Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/27/01. The contractual start date was in October 2012. The draft report began editorial review in May 2014 and was accepted for publication in January 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Alice Turner’s clinic has been loaned sleep monitors by ResMed Inc., which also produces non-invasive ventilation equipment. Rahul Mukherjee reports receiving non-financial support from ResMed Inc. and Breas Medical in the form of training sessions for the non-invasive ventilation equipment supplied to his non-invasive ventilation multidisciplinary team. David Moore and Janine Dretzke acted as peer reviewers for the Cochrane systematic review by Struik et al. which is included in this report. Rachel Jordan was awarded a grant in respect of a National Institute for Health Research postdoctoral fellowship during the conduct of the study. The fellowship relates to a different project (‘Towards a better understanding of the definition, characteristics and health service requirements of patients with COPD in the UK’).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Dretzke et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a chronic progressive lung disease characterised by non-reversible airflow obstruction, mostly affecting middle-aged or elderly people who have smoked. The most important cause of COPD is cigarette smoking, although other risk factors are thought to be indoor and outdoor air pollution, occupational exposures and diet. 1 Over time, patients experience increasing breathlessness, leading to increasing disability and loss of health-related quality of life (HRQoL). Many COPD patients will experience worsening of their symptoms, often precipitated by infection, on a regular basis. 2 These are known as exacerbations. COPD accounts for one in eight medical hospital admissions in the UK, most of which will be for exacerbations, and costs the NHS in excess of £800M per annum. 3 Currently, treatment is mainly symptomatic, although it also aims to slow down disease progression. The main evidence-based treatments are inhaled agents, such as bronchodilators4 and pulmonary rehabilitation. 5
Diagnosis
Airflow obstruction is diagnosed by spirometry, performed after use of a bronchodilator. Spirometry generates a forced expiratory volume in 1 second (FEV1) and a forced vital capacity (FVC); it is the ratio between these two figures that defines airflow obstruction. Although definitions of airflow obstruction are inconsistent and controversial,6 National Institute for Health and Care Excellence (NICE) guidance currently defines airflow obstruction as FEV1/FVC< 0.7. 4 This is one of two common definitions; the other is to define it as any value of FEV1/FVC that is less than the lower limit of normal for the patient’s age. 6
Severity
Severity of airflow obstruction is graded using categories of FEV1 as a percentage of predicted normal values of a healthy reference population. 1,4 Table 1 outlines some severity categories. The definitions of different grading categories vary somewhat and have changed over time. For example, the Global Initiative for Chronic Obstructive Lung Disease (GOLD)1 has proposed a revised COPD grading system that incorporates symptoms and HRQoL into the severity categories.
| GOLD category | NICE category | FEV1 % predicted |
|---|---|---|
| 1 | Mild | > 80 |
| 2 | Moderate | 50–79 |
| 3 | Severe | 30–59 |
| 4 | Very severe | < 30 |
Most research studies evaluating treatments use FEV1 % predicted to select and describe patients. FEV1 is also often used as an outcome measure to describe prognosis of patients, as are clinical measures such as dyspnoea and exacerbations, HRQoL and health-service utilisation (e.g. hospital admissions1).
Severity of airflow obstruction does not necessarily reflect either the level of disability experienced or the frequency of exacerbation, and composite measures to capture the global impact of the disease have been proposed. 1,7 However, they are not yet widely used as the basis for treatment decisions.
In more severe disease, respiratory failure can occur [hypoxia with resting pO2 (partial pressure of oxygen) < 8 kPa]. Respiratory failure may be either type 1, in which arterial carbon dioxide is normal or low, or type 2, in which the patient is hypercapnic (elevated arterial CO2). It is not yet clear which factors predispose to the different types of respiratory failure or how quickly it will develop or progress. Type 2 respiratory failure can also result in acidosis of the circulating blood; this is known as acute hypercapnic respiratory failure (HRF). Acidosis is not present in the stable state, as it is compensated for by renal mechanisms of acid–base balance.
End-stage COPD could be defined in several ways; classically, it would concern those patients in the terminal stage of their disease who were likely to die within months, a situation which is not always clear. Attempts are being made to define the disease trajectory at the end of life, but it remains hard to predict. 8 Alternatively, patients with end-stage COPD might be defined as those who have developed chronic respiratory failure and remain symptomatic on maximal therapy, with no hope of cure. This is a significant population, who could potentially be stabilised for years. For the purposes of this report, the latter, broader definition prevails, as this encompasses the wide population for whom domiciliary non-invasive ventilation (NIV) has been considered in some studies and in practice.
Exacerbations
Exacerbations or ‘flare-ups’ of COPD occur in approximately 50–60% of moderate/severe COPD patients per year. 2,9 An exacerbation can be defined as an acute event characterised by a worsening of the patient’s respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication. 1 The most common causes are respiratory tract infections, either viral or bacterial, although other factors, including pollution,10 can precipitate them. Exacerbations play a very important role in COPD, as they are a key cause of increased morbidity, mortality and poor health status and place a considerable burden on the health-care system. 11
It is estimated that approximately 75% of exacerbations can be managed in the community with antibiotics, corticosteroids and bronchodilators. 12 Half of exacerbations treated in the community result in patients recovering to their baseline level in about 7 days; however, in 14% of such events patients do not reach their baseline level after 35 days, and a few never return to baseline. 13
In general, a severe exacerbation is defined as one that results in hospitalisation (Table 2). Approximately 15% of COPD patients per year have exacerbations severe enough to lead to hospital admission, with a median length of stay in the UK of 8 days. This contributes to over half of the total direct costs of COPD to the NHS. 3,15 Between 10% and 25% of patients admitted with HRF caused by COPD die in hospital. 16 Readmission for an exacerbation within 3 months is high, at over 30%, as is 30-day mortality. In those requiring artificial ventilation in hospital (intubation or NIV), mortality may be as high as 40% at 1 year after discharge, and the all-cause mortality 3 years after hospitalisation is higher still. 16 It is therefore evident that hypercapnic COPD patients, and those who have previously used ventilation in hospital, may have a poor prognosis.
| Severity of exacerbation | Defining features |
|---|---|
| Mild | Uses normal medication more than usual |
| Moderate | Requires antibiotics and/or steroids |
| Severe | Admitted to hospital |
Exacerbations have an adverse effect on a patient’s quality of life (QoL). 11 It may take many weeks for the symptoms to abate and lung function to recover; they have also been shown to lead to a rapid overall decline in lung function. 17 Some patients appear to suffer from frequent exacerbations while others do not. Those reporting two or more exacerbations of COPD per year are classified as ‘frequent exacerbators’. 2
Exacerbations are important outcome measures in COPD and a reduction in exacerbation frequency is an important target to achieve for any intervention.
Quality of life
Health-related QoL is known to be impaired by COPD, even at relatively early stages,18 and gets worse with more severe disease and with exacerbations. 19 Many tools are available to monitor HRQoL in COPD, and have been reviewed elsewhere;20 they may be generic (i.e. used in any health problem), respiratory-specific or COPD-specific (e.g. COPD assessment test). 21 Tools commonly used in clinical trials include the St George’s Respiratory Questionnaire (SGRQ)22 and the Chronic Respiratory Questionnaire (CRQ). As with all questionnaires, it is possible that scores vary between the tools used; indeed, this has been the case in COPD when comparing a generic tool [Short Form questionnaire-36 items (SF-36)], the SGRQ and the CRQ. 23 Other tools include the Severe Respiratory Insufficiency Questionnaire (SRI) and Maugeri Foundation Respiratory Failure questionnaire (MRF-28). 24
Management of chronic obstructive pulmonary disease
Pharmacotherapy in stable COPD is mainly directed at airflow obstruction and inflammation. It is recommended that short- and long-acting bronchodilators be used in a stepwise manner, with the addition of inhaled corticosteroids later in the disease. In addition to pharmacotherapy, pulmonary rehabilitation is recommended for breathless patients. 4 This is a programme of exercise and education which has been shown to be very beneficial in COPD patients. 25 Patients may also be taught various self-management techniques, the efficacy of which has recently been reviewed by some of the authors of this report. 26
In general, treatment is aimed at improving lung function, defined by FEV1, and/or HRQoL and exacerbation frequency. Many drug treatments have also tried to improve longer-term outcomes, such as lung function decline12 and mortality,27 with limited success. In addition to the general treatments described above, long-term oxygen therapy (LTOT) should be considered28 in patients with pO2 < 7.3 kPa, or < 8 kPa in the presence of cor pulmonale,29 because it improves mortality. Other forms of oxygen use in COPD are more controversial; oxygen use just on walking (ambulatory oxygen) is used in selected patients not on LTOT if their oxygen levels drop on objective testing. 30 Short-burst oxygen therapy, defined as oxygen used outside the context of LTOT or ambulatory oxygen, is not recommended because of lack of benefit. 31 Another treatment that has been proposed for selected COPD patients is domiciliary use of NIV, which is the subject of this review.
Non-invasive ventilation
Non-invasive ventilation is a method of providing ventilatory support via a mask, without the placement of an endotracheal tube. In contrast to traditional intensive care unit (ICU) mechanical ventilators, NIV machines are generally compact and lightweight and can be applied by a patient with minimal or no assistance; it is therefore possible to use them outside the hospital. Machines will deliver pressure support during the breathing cycle and will sense the respiratory cycle such that, if no breaths are taken, back-up breaths can be delivered. NIV aims to improve tidal volumes while breathing and reduce ventilatory effort by resting tired respiratory muscles, as well as to improve fatigue, lung function and gas exchange, particularly during disease exacerbations.
Types of non-invasive ventilation
Non-invasive ventilation delivers two different pressures; that is, a different pressure on inspiration [inspiratory positive airway pressure (IPAP)] from expiration [expiratory positive airway pressure (EPAP)]. It differs from continuous positive airway pressure (CPAP), another non-invasive form of respiratory support, which aims to maintain a continuous level of positive airway pressure. The type of NIV is generally described by the way in which the machine is set up, such that it will be either pressure controlled or volume controlled. In pressure-controlled settings, the main descriptors for treatment will be the IPAP and EPAP, although a back-up rate will usually also be set. The mask type may also be described, with most patients using a full-face mask, although nasal-only devices are also available. Once patients start NIV, the parameters can be varied according to their ability to tolerate treatment and their response; for instance, an in-patient NIV protocol might start at relatively low IPAP and titrate upwards until the patient’s blood gases improve, stopping any further elevation in pressure if the patient finds it uncomfortable. While there is no widely accepted definition of low-pressure and high-pressure NIV, there has been work to suggest that degree of IPAP may relate to outcome, and there has been an increasing trend towards the use of higher pressures in more recent studies32,33 compared with older studies. 34,35 The type of NIV most useful to COPD is not clear; neither are the optimal pressure setting and optimisation protocol. It is likely that there will be significant patient variability and individualised protocols will be required.
Domiciliary NIV is usually used overnight during sleep, although hours of use may gradually climb with time until daytime use is also needed.
Use of non-invasive ventilation for chronic obstructive pulmonary disease in hospital settings
There is good evidence that HRF during an acute exacerbation should be treated with NIV;4 a Cochrane review (Ram et al. 200436) of NIV found statistically significant benefits in favour of NIV for a wide range of outcomes, including treatment failure (based on eight trials), risk of intubation (based on 14 trials), length of hospital stay (based on eight trials), complications (based on three studies) and mortality (based on 10 trials).
Use of non-invasive ventilation in stable chronic obstructive pulmonary disease patients
The evidence from NIV use in a hospital setting led to trials of domiciliary NIV, aimed at reducing mortality and readmission rates; this is the subject of this report.
Increasingly employed for longer-term treatment of patients suffering from chronic HRF due to thoracic cage disorders, neuromuscular disorders and various other causes of nocturnal hypoventilation syndrome,37 long-term NIV offers theoretical benefits in certain groups of chronic end-stage COPD patients. Clinically, despite the success of NIV in acute HRF with COPD, survivors continue to suffer from further episodes/exacerbations after discharge. 38 Thus, a utility of domiciliary NIV is thought to lie in preventing recurrent admission to hospital and slowing declining health.
The National COPD strategy consultation in 2010 highlighted NIV as an area of COPD therapy that warranted review and/or further research. 39
Domiciliary non-invasive ventilation: existing clinical effectiveness evidence
Despite the existence of a number of randomised controlled trials (RCTs) and systematic reviews of RCTs, there remains uncertainty around the clinical effectiveness of domiciliary NIV in COPD, particularly with regard to patient and health service-related outcomes likely to be important in an economic model (e.g. survival, hospital admissions, exacerbations and QoL). See Chapter 5, Review of systematic reviews, for a more detailed assessment of existing systematic reviews.
Domiciliary non-invasive ventilation: existing cost-effectiveness evidence
Currently, there are no cost-effectiveness studies of domiciliary NIV in patients with stable end-stage COPD, only cost studies (see Chapter 6, Results).
In the UK, domiciliary NIV is considered on health economic grounds if a patient has had three hospital admissions with acute HRF,40 although, given the absence of a full economic evaluation, such use could be considered not fully evidence based. Given this, an economic evaluation of domiciliary NIV in stable end-stage COPD is warranted.
Decision problem
This project was commissioned to determine the cost-effectiveness of community-based NIV in patients with stable end-stage COPD plus chronic HRF. However, a scoping review of the existing clinical effectiveness evidence, in particular existing systematic reviews, found that there was a lack of reporting on outcomes likely to be important for informing economic modelling; therefore, it was decided to additionally undertake a systematic review of the clinical effectiveness with the aim of using the findings to populate the economic model where possible.
Population
The National Institute for Health Research (NIHR) call relating to this project (11/27/01) specified ‘adult patients with stable end-stage COPD plus chronic HRF, who have required assisted ventilation (whether invasive or non-invasive) during an exacerbation or who are hypercapnic or acidotic on LTOT.’
The following points are worth considering:
-
This specification includes a number of types of patient, that is:
-
stable patients who have required assisted ventilation during an exacerbation
-
stable patients who are hypercapnic on LTOT (regardless of whether or not they have required assisted ventilation during an exacerbation)
-
patients who are acidotic on LTOT.
-
-
‘Acidotic on LTOT’ implies the absence of stable disease, as it usually requires acute treatment in hospital.
-
The determination of whether or not a patient has stable disease and/or end-stage disease is, to some extent, subjective.
In order to capture all relevant groups, studies with both stable patients and post-hospital (exacerbation) patients were eligible for inclusion into separate analyses.
This report therefore necessarily considers a broad definition of patients, that is any adult patient with COPD with or without HRF (however defined), and includes any patients recently discharged from hospital (following an exacerbation) or more stable patients, without restriction to a specific disease severity.
Setting
The NIHR brief specified a community setting and this was taken to mean any setting where NIV was not used in an acute setting or a research setting. Therefore, evidence was not considered when NIV was given in hospital or immediately before hospital during an exacerbation or, for example, when NIV was given during exercise assessment as part of a pulmonary rehabilitation programme. In practice, only studies where patients used NIV in their own home were identified, although other domiciliary settings, such as a care home, would have been eligible.
Intervention
Any form of NIV, whether continuous or intermediate, added to (any form of) usual care was considered. There were no restrictions according to length of daily use.
While the term ‘NIV’ in its broadest sense refers to any type of NIV, as opposed to invasive techniques, in this context it is used to mean a system that delivers two different pressures. The following terms can all mean NIV (i.e. systems with two positive pressures), provided they are non-invasive: bilevel positive airway pressure (BIPAP), non-invasive positive-pressure ventilation or nasal intermittent positive pressure ventilation (both referred to as NIPPV), non-invasive mechanical ventilation, positive pressure ventilation and (nasal) proportional assist ventilation.
Negative pressure ventilation (NPV) is rarely used routinely but also delivers two pressures (although one relates to ambient pressure); studies using NPV would have been eligible for inclusion and would have been considered separately (none was identified). CPAP was excluded, as it delivers constant pressure and is thus a distinct therapy; it is primarily used to treat sleep apnoea.
Comparator
While the NIHR brief did not specify looking at different types of NIV, it is becoming apparent that higher pressures are increasingly being used (see Chapter 1, Types of non-invasive ventilation). Therefore, studies with head-to-head comparisons of different NIV settings (particularly pressure, or pressure vs. volume controlled) were of interest and considered. Other differences include mask type and number of hours of use per day. Length of daily use and a need for longer periods of use, for example during the day as well as during the night may also be related to severity of disease.
Discontinuing domiciliary NIV was not covered by the remit for this report. Therefore it is not considered. However, the availability of evidence on the effect of discontinuing was noted (see Chapter 4, Discontinuation studies).
Outcomes
As the NIHR brief was for an economic evaluation, the primary effectiveness outcomes were considered to be those relating to survival, health-care service utilisation (as a results of exacerbations), patient QoL and adherence to NIV/discontinuations. Secondary outcomes {relating to lung function, blood gases, exercise capacity [e.g. 6-minute walking distance (6MWD)], quality of sleep, activities of daily living and acceptability} were not relevant for the economic model, as cost and utility changes associated with changes in these outcomes cannot be measured. However, in order to present a full clinical picture of the effect of NIV, and to be consistent with previous systematic reviews, data on key secondary outcomes were extracted and presented. A decision was made to focus on lung function (FEV1, FVC), blood gases [partial pressure of carbon dioxide in the arterial blood (PaCO2), partial pressure of oxygen in the arterial blood (PaO2)] and 6MWD. Hydrogen carbonate (HCO3–) and pH are to some extent reflected in other blood gas measures, and measures of activity are reflected to some extent in QoL measures and 6MWD. Further, there is a lack of validated questionnaires for activities of daily living and also for sleep quality; hence, data were not extracted for these outcomes.
Study design
Based on an overview of existing clinical effectiveness reviews of RCTs, it was apparent that a lack of large, long-term studies, variations between study methods and physiological or clinical outcomes measured, and a lack of adjustment for clinical variables (such as oxygen use or prior acute NIV use) have limited the conclusions that can be drawn. In particular, RCTs appear to have insufficiently long follow-up periods to capture outcomes relating to survival, long-term HRQoL, exacerbations over the long term, adverse events or adherence rates. Further, the clinical perception is that inclusion criteria for the RCTs may be narrow and restricted to patients with very specific characteristics, calling into question their applicability to a wider population. There may also be clear subgroups within current trials which warrant formal meta-analysis not conducted in prior reviews.
As a further review based on RCTs alone might be insufficient to derive all the necessary (long-term) parameters needed to adequately populate an economic model, non-randomised controlled studies and uncontrolled studies are also considered in this report.
Chapter 2 Aims of the review
Given the remit and the above context, the aims of this report were to undertake:
-
a systematic review of RCTs and non-RCTs comparing domiciliary NIV with usual care, or different types of NIV, in COPD patients either who are in a stable state of disease (stable population) or who commence NIV immediately after a period of hospitalisation/exacerbation (post-hospital population)
-
an overview of existing systematic reviews of RCTs comparing domiciliary NIV with usual care, or different types of NIV, in COPD patients
-
a systematic review of uncontrolled studies (patients on domiciliary NIV only) with the aim of supplementing the findings from the controlled studies where evidence was lacking
-
a systematic review of the evidence on the cost-effectiveness of domiciliary NIV compared with usual care only in COPD patients
-
a model-based cost–utility analysis to determine the cost-effectiveness of domiciliary NIV compared with usual care only in COPD patients (stable and post-hospital populations).
Aims 1 to 3 relate to the systematic review of clinical effectiveness and are addressed in Chapters 4 and 5 of this report; aims 4 and 5 relate to the systematic review of cost-effectiveness and the economic model and are addressed in Chapters 6 and 7.
Chapter 3 Clinical effectiveness review methods
This chapter outlines the methods for the review of clinical effectiveness as detailed in the aims (see Chapter 2).
The protocol for this project was registered with PROSPERO (2012:CRD42012003286) and has been published on the NIHR Health Technology Assessment programme website (www.nets.nihr.ac.uk/projects/hta/112701). A version of the protocol was also published in the journal BioMed Central Systematic Reviews. 41
Searches
Searches for studies to populate the systematic review were devised to incorporate a combination of text words and index terms relating to NIV (intervention) and COPD (population). Given that NIV has been in routine use since the early 1990s, and one of the main co-interventions/comparators (LTOT) since the 1980s, searches were run from 1980 to September 2014, inclusive, to ensure that no relevant studies were missed. No study design or language restrictions were imposed.
The following sources were searched:
-
bibliographic databases – MEDLINE, MEDLINE In Process & Other Non-Indexed Citations and EMBASE via Ovid, Cochrane Central Register of Controlled Trials, Cumulative Index to Nursing and Allied Health Literature (EBSCOhost) and Science Citation Index Expanded (ISI)
-
MEDLINE and EMBASE via Ovid and NHS Economic Evaluation Database (Cochrane Wiley) for studies relating to QoL, cost and cost-effectiveness
-
Applied Social Sciences Index and Abstracts (ProQuest), Social Sciences Citation Index (ISI), International Bibliography of the Social Sciences (ProQuest), PsycINFO (Ovid) and Sociological Abstracts (ProQuest) for qualitative studies
-
metaRegister of Current Controlled Trials, International Standard Randomised Controlled Trial Number (ISRCTN) database, UK Clinical Research Network, the World Health Organization’s International Clinical Trials Registry Platform Portal and ClinicalTrials.gov for ongoing studies
-
the Cochrane Airways specialised register
-
The British Library’s Electronic Table of Contents and ISI Conference Proceedings Citation Index for abstracts and proceedings.
In addition to the above, experts in the field were consulted, citation lists of included studies and relevant reviews were checked and selected websites were searched for grey literature. For full search strategies, see Appendix 1.
Study selection
Screening and study selection strategy
Titles (and abstracts, where available) of articles identified by the searches were screened by two reviewers for relevance to the review question using prespecified screening criteria. Hard copies of potentially relevant articles were acquired and assessed against the full inclusion criteria (Table 3) by two reviewers independently. Discrepancy between reviewers was resolved by discussion or by referring to a third reviewer. Where necessary, translation (full/part) of non-English-language articles was undertaken to facilitate this process and subsequent reviewing. The study selection process was illustrated using a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. Reference management software (Reference Manager version 11, Thomson ResearchSoft, San Francisco, CA, USA) was used to record reviewer decisions, including reasons for exclusion.
| Study selection criteria | Included | Excluded |
|---|---|---|
| Study design | RCTs, observational studies with n > 1, systematic reviews (at least one database searched) | Narrative reviews, commentaries, non-human/laboratory studies, single-case reports |
| Publication type | Full articles, letters, conference abstracts 2010–present, ongoing study reports | Conference abstracts from 2009 or older |
| Population | COPD patients. Studies where only a proportion had COPD were included. There were no restrictions by disease severity, history of exacerbations or comorbidities (except where patients also had sleep apnoea) | Studies where the primary condition was not COPD; patients with overlap syndrome (COPD and obstructive sleep apnoea) |
| Intervention | Any type of NIV added to (any) usual care. NIV here means any non-invasive system delivering two different positive pressures. Studies using NPV were eligible for inclusion | Any invasive system, or non-invasive system delivering continuous pressure, such as CPAP |
| Setting | Any domiciliary setting (e.g. own home, nursing home) | Studies where:
|
| Outcomes for analysisa | Included | Excluded |
| Primary |
|
Quality-of-life questionnaires that were not disease specific (e.g. SGRQ) and not generic (e.g. SF-36), for example relating to anxiety/depression (HADS) |
| Secondary |
|
Other secondary outcomes such as:
|
Selection criteria
Study design
Systematic reviews, RCTs and controlled or uncontrolled observational studies with n > 1 were included. There were no restrictions on the type of RCT (e.g. parallel, crossover).
Patient group
Adult patients with COPD, with or without hypercapnia and with or without LTOT. When only a proportion of patients in a study met the inclusion criteria, the study was eligible for inclusion. Data were extracted only where results for relevant patients could be separated. Patients with any comorbidities were included, except where they had overlap syndrome (COPD and obstructive sleep apnoea).
Technology
Any form of NIV, whether continuous or intermediate, added to (any form of) usual care.
Setting
Any domiciliary setting (e.g. own home, care home).
Comparators/control (for controlled studies)
-
Any form of usual care with no NIV (or with sham NIV); it is noted that both the setting and the nature of usual care in the absence of treatment with NIV may be different from those of treatment with NIV; such differences did not affect inclusion/exclusion decisions, but were noted and commented upon.
-
Studies comparing alternative methods of NIV.
Outcomes
Study selection was not restricted by outcomes. Based primarily on the need to inform the economic evaluation model, outcomes to be analysed from included studies were:
-
mortality
-
hospitalisations, including accident and emergency (A&E) admissions or other health-care resource use
-
exacerbations (and requirements for associated medication)
-
QoL
-
adverse events
-
adherence to NIV/discontinuations.
These were considered to be the primary outcomes for this report.
Other outcomes likely to be reported in included studies were:
-
lung function (FEV1, FVC)
-
blood gases (PaO2, PaCO2)
-
pH
-
HCO3–
-
dyspnoea
-
exercise capacity (e.g. 6MWD)
-
other patient-related outcomes such as quality of sleep, activities of daily living and acceptability.
These were considered to be secondary outcomes, as they could not be used to inform the economic evaluation model. However, data were extracted for FEV1, FVC, PaO2, PaCO2 and 6MWD (see Chapter 1, Decision problem).
Publication type
Identified ongoing and/or unpublished studies were included if they met the above criteria. The triallists were contacted for additional information and data. Conference abstracts from 2010 were considered, as these may relate to studies that have not yet been fully published.
A summary of study selection criteria is shown in Table 3 (see also Chapter 1, Decision problem).
Assessment of risk of bias
Data were extracted to allow quality assessment of the included studies. For systematic reviews, the AMSTAR (A Measurement Tool to Assess Systematic Reviews) checklist was used. 42 The risk of bias tool from the Cochrane Handbook43 was used for RCTs. The domains relate to selection bias, performance bias, detection bias, attrition bias and reporting bias. For crossover trials, the following additional areas of risk of bias were assessed:44 (1) if there was a carryover effect; (2) if only first-period data were available; (3) if analysis was appropriate to crossover trials; and (4) the comparability of results with those from parallel-group trials.
For controlled observational studies, risk-of-bias assessment criteria were based on the guidelines outlined in chapter 13 of the Cochrane Handbook;45 these were adapted in consultation with the wider review team. The most relevant criteria relate to how the groups were selected and differences in patient characteristics, as well as potential differences in usual care and differential loss to follow-up or outcome assessment. Uncontrolled observational studies were not formally quality-assessed. See Appendix 2 for all quality-assessment criteria.
The GRADE (Grading of Recommendations Assessment, Development and Evaluation) framework46 was used (for primary outcomes of controlled studies only) to consider inconsistency (or heterogeneity) between studies, precision (uncertainty) of results, likelihood of publication bias and applicability of results to population(s) of interest.
Data extraction
Data relating to all aspects of the study were extracted by one reviewer using a standardised, piloted data extraction form in Microsoft Excel® version 2010 (Microsoft Corporation, Redmond, WA, USA); a second reviewer checked all numerical data. For foreign-language papers, independent checking was not always possible. Data were extracted on study and population characteristics, intervention and comparator characteristics, study quality and results.
In particular, details were sought on factors that could potentially inform subgroup analysis and economic model parameterisation, including severity of disease (GOLD stage), level of hypercapnia, number of patients on LTOT, history of exacerbations (frequent vs. non-frequent exacerbators), obesity, ruling out of sleep apnoea (overlap syndrome), whether or not patients were in a stable state or commenced the study immediately after a hospital admission, details on type of ventilation (e.g. different pressure settings) and number of hours of use per day of NIV.
Not all studies reported exacerbations as a predefined outcome; instead, some reported it as an adverse event or reason for discontinuation. In this case, the data were still extracted and the potential for selective reporting was assessed.
Authors were contacted for additional information on primary outcomes where results could potentially be included in a meta-analysis and/or inform the economic model (hospitalisation and survival data).
Uncontrolled studies were explored when relevant data were not (sufficiently) available from controlled studies; in particular, larger uncontrolled studies with longer follow-up times were considered. See Chapter 4, Survival, Hospitalisations, Exacerbations and Quality of Life, for more details on selection of uncontrolled studies. Formal data extraction was not performed.
Analysis
Primary outcomes
Narrative synthesis of evidence was undertaken for all included (controlled) studies. For primary outcomes, meta-analysis was undertaken in Stata (Version 10, StataCorp LP, College Station, TX, USA) where there was clinical and methodological homogeneity between studies reporting the same outcome and using the same outcome statistic (reported or calculable, see Appendix 3 for more detailed methods). As outlined in the decision problem (see Chapter 1, Decision problem), a distinction was made between stable and post-hospital populations, and studies were subgrouped accordingly for meta-analyses. Studies were also subgrouped according to study design (RCTs or non-randomised controlled studies).
Given probable residual clinical heterogeneity, a random-effects model was deemed most appropriate. The I2 statistic (which gives the percentage of the total variability in the data caused by between-study heterogeneity) was reported and commented on where appropriate. Further subgroup analysis (beyond stable and post-hospital populations or study design) was considered, subject to sufficient numbers of studies being available.
Construction of funnel plots to aid assessment of potential publication bias was planned where there were at least 10 studies in a meta-analysis. Sensitivity analyses, that is assessments of the robustness of any meta-analysis conclusions to the inclusion/exclusion of low-quality studies (those at most risk of bias), were planned, subject to sufficient numbers of studies and adequately reported quality criteria. Heterogeneity between studies (in terms of population and intervention characteristics) was explored in order to assess the feasibility of an indirect comparison. Small numbers of studies and/or clinical and methodological heterogeneity precluded the undertaking all of these analyses.
Secondary outcomes
Secondary outcome results (for FEV1, FVC, 6MWD, PaCO2, PaO2) were presented in forest plots to show the overall direction of effect, but were not pooled (see Chapter 1, Decision problem, for choice of which secondary outcomes to analyse). The following factors potentially leading to between-study heterogeneity were explored when considering whether or not to undertake meta-analyses: baseline imbalances, adjusted or unadjusted results, time points presented, type of RCT (parallel or crossover) and adequately presented (or calculable) data.
Presentation of results
In the following chapters, results have been presented separately for (1) studies comparing NIV and usual care (with or without sham NIV) and (2) studies comparing different types of NIV. When possible, results are reported by population and study design.
Chapter 4 Clinical effectiveness review results
This chapter presents the results of the clinical effectiveness review.
Volume of evidence
A total of 7402 titles (and abstracts where available) were screened independently by two reviewers using a screening form. Overall, 432 full texts were obtained, and full inclusion and exclusion criteria (see Table 3) were applied by two reviewers independently. Full texts of three further studies, identified through citation checking, were also obtained and assessed for inclusion. All discrepancies were resolved through discussion. Twenty-one articles could not be obtained (see Appendix 4, see Table 54) and further details to aid decision-making on four articles were requested from study authors without reply (see Appendix 4, see Table 55).
A total of 158 articles relating to 140 individual studies met the full inclusion criteria. The article selection process is outlined in a flow diagram in Figure 1 and a listed of excluded studies can be found in Appendix 4 (see Table 49). Twenty-nine studies were not further analysed, despite meeting the inclusion criteria, because results were not separable for COPD patients where studies included mixed populations (25 studies),47–70 two studies addressed a different question71,72 (discontinuation of NIV; see Discontinuation studies) and one was a study protocol of an RCT. 73 In the case of one further study67 in a COPD population, results were not presented for the usual-care group and the information was not obtainable from the authors.
FIGURE 1.
Flow diagram depicting article selection for clinical effectiveness review.
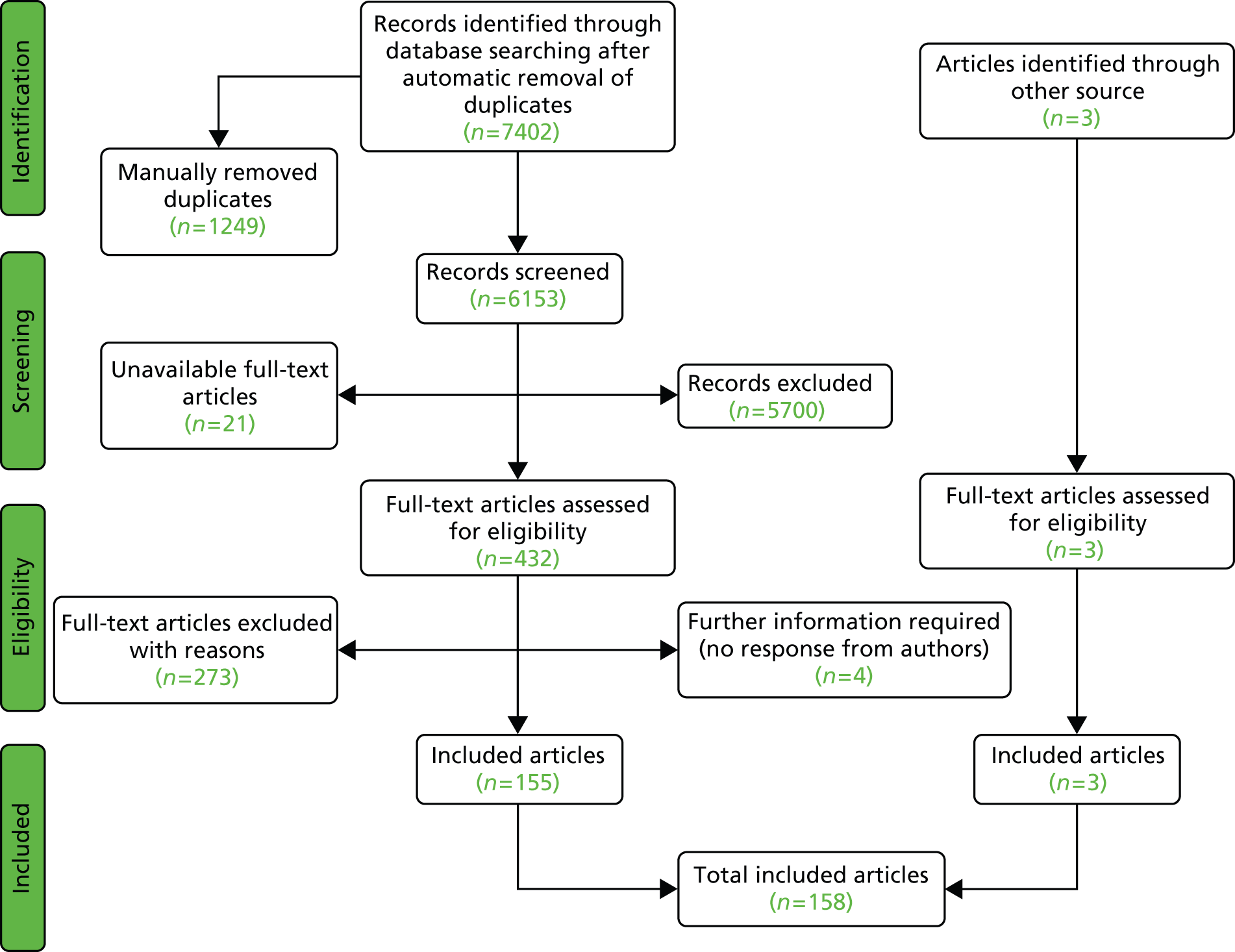
Of the remaining 111 studies, there were 21 RCTs (18 comparing NIV with usual care and three comparing different types of NIV), 10 non-randomised controlled studies (five prospective and five non-prospective), eight systematic reviews and 72 uncontrolled studies. Of the 31 controlled studies, six (19%) were available only in a language other than English (Chinese, German, French and Polish) and required partial or full translation.
The division of the included articles is outlined in Figure 2.
FIGURE 2.
Outline of included studies. a, Including data analyses and cross-sectional studies.
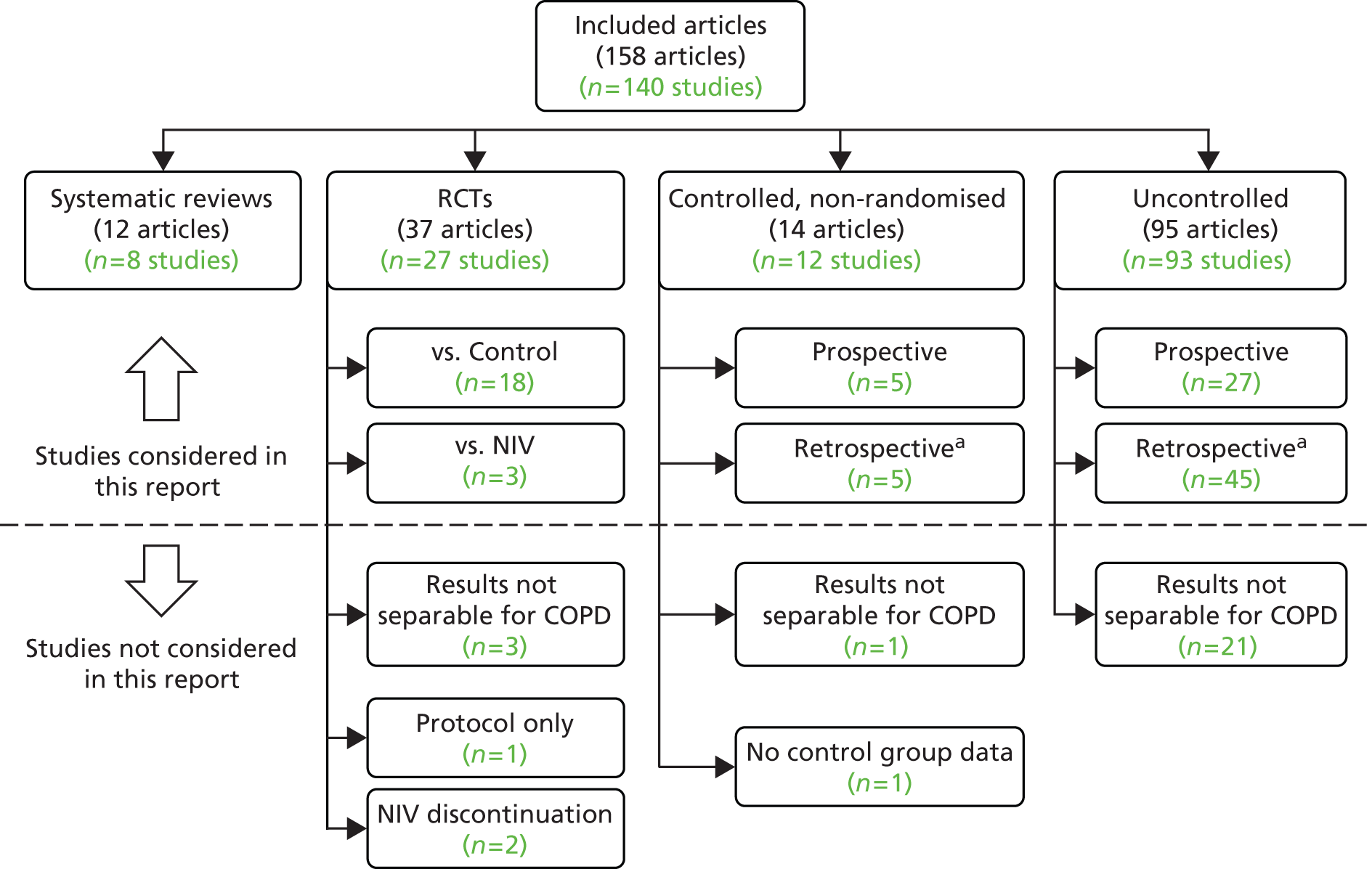
Appendix 5 gives brief details on all 72 uncontrolled studies; systematic reviews are discussed in Review of systematic reviews, and controlled studies are reviewed in the sections below.
Clinical effectiveness results: non-invasive ventilation versus usual care
This section details the studies (18 RCTs and 10 non-randomised) that compared NIV with usual care.
Study and population characteristics
Patient numbers across the 18 RCTs varied between 13 and 201 (total number of patients 1095). Only three RCTs had > 100 patients (by McEvoy et al. in 2009,74 n = 144; Struik et al. in 2014,75 n = 201; and Köhnlein et al. in 2014,76 n = 195). Length of follow-up varied between 3 and 24 months. There were two crossover trials34,77 (3 months’ follow-up). One RCT was reported as an abstract only;78 this was an interim (3 month) report of the ongoing HOT-HMV (Home Oxygen Therapy versus Home Mechanical Ventillation) trial (see Ongoing studies). One RCT was reported in two publications (by Duiverman et al. in 200879 and 201180), the first79 reporting outcomes after 3 months, during which time usual care in all patients took the form of a rehabilitation programme, and the second80 reporting over a longer follow-up period, with usual care now switched to less-intensive home-based rehabilitation.
Patient numbers across the 10 non-randomised controlled studies varied between 34 and 140 (total number of patients 664). Five were prospective studies, in which a group of patients on NIV and a group of patients receiving only usual care (usually those who refused NIV) were followed up over a period of between 12 and 35 months. Five were retrospective analyses of previously gathered data, with follow-up/analysis periods of 6 months, 12 months and 4, 8 and 10 years (see Study quality on quality assessment for potential selection bias in non-randomised studies).
The average age of patients across RCTs and non-randomised controlled studies varied between 60 and 73 years, and there were typically more men than women (between 41% and 98% men).
Most RCT populations were described as having GOLD stage 3 or 4, had mean FEV1 % predicted values that were indicative of severe/very severe COPD and/or were described as severe (with no indication of the classification system used). One RCT81 reported no details. There are unlikely to be major differences between RCTs in terms of severity, although the relative proportions of patients with stage 3 and 4 disease are unknown. Most RCTs described populations as being hypercapnic, although the PaCO2 cut-off points for inclusion varied (e.g. > 6 kPa, > 6.6 kPa). Three RCTs34,81,82 presented mean PaCO2 levels only, which were suggestive of at least a proportion of patients being hypercapnic. Two RCTs83,84 included normocapnic patients, while one85 stated that the number of hypercapnic patients included was small.
Seven of the non-randomised controlled studies also appeared to include mainly GOLD stage 3 and 4 patients; three studies86–88 provided no details. Eight studies included hypercapnic patients (based on PaCO2 cut-off points for seven), one86 presented mean PaCO2 levels only, which were suggestive of at least a proportion of patients being hypercapnic, and a further study87 provided no details.
A distinction has been made between ‘stable’ patient populations and those who commenced NIV after hospitalisation. In the stable populations, authors of the studies have specified that patients should not have been hospitalised within a certain time period. This time period varies between studies (from 4 weeks to 3 months). Not all studies with stable populations have defined a time period but may merely have described patients as stable. For populations described as ‘post hospital’, there was clear evidence in the study report that treatment with NIV commenced after an episode of hospitalisation (because of an exacerbation). There is no information, however, on timings, for example how soon after an exacerbation were patients admitted or how long the period in hospital was before NIV was initiated. This classification has informed the presentation of results (see Clinical effectiveness results: non-invasive ventilation versus usual care and Clinical effectiveness results: non-invasive ventilation versus non-invasive ventilation) and the economic evaluation scenarios (see Clinical effectiveness review discussion). Note that there is limited information on time since last exacerbation (for either population), with the exception of the controlled study by Paone et al. (2014)89 where all patients were enrolled 3 months after discharge from hospital after an exacerbation; they were free from exacerbations for at least 4 weeks and, therefore classified as stable.
Most RCTs included stable populations (13 out of 18), with five75,78,90–92 including a post-hospital population. Four non-randomised controlled studies89,93–95 included stable populations, four86,96–98 included post-hospital populations and there was no description for two. 87,88
Data were sought on patients’ exacerbation history in order to make an assessment of whether any could be described as frequent exacerbators. Most studies provided no information. One RCT92 stated that all patients had had previous exacerbations and three non-randomised controlled studies in stable populations89,93,94 specified at least one previous admission because of severe exacerbation.
Most RCTs (13 out of 18) provided details on assessing patients for obstructive sleep apnoea, for the purpose of ruling out patients with co-existing disorders (overlap syndrome); there were no details for five. 76,78,81,84,92 Only four out of nine89,93–95 non-randomised controlled studies provided clear details.
Details of the study and population characteristics of the RCTs can be found in Table 4 and for the non-randomised studies in Table 5.
| Study | Total n (n, % male) | Length of follow-up | GOLD stage (or other description of severity) | History of exacerbations | Stable or post-hospital/post-exacerbation population | Hypercapnia | Mean age (years) (SD) | Proportion smokers | BMI, mean (SD) | Overlap syndrome ruled out? |
|---|---|---|---|---|---|---|---|---|---|---|
| Bhatt et al. 201383 | 30 (20/27, 74%) | 6 months | FEV1/FVC < 0.70. Based on mean (SD) FEV1 % predicted NIV group 30.3 (7), usual-care group 29.6 (7.4), likely GOLD stage 3 or 4 for most patients | No details | Stable: no exacerbations in 4 weeks prior to study | Patients described as normocapnic. Inclusion criterion: PaCO2 < 52 mmHg (or < 6.93 kPa) | Median (IQR), NIV group 70 (66–73), usual-care group 68 (65–78) | Not stated. Could be active or ex-smokers (providing stable smoking status in last 6 months). Mean (SD), NIV group 59 pack-years (29 pack-years), usual-care group 61 pack-years (30 pack-years) | NIV group 24.8 kg/m2 (2.8 kg/m2), usual-care group 24.8 kg/m2 (4.8 kg/m2) | Yes. Only patients with a low clinical probability of having obstructive sleep apnoea as assessed using the Berlin Questionnaire were included |
| Casanova et al. 200084 | 52 (43/44, 98%) | 12 months | Based on inclusion criteria (FEV1/FVC < 70%; FEV1 < 45% predicted), GOLD stage 3 or 4 | No details | Stable: no acute exacerbation in previous 3 months | No details, but stated in the discussion that ‘The number of hypercapnic patients in our series was small’.84 Mean (SD), PaCO2 in NIV group 50.7 mmHg (7.9 mmHg) (or 6.76 kPa), usual-care group 53.2 mmHg (8.6 mmHg) (or 7.09 kPa) | NIV group 64 (5), usual-care group 68 (4) | No active smokers (smoking history of > 20 pack-years was an inclusion criterion) | NIV group 25 kg/m2 (4 kg/m2), usual-care group 25 kg/m2 (4 kg/m2) | Yes. To rule out the coexistence of obstructive sleep apnoea, patients were screened with a nocturnal respiratory polysomnography |
| Cheung et al. 201090 | 47 (43/47, 91%) | 12 months | Mean (SD) FEV1 % predicted NIV group 28.1 (8.5), usual-care group 31.3 (9.3), likely GOLD stage 3 or 4 for most patients | No details | Post-hospital: patients who were admitted with a severe exacerbation with persistent respiratory acidosis despite initial treatment with bronchodilators, corticosteroids and antibiotics and who required treatment with NIV. Those who survived after treatment with acute NIV were the target study population | Inclusion criterion: PaCO2 > 6 kPa | NIV group 69.5 (7.8), usual-care group 71 (7.7) | No active smokers. Mean (SD): NIV 48.7 pack-years (30.7 pack-years), usual care 53.1 pack-years (29.4 pack-years) | NIV group 19.2 kg/m2 (3.6 kg/m2), usual-care group 19.2 kg/m2 (3.6 kg/m2) | Yes. Polysomnography was performed in all eligible patients to exclude obstructive sleep apnoea |
| Clini et al. 200299 | 90 (69/86, 80%) | 24 months | Severe as defined by American Thoracic Society criteria. FEV1/FVC ratio < 60%. Mean (SD) FEV1 % predicted NIV group 27(8), usual-care group 31(11), likely GOLD stage 3 or 4 for most patients | No details | Stable clinical condition, as assessed by an arterial pH > 7.35, and free from exacerbation in the 4 weeks preceding recruitment | Inclusion criterion: PaCO2 > 6.6 kPa | NIV group 64 (7), usual-care group 66 (14) | No active smokers. Mean (SD), NIV 29 pack-years (6 pack-years), usual care 26 pack-years (5 pack-years) | NIV group 26 kg/m2 (5 kg/m2), usual-care group 25 kg/m2 (6 kg/m2) | Yes. Patients excluded if documented history of obstructive sleep apnoea syndrome as defined by an Apnoea–Hypopnoea Index > 10 episodes/hour – one during polysomnography |
| De Backer et al. 201191 | 15 (10/15, 67%) | 6 months | GOLD stage 3 or 4 | No details | Post-hospital: hospitalised because of a hypercapnic exacerbation | Inclusion criterion: PaCO2 > 45 mmHg (or 6.0 kPa) | All 65.6 (SD given as 6854?) | No active smokers, no details on pack-years | No details | Yes. Patients with obstructive sleep apnoea syndrome excluded (no details on assessment) |
| aDuiverman et al. 200879 | 72 (35/66, 53% first study period; 33/56, 59% second study period) | 3 months | GOLD stage 3 or 4 | No details | Stable clinical condition (no exacerbation in the 4 weeks prior to study participation together with a pH of > 7.35) | Inclusion criterion: PaCO2 > 6.0 kPa | NIV group 63 (10), usual-care group 61 (7) | No details. Median (IQR), NIV 42 pack-years (31–57 pack-years), usual care 43 pack-years (24–58 pack-years) | NIV group 27.1 kg/m2 (6.4 kg/m2), usual-care group 27.5 kg/m2 (6.3 kg/m2) | Yes. Apnoea–Hypopnoea Index ≥ 10 events/hour was an exclusion criterion |
| aDuiverman et al. 201180 | 72 (35/66, 53% first study period; 33/56, 59% second study period) | 24 months | GOLD stage 3 or 4 | No details | Stable clinical condition (no exacerbation in the 4 weeks prior to study participation together with a pH of > 7.35) | Inclusion criterion: PaCO2 > 6.0 kPa | NIV group 63 (10), usual-care group 61 (8) | NIV 5/24 (21%). Median (IQR) pack-years 42 (31–57). Usual care: 11/32 (34%). Median (IQR) 43 pack-years (24–58 pack-years) | NIV group 27.2 kg/m2 (5.1 kg/m2), usual-care group 27.0 kg/m2 (5.8 kg/m2) | Yes. Apnoea–Hypopnoea Index ≥ 10 events/hour was an exclusion criterion |
| Garrod et al. 200084 | 45 (28/45, 62%) | 3 months | No details on FEV1/FVC, FEV1 < 50% predicted indicative of GOLD stages 3 and 4 | No details | Stable severe COPD. Patients had no reported exacerbations in the past 4 weeks | Patients described as normocapnic. Mean (SD), NIV group PaCO2 44.2 mmHg (6.68 mmHg) (or 5.89 kPa) usual-care group 46.1 mmHg (9.07 mmHg) (or 6.15 kPa) | NIV group 63 (range 38–84), usual care 67 (range 55–79) | No details | No details | No details. (Polysomnography was performed on 6/45 patients for purposes of assessing sleep quality) |
| Gay et al. 1996100 | 13 (10/13, 77%) | 3 months | No details on FEV1/FVC, FEV1 < 40% predicted indicative of GOLD stages 3 and 4 | No details | Clinically stable, severe COPD. No major changes in FEV1, PaCO2, hospitalisation or change in medications over a 6-week period | Inclusion criterion: PaCO2 > 45 mmHg (or 6.0 kPa) | NIV group 71 (4.5), usual-care group 66.5 (9.1) | No details | NIV group 23 kg/m2 (4.5 kg/m2), usual-care group 26.5 kg/m2 (2.2 kg/m2) | Yes. Sleep-related breathing disorders were an exclusion criterion. Polysomnography performed to assess sleep quality, but no patient was later found to have obstructive or central apnoeas (no patient had more than 6 episodes of hypopnoea per hour) |
| Kaminski et al. 1999101 | 19 (16/19, 84%) | NIV mean 16 (SD 10) months, usual care mean 23 (SD 13) months | Advanced, stable, hypercapnic COPD. No details on FEV1/FVC, FEV1 < 50% predicted indicative of GOLD stages 3 and 4 | No details | Stable: exacerbation of COPD during last 3 months was an exclusion criterion | Inclusion criterion: PaCO2 > 50 mmHg (or 6.6 kPa) | All 60 (8) | No details | No details | Yes. Sleep apnoea excluded using polysomnography |
| Köhnlein et al. 201476 | 195 (121/195, 62%) | 12 months | Severe stable COPD, GOLD stage 4 | No details | Stable: no exacerbations in 4 weeks prior to study | Yes (PaCO2) of ≥ 7 kPa (51.9 mmHg) | NIV group 62.2 (8.6), usual-care group 64.4 (8.0) | No details | NIV group 24.8 kg/m2 (5.8 kg/m2), usual-care group 24.5 kg/m2 (5.8 kg/m2) | No details (not listed in exclusion criteria) |
| McEvoy et al. 200974 | 144 (94/144, 65%) | 12 months | GOLD stage 3 or 4 based on FEV1/FVC < 60%, FEV1 < 50% predicted | No details | Stable hypercapnic COPD | All described as hypercapnic. PaCO2 > 46 mmHg (or 6.13 kPa) at least twice in the previous 6 months during periods of clinical stability | NIV group 67.2 (65.3 to 69.1); usual-care group 68.8 (67.1 to 70.5) | No active smokers (inclusion criterion). No details on pack-years | NIV group 25.5 kg/m2 (24.3 to 26.7 kg/m2), usual-care group 25.4 kg/m2 (24.0 to 26.8 kg/m2) | Yes. Polysomnographic evidence of sleep apnoea (> 20 apnoeas plus hypopnoeas per hour of sleep) |
| Meecham-Jones et al. 199577 | 18 (15/18, 83%) | 3 months (crossover RCT) | No details on FEV1/FVC, FEV1 < 50% predicted indicative of GOLD stages 3 and 4 | No details | Stable clinical state for at least 1 month prior to entry into the study, with no recent deterioration in clinical state, spirometric values or resting blood gases | Inclusion criterion: PaCO2 > 45 mmHg (or 6.0 kPa) | Median (range), all 69 (43–74) | No details | All 25.3 kg/m2 (4.1 kg/m2) | Yes. Obstructive sleep apnoea an exclusion criterion (Sleepmaster computerised polysomnography system; patients with more than five apnoeic episodes per hour were excluded) |
| bMurphy et al. 201178 | 36 (no details) | 3 months (interim) | No details on FEV1/FVC. Mean (SD) FEV1 % predicted NIV group 31(7), usual-care group 22 (12) indicative of GOLD stages 3 and 4 | No details | Post-hospital: patients admitted for acute HRF due to an exacerbation of COPD with persistent hypercapnia (PaCO2 > 7 kPa) 2–4 weeks following resolution of the acute episode | PaCO2 > 7 kPa | NIV group 70 (10), usual-care group 68 (9) | No details | NIV group 21 kg/m2 (3 kg/m2), usual-care group 26 kg/m2 (6 kg/m2) | No details |
| Sin et al. 200782 | 23 (10/21, 48%) | 3 months | FEV1/FVC ratio < 70%. Inclusion criterion specified at least GOLD 2; mean FEV1 % predicted values imply patients more likely to be stage 3 or 4 | No details | Advanced stable COPD (no further details) | Based on mean PaCO2, NIV 43.1 mmHg (4.9 mmHg) (or 5.7 kPa), usual care 45.2 mmHg (13.5 mmHg) (or 6.0 kPa), a proportion of patients with hypercapnia | NIV group 64.1 (10.6), usual-care group 66.6 (9.7) | No details. Inclusion criterion: ≥ 10-pack-year history of cigarette smoking | NIV group 28.2 kg/m2 (7.2 kg/m2), usual-care group 26.2 kg/m2 (6.4 kg/m2) | Yes. Apnoea–Hypopnea Index ≥ 20 events/hour on a home-based sleep apnoea test (Embletta PDS; Medcare; Reyjkavik, Iceland) |
| Struik et al. 201475 | 201 (83/201, 41%) | 12 months | Patients with prolonged hypercapnia after ventilatory support, GOLD stages 3 and 4 | No details | Post-hospital: patients included after episode of acute respiratory failure | Yes (PaCO2 > 6.0 kPa) | NIV group 63.9 (8.6), usual-care group 63.5 (7.9) | No details | NIV group 24.6 kg/m2 (5.4 kg/m2), usual-care group 24.7 kg/m2 (5.5 kg/m2) | Obstructive sleep apnoea an exclusion criterion (Apnoea–Hypopnoea Index > 15 events/hour) |
| Strumpf et al. 199134 | 19 (19/23, 83%) | 3 months (crossover RCT) | FEV1/FVC ratio of < 0.75 | No details | Severe, stable COPD. No exacerbation of airway disease within the previous month | Mean PaCO2 49 mmHg (2 mmHg), range 35–67 mmHg. (range 4.7 to 8.9 kPa). Likely to include a proportion of patients with hypercapnia | All mean (SE), 66 (1) (range 57–76) | No details | No details | Yes. Obstructive sleep apnoea ruled out through polysomnography |
| Xiang et al. 200792 | 40 (31/40, 77%) | 24 months | FEV1/FVC< 70%, FEV1 % predicted < 30% or < 50%, consistent with GOLD stage 3 or 4 | All patients had previous exacerbations (other than the one immediately preceding the study) | Post-hospital: after discharge from hospital. All admitted with acute exacerbation and type II respiratory failure. Discharged once stable | Inclusion criterion: PaCO2 ≥ 55 mmHg (or 7.33 kPa) | NIV group 71 (9), usual-care group 69 (10) | No details | No details | No details (not listed as an exclusion criterion) |
| Zhou et al. 200881 | 36 (29/36, 81%) | 12 months | No description of severity. No details on FEV1/FVC or FEV1 % predicted | No details | Stable: no exacerbations within the last month | Baseline PaCO2 NIV 57.42 mmHg (7.64) (or 7.6 kPa), usual care 56.89 mmHg (8.26 mmHg) (or 7.6 kPa). Likely to include proportion of patients with hypercapnia | NIV group 72.81(4.16), usual-care group 69.76 (6.83) | No details | No details | No details (not listed as an exclusion criterion) |
| Study | Total n (n, % male) | Study type | Length of follow-up | GOLD stage (or other description of severity) | History of exacerbations/frequent exacerbators | Stable or post-hospital/post-exacerbation population | Hypercapnia | Mean age (years), (SD) | % smokers | BMI | Overlap syndrome ruled out? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Budweiser et al. 200796 | 140 (91/140, 65%) | Prospective controlled | Mean (SD), NIV group 19.8 months (12.9 months), usual-care group 12.9 months (9.9 months) | GOLD stage 4. FEV1/FVC < 70% and FEV1 < 50% | No details | Both stable and post-hospital patients: patients with immediately preceding exacerbation eligible for inclusion (proportion of patients not stated) | Inclusion criterion: PaCO2 ≥ 50 mmHg (or 6.6 kPa) | NIV group 64.2 (8.4), usual-care group 66.6 (8.6) | NIV group 17/99 (17%), mean pack-years (SD) 24.8 (27.5); usual-care group 11/41 (27%), mean pack-years (SD) 31.5 (29.2) | NIV group 25.4 kg/m2 (6.6 kg/m2), usual-care group 23.5 kg/m2 (6.5 kg/m2) | No details |
| Clini et al. 199893 | 49 (36/49, 73%) | Prospective controlled | Mean (SD), 35 months (7 months) | Severe as defined by American Thoracic Society criteria. Based on mean FEV1/FVC and FEV1 % predicted, likely to be GOLD stages 3 and 4 | At least one ICU admission because of severe exacerbation in the 2 years preceding the study | Stable clinical state i.e. stability in blood gas values and pH (> 7.35), and lack of exacerbation in the preceding 4 weeks | Inclusion criterion: PaCO2 > 6 kPa | NIV group 66 (6), usual-care group 66 (8) | No active smokers. States that previous smoking habit did not differ between the groups | NIV group 23 kg/m2 (4 kg/m2), usual-care group 23 kg/m2 (1 kg/m2) | Yes. Patients excluded on suspicion of sleep apnoea as assessed by nocturnal monitoring of arterial oxygen saturation |
| Clini et al. 199694 | 34 (21/34, 62%) | Prospective controlled | 18 months | Severe as defined by American Thoracic Society criteria. Based on mean FEV1/FVC and FEV1 % predicted, likely to be GOLD stages 3 and 4 | At least one hospital admission because of severe exacerbation in the preceding 18 months | Stable: non-invasive mechanical ventilation was initiated during a preliminary hospital trial when patients were in a stable state | Inclusion criterion: PaCO2 > 6.7 kPa | NIV group 62 (5), usual-care group 67 (7) | No details | No details | Yes. Patients excluded on suspicion of sleep apnoea as assessed by arterial saturation monitoring |
| Heinemann et al. 201197 | 82 (59/82, 72%) | Retrospective analysis of data (controlled) | 12 months | Based on mean FEV1 % predicted [NIV group mean (SD) 32.3 (10.1), usual-care group mean (SD) 43.4 (13.2)] and FEV1/FVC % [NIV group mean (SD) 53.1 (15.1), usual-care group mean (SD) 63.5 (21.4)], patients likely to be GOLD stage 3 and 4. All required prolonged weaning | No details | Post-hospital: patients with severe COPD who required prolonged weaning from invasive mechanical ventilation because of acute exacerbation, pneumonia or post-operative respiratory failure | Inclusion criterion: PaCO2 > 52.5 mmHg (or 6.9 kPa) for those receiving NIV | NIV group 64.6 (10.8), usual-care group 72.8 (8.6) | No details | NIV group 26 kg/m2 (5.9 kg/m2), usual-care group 23.7 kg/m2 (5.5 kg/m2). Based on 64/82 | No details (not listed as an exclusion criterion) |
| Laier-Groeneveld and Criee 199588 | 100 (no details on per cent male) | Retrospective analysis of data (controlled) | Up to 4 years | No details | No details | No details | Hypercapnia a prerequisite for treatment with NIV (no cut-off point stated) | No details | No details | No details | Unclear. Mixed population including those with obstructive sleep apnoea; results for COPD patients presented separately |
| Lu et al. 201298 | 44 (31/44, 70%) | Retrospective analysis of data (controlled) | 6 months | FEV1/FVC < 70%, FEV1 predicted < 50%, consistent with GOLD stages 3 and 4 | No details | Post-hospital: patients who were discharged once they were stable following hospitalisation | Inclusion criterion: PaCO2 ≥ 55 mmHg (or 7.33 kPa) | NIV group 72 (10), usual-care group 70 (9) | No details | No details | No details (not listed as an exclusion criterion) |
| Milane and Jonquet 198586 | 66 (62/66, 94%) | Retrospective analysis of data (controlled) | Up to 10 years | Described as severe; no further details | No details | Post-hospital: patients hospitalised during 1973–1983 because of an exacerbation | ‘Blood gas measurements determined eligibility for NIV’.86 Mean (SD) PaCO2, NIV group 56.1 (5.3) mmHg (or 7.45 kPa), usual-care group 48 mmHg (6.6 mmHg) (or 6.4 kPa) | All group 66, median IQR (48–81) | No details | No details | No details (not listed as an exclusion criterion) |
| aPahnke et al. 199787 | 40 (no details on per cent male) | Retrospective analysis of data (controlled) | Up to 8 years | No details | No details | No details | No details | No details | No details | No details | No details |
| Paone et al. 201489 | 60 (31/60, 52%) | Prospective controlled with matching | 24 months | GOLD stages 3 and 4 | All admitted for acute exacerbation | Stable: patients enrolled 3 months after discharge from hospital (for exacerbation); free from exacerbations for at least 4 weeks | Yes (PaCO2 > 50 mmHg) (6.6 kPa) | NIV group 70 (64–73), usual-care group 71 (66–77) | 5% (3/60) | Exclusion criterion: body mass index > 40 kg/m2 | Exclusion criterion: history of obstructive sleep apnoea syndrome |
| Tsolaki et al. 200895 | 49 (31/46, 67%) | Prospective controlled | 12 months | FEV1 < 50% predicted and FEV1/FVC < 70% consistent with GOLD stages 3 and 4 | No details | Stable clinical state, as assessed by a pH > 7.35, and free from exacerbations for at least 4 weeks preceding recruitment | Inclusion criterion: PaCO2 > 50 mmHg (6.6 kPa) | NIV group 65.2 (8.9), usual-care group 68.9 (5.6) | No details. More than 20 pack-years (inclusion criterion) | NIV group 30.4 kg/m2 (5.7 kg/m2), usual-care group 27.8 kg/m2 (3.4 kg/m2) | Yes. Patients screened with screened with nocturnal polysomnography and excluded if they presented an Apnoea– Hypopnea Index ≥ 10 episodes/hour |
Intervention and comparator characteristics
A number of devices were used to administer NIV, reflecting preferences of different countries and changes in devices over time (see Appendix 6). Masks were nasal, oronasal or full-face, sometimes depending on patient choice.
Inspiratory (IPAP) and expiratory pressures (EPAP) were set according to target pressures, target volumes or target blood gases. IPAP settings were compared across studies to ascertain whether or not there were any differences in pressure (see Appendix 6). Pressure was inconsistently reported, for example as mean or median, at the start of the study or at discharge, or described as ‘highest tolerated pressure’, adjusted to patient, or at a level to achieve specific blood gas pressures. Not all studies gave numerical values. Given this lack of consistency and the absence of an agreed cut-off point, it was not possible to dichotomise studies according to high/low pressure (see Clinical effectiveness results: non-invasive ventilation versus non-invasive ventilation for RCTs comparing different NIV settings directly). RCTs with the highest IPAP (mean ≥ 20, where described) in the current set of studies were Duiverman et al. (in 200879 and 201180), Murphy et al. (2011)78 (interim results from the ongoing HOT-HMV study) and Köhnlein et al. (2014). 76 The non-randomised controlled studies with the highest pressures (mean ≥ 20 cm H2O, where described) were Budweiser et al. (2007)96 and Heinemann et al. (2011). 97
Patients in both treatment arms continued to receive usual care, which was normally standard medical therapy, including LTOT where required, to optimise symptom control (Table 6 shows proportion of patients on LTOT in the RCTs/controlled studies). In 10 studies most (> 90%) or all patients were on LTOT, a smaller or unknown proportion of patients were on LTOT in 11 studies, six studies gave no details and in one study only 4% were on LTOT. 84 It is likely that there were regional variations in what constitutes usual care.
| Study | NIV arm, n/N (%) | Usual-care arm, n/N (%) |
|---|---|---|
| RCTs | ||
| Bhatt et al. 201383 | No details | |
| Casanova et al. 200085 | 19/20 (95) | 22/24 (91.7) |
| Cheung et al. 201090 | Before admission 10/23 (43.5) | Before admission 11/24 (45.8) |
| Clini et al. 200299 | All (100) | All (100) |
| De Backer et al. 201191 | No details | |
| Duiverman et al. 200879 | 14/31 (45) | 16/35 (46) |
| Duiverman et al. 201180 | 14/24 (58) | 18/32 (56) |
| Garrod et al. 200084 | 2/45 (4, unclear which treatment arm) | |
| Gay et al. 1996100 | 4/7 (57); 24-hour oxygen use (four patients), nocturnal oxygen (two patients) | 6/6 (100); all on 24-hour oxygen |
| Kaminski et al. 1999101 | All (100) | All (100) |
| Köhnlein et al. 201476 | 67/102 (66) | 60/93 (65) |
| McEvoy et al. 200974 | All (100) | All (100) |
| Meecham-Jones et al. 199577 | All (100) | All (100) |
| Murphy et al. 201178 | No details | |
| Sin et al. 200782 | Unclear. For patients with arterial oxyhemoglobin saturation of < 90% despite nocturnal non-invasive (positive) mechanical ventilation, supplemental oxygen was entrained to maintain arterial oxyhaemoglobin saturation of ≥ 90% | |
| Struik et al. 201475 | 76/101 (75) | 78/100 (78) |
| Strumpf et al. 199134 | Only stated for 7/19 completers. 6/7 (86) completers on oxygen | |
| Xiang et al. 200792 | All (100) | All (100) |
| Zhou et al. 200881 | No details (likely to be at least some given random treatment allocation) | All (100) |
| Non-randomised controlled studies | ||
| Budweiser et al. 200796 | Prior to study 54/99 (54); upon discharge 94/99 (95) | Prior to study 23/41 (56); upon discharge 33/41 (81) |
| Clini et al. 199893 | All (100) | All (100) |
| Clini et al. 199694 | All (100) | All (100) |
| Heinemann et al. 201197 | Oxygen therapy prescribed according to guidelines – proportion on oxygen unclear | |
| Laier-Groeneveld and Criee 199588 | No details | |
| Lu et al. 201298 | All (100) | All (100) |
| Milane and Jonquet 198586 | No details | |
| Pahnke et al. 199787 | No details | |
| Paone et al. 201489 | All (100) | All (100) |
| Tsolaki et al. 200895 | Part of usual care ‘as indicated’ | |
Three studies had what could be considered to be more intensive usual care: the RCT by Duiverman et al. (200879 and 201180) started with patients who were in a 12-week (in-hospital or outpatient) multidisciplinary rehabilitation programme, followed by a long-term home-based rehabilitation programme (one or two sessions per week of physiotherapy at community practice); the patients in the study by Garrod et al. (2000)84 were also in a pulmonary rehabilitation programme for part of the RCT; and the non-randomised controlled study by Clini et al. (1996)94 included as usual care a ‘home supervision programme’ [including physical, occupational and dietary information, a link service between hospital and community health service via telephone contact with general practitioners (GPs) and with patients, monthly physician visits to assess treatment and give further advice and the checking of equipment and decisions on hospitalisation]. It is unclear how additional usual care might influence the effect of NIV.
Study quality
Full quality assessment details can be found in Appendix 2. For the 18 RCTS, risk of bias regarding random sequence generation was low (10 studies) or unclear (eight studies); risk of bias regarding allocation concealment was also low (seven studies) or unclear (11 studies). Blinding of patients was not possible unless a ‘placebo NIV’ was used; this was the case in three studies. Cheung et al. (2010)90 carried out an open-label study which used CPAP in the non-NIV arm and stated that ‘care had been taken to avoid biasing the patients into believing either mode was superior’. Sin et al. (2007)82 also used CPAP as sham therapy, while Gay et al. (1996)100 used the same NIV equipment in the usual-care arm but ‘ventilated’ with lowest EPAP level and had no added IPAP or timed breaths.
There was little information overall on blinding of outcome assessors, with only three RCTs giving some detail (Clini et al. 2002,99 Sin et al. 200782 and Köhnlein et al. 201476). For more objective outcomes, such as survival and hospitalisations, a lack of blinding may be less important than for more subjective assessments such as QoL questionnaires. However, a lack of blinding may also inadvertently lead to performance bias, with a potential for NIV patients to receive more medical attention, which could in turn have an effect on any outcome.
A risk-of-bias rating for incomplete data/handling of missing data was derived as described in the methods (see Chapter 3, Assessment of risk of bias). As noted in the methods section, the cut-off points are arbitrary and a lack of reporting may in some cases be contributing to a high risk-of-bias rating. A high loss to follow-up for outcome assessments which require clinic attendance may to some extent be a result of the nature of severe COPD, with patients finding it difficult to travel. There were nine RCTs34,75–77,79–83,100 with a high risk of bias for incomplete data in the NIV and/or the usual-care arm (for at least one outcome). None of these contributed to the primary outcome meta-analyses. However, it was noted in the relevant results sections where RCTs had a high risk of bias for incomplete data.
Two34,77 of the 15 RCTs had a crossover design. Both appeared to use appropriate statistical methods, although the possibility of a carry-over effect was not explored for one. 77 Both were at high risk of bias for incomplete data, and it in some cases it was unclear during which treatment period patients dropped out.
No sensitivity analysis was performed on the basis of the quality assessment, as (1) a lack of reporting hampered classifying studies according to quality and (2) a lack of reporting is not necessarily an indication of poor study quality. Ideally, a risk-of-bias rating would have been generated for each outcome within each study. Where reported, a distinction has been made for blinding and incomplete outcome data for the different outcomes (see Appendix 2); however, this information was frequently not available. Overall, incomplete data are less likely to be an issue for mortality and hospitalisations/severe exacerbations, while outcome measurements that required patients to attend a clinic are more prone to missing data.
The non-randomised controlled studies were not given a risk-of-bias rating. Of the 10 controlled studies, five89,93–96 had a prospective design and five appeared to be retrospective analyses of data, although this was not always clearly described. One prospective study89 used a matched design, which may result in more similar groups at baseline and thus less biased results. Retrospective studies are more prone to bias, as outcome measurements and usual care cannot be retrospectively standardised for both groups. Most usual-care groups were made up of patients who were eligible for NIV, but could not adhere (e.g. because of mask intolerance) or did not want to continue; however, in three retrospective studies,86,88,97 levels of blood gases determined eligibility for NIV, and one retrospective study98 gave no details. All prospective studies89,93–96 and one of the retrospective studies98 gave details on similarity between NIV and usual-care groups in terms of baseline characteristics, and there appeared to be no major differences. There were no details in one retrospective study,87 and the other three may have differences in blood gas levels as these determined eligibility for NIV. Three studies86,96,97 report that NIV patients had more follow-up visits (at hospital or home) than usual-care patients, which may have impacted on the effectiveness of their usual care.
There were very few details on blinding and frequently incomplete details on losses to follow-up, whether or not intention-to-treat (ITT) analysis was performed and/or how many patients were contributing to results at different time points. Four of the five retrospective studies reported only survival, in which case loss to follow-up may be less of a problem in terms of accessing outcome data.
The potential impact of quality findings are discussed in the individual results sections; however, a formal sensitivity analysis based on quality was not undertaken for non-randomised studies (because of the small number of studies in meta-analyses and difficulties in ascertaining quality cut-off points).
Results from RCTs and non-randomised studies were not pooled because the inherent differences in susceptibility to bias.
Overview of outcomes
Table 7 shows the primary and secondary outcomes (as defined in this report, not by individual study authors) where data have been extracted and analysed. Where studies have reported hospitalisation as a result of an exacerbation, this has been included in the results only once (under hospitalisation) in order to avoid double-counting (e.g. Tsolaki et al. 200895). Where a study has reported severe exacerbations (Cheung et al. 201090), it has been assumed that patients will be hospitalised and this has also been counted as hospitalisation. Where exacerbations have been reported without any indication of severity, they have been presented separately.
| Study | Primary outcomes | Secondary outcomes | Included in meta-analyses |
|---|---|---|---|
| RCTs | |||
| Bhatt et al. 201383 | QoL, adherence | FEV1 % predicted, FVC % predicted, PaCO2, PaO2 | – |
| Casanova et al. 200085 | Survival, exacerbations, hospitalisations, adherence | FEV1 % predicted, FVC % predicted, PaCO2, PaO2 | Survival |
| Cheung et al. 201090 | Survival, exacerbations, hospitalisations, adherence | PaCO2 | Hospitalisations |
| Clini et al. 200299 | Survival, hospitalisations, QoL, adherence | FEV1 % predicted, PaCO2, PaO2, 6MWD | Survival, hospitalisations |
| De Backer et al. 201191 | Adherence | FEV1, PaCO2, 6MWD | – |
| Duiverman et al. 200879 | QoL | FEV1 % predicted, PaCO2, PaO2, 6MWD | – |
| Duiverman et al. 201180 | Exacerbations, hospitalisations, adherence | ||
| Garrod et al. 200084 | Exacerbations, QoL, adherence | FEV1 % predicted, FVC, PaCO2, PaO2 | – |
| Gay et al. 1996100 | Adherence | FEV1 % predicted, PaCO2, PaO2, 6MWD | – |
| Kaminski et al. 1999101 | Survival, hospitalisations, adherence | FEV1, FVC, PaCO2, PaO2, 6MWD | Survival, hospitalisations |
| Köhnlein et al. 201476 | Survival, hospitalisations, QoL, adherence | FEV1, FVC, PaCO2, PaO2, 6MWD | Survival, hospitalisations |
| McEvoy et al. 200974 | Survival, hospitalisations, QoL, adherence | FEV1 % predicted, FVC, PaCO2, PaO2 | Survival |
| Meecham-Jones et al. 199577 | QoL, adherence | FEV1, FVC, PaCO2, PaO2, 6MWD | – |
| Murphy et al. 201178 | Adherence | – | – |
| Sin et al. 200782 | Adherence | FEV1, PaCO2, 6MWD | – |
| Struik et al. 201475 | Survival, hospitalisations, exacerbations, QoL, adherence | FEV1, FVC, PaCO2, PaO2 | Survival, hospitalisations |
| Strumpf et al. 199134 | Hospitalisations, exacerbations, adherence | FEV1, FVC, PaCO2, PaO2 | – |
| Xiang et al. 200792 | Survival, hospitalisations | FEV1, FVC, PaCO2, PaO2, dyspnoea, 6MWD | Survival, hospitalisations |
| Zhou et al. 200881 | Survival, exacerbations, hospitalisations, adherence | PaCO2, PaO2, pH | Survival, hospitalisations |
| Non-randomised controlled studies | |||
| Budweiser et al. 200796 | Survival, adherence | – | Survival |
| Clini et al. 199893 | Survival, hospitalisations adherence | FEV1, FVC, PaCO2, PaO2, 6MWD | Survival, hospitalisations |
| Clini et al. 199694 | Survival, hospitalisations | PaCO2, PaO2 | Survival, hospitalisations |
| Heinemann et al. 201197 | Survival, adherence | – | Survival |
| Laier-Groeneveld and Criee 199588 | Survival | – | Survival |
| Lu et al. 201298 | Survival, hospitalisations adherence | FEV1, FVC, PaCO2, PaO2, 6MWD | Survival |
| Milane and Jonquet 198586 | Survival | – | Survival |
| Pahnke et al. 199787 | Survival, adherence | – | Survival |
| Paone et al. 201489 | Survival, hospital admissions | Blood and sputum inflammatory biomarkers | Survival |
| Tsolaki et al. 200895 | Survival, exacerbations, hospitalisations, QoL, adherence | PaCO2, PaO2 | Survival, hospitalisations |
There were only very limited data contained within RCTs or non-randomised controlled studies that linked exacerbation, hospitalisation, QoL and survival data (i.e. number of exacerbations leading to hospitalisation, number of exacerbations resulting in death, number of hospitalisations resulting in death or effect of exacerbation and/or hospitalisation on QoL). These outcomes have thus been considered separately. Uncontrolled studies were not searched for linked data.
Primary outcomes were less frequently measured than secondary outcomes (with the exception of adherence to NIV) in the prospective controlled studies, and only a proportion of results could be incorporated into meta-analyses (see Chapter 4, Survival and Hospitalisations individual results sections for more details). Most prospective studies (except two78,96) reported secondary outcomes (one or more of FEV1, FVC, PaO2, PaCO2 or 6MWD). Secondary outcome results from RCTs only are presented in forest plots (see Secondary outcomes). These outcomes were not measured/reported by the retrospective controlled studies.
Survival
Ten RCTs reported survival, seven in stable populations and three in post-hospital populations. All were meta-analysed or presented in a forest plot. All 10 non-randomised controlled studies reported survival, and all were presented in one or more forest plots. Follow-up times for all but one of the RCTs was between 6 and 24 months only; where follow-up was beyond 24 months (in one RCT and four controlled studies), the results have been presented in a separate plot. Relative risks (RRs) and hazard ratios (HRs) were taken directly from the published article where possible. Otherwise they were calculated as described in Chapter 3, Analysis (RR for all populations, HR for stable populations only). A footnote indicates where RR or HR was calculated from event rates reported in the published article.
Figure 3 shows the RR for mortality for all studies, subgrouped by RCTs/non-randomised studies and stable/post-hospital populations. Pooling was undertaken only within subgroups. There was no difference in mortality between stable populations treated or not treated with NIV, based on seven RCTs and four controlled studies. One study88 which reported no details on the population has been categorised separately; this also found no difference. There was also no difference in mortality for the post-hospital population based on three RCTs, but a statistically significant difference favouring NIV based on four non-randomised studies in post-hospital populations.
FIGURE 3.
Mortality (RR). CI, confidence interval. a, Calculated by authors of this report; b, controlled study with matching.
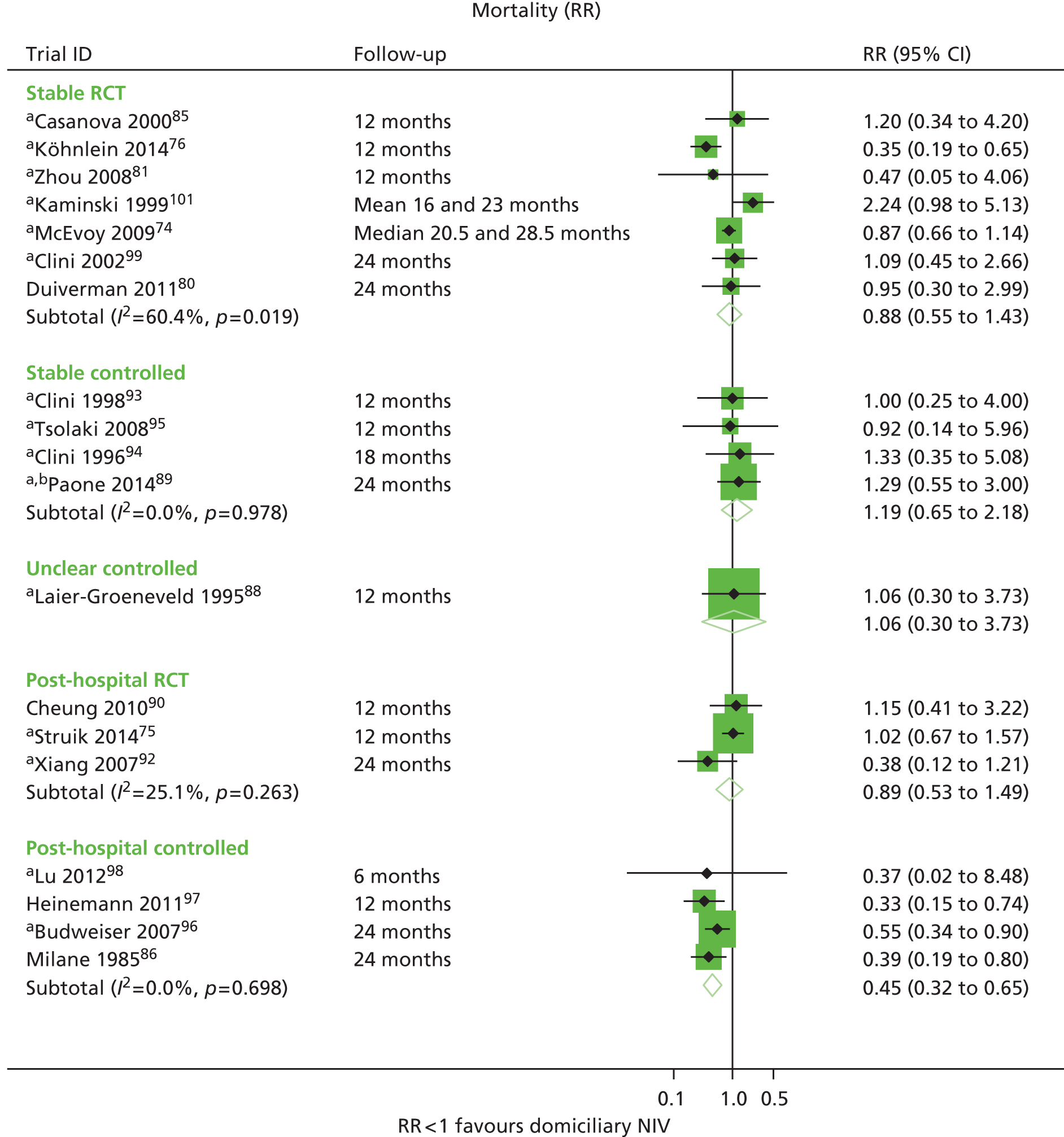
Between 57% and 100% of patients within the stable populations were on LTOT (unclear for NIV arm in Zhou et al. 200881); this was also the case for the three post-hospital studies. 75,92,98 One post-hospital study (Budweiser et al. 200796) included around 50% patients on LTOT at the start of the study; however, at discharge most were on LTOT. A subgroup analysis for patients with or without LTOT found no difference. Two studies86,97 gave no details on LTOT.
Figure 4 shows the HRs, again pooled by subgroup (RCT/controlled study, stable/post hospital). Unadjusted HRs showed no difference between treatment arms for the stable populations, while the adjusted HR from the McEvoy et al. 2009 RCT74 was significantly in favour of NIV (adjusted for those variables found to be confounders based on changing the HR by 10% or more when added to the model: PaCO2, PaO2 and SGRQ total score). It should be noted that the upper confidence limit for the Köhnlein et al. RCT76 differs slightly from that in the published article, as the standard error (SE) had to be approximated from the data given.
FIGURE 4.
Mortality (HR). CI, confidence interval. a, Calculated by authors of this report. b, Controlled study with matching.
