Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/14/03. The contractual start date was in April 2010. The draft report began editorial review in September 2014 and was accepted for publication in May 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
The authors have no financial or non-financial interests relevant to the submitted work except that Covidien Ltd (Mansfield, MA, USA) provided free supplies of its intermittent pneumatic compression devices and sleeves to hospitals participating in the trial. Neither Covidien Ltd nor the funders of the study had any role in data collection, data storage, data analysis, drafting of reports or the decision to publish, although we did allow Covidien Ltd to comment on the draft manuscripts prior to final submission to ensure that we described and used trademarks, etc., appropriately for their products.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Dennis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
In 2005, a House of Commons Health Committee highlighted the very large number of patients dying in UK hospitals from venous thromboembolism (VTE) and called for more effective prophylaxis. 1 Studies reported since 1985 have shown that deep vein thrombosis (DVT) is particularly common in patients who have suffered a recent stroke. 2 Patients with a history of VTE and significant weakness of the leg and who are immobile appear to be at the greatest risk. 3 Studies with magnetic resonance imaging demonstrated DVT in 40% of stroke patients and above-knee DVT in 18% of patients within 3 weeks of stroke. 4 Studies using less sensitive screening techniques, such as compression duplex ultrasound (CDU), suggest a lower frequency of above-knee DVT (about 10%), although the types of patients included and the duration and timing of follow-up influences these estimates. 5 Clinically apparent DVT, confirmed on investigation, is less common than DVT identified through screening; however, DVT may not be recognised and may cause important complications including pulmonary embolism (PE), sudden death and, in the longer term, post-phlebitis leg syndrome. PE is an important cause of preventable death after stroke. 2 Clinically evident PE has been variably estimated to affect 1–16% of patients in prospective trials6 and 3–30% of patients in observational studies. 7 In the Clots in Legs Or sTockings after Stroke (CLOTS) 1 trial, about 5% of patients developed symptomatic DVT and 1.5% had a confirmed PE in the first month after stroke. 8 The rate of PE is likely to be underestimated because PEs are not routinely screened for and autopsies are rarely performed. Many patients who have pneumonia or unexplained fever may actually have PEs. 9 Fifty per cent of patients who die after an acute stroke showed evidence of PE on autopsy. 10 Studies, such as the CLOTS 1 trial, which screen for DVT may underestimate the clinical importance of VTE because patients are usually treated while still asymptomatic and so their risk of developing symptomatic DVT and PE is reduced.
A number of interventions have been used to reduce the risk of DVT, as summarised in the following sections.
Anticoagulants
A Cochrane systematic review showed that both low- and medium-dose subcutaneous heparin reduces the risk of DVT, and probably PE, in patients with acute ischaemic stroke. 6 However, evidence from the International Stroke Trial11 showed that even low-dose heparin (5000 units twice daily) is associated with a significant excess of symptomatic intracranial and extracranial bleeds which offset any other benefits heparin may have on recurrent ischaemic stroke and fatal PE. A systematic review of randomised trials testing anticoagulation for VTE prophylaxis in stroke patients focused on only symptomatic events: deaths, DVT, PE, fatal PE and bleeds. 12 The estimates of treatment effect, expressed as events avoided or caused per 1000 patients treated, are shown in Table 1. This review also compared the effect of low-molecular-weight heparins (LMWHs) with the effect of unfractionated heparin. The LMWH was associated with fewer DVTs, but no significant differences in other clinical outcomes.
| Outcome | Number of trials | Number of outcomes/number treated | Outcomes avoided (–) or caused (+) per 1000 patients treated | 95% CI | |
|---|---|---|---|---|---|
| Heparin | No heparin | ||||
| Death | 8 | 496/5276 | 990/10,129 | –9 | –29 to 18 |
| PE | 5 | 39/5015 | 95/9847 | –3 | –5 to 0 |
| Symptomatic DVT | 1 | 0/101 | 1/105 | –9 | –10 to 57 |
| Major bleeding | 8 | 79/5276 | 89/10,129 | +6 | 2 to 12 |
Graduated compression stockings
Although graduated compression stockings (GCSs) seem to reduce the risk of DVT in patients undergoing surgery,13,14 the CLOTS 1 trial showed that thigh-length GCSs were not associated with a clinically useful reduction in the risk of post-stroke DVT [absolute reduction in risk of proximal DVT 0.5%, 95% confidence interval (CI) –1.9% to 2.9%]. 8 Moreover, use of GCSs was associated with an increase in the risk of skin breaks on the legs. A Cochrane review of the effectiveness of physical means of reducing the risk of VTE after stroke identified only one other trial of GCSs. 15,16 The two trials included a total of 2615 patients with stroke. The use of GCSs was not associated with any significant effect on DVT [odds ratio (OR) 0.88, 95% CI 0.72 to 1.08] or death (OR 1.13, 95% CI 0.87 to 1.47).
Intermittent pneumatic compression
Intermittent pneumatic compression (IPC) is achieved by means of a pair of inflatable sleeves which are wrapped around the legs and are secured by hoop-and-loop fastener and attached via flexible tubing to a small bedside electric pump. Different manufacturers produce systems with different characteristics, but it is unclear whether or not these influence the effect on risk of VTE. 17 The sleeves may be short (or below knee), wrapping around just the lower leg, or long (thigh length) to wrap around the thigh as well. Some types provide compression to the foot only. They are inflated one side at a time to compress the legs at intervals. Some types inflate sequentially, first around the lower leg and then the upper, to ‘milk’ the blood from the leg and increase venous flow. Others compress along the length of the sleeves at the same time (so-called single compression). Some inflate rapidly, others slowly. The frequency of inflation can be fixed or may vary depending upon the rate of venous refill. IPC is thought to reduce the risk of venous thrombosis by:
-
increasing the flow of venous blood through the deep veins of the leg to reduce the likelihood of thrombosis
-
stimulating release of intrinsic fibrinolytic substances. 18
Intermittent pneumatic compression has mainly been used in surgical patients during and immediately after operations. One systematic review identified 22 randomised controlled trials (RCTs) of IPC, which included a total of 2779 patients. 13 Use of IPC was associated with a 64% reduction in the odds of DVT (p < 0.00001). This review concluded that a priority for future research was trials of ‘prevention of venous thromboembolism with mechanical methods among high-risk medical patients (such as those with stroke)’. 13 Another, more recently published, systematic review identified 40 RCTs comparing IPC with No IPC. 19 These trials included a total of 7252 patients, were of very variable quality and included mostly surgical patients. IPC was more effective than no-IPC prophylaxis in reducing DVT [7.3% vs. 16.7%; absolute risk reduction (ARR) 9.4%, 95% CI 7.9% to 10.9%; relative risk 0.43, 95% CI 0.36 to 0.52; p < 0.01; I2 = 34%] and PE (1.2% vs. 2.8%; ARR 1.6%; 95% CI 0.9% to 2.3%; relative risk 0.48; 95% CI 0.33 to 0.69; p < 0.01; I2 = 0%). This review also showed that IPC was also more effective than GCS in reducing DVT and appeared to be as effective as pharmacological thromboprophylaxis but with a reduced risk of bleeding (relative risk 0.41, 95% CI 0.25 to 0.65; p < 0.01; I2 = 0%). Adding pharmacological thromboprophylaxis to IPC further reduced the risk of DVT (relative risk 0.54, 95% CI 0.32 to 0.91; p = 0.02; I2 = 0%) compared with IPC alone.
The Cochrane systematic review of the effectiveness of physical means of reducing the risk of VTE after stroke identified only two small RCTs of IPC including just 177 patients in total. 15 IPC was associated with a non-significant trend towards a lower risk of DVTs (OR 0.45, 95% CI 0.19 to 1.10) with no evidence of an effect on deaths (OR 1.04, 95% CI 0.37 to 2.89).
Thus, the available evidence confirmed that after stroke, even applying current prophylactic strategies, the risk of VTE was substantial. The available data suggested that IPC is a promising but unproven and rarely used intervention in the UK. The CLOTS 3 trial aimed to:
-
establish whether or not the routine application of IPC to the legs of immobile stroke patients reduces their risk of DVT and PE
-
determine whether or not IPC adds to the benefits of routine care, which often includes good hydration, early use of aspirin and mobilisation
-
quantify any risks of IPC when applied to stroke patients
-
estimate the cost-effectiveness of IPC which would help health service planners decide whether or not IPC should be offered routinely in the UK NHS
-
provide robust estimates of the effectiveness of IPC in stroke patients which might be extrapolated to other groups of medical (rather than surgical) patients at high risk of VTE.
Chapter 2 Methods
Design overview
The CLOTS 3 trial was a multicentre, parallel-group trial with a centralised randomisation system to allocate treatment in a 1 : 1 ratio, which ensured allocation concealment. Its methods were very similar to those of CLOTS trials 1 and 2. 3,20 We aimed to blind the ultrasonographers who carried out the imaging to detect DVTs but were unable to blind the patients and their caregivers to allocation group because of the nature of the intervention. The multicentre research ethics committees in the UK and the local ethics committees in all contributing centres approved our protocol. We obtained written informed consent from all patients or, for patients lacking mental capacity, from the patients’ personal legal representatives. The trial was registered as [International Standard Randomised Controlled Trial Number (ISRCTN) 93529999].
Setting
Our collaborators in 105 centres in the UK aimed to enrol and/or follow up at least 2800 patients. To participate, hospitals had to have a local principal investigator who took responsibility for the trial governance; a well-organised inpatient stroke service; nursing staff trained in the use of IPC; and a diagnostic ultrasound department which routinely performed CDU.
Participants
Inclusion criteria
Any patient admitted to hospital within 3 days of a clinical stroke fulfilling the World Health Organization criteria and who was not able to get up from a chair/out of bed and walk to the toilet without the help of another person.
Patients could be randomised from day 0 (day of admission) to day 3 of hospital admission. Any patient who had a stroke during hospital admission was eligible until day 3 from the stroke onset (day 0). Stroke should have been the most likely clinical diagnosis, but it was not necessary for a visible infarction to be seen on a brain scan.
Exclusion criteria
-
Patients under 16 years of age.
-
Patients with stroke due to subarachnoid haemorrhage.
-
Patients who, in the opinion of the responsible clinician/nurse, were unlikely to benefit from IPC – for instance, this would include patients who were expected to mobilise or die within the next day.
-
Patients with contraindications to the use of IPC. Contraindications included:
-
local leg conditions with which the IPC sleeves would interfere such as leg ulcers or dermatitis
-
severe arteriosclerosis, as indicated by absence of pedal pulses or history of definite intermittent claudication
-
massive leg oedema or pulmonary oedema from congestive heart failure.
-
-
Patients who already had swelling or other signs of an existing DVT. Such patients could be recruited once a DVT had been excluded by normal D-dimers or CDU. There was a concern that the application of IPC to patients who may already have a DVT might displace the thrombus and increase the risk of PE. However, this potential risk has not been documented in the RCTs so far. We have not identified any case reports that provide convincing evidence that this has occurred.
Inclusion in another research study, including another RCT, did not automatically exclude a patient from participating in CLOTS 3. As long as inclusion in the other study would not confound the results of CLOTS 3, co-enrolment was permissible. In addition, local researchers had to avoid overburdening their patients. Patients were not co-enrolled in another research study that aimed to test an intervention intended to reduce the risk of VTE.
We did not require local research teams to maintain a screening log, although many did because it was required by the UK National Institute for Health Research stroke research networks. First, our view is that maintenance of a screening log diverts valuable resource away from enrolment, data collection and other important aspects of the trial procedures. Second, given that almost inevitably only a small proportion of potentially eligible patients are enrolled, screening logs do not provide useful data on which to judge the generalisability (external validity) of trial results. Finally, if screening logs are to be robust they need to include patient identifiers, to avoid double counting; the legality of collecting and storing this personal and sensitive information (patient identifier and name of trial is enough to indicate the patient’s medical diagnosis) without obtaining informed consent is questionable.
Consent
The patients, or their legal representatives, were approached by a member of the clinical team looking after that patient to ascertain their interest in participating in the CLOTS 3 trial or to obtain their permission to pass their details onto any research staff involved. Written informed consent was sought wherever possible. If this was not possible, the randomising clinician or nurse could obtain witnessed verbal consent. Patients or legal representatives were given enough time to consider the trial fully and ask any questions they may have had about the implications of the trial.
Randomisation
Having obtained consent, the clinician entered the patient’s baseline data onto a randomisation form (see Appendix 2) and then into a computerised central randomisation service via a secure web interface or a touch-tone telephone system. We encouraged clinicians to enrol patients as early as possible, as prophylaxis for DVT is likely to have a greater effect if started early. Once the computer program had checked these baseline data for completeness and consistency, it generated that patient’s treatment allocation to either ‘routine care plus IPC‘ or ‘routine care alone’. The system applied a minimisation program to achieve balance for four prognostic factors:
-
delay since stroke onset (day 0 or 1 vs. days 2–7)
-
stroke severity (using a validated prognostic model21 which includes the following factors: age; prestroke dependency in activities of daily living; living with another person prior to stroke; able to talk and orientated in time, place and person; and able to lift both arms to horizontal position against gravity)
-
severity of leg paresis (able or not to lift leg off the bed)
-
use of heparin, warfarin or thrombolysis at the time of enrolment.
As simple minimisation can theoretically lead to alternation of treatment allocation, our system also incorporated a degree of random allocation, that is it allocated patients to the treatment group that minimises the difference between the groups with a probability of 0.8 rather than 1.0. 22 This helped to guarantee allocation concealment.
The interventions
In the IPC group we applied the Kendall SCD™ Express system (Covidien Ltd, Mansfield, MA, USA) using thigh-length sleeves only (Figure 1). Initially, we used the standard sleeves, but during the study we switched to the Comfort™ sleeves (Covidien Ltd, Mansfield, MA, USA), which were designed to improve adherence while delivering the same pattern of compression. These devices deliver sequential compression, first around the distal calf, then proximal calf and then the thigh. Compression is circumferential (i.e. around the whole circumference of the leg) and sleeve inflation is gradual compared with some types of IPC. The maximum pressure is 45 mmHg. The compression is delivered to one leg at a time at a frequency which depends on the ‘venous refill time’. When the leg is compressed, its volume is reduced. Between compressions the veins refill and the volume of the leg increases. The changes in volume are detected by the IPC controller, which detects movement of air between the sleeves and the controller. The frequency of compression is higher if venous refill is faster, such as when the legs are dependent. This system aims to maximise venous flow.
FIGURE 1.
The Kendall SCD™ Express system used in the trial. This patient is wearing thigh-length Comfort™ sleeves.
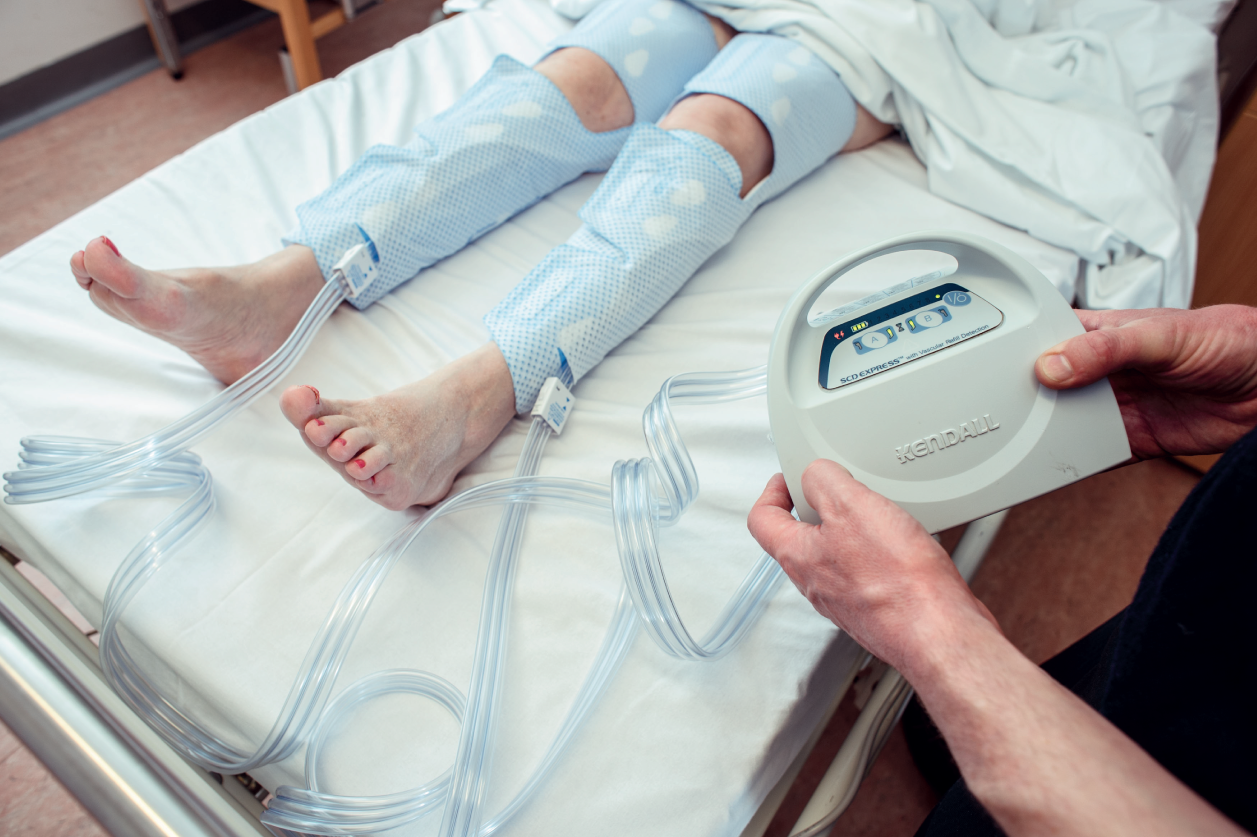
If allocated IPC, nursing staff sized, fitted and applied the sleeves, based on the manufacturer’s instructions, to both legs as soon as possible after the randomisation telephone call. The IPC sleeves were meant to be worn both day and night, while the patient was in the bed or chair for 30 days from randomisation, or until a second-screening CDU had been performed (if after 30 days). They could be removed earlier for any of the following reasons:
-
The patient was independently mobile around the ward (i.e. could get up from a chair/out of bed and walk to the toilet without the help of another person).
-
The patient was discharged from the participating hospital. If the patient was transferred to a rehabilitation unit where it was practical to continue the IPC and monitor its use appropriately, then IPC could be continued until the patient was independently mobile, or declined to continue or until an adverse effect of IPC occurred. If IPC could not be continued after transfer to a rehabilitation unit, a discharge form was completed at the time of transfer to the rehabilitation unit.
-
The patient declined to continue to have IPC applied.
-
Health-care staff identified any adverse effect of the IPC (such as pressure ulcers on the legs, falls due to the IPC) which they judged made continued application of the IPC unwise.
If the IPC was removed for any other reason, for example to check the legs, or for bathing or for screening CDU, then the IPC was to be re-applied as soon as possible. If the sleeves became soiled, they were to be replaced with new sleeves as soon as possible. If a reason to remove the IPC sleeve from one leg developed, treatment to the other leg could continue; however, information on how often this occurred was not captured systematically.
Our recruitment co-ordinator (CW) and representatives of Covidien Ltd provided onsite training to nursing staff in the correct sizing, fitting and monitoring of IPC. This was supplemented by a training video and web-based training. We asked nursing staff to record their use of IPC on the medication chart to increase compliance and to aid monitoring. We stipulated that both treatment groups should receive the same medical care, which could include, depending on local protocols, early mobilisation, hydration and antiplatelet or anticoagulant drugs. The local co-ordinator, who was not blinded to treatment allocation, extracted information from the medication charts on the compliance with IPC and use of antiplatelet and anticoagulant drugs during the admission and recorded this on our hospital discharge form (see Appendix 2); therefore, we could check that these aspects of medical care were used equally in the treatment groups.
Primary outcome
The primary outcome was the occurrence of either a symptomatic or asymptomatic DVT in the popliteal or femoral veins (detected on the first or second CDU performed as part of the trial protocol) or a symptomatic DVT in the popliteal or femoral veins, confirmed on imaging (either CDU or venography) within 30 days of randomisation. We focused on proximal DVTs because they are much more reliably detected by ultrasound and are generally regarded as clinically more important. 23–25
Secondary outcomes
In hospital or within 30 days:
-
death within 30 days
-
presence of definite or probable DVT in the popliteal or femoral veins detected on a screening CDU scan which had not been suspected clinically before the scan
-
definite (i.e. excluding probable DVTs) symptomatic or asymptomatic DVT in the popliteal or femoral veins detected on either a CDU scan or contrast venography or magnetic resonance direct thrombus imaging within 30 days of randomisation
-
any definite or probable symptomatic or asymptomatic DVT (i.e. including DVTs which only involve the calf veins)
-
confirmed fatal or non-fatal PE
-
adherence to allocated treatment
-
adverse events related to IPC within 30 days of randomisation.
At 6 months:
-
death from any cause
-
any confirmed symptomatic or asymptomatic DVT or PE occurring between randomisation and final follow-up
-
any symptomatic DVT or PE occurring between randomisation and final follow-up
-
functional status (as measured by the Oxford Handicap Scale)26
-
place of residence
-
health-related quality of life [European Quality of Life-5 Dimensions 3 Level (EQ-5D-3L) questionnaire]. 27,28
Adverse events
Stroke is a serious medical condition. About 20% of hospitalised patients are expected to die. Serious medical complications are common. The CLOTS 3 trial evaluated IPC, a non-drug intervention which has a CE mark and has been approved for the purpose of reducing the risks of VTE. The risks associated with IPC and participation in the trial were judged to be very small and generally predictable, for example skin problems on legs or falls resulting in injury. It would be relatively straightforward to attribute any serious adverse event to the IPC. In this trial we therefore did not require routine reporting of any adverse events, as this was unlikely to be informative and would have placed an unnecessary burden on the local researchers, which would have compromised the practicality of the trial. We did require prompt reporting of primary and secondary outcomes on the radiology report form (within 2 working days), discharge form (within 10 working days), general practitioner (GP) questionnaire and hospital follow-up forms (see Appendix 2).
