Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/80/01. The contractual start date was in January 2010. The draft report began editorial review in November 2014 and was accepted for publication in May 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Caroline Fairhurst, Sarah Cockayne, Sara Rodgers and David Torgerson all report other grants from the National Institute for Health Research Health Technology Assessment programme.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Clark et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Heart failure
Heart failure is a clinical syndrome that arises when the heart fails to pump in a manner adequate to meet the body’s needs. It is increasingly common and affects between 1% and 2% of the UK population. Its incidence and prevalence rise markedly with age. 1 The most common cause of heart failure is myocardial infarction and, as more people survive acute myocardial infarction with modern therapy, so the population of patients with damaged heart muscles grows. 2
Heart failure is the single most common cause for admission to hospital in England and Wales and, following admission to hospital, there is a 25% chance of readmission or death within 12 weeks. By 1 month after an index admission, 15% of patients have died, either as an inpatient or during the days following discharge. 3 The prognosis of heart failure is bleak if it is not well treated. However, one of the greatest success stories of modern medicine is the dramatic improvement in prognosis for patients with chronic heart failure (CHF). Good medical management largely consists of medicines designed to block the adverse consequences of neuroendocrine activation such as beta-blockers, angiotensin-converting enzyme inhibitors and mineralocorticoid receptor antagonists. In selected patients, treatment with cardiac resynchronisation therapy also improves prognosis and, taken together, these treatments approximately double life expectancy. 4
Chronic heart failure has been recognised for many years as having the greatest symptomatic burden of any chronic medical condition. 5 The cardinal symptoms of heart failure are breathlessness and fatigue, particularly on exertion. Worsening breathlessness is part of the cause of most admissions to hospital with heart failure, although many patients also complain of ankle swelling due to fluid retention. Drug therapy is very successful in controlling symptoms, and can induce ‘remission’ in a number of patients; that is, their symptoms can remit almost entirely. However, the clinical course for most patients with heart failure tends to be one of gradual decline, often punctuated with episodes of severe deterioration resulting in hospitalisation.
Symptom severity is most commonly measured using the New York Heart Association (NYHA) classification of symptoms (Table 1).
| NYHA class | Symptoms |
|---|---|
| I | Breathless on severe exertion (normal) |
| II | Breathless and/or fatigue on moderate exertion |
| III | Breathless and/or fatigue on mild exertion |
| IV | Breathless and/or fatigue at rest |
Towards the end of their lives, many patients with CHF become very symptomatic, with limiting breathlessness on minimal exertion (class III) and even at rest (class IV). Once they have reached this stage, although patients need continued treatment with drugs known to improve prognosis, the emphasis of treatment becomes the relief of symptoms, that is, palliative care. However, although drug treatment with diuretics (which relieve fluid congestion), other drugs and pacing devices may relieve symptoms, for many the last few months and even years of life can be miserable, with persisting severe breathlessness on minimal exertion and episodic hospitalisation.
Although there is some evidence that opioids may relieve breathlessness in patients with chronic airways disease and cancer,6–8 the evidence is mixed in heart failure,9–11 and there is no specific intervention for the intractable breathlessness of severe CHF. Another frequently encountered group with severe breathlessness is those patients with chronic airways disease. In patients with chronic airways disease who also have hypoxia, there is reasonably robust evidence that long-term oxygen therapy (LTOT) can improve prognosis as well as symptoms (see Pulmonary disease and oxygen). By extension from these data, physicians often use home oxygen therapy (HOT) for patients with severely symptomatic heart failure. There is, however, no evidence that LTOT is helpful in CHF, either for the relief of symptoms or to improve prognosis.
Pulmonary disease and oxygen
For many years, patients with chronic airways disease or chronic obstructive pulmonary disease (COPD) have been treated with LTOT, particularly if they have evidence of hypoxia at rest. Treatment is given for at least 15 hours a day (including overnight). The evidence for the benefit of oxygen therapy comes from randomised clinical trials; the Medical Research Council’s (MRC’s) oxygen trial12 and the Nocturnal Oxygen Therapy (NOT) trial13 are perhaps the best known. A Cochrane review of oxygen therapy for patients with chronic airways disease suggests that ‘long[-]term home oxygen therapy improved survival in . . . COPD patients with severe hypoxaemia (PaO2 [partial pressure of arterial oxygen] less than 55 mmHg (8.0 kPa))’. 14 In the MRC’s oxygen trial,12 treating five patients with severe hypoxaemic COPD with LTOT saved one life over the 5-year study period. 14 The prognostic benefits were not apparent until after more than 1 year of therapy.
Although it does not affect prognosis in people with more modest hypoxaemia, LTOT does appear to help by reducing the severity of breathlessness. 15 There is no evidence that NOT alone (in other words, giving oxygen only at night) improves prognosis. 14 The authors of the systematic review and meta-analysis observed significant heterogeneity in most of their analyses and pointed out that most studies were either single blinded or not blinded at all. They therefore recommended an individual approach to care until data from large randomised controlled trials (RCTs) are available.
Data on the effects of oxygen therapy on quality of life (QoL) in patients with chronic airways disease are not clear-cut. Although the MRC reported that symptoms improved, few data were given. In patients with moderate hypoxaemia, the Cochrane meta-analysis reported a reasonably robust improvement in breathlessness equivalent to a reduction of 0.78 cm on a 10-cm visual analogue scale. 15
It is difficult to estimate adherence to LTOT in people with airways disease. Using the oxygen delivery system for 15 hours per day is clearly burdensome, and most studies suggest that adherence to this demand is less than 50%. 16 The summary figure of 45–70% is commonly quoted,17 but the studies from which the figures are derived are now quite old (see Chapter 9 for discussion). The only recent study suggests that adherence remains poor. 18
Although the original studies demonstrated a positive relationship between benefit and duration of oxygen, the mechanism is not clear. People who used oxygen for a longer period of time would have been more likely to have prevented worse desaturations during sleep or exertion, and the same benefit could have been achieved by supplemental oxygen at night only, or during exertion. Furthermore, if the prevention of exertion-induced desaturation helped exercise tolerance, then increased physical activity and reconditioning over time could have been the mechanism of improved symptoms and prognosis. 19 However, given that the only studies to show an improvement in prognosis had a target of oxygen use for long periods of time during the 24 hours, 15 hours per day remains the recommended prescription for prognostic benefit.
Heart disease and oxygen
Oxygen is commonly prescribed for patients with heart disease. There is a widespread perception that oxygen therapy can do no harm and may possibly be helpful and, thus, patients are often given high concentrations of inspired oxygen immediately following acute myocardial infarction or when they are admitted with acute pulmonary oedema. It is also commonly used during an admission for CHF.
Patients with severe (or even end-stage) CHF can appear very similar to patients with severe chronic airways disease. They are breathless at rest or on minimal exertion despite maximal medical therapy. A consequence is that HOT is often prescribed for severely breathless patients with heart failure, even in the absence of hypoxia, particularly towards the end of life.
There is only very limited evidence for the use of oxygen in heart disease, and much of the evidence suggests that oxygen might be harmful. In a study of patients with acute myocardial infarction, oxygen was given at as near to 100% as possible. Oxygen therapy was associated with a fall in cardiac output and stroke volume, together with a rise in heart rate. 20
The failing heart requires a higher filling pressure. The higher the filling pressure, the worse the cardiac function; hence an intervention causing a rise in filling pressure is deleterious. In a study of 10 patients with CHF,21 100% oxygen caused a rise in cardiac filling pressure, a fall in cardiac output and an increase in systemic vascular resistance (SVR). The SVR represents the load against which the heart has to work: the higher the SVR, the greater the work required of the heart. In another study of 12 patients with CHF,22 high-dose oxygen was associated with an increase in left ventricular (LV) filling pressure.
In contrast, in a study of patients with CHF given lower doses of oxygen (50%), exercise capacity increased and patients were less breathless and had a lower level of ventilation during exercise than during exercise with room air. 23 Findings from studies are inconsistent; another study has suggested that supplementary oxygen has little effect on exercise performance. 24 There is little evidence of the effect of oxygen when given to patients with heart failure at the much more modest levels used for treating chronic airways disease.
There is no evidence on whether or not the low-dose oxygen delivered by home oxygen concentrators is safe in patients with heart failure. There is no evidence about the effects of low-dose oxygen on haemodynamics in patients with severe heart failure.
The equivocal findings are perhaps not surprising. Oxygen is perhaps likely to be helpful only to people who are hypoxic (i.e. have low levels of arterial oxygen). Where it has been measured, oxygen has been found to be normal, or even high, during exercise in patients with CHF. 25 When patients with CHF are found to be hypoxic, there is usually an alternative explanation, such as coincident lung disease or congenital heart disease. 26 Thus, although patients with CHF may resemble patients with chronic airways disease clinically, they are much less likely to be hypoxic, and so might be thought, a priori, to be less likely to gain benefit from oxygen treatment.
Sleep apnoea
One complicating issue in patients with heart failure is the possible contribution of sleep-disordered breathing (SDB). Depending on the population studied, approximately one-third of patients with heart failure have SDB. 27 SDB happens when breathing stops during sleep. There are two kinds of SDB: obstructive and central sleep apnoea. In obstructive sleep apnoea, there is upper airways obstruction from soft tissues; respiratory efforts continue but there is no movement of air into the lungs. The patient usually wakes and breathing restarts. In central sleep apnoea, the central drive to breathe stops, usually in a cyclical manner alternating with periods of hyperventilation.
Patients with SDB, of either kind, become hypoxic during periods of apnoea. It thus might be that patients with heart failure might benefit from oxygen therapy even in the absence of daytime hypoxia, because it may relieve hypoxia at night.
Oxygen therapy might be helpful for periodic respiration. 28 There are some studies in a small number of patients investigating the effects of short-term overnight oxygen therapy which suggest that there may be some beneficial effect. For example, Toyama et al. 29 studied 20 patients and found improvements in exercise capacity and cardiac function in the 10 patients randomised to overnight oxygen. Other small studies have found similar beneficial effects, but there is no systematic review available.
In the largest available study, Sasayama et al. 30 reported on 52 patients with CHF and a positive overnight sleep test randomised to receive NOT or conventional therapy for 1 year. The group with NOT had better sleep patterns and a slight improvement in left ventricular ejection fraction (LVEF) and daytime activity level but no reduction in cardiac events.
Home oxygen therapy for breathlessness
There is surprisingly little evidence that oxygen is effective in treating breathlessness per se. A large observational study of patients with a variety of causes of breathlessness suggested that oxygen therapy was of no benefit,31 and apart from the review and a meta-analysis in people with COPD discussed in Pulmonary disease and oxygen,15 other systematic reviews identified no evidence that supplemental oxygen helped in the relief of breathlessness in patients with heart failure or patients with breathlessness from other causes in the absence of hypoxia. 32–35
A subsequent RCT compared LTOT via concentrator with a sham concentrator for refractory breathlessness due to a mixture of aetiologies (mainly COPD or cancer). Although breathlessness improved over 7 days in both groups, neither was superior. The authors suggested that it was simply airflow over the nasal mucosa, and not oxygen, that might have been the therapeutic intervention. 36
Despite the absence of any evidence in favour of using oxygen, HOT is commonly prescribed for severely symptomatic patients with end-stage heart disease: indeed, HOT in the UK is most commonly prescribed for conditions other than COPD (Department of Health, 2011, unpublished data).
The National Service Framework for coronary disease specifically recommends considering the potential benefit from ‘palliative care services and palliation aids (e.g. home oxygen)’. A Canadian study demonstrated that nearly 30% of oxygen prescribing costs were a result of palliative oxygen prescription (i.e. the patients did not meet the formal criteria for oxygen prescription for respiratory disease). 37 In another survey, 77% palliative physicians gave ‘intractable dyspnoea’ as the most common reason for prescription of home oxygen. 38 Fifty per cent of heart failure patients have breathlessness that markedly limits exercise in their last year of life,35 and in an Australian study between 10% and 20% of patients receiving palliative care were treated with HOT. 39 A small UK study found that 25% of patients receiving HOT had heart failure as their underlying pathology. 40 Finally, it is important to note that data from the Department of Health suggest that between 24% and 43% of the home oxygen prescribed to approximately 85,000 patients in England is not used or leads to no clinical benefit (Department of Health, 2011, unpublished data).
The home oxygen therapy trial rationale
Home oxygen therapy is potentially burdensome for patients and their carers. The concentrator has to be fitted to the home, usually requiring some drilling through walls. The device consumes electricity, although the costs are met by the NHS. The patient is encumbered to a degree by the oxygen. It is delivered through a nasal cannula, usually via a long length of piping, which limits movement. For some, oxygen cylinders to supply oxygen when the patient leaves the house are needed. The oxygen can leave the nose and throat feeling very dry. In addition, the stream of oxygen-enriched air is a potential fire hazard, particularly for patients who continue to smoke.
Home oxygen therapy is thus expensive to the health service and burdensome to the patient, and there is little evidence of its effectiveness. In the absence of any evidence-based guidance on the use of oxygen therapy for patients with CHF despite its widespread use, there is a need for a trial of HOT to find out if patients gain any benefit from its use.
Therefore, the HOT trial was designed to address the question of whether or not there is a role for HOT in patients with severely symptomatic heart failure, in terms of its effect on the symptoms for which it is usually prescribed (e.g. breathlessness), QoL and prognosis. We also planned to conduct a cost-effectiveness analysis.
The study consisted of three parts. The main study was designed to measure the impact of HOT on QoL in severely symptomatic patients. A qualitative substudy was designed to assess the burden on patients and their carers, and an acute oxygen substudy was designed to study whether or not there was any effect on haemodynamics of oxygen given in the same concentration as used by concentrators at home.
Chapter 2 Synopsis of trial evolution
Trial structure and protocol
The trial was designed in response to a call from the NHS research and development Health Technology Assessment (HTA) programme (HTA number 06/80; see Appendix 1). The grant for the trial was originally awarded in March 2008.
Stage 1: the North East Oxygen Network trial
The HOT trial was originally named the North East Oxygen Network (NEON) trial. The original conception of the trial was an attempt to provide evidence from a double-blind trial of the effectiveness of HOT in patients with severely symptomatic CHF. Four centres in the UK were involved: Hull, Leeds, Darlington and Leicester.
In the absence of any data from adequately powered studies, it was impossible to know what duration of oxygen therapy might be useful. Whether long-term therapy, following the example of successful treatment for patients with chronic airways disease, or shorter-term overnight oxygen therapy alone was the better treatment was unknown.
The study was thus designed in two phases. In the first phase (Figure 1), patients were to be randomised to receive either NOT overnight, assumed to be around 8 hours per night, or LTOT, aiming for at least 15 hours per day. A second randomisation would allocate patients within the two arms to receive either a true or a sham oxygen concentrator. The design of the second phase depended on the results of the first; whichever of NOT or LTOT appeared the better in phase 1 would then be formally tested in the second phase.
FIGURE 1.
The original design for the NEON trial.
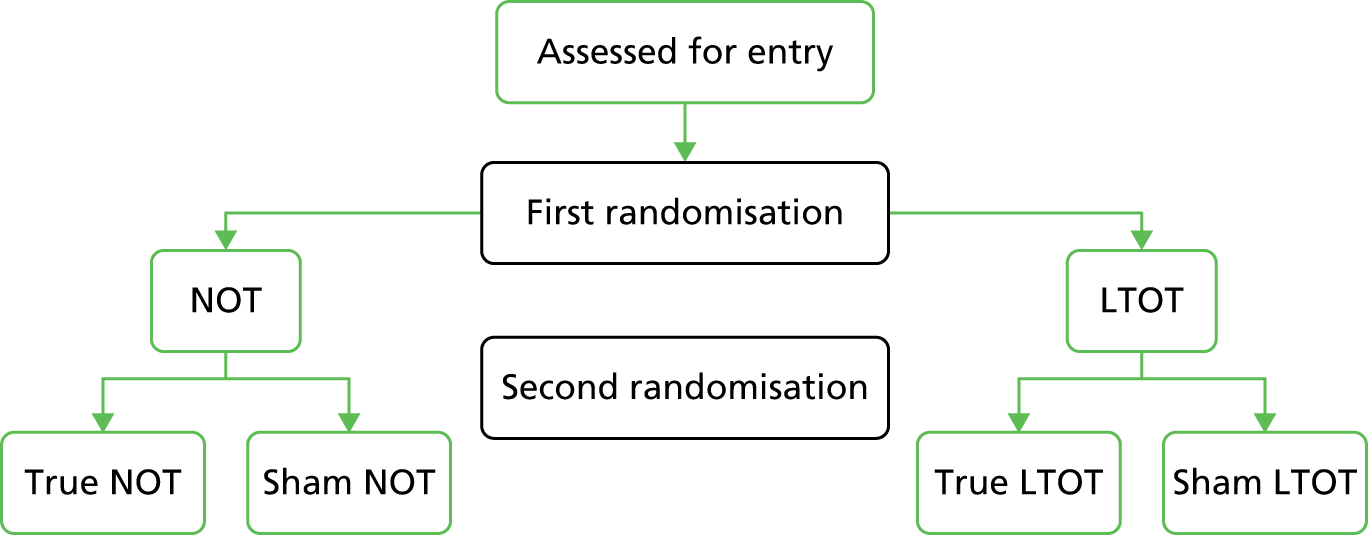
There was discussion among the research group about the optimum design. Some felt that an open pragmatic trial was best, as this would estimate treatment effectiveness rather than efficacy, which is an important question for the NHS. On the other hand, a placebo or sham trial would estimate the true treatment efficacy of oxygen and ensure blinded assessment of outcomes. Indeed, there is some evidence to suggest that the symptom of breathlessness is partially relieved by air blowing over the face. 36,41 Consequently, the original trial design was to use sham concentrators for the control treatment. HOT was to be delivered in the trial using oxygen concentrators rather than oxygen cylinders. A concentrator uses room air and removes a fraction of the nitrogen catalytically, so that the resulting inhaled gas is room air with some nitrogen removed; the concentration of inspired oxygen thus increases from 20.9% to approximately 28%. The sham machines would have delivered only room air.
The aim of the pilot was to recruit 120 patients, 30 in each of the four potential arms (see Figure 1). The results of the initial pilot study were then to inform the second phase study. Whichever of the two oxygen delivery schedules was the more successful would be used in a three-way comparison of oxygen therapy, sham therapy and open-label best medical therapy (BMT).
The investigators negotiated with Air Products Healthcare (Air Products and Chemicals, Inc., Allentown, PA, USA), which was able to manufacture sham machines which delivered room air. At this stage, recruiting sites were thus restricted to those whose contract for home oxygen supplies was with Air Products Healthcare. Plans were developed for the two-stage randomisation.
The original grant application to the HTA programme was with this study design.
Problems with the North East Oxygen Network trial
Trial management
The University of Hull initially agreed to sponsor the trial; however, after a period of some months it decided not to sponsor the study because of the potential issue with indemnity which could arise if a sham oxygen concentrator machine was accidentally given to a patient outside the trial. In addition, a full-time trial manager had not been included in the original submission to the HTA programme.
These problems were rectified by the Hull and East Yorkshire NHS Hospitals Trust agreeing to sponsor the study and through the establishment of a trial management function.
Sham machines
A crucial component of the trial, as originally conceived, was the use of sham machines to deliver room air. However, although it was relatively straightforward for Air Products Healthcare to deliver the sham machines, their use led to two insuperable logistical problems.
As the sham machines had to look as similar to the real devices as possible, it was felt by the company and the sponsor to be a substantial problem that the sham machines might not be kept out of the general pool of machines intended for use in delivering HOT to non-trial patients.
In addition, as the sham machines had to have their alarm functions disabled, there was a risk that they would not detect electrical faults that could potentially be a fire hazard. Specific trial insurance for their use was required, but was too costly to allow the trial to proceed.
Eventually, the trial management group accepted that the problems being raised made the trial as originally conceived impossible to run. Following a HTA site monitoring visit by Professor Jenny Hewison and Dr Vaughan Thomas on 24 September 2010, the group redesigned the study and approached the HTA programme to approve an alternative trial design.
Stage 2: the three-arm home oxygen therapy trial
The redesigned trial was named the HOT trial. We chose a pragmatic study design avoiding blinding and did not include a sham oxygen arm. In turn, this meant that more oxygen supply companies could be used and the number of recruiting centres could be expanded. An additional benefit was to avoid the need for special insurance related to dummy devices. Furthermore, the answer to a pragmatic trial is arguably of more relevance to patients and clinicians than the original study design. The original research question, however, remained unchanged: to assess the effects of HOT on QoL in patients with CHF (Figure 2).
FIGURE 2.
The original design for the three-arm HOT trial.
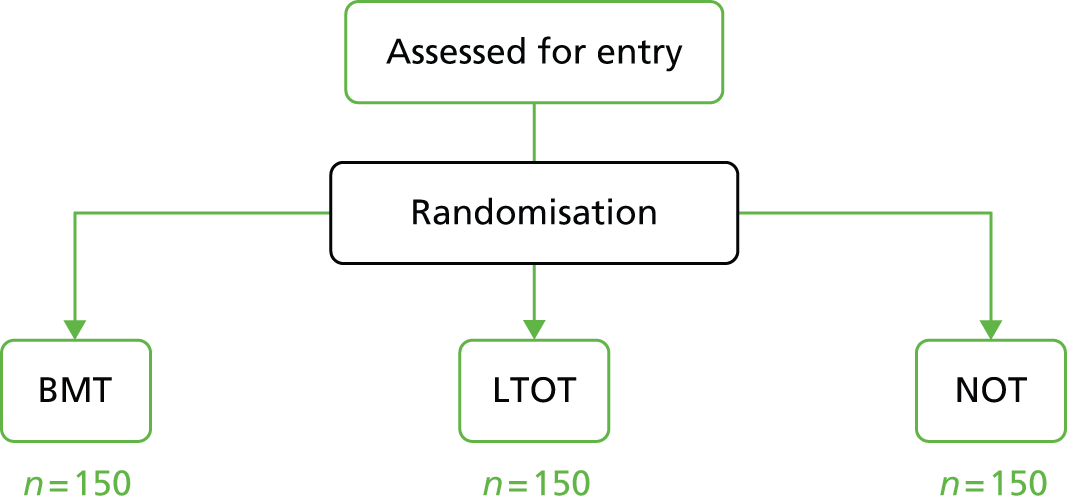
Patients were randomised to receive BMT, BMT plus NOT or BMT plus LTOT for 15 hours per day. The primary end point was QoL at 12 months as measured by the Minnesota Living with Heart Failure (MLwHF) questionnaire.
The trial started recruitment in April 2012. However, recruitment to the trial was much slower than expected and recruitment targets were not met. There were two major reasons.
First, it is a particular challenge to recruit patients to studies of palliative care,42–44 and the patients being recruited into the HOT study were necessarily very unwell and reaching the end of their lives. Some centres found it difficult to approach such patients and, in some centres, the patients being recruited were predominantly cared for by other groups of health-care workers in their area, commonly in the areas of palliative care, elderly care and primary care. Simply put, frail patients characterised by instability needed to be sufficiently fit and stable to participate in the trial.
The second, and more intractable, problem was the time it took to recruit centres to the trial. Multiple individual site approvals were needed, as for any clinical study, but the peculiar problem for HOT was the need for approval of HOT. Many sites took the view that this should be an NHS excess treatment cost. Hospital trust research and development departments naturally needed approval to prescribe HOT from the relevant primary care providers, leading in many instances to prolonged delays. During the trial set-up period of HOT, there were major upheavals in NHS structures. The replacement of primary care trusts with clinical commissioning groups made it extremely challenging to identify who was responsible for HOT. In some instances, clinical commissioning groups refused permission for the study to go ahead, and sometimes it proved impossible to find the responsible party with whom to negotiate. The median length of time taken from a centre expressing interest in the study to final approval was 9 months.
Stage 3: the two-arm home oxygen therapy trial
The trial management group realised that, with the difficulties in recruiting both patients and centres, it would prove impossible to complete the study within a reasonable time frame and budget. We again approached the HTA programme to ask permission for a modestly redesigned study. We removed the NOT arm, reducing the study from a three- to a two-armed trial comparing BMT with BMT plus LTOT (15 hours per day). LTOT was chosen over NOT as being the only form of oxygen therapy shown to improve prognosis, albeit in patients with COPD. The simplification of the study design allowed the sample size to be greatly reduced (Figure 3).
FIGURE 3.
The final design for the two-arm HOT trial.

In addition, we brought forward the timing of the primary end point from 12 to 6 months. This had the effect of reducing the time required to follow up patients and increasing the time available for analysing the data and writing the report for the HTA. After discussion with the HTA programme, it was agreed that the cost-effectiveness analysis for the trial was not to be included.
Chapter 3 The home oxygen therapy trial: methods
The HOT trial was a Phase III, prospective, open, pragmatic, multicentred, randomised controlled parallel-group trial with equal randomisation. The study was designed after extensive consultations with patients in the Department of Academic Cardiology in Hull. Patients gave very helpful advice about (1) the design of the study and (2) the format and wording of the patient information leaflet. This was particularly useful, as we were aware of the need to be clear about the requirements of the study, but did not want to cause alarm (particularly about fire risk). The patients also advised about the number and type of study measures with regard to what was an acceptable participant burden.
We particularly acknowledge the help and advice we received in designing the study from Mr Patrick Foulk, who generously agreed to take part in the trial steering committee. Mr Patrick Foulk is an experienced patient representative who has been part of many trial management and trial steering groups. He has been particularly helpful in ensuring that the trial maintained patient relevance, was not afraid to ask the pertinent, and sometimes difficult, questions during meetings, and gave useful ongoing advice about recruitment from the patients’ viewpoint.
The aim of the study was to determine whether or not the addition of long-term HOT (given for at least 15 hours per day) improved the QoL for patients with stable, severely symptomatic CHF who were already receiving BMT. Such patients are usually thought of as being in need of palliative care: their outlook is limited and they are severely symptomatic. Any intervention which can relieve symptoms is to be welcomed; conversely, if a treatment is simply burdensome, its use should be abandoned.
Participants with NYHA class III or IV LV systolic dysfunction receiving optimal medical therapy were randomised to receive either:
-
BMT plus at least 15 hours’ LTOT or
-
BMT.
The original trial had a third arm with patients allocated to a NOT-only group. Twenty-five patients were randomised into the NOT arm before the decision was made to drop the arm in April 2013.
Approvals obtained
The Northern and Yorkshire Research Ethics Committee [the Medical Research and Ethics Committee (MREC)] approved the study originally known as the NEON trial on 24 August 2009. Further approvals were received on 18 May 2011 and 2 April 2013 for changes to the design of the study that were implemented to improve recruitment.
The Medicines and Healthcare products Regulatory Agency reviewed the trial protocol and on 28 June 2008 confirmed that oxygen concentrators were medical devices and not medicines. The study, therefore, did not fall under the Clinical Trials Directives and so a clinical trial authorisation was not required.
The details of MREC and research and development department approvals are provided in Appendix 2.
Patient study group
To make the findings of the study as widely applicable as possible, the inclusion criteria were very broad, with few exclusions.
Inclusion criteria
To be included in the study, patients had to:
-
be willing to provide written informed consent and be able to complete patient assessments
-
be aged 18 years or over
-
have heart failure from any aetiology
-
have severe symptoms of heart failure (NYHA class III/IV)
-
have LV systolic dysfunction confirmed by echocardiography, with LVEF less than 40% or graded as at least ‘moderately’ impaired on visual inspection if an accurate ejection fraction could not be calculated
-
be receiving maximally tolerated medical management of their heart failure as
-
reached target dose of (or be on maximally tolerated dose of, or be intolerant of) an inhibitor of the renin–angiotensin system shown to improve prognosis
-
reached target dose of (or be on maximally tolerated dose of, or be intolerant of) a beta-adrenoceptor antagonist shown to improve prognosis
-
reached target dose of (or be on maximally tolerated dose of, or be intolerant of) an aldosterone antagonist.
-
Exclusion criteria
Patients were excluded from the study if they:
-
were unable to provide written informed consent
-
had had a cardiac resynchronisation therapy device implanted within the previous 3 months
-
had coexisting malignant disease if this would affect the study in the investigators’ opinion
-
had interstitial lung disease
-
had COPD likely to fulfil criteria for LTOT; forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 70% and FEV1 < 40% predicted and hypoxia [partial pressure of arterial oxygen (PaO2) < 7.3kPa or saturations < 90%]
-
were using any device or medication that would impede their ability to use LTOT or NOT, such as continuous positive airway pressure
-
were unwilling or unable to comply with safety regulations regarding oxygen use, particularly smoking
-
were unable to complete patient-related information on entry.
Randomisation
Allocations were centrally generated by the York Trials Unit. Patients were initially randomised into the trial on a 1 : 1 : 1 basis using block randomisation, with randomly permuted block sizes of three, six and nine. Subsequently, after the NOT arm was dropped, 1 : 1 allocation was used, with randomly permuted block sizes of four, six and eight. Patients were randomised by a member of the research team at the recruiting site using the secure, telephone-based randomisation service at the York Trials Unit.
Primary outcome
The primary end point was the total score from the MLwHF questionnaire at 6 months from baseline. As the patient group is highly symptomatic and has a limited prognosis, the 6-month primary outcome is highly clinically meaningful.
The MLwHF questionnaire is a validated, disease-specific QoL instrument widely used in heart failure research to assess both symptom severity and response to treatment. 45,46 It consists of 21 questions focusing on the impact of heart failure on QoL. Patients are asked to rate the extent to which their heart failure has prevented them from living as they wanted during the past month using questions rated on a scale of 0 (no effect) to 5 (very much). The questionnaire is scored by summing the responses to all 21 questions, thus resulting in a score from 0 to 105, with a higher score reflecting poorer QoL. The MLwHF questionnaire-validated QoL score, version 2, is easy to complete and has been shown to be especially effective in older patients with comorbidities. 47
An improvement in the score of 5 is sometimes taken to be a minimum clinically important difference,48 but others have suggested that a change of 1 standard error (SE) around the mean score is needed (around 6 or 7, depending on the population studied). 49 There are no studies of LTOT to help guide us. The MIRACLE (Multicenter InSync RAndomized CLinical Evaluation) trial of biventricular pacing was powered for a 13-point improvement in MLwHF questionnaire score and found a score difference of 9 between treatment groups. 50 The CARE-HF (CArdiac REsynchronization-Heart Failure) study of biventricular pacing found a score improvement of 10.6 with intervention. 51 In the absence of data on which to base study size, we took a MLwHF questionnaire score of 10 as an arbitrary indicator of the minimum improvement necessary to justify the cost and inconvenience of oxygen therapy for patients.
Power calculation
To detect a difference in MLwHF questionnaire score between the two groups of 10 points, with a standard deviation (SD) of 25, at 80% power and 5% significance, we required 100 patients per group. Assuming 10% attrition at 6 months, this equated to 111 per group, a total sample size of 222 patients.
Secondary outcomes
Other indices of QoL, exercise performance and severity of heart failure were also measured as part of the study.
Measures of quality of life
Health status as measured by the European Quality of Life-5 Dimensions instrument
The European Quality of Life-5 Dimensions (EQ-5D) is a self-administered, validated measure of health status and consists of a five-question multiattribute questionnaire and a visual analogue self-rating scale. 52,53 Respondents are asked to rate severity of their current problems (level 1, no problems; level 2, some/moderate problems; level 3, severe/extreme problems) for five dimensions of health: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Patients can be classified into 243 health states plus two further additional states (unconscious and dead).