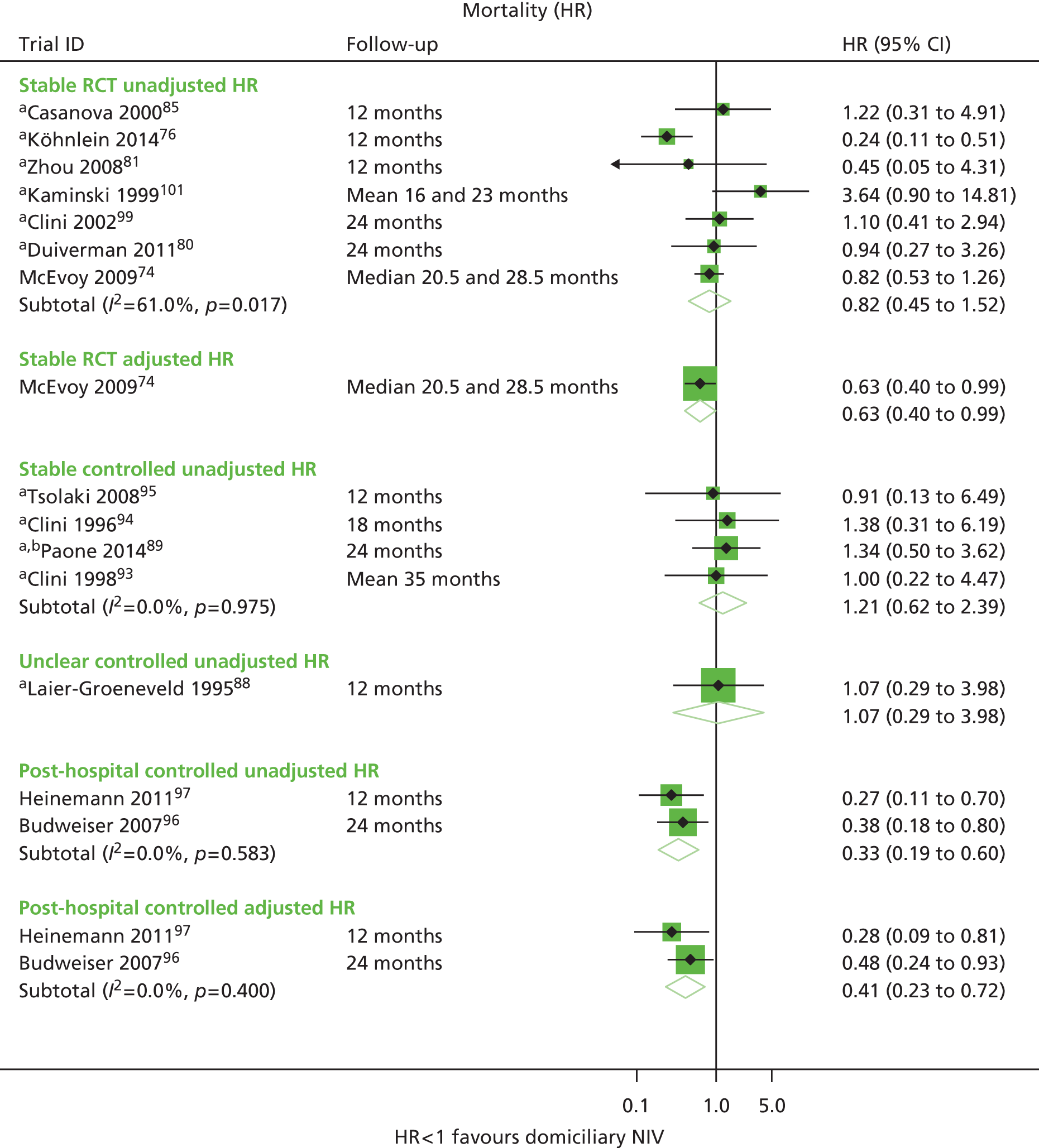
The adjusted and unadjusted HRs reported in two controlled studies in post-hospital populations were significantly in favour of NIV (adjusted for LTOT, haemoglobin, age and body mass index96 and for age, PaO2, haemoglobin and haematocrit at discharge97). However, these are observational studies and there is no way of knowing whether or not the estimated HRs are biased because of unmeasured or inappropriate adjustment for confounders.
Of the above studies, those with the highest IPAP were by Budweiser et al. (2007)96 and Heinemann (2011),97 both non-randomised studies. The fact that these studies showed a significant difference in favour of NIV may be a result of the higher pressure and/or of a greater benefit from NIV in a post-hospital population. Patients in both studies had more frequent evaluations in the NIV arm, and there may be additional confounders which have not been adjusted for.
Figure 5 shows mortality at longer follow-up times (> 2 years). Data are not pooled as multiple time points are presented. One RCT in a post-hospital population found no significant difference. 75 Three controlled studies, two with no details on the population and one in a stable population,93 also found no difference. The one post-hospital study86 showed a statistically significant benefit from NIV at 2 and 5 years, with less of a benefit at 10 years (although this was still statistically significant). It is conceivable that any potential benefit from NIV would not be sustained for the duration of a patient’s life given the progressive worsening of COPD. There were no details on IPAP settings for this study. The schedule of NIV was also unusual in this study and is not consistent with all other studies, as it was used intermittently for 15 minutes every hour, up to a minimum of 4 hours. For all studies an assumption has been made that the survival rates are based on no loss to follow-up; patient numbers have been calculated from percentages and rounding errors may have occurred. Given that the population details were unclear in two studies, it was difficult to draw any conclusions. It could be speculated that if the study had specifically included a post-hospital population this may have been highlighted.
FIGURE 5.
Mortality (RR) longer follow-up. CI, confidence interval. a, Calculated by authors of this report.
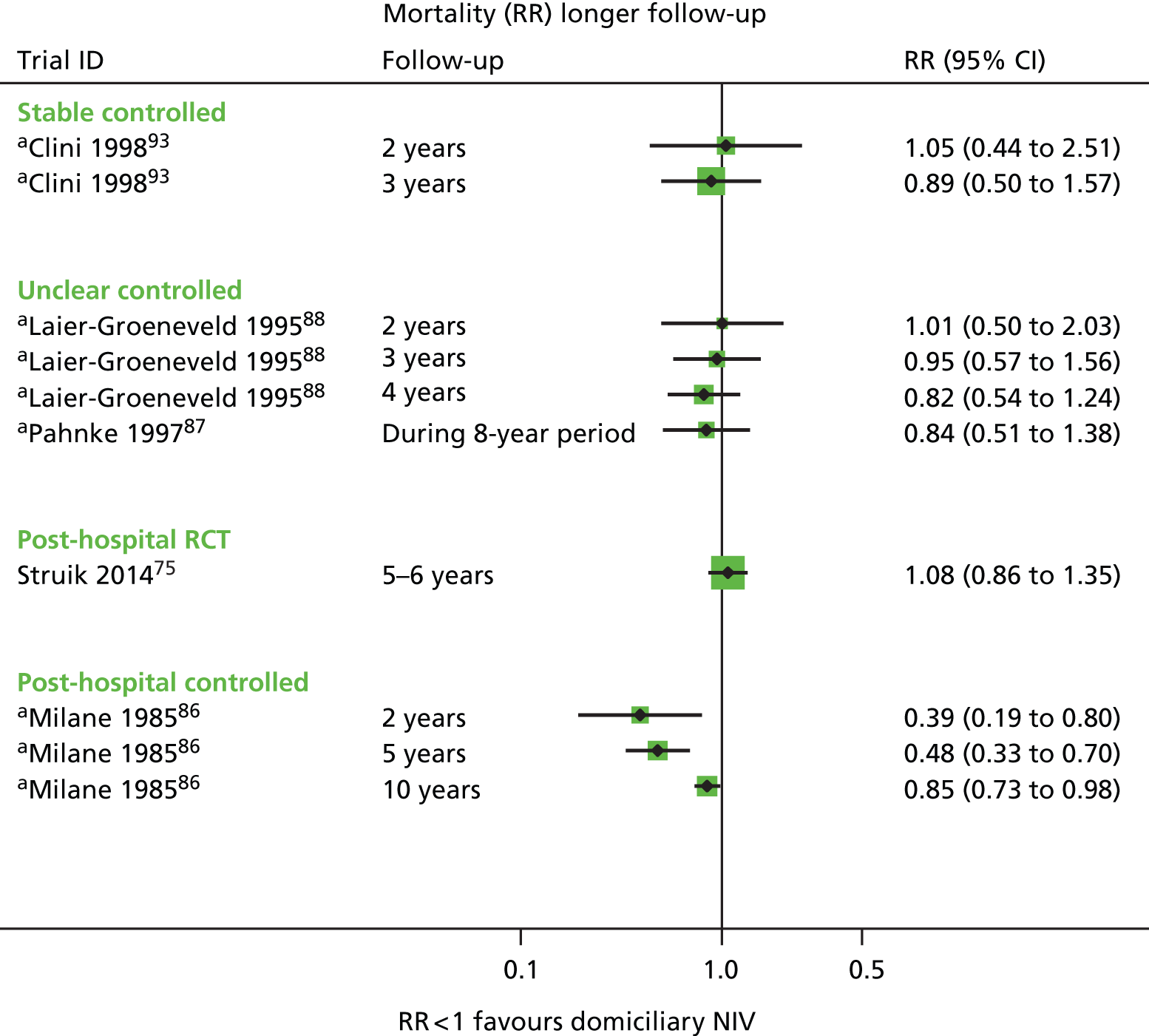
Overall, the results on mortality (RR, unadjusted HR) suggest that, on average, there is no benefit from starting NIV in a stable COPD population, and this is consistent for RCTs and non-randomised controlled studies (n = 770 in total). This is based on a fairly limited follow-up period for mortality (up to 24 months for RCTs), and it is possible that small differences might not manifest themselves within this time frame. One exception is the adjusted HR (McEvoy et al. 200974), which shows a statistically significant benefit for NIV.
The results from three RCTs in a post-hospital population also show that, on average, there is no benefit to home NIV. One further RCT (Xiang et al. 2007,92 n = 40; not represented in the forest plots) also found no statistically significant difference. This is in contrast to the non-randomised controlled studies in this population, which show a statistically significant pooled result favouring NIV. Follow-up in the RCTs was 1290 and 2492 months, arguably not long enough to measure mortality as an outcome. The sample size was small in two RCTs (40 patients in each) and larger in one RCT (Struik et al. 2014,75 n = 201).
Further subgroup analyses (e.g. by level of hypercapnia or history of exacerbations) were not possible given the small number of studies and the inconsistent reporting of the relevant variables. Based on the inclusion criteria (CO2 threshold) and description of populations, most studies appeared to be on hypercapnic patients, with the exception of that by Casanova et al. 2000,85 who stated that ‘The number of hypercapnic patients in our series was small’. Performing subgroup analysis based on reported mean baseline CO2 values would have meant dichotomising trials based on an arbitrary threshold, and this was not considered appropriate. However, in a separate analysis, CO2 levels at baseline and change in CO2 levels were plotted against mortality, in order to determine if baseline CO2 can predict response to NIV and whether or not the effect of NIV on CO2 correlates with the effect on mortality (see Figure 42 and Figure 44, Appendix 7). There was no discernible relationship either between CO2 levels at baseline and mortality or between change in CO2 levels and mortality. This was an exploratory post-hoc analysis and subject to a number of limitations; as such the findings should be considered to be speculative only and should be interpreted with caution (see Appendix 7 for caveats).
Sensitivity analyses by study quality were also not performed, again because of the small number of studies, and the lack of reporting of details on quality. Only one of the RCTs80 was flagged as having a high risk of bias regarding incomplete data. Only one RCT90 used a form of sham NIV, so patients were not blinded in most studies. There may also be a risk of performance bias if the use of NIV is associated with additional check-ups or follow-up visits. Assessment of potential publication or other bias through funnel plots was not undertaken given that there was a maximum of seven studies in any given meta-analysis.
Non-randomised studies are more prone to overestimating effect size; this does not seem to be the case here for stable populations, as the results of RCTs and non-randomised controlled studies are consistent. The non-randomised studies in the post-hospital population do show a benefit of NIV, in contrast to the pooled result from RCTs in this population (which shows no difference).
The potential for selection bias was explored for the non-randomised studies. NIV and usual-care arms appeared to be similar for the stable populations (see Appendix 2 for full details), whereas Laier-Groeneveld and Criee (1995)88 (population unclear) reported that patients in the usual-care group were normocapnic and those in the NIV group were hypercapnic. For the post-hospital populations, baseline characteristics also appeared to be similar in two studies,96,98 although one98 gave no detail on how groups were selected. In the other two studies,86,97 blood gas levels determined the eligibility for NIV; that is, only patients with poorer blood gas levels/lower level of hypercapnia received NIV.
Differences in usual care were also explored. In the studies by Budweiser et al. (2007),96 Heinemann et al. (2011)97 and Milane and Jonquet (1985)86 (all post-hospital populations), patients in the NIV groups received more frequent hospital evaluations or home visits, which may have led to better usual care. These studies found a statistically significant benefit for NIV.
Uncontrolled observational studies were explored in order to determine whether there were additional useful data from larger and/or longer-term studies (see Appendix 8 for further details). There were two larger prospective studies102,103 following stable patients on NIV. Survival rates in one103 of these were similar to those reported in the NIV arm of Laier-Groeneveld and Criee (1995),88 while the other102 reported slightly lower survival rates. An analysis of patient survival depending on haemoglobin level (Kollert et al. 2013104) reported survival rates for normocythaemic patients (n = 207) of 72%, 50%, 47% and 18% respectively at 2, 4, 5 and 10 years (approximate, estimated from graph); these were similar at 2 years and slightly lower at 4 years compared with Laier-Groeneveld and Criee (1995),88 although a comparison is difficult as nothing is known about the haemoglobin status in the controlled studies included in this report. One small study105 followed both stable (n = 16) and post-hospital (n = 31) patients on NIV. This found significantly poorer median survival in post-hospital patients compared with stable patients, which is consistent with the population differences observed in the controlled studies.
Hospitalisations
Hospitalisation data were reported in a number of ways, for example the number of hospitalisations (general or ICU) per patient per year, mean number of days in hospital, proportion of patients affected by hospitalisation, time to first readmission, etc. Only the first two outcomes are represented in Figures 6 and 7, based on eight RCTs (five76,80,81,99,101 in stable and two90,92 in post-hospital populations) and three controlled studies93–95 (all stable populations). Severe exacerbations have been assumed to lead to hospitalisation and have been included in the hospitalisation analysis. The hospital admissions and days in hospital were all presented per patient per year, except for the Clini et al. (1996)94 study, which reported hospitalisation rates over a period of 18 months; the results have been adjusted to 1 year (which assumes a constant rate). Additional hospitalisation data not represented in the forest plots are shown in Table 8.
| Study | Design | Length of follow-up (months) | Outcome | Results | Direction of effect |
|---|---|---|---|---|---|
| Stable population | |||||
| Casanova et al. 200085 | RCT | 12 | Proportion of patients affected by hospital admissions | At 3 months: NIV group, approximately 4%; usual-care group, approximately 15%. At 12 months: NIV group, approximately 19%; Usual-care group, approximately 18% | Significantly in favour of NIV at 3 months, no significant difference at 12 months. Results approximate as read off graph; no CIs |
| Duiverman et al. 201180 | RCT | 24 | Hospitalisation frequency (per year) | NIV group, between 0 and 2 per year; usual-care group, between 0 and 2 per year | No significant difference |
| 24 | Hospitalisation days (per year) | Not stated | No significant difference | ||
| McEvoy et al. 200974 | RCT | Median 28.5 (NIV group) and 20.5 (usual-care group) | Hospitalisation rates (days in hospital/days on trial across all patients) | NIV group, 0.032 days; usual-care group, 0.031 days; rate ratio 0.96 days (95% CI 0.9 to 1.02 days) | No significant difference |
| Strumpf et al. 199134 | Crossover RCT | 3 | Number of hospitalisations | One patient required two brief hospitalisations because of exacerbations, once during the NIV period and once during the usual-care period | No significant difference |
| Clini et al. 199694 | Controlled | 18 | Percentage time spent in hospital | NIV group, approximately 6%; usual-care group, approximately 3% | No details on statistical significance between groups. Results approximate as read off graph; no CIs |
| Paone et al. 201489 | Controlled (matched) | 24 | Number of hospitalisations | NIV group, median 1 hospitalisation (range 0–1.3 hospitalisations); usual-care group, median 2.5 hospitalisations (range 1.0–4.0 hospitalisations) | Significant difference in favour of NIV |
| Post-hospital population | |||||
| Cheung et al. 201090 | RCT | 12 | Time to first readmission because of any COPD exacerbation | NIV group, 71 days; usual-care group, 56 days | No statistically significant difference |
| Struik et al. 201475 | RCT | 12 | Median (range) number of readmissions during 12 months | NIV group, median 1.0 (range 0–9); usual-care group, 1.0 (range 0–6) | No statistically significant difference (p = 0.23) |
| Percentage | NIV group, 56%; usual-care group, 57% | No further details | |||
| Hospital admissions [median, (range) number of total days spent in hospital] | NIV group, median 7 days (range 0–107 days); usual-care group, median 3.5 days (range 0–77 days) | No statistically significant difference (p = 0.087) | |||
| Lu et al. 201298 | Controlled | 6 | Hospital admission because of exacerbation, n/N | NIV group, 0/22, usual-care group, 2/24 | Unclear if this relates to ALL hospitalisations. No details on statistical significance between groups |
For stable populations, there appeared to be a non-significant trend towards fewer hospital admissions with NIV (based on five RCTs) and ICU admissions (based on one RCT and two controlled studies); no difference in hospital admissions was found based on two controlled studies (Figure 6).
FIGURE 6.
Hospital admissions per patient per year. CI, confidence interval; WMD, weighted mean difference. a, Calculated by authors if this report; b, individual mean differences (95% confidence interval) presented for this outcome.
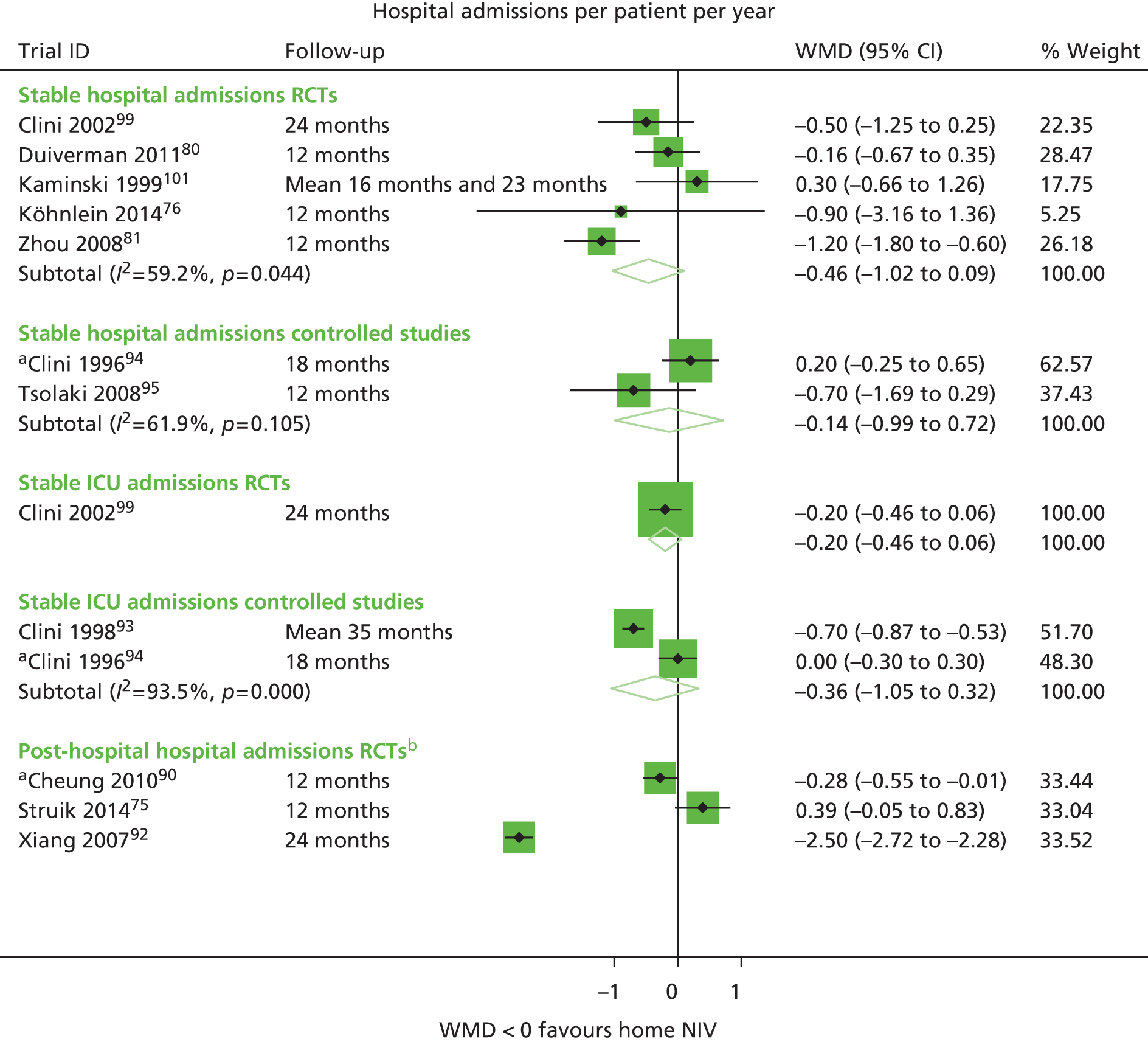
Note that the forest plot does not include results from all RCTs; McEvoy et al. (2009),74 who carried out a fairly large RCT (n = 144), and Casanova et al. (2000),85 who studied 52 patients, reported very similar hospitalisation rates74 and proportion of patients affected by hospitalisations85 in the NIV and usual-care groups. Had it been possible to incorporate these results, the overall pooled estimate may have shifted more towards an equivocal effect (see Table 8 for additional results). It should be noted that the Köhnlein et al. 76 study in 2014 is given a relatively small weighting in the meta-analysis, despite it being a larger study (n = 195); this is because the standard deviation (SD) (and thus the SE) was much higher. It could be argued that hospital admissions data are by their nature skewed (with some patients having repeated admissions and one admission making a further one more likely) and that the median may be a better metric to compare groups. However, all but two of the studies in the meta-analysis reported the mean in their publications; the authors of two studies75,80 reported the median but provided the mean (SD) on request.
For the post-hospital population, the results from the three RCTs give disparate results (significant difference in favour of NIV92, non-significant difference in favour of NIV90 and non-significant difference on favour of usual care75). The results have therefore not been pooled. Note that the (larger) study by Struik et al. 75 in 2014 has a similar weight in the meta-analysis compared with the smaller studies; this is because the SD (and thus the SE) was much higher. The study by Cheung et al. (2010)90 randomised patients to NIV or CPAP and, while this was an open-label study, the authors stressed that ‘care had been taken to avoid biasing the patients into believing either mode was superior’. There was no sham NIV in the studies by Xiang et al. (2007)92 and Struik et al. (2014),75 and arguably the results from the Cheung et al. 90 study in 2010 could be considered more robust. However, it is likely that there are additional clinical or methodological differences between the studies.
Days in hospital (per patient per year) were reported, only for stable populations, in one RCT and three controlled studies (Figure 7). There was no significant difference, although a very slight trend towards benefit with NIV. Again, this is not based on the totality of the evidence and firm conclusions cannot be drawn.
FIGURE 7.
Days in hospital per patient per year. CI, confidence interval; WMD, weighted mean difference
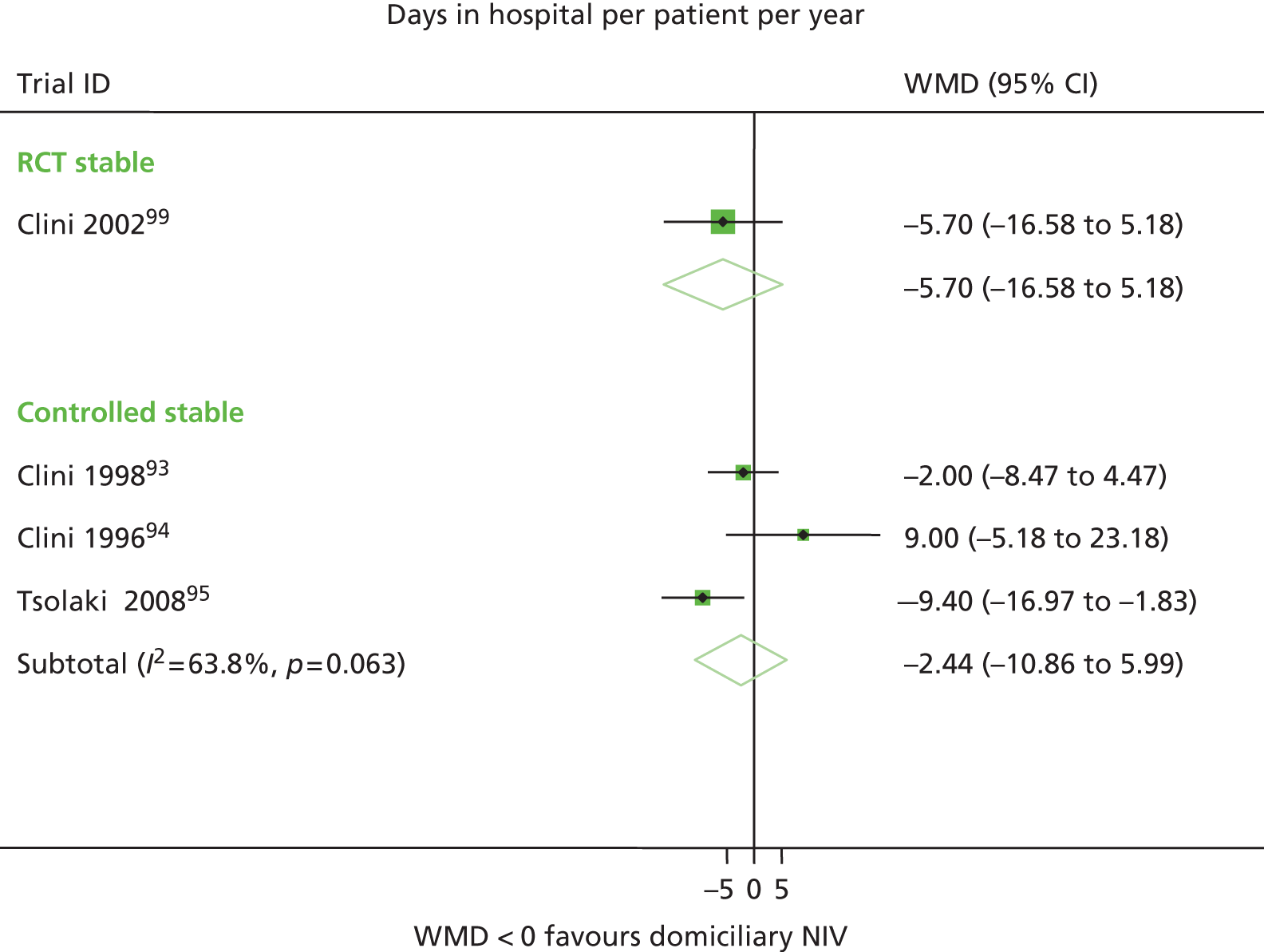
Additional data on hospitalisation are reported in Table 8 (note that some studies have used more than one outcome measure and are represented in both Figure 6 and Table 8). There were no significant differences between NIV and usual care based on three RCTs (and one small crossover RCT) and one controlled study (stable population), with the exception of proportion of patients affected by hospitalisations at 3 months in one study (Casanova et al. 200085). One matched controlled study89 found a significant difference in favour of NIV at 24 months.
Three RCTs in post-hospital populations also found no significant differences, although there appeared to be a trend for a longer time to first readmission due to any COPD exacerbation with NIV (Cheung et al. 201090).
Based on the meta-analyses, there appeared to be an overall trend towards fewer hospitalisations with NIV (for a stable population); however, this is not based on the totality of the evidence. For a post-hospital population, the results are quite disparate and likely to be because of clinical and/or methodological heterogeneity or, particularly in the case of the two smaller studies (n = 40 and n = 47), chance. Arguably, the Struik et al. (2014)75 study, which suggests no benefit from NIV in this population, could be given more consideration, as it is based on a much larger sample (n = 201). Looking at all the evidence, no firm conclusions could be drawn as to whether or not stable or post-hospital patients are likely to benefit from NIV in terms of fewer hospital admissions or days in hospital, although there may be patients with specific characteristics who would benefit.
None of the five included RCTs (stable population) had a sham NIV arm, so lack of blinding may be a source of bias. Sensitivity analyses around study quality were not undertaken because of the small number of trials and the lack of reporting of some quality criteria. Similarly, funnel plots were not constructed to assess potential for publication or other bias, as there were a maximum of five studies in any given meta-analysis.
Further subgroup analyses (e.g. by level of hypercapnia, history of exacerbations, proportion on LTOT or IPAP) were not possible given the small number of studies and the inconsistent reporting of the relevant variables. As with mortality data, baseline CO2 levels and change in CO2 levels were plotted against mean difference in hospital admissions in order to determine whether baseline CO2 can predict response to NIV and whether or not the effect of NIV on CO2 correlates with the effect on admissions (see Figure 43 and Figure 45, Appendix 7). It was unclear whether there was a trend towards an association between higher mean CO2 levels at baseline and a greater mean difference in hospital admissions, but an apparent trend was observed for change in CO2 levels and admissions. Such a trend would indicate that a greater effect in terms of reducing CO2 levels correlates with a greater reduction in hospital admissions. However, as the analysis is using aggregate data for change in CO2 and also for mean difference in hospital admissions, a causal association cannot be inferred, even if there is potential biological plausibility. Further, this was an exploratory post-hoc analysis and subject to a number of limitations; as such, the findings should be considered speculative only and should be interpreted with caution (see Appendix 7 for caveats).
There were three small prospective uncontrolled observational studies, two106,107 in stable populations (n = 11 and n = 35) and one108 in a post-hospital population (n = 27) (see Appendix 8 for further details). The study in a post-hospital population did not report before and after (NIV) data and so did not add useful information. The studies in stable populations found a reduction in admissions/days in hospital over time with NIV (one pre–post NIV106 and one after 1 year compared with after 2 years with NIV107). This needs to be interpreted in the context of the small number of patients and the lack of a usual-care group, but might be consistent with the trend observed in some of the controlled studies.
Exacerbations
Severe exacerbations (assumed to be exacerbations leading to hospitalisation) are described previously (see Chapter 4, Hospitalisations). This section has attempted to identify additional exacerbations (mild or moderate) which do not lead to hospitalisation but could potentially incur a cost and/or have an impact on QoL (Table 9). However, this was hampered by a lack of reporting of severity. In some cases, exacerbations were not a predefined outcome but instead were listed as an adverse event (Garrod et al. 2000,84 Meecham-Jones et al. 199577). As it was unclear whether or not all exacerbations were reported (rather than only those in the context of not completing final assessment84 or in the context of cause of death77), the data from these two studies has not been further considered.
| Study | Design | Length of follow-up | Outcome | Results | Direction of effect | Indication of severity/comment |
|---|---|---|---|---|---|---|
| Stable population | ||||||
| Bhatt et al. 201383 | RCT | 6 months | Number of exacerbations | NIV group, 1/15; usual-care group 1/12 or 1/15 (unclear) | No difference. Not pre-defined outcome but all reported | No details on severity |
| Casanova et al. 200085 | RCT | 3 and 12 months | Percentage of patients affected by exacerbation | 3 months: NIV group, 52%; usual-care group, 56%. 12 months: NIV group, 66%; usual-care group, 69% | Slight trend favouring NIV, but no significant differences. Unclear if % relates to ITT population or completers | No details on severity |
| Duiverman et al. 201180 | RCT | 24 months | Number of exacerbations | Median of three exacerbations per year for both NIV and usual care | No significant difference. No other outcome statistics reported | No details on severity |
| Zhou et al. 200881 | RCT | 12 months | Exacerbations per patient per year | NIV group, mean 3.73 (SD 1.03); usual-care group, mean 4.86 (SD 1.71) | Significant difference in favour of NIV | No details on severity. This study also reports hospitalisations and it is likely that some of the exacerbations will have led to hospitalisation |
| Tsolaki et al. 200895 | Controlled | 12 months | Exacerbations per patient per year | NIV group, mean 1.4 (SD 2.1); usual-care group, mean 1.8 (SD 1.4) | No significant difference | This includes all exacerbations, including those leading to hospitalisations |
| Exacerbations per patient per year leading to hospitalisation | NIV group, mean 1.0 (SD 2.2); usual-care group, mean 1.7 (SD 1.3) | No significant difference | Exacerbations assumed to be severe, as resulted in hospitalisations (included in Chapter 4, Hospitalisations) | |||
| Post-exacerbation population | ||||||
| Cheung et al. 201090 | RCT | 12 months | Exacerbation without acute HRF, n/N | NIV group, 5/23; usual-care group, 4/24 | No details on statistical significance | Outcome listed as adverse event, not predefined outcome (but all reported) |
| Recurrent severe COPD exacerbation with acute HRF (primary outcome) | NIV group, 7/23; usual-care group: 14/24. HR 0.39 (95% CI 0.16 to 0.98) | Statistically significant difference favouring NIV | Severe, therefore assumed to result in hospitalisation (included in Chapter 4, Hospitalisations) | |||
| Struik et al. 201475 | RCT | 12 months | Annual number of exacerbations at home [median (range)] | NIV group, median 1 (range 0–9); usual-care group, median 2 (range 0–14) | No statistically significant difference (p = 0.26) | Exacerbation defined as an event in the natural course of the disease characterised by a change in the patient’s baseline dyspnoea, cough, and/or sputum that is beyond day to day variations, is acute in onset, and treated with antibiotics and/or prednisolone |
Pooling of exacerbations data was not possible, as a number of different outcome statistics were used; these could not be converted to make them more consistent.
There was little information on the frequency of exacerbations of differing severity (mild, moderate or severe). Cheung et al. (2010)90 noted exacerbations without acute HRF, which may be less severe, while Tsolaki et al. (2008)95 made a distinction between all exacerbations and those leading to hospitalisation. Struik et al. (2014)75 reported exacerbations occurring at home.
Overall there were no significant differences in exacerbations between NIV and usual-care arms, based on three RCTs and one controlled study in stable populations and two RCTs in a post-hospital population. One exception was the study by Zhou et al. (2008)81 (n = 36, stable population), which reported significantly fewer exacerbations with NIV (at 12 months). Subgroup analyses were not possible. IPAP was highest in the study by Duiverman et al. (2011)79 (mean 23 at start of study), and between 12 and 20 in the other studies (where reported). The study by Duiverman et al. (2011)79 also had a more intensive usual-care arm. The study by Bhatt et al. (2013)83 differed from the others in that it included normocapnic patients. Around half of80,90 or most85 patients were on LTOT, with no details in two studies. 83,95
Given the sparsity of information, the uncontrolled studies were also considered. Only three studies (no restriction on sample size or length of follow-up) reported exacerbations. Neither the prospective uncontrolled study by Tsolaki107 in 2011 nor the retrospective study by Windisch109 in 2009, both in stable populations, made a distinction between different severities of exacerbation. The report of a further small (n = 20) Japanese study64 was not translated. It is possible that reporting of exacerbations was included within hospitalisation and/or adverse outcome data; however, this would have necessitated reading the full texts of all the uncontrolled studies, which was not undertaken.
Quality of life
Seven RCTs and one prospective controlled study (Tsolaki et al. 200895), all with stable populations, reported QoL using a variety of instruments (SF-36, SGRQ, SRI, the Chronic Respiratory Disease Questionnaire, the MRF-28 and the Profile of Mood States). Note that for the SGRQ, the MRF-28 and the Profile of Mood States, a lower score is indicative of better QoL (or more stable mood profile for Profile of Mood States). For the other instruments, a higher score is indicative of better QoL.
There was heterogeneity regarding the number of QoL instruments used (between one and three), time points of reporting QoL (between 3 and 24 months), and in the way results were reported, for example for subscales only rather than the total score or as a mean difference between final scores rather than a change score or adjusted score. Some studies did not report numerical data but only commented on statistical significance or only presented results on a graph. Further, in order to pool, an assumption would have had to have been made that all instruments were measuring QoL in the same way and were equally valid. For these reasons it was not considered feasible to pool any QoL data.
Table 10 presents the findings. Some studies used several QoL instruments, thus contributing to a greater proportion of the findings (e.g. Duiverman in 2008 and 2011,79,80 which also had a more intensive usual-care arm compared with the usual care employed in most studies). Note that, for the study by Köhnlein et al. (2014),76 results were available only for a small subgroup of patients.
| Study | Design | Length of follow-up | Time points for assessment | Results | Direction of effect |
|---|---|---|---|---|---|
| Stable populations | |||||
| SF-36 | |||||
| Köhnlein et al. 201476 | RCT | 3, 6, 9 and 12 months | 12 months | General health perception subscore only: mean 8.6 points (95% CI 1.8 to 13.3 points) greater improvement in the NIV group | No significant difference for summary score (results not reported). Significant difference in favour of NIV for general health perception subscore (p = 0.013). Results based on small subgroup of patients only |
| McEvoy et al. 200974 | RCT | Median 28.5 months (NIV group) and 20.5 months (usual-care group); up to 5 years | 12 months | Results presented separately for the eight subscales of SF-36. No summary scores | Statistically significant difference for 2/8 subscales (general health and mental health) favouring the usual-care group. No significant differences for other subscales |
| Tsolaki et al. 200895 | Prospective controlled | 12 months | 1, 3, 6, 9 and 12 months | Results for mental and physical summary scores | Statistically significant difference favouring NIV for mental and physical scores at 6, 9 and 12 months |
| SGRQ | |||||
| Clini et al. 200299 | RCT | 24 months | 24 months | Score changes: –5% in NIV group; –4% in usual-care group. (Increase in QoL in both arms) | No significant difference |
| Köhnlein et al. 201476 | RCT | 3, 6, 9 and 12 months | 12 months | Mean 6.2 points (95% CI 0.7 to 11.8 points) greater improvement in NIV group | Statistically significant difference in favour of NIV (p = 0.029), but results based on small subgroup of patients only |
| McEvoy et al. 200974 | RCT | Median 28.5 months (NIV group) and 20.5 months (usual-care group) months; up to 5 years | 12 months | No data reported | No significant difference |
| Clini et al. 200299 and McEvoy et al. 200974 | IPD data from both RCTsa | See McEvoy et al. 200974 and Clini et al. 200299 | 12 months | Mean difference of 0.9 points (95% CI –19.21 to 21.01 points) | No significant difference (small benefit in favour of usual-care arm) |
| Meecham-Jones et al. 199577 | RCT | 3 months | 3 months | Only individual results presented in graph; no summary data | Significant difference for symptom, activity and total score in favour of NIV; no significant difference for activity scale |
| SRI | |||||
| Duiverman et al. 2008 79 | RCT | 3 months | 3 months | NIV group, mean 60.1 points (SD 11 points); usual-care group mean 55.7 points (SD 15 points). between-group difference adjusted for baseline: mean 3.1 points (95% CI –2 to 8.2 points) | Trend for better QoL in NIV group but not statistically significant |
| Duiverman et al. 201180 | RCT | 24 months | 6, 12, 18, 24 months | 6 months: NIV group, mean 59.5 points (SD 14.4 points); usual-care group, mean 55.6 points (SD 915.2 points). 12 months: NIV group, mean 60.5 points (SD 10.9 points); usual-care group, mean 55.8 points (SD 13.4 points). 18 months: NIV group, mean 56.8 points (SD 12.7 points); usual-care group, mean 54.4 points (SD 11.8 points). 24 months: adjusted difference in change 2.9 points (95% CI –1.9 to 7.8 points) | Trend for better QoL in NIV group at all time points but not statistically significant |
| Köhnlein et al. 201476 | RCT | 3, 6, 9 and 12 months | 12 months | Mean 5.6 points (95% CI 0.1 to 11.1 points) greater improvement in NIV group | Statistically significant difference in favour of NIV (p = 0.0445), but results based on small subgroup of patients only |
| Chronic Respiratory Disease Questionnaire | |||||
| Bhatt et al. 201383 | RCT | 6 months | 6 weeks, 3 months, 6 months | No total score given, only for subscales at 6 weeks, 3 months and 6 months | Significant difference at 6 months for mastery subscore in favour of NIV, but no significant difference for other three subscales. No significant improvement in total score |
| Duiverman et al. 200879 | RCT | 3 months | 3 months | NIV group, mean 96.8 points (SD 15 points), usual care mean 87.9 points (SD 20 points). Mean between-group difference adjusted for baseline: mean 7.5 points (95% CI –1 to 16 points) | Trend for better QoL in NIV group but not statistically significant |
| Duiverman et al. 201180 | RCT | 24 months | 6, 12, 18 and 24 months | 6 months: NIV group, mean 94.4 points (SD 20.3 points); usual-care group, mean 86.3 points (SD 18.4 points). 12 months: NIV group, mean 93.5 points (SD 16.5 points); usual-care group, mean 87.7 points (SD 19.14 points). 18 months: NIV group, mean 89.9 points (SD 17.3 points); usual-care group, mean 88.7 points (SD 21.5 points). 24 months: mean adjusted difference in change –1.3 points (95% CI –9.7 to 7.4 points) | Trend for better QoL in NIV group at all time points but not statistically significant |
| Garrod et al. 200084 | RCT | 3 months | 1, 2 and 3 months | 1- and 2-month data in graph only. 3 months: NIV group, mean 92.2 points (SD 17 points); usual-care group, mean 85.1 points (SD 23.9 points). Mean adjusted difference in change mean 12.3 points (95% CI 1.19 to 23.4 points), p = 0.03 | Statistically significant difference in favour of NIV at 3 months |
| MRF-28 | |||||
| Clini et al. 200299 | RCT | 24 months | 24 months | Mean difference (adjusted for baseline) 7.1 (95% CI 0.13 – 4.07); p = 0.041 | Statistically significant difference in favour of NIV at 24 months |
| Duiverman et al. 200879 | RCT | 3 months | 3 months | Mean difference (adjusted for baseline) –9.7 (95% CI –18 to –1); p < 0.05 | Statistically significant difference in favour of NIV at 3 months |
| Duiverman et al. 201180 | RCT | 24 months | 6, 12, 18 and 24 months | Mean difference (adjusted for baseline) 12 months: –13.4 (95% CI –22.7 to –4.2); p < 0.05 | Statistically significant difference in favour of NIV at 24 months (statistical significance not reported for earlier time points) |
| POMS | |||||
| McEvoy et al. 200974 | RCT | Median 28.5 months (NIV) and 20.5 months (usual care); up to 5 years | 12 months | NIV group, total mood score median 22 (IQR 48), usual-care group median 5 (IQR 21); p = 0.318 | No statistically significant difference for total score; statistically significant difference in favour of usual care for two subgroups on Profile of Mood States (vigour, confusion and bewilderment) |
| Post-hospital population | |||||
| Clinical COPD Questionnaire | |||||
| Struik et al. 201475 | RCT | 12 months | 12 months | Mean difference in change –0.04 (95% CI –0.5 to 0.4) | Not statistically significant between groups. Based on completers only |
| MRF-28 | |||||
| Struik et al. 201475 | RCT | 12 months | 12 months | Mean difference in change –1.5 (95% CI –8.6 to 5.7) | Not statistically significant between groups. Based on completers only |
| Chronic Respiratory Disease Questionnaire | |||||
| Struik et al. 201475 | RCT | 12 months | 12 months | Mean difference in change 0.01 (95% CI –0.4 to 0.4) | Not statistically significant between groups. Based on completers only |
| SRI | |||||
| Struik et al. 201475 | RCT | 12 months | 12 months | Mean difference in change 4.8 (95% CI –0.1 to 9.7) | Not statistically significant between groups. Based on completers only |
The overall results were suggestive of trend towards better QoL with NIV compared with usual care, with some results statistically significant (in favour of NIV). This is not consistent across all studies, however, with one of the larger RCTs (McEvoy et al. 200974) finding statistically significant results in favour of usual care, albeit for only some subscales. The one non-randomised study may have been prone to greater bias than the RCTs; however, the authors stated that there were no statistically significant differences between groups for baseline characteristics (thus making selection bias less probable).
The 2013 Cochrane review110 pooled 12-month SGRQ results for McEvoy et al. 200974 and Clini et al. 200299 based on individual patient data (IPD) (which were not available for this report). They found a very small, and not statistically significant, effect in favour of the usual-care group [mean difference of 0.9, 95% confidence interval (CI) –19.21 to 21.01]. This result is based on one QoL instrument only. See also Chapter 5, Review of systematic reviews.
Only one RCT (Struik et al. 201475) in a post-hospital population reported QoL; there were no statistically significant differences between groups at 12 months, based on four different measurement scales.
None of the studies reporting QoL had a sham NIV arm, so there may be a risk of patients receiving NIV being more optimistic in their assessment of QoL. However, NIV is also known to have an adverse impact on QoL in the short term (while patients are adapting).
Given the paucity of QoL data in a post-hospital population, the uncontrolled studies were explored (only studies with at least 10 patients considered, using one of the QoL instruments used by the RCTs/controlled studies). There was only one small prospective uncontrolled study (Skobel et al. 2011108) which reported QoL using a German version of the SGRQ at 3 months in 27 patients. This study found a significant improvement in the symptom and impact domains, as well as in overall score compared with the baseline; after further follow-up the symptom score showed a deterioration (while the scores for the other domains remained stable). There are too few data to make an assessment of how QoL changes might differ in stable and post-hospital populations.
There were no retrospective uncontrolled studies conducted in post-hospital populations using one of more of the relevant QoL instruments.
Adherence and adverse events
Table 11 shows details of the recommended schedule of NIV, any support provided for adapting to NIV, the extent to which patients adhered to NIV and any adverse events associated with NIV, including where these led to treatment discontinuation. Length of hours that NIV is used for per night and the percentage of nights NIV is used for may affect the potential benefit. Overall, there was a lack of consistency and/or detail in terms of how these parameters were reported across studies. Generally there was less detail in the retrospective studies.
| Study | Period of adaptation/help in adapting to NIV | Recommended period of use | Adherence | Adverse events associated with NIV (compared with usual care if reported) | Adverse events leading to NIV discontinuation | ||
|---|---|---|---|---|---|---|---|
| How measured | Mean hours of use | % of patients or other | |||||
| RCTs | |||||||
| Bhatt et al. 201383 | Respiratory therapists called the subjects every day during the first week to ensure optimal usage and to troubleshoot complications; also home visit during first week and as needed thereafter | All night or for at least 6 hours every night for 6 months | Patient reported hours of use and machine downloaded data (machine downloaded data used for analyses) | Patient report, 3.9 hours/night (SD 3.4 hours/night); machine report, 3.1 hours/night (SD 3.3 hours/night) | No patients discontinued NIV. % days used [42 (SD 36)], % use greater than 4 hours per night (40%), initial compliance [usage > 4 hours/night in first week: 8 (53%)] | 13 patients experienced symptoms in the NIV group (1/13 dryness of eyes, 5/13 sinus congestion, 1/13 nose bleed, 5/13 discomfort, 1/13 skin break). Two patients experienced symptoms in the usual-care group (1/2 dryness of eyes, 1/2 nose bleed) | None |
| Casanova et al. 200085 | Two nights in hospital to optimise settings, and to instruct patients in use; ‘close contact’ with the patient during the first 3 weeks to ensure good coupling with the ventilator during sleep | Nocturnal. No details regarding recommended times, but used ≥ 5 hours/day as cut-off point for subgroup analysis (compliant vs. non-compliant) | Electrical time counters | Average of 6.2 hours/day (months 3 and 6), 5.9 hours/day during month 6–12 | 5/26 (19%) discontinued during the first 3 weeks | High pressure. No other adverse events reported | 5/26 had high pressure |
| Cheung et al. 201090 | No details on length. Patients and/or family members were educated by specialist respiratory nurses on the use of NIV/CPAP and interfaces; and proficiency was assessed before discharge | 8 hours during sleep every night | Built-in timer | 3 months: 8.1 hours (SD 1.8 hours), based on n = 14; 6 months: 8.5 hours (SD 2.2 hours), based on 12 patients; 12 months: 8.7 hours (SD 1.3 hours) based on eight patients | 4/23 (17%) withdrew consent and a further 4/23 were withdrawn because of significant concurrent illness (both NIV arm). 1/24 withdrew consent in usual-care arm (CPAP as placebo NIV), 1/24 was withdrawn because of protocol violation, and a further 2/24 were withdrawn because of significant concurrent illness and inadequate home support | 4/23 (17%) withdrew consent because of discomfort associated with treatment. 1/24 withdrew consent in usual-care arm because of discomfort associated with treatment | 4/23 had discomfort associated with treatment |
| Clini et al. 200299 | Patients assigned to NIV treatment were admitted to hospital for 3 to 4 days for education and familiarisation with the device | ≥ 5 hours per night | Time counter and daily cards | 9 hours (SD 2 hours) (in compliant patients) | 12/43 dropouts in total. 4/12 early dropouts because of non-compliance with NIV, 3/12 dropouts because of non-compliance after discharge. Reasons for non-compliance not stated | No details | No details |
| De Backer et al. 201191 | No details | Stated that ‘NIV was supplied for > 5 hours a day’91 | Ventilator memory card | No details | No details | No details | No details |
| Duiverman et al. 200879 | Patients hospitalised to practice NIV under supervision; the practice period lasted until patients could sleep at least 6 hours with NIV [mean days necessary 5 (SD 6)] | Patients discharged from hospital once they could sleep for 6 hours with NIV | Ventilator counter readings | No details | 5/31 could not adapt to NIV (this excludes early dropouts). Completers used NIV on average 96% of the days with a median daily use of 7.7 hours (IQR 5.8–8.5 hours/day) | 5/31 could not adapt to NIV (no further details) | |
| Duiverman et al. 201180 | Patients hospitalised to practice NIV under supervision; the practice period lasted until patients could sleep at least 6 hours with NIV [mean days necessary 5 (SD 6)] | Patients discharged from hospital once they could sleep for 6 hours with NIV | No details | Median use per day 6.9 hours (range 40 minutes–11.4 hours/24 hours) | After 2 years, patients used their ventilator for 94% of the days (range 75–100%) | No details | No details |
| Garrod et al. 200084 | 4-week run-in period (with twice-weekly contact to encourage compliance) | At least 8 hours/day. Where patients reported being unable to sleep with ventilation, they were advised to use the machines for at least 6 hours during the day | Time counter and daily diary cards | Median 2.08 hours/day (range 0–11.4 hours/day) from counter readings. Sixteen patients returned completed diary cards with median reported use of 3.8 hours/day (range 0–9.8 hours/day) | Of 17 patients who reached the end of the study, 5 used the ventilator for more than 4 hours/day, and 8 for more than 3 hours. 2/23 patients lost from the study because of non-compliance | 4/23 patients complained of dry mouth and throat (humidification provided). Reasons for poor compliance overall ranged from upper airway problems to complaints regarding disturbance to spouse and inability to sleep | 2/23 patients lost from the study because of non-compliance (unclear if because of adverse events) |
| Gay et al. 1996100 | 1.5 days in hospital | Throughout night | Concealed counter on the ventilator and patient diary | NIV group, 5.1 hours/night (SD 3.8 hours/night); sham NIV group, 4.8 hours/night (SD 3.5 hours/night) | NIV group, missed nights 20.8 (SD 12.6); sham NIV group, missed nights 16 (SD 10.3) | 3/7 stopped using the device and did not return for follow-up assessments (primary reason was an inability to sleep) | |
| Kaminski et al. 1999101 | Adaptation period in hospital before discharge. Length of time not stated | During night-time | No details | 7.2 hours/day (SD 4 hours/day) | 2/7 discontinued NIV because of intolerance. No further details | ||
| Köhnlein et al. 201476 | Patients trained by specialist nurses in use of equipment. At study entry, NIV patients were admitted to hospital for a mean of 5.6 days (SD 1.1) [usual-care group, 2.5 days (SD 0.2)] | 6 hours per day, preferably during the night, but daytime use permitted | Internal time meters on ventilators | Based on 48/102 patients and 3-month follow-up period. Mean NIV usage was 5.9 hours/day | Based on 48/102 patients and 3-month follow-up period: 65% exceeded the prescribed time of 6 hours; usage time was less than 3 hours in 18.8% | 14/102 (14%) with skin rash (managed by changing mask type); no other AEs that could be attributed to intervention | 9/102 discontinued NIV; mask intolerance (5/102) and perceived lack of effect of NIV (4/102) |
| McEvoy et al. 200974 | 3 to 4 days in hospital. NIV considered to be successfully established when at least 3 hours sleep were confirmed on NIV with an IPAP–EPAP difference of at least 5 cm H2O | Consistent use defined as an average of > 4 hours per night | Hour meter values on the NIV and oxygen concentrator devices read out by patient or family member and recorded. Nurses read the hour meter at vistis every 6 months | 4.5 hours/night (SD 3.2 hours/night) | 4/72 lost to follow-up (not contactable or withdrew consent). 41/72 (60%) used NIV for > 4 hours/night | No details – reasons for withdrawals not stated | |
| Meecham-Jones et al. 199577 | 2 to 4 days in hospital | During night-time | Patient diary cards recording total daily hours of nasal ventilation. Also timers on ventilators | Patient reported median of 7.1 hours (range 4.3 to 11 hours), measured median of 6.9 hours (range 4.2 to 10.8 hours) | One patient (1/18) withdrawn from study because of inability to tolerate equipment | ||
| Murphy et al. 201178 (abstract) | No details | No details | No details | 6 weeks: 3 hours 41 minute (SD 1 hour 41 minute). 3 months: 4 hours 30 minute (SD 1 hour 44 minute) | No details | No details | No details |
| Sin et al. 200782 | At least 4 hours training at university sleep laboratory | During night-time | Hours recorded by ventilator counter | NIV group,3.7 (3.4) hours per night; sham NIV group, 5.3 (4.4) hours per night. Not clear whether this relates to total study period | 2/13 refused NIV after randomisation (reasons not stated) | No details | No details |
| Struik et al. 201475 | Experienced nurse practitioners started NIV. No details on length of time | Use during night and during day/nap times if desired | Time counter on NIV machine | Mean duration of NIV until death or last follow-up. Total group, 6.3 hours/night (SD 2.4 hours/night); completers, 7.7 hours/night (SD 1.5 hours/night) | 25/101 (25%) discontinued NIV. Reasons: lack of motivation (n = 15), discomfort associated with treatment (n = 8), dementia (n = 1), cerebrovascular accident (n = 1) | 8/101 (8%) discontinued treatment because of discomfort associated with treatment | |
| Strumpf et al. 199134 | 2 to 3 hours in hospital, then three once-weekly visits at home until patient had adapted to ventilator, less frequent visits thereafter | Patients asked to use device every evening and to gradually extend periods of use until they could sleep using it throughout the night | Electronic timer | Average 6.7 hours/night (SD 6 hours/night) for 7 completers | 7/23 could not tolerate mask. Unclear how many withdrew during first/second treatment period. Of seven completers, no patients interrupted use of ventilator for more than three consecutive nights | Complaints included intolerable nasal mucosal irritation unresponsive to corticosteroids or humidification, inability to sleep and excessive anxiety associated with ventilator use | |
| Xiang et al. 200792 | Patients discharged with NIV once patients and relatives were used to the treatment (time not stated) | At least 8 hours/day during night or daytime nap | No details | No details | 1/20 patients discontinued NIV | Abdominal distension (5/20), localised skin pressure damage (2/20), suspected pulmonary barotrauma/pneumothorax (1/20) | Suspected pulmonary barotrauma/pneumothorax (1/20) |
| Zhou et al. 200881 | No details | Intermittent ventilation. Either three times a day for 3 hours or twice a day for 2 hours and 5 hours at night depending on patient characteristics. At least 9 hours in total | No details | No details | No details | No details | No details |
| Controlled studies NIV vs. usual care | |||||||
| Budweiser et al. 200796 | No details | Nocturnal NIV was offered during hospital stay | Counter readings on ventilator for hours of daily use | 6.5 hours/day (SD 2.5 hours/day) | 12/99 (12%) discontinued NIV within 2 to 27 months (mean 6.3 months). Reasons were mask intolerance (3/99), decreased motivation (3/99), reported improvement in symptoms (4/99), lung transplantation (1/99) or not specified (1/99). 6/99 (6%) used NIV for < 3 hours/day | Mask intolerance (3/99) | |
| Clini et al. 199893 | All patients received an in-hospital trial of NIV, spending at least 15 days in hospital. Effects of NIV tested during two daily practice trials | At the end of trial period, patients instructed to use NIV for at least 5 consecutive hours per night | Patient and relative interviews and device time counter | 7.4 hours/night (SD 1.3 hours/night) | 21/49 initially did not comply with NIV during adaptation period and formed the usual-care group (lack of compliance defined as the patient’s inability to use NIV properly for at least 5 hours for even one night (subjective intolerance, excessive air leaks) | Nasal skin lesion, n = 6/28 (21%); gastric distension, n = 4/28 (14%); rhinorrhoea, n = 4/28 (14%), mucosal dryness, n = 2/28 (7%); skin inflammation, n = 1/28 (4%) | No details |
| Clini et al. 199694 | Patients received an in-hospital trial for at least 15 days | Minimum of 8 hours at night | No details | No details | No details | No details | No details |
| Heinemann et al. 201197 | Unclear, but NIV initiated in hospital after weaning | No details | Patients using NIV admitted regularly at 3, 6 or 12 months to verify adherence | No details | No details | Stated only that patients with dryness of the mucosa used a passive heat and moisture exchanger, which was switched to a heated humidification system if dryness persisted. Number of patients experiencing this not stated | No details |
| Laier-Groeneveld and Criee 199588 | No details | Nocturnal (whole night) and if required during the day (length according to normalisation of blood gases) | No details | No details | No details | No details | No details |
| Lu et al. 201298 | Patients discharged with NIV once patients and relatives were used to the treatment | At least 8 hours (mainly at night and during mid-day rest) | No details | No details | Stated that patients had ‘good’ tolerance and compliance to NIV. No patients appeared to discontinue. There was no pulmonary barotrauma | ||
| Milane and Jonquet 198586 | No details | 15 minutes per hour when awake, up to a minimum of 4 hours per day | No details | No details | No details | No details | No details |
| Pahnke et al. 199787 | No details | No details | No details | No details | 15/40 patients refused NIV or discontinued within first 3 months. Reasons include social (flat too small, disturbs partner/neighbours or too technical) rational (because of age reached with COPD) or fear of mask/claustrophobia or of becoming dependent | ||
| Paone et al. 201489 | No details, but allocation to NIV group on the basis of compliance and/or willingness to be trained | No details but compliance defined as ≥ 5 hours/night | No details | Mean, 7.4 hours/day (SD 1.3 hours/day) | No details | No details (patients discontinuing NIV during an initial trial were allocated to the usual-care group) | |
| Tsolaki et al. 200895 | 2 to 3 days in hospital until patients were confident in use | At least 5 hours/night | No details | 9 hours/day (SD 2.2 hours/day) | 3/27 dropouts from NIV group because of poor compliance (< 5 hours/day) | No details | No details |
Most studies reported some detail on how patients were accustomed to NIV; patients usually had a run-in period in hospital [between 2 and 6 days where stated, with the exception of Clini et al. (1998)93 with 15 days], and some studies gave details of follow-up support in the home. NIV was generally recommended for nocturnal use, with the exception of Milane and Jonquet (1985),86 who specified a schedule of 15 minutes per hour when awake, up to a minimum of 4 hours per day, and Zhou et al. (2008),81 who also specified a more intermittent schedule (three sessions over a 24-hour period). Some studies recommended a minimum number of hours of use (between 5 and 9); one study74 defined consistent use as an average of > 4 hour per night.
Most of the prospective studies reported the average number of hours during which NIV was used each day (assessed by ventilator recordings), but only three76,83,84 gave details on the percentage of patients using NIV for a minimum number of hours on average. Average use may not be informative regarding the proportion of patients likely to benefit from adequate use of NIV. The mean use varied between 3.1 and 9.2 hours per night, although it was not always stated if this referred to completers only. Variations may be a reflection of recommendations given by clinical staff, disease severity or patient comfort (e.g. different NIV pressures, ability to sleep with equipment). No retrospective controlled studies had details on average NIV use, as this information would likely not have been captured.
There was variation in how studies reported adverse events (e.g. all associated with NIV or only those associated with discontinuation), hampering comparisons across studies. Reasons for discontinuation due to NIV included high pressure, inability to sleep, disturbance of partner, mask intolerance/claustrophobia, perceived lack of effect or general discomfort or intolerance. There was one case of suspected barotrauma. 92 Rates of discontinuation from NIV varied between 5% and 43%; these withdrawal rates were not all because of adverse events but may have included other reasons (e.g. other illness). Differing discontinuation rates may be a reflection of length of follow-up, although most discontinuation would be expected soon after starting NIV. Other adverse events, not necessarily leading to discontinuation, included skin lesion/inflammation, dry mouth/throat/eyes (humidification was provided in some cases), rhinorrhoea, gastric distension and anxiety.
Secondary outcomes
Results for key secondary outcomes are presented below. All results are based on RCTs only; non-randomised controlled studies were not considered. Results are presented in forest plots in order to show the overall direction of effect; however, pooling was not undertaken owing to baseline imbalances between arms in many of the RCTs and a lack of reporting of adjusted results. Additional reasons for not pooling were differences between studies in terms of time points presented, the type of RCT (e.g. parallel or crossover) and, in some cases, uncertainty around patient numbers or exact results (where estimated from graphs or units were converted; see also Appendix 3 for details on calculation/assumptions made). Some studies are represented more than once (results at different time points or reported using different metrics). All extracted (or calculated) data relating to secondary outcomes are presented in Appendix 9.
Only one study32,79 presented adjusted results using appropriate methods (linear regression analysis). Some studies presented change scores; for most studies mean difference between post-treatment scores was calculated for this report. Studies were separated in forest plots depending on the method used for calculating a mean difference (adjusted, change score or mean difference based on final scores). The Köhnlein et al. 76 RCT presented all results for secondary outcomes as percentage change only, therefore results could not be incorporated into forest plots. Findings have been described narratively for relevant outcomes.
Forced expiratory volume in 1 second
Five RCTs,34,74,83,85,99 all in a stable population, reported FEV1 (% predicted), at time points between 3 and 24 months (Figure 8). Two studies34,99 reported a change score; for the other three a mean difference was calculated from aggregate final scores. There were no significant differences in any studies and no overall trend was observed.
FIGURE 8.
The FEV1 % predicted. a, Calculated by authors of this report.
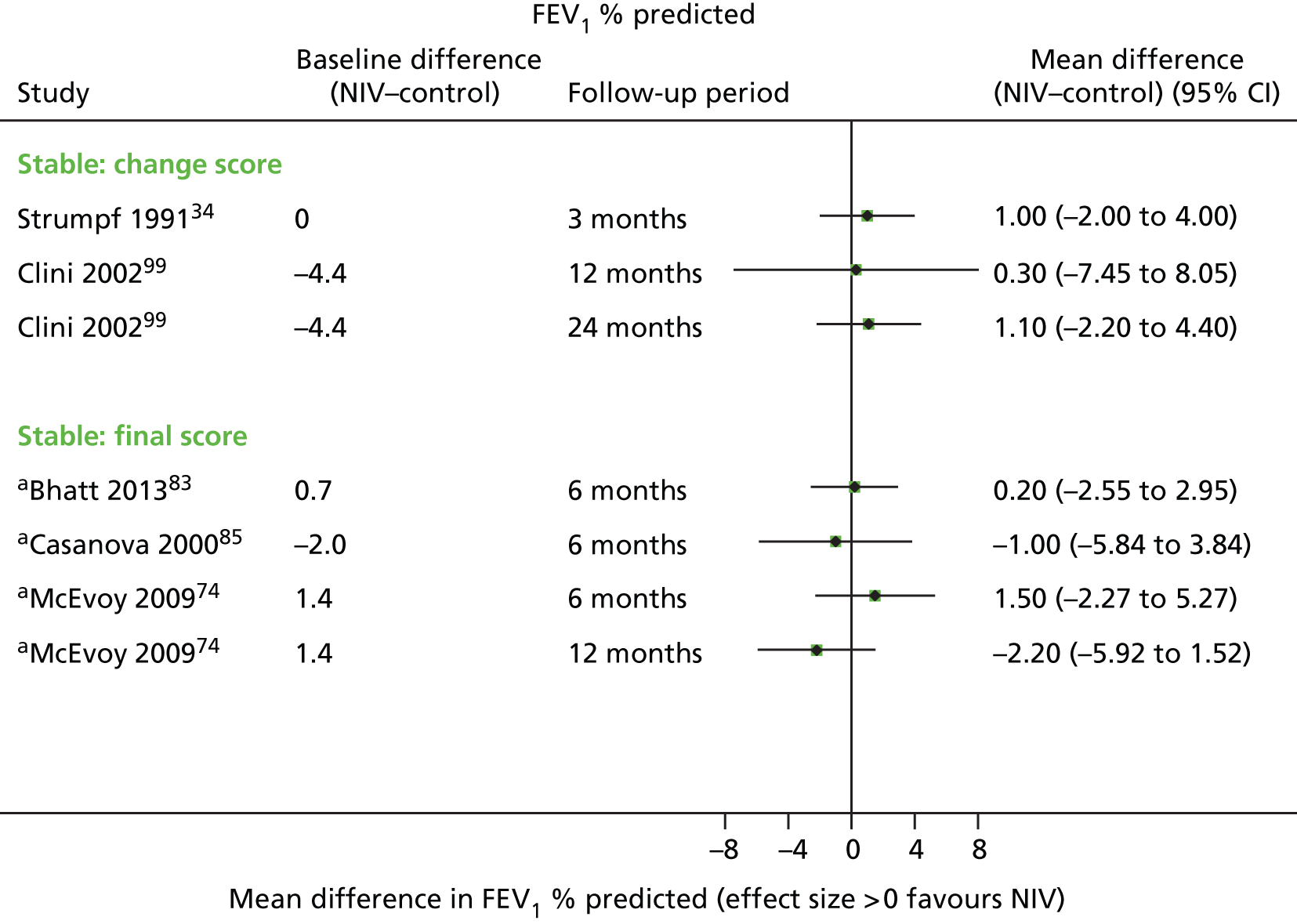
Six RCTs32,34,77,79,84,100,101 in stable populations and two75,92 in a post-hospital population reported FEV1 (l), at time points between 3 and 24 months. All but one,84 which did not report sufficient data, have been represented in a forest plot (Figure 9). There may be a very slight trend across studies favouring NIV, with three statistically significant results, all of which show a clinically important change (of at least 120 ml). However, two of these were from the same study79,80 in a stable population; this study had more intensive usual care in both arms than in the other studies. The other significant result was in a post-hospital population92 (based on post-treatment scores). Overall, there is a lack of consistency in terms of direction of effect, for both populations.
FIGURE 9.
The FEV1 (l). a, Calculated by the authors of this report.
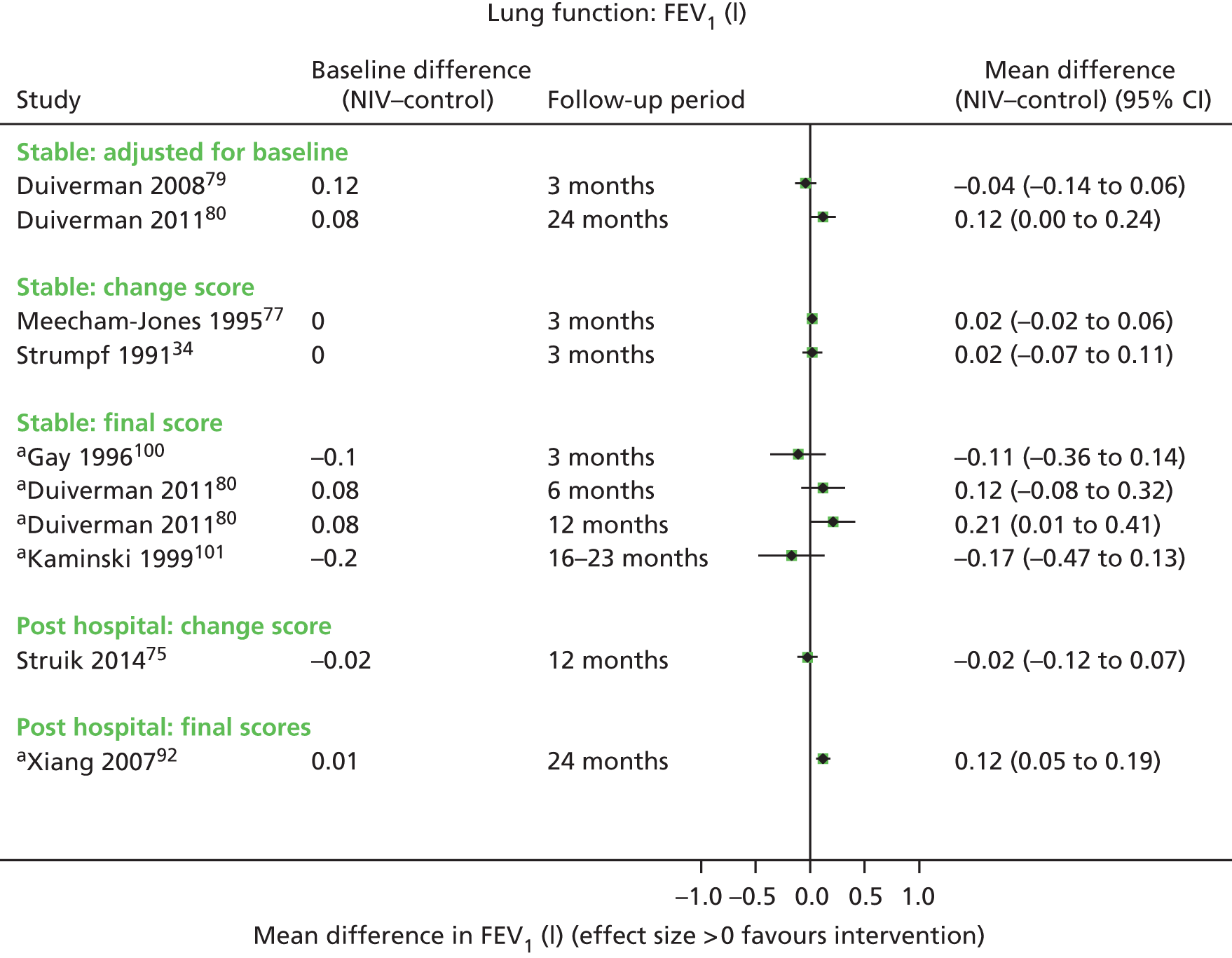
Forced expiratory volume in 1 second was measured in two further studies; however, in one82 no post-treatment data were presented (it was stated that there were no significant changes in either arm), and in the other91 the data presented for SDs appeared to be incorrect. The RCT by Köhnlein et al. 76 measured FEV1 and found a statistically significant difference in favour of NIV.
Forced vital capacity
Two studies in stable populations reported FVC (% predicted; Figure 10). Both results were calculated from post-treatment scores. Neither was statistically significant and the direction of effect is not consistent. Note that the population on the study by Bhatt et al. 201383 was normocapnic and thus not representative of the majority of NIV patients across studies.
FIGURE 10.
The FVC (% predicted). a, Calculated by authors of this report.
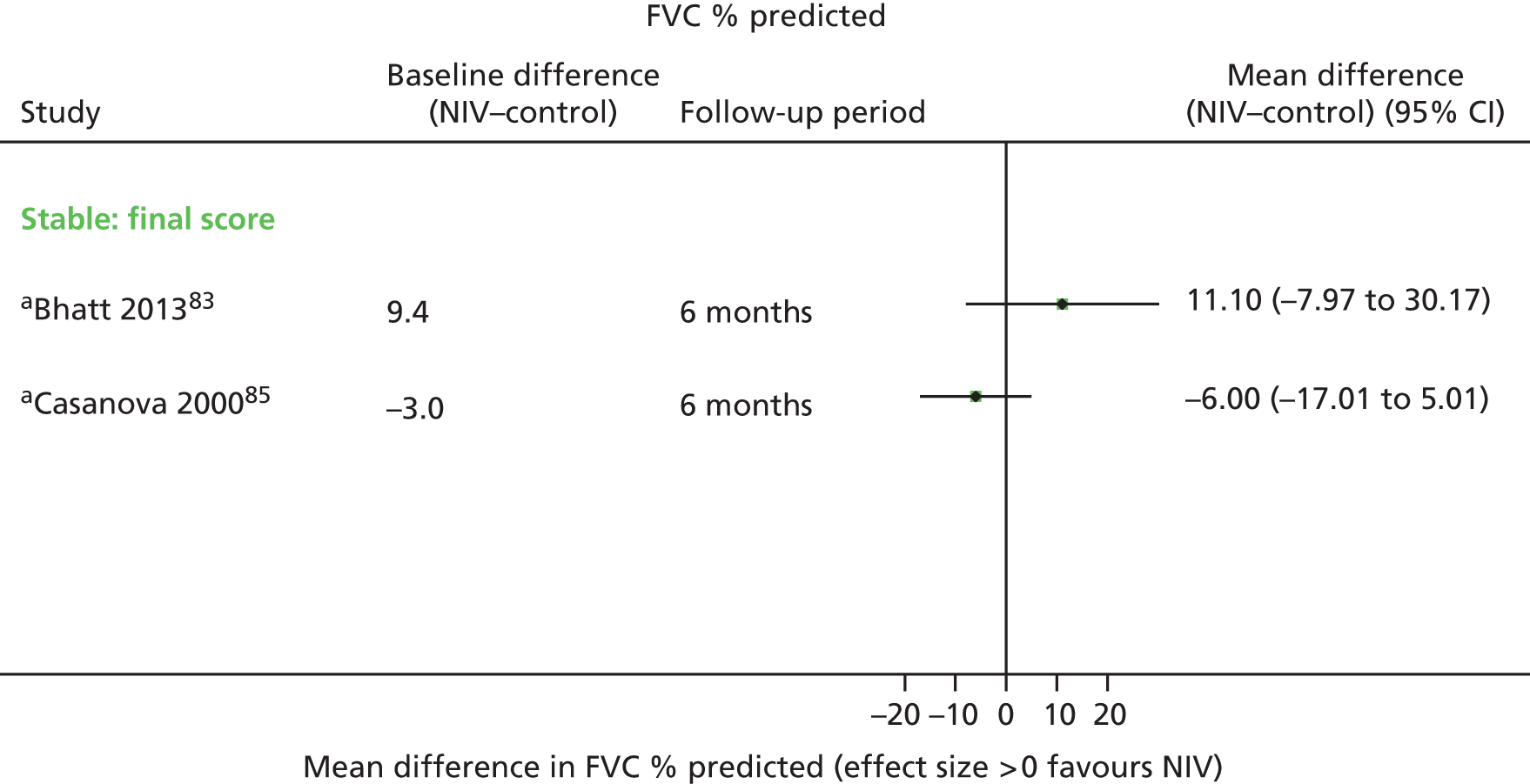
Five studies reported FVC (l), which in four cases is presented in a forest plot (Figure 11; one study84 did not report sufficient data to be included). Two studies reported a change score75,77 and two a mean difference calculated from post-treatment scores. Only one study (post-hospital population)92 found a significant difference in favour of NIV. Overall there were few data, and no consistency in terms of direction of effect. The study by Köhnlein et al. 76 reported data for FVC (unclear if % predicted or litres) and found no significant difference.
FIGURE 11.
The FVC (l). a, Calculated by authors of this report.
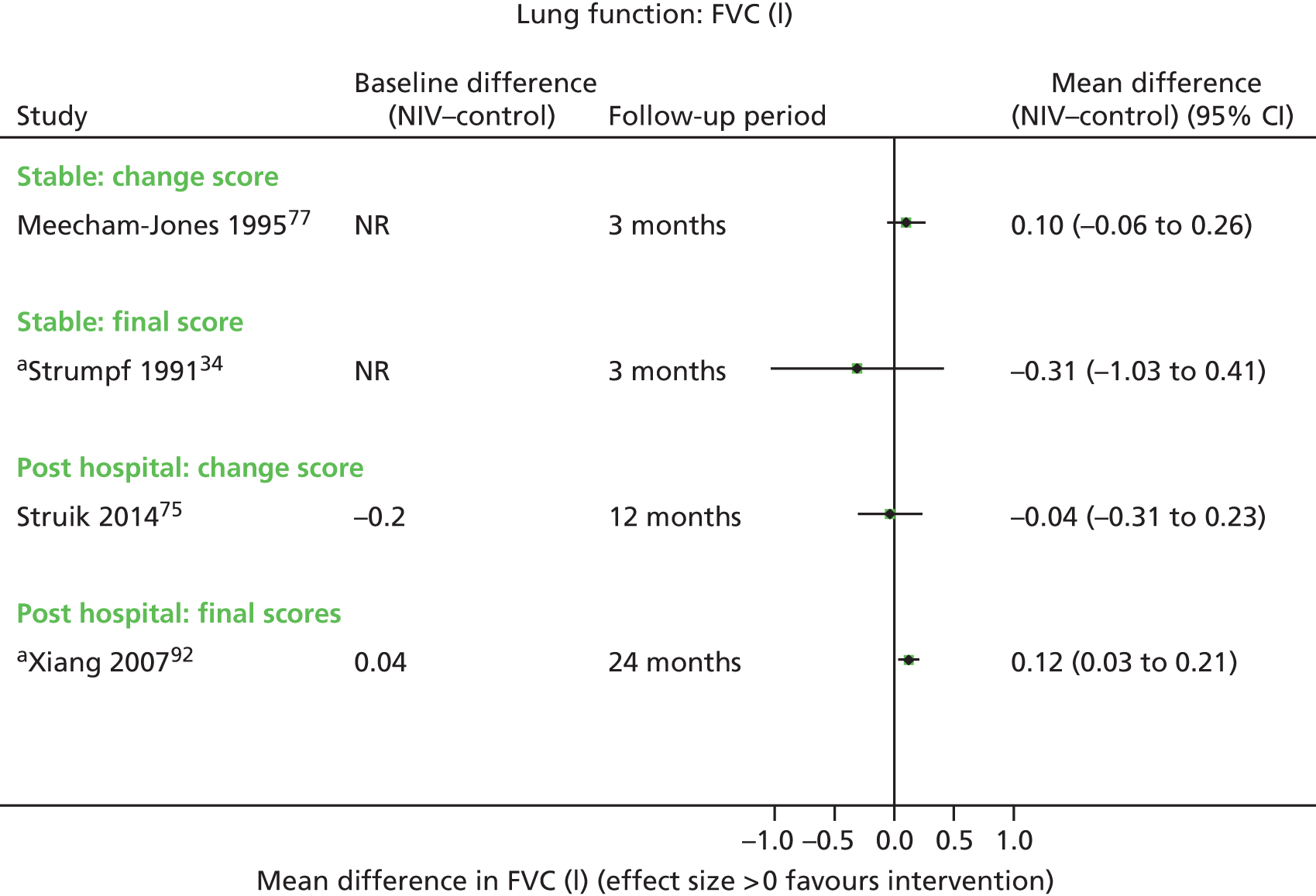
Partial pressure of carbon dioxide in the arterial blood
Partial pressure of carbon dioxide in the arterial blood was measured in 16 studies, of which 13 are represented in Figure 12. There was an overall trend favouring NIV for reduction in PaCO2, with seven statistically significant results, one of which was an adjusted result. Mean difference in favour of NIV was between 0.4 and 1.60 (the latter in the post-hospital population). A reduction of 1 kPa might make a clinical difference, but this also depends on the individual patient and their starting value. The proportion of patients within the individual studies achieving a (for them) meaningful change in PaCO2 is unknown, as is the proportion reaching a threshold values of what might be considered to be ‘normal’ (around 6.5 kPa).
FIGURE 12.
The PaCO2. a, Calculated by authors of this report; b, measurement performed regardless of oxygen use; c, measurements both on room air or both on oxygen at the same flow rate.
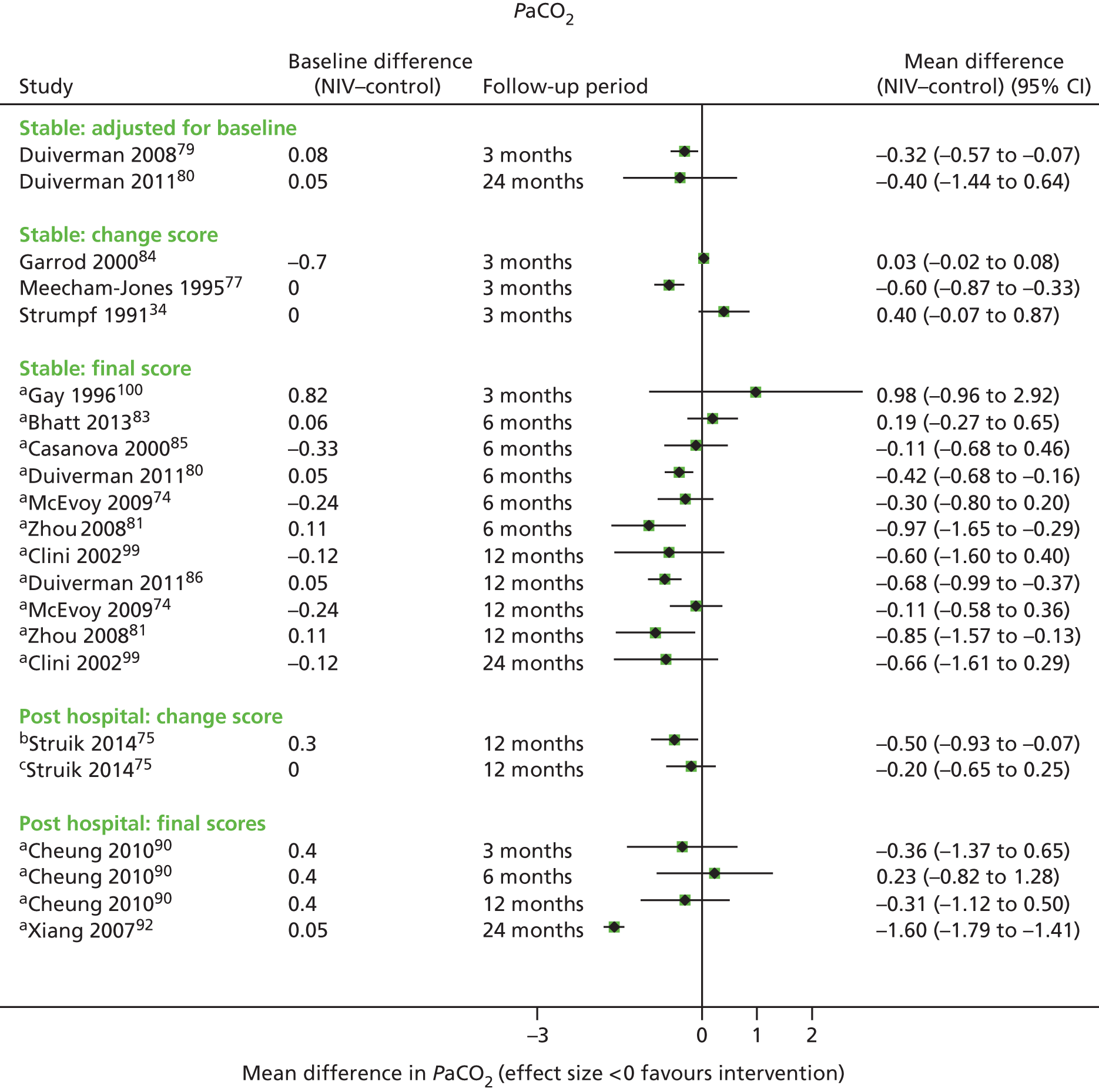
The three studies not represented found a statistically significant difference in favour of NIV,76 found no significant difference82 or appeared to have errors in the data. 111
Partial pressure of oxygen in the arterial blood
Nine RCTs34,77,79–81,83–85,99,100 in a stable population and two75,92 in a post-hospital population reported PaO2 and could be included in a forest plot (Figure 13). There was a clear trend in favour of NIV for the stable population, with some results statistically significant. There were fewer data for a post-hospital population and no consistent trend. The difference in change for the statistically significant results was between 0.5 and 1.0. Again, a 1 kPa difference might be important but the same caveats as for PaCO2 apply. Results from only one study79,80 with a stable population were adjusted for baseline (this study had more intensive usual care in both arms). Three further studies (in a stable population) measured PaO2 but were not included: two74,76 found no significant difference and the other91 appeared to have errors in the reported SD.
FIGURE 13.
The PaO2. a, Calculated by authors of this report; b, measurement performed regardless of oxygen use; c, measurements both on room air or both on oxygen at the same flow rate.