The following were reported on the radiology report form, discharge form or GP questionnaire (if patient had been discharged) or hospital 6-month follow-up form (if the patient was still in hospital):
-
any confirmed DVT
-
any confirmed PE
-
any fall associated with significant injury occurring within 30 days of enrolment (when IPC might still be applied); an injury requiring investigation or specific treatment, which prolongs hospitalisation, or which leads to death, temporary or permanent disability
-
any damage to the skin of the legs including necrosis or ulcers occurring within 30 days of enrolment
-
reason(s) for prematurely stopping the IPC.
The following were expected complications of stroke and its comorbidities and did not need to be reported as adverse events:
-
deaths, which were reported as outcome events on the discharge or 6-month follow-up forms
-
infections other than those affecting the skin of the legs
-
further vascular events (including recurrent strokes, myocardial infarction and bowel ischaemia)
-
cardiac, renal or liver problems
-
epileptic seizures
-
spasticity or contractures
-
painful shoulder and other joint problems
-
mood disturbances.
Any other adverse events which the investigator felt might be a result of either the treatment or participation in the trial were to be reported within 10 working days to the co-ordinating centre. A serious adverse event (i.e. one resulting in death, is life-threatening or which results in significant disability or incapacity or prolongation of hospitalisation) was to be reported immediately on a serious adverse events form online or by fax. Serious adverse events attributed to the trial treatment or participation in the trial were reported to the Data Monitoring Committee (DMC), trial sponsors and ethics committees.
Follow-up
Detection of deep vein thrombosis
Patients should have had a CDU of the veins in both legs between day 7 and day 10 and usually between day 25 and day 30. We defined minimum acceptable technical standards for ultrasound equipment and stipulated that the trial ultrasonographers should have performed CDU to diagnose DVTs as part of a clinical service. We asked them to visualise the popliteal and femoral veins in both legs but did not insist that they routinely visualised the six deep veins in the calf, as detecting thrombosis in these is far less reliable. In addition, insisting on scanning of calf veins increases the time required for scanning, which might have impacted on the ability of centres to participate. We obtained a hard copy of scans in those patients with our primary outcome in order to enable our trial radiologist (JR), who was blinded to group allocation, to verify each primary outcome. We did not perform central verification of negative scans because, with ultrasound techniques, meaningful verification of static images is difficult. If no image was available we verified the scan result by obtaining a local radiologist’s clinical report. If the second ultrasound was delayed to more than 30 days and showed a popliteal or femoral DVT, it was included in the primary outcome. However, we did not include in our primary outcome a proximal DVT that came to attention only because symptoms started more than 30 days after enrolment, as this might have introduced bias because researchers unblinded to treatment allocation might theoretically include later clinical outcomes in one group preferentially.
Where the randomising person judged that it was likely to be impractical to perform a CDU between day 25 and day 30, he or she could, prior to randomisation, stipulate that a CDU would be performed only between day 7 and day 10. This might have been the case if the patient was likely to be discharged home to another region or transferred to a rehabilitation facility that did not have use of CDU facilities and was remote from the randomising centre. This was done to minimise losses to follow-up which, if unequal between the two treatment groups, could bias the trial results.
If a definite above-knee DVT was detected on the first screening CDU, that is the patient had our primary outcome, then the second screening CDU was no longer required.
Intermittent pneumatic compression was removed completely before the patient left the ward to undergo CDU, to ensure optimal blinding of the primary outcome measure. The CDU operator was asked to guess which treatment group the patient was in prior to the examination to estimate the effectiveness of blinding. In those allocated IPC, the sleeves were immediately re-applied on the patient’s return to the ward after the screening CDU.
In-hospital follow-up
The local co-ordinators completed a discharge form (see Appendix 2) for all randomised patients on discharge from the randomising centres or in the event of earlier death. We could not blind the local co-ordinators to group allocation. If a patient was transferred to a rehabilitation unit on a different site to the randomising centre, and it was impractical to continue the allocated treatment or its monitoring while the patient was in that unit, a discharge form was completed on transfer to that unit.
The data collected at hospital discharge by the unblinded local co-ordinator included:
-
Use of heparin, LMWHs, warfarin and antiplatelet drugs during admission to monitor the components of routine care and to ensure equal use in the two treatment arms. However, an imbalance of heparin or LMWH and warfarin might occur if IPC is effective as more patients in the control arm would receive these drugs to treat the excess of VTE. The indications for their use were therefore recorded. The timing of starting and duration of anticoagulation were not recorded.
-
Use of full-length or below-knee GCSs to monitor the components of routine care and to ensure equal use in the two treatment arms. Again the timing of initiation and duration of use were not recorded.
-
Adherence to treatment allocation and use of IPC. We captured the date any IPC was first applied and the date it was last applied. We also asked the local researcher to record the number of days between these dates when the IPC was not applied at all. We did not systematically record whether or not the IPC was applied to one or both legs and how many hours per day it was worn.
-
Any clinical DVT or PE requiring treatment.
-
Any complications of treatments, in particular skin problems on the legs and falls resulting in injuries and fractures occurring within the first 30 days after randomisation. Local researchers were asked to provide the date that each event occurred, and whether or not it was thought likely to be because of the IPC on the basis of the type of event and its timing.
The discharge form included checkboxes to record these secondary outcomes and adverse events (see Appendix 2). The date of occurrence of any secondary outcome was recorded along with a free-text description of the problem. The chief investigator (MD) reviewed these data centrally and coded the events as far as possible blind to the group allocation.
Later outcomes
The co-ordinating centre telephoned and sent a postal questionnaire to the GPs of all patients who survived to discharge from hospital about 24 weeks after randomisation. This established that the patient was alive prior to sending out a follow-up form and to ascertain whether he or she had had any DVT or PE since discharge from the randomising centre.
Six-month follow-up
The co-ordinating centre sent a postal questionnaire (and one postal reminder and then a telephone follow-up for non-responders) to those surviving patients who had been discharged. The 6-month questionnaire (see Appendix 2) aimed to establish:
-
the place of residence (own home, with relatives, residential care or nursing home) (as a guide to resource use)
-
their functional status [as measured by the Oxford Handicap Scale (OHS)]
-
their current antithrombotic medication regimen
-
presence of leg swelling or ulcers which might reflect post-DVT syndrome.
The questionnaire was completed by the patient or, in the case of those who had difficulties which prevented them doing it themselves, it could be completed by someone close to them. If there was a delay in completing the questionnaire, no attempt was made to judge retrospectively what the patient’s status had been at 6 months.
If the patient was still in hospital when the 6-month follow-up was due, the randomising clinician/nurse was sent a 6-month ‘in-hospital’ follow-up form, which was completed through consultation with the patient. We checked data centrally for completeness and consistency and sent monthly reports to each centre with data queries.
Management of deep vein thrombosis in the trial
If the clinician was satisfied that the patient had a proximal DVT (with or without a confirmatory venogram), the patient was anticoagulated using subcutaneous heparin/LMWH according to local protocols as long as there was no contraindication. If only calf vein thrombus was detected (by screening CDU and/or venography), the responsible clinician could anticoagulate the patient according to local protocols or, alternatively, arrange follow-up CDU approximately 7 days later to identify any propagation into the popliteal vein. If definite popliteal or femoral vein thrombus was detected, the patient was anticoagulated unless contraindicated. Any patient who developed symptoms or signs suggestive of DVT during their admission was investigated by either CDU and/or venography or magnetic resonance direct thrombus imaging and treated in accordance with local protocols if the diagnosis was confirmed. Use of heparin, LMWHs and warfarin to treat DVT and/or PE during admission was recorded on the discharge form. Timing of any use of anticoagulants during admission but after randomisation was not recorded. Continued use of IPC in patients with DVT was at the discretion of the responsible clinician.
Sample size
We originally planned to enrol at least 2000 patients. This aimed to give the trial a > 90% power (α = 0.05) to identify an absolute reduction in risk of our primary outcome of 4% (i.e. a reduction in risk of our primary outcome from 10% in the no IPC group to 6% in the IPC group). The frequency of our primary outcome was estimated from the CLOTS trials 1 and 2. We aimed to enrol at least 75% of patients from day 0 to day 2 after stroke onset. If the proportion enrolled after day 2 exceeded 25% of the total, then the Trial Steering Committee (TSC) could consider raising the overall target. This aimed to ensure that we did not miss a real treatment effect because of delays in recruitment.
In October 2010, the frequency of the primary outcome in both groups combined was 12.2%, rather than the expected 8%. The TSC therefore revised the sample size to 2800 to ensure that the trial maintained power to detect a 4% absolute difference in proximal DVT (i.e. a reduction from 14% in the routine care group to 10% in the routine care plus IPC group). The TSC was not aware of any results split by treatment group.
Statistical analyses
The trial statistician (CG) prepared analyses of the accumulating data which the independent DMC reviewed in strict confidence at least once per year. No other members of the trial team, TSC or participants had access to these analyses.
A detailed analysis plan was prepared by the members of the TSC and then published prior to the completion of enrolment without input from the trial statistician or reference to the unblinded data. 29,30 For the purposes of all primary analyses, we retained participants in the treatment group to which they were originally assigned irrespective of the treatment they actually received. We strived to obtain full follow-up data on every patient to allow a full intention-to-treat analysis. Inevitably, some patients were lost to follow-up. If data were unavailable, we excluded these patients from the analyses and conducted sensitivity analyses to see the effect of these exclusions on our conclusions. For binary outcomes (e.g. occurrence of a primary or secondary outcome or not), outcomes are presented as ORs and 95% CIs, adjusted using logistic regression for the factors used in the minimisation algorithm. We calculated absolute reductions in risk from these values. We converted ORs into relative risks using a standard method. 31 We analysed survival with Cox regression, adjusting for baseline variables included in our minimisation algorithm. We analysed the OHS in two ways: dichotomised into OHS score of 0–2 versus 3–6 (by logistic regression) and as an ordinal scale (by ordinal regression). The utility based on the EQ-5D-3L score was compared by a two-sample Wilcoxon test given the non-normal distribution of the data.
Preplanned subgroup analyses
Preplanned subgroup analyses included the effect of treatment allocation on the primary outcome subdivided by key baseline variables:
-
Time from stroke onset to randomisation (day 0 or day 1 vs. days 2–7, and days 0–2 vs. days 3–7). As DVT may develop very soon after stroke onset and IPC may not influence propagation of thrombus which has already started, it is plausible that IPC would be more effective if started earlier after stroke.
-
Paralysis of leg (able to lift both legs or not?).
-
Stroke severity [using a validated prognostic model. Probability of independent survival (OHS score of < 3) = 0–0.15 vs. > 0.15 (simple six-variable model21)].
-
High or low risk of DVT based on the presence of any one or more of the following indicators of a higher risk of DVT: dependent in activities of daily living (ADL) prior to stroke; prior history of DVT; unable to lift both arms or unable to lift both legs. 3
-
Use of heparin, LMWHs, warfarin or thrombolysis at the time of enrolment.
-
Haemorrhagic stroke versus ischaemic or unknown pathology.
-
Standard versus Comfort™ sleeves.
Subgroup analyses were performed by observing the change in log-likelihood when the interaction between the treatment and the subgroup was added into a logistic regression model.
Secondary analyses
Intermittent pneumatic compression has usually been used for a relatively short time on perioperative patients and patients in high-dependency units. In the CLOTS 3 trial, we aimed to maintain the IPC treatment for up to 30 days, if the patient remained in hospital and was still immobile. However, for a variety of reasons, the IPC was often terminated earlier than this or it was not applied continuously. In the CLOTS 1 and 2 trials, 79% of proximal DVTs were detected on the first CDU scan. 5 The risk of DVT seemed to be highest early on, and therefore any prophylaxis might be more effective during this period. We carried out additional analyses (including all those subgroups identified in the primary outcome) of the effect of allocation to IPC on the primary outcome occurring within 14 days of randomisation, rather than 30 days. This would, we hoped, to some extent, reduce the impact of patients who required IPC for only a few days before becoming mobile or adhered to IPC for only a short time. In addition, it would reflect the practice in many places where acute hospital admission for stroke is short and prophylaxis is applied for only the first few days. However, it is also possible that prophylaxis for the first 7, 14 or 30 days may simply defer the onset of DVT. We therefore analysed whether or not there is a difference in frequency of any symptomatic and/or asymptomatic DVT or PE within 6 months between patients allocated to IPC and those not.
These statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC, USA).
Research governance
The principal investigator in each centre was responsible for:
-
discussing the trial with medical and nursing staff who see stroke patients and ensure that they remained aware of the state of the current knowledge, the most recent trial protocol and its procedures
-
delegating roles to those with appropriate knowledge and skills
-
ensuring that patients admitted with stroke were considered promptly for the trial
-
ensuring that the randomisation forms, radiology report forms and discharge forms were completed and either entered on line or sent to the co-ordinating centre promptly and that copies were kept in a site file and patient notes
-
ensuring that the trial was conducted in accordance with good clinical practice and fulfilled all national and local regulatory requirements
-
ensuring that the patients’ confidentiality was not breached
-
allowing access to source data for audit and verification.
The co-ordinating centre was responsible for:
-
providing study materials, a 24-hour randomisation service and helpline
-
giving collaborators regular information about the progress of the study
-
helping ensure complete data collection at discharge
-
responding to any questions (e.g. from collaborators) about the trial
-
assuring data security and quality in accordance with good clinical practice and local guidelines
-
ensuring trial was conducted in accordance with good clinical practice.
Monitoring
Intermittent pneumatic compression devices carry a CE mark and are licensed for use as prophylaxis for VTE. In surgical practice, use appears to be associated with a low risk of adverse effects. The trial procedures were relatively simple and placed only a small burden on the patients. No significant financial inducements were offered to collaborating centres to encourage their active participation or to reward high recruitment rates. Central follow-up of all patients at about 6 months after enrolment provided confirmation of the trial participants identity (hence avoided the need for detailed on-site source data verification of patient identity, a very resource-intensive activity). After an appropriate risk assessment process, the Trial Management Group and the trial sponsors judged that the risks of patients being harmed by the trial interventions were small, any hazard associated with participation in the trial was very small and the risk of research misconduct was also small. The intensity of on-site monitoring which we undertook was based on this risk assessment. The co-ordinating centre monitored the completeness, internal consistency and validity of the data from all trial sites. We used the trial data collected to monitor adherence to the trial protocol. Our study monitor carried out source data verification in a small random sample of patients during site visits. If concerns arose as a result of the routine central statistical monitoring, a more detailed investigation including on-site verification of source data was carried out. 32
The trial was jointly sponsored by NHS Lothian and the University of Edinburgh [ACCORD (Academic and Clinical Central Office for Research and Development)].
Patient and public involvement
Two patient representatives on the TSC provided feedback on our trial materials, procedures and results. Both had had a stroke and both had worked in nursing in the past; therefore, both were familiar with the problem of VTE. One worked for the NHS in procurement and was very knowledgeable about NHS purchasing of GCSs and IPC devices. This proved very valuable to us. She had been eligible for the CLOTS 1 trial but had declined to be enrolled, although she had then agreed to work with us on the TSC of each of the CLOTS trials. The other had been enrolled in the CLOTS 3 trial during the start-up phase and could therefore provide personal experience of being a trial participant.
Chapter 3 Trial conduct
Between 8 December 2008 and 6 September 2012, 2876 patients were enrolled in 94 centres in the UK, and an additional 11 centres took responsibility for delivering the allocated treatment and follow-up for patients who were transferred from the randomising hospital (see Appendix 1).
Recruitment
Funded by the Chief Scientist Office in Scotland, recruitment at the Western General Hospital, Edinburgh, started in the start-up phase once the CLOTS 1 trial had completed recruitment (in September 2008), although recruitment into the CLOTS 2 trial continued in other hospitals. The CLOTS 2 trial completed recruitment in May 2009, after which many centres switched to recruiting into the CLOTS 3 trial. The CLOTS 3 collaboration was expanded after funding from the Health Technology Assessment programme was secured in April 2010. The rate of recruitment over time is shown in Figure 2. The recruiting hospitals, and the number each recruited, are listed in Appendix 1.
FIGURE 2.
Recruitment in the CLOTS 3 trial over time.

The rate of recruitment exceeded our expectations; therefore, we reached our revised target of 2800 approximately 1 year before we had expected to reach our original target of 2000 patients.
Baseline characteristics of patients recruited
The patients’ baseline characteristics are shown in Table 2 and were well balanced between treatment groups. The patients’ progress through the trial is shown in the CONSORT (Consolidated Standards of Reporting Trials) diagram (Figure 3).
| Baseline variable | IPC (n = 1438) | No IPC (n = 1438) |
|---|---|---|
| Median age,a years (IQR) | 76 (67–83) | 77 (68–84) |
| Mean age, years (SD) | 74.2 (12.3) | 74.9 (11.9) |
| Males, n (%) | 695 (48.3) | 688 (47.8) |
| Final diagnosis at hospital discharge | ||
| Stroke/TIA (definite/probably ischaemic), n (%) | 1211 (84.2) | 1217 (84.6) |
| Confirmed haemorrhagic stroke, n (%) | 187 (13.0) | 189 (13.1) |
| Unknown type, n (%) | 19 (1.3) | 14 (1.0) |
| Non-strokes (included in primary analysis), n (%) | 19 (1.3) | 18 (1.3) |
| Missing (no discharge form), n (%) | 2 (0.1) | 0 (0.0) |
| Past history and risk factors | ||
| Previous DVT or PE, n (%) | 66 (4.6) | 74 (5.1) |
| Diabetes mellitus, n (%) | 256 (17.8) | 247 (17.2) |
| Peripheral vascular disease, n (%) | 24 (1.7) | 31 (2.2) |
| Overweight, n (%) | 417 (29.0) | 457 (31.8) |
| Current cigarette smoker, n (%) | 250 (17.4) | 228 (15.9) |
| Independent before stroke,a n (%) | 1301 (90.5) | 1295 (90.1) |
| Lives alone before stroke,a n (%) | 500 (34.8) | 503 (35.0) |
| Indicators of stroke severity | ||
| Able to lift both arms off bed,a n (%) | 499 (34.7) | 502 (34.9) |
| Able to talk and orientated,a n (%) | 886 (61.6) | 845 (58.8) |
| Able to lift both legs off bed, n (%) | 494 (34.4) | 493 (34.3) |
| Able to walk without help,a n (%) | 0 (0) | 0 (0) |
| Stroke severity – probability of being alive and independent = 0 to 0.15), n (%) | 898 (62.4) | 892 (62.0) |
| Stroke severity – median (IQR) probability of being alive and independent | 0.09 (0.02–0.31) | 0.09 (0.01–0.31) |
| On warfarin at recruitment, n (%) | 25 (1.7) | 29 (2.2) |
| On heparin or LMWHs at recruitment, n (%) | 86 (6.0) | 78 (5.4) |
| Taken antiplatelet drug in the last 24 hours, n (%) | 970 (67.5) | 971 (67.5) |
| Received thrombolysis since admission, n (%) | 249 (17.3) | 255 (17.7) |
| On heparin or LMWH or warfarin at recruitment or received thrombolysis since admission, n (%) | 347 (24.1) | 352 (24.5) |
| Delay from stroke to randomisation | ||
| 0 to 1 day, n (%) | 624 (43.4) | 620 (43.1) |
| 2 days, n (%) | 478 (33.2) | 457 (31.8) |
| ≥ 3 days, n (%) | 336 (23.4) | 361 (25.1) |
| Second CDU considered unlikely to be practical at time of randomisation, n (%) | 225 (15.6) | 215 (15.0) |
FIGURE 3.
The CONSORT diagram (the number screened for eligibility was not collected). a, The number of patients who withdrew consent is less than the number published in The Lancet. 33 The figure includes data only up to the point at which permission to follow up the patients or to use data was explicitly withdrawn. F/u, follow-up.

Withdrawal
Patients, or their proxies, were informed at the time they gave consent that they could stop the allocated treatment at any time, and without giving a reason. They were also told that they could withdraw completely from the study, although the data collected up to that point would be used in analyses. Unfortunately, local research teams did not always clearly establish the precise wishes of the patient or proxy. For instance, if the patient wished to stop receiving the allocated treatment, then this was sometimes interpreted as wishing to withdraw from the trial completely (implying that the patient would not be followed up). In addition, even when a patient expressed a wish not to be contacted directly by mail or telephone for follow-up, this did not necessarily preclude us from establishing their follow-up status through their GP or routine data sources. As a result, in some cases there was some uncertainty about whether or not we could use certain data items, such as date of death. In general, we included patients in the analysis unless it was clear from the correspondence that they did not want anything more to do with the trial. Based on this experience, it is clearly important that trials are rigorous in defining exactly what the patient wishes to do when they stop the intervention and whether or not they are also refusing any further contact for follow-up.
Background treatment
The use of antiplatelet medications, prophylactic- and treatment-dose heparin or LMWHs, oral anticoagulants and GCSs was recorded in both treatment groups on the discharge form by the local co-ordinator. However, we did not collect the dates that these treatments were given; therefore, we cannot tell if they were given concomitantly with IPC, if they followed IPC and if any anticoagulation was used in response to the diagnosis of a DVT or PE. We did not record timing of mobilisation, use of parenteral fluids or any measure of hydration.