Numerical Rating Scale for breathlessness
The severity of and distress caused by breathlessness was measured by the Numerical Rating Scale (NRS) for breathlessness (average and worse over past 24 hours and global change in breathlessness). Patient satisfaction was also measured by the NRS (in addition to a qualitative substudy to assess patient experience). The NRS measures symptoms and satisfaction on a 10-point scale, anchored at 0 and 10. It is highly correlated with visual analogue scale scores, but is more repeatable. 31,54 Average and worst breathlessness over the past 24 hours were anchored with ‘not breathless’ and ‘worst breathlessness imaginable’. 55 Distress due to breathlessness was anchored with ‘no distress at all’ and ‘the worst imaginable distress’. The minimum clinically important difference in the scale is 1 point. 56,57 How well a patient had coped with their breathlessness over the past 24 hours was anchored on ‘not coping at all’ and ‘coping very well’. Satisfaction with treatment for breathlessness was anchored on ‘not at all satisfied’ and ‘very satisfied’.
Epworth Sleepiness Scale score to assess daytime somnolence
The Epworth Sleepiness Scale (ESS) is a standard scale for screening for, and assessing the severity of, daytime sleepiness as part of the SDB syndrome. Patients are asked to rate their chance of dozing in eight different scenarios, such as being a passenger in a car or watching TV. Each is measured on a Likert-type scale from 0, would never doze, to 3, high chance of dozing, and a total score is obtained from summing the 8 items out of 24.
Mood assessment using the Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) is a well-validated and easy-to-complete 14-item screening tool for depression and anxiety. 58 Each item is measured on a Likert-type scale from 0 to 3, and seven non-overlapping items make up each of the two subscales. For each of the two continuous subscales (anxiety and depression), patients are categorised as ‘normal’ (0–7), ‘borderline’ (8–10) or ‘clinically anxious/depressed’ (11–21).
Severity of heart failure
The severity of the patient’s heart failure was assessed by measuring LV dysfunction on echocardiography and the N-terminal pro-B-type natriuretic hormone (NT-proBNP) levels.
Exercise capacity
The 6-minute walk test
The 6-minute walk test (6MWT) followed a standardised protocol. 59–61 A flat, obstacle-free corridor with chairs placed at either end was used. Patients were instructed to walk as far as possible, turning 180° every 15 metres in the allotted time of 6 minutes. Patients were able to rest, if needed, and the time remaining called every second minute. Patients walked unaccompanied so as not to influence walking speed. After 6 minutes, patients were instructed to stop and the total distance covered was measured to the nearest metre.
Performance
Karnofsky Performance Status scale
This validated scale incorporates the components of physical activity, work and self-care of patients. 62,63 Patients are classified according to their functional impairment, with the status categories ranging from 0% (dead) to 100% (normal with no complaints and no evidence of disease) in steps of 10%. Although the Karnofsky Performance Status (KPS) scale is categorical, it is of an ordinal nature and was, therefore, treated as continuous data in our analysis. The scale can be further classified as ‘died’ (0); ‘unable to care for self; requires equivalent of institutional or hospital care; disease may be progressing rapidly’ (10–40); ‘unable to work; able to live at home and care for most personal needs; varying amount of assistance needed’ (50–70); and ‘able to carry on normal activity and to work; no special care needed’ (80–100).
Comorbidity
Charlson Comorbidity Index
This is a validated age–comorbidity index used to estimate relative risk of death from prognostic clinical covariates, and is useful in studies with 1 to 2 years’ follow-up. 64 [See also http://touchcalc.com/calculators/cci_js (accessed June 2015).]
Prevalence of hypoxia
The prevalence of hypoxia was assessed by measuring:
-
resting oxygen saturation
-
oxygen saturation at peak exercise during 6MWT
-
oxygen saturation 5 minutes after the end of the 6MWT
-
nocturnal oxygen saturation and the presence of SDB during an overnight sleep test using an Embletta (Natus Medical Inc., San Carlos, CA, USA).
Safety and adherence
We recorded the patients’ own report of their adherence with the oxygen concentrator, and the number of hours of oxygen used measured by the concentrator meter.
Participant death was recorded as a serious adverse event (SAE), with the date of death where possible.
The number of days the patient was alive and out of hospital was calculated.
Other measurements
Blood analysis
Standard biochemistry and full blood count were undertaken. Results from these tests undertaken within a month of baseline assessment could be used.
Electrocardiogram
A 12-lead electrocardiogram was undertaken to determine cardiac rhythm and electrocardiography intervals.
Echocardiogram
Routine echocardiographic assessment was performed including M-mode, 2-dimensional images and colour flow Doppler recordings by trained operators. Measurements were taken in accordance with American Echocardiography Society or European Association of Echocardiography guidelines. LV systolic function was assessed by attempted measurement of LVEF using Simpson’s biplane method in all subjects, and by estimation on a scale of LV systolic impairment as follows: normal, mild, mild to moderate, moderate, moderate to severe or severe systolic impairment. Results from echo assessments within 3 months of the baseline assessment were used.
New York Heart Association class
Assessment of NYHA grade was made using the following classification:
-
class I – breathless on severe exertion (normal)
-
class II – breathless and/or fatigue on moderate exertion
-
class III – breathless and/or fatigue on mild exertion
-
class IV – breathless and/or fatigue at rest.
Spirometry
Spirometry was undertaken to determine the FVC and forced expiratory volume in the first 3 seconds. Results from spirometry tests undertaken within 3 months of the baseline assessment were used.
Other assessments
Resting pulse rate, blood pressure and respiratory rate, peripheral oedema and lung crackles, if any, were measured and recorded.
Other data
Data recorded included height, weight and date of birth, which allowed age at recruitment to be calculated. Sex, aetiology of heart failure, current medication and adverse events were also recorded.
Cost-effectiveness
In addition to the EQ-5D instrument, the Health Service Use Questionnaire was used to measure the level of health resource use. Respondents were asked to recall the amount of use they had made of the specified services over the previous 6 months.
Adverse events
In this study an adverse event was defined as any untoward medical occurrence in a patient which did not necessarily have a causal relationship with the study treatments or procedures.
Health-care professionals were asked to report any adverse event occurring in participants in both groups using either the SAE form or the adverse event form within 24 hours of becoming aware of the event. A SAE was defined as an event that resulted in death, was life-threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, consisted of a congenital anomaly or birth defect or was otherwise considered to be medically significant by the investigator. The reporting health-care professional was asked to indicate whether or not, in his or her opinion, the event was related to the treatment, to indicate if it was expected and to grade the intensity of the event. Further follow-up reports were completed if necessary or until the local principal investigator considered the event to be resolved or to have become a chronic ongoing condition.
Chapter 4 Statistical analysis
Analyses mainly compared the LTOT intervention group with the control (BMT) group on an intention-to-treat basis, including all randomised patients in the groups to which they were originally allocated. We performed exploratory analyses including contemporaneously recruited patients in the NOT arm for the primary outcome and survival analysis. Analyses were conducted in Stata version 13 (StataCorp LP, College Station, TX, USA), using two-sided significance tests at the 5% significance level.
Trial completion
The flow of participants through the trial is presented in a CONSORT (Consolidated Standards of Reporting Trials) diagram. The numbers of participants withdrawing from treatment and/or the trial are summarised with the reasons where applicable (see Figure 6).
Baseline data
All participant baseline data are summarised descriptively by treatment group. Continuous measures are reported using summary statistics (mean, SD, median, interquartile range, minimum, maximum) and categorical data are reported as counts and percentages. Comparisons were made between trial groups ‘as randomised’ and ‘as analysed’ in the primary analysis. No formal statistical comparisons were undertaken.
Primary analysis
The primary outcome was health-related quality of life (HRQoL) as measured by the MLwHF questionnaire scores at 6 months. A lower MLwHF questionnaire score indicates a better QoL. The MLwHF questionnaire consists of 21 items and a total score is obtained by summing the item scores, where all 21 items have a response. The number of missing MLwHF questionnaire responses was examined at each time point. Multiple imputation using chained equations was used to fill in missing questionnaire items, where there were fewer than four missing item responses, using other items in the questionnaire (http://178.23.156.107:8085/Instruments_files/USERS/mlhf.pdf). Linear regression was used to perform the imputation and five imputed data sets were created. The mean of the imputed values for each patient was used to replace the missing item. Where the mean was less than 0 or greater than 5 (the range of permitted scores for the MLwHF questionnaire) it was replaced with the nearest permitted value.
Our primary analysis compared MLwHF questionnaire scores between the LTOT and BMT treatment groups using a covariance pattern mixed model, where effects of interest and baseline covariates are specified as fixed effects, and the correlation of observations within patients is modelled by a covariance structure. The outcome modelled was total MLwHF questionnaire scores at 3, 6 and 12 months. The model included as fixed effects baseline MLwHF questionnaire score, age, log-NT-proBNP level, creatinine level, treatment group, time and a treatment group–time interaction term. Age, NT-proBNP levels and creatinine levels were all continuous variables as assessed at baseline. NT-proBNP data were found to be significantly positively skewed and so were log transformed.
Different covariance structures for the repeated measurements, which are available as part of Stata version 13, were explored and the most appropriate pattern used for the final model. Diagnostics including Akaike’s information criterion65 were compared for each model (smaller values are preferred).
Participants were naturally included in the model only if they had full data for the baseline covariates and outcome data for at least one post-randomisation time point (3, 6 or 12 months).
Estimates of the adjusted mean difference (AMD) between treatment groups in MLwHF questionnaire scores were extracted from the model for all time points with 95% confidence intervals (CIs) and p-values. The primary end point is the treatment effect estimate at 6 months. Estimates for the other time points serve as secondary outcomes. An overall effect of the intervention across all included time points was not extracted.
Sensitivity analyses
Patients were recruited from multiple centres. To investigate whether or not centre affected the outcome, centre was included as a random effect in the primary analysis model. 66
Secondary analysis
Comparisons with the nocturnal oxygen therapy subgroup
Initially, the HOT trial recruited patients to three trial arms: BMT, LTOT and NOT. In April 2013, the decision was made to stop recruitment to the NOT arm, although patients in this group continued to be followed up. We conducted an exploratory analysis on the primary outcome including all three treatment arms, which included only contemporaneously recruited patients, that is only patients in any arm randomised up to the date at which the randomisation to the NOT arm was closed. This was to ensure comparability of the treatment groups. A covariance pattern mixed model was used similar to that described for the primary analysis including a variable for treatment with the three levels.
A non-significant difference was observed between the NOT and LTOT arms in a pairwise comparison from this model; therefore, these arms were combined and compared against the BMT arm on the primary outcome using a similar analysis, and including only patients randomised up to the time that the NOT arm was dropped from the study.
Secondary outcomes
The following outcomes were analysed using the same method as the primary outcome but just adjusting for the baseline value of the dependent variable: the six questions (separately) of the NRS for breathlessness; ESS; HADS scores for anxiety and depression; KPS of physical activity; metres walked as part of the 6MWT; the Charlson Comorbidity Index (CCI); and NT-proBNP levels.
Scoring of the secondary outcomes
-
A total score for the ESS was calculated only when all items had a valid response, in accordance with the scoring instructions detailed at http://epworthsleepinessscale.com/about-epworth-sleepiness/.
-
For the HADS, as is recommended, the score for a single missing item from a subscale was inferred using the mean of the remaining six items. If more than one item was missing, then the subscale was judged as invalid (www.gl-assessment.co.uk/products/hospital-anxiety-and-depression-scale/hospital-anxiety-and-depression-scale-faqs#FAQ4).
-
A total score for the CCI was computed by applying a certain number of points for each comorbidity present and adding a score for age (≤ 40 years, 0 points; 41–50 years, 1 point; 51–60 years, 2 points; 61–70 years, 3 points; and 71–80 years, 4 points) as detailed at http://touchcalc.com/calculators/cci_js (accessed June 2015).
Mortality
Mortality was analysed as a time-to-event outcome. For each group, the distribution of time from randomisation to death was described using Kaplan–Meier survival estimates. Kaplan–Meier survival curves are presented for the two groups. The statistical equivalence of the two curves was tested using the log-rank test. Right censoring occurred if the patient was still alive at the end of follow-up or if they withdrew or were lost to follow-up.
We compared the survival of the LTOT and the BMT groups using a Cox proportional hazards regression model adjusting for baseline CCI score. Hazard ratios (HRs) are presented with p-values and 95% CIs.
Similar survival analyses were also conducted by combining the LTOT and NOT arms, and comparing them with the BMT arm, including only those patients contemporaneously recruited.
Number of days alive and out of hospital
The number of days alive and out of hospital (DAOH) was calculated for each patient as follows:67 the total potential follow-up time was determined as the number of days from randomisation until the date of the final follow-up time point (if alive) or the end of study date, 30 May 2014, if the patient had died. Patients who were lost to follow-up had a censoring date of 30 May 2014 to determine potential follow-up time. The number of nights spent in hospital over the previous 6 months was captured on the patient questionnaire at 6, 12, 18 and 24 months. The total time spent in hospital was computed by adding the durations of each individual hospital stay. If a patient died, the number of days from their death to the end of the study was assigned as days dead. Days in hospital and days dead were then subtracted from the total potential follow-up time to arrive at DAOH for each patient. Summaries of the number and percentage of days the patients in the two groups are alive and out of hospital are presented.
Health-related quality of life as measured by the European Quality of Life-5 Dimensions
Data are summarised for the two treatment groups and a simple analysis of variance was used to compare each treatment at each time point. In addition, an unadjusted t-test compared baseline scores at each time point for each treatment group.
Prevalence of hypoxia
The prevalence of saturation at the thresholds of 90% and 95% are summarised for O2 saturation at rest, at peak and at 5 minutes after the 6MWT.
Patient-reported adherence
Patient-reported adherence to the oxygen machine is summarised at 3, 6, 12, 18 and 24 months in the LTOT arm and NOT subgroup.
Number of hours of oxygen used measured by concentrator meter
Summary statistics for the mean number of hours that the machine was used per day by patients in the LTOT arm and NOT subgroup are reported.
Adverse events
The number of adverse events experienced by each participant and total number of events overall are summarised for each treatment group. The severity of the event and whether or not it was considered related to treatment is summarised.
Chapter 5 Study procedures
Members of the research team participating in the study received good clinical practice training as well as training in all aspects of the trial. Training included participant recruitment, eligibility criteria, trial protocol, adverse event reporting procedures and trial documentation. In order to standardise the study prior to commencement, each study site also received a trial handbook.
Potential participants for the trial were identified from NHS heart failure, cardiology or general medical clinics. Existing lists of likely eligible patients held within the NHS hospitals were also reviewed. The study was introduced to patients by the clinician responsible for their treatment (usually a consultant cardiologist). Patients then had the opportunity to discuss study participation with the research nurse. In order to aid recruitment some sites used patient identification centres. Potential participants were sent an introduction letter with an invitation to contact the study team if they were interested in taking part in the study. Alternatively, the research nurse could contact the patient directly by telephone.
Each potential participant was informed of the aims, methods, expected benefits, potential hazards and discomforts of the study verbally and through a patient information sheet (see Appendix 3). Participants were given at least 24 hours to consider participation in the study. Participants who wished to take part in the study provided written informed consent (see Appendix 4). Baseline data were then collected. Each participant’s general practitioner was notified of the patient’s involvement in the HOT trial and their group allocation after recruitment. The flow of participants through the trial is presented in a CONSORT68 diagram (see Figure 6).
Baseline and follow-up assessments
After written informed consent had been obtained, baseline data were collected using the nurse and patient baseline questionnaires.
After giving consent and completing baseline assessments, patients were randomised by a member of the research team at the recruiting site using the secure, telephone-based randomisation service at the York Trials Unit.
In the event that the local clinical team felt that a patient needed to be assessed for obstructive sleep apnoea, the patient was not randomised until a clinical decision was made.
Home oxygen therapy
If the patient was randomised to receive HOT, a clinical prescription was completed and sent to the local oxygen supply company holding the standard NHS contract within that particular region. These were Air Liquide UK (Birmingham, UK), Dolby Vivisol (Stirling, UK), BOC Healthcare (Manchester, UK) and Air Products (Crewe, UK). Concentrators were delivered to the patient’s home by the recruiting hospital’s usual oxygen supplier, in accordance with existing NHS agreements. The oxygen supply company typically installed the equipment within 3 days of the prescription being issued. The installing engineer instructed the participant about the safety requirements of using the machines and gave details of how to claim for the electricity costs of running the machine. In accordance with NHS agreements, the concentrators should have been serviced approximately every 6 months.
At the end of an individual patient’s trial participation, oxygen concentrators were removed from the patients’ homes unless they wished to continue treatment.
Best medical therapy
Patients allocated to this arm received the BMT currently available. Patients received the maximally tolerated medical management for their heart failure and reached their target dose of inhibitors of the renin–angiotensin system, a beta-adrenoceptor antagonist and an aldosterone antagonist.
Follow-up
Patients with CHF usually attend clinic every 6 months and follow-up in the study was arranged around standard attendances to prevent patients being unduly burdened with additional hospital visits. At the conclusion of the trial, final clinical data were collected using existing hospital records, including admission and mortality. The data collected are summarised in Table 2, together with the schedule for data collection.
| Assessment | Months after recruitment | |||||
|---|---|---|---|---|---|---|
| Baseline: clinic | 3: home or clinic | 6: clinic | 12: clinic | 18: clinic | 24: clinic | |
| Clinical | ||||||
| Age, sex, aetiology, height | ✗ | – | – | – | – | – |
| Weight | ✗ | – | ✗ | ✗ | ✗ | ✗ |
| Current medication | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Resting pulse rate and blood pressure, respiratory rate | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Assessment of peripheral oedema and lung crackles | ✗ | – | ✗ | ✗ | ✗ | ✗ |
| ECG | ✗ | – | ✗ | ✗ | ✗ | ✗ |
| Blood test – BCP, FBC (standard biochemistry and haematology) | ✗ | – | ✗ | ✗ | ✗ | ✗ |
| Blood test – NT-proBNP | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Spirometry | ✗ | – | – | ✗ | – | ✗ |
| Echocardiogram | ✗ | – | – | ✗ | – | ✗ |
| 6MWT and pre/post O2 saturation | ✗ | – | ✗ | ✗ | ✗ | ✗ |
| Overnight sleep test (if locally accessible) | ✗ | – | ✗ | ✗ | ✗ | ✗ |
| CCI | ✗ | – | ✗ | ✗ | ✗ | ✗ |
| KPS score | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| QoL | ||||||
| MLwHF questionnaire | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| NRS – breathlessness | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| HADS | ✗ | – | ✗ | ✗ | ✗ | ✗ |
| ESS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Health economics | ||||||
| EQ-5D | ✗ | – | ✗ | ✗ | ✗ | ✗ |
| Health Service Use Questionnaire (not all questions) | ✗ | – | ✗ | ✗ | ✗ | ✗ |
Trial completion
Participants were deemed to have exited the trial when:
-
they had been in the trial for 24 months or 6 months if there was insufficient time to follow the patient further
-
they wished to withdraw from the trial
-
their treating physician or medical researcher withdrew them from the trial
-
they were lost to follow-up
-
they died.
As well as withdrawing fully from the trial, participants had the option of:
-
withdrawing from receiving oxygen (if that had been their allocation)
-
withdrawing from the collection of data via patient questionnaires
-
withdrawing from the collection of data via nurse questionnaires.
Chapter 6 Results
Trial recruitment
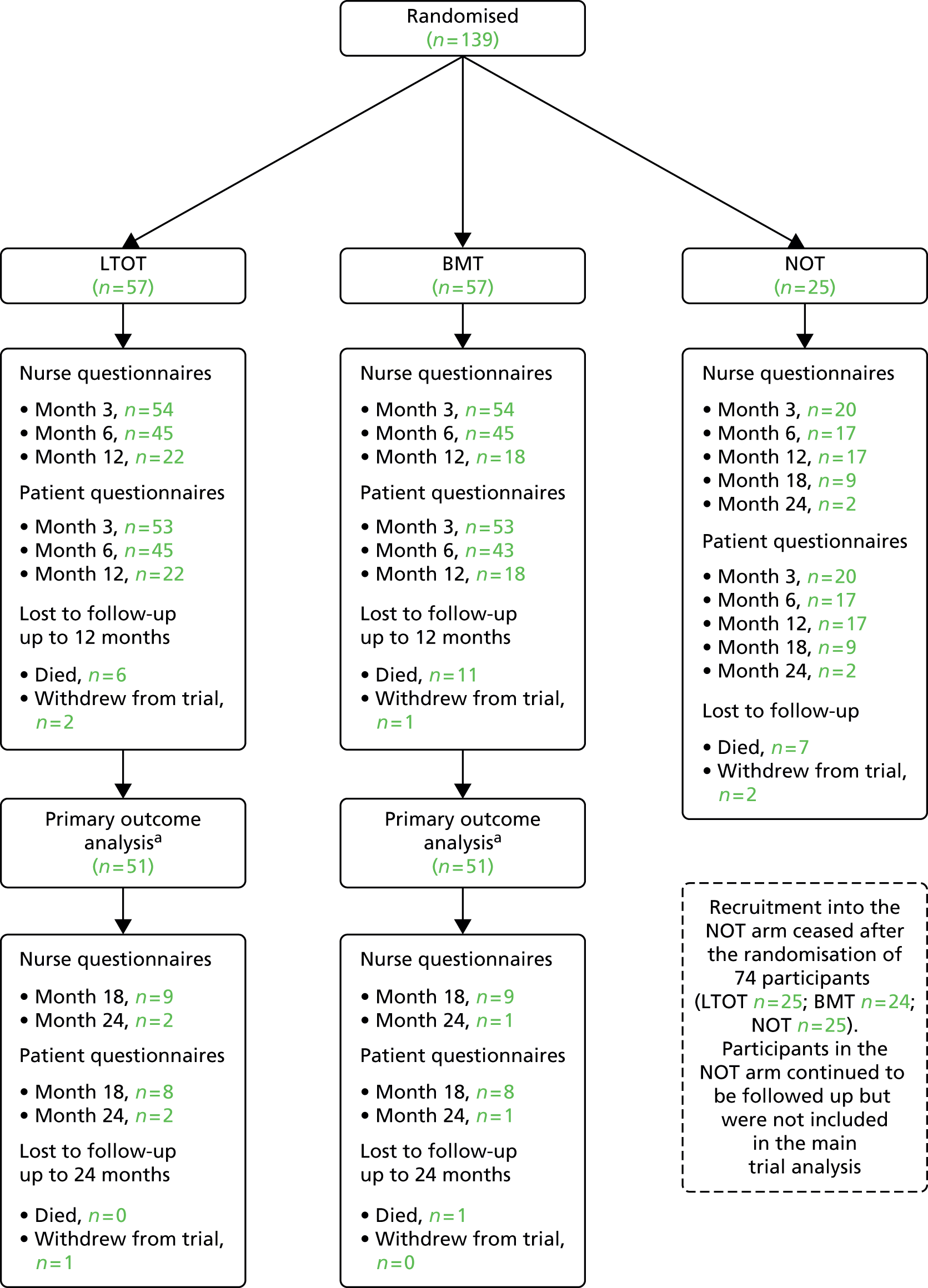
The HOT study was stopped early by the funders because of poor patient adherence to the oxygen prescription. Recruitment began in April 2012 and stopped in February 2014. Randomisation to the NOT arm was stopped in April 2013. In total, 139 patients were randomised, 57 to each of the LTOT and BMT arms and 25 to the NOT arm. The overall rate of recruitment is shown in Figure 4.
FIGURE 4.
The overall rate of recruitment in the HOT trial.

The original sample size for the three-arm trial was 450 patients. The aim was to recruit these patients in 12 months; however, recruitment was slower than expected and, by the end of April 2013, 74 patients had been recruited into the trial (25 to each of the LTOT and NOT arms and 24 to the BMT arm). The decision was made to drop the NOT arm and continue the trial with two arms with a revised sample size of 222 patients. The recruitment period was extended to August 2014. When the trial was closed at the end of February 2014, a total of 139 participants had been randomised.
Over the course of the trial, a total of 15 participating sites joined, all in the UK (Table 3). Recruitment was staggered, with sites joining over the course of the trial. At least one trial participant was recruited in 13 out of the 15 sites (Figure 5 and Table 3). The median number of participants recruited per site was 4 (range 1–76). Over half of the participants (n = 76) were recruited from the Hull site, where the chief investigator was based.
| Site | Principal investigator | Started recruiting | LTOT (n = 57) | NOT (n = 25) | BMT (n = 57) | Total (n = 139) |
|---|---|---|---|---|---|---|
| Hull | Professor Andrew Clark | April 2012 | 26 (45.6) | 18 (72.0) | 32 (56.1) | 76 (54.7) |
| Chesterfield | Dr Justin Cooke | October 2012 | 7 (12.3) | 0 (0.0) | 5 (8.8) | 12 (8.6) |
| Oldham | Dr Jolanta Sobolewska | January 2013 | 6 (10.5) | 3 (12.0) | 3 (5.3) | 12 (8.6) |
| Darlington | Professor Jerry Murphy | September 2012 | 4 (7.0) | 0 (0.0) | 4 (7.0) | 8 (5.8) |
| Dundee | Dr Miles Witham | November 2012 | 6 (10.5) | 1 (4.0) | 0 (0.0) | 7 (5.0) |
| Leicester | Professor Iain Squire | November 2012 | 2 (3.5) | 0 (0.0) | 5 (8.8) | 7 (5.0) |
| Barnet | Dr Ameet Bakhai | October 2012 | 1 (1.7) | 2 (8.0) | 1 (1.8) | 4 (2.9) |
| Durham | Dr Mohamed El-Harari | January 2013 | 0 (0.0) | 1 (4.0) | 3 (5.3) | 4 (2.9) |
| Bradford | Dr Paul Smith | January 2013 | 2 (3.5) | 0 (0.0) | 1 (1.8) | 3 (2.2) |
| Ealing | Dr Stuart Rosen | May 2013 | 0 (0.0) | 0 (0.0) | 2 (3.5) | 2 (1.4) |
| Sunderland | Dr John Baxter | June 2013 | 1 (1.8) | 0 (0.0) | 1 (1.7) | 2 (1.4) |
| Pinderfields | Dr Paul Brooksby | July 2013 | 1 (1.8) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| Plymoutha | Dr Andrew Stone | June 2013 | 1 (1.8) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
FIGURE 5.
Participant recruitment by site (two sites did not recruit any patients).

Two sites did not recruit any patients. Significant attempts were made to recruit a patient in Aneurin Bevan University Health Board but no eligible, consenting patients were identified; East Cheshire NHS Trust was granted research and development approval only shortly before recruitment ceased and, therefore, did not have time to recruit a patient.
Figure 6 shows the CONSORT flow diagram of participants through the trial.
FIGURE 6.
The CONSORT flow diagram. a, Based on patients who provided full covariate data for the primary analysis model and primary outcome data at at least one of the 3-, 6- or 12-month time points.
Withdrawals
A greater proportion of patients in the LTOT arm (n = 8, 14%) than the NOT arm (n = 2, 8%) formally withdrew from their allocated trial treatment. One patient in the NOT arm withdrew from completing the postal patient questionnaires and one patient in each of the NOT and BMT arms withdrew from completing the nurse questionnaires. A total of four patients (one LTOT, one BMT and two NOT) requested full trial withdrawal and two further patients (one LTOT, one NOT) were withdrawn by a health professional. The reasons given for each of the change of circumstances are shown in Table 4. There were 25 recorded deaths during the course of the trial (LTOT, 6; BMT, 12; and NOT, 7).
| Reason | LTOT, n (%) | NOT, n (%) | BMT, n (%) | Overall, n (%) |
|---|---|---|---|---|
| Withdrew from treatment | n = 8 | n = 2 | – | n = 10 |
| Did not feel oxygen was helping | 3 (37.5) | 0 (0) | – | 3 (30) |
| Was not using oxygen | 2 (25.0) | 1 (50) | – | 3 (30) |
| Problems sleeping/at night | 0 (0) | 1 (50) | – | 1 (10) |
| Worried about tripping over tubing | 1 (12.5) | 0 (0) | – | 1 (10) |
| Cannula and mask uncomfortable | 1 (12.5) | 0 (0) | – | 1 (10) |
| No reason given | 1 (12.5) | 0 (0) | – | 1 (10) |
| Withdrew from patient questionnaires | n = 0 | n = 1 | n = 0 | n = 1 |
| No reason given | 0 (0) | 1 (100) | 0 (0) | 1 (100) |
| Withdrew from nurse questionnaires | n = 0 | n = 1 | n = 1 | n = 2 |
| Did not wish to attend hospital for visits | 0 (0) | 1 (100) | 1 (100) | 2 (100) |
| Withdrew from trial | n = 1 | n = 2 | n = 1 | n = 4 |
| Did not feel study was beneficial | 1 (100) | 0 (0) | 0 (0) | 1 (25) |
| Did not want assessments/site visits | 0 (0) | 0 (0) | 1 (100) | 1 (25) |
| Had not used oxygen at all as was afraid of it, and did not want to be followed up | 0 (0) | 1 (50) | 0 (0) | 1 (25) |
| No reason given | 0 (0) | 1 (50) | 0 (0) | 1 (25) |
| Withdrawn by health-care professional | n = 1 | n = 1 | n = 0 | n = 2 |
| Patient in hospice and unwell | 0 (0) | 1 (100) | 0 (0) | 1 (50) |
| Patient incapacitated due to stroke | 1 (100) | 0 (0) | 0 (0) | 1 (50) |
Baseline participant characteristics
Completed baseline questionnaires were received from 139 (100%) randomised participants. Participant baseline characteristics are summarised by treatment group (LTOT and BMT arms only) in Tables 5 and 6.
| Characteristic | LTOT (n = 57) | BMT (n = 57) | Total (n = 114) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 40 (70.2) | 40 (70.2) | 80 (70.2) |
| Female | 17 (29.8) | 17 (29.8) | 34 (29.8) |
| Age (years), n (%) | |||
| Mean (SD) | 73.1 (10.6) | 71.4 (11.9) | 72.3 (11.3) |
| Median (min., max.) | 74.7 (51.7, 94.0) | 74.4 (38.9, 87.4) | 74.7 (38.9, 94.0) |
| Height (m) | |||
| Mean (SD) | 1.68 (0.10) | 1.68 (0.08) | 1.68 (0.09) |
| Median (min., max.) | 1.67 (1.43, 1.85) | 1.67 (1.48, 1.90) | 1.67 (1.43, 1.90) |
| Weight (kg) | |||
| Mean (SD) | 83.0 (20.7) | 84.1 (21.8) | 83.5 (21.2) |
| Median (min., max.) | 83.2 (41.5, 165.1) | 82.0 (50.0, 140.0) | 82.1 (41.5, 165.1) |
| Aetiology, n (%) | |||
| IHD | 51 (89.5) | 45 (79.0) | 96 (84.2) |
| Arrhythmia | 15 (26.3) | 21 (36.8) | 36 (31.6) |
| Dilated cardiomyopathy | 4 (7.0) | 10 (17.5) | 14 (12.3) |
| Valvular heart disease | 2 (3.5) | 3 (5.3) | 5 (4.4) |
| Amyloidosis | 0 (0.0) | 1 (1.8) | 1 (0.9) |
| Hypertension | 0 (0.0) | 1 (1.8) | 1 (0.9) |
| NYHA class, n (%) | |||
| III | 57 (100.0) | 51 (89.5) | 108 (94.7) |
| IV | 0 (0.0) | 6 (10.5) | 6 (5.3) |
| NT-proBNP (ng/l) | |||
| Mean (SD) | 5463.2 (8402.0) | 3558.4 (4026.7) | 4510.8 (6627.3) |
| Median (min., max.) | 2243 (118, 35,000) | 1931 (82, 15,594) | 2202.5 (82, 35,000) |
| Creatinine (µmol/l) | |||
| Mean (SD) | 126.0 (44.1) | 132.0 (50.2) | 129.0 (47.1) |
| Median (min., max.) | 113 (66, 252) | 117.5 (63, 235) | 114 (63, 252) |
| LV ejection fraction (%) | |||
| Mean (SD) | 28.0 (7.7) | 28.2 (8.1) | 28.1 (7.9) |
| Median (min., max.) | 29 (7, 39) | 28 (11, 50) | 28 (7, 50) |
| LV impairment, n (%) | |||
| Mild to moderate | 1 (1.8) | 0 (0.0) | 1 (0.9) |
| Moderate | 11 (19.3) | 9 (15.8) | 20 (17.5) |
| Moderate to severe | 10 (17.6) | 12 (21.1) | 22 (19.3) |
| Severe | 35 (61.4) | 36 (63.2) | 71 (62.3) |
| ACE inhibitors/ARBs, n (%) | 50 (87.7) | 48 (84.2) | 98 (86.0) |
| Beta-blockers, n (%) | 53 (93.0) | 49 (86.0) | 102 (89.5) |
| Aldosterone antagonists, n (%) | 47 (82.5) | 38 (66.7) | 85 (74.6) |
| Characteristic | LTOT (n = 57) | BMT (n = 57) | Total (n = 114) |
|---|---|---|---|
| Pulse rate (beats per minute) | |||
| Mean (SD) | 68.7 (13.0) | 70.5 (12.8) | 69.6 (12.9) |
| Median (min., max.) | 69 (18, 99) | 72 (15, 97) | 70 (15, 99) |
| Systolic blood pressure (mmHg) | |||
| Mean (SD) | 120.1 (20.8) | 116.6 (20.8) | 118.3 (20.8) |
| Median (min., max.) | 117 (78, 180) | 114 (65, 194) | 115 (65, 194) |
| Diastolic blood pressure (mmHg) | |||
| Mean (SD) | 65.1 (9.8) | 65.1 (13.3) | 65.1 (11.6) |
| Median (min., max.) | 63 (46, 100) | 63 (25, 111) | 63 (25, 111) |
| Respiratory rate (breaths per minute) | |||
| Mean (SD) | 16.6 (4.7) | 15.6 (3.4) | 16.1 (4.1) |
| Median (min., max.) | 16 (10, 36) | 16 (9, 24) | 16 (9, 36) |
| FVC (l) | |||
| Mean (SD) | 2.4 (0.8) | 2.6 (0.9) | 2.5 (0.9) |
| Median (min., max.) | 2.34 (0.89, 4.42) | 2.48 (0.73, 5.28) | 2.37 (0.73, 5.28) |
| FEV1 (l) | |||
| Mean (SD) | 1.7 (0.8) | 1.8 (0.7) | 1.8 (0.7) |
| Median (min., max.) | 1.65 (0.55, 3.49) | 1.78 (0.45, 3.80) | 1.74 (0.45, 3.80) |
| Rhythm, n (%) | |||
| Sinus rhythm | 29 (50.9) | 25 (43.9) | 54 (47.4) |
| Atrial fibrillation | 14 (24.6) | 16 (28.1) | 30 (26.3) |
| Atrial flutter | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Biventricular pacing synchronous | 12 (21.1) | 12 (21.1) | 24 (21.1) |
| Biventricular pacing asynchronous | 0 (0.0) | 3 (5.3) | 3 (2.6) |
| RV pacing synchronous | 1 (1.8) | 0 (0.0) | 1 (0.9) |
| RV pacing asynchronous | 1 (1.8) | 0 (0.0) | 1 (0.9) |
| Other | 7 (12.3) | 3 (5.3) | 10 (8.8) |
| Haemoglobin (g/l) | |||
| Mean (SD) | 129.5 (16.7) | 129.5 (19.6) | 129.5 (18.2) |
| Median (min., max.) | 128 (96, 170) | 127 (92, 179) | 127.5 (92, 179) |
The majority of patients in the study were male (n = 80, 70%) and the mean age was 72 years (range 38–94 years). The most common cause of the participants’ heart failure was ischaemic or coronary heart disease (n = 96, 84%), and the vast majority of participants were in NHYA class III (n = 108, 95%).