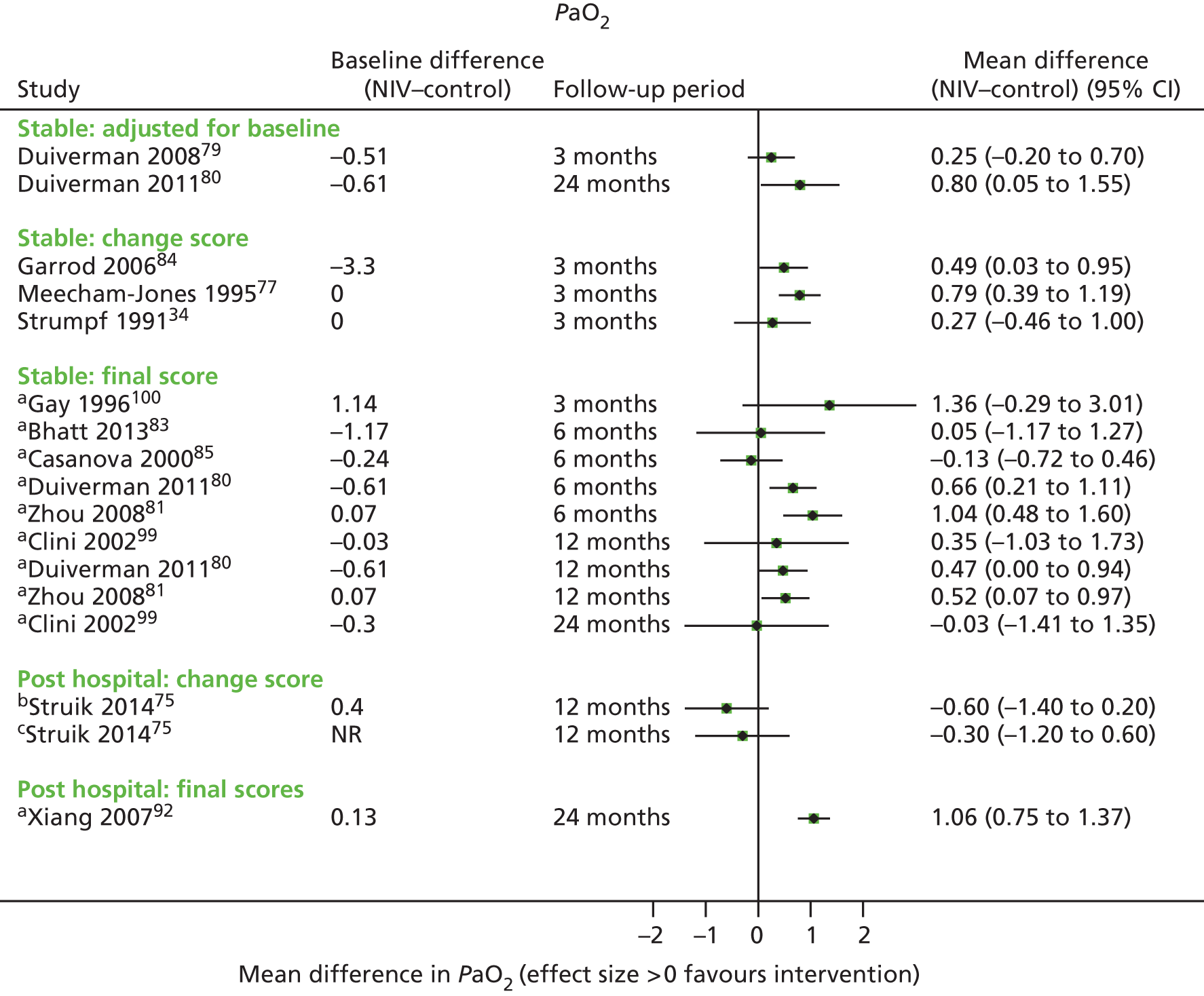
Six-minute walking distance
Eight RCTs32,77,79,82,83,91,99–101 in a stable population and one92 in a post-hospital population reported 6MWD at baseline and at follow-up times between 3 and 24 months. There was one statistically significant result for the stable population (in favour of NIV, adjusted result), but, overall, the results for the stable population are inconsistent in terms of direction of effect, which may be a reflection of differences in baseline values. The one post-hospital study shows a statistically significant result in favour of NIV (improvement of 86 m), but this is not based on an adjusted result. One study (stable population)77 reported medians (and ranges) only and is not represented in the forest plot (Figure 14). A further study (stable population),76 also not represented, found a statistically significant difference in favour of NIV.
FIGURE 14.
The 6MWD. a, Calculated by the authors of this report.
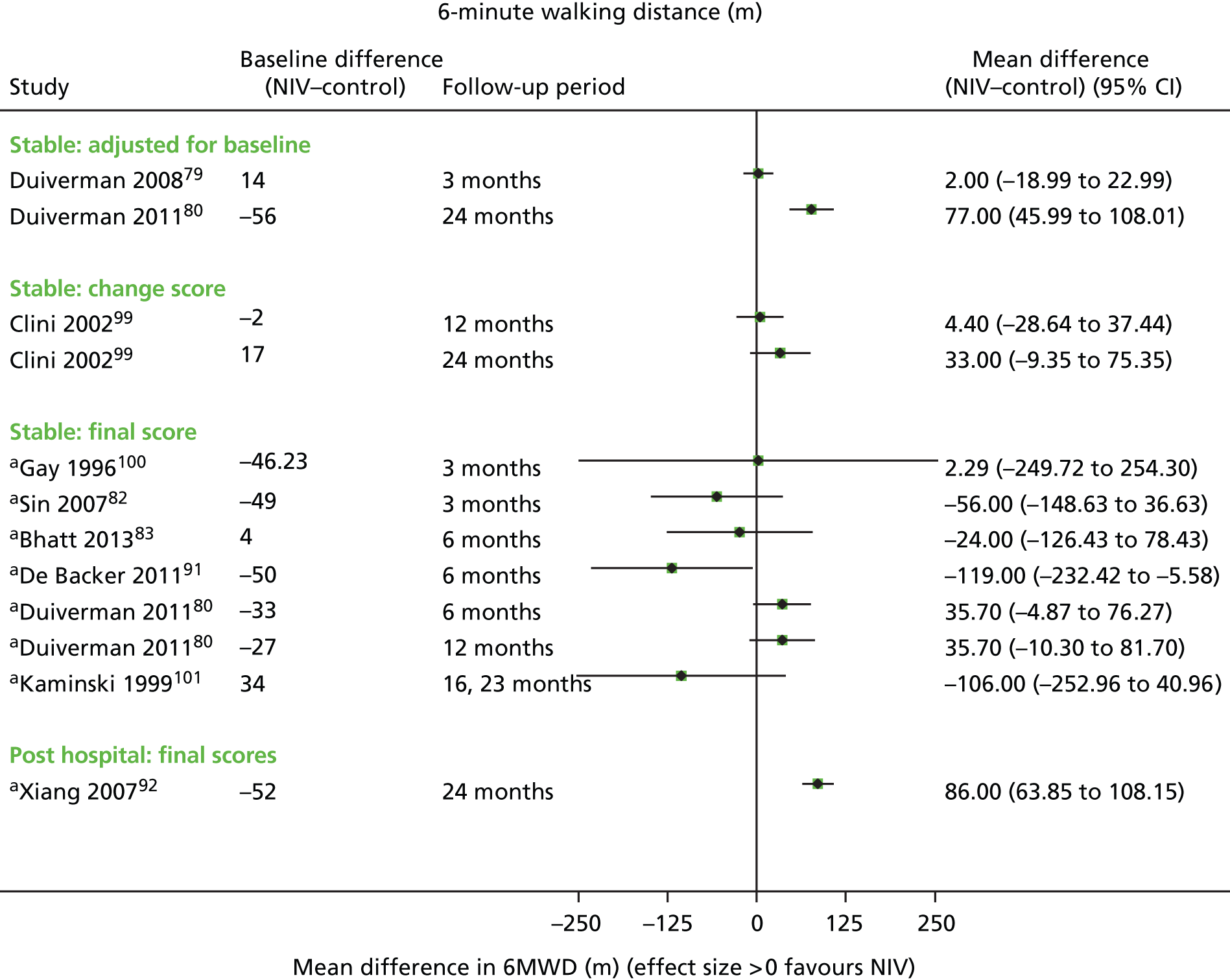
Summary secondary outcomes
Overall, there is no evidence to suggest a difference between NIV and usual care for FEV1 % predicted and FVC % predicted (measured in stable populations only). For FEV1 (change in litres), there may be a slight trend towards benefit from NIV for a stable population, Measuring the predicted values may be more meaningful, as this takes into account patient age.
A clear trend towards a benefit from NIV was apparent for PaCO2, for both stable and post-hospital populations, and for PaO2, for the stable population. Some results were statistically significant. However, most results are based on post-treatment scores only, which do not adjust for baseline. It is possible that baseline imbalances are likely to be evened out across several RCTs; nonetheless, using appropriately adjusted data would give a more robust result. The results from the one adjusted study79,80 are consistent with the overall findings. Even if the results are taken at face value, it remains uncertain whether or not an improvement of between 0.5 and 1.6 kPa (increase for O2, reduction for CO2) translates into clinically important benefits for patients. Any improvement also needs to be considered in the context of the starting values, as there are likely to be ceiling effects. Most of the results were reported for completers only; it is uncertain how many of the non-completers were still on NIV or had discontinued. Sensitivity analyses for missing data may have been appropriate in some cases. Two of the studies in stable populations included a sham NIV arm (Gay et al. 1996100 and Sin et al. 200782). Neither found any statistically significant differences.
Two of the RCTs34,77 used a crossover design (both stable populations), and there are less likely to be baseline imbalances as each patient acts as their own control; findings from these two studies are inconsistent for PaCO2, and only one of the results is statistically significant for PaO2. (Note that while a crossover design cancels out baseline imbalances, there may be other issues relating to interaction effects, and differences arising from the order of giving treatments.)
The results for 6MWD were more inconsistent, possibly because of baseline imbalances, and it is difficult to draw firm conclusions. In interpreting 6MWD, the possibility of a ceiling effect needs to be considered where patients had high baseline values. The fact that the one study79,80 that adjusted for baseline shows a benefit at the later follow-up time80 (difference of 77 m at 24 months) is potentially promising, and consistent with benefits for other outcomes in this study, but this finding would need to be confirmed. This study also had more intensive usual care underpinning both arms.
An assessment of overall (pooled) direction of effect for secondary outcomes was hampered by the fact that studies reported outcomes using different methods (e.g. adjusted, change score or mean difference calculated from final scores) and different study designs (crossover or parallel). It is possible that the pattern of results observed may change with the use of different metrics and/or sensitivity analyses around missing assessment data.
Discontinuation studies
Two RCTs met the inclusion criteria of this report but addressed a question that was beyond the remit of this report, namely to look at the effect of discontinuing NIV. Both RCTs (Funk et al. 201171 and Oscroft et al. 201072) included patients on NIV who, after a period of withdrawal, were randomised to continue withdrawal or restart NIV. The results have not been further explored here.
Summary clinical effectiveness (non-invasive ventilation versus usual care)
Stable population
-
Evidence based on seven RCTs and four non-randomised controlled studies suggests that there is no survival benefit from NIV (follow-up times between 6 and 24 months).
-
There is some evidence to suggest a trend (not statistically significant) for fewer hospital admissions/days in hospital based on five RCTs and two non-randomised controlled studies; not all available data could be meta-analysed and three of four other studies reported no significant differences overall for hospitalisation outcomes.
-
There was little information on exacerbations not leading to hospitalisation and no significant differences were reported.
-
There was a trend towards better QoL with NIV, with some statistically significant results; not all studies used appropriate methods of adjusting for baseline and use of different instruments hampered comparisons.
-
None of the studies contributing to primary outcomes used a sham NIV arm and so they were potentially more susceptible to bias.
-
No subgroup analyses for other important parameters (e.g. LTOT, level of hypercapnia, etc.) were possible.
-
Sensitivity analyses around study quality and construction of funnel plots were not feasible.
-
Results for secondary outcomes suggested a benefit from NIV for improving blood gases (some statistically significant results); this was based mainly on post-treatment scores (unadjusted for baseline).
Post-hospital population
-
Evidence from three RCTs suggested no benefit from NIV in terms of survival (follow-up times between 12 and 24 months), while four non-randomised controlled studies suggested a benefit from NIV (statistically significant pooled result follow-up times between 6 and 24 months).
-
One longer-term (retrospective) non-randomised study suggested benefit from NIV up to 10 years; this study used an intermittent daytime NIV schedule.
-
Three RCTs found inconsistent results in terms of hospital admissions: one was statistically significant in favour of NIV, one (with a sham NIV arm) was marginally in favour of NIV and one (the largest study) was marginally in favour of usual care. Data on hospital admissions may be skewed. Only one RCT reported exacerbations (without acute HRF) and found no significant difference.
-
Quality of life was reported in only one post-hospital study, which found no statistically significant difference.
-
Sensitivity analyses around study quality and construction of funnel plots were not feasible.
There was too few data to draw firm conclusions regarding secondary outcomes, but there was a trend towards improvement in PaCO2 based on three RCTs.
Grading of Recommendations Assessment, Development and Evaluation
The study quality and findings were also presented in GRADE tables (Tables 12 and 13). Only primary outcomes were considered (mortality, hospitalisations, exacerbations and QoL) and only studies comparing NIV to usual care. In line with the whole report, stable and post-hospital populations were considered separately. Note that results presented in the GRADE tables are to some extent subjective and should not be considered in isolation but alongside the more detailed discussions in the report. This is particular relevant for the risk-of-bias rating. In interpreting the GRADE tables the following considerations were undertaken:
| Domiciliary NIV vs. usual care only in COPD patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population: stable COPD patients (GOLD stage 3 or 4) | ||||||||||||
| Setting: patient’s home | ||||||||||||
| Intervention: NIV (continuous or intermittent, any length of time) with or without LTOT | ||||||||||||
| Comparison: usual care only, with or without LTOT | ||||||||||||
| Quality assessment | No. of patients | Effect | Quality | Importance | ||||||||
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | NIV | Usual care | Relative (95% CI) | Absolute | ||
| All-cause mortality (up to 24 months) | ||||||||||||
| 7 | RCT | Unclear | Potentially moderate to serious inconsistency (I2 = 60%) | No serious indirectness | No serious imprecision | Not assessed | 302 | 306 | RR 0.88 (0.55 to 1.43) | – | Not given | Critical |
| 4 | Controlled | Potentially serious | No serious inconsistency (I2 = 0%) | No serious indirectness | No serious imprecision | Not assessed | 102 | 90 | RR 1.19 (0.65 to 2.18) | – | Not given | |
| Hospital admissions per patient per year | ||||||||||||
| 5 | RCT | Unclear | Potentially moderate to serious inconsistency (I2 = 59%) | No serious indirectness | Potentially serious imprecision | Not assessed | 204 | 208 | WMD –0.46 (–1.02 to 0.09) | – | Not given | Critical |
| 2 | Controlled | Potentially serious | Potentially serious inconsistency (I2 = 62%) | No serious indirectness | Potentially serious imprecision | Not assessed | 44 | 39 | WMD –0.14 (–0.99 to 0.72) | – | Not given | |
| ICU admissions | ||||||||||||
| 1 | RCT | Unclear | N/A | No serious indirectness | No serious imprecision | Not assessed | 43 | 47 | WMD –0.20 (–0.46 to 0.06) | – | Not given | Critical |
| 2 | Controlled | Potentially serious | Potentially serious inconsistency (I2 = 94%) | No serious indirectness | Potentially serious imprecision | Not assessed | 45 | 38 | WMD –0.36 (–1.05 to 0.32) | – | Not given | |
| Days in hospital per patient per year | ||||||||||||
| 1 | RCT | Unclear | N/A | No serious indirectness | Potentially serious imprecision | Not assessed | 43 | 47 | WMD –5.7 (–16.58 to 5.18) | – | Not given | Critical |
| 3 | Controlled | Potentially serious | Potentially serious inconsistency (I2 = 64%) | No serious indirectness | Potentially serious imprecision | Not assessed | 72 | 60 | WMD –2.44 (–10.86 to 5.99) | – | Not given | |
| Exacerbations | ||||||||||||
| 4 | RCT | Unclear | N/A | No serious indirectness | No summary estimates reported for individual studies | Not assessed | 87 | 95 | Not meta-analysable. Results reported using different outcome measures relating to exacerbations. No overall significant differences for three of the four RCTs; one in favour of NIV | Not given | Critical | |
| 1 | Controlled | Potentially serious | N/A | No serious indirectness | No summary estimates reported | Not assessed | 23 | 24 | Not given | |||
| QoL | ||||||||||||
| 7 | RCT | Unclear | N/A | No serious indirectness | Reporting heterogeneous (see Table 10) | Not assessed | 247 | 236 | Not meta-analysable. Heterogeneity in (number of) instruments, time points and reporting. Trend towards improved QoL in some studies but not consistent | Not given | Critical | |
| 1 | Controlled | Potentially serious | N/A | No serious indirectness | Not assessed | 27 | 22 | Not given | ||||
| Serious adverse events | ||||||||||||
| 9 | RCT | Unclear | N/A | No serious indirectness | N/A | Not assessed | 238 | 230 | None reported. Not all stable population studies reported adverse events | Not given | Critical | |
| 2 | Controlled | Potentially serious | N/A | No serious indirectness | N/A | Not assessed | 45 | 22 | Not given | |||
| Domiciliary NIV versus usual care only in COPD patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | Post-hospital COPD patients (GOLD stage 3 or 4) | |||||||||||
| Setting | Patient’s home | |||||||||||
| Intervention | NIV (continuous or intermittent, any length of time) with or without LTOT | |||||||||||
| Comparison | Usual care only, with or without LTOT | |||||||||||
| Quality assessment | No. of patients | Effect | Quality | Importance | ||||||||
| Number of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | NIV | Usual care | Relative (95% CI) | Absolute | ||
| All-cause mortality (up to 24 months) | ||||||||||||
| 3 | RCT | Unclear | N/A | No serious indirectness | Potentially serious imprecision | Not assessed | 144 | 144 | RR = 0.89 (0.53 to 1.49) | – | Not given | Critical |
| 4 | Controlled | Potentially serious | No serious inconsistency (I2 = 0%) | No serious indirectness | No serious imprecision | Not assessed | 196 | 134 | RR = 0.45 (0.32 to 0.65) | – | Not given | |
| Hospital admissions per patient per year | ||||||||||||
| 3 | RCT | Unclear (one study used sham NIV) | Potentially serious inconsistency | No serious indirectness | No summary estimate | Not assessed | 144 | 144 | Not pooled because of inconsistency of results. One statistically significant result in favour of NIV, one marginally favourable result in favour of NIV and one marginally favourable result in favour of usual care | – | Not given | Critical |
| Exacerbations | ||||||||||||
| 2 | RCT | Unclear | N/A | No serious indirectness | No summary estimate | Not assessed | 124 | 124 | Pooling not possible. No significant difference between NIV and usual care | – | Not given | Critical |
| 0 | Controlled | – | – | – | – | – | – | – | – | – | Not given | |
| QoL post-hospital population | ||||||||||||
| 1 | RCT | Unclear | N/A | No serious indirectness | Potentially serious imprecision | Not assessed | 101 | 100 | No significant differences based on four QoL measures | – | Not given | Critical |
| Serious adverse events | ||||||||||||
| 3 | RCT | Unclear | N/A | No serious indirectness | N/A | Not assessed | 144 | 144 | One suspected pulmonary barotrauma/pneumothorax. Not all post-hospital studies reported adverse events | Not given | Critical | |
| 3 | Controlled | Potentially serious | N/A | No serious indirectness | N/A | Not assessed | 158 | 106 | Not given | |||
Risk of bias
Risk-of-bias assessment was complicated by the fact that not all quality criteria were reported in studies, and also by the fact that NIV is a difficult intervention to blind. Some studies did include a sham NIV arm, which may reduce performance bias, but could arguably also lead to an overestimation of benefit for NIV, as the sham intervention may be associated with some disbenefit. As the effect of a lack of blinding is not completely certain, an unclear risk-of-bias rating has been given for all outcomes unless there were other serious risks of bias. Further, in assessing (differential) loss to follow-up, the cut-off points for low/unclear/high risk-of-bias rating were to some extent arbitrary. Non-randomised studies have an inherently greater risk of bias and have therefore been given a rating of ‘potentially serious risk of bias’, unless there was clear evidence of more serious risk.
Inconsistency
The meta-analyses for mortality in both stable and post-hospital populations were associated with low to moderate levels of heterogeneity (I2 of between 0% and 61%), and results may therefore not be consistent. Meta-analyses for outcomes relating to hospital admissions were associated with moderate to substantial/considerable heterogeneity, potentially a reflection of population differences or thresholds for hospital admission.
Indirectness
It was assumed that there were no serious risks of indirectness, as (1) RCTs undertake direct comparisons of relevant comparators (NIV vs. usual care), (2) the populations in included studies were eligible in terms of the review question (with an additional distinction made for stable and post-hospital populations), (3) intervention and comparators were considered to be usual treatments (albeit potentially with differences between countries) and (4) at least some outcomes were patient related (i.e. not surrogate).
Imprecision
Confidence intervals around pooled estimates for mortality were fairly narrow and thus not considered to be imprecise. There were broader CIs on some of the pooled estimates for hospitalisation-related outcomes and for individual studies (with a small sample size). It needs to be considered that CIs will be narrower where a greater number of studies (patients) are contributing to the results, so it is important that heterogeneity (inconsistency) is considered alongside imprecision.
Publication bias
Publication bias was not assessed as there were too few studies in meta-analyses (five at most).
Quality
An overall quality rating was not given as this was considered to be too subjective and may be taken out of context.
Importance
As only primary outcomes are considered, all outcomes were deemed to be of critical importance, as they are directly related to COPD progression and/or mortality.
Absolute risks
Absolute risk reductions were not calculated, as none of the results (pooled or individual) was statistically significant (except for survival in a post-hospital population). However, this result was based on non-randomised studies and calculating an absolute effect without being able to compare this with potentially more robust studies may be misleading.
Chapter 5 Clinical effectiveness results: non-invasive ventilation versus non-invasive ventilation
This section details three studies that directly compare one form of NIV with another. All were RCTs.
Main study and population characteristics
Patient numbers in the three RCTs were very small (between 12 and 25), and follow-up was short (6 or 8 weeks in a crossover design, Table 14). Average patient age was 65 and 71 years in two trials and not reported in the third. There were more men than women (between 52% and 69% men). Patients had GOLD stage 3 or 4, or at least stage 2112 (although based on mean FEV1 % predicted likely to be more severe stages). Proportions of the different stages are unknown. Patients in all trials were hypercapnic, although the cut-off points for inclusion varied (higher at PaCO2 > 7.5 kPa in one trial112). All populations were described as stable, although one study113 did not give details on length of time without exacerbations. There were no details on past history of exacerbations, and one study112 did not state whether or not sleep apnoea had been ruled out.
| Study | n (n, % male) | Length of follow-up | GOLD stage (or other description of severity) | History of exacerbations | Stable or post-hospital/post-exacerbation population | Hypercapnia | Mean age (years), (SD) | Proportion smokers | BMI, mean (SD) | Overlap syndrome ruled out? |
|---|---|---|---|---|---|---|---|---|---|---|
| Dreher et al. 201032 | 17 (9/13 completers, 69%) | 6 weeks (crossover trial) | GOLD stage 4 | No details | Stable. Patients enrolled during stable phase of disease. Excluded if weaned from invasive ventilation or intubated during the last 3 months | PaCO2 > 45 mmHg (or 6.0 kPa) | No details | No details | 24.1 (5.7) | Yes. Based on clinical evidence (snoring, reported apnoeas) |
| Oscroft et al. 2010112 | 25 (13/25, 52%) | 8 weeks (crossover trial) | Based on FEV1/FVC and FEV1 predicted specified as inclusion criteria, at least GOLD stage 2 or worse [mean FEV1 value at enrolment 24% (9%) predicted indicating more severe COPD] | No details | Stable. (No exacerbations in preceding 4 weeks; clinical stability confirmed during overnight assessment) | PaCO2 > 7.5 kPa | 65 (9.2) | Smoking history of over 20 pack-years | 31.5 (9.5) | No details |
| Murphy et al. 2012113 | 12 (8/12, 66.7%) | 6 weeks (crossover trial) | Based on FEV1/FVC and FEV1 predicted, GOLD stage 3 and 4 | No details | Stable. (No details on length of time without exacerbations) | PaCO2 > 6.0 kPa | 71 (8) | No details | No details | Yes. Based on limited attended respiratory polygraphy data |
Intervention and comparator characteristics
The three crossover RCTs compared different ventilator settings (Table 15). Dreher et al. 201032 compared high-pressure (assist/control mode) and low-pressure NIV (pressure support mode). This is similar to the comparison in the study by Oscroft et al. 2010,112 which also compares a high-pressure mode with a low-pressure mode.
| Author | Mask | Target | Modes being compared | IPAP | EPAP |
|---|---|---|---|---|---|
| Dreher et al. 201032 | Nasal or oronasal | Blood gases | High intensity: high pressure with respiratory rates beyond the spontaneous breathing frequency | High intensity, 28.6 mbar (1.9 mbar); low intensity, 14.6 mbar (0.8 mbar) | High intensity, 4.5 mbar (0.7 mbar); low intensity, 4.0 mbar (0 mbar) |
| Low intensity: low pressure with back-up respiratory rates of 8 bpm | |||||
| Oscroft et al. 2010112 | Unclear if nasal or full-face mask | Pressure | Volume assured: set to enable adjustment of inspiratory pressure up to 25, the maximum possible with this ventilator. Back-up rate as previously used | Volume assured-NIV: up to 25 cmH2O | Settings that patients had previously used |
| Pressure preset: set at similar pressure settings that subject had previously used. Back-up rate as previously used | Pressure preset-NIV: as previously used | ||||
| Murphy et al. 2012113 | Nasal or oronasal | Pressure | High intensity: high pressure and high back-up rate (–2 bpm) | Mean 29 cmH2O (SD 2 cmH2O) | Mean 5 cmH2O (SD 3 cmH2O) |
| High pressure: high-pressure and low back-up rate (6 bpm) |
The study by Murphy et al. (2012)113 compares two high-pressure modes, with high and low back-up rates, the hypothesis being that high inspiratory pressures with low back-up rate would result in levels of ventilator usage equivalent to those of high inspiratory pressures with high back-up rate.
Use of LTOT in the three studies was 100%,32 67%112 or unknown113 (no details provided).
Quality assessment
Full quality assessment details can be found in Appendix 2. Risk of bias for the generation of the randomisation sequence was unclear for all three RCTs; two112,113 provided details of allocation concealment. One study112 stated that trial subjects and technicians were blinded to the ventilation mode, one113 was described as single blind and one33 was open label. Given that all patients received an active treatment, lack of blinding may have been less important than in the studies comparing NIV with usual care only.
Risk of bias from incomplete outcome data was rated as high for two studies. In the study by Murphy et al. (2012),113 42% (5 out of 12) of patients withdrew, four of those during the high-pressure ventilation period; however, it was reported that there were no differences between completers and withdrawers, with the exception of FVC. The other study32 had a loss to follow-up of 23% (4 out of 17) and no details on differences in characteristics between completers and dropouts. Dropouts did not appear to be included in the analysis.
All were crossover trials and appeared to use appropriate forms of analysis; however, only one study32 performed a period-effect test for carryover effects.
Primary outcomes
Given the short duration of the head-to-head trials, the only primary outcome reported was QoL. The studies by Dreher et al. (2010)32 and Oscroft et al. (2010)112 comparing different pressures found no differences in total SRI score32 or SF-36 and SGRQ scores,112 although there was a trend for better QoL on the SGRQ with volume-assured NIV. The first32 of these two studies was rated as having a high risk of bias regarding incomplete outcome data. Neither of these studies was designed to look at the main outcomes of interest for this report as a primary outcome (their main outcomes as defined by the authors of the study were blood gases).
Murphy et al. (2012),113 which compared different breathing frequencies, also found no significant differences in total SRI scores, although there was a statistically significant difference for the respiratory symptom domain in favour of high-pressure ventilation (pressure support ventilation). The risk of bias for incompleteness of data was rated as high. It should be noted that the main aim of this study was to compare ventilator adherence rates. No conclusions can be drawn from these small, short-term trials regarding the effect on QoL of different ventilator settings.
Given the short follow-up times, small numbers of patients, differences in instruments and the inconclusive results from the larger (parallel) trials, it was not feasible to assess the consistency of results across trial design.
Adherence and adverse events
Table 16 gives details of the recommended schedule of NIV, any support provided for familiarisation, the extent to which patients adhered to NIV, and any adverse events associated with NIV, including where these led to treatment discontinuation.
| Study | Modes being compared | Period of adaptation/help in adapting to NIV | Recommended period of use | Adherence | Adverse events associated with NIV | ||
|---|---|---|---|---|---|---|---|
| How measured | Mean hours use (SD) | % of patients or other | |||||
| Dreher et al. 201032 | High intensity | Number of days needed for initiation of NIV: 4.6 (1.0) high, period 1, 3.7 (1.0) high, period 2 | Nocturnal use (no further details) | Ventilator counter reading | Period 1, 10.8 hours/day (4.7 hours/day); period 2, 8.9 hours/day (6.4 hours/day) | Two dropouts from low-intensity treatment arm during first period. Two patients refused to swap over to low intensity after first treatment period with high intensity | One patient refused low-intensity NIV in hospital because of intolerance. No further details |
| Low intensity | Number of days needed for initiation of NIV: 1.7 days (1.6 days) low, period 1, 1.6 days (0.8 days) low, period 2 | Period 1, 7.7 hours/day (3.0 hours/day); period 2, 4.6 hours/day (1.8 hours/day) | |||||
| Oscroft et al. 2010112 | Volume assured | Patients already established on pressure preset-NIV, mean use at study entry 7.8 hours/day (2.2 hours/day) | Nocturnal use (no further details) | Ventilator download data | 8.2 hours/day (3.6 hours/day) | 1/25 withdrew from study because of exacerbation | Appear to be none |
| Pressure preset | 7.7 hours/day (2.4 hours/day) | ||||||
| Murphy et al. 2012113 | High intensity | No details | Nocturnal use (no further details) | Ventilator download data | Mean nightly use 6 hours 33 minutes (2 hours 14 minutes) | 1/12 withdrawal (reason: acute exacerbation) | Appear to be none |
| High pressure | Mean nightly use 6 hours 37 minutes (SD 1 hour 45 minutes); mean difference 4 minutes (95% CI –45 minutes to 53 minutes) | 4/12 withdrawals (reasons: 1/12 claustrophobia, 2/12 intolerant of therapy, 1/12 prolonged central sleep apnoeas) | 1/12 claustrophobia, 2/12 intolerant of therapy | ||||
In the study by Oscroft et al. (2010)112 patients were already established on NIV, and in Dreher et al. (2010)32 there was an indication of the number of days patients had needed for initiation of NIV. There were no details in Murphy et al. (2010). 113 All three trials stated that NIV use was nocturnal but there were no details on minimum number of hours recommended. Data were recorded by the ventilators. Treatment compliance was similar between study arms in two studies,112,113 and was higher in the high-intensity arm in one. 32
Adverse events from NIV leading to dropouts included intolerance and claustrophobia; no conclusion could be drawn from this small data set on whether or not (specific) adverse events and/or discontinuations are more common with certain ventilator settings.
Secondary outcomes
The same secondary outcomes were considered for the head-to-head trials (FEV1, FVC, PaCO2, PaO2 and 6MWD). Table 17 shows the outcomes reported by the three trials and the main findings. Overall, there appear to be few significant differences in outcomes, although this may be in part a reflection of the small sample sizes and short follow-up times. There may be a slight trend for a benefit from high-pressure versus low-pressure NIV, with one statistically significant result (reduction in PaCO2) shown in Dreher et al. (2010). 32 Note that not all outcomes measured in the trials have been reported here.
| FEV1 (l or % predicted) | ||
|---|---|---|
| Study | Comparison | Main findings |
| Dreher et al. 201032 | High intensity (pressure) vs. low intensity (pressure) | Significant increase from baseline to 6 weeks in high intensity group. No significant between-group differences at 6 weeks for FEV1 (l) |
| Oscroft et al. 2010112 | Volume assured (higher pressure) vs. pressure preset (lower pressure) | Slightly higher values with volume assured NIV. No significant between-group differences at 6 weeks for FEV1 (l) or FEV1 (% predicted) |
| FVC (l or % predicted) | ||
| Oscroft et al. 2010112 | Volume assured (higher pressure) vs. pressure preset (lower pressure) | No significant between-group differences at 8 weeks for FVC (l) or FVC (% predicted) |
| PaCO2 | ||
| Dreher et al. 201032 | High intensity (pressure) vs. low intensity (pressure) | Statistically significant difference in reduction in favour of high-intensity NIV (p = 0.001) |
| Oscroft et al. 2010112 | Volume assured (higher pressure) vs. pressure preset (lower pressure) | No significant between-group differences at 8 weeks |
| Murphy et al. 2012113 | High intensity (high pressure + high back-up rate) vs. high pressure (high pressure + low back-up rate) | No significant between-group differences at 6 weeks |
| 6MWD | ||
| Dreher et al. 201032 | High intensity (pressure) vs. low intensity (pressure) | No significant between-group differences at 6 weeks |
Summary clinical effectiveness (non-invasive ventilation versus non-invasive ventilation)
-
Three small crossover trials with stable populations were identified, two32,112 comparing higher versus lower pressure, and one113 comparing similarly high pressures, with settings differing in back-up rates.
-
All trials were short term (6–8 weeks) and did not have the outcomes of primary interest for this report as their primary aim. Dropout rates were high in two32,113 of the studies.
-
Treatment compliance was similar between study arms in two studies112,113 and was higher in the high-intensity arm in one. 32
-
Quality of life was the only primary outcome measure (based variously on SRI, SF-36 and SGRQ). No firm conclusions could be drawn from the limited data on whether certain settings are more beneficial.
-
There was one statistically significant result (Dreher et al. 201032) in terms of PaCO2 reduction with higher-pressure NIV.
Review of systematic reviews
Eight systematic reviews/guidelines met the selection criteria for the review of clinical effectiveness of domiciliary NIV. 110,114–120 There were multiple publications relating to the authors of the Cochrane Review,110,121–124 with only the most recent update (2013/14) being considered here110,124 (reported in two publications).
This section concentrates on the more recently published systematic reviews: the Cochrane review (2013/14),110,124 Shi et al. (2013)119 and an evidence-based analysis for the Ontario Health Technology Assessment series (2012). 118 The reviews not mentioned further here are by Sin et al. (2003),117 Kolodziej et al. (2007),115 McKim et al. (2011)116 and Chen et al. (2011). 114 The recent guidelines120 are also not considered further, as their findings are based on systematic reviews discussed here. However, all the reviews had reasonably similar aims to the current review with, in most cases, a focus more on the physiological outcomes and less on those outcomes that might more readily populate an economic model to determine cost-effectiveness from a health-service provision perspective.
The Cochrane review110,124 was fairly robustly conducted and it had narrow inclusion criteria relative to the current review in that NIV use was required to be used for a minimum of 5 hours per night (for at least 3 weeks). Seven RCTs were included; of note is that a further seven RCTs were included in the current report and, although these may not have met the narrower inclusion criteria of the Cochrane review, they were not listed in the ‘excluded studies’ section of the Cochrane review. Some studies in abstract form were excluded from the Cochrane review, but this does not appear to have been an exclusion criterion. One study was listed as awaiting assessment because of clarification requests made to the authors, even although it had been published in 2007; this study is included in the current report (Xiang et al. 92 in 2007). Survival and exacerbations were not included as outcomes in the Cochrane review, although one of the publications124 mentions these outcomes in the discussion and states that there is insufficient evidence to draw any conclusions (based on three RCTs). The review, however, did obtain IPD and undertake IPD analysis for several outcomes, including for HRQoL. For the HRQoL, analysis was undertaken with data from two studies, both of which enrolled a relatively stable COPD population and used the SGRQ. The main conclusions from Cochrane review were that nocturnal domiciliary NIV for at least 5 hours per night and for at least 3 months in hypercapnic patients with COPD had no consistent clinically or statistically significant effect on gas exchange, exercise tolerance, HRQoL, lung function, respiratory muscle strength or sleep efficiency. The conclusion highlights the small sample size and the effect on drawing conclusions.
The Ontario Health Technology Assessment Series118 aimed to review both the clinical effectiveness and cost-effectiveness evidence of NIV compared with no ventilation in stable COPD patients. There appears to have been no restriction on the location or duration of use of NIV. The effectiveness review aimed to include only systematic reviews, meta-analyses and RCTs, published in English. Studies beyond RCTs were excluded. The review included eight RCTs, three of which were excluded from the current report, as the setting for NIV administration was deemed not to be domiciliary and this difference might be related to the breadth of the review question (see Decision problem). Primary effectiveness outcomes were similar to this report and the review was of a fairly good standard. A conclusion of the review was that there were no long-term beneficial effects of NIV on mortality, FEV1, O2 and CO2 levels, exercise tolerance and hospitalisations, and that HRQoL data were not substantial enough to allow the formation of conclusions.
The cost-effectiveness section of the Ontario Health Technology Assessment Series118 is reported in the systematic review of cost-effectiveness studies section of this report (see Chapter 6, Commentary on excluded studies).
The study by Shi et al. 119 in 2013 was a meta-analysis of RCTs of NIV in patients with severe stable COPD. The follow-up period had to be 2 weeks or longer; however, there was no restriction on setting of NIV use, and thus some studies included in this meta-analysis (n = 2) are excluded from the current report, as the setting was not domiciliary NIV use. Low-quality studies (Jadad score of < 2) were also excluded from the meta-analysis (n = 2); however, a list of excluded studies was not provided. The meta-analysis included eight RCTs and three crossover RCTs. One of these was not included in any other review or identified through searches of bibliographic databases for the current report. This RCT by Zhou et al. 81 in 2008 was undertaken and published in China and was included in the current report through citation checking of the Shi et al. meta-analysis. Primary effectiveness outcomes in the meta-analysis were similar to this report and the review was of a fairly good standard. The main conclusions were that, although NIV may improve gas exchange and dyspnoea, it has no effect on mortality, pulmonary function or exercise tolerance in severe stable COPD and that unbiased medium-term studies are required.
In summary, although there are a number of existing systematic reviews on NIV in COPD in what could be considered a non-emergency setting, there are some subtle differences in the review aims of the more recent reviews and thus differences in their study selections. However, the findings from these reviews are fairly uniform in highlighting the absence of consistent effects of NIV across outcomes, the limitations of the data and thus the difficulties in drawing firm conclusions of effectiveness.
None of the included systematic reviews subdivided studies by the proximity of the population to an exacerbation requiring hospitalisation, therefore the current report adds to the current knowledge base in this regard, as well as in relation to a primary focus on outcomes related to determination of cost-effectiveness.
Ongoing studies
Searches (undertaken September 2014) of clinical trials registers identified 416 potentially relevant records of apparent ongoing studies (see Chapter 3, Searches, and Appendix 1 for search strategies). Screening of these records and application of review selection criteria was undertaken. The key difficulty with the selection process was the absence of information in the records, given that these were not protocols for studies but only brief outlines of study aims with limited associated methodological information provided. Therefore, the relevance of some records to this report could not be ascertained. Despite this, it was possible to identify a number of records that met the selection criteria. However, some of these had trial registry records that had not been updated for several years (since April 2011), had not commenced (e.g. because of lack of funding) or were terminated because of recruitment issues. These studies are not considered further here. Table 18 details the remaining studies meeting the inclusion criteria.
| Trial codea | Research question | Design | Statusa/Details |
|---|---|---|---|
| NCT00990132 | To test the hypothesis that home mechanical ventilation and LTOT increases admission-free survival compared with LTOT alone in COPD patients who remain persistently hypercapnic following an acute exacerbation requiring NIV | RCT, open label, nocturnal NIV + LTOT vs. LTOT. Follow-up: 1 year | Eight UK university centres |
| Estimated enrolment: 116 | |||
| Study start: October 2009 | |||
| Estimated completion: December 2014 | |||
| Record last updated: May 2012 | |||
| Known as: HOT-HMV trial | |||
| Status: this study is enlisting participants at present | |||
| NCT01481727 | To evaluate the efficacy of high intensity non-invasive mechanical ventilation (during 1 year) to reduce the severity and frequency of acute exacerbations of COPD in patients with frequent exacerbations and in GOLD stage 4 | RCT, double blind, BIPAP vs. CPAP sham control. Follow-up: 1 year | National Institute of Respiratory Diseases, Mexico |
| Estimated enrolment: 25 | |||
| Study start: December 2011 | |||
| Estimated completion: December 2013 | |||
| Record last updated: May 2012 | |||
| Status: unknown recruitment status as information has not recently been verified | |||
| NCT01526642 | To determine whether or not home NIV can reduce recurrent acute HRF in COPD patients who survived an episode of acute HRF treated by NIV | RCT, open label, home NIV vs. LTOT. Follow-up: 2 years | Six French hospitals |
| Estimated enrolment: 86 | |||
| Study start: December 2011 | |||
| Estimated completion: December 2013 | |||
| Record last updated: February 2012 | |||
| Status: terminated | |||
| ISRCTN15832381 | To assess the application of NIV during an exercise programme following acute acidotic exacerbation of COPD in hospital and follow-up use at home, feasibility of using the Trilogy Ventilator | RCT, open label, exercise + oxygen versus exercise + NIV + oxygen vs. continued supervised exercise program + NIV + oxygen, if indicated, at home. Follow-up: 3 months | Bristol, UK |
| Estimated enrolment: 45 | |||
| Study start: February 2013 | |||
| Estimated completion: February 2015 | |||
| Record last updated: February 2013 | |||
| Status: study is ongoing | |||
| NCT01513655 | To investigate whether or not home NIV, as an exacerbation prophylactic treatment, may reduce mortality in COPD patients after first acute NIV treatment and secondarily reduce both exacerbations and hospitalisations and improve the QoL | RCT, single blind (outcome assessor), NIV + usual care vs. usual care. Follow-up: 1 year | Copenhagen, Denmark |
| Estimated enrolment: 150 | |||
| Study start: July 2013 | |||
| Estimated completion: August 2015 | |||
| Record last updated: July 2013 | |||
| Status: this study is enlisting participants at present |
For the reasons mentioned, the selection process could not provide definitive sets of included and excluded records in regard to ongoing studies, and as such no lists are given in this report. Therefore, this section serves to indicate that there are a number of ongoing studies which may add further data in the future.
Of the identified ongoing studies, one was known of prior to the start of this project.
A UK-based open-label trial (NCT00990132), commonly known as the HOT-HMV trial, aims to test the hypothesis that home mechanical ventilation and LTOT increases admission-free survival compared with LTOT alone in COPD patients who remain persistently hypercapnic following an acute exacerbation requiring NIV. Patients 2 to 4 weeks post acute hypercapnic exacerbation are recruited, randomised and then followed up for up to 12 months, with admission-free survival being the primary outcome measure. This study is thus of relevance to the post-hospital subgroup considered in this report. The target recruitment number is 116 patients across eight UK centres. An abstract by Murphy et al. 78 in 2011 from this trial was identified and included in the review of effectiveness (see Chapter 4, Clinical effectiveness results: non-invasive ventilation versus usual care). This abstract contained interim (3-month) data on issues related to sleep and adherence. Further details on the latter can be found in Chapter 4, Adherence and adverse events.
Contact with the principal investigator of the HOT-HMV trial in December 2014 identified that the study was still recruiting patients (105 out of 116 recruited so far), with full results expected in spring 2016. While wishing to be helpful to the current report, the principal investigator was concerned that premature analysis on an incomplete and underpowered data set would be meaningless and potentially misleading in comparison to the final data from the study. As such, no early data were supplied for this report. Enquiries about ways in which baseline patient data might be shared to additionally inform assumptions made in the economic modelling carried out in this report were also undertaken, but time scale prevented full pursuit of this.
Two further studies are appearing completed or near completion according to trial registry records. A small RCT (n = 25) to evaluate the efficacy of high-intensity non-invasive mechanical ventilation (during 1 year) to reduce the severity and frequency of acute exacerbations of COPD in patients with frequent exacerbations and in GOLD stage 4, appears to be ongoing in Mexico (NCT01481727). This trial is described as comparing BIPAP and CPAP sham control in a double-blind fashion. The duration since last exacerbation at enrolment is not stated for this trial. The completion date was stated in May 2012 to be December 2013. Contact with the investigators of this study (October 2014) identified that the study is still ongoing and recruitment was due to finish in November 2014 because of issues related to the strict inclusion criteria; thus no data are currently available. The other study near completion is an open-label RCT of home NIV versus LTOT to determine whether or not home NIV can reduce recurrent acute HRF in COPD patients who survived an episode of acute hypoxaemic respiratory failure treated by NIV. This French study has an estimated enrolment of 86 patients and the stated estimated completion date was December 2013; however, the trials register indicates that the study has been terminated. For this report contact was attempted with the investigators in this study, but no response was received (October 2014).
Finally, recently commenced studies are due to be completed in 2015. One is listed as being conducted in Bristol, UK (ISRCTN15832381), and aims to assess the application of NIV during an exercise programme following acute acidotic exacerbation of COPD in hospital and with patients in one arm of the three-armed study being followed up after 3 months’ use of home ventilation. The other study (NCT01513655) aims to investigate whether or not home NIV, as an exacerbation prophylactic treatment, may firstly reduce mortality in COPD patients after first acute NIV treatment and secondarily reduce both exacerbations and hospitalisations and improve QoL. This is a single-blind (outcome assessors blinded) RCT comparing domiciliary NIV added to usual care, with usual care and it appears that patients will be recruited while hospitalised for an exacerbation and will be followed up for 1 year. The study is being conducted in Denmark and the enrolment target is 150 patients.
Based on the information available for these studies, it appears that the HOT-HMV, Danish and, possibly, Bristolian studies might provide further evidence in a population commencing domiciliary NIV post-hospitalisation for an exacerbation. The Mexican study may provide evidence on a population of more frequent exacerbators.
None of the ongoing trials documented appear to be comparing two or more types or modes of NIV in a domiciliary setting.
Clinical effectiveness review discussion
Main findings
Eighteen RCTs and 10 controlled studies were identified that compared domiciliary NIV with usual care. Despite this fairly high number of studies, sample sizes were generally small (between 13 and 201 patients) and not all reported on the primary outcomes relevant to this report. The main findings of studies comparing NIV to usual care, for the two relevant COPD populations, are described below.
Stable population
For the stable population there was evidence to suggest that there was no difference between NIV and usual care in terms of survival, for a follow-up time of between 12 and 24 months. This was based on seven RCTs and four controlled studies. Longer-term survival data were available from three uncontrolled studies,87,88,93 although there was no information to confirm whether or not two87,88 of these were in a stable population. No longer-term study found a significant difference between NIV and usual care, although there were slightly fewer deaths with NIV at later time points (4 and 8 years). There appeared to be a trend towards fewer hospital admissions/days in hospital with NIV (based on five RCTs and three controlled studies with follow-up times up to 24 months) but there were no statistically significant differences. Heterogeneity in use of outcome measures meant that not all results relating to hospital admissions could be pooled; studies not included in the meta-analyses found no statistically significant differences overall. There were no significant differences in exacerbations based on three RCTs and one controlled study. QoL reporting was very heterogeneous in terms of use of instruments, time points and presentation of results. There appeared to be a trend favouring NIV, but a consistent benefit could not be demonstrated, and not all studies used, or reported on, appropriate methods for adjusting for baseline differences.
Post-hospital population
For the post-hospital population, evidence from three RCTs suggested that, on average, there was no difference between NIV and usual care (follow-up between 12 and 24 months) in terms of survival. Evidence from four non-randomised controlled studies found a statistically significant pooled result in favour of NIV in terms of survival. One of the non-randomised controlled studies86 found additional statistically significant benefits with NIV at 5 years, and, with a smaller effect size, at 10 years. This study had a different NIV schedule to all other studies (NIV intermittently for 15 minutes every hour during the daytime). Three RCTs75,90,92 reported disparate results regarding hospital admissions: one found a statistically significant result in favour of NIV,92 one marginally favoured NIV90 and one marginally favoured usual care. 75
Time to first hospital readmission due to any COPD exacerbation, reported in the one study,90 was not significantly different for the NIV and usual-care arms, although admissions occurred sooner in the usual-care arm. This study also found no significant difference for exacerbations without acute HRF.
Only one RCT (Struik et al. 201475) in a post-hospital population reported quality of life; there were no statistically significant differences between groups at 12 months, based on four different measurement scales.
Exploratory analyses
Subgroup analyses by level of hypercapnia were not possible given the small number of studies and the inconsistent reporting of the relevant variables. Performing a subgroup analysis based on reported mean baseline CO2 values would also have meant dichotomising trials based on an arbitrary threshold, and this was not considered appropriate. However, in a separate analysis, CO2 levels at baseline and change in CO2 levels were plotted against mortality in order to determine if baseline CO2 can predict response to NIV and whether or not the effect of NIV on CO2 correlates with the effect on mortality. These analyses, undertaken across both stable and post-hospital populations, did not suggest a relationship between CO2 levels at baseline and mortality; nor did changes in CO2 appear to correlate with mortality. It was unclear if there was a trend towards a correlation between CO2 levels at baseline and hospital admissions. While there does appear to be a trend towards a correlation between changes in CO2 and hospital admissions, the analysis is using aggregate data for change in CO2 and also for mean difference in hospital admissions, therefore a causal association cannot be inferred even if there is potential biological plausibility. Further, this was a post-hoc analysis, which is subject to a number of limitations (see Appendix 7).
Uncontrolled studies
Uncontrolled studies were explored where there was a lack of data from controlled studies, for example for long-term survival and QoL in a post-hospital population. Survival data were broadly consistent with those found in the NIV arms of controlled studies, although comparisons were difficult because of different time points of reporting and potential population differences. No useful QoL data were found in the uncontrolled studies for the post-hospital population. Overall there were no findings from uncontrolled studies that either contradicted or added meaningfully to data from controlled studies.
Quality of included studies (randomised controlled trials and controlled)
Overall, the RCTs comparing NIV with usual care appeared to be of reasonably good quality, although there was a lack of reporting of some details relevant to quality. Only three RCTs included a ‘sham NIV’ arm, and only one of these reported a primary outcome of interest (Cheung et al. 201090 – hospitalisations/exacerbations). It is possible that studies with no ‘sham NIV’ may have been subject to performance bias, with patients in the NIV arms (inadvertently) receiving more medical attention; this in turn may have led to more positive outcomes overall. However, the use of sham NIV may also enhance the apparent benefit of NIV by resulting in side effects without benefit in the control arm. A lack of blinding may also have affected QoL reporting by patients. Some studies suffered from fairly substantial loss to follow-up (up to 59%), and it was not always clear if an ITT analysis had been undertaken. Particularly for secondary outcomes (e.g. blood gases), it was more common for completers only to be included, possibly as a consequence of people with severe COPD finding it difficult to attend follow-up appointments.
Non-randomised studies are likely to be more prone to bias, particularly in terms of patient selection. While the prospective controlled studies did not appear to have major differences in terms of patient characteristics, some of the retrospective studies did allocate patients to NIV and usual-care groups on the basis of different criteria (dependent on blood gases), thus complicating the interpretation of results. Three retrospective studies also reported more frequent follow-up visits in the NIV arm. Overall, there were fewer details on blinding, adherence and how incomplete data were dealt with in the non-randomised (particularly retrospective) studies. Four of the five retrospective studies reported only survival, in which case loss to follow-up may be less of a problem in terms of accessing outcome data. There does, however, appear to be consistency in terms of direction of effect between non-randomised and randomised studies for the stable population (survival and hospitalisation results) despite potential differences in quality and robustness of data. For the post-hospital population, the controlled studies appeared to show more of a benefit from NIV than the RCTs for survival. There were no controlled studies in this population reporting hospital admissions.
Effect of patient characteristics on effectiveness of non-invasive ventilation
There are a number of patient characteristics that could potentially influence the effectiveness of NIV, including comorbidities, level of hypercapnia, and whether or not a patient is on LTOT or suffers frequent exacerbations. Formal subgroup analysis for these parameters was not feasible given the small number of studies, lack of reporting detail and other study heterogeneity. Based on the data in this report, it is currently not possible to make a judgement on the type of patient who is most or least likely to benefit from NIV. COPD is being recognised as an increasingly diverse disease, and individuals may benefit from more tailored treatment approaches applied to NIV. Examples of tailored treatment in COPD include roflumilast for patients with chronic bronchitis125 and lung volume reduction surgery for patients with both predominantly upper-lobe emphysema and low baseline exercise capacity. 126
Effect of different pressure settings on effectiveness of non-invasive ventilation
Non-invasive ventilation pressure settings may influence effectiveness, and this report identified three RCTs directly comparing different settings. The main outcomes of interest of these small, short-term crossover RCTs were lung function, blood gases and adherence. QoL was also measured, but no firm conclusions could be drawn from the available data. One study32 comparing high pressure with low pressure reported a statistically significant result in favour of NIV (reduction of PaCO2) with the higher-pressure setting.
Pressure may be important not only in terms of effectiveness, but also in terms of adherence, as higher pressure may be experienced as less comfortable by the patient and thus lead to discontinuation. There were no major differences between pressure settings regarding adherence in the three small head-to-head trials. For RCTs comparing NIV versus usual care only, it was not possible to undertake formal subgroup analyses based on pressure, given the small number of studies, the inconsistency in how pressure was reported and other potential sources of heterogeneity. It is conceivable that discontinuation rates may differ between stable and post-hospital populations, with, for example, post-hospital patients being more motivated to use NIV. It was, however, not possible to make a judgement on this given the available data.
Adverse events
Adverse events were inconsistently reported but in line with those known to affect NIV patients and generally not serious (e.g. mask discomfort, pressure experienced as too high, inability to sleep, etc.). There was only one potentially more serious adverse event, a suspected barotrauma. 92
Secondary outcomes
There was a clear trend among stable populations towards a benefit from NIV for improving blood gases (PaO2 and PaCO2) and, based on more limited data, in the case of the post-hospital population, for reduction in PaCO2. There was no consistent trend for the other secondary outcomes (lung function parameters, 6MWD). Only one study79,80 presented appropriately adjusted results; it would be of interest to undertake an analysis across all trials using appropriately adjusted results in order to examine the potential impact. For 6MWD, in particular, there appeared to be substantial baseline imbalances, which may be a reason for the inconsistent results found for this outcome.
Stable versus post-hospital patients
In this report a distinction was made between stable and post-hospital populations in order to capture baseline imbalances in risk of future exacerbations. It is recognised, however, that there are likely to be not two distinct groups, but, rather, a continuum of patients with differing baseline risks (which change over time). The population in the RCT by Zhou et al. (2008)81 was defined as stable, but the rate of hospital admissions in the usual-care arm was higher than that of the other stable populations and similar to the usual-care arm rates in one post-hospital study. Benefit from NIV (in terms of reduction of hospital admissions and a trend towards survival) appeared to be greater in this study than in the other studies with stable populations. Given the lack of information in most studies on history of exacerbations, the dichotomisation into stable and post-hospital patients was a pragmatic one. However, it is also known that patients with a recent exacerbation are more likely than patients who have been stable for a period of time to experience a further exacerbation. More recent GOLD criteria (from 2011)127 take into account the risk of exacerbations, but this classification was not used in the included studies.
Generalisability of findings
In terms of applicability, RCTs have a tendency to use more stringent inclusion criteria than non-randomised studies. A broader, potentially less well population might therefore be expected to be included in the non-randomised studies. Given the relative consistency in the results between RCTs and non-randomised studies (at least for the stable population), there is nothing to suggest significant differences between the populations, although there may be other sources of heterogeneity obscuring these. Most of the RCTs and the prospective non-randomised studies specifically excluded patients with obstructive sleep apnoea, so results are not applicable to patients with overlap syndrome. While primary obstructive sleep apnoea is treated with CPAP, COPD patients with overlap syndrome may benefit from NIV, and could be considered for future trials. 128
A majority of patients in the post-hospital studies had a form of ventilation in hospital before commencing domiciliary NIV. This might indicate a more severe population than a general COPD population being admitted to hospital, where one might expect around 15% to be treated with ventilation during their stay. 129 However, previously ventilated patients might be the type of patient most likely to be selected for NIV.
A recent analysis130 of UK COPD patients followed after one or more hospital admissions requiring NIV found mortality rates of between 45% and 57% at 1 year, between 62% and 68% at 3 years and between 72% and 75% at 5 years. This was broadly consistent with the 1-year mortality rates in the usual-care arm of one post-hospital study97 included in this report. There were some differences between the UK data and the study populations in this report at later time points, but comparisons were hampered because of reporting of mortality at different time points.
Strengths and limitations of the available data
A number of RCTs of reasonably good methodological quality were available, particularly for the stable population. However, there were several limitations, particularly concerning the outcomes relevant to the economic model. Hospital admissions data were not reported consistently across trials, so not all available evidence contributed to the pooled estimate for the stable population; further, admissions data may be skewed and the mean (SD) may not be the most appropriate metric to use, although it was frequently reported. QoL was inconsistently measured in studies of stable patients, and in only one study of post-hospital patients. As a result, the impact of domiciliary NIV on QoL remains uncertain for both populations. A trend of improved QoL needs to be confirmed in stable patients. While a reduction in hospital admissions will likely have a temporary impact on QoL, the effect of NIV on daily living (when COPD is stable) remains uncertain. Further research on which instruments best capture QoL in this patient group and whether scores are translatable between different instruments is necessary. Qualitative research may help to inform which aspects of QoL should be reflected in assessment tools. The timing of QoL measurements is important. Patients may experience a decrease in QoL early on (while they are adapting to the equipment) but an increase at later stages, provided the treatment is effective. A potential survivor effect also needs to be taken into account; QoL may eventually decrease in the later stages of COPD, but this could be in patients who might not have survived without NIV.
For secondary outcomes, most studies did not present adjusted results, despite there being baseline imbalances. Only one study79,80 used an appropriate method of adjusting. Baseline imbalance should be accounted for using an analysis of covariance model. 131 In the absence of this, analyses that use the summary results to adjust for baseline imbalance will not account for the within-study correlations between these repeated measures. More sophisticated analyses may be possible; for example, external evidence could be used to provide an informative prior for the within-study change variance, but this was outside the scope of the current report.
Other limitations in the available data included poor description of when and where NIV was initiated; a lack of detail to allow for a complete judgement on study quality; a lack of reporting of time-to-event data and HRs (even for survival data); inconsistent reporting of adverse events; and a lack of data explicitly linking the number of exacerbations to subsequent hospitalisations and survival. This last point has potential implications for double-counting data, as these outcomes are not independent of each other.
Strengths and limitations of this report
A comprehensive search strategy (including citation checking) meant that this report identified more relevant studies than previous systematic reviews (even taking into account different search periods).
The fact that no language restrictions were applied meant that 6 out of 31 (19%) of the included RCTs or controlled studies were non-English language, constituting a substantial proportion of the overall evidence base.
This is the first systematic review to attempt to account for differing baseline risks of exacerbation/hospitalisation by categorising populations into stable and post hospital. This is also the first systematic review to both focus on patient-related outcomes (survival, hospitalisation, exacerbations and QoL) and incorporate data from non-randomised studies in separate analyses. Further, by calculating summary measures from raw data or converting data, the number of results that could be presented in forest plots was maximised.
In contrast to some previous systematic reviews, secondary outcome data (lung function, blood gases and 6MWD) were not pooled because of a lack of reporting of results which were appropriately adjusted for baseline. A failure to do this can result in misleading results (e.g. where the usual-care arm shows a higher final result but the improvement has been greater in the NIV arm). Instead, results were presented in forest plots with the method of calculating the effect estimate highlighted, so that findings could be put into the context of the robustness of the methods.
Heterogeneity in the way some outcomes were reported meant that not all studies could be incorporated into meta-analyses (e.g. for exacerbations and some hospitalisation data). Small numbers of studies in meta-analyses (up to seven maximum) precluded the construction of funnel plots, and the small number, together with a lack of reporting of some quality criteria, meant that sensitivity analyses around study quality were also not feasible.
Chapter 6 Cost-effectiveness review
This chapter describes the methods and results of a systematic review of the literature on the cost-effectiveness of domiciliary NIV in patients with end-stage COPD.
Methods
The methods of this systematic review were broadly similar to those presented in Chapter 3 and thus only key details and differences are given here.
Search strategy
Searches for economic studies were run on MEDLINE, EMBASE via Ovid and NHS Economic Evaluation Database using, where appropriate, relevant terms for economic studies along with terms for clinical populations. Examples of these strategies can be found in Appendix 1. These searches were supplemented with any further economic evaluations and cost studies identified during screening of the search yield in the clinical effectiveness review.
Study selection
All records were screened by two reviewers independently, and copies of potentially relevant articles were obtained for scrutiny against the full selection criteria, with any disagreements resolved by discussion. The inclusion criteria were the same as those for the clinical effectiveness review except that the study designs were full economic evaluations, partial economic evaluations and cost-effectiveness analyses alongside trials and economic modelling studies.
Data extraction and quality assessment strategy
Data on the following, where available, were extracted from included studies by one reviewer and checked by another:
-
study characteristics, such as study question, form of economic analysis, population, interventions, comparators, perspective, time horizon and form of modelling used
-
clinical effectiveness and cost parameters, such as effectiveness data, health state valuations (utilities), resource-use data, unit-cost data, price year, discounting and key assumptions
-
results and sensitivity analyses.
Studies were to be quality-assessed using the Drummond checklist132 for economic evaluations and the checklist by Philips133 for model-based analyses.
Results
A total of 342 records were identified from the searches and, following the removal of duplicates, there were 247 unique records. Eight additional articles were identified from the systematic review of clinical effectiveness, three of which were duplicates.
Thirty-one records were deemed relevant to this economic review and hard copies were obtained for scrutiny against the inclusion criteria for the review. Two of these met the inclusion criteria. 40,134 A list of excluded studies can be found in Appendix 10. A flow diagram summarising the selection process can be found in Figure 15.
FIGURE 15.
Flow diagram depicting article selection for cost-effectiveness review.
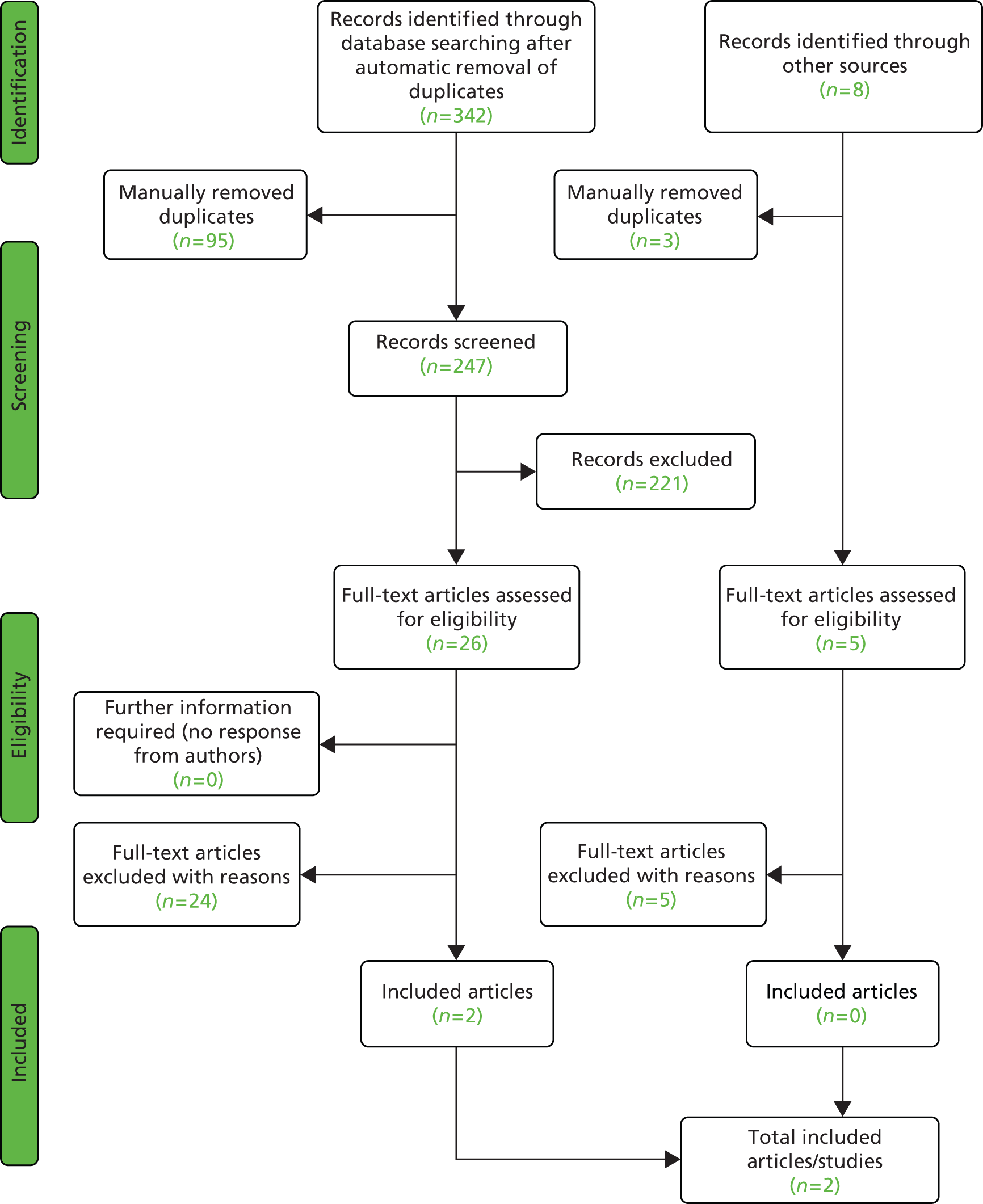
Summary of included studies
Both included studies conducted a cost analysis alongside a clinical study of domiciliary NIV in COPD patients. 40,134 Each study met at least eight of the 10 Drummond and Jefferson quality-assessment criteria (see Appendix 11). 132 Neither study was sponsored by industry, although some potential conflicts of interested were declared by Tuggey et al. (2003). 40 The characteristics and main results of each study are summarised in Table 19.
| Economic analysis features | Tuggey et al. (2003)40 | Clini et al. (2009)134 |
|---|---|---|
| Country | UK | Italy |
| Sponsor | Research was funded by the Northern and Yorkshire NHS Executive. Ventilator equipment was provided on loan by ventilator companies. One of the authors received an honoraria from a ventilator company for lecturing | Research was funded by the Italian Association of Pulmonologists |
| Choice of therapy | Patients admitted frequently to hospital who responded well to NIV and were offered or requested home NIV | Home NIV added to LTOT |
| Comparators | Care in the year prior to treatment with home NIV | LTOT alone |
| Form of analysis | Retrospective cost analysis from a hospital perspective | Retrospective cost analysis of NIV alongside randomised trial |
| Sample size | 13 monitored before and after commencing home NIV | 35 on LTOT and home NIV; 42 on LTOT alone |
| Patient characteristics | 69% male, mean age 55 years, FEV1 0.58 l (SD 0.24 l) | 78% male, mean age 66 years ± 6 years, FEV1 % predicted 30% ± 10%, BMI median 23 kg/m2 ± 4 kg/m2 (range 18–28 kg/m2) |
| Model used | N/A | N/A |
| Inclusion criteria | History of recurrent severe exacerbations. Good tolerance of NIV during acute exacerbation | Prescription for LTOT. Diagnosis of COPD |
| Time horizon | 1 year | 2 years |
| Type of costs considered | Ward costs: capital, nursing and pharmacy, intensive care, acute NIV, home NIV, staff training, outpatient appointments | Hospital admission because of exacerbation, drug therapy, LTOT supplementation, domiciliary ventilators including accessories |
| Cost year and currency | 2003, GBP | 2008, EUR |
| Base-case results | Cost per patient on home NIV was £4909. Cost per patient on usual care was £13,163 | Cost per patient per day in NIV and LTOT was €23.72 ± €16.18. Cost per patient per day in LTOT alone is €21.42 ± €20.38 |
| Conclusions | The provision of home NIV in patients subject to frequent exacerbations who tolerate acute NIV well was associated with cost savings because of reduced hospital admissions and reduced lengths of stay | The addition of home NIV to COPD patients on LTOT did not result in an increase in cost as the cost of home NIV was equalised by the savings from reduced hospital admissions |
Tuggey et al. (2003)
Tuggey et al. ’s (2003)40 cost analysis was based on a before-and-after study of 13 patients offered domiciliary NIV in one UK hospital between 1995 and 2000. Patients were started on NIV if they had a history of recurrent COPD-related hospital admissions (mean of five per year) and had demonstrated prior tolerance of NIV during treatment of an acute exacerbation. As the clinical study enrolled a highly select group of COPD patients, the results are not necessarily transferable to a wider COPD population.
Outcomes were collected from case notes and as a result are potentially biased because of the risk of reporting errors. The study found a statistically significant reduction in admissions, which was the primary measure of effect considered in the subsequent cost analysis. Costs were collected retrospectively and the cost analysis was conducted from a hospital perspective, and thus did not include costs incurred by patients, carers, primary care or the wider society. Costs included acute and conventional hospital admissions, intensive care treatment, outpatient appointments and the cost of domiciliary NIV. The cost of trialled patients on NIV in hospital was not considered as an additional cost, as this was considered part of usual care.
A number of assumptions regarding the costs of acute and domiciliary NIV were drawn from Plant et al. (2003),135 a published economic evaluation of NIV for managing acute exacerbation in COPD patients. In this analysis the provision of domiciliary NIV was estimated to cost £1060 per patient per year in 2003 NHS prices. This included £570 for ventilator equipment discounted over 5 years, £224 for mask and tubing with an assumed lifespan of 8 months, £179 for a warm air humidifier with an assumed lifespan of 1 year, £28 for annual servicing and £60 for access to a respiratory nurse specialist.
The results of this analysis found domiciliary NIV in COPD patients with recurrent exacerbation, implemented at discharge, to be cost-saving, resulting in a net saving of £8254 (95% CI £4013 to £12,495) per patient per year. A one-way sensitivity analysis was conducted by varying key assumptions from baseline between the 5th and 95th centiles, and in all scenarios domiciliary NIV was cost-saving compared with usual care alone.
In summary, this study suggests domiciliary NIV may be cost-effective in NIV patients with a history of readmission to hospital. Caution should be taken in interpreting these results, as they are based on a very small sample size and highly select group of patients.
Clini et al. (2009)
Clini et al. (2009)134 presented the results of an Italian cost analysis, conducted alongside a RCT of domiciliary NIV, where 77 patients were followed up for 2 years. Patients with stable COPD, with a prior prescription of LTOT, were enrolled on the trial and randomised to NIV or usual care alone.
This cost analysis applied the mean reduction in hospital admissions and length of stay derived from the RCT. It should, however, be noted that, while the outcomes found in the RCT were in favour of NIV, the results applied were not statistically significant. The resources considered in the analysis included hospital admissions, drug therapy, LTOT and domiciliary NIV equipment and training. Neither the other primary-care resources and costs incurred by patients and their families nor the additional hospital and set-up costs to familiarise patients with NIV were included.
The study estimated the cost of domiciliary NIV to be €160 per month or €1920 per year based on 2008 prices. This estimate was based on a contract with a regional health-care provider and included the provision of equipment, a Respironics BiPAP® ST-30 (Murrysville, PA, USA) device and warm air humidifier, as well as tubing and masks replaced every 6 months. The results suggest that the addition of domiciliary NIV to LTOT results in a similar cost per patient per day of €23.73 ± €16.18, compared with €21.42 ± €20.38 in the group with LTOT alone, from which the authors concluded that domiciliary NIV was likely to be cost-neutral, as the additional costs were offset by savings from reduced hospital admissions and shorter hospital stays.
In summary, this study suggests that domiciliary NIV may be cost-neutral in stable COPD patients. However, as this study was conducted in Italy, the costs and outcomes are not directly transferable to UK patients. The results should also be considered cautiously because of uncertainty around the effect.
Commentary on excluded studies
Two studies excluded from this review warrant comment.
Criner et al. (1995)136 conducted a retrospective cost analysis of providing NIV to patients admitted to a ventilator rehabilitation unit for moderately severe respiratory failure in the USA. There was some ambiguity as to whether or not this met the inclusion criterion, as it was unclear if patients were discharged with NIV for home use. On balance, the decision was made to exclude the study, as it appeared to primarily assess the effect of NIV delivered in an acute setting. This study demonstrated the interdependence between acute and domiciliary NIV services when considering costs and outcomes.
Chandra et al. (2012)137 published a Markov model developed as part of a series of papers to evaluate the effectiveness and cost-effectiveness of interventions in COPD populations sponsored by the Medical Advisory Secretariat, Canada. NIV in stable COPD patients was included in this series and the authors documented their intent to conduct a clinical review and economic evaluation. 137 However, after conducting the clinical review, they concluded that there was no evidence that domiciliary NIV was effective in COPD patients and thus did not conduct an economic analysis.
Conclusions
This systematic review identified two studies that met the inclusion criterion. Tuggey et al. (2003)40 conducted a cost analysis alongside a before-and-after study and found that domiciliary NIV, provided in a highly select COPD population prone to severe exacerbation, may be cost-effective in this subpopulation. However, there was a lot of uncertainty around their results because of the nature of the study design and the small population size. Similarly, the cost analysis by Clini et al. (2009)134 suggested that the additional cost of NIV may be offset by cost savings resulting from a reduction in hospital admissions in ambulatory COPD patients. These results were also not generalisable to the UK population, as the costs were estimated from an Italian hospital perspective, and the results should be considered cautiously because of the uncertainty regarding clinical effectiveness estimates applied.
While both studies provided useful estimates on the cost of providing domiciliary NIV to inform an economic model, neither addressed lifetime cost-effectiveness of NIV in a UK population. This suggests there is a need for a de novo study of the cost-effectiveness of domiciliary NIV in end-stage COPD patients.
Chapter 7 Economic evaluation
This section provides a description of the economic model developed and used to evaluate the cost-effectiveness (cost–utility) of domiciliary NIV in patients with end-stage COPD, when compared with usual care alone. The two COPD populations considered in the systematic review of clinical effectiveness (see Chapter 4) were also considered in this economic evaluation:
-
patients started on domiciliary NIV when stable in the community, referred to as the ‘stable’ population
-
patients started on domiciliary NIV following an admission to hospital for exacerbation, referred to as the ‘post-hospital’ population.
The underlying assumption in differentiating between these two populations was that COPD patients with a recent history of hospital admission were more likely to have a higher baseline risk of COPD events and a shorter life expectancy.
Using appropriate evidence where possible from the systematic review of clinical effectiveness, a speculative model was built to estimate the cost-effectiveness of domiciliary NIV if it were to result in a reduction in the risk of hospital admissions. Hospital admission was chosen as the primary outcome for modelling, as severe exacerbations resulting in hospital admission were expected to be the main cause of COPD-related morbidity and mortality. Using this outcome would capture the potential costs and utility associated with any change in the risk of readmission. There was little evidence of an improvement in QoL for the stable population and none for the post-hospital population; therefore, an assumption was made that the only improvement in QoL would be based on avoided hospital admission. A potential additional change in utility in either direction was considered in a sensitivity analysis.
The model was run using hospital admissions data from RCTs in the two populations. The base-case risk reduction for hospital admission for the stable population was based on the rate ratio derived from the pooled results of the five RCTs76,80,81,99,101 suitable for meta-analysis (see Chapter 4, Hospitalisations). For the post-hospital population, three RCTs75,90,92 provided evidence on hospitalisations (see Chapter 4, Hospitalisations). As the size and direction of the effects reported were very different, three base cases were considered by applying three different rate ratios derived from the RCT data.
The long-term effect of NIV on hospitalisations is unclear, as there is no evidence beyond 24 months for either population. However, for the purposes of the model base cases, it was assumed that the effect on hospitalisation lasted for up to 10 years, and the costs were assumed to last for the cohort lifetime. The uncertainty around this assumption was considered in sensitivity analyses in which different assumptions were applied for the duration of the effect.
Methods
Model description
A Markov decision model was built in TreeAgePro (TreeAge Software, Inc., 2013) to compare the cost-effectiveness of domiciliary NIV with usual care from a UK perspective.
The model was developed subsequent to a review of published Markov decision models138–147 in stable and post-admission COPD populations, and considers stable and post-admission populations separately. The model incorporated both the short-term increased risk of readmission and subsequent mortality after a hospital admission, and the long-term natural history of the disease, taking into account exacerbations, increasing COPD severity and mortality (Figure 16). Health states are linked to GOLD severity (using the pre-2011 definition of GOLD because of a lack of available data for the current classification). It incorporates evidence on the increased risk of mortality and readmission in those recently discharged after admission for COPD exacerbation. Tunnel states were added to incorporate the increased risk of mortality and readmission in those recently discharged after admission for COPD exacerbation. 129
FIGURE 16.
Model health states.
The model considers stable or post-discharge health states (at months 1, 2 and 3); that is, patients can be a stable condition or in month 1, 2 or 3 of recovery after a recent exacerbation. Patients can move between stable and post-discharge health states, and there is a risk of death while in any health state. The two populations, stable and post-hospital, differ in where they enter the model and in the probability of moving between the different health states.
The model had a time cycle of 1 month and a lifetime time horizon (30 years) was used. All costs and outcomes were considered from a UK NHS perspective for a price year of 2012.
The model had three transition health states and one stable health state, with each state subdivided into two disease severity levels (see Figure 16). The three transition health states captured patients’ elevated risk of readmission or death in each of the 3 months subsequent to a hospital admission and were referred to as the ‘post-discharge’ health states. A post-discharge period of 3 months was chosen as there are consistent data to indicate elevated risk of readmission and death during this period (2011 audit129). The ‘stable’ health state represented patients’ ongoing risks of events (e.g. exacerbation, hospital admission, death) beyond this period. The subdivision of these states represented GOLD stages 3 and 4. At any point patients could transition to a worse disease severity or die. As COPD is a progressive disease, patients could not transition to an improved health state (e.g. from GOLD stage 4 to 3).
The two COPD severity levels considered were defined according to the traditional GOLD classification stages 1–4. GOLD stage 3 (severe COPD) was defined as having a predicted FEV1 ≥ 30% but < 50% and GOLD stage 4 (very severe COPD) a predicted FEV1 ≤ 30%. Health states for GOLD stage 1 (mild COPD) and GOLD stage 2 (moderate) were excluded, as it was assumed that the majority of patients with end-stage COPD were at GOLD stage 3 or 4. After any cycle, patients in GOLD stage 3 could move to a GOLD stage 4 health state as their disease progressed, but GOLD stage 4 patients could not move back to GOLD stage 3.
The stable health states for the stable population reflected outcomes (e.g. exacerbation, readmission, death) reported in large cohorts of COPD patients who were stable for at least 12 weeks, and the post-discharge health states reflected short-term outcomes immediately after admission for severe exacerbation.
The stable health states for the post-hospital population were adapted slightly and populated using different sources to reflect outcomes reported in cohorts of COPD patients followed up after admission to hospital. The post-discharge health states for the post-hospital population were identical to those applied in the stable population (further details on parameterisation of the model are given in Estimation of model parameters).
Model entry
The stable cohort entered the model in one of the two stable health states (GOLD 3 or 4) and the post-hospital population entered the model in one of the two first month post-admission health states.
Transitions within the stable health states
In the stable health state, patients could die from a non-COPD-related cause, live exacerbation-free or experience an exacerbation. The exacerbation could be moderate (managed at home) or severe (requiring admission) or be fatal. Those who survived a severe exacerbation were discharged and moved to a first month post-discharge health state. Those who lived without experiencing an exacerbation or experienced a moderate exacerbation re-entered a stable health state. An example of the pathway within a stable health state is illustrated with reference to GOLD stage 3 for the stable population in Figure 17.
FIGURE 17.
Example of pathway within stable health state with reference to GOLD stage 3 for the stable population.
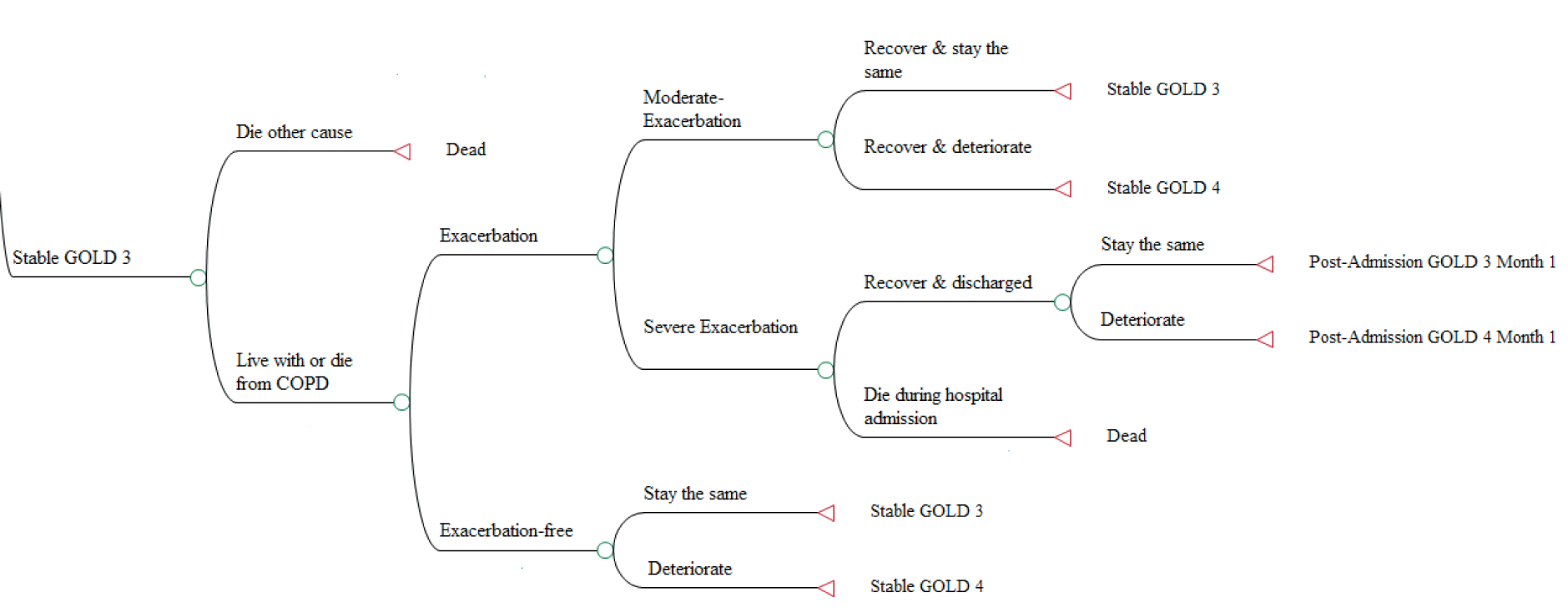
This pathway was almost identical for GOLD stage 4 in the stable population, but patients were not able to move to a GOLD stage 3 health state.
The pathways for the GOLD stage 3 and 4 stable health for the post-hospital population were similar but populated using different sources. As the risk of a moderate exacerbation (managed at home) was not reported for this population, the risk of exacerbation without admission was not considered for the post-hospital population.
Transitions within the post-discharge health states
Patients who entered the post-discharge health state could die at home, continue their recovery or be readmitted, where they could die during admission. If they survived the hospital admission, they re-entered one of the first month post-admission health states. If recovery continued without being readmitted, they moved to the second and then third post-admission health states where they faced similar pathways. The additional costs and utility losses associated with a non-severe exacerbation during the recovery period were considered negligible, as patients were already assumed to have incurred higher costs and utility loss.
The pathways within the post-discharge health states were almost identical for months 1–3, but in month 3 patients could transition to a stable health state and differed only in disease severity, allowing GOLD stage 3 patients to transition to a parallel GOLD stage 4 state. As noted above, the post-discharge health states were identical for the stable and post-hospital populations. An example of the pathway within a post-discharge health state is illustrated with reference to the first month post discharge for GOLD stage 3 in the stable population in Figure 18.
FIGURE 18.
Example of pathway within a post-discharge health state with reference to GOLD stage 3 for the stable population.
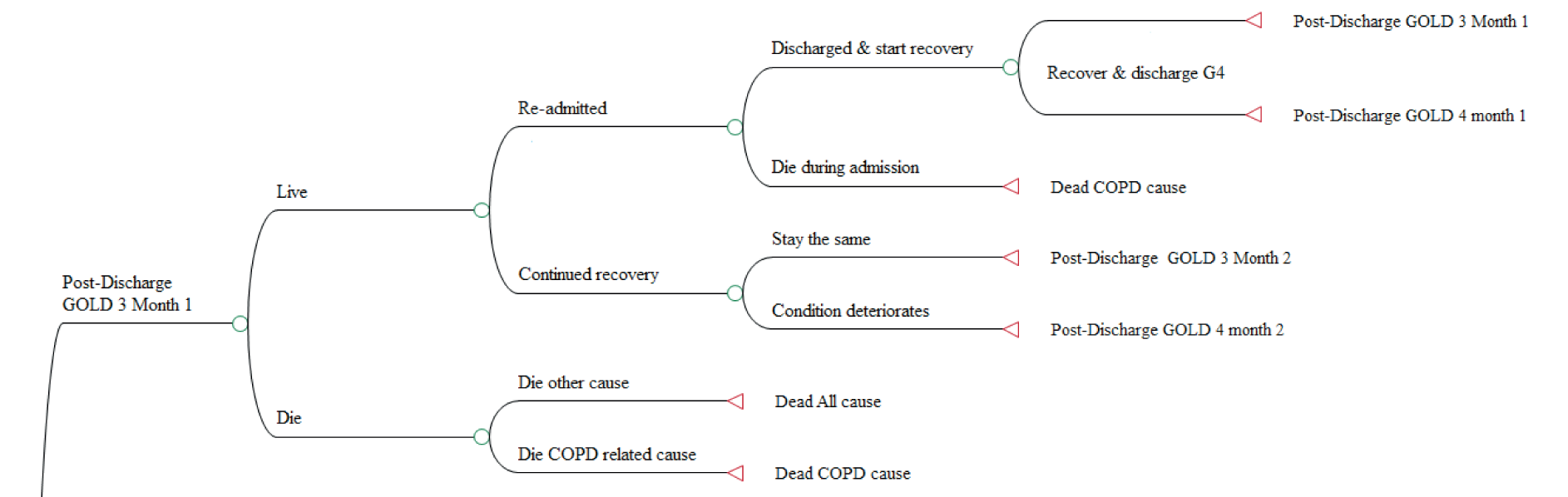
Population demographics
The stable COPD population was assumed to have similar demographics to those patients enrolled in the studies included in the clinical effectiveness systematic review. The 2011 COPD hospital admissions audit was considered to be a more appropriate source of UK demographics data for the post-hospital population, as the studies included in the systematic review were small and non-UK based. It should be noted that a relatively small proportion of patients included in the audit received in-hospital ventilation (before commencing domiciliary NIV), while the studies included in the systematic review had larger proportions of patients receiving this treatment, indicating a potentially more severe population compared with the one audited.
