Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 12/60/01. The protocol was agreed in March 2013. The assessment report began editorial review in September 2013 and was accepted for publication in October 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor Ian Pavord received speaker and travel fees from GlaxoSmithKline, Astra Zeneca, Napp and Boehringer Ingelheim and speaker fees from Aerocrine and Boston Scientific, all outside this work. Dr Rod Lawson received research support in the form of a grant from GSK and Novartis for diagnostic imaging, personal fees from GlaxoSmithKline and Novartis for advisory board meetings and educational meetings and personal fees from AstraZeneca, Almirall and Boehringer Ingelheim for educational meetings, all outside this work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Harnan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Condition and aetiology
Introduction
Asthma is a chronic disorder of the airways, caused primarily by inflammatory processes and constriction of the smooth muscle in airway walls (bronchoconstriction). It is characterised by airflow obstruction and increased responsiveness of the airways to various stimuli. Symptoms include recurrent episodes of wheezing, breathlessness, chest tightness and coughing. Typical asthma symptoms tend to be variable, intermittent and worse at night. Asthma is commonly triggered by viral respiratory infections, exercise or external factors such as smoke, a change in weather conditions and allergens, for instance pollen, mould and house dust mites.
Asthma usually develops in childhood but may start at any age. It runs in some families but many people with asthma have no other family members affected. In adults, asthma is more common in women than in men. 1 There is no cure for asthma, although people may experience long periods of remission. Poorly controlled asthma can have a significant impact on the quality of life of the affected individual and his or her family. However, there may be variation in an individual’s perception of the symptoms and how he or she adapts to the condition over time. Clinical measures such as lung function may not correlate with an individual’s quality of life scores, but if asthma is well controlled near-maximal scores on quality of life instruments can be achieved.
Classification of asthma
There are several ways of categorising different types of asthma, including:
-
Intrinsic and extrinsic asthma. Asthma can be divided into extrinsic (external cause) and intrinsic (when no causative agent can be found) asthma. Extrinsic asthma is triggered by allergens and hence it is also termed ‘allergic asthma’. In extrinsic asthma the immune system reacts to substances such as pollen and produces antibodies. Individuals with a predisposition to developing such allergies are said to be atopic and may develop any combination of the triad of hay fever, eczema and asthma. In the case of asthma, the allergic reaction is observed in bronchi and bronchioles, which results in the production of excess mucus that obstructs the air passages. Extrinsic asthma is commonly seen in children. About 90% of childhood asthma cases are caused by specific allergens. Individuals with a family history of atopy are at a higher risk of developing extrinsic asthma. In contrast, intrinsic asthma is a non-seasonal, non-allergic form of asthma, which usually first occurs at a later point in life than allergic asthma. Intrinsic asthma tends to be chronic and persistent rather than episodic. It is not related to specific allergens and may be provoked by the inhalation of chemicals such as cigarette smoke or cleaning agents, non-steroidal anti-inflammatory drugs, chest infections, emotion, exercise, cold air, food preservatives or various other non-specific irritants.
-
Eosinophilic and non-eosinophilic asthma (neutrophilic asthma). Asthma can also be categorised as eosinophilic or non-eosinophilic. There is some evidence that eosinophils may play an important proinflammatory role in the pathogenesis of asthma,2,3 although there remains some uncertainty around this and other pathogenic mechanisms associated with asthma. Eosinophils are found in the airways of asthmatics but not healthy subjects and are believed to be related to exacerbations. It has also been noted that suppression of eosinophil infiltration is often associated with amelioration of symptoms2 but that the relationship is not close. Poor inflammation control is most closely related to the risk of future exacerbations. The presence of eosinophils may be used to direct treatment as patients without eosinophilic inflammation are thought to be less responsive to inhaled corticosteroid (ICS) treatment. 4 High levels of eosinophils are correlated with high levels of fractional exhaled nitric oxide (FeNO) and it is thought that FeNO could be used as a biomarker of eosinophilic inflammation and therefore of ICS responsiveness. 5,6 However, the presence of eosinophils is not always a marker of severity of disease; fatal asthma may be associated with neutrophilia rather than eosinophilia. 7 Targeting the type of inflammation may be a better guide to treatment than measures of disease severity alone. For instance, glucocorticosteroids are typically very effective in eosinophilic inflammation but less so if the inflammation is neutrophilic.
-
Eosinophilic and non-eosinophilic airway disease. Eosinophilic inflammation occurs in both asthma and chronic obstructive pulmonary disease (COPD) and in both cases the appropriate treatment is ICSs. 6 There is a view held by some clinicians that, rather than a diagnosis of asthma, a diagnosis of responsiveness to ICSs [irrespective of diagnostic label (asthma or COPD)] may be a more helpful approach in terms of directing treatment, reducing costs and reducing exacerbations. 6 However, this form of classification has not yet been officially adopted in the British Thoracic Society (BTS) and Scottish Intercollegiate Guidelines Network (SIGN) guidelines8 and this report will focus on the diagnosis of asthma as described in these guidelines.
-
Molecular approaches to classifying asthma phenotypes. There is an increasing trend to characterise asthma by molecular and cellular factors to enable more targeted and personalised therapy. Such efforts are ongoing and specific phenotypes and the implications of these are not yet fully elucidated. 9
-
Exercise-induced bronchoconstriction (EIB). Most patients with asthma will experience EIB but approximately 11% of the population without other forms of asthma also experience this. It is characterised by a reduction in the forced expiratory volume in the first second (FEV1) of > 10% after exercise and can be treated pharmacologically with short-acting beta2-agonists (SABAs) or leukotriene receptor antagonists (LTRAs) and non-pharmacologically with a light warm-up before vigorous exercise for example. The exact mechanisms behind EIB are not fully understood but may include neural and biochemical mediators. 10
Prevalence of asthma
It is estimated that 5.4 million people in the UK are receiving treatment for asthma. Of these, 1.1 million are children (one in 11) and 4.3 million are adults (one in 12) [see www.asthma.org.uk/asthma-facts-and-statistics (accessed 21 May 2015)]. The UK has one of the highest prevalence rates of asthma symptoms in children worldwide. In adults, occupational asthma, for instance because of allergens from animals, flour or grain, may affect up to 20% of the workforce exposed to the sensitiser. An analysis of routine UK databases undertaken by Anderson et al. 11 indicates that the prevalence of asthma in all age groups has risen substantially between 1955 and 2004 (Figure 1).
FIGURE 1.
Patients consulting general practitioners for asthma per 100,000 population, England and Wales, 1955–88. 11 GPRD, General Practice Research Database; MSGP, Mortality Statistics in General Practice. Reproduced from Thorax, 50 years of asthma: UK trends from 1955 to 2004, Anderson H, Gupta R, Strachan D, Limb E, vol. 62, pp. 85–90, 2007, with permission from BMJ Publishing Group Ltd.

Estimates of the prevalence of doctor-diagnosed asthma by age and sex are presented in Table 1, taken from the Health Survey for England 2011. 12
| Age group (years) | Total | |||||||
|---|---|---|---|---|---|---|---|---|
| 16–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75+ | ||
| Men (%) | ||||||||
| Ever | ||||||||
| Self-reported doctor-diagnosed asthma | 25 | 20 | 16 | 12 | 13 | 13 | 9 | 16 |
| Doctor-diagnosed asthma and in last 12 months | ||||||||
| Symptoms of asthma | 6 | 7 | 7 | 4 | 5 | 5 | 4 | 5 |
| No symptoms, asthma controlled with medications | 4 | 3 | 3 | 3 | 3 | 5 | 4 | 3 |
| Current asthma: with symptoms of asthma or taking medication | 10 | 10 | 10 | 7 | 8 | 9 | 8 | 9 |
| No symptoms and no medication for asthma | 90 | 90 | 90 | 93 | 92 | 91 | 92 | 91 |
| Women (%) | ||||||||
| Ever | ||||||||
| Self-reported doctor-diagnosed asthma | 21 | 20 | 17 | 16 | 15 | 16 | 14 | 17 |
| Doctor-diagnosed asthma and in last 12 months | ||||||||
| Symptoms of asthma | 7 | 7 | 7 | 7 | 8 | 5 | 5 | 7 |
| No symptoms, asthma controlled with medications | 4 | 3 | 4 | 3 | 2 | 7 | 5 | 4 |
| Current asthma: with symptoms of asthma or taking medication | 10 | 10 | 11 | 10 | 10 | 12 | 10 | 10 |
| No symptoms and no medication for asthma | 90 | 90 | 89 | 90 | 90 | 88 | 90 | 90 |
| Bases (unweighted) | ||||||||
| Men | 378 | 493 | 642 | 624 | 642 | 518 | 402 | 3699 |
| Women | 476 | 695 | 820 | 874 | 722 | 566 | 563 | 4716 |
| Bases (weighted) | ||||||||
| Men | 644 | 701 | 754 | 720 | 608 | 429 | 318 | 4174 |
| Women | 610 | 686 | 760 | 730 | 630 | 470 | 441 | 4327 |
Based on data from the 2010 Health Survey for England,12 the prevalence of lifetime doctor-diagnosed asthma was 16% among men and 17% among women and decreased with age for both sexes. At the time of the survey, approximately 9% of men and 10% of women were classed as currently having asthma as they had experienced symptoms of asthma or were controlling their symptoms with medication in the previous 12 months. The proportion of respondents with asthma in the last 12 months did not vary by age group in either sex. Of those individuals who had doctor-diagnosed asthma, 30% of men and 39% of women had experienced an asthma attack in the previous 12 months. Of these patients, 42% of men and 52% of women had experienced symptoms during the day in the last week, 22% of men and 29% of women reported that their symptoms had interfered with their usual activities in the last week and 19% of men and 28% of women reported difficulties with sleep in the last week. 12
Any data on the prevalence of asthma are subject to the problems associated with diagnosing asthma. As there is no definitive, objective test, there is significant over- and underdiagnosis of the condition.
Asthma mortality
In England and Wales, deaths resulting from asthma are rare. In 2011, the Office for National Statistics (ONS) reported that there were 1041 reported deaths from asthma in England and Wales [see www.ons.gov.uk/ons/rel/vsob1/death-reg-sum-tables/2011--provisional-/deaths-summary-tables---2011.xls (accessed 21 May 2015)]. Approximately two-thirds (67.2%) of these were in women and almost 79% of all asthma deaths were in adults aged > 65 years (Figure 2).
FIGURE 2.
Registered deaths from asthma, England and Wales, 2011. Source: ONS [see www.ons.gov.uk/ons/rel/vsob1/death-reg-sum-tables/2011--provisional-/deaths-summary-tables---2011.xls (accessed 21 May 2015)].
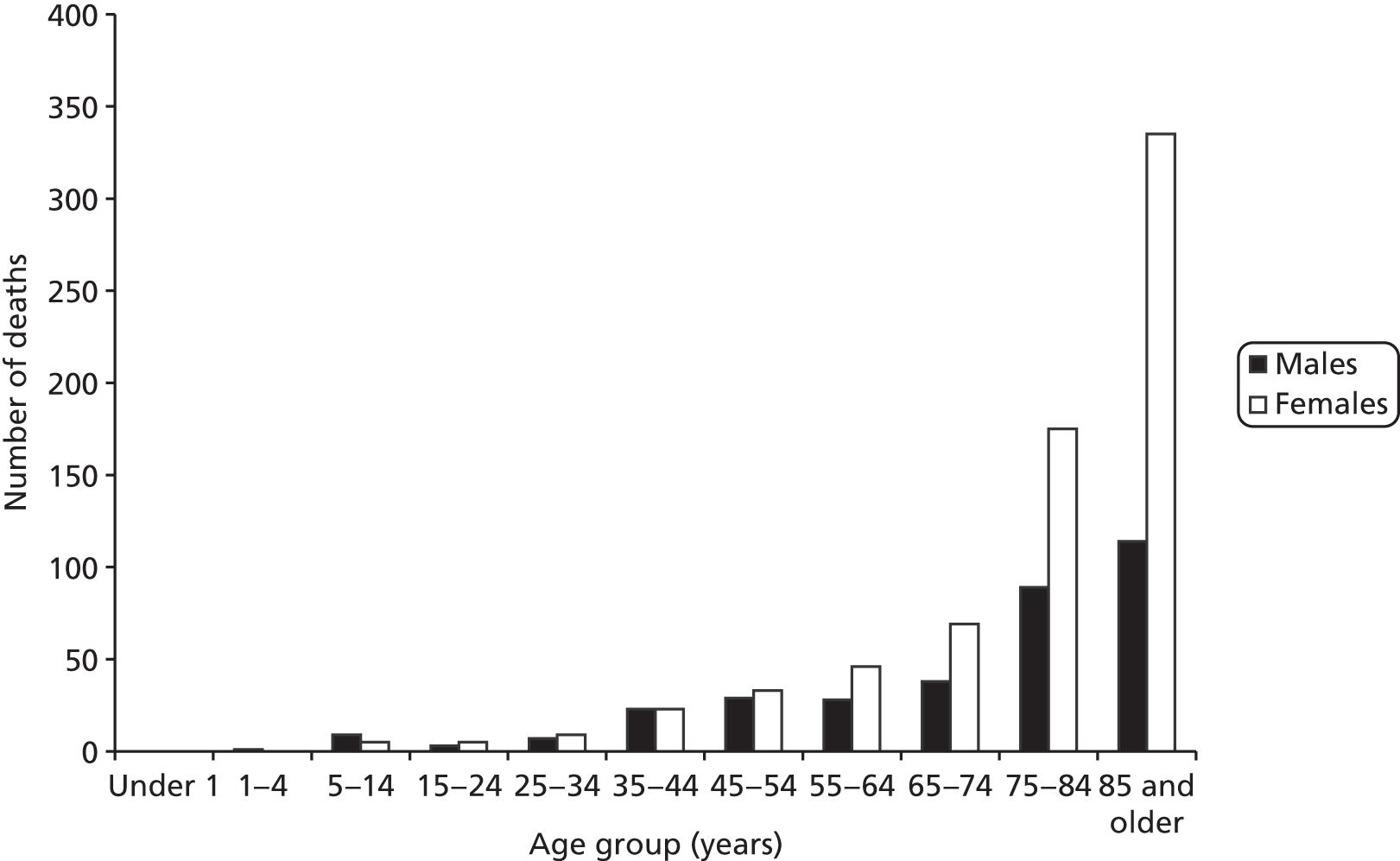
As noted elsewhere,13 audit and case–control studies14–18 indicate that risk factors for death can be separated into four categories: (1) disease severity, (2) medical care factors both before and during the fatal episode, (3) health behaviour such as reduced concordance with prescribed medication, poor inhaler technique and reduced contact with primary care services and (4) adverse psychosocial factors. Shepherd et al. 13 suggest that, given this categorisation, a proportion of asthma-related deaths are preventable, especially in patients aged < 65 years.
Impact of the health problem
Impact of asthma on patients
The principal symptoms of asthma are wheezing attacks and episodic shortness of breath. An acute onset of symptoms is known as an exacerbation. Coughing, which worsens at night, may also be a symptom. Asthma exacerbations tend to vary considerably in terms of frequency and duration. Some people experience one or two per year lasting for a few hours, whereas others have exacerbations lasting for weeks or experience them more frequently. Exacerbations may be precipitated by a wide range of triggers, as described in Classification of asthma. Asthma is a major cause of impaired quality of life and may impact on a patient’s work, recreational activities, physical activities and emotions. However, although patients’ health-related quality of life (HRQoL) may be impacted on by poor asthma control and the incidence of exacerbations, it has been noted elsewhere that meeting clinical treatment goals may not result in noticeable changes in a patient’s quality of life. 13
In the long term, asthma may lead to permanent airflow obstruction and associated loss of quality of life, especially when it is persistent or poorly controlled. 19 Asthma also has a substantial impact on a patient’s ability to work and study and has been estimated to result in at least 12.7 million lost working days per year. 12 Many patients will undergo regular monitoring and will be required to take medication for the rest of their life. There have been concerns that long-term ICS use may reduce growth rates in children, although evidence is conflicting and it appears that any reduction in growth may be transient, with patients eventually achieving a normal adult height. 20,21
Burden on the NHS
Given the high prevalence of people with asthma, asthma treatment represents a significant cost to the NHS. The Health Survey for England 2010 estimated that direct health-care costs associated with asthma are £1B per year. 22 In addition, estimates from 2002 indicate that general practitioner (GP) prescriptions alone are worth approximately £600M per year. 22
As asthma is an incurable condition, treatment, or at the least monitoring, is usually required for the remainder of the patient’s lifetime. However, as the diagnosis of asthma is not definitive there is the potential for misdiagnoses to go undetected for many years or even an entire lifetime. Misdiagnosis can occur when a patient appears to respond to treatment but in fact has experienced a natural resolution of the symptoms of another underlying condition such as a cold, a respiratory infection or allergy. In these cases, patients will appear well controlled and a treating physician may simply assume that the treatment is working. The BTS/SIGN guidelines8 recommend that patients who are well controlled should ‘step down’ their therapy dose. This could result in a patient being taken off treatment altogether and their diagnosis being reconsidered. However, clinical input to this review suggests that step down of doses does not always occur as treatment is relatively cheap per patient and physicians are cautious not to risk exacerbations. As such, there may be long-term unnecessary NHS expenditure associated with these misdiagnoses. Similarly, both overtreatment and undertreatment of patients who have been correctly diagnosed with asthma may be sources of substantial NHS expenditure. Undertreatment may increase costs to the NHS as poor control may lead to an increased rate of severe exacerbations, which require additional primary care management and acute hospital admissions. Overtreatment may increase costs to the NHS because a patient may be able to receive the same level of symptom control with less medication and so the condition could have been treated as effectively at a lower cost.
Guidelines for the diagnosis and management of asthma
Detailed guidelines on the diagnosis and management of asthma have been published and updated. 8 These guidelines are referred to as the BTS/SIGN guidelines throughout the remainder of this report.
Diagnosis of asthma
The diagnosis of asthma is a clinical one and there is no standardised definition of the condition. Central to all definitions in adults is the presence of symptoms (wheezing, breathlessness, chest tightness and cough) and of variable airflow obstruction measured through objective tests of lung function [such as peak expiratory flow rate (PEFR) and FEV1 divided by forced vital capacity (FVC), known as the Tiffeneau–Pinelli index (FEV1/FVC)] and percentage of predicted FEV1 (FEV1%; calculated as the percentage of the predicted FEV1 for a person of the same height, sex and age without diagnosed asthma). Variability in PEFR and FEV1, either spontaneously or in response to therapy, is a characteristic feature of asthma. The BTS/SIGN guidelines8 indicate that the severity of asthma should be judged according to symptoms and the amount of medication required to control symptoms.
More recently, descriptions of asthma have included airway hyper-responsiveness and airway inflammation. It is unclear how these features relate to each other, how they are best measured and how they contribute to the clinical manifestations of asthma.
Figures 3 and 4 present the diagnostic pathways for children and adults, respectively, as they currently stand. 8
FIGURE 3.
Diagnosis of asthma in children according to BTS/SIGN guidelines. 8 –ve, negative; +ve, positive. a, Lung function tests include spirometry before and after bronchodilator (test of airway reversibility) and possible exercise or methacholine challenge (tests of airway responsiveness). Most children over the age of 5 years can perform lung function tests. Reproduced with permission from BTS/SIGN. British Guideline on the Management of Asthma: a National Clinical Guideline. Edinburgh and London: BTS/SIGN; 2012. 8

FIGURE 4.
Diagnosis of asthma in adults according to BTS/SIGN guidelines. 8 PEF, peak expiratory flow. a, See section 2.5.1 of the BTS/SIGN guidelines; b, see table 6 of the BTS/SIGN guidelines. Reproduced with permission from BTS/SIGN. British Guideline on the Management of Asthma: a National Clinical Guideline. Edinburgh and London: BTS/SIGN; 2012. 8

Diagnosis in children is clinically based on recognising a characteristic pattern of episodic symptoms in the absence of an alternative explanation. Lung function tests are less useful because of variability and the inability of very young children to perform these tests reliably. According to the BTS/SIGN guidelines,8 clinical features that increase the probability of asthma include:
More than one of the following symptoms: wheeze, cough, difficulty breathing, chest tightness, particularly if these symptoms:
are frequent and recurrent
are worse at night and in the early morning
occur in response to, or are worse after, exercise or other triggers, such as exposure to pets, cold or damp air, or with emotions or laughter
occur apart from colds
Personal history of atopic disorder
Family history of atopic disorder and/or asthma
Widespread wheeze heard on auscultation
History of improvement in symptoms or lung function in response to adequate therapy.
Reproduced with permission from BTS/SIGN guidelines8
If asthma is suspected, an initial clinical assessment should be carried out to estimate the probability of asthma. According to the BTS/SIGN guidelines,8 based on initial clinical assessment a child can be classified according to their risk of having asthma as:
-
high probability, where an asthma diagnosis is likely
-
low probability, where a diagnosis other than asthma is likely
-
intermediate probability, where the likely diagnosis is uncertain.
For children identified as having a low probability of asthma, a more detailed investigation and specialist referral should be considered. For children with a high probability of asthma, a trial of treatment should be started immediately, with review at 6–8 weeks. When the response is good, the ICS dose should be reassessed every 6 months. Those with a poor response to treatment should undergo more detailed investigations.
There is insufficient evidence at first consultation to make a firm diagnosis of asthma in some children, particularly those aged < 4–5 years. 8 For those children who can perform spirometry and for whom airway obstruction is evident, change in forced expiratory flow volume or peak expiratory flow monitoring should be assessed in response to an inhaled bronchodilator and/or in response to a trial of treatment for a specified period.
In children with an intermediate probability of asthma who can perform spirometry and who have no evidence of airway obstruction, tests for atopic status, assessment of bronchodilator reversibility and, if possible, assessment of bronchial hyper-responsiveness using methacholine, exercise or mannitol should be considered, although these last three would be performed in secondary care. In such cases specialist referral should always be considered.
Other investigations to support a diagnosis of, or alternatively rule out, asthma in children include tests of eosinophilic airway inflammation using induced sputum or exhaled nitric oxide concentrations, tests of atopy by skin prick test or blood eosinophilia and chest radiography or other imaging techniques to investigate other causes.
Diagnosis in adults is also based on clinical history and includes the recognition of a characteristic pattern of symptoms and signs and the absence of an alternative explanation for them. However, in contrast to the diagnostic pathway for children, in adults spirometry is performed at the first consultation to assess the presence and severity of airflow obstruction.
As in the diagnosis of children, adults are also classified as having a high, low or intermediate probability of asthma. Chest radiography and specialist referral may be considered in any patient presenting atypically or with additional symptoms or signs.
Monitoring and management of diagnosed asthma
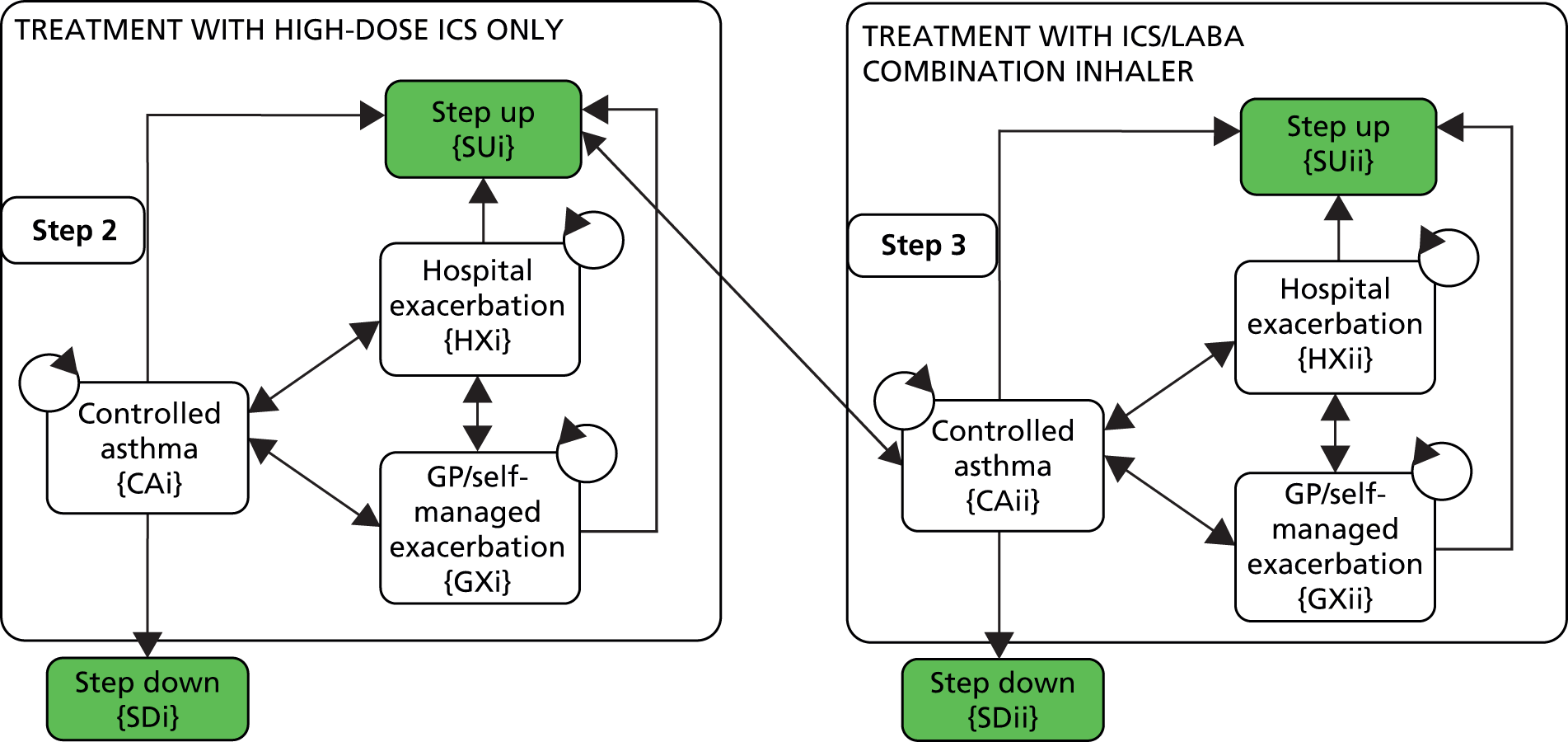
Asthma management aims to control symptoms (including nocturnal symptoms and exercise-induced asthma), prevent exacerbations and achieve the best possible lung function, with minimal side effects of treatment. For both children and adults, asthma is monitored and managed in primary care by routine clinical review on at least an annual basis. These reviews include (but are not limited to) assessment of a patient’s symptom score (using a validated questionnaire), exacerbations, oral corticosteroid (OCS) use, time off school or work, growth and inhaler technique; in adults, lung function is also assessed by spirometry of peak expiratory flow. Patients are managed in a stepwise manner, with escalation of medication until control is reached. This approach to pharmacological management for children and adults is represented in Figures 5 and 6 respectively. 8 Treatment is started at the step most appropriate to the initial severity of the asthma, with the aim of achieving early control of symptoms and optimising respiratory function. Control is maintained by stepping up treatment as necessary and stepping down when control is good.
FIGURE 5.
Management of asthma in children. BDP, beclomethasone dipropionate. Reproduced with permission from BTS/SIGN. British Guideline on the Management of Asthma: a National Clinical Guideline. Edinburgh and London: BTS/SIGN; 2012. 8
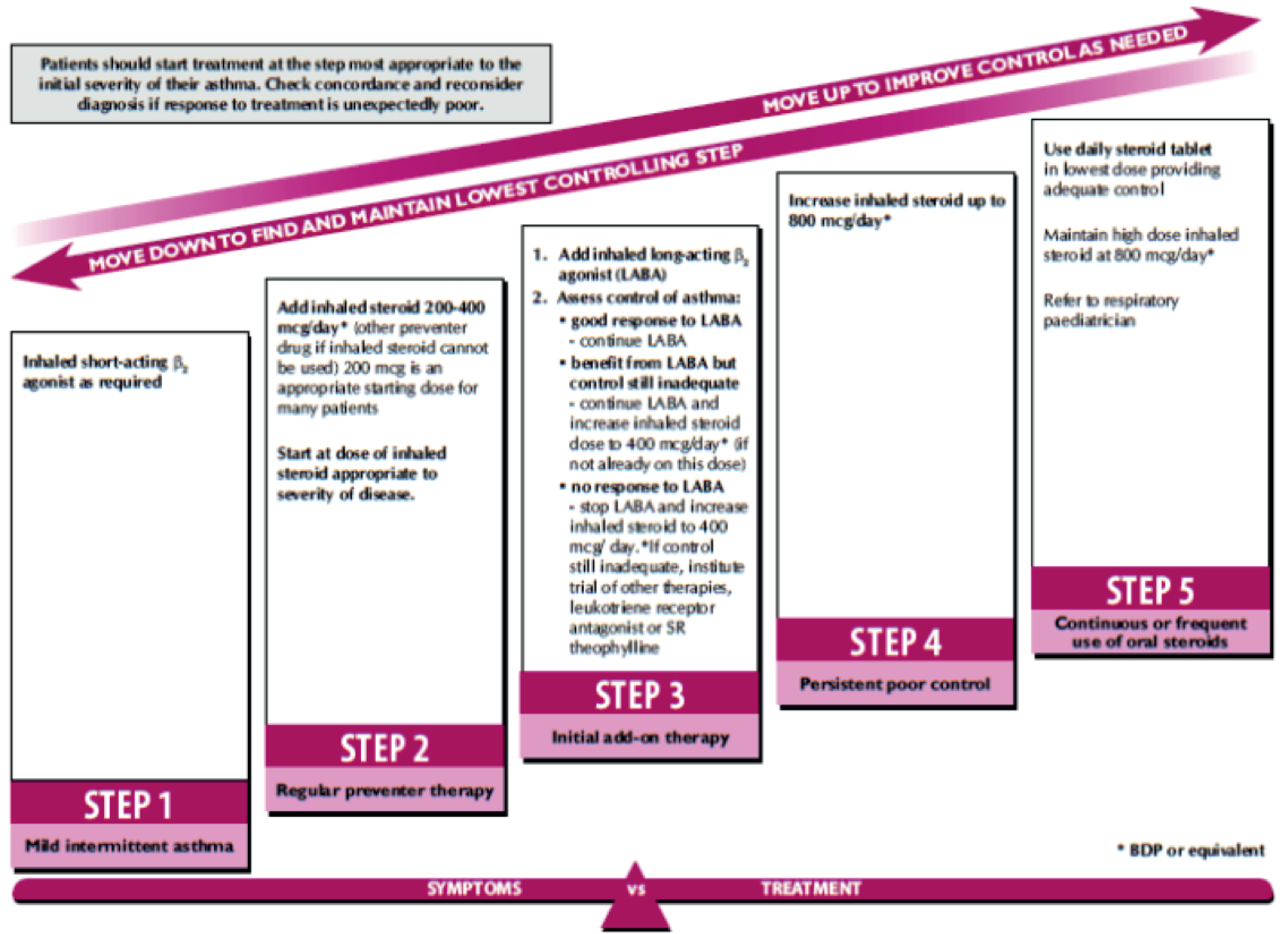
FIGURE 6.
Management of asthma in adults. BDP, beclomethasone dipropionate. Reproduced with permission from BTS/SIGN. British Guideline on the Management of Asthma: a National Clinical Guideline. Edinburgh and London: BTS/SIGN; 2012. 8
Monitoring asthma in children
The BTS/SIGN guidelines8 on the management of asthma state that the monitoring of asthma in children should include the assessment and recording of:
symptom score, e.g. Children’s Asthma Control Test, Asthma Control Questionnaire
exacerbations, oral corticosteroid use and time off school/nursery due to asthma since last assessment
inhaler technique
adherence, which can be assessed by reviewing prescription refill frequency
possession of and use of self management plan/personalised asthma action plan
exposure to tobacco smoke
growth (height and weight centile).