To be eligible for the trial, participants had to have a LVEF < 40% or be graded as at least ‘moderately’ impaired on visual inspection. LVEF was not recorded in eight participants in the LTOT or BMT arm, and all of these participants had either ‘moderate’ or ‘severe’ LV impairment. One participant with a LVEF > 40% was randomised as he or she was visually assessed as having ‘severe’ LV impairment. NT-proBNP level was not an entry criterion to the study, but the very high levels suggest that patients with severe heart failure were recruited.
In general, the two treatment groups were comparable across baseline participant characteristics; however, there was a slight imbalance in NT-proBNP level and proportion of patients taking aldosterone antagonists. The mean NT-proBNP level was higher in the LTOT arm than in the BMT arm, and the proportion of patients taking an aldosterone antagonist was greater in the LTOT arm. NT-proBNP level was pre-specified as a covariate in the primary analysis and so this imbalance was controlled for.
Primary outcome
The primary outcome was MLwHF questionnaire score at 6 months post randomisation. At baseline, a response to one item was missing in two patients and responses to two items were missing in two patients. At 3 months, among those for whom the MLwHF questionnaire was returned, there were no missing data. At 6 months, seven patients had a missing response to one item. At 12 months, one questionnaire was returned not completed, with a note to say that the patient was too unwell to complete the questionnaire; otherwise there were no missing data. At 12, 18 and 24 months, where patients returned a questionnaire, there were complete item data for the MLwHF questionnaire. Imputation of missing items was, therefore, necessary only on baseline and 6-month data. Summaries of the MLwHF questionnaire score (post imputation) by treatment group at each time point are presented in Table 7.
| Time point | Unadjusted | Adjusted | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | LTOT | BMT | Mean difference (95% CI); p-value | |||||||||
| n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | Mean (SE) | 95% CI | Mean (SE) | 95% CI | ||
| Baseline | 57 | 54.0 (18.4) | 52 (13, 95) | 57 | 54.0 (17.9) | 50 (20, 93) | 114 | 54.0 (18.1) | 52 (13, 95) | – | – | – | – | – |
| Month 3 | 53 | 45.3 (16.2) | 46 (7, 84) | 53 | 52.4 (18.2) | 52 (2, 99) | 106 | 48.5 (19.2) | 48 (2, 91) | 46.5 (1.8) | 42.9 to 50.1 | 52.0 (1.8) | 48.4 to 55.6 | –5.5 (–10.5 to –0.4); p = 0.03 |
| Month 6 | 45 | 48.1 (18.5) | 49 (2, 81) | 43 | 49.0 (20.2) | 47 (10, 91) | 88 | 48.5 (19.2) | 48 (2, 91) | 49.2 (2.4) | 44.5 to 54.0 | 49.3 (2.5) | 44.5 to 54.2 | –0.1 (–6.9 to 6.7); p = 0.98 |
| Month 12 | 21 | 48.0 (16.0) | 46 (20, 79) | 18 | 47.7 (18.8) | 51 (11, 77) | 39 | 47.8 (17.1) | 49 (11, 79) | 46.5 (2.8) | 40.9 to 52.1 | 49.1 (3.2) | 42.9 to 55.4 | –2.6 (–11.0 to 5.8); p = 0.54 |
| Month 18 | 8 | 43.4 (18.6) | 35.5 (25, 72) | 8 | 36.3 (20.4) | 30.5 (12, 72) | 16 | 39.8 (19.2) | 33 (12, 72) | – | – | – | – | – |
| Month 24 | 2 | 43.5 (13.4) | 43.5 (34, 53) | 1 | 51 | – | 3 | 46.0 (10.4) | 51 (34, 53) | – | – | – | – | – |
A covariance pattern model was used to compare MLwHF questionnaire score between the LTOT and BMT arms, adjusting for baseline MLwHF questionnaire score, age, log-NT-proBNP level, creatinine level, treatment group, time and a treatment group–time interaction. The model included all patients who provided full data for each of the included covariates, and MLwHF questionnaire outcome data at one or more post-baseline time points up to 12 months, and so was based on 102 out of 114 patients (89.5%): 51 (89.5%) in each group. Baseline characteristics of participants as included in primary analysis model are compared between the treatment arms in Table 8. It does not appear that the loss of the 12 patients for whom covariate or outcome data were missing significantly impacted on the balance between the treatment arms achieved at randomisation.
| Characteristic | LTOT (n = 51) | BMT (n = 51) | Total (n = 102) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 35 (68.6) | 36 (70.6) | 71 (69.6) |
| Female | 16 (31.4) | 15 (29.4) | 31 (30.4) |
| Age (years) | |||
| Mean (SD) | 72.0 (9.8) | 71.5 (11.9) | 71.8 (10.9) |
| Median (min., max.) | 74.1 (51.7, 91.8) | 74.9 (38.9, 86.4) | 74.5 (38.9, 91.8) |
| Height (m) | |||
| Mean (SD) | 1.68 (0.10) | 1.68 (0.08) | 1.68 (0.09) |
| Median (min., max.) | 1.68 (1.43, 1.85) | 1.67 (1.48, 1.90) | 1.67 (1.43, 1.90) |
| Weight (kg) | |||
| Mean (SD) | 83.1 (20.9) | 83.8 (21.9) | 83.5 (21.3) |
| Median (min., max.) | 84.2 (41.5, 165.1) | 82.0 (50.0, 140.0) | 82.4 (41.5, 165.1) |
| Aetiology, n (%) | |||
| IHD | 47 (92.2) | 39 (76.5) | 86 (84.3) |
| Arrhythmia | 14 (27.5) | 17 (33.3) | 31 (30.4) |
| Dilated cardiomyopathy | 4 (7.8) | 10 (19.6) | 14 (13.7) |
| Valvular heart disease | 1 (2.0) | 3 (5.9) | 4 (3.9) |
| Amyloidosis | 0 (0.0) | 1 (2.0) | 1 (1.0) |
| Hypertension | 0 (0.0) | 1 (2.0) | 1 (1.0) |
| NYHA class, n (%) | |||
| III | 51 (100.0) | 45 (88.2) | 96 (94.1) |
| IV | 0 (0.0) | 6 (11.8) | 6 (5.9) |
| NT-proBNP (ng/l) | |||
| Mean (SD) | 4483.6 (7288.2) | 3476.7 (4081.2) | 3980.1 (5898.9) |
| Median (min., max.) | 2198 (118, 35,000) | 1900 (82, 15,594) | 1915.5 (82, 35,000) |
| Creatinine (µmol/l) | |||
| Mean (SD) | 125.3 (46.1) | 134.7 (51.6) | 130.0 (48.9) |
| Median (min., max.) | 112 (66, 252) | 127 (63, 235) | 113.5 (63, 252) |
| LV ejection fraction (%) | |||
| Mean (SD) | 28.5 (7.8) | 28.7 (8.3) | 28.6 (8.0) |
| Median (min., max.) | 30 (7, 39) | 29 (11, 50) | 29.5 (7, 50) |
| LV impairment, n (%) | |||
| Mild to moderate | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Moderate | 10 (19.6) | 9 (17.7) | 19 (18.6) |
| Moderate to severe | 10 (19.6) | 11 (21.6) | 21 (20.6) |
| Severe | 30 (58.8) | 31 (60.8) | 61 (59.8) |
| ACE inhibitors/ARBs, n (%) | 44 (86.3) | 43 (84.3) | 87 (85.3) |
| Beta-blockers, n (%) | 47 (92.2) | 43 (84.3) | 90 (88.2) |
| Aldosterone antagonists, n (%) | 41 (80.4) | 35 (68.6) | 76 (74.5) |
The assumptions of the linear model were checked visually. The normality of the standardised residuals was assessed via a histogram and Q–Q plot (see Appendix 5), and the homoscedasticity of the errors was checked by plotting the residuals against the fitted values. These plots gave no reason to be concerned about the validity of the assumptions.
In total, 88 participants provided valid MLwHF questionnaire data at 6 months (LTOT n = 45; BMT n = 43); however, five of these participants did not provide data for at least one of the included baseline covariates (MLwHF questionnaire score, age, NT-proBNP level, creatinine level) so they were not included in the model. A further 19 participants (LTOT n = 8; BMT n = 11) were included in the model, as they provided valid MLwHF questionnaire data at 3 and/or 12 months, resulting in an analysed sample of 102 participants. An estimate of the treatment effect at 6 months was extracted from the model. As well as the 85 participants in the model who provided primary outcome data at 6 months, participants who provided data at 3 and/or 12 months but not at 6 months are taken into account when the treatment effect at 6 months is estimated owing to the specification of the covariance pattern between the within-patient repeated measures.
There was no evidence of a difference in MLwHF questionnaire score between the LTOT and BMT groups at 6 months (AMD –0.10, 95% CI –6.88 to 6.69; p = 0.98) (see Table 7); the LTOT group had a slightly lower MLwHF questionnaire score at 6 months, but the difference was not statistically significant. Figure 7 plots the adjusted means obtained from the model by treatment group over time.
FIGURE 7.
Adjusted means for MLwHF questionnaire score by treatment group over time from the primary analysis model.
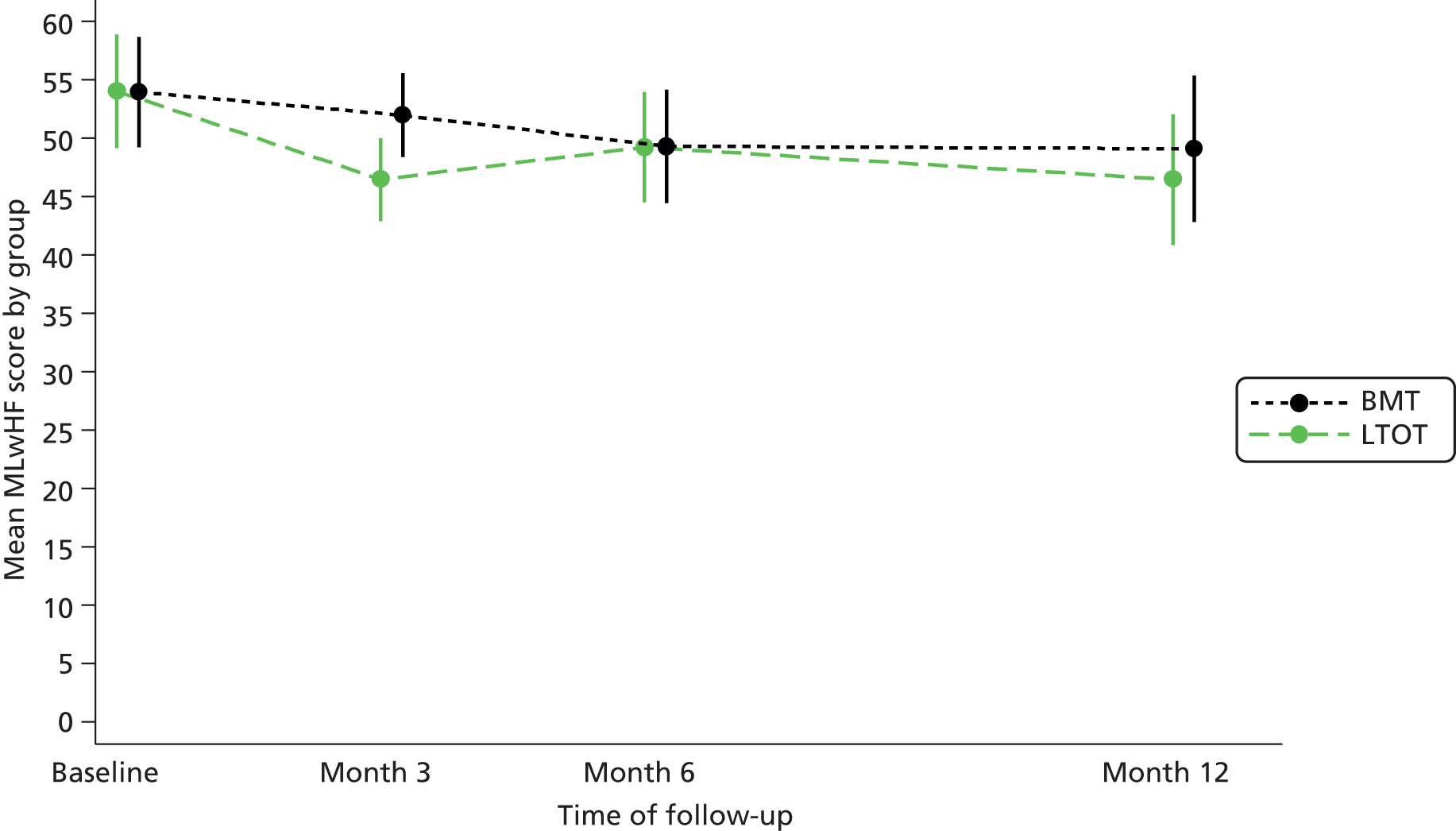
Predictors of Minnesota Living with Heart Failure questionnaire score at 6 months
Table 9 presents the coefficients from the primary analysis model. The baseline MLwHF questionnaire score, creatinine level and log-NT-proBNP level were significant predictors of the outcome MLwHF questionnaire score.
| Variable | Coefficient | SE | 95% CI | p-value |
|---|---|---|---|---|
| Baseline MLwHF questionnaire score | 0.58 | 0.08 | 0.43 to 0.73 | < 0.001 |
| Age | –0.04 | 0.12 | –0.29 to 0.20 | 0.73 |
| Creatinine | 0.06 | 0.03 | 0.01 to 0.11 | 0.02 |
| Log-NT-proBNP | –2.92 | 1.05 | –4.97 to –0.87 | 0.01 |
| Allocation | ||||
| LTOT | –5.47 | 2.58 | –10.54 to –0.41 | 0.03 |
| Time point | ||||
| 6 months | –2.63 | 2.43 | –7.39 to 2.14 | 0.28 |
| 12 months | –2.85 | 3.33 | –9.38 to 3.68 | 0.39 |
| Allocation (time point) | ||||
| LTOT (6 months) | 5.38 | 3.37 | –1.23 to 11.99 | 0.11 |
| LTOT (12 months) | 2.84 | 4.48 | –5.95 to 11.62 | 0.53 |
| Constant | 38.38 | 12.38 | 14.12 to 62.65 | 0.002 |
Minnesota Living with Heart Failure questionnaire score at 3 and 12 months
From the primary analysis model, the mean difference between groups for the outcome at 3 and 12 months was extracted. At 3 months, there was evidence to suggest that LTOT patients had a lower MLwHF questionnaire score (AMD –5.47, 95% CI –10.54 to –0.41; p = 0.03); however, this difference did not persist to 6 or 12 months (12-month AMD –2.64, 95% CI –11.02 to 5.75; p = 0.54) (see Table 3).
Minnesota Living with Heart Failure questionnaire score at 6 months adjusting for centre
Analysis was undertaken to adjust for possible correlation between MLwHF questionnaire score within a centre, by including centre as a random effect in the primary analysis model. No evidence of a difference between the LTOT and BMT arms was found at 6 months (AMD –0.21, 95% CI –6.98 to 6.57; p = 0.95) (see Table 7).
Note that an unstructured covariance pattern was used for all analyses of the MLwHF questionnaire scores.
The nocturnal oxygen therapy subgroup
Patients were recruited to the NOT arm of the trial until 30 April 2013. The baseline participant characteristics of all patients randomised up to this date are shown in Table 10 by treatment group. We conducted an exploratory analysis on the primary outcome including all three treatment arms, which included only contemporaneously recruited patients. That is, patients allocated to the NOT arm were compared against other patients randomised up to 30 April 2013. This was to ensure comparability of the treatment groups. A covariance pattern mixed model (unstructured correlation) was used to compare MLwHF questionnaire scores between the three treatment groups. The outcome modelled was total MLwHF questionnaire scores up to 12 months, and the model was adjusted for baseline MLwHF questionnaire score, age, creatinine level, log-NT-proBNP level, treatment group, time and a treatment group–time interaction. The overall treatment effect was not found to be significant [χ2 = 2.94, degrees of freedom (df) = 2; p = 0.23] so no pairwise comparisons were employed.
| Characteristic | LTOT (n = 25) | NOT (n = 25) | BMT (n = 24) | Total (n = 74) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 17 (68.0) | 22 (88.0) | 17 (70.8) | 56 (75.7) |
| Female | 8 (32.0) | 3 (12.0) | 7 (29.2) | 18 (24.3) |
| Age (years) | ||||
| Mean (SD) | 70.7 (10.9) | 75.8 (9.1) | 71.6 (11.2) | 72.7 (10.5) |
| Median (min., max.) | 73.7 (51.7, 89.7) | 77.4 (60.3, 89.9) | 73.9 (38.9, 84.5) | 74.5 (38.9, 89.9) |
| Height (m) | ||||
| Mean (SD) | 1.69 (0.09) | 1.71 (0.08) | 1.70 (0.09) | 1.70 (0.08) |
| Median (min., max.) | 1.65 (1.52, 1.82) | 1.71 (1.54, 1.83) | 1.69 (1.49, 1.90) | 1.70 (1.49, 1.90) |
| Weight (kg) | ||||
| Mean (SD) | 88.5 (21.6) | 80.4 (14.6) | 86.9 (22.6) | 85.2 (19.9) |
| Median (min., max.) | 85.5 (56.2, 165.1) | 79.3 (49.6, 105.6) | 85.5 (50.0, 128.0) | 84.6 (49.6, 165.1) |
| Aetiology, n (%) | ||||
| IHD | 23 (92.0) | 22 (88.0) | 17 (70.8) | 62 (83.8) |
| Arrhythmia | 7 (28.0) | 11 (44.0) | 9 (37.5) | 27 (36.5) |
| Dilated cardiomyopathy | 2 (8.0) | 3 (12.0) | 5 (20.8) | 10 (13.5) |
| Valvular heart disease | 0 (0.0) | 1 (4.0) | 3 (12.5) | 4 (5.4) |
| NYHA class, n (%) | ||||
| III | 25 (100.0) | 17 (68.0) | 21 (87.5) | 63 (85.1) |
| IV | 0 (0.0) | 8 (32.0) | 3 (12.5) | 11 (14.9) |
| NT-proBNP (ng/l) | ||||
| Mean (SD) | 4059.6 (6738.0) | 5671.8 (7223.5) | 4163.9 (4817.6) | 4636.9 (6342.7) |
| Median (min., max.) | 1662 (118, 28,504) | 3276 (329, 25,674) | 2035.5 (82, 15,594) | 2177 (82, 28,504) |
| Creatinine (µmol/l) | ||||
| Mean (SD) | 127.5 (46.7) | 146.3 (43.7) | 137.7 (50.4) | 137.2 (46.9) |
| Median (min., max.) | 112 (67, 213) | 148 (83, 235) | 134.5 (70, 235) | 132 (67, 235) |
| LV ejection fraction (%) | ||||
| Mean (SD) | 27.7 (6.9) | 27.8 (6.4) | 29.4 (6.1) | 28.3 (6.4) |
| Median (min., max.) | 29 (7, 37.5) | 29.5 (15, 40) | 29.5 (18, 40) | 29 (7, 40) |
| LV impairment, n (%) | ||||
| Mild to moderate | 1 (4.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| Moderate | 5 (20.0) | 3 (12.0) | 5 (20.8) | 13 (17.6) |
| Moderate to severe | 5 (20.0) | 6 (24.0) | 5 (20.8) | 16 (21.6) |
| Severe | 14 (56.0) | 16 (64.0) | 14 (58.3) | 44 (59.5) |
| ACE inhibitors/ARBs, n (%) | 22 (88.0) | 21 (84.0) | 22 (91.7) | 65 (87.8) |
| Beta-blockers, n (%) | 25 (100.0) | 22 (88.0) | 22 (91.7) | 69 (93.2) |
| Aldosterone antagonists, n (%) | 18 (72.0) | 18 (72.0) | 16 (66.7) | 52 (70.3) |
As there was a non-significant difference between the NOT and LTOT arms (contrast –5.28, 95% CI –14.17 to 3.60; p = 0.24), these arms were combined and compared against the BMT arm on the primary outcome using an analysis similar to that described for the primary analysis and including only patients randomised up to the time that the NOT arm was dropped from the study. There was no evidence of a difference between the combined HOT groups and the BMT group at 3, 6 or 12 months (Table 11).
| Time point | Unadjusted | Adjusted | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 25) | NOT (n = 25) | BMT (n = 24) | Total (n = 74) | LTOT + NOT | BMT | Mean difference (95% CI); p-value | |||||||||||
| n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | Mean (SE) | 95% CI | Mean (SE) | 95% CI | ||
| Baseline | 25 | 60.2 (18.5) | 58 (23, 95) | 25 | 61.8 (16.5) | 57 (35, 90) | 24 | 59.0 (18.5) | 55.5 (21, 93) | 74 | 60.4 (17.7) | 57 (21, 95) | – | – | – | – | – |
| Month 3 | 23 | 49.2 (17.2) | 52 (7, 84) | 20 | 53.3 (20.8) | 56.5 (7, 83) | 23 | 52.7 (18.4) | 51 (2, 86) | 66 | 51.7 (18.6) | 52 (2, 86) | –9.7 (2.4) | – | 52.7 (3.4) | – | –3.0 (–11.0 to 5.0); p = 0.46 |
| Month 6 | 21 | 49.6 (19.2) | 49 (11, 81) | 17 | 39.2 (20.9) | 37 (0, 80) | 20 | 48.9 (23.6) | 46 (10, 91) | 58 | 46.3 (21.4) | 45 (0, 91) | 45.8 (3.0) | 39.9 to 51.8 | 50.1 (4.3) | 41.6 to 58.6 | –4.3 (–14.7 to 6.1); p = 0.42 |
| Month 12 | 20 | 47.9 (16.4) | 46 (20, 79) | 17 | 38.1 (15.3) | 37 (12, 66) | 16 | 47.9 (19.4) | 51 (11, 77) | 53 | 44.8 (17.3) | 42 (11, 79) | 43.6 (2.7) | 38.4 to 48.9 | 50.7 (4.2) | 42.6 to 58.9 | –7.1 (–16.8 to 2.6); p = 0.15 |
| Month 18 | 8 | 43.4 (18.6) | 35.5 (25, 72) | 9 | 36.1 (20.7) | 37 (1, 76) | 8 | 36.3 (20.4) | 30.5 (12, 72) | 25 | 38.5 (19.4) | 35 (1, 76) | – | – | – | – | – |
| Month 24 | 2 | 43.5 (13.4) | 43.5 (34, 53) | 2 | 65.0 (1.4) | 65 (64, 66) | 1 | 51 | – | 5 | 53.6 (12.8) | 53 (34, 66) | – | – | – | – | – |
Secondary outcomes
For each of the following models, assumptions were checked in the same way as for the primary analysis and no significant violations were observed.
Epworth Sleepiness Scale
Summaries of the observed ESS score by treatment group across all time points are presented in Table 12. A lower score indicates a lower general level of daytime somnolence. There was no evidence of a difference in mean score between the treatment groups at 6 months (AMD –0.85, 95% CI –2.41 to 0.71; p = 0.28). Participants in the LTOT group were predicted to have a slightly lower ESS score at 6 months, but not statistically significantly so. A banded (1) covariance structure was used in this model.
| Time point | Unadjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |||||||
| n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | |
| Baseline | 57 | 10.5 (5.3) | 10 (3, 24) | 56 | 9.9 (4.9) | 10 (0, 22) | 113 | 10.2 (5.1) | 10 (0, 24) |
| Month 3 | 53 | 10.1 (5.2) | 10 (1, 24) | 53 | 10.5 (5.7) | 10 (0, 24) | 106 | 10.3 (5.4) | 10 (0, 24) |
| Month 6 | 43 | 9.9 (4.9) | 10 (0, 18) | 43 | 10.1 (4.6) | 11 (1, 22) | 86 | 10.0 (4.7) | 10 (0, 22) |
| Month 12 | 21 | 9.4 (6.2) | 8 (1, 23) | 18 | 8.7 (4.6) | 9 (2, 20) | 39 | 9.1 (5.5) | 9 (1, 23) |
| Month 18 | 8 | 7.0 (6.0) | 5 (2, 21) | 8 | 10.6 (5.3) | 10.5 (3, 21) | 16 | 8.8 (5.8) | 7.5 (2, 21) |
| Month 24 | 2 | 12.0 (4.2) | 12 (9, 15) | 1 | 4 | – | 3 | 9.3 (5.5) | 9 (4, 15) |
Numerical Rating Scale for breathlessness
Participants were asked to score six questions relating to their breathlessness using a NRS at baseline and at 3, 6, 12, 18 and 24 months. Summaries of observed scores are presented in Table 13. The scores for each question were compared between the two treatment groups using a covariance pattern model with an exchangeable covariance pattern.
| Time point | Unadjusted | |||||
|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | ||||
| n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | |
| Q1 | ||||||
| Baseline | 57 | 5 (4–7) | 57 | 5 (3–7) | 114 | 5 (4–7) |
| Month 3 | 53 | 4 (2–5) | 53 | 5 (3–6) | 106 | 5 (3–6) |
| Month 6 | 45 | 5 (2–6) | 43 | 5 (3–7) | 88 | 5 (3–7) |
| Month 12 | 21 | 5 (4–8) | 18 | 6 (4–7) | 39 | 6 (4–7) |
| Month 18 | 8 | 5 (5–5) | 8 | 2 (0–6) | 16 | 5 (2–5) |
| Month 24 | 2 | 4.5 (3–6) | 1 | 2 | 3 | 3 (2–6) |
| Q2 | ||||||
| Baseline | 57 | 6 (5–8) | 57 | 5 (4–7) | 114 | 5.5 (4–7) |
| Month 3 | 53 | 5 (2–6) | 53 | 5 (4–8) | 106 | 5 (3–7) |
| Month 6 | 45 | 6 (3–8) | 43 | 6 (4–7) | 88 | 6 (4–8) |
| Month 12 | 21 | 7 (4–8) | 18 | 6 (4–7) | 39 | 6 (4–8) |
| Month 18 | 8 | 6 (5–7.5) | 8 | 3 (0–6) | 16 | 5 (3–7) |
| Month 24 | 2 | 4 (1–7) | 1 | 2 | 3 | 2 (1–7) |
| Q3 | ||||||
| Baseline | 57 | 3 (1–4) | 57 | 2 (1–5) | 114 | 3 (1–5) |
| Month 3 | 53 | 2 (0–4) | 53 | 2 (1–4) | 106 | 2 (1–4) |
| Month 6 | 45 | 3 (1–5) | 43 | 2 (0–3) | 88 | 2 (1–4.5) |
| Month 12 | 21 | 2 (1–6) | 18 | 2 (1–4) | 39 | 2 (1–6) |
| Month 18 | 8 | 2 (1–5) | 8 | 1 (0.5–3) | 16 | 1.5 (1–4.5) |
| Month 24 | 2 | 1.5 (1–2) | 1 | 0 | 3 | 1 (0–2) |
| Q4 | ||||||
| Baseline | 57 | 5 (2–7) | 56 | 4 (1.5–6) | 113 | 5 (2–7) |
| Month 3 | 53 | 2 (0–5) | 53 | 5 (3–6) | 106 | 4 (1–5) |
| Month 6 | 45 | 4 (2–7) | 43 | 4 (1–5) | 88 | 4 (1–6) |
| Month 12 | 21 | 5 (2–8) | 18 | 5 (4–7) | 39 | 5 (2–7) |
| Month 18 | 8 | 4 (0.5–5) | 8 | 2.5 (0–7.5) | 16 | 4 (0–5) |
| Month 24 | 2 | 2 (0–4) | 1 | 9 | 3 | 4 (0–9) |
| Q5 | ||||||
| Baseline | 57 | 7 (5–9) | 56 | 7 (5–9) | 113 | 7 (5–9) |
| Month 3 | 53 | 8 (4–9) | 53 | 5 (5–7) | 106 | 6 (5–9) |
| Month 6 | 45 | 7 (5–9) | 43 | 5 (5–9) | 88 | 6.5 (5–9) |
| Month 12 | 21 | 7 (5–8) | 18 | 6 (5–9) | 39 | 7 (5–9) |
| Month 18 | 8 | 6 (5–10) | 8 | 9 (7–10) | 16 | 7.5 (5–10) |
| Month 24 | 2 | 9 (8–10) | 1 | 10 | 3 | 10 (8–10) |
| Q6 | ||||||
| Baseline | 57 | 7 (5–10) | 57 | 8 (5–10) | 114 | 7.5 (5–10) |
| Month 3 | 53 | 9 (6–10) | 53 | 7 (5–10) | 106 | 8 (5–10) |
| Month 6 | 45 | 8 (6–10) | 43 | 7 (5–9) | 88 | 8 (5–10) |
| Month 12 | 21 | 8 (7–9) | 18 | 8 (5–10) | 39 | 8 (5–10) |
| Month 18 | 8 | 10 (8.5–10) | 8 | 10 (8–10) | 16 | 10 (8–10) |
| Month 24 | 2 | 9 (8–10) | 1 | 10 | 3 | 10 (8–10) |
Q1: How bad has your breathlessness felt on average over the past 24 hours?