The base-case age, sex, smoking status and disease severity applied in both cohorts are detailed in Table 20.
Estimation of model parameters
This section outlines in detail the assumptions applied and sources used to populate the base-case parameters for usual care and domiciliary NIV in both populations. For those readers wishing to just read a summary of the assumptions and data sources applied in the model, these are provided in Chapter 7, Assessment of cost-effectiveness.
Exacerbation and hospitalisation risks for the stable chronic obstructive pulmonary disease population
The probabilities of exacerbation and hospital admission for the stable population in the stable health states were obtained from the TORCH129,148 and Eclipse2,129 studies respectively and are reported in Tables 21 and 22. These studies were chosen as they reported outcomes in large cohorts of COPD patients followed up for at least 3 years. Therefore, the mean exacerbation and hospitalisation risks were expected to reflect COPD-related mortality in stable GOLD stage 3 and 4 patients.
| Treatment and exacerbation rates | Treatment type | |||
|---|---|---|---|---|
| Othera | ICS | LABA | ICS/LABA | |
| GOLD stage 3 | ||||
| Proportion on treatment (%) (BLISS149) | 26.27 | 0.85 | 5.08 | 67.80 |
| Annual exacerbation rate (TORCH148) | 1.24 | 0.99 | 1.08 | 0.91 |
| GOLD stage 4 | ||||
| Proportion on treatment (%) (BLISS149) | 16.28 | 4.65 | 2.33 | 76.74 |
| Annual exacerbation rate (TORCH148) | 1.79 | 1.53 | 1.40 | 1.54 |
| Severity stage | Overall weighted exacerbation rate148 | Proportion of exacerbations hospitalised2 | |
|---|---|---|---|
| % | Beta distribution | ||
| GOLD stage 3 | 1.00 | 25 | α = 225, β = 675 |
| GOLD stage 4 | 1.57 | 54 | α = 158, β = 135 |
As exacerbation rates from the TORCH trial148 were reported by type of treatment, assumptions were required for the proportion of patients on each type of treatment in each GOLD stage health state. These proportions were obtained from unpublished data collected from a cohort of UK COPD patients recruited as part of the Birmingham Lung Improvement (BLISS) studies149 in the West Midlands. Exacerbation rates were then weighted by the proportion of patients on each treatment, in each GOLD stage severity group. As the TORCH148 study did not report the proportion admitted to hospital for an exacerbation, this was obtained from the Eclipse Study. 2
Hospitalisation and mortality risks in the post-discharge health states (for both post-hospital and stable populations)
The majority of the transition probabilities for post-discharge health states were obtained from the European Audit129 and are reported in Table 23. This study was chosen as it included recent UK audit data of patients admitted to hospital. Risks of readmission and mortality were assumed (1) not to differ by GOLD stage and (2) to be evenly distributed over the 3-month period. These risks were applied to the post-discharge health states for both the stable and post-hospital populations. While it may be the case that GOLD stage 4 patients are at high risk of readmission compared with less severe patients,150 no prior publications have been able to differentiate GOLD stage 3 and GOLD stage 4, hence the assumption employed in the model. 151
| Definition | Probability | Beta distribution |
|---|---|---|
| COPD-related death during admissiona | ||
| Men | 0.050 | α = 118, β = 2243 |
| Women | 0.051 | α = 133, β = 2490 |
| 90-day COPD-related death post admission | ||
| Men | 0.062 | α = 203, β = 1998 |
| Women | 0.067 | α = 124, β = 2320 |
| 90-day COPD-related readmissiona | ||
| Men | 0.052 | α = 932, β = 1269 |
| Women | 0.051 | α = 954, β = 1490 |
| All-cause COPD mortality post admission | ||
| All | 0.023 | α = 201, β = 4785 |
Mortality risks for the stable population
Age- and sex-specific all-cause mortality among stable COPD rates were obtained from Office for National Statistics life-tables and adjusted to avoid double counting of COPD-related mortality (see Appendix 12). These were applied only to the stable health states for the stable population, as all-cause mortality was expected to be higher in the post-hospital population.
Chronic obstructive pulmonary disease-related mortality was captured through the risk of death during admission, applying the risk of death during admission and in the 90 days following an admission reported in the COPD European audit129 (see Table 23).
Hospitalisation and mortality risks for the post-hospital population
Beyond 3 months post discharge, there was greater uncertainty regarding baseline risks for admission rates and mortality in the post-hospital population. The health status may be underestimated by using airflow limitation as a definition of disease severity. Furthermore, long-term survival was expected to be lower than that reported in large cohort studies (TORCH, Eclipse) because of the recruitment criteria for inclusion in clinical trials. To investigate this, survival rates were extracted from five clinical studies of domiciliary NIV in cohorts discharged from hospital and five studies that followed up cohorts of COPD patients admitted for exacerbation, which are reported in Appendix 13 and Appendix 14. Annual mortality rates in the year following admission were very variable, ranging from 10% to 50%.
In the base-case analysis, the risks of annual all-cause mortality and COPD-related admission were obtained from Garcia-Aymerich et al. (2003)152 and are reported in Table 24. All annual rates were converted to monthly probabilities. This study also reported COPD admission rates as well as all-cause and COPD-related mortality.
| Definition | Rate | Sample size |
|---|---|---|
| Base case (Garcia-Aymerich et al. 2003)152 | ||
| COPD admissions per year | 1.60 | 340 |
| Non-COPD-related mortality | 0.071 | 340 |
| Sensitivity analysis 1 (Bucknall et al. 2011)153 | ||
| COPD admissions per year | 0.47 | 464 |
| Non-COPD-related mortality | 0.194 | 464 |
| Sensitivity analysis 2 (higher rates) | ||
| All mortality risks increased by a factor of 2 | N/A | N/A |
Chronic obstructive pulmonary disease-related mortality was captured through the risk of a COPD-related death either during admission or in the 3-month post-discharge period, as reported in the COPD audit. 129 Applying these risks and the all-cause mortality risk reported in Garcia-Aymerich et al. (2003),152 and assuming they remained constant for the cohort’s lifetime, the model projected cumulative mortality similar to that reported in long-term (5-year) studies. 86,130 The mortality rates reported in post-hospital studies and cohorts of COPD patients discharged from hospital are reported in Appendix 13 and the baseline survival curves for the stable and post-hospital populations are illustrated in Appendix 15.
As there was a lot of uncertainty around baseline survival in this group, and the model was expected to be sensitive to this uncertainty, two one-way sensitivity analyses were applied using lower and higher baseline mortality. The first applied the admission and mortality rates reported in Bucknall et al. (2012)153 to show outcomes in cohorts admitted to hospital but had lower readmission and mortality rates than those reported in Garcia-Aymerich. 152
The second sensitivity analysis considered cohorts with shorter survival. Two of the non-randomised studies96,97 of domiciliary NIV in post-hospital populations reported that over 40% in the usual-care arm had died during the first year and one other non-randomised study86 reported that 55% had died by the second year. To reflect costs and outcomes in similar populations with shorter survival, the risks of all-cause mortality, death during admission and death 90 days post-admission were increased by a factor of 2. This increased the proportion expected to die in the first year in the usual-care arm from 27% to 47%.
The base-case and usual-care arm survival curves for the stable and post-hospital populations, and the alternative survival curves based on lower and higher mortality (in sensitivity analyses) for the post-hospital population are illustrated in Appendix 15.
Disease progression applicable to stable and post-hospital population
The probabilities of progressing to a more severe GOLD stage, applied to all living health states for both the stable and post-hospital populations, were taken from a published model141 (see Appendix 16). This model was selected as it represented disease progression in a UK population and reported the annual risk of disease progression by age, sex and smoking status, which were converted to monthly probabilities.
Estimate for effectiveness of domiciliary non-invasive ventilation
The modelled estimate of the effectiveness of NIV was based on an assumed reduced risk of hospitalisation which was expected to result in cost savings and short-term utility improvements. Owing to limited QoL data, any effect of NIV on utility was not modelled in the base case but explored in sensitivity analyses.
Hospital admission data from the systematic review of clinical effectiveness were available mainly as (pooled) mean differences. In order to be able to use these data in the economic model, all means (SD) were converted to rate ratios (CI) by converting the data into the estimated numbers of events and total time at risk assuming complete follow-up. The rate ratio and its SE were then calculated using Poisson regression. Any reduction in hospital admission in the model was assumed to result in improved mortality; however, it should be noted that there was no evidence of reduced mortality with NIV in the studies included in the effectiveness review for the stable population (RCTs and controlled studies) or the post-hospital population (RCTs only); evidence from non-randomised controlled studies in the post-hospital population suggested a survival benefit with NIV. As such, the estimates of hospitalisations applied in the model contain uncertainty that gives rise to both reduced and increased hospitalisation (and thus mortality) from the use of NIV.
The base-case risk reduction for hospital admission for the stable population was based on the rate ratio derived from the pooled effect estimate of five RCTs76,80,81,99,101 suitable for meta-analysis (see Chapter 4, Hospitalisations). Four further RCTs74,85,89,94 in this population did not report the number of admissions per patient specifically and thus could not be included in this pooled analysis. These studies reported the proportion of patients affected by hospital admissions and hospitalisation rates and found no difference between NIV and usual-care patients. Such data added to the meta-analysis would be likely to have brought the pooled rate ratio for readmission in the stable population closer to the line of no effect.
For the post-hospital population, three RCTs75,90,92 reported hospital admissions data (see Chapter 4, Hospitalisations). As the size and direction of the effects reported were very different, it was not appropriate to pool these results. One small study92 found a statistically significant difference in favour of NIV and the two remaining studies found no significant differences; however, one marginally favoured NIV90 and the other, the largest study, marginally favoured usual care. 75 As there was no clear choice for a single base case, three base cases were considered, using three different rate ratios derived from the RCT data.
The rate ratios for the base cases are reported in Table 25. As there was a high level of uncertainty around rate ratios for admission, an estimate that strongly favoured NIV and an estimate that marginally favoured usual care were applied in the sensitivity analysis for the stable population. For the post-hospital population, no further rate ratios were applied in a sensitivity analysis, as three rate ratios were applied in the base case. The assumptions applied are reported in Table 26.
| Definition | Rate ratio (95% CI) | Source |
|---|---|---|
| Stable population | ||
| Marginally favours NIVa | 0.751 (0.583 to 0.968) | Pooled result of 5 RCTs76,80,81,99,101 |
| Post-hospital population | ||
| Marginally favours usual carea | 1.36 (1.061 to 1.756) | Struik et al. 201475 |
| Marginally favours NIV | 0.5981 (0.2208 to 1.273) | Cheung et al. 201090 |
| Most favourable for NIV | 0.3717 (0.235 to 0.3645) | Xiang et al. 200792 |
Discontinuation rates
It was expected that a proportion of patients starting on domiciliary NIV would discontinue treatment after an initial trial period, either because they suffered adverse events or because the discomfort outweighed the benefit, or for other reasons. The non-adherence and discontinuation rates reported across both stable and post-hospital populations varied from 5% to 43% (see Chapter 4, Adherence and adverse events). This variability may have been partly explained by study design, but factors such as the recruitment criteria, initial patient training and motivations of the patient were also expected to have contributed. In the base case it was assumed that 15% of patients would discontinue using NIV after an initial trial period of 3 months, with a distribution varying this between 10% and 20%. This assumption was tested in a one-way sensitivity analysis by varying values from 5% to 45% for both populations. Patients who discontinued using domiciliary NIV were expected to incur the cost but not the benefits of NIV in the first 3 months and incur neither the costs nor benefits associated with NIV beyond 3 months.
Estimation of quality-adjusted life-years
Utility values were required for all health states and exacerbation events, and were combined with information on survival in order to calculate quality-adjusted life-years (QALYs). The model health states were based on COPD severity defined by GOLD stages 3 and 4. Utility values for these health states were calculated from unpublished data collected from the BLISS cohort. 149 Utility scores for GOLD stages 3 and 4 were derived from the EQ-5D-5L, a revised version of the EQ-5D™ questionnaire. The EQ-5D-5L was completed by 336 participants enrolled in the BLISS study with a confirmed diagnosis of COPD at GOLD stages 3 or 4. This data source was chosen as it reflected a UK COPD population in a real-life setting. Data from this cohort were deemed suitable for stable health states in the model, as participants were not recovering from an exacerbation at the time of questionnaire completion, and therefore the utility scores were expected to reflect QoL in the stable condition. EQ-5D-5L responses were converted to utility scores using the interim crosswalk value set for a UK population reported by EuroQol154 (Table 27).
| Sample size and utility score | GOLD stage 3 | GOLD stage 4 |
|---|---|---|
| Sample size (n) | 299 | 37 |
| Mean utility score (SE) | 0.6765 (0.01521) | 0.6014 (0.0421) |
Data on utility loss suffered immediately after a moderate or severe exacerbation were extracted from previously published models, although estimates varied greatly and the evidence underpinning these was poor. 138–140,143,147 It was assumed that there was a loss of utility for 1 month for moderate exacerbations and a utility loss for 3 months with an improvement after 1 month for severe exacerbations as a result of full recovery taking a longer period of time. 13,155
The utility loss estimate of 15% for moderate exacerbation and 50% in the first month for severe exacerbation was obtained from Rutten-van Molken et al. ,139 reducing to 25% in the second and third months for a severe exacerbation. 31 An alternative assumption, obtained from Hertel et al. 140 was applied in a one-way sensitivity analysis. This assumed a loss of utility for 1 month: mean utility loss of 0.12 for a moderate exacerbation and a utility loss of 0.54 for a severe exacerbation for 1 month. Utility scores taking into account exacerbations that were applied in the model are shown in Table 28.
| Severity of exacerbation and utility loss | GOLD stage 3 | GOLD stage 4 |
|---|---|---|
| Moderate exacerbation | 0.5750 (1 month) | 0.5112 (1 month) |
| Severe exacerbation | 0.3383 (first month); 0.5074 (months 2 and 3) | 0.2556 (first month); 0.4511 (months 2 and 3) |
| Sensitivity analysis | ||
| U – 0.12 for 1 month | 0.5565 (1 month) | 0.4814 (1 month) |
| U – 0.54 for 1 month | 0.1365 (1 month) | 0.0614 (1 month) |
Resource use and costs
The resource use considered within the model was broadly concerned with primary and secondary health-care professional contacts and pharmacotherapy for usual care and the additional costs associated with domiciliary NIV. Health-care contacts for each GOLD severity state were estimated with reference to NICE guidelines4 and expert opinion. Use of pharmacotherapy was estimated from data provided by the BLISS cohort. 149 Unit costs were primarily obtained from NHS reference costs156 and Unit Costs of Health and Social Care. 157 Where appropriate, unit costs were inflated to 2012 prices using NHS health index inflation rates. Annual costs were divided by 12 to derive a monthly cost. Moderate and severe exacerbations were treated as additional one-off costs and assumed to be the same irrespective of the underlying GOLD stage.
Routine health-care visits
The National Institute for Health and Care Excellence28 recommends that stable patients with COPD be followed up at least once a year and those with very severe COPD at least twice a year, with rapid access to hospital assessment where necessary. Based on these guidelines it was assumed that patients at GOLD stages 3 and 4 would attend, respectively, two and two and a half assessments per year in secondary care.
The costs of follow-up and spirometry in secondary care were obtained from NHS reference costs. 156 Costs for follow-up in primary care were based on the cost of a home visit by a community nurse published in the Personal Social Services Research Unit (PSSRU)157 and the cost of spirometry was extracted from a costing document publish by NHS Commissioning Support for London. 158 Additional health-care costs included were the provision of annual flu vaccinations, home oxygen therapy and the cost of the process of prescribing. As the mean age of both populations was over 65 years, it was assumed that 75%159 of patients in each severity group received the vaccination at the current estimated cost of £6.21. 160 This assumption was based on vaccination rates of 50% and between 80% and 93% reported in Spanish and UK studies. 161,162
The average number of days and cost of home oxygen therapy received in each severity group was derived from expert opinion reported in Hertel et al. (2012). 140
Smoking-cessation advice and pulmonary rehabilitation are also recommended by NICE as usual care for COPD patients. 4 However, these costs were assumed to be the same for both strategies, thus cancelling each other out, and were omitted from the model.
The annual costs of health-care visits in GOLD stages 3 and 4 were estimated to be £358 and £486 respectively. A summary of the assumptions and reference costs applied to derive these estimates is provided in Table 29.
| Health care | GOLD stage 3 | GOLD stage 4 | Unit cost (£) | Source |
|---|---|---|---|---|
| Secondary care follow-up | 2 visit | 2.5 visits | 111 | NHS reference costs 2010-11, inflated to 2012156 |
| Secondary care spirometry | 1 test | 1 test | 52 | NHS reference prices 2010–11, inflated to 2012 prices156 |
| Primary care spirometry | 1 test | 1 test | 18 | NHS Commissioning for London costing report for a community-led COPD pathway158 |
| Flu vaccination | 75% take up | 75% take up | 6.21 | Department of Health 2011160 |
| Oxygen therapy | 1.22 days | 6.08 days | 15 | Hertel et al. 2012140 |
| Prescription costs per consultation | £42.70 (assuming one per annum) | PSSRU 2012157 | ||
| Annual cost (£) | 357.66 | 486.01 | – | – |
| Monthly cost (£) | 29.80 | 40.50 | – | – |
Routine pharmacotherapy
NICE guidance is not prescriptive for each GOLD stage, and suggests that the number and type of treatments prescribed should be determined by patient symptoms and response. Therefore, the model used data from the previously described BLISS cohort149 for the proportion of patients on each line of therapy by GOLD stage. 4 As 100% of patients were reported to be on an inhaled short-acting β2-agonist (SABA), assumptions on the number of delivery devices in each severity stage were made by the clinical experts on this project. Drug reference costs reported by NICE (2011)163 (as in Table 30) were compared with current unit costs listed on the NHS Drug Tariff database164 in 2014. Most of the drug prices were consistent with those listed in the NICE 2011163 report, although some were higher and some were lower. As there did not appear to be a consistent drug inflation rate during this period (2011–14) it was not appropriate to inflate the 2011 prices or deflate the 2014 prices to estimate the costs in 2012; thus, the prices listed in the NHS Drug Tariff database164 were applied. Annual and monthly costs were calculated by applying the same unit cost to the annual cost reported by NICE. Where there was more than one drug in each treatment class, an overall average cost was applied. Monthly costs by GOLD stage are shown in Table 31.
| Class | Drug formulation & dose | Price per pack (NICE 2011163) | Price per pack (NHS 2012164) | Annual cost estimated by NICE | Annual cost adjusted to drug tariff (£) | Monthly cost (£) |
|---|---|---|---|---|---|---|
| SABA | Salbutamol 100 µg metered inhalation (generic) | 3.52 | 3.31 | 25.70 | 24.17 | 2.01 |
| Terbutaline 500 µg metered inhalation (Bricanyl®, AstraZeneca) | 6.92 | 6.92 | 101.03 | 101.03 | 8.42 | |
| SABA average cost | 5.22 | |||||
| ICS | Beclometasone 250 µg metered inhalation (generic) | 18.74 | 12.31 | 34.20 | 22.45 | 1.87 |
| SAMA | Ipratropium 20 µg metered inhalation (Atrovent®, Boehringer Ingelheim) | 5.05 | 5.05 | 27.65 | 27.65 | 2.30 |
| LABA | Salmeterol 25 µg metered inhalation (Serevent) | 29.26 | 29.26 | 356.00 | 356.00 | 29.67 |
| LAMA | Tiotropium 18 µg inhalation capsule (Spiriva) | 32.49 | 33.50 | 395.30 | 407.58 | 33.97 |
| LABA and ICS | Budesonide 200 µg + formoterol 6 µg metered inhalation (Symbicort® turbohaler, Astrazeneca) | 38.00 | 11.84 + 24.80 | 462.33 | 445.78 | 37.15 |
| Budesonide 400 µg + formoterol 12 µg metered inhalation (Symbicort turbohaler) | 38.00 | 13.86 + 30.06 | 462.33 | 534.36 | 44.53 | |
| Fluticasone propionate 500 µg + salmeterol 50 µg metered inhalation (Seretide® accuhaler, Allen & Hanburys Ltd) | 40.92 | 40.92 | 497.86 | 497.92 | 41.49 | |
| LABA + ICS average cost | 41.06 |
| GOLD stage and costs | Assumed no. of SABAs used per month | Proportion on type of pharmacotherapy | |||||
|---|---|---|---|---|---|---|---|
| SABA | ICS | LABA | Combo | LAMA | SAMA | ||
| GOLD stage 3 (n = 216)149 | 2 | 1.00 | 0.01 | 0.05 | 0.68 | 0.62 | 0.04 |
| GOLD stage 4 (n = 37)149 | 2.5 | 1.00 | 0.05 | 0.02 | 0.77 | 0.65 | 0.05 |
| Monthly cost GOLD stage 3 (£) | 59.36 | ||||||
| Monthly cost GOLD stage 4 (£) | 65.91 | ||||||
Cost of exacerbations
Moderate exacerbations were assumed to be predominantly managed in primary care through GP appointments, with a proportion of patients attending A&E without admission. As no data were found on the split between GP and A&E visits, assumptions were derived from expert opinion reported in Hertel et al. ,140 which assumed that two-thirds would see a GP and one-third would attend A&E. Prescribed additional medication for a moderate exacerbation was assumed to be a course of prednisolone (six 5-mg tablets per day for 5 days) and antibiotics if exacerbations were associated with a history of purulent sputum. 4 The total cost of treating a moderate exacerbation was estimated to be £114, and a breakdown of how this cost was calculated is presented in Table 32.
| Resource use/cost | % requiring resource | Unit cost (£) | Source of cost estimate |
|---|---|---|---|
| GP visit (12 minutes) | 66.7 | 44.40 | PSSRU157 |
| A&E visit without admission | 33.3 | 112.00 | PSSRU157 |
| Prednisolone 5 mg tablets (six times a day for 5 days) | 100 | 0.11 | NHS drug tariff database164 |
| Amoxicillin 500 mg capsules (three times a day for 5 days) | 100 | 0.09 | NHS drug tariff database164 |
| Prescription costs per consultation | 100 | 42.70 | PSSRU157 |
| Estimated cost of moderate exacerbation | £114.28 | ||
The majority of severe exacerbations were assumed to be managed in hospital, but 20% were assumed to be managed through hospital-at-home or early discharge schemes. The 2011 NICE163 costing study estimated the average cost of a COPD hospital admission to be £1978. These costs were not inflated, as the current NHS tariff prices applied appeared similar to those listed in 2012. No data were available on the tariffs for hospital-at-home or early discharge, although a UK-based cost analysis estimated the costs incurred in a similar scheme to be £1653 in 2009 prices,165 inflated to £1769 for 2012. Following discussion with clinical experts, it was assumed that 20% of those that suffered an exacerbation requiring admission accessed services other than in-patient.
National Institute for Health and Care Excellence guidance4 also recommends that patients should be followed up after discharge, and therefore this cost was included in the average cost of a severe exacerbation and was assumed to include one follow-up visit, 30% being seen by a community nurse, 30% attending a GP appointment and 40% attending an outpatient appointment. The total cost of managing a severe exacerbation was estimated to be £2053 (Table 33).
| Resource use/cost | Proportion requiring resource (%) | Unit cost (£) | Source |
|---|---|---|---|
| Average cost of COPD hospital stay | 80 | 1978 | NICE 2011163 |
| Average cost of hospital-at-home programme | 20 | 1769 | Bakerley et al. 2009.165 Inflated to 2012 prices |
| Community nurse follow-up | 30 | 57 | PSSRU157 |
| GP follow-up (12-minute visit) | 30 | 44 | PSSRU157 |
| Outpatient appointment follow-up | 40 | 139 | NHS tariff prices156 |
| Estimated cost of severe exacerbation (£) | – | 2053 | – |
As the cost of admission in the populations considered in this analysis may be higher if acute ventilation was necessary, particularly in the post-hospital patients, higher costs for severe exacerbation were considered in the sensitivity analysis.
Cost of non-invasive ventilation
The cost of providing NIV was assumed to be the same irrespective of whether patients were started on NIV post-hospital or when stable. In the base case, information from a domestic NIV service provided by an established regional specialist centre in England was used to estimate resource use. Costs were estimated using published Healthcare Resource Group (HRG) tariffs, costs reported in NIV costing studies166 and clinical opinion within the project team. One-way sensitivity analysis was used to consider alternative estimates reported in other NIV studies.
The resources considered included the cost of NIV equipment, set-up and monitoring activities in the first 3 months, and ongoing activities to monitor patients and service the NIV equipment. The NIV device was treated as a one-off cost and applied in the model as a monthly cost, depreciated over 5 years at a rate of 3.5%. The NIV device and humidifier were estimated to cost £3600, varying between £3000 and £4000 as reported in the NICE 2010 costing study of NIV in motor neuron disease. 166
The initial set-up appointment was treated as an outpatient appointment where respiratory tests were conducted and patients were started on domiciliary NIV. This was conducted in an NIV clinic led by a respiratory team and expected to last up to 4 hours. The cost of this service was assumed to be covered by the tariff ‘DZ37 A: NIV Support Assessment 19yrs & over’. 167 After 8–12 weeks, patients on domiciliary NIV attended a follow-up clinic, where their usage was monitored, their blood gases were checked and their NIV pressure settings or masks were adjusted if required. The cost of this service was assumed to be covered by the HRG tariff for a consultant-led outpatient appointment and the HRG tariff for conducting blood gas tests. The set-up and follow-up costs were applied as monthly costs spread evenly over the first 3 months of starting NIV.
Follow-up care beyond 3 months included a bi-annual check of a patient’s usage of NIV and blood gases and an annual NIV equipment check, in which consumables were replaced. As patients were already expected to attend two respiratory appointments a year, the only additional cost for monitoring patients was that of conducting a blood gas check. The cost for an annual NIV equipment check that included device verification, consumable replacement and technical support within normal working hours was estimated to be between £500 and £600 (based on knowledge of current service costs); thus, a point estimate of £550 was applied.
The estimated costs of providing a domiciliary NIV service are reported in Table 34 and for NIV were £2373 in the first year and £1536 in subsequent years. This estimate was in between cost estimates reported in the two studies identified in the clinical review. Tuggey et al. (2003)40 estimate domiciliary NIV to cost £1060 per year in 2003 prices, which converts to £1344 in 2012 prices (assuming a 3% inflation rate), and Clini et al. (2009)134 estimated NIV to cost €1920 in 2008 prices, which converts to £2727 (converting to GBP at the mid-year conversion rate of 1.263168 and inflating to 2012 prices at a rate of 3%).
| Resource | Cost (£) (range used in sensitivity analysis) | Source of cost estimate |
|---|---|---|
| Equipment costs | ||
| NIV equipment for domiciliary use | 3600 (3000–4000) | NICE costing report166 |
| NIV equipment for home use monthly cost (depreciated over 5 years) | 66.66 | – |
| Set-up costs | ||
| NIV set-up and assessment in month 1 | 354 | HRG 2012167 |
| NIV follow-up in month 3: 1 × consultant-led outpatient appointment + 1 × blood gas test | 189 + 95 | HRG 2012167 (OPCS code for blood gases is E92.4) |
| Annual costs thereafter | ||
| 2 × blood gas test conducted at routine follow-up | 95 | HRG 2012167 (OPCS code for blood gases is E92.4) |
| 1 × annual NIV assessment and consumable provision | 550 (500–600) | Estimate |
| Monthly costs | ||
| Monthly costs in the first 3 months | 279 | Includes equipment and set-up costs |
| Monthly costs beyond 3 months | 128 | Includes equipment and annual monitor and service costs |
Assessment of cost-effectiveness
The incremental analysis was designed to generate the cost per additional QALY gained for the addition of domiciliary NIV to usual care in two populations (stable and post-hospital), when compared with usual care alone, in a cohort of COPD patients.
Where available, data were entered into the model as distributions in order to fully incorporate the uncertainty around parameter values so that a probabilistic sensitivity analysis could be undertaken. Where distributions were not available for cost estimates, a normal distribution was applied, assuming a 10% variation either side of the point estimate. Beta distributions were applied to the proportion on different treatments and the proportion accessing services in primary and secondary care. Beta distributions were also applied to annual exacerbation rates and the proportion resulting in hospital admissions, as well as the reduced risk of hospitalisation expected in the domiciliary NIV arm. Normal distributions were applied to utilities and utility losses, as well as the utility improvement associated with NIV in the stable population. The probabilistic sensitivity analysis was run with 1000 simulations, and cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs) were produced.
Summary of key assumptions in the model
In summary, the key assumptions applied in the model were:
-
The starting cohort for the stable population was assumed to be aged 65 years, 70% male, 10% current smokers and 50% with GOLD stage 3 and the remainder with GOLD stage 4.
-
The starting cohort for the post-hospital population was assumed to be aged 72 years, 47% male, 39% current smokers with 50% being in GOLD stage 3 and the remainder GOLD stage 4.
-
Long-term exacerbation and hospitalisation risks for the stable population were taken from large cohort studies of 3 years or more. 2,148 (See Chapter 7, Exacerbation and hospitalisation risks for the stable chronic obstructive pulmonary disease population.)
-
Mortality and readmission risks during admission and immediately after discharge were taken from a European audit129 and applied for 3 months post admission. (See Chapter 7, Hospitalisation and mortality risks for the post-hospital population.)
-
Long-term admission rates and all-cause mortality rates for the post-hospital population were taken from a cohort study of patients admitted to hospital followed up for 1 year. 152
-
Utility values were obtained from the BLISS149 cohort and an estimate was applied for the utility loss associated with exacerbation obtained from a published model. 139 (See Chapter 7, Estimation of quality-adjusted life years.)
-
The cost of usual care was estimated with reference to pharmacotherapy use among the BLISS cohort, best practice guidance, expert opinion and NHS reference prices. (See Chapter 7, Resource use and costs.)
-
The estimate for the risk reduction (rate ratio) for hospital admissions for the stable population was derived from the pooled weighted mean difference based on five RCTs. 76,80,81,99,101
-
The estimates for the risk reduction (rate ratio) for hospital admission for the post-hospital population were derived from the mean differences reported in three RCTs75,90,92 and applied as three separate base cases.
-
No additional improvement in baseline utility was applied to either the stable or post-hospital population.
-
The cost of domiciliary NIV was estimated to be £279 per month in the first 3 months and £128 per month thereafter, inclusive of NIV device and based on NHS tariff prices and expert opinion.
-
The effect of domiciliary NIV was assumed to last for up to 10 years and be driven primarily by a reduced risk of hospital admission and associated mortality.
-
Fifteen per cent of patients were assumed to discontinue using domiciliary NIV after 3 months. These patients were assumed to incur costs but no benefits in the first 3 months and neither costs nor benefits beyond 3 months.
Sensitivity analysis
Additional model runs were undertaken to determine the impact of changing key parameters on the model results for each population. Those parameters where the incremental cost-effectiveness ratio (ICER) was demonstrated to be particularly sensitive to change were explored in more detail. The following analyses were undertaken for the stable population:
-
The effect of domiciliary NIV on hospital admissions (rate ratio) was varied, applying high [0.6219 (95% CI 0.384 to 0.937581] and low [1.794101 (95% CI 0.878 to 1.707)] estimates from the three RCTs that reported this outcome. The rate ratio required for domiciliary NIV to be cost-effective at a threshold of £30,000 per QALY gained was then identified through a one-way sensitivity analysis of this parameter.
-
The effect domiciliary NIV had on baseline utility (QoL) was varied, assuming that it resulted in a 0.025 change in either direction. The utility improvement required for domiciliary NIV to be cost-effective at a threshold of £30,000 per QALY gained was identified through a one-way sensitivity analysis of this parameter.
-
The duration of effect was tested, assuming the effect lasted for 2 years, 5 years, 20 years and a lifetime (30 years). The duration of effect required for domiciliary NIV to be cost-effective at a threshold of £30,000 per QALY gained was identified through a one-way sensitivity analysis of this parameter.
-
The time horizon was varied, changing from the base-case assumption of 30 years, to 6 months, 2 years, 5 years, 10 years and 20 years.
-
The monthly cost of domiciliary NIV was tested, varying the cost of the equipment between £2000 and £6000 and the lifespan between 4 years and 8 years.
-
The cost of NIV was tested, varying the cost of annual servicing and consumable replacement from £200 to £1000.
-
The cost of severe exacerbation was varied between £2000 and £4000.
-
An alternative set of utility scores obtained from Borg et al. (2004)138 were applied (higher utility scores for each severity stage and decreased utility loss from moderate or severe exacerbation) (see Table 28).
-
Subgroup analysis was conducted to test whether the decision rules changed if targeted at different subpopulations. This was tested by assuming patients were (1) all GOLD stage 3, (2) all GOLD stage 4, (3) a cohort with a higher baseline risk of admission and mortality, (4) aged 55 years, (5) aged 85 years, (6) male (entire cohort) and (7) female (entire cohort).
Base cases were run for the post-hospital population, each using rate ratios from one of three relevant RCTs. No further rate ratios were applied in the sensitivity analysis. The same one-way sensitivity analyses were conducted for each of the three base cases and for the post-admission cohort, albeit with the following changes made:
-
Age and sex were not considered in a sensitivity analysis for this population, as all-cause mortality was obtained from a cohort recently admitted for COPD exacerbations which did not report outcomes by age and sex. As only the risk of readmission and death immediately after discharge differentiated by sex, this was unlikely to represent the difference in mortality rates by sex over the lifetime of the model and may thus be misleading.
-
A sensitivity analysis was run in this population, assuming that they had a lower baseline risk of mortality and readmission. This was done by substituting the baseline risks reported in Garcia-Aymerich et al. (2003)152 with those reported in Bucknall et al. (2012),153 as patients in the latter, while recently admitted for exacerbation, were expected to be a slightly healthier population, as they were recruited to be enrolled on a self-management trial.
-
A sensitivity analysis was run, assuming a higher baseline risk of mortality and readmission by adjusting the baseline mortality risks reported in Garcia-Aymerich et al. (2003)152 by a factor of 2.
Expected value of perfect information
Expected value of perfect information (EVPI) analysis quantifies the economic value of removing all uncertainty in a decision model. 169 EVPI analyses were conducted based on the methods described by Claxton and Posnett (1996)170 and applied to estimate the value of perfect information per patient and per population in stable and post-hospital COPD populations in the UK.
The sources used to estimate the size of the stable and post-hospital populations are reported in Table 35. The UK COPD population was estimated by applying the prevalence rate of 1.7% reported in Haughney et al. (2013)172 to the mid-year population projections171 for the UK in 2014. The stable COPD population was estimated from this, assuming that 25.5% and 5.2% were in GOLD stages 3 and 4, respectively, as reported in Haughney et al. (2013). 172
| Definition of population | Prevalence | Population estimate (000s) | Source of estimate |
|---|---|---|---|
| UK | – | 64,487 | ONS mid-2014 projection171 |
| Diagnosed with COPD | 1.7% of the UK population | 1096 | Haughney et al. 2013172 |
| Stable end-stage COPD (GOLD stage 3 and stage 4) | 30.6% of COPD population | 329 | Haughney et al. 2013172 |
| Post-hospital end-stage COPD | 8.8% of COPD population | 96 | Haughney et al. 2013172 |
The same study also reported the proportion of patients in each of the new GOLD classifications (A–D1) admitted to hospital at least once. This was used to estimate the post-hospital population, assuming that only GOLD stage C and D patients were likely to be in end-stage COPD.
As there is a lot of uncertainty regarding both the prevalence of COPD and the proportion of COPD patients who could be defined as end-stage stable patients and end-stage post-hospital patients, the estimated value of perfect information per population reported should be interpreted cautiously and considered indicative of the value of perfect information in decisions that affect these populations.
Results
This section presents the results for the base-case, sensitivity and subgroup analysis for the stable population, followed by the results for the base case post-hospital population and accompanying sensitivity and subgroup analysis.
Results for the stable population
Base-case analysis
The base-case results for the stable population presented in Table 36 show that, compared with usual care alone, the addition of domiciliary NIV was more costly and resulted in better outcomes. The difference in cost was £12,769, with 0.4534 QALYs gained, resulting in an ICER of £28,162 per QALY gained.
| Strategy | Mean cost (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|---|---|
| Usual care | 23,969 | – | 5.3360 | – | – | – | – |
| Domiciliary NIV + usual care | 36,737 | 12,769 | 5.7894 | 0.4534 | 28,162 | 13 | 55 |
Results from the probabilistic sensitivity analysis can be found in the cost-effectiveness plane in Figure 19, which shows the distribution of 1000 resampled cost and effect difference pairs. In all samples, domiciliary NIV was more costly than usual care, but there was uncertainty regarding its effect. The samples to the left of the line of no effect represent where domiciliary NIV results in more admissions and higher mortality, in which case usual care is more favourable. The majority of the samples are to the right of the line of no effect where cost-effectiveness is determined by the decision-maker’s willingness-to-pay threshold for a QALY gained. The CEAC in Figure 20 shows that domiciliary NIV has a 55% probability of being cost-effective at a threshold of £30,000 per QALY gained.
FIGURE 19.
Cost-effectiveness plane for NIV vs. usual care in the base case for a stable population.
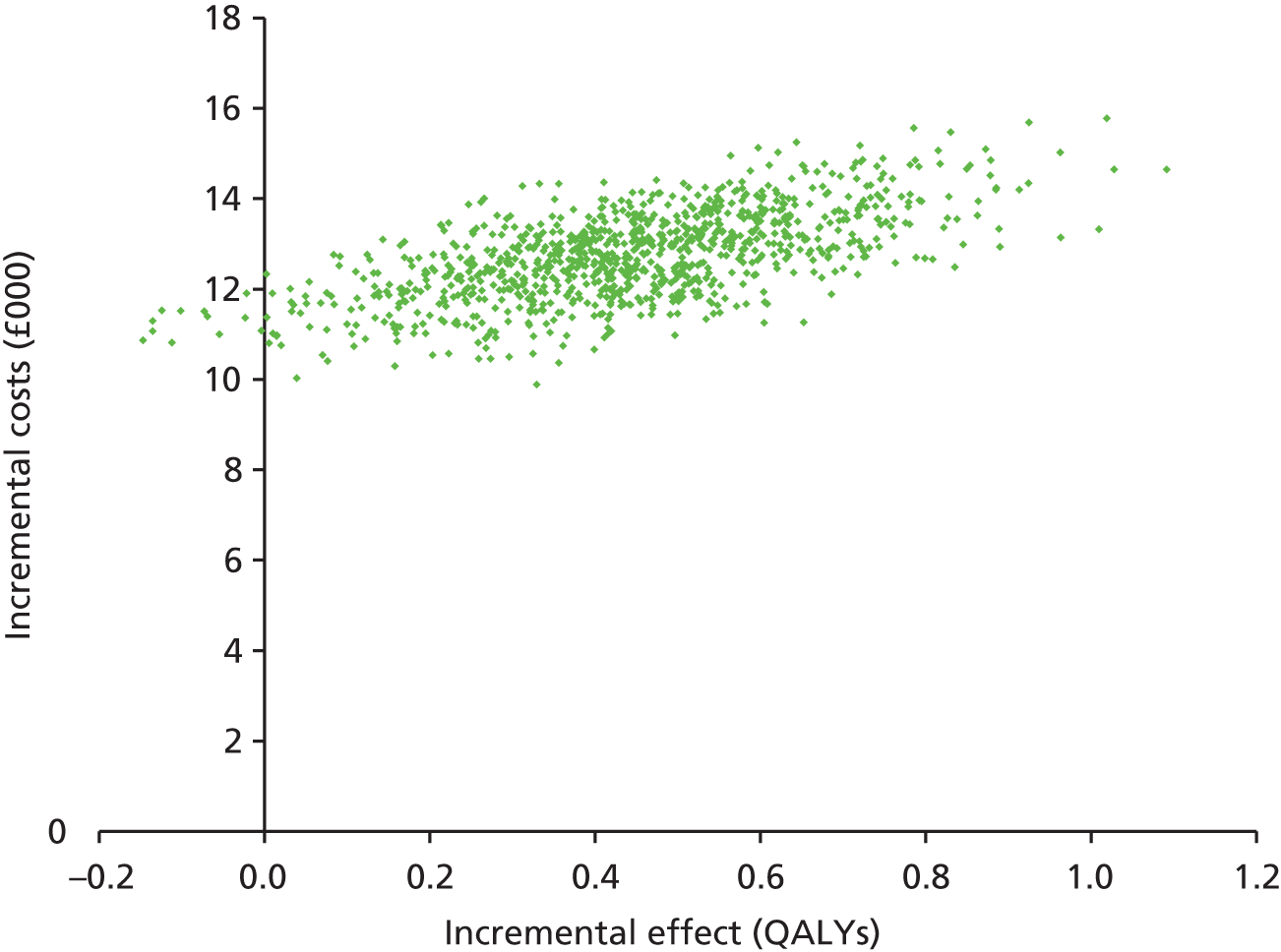
FIGURE 20.
Cost-effectiveness acceptability curve for NIV vs. usual care in for the base case for a stable population.
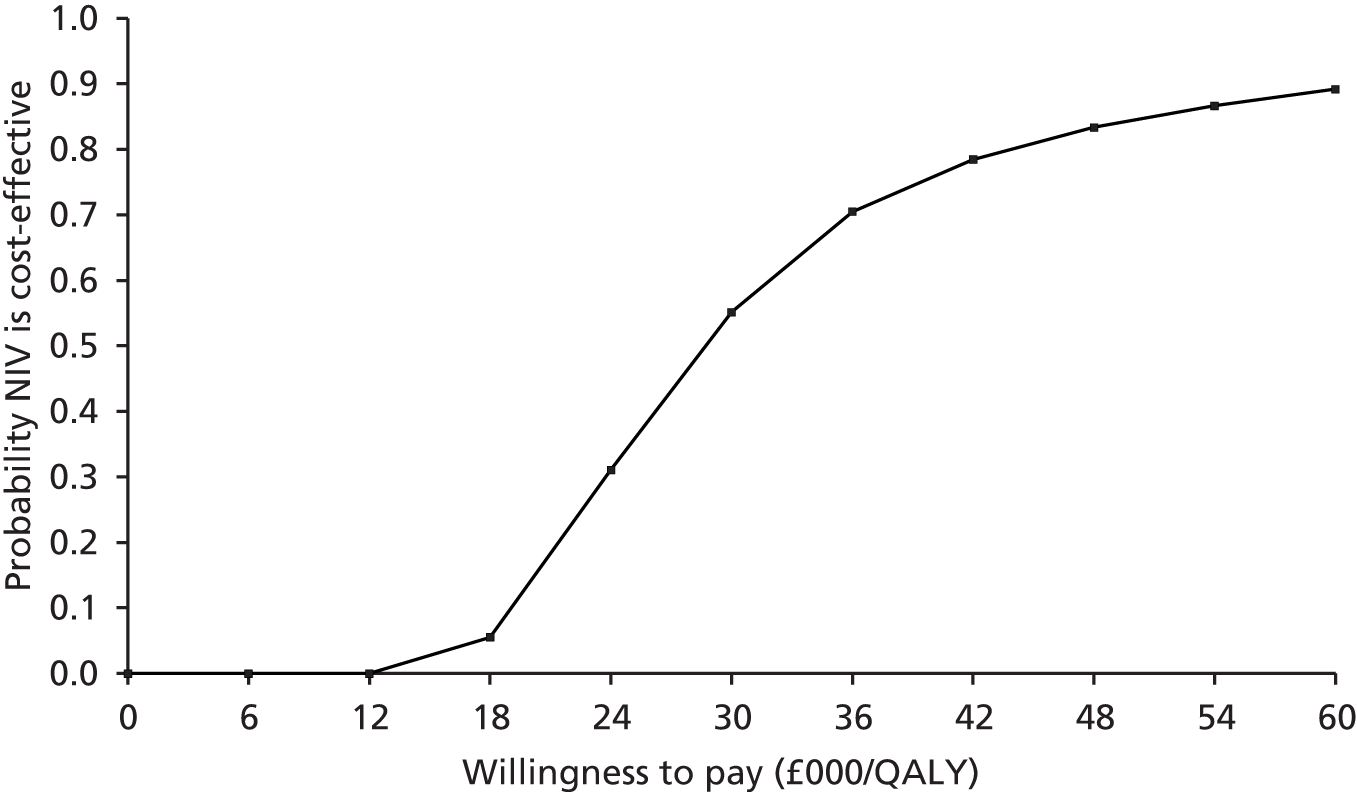
Sensitivity analysis for the stable population
This section presents the results of the sensitivity analysis conducted on the base-case assumptions for the stable COPD population.
Alternative rate ratios for hospital admissions
Owing to considerable uncertainty around the estimate for the primary outcome, the rate ratio for hospital admission, this parameter was expected to be the biggest driver of the cost-effectiveness results. High and low rate ratio estimates were applied, obtained from the RCTs showing the most positive and negative effect on admissions, and the results are reported in Table 37. The most favourable rate ratio increased the difference in cost and effect to £13,593 and 0.7285 QALYs, respectively, and the ICER was £18,660 per QALY gained, increasing the probability of domiciliary NIV being cost-effective at a threshold of £30,000 per QALY gained to 82%.
| Rate ratio | Cost difference (£) | QALY difference (QALY) | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|
| Base | |||||
| Pooled estimate | 12,769 | 0.4534 | 28,162 | 13 | 55 |
| High estimate | |||||
| Zhou et al. 2008,81 0.6219 (0.1425) | 13,593 | 0.7285 | 18,660 | 57 | 82 |
| Low estimate | |||||
| Kaminski et al. 1999,101 1.794 (0.6031) | 8735 | –1.0507 | Usual care dominates | < 1 | 1 |
Conversely, applying the rate ratio that marginally favoured usual care decreased the difference in costs to £8735, and domiciliary NIV was less effective than usual care, resulting in QALY difference of –1.0507 QALYs. Under this assumption, usual care dominated NIV and the probability of this being cost-effective reduced to 1% at a threshold of £30,000 per QALY gained.
The ICERs obtained from varying the rate ratios between the low and most favourable estimates are illustrated in Figure 21. The points where the trend line is not on the graph represent ICERs above £60,000 or below –£60,000 per QALY gained and the negative values signify scenarios where usual care dominates domiciliary NIV (usual care costs less and is more effective). For the ICER to be below a threshold of £30,000 per QALY gained, the rate ratio for hospital admissions needs to be 0.76 or lower; that is, a 24% reduction in rate of admissions per patient per year with NIV.
FIGURE 21.
One-way sensitivity analysis of the rate ratio for admissions in a stable population.
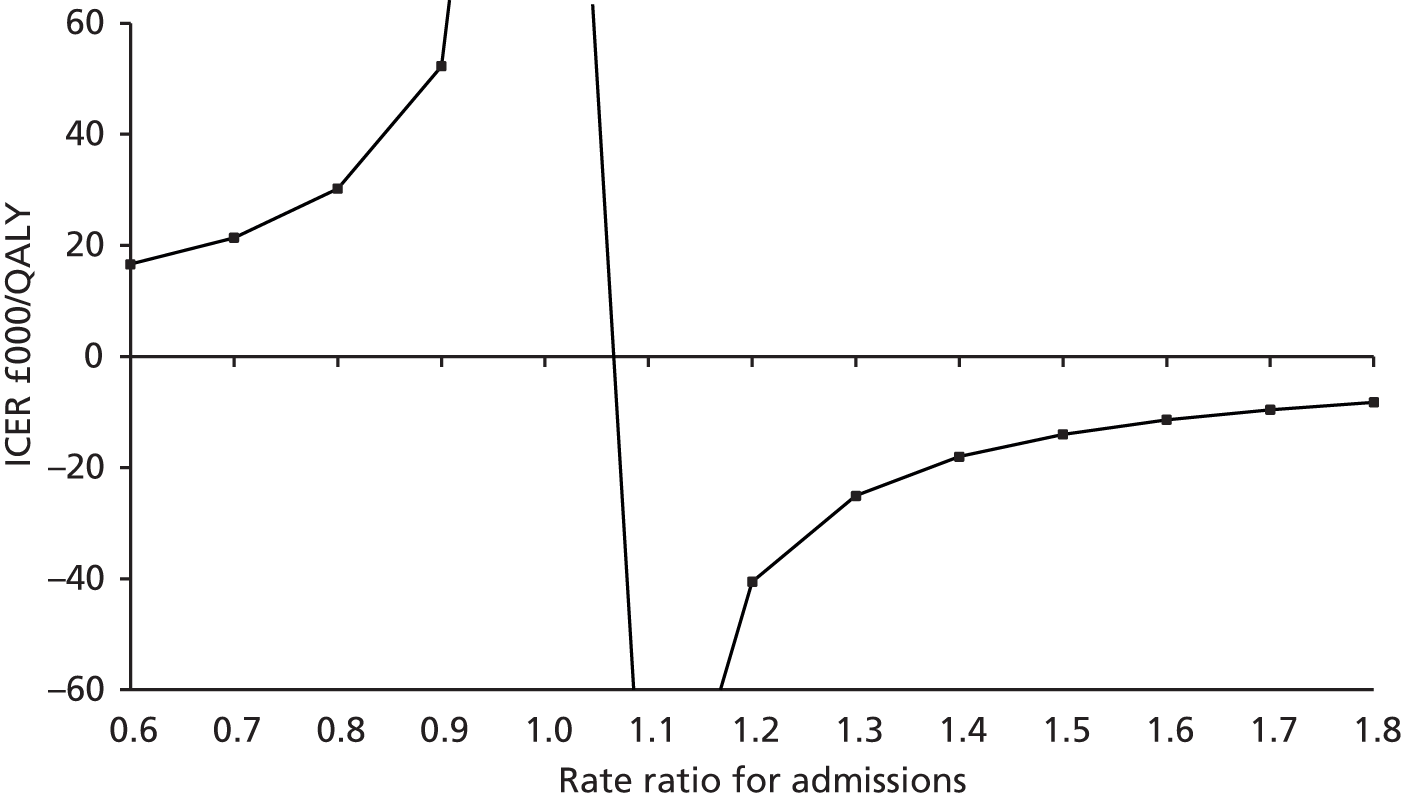
Alternative improvements in utility
The assumption that domiciliary NIV has no effect on utility (outside of utility loss avoided through lower risk of admission) was tested by varying an assumption that NIV would lead to a change in utility score of 0.025 (on a QoL scale of 0 to 1) in either direction. Although not directly mappable, this could be considered comparable to a 2.5 point variation on the SGRQ (scale 0–100 points). The effect this utility variation has on the ICER is illustrated in Figure 22. Assuming that domiciliary NIV improved QoL and increased the utility score by 0.025, the ICER decreased to £20,462, close to the threshold of £20,000 per QALY gained. Conversely, assuming that domiciliary NIV decreased patients’ QoL, reducing the utility score by 0.025 increased the ICER to above £40,000/QALY.
FIGURE 22.
One-way sensitivity analysis of QoL for the stable population.
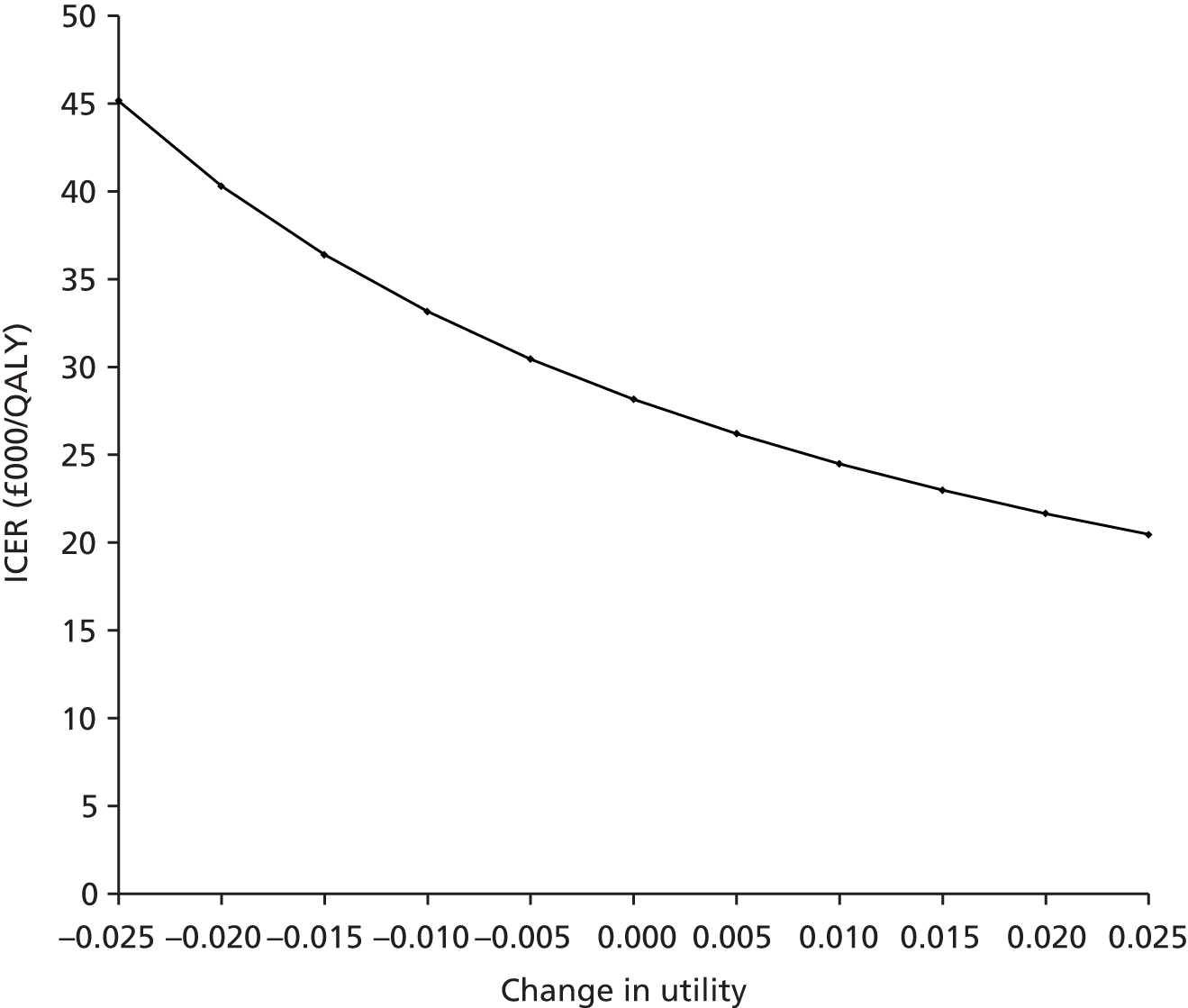
Alternative duration of effect
The duration of the effect of domiciliary NIV in reducing the risk of hospital admission and improving utility was varied from 10 years to 2, 5, 20 and 30 years, with 30 years representing the lifetime of the population. Table 38 shows that assuming that the effect lasted for only 2 years results in an ICER of £93,091 per QALY gained and assuming it lasted for 5 years results in an ICER of £43,510 per QALY gained, with the probability of this being cost-effective at a threshold of £30,000 per QALY gained being less than 0% and 12%, respectively.
| Duration of effect | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|
| 2 years | 11,865 | 0.1275 | 93,091 | 0 | 0 |
| 5 years | 12,328 | 0.2833 | 43,510 | 10 | 12 |
| Base case (10 years) | 12,769 | 0.4534 | 28,162 | 13 | 55 |
| 20 years | 12,944 | 0.5817 | 22,252 | 37 | 73 |
| 30 years | 12,888 | 0.5969 | 21,592 | 42 | 75 |
Conversely, assuming that these effects lasted for 20 years or more reduced the ICER towards £20,000 per QALY gained, and the probability of domiciliary NIV being cost-effective at a threshold of £30,000 per QALY gained was greater than 70%.
The effect of varying the duration of effect between 2 years and 30 years is illustrated in Figure 23. This shows that the ICER decreases at longer durations of effect but, even assuming that the effects last for up to 30 years, the ICER is marginally higher than £20,000 per QALY gained.
FIGURE 23.
One-way sensitivity analysis of the duration of the effects.
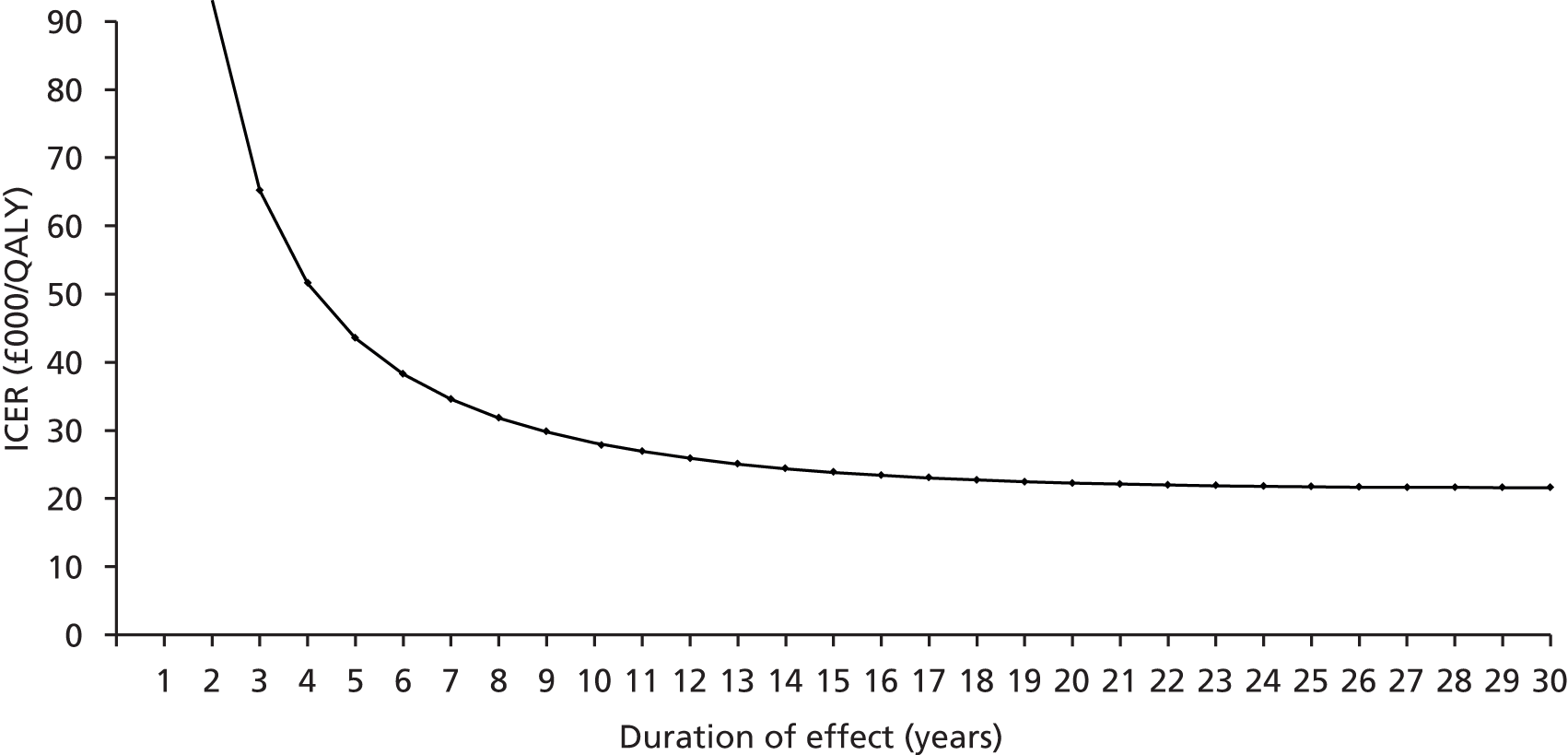
Alternative model time horizon
Table 39 presents the results of the model when varying the time horizon of the model. At a short time horizon of 6 months, the ICER was above £475,000 per QALY gained, and using a time frame of 2 years it gave an ICER above £116,000 per QALY gained, both well above the threshold of £30,000 per QALY gained. While these are unlikely to represent realistic time frames, there were very few studies that evaluated domiciliary NIV in this population beyond 2 years; therefore, projecting costs and outcomes beyond this point is associated with uncertainty.
| Time horizons | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £30,000/QALY (%) | Probability cost-effective at £60,000/QALY (%) |
|---|---|---|---|---|---|
| 6 months | 928 | 0.0019 | 476,982 | 0 | 0 |
| 2 years | 2346 | 0.0201 | 116,857 | 0 | 0 |
| 5 years | 4897 | 0.0908 | 53,910 | 0 | 5 |
| 10 years | 8273 | 0.2526 | 32,757 | 7 | 41 |
| 20 years | 12,604 | 0.4466 | 28,222 | 12 | 55 |
| Base case (30 years) | 12,769 | 0.4534 | 28,162 | 13 | 55 |
There was very little difference between applying a time horizon of 20 years and one of 30 years, as the base case assumed that domiciliary NIV was effective for 10 years, and beyond this the monthly costs would continue to accrue with no further benefit other than continued survival. Thus, at all time horizons the ICER is above £30,000 per QALY gained and the probability of being below £30,000 per QALY gained is less than 55%.
Alternative continuation rates
Varying the assumption that 85% of those started on NIV would benefit from and continue using NIV beyond 3 months between 55% and 95% (representing low and most favourable estimates from selected studies) suggested that the model was not very sensitive to this variable, as illustrated in Figure 24. While a high dropout rate was expected to increase the costs and lower QALYs gained in the short term, these costs were minimal compared with the costs and benefits that accrued in the population that continued with NIV beyond 3 months.
FIGURE 24.
One-way sensitivity analysis of alternative continuation rates beyond 3 months in the stable population.
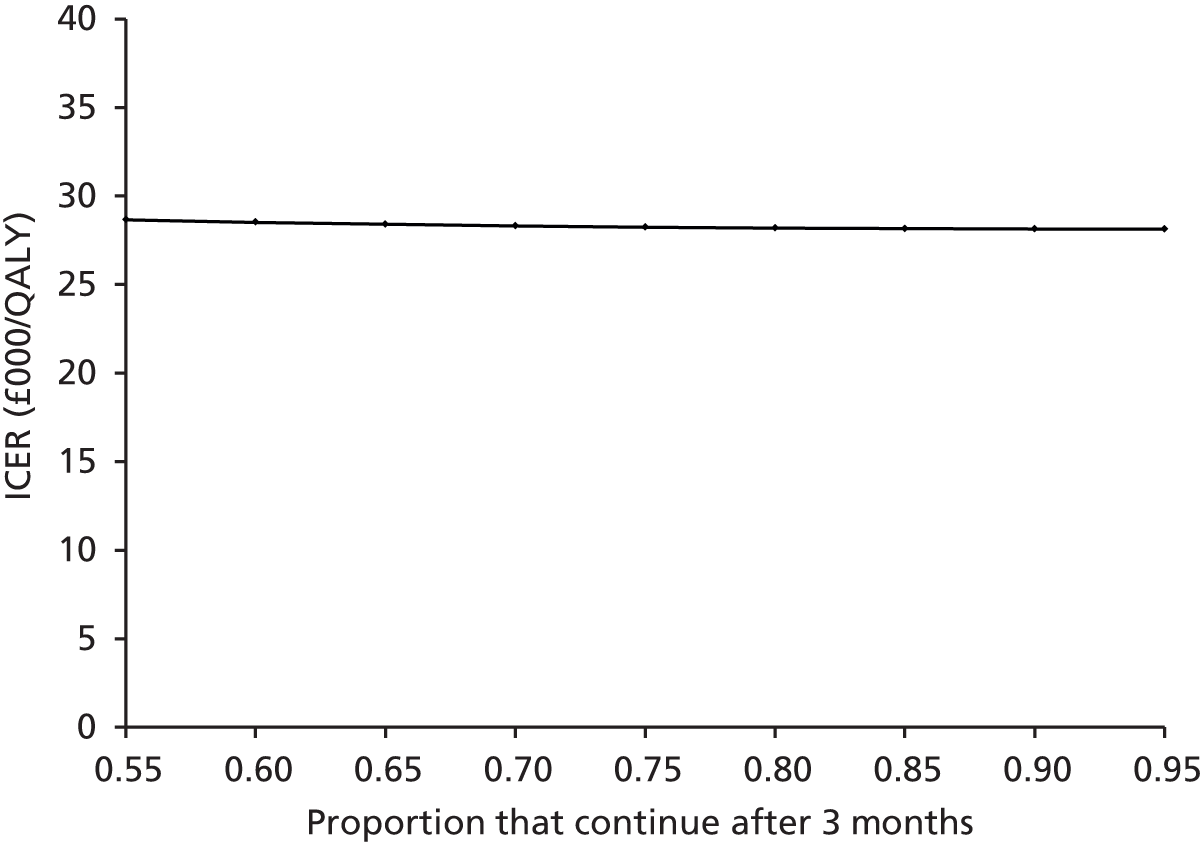
Alternative costs of the non-invasive ventilation device
In the base case, it was assumed that the device would cost £3600 and a monthly cost was derived assuming that it would be used continuously for 5 years. Varying the cost of the NIV device demonstrated that, if over £4000, the ICER is above the threshold of £30,000 per QALY gained, as illustrated in Figure 25 (keeping the lifespan constant).
FIGURE 25.
One-way sensitivity analysis of alternative costs of device in a stable population.
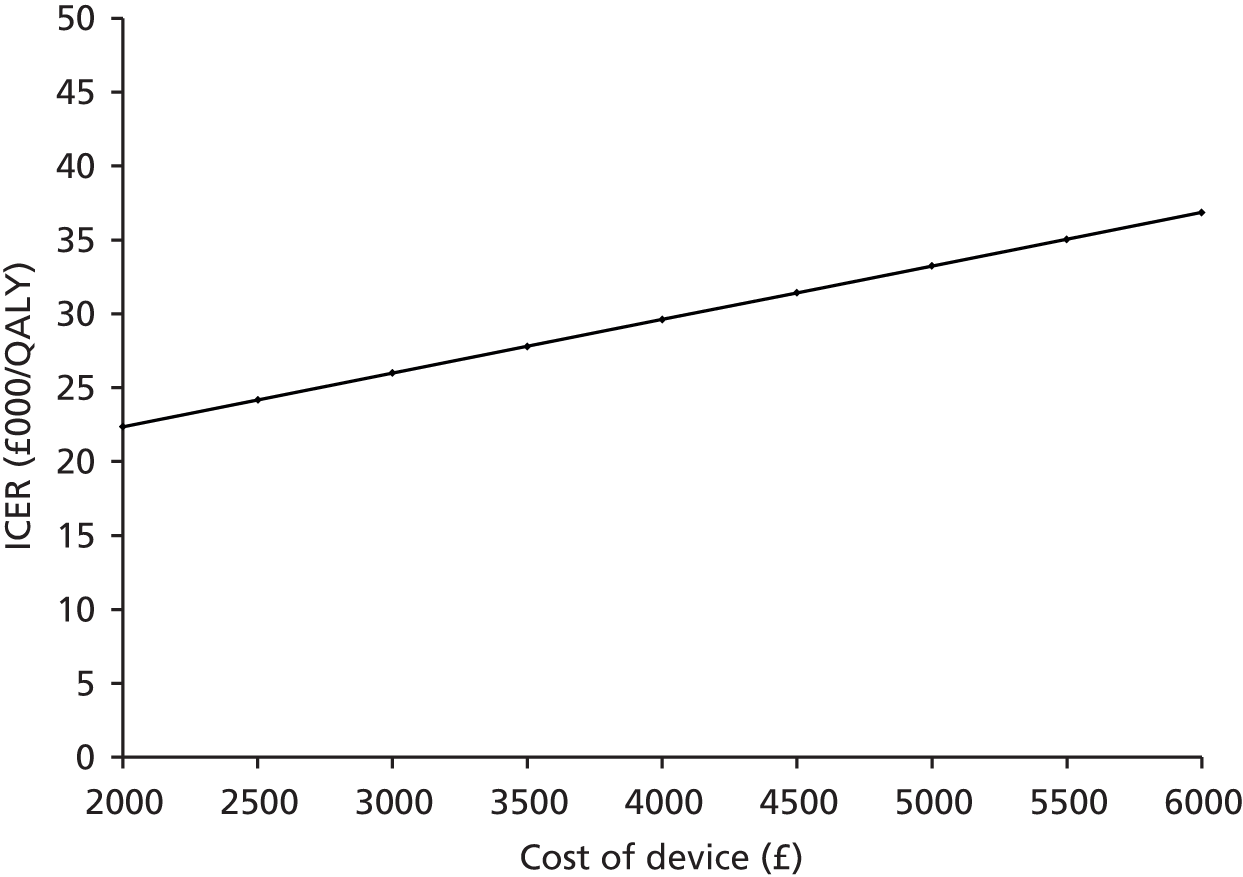
Keeping the cost of the device constant at £3600, the ICER rises above £30,000 per QALY gained when the device is assumed to be used for 4 years (rather than 5 years), as illustrated in Figure 26.
FIGURE 26.
One-way sensitivity analysis of alternative lifespan of device in a stable population.
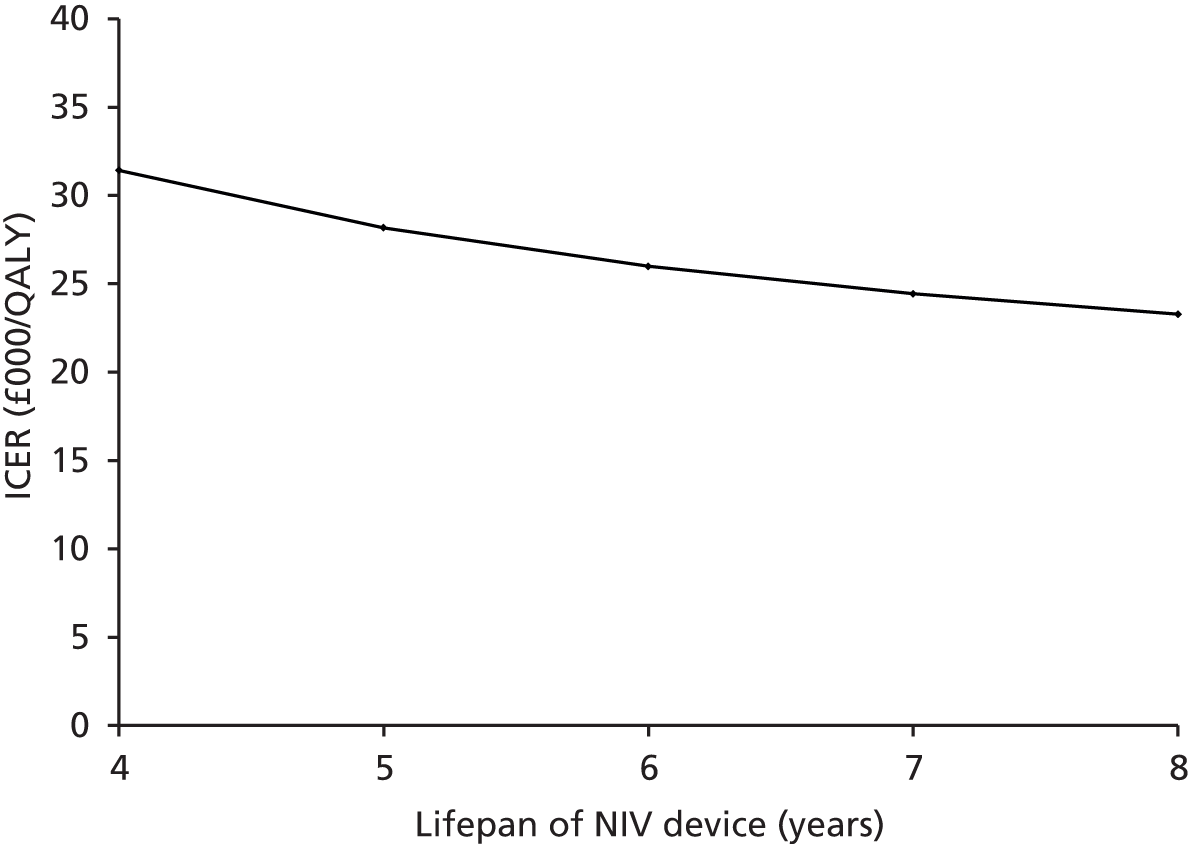
Alternative costs of the service aspects of non-invasive ventilation provision
The other costs involved in the provision of a domiciliary NIV service were tested by varying the tariff for a NIV assessment and set-up (base case £354) and the cost of annual maintenance (base case £550) between £200 and £1000. Changing the tariff for NIV assessment and set-up had minimal impact on the cost-effectiveness of NIV, as this was a one-off cost incurred in the first 3 months of use (Figure 27). The model was more sensitive to changes in the annual service cost; however, even when applying a very low cost of £200 the ICER was above £20,000 per QALY gained (Figure 28).
FIGURE 27.
One-way sensitivity analysis of alternative NIV assessment costs in a stable population.
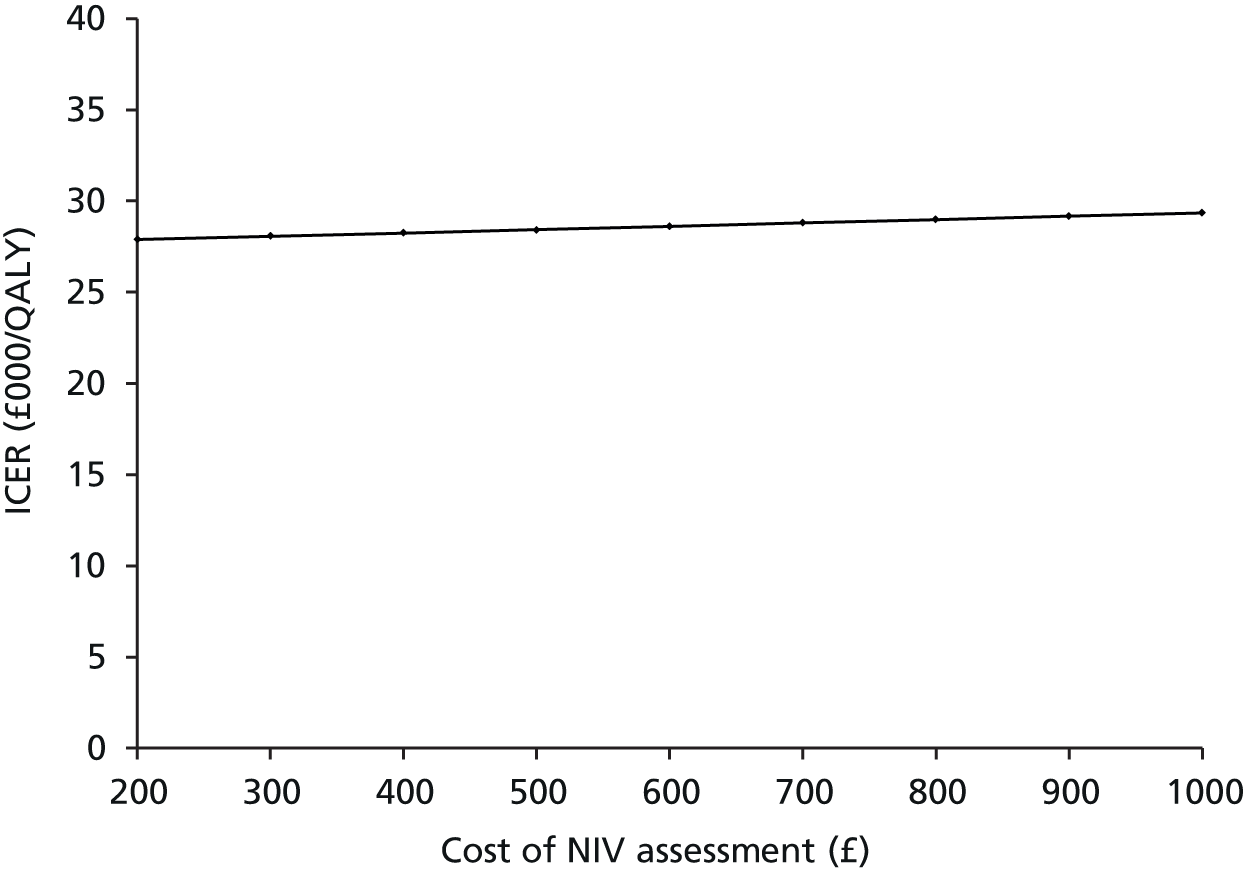
FIGURE 28.
One-way sensitivity analysis of annual maintenance costs in a stable population.
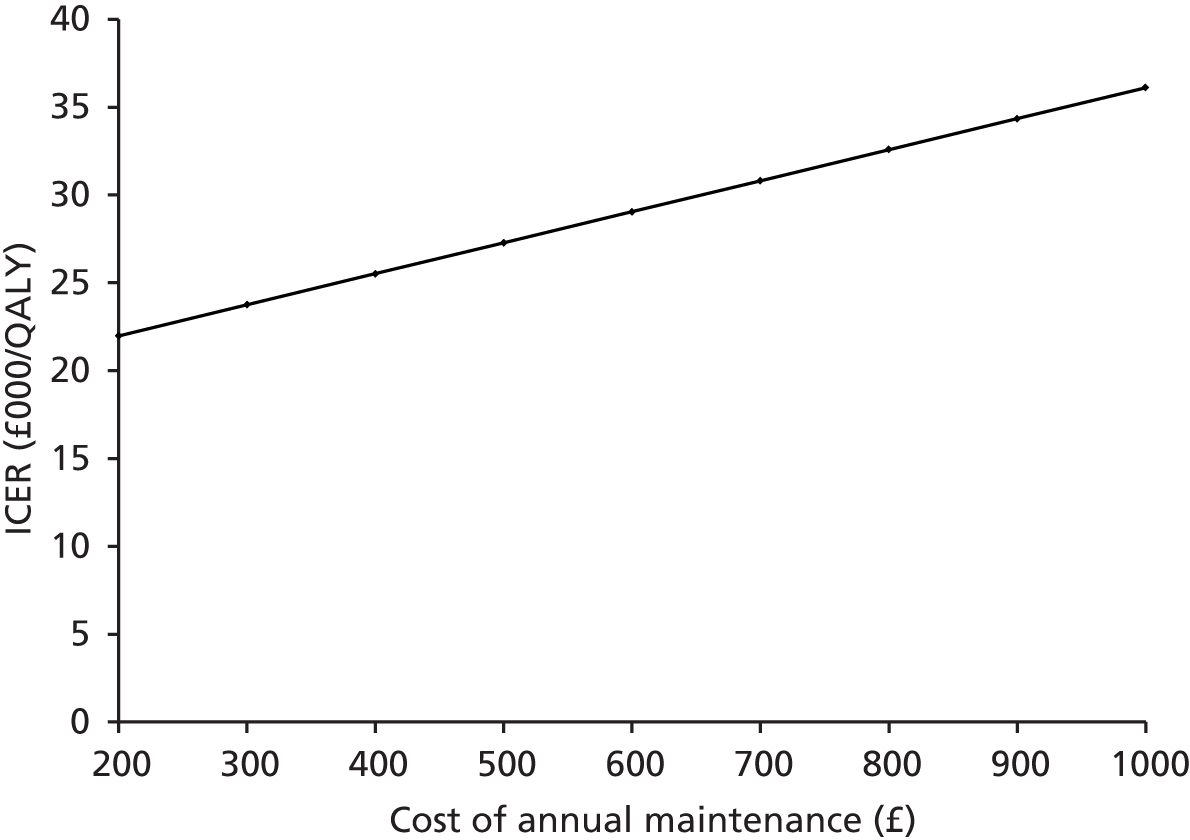
Alternative assumption for cost of admission
The results of varying the cost of an admission from the base-case assumption of £2053 between values of £1500 and £3000 are shown in Figure 29 and demonstrate that the model was not very sensitive to changes in this cost.
FIGURE 29.
One-way sensitivity analysis of cost of admission in a stable population.
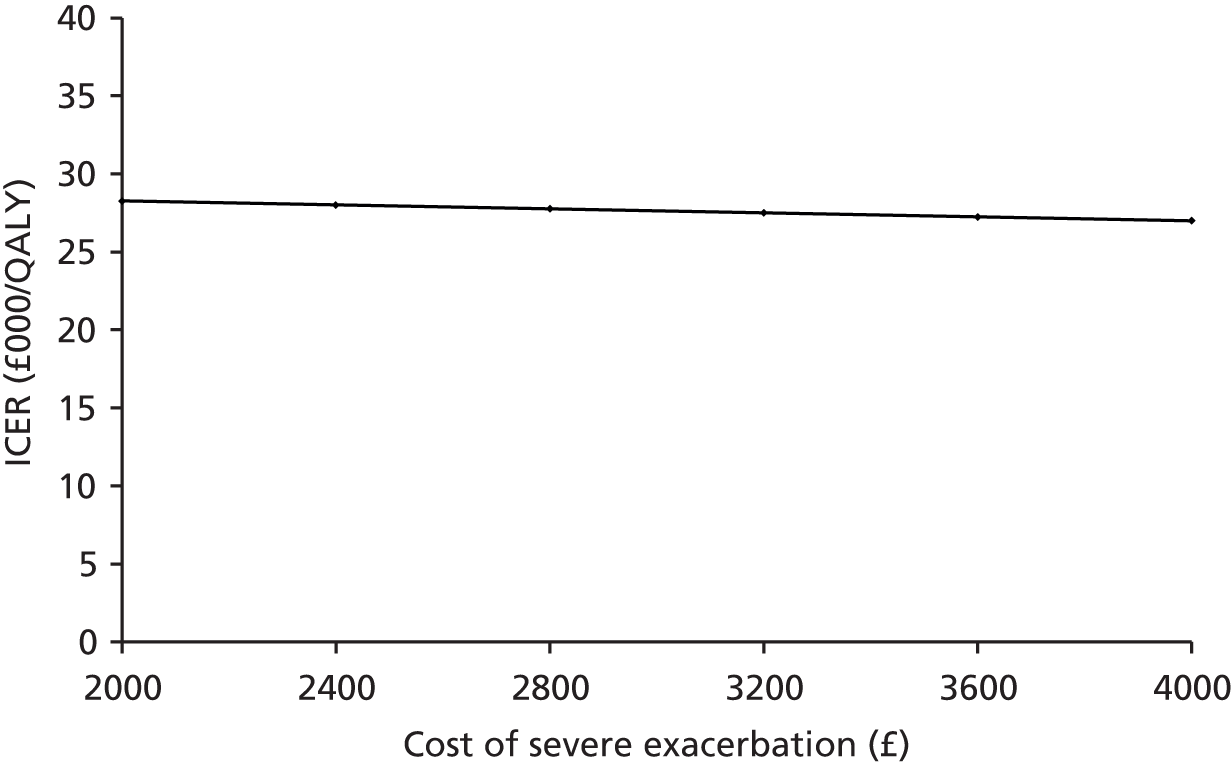
Alternative utility assumptions applied to the stable population
The difference in costs and QALYs when using the utility assumptions applied in Borg et al. (2004)138 are reported in Table 40. As these were similar to the values in the base case, it is unsurprising that this made very little difference to the ICER and cost-effectiveness of domiciliary NIV.
| Strategy | Mean cost (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|---|---|
| Usual care | 23,969 | – | 5.3330 | – | – | – | – |
| Domiciliary NIV + usual care | 36,737 | 12,769 | 5.7888 | 0.4558 | 28,013 | 14 | 55 |
Subgroup analysis for the stable population
Global Initiative for Chronic Obstructive Lung Disease severity stage
Table 41 presents the ICERs when assuming stable end-stage COPD patients were started on NIV at a specified severity stage. Assuming all patients entering the model were in GOLD stage 3, the ICER increased to £33,490 per QALY gained and the probability of domiciliary NIV being cost-effective reduced to 38%. Conversely, assuming that all stable patients were started on NIV in GOLD stage 4, the ICER decreased to £23,124 per QALY gained and the probability of domiciliary NIV being cost-effective at a threshold of £30,000 per QALY gained increased to 72%.
| Severity | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|
| Base case (50 : 50 split between GOLD stage 3 and stage 4) | 12,769 | 0.4534 | 28,162 | 13 | 55 |
| GOLD stage 3 | 14,761 | 0.4407 | 33,490 | 2 | 38 |
| GOLD stage 4 | 10,777 | 0.4661 | 23,124 | 32 | 72 |
This result is most likely to be because of the higher baseline risk of exacerbation, admission and COPD-related mortality applied to GOLD stage 4 health states than to GOLD stage 3. This suggests that domiciliary NIV is more likely to be cost-effective in patients with higher risk of admission if NIV is effective at reducing this risk of admission.