Reproduced with permission from BTS/SIGN guidelines8
The guideline is indistinct with respect to the use of biomarkers such as FeNO in the monitoring of asthma. It states that ‘a better understanding of the natural variability of biomarkers independent of asthma is required and studies are needed to establish whether subgroups of patients can be identified in which biomarker guided management is effective’ (reproduced with permission from BTS/SIGN guidelines). 8
Monitoring asthma in adults
According to the BTS/SIGN guidelines,8 symptom-based monitoring is adequate in the majority of adults with asthma. Those patients with poor lung function and a history of exacerbations in the previous year may be at a greater risk of future exacerbations for a given level of symptoms. For adults, the factors that should be assessed and recorded include:
symptomatic asthma control: best assessed using directive questions such as the . . . Asthma Control Questionnaire or Asthma Control Test
lung function, assessed by spirometry or PEF [peak expiratory flow]
exacerbations, oral corticosteroid use and time off work or school since last assessment
inhaler technique
adherence, which can be assessed by reviewing prescription refill frequency
bronchodilator reliance, which can be assessed by prescription refill frequency
possession of and use of self management plan/personal action plan.
Reproduced with permission from BTS/SIGN guidelines8
Description of technologies under assessment
The potential role of FeNO devices in the diagnosis and management of asthma
Nitric oxide monitors measure FeNO. High FeNO levels in a patient with symptoms suggestive of asthma, such as coughing and wheezing, may suggest that the patient has eosinophilic asthma that could be treated with ICSs (see Classification of asthma). In individuals already diagnosed with asthma, changes in FeNO levels may indicate how well a patient is responding to ICS-based medication, whether medication is being adhered to and whether the dosage of medication should be increased or decreased (titrated or step-up/step-down adjustment). Consequently, FeNO monitors may have a role in the diagnosis, monitoring and management of patients with asthma.
However, current opinion is divided as to the utility of this measurement, in large part because of the potential for various factors to confound FeNO levels. Amongst these are age, sex, smoking status, exposure to environmental tobacco, pregnancy, height, measurement technique and atopic status and medication. 23,24 A further consideration is the observation that the dose–response plateaus within the therapeutic range of ICSs,25,26 although doses up to 800 µg of beclomethasone dipropionate have been reported to be distinguishable from placebo. 27
Current service provision
A number of FeNO devices have been developed. Some of these are hand-held portable devices (such as the devices that are the focus of this assessment) and others are stationary devices that measure FeNO through chemiluminescent techniques. Both types of FeNO monitor have been available for use in the NHS for a number of years. However, they are not available in all secondary care settings and their use in primary care is extremely rare. There are a number of possible reasons why FeNO devices have not had a more widespread diffusion into care, including the lack of clear guidance in the BTS/SIGN guidelines8 on how they should be used, which itself is a consequence of contradictory research, and the previously prohibitive cost and operational requirements of large chemiluminescent devices.
A number of other diagnostic interventions are commonly used in the diagnosis of asthma in England and Wales, as described in Diagnosis of asthma. Some of these are performed in primary care, such as spirometry, reversibility testing and trials of treatment, whereas others are performed in secondary care, such as airway hyper-responsiveness [methacholine challenge test (MCT)] and sputum induction. As noted earlier, monitoring and management of asthma in diagnosed patients is guided by BTS/SIGN guidelines. 8
Technologies under assessment
The three hand-held FeNO devices included in this assessment are NIOX MINO® (Aerocrine, Solna, Sweden), NIOX VERO® (Aerocrine) and NObreath® (Bedfont Scientific, Maidstone, UK).
NIOX MINO
The NIOX MINO device determines FeNO concentration in a breath sample. The device is small, hand-held and portable and it can be used by both adults and children. It requires a 10-second exhalation of breath by the patient at an exhalation pressure of 10–20 cmH2O to maintain a fixed flow rate of 50 ± 5 ml/second. The last 3 seconds of the 10-second exhalation are analysed by a calibrated electrochemical sensor to give a definitive result in parts per billion (ppb). Clinical cut-off values can be applied to the FeNO values to categorise readings as low, intermediate or high, according to the reference ranges for ages < 12 years and ≥ 12 years, as detailed in the sponsor’s submission (Aerocrine. Clinical Guide to Interpretation of FeNO Values. Sponsor’s submission, 2013).
The NIOX MINO device is precalibrated and designed to ensure a service- and calibration-free system. It can be used as a stand-alone device or connected to a PC for monitoring with the NIOX MINO Data Management Program and for use with electronic medical record systems.
The device is Conformité Européenne (CE) marked and was launched in the UK in November 2004. According to information provided by the manufacturer,28 there are currently 18 units available in primary care settings, including general practices and nurse outreach projects, and 197 units in 127 hospitals across the UK. 29 The manufacturer claims that NIOX MINO is indicated for use as follows:
-
to diagnose the specific type of airway inflammation to guide treatment
-
to predict the onset of asthma symptoms or loss of asthma control as a result of eosinophilic airway inflammation
-
to monitor compliance to corticosteroid therapy and the effectiveness of treatment (frequency of exacerbations).
NIOX VERO
During the assessment, Aerocrine began launching a new FeNO device that is intended to replace the NIOX MINO device. The new device is called the NIOX VERO. This is a battery-powered device that features a longer operational life and extended test volume life than the NIOX MINO device.
NObreath
The NObreath device is a diagnostic monitoring device that measures FeNO. The reading is presented in ppb and is claimed to be directly related to the severity of inflammatory disease (e.g. asthma). NObreath requires 12 seconds of exhalation of breath in adults and 10 seconds in children. The device weighs approximately 400 g (including batteries). It has a battery life that lasts for up to 120 tests. The device is CE marked. The device does not have a set lifetime as sensor cells can, and should, be replaced every 2 years.
Anticipated costs associated with the intervention
The marginal per-test costs of each of the three technologies considered within this assessment depend on both fixed costs, such as the initial cost of the devices, and variable costs, such as the costs of consumables.
The NIOX MINO device has a unit cost of £2100 and has an effective unit lifetime of 3 years or 3000 tests (whichever comes first). The NIOX VERO device has a unit cost of £2310 and has an effective unit lifetime of 5 years or 5000 tests (whichever comes first). The NObreath device costs £1995 and has an unlimited unit lifetime. Maintenance for the NObreath device is provided free of charge by Bedfont Scientific.
Test kits for NIOX MINO are available in packs of 300 at a price of £1350, packs of 500 at a price of £2100 or packs of 1000 at a price of £3950. Test kits for NIOX VERO are available in packs of 300 at a price of £1500, packs of 500 at a price of £2200 or packs of 1000 at a price of £4200. Mouthpieces for NObreath are available in packs of 50, 100, 300 or 1000 at prices of £195, £365, £995 and £2995 respectively.
The NObreath device requires replacement of the sensor unit every 2 years at a cost of £295. Besides test kits, there are no other replacement costs for the NIOX MINO and NIOX VERO devices.
This information is summarised in Table 2.
| Item | NIOX MINO (£) | NIOX VERO (£) | NObreath (£) |
|---|---|---|---|
| Lifetime | 3 years or 3000 tests | 5 years or 5000 tests | Unlimited |
| Equipment cost | 2100 | 2310 | 1995 |
| Test kits – 100 | NA | NA | 365 |
| Test kits – 300 | 1350 | 1500 | 995 |
| Test kits – 500 | 2100 | 2200 | NA |
| Test kits – 1000 | 3950 | 4200 | 2995 |
| Sensor replacement | NA | NA | 295 |
| Maintenance | NA | NA | Provided free by Bedfont Scientific |
Chapter 2 Definition of the decision problem
Purpose of the decision to be made
The aim of the assessment was to assess the clinical effectiveness and cost-effectiveness of FeNO measurement in people with asthma. This was separated into two distinct questions:
-
What is the clinical effectiveness and cost-effectiveness of FeNO testing in the diagnosis of asthma in adults and children?
-
What is the clinical effectiveness and cost-effectiveness FeNO testing in the management and monitoring of asthma in adults and children?
The cut-off values used in diagnostic technologies affect their sensitivity and specificity and result in different proportions of patients being true positives (TPs), true negatives (TNs), false positives (FPs) and false negatives (FNs). The consequences of being TP, TN, FP and FN are different in terms of costs and health impacts; hence, the highest sum of sensitivity and specificity may not necessarily lead to optimal health outcomes. This is relevant to the use of FeNO in the diagnosis of asthma and also to its use in guiding asthma management.
Definition of the scope of the assessment
The scope of this assessment was informed by two scoping workshops attended by specialist committee members (SCMs), the External Assessment Group (EAG), the manufacturers, the National Institute for Health and Care Excellence (NICE) and patient stakeholders. The definition of the decision problem reflects the initial NICE scope29 and the subsequent discussions in the second workshop.
Definition of the interventions
Two monitors were identified at the scoping stage for this appraisal: NIOX MINO, which is manufactured by Aerocrine, and NObreath, which is manufactured by Bedfont Scientific. During the latter stages of the assessment, Aerocrine alerted the EAG to a follow-up device to NIOX MINO, the NIOX VERO device. This device is also considered within this assessment although the evidence base is limited. All three interventions are evaluated in the context of the diagnosis and management of asthma.
Populations and relevant subgroups
Relevant population for the assessment of FeNO in the diagnosis of asthma
The population of interest is people with clinical characteristics suggestive of asthma. Relevant subgroups are:
-
any patient aged ≥ 5 years presenting to primary care with symptoms of asthma
-
people with clinical characteristics suggestive of asthma who are difficult to diagnose
-
patients who may experience different outcomes from those of the main population under assessment with the use of FeNO, defined as smokers, the elderly and pregnant women.
Relevant population for the assessment of FeNO in the management of asthma
The population of interest is patients aged ≥ 5 years and diagnosed with asthma. There are two subgroups of particular interest:
-
those with good asthma control who are being considered for a dose reduction
-
those with uncontrolled asthma who are experiencing exacerbations or worsening of symptoms and who are being considered for a dose increase of ICSs or who are being checked for compliance with treatment.
Comparators
The relevant comparators are diagnosis or management according to the current UK guidelines, as described in Chapter 3. In the diagnostic setting, the relevant comparator consists of the current diagnostic pathway without the use of FeNO measurements; this is different for children and adults (see Guidelines for the diagnosis and management of asthma). In the management setting, the relevant comparator is management according to current guidelines without the use of FeNO.
Relevant outcomes for the assessment
The assessment includes consideration of the available evidence across a wide range of clinical and economic outcomes.
Clinical considerations
The intermediate measures for consideration include:
-
diagnostic test accuracy
-
test failure rate.
The clinical outcomes for consideration include:
-
asthma control, which includes asthma symptoms
-
exacerbation rates, which includes the frequency of exacerbations requiring unscheduled contact with health-care professionals, visits to accident and emergency departments or hospitalisations
-
clinical complications associated with acute exacerbations
-
levels of ICSs
-
use of OCSs
-
adverse effects of treatment (including bronchodilators and steroids)
-
HRQoL
-
mortality.
Cost considerations
-
Costs of equipment, reagents and consumables.
-
Maintenance and renewal of equipment.
-
Costs associated with asthma medication.
-
Cost associated with acute exacerbations.
-
Cost of further investigations avoided.
Place of the intervention in the diagnostic/treatment pathways
During the scoping phase of this appraisal, workshop attendees considered that the interventions should be assessed when added to current practice. There are a number of potential places within the diagnostic/treatment pathways where FeNO may be of clinical use and each is likely to have different consequences for clinical effectiveness and cost-effectiveness.
Position of FeNO in the diagnostic pathway: children
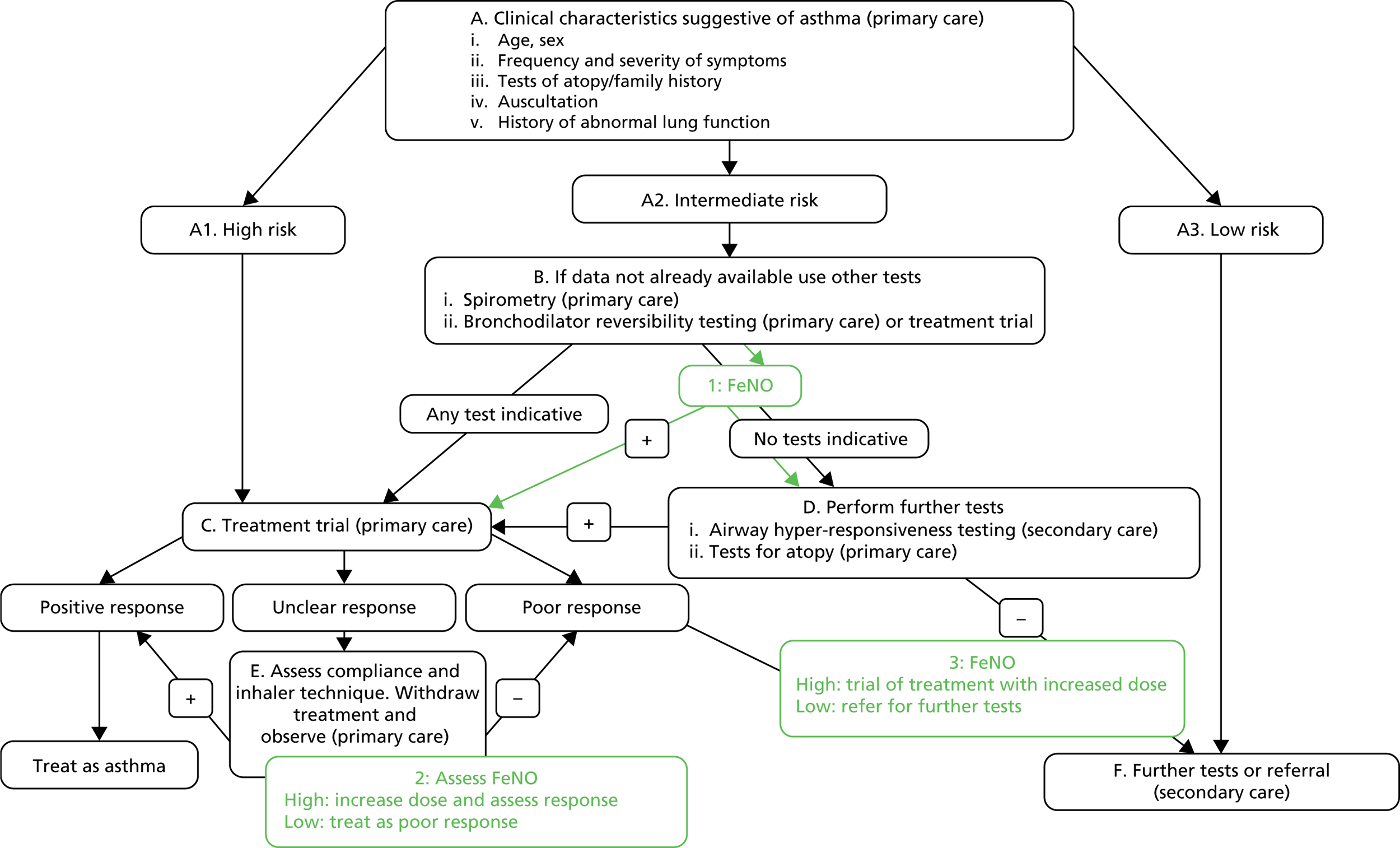
During the scoping workshop it was agreed that FeNO is likely to be of most use in positions 1, 2 and 3 in Figure 7. This figure is based on the BTS/SIGN clinical guidelines,8 with input from a clinician about how the tests are used in practice (Dr John White, York Teaching Hospital NHS Foundation Trust, 17 July 2013, personal communication). This equates to patients who are difficult to diagnose. Depending on whether FeNO is used as a direct replacement for a test or as a rule-in or rule-out test at these positions in the pathway, it may have the ability to prevent expensive secondary care visits if used in primary care. In secondary care it may have additional value alone or in conjunction with existing secondary care tests. FeNO could also be considered to replace the whole pathway or be inserted at other points along the pathway. Tables 3–5 detail the actions and consequences associated with some different replacement and rule-in/rule-out scenarios. In rule-in scenarios, patients testing positive are assumed to have asthma and those testing negative go on to have further tests for asthma. In rule-out scenarios, those who test negative are assumed not to have asthma and those who test positive go on to have further tests for asthma.
FIGURE 7.
Potential positions for FeNO in the diagnostic pathway: children. Source: BTS/SIGN guidelines8 with clinical input from Dr John White (17 July 2013, personal communication).
| Replacement scenario | FeNO measurement | Action taken | Consequence 1 | Consequence 2 |
|---|---|---|---|---|
| TP | High FeNO measurement | Treat as asthma | Correct diagnosis of asthma reached | None |
| FP | Patient’s misdiagnosis goes undetected until worsening of symptoms or routine review or continues lifelong | None | ||
| TN | Low FeNO measurement | Treat as not asthma | Further tests for other conditions | Correct diagnosis reached |
| FN | Further tests negative, re-enter asthma pathway or remain misdiagnosed until exacerbation or return to GP with ongoing symptoms |
| Rule-out scenario | FeNO measurement | Action taken | Consequence 1 | Consequence 2 |
|---|---|---|---|---|
| TP | High FeNO measurement | Treat as possibly asthma and undertake further confirmatory tests | Further tests confirm asthma diagnosis | Treat as asthma |
| FP | Further tests reject asthma diagnosis | Further tests for other conditions or diagnose as non-specific symptoms | ||
| TN | Low FeNO measurement | Treat as not asthma | Further tests for other conditions | Correct diagnosis reached |
| FN | Further tests negative, re-enter asthma pathway or remain misdiagnosed until exacerbation or return to GP with ongoing symptoms |
| Rule-in scenario | FeNO measurement | Action taken | Consequence 1 | Consequence 2 |
|---|---|---|---|---|
| TP | High FeNO measurement | Treat as asthma | Correct diagnosis of asthma reached | None |
| FP | Patient’s misdiagnosis goes undetected until worsening of symptoms or routine review or continues lifelong | None | ||
| TN | Low FeNO measurement | Further tests for asthma | Tests for asthma negative | Further tests for other conditions or diagnose as non-specific symptoms |
| FN | Correct diagnosis of asthma reached | None |
Position of FeNO in the diagnostic pathway: adults
For the diagnostic pathway in adults, FeNO is thought to be of most use in positions 1 and 2 in Figure 8. This equates to patients who are difficult to diagnose. This figure is based on the BTS/SIGN clinical guidelines,8 with input from a clinician about how the tests are used in practice (Dr John White, 17 July 2013, personal communication). This led to the understanding that, in nearly all or at least most cases, patients would undergo a trial of treatment or airway reversibility testing before being referred to secondary care, regardless of their FEV1/FVC ratio. This is slightly different from our initial reading of the BTS/SIGN guidelines, in which only patients with a FEV1/FVC ratio of < 0.7 would undergo these tests, with those with a FEV1/FVC ratio of > 0.7 going on to secondary care for airway hyper-responsiveness testing. Our initial diagrammatic representation of the adult pathway can be viewed on the NICE website (www.nice.org.uk/guidance/dg12/documents/measurement-of-exhaled-nitric-oxide-concentration-in-asthma-niox-mino-and-nobreath-final-protocol2).
FIGURE 8.
Potential positions for FeNO in the diagnostic pathway: adults. PEF, peak expiratory flow. Source: BTS/SIGN guidelines8 with clinical input from Dr John White (17 July 2013, personal communication).

Depending on whether FeNO is used as a direct replacement for an existing test or as a rule-in or rule-out test at these positions in the pathway, it may have the ability to prevent expensive secondary care visits if used in primary care. In secondary care it may have additional value alone or in conjunction with existing secondary care tests. FeNO could also be considered to replace the whole pathway or be inserted at other points along the pathway.
Position of FeNO in the management pathway
The measurement of FeNO may be helpful in individuals diagnosed with asthma to facilitate titration of corticosteroid therapy, to check for compliance with medication and ultimately to lead to better asthma control. It is likely that management decisions would be based on a combination of the monitoring information collected at review and FeNO measurements. In these scenarios, high levels of FeNO could indicate that a patient’s asthma is not fully treated and may be interpreted in combination with symptoms and medication use. A lack of control could be the result of worsening of the disease or it could be the result of failure to comply with medication. The latter could be ascertained through additional checks on the collection of prescriptions or the number of doses used, as measured by a dose-counter inhaler. Low levels of FeNO could indicate that asthma is well controlled and may be interpreted in combination with symptoms and medication use; this could guide a step down of medication and subsequent monitoring of control.
Structure of the assessment report
The assessment report consists of two main parts: (1) an assessment of the clinical evidence relating to FeNO in the diagnosis and management of asthma and (2) an assessment of the cost-effectiveness of FeNO compared with standard care in the diagnosis and management of asthma.
Clinical evidence review
Two systematic reviews and one rapid review were conducted concurrently to identify clinical evidence relevant to the decision problem:
-
Rapid review of the equivalence of FeNO devices. It was not clear at the outset if there would be sufficient primary research evidence relating to the three devices to inform the appraisal. As such, a review of the equivalence of these devices to other FeNO measurement devices was anticipated and appropriate searches were conducted. The review of equivalence was conducted in full when it became apparent that sufficient evidence was not available from the diagnostic accuracy review and management efficacy review. The equivalence review aimed to establish whether measurements from different FeNO measurement devices could be considered to be equivalent to one another and therefore whether studies that used other devices could helpfully inform this appraisal. This review was thought to be the least critical in terms of informing key model inputs and a rapid review using systematic methods was therefore conducted because of time and resource constraints. This represents a change to the published assessment protocol. 30 (www.nice.org.uk/guidance/dg12/documents/measurement-of-exhaled-nitric-oxide-concentration-in-asthma-niox-mino-and-nobreath-final-protocol2.)
-
Systematic review of the diagnostic accuracy of FeNO measurements for asthma. The ideal study would recruit patients with symptoms of asthma, have a cohort design or randomise patients to diagnosis using FeNO or diagnosis using other methods and follow them to clinical outcomes. Such studies are known as end-to-end studies and demonstrate the ability of the test to improve patient outcomes. In the absence of such studies, diagnostic cohort studies represent the next best level of evidence, with modelling of clinical outcomes based on the numbers of patients classed as TP, TN, FP and FN. Below this are correlation studies. All levels of evidence were searched for in this review; lower levels of evidence were consulted when the higher levels of evidence were not identified. When available, three pairs of sensitivity and specificity values were selected: those that produced the highest sum of sensitivity and specificity; those that had the highest sensitivity for rule-in scenarios; and those that had the highest specificity for rule-out scenarios. In rule-in scenarios, patients testing positive are assumed to have asthma and those testing negative go on to have further tests for asthma. In rule-out scenarios, those who test negative are assumed not to have asthma and those who test positive go on to have further tests for asthma.
-
Systematic review of the efficacy of FeNO-guided management of asthma. Existing systematic reviews of randomised controlled trial (RCT) evidence in adults31 and children32,33 meant that only RCT evidence was searched for in this review, with additional interrogation of the database for data on subgroups when RCT evidence was not found.
Cost-effectiveness assessment
The cost-effectiveness assessment of FeNO includes two components: a systematic review of existing economic analyses and the development of two de novo health economic models:
-
Systematic review of the cost-effectiveness of FeNO for the diagnosis and/or management of asthma. A systematic review was undertaken to identify all existing economic analyses of FeNO testing for asthma; this includes published studies as well as evidence submitted by the manufacturers of NIOX MINO, NIOX VERO and NObreath. This included a critical appraisal of the available evidence and a summary of methodological problems and concerns relating to these analyses.
-
Development of two de novo models. Independent health economic models were developed to assess the incremental cost-effectiveness of FeNO compared with standard care in the diagnosis and management of asthma.
Chapter 3 Clinical review
Methods
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
As described in Chapter 2, Clinical evidence review, two systematic reviews and one rapid review were conducted concurrently to identify clinical evidence relevant to the decision problem:
-
rapid review of the equivalence of FeNO devices
-
systematic review of the diagnostic accuracy of FeNO testing for asthma
-
systematic review of the efficacy of FeNO-guided management of asthma.
The protocol is registered with PROSPERO (reference number CRD42013004149) and can be accessed at www.crd.york.ac.uk/NIHR_PROSPERO/.
Search methodology for the clinical reviews
Systematic searches were carried out between March 2013 and April 2013. Update searches were conducted in September 2013 for the diagnostic and management reviews. For the review of device equivalence and for both diagnostic and management reviews, the following databases were searched:
-
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid): 1948–present
-
EMBASE (Ovid): 1974–present
-
The Cochrane Library (Wiley Interscience):
-
Cochrane Database of Systematic Reviews (CDSR): 1996–present
-
Database of Abstracts of Reviews of Effects (DARE): 1995–present
-
Cochrane Central Register of Controlled Trials (CCRCT): 1898–present
-
Health Technology Assessment (HTA) database: 1995–present
-
NHS Economic Evaluation Database (NHS EED): 1995–present
-
-
Science Citation Index Expanded (SCIE) (Web of Science): 1899–present
-
Conference Proceedings Citation Index – Science (CPCI-S) (Web of Science): 1990–present.
The search strategies used in MEDLINE are provided in Appendix 1.
The following trial registers and websites were searched in March 2013 for all three reviews and again in September 2013 for the diagnostic and management reviews (search terms used are provided in Appendix 1):
-
ClinicalTrials.gov (http://clinicaltrials.gov/)
-
metaRegister of Controlled Trials (www.controlled-trials.com/mrct/)
-
US Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database (www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm)
-
EuroScan International Network (http://euroscan.org.uk/).
Management review searches
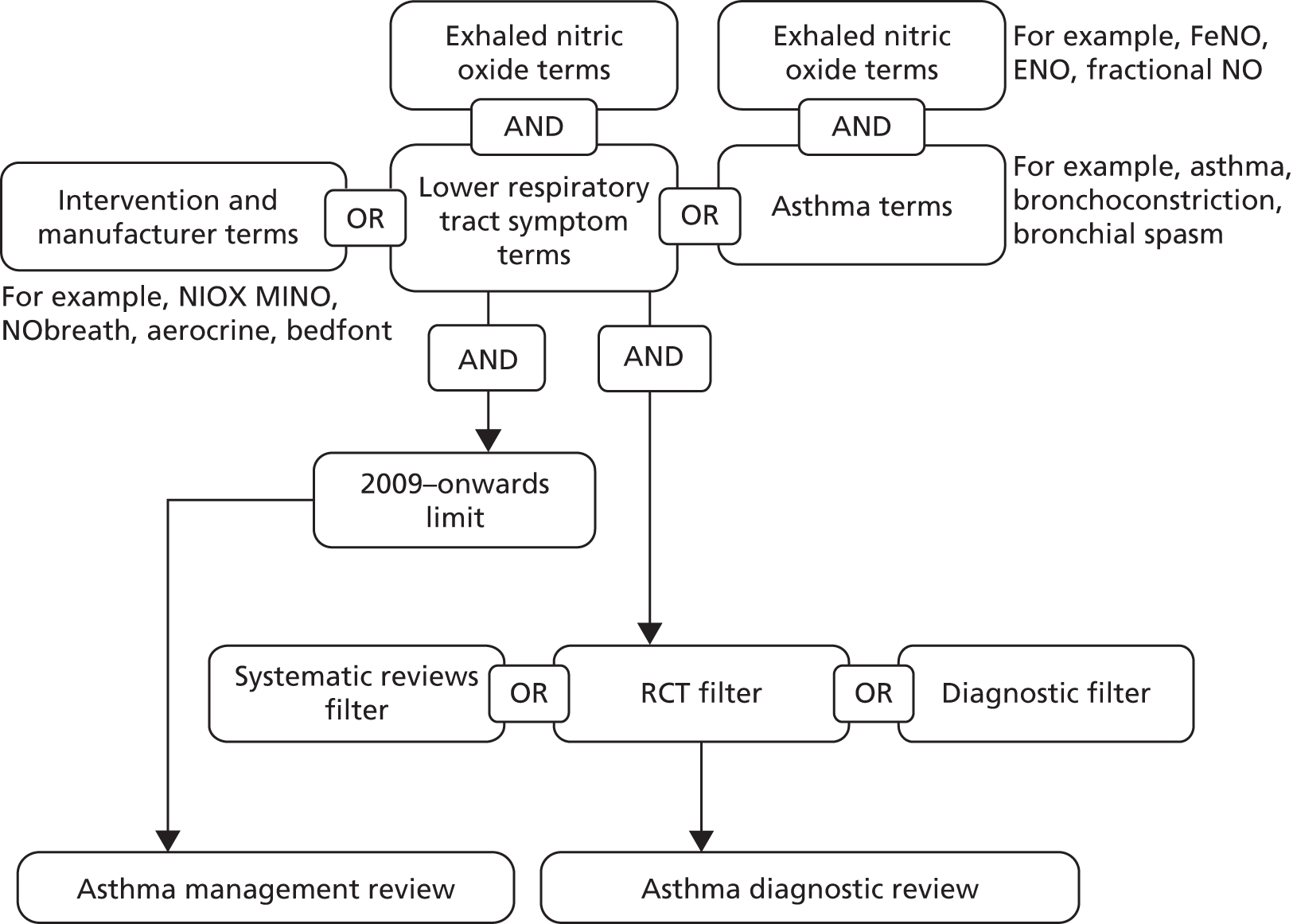
Searches for the management review were developed following the identification of a 2009 Cochrane review. 31 Study design filters were not applied to the strategy in case lower levels of evidence were needed for the subgroups defined a priori in the protocol. The strategy (Figure 9) was made up of (1) free-text terms for NIOX MINO and NObreath (including manufacturer names), (2) subject heading and free-text terms for asthma (e.g. respiratory hypersensitivity, bronchoconstriction) and (3) subject heading and free-text terms for lower respiratory tract symptoms (e.g. coughing, wheezing, chest pain). Search strings (2) and (3) were combined with subject heading and free-text terms for exhaled nitric oxide and the results were added to the results for search string (1). Searches were limited to publications since 2009.
FIGURE 9.
Management and diagnostic studies of FeNO devices.
A summary of the search records retrieved from the searches is provided in Table 6.
| Database | Number of records |
|---|---|
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 991 |
| EMBASE | 2269 |
| CDSR | 44 |
| DARE | 1 |
| CCRCT | 117 |
| HTA database | 8 |
| SCIE | 1387 |
| CPCI-S | 70 |
| Total unique references | 2747 |
Diagnostic review searches
Similar to the management review search strategy, the diagnostic search consisted of terms for NIOX MINO and NObreath, including manufacturer names, and subject heading and free-text terms for asthma and lower respiratory tract symptoms combined with terms for exhaled nitric oxide (see Figure 9). The strategy was combined with three filters: (1) a systematic reviews filter, (2) a RCT filter and (3) a diagnostic filter. No date limits were applied to the searches.
A summary of the search records retrieved from the searches is provided in Table 7.
| Database | Search by study design | Equivalence review | ||
|---|---|---|---|---|
| Systematic reviews | RCTs | Diagnostic studies | ||
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 26 | 958 | 377 | 97 |
| EMBASE | 114 | 1386 | 452 | 282 |
| CDSR | 44 | – | – | 0 |
| DARE | 1 | – | – | 0 |
| CCRCT | – | 509 | – | 10 |
| HTA database | 8 | – | – | 4 |
| NHS EED | 2 | – | – | 1 |
| SCIE | 76 | 637 | 284 | 92 |
| CPCI-S | 3 | 17 | 10 | 8 |
| Total unique references | 227 | 1635 | 680 | 309 |
Equivalence of devices review searches
The analytical validity study searches for NIOX MINO and NObreath were carried out using terms for NIOX MINO and NObreath and the manufacturer names without any application of filters and limits in the databases listed (Figure 10). The numbers of records retrieved by database are provided in Table 7 (final column).