Breathlessness was scored from 0 (not breathless at all) to 10 (the worst imaginable breathlessness). There was no evidence of a difference in score at 6 months (AMD –0.63, 95% CI –1.57 to 0.31; p = 0.19).
Q2: What is the worst that your breathlessness has been over the past 24 hours?
Breathlessness was scored from 0 (not breathless at all) to 10 (the worse imaginable breathlessness). There was no evidence of a difference in score at 6 months (AMD –0.16, 95% CI –1.25 to 0.94; p = 0.78).
Q3: How bad is your breathlessness right now?
Breathlessness was scored from 0 (not breathless at all) to 10 (the worse imaginable breathlessness). There was no evidence of a difference in score at 6 months (AMD 0.75, 95% CI –0.18 to 1.68; p = 0.12).
Q4: How much distress has your breathlessness caused you on average over the past 24 hours?
Distress was scored from 0 (no distress at all) to 10 (the worse imaginable distress). There was no evidence of a difference in score at 6 months (AMD –0.07, 95% CI –1.20 to 1.06; p = 0.90).
Q5: How well have you coped with your breathlessness on average over the past 24 hours?
Coping was scored from 0 (I have not coped at all) to 10 (I have coped very well). There was no evidence of a difference in score at 6 months (AMD 0.79, 95% CI –0.34 to 1.93; p = 0.17).
Q6: How satisfied have you felt with the treatment you have received for your breathlessness?
Satisfaction was scored from 0 (not satisfied at all) to 10 (completely satisfied). There was no evidence of a difference in score at 6 months (AMD 0.69, 95% CI –0.39 to 1.78; p = 0.21).
Hospital Anxiety and Depression Scale
The HADS was measured at baseline and at 6, 12, 18 and 24 months. The raw summary scores for the anxiety and depression subscales are summarised in Tables 14 and 15. The anxiety and depression subscale scores were compared between the two groups using separate covariance pattern models both using an exchangeable covariance pattern. No evidence of a difference was observed at 6 months in anxiety score (AMD –0.19, 95% CI –1.47 to 1.10; p = 0.77) or depression score (AMD –0.34, 95% CI –1.49 to 0.81; p = 0.56).
| Time point | Unadjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |||||||
| n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | |
| Baseline | 57 | 7.8 (4.6) | 7 (0, 20) | 57 | 7.2 (4.5) | 7 (0, 18) | 114 | 7.5 (4.5) | 7 (0, 20) |
| Month 6 | 45 | 7.0 (4.5) | 7 (0, 16) | 43 | 6.5 (4.6) | 6 (0, 16) | 88 | 6.8 (4.6) | 6 (0, 16) |
| Month 12 | 21 | 6.0 (4.2) | 5 (0, 13) | 18 | 4.9 (3.6) | 3 (0, 10) | 39 | 5.5 (3.9) | 4 (0, 13) |
| Month 18 | 8 | 3.2 (1.6) | 3.3 (0, 5) | 8 | 2.9 (4.6) | 2 (0, 14) | 16 | 3.0 (3.4) | 2.5 (0, 14) |
| Month 24 | 2 | 1.5 (0.7) | 1.5 (1, 2) | 1 | 3 | – | 3 | 2.0 (1.0) | 2 (1, 3) |
| Time point | Unadjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |||||||
| n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | |
| Baseline | 57 | 7.8 (3.5) | 7 (2, 17) | 57 | 7.5 (3.6) | 7 (1, 20) | 114 | 7.7 (3.5) | 7 (1, 20) |
| Month 6 | 45 | 7.3 (3.3) | 7 (1, 15) | 43 | 7.7 (3.5) | 7 (1, 16) | 88 | 7.5 (3.4) | 7 (1, 16) |
| Month 12 | 21 | 7.0 (3.2) | 7 (1, 13) | 18 | 7.7 (4.4) | 7 (1, 18) | 39 | 7.3 (3.8) | 7 (1, 18) |
| Month 18 | 8 | 6.5 (2.2) | 6.5 (3, 9) | 8 | 5.6 (2.5) | 6.5 (1, 8) | 16 | 6.1 (2.3) | 6.5 (1, 9) |
| Month 24 | 2 | 5.0 (5.7) | 5 (1, 9) | 1 | 6 | – | 3 | 5.3 (4.0) | 6 (1, 9) |
Karnofsky Performance Status scale of physical activity
Karnofsky Performance Status is measured from 0% (death) to 100% (normal, no complaints, no signs of disease). Baseline data for the distribution of KPS scores are shown in Tables 16 and 17. A score of 0 was given to patients for the time points after which they were known to have died. No significant difference was observed between the treatment groups at 6 months (AMD 4.97, 95% CI –2.18 to 12.13; p = 0.17). An unstructured covariance structure was used in this model. The KPS score can be categorised as follows:
-
0–40 – unable to care for self
-
50–70 – some assistance needed
-
80–100 – no special care needed.
| Distribution of KPS score (%) | LTOT (N = 57), n (%) | BMT (N = 57), n (%) | Total (N = 114), n (%) |
|---|---|---|---|
| All patients | |||
| 100 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 90 | 0 (0.0) | 1 (1.8) | 1 (0.9) |
| 80 | 5 (8.8) | 4 (7.0) | 9 (7.9) |
| 70 | 12 (21.1) | 11 (19.3) | 23 (20.2) |
| 60 | 28 (49.1) | 25 (43.9) | 53 (46.5) |
| 50 | 11 (19.3) | 13 (22.8) | 24 (21.1) |
| 40 | 1 (1.8) | 3 (5.3) | 4 (3.5) |
| 30 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 20 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Patients in primary analysis | |||
| 100 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 90 | 0 (0.0) | 1 (2.0) | 1 (1.0) |
| 80 | 4 (7.8) | 3 (5.9) | 7 (6.9) |
| 70 | 12 (23.5) | 10 (19.6) | 22 (21.6) |
| 60 | 26 (51.0) | 22 (43.1) | 48 (47.1) |
| 50 | 9 (17.7) | 12 (23.5) | 21 (20.6) |
| 40 | 0 (0.0) | 3 (5.9) | 3 (2.9) |
| 30 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 20 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Distribution of KPS score (%) | LTOT (N = 25), n (%) | NOT (N = 25), n (%) | BMT (N = 24), n (%) | Total (N = 114), n (%) |
|---|---|---|---|---|
| 100 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 90 | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (1.4) |
| 80 | 2 (8.0) | 1 (4.0) | 2 (8.3) | 5 (6.8) |
| 70 | 7 (28.0) | 4 (16.0) | 4 (16.7) | 15 (20.3) |
| 60 | 12 (48.0) | 10 (40.0) | 10 (41.7) | 32 (43.2) |
| 50 | 3 (12.0) | 8 (32.0) | 5 (20.8) | 16 (21.6) |
| 40 | 1 (4.0) | 1 (4.0) | 2 (8.3) | 4 (5.4) |
| 30 | 0 (0.0) | 1 (4.0) | 0 (0.0) | 1 (1.4) |
| 20 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
The categorised data are shown in Table 18. There was no notable difference in the distribution of scores at each time point between the trial arms.
| KPS summary | LTOT (n = 57) | BMT (n = 57) | Total (n = 114) |
|---|---|---|---|
| Baseline | |||
| Dead, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unable to care for self, n (%) | 1 (1.8) | 3 (5.3) | 4 (3.5) |
| Some assistance needed, n (%) | 51 (89.5) | 49 (86.0) | 100 (87.7) |
| No special care needed, n (%) | 5 (8.8) | 5 (8.8) | 10 (8.8) |
| Median (min., max.) | 60 (40, 80) | 60 (40, 90) | 60 (40, 90) |
| Month 3 | |||
| Dead, n (%) | 3 (5.4) | 3 (5.4) | 6 (5.4) |
| Unable to care for self, n (%) | 2 (3.6) | 5 (8.9) | 7 (6.3) |
| Some assistance needed, n (%) | 46 (82.1) | 43 (76.8) | 89 (79.5) |
| No special care needed, n (%) | 5 (8.9) | 5 (8.9) | 10 (8.9) |
| Median (min., max.) | 60 (0, 90) | 60 (0, 80) | 60 (0, 90) |
| Month 6 | |||
| Dead, n (%) | 4 (8.2) | 6 (12.5) | 10 (10.3) |
| Unable to care for self, n (%) | 2 (4.1) | 3 (6.3) | 5 (5.2) |
| Some assistance needed, n (%) | 36 (73.5) | 35 (72.9) | 71 (73.2) |
| No special care needed, n (%) | 7 (14.3) | 4 (8.3) | 11 (11.3) |
| Median (min., max.) | 60 (0, 80) | 60 (0, 80) | 60 (0, 80) |
| Month 12 | |||
| Dead, n (%) | 6 (22.2) | 11 (37.9) | 17 (30.4) |
| Unable to care for self, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Some assistance needed, n (%) | 18 (66.7) | 17 (58.6) | 35 (62.5) |
| No special care needed, n (%) | 3 (11.1) | 1 (3.5) | 4 (7.1) |
| Median (min., max.) | 60 (0, 80) | 60 (0, 80) | 60 (0, 80) |
| Month 18 | |||
| Dead, n (%) | 6 (40.0) | 12 (60.0) | 18 (51.4) |
| Unable to care for self, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Some assistance needed, n (%) | 9 (60.0) | 7 (35.0) | 16 (45.7) |
| No special care needed, n (%) | 0 (0.0) | 1 (5.0) | 1 (2.9) |
| Median (min., max.) | 60 (0, 70) | 0 (0, 80) | 0 (0, 80) |
| Month 24 | |||
| Dead, n (%) | 6 (75.0) | 12 (92.3) | 18 (85.7) |
| Unable to care for self, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Some assistance needed, n (%) | 1 (12.5) | 1 (7.7) | 2 (9.5) |
| No special care needed, n (%) | 1 (12.5) | 0 (0.0) | 1 (4.8) |
| Median (min., max.) | 0 (0, 80) | 0 (0, 60) | 0 (0, 80) |
Charlson Comorbidity Index
The CCI was assessed at baseline and at 6, 12, 18 and 24 months (a lower score is preferable). The observed CCI scores are summarised descriptively by treatment group at each time point in Table 19. There was weak evidence of a difference between the treatment groups in CCI score at 6 months (AMD 0.45, 95% CI –0.01 to 0.91; p = 0.06), that is the BMT group were predicted as having a lower CCI score, but not statistically significantly so. An exchangeable covariance structure was used in this model.
| Time point | Unadjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |||||||
| n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | |
| Baseline | 57 | 6.7 (2.0) | 6 (3, 11) | 57 | 7.0 (2.3) | 7 (1, 12) | 114 | 6.8 (2.1) | 7 (1, 12) |
| Month 6 | 45 | 6.9 (2.2) | 7 (3, 15) | 43 | 6.9 (2.1) | 7 (2, 12) | 88 | 6.9 (2.1) | 7 (2, 15) |
| Month 12 | 22 | 6.4 (1.9) | 6 (3, 11) | 18 | 7.7 (1.7) | 8 (5, 11) | 40 | 7.0 (1.9) | 7 (3, 11) |
| Month 18 | 9 | 6.3 (2.3) | 6 (4, 11) | 9 | 7.2 (1.8) | 6 (6, 11) | 18 | 6.8 (2.0) | 6 (4, 11) |
| Month 24 | 2 | 5.5 (2.1) | 5.5 (4, 7) | 1 | 5 | – | 3 | 5.3 (1.5) | 5 (4, 7) |
The 6-minute walk test
The distance a patient walked in metres over a 6-minute time period was recorded at baseline and at 6, 12, 18 and 24 months (Table 20). The distance walked was compared between the LTOT and BMT arms using a covariance pattern model with an unstructured correlation. No evidence of a difference in distance walked was observed between the two groups at 6 months (AMD 0.64, 95% CI –34.54 to 35.83; p = 0.97).
| Time point | Unadjusted | |||||
|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | ||||
| n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | |
| Baseline | 55 | 130 (54 –210) | 56 | 100 (52.5–196) | 111 | 120 (54–210) |
| Month 6 | 41 | 120 (60–240) | 33 | 156 (60–250) | 74 | 123 (60–248) |
| Month 12 | 18 | 180 (90–230) | 13 | 200 (50–245) | 31 | 195 (70–245) |
| Month 18 | 6 | 184.5 (140–305) | 6 | 162.5 (70–340) | 12 | 184.5 (100–307.5) |
| Month 24 | 1 | 450 | 0 | – | 1 | 450 |
Prevalence of hypoxia
The arterial oxygen saturation measured as part of the 6MWT protocol (i.e. at rest, during peak exercise and then during recovery from exercise) is shown in Table 21. The prevalences of oxygen saturation in the ranges < 90%, 90% to < 95% and ≥ 95% at rest, at peak and 5 minutes after the 6MWT are summarised Tables 22, 23 and 24, respectively.
| Time point | LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |||
|---|---|---|---|---|---|---|
| n | Median (min., max.) | n | Median (min., max.) | n | Median (min., max.) | |
| SaO2 at rest (%) | ||||||
| Baseline | 55 | 96 (91, 99) | 57 | 97 (92, 99) | 112 | 97 (91, 99) |
| Month 6 | 41 | 96 (92, 99) | 33 | 97 (91, 99) | 74 | 96 (91, 99) |
| Month 12 | 19 | 96 (93, 100) | 14 | 96.5 (95, 100) | 33 | 96 (93, 100) |
| Month 18 | 6 | 96 (93, 98) | 6 | 96 (95, 98) | 12 | 96 (93, 98) |
| Month 24 | 1 | 97 | 0 | – | 1 | 97 |
| SaO2 at peak (%) | ||||||
| Baseline | 51 | 97 (90, 100) | 52 | 97 (89, 100) | 103 | 97 (89, 100) |
| Month 6 | 38 | 96 (78, 100) | 32 | 97 (89, 100) | 70 | 97 (78, 100) |
| Month 12 | 18 | 95 (93, 100) | 14 | 97.5 (93, 100) | 32 | 96.5 (93, 100) |
| Month 18 | 6 | 96 (94, 100) | 6 | 97 (96, 99) | 12 | 96.5 (94, 100) |
| Month 24 | 1 | 99 | 0 | – | 1 | 99 |
| SaO2 post test (%) | ||||||
| Baseline | 55 | 97 (91, 100) | 56 | 97 (91, 100) | 111 | 97 (91, 100) |
| Month 6 | 40 | 96 (92, 100) | 33 | 97 (92, 100) | 73 | 97 (92, 100) |
| Month 12 | 18 | 96 (89, 100) | 14 | 97 (95, 100) | 32 | 97 (89, 100) |
| Month 18 | 6 | 96 (94, 98) | 6 | 96 (94, 99) | 12 | 96 (94, 99) |
| Month 24 | 1 | 97 | 0 | – | 1 | 97 |
| Oxygen saturation before 6MWT | Unadjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |||||||
| < 90%, n (%) | 90% to < 95%, n (%) | ≥ 95%, n (%) | < 90%, n (%) | 90% to < 95%, n (%) | ≥ 95%, n (%) | < 90%, n (%) | 90% to < 95%, n (%) | ≥ 95%, n (%) | |
| Baseline | 0 (0.0) | 10 (18.2) | 45 (81.8) | 0 (0.0) | 3 (5.3) | 54 (94.7) | 0 (0.0) | 13 (11.6) | 99 (88.4) |
| Month 6 | 0 (0.0) | 13 (31.7) | 28 (68.3) | 0 (0.0) | 2 (6.1) | 31 (93.9) | 0 (0.0) | 15 (20.3) | 59 (79.7) |
| Month 12 | 0 (0.0) | 2 (10.5) | 17 (89.5) | 0 (0.0) | 0 (0.0) | 14 (100.0) | 0 (0.0) | 2 (6.1) | 31 (93.9) |
| Month 18 | 0 (0.0) | 1 (16.7) | 5 (83.3) | 0 (0.0) | 0 (0.0) | 6 (100.0) | 0 (0.0) | 1 (8.3) | 11 (91.7) |
| Month 24 | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Time point | Unadjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |||||||
| < 90%, n (%) | 90% to < 95%, n (%) | ≥ 95%, n (%) | < 90%, n (%) | 90% to < 95%, n (%) | ≥ 95%, n (%) | < 90%, n (%) | 90% to < 95%, n (%) | ≥ 95%, n (%) | |
| Baseline | 0 (0.0) | 12 (23.5) | 39 (76.5) | 1 (1.9) | 6 (11.5) | 45 (86.5) | 1 (1.0) | 18 (17.5) | 39 (76.5) |
| Month 6 | 2 (5.3) | 8 (21.1) | 28 (73.7) | 1 (3.1) | 3 (9.4) | 28 (87.5) | 3 (4.3) | 11 (15.7) | 56 (80.0) |
| Month 12 | 0 (0.0) | 4 (22.2) | 14 (77.8) | 0 (0.0) | 1 (7.1) | 13 (92.9) | 0 (0.0) | 5 (15.6) | 27 (84.4) |
| Month 18 | 0 (0.0) | 1 (16.7) | 5 (83.3) | 0 (0.0) | 0 (0.0) | 6 (100.0) | 0 (0.0) | 1 (8.3) | 11 (91.7) |
| Month 24 | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Time point | Unadjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |||||||
| < 90%, n (%) | 90% to < 95%, n (%) | ≥ 95%, n (%) | < 90%, n (%) | 90% to < 95%, n (%) | ≥ 95%, n (%) | < 90%, n (%) | 90% to < 95%, n (%) | ≥ 95%, n (%) | |
| Baseline | 0 (0.0) | 7 (12.7) | 48 (87.3) | 0 (0.0) | 7 (12.5) | 49 (87.5) | 0 (0.0) | 14 (12.6) | 97 (87.4) |
| Month 6 | 0 (0.0) | 9 (22.5) | 31 (77.5) | 0 (0.0) | 3 (9.1) | 30 (90.9) | 0 (0.0) | 12 (16.4) | 61 (83.6) |
| Month 12 | 1 (5.6) | 1 (5.6) | 16 (88.9) | 0 (0.0) | 0 (0.0) | 14 (100.0) | 1 (3.1) | 1 (3.1) | 30 (93.8) |
| Month 18 | 0 (0.0) | 1 (16.7) | 5 (83.3) | 0 (0.0) | 1 (16.7) | 5 (83.3) | 0 (0.0) | 2 (16.7) | 10 (83.3) |
| Month 24 | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
N-terminal pro-B-type natriuretic hormone
Patients’ NT-proBNP level was measured at baseline and at 3, 6, 12, 18 and 24 months. The NT-proBNP levels are summarised descriptively by treatment group at each time point in Table 25. There was a wide range of NT-proBNP levels at all time points; for example, at baseline, the range was 82–35,000. NT-proBNP level was compared between the two treatment groups at 6 months with a covariance pattern model, using an unstructured correlation, adjusting for baseline level. NT-proBNP level data were highly positively skewed at all time points and so were log transformed. There was no evidence of a difference between the treatment groups at 6 months (p = 0.80). As the outcome was log transformed, interpretation of the estimated difference between the two treatment groups is a little more difficult. The LTOT group was predicted to have a decrease in log-NT-proBNP of 0.04 (95% CI –0.31 to 0.24) or, in other words, patients in the LTOT group were expected to have a NT-proBNP level 0.96 times that of a BMT patient (95% CI 0.73 to 1.27).
| Time point | Unadjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |||||||
| n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | n | Mean (SD) | Median (min., max.) | |
| Baseline | 55 | 5463.2 (8402.0) | 2243 (118, 35,000) | 55 | 3558.4 (4026.7) | 1931 (82, 15,594) | 110 | 4510.8 (6627.3) | 2202.5 (82, 35,000) |
| Month 3 | 47 | 4198.0 (6965.9) | 1713 (138, 35,000) | 47 | 3802.2 (5714.9) | 1984 (25.4, 29,331) | 94 | 4000.1 (6340.0) | 1921.5 (25.4, 35,000) |
| Month 6 | 41 | 4089.5 (7507.4) | 1916 (94, 35,000) | 38 | 2969.6 (5401.1) | 1554 (38.3, 32,621) | 79 | 3550.8 (6561.9) | 1608 (38.3, 35,000) |
| Month 12 | 22 | 3531.6 (7358.9) | 1622 (91, 35,000) | 17 | 2689.3 (3297.3) | 1803 (140.4, 14,208) | 39 | 3164.4 (5889.3) | 1670.85 (91, 35,000) |
| Month 18 | 8 | 1787.9 (1559.1) | 1416.3 (264, 4639) | 7 | 2618.3 (31,78.5) | 1650 (222, 9367) | 15 | 2175.4 (2393.5) | 1650 (222, 9367) |
| Month 24 | 1 | 3515 | – | 0 | – | – | 1 | 3515 | – |
Left ventricular ejection fraction
Patients had an echocardiogram at baseline and at 12 and 24 months. Where possible, the LVEF was calculated and expressed as a percentage. To be eligible for the HOT trial, participants had to have a LVEF < 40% or have at least ‘moderate’ LV impairment. LVEF and LV impairment at the three time points are summarised in Table 26. One participant had a LVEF above 40% at baseline; however, this participant had LV impairment graded ‘severe’ and was thus still eligible for the trial. Only one patient at 12 months had a LVEF about 40%. At 24 months, echocardiography findings were available for only two participants, both in the LTOT group. These two patients were graded as having ‘severe’ LV impairment, but the LVEF could be calculated for only one patient (a value of 25).
| Time point | Unadjusted | ||
|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |
| Baseline | |||
| LVEF (%) | |||
| n | 48 | 57 | 105 |
| Mean (SD) | 28.0 (7.7) | 28.2 (8.1) | 28.1 (7.9) |
| Median (min., max.) | 29 (7, 39) | 28 (11, 50) | 28 (7, 50) |
| Severity of LV dysfunction, n (%) | |||
| Mild | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mild to moderate | 1 (1.8) | 0 (0.0) | 1 (0.9) |
| Moderate | 11 (19.3) | 9 (15.8) | 20 (17.5) |
| Moderate to severe | 10 (17.5) | 12 (21.1) | 22 (19.3) |
| Severe | 35 (61.4) | 36 (63.2) | 71 (62.3) |
| Month 12 | |||
| LVEF (%) | |||
| n | 18 | 13 | 31 |
| Mean (SD) | 30.5 (7.5) | 25.2 (9.1) | 28.3 (8.5) |
| Median (min., max.) | 30 (17, 47.5) | 23 (8, 38) | 30 (8, 47.5) |
| Severity of LV dysfunction, n (%) | |||
| Mild | 0 (0.0) | 1 (6.3) | 1 (2.8) |
| Mild to moderate | 1 (5.0) | 0 (0.0) | 1 (2.8) |
| Moderate | 4 (20.0) | 2 (12.5) | 6 (16.7) |
| Moderate to severe | 3 (15.0) | 1 (6.3) | 4 (11.1) |
| Severe | 12 (60.0) | 12 (75.0) | 24 (66.7) |
| Month 24 | |||
| LVEF (%) | |||
| n | 1 | 0 | 1 |
| Mean (SD) | 25 | – | 25 |
| Median (min., max.) | – | – | – |
| Severity of LV dysfunction, n (%) | |||
| Mild | 0 (0.0) | – | 0 (0.0) |
| Mild to moderate | 0 (0.0) | – | 0 (0.0) |
| Moderate | 0 (0.0) | – | 0 (0.0) |
| Moderate to severe | 0 (0.0) | – | 0 (0.0) |
| Severe | 2 (100.0) | – | 2 (100.0) |
Mortality
At 6 months, 15 patients had died (LTOT n = 4, NOT n = 5 and BMT n = 6). A further 10 deaths were reported after 6 months of follow-up (LTOT n = 2, NOT n = 2 and BMT n = 6). Mortality was analysed as a time-to-event outcome. The main analysis compared the BMT and LTOT treatment groups. For each group, the distribution of time from randomisation to death is described using Kaplan–Meier survival curves (Figure 8).
FIGURE 8.
Kaplan–Meier survival curve.

Unadjusted Cox regression gave a HR of 2.03 (95% CI 0.76 to 5.40) for BMT relative to LTOT, but this was not statistically significant (p = 0.16). Adjusting for baseline CCI score, the HR was slightly lower (1.84, 95% CI 0.68 to 4.96; p = 0.30), indicating, again, that the risk of death was higher in the BMT group than in the LTOT group, but the difference was not statistically significant (p = 0.30). The CCI was not a significant predictor in the survival model (p = 0.19)
Additional survival analyses were undertaken comparing the NOT and LTOT arms only including patients randomised up to the time that the NOT arm was dropped from the study.
The number of patients recruited to the LTOT, NOT and BMT arms was 25, 25 and 24, respectively (recruited up to the end of April 2013). There was no significant difference in survival between the groups (Kaplan–Meier, χ2 = 2.07, df = 1; p = 0.15). The oxygen arms were combined and compared against the BMT arm including only those patients randomised up to the time that the NOT arm was dropped. Figure 9 shows the survival curve for BMT against LTOT and NOT. There was no significant difference in survival between the groups (Kaplan–Meier, χ2 = 1.12, df = 1; p = 0.29). The 75th percentile for the BMT group was 269.0 days (SE 79.3 days) and for the combined LTOT and NOT group it was 655.0 days (SE 0.98 days). Unadjusted Cox regression gave a HR of 1.64 (95% CI 0.65 to 4.17), indicating that the risk of death was higher in the BMT group than in the LTOT and NOT groups, but the difference was not statistically significant (p = 0.30). The model adjusting for baseline CCI score gave very similar estimates and CCI was not a significant predictor of mortality.
FIGURE 9.
Kaplan–Meier survival curve for BMT vs. LTOT plus NOT.
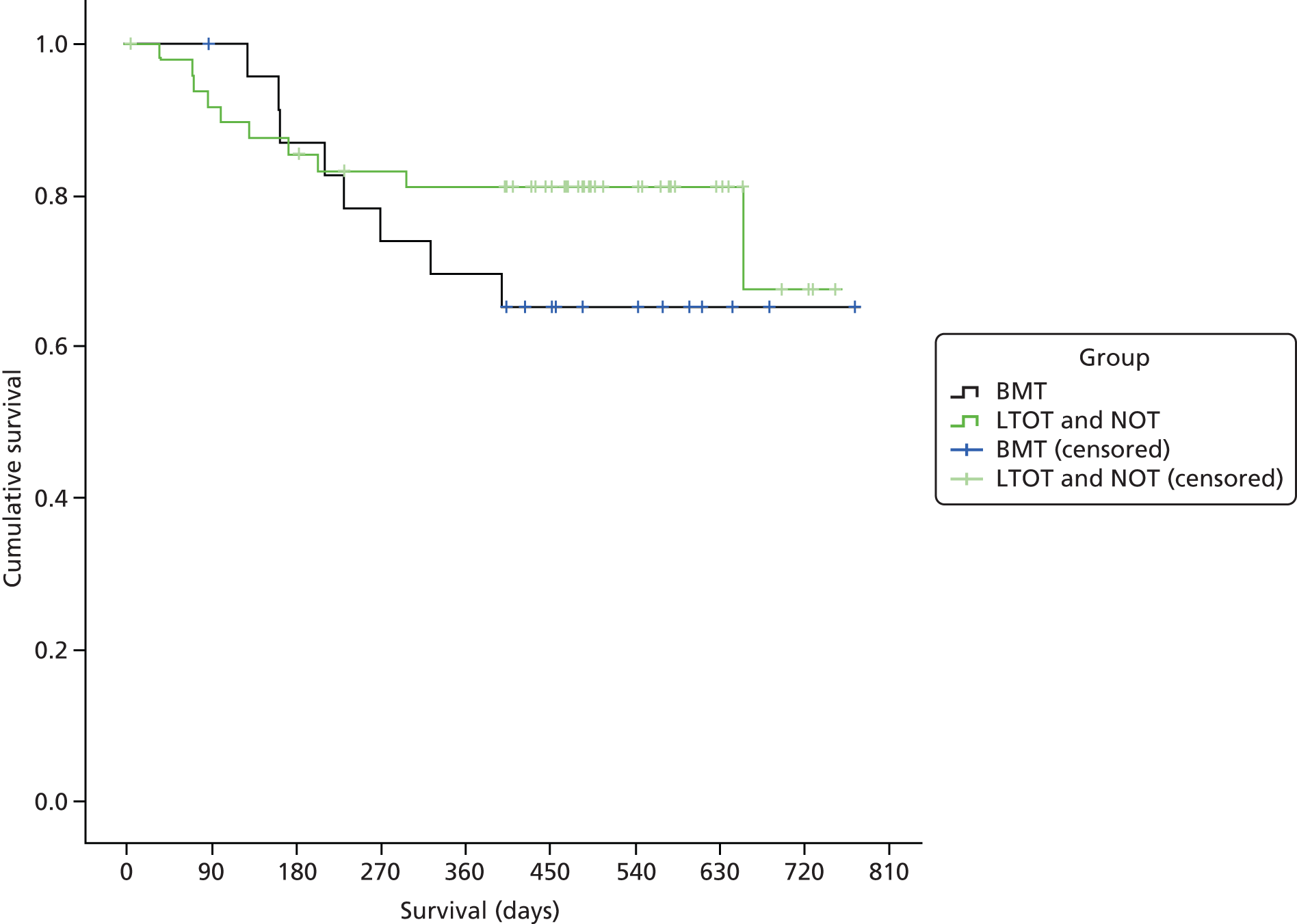
The proportional hazards assumption was checked using log–log plots of the estimated survivor function, plotting scaled Schoenfeld residuals and by the Grambsch and Therneau test. Visual inspection of the log–log and Schoenfeld plots indicated potential non-proportionality of the treatment group variable in each model; however, the Grambsch and Therneau test did not indicate evidence of non-proportionality.