Background treatment with antiplatelet medications, anticoagulants and GCSs is shown in Table 3. Use of prophylactic-dose heparin or LMWHs after randomisation was very similar in the treatment arms (IPC 17.2% vs. no IPC 16.7%). There was a small excess of treatment-dose heparin or LMWHs in the control arm, probably because DVTs occurred more commonly in that group. The low overall use of GCSs suggests that most centres were aware of, and accepted, the evidence from the CLOTS 1 trial that GCS use is not associated with lower risk of DVT. There was a small excess of GCS use in the IPC group, perhaps because the manufacturer of IPC previously recommended using IPC and GCSs in combination. However, it is possible that the GCSs were applied after the IPC had been removed or to prevent post-phlebitis leg syndrome.
| Background treatment prescribed | IPC (n = 1438) | No IPC (n = 1438) |
|---|---|---|
| Antiplatelet medication, n (%) | ||
| Aspirin | 1039 (72.4) | 1033 (71.8) |
| Dipyridamole | 152 (10.6) | 155 (10.8) |
| Clopidogrel | 526 (36.6) | 524 (36.4) |
| Anticoagulation, n (%) | ||
| Prophylactic-dose heparin/LMWHs | 248 (17.2) | 240 (16.7) |
| Treatment-dose heparin/LMWHs | 182 (12.7) | 219 (15.2) |
| Warfarin | 292 (20.3) | 294 (20.5) |
| Other oral anticoagulant | 10 (0.7) | 8 (0.6) |
| GCSs, n (%) | ||
| GCSs worn (any length) | 118 (8.2) | 42 (2.9) |
| Thigh-length GCSs worn only | 90 (6.3) | 22 (1.5) |
| Below-knee GCSs worn only | 17 (1.2) | 19 (1.3) |
| Both thigh-length and below-knee GCSs worn | 10 (0.7) | 1 (0.1) |
| GCSs of unknown length | 1 (0.7) | 0 |
Adherence
The measurement of adherence to IPC was based on data provided on the hospital discharge form by the local co-ordinators. In most cases it was based on the recording of IPC use on medication charts completed by the nurses on the stroke unit. It was not always complete. We attempted to collect the date the IPC was first applied, the date it was permanently taken off, the number of days between when it was not worn at all, and the reason why the IPC was stopped, especially if it was stopped prematurely.
In total, 1424 (99.2%) patients were allocated to the IPC group and were treated with IPC at some point in the first 30 days after randomisation. In the group allocated to no IPC, four patients received IPC at some point in the first 30 days. Three patients received IPC having been transferred to an intensive care or high-dependency unit where IPC was standard care, and one received IPC for 3 days because of a mistake in recording the allocated treatment.
Ideally, we would have defined 100% adherence as wearing IPC sleeves every day (i.e. no days on which they were not worn) from randomisation until the patient regained mobility, was discharged from a participating hospital or died or until 30 days or until a delayed second screening CDU. However, the number of days on which the IPC sleeves were not worn was not well recorded and was sometimes inconsistent with other data items. We therefore defined 100% adherence as wearing IPC sleeves from randomisation until the patient regained mobility, was discharged from a participating hospital or died, or until 30 days, or until a delayed second screening CDU, ignoring the likelihood that some patients would have had the sleeves removed for some intervening days and may not have worn them on both legs day and night. One hundred per cent adherence was achieved in 378 (26.3%) of patients in the IPC group. The distribution of percentage adherence is shown in Figure 4. The median adherence was 55.7% [interquartile range (IQR) 16.7–100%]. The adherence to the standard and Comfort™ sleeves are compared in Table 4.
FIGURE 4.
Distribution of percentage adherence.

| Adherence measure | Standard sleeves (n = 834) | Comfort™ sleeves (n = 604) |
|---|---|---|
| Duration IPC applied (stop date–start date), n (%) | ||
| Missing | – | – |
| ≤ 1 day | 140 (16.8) | 96 (15.9) |
| 2–5 days | 180 (21.6) | 145 (24.0) |
| 6–10 days | 169 (20.3) | 104 (17.2) |
| 11–20 days | 138 (16.5) | 109 (18.0) |
| 21–30 days | 172 (20.6) | 138 (22.8) |
| > 30 days | 35 (4.2) | 12 (2.0) |
| Mean duration of IPC use (SD) in days | 11.6 (10.7) | 11.7 (10.4) |
| Median duration of IPC use (IQR) in days | 8 (3–20) | 8 (3–20) |
| Wore IPC sleeves until death/discharge/mobile/30 days or later second CDU (i.e. 100% adherence) | 222 (26.6) | 156 (25.8) |
| Mean adherence (SD), % of days applied per protocol | 55.5 (39.0) | 55.9 (37.9) |
| Median adherence (IQR) | 54.4 (16.7–100.0) | 55.9 (16.9–100.0) |
The distribution of duration of use in the IPC group is shown in Figure 5. The mean and median duration of IPC use was 11.7 days [standard deviation (SD) 10.6 days] and 8 days (IQR 3–20 days) respectively.
FIGURE 5.
The distribution of duration of use of IPC in the group allocated IPC.
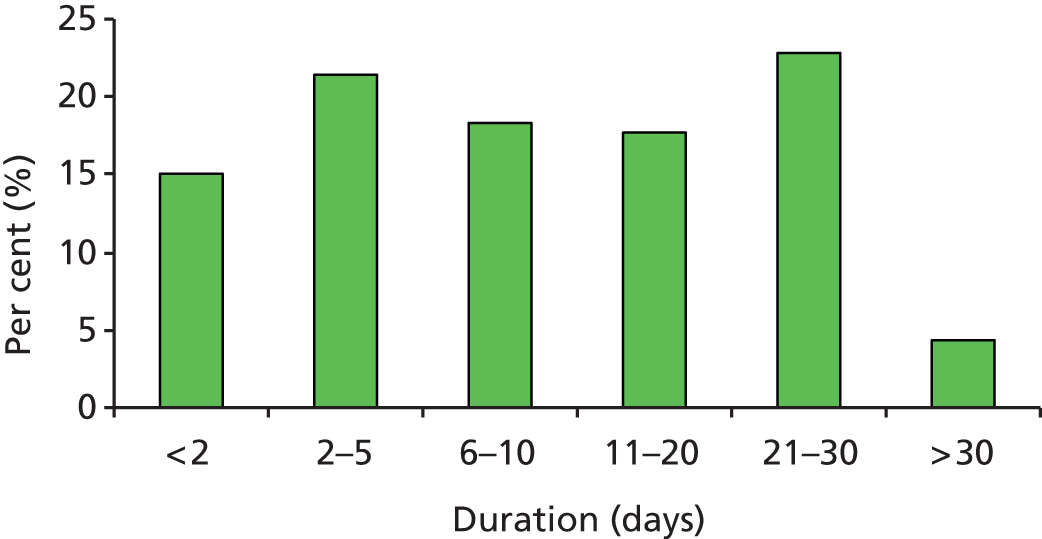
Table 5 shows the adherence and reason(s) for removal of IPC in the 1438 patients allocated to IPC (reasons given are not always mutually exclusive).
| Use of IPC | n (%) |
|---|---|
| Total number of patients with a completed discharge form | 1436 (100) |
| Wore IPC sleeves at any time in the first 30 days after randomisation | 1424 (99.2) |
| Wore IPC sleeves every day was supposed to according to protocol | 378 (26.3) |
| Removed according to protocol | |
| Patient regained mobility | 142 (9.9) |
| Second CDU delayed after 30 days | 33 (2.3) |
| Completed 30 days of IPC | 66 (4.6) |
| Removed early | |
| Concerns about patient’s skin | 62 (4.3) |
| Patient refused to wear IPC any longer | 474 (33.0) |
| Patient complained of discomfort | 189 (13.2) |
| Other reasons specified by local researchers | 535 (37.3) |
| Change in view of staff | 142 (26.5) |
| Patient/family refused | 107 (20.0) |
| Intermediate-severity skin problems | 3 (0.6) |
| Low-severity skin problems | 18 (3.4) |
| Other leg problems | 4 (0.8) |
| Technical fault with IPC | 7 (1.3) |
| Fall | 1 (0.2) |
| Other events – fractures, stroke, palliative care | 3 (0.6) |
| Confirmed PE | 3 (0.6) |
| Symptomatic/confirmed DVT | 3 (0.6) |
| Change of use because of DVT | 41 (7.7) |
| Incontinence/diarrhoea | 18 (3.4) |
| Transferred/discharged to another unit | 79 (14.8) |
| Administrative error | 56 (10.5) |
| Other – including no reason | 50 (9.3) |
Screening compression duplex ultrasounds
The number and proportions of patients having a CDU in each treatment group are shown in the CONSORT diagram (see Figure 3). The reasons for a missing CDU are also given. Our protocol stated that CDU should be performed between day 7 and day 10 after randomisation and between day 25 and day 30. The median delay from randomisation to the first CDU was 8 days (IQR 7–10 days) in both treatment groups. The median delay from randomisation to the second CDU was 28 days (IQR 26–30 days) in both treatment groups. The actual timing of CDU is shown in Table 6.
| Delay from randomisation to CDU | IPC, n (%) | No IPC, n (%) |
|---|---|---|
| Timing of first CDU | 1315 (100.0) | 1305 (100.0) |
| < 7 days | 17 (1.3) | 14 (1.1) |
| 7–10 days | 1091 (83.0) | 1060 (81.2) |
| > 10 days | 207 (15.7) | 231 (17.7) |
| Timing of second CDU | 955 (100.0) | 938 (100.0) |
| < 25 days | 9 (0.9) | 6 (0.6) |
| 25–30 days | 721 (75.5) | 737 (78.6) |
| > 30 days | 225 (23.6) | 195 (20.8) |
Compression duplex ultrasound scans were not available for all surviving randomised patients (see Figure 3). In addition, we did not stipulate in our protocol that all patients should undergo CDU of the calf veins. The minimum acceptable CDU image set was one scan visualising the popliteal and more proximal veins. Therefore, calf veins were not visualised fully in all patients. Among patients who underwent first CDU, we were unable to exclude an isolated calf DVT in 615 out of 1315 (46.8%) in the IPC group and in 596 out of 1305 (45.7%) in the non-IPC group. Among those who underwent second CDU, we were unable to exclude an isolated calf DVT in 453 out of 955 (47.4%) in the IPC group and in 451 out of 938 (48.1%) in the non-IPC group.
In the IPC arm, 117 of 1315 (8.9%) patients were wearing IPC sleeves when they attended for their first CDU and 37 of 995 (3.7%) were wearing IPC sleeves when they attended for their second CDU. Therefore, the technician was not blinded in 154 (6.7%) of these 2310 CDUs. In addition, among the remaining 1198 patients who did not attend their first CDU wearing IPC, the technician guessed correctly they were allocated to IPC in 48 of cases (4%), had no idea in 916 (76.5%) and guessed incorrectly in 219 (18.3%). Of the 918 patients who were not wearing IPC at their second CDU, the technician correctly guessed the treatment allocated in 33 (3.6%), had no idea in 702 (76.5%) and guessed incorrectly in 171 (18.6%).
In the no-IPC arm, 4 of 1305 (0.3%) patients were wearing IPC sleeves when they attended for their first CDU and 4 of 938 (0.4%) were wearing IPC sleeves when they attended for their second CDU. At the first CDU the technician correctly guessed IPC status in 296 (22.7%) patients, had no idea in 970 (74.3%) patients and guessed incorrectly in 22 (1.7%) patients. At the second CDU, the technician correctly guessed the treatment allocation in 167 of cases (17.8%), had no idea in 739 (78.8%) and guessed incorrectly in 17 (1.8%) cases.
These data suggest that, where centres followed the protocol which required that they remove any IPC device from the patient before sending them for CDU, blinding was reasonably effective, that is the vast majority of observers had little idea of the treatment allocation.
Confirmation of primary outcome
Our primary outcome was confirmed in 276 of 296 (93.2%) cases by central review of CDU images by our radiologist (JR). In the remaining 20 cases (6.8%), the centres were unable to provide an image for central review. In these cases, the presence of a proximal DVT was confirmed by obtaining the local radiology report produced for clinical purposes. In the case of some patients, the local co-ordinator or technician had recorded a proximal DVT on the CDU report form which was entered into our database. These were not counted as primary outcomes unless the central review or local clinical report confirmed it.
Six-month follow-up
We aimed to follow up all surviving patients at about 6 months after randomisation. The number and proportions of patients in whom this was achieved in each treatment group are shown in our CONSORT diagram (see Figure 3). Patients and GPs were initially followed up by postal questionnaire, with a repeat mailing for non-responders. If no response, or an incomplete response to the postal questionnaire was received, then the chief investigator (MD) telephoned the respondent to obtain as much information as possible. The method of follow-up is shown in Table 7.
| Method of follow-up | IPC (n = 1098), n (%) | No IPC, (n = 1058), n (%) |
|---|---|---|
| Patient completed postal questionnaire | 359 (32.7) | 336 (31.6) |
| Proxy completed postal questionnaire | 474 (43.2) | 440 (41.6) |
| Postal completer not recorded | 20 (1.8) | 24 (2.3) |
| Telephone interview with patient | 112 (10.2) | 115 (10.7) |
| Telephone interview with proxy | 108 (9.8) | 117 (11.1) |
| Telephone interview not recorded if patient or proxy | 15 (1.4) | 15 (1.4) |
The timings of the 6-month patient follow-ups are shown in Table 8. As, inevitably, many of the follow-up questionnaires were not obtained until much more than 6 months later, some patients had died in the period from 6 months to the time when a follow-up questionnaire was obtained. We did not try to retrospectively code the functional status in such cases. However, it does mean that the number of patients recorded as having an OHS score of 6 (i.e. dead) at their 6-month follow-up is greater than the number of deaths which had occurred by 6 months (based on an actuarial analysis).
| Delay since randomisation (days) | IPC, n (%) | No IPC, n (%) |
|---|---|---|
| < 167 | 6 (0.5) | 14 (1.3) |
| 167–183 | 659 (60.0) | 588 (55.6) |
| 184–199 | 122 (11.1) | 119 (11.2) |
| 200–219 | 224 (20.4) | 237 (22.4) |
| 220–239 | 66 (6.0) | 79 (7.5) |
| ≥ 240 | 20 (1.8) | 21 (2.0) |
| Missing time | 1 (0.1) | 0 (0) |
| Total received | 1098 | 1058 |
Causes of death
For patients who died during their initial hospital admission, the local researcher coded the cause of death on the discharge form. For patients who died after hospital discharge, we aimed to obtain a copy of their death certificate, supplemented, where possible, by their GP notes or relevant hospital notes. Whenever an autopsy was carried out we tried to obtain the report. Final cause of death was coded by the Chief Investigator, usually blind to the treatment allocation. However, attribution of cause of death is notoriously difficult unless autopsies are performed. These data are reported for completeness, but we do not believe that any robust conclusions can be drawn from them.
We attempted to detect post-phlebitic leg syndrome by asking about leg swelling and ulcers at the 6-months follow-up. However, these questions were not validated and unlikely to be specific given the high frequency of swelling in stroke-affected limbs and of leg ulcers of other types.
Close out
After the final follow-up questionnaire had been completed, we sent each principal investigator a close-out checklist (see Appendix 3) to complete and send back to us. This checklist had been approved by ACCORD, the sponsor of the trial. Completed checklists were received from 93 (99%) of the 94 randomising hospitals. The remaining hospital, which had withdrawn from the trial before its completion, had lost all documentation and has instigated an internal inquiry. The trial master file was archived.
Chapter 4 Results
Results: primary and secondary outcomes – effectiveness and safety
Primary outcomes
The patients’ outcomes, with respect to our primary outcome, within 30 days of enrolment are shown in Table 9. The primary outcome occurred in 122 of 1438 patients (8.5%) allocated to IPC and in 174 of 1438 (12.1%) allocated to no IPC, with an OR of 0.65 (95% CI 0.51 to 0.84; p = 0.001) after adjustment for baseline variables and an ARR of 3.6% (95% CI 1.4% to 5.8%). To allow for any observer bias in detecting symptomatic DVTs not detected on routine-screening CDU, we repeated the primary analysis excluding those primary outcomes where a DVT was suspected prior to the CDU (n = 22). The estimates of effect were unchanged.
| Outcome | IPC (n = 1438), n (%) | No IPC (n = 1438), n (%) | Absolute risk difference (95% CI) | OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Primary outcome (proximal DVT) | 122 (8.5) | 174 (12.1) | –3.6 (–5.8 to –1.4) | – | – |
| Alive and free of primary outcome | 1145 (79.6) | 1071 (74.5) | – | – | – |
| Died prior to any primary outcome | 147 (10.2) | 176 (12.2) | – | – | – |
| Missing | 24 (1.7) | 17 (1.2) | – | – | – |
| Dead and missing excluded (unadjusted) | 122/1267 (9.6) | 174/1245 (14.0) | –4.3 (–6.9 to –1.8) | 0.66 (0.51 to 0.84) | 0.001 |
| Primary analysis (dead and missing excluded) | – | – | – | 0.65 (0.51 to 0.84) | 0.001 |
| Dead included with DVT and missing included with no DVT (unadjusted) | 269/1438 (18.7) | 350/1438 (24.3) | –5.6 (–8.6 to –2.6) | 0.71 (0.60 to 0.86) | 0.0002 |
| Dead included with DVT and missing included with no DVT | – | – | – | 0.71 (0.59 to 0.85) | 0.0002 |
Secondary outcomes within 30 days
The frequencies of deaths and other VTE outcomes within 30 days of randomisation are shown in Table 10. There were significant reductions in ‘any DVT’ (symptomatic or asymptomatic involving proximal or calf veins) (ARR 4.9%, 95% CI 2.1 to 7.8; p < 0.001) and symptomatic DVT (including proximal or calf) (ARR 1.7%, 95% CI 0.0 to 3.3; p = 0.045).
| Secondary outcomes by 30 days or later second CDU | IPC (n = 1438), n (%) | No IPC (n = 1438), n (%) | Absolute risk difference (95% CI) | OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Dead by 30 days | 156 (10.8) | 189 (13.1) | –2.3 (–4.7 to 0.1) | 0.80 (0.63 to 1.01) | 0.057 |
| Symptomatic proximal DVT | 39 (2.7) | 49 (3.4) | –0.7 (–2.0 to 0.6) | 0.79 (0.51 to 1.21) | 0.269 |
| Asymptomatic proximal DVT | 83 (5.8) | 125 (8.7) | –2.9 (–4.8 to –1.0) | 0.65 (0.48 to 0.86) | 0.003 |
| Symptomatic DVT (proximal or calf) | 66 (4.6) | 90 (6.3) | –1.7 (–3.3 to –0.0) | 0.72 (0.52 to 0.99) | 0.045 |
| Any DVT (symptomatic or asymptomatic, proximal or calf) | 233 (16.2) | 304 (21.1) | –4.9 (–7.8 to –2.1) | 0.72 (0.60 to 0.87) | 0.001 |
| All confirmed PE (imaging or autopsy) | 29 (2.0) | 35 (2.4) | –0.4 (–1.5 to 0.7) | 0.83 (0.50 to 1.36) | 0.453 |
| Any DVT or confirmed PE | 248 (17.2) | 325 (22.6) | –5.4 (–8.3 to –2.4) | 0.72 (0.59 to 0.86) | 0.00035 |
| Any DVT or dead | 377(26.2) | 472 (32.8) | –6.6 (–9.9 to –3.3) | 0.72 (0.61 to 0.85) | 0.00009 |
| Any DVT, PE or dead | 391 (27.2) | 491 (34.1) | –7.0 (–10.3 to –3.6) | 0.72 (0.61 to 0.84) | 0.00005 |
There were fewer deaths from all causes within 30 days among those allocated to IPC, but the difference was non-significant: IPC, 156 (10.8%), versus no IPC, 189 (13.1%; OR 0.80, 95% CI 0.63 to 1.01; ARR 2.3%, 95% CI to 0.1 to 4.7%; p = 0.057).
