there?
Nurse participant 9
Primary care management of otitis media with effusion
This theme is defined as how families and nurses understand the role of primary care in the early diagnosis and management of OME.
Screening for otitis media with effusion
Being invited for screening was viewed as positive by parents, although some nurses described certain parents as ‘overly worried’ rather than having real concerns about their child’s hearing. Parents were advised one way or another if glue ear was present or absent and this helped with their future treatment or management decisions.
If it was – if it showed that they did have glue ear, possibly, the parents were quite relieved. I said, oh, you know, there could be – and they said, thank goodness, you know, there is something wrong.
Nurse participant 12
Practice nurse as case manager for otitis media with effusion
Nurses were sufficiently informed and skilled to screen children with tympanometry as part of the study, although some nurses reported anxiety with interpretation of the results. Nurses were considered by parents to be competent in screening and managing OME. They were described as accessible to families and, while knowing the whole family, could provide continuous, co-ordinated management in the wider family context. Nurses reported that it was feasible to provide screening in primary care, although workload management and financial constraints were suggested as potential barriers.
There’s always a huge time pressure and more and more and stuff is being moved from hospital into general practice; we are all up-skilling all the time, so it would be a financial consideration.
Nurse participant 17
Some nurses also reported a need for additional training in tympanometry and interpretation to provide ongoing screening at their surgery. Others reported concerns about not seeing sufficient children with glue ear to maintain their skill level.
I think if it’s just basic tympanometry I’d be happy to do it. I think – on having said that – I think if I am doing it, I would like more training just so – because – you know – it’s nice to tell people – have information and knowledge so that you know what you’re telling them.
Nurse participant 18
Referral expectations
Having their concerns listened to by GPs was very important to parents. Some parents reported that their concerns were not always recognised, and this resulted in repeat consultation and requests for onward referral.
Quite often they expected to be referred . . . and, you know, often – not often, but a few times I would get – the GP to come in just for – for reassurance, to say this is the glue ear season and even if we referred now, then maybe we would wait for a few months to see if things cleared naturally.
Nurse participant 2
Engaging with monitoring and treatment
This theme is defined as the importance of engaging parents and children in the screening process, AM and autoinflation for OME in primary care.
Interactions between nurses and families
Nurse–parent–child interactions were important for engaging families with primary care screening and compliance with the nasal balloon. Nurses reported good relationships with the children and their families. Parents often reported nurses to be more accessible than their GP colleagues, and having more time to spend with the children.
A good demonstration by the nurse, together with involvement of the parents, ensured that the children engaged with autoinflation treatment.
I demonstrated and they would then have a go and they – obviously weren’t particularly good at it so I said to the mum – oh – you have a go and if you can do it, that helps the child.
Nurse participant 12
Some children had initial problems inflating the balloon, but in most cases this was overcome quickly and almost all children mastered the technique within a few days.
A couple were just scared of the idea but once they were shown whatever – and even if they just blew it a bit, then we sort of said – oh that’s brilliant. And then, of course, the next time you saw them, they’d been blowing it up to the size of an orange.
Nurse participant 2
The ‘fun’ element of the balloon was often reported as appealing to the children. This led to the children taking ownership of the treatment:
Well, the girls thought that was great fun, anything to do with balloons isn’t it? They think it’s great and the gross factor of blowing it up with your nose is a real hit with the little ones. They love it.
Parent participant 5
Compliance with autoinflation
Overall, compliance was good during the first month of treatment. Making the balloon part of the daily routine made it easier for families to adhere to the treatment regimen.
in the morning whatever we were doing, and then at bedtime, so it was just like cleaning your teeth, just brought it in as an extra thing to do as part of the routine.
Parent participant 6
Positive feedback with reward sticker charts and the ‘fun’ element of the nasal balloon helped towards adherence over a longer period.
I think the sticker chart – I mean that definitely – having their reward book and different bits and pieces, I think that was – yes – that was a bit of an incentive.
Nurse participant 2
By contrast, some parents reported the novelty wearing off and others became frustrated with their children for not continuing. Unlike a medication that needs to be swallowed, autoinflation requires the child’s active participation and this could become a battleground for parents.
So we staggered along for a few weeks with her not really trying to do it and, yes, it was just becoming such a pain, really. It was so painful to try and get her to do it and my husband was very supportive and we were both trying to encourage her to do it and I tried everything.
Parent participant 4
Discussion
This nested qualitative study of primary care monitoring and autoinflation in children with OME highlights the potential for an improved and more proactive role for general practice in the earlier diagnosis and treatment of this common childhood condition.
Primary care management of glue ear
The first point of contact for parents who have concerns about their child’s hearing is usually primary care; they often present with a range of concerns, background knowledge and expectations for the diagnosis and treatment of their child.
This study found that parents wanted to take action once they had received a diagnosis, and that waiting was not always acceptable to them. For them, action involved taking medications, surgery and autoinflation. In a study of AOM, parents with more knowledge and who felt included in medical decisions were more likely to accept watchful waiting, rather than immediate antibiotic treatment. 97 OME naturally shows some improvement in ≈50% of cases by 3 months, rising to 75% at 6 months depending on the health-care system and on tympanometric criteria used to define improvement,30 so there is a valid case for waiting for natural resolution of OME to occur.
Access to good-quality information about the natural history, causes and risk factors, treatments and preventative measures may help parents to rationalise and make informed choices concerning the management of their child. Written information has been found to increase the trust in verbal medical advice and reduce the need to obtain additional information elsewhere. 98 Ensuring that information fulfils the needs of parents with children with OME may be of particular importance considering the evidence of a link between parent views and treatment-seeking behaviours. 99
Nurses were competent and skilled in managing children with glue ear, providing information, diagnosis with otoscopy and tympanometry, monitoring during the initial 3-month period and managing their treatment with the nasal balloon. It has been argued that nurses do not have the skills or sufficient training to conduct tympanometry. 100 Most of this research has been conducted in secondary care, where tympanometry diagnosis has been compared directly with the best relative standard of myringotomy (‘relative’ because of substantial dry tap rates at myringotomy), giving a direct measure of specificity and sensitivity for detecting middle ear effusions. 101 In the secondary care environment, multiple rigorous measures of bilateral OME causing persistent hearing loss are required as part of the AM process prior to undertaking grommet surgery, a requirement of the NICE guidelines. 1 However, in primary care, it is more useful to improve the early diagnosis of glue ear, to be able to start treatment as problems arise rather than allowing the condition to develop to the point of needing an operation. The latter may be considered a more substantial intervention from the child and family perspective.