Number of days alive and out of hospital
The number of DAOH was calculated for each patient. Summaries of the number and percentage of days the patients in the groups were alive and out of hospital are presented in Table 27.
| Number of DAOH | BMT | LTOT | NOT | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Number of days | % | Number of days | % | Number of days | % | Number of days | % | |
| Mean (SD) | 312.8 (171.1) | 85.4 (27.7) | 329.0 (182.0) | 89.8 (25.6) | 415.0 (236.6) | 74.3 (38.2) | 337.8 (190.8) | 85.2 (29.3) |
| Min., max. | 21, 773 | 13, 100 | 15, 752 | 4, 100 | 3, 730 | 1, 100 | 3, 773 | 1, 100 |
European Quality of Life-5 Dimensions
The primary objective of the HOT trial was to assess the HRQoL benefits of HOT in the management of patients with stable CHF who are still severely symptomatic despite maximally tolerated medical therapy. A preference-based measure of health status is the EQ-5D instrument (EQ-5D-3L). The EQ-5D is a descriptive system that allows patients to indicate their current health state across five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. In the EQ-5D-3L questionnaire provided to patients in the HOT study, each dimension has three levels: no problems, some problems or extreme problems. Patients select a level for each of the five dimensions in order to generate a health state description, which can then be converted into a single summary score. The summary score is anchored at 1, which indicates full health, and 0, which is equivalent to death. States worse than death are possible.
Patients were asked to complete the EQ-5D-3L at baseline and at 6, 12, 18 and 24 months. The EQ-5D-3L health state descriptions collected in the HOT trial were converted to summary scores by applying a formula based on values collected in the UK general population. 69 Table 28 shows the summary EQ-5D-3L scores in the HOT trial overall and by trial arm at each time point.
| Time point | Unadjusted | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTOT (n = 57) | NOT (n = 25) | BMT (n = 57) | Total (n = 114) | |||||||||
| n | Mean (SD) | Min., max. | n | Mean (SD) | Min., max. | n | Mean (SD) | Min., max. | n | Mean (SD) | Min., max. | |
| Baseline | 57 | 0.52 (0.24) | –0.09, 0.88 | 25 | 0.49 (0.32) | –0.24, 0.81 | 57 | 0.53 (0.27) | –0.18, 0.85 | 139 | 0.52 (0.26) | –0.24, 0.88 |
| Month 6 | 45 | 0.55 (0.23) | 0.00, 1 | 17 | 0.62 (0.28) | –0.02, 1 | 43 | 0.54 (0.30) | –0.07, 1 | 105 | 0.56 (0.27) | –0.07, 1 |
| Month 12 | 21 | 0.46 (0.36) | –0.18, 1 | 17 | 0.59 (0.25) | –0.10, 1 | 18 | 0.57 (0.25) | –0.07, 1 | 56 | 0.53 (0.30) | –0.18, 1 |
| Month 18 | 8 | 0.63 (0.30) | 0.13, 0.88 | 9 | 0.50 (0.34) | –0.06, 1 | 8 | 0.65 (0.12) | 0.52, 0.85 | 25 | 0.59 (0.27) | –0.06, 1 |
| Month 24 | 5 | 0.83 (0.02) | 0.81, 0.85 | 2 | 0.26 (0.36) | 0.00, 0.52 | 1 | 0.73 | – | 5 | 0.58 (0.35) | 0.00, 0.85 |
On average, participants in the HOT trial had a low HRQoL. A mean score of 0.52 at baseline indicates that patients would exchange 10 years of life in their current health state for 5.2 years in full health and, thus, are willing to sacrifice 4.8 years of life. An unadjusted t-test comparison of overall baseline scores to each time point suggested that there was a statistical significant increase in mean HRQoL (p < 0.05) between 18 months and baseline, and between 24 months and baseline. However, by 18 months’ follow-up few patients provided data for the EQ-5D (n = 5).
A simple analysis of variance comparing each treatment at each time point suggested that there was no evidence of a difference in scores between treatment arms (p > 0.05). An unadjusted t-test comparison of baseline scores to each time point for each treatment showed only one statistically significant result (p < 0.05): for patients allocated to NOT at 6 months compared with baseline. However, the total number of patients in the NOT arm was small (n = 25), and only two-thirds (n = 17) completed the EQ-5D-3L questionnaire at 6 months.
Adverse events
The following sections detail the SAEs and non-SAEs for all three groups.
Serious adverse events
In total, 67 participants had 123 SAEs. More participants in the BMT arm than in the LTOT and NOT arms experienced one or more SAEs (52.6% compared with 45.6% and 44.0% respectively). However, these differences were not statistically significant (χ2 = 0.78, df = 2; p = 0.68).
Of these SAEs, 1.6% were classified as life-threatening and 19.5% were deaths. In total, there were 25 deaths but a SAE form was not submitted for one of these; instead, it was recorded as the outcome of a SAE for hospitalisation. The majority of the deaths were not deemed to be related to treatment (80.5%) and 41.5% were expected. Details of all SAEs reported are shown in Table 29.
| Events | LTOT (n = 57) | NOT (n = 25) | BMT (n = 57) | Total (n = 139) |
|---|---|---|---|---|
| Number of participants with one or more adverse events, n (%) | 26 (45.6) | 11 (44.0) | 30 (52.6) | 67 (48.2) |
| Total number of adverse events | 44 | 23 | 56 | 123 |
| Events per participant, n (%) | ||||
| 1 | 17 (65.4) | 5 (45.5) | 19 (63.3) | 41 (61.2) |
| 2 | 3 (11.5) | 3 (27.3) | 5 (16.7) | 11 (16.4) |
| 3 | 3 (11.5) | 2 (18.2) | 2 (6.7) | 7 (10.4) |
| 4 | 3 (11.5) | 0 (0.0) | 1 (3.3) | 4 (6.0) |
| 5 | 0 (0.0) | 0 (0.0) | 2 (6.7) | 2 (3.0) |
| 6 | 0 (0.0) | 1 (9.0) | 0 (0.0) | 1 (1.5) |
| 7 | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (1.5) |
| Event details (all), n (%) | ||||
| Death | 5 (11.4) | 7 (30.4) | 12 (21.4) | 24 (19.5) |
| Hospital prolonged | 2 (4.6) | 1 (4.4) | 2 (3.6) | 5 (4.1) |
| Hospitalisation | 35 (79.5) | 15 (65.2) | 41 (73.2) | 91 (74.0) |
| Life-threatening | 1 (2.3) | 0 (0.0) | 1 (1.8) | 2 (1.6) |
| Other | 1 (2.3) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Outcome, n (%) | ||||
| Died | 6 (13.6) | 7 (30.4) | 12 (21.4) | 25 (20.3) |
| Ongoing | 10 (22.7) | 3 (13.0) | 18 (32.1) | 31 (25.2) |
| Recovered fully | 24 (54.5) | 10 (43.5) | 25 (44.6) | 59 (48.0) |
| Recovered partially | 4 (9.1) | 3 (13.0) | 1 (1.8) | 8 (6.5) |
| Relationship to treatment, n (%) | ||||
| Not able to assess | 0 (0.0) | 0 (0.0) | 1 (1.8) | 1 (0.8) |
| Not related | 32 (72.7) | 21 (91.3) | 46 (82.1) | 99 (80.5) |
| Unlikely related | 12 (27.3) | 2 (8.7) | 9 (16.1) | 23 (18.7) |
| Expected or unexpected, n (%) | ||||
| Expected | 19 (43.2) | 11 (47.8) | 21 (37.5) | 51 (41.5) |
| Unexpected | 25 (56.8) | 12 (52.2) | 35 (62.5) | 72 (58.5) |
Follow-up for serious adverse events
In total, 17 participants had follow-up for their severe adverse event. This is summarised in Table 30.
| Events | LTOT (n = 57) | NOT (n = 25) | BMT (n = 57) | Total (n = 139) |
|---|---|---|---|---|
| Number of participants with one or more follow-ups | 7 | 1 | 9 | 17 |
| Total number of events | 9 | 1 | 13 | 23 |
| Events per participant, n (%) | ||||
| 1 | 5 (71.4) | 1 (100.0) | 7 (77.8) | 13 (76.4) |
| 2 | 2 (28.6) | 0 (0.0) | 0 (0.0) | 2 (11.8) |
| 3 | 0 (0.0) | 0 (0.0) | 2 (22.2) | 2 (11.8) |
| Intensity, n (%) | ||||
| Mild | 4 (44.4) | 0 (0.0) | 1 (7.7) | 5 (21.7) |
| Moderate | 0 (0.0) | 0 (0.0) | 8 (61.5) | 8 (34.8) |
| Severe | 5 (55.6) | 1 (100.0) | 4 (30.8) | 10 (43.5) |
| Outcome, n (%) | ||||
| Ongoing | 3 (33.3) | 0 (0.0) | 5 (38.5) | 8 (34.8) |
| Resolved | 6 (66.7) | 1 (100.0) | 8 (61.5) | 15 (65.2) |
Non-serious adverse events
In total, 32 participants had 139 non-SAEs. More participants in the BMT and LTOT arms than in the NOT arm experienced one or more non-SAEs (26.3%, 26.3% and 8.0% respectively). Of these adverse events, 72.7% were classified as mild, 50.9% resolved within the course of the trial, all were deemed not, or unlikely to be, related to treatment and 17.5% were expected. Details of all adverse events reported are shown in Table 31.
| Non-SAEs | LTOT (n = 57) | NOT (n = 25) | BMT (n = 57) | Total (n = 139) |
|---|---|---|---|---|
| Number of participants with one or more adverse events, n (%) | 16 (28.1) | 2 (8.0) | 15 (26.3) | 33 (23.7) |
| Total number of adverse events | 31 | 6 | 20 | 57 |
| Events per participant, n (%) | ||||
| 1 | 9 (52.9) | 1 (50.0) | 11 (73.3) | 21 (63.6) |
| 2 | 3 (18.8) | 0 (0.0) | 3 (20.0) | 6 (18.2) |
| 3 | 1 (6.3) | 0 (0.0) | 1 (6.7) | 2 (6.1) |
| 4 | 2 (12.5) | 0 (0.0) | 0 (0.0) | 2 (6.1) |
| 5 | 1 (6.3) | 1 (50.0) | 0 (0.0) | 2 (6.1) |
| Intensity, n (%) | ||||
| Missing | 0 (0.0) | 0 (0.0) | 1 (5.0) | 1 (1.8) |
| Mild | 24 (77.4) | 6 (100.0) | 12 (60.0) | 42 (73.7) |
| Moderate | 7 (22.6) | 0 (0.0) | 6 (30.0) | 13 (22.8) |
| Severe | 0 (0.0) | 0 (0.0) | 1 (5.0) | 1 (1.8) |
| Outcome, n (%) | ||||
| Ongoing | 12 (38.7) | 0 (0.0) | 7 (35.0) | 19 (33.3) |
| Ongoing with sequelae | 2 (6.5) | 0 (0.0) | 2 (10.0) | 4 (7.0) |
| Resolved | 16 (51.6) | 4 (66.7) | 9 (45.0) | 29 (50.9) |
| Resolved with sequelae | 1 (3.2) | 2 (33.3) | 2 (10.0) | 5 (8.8) |
| Relationship to treatment, n (%) | ||||
| Not related | 27 (87.1) | 6 (100.0) | 20 (100.0) | 53 (93.0) |
| Unlikely related | 4 (12.9) | 0 (0.0) | 0 (0.0) | 4 (7.0) |
| Expected or unexpected, n (%) | ||||
| Expected | 4 (12.9) | 0 (0.0) | 6 (30.0) | 10 (17.5) |
| Unexpected | 27 (87.1) | 6 (100.0) | 14 (70.0) | 47 (82.5) |
Adherence
The oxygen concentrators were generally installed in the homes of patients allocated to receive HOT within 2 weeks of randomisation (median 5 days). Two participants did not receive a machine as one died and one withdrew from the trial very shortly after randomisation. Eight (14%) patients in the LTOT arm formally withdrew from the trial treatment throughout follow-up and requested that the oxygen concentrator be removed from their home. Here, we present data on patient-reported adherence and data from the oxygen suppliers’ meter readings.
Patient-reported adherence
Patients allocated to LTOT were asked to use their home oxygen concentrators for at least 15 hours per day including overnight hours, and patients in the NOT arm to use it overnight (8 hours). At 3, 6, 12, 18 and 24 months, patients allocated to either of the HOT arms were asked the following question: ‘Thinking about the past month, typically for how many hours a day did you use your HOT?’ Of the patients who provided a response to this question in the LTOT arm, the proportion reporting that they did not use the oxygen therapy increased at each subsequent time point (Table 32). Among patients who reported that they used the oxygen, the majority used it for less than the recommended 15 hours. In the NOT arm, between 44% and 100% of patients reported using the oxygen for the recommended duration or more (at least 8 hours) at each time point.
| Adherence | LTOT (n = 57) | NOT (n = 25) |
|---|---|---|
| Month 3, n | n = 54 | n = 20 |
| None, I do not use it | 8 (14.8) | 2 (10.0) |
| Less than 8 hours a day | 22 (40.7) | 6 (30.0) |
| Overnight but less than 15 hours a day | 19 (35.2) | 10 (50.0) |
| 15 hours a day | 5 (9.3) | 2 (10.0) |
| Month 6, n | n = 44 | n = 17 |
| None, I do not use it | 9 (20.5) | 3 (17.7) |
| Less than 8 hours a day | 16 (36.4) | 5 (29.4) |
| Overnight but less than 15 hours a day | 14 (31.8) | 8 (47.1) |
| 15 hours a day | 5 (11.4) | 1 (5.9) |
| Month 12, n | n = 22 | n = 17 |
| None, I do not use it | 6 (27.3) | 5 (29.4) |
| Less than 8 hours a day | 9 (40.9) | 3 (17.7) |
| Overnight but less than 15 hours a day | 4 (18.2) | 8 (47.1) |
| 15 hours a day | 3 (13.6) | 1 (5.9) |
| Month 18, n | n = 9 | n = 9 |
| None, I do not use it | 5 (55.6) | 2 (22.2) |
| Less than 8 hours a day | 1 (11.1) | 3 (33.3) |
| Overnight but less than 15 hours a day | 2 (22.2) | 4 (44.4) |
| 15 hours a day | 1 (1.1) | 0 (0.0) |
| Month 24, n | n = 2 | n = 2 |
| None, I do not use it | 2 (100.0) | 0 (0.0) |
| Less than 8 hours a day | 0 (0.0) | 0 (0.0) |
| Overnight but less than 15 hours a day | 0 (0.0) | 0 (0.0) |
| 15 hours a day | 0 (0.0) | 2 (100.0) |
Oxygen suppliers’ meter readings
The oxygen concentrators installed in the homes of the participants allocated to the LTOT and NOT arms contained meters which recorded the number of hours the machine was used for. Meter readings were taken when the concentrators were installed and again at intermittent intervals (range of days between visits, 8–469). From these data, an average daily usage of the machines in hours per participant could be calculated. Data were supplied for 57 participants in the LTOT arm and for 24 patients in the NOT arm. In the case of four patients, no visit was recorded after the initial installation and so average daily usage could not be calculated.
It was hoped that the oxygen concentrators would be installed in the participant’s home as quickly as possible after they were randomised. One patient already had a machine in their home that they had previously been using and so continued to use this machine during the trial follow-up. Two participants did not receive a machine, as one died and one withdrew from the trial very shortly after randomisation. Of the other patients, the median time between randomisation and installation of the machine was 5 days. The vast majority of patients received the machine between 0 and 16 days after randomisation, one received it after 35 days and another after 91 days (owing to a supply issue at the Dundee site). It was recorded that one patient only received their machine 420 days after randomisation. This was queried with the oxygen supplier (Air Products) who suggested the patient may have lived at another address at randomisation and only the records relating to the second address were sent to us for analysis.
For those patients who had a second visit (n = 77 patients), the machine-use data are presented in Table 33, which shows the number of patients (%) who, on average, per day, used the machine in the following categories: < 8 hours, 8–15 hours and >15 hours. Summary statistics of the average daily use are presented, for each oxygen therapy group and overall. The figures are based on average hourly use between first and last meter readings. Average usage between readings fluctuated, indicating that patients might not have used the machine consistently throughout the follow-up.
| Oxygen machine usage data | LTOT (n = 55) | NOT (n = 23) | Overall (n = 77) |
|---|---|---|---|
| Average hours of use per day | |||
| < 8, n (%) | 37 (67.3) | 19 (82.6) | 56 (71.8) |
| 8–15, n (%) | 17 (30.9) | 3 (13.0) | 20 (25.6) |
| > 15, n (%) | 1 (1.8) | 1 (4.4) | 2 (2.6) |
| Total hours | |||
| Mean (SD) | 5.4 (5.0) | 5.4 (4.4) | 5.4 (4.8) |
| Median (min., max.) | 3.8 (0.1, 15.5) | 4.9 (0, 19.6) | 4.2 (0, 19.6) |
In both arms, the majority of participants were using the machine for, on average, less than 8 hours per day. The patients in the NOT arm, despite being asked to use the oxygen machine for only 8 hours (overnight), tended to use the machine for longer than those allocated to LTOT, who were asked to use the machine for 15 hours per day (including overnight) (median daily usage 4.9 hours compared with 3.8 hours). No formal statistical tests were conducted on these data and figures are for information only.
Overnight Embletta sleep study
If an Embletta device was locally accessible, participants were asked to complete an overnight sleep test at baseline and at 6, 12, 18 and 24 months. The Embletta records multiple signals to detect whether or not a patient has sleep apnoea, either obstructive or central. A pair of nasal prongs records air flow and a pulse oximeter worn on the finger records arterial oxygen saturation. Together, these signals detect episodes of reduced breathing (hypopnoea) or arrested breathing (apnoea). A band is worn across the chest and upper abdomen: this channel records respiratory effort. If decreased nasal air flow (and decrease in arterial oxygen saturation) suggests apnoea or hypopnoea, and if respiratory efforts are detected, an obstructive event is recorded. If, on the other hand, no respiratory efforts are detected, a central event is recorded.
The apnoea–hypopnoea index (AHI) is used to indicate the severity of sleep apnoea and represents the number of apnoea and hypopnoea events per hour of sleep. AHI values are categorised as normal (0–4), mild sleep apnoea (5–14), moderate sleep apnoea (15–29) and severe sleep apnoea (30+). Considering that both AHI and oxygen saturation can give an overall assessment of the sleep apnoea severity. The AHI, the number of desaturations per hour and the total sleep time with saturation less than 90% at each time point are summarised by treatment group in Table 34.
| Time point | Unadjusted | ||
|---|---|---|---|
| LTOT (n = 57) | BMT (n = 57) | Total (n = 114) | |
| Baseline | |||
| n | 32 | 38 | 70 |
| AHI, n (%) | |||
| Normal | 15 (46.9) | 20 (52.6) | 35 (50.0) |
| Mild | 8 (25.0) | 11 (29.0) | 19 (27.1) |
| Moderate | 6 (18.8) | 6 (15.8) | 12 (17.1) |
| Severe | 3 (9.4) | 1 (2.6) | 4 (5.7) |
| Desaturations per hour | |||
| Mean (SD) | 8.9 (10.6) | 10.6 (12.4) | 9.8 (11.6) |
| Median (min., max.) | 5.1 (0, 43.6) | 5.7 (0, 59.6) | 5.4 (0, 59.6) |
| Sleep time with SaO2 < 90% (minutes) | |||
| Mean (SD) | 0.03 (0.1) | 0.03 (0.1) | 0.03 (0.1) |
| Median (min., max.) | 0 (0, 0.8) | 0 (0, 0.6) | 0 (0, 0.8) |
| Month 6 | |||
| n | 17 | 23 | 40 |
| AHI, n (%) | |||
| Normal | 6 (35.3) | 9 (39.1) | 15 (37.5) |
| Mild | 7 (41.2) | 8 (34.8) | 15 (37.5) |
| Moderate | 3 (17.7) | 5 (21.7) | 8 (20.0) |
| Severe | 1 (5.9) | 1 (4.4) | 2 (5.0) |
| Desaturations per hour | |||
| Mean (SD) | 9.3 (11.4) | 10.5 (11.0) | 10.0 (11.0) |
| Median (min., max.) | 4 (0, 36.4) | 7.6 (0, 37) | 5.2 (0, 37) |
| Sleep time with SaO2 < 90% (minutes) | |||
| Mean (SD) | 0 (0.0) | 0.02 (0.1) | 0.02 (0.1) |
| Median (min., max.) | 0 (0, 0) | 0 (0, 0.6) | 0 (0, 0.6) |
| Month 12 | |||
| n | 10 | 8 | 18 |
| AHI, n (%) | |||
| Normal | 7 (70.0) | 4 (50.0) | 11 (61.1) |
| Mild | 2 (20.0) | 1 (12.5) | 3 (16.7) |
| Moderate | 1 (10.0) | 3 (37.5) | 4 (22.2) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Desaturations per hour | |||
| Mean (SD) | 4.4 (3.5) | 11.7 (11.4) | 7.6 (8.6) |
| Median (min., max.) | 3.2 (0.4, 11) | 7.0 (0.4, 27.7) | 3.8 (0.4, 27.7) |
| Sleep time with SaO2 < 90% (minutes) | |||
| Mean (SD) | 0 (0.0) | 0.01 (0.03) | 0.01 (0.02) |
| Median (min., max.) | 0 (0, 0) | 0 (0, 0.1) | 0 (0, 0.1) |
| Month 18 | |||
| n | 5 | 3 | 8 |
| AHI, n (%) | |||
| Normal | 2 (40.0) | 1 (33.3) | 3 (37.5) |
| Mild | 0 | 1 (33.3) | 1 (12.5) |
| Moderate | 2 (40.0) | 1 (33.3) | 3 (37.5) |
| Severe | 1 (20.0) | 0 (0.0) | 1 (12.5) |
| Desaturations per hour | |||
| Mean (SD) | 8.3 (7.8) | 7.7 (11.3) | 8.0 (8.5) |
| Median (min., max.) | 8.2 (0.2, 16.4) | 2.2 (0.2, 20.7) | 3.2 (0.2, 20.7) |
| Sleep time with SaO2 < 90% (minutes) | |||
| Mean (SD) | 0 (0.0) | 0.03 (0.06) | 0.01 (0.04) |
| Median (min., max.) | 0 (0, 0) | 0 (0, 0.1) | 0 (0, 0.1) |
| Month 24 | |||
| n | 1 | 0 | 1 |
| AHI, n (%) | |||
| Normal | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| Mild | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Desaturations per hour | |||
| Mean (SD) | – | – | – |
| Median (min., max.) | – | – | – |
| Sleep time with SaO2 < 90% (minutes) | |||
| Mean (SD) | – | – | – |
| Median (min., max.) | – | – | – |
The sleep test was conducted in only five sites (Barnet, Darlington, Dundee, Durham and Hull), and only if the patient consented to the test, hence the low number of tests conducted. There was a variety of potential problems with the sleep study. Some patients found the Embletta difficult to set up and use despite training at the hospital; some found that it interfered with sleep and were unwilling to use it again in the event of a poor-quality recording. As there is no currently recognised indication for sleep studies in heart failure patients per se, it is likely that some investigators did not encourage patients to take part in this substudy.
Chapter 7 Acute oxygen substudy
Background
As there is some evidence that oxygen administration to patients with heart failure might be harmful, and that, in particular, oxygen administration might be associated with adverse haemodynamic consequences, we undertook an acute haemodynamic substudy as part of the HOT trial.
The evidence for harm with hyperoxia is limited to small-scale studies, the main ones being by Haque et al. 21 and Mak et al. 22
Haque et al. 21 studied 10 patients with class III and IV CHF who breathed 100% oxygen for 20 minutes. The administration of 100% oxygen reduced cardiac output from 3.7 l/minute to 3.1 l/minute and increased pulmonary capillary wedge pressure (from 25 mmHg to 29 mmHg). Systemic vascular resistance increased from 1628 dyn × s/cm5 to 2203 dyn × s/cm5, and there was no significant change in pulmonary vascular resistance. In a smaller substudy of only seven patients, similar changes were seen in patients breathing 24% oxygen.
Mak et al. 22 studied 16 patients with slightly milder stable CHF (NYHA class II and III) and 12 subjects with normal LV function. Subjects again received 100% oxygen for 20 minutes. In the patients, LV end-diastolic pressure (equivalent to pulmonary capillary wedge pressure in Haque’s study21) increased from 21 mmHg to 25 mmHg, cardiac output fell from 4.6 l/minute to 4.1 l/minute and SVR increased from 1626 dyn × s/cm5 to 1901 dyn × s/cm5. There were similar changes to cardiac output and SVR in the control subjects.
Other evidence for the haemodynamics effects of oxygen is limited to very old studies of small numbers of patients (such as a study of six patients20 with acute myocardial infarction, in whom 100% oxygen caused a fall in cardiac output). There is no literature suggesting harm from LTOT in patients with CHF.
The oxygen dose that patients received in the main HOT trial was 2 l/minute, equivalent to 28% oxygen and much lower than the 40% threshold for harm suggested by Haque et al. 21 There is no formal study assessing the effects of this dose of oxygen on haemodynamics in the type of patient included in the HOT study.
We therefore assessed the haemodynamic effects of oxygen delivered for 10 minutes at a similar concentration to that received by patients in the main HOT study (28%), with the aim of establishing at least the short-term safety of low-dose oxygen.
Methods
Inclusion criteria
To be included in the substudy, patients had to be undergoing cardiac catheterisation as part of the standard management of their CHF and meet the following criteria:
-
suffer from heart failure NYHA class III/IV with LV systolic dysfunction confirmed by echocardiography (with < 40% or graded as at least ‘moderately’ impaired on visual inspection if an accurate ejection fraction could not be calculated)
-
suffer from heart failure from any aetiology
-
be receiving maximally tolerated (and unchanged over previous 1 month) medical management of their heart failure.
-
be aged 18 years or over
-
provide written informed consent and be able to complete patient assessments.
Exclusion criteria
Patients did not undergo cardiac catheterisation merely to be included in the study. Patients were excluded if they:
-
were unable to provide informed consent
-
had severe chronic airways disease – FEV1/FVC < 70% and FEV1 < 40% predicted and hypoxia (PaO2 < 7.3 kPa or saturations < 90%).
Procedures
Cardiac catheterisation is a routine procedure taking around 20 minutes. The patient is usually in hospital as a day case, arriving in the morning, having the test in the afternoon and going home in the evening. Patients were asked if they wished to take part in the substudy either at the time they were listed for cardiac catheterisation or on the day-case ward before the procedure, and provided written consent. As this was an observational study, there were no randomisation or blinding procedures.
Before undergoing coronary angiography to assess coronary artery anatomy and left ventriculography to assess LV function, patients underwent standard right heart catheterisation to measure:
-
pressure in all cardiac chambers
-
oxygen consumption
-
oxygen content in pulmonary artery and left ventricle
-
cardiac output
-
pulmonary and SVRs.
Standard measures to ensure accuracy were as follows:
-
the pressure recording equipment was zeroed at mid-chest, with rezeroing prior to each pressure measurement
-
pressure readings were made at the end of normal expiration
-
oxygen saturations were measured after calibrating the oximeter.
After a complete set of data was acquired, the patient breathed 28% oxygen instead of room air (20.9% oxygen). After 10 minutes of oxygen breathing, the pressure, oxygen consumption and oxygen content measurements were repeated.
Sample size
As there are few data available from previous studies, the sample size was largely empirical. The study was stopped after 19 patients when it became apparent that oxygen had very few effects.
Calculations
The intracardiac pressures and oxygen saturations were directly measured. Cardiac output was measured using the Fick principle from the oxygen consumption and the difference between the systemic arterial and pulmonary arterial oxygen content, using the equation:
The pulmonary vascular resistance was calculated from (mean pulmonary arterial pressure – mean pulmonary capillary wedge pressure)/cardiac output and given in Wood units. Similarly, SVR was calculated from (mean systemic arterial pressure – mean right atrial pressure)/cardiac output and given in Wood units.
Results
Demographic data are shown in Table 35. The patients were younger than those in the main study, reflecting the fact that most were undergoing assessment for possible heart transplantation. The patients were well treated and had severe LV systolic dysfunction. Most had ischaemic heart disease and 32% were in atrial fibrillation.
| Characteristic | Baseline data (n= 19) |
|---|---|
| Age (years) | |
| Mean (SD) | 59.2 (14.7) |
| Min., max. | 27.1, 84.7 |
| Male (n) | 7 |
| Height (cm) | |
| Mean (SD) | 172.2 (5.9) |
| Min., max. | 159, 183 |
| Weight (kg) | |
| Mean (SD) | 89.5 (14.6) |
| Min., max. | 67,117 |
| BSA (m2) | |
| Mean (SD) | 2.03 (0.18) |
| Min., max. | 1.69, 2.37 |
| IHD/DCM (n) | 15/4 |
| NYHA III/IV (n) | 10/9 |
| ACEI/ARB (n) | 19 |
| BB (n) | 16 |
| MRA (n) | 16 |
| Loop diuretic (n) | 17 (median 80 mg/day, range 0–200) |
| Sinus rhythm/AF | 13/6 |
| Haemoglobin (g/dl) | |
| Mean (SD) | 13.8 (2.0) |
| Min., max. | 9.8, 17.3 |
| LVEF (%) | |
| Mean (SD) | 24.9 (10.2) |
| Min., max. | 5, 40 |
There were no complications during any of the procedures, but one patient experienced an episode of pulmonary oedema 1 hour after catheterisation and was hospitalised overnight.