FIGURE 10.
Equivalence studies of FeNO devices.
Additional search for NIOX VERO
Aerocrine’s new device, NIOX VERO, was brought to the attention of the EAG in July 2013. An additional search was conducted on 13 August 2013 to check for any publications relating to this device that would have been missed by the original search. This search consisted of simply the term ‘Niox Vero’. A summary of the search records retrieved from the searches is provided in Table 8.
| Database | Number of records |
|---|---|
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 0 |
| EMBASE | 0 |
| CDSR | 0 |
| DARE | 0 |
| CCRCT | 0 |
| HTA database | 0 |
| NHS EED | 0 |
| SCIE | 0 |
| CPCI-S | 0 |
| ClinicalTrials.gov | 0 |
| metaRegister of Controlled Trials | 0 |
| MAUDE database | 0 |
| EuroScan International Network | 2 |
Management and diagnostic review update searches: September 2013
In response to stakeholder comments received as part of the appraisal process, an update to the management and diagnostic reviews was undertaken in September 2013. Table 9 summarises the search records retrieved. Searches were limited to papers published in 2013.
| Database | Number of records |
|---|---|
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 206 |
| EMBASE | 341 |
| CDSR | 12 |
| DARE | 0 |
| CCRCT | 0 |
| HTA database | 0 |
| SCIE | 250 |
| CPCI-S | 8 |
| Total | 817 |
| Total unique | 464 |
| Clinical trials.gov | 9 |
| metaRegister of Controlled Trials | 32 |
| MAUDE database | 0 |
| EuroScan International Network | 15 |
Reference management
All retrieved citations were downloaded into Reference Manager bibliographic software version 12 (Thomson ResearchSoft, San Francisco, CA, USA) and deduplicated to include only unique citations. The update searches were also deduplicated but not against the original searches.
Study selection
Retrieved citations were considered for inclusion in several stages. First, titles were considered and any studies obviously not relevant were excluded. Second, abstracts were consulted. At this stage, tags were applied to studies in Reference Manager to identify the device used, the age group of the participants and the study design. In instances in which it was obvious which review the study was likely to inform, this tag was also applied. In the third stage, articles tagged as the highest levels of evidence for each review were retrieved and the full texts were compared against the inclusion and exclusion criteria.
Once the full-text selection process was complete, a decision was made whether there were gaps in the evidence that would require lower levels of evidence to be consulted. This was the case for the diagnostic review, in which no end-to-end studies were identified; for the management review, in which only limited evidence was identified for NIOX MINO and no evidence was identified for NObreath; and for some of the subgroups of interest to the review. For the diagnostic review, studies including any device were included rather than just those using NIOX MINO, NIOX VERO or NObreath (see Review of the diagnostic accuracy of FeNO testing for asthma) and, for the management review, studies using any FeNO device were included (see Review of the efficacy of FeNO-guided management of asthma); the rapid review of the equivalence of devices was conducted in full (see Review of the equivalence of devices). To retrieve relevant titles from the database for the subgroups of interest to the review, the keyword search facility in Reference Manager was used to search for the following keywords:
-
elderly asthmatics: elderly, old, older and elderly care
-
smokers: smoke, smoking, smoking.adverse effects, smoking.epidemiology, smoking cessation, smoking cessation programme, smoking habit, smoking/ae [adverse drug reaction] and smoking: epidemiology
-
pregnant women: pregnant, pregnancy, expectant, pregnancy complication/co [complication], pregnancy complication/si [side effect], pregnancy complications, pregnancy diabetes mellitus, pregnancy diabetes mellitus/dt [drug therapy], pregnancy outcome, pregnancy test and pregnant women.
These titles were then sifted by title, abstract and full text for inclusion in the review with relation to criteria for population, intervention and comparator. Criteria on study design and specific outcomes were relaxed and studies of the next best level of evidence that provided data evaluating the use of FeNO measurements in appropriate subgroups were included. The hierarchy of evidence used was as described in the NICE guidelines methods guide. 34
Review of equivalence of devices
Table 10 describes the inclusion and exclusion criteria for this review.
| Criterion | Inclusion | Exclusion | Change from protocol |
|---|---|---|---|
| Population | Studies conducted in humans only, regardless of asthmatic status or recruitment methods | Studies performed in vitro on gas samples unless no test evidence was found in humans | None |
| Primary device | NIOX MINO, NIOX VERO or NObreath operated in accordance with ATS 2005 guidelines:35
|
NIOX VERO added | |
| Comparator | Other chemiluminescent devices operated in accordance with ATS 2005 guidelines:35
|
None | |
| Outcomes | Studies of analytical validity were included if they reported the ability of the test to measure FeNO accurately, by any statistical method, compared with chemiluminescent devices | Studies of inter-rater reliability or intersubject repeatability were excluded | None |
| Study design | Any | None |
Review of the diagnostic accuracy of FeNO testing for asthma
Table 11 describes the inclusion and exclusion criteria for this review and any differences from the published protocol (www.nice.org.uk/guidance/dg12/documents/measurement-of-exhaled-nitric-oxide-concentration-in-asthma-niox-mino-and-nobreath-final-protocol2). At full-text sift stage, some unforeseen questions about the scope were sent to SCM clinicians for clarification. This is documented in Appendix 2.
| Criterion | Inclusion | Exclusion | Change from protocol |
|---|---|---|---|
| Population | Primary population is patients presenting with clinical characteristics suggestive of asthma. The main relevant subgroups within this population are:
|
|
None |
| Intervention | Use of NIOX MINO or NObreath in the diagnosis of asthma, either with or without another test or tests. NIOX MINO and NObreath devices are set to record according to American Thoracic Society 2005 criteria:35
|
|
The protocol stated that studies using the following cut-off values would be included:
Because no studies using NObreath or NIOX VERO were found, the contingency to include any chemiluminescent device was affected |
| Comparator | Any combination or selection of tests and clinical characteristics described in the BTS/SIGN guidelines8 for the diagnosis of asthma | Uses tests to diagnose asthma that are not included in the BTS/SIGN guidelines8 or if the comparator includes the use of FeNO measurements | Studies using tests not in routine use in the UK but mentioned in the BTS/SIGN guidelines8 were included in the review |
| Outcome |
|
Does not report useable diagnostic validity data (i.e. extraction of the numbers of patients who are TP, TN, FP and FN against the reference standard) | None |
| Study design |
|
|
Studies published as abstracts and not reporting sufficient methodological details to allow critical appraisal of study quality were not excluded |
| Setting | Primary care, secondary care, outpatient clinic or specialist clinic | Emergency care diagnosis of exacerbation | None |
Review of the efficacy of FeNO-guided management of asthma
Table 12 describes the inclusion and exclusion criteria for this review and any differences from the published protocol (www.nice.org.uk/guidance/dg12/documents/measurement-of-exhaled-nitric-oxide-concentration-in-asthma-niox-mino-and-nobreath-final-protocol2).
| Criterion | Inclusion | Exclusion | Change from protocol |
|---|---|---|---|
| Population | Patients diagnosed with asthma. The two subgroups of particular interest were:
|
|
None |
| Intervention | Use of NIOX MINO or NObreath in the diagnosis of asthma, either with or without another test. NIOX MINO and NObreath devices are set to record according to American Thoracic Society 2005 criteria:35
Studies monitoring at intervals of > 2 weeks were included Any protocols and cut-off values for management decisions or compliance monitoring were included |
Device that is not validated for measuring FeNO:
Studies in which FeNO is measured on a more regular basis (i.e. not during routine annual review) were excluded |
Studies that did not report any details about the device or measurement methods were included and discussed in the narrative review The protocol stated that ‘only studies using FeNO measurements in:
|
| Comparator |
|
Includes the use of FeNO measurements as part of the management strategy | None |
| Outcome |
|
|
None |
| Study design |
|
|
Studies published as abstracts and not reporting sufficient methodological details to allow critical appraisal of study quality were not excluded |
| Setting | Primary care, secondary care, outpatient clinic or specialist clinic | Emergency care | None |
Data extraction
A different standardised data extraction form was developed for each review following the guidelines given in the Cochrane Handbook for Systematic Reviews of Interventions36 and the Centre for Reviews and Dissemination (CRD) Guidance for Undertaking Reviews in Healthcare;37 these forms were piloted using two studies per review. Missing fields were added as appropriate and backfilled where necessary. Appendix 3 lists the fields that were data extracted for each review. Data were extracted from the studies by one of three reviewers and checked by a second reviewer (SH, ME, TG), except for the rapid review of the equivalence of devices for which a sole reviewer (SH) extracted all relevant data. Any discrepancies were resolved by discussion, with involvement of a third reviewer when necessary. When appropriate, authors were contacted for missing or unclear data. Data from multiple publications of the same study were extracted and quality assessed as a single study. In a change from the protocol, data were not extracted from existing systematic reviews, but directly from the primary research journal articles and conference abstracts.
Quality assessment
As it was a rapid review, quality assessment was not conducted for the review of the equivalence of devices.
Diagnostic cohort studies were assessed using the Quality Assessment of Diagnostic Accuracy Studies – second revision (QUADAS-2) tool. 38 The tool was adapted to the specifics of this appraisal and the scoring scheme can be found in Appendix 4. Because of the complexity of this assessment, items within QUADAS-2 that related to applicability were omitted and this was addressed in detail as follows:
-
Are there concerns that the included patients do not match the review question? – addressed through the subcategorisation of studies according to patient characteristics.
-
Are there concerns that the index test, its conduct or its interpretation differs from the review question? – addressed through a review of the equivalence of devices and through the selection of studies that recorded FeNO according to American Thoracic Society (ATS) guidelines. 35
-
Are there concerns that the target condition as defined by the reference standard does not match the review question? – addressed through the subcategorisation of studies according to the reference standard used.
Management RCT studies were assessed using domains listed in the Cochrane risk of bias tool. 36 The scoring scheme can also be found in Appendix 4.
Studies of lower quality were not formally quality assessed but were considered on their individual merits.
Quality assessment was conducted by one reviewer and checked by a second. A third reviewer was consulted in cases of disagreement.
Analysis and synthesis
A narrative synthesis was conducted for the rapid review of the equivalence of devices and no meta-analysis was planned or attempted.
A narrative synthesis was conducted for the review of diagnostic studies. A meta-analysis was planned if sufficient studies of acceptable clinical heterogeneity in terms of patient populations, devices, cut-off points and reference standards were available. A meta-regression to allow the use of multiple cut-off points in the modelling was planned, again if the necessary data were available with appropriate levels of heterogeneity between studies. However, data were not suitable for meta-analysis or meta-regression.
A narrative synthesis was conducted for the review of management studies. A meta-analysis was planned if enough studies of acceptable clinical heterogeneity in terms of patient populations, devices, cut-off points, interventions, comparators and outcomes were available. Clinical heterogeneity indicated that such an analysis was unlikely to produce meaningful results, but exploratory analyses and sensitivity analyses in relation to elements of study design were conducted in the review of adult studies, even though clinical heterogeneity was high. For rate outcomes, the generic inverse variance method was used in Review Manager version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) to meta-analyse rate ratios. For continuous outcomes, a standardised mean difference analysis was conducted as metrics for ICS use were different.
In all cases, fixed effects were used first and random effects were applied if the I2 statistic indicated that heterogeneity was moderate or high. This was judged to be the case at > 40%.
Results
A total of 4859 citations were retrieved and considered for inclusion in the review. After scrutiny of the titles and abstract, 4454 studies were excluded and the full texts of 405 citations were obtained and consulted. Of these, 338 were excluded (see Appendix 5), one additional study was retained for the subgroup reviews of management in pregnant women and 58 other studies (66 citations) were included in the review. The update search yielded 495 citations. These were not deduplicated against the original search. Of these, 489 were excluded and six studies (six citations) were included in the review. As such, in total, 65 studies (71 citations39–109 plus two sponsors’ submissions: Fukuhara A, Sato S, Saito J, Sato Y, Nikaido T, Inokoshi Y, et al. Conversion equations of FeNO levels measured by two portable and a stationary analyzers. Sponsor’s submission, 2013. Unpublished abstract submitted by Bedfont, 2013. Hedlund A. A Randomized, Multi-center Study to Determine the Agreement between the NIOX MINO® Nitric Oxide Monitoring System and the NIOX VERO@ Device Using the 10-Second Exhalation Mode. Sponsor’s submission received 18 July 2013) were included in the main equivalence, diagnostic and management reviews, including one study on the management of pregnant women with asthma. 102
For the review of subgroups, a total of 162 citations were identified of which 14 studies (14 citations) were included. 110–123 A further three references124–126 (two new studies and one update of a study already included in the main management review) were identified during the update search. Appendices 5 (update search) and 6 (subgroup searches) summarise the process of identifying and selecting relevant literature. As such, a total of 17 studies (17 citations) were included in the subgroup reviews.
No end-to-end studies were identified within the review.
As previously described, a review of the equivalence between FeNO devices was undertaken, alongside a review of diagnostic validity (cohort study design) and a review of management (RCT study design), with data for subgroups of interest to the review taken from lower levels of evidence when necessary. This report considers each review separately in the following order:
-
rapid review of the equivalence of devices [analytical validity; see Equivalence of devices (analytical validity)]
-
systematic review of diagnostic studies (diagnostic validity; see Diagnostic review)
-
systematic review of management studies (see Management review).
Equivalence of devices (analytical validity)
A total of 27 studies [30 citations39–68 plus two sponsors’ submissions (Fukuhara 2013 and Hedlund 2013)] comparing the intervention devices (NIOX MINO, NIOX VERO and NObreath) with other devices were included in the review. One additional study127 was excluded as it compared NIOX MINO with another hand-held device (NoVario; FILT, Berlin, Germany) not in the scope of this appraisal. The studies have been categorised for presentation and discussion according to the devices compared and population age ranges as follows:
-
NIOX MINO compared with the Niox chemiluminescent device (Aerocrine) in adults
-
NIOX MINO compared with the Niox chemiluminescent device in children
-
NIOX MINO compared with other stationary chemiluminescent devices in adults and/or children
-
NIOX VERO compared with NIOX MINO
-
NObreath compared with other stationary chemiluminescent devices in adults and/or children
-
NIOX MINO compared with NObreath in adults and/or children
-
area under the curve (AUC), cut-off points and correction equations
-
test failure rates
-
conclusions.
Three main comparisons were considered in this review:
-
Comparison of means – comparison between reported mean FeNO values as measured by each device in the same cohort. This comparison may be confounded by natural within-patient variance between measurements by the two devices.
-
Correlation coefficients – these show whether measurements by the two devices are correlated but not whether the actual values produced are the same (agreement). Highly correlated devices might produce slopes on a graph (plotting FeNO measurement against a known FeNO concentration) of the same gradient but at different heights, indicating that one device measures consistently higher or lower than another. Correlation coefficients can be confounded by the fact that comparison over wider ranges of values can lead to higher correlation values. 128
-
Bland–Altman analysis – produces a number of useful comparison statistics that assess agreement between devices rather than just correlation. Bland–Altman plots128 plot the mean of two measurements by two devices (x-axis) against the difference between the measurements (y-axis). If the devices agreed perfectly across the whole range of measurements, all points would be at point zero on the y-axis across the range of measurements. However, if agreement is not perfect, the points will fall above and below zero. If there is a systematic bias in the results, such as one device consistently reading higher than the other, the mean of the points will be clustered either above or below zero on the y-axis and this will be evident both visually and by the mean difference value produced. If this deviation is consistent and can be relied on, readings between devices can be corrected by subtracting or adding the mean difference. However, if there is also variance in the difference between devices, points will be more scattered and there will be a wider ‘limit of agreement’, which is calculated as ± 2 standard deviations (SDs). If this limit of agreement is wide by clinical standards, it may be concluded that the devices are not clinically interchangeable, even if the mean difference is relatively small.
NIOX MINO compared with the Niox chemiluminescent device in adults
Eight studies compared NIOX MINO with the Niox chemiluminescent device (Table 13), of which five studies were carried out exclusively with adults39–43 and three studies were undertaken with a mix of adults and other age groups. 44–46 When considering the mean values recorded in each study, differences between studies ranged from 0.3 to 9 ppb. NIOX MINO was found to give largely similar results to the Niox chemiluminescent device in five studies39,41,43,45,46 but gave higher FeNO readings in two other studies. 42,44 One further study40 tested two NIOX MINO devices side by side and found that the mean FeNO recorded was higher for one device than for the Niox chemiluminescent device but similar for the other device and the Niox chemiluminescent device. Another study45 tested three devices and found excellent correlation between them and no statistically significant difference between them. This may indicate that there is some variation between NIOX MINO devices themselves, which may account for some of the heterogeneity in estimates of equivalence with other devices in other studies. In summary:
-
When the cohort mean FeNO value was < 30 ppb as measured by the Niox chemiluminescent device, studies showed small differences between the cohort means for devices,39,40,46 whereas when the mean FeNO value was > 35 ppb as measured by the Niox chemiluminescent device, larger and statistically significant differences in cohort means were seen. 42,44
-
Correlation coefficients ranged from 0.73 to 0.998.
-
Bland–Altman analyses were not reported in a consistent way, with some studies using proportions, some using absolute values and some using log values. It is not clear whether log transformation is appropriate as results varied across studies and were apparently conflicting on this point. When the relationship between devices was multiplicative, differences between devices became greater at higher values. Studies saw limits of agreement (when reported on the absolute scale) of around 10 ppb in both directions. 45,46 These large limits of agreement may be due to an assumption that the relationship is additive rather than multiplicative. The difference in percent reported by Korn et al. 41 is large, with limits of agreement of –46% to 73%, and it is assumed that a log transformation was performed. However, the log values reported by Menzies et al. 39 indicate tighter limits of agreement, but the Bland–Altman plot did not suggest a multiplicative relationship on the absolute scale. It is therefore unclear if the upper and lower limits of agreement between devices are of clinical importance and whether this is a multiplicative or an additive relationship. It seems likely that a range of 20 ppb could be important even at high FeNO values.
| Author, year | Sponsored? | Population | Measurement according to guidelines 200535? | n a | FeNO NIOX MINO (ppb) | FeNO chemiluminescence (ppb) | Comparison data | Correlation coefficient | Bland–Altman analysis (95% limits of agreement) | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Adults: asthmatics or mixed including some asthmatics | ||||||||||
| Grob 200840 | NR | AS | NR | 1 | Mean 32.5 | Mean 26.9 | NIOX MINO device A: no statistically significant difference NIOX MINO device B: 7.2 ppb greater (p = 0.26) |
NIOX MINO device A: r2 = 0.73, p < 0.0001; NIOX MINO device B: r2 = 0.74, p < 0.0001 | NIOX MINO device A: similar (NSD); NIOX MINO device B: trend to read higher (NSD) | |
| Korn 201041 | NR | AS, COPD, HE | Yes | 85 | Median (95% CI) 16.3 (5.0 to 208.3) | Median (95% CI) 14.5 (0 to 196.6) | NIOX MINO 9% (SEM 3%) higher (range –32% to 38%) | Spearman: r = 0.860 | 2% (73% to –46%) | Similar, but wide upper and lower limits of BA plot |
| Menzies 200739 | Yes | AS | Yes | 101 | GM (95% CI) 26.6 (24.5 to 28.9) | GM (95% CI) 26.9 (24.8 to 29.6) | NR | Pearson: r = 0.94, p < 0.001; Spearman: lack of bias at either end of values | Log scale: –0.0 (0.2 to –0.2);b bigger differences at higher valuesc,d | Similar (SSNR) |
| Adults: non-asthmatics | ||||||||||
| Chen 200742 | NR | AL | Yes | 27 | Mean 43.1 | Mean 36.9 | NIOX MINO 7.2 ppb higher (range 6.5–8.0 ppb), p < 0.0001 | r = 0.94–0.99 | NR | NIOX MINO reads higher (SS) |
| Adults: healthy participants | ||||||||||
| Hemmingsson 200443 | Yes | HE | NR | 19 | NR | NR | 0.5 (SD 3.8) ppb | NR | NR | Similar (SSNR) |
| Menzies 200739 | Yes | HE | Yes | 50 | GM (95% CI) 19.3 (17.6 to 21.1) | GM (95% CI) 17.7 (16.1 to 19.4) | NR | Pearson: r = 0.96, p < 0.001; Spearman: lack of bias at either end of values | Log scale: –0.0 (0.1 to –0.2);b bigger differences at higher valuesc,d | Similar (SSNR) |
| Adolescents and adults: asthmatics or mixed including some asthmatics | ||||||||||
| Pizzimenti 200844 | No | AS, HE | Yes | 32 | Mean (95% CI) 47.1 (35.2 to 59.1) | Mean (95% CI) 36.9 (25.0 to 49.0) | NIOX MINO higher, p < 0.05 | r = 0.998, p < 0.001 | NR | NIOX MINO reads higher (SS) |
| No age restrictions: asthmatics or mixed including some asthmatics | ||||||||||
| Alving 200646 | Yes | AS, HE | Yes | 71 | Mean (SD) 27.5 (23.2), n = 62 | Mean (SD) 26.5 (24.2), n = 63 | r = 0.97 | 1.5 (10.2 to –13.2) ppb; bigger differences at higher valuesc | Similar (SSNR); NIOX MINO slightly higher than Niox chemiluminescent device | |
| Khalili 200745 | Yes | AS | Yes | 110 | NR | NR | NIOX MINO higher, –0.5 ppb (p = 0.21) | Spearman: r = 0.98, p < 0.0001 | 0.5 (8.3 to –9.4) ppb; bigger differences at higher valuesc | Similar, but with wide range in BA analysis (NSD) |
NIOX MINO compared with the Niox chemiluminescent device in children
Three studies compared NIOX MINO with the Niox chemiluminescent device in children (Table 14). All cohorts included children with asthma.
-
One study47 reported statistically significantly higher mean FeNO values with NIOX MINO whereas two studies48,49 reported statistically significantly lower values. One of these studies49 had low mean values (< 10 ppb).
-
All studies reported good correlation between the devices.
| Author, year | Sponsored? | Population | Measurement according to ATS 2005 guidelines35? | n a | FeNO NIOX MINO (ppb) | FeNO chemiluminescence (ppb) | Comparison data | Correlation coefficient | Bland–Altman analysis (95% limits of agreement) | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Children: asthmatics or mixed including some asthmatics | ||||||||||
| Kalliola 201149 | No | AS and HE | Yes | 40 | GM 7.8 | GM 9.9 | NIOX MINO lower, p = 0.002 | Pearson: r = 0.972, p < 0.001 | 1.1 (–4.4 to 6.7) ppb; no obvious difference at higher valuesb | NIOX MINO slightly lower (SS) |
| McGill 200648 | Yes | AS and others | Yes | 34 | NR | NR | NIOX MINO lower; greater difference at higher values (p < 0.001) | 0.986 (95% CI 0.972 to 0.993) | 3.9 (–1.1 to 8.9) ppb; mean difference greater with higher FeNO valuesb | NIOX MINO lower (SS) |
| Vahlkvist 200647 | Yes | AS with AL | Yes | 11 | Mean 30 | Mean 26 | NIOX MINO higher, p = 0.004 | r = 0.977; unclear which method | Correlation independent of level | NIOX MINO higher (SS) |
Bland–Altman statistics were reported in two studies48,49 and indicated that NIOX MINO gave lower readings in both cases, by 1.1 ppb (limits of agreement –4.4 to 6.7 ppb) and 3.9 ppb (limits of agreement –1.1 to 8.9 ppb) respectively.
NIOX MINO compared with other chemiluminescent devices in adults and children
Twelve studies compared NIOX MINO with chemiluminescent devices other than the Niox chemiluminescent device and were included in the review (Table 15). Six studies [reported across nine studies and one sponsor’s submission (Fukuhara 2013)] were carried out in adults,50–57,64 three were carried out in an unspecified age group58–60 (two of which had potentially largely overlapping cohorts and will be considered as one study)58,59 and three were carried out in children. 61–63 All studies included at least some asthmatic patients, except that by de Laurentiis et al. 57 Mean FeNO values varied across the studies (from 7 ppb in a healthy cohort of patients52,53 to 64.3 ppb in an asthmatic cohort54). Devices studied in adults/unspecified age groups were the EndoNO (SERES, Aix en Provence, France), N-6008 (SIR, Madrid, Spain), NA623N (CHEST Inc., Tokyo, Japan), NOA 280i (Sievers, Boulder, CO, USA), CLD 88sp (ECO MEDICS, Dürnten, Switzerland), NOA (Sievers, Boulder, CO, USA) and LR2000 (Logan Research Ltd, Rochester, UK), whereas devices tested in children were the CLD 88 and CLD 77 (ECO MEDICS). Within subgroups no chemiluminescence device was tested in more than one study apart from LR2000, which was tested in one comparatively large study (n = 118)58,59 and one small study (n = 16) within the subgroup mixed including some asthmatics. 60 In summary:
-
correlation coefficients (r) in adults/unspecified age groups ranged from 0.876 to 0.96, indicating a good level of correlation between devices.
| Author, year | Sponsored? | Population | Comparator device | Measurement according to ATS 2005 guidelines35? | n a | FeNO NIOX MINO (ppb) | FeNO chemiluminescence (ppb) | Comparison data | Correlation coefficient | Bland–Altman analysis (95% limits of agreement) | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults: asthma or mixed including some asthmatics | |||||||||||
| Boot 200864 | No | AS, HE | CLD 88sp | Yes | 37 | HE: mean (range) 20.3 (8.0 to 39.0); HE smokers:mean (range) 12.2 (5 to 23); AS: mean (range) 63.8 (13 to 172) | HE: mean (range) 18 (7.4 to 35.5); HE smokers: mean (range) 11.1 (4.7 to 20.5); AS: mean (range) 60.8 (10.9 to 184.6) | Non-significant difference | r = 0.975, p < 0.0001 | –10% (–36% to 28%); bigger differences at higher valuesb,c | Similar (NSD) but with wide range in BA analysis |
| Fortuna 2006,52 Fortuna 200753 | NR | AS | N-6008 | Yes | 11 | Mean (SD) 79 (55) | Mean (SD) 40 (30) | Mean (SD) 47 (30) ppb | r = 0.9 | NR | NIOX MINO higher (SS NR) |
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | AS – treated | NA623N | NR | 13 | Treated AS: GM (95% CI) 50.0 (26.5 to 73.4) | Treated AS: GM (95% CI) 64.5 (33.4 to 95.6) | p = 0.009 | NR | NR | NIOX MINO lower (SS) |
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | AS – untreated | NA623N | NR | 14 | Non-treated AS: GM (95% CI) 52.3 (28.8 to 75.7) | Non-treated AS: GM (95% CI) 64.3 (39.6 to 89.0) | p = 0.0167 | NR | NR | NIOX MINO lower (SS) |
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | AS, HE | NA623N | NR | 32 | NR | NR | p < 0.001 | NR | NR | NIOX MINO lower (SS) |
| Ozier 2010,50 Ozier 201151 | Yes | AS | EndoNO | Yes | NIOX MINO: 78, Endo-NO: 89 | Controlled AS: approx. 23;d uncontrolled AS: approx. 38d | Controlled AS: approx. 19;d uncontrolled AS: approx. 30d | 9 ppb difference, p < 0.0001 | Pearson, log-transformed data: r = 0.96, p ≤ 0.001 | Log scale: 0.12 (0.3 to –0.6);d no obvious difference at higher valuesb | NIOX MINO higher (SS) |
| Yoon 2011,56 Kim 201255 | No | AS, HE | NOA 280i | Yes | 100 | Mean (SEM, 95% CI) 18.8 (0.9, 17.0 to 20.6) | Mean (SEM, 95% CI) 22.1 (1.2, 19.8 to 24.5) | 14.5% (2.5%),e range –61.7% to 111.1% | Spearman: r = 0.876, p < 0.001 | 3.3 (–7.0 to 13.6) ppb; mean difference greater with higher FeNO valuesb | NIOX MINO lower (SS) |
| Adults: non-asthmatics | |||||||||||
| de Laurentiis 200857 | Yes | COPD, HE | NOA | Yes | 20 | Mean (SD) 14.8 (5.7) | Mean (SD) 14.2 (5.9) | NR | r = 0.96, p < 0.0001 | –0.4 (– 2.7 to 1.9) ppb; bigger differences at higher valuesb | Similar (SSNR) |
| Adults: healthy participants | |||||||||||
| Fortuna 2006,52 Fortuna 200753 | NR | HE | N-6008 | Yes | 28 | Mean (SD) 20 (8) | Mean (SD) 7 (5) | Mean (SD) 13 (14) ppb | r = 0.92, p = 0.00153 | NR | NIOX MINO higher (SSNR) |
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | HE | NA623N | NR | 5 | GM (95% CI) 23.4 (7.18 to 39.6) | GM (95% CI) 29.3 (7.77 to 50.8) | p = 0.073 | NR | NR | NIOX MINO lower (trend, NSD) |
| Population unclear: mix including some asthmatics | |||||||||||
| Logan Research Ltd 200960 | Yes | AS, HE | LR2000 | NR | 16 | NR | NR | +13.1 ppb | NR | NIOX MINO higher (SSNR) | |
| fMichils 200859 | NR | AS, HE | LR2000 | Yes | 102 | NR | NR | Mean log difference 0.144; NIOX MINO average 39% higher | r = 0.957, p < 0.001; bigger differences at higher valuesc,g | NR | NIOX MINO higher (SSNR) |
| fPeche 200758 | NR | AS, HE, lung transplant patients | LR2000 | Yes | 118 | NR | NR | NIOX MINO average 35% higher | NR highly correlated (p < 0.001) | NR | NIOX MINO higher (SSNR) |
| Children: mix including some asthmatics | |||||||||||
| Chladkova 200863 | No | AS, AL Rh | CLD 88sp | Yes | 82 | > 12 years: GM (95% CI) 17.4 (7.05 to 43.4); < 12 years: GM (95% CI) 11.9 (6.87 to 21.9) | > 12 years: GM (95% CI) 19.6 (7.43 to 51.6); < 12 years: GM (95% CI) 9.59 (4.74 to 19.4) | > 12 years: CLD 88sp 11% higher; < 12 years: CLD 88sp 11% lower | NR | Median ratio: > 12 years: 1.11 (0.59 to 2.08), < 12 years: 0.89 (0.52 to 1.52); bigger difference at higher valuesb | > 12 years: NIOX MINO lower; < 12 years: NIOX MINO higher |
| Park 201161 | NR | AS, HE | CLD 88 | NR | 188 | Mean (SD) 30.8 (23.4) | Mean (SD) 42.8 (30.1) | NR | 0.690 (p < 0.001) | NR | NIOX MINO lower (SSNR) |
| Schiller 200962 | NR | AS, HE | CLD 77 | Yes | 66 | Three measurements: mean 23.7; first measurement: mean 23.6 | Three measurements: mean 20.1; first measurement: mean 20.3 | NR | 0.98, p < 0.001 | Ratio: 0.79 (0.44 to 1.42) | NIOX MINO higher (SSNR); larger difference at higher values (SSNR) |
However, comparison of mean FeNO levels between devices and Bland–Altman statistics within studies recruiting adult or mixed populations show a more variable picture:
-
NIOX MINO appeared to give higher readings than the comparator device according to the mean FeNO values in two studies50–53 and lower readings in a further two studies. 54–56 Devices appeared to be comparable in only two studies. 57,64 Absolute differences in mean FeNO values on the natural scale were not always reported, but when they were they ranged from 9 ppb50,51 to 47 ppb,52,53 which could represent a clinically meaningful difference.