Table 11 shows the causes of death among the patients who died within 30 days of randomisation. This was based on the information provided by the local researchers. Autopsy to confirm the cause of death was carried out in only 10 (3%) of the patients who died within 30 days (three in the IPC group and seven in the no-IPC group).
| Cause of death | IPC, n (%) | No IPC, n (%) |
|---|---|---|
| Initial stroke | ||
| Neurological | 56 (36) | 64 (34) |
| Pneumonia | 43 (28) | 61 (32) |
| PE | 4 (3) | 5 (3) |
| Recurrent stroke | 18 (12) | 16 (8) |
| Coronary heart disease | 12 (8) | 8 (4) |
| Other vascular | ||
| Cerebrovascular | 10 (6) | 12 (6) |
| Bowel ischaemia | 1 (1) | 0 (0) |
| Other | 0 (0) | 7 (4) |
| Non-vascular | ||
| Fall | 0 (0) | 1 (1) |
| Carcinoma | 2 (1) | 2 (1) |
| Renal failure | 1 (1) | 1 (1) |
| Respiratory failure | 2 (1) | 4 (2) |
| Sepsis | 2 (1) | 2 (1) |
| Other | 2 (1) | 5 (3) |
| Uncertain | 1 (1) | 0 (0) |
| Missing | 2 (1) | 1 (1) |
| Total deaths | 156 (100) | 189 (100) |
Adverse events
Adverse events, including skins breaks, leg ischaemia, falls and fractures, which might have resulted from wearing IPC sleeves are shown in Table 12. There was a statistically significant excess of skin breaks on the legs of patients allocated to IPC [44 (3.1%) vs. 20 (1.4%), p = 0.002] but no significant differences in the risk of falls with injury or fractures within 30 days. Few of the skin breaks or falls with injury were attributed by the local researchers to the IPC. The majority of adverse events occurred either when IPC sleeves had been removed or when skin breaks affected the heels (which are not covered by the IPC sleeves); therefore, they were unlikely to be caused by the IPC. However, the reporting of secondary outcomes in hospital and adverse effects was based on case note review and was not blinded to treatment allocation. These adverse event data are therefore prone to ascertainment bias.
| Outcome | IPC (n = 1438), n (%) | No IPC (n = 1438), n (%) | Absolute risk difference (95% CI) | OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Potential adverse effects of IPC | |||||
| Skin breaks | 44 (3.1) | 20 (1.4) | 1.7 (0.6 to 2.7) | 2.23 (1.31 to 3.81) | 0.002 |
| Skin breaks attributed to IPC | 10 (0.7) | 0 (0.0) | 0.7 (0.3 to 1.1) | – | – |
| Lower limb ischaemia/amputation | 0 (0.0) | 2 (0.1) | –0.1 (–0.3 to 0.1) | – | – |
| Falls with injury in 30 days | 33 (2.3) | 24 (1.7) | 0.6 (–0.4 to 1.6) | 1.39 (0.82 to 2.37) | 0.221 |
| Falls with injury in 30 days attributed to IPC | 1 (0.1) | 0 (0.0) | 0.1 (–0.1 to 0.2) | – | – |
| Fractures within 30 days | 4 (0.3) | 4 (0.3) | 0.0 (–0.4 to 0.4) | – | – |
Deaths and venous thromboembolism events up to six months
The deaths and VTE events up to 6 months, including those arising during the first 30 days, are shown in Table 13. There was no evidence of an excess of VTE events in the post-treatment period to indicate that IPC simply deferred VTE events.
| Outcome | IPC (n = 1438), n (%) | No IPC (n = 1438), n (%) | Absolute risk difference (95% CI) | OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Dead by 6 months | 320 (22.3) | 361 (25.1) | –2.9 (–6.0 to 0.3) | 0.85 (0.70 to 1.01) | 0.059 |
| Any DVT | 240 (16.7) | 312 (21.7) | –5.0 (–7.9 to –2.1) | 0.72 (0.60 to 0.87) | 0.001 |
| Any symptomatic DVT | 77 (5.4) | 101 (7.0) | –1.7 (–3.4 to 0.1) | 0.75 (0.55 to 1.02) | 0.061 |
| Any confirmed PE | 42 (2.9) | 49 (3.4) | –0.5 (–1.8 to 0.8) | 0.86 (0.56 to 1.30) | 0.463 |
| Any death, DVT or PE | 526 (36.6) | 626 (43.5) | –7.0 (–10.5 to –3.4) | 0.74 (0.63 to 0.86) | < 0.001 |
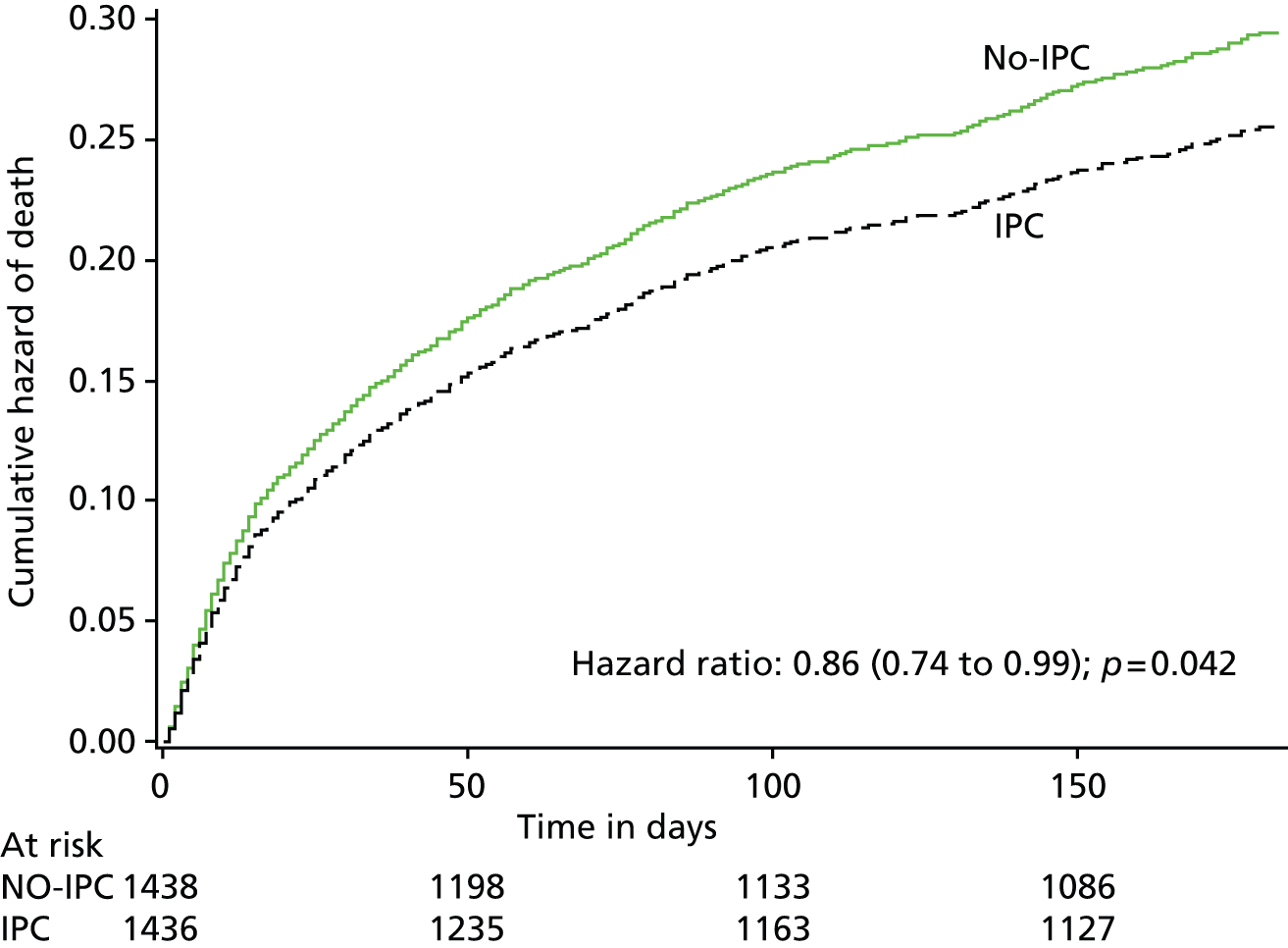
By 6 months from randomisation, there had been 320 deaths in the IPC group and 361 in the no-IPC group. The probability of death over the 6 months after randomisation in the two treatment groups is shown in Figure 6. The Cox model, adjusted for the factors included in our minimisation algorithm, showed a reduced hazard ratio of 0.86 (95% CI 0.74 to 0.99; p = 0.042) for death up to 6 months after randomisation in those allocated IPC.
FIGURE 6.
Cumulative hazard of death over the 6 months after randomisation.
The patients’ OHS scores are shown in Table 14. The numbers of deaths on the OHS were greater than the number of deaths by 6 months, because some patients died between 6 months and the completion of their OHS score.
| Outcomes | IPC, n (%) | No IPC, n (%) | p-value |
|---|---|---|---|
| OHS score | |||
| 0 | 45 (3.1) | 45 (3.1) | 0.375a |
| 1 | 94 (6.5) | 92 (6.4) | |
| 2 | 156 (10.8) | 156 (10.8) | |
| 3 | 306 (21.3) | 320 (22.3) | |
| 4 | 181 (12.6) | 185 (12.9) | |
| 5 | 309 (21.5) | 255 (17.7) | |
| Deadb | 330 (22.9) | 367 (25.5) | |
| Missing | 17 (1.2) | 18 (1.3) |
In total, 295 (20.5%) patients in the IPC group had an OHS score of 0–2, compared with 293 (20.4%) in the no-IPC group, an absolute risk difference of only 0.1% (95% CI –2.8% to 3.1%), a relative risk of 0.98 (95% CI 0.84 to 1.14) and an OR adjusted for baseline imbalance of 0.98 (95% CI 0.80 to 1.19; p = 0.83). Figure 7 illustrates the differences in functional outcomes in the two treatment groups at the final follow-up.
FIGURE 7.
The differences in functional outcomes (OHS) at 6 months between treatment groups (excluding the missing data so % values do not match those in Table 14).
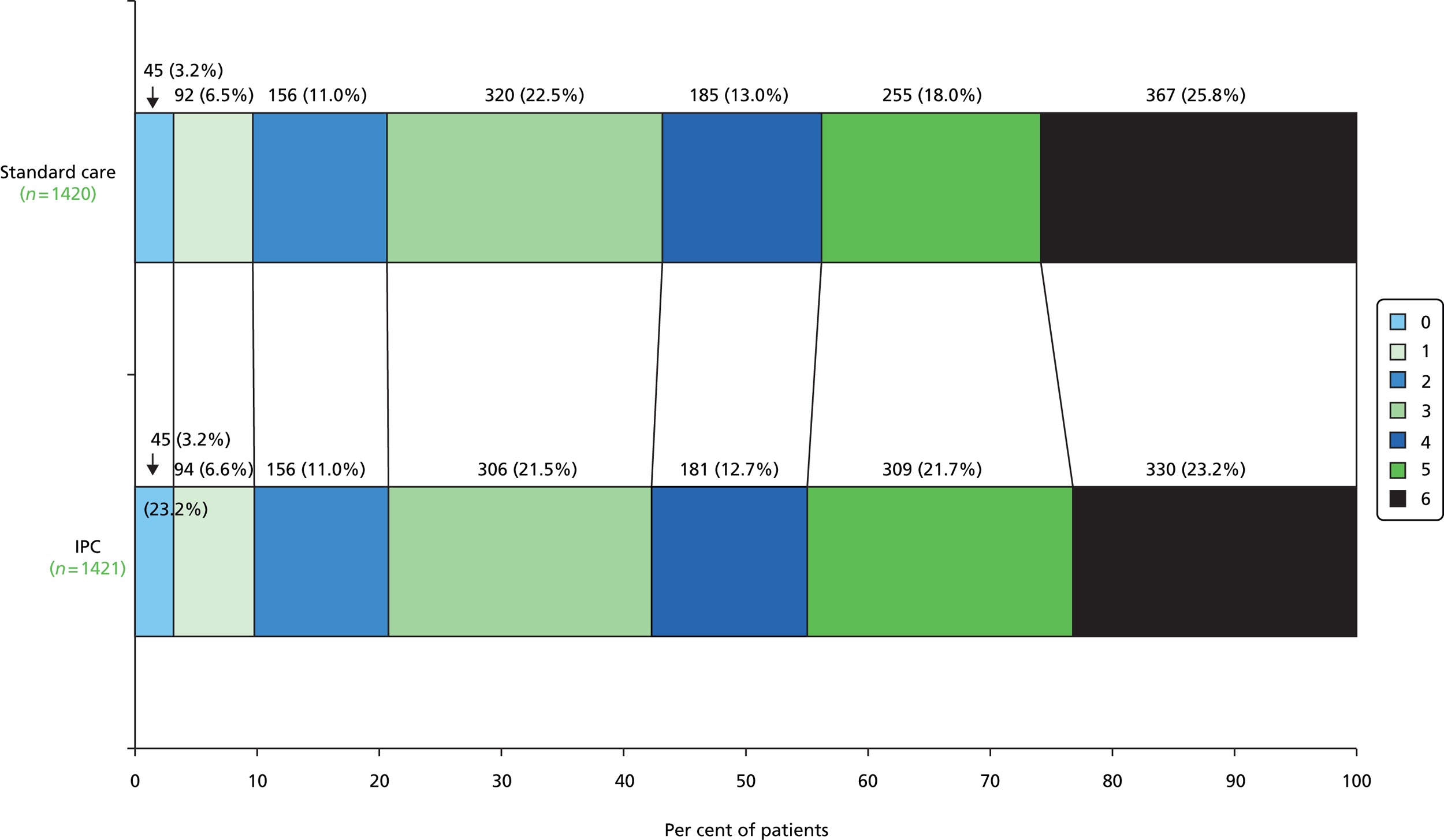
We carried out an ordinal analysis of the OHS using ordinal regression (results in Table 15). Neither the adjusted nor the unadjusted analysis showed a difference in the common odds between those treated with IPC or standard care.
| Comparing standard care with IPC | OR for poor outcome | p-value | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|
| Adjusted | 0.97 | 0.71 | 0.86 | 1.11 |
| Unadjusted | 0.98 | 0.80 | 0.86 | 1.12 |
Table 16 shows the individual dichotomies of the OHS scores. None of the differences between the treatment groups was statistically significant. However, a post-hoc exploratory analysis of the OHS scores shows that a 2.6% increase in the proportion of patients surviving at 6 months (p = 0.11) is more than offset by a 3.8% increase in the proportion of patients surviving with an OHS score of 5 (p = 0.013). However, this p-value needs to be interpreted with caution given that this analysis was not prespecified, was exploratory and might be spurious because of this.
| OHS score dichotomy | No IPC | IPC | OR | 95% CI |
|---|---|---|---|---|
| 0 | 45 | 45 | 1.00 | 0.70 to 1.64 |
| 1–6 | 1375 | 1376 | ||
| 0–1 | 137 | 139 | 0.98 | 0.77 to 1.26 |
| 2–6 | 1283 | 1282 | ||
| 0–2 | 293 | 295 | 0.99 | 0.83 to 1.19 |
| 3–6 | 1127 | 1126 | ||
| 0–3 | 613 | 601 | 1.04 | 0.89 to 1.20 |
| 4–6 | 807 | 820 | ||
| 0–4 | 798 | 782 | 1.05 | 0.90 to 1.22 |
| 5–6 | 622 | 639 | ||
| 0–5 | 1053 | 1091 | 0.87 | 0.73 to 1.03 |
| 6 | 367 | 330 | ||
| Total | 1420 | 1421 | – | – |
Living circumstances at 6 months
There were two questions on the 6-month follow-up form concerning patients’ living circumstances. Table 17 shows the cross-tabulation of the two variables where follow-up was completed.
| Where do you live now? | How do you live now? | ||||
|---|---|---|---|---|---|
| Not applicable | On my own | With partner or relative | Missing | Total | |
| In a nursing home | 120 | 48 | 6 | 171 | 345 |
| In a residential home | 32 | 34 | 10 | 43 | 119 |
| In my own home | 3 | 413 | 1101 | 0 | 1517 |
| In the home of a relative | 0 | 2 | 97 | 0 | 99 |
| Other | 28 | 5 | 4 | 0 | 37 |
| Unknown | 2 | 0 | 1 | 0 | 3 |
| Missing | 0 | 0 | 0 | 36a | 36 |
| Total | 185 | 502 | 1219 | 250 | 2156 |
To determine if there is a relationship between use of IPC and living circumstances at final follow-up, we dichotomised the location of the patients at follow-up as living at home (in my own home or in the home of a relative) or living in an institution/still in hospital (in a nursing home, in a residential home, in-hospital patient). Those whose living circumstances were classed as ‘other’ or ‘unknown’ were not included in this analysis. Of those allocated to IPC, 24.7% (266 out of 1076) were living in an institution or were still in hospital at 6 months, compared with 22.4% (233 out of 806) of those allocated to standard care.
A summary of the logistic regression models is shown in Table 18. In both the unadjusted and adjusted analysis the CI for the ORs indicate that treatment is not significantly related to location at 6 months in those who responded at 6 months.
| Comparing home with institution at 6 months (excluding dead and missing) | OR for poor outcome | p-value | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|
| Adjusted | 1.11 | 0.358 | 0.89 | 1.37 |
| Unadjusted | 1.14 | 0.214 | 0.93 | 1.39 |
Post-phlebitis leg syndrome
The reported frequency of leg swelling and leg ulcers at the 6-month follow-up is shown in Table 19. However, it is unclear whether or not these reported symptoms indicate the development of post-phlebitis leg syndrome, or if they simply reflect comorbidities such as heart failure or ulcers due to other causes.
| Completed 6-month follow-up form | IPC (n = 1098), n (%) | No IPC (n = 1058), n (%) |
|---|---|---|
| Leg swollen since stroke | 388 (35.3) | 396 (37.4) |
| Leg ulcer since stroke | 24 (2.2) | 19 (1.8) |
Subgroup analyses
Subgroup analyses were performed to determine if particular types of patients might gain more or less benefit from IPC. We estimated the effect of treatment allocation on our primary outcome subdivided by key baseline variables and adjusted for the other factors included in our minimisation algorithm. Subgroup analyses were performed by observing the change in log-likelihood when the interaction between the treatment and the subgroup is added into a logistic regression model. We determined whether or not there was any significant heterogeneity between these subgroups. The lack of any significant interaction between the subgroups and treatment effect has to be interpreted with caution, given that the trial was not large enough to identify small to moderate interactions.
Does delay in applying intermittent pneumatic compression impact on its effectiveness?
There is evidence that DVTs may develop within the first 3 days after a stroke. 34 There is often a delay in presenting with a stroke and there will always be some delay in applying VTE prophylaxis because the patients need to be assessed and the treatment started. In addition, in a randomised trial there may be additional delays because of the time taken to obtain consent and enrol the patients. To reduce the possible impact of this delay, we encouraged collaborators to enrol patients as early as possible after admission and stipulated that we would ensure that at least 75% of patients are enrolled by day 2, with day 0 being the day of the stroke.
We hypothesised that IPC would reduce the frequency of our primary outcome to a greater extent among patients enrolled earlier than among those enrolled later. We therefore examined the effect of treatment among patients enrolled early compared with those who were enrolled later. We assessed the effect of delay by applying two different definitions of delay (measured in days from stroke onset to enrolment):
-
day 0 or 1 versus days 2–7 (the split on which our minimisation algorithm is based)
-
days 0–2 versus days 3–7 (as stipulated in our sample size estimates).
The results are shown in Table 20 and indicate that, surprisingly, the effect of IPC was not greater in those in whom it was applied earlier.
| Comparing IPC with no IPC, DVT with no DVT (dead and missing excluded) | OR for poor outcome | p-value | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|
| Adjusted: delay 0 or 1 day | 0.71 | 0.572 | 0.48 | 1.04 |
| Adjusted: delay ≥ 2 days | 0.61 | 0.44 | 0.85 | |
| Unadjusted: delay 0 or 1 day | 0.69 | 0.696 | 0.47 | 1.02 |
| Unadjusted: delay ≥ 2 days | 0.63 | 0.46 | 0.87 | |
| Adjusted: delay 0–2 days | 0.67 | 0.758 | 0.50 | 0.89 |
| Adjusted: delay ≥ 3 days | 0.61 | 0.37 | 1.01 | |
| Unadjusted: delay 0–2 days | 0.66 | 0.839 | 0.50 | 0.88 |
| Unadjusted: delay ≥ 3 days | 0.63 | 0.38 | 1.03 |
Does the severity of stroke impact on the effect of intermittent pneumatic compression?
Our analyses divided patients into subgroups with different stroke severities including:
-
paralysis of leg (able vs. unable to lift the leg) at randomisation
-
probability of survival free of dependency (OHS score of < 3) based on the predictive model used in minimisation.
We hypothesised that those with more severe weakness, or a predicted worse outcome, would have higher rates of DVT because of more prolonged immobility and the co-occurrence of infections, etc. In addition, this group may tend to have better adherence to IPC because often they are not so able to express a wish to have them removed. The results are shown in Table 21 and do not suggest any significant interaction between these subgroups and the overall treatment effect, although this may reflect the moderate numbers of patients included in the subgroups as there are marked differences in the effect sizes in the two subgroups.
| Comparing IPC to no IPC, DVT to no DVT (dead and missing excluded) | OR for poor outcome | p-value | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|
| Adjusted: lift both legs – no | 0.58 | 0.164 | 0.43 | 0.78 |
| Adjusted: lift both legs – yes | 0.84 | 0.53 | 1.33 | |
| Unadjusted: lift both legs – no | 0.59 | 0.169 | 0.44 | 0.79 |
| Unadjusted: lift both legs – yes | 0.86 | 0.54 | 1.36 | |
| Adjusted: probability 0–0.15 | 0.64 | 0.793 | 0.47 | 0.86 |
| Adjusted: probability 0.16–1 | 0.68 | 0.45 | 1.05 | |
| Unadjusted: probability 0–0.15 | 0.63 | 0.705 | 0.46 | 0.85 |
| Unadjusted: probability 0.16–1 | 0.70 | 0.45 | 1.06 |
Is intermittent pneumatic compression more effective in patients at higher risk of deep vein thrombosis?
It seemed likely that individuals at higher risk of DVT might gain more from IPC than those at lower risk. In the CLOTS 1 and 2 trials, we showed that immobile stroke patients with the following features had a greater risk of proximal DVT:3
-
dependent in ADL prior to stroke
-
prior history of DVT
-
unable to lift both arms
-
unable to lift both legs.
We undertook subgroup analyses of the effect of allocation to the IPC group on the primary outcome among individuals with and without at least one of these prognostic factors at baseline. There was no statistically significant interaction between the baseline risk of DVT and the treatment effect (Table 22), although this may reflect the moderate numbers of patients included in the subgroups because there are marked differences in the effect sizes in the two subgroups. The risk of DVT was higher in those with at least one of the predefined risk factors (13.3%; 257/1927) than in those without (6.7%; 39/585).
| Comparing IPC with no IPC and DVT with no DVT (dead and missing excluded) | OR for poor outcome | p-value | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|
| Adjusted: high-risk DVT – no | 0.87 | 0.294 | 0.45 | 1.67 |
| Adjusted: high-risk DVT – yes | 0.61 | 0.47 | 0.80 | |
| Unadjusted: high-risk DVT – no | 0.89 | 0.306 | 0.46 | 1.71 |
| Unadjusted: high-risk DVT – yes | 0.61 | 0.47 | 0.80 |
Is intermittent pneumatic compression more effective in those patients in whom anticoagulation is not given or advisable?
In some health systems anticoagulation is used widely for the prophylaxis of DVT in those who have experienced ischaemic stroke, despite the lack of evidence that this improves overall outcome. 12 As a result of this, IPC and other forms of external compression have often been used in individuals with haemorrhagic stroke. Therefore, we carried out subgroup analyses among individuals:
-
on anticoagulants (as defined in our protocol) versus not, at baseline
-
with confirmed haemorrhagic versus ischaemic or unknown pathological type of stroke.