Table 36 shows the effects of 10 minutes of 28% inspired oxygen on the directly measured intravascular pressures during cardiac catheterisation. The patients had mild pulmonary arterial hypertension and high left heart filling pressures but there was no effect of oxygen on any of the variables measured.
| Variable | Air | Oxygen | Paired t-test (p-value) |
|---|---|---|---|
| Heart rate (beats per minute), mean (SD) | 75.58 (13.41) | 77.05 (14.04) | 0.35 |
| RAP (mmHg), mean (SD) | 6.95 (3.36) | 6.79 (3.95) | 0.67 |
| RV systolic (mmHg), mean (SD) | 45.32 (16.17) | 43.47 (17.82) | 0.11 |
| RV EDP (mmHg), mean (SD) | 9.05 (3.60) | 8.74 (4.62) | 0.32 |
| PA systolic (mmHg), mean (SD) | 44.32 (17.02) | 42.95 (16.86) | 0.26 |
| PA diastolic (mmHg), mean (SD) | 16.16 (7.46) | 15.47 (8.28) | 0.53 |
| PA mean (mmHg), mean (SD) | 27.53 (11.05) | 26.84 (11.04) | 0.51 |
| PCWP (mmHg), mean (SD) | 18.05 (8.88) | 19.26 (9.75) | 0.17 |
| LV systolic (mmHg), mean (SD) | 111.79 (19.56) | 109.42 (17.47) | 0.12 |
| LV EDP (mmHg), mean (SD) | 17.84 (7.46) | 18.11 (7.07) | 0.78 |
| Aorta systolic (mmHg), mean (SD) | 111.58 (17.29) | 112.00 (18.65) | 0.85 |
| Aorta diastolic (mmHg), mean (SD) | 62.42 (11.98) | 62.79 (12.33) | 0.61 |
| Aorta mean (mmHg), mean (SD) | 81.47 (12.78) | 82.00 (13.19) | 0.70 |
Table 37 shows the effects of 28% inspired oxygen on the oxygen saturation in pulmonary artery and aorta together with the effects on derived haemodynamic variables. The increase in the inspired oxygen led to an increase in pulmonary and systemic arterial saturation. There was a small fall in pulmonary vascular resistance and an increase in cardiac output.
| Variable | Air | Oxygen | Paired t-test (p-value) |
|---|---|---|---|
| PA saturation (%), mean (SD) | 61.24 (9.77) | 65.35 (9.73) | 0.009 |
| LV saturation (%), mean (SD) | 96.67 (1.89) | 98.46 (1.24) | < 0.0001 |
| Oxygen consumption (l/minute), mean (SD) | 0.24 (0.04) | 0.24 (0.04) | 0.56 |
| PVR (Wood units), mean (SD) | 2.78 (2.34) | 2.12 (1.94) | 0.02 |
| SVR (Wood units), mean (SD) | 19.94 (4.78) | 18.69 (3.58) | 0.07 |
| CO (l/minute), mean (SD) | 3.89 (0.95) | 4.16 (1.03) | 0.03 |
| Cardiac index (l/minute/m–2), mean (SD) | 1.92 (0.42) | 2.06 (0.46) | 0.02 |
| SV (ml), mean (SD) | 51.02 (16.80) | 55.50 (17.40) | 0.11 |
The change in pulmonary vascular resistance correlated with change in mean pulmonary artery pressure rather than with pulmonary capillary wedge pressure, and the change in cardiac output correlated with change in pulmonary artery saturation (Figure 10), suggesting that what small changes to central haemodynamics were seen were driven by changes in the pulmonary rather than the systemic circulation.
FIGURE 10.
Relations between haemodynamic variables. CO, cardiac output; PA, pulmonary artery; PVR, pulmonary vascular resistance.
Chapter 8 The home oxygen therapy trial qualitative substudy
Background and rationale
Despite a survival benefit in COPD, patient adherence to LTOT is known to be problematic. 17,18 Adherence appears to be related to symptom burden and performance status, and is better in patients who have had training in the use of the concentrator. 18,70–72 Given the lack of data about the use of home oxygen for people with symptomatic heart failure, this substudy explored the experience of patients and their informal carers regarding the use of HOT during participation in the main study to gain a deeper understanding of adherence issues than could be gained from monitoring concentrator use alone.
Breathless patients may have mixed feelings about oxygen therapy. 73–75 Positive feelings include having ‘treatment’ and an instinctive welcome of oxygen as it is routinely used during episodes of severe decompensated heart failure by emergency health professionals. The presence of an oxygen concentrator in the home and regular attention from professionals may be reassuring. Patients with prior experience of cylinders may find the concentrator easier to handle with less need for deliveries. However, the presence of the concentrator and associated paraphernalia can cause home disruption, limitations to activity, problems with tubing and anxieties about it ‘going wrong’. The fixed nature of the concentrator in the house may restrict outside activities and hence adherence is lower in patients able to do activities outdoors. 74,75
In general, adherence is lower for treatments given for maintenance or prevention than for those which are directed at an acute symptom with a clear temporally related dose–response relationship. In a RCT of oxygen of sham concentrators for people with advanced disease and symptomatic breathlessness, 50% of patients reported no benefit (for their breathlessness) and did not want further oxygen therapy. 36,76
The sensation of breathlessness correlates poorly with measures of lung function or arterial oxygen saturation and has a complex genesis in heart failure. Two factors may make it difficult to identify the possible role of oxygen therapy for people with advanced heart failure, particularly if they are not hypoxaemic enough to warrant HOT in order to gain a survival benefit. The first is the lack of immediate relief of breathlessness (particularly when patients intuitively believe that they should be helped by oxygen) and the second is the fact that it might be a benefit that is mediated by airflow rather than by the oxygen.
Aims and objectives
This planned substudy aimed to explore the views of patients with CHF using a home oxygen concentrator, and their carers, with regard to benefits and burdens. In addition, we wished to understand their experience of this in the context of participation in the HOT trial.
Methods
Ethics approval was given by Northern and Yorkshire Research Ethics Committee (reference 09/H0903/43).
Design
This was a single-centre nested qualitative study. A modified grounded theory approach using semistructured interviews was chosen, with the option of dyad or separate interviews with a carer, if there was one, rather than focus groups, to explore the experience of the participant within the context of their immediate family and home.
Sampling strategy
The participants were purposively sampled, in order to gain maximum variation, from patients in Hull recruited into the main HOT trial who had been allocated to an oxygen arm. Participants were sampled for a varying time on the study: some within a few months, some after about 6 months and others after they had been allocated oxygen for a year. We anticipated that this approach would allow for a spread of age, sex, types of living arrangement (alone, with spouse, etc.), severity of CHF and comorbidities, which could all potentially affect a patient’s experience of the oxygen concentrator.
Potential participants were identified and invited by the HOT research nurse according to the sampling grid. Interested patients were given an information sheet and then contacted by the qualitative substudy researchers and an interview arranged. Written consent was obtained just prior to the interview. If participants’ informal carers wished to be interviewed as well, they received their own information sheet and signed their own consent.
Data collection
Background demographic data were collected from the baseline assessment of the main trial and included age, sex, severity of HF and length of time on the HOT study. Semistructured dyad (if carer present) interviews then took place with participants using a topic guide which was developed, based on the study aims, by the expert research group and informed by the published literature about LTOT adherence and service users. Dyad interviews, in which patient and carer are interviewed together, have been used in a range of clinical specialties, including palliative care, and can generate a richer understanding of needs and experiences than a single interview. However, if the patient did not wish the carer to be interviewed, did not wish to be interviewed together with his or her carer or did not have a carer, he or she was interviewed alone. Participants were interviewed once in order to minimise burden.
Interviews were conducted by Professor Miriam Johnson (MJ) and Dr Samantha Nabb (SN), neither of whom was involved in seeing patients as part of the main study. The interviews were audio recorded and transcribed verbatim. An iterative approach was used; transcriptions were read, and any issues presented by patients and carers which were not included in the topic guide were added so that they may be specifically addressed in future interviews. Participants were interviewed at home or in the hospital clinic according to preference as long as confidentiality could be maintained and the setting was as free as possible from potential interruption. Field notes were taken by the researcher during and immediately after the interview to record any observations about the body language of participants or the physical environment, particularly in relation to the presence of the oxygen concentrator.
Data analysis
Thematic framework analysis was used. This is an approach developed for conducting applied qualitative research,77 and involves moving through the stages of familiarisation with the data, identification of a thematic framework, indexing the data using the thematic framework, arranging the data into charts, mapping and interpretation of the data. SN, MJ and Lesley Jones carried out the preliminary coding of interview transcripts, checking each other’s coding for similarity to increase rigor. SN used the NVivo computer software (QSR International, Warrington, UK) to manage the data.
All transcripts were coded by at least two researchers. The coding strategies and emerging themes were then agreed between all three researchers. Once the thematic framework had been drawn up, the findings were then synthesised to provide a summary of patient and carer views with particular regard to the perceived benefits and burdens of HOT using a concentrator, and their experience of participation in the HOT trial. Data from informal carers and patients were analysed together, as we considered the dynamics of the family unit to be integral to the use of oxygen therapy in the home.
Results
Participants
Characteristics of the participants are summarised in Table 38. Two had stopped the oxygen therapy by the time of the interview but had continued providing data for the trial. All were interviewed in their own homes except for one who chose to be interviewed in the research clinic while attending for an assessment for another study. Most who lived with a carer chose to be interviewed with them. Interviews took between 20 minutes and 1 hour.
| ID | Age (years) | Sex | KPS score | Comorbidites | Lives alone/with | Interviewed with carer (if applicable) | Months in main study prior to interview |
|---|---|---|---|---|---|---|---|
| 1 | 75 | M | 80 | BiV PPM + ICD, DCM, hypercholesterolaemia, IDDM, IHD | With wife and daughter | Wife and daughter | 1 |
| 2 | 64 | M | 60 | AF, CABG, IHD, osteoarthritis, PVD, T2DM | With wife | Wife | 2 |
| 3 | 74 | F | 60 | AF, arthritis, ICD, IHD, MI | Alone | n/a | 2 |
| 4 | 76 | M | 50 | Anaemia, BiV PPM, CKD, HTN, T2DM | Alone | n/a | 3 |
| 5 | 73 | F | 60 | AF, CABG, ICD, MI, PVD, T1DM | Alone | n/a | 4; stopped O2 |
| 6 | 73 | M | 70 | CKD, gout, HTN, MI | With wife | Wife | 6 |
| 7 | 68 | F | 60 | Arthritis, HTN, IHD, T2DM | With daughter | Daughter | 7 |
| 8 | 78 | M | 60 | AAA, AF, asthma, BiV PPM, IHD | With wife | Wife | 6 |
| 9 | 85 | F | 60 | COPD, IHD, trigeminal neuralgia | Alone | n/a | 9 |
| 10 | 76 | M | 60 | Anaemia, BiV PPM + ICD, CABG, MI, VT | Alone | n/a | 12; stopped O2 at 6 |
| 11 | 80 | M | 50 | AF, BiV PPM + ICD, COPD, IHD, T2DM | With wife | Wife | 9 |
| 12 | 75 | F | 60 | CKD, IHD, MI, T2DM | Alone | n/a | 14 |
| 13 | 90 | F | 50 | AF, BiV PPM, COPD, IHD, MVR | With husband | Husband and son | 7 |
| 14 | 60 | F | 60 | BiV PPM, CVA, IHD | With husband | Husband | 13 |
| 15 | 75 | M | 60 | AF, CABG, depression, IBS, ICD, IHD, HTN | With wife | Wife | 7 |
| 16 | 66 | M | 50 | BiV PPM, Ca bowel, COPD, T2DM | With wife | Wife | 12 |
| 17 | 56 | F | 60 | AF, BiV PPM, CKD, HTN, IHD, MI | Alone | n/a | 7 |
Findings
The agreed thematic framework used in the analysis is presented as major and subthemes arising from the data (Table 39). The themes and subthemes are discussed in the following text with illustrative quotes only for sake of brevity. Further quotation data for each theme can be provided if requested.
| Major theme | Subthemes |
|---|---|
| Self | Coping
|
| Biographical disruption and sense of identity | |
| Ambivalence | |
| Narrative | Illness
|
Life space
|
|
Health beliefs
|
|
| Impact on others | |
| Life narrative | |
| Trial | Motivation
|
Practicalities
|
|
| Adherence with intervention | Benefit–burden balance
|
Incorporation into daily routine
|
|
Stigma
|
Major theme 1: sense of self and effect of chronic heart failure
The first area we examined was to understand the experience of living with CHF, including participants’ understanding, their limitations and their ways of managing their condition in order to gain background for how an additional factor, HOT, would be received. Two related major themes were apparent: a sense of self and how this had been affected by the CHF, and the story (narrative) of participants’ lives in the context of their medical condition.
Self
Participants talked about the need to still be themselves, both as an individual and in terms of family relationships, despite the challenges of living with chronic ill health. Maintaining ‘normality’ as much as possible was part of that aim. When maintaining their notion of self became more difficult to do, because of increasing limitations, it was a source of distress. Intrinsic personal qualities and external supports were clearly important in this process.
Participants coped using a variety of strategies, in particular adaptation, humour (often linked with stoicism) and family support.
I try to not let it affect my everyday life. I just do what I want to do, within reason. You just have to cut your cloth, and that’s what I’ve done. It is frustrating, it is frustrating, cause I’ve always been a person that just got on and got it done and, and I can’t do that any more. But I don’t dwell on what I can do, what I can’t do, I actually just think about what I can do, and I, it could be a lot worse.
Participant 17, woman
And they [family] all come . . .
They keep us going, that’s my belief anyway . . .
Yeah, they do.
Participant 11, man with wife
Often the coping strategies were ways in which participants had coped with adversity for many years and had served their purpose well, helping to preserve their identity as someone who could manage.
It’s a blasted nuisance [laughter]. It, but it doesn’t, it rarely makes me feel ill, I may be out of breath, I may be fatigued, but inside my head I’m exactly the same person that I was before . . . But heart failure, one lives with it, one lives round it.
Participant 5, woman (withdrew from oxygen)
However, sometimes, coping was overwhelmed by ill health, particularly for those who saw themselves as the person who helped and organised others, rather than as the person receiving assistance. Family supports were tested and altered as a result of the effect of the patient’s condition, changing the relationship and role within that unit, and not always constructively.
When coping strategies were overwhelmed or unhelpful, and patients were unable to adapt or accept the change in circumstances, the loss of confidence experienced with social interaction (both in the home and more widely) was reflected in a loss of sense of self. The term ‘biographical disruption’ has been used to describe this result of chronic illness and disability.
So heart failure is a blasted nuisance in the fact that it prevents John and I doing together the things that we, we, if we weren’t, if I hadn’t got it we’d be going on rallies, we’d be going abroad, we’d be visiting our friends in the south of England and our friends in Scotland. But it is difficult because you are socially unacceptable if you cannot, and our friends in Ireland, if you cannot spend a day awake, you can’t, have to have 2 hours gone in the afternoon.
Participant 5, woman
I was the driver. And of course the work did take me round a lot but on, in driving . . . Yeah, and, and now, you know, I’m totally dependent and it does, it does affect one, you know.
Participant 15, man
But I’m very independent, I keep to meself a lot, I am maybe me own worst enemy, but there you are . . . I was very active, I was always the carer, I looked after other people . . .
Participant 9, woman
Major theme 2: the story (narrative) of the patient’s life in the context of his or her medical condition
Narrative
All participants related their experience in a time-framed story of their life, their illness and its effect in terms of the shrinking world, or space within which they lived their life, which was imposed on them and their families. This story developed and contributed to beliefs about CHF and its treatment and about health professionals, beliefs that were held by themselves and their family members. Most had stories of very serious episodes of illness.
Cause I did have a slight heart attack when I lived in [town], that were in ‘93, and then some, when I moved here, oh it was 2008, cause I were in [supermarket] at [town] and I had a massive heart attack and I couldn’t move or anything, sweat poured off me, and all me limbs went numb, and when they took me to hospital I’d had a cardiac arrest, whatever they call it, and I’ve never been right since, so . . .
Participant 7, woman
Well I’ve had three heart attacks that they said they were heart attacks, and then two that were, they were mm er, mm um, mm, so five in all. I’ve had a stroke, I’m diabetic, insulin dependent, I’ve had asthma since 60s, late 60s, I’ve had an underactive thyroid for 30/40 years, so. And I’ve got a defib [defibrillator] and a pacemaker, so. Sometimes it, you get a bit fed up about it, but I, I think of it this way, at least I’m around.
Participant 12, woman
Part of the illness narrative included the influence of both good and bad experiences of health services in general and health-care professionals in particular.
. . . when I first had the heart attack I was, I was told, quite, quite wrongly by a, a very young medic at one of the outpatients’ appointments. I’d had an angiogram and the, the news was bad, that I’d shortened my lifespan, there was too much damage to operate. So at the outpatients’ appointment I asked the, the doctor if he could clarify then, the phrase, what was it? It’s shortened your lifespan. And he said ‘Oh you’ll be all right for 2 to 3 years’ . . .
Participant 3, woman
. . . so Professor [name]’ll get me right again . . . me and Mary, never mind doctors, what, what, Professor [name] that, that side’s, because they’re, they’re monitoring, they’re doing it, you know, but when you get called into your own doctor after you’ve had a blood test saying your, your, your, your, your whatsits is all airy-fairy and all over the place and they start knocking tablets off and they have, they, and they’re doctors that don’t know your history, you know, you know?
Participant 4, man
. . . I can’t, I can’t compliment the, the, what can I say, the care that I’ve had from the hospitals and the [hospice] and the [academic heart care clinic], all, all of them have been fantastic for me . . . and whenever I’ve gone in hospital its unbelievable how, how, how the care has been for me and I’m very grateful for that.
Participant 8, man
Yeah. I found, and then he went on to the, the [accident and emergency] ward. That was just awful, they were so, and I, and I did say it, it was so bad, I mean, in so much as they were allowing him to self-medicate and they were also medicating him, so his blood pressure, he didn’t know whether he was on this earth or Fuller’s earth.
Fuller’s earth [laughs].
It was a really frightening experience, but then that’s when you went from there to [inpatient hospital ward], wasn’t it? And then it was, it was like an entirely di–, it was like, well it was, it was just amazing, the difference was just amazing . . .
Participant 16, man
Sometimes I have breathing problems, but the la–, the lady at the [hospice] gave me some exercises to do and she also gave me one of these battery operated fans, which if I, if I get any problems with me breathing it does help . . .
Participant 8, man
In general, there was overt recognition of the limited treatment options at this stage of their illness.
I know that I am getting towards the end now, there’s no way I’ve got much longer to go, so what do you do? Do you, you know, stop the heart tablets or do you con–, continue with your diabetic fluctuations? And I’m happy to do that. I’ve had it for such a long time.
Participant 5, woman (withdrew from oxygen)
But everybody, everybody I’ve, I’ve come across, like even [Professor] he says ‘You’re on the right medi, there’s not’ he says ‘there’s only oxygen left for you after, after that.’ You know, there’s nothing . . .
Participant 2, man
Participants also gave a narrative of their life as part of maintaining sense of self, trying to adapt and incorporate the limitations due to their illness with varying degrees of success.
. . . but then things like domestic chores, everyone tells you as they get older that you realise it’s a, it’s a total waste of time because [interviewer laughs] tomorrow’s as bad as it was today before you did it, so . . . since I retired, got a lot of increased hobbies and I, I, I centre on those really, as being my priorities, because those are the things I enjoy doing. I can pick up and leave off as I want . . .
Participant 3, woman
I didn’t run a marathon but just, just quickly, I was a excellent 800 metre runner . . . Mm. Lan–, Lancashire champion . . . Trials for England. Yeah, I was very athletic.
Participant 16, man
I’m a very independent person. My husband was 37 years in the Royal Air Force and I travelled the world with him, but I had four children and I took them with me. Many the times I’ve travelled on my own with four children and he’d meet me at the other end, you know, if I was lucky. So I’m very independent but I’ve lost me, me independence with me heart troubles.
Participant 12, woman
He’s not a big one for walking.
I’m not a big one for walking now.
But he, he was a big one for walking when he was 23 . . .
. . . 24 years in the prison service.
Well yeah, he . . .
20 of those years were a dog-handler . . . Lot of walking involved there.
Participant 16, man
The second area was that of the participants’ experiences of the trial and issues relating to adherence with oxygen as major themes. We looked particularly to see if issues related to the trial had an influence over and above the issues related to oxygen as possible therapy but these were not apparent. Therefore, we report them as two separate themes.
Experiences of the trial
Trial
Issues relating to the trial could be divided into two subthemes: motivation for participating in the trial and factors helping or hindering continuation in the trial. Factors continuing with the trial related solely to perceived burdens or benefits of the trial oxygen therapy and are discussed under adherence with oxygen, as we were unable to identify whether or not the fact that patients were receiving the intervention as part of a trial (rather than for an expected clinical benefit) made a difference to their decision to continue with the oxygen.
Motivation
I’d say give it a go, give something back, it, it’s no imposition, and it is voluntary, you can stop at any time. If you don’t like it when you put it on you can always take it off, it’s not, nobody’s welding it to your face.
Participant 2, man
They aren’t gonna cure my heart failure now, it’s too far gone, it’s, you know, too far and it’ll help somebody else get some comfort, I don’t mind at all.
Participant 12, woman
Motives to enter the trial were mixed. Although altruism was a feature, and expressed clearly by several participants, there was a strong perception that participation in a trial gave the patient better support (e.g. from the research nurse and clinical academic team), better clinical management (e.g. more chance of being reviewed regularly by a senior doctor, if not the professor himself), and access to the latest technology/treatments.
. . . he said I’d been painted a very black, black picture and would I consider going to the academic cardiology because that way I would be monitored on a regular basis and they, at the same time, would use my, my records, etc., to their advantage, and I would, it was mutually beneficial.
Participant 3, woman
There was also a significant influence from the beliefs held by the family carers, some almost instructing the patient to participate, for the reasons given above, especially that this was seen as the best way of getting special treatment, with one participant’s wife seeing it as a way of getting her husband reinstated at the professorial unit, as his clinical care had been transferred to a district general hospital.
. . . because in the hospital in [district general hospital] he was like this, every day came a new doctor to say ‘Ah, we’re going to put a pacemaker on you’. And next day came another doctor and said ‘No, we’re going to do this to you’. And then we have three different . . . But the cardiac doctors, they are all over the place, they don’t know what they are talking about, and they make us very insecure.
Participant 15, man
I’ve joined every study that I’ve been offered, since, that I was eligible for, and so when the oxygen came up my automatic reaction was if I’m the right person then sure, you know, I’m game for anything . . . And like this trial, I mean I know they’re going to send for me at regular intervals, during the course, so. So no, I, I just get involved in, in anything they ask me, with pleasure really.
Participant 3, woman
I like these trials. The reason I like ‘em is they keep their eye on yer. Their eye on summat . . . cause sometimes if you’re just in the routine system, you know, it can be 4 months, it can be 12 months before . . .
Participant 6, man
There were some specific comments relating to the initial entry on the trial, with some participants commenting that the oxygen concentrator was larger and noisier than they felt they had been led to believe and that the initial disruption in the house to fit tubing was more than they had expected. However, that in itself did not have an impact on perseverance with the study at that stage, and the initial invitation discussions with the site investigator were recounted with good humour.
. . . the machine is, in itself, is quite noisy and, again, Professor [name] thinks it’s just a mild hum, so I said ‘Well I, have you actually listened to one?
Participant 3, woman
So I said, I said, ‘Fancy getting you again’ [Interviewer laughs]. So he [Professor] said ‘Oh, come on, what’s the matter?’ So I forgot to say about the cylinder, so I said ‘You didn’t tell me that it had to be piped downstairs as well so that you’d access to the oxygen’. He, I’d made him laugh because he said that there was gonna be 50 yards of tubing from here to kingdom come, from the machine upstairs, you see, which again could’ve been an accident waiting to happen. And he said ‘Oh I must remember to ask people, next time then, if they live in a house or a bungalow’. So I don’t think it had entered his head that, you know, that the piping would have to go through your walls and down your boards, etc., but . . .
Participant 3, woman
And I was rather shocked [laughter] by the size of it, cause Professor [name] said it’s the size of a cardboard box.
Participant 17, woman
Adherence
The overwhelming majority of the issues influencing adherence were those practical issues already well documented: noise and heat from the concentrator and a wide range of tube-related challenges. Tubes caused practical obstacles such as sore nose or ears, tying people up, coming adrift (providing excellent oxygenation of the participant’s ear or face) in bed at night or disrupting the stairway.
Participants varied in their response to these difficulties within the context of how they managed their chronic condition overall and depending upon their character. Although family and friends were seen by all as a source of support, participants who had maintained a sense of their role within the family and of themselves more easily incorporated the presence of the oxygen concentrator and tubing within their everyday life with relative ease.
The family come in and say ‘Have you used that [oxygen] today?’ you know [laughs]. ‘No, I haven’t’. ‘Well you should do’. . .
Participant 11, man
However, those who seemed to be struggling with adapting to their current circumstances and still spending a lot of energy in ‘keeping up appearances’, or who were finding it hard to accept how life was, were acutely aware of the presence of the concentrator, actively keeping it out of sight, out of the way of family and friends to the extent that if the grandchildren were visiting, they did not use it, ostensibly so as not to concern the grandchildren or to prevent adverse comments which they found embarrassing and stigmatising.
The, well my missus, she’s got ears like a hawk [interviewer laughs] you know, and she wouldn’t put up with it, so . . . nobody talks about it, they know I’m on it but they, they, they, they don’t want to know . . . Well my missus gets on about it saying ‘How long is that blinking thing going to be there?’ but, you know.
Participant 4, man
Yeah, my, my granddaughters come, sometimes they come for a weekend, and ‘Oh that machine, nan’ they say. So sometimes I switch it off and when they’ve gone I (. . .) then, you see. But I’m lucky, I suppose, I live on me own so I can cope with it . . . Well they’re young aren’t they? [interviewer laughs] It’s an hindrance to them [laughs].
Participant 9, woman
Such participants appeared to be ambivalent about the oxygen concentrator and tubing, stating first that it was no problem, but then immediately presenting issues that they found difficult.
. . . apart from it’s another wire down the skirting board and everything else, it’s really not a problem, except, to switch it on I have to go upstairs, and likewise, downstairs to switch it off, back up again . . . it becomes a major ordeal of having to lock the back door, go upstairs and lay on the bed, or switch it on there and then come downstairs. So that is the only inconvenience for, at home, which isn’t really such a bad thing.
Participant 3, woman
One participant in particular demonstrated an ambivalent attitude, trying to be positive, but then describing almost simultaneously catastrophic responses to her condition and her current life including the oxygen therapy.
The oxygen concentrator had caused some married couples to start sleeping in separate rooms because of the disturbance of the noise.
But, as I say, it makes a lot of row and, you know, I’ve got to sleep in a back bedroom . . .
Participant 6, man
So I shifted meself into the back bedroom, left [wife] in the front bedroom . . . No, no, don’t want to upset [wife], so I’ll stay in the back bedroom.
Participant 16, man
Some commented that this was a reasonable option for them as they had a spare bedroom, but they felt that it would be difficult for more deprived families for whom this might not be possible.
I don’t have it in the room I sleep in. I, I’m lucky because I have three bedrooms . . . and I live on me own, you see, so I put it in the other bedroom and close the door. I worry about if the neighbours can hear it, but nobody’s said anything now so they can’t do.
Participant 9, woman
We put the machine in the dressing room . . . very fortunate we have a dressing room and the machine was in the dressing room and we could still, I could, [husband’s] a bit deaf but I could still hear it. Yes, it is noisy, yeah.
Participant 5, woman (withdrew from oxygen)
Others had found other ways of dealing with it (with one family placing the concentrator in the hallway during the day and in the lounge overnight with the door shut). This family came to that arrangement to avoid disturbing their adult daughter, as they were both hard of hearing and this was not a problem for them.
. . . the only thing is when I do, I run it through the door, I leave the machine there . . . so we can, if it . . . we can shut that door if we want to cut the noise down a bit and put the piping across here [gesticulating behind the sofa]. A at night-time, we fetch the machine, in, in here [lounge] and then we can close up that door nearly to. We’re in the bedroom along on the ground floor, so we go in there, put our door close and . . .cause when we had it out there, [daughter]’s upstairs and she could hear it, so we cut that down and it helps her – we can’t hear it.
Participant 1, man
Conversely, one wife commented that the oxygen had helped her to sleep at night because her husband’s breathing was more settled at night than it had been when he was not taking oxygen.
The concentrator was a significant limitation to mobility which had some serious consequences. Going away on holiday either became impossible or had to be reframed. One family had still managed to find a way to go away on holiday by using a facility owned by the oxygen company to rent out to clients but, although they appreciated the opportunity to be away, they still felt that it was restricting.
All found the oxygen installation and maintenance service to be excellent, responsive and reassuring. There were some concerns about the risk of fire, but these were in relation to the back-up oxygen cylinder which came with the concentrator and did not appear to be a major concern, mentioned only by one adult daughter. Despite specific enquiry, concerns regarding smoking were not elicited.
One participant stopped the oxygen therapy after an acute intercurrent illness which necessitated admission to a coronary care unit. She was severely ill and unable to speak for herself. However, she remained aware enough to feel that, although it was clear she was taking the oxygen as part of a clinical trial, the presence of HOT was perceived by the attending health-care professionals (in particular the ambulance staff and coronary care doctor) to be a marker for end-stage disease and that she would be ‘not for cardiopulmonary resuscitation’. When she was sufficiently recovered to go home, she was so disturbed by this, and worried that should a similar event happen she would be ‘labelled’ as ‘not for treatment’, that she opted to stop the oxygen therapy although she continued to provide data for the trial.
In the hospital, they were handing me on to somebody else, obviously, and one of them said ‘She’s on home oxygen.’ And I said ‘Just a minute. It’s just a clinical trial’. But I don’t think that bit was heard . . . But one of the doctors in the intensive care unit, I don’t know whether he thought I couldn’t hear him, but I could [laughs]. So the things he was saying at the end of the bed was that it’s end-stage heart failure and end-stage COPD . . . It affected their judgement of me, but I felt that I really needed the oxygen that morning, because I was so breathless, and as soon as I put the oxygen on I was obviously less breathless because I’d got the oxygen on. But I really felt that the perception of me, as a patient, was, was completely wrong, as soon as they realised I had, I, because as they walked in, I’ve got the . . . [oxygen on] . . .
Participant 17, woman (withdrew from oxygen)
Although some participants were very strict with attempting to fit in the full 15 hours of therapy by planning hours of oxygen around the other things they wanted to do, most freely admitted to poor adherence. This was often inadvertent, simply forgetting to use it if they were busy going out, or doing things around the house. Others deliberately chose to use it only at night, or when they did not have visitors or if they felt more breathless. Most admitted that they liked the idea of the concentrator in the house as a ‘safety net’ should they have a bad day with breathlessness, feeling this might prevent them having to call the emergency services for the oxygen that had been given on previous occasions. On the other hand, there was a concern that they would become too reliant on it, which contributed to a feeling that they should restrict its use.
But, you know, I’m, I find it reassuring to have it, though sometimes I, I feel I might be dependent on it too . . .
Participant 15, man
Peace of mind I think, because I’ve always found when I have had the, the very, very bad uncontrollable angina, the first thing they do is give me oxygen, it calms you down . . . And it, it dulls the pain knowing that it’s there. If it, I’ve got a real bad go of angina I could shove it on while we’re waiting for the ambulance people to come . . .
Participant 15, man
. . . and then they’ve brought me on the machine to, to give it a go, and it helped me a lot . . . I weren’t gasping for breath, I weren’t emergency, but it did calm me down, just calm me down, and after about half an hour I’d, I were back to normal . . . Yes, I don’t want to get too reliant on it, so that if I do, don’t go on it I haven’t any problems; but it helps me a lot, it helps me sleep and; this is what I like about it really, it calms me breathing down and it helps me sleep through the night.
Participant 14, woman
There is a strong layperson and health-care professional belief that oxygen is an important treatment for acute breathlessness, which was a major barrier to adherence to a 15-hour-per-day regime. Many of the study participants had experience of repeated heart failure decompensations and other acute events necessitating unscheduled health care for which administration of oxygen is a common first intervention. Thus, the use of oxygen for long periods of time during the day and overnight, when at rest and when their breathing was usually comfortable, was difficult for many to understand.
Switch it off, we have breakfast, and he goes all day without it, don’t yer, till . . . think, to be really truthful we didn’t think we needed that, because he’s not that poorly that he had to go, oh me oxygen, he never puts it on even . . .
No I’ve never ever.
. . . and he’s busy during the day aren’t, you know it . . .
Participant 8, man
This was further complicated by the beliefs of family members. One participant’s wife had ambulatory oxygen for exertion-induced desaturation. This was immediately beneficial for her and made intuitive sense. She and her husband found it hard to grasp why restricting mobility with oxygen tubing for hours when one is comfortable at rest, for example watching the television, should have any benefit – especially if the patient could not perceive any immediate benefit.