Age
The starting age of the stable population entering the model was varied and results are presented in Table 42. There was very little difference by changing the start age from 65 to 55 years; the ICER was slightly lower, but this made little difference to the probability of domiciliary NIV being cost-effective at a threshold of £30,000 per QALY gained.
| Start age (years) | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|
| 65 (base case) | 12,769 | 0.4534 | 28,162 | 13 | 55 |
| 55 | 14,621 | 0.5367 | 27,242 | 14 | 58 |
| 85 | 5895 | 0.1379 | 42,758 | < 1 | 8 |
By increasing the start age to 85 years, the ICER increased to £42,785/QALY gained and the probability of domiciliary NIV being cost-effective decreased to 8%. This is most likely because of the shorter time horizon for the effects of domiciliary NIV to accumulate as the risk of all-cause mortality increased significantly with age. It should, however, be noted that the risk of a COPD-related event (exacerbation, admission or death) was not linked to age because of lack of age-specific data available. Therefore, while this result may suggest that domiciliary NIV is less effective in an older stable population, this may not be the case if the risk of having a COPD-related event also increases with age.
Sex
The results for separate male and female cohorts are shown in Table 43 and show very little difference in the ICERs or probability of NIV being cost-effective when targeting solely men or women. This may be because only a limited number of parameters differentiated between sex, namely mortality and readmission rates post-discharge and all-cause mortality. Until more gender-specific data on COPD-related events in a stable population become available, it is assumed the cost-effectiveness of NIV is not influenced by sex.
| Sex | Cost difference (£) | QALY difference | ICER (£/QALY) | % cost-effective at £20,000/QALY | % cost-effective at £30,000/QALY |
|---|---|---|---|---|---|
| Base case | 12,769 | 0.4534 | 28,162 | 13 | 55 |
| Male | 12,500 | 0.4450 | 28,089 | 12 | 56 |
| Female | 13,444 | 0.4747 | 28,323 | 12 | 57 |
Higher baseline risk of admission risk
Substituting the baseline admission and mortality risk in the stable health states (obtained from the TORCH and Eclipse trials and the Office for National Statistics) with baseline risks applied in stable health states for the post-hospital population (obtained from Garcia-Aymerich et al. 2003152) reduced the ICER to below the threshold of £20,000 per QALY gained and increased the probability of being cost-effective to 86% at £30,000 per QALY gained and 65% at £20,000 per QALY gained (Table 44).
| Risks in stable health states | Cost difference (£) | QALY difference | ICER (£/QALY) | % cost-effective at £20,000/QALY | % cost-effective at £30,000/QALY |
|---|---|---|---|---|---|
| Base case | 12,769 | 0.4534 | 28,162 | 13 | 55 |
| Higher readmission and mortality risks | 6222 | 0.3594 | 17,313 | 65 | 86 |
Expected value of perfect information for the stable population
The EVPI per patient for the stable population was estimated to be £204 at a threshold of £20,000 per QALY gained and £1812 per patient at a threshold of £30,000 per QALY gained. When this is multiplied by the estimated population size (329,000 stable end-stage COPD patients in the UK), it results in an EVPI per population estimate of £67M and £596M respectively. Figure 30 shows the EVPI per population at all willingness-to-pay thresholds as between £0 and £60,000 per QALYs gained. These estimates reflect the value of research that removes all uncertainty in the decisions to adopt NIV at these willingness-to-pay thresholds. The high value at all thresholds above £20,000 per QALY gained reflects the fact that removing all uncertainty in the decision to offer domiciliary NIV to all end-stage COPD potentially affects a large patient base.
FIGURE 30.
Expected value of per information per population for the stable COPD population.
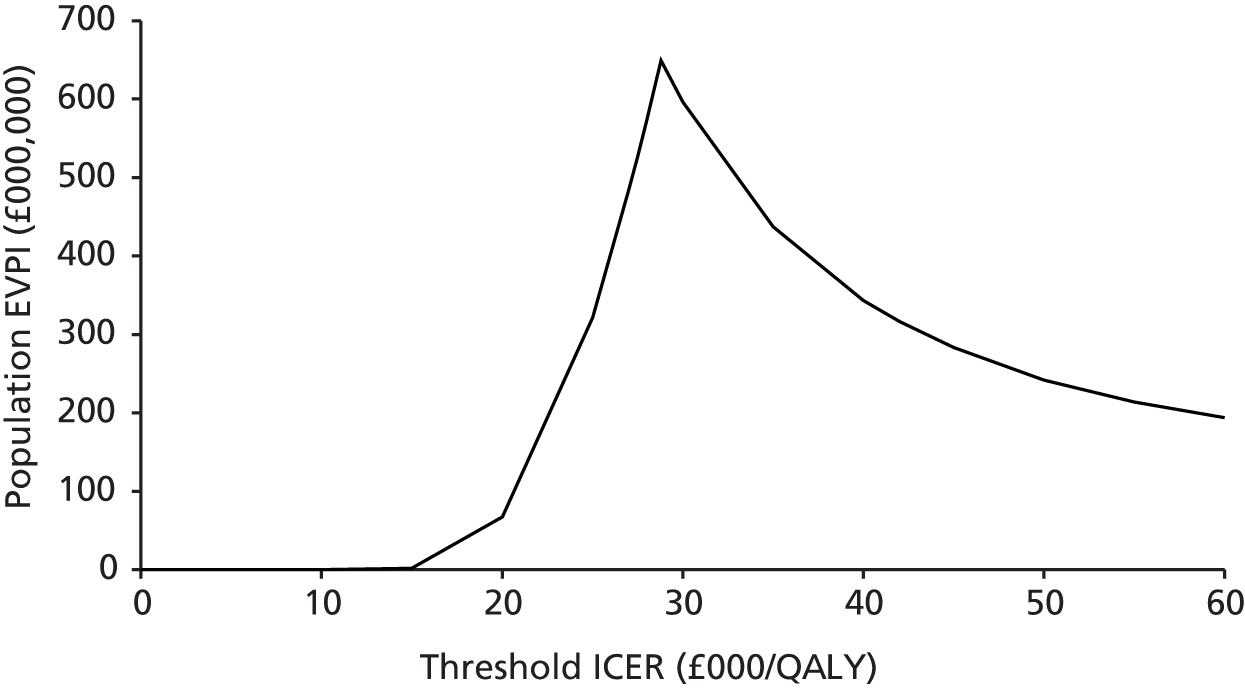
At a threshold of £20,000 per QALY gained, usual care is the preferred option. The EVPI per population increases above the threshold of £20,000 per QALY gained and reaches a peak at around a little over £28,000/QALY gained, where the decision changes and domiciliary NIV becomes the preferred option. This sharp increase is because, at thresholds close to the base-case ICER, perfect information becomes more important, as it is likely to change the decision to offer domiciliary NIV.
Results for the post-hospital population
This section presents the results of the base-case, sensitivity and subgroup analyses for the post-hospital population.
Base-case analysis
The three base-case results for the post-hospital population presented in Table 45 show that, compared with usual care alone, the addition of domiciliary NIV was more costly and resulted in better outcomes where the rate ratios favoured NIV and resulted in higher costs and worse outcomes with the rate ratio that favoured usual care; therefore, domiciliary NIV was dominated. Where the evidence marginally favoured NIV, the ICER was £10,107 per QALY gained and this reduced to £6281 per QALY gained where the rate ratio strongly favoured NIV. Therefore, both of the rate ratios that favoured NIV produced ICERs that were well under the willingness-to-pay threshold of £20,000 per QALY gained; however, if NIV is not more effective than usual care, it would not be considered cost-effective irrespective of the willingness-to-pay threshold value.
| Strategy | Mean cost (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|---|---|
| Usual care | 17,048 | – | 2.1452 | – | – | – | – |
| Domiciliary NIV + usual care | |||||||
| Marginally favoured usual care (Struik et al.75) | 21,912 | 4864 | 1.8196 | –0.3255 | Dominated | 0 | 0 |
| Marginally favoured NIV (Cheung et al.90) | 22,879 | 5830 | 2.722 | 0.5769 | 10,107 | 72 | 79 |
| Most favourable (Xiang et al.92) | 23,533 | 6485 | 3.177 | 1.0325 | 6281 | 100 | 100 |
The results of the probabilistic sensitivity analysis, showing the distribution of 1000 resampled cost–effect difference pairs for each base case, are shown on three cost-effectiveness planes in Figure 31.
FIGURE 31.
Cost-effectiveness plane of NIV vs. usual care in the three base-case post-hospital populations. (a) Base case marginally favours control; (b) base case marginally favours NIV; and (c) base case most favourable to NIV.
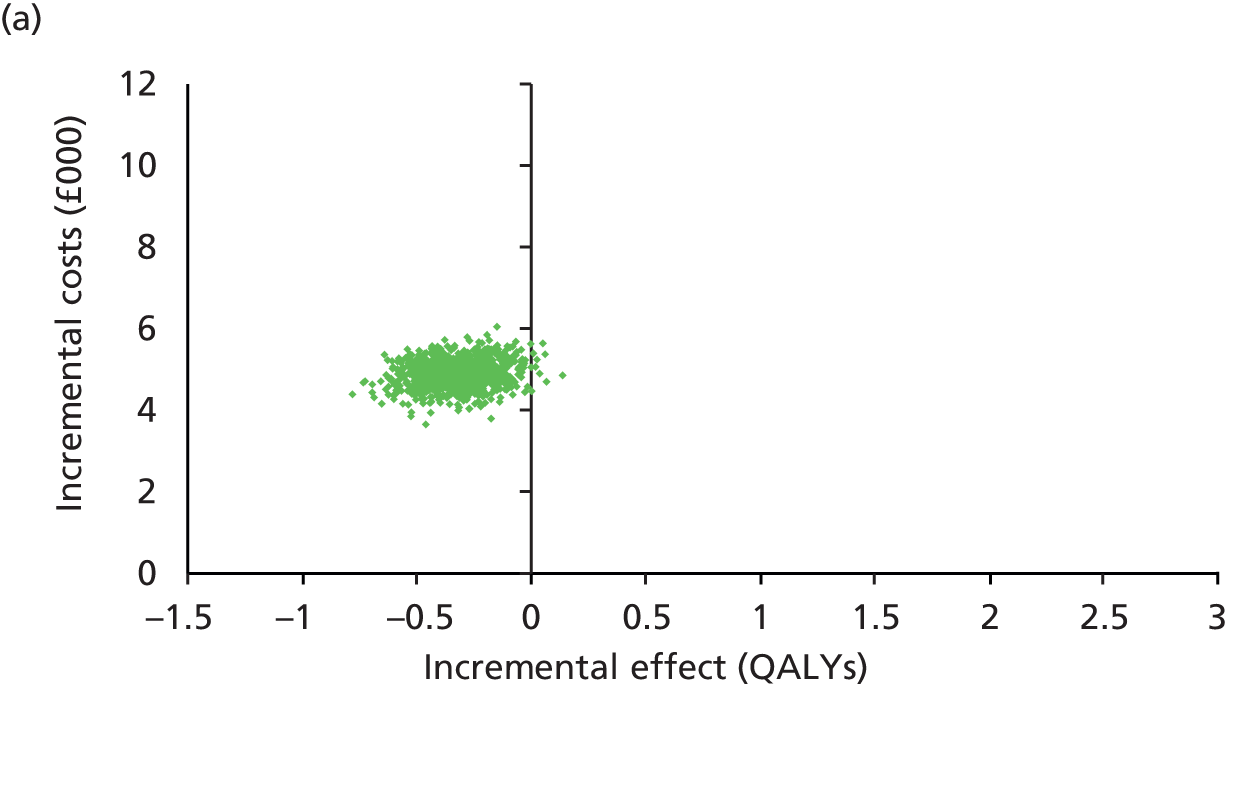
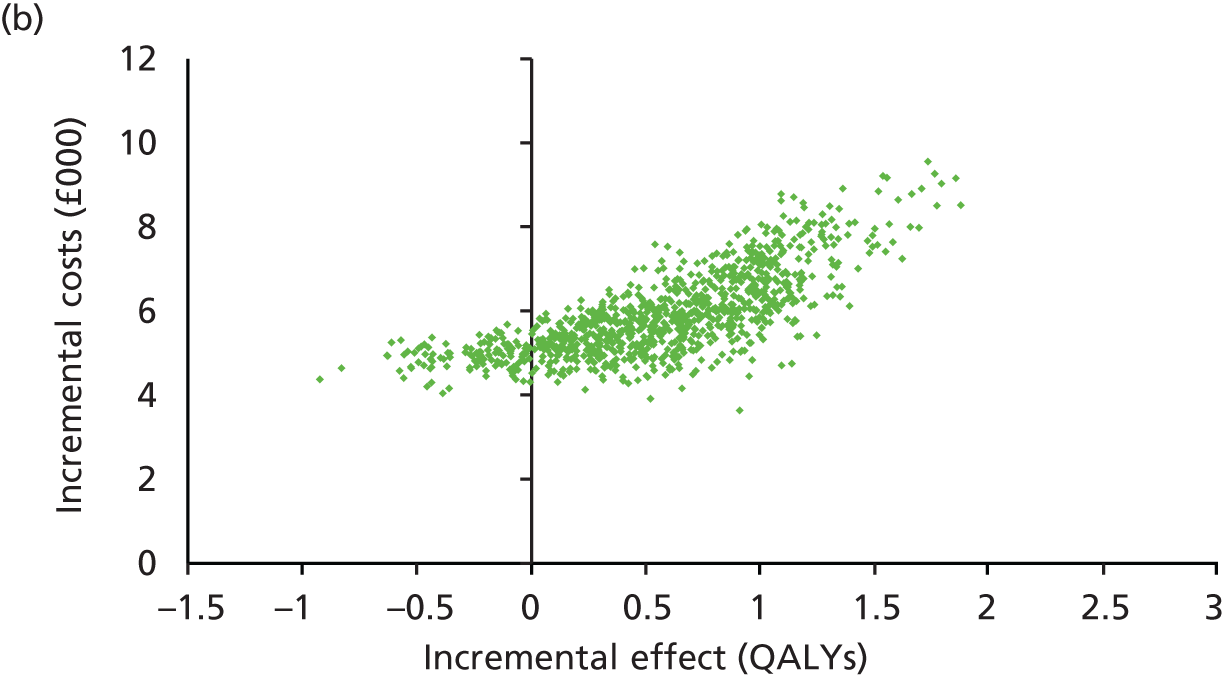
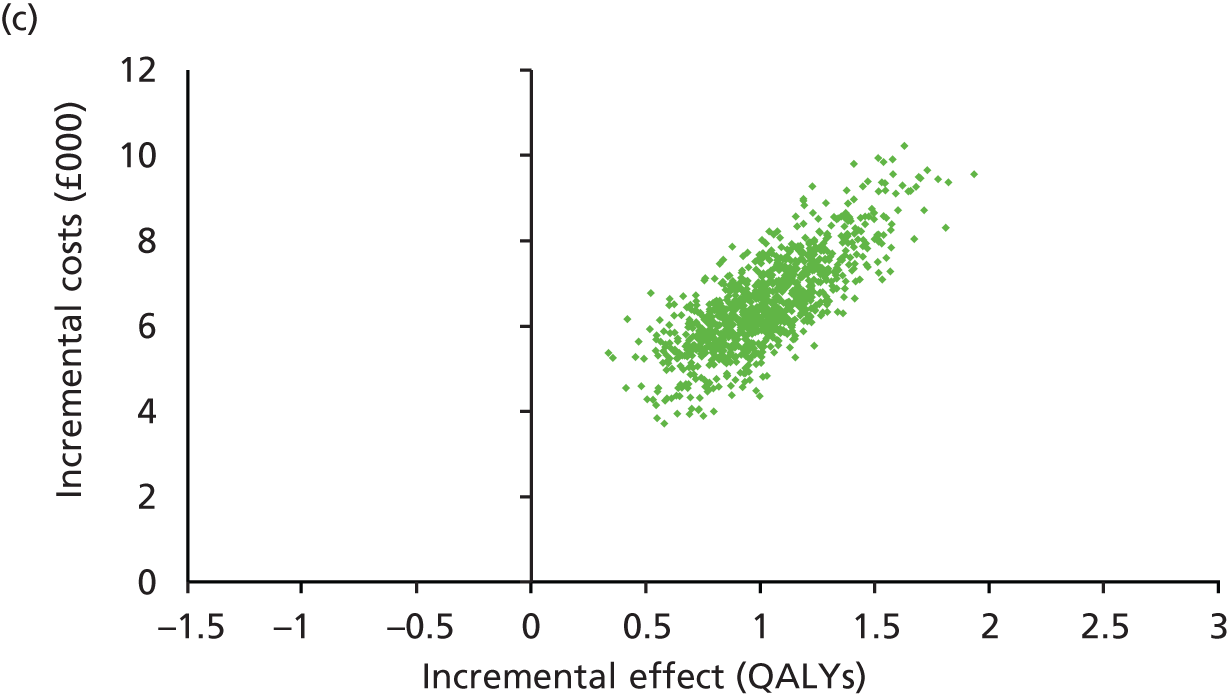
In all three base cases, domiciliary NIV was more costly; however, the results differed widely across the three rate ratios with respect to the effect. For the base case that used evidence that marginally favoured usual care, the majority of the samples are on or to the left of the line of no effect, but there was some uncertainty around this. Similarly, for the base case that used evidence that marginally favoured NIV, in most samples domiciliary NIV was more effective, but there was a lot of uncertainty regarding effectiveness. In the most favourable base case, NIV was more costly and more effective in all samples.
The CEAC in Figure 32 show that, at a threshold of £20,000 per QALY gained, domiciliary NIV has a 0%, 72% and 100% probability of being cost-effective in the base case that marginally favoured usual care, the base case that marginally favoured NIV and the base case that was most favourable to NIV, respectively. This suggests that the considerable uncertainty around the effect of NIV on admissions follows through into the cost-effectiveness analysis findings.
FIGURE 32.
Cost-effectiveness acceptability curve for NIV vs. usual care in the three base-case post-hospital populations.
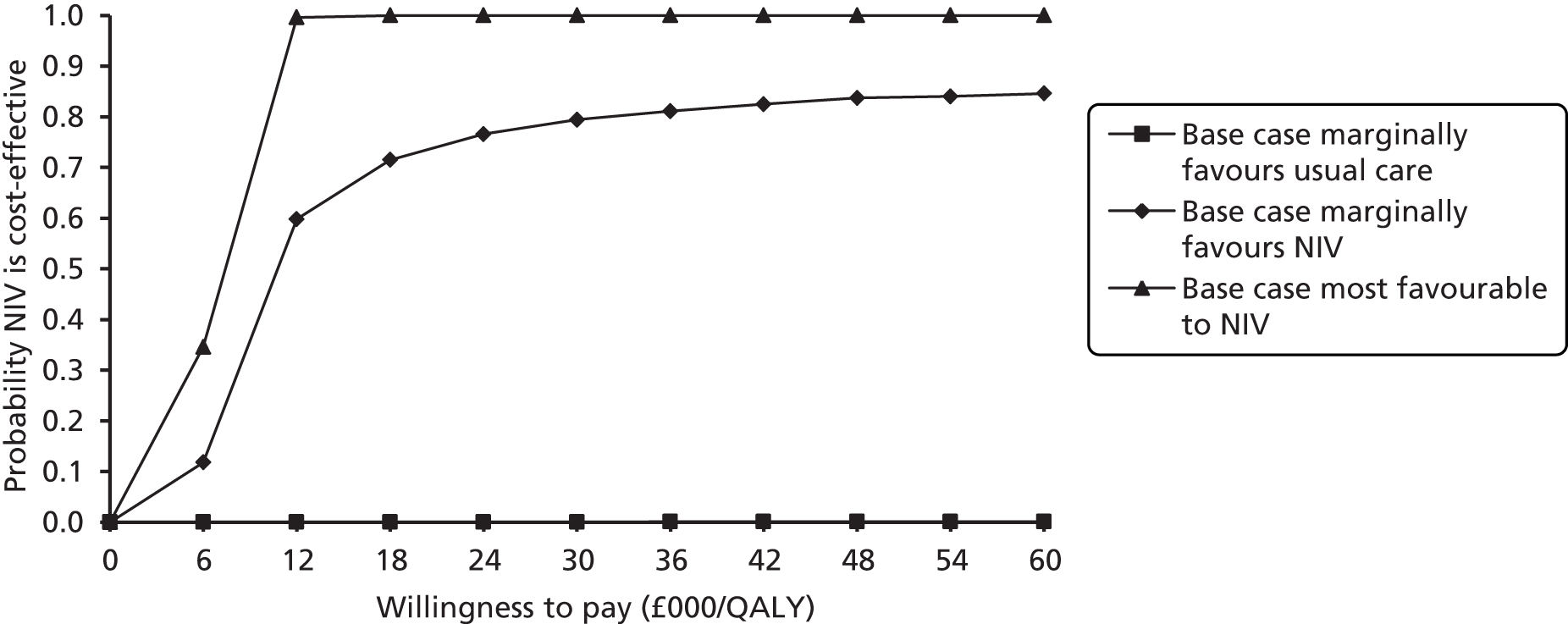
Sensitivity analysis
This section presents the results of the sensitivity analysis conducted on the base-case assumptions applied to the post-hospital COPD population.
Alternative rate ratios for hospital admissions
Varying the rate ratio for admission in this population between 0.05 and 0.95, as illustrated in Figure 33, shows that the ICER for NIV is very sensitive to this parameter. At rate ratios of 0.85 and above (i.e. a 15% reduction in the rate of admissions per patient per year with NIV), the ICER is above £30,000 per QALY gained, and at rate ratios of 0.75 and below, the ICERs are below £20,000 per QALY gained.
FIGURE 33.
One-way sensitivity analysis varying the rate ratio for admissions in a post-hospital population.
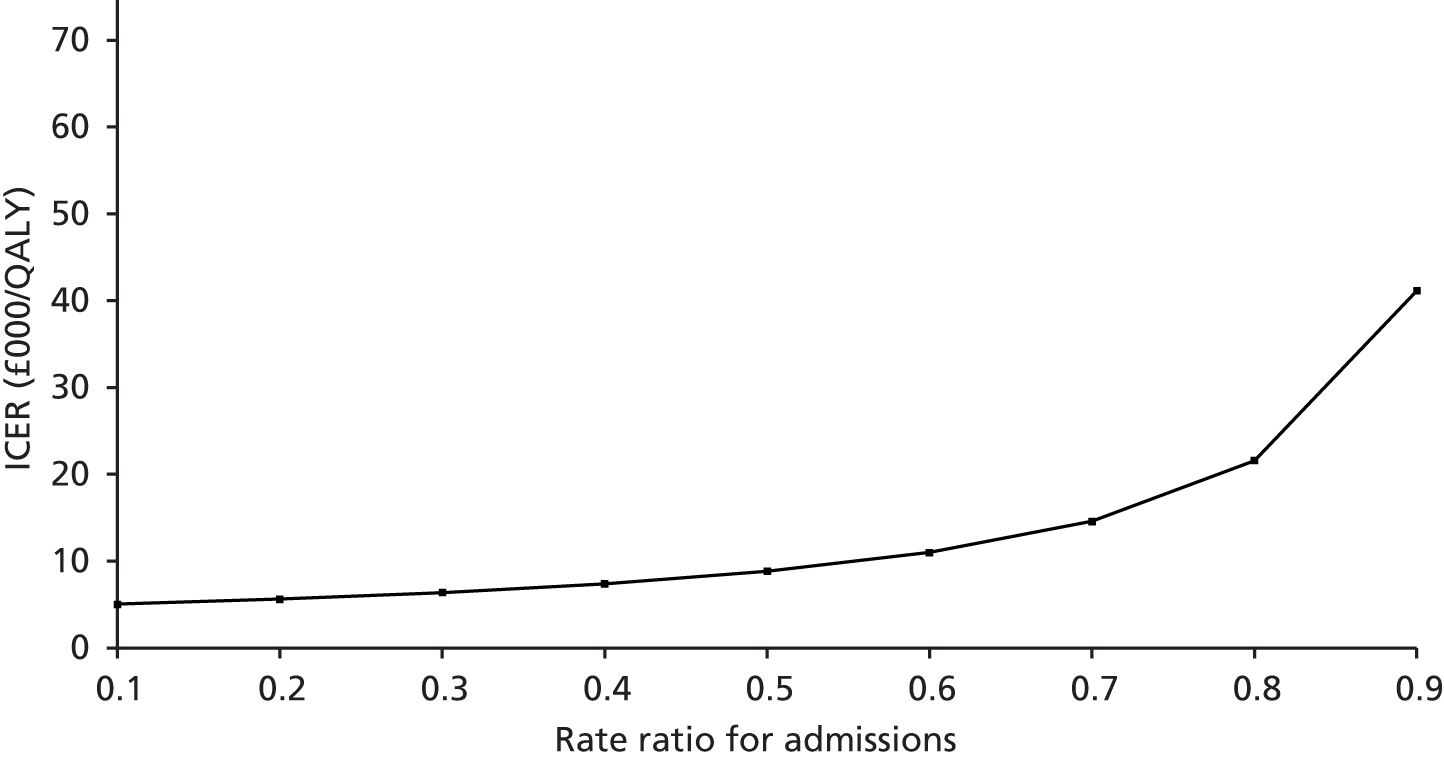
Alternative improvements in utility
While there was no evidence to suggest that domiciliary NIV had an effect on utility, the assumption that domiciliary NIV may have a positive or negative effect on QoL was tested by applying a change of 0.025 in the utility score (on a scale of 0 to 1) in either direction to the three base cases. The effects on the ICERs are illustrated in Figure 34. Assuming that NIV had a small positive or negative effect on the utility score (by 0.025), this had little impact on cost-effectiveness. Usual care dominated NIV in all cases in which the base case marginally favoured usual care. Conversely, where the base cases favoured domiciliary NIV, in all cases the ICER remained below £20,000 per QALY gained.
FIGURE 34.
Alternative assumption for effect on utility in a post-hospital population.
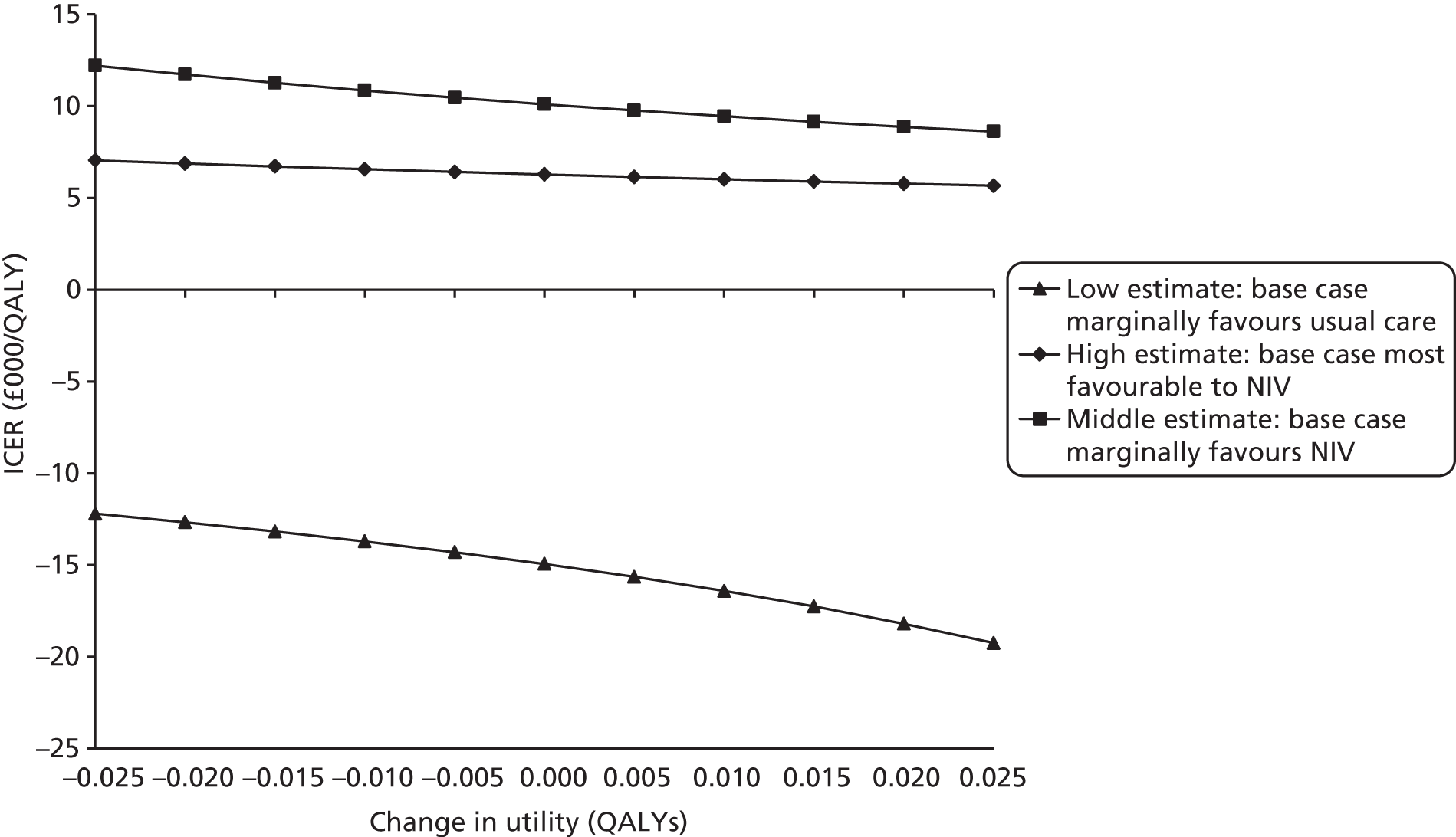
Alternative durations for effect
Figure 35 presents the results, varying the duration of the effect relating to the three base cases from 10 years to between 1 and 30 years. In both of the base cases in which the effectiveness estimate favoured NIV, the ICERs were below £20,000 if the effect of NIV lasted for 2 years or more. Conversely, in the base case in which the evidence marginally favoured usual care, changing the duration of this effect, unsurprisingly, did not change the likelihood of NIV being cost-effective and, in all cases, usual care dominated NIV.
FIGURE 35.
One-way analysis changing the duration of effect in a post-hospital population.
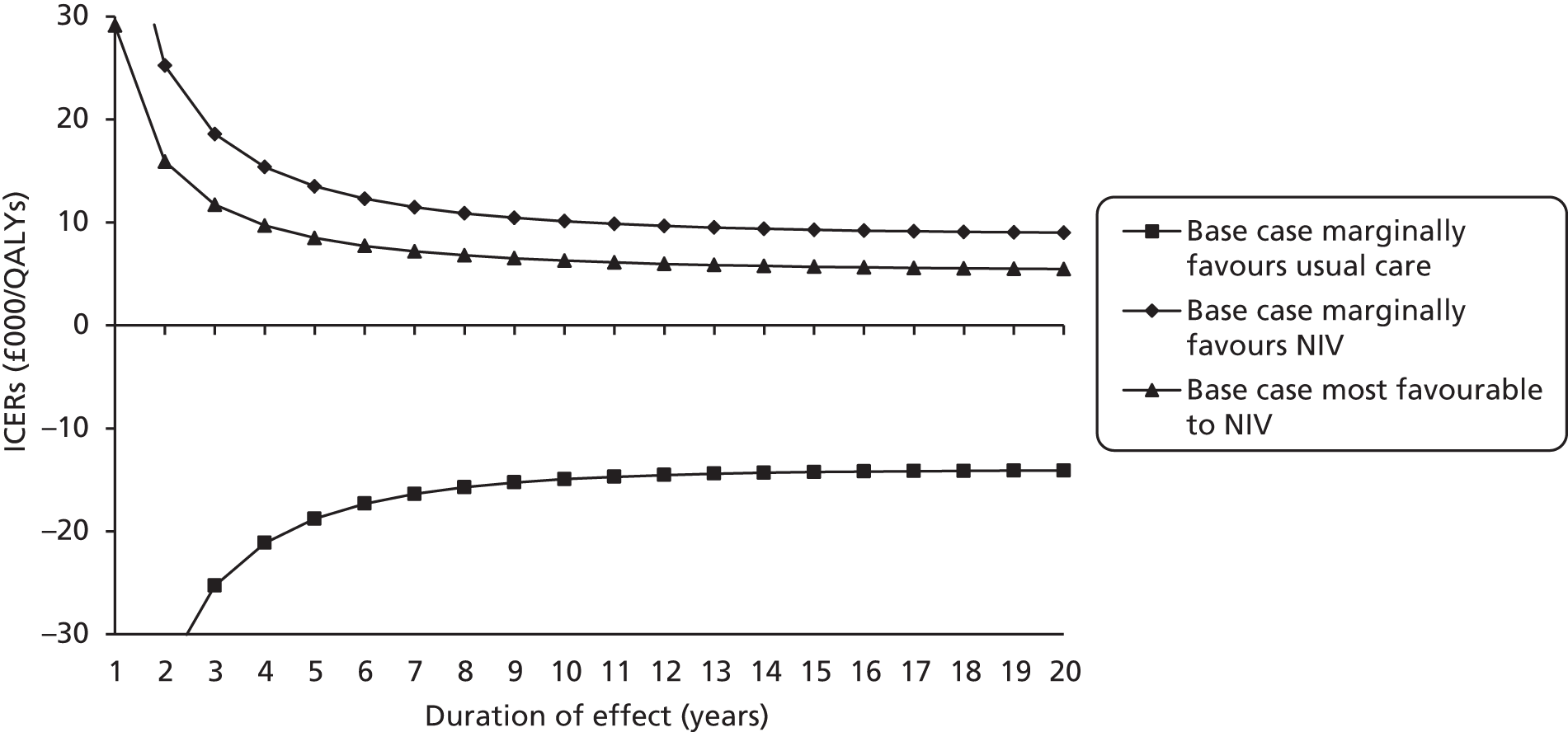
This suggests that it is important to verify the effects of NIV beyond 2 years, as this assumption is an important determinant of the cost-effectiveness of this intervention in this population, but it is less important if NIV is effective beyond 5 years in this population.
Alternative model time horizons
Table 46 presents the results when varying the time horizon of the model. Usual care dominated NIV for all possible time horizons. With a time horizon of 6 months, the ICERs for marginally favouring NIV and strongly favouring NIV were £59,775 per QALY gained and £18,805 per QALY gained, respectively. After 2 years, the ICERs decreased to £14,806 per QALY gained and £2023 per QALY gained, both with an increasing probability of being cost-effective. At all time horizons beyond 5 years, the ICER was £10,107 per QALY gained or less for both the cases that favoured NIV and there was only a small change to the probability of domiciliary NIV being cost-effective, varying between 75% and 80% at a threshold of £30,000 per QALY gained in the case that marginally favoured NIV and staying at 100% in the most favourable case.
| Direction of effect | Time horizon | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|---|
| Marginally favoured usual care | 6 months | 1289 | –0.0057 | Dominated | 0 | 0 |
| 2 years | 3164 | –0.0465 | Dominated | 0 | 0 | |
| 5 years | 4719 | –0.1476 | Dominated | 0 | 0 | |
| 10 years | 5085 | –0.2655 | Dominated | 0 | 0 | |
| 20 years | 4881 | –0.3209 | Dominated | 0 | 0 | |
| 30 years (base case) | 4865 | –0.3255 | Dominated | 0 | 0 | |
| Marginally favoured NIV | 6 months | 504 | 0.0084 | 59,775 | 17 | 31 |
| 2 years | 995 | 0.0672 | 14,806 | 60 | 68 | |
| 5 years | 2301 | 0.2277 | 10,106 | 67 | 75 | |
| 10 years | 4119 | 0.4509 | 9135 | 69 | 77 | |
| 20 years | 5697 | 0.5673 | 10,043 | 72 | 78 | |
| 30 years (base case) | 5830 | 0.5769 | 10,107 | 71 | 77 | |
| Most favourable | 6 months | 249 | 0.0132 | 18,805 | 53 | 80 |
| 2 years | 221 | 0.1092 | 2023 | 98 | 100 | |
| 5 years | 1285 | 0.3828 | 3357 | 100 | 100 | |
| 10 years | 3581 | 0.7918 | 4523 | 100 | 100 | |
| 20 years | 6259 | 1.0141 | 6171 | 100 | 100 | |
| 30 years (base case) | 6485 | 1.0325 | 6281 | 100 | 100 |
Similar to varying the duration of effect, the model was less sensitive to changes in the time-horizon beyond 2 years compared with the stable population because of higher baseline all-cause and COPD-related mortality risks.
Alternative continuation rates
The effect on the ICER of varying the proportion of those who benefit from domiciliary NIV and continue using it from 55% to 95% in the three base cases is shown in Figure 36. As with the stable population, the model was not very sensitive to this assumption as the short-term costs of trialled domiciliary NIV on those who discontinue without benefiting was minimal compared with the costs and benefits that accrue over the lifetime of the population that continues with NIV.
FIGURE 36.
One-way sensitivity analysis of alternative continuation rates beyond 3 months in a post-hospital population.
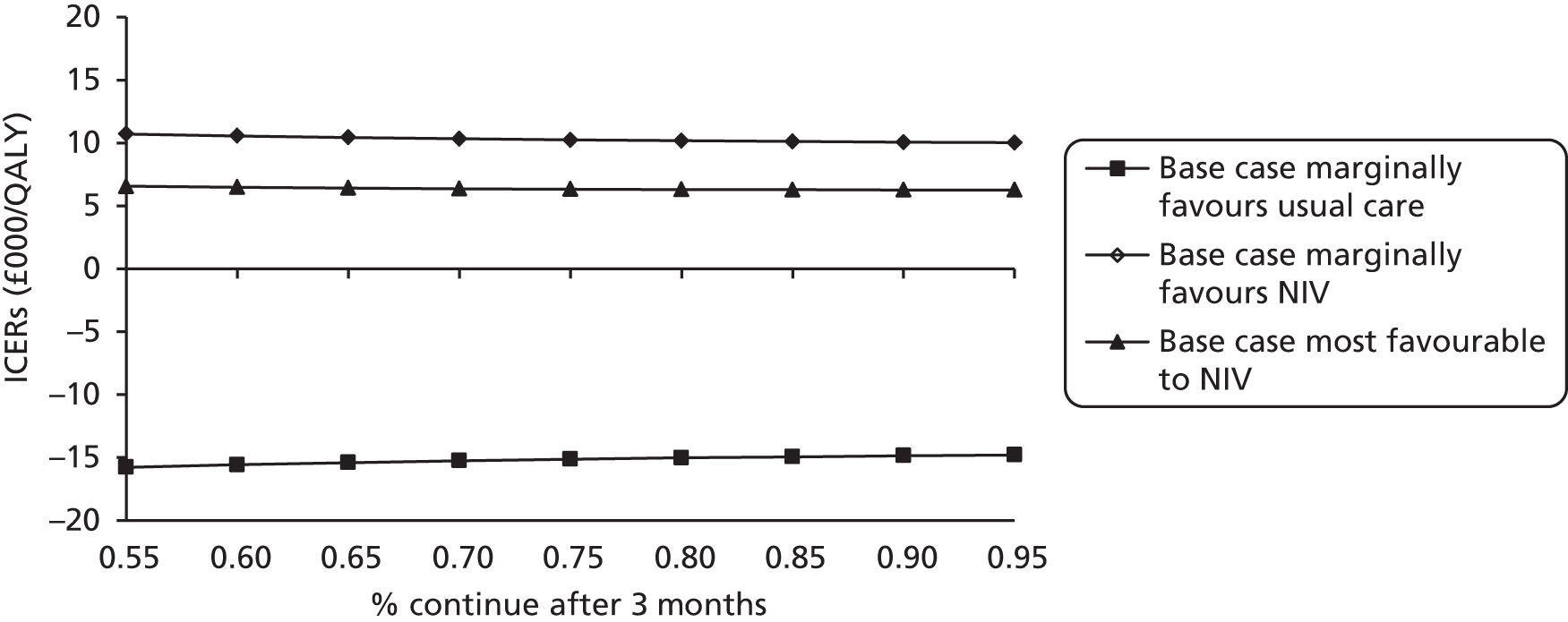
Alternative costs of the non-invasive ventilation device
In the base cases it was assumed that the NIV device would cost £3600 and the monthly cost was derived on the assumption that it would be used continuously for 5 years. The results of varying the cost of the NIV device are illustrated in Figure 37 and show that at all costs of the device between £2000 and £6000 the ICER was below the lower threshold of £30,000 per QALY gained for the base cases in which the effectiveness estimate favoured NIV, and was dominated at all costs for the base case that favoured usual care.
FIGURE 37.
One-way sensitivity analysis of alternative costs of device in a post-hospital population.
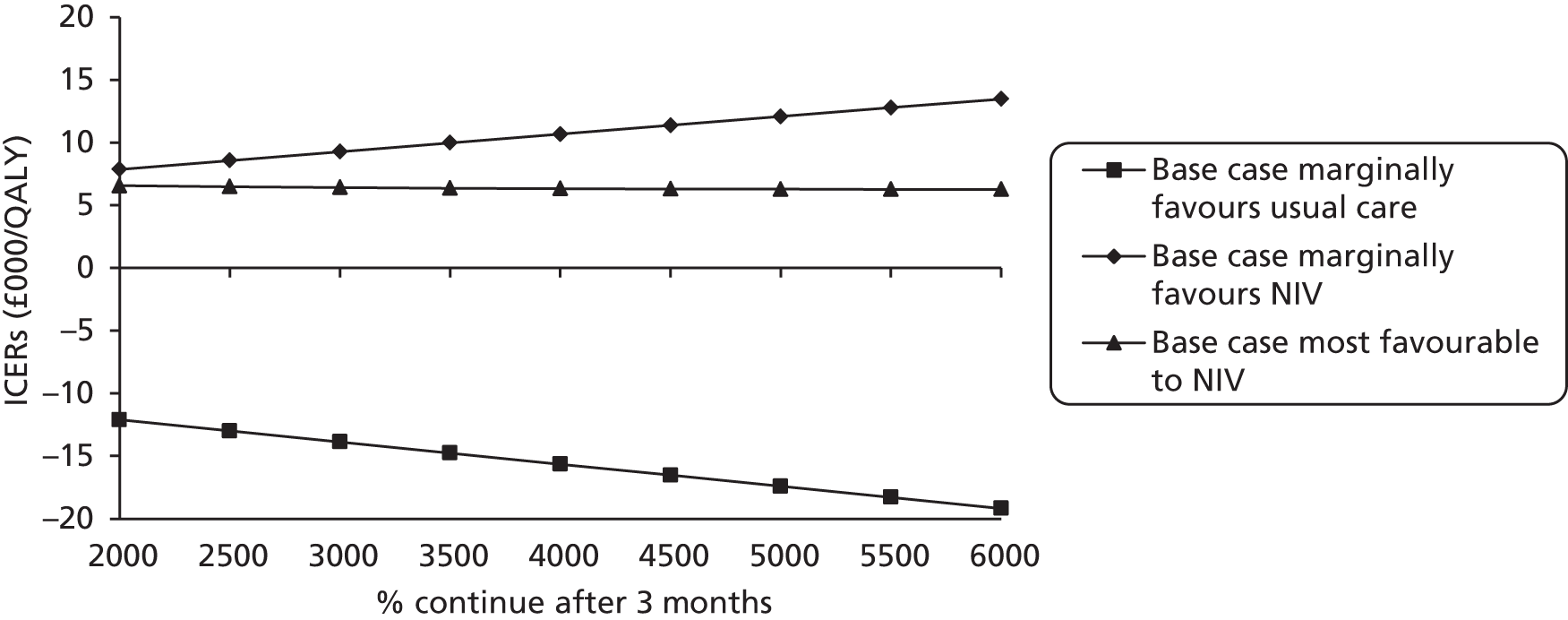
Similarly, the assumption for the lifespan of the device was varied between 4 and 8 years and, as illustrated in Figure 38, in all cases the ICER was below £30,000 per QALY gained for the base cases in which the effectiveness estimate favoured NIV, and was dominated at all lifespans for the case that favoured usual care.
FIGURE 38.
One-way sensitivity analysis of alternative lifespan of device in a post-hospital population.
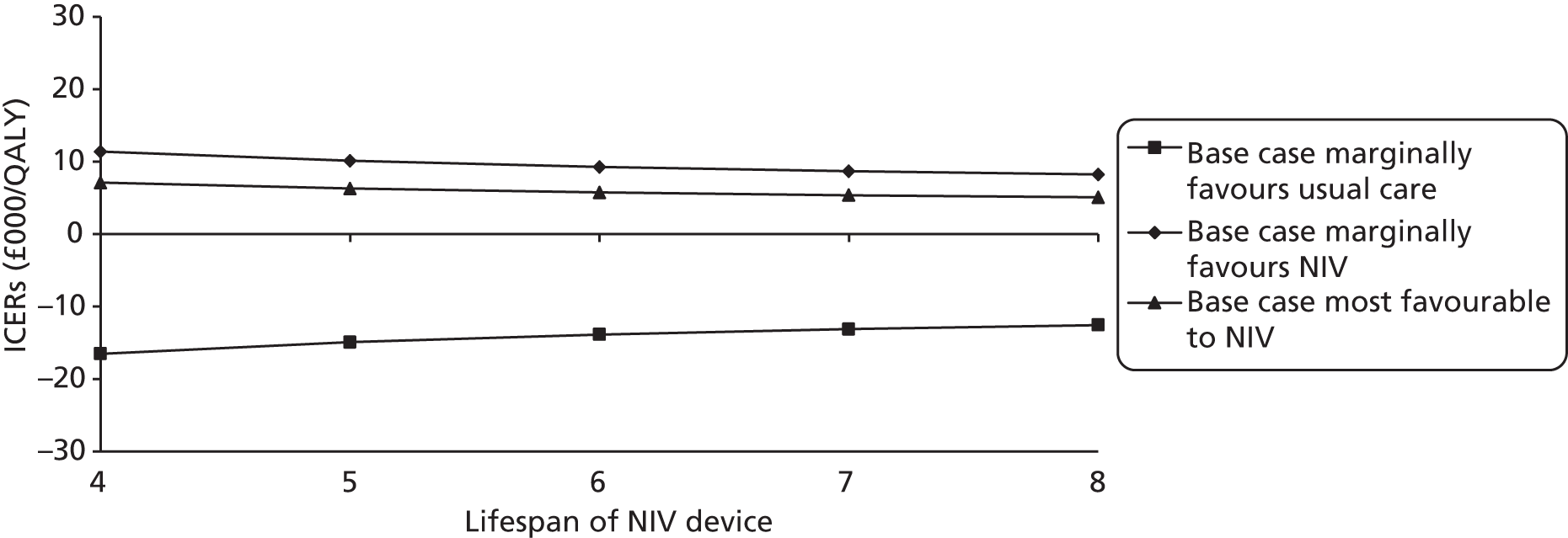
Alternative costs of the service aspects of non-invasive ventilation provision
The tariff for an NIV assessment and set-up varied between £200 and £1000. The results in Figure 39 show that the model was not sensitive to variation in this cost in any of the cases, as it was a one-off cost and had a minimal impact on the cost difference over the cohort’s lifetime. The model was more sensitive to changes to the annual service cost (Figure 40). However, as with the cost of the NIV device, the ICERs were below £15,000 per QALY gained for the two base cases in which the effectiveness estimate favoured NIV and were dominated in all cases where usual care was favoured.
FIGURE 39.
One-way sensitivity analysis of alternative NIV assessment costs in a post-hospital population.
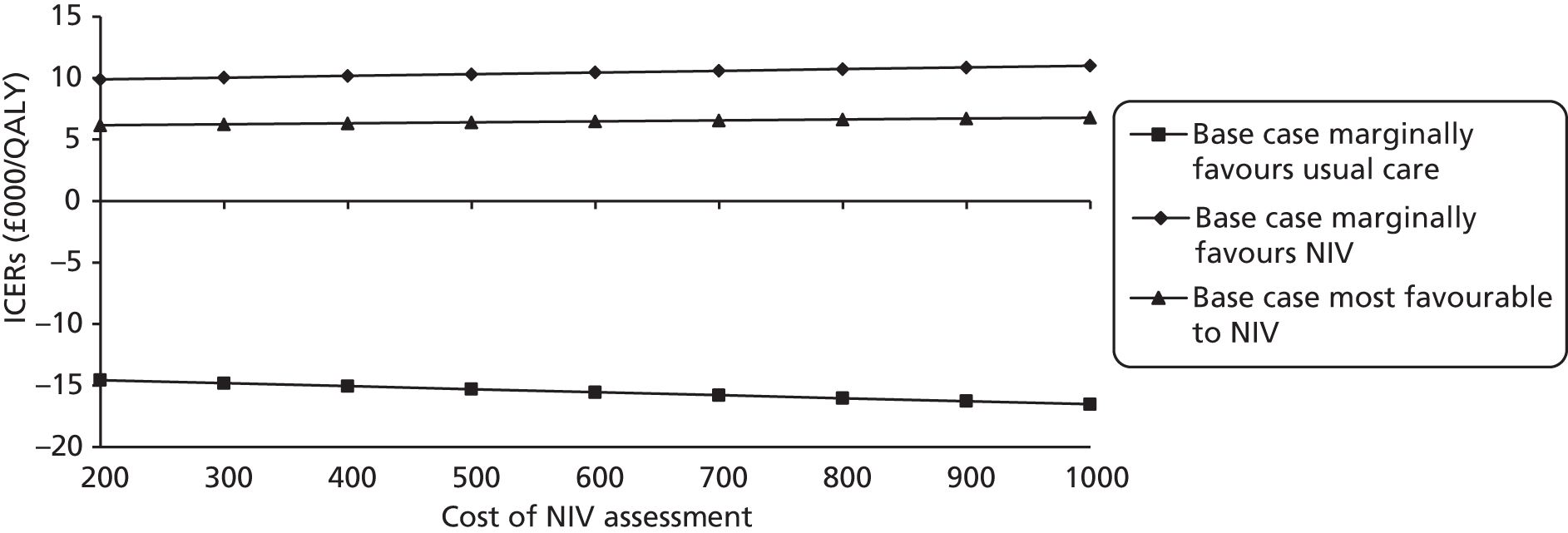
FIGURE 40.
One-way sensitivity analysis of annual maintenance costs in a post-hospital population.
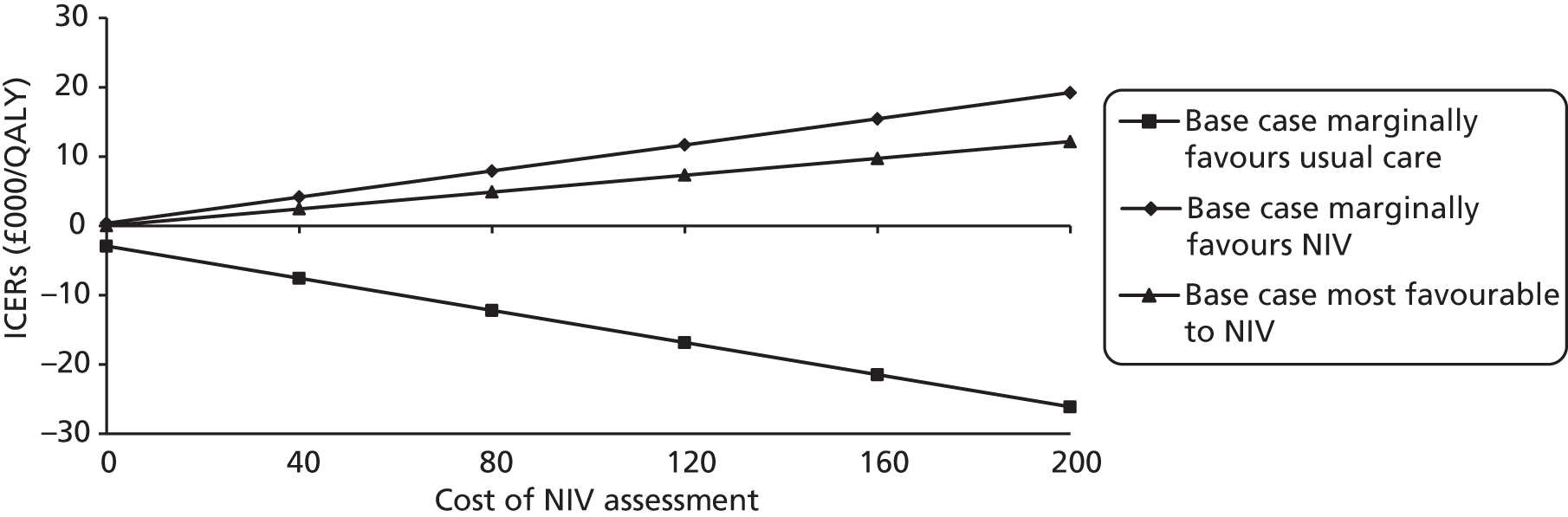
Alternative utility assumptions applied in the post-hospital population
The difference in costs and QALYs when using the utility assumptions applied in Borg et al. (2004)138 are reported in Table 47. Similarly to when these were applied to the stable population, this made very little difference to the cost-effectiveness of domiciliary NIV in any of the three post-hospital population base cases.
| Strategy | Mean cost (£) | Cost difference (£) | Mean QALYs | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|---|---|
| Usual care | 17,048 | – | 2.2715 | – | – | – | – |
| Domiciliary NIV + usual care | |||||||
| Marginally favoured usual care | 21,912 | 4864 | 2.1614 | –0.3294 | Dominated | 0 | 0 |
| Marginally favoured NIV | 22,879 | 5830 | 2.7447 | 0.5833 | 9996 | 72 | 79 |
| Most favourable | 23,533 | 6485 | 3.2014 | 1.04 | 6236 | 0 | 0 |
Alternative baseline risks applied in the post-hospital population
Replacing the parameters for hospitalisation and mortality obtained from Garcia-Aymerich et al. (2003)152 with those obtained from Bucknall et al. (2011)153 increased the ICER to £23,211 per QALY gained in the case in which the effectiveness estimate marginally favoured NIV and to £13,101 per QALY gained with the most favourable effectiveness estimate (Table 48). It reduced the probability of NIV being cost-effective at a threshold of £30,000 per QALY gained to 61% in the case that the effectiveness estimate marginally favoured NIV, but had no effect on the probabilities of the other cases. This suggests that domiciliary NIV is less cost-effective in patients with lower baseline risk of hospitalisation and/or mortality.
| Severity | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|
| Applying baseline risks from Bucknall et al.153 | |||||
| Marginally favoured usual care | 5613 | –0.1346 | Dominated | 0 | 0 |
| Marginally favoured NIV | 4034 | 0.1738 | 23,211 | 34 | 61 |
| Most favourable | 3562 | 0.2719 | 13101 | 99 | 100 |
| Doubling all mortality risks | |||||
| Marginally favoured usual care | 2567 | –0.1637 | Dominated | 0 | 0 |
| Marginally favoured NIV | 3019 | 0.3147 | 9593 | 75 | 80 |
| Most favourable | 3379 | 0.587 | 5756 | 100 | 100 |
Adjusting all mortality risks (risk of death during exacerbation, risk of death post-exacerbation and risk of all-cause mortality) by a factor of two decreased both the costs and outcomes compared with the base case, reflecting shorter survival in both arms. This reduced the ICER to £9593 per QALY gained in the case where the effectiveness estimate marginally favoured NIV and £5756 per QALY gained with the most favourable effect estimate and had a minimal impact on the likelihood of domiciliary NIV being cost-effective at £20,000 or £30,000 per QALY gained. For all analyses, usual care remained dominant over NIV with the case that used an effect estimate that favoured usual care.
Subgroup analysis for the post-hospital population
Global Initiative for Chronic Obstructive Lung Disease severity stage
Table 49 presents the ICERs when assuming that only one GOLD-stage severity group entered the model. The post-hospital model was not expected to be as sensitive to changes in the proportion of each GOLD stage severity group, as the same probability of admission, COPD and all-cause mortality were applied to GOLD stages 3 and 4. The only differences between these health states by severity were the costs of usual care and utility values.
| Direction of effect | Severity | Cost difference (£) | QALY difference | ICER (£/QALY) | Probability cost-effective at £20,000/QALY (%) | Probability cost-effective at £30,000/QALY (%) |
|---|---|---|---|---|---|---|
| Marginally favoured usual care | Base case (50 : 50 split between GOLD stages 3 and 4) | 4865 | –0.3255 | Dominated | 0 | 0 |
| GOLD stage 3 | 4913 | –0.334 | Dominated | 0 | 0 | |
| GOLD stage 4 | 4814 | –0.3171 | Dominated | 0 | 0 | |
| Marginally favoured NIV | Base case (50 : 50 split between GOLD stages 3 and 4) | 5830 | 0.5769 | 10,107 | 71 | 77 |
| GOLD stage 3 | 5785 | 0.6001 | 9641 | 73 | 78 | |
| GOLD stage 4 | 5876 | 0.5537 | 10,611 | 71 | 78 | |
| Marginally favoured NIV | Base case (50 : 50 split between GOLD stages 3 and 4) | 6485 | 1.0325 | 6281 | 100 | 100 |
| GOLD stage 3 | 6394 | 1.0706 | 5972 | 100 | 100 | |
| GOLD stage 4 | 6576 | 0.9945 | 6613 | 100 | 100 |
Assuming that 100% of the population were in GOLD stage 3, the ICER decreased to £9641 per QALY in the case in which effectiveness evidence marginally favoured NIV and to £5972 per QALY gained with the most favourable effect estimate, and, assuming that 100% of the population were at GOLD stage 4, the ICER increased to £10,611 per QALY gained, and to £6613 per QALY gained, respectively. There was almost no change in the probability of either being cost-effective. Where the effectiveness estimate was more favourable for usual care, this option still dominated NIV.
Expected value of perfect information analysis for the post-hospital population
The EVPI per patient for the post-hospital population was estimated to be £1541 and £1694 at a threshold of £20,000 and 30,000 per QALY gained, respectively, for the case in which effectiveness evidence marginally favoured NIV. This reflects the high value of removing the uncertainty in this case, in which there was considerable uncertainty around the likelihood of NIV being cost-effective at these thresholds. Conversely, the value of EVPI for the case in which the effectiveness estimate that marginally favoured usual care and in which the intervention was most favourable for NIV was zero in both cases, as the probability of NIV being cost-effective was 0% and 100%, respectively.
Multiplying the EVPI per patient to the estimated population size (77,000 post-hospital patients in the UK) generated an estimate for the EVPI per population of £119M and £130M, respectively, for the case that marginally favoured NIV. The EVPIs per population at thresholds between £0 and £60,000 per QALY gained are illustrated in Figure 41. This EVPI curve reaches a peak at thresholds close to the base-case ICER, at which point the decision changes and domiciliary NIV becomes the preferred option. These estimates reflect the value of conducting research that would remove all uncertainty from this decision and are high because of the uncertainty around the effect and the potentially large population that would be affected by the decision to adopt this technology (albeit a smaller population than all stable end-stage COPD patients).
FIGURE 41.
Expected value of perfect information analysis for the three post-hospital populations.
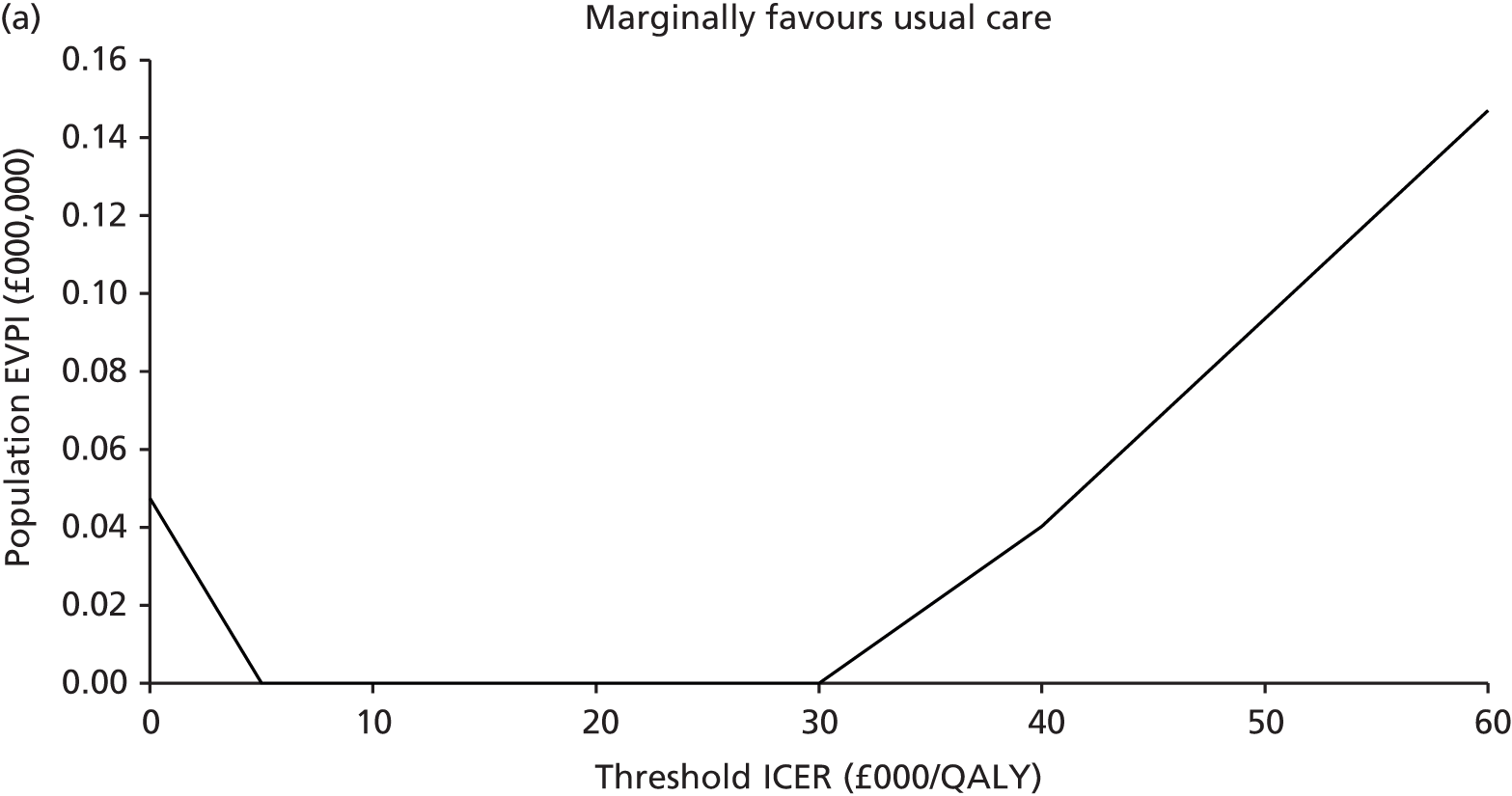
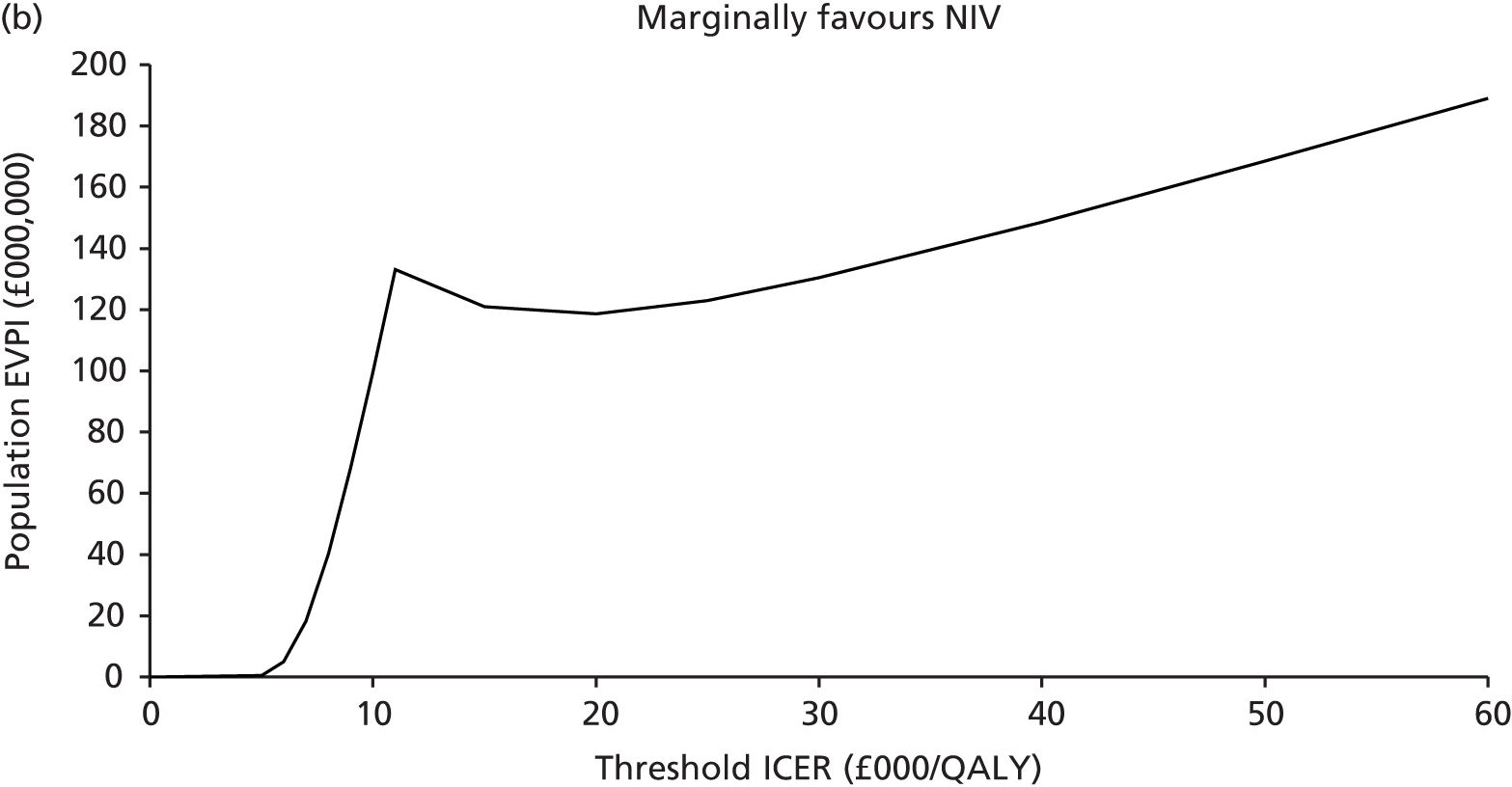
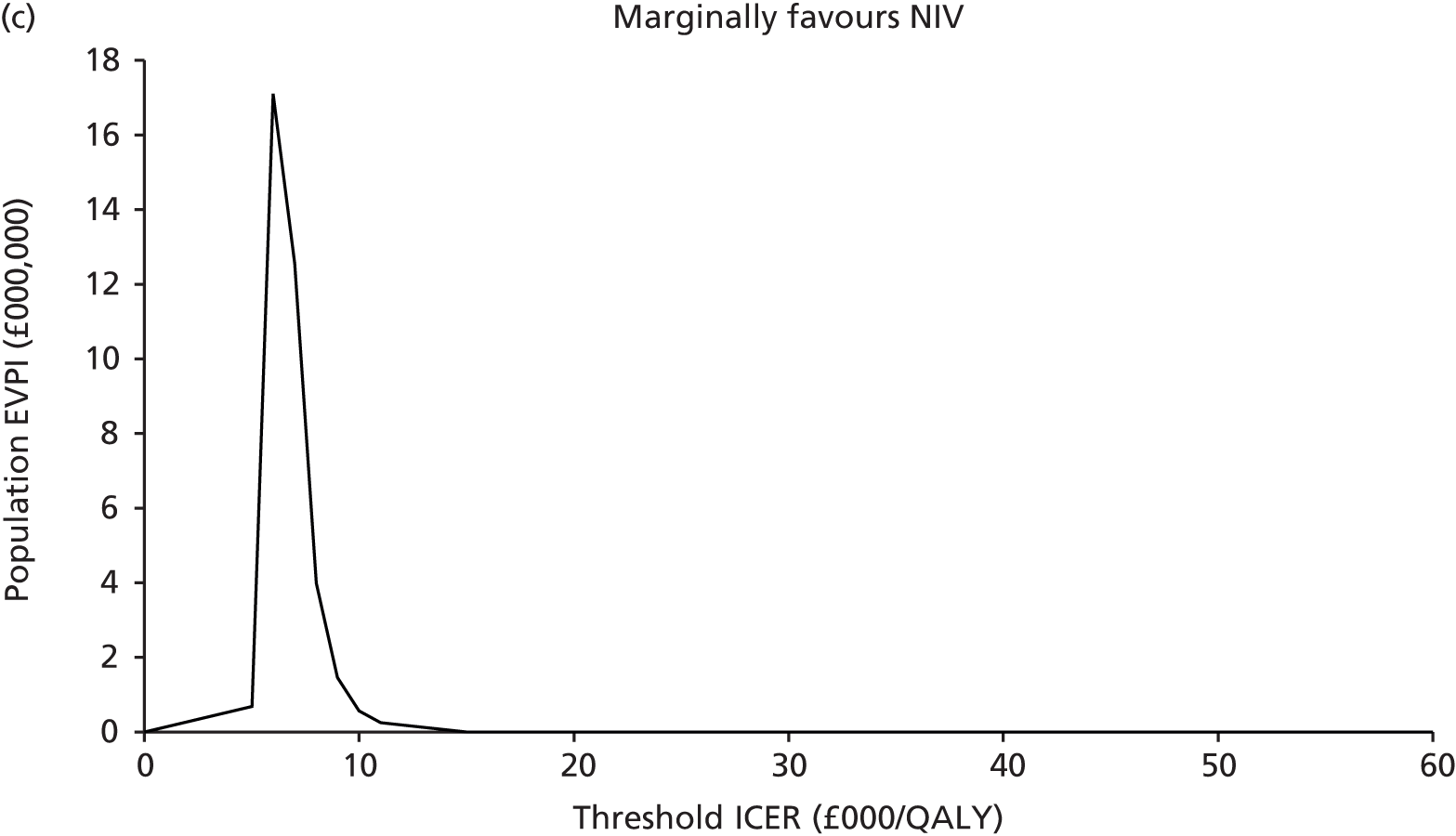
In the case where effectiveness evidence marginally favoured usual care, the EVPI decreases between £0 and £6000 per QALY gained, then remains at zero until the £30,000 threshold. The EVPI increases above this point, reflecting the small probability that NIV results in higher costs and better outcomes.
At most willingness-to-pay thresholds the EVPI for the case that most favours NIV is zero, reflecting the higher probability of NIV being cost-effect at all thresholds considered. There is a small peak around the point estimate, suggesting that, if the willingness-to-pay per QALY gained threshold were close to £6000, there would be some value to further research of knowing with greater certainty that NIV was cost-effective.
Discussion
This is the first economic model to consider the cost-effectiveness of domiciliary NIV in two end-stage COPD populations, namely stable end-stage COPD patients started on domiciliary NIV in the community and end-stage COPD patients started on domiciliary NIV when recovering from an exacerbation requiring hospital admission. In both populations the model-based analyses should be viewed as speculative, owing to uncertainties in the base-case effects applied. For the stable population, the effect on admissions was uncertain; while four of five RCTs included in the meta-analysis found a reduction in hospital admissions, only one of these findings was statistically significant and the overall pooled result was also not statistically significant. Further, not all available evidence could be included in the meta-analysis. This finding of a trend towards a reduction in hospital admissions was not reflected in the available (short-term) mortality data, which found no evidence of a difference in effect between NIV and usual care. However, the uncertainty around the impact on admissions is captured in the analysis, as the 95% CI around the point estimate of risk reduction is used in the probabilistic sensitivity analysis.
There was also considerable uncertainty regarding the effect in a post-hospital population, as the only three RCTs reporting this outcome had inconsistent findings.
Stable population
Applying the assumption that domiciliary NIV results in a reduction in hospital admissions, the base-case results for the stable population suggest that domiciliary NIV may be a cost-effective intervention at a threshold of £30,000 per QALY gained. However, probabilistic sensitivity analysis estimates that the probability of being cost-effective at this threshold is only 55%, demonstrating the uncertainty around the impact of domiciliary NIV on admission and utility in this population. If a £20,000/QALY gained threshold is applied, the intervention is not cost-effective. The impact of a reduced risk of admission led to lower mortality and morbidity; however, as these effects were expected to last for 10 years and the costs were assumed to accumulate over the cohort’s lifetime, it is uncertain whether or not benefits outweigh the higher lifetime costs of domiciliary NIV provision. The one-way sensitivity analyses undertaken were informative in highlighting the key drivers of the model results. As expected, cost-effectiveness was affected by the estimate of effect on admissions and utility improvement, the duration of effect and elements of the cost of domiciliary NIV provision. Applying the high effectiveness estimate reduced the ICER to £18,660 per QALY gained, and the probability of NIV being cost-effective at £30,000 per QALY gained increased to 82%. However, when applying the effectiveness estimate (which marginally favoured usual care), the intervention was dominated by usual care and this probability was reduced to less than 1%, highlighting the uncertainty around the effect. Varying the rate ratio for hospital admissions found that for NIV to be cost-effective at this threshold, the rate ratio would need to be 0.76 or lower (i.e. a 24% reduction in the rate of admissions per patient per year with NIV). Similarly, assuming domiciliary NIV improved QoL and increased the utility score by 0.025, the ICER decreased to £26,462, closer to the threshold of £20,000 per QALY gained.
It is unsurprising that the intervention becomes more cost-effective when extending the duration of effect beyond 10 years, and is less effective at shorter durations. While it is reasonable to conclude that the effects are likely to last beyond those reported in trials (1 or 2 years) the model becomes more speculative when assuming that the effects extend beyond 10 years. Taking into account all the uncertainty around the effect estimate for hospital admission and effect on utility, it is plausible that the intervention may be cost-effective at £30,000 per QALY gained if the effects lasted for a patient’s lifetime. As the base-case ICER was between the willingness-to-pay thresholds of £20,000 and £30,000 per QALY gained, the model was sensitive to costs that accumulated over the long term, such as the monthly equipment costs and annual maintenance costs. If these costs were lower than those applied in the base-case analysis, the ICER comes closer to £20,000 per QALY gained; however, the overall uncertainty around the results would remain.
The population EVPI was £596M, which reflects the value of removing all uncertainty regarding the decision to adopt domiciliary NIV at a willingness-to-pay threshold of £30,000 per QALY gained. This value is high because of the large population potentially affected by this decision, and should be considered indicative because of uncertainties regarding the prevalence of COPD and the proportion considered end stage and stable.
Post-hospital population
Three base cases were explored in order to reflect the considerable uncertainty of the effect of NIV on hospital admissions. For the case in which the effectiveness estimate marginally favoured usual care, NIV was more costly and resulted in worse outcomes; therefore, usual care was dominant. While there was some uncertainty around the effect, there was a zero likelihood of NIV being cost-effective, and this conclusion was not sensitive to any of the other assumptions tested in sensitivity analysis.
Conversely, the opposite conclusion could be drawn from the case in which the effectiveness estimate was most favourable to NIV. Here NIV was more costly but more effective and, as the ICER was below £10,000 per QALY gained, there was a 100% probability of NIV being cost-effective at a threshold of £20,000 per QALY gained. The case in which the effectiveness estimate marginally favoured NIV also produced an ICER close to £10,000 per QALY, but there was a lot more uncertainty around the probability of NIV being cost-effective, driven primarily by the uncertainty around the effect.
The disparity in the findings from the three base cases highlights the need for more studies to evaluate the effect in this population. Although two out of the three studies informing the base cases favoured NIV and thus found NIV to be cost-effective, the sample size was very small in both cases (n = 40 and 47) and there was also a large difference in size of effect. The study that marginally favoured usual care was larger (n = 201), but the direction of effect was not consistent with that of the other two RCTs.
The results from the EVPIs conducted for each case, not surprisingly, also gave very mixed values for perfect information to inform the decision to offer domiciliary NIV to this population. The value of perfect information in the case in which the effectiveness estimate marginally favoured usual care (obtained from a large study) and the case in which the effectiveness estimate favoured NIV (obtained from a small study) was much smaller than the value of perfect information in the case in which the effectiveness estimate marginally favoured NIV. This was associated with a lot of uncertainty. As a pooled rate ratio for hospital admissions is likely to be closer to the middle estimate (i.e. marginally favouring NIV) than the other two cases, this suggest that there is a strong argument in favour of conducting further research to remove this uncertainty.
Sensitivity analyses
The sensitivity analysis conducted for both populations found that the relative cost-effectiveness of domiciliary NIV was not particularly sensitive to the initial costs of set-up or to the proportion that discontinued using domiciliary NIV in the first 3 months (assuming that only those who benefited continued using it and incurred costs beyond this point). Conversely, the model was sensitive to changes in the size of the effects (rate ratio for admission and change in utility score) and duration of these effects in both populations.
In the base cases for both populations it was assumed that there was no improvement in QoL associated with domiciliary NIV outside of the short-term decrements to QoL avoided from a reduced risk of readmission. This was based on limited QoL data being reported in the studies identified. As this was a conservative assumption, it may have underestimated the relative cost-effectiveness of domiciliary NIV and points to the importance of gathering more robust evidence QoL.
The one-way sensitivity analysis varying the baseline mortality risks applied to the usual-care arm in both populations found that the model was sensitive to this variable and that domiciliary NIV was more cost-effective in patients at greater risk of admission and death. This is logical, demonstrating that if domiciliary NIV is effective in reducing admissions and associated mortality, it will be more effective and cost-effective in patients most at risk of these events. This also highlights the importance of collecting more robust data on patient characteristics that determine the risk of admission and mortality. It also suggests that prioritising those most at risk of these events within NIV services could be considered.
Strengths and limitations
A key strength of this analysis is that it is the first economic model to consider the cost-effectiveness of domiciliary NIV in both stable and post-hospital COPD patients and illustrates the key variables that impact on the results. A further strength of this study was the model structure applied, which is a modified version of previously published decision model where additional post-admission health states were added to incorporate evidence on the higher risks in COPD patients immediately after discharge. This was particularly useful when modelling two different COPD populations with different baseline risks of admission and mortality.
Although there was a great deal of uncertainty around effectiveness data and assumptions applied to this model, distributions were applied to reflect this uncertainty. A probabilistic sensitivity analysis was undertaken, and this was supplemented with an extensive one-way sensitivity analysis of key parameters, thus demonstrating which parameters were mostly likely to influence decisions to implement domiciliary NIV in each patient population. Furthermore, value of information analysis was conducted to quantify the value of conducting further research to eliminate this uncertainty.
However, caution should be applied when interpreting the results of the analyses for both populations modelled. This is a speculative decision model and should only be considered as indicative of the potential cost-effectiveness of domiciliary NIV if it is effective at reducing hospital admissions. For the stable population, there was some evidence of a reduced risk of admissions in some studies reporting this outcome, but there was no evidence of a reduced risk of mortality (up to 2 years). There was also a lot of uncertainty for the post-hospital population, as studies reported very different effect estimates for hospital admissions. A further limitation is the lack of long-term admissions data (up to 2 years). However, both models have assumed an effect on admissions over the first 10 years.
In addition to the uncertainty around the effect of domiciliary NIV, there was also uncertainty around parameters and assumptions for usual care in both populations. The model was populated using some trial evidence but, as the studies identified in the clinical effectiveness systematic review reported limited data, parameters were additionally obtained from large cohort studies. Furthermore, while the model was able to reflect mortality and readmission risks in the first 3 months after discharge, it was assumed that, after those 3 months, those who were not readmitted would move to a stable health state. The baseline admission and mortality rates were assumed to be different for each population. In the post-hospital group, these risks were obtained from a cohort study that followed up patients admitted for an exacerbation. 152 In the stable population, data were extracted from the TORCH148 and Eclipse2 studies, representing average exacerbation and hospitalisations rates in stable COPD cohorts over a 3-year period, and these data were applied over a 30-year time horizon. In reality, these rates may vary over time in both populations.
While a relatively simple EVPI analysis was conducted, which reflected the value of perfect information across all parameters, as opposed to a more complex analysis of perfect information associated with specific parameters, the high values (>£100M) for both populations at £30,000 per QALY gained are indicative of the value of conducting further research to remove all uncertainty from adoption decisions. This suggests that it is worth spending money on research up a maximum of these high values in order to further inform all the model parameters, and eliminate uncertainty. Conducting further expected value of perfect parameter information analysis would help quantify the value of removing uncertainty around particular parameters, but it is expected that continued evaluation of the effect of domiciliary NIV on admissions, mortality and QoL should be a research priority for both populations. Furthermore, the values for EVPI per population analyses reported assumed a COPD prevalence rate of 1.7%171 and that 30.8% and 8.5% of COPD patients would meet the criteria of being end stage and stable, and end stage and post hospital, respectively. 172 As there is a lot of uncertainty around both the true prevalence of COPD and how end-stage patients should be defined, the values for the EVPI per population reported should be interpreted cautiously and considered indicative of the value of perfect information to inform decisions that affect these populations.
The model highlights a number of areas where further research is required. Crucially, further evidence is needed on the effectiveness of domiciliary NIV in reducing admissions and improving QoL. There is a lot of uncertainty around the effectiveness and cost-effectiveness of domiciliary NIV in a stable population, and the intervention may be cost-effective at £30,000 per QALY gained when applying current evidence, but with only a 55% probability of being cost-effective. If NIV is effective in reducing the risk of admissions in a post-hospital population, then it is likely to be below the willingness-to-pay threshold of £20,000. This must be tempered with the fact that the largest study undertaken so far in this population found no benefit of NIV in terms of hospital admissions. More evidence is required on what patient characteristics predict exacerbations, hospitalisation and mortality in COPD populations. It is clear that these events differ by severity of COPD and by recent experience of a hospital admission, although more data are needed on how these two variables interact over time. Finally, while utility values in COPD populations are relatively consistent by GOLD severity, there is a dearth of data available on the impact of exacerbations on QoL.
Summary
-
Currently, there are no published economic models on the cost-effectiveness of domiciliary NIV versus usual care in end-stage COPD either starting on NIV when stable in the community or at discharge when recovering from an exacerbation.
-
This is the first economic model to attempt to estimate the cost-effectiveness of domiciliary NIV in these patient groups.
-
This speculative model indicates that domiciliary NIV may be cost-effective in the stable population at a threshold of £30,000 per QALY gained, but there is considerable uncertainty around this result. Changing parameter estimates around the duration of effect, cost of NIV and target group (e.g. stable patients in GOLD stage 4 or those with a higher risk of repeat exacerbation and mortality) reduces the ICER further to values closer to £20,000 per QALY gained. However, there is a lot of uncertainty around these results.
-
There is a lot of uncertainty around the cost-effectiveness of NIV in the post-hospital population. Two cases based on effectiveness estimates from single RCTs with small sample sizes indicated that domiciliary NIV might be cost-effective in a post-hospital population at a willingness-to-pay threshold of £30,000 per QALY gained. This is consistent with the findings above, that if NIV is more effective in reducing the risk of admission it is more likely to be cost-effective in patients with a higher risk of admission and mortality. However, the opposite conclusion was drawn from a third base case, which used data from a larger RCT that favoured usual care with regard to the effect on hospitalisations. More evidence is thus required to investigate the reason for this disparity in outcome between studies in this population.
-
The model has a number of limitations, the most important relating to the large amount of uncertainty around the effectiveness estimate driving the model results.
-
The analysis highlights the importance of conducting further research on the effect and duration of effect of domiciliary NIV, and on whether or not a population of COPD patients not likely to benefit can be identified, to allow a more robust analysis of cost-effectiveness.
-
It also highlights the importance of estimates of baseline risk of admission and COPD-related mortality when assessing the cost-effectiveness of NIV.
Chapter 8 Overarching discussion and future research recommendations
Overarching discussion
Two COPD populations were considered in this report, stable and post-hospital, in order to account for underlying risk of future exacerbations. Recent hospitalisation (due to exacerbation) was used as a proxy for a higher risk of recurring exacerbation. This division could be seen as artificial, as it does not take into account overall exacerbation history, but was the only possible approach given the information reported in the included studies. In order to be able to gauge the totality of the evidence, clinical effectiveness results for stable and post-hospital populations were presented, where possible, in one forest plot.
For the stable population, there was evidence available to suggest no difference between NIV and usual care in terms of survival (up to 24 months); however, there was a lack of longer-term controlled data on survival. There was a trend (not statistically significant) towards fewer hospital admissions with NIV where studies had reported this outcome, and possibly for improved QoL, although this was not consistent. Not all available evidence on admissions in a stable population could be incorporated into the meta-analysis (and subsequent economic modelling), and it is possible that, based on all evidence, the effect may have been more equivocal. There was less evidence available for the post-hospital population. A benefit from NIV in terms of survival was demonstrated in non-RCTs but not confirmed by RCTs. Hospital admissions findings were inconsistent across three RCTs in this population and there was a lack of QoL data.
In line with these results, the speculative economic model found that NIV may be cost-effective in a stable population at a willingness-to-pay threshold of £30,000 per QALY gained. This is driven by the trend towards fewer hospital admissions but is associated with large uncertainty. The disparity between effect estimates for the post-hospital population is reflected in the cost-effectiveness estimates which range from NIV being dominated by usual care, to ICERs for NIV of near or below £10,000 per QALY gained. This is the first model-based economic evaluation in domiciliary NIV; however, it remains speculative because of the uncertainty around a potential benefit from NIV for a stable population and because of limited and inconsistent evidence for the post-hospital population. The main drivers of the model were clinical effectiveness estimates, duration of benefit (which is currently unknown) and, to a lesser extent, NIV costs. Speculative modelling indicated that a reduction of 24% and 15% (for stable and post-hospital populations respectively) in rate of hospital admissions (per patient per year) would result in NIV being cost-effective at a threshold of £30,000 per QALY gained. Similarly, a 2.5% utility improvement would also hypothetically make NIV cost-effective in a stable population, while a similar change in the post-hospital population does not much affect the current cost-effectiveness estimates. A 2.5% improvement in utility is, however, difficult to translate into a clinically meaningful measure. Given the limited QoL evidence, no changes in utility were included in the base case (other than those caused by avoided hospital admissions), which is contrary to anecdotal patient reports of improvement in daily living with NIV. Assuming there was an improvement in QoL (of daily living), which has not been adequately captured by studies so far, this may increase cost-effectiveness of NIV.