-
Bland–Altman statistics were reported in only four studies,50,51,55–57,64 and were not reported consistently. Mean values were reported as relative values, log-transformed data and absolute data. Interpretation would suggest that mean differences were small, 0–5 ppb, but that limits of agreement were much larger, with ranges of around 10 ppb above and below the mean. The studies with the largest mean differences in absolute FeNO values did not report Bland–Altman statistics.
In children:
-
Correlation coefficients (r) in children ranged from 0.69 to 0.98, indicating variable correlation. The study with the poorest correlation61 also had higher mean FeNO levels and it would be tempting to suggest that the poorer correlation is the result of the greater variability at higher FeNO values. However, the study authors state that correlation improved at higher values. One study63 noted that the direction of disagreement was different in children aged over and under 12 years.
-
The back-transformed Bland–Altman statistics63 and range of ratios reported62 indicate a wide range of agreement and suggest that the devices are not interchangeable.
NIOX VERO compared with NIOX MINO
(Academic-in-confidence information has been removed.) The results are summarised in Table 16.
| (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) |
|---|---|---|---|---|---|---|---|
| (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) | (Academic-in-confidence information has been removed) |
NObreath compared with chemiluminescent devices in adults and children
Four studies compared NObreath with chemiluminescent devices (Table 17). 54,60,65,66 All studies were carried out in adults or in an unspecified age group likely to be adults and all included some asthmatic patients and reported good correlation coefficients. Only one study compared NObreath with the Niox chemiluminescent device;65 this study reported a small statistically significant difference between the devices [intervention arm: geometric mean 22.6 ppb (geometric standard error of the mean 1.075), control arm: geometric mean 24.6 ppb (geometric standard error of the mean 1.073 ppb); p = 0.0002] and a good level of agreement with Bland–Altman analysis. However, the cut-off points with the best combination of sensitivity and specificity derived in this study for each device differed by 10 ppb (25 ppb for NObreath, 15 ppb for Niox; see Areas under the curve, cut-off points and correction equations), indicating that even small differences in agreement may have potentially large effects on derived sensitivity and specificity.
| Author, year | Sponsored? | Population | Comparator device | Measurement according to ATS 2005 guidelines35? | n a | FeNO NObreath (ppb) | FeNO chemiluminescence (ppb) | Comparison data | Correlation coefficient | Bland–Altman analysis (95% limits of agreement) | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults: asthmatics or mix including some asthmatics | |||||||||||
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | AS – treated | NA623N | NR | 13 | Mean (95% CI) 55.6 (28.1 to 83.2) | Mean (95% CI) 64.5 (33.4 to 95.6) | p = 0.015 | NR | NR | NObreath lower (SS) |
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | AS – untreated | NA623N | NR | 14 | Mean (95% CI) 66.8 (38.3 to 95.4) | Mean (95% CI) 64.3 (39.6 to 89.0) | p = 0.413 | NR | NR | Similar (NSD) |
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | AS, HE | NA623N | NR | 32 | NR | NR | p = 0.138 | r = 0.969, p < 0.001 | NR | Similar (NSD) |
| Pisi 201065 | No | AS | Niox | Yes | 154 | GM (GSEM) 22.6 (1.075) | GM (GSEM) 24.6 (1.073) | p = 0.0002 | Pearson: r = 0.95, p < 0.001; Spearman: r = −0.088, p = 0.275) | Log scale: +0.0 (0.5 to –0.5);b no obvious difference at higher valuesc | NObreath lower (SS) but small difference |
| Adults: healthy participants | |||||||||||
| dAntus 201066 | Yes | HE | LR2500 | Yes | 18 | GM (95% CI) 15.7 (11.7 to 21.9) | GM (95% CI) 13.0 (10.1 to 16.7) | p = 0.299 | r = 0.897, p < 0.001 | –3.95 (–10.98 to 4.08) ppb; plot suggestive of systematic biasc | Similar (NSD) |
| GM (95% CI) 14.8 (10.4 to 21.3) | GM (95% CI) 13.5 (10.4 to 17.4) | p = 0.351 | r = 0.913, p < 0.001 | ||||||||
| GM (95% CI) 16.4 (12.3 to 21.9) | GM (95% CI) 12.9 (9.9 to 16.6) | p = 0.179 | r = 0.938, p < 0.001 | ||||||||
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | HE | NA623N | NR | 5 | Mean (95% CI) 25.0 (7.87 to 42.1) | Mean (95% CI) 29.3 (7.77 to 50.8) | p = 0.233 | NR | NR | NObreath lower (trend, NSD) |
| Population unclear: mix including some asthmatics | |||||||||||
| Logan Research Ltd 200960 | Yes | AS, HE | LR2000 | NR | 16 | NObreath compared with LR2000: –3.81 ppb | NObreath lower (SSNR) | ||||
The NObreath device was compared with three other chemiluminescent devices: NA623N, LR2500 and LR2000. Bland–Altman analysis was reported in only one study66 and showed a mean difference of –3.95 ppb compared with the LR2500 device in a healthy cohort with low FeNO values, with wide limits of agreement (–10.98 to 4.08 ppb). Similarly, another study using a Logan device (LR2000) reported an absolute mean difference in FeNO measurements of –3.81 ppb. 60 Comparison with the NA623N device54 showed small differences between mean FeNO values for the cohorts, with NObreath giving lower values in some cohorts.
NObreath compared with NIOX MINO in adults and children
Table 18 details the two studies that compared NObreath with NIOX MINO in adults. 54,66 Both studies found that in most analyses NIOX MINO provided lower mean FeNO values than NObreath. This contradicts the available evidence for comparisons between NIOX MINO and the Niox chemiluminescent device and NObreath and the Niox chemiluminescent device, for which NIOX MINO > Niox > NObreath. This would predict that NIOX MINO should provide higher readings than NObreath. However, it should be noted that there is only one study comparing NObreath with Niox65 and the difference observed was small. The two direct comparisons between NObreath and NIOX MINO include small numbers of patients; only one includes asthmatic patients54 and this study does not provide a Bland–Altman analysis to assess agreement. As such, it is unclear whether the two devices are interchangeable and, if not, in which direction the difference may be.
| Author, year | Sponsored? | Population | Measurement according to ATS 2005 guidelines35? | n a | FeNO NIOX MINO (ppb) | FeNO NObreath (ppb) | Comparison data | Correlation coefficient | Bland–Altman analysis (95% limits of agreement) | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Adults: asthmatics or mix including some asthmatics | ||||||||||
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | AS – treated | NR | 13 | Mean (95% CI) 50.0 (26.5 to 73.4) | Mean (95% CI) 55.6 (28.1 to 83.2) | p = 0.135 | NR | NR | NIOX MINO lower (trend, NSD) |
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | AS – untreated | NR | 14 | Mean (95% CI) 52.3 (28.8 to 75.7) | Mean (95% CI) 66.8 (38.3 to 95.4) | p ≤ 0.001 | NR | NR | NIOX MINO lower (SS) |
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | AS, HE | NR | 32 | NR | NR | p < 0.001 | r = 0.973, p < 0.001 | NR | NIOX MINO lower (SS) |
| Adults: healthy participants | ||||||||||
| bAntus 201066 | Yes | HE | Yes | 18 | GM (95% CI) 12.6 (9.3 to 17.1) | GM (95% CI) 14.9 (11.9 to 18.8) | p = 0.409 | r = 0.661, p = 0.004 | 4.36 (–7.38 to 16.1) ppb | NIOX MINO lower (SS in two analyses); correlation relatively poor |
| GM (95% CI) 10.3 (7.8 to 13.4) | GM (95% CI) 16.1 (13.3 to 19.6) | p = 0.010 | r = 0.750, p < 0.001 | |||||||
| GM (95% CI) 10.9 (8.4 to 14.2) | GM (95% CI) 16.3 (12.8 to 20.6) | p = 0.043 | r = 0.654, p = 0.003 | |||||||
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Yes | HE | NR | 5 | Mean (95% CI) 23.4 (7.18 to 39.6) | Mean (95% CI) 25.0 (7.87 to 42.1) | p = 0.669 | NR | NR | Similar (NSD) |
| Children: asthmatics | ||||||||||
| Kapande 2011,68 201267 | No | AS | Yesc | 109 | NR | NR | NR | Lin’s CC (rho) = 0.65 with reduced major axis slope of 1.32 and intercept of 5.03 | 7.8 (–11.5 to 27.52) ppb; NIOX MINO higher | NIOX MINO higher (SS) |
One study compared NIOX MINO and NObreath in children with asthma67,68 and found that NIOX MINO measured statistically significantly higher than NObreath with a mean difference of 7.8 ppb (95% limits of agreement –11.5 to 27.52 ppb; p < 0.001) in Bland–Altman analysis.
Areas under the curve, cut-off points and correction equations
The mean FeNO values, correlations and Bland–Altman data for each of these trials have already been considered in the previous narrative synthesis; this section considers the impact that differences between devices can have on cut-off points and reports the attempts that researchers have made to provide correction equations for measurements between devices.
Six studies reported other comparative data between devices (Table 19). One study39 demonstrated that the AUC and cut-off points derived to diagnose asthma using the Niox chemiluminescent device or NIOX MINO were very similar (this study used a case–control design and so data were not includable in the diagnostic review), supporting the conclusion that the Niox chemiluminescent device and NIOX MINO are roughly interchangeable. However, another study44 reported a correction factor that should be used to convert NIOX MINO values to Niox values. Three50,51,54,65 of the remaining four studies demonstrate how cut-off points derived using measurements from different devices can be very different, with 7 ppb, 9 ppb and 10 ppb differences. One of these studies65 compared NObreath with the Niox chemiluminescent device directly and found a 10-ppb difference between cut-off points that provide the highest AUC (15 ppb and 25 ppb respectively) and very different sensitivity and specificity values at these cut-off points. Another study54 compared NObreath with NIOX MINO and found a 7-ppb difference in derived cut-off points. In this case, the cut-off point for NObreath was higher at 36 ppb. Two studies52–54 also reported correction equations between various devices, indicating that measurements from these devices are not directly interchangeable.
| Author, year | Population | Comparator device | AUC | Sensitivity, specificity and cut-off values | Correction equation |
|---|---|---|---|---|---|
| NIOX MINO vs. Niox: adults/adolescents – mix including some asthmatics | |||||
| Menzies 200739 | AS, HE | Niox | Niox: 0.654 (95% CI 0.565 to 0.744, p = 0.002); NIOX MINO: 0.619 (95% CI 0.527 to 0.711, p = 0.018) Pairwise comparison difference in AUC of 0.036 (95% CI –0.002 to 0.073, p = 0.061) |
Sens 83.2%, spec 27%; Niox 13 ppb, NIOX MINO 12.5 ppb | NR |
| Pizzimenti 200844 | AS, HE | Niox | NR | NR | FeNO Niox = –1.656 (SE 0.61) + 0.808 (SE 0.009) × FeNO NIOX MINO Correction factor = approximately 0.81 to convert NIOX MINO values to Niox values |
| NIOX MINO vs. other chemiluminescent devices: adults – mix including some asthmatics | |||||
| Fortuna 2006,52 Fortuna 200753 | AS, HE | N-6008 | NR | NR | Correction factor = 3 For HE people: FeNO NIOX MINO = 10 + (1.5 × FeNO N6008) |
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | AS, HE | NObreath, NA623N | NR | NObreath: > 36 ppb; NIOX MINO: > 29 ppb | FeNO NA623N = FeNO NIOX MINO × 1.278 + 3.065; FeNO NA623N = FeNO NObreath × 0.953 + 5.779 |
| Ozier 2010,50 Ozier 201151 | AS | EndoNO | NR | To identify patients who will lose control: NIOX MINO: 40 ppb (sens 85.7%, spec 87.8%); EndoNO: 31 ppb (sens 80.0%, spec 91.1%) | NR |
| NObreath vs. Niox: adults, asthmatics | |||||
| Pisi 201065 | AS | Niox | To identify patients who have ACT ≥ 20 (uncontrolled asthma): NObreath: 0.607 (95% CI 0.525 to 0.684); Niox: 0.644 (95% CI 0.562 to 0.719) Pairwise comparisons of difference in AUC of 0.0369 (95% CI 0.004 to 0.0697, p = 0.028) |
To identify patients who have ACT ≥ 20 (uncontrolled asthma): NObreath: 15 ppb (sens 84%, spec 42%); NIOX: 25 ppb (sens 53%, spec 69%) | NR |
Test failure rates
Nine studies39,45,46,48,49,63–65,67 were included in the review of test failure rates and are described in Table 20. The review intended to draw evidence from studies included in the review of equivalence of devices, the diagnostic accuracy review and the review of the efficacy of FeNO-guided management for asthma. However, all nine of the studies that provided some relevant information with respect to test failure rates came from the review of the equivalence of devices. Eight studies39,45,46,48,49,63,64,67 examined NIOX MINO and two studies65,67 used NObreath. The definition of a test failure was reasonably consistent across the body of literature. Boot et al. ,64 Kalliola et al. ,49 Kapande et al. ,67 Khalili et al. ,45 Pisi et al. 65 and Menzies et al. 39 all defined test failure rates in terms of the number of patients who could not perform acceptable measurements. However, McGill et al. 48 specified a test failure as the inability to provide a successful reading from six attempts; Chladkova et al. 63 defined a test failure as three unsuccessful attempts; and Alving et al. 46 defined a test failure as three invalid readings out of six or one failed single first attempt, depending on the device used.
| Author, year | Patient sample and no. of participants | Relevant device(s) used | Definition of test failure | Test failure rates |
|---|---|---|---|---|
| Alving 200646 | Asthmatic and healthy, all ages (n = 75) | NIOX MINO | Successful test defined as three valid readings out of six or one single first attempt | All: 92% (65/71) successful; children: 84% (31/37) successful; adults: 100% (34/34) successful |
| Boot 200864 | Asthmatic and healthy adults (n = 50) | NIOX MINO | Number of patients who could not perform acceptable measurement | 0/50 |
| Chladkov 200863 | Children with asthma (n = 82) | NIOX MINO | Number of patients who could not perform acceptable measurement within three attempts | 2/36 |
| Kalliola 201149 | Children referred because of asthma symptoms and healthy age-matched children (total n = 55) | NIOX MINO | Number of patients who could not perform acceptable measurement | 15/55 (younger than successful measurement group, p = 0.004) |
| Kapande 201267 | Children (aged 4–14 years) with asthma (n = 109) | NIOX MINO, NObreath | Number of patients who could not perform acceptable measurement | NIOX MINO: 7/109; NObreath: 0/109 |
| Khalili 200745 | Patients (all ages) with asthma (n = 115) | NIOX MINO | Number of patients who could not perform acceptable measurement | 1/115; a few subjects needed to perform a test four to seven times |
| McGill 200648 | Children attending respiratory clinic aged > 5 years (n = 55) | NIOX MINO | Number of children unable to provide a single measurement in of six attempts | 11/55 (therefore at least 66 failed tests out of 330 = 20% test failure rate) |
| Menzies 200739 | Patients known to have mild to moderate asthma (n = 101) and healthy volunteers (n = 50) | NIOX MINO | Number of patients who could not perform acceptable measurement | 0 |
| Pisi 201065 | Patients aged ≥ 14 years diagnosed with asthma according to GINA guidelines;129 included only patients able to perform at least two acceptable measurements with both devices | NObreath | Number of patients who could not perform acceptable measurement | NObreath: 5/154; both (Niox and NObreath): 1; unapproved values: 2 |
All studies included patients with confirmed asthma or symptoms suggestive of asthma; however, the criteria for establishing this diagnosis varied across the literature. For instance, Pisi et al. 65 included those who met the criteria for a diagnosis of asthma according to Global Initiative for Asthma (GINA) guidelines129 whereas Menzies et al. 39 stated that they included those with mild to moderate persistent asthma, McGill et al. 48 included children attending a respiratory clinic and Kalliola et al. 49 included children who had been referred to a specialist clinic because of asthma-like symptoms. In terms of the age range of study samples, the studies by Kalliola et al. ,49 Kapande et al. ,67 Chladkova et al. 63 and McGill et al. 48 were all conducted with children whereas the study by Boot et al. 64 was conducted with adults. In addition, although Menzies et al. 39 did not report any cut-off ages for inclusion, the mean age of the study sample suggests that it was conducted with adults only. Alving et al. 46 included all ages and provided separate data for children and adults whereas Khalili et al. 45 included all ages and reported test failures for the whole study cohort. Pisi et al. 65 included adolescents and adults (the cut-off age for inclusion was ≥ 14 years of age).
NIOX MINO
Eight studies39,45,46,48,49,63,64,67 reported test failure rates with NIOX MINO. The studies by Kalliola et al. ,49 Kapande et al. ,67 Chladkova et al. 63 and McGill et al. 48 were conducted with children whereas the studies by Boot et al. 64 and Menzies et al. 39 were conducted with adults only. Alving et al. 46 and Khalili et al. 45 included all age ranges; however, only Alving provided separate data for adults and children. Although the data sets were limited in both age cohorts, the test failure rates for NIOX MINO were consistently higher in the studies of children. In the adult-only studies of Boot et al. 64 and Menzies et al. ,39 no test failures were observed in cohorts of 50 and 151 participants, respectively, and, similarly, the adult cohort in Alving et al. 46 showed a test failure rate of 0% (0/34 participants). The overall test failure rate in adults is therefore likely to be close to 0%. However, data were unavailable on how many attempts were required on average to obtain a successful reading.
In the children’s cohorts, however, there were test failures in each study. The rate ranged from two out of 36 (5.6%) in Chladkova et al. 63 to 27% (15/55 participants) in Kalliola et al. 49 Alving et al. 46 reported a failure rate of 16% (6/37 participants). McGill et al. 48 classified failures as those who were unable to provide a successful reading from six attempts. They reported 11 patients who fell into this category (20%). In terms of overall FeNO measurement attempts, there were at least 66 failed tests out of 330, that is, also a 20% test failure rate. It was also notable that, in the study with the highest incidence of failure,49 the age of the children who failed was significantly lower than the age of those who successfully provided a measurement (p = 0.004). In Khalili et al. ,45 a failure rate of approximately 0.9% was observed (one failure out of 115 participants); however, as data were not presented separately for adults and children, the age of this participant was not clear.
NObreath
Only two studies65,67 reported test failure rates with the NObreath device. In the study by Pisi et al. 65 of adolescents and adults there were five failures in a cohort of 154 patients (3.2%) and a single patient who failed with both NObreath and the Niox chemiluminescent device. Two patients were said to have provided ‘unapproved values’; however, it was unclear whether this was with Niox chemiluminescence, NObreath or both. The study by Kapande et al. 67 of children only reported no test failures in a cohort of 109.
NIOX VERO
(Academic-in-confidence information has been removed.)
Based on the available data, FeNO test failure rates appeared to generally be low. Most studies reported test failure rates in terms of the number of patients who were unable to provide a satisfactory reading; however, the data also appeared to indicate that multiple readings would be needed for some patients. As this data were not quantified, and usually not reported at all, it is likely that the review underestimates the number of test failures. Moreover, variations in failure rates may be a result of individual differences in operator skills and techniques. Notably, the highest rate of test failure for both NIOX MINO and chemiluminescence was observed in the same study. 49
There may also be important variations in test failure rates depending on age, particularly when using NIOX MINO. Three of the four NIOX MINO studies of adults reported failure rates of 0% whereas one study reported a withdrawal rate of 13.3%; however, it was unclear whether withdrawals could be treated as synonymous with test failures. By contrast, the failure rate in children’s studies ranged from 5.5%to 27%. Indeed, in the study that reported the latter figure,49 the children who failed the test were significantly younger than those who provided a successful reading. Although the study by Khalili et al. 45 included all age groups and reported a much lower failure rate (approximately 0.9%), the mean age of the study cohort (41.9 years, range 6–86 years) may indicate that few children took part. Hence, although the data are too limited to make any definitive conclusions, it seems likely that higher test failure rates may be encountered when using NIOX MINO with children. Finally, with respect to NObreath, the data were particularly sparse, although a low failure rate was apparent. Pisi et al. 65 reported six failures in a cohort of 154 adults and adolescents (3.9%) whereas Kapande et al. 67 saw no failures in a cohort of 109 children.
Discussion
It is worth noting that there were data available within some of these studies on which device was used first. However, because of time constraints, these data were not formally analysed. In some cases the order was random, in others the order was fixed and in yet others this information was not provided. We therefore cannot rule out the possibility that the order of device use may have confounded the results.
NIOX MINO
The comparability of NIOX MINO to chemiluminescent devices appears to be influenced by several factors. There may be some variability between NIOX MINO devices themselves,40 although the extent of this is unclear and may be small. 45 There seems to be a generally consistent observation of poorer equivalence between FeNO devices at higher FeNO levels. There also appears to be a lack of comparability between other chemiluminescent devices themselves, as concluded by one study,41 which leads to heterogeneity in estimates of the comparability of NIOX MINO to chemiluminescent devices. Comparability studies gave different estimates of equivalence between NIOX MINO and other devices and it is therefore unclear if equivalence can be assumed.
NIOX VERO
Only one study provided data on this device. (Academic-in-confidence information has been removed.)
NObreath
There are not enough data and too much apparently conflicting data on the comparability of NObreath to other devices to draw any specific conclusions about its comparability with other devices in asthma populations. However, based on the available evidence it would seem likely that any differences in absolute values between NObreath and other devices are relatively small, although derived cut-offs and maximum sensitivity and specificity may be quite different.
Test failure rates
Because of the small number of studies using NObreath and NIOX VERO, it is not possible to state definitively whether any FeNO measurement device has advantages over any other in terms of test failure rates. In all three studies of adults using NIOX MINO,39,46,64 the test failure rate was 0%, whereas none of the studies using NIOX MINO in children reported a 0% test failure rate, with the lowest being 5.5%63 and the highest 27%. 49 As such, there may be some problems with using NIOX MINO with children, although further research would be needed to confirm this pattern. Conversely, with regard to NObreath, the study in adults reported a 3.3% failure rate65 whereas the study in children reported a 0% failure rate. 67 (Academic-in-confidence information has been removed.)
In summary, the overall test failure rate for FeNO measurement in adults was generally low across all devices and most patients appear to be able to provide FeNO readings, provided that they are permitted sufficient measurement attempts. There may be a higher test failure rate in children using NIOX MINO.
Conclusions
Overall, it cannot be concluded that any two devices are equivalent in all situations. Although there may be situations when they are similar, it appears to depend on the characteristics of the studies and cannot be generalised to all situations. Further research is required to identify what is driving the variability between studies and devices. However, as there is mostly a high degree of correlation between measurements across all devices, estimates of sensitivity and specificity are likely to be a reasonable indication of the potential diagnostic accuracy of using FeNO to guide diagnosis and management, but the derived cut-off points are not likely to be interchangeable between devices. As such, for the purpose of this assessment sensitivities and specificities will be assumed to be interchangeable but it cannot be assumed that the cut-off points that should be used to achieve them will be the same for each device, and there is still some doubt whether the same diagnostic accuracy would be achievable with all devices. The committee will need to consider this in their recommendations.
Diagnostic review
In the absence of an end-to-end study, the next best study design is a cohort study. The ideal cohort study would have recruited patients presenting to their GP with symptoms of asthma and would have assessed the standard UK diagnostic pathway8 as well as this pathway with the addition of FeNO against a reference standard of long-term follow-up. No studies of this design were found. Instead, studies that compared FeNO with or without another test against a reference standard of any test or combination of tests in the UK guidelines (see Figures 3 and 4) were included. UK guideline tests include:
-
Spirometry and lung function tests (mostly FEV1%, FEV1/FVC, PEFR).
-
Airway reversibility: airway obstruction that shows reversibility when a bronchodilator is taken.
-
ICS responsiveness: response to a trial of treatment with ICSs.
-
Airway hyper-responsiveness to methacholine, histamine, exercise or mannitol.
-
Tests for airway inflammation (FeNO or sputum eosinophil counts), although these are currently restricted to a few specialist centres. Studies that use sputum eosinophils within the reference standard are not considered to be similar to UK practice as this test is not widely available.
Twenty-three cohort studies [across 26 publications54,69–79,82–91,93–96 and one sponsor’s submission (Fukuhara 2013)] that reported the sensitivity and specificity of FeNO testing (alone or in combination with another test or tests) compared with the sensitivity and specificity of some or all of the tests within the UK diagnostic pathway were identified and included in the review. A further three studies80,81,92 were identified from the update search. Four of these studies70,77,87,95 included data for FeNO testing in conjunction with another test.
Studies were not similar enough to warrant a meta-analysis, with substantial heterogeneity in populations, cut-off values, devices used and reference standards. We decided to instead focus on key studies that most closely resembled UK practice and resifted the included studies to separate out these studies. We did not, however, want to exclude completely the other studies in case they might prove useful to the committee in their decision-making, especially as some were studies that the SCMs had indicated might be of use when consulted during the clarification of the inclusion and exclusion criteria (see Appendix 2).
This review is subdivided into a number of sections and to aid reading a summary is given here:
-
Studies including adults, adults plus adolescents, all age groups and unspecified age groups.
-
Studies meeting the inclusion criteria – this section tabulates all included studies and assesses their relevance to the decision problem.
-
Studies relevant to the decision problem using only FeNO as the index test – this section provides an appraisal of study quality, a narrative synthesis and greater detail relating to these studies, along with estimates of sensitivity and specificity.
-
Studies using FeNO in conjunction with another test as the index test – this section provides a narrative synthesis and greater detail relating to these studies, along with estimates of sensitivity and specificity.
-
-
Studies including children or children and adolescents.
-
Studies using only FeNO as the index test – because of the smaller number of studies relating to children, all studies are included without selection based on their relevance to the decision problem. This section provides a narrative synthesis and greater detail relating to these studies, along with estimates of sensitivity and specificity.
-
Studies using FeNO in conjunction with another test as the index test – this section provides greater detail relating to one study, along with estimates of sensitivity and specificity.
-
-
Studies providing data on subgroups of interest to the review:
-
adult smokers
-
children exposed to tobacco smoke
-
pregnant women
-
the elderly.
-
More detailed descriptions of the study characteristics for studies that were judged to be less relevant, along with sensitivity and specificity data, are included in Appendices 7 and 8 for reference.
Adults, adults plus adolescents, all age groups and unspecified age groups
Of the 26 studies included in the review, 22 (26 citations) were conducted in adults (13 studies, 16 citations54,69–81,91), adults plus adolescents (three studies, four citations82–85), all age groups (three studies, three citations86–88) or an unspecified age range (three studies, three citations89,90,92). Table 21 summarises the characteristics of the studies and provides a brief description explaining their relevance to the decision problem (note: Schneider et al. 71,72 appears twice in this table as this study reported two differently defined populations). This table should be read alongside Figure 8, which ascribes a letter to positions that already exist in the current diagnostic pathway and a number to the positions where FeNO could be added to the pathway, as agreed during the scoping workshop.
| Author, year | Population | Age group | Device | Cut-off values (ppb) | Reference standard | Position of FeNO in the pathway | Relevance to decision problem |
|---|---|---|---|---|---|---|---|
| Position A | |||||||
| Fortuna 200770 | Position A | Adults | N-6008 | 20 | FEV1, FEV1/FVC, airway responsiveness, WBP, airway hyper-responsiveness (MCT) | No equivalent position in UK pathway | Not relevant – uses WBP and device of unknown equivalence |
| Fukuhara 2011,54 Fukuhara 2013 (sponsor’s submission) | Position A | Adults | NA623 | 40 | At least two of the three criteria of induced sputum eosinophilia, airway hyper-responsiveness and reversibility. Exclusion of other lung diseases | No equivalent position in UK pathway (sputum eosinophilia) | Not relevant – reference standard not similar enough to common UK practice (sputum eosinophilia) |
| Schneider 200971,72 | Position A | Adults | NIOX MINO | 12, 16, 20, 35, 46, 76 | FEV1, FEV1/FVC, airway reversibility, airway hyper-responsiveness (MCT) in sequence similar to that in UK guidelines | FeNO replaces whole pathway (excluding trial of treatment) | Relevant |
| Schneider 201369 | Position A | Adults | NIOX MINO | 9, 12,16, 20, 25, 35, 41, 42, 43, 44, 45, 46, 71 | FEV1, FEV1/FVC, airway reversibility, hyper-responsiveness (MCT) | FeNO replaces whole pathway (excluding trial of treatment) | Relevant |
| Smith 200486 | Position A | All ages | NR | 20 | ATS 1987130 symptoms plus one of airway reversibility, positive response to ICSs, airway hyper-responsiveness (MCT) | FeNO replaces whole pathway | Relevant although uses unknown device of unknown equivalence |
| Smith 200583 | Position A | Adults and adolescents | Niox | ≥ 15, > 47, < 15 | ATS 1987130 symptoms plus one of airway reversibility, positive response to ICSs, airway hyper-responsiveness (MCT) | FeNO replaces whole pathway | Relevant |
| de La Barra 201184 | 25, 40, 50, 70, 90, 110, 130, 150 | Airway reversibility | FeNO replaces airway reversibility | Relevant | |||
| Subset of position A | |||||||
| Cordeiro 201187 | Position A with high prevalence of atopy | All ages | NIOX Flex (Aerocrine, Solna, Sweden) | 27 | Airway reversibility, airway hyper-responsiveness (histamine) | FeNO replaces whole pathway | Relevant although population not whole spectrum |
| NIOX Flex, airway reversibility | FeNO 27 and/or > 12% and 200-ml improvement in FEV1 with bronchodilator | Airway reversibility, airway hyper-responsiveness (histamine) | Combination replacing FEV1/FVC and airway hyper-responsiveness | Relevant although population not whole spectrum | |||
| Heffler 200682 | Position A with rhinitis | Adults and adolescents | Niox | 10, 15, 20, 25, 30, 34, 36, 40, 454, 50, 55, 60, 65, 75, 80, 85, 100 | Airway hyper-responsiveness (MCT) or airway reversibility | FeNO replaces pathway (not including ICS responsiveness but including airway reversibility) | Relevant – rhinitis population generalisable to whole asthma population |
| Pizzimenti 200990 | Position A with chronic cough | Unclear age group | NIOX MINO | 55 | Airway hyper-responsiveness (MCT) | No equivalent position in UK practice | Not relevant – not equivalent to UK practice |
| Difficult to diagnose | |||||||
| Bobolea 201288 | Position H | All ages | NIOX MINO | 30 | Adenosine challenge test | FeNO at position 2 | Relevant |
| Katsoulis 201381 | Before position G (via negative bronchodilator response) | Adults | NIOX MINO | 32 | Hyper-responsiveness (MCT) | FeNO precedes MCT | Relevant |
| Mathew 201191 | Patients at E or F | Unclear age group | NR | NR | Airway hyper-responsiveness (MCT) | Equivalent to FeNO at position 1 or 2 | Not relevant – device and cut-off values not reported and uses unknown device of unknown equivalence |
| Pedrosa 201085 | Before position G | Adults and adolescents | NIOX MINO | 40 | Airway hyper-responsiveness (MCT) | FeNO at position 1 | Relevant |
| Schleich 201277 | Before position G | Adults | Niox | 34 | Airway hyper-responsiveness (MCT) | FeNO at positions 1 and 2 (in place of MCT) | Relevant |
| Niox, FEV1 101% | FeNO > 34 and FEV1 ≤ 101% | Airway hyper-responsiveness (MCT) | Combination replaces FEV1/FVC and airway hyper-responsiveness (MCT) | Relevant | |||
| Schneider 200971,72 | Position Bii | Adults | NIOX MINO | 12, 46 | Airway hyper-responsiveness (MCT) | NA | Not relevant – in the UK these patients are more likely to receive trial of treatment than MCT |
| Difficult to diagnose with chronic cough | |||||||
| Hahn 200774 | Position F with chronic cough | Adults | NOA 280i | 35, 38 | ICS responsiveness | FeNO replaces trial of treatment (whole treatment pathway) | Relevant although diagnosis is of ICS responsiveness not asthma and uses device of unknown equivalence |
| Hsu 201373 | Position F with chronic cough | Adults | NOA 280i | 30, 33.9 | ICS responsiveness | FeNO replaces trial of treatment (whole treatment pathway) | Relevant although diagnosis is of ICS responsiveness not asthma and uses device of unknown equivalence |
| Prieto 200976 | Position F with chronic cough | Adults | Niox | 20 | ICS responsiveness | FeNO replaces trial of treatment | Relevant although diagnosis is of ICS responsiveness not asthma |
| Sato 2008,75 Mathew 201191 | Position F with chronic cough | Adults | Chemiluminescence analyser (Kimoto; Osaka, Japan) | 38.8 | Cough with/without wheeze, sputum eosinophilia, airway reversibility, airway hyper-responsiveness (MCT) | No equivalent position in UK pathway (sputum eosinophilia) | Not relevant – reference standard not similar to common UK practice (sputum eosinophilia) and uses device of unknown equivalence |
| Zhang 201189 | Position F with chronic cough | Unclear age group | NIOX MINO | 31, 40 | Sputum eosinophilia, pulmonary function test, airway hyper-responsiveness, 24-hour oesophageal pH monitoring, skin prick test and serum IgE | No equivalent position in UK practice | Not relevant – reference standard uses tests not used in UK standard practice |
| EIB | |||||||
| El Halawani 200378 | Suspected EIB | Adults | NOA/Sievers 280A | 12 | Exercise challenge | NA | Relevant but unclear if patients similar to those who would be referred to exercise challenge test in the UK and uses device of unknown equivalence |
| Other | |||||||
| Arora 200679 | Mix of undiagnosed and diagnoseda | Adults | Niox | 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 25, 30, 40, 46 | Airway hyper-responsiveness (histamine) | Equivalent to MCT or later? | Not relevant – too different from UK population; reference standard would not be applied to all UK patients |
| Brannan 201392 | Unclear, referred for mannitol challenge in Australia | Unclear age group | HypAir (Medisoft, Leeds, UK) with 0.6-ppb correction to match Niox | 47 | Hyper-responsiveness – mannitol challenge | Unclear if comparable as population unknown | Unclear |
| Chancafe-Morgan 201380 | Unclear, referred for bronchial hyper-responsiveness testing in Spain | Adults | NR | 35 | Hyper-responsiveness (MCT) | Unclear if comparable as population unknown | Unclear |
Studies meeting the inclusion criteria
Of the 22 studies conducted in adults that met the inclusion criteria for the review:
-
Nine studies54,69–72,82–84,86,87,90 recruited patients with symptoms of asthma who were broadly equivalent to patients entering the UK pathway at position A (see Figure 8). Of these, six69,71,72,82–84,86,87 were considered to be of most relevance to the decision problem, although the studies had not necessarily been conducted in the UK.