Those taking nocturnal oxygen seemed to find adherence easier as it became part of the rhythm of going to bed. In addition, some (or their spouses) felt they slept better (when not having tube trouble).
Well I think it helps me with me breathing at night-time, and I can have it on daytime if I think I need it, but I don’t often need it.
Participant 13, woman
In general, there was no clear pattern of adherence becoming easier or more difficult over time, although some who had been on oxygen for some months commented that they felt that they were steadily improving and put the improvement down to the oxygen. One participant described how he felt he had his role back in the family again, as the family ‘taxi driver’, helping collect his grandson from college. This helped problems seem less significant in comparison with perceived benefit.
Overall, patients weighed up the benefit–burden balance of the oxygen therapy.
. . . you get a bit, a bit sore and a bit resentful of its irritation, to some extent. But then give it 5 minutes and you don’t even know it’s there really. I think it’s too early days for me to say that it, it’s beneficial. It, it appears to be going that way. But, as I say, it’s, it’s too early days to say. I do get good spells but it’s a long time since I’ve had a, a really good spell so it could be prompted by the oxygen. I would like to think it was, because I would then opt to continue with it at some later date.
Participant 3, woman
I’ve not used a, a mask long term so I don’t know. I might have coped, after a while got used to it and coped very well, you know. I mean I was a bit of wimp, I think, letting go so quickly. The physical, immediate, almost immediate physical problem took any long-term thoughts from me, it was simply that I couldn’t manage with it, and if, later, if you had said to me we know that oxygen therapy is good for you then I would have said, fine, but I’ll have to use a mask not these, yes.
Participant 5, woman (withdrew from oxygen)
As the concept of long-term improvement in QoL (rather than specific and immediate symptom improvement) was difficult to accept, even for those who implicitly trusted medical opinion, the practical and obvious burdens relating to equipment and restrictions were important. Again, the burdens were seen in the context of the participants’ personality and ability to adapt and accept their chronic illness. Thus, for some, the practical difficulties loomed large, whereas, for others, the same issue was incorporated into everyday life and was seen as a much smaller issue.
See I’m used to it now, it gets on [wife’s] nerves a little bit at times, but I can sit in here with it on . . .
You don’t hear it [laughs].
. . . with, with the telly, with watching the sport and so . . .
Participant 8, man
Chapter 9 Discussion
The findings of the HOT study suggest that there is no symptomatic benefit at 6 months for patients with severe heart failure from treatment with long-term HOT when prescribed for 15 hours per day.
Chronic heart failure affects approximately 2% of the adult population in the UK. Although medical therapy has advanced dramatically in the last 20 years, patients with CHF tend to deteriorate with time and usually eventually die from the condition. As their condition worsens, so they become more and more breathless on minimal exertion. In an attempt to relieve breathlessness, HOT is commonly prescribed, and yet there is no evidence that it is effective.
The HOT study is the largest randomised trial to date of long-term HOT for patients with CHF. The study is certainly needed; there is a proven mortality benefit with HOT only for patients with severe lung disease and hypoxia, yet most NHS prescriptions for home oxygen are given for indications other than lung disease. 39 HOT is not only burdensome for patients but also expensive to deliver. If it serves no purpose for patients in terms of symptoms relief, it should not be used.
Clinical effectiveness
Although there was no difference in MLwHF questionnaire score between the LTOT and BMT arms at 6 months, we did see evidence for an improvement at 3 months. The LTOT group had a statistically significant lower MLwHF questionnaire score (difference –5.47, 95% CI –10.54 to –0.41; p = 0.03). This represents a clinically important difference;48 however, a difference of 10 was used to power the trial. The improvement may reflect a placebo effect and represent the effect of receiving active treatment, and having the oxygen available as a possible acute treatment in times of severe symptoms. That the improvement disappeared at 6 months might be a result of the waning of the placebo effect coupled with the burden of the oxygen usage. However, 3 months seems to be a long time for a placebo effect. Although placebo responses can last up to 12 weeks, it is unusual and would be particularly so in the situation where the patient is expecting an immediate benefit.
There were twice as many deaths in the BMT arm (n = 12) as in the LTOT arm (n = 6), although the impact of oxygen therapy on survival was not statistically significant (p = 0.16). Hazard rates were similar up to around 6 months post randomisation, after which only two more deaths were recorded in the LTOT arm but a further six were reported in the BMT arm.
As we observed a significant difference in the MLwHF questionnaire score at 3 months (favouring LTOT), we hypothesised that LTOT might be keeping sicker patients alive longer and so be artificially pulling the MLWHF score up at 6 months (lower scores indicate a better QoL) relative to BMT. In the BMT arm, the higher mortality of the more symptomatic patients would have the effect that the average QoL of the group would tend to improve as the more severely symptomatic patients died.
To investigate this hypothesis, we repeated the primary analysis model restricted to patients who returned data at 3 and 6 months (and so necessarily were alive at 6 months). However, restricting the analysis to those patients alive at all three time points showed no evidence of such an effect.
Quality of life measures
Patients in the LTOT arm at 3 months reported an improvement in average breathlessness over the past 24 hours on the numerical rating score by a clinically important amount57 (which was no longer apparent at 6 months). The improvement coincided with an improvement in the distress caused by breathlessness, reporting of having coped better with breathlessness, and a higher level of satisfaction with the treatment received for breathlessness. At 6 months, there was no difference in average or worse breathlessness experienced over the last 24 hours, but patients in the LTOT arm continued to have increased median scores for coping and satisfaction with treatment.
There are two potential explanations. First, it seems from other work that airflow (whether or not it is oxygen enriched) can reduce the sensation of breathlessness; therefore, there may be a ‘real’ symptom improvement from using the flow of gas across the nasal mucosae,36 which may not persist to 6 months, particularly in patients who are less well. Second, the improvement in distress due to breathlessness at 3 months may be because of the ‘safety net’ issue seen in the qualitative substudy. In other words, patients may have had increasing self-efficacy, that is the oxygen was something they had in reserve for a bad day. That effect, too, might disappear over time as the underlying condition progresses or the therapy does not lead to hoped-for improvements. However, it is notable that improvements in self-rated ‘coping with breathlessness’ persisted for the first 12 months and ‘satisfaction with treatment of breathlessness’ until 6 months.
Safety
There were no safety concerns with the use of HOT. There were theoretical concerns related to possible mechanical complications from tubing, and at a very early stage there was a suggestion of an increase in admissions because of peripheral vascular disease in the oxygen-treated patients. However, as the study progressed, there were no significant differences in event rates between the two groups. Although more participants in the BMT arm than in the LTOT and NOT arms experienced one or more SAEs, the difference was not statistically significant.
Might oxygen be associated with an increase in survival? Although the difference between the survival curves is visually striking (see Figure 8), the difference in survival between the two groups was not statistically significant. Coupled with the results from the acute oxygen substudy, which did detect a favourable haemodynamic response to oxygen, the evidence is reassuring that HOT, at least when the fraction of inspired oxygen is < 30%, is safe.
However, the study was greatly underpowered to detect a difference in mortality. The premature end to the study reduced the chances of detecting a survival effect even further. It should be borne in mind that the target dose of oxygen at 15 hours per day is an almost arbitrary figure arrived at from analysis of patients in long-term trials of another condition. It remains possible that oxygen used for shorter periods of time might be beneficial for people with heart failure.
Why was the home oxygen therapy trial neutral?
The most likely explanation for the apparent lack of effect of LTOT for heart failure in the HOT trial is that oxygen has no effect on breathlessness in this clinical situation. There are several possible confounding issues.
Patient selection
Were the patients in the HOT study representative of patients with severe heart failure? If the patients were insufficiently ill at baseline, it is unlikely that they would benefit from treatment directed at palliative care. The study protocol was designed to ensure that only severely symptomatic patients were recruited, based on their NYHA status. However, the NYHA scale is relatively crude; those in class IV are breathless at rest (and are thus largely either bed-bound or in hospital) and therefore most of the patients recruited into HOT were class III patients (that is they were breathless on mild exertion). It may be that this level of symptom was simply insufficiently severe to allow any benefit from home oxygen. However, the KPS gives finer gradations of performance status and show that almost all the patients needed help with activities of daily living, consistent with a group of patients who were significantly limited by their condition.
On average, patients recruited to the HOT study were nearly 10 years older than patients in most studies of CHF (whose average age is typically around 60 years), suggesting that the patients were representative of patients seen in day-to-day practice. That the patients had severe heart failure is confirmed by a number of the baseline variables. Two-thirds had severe LV systolic dysfunction on echocardiogram, and the mean NT-proBNP at 4500 ng/l was very high.
The 6MWT distance was grossly reduced, at 120 m. The average distance covered by a similarly aged population in 6 minutes is around 660 m,78 but falls with age. 79 In an unselected population of patients with CHF of similar age, the median (interquartile range) 6MWT distance was 345 m (240–420 m). 80 Patients with a 6MWT distance of 120 m had an 11-fold higher risk of death than patients with a 6MWT distance above 376 m. 80
It does thus seem that patients recruited to HOT did have severe heart failure.
Prevalence of hypoxia
The patients with chronic airways disease who gain a survival advantage from home oxygen are severely hypoxic (PaO2 < 55 mmHg14). None of the patients in HOT was so severely hypoxaemic.
There is a persistent belief that breathlessness equates with hypoxia. Patients with treated chronic stable heart failure are commonly assumed to be hypoxic at rest or during exertion, but the overwhelming body of evidence demonstrates that they are not. 25,26,81–85 In the patients recruited to the HOT trial, arterial oxygen saturation was normal and there was no significant change in arterial oxygen saturation during exercise or during recovery from exercise.
The HOT trial was designed to assess the prevalence of hypoxia as part of the SDB syndrome. Epidemiological evidence suggests that SDB is very common in patients with heart failure when a cut-off of 15 episodes of apnoea/hypopnoea per hour of sleep is used as the diagnostic standard. 27 However, even though the patients in the trial had severe heart failure, the mean AHI at baseline was only 9.8 per hour, with more than 75% having an AHI below the diagnostic threshold of 15.
The lack of symptomatic benefit seen in the HOT trial may thus simply reflect the very low prevalence of hypoxia at rest, during exercise or overnight. There may be a very small subset of patients with severe hypoxia as a result of CHF alone, but we did not detect such patients despite specifically targeting the most severely symptomatic patients.
Adherence
We found that adherence to the 15 hours-per-day prescription of home oxygen was poor. In the LTOT arm, the mean usage was only 5.4 hours per day, with two-thirds of patients using the oxygen for < 8 hours per day. Only a small minority of patients used the oxygen as prescribed, although average use between readings often fluctuated, indicating that patients often had periods when they used the machine more or less.
These findings are broadly in line with reports from the use of HOT in patients with chronic airways disease (Table 40). There is a variety of reasons for poor adherence:
| Source | Subjects | Adherence |
|---|---|---|
| Evans et al.73 | 14 concentrator patients prescribed 15 hours per day | Mean 13.3 (SD 2) hours per day |
| Vergeret et al.86 | 159 hypoxic COPD patients | Fixed: mean 14 (SD 3) hours per day |
| Randomly assigned to fixed unit only or fixed + portable | Portable: mean 17 (SD 3.5) hours per day | |
| Walshaw et al.72 | 61 patients reassessed for use and prescription appropriateness | 45.9% inadequate prescription |
| 29.5% adherence with correct prescription | ||
| Howard et al.87 | 531 concentrators post use | If prescription < 15 hours per day then 9.9 hours per day |
| Compared prescription and concentrator clocks | If prescription > 15 hours per day then 13.4 hours per day | |
| Restrick et al.88 | 176 patients interviewed and followed up | 74% used > 12 hours per day |
| Morrison et al.89 | 630 LTOT patients (79% COPD): 3-year data | Mean 14.9 (SD 6) hours per day |
| 44% < 15 hours per day | ||
| Granados et al.90 | 62 LTOT patients (70% COPD) | 31% met all criteria for adherence to adequate prescription |
| 61% compliant | ||
| Pépin et al.16 | 930 COPD patients | 45% achieved > 15 hours per day |
| Compared prescription and actual use | Prescription mean 16 (SD 3) hours per day | |
| Actual use mean 14.5 (SD 5) hours per day | ||
| Ringbaek et al.91 | 125 of 182 LTOT patients | 65% had ‘acceptable adherence’ |
| Ambulatory use positively affected adherence | ||
| Atis et al.92 | 379 of 1100 patients responded to questionnaire | 28.2% self-reported use was > 15 hours per day |
| Mean 9 (SD 6.8) hours per day |
-
There was equipoise in prescribing the oxygen: because there is no evidence that oxygen prolongs survival, the investigators could not impress any mortality benefit on the patients, perhaps lessening the patients’ enthusiasm for oxygen compared with patients with chronic airways disease oxygen therapy.
-
As the findings from the qualitative substudy suggest, oxygen therapy is certainly burdensome. The physical limitations imposed by the oxygen tubing and the symptoms caused by the oxygen delivery (dry nose and mouth in particular) caused problems for some patients. Although patients were told that it was unnecessary to take oxygen with them (in cylinders) when they left the house, some were concerned at stopping oxygen when they left home. It’s possible that making oxygen cylinders available might improve adherence.
-
Patients had mixed reasons for taking part in the study, some thinking that they would receive better care when taking part in the study and who were perhaps less well motivated to use the oxygen. It is certainly the case that patients taking part in studies have a better prognosis generally. 93
-
Oxygen did not necessarily cause an immediate improvement in patients’ symptoms: as time on treatment passed, patients became less enthusiastic about oxygen use because of lack of perceived benefit, particularly in comparison with hoped-for benefit.
The poor adherence with the 15 hours per day prescription was the ultimate reason that the HOT trial came to a premature end. It is possible that oxygen may have conferred some benefit when used as short-burst therapy to relieve acute breathlessness: however, there was no evidence of sustained improvement in symptoms with oxygen therapy. Although there was some evidence of short-term reduction in breathlessness intensity as measured by the NRS, and longer-term improvement in subjective coping with breathlessness, we cannot be sure that these findings are attributable to oxygen and not simply to increased airflow over the nasal mucosae.
Substudies
Acute oxygen substudy (see Chapter 7)
The major aim of the oxygen substudy was to demonstrate the safety of oxygen given at the level to be used in the main HOT study. There had previously been concern that high concentration of inspired oxygen might be deleterious in people with heart disease, and there had never been any studies to show whether or not 28% oxygen had any haemodynamic effect. Some previous studies have suggested that there may be a small fall in pulmonary vascular resistance with oxygen given at > 90%,94 but other studies have suggested that 100% has no haemodynamic effect. 95 This is the first study to examine the effects of 28% oxygen on haemodynamic variables in patients with heart failure.
We found no evidence of a deleterious haemodynamic effect of 28% oxygen. There was, in fact, a small fall in pulmonary vascular resistance and a small increase in cardiac output with oxygen, findings suggesting small improvements in haemodynamics. The mechanisms behind the apparent improvement cannot be assessed from this experiment.
There are two major limitations to the findings. First, the patient group was a highly selected one, namely patients having cardiac catheterisation for clinical reasons, in large part for transplant assessment. Patients in the substudy tended to be younger than the patients recruited into the main HOT study, and tended to have only a single (cardiac) pathology. Second, we were able to give the oxygen for only a relatively brief period, and it is possible that longer exposure to increased inspired oxygen may have had later harmful effects.
The order of the two tests, namely air then oxygen, was not randomised and the patients were not blinded to the administration of oxygen.
Conclusion
The results of the acute haemodynamic substudy suggested that it is reasonable to use 28% oxygen in patients with severe heart failure and that there was no evidence that patients in the main HOT study were being exposed to a dangerous intervention.
Qualitative substudy (see Chapter 8)
Work in other areas of treatment adherence has resulted in the development of the Common Sense Model,96–98 which explains a patient’s adherence to treatment as the combined result of two main aspects of the way in which they perceive their illness: cognitive illness perception and emotional illness perception. Information and understanding about their medical condition, the way in which treatment may help and the aims of therapy are important in developing patients’ cognitive illness perception. For many, information comes from sources other than their health-care professional. Likewise, patients’ emotional response to their health problems will result from interplay between their life experience, their psychological make-up and coping mechanisms. If health-care professionals are not aware of such health and personal beliefs, then attempts to improve adherence may be unsuccessful.
The difficulties of LTOT, and patient concerns about overdependence, or even addiction, which contribute to poor adherence in clinical practice are described in Adherence70–75 and can be related easily to the Common Sense Model. Although the model, and others similar to it, refers to self-regulation in the context of chronic illness and its treatment rather than in the context of a clinical trial for people with chronic illness, we found no trial-specific issues which affected a participant’s adherence to oxygen therapy. Even though there is altruism associated with giving trial consent, there was a strong belief that participation in a clinical trial was a way of getting access to the best management for their heart failure.
Once in the trial, patients’ adherence to oxygen therapy was heavily influenced by their beliefs about the therapeutic target of oxygen (as immediate symptom benefit for breathlessness or for end-stage disease). Despite careful explanation to the contrary in the trial patient information leaflet, reinforced by discussion with the site principal investigators and research nurses, these beliefs were firmly held by some, learned through past experience of their chronic illness, past health-care interventions for that illness and lay wisdom. A further complication in some of those who did feel they were benefiting was a worry that they should become dependent upon the oxygen if they used it too much, even though there was explicit instruction to use it for at least 15 hours per day.
The strong belief as to the purpose of oxygen in its expected relationship to immediate symptom relief led to an apparent cognitive and emotional dissonance, which resulted in some participants preferring to have the concentrator in the house but using it only as a safety net for bad symptom days.
The participants’ response to the practical challenges and difficulties encountered by the oxygen mirrored their emotional response to their chronic illness, how they coped with it and how they had functioned, or not, within their family relationships and relationships with health professionals over many years. Thus, the same practical issues (noise, tubes), and same apparent sources of coping (e.g. family), resulted in different rates of adherence, which were consistent with the individual’s pattern of approach to stressful situations. The best adherence was seen in those, particularly those who were allocated NOT only, who found a way to incorporate the oxygen therapy into the routine of their daily lives (e.g. part of the routine of going to bed), and managed the disruptions to daily life in the home in a practical manner (e.g. moving bedrooms). Those who were unable to be flexible in their view of what was normal life appeared to be less compliant.
Conclusions
Participants viewed study participation in the trial both as an altruistic act and as a way of accessing optimal clinical care. Adherence or not in the oxygen arm did not appear to be specifically related to the context of a clinical trial.
There was a deep-seated belief that oxygen is a therapy for acute deterioration or for those with end-stage disease. Thus, the use of LTOT was counterintuitive despite clear explanation of the trial’s aim. This misunderstanding formed a poor basis for subsequent weighing up by the participant of the benefit–burden balance of the LTOT. In addition, the individual’s psychological make-up, ways of coping, and previous illness and life narrative influenced their emotional response to the burden imposed by oxygen therapy and affected whether or not they could incorporate oxygen therapy into the rhythm of daily life. Those who could appeared to use the oxygen more often than those who could not.
Limitations
We were unable to blind oxygen delivery as the original study design had planned. However, the open trial was a pragmatic one which addressed the important question of treatment effectiveness. The trial was stopped early because of poor adherence to the 15 hours per day prescription. The prescription was based on extrapolation from studies of patients with a different pathology, chronic airways disease, who had severe hypoxia. It may be that shorter periods of exposure might have been effective, in terms of either symptom relief or preventing hospitalisation.
The a priori sample size calculation was based on the independent-sample t-test at 6 months, giving a conservative estimate of 200 participants to detect a difference in a MLwHF questionnaire score of 10 points, assuming a SD of 25 points (effect size of 0.4), at 80% power with 5% significance. Allowing for 10% attrition at 6 months, we needed to recruit and randomise 222 participants.
The primary result was the treatment effect at 6 months extracted from an adjusted covariance pattern model. Adjusting for baseline covariates by using an analysis of covariance (ANCOVA) increases the statistical power compared with a t-test for the same number of participants. With an ANCOVA, the sample size required to detect the same difference can be reduced by a factor of (1 – ρ2), where ρ is the correlation between the baseline covariates and the outcome. For example, with a sample size of 200 at 6 months, assuming a moderate correlation of 0.5 between the baseline covariates and MLwHF questionnaire score at 6 months, we would have had 90% power to detect an effect of magnitude 0.4 with an ANCOVA compared with 80% power with the t-test.
An ANCOVA can use data available only for the time point of interest, here 6 months; however, in the covariance pattern model, we could include the 3- and 12-month post-randomisation assessments. In a repeated-measures model, missing data are less problematic than when using a t-test, as observations at each time point influence estimates of treatment effects at every other time point, owing to the specification of a covariance pattern for the within-patient repeated measures. Thus, patients whose observations are limited to the 3- and/or 12-month time points will nevertheless be taken into account when the estimate at 6 months is made. Clearly, such individuals will not influence the estimates as greatly as individuals whose data are complete, but the philosophy is that some data from a patient is better than none. The extra information further increases the power available from the ANCOVA; however, it is beyond our knowledge to quantify this extra benefit and we have found no literature that discusses this exact problem.
Calculating a sample size based on an adjusted repeated-measures analysis is complicated and requires knowledge of parameters that are not known in advance (particularly the correlation between baseline covariates and the outcome). The calculations are sensitive to initial assumptions and so we used a t-test to provide a conservative estimate to minimise the risk of underpowering the trial.
A total of 88 participants provided usable primary outcome data at 6 months; 83 also had complete baseline data for MLwHF questionnaire score, age, NT-proBNP level and creatinine level. To detect a 10-point difference with a SD of 25 at 6 months, the estimated power available in a two-sided test of two independent means is 46% but by ANCOVA (adjusting for the covariates and assuming a correlation between baseline covariates and 6 months MLwHF questionnaire score of 0.5) is 55%.
As a result of the trial being underpowered, the risk of a type II error is increased and any of the results, especially those with an associated p-value greater than 0.05, are inconclusive. The low sample size means it is not possible to distinguish whether the lack of a detected effect is a result of the trial being underpowered or whether there is a true lack of effect. In addition, a lack of adherence with oxygen may have diluted any effect of long-term home oxygen.
The MLwHF questionnaire is the most widely used instrument for the assessment of QoL in patients with heart failure. It asks patients to rate the severity of their symptoms on a 6-point scale (from 0 to 5) but does not assess the impact of those symptoms on the patient’s life directly. It might be that another scale, such as the Kansas City Cardiomyopathy Questionnaire,99 would have been more sensitive to changes in health state. The Kansas City Cardiomyopathy Questionnaire asks patients to record the impact that a symptom has on their life rather than merely the presence or absence of a symptom. It might be that the patients in the HOT study were sufficiently habituated to their symptoms that although they were, perhaps severely, breathless, they were used to it and thus felt that the breathlessness had little impact on their life.
Conclusions
The prevalence of hypoxia in patients with severe heart failure, at rest, following exercise and during an overnight sleep test, is low. There is no evidence that LTOT, although it is safe, improves the symptoms, prognosis or severity of heart failure in patients with severe CHF at 6 months. There is no evidence to support the use of HOT in patients with heart failure but, as the study was terminated early, we cannot exclude the possibility of a type II error.
Recommendations for future research
We suggest that two further studies might be appropriate:
-
a trial of patients with severe heart failure randomised to have emergency oxygen supply in the house, supplied by cylinders rather than oxygen concentrator, powered to detect a reduction in admissions to hospital
-
a study of bed-bound patients with heart failure who are in the last few weeks of life, powered to detect changes in symptom severity.
However, given the problems with the conduct of HOT encountered in the present study, mounting such a trial would face similar problems with both blinding (and the use of a sham device) and adherence.
The HOT investigators are in close contact with the investigators of the OXYGEN-HF trial (ACTRN12609000103268). OXYGEN-HF is a randomised trial of 285 patients comparing the effects of HOT, medical air (placebo) and no treatment (control) on all-cause admissions to hospital at 6 months. Many of the trial’s secondary end points are the same as ours, and the findings of both studies will be of value in a meta-analysis.
Acknowledgements
We would particularly like to acknowledge the contribution made to the study by the patients. The trial was demanding for the patients in terms of both disruption to their lives (and to their houses) and data collection. We are particularly grateful to Mr Patrick Foulk whose help and advice was vital. Mr Foulk also helped with drafting the Plain English summary. Thank you.
The trial could not have happened without the support of the recruiting centres, and we gratefully acknowledge the support we received from each one. We particularly acknowledge Mr Dominic Fellows and Mr Paul Atkin, and the nurses at the Hull centre, who provided a great deal of support to the other recruiting centres.
The trial owes a large debt of gratitude to Dr Ian Harvey, the trial manager, without whose energy and support the trial would have been impossible. Mr James Illingworth on behalf of the trial sponsors, the Hull and East Yorkshire NHS Hospitals Trust, also provided invaluable support.
We also acknowledge the help, support and advice of the HTA programme, particularly Mrs Lesley Dodd, the programme manager, who was unfailingly helpful and encouraging. The investigators have always felt well supported by the HTA programme.
The home oxygen therapy trial
Chief investigator
Professor Andrew Clark, Honorary Consultant Cardiologist, Department of Academic Cardiology, University of Hull, Castle Hill Hospital, Cottingham, UK.
Trial manager
Dr Ian Harvey, Academic Cardiology, Castle Hill Hospital, Cottingham, UK.
Trial co-ordinators
Sarah Cockayne, York Trials Unit, University of York, York, UK.
Sara Rodgers, York Trials Unit, University of York, York, UK.
Statistician
Caroline Fairhurst, Statistician, York Trials Unit, University of York, York , UK.
Co-investigators
Dr Victoria Allgar, Senior Lecturer in Medical Statistics, Hull York Medical School, University of York, Heslington, York, UK.
Dr Michael Greenstone, Consultant Chest Physician and Honorary Clinical Senior Lecturer, Medical Chest Unit, Castle Hill Hospital, Cottingham, East Yorkshire, UK.
Dr Susan Griffin, Senior Research Fellow, Centre for Health Economics, University of York, York, UK.
Professor Miriam Johnson, Professor of Palliative Medicine, Hull York Medical School, University of Hull, Hull, UK.
Professor Jerry Murphy, Consultant Cardiologist, Darlington Memorial Hospital, Darlington, UK.
Professor Iain Squire, Cardiovascular Medicine, Department of Cardiovascular Sciences, and National Institute for Health Research Cardiovascular Biomedical Research Unit, Glenfield Hospital, Leicester, UK.
Professor David Torgerson, Director of York Trials Unit, University of York, York, UK.
The study committees
Trial Steering Committee
Independent chairperson
Professor Henry J Dargie, Honorary Senior Research Fellow, University of Glasgow, Glasgow, UK.
Independent members
Professor Andrew Coats, Faculty of Medicine, University of Sydney, Australia.
Professor Martin Cowie, National Heart and Lung Institute, Dovehouse Street, London, UK.
Patient representative
Patrick Foulk.
Researchers
Professor Andrew Clark, Professor Miriam Johnson and Dr Ian Harvey.
Data Monitoring and Ethics Committee
Professor Theresa McDonagh, Consultant Cardiologist, King’s College Hospital, London, UK.
Dr Roy S Gardner, Consultant Cardiologist, Scottish Advanced Heart Failure Service, Golden Jubilee National Hospital, Clydebank, UK.
Dr Suzanna Hardman, Consultant Cardiologist with an interest in Community Cardiology, The Whittington and University College London Hospitals, London, UK.
Gillian Worthy, Statistician, Kleijnen Systematic Reviews Ltd, Escrick Business Park, Escrick, York, UK.
Contributions of authors
Professor Andrew L Clark (Professor of Clinical Cardiology) conceived the study and applied for, and received, the original grant. He was principal investigator for the study. He conducted the review of the data for HOT in heart failure and is the principle author of this report.
Miriam Johnson (Professor of Palliative Care) cowrote the initial grant application, trial protocol (main study and qualitative substudy) and ethics application (main study and qualitative substudy), was a member of the trial management group, provided palliative care and symptom outcome assessment expertise, was principal investigator for the qualitative substudy, conducted data collection and analysis for the qualitative substudy and contributed to writing the report.
Caroline Fairhurst (Statistician) conducted the statistical analysis of the main trial, prepared the results for publication, and contributed to the preparation and review of the final report.
David Torgerson (Director of York Trials Unit, Health Sciences) helped design the study, gave trial methodological advice and was a co-applicant.
Sarah Cockayne (Research Fellow, Health Sciences) was a trial co-ordinator on the study, and contributed to the design of the study and to the preparation and review of the final report.
Sara Rodgers (Research Fellow, Health Sciences) was a trial co-ordinator on the study, and contributed to the preparation and review of the final report.
Susan Griffin (Senior Research Fellow, Health Economics) analysed the EQ-5D data, and contributed to the preparation and review of the final report.
Victoria Allgar (Statistician) provided statistical support during the design and conduct of the main trial.
Lesley Jones (Senior Lecturer, Social Sciences) cowrote the qualitative substudy protocol and provided senior qualitative expertise regarding data analysis and report writing.
Samantha Nabb (Health Psychologist, University of Hull) helped to create the interview schedule investigating the patient and carer experience of the oxygen concentrator, interviewed patients and carers and analysed the data.
Ian Harvey (Trial Manager) contributed to the design, development and delivery of the trial, and to the preparation and review of the final report.
Iain Squire (Professor of Cardiovascular Medicine) helped design the study, contributed to the writing of the report and was a co-applicant.
Jerry Murphy (Professor of Cardiovascular Medicine) helped design the study, contributed to the writing of the report and was a co-applicant.
Michael Greenstone (Consultant Chest Physician and Honorary Clinical Senior Lecturer) co-wrote the initial grant application and trial protocol, and contributed to the writing of the report.
Publications
Clark AL, Johnson MJ, Squire I. Does home oxygen benefit people with chronic heart failure? BMJ 2011;342:d234.
Davidson PM, Johnson J. Update on the role of palliative oxygen. Curr Opin Support Palliat Care 2011;5:87–91.
Gadoud A, Johnson MJ. What palliative care clinicians need to know about heart failure. Progress Palliat Care 2014;22:26–31.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Cowie MR, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Suresh V, et al. Incidence and aetiology of heart failure; a population-based study. Eur Heart J 1999;20:421-8. http://dx.doi.org/10.1053/euhj.1998.1280.
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606-19. http://dx.doi.org/10.1161/HHF.0b013e318291329a.
- National Heart Failure Audit April 2012–March 2013. London: National Centre for Cardiovascular Prevention and Outcomes, University College London; 2013.
- Cleland JG, Clark AL. Delivering the cumulative benefits of triple therapy to improve outcomes in heart failure: too many cooks will spoil the broth. J Am Coll Cardiol 2003;42:1234-7. http://dx.doi.org/10.1016/S0735-1097(03)00948-3.
- Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, et al. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA 1989;262:907-13. http://dx.doi.org/10.1001/jama.1989.03430070055030.
- Currow DC, McDonald C, Oaten S, Kenny B, Allcroft P, Frith P, et al. Once daily opioids for chronic dyspnoea: a dose increment and pharmacovigilance study. J Pain Symptom Manage 2011;42:388-99. http://dx.doi.org/10.1016/j.jpainsymman.2010.11.021.
- Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003;327:523-8. http://dx.doi.org/10.1136/bmj.327.7414.523.
- Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax 2002;57:939-44. http://dx.doi.org/10.1136/thorax.57.11.939.
- Johnson MJ, McDonagh T, Harkness A, MacKay S, Dargie H. Morphine for breathlessness in chronic heart failure. Eur J Heart Fail 2002;4:753-6. http://dx.doi.org/10.1016/S1388-9842(02)00158-7.