The analyses show (Table 23) that there was no significant interaction between the presence or absence of these factors at baseline and the treatment effect. The patients who received alteplase, heparin, LMWHs or oral anticoagulants at baseline had a higher risk of our primary outcome than those who did not [14.9% (92/619) vs. 10.8% (204/1890)]. However, there was a trend for IPC to have a greater effect in patients with haemorrhagic stroke, although, overall, the risk of our primary outcome was similar in those with haemorrhagic and ischaemic stroke [11.8% (38/322) vs. 11.8% (258/2190)]. The lack of a significant interaction may reflect the moderate numbers of patients included in the subgroups, as there are marked differences in the effect sizes in the two subgroups.
| Comparing IPC with no IPC and DVT with no DVT (dead and missing excluded) | OR for poor outcome | p-value | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|
| Adjusted: anticoagulants – no | 0.65 | 0.897 | 0.48 | 0.87 |
| Adjusted: anticoagulants – yes | 0.67 | 0.43 | 1.06 | |
| Unadjusted: anticoagulants – no | 0.65 | 0.870 | 0.48 | 0.87 |
| Unadjusted: anticoagulants – yes | 0.68 | 0.43 | 1.06 | |
| Adjusted: haemorrhage – no | 0.71 | 0.057 | 0.55 | 0.93 |
| Adjusted: haemorrhage – yes | 0.35 | 0.17 | 0.75 | |
| Unadjusted: haemorrhage – no | 0.71 | 0.071 | 0.55 | 0.93 |
| Unadjusted: haemorrhage – yes | 0.35 | 0.17 | 0.74 |
Is intermittent pneumatic compression using the Comfort™ sleeves more effective than that using the standard sleeves?
During the CLOTS 3 trial, it was noted that adherence to IPC was suboptimal. The manufacturer responded to this information by introducing a modified IPC sleeve, the Comfort™ sleeve, intended to improve acceptability and thus adherence. We switched to using this new sleeve on 17 October 2011. Therefore, the first 1679 (59%) patients enrolled in the CLOTS 3 trial were allocated to the original sleeve or not, and the subsequent patients to Comfort™ sleeve or not. There was little difference in adherence between the two types of sleeves (see Table 4). We examined the effect on our primary outcome of the original and the Comfort™ sleeves separately. There was no significant interaction between the sleeve type and the treatment effect (Table 24).
| Comparing IPC with no IPC and DVT with no DVT (dead and missing excluded) | OR for poor outcome | p-value | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|
| Adjusted: sleeves – Comfort™ | 0.59 | 0.510 | 0.41 | 0.87 |
| Adjusted: sleeves – standard | 0.70 | 0.51 | 0.98 | |
| Unadjusted: sleeves – Comfort™ | 0.60 | 0.550 | 0.41 | 0.87 |
| Unadjusted: sleeves – standard | 0.70 | 0.50 | 0.97 |
The results of these subgroup analyses are summarised in the forest plot shown in Figure 8.
FIGURE 8.
Forest plot showing the results of our prespecified subgroup analyses.

Other prespecified secondary analyses
Intermittent pneumatic compression has usually been used for a relatively short duration in perioperative patients and those in high-dependency units. In the CLOTS 3 trial, we aimed to maintain the IPC treatment for up to 30 days, if the patient remained in hospital and was still immobile. However, for a wide variety of reasons, IPC was often terminated earlier than this or was not applied continuously. In the CLOTS 1 and 2 trials, 79% of proximal DVTs were detected on the first CDU. 5 Thus, the risk of DVT seemed to be highest early on; therefore, any prophylaxis might be more effective during this earlier period when the risks are highest and the adherence is best. We carried out additional prespecified analyses (including all those subgroups identified for our primary outcome) of the effect of allocation to IPC on the primary outcome occurring within 14 days of randomisation, rather than 30 days. This aimed to reduce the impact of those patients who required IPC for only a few days before becoming mobile or adhered to IPC for only a short time. In addition, it reflects the practice in many places where acute hospital admissions for stroke are short and thus prophylaxis is applied only for the first few days.
The number of patients with our primary outcome within 14 days is shown in Table 25. A total of 184 (62%) out of 296 patients with a primary outcome experienced the primary outcome within 14 days of randomisation. The effect of IPC on the rate of the primary outcome within 14 days is shown in Table 26. The observed effect size based on the 14-day outcomes was slightly larger (OR 0.58 compared with 0.65) than that for the 30-day outcomes, but the difference was not statistically significant.
| Outcome | Randomised treatment | |
|---|---|---|
| IPC, n (%) | Standard care, n (%) | |
| Number of patients randomised | 1438 (100.00) | 1438 (100.00) |
| Alive no prior DVT | 1229 (85.5) | 1171 (81.4) |
| DVT | 70 (4.9) | 114 (7.9) |
| Dead without prior DVT | 104 (7.2) | 122 (8.5) |
| Missing | 35 (2.4) | 31 (2.2) |
| Comparing IPC with no IPC and DVT with no DVT (dead and missing excluded) | OR for a proximal DVT | p-value | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|
| Unadjusted | 0.58 | 0.001 | 0.43 | 0.80 |
| Adjusted | 0.58 | 0.001 | 0.43 | 0.80 |
Chapter 5 The cost-effectiveness of intermittent pneumatic compression in stroke patients
Introduction
We incorporated a health economic analysis to provide some estimates of the costs of IPC and, therefore, its cost-effectiveness and cost utility. These data aim to inform decisions about whether or not IPC should be offered routinely by the NHS.
Methods
We performed a within-trial cost–utility analysis to estimate the cost-effectiveness of IPC with an intention-to-treat analysis. We measured patient resource use using the duration of stay for the index stroke following randomisation. Resource consequences included hospital length of stay as well as the direct costs of IPC capital and equipment. We converted length-of-stay distributions into cost estimates based on a per-diem hospital cost. Trial centre-/region-specific per-diem hospital costs were based on NHS Reference Costs in England35 and cost information for NHS Scotland derived from the Scottish Health Service Costs resource. 36 Per-diem hospital costs were derived using 2012/13 tariff information for Healthcare Resource Group code AA22Z (non-transient stroke or cerebrovascular accident, nervous system infections or encephalopathy), which is specific for admitted patient care for stroke. The non-elective tariff spell for hospital reimbursement was £4208 for Healthcare Resource Group code AA22Z up to the non-elective trim point of 53 days. For length of stay beyond the trim point, a per-diem cost of £210 was added to the non-elective spell tariff. Conversely, the tariff was reduced by 25% if patient length of stay was less than 2 days. A per-diem-based cost estimate was used in all economic analyses by calculating the monetary value of the National Tariff estimates35,36 and national average length of stay for stroke. This resulted in a per-diem cost of £216. To relax the assumption of a constant rate of activity and use of hospital resources, a gamma distribution was used in sensitivity analyses to simulate patient cost distributions as a function of length of stay. Within this scenario, per-diem cost estimates were greater for initial days in hospital. The results from the sensitivity analysis were quantitatively and qualitatively similar to the base-case analysis, so are not reported. A NHS hospital perspective was adopted for assessing resource use and costs. We did not assess the cost of nursing home, social care or the cost of readmissions to hospital within the first 6 months after randomisation.
Our economic analyses aimed to estimate the costs of preventing a proximal DVT (our primary outcome) and other adverse outcomes (VTE and deaths). We had originally planned (see protocol) to carry out a within-trial, short- and long-run cost-effectiveness analysis. However, the short-run analysis demonstrated that there was little significant difference in hospital costs up to 6 months following randomisation and no significant difference in the average quality-adjusted survival times, again up to 6 months following randomisation. We were unable to use these negligible and imprecise estimates of cost-effectiveness as a foundation for the long-run state-transition modelling that we had originally planned. Unfortunately, the consent procedures did not include obtaining participant or proxy consent to long-term data linkage which would provide data on long-term survival and hospital admissions. It is possible that in the future a Caldicott guardian might provide permission for data linkage. If long-run survival data (up to 5 years) are obtained, we will be able to calibrate a long-run model of hospital costs and survival and estimate the cost-effectiveness of using IPC as a means of reducing the risk of proximal DVT in immobilised stroke patients.
As described in our protocol, we estimated the average (mean) incremental costs and incremental quality-adjusted survival, expressed as quality-adjusted life-days (QALDs) rather than years. With only a 6-month follow-up and no direct measure of baseline quality of life we were unable to reliably estimate quality-adjusted life-years.
We estimated the direct cost of preventing VTE events or death in the treatment period with IPC in immobile stroke patients based on the ARR (see Table 10) and the numbers needed to treat derived from this. We produced estimates which took into account only the direct cost of providing IPC. We did not take account of any possible increased hospital costs in the first 30 days associated with IPC use because these were low and not statistically significant.
We used a standard multiplicative model to estimate QALDs by calculating the area under a linear interpolation of the EQ-5D-3L index trajectory for each individual using survival times, the EQ-5D-3L utility index score at 6 months and a modelled baseline EQ-5D-3L utility index score. We used multiple imputation by chained equations to impute missing health-related quality-of-life data on the EQ-5D-3L questionnaire using the multiple imputations suite of commands in Stata version 12 (StataCorp LP, College Station, TX, USA). 37
The EQ-5D-3L (see Appendix 2) aims to assess patients’ quality of life. In the CLOTS 3 trial, it was measured at the final follow-up by asking patients or carers to rate them on five domains. Each domain can be scored from 1 (best) to 3 (worst). The EQ-5D-3L score was not collected at the time of randomisation because the validity of asking patients or carers to rate patients’ quality of life shortly after admission to hospital with a severe stroke is questionable. We therefore used a Bayesian (belief) network38 to learn the pattern of responses across the five domains of EQ-5D: mobility (A), self-care (B), usual activities (C), pain or discomfort (D) and anxiety or depression (E). The pooled 6-month EQ-5D-3L data from the CLOTS 1,8 220 and 3 and FOOD trials39,40 were used to predict and inform baseline EQ-5D-3L scores for the CLOTS 3 trial patients. Only cases with complete responses across all five domains were included in the learning algorithm. This resulted in a total sample of 12,282, of which the CLOTS 3 trial contributed 2102 cases. Any EQ-5D-3L responses weighted as zero resulting from death at 6 months were excluded from the sample because of the explicit assumption that patients need to be alive at baseline to be randomised.
Bayesian networks are constructed through a two-stage process involving both qualitative and quantitative components. The qualitative component represents the initial stage and considers the relationship among key variables to define the structure of the quantitative component. Constraint- and score-based learning algorithms were evaluated using the bnlearn package in R, version 3.0.1 (The R Foundation for Statistical Computing, Vienna, Austria). Figure 9 presents the results from the graphical language of the qualitative component for both types of learning algorithms. Figure 9a represents the constraint-based algorithm based on a grow–shrink Markov blanket. This naive Bayesian network can be considered one of the simplest learning algorithm structures. Nonetheless, results were not sensitive to more sophisticated constraint-based algorithms, such as incremental association classifiers. Instead, the qualitative component was sensitive to the choice between a constraint- and score-based learning algorithm, as depicted in Figure 9b. The score-based learning algorithm based on a hill-climbing search was selected in favour of the constraint-based approach in order to account for the dependency between the five domains of EQ-5D. A tree-augmented naive Bayes network classifier was used to predict and inform baseline EQ-5D-3L scores using a Chow–Liu algorithm to approximate the dependency structure between the EQ-5D-3L domains.
FIGURE 9.
Diagram representing (a) the constraint-based algorithm; and (b) the hill-climbing learning algorithm used to model the EQ-5D-3L scores at baseline for patients in the trial. The arrows indicate a causal relationship in the hill-climbing algorithm.

Mobility (A) is defined as the root node within the hill-climbing algorithm in Figure 9. The consequence of this categorisation ensures that a particular response to the mobility domain influences responses across self-care (B), usual activities (C) and pain (D). The graphical language presented in Figure 9 has a causal interpretation so that a decline in mobility is considered to cause reductions in self-care and usual activities and worsening of pain. Anxiety/depression, denoted by (E) in Figure 9, represents the leaf node within the hill-climbing algorithm because of the absence of any causal link from (E) to the other domains of EQ-5D. Instead, responses for anxiety/depression (E) are considered to be determined by self-care (B), usual activities (C) and pain (D).
To derive baseline EQ-5D-3L, the score-based learning algorithm was used to predict discrete responses to the domains of EQ-5D-3L using an informative prior based on expert opinion for the mobility, self-care and usual activities domains. A prior value of 3 was attached to the mobility domain because the eligibility criteria for the trial meant that patients had to be immobile on entry. Similarly, we allocated a value of 3 (unable) to self-care (B) and usual activities (C) given that immobile stroke patients who are in hospital are unable to wash and dress themselves or carry out their usual activities. The prediction of baseline EQ-5D-3L scores within the Bayesian network was, therefore, confined to pain (D) and anxiety/depression (E). A sensitivity analysis was undertaken in which a discrete distribution was also used to simulate responses for the pain and anxiety/depression domains based on an imputed value of 3 for the other categories. The Bayesian network approach represents the primary method for estimating baseline EQ-5D-3L scores and the discrete distribution was explored in sensitivity analyses with results which were both qualitatively and quantitatively similar.
We estimated the direct cost of the intervention to the NHS based on a range of prices provided by Covidien Ltd, the manufacturer and supplier of the Kendall SCD™ Express devices. Covidien Ltd usually lends hospitals the controllers (pumps) and tubing, and includes the cost of these in the cost of the sleeves. The price that the NHS pays for Covidien Ltd’s single-patient use standard and Comfort™ sleeves depends on the volume purchased and local negotiation. We used a price at the upper end of this range (£25 per pair), which would subsume the estimated cost of the small amount of time required by nursing staff to size and fit the sleeves (15 minutes) and to monitor the use of IPC (5 minutes per day). The per-patient cost of the intervention was based on the average duration of use (about 12 days) and the average number of pairs of sleeves used by a patient (2.5, range 1–5). The latter was estimated by subtracting the numbers of sleeves remaining in the centres at the end of the trial (based on a stocktake) from the total number provided during the trial, divided by the number of patients allocated to the IPC group. This assumes that the IPC sleeves were used only on patients allocated IPC in the CLOTS 3 trial and that supplies were not lost by the hospital.
We estimated the average (mean) incremental costs, expressed in 2013 UK pounds, and incremental QALDs. We also estimated direct costs of preventing VTE events and deaths within 30 days of randomisation. We used generalised linear models to analyse the distribution of costs and QALDs separately using a general specification that allowed for different parametric distributions. We also assessed differences in costs and effects using econometric methods that account for the dependency between each outcome. We used simultaneous equation individual-level regression models to estimate the joint distribution of costs and QALDs. We performed non-parametric bootstrapping to assess the joint densities of incremental costs and incremental effects and to explore uncertainty in the cost-effectiveness results based on 10,000 bootstrap replications using Stata version 12. All simulations were undertaken within R, version 3.0.1, and exported to Stata, version 12, for analysis. 41 We also performed probabilistic sensitivity analysis to assess the robustness of the reported results for both short-run QALD estimates and hospital cost distributions. The utilities were based on published preferences for a UK population. 42
We had planned to estimate the averted costs arising from the effects of IPC on expected DVT/PE incidence. However, the marginal effect of a change in DVT/PE incidence would be observed only for symptomatic and treated DVT/PE. These were relatively rare events. We did not see a material difference in hospital resource use between the treatment groups that could be attributed to a change in DVT/PE incidence; therefore, we did not enter this into our modelling.
We assessed differences in costs and effects using econometric methods based on a copula framework that is particularly useful and straightforward when modelling joint parametric distributions. We also summarised our cost-effectiveness results within a net-benefit approach using incremental net (monetary) benefit and cost-effectiveness acceptability curves.
Costs and benefits of an effective approach to preventing DVT following stroke will accrue over time. However, in these analyses we estimated only the short-run costs over the first 6 months after randomisation.
Results
Costs of intermittent pneumatic compression
The price of sleeves provided by Covidien Ltd was £14 for a medium pair of standard sleeves and £26 to £31.50 for Comfort™ sleeves depending on size (extra small, small, medium or large). For our analyses we estimated an average cost of £64.10 per patient, including the cost of sleeves, fitting and monitoring.
We estimated the direct cost of preventing VTE events or death in the treatment period with IPC in immobile stroke patients (Table 27).
| Prevention of | Cost (£) | 95% CI |
|---|---|---|
| Proximal DVT (symptomatic or asymptomatic) | 1795 | 1089 to 4551 |
| Any DVT (proximal or distal, symptomatic or asymptomatic) | 1282 | 785 to 3077 |
| Any symptomatic DVT | 3546 | 1923 to ∞ |
| Confirmed PE | 16,025 | 4295 to ∞ |
| Death within 30 days | 2756 | 1346 to ∞ |
Costs of hospital stay
Table 28 outlines the descriptive statistics for length of stay and the use of IPC sleeves. The average length of stay for the CLOTS 3 trial sample is substantially higher than the UK national average for stroke, probably because the patients who were recruited into the trial generally had had more severe strokes. The UK mean is 19.5 days, with a median of 9 days. A discrete distribution was used to simulate the number of sleeves used by each patient based around a mean value of 2.5 pairs of sleeves. The discrete distribution assumed a minimum number of one pair of sleeves, with a maximum number of five pairs. The cost estimates of IPC and hospitalisation are shown in Table 29.
| Resources | IPC (n = 1438) | No IPC (n = 1438) | ||
|---|---|---|---|---|
| Mean (SD) | Median (range) | Mean (SD) | Median (range) | |
| Truncated length of stay (days) | 44.5 (37.6) | 34.0 (1–184) | 42.8 (37.2) | 32.0 (1–184) |
| Number of sleeves per patient | 2.5 (0.9) | 2.5 (1–5) | 2.5 (0.9) | 3.0 (1–5) |
| Number of days IPC should have been worn | 22.9 (11.2) | 30.0 (0–91) | 0 (0) | 0 (0) |
| Number of days actually worn | 11.7 (10.6) | 8 (0–65) | 0.01 (0.3) | 0 (0–12) |
| Costs | IPC (n = 1438) | No IPC (n = 1438) | ||
|---|---|---|---|---|
| Mean (SD) | Median (range) | Mean (SD) | Median (range) | |
| Cost of IPC | 64.10 (28.3) | 60.5 (0–159.9) | 0.19 (3.8) | 0 (0–103.5) |
| Cost of hospital days | 12,503 (8263) | 10,283 (1579–43,093) | 12,116 (8163) | 9694 (1595–43,129) |
| Cost of IPC plus hospital days | 12,567 (8264) | 10,338 (1579–43,153) | 12,116 (8163) | 9694 (1595–43,129) |
Health-related quality of life at the 6-month follow-up
We assessed the health-related quality of life at the 6-month follow-up with the EQ-5D-3L. We derived utilities based on the preferences of a UK population, with values varying from –0.594 to 1.0. 42 Figure 10 shows the distribution of EQ-5D-3L scores split by treatment allocation. In these plots, patients who have died are included with a utility of 0 and those with missing data (IPC, n = 35; no IPC, n = 42) have been excluded. The descriptive statistics are shown in Table 30, which also shows the results with imputed outcome data. Given the distribution of the data, we used a two-sample Wilcoxon test to compare the groups and there is no evidence of a statistically significant difference in the distributions [IPC, median 0.028 (IQR 0.000–0.587); standard care, median 0.024 (IQR 0.000–0.587); p = 0.62].
FIGURE 10.
Distributions of utilities based on EQ-5D-3L and UK population preferences at about 6 months after randomisation in the IPC and standard care groups. (a) IPC; and (b) standard care.