-
Nine studies73–77,81,85,88,89,91 recruited patients who could be considered ‘difficult to diagnose’ and are located at other points along the diagnostic pathway. These patients had already undergone some of the tests in the UK pathway and had tested negative for asthma thus far. One further study71,72 reported a subset of difficult-to-diagnose patients from a larger cohort of patients at position A (see Figure 8). Of these, seven studies73,74,76,77,81,85,88 were considered to be of most relevance to the decision problem, although the studies had not necessarily been conducted in the UK.
-
One study78 recruited patients with suspected EIB. This study was considered to be relevant to the decision problem, although it had not been conducted in the UK.
-
One study79 recruited army recruits, amongst whom a high proportion are thought to have lied about their asthmatic status. This study was not considered to be relevant to the decision problem and it had not been conducted in the UK.
-
Two studies80,92 did not describe the populations they included and their relevance to the decision problem is therefore unknown.
Reasons for considering a study not relevant to the decision problem are given in Table 21. A total of 14 studies69,71–74,76–78,81–88 were considered relevant and a further two80,92 were considered to be of unknown relevance and are considered in greater detail in the following section. Full study details and results for the six studies54,70,75,79,89–91 considered not relevant are provided in Appendices 7 and 8.
Studies relevant to the decision problem using only FeNO as the index test
From the initial 22 studies conducted in adults, adults plus adolescents, all age groups and unspecified age groups, 14 studies were considered to be of most or of some relevance to the decision problem. These studies are Schneider et al. ,69 Schneider et al. ,71,72 Schleich et al. ,77 Prieto et al. ,76 El Halawani et al. ,78 Hsu et al. ,73 Hahn et al. ,74 Pedrosa et al. ,85 Heffler et al. ,82 Smith et al. 83 (de la Barra et al. 84 reports additional analyses to Smith et al. 83) Smith et al. ,86 Bobolea et al. ,88 Cordeiro et al. 87 and Katsoulis et al. 81 Two further studies80,92 had unclear relevance to the decision problem and are also considered.
Table 22 groups the 14 studies considered to be of relevance to the decision problem according to the position on the pathway and the reference standard and tabulates the study and patient characteristics. Appendix 9 provides more detail about the specifics of the reference standards used and Appendix 10 provides more detail about the patient inclusion and exclusion criteria. There are several main sources of heterogeneity amongst these studies that preclude meta-analysis of the results. These include: the age groups recruited; the spectrum of patients in terms of their position in the pathway and other restrictions in recruitment such as having rhinitis or chronic cough; the device used to measure FeNO; the reference standards used; and the cut-offs reported.
| Author, year | Study design, funding | Country, setting, recruitment dates | Population | Device | Reference standard | n analysed/N recruited (%), reasons for withdrawals | Mean (SD) age (years) | Sex male, n/N (%) | Severity, mean (SD) FEV1% | Mean (SD) FeNO (ppb) | Smokers, n/N (%) | Atopic, n/N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position A vs. whole pathway | ||||||||||||
| Schneider 200971,72 | Prospective, consecutive cohort study Funding: GOVERNMENT |
Germany Primary care – 14 GPs across 10 practices February 2006–June 2007 |
Adults Position A |
NIOX MINO | FEV1, FEV1/FVC, airway reversibility, airway hyper-responsiveness (MCT) | 160/160 (100) | 43.9 | 72/160 (45) | Asthmatics (n = 75) 100 (12.2); COPD (n = 25) 67.8 (18.5); overlap (n = 8) 68.8 (18.4); no OAD (n = 52)107.4 (12.8) | Asthmatics (n = 75) 42.6 (47.9); COPD (n = 25) 16.2 (11.1); overlap (n = 8) 20.4 (18.6); no OAD (n = 52) 24.7 (16.0) | Current and ex-smokers 86/160 (54) | NR |
| Schneider 201369 | Prospective, consecutive cohort study Funding NR: authors report no conflicts |
Germany Private practice run by five pneumologists June 2010–October 2011 |
Adults, position A | NIOX MINO | FEV1, FEV1/FVC, airway reversibility, hyper-responsiveness (MCT) | 393/400 (98) Lack of data: n = 7 |
Asthma: 40.5 (15.4); COPD: 60.8 (17.0); no OAD 44.6 (16.5) | 158/393 (40.2) | Asthmatics 101.3 (17.0); COPD 74.1 (12.3); no OAD 107.7 (16.3) | Asthmatics 42.4 (46.4); COPD 16.6 (6.8); no OAD 22.0 (16.5) | Current smoker 39/393 (9.9); ex-smoker 139/393 (35.4) | NR |
| Smith 200486 | Prospective, consecutive cohort study Funding: mix of industry and non-industry but not device manufacturer |
New Zealand Secondary care, one centre Dates NR |
All patients | NR | Airway reversibility, positive response to ICSs, airway hyper-responsiveness (MCT) | 44/51 (86) Withdrew: n = 7; withdrew consent (time): n = 4; technical difficulties n = 3 |
Asthmatics (n = 17) 41.6 (range 9–72); non-asthmatics (n = 30) 31.8 (range 9–64) | 20 (42.6) | Asthmatics (n = 17) 90.5 (18.4); non-asthmatics (n = 30) 110.0 (13.5) | Asthmatics (n = 17) 52 (34.0); non-asthmatics (n = 30) 15.7 (12.9) | Ex-smokers 5/47 (10.6) | NR |
| Smith 200583 | Prospective, consecutive cohort study Funding: mix of industry and non-industry but not device manufacturer |
New Zealand Secondary care, one centre Dates NR |
Adults and adolescents Position A |
Niox | Airway reversibility, positive response to ICSs, airway hyper-responsiveness (MCT) | 52/60 (87) Withdrew: n = 8; withdrew consent: n = 3; respiratory tract infection n = 1; acute rhinitis n = 1, LTFU n = 3 |
40.5 (range 14–71) | 20/52 (38.5) | 97.8 (14.2) | Range 6.3–242.084 | Current smoker 3/52 (5.8); ex-smoker 10/52 (19.2) | 40/52 (77)84 |
| Position A vs. airway reversibility | ||||||||||||
| de La Barra 201184 | Prospective, consecutive cohort study Funding: Aerocrine and lottery grants New Zealand |
New Zealand Secondary care, one centre Dates NR |
Adults and adolescents Position A |
Niox | Airway reversibility | 52/60 (87) Withdrew: n = 8; withdrew consent: n = 3; respiratory tract infection: n = 1; acute rhinitis: n = 1; LTFU: n = 3 |
40.5 (range 14–71)83 | 20 (38.5)83 | 97.8 (14.2)83 | Range 6.3–242.0 | Current smokers 3/52 (5.8); ex-smokers 10/52 (19.2) | 40/52 (77) |
| Subset of Position A vs. airway reversibility or airway hyper-responsiveness | ||||||||||||
| Cordeiro 201187 | Retrospective (analysis of prospective database) Funding NR, authors reported no conflicts |
Netherlands Secondary care January 2007–September 2007 |
All ages with high prevalence of atopy Position A |
NIOX Flex | Airway reversibility, airway hyper-responsiveness (histamine) | 114/114 (100) | Median (range): asthmatics (n = 42) 39 (7–83); non-asthmatics (n = 72): 38 (7–87) | 43/114 (37.7) | FEV1/FVC%, median (range): asthmatics 70 (42–95); non-asthmatics 77 (69–95) | Median (range): asthmatics (n = 42) 44 (6–290); non-asthmatics (n = 72) 17 (5–45) | 11/114 (9.6) | 81/114 (71.1) |
| Heffler 200682 | Prospective, consecutive cohort study Funding: government/non-industry |
Italy Allergy and immunity clinic Dates NR |
Adults and adolescents with rhinitis Position A |
Niox | Airway hyper-responsiveness (MCT) or airway reversibility | 48/48 (100) | 40.08 (SD NR) | 21 (43.75) | 89.2 (95% CI 80.1 to 98.4) | 59.7 (95% CI 50.2 to 89) | 0 | 35/38 (92.1) |
| Difficult to diagnose vs. airway hyper-responsiveness | ||||||||||||
| Bobolea 201288 | Prospective, consecutive cohort study Funding: NR |
Spain Assuming secondary care Dates NR |
All ages Position H |
NIOX MINO | Adenosine challenge test | 30/30 (100) | 37.3 (range 13–69) | 13/30 (43.3) | NR | NR | NR | NR |
| Katsoulis 201381 | Assume prospective cohort Funding NR |
Greece Army general hospital and local general hospital Dates NR |
Adults Position G |
NIOX MINO | Airway hyper-responsiveness (MCT) | 112 | Median (IQR) 25 (22 to 37) | 95/112 (84.8) | Median (IQR) 89 (83 to 99) | Median (IQR) 20.5 (12 to 34) | NR Recent ex-smokers excluded |
51/112 (45.5) |
| Pedrosa 201085 | Prospective, consecutive cohort study Funding: NR, authors report no conflicts |
Spain Secondary care Dates NR |
Adults and adolescents Position G |
NIOX MINO | Airway hyper-responsiveness (MCT) | 114/115 (99) Withdrawal n = 1, reason NR |
34 (13) | 72/115 (62.6) | (n = 115) 104.29 (14.95) | (n = 115) 34 | Current smokers 17/115 (14.8); ex-smokers 11/115 (9.6) | 100/115 (87) |
| Schleich 201277 | Prospective cohort study Funding: non-industry |
Belgium Secondary care March 2009–December 2009 |
Adults with chronic cough Position G |
Niox | Airway hyper-responsiveness (MCT) | 174/237 (73) n = 63 did not meet inclusion criteria |
41 (16) | 72/174 (41) | 97 (13) | Median (range) 17 (4–271) | 59/174 (33.9) | 84/174 (48) |
| Suspected EIB vs. exercise challenge test | ||||||||||||
| El Halawani 200378 | Prospective, consecutive cohort study Funding: NR |
USA Naval medical centre Dates NR |
Adults Suspected EIB |
NOA/Sievers 280A | Exercise challenge | 49/50 (98) Inability to complete spirometry n = 1 |
27.9 (SD NR) | 35 (71.4) | NR | EIB group (n = 7) 41; non-EIB group (n = 42) 25.6 | 0 | 0 |
| Position H with chronic cough vs. ICS responsiveness | ||||||||||||
| Hahn 200774 | Retrospective cohort study Funding: NR, authors reported no conflicts |
Mayo Clinic, Rochester, MN, USA Secondary care December 2004–November 2005 |
Adults with chronic cough Position H |
NOA 280i | ICS responsiveness | 64/64 (100) | Pooled weighted mean 46.8 (NR) | 26/64 (40.6) | ICS unresponsive group 98; ICS responsive group 94 | ICS unresponsive group 26.0 ± 16.5; ICS responsive group 51.25 ± 20.1 | Current smokers 0/64 (0); ex-smokers 10/64 (15.6) | NR |
| Hsu 201373 | Retrospective cohort study Funding: NR |
Taiwan Asthma and cough-specific clinic June 2007–May 2008 |
Adults with chronic cough Position H |
NOA 280i | ICS responsiveness | 81/114 (71) n = 33 (26 lost after first visit, 7 stopped coughing after 1–2 weeks of treatment for UACS and GORD) |
49 (14) | 33/81 (40.7) | 91.8 (15.3) | Mean rank FeNO by Kruskal–Wallis test: 47 | 0/81 (0) | NR |
| Prieto 200976 | Prospective cohort study, unclear if consecutive Funding: none |
Spain Allergy or respiratory clinics Dates NR |
Adults with chronic cough Position H |
Niox | ICS responsiveness | 43/43 (100) | 48 (95% CI 43 to 52) | 18/43 (41.9) | 113.2 (95% CI 108.0 to 118.3) | GM (95% CI): responders 23.2 (17.5 to 30.7); non-responders 18.6 (14.7 to 24.0) | 0/43 (0) | 43/43 (100) |
| Unclear population vs. airway hyper-responsiveness | ||||||||||||
| Brannan 201392 | Retrospective cohort study Funding NR |
Australia Pulmonary function laboratory (secondary care) Dates NR |
Unclear, referred for mannitol challenge in Australia | HypAir with 0.6-ppb correction to match Niox | Mannitol challenge | 401 | NR | NR | NR | NR | NR | NR |
| Chancafe-Morgan 201380 | Prospective, consecutive cohort study Funding NR |
Spain Pulmonary function laboratory (secondary care) Dates NR |
Unclear, referred for bronchial hyper-responsiveness testing in Spain | NR | Hyper-responsiveness (MCT) | 30 | 44.2 (16.7) | 10/30 (33) | NR | 33.6 (18.7) | NR | NR |
For each study we have selected and presented in tables three sets of sensitivity and specificity estimates. These are:
-
The highest sum of sensitivity and specificity as reported by the authors of the study.
-
The highest sensitivity – in this scenario a negative test result rules out a diagnosis of asthma (see Tables 3–5 for details). This was selected as the cut-off that provided the highest sensitivity. When 100% sensitivity was reported for more than one cut-off, the cut-off that maintained the highest specificity was selected. It should be noted that some studies did not report 100% sensitivity, although this may have been achievable at lower cut-off points. When the cut-off with the highest sensitivity was not also the cut-off with the highest positive predictive value (PPV), this latter cut-off was also presented.
-
The highest specificity – in this scenario a positive test result rules in a diagnosis of asthma (see Tables 3–5 for details). Selected as for the highest sensitivity but for specificity. When the cut-off with the highest specificity was not also the cut-off with the highest negative predictive value (NPV), this latter cut-off was also presented.
It should be noted that superior sets of sensitivity and specificity values may in fact have been achieved but selection was limited to the range of cut-off points reported within studies.
Sixteen studies (18 citations69,71–74,76–78,80–88,92) exploring FeNO measurement for the diagnosis of asthma in adults were assessed for quality according to QUADAS-2 criteria for diagnostic accuracy studies. 38 Although based on the same data as the study by Smith et al. ,83 the study by de la Barra et al. 84 was assessed separately as the analysis was different.
The overall study quality was variable, with the study by Smith et al. 83 scoring well on all of the domains and thus being at least risk of bias. The studies at highest risk appeared to be those by Hsu et al. 73 and Cordeiro et al. ,87 neither of which provided sufficient information on the nature of blinding for the index and reference standard tests. There were also some issues in terms of patient flow in both studies. In the study by Cordeiro et al. ,87 patients did not all receive the same reference test (MCT was provided only if asthma was suspected from other tests). Similarly, in the study by Hsu et al. ,73 the reference standard was allocated based on an algorithm rather than on an a priori set of tests (Figure 11). The studies by Katsoulis et al. 81 and Brannan et al. 92 were poorly reported and at unknown risk of bias. The risk of bias from the conduct of the index test scored worst overall, with only one study scoring positively for this domain. Studies scored poorly for risk of bias from the conduct of the reference standard, with 12 having a score of ‘unclear’ for this domain.
FIGURE 11.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. Dark green circles with + sign, low risk of bias; light green circles with – sign, high risk of bias; medium green circles with ?, unclear risk of bias.

As far as we could ascertain, patient selection did not appear to be a source of bias in the body of literature. All studies avoided a case–control design and recruited appropriately (i.e. those patients presenting with clinical signs of asthma or a subset thereof or patients at a definable point in the UK pathway). However, it was unclear in seven cases73,74,76,77,81,87,92 whether a consecutive sample was recruited.
The conduct of the index test was a potentially important source of bias, with only the study by Smith et al. 83 being free from bias in this domain as a whole. There were two component questions for this domain, one relating to blinding and one to whether the study was a derivation study or a validation study. Ten studies were unclear whether the index test was interpreted blind to the reference standard. 71,73,74,77,80–82,85,88,92 The studies by de la Barra et al. 84 and Cordeiro et al. 87 were not explicit with regard to the blinding of the reference standard results; however, as the index test was performed before the reference standard, it would not have been possible for the investigator to be aware of the reference results at the time of the index test unless interpretation was not performed at the time of the test. This would seem unlikely as FeNO measurement carried out according to standardised protocols is objective and interpretation is not required.
Several further studies were at potential risk of bias in that they were derivation studies that fitted cut-off points to the data post hoc and were thus likely to overestimate accuracy. 69,74,76–78,82,84,85,87
The reference standard and its interpretation was a further source of bias among much of the literature, with only the study by Prieto et al. ,76 the two studies by Schneider et al. 69,71 and the study by Smith et al. 83 being free of bias. It was not possible to ascertain in any of the remaining literature whether the operator conducting the reference standard had been blinded to the results of the index test.
For the most part there was little concern about the patient flow and study timing. However, at least two studies did not provide an identical reference standard for all patients. In the study by Cordeiro et al. 87 patients received MCT only if asthma was suspected based on other tests, whereas in the study by Hsu et al. 73 reference standard provision was algorithm based, with some patients not receiving ICS treatment. It was not necessarily clear in all other studies whether patients received all reference standard tests or a sequence. In other respects, the patient flow and study timing were satisfactory. Dropout rates were low and, when dropouts occurred, these were adequately accounted for in the study reports.
The corpus of included literature was of variable quality, with the study by Smith et al. 83 being at the least risk of bias and the two Schneider studies69,71 also performing well. The conduct of the index test was identified as a potentially serious source of bias among the literature, with few studies providing adequate information on how blinding to the reference test results was achieved. Most studies were derivation studies and, in fitting cut-off points to the data post hoc, are likely to overestimate the accuracy of FeNO as a diagnostic test. In terms of the conduct of the reference standard, few studies provided satisfactory information on how operators were blinded to the results of the index test. However, it is important to stress that this may reflect lack of clarity in the study reports rather than in the conduct of the reference test itself. The likelihood of unblinding biasing the results is therefore unclear.
Position A is the start of the UK pathway. Patients will have undergone no other tests. The reference standard used in studies that recruit patients at this position will determine whether the results relate to a scenario in which FeNO is replacing the whole pathway or a scenario in which it is replacing just one test within the pathway. When replacing just one test it could be used as a rule-in scenario; patients testing positive would go on to be treated as asthmatic and patients testing negative would go on to have further tests for asthma. When a rule-out scenario is used, patients testing positive would go on to have further tests and patients testing negative would go on to be treated as not asthmatic.
Population: Four studies69,71,72,83,86 recruited patients with symptoms of asthma who had not undergone any other tests. These studies are unlikely to have recruited the full spectrum of patients at this point in the pathway because of common exclusions such as those who had experienced a respiratory infection in the last month and those taking ICSs (see Appendix 10). As in many cases a GP may provide a patient with ICSs before confirmation of asthma; these exclusions may result in a patient spectrum that does not reflect UK practice.
Of the four studies, those by Schneider et al. 69,71,72 recruited adults, that by Smith et al. 83 recruited adults and adolescents and that by Smith et al. 86 recruited patients of any age. The largest study was that by Schneider et al. 69 with 393 participants and the smallest was that by Smith et al. 86 with 44 participants. Mean age and FEV1% and FeNO values were not always reported for the whole cohort, making it difficult to compare across studies. All studies recruited more females than males, with the proportion of males ranging from 38.5% to 45%. Schneider et al. 69,71,72 and Smith et al. 83 recruited a mix of smokers, ex-smokers and non-smokers whereas Smith et al. 86 did not recruit any smokers, although this was not listed as an exclusion criteria and may be a result of the small sample size. Only Smith et al. 83 reported how many participants were atopic, with a high prevalence of 77%; the other three studies did not list atopy as an exclusion criterion, making it likely they included a proportion of atopic patients. The study by Schneider et al. 69 excluded pregnant women.
Intervention: The two Schneider studies69,71,72 both used NIOX MINO. The study by Smith et al. 83 used the Niox chemiluminescent device and that by Smith et al. 86 did not report the device used.
Reference standard: The reference standard for both studies by Schneider et al. 69,71,72 was airway reversibility or airway hyper-responsiveness (depending on spirometric test results), whereas in both studies by Smith et al. 83,86 the reference standard also incorporated ICS responsiveness. Although these reference standards do differ, bronchodilator reversibility and ICS responsiveness appear to be used interchangeably in the UK pathway and so both reference standards are equivalent to the whole pathway. However, it is likely that these reference standards may differentially influence estimates of FeNO diagnostic accuracy as FeNO would be expected to correlate better with ICS responsiveness than airway reversibility testing with a bronchodilator.
Study design and setting: All studies were prospective, consecutive cohort studies and none of the studies was funded by the manufacturers of a FeNO device. Both studies by Schneider et al. 69,71,72 were conducted in Germany in primary care or private practice whereas both studies by Smith et al. 83,86 were conducted in New Zealand in secondary care.
Estimates of diagnostic accuracy: Table 23 details the estimates of sensitivity and specificity for these studies. The results do not appear to be similar between studies. The cut-off for the highest sum of sensitivity and specificity varied from 20 ppb to 47 ppb and this did not appear to be dependent on any variable. The studies by Schneider et al. 69,71,72 and Smith et al. 83 all reported higher specificity values than sensitivity values, whereas the study by Smith et al. 86 reported the opposite. This study recruited a mixed population of adults and children and also did not report the device used to measure FeNO. Sensitivities varied greatly across studies, ranging from 32% to 88%. Specificities were more consistent across studies, ranging from 75% to 93%.
| Author, year | Population | Device | n | Reference standard | Highest sum of sensitivity and specificity | Rule out | Rule in | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||||
| Position A vs. whole pathway | |||||||||||||||||||
| Schneider 200971,72 | Adults Position A |
NIOX MINO | 160 | FEV1, FEV1/FVC, airway reversibility, airway hyper-responsiveness (MCT) | 46 | 32 | 93 | 77.3 | 59.5 | 12 | 85 | 24 | 49.6 | 64.5 | 76 | 13 | 100 | 100 | 56.7 |
| 16 | 69 | 53 | 56.5 | 66.2 | |||||||||||||||
| Schneider 201369 | Adults Position A |
NIOX MINO | 393 | FEV1, FEV1/FVC, airway reversibility, hyper-responsiveness (MCT) | 25 | 49 | 75 | 56.0 | 69.5 | 9 | 96 | 13 | 41.6 | 83.8 | 71 | 18 | 97 | 79.5 | 64.6 |
| Smith 200486 | All patients | NR | 44 | Airway reversibility, positive response to ICSs, airway hyper-responsiveness (MCT) | 20 | 88 | 79 | 70 | 91.7 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Smith 200583 | Adults and adolescents Position A |
Niox | 52 | Airway reversibility, positive response to ICSs, airway hyper-responsiveness (MCT) | 47 | 55.6 | 92 | 88.2 | 65.7 | 15 | 81.5 | 48 | 29.4 | 37.1 | As highest sum | ||||
| Position A vs. airway reversibility | |||||||||||||||||||
| de la Barra 201184 | Adults and adolescents Position A |
Niox | 52 | Airway reversibility | 41.7 | NR | NR | 25 | 83.3 | 57.5 | 37.0 | 92 | 110 | 25 | 95 | 60 | 80.9 | ||
| 90 | 41.7 | 92.5 | 62.5 | 84.1 | |||||||||||||||
| Subset of Position A vs. airway reversibility or airway hyper-responsiveness | |||||||||||||||||||
| Cordeiro 201187 | All ages with high prevalence of atopy Position A |
NIOX Flex | 114 | Airway reversibility, airway hyper-responsiveness (histamine) | 27 | 78 | 92 | 84.6 | 88 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| NIOX Flex, airway reversibility | Airway reversibility, airway hyper-responsiveness (histamine) | 27 | 87 | 90 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||||
| Heffler 200682 | Adults and adolescents with rhinitis Position A |
Niox | 48 | Airway hyper-responsiveness (MCT) or airway reversibility | 36 | 77.8 | 60 | 53.8 | 81.8 | 25 | 100 | 46.7 | 52.9 | 100 | 100 | 27.8 | 100 | 100 | 69.8 |
| Difficult to diagnose vs. airway hyper-responsiveness | |||||||||||||||||||
| Bobolea 201288 | All ages Position H |
NIOX MINO | 30 | Adenosine challenge test | 30b | 100 | 29.2 | 26 | 100 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Katsoulis 201381 | Adults Position G |
NIOX MINO | 112 | Hyper-responsiveness (MCT) | 32 | 47 | 85 | 70.1c | 68.1c | 10d | 81 | 39 | 49.9c | 73.2c | 30d | 49 | 82 | 67.1c | 68.2c |
| Atopics | 51 | 26 | 55 | 85 | NR | NR | 10d | 90 | 10% | NR | NR | 30d | 48 | 85 | NR | NR | |||
| Pedrosa 201085 | Adults and adolescents Position G |
NIOX MINO | 114 | Airway hyper-responsiveness (MCT) | 40 | 74.3 | 72.5 | 54.1 | 86.3 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Schleich 201277 | Adults with chronic cough Position G |
Niox | 174 | Airway hyper-responsiveness (MCT) | 34 | 35 | 95 | 87.8 | 62.4 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Niox, FEV1 ≤ 101% | Airway hyper-responsiveness (MCT) | 34 | 24.4 | 98.9 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||||
| Suspected EIB vs. exercise challenge test | |||||||||||||||||||
| El Halawani 200378 | Adults Suspected EIB |
NOA/Sievers 280A | 49 | Exercise challenge | 12b | 100 | 31 | 19.4 | 100 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Position H with chronic cough vs. ICS responsiveness | |||||||||||||||||||
| Hahn 200774 | Adults with chronic cough Position H |
NOA 280i | 64 | ICS responsiveness | 38 | 90 | 85 | 89.5 | 84.6 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Hsu 201373 | Adults with chronic cough Position H |
NOA 280i | 81 | ICS responsiveness | 33.9 | 94.7 | 76.3 | 80 | 94 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Prieto 200976 | Adults with chronic cough Position H |
Niox | 43 | ICS responsiveness | 20 | 53 | 63 | 52.6 | 62.5 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Unclear position in the pathway vs. airway hyper-responsiveness | |||||||||||||||||||
| Brannan 201392 | Unclear | HypAir | 401 | Mannitol challenge | 47 | 30.2c | 96.3c | 65.7c | 85.5c | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Chancafe-Morgan 201380 | Unclear | NR | 30 | Airway hyper-responsiveness (MCT) | 35 | 75 | 83.3 | 75 | 83.3 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
Rule-out cut-off points varied from 9 ppb to 16 ppb with sensitivities between 69% and 96%, specificities between 13% and 53%, PPVs between 29.4% and 56.5% and NPVs between 37.1% and 83.8%. Rule-in cut-off points varied from 47 ppb to 76 ppb, with specificities between 92% and 100%, sensitivities between 13% and 55.6%, PPV between 79.5% and 100% and NPV between 56.7% and 65.7%. The two studies by Schneider et al. 69,71,72 reported very similar rule-in (71 ppb and 76 ppb respectively) and rule-out (9 ppb and 12 ppb respectively) cut-off points, but the cut-off providing the highest sum of sensitivity and specificity was not similar between these two studies (25 ppb and 46 ppb respectively). Smith et al. 83 reported a similar rule-out cut-off point (15 ppb) to these studies but a quite different rule-out cut-off point (47 ppb). Only Schneider et al. 71,72 reported a 100% PPV that would reliably rule patients in, with no studies reporting a 100% NPV.
De la Barra et al. 84 performed a secondary analysis of the data from Smith et al. 83 against a reference standard of airway reversibility only. This is equivalent to replacing airway reversibility with FeNO, or placing FeNO before airway reversibility as a rule-in test or rule-out test, with patients going on to receive this and further tests as appropriate. The cut-off point with the best sum of sensitivity and specificity (41.7 ppb) seemed fairly similar to that reported in Smith et al. 83 (47 ppb). The rule-out cut-off point was somewhat higher at 25ppb, compared with 15 ppb in Smith et al. ,83 and the rule-in cut-off point was higher at 110 ppb (or 90 ppb if selecting the cut-off with the highest NPV) compared with 47 ppb respectively. Sensitivity and specificity values were also different (see Table 23).
Population: These studies recruited patients who may represent a narrower selection of the full spectrum of patients who present with symptoms of asthma than described in the previous studies. Heffler et al. 82 recruited 48 adults and adolescents with rhinitis and symptoms of asthma whereas Cordeiro et al. 87 recruited 114 patients with a ‘high prevalence of atopy’. However, it would appear that these two studies are in fact reasonably comparable to the studies that recruited a full spectrum of patients at position A. The prevalence of atopy in the study by Heffler et al. 82 was higher than that in the study by Cordeiro et al. ,87 at 92% compared with 71%. The study by Smith et al. 83 was the only study to report the prevalence of atopy among the studies that recruited the fuller spectrum of patients at position A and this study reported a similar prevalence of 77%. Similar to the previous studies, Heffler et al. 82 did not recruit any smokers whereas 9.6% of the participants in the study by Cordeiro et al. 87 were smokers. Mean age, severity and FeNO values were not reported in a way that allowed comparison between studies.
Intervention: Heffler et al. 82 used the Niox chemiluminescent device and Cordeiro et al. 87 used NIOX Flex.
Reference standard: Both studies used a combination of airway reversibility and airway hyper-responsiveness as the reference standard, which is equivalent to the whole UK pathway.