Interactions
Building alliances in health care is an important part of helping towards a positive outcome and an important element of self-care. 102 Therapeutic alliances, most commonly reported in psychotherapy, may be a useful way of looking at the relationships between the nurse, parent and child in the case of primary care monitoring of OME. The model of therapeutic alliance was developed from the early work of Bordin,103 who described the relationship between the practitioner and patient in terms of personal and collaborative relationships, and the effect they have on patient outcomes. 104 Our study has shown that the personal relationships between nurses and families can affect parental confidence in the information and diagnosis they receive and, consequently, the care that their child is receiving in primary care. The nasal balloon demonstration draws on the task element of this collaborative relationship and the combination of the nurse demonstration, parental involvement and engaging the child in the process has been shown to be important in children mastering the technique of autoinflation. The triadic relationship between the nurse, parent and child has been explored in asthma review consultations, which found that the individual dyadic relationships between nurse–parent, nurse–child and parent–child needed to be taken into account where there could be potential areas of conflict and lack of co-operation. 105 In this study, nurses reported focusing their attention on the relationship with the child, and seeing that co-operation at an early stage would be important for compliance with the procedure.
Acceptability and compliance
There have been both trial and anecdotal concerns from ENT centres that children may not be able to reliably perform autoinflation and that adherence to the treatment regimen may be a problem, especially in younger children. This study reported that the nasal balloon was perceived as an acceptable technique. School children mastered the technique relatively quickly, and adherence over the period of a month was achievable for most parents.
Acceptability to families of the nasal balloon has been reported in three previous secondary care studies, in which the technique was described as ‘acceptable’58 and ‘fun’ or ‘amusing’ for the children. 56,61 This is consistent with the findings of this study, where parents described the nasal balloon as a natural and holistic treatment, found it acceptable as a treatment and children are reported to enjoy the novelty of the technique. However, some children reported initial anxieties around the use of the balloon that were overcome with parental support and encouragement.
Previous studies have reported that young children had difficulty in mastering the technique of autoinflation, especially at the beginning. One study, which evaluated the use of Otovent after flying, found that just 53% of children aged 2–6 years could inflate the balloon. 106 However, the authors suggested that the children could have learnt the technique from their parents if they had commenced training 1–2 days before the flight. Accounts from this qualitative study suggest that most children became proficient at autoinflation after some practice. Stretching the balloon beforehand by oral inflation (by child or parent) helped with initial inflation, together with the encouragement and support of the parents.
Parents reported that the key to remembering to use the nasal balloon was to make it part of the child’s everyday routine, such as after cleaning their teeth or using their asthma inhaler. Routines and rituals are important organisers of family life. 107 Children naturally adopt routines such as eating meals, daily homework and bedtime routines. It has been theorised that adopting good routines can improve the likelihood of compliance with certain medical treatments108 and minimise the burden to families. 109 Adopting autoinflation as part of a routine may be very important for the longer-term use of the nasal balloon up to 3 months.
Strengths and limitations of the qualitative study
This research is the first to provide pragmatic, experiential data about use of autoinflation from a primary care setting, and includes both nurse and parent participant perspectives. It covers screening, AM and the use of the nasal balloon from the views of both the parents and primary care nurses. Using more than one data source to obtain different perspectives allows triangulation of the findings to check and establish study validity. 110
The study has also given insight into day-to-day, real-life experiences of children using the nasal balloon, which has not hitherto been formally captured in previous studies of autoinflation. This study information should help identify the common enablers and barriers to the wider implementation of autoinflation in a community setting.
It was not possible to recruit parents of children who withdrew or dropped out of the AIRS. Their experiences of screening, monitoring and treatment may well have differed to the study group, such that possible problems associated with AM and compliance with the autoinflation treatment may be missing. It might have also been useful to gather views and opinions from the participating GPs, especially regarding primary care-led services. Also missing were the direct voices of the children themselves. Including children in research can enhance the scope and findings of a study;111 however, in this instance the children were individually considered too young to be able to separately contribute to this study (predominantly 4–6 years).
Implications for clinical practice
The findings suggest that primary care professionals are eminently capable of engaging families early on in the process of AM with autoinflation and can provide good-quality information while drawing in parents and children in co-operative management decisions. Good demonstration/training with the autoinflation method, together with positive reinforcement by the health professional, will enhance child co-operation and improve overall adherence to the treatment schedule. Parents reported autoinflation to be acceptable to their children and compliance was improved by making the treatment part of the daily routine. However, the sample of parents had a somewhat higher than average educational level and were from areas of low social deprivation. Therefore, it would be of much interest to explore the potential barriers to autoinflation in lower socioeconomic groups where OME may have disadvantageous impacts.
Parents viewed practice nurses as accessible, local and able to provide continuity of care for OME. However, it remains uncertain as to exactly how a nurse-led service would work in the wider context of general practice, and this requires further research.
Chapter 7 Discussion
Principal findings
We report the results of the first pragmatic trial of the clinical effectiveness and cost-effectiveness of autoinflation that is generalisable to primary care, that is, to the majority of children attending practices with typical symptom clusters and impaired QoL linked to OME. It is the largest of the relatively few RCTs to date reported from either primary care or the community for any type of medical/non-surgical intervention,1,45 and the largest trial on autoinflation to date performed in any health setting. 35 There are currently no proven non-surgical interventions for glue ear, which often leads to inappropriate treatment with antibiotics and other ineffective remedies. 1 A NNT of 9 for autoinflation shows it to be a reasonably effective method for clearing middle ear effusions when using stringent tympanometric criteria of resolution. The method also significantly and importantly reduces the level of ear concerns and symptoms that include reported hearing loss, earache, difficulty concentrating and consequent impaired QoL for both child and family over a 3-month period.
We found autoinflation to be a simple, low-cost procedure that appears to be moderately easy to teach to appropriate-age selected children (attending school) in a primary care setting, with good reported compliance. Blowing up a balloon through the nose is an acceptable relatively non-invasive option with potential to add benefit to guideline recommended ‘watch and wait’ or surveillance processes, which are commonly performed in primary care and the community. It is thus an intervention with considerable potential to be used at scale in the NHS.