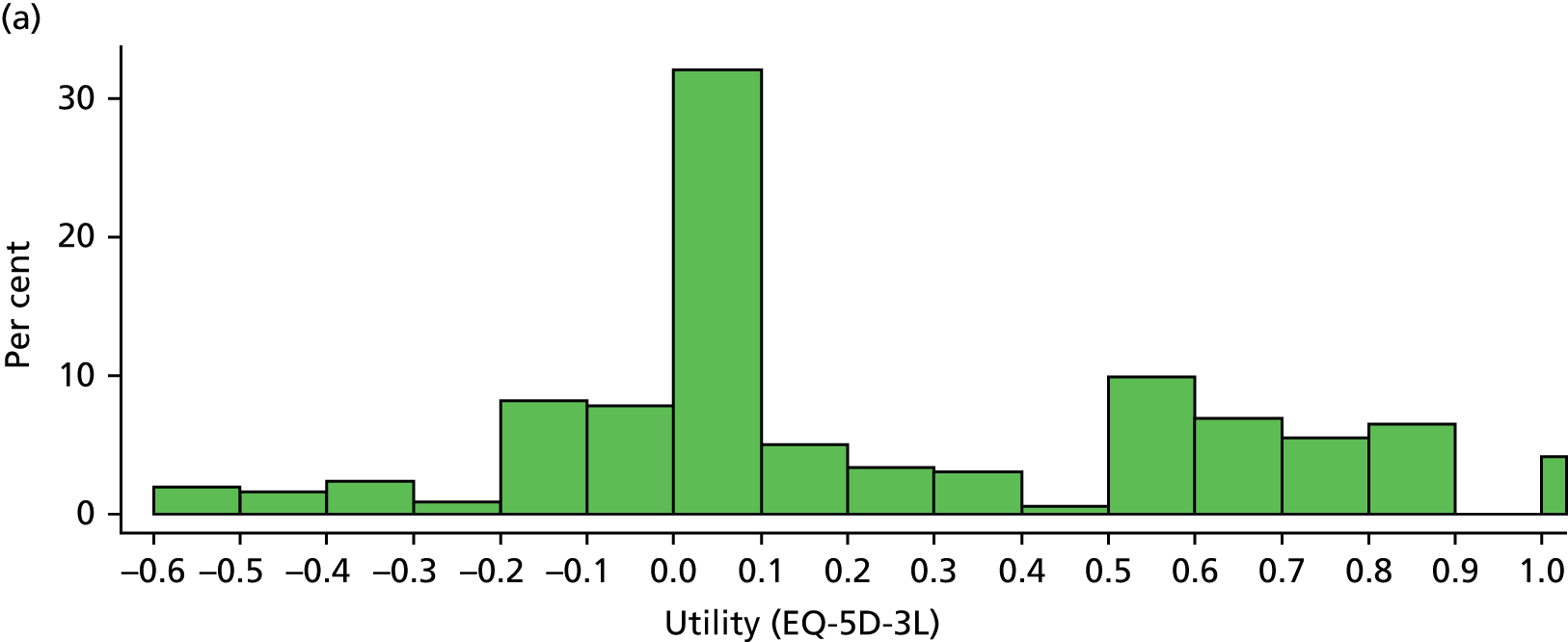

| Outcome | IPC | No IPC | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (range) | n | Mean (SD) | Median (range) | |
| 6-month EQ-5D | 1403 | 0.22 (0.39) | 0.03 (–0.59 to 1) | 1396 | 0.22 (0.37) | 0.02 (–0.59 to 1) |
| 6-month EQ-5D-3L score multiple imputations | 1438 | 0.23 (0.39) | 0.03 (–0.59 to 1) | 1438 | 0.22 (0.38) | 0.03 (–0.59 to 1) |
The effect of treatment on health-related quality of life
We defined effect as the change in utility based on EQ-5D-3L score from baseline (modelled as in Figure 9 and Table 31) to 6 months, divided by 2. We then multiplied this value by survival in days to generate the QALDs over the 6-month period. We derived the baseline EQ-5D-3L score index from the Bayesian network rather than the discrete simulation approach (see Table 31). The results are shown in Table 32.
| Outcome | IPC (n = 1438) | No IPC (n = 1438) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Minimum | Maximum | Mean (SD) | Median | Minimum | Maximum | |
| Baseline EQ-5D-3L score Bayesian network | –0.127 (0.13) | –0.095 | –0.594 | 0.028 | –0.130 (0.13) | –0.095 | –0.594 | 0.028 |
| Baseline EQ-5D-3L score discrete | –0.188 (0.17) | –0.166 | –0.594 | 0.028 | –0.193 (0.18) | –0.166 | –0.594 | 0.028 |
| Outcome | IPC | No IPC | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (range) | n | Mean (SD) | Median (range) | |
| Survival, days (complete cases) | 1403 | 152.5 (60.6) | 183 (0 to 183) | 1396 | 148.1 (64.3) | 183 (1 to 183) |
| Survival, days (multiple imputations) | 1438 | 153.3 (60.0) | 183 (0 to 183) | 1438 | 149.1 (63.6) | 183 (1 to 183) |
| QALDs (complete cases) | 1403 | 27.6 (40.6) | 16.6 (–84.3 to 145.9) | 1396 | 26.7 (39.6) | 15.4 (–84.3 to 145.9) |
| QALDs (multiple imputations) | 1438 | 28.2 (40.7) | 17.6 (–84.3 to 145.9) | 1438 | 27.5 (39.8) | 15.9 (–84.3 to 145.9) |
Tables 33 and 34 present the seemingly unrelated regression results for cost and effect. The dependent variable for the cost equation is cost of sleeves plus hospital days (see Table 29). The standard errors were derived from bootstrapping based on 10,000 replications in Stata, version 12.1. The results from the Breusch–Pagan test of independence provide support for the simultaneous estimation of costs and effects.
| Equation | Coefficient | Standard error | Lower CI | Upper CI |
|---|---|---|---|---|
| Cost equation | ||||
| Treatment | 544.2 | 303.7 | –51.4 | 1139.4 |
| Constant | 12048.0 | 212.9 | 11,630.7 | 12,465.2 |
| Health-related quality-of-life equation | ||||
| Treatment | 0.901 | 1.52 | –2.083 | 3.885 |
| Constant | 26.722 | 1.05 | 24.654 | 28.790 |
| Equation | Coefficient | Standard error | Lower CI | Upper CI |
|---|---|---|---|---|
| Cost equation | ||||
| Treatment | 450.6 | 306.4 | –150.0 | 1051.1 |
| Constant | 12116.3 | 217.6 | 11,689.9 | 12,542.7 |
| Health-related quality-of-life equation | ||||
| Treatment | 0.636 | 1.49 | –2.286 | 3.558 |
| Constant | 27.535 | 1.04 | 25.491 | 29.580 |
Cost-effectiveness analysis (complete cases)
Figure 11 summarises the cost-effectiveness results based on complete cases (n = 2799) based on a maximum willingness to pay (K) of £1000 per QALD. Essentially, despite the improved survival in the IPC group there was a gain of less than 1 day in QALDs over the 6-month follow-up period. The incremental cost-effectiveness ratio indicates that the optimal decision is to select IPC if willingness to pay is greater than £611 per QALD and the no-IPC treatment pathway if a decision-maker’s willingness to pay is less than this value.
FIGURE 11.
Cost-effectiveness plane. (Incremental cost-effectiveness ratio = 610.88.)
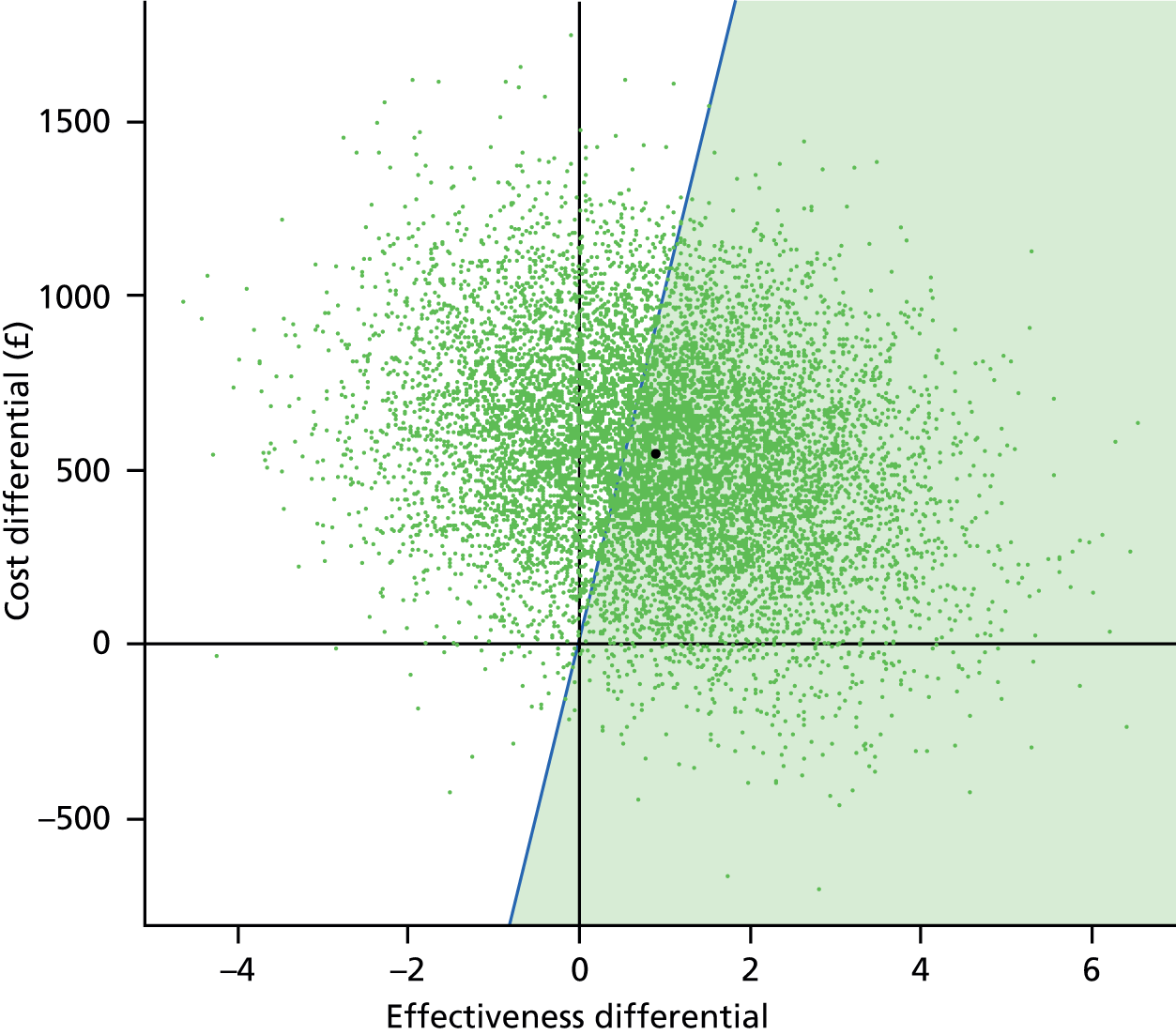
Figure 12 presents the cost-effectiveness acceptability curve to assess the uncertainty in the cost-effectiveness results of the cost per QALD. At a maximum willingness to pay of £1000 per QALD, the probability that IPC is cost-effective relative to standard care is just under 0.6.
FIGURE 12.
Cost-effectiveness acceptability curve.

The CLOTS 3 trial had more than 90% power to detect an ARR of 4% for proximal DVTs. Although the study successfully recruited 2876 patients, it was not powered to detect differences in costs or health-related quality of life, as the required sample size would ensure that the proposed study would be prohibitively expensive. As a result, decision-making should focus on the additional costs associated with the implementation of IPC across all net benefits, including the primary clinical outcome of the CLOTS 3 trial. The cost–consequence analysis should, therefore, consider the budget impact of implementing IPC in routine care rather than prioritising a ratio measure of a cost per QALD in the light of the trivial changes in QALD reported. This would ensure that there is a more transparent synthesis of the existing evidence rather than standard threshold interpretations of ratio measures which are undefined when there is no difference in quality-adjusted survival.
Chapter 6 Discussion
The CLOTS 3 trial has shown that IPC (delivering sequential circumferential compression via thigh-length sleeves at a frequency determined by the venous refill time) applied to immobile stroke patients significantly reduces the risk of proximal DVTs (our primary outcome), symptomatic DVTs (proximal or calf) and all DVTs (symptomatic or asymptomatic, proximal or calf). We were unable to demonstrate a statistically significant reduction in PE. Although there was a significant excess of skin breaks and a non-significant excess of falls with injury, the absolute risks were low and most adverse events were not attributed to the IPC.
The number of deaths within 30 days and within 6 months was lower in the IPC group than in the no-IPC group, although the differences were not statistically significant. However, our prespecified analysis of the hazard of death over the first 6 months, adjusted for any baseline imbalance, and which one would expect to be a more sensitive test of the effect on survival than the dichotomous data and fixed time points, demonstrated that the relative hazard of death was reduced by about 14% and this was statistically significant (p = 0.042).
Intermittent pneumatic compression is an inexpensive intervention. The actual cost per pair of Comfort™ sleeves since completion of the trial and implementation of IPC into practice is currently about £20, even less than the costs we used in the health economic analyses. The cost of preventing DVTs and deaths appears to be modest, although there are no generally accepted standards to indicate whether or not these costs represent good value for money. There was no statistically significant difference in the functional status (OHS score) of patients allocated to IPC or not. However, a post-hoc analysis of the OHS scores (see Table 13) shows that a 2.6% increase in the proportion of patients surviving at 6 months (p = 0.11) is more than offset by a 3.8% increase in the proportion of patients surviving with an OHS score of 5 (p = 0.013), indicating that they are bed bound or chair bound and require all care. This possibly suggests that many of the deaths which might result from PEs and which can be prevented by IPC occur in patients with severe strokes and therefore poor functional outcomes. We have previously shown that dependency in activities of daily living prior to the stroke, greater limb weakness and prior DVT/PE are independently associated with a greater risk of DVT after stroke3 and, further, that prior dependency and greater weakness are associated with worse functional outcomes. 21 It is therefore not surprising that, if IPC effectively reduces the risk of DVT and improves survival by preventing fatal VTE, many of the patients who survive because of IPC will have poor functional status. Patients with poor functional status have a utility, as determined by the EQ-5D-3L score, which is little different from death. For this reason there is little gain in quality-adjusted survival.
Perhaps because of the improved survival, the use of IPC was associated with a non-significant increase in length of stay (mean 1.7 days) and a non-significant increase in NHS hospital costs. Therefore, IPC use is associated with modest increase in direct hospital-based costs with only a very small gain in QALDs. This results in an incremental cost-effectiveness ratio of £611 per QALD. However, with a negligible difference in QALDs, any cost will result in a high incremental cost-effectiveness ratio.
Internal validity
The CLOTS 3 trial protocol was designed to minimise bias and confounding and thus to provide a robust estimate of the effectiveness of IPC in immobile stroke patients. The CLOTS 3 trial exceeded its recruitment target and, therefore, had at least 87% power to detect the 3.6% absolute reduction in risk of proximal DVT observed. The central web-based randomisation system achieved excellent balance for all baseline characteristics measured and excluded the possibility of any foreknowledge of group allocation which could have resulted in selection bias.
The CLOTS 3 trial focused on prevention and identification of proximal DVTs, which are detected more reliably with CDU and are considered clinically more important than DVTs restricted to the calf. 23–25 Calf DVTs are the most frequent component of the cluster of VTE events used in previous trials of VTE prophylaxis, yet their detection with CDU is technically challenging and results are inconsistent. The ultrasound technicians and radiographers were mainly blinded in their assessment of our primary outcome.
We achieved excellent levels of follow-up and we were able to account for all patients at 6 months who had not withdrawn from the study. All primary analyses kept patients in the treatment group they were allocated to, irrespective of the treatment they actually received.
Limitations
The trial had some methodological limitations as detailed below.
-
The primary outcome included asymptomatic DVTs, which are not of huge clinical significance but are widely used as surrogate outcomes in trials of VTE prophylaxis. Ideally, our trial would have focused on survival, function and quality of life, although this would have required a much larger sample size.
-
We included a small number of patients who turned out to have a diagnosis other than stroke. However, given their small numbers, excluding these patients from the analyses did not alter the conclusions.
-
Despite only moderate adherence to IPC, the trial demonstrated beneficial effects on VTE and survival. Data on adherence were sometimes incomplete and not always internally consistent, but it is unlikely that this would influence our conclusions. We are unable to reliably estimate the effect that imperfect adherence might have on the observed effect size.
-
Imperfect blinding of the radiographers could have biased detection of our primary outcome. However, given that in the majority of cases primary outcomes were confirmed by a blinded central review of stored images and in the remainder they were confirmed by local clinical reporting by a radiologist, it seems unlikely that this would have explained the results.
-
In some cases scheduled CDU did not include the calf veins and some scans were missing. Ideally, all radiographers would have adhered to a standardised protocol for carrying our CDU which would have optimised their sensitivity and specificity.
-
We did not systematically screen for PE with computerised tomography, pulmonary angiography or ventilation–perfusion isotope scans. It is therefore likely that we underestimated the frequency of VTE.
-
As we systematically screened for DVT, many patients found to have asymptomatic DVT were then treated with anticoagulants to reduce the risk of symptomatic events (DVTs, PEs and deaths). This might bias the estimate of the effect of IPC towards the null.
-
There was lack of central verification of negative CDU scans. This was not done because it was impractical and, given the difficulty of interpreting ultrasound retrospectively from recorded images, we think that it is unlikely that this had an important effect on our results.
-
There was lack of blinding of nursing staff. It would have been impossible to achieve in practice because we did not have a practical sham IPC. This might bias nursing staff’s use of background treatment and assessment of some of the secondary outcomes. However, our data suggest no major difference in the background treatments that are likely to have resulted in an important bias in favour of IPC. Although there was an excess of GCS use in the IPC arm, the CLOTS 1 trial showed that GCS use is not associated with reduced risk of DVT. The lack of blinding of nursing staff may have partly explained the observed excess of reported skin breaks seen in the IPC group.
-
There was lack of blinding of patients and their relatives which may have biased their responses to the OHS and EQ-5D-3L scores at the 6-month follow-up, but it seems unlikely that this would have affected our conclusions.
-
A manufacturer of IPC devices was involved in the trial. This might have in theory led to a conflict of interest among the researchers and distortion of the reporting of the trial results. However, Covidien Ltd only provided centres with their equipment and had no other role within the trial. They were not involved in its design, conduct (other than provision of the devices), data collection, storage, analysis or reporting of the results.
-
The economic evaluation is confined to a short time horizon of 6 months and does not include the costs of readmissions to hospital within the first 6 months after randomisation or include direct measurement of baseline health-related quality of life. For these reasons, no reliable estimate of the cost/quality-adjusted life-year could be provided.
External validity
The patients in the CLOTS 3 trial were enrolled by a large number of hospitals in the UK. We did not require centres to maintain screening logs; therefore, we are unable to report how many patients were screened for eligibility and what proportion of eligible patients agreed to participate. However, we purposefully kept our eligibility criteria wide so that the trial would be able to recruit a wide range of immobile stroke patients who might benefit from IPC. Anecdotally, the main reason for excluding eligible patients was the limit on the number of CDUs which could be done placed on many of our centres by their radiology departments.
However, Table 35 compares the characteristics of patients randomised into the CLOTS 3 trial with those of unselected patients admitted to all 32 Scottish hospitals as acute stroke patients in 2011. 43 It also shows the characteristics of all immobile patients. These data provide a basis for assessing the extent to which the trial results might apply to future NHS patients in the UK.
| Modelling method | CLOTS 3 trial | Unselected stroke patients in SSCA | Immobile stroke patients in SSCA |
|---|---|---|---|
| n | 2876 | 10,838 | 4292 |
| Age (years), mean | 74.6 | 72.4 | 75.6 |
| Male (%) | 48.0 | 47.2 | 43.3 |
| Haemorrhagic stroke (%) | 13.1 | 8.5 | 13.1 |
| Lives alone before stroke (%) | 34.9 | 32.8 | 38.5 |
| Independent in ADL before stroke (%) | 90.3 | 69.2 | 67.7 |
| Able to talk and orientated (%) | 60.2 | 60.9 | 49.1 |
| Able to lift both arms off bed (%) | 34.8 | 59.7 | 36.0 |
| Unable to walk independently (%) | 100 | 39.6 | 100 |
| Thrombolysed (%) | 17.0 | 5.8 | 10.3 |
| Discharged home (%) | 48.0 | 68.5 | 49.6 |
| Died in hospital (%) | 15.6 | 10.5 | 19.4 |
| Mean length of stay (days) | 43.6 | 18.0 | 28.0 |
| Median length of stay (days) | 33 | 6 | 12 |
About 40% of acute stroke patients admitted to hospital in Scotland in 2011 were initially immobile and the vast majority would have been eligible for the trial. It therefore seems reasonable to assume that IPC would potentially benefit very large numbers of patients each year: perhaps 50,000 in the UK. However, the in-hospital case fatality of patients enrolled was lower than that of unselected immobile patients. This reflects that patients were not entered into the trial if they had a very high early risk of dying, indeed we discouraged enrolment of patients where palliation was the main aim of treatment or where the patient had a comorbidity which was likely to severely restrict their survival. Patients enrolled in the trial tended to be more independent before their stroke and to be more often orientated and able to talk after the stroke. This probably reflects the difficulty of obtaining informed consent from patients who are unable to communicate or who have cognitive problems. Some research staff were less keen to obtain consent from proxies even though this was encouraged by our protocol. The much longer lengths of stay in the CLOTS 3 trial patients probably indicate that research staff preferentially enrolled patients who were likely, because of their stroke severity, to be immobile for a significant period.
Our subgroup analyses suggest that the effects of IPC on our primary outcome was similar across a broad range of patients, and was not significantly affected by patient demographics, delays in its application, stroke severity and background use of antithrombotics. Importantly, IPC appeared to be at least as effective in patients with haemorrhagic stroke as in those with ischaemic stroke (see Figure 8).
Although adherence to IPC was only modest within the trial, one could speculate whether or not this might differ from routine practice. In the trial, patients were explicitly told that there was no definite evidence that IPC would benefit them and also that they could remove the devices without giving a reason. IPC was a novel intervention for most of the nursing staff caring for patients, and nurses were also aware that the IPC was being tested, and that it was not of proven benefit. If IPC were to be introduced into routine practice, staff and patients could be told that it would definitely reduce the risk of DVT and would probably increase their chances of surviving. This might encourage better adherence than in the trial. However, we do not know the extent to which patients, their families and nursing staff encouraged adherence in the trial citing the potential benefits for the trial and future patients with stroke.
The IPC devices used in the trial were provided by one manufacturer. IPC devices vary in their characteristics, for example in the length of the sleeves (calf only or thigh length), the frequency of compression, whether inflation is rapid or gradual, sequential (distal before proximal) or single (distal and proximal at the same time), and whether compression is circumferential or applied to only the back of the calf. It is unclear if other types of IPC device in widespread use in the UK would achieve greater, lesser or the same effects as those observed in the CLOTS 3 trial.
Putting the results in context
The CLOTS 3 trial aimed to establish whether or not IPC reduces the risk of DVT in patients who are initially immobile after being admitted to hospital with an acute stroke. Prior to starting the trial, we searched for other trials which had addressed this question in stroke patients. We updated this search in March 2013. We searched the Cochrane Stroke Group Trials Register, the Cochrane Central Register of Controlled Trials, MEDLINE (1966 to March 2013), EMBASE (1980 to March 2013), the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to March 2013) and the British Nursing Index (1985 to March 2013) using the search terms listed in Appendix 4. We screened reference lists of all relevant papers, searched ongoing trials registers (March 2013) and contacted experts in the field. We included unconfounded RCTs comparing IPC for reducing the risk of DVT with a control and in which trial treatment was started within 7 days of the onset of stroke. In late 2014, the National Institute for Health and Care Excellence completed a systematic review of the evidence of IPC in stroke patients and did not identify any additional randomised trials.