Study design and study setting: The study by Heffler et al. 82 was a prospective consecutive cohort study conducted in Italy in an allergy and immunity clinic. The study by Cordeiro et al. 87 was a retrospective analysis of a prospective database conducted in the Netherlands in secondary care. Neither study was funded by the manufacturers of the FeNO devices.
Estimates of diagnostic accuracy: Table 23 details the estimates of sensitivity and specificity for these studies. In Heffler et al. ,82 the sensitivity and specificity for the highest sum of sensitivity and specificity do not seem noticeably different from the values in studies recruiting the fuller spectrum of patients with symptoms of asthma (77.8% and 60% respectively), although the rule-in scenario achieved 100% for specificity and the rule-out scenario achieved 100% for sensitivity, which was not achieved by the studies with a full spectrum of patients at position A. This study also reported higher values for the paired sensitivity and specificity in the rule-in and rule-out scenarios than the studies with a fuller spectrum of patients at position A. In the study by Cordeiro et al. ,87 sensitivity and specificity were 78% and 92%, which has a similar sum to the highest pair of sensitivity and specificity values reported for studies recruiting the full spectrum of patients, reported by Smith 200486 at 88% and 79% respectively, but with the balance between sensitivity and specificity inverted.
The most appropriate reference standard in the difficult-to-diagnose population in relation to UK guidelines varies according to where in the pathway the group is recruited from, in other words, which tests they have already undergone and which test they would get next. As previously described, when a rule-in scenario is used, patients testing positive would go on to be treated as asthmatic and patients testing negative would go on to have further tests for asthma. When a rule-out scenario is used, patients testing positive would go on to have further tests and patients testing negative would go on to be treated as not asthmatic.
Four studies recruited patients who fall into the difficult-to-diagnose category and were of relevance to the decision problem, but none recruited the same spectrum of patients at exactly the same point in the pathway (see Appendix 10 for more details of study inclusion criteria). Schleich et al. 77 recruited adults with chronic cough who had a negative test for airway reversibility and normal spirometry (position G in the UK pathway). Pedrosa et al. 85 and Katsoulis et al. 81 also recruited patients at position G but in the study by Pedrosa et al. 85 this was not restricted to those with chronic cough and included adolescents as well as adults. Bobolea et al. 88 recruited a somewhat different spectrum of patients who were of all ages and who had a negative test for airway reversibility, normal spirometry and a negative MCT. Mean ages were between 34 and 41 years and FEV1% when reported was similar at 97% and 104%; however, FeNO values were not reported in a way that allowed comparison between studies. The studies by Schleich et al. ,77 Katsoulis et al. 81 and Pedrosa et al. 85 recruited smokers and atopic patients, although the prevalence of atopy was higher in the study by Pedrosa et al. 85 at 87%, compared with 48% and 45.5% in the studies by Schleich et al. 77 and Katsoulis et al. 81 respectively.
The studies by Katsoulis et al. ,81 Pedrosa et al. 85 and Bobolea et al. 88 used NIOX MINO, whereas the study by Schleich et al. 77 used the Niox chemiluminescent device.
All four studies77,81,85,88 used airway hyper-responsiveness as the reference standard, which was appropriate to the UK pathway for the patients selected. The studies by Katsoulis et al. ,81 Schleich et al. 77 and Pedrosa et al. 85 used MCT whereas Bobolea et al. 88 used an adenosine challenge test as patients had already had a negative MCT test.
Table 23 details the estimates of sensitivity and specificity for these studies. Katsoulis et al. ,81 Schleich et al. 77 and Pedrosa et al. 85 reported quite similar cut-offs for the highest sum of sensitivity and specificity at 32, 34 and 40 ppb respectively. The paired estimates of sensitivity and specificity were similar in Katsoulis et al. 81 and Schleich et al. ,77 with sensitivities of 47% and 35% and specificities of 85% and 95% respectively. Pedrosa et al. 85 reported a sensitivity of 74.3% and a specificity of 72.5%. There is no one obvious characteristic of the studies that correlates with the differences in results. Bobolea et al. 88 reported 100% sensitivity but only 29.2% specificity, indicating that FeNO measurement in this position would be most likely to be useful as a rule-out test. No data were available for other cut-off points.
In comparison to studies that recruited patients at position A in the pathway, patient populations are perhaps somewhat younger. Other patient spectrum characteristics look comparable. The range of estimates of sensitivity and specificity also look largely comparable. A sensitivity of 100% was achieved by Bobolea et al. ,88 although it is not clear if this was for the highest sum of sensitivity and specificity or if the cut-off was selected so that FeNO could perform as a rule-out test with high sensitivity.
The studies by Prieto et al. ,76 Hsu et al. 73 and Hahn et al. 74 all recruited adults with chronic cough who were negative for some other causes of cough (see Appendix 10 for more details on the inclusion and exclusion criteria). Prieto et al. 76 recruited patients with a FEV1 of at least 80% predicted with chronic cough and no signs of other lung disease. Hsu et al. 73 recruited patients who were negative for upper airway cough syndrome and gastro-oesophageal reflux disease (GORD) and who had no obvious chest radiograph abnormalities. Hahn et al. 74 recruited patients with normal chest radiographs. All three patient groups appear to be equivalent to patients at position H in the UK pathway, who were classed as being at low risk for asthma, who have undergone tests for other conditions and for whom an asthma diagnosis is being reconsidered. All three studies recruited no current smokers and only the study by Prieto et al. 76 reported the prevalence of atopy and this was 100%. Cohorts were perhaps somewhat older than in other studies, with all averaging in the mid- to high 40s.
The device used was the NOA 280i in Hsu et al. 73 and Hahn et al. 74 and the Niox chemiluminescent device in Prieto et al. 76
The reference standard was ICS responsiveness, which would be the next test in UK practice for some or all of these patients.
Table 23 details the estimates of sensitivity and specificity for these studies. In the study by Prieto et al. ,76 sensitivity and specificity were poor at 53% and 63%, respectively, although the studies by Hsu et al. 73 and Hahn et al. 74 both report high sensitivities (94.7% and 90% respectively) and fairly high specificities (76.3% and 85% respectively), indicating that FeNO could be a useful rule-out test. It is not clear why the estimates reported by Prieto et al. 76 differ from those reported by the other two similar studies, but it may be the result of differences in patient selection. Although the device used by Prieto et al. 76 was Niox, it is not thought that this would alter estimates of diagnostic accuracy, rather the cut-off points derived.
Table 23 details the estimates of sensitivity and specificity for other studies of some interest to the assessment. The study by El Halawani et al. 78 recruited adults with suspected EIB. As in the study by Arora et al. ,79 which was not considered relevant to the review because of the reference standard used, this group of patients was made up of army recruits. It is not clear what previous tests these patients had undergone, if any. None of the patients was a smoker or atopic. The reference standard was an exercise challenge test, which will identify only patients with EIB rather than other forms of asthma. The device used (NOA/Sievers 280A) is of unknown equivalence to NIOX MINO and NObreath. The study reports 100% sensitivity and 31% specificity, indicating that this test could be used as a rule-out test.
The studies by Brannan et al. 92 and Chancafe-Morgan et al. 80 both used airway hyper-responsiveness (MCT or mannitol challenge) as the reference standard but it is not clear if the patient spectrum matches UK practice for patients being referred for such testing. The next most similar studies are those by Schleich et al. ,77 Pedrosa et al. 85 and Katsoulis et al. ,81 all of which use MCT challenge testing as the reference standard, in patients with asthma symptoms. In the study by Chancafe-Morgan et al. ,80 the cut-off point was comparable to that in the other studies at 35 ppb, whereas in the study by Brannan et al. 92 the cut-off point was higher than in any of the other studies at 47 ppb (next highest 40 ppb85), which may reflect the use of a device known to read higher than the Niox chemiluminescent device (despite a correction of 0.6 ppb). Brannan et al. ,92 Katsoulis et al. 81 and Schleich et al. 77 all report sensitivities and specificities in similar ranges, with low sensitivity but high specificity, whereas Chancafe-Morgan et al. 80 and Pedrosa et al. 85 report a more even split between sensitivity and specificity (sensitivities of 75% and 74.3% and specificities of 83.3% and 72.5% respectively).
From Table 22 it can be seen that only two sets of two studies are similar enough to each other to warrant meta-analysis:
-
Schneider et al. 71,72 and Schneider et al. 69 – two studies conducted by the same research group with populations recruited in 2006–7 and 2010–11 respectively
-
Hsu et al. 73 and Hahn et al. 74 – these studies recruited in 2009–10 and 2004–5, respectively, and were conducted in China and the USA respectively.
However, the value of such a meta-analysis is limited given that these studies are no more or less relevant to the decision problem than any of the other studies found.
Studies using FeNO in conjunction with another test as the index test
From the initial 22 studies conducted in adults, adults plus adolescents, all age groups and unspecified age groups, two77,87 reported diagnostic accuracy data for FeNO in conjunction with another test as the index test. One further sudy70 did not report actual data but did state that the addition of certain tests (see next paragraph) did not increase accuracy.
The study characteristics of the studies by Schleich et al. ,77 Cordeiro et al. 87 and Fortuna et al. 70 are presented in Tables 21 and 22 and the diagnostic accuracies in the studies by Schleich et al. 77 and Cordeiro et al. 87 are presented in Table 23. Neither the study by Schleich et al. 77 nor that by Cordeiro et al. 87 reported a change in the optimum cut-off for FeNO when using it in conjunction with another test, but sensitivities and specificities did change. The study by Fortuna et al. 70 was not judged to be of high relevance to the decision problem as it used sputum eosinophilia as part of the reference standard. This test is not widely available in the UK and so this study has low generalisability. The study reported that the addition of sputum eosinophilia to FeNO measurements increased specificity from 64% to 76%; sensitivity was not reported for the two tests together. The authors also stated that the addition of lung function tests and bronchodilator tests did not increase accuracy, but actual data were not provided.
Cordeiro et al. 87 used FeNO with a cut-off of 27 ppb in conjunction with airway reversibility in a population of patients at position A in the UK pathway. If patients were positive by either test they were considered to have tested positive. Compared with using FeNO at a cut-off of 27 ppb alone, sensitivity increased from 78% to 87% whereas specificity decreased from 92% to 90%. However, it should be noted that the reference standard for this study was airway reversibility or airway hyper-responsiveness to histamine. As such, the study results are at high risk of incorporation bias as the reference standard incorporates some of the same results as the index test. This is likely to overestimate the actual diagnostic accuracy of this combination of tests. 131
Schleich et al. 77 used FeNO with a cut-off of 34 ppb in conjunction with a FEV1% predicted of ≤ 101% in a population of patients with chronic cough and at position E (difficult to diagnose) in the diagnostic pathway. Patients were required to have both a FeNO > 34 ppb and an FEV1% predicted ≤ 101% to be judged positive by this combination of tests. This resulted in an increase in specificity from 95% to 98.9%, but a decrease in sensitivity from 35% to 24.4%. In this case the reference standard was airway hyper-responsiveness to MCT and so incorporation bias was avoided.
In both cases the improvements in diagnostic accuracy are modest (or negative when considering the sum of sensitivity and specificity) and necessitate the usual trade-off between sensitivity and specificity. As both studies are derivation studies rather than validation studies (in which the cut-off points are pre-set), it is possible that the gains seen are an overestimate of increases in diagnostic accuracy. However, it would seem that using a combination of tests may have additional benefit to using FeNO on its own, and these studies equate more accurately to adding FeNO into the pathway than studies that do not use FeNO in conjunction with other tests.
Studies including children or children and adolescents
Studies using only FeNO as the index test
Four studies that recruited children (plus adolescents and/or young adults) and compared FeNO- guided diagnosis to non-FeNO-guided diagnosis were identified. 93–96 All of the studies were based in secondary care and each study was undertaken in a different country: Finland,93 Switzerland,94 Israel95 and Korea. 96 Funding sources were reported only in the studies by Linkosalo et al. 93 and Woo et al. :96 these were the Tampere Tuberculosis Foundation/Medical Research Fund of Tampere University Hospital and the National Research Foundation of Korea respectively. The study by Sivan et al. 95 declared that there were no conflicts of interest in the research.
The four studies exploring FeNO measurement for the diagnosis of asthma in children were assessed for quality according to QUADAS-2 criteria for diagnostic accuracy studies. 38 The overall quality was variable, with no one study being free from potential bias in all domains and no single domain being free from bias in all studies. The Woo et al. study96 appeared to be at the lowest risk of bias whereas the studies by Ramser et al. 94 and Linkosalo et al. 93 displayed the highest risk of bias (Figure 12).
FIGURE 12.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. Dark green circles with + sign, low risk of bias; light green circles with – sign, high risk of bias; medium green circles with ?, unclear risk of bias.
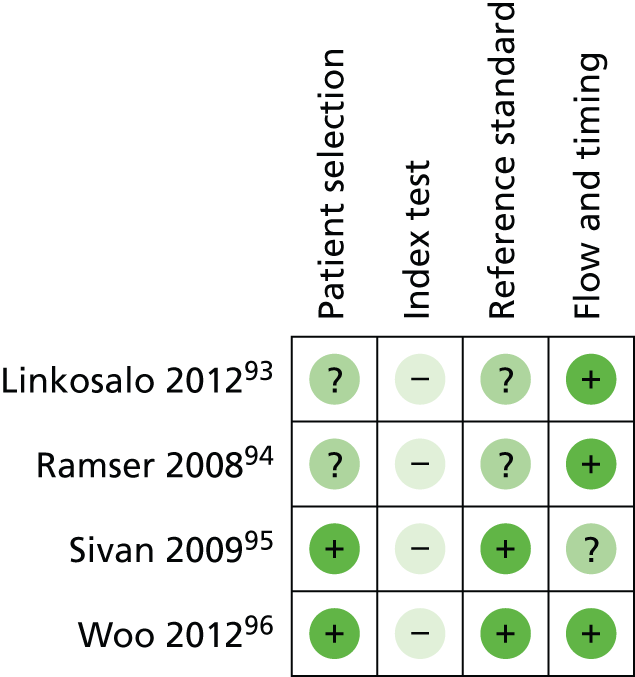
The studies by Sivan et al. 95 and Woo et al. 96 both appeared to be free of bias in terms of patient selection. Both studies enrolled consecutive samples, avoided a case–control design and recruited appropriately (i.e. those patients presenting with clinical signs of asthma). However, there were potential sources of bias in the studies by Linkosalo et al. 93 and Ramser et al. 94 in that neither study explicitly clarified whether it had enrolled patients consecutively.
There was potential bias in the conduct of the index test throughout the corpus of literature. All four studies93–96 were derivation studies; hence, in fitting the cut-off points to the data post hoc they are likely to provide liberal estimates of diagnostic accuracy. There was an additional source of possible bias in the Ramser et al. study94 in that it was not clear whether the index test was interpreted blind to the results of the reference standard.
The studies by Sivan et al. 95 and Woo et al. 96 both appeared to provide a satisfactory reference standard in that both adhered to all or part of the UK guidelines and clearly stated that the results were interpreted by a blinded investigator. Neither the study by Linkosalo et al. 93 nor that by Ramser et al. 94 provided sufficient information to confirm whether the result was interpreted blind to the index test results.
The patient flow and test timing appeared to be broadly satisfactory. The studies by Linkosalo et al. ,93 Ramser et al. 94 and Woo et al. 96 each conducted tests consecutively, provided the same reference standard to all patients and included all enrolled patients in the final analysis. The one study that did display a potential source of bias in this domain was that by Sivan et al. 95 These investigators provided a list of criteria that may have been used to confirm a diagnosis of asthma, but it was not clear precisely which of these tests in which combination(s) were given to which patients.
The small body of research was of variable quality, with the study by Woo et al. 96 displaying the least risk of bias and that by Ramser et al. 94 being at the highest risk of bias. The most important source of potential bias in this literature is concerned with the conduct and interpretation of the index test. All studies fitted FeNO cut-off points to the data post hoc and are thus likely to overestimate diagnostic accuracy. In addition, the study by Ramser et al. 94 did not provide sufficient information to judge whether the index test results had been interpreted blind to the reference standard. Study flow and timing was the least likely domain to contain sources of bias in that only the study by Sivan et al. 95 did not provide sufficient clarity on whether all patients received the same reference standard.
Study characteristics and timelines are provided in Tables 24 and 25. All four studies had a prospective cohort design and, with the exception of the study by Linkosalo et al. 93 (when the relevant information was not reported), they each enrolled consecutive patients. The timing of diagnostic procedures among the studies also appeared broadly comparable. Linkosalo et al. 93 performed FeNO measurement before an exercise challenge test, after which spirometric testing was used at 4, 10 and 15 minutes. Final spirometry occurred at 20 minutes, after salbutamol inhalation. Ramser et al. 94 likewise performed FeNO measurement before pulmonary function assessment and spirometric testing. In addition, patients who did not react to the exercise testing were provided with an additional MCT at 1 hour. Sivan et al. 95 also assessed FeNO first and followed up with spirometry and sputum induction 1–2 hours later, although sputum induction did not contribute to the diagnosis of asthma, which was based on assessment by a certified paediatric pulmonologist after at least 18 months’ follow-up and treatment. Finally, Woo et al. 96 asked all participants to fill in an ISAAC (International Study of Asthma and Allergies in Childhood) questionnaire132 and undergo clinical assessment. FeNO measurements were then taken, followed by spirometry and a MCT.
| Author, year | Study details | Age group | Inclusion/exclusion criteria | No. analysed/no. recruited | Age (years), sex | FEV1% predicted | FeNO (ppb) | Atopic, n/N (%) |
|---|---|---|---|---|---|---|---|---|
| Linkosalo 201293 | Setting: paediatric allergist, Finland Funding: non-industry Design: prospective cohort study, unclear if consecutive |
Children and adolescents | Children and adolescents aged 6–19 years; those with confirmed atopy referred to an allergist with asthma-like symptoms | 30/30 | Mean age (range): EIB +ve: 10.7 (8–19); EIB –ve: 9.6 (6–13) Code for population: children and adolescents Male, n (%): 20 (66.7) |
Mean ± SE: EIB +ve: 97 ± 2; EIB –ve: 96 ± 3 (p = 0.723) | Mean ± SE: EIB +ve: 31.3 (SD 4.1); EIB –ve: 15.6 (SD 3.6) | 30/30 (100) |
| Ramser 200894 | Setting: secondary care, Switzerland Funding: NR Design: prospective, consecutive cohort study |
Children and adolescents | Children aged 6–16 years referred to an outpatient clinic for diagnostic work on possible reactive airway disease. SABAs must have been withheld on the day of testing and long-acting beta2-agonists withheld for at least 24 hours before testing | 169/169 | Mean age: NR Code for population: young children Male, n/N (%): 96/169 (57) |
Mean (SD): atopic (n = 104): 97 (12); non-atopic (n = 57): 102 (13) | Mean (SD): atopic (n = 104): 35 (36); non-atopic (n = 57): 13 (16) | 104/169 (61.5) |
| Sivan 200995 | Setting: secondary care, outpatient clinic, Israel Funding: authors declared no conflict of interests Design: prospective, consecutive patients |
Children and adolescents | Children and adolescents Inclusion criteria: (1) non-specific respiratory symptoms suggestive of asthma for ≥ 3 months’ duration, including cough, wheezing, and shortness of breath with or without trials of treatment with bronchodilators and ICSs; (2) children were co-operative and successfully completed all three tests; (3) follow-up at clinic for at least 1 year Exclusion criteria: patients with other conditions that could affect FeNO or sputum eosinophil count, including subjects with symptoms of unresolved respiratory tract infection, with systemic clinical manifestations of atopy such as anaphylaxis, angioedema, food allergy or urticaria or with an underlying systemic or inflammatory disease |
150/156 (n = 6 unable to produce sputum) | Mean age (range): steroid-naive asthmatics (n = 69): 12.6 (5–18); asthma treated with ICSs (n = 37): 12.3 (6–18); non-asthmatics (n = 44): 12.0 (7–18) Code for population: children and adolescents Male, n/N (%): steroid-naive asthmatics: 40/69 (58); asthma treated with ICSs: 19/37 (51); non-asthmatics: 24/44 (55) |
Mean (SD): steroid-nave asthmatics: 79.3 (44.4); asthma treated with ICSs: 75.0 (16.0); non-asthmatics: 86.1 (17.1) | Mean (SD): steroid-naive asthmatics: 69 (17); asthma treated with ICSs: 36 (57); non-asthmatics: 12.6 (9) | NR |
| Woo 201296 | Setting: secondary care (outpatient clinic), Korea Funding: non-industry Design: prospective, consecutive cohort study |
Children and adolescents | Children and adolescents aged 8–16 years Inclusion criteria: children presenting with non-specific respiratory symptoms suggestive of asthma, including cough, wheezing, and shortness of breath. All included patients did not receive inhaled SABAs in the 8 hours before the measurements and were also not receiving a regular treatment with controller medications for ≥ 3 months before evaluation of FeNO and lung function |
245/245 | Mean (SD) age: 11.7 ± 2.2 Non-atopic asthmatic (n = 38): 11.6 (2.7); non-atopic non-asthmatic (n = 18): 11.4 (2.0); atopic asthmatic: 11.7 (2.4) (n = 129); atopic non-asthmatic (n = 60): 12.6 (2.6) Code for population: children and adolescents Male, n/N (%): non-atopic asthmatic 20/38 (52.6); non-atopic non-asthmatic 9/18 (50.0); atopic asthmatic 92/129 (71.3); atopic non-asthmatic 42/60 (70.0) |
Mean (SD): 87.6 (11.6) | GM (95% CI): asthmatic: 23.4 (20.9 to 26.2); non-asthmatic: 12.6 (10.9 to 14.5) | 189/245 (77) |
| Author, year | Population | Age group | Device | Cut-off values (ppb) | Reference standard | Details of reference standard | Position of FeNO in the pathway | Relevance to decision problem |
|---|---|---|---|---|---|---|---|---|
| Linkosalo 201293 | Position A with confirmed atopy | Children and adolescents | NOA 280i (chemiluminescence) | 10, 20, 30, 40, 50 | Airway hyper-responsiveness to exercise | EIB – free-running test with goal of 80% maximum heart rate according to age. Spirometry 4, 10 and 15 minutes after exercise and after salbutamol inhalation given 20 minutes after exercise. EIB positive if maximal decrease in FEV1 ≥ 12% | FeNO at position 1 | Relevant |
| Ramser 200894 | Position A | Children and adolescents | CLD 77 AM (chemiluminescence) | 10, 20, 30, 40, 50 | Airway hyper-responsiveness (MCT or exercise) | Spirometry, body pleythysmography and MTC challenge according to ATS/ERS guidelines.35 EIB was defined by decrease in FEV1 by ≥ 15% of baseline. MCT challenge was carried out using a panel of incremental dosages of MCT and a dose of 1.8 mg was defined as the threshold of PD20 to differentiate normal airway hyper-responsiveness from BHR | FeNO replaces whole pathway | Not relevant – uses reference standard not used in the UK |
| Sivan 200995 | Position A | Children and adolescents | CLD 88 (chemiluminescence) | 15, 18, 19, 25, > 20 or < 15 | Exacerbation history, airway reversibility, airway hyper-responsiveness | Patient’s history of two or more clinical exacerbations of wheezing documented by a physician, dyspnoea or cough relieved by bronchodilators, documented variability in FEV1 ≥ 15% in response to bronchodilators at any time during the follow-up period (reversibility), documented variability in FEV1 ≥ 15% over time with or without controller medications (ICSs or montelukast). Results of provocation tests were included when available. Children in whom asthma did not manifest within 18 months of follow-up were considered as not having asthma | FeNO replaces whole pathway | Relevant – uses long-term follow-up |
| Woo 201296 | Position A | Children and adolescents | NIOX MINO | 5, 10, 15, 20, 25, 30, 34, 40, 45, 50 (optimum at 22) | Airway reversibility, airway hyper-responsiveness (MCT) | Relevant symptom history and reversible airflow obstruction (≥ 12% improvement in FEV1 in response to inhaled beta2-agonist) and/or airway hyper-responsiveness | FeNO replaces whole pathway | Relevant |
The study populations were broadly similar in terms of their position on the diagnostic pathway and all recruited children and adolescents, although upper and lower age cut-offs varied a little, with the most inclusive being those in the study by Sivan et al. 95 at 5–18 years and Linkosalo et al. 93 at 6–19 years and the least inclusive being those in the study by Ramser et al. 94 at 6–16 years. There were some further differences in the inclusion criteria. Linkosalo et al. 93 included only children and adolescents with confirmed atopy whereas Ramser et al. 94 and Woo et al. 96 included a mix of atopic and non-atopic patients. Sivan et al. 95 did not report the number of patients with atopy but did not specifically include on this basis and the study is therefore likely to have included a mix of atopic and non-atopic patients. All studies recruited patients at position A in the UK pathway; Linkosalo et al. 93 recruited patients who had been referred to an allergist with asthma-like symptoms (position A); Ramser et al. 94 included children in position A who had been referred to an outpatient clinic for diagnostic assessment of possible reactive airway disease; Woo et al. 96 included children presenting with non-specific respiratory symptoms suggestive of asthma and who had not been receiving controller medications for at least 3 months prior to FeNO testing (position A); and Sivan et al. 95 also recruited from position A – in this case those with non-specific respiratory symptoms suggestive of asthma for at least 3 months. This study also excluded patients with any other conditions that may have interfered with FeNO testing or sputum eosinophil count, especially unresolved respiratory tract infection or underlying systemic or inflammatory disease.
Sample size ranged from 3093 to 24596 and the mean age (often reported for subgroups rather than whole cohorts) ranged from 9.693 to 12.6 years,95 although mean age was not provided by Ramser et al. 94 Unlike adult studies in which male participants were in the minority, there was a preponderance towards male participants in all four studies, with the lowest percentage being observed in the Sivan et al. 95 study (55.3%).
Three of the four studies measured FeNO via chemiluminescence, although each used a different device: Linkosalo et al. 93 used the NOA280i; Ramser et al. 94 used the CLD 77 AM; and Sivan et al. 95 used the CLD 88. Woo et al. 96 was the only study to use NIOX MINO for FeNO evaluation. In terms of FeNO cut-off points, Linkosalo et al. 93 and Ramser et al. 94 both used the same prespecified cut-off points of 10, 20, 30, 40 and 50 ppb. Sivan et al. 95 used cut-offs of 15, 18, 19, 25 and > 20/< 15 ppb. Woo et al. 96 reported a large number of cut-off values, ranging from 5 ppb to 50 ppb.
None of the studies fully replicated the UK guidelines. Linkosalo et al. 93 used an exercise challenge test (free-running test) with spirometric tests before and after exercise and after salbutamol inhalation. Sivan et al. 95 based the diagnosis of asthma on a history of two or more exacerbations, evidence of airway reversibility in response to ICSs or bronchodilators or airway hyper-responsiveness at any time during a period of 18 months’ follow-up. Woo et al. 96 performed a battery of tests similar to those in the UK treatment pathway (spirometry, MCT and atopy assessment), with FeNO being measured before these other tests. Ramser et al. 94 reported results against a reference standard of MCT or exercise challenge testing.
The study of greatest relevance for this assessment is that by Woo et al.,96 which recruited patients in position A on the pathway and used the NIOX MINO device compared with a reference standard that roughly equates to UK practice. In this study FeNO replaces the whole pathway prior to ICS use.
The three remaining studies were of varying relevance to the UK context:
-
Sivan et al. 95 used an ECO MEDICS CLD 88 device in patients at position A in the pathway compared with a reference standard similar to UK practice. In this study FeNO replaces the whole pathway prior to ICS use.
-
The study by Ramser et al. ,94 used an Eco Physics CLD 77 AM device for patients in position A on the pathway with a reference standard of airway hyper-responsiveness to exercise or methacholine. As MCT is a very good test for asthma, this study can be seen as similar to testing FeNO against the whole UK diagnostic pathway.
-
Linkosalo et al. ,93 used a Sievers NOA280i chemiluminescence device for patients at position A on the pathway with a reference standard of an exercise challenge test. In the UK this test is reserved for those with symptoms of EIB.
No studies in children were identified that incorporated ICS responsiveness in the reference standard.
The sensitivity and specificity values for each of the studies are presented in Appendix 12.
Table 26 also displays three sets of sensitivity and specificity values for each of the studies. These are:
-
The highest sum of sensitivity and specificity as reported by the authors of the studies.
-
The highest sensitivity – in this scenario a negative test result rules out a diagnosis. This was selected as the cut-off that provided the highest sensitivity. When 100% sensitivity was reported for more than one cut-off, the cut-off that maintained the highest specificity was selected. When the cut-off with the highest sensitivity was not also the cut-off with the highest PPV, this latter cut-off was also presented.
-
The highest specificity – in this scenario a positive test result rules in a diagnosis of asthma. Selected as for the highest sensitivity but for specificity. When the cut-off with the highest specificity was not also the cut-off with the highest NPV, this latter cut-off was also presented.
| Author, year | Population | Device | Reference standard | No. analysed | Highest sum of sensitivity and specificity | Rule out | Rule in | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||||
| Position A vs. whole pathway | |||||||||||||||||||
| Linkosalo 201293 | Position A with confirmed atopy | NOA280i (chemiluminescence) | Airway hyper-responsiveness to exercise | 30 | 20 | 72 | 83 | 86.7 | 66.7 | 10 | 89 | 33 | 66.7 | 66.7 | 30 | 50 | 92 | 90 | 55 |
| Ramser 200894 | Position A | CLD 77 AM (chemiluminescence) | Airway hyper-responsiveness (MCT or exercise) | 169 | 20 | 49 | 76 | 74 | 51 | 10 | 76 | 36 | 63 | 51 | 50 | 20 | 93 | 80 | 45 |
| 20 | 49 | 76 | 74 | 51 | |||||||||||||||
| Sivan 200995 | Position A | CLD 88 (chemiluminescence) | Exacerbation history, airway reversibility, airway hyper-responsiveness | 150 | 19 | 86 | 89 | 92.2 | 79.6 | 15 | 90 | 70 | 82.7 | 81.6 | As highest sum | ||||
| > 20 or < 15 | 89 | 88 | 93.5 | 82.1 | |||||||||||||||
| Woo 201296 | Position A | NIOX MINO | Airway reversibility, airway hyper-responsiveness (MCT) | 245 | 21 | 56.9 | 87.2 | 90.5 | 50.0 | 5 | 94 | 14.1 | 70.0 | 50 | 41 | 23.4 | 100.0 | 100 | 37.9 |
It should be noted that superior sets of sensitivity and specificity values may have in fact been achieved but selection was limited to the range of cut-off points reported within studies.
There was a high degree of agreement as to the cut-off point that produces the highest sum of sensitivity and specificity, despite the heterogeneity in devices and reference standards, with values between 19 and 21 ppb (see Table 26). However, estimates of sensitivity at these cut-off points were not similar across studies, ranging from 49% to 86%; specificity was more similar between studies, ranging from 76% to 89%. Rule-out cut-off points were not similar and varied from 5 to 20 ppb; rule-in cut-off points similarly ranged from 30 to 50 ppb. For ruling out, the highest sensitivity was reported by Woo et al. ,96 at 100%, with a paired specificity of 14.1%, PPV of 70% and NPV of 50%. Sensitivities ranged from 76% to 94%. For ruling in, the highest specificity was also reported by Woo et al. 96 at 100%, with a paired sensitivity of 23.4%, PPV of 100% and NPV of 37.9%. Specificities varied less than sensitivities, from 89% to 100%. It should be noted that superior rule-in and rule-out sets of sensitivity and specificity may in fact have been achieved but selection was limited to the range of cut-off points reported within the studies.
No meta-analysis was performed on these data because of heterogeneity in FeNO measurement devices and reference standards.