Research in context of other studies
The most recent Cochrane review of autoinflation,35 which highlighted the need for a large primary care trial, included three hospital studies of the same low-cost device trialled here. Adding our data to the meta-analysis more than doubles the available sample size of studies using similar outcomes with an estimated aggregate effect size (RR of improvement) of 1.61 (95% CI 1.26 to 2.06; I2 heterogeneity 0.0%).
Tympanometry findings can be misleading when comparing studies, particularly because different studies can class type C2 as resolved or improved. As we regard C2 as poorly predictive of effusion status, we have not considered such cases as sufficiently resolved and hence our resolution rates will be lower than in studies that present C2 as improved/resolved.
Effect on quality of life
Although clearance of effusions is a necessary and important physiological outcome, it is known that there is only poor correlation between tympanometry and audiometry (hearing level),2 and between audiometry and QoL. 19,21 For the child and parent, the most important issues are the expressed concerns about the consequential impacts caused by the OME. 75 Significant impacts of OME have been found in general practice settings to rival the impacts seen in UK secondary care settings. 21,30 These impacts include recurrent physical illness, hearing, speech and developmental impairments, and total effects on child and parent QoL. Taken from this perspective, the improved difference in the global OMQ-14 score of –0.42 points between arms (representing a moderate effect size) is both important and encouraging.
Feasibility and compliance
Children found autoinflation fun to do. In addition, the training method of demonstration by the nurse, then parent and then the child, was associated with good acceptability and engagement. With practice, nearly all the children mastered the technique: 89% of parents reported good compliance in their child’s use of the balloon at 1 month, and 80% at 3 months. Making the treatment part of daily routine could enhance compliance, especially over a longer period.
Adverse events and safety
Parent-reported adverse events were similar between groups. There were, however, more mild URTIs and mild to moderate otalgia, which usually settled quickly, in the treated arm. This contrasts with fewer URTIs found in two hospital studies. 55,56 A single case of mastoiditis in the treatment arm was reported to the DMEC, who conducted a full, independent review. They concluded that the case of mastoiditis could not be attributed to autoinflation and that it was safe to continue the trial (see Appendix 7).
Strengths
A key strength is that the study population is representative of typical cases of OME seen in primary care, that is, in most cases parents had recently expressed relevant concerns that suggested OME, and OME had been clinically confirmed at the point of initiating treatment. The findings are therefore likely to be generalisable to a majority of affected children. We think that the observed aggregate effects, in terms of both consistent direction and the magnitude of effect sizes, across a range of repeated tympanometric and clinical outcomes, strengthen the plausibility and reliability of our findings.
The trial methodology was rigorous in other ways; for example, a power calculation was performed and a large sample achieved within the allocated time frame, web randomisation was used, and the executor and generator of randomisation were kept entirely separate. The execution and generation of randomisation was done by a company using computer-generated randomised sequences and stratified according to an analysis plan. The trial was analysed on an ITT basis (and PP), with objective evidence of OME (both at trial entry and as an outcome), good treatment compliance ≥ 80%, and a very modest loss to follow-up of ≈10%. Patient and public involvement contributed to various aspects of the trial. Feedback about the practicalities and training of the treatment method from parents whose children participated in the pilot was incorporated into the main study. A lay member of the Trial Steering Committee, also a parent of children with glue ear, contributed to study recruitment strategies and had input into the qualitative evaluation.
All practice nurses had training in trial protocol and methods used, for example otoscopy and tympanometry. Two authors and one external audiologist, who were all blind to treatment allocation, independently reviewed the outcome assessments at 1 and 3 months. Nurses showed a substantial level of agreement with expert interpretation of tympanometry as a relative standard (κ > 0.7), which improved as the study progressed. Completion rates of trial forms (case report forms) were very high and multiple imputation methods were used for all missing data both at 1 and 3 months. A CONSORT diagram is provided (see Figure 5), with separate baseline tables for both the screened and entered populations.
Limitations
The main limitation of the study was that using a nasal balloon is a method that cannot be blinded and Hawthorne effects are possible. 112 However, the lack of blinding is unlikely to affect the primary tympanometric outcomes and, even if symptom and mapped QoL scores are affected by performance bias, the effects observed are still likely to be commensurate with those found in routine clinical practice. The HE analysis suggests that trial behaviour was very similar in both arms, and the PP analysis was not different from the ITT analysis in terms of effects. The study population included children who were deemed likely to be able to reliably perform autoinflation (≥ 4.5 years), whereas the usual presentation pattern to primary care and ENT clinics in the UK is from approximately 3.5 to 8 years. This does not mean that younger children cannot perform the technique – some children as young as 3 years have been able to use the device in secondary care,28,29 and even younger than 3 years when a novel counter-pressure method is used. 65
Clinical implications
At the time of writing there are no known effective non-surgical treatments that have been proven to be satisfactory to apply in primary care and the community for children with the tenacious cluster of symptoms and impacts that characterise OME. 2,7,12,23 The study findings of a treatment, a device, that actually works for OME in primary care has potential when judiciously applied to fill the ‘management gap’ in current practice that exists between either doing nothing effective or referring the worst cases for surgery (often after incurring long delays). Temporising strategies, an attempt to let nature take its course, are often seen as unreasonable delay(s) by parents. Some strategies, such as prescribing antibiotics, are a misplaced attempt to fill the therapeutic vacuum, because they are inappropriate, ineffective and harmful, and contribute a major threat to public health in the form of antibiotic resistance.
Although finding the method effective over 3 months, because fluid in the ear does not fully resolve in about half of all children who use autoinflation in the short term, and with the known tendency to recur, continued vigilance with consideration of surgical referral must remain a central consideration in evidence-based management of children presenting with OME.
In this study we have used relatively rigorous measures of diagnosis, impact and outcome, but there is no reason to suppose that GPs’ and practices’ own routine criteria for identifying cases of OME are generally inadequate or insufficient, given the time and demand pressures on the NHS. Like many areas of current health care, however, there is always scope for better definition of the problem needing fixing. The characteristic symptoms and concerns of OME are presented in Box 1 and the OMQ-14 that was used in the study (see Appendix 5). They are distinct from acutely presenting otitis media with fever and pain, and more problematic to child and family than those of simple self-limiting viral illnesses. The more frequently the child attends with relevant ear symptoms and concerns, the clearer that the case becomes for treatment. A secondary analysis found three symptoms/concerns: any ear concern in the previous 3 months; mis-hearing what is said; and needs the television turning up. These three symptoms together produce 70% of the area under the ROC curve, which is a reasonably good indication for management purposes where tympanometry is not available. However, it must be accepted that tympanometry is only a relative and not a gold standard. Treatment criteria clearly depend on the case being considered, and secondary care criteria for intervention, somewhat embedded in current clinical culture (perhaps because grommets are the only known effective treatment), cannot be used as a basis for any treatment applicable to an earlier case stage in a primary care context, where the therapeutic aim is timely and proportionate remediation.