Only the two small RCTs included in the original review were identified. 44,45 When the results of the CLOTS 3 trial are incorporated, the estimates of treatment effects are an OR of 0.66 (95% CI 0.52 to 0.84) for proximal DVT, an OR of 0.71 (95% CI 0.59 to 0.85) for any DVT and an OR of 0.81 (95% CI 0.65 to 1.01) for deaths by the end of the treatment period. The two other trials did not report any symptomatic DVTs or PEs. 44,45
The reduction in DVT observed in the CLOTS 3 trial is likely to be because of the reduced venous stasis and possibly the effects on intrinsic fibrinolysis observed with IPC. 18 The improved survival to 6 months observed in those allocated to IPC is potentially of clinical significance. Unfortunately, the autopsy rate was very low; therefore, we were unable to reliably assign a cause to most deaths, especially given the difficulty of distinguishing PE from other cardiorespiratory problems in stroke patients. 9 There was an intriguingly lower frequency of deaths due to pneumonia in the IPC group. Therefore, taken with the pattern of benefits across all the secondary outcomes, it seems plausible that the difference in survival may be real and attributable, at least in part, to IPC. The most probable mechanism is a reduction in undiagnosed PE which contributed to death.
Previous meta-analyses of trials of heparins/LMWHs in stroke patients have demonstrated significant reductions in PE (3 out of 1000, 95% CI 1 to 3) but only a non-significant reduction in deaths (9 out of 1000, 95% CI –29 to 18), perhaps in part because any reduction in major VTE was offset by a significant increase in major bleeds (6 out of 1000, 95% CI 2 to 12). 12 In contrast, IPC was not associated with an excess of any major adverse effects that might offset the benefits. The observed effect of IPC on survival in the CLOTS 3 trial is also reassuring about its safety in this high-risk vulnerable population.
The CLOTS 3 trial has shown that application of IPC to the legs of patients admitted to hospital with stroke who are initially immobile reduces their risk of DVT, and also appears to increase their chances of surviving to 6 months. However, similar to other interventions which aim to prevent or treat complications after stroke (e.g. tube feeding,40 antibiotics46), IPC does not appear to improve the functional outcomes of survivors.
The results of the CLOTS 3 trial have already been widely disseminated. 33,47 IPC is relatively inexpensive and is already being widely implemented in stroke units in the UK. 47,48 The national audits of stroke care patients in Scotland (Scottish Stroke Care Audit43) and the rest of the UK (Sentinel Stroke National Audit Programme49) have been monitoring the use of IPC in stroke patients prospectively since January 2014 to determine the speed of implementation.
Chapter 7 Conclusions
Guidelines
The National Institute for Health and Care Excellence, Scottish Intercollegiate Guidelines Network, the European Stroke Organisation and the Danish Stroke Society have all taken account of the CLOTS 3 results and recommend that IPC should be considered for VTE prophylaxis after stroke. Other guidelines groups are updating their recommendations to take account of these data. If guidelines recommend the use of IPC in immobile stroke patients admitted to hospitals this will have important implications for health care and raise important questions for service providers.
Implications for health-care providers
How to train the staff to implement the guideline recommending intermittent pneumatic compression use in stroke patients?
Medical, nursing and therapy staff working in UK stroke units, and those in many other parts of the world, have little experience of applying IPC to their patients. They require training in:
-
selecting appropriate patients who might benefit
-
providing information about IPC to the patients and/or their carers
-
sizing the sleeves
-
fitting the sleeves
-
monitoring their use, including being alert to complications
-
dealing with technical problems, for example high- and low-pressure alarms.
Manufacturers of IPC devices do provide some training in the use of their products, but this rarely keeps up with the turnover in staff. As a minimum, all staff coming into contact with patients receiving IPC (e.g. physiotherapists or care assistants) will need to know how to switch the systems on and off and how to reapply the sleeves. Online training is now freely available at www.stroketraining.org.
Further research questions
Should intermittent pneumatic compression be used in a broader group of medical patients?
Given the demonstrated effectiveness of IPC in surgical19 and stroke patients, it seems very likely that it would also reduce the risk of VTE in other groups of medical patients at high risk of VTE. Those responsible for writing national guidelines might extrapolate from surgery and stroke to medical patients. However, if they do not consider this appropriate, IPC should probably be tested in high-risk medical patients. The even more important question is whether or not it would significantly improve survival in this huge group of patients, which is estimated to be 750,000 per year in the UK alone. 50 However, given the uncertainty about the effect of prophylactic anticoagulation with heparin in medical patients on survival, there is a case for a very large factorial design trial testing both anticoagulants and IPC with survival as the primary outcome. This would be practical only if an effect size of perhaps 10 deaths avoided per thousand patients treated were considered the minimal clinically important difference. Preliminary studies of the size of the benefit which would be considered worthwhile by patients, carers, clinicians and commissioners and a value of information modelling exercise could further guide whether or not such very large trials are a sufficiently high research priority for the NHS.
Can adherence to intermittent pneumatic compression be improved, which in turn might increase its effect?
In trial conditions, adherence to IPC was modest and it is likely that this reduced the size of the effect observed. Research into methods to improve adherence to IPC might provide information which would increase the benefits of IPC in stroke patients.
Acknowledgements
The CLOTS 3 trial acknowledges the financial support of NHS Research Scotland through NHS Lothian and the Scottish Stroke Research Network. The start-up phase of the CLOTS trial 3 was funded by the Chief Scientist Office of the Scottish Government. The main phase of the trial was funded by the National Institute for Health Research Health Technology Assessment programme. Covidien Ltd (Mansfield, MA, USA) provided free supplies of its intermittent pneumatic compression devices and sleeves to hospitals participating in the trial. The UK Stroke Research Network adopted the trial and network-funded staff were responsible for enrolment and data collection in many of our centres. We would also like to acknowledge all the patients and their families who participated in the CLOTS trial and nursing and radiology staff who assisted at collaborating sites without whom the trial would not have been possible.
We also acknowledge the input from all of the current and past members of the CLOTS trial collaboration as detailed in Appendix 1.
Contributions of authors
Martin Dennis is a consultant stroke physician and Professor of Stroke Medicine at the University of Edinburgh. He was the chief investigator of the CLOTS 3 trial, wrote the protocol, was the principal applicant for grant funding, managed the trial on a day-to-day basis, enrolled patients to one centre and carried out all 6-month telephone follow-ups. He drafted this manuscript.
Peter Sandercock is a consultant neurologist and Professor of Medical Neurology at the University of Edinburgh and has extensive experience in large randomised trials as a chief investigator, chairperson and member of many TSCs and DMCs. He was involved in design and grant applications, was a member of the steering committee and constructively commented on drafts of this manuscript.
Catriona Graham is a biostatistician in the Wellcome Trust Clinical Research Facility at the University of Edinburgh. She was involved in the trial design and grant applications and constructively commented on this manuscript. She provided statistical support to the trial, carrying out both interim analyses for the DMC and the final analyses for this report.
John Forbes is a health economist and Professor of Health Economics at the University of Limerick. He designed the health economic analyses, oversaw their completion, drafted that section of this report, was a member of the steering committee and constructively commented on this manuscript.
Data sharing statement
Once we have finished publishing the papers based on the analyses described in our statistical analysis plan, we will prepare an anonymised data set which will be shared with other researchers. We will lodge a full CLOTS 3 trial data set with data dictionary and other relevant metadata with University of Edinburgh DataShare, which is free to the data owners and to those who wish to access it in the future.
Publications
Dennis M, Sandercock P, Reid J, Graham C, Forbes J. Does intermittent pneumatic compression reduce the risk of post stroke deep vein thrombosis? The CLOTS 3 trial: study protocol for a randomised controlled trial. Trials 2012;13:26.
Dennis M, Sandercock P, Murray G, Forbes J, CLOTS Trials Collaboration. Does intermittent pneumatic compression reduce the risk of post stroke deep vein thrombosis. The CLOTS 3 trial: statistical analysis plan. Trials 2013;14:66.
CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet 2013;382:516–24.
CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration. Effect of intermittent pneumatic compression on disability, living circumstances, quality of life and hospital costs after stroke: a randomised trial. Lancet Neurol 2014;13:1186–92.
CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration. Which stroke patients gain most from intermittent pneumatic compression: further analyses of the CLOTS 3 trial [published online ahead of print 26 August 2015]. Int J Stroke 2015. doi: http://dx.doi.org/10.1111/ijs.12598
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- The Prevention of Venous Thromboembolism in Hospitalised Patients. Second Report of Session 2004–05 Report, HC 99. London: The Stationery Office; 2005.
- Kelly J, Rudd A, Lewis RR, Hunt BJ. Venous thromboembolism after acute stroke. Stroke 2001;32:262-7. http://dx.doi.org/10.1161/01.STR.32.1.262.
- The CLOTS Trials Collaboration . Can clinical features distinguish between immobile stroke patients at high and low risk of deep vein thrombosis (DVT)? Statistical modelling based on the CLOTS trials cohorts. J Neurol Neurosurg Psychiatry 2011;82:1067-73. http://dx.doi.org/10.1136/jnnp.2010.235945.
- Kelly J, Rudd A, Lewis RR, Coshall C, Moody A, Hunt BJ. Venous thromboembolism after acute ischemic stroke: a prospective study using magnetic resonance direct thrombus imaging. Stroke 2004;35:2320-5. http://dx.doi.org/10.1161/01.STR.0000140741.13279.4f.
- Dennis M, Mardi N, Graham C, Sandercock P. CLOTS trials collaboration . The timing, extent, progression and regression of DVT in immobile stroke patients: observational data from the CLOTS randomized trials. J Thromb Haemost 2011;9:2193-200. http://dx.doi.org/10.1111/j.1538-7836.2011.04486.x.
- Sandercock PAG, Counsell C, Kamal AK. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.cd000024.pub3.
- Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications following acute stroke. Stroke 1996;27:415-20. http://dx.doi.org/10.1161/01.STR.27.3.415.
- CLOTS Trials Collaboration . Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS Trial 1): a multicentre, randomised controlled trial. Lancet 2009;373:1958-65. http://dx.doi.org/10.1016/S0140-6736(09)60941-7.
- Kelly J, Hunt BJ, Rudd A, Lewis RR. Pulmonary embolism and pneumonia may be confounded after acute stroke and may co-exist. Age Ageing 2002;31:235-9. http://dx.doi.org/10.1093/ageing/31.4.235.
- Warlow C, Ogston D, Douglas AS. Deep venous thrombosis of the legs after strokes. Part 1. Incidence and predisposing factors. Br Med J 1976;1:1178-81. http://dx.doi.org/10.1136/bmj.1.6019.1178.
- International Stroke Trial Collaborative Group . The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both or neither among 19435 patients with acute ischaemic stroke. Lancet 1997;349:1569-81. http://dx.doi.org/10.1016/S0140-6736(97)04011-7.
- Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2011;155:602-15. http://dx.doi.org/10.7326/0003-4819-155-9-201111010-00008.
- Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al. Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9490.
- Agu O, Hamilton G, Baker D. Graduated compression stockings in the prevention of venous thromboembolism. Br J Surg 1999;86:992-1004. http://dx.doi.org/10.1046/j.1365-2168.1999.01195.x.
- Naccarato M, Chiodo Grandi F, Dennis M, Sandercock PAG. Physical methods for preventing deep vein thrombosis in stroke. Cochrane Database Syst Rev 2010;8. http://dx.doi.org/10.1002/14651858.cd001922.pub3.
- Muir KW, Watt A, Baxter G, Grosset DG, Lees KR. Randomised trial of graded compression stockings for prevention of deep vein thrombosis after acute stroke. QJM 2000;93:359-64. http://dx.doi.org/10.1093/qjmed/93.6.359.
- Morris R. Intermittent pneumatic compression – systems and applications. J Med Eng Technol 2008;32:179-88. http://dx.doi.org/10.1080/03091900601015147.
- Kohro S, Yamakage M, Sato K, Sato JI, Namiki A. Intermittent pneumatic foot compression can activate blood fibrinolysis without changes in blood coagulability and platelet activation. Acta Anaesthesiol Scand 2005;49:660-4. http://dx.doi.org/10.1111/j.1399-6576.2005.00661.x.
- Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation 2013;128:1003-20. http://dx.doi.org/10.1161/CIRCULATIONAHA.113.002690.
- The CLOTS Trials Collaboration . Thigh-length versus below-knee stockings for DVT prophylaxis after stroke: a randomised trial. Ann Intern Med 2010;153:553-62. http://dx.doi.org/10.7326/0003-4819-153-9-201011020-00280.
- Counsell C, Dennis M, McDowall M, Warlow C. Predicting outcome after acute stroke: development and validation of new models. Stroke 2002;33:1041-7. http://dx.doi.org/10.1161/hs0402.105909.
- Altman DG, Bland JM. Treatment allocation by minimisation. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.330.7495.843.
- Davidson BL. What are the most reliable detection methods for deep vein thrombosis and pulmonary embolism to be used as endpoints in trials of venous thromboprophylaxis?. Haemostasis 1998;28:S113-19. http://dx.doi.org/10.1159/000022388.
- Galanaud JP, Sevestre-Pietri MA, Bosson JL, Laroche JP, Righini M, Brisot D, et al. OPTIMEV-SFMV Investigators. Comparative study on risk factors and early outcome of symptomatic calf versus proximal deep vein thrombosis: results from the OPTIMEV study. Thromb Haemost 2009;102:493-500. http://dx.doi.org/10.1160/TH09-01-0053.
- Kearon C. Natural history of venous thromboembolism. Circulation 2003;107:I-22-I-30. http://dx.doi.org/10.1161/01.CIR.0000078464.82671.78.
- Bamford JL, Sandercock PAG, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989;20. http://dx.doi.org/10.1161/01.STR.20.6.828.
- EUROQoL Group . EUROQoL – a new facility for the measurement of health related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: University of York; 1995.
- Dennis M, Sandercock P, Reid J, Graham C, Forbes J. Does intermittent pneumatic compression reduce the risk of post stroke deep vein thrombosis? The CLOTS 3 trial: study protocol for a randomised controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-26.
- Dennis M, Sandercock P, Murray G, Forbes J. Does intermittent pneumatic compression reduce the risk of post stroke deep vein thrombosis? The CLOTS 3 trial: statistical analysis plan. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-66.
- Schunemann HJ, Oxman AD, Gunn EV, Higgins JPT, Deeks JJ, Glasziou P, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2011.
- Buyse M, George SL, Evans S, Geller NL, Ranstam J, Scherrer B, et al. The role of biostatistics in the prevention, detection and treatment of fraud in clinical trials statist. Med 1999;18:3435-51.
- CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration . Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet 2013;382:516-24. http://dx.doi.org/10.1016/S0140-6736(13)61050-8.
- Bembenek J, Karlinski M, Kobayashi A, Czlonkowska A. Early stroke-related deep venous thrombosis: risk factors and influence on outcome. J Thromb Thrombolysis 2011;32:96-102. http://dx.doi.org/10.1007/s11239-010-0548-3.
- NHS Reference Costs 2011–2012. London: Department of Health; 2013.
- Scottish Health Service Costs. Edinburgh: NHS Scotland; 2013.
- Stata 12 Multiple-Imputation Reference Manual. College Station, TX: Stata Press; 2011.
- Baio G. Bayesian Methods in Health Economics. London: CRC/Chapman & Hall; 2012.
- The FOOD Trial Collaboration . Routine oral nutritional supplementation for stroke patients in hospital (FOOD): a multicentre randomised controlled trial. Lancet 2005;365:755-63. http://dx.doi.org/10.1016/S0140-6736(05)70998-3.
- The FOOD Trial Collaboration . Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet 2005;365:764-72. http://dx.doi.org/10.1016/S0140-6736(05)70999-5.
- R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2014.
- Szende A, Oppe M, Devlin N. EQ-5D Value Sets: Inventory, Comparative Review and User Guide. Dordrecht: Springer; 2007.
- Scottish Stroke Care Audit n.d. www.strokeaudit.scot.nhs.uk/ (accessed 9 April 2013).
- Prasad BK, Banerjee AK, Howard H. Incidence of deep vein thrombosis and the effect of pneumatic compression of the calf in elderly hemiplegics. Age Ageing 1982;11:42-4. http://dx.doi.org/10.1093/ageing/11.1.42.
- Lacut K, Bressollette L, Le GG, Etienne E, De TA, Renault A, et al. Prevention of venous thrombosis in patients with acute intracerebral hemorrhage. Neurology 2005;65:865-9. http://dx.doi.org/10.1212/01.wnl.0000176073.80532.a2.
- Westendorp WF, Vermeij JD, Vermeij F, Hertog HMD, Dippel DWJ, van de Beek D, et al. Preventive antibiotics in acute stroke: summary of a Cochrane systematic review and meta-analysis. Stroke 2012;43:e113-14. http://dx.doi.org/10.1161/STROKEAHA.112.671420.
- Intermittent Pneumatic Compression (IPC) Sleeves Programme FAQ. Leeds: NHS Improving Quality; 2014.
- Scottish Government . Stroke Improvement Plan 2014. www.scotland.gov.uk/Publications/2014/08/9114 (accessed 1 September 2014).
- Sentinel Stroke National Audit Programme. London: Royal College of Physicians; n.d.
- Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe: the number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756-64. http://dx.doi.org/10.1160/th07-03-0212.
Appendix 1 Membership and roles within the CLOTS trial collaboration
Chief investigator
M Dennis.
CLOTS trial Co-ordinating Centre
G Cranswick, A Deary, A Fraser, C Graham, S Grant, A Gunkel, J Hunter, A Mcgrath, D Perry, V Soosay, C Williams and A Young.
Writing group
M Dennis (chairperson), P Sandercock, C Graham, J Smith and J Forbes.
Trial Steering Committee
M Dennis, C Graham, S Lewis, G Murray, J Reid, A Rudd, PAG Sandercock, G Venables (chairperson), J Forbes, G Lowe, G Bowler (patient representative), Y Henderson (patient representative) and observers from the joint Sponsor (University of Edinburgh and NHS Lothian) and Covidien Ltd.
Co-applicants on funding applications
Chief Scientist Office, Scotland: M Dennis, P Sandercock, J Reid and S Lewis.
Health Technology Assessment programme: M Dennis, P Sandercock, J Reid, S Lewis, J Forbes, D Perry and P Taylor.
Independent Data Monitoring Committee
C Baigent (Oxford), J Bamford (Leeds, chairperson) and J Slattery (London).
Central verification of compression duplex ultrasounds
J Reid.
Health economic analyses
J Smith and J Forbes.
Updating systematic review of randomised controlled trials of intermittent pneumatic compression in stroke
M Naccarato, F Chiodo Grandi, B Thomas and H Fraser.
Extraction of data from Scottish Stroke Care Audit
M Turner and MJ Mcleod.
Participating centres
We have listed each hospital with the names of the local principal investigator, local co-ordinator and other significant contributors who have enrolled patients into the CLOTS 3 trial. The hospitals are ordered depending on the numbers recruited. The figure in brackets represents number of patients recruited.
Recruiting centres
Western General Hospital (161): M Dennis, S Connolly, J Dyce, E Eadie, S Keir, K Peters, L Smith, P Taylor, A Thomas, J Wardlaw and S Young.
Countess of Chester Hospital NHS Foundation Trust (128): K Chatterjee, J Almansoor, S Booth, H Eccelson, C Kelly, S Leason and A Nallasivan.
Leeds General Infirmary (128): P Wanklyn, J Bamford, R Bellfield, J Cooper, C Coulson, J Greig, K Harrison, A Hassan and L Mandizvida.
Pinderfields General Hospital (118): A Stanners, R Barr, G Bateman, M Carpenter, R Davey, T Hendra, A Keeney, A McGuinness, S Morrissey and A Needle.
York Health Services NHS Trust (92): J Coyle, L Boyes, C Croser, N Dyer, S Howard, E Iveson, G Johnson, M Keeling, S Limaye, N Marshall and P Willcoxson.
Royal Infirmary of Edinburgh (87): S Hart, S Broadbent, P Brown, S Carroll, B Chapman, A Cormack, A Coull, T Elder, C Green, S McManus, G Mead, B Morrow, W O’Dea, C Stirling and M Watson.
Luton and Dunstable Hospital (80): L Sekaran, J Griffiths, T Iyngkaran, F Justin, G Jutlla, D Phiri, S Ramkumar, S Sethuraman and L Tate.
Harrogate District Hospital (78): S Brotheridge, S Appleby, J Crabtree, C Hare, S Lee, J Strover, L White, G Wihl and S Wood.
Bradford Teaching Hospitals NHS Foundation Trust (75): C Patterson, D Beresford, I Green, B Hairsine, L Johnston, V Lodge, S Maguire and P Sharratt.
Royal Bournemouth and Christchurch NHS Trust (66): D Jenkinson, J Bell, O David, J Kwan, A Orpen, C Ovington, S Smith and A Sturgess.
St Peter’s Hospital (66): R Nari, B Mandal, A Moth, C Potter, G Rai-Tidbury and E Young.
Airedale General Hospital (65): M Mawer, A Catto, P Sharratt, H Shaw, K Smith, M Smith and K Spencer.
Southend University Hospital (63): P Guyler, G Chaudhary, P Harman, C Khuoge, A Kundu, A O’Brien, O Sinha, P Terrazzano, V Thompson and S Tysoe.
Royal Preston Hospital (62): S Punekar, S D’Souza, S Duberley, C Gilmore, B Gregory, S Philip, S Raj and D Seriki.
Basingstoke and North Hampshire NHS Foundation Trust (62): E Giallombardo, D Dellafera, G Goodwin, S Pitchell and T Solomon.
Yeovil District Hospital (60): K Rashed, S Board, C Buckley, S Bulley, D Hayward, K Jenkins, S More, W Parsons, M Quadiri and R Sophia.
King’s College Hospital (56): L Kalra, E Cattermole, K Harvey, E Khoromana, R Lewis, V Licence, D Manawadu, B Mistry and C Witehead.
Royal Liverpool University Hospital (54): P Fitzsimmons, G Fletcher, F Hussain, S Loharuka, P Lopez and A Manoj.
University Hospital Aintree (52): R Durairaj, T Fluskey, R Kumar, Z Mellor, A Sharma, H Simmons, V Sutton, J Webb and A Wilcox.
Torbay Hospital (51): D Kelly, C Bailey, H Bearne, P Fitzell, M Hanley, C Hilaire, I Salih, P Sleight, S Szabo and R Walker.