Studies using FeNO in conjunction with another test as the index test
One study recruiting children reported estimates of diagnostic accuracy for FeNO in conjunction with another test. 95 This study was described in more detail in the previous section but in summary recruited children at position A in the pathway and used FeNO in conjunction with sputum eosinophilia against a reference standard of evidence of airway reversibility in response to ICSs or bronchodilators or airway hyper-responsiveness at any time during 18 months’ follow-up. Sputum eosinophilia is not a test in widespread use in the UK and the combination of it and FeNO as a diagnostic test is of low relevance to the UK pathway. The results showed that improvements in diagnostic accuracy were very small: sensitivity increased from 86% to 87% and specificity remained the same at 89%.
Studies providing data on subgroups of interest to the review
Adult smokers
Results are presented in Table 27. Malinovschi et al. 110 and Katsoulis et al. 81 investigated the effects of smoking on the usefulness of FeNO in diagnosing asthma. The study by Malinovschi et al. 110 was not included in the main diagnostic review as the method of recruitment was unusual.
| Author, year | Device | Reference standard | Population | No. analysed | Highest sum of sensitivity and specificity | Rule out | Rule in | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||||
| Katsoulis 201381 | NIOX MINO | Airway hyper-responsiveness (MCT) | All (excluded recent ex-smokers) | 112 | 32 | 47 | 85 | 70.1 | 68.1 | 10 | 81 | 39 | 49.9 | 73.2 | 30 | 49 | 82 | 67.1 | 68.2 |
| Smokers | NR | 11 | 85 | 50 | NR | NR | 10 | 84 | 55 | NR | NR | 30 | 12 | 95 | NR | NR | |||
| Malinovschi 2012110 | NIOX MINO | Symptoms plus one of airway reversibility, airway hyper-responsiveness (MCT), prescribed steroids or SABAs, symptoms in pollen season plus allergic rhinitis | All | 282 | 20 | 52.08 | 82.8 | 61 | 77 | 10 | 87.5 | 33.3 | 40.4 | 83.8 | 50 | 15.6 | 96.8 | 71.4 | 69.0 |
| Smokers | 112 | 17 | 56.3 | 82.5 | 56.3 | 82.5 | 7 | 90.6 | 15.0 | 29.9 | 80.0 | 50 | 9 | 98 | 60 | 72.9 | |||
| 10 | 78 | 48 | 37.3 | 84.4 | 35 | 16 | 96 | 62.5 | 74.0 | ||||||||||
| Never smokers | 108 | 15 | 77.8 | 63.5 | 60.3 | 80.0 | 10 | 91 | 27 | 47.1 | 81.0 | 50 | 20 | 95 | 75 | 62.5 | |||
| Ex-smokers | 62 | 22 | 62.2 | 86.1 | 66.7 | 84.1 | 10 | 95 | 16 | 33.3 | 87.5 | 35 | 37 | 98 | 87.5 | 77.8 | |||
Both studies were prospective cohort studies, with the study by Malinovschi et al. 110 conducted in Denmark and that by Katsoulis et al. 81 conducted in Greece in an army hospital.
Neither study recruited an ideal spectrum of patients. Both recruited patients on the basis of the presence of symptoms of asthma as reported in a questionnaire rather than through presentation to a GP. In the study by Malinovschi et al. 110 the questionnaire was mailed to the general population whereas in the study by Katsoulis et al. 81 the reason for patients filling in the questionnaire is not clear but may be for the purpose of army recruitment (army general hospital).
Both studies used NIOX MINO to measure FeNO values.
In the study by Malinovschi et al. ,110 to be diagnosed with asthma patients had to exhibit symptoms and test positive by one of MCT; airway reversibility to bronchodilator use; daily use of steroids or SABAs; or asthma symptoms during but not outside the pollen season supported by allergic rhinitis. As the cohort was recruited from a random sample of the population with asthma symptoms and did not exclude existing asthmatics, the reference standard in part depends on a previous diagnosis of asthma in that a patient already prescribed steroids or SABAs is automatically classed as asthmatic. In practice, this reference standard may therefore include patients who have been wrongly diagnosed in primary care. In the study by Katsoulis et al. 81 the reference standard was MCT.
In both studies,81,110 cohorts of smokers alone had lower cut-off values than mixed cohorts (11 ppb compared with 32 ppb81 and 17 ppb compared with 20 ppb110) but the difference was small in one study. 110 Differences in cut-off values were not as apparent for rule-in and rule-out scenarios between these two subgroups. Malinovschi et al. 110 also reported data for never smokers and ex-smokers. Surprisingly, ex-smokers had a higher derived cut-off (22 ppb) than all other subgroups, but this difference did not hold true in rule-in and rule-out scenarios.
Children exposed to tobacco smoke
We were unable to identify any studies that evaluated the diagnostic accuracy of FeNO testing in children exposed to tobacco smoke. However, evidence from the review on the use of FeNO measurements in the management of asthmatic children exposed to tobacco smoke may provide some insight (albeit limited) on how environmental tobacco smoke may impact on mean FeNO values and therefore FeNO cut-off points. Mahut et al. 111 and Hanson et al. 112 both reported that FeNO levels were not statistically significantly different between those exposed and those not exposed to tobacco smoke whereas de la Riva-Velasco113 reported that FeNO values were lower in ICS-treated children who were exposed to tobacco smoke. Similarly, evidence from a diagnostic cohort study110 (see the previous section) that investigated the effects of smoking on the usefulness of FeNO in diagnosing asthma in adults and adolescents (rather than children) suggested that FeNO could differentiate asthmatic subjects from non-asthmatic subjects with asthma-like symptoms equally well in both never and current smokers. However, the FeNO cut-off levels were lower in current and ex-smokers.
The findings from the above studies suggest that it may be necessary to consider a child’s exposure status when interpreting the results of FeNO testing for the diagnosis of asthma, as FeNO levels may be lower in children exposed to tobacco smoke.
Pregnant women
Although no studies were identified that evaluated the diagnostic accuracy of FeNO testing in pregnant women, a cross-sectional study by Tamasi et al. ,114 conducted in Hungary, compared FeNO levels in pregnant and non-pregnant asthmatic and healthy women. A total of 102 women were recruited from an outpatient clinic, of whom 35 were healthy non-pregnant women, 27 were healthy pregnant women, 20 were asthmatic non-pregnant women and 20 were asthmatic pregnant women. The exclusion criteria were as follows: current smokers or > 5 pack-years of smoking history, other chronic diseases (e.g. chronic rhinitis, hypertension), acute infection within 3 weeks of measurement or a body mass index > 30 kg/m2. Asthma was diagnosed using the GINA guidelines129 and all asthmatic patients had persistent disease. All asthmatic patients were receiving ICSs. In addition, 14 patients were on long-acting beta2-agonists (LABAs) and seven patients received additional LTRA therapy. Mean age ranged from 27 years for non-pregnant healthy women to 31 years for non-pregnant asthmatic women. FeNO was measured using the NIOX MINO device.
The authors found no significant difference in median FeNO levels between healthy pregnant subjects [16 ppb, interquartile range (IQR) 9–35 ppb] and healthy non-pregnant subjects (16, IQR 8–31 ppb). Similarly, no significant difference was observed in the level of asthma control between pregnant and non-pregnant asthmatic subjects and there was no significant difference in the total Asthma Control Test (ACT) scores (20.78 ± 2.96 vs. 19.17 ± 3.1 respectively; p = 0.17). In contrast, FeNO levels in pregnant asthmatic women were significantly higher than those in pregnant healthy women (28 ppb, IQR 10–56 ppb vs. 16 ppb, 9–35 ppb respectively; p < 0.05). Similarly, the FeNO levels in non-pregnant asthmatic women were significantly higher than those in non-pregnant healthy women (38 ppb, IQR 9–54 ppb vs. 16 ppb, 8–31 ppb respectively; p < 0.0001). In addition, the authors reported that there was no significant difference between the two groups of asthmatic women: the mean FeNO value (estimated from a graph in the published paper) for pregnant asthmatic women was 29 ppb and that for non-pregnant asthmatic women was 32 ppb.
Overall, the study authors concluded that pregnancy itself does not alter FeNO levels either in healthy patients or in asthmatic patients and that FeNO levels in pregnant asthmatic patients correlate with asthma control levels.
The elderly
No diagnostic studies that used FeNO to diagnose asthma in the elderly were identified; however, one study115 that examined FeNO levels and eosinophilic airway inflammation in elderly subjects with airflow obstruction was identified. In asthma diagnosis, the main use of FeNO testing is to identify patients with eosinophilic airway inflammation who are likely to respond to ICSs, as a surrogate for other methods of ascertaining eosinophilic inflammation such as sputum counts. As such, this study should provide some evidence on whether FeNO still acts as a surrogate marker for eosinophilic inflammation in the elderly. This observational case–control study was conducted in Australia and was reported in abstract form only and thus provides limited data. The study recruited 65 elderly patients with or without fixed airflow obstruction and 32 healthy control subjects. The setting from which the patients were recruited is unclear and the majority of patients (86%) with air flow obstruction were on ICS treatment.
The authors found that participants with eosinophilic airway inflammation (sputum eosinophil count > 3%) had similar FeNO levels to those with non-eosinophilic inflammation (16.1 ppb vs.19.1 ppb respectively; p = 0.762). Those with a diagnosis of asthma had similar FeNO levels to those with COPD. There was no correlation between FeNO level and sputum eosinophils or any clinical markers. The authors concluded that FeNO was not a surrogate marker for eosinophilic airway inflammation in older people and showed no relationship with clinical outcomes.
Management review
This section is broken down into a number of subsections by population age and subgroup. Briefly, these are:
-
FeNO-guided management in adults:
-
quality assessment
-
study details
-
estimates of efficacy.
-
-
FeNO-guided management in children:
-
quality assessment
-
study details
-
estimates of efficacy.
-
-
FeNO-guided management in subgroups defined in the scope:
-
pregnant women
-
the elderly
-
adult smokers
-
children exposed to tobacco smoke.
-
Adults
Four studies that recruited adults and compared FeNO-guided management with non-FeNO-guided management were included in the review. 97–100 One additional study101 was identified from the update search. The study by Shaw et al. 98 was based in the UK, that by Smith et al. 97 was based in New Zealand, that by Syk et al. 99 was based in Sweden, that by Calhoun et al. 100 was based in the USA and that by Honkoop et al. 101 was based in the Netherlands. The studies by Smith et al. ,97 Syk et al. ,99 Calhoun et al. 100 and Honkoop et al. 101 were at least partly supported by Aerocrine and the study by Syk et al. 99 was submitted as part of Aerocrine’s sponsor submission. An additional study by Powell et al. 102 was conducted in adult pregnant women and is discussed separately as this group was defined a priori as a distinct group.
Quality assessment
The quality of the five adult management studies was assessed according to criteria proposed in the Cochrane Handbook for Systematic Reviews of Interventions36 and Systematic Reviews – CRD’s Guidance for Undertaking Reviews in Healthcare. 37 The studies by Powell et al. 102 and Shaw et al. 98 appeared to be the highest-quality articles, with each containing only one potential source of bias (industry sponsorship and uncertain outcome assessor blinding respectively) (Figure 13). The study at highest risk of bias was the unpublished study by Syk et al. ;99 this was because of the lack of participant/personnel blinding, incomplete outcome data and selective reporting. In addition, Calhoun et al. 100 was largely at unclear or high risk, making this study a potential source of bias. The study by Honkoop et al. 101 was published as an abstract and some methodological data were available from the published protocol. However, it is unclear if the execution of the study was per protocol and so quality assessment items were scored as unclear.
FIGURE 13.
Methodological quality summary: review authors’ judgements about each methodological quality item for all included studies. Dark green circles with + sign, low risk of bias; light green circles with – sign, high risk of bias; medium green circles with ?, unclear risk of bias.

All of the included studies were described as randomised and two of the five studies provided satisfactory information on both random sequence generation and allocation concealment. In the study by Shaw et al. ,98 allocation was performed by an independent individual; Syk et al. 99 drew lots from sealed envelopes. There may have been adequate randomisation procedures in the remaining three studies97,100,101 but this could not be confirmed on the basis of the reports.
The study at highest risk from lack of blinding was that by Syk et al. ,99 which was described as an open-label study. The study by Smith et al. 97 was rated as ‘unclear’ on this item as the study was single blind (participants only). As many of the outcomes were patient reported, patient blinding may have been the most important source of bias to avoid, although the blinding of other study personnel who were deciding whether to step patients up or down may also have been important. The study by Honkoop et al. 101 was also scored as ‘unclear’. The remaining two studies98,100 were double-blind and therefore at low risk of performance bias.
The poor reporting of outcome assessment blinding in the studies means that unblinded outcome assessment may be a potentially important source of bias throughout this body of literature. However, as outcome assessment blinding often goes unreported in journal articles, it was unclear whether any potential bias was the result of reporting practices or methodological shortcomings in the conduct of the studies themselves.
The studies by Shaw et al. 98 and Smith et al. 97 appeared to be at low risk of attrition bias. Dropout rates from these studies were low, adequately reported and corrected for in the statistical analyses. In addition, Smith et al. 97 performed analysis by intention to treat and extrapolated missing data. There was a potentially high risk of bias in the Syk et al. study99 in that patients were missing and not corrected for in multiple analyses. However, Syk et al. 99 did consistently report the number of patients included in each analysis and so the attrition rate was transparent. There were two possible sources of bias in the Calhoun et al. study:100 it was unclear how missing data were corrected for and there were more dropouts in the intervention arm. If these patients were dropping out because of unsatisfactory outcomes (which was not clear from the report), this could skew the results in favour of FeNO. Finally, the study by Honkoop et al. 101 was scored as ‘unclear’ for this item.
Two of the five studies appeared to have provided data on all of the prespecified outcomes. 97,98 However, there was some evidence of selective reporting in Calhoun et al. 100 and Syk et al. 99 Calhoun et al. 100 failed to report oral prednisone levels, although this had been specified as an outcome in the study protocol, and Syk et al. 99 did not report the number of severe exacerbations. Syk et al. 99 also used medians rather than means in several of the outcomes, precluding these from meta-analysis. However, these data were supplied by the manufacturer (Aerocrine) on request. Finally, the study by Honkoop et al. 101 was scored as ‘unclear’ for this item.
There were a number of further potential sources of bias in the studies. Smith et al. 97 reported receipt of commercial sponsorship whereas Syk et al. ,99 Calhoun et al. 100 and Honkoop et al. 101 reported at least partial commercial funding. Three studies97,99,100 also conducted a run-in period before randomisation. It is unclear whether this may have introduced bias to the results in the studies by Smith et al. 97 and Syk et al. 99 In the study by Calhoun et al. 100 patients were excluded if their asthma did not remain controlled when administered two puffs twice a day of beclomethasone HFA (40 µg/puff). This is likely to have influenced the spectrum of patients recruited to this trial towards those with less severe asthma. However, this is likely to affect external validity rather than internal validity as both arms are subject to the same run-in period. No further sources of bias were identified in the remaining studies.
The quality of the sampled literature was variable, with the study by Shaw et al. 98 being at the lowest risk of bias. The only potential source of bias that we identified in this study pertained to the failure to explicitly state the blinding of outcome assessors. However, it was unclear whether this was an actual methodological flaw or merely inadequate reporting, and at least some of the outcomes were patient reported (patients were blinded). Among the remaining literature, the most important potential source of bias was selective reporting in the Calhoun et al. 100 and Syk et al. 99 studies, both of which failed to report some prespecified outcomes. The study at highest overall risk of bias was the open-label Syk et al. 99 investigation. In addition to lack of blinding, and the aforementioned selective reporting, this study may have been subject to attrition bias. In the absence of information on why data were missing from this study, it is difficult to ascertain how this may have biased the results and in what direction. However, there may be debate about the impact of blinding as a source of bias in these studies. For example, patients could use knowledge of their FeNO readings to guide their self-management, which may capture real-world clinical benefits that would not be observable in double-blind studies.
Study details
Unlike other reviews of FeNO for asthma management, in this review our primary analysis of studies that assess the efficacy of guiding treatment by FeNO measurement in adults considers the study in pregnant women102 separately, as this subgroup of patients was defined a priori as a separate group. This study is described and discussed later. This current section considers the other five studies in adults.
Table 28 provides details of study design and the timelines of the studies. All five studies were RCTs. The studies by Smith et al. 97 and Shaw et al. 98 were both single blind whereas that by Syk et al. 99 was an open-label study. The study by Calhoun et al. 100 was described as ‘multiply blinded’, although it is not entirely clear who was blinded. The study by Honkoop et al. 101 did not report blinding. No two studies followed the same timeline exactly. Smith et al. ,97 Syk et al. 99 and Calhoun et al. 100 had a run-in period pre randomisation in which LABAs were reduced or withdrawn and/or doses of ICSs were standardised. Post randomisation, all studies except that by Honkoop et al. 101 had an initial period of time in which visits were more frequent. In the study by Calhoun et al. ,100 visits were made every 2 weeks for the first 6 weeks post randomisation and then were 6-weekly after that. In Shaw et al. 98 initial visits were monthly for 4 months and then every 2 months up to 12 months. In the study by Smith et al. ,97 treatment consisted of two phases: a optimisation phase of 3–12 months and a titration phase of a further 12 months. Patients were randomised before both phases and both phases managed patients according to protocols that either did or did not incorporate FeNO measurements. However, data on exacerbations were reported only for the titration phase and it was these data that were incorporated into the analysis. In the study by Syk et al. 99 participants had an initial visit 2–4 weeks after the initial titration visit, followed by every 2 months up to 4 months and then every 4 months up to 12 months. All studies titrated doses for at least 12 months except that by Calhoun et al. 100 in which doses were titrated for 9 months only. Calhoun et al. 100 included a third intervention arm that was not relevant to this review in which the ICS dose was controlled by matching ICS use on a puff-by-puff basis to the rescue use of albuterol in response to the occurrence of symptoms.
| Author, year | Study design | Timeline of study |
|---|---|---|
| Calhoun 2012100 | RCT – multiply blinded, multicentre study | Visit 1 (week 0): consent and start of run-in period of 2 weeks – two puffs b.i.d. of beclomethasone HFA (40 µg/puff). If asthma acceptably controlled at this level, enrolled in trial. Visits 2 and 3 (weeks 2–8): pre-randomisation period – patients given two pairs of inhalers to facilitate blinding, one with beclomethasone (2 × 40 µg b.i.d.) and a placebo counterpart and one with albuterol and a placebo counterpart (taken together on demand). Visit 4 (week 8): randomisation to group 1 or group 2. Visits 5–12 (titration): 2, 4, 6, 12, 18, 24, 30 and 36 weeks post randomisation – dose adjustments made at time of clinic visits, monitoring of secondary outcomes |
| Honkoop 2013101 | RCT – cluster design | From protocol: Visit 1: introduction session run by practice nurses; randomisation by GP cluster with stratification on postcode; baseline measurement of IPQ, MARS, BMQ, ICQ, FACCT, SF-36, AQLQ, TTO, ASUI, EQ-5D and CostQ, all at home. Visit 2 (titration): GP measures ACQ, FEV1 and FeNO and titrated dose according to algorithm. Visit 3 (titration): 3 months – GP measures ACQ, FEV1 and FeNO; patients measure AQLQ, EQ-5D and CostQ at home. Visit 4 (titration): 6 months – GP measures ACQ, FEV1 and FeNO; patients measure IPQ, MARS, BMQ, ICQ, FACCT, SF-36, AQLA, TTO, ASUI, EQ-5D and CostQ, all at home. Visit 5 (titration): 9 months – as visit 3. Visit 6 (titration): 12 months – as visit 4 |
| Shaw 200798 | RCT – single blind, parallel group | Titration at each visit Visit 0: randomisation – FeNO, FEV1, FVC, PC20, induced sputum analysis, skin prick test, Juniper score. Visit 1: 2 weeks after visit 0 – FEV1, FeNO, Juniper score. Visits 2–5: monthly visits to 4 months – FEV1, FeNO, Juniper score. Visit 6: at 6 months – FEV1, FeNO, Juniper score, PC20, sputum analysis. Visits 7 and 8: at 8 and 10 months respectively – FEV1, FeNO, Juniper score. Visit 9: 12 months – FEV1, FeNO, Juniper score, PC20, sputum analysis |
| Smith 200597 | RCT – single blind, single centre, placebo controlled | Visit 1: enrolment, start of 2-week run-in period in which LABA withdrawn, reinstated at fixed dose if not tolerated. Visit 2 (week 2): FeNO and spirometry; patients begin 4 weeks of 750 µg/day fluticasone or 500 µg/day if previous dose < 200 µg/day. Visit 3 (week 6): randomisation and start of phase 1 Titration phase 1 (3–12 months after randomisation): visits every 4 weeks, FeNO and spirometry, dose adjustment to optimal dose by downwards titration until FeNO ≥ 15 ppb (equivalent to 35 ppb at 50-ml flow rate) or uncontrolled, then uptitrated until controlled/≤ 15 ppb. This dose deemed the ‘optimal dose’ Titration phase 2 (12 months after completion of phase 1): visits every 2 months, upwards adjustments when control lost/FeNO > 15 ppb, downwards adjustment if controlled/FeNO ≤ 15 ppb for two consecutive visits, but not below optimal dose. Treatment orders assigned by blinded investigator. Compliance assessed by inhaler weight |
| Syk 201399 | RCT – open label, parallel group, multicentre | Visit 1: eligibility and consent – capillary blood for IgE confirmation, LABA withdrawn, ICSs continued (salbutamol inhaler with dose counter). Visit 2 (titration): 2–4 weeks later – FeNO, spirometry, reversibility, Juniper mini-AQLQ, generic quality of life, Juniper six-item ACQ, questionnaire on allergen exposure, venous blood for IgE analysis. ICSs and LTRAs altered according to (a) FeNO levels and six fixed treatment steps in FeNO group and (b) usual care (patient report, SABA use, physical examination, pulmonary function tests) in the control group, with FeNO measured but not revealed to treatment decision-maker or patient. Visit 3 (titration): 2 months – ACQ, FeNO and treatment altered. Visit 4 (titration): 4 months – mini-AQLQ, ACQ, FeNO and treatment altered. Visit 5 (titration): 8 months – as visit 3. Visit 6 (titration): 12 months – identical to visit 2. Outcomes recorded at visits 2–6 |
Table 29 provides details of patient characteristics across studies. All studies were of a moderate size, with numbers analysed ranging from 9497 to 611. 101 All patients were recruited from primary care, except in the study by Calhoun et al. ,100 in which it was not clear whether patients were recruited from primary or secondary care settings. All had either a doctor’s diagnosis of asthma or asthma diagnosed according to guidelines. In the study by Calhoun et al. ,100 the doctor’s diagnosis was confirmed with either a positive MCT or demonstration of airway reversibility. Inclusion and exclusion criteria varied across studies but, when compatible data are reported, study populations seem broadly similar in terms of age (mean ranged from around 34.5100 to 4597 years), FEV1% (mean ranged from 81.4% to 87.7%) and FeNO values (range of geometric means 18.88–29.0 ppb). It is difficult to determine the comparability of study populations in terms of severity at baseline as different scales for severity and different metrics for medication use have been used. Inclusion and exclusion criteria suggest that at least three studies97,99,100 recruited populations with mild to moderate asthma. Smith et al. 97 excluded those with four or more severe exacerbations in the previous 12 months and those ever admitted to intensive care for asthma, whereas Syk et al. 99 and Calhoun et al. 100 stated that all patients were mild to moderate asthmatics. Smith et al. 97 and Syk et al. 99 also required patients to have been receiving ICS treatment for > 6 months and Calhoun et al. 100 recruited only patients who were well controlled when prescribed two puffs twice a day of beclomethasone HFA (40 µg/puff) and who were ≥ 75% compliant with medication during the 2-week run-in period. Shaw et al. 98 on the other hand may have recruited patients with a wider spectrum of severity. Patients were required only to have had one prescription for asthma medication in the previous 12 months, making it possible that patients with comparatively less severe or less well-documented asthma were included, but excluded only those with severe exacerbations in the previous 4 weeks, making it possible that severe asthmatics were included. Honkoop et al. 101 included patients with a broad spectrum of severity and excluded only very mild asthmatics. Unlike other studies, that by Honkoop et al. 101 had an upper age limit of 50 years.
| Author, year | Study details | Inclusion/exclusion criteria | No. analysed/no. recruited | Age (years), sex, n/N (%) | Spirometry, mean (SD) | Severity, mean (SD) | FeNO (ppb) | Smokers, atopic | Medication use |
|---|---|---|---|---|---|---|---|---|---|
| Calhoun 2012100 | Setting: care level NR, USA Funding: mixed;a equipment from Aerocrine |
Patients with mild to moderate, well-controlled persistent asthma with compliance rates ≥ 75% who could tolerate treatment of two puffs b.i.d. of beclomethasone HFA (40 µg/puff) during the 2-week run-in period | 363 recruited to trial WBR: 21; I: 115/115;b C: 114/114c Other study arm (not included in review): 113/113 |
Adults (assumed from mean age) Mean (SD) age: I: 34.8 (11.3); C: 34.2 (11.9) Male 75/229 (32.8) |
FEV1%: I: 86.3 (10.4); C: 87.7 (12.1) | ACQ: I: 0.79 (0.54); C: 0.72 (0.50) AQLQ: I: 6.16 (0.77); C: 6.27 (0.76) ASUI: I: 0.88 (0.12); C: 0.90 (0.10) |
FeNO, GM (SD): I: 18.88 (0.66); C: 21.38 (0.62) | Smokers NR Atopic: 196/229 (85.6%) |
Albuterol rescue use, median (IQR): I: 0.07 (0 to 0.43); C: 0.04 (0 to 0.29) |
| Honkoop 2013101 | Setting: Netherlands Funding: mixeda |
From protocol: age 18–50 years; doctor’s diagnosis of asthma; patients who need ICSs as controller medication (steps 2–4 GINA guidelines112); ICSs for ≥ 3 months in the previous year; written informed consent; no exacerbation of asthma within 1 month before entry. Exclusions: daily or alternate day OCS therapy for at least 1 month before entering into the study; inability to understand written and oral Dutch instructions; active diseases likely to interfere with the purpose of the study, such as end-stage disease or inability to visit GP | 611 randomised Other data NR |
43 Male: 32% |
NR | NR | NR | NR | NR |
| Shaw 200798 | Setting: recruited from primary care UK Funding: Asthma UK grant; speakers’ fees but not from Aerocrine |
Included: patients with GP diagnosis of asthma who received one or more prescriptions for antiasthma medication in the last 12 months; current non-smokers with a past smoking history of < 10 pack-years. Excluded: those poorly compliant; those with a severe asthma exacerbation (needing prednisolone) in the last 4 weeks | 118 (ITT LOCF)/119 WBR: 1; I: 58; C: 60 |
Adults > 18 years Mean age NR Male 54/118 (46) |
FEV1%: I: 81.4 (20.9); C: 84.9 (20.1) FEV1/FVC: I: 71 (10.7); C: 72 (9.9) |
Juniper score: I: 1.32 (0.65); C: 1.26 (0.75) | Log FeNO, GM (68% CI): I: 29.2 (14.0 to 61.0); C: 31.2 (13.3 to 73.1) | Ex-smokers: I: 22%; C: 25% Atopic: 78/118 (66.1%) |
Mean (SD) daily dose of ICS: I: 697 (708) µg; C: 652 (533) µg |
| Smith 200597 | Setting: primary care, New Zealand Funding: mixed;a equipment from Aerocrine |
Included: chronic asthma130 managed in primary care; regular ICSs for ≥ 6 months, no dose change in previous 6 weeks. If could not tolerate removal of LABA during run-in allowed to participate if could tolerate a fixed dose. Excluded: four or more courses of oral prednisone in previous 12 months, admission to hospital for asthma in previous 6 months, ever admitted to IC unit for asthma, smokers (current or ex) with history of > 10 pack-years | 94/110 WBR: 13; I: 46/48; C: 48/49 |
Adolescents and adults 12–75 years Mean age (range): 44.8 (12–73) Male 41/110 (37.3) |
FEV1%, mean (95% CI): I: 86.4 (80.6 to 92.2); C: 83.1 (76.5 to 89.7) | Symptom score,d mean (95% CI): I: 0.6 (0.4 to 0.8); C: 0.8 (0.6 to 1.1) | FeNO 250 ml/second,e GM (95% CI): I: 7.8 (6.6 to 9.3); C: 6.4 (5.5 to 7.5) | Smokers NR Atopic NR |
Bronchodilator use, mean per day previous 7 days (95% CI): I: 0.5 (0.2 to 0.8); C: 0.6 (0.3 to 0.8) ICS NR |
| Syk 201399 | Setting: primary care, Sweden Funding: mixed;a some from Aerocrine |
Doctor’s diagnosis of asthma and ICS treatment for ≥ 6 months; IgE sensitisation to at least one major airborne perennial allergen (dog, cat or mite); non-smoker for ≥ 1 year and with smoking history of < 10 pack-years. Patients all had mild to moderate asthma | 165/187 WBR: 6; I: 87/93; C: 78/88 |
Adults (18–64 years) Mean (SD) age 41 (12.4) Male 94/181 (51.9) |
FEV1%, mean (SD): I: 84.3 (14.1); C: 83.7 (12.5) FEV1/FVC, mean (SD): I: 0.78 (0.08); C: 0.79 (0.08) |
NR | FeNO, GM (95% CI): I: 22.0 (19.3 to 25.2); C: 21.6 (18.7 to 25.0) | Smokers: 0/165 (0%) Atopic: 165/165 (100%) |
Median (IQR) budesonide-equivalent ICS dose (µg/day): 400 (400 to 800) LABA before study entry: 54/180 (30.0%) |
The study by Smith et al. 97 included smokers (current or ex) with a history of < 10 pack-years whereas the studies by Shaw et al. ,98 Syk et al. 99 and Calhoun et al. 100 all excluded current smokers but included ex-smokers with a past smoking history of < 10 pack-years. Smith et al. ,97 Shaw et al. 98 and Calhoun et al. 100 all included a mix of atopic and non-atopic patients whereas Syk et al. 99 included only atopic patients. Honkoop et al. 101 included atopic patients and smokers. It is unclear whether studies in atopic patients will over- or underestimate efficacy or have no impact at all, although clinical input to the assessment suggested that it would be expected to increase estimates of efficacy as atopy is correlated with ICS responsiveness.
Overall, patient populations recruited by Honkoop et al. ,101 Smith et al. 97 and Shaw et al. 98 are likely to be the most representative of the general asthma population in the UK as these studies included atopic and non-atopic patients. Honkoop et al. 101 included smokers and a broad spectrum of patients from mild to severe, Smith et al. 97 included some smokers and Shaw et al. 98 included a potentially broader spectrum of patients than Smith et al. 97 Calhoun et al. 100 also recruited a mix of atopic and non-atopic asthmatics, but the run-in requirements for treatment tolerance and compliance may mean that generalisation to a wider population is difficult. However, were the application of FeNO management to be limited in the UK to certain populations (e.g. only atopic patients, only stable patients, only mild to moderate patients), data from Calhoun et al. 100 or Syk et al. 99 may be more appropriate.