Although autoinflation is generally acceptable with brief instruction, it may not be suitable for all, particularly children aged under 4 years, and does require regular ongoing commitment to treatment. The method is deemed to have scope to be used in the majority of routine symptomatic cases, and thus is capable of improving satisfaction with management and outcomes in primary care. It should be more widely used.
Chapter 8 Conclusion
Implications for practice
This clinical trial is the first of its type in a primary care setting. It is one of the largest trials reported for interventions for OME from any setting. Considering the current evidence base for non-surgical interventions systematically, one is led to the conclusion that there is no prior justification for any cost-effective treatment for OME, at the point in the NHS where the majority of children are initially identified and treated. Furthermore, medical treatments that are presently applied as part of temporising management are not only ineffective but also harmful. The latter is particularly the case for antibiotics.
Our findings reveal that autoinflation using the balloon method is feasible in primary care. A NNT of 9 at both 1 and 3 months was found for improved clearance of middle ear effusions, beyond what can be expected from natural resolution effects alone (standard care). The symptom diaries (hearing loss, earache, etc.) showed significant and encouraging improvements by 3 months, a recommended waiting time, as did the mapped ear-related QoL measure (PROM). The effect size for effusion clearance is comparable to that achieved when smaller secondary care studies are combined in Cochrane and makes the case for wide use of autoinflation more robust.
Our sample characteristics are considered generalisable to primary care populations, of which they are reasonably representative in terms of both the baseline severity of the effusions and the prior number of typical symptoms and concerns expressed. There are relatively few exclusion criteria and the sample, although heterogeneous, has demonstrated important clinical effects. Baseline severity markers and method of recruitment were shown in the statistical models to have no effect on the primary treatment outcomes, which is an important clinical finding. The main limitation in terms of generalisability is the age of the children recruited in to the study because of age-related limitations with the method. Surgical studies have demonstrated effectiveness when recruiting children as young as 3 years,55,56 but compliance is likely to be poorer in such age groups.
In terms of capacity to change clinical practice, we have demonstrated that this method can augment the current natural resolution process in a beneficial, inexpensive, safe and timely fashion for the majority of children with symptomatic OME. It should therefore be an attractive initial option when one considers the unsatisfactory nature of present ‘temporising’ or available management options, which include usually either offering advice only or giving a ‘known’ ineffective and harmful treatment such as antibiotics or a decongestant, or referring the child prematurely for further evaluation for surgery.
Clinically, assuming the status quo of children identified in primary care across the UK with a working diagnosis of OME, the majority will be eligible for empirical management – the modus vivendi of primary care practitioners. Thus, although there are inevitable limits to what one can conclude from a large open pragmatic trial, the sum of the new evidence appears sufficiently strong to justify far wider use of this intervention than is currently the case.
Recommendations for future research
-
The findings from this study should be reviewed in wider terms, for example relevance to current practice by multiprofessional groups to aid positioning and prudent application. There is potential to be considered in updates to the Cochrane meta-analysis of autoinflation and review of future NICE guidelines for OME.
-
Further pragmatic research should be undertaken to evaluate the relative benefits of:
-
usual care in practices plus or minus autoinflation using HE and standardised outcomes
-
improved diagnostic care for recurrent otitis media/OME using trial-developed symptom predictors of effusions/impact measures or by selective use of tympanometry in primary care practices
-
shared care with other agencies.
Such research work should aim to offer more effective integrated approaches for children recognised with OME symptoms and concerns in primary care.
-
-
To improve the management of OME in primary care by the development of tools that encourages and promotes self-efficacy. Development of a web-based support intervention could provide evidence-based patient information, practical support for use of nasal balloon autoinflation to enhance uptake and compliance.
-
To evaluate selective screening and monitoring of at-risk children in primary care, using age, attendance records, near patient hearing tests and/or short-form QoL questionnaires. This has potential to address Tudor Hart’s inverse care law,33 but needs to be shown to be cost-effective.
-
Treatment failures are an important group for further research in primary and secondary care settings. Randomised trials would be helpful to determine the probable effectiveness of autoinflation as a re-treatment, treatment before surgery in hospital and also effectiveness in prevention of second operations for grommets.
-
The youngest children are an important group for further research. To evaluate different autoinflation methods that evaluate comparative effectiveness and feasibility in relation to the age-related competence of the child. New methods using counter pressure may be promising for the very youngest children.
-
There is potential scope for basic science technical development of the study device in relation to drug delivery to the Eustachian tubes.
-
To evaluate the clinical effectiveness of oral steroids, which may be considered appropriate for young age groups or for treatment failures (of autoinflation), or for children seen in hospital prior to surgery. [Current research in progress includes a randomised trial funded by the NIHR HTA programme that addresses relevant issues (HTA reference number 11/01/26).]
-
To update the epidemiology of otitis media with use of databases, for example Clinical Practice Research Database, with better differentiation of OME from recurrent AOM.
Acknowledgements
The research team would like to thank the following:
The HTA programme/NIHR funders: Oxford Primary Care CTU, especially Sarah Benge, for protocol development, logistical help and pilot work set-up; Michelle Caneppele for help with the pilot study; Jing Jin for developing the statistical analysis plan; David Turner, for assistance with the HE protocol; Sharon Charnock, of Starkey Laboratories Ltd, for audiological review and assistance with nurse training; Mark Haggard and Helen Spencer for use of the OMQ-14 with advice; and Beth Stuart for supplementary statistical analysis.
We also thank the South East and South West PCRNs and all participating practices and Kestrel Medical Ltd for the trial supply of Otovent and providing the photograph.
Finally, and most importantly, we would like to thank all the children and families who took part in the study.
Contributions of authors
Ian Williamson (Associate Professor, University of Southampton and chief investigator) conceived and designed the study, led protocol development and the funding application. Also contributed to the analysis and interpretation, and led the drafting of all chapters of the report.