John Radcliffe Hospital (49): G Pope, C Barker, M Bratby, C Ferrett, C Hadyn, R Hannah, E James, J Kennedy, A McCulloch, K Michael, J Price, I Reckless, K Shah, S Singh, S Smith, R Teal, C Tiedenman, M Webb, M Westwood and S Winner.
Gloucestershire Royal Hospital (47): D Dutta, P Brown, F Davis, P Dix, J Hapeshi, K Hellier, L Jelly, A Radford and M Walker.
Blackpool Victoria Hospital (44): J McIlmoyle, I Ellwood, H Goddard, S Holmes, C Jeffs, M O’Donnell, L Pett and A Wilkinson.
University Hospital of North Staffordshire (43): C Roffe, K Amor, A Aurora, K Castro, K Finney, S Gomm, M Hughes, J Lucas, H Maguire, I Natarajan, K Preece and J Yates.
Derriford Hospital (43): A Mohd Nor, S Allder, M Andrews, C Brown, R Craven, S Edwards, A Ellison, J George, B Hyams, N Persad, M Sadler, R Truscott and S Weatherby.
Salford Royal Hospital Foundation NHS (42): P Tyrrell, A Bell, C Diment, C Douglass, J Ford, R Grue, J Haricoe, A Ingham, R Jarapa, A Majid, E Quick, C Sherrington, A Singh, C Smith, L Tew and J Wainwright.
Calderdale Royal Hospital (40): P Rana, C Button, S Depledge, B Hairsine, A Nair, S Prasad, M Rodgers, I Shakir and J Wilkinson.
Royal Sussex County Hospital (35): C Rajkumar, J Breeds, N Gainsborough and I Kane.
Eastbourne District General Hospital (33): C Athulathmudali, E Barbon, L Barsley, B Betts, L Leleu, C Parter and S Wickens.
Scarborough Hospital (30): J Paterson, K Deighton, C Dimopoulos, S Jamieson, K Khadjool, J Major, E Tranter, J Wood and T Zuromskis.
Royal Hampshire County Hospital (30): N Smyth, J Duffy, C Eglinton and S Kidd.
Queen’s Hospital Romford (29): S Andole, J Cando, K Darawil, K Dunne and J Farrah.
Whiston Hospital (28): V Gowda, S Connolly, S Dealing, J Holt, V Mahoney, J Martin, S Meenakshisundaram, G Navis and T Smith.
Charing Cross Hospital (28): A Kar, S Banerjee, E Beranova, E Beranova, L Honeyfield, H Jenkins, A Lacey, H Lee, D Patel, H Reid, T Sachs, J Scott, M Teklay, S Wakely and L Wilding.
Doncaster Royal Infirmary (27): D Chadha, P Anderton, L Holford and D Walstow.
St Thomas’ Hospital (26): A Bhalla, J Birns, E Cattermole, G Cluckie, I Davis, A Jones, L MacDonald, N Mitchell, A Rudd and E White.
University Hospitals Coventry and Warwickshire NHS Trust (26): P Ray, L Aldridge, S Bera, T Goodfellow, S Nyabadza and C Randall.
Warrington & Halton Hospitals NHS Foundation Trust (26): O Otaiku, G Barton, K Bunworth, C Cecchini, L Connell, C Coster, G Delaney-Segar, M Kidd, A Maloney and H Whittle.
University Hospital North Durham (24): B Esisi, A Baggett, V Baliga, E Brown, S Clayton, J Dent, P Earnshaw, R Hayman, J Kent, C McGrath and M Myint.
Ayr Hospital (23): S Ghosh, E Barrie, L Belkhiri, A Burinski, S Cooper, T Flannighan, K Hockings, C Hutton, L McGibbon, C Somerville, J Thomson, C Wells and K Whyte.
Royal Berkshire Hospital (23): P Tun, M Adamson, B Elba, J Foxton, G Grimwood, S Heaton, J King, G Nicholas, S Panchalingam, P Rodriguez-Osorio and A Van Wyke.
Forth Valley Royal Hospital (21): M MacLeod, Byrne, A Grant, C McGhee, A McKenzie and A Smart.
Nottingham City Hospital (21): W Sunman, A Andrew, P Bath and D Havard.
West Cumberland Hospital (21): O Orugun, C Blackcock, A Henderson, R Jolly, D McCafferty, U Poultney, T Riley and N Russell.
North Devon District Hospital (21): M Dent, N Batho, F Hammonds, J Hunt, C Vernon and L Wilcox.
Dewsbury and District Hospital (21): P Datta, G Bateman, C Colabella, A Das, A McGuinness and A Needle.
Perth Royal Infirmary (20): S Johnston, M Barta, K Fowler, A Kelly, P Nair and M Stirling.
Salisbury District Hospital (20): T Black, J Cronan, L Harris, M Skelton and D Walters.
Pilgrim Hospital (20): D Mangion, T Ashraf, M Aslam and A Hardwick.
Weston General Hospital (19): H Bhakri, Chambers, P Easton, F Henchie, Lacy, G Saunders and Sparks.
Leighton Hospital (19): M Salehin, D Bailey, L Garcia-Alen, N Gautam, K Harvey, J Jardine, R Lea, L Marshall and R Miller.
North Tees and Hartlepool NHS Foundation Trust (18): D Bruce, B Allan, I Anwar, B Kumar, M Platton, E Reade and H Skinner.
Queen Alexandra Hospital (18): J Tandy, K Atkins, A Charig, R Deadman, T Dobson, C Edwards, P Gill, J Hewitt, L Hyatt, D Jarrett, A Ravindrane, D Veal, J Ward and J Williams.
East Lancashire Hospitals, Royal Blackburn Hospital (18): N Goorah, A Bell, C Berry, D Gavan, S Mellor, Y Potts and A Sangster-Drysdale.
Southampton General Hospital (18): N Weir, S Archer, M Brown, P Crawford, G Durward, S Evans, H Forder, W Foster, M Harris, R Mani, V Pressly and G Roberts.
Queen Margaret Hospital NHS Trust (17): N Chapman, H Fraser, K McCormick and D Wilkinson.
Broomfield Hospital (17): V Umachandran, A Lyle, F McNeela, C Mitchell-Inwang, S Smolen, K Swan, J Topliffe, H Walsh, S Williams and G Zacharaiah.
Birmingham Heartlands Hospital (16): D Sandler, P Carr, S Dudley, A Ganeshan, W Gregory, E Hoey, A Majeed, J McCormack, B Miller, C Nevin, C Pearsall, C Stretton and K Warren.
Cheltenham General Hospital (16): A Kumar, P Brown, C Davies, F Davis, A Deering, J Hapeshi, L Marsh, W Sylvester and M Walker.
St Mary’s Hospital, Isle of Wight (15): E Hakim, D Beare and T Norman.
Norfolk and Norwich University Hospital (15): A Metcalf, S Anderson, N Gange, J Jagger, P Myint, J Potter, G Ravenhill, E Thomas and T Vaughan.
Wycombe General Hospital (14): M Burn, A Benford, D Briley, C Durkan, M Ezad, D Hilton, M Jackson, S Manchanda and K Misra.
The Princess Royal Hospital Haywards Heath (13): K Ali, H Bowra, E Elks, J Gaylard, M Jones, J Knight and G Spurling.
Hairmyres Hospital (13): B Yip, A Anderson, D Bell, C Brady, C Forman, F Gardner, B MacInnes, L Macliver, B Martin and J Santamaria.
Milton Keynes Hospital (13): Y Duodo, T Antoine, L Bartlett, Y Behnam, E Bullens, S Cole, D Dolling, S El Tawil, C Keyes, K Lloyd, C Padilla-Harris, D Pereira and M Toomey.
Lincoln County Hospital (11): S Leach, S Arif, R Brown, J Chambers, S Chapman, L Parkes, J Sharma, V Sherburn, I Wahisi and L Wright.
Royal Surrey County Hospital (11): A Blight, A Carne, H Lapham, C Lawlor and K Pasco.
Watford General Hospital (10): D Collas, T Attygalle, P Botten, K Butchard, M Caton, S Daniels, A Divers, M Hoare, P Jacob, C Merrill, J Napper, S Sarin, A Sinclair, S Sundayi and E Walker.
Fairfield General Hospital (9): K Kawafi, S Bhat, L Harrison, L Johnson, R Namushi, A Picton and L Smith.
Darlington Memorial Hospital (9): B Esisi, V Baliga, E Brown, J Kent, C McGrath and A Mehrzad.
The Princess Alexandra Hospital (9): S Mansoor, M Bone, L Brown, A Daniel, S Hameed and N Walsh.
Solihull Hospital, Heart of England NHS Trust (8): K Elfandi, A Britton, Gregory, U Khan, R Morris, M Sandler and K Warren.
Royal Lancaster Infirmary (8): P Kumar, C Culmsee, M Schofield and S Timperley.
Victoria Hospital (8): N Chapman, V Cvoro, K McBride, K McCormick and S Pound.
Monklands Hospital (7): M Barber, D Esson, J Guse, C Maguire and A Talbot.
Darent Valley Hospital, Dartford and Gravesham NHS Trust (7): P Aghoram, A Anstead, T Daniel, S Hussein and P Mellor.
Princess Royal University Hospital (7): B Piechowski-Jozwiak, D Bettesworth, D Jayasinghe, E Khoromana, R Lewis, D Ramsey and K Rhodes.
Chesterfield Royal Hospital (6): M Sajid, M Ball, M Beardshall, S Glenn, A Rashid and S Southern.
East Surrey Hospital (5): Y Abouslieman, S Collins, A Jolly, M Mabbutti, M McDade and N Powell.
Frimley Park Hospital (5): O Speirs, S Atkinson, B Clarke, Manock, L Moore and P Ramsey.
Kent and Canterbury Hospital (4): H Baht, I Burger, J Burt, L Cowie, Nair, F Smith and A Thomson.
Medway Maritime Hospital (4): S Sanmuganathan, P Akhurst, S Burrows, T France, M Mamun and F Williams.
Southport District General Hospital (3): P McDonald, J Horsley, J Murray, H Terrett and S Wareing.
University Hospital of South Manchester NHS Foundation Trust (3): E Gamble, K Keating, S Mawn, K Norse, P O’Neill and R Pole.
Ninewells Hospital and Medical School (3): R MacWalter, A Doney, N Duffy, A Kelly, S Pillai and M Stirling.
Borders General Hospital (2): S Kerr, A Brown, S Haines, M Mackay and J Reid.
Hull Royal Infirmary (2): A Abdul-Hamid, R Conet-Baldwin, J Greig, K Mitchelson, P Parker, R Rayessa and L Sunman.
Royal London Hospital (2): P Gompertz, P Daboo, M Farrugia, E Friedman, J Richards, K Saastamoinen, A Salek-Haddadi and R Yadava.
Cumberland Infirmary (2): P Davies, Z Ferguson, C Hagon and S Holliday.
Royal Albert Edward Infirmary (2): A Suman, P Farren and U Skulbedau.
Scunthorpe General Hospital (2): A Banerjee, D Briggs, B Evans, K Kent, N Nadeem and K Short.
University Hospital of Hartlepool (1): D Bruce, O Bowman, B Kumar and M Platton.
Kings Mill Hospital (1): M Cooper, J Burkitt, L Cordon, A Feely, K Hannah, P Hill, M Nasar, A Rajapakse and I Wynter.
Diana Princess of Wales Hospital (1): J Adiotomre, A Ali, D Briggs, K Short, J Wallhead and J Wivell.
Follow-up centres
Bexhill Hospital: C Athulathmudali, M Barbon, S Holmes, A Mason and C Parter.
Chelsea and Westminster Hospital: M Pelly, E Beranova, H Lee, H Reid and T Sachs.
Lymington New Forest Hospital: G Durward, S Evans, W Foster, M Harris and V Pressly.
Newham General Hospital: P Gompertz, P Daboo, M Farrugia, A Jackson, J Richardson and H Syed.
Pontefract Hospital: A Keeney, R Barr, G Bateman, MA Carpenter, R Davey, T Hendra, A Needle, A McGuinness and Stanners.
St Bartholomews Hospital, Rochester: T France, P Akhurst, S Burrows and F Williams.
West Middlesex University Hospital NHS Trust: R Singh, J Platt, M Teklay and S Wakely.
Whipps Cross University Hospital: R Yadav, E Clough, D Maguire, P Purdy and R Simister.
Appendix 2 Data collection forms
Appendix 3 Close out checklist
Appendix 4 Search strategies used
MEDLINE (via Ovid)
We used the following search strategy for MEDLINE (via Ovid) and adapted it to search the Cochrane Central Register of Controlled Trials:
-
cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp “intracranial embolism and thrombosis”/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/
-
(stroke or poststroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
-
((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$)).tw.
-
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
-
1 or 2 or 3 or 4
-
thrombosis/
-
thromboembolism/
-
venous thrombosis/ or venous thromboembolism/
-
thrombophlebitis/
-
deep venous thrombo$.tw.
-
deep vein thrombo$.tw.
-
((venous or vein) adj5 thrombo$).tw.
-
(DVT or VTE).tw.
-
thromboprophylaxis.tw.
-
phlebothrombosis.tw.
-
exp pulmonary embolism/
-
pulmonary artery/ and embolism/
-
((pulmonary or lung) adj5 (embol$ or thrombo$ or infarct$)).tw.
-
or/6-18
-
Intermittent Pneumatic Compression Devices/
-
bandages/ or stockings, compression/ or gravity suits/
-
(pneumatic adj5 (compression or device$ or appliance$ or stocking$ or hose or boot$ or suit or suits)).tw.
-
((pneumatic or electric$ or compression) adj5 pump$).tw.
-
(intermittent adj5 (compression or impulse device$)).tw.
-
(compression adj5 (device$ or system$ or stocking$ or hose or boot$)).tw.
-
((elastic or antiembolic or anti-embolic) adj5 (stocking$ or hose)).tw.
-
(mechanical adj5 (prophylaxis or compression)).tw.
-
(inflatable adj5 (device$ or garment$ or stocking$ or hose or boot$)).tw.
-
IPC.tw.
-
(sequential adj5 compression).tw.
-
exp Electric Stimulation/
-
exp Electric Stimulation Therapy/
-
(electric$ adj10 stimulat$).tw.
-
electrostimulation.tw.
-
or/20-34
-
5 and 19 and 35
EMBASE (via Ovid)
We used the following search strategy to search EMBASE (via Ovid):
-
cerebrovascular disease/ or basal ganglion hemorrhage/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or exp carotid artery disease/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/
-
stroke patient/
-
(stroke or poststroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
-
((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$)).tw.
-
((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
-
1 or 2 or 3 or 4 or 5
-
thromboembolism/ or thrombosis/ or leg thrombosis/ or vein thrombosis/ or deep vein thrombosis/ or leg thrombophlebitis/ or thrombophlebitis/ or venous thromboembolism/
-
thrombosis prevention/ or postoperative thrombosis/
-
deep venous thrombo$.tw.
-
deep vein thrombo$.tw.
-
((venous or vein) adj5 thrombo$).tw.
-
(DVT or VTE).tw.
-
thromboprophylaxis.tw.
-
phlebothrombosis.tw.
-
lung embolism/
-
lung artery/ or pulmonary artery/
-
embolism/ or artery embolism/ or embolism prevention/
-
16 and 17
-
((pulmonary or lung) adj5 (embol$ or thrombo$ or infarct$)).tw.
-
7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 18 or 19
-
intermittent pneumatic compression device/
-
compression/ or compression therapy/ or leg compression/ or pneumatic tool/
-
clothing/ or protective clothing/ or cuff/ or elastic stockings/
-
(pneumatic adj5 (compression or device$ or appliance$ or stocking$ or hose or boot$ or suit or suits)).tw.
-
((pneumatic or electric$ or compression) adj5 pump$).tw.
-
(intermittent adj5 (compression or impulse device$)).tw.
-
(compression adj5 (device$ or system$ or stocking$ or hose or boot$)).tw.
-
((elastic or antiembolic or anti-embolic) adj5 (stocking$ or hose)).tw.
-
(mechanical adj5 (prophylaxis or compression)).tw.
-
(inflatable adj5 (device$ or garment$ or stocking$ or hose or boot$)).tw.
-
IPC.tw.
-
electrostimulation/ or electrostimulation therapy/
-
(electric$ adj10 stimulat$).tw.
-
electrostimulation.tw.
-
or/21-34
-
6 and 20 and 35
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
We used the following search strategy to search CINAHL (via EBSCOhost):
S52 .S9 and S23 and S51
S51 .S24 or S27 or S30 or S33 or S36 or S39 or S42 or S45 or S46 or S47 or S48 or S49 or S50
S50 .TI electrostimulation or AB electrostimulation
S49 .TI (electric* N10 stimulat*) or AB (electric* N10 stimulat*)
S48 .MH “electrical stimulation" or MH “electrotherapy"
S47 .TI (sequential N5 compression) or AB (sequential N5 compression)
S46 .TI IPC or AB IPC
S45 .S43 and S44
S44 .TI (device* or garment* or stocking* or hose or boot*) or AB (device* or garment* or stocking* or hose or boot*)
S43 .TI inflatable or AB inflatable
S42 .S40 and S41
S41 .TI (prophylaxis or compression) or AB (prophylaxis or compression)
S40 .TI mechanical or AB mechanical
S39 .S37 and S38
S38 .TI (stocking* or hose) or AB (stocking* or hose)
S37 .TI (elastic or antiembolic or anti-embolic) or AB (elastic or antiembolic or anti-embolic)
S36 .S34 and S35
S35 .TI (device* or system* or stocking* or hose or boot*) or AB (device* or system* or stocking* or hose or boot*)
S34 .TI compression or AB compression
S33 .S31 and S32
S32 .TI (compression or impulse device*) or AB (compression or impulse device*)
S31 .TI intermittent* or AB intermittent*
S30 .S28 and S29
S29 .TI pump* or AB pump*
S28 .TI (pneumatic or electric* or compression)or AB (pneumatic or electric* or compression)
S27 .S25 and S26
S26 .TI (compression or device* or appliance* or stocking* or hose or boot* or suit or suits) or AB (compression or device* or appliance* or stocking* or hose or boot* or suit or suits)
S25 .TI pneumatic or AB pneumatic
S24 .(MH “Compression Garments”) or (MH “Compression Therapy”)
S23 .S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S22
S22 .S20 and S21
S21 .TI (embol* or thrombo* or infarct*) or AB (embol* or thrombo* or infarct*)
S20 .TI (pulmonary or lung) or AB (pulmonary or lung)
S19 .MH “pulmonary artery” and MH “embolism”
S18 .MH “pulmonary embolism”
S17 .TI phlebothrombosis or AB phlebothrombosis
S16 .TI thromboprophylaxis or AB thromboprophylaxis
S15 .TI (DVT OR VTE) or AB (DVT OR VTE)
S14 .TI (vein N5 thrombo*) or AB (vein N5 thrombo*)
S13 .TI (venous N5 thrombo*) or AB (venous N5 thrombo*)
S12 .TI deep vein thrombo* or AB deep vein thrombo*
S11 .TI deep venous thrombo* or AB deep venous thrombo*
S10 .(MH “Thrombosis”) or (MH “thromboembolism”) or (MH “venous thrombosis”) or (MH “thrombophlebitis”)
S9 .S1 or S2 or S5 or S8
S8 .S6 and S7
S7 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)
S6 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid)
S5 .S3 and S4
S4 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)
S3 .TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral)
S2 .TI ( stroke or poststroke or post-stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post-stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH)
S1 .(MH “Cerebrovascular Disorders+”) or (MH “stroke patients”) or (MH “stroke units”)
British Nursing Index (via Ovid)
We used the following search strategy to search the British Nursing Index (via Ovid):
-
“equipment and supplies”/ and dressings/
-
(pneumatic adj5 (compression or device$ or appliance$ or stocking$ or hose or boot$ or suit or suits)).tw.
-
((pneumatic or electric$ or compression) adj5 pump$).tw.
-
(intermittent adj5 (compression or impulse device$)).tw.
-
(compression adj5 (device$ or system$ or stocking$ or hose or boot$)).tw.
-
((elastic or antiembolic or anti-embolic) adj5 (stocking$ or hose)).tw.
-
(mechanical adj5 (prophylaxis or compression)).tw.
-
(inflatable adj5 (device$ or garment$ or stocking$ or hose or boot$)).tw.
-
ipc.tw.
-
(sequential adj5 compression).tw.
-
((electric$ adj10 stimulat$) or electrostimulation).tw.
-
or/1-11
-
thrombosis/
-
deep venous thrombo$.tw.
-
deep vein thrombo$.tw.
-
((venous or vein) adj5 thrombo$).tw.
-
(DVT or VTE).tw.
-
thromboprophylaxis.tw.
-
phlebothrombosis.tw.
-
((pulmonary or lung) adj5 (embol$ or thrombo$ or infarct$)).tw.
-
or/13-20
-
stroke services/ or stroke/ or stroke rehabilitation/
-
(stroke or poststroke or post-stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
-
((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
-
((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
-
or/22-25
-
12 and 21 and 26
List of abbreviations
- ACCORD
- Academic and Clinical Central Office for Research and Development
- ADL
- activities of daily living
- ARR
- absolute risk reduction
- CDU
- compression duplex ultrasound
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CLOTS
- Clots in Legs Or sTockings after Stroke trial
- CONSORT
- Consolidated Standards of Reporting Trials
- DMC
- Data Monitoring Committee
- DVT
- deep vein thrombosis
- EQ-5D-3L
- European Quality of Life-5 Dimensions 3 Level
- GCS
- graduated compression stocking
- GP
- general practitioner
- IPC
- intermittent pneumatic compression
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LMWH
- low-molecular-weight heparin
- OHS
- Oxford Handicap Scale
- OR
- odds ratio
- PE
- pulmonary embolism
- QALD
- quality-adjusted life-day
- RCT
- randomised controlled trial
- SD
- standard deviation
- TSC
- Trial Steering Committee
- VTE
- venous thromboembolism