- Oxberry SG, Torgerson DJ, Bland JM, Clark AL, Cleland JG, Johnson MJ. Short-term opioids for breathlessness in stable chronic heart failure: a randomized controlled trial. Eur J Heart Fail 2011;13:1006-12. http://dx.doi.org/10.1093/eurjhf/hfr068.
- Oxberry SG, Bland JM, Clark AL, Cleland JG, Johnson MJ. Repeat dose opioids may be effective for breathlessness in chronic heart failure if given for long enough. J Palliat Med 2013;16:250-5. http://dx.doi.org/10.1089/jpm.2012.0270.
- Medical Research Council Working Party . Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet 1981;1:681-6.
- Nocturnal Oxygen Therapy Trial Group . Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980;93:391-8. http://dx.doi.org/10.7326/0003-4819-93-3-391.
- Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005;4. http://dx.doi.org/10.1002/14651858.cd001744.pub2.
- Uronis H, McCrory DC, Samsa G, Currow D, Abernethy A. Symptomatic oxygen for non-hypoxaemic chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011;6. http://dx.doi.org/10.1002/14651858.cd006429.pub2.
- Pépin JL, Barjhoux CE, Deschaux C, Brambilla C. Long term oxygen therapy at home. Compliance with medical prescription and effective use of therapy. Chest 1996;109:1144-50. http://dx.doi.org/10.1378/chest.109.5.1144.
- Avdeev SN, Aisanov ZR, Chuchalin AG. Compliance as a critical issue in long term oxygen therapy. Mondali Arch Chest Dis 1999;54:61-6.
- Katsenos S, Froudarakis ME, Charisis A, Vassiliou MP, Constantopoulos SH. Long term oxygen therapy in Ioannina. Respiration 2004;71:619-24. http://dx.doi.org/10.1159/000081763.
- Croxton TL, Bailey WC. Long term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med 2006;174:373-8. http://dx.doi.org/10.1164/rccm.200507-1161WS.
- Thomas M, Malmcrona R, Shillingford J. Haemodynamic effects of oxygen in patients with acute myocardial infarction. Br Heart J 1965;27:401-7. http://dx.doi.org/10.1136/hrt.27.3.401.
- Haque WA, Boehmer J, Clemson BS, Leuenberger UA, Silber DH, Sinoway LI. Hemodynamic effects of supplemental oxygen administration in congestive heart failure. J Am Coll Cardiol 1996;27:353-7. http://dx.doi.org/10.1016/0735-1097(95)00474-2.
- Mak S, Azevedo ER, Liu PP, Newton GE. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest 2001;120:467-73. http://dx.doi.org/10.1378/chest.120.2.467.
- Moore DP, Weston AR, Hughes JM, Oakley CM, Cleland JG. Effects of increased inspired oxygen concentrations on exercise performance in chronic heart failure. Lancet 1992;339:850-3. http://dx.doi.org/10.1016/0140-6736(92)90288-E.
- Russell SD, Koshkarian GM, Medinger AE, Carson PE, Higginbotham MB. Lack of effect of increased inspired oxygen concentrations on maximal exercise capacity or ventilation in stable heart failure. Am J Cardiol 1999;84:1412-16. http://dx.doi.org/10.1016/S0002-9149(99)00587-1.
- Clark AL, Volterrani M, Swan JW, Coats AJ. The increased ventilatory response to exercise in chronic heart failure: relation to pulmonary pathology. Heart 1997;77:138-46. http://dx.doi.org/10.1136/hrt.77.2.138.
- Clark AL, Coats AJ. Usefulness of arterial blood gas estimations during exercise in patients with chronic heart failure. Br Heart J 1994;71:528-30. http://dx.doi.org/10.1136/hrt.71.6.528.
- Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 2007;9:251-7. http://dx.doi.org/10.1016/j.ejheart.2006.08.003.
- Pembrey MS. Observations on Cheyne–Stokes respiration. J Pathol Bacteriol 1908;12:259-66. http://dx.doi.org/10.1002/path.1700120211.
- Toyama T, Seki R, Kasama S, Isobe N, Sakurai S, Adachi H, et al. Effectiveness of nocturnal home oxygen therapy to improve exercise capacity, cardiac function and cardiac sympathetic nerve activity in patients with chronic heart failure and central sleep apnea. Circ J 2009;73:299-304. http://dx.doi.org/10.1253/circj.CJ-07-0297.
- Sasayama S, Izumi T, Matsuzaki M, Matsumori A, Asanoi H, Momomura S, et al. Improvement of quality of life with nocturnal oxygen therapy in heart failure patients with central sleep apnea. Circ J 2009;73:1255-62. http://dx.doi.org/10.1253/circj.CJ-08-1210.
- Currow DC, Agar M, Smith J, Abernethy AP. Does palliative home oxygen improve dyspnoea? A consecutive cohort study. Palliat Med 2009;23:309-16. http://dx.doi.org/10.1177/0269216309104058.
- Uronis HE, Currow DC, McCrory DC, Samsa GP, Abernethy AP. Oxygen for relief of dyspnoea in mildly- or non-hypoxaemic patients with cancer: a systematic review and meta-analysis. Br J Cancer 2008;98:294-9. http://dx.doi.org/10.1038/sj.bjc.6604161.
- Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.cd004769.pub2.
- Ben-Aharon I, Gafter-Gvili A, Paul M, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review. J Clin Oncol 2008;26:2396-404. http://dx.doi.org/10.1200/JCO.2007.15.5796.
- Booth S, Wade R, Johnson M, Kite S, Swannick M, Anderson H. The use of oxygen in the palliation of breathlessness. A report of the expert working group of the Scientific Committee of the Association of Palliative Medicine. Respir Med 2004;98:66-77. http://dx.doi.org/10.1016/j.rmed.2003.08.008.
- Abernethy AP, McDonald CF, Frith PA, Clark K, Herndon JE, . Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010;376:784-93. http://dx.doi.org/10.1016/S0140-6736(10)61115-4.
- Guyatt GH, McKim DA, Austin P, Bryan R, Norgren J, Weaver B, et al. Appropriateness of domiciliary oxygen delivery. Chest 2000;118:1303-8. http://dx.doi.org/10.1378/chest.118.5.1303.
- Abernethy AP, Currow DC, Frith P, Fazekas BS. Prescribing palliative oxygen: a clinician survey of expected benefit and patterns of use. Palliat Med 2005;19.
- Currow DC, Christou T, Smith J, Carmody S, Lewin G, Aoun S, et al. Do terminally ill people who live alone miss out on home oxygen treatment? An hypothesis generating study. J Palliat Med 2008;11:1015-22. http://dx.doi.org/10.1089/jpm.2008.0016.
- Chapman TH, Seymour J, Ho TBL. Home oxygen prescribing practices at a UK district general hospital. Am J Respir Crit Care Med 2009;179. http://dx.doi.org/10.1164/ajrccm-conference.2009.179.1_meetingabstracts.a2299.
- Schwartzstein RM, Lahive K, Pope A, Weinberger SE, Weiss JW. Cold facial stimulation reduces breathlessness induced in normal subjects. Am Rev Respir Dis 1987;136:58-61. http://dx.doi.org/10.1164/ajrccm/136.1.58.
- Wohleber AM, McKitrick DS, Davis SE. Designing research with hospice and palliative care populations. Am J Hosp Palliat Care 2012;29:335-45. http://dx.doi.org/10.1177/1049909111427139.
- LeBlanc TW, Lodato JE, Currow DC, Abernethy AP. Overcoming recruitment challenges in palliative care clinical trials. J Oncol Pract 2013;9:277-82. http://dx.doi.org/10.1200/JOP.2013.000996.
- Jordhøy MS, Kaasa S, Fayers P, Ovreness T, Underland G, Ahlner-Elmqvist M. Challenges in palliative care research; recruitment, attrition and adherence: experience from a randomized controlled trial. Palliat Med 1999;13:299-310. http://dx.doi.org/10.1191/026921699668963873.
- Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart questionnaire: reliability and validity during a randomized, double blind, placebo-controlled trial of pimobendan. Am Heart J 1992;124:1017-25. http://dx.doi.org/10.1016/0002-8703(92)90986-6.
- Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol 1993;71:1106-7. http://dx.doi.org/10.1016/0002-9149(93)90582-W.
- Witham MD, Crighton LJ, McMurdo MET. Using an individualised quality of life measure in older heart failure patients. Int J Cardiol 2007;116:40-5. http://dx.doi.org/10.1016/j.ijcard.2006.03.026.
- Rector TS, Tschumperlin LK, Kubo SH, Bank AJ, Francis GS, McDonald KM, et al. Use of the Living With Heart Failure questionnaire to ascertain patients’ perspectives on improvement in quality of life versus risk of drug-induced death. J Card Fail 1995;1:201-6. http://dx.doi.org/10.1016/1071-9164(95)90025-X.
- Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999;52:861-73. http://dx.doi.org/10.1016/S0895-4356(99)00071-2.
- Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845-53. http://dx.doi.org/10.1056/NEJMoa013168.
- Cleland JG, Calvert MJ, Verboven Y, Freemantle N. Effects of cardiac resynchronization therapy on long term quality of life: an analysis from the Cardiac Resynchronisation-Heart Failure (CARE-HF) study. Am Heart J 2009;157:457-66. http://dx.doi.org/10.1016/j.ahj.2008.11.006.
- Brooks R. EuroQoL Group . EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- EuroQol Group . EQ-5D A Measure of Health-Related Quality of Life Developed by the EuroQol Group. User Guide 2001. www.euroqol.org (accessed 24 June 2015).
- Wilcock A, Crosby V, Clarke D, Tattersfield A. Repeatability of breathlessness measurements in cancer patients. Thorax 1999;54. http://dx.doi.org/10.1136/thx.54.4.374b.
- Gift AG, Narsavage G. Validity of the numerical rating scale as a measure of dyspnoea. Am J Crit Care 1998;7:200-4.
- Oxberry SG, Bland JM, Clark AL, Cleland JG, Johnson MJ. Minimally clinically important difference in chronic breathlessness: every little helps. Am Heart J 2012;164:229-35. http://dx.doi.org/10.1016/j.ahj.2012.05.003.
- Johnson MJ, Bland JM, Abernethy A, Currow DC. Clinically important differences in chronic refractory breathlessness. J Pain Symptom Manage 2013;46:957-63. http://dx.doi.org/10.1016/j.jpainsymman.2013.01.011.
- Haworth JE, Moniz-Cook E, Clark AL, Wang M, Waddington R, Cleland JG. Prevalence and predictors of anxiety and depression in a sample of chronic heart failure patients with left ventricular systolic dysfunction. Eur J Heart Fail 2005;7:803-8. http://dx.doi.org/10.1016/j.ejheart.2005.03.001.
- Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The six minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919-23.
- Olsson LG, Swedberg K, Clark AL, Witte KK, Cleland JG. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review. Eur Heart J n.d.;26:778-93. http://dx.doi.org/10.1093/eurheartj/ehi162.
- Ingle L, Shelton RJ, Rigby AS, Nabb S, Clark AL, Cleland JG. The reproducibility and sensitivity of the 6-min walk test in elderly patients with chronic heart failure. Eur Heart J 2005;26:1742-51. http://dx.doi.org/10.1093/eurheartj/ehi259.
- Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity and guidelines. J Clin Oncol 1984;2:187-93.
- Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer 1984;53:2002-7. http://dx.doi.org/10.1002/1097-0142(19840501)53:9<2002::AID-CNCR2820530933>3.0.CO;2-W.
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. http://dx.doi.org/10.1016/0895-4356(94)90129-5.
- Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control 1974;19:716-23. http://dx.doi.org/10.1109/TAC.1974.1100705.
- Pickering RM, Weatherall M. The analysis of continuous outcomes in multi-centre trials with small centre sizes. Stat Med 2007;26:5445-56. http://dx.doi.org/10.1002/sim.3068.
- Ariti CA, Cleland JGF, Pocock SJ, Pfeffer MA, Swedberg K, Granger CB, et al. Days alive and out of hospital and the patient journey in patients with heart failure: Insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J 2011;162:900-6. http://dx.doi.org/10.1016/j.ahj.2011.08.003.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726-32. http://dx.doi.org/10.7326/0003-4819-152-11-201006010-00232.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Hayashi A, Tatsumi K, Kato K, Sakuma T, Okada O, Kimura H, et al. Compliance with long term home oxygen therapy. Nihon Kyobu Shikkan Gakkai Zasshi 1996;34:45-51.
- Peckham DG, McGibbon K, Tonkinson J, Plimbley G, Pantin C. Improvement in patient adherence with long term oxygen therapy following formal assessment with training. Respir Med 1998;92:1203-6. http://dx.doi.org/10.1016/S0954-6111(98)90422-X.
- Walshaw MJ, Lim R, Evans CC, Hind CRK. Factors influencing the adherence of patients using oxygen concentrators for long term home oxygen therapy. Respir Med 1990;84:331-3. http://dx.doi.org/10.1016/S0954-6111(08)80062-5.
- Evans TW, Waterhouse J, Howard P. Clinical experience with the oxygen concentrator. BMJ 1983;287:459-61. http://dx.doi.org/10.1136/bmj.287.6390.459.
- Dilworth JP, Higgs CMB, Jones PA, White RJ. Acceptability of oxygen concentrators: the patients’ views. Br J Gen Pract n.d.;40:415-17.
- Heaton RK, Grant I, McSweeny AJ, Adams KM, Petty TL. Psychologic effects of continuous and nocturnal oxygen therapy in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med 1983;143:1941-7. http://dx.doi.org/10.1001/archinte.1983.00350100121023.
- Currow DC, Fazekas B, Abernethy AP. Oxygen use – patients define symptomatic benefit discerningly. J Pain Symptom Manage 2007;34:113-14. http://dx.doi.org/10.1016/j.jpainsymman.2007.03.004.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analyzing Qualitative Data. London: Routledge; 1994.
- Camarri B, Eastwood PR, Cecins NM, Thompson PJ, Jenkins S. Six minute walk distance in healthy subjects aged 55–75 years. Respir Med 2006;100:658-65. http://dx.doi.org/10.1016/j.rmed.2005.08.003.
- Casanova C, Celli BR, Barria P, Casas A, Cote C, de Torres JP, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J 2011;37:150-6. http://dx.doi.org/10.1183/09031936.00194909.
- Ingle L, Rigby AS, Carroll S, Butterly R, King RF, Cooke CB, et al. Prognostic value of the 6 min walk test and self-perceived symptom severity in older patients with chronic heart failure. Eur Heart J 2007;28:560-8. http://dx.doi.org/10.1093/eurheartj/ehl527.
- Sullivan MJ, Higginbotham MB, Cobb FR. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation 1988;77:552-9. http://dx.doi.org/10.1161/01.CIR.77.3.552.
- Rubin SA, Brown HV, Swan HJ. Arterial oxygenation and arterial oxygen transport in chronic myocardial failure at rest, during exercise and after hydralazine treatment. Circulation 1982;66:143-8. http://dx.doi.org/10.1161/01.CIR.66.1.143.
- Munger MA, Stanek EJ, Nara AR, Strohl KP, Decker MJ, Nair RN. Arterial oxygen saturation in chronic congestive heart failure. Am J Cardiol 1994;73:180-5. http://dx.doi.org/10.1016/0002-9149(94)90211-9.
- Herrlin B, Sylvén C. Increased arterial oxygen content – an important compensatory mechanism in chronic moderate heart failure. Cardiovasc Res 1991;25:384-90. http://dx.doi.org/10.1093/cvr/25.5.384.
- Agostini PG, Wasserman K, Perego GB, Marenzi GC, Guazzi M, Assanelli E, et al. Oxygen transport to muscle during exercise in chronic congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol 1997;79:1120-4. http://dx.doi.org/10.1016/S0002-9149(97)00061-1.
- Vergeret J, Brambilla C, Mounier L. Portable oxygen therapy: use and benefit in hypoxaemic COPD patients on long term oxygen therapy. Eur Respir J 1989;2:20-5.
- Howard P, Waterhouse JC, Billings CG. Compliance with long term oxygen therapy by concentrator. Eur Respir J 1992;5:128-9.
- Restrick LJ, Paul EA, Braid GM, Cullinan P, Moore-Gillon J, Wedzicha JA. Assessment and follow-up of patients prescribed long term oxygen treatment. Thorax 1993;48:708-13. http://dx.doi.org/10.1136/thx.48.7.708.
- Morrison D, Skwaraski K, MacNee W. Review of the prescription of domiciliary long term oxygen therapy in Scotland. Thorax 1995;50:1103-5. http://dx.doi.org/10.1136/thx.50.10.1103.
- Granados A, Escarrabill J, Borras JM, Rodriguez-Roisin R. The importance of process variables analysis in assessment of long term oxygen therapy by concentrator. Respir Med 1997;91:89-93. http://dx.doi.org/10.1016/S0954-6111(97)90073-1.
- Ringbaek T, Lange P, Viskum K. Compliance with LTOT and consumption of mobile oxygen. Respir Med 1999;93:333-7. http://dx.doi.org/10.1016/S0954-6111(99)90314-1.
- Atis S, Tutluoglu B, Bugdayci R. Characteristics and adherence of patients receiving long term oxygen therapy in Turkey. Monaldi Arch Chest Dis 2001;56:105-9.
- Clark AL, Lammiman MJ, Goode K, Cleland JG. Is taking part in clinical trials good for your health? A cohort study. Eur J Heart Fail 2009;11:1078-83. http://dx.doi.org/10.1093/eurjhf/hfp133.
- Semigran MJ, Cockrill BA, Kacmarek R, Thompson BT, Zapol WM, Dec GW, et al. Hemodynamic effects of inhaled nitric oxide in heart failure. J Am Coll Cardiol 1994;24:982-8. http://dx.doi.org/10.1016/0735-1097(94)90859-1.
- Mahajan A, Shabanie A, Varshney SM, Marijic J, Sopher MJ. Inhaled nitric oxide in the preoperative evaluation of pulmonary hypertension in heart transplant candidates. J Cardiothorac Vasc Anesth 2007;21:51-6. http://dx.doi.org/10.1053/j.jvca.2006.01.028.
- Leventhal H, Brissette I, Leventhal EA, Cameron LD, Leventhal H. The Self-Regulation of Health and Illness Behaviour. London: Routledge; 2003.
- Burish TG, Bradley LA, Nerenz DR, Leventhal H, Burish TG, Bradley LA. Coping with Chronic Disease: Research and Applications. New York, NY: Academic Press; 1983.
- Hagger MS, Orbell S. A meta-analytic review of the common-sense model of illness representations. Psychol Health 2003;18:141-82. http://dx.doi.org/10.1080/088704403100081321.
- Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245-55. http://dx.doi.org/10.1016/S0735-1097(00)00531-3.
Appendix 1 Original Health Technology Assessment commissioning brief
NHS R&D Health Technology Assessment programme HTA number 06/80
Home oxygen therapy for chronic heart failure
Introduction
The aim of the HTA programme is to ensure that high quality research information on the costs, effectiveness and broader impact of health technologies is produced in the most efficient way for those who use, manage, provide care in or develop policy for the NHS. Topics for research are identified and prioritised to meet the needs of the NHS. Health technology assessment forms the largest portfolio of work in the NHS Research and Development Programme and each year about fifty new studies are commissioned to help answer questions of direct importance to the NHS. The studies include both primary research and evidence synthesis.
Question
What is the clinical and cost-effectiveness of home oxygen therapy in addition to standard care for patients with chronic heart failure?
1 Technology
Home oxygen therapy plus standard care.
2 Patient group
Patients with chronic heart failure and chronic arterial hypoxia. Further inclusion criteria to be established in Phase I of study.
3 Setting
Home.
4 Control or comparator treatment
Standard care.
5 Design
Two stage
Stage 1: a feasibility study: An assessment of the practical and ethical issues involved in carrying out a RCT, and the determination of the most appropriate inclusion criteria for a RCT including at least a definition of hypoxia in terms of PaO2 and of heart failure in terms of ejection fraction (if researchers feel other definitions are more clinically appropriate they should justify their decision), the most appropriate oxygen flow rate and timing, and the most appropriate mechanism of delivery. Stage 2: a randomised controlled trial to assess the use of home oxygen therapy in addition to standard care for patients with heart failure. Results for patients with different grades of chronic heart failure will be modelled.
6 Primary outcomes
Stage 1
The applicants should propose appropriate objective success criteria to move onto stage 2. These might include approval from ethics committees, centres signed up to participate, demonstration of equipoise among clinicians likely to enter patients into a trial and the ability to recruit adequate numbers of patients to the trial.
Stage 2
Mortality, QoL and quality-adjusted life-years (QALYs) gained. Secondary outcomes: breathlessness, drowsiness, mortality, adverse effects, functional capacity, cost-effectiveness.
7 Minimum duration of follow-up
Two years.
8 Note to applicants
Please submit separate costings for Stage 1 and Stage 2 in the outline application: the costs should be detailed in the ‘Summary of Project’ section on page 2 of the electronic application form. The combined cost of both stages should be entered in the ‘Research grant’ box on page 1 of the form. Stage 2 will only proceed if the pre-agreed success criteria for stage 1 are met. If full proposals are requested, applicants will need to submit protocols for both stages of the study. In the event of a small gap between stage 1 and stage 2 funding may be available to ensure continuity.
Background to commissioning brief
Patients with chronic heart failure may develop arterial hypoxaemia, which can have acute and chronic adverse effects on cardiac function. Patients with grade III(b) or IV heart failure and chronic arterial hypoxaemia have particular disturbance in breathing at night, which causes disturbed sleep, daytime sleepiness and reduced ability to function. Up to 50% die within 1 year.
Oxygen therapy may be provided for continuous use by patients with chronic hypoxaemia at home. However, current recommendations by the Royal College of Physicians are not based on clear evidence of effectiveness or cost-effectiveness. There is a lack of evidence for oxygen therapy in patients with chronic heart failure.
Notes to applicants
For many of the questions posed by the HTA programme, a randomised controlled trial is likely to be the most appropriate method of providing an answer. However, there may be practical or ethical reasons why this might not be possible. Applicants proposing other research methods are invited to justify these choices.
Applicants are asked to:
-
Follow the Medical Research Council’s Good Clinical Practice guidelines (http://www.mrc.ac.uk/pdf-ctg.pdf) when planning how studies, particularly RCTs, will be supervised. Further advice specific to each topic will be given by the HTA programme at full proposal and contract stages.
-
Note that trials involving medicinal products must comply with ‘The Medicines for Human Use (Clinical Trials) Regulations 2004’. In the case of such trials, the Department of Health (DH) expects the employing institution of the chief investigator to be nominated as the sponsor. Other institutions may wish to take on this responsibility or agree co-sponsorship with the employing institution. The DH is prepared to accept the nomination of multiple sponsors.
Applicants who are asked to submit a full proposal will need to obtain confirmation of a sponsor(s) to complete their application. The DH reserve the right to withdraw from funding the project if they are not satisfied with the arrangements put in place to conduct the trial.
The MHRA (info@mhra.gsi.gov.uk, http://www.mhra.gov.uk) can provide guidance whether your trial would be covered by the regulations. The DH/MRC website (http://www.ct-toolkit.ac.uk/) also contains the latest information about Clinical Trials regulations and a helpful FAQ page.
Making an application
If you wish to submit an outline proposal on this topic, complete the electronic application form and return it to the HTA Commissioning Manager at the National Coordinating Centre for Health Technology Assessment, Mailpoint 728 Boldrewood, University of Southampton, Southampton SO16 7PX by 7 February 2007. Outline applications will be considered by the HTA Commissioning Board at its meeting in July 2007. If they are acceptable, investigators will be given a minimum of eight weeks to submit a full proposal.
Applications received after 1300 hours on the due date will not be considered.
Appendix 2 Regulatory approvals and details of study sites
| MREC approvals | Change |
|---|---|
| Original ethics approval, 24 August 2009 | Approval for the NEON study |
| Substantial amendment 1, 11 April 2011 | Change in study design to an open three-arm trial |
| Substantial amendment 2, 20 February 2013 | Change in study design from three-arm to two-arm trial (BMT only vs. LTOT plus BMT). Primary outcome time point was changed from 12 months to 6 months |
| Substantial amendment 3, 21 March 2013 | Permission to use invitation letter for potential participants from patient lists held at NHS general practitioner practices (patient identification centres), or via existing lists of likely eligible patients held within NHS Hospitals |
| Substantial amendment 4, 2 August 2013 | Permission for researchers and study nurses to be able to contact patients via phone after initial introductory letter from the principal investigator |
| Substantial amendment 5, 26 March 2014 | New patient letter informing them of end of trial |
| Research site | Principal investigator | Research and design approval |
|---|---|---|
| County Durham & Darlington NHS Foundation Trust (Darlington Site) | Professor Jerry Murphy | 22 August 2011 |
| Hull and East Yorkshire NHS Trust | Professor Andrew Clark | 24 February 2012 |
| The Mid Yorkshire Hospitals NHS Trust | Dr Paul Brooksby | 11 April 2012 |
| NHS Tayside | Dr Miles Witham | 15 June 2012 |
| University Hospitals of Leicester | Professor Iain Squire | 9 July 2012 |
| Chesterfield Royal Hospital | Dr Justin Cooke | 5 July 2012 |
| Barnet and Chase Farm Hospitals NHS Trust | Dr Ameet Bakhai | 2 August 2012 |
| Ealing Hospital NHS Trust | Dr Stuart Rosen | 6 September 2012 |
| County Durham & Darlington NHS Foundation Trust (Durham site) | Dr Mohamed El-Harari | 25 September 2012 |
| Penine Acute Hospitals NHS Trusta | Dr Jolanta Sobolewska | 19 October 2012 |
| NHS Bradford and Airedale | Dr Paul Smith | 15 November 2012 |
| Aneurin Bevan University Health Board | Dr Jackie Austin | 9 January 2013 |
| City Hospitals Sunderland NHS Foundation Trust | Dr John Baxter | 21 March 2013 |
| Plymouth Hospitals NHS Trust | Dr Andrew Stone | 3 June 2013 |
| East Cheshire NHS Trust | Dr Robin Egdell | 13 December 2014 |
Appendix 3 Home oxygen therapy patient information sheet version 7
Appendix 4 Patient consent form
Appendix 5 Graphical checks of the assumptions for the primary analysis model
FIGURE 11.
Histogram of the standardised residuals.
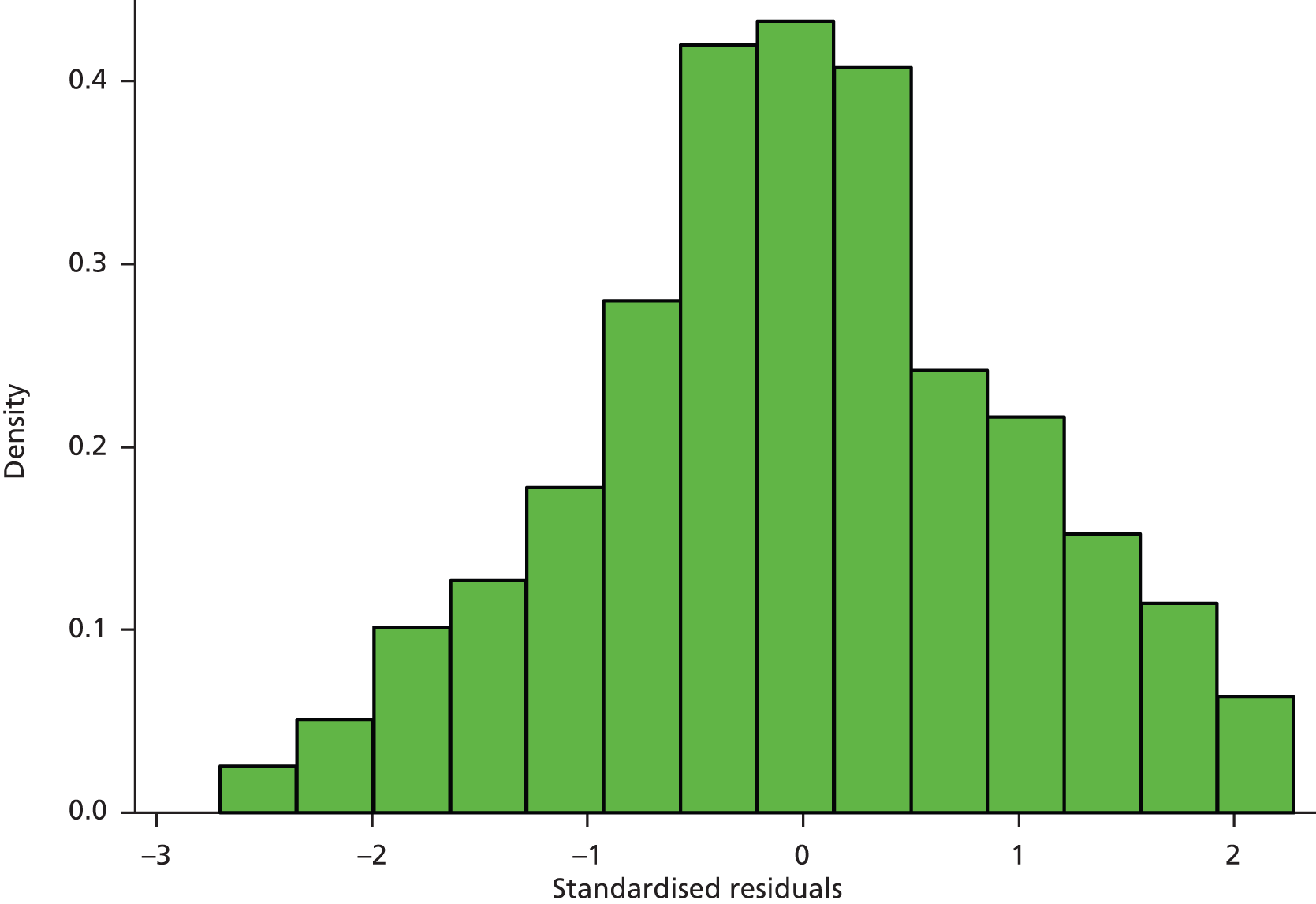
FIGURE 12.
Q–Q plot of the standardised residuals.
FIGURE 13.
Scatterplot of residuals vs. predicted values.

List of abbreviations
- 6MWT
- 6-minute walk test
- AHI
- apnoea–hypopnoea index
- AMD
- adjusted mean difference
- ANCOVA
- analysis of covariance
- BMT
- best medical therapy
- CCI
- Charlson Comorbidity Index
- CHF
- chronic heart failure
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- DAOH
- days alive and out of hospital
- df
- degree of freedom
- ECG
- electrocardiography
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 Levels
- ESS
- Epworth Sleepiness Scale
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced vital capacity
- HADS
- Hospital Anxiety and Depression Scale
- HOT
- home oxygen therapy
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- KPS
- Karnofsky Performance Status scale
- LTOT
- long-term oxygen therapy
- LV
- left ventricular
- LVEF
- left ventricular ejection fraction
- MLwHF
- Minnesota Living with Heart Failure
- MRC
- Medical Research Council
- MREC
- Medical Research and Ethics Committee
- NEON
- North East Oxygen Network
- NOT
- nocturnal oxygen therapy
- NRS
- Numerical Rating Scale
- NT-proBNP
- N-terminal pro-B-type natriuretic hormone
- NYHA
- New York Heart Association
- PaO2
- partial pressure of arterial oxygen
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SDB
- sleep-disordered breathing
- SE
- standard error
- SVR
- systemic vascular resistance