Jane Vennik (Trial Manager, University of Southampton) provided day-to-day management, co-ordinated recruitment and contributed to the data collection, analysis and interpretation. Also led the design, data collection, analysis and reporting of the qualitative study. Contributed to drafting of the all chapters of the report.
Anthony Harnden (Professor of General Practice, University of Oxford) contributed to protocol development and funding application, and contributed to the drafting of all chapters.
Merryn Voysey (Senior Statistician, Oxford PCVC-CTU) led the statistical analysis of the study and contributed to drafting Chapters 3 and 4.
Rafael Perera (Associate Professor, University of Oxford) contributed to the protocol statistics section and advised on drafts.
Maria Breen, Brendan Bradley and Sadie Kelly (Data Officer, Senior Data Specialist and Head of Trials, Oxford PCVC-CTU, respectively) provided CTU oversight for both the pilot and the main study, and contributed to the main trial design. Also supervised and managed the randomisation process, data collection, cleaning and validation, and commented on drafts of all chapters of the report.
Guiqing Yao (Associate Professor of Health Economists, University of Southampton) co-led the HE analysis and drafting of Chapter 5.
James Raftery (Professor of Health Economists, University of Southampton) co-led the HE analysis and drafting of Chapter 5.
David Mant (Emeritus Professor of General Practice, University of Oxford) contributed to protocol development and funding application, contributed to the drafting of all chapters.
Paul Little (Professor of Primary Care Research, University of Southampton) contributed to protocol development and funding application, contributed to the drafting of all chapters.
Contributors to protocol and pilot study
Dr Sarah Benge (Trials Unit Manager, Oxford PCVC-CTU) contributed to protocol development for the funding application.
Ms Michelle Caneppele (Trial Co-ordinator, PCVC-CTU) provided day-to-day management of the pilot study, including interpretation and reporting.
David Turner (Health Economist, University of Southampton) developed the HE plan.
Jing Jin (Statistician, Oxford PCVC-CTU) contributed to trial design and developed the statistical analysis plan.
Members of the Trial Steering Committee
Mr James Ramsden (chairperson), Professor Chris Frost, Dr Anthony Harnden, Dr Tim Whelan, Dr Ian Williamson (chief investigator) and Dr Rachel MacDonald (lay member).
Members of the Data Monitoring and Ethics Committee
Professor Kerry Hood (chairperson) and Mr Emeka Okpala.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- National Institute for Health and Care Excellence . Surgical Management of Otitis Media With Effusion in Children (OME). CG60 n.d. www.nice.org.uk/guidance/cg60 (accessed 4 March 2014).
- Stool SE, Berman S, Carney CJ, Cooley JR, Culpepper L, Eavey RD, et al. Otitis Media with Effusion in Children. Rockville, MD: US Department of Health and Human Services; 1994.
- Zielhuis GA, Rach GH, van den Broek P. Screening for otitis media with effusion in preschool children. Lancet 1989;1:311-14. http://dx.doi.org/10.1016/S0140-6736(89)91317-2.
- Fry J, Sandler G. Common Diseases: Their Nature, Prevalence and Care. Dordrecht: Kluwer Academic Publishers; 1993.
- Hogan SC, Stratford KJ, Moore DR. Duration and recurrence of otitis media with effusion in children from birth to 3 years: prospective study using monthly otoscopy and tympanometry. BMJ 1997;314:350-3. http://dx.doi.org/10.1136/bmj.314.7077.350.
- Williamson IG, Dunleavey J, Bain J, Robinson D. The natural history of otitis media with effusion – a three-year study of the incidence and prevalence of abnormal tympanograms in four South West Hampshire infant and first schools. J Laryngol Otol 1994;108:930-4. http://dx.doi.org/10.1017/S0022215100128567.
- Higson J, Haggard M. Parent versus professional views of the developmental impact of a multi-faceted condition at school age: otitis media with effusion (‘glue ear’). Br J Educ Psychol 2005;75:623-43. http://dx.doi.org/10.1348/000709905X41906.
- Maw AR, Bax M. Glue Ear in Childhood. Clinics in Developmental Medicine No. 135. London: Mac Keith Press; 1995.
- Browning GG. Two-year outcome of ventilation tubes in a randomized controlled trial of persistent childhood otitis media with effusion. Clin Otolaryngol Allied Sci 2001;26:342-4. http://dx.doi.org/10.1046/j.1365-2273.2001.00479-13.x.
- Browning GG. Watchful waiting in childhood otitis media with effusion. Clin Otolaryngol Allied Sci 2001;26:263-4. http://dx.doi.org/10.1046/j.1365-2273.2001.00471.x.
- Casselbrant ML, Brostoff LB, Canekin EI, Flahert MR, Doyle WJ, Bluestone CD, et al. Otitis media with effusion in preschool children. Laryngoscope 1985;95:428-36. http://dx.doi.org/10.1288/00005537-198504000-00011.
- Williamson I, Benge S, Mullee M, Little P. Consultations for middle ear disease, antibiotic prescribing and risk factors for reattendance: a case-linked cohort study. Br J Gen Pract 2006;56:170-5.
- Cullen KA, Hall MJ, Golosinskiy A. Ambulatory Surgery in the United States, 2006. Hyattsville, MD: National Center for Health Statistics; 2009.
- Rosenfeld RM, Schwartz SR, Pynnonen MA, Tunkel DE, Hussey HM, Fichera JS, et al. Clinical practice guideline: tympanostomy tubes in children. Otolaryngol Head Neck Surg 2013;149:S1-35. http://dx.doi.org/10.1177/0194599813487302.
- van Dongen TM, van der Heijden GJ, Freling HG, Venekamp RP, Schilder AG. Parent-reported otorrhea in children with tympanostomy tubes: incidence and predictors. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0069062.
- Grommet Insertion and Adenoidectomy for Otitis Media with Effusion. Health Technology Assessment of Scheduled Surgical Procedures. Dublin: Health Information and Quality Authority; 2013.
- American Academy of Family Physicians, American Academy of Otolaryngology–Head Neck Surgery, American Academy of Pediatrics Subcommittee on Otitis Media with Effusion . Otitis media with effusion. Pediatrics 2004;113:1412-29. http://dx.doi.org/10.1542/peds.113.5.1412.
- Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al. Topical intranasal corticosteroids in 4–11 year old children with persistent bilateral otitis media with effusion in primary care: double blind randomised placebo controlled trial. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4984.
- Haggard MP, Smith SC, Rosenfeld RBC. Evidence Based Otitis Media. Hamilton, ON: BC Decker Inc.; 1999.