One potential drawback of the model is that it had to consider the two populations separately, while in a real-life setting there is likely to be much more of a continuum of risk. It is possible that the post-hospital population considered represents only a small proportion of COPD patients at the more severe end of the disease spectrum. There was limited information in the included studies on baseline risk in relation to exacerbation history. The model did, however, account (for both populations) for the fact that baseline risk changes, both over time and dependent on exacerbations. Further uncertainty in the model stemmed from the fact that estimates for clinical parameters for the NIV and usual-care arms were in some cases obtained from different sources, and it is unclear how potential differences in populations may affect model outputs.
The remit for this report specified ‘patients with stable end-stage COPD plus chronic HRF, who have required assisted ventilation (whether invasive or non-invasive) during an exacerbation or who are hypercapnic or acidotic on long term oxygen therapy (LTOT).’ Based on the existing clinical effectiveness evidence, no conclusions could be drawn on whether or not a certain type of patient is more likely to benefit from NIV (e.g. in terms of LTOT use or level of hypercapnia), or whether or not a certain type of NIV is more favourable (e.g. higher pressure), and the economic model has not considered any such differences. Most included populations were hypercapnic and in many studies a majority of patients were on LTOT. Performing subgroup analysis based on reported mean baseline CO2 values would have meant dichotomising trials based on an arbitrary threshold and this was not considered appropriate. Exploratory analyses undertaken across both stable and post-hospital populations suggested a trend towards a correlation between changes in CO2 and hospital admissions. Such a potential correlation was not observed for mortality. However, the analysis is using aggregate data for change in CO2 and also for mean difference in hospital admissions, and a causal association therefore cannot be inferred even if there is potential biological plausibility. Further, this was a post-hoc analysis, which is subject to a number of limitations (see Appendix 7). It does suggest that there needs to be further investigation into the association between CO2 and clinical outcomes such as hospital admissions.
The evidence overall indicates that some patients may be more likely to benefit from NIV than others, but it is difficult to relate this to underlying risk of future exacerbations. It therefore remains uncertain at which level of risk it might be beneficial to commence domiciliary NIV, if indeed such a risk could be determined accurately for an individual patient during the course of their disease. The current recommendations in the UK suggest that domiciliary NIV is considered on health-economic grounds if a patient has had three hospital admissions with acute HRF. 40 There may, however, be other, as yet undetermined, patient characteristics which could influence the effectiveness of NIV.
Uncertainty also remains regarding the length of time NIV may provide benefit for. There are at least two RCTs looking at the effect of discontinuing NIV (see Chapter 5, Discontinuation studies), but it was beyond the scope of this report to explore this question.
Research recommendations
Based on the current evidence, it is possible that there are some patients who may derive greater benefit from NIV, but at present it is not possible to define those patients’ characteristics.
A number of currently ongoing studies may go some way to adding to the evidence base, but this may depend to some extent on how the study populations are defined (see Chapter 4, Ongoing studies, for full details). There is at least one ongoing trial (the UK HOT-HMV trial) which includes a population with a higher underlying risk of recurrent events similar to the post-hospital populations in this report. This study had almost finished recruitment, but no results were available to include in this report. Recruitment appears to have been slower than expected in at least some ongoing studies, which may be explained by narrow inclusion criteria.
No further ongoing trials in a stable population were identified, which is perhaps a reflection of the lack of evidence of benefit in this population. Uncertainty remains regarding a potential effect on QoL and long-term effects on survival.
The results from this report will need to be re-examined in the light of any new results from the ongoing trials, particularly in terms of reducing some of the uncertainty in the economic model. As such, recommendations for additional RCTs would be premature.
Should there be a need for additional new RCTs, these could include a sham NIV arm in order to minimise potential bias, as well a higher-pressure and lower-pressure NIV arm, to enable further exploration of the relationship between pressure and effectiveness. However, some argue that sham NIV may lead to an overestimate of the potential benefit of NIV, because of its engendered disbenefits in terms of QoL, in which case a control arm based on usual care only may be more appropriate. A three-arm trial (NIV vs. sham NIV vs. usual care) may be required. In view of the small sample sizes in published and ongoing trials, as well as recruitment issues, broader inclusion criteria could be considered, together with planned subgroup analyses.
Future studies should consider measuring and reporting outcomes in a way that could usefully inform an economic model. So QoL should be measured in way that enables conversion into utilities, exacerbations should be reported with an indication of severity and the relationship between exacerbations, hospitalisations and mortality should be clearly reported. Where continuous data are reported, analysis of covariance should be used for adjusting for baseline imbalances. Alternatively, the proportion of patients achieving a level of clinically important improvement could also be reported.
An appropriately conducted IPD analysis of all study data may help to answer some of the outstanding questions about the type of patient that might benefit most from NIV. A recent Cochrane review110,124 has incorporated IPD analyses but based on a smaller group of studies and without considering hospitalisations or survival. The Cochrane analysis was not aimed at identifying potential effect modifiers. Feasibility of an IPD analysis would depend on the availability of information on potential effect modifiers for all patients and differences in methodological quality between studies. Small patient numbers and potentially insufficiently high event rates in the trials published so far may also preclude analysis of all effect modifiers of potential interest (e.g. age, GOLD severity, level of hypercapnia, use of LTOT, history of exacerbations).
Chapter 9 Conclusions
Overall, the evidence from RCTs could not demonstrate a benefit from NIV compared with usual care in either stable or post-hospital populations, although there was a trend towards fewer hospital admissions and, to a lesser extent, improved QoL for the stable population. A benefit in terms of survival for the post-hospital population was shown in non-randomised controlled studies only and the findings for hospital admissions (from RCTs) were inconsistent. There was also too little evidence to draw any conclusions on the potential benefits of higher-pressure NIV settings. A speculative economic model found that NIV may be cost-effective in a stable population at a threshold of £30,000 per QALY gained, but this is associated with a large amount of uncertainty. It is not possible to draw any overall conclusions regarding cost-effectiveness in a post-hospital population, as the results based on three different base cases are too disparate and also based on limited evidence. It is likely that the broad categorisation into stable and post-hospital patients has not been able to capture more subtle differences between patients, who may derive more or less benefit from NIV. Further evidence, potentially from currently ongoing trials but more likely from IPD analyses, is required to determine whether or not there are any patient characteristics (such as baseline hypercapnia level) or equipment settings that are predictive of a benefit of NIV and to establish optimum time points for starting (and potentially discontinuing) NIV.
Acknowledgements
The authors thank the following:
-
Simon Stevens for his invaluable administrative support and excellent organisational skills
-
all members of the patient group for supporting and contributing to this project: Maireade Bird, Michael Darby, Don Etheridge, Chris Huckle, Jan Turner and Anne Yeomans
-
Pelham Barton for economic modelling guidance
-
Chris Cates, Peymane Adab, Brendan Cooper and Rob Stockley for contributions to wider team meetings
-
all the people who kindly gave their time to help translate articles.
Contributions of authors
Janine Dretzke was the lead systematic reviewer, wrote and edited sections of the report and undertook study selection, data extraction and analysis and quality assessment for the clinical effectiveness review.
Deirdre Blissett undertook the systematic review of cost-effectiveness, contributed to the development of the economic model, undertook cost-effectiveness analyses and wrote sections of the report.
Chirag Dave advised on clinical aspects of the project, contributed to many parts of the project and undertook study selection.
Rahul Mukherjee advised on clinical aspects of the project, undertook study selection and provided input into the costs aspect of the economic evaluation.
Malcolm Price advised on statistical aspects, analysed data and edited statistical methodological sections of the report.
Sue Bayliss devised the search strategies and ran the searches in electronic databases.
Xiaoying Wu translated Chinese papers, undertook data extraction and data checking.
Rachel Jordan undertook study selection and contributed to methodological aspects of the project.
Sue Jowett led the economic section of this report and contributed to all parts of the economic review and development of the economic model and associated analysis.
Alice M Turner was co-principal investigator and clinical lead, oversaw all clinical aspects of the project, undertook study selection and wrote and commented on sections of the report.
David Moore was co-principal investigator and methodological lead, led all aspects of the project, contributed to all aspects of the project, undertook study selection, and wrote and edited sections of the report.
All authors contributed to patient and public involvement and team meetings and read and approved a draft of the report.
Data sharing statement
All available data is either available in the public domain or can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. http://dx.doi.org/10.1164/rccm.201204-0596PP.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. http://dx.doi.org/10.1056/NEJMoa0909883.
- Britton M. The burden of COPD in the U.K.: results from the Confronting COPD survey. Respir Med 2003;97:S71-9. http://dx.doi.org/10.1016/S0954-6111(03)80027-6.
- CG101 Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care (Partial Update). London: NICE; 2010.
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest 2007;131:S4-42. http://dx.doi.org/10.1378/chest.06-2418.
- Miller MR, Quanjer PH, Swanney MP, Ruppel G, Enright PL. Interpreting lung function data using 80% predicted and fixed thresholds misclassifies more than 20% of patients. Chest 2011;139:52-9. http://dx.doi.org/10.1378/chest.10-0189.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes O, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease 1. N Engl J Med 2004;350:1005-12. http://dx.doi.org/10.1056/NEJMoa021322.
- Trueman J, Trueman I. COPD: criteria to assist in the identification of the palliative phase. Br J Nurs 2011;20:635-9. http://dx.doi.org/10.12968/bjon.2011.20.10.635.
- Jenkins CR, Celli B, Anderson JA, Ferguson GT, Jones PW, Vestbo J, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J 2012;39:38-45. http://dx.doi.org/10.1183/09031936.00194610.
- Ling SH, van Eeden SF. Particulate matter air pollution exposure: role in the development and exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2009;4:233-43. http://dx.doi.org/10.2147/COPD.S5098.
- Seemungal T, Donaldson G, Paul E, Bestall J, Jeffries D, Wedzicha JADW. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418-22. http://dx.doi.org/10.1164/ajrccm.157.5.9709032.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543-54. http://dx.doi.org/10.1056/NEJMoa0805800.
- Aaron SD, Donaldson GWJ, Hurst G, Ramsay T, Wedzicha J. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:1608-13. http://dx.doi.org/10.1164/ajrccm.161.5.9908022.
- Jones P, Higenbottam T. Quantifying of severity of exacerbations in chronic obstructive pulmonary disease: adaptations to the definition to allow quantification. Proc Am Thorac Soc 2007;4:597-601. http://dx.doi.org/10.1513/pats.200707-115TH.
- Roberts CM, Lowe D, Bucknall CE, Ryland I, Kelly Y, Pearson MG. Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax 2002;57:137-41. http://dx.doi.org/10.1136/thorax.57.2.137.
- Connors AF, Dawson NV, Thomas C, Harrell FE, Desbiens N, Fulkerson WJ, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959-67. http://dx.doi.org/10.1164/ajrccm.154.4.8887592.
- Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. http://dx.doi.org/10.1136/thorax.57.10.847.
- Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, et al. Health-related quality of life in patients by COPD severity within primary care in Europe. Respir Med 2011;105:57-66. http://dx.doi.org/10.1016/j.rmed.2010.09.004.
- Solem CT, Sun SX, Sudharshan L, Macahilig C, Katyal M, Gao X. Exacerbation-related impairment of quality of life and work productivity in severe and very severe chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2013;8:641-52. http://dx.doi.org/10.2147/COPD.S51245.
- Reardon JZ, Lareau SC, Zuwallack R. Functional status and quality of life in chronic obstructive pulmonary disease. Am J Med 2006;119:S32-7. http://dx.doi.org/10.1016/j.amjmed.2006.08.005.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648-54. http://dx.doi.org/10.1183/09031936.00102509.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321-7. http://dx.doi.org/10.1164/ajrccm/145.6.1321.
- Desikan R, Mason HL, Rupp MT, Skehan M. Health-related quality of life and healthcare resource utilization by COPD patients: a comparison of three instruments. Qual Life Res 2002;11:739-51. http://dx.doi.org/10.1023/A:1020836719321.
- Struik FM, Kerstjens HA, Bladder G, Sprooten R, Zijnen M, Asin J, et al. The Severe Respiratory Insufficiency Questionnaire scored best in the assessment of health-related quality of life in chronic obstructive pulmonary disease. J Clin Epidemiol 2013;66:1166-74. http://dx.doi.org/10.1016/j.jclinepi.2013.04.013.
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;18. http://dx.doi.org/10.1002/14651858.cd003793.pub2.
- Jordan RE, Majothi S, Heneghan NR, Blissett DB, Riley RD, Sitch AJ, et al. Supported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysis. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta19360.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89. http://dx.doi.org/10.1056/NEJMoa063070.
- National Collaborating Centre for Chronic Conditions . Chronic obstructive pulmonary disease: national clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59:S1-232.
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema . Report of the Medical Research Council Working Party. Lancet 1981;1:681-6.
- Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest 2010;138:179-87. http://dx.doi.org/10.1378/chest.09-2555.
- O’Neill B, Bradley JM, Heaney L, O’Neill C, MacMahon J. Short burst oxygen therapy in chronic obstructive pulmonary disease: a patient survey and cost analysis. Int J Clin Pract 2005;59:751-3. http://dx.doi.org/10.1111/j.1368-5031.2005.00574.x.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010;65:303-8. http://dx.doi.org/10.1136/thx.2009.124263.
- Windisch W, Kostic S, Dreher M, Virchow JC, Sorichter S. Outcome of patients with stable COPD receiving controlled noninvasive positive pressure ventilation aimed at a maximal reduction of Pa(CO2). Chest 2005;128:657-62. http://dx.doi.org/10.1378/chest.128.2.657.
- Strumpf DA, Millman RP, Carlisle CC, Grattan LM, Ryan SM, Erickson AD, et al. Nocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1991;144:1234-9. http://dx.doi.org/10.1164/ajrccm/144.6.1234.
- Lin C-C. Comparison between nocturnal nasal positive pressure ventilation combined with oxygen therapy and oxygen monotherapy in patients with severe COPD. Am J Respir Crit Care Med 1996;154:353-8. http://dx.doi.org/10.1164/ajrccm.154.2.8756806.
- Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004;3. http://dx.doi.org/10.1002/14651858.cd004104.pub3.
- Ozsancak A, D’Ambrosio C, Hill NS. Nocturnal noninvasive ventilation. Chest 2008;133:1275-86. http://dx.doi.org/10.1378/chest.07-1527.
- Chu CM, Chan VL, Lin AW, Wong IW, Leung WS, Lai CK. Readmission rates and life threatening events in COPD survivors treated with non-invasive ventilation for acute hypercapnic respiratory failure. Thorax 2004;59:1020-5. http://dx.doi.org/10.1136/thx.2004.024307.
- Department of Health . Consultation on a Strategy for Services for Chronic Obstructive Pulmonary Disease (COPD) in England. n.d. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/consultations/liveconsultations/dh_112977 (accessed 1 January 2014).
- Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax 2003;58:867-71. http://dx.doi.org/10.1136/thorax.58.10.867.
- Dave C, Turner A, Dretzke J, Bayliss S, O’Brien D, Jowett S, et al. Protocol for a systematic review and economic evaluation of the clinical and cost-effectiveness of non-hospital-based non-invasive ventilation (NIV) in patients with stable end-stage COPD with hypercapnic respiratory failure. Syst Rev 2014;3. http://dx.doi.org/10.1186/2046-4053-3-32.
- Shea BJ, Bouter LM, Peterson J, Boers M, Andersson N, Ortiz Z, et al. External validation of A Measurement Tool to Assess Systematic Reviews (AMSTAR). PLOS ONE 2007;2. http://dx.doi.org/10.1371/journal.pone.0001350.
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions 2011. http://handbook.cochrane.org/ (accessed 18 March 2014).
- Higgins JPT, Deeks JJ, Altman DG, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2011.
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2011.
- The GRADE working group . Grading of Recommendations Assessment, Development and Evaluation (GRADE) n.d. www.gradeworkinggroup.org/ (accessed 23 October 2012).
- Ali S. Domiciliary non-invasive ventilation and the quality of life outcome of patients suffering from chronic respiratory failure. Ir Med J 2007;100:336-8.
- Cano M, Martin C, Zamora E, Pinedo C, Segrelles G, Rajas O, et al. Non-invasive home ventilation: Indications are changing. Am J Respir Crit Care Med 2010.
- Cheng SL, Chan VL, Chu CM. Compliance with home non-invasive ventilation. Respirology 2012;17:735-6. http://dx.doi.org/10.1111/j.1440-1843.2012.02169.x.
- Fernandez R, Cabrera C, Rubinos G, Pando A, Galindo R, Rodriguez F, et al. Nasal versus oronasal mask in home mechanical ventilation: The preference of patients as a strategy for choosing the interface. Respir Care 2012;57:1413-17. http://dx.doi.org/10.4187/respcare.01500.
- Hein H, Schucher B, Kirsten D, Magnussen H. Prospective study of the quality of life in intermittent self-ventilation. Med Klin (Munich) 1997;92:S93-4.
- Janssens J-P, Kehrer P, Chevrolet J-C, Rochat T. Non-invasive home ventilation (NIHV): Analysis of 32 cases with an average 41-month follow-up. Rev Mal Respir 1999;16:511-20.
- Heindl S, Bullemer F, Karg O, Kroworsch B. Physical performance of patients with non-invasive ventilation (Nocturnal Mechanical Ventilation) (NMV). Effects of ventilatory therapy – effects of physical training. Pneumologie 1999;53:S107-8.
- Lewis KE, Bailes J, Hughes R, Morris J, Jones B. Improvements in Epworth Sleepiness Scores after using bilevel non-invasive ventilation for stable hypercapnic COPD. Am J Respir Crit Care Med 2011. http://dx.doi.org/10.1164/ajrccm-conference.2011.183.1_meetingabstracts.a4580.
- Naeck R, Bounoiare D, Portmann A, Freitas U, Portier F, Muir JF, et al. Modifications of sleep quality and cardiac variability during initiation of domiciliary noninvasive ventilation in patients with chronic respiratory failure. Fundam Clin Pharmacol 2012.
- Muller-Pawlowski H, Raffenberg M, Schaberg T, Petri M, Lode H. Nasal intermittent positive pressure ventilation (nIPP) with a respirator designed according to the third version of the German regulations governing the use of medical apparatus and equipment (= MedGV III). Pneumologie 1996;50:518-22.
- Raffenberg M, Geerdes-Fenge H, Muller-Pawlowski H, Petri M, Schaberg T, Loddenkemper R, et al. Invasive and non-invasive home ventilation – changes between 1982 and 1996. Med Klin (Munich) 1999;94:18-21.
- Schonhofer B, Zimmermann C, Abramek P, Suchi S, Kohler D, Polkey MI. Non-invasive mechanical ventilation improves walking distance but not quadriceps strength in chronic respiratory failure. Respir Med 2003;97:818-24. http://dx.doi.org/10.1016/S0954-6111(03)00037-4.
- Schonhofer B, Geibel M, Jones P, Kohler D. Daily activity improves following nocturnal mechanical ventilation due to chronic respiratory failure. Med Klin (Munich) 1997;92:26-30.
- Bach JR, Rajaraman R, Ballanger F, Tzeng AC, Ishikawa Y, Kulessa R, et al. Neuromuscular ventilatory insufficiency: effect of home mechanical ventilator use v oxygen therapy on pneumonia and hospitalization rates. Am J Phys Med Rehabil 1998;77:8-19. http://dx.doi.org/10.1097/00002060-199801000-00003.
- Chailleux E, Fauroux B, Binet F, Dautzenberg B, Polu JM. Predictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation – a 10-year analysis of ANTADIR Observatory. Chest 1996;109:741-9. http://dx.doi.org/10.1378/chest.109.3.741.
- Chailleux E, Binet F, Ludot A, Polu J-M. Home treatment for chronic respiratory failure, the ANTADIR experience. Rev Med Suisse 1995;53:825-32.
- Chang AY, Marsh S, Smith N, Neill A. Long-term community non-invasive ventilation. Intern Med J 2010;40:764-71. http://dx.doi.org/10.1111/j.1445-5994.2010.02171.x.
- Iwanaga T, Takata S, Higashi K, Kitahara Y, Fukushima K, Kawakami K, et al. A clinical survey on non-invasive positive pressure ventilation in patients with stable chronic respiratory failure covering the past two years; National Hospital Network of respiratory diseases in Kyushu area. IRYO 2004;58:425-30.
- Schonhofer B, Von SK, Bucher T, Nietsch M, Suchi S, Kohler D, et al. Sexuality in patients with noninvasive mechanical ventilation due to chronic respiratory failure. Am J Respir Crit Care Med 2001;164:1612-17. http://dx.doi.org/10.1164/ajrccm.164.9.2103020.
- Tsara V, Serasli E, Voutsas V, Lazarides V, Christaki P. Burden and coping strategies in families of patients under noninvasive home mechanical ventilation. Respiration 2006;73:61-7. http://dx.doi.org/10.1159/000087460.
- Windisch W, Dreher M, Storre JH, Sorichter S. Nocturnal non-invasive positive pressure ventilation: physiological effects on spontaneous breathing. Respir Physiol Neurobiol 2006;150:251-60. http://dx.doi.org/10.1016/j.resp.2005.05.017.
- Kamei M. Effectiveness of noninvasive positive-pressure ventilation for patients with chronic stable hypercapnic respiratory failure. Nihon Kokyuki Gakkai Zasshi 1999;37:886-92.
- Chiang L-L, Liu C-Y, Ho S-C, Sheng T-F, Yu C-T, Lin H-C, et al. Efficacy of nocturnal nasal positive pressure ventilation in hypercapnic patients with severe obstructive lung diseases. Chang Gung Med J 2004;27:98-106.
- Windisch W, Storre JH, Sorichter S, Virchow JC. Comparison of volume- and pressure-limited NPPV at night: a prospective randomized cross-over trial. Respir Med 2005;99:52-9. http://dx.doi.org/10.1016/j.rmed.2004.05.009.
- Funk GC, Breyer MK, Burghuber OC, Kink E, Kirchheiner K, Kohansal R, et al. Long-term non-invasive ventilation in COPD after acute-on-chronic respiratory failure. Respir Med 2011;105:427-34. http://dx.doi.org/10.1016/j.rmed.2010.09.005.
- Oscroft NS, Quinnell TG, Shneerson JM, Smith IE. The effects of withdrawing long-term nocturnal non-invasive ventilation in COPD patients. COPD 2010;7:111-16. http://dx.doi.org/10.3109/15412551003631725.
- Kohnlein T, Criee CP, Kohler D, Welte T, Laier-Groeneveld G. Multicenter study on ‘non-invasive ventilation in patients with severe chronic obstructive pulmonary disease and emphysema (COPD)’. Pneumologie 2004;58:566-9.
- McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009;64:561-6. http://dx.doi.org/10.1136/thx.2008.108274.
- Struik FM, Sprooten RT, Kerstjens HA, Bladder G, Zijnen M, Asin J, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax 2014;69:826-34. http://dx.doi.org/10.1136/thoraxjnl-2014-205126.
- Köhnlein T, Windisch W, Kohler D, Drabik A, Geiseler J, Hartl S, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014;2:698-705. http://dx.doi.org/10.1016/S2213-2600(14)70153-5.
- Meecham-Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med 1995;152:538-44. http://dx.doi.org/10.1164/ajrccm.152.2.7633704.
- Murphy PB, Moxham J, Polkey MI, Hart N. UK HOT-HMV trial: Acceptability and tolerability of high pressure domiciliary non-invasive ventilation (NIV) in COPD. Thorax 2011.
- Duiverman ML, Wempe JB, Bladder G, Jansen DF, Kerstjens HA, Zijlstra JG, et al. Nocturnal non-invasive ventilation in addition to rehabilitation in hypercapnic patients with COPD. Thorax 2008;63:1052-7. http://dx.doi.org/10.1136/thx.2008.099044.
- Duiverman ML, Wempe JB, Bladder G, Vonk JM, Zijlstra JG, Kerstjens HA, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respir Res 2011;12. http://dx.doi.org/10.1186/1465-9921-12-112.
- Zhou X, Yang J, Shen C. Effect of non-invasive positive pressure ventilation and long-term oxygen therapy in patients with stable COPD. Clinical Medical Journal of China 2008;15:486-8.
- Sin DD, Wong E, Mayers I, Lien DC, Feeny D, Cheung H, et al. Effects of nocturnal noninvasive mechanical ventilation on heart rate variability of patients with advanced COPD. Chest 2007;131:156-63. http://dx.doi.org/10.1378/chest.06-1423.
- Bhatt SP, Peterson MW, Wilson JS, Durairaj L. Noninvasive positive pressure ventilation in subjects with stable COPD: a randomized trial. Int J Chron Obstruct Pulmon Dis 2013;8:581-9. http://dx.doi.org/10.2147/COPD.S53619.
- Garrod R, Mikelsons C, Paul EA, Wedzicha JA. Randomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:1335-41. http://dx.doi.org/10.1164/ajrccm.162.4.9912029.
- Casanova C, Celli BR, Tost L, Soriano E, Abreu J, Velasco V, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000;118:1582-90. http://dx.doi.org/10.1378/chest.118.6.1582.
- Milane J, Jonquet O. Intermittent positive pressure breathing in the treatment of respiratory insufficiency by chronic lung disease. Agressologie 1985;26:651-5.
- Pahnke J, Bullemer F, Heindl S, Karg O. Patient-related rejection of nasal IPPV therapy. Patients, reasons, follow-up. Med Klin (Munich) 1997;92:S73-4.
- Laier-Groeneveld G, Criee CP. Long-term effects and life expectancy after six years intermittent self ventilation. Med Klin (Munich) 1995;90:S62-3.
- Paone G, Conti V, Biondi-Zoccai G, De FE, Chimenti I, Peruzzi M, et al. Long-term home noninvasive mechanical ventilation increases systemic inflammatory response in chronic obstructive pulmonary disease: a prospective observational study. Mediators Inflamm 2014;2014. http://dx.doi.org/10.1155/2014/503145.
- Cheung AP, Chan VL, Liong JT, Lam JY, Leung WS, Lin A, et al. A pilot trial of non-invasive home ventilation after acidotic respiratory failure in chronic obstructive pulmonary disease. Int J Tuberc Lung Dis 2010;14:642-9.
- De Backer L, Vos W, Dieriks B, Daems D, Verhulst S, Vinchurkar S, et al. The effects of long-term noninvasive ventilation in hypercapnic COPD patients: a randomized controlled pilot study. Int J Chron Obstruct Pulmon Dis 2011;6:615-24. http://dx.doi.org/10.2147/COPD.S22823.
- Xiang PC, Zhang X, Yang JN, Zhang EM, Guo WA, Ju LX, et al. The efficacy and safety of long term home noninvasive positive pressure ventilation in patients with stable severe chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 2007;30:746-50.
- Clini E, Sturani C, Porta R, Scarduelli C, Galavotti V, Vitacca M, et al. Outcome of COPD patients performing nocturnal non-invasive mechanical ventilation. Respir Med 1998;92:1215-22. http://dx.doi.org/10.1016/S0954-6111(98)90424-3.
- Clini E, Vitacca M, Foglio K, Simoni P, Ambrosino N. Long-term home care programmes may reduce hospital admissions in COPD with chronic hypercapnia. Eur Respir J 1996;9:1605-10. http://dx.doi.org/10.1183/09031936.96.09081605.
- Tsolaki V, Pastaka C, Karetsi E, Zygoulis P, Koutsokera A, Gourgoulianis KI, et al. One-year non-invasive ventilation in chronic hypercapnic COPD: effect on quality of life. Respir Med 2008;102:904-11. http://dx.doi.org/10.1016/j.rmed.2008.01.003.
- Budweiser S, Hitzl AP, Jorres RA, Heinemann F, Arzt M, Schroll S, et al. Impact of noninvasive home ventilation on long-term survival in chronic hypercapnic COPD: a prospective observational study. Int J Clin Pract 2007;61:1516-22. http://dx.doi.org/10.1111/j.1742-1241.2007.01427.x.
- Heinemann F, Budweiser S, Jorres RA, Arzt M, Rosch F, Kollert F, et al. The role of non-invasive home mechanical ventilation in patients with chronic obstructive pulmonary disease requiring prolonged weaning. Respirology 2011;16:1273-80. http://dx.doi.org/10.1111/j.1440-1843.2011.02054.x.
- Lu P, Wu XM, Li ZG, Yang CC. Clinical observation of home noninvasive positive pressure ventilation in hypercapnic patient with stable severe chronic obstructive pulmonary disease. Chin Med J (Engl) 2012;92:401-4.
- Clini E, Sturani C, Rossi A, Viaggi S, Corrado A, Donner CF, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002;20:529-38. http://dx.doi.org/10.1183/09031936.02.02162001.
- Gay PC, Hubmayr RD, Stroetz RW. Efficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a 3-month controlled trial. Mayo Clin Proc 1996;71:533-42. http://dx.doi.org/10.4065/71.6.533.
- Kaminski D, Sliwinski P, Bieleń P, Zieliński J. Noninvasive positive pressure ventilation in COPD patients with hypercapnic respiratory failure. Pneumonol Alergol Pol 1999;67:45-52.
- Budweiser S, Jorres RA, Riedl T, Heinemann F, Hitzl AP, Windisch W, et al. Predictors of survival in COPD patients with chronic hypercapnic respiratory failure receiving noninvasive home ventilation. Chest 2007;131:1650-8. http://dx.doi.org/10.1378/chest.06-2124.
- Budweiser S, Hitzl AP, Joerres RA, Schmidbauer K, Heinemann F, Pfeifer M. Health-related quality of life and long-term prognosis in chronic hypercapnic respiratory failure: a prospective survival analysis. Respir Res 2007;8. http://dx.doi.org/10.1186/1465-9921-8-92.
- Kollert F, Tippelt A, Muller C, Jorres RA, Porzelius C, Pfeifer M, et al. Hemoglobin levels above anemia thresholds are maximally predictive for long-term survival in COPD with chronic respiratory failure. Respir Care 2013;58:1204-12. http://dx.doi.org/10.4187/respcare.01961.
- Oscroft NS, Quinnell TG, Shneerson JM, Smith IE. Long-term non-invasive ventilation to manage persistent ventilatory failure after COPD exacerbation. Respirology 2010;15:818-22. http://dx.doi.org/10.1111/j.1440-1843.2010.01787.x.
- Jones SE, Packham S, Hebden M, Smith AP. Domiciliary nocturnal intermittent positive pressure ventilation in patients with respiratory failure due to severe COPD: long-term follow up and effect on survival. Thorax 1998;53:495-8. http://dx.doi.org/10.1136/thx.53.6.495.
- Tsolaki V, Pastaka C, Kostikas K, Karetsi E, Dimoulis A, Zikiri A, et al. Noninvasive ventilation in chronic respiratory failure: effects on quality of life. Respiration 2011;81:402-10. http://dx.doi.org/10.1159/000317138.
- Skobel E, Norra C, Randerath W, Jendralski A. Influence of pulmonary rehabilitation on quality of life and anxiety in patients with severe COPD and long-term ventilation. Atemweg Lungenkrank 2011;37:257-66. http://dx.doi.org/10.5414/ATP37257.
- Windisch W, Haenel M, Storre JH, Dreher M. High-intensity non-invasive positive pressure ventilation for stable hypercapnic COPD. Int J Med Sci 2009;6:72-6. http://dx.doi.org/10.7150/ijms.6.72.
- Struik FM, Lacasse Y, Goldstein R, Kerstjens HM, Wijkstra PJ. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013;6. http://dx.doi.org/10.1002/14651858.cd002878.pub2.
- Ambrosino N, Clini E. Noninvasive ventilation in COPD patients with chronic respiratory failure–pro. Monaldi Arch Chest Dis 2000;55:54-7.
- Oscroft NS, Ali M, Gulati A, Davies MG, Quinnell TG, Shneerson JM, et al. A randomised crossover trial comparing volume assured and pressure preset noninvasive ventilation in stable hypercapnic COPD. COPD 2010;7:398-403. http://dx.doi.org/10.3109/15412555.2010.528084.
- Murphy PB, Brignall K, Moxham J, Polkey MI, Davidson AC, Hart N. High pressure versus high intensity noninvasive ventilation in stable hypercapnic chronic obstructive pulmonary disease: a randomized crossover trial. Int J Chron Obstruct Pulmon Dis 2012;7:811-18. http://dx.doi.org/10.2147/COPD.S36151.
- Chen H, Liang BM, Xu ZB, Tang YJ, Wang K, Xiao J, et al. Long-term non-invasive positive pressure ventilation in severe stable chronic obstructive pulmonary disease: a meta-analysis. Chin Med J (Engl) 2011;124:4063-70.
- Kolodziej MA, Jensen L, Rowe B, Sin D. Systematic review of noninvasive positive pressure ventilation in severe stable COPD. Eur Respir J 2007;30:293-306. http://dx.doi.org/10.1183/09031936.00145106.
- Mckim DA, Road J, Avendano M, Abdool S, Cote F, Duguid N, et al. Home mechanical ventilation: A Canadian Thoracic Society clinical practice guideline. Can Respir J 2011;18:197-215.
- Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 2003;290:2301-12. http://dx.doi.org/10.1001/jama.290.17.2301.
- COPD Working Group . Noninvasive Positive Pressure Ventilation for Chronic Respiratory Failure Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis. Ont Health Technol Assess Ser 2012;12:1-51.
- Shi JX, Xu J, Sun WK, Su X, Zhang Y, Shi Y. Effect of noninvasive, positive pressure ventilation on patients with severe, stable chronic obstructive pulmonary disease: a meta-analysis. Chin Med J (Engl) 2013;126:140-6. http://dx.doi.org/10.3901/JME.2013.18.140.
- Gupta D, Agarwal R, Aggarwal AN, Maturu VN, Dhooria S, Prasad KT, et al. Guidelines for diagnosis and management of chronic obstructive pulmonary disease: joint recommendations of Indian Chest Society and National College of Chest Physicians (India). Indian J Chest Dis Allied Sci 2014;56:5-54.
- Wijkstra PJ. Non-invasive positive pressure ventilation (NIPPV) in stable patients with chronic obstructive pulmonary disease (COPD). Respir Med 2003;97:1086-93. http://dx.doi.org/10.1016/S0954-6111(03)00163-X.
- Wijkstra PJ, Lacasse Y, Guyatt GH, Casanova C, Gay PC, Meecham JJ, et al. A meta-analysis of nocturnal noninvasive positive pressure ventilation in patients with stable COPD. Chest 2003;124:337-43. http://dx.doi.org/10.1378/chest.124.1.337.
- Wijkstra PJ, Lacasse Y, Guyatt GH, Goldstein RS. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002;3. http://dx.doi.org/10.1002/14651858.cd002878.
- Struik FM, Lacasse Y, Goldstein RS, Kerstjens HA, Wijkstra PJ, Struik FM, et al. Nocturnal noninvasive positive pressure ventilation in stable COPD: a systematic review and individual patient data meta-analysis. Respir Med 2014;108:329-37. http://dx.doi.org/10.1016/j.rmed.2013.10.007.
- Kim V, Criner G. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:228-37. http://dx.doi.org/10.1164/rccm.201210-1843CI.
- National Emphysema Treatment Trial Research Group . A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. http://dx.doi.org/10.1056/NEJMoa030287.
- Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med 2012;186:975-81. http://dx.doi.org/10.1164/rccm.201207-1299OC.
- Owens RL, Malhotra A. Sleep-disordered breathing and COPD: the overlap syndrome. Respir Care 2010;55:1333-46.
- An International Comparison of COPD Care in Europe: Results of the First European COPD Audit. Lausanne: European Respiratory Society; 2012.
- Dave C, Turner A, Thomas A, Beauchamp B, Chakraborty B, Ali A, et al. The utility of respiratory ward based NIV in acidotic hypercapnic respiratory failure. Respirology 2014;19:1241-7. http://dx.doi.org/10.1111/resp.12366.
- Riley R, Kauser I, Bland M, Thijs L, Staessen JA, Wang J, et al. Meta-analysis of randomised trials with a continuous outcome according to baseline imbalance and availability of individual participant data. Stat Med 2013;32:2747-66. http://dx.doi.org/10.1002/sim.5726.
- Drummond MF, Sculpher M, Torrance GW, O’Brien B, Stoddart DL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. PharmacoEconomics 2006;24:355-71. http://dx.doi.org/10.2165/00019053-200624040-00006.
- Clini EM, Magni G, Crisafulli E, Viaggi S, Ambrosino N. Home non-invasive mechanical ventilation and long-term oxygen therapy in stable hypercapnic chronic obstructive pulmonary disease patients: comparison of costs. Respiration 2009;77:44-50. http://dx.doi.org/10.1159/000127410.
- Plant PK, Owen JL, Parrott S, Elliott MW. Cost effectiveness of ward based non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7396.956.
- Criner GJ, Kreimer DT, Tomaselli M, Pierson W, Evans D. Financial implications of noninvasive positive pressure ventilation (NPPV). Chest 1995;108:475-81. http://dx.doi.org/10.1378/chest.108.2.475.
- Chandra K, Blackhouse G, McCurdy B, Bornstein M, Campbell K, Costa V, et al. Cost-effectiveness of interventions for Chronic Obstructive Pulmonary Disease (COPD) using an ontario policy model. Ont Health Technol Assess Ser 2012;12:1-61.
- Borg S, Ericsson A, Wedzicha J, Gulsvik A, Lundback B, Donaldson GC, et al. A computer simulation model of the natural history and economic impact of chronic obstructive pulmonary disease. Value Health 2004;7:153-67. http://dx.doi.org/10.1111/j.1524-4733.2004.72318.x.
- Rutten-van Molken MP, Oostenbrink JB, Miravitlles M, Monz BU. Modelling the 5-year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. Eur J Health Econ 2007;8:123-35. http://dx.doi.org/10.1007/s10198-007-0039-4.
- Hertel N, Kotchie RW, Samyshkin Y, Radford M, Humphreys S, Jameson K. Cost-effectiveness of available treatment options for patients suffering from severe COPD in the UK: a fully incremental analysis. Int J Chron Obstruct Pulmon Dis 2012;7:183-99. http://dx.doi.org/10.2147/COPD.S29820.
- Atsou K, Chouaid C, Hejblum G. Simulation-based estimates of effectiveness and cost-effectiveness of smoking cessation in patients with chronic obstructive pulmonary disease. PLoS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0024870.
- Oba Y. Cost-effectiveness of long-term oxygen therapy for chronic obstructive disease. Am J Manag Care 2009;15:97-104.
- Earnshaw SR, Wilson MR, Dalal AA, Chambers MG, Jhingran P, Stanford R, et al. Cost-effectiveness of fluticasone propionate/salmeterol (500/50 microg) in the treatment of COPD. Respir Med 2009;103:12-21. http://dx.doi.org/10.1016/j.rmed.2008.10.005.
- Chuck A, Jacobs P, Mayers I, Marciniuk D. Cost-effectiveness of combination therapy for chronic obstructive pulmonary disease. Can Respir J 2008;15:437-43.
- Oostenbrink JB, Rutten-van Molken MP, Monz BU, FitzGerald JM. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health 2005;8:32-46. http://dx.doi.org/10.1111/j.1524-4733.2005.03086.x.
- Liu SX, Lee MC, Atakhorrami M, Tatousek J, McCormack M, Yung R, et al. Economic assessment of home-based COPD management programs. COPD 2013;10:640-9. http://dx.doi.org/10.3109/15412555.2013.813447.
- Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. PharmacoEconomics 2005;23:619-37. http://dx.doi.org/10.2165/00019053-200523060-00008.
- Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009;10. http://dx.doi.org/10.1186/1465-9921-10-59.
- Adab P, Fitzmaurice D. COPD in Primary Care: From Case Finding to Improving Patient Outcomes: NIHR Programme Grant 2011–16. Birmingham: Birmingham Lung Improvement Studies; 2014.
- Almagro P, Cabrera FJ, Diez J, Boixeda R, Alonso Ortiz MB, Murio C, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: The epoc en servicios de medicina interna (esmi) study. Chest 2012;142:1126-33. http://dx.doi.org/10.1378/chest.11-2413.
- Ozyilmaz E, Kokturk N, Teksut G, Tatlicioglu T. Unsuspected risk factors of frequent exacerbations requiring hospital admission in chronic obstructive pulmonary disease. Int J Clin Pract 2013;67:691-7. http://dx.doi.org/10.1111/ijcp.12150.
- Garcia-Aymerich J, Farrero E, Felez MA, Izquierdo J, Marrades RM, Anto JM, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003;58:100-5. http://dx.doi.org/10.1136/thorax.58.2.100.
- Bucknall CE, Miller G, Lloyd SM, Cleland J, McCluskey S, Cotton M, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e1060.
- EuroQol Group . EQ-5D-5L Value Sets n.d. www.euroqol.org/about-eq-5d/valuation-of-eq-5d/eq-5d-5l-value-sets.html (accessed 1 February 2014).
- Perera WR, Hurst JR, Wilkinson TM, Sapsford RJ, Mullerova H, Donaldson GC, et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J 2007;29:527-34. http://dx.doi.org/10.1183/09031936.00092506.
- NHS 2010–11 Reference Costs. London: DH; 2011.
- Unit Cost of Health & Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- NCL Care Pathway Review – Proposed NCL Community COPD Contracting Model. London: NHS Commissioning for London; 2010.
- Department of Health . The Flu Immunisation Programme 2013 14 n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/207008/130613_Flu_Letter_v_29_Gateway_GW_signed.pdf (accessed 1 February 2014).
- The Seasonal Influenza Immunisation Programme. Consultation: A Review of the Procurement of Seasonal Flu Vaccine. London: Department of Health; 2013.
- Santos-Sancho JM, Jimenez-Trujillo I, Hernandez-Barrera V, Lopez-de AA, Carrasco-Garrido P, Ortega-Molina P, et al. Influenza vaccination coverage and uptake predictors among Spanish adults suffering COPD. Hum Vaccin Immunother 2012;8:938-45. http://dx.doi.org/10.4161/hv.20204.
- Kontopantelis E, Doran T, Gravelle H, Goudie R, Siciliani L, Sutton M. Family Doctor Responses to Changes in Incentives for Influenza Immunization under the U.K. Quality and Outcomes Framework Pay-for-Performance Scheme. Health Serv Res 2012;47:1117-36. http://dx.doi.org/10.1111/j.1475-6773.2011.01362.x.
- Chronic Obstructive Pulmonary Disease: Costing report – Implementing NICE Guidance. NICE Clinical Guideline 101. NICE; 2011.
- Electronic Drug Tariff. London: Department of Health; 2014.
- Bakerly ND, Davies C, Dyer M, Dhillon P. Cost analysis of an integrated care model in the management of acute exacerbations of chronic obstructive pulmonary disease. Chron Respir Dis 2009;6:201-8. http://dx.doi.org/10.1177/1479972309104279.
- Motor Neurone Disease: The Use of Non-Invasive Ventilation in the Management of Motor Neurone Disease. NICE Clinical Guideline 105. NICE; 2010.
- Payment by Results in the NHS: Tariff for 2012 to 2013. London: Department of Health; 2013.
- Monthly Euro Conversion Rates. London: HM Revenue & Customs; 2014.
- Claxton K, Egginton S, Ginnelly L, Griffin S, McCabe C, Philips Z, et al. A Pilot Study of Value of Information Analysis to Support Research Recommendations for the National Institute for Health and Care Excellence. York: Centre for Health Economics, University of York; 2014.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24. http://dx.doi.org/10.1002/(SICI)1099-1050(199611)5:6<513::AID-HEC237>3.0.CO;2-9.
- 2012-based National Population Projections. Newport: Office for National Statistics; 2012.
- Haughney J, Gruffydd-Jones K, Roberts J, Lee AJ, Hardwell A, McGarvey L. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J 2014;43:993-1002. http://dx.doi.org/10.1183/09031936.00065013.
- Perneger TV. Estimating the relative hazard by the ratio of logarithms of event-free proportions. Contemp Clin Trials 2008;29:762-6. http://dx.doi.org/10.1016/j.cct.2008.06.002.
- Kirkwood B, Sterne J, Kirkwood B, Sterne J. Essential Medical Statistics. Oxford: Wiley-Blackwell; 2003.
- Blankenburg T, Roloff D, Schadlich S, Criee CP, Schutte W. Hypercapnic failure in patients with COPD under 4 weeks non-invasive, home mechanical ventilation. Pneumologie 2008;62:126-31. http://dx.doi.org/10.1055/s-2007-993036.
- Borel JC, Pepin JL, Pison C, Vesin A, Gonzalez-Bermejo J, Court-Fortune I, et al. Long-term adherence with non-invasive ventilation improves prognosis in obese COPD patients. Respirology 2014;19:857-65. http://dx.doi.org/10.1111/resp.12327.
- Budweiser S, Heinemann F, Meyer K, Wild PJ, Pfeifer M. Weight gain in cachectic COPD patients receiving noninvasive positive-pressure ventilation. Respir Care 2006;51:126-32.
- Carroll N, Branthwaite MA. Control of nocturnal hypoventilation by nasal intermittent positive pressure ventilation. Thorax 1988;43:349-53. http://dx.doi.org/10.1136/thx.43.5.349.
- Chatwin M, Heather S, Hanak A, Polkey MI, Simonds AK. Analysis of home support and ventilator malfunction in 1,211 ventilator-dependent patients. Eur Respir J 2010;35:310-16. http://dx.doi.org/10.1183/09031936.00073409.
- Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest 1999;116:667-75. http://dx.doi.org/10.1378/chest.116.3.667.
- Duiverman ML, Bladder G, Struik F, Wijkstra PJ. Decrease in respiratory muscle activity with long-term nocturnal non-invasive ventilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010. http://dx.doi.org/10.1164/ajrccm-conference.2010.181.1_meetingabstracts.a5051.
- Elliott MW, Mulvey DA, Moxham J, Green M, Branthwaite MA. Domiciliary nocturnal nasal intermittent positive pressure ventilation in COPD: mechanisms underlying changes in arterial blood gas tensions. Eur Respir J 1991;4:1044-52.
- Elliott MW, Simonds AK, Carroll MP, Wedzicha JA, Branthwaite MA. Domiciliary nocturnal nasal intermittent positive pressure ventilation in hypercapnic respiratory failure due to chronic obstructive lung disease: effects on sleep and quality of life. Thorax 1992;47:342-8. http://dx.doi.org/10.1136/thx.47.5.342.
- Khatun Y, Murphy PB, Davidson AC, Hart N. Seasonal variation in initiation and discontinuation of domiciliary non-invasive ventilation: A 12-month cohort study. Thorax 2011:A179-80.
- Kobayashi N, Miyazawa N, Ogura T, Watanuki Y, Nakamura M, Hashizume T, et al. Eighty cases of chronic respiratory failure treated with home noninvasive positive pressure ventilation. Nihon Kokyuki Gakkai Zasshi 2005;43:3-9.
- Nickol AH, Hart N, Hopkinson NS, Hamnegard CH, Moxham J, Simonds A, et al. Mechanisms of improvement of respiratory failure in patients with COPD treated with NIV. Int J Chron Obstruct Pulmon Dis 2008;3:453-62.
- Perrin C, El Far Y, Vandenbos F, Tamisier R, Dumon MC, Lemoigne F, et al. Domiciliary nasal intermittent positive pressure ventilation in severe COPD: effects on lung function and quality of life. Eur Respir J 1997;10:2835-9. http://dx.doi.org/10.1183/09031936.97.10122835.
- Schönhofer B, Polkey MI, Suchi S, Kohler D. Effect of home mechanical ventilation on inspiratory muscle strength in COPD. Chest 2006;130:1834-8. http://dx.doi.org/10.1378/chest.130.6.1834.
- Schönhofer B, Barchfeld T, Wenzel M, Kohler D. Long term effects of non-invasive mechanical ventilation on pulmonary haemodynamics in patients with chronic respiratory failure. Thorax 2001;56:524-8. http://dx.doi.org/10.1136/thorax.56.7.524.
- Schönhofer B, Barchfeld T, Wenzel M, Kohler D. Effect of nocturnal intermittent non-invasive ventilation on pulmonary hypertension in chronic respiratory failure. Pneumologie 1999;53:S113-15.
- Simonds AK, Elliott MW. Outcome of domiciliary nasal intermittent positive pressure ventilation in restrictive and obstructive disorders. Thorax 1995;50:604-9. http://dx.doi.org/10.1136/thx.50.6.604.
- Storre JH, Matrosovich E, Ekkernkamp E, Walker DJ, Schmoor C, Dreher M, et al. Home mechanical ventilation for COPD: high-intensity versus target volume noninvasive ventilation. Respir Care 2014;59:1389-97. http://dx.doi.org/10.4187/respcare.02941.
- Windisch W. Impact of home mechanical ventilation on health-related quality of life. Eur Respir 2008;32:1328-36. http://dx.doi.org/10.1183/09031936.00066407.
- Windisch W, Vogel M, Sorichter S, Hennings E, Bremer H, Hamm H, et al. Normocapnia during nIPPV in chronic hypercapnic COPD reduces subsequent spontaneous PaCO2. Respir Med 2002;96:572-9. http://dx.doi.org/10.1053/rmed.2002.1326.
- Adams AB, Shapiro R, Marini JJ. Changing prevalence of chronically ventilator-assisted individuals in Minnesota: Increases, characteristics, and the use of noninvasive ventilation. Respir Care 1998;43:643-9.
- Budweiser S, Heidtkamp F, Jorres RA, Heinemann F, Arzt M, Schroll S, et al. Predictive significance of the six-minute walk distance for long-term survival in chronic hypercapnic respiratory failure. Respiration 2008;75:418-26. http://dx.doi.org/10.1159/000109662.
- Budweiser S, Heinemann F, Fischer W, Dobroschke J, Pfeifer M. Long-term reduction of hyperinflation in stable COPD by non-invasive nocturnal home ventilation. Respir Med 2005;99:976-84. http://dx.doi.org/10.1016/j.rmed.2005.02.007.
- Bullemer F, Heindl S, Pahnke J, Karg O. Noninvasive intermittent ventilation. A prospective data collection in patients with hypoventilation syndrome. Med Klin (Munich) 1996;91:S14-16.
- Charbonnier F, Pasquina P, Janssens J-P, Adler D. Changes in Patient Demographics and Ventilator Settings in a Home Mechanical Ventilation (HMV) Clinic over 6 Years 2014.
- Chu CM, Yu WC, Tam CM, Lam CW, Hui DS, Lai CK, et al. Home mechanical ventilation in Hong Kong. Eur Respir J 2004;23:136-41. http://dx.doi.org/10.1183/09031936.03.00017803.
- Cuvelier A, Viacroze C, Benichou J, Molano LC, Hellot MF, Benhamou D, et al. Dependency on mask ventilation after acute respiratory failure in the intermediate care unit. Eur Respir J 2005;26:289-97. http://dx.doi.org/10.1183/09031936.05.00117104.
- Dale L, Brocklebank D. An outcome evaluation of a local respiratory support service. J Sleep Res 2012;21.
- Dellweg D, Schonhofer B, Haidl PM, Barchfeld T, Wenzel MD, Appelhans P, et al. Short-term effect of controlled instead of assisted noninvasive ventilation in chronic respiratory failure due to chronic obstructive pulmonary disease. Respir Care 2007;52:1734-40.
- Ekkernkamp E, Kabitz HJ, Walker DJ, Schmoor C, Storre JH, Windisch W, et al. Minute ventilation during spontaneous breathing, high-intensity noninvasive positive pressure ventilation and intelligent volume assured pressure support in hypercapnic COPD. COPD 2014;11:52-8. http://dx.doi.org/10.3109/15412555.2013.829437.
- Eller J, Priesnitz M, Lode H. Results of domiciliary treatment with IPPB in COLD patients. Atemweg Lungenkrank 1989;15:340-1.
- Fernandez Alvarez R, Rubinos CG, Rodriguez JF, Garcia GA, Rodriguez MP, Casan CP. Home mechanical ventilation through mask: Monitoring leakage and nocturnal oxygenation at home. Respiration 2013;85:132-6. http://dx.doi.org/10.1159/000341983.
- Ülger AF, Poyraz B, Gulec BE, Binay S, Ulger AF, Poyraz B, et al. Our experience of 200 patients: usage and maintenance of long-term oxygen therapy and non-invasive ventilation devices at home. Int J Clin Exp Med 2014;7:170-6.
- Garner DJ, Berlowitz DJ, Douglas J, Harkness N, Howard M, McArdle N, et al. Home mechanical ventilation in Australia and New Zealand. Eur Respir J 2013;41:39-45. http://dx.doi.org/10.1183/09031936.00206311.
- Gay PC, Patel AM, Viggiano RW, Hubmayr RD. Nocturnal nasal ventilation for treatment of patients with hypercapnic respiratory failure. Mayo Clin Proc 1991;66:695-703. http://dx.doi.org/10.1016/S0025-6196(12)62081-3.
- González Rodríguez CI, Jimenez BF, Rubio T, Godia S, Salinas U. Non-invasive home mechanical ventilation in the COPD patient. An Sist Sanit Navar 2005;28:345-50.
- Janssens J-P, Derivaz S, Breitenstein E, De Muralt B, Fitting JW, Chevrolet JC, et al. Changing patterns in long-term noninvasive ventilation: A 7-year prospective study in the Geneva Lake area. Chest 2003;123:67-79. http://dx.doi.org/10.1378/chest.123.1.67.
- Laier-Groeneveld G, Criee C-P, Bansch S, Huttemann U. Noninvasive intermittent ventilation. Atemweg Lungenkrank 1989;15:339-40.
- Criee C-P, Laier-Groeneveld G, Bansch S, Huttemann U. Noninvasive intermittent ventilation – effects on ventilation and respiratory muscle function. Atemweg Lungenkrank 1989;15:337-8.
- Leger P, Bedicam JM, Cornette A, Reybet-Degat O, Langevin B, Polu JM, et al. Nasal intermittent positive pressure ventilation. Long-term follow-up in patients with severe chronic respiratory insufficiency. Chest 1994;105:100-5. http://dx.doi.org/10.1378/chest.105.1.100.
- López-Campos JL, Failde I, Masa JF, Benitez-Moya JM, Barrot E, Ayerbe R, et al. Factors related to quality of life in patients receiving home mechanical ventilation. Respir Med 2008;102:605-12. http://dx.doi.org/10.1016/j.rmed.2007.11.005.
- McLaughlin P, Keatings V. Audit on use of domiciliary niv in patients with a diagnosis of COPD. Ir J Med Sci 2011.
- Örnek T, Erboy F, Atalay F, Altinsoy B, Tanriverdi H, Uygur F, et al. Evaluation of clinical data and mortality among COPD patients receiving domiciliary NIMV therapy. Turk Toraks Dergisi 2014;15:112-16. http://dx.doi.org/10.5152/ttd.2014.3852.
- Pasquina P, Adler D, Farr P, Bourqui P, Bridevaux PO, Janssens JP. What does built-in software of home ventilators tell us? An observational study of 150 patients on home ventilation. Respiration 2012;83:293-9. http://dx.doi.org/10.1159/000330598.
- Perrin C, Vandenbos F, Tamisier R, Lemoigne F, Blaive B. Impact of acute respiratory failure on survival of COPD patients managed with long-term non-invasive ventilation and oxygen therapy. Rev Mal Respir 2000;17:91-7.
- Schönhofer B, Kohler D. Acceptance and efficiency in the initial phase of non-invasive mechanical ventilation in COPD. Atemweg Lungenkrank 1997;23:517-22.
- Schucher B, Hein H, Kirsten D, Magnussen H. Use of home mechanical ventilation in patients with high grade chronic obstructive lung disease (COPD). Pneumologie 1999;53:S103-6.
- Schucher B, Hein H, Magnussen H. Acceptance and long-term results of home mechanical ventilation in various thoracic diseases. Med Klin (Munich) 1999;94:22-6.
- Sivasothy P, Smith IE, Shneerson JM. Mask intermittent positive pressure ventilation in chronic hypercapnic respiratory failure due to chronic obstructive pulmonary disease. Eur Respir J 1998;11:34-40. http://dx.doi.org/10.1183/09031936.98.11010034.
- Sugino K, Tsuboi E, Miyamoto A, Takaya H, Sakamoto S, Kishi K, et al. Efficacy of domiciliary noninvasive positive pressure ventilation in chronic obstructive pulmonary disease patients. Nihon Kokyuki Gakkai Zasshi 2008;46:432-7.
- Takada K, Matsumoto S, Hiramatsu T, Kojima E, Shizu M, Okachi S, et al. COPD patients with chronic hypercapnic respiratory failure receiving domiciliary noninvasive positive pressure ventilation – Analysis of prognostic factors and suggestion for supportive system. Ther Res 2009;30:1491-7.
- Takada K, Matsumoto S, Hiramatsu T, Kojima E, Watanabe H, Sizu M, et al. Predictors of survival in patients with chronic hypercapnic respiratory failure receiving domiciliary NPPV. Nihon Kokyuki Gakkai Zasshi 2008;46:614-19.
- Thibout Y, Philit F, Freymond N, Petitjean T, Nesme P, Guerin C. Outcome in COPD patients treated with at-home, long-term, non-invasive ventilation. Rev Mal Respir 2006;23:438-44. http://dx.doi.org/10.1016/S0761-8425(06)71814-9.
- Tollefsen E, Gulsvik A, Bakke P, Fondenes O. Prevalence of home ventilation therapy in Norway. Tidssk Nor Laegeforen 2009;129:2094-7. http://dx.doi.org/10.4045/tidsskr.08.0716.
- Tsuboi T, Oga T, Machida K, Sumi K, Oguri S, Sato A, et al. PaCO2 six months after the initiation of long-term noninvasive ventilation in patients with COPD. Intern Med 2011;50:563-70. http://dx.doi.org/10.2169/internalmedicine.50.4310.
- Tsuboi T, Ohi M, Chin K, Noguchi T, Kita H, Otsuka N, et al. Outcome of long-term nasal intermittent positive pressure ventilation in 41 patients with hypercapnic respiratory failure. Nihon Kyobu Shikkan Gakkai Zasshi 1996;34:959-67.
- Vitacca M, Nava S, Confalonieri M, Bianchi L, Porta R, Clini E, et al. The appropriate setting of noninvasive pressure support ventilation in stable COPD patients. Chest 2000;118:1286-93. http://dx.doi.org/10.1378/chest.118.5.1286.
- Waugh JB, Spratt G, Causey DE, Lain DC, Waugh SD. NPPV & COPD: the quality of life of COPD patients with chronic hypercapnia can be improved by providing 20 hours per week of NPPV home use. noninvasive positive-pressure ventilation. J Respir Care Pract 2001;14:43-5.
- Wiebel M, Rossbach L, Herth F, Schulz M, Schulz V. Ventilatory failure in COPD: follow-up under intermittent positive pressure ventilation (IPPV). Pneumologie 1999;53:100-2.
- Wiebel M, Schulz M, Herth F, Schulz V. Follow-up of intermittent self-ventilation (ISB). Mortality and causes. Med Klin 1997;92:S63-7.
- Windisch W, Budweiser S, Heinemann F, Pfeifer M, Rzehak P. The Severe Respiratory Insufficiency Questionnaire was valid for COPD patients with severe chronic respiratory failure. J Clin Epidemiol 2008;61:848-53. http://dx.doi.org/10.1016/j.jclinepi.2007.09.009.
- Windisch W, Freidel K, Schucher B, Baumann H, Wiebel M, Matthys H, et al. Evaluation of health-related quality of life using the MOS 36-Item Short-Form Health Status Survey in patients receiving noninvasive positive pressure ventilation. Intensive Care Med 2003;29:615-21. http://dx.doi.org/10.1007/s00134-003-1839-3.
- Windisch W, Petermann F, Laier-Groeneveld G, Fischer S, Criee CP. Quality of life in home ventilation. Med Klin (Munich) 1997;92:S95-100.
Appendix 1 Search strategies
Clinical effectiveness searches
Cochrane Central Register of Controlled Trials Issue 9 of 12 2014
#1 copd
#2 “chronic obstructive pulmonary disease”
#3 “chronic obstructive lung disease”
#4 “chronic obstructive airway disease”
#5 “chronic respiratory disorder*”
#6 “smoking related lung disease*”
#7 MeSH descriptor: [Pulmonary Disease, Chronic Obstructive] explode all trees
#8 emphysema
#9 MeSH descriptor: [Emphysema] explode all trees
#10 MeSH descriptor: [Bronchitis] explode all trees
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#12 non-invasive near/2 ventilation
#13 noninvasive near/2 ventilation
#14 MeSH descriptor: [Positive-Pressure Respiration] explode all trees
#15 MeSH descriptor: [Intermittent Positive-Pressure Ventilation] explode all trees
#16 cpap
#17 bipap
#18 “bi-level ventilation”
#19 “bilevel ventilation”
#20 niv
#21 nippv
#22 nppv
#23 “positive pressure ventilation”
#24 “positive airway pressure”
#25 #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24
#26 #11 and #25 from 1980 to 2014
MEDLINE (Ovid) 1946 to August Week 4 2014
-
chronic obstructive pulmonary disease.mp. or exp Pulmonary Disease, Chronic Obstructive/
-
copd.mp.
-
chronic obstructive lung disease.mp.
-
chronic obstructive airway disease.mp.
-
chronic respiratory disorder$.mp.
-
smoking-related lung disease$.mp.
-
Pulmonary Emphysema/
-
exp Bronchitis/
-
emphysema.mp.
-
or/1-9
-
exp positive-pressure respiration/ or intermittent positive-pressure ventilation/
-
cpap.mp.
-
bipap.mp.
-
bi-level ventilation.mp.
-
niv.mp.
-
nippv.mp.
-
positive pressure ventilation.mp.
-
positive airway pressure.mp.
-
((noninvasive or non-invasive) adj2 ventilation).mp.
-
nppv.mp.
-
or/11-20
-
10 and 21
-
limit 22 to yr=“1980–2014”
MEDLINE (Ovid) In-Process & Other Non-Indexed Citations 8 September 2014
-
copd.mp.
-
chronic obstructive lung disease.mp.
-
chronic obstructive airway disease.mp.
-
chronic respiratory disorder$.mp.
-
smoking-related lung disease$.mp.
-
emphysema.mp.
-
chronic obstructive pulmonary disease$.mp.
-
bronchitis.mp.
-
or/1-8
-
cpap.mp.
-
bipap.mp.
-
bi-level ventilation.mp.
-
niv.mp.
-
nippv.mp.
-
positive pressure ventilation.mp.
-
positive airway pressure.mp.
-
((noninvasive or non-invasive) adj2 ventilation).mp.
-
nppv.mp.
-
positive pressure respiration.mp.
-
or/10-19
-
9 and 20
-
limit 21 to yr=“1980–2014”
EMBASE (Ovid) 1980 to 8 September 2014
-
chronic obstructive lung disease/
-
chronic obstructive pulmonary disease.mp.
-
copd.mp.
-
chronic obstructive lung disease.mp.
-
chronic obstructive airway disease.mp.
-
chronic respiratory disorder$.mp.
-
smoking-related lung disease$.mp.
-
lung emphysema/
-
emphysema.mp.
-
exp bronchitis/
-
or/1-10
-
noninvasive ventilation.mp. or exp noninvasive ventilation/
-
positive end expiratory pressure/
-
positive pressure respiration.mp.
-
positive pressure ventilation.mp.
-
cpap.mp.
-
bipap.mp.
-
bi-level ventilation.mp.
-
niv.mp.
-
nippv.mp.
-
positive airway pressure.mp.
-
nppv.mp.
-
((noninvasive or non-invasive) adj2 ventilation).mp.
-
or/12-23
-
11 and 24
-
limit 25 to yr=“1980- 2014”
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost) 1981: September 2014
S1 MH “pulmonary disease, Chronic Obstructive+”
S2 chronic obstructive pulmonary disease
S3 chronic obstructive lung disease
S4 copd
S5 chronic obstructive airway disease
S6 chronic respiratory disorder*
S7 smoking-related lung disease
S8 MH “Emphysema”
S9 MH “bronchitis+”
S10 emphysema
S11 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10
S12 MH “positive pressure ventilation+”
S13 cpap
S14 bipap
S15 bi-level ventilation
S16 niv
S17 nippv
S18 nppv
S19 positive pressure ventilation
S20 positive airway pressure
S21 non-invasive ventilation
S22 “noninvasive N2 ventilation”
S23 S12 or S 13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22
S24 S11 and S23
S25 S11 and S23 limited by years 1980-2014
Science Citation Index (Web of Knowledge) 1900–8 September 2014
Conference Proceedings Citation Index (Web of Knowledge) 1900–8 September 2014
#1 Topic=(copd) OR Topic=(chronic obstructive pulmonary disease) OR Topic=(chronic obstructive lung disease) OR Topic=(chronic obstructive airway disease) OR Topic=(chronic respiratory disorder*) OR Topic=(smoking related lung disease) OR Topic=(emphysema) OR Topic=(bronchitis)
#2 Topic=(cpap or bipap or niv or nippv or nppv) OR Topic=(positive pressure respiration) OR Topic=(positive pressure ventilation) OR Topic=(bi-level ventilation) OR Topic=(positive airway pressure) OR Topic=(noninvasive ventilation) OR Topic=(non-invasive ventilation)
#3 #1 and #2
Limited to time span 1980–2014 (searched 8 September 2014).
Clinical Trials Registers Searches for ongoing studies
Search strategies run 8 September 2014.
World Health Organization International Clinical Trials Registry Platform
Search terms
“NIV” AND “COPD”
“Home” AND “ventilation”
“Chronic” AND “Pulmonary”
“Non invasive ventilation” AND “outpatients”
All limited to status: “Recruiting”
ClinicalTrials.gov
Search terms
“Non-invasive ventilation” AND “home”
Limited to “Recruiting” OR “Active, non-recruiting”
Current Controlled Trials
Search terms
“Ventilation” AND “Home” AND “Pulmonary”
“COPD” AND “Home” AND “Ventilation”
Limited to “Recruiting” OR “Active, non-recruiting”
UK Clinical Research Network Portfolio
Search terms
“COPD”
Limited to “Open” OR “In set-up”
Economic models
MEDLINE (Ovid ) 1946 to August Week 4 2014
Search strategy
-
chronic obstructive pulmonary disease.mp. or exp Pulmonary Disease, Chronic Obstructive/
-
copd.mp.
-
chronic obstructive lung disease.mp.
-
chronic obstructive airway disease.mp.
-
chronic respiratory disorder$.mp.
-
smoking-related lung disease$.mp.
-
Pulmonary Emphysema/
-
exp Bronchitis/
-
emphysema.mp.
-
or/1-9
-
decision support techniques/
-
markov.mp.
-
exp models economic/
-
decision analysis.mp.
-
cost benefit analysis/
-
or/11-15
-
10 and 15
-
limit 18 to yr=“1980- 2014”
EMBASE (Ovid) 1980 to 9 September 2014
-
chronic obstructive lung disease/
-
chronic obstructive pulmonary disease.mp.
-
copd.mp.
-
chronic obstructive lung disease.mp.
-
chronic obstructive airway disease.mp.
-
chronic respiratory disorder$.mp.
-
smoking-related lung disease$.mp.
-
lung emphysema/
-
emphysema.mp.
-
exp bronchitis/
-
or/1-10
-
noninvasive ventilation.mp. or exp noninvasive ventilation/
-
(non-invasive adj2 ventilation).mp.
-
positive end expiratory pressure/
-
positive pressure respiration.mp.
-
positive pressure ventilation.mp.
-
npvv.mp.
-
cpap.mp.
-
bipap.mp.
-
bi-level ventilation.mp.
-
niv.mp.
-
nippv.mp.
-
positive pressure ventilation.mp.
-
or/12-23
-
11 and 24
-
decision support.ti,ab.
-
decision analysis.ti,ab.
-
markov.ti,ab.
-
model$.ti,ab.
-
or/26-29
-
25 and 30
-
limit 31 to yr=“1980–2014”
Economic evaluations
The Cochrane Library (Wiley) NHS Economic Evaluation Database 2014 Issue 3 of 4
#1 copd
#2 “chronic obstructive pulmonary disease”
#3 “chronic obstructive lung disease”
#4 “chronic obstructive airway disease”
#5 “chronic respiratory disorder*”
#6 “smoking related lung disease*”
#7 MeSH descriptor: [Pulmonary Disease, Chronic Obstructive] explode all trees
#8 emphysema
#9 MeSH descriptor: [Emphysema] explode all trees
#10 MeSH descriptor: [Bronchitis] explode all trees
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#12 non-invasive near/2 ventilation
#13 noninvasive near/2 ventilation
#14 MeSH descriptor: [Positive-Pressure Respiration] explode all trees
#15 MeSH descriptor: [Intermittent Positive-Pressure Ventilation] explode all trees
#16 cpap
#17 bipap
#18 “bi-level ventilation”
#19 “bilevel ventilation”
#20 niv
#21 nippv
#22 nppv
#23 “positive pressure ventilation”
#24 “positive airway pressure”
#25 #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24
#26 #11 and #25 from 1980 to 2014
MEDLINE (Ovid) 1946 to August Week 4 2014
-
economics/
-
exp “costs and cost analysis”/
-
cost of illness/
-
exp health care costs/
-
economic value of life/
-
exp economics medical/
-
exp economics hospital/
-
economics pharmaceutical/
-
exp “fees and charges”/
-
(econom$ or cost or costs or costly or costing or price or pricing or pharmacoeconomic$).tw.
-
(expenditure$ not energy).tw.
-
(value adj1 money).tw.
-
budget$.tw.
-
or/1-13
-
chronic obstructive pulmonary disease.mp. or exp Pulmonary Disease, Chronic Obstructive/
-
copd.mp.
-
chronic obstructive lung disease.mp.
-
chronic obstructive airway disease.mp.
-
chronic respiratory disorder$.mp.
-
smoking-related lung disease$.mp.
-
Pulmonary Emphysema/
-
exp Bronchitis/
-
emphysema.mp.
-
or/15-23
-
exp positive-pressure respiration/ or intermittent positive-pressure ventilation/
-
cpap.mp.
-
bipap.mp.
-
bi-level ventilation.mp.
-
niv.mp.
-
nippv.mp.
-
positive pressure ventilation.mp.
-
positive airway pressure.mp.
-
((noninvasive or non-invasive) adj2 ventilation).mp.
-
nppv.mp.
-
or/25-34
-
14 and 35
-
14 and 24 and 35
-
limit 37 to yr=“1980–2014”
EMBASE (Ovid) 1980 to 9 September 2014
-
chronic obstructive lung disease/
-
chronic obstructive pulmonary disease.mp.
-
copd.mp.
-
chronic obstructive lung disease.mp.
-
chronic obstructive airway disease.mp.
-
chronic respiratory disorder$.mp.
-
smoking-related lung disease$.mp.
-
lung emphysema/
-
emphysema.mp.
-
exp bronchitis/
-
or/1-10
-
noninvasive ventilation.mp. or exp noninvasive ventilation/
-
(non-invasive adj2 ventilation).mp.
-
positive end expiratory pressure/
-
positive pressure respiration.mp.
-
positive pressure ventilation.mp.
-
npvv.mp.
-
cpap.mp.
-
bipap.mp.
-
bi-level ventilation.mp.
-
niv.mp.
-
nippv.mp.
-
positive pressure ventilation.mp.
-
or/12-23
-
11 and 24
-
cost benefit analysis/
-
cost effectiveness analysis/
-
cost minimization analysis/
-
cost utility analysis/
-
economic evaluation/
-
(cost or costs or costed or costly or costing).tw.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
(technology adj assessment$).tw.
-
or/26-33
-
25 and 34
-
limit 35 to yr=“1980–2014”
Appendix 2 Quality assessment of studies included in clinical effectiveness review
| Study | Random sequence generation | Allocation concealment | Blinding of patients | Blinding of outcome assessment | Incomplete outcome dataa | Selective outcome reporting | |
|---|---|---|---|---|---|---|---|
| NIV group | Control group | ||||||
| Bhatt et al. 201383 | LOW | LOW | HIGH | UNCLEAR | LOW | HIGH | LOW |
| Random number generator | Opaque sealed envelopes which were opened during screening visits | No sham NIV arm | No details | No loss to follow-up | 3/15 (20%) early withdrawals; analysis in 12/15 (no ITT and no reasons given for withdrawal or on similarity to completers) | No apparent selective reporting | |
| Casanova et al. 200085 | LOW | LOW | HIGH | UNCLEAR | UNCLEAR | LOW | LOW |
| Random numbers table | Randomisation by independent office, so likely that concealment adequate | No sham NIV arm | No details | 6/26 (23%) withdrawals: 5/6 because of ‘pressure being too high’, 1/6 after diagnosis of significant aortic stenosis. Results for completers only. No details on baseline differences between dropouts and completers. Stated that ‘inclusion of the patients who did not complete the trial (intent-to-treat) did not affect any of the outcomes’85 | 2/26 (8%) withdrawals because of abnormal echocardiographic findings detected during routine follow-up. Results for completers only. No details on baseline differences between dropouts and completers. Stated that ‘inclusion of the patients who did not complete the trial (intent-to-treat) did not affect any of the outcomes’85 | Not all results reported at all time points, although it was mentioned in the text whether or not there were any significant differences | |
| Cheung et al. 201090 | LOW | LOW | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR | LOW |
| Computer-generated random numbers | Drawing of sequentially numbered and sealed opaque envelopes by non-study personnel | CPAP as ‘placebo NIV’. This was an open-label study, but ‘care had been taken to avoid biasing the patients into believing either mode was superior’90 | No details | Withdrawals reported/accounted for (8/23, 35%). The main results in both arms of the study were analysed by an ITT approach. Non-completers were included in the final analysis, with their timed data censored on the withdrawal dates. Varying (reducing) numbers of patients included for arterial pH and PaCO2. No details on characteristics of dropouts and completers | Withdrawals reported/accounted for (4/24, 17%). The main results in both arms of the study were analysed by an ITT approach. Non-completers were included in the final analysis, with their timed data censored on the withdrawal dates. Varying (reducing) numbers of patients included for arterial pH and PaCO2. No details on characteristics of dropouts and completers | No apparent selective reporting | |
| Clini et al. 200299 | LOW | LOW | HIGH | LOW | UNCLEAR | UNCLEAR | LOW |
| Centralised block randomisation | Centralised randomisation likely to ensure allocation concealment | No sham NIV arm | All physiological measurements were performed by personnel blind to treatment and not involved in the study | Numbers and reasons given for dropouts in both NPPV and control groups. The numbers of those lost to follow-up in each group were also recorded. Similar number of dropouts/losses in both groups [12/43 (28%) NIV, 15/47 (32%) LTOT] if early dropouts were included. Slightly more patients lost to follow-up from LTOT group compared with NIV group (7/47 vs. 1/43) and more non-compliers in NIV group (7/43 vs. 1/47). Baseline characteristics of dropouts stated to be similar to those of completers. ‘The main parameters were evaluated both in terms of patient completers and in terms of the ITT approach. The last observation carried forward was used as a method of ITT and data are presented accordingly. Data on patients’ compliance were evaluated only in terms of patient completers in order to document “per protocol” analysis’99 | It appears that all of the study’s prespecified outcomes have been reported | ||
| De Backer et al. 201191 | UNCLEAR | UNCLEAR | HIGH | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR |
| Stated only that patients were randomised | No details | No sham NIV arm | No details | No details on loss to follow-up | No details on loss to follow-up | Additional measurements were taken but not reported. Blood gases at 1, 3 and 12 months, 6MWD at 3 months. Hospital admission, morbidity/mortality and compliance recorded but not reported (these were not predefined outcomes) | |
| Duiverman et al. 200879 | LOW | UNCLEAR | HIGH | UNCLEAR | HIGH | UNCLEAR | LOW |
| Computerised randomisation (with minimisation for FEV1, PaCO2 and body mass index) | Randomisation performed by independent statistician | No sham NIV arm | No details | 6/37 early dropouts before baseline measurements (two died, two withdrew and two had other diseases); seven further dropouts during 3 months’ study (5 intolerance to NIV, 1 non-compliant with rehabilitation, 1 death). Total 35% dropouts. Non-completers had a lower FEV1 (p < 0.05), lower vital capacity (p < 0.05) and higher residual volume as a percentage of total lung capacity (p < 0.05). Stated that main outcomes were evaluated for completers (not clear how many patients assessed for each outcome) | 3/35 (8.6%) dropouts because of non-compliance. Non-compliers had a higher total lung capacity and residual volume than completers (p < 0.01). Stated that main outcomes evaluated for completers (not clear how many patients were assessed for each outcome) | No apparent selective reporting | |
| Duiverman et al. 201180 | LOW | UNCLEAR | HIGH | UNCLEAR | HIGH | HIGH | LOW |
| Computerised randomisation (with minimisation for FEV1, PaCO2 and body mass index) | Randomisation performed by independent statistician | No sham NIV arm | No details (but analyses performed by an independent statistician) | 6/37 early dropouts (during in-hospital rehabilitation programme, Duiverman 200879); further seven dropouts before start period of this follow-on study. 24 started home-based follow-up period, 9/24 dropouts (3 withdrew, 1 aorta dissection, 5 deaths); total dropout 59% (22/37). significantly lower baseline PaO2 in dropouts compared with completers. ‘All data of all patients available at the start of the home-based period included for analyses and all available data used for analyses until patients dropped out.’80 Patient numbers stated for different outcomes at different time points | 3/35 dropouts (during in-hospital rehabilitation programme, Duiverman 200879). 32 started home-based follow-up period, 12/32 (37%) dropouts (3 non-compliant, 1 lung transplantation, 1 stroke, 1 deterioration in condition, 1 treated with CPAP, 5 deaths); significantly worse CRQ score and 6MWD in dropouts compared with completers. ‘All data of all patients available at the start of the home-based period included for analyses and all available data used for analyses until patients dropped out.’80 Patient numbers stated for different outcomes at different time points | No apparent selective reporting | |
| Garrod et al. 200084 | UNCLEAR | LOW | HIGH | UNCLEAR | UNCLEAR | UNCLEAR | LOW |
| Randomisation using sealed envelopes. No further details | Sealed envelopes suggest that allocation was likely concealed | No sham NIV arm | No details | 3/24 (12%) withdrawals (one transient ischaemic attack, two non-compliance). Available for assessments: 17/23 (after 4-week run-in), 18/23 at 8 weeks, 17/23 at 12 weeks (between 22% and 27% loss to follow-up). There were no significant differences in baseline variables between patients who completed all assessments compared with those who withdrew or were unable to attend an assessment | 1/22 (4%) withdrawal (refusal to attend training sessions). Available for assessments: 18/22 (after 4-week run-in), 21/22 at 8 weeks, 20/22 at 12 weeks (between 9% and 18% loss to follow-up). There were no significant differences in baseline variables between patients who completed all assessments compared with those who withdrew or were unable to attend an assessment | It appears that all of the study’s prespecified outcomes have been reported | |
| Gay et al. 1996100 | UNCLEAR | UNCLEAR | LOW | UNCLEAR | HIGH | LOW | LOW |
| Stated only that patients were randomised | No details | Sham NIV (same equipment, but ‘ventilated’ with lowest EPAP level and had no added IPAP or timed breaths). All patients were told that they may be randomised to a ‘low-pressure’ setting | No details | 3/7 (43%) discontinued after a median of 1 month. Significantly more than in sham group (main reason was difficulty sleeping). Results based on completers only | 6/6 completed study; no losses to follow-up | No apparent selective reporting | |
| Kaminski et al. 1999101 | UNCLEAR | UNCLEAR | HIGH | UNCLEAR | UNCLEAR | LOW | LOW |
| Stated that allocated randomly | No details | No sham NIV arm | No details | 2/7 (29%) discontinued NIV and crossed over to control arm, four deaths, no further losses to follow-up. Last assessment before death included | Five deaths (5/12), no further losses to follow-up. Last assessment before death included | No apparent selective reporting | |
| Köhnlein et al. 201476 | LOW | LOW | HIGH | LOW | LOW for survival, HIGH for QoL; UNCLEAR for remaining outcomes | LOW for survival; HIGH for QoL; UNCLEAR for remaining outcomes | LOW |
| Computer-generated block randomisation | Randomisation hotline, so assume allocation concealed | No sham NIV arm | Outcome assessors unaware of treatment assignment throughout the study | 2/102 lost to follow-up. ITT for primary outcome survival. Patient numbers not always clear for other outcome assessments at different time points. HRQoL assessments in subgroups of patients only | No losses to follow-up. ITT for primary outcome survival. Patient numbers not always clear for other outcome assessments at different time points. HRQoL assessments in subgroups of patients only | No obvious selective reporting. QoL and compliance reported for only a subset of patients but made explicit | |
| McEvoy et al. 200974 | LOW | LOW | HIGH | UNCLEAR | UNCLEAR (LOW for survival) | UNCLEAR (LOW for survival) | LOW |
| The central study co-ordinator generated a random sequence of treatment assignments that were stratified by centre | Sealed opaque envelopes; central co-ordinator verified that the patient met all eligibility criteria before the site research nurse broke the envelope seal | No sham NIV arm | Sleep studies were scored by experienced sleep scorers who were blinded to treatment allocation. No details for other outcomes | 4/72 (5%) lost to follow-up (not contactable or withdrawal of consent). Varying number of patients attended for repeat measurements (high mortality rate and reluctance of patients to attend; therefore, not ITT and for first 12 months only). ITT and PP analysis for survival | 4/72 (5%) lost to follow-up (not contactable or withdrawal of consent). Varying number of patients attended for repeat measurements (high mortality rate and reluctance of patients to attend; therefore, not ITT and for first 12 months only). ITT and PP analysis for survival | Main outcomes appear to be reported. Results for FVC appear not to be reported | |
| Murphy et al. 201178 (abstract, interim trial report) | UNCLEAR | UNCLEAR | HIGH | UNCLEAR | UNCLEAR | UNCLEAR | UNCLEAR |
| Stated only that patients were randomised | No details | No sham NIV arm | No details | No details – data on 20 (of 36 randomised) that have been followed up for 3 months at time of writing | No details – data on 20 (of 36 randomised) that have been followed up for 3 months at time of writing | Results reported only for sleep-related outcomes and compliance. However, blood gases and HRQoL measures also mentioned in methodology | |
| Sin et al. 200782 | UNCLEAR | UNCLEAR | LOW | LOW | HIGH | LOW | LOW |
| Randomisation occurred at a central site | Randomisation undertaken at central site by one individual who was unaware of patients’ clinical status | Subjects blinded by using sham therapy; authors state that ‘complete blinding may not have been present and we cannot completely eliminate the possibility of a “placebo effect”, although this seems unlikely in view of the excellent compliance observed in those assigned to sham therapy’82 | All outcome measurements performed and interpreted by personnel who were blinded to treatment allocation | 2/13 (15%) refused NIV after randomisation. Not included in analysis. No details on whether or not patient characteristics were similar | No loss to follow-up/no dropouts | No apparent selective reporting | |
| Struik et al. 201475 | LOW | UNCLEAR | HIGH | UNCLEAR | LOW for survival, HIGH for blood gases and QoL, UNCLEAR for remaining outcomes | LOW for survival, HIGH for blood gases and QoL, UNCLEAR for remaining outcomes | LOW |
| Computer-generated randomisation with minimisation | No details | No sham NIV arm | No details | 25/101 dropouts. Lack of motivation (15/25), discomfort associated with treatment (8/25), dementia (1/25), cerebrovascular accident (1/25). ITT analysis for survival, unclear for hospital admissions and exacerbations, completers only for QoL and blood gases | 24/100 dropouts. Lack of motivation (14/24), unable to come for testing (6/24), switch to NIV (4/24). ITT analysis for survival, unclear for hospital admissions and exacerbations, completers only for QoL and blood gases | No apparent selective reporting | |
| Xiang et al. 200792 | LOW | UNCLEAR | HIGH | UNCLEAR | UNCLEAR | UNCLEAR | LOW |
| Random number table used to generate randomisation sequence | No details | No sham NIV arm | No details | All results appear to be based on all patients (ITT) but no details on how missing values dealt with | All results appear to be based on all patients (ITT) but no details on how missing values dealt with | No apparent selective reporting | |
| Zhou et al. 200881 | LOW | UNCLEAR | HIGH | UNCLEAR | LOW (primary), HIGH (secondary) | LOW (primary), HIGH (secondary) | LOW |
| Random number table used to generate randomisation sequence | No details | No sham NIV arm | No details | Results for primary outcomes appear to be based on all patients. Between 7% and 14% loss to follow-up for secondary outcomes (results for completers only, no details on similarities) | Results for primary outcomes appear to be based on all patients. Between 7% and 14% loss to follow-up for secondary outcomes (results for completers only, no details on similarities) | No apparent selective reporting | |
| Study | Random sequence generation | Allocation concealment | Blinding of patients | Blinding of outcome assessment | Incomplete outcome data NIV groupa | Incomplete outcome data control groupa | Selective outcome reporting | Is it clear that the order of receiving treatments was randomised? | Can it be assumed that the trial was not biased from carry-over effects? | Dropouts after first treatment period (how incorporated into analysis?) | Are data available from both treatment periods? | Was a form of paired analysis used? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meecham-Jones et al. 199577 | UNCLEAR | UNCLEAR | HIGH | UNCLEAR | HIGH | LOW | No Cochrane guidelines for rating risk of bias | |||||
| Stated that randomisation was achieved with a previously generated randomised sequence | No details | No sham NIV arm | No details | 4/18 (22%) did not complete all stages of the study. 1/4 was withdrawn because of lung transplantation (during second study period), 1/4 because of development of bronchial carcinoma, 1/4 died at home during acute exacerbation 2 weeks after entering second study period (NIV), 1/4 was withdrawn because of inability to tolerate equipment. Time point of withdrawal was not clear for all. Results based on 14/18 completers | No apparent selective reporting | Yes | No statistical tests for carryover performed | Analysis based on completers only. Of the 4 withdrawals, 2/4 were during the second treatment period and it was unclear for the other 2/4 | Yes | Yes | ||
| Strumpf et al. 199134 | UNCLEAR | UNCLEAR | HIGH | UNCLEAR | HIGH | LOW | No Cochrane guidelines for rating risk of bias | |||||
| Stated only that patients were randomised | No details | No sham NIV arm | No details | 23 initially enrolled, 4/23 of whom did not meet eligibility criteria. 7/23 could not tolerate the mask (complaints included intolerable nasal mucosal irritation unresponsive to corticosteroids or humidification, inability to sleep, excessive anxiety associated with ventilator use). Unclear how many withdrew during first/second treatment period. 5/23 patients withdrew because of other illnesses (3/5 during the NIV treatment period and 2/5 during the control period). Results presented for 7 patients who completed both treatment periods. Total dropout 70%. Stated that baseline pulmonary functions did not differ significantly between the 7 patients who completed both arms and the 23 patients initially enrolled | No apparent selective reporting | Yes | ANOVA performed to determine whether or not results may have been affected by sequence effects-no significant trends revealed | Unclear when patients dropped out, but dropouts not included in analysis | Yes, but only for 7/19 randomised patients | Yes | ||
| Study | Prospective or retrospective | How were NIV and control groups selected (e.g. from the same source, at the same time)? | Were NIV and control groups similar at baseline? | Blinding of outcome assessment | Was blinding of outcome assessment the same for both groups? | NIV group incomplete outcome data | Control group incomplete outcome data | Was follow-up time and method of follow-up the same in both groups? | Selective reporting |
|---|---|---|---|---|---|---|---|---|---|
| Budweiser et al. 200796 | Prospective | NIV initiated or attempted in most patients. Those who refused NIV from the beginning or could not tolerate NIV during hospital stay (mostly because of mask intolerance) formed the control group | Most baseline characteristics appear to be similar. There was a difference in LTOT at discharge (95% NIV group and 81% in control group); a subgroup analysis was performed for patients on LTOT (with or without NIV) only; also used as variable in adjusted HR | No details. Only survival as an outcome measure (objective measure, blinding not as relevant) | No details. As survival is an objective measure, blinding is not as relevant | Not specifically stated. Details on those who died or discontinued but no mention of losses to follow-up. 12/99 (12%) discontinued NIV (3/12 because of mask intolerance, 3/12 because of decreased motivation, 4/12 reported improvement of symptoms, 1/12 had lung transplantation, 1/12 was not specified). No details on discontinuation rates of LTOT | Not specifically stated. Details on those who died given but no mention of losses to follow-up. No details on discontinuation rates of LTOT | Follow-up time was slightly longer in the NIV group (19.8 months vs. 12.9 months); as a result of earlier deaths in non-NIV group? Patients undergoing long-term NIV were regularly admitted for re-evaluation to hospital and thus may have had more intense contact than the control group | No apparent selective reporting |
| Clini et al. 199893 | Prospective | Those not complying with NIV during in-hospital adaptation period. Lack of compliance defined as the patient’s inability to use NIV properly for at least 5 hours for even 1 night (subjective intolerance, excessive air leaks) | Stated that the two groups were not different for anthropometric and functional characteristics; similar severity of airway obstruction and hyperinflation; previous smoking habit and medical therapy did not differ between the two groups, neither did numbers of acute exacerbations over previous 2 years and rates of endotracheal intubation | 6MWT performed and recorded under supervision of a nurse not involved in the study | Appears to be | 21/49 did not tolerate NIV in adaptation period and formed the control group. No further losses to follow-up reported apart from deaths. Unclear how many patients are contributing to results at different time points. No mention of ITT analysis or how missing data were handled | Yes | No apparent selective reporting | |
| Clini et al. 199694 | Prospective (and also a historical control – data not extracted) | Patients matched for anthropometric, functional and blood gas data. Patients in NIV group had suffered from at least one episode of acute respiratory failure needing non-invasive mechanical ventilation or had undergone at least two admissions to respiratory units for severe exacerbations not requiring ventilatory support; 7/17 patients included in the control group had undergone ICU admissions needing mechanical ventilation but were not able to perform long-term NIV | No significant differences at baseline | No details | No details | No details (except deaths). Blood gases based on varying numbers of patients. Numbers not stated for other hospital-related outcomes | Yes | No apparent selective reporting | |
| Heinemann et al. 201197 | Retrospective | Those not meeting criteria for NIV (i.e. PaCO2 > 52.5 and/or pH 7.35) formed the control group | No. Those discharged without NIV were significantly older and had higher SAPS-II (simplified acute physiology score-II) scores (simplified acute physiology score-II) at admission, but better pulmonary function and showed a trend towards lower severity of hypercapnia | No details | No details | No details (only on deaths) | No. Those on NIV received more intensive medical care as they went for additional check-ups | No apparent selective reporting | |
| Laier-Groeneveld and Criee 199588 | Retrospective | Control group were treated during same time period in same clinic | No. Control group were normocapnic, NIV group were hypercapnic. Control group patients would not have been able to receive NIV. Higher pO2 and FEV1 in control group (although not clear if statistically significant difference) | Stated that investigator was blinded regarding NIV but in context of measuring arrhythmias, so not relevant. Not relevant for survival | No details | No details | Appears yes | No apparent selective reporting | |
| Lu et al. 201298 | Retrospective | All groups are selected from the patients who were in hospital from January 2009 to December 2010 and with stable COPD (PaCO2 ≥ 55 mmHg) after treatment. No details on how control group was selected vs. NIV group | All baseline characteristics appear to be similar | No details | No details | No losses to follow-up | Not specifically stated. Details on those who were lost to follow-up and died | Yes | No apparent selective reporting |
| Milane and Jonquet 198586 | Retrospective | Group selected from patients hospitalised during 1973–1983 because of an exacerbation (same centre). Blood gas measurements determined eligibility for NIV or not | Stated that similar for age. Slightly better blood gas values in those not receiving home NIV | No details | No details | No details | No details | NIV patients received additional home visits to check medication and ventilator technique | No apparent selective reporting |
| Pahnke et al. 199787 | Retrospective | Control group – those who refused NIV a priori or within first 3 months | No details | No details | No details | No details | No details | No details | No apparent selective reporting |
| Paone et al. 201489 | Prospective | Patients allocated to NIV or control group on basis of compliance (during an NIV trial) and/or willingness to be trained | Propensity matched scores obtained and used for adjusted analyses. No obvious difference between groups at baseline | No details (only hospitalisations and survival as outcome measures, both objective so blinding less relevant) | No details | No details on loss to follow-up during the 24-month follow-up period. Four patients crossed over to NIV, but they are included in the main analysis | Appears to be. All had regular clinical evaluations every 2 months | No apparent selective reporting | |
| Tsolaki et al. 200895 | Prospective | Those who had good compliance with ventilator during hospital stay but refused to continue NIV at home on a long-term basis | No statistically significant differences between groups for baseline characteristics; trend towards higher BMI in NIV group | No details | No details | 3/27 early dropouts because of poor compliance with ventilator (< 5 hours/day). Appear to be no further dropouts (except deaths). Numbers assessed for outcomes at different time points not specifically stated. Early dropouts not included in analysis | Appear to be no losses to follow-up (except deaths). Numbers assessed for outcomes at different time points not specifically stated | Stated that all patients followed up in an identical pattern and closely supervised for adherence to medical treatment | Not all time points presented for PaO2 and HCO3. Otherwise no selective reporting |
| Study | Random sequence generation | Allocation concealment | Blinding of patients | Blinding of outcome assessment | Incomplete outcome data NIV group | Incomplete outcome data control group | Selective outcome reporting | Is it clear that the order of receiving treatments was randomised? | Can it be assumed that the trial was not biased from carry-over effects? | Dropouts after first treatment period (how incorporated into analysis?) | Are data available from both treatment periods? | Was a form of paired analysis used? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dreher et al. 201032 | UNCLEAR | UNCLEAR | HIGH | UNCLEAR | HIGH | LOW | No Cochrane guidelines for rating risk of bias | |||||
| Randomised two-period crossover design – no further details | No details | No. Open label | No. Described as open-label study. Investigators not blinded | Two dropouts from low intensity treatment arm during first period (1/2 refused treatment in hospital because of intolerance, another stopped at home despite successful establishment in hospital). 2 patients refused to swap over to low intensity after first treatment period with high intensity. Four fewer patients during treatment period 2 (23%). No details on whether or not characteristics of dropouts were similar to completers. 17 patients during treatment period 1, 13 patients during treatment period 2 | No apparent selective reporting | Yes (patients randomised to receive a specific sequence) | No effect found based on period effect tests and test for carryover effects | 4/17 during first treatment period. Only patients included who received both treatments (?) | Yes | Yes. Paired analyses for all patients who received the respective treatments | ||
| Oscroft et al. 2010112 | UNCLEAR | LOW | LOW | LOW | UNCLEAR | LOW | No Cochrane guidelines for rating risk of bias | |||||
| No details on method of randomisation | Sealed opaque envelopes | Trial subjects were blinded to the ventilators’ mode | Spirometry, assessment of lung volumes, gas diffusion and shuttle walk test assessments performed by technicians blinded to the treatment group | 1/25 lost to follow-up (4%). 1/25 withdrew consent after exacerbation (during volume-assured NIV period). Not all patients were able to participate in the shuttle walk test (results based on 18/24). FEV1 and FVC were based on 23 participants. Unclear for SGRQ and SF-36 | No apparent selective reporting | Yes | No details | One withdrew during volume assured NIV (not clear if treatment period 1 or 2). Not included in analysis | Yes | Yes | ||
| Murphy et al. 2012113 | UNCLEAR | LOW | UNCLEAR | UNCLEAR | HIGH | LOW | No Cochrane guidelines for rating risk of bias | |||||
| Randomised, two–treatment crossover design | ‘Randomisation via sealed envelope allocation’113 | Trial described as single blind (no further details) | Trial described as single blind (no further details) | 5/12 (42%) patients withdrew during trial period, 4/5 of those during high-pressure ventilation. Stated that there were no significant differences between completers and withdrawers in terms of age, sex, anthropometrics, gas exchange, spirometry or ventilator settings, with the exception of FVC (46% completers, 69% withdrawers) | No apparent selective reporting | No details | Carryover effect could not be excluded owing to a relatively short washout period | No details, it appears dropouts were not included in analysis | Yes | Yes | ||
Appendix 3 Analysis methods
Meta-analyses primary outcomes
Calculation of relative risks
Most studies presented numbers of events but none reported the RR; these were therefore calculated from the raw data. Where numbers of events were calculated from percentages (for survival data), an assumption of an ITT analysis was made; that is, that proportions were based on total number of patients at the start of the study (no loss to follow-up). When graphs were presented, percentages were estimated from these.