Table 30 provides details of the interventions used in each study. Syk et al. 99 and Honkoop et al. 101 used NIOX MINO. It is not possible to determine whether the other studies used the same devices as this information is not clearly reported. 97,98,100 Smith et al. 97 used an unusual flow rate but justified their conversion to 35 ppb equivalent at 50 ml/second. None of the studies used the same protocol or cut-off points for the management of asthma with FeNO. Syk et al. 99 and Calhoun et al. 100 used FeNO only to guide management, Smith et al. 97 used FeNO only, with a safety measure based on symptoms, bronchodilator use and spirometry, and Shaw et al. 98 used FeNO in addition to the Juniper score, which gauges control through symptoms. The study by Honkoop et al. 101 was similar to that by Shaw et al. 98 in that it used FeNO in conjunction with symptoms, spirometry and medication use [all captured in the Asthma Control Questionnaire (ACQ)]. Doses and medications used also varied from study to study, with Smith et al. 97 and Calhoun et al. 100 titrating only ICSs, Shaw et al. 98 titrating ICSs, LTRAs and bronchodilators and Syk et al. 99 titrating ICSs and LTRAs. Honkoop et al. 101 controlled multiple treatment doses rather than just ICSs. There were some differences between the doses and combinations of treatments indicated and the study allowed for a step-down in treatment on the basis of low FeNO in the presence of moderate symptoms (when FeNO was low for > 3 months) but not in the presence of a high ACQ score. This is in contrast to Shaw et al. 98 and Smith et al. 97 which did not allow step-down if moderate symptoms were present,98 or did not allow the dose to fall below the optimum derived in the first titration phase,97 thus placing a limit on how far ICS could be decreased. The number of cut-off points also varied. Smith et al. 97 used only one cut-off of 35 ppb (equivalent). Honkoop et al. ,101 Shaw et al. 98 and Calhoun et al. 100 each used two cut-offs but at different cut-points, one for titrating down (< 25 ppb, < 16 ppb and < 22 ppb respectively) and one for titrating up (50 ppb, > 26 ppb and > 35 ppb respectively), with an intermediate area in between where symptoms also guided treatment98,101 or the dose remained the same. 100 Syk et al. 99 used three cut-offs, with different values for men and women (< 19 ppb, ≥ 24 ppb and ≥ 30 ppb for men; < 21 ppb, ≥ 25 ppb and ≥ 32 ppb for women). Given the uncertain comparability in FeNO measurements between devices, it is difficult to assess how similar these cut-off points may in fact be.
| Author, year | Decisions based on flow rate, device and cut-off points | Step-up/step-down protocol | Doses |
|---|---|---|---|
| Calhoun 2012100 | Based on FeNO only Flow rate; device: flow rate NR; device NR (protocol states Niox) Cut-offs: well controlled < 22 ppb; controlled 22–35 ppb; undercontrolled > 35 ppb |
< 22 ppb = well controlled = down one level; 22–35 ppb = controlled = maintain current level; > 35 ppb = undercontrolled = up one level | Dosing beclomethasone HFA: level 1 = 0 µg/day; level 2 = 80 µg q.d.; level 3 = 160 µg b.i.d.; level 4 = 320 µg b.i.d.; level 5 = 640 µg b.i.d. |
| Honkoop 2013101 | Based on ACQ and FeNO Flow rate; device: protocol states NIOX MINO Cut-offs: FeNO low ≤ 25 ppb, FeNO intermediate > 25 ppb and < 50 ppb, FeNO high ≥ 50 ppb; ACQ strictly controlled ≤ 0.75, ACQ sufficiently controlled > 0.75 and < 1.50, ACQ uncontrolled ≥ 1.50 |
When ACQ ≤ 0.75:
|
Step 1: SABA as needed; step 2: low-dose ICS or LTRA; step 3: low-dose ICS + LABA or medium- or high-dose ICS or low-dose ICS + LTRA; step 4: add one or both of medium- or high-dose ICS + LABA and LTRA; step 4: add one or both of OCS (lowest dose) and anti-IgE treatment |
| Shaw 200798 | Based on FeNO plus symptoms (Juniper score) Flow rate; device: 50 ml/second; device NR Cut-offs: intermediate 16–26 ppb; high > 26 ppb |
Exhaled NO < 16 ppb on first occasion or exhaled NO 16–26 ppb on second occasion:
|
Hierarchy of anti-inflammatory treatment: (1) low-dose inhaled steroid (100–200 µg BDP b.i.d.), (2) moderate-dose inhaled steroid (200–800 µg BDP b.i.d.), (3) high-dose inhaled steroid (800–2000 µg BDP b.i.d.), (4) high-dose inhaled steroid (800–2000 µg BDP b.i.d.) plus LRTA, (5) higher-dose inhaled steroid (2000 µg BDP b.i.d.) plus leukotriene antagonist, (6) higher-dose inhaled steroid (2000 µg BDP b.i.d.) plus leukotriene antagonist plus oral prednisolone 30 mg for 2 weeks, then titrate dose, reducing by 5 mg/week Hierarchy of bronchodilator treatment: (1) PRN SABAs, (2) LABAs, (3) LABAs plus theophylline, (4) LABAs plus theophylline plus nebulised bronchodilator |
| Smith 200597 | Based on FeNO, with a safety measure based on symptoms, bronchodilator use and spirometry Flow rate; device: 250 ml/second according to ATS 1999 guidelines;133 assume Niox devicea Cut-offs: equivalent to 35 ppb at 50 ml/second (≥ 15 ppb at 250 ml/second)b |
FeNO < 35 ppb (equivalent at 50 ml/second) = asthma controlled FeNO ≥ 35 ppb = asthma uncontrolled. Safety measure: if one or more of the following clinical criteria are met, increase one step: (1) symptom score for previous 7 days ≥ 1 point more than mean run-in and minimum score of 2/5; (2) nocturnal wakening on ≥ 3 nights/week more than mean run-in; (3) mean daily bronchodilator use three or more times that of mean run-in and minimum use 15 occasions during previous 7 days; (4) diurnal peak flow variation ≥ 30% and/or FEV1 of < 85% of baseline |
Dose steps: placebo and inhaled fluticasone at 100 µg, 250 µg, 500 µg, 750 µg and 1000 µg Phase 1: until optimal dose reached. Optimal dose = one step higher than that at which control lost Phase 2: up-titrate one step at a time; down-titrate if controlled for two visits but not lower than optimal dose Patients had a personalised self-management plan that instructed them to take oral prednisone 40 mg/day when the morning peak flow fell below 70% of the mean run-in value, until it reached > 85%, at which time they took 20 mg/day for the same number of days |
| Syk 201399 | Based on FeNO only Flow rate; device: according to 2005 guidelines;35 NIOX MINO Cut-offs: < 19 ppb (men), < 21 ppb (women); 19–23 ppb (men), 21–25 ppb (women); ≥ 24 ppb (men), ≥ 26 ppb (women); ≥ 30 ppb (men), ≥ 32 ppb (women) |
FeNO < 19 ppb (men), < 21 ppb (women): decrease one step; FeNO 19–23 ppb (men), 21–25 ppb (women): no change; FeNO ≥ 24 ppb (men), ≥ 26 ppb (women): increase one step (no change in treatment step if on step 4 or 5 and using two or fewer inhalations of SABA per week); FeNO ≥ 30 ppb (men), ≥ 32 ppb (women): increase two steps (only if on treatment step 1). Grey zone of 5 ppb applied to avoid frequent dose changes | Steps 1–6: budesonide (µg/day): 0, 200, 400, 800, 800 + LTRA, 1600 + LTRA respectively; fluticasone (µg/day): 0, 100, 250, 500, 500 + LTRA, 1000 + LTRA respectively; mometasone (µg/day): 0, 100, 200, 400, 400 + LTRA, 800 + LTRA respectively |
Table 31 provides details of the control interventions used in each study. As with the interventions, none of the studies used the same criteria, protocols or treatment doses for the management of asthma in the control arm of the studies. Generally speaking, the control arms considered symptoms, self-reported medication use and sometimes lung function to guide titration. In terms of similarity to UK practice, Shaw et al. 98 state that BTS/SIGN guidelines8 were followed, using the Juniper scale to score symptoms. It is not clear how similar to UK practice other studies may be.
| Author, year | Decisions based on | Step-up/step-down protocol | Doses |
|---|---|---|---|
| Calhoun 2012100 | NHLBI guidelines134 (US version of the SIGN guidelines) | Use severity classification chart, assessing both domains of impairment and risk, to determine initial treatment. Use asthma control chart, assessing both domains of impairment and risk, to determine if therapy should be maintained or adjusted (step up if necessary, step down if possible). Use multiple measures of impairment and risk: different measures assess different manifestations of asthma; they may not correlate with each other; and they may respond differently to therapy. Obtain lung function measures by spirometry at least every 1–2 years, more frequently for not well-controlled asthma. Asthma is highly variable over time and periodic monitoring is essential. In general, consider scheduling patients at 2- to 6-week intervals while gaining control; at 1–6 month intervals, depending on step of care required or duration of control, to monitor if sufficient control is maintained; at 3-month intervals if a step down in therapy is anticipated. Assess asthma control, medication technique, written asthma action plan, patient adherence and concerns at every visit | As for intervention |
| Honkoop 2013101 | |||
| Strict strategy | ACQ scores135 | When ACQ ≤ 0.75: if < 3 months, no change; if > 3 months, step down When ACQ > 0.75 and < 1.50: step up (choice of treatments) When ACQ ≥ 1.50: step up (choice of treatments) |
As for intervention |
| Sufficient strategy | When ACQ ≤ 0.75: step down When ACQ > 0.75 and < 1.50: no change When ACQ ≥ 1.50: step up (choice of treatments) |
As for intervention | |
| Shaw 200798 | BTS/SIGN guidelines8 using the Juniper scale to score symptoms | Scored by Juniper scale. Treatment doubled if score > 1.57, treatment halved if score < 1.57 for 2 consecutive months | Step 1: SABA as required; step 2: add inhaled steroid 200–800 µg/day BDP equivalent; step 3: add inhaled LABA; step 4: increase ICS up to 2000 µg/day and addition of fourth drug, e.g. LTRA, theophylline, LABA; step 5: oral prednisolone, high-dose ICS, refer to specialist care |
| Smith 200597 | GINA guidelines;136 symptoms, bronchodilator use, spirometry | GINA uncontrolled asthma criteria:125 (1) symptoms present on > 2 days/week with 24-hour asthma score ≥ 2/5; (2) More than one night-time waking/week; (3) bronchodilator use on more than four occasions/week or on > 2 days per week; (4) variation in PEFR > 20 (amplitude % of mean over previous 7 days); (5) FEV1 < 90% of baseline | As for intervention but without the personalised management plan |
| Syk 201399 | Symptoms, lung function, beta-agonist use | Usual care (patient symptom report, SABA use, physical examination, pulmonary function tests) | Assume same doses as for intervention |
Estimates of efficacy
Exacerbations were reported in all studies but definitions varied (Table 32) and results were not entirely consistent across studies.
| Author, year, time of outcome | Definition of outcomes | n | Intervention per person-year | Control per person-year | Between-group comparison |
|---|---|---|---|---|---|
| Calhoun 2012100 | Exacerbation: unscheduled medical contact for increased asthma symptoms that results in the use of OCSs, increased ICSs or additional medication for asthma | 229 | 0.21 (97.5% CI 0.1 to 0.32) | 0.23 (97.5% CI 0.1 to 0.37) | ‘Did not differ’ |
| Treatment failure defined as exacerbation or loss of controla | 0.27 (97.5% CI 0.14 to 0.39) | 0.43 (97.5% CI 0.23 to 0.64) | ‘Were not different’ | ||
| Honkoop 2013101 12 months |
Course of oral prednisone | 611 | 0.20 per person-year | Strict: 0.29 per person-year; sufficient: 0.29 per person-year | Odds ratio: vs. strict: 0.52 (95% CI 0.20 to 1.30); vs. sufficient: 0.73 (95% CI 0.28 to 1.85) |
| Shaw 200798 12 months |
Course of oral steroids or antibiotics | 118 | 0.33 (SD 0.69) | 0.42 (SD 0.79) | –21% (95% CI –57% to 43%; p = 0.43) |
| Smith 200597 3–12 months optimisation (exacerbation rates not reported for this period) plus 12 months titration |
Minor: global daily asthma scoreb of 2 on ≥ 2 consecutive days | 94 | Minor:c 0.36 | Minor:c 0.75 | Minor: p = 0.24 |
| Major: global daily asthma scoreb of 3 on ≥ 2 consecutive days (or in one day, in the context of a minor exacerbation). Major exacerbation or medical emergency: global daily asthma scoreb of 4 in one day | Major:c 0.13 | Major:c 0.14 | Major: p = 0.91 | ||
| Any minor or major exacerbation | 0.49 (95% CI 0.20 to 0.78) | 0.90 (95% CI 0.31 to 1.49) | –45.6% (95% CI –78.6 to 54.5; p = 0.27) | ||
| Course of oral prednisone | 22 events in 46 patients (0.48 events per patient) | 29 events in 48 patients (0.60 events per patient) | p = 0.60 | ||
| Syk 201399 End points analysed from visit 2 to visit 6 (2–4 weeks to 12 months) |
Moderate exacerbationd – need to step up controller treatment for at least 2 days with or without clinic visit. Prophylactic use before pollen season excluded | 165 | 0.1 | 0.325 | NR |
| Severe exacerbationd – worsening requiring a course of OCSs | 0.113 | 0.0875 | Not significant | ||
| Moderate or severe exacerbation | 0.22 | 0.41 | Total: p = 0.024 |
This outcome was defined differently across studies. Smith et al. 97 reported two such outcomes: ‘major exacerbations’ defined according to global daily asthma scores and exacerbations leading to a course of oral prednisone. A similar outcome, ‘worsening requiring a course of oral prednisone’, was also reported in Syk et al. 99 Shaw et al. 98 did not report rates of oral prednisone use alone but did report a composite outcome of ‘exacerbations resulting in the use of oral prednisone or antibiotics’. Calhoun et al. 100 reported an outcome called ‘exacerbations’, which included exacerbations leading to oral prednisone use, increased ICS use or additional medication for asthma. This last definition may incorporate exacerbations that other studies would have classified as moderate or minor, although the study does define an additional outcome called ‘treatment failure’, which is likely to incorporate minor, moderate and major exacerbations. As such, the outcome ‘exacerbations’ in the study by Calhoun et al. 100 will be considered in this analysis. Honkoop et al. 101 reported courses of oral prednisone, as in Smith et al. 97 and Syk et al. 99
Honkoop et al. 101 and Shaw et al. 98 reported lower rates per person-year of major/severe exacerbations in the intervention arm and Smith et al. 97 reported lower rates per person (data per person-year not reported) but the difference did not reach statistical significance compared with the control arm in any of the studies (these data were available only as odds ratios for Honkoop et al. 101). Syk et al. 99 reported higher rates per person-year of oral prednisone use in the intervention arm, but the level of significance was not reported. Calhoun et al. 100 showed very similar rates per person-year of exacerbations in both arms of the trial, with no statistically significant difference between them. The best improvement in major/severe exacerbations per person-year was seen in the study by Shaw et al. ,98 at –21% (95% CI –57% to 43%; p = 0.43) (reviewer-calculated rate ratio 0.79, 95% CI 0.66 to 0.94), and the worst improvement was seen in the study by Syk et al. ,99 which reported a higher rate per person-year, although not statistically significantly so, in the intervention arm (0.113) than in the control arm (0.0875) (p-value not reported) (reviewer-calculated rate ratio 1.29, 95% CI 0.83 to 2.03).
Despite the high level of heterogeneity in study characteristics, an exploratory meta-analysis of the rates of major/severe exacerbations was performed. As data per person-year were not reported in or calculable for Smith et al. ,97 this study was excluded from the meta-analysis. The standard error (SE) was not reported in the study by Honkoop et al. 101 and analyses were performed without these data and with various imputed SEs. The pooled estimate of the rate ratio without the data from Honkoop et al. ,101 using random effects methods (Figure 14a), was 0.94 (95% CI 0.66 to 1.34), with a p-value of 0.73. This indicated no difference between the two intervention groups in major or severe exacerbations. The I2 statistic was 52%, however, indicating moderate heterogeneity between studies.
A SE of 0.1, 0.2, 0.3 or 0.4 was imputed for Honkoop et al. ,101 based on the range of errors observed in other studies. Depending on the error imputed, rate ratios ranged from 0.82 (95% CI 0.64 to 1.05; p = 0.11) to 0.89 (95% CI 0.67 to 1.17; p = 0.40), which is not statistically significant. Figure 14b presents the analysis imputing an error of 0.2.
In a prespecified sensitivity analysis, only studies that reported data relating to exacerbations resulting in the use of OCSs were included. Only Syk et al. 99 and Honkoop et al. 101 reported this outcome although Honkoop et al. 101 did not report SEs, leaving only the study by Syk et al. 99 This study reported a rate ratio of 1.29 (95% CI 0.83 to 2.03), indicating no significant difference between the intervention groups (p = 0.26) (Figure 15).
FIGURE 15.
Sensitivity analysis removing studies with wider definitions of major/severe exacerbations: all studies with data available, excluding Honkoop et al. 101 (unknown SE). IV, inverse variable.
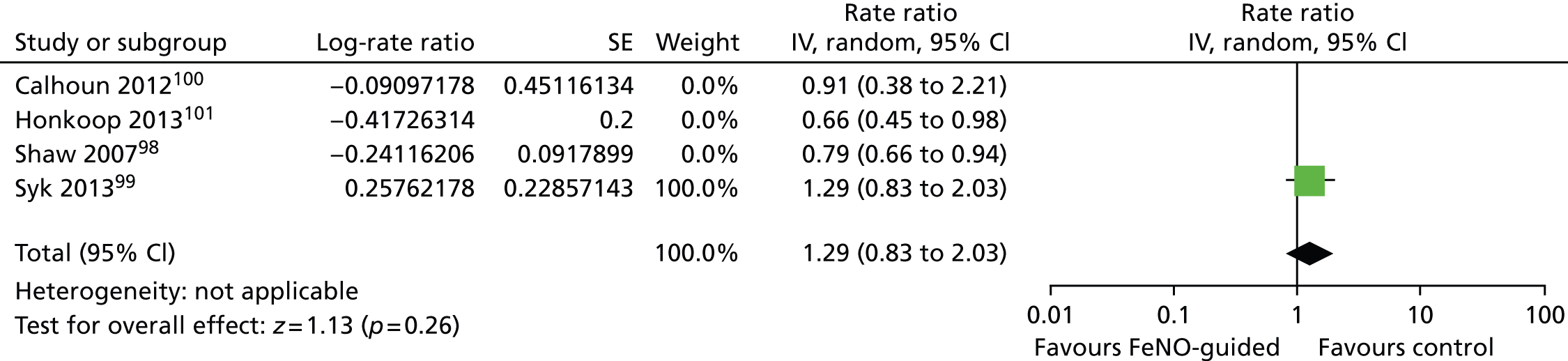
In further sensitivity analyses, SEs of 0.1, 0.2, 0.3 and 0.4 were imputed for Honkoop et al. 101 based on the range of errors observed in other studies. Depending on the error imputed, rate ratios ranged from 0.91 (95% CI 0.47 to 1.77; p = 0.79) to 1.00 (95% CI 0.53 to 1.90; p = 1.00), indicating no significant differences between the intervention groups. Heterogeneity statistics were high, ranging from 80% to 53% and reflecting the opposite direction of effect reported in these two studies.
Sensitivity analyses including imputed data for Smith et al. 97 were not conducted and it is unclear how the exclusion of these data affects the meta-analyses.
When considering other, wider definitions of exacerbation, as described in Table 32, three studies report composite outcomes that can be considered to be broadly similar and which represent what may be termed ‘treatment failure’. In the studies by Smith et al. 97 and Syk et al. 99 this was ‘any major or minor exacerbation’, whereas in the study by Calhoun et al. 100 it was exacerbation or any loss of control by a variety of measures (see footnotes to Table 32 for details). In the studies by Smith et al. 97 and Calhoun et al. ,100 FeNO-guided groups showed numerically but not statistically significantly lower rates of treatment failure. In the study by Syk et al. 99 the improvement was statistically significant, with a rate of 0.22 in the intervention arm compared with 0.41 in the control arm (p = 0.024) (reviewer-calculated rate ratio 0.52, 95% CI 0.44 to 0.61).
Despite the high level of heterogeneity in study characteristics, an exploratory meta-analysis of these rates using random effects methods (the I2 statistic was 0%) was conducted (Figure 16). The pooled relative risk (RR) was 0.53 (95% CI 0.46 to 0.61), which represents a statistically significant effect in favour of using FeNO-guided management in asthmatics for this outcome (p < 0.00001).
FIGURE 16.
Meta-analysis of the effects of FeNO-guided asthma management on the composite outcome of major/severe, moderate and minor exacerbation rates/treatment failures. IV, inverse variable.
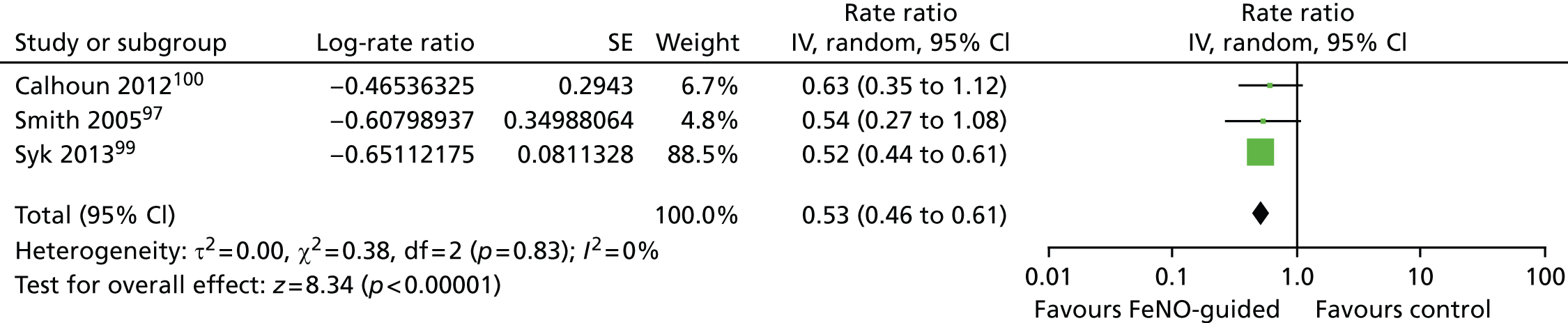
Smith et al. 97 and Syk et al. 99 both reported the rates of less severe exacerbations separately from the rates of all exacerbations and from the rates of major/severe exacerbations (see Table 32). In both cases the point estimate reduction in minor/moderate exacerbations was far greater than the reduction in severe/major exacerbations. Smith et al. 97 reported 0.36 minor exacerbations per person-year in the intervention arm and 0.75 per person-year in the control arm, with a p-value of 0.24. Syk et al. 99 reported 0.1 moderate exacerbations per person-year in the intervention arm and 0.325 in the control arm. The p-value was not reported. When considering the results reported by Calhoun et al. 100 for exacerbations alone and the composite outcome treatment failure, it can be seen that the larger difference in rates of treatment failure in favour of the intervention arm is not driven by the exacerbation rates, which are very similar at 0.21 (97.5% CI 0.1 to 0.32) and 0.23 (97.5% CI 0.1 to 0.37), and it must therefore be due to a decrease in less severe exacerbations/loss of control in the intervention arm. The impact on quality of life and the costs of such exacerbations are much lower than for major/severe exacerbations.
All studies except that by Honkoop et al. 101 provided some data on ICS use and these are presented in Table 33. Smith et al. 97 and Shaw et al. 98 reported ICS use as a mean per day at the end of the study, with mean differences of –270 µg/day (95% CI –430 µg/day to –112 µg/day; p = 0.003) and –338 µg/day (95% CI –640 µg/day to –37 µg/day; p = 0.028), respectively, in favour of FeNO-guided management. Syk et al. 99 reported median values that were not statistically significantly different. Means were supplied on request but without significance tests and showed a small increase in ICS use in the intervention arm [586 µg (SE 454 µg) vs. 540 µg (SE 317 µg) in the control arm]. Calhoun et al. 100 reported mean use per month, although it is unclear if this was an average over the whole course of the study or the mean for the final month of the study. The means were very similar at 1617 µg/month in the intervention group and 1610 µg/month in the control group. It should also be noted that this study managed and followed patients for only 9 months whereas the other studies did so for 12 months.
| Author, year | ICS measurement | Intervention | Control | Between-group difference expressed as intervention minus controla |
|---|---|---|---|---|
| Calhoun 2012100 | ICS use (unclear if mean over whole study or final value)b | Mean 1617 µg/month | Mean 1610 µg/month | NR |
| Shaw 200798 | Final value ICS useb | 557 µg | 895 µg | Mean difference –338 µg/day (95% CI –640 µg to –37 µg; p = 0.028) |
| Total used in study (AUC) | 11% greater use in FeNO group (95% CI –15% to 37%) | |||
| Smith 200597 | Final value ICS usec | Baseline: mean 411 µg/day (95% CI 344 µg to 478 µg); end of phase 2: mean 370 µg/day (95% CI 263 µg to 477 µg) | Baseline: mean 491 µg/day (95% CI 403 µg to 579 µg); end of phase 2: mean 641 µg/day (95% CI 526 µg to 756 µg) | Mean difference –270 µg/day (95% CI –112 µg to –430 µg; p = 0.003) |
| Syk 201399 | ICS used | Median 0 (IQR –400 to 400) µg; baseline: mean 604 (SE 370) µg; final value: 586 (SE 454) µg | Median 0 (IQR –200 to 200) µg; baseline: mean 626 (SE 391) µg; final value: 540 (SE 317) µg | 0.945 |
When looking at mean use over time (graphical data not reproduced here) in Smith et al. 97 and Syk et al. ,99 ICS use fell initially in the FeNO arm (both when compared with baseline and in comparison to the control arm) and then rose at the final measurement to a level above that in the control arm in Syk et al. 99 but staying below that in the control arm in Smith et al. 97 Conversely, in the Shaw et al. 98 study, ICS use initially rose and then fell at the final two measurement points to below the baseline level and below the control arm level. Only Shaw et al. 98 reported the AUC for ICS use and this showed an 11% greater use of ICSs in the FeNO group. Based on the ‘mean use over time’ figures, this is unlikely to be true for the study by Syk et al. ,99 in which a visual interpretation of the AUC would suggest very similar levels of total ICS use in both arms, with little change over time. Appropriate data were not available for the study by Calhoun et al. 100 or that by Smith et al. 97 These differences may be the result of the different titration protocols and cut-off values used in the studies and it is difficult to draw a generalised conclusion as to the direction of effect and the trends over time for ICS use. However, it would seem most likely that ICS use will either remain the same or fall in FeNO-managed groups when taken as an average over the course of the first year. The first year of titration is likely to be when the greatest gains are made, as patients reach a stable dose. It is unclear how ICS use will change in the following years as no study reported results beyond 1 year of follow-up, as the severity of the disease may progress, stay stable or remiss over time.
Despite the high level of heterogeneity in study characteristics, an exploratory meta-analysis of ICS use incorporating data from all four studies was conducted (Figure 17). As studies reported values for different ICSs (fluticasone, beclomethasone and budesonide), a standardised mean difference analysis was performed. A random-effects model was used as both clinical and statistical heterogeneity were high, but the I2 statistic remained high at 75%. SDs for Calhoun et al. 100 were imputed based on consideration of the other three studies. Sensitivity analyses in which the imputed SDs were altered by an order of magnitude in either direction, and in which a value of 10,000 was used for the intervention arm and 5000 for the control arm (to mirror the SDs of Syk et al. 99), did not have a big effect, with the pooled-analysis CIs crossing the line of no effect in every case and the pooled mean value ranging from –0.25 to –0.23 standardised mean difference. The results of the meta-analysis agree with the conclusions drawn from the narrative consideration of the data; it would seem most likely that ICS use will either remain the same or fall in FeNO-managed groups, probably depending on factors such as step-up/step-down protocols, cut-off values selected, treatments incorporated in the treatment protocol and comparator interventions.
FIGURE 17.
Meta-analysis of the effects of FeNO-guided asthma management on mean ICS use (standardised mean difference analysis). IV, inverse variable.
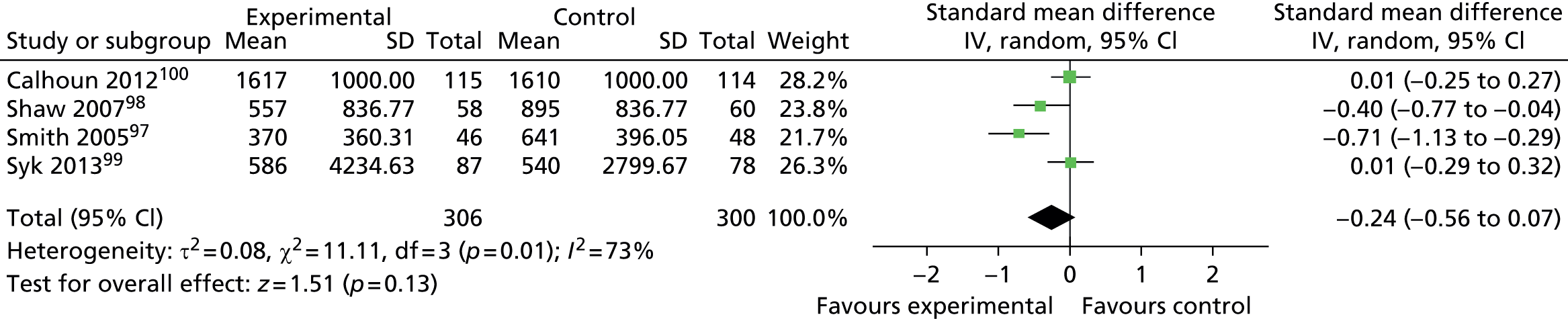
Syk et al. 99 Calhoun et al. 100 and Honkoop et al. 101 reported quality of life data. This was measured by the mini Asthma Quality of Life Questionnaire (mAQLQ) in Syk et al. 99 and the Asthma Quality of Life Questionnaire (AQLQ) in Calhoun et al. 100 and Honkoop et al. 101 In all studies the overall score, and in Syk et al. 99 three of four domains, did not show a statistically significant change over time. The symptoms domain did, however, show a relatively small but statistically significant between-group difference in change from baseline of 0.10 (Table 34) in Syk et al. 99 An exploratory meta-analysis of the overall scores (Figure 18) showed no effect, with a standardised mean difference of 0.00 (95% CI –0.20 to 0.20). In this case, data from the study by Honkoop et al. 101 were not included as there was not enough information provided to calculate a mean AQLQ score across the two control groups.
| Author, year | Intervention | Control | Between-group difference |
|---|---|---|---|
| Calhoun 2012100 | AQLQ change from baseline 0.02 (97.5% CI –0.14 to 0.18), p = 0.75 | AQLQ change from baseline 0.02 (97.5% CI –0.14 to 0.17), p = 0.80 | AQLQ between-group difference 0.00 (97.5% CI –0.22 to 0.23), p = 0.96 |
| Syk 201399 | Appears to be some data missing (n = 78–86) Total change over time in mAQLQ (n = 80/87), median (IQR): 0.23 (0.07 to 0.73); final mean (SE) value 6.07 (0.90) Visit 2 and visit 6 data, median (IQR): mAQLQ symptoms – visit 2: 5.60 (4.80 to 6.20), visit 6: 6.00 (5.60 to 6.60); activity limitation – visit 2: 6.50 (5.75 to 6.75), visit 6: 6.75 (6.00 to 7.00); emotional function – visit 2: 6.00 (4.67 to 6.67), visit 6: 6.33 (5.67 to 7.00); environmental stimuli – visit 2: 6.00 (5.00 to 6.67), visit 6: 6.33 (5.67 to 6.67) GQLI change (n = 85/88): 0.06 (–0.22 to 0.28) |
Appears to be some data missing (n = 77–85) Total change over time in mAQLQ (n = 77/78), median (IQR): 0.07 (–0.20 to 0.80); final mean (SE) value 5.98 (0.83) Visit 2 and visit 6 data, median (IQR): mAQLQ symptoms – visit 2: 5.70 (4.80 to 6.40), visit 6