- Dakin H, Petrou S, Haggard M, Benge S, Williamson I. Mapping analyses to estimate health utilities based on responses to the OM8-30 Otitis Media Questionnaire. Qual Life Res 2010;19:65-80. http://dx.doi.org/10.1007/s11136-009-9558-z.
- Marchisio P, Grasso D. Eurotitis-2 study group . Using Differences and Similarities Between Centres for Power and Generality. Special Eurotitis-2 Session n.d.
- Maw AR. Using tympanometry to detect glue ear in general practice. BMJ 1992;304:67-8. http://dx.doi.org/10.1136/bmj.304.6819.67.
- Diagnosis and Management of Childhood Otitis Media in Primary Care. Edinburgh: Scottish Intercollegiate Guidelines Network; 2003.
- Green LA, Culpepper L, de Melker RA, Froom J, van Balen F, Grob P, et al. Tympanometry interpretation by primary care physicians. A report from the International Primary Care Network (IPCN) and the Ambulatory Sentinel Practice Network (ASPN). J Fam Pract 2000;49:932-6.
- Bennett K, Higson J, Haggard M. Do GPs have the techniques for ‘watchful waiting’ in glue-ear?. Br J Gen Pract 1998;48:1079-80.
- Williamson I, Little P. Otitis media with effusion: the long and winding road?. Arch Dis Child 2008;93:268-9. http://dx.doi.org/10.1136/adc.2006.112383.
- Williamson IG, Sheridan C, Galker E, Lous J. A video-based performance in noise test for measuring audio-visual disability in young school children: test development, with validation by trained teachers, parents and audiometry as relative standards for disability. Int J Pediatr Otorhinolaryngol 1999;49:127-33. http://dx.doi.org/10.1016/S0165-5876(99)00110-X.
- Black N. Glue ear: the new dyslexia?. BMJ 1985;290:1963-5. http://dx.doi.org/10.1136/bmj.290.6486.1963.
- Rosenfeld RM, Goldsmith AJ, Tetlus L, Balzano A. Quality of life for children with otitis media. Arch Otolaryngol Head Neck Surg 1997;123:1049-54. http://dx.doi.org/10.1001/archotol.1997.01900100019002.
- Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al. A double-blind randomised placebo-controlled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13370.
- Browning G, Browning G. Scott-Brown’s Otorhinolaryngology, Head and Neck Surgery. London: Hodder Arnold; 2008.
- Williamson IG, Dunleavey J, Robinson D. Risk factors in otitis media with effusion. A 1 year case control study in 5–7 year old children. Fam Pract 1994;11:271-4. http://dx.doi.org/10.1093/fampra/11.3.271.
- Tudor Hart J. Inverse care law. Lancet 1971;1:405-12. http://dx.doi.org/10.1016/S0140-6736(71)92410-X.
- Williamson I. Otitis Media With Effusion in Children 2011. www.clinicalevidence.com/x/systematic-review/0502/overview.html (accessed 4 March 2014).
- Perera R, Glasziou PP, Heneghan CJ, McLellan J, Williamson I. Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev 2013;5. http://dx.doi.org/10.1002/14651858.cd006285.pub2.
- van Balen FA, de Melker RA, Touw-Otten FW. Double-blind randomised trial of co-amoxiclav versus placebo for persistent otitis media with effusion in general practice. Lancet 1996;348:713-16. http://dx.doi.org/10.1016/S0140-6736(96)02511-1.
- Browning GG, Rovers MM, Williamson I, Lous J, Burton MJ. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2010;10. http://dx.doi.org/10.1002/14651858.cd001801.pub3.
- van Zon A, van der Heijden GJ, van Dongen TM, Burton MJ, Schilder AG. Antibiotics for otitis media with effusion in children. Cochrane Database Syst Rev 2012;9. http://dx.doi.org/10.1002/14651858.cd009163.pub2.
- Choung YH, Shin YR, Choi SJ, Park K, Park HY, Lee JB. Management for the children with otitis media with effusion in the tertiary hospital. Clin Exp Otorhinolaryngol 2008;1:201-5.
- Leach AJ, Morris PS, Mathews JD. Chronic Otitis Media Intervention Trial - One (COMIT1) group . Compared to placebo, long-term antibiotics resolve otitis media with effusion (OME) and prevent acute otitis media with perforation (AOMwiP) in a high-risk population: a randomized controlled trial. BMC Pediatrics 2008;8.
- Otten FW, Grote JJ. Otitis media with effusion and chronic upper respiratory tract infection in children: a randomized, placebo-controlled clinical study. Laryngoscope 1990;100:627-33.
- Ozmen OA, Genc A, Ozmen S, Kayikci EMK, Sarac S, Sennaroglu L. Successive medical treatment versus watchful waiting in chronic otitis media with effusion. J Int Adv Otol 2010;6:11-7.
- Principi N, Marchisio P, Massironi E, Grasso RM, Filiberti G. Prophylaxis of recurrent acute otitis media and middle and middle-ear effusion. Comparison of amoxicilin with sulfamethoxazole and trimethoprim. Am J Dis Child 1989;143:1414-18.
- Thomsen J, Sederberg Olsen J, Balle V, Vejlsgaard R, Stangerup SE, Bondesson G. Antibiotic treatment of children with secretory otitis media. A randomized, double-blind, placebo-controlled study. Arch Otolaryngol Head Neck Surg 1989;115:447-51.
- Goossens H, Ferech M, Vander SR, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005;365:579-87. http://dx.doi.org/10.1016/S0140-6736(05)70799-6.
- Great Britain . Resistance to Antibiotics and Other Antimicrobial Agents. House of Lords Select Committee on Science and Technology Seventh Report 1998.
- Davies SC, Grant J, Catchpole M. The Drugs Don’t Work: Global Threat. London: Penguin; 2013.
- World Health Organization . Antimicrobial Resistance: Global Report on Surveillance 2014. www.who.int/drugresistance/documents/surveillancereport/en (accessed 6 May 2014).
- The Path of Least Resistance. London: HMSO; 1998.
- Fauci AS, Marston HD. The perpetual challenge of antimicrobial resistance. JAMA 2014;311:1853-4. http://dx.doi.org/10.1001/jama.2014.2465.
- Bradley-Stevenson C, O’Neill P, Roberts T. Otitis media in children (acute). BMJ Clin Evid 2011;6:124-6.