Calculation of hazard ratios
Where HRs were reported in the published papers, these were presented in forest plots. Where a HR was reported showing the ratio of an increased risk from no NIV rather than a benefit from NIV, the HR and upper and lower confidence limits were reversed by taking the reciprocal values. When data on numbers of events and patients (aggregated interval-censored survival data) were reported, the log-HR was calculated using complementary log-log (Clog-log) regression. 173 This method assumes a constant event rate in each arm and hence proportional hazards. As the proportional hazards assumption is made, meta-analysis was only undertaken for studies with stable populations. One post-hospital population study (Budweiser et al. 200796) presents HRs for multiple follow-up times and it is clear from these and the presented Kaplan–Meier curve that the assumption of proportional hazards does not hold in this study.
In order to check the assumption of constant baseline rates, HRs calculated from Clog-log regression were compared with reported HRs when these were presented. This was the case for one RCT (McEvoy et al. 2009,74 stable population). Both HRs were found to be very similar (0.8 calculated and 0.82 reported) lending some credibility to this assumption in a stable population, albeit based on only one estimate.
Means and rates
Most hospital admissions data were presented as mean (SD) number of admissions per patient. When only the proportion was presented, the rates and SD were calculated. 174 An assumption of ITT analysis was made where patient numbers were not given.
Secondary outcomes
In order to use as many data as possible, SDs were calculated from SEs or CIs. Where units had to be converted (e.g. feet to metres, or mmHg to kPa), the correct mean and SD were found using a simple linear transformation. For some studies,80,99 results were only available by estimating from graphs.
Most studies reported results for completers only, and most analyses were therefore not on an ITT basis. In some studies it was uncertain whether baseline and/or follow-up values referred to completers only or all patients.
Note that for the study by Clini et al. (2002)99 the lower CI limit presented for the adjusted mean difference at 12 months (FEV % predicted) was thought to be inaccurate (–13.1) and was assumed to be –3.1. Further, the results are presented as usual care minus control (so a negative score indicates benefit from NIV); this has been inverted for the forest plot for consistency with the other studies. It is unclear what the treatment effect estimates for PaCO2 at 12 and 24 months reported in the paper refer to, so mean difference has been calculated from final scores.
The study by Kaminski et al. (1999)101 reports a SD of 1591 for 6MWD. This has been assumed to be inaccurate and 159 used instead.
Appendix 4 Excluded studies from the clinical effectiveness review
This appendix includes reference lists of excluded studies from the clinical effectiveness review, studies that could not be obtained and studies where further information was sought but not obtained.
Excluded studies
This section details the articles excluded from the review of clinical effectiveness with the reason they were not selected.
| Article | Reason for exclusion |
|---|---|
| Abramson MJ, Crockett AJ, Frith PA, McDonald CF. COPDX: an update of guidelines for the management of chronic obstructive pulmonary disease with a review of recent evidence. [Review]. Med J Aust 2006;184:342–5 | A |
| Adler D, Perrig S, Takahashi H, Espa F, Rodenstein D, Pepin JL, et al. Polysomnography in stable COPD under non-invasive ventilation to reduce patient-ventilator asynchrony and morning breathlessness. Sleep Breath 2012;16:1081–90 | E |
| Agusti AG, Barbe F, Togores B. Noninvasive ventilation. N Engl J Med 1998;338:1388–9 | A |
| Al JE, Fiorentino F, Reeves BC, Ind PW, Angelini GD, Kemp S, et al. Reply to the editor. J Thorac Cardiovasc Surg 2013;146:1299–300 | A |
| Alkhuja S. Effects of expiratory positive airway pressure on dynamic hyperinflation during exercise in patients with COPD. Respir Care 2013;58:e34 | A |
| Allan PF, Thomas KV, Ward MR, Harris AD, Naworol GA, Ward JA. Feasibility study of noninvasive ventilation with helium-oxygen gas flow for chronic obstructive pulmonary disease during exercise. Respir Care 2009;54:1175–82 | E |
| Ambrosino N, Clini E. Noninvasive ventilation in COPD patients with chronic respiratory failure – pro. Monaldi Arch Chest Dis 2000;55:54–7 | A |
| Ambrosino N, Nava S, Bertone P, Fracchia C, Rampulla C. Physiologic evaluation of pressure support ventilation by nasal mask in patients with stable COPD. Chest 1992;101:385–91 | E |
| Ambrosino N, Nava S, Torbicki A, Riccardi G, Fracchia C, Opasich C, et al. Haemodynamic effects of pressure support and PEEP ventilation by nasal route in patients with stable chronic obstructive pulmonary disease. Thorax 1993;48:523–8 | E |
| Ambrosino N, Vitacca M, Polese G, Pagani M, Foglio K, Rossi A. Short-term effects of nasal proportional assist ventilation in patients with chronic hypercapnic respiratory insufficiency. Eur Respir J 1997;10:2829–34 | E |
| Anon. Noninvasive positive pressure ventilation improves COPD patient outcomes. AHRQ Research Activities 2012;(386):18–19 | A |
| Anon. [NIPPV use increases, but mortality for some is also up]. AARC Times 2012;36:77 | A |
| Anon. Noninvasive ventilation has been shown to be ineffective in stable COPD – rebuttal. Am J Respir Crit Care Med 2000;161:691 | A |
| Anon. Summaries for patients. Noninvasive positive-pressure ventilation for severe worsening of chronic obstructive pulmonary disease. Ann Intern Med 2003;138:I27 | A |
| Anton A, Guell R, Tarrega J, Sanchis J. Non-invasive ventilation. Thorax 2002;57:919 | A |
| Anton A, Guell R. Home mechanical ventilation in COPD: do we know when and how to use it? Chest 2000;118:1525–6 | A |
| Aron C, Moutaux G. Non-invasive ventilation in acute or chronic respiratory failure: a comparison of volumetric ventilation. Rev Mal Respir 1999;16:181–7 | E |
| Aubier M, Muir JF, Robert D, Leger P, Langevin B, Benhamou D. [Chronic respiratory failure. Non-invasive methods of long-term ventilation]. Rev Mal Respir 1993;10:385–400 | A |
| Aufiero A, Citarella A, Cilotaj V, Cogliati A, Curto P, Damonte L, et al. Remeo Lago Maggiore: The first Italian specialized nursing home for ventilated patients’ long term care. Italian Journal of Medicine Conference: 19th Congresso Nazionale della Societa Scientifica FADOI, 10–13 May 2014, Bologna. Conference Publication: pp. 6–8 | E |
| Bai C. [Efficacy and safety of non-invasive ventilation in the management of chronic airway diseases]. Acta Anaesthesiol Ital 2008;59:6–17 | A |
| Bai CX. [A comparative study of the effects of control ventilation and synchronized intermittent mandatory ventilation on hemodynamic and blood gases in patients with chronic obstructive pulmonary disease]. Zhonghua Jie He He Hu Xi Za Zhi 1992;15:220–1 | E |
| Baliko Z. [Noninvasive mechanical ventilation in chronic obstructive lung diseases with acute or chronic respiratory failure]. Lege Artis Med 2006;16:625–30 | A |
| Barakat S, Michele G, Nesme P, Nicole V, Guy A. Effect of a noninvasive ventilatory support during exercise of a program in pulmonary rehabilitation in patients with COPD. Int J Chron Obstruct Pulmon Dis 2007;2:585–91 | E |
| Barbano L, Bertella E, Vitacca M. Episodic medical home interventions in severe bedridden chronic respiratory failure patients: a 4 year retrospective study. Monaldi Arch Chest Dis 2009;71:113–18 | D |
| Benditt JO. Noninvasive ventilation at the end of life. Respir Care 2000;45:1376–84 | A |
| Benhamou D, Muir JF, Raspaud C, Cuvelier A, Girault C, Portier F, et al. Long-term efficiency of home nasal mask ventilation in patients with diffuse bronchiectasis and severe chronic respiratory failure – a case–control study. Chest 1997;112:1259–66 | C |
| Berzin A, Chopin C, Fourrier F. Study of efficacy of ventilation in intermittent positive pressure with a mouth piece in patients with chronic obstructive lung disease. Lyon Med 1981;245:533–5 | E |
| Bianchi L, Foglio K, Pagani M, Vitacca M, Rossi A, Ambrosino N. Effects of proportional assist ventilation on exercise tolerance in COPD patients with chronic hypercapnia. Eur Respir J 1998;11:422–7 | E |
| Birnbaumer DM. Noninvasive ventilatory support – saving a life without intubation. West J Med 1998;168:182–3 | A |
| Boix JH, Tejeda M, Alvarez F, Ernesto E, Bertomeu F, Bano M. [Noninvasive ventilator support in patients with chronic obstructive pulmonary disease. A comparison of 2 methods]. Rev Clin Esp 1995;195:678–83 | E |
| Borel J-C, Burel B, Tamisier R, as-Domingos S, Baguet J-P, Levy P, et al. Comorbidities and mortality in hypercapnic obese under domiciliary noninvasive ventilation. PLOS ONE 2013;8:e52006 | C |
| Borghi SA, Mendes RG, Sampaio LMM, de-Souza HCD, Tania ST, Costa D. Noninvasive ventilation is better than oxygen supplementation to improve performance during a physical training program in COPD patients – a randomized study. [Abstract]. European Respiratory Society Annual Congress, Barcelona, 18–22 September 2010; 3651 | E |
| Borghi-Silva A, Di TL, Pantoni CB, Mendes RG, Salvini TF, Costa D. Non-invasive ventilation improves peripheral oxygen saturation and reduces fatigability of quadriceps in patients with COPD. Respirology 2009;14:537–44 | E |
| Borghi-Silva A, Mendes RG, Toledo AC, Malosa Sampaio LM, da Silva TP, Kunikushita LN, et al. Adjuncts to physical training of patients with severe COPD: oxygen or noninvasive ventilation? Respir Care 2010;55:885–94 | E |
| Borghi-Silva A, Mendes RG, Toledo AC, Sampaio LMM, da Silva TP, Kunikoshita LN, et al. Different adjuncts during physical training in severe chronic obstructive pulmonary disease patients: oxygen or non-invasive ventilation? Am J Respir Crit Care Med 2010;181:A6502. American Thoracic Society International Conference, ATS 2010, New Orleans, LA, 14–19 May 2010; various page numbers | E |
| Borghi-Silva A, Reis MS, Mendes RG, Pantoni CB, Simoes RP, Martins LE, et al. Noninvasive ventilation acutely modifies heart rate variability in chronic obstructive pulmonary disease patients. Respir Med 2008;102:1117–23 | E |
| Braghiroli A, Sacco C, Erbetta M, Ruga V, Donner CF. Overnight urinary uric acid: creatinine ratio for detection of sleep hypoxemia. Validation study in chronic obstructive pulmonary disease and obstructive sleep apnea before and after treatment with nasal continuous positive airway pressure. Am J Respir Crit Care Med 1993;148:173–8 | D, E |
| Branthwaite MA. Mechanical ventilation for stable COPD: Alternative, adjunct or sequel to LTOT. Eur Respir Rev 1992;2:346–7 | A |
| Briones Claudett KH, Briones Claudett M, Chung Sang Wong M, Nuques Martinez A, Soto Espinoza R, Montalvo M, et al. Noninvasive mechanical ventilation with average volume assured pressure support (AVAPS) in patients with chronic obstructive pulmonary disease and hypercapnic encephalopathy. BMC Pulm Med 2013;12 | E |
| Brooker AS, Carcone S, Witteman W, Krahn M. Quantitative patient preference evidence for health technology assessment: a case study. Int J Technol Assess Health Care 2013;29:290–300 | E |
| Bruge P, Jabre P, Dru M, Jbeili C, Lecarpentier E, Khalid M, et al. An observational study of noninvasive positive pressure ventilation in an out-of-hospital setting. Am J Emerg Med 2008;26:165–9 | E |
| Cabrini L, Moizo E, Nicelli E, Licini G, Turi S, Landoni G, et al. Noninvasive ventilation outside the intensive care unit from the patient point of view: a pilot study. Respir Care 2012;57:704–9 | E |
| Calverley PM. Domiciliary ventilation in chronic obstructive lung disease. Thorax 1992;47:334–6 | A |
| Carrey Z, Gottfried SB, Levy RD. Ventilatory muscle support in respiratory failure with nasal positive pressure ventilation. Chest 1990;97:150–8 | E |
| Carron M, Freo U, Bahammam AS, Dellweg D, Guarracino F, Cosentini R, et al. Complications of non-invasive ventilation techniques: A comprehensive qualitative review of randomized trials. Br J Anaesth 2013;110:896–914 | E |
| Chakrabarti B, Sulaiman MI, Davies L, Calverley PM, Warburton CJ, Angus RM. A study of patient attitudes in the United Kingdom toward ventilatory support in chronic obstructive pulmonary disease. J Palliat Med 2009;12:1029–35 | D, E |
| Chandra K, Blackhouse G, McCurdy B, Bornstein M, Campbell K, Costa V, et al. Cost-effectiveness of interventions for chronic obstructive pulmonary disease (COPD) using an Ontario policy model. Ont Health Technol Assess Ser 2012;12:1–61 | E |
| Chandramouli S, Molyneaux V, Angus RM, Calverley PMA, Chakrabarti B. Insights into chronic obstructive pulmonary disease patient attitudes on ventilatory support. Curr Opin Pulm Med 2011;17:98–102 | A |
| Chatwin M, Heather S, Hanak A, Polkey MI, Wilson B, Simonds AK. Analysis of emergency helpline support for home ventilator dependent patients: Risk management and workload. Eur Respir Rev 2008;17:33–5 | B |
| Chevrolet JC, Rossi JM, Chatelain G, Pahud C, Rochat T, de Haller R, et al. [Intermittent mechanical ventilation as home care]. Ther Umsch 1989;46:697–708 | C |
| Chodri TA, Groth ML. Nocturnal ventilation with BiPAP in severe COPD. Clin Pulm Med 2001;8:190–1 | A |
| Christensen HR, Simonsen K, Lange P, Clementsen P, Kampmann JP, Viskum K, et al. PEEP-masks in patients with severe obstructive pulmonary disease: A negative report. Eur Res J 1990;3:267–72 | D |
| Claman DM, Piper A, Sanders MH, Stiller RA, Votteri BA. Nocturnal noninvasive positive pressure ventilatory assistance. Chest 1996;110:1581–8 | A |
| Clini E, Ambrosino N. Noninvasive ventilation in stable chronic obstructive pulmonary disease. Eur Res J 2003;21:558–9 | A |
| Clini E, Sturani C, Rossi A, Viaggi S, Corrado A, Donner CF, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. [Erratum published in Eur Res J 2002;20:529–38.] Eur Respir J 2002;20:1617 | A |
| Clini EM, Ambrosino N. Nonpharmacological treatment and relief of symptoms in COPD [Review]. Eur Res J 2008;32:218–28 | A |
| Clini EM, Magni G, Crisafulli E, Viaggi S, Ambrosino N. Home non-invasive mechanical ventilation and long-term oxygen therapy in stable hypercapnic chronic obstructive pulmonary disease patients: comparison of costs. Respiration 2009;77:44–50 | F |
| Collett AS, Rees PJ. Non-invasive ventilation at home in chronic obstructive pulmonary disease. Int J Clin Pract 2007;61:1434–6 | A |
| Cooper BG. Domiciliary NIPPV in COPD. Thorax 1999;54:91 | A |
| COPD Working Group. Long-term oxygen therapy for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012;12:1–64 | D |
| COPD Working Group. Pulmonary rehabilitation for patients with chronic pulmonary disease (COPD): An evidence-based analysis. Ont Health Technol Assess Ser 2012;12:1–75 | D |
| Corner E, Garrod R. Does the addition of non-invasive ventilation during pulmonary rehabilitation in patients with chronic obstructive pulmonary disease augment patient outcome in exercise tolerance? A literature review. Physiother Res Int 2010;15:5–15 | E |
| Cornil A. Long-term ventilatory assistance at home. Acta Clin Belg 1988;43:329–34 | A |
| Corrado A, Gorini M. Long-term negative pressure ventilation. Respir Care Clin N Am 2002;8:545–57 | A |
| Costa D, Toledo A, Silva AB, Sampaio LM. Influence of noninvasive ventilation by BiPAP on exercise tolerance and respiratory muscle strength in chronic obstructive pulmonary disease patients (COPD). Rev Latino-Am Enfermagem 2006;14:378–82 | E |
| Costes F, Agresti A, Court-Fortune, Roche F, Vergnon JM, Barthelemy JC. Noninvasive ventilation during exercise training improves exercise tolerance in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2003;23:307–13 | E |
| Criee C-P. Non-invasive ventilation (NIV) in COPD. Atemweg Lungenkrank 2008;34:69–72 | A |
| Criner GJ, Kreimer DT, Tomaselli M, Pierson W, Evans D. Financial implications of noninvasive positive pressure ventilation (NPPV). Chest 1995;108:475–81 | F |
| Criner GJ. Cost/benefit of noninvasive mechanical ventilation. Monaldi Arch Chest Dis 1998;53:358–9 | A |
| Crisafulli E, Manni G, Kidonias M, Trianni L, Clini EM. Subjective sleep quality during average volume assured pressure support (AVAPS) ventilation in patients with hypercapnic COPD: A physiological pilot study. Lung 2009;187:299–305 | E |
| Cuny J, Campagne G, Goldstein P, Assez N, Wiel E. Analysis of management of noninvasive ventilation support in prehospital care for COPD patients and short-term outcome. Chest 2013;888A. Conference: CHEST 2013 Chicago, IL, 26–31 October 2013 | E |
| Cuvelier A, Molano LC, Muir JF. [Domiciliary ventilation in patients with COPD]. Rev Mal Respir 2005;22:615–33 | A |
| Cuvelier A, Muir JF. [Instrumental treatment of chronic obstructive bronchopneumonia. The place of non-invasive ventilation]. Presse Med 2003;32:1283–90 | A |
| Cuvelier A, Muir J-F. [Indications of NPPV during chronic respiratory failure]. Rev Med Suisse 2003;61:650–5 | A |
| Cuvelier A, Muir J-F. Noninvasive ventilation and chronic respiratory failure: Indications and obstructive lung diseases. ERS 2001;6:187–203 | A |
| Cuvelier A, Muir JF. Noninvasive ventilation and obstructive lung diseases. Eur Respir J 2001;17:1271–81 | A |
| Diaz LS, Mayoralas AS. A network of networks for noninvasive mechanical ventilation. Arch Bronconeumol 2008;44:507 | A |
| Diaz O, Begin P, Andresen M, Prieto ME, Castillo C, Jorquera J, et al. Physiological and clinical effects of diurnal noninvasive ventilation in hypercapnic COPD. Eur Respir J 2005;26:1016–23 | E |
| Diaz O, Begin P, Torrealba B, Jover E, Lisboa C. Effects of noninvasive ventilation on lung hyperinflation in stable hypercapnic COPD. Eur Respir J 2002;20:1490–8 | E |
| Diaz O, Ramos J, Gallardo J, Torrealba B, Lisboa C. Non-invasive mechanical ventilation in patients with severe stable COPD: mechanisms underlying their clinical and physiologic effects. Rev Med Chil 1999;127:647–54 | E |
| Dreher M, Doncheva E, Schwoerer A, Walterspacher S, Sonntag F, Kabitz HJ, et al. Preserving oxygenation during walking in severe chronic obstructive pulmonary disease: noninvasive ventilation versus oxygen therapy. Respiration 2009;78:154–60 | E |
| Dreher M, Dreher M. Author’s reply: High intensity noninvasive positive pressure ventilation and long-term pulmonary function responses in severe stable COPD. A delicate and difficult balance. COPD 2014;11:361–2 | A |
| Dreher M, Ekkernkamp E, Walterspacher S, Walker D, Schmoor C, Storre JH, et al. Noninvasive ventilation in COPD: impact of inspiratory pressure levels on sleep quality. Chest 2011;140:939–45 | E |
| Dreher M, Storre JH, Windisch W. Noninvasive ventilation during walking in patients with severe COPD: a randomised cross-over trial. Eur Respir J 2007;29:930–6 | E |
| Dres M, Tran T-C, Aegerter P, Rabbat A, Guidet B, Huchon G, et al. The authors’ reply. Crit Care Med 2014;42:e250 | A |
| Duiverman ML, Struik FM, Wijkstra PJ. Noninvasive ventilation in severe stable COPD: is it effective, and if so, in what way? Eur Respir J 2008;31:1136–7 | A |
| Dvorakova V, Sedlak J, Cimbalova J. Possible use of positive pressure oxygen inhalation in chronic bronchopulmonary obstruction. Vnitr Lek 1988;34:807–12 | E |
| Dwarakanath A, Elliott MW. Noninvasive ventilation in the management of acute hypercapnic respiratory failure. Breathe 2013;10:339–48 | A |
| Dyer F, Flude L, Bazari F, Jolley C, Englebretsen C, Lai D, et al. Non-invasive ventilation (NIV) as an aid to rehabilitation in acute respiratory disease. BMC Pulm Med 2011;11:58 | E |
| Elliott M. Domiciliary NIV for COPD: where are we now? Lancet 2014;2:672–3 | A |
| Elliott MW, Aquilina R, Green M, Moxham J, Simonds AK. A comparison of different modes of noninvasive ventilatory support: effects on ventilation and inspiratory muscle effort. Anaesthesia 1994;49:279–83 | E |
| Elliott MW, Mulvey DA, Moxham J, Green M, Branthwaite MA. Inspiratory muscle effort during nasal intermittent positive pressure ventilation in patients with chronic obstructive airways disease. Anaesthesia 1993;48:8–13 | E |
| Elliott MW. Domiciliary non-invasive ventilation in stable COPD? Thorax 2009;64:553–6 | A |
| Elliott MW. Non-invasive ventilation during sleep. Sleep 2000;23:S209–12 | A |
| Elliott MW. Non-invasive ventilation in chronic obstructive pulmonary disease. Br J Hosp Med (Lond) 1997;57:83–6 | A |
| Elliott MW. Noninvasive ventilation in chronic obstructive pulmonary disease. N Engl J Med 1995;333:870–1 | A |
| Elliott MW. Noninvasive ventilation in chronic ventilatory failure due to chronic obstructive pulmonary disease. Eur Respir J 2002;20:511–14 | A |
| Elliott MW. Noninvasive ventilation in COPD patients with chronic respiratory failure--con. Monaldi Arch Chest Dis 2000;55:58–61 | A |
| Esquinas AM, Malacarne P, Mina B. Noninvasive ventilation at the end of life: and now? Intensive Care Med 2013;39:2063–4 | A |
| Esquinas AM, Matsuoka Y, Stieglitz S. Predicting survival after acute exacerbation chronic obstructive pulmonary disease (ACOPD): is long-term application of noninvasive ventilation the last life guard? Int J Chron Obstruct Pulmon Dis 2013;8:379–81 | A |
| Esquinas AM, Petroianni A. High intensity positive pressure ventilation and long term pulmonary function responses in severe stable COPD. A delicate and difficult balance. COPD 2014;11:359–60 | A |
| Esquinas AM, Scala R, Nasilowski J. Inspiratory pressure during noninvasive ventilation in stable COPD: help the lungs, but do not forget the heart. Eur Respir J 2013;41:764–5 | A |
| Esquinas AM, Siscaro G, Clini EM. Noninvasive mechanical ventilation with high pressure strategy remains a ‘double edged sword’? Int J Chron Obstruct Pulmon Dis 2013;8:255–6 | A |
| Esquinas AM, Ucar ZZ, Kirakli C. Deventilation syndrome in severe COPD patients during long-term noninvasive mechanical ventilation: poor sleep pattern, hyperinflation, or silent chronic muscular fatigue? Sleep Breath 2014;18:225–6 | A |
| Esquinas Rodriguez AM, Kirakli C. Algorithm for NIV in chronic obstructive pulmonary disease: could this be applicable for every scenario? Physiotherapy 2013;99:181 | A |
| Esquinas Rodriguez AM, Scala R, Ambrosino N. Shrinking the room for invasive mechanical ventilation in acute chronic hypercapnic respiratory failure: yes, but must be sure to have opened windows for noninvasive ventilation. Int J Chron Obstruct Pulmon Dis 2013;8:313–14 | A |
| Fabbri M, Fasano L, Rocca A, Gunella G. Setting the iron lung in hypercapnic patients with COPD. Lotta Contro Tuberc Malat Polm Sociali 1993;63:346–9 | E |
| Fanfulla F, Taurino AE, Lupo ND, Trentin R, D’Ambrosio C, Nava S. Effect of sleep on patient/ventilator asynchrony in patients undergoing chronic non-invasive mechanical ventilation. Respir Med 2007;101:1702–7 | E |
| Fiorenza D, Vitacca M, Clini E. Hospital monitoring, setting and training for home non invasive ventilation. Monaldi Arch Chest Dis 2003;59:119–22 | A |
| Funk GC, Breyer MK, Burghuber OC, Kirchheiner K, Schmidt I, Hartl S. Nocturnal non-invasive positive pressure ventilation (NIPPV) in stable hypercapnic COPD patients – a randomized controlled trial. Wien Klin Wochenschr 2008;120:A19 | B |
| Gaber KA, Barnett M, Planchant Y, McGavin CR. Attitudes of 100 patients with chronic obstructive pulmonary disease to artificial ventilation and cardiopulmonary resuscitation. Palliat Med 2004;18:626–9 | D, E |
| Gabrielli A, Caruso LJ, Layon AJ, Antonelli M. Yet another look at noninvasive positive-pressure ventilation. Chest 2003;124:428–31 | A |
| Garcia-Delgado M, Colmenero M. Response to Rodriguez and Feltracco: Effectiveness of noninvasive ventilation: The mystery is in the bottom line. J Cardiothorac Vasc Anesth 2012;26:e74–5 | A |
| Garvey C. Sleep waves. Treating sleep disorders in the COPD patient. AARC Times 2007;31:12,14,16,17 | A |
| Giacomini M, Dejean D, Simeonov D, Smith A. Experiences of living and dying with COPD: a systematic review and synthesis of the qualitative empirical literature. Ont Health Technol Assess Ser 2012;12:1–47 | D |
| Gigliotti F, Spinelli A, Duranti R, Gorini M, Goti P, Scano G. Four-week negative pressure ventilation improves respiratory function in severe hypercapnic COPD patients. Chest 1994;105:87–94 | E |
| Girault C. [Indications for noninvasive ventilation]. Rev Mal Respir 2004;21:7S168–70 | A |
| Goldstein RS, Psek JA, Gort EH. Home mechanical ventilation – demographics and user perspectives. Chest 1995;108:1581–6 | F |
| Gulati A, Ali M, Oscroft N, Davies MG, Quinnell T, Shneerson JM, et al. A randomised trial of autovariable positive airway pressure vs standard non-invasive ventilation in patients with COPD with chronic ventilatory failure. Thorax 2009;A29. British Thoracic Society, BTS Winter Meeting 2009 London, 2–4 December 2009 | B |
| Gungor G, Karakurt Z, Adiguzel N, Aydin RE, Balci MK, Salturk C, et al. The 6-minute walk test in chronic respiratory failure: does observed or predicted walk distance better reflect patient functional status? Respir Care 2013;58:850–7 | D |
| Hannink JDC, Hees HWH, Dekhuijzen PNR, Helvoort HAC, Heijdra YF. Non-invasive ventilation abolishes the IL-6 response to exercise in muscle-wasted COPD patients: A pilot study. Scand J Med Sci Sports 2014;24:136–43 | E |
| Herth F, Schulz MR, Schulz V. [Course of pulmonary hemodynamics in intermittent ventilation]. Pneumologie 1999;53:S120–1 | E |
| Heyer L, Lorino H, Delclaux C, Isabey D, Harf A, Lofaso F. Carbon dioxide respiratory response during positive inspiratory pressure in COPD patients. Respir Physiol 1997;109:29–37 | E |
| Highcock MP, Shneerson JM, Smith IE. Increased ventilation with NiIPPV does not necessarily improve exercise capacity in COPD. Eur Respir J 2003;22:100–5 | E |
| Hill N. Noninvasive ventilation for COPD. J Respir Care Pract 1997;10:45–6 | A |
| Hill NS. Noninvasive ventilation has been shown to be ineffective in stable COPD. Am J Respir Crit Care Med 2000;161:689–90 | A |
| Hodgkin JE, Zorn EG. Intermittent positive pressure breathing (IPPB) in the outpatient management of chronic obstructive pulmonary disease: description of the NIH clinical trial. Respir Care 1981;26:1095–104 | D |
| Hormann C. [Noninvasive ventilation]. Journal für Kardiologie 2004;11:11–15 | A |
| Idell S. Managing end-stage COPD with positive-pressure ventilation. J Crit Illn 1998;13:731–4 | A |
| Jarry J, Hournau M, Rault A, Collet D. Gastric rupture secondary to non invasive ventilation. Gastroenterol Clin Biol 2009;33:492–3 | A |
| Ji S. [Objective evaluation of non-invasive mechanical ventilation]. Zhonghua Jie He He Hu Xi Za Zhi 1996;19:259–60 | E |
| Johnson JE, Gavin DJ, Adams-Dramiga S. Effects of training with heliox and noninvasive positive pressure ventilation on exercise ability in patients with severe COPD. Chest 2002;122:464–72 | E |
| Jones SE, Packham SM, Hebden MH, Smith AP. Domiciliary NIPPV in COPD – reply. Thorax 1999;54:91 | A |
| Kallet RH, Diaz JV. The physiologic effects of noninvasive ventilation. Respir Care 2009;54:102–15 | E |
| Karakurt S, Fanfulla F, Nava S. Is it safe for patients with chronic hypercapnic respiratory failure undergoing home noninvasive ventilation to discontinue ventilation briefly? Chest 2001;119:1379–86 | E |
| Karg O, Geiseler J. [Hot topics in COPD: Home mechanical ventilation – What are the facts?] Atemweg Lungenkrank 2012;38:376–80 | A |
| Karg O. [Long-term home mechanical ventilation: Indications and results]. Atemweg Lungenkrank 2002;28:482–6 | A |
| Katz-Papatheophilou E, Heindl W, Gelbmann H, Hollaus P, Neumann M. Effects of biphasic positive airway pressure in patients with chronic obstructive pulmonary disease. Eur Respir J 2000;15:498–504 | E |
| Khan N, Monday D. Non-invasive ventilation in management of end-stage COPD: the implications for palliative care. Eur J Palliat Care 2012;19:218–21 | A |
| Klein F. [Noninvasive ventilation makes training in COPD patients more effective]. Pneumologie 2007;61:623 | A |
| Klein G, Matthys H, Costabel U. [Oxygen therapy vs. intermittent positive pressure respiration in the long-term treatment of chronic obstructive pulmonary disease (author’s transl)]. Pneumologie 1981;35:528–31 | D |
| Klein G, Ruhle KH, Matthys H. Long-term oxygen therapy vs. IPPB therapy in patients with COLD and respiratory insufficiency: survival and pulmonary hemodynamics. Eur J Respir Dis 1986;146:S409–15 | D |
| Kohnlein T, Schonheit-Kenn U, Winterkamp S, Welte T, Kenn K. Noninvasive ventilation in pulmonary rehabilitation of COPD patients. Respir Med 2009;103:1329–36 | E |
| Kohnlein T, Welte T. Non-invasive ventilation as long-term treatment for respiratory insufficiency in severe COPD. Nothing is really proven. Pneumologie 2002;56:770–2 | A |
| Kohnlein T, Welte T. Noninvasive ventilation in stable chronic obstructive pulmonary disease. Eur Respir J 2003;21:558–9 | A |
| Kollef MH. Non-invasive ventilation for chronic obstructive pulmonary disease. Lancet 2000;356:956–7 | A |
| Kolodziej MA, Jensen L, Rowe B, Sin D. Noninvasive ventilation in severe stable COPD: is it effective, and if so, in what way? Eur Respir J 2008;31:1136–7 | A |
| Koutsenko M, Shogenova L, Novikov K, Chuchalin A. Comparison of heliox therapy and non invasive positive pressure ventilation (NPPV) in patients with COPD and hypercapnic respiratory failure (HRF). Eur Respir J 2000;16:14s | B |
| Koutsogiannidis C-P, Ananiadou OG. Tailoring noninvasive ventilation management in non-ICU settings and the cardiac surgery context. Respir Care 2014;59:303–4 | A |
| Krishna B, Sampath S, Moran J. Authors’ reply. Indian J Crit Care Med 2014;18:51 | A |
| Kruse N, Bulow HH. [Non-invasive ventilation of patients with respiratory insufficiency. Experiences after the first six months’ use]. Ugeskr Laeger 2003;165:4020–5 | E |
| Kyroussis D, Polkey MI, Hamnegard CH, Mills GH, Green M, Moxham J. Respiratory muscle activity in patients with COPD walking to exhaustion with and without pressure support. Eur Respir J 2000;15:649–55 | E |
| Laier-Groeneveld G, Criee CP. [Intermittent ventilation in chronic obstructive pulmonary disease]. Med Klin (Munich) 1996;91:2–6 | A |
| Laier-Groeneveld G, Huttemann U, Criee CP. [Non-invasive intermittent self-ventilation as therapy of chronic respiratory failure]. Med Klin (Munich) 1991;86:229–33 | E |
| Laier-Groeneveld G, Schucher B, Criee CP. [The etiology of chronic hypercapnia]. Med Klin (Munich) 1997;92:S33–8 | E |
| Laier-Groeneveld G. [Non-invasive ventilation in chronic obstructive lung disease]. Intensiv- Notfallbehandl 1994;19:103–4 | A |
| Langmack EL, Make BJ. Survival of individuals receiving long-term mechanical ventilation. Respir Care Clin N Am 2002;8:355–77 | A |
| Lavergne F, Bravard A, Molano L, Portier F, Muir J, Cuvelier A. Clinical profile and characteristics of domiciliary ventilation in patients with overlap syndrome (COPD+OSAS). Am J Respir Crit Care Med 2010;A3057. American Thoracic Society International Conference, ATS 2010 New Orleans, LA, 14–19 May 2010 | C |
| Leger P, Bach JR, Sortor L, Kacmarek RM, Hill NS, Decker, et al. Noninvasive positive pressure ventilation at home. Respir Care 1994;39:501–14 | A |
| Liebler JM. Noninvasive positive pressure mechanical ventilation. West J Med 1998;168:529 | A |
| Lien TC, Wang JH, Huang SH, Chen SD. Comparison of bilevel positive airway pressure and volume ventilation via nasal or facial masks in patients with severe, stable COPD. Chin Med J 2000;63:542–51 | E |
| Lin C-C. Comparison between nocturnal nasal positive pressure ventilation combined with oxygen therapy and oxygen monotherapy in patients with severe COPD. Am J Respir Crit Care Med 1996;154:3EX SET | E |
| Lloyd-Owen SJ, Donaldson GC, Ambrosino N, Escarabill J, Farre R, Fauroux B, et al. Patterns of home mechanical ventilation use in Europe: Results from the Eurovent survey. Eur Respir J 2005;25:1025–31 | A |
| Lukacsovits J, Nava S. Inspiratory pressure during noninvasive ventilation in stable COPD: help the lungs, but do not forget the heart. Eur Respir J 2013;41:765–6 | A |
| Luo H, Cheng P, Zhou R. [Sequential BiPAP following invasive mechanical ventilation in COPD patients with hypercapnic respiratory failure]. Hunan Yi Ke Da Xue Xue Bao 2001;26:563–5 | E |
| Ma Z, Huai Y-J, Zhang S-M. [Clinical analysis of the effect of non-invasive mechanical ventilation in 519 cases of respiratory failure]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2009;21:632–3 | E |
| Manning SA. Both sides now. J Am Geriatr Soc 2014;62:777 | A |
| Marangoni S, Vitacca M, Quadri A, Schena M, Clini E. Non-invasive haemodynamic effects of two nasal positive pressure ventilation modalities in stable chronic obstructive lung disease patients. Respiration 1997;64:138–44 | E |
| Marrades RM, Rodriguez RR. COPD and non invasive ventilation. Arch Bronconeumol 2001;37:88–95 | A |
| Mauri T, Bellani G, Salerno D, Mantegazza F, Pesenti A. Regional distribution of air trapping in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;188:1466–7 | A |
| McCloskey M, Sharkey R, Gallagher S, Kelly MG, Daly JG. The two year survival for patients with chronic obstructive pulmonary disease who undergo non-invasive ventilation. Thorax 2005;60:II69 | E |
| McCurdy B. Action plans for individuals with chronic obstructive pulmonary disease (COPD): a rapid review. Health Quality Ontario; 2013. URL: www.hqontario.ca/Portals/0/Documents/eds/rapid-reviews/action-plans-copd-130111-en.pdf (accessed September 2015) | D |
| Menadue C, Alison JA, Piper AJ, Flunt D, Ellis ER. Non-invasive ventilation during arm exercise and ground walking in patients with chronic hypercapnic respiratory failure. Respirology 2009;14:251–9 | E |
| Menadue C, Piper AJ, Van’t Hul AJ, Wong KK. Non-invasive ventilation during exercise training for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2009;2:CD007714 | A |
| Mihaltan F, Ulmeanu R, Ungureanu D, Stoica R, Macri A, Halic E, et al. [First experiences of non-invasive positive-pressure ventilation at home in chronic respiratory failure in Romania]. Pneumologia 2002;51:51–3 | D |
| Mikelsons C, Muncey TK, Wedzicha JA. Chronic obstructive pulmonary disease patients’ experience of using non-invasive ventilation at home: a case study approach. Thorax 2006;61:II116 | B |
| Miro RE, Fernandez-Montes CV, Ramos PD, Martinez LPDL, Anon MM, Jimenez JFM, et al. Guidelines for domiciliary mechanical ventilation. Arch Bronconeumol 2001;37:142–9 | A |
| Molfino NA. Treatment of patients with chronic obstructive pulmonary disease (COPD) by primary care physicians. Medicina (B Aires) 2004;64:445–54 | A |
| Moloney E, Kiely JL, McDonnell T, McNicholas WT. Nocturnal nasal intermittent positive pressure ventilation (NIPPV) therapy for chronic respiratory failure: long-term effects. Ir Med J 1999;92:401–3 | C |
| Muir J-F, Cuvelier A, Heliot P, Ozenne G, Paillotin D, Sauvage B, et al. [Ambulatory treatment of hypercapnic chronic obstructive bronchopneumopathy with intermittent negative pressure breathing]. Rev Kinesither 1990;142:43–4 | E |
| Muir J-F, Voisin C, Ludot A. Organization of home respiratory care: the experience in France with ANTADIR. Monaldi Arch Chest Dis 1993;48:462–7 | A |
| Muir J-F. [Home mechanical ventilation in France]. Rev Mal Respir 2004;21:461–4 | A |
| Muir J-F. Home mechanical ventilation. Thorax 1993;48:1264–73 | A |
| Murphy P, Hart N. Who benefits from home mechanical ventilation? Clin Med 2009;9:160–3 | A |
| Murphy PB, Brignall K, Moxham J, Polkey MI, Davidson AC, Hart N. Authors’ reply. Int J Chron Obstruct Pulmon Dis 2013;8:257–8 | A |
| Murphy PB, Brignall K, Moxham J, Polkey MI, Davidson AC, Hart N. Re: Noninvasive mechanical ventilation with high pressure strategy remains a ‘double edged sword’? Int J Chron Obstruct Pulmon Dis 2013;8:257–8 | A |
| Nava S, Ambrosino N, Rubini F, Fracchia C, Rampulla C, Torri G, et al. Effect of nasal pressure support ventilation and external PEEP on diaphragmatic activity in patients with severe stable COPD. Chest 1993;103:143–50 | E |
| Nava S, Ceriana P. Patient–ventilator interaction during noninvasive positive pressure ventilation. Respir Care Clin N Am 2005;11:281–93 | A |
| Nava S, Fanfulla F, Frigerio P, Navalesi P. Physiologic evaluation of 4 weeks of nocturnal nasal positive pressure ventilation in stable hypercapnic patients with chronic obstructive pulmonary disease. Respiration 2001;68:573–83 | E |
| Neme JY, Gutierrez AM, Santos MC, Beron M, Ekroth C, Arcos JP, et al. Physiologic effects of noninvasive ventilation in patients with chronic obstructive pulmonary disease. Arch Bronconeumol 2007;43:150–5 | D, E |
| Nuila MDL, Ho TBL. Re-admission rate for non-invasive ventilation in patients with acute hypercapnic respiratory failure in a UK district general hospital. Am J Respir Crit Care Med 2010;A3051. American Thoracic Society International Conference, ATS 2010 New Orleans, LA, 14–19 May 2010 | E |
| O’Donoghue FJ, Howard ME, O’Donoghue FJ, Howard ME. Obesity, COPD, NIV and reverse epidemiology. Respirology 2014;19:777–9 | A |
| Oh S, O’Carroll G, Akintola A, Byrne D. BIPAP – Too little, too late? Ir Med J 2014:107:1 | E |
| Oliveira CC, Carrascosa CR, Borghi-Silva A, Berton DC, Queiroga F, Jr., Ferreira EM, et al. Influence of respiratory pressure support on hemodynamics and exercise tolerance in patients with COPD. [Erratum published in Eur J Appl Physiol 2010;109:1219. Note: Alberto Neder, J (corrected to Neder, J Alberto)]. Eur J Appl Physiol 2010;109:681–9 | E |
| Olofson J, Dellborg C, Sullivan M, Midgren B, Caro O, Bergman B. Qualify of life and palliation predict survival in patients with chronic alveolar hypoventilation and nocturnal ventilatory support. Qual Life Res 2009;18:273–80 | C |
| Ortega GA, Peces-Barba RG, Fernandez OI, Chumbi FR, Cubero de FN, Gonzalez MN. [Evolution of patients with chronic obstructive pulmonary disease, obesity hypoventilation syndrome or congestive heart failure in a respiratory monitoring unit]. Arch Bronconeumol 2006;42:423–9 | E |
| Osadnik C, Stuart-Andrews C, Ellis S, Thompson B, McDonald CF, Holland AE. Positive expiratory pressure does not improve ventilation inhomogeneity in individuals with copd and chronic sputum expectoration. Respirology 2013;18:55 | E |
| Oscroft N, Pilsworth S, Quinnell T, Shneerson J, Smith I. Does long term domiciliary non-invasive ventilation improve survival in severe hypercapnic chronic obstructive pulmonary disease? Thorax 2005;60:II21–2 | B |
| Padkao T, Boonsawat W, Jones CU. Conical-PEP is safe, reduces lung hyperinflation and contributes to improved exercise endurance in patients with COPD: a randomised cross-over trial. J Physiother 2010;56:33–9 | D, E |
| Pankow W, Becker H, Kohler U, Schneider H, Penzel T, Peter JH. [Patient–ventilator interaction during noninvasive pressure supported spontaneous respiration in patients with hypercapnic COPD]. Pneumologie 2001;55:7–12 | E |
| Pastaka C, Kostikas K, Karetsi E, Tsolaki V, Antoniadou I, Gourgoulianis KI. Non-invasive ventilation in chronic hypercapnic COPD patients with exacerbation and a pH of 7.35 or higher. Eur J Int Med 2007;18:524–30 | E |
| Perrin C, Wolter P, Berthier F, Tamisier R, Jullien V, Lemoigne F, et al. [Comparison of volume preset and pressure preset ventilators during daytime nasal ventilation in chronic respiratory failure]. Rev Mal Respir 2001;18:41–8 | E |
| Pessoa IM, Costa D, Velloso M, Mancuzo E, Reis MA, Parreira VF. Effects of noninvasive ventilation on dynamic hiperinflation of patients with COPD during activities of daily living with upper limbs. Revista Brasileira de Fisioterapia 2012;16:61–7 | E |
| Poggi R, Appendini L, Polese G, Colombo R, Donner CF, Rossi A. Noninvasive proportional assist ventilation and pressure support ventilation during arm elevation in patients with chronic respiratory failure. A preliminary, physiologic study. Respir Med 2006;100:972–9 | E |
| Polese G, Vitacca M, Bianchi L, Rossi A, Ambrosino N. Nasal proportional assist ventilation unloads the inspiratory muscles of stable patients with hypercapnia due to COPD. Eur Respir J 2000;16:491–8 | E |
| Polkey MI, Hawkins P, Kyroussis D, Ellum SG, Sherwood R, Moxham J. Inspiratory pressure support prolongs exercise induced lactataemia in severe COPD. Thorax 2000;55:547–9 | E |
| Polkey MI, Kyroussis D, Mills GH, Hamnegard CH, Keilty SE, Green M, et al. Inspiratory pressure support reduces slowing of inspiratory muscle relaxation rate during exhaustive treadmill walking in severe COPD. Am J Respir Crit Care Med 1996;154:1146–50 | E |
| Porszasz J, Cao R, Morishige RJ, Eykern LA, Stenzler A, Casaburi R. Effect of a portable noninvasive ‘open’ ventilation system on respiratory muscle activity during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:A2565 | E |
| Porta R, Appendini L, Vitacca M, Bianchi L, Donner CF, Poggi R, et al. Mask proportional assist vs pressure support ventilation in patients in clinically stable condition with chronic ventilatory failure. Chest 2002;122:479–88 | E |
| Porta R, Vitacca M, Clini E, Ambrosino N. Physiological effects of posture on mask ventilation in awake stable chronic hypercapnic COPD patients. Eur Respir J 1999;14:517–22 | E |
| Qiao H, Li W-Y, Hou T-H, Chen Y-H. Influence of continuous positive airway pressure treatment on the hemorheology of patients with chronic obstructive pulmonary disease and sleep apnea syndrome. Chin J Clin Rehabil 2005;9:64–5 | D |
| Qu Y, Peng H, Chen P, Xiang X. Combination of chest physiotherapy and intermittent non-invasive mechanical ventilation for chronic obstructive pulmonary disease patients with respiratory failure. Zhong Nan da Xue Xue Bao Yi Xue Ban 2009;34:655–8 | E |
| Quill CM, Quill TE. Palliative use of noninvasive ventilation: navigating murky waters. J Palliat Med 2014;17:657–61 | A |
| Racca F, Squadrone V, Ranieri VM. Patient–ventilator interaction during the triggering phase. Respir Care Clin N Am 2005;11:225–25 | A |
| Rasche K, Hader C, Leidag M, Duchna H-W, Bauer TT, Orth M. Noninvasive ventilation in COPD. Atemweg Lungenkrank 2004;30:579–83 | A |
| Renston JP, DiMarco AF, Supinski GS. Respiratory muscle rest using nasal BiPAP ventilation in patients with stable severe COPD. Chest 1994;105:1053–60 | E |
| Reuveny R, Ben-Dov I, Gaides M, Reichert N. Ventilatory support during training improves training benefit in severe chronic airway obstruction. IMAJ 2005;7:151–5 | E |
| Rochester CL, Maltais F. Innovate to ambulate: creating opportunities for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;188:265–7 | A |
| Rodriguez AME, Ioannis P. A link of non-invasive ventilation in chronic hypercapnic COPD patients with pulmonary hypertension. A new index of prognosis. Eur Rev Med Pharmacol Sci 2012;16:1133–4 | A |
| Rolin S. What’s hot that the other lot got. Thorax 2014;69:304 | A |
| Rossi A, Ambrosino N. Non-invasive ventilatory support in COPD patients: Benefits or harm? Acta Anaesthesiol Scand 1998;42:21–2 | A |
| Sauret JM, Guitart AC, Rodriguez-Frojan G, Cornudella R. Intermittent short-term negative pressure ventilation and increased oxygenation in COPD patients with severe hypercapnic respiratory failure. Chest 1991;100:455–9 | E |
| Scarpazza P, Incorvaia C, Melacini C, Cattaneo R, Bonacina C, Riario-Sforza GG, et al. Shrinking the room for invasive ventilation in hypercapnic respiratory failure. Int J Chron Obstruct Pulmon Dis 2013;8:135–7 | E |
| Scarpazza P, Incorvaia C, Melacini C, Cattaneo R, Bonacina C, Riario-Sforza GG, et al. Authors’ response. Int J Chron Obstruct Pulmon Dis 2013;8:315 | A |
| Schaefer IL, Dorschner S. [‘Quality-of-life means acting independently’. How do COPD-patients experience non-invasive ventilation? A qualitative study.] Pflege 2005;18:159–68 | F |
| Schaefer IL. What does quality-of-life mean to artificially ventilated COPD outpatients in respect of their everyday life? Pneumologie 2004;58:865–6 | F |
| Schmidt MJ, Laier-Groeneveld G, Criee CP. Fitness training during nasal ventilation in diseases limited by dyspnea. Pneumologie 1999;53:S109–12 | E |
| Schonhofer B, Dellweg D, Suchi S, Kohler D. Exercise endurance before and after long-term noninvasive ventilation in patients with chronic respiratory failure. Respiration 2008;75:296–303 | E |
| Schonhofer B. Choice of ventilator types, modes, and settings for long-term ventilation. Respir Care Clin N Am 2002;8:419–45 | A |
| Schonhofer B. Non-invasive positive pressure ventilation in patients with stable hypercapnic COPD: light at the end of the tunnel? Thorax 2010;65:765–7 | A |
| Schwartz SW, Rosas J, Iannacone MR, Foulis PR, Anderson WM. Correlates of a prescription for Bilevel positive airway pressure for treatment of obstructive sleep apnea among veterans. J Clin Sleep Med 2013;9:327–35 | C |
| Segrelles CG, Zamora GE, Ancochea J. Reply. Arch Bronconeumol 2014;50:80–1 | A |
| Shaikh H, Laghi F. Eating on noninvasive ventilation*. Crit Care Med 2014;42:737–8 | A |
| Shoikhet I, Markin AV. [The specific features of respiratory support in patients with obstructive sleep apnoea syndrome concurrent with chronic obstructive pulmonary disease (crossing-syndrome)]. Ter Arkh 2010;82:26–8 | C, E |
| Simoes Saldanha MM, Ferreira C, Dias C, Moita J. Ethical limits for noninvasive ventilation prescription. Rev Port Pneumol 2014;20:281–2. | C, E |
| Simonds AK. Home ventilation. Eur Respir J 2003;47:S38–46 | A |
| Simonds AK. Home ventilation. Curr Anaesth Crit Care 1994;5:236–42 | A |
| Simonds AK. Long-term ventilation in obstructive ventilatory disorders. Respir Care Clin N Am 2002;8:533–44 | A |
| Smith R. NPPV in end-stages of COPD. J Respir Care Pract 2000;13:51–2 | A |
| Sorensen D, Frederiksen K, Groefte T, Lomborg K. Nurse-patient collaboration: a grounded theory study of patients with chronic obstructive pulmonary disease on non-invasive ventilation. Int J Nurs Stud 2013;50:26–33 | E |
| Stanton MW. Increased use of noninvasive ventilation could save more lives for patients with chronic obstructive pulmonary disease. Ala Nurse 2014;41:5. URL: www.ahrq.gov/news/newsletters/research-activities/14feb/0214RA13.html (accessed September 2015) | A |
| Steier J, Teschler H. Non-invasive ventilation should be initiated in chronic hypercapnic COPD – Pro. Atemweg Lungenkrank 2005;31:255–61 | A |
| Stevens P, Abeles R, Hodgkin JE. Intermittent positive pressure breathing therapy of chronic obstructive pulmonary disease. A clinical trial. Ann Intern Med 1983;99:612–20 | D |
| Storre JH, Bohm P, Dreher M, Windisch W. Clinical impact of leak compensation during non-invasive ventilation. Respir Med 2009;103:1477–83 | E |
| Storre JH, Huttmann SE, Ekkernkamp E, Walterspacher S, Schmoor C, Dreher M, et al. Oxygen supplementation in noninvasive home mechanical ventilation: the crucial roles of CO2 exhalation systems and leakages. Respir Care 2014;59:113–20 | E |
| Struik FM, Duiverman ML, Bladder G, Wijkstra PJ. Effects of non-invasive positive pressure ventilation (NIPPV) in stable chronic obstructive pulmonary disease (COPD). Respir Med 2008;4:94–100 | A |
| Strumpf DA, Carlisle CC, Millman RP, Smith K, Hill N. An evaluation of the respironics BiPAP bi-level CPAP device for delivery of assisted ventilation. Respir Care 1990;35:415–22 | A |
| Summers RL, Patch J, Kolb JC. Effect of the initiation of noninvasive bi-level positive airway pressure on haemodynamic stability. Eur J Emerg Med 2002;9:37–41 | E |
| Sun J-H, Han N, Wu X-Y. Noninvasive positive pressure ventilation in exacerbations of chronic obstructive pulmonary disease: a systematic review. Chinese Journal of Evidence-Based Medicine 2008;8:1112–21 | E |
| Sunwoo BY, Mulholland M, Rosen IM, Wolfe LF. The changing landscape of adult home noninvasive ventilation technology, use, and reimbursement in the United States. Chest 2014;145:1134–40 | A |
| Takechi Y, Endo N, Fuse M, Maruyama Y, Matsunaga A. Domiciliary non-invasive positive pressure ventilation (NPPV) care. Gan to Kagaku Ryoho 2009;36:S110–12 | A |
| Tintignac A, Gonzalez-Bermejo J. Follow-up and management of non-invasive home mechanical ventilation. Rev Pneumol Clin 2009;65:237–247 | A |
| Titlestad IL, Lassen AT, Vestbo J. Long-term survival for COPD patients receiving noninvasive ventilation for acute respiratory failure. Int J Chron Obstruct Pulmon Dis 2013;8:215–19 | E |
| Titlestad IL, Lassen AT, Vestbo J. Re: Predicting survival after acute exacerbation chronic obstructive pulmonary disease (ACOPD): Is long-term application of noninvasive ventilation the last life guard? Int J Chron Obstruct Pulmon Dis 2013;8:381 | A |
| Toledo A, Borghi-Silva A, Sampaio LM, Ribeiro KP, Baldissera V, Costa D. The impact of noninvasive ventilation during the physical training in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD). Clinics (Sao Paulo, Brazil) 2007;62:113–120 | E |
| Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax 2003;58:867–71 | E |
| Tuggey JM, Plant PK, Elliott MW. Economic analysis of the use of home NIV for severe COPD. Thorax 2001;56:10–11 | B |
| van Kesteren RG, Kampelmacher MJ, Dullemond-Westland AC, van Leyden LW, Verwey-van den Oudenrijn LP, Douze JM. [Favorable results of nocturnal nasal positive-pressure ventilation in 64 patients with neuromuscular disorders; 5-year experience]. Ned Tijdschr Geneeskd 1994;138:1864–8 | C |
| Vanpee D, Clause D, Delaunois L, Nava S. Non-invasive mechanical ventilation. Thorax 2001;56:666 | A |
| Vitacca M, Lanini B, Nava S, Barbano L, Portal R, Clini E, et al. Inspiratory muscle workload due to dynamic intrinsic PEEP in stable COPD patients: effects of two different settings of non-invasive pressure-support ventilation. Monaldi Arch Chest Dis 2004;61:81–5 | E |
| Waldhorn RE. Nocturnal nasal intermittent positive pressure ventilation with bi-level positive airway pressure (BiPAP) in respiratory failure. Chest 1992;101:516–21 | C |
| Wang X, Zhu H. [Effect of nasal bi-level positive airway pressure on 29 chronic obstructive pulmonary disease patients with chronic respiratory failure]. Medical Journal of Wuhan University 2006;27:664–6 | E |
| Ward K. Algorithm for NIV in chronic obstructive pulmonary disease: could this be applicable for every scenario? Response. Physiotherapy 2013;99:181–2 | A |
| Wedzicha JA, Meecham-Jones DJ. Domiciliary ventilation in chronic obstructive pulmonary disease: where are we? Thorax 1996;51:455–7 | A |
| Wedzicha JA. Long-term ventilatory support. Monaldi Arch Chest Dis 1998;53:317–20 | A |
| Wedzicha JA. Noninvasive positive pressure ventilation for chronic obstructive lung diseases. Monaldi Arch Chest Dis 1999;54:79–82 | A |
| Whyte KF. Home nocturnal ventilation for chronic respiratory failure – a proven treatment? N Z Med J 1998;111:155–6 | A |
| Winck JC, Vitacca M, Morais A, Barbano L, Porta R, Teixeira-Pinto A, et al. Tolerance and physiologic effects of nocturnal mask pressure support vs proportional assist ventilation in chronic ventilatory failure. Chest 2004;126:382–8 | E |
| Windisch W. Home mechanical ventilation: who cares about how patients die? Eur Respir J 2010;35:955–7 | A |
| Windisch W. [Noninvasive ventilation in patients with COPD]. Journal fur Anasthesie und Intensivbehandlung 2003;10:269–70 | A |
| Yazici M, Uzun K, Ulgen MS, Teke T, Maden E, Kayrak M, et al. The acute effect of bi-level positive airway pressure on heart rate variability in chronic obstructive pulmonary disease patients with hypercapnic respiratory failure. Anadolu Kardiyol Derg 2008;8:426–30 | E |
| Zhang YZ. Treatment of BiPAP breathing machine for COPD combined with respiratory failure of type 2. Chin J Integr Med 2008;17:358–9 | E |
| Zhou M-H, Zhou S-L, Zhou M. [A retrospective study of noninvasive positive-pressure ventilation in chronic obstruction pulmonary disease combined with respiratory failure]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2009;21:247 | E |
Unobtainable studies
This section details the 16 articles which could not be obtained to allow assessment against the selection criteria for the review of clinical effectiveness.
| Article |
|---|
| Anon. Non-invasive Positive Pressure Ventilation in Patients with Chronic Respiratory Failure. Switzerland: Lausanne; 2007 |
| Borghi SA, Sampaio LMM, Toledo A, Pincelli MP, Costa D. [Acute effects of BiPAP application on physical exercise tolerance among chronic obstructive pulmonary disease patients]. Revista Brasileira de Fisioterapia 2005;9:273–80 |
| Briones Claudett KH. Influence of F(IO2) on Pa(CO2) during noninvasive ventilation in patients with COPD: what will be constant over time? Respir Care 2014;59:e104–5 |
| Chatburn RL. Simulation studies for device evaluation. Respir Care 2014;59:e61–6 |
| Ishihara H. [Non-invasive ventilation for chronic respiratory failure]. Respiration and Circulation 2003;51:15–20 |
| Jones LD, Park JG. Simulation studies for device evaluation. Hosp Med Clin 2014;3:e149–61 |
| Lierl D, Chaney M. Nocturnal bilevel ventilation for the COPD patient. J Respir Care Pract 2000;13:59–60,62,64,(19 ref) |
| Liu J, Xiong SD. Effectiveness of BiPaP for pulmonary hemodynamics and output of sodium and potassium in urine in chronic obstructive pulmonary disease. J Clin Internal Med 1999;15:73 |
| Mihaltan F, Deleanu O, Ulmeanu R, Nemes R, Mihaltan F, Deleanu O, et al. Non-invasive positive pressure ventilation in stable COPD: has it any role? [Review]. Pneumologia 2012;61:183–7 |
| Muir J-F, Cuveller A. Long-term invasive ventilation in the home. Int J Respir Care 2006;2:48–53 |
| Perruchoud A, Tschan M, Kopp C, Blum V, Herzog H. [Rehabilitation program with IPPB-home treatment in severe chronic obstructive lung disease: hospitalizations and cost analysis]. Schweizerische Medizinische Wochenschrift 1980;110:229–31 |
| Pires DL, V, Silva AB, Sampaio LMM, Jamami M, Oishi J, Costa D. Effects of the physical and respiratory muscular training in patients suffering from severe chronic obstructive pulmonary disease submitted to BiPAP. Revista Brasileira de Fisioterapia 2003;7:69–76 |
| Raffenberg M, Muller-Pawlowski H, Geerdes-Fenge H, Petri M, Schaberg T, Loddenkemper R, et al. [Indications, methods and results of intermittent positive pressure ventilation at home]. Atemwegs- und Lungenkrankheiten 1997;23:138–41 |
| Servera E, Crescencio V, Vergara P, Pasrez D, Gonzalez M, Diaz J, et al. Non-invasive ventilation in patients with decompensated COPD: initial daily administration versus day and night. Archivos de Bronconeumología 1997;33:4 |
| Servera E. Chronic obstructive lung disease: Non-invasive mechanical ventilation. Archivos de Bronconeumología 2003;39:S35–38 |
| Singh S. Is non-invasive positive pressure ventilation the answer to chronic obstructive pulmonary disease? British Journal of Intensive Care 2003;13:114–18 |
| Teixeira C, Savi A, Tonietto TF, Teixeira C, Savi A, Tonietto TF. Influence of F(IO2) on Pa(CO2) during noninvasive ventilation in patients with COPD: what will be constant over time?--Reply. Influence of F(IO2) on Pa(CO2) in COPD patients with chronic CO2 retention--reply. Respir Care 2014;59:e106-e107 |
| Tsuboi T. Evidence based medicine in long-term NPPV. Respiration and Circulation 2003;51:47–56 |
| Tuggey JM, Elliott MW. Titration of non-invasive ventilation in COPD. Thorax 2002;57:S81 |
| Vargas F, Weissheimer KV, da Cunha LS, Filippin LI. [Noninvasive mechanical ventilation improves exercise tolerance in patients with severe-to-very severe chronic obstructive pulmonary disease]. Revista Inspirar Movimento Saude 2011;3:47–52 |
| Zhang JH, Gong L, Li X, Xin DG. Effect of Shenmai injection and bi-level positive airway pressure ventilation for daphragmatic activities in chronic obstructive pulmonary disease. Chin J Integr Med 1995;15:726–7 |
Studies where additional information was sought in order to aid selection decisions but not obtained
This section details the four articles for which the authors were contacted for additional information to aid selection decisions for the review of clinical effectiveness and where responses were not received.
| Article | Feature on which further information was required |
|---|---|
| Duan Y. Observation of non-invasive positive ventilation united inhalation treatment therapeutic effect on chronic obstructive pulmonary disease with type II respiratory failure. Respirology 2011;93–4. 16th Congress of the Asian Pacific Society of Respirology, Shanghai, 3–6 November, China. Conference | Setting |
| Ishihara, H. NPPV for chronic respiratory failure. Jpn J Chest Dis 2003;62:333–43 | Study design |
| Laier-Groeneveld G, Gietl C, Bauer JU. Normocapnia following noninvasive ventilation in acute exacerbations and chronic state of obstructive pulmonary disease. J Physiol Pharmacol 2007;58:S339–44 | Setting |
| Schucher B, Laier-Groeneveld G, Huttemann U, Criee CP. [Effects of intermittent self-ventilation on ventilatory drive and respiratory pump function]. Medizinische Klinik 1995;90:S13–16 | Setting |
Appendix 5 Uncontrolled studies meeting inclusion criteria for review of clinical effectiveness
This appendix details the characteristics of the 72 uncontrolled studies meeting the inclusion criteria for the review of clinical effectiveness.
| Study | Follow-up | Number of patients | Outcomes |
|---|---|---|---|
| Blankenburg et al. 2008175 | At least 4 weeks | 40 | Lung function, blood gases, 6MWD, SF-36 |
| Borel et al. 2014176 | Median 47.7 months | 118 | Composite of survival and hospitalisation and event-free survival |
| Budweiser et al. 2007102 | Mean 32 months | 188 | Survival, lung function, blood gases |
| Budweiser et al. 2007103 | 2 to 4 years | 98/231 | Survival, HRQoL (SRI), blood gases, lung function |
| Budweiser et al. 2006177 | Up to 12 months | 141 | BMI, blood gases, lung function |
| Carroll and Branthwaite 1988178 | Median 4 months | 4 | Blood gases, sleep time, spirometric values, exercise tolerance |
| Chatwin et al. 2010179 | 6 months | Approximately 182 (15% of 1211) | Number of calling emergency helpline, number of home visits, nature of technical issue |
| Criner et al. 1999180 | 6 months | 20/40 with COPD | Complications, discontinuation rates, compliance, FEV1, FVC |
| Duiverman et al. 2010181 (abstract) | 3 months | 11 | Respiratory muscle activity |
| Elliot et al. 1991182 | 6 months | 8 | Blood gases and respiratory parameters |
| Elliot et al. 1992183 | 1 year | 12 | Blood gases and respiratory parameters, QoL, sleep efficiency |
| Jones et al. 1998106 | Up to 2 years | 11 | Blood gases, hospital admissions, use of GP resources, survival |
| Khatun et al. 2011184 (abstract) | 12 months | 52/200 with COPD | Discontinuation rates and patterns |
| Kobayashi et al. 2005185 | Unclear (at least 3 months) | 8/80 with COPD | Survival, QoL (unclear what measure) |
| Nickol et al. 2008186 | 3 months | 19 | Blood gases, respiratory parameters |
| Oscroft et al. 2010105 | Up to 2 years | 35 | Blood gases, ventilatory parameters, compliance, survival |
| Perrin et al. 1997187 | 6 months | 14 | Lung function, QoL |
| Schönhofer et al. 2006188 | 2 months | 25 | Blood gases, compliance, inspiratory muscle strength |
| Schönhofer et al. 2003189 | 1 year | 13/33 with COPD | Pulmonary haemodynamics |
| Schönhofer et al. 1999190 | 1 year | 13/33 with COPD | Blood gases, pulmonary arterial pressure |
| Simonds and Elliott 1995191 | Up to 5 years | 33/180 with COPD | Pulmonary function, survival, discontinuation, SF-36 (cross-sectional survey in subset only) |
| Skobel et al. 2011108 | 3 months | 27/37 | Survival, hospital stay, acceptance of NIV, SGRQ, HADS |
| Storre et al. 2014192 (RCT, but only uncontrolled element relevant) | 3 months | 12 | Sleep quality, overnight gas exchange, tolerance, overnight pneumotachygraphic measurements, HRQoL, exercise capacity, lung function |
| Tsolaki et al. 2011107 | Up to 2 years | 35/91 with COPD | SF-36, exacerbations, hospital days, survival |
| Windisch 2008193 | 1 year | 27/85 | Blood gases, SF-36, SRI, side effects |
| Windisch et al. 2002194 | 6 months | 14 | Blood gases, respiratory muscle function, discontinuations |
| Study | Follow-up | Number of patients | Outcomes |
|---|---|---|---|
| Adams et al. 1998195 | Unclear | 40 | Prevalence, usage pattern-no clinical outcomes |
| Budweiser et al. 2008196 | Mean 25 months | 197 | 6MWD, survival |
| Budweiser et al. 2005197 | Up to 12 months | 46 | Blood gases, respiratory parameters, compliance, survival |
| Bullemer et al. 1996198 | Up to 1 year | 11/115 | Blood gases, survival |
| Charbonnier et al. 2014199 | 6-year period | Unclear for COPD and NIV | Pattern of NIV use and settings |
| Chu et al. 2004200 | Covers up to 36 months of NIV use | 121/249 with COPD (119 on NIV) | (Dis)continuation rates, deaths |
| Cuvelier et al. 2005201 | 1 year | 42/100 with COPD but only proportion with home NIV (15?) | Hospitalisation, mortality |
| Dale and Brocklebank 2012202 | 1 year | 20 | Blood gases, hospitalisation rates |
| Dellweg et al. 2007203 | 3 months | 305 | Blood gases, respiratory and lung function |
| Ekkernkamp et al. 2014204 | Median 22 months | 27 | Minute ventilation |
| Eller et al. 1989205 | Mean 64 months | 215 (results only for 78) | Dyspnoea, cough, sputum production, QoL, hospitalisation |
| Fernandez Alvarez et al. 2013206 | Cross-sectional | 7 | Leakage and blood gases |
| Ülger et al. 2014207 | Survey | Unclear (for NIV + COPD) | Usage and maintenance of devices |
| Garner et al. 2013208 | Cross-sectional | 218 (approximately) | Prevalence of home mechanical ventilation |
| Gay et al. 1991209 | Mean 14 months | 4/26 with COPD | Arterial blood gases, pulmonary function tests, discontinuation, adverse events |
| González-Rodríguez et al. 2005210 | 12 months | 23 | Blood gases, hospital admissions |
| Janssen et al. 2003211 | Median 24 months (up to 7 years) | 58/211 with COPD | Blood gases, days in hospital, discontinuation, compliance, survival |
| Kollert et al. 2013104 | Up to 12.5 years | 309 | Haemoglobin levels, survival |
| Laier-Groeneveld and Criee 1989212 and Criée et al. 1989213 | Up to 6 months | 4/15 with COPD | Hospital days, medication, exercise capacity |
| Leger et al. 1994214 | up to 3 years | 50/276 with COPD | Arterial blood gases, discontinuation, hospital days |
| López-Campos et al. 2008215 | 12 months | 15/115 | Dyspnoea, hospitalisation, HRQoL |
| McLaughlin and Keatings 2011216 (abstract) | 1 year | 30 | Admissions, bed-days, blood gases |
| Örnek et al. 2014217 | Median 17 months | 34 | Survival, hospitalisations |
| Pasquina et al. 2012218 | 2.5 years | 32/150 | Data recorded by ventilator software, leaks, compliance |
| Perrin et al. 2000219 | Mean 35 months | 24 | Blood gases, survival |
| Schönhofer and Kohler 1997220 | 3 months | 78 | Blood gases, acceptance/compliance, subjective improvement |
| Schucher et al. 1999221 | mean 13 months | 25 | Blood gases, lung function, acceptance |
| Schucher et al. 1999222 | 1 year | 54/111 | Blood gases, lung function, discontinuation/acceptance |
| Sivasothy et al. 1998223 | Median 17.5 months | 26 | Blood gases, survival, compliance, SF-36 |
| Sugino et al. 2008224 | Mean 2.3 years | 16 | Blood gases, survival |
| Takada et al. 2009225 | 11 years | 28 | Survival, days in hospital, risk factors for death/survival |
| Takada et al. 2008226 | Unclear (1 and 5 year survival rates) | 26/54 with COPD | Hospitalisation, survival |
| Thibout et al. 2006227 | Up to 5 years (?) | 37 | Blood gases, survival |
| Tollefsen et al. 2009228 | Cross-sectional | 272 | Prevalence of COPD patients using home NIV in different regions of Norway |
| Tsuboi et al. 2011229 | 2 years | 54 | Blood gases, hospitalisation rates |
| Tsuboi et al. 1996230 | Unclear (up to 11 year survival rates) | 6/41 with COPD | Survival, probability of continuation |
| Vitacca et al. 2000231 | Home NIV for 31 months | 23 | Blood gases, respiratory parameters |
| Waugh et al. 2001232 | Over previous 6 months | 36 | QoL (RQL) |
| Wiebel et al. 1999233 | Over 9 years | 29 | Blood gases, lung function parameters, survival |
| Wiebel et al. 1997234 | Over 8 years | 10/108 with COPD | Survival |
| Windisch et al. 2009109 | Up to 5 years | 73 | Physiological parameters, exacerbation rates, survival, hospitalisation |
| Windisch et al. 2008235 | Questionnaire refers to preceding week | 162 | SRI |
| Windisch et al. 200533 | 2 months | 34 | Blood gases, lung function |
| Windisch et al. 2003236 | Cross-sectional | 78/226 COPD | Blood gases, SF-36 |
| Windisch et al. 1997237 | Questionnaire looks at changes retrospectively but length of follow-up unclear | 20/72 COPD | QoL questionnaire, HADS, AQLQ |
Appendix 6 Details of non-invasive ventilation used in studies included in clinical effectiveness review
| Author | Mask | Target | IPAP (cmH2O) | EPAP (cmH2O) | NIV kit |
|---|---|---|---|---|---|
| Bhatt et al. 201383 | Full face | Pressure | Titrated to final pressure of 15 | Titrated to final pressure of 5 | BiPAP® Synchrony Ventilator (Respironics Inc, Murrysville, PA, USA) |
| Casanova et al. 200085 | Nasal | Pressure | At least 8 targeted, mean achieved 12 (SD 2) | At least 4 targeted (minimum of 4 achieved) | DP-90 (Bilevel pressure ventilation system) (Taema, Antony Cedex, France) |
| Cheung et al. 201090 | No details | Volume | 10–20 (as tolerated to target a tidal volume of 7–10 ml/kg) | 5 at start | BiPAP® Synchrony® (Respironics Inc, Murrysville, PA, USA) |
| Clini et al. 200299 | Nasal | Pressure | Set at maximum tolerated, average 14 (SD 3) | Set in range of 2–5, average 2 (SD 1) | BiPAP® ST30® ‘Auto-Trak’™ ventilator (Respironics Inc, Murrysville, PA, USA, distributed in Italy by Markos-Mefar, Air Liquide Group) |
| De Backer et al. 201191 | Full face | Blood gases | ‘Modes were adapted until O2 saturation was > 90% during 90% of the time and PaCO2 was decreased 5% in 1 hour’91 | ‘Modes were adapted until O2 saturation was > 90% during 90% of the time and PaCO2 was decreased 5% in 1 hour’91 | BiPAP® Synchrony® device (Respironics, Inc, Murrsville, PA, USA) |
| Duiverman et al. 200879 | Nasal (30%) or full face (70%) | Blood gases | Up to maximal tolerated pressure titrated towards an optimal correction of nocturnal arterial blood gases [mean 20 (SD 4) in completers and 18 (SD 1) in dropouts] | EPAP titrated on patient comfort. Mean 6 (SD 2) in completers and 5 (SD 1) in dropouts | BiPAP® spontaneous/timed mode (Respironics, Inc., Murrysville, PA, USA) (no further details) |
| Duiverman et al. 201180 | One patient with nasal mask, remaining with full-face mask | Blood gases | 23 (SD 4) at start of study | 6 (SD 2) at start of study | BiPAP® Synchrony® (Respironics, Inc., Murrysville, PA, USA) |
| Garrod et al. 200084 | Nasal | Pressure | Median 16 (range 13–24) | Median 4 (range 4–6) | BiPAP® ST30® ventilator (Respironics, Inc., Murrysville, PA, USA) |
| Gay et al. 1996100 | Nasal | Pressure | 10 (target level) | 2 (lowest possible) | BiPAP® (Respironics, Inc., Murrysville, PA, USA) |
| Kaminski et al. 1999101 | Nasal | Blood gases | Settings adjusted to decrease PaCO2, to increase SaO2 > 90% and to obtain maximum comfort for patients | Settings adjusted to decrease PaCO2, to increase SaO2 > 90% and to obtain maximum comfort for patients | Monnal D ventilator (Air Liquide Medical Systems, Antony,France) |
| Köhnlein et al. 201476 | Face or nasal mask according to judgement of investigator | Blood gases | Mean 21.6 (SD 4.7) | Mean 4.8 (SD 1.6) | Ventilators marketed post 2004 (ResMed, Martinsried, Germany; Weinmann, Hamburg, Germany; or Tyco Healthcare Neuburg, Germany) |
| McEvoy et al. 200974 | Choice of nasal or full-face mask and humidification | Pressure | Gradually increased to maximum tolerated (target of IPAP–EPAP difference of 10 or greater) | Lowest possible level (approximately 3) | VPAP™ S mode (ResMed, Sydney, Australia) |
| Meecham-Jones et al. 199577 | Nasal | Pressure | Median 18 (range 16–22) | Median 2 (none exceeding 4) | BiPAP® in S mode (Respironics, Inc., Murrysville, PA) |
| Murphy et al. 201178 | No details | Pressure | Discharge setting 26 (SD 3) | Discharge setting 5 (SD 1) | No details |
| Sin et al. 200782 | Choice of nasal or full-face mask | Pressure | Patients started on 8, then titrated up until the highest tolerated level or 20 was reached (whichever came first) | Set at 4 | VPAP II™ with heated humidifier (HumidAire, ResMed, Martinsried, Germany) |
| Struik et al. 201475 | Full-face mask | Pressure | Mean 19.2 (SD 3.4) at discharge | Mean 4.8 (SD 1.0) at discharge | BiPaP® Synchrony, (Respironics Inc., Murrysville, PA, USA) |
| Strumpf et al. 199135 | Nasal mask | Blood gases | Sufficient to maintain PETCO2 at least 5 mmHg below the spontaneous resting level. Mean 15 (SD 1) in completers | Set at 2 (lowest possible) | BiPaP® ventilator (Respironics, Inc.) |
| Xiang et al. 200792 | Nasal | Pressure | 16–20 at start then adjusted to patient | 2–4 at start then adjusted to patient | BiPAP® (Hoffrichter GmbH, Schwerin, Germany, or US-based company – unable to translate) |
| Zhou et al. 200881 | Full-face mask | Pressure | Mean 12–16 | Mean 2–4 | BiPAP® (Respironics, Inc., Murrysville, PA) |
| Author | Mask | Target | IPAP (cmH2O) | EPAP (cmH2O) | NIV kit |
|---|---|---|---|---|---|
| Budweiser et al. 200796 | Nasal, full face or custom made | Blood gases | Mean 21 (SD 4) | Mean 4.5 (SD 1.4) | Twin Air® (Airox Inc., Pau, France) (13/99), Smart Air® (Airox Inc., Pau, France) (14/99) or BiPAP® Synchrony ST® devices (Respironics Inc., Murrysville, PA) (51/99) or other |
| Clini et al. 199893 | Nasal | Volume | Minimal pressure to achieve an expiratory tidal volume > 8 ml/kg (range 10–16 ml/kg) | Set in order not to overcome the supposed intrinsic positive expiratory pressure (range 2–4) | BiPAP® (Respironics, Inc., Murrysville, PA, USA) |
| Clini et al. 199694 | Nasal | Volume | Minimal pressure to achieve an expiratory tidal volume > 8 ml/kg (range 10–16) | Range 0–2 | BiPAP® (Respironics, Inc., Murrysville, PA, USA) |
| Heinemann et al. 201197 | Nasal, oronasal or individual | Blood gases | Mean 22.7 (SD 4.3) mbar (= 23.15 cmH2O) | Mean 5 (SD 1.3) mbar (= 5.1 cmH2O) | No details |
| Laier-Groeneveld and Criee 199588 | Nasal or oronasal | Blood gases | To achieve adequate pO2 | No details | No details |
| Lu et al. 201298 | No details | Blood gases | Mean 18.0 (SD 2.0) | Mean 5.0 (SD 1.0) | BiPAP® Harmony (Respironics, Inc., Murrysville, PA, USA) |
| Milane and Jonquet 198586 | No details | No details | No details | No details | Bird® Mark 1, Bird® Mark 7, Portabird (Bird Products Corporation, Palm Springs, CA, USA) |
| Pahnke et al. 199787 | No details | No details | No details | No details | No details |
| Paone et al. 201489 | Nasal or full-face mask | Volume | Maximum inspiration pressure value tolerated by patients, able to ensure an exhaled tidal volume of 6 ml/kg (measured body weight) | Between 2 and 8 cmH2O | Neftis (Linde, Munich, Germany) or Synchrony (Philips Respironics, Andover MA, USA) |
| Tsolaki et al. 200895 | Full face | Pressure | Adjusted according to patient’s comfort and synchrony with the ventilator and a marked reduction in use of accessory muscles | Adjusted according to patient’s comfort and synchrony with the ventilator and a marked reduction in use of accessory muscles | VPAP™ III ST (ResMed, Sydney, Australia) |
| Author | Mask | Target | IPAP (cmH2O) | EPAP (cmH2O) | NIV kit |
|---|---|---|---|---|---|
| Murphy et al. 2012113 | Nasal or oronasal | Pressure | Mean 29 (SD 2) | Mean 5 (SD 3) | NIPPY3, NIPPY3 + (B&D ElectroMedical, Warwickshire, UK) |
| Dreher et al. 201032 | Nasal or oronasal | Blood gases | Mean 14.6 (SD 0.8) low intensity, mean 28.6 (SD 1.9) high intensity (mbar) | Mean 4.0 (SD 0) low intensity, mean 4.5 (SD 0.7) high intensity (mbar) | VIVO 40 (Breas Medical AB, Molnlycke, Sweden) or modified Smart Air® (Airox, Pau Cedex, France). Two patients used passive humidification |
| Oscroft et al. 2010112 | Unclear if nasal or full-face mask | Pressure | Va-NIV set to enable adjustment of inspiratory pressure up to 25, the maximum possible with this ventilator; pp-NIV set at similar pressure settings that the subject had previously used | Settings that the patients had previously used | Va-NIV: iVAPs (ResMed, Bella Vista, Australia); pp-NIV: VPAP™ III STA (ResMed, Bella Vista, Australia) |
Appendix 7 Exploratory analysis of carbon dioxide data and clinical outcomes
There is a discussion around whether or not the extent of hypercapnia at baseline or the change in hypercapnia status is related to the effectiveness of NIV. Performing subgroup analysis based on reported mean baseline CO2 values would have meant dichotomising trials based on an arbitrary threshold, and this was not considered appropriate. Therefore, CO2 levels at baseline and change in CO2 levels were plotted against mortality in a separate analysis in order to determine if baseline CO2 can predict response to NIV, and whether or not the effect of NIV on CO2 correlates with the effect on clinical outcomes.
Figures 42–45 show scatterplots of trial summary treatment effects (with 95% CIs) versus mean baseline CO2 and change in CO2 levels for stable and post-hospital populations. It should be noted that these are exploratory analyses only and as such results should be considered speculative and interpreted very cautiously. The analysis uses aggregate data for change in CO2 and also for clinical outcomes, and a causal association therefore cannot be inferred even if there is potential biological plausibility. Further caveats relate to the fact that not all trials contributed data to these analyses (particularly for the hospital admissions data) and that CO2 change scores were mostly not adjusted for baseline differences.
FIGURE 42.
Mortality (RR) and baseline CO2.
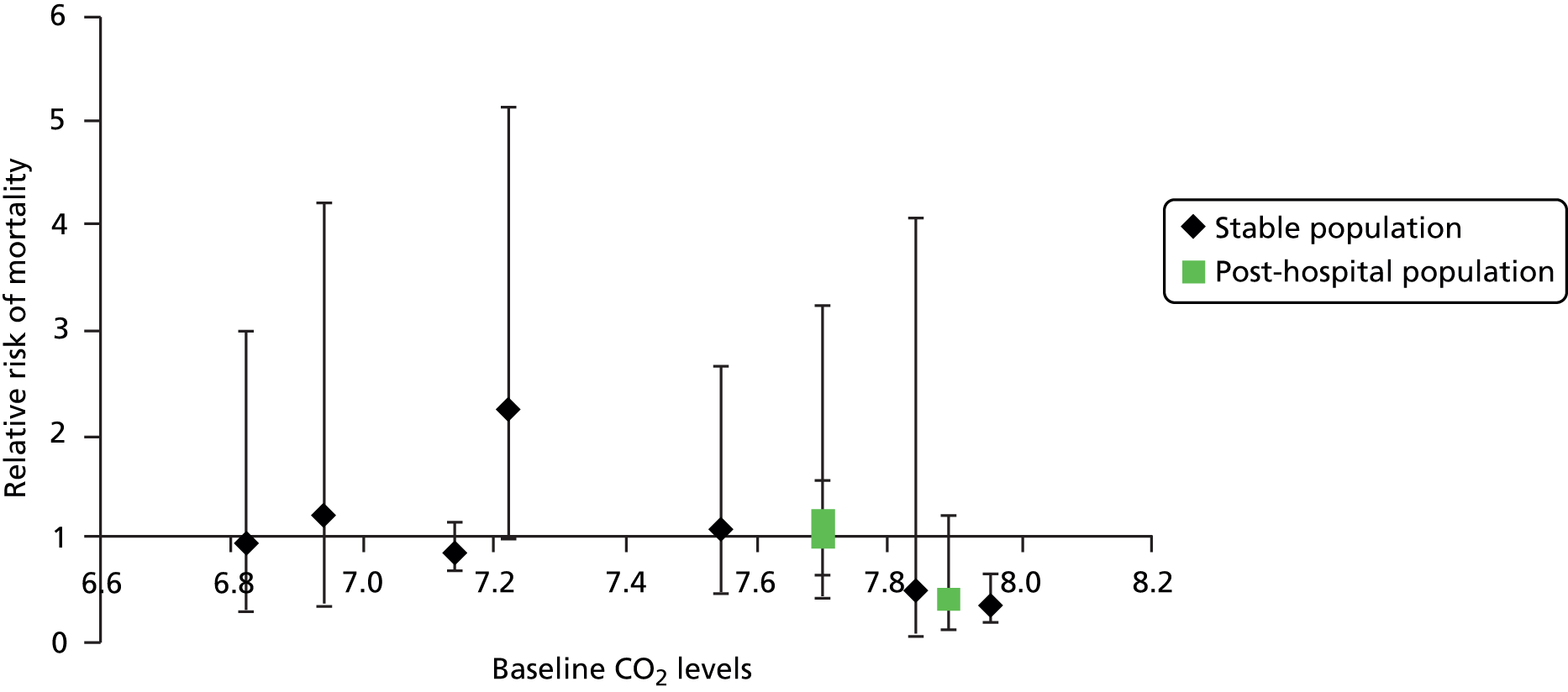
FIGURE 43.
Hospital admissions and baseline CO2.
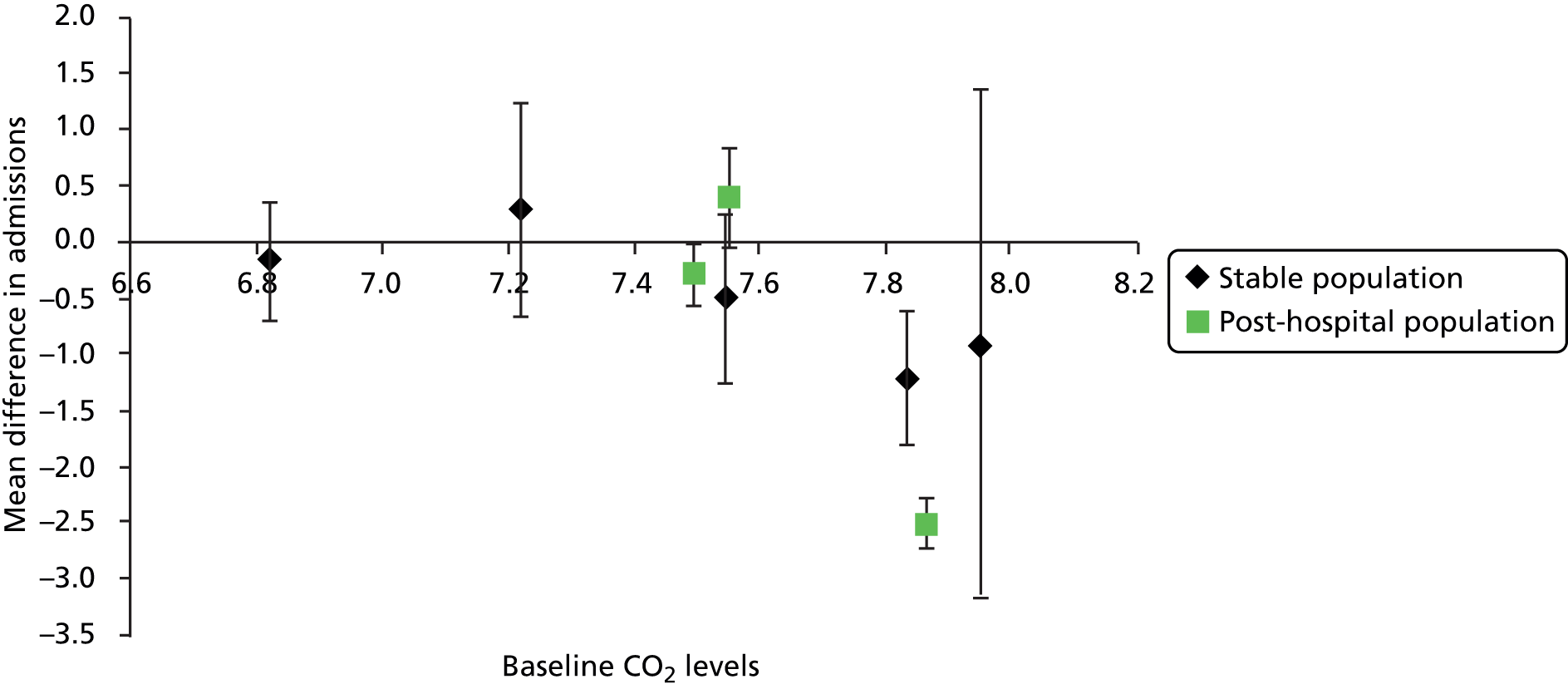
FIGURE 44.
Mortality (RR) and change in CO2.
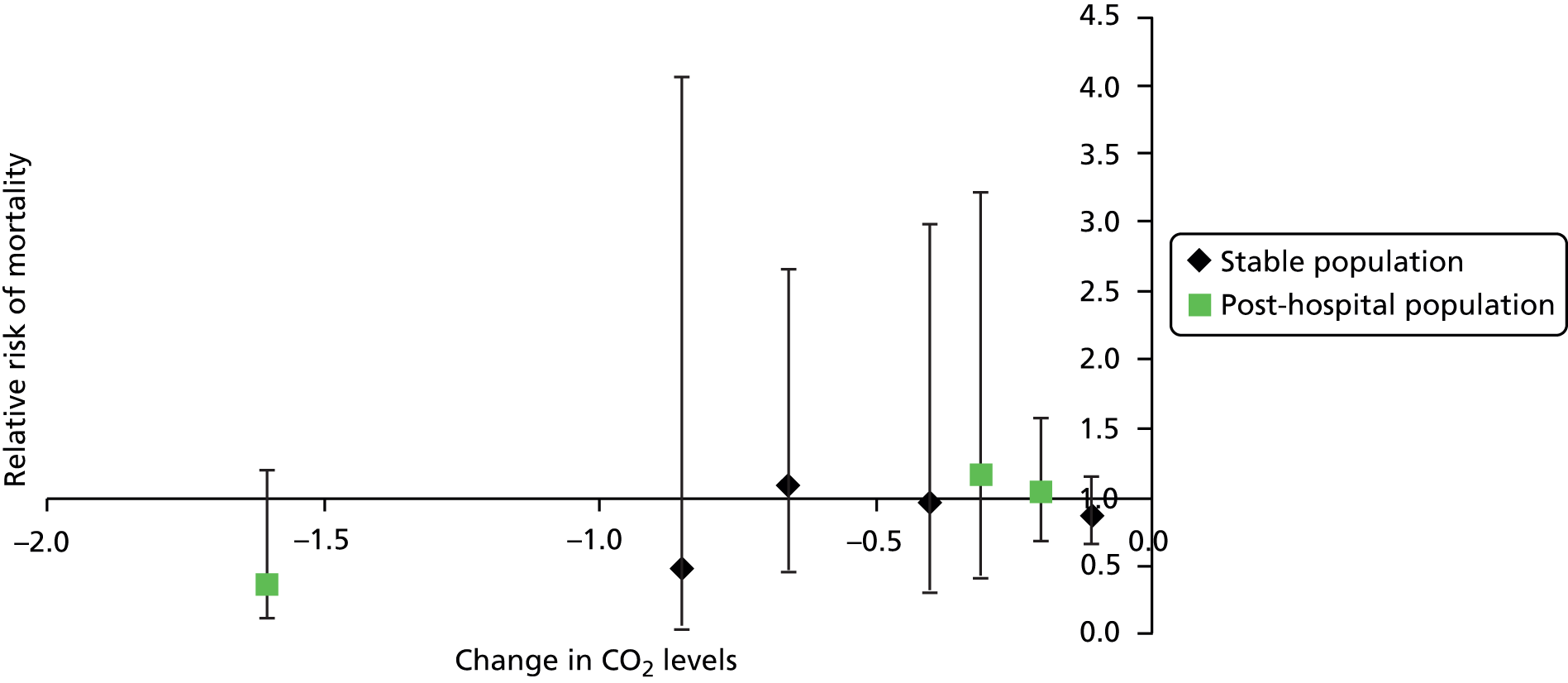
FIGURE 45.
Hospital admissions and change in CO2.
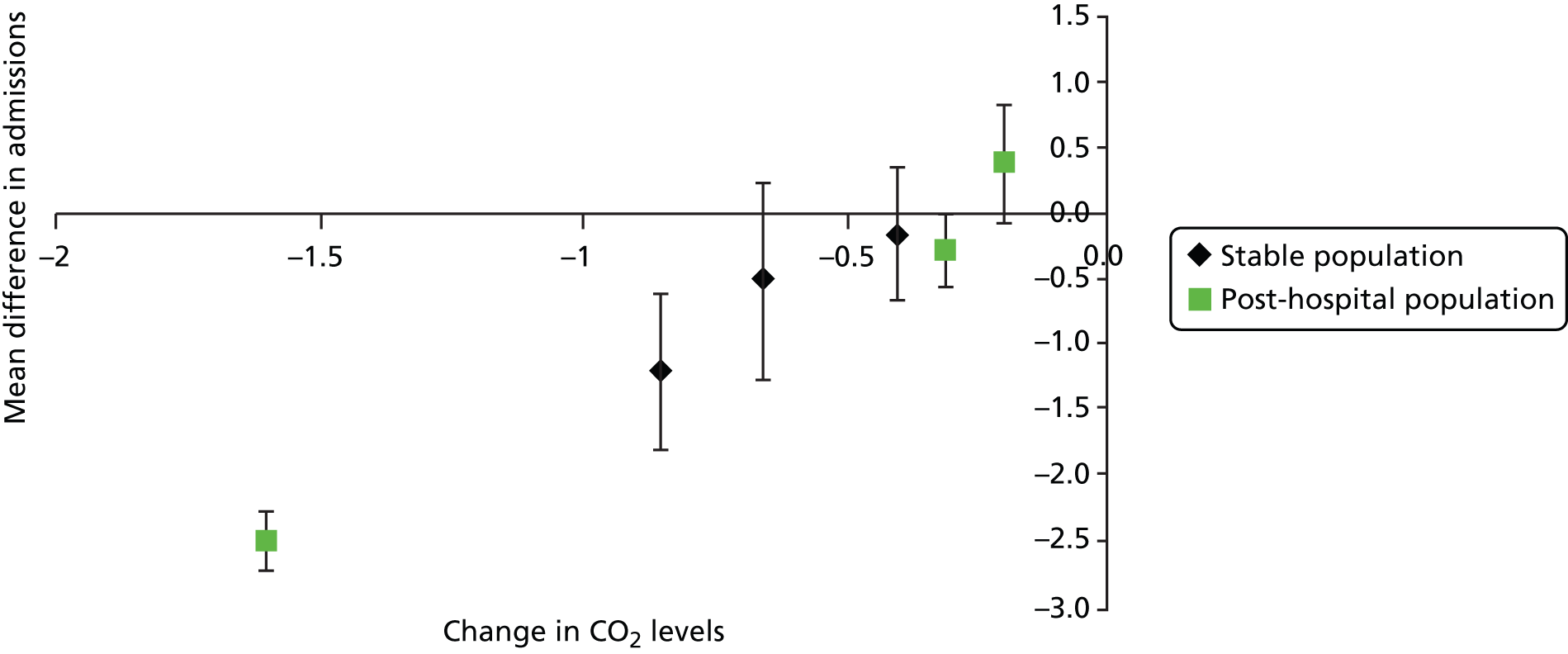
Appendix 8 Clinical effectiveness findings from selected uncontrolled studies
| Study | Patient numbers | Population | Length of follow-up | Survival rate |
|---|---|---|---|---|
| Budweiser et al. 2007102 | n = 188 | Stable. Only subjects with an event-free observation time ≥ 12 months were included | Up to 5 years. Mean 32 months | 1-year survival rate, 84.0% (n = 158 of 188 patients; 95% CI 69.3% to 96.3%); 2-year survival rate, 65.3% (n = 98 of 150 patients; 95% CI 49.5% to 82.8%); and 5-year survival rate, 26.4% (n = 28 of 106 patients; 95% CI 16.5% to 42.2%) |
| Budweiser et al. 2007103 | n = 98 | Participants had to be in a stable clinical condition without signs of current exacerbation or respiratory tract infection | 2–4 years. Mean observation 28.9 months (SD 8.8) months. Range 0.2–45.8 months | Overall mortality, 31.6% (31/98). Survival rates at 1 year 85.7% (SE 3.5%), 2 years 72.4% (SE 4.5%) and 3 years 65.3% (SE 5.3%) |
| Jones et al. 1998106 | n = 11 | Stable | Up to 2 years | Two patients died, one (patient 4) after 820 days and the other (patient 5) after 1522 days of NIPPV treatment, both of acute respiratory failure. Median survival is 920 days (range 686–1240 days). Small sample size |
| Oscroft et al. 2010105 | n = 31 (post-exacerbation), n = 16 (stable) | Post-exacerbation. Patients transferred to the unit from other hospitals following emergency admission and treatment for a severe exacerbation of COPD. Also compared with 16 stable patients on NIV | Up to 2 years | Median survival in the post-exacerbation patients was 28.6 months (95% CI 10.9 to 46.8 months), which was significantly poorer than that of the stable patients (28.6 vs. 52.4, p = 0.03). Survival in the post-exacerbation group was 68% at 1 year and 55% at 2 years. Those who were intolerant of NIPPV (n = 4) had a survival of 0.5 years (95% CI 0 to 1 years). An ITT analysis of all post-exacerbation patients offered NIPPV following exacerbation (n = 35) shows a 1-year survival of 63% and median survival of 21.1 months (95% CI 5.7 to 36.6 months) |
| Tsolaki et al. 2011107 | n = 35 | Stable. Patients with acute respiratory failure (pH < 7.35 and symptoms such as increasing cough, purulent sputum, need for antibiotics) or patients with an exacerbation during 4 weeks preceding recruitment were excluded | Up to 2 years | The survival rate after 2 years of follow-up was 88.6% in the COPD group |
| Study | Patient numbers | Population | Length of follow-up | Survival rate |
|---|---|---|---|---|
| Kollert et al. 2013104 | n = 309 (207 normocythaemic) | Appear to be stable (no mention of hospitalisation) | Up to 12.5 years | Survival rates for normocythaemic patients (n = 207) were 72% at 2 years, 50% at 4 years, 47% at 5 years and 18% at 10 years (approximate, estimated from graph) |
| Study | Patient numbers | Population | Length of follow-up | Hospitalisations |
|---|---|---|---|---|
| Jones et al. 1998106 | n = 11 | Stable | Up to 2 years | The median number of admissions to hospital before NIPPV was 2 per year (range 0–5); the median difference in admissions to hospital before and after NIPPV was 1 (95% CI 0 to 2). The duration of stay in hospital decreased from a median of 16 days (range 0–47 days) to 7 days (range 0–15 days); p = 0.023; median difference 1 (95% CI 2 to 25) |
| Skobel et al. 2011108 | n = 27 | Post-hospital. Recruited from weaning clinics or ICUs | 3 months | 22% of patients had to be readmitted to hospital while on NIV. No details on admission rates before NIV |
| Tsolaki et al. 2011107 | n = 35 | Stable. Patients with acute respiratory failure (pH < 7.35 and symptoms such as increasing cough, purulent sputum, need for antibiotics) or patients with an exacerbation during 4 weeks preceding recruitment were excluded | Up to 2 years | 357 days of hospitalisation (5.35/patient/year) (210 in the first year and 147 in the second, 6.00 ± 13.86 vs. 4.31 ± 10.91/patient/year; p = 0.574) |
Appendix 9 Secondary outcome data for clinical effectiveness review
| Study | Population | Follow-up | NIV baseline n | NIV baseline mean (SD) | NIV follow-up n | NIV follow-up mean (SD) | Control baseline n | Control baseline mean (SD) | Control follow-up n | Control follow-up mean (SD) | Between-group difference in change | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duiverman et al. 200879 | Stable | 3 months | 31 | 0.9 l (0.38 l) | 24 | 0.89 l (0.39 l) | 35 | 0.78 l (0.3 l) | 32 | 0.81 l (0.29 l) | –0.04 l (95% CI –0.1 l to 0.1 l) | Between-group difference in change, adjusted for baseline values (linear regression analysis). No significant difference |
| Garrod et al. 200084 | Stable | 3 months | 17 | 0.97 l (not stated) | 17 | 0.94 l (not stated) | 20 | 0.92 l (not stated) | 20 | 0.88 l (not stated) | Not stated | NIV pre–post difference –0.03 (95% CI –0.93 to 0.15); control pre–post difference –0.04 (95% CI –0.57 to 0.13). Completers only. No significant changes |
| Gay et al. 1996100 | Stable | 3 months | 7 | 0.62 l (0.25 l) | 4 | 0.6 l (0.24 l) | 6 | 0.72 l (0.06 l) | 6 | 0.71 l (0.12 l) | Not stated | Completers only for follow-up results NIV group |
| Meecham-Jones et al. 199577 | Stable | 3 months | 14 | 0.86 l (0.32 l) | 14 | 0.83 l (0.4 l) | 14 | 0.86 l (0.32 l) | 14 | 0.81 l (0.4 l) | 0.02 l (95% CI –0.005 l to 0.08 l) | No significant difference. Completers only. Crossover trial |
| Strumpf et al. 199134 | Stable | 3 months | 7 | 0.54 l (SE 0.03 l, SD 0.08 l) | 7 | 0.55 l (SE 0.05 l, SD 0.13 l) | 7 | 0.54 l (SE 0.03 l, SD 0.08 l) | 7 | 0.53 l (SE 0.06 l, SD 0.16 l) | 0.02 l (95% CI –0.07 l to 0.11 l) | No significant difference. Completers only |
| Duiverman et al. 201180 | Stable | 6 months | 24 | 0.89 l (0.39 l) | 23 | 0.92 l (0.43 l) | 32 | 0.81 l (0.29 l) | 29 | 0.8 l (0.27 l) | Not stated | Completers only (follow-up). Not clear if the baseline values used in analysis were based on starting numbers or completers only |
| Stable | 12 months | 24 | 0.89 l (0.39 l) | 18 | 0.92 l (0.35 l) | 32 | 0.81 l (0.29 l) | 27 | 0.74 l (0.33 l) | Not stated | Completers only (follow-up). Not clear if the baseline values used in analysis were based on starting numbers or completers only | |
| Kaminski et al. 1999101 | Stable | 16–23 months | 7 | 0.7 l (0.3 l) | 7 | 0.69 l (0.29 l) | 12 | 0.9 l (0.38 l) | 12 | 0.86 l (0.38 l) | Not stated | No significant difference (appears to be based on comparison of values at follow-up). Last observation before death included. Patient numbers uncertain |
| Duiverman et al. 201180 | Stable | 24 months | 24 | 0.89 l (0.39 l) | 15 | 0.95 l (0.48 l) | 32 | 0.81 l (0.29 l) | 20 | 0.69 l (0.25 l) | 0.12 l (95% CI 0.02 l to 0.21 l) | Completers only (follow-up). Not clear if baseline values used in analysis based on starting numbers or completers only. Between-group difference in change, adjusted for baseline values (linear regression analysis). Significant difference in favour of NIV |
| Struik et al. 201475 | Post hospital | 12 months | 48 | 0.70 l (0.24 l) | 48 | 0.71 l (0.30 l) | 46 | 0.72 l (0.26 l) | 46 | 0.76 l (0.32 l) | –0.024 l (95% CI –0.12 l to 0.07 l) | No significant difference. Mean change (95% CI). Based on completers only |
| Xiang et al. 200792 | Post hospital | 24 months | 20 | 0.59 l (0.11 l) | 20 | 0.58 l (0.14 l) | 20 | 0.58 l (0.06 l) | 20 | 0.46 l (0.06 l) | Not stated | Significant difference (p < 0.05) in favour of NIV. Results appear to be based on all patients but no details on how missing data dealt with |
| Study | Population | Follow-up | NIV baseline n | NIV baseline mean (SD) | NIV follow-up n | NIV follow-up mean (SD) | Control baseline n | Control baseline mean (SD) | Control n | Control follow-up mean (SD) | Between-group difference in change | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strumpf et al. 199134 | Stable | 3 months | 7 | 31% (SE 1%, SD 2.65%) | 7 | 34 % (SE 2%, SD 5.3%) | 7 | 31% (SE 1%, SD 2.65%) | 7 | 33% (SE 2%, SD 5.3%) | 1% (95% CI –2% to 4%) | No significant difference. Completers only |
| Bhatt et al. 201383 | Stable | 6 months | 15 | 30.3% (7%) | 15 | 31.2% (11.9%) | 12 | 29.6 % (7.4%) | 12 | 31% (7.8%) | Not stated | No significant difference (p = 0.84). Normocapnic population |
| Casanova et al. 200085 | Stable | 6 months | 20 | 29% (8%) | 20 | 30% (9%) | 24 | 31% (7%) | 24 | 31% (7%) | Not stated | Completers only. No significant difference |
| McEvoy et al. 200974 | Stable | 6 months | 58 | 25.3% (95% CI 22.3% to 28.3%, SD 11.66%) | 56 | 24.9% (95% CI 21.7% to 28.1%, SD 12.22%) | 51 | 23.9% (95% CI 21.9% to 25.9%, SD 7.29%) | 47 | 23.4% (95% CI 21.4% to 25.4%, SD 7%) | Not stated | No significant difference. Based on available data |
| Clini et al. 200299 | Stable | 12 months | 39 | 26.6% (8.7%) | 39 | 26.8% (8.9%) | 46 | 31% (11.4%) | 46 | 30.9% (11.3%) | –0.3% (95% CI –3.1%a to 2.4%) | Adjusted for baseline values (ANOVA model). No significant difference |
| Köhnlein et al. 201476 | Stable | 12 months | 71 | Not stated | 71 | Adjusted change from baseline: –2.0% (0.2% to 3.8%) | 66 | Not stated | 66 | Adjusted change from baseline: –0.8% (95% CI –2.6% to 1.0%) | Adjusted difference in change 2.8% (95% CI 0.2% to 5.4%) | 66/78 patients in the control group and 71/87 patients in the NIV group contributed to the estimate at 12 months. Unclear if FEV1 (l) or FEV1 (% predicted), as all of the secondary outcomes were presented as % change. Mixed models for calculation of adjusted mean difference in change. p = 0.034 for difference in change (in favour of NIV) |
| McEvoy et al. 200974 | Stable | 12 months | 58 | 25.3% (95% CI 22.3% to 28.3%, SD 11.66%) | 47 | 24.1% (95% CI 21.1% to 27.1%, SD 10.49%) | 51 | 23.9% (95% CI 21.9% to 25.9%, SD 7.29%) | 38 | 26.3% (95% CI 24.1% to 28.5%, SD 6.92%) | Not stated | No significant difference. Based on available data |
| Clini et al. 200299 | Stable | 24 months | 39 | 26.6% (8.7%) | 39 | 27.5% (10.6%) | 46 | 31% (11%) | 46 | 30.8% (11.1%) | –1.1% (95% CI –4.5% to 2.1%) | Adjusted for baseline values (ANOVA model) |
| Study | Population | Follow-up | NIV baseline n | NIV baseline mean (SD) | NIV follow-up n | NIV follow-up mean (SD) | Control baseline n | Control baseline mean (SD) | Control n | Control follow-up mean (SD) | Between-group difference in change | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Garrod et al. 200084 | Stable | 3 months | 17 | 2.28 l (not stated) | 17 | 2.52 l (not stated) | 20 | 2.32 l (not stated) | 20 | 2.27 l (not stated) | Not stated | NIV pre–post difference 0.24 (95% CI –0.45 to 0.05); control pre–post difference –0.05 (95% CI –0.63 to 0.25) Completers only. No significant changes |
| Meecham-Jones et al. 199577 | Stable | 3 months | 14 | 2.03 l (0.62) | 14 | 2.01 l (0.6 l) | 14 | 2.03 l (0.62 l) | 14 | 1.91 l (0.6 l) | 0.1 l (95% CI –0.06 l to 0.26 l) | No significant difference. Completers only. Crossover trial |
| Strumpf et al. 199134 | Stable | 3 months | 7 | 1.71 l (SE 0.23 l, SD 0.61 l) | 7 | 1.6 l (SE 0.20 l, SD 0.53 l) | 7 | 1.71 l (SE 0.23 l, SD 0.61 l) | 7 | 1.57 l (SE 0.31 l, SD 0.82 l) | Not stated | No significant difference. Completers only. Crossover trial |
| Struik et al. 201475 | Post hospital | 12 months | 49 | 2.1 l (0.70 l) | 49 | 2.2 l (0.78 l) | 46 | 2.3 l (0.75 l) | 46 | 2.4 l (0.74 l) | –0.036 l (95% CI –0.31 l to 0.23 l) | No significant difference. Mean change (95% CI). Based on completers only |
| Xiang et al. 200792 | Post hospital | 24 months | 20 | 1.38 l (0.15 l) | 20 | 1.30 l (0.16 l) | 20 | 1.34 l (0.12 l) | 20 | 1.18 l (0.12 l) | Not stated | Significant difference in favour of NIV. Results appear to be based on all patients but no details on how missing data were dealt with |
| Study | Population | Follow-up | NIV baseline n | NIV baseline mean (SD) | NIV follow-up n | NIV follow-up mean (SD) | Control baseline n | Control baseline mean (SD) | Control follow-up n | Control follow-up mean (SD) | Between-group difference in change | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bhatt et al. 201383 | Stable | 6 months | 15 | 68.8% (14.1%) | 15 | 71.9% (16.7%) | 12 | 59.4% (8%) | 12 | 60.8% (30.2%) | Not stated | No significant difference (p = 0.74). Normocapnic population |
| Casanova et al. 200085 | Stable | 6 months | 20 | 59% (14%) | 20 | 59% (19%) | 24 | 62% (17%) | 24 | 65% (18%) | Not stated | Completers only. No significant difference |
| Köhnlein et al. 201476 | Stable | 12 months | 72 | Not stated | 72 | Adjusted change from baseline –0.2% (95% CI –2.1% to 1.8%) | 66 | Not stated | 66 | Adjusted change from baseline 0.1% (95% CI –3.1% to 2.5%) | Adjusted difference in change –0.3% (95% CI –1.6% to 3.1%) | 66/78 patients control group, 72/87 NIV group who contributed to estimate at 12 months. NB-unclear if FVC (l) or FVC (% predicted), as all secondary outcomes presented as % change. Mixed models for calculation of adjusted mean difference in change. p = 0.83 for difference in change |
| Study | Population | Follow-up | NIV baseline n | NIV baseline mean (SD) | NIV follow-up n | NIV follow-up mean (SD) | Control baseline n | Control baseline mean (SD) | Control follow-up n | Control follow-up mean (SD) | Between-group difference in change | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duiverman et al. 200879 | Stable | 3 months | 31 | 318 m (131 m) | 24 | 340 m (119 m) | 35 | 304 m (112 m) | 32 | 325 m (108 m) | 2 m (95% CI –19 m to 23 m) | Adjusted for baseline values (linear regression analysis) |
| Gay et al. 1996100 | Stable | 3 months | 4 | 862.1 feet (497.6 feet); 262.77 m (151.7 m) | 4 | 1014.3 feet (782.1 feet); 309.16 m (238.4 m) | 6 | 980.5 feet (294.8 feet); 298.86 m (89.88 m) | 6 | 1006.8 feet (360.9 feet); 306.87 m (110.0 m) | Not stated | Based on completers only |
| Meecham-Jones et al. 199577 | Stable | 3 months | 14 | Median 250 m, range 100–425 m | 14 | Median 240 m, range 100–450 m | 14 | Median 250 m, range 100–425 m | 14 | Median 235 m, range 80–440 m | Not stated | Based on completers only. Crossover trial |
| Sin et al. 200782 | Stable | 3 months | 11 | 262 m (100 m) | 11 | 311 m (144 m) | 10 | 343 m (48 m) | 10 | 367 m (59 m) | Not stated | No significant difference. Completers only for NIV group |
| Bhatt et al. 201383 | Stable | 6 months | 15 | 284 m (101 m) | 15 | 280 m (142 m) | 12 | 321 m (91 m) | 12 | 304 m (129 m) | Not stated | No significant difference. Completers only for NIV group |
| De Backer et al. 201191 | Stable | 6 months | 10 | 232 m (151 m) | 10 | 282 m (146 m) | 5 | 408 m (34 m) | 5 | 401 m (78 m) | Not stated | No details on loss to follow-up, have assumed none |
| Duiverman et al. 201180 | Stable | 6 months | 24 | 317 m (80.78 m) | 23 | 350 m (73.12 m) | 32 | 304 m (68.63 m) | 29 | 314.3 m (75.39 m) | Not stated | Completers only (follow-up). Not clear if baseline values used in analysis based on starting numbers or completers only. Have assumed completers. All results approximate, read off graph and SD calculated from CI |
| Clini et al. 200299 | Stable | 12 months | 39 | 200 m (125 m) | 37 | 202 m (120 m) | 47 | 247 m | 42 | 244 m (108 m) | –4.4 m (95% CI –37.5 m to 28.6 m) | Adjusted for baseline value (ANOVA model). No significant difference. Completers only (stated completers no different from dropouts) |
| Duiverman et al. 201180 | Stable | 12 months | 24 | 317 m (80.78 m) | 18 | 344.3 m (77.87 m) | 32 | 304 m | 27 | 308.6 m (76 m) | Not stated | Completers only (follow-up). Not clear if baseline values used in analysis based on starting numbers or completers only. Have assumed completers. All results approximate, read off graph and SD calculated from CI |
| Köhnlein et al. 201476 | Stable | 12 months | 65 | Not stated | 65 | Adjusted change from baseline 7.6% (95% CI 1.9% to 13.6%) | 60 | Not stated | 60 | Adjusted change from baseline 0.0% (95% CI –5.5% to 5.8%) | Adjusted difference in change 7.6% (95% CI –0.5% to 16.2%) | 60/71 patients in the control group and 65/79 patients in the NIV group who contributed to estimate at 12 months. Mixed models for calculation of adjusted mean difference in change. p = 0.07 for difference in change. Change in % not m |
| Kaminski et al. 1999101 | Stable | 16–23 months | 5 | 234 m (154 m) | 5 | 200 m (1591 m) | 14 | 286 m | 14 | 306 m (89 m) | Not stated | Appears to be ITT (last observation before death carried forward) |
| Clini et al. 200299 | Stable | 24 months | 39 | 200 m (125 m) | 37 | 183 m (118 m) | 47 | 247 m | 42 | 232 m (111 m) | 3.3 m (95% CI –39.0 m to 45.7 m) | Adjusted for baseline value (ANOVA model). No significant difference. Completers only (stated completers no different). Assuming patient numbers are the same as for 12 months (no distinction made in paper) |
| Duiverman et al. 201180 | Stable | 24 months | 24 | 317 m (80.78 m) | 15 | 372.9 m (81.93 m) | 32 | 304 m | 20 | 277.1 m (68.17 m) | 77 m (95% CI 46 m to 108 m) | Completers only (follow-up). Not clear if baseline values used in analysis based on starting numbers or completers only. All results for individual groups approximate, read off graph and SD calculated from CI. Mean change in difference reported. Adjusted for baseline values (linear regression analysis). Significant difference in favour of NIV |
| Xiang et al. 200792 | Post hospital | 24 months | 20 | 161 m (38 m) | 20 | 213 m (45 m) | 20 | 158 m | 20 | 127 m (23 m) | Not stated | Significant difference in favour of NIV. Results appear to be based on all patients but no details on how missing data dealt with |
| Study | Population | Follow-up | NIV baseline n | NIV baseline mean (SD) | NIV follow-up n | NIV follow-up mean (SD) | Control baseline n | Control baseline mean (SD) | Control follow-up n | Control follow-up mean (SD) | Between-group difference in change | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duiverman et al. 200879 | Stable | 3 months | 31 | 6.8 kPa (0.68 kPa) | 24 | 6.44 kPa (0.69 kPa) | 35 | 6.81 kPa (0.81 kPa) | 32 | 6.71 kPa (0.58 kPa) | –0.32 kPa (95% CI –0.6 kPa to –0.1 kPa) | Adjusted for baseline values (linear regression analysis). Significant difference in favour of NIV |
| Garrod et al. 200084 | Stable | 3 months | 17 | 44.6 mmHg (not stated) | 17 | 43.3 mmHg (not stated) | 20 | 45.3 mmHg (not stated) | 20 | 44.2 mmHg (not stated) | 0.2 mmHg (95% CI –0.41 mmHg to 0.41 mmHg) | Based on completers only. No significant differences in baseline variables between patients who completed all assessments compared with those who withdrew or were unable to attend an assessment. No statistically significant difference |
| Gay et al. 1996100 | Stable | 3 months | 7 | 54.7 mmHg (8.8 mmHg); 7.29 kPa (1.73 kPa) | 4 | 57.5 mmHg (14.4 mmHg); 7.67 kPa (1.92 kPa) | 6 | 48.5 mmHg (2.5 mmHg); 6.47 kPa (0.33 kPa) | 6 | 50.2 mmHg (4.3 mmHg); 6.69 kPa (0.57 kPa) | Not stated | Completers only at follow-up |
| Köhnlein et al. 201476 | Stable | 3 months | Unclear | 8.0 kPa (0.8 kPa) | Unclear | 6.6 kPa (1.1 kPa) | Unclear | 7.9 kPa (0.7 kPa) | Unclear | 7.4 kPa (0.9 kPa) | Not stated | Proportion included in outcome assessments unclear |
| Meecham-Jones et al. 199577 | Stable | 3 months | 14 | 55.8 mmHg (3.6 mmHg); 7.44 kPa (0.48 kPa) | 14 | 52.5 mmHg (4.7 mmHg); 7.00 kPa (0.63 kPa) | 14 | 55.8 mmHg (3.6 mmHg); 7.44 kPa (0.48 kPa) | 14 | 57 mmHg (5.7 mmHg); 7.60 kPa (0.76 kPa) | –4.5 mmHg (95% CI –2.2 mmHg to –6.3 mmHg) | Based on completers only. Crossover trial. Statistically significant difference in favour of NIV |
| Strumpf et al. 199134 | Stable | 3 months | 7 | 46 mmHg (SE 2 mmHg, SD 5.29 mmHg); 6.13 kPa (0.71 kPa) | 7 | 50 mmHg (SE 2 mmHg, SD 5.29 mmHg); 6.67 kPa (0.71 kPa) | 7 | 46 mmHg (SE 2 mmHg, SD 5.29 mmHg); 6.13 kPa (0.71 kPa) | 7 | 47 mmHg (SE 3 mmHg, SD 7.94 mmHg); 6.27 kPa (1.06 kPa) | 3 mmHg (95% CI –1 mmHg to 6 mmHg) | Crossover trial. Based on completers only |
| Bhatt et al. 201383 | Stable | 6 months | 15 | 42.4 mmHg (5.6 mmHg); 5.65 kPa (0.75 kPa) | 15 | 44.3 mmHg (8.5 mmHg); 5.91 kPa (1.13 kPa) | 12 | 41.9 mmHg (4.6 mmHg); 5.59 kPa (0.61 kPa) | 12 | 42.9 mmHg (4.5 mmHg); 5.72 kPa (0.6 kPa) | Not stated | Based on completers-only NIV arm. Normocapnic population. No statistically significant difference |
| Casanova et al. 200085 | Stable | 6 months | 20 | 50.7 mmHg (7.9 mmHg); 6.76 kPa (1.05 kPa) | 20 | 51.1 mmHg (8.8 mmHg); 6.81 kPa (1.17 kPa) | 24 | 53.2 mmHg (8.1 mmHg); 7.09 kPa (1.08 kPa) | 24 | 52.3 mmHg (6.1 mmHg); 6.92 kPa (0.81 kPa) | Not stated | Based on completers only. Stated that no significant difference |
| Duiverman et al. 201180 | Stable | 6 months | 24 | 6.85 kPa (0.40 kPa) | 23 | 6.38 kPa (0.40 kPa) | 32 | 6.80 kPa (0.47 kPa) | 29 | 6.8 kPa (0.47 kPa) | Not stated | Completers only (follow-up). Not clear if baseline values used in analysis based on starting numbers or completers only. Results estimated from graph and SD calculated from CI |
| Köhnlein et al. 201476 | Stable | 6 months | Unclear | 8.0 kPa (0.8 kPa) | Unclear | 6.4 kPa (0.9 kPa) | Unclear | 7.9 kPa (0.7 kPa) | Unclear | 7.1 kPa (1.0 kPa) | Not stated | Proportion included in outcome assessments unclear |
| McEvoy et al. 200974 | Stable | 6 months | 61 | 52.6 mmHg (51.0 mmHg to 54.2 mmHg, SD 2.5 mmHg); 7.01 kPa (0.33 kPa) | 44 | 52.9 mmHg (50.5 mmHg to 55.3 mmHg, SD 8.1 mmHg); 7.05 kPa (1.08 kPa) | 72 | 54.4 mmHg (95% CI 52.6 mmHg to 56.2 mmHg, SD 7.8 mmHg); 7.25 kPa (1.04 kPa) | 34 | 55.1 mmHg (52.1 mmHg to 58.1 mmHg, SD 8.9 mmHg); 7.35 kPa (1.19 kPa) | Not stated | SD calculated from CI |
| Zhou et al. 200881 | Stable | 6 months | 15 | 59.28 mmHg (6.43 mmHg); 7.90 kPa (0.86 kPa) | 15 | 54.07 mmHg (5.51 mmHg); 7.21 kPa (0.73 kPa) | 21 | 58.40 mmHg (9.21 mmHg); 7.79 kPa (1.23 kPa) | 20 | 61.38 mmHg (4.58 mmHg); 8.18 kPa (0.61 kPa) | Not stated | Significant difference in favour of NIV |
| Köhnlein et al. 201476 | Stable | 9 months | Unclear | 8.0 kPa (0.8 kPa) | Unclear | 6.4 kPa (0.9 kPa) | Unclear | 7.9 kPa (0.7 kPa) | Unclear | 7.3 kPa (0.8 kPa) | Not stated | Proportion included in outcome assessments unclear |
| Clini et al. 200299 | Stable | 12 months | 39 | 7.48 kPa (2.27 kPa) | 39 | 7.36 kPa (2.42 kPa) | 47 | 7.60 kPa (2.15 kPa) | 47 | 7.96 kPa (2.28 kPa) | 2.997 kPa (95% CI 0.94 kPa to 5.05 kPa) | Individual group results estimated from graph, and SD calculated from CI. Results for patients on usual oxygen (results for ambient air also presented in paper). Adjusted for baseline value (ANOVA model) p = 0.005. Significant difference in favour of NIV. Stated that ITT (LOCF) |
| Duiverman et al. 201180 | Stable | 12 months | 24 | 6.85 kPa (0.40 kPa) | 18 | 6.17 kPa(0.47 kPa) | 32 | 6.80 kPa (0.47 kPa) | 27 | 6.85 kPa (0.57 kPa) | Not stated | Completers only (follow-up). Not clear if baseline values used in analysis based on starting numbers or completers only. Results estimated from graph and SD calculated from CI |
| Köhnlein et al. 201476 | Stable | 12 months | 79 | 8.0 kPa (0.8 kPa) | 79 | 6.5% (0.9%). Adjusted change from baseline 7.4% (95% CI –8.6% to –6.2%) | 69 | 7.9 kPa (0.7 kPa) | 69 | 7.4 kPa (1.2 kPa). Adjusted change from baseline –2.4% (95% CI –3.7% to –1.1%) | Adjusted difference in change –5.1% (95% CI –6.8% to 3.4%) | 69/83 patients control group, 79/89 NIV group who contributed to estimate at 12 months. Mixed models for calculation of adjusted mean difference in change. p < 0.0001 for difference in change. Change in % rather than kPa |
| McEvoy et al. 200974 | Stable | 12 months | 61 | 52.6 mmHg (51.0 mmHg to 54.2 mmHg, SD 6.4 mmHg); 7.01 kPa (0.85 kPa) | 43 | 53.2 mmHg (50.6 mmHg to 55.8 mmHg, SD 8.7 mmHg); 7.09 kPa (1.16 kPa) | 72 | 54.4 mmHg (52.6 mmHg to 56.2 mmHg, SD 7.8 mmHg); 7.25 kPa (1.04 kPa) | 29 | 52.2 mmHg (95% CI 49.5 mmHg to 54.9 mmHg, SD 7.4 mmHg); 7.0 kPa (0.99 kPa) | Not stated | SD calculated from CI. Stated that no significant difference |
| Zhou et al. 200881 | Stable | 12 months | 15 | 59.28 mmHg (6.43 mmHg); 7.90 kPa (0.86 kPa) | 14 | 57.36 mmHg (2.73 mmHg); 7.65 kPa (0.36 kPa) | 21 | 58.40 mmHg (9.21 mmHg); 7.79 kPa (1.23 kPa) | 18 | 63.76 mmHg (5.29 mmHg); 8.50 kPa (0.71 kPa) | Not stated | Significant difference in favour of NIV |
| Clini et al. 200299 | Stable | 24 months | 39 | 7.48 kPa (2.27 kPa) | 39 | 7.23 kPa (2.27 kPa) | 47 | 7.60 kPa (2.15 kPa) | 47 | 7.89 kPa (2.19 kPa) | 4.27 kPa (95% CI 1.58 kPa to 9.96 kPa) | Individual group results estimated from graph, and SD calculated from CI. Results for patients on usual oxygen (results for ambient air also presented in paper). Adjusted for baseline value (ANOVA model) p = 0.002. Significant difference in favour of NIV. Stated that ITT (LOCF) |
| Duiverman et al. 201180 | Stable | 24 months | 24 | 6.85 kPa (0.40 kPa) | 15 | 6.13 kPa (0.46 kPa) | 32 | 6.80 kPa (0.47 kPa) | 20 | 6.85 kPa (0.44 kPa) | –0.4 kPa (95% CI –0.8 kPa to –0.2 kPa) | Completers only (follow-up). Not clear if baseline values used in analysis based on starting numbers or completers only. Individual group results estimated from graph and SD calculated from CI. Adjusted for baseline values (linear regression analysis). Significant difference in favour of NIV |
| Cheung et al. 201090 | Post-hospital. | 3 months | 23 | 7.7 kPa (1.0 kPa) | 14 | 6.53 kPa (0.8 kPa) | 24 | 7.3 kPa (1.0 kPa) | 11 | 6.89 kPa (1.55 kPa) | Not stated | Completers only at follow-up. No significant difference |
| Cheung et al. 201090 | Post-hospital. | 6 months | 23 | 7.7 kPa (1.0 kPa) | 12 | 6.64 kPa (1.42 kPa) | 24 | 7.3 kPa (1.0 kPa) | 7 | 6.41 kPa (0.92 kPa) | Not stated | Completers only at follow-up. No significant difference |
| Cheung et al. 201090 | Post-hospital | 12 months | 23 | 7.7 kPa (1.0 kPa) | 8 | 6.05 kPa (0.4 kPa) | 24 | 7.3 kPa (1.0 kPa) | 6 | 6.36 kPa (0.95 kPa) | Not stated | Completers only at follow-up. No significant difference |
| Struik et al. 201475 | Post-hospital | 12 months | 50 | 7.7 kPa (1.1 kPa) | 50 | 6.4 kPa (1.0 kPa) | 48 | 7.4 kPa (0.9 kPa) | 48 | 6.6 kPa (1.0 kPa) | –0.5 kPa (95% CI –0.9 kPa to –0.04 kPa) | Mean change (95% CI). Based on completers only. Significant difference in change (p < 0.05). Measurement performed regardless of oxygen use |
| Struik et al. 201475 | Post-hospital | 12 months | 38 | Not stated | 38 | Not stated. Change –1.3 (95% CI –1.6 to –0.9) | 37 | Not stated | 37 | Not stated. Change –0.9 (95% CI –1.2 to 0.6) | –0.2 kPa (95% CI –0.6 kPa to 0.3 kPa) | Mean change (95% CI). Based on completers only. Based only on patients where measurements were both on room air or both on oxygen at the same flow air. No significant difference |
| Xiang 200792 | Post-hospital | 24 months | 20 | 59.2 mmHg (2.8 mmHg); 7.89 kPa (0.37 kPa) | 20 | 49.5 mmHg (2.2 mmHg); 6.60 kPa (0.29 kPa) | 20 | 58.8 mmHg (1.9 mmHg); 7.84 kPa (0.25 kPa) | 20 | 61.5 mmHg (2.3 mmHg); 8.20 kPa (0.31 kPa) | Not stated | Results appear to be based on all patients but no details on how missing data dealt with. Significant difference in favour of NIV |
| Study | Population | Follow-up | NIV baseline n | NIV baseline mean (SD) | NIV follow-up n | NIV follow-up mean (SD) | Control baseline n | Control baseline mean (SD) | Control follow-up n | Control follow-up mean (SD) | Between-group difference in change | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duiverman et al. 200879 | Stable | 3 months | 31 | 7.82 kPa (1.0 kPa) | 24 | 8.26 kPa (1.2 kPa) | 35 | 8.33 kPa (1.25 kPa) | 32 | 8.33 kPa (0.93 kPa) | 0.25 kPa (–0.2 kPa/mmHg to 0.7 kPa) | Adjusted for baseline values (linear regression analysis). No significant difference |
| Garrod et al. 200084 | Stable | 3 months | 17 | 63.5 mmHg (not stated) | 17 | 66.1 mmHg (not stated) | 20 | 66.8 mmHg (not stated) | 20 | 66.8 mmHg (not stated) | 3.7 mmHg (0.37 kPa/mmHg to 7.27 mmHg) | Based on completers only. No significant differences in baseline variables between patients who completed all assessments compared with those who withdrew or were unable to attend an assessment. Statistically significant difference in favour of NIV (p = 0.03) |
| Gay et al. 1996100 | Stable | 3 months | 7 | 66.4 mmHg (15.1 mmHg); 8.85 kPa (2.01 kPa) | 4 | 70.5 mmHg (4.7 mmHg); 9.40 kPa (0.63 kPa) | 6 | 57.8 mmHg (11.5 mmHg); 7.71 kPa (1.53 kPa) | 6 | 60.3 mmHg (14.4 mmHg); 8.04 kPa (1.92 kPa) | Not stated | Completers only at follow-up |
| Meecham-Jones et al. 199577 | Stable | 3 months | 14 | 45.3 mmHg (5.7 mmHg); 6.04 kPa (0.76 kPa) | 14 | 50.2 mmHg (7.3 mmHg); 6.69 kPa (0.97 kPa) | 14 | 45.3 mmHg (5.7 mmHg); 6.04 kPa (0.76 kPa) | 14 | 44.3 mmHg (7 mmHg); 5.91 kPa (0.93 kPa) | 5.9 mmHg (2.9 mmHg to 8.9 mmHg) | Based on completers only. Crossover trial. Statistically significant difference in favour of NIV |
| Strumpf et al. 199134 | Stable | 3 months | 7 | 64 mmHg (SE 3 mmHg, SD 7.94 mmHg); 8.53 kPa (1.06 kPa) | 7 | 62 mmHg (SE 4 mmHg, SD 10.58 mmHg); 8.26 kPa (1.41 kPa) | 7 | 64 mmHg (SE 3 mmHg, SD 7.94 mmHg); 8.53 kPa (1.06 kPa) | 7 | 60 mmHg (SE 4 mmHg, SD 10.58 mmHg); 8.00 kPa (1.41 kPa) | 2 mmHg (–4 mmHg to 7 mmHg) | Crossover trial. Based on completers only |
| Bhatt et al. 201383 | Stable | 6 months | 15 | 65.1 mmHg (13.3 mmHg); 8.68 kPa (1.77 kPa) | 15 | 67.2 mmHg (14.4 mmHg); 8.96 kPa (1.92 kPa) | 12 | 73.9 mmHg (9.9 mmHg); 9.85 kPa (1.32 kPa) | 12 | 66.8 mmHg (9.9 mmHg); 8.91 kPa (1.32 kPa) | Not stated | Based on completers only NIV arm. Normocapnic population. Statistically significant difference (p = 0.02) in favour of NIV |
| Casanova et al. 200085 | Stable | 6 months | 20 | 55.7 mmHg (8.6 mmHg); 7.43 kPa (1.15 kPa) | 20 | 56.3 mmHg (8.2 mmHg); 7.51 kPa (1.09 kPa) | 24 | 57.5 mmHg (7.2 mmHg); 7.67 kPa (0.96 kPa) | 24 | 57.3 mmHg (6.5 mmHg); 7.64 kPa (0.87 kPa) | Not stated | Based on completers only. Stated that no significant difference |
| Duiverman et al. 201180 | Stable | 6 months | 24 | 7.76 kPa (0.65 kPa) | 23 | 8.66 kPa (0.77 kPa) | 32 | 8.37 kPa (0.75 kPa) | 29 | 8.0 kPa (0.88 kPa) | Not stated | Completers only (follow-up). Not clear if baseline values used in analysis based on starting numbers or completers only. Results read off graph and SD calculated from CI |
| Köhnlein et al. 201476 | Stable | 12 months | 79 | Not stated | 79 | Adjusted change from baseline 2.2% (95% CI 0.6% to 3.8%) | 69 | Not stated | 69 | Adjusted change from baseline –1.4% (–0.3% to 3.2%) | Adjusted difference in change 0.8% (–1.6% to 3.1%) | 69/83 patients control group, 79/89 NIV group who contributed to estimate at 12 months. Mixed models for calculation of adjusted mean difference in change. p = 0.53 for difference in change. Change in % rather than kPa |
| Zhou et al. 200881 | Stable | 6 months | 15 | 57.42 mmHg (7.64 mmHg); 7.65 kPa (1.02 kPa) | 15 | 62.53 mmHg (6.06 mmHg); 8.34 kPa (0.88 kPa) | 21 | 56.89 mmHg (8.26 mmHg); 7.58 kPa (1.10 kPa) | 20 | 54.75 mmHg (6.54 mmHg); 7.30 kPa (0.87 kPa) | Not stated | Significant difference in favour of NIV |
| Clini et al. 200299 | Stable | 12 months | 39 | 8.9 kPa (1.88) kPa | 39 | 9.25 kPa (3.46 kPa) | 47 | 9.2 kPa (2.80 kPa) | 47 | 8.9 kPa (2.96 kPa) | Not stated | Estimated from graph and SD calculated from CI (estimated from graph). Adjusted for baseline value (ANOVA model) |
| Duiverman et al. 201180 | Stable | 12 months | 24 | 7.76 kPa (0.65 kPa) | 18 | 8.57 kPa (0.79 kPa) | 32 | 8.37 kPa (0.75 kPa) | 27 | 8.10 kPa (0.77 kPa) | Not stated | Completers only (follow-up). Not clear if baseline values used in analysis based on starting numbers or completers only. Results read off graph and SD calculated from CI |
| Zhou et al. 200881 | Stable | 12 months | 15 | 57.42 mmHg (7.64 mmHg); 7.65 kPa (1.02 kPa) | 14 | 56.36 mmHg (5.65 mmHg); 7.51 kPa (0.75 kPa) | 21 | 56.89 mmHg (8.26 mmHg); 7.58 kPa (1.10 kPa) | 18 | 52.41 mmHg (3.64 mmHg); 6.99 kPa (0.49 kPa) | Not stated | Significant difference in favour of NIV |
| Clini et al. 200299 | Stable | 24 months | 39 | 8.9 kPa (1.88 kPa) | 39 | 8.57 kPa (3.53 kPa) | 47 | 9.2 kPa (2.80 kPa) | 47 | 8.6 kPa (2.86 kPa) | Not stated | Estimated from graph and SD calculated from CI (estimated from graph). Adjusted for baseline value (ANOVA model) |
| Duiverman et al. 201180 | Stable | 24 months | 24 | 7.76 kPa (0.65 kPa) | 15 | 9.13 kPa (0.59 kPa) | 32 | 8.37 kPa (0.75 kPa) | 20 | 8.0 kPa (0.73 kPa) | 0.8 kPa (0 kPa to 1.5 kPa) | Adjusted for baseline values (linear regression analysis). Significant difference. Completers only (follow-up). Not clear if baseline values used in analysis based on starting numbers or completers only. Individual group results read off graph and SD calculated from CI |
| Struik et al. 201475 | Post-hospital | 12 months | 45 | 8.3 kPa (1.6 kPa) | 45 | 8.3 kPa (1.6 kPa) | 48 | 7.9 kPa (1.9 kPa) | 48 | 8.4 kPa (1.4 kPa) | –0.6 kPa (–1.4 kPa to 0.2 kPa). 0.6 kPa stated in paper, but not consistent with CI, so –0.6 kPa assumed | Mean change (95% CI). Based on completers only. Measurement performed regardless of oxygen use. No significant difference |
| Struik et al. 201475 | Post-hospital | 12 months | 35 | Not stated | 35 | Not stated. Change 0.3 kPa (–0.3 kPa to 0.9 kPa) | 37 | Not stated | 37 | Not stated. Change 0.6 kPa (–0.1 kPa to 1.3 kPa) | –0.3 kPa (–1.2 kPa to 0.6 kPa) | Mean change (95% CI). Based on completers only. Based only on patients where measurements were both on room air or both on oxygen at the same flow air. No significant difference |
| Xiang et al. 200792 | Post-hospital | 24 months | 20 | 55 mmHg (4 mmHg); 7.33 kPa (0.53 kPa) | 20 | 60.8 mmHg (4.7 mmHg); 8.10 kPa (0.63 kPa) | 20 | 54 mmHg (4 mmHg); 7.20 kPa (0.53 kPa) | 20 | 52.8 mmHg (2.4 mmHg); 7.04 kPa (0.32 kPa) | Not stated | Results appear to be based on all patients but no details on how missing data dealt with. Significant difference in favour of NIV |
Appendix 10 Excluded studies from the cost-effectiveness review
This section contains a list of the excluded studies from the cost-effectiveness review.
| Article | Reason for exclusion |
|---|---|
| From cost-effectiveness searches | |
| Acton RD, Hotchkiss J, Dries DJ. Noninvasive ventilation. J Trauma 2002;53:593–601 | A, E |
| Barbano L, Bertella E, Vitacca M. Episodic medical home interventions in severe bedridden chronic respiratory failure patients: a 4 year retrospective study. Monaldi Arch Chest Dis 2009;71:113–18 | A, D |
| Chandra K, Blackhouse G, McCurdy B, Bornstein M, Campbell K, Costa V, et al. Cost-effectiveness of interventions for chronic obstructive pulmonary disease (COPD) using an Ontario policy model. Ont Health Technol Assess Ser 2012;12:1–61 | A |
| Collett AS, Rees PJ. Non-invasive ventilation at home in chronic obstructive pulmonary disease. Int J Clin Pract 2007;61:1434–6 | A |
| Connolly MJ. Non-invasive ventilation in elderly patients with acute exacerbations of COPD: Bringing pressure to bear. Age Ageing 2006;35:1–2 | A, E |
| Cooke CR. Economics of mechanical ventilation and respiratory failure. Crit Care Clin 2012;28:39–55 | A |
| COPD Working Group. Noninvasive positive pressure ventilation for chronic respiratory failure patients with stable chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012;12:1–51 | A |
| Criner GJ, Kreimer DT, Tomaselli M, Pierson W, Evans D. Financial implications of noninvasive positive pressure ventilation (NPPV). Chest 1995;108:475–81 | E |
| Criner GJ. Cost/benefit of noninvasive mechanical ventilation. Monaldi Arch Chest Dis 1998;53:358–9 | A |
| Decramer M, Sibille Y, Bush A, Carlsen K-H, Rabe KF, Clancy L, et al. The European Union conference on chronic respiratory disease: Purpose and conclusions. Eur Respir J 2011;37:738–42 | A, D |
| Dellweg D, Schonhofer B, Haidl PM, Barchfeld T, Wenzel MD, Appelhans P, et al. Short-term effect of controlled instead of assisted noninvasive ventilation in chronic respiratory failure due to chronic obstructive pulmonary disease. Respir Care 2007;52:1734–40 | A |
| Diez AR, Abbona H, Ferrero G, Figueroa Casas JC, De VM, Lisanti R, et al. Argentine consensus of non-invasive ventilation. Medicina (B Aires) 2005;65:437–57 | A |
| Dwarakanath A, O’Flynn H. Impact of domiciliary non-invasive ventilation (NIV) service in chronic ventilatory disorder on hospitalisation and quality-of-life: a Grimsby experience. Thorax 2009; Conference: British Thoracic Society, BTS Winter Meeting 2009, London: A76 | A |
| Ferrer M, Sellares J, Torres A. The use of non-invasive ventilation by Italian physicians in the clinical practice. Minerva Anestesiol 2011;77:941–2 | A, E |
| Franek J. Home telehealth for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012;12:1–58 | D |
| Janssens J-P, Kehrer P, Chevrolet J-C, Rochat T. Non-invasive home ventilation (NIHV): analysis of 32 cases with an average 41-month follow-up. Rev Mal Respir 1999;16:511–20 | A |
| Janssens J-P, Derivaz S, Breitenstein E, De MB, Fitting J-W, Chevrolet J-C, et al. Changing patterns in long-term noninvasive ventilation: A 7-year prospective study in the Geneva Lake Area. Chest 2003;123:67–79 | A |
| Mehta B, Goyal R, Reznik R, Fayaaz J, Allred C, DiFabrizio L, et al. Hospital utilization of bilevel non-invasive positive pressure ventilation could become more cost-effective. Am J Respir Crit Care Med 2010; Conference: American Thoracic Society International Conference, ATS 2010 New Orleans, LA | E |
| Muir JF, Voisin C, Ludot A. Organization of home respiratory care: the experience in France with ANTADIR. Monaldi Arch Chest Dis 1993;48:462–7 | A |
| Palmer JM, Hughes PD. Retrospective evaluation of home initiation for long-term non-invasive ventilatory support. Thorax 2009; Conference: British Thoracic Society, BTS Winter Meeting 2009, London. Conference Publication:(var.pagings):A79 | A |
| Pandey R, Chokhani R. Use of non invasive ventilation in patients with respiratory failure in Nepal. Kathmandu Univ Med J 2011;9:256–9 | A, E |
| Rossi A. European respiratory monograph: Preface. European Respiratory Monograph 2001;6:a-b | A |
| Schilling JP, Kasik JE. Intermittent positive pressure breathing: a continuing controversy. Iowa Med 102;70:99–100 | A |
| Sikich N. Community-based multidisciplinary care for patients with stable chronic obstructive pulmonary disease (COPD): An evidence-based analysis. Ont Health Technol Assess Ser 2012;12:1–51 | D |
| From clinical effectiveness review | |
| Budweiser S, Heinemann F, Meyer K, Wild PJ, Pfeifer M. Weight gain in cachectic COPD patients receiving noninvasive positive-pressure ventilation. Respir Care 2006;51:126–32 | A, C, D |
| Chatwin M, Heather S, Hanak A, Polkey MI, Wilson B, Simonds AK. Analysis of emergency helpline support for home ventilator dependent patients: Risk management and workload. Eur Respir Rev 2008;17:33–5 | A |
| Chatwin M, Heather S, Hanak A, Polkey MI, Simonds AK. Analysis of home support and ventilator malfunction in 1211 ventilator-dependent patients. Eur Respir J 2010;35:310–16 | A, C |
| Lloyd-Owen SJ, Donaldson GC, Ambrosino N, Escarabill J, Farre R, Fauroux B, et al. Patterns of home mechanical ventilation use in Europe: Results from the Eurovent survey. Eur Respir J 2005;25:1025–31 | A |
| Tsolaki V, Pastaka C, Karetsi E, Zygoulis P, Koutsokera A, Gourgoulianis KI, et al. One-year in chronic hypercapnic COPD: effect on quality-of-life. Respir Med 2008;102:904–11 | A |
Appendix 11 Quality assessment of included studies in cost-effectiveness review
| Quality criteria | Tuggey et al. (2003)40 | Clini et al. (2009)134 |
|---|---|---|
| Was a well-defined question posed in answerable form? | Yes | Yes |
| Was a comprehensive description of the competing alternatives given? | Yes | Yes |
| Was the effectiveness of the programme or services established? | Yes | Yes |
| Were all the important and relevant costs and consequences for each alternative identified? | No | No |
| Were costs and consequence measured accurately in appropriate physical units? | Yes | Yes |
| Were cost and consequences valued credibly? | Yes, although some ambiguity | Yes |
| Were costs and consequences adjusted for differential timing? | Yes | Yes |
| Was an incremental analysis of costs and consequences of alternatives performed? | No | No |
| Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | Yes |
| Did the presentation and discussion of study results include all issues of concern to users? | Yes | Yes |
Appendix 12 All-cause mortality rates applied in the economic model
Table 74 lists the COPD-adjusted all-cause mortality rates applied in the economic model. These were derived from all-cause and COPD-related mortality rates by sex and age for a UK population and were obtained from the Office for National Statistics.
| Age (years) | All-cause mortality | Deaths caused by COPD | COPD-adjusted mortality | |||
|---|---|---|---|---|---|---|
| Male (%) | Female (%) | Male (%) | Female (%) | Male (%) | Female (%) | |
| 60 | 0.8342 | 0.5361 | 4.2052 | 5.1115 | 0.7828 | 0.5095 |
| 61 | 0.8871 | 0.5810 | 0.8325 | 0.5522 | ||
| 62 | 0.9507 | 0.6165 | 0.8921 | 0.5859 | ||
| 63 | 1.0509 | 0.6812 | 0.9862 | 0.6474 | ||
| 64 | 1.1558 | 0.7478 | 1.0846 | 0.7107 | ||
| 65 | 1.2725 | 0.8201 | 6.1850 | 7.2580 | 1.1941 | 0.7794 |
| 66 | 1.4205 | 0.9119 | 1.3330 | 0.8666 | ||
| 67 | 1.5369 | 0.9737 | 1.4422 | 0.9254 | ||
| 68 | 1.7243 | 1.0949 | 1.6181 | 1.0405 | ||
| 69 | 1.9125 | 1.2158 | 1.7947 | 1.1554 | ||
| 70 | 2.1149 | 1.3856 | 1.9846 | 1.3168 | ||
| 71 | 2.3225 | 1.4768 | 2.1794 | 1.4035 | ||
| 72 | 2.5652 | 1.6469 | 2.4072 | 1.5651 | ||
| 73 | 2.7907 | 1.8063 | 2.6188 | 1.7166 | ||
| 74 | 3.1141 | 2.0492 | 2.9223 | 1.9475 | ||
| 75 | 3.3999 | 2.2567 | 6.6549 | 6.4764 | 3.1905 | 2.1447 |
| 76 | 3.8443 | 2.5538 | 3.6075 | 2.4270 | ||
| 77 | 4.2217 | 2.8839 | 3.9616 | 2.7407 | ||
| 78 | 4.7005 | 3.2547 | 4.4109 | 3.0931 | ||
| 79 | 5.2482 | 3.6732 | 4.9249 | 3.4908 | ||
| 80 | 5.9440 | 4.1742 | 5.5778 | 3.9670 | ||
| 81 | 6.6343 | 4.6620 | 6.2256 | 4.4305 | ||
| 82 | 7.4283 | 5.3215 | 6.9707 | 5.0573 | ||
| 83 | 8.1907 | 6.0585 | 7.6861 | 5.7577 | ||
| 84 | 9.2142 | 6.7739 | 8.6466 | 6.4376 | ||
| 85 | 10.2895 | 7.5849 | 5.5753 | 3.4135 | 9.6556 | 7.2083 |
| 86 | 11.2992 | 8.5749 | 10.6031 | 8.1492 | ||
| 87 | 12.7193 | 9.5838 | 11.9358 | 9.1080 | ||
| 88 | 14.0875 | 10.7790 | 13.2197 | 10.2438 | ||
| 89 | 16.0713 | 12.1602 | 15.0813 | 11.5565 | ||
| 90 | 16.6367 | 13.5352 | 15.6118 | 12.8632 | ||
| 91 | 17.8196 | 14.6525 | 16.7219 | 13.9250 | ||
| 92 | 18.8878 | 16.0748 | 17.7243 | 15.2767 | ||
| 93 | 21.4681 | 18.0517 | 20.1456 | 17.1555 | ||
| 94 | 23.7662 | 20.2789 | 22.3021 | 19.2721 | ||
| 95 | 25.6292 | 22.3947 | 24.0504 | 21.2828 | ||
| 96 | 27.5704 | 24.0167 | 25.872 | 22.8243 | ||
| 97 | 29.4811 | 25.9706 | 27.6650 | 24.6811 | ||
| 98 | 31.5556 | 27.8215 | 29.6123 | 26.4402 | ||
| 99 | 32.7281 | 29.6932 | 30.7120 | 28.2190 | ||
| 100 | 34.4584 | 31.8222 | 32.3357 | 30.2422 | ||
Appendix 13 Survival reported in clinical studies of domiciliary non-invasive ventilation in cohorts discharged from hospital
| Author, year | Type of study | Country | Control arm size | Mortality at 6 months | Mortality at 1 year | Mortality at 2 year | Mortality at 5 years | Mortality at 10 years |
|---|---|---|---|---|---|---|---|---|
| Cheung et al. 201090 | Randomised controlled trial | China (Hong Kong) | 23 | – | Eight withdrew, four for critical illness | – | – | – |
| Xiang et al. 200792 | Randomised controlled trial | China | 20 | – | – | 40% | – | – |
| Budweiser et al. 200796 | Non-randomised controlled trial | Germany | 41 | – | 43.9%, of which 72% were COPD related | – | – | – |
| Heinemann et al. 201197 | Non-randomised controlled trial | Germany | 43 | – | 45.7% | – | – | – |
| Milane and Jonquet 198586 | Non-randomised controlled trial | France | – | – | – | 55% | 93.4% | 0% |
Appendix 14 Studies reporting outcomes in patients followed up post hospital
| Author, year | Type of study | Country; cohort size | % dead at < 3 months | % dead at 6 months | % dead at 1 year | % dead at 2 year | % dead at 3 year | % dead at 5 years |
|---|---|---|---|---|---|---|---|---|
| European Audit129 | Cohort study of patients admitted to hospital for exacerbation, reports outcomes at 90 days post admission by hospital | UK patients; 4986 | 15.8 at 90 days; 53% of these deaths were COPD related and a further 22.6% were of unknown cause | – | – | – | – | – |
| Bucknall et al. 2011153 | Randomised control trial of a self-management intervention delivered at discharge following admission for exacerbation | Scotland; 464 across both the intervention and control | – | – | 11.2; 75% of these deaths were COPD-related | – | – | – |
| Garcia-Aymerich et al. 2003152 | Prospective cohort study to determine risk factors for readmission; patients followed up for a mean of 1.1 years after admission to hospital for an exacerbation | Spain | – | – | 29; 74% from COPD exacerbation | – | – | – |
| Dave et al. 2014130 | Prospective observational cohort study of admissions to the respiratory ward, ITU and HDU with acute HRF and COPD | UK; 448 patients in the first-time admission cohort | – | – | 45 | – | 65 | 75 |
| Dave et al. 2014130 | As above | UK; 230 patients in the second-time admission cohort | – | – | 57 | – | 68 | 72 |
| Connors et al. 199616 | Prospective observational cohort study of patients admitted to five US hospitals for COPD exacerbation followed up for 5 years | USA; 1016 | 20 | 33 | 43 | 49 | – | – |
Appendix 15 Usual-care survival curves
FIGURE 46.
Base-case survival curve for usual care in the stable population.
FIGURE 47.
Base-case survival curve for usual care in the post-hospital population.
FIGURE 48.
Survival curve for sensitivity analysis assuming lower risk of admission and all-cause mortality for usual care in the post-hospital population. Risks obtained from Bucknall et al. 153
FIGURE 49.
Survival curve for sensitivity analysis increasing the base-case mortality risk for usual care in the post-hospital population. Risk of all-cause mortality, death during admission and death post admission increased by a factor of 2.
Appendix 16 Disease progression rates applied in the economic model
Table 77 lists the annual disease progression rates applied in the economic model. These were obtained from a published COPD Markov model by Atsou et al. 2011. 141
| Age (years) | GOLD stage 2 to GOLD stage 3 | GOLD stage 3 to GOLD stage 4 | GOLD stage 2 to GOLD stage 3 | GOLD stage 3 to GOLD stage 4 |
|---|---|---|---|---|
| Ex-smoker (%) | Ex-smoker (%) | Smoker (%) | Smoker (%) | |
| 60 | 5.803 | 5.120 | 9.338 | 7.823 |
| 61 | 5.926 | 5.229 | 9.535 | 7.989 |
| 62 | 6.049 | 5.338 | 9.733 | 8.155 |
| 63 | 6.104 | 5.386 | 9.822 | 8.229 |
| 64 | 6.159 | 5.434 | 9.912 | 8.304 |
| 65 | 6.213 | 5.482 | 10.001 | 8.379 |
| 66 | 6.268 | 5.530 | 10.091 | 8.454 |
| 67 | 6.322 | 5.579 | 10.180 | 8.529 |
| 68 | 6.367 | 5.618 | 10.252 | 8.589 |
| 69 | 6.412 | 5.658 | 10.324 | 8.650 |
| 70 | 6.457 | 5.698 | 10.396 | 8.710 |
| 71 | 6.502 | 5.737 | 10.468 | 8.770 |
| 72 | 6.547 | 5.777 | 10.540 | 8.831 |
| 73 | 6.561 | 5.789 | 10.562 | 8.849 |
| 74 | 6.575 | 5.801 | 10.584 | 8.868 |
| 75 | 6.589 | 5.814 | 10.607 | 8.887 |
| 76 | 6.603 | 5.826 | 10.629 | 8.905 |
| 77 | 6.617 | 5.838 | 10.651 | 8.924 |
| 78 | 6.638 | 5.857 | 10.686 | 8.953 |
| 79 | 6.659 | 5.876 | 10.720 | 8.982 |
| 80 | 6.681 | 5.895 | 10.755 | 9.011 |
| 81 | 6.702 | 5.914 | 10.789 | 9.040 |
| 82 | 6.724 | 5.933 | 10.824 | 9.069 |
| 83 | 6.792 | 5.993 | 10.935 | 9.161 |
| 84 | 6.861 | 6.054 | 11.045 | 9.254 |
| 85 | 6.930 | 6.114 | 11.156 | 9.347 |
| 86 | 6.998 | 6.175 | 11.266 | 9.439 |
| 87 | 7.067 | 6.236 | 11.377 | 9.532 |
| 88 | 7.136 | 6.296 | 11.487 | 9.624 |
| 89 | 7.204 | 6.357 | 11.598 | 9.717 |
| 90 | 7.273 | 6.417 | 11.708 | 9.810 |
| 91 | 7.342 | 6.478 | 11.819 | 9.902 |
| 92 | 7.410 | 6.538 | 11.929 | 9.995 |
| 93 | 7.479 | 6.599 | 12.040 | 10.088 |
| 94 | 7.547 | 6.659 | 12.150 | 10.180 |
| 95 | 7.616 | 6.720 | 12.261 | 10.273 |
| 96 | 7.685 | 6.781 | 12.372 | 10.365 |
| 97 | 7.753 | 6.841 | 12.482 | 10.458 |
| 98 | 7.822 | 6.902 | 12.593 | 10.551 |
| 99 | 3.610 | 7.891 | 12.703 | 10.643 |
| 100 | 3.610 | 7.891 | 12.703 | 10.643 |
Glossary
- Airflow obstruction
- A ratio of forced expiratory volume in 1 second to forced vital capacity that is less than 0.7.
- Chronic hypercapnic respiratory failure
- Evidence of chronic respiratory disease, high partial pressure of carbon dioxide in the arterial blood, normal pH, high bicarbonate (British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax 2002;57:192–211).
- Chronic obstructive pulmonary disease
- A chronic progressive lung disease characterised by airflow obstruction that is not fully reversible. The airflow obstruction does not change markedly over several months and is usually progressive in the long term.
- Cost-effectiveness acceptability curve
- A graph that illustrates the probability of an intervention being cost-effective at varying willingness-to-pay per quality-adjusted life-year gained thresholds.
- Domiciliary setting
- Any non-acute/non-hospital setting. Most likely to be the patient’s home, although it could also be, for example, a care home.
- End-stage chronic obstructive pulmonary disease
- A state of chronic respiratory failure that occurs in patients who remain symptomatic despite maximal therapy and in whom there no hope of cure, irrespective of predicted length of remaining lifespan.
- Exacerbation
- A sustained worsening of a patient’s symptoms compared with the usual stable state which is beyond normal day-to-day variations and which is acute in onset [National Institute for Health and Care Excellence. CG101 Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care (Partial Update). London: NICE; 2010].
- Incremental cost-effectiveness ratio
- The ratio between the difference in costs and the difference in outcomes between one or more intervention.
- Non-invasive ventilation
- Ventilatory support to a patient through the upper airways. It enhances the breathing process by giving the patient a mixture of air and oxygen from a flow generator through a tightly fitted facial or nasal mask. Also known as non-invasive positive-pressure ventilation, non-invasive ventilation assists the patient in taking a full breath and helps to maintain an adequate oxygen supply to the body.
- Post-hospital population
- For the purposes of this report, the population of chronic obstructive pulmonary disease patients who commence domiciliary non-invasive ventilation immediately after an admission to hospital/exacerbation.
- Probabilistic sensitivity analysis
- A type of analysis that demonstrates the uncertainty in model parameters and is used to provide an estimate of the level of confidence decision-makers should have in the conclusions of an economic evaluation. Conducting a probabilistic sensitivity analysis involves applying distributions to all uncertain parameters and then running the model multiple times, randomly using values from the distributions in the model to produce multiple pairs of costs and effects.
- Severity of chronic obstructive pulmonary disease
- This can be defined by the Global Initiative for Chronic Obstructive Lung Disease criteria.
- Stable population
- For the purposes of this report, chronic obstructive pulmonary disease patients described as being in a stable state, with no recent exacerbations/hospital admissions or other major change in clinical parameters over a defined period (e.g. 4 weeks).
- Usual care
- Standard approaches to disease management. This is multidisciplinary in nature, and will depend on severity of disease and effectiveness of individual treatments. The full treatment pathway is outlined in the National Institute for Health and Care Excellence clinical guidelines. In brief, smoking-cessation support is offered to all chronic obstructive pulmonary disease patients, pulmonary rehabilitation is offered where appropriate, short-acting bronchodilators and short-acting muscarinic antagonists as necessary are the initial treatment for the relief of breathlessness and exercise limitation and, where necessary, long-acting muscarinic antagonists and/or long-acting β2-agonists with or without inhaled corticosteroids are offered. Other treatments may include oral corticosteroids, oral theophylline, oral mucolytic therapy, combined oral and inhaled therapy, long-term oxygen therapy and lung surgery.
List of abbreviations
- 6MWD
- 6-minute walking distance
- A&E
- accident and emergency
- BIPAP
- bilevel positive airway pressure
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CPAP
- continuous positive airway pressure
- CRQ
- Chronic Respiratory Questionnaire
- EPAP
- expiratory positive airway pressure
- EVPI
- expected value of perfect information
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced vital capacity
- GOLD
- Global Initiative for Chronic Obstructive Lung Disease
- GP
- general practitioner
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- HR
- hazard ratio
- HRF
- hypercapnic respiratory failure
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IPAP
- inspiratory positive airway pressure
- IPD
- individual patient data
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- LTOT
- long-term oxygen therapy
- MRF-28
- Maugeri Foundation Respiratory Failure questionnaire
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NIPPV
- non-invasive positive-pressure ventilation
- NIV
- non-invasive ventilation
- NPV
- negative pressure ventilation
- PaCO2
- partial pressure of carbon dioxide in the arterial blood
- PaO2
- partial pressure of oxygen in the arterial blood
- pO2
- partial pressure of oxygen
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SABA
- short-acting β2-agonist
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SGRQ
- St George’s Respiratory Questionnaire
- SRI
- Severe Respiratory Insufficiency Questionnaire