- Cantekin EI, McGuire TW. Antibiotics for otitis media with effusion: re-analysis of meta-analysis. Otorhinolaryngol Nova 1998;8:214-22. http://dx.doi.org/10.1159/000027879.
- Simpson SA, Lewis R, van der Voort J, Butler CC. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2011;5. http://dx.doi.org/10.1002/14651858.cd001935.pub3.
- Reidpath DD, Glasziou PP, Del Mar C. Systematic review of autoinflation for treatment of glue-ear in children. BMJ 1999;318:1177-8. http://dx.doi.org/10.1136/bmj.318.7192.1177.
- Blanshard J, Maw A, Bawden R. Conservative treatment of otitis media with effusion in children by autoinflation of the middle ear. Clin Otolaryngol 1993;18:188-92. http://dx.doi.org/10.1111/j.1365-2273.1993.tb00827.x.
- Stangerup SE, Sederberg-Olesen J, Balle V. Autoinflation as a treatment of secretory otitis media. A randomized controlled study. Arch Otolaryngol Head Neck Surg 1992;118:149-52. http://dx.doi.org/10.1001/archotol.1992.01880020041013.
- Arick DS, Silman S. Nonsurgical home treatment of middle ear effusion and associated hearing loss in children. Part I: clinical trial. Ear Nose Throat J 2005;84:567-76.
- Brooker D, McNiece A. Autoinflation in the treatment of glue ear in children. Clin Otolaryngol Allied Sci 1992;17:289-90. http://dx.doi.org/10.1111/j.1365-2273.1992.tb00997.x.
- Fraser J, Mehta M, Fraser P. The medical treatment of secretory otitis media. A clinical trial of three commonly used regimes. J Larngol Otol 1977;91:757-65. http://dx.doi.org/10.1017/S0022215100084334.
- Lesinskas E. Factors affecting the results of non surgical treatment of secretory otitis media in adults. Auris Nasus Larynx 2003;30:7-14. http://dx.doi.org/10.1016/S0385-8146(02)00100-1.
- Ercan I, Cakir B, Kayaoglu S, Turgut S, Cerrahisi K. Long term effect of autoinflation in the treatment of otitis media with effusion. KBB Forum 2005;4:166-70.
- De Nobili and A Bellomo . Comparative evaluation of efficacy of crenotherapeutic Politzer with sulphurous water versus crenotherapeutic Politzer and autoinsufflation (Otovent) in patients with tubaric dysfunction and secretory otitis media. Med Clin Termale 2008;20:30-4.
- Bidarian-Moniri A, Ramos MJ, Ejnell H. Autoinflation for treatment of persistent otitis media with effusion in children: a cross-over study with 12 month follow-up. Int J Pediatr Otorhinolaryngol 2014;78:1298-305. http://dx.doi.org/10.1016/j.ijporl.2014.05.015.
- Griffin G, Flynn CA. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev 2011;9. http://dx.doi.org/10.1002/14651858.cd003423.pub3.
- Hospital Episode Statistics. Grommets. London: Department of Health; 2004.
- Kay DJ, Nelson M, Rosenfeld RM. Mata-analysis of tympanostomy tube sequelae. Otolaryngol Head Neck Surg 2001;124:374-80. http://dx.doi.org/10.1067/mhn.2001.113941.
- Haggard M. Commentary: plausible candidates for treatment of glue ear: is one issue really three?. BMJ 1999;318.
- Thabane L, Ma J, Chu, R, Cheng J, Ismaila A, Lorena P, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-1.
- Jerger J. Clinical experience with impedance audiometry. Arch Otolaryngol 1970;92:311-24. http://dx.doi.org/10.1001/archotol.1970.04310040005002.
- Fiellau-Nikolajsen M. Epidemiology of secretory otitis media. A descriptive cohort study. Ann Otol Rhinol Laryngol 1983;92:172-7. http://dx.doi.org/10.1177/000348948309200215.
- Cantekin EI, Bluestone CD, Fria TJ, Stool SE, Beery QC, Sabo DL. Identification of otitis media with effusion in children. Ann Otol Rhinol Laryngol Suppl 1980;89:190-5.
- Deafness Research UK . Glue-Ear: Signs and Tests n.d. www.nhs.uk/Conditions/Glue-ear/Pages/Symptoms.aspx (accessed 12 February 2013).
- Stangerup SE, Klokker M, Vesterhauge S, Jayaraj S, Rea P, Harcourt J. Point prevalence of barotitis and its prevention and treatment with nasal balloon inflation: a prospective controlled study. Otol Neurotol 2004;25:89-94. http://dx.doi.org/10.1097/00129492-200403000-00001.
- Doyle WJ, Alper CM. A model to explain the rapid pressure decrease after air-inflation of diseased middle ears. Laryngoscope 1999;109:70-8. http://dx.doi.org/10.1097/00005537-199901000-00015.
- Umansky AM, Jeffe DB, Lieu JE. The HEAR-QL: quality of life questionnaire for children with hearing loss. J Am Acad Audiol 2011;10:644-53. http://dx.doi.org/10.3766/jaaa.22.10.3.
- Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, DePauw S, et al. Multi-attribute and single attribute utility functions for the Health Utilities Index Mark 3 system. Med Care 2002;40:113-28. http://dx.doi.org/10.1097/00005650-200202000-00006.
- Furlong WJ, Feeny D, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health related quality of life in clinical studies. Ann Med 2001;33:375-84. http://dx.doi.org/10.3109/07853890109002092.
- Timmerman AA, Angelique A, Meesters CMG, Anteunis, LJC, Chenault MN, Haggard MP. Psychometric evaluation of the OM8–30 questionnaire in Dutch children with otitis media. Eur Arch Otorhinolaryngol 2008;265:1047-56. http://dx.doi.org/10.1007/s00405-008-0591-2.
- Williamson I, Sheridan C. The development of a test of speech reception disability for use in 5- to 8-year-old children with otitis media with effusion. Eur J Disord Commun 1994;29:27-3. http://dx.doi.org/10.3109/13682829409041479.
- Robb PJ, Williamson I. Otitis media with effusion in children: current management. Paediatr Child Health 2011;22:9-12. http://dx.doi.org/10.1016/j.paed.2011.03.002.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. http://dx.doi.org/10.2307/2529310.
- Nelder J, Wedderburn R. Generalized linear models. J Royal Stat Soc 1972;135:370-84. http://dx.doi.org/10.2307/2344614.
- Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;154:702-6. http://dx.doi.org/10.1093/aje/kwh090.
- Royston P. Multiple imputation of missing values. Stata J 2004;4:227-41.
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13-22. http://dx.doi.org/10.1093/biomet/73.1.13.
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121-30. http://dx.doi.org/10.2307/2531248.
- Hosmer DW, Lemeshow S. Applied Logistic Regression. Hoboken, NJ: John Wiley and Sons Inc.; 2000.
- Cox CL, Lensing S. 2011 Census. Table KS501UK Qualifications and Students, Local Authorities in the United Kingdom n.d. www.ons.gov.uk (accessed 12 January 2014).
- Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI®) system for assessing health-related quality of life in clinical studies. Ann Med 2001;33:375-84. http://dx.doi.org/10.3109/07853890109002092.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Curtis L. Unit Costs of Health Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Primary Care Foundation 2013. www.primarycarefoundation.co.uk/ (accessed 30 July 2013).
- NHS Reference Costs 2013. London: Department of Health; 2013.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 2009.
- Patton M. Qualitative Evaluation and Research Methods. Beverly Hills, CA: Sage; 1990.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Finkelstein JA, Stille CJ, Rifas-Shiman SL, Goldmann D. Watchful waiting for acute otitis media: are parents and physicians ready?. Pediatrics 2005;115:1466-73. http://dx.doi.org/10.1542/peds.2004-1473.
- Trevena LJ, Davey HM, Barratt A, Butow P, Caldwell P. A systematic review on communicating with patients about evidence. J Eval Clin Pract 2006;12:13-2. http://dx.doi.org/10.1111/j.1365-2753.2005.00596.x.
- Black N. The ‘health culture’ of families as an influence on the use of surgery for glue ear: a case–control study. Int J Epidemiol 1985;14:594-9. http://dx.doi.org/10.1093/ije/14.4.594.
- Blomgren K, Haapkyla J, Pitkaranta A. Tympanometry by nurses – can allocation of tasks be optimised?. Int J Pediatr Otorhinolaryngol 2007;71:7-10. http://dx.doi.org/10.1016/j.ijporl.2006.08.010.
- Fields MJ, Allison RS, Corwin P, White PS, Doherty J. Microtympanometry, microscopy and tympanometry in evaluating middle ear effusion prior to myringotomy. N Z Med J 1993;106:386-7.
- Heritage J, Maynard DW. Communication in Medical Care: Interaction between Primary Care Physicians and Patients. New York, NY: Cambridge University Press; 2006.
- Bordin ES. The generalizablity of the psychoanalytic concept of the working alliance. Psychother Theor Res Pract 1979;16:252-60. http://dx.doi.org/10.1037/h0085885.
- Hougaard E. The therapeutic alliance – a conceptual analysis. Scand J Psychol 1994;35:67-85. http://dx.doi.org/10.1111/j.1467-9450.1994.tb00934.x.
- Callery P, Milnes L. Communication between nurses, children and their parents in asthma review consultations. J Clin Nurs 2012;21:1641-50. http://dx.doi.org/10.1111/j.1365-2702.2011.03943.x.
- Stangerup SE, Tjernstrom O, Harcourt J, Klokker M, Stokholm J. Barotitis in children after aviation; prevalence and treatment with Otovent. J Laryngol Otol 1996;110:625-8. http://dx.doi.org/10.1017/S0022215100134450.
- Bossard J, Boll E. Ritual in Family Living. Pennsylvania, PA: University of Pennsylvania Press; 1950.
- Fiese BH, Tomcho TJ, Douglas M, Josephs K, Poltrock S, Baker T. A review of 50 years of research on naturally occurring family routines and rituals: cause for celebration?. J Fam Psychol 2002;16:381-90. http://dx.doi.org/10.1037/0893-3200.16.4.381.
- Fiese BH, Wamboldt FS, Anbar RD. Family asthma management routines: connections to medical adherence and quality of life. J Pediatr 2005;146:171-6. http://dx.doi.org/10.1016/j.jpeds.2004.08.083.
- Silverman D. Doing Qualitative Research: A Practical Handbook. London: Sage; 2000.
- Coyne I. Accessing children as research participants: examining the role of gatekeepers. Child Care Health Dev 2010;36:452-4. http://dx.doi.org/10.1111/j.1365-2214.2009.01012.x.
- Mangione-Smith R, Elliott MN, McDonald L, McGlynn EA. An observational study of antibiotic prescribing behaviour and the Hawthorne effect. Health Serv Res 2002;37:1603-23. http://dx.doi.org/10.1111/1475-6773.10482.
Appendix 1 National Research Ethics Service approval and amendments to the study
Appendix 2 Recruitment materials
Appendix 3 Parent and child information sheets
Appendix 4 Consent forms
Appendix 5 The 14-point questionnaire on the impact of OME (OMQ-14)
Appendix 6 Data collection forms
Appendix 7 Serious adverse event report
Appendix 8 Qualitative interview guide
List of abbreviations
- AIRS
- AutoInflation Randomised Study
- AM
- active monitoring
- AOM
- acute otitis media
- CI
- confidence interval
- CONSORT
- Consolidated Standards Of Reporting Trials
- CTU
- clinical trials unit
- DMEC
- Data Monitoring and Ethics Committee
- ENT
- ear, nose and throat
- GCP
- good clinical practice
- GNOME
- General practice Nasal steroid trial of Otitis Media with Effusion trial
- GP
- general practitioner
- HE
- health economic
- HTA
- Health Technology Assessment
- HUI
- Health Utilities Index
- HUI3
- Health Utilities Index, version 3
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NNT
- number needed to treat
- NRES
- National Research Ethics Service
- OM8-30
- 30-point questionnaire on the impact of OME
- OME
- otitis media with effusion (or glue ear)
- OMQ-14
- 14-point questionnaire on the impact of OME
- OR
- odds ratio
- PCRN
- primary care research network
- PCT
- primary care trust
- PCVC-CTU
- Primary Care and Vaccines Collaborative Clinical Trials Unit
- PP
- per protocol
- PROM
- patient-reported outcome measure
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RN
- research nurse
- ROC
- receiver operator characteristic
- RR
- relative risk
- RTI
- respiratory tract infection
- SD
- standard deviation
- TADAST
- Two Alternative Auditory Disability and Speech Reception Test
- URTI
- upper respiratory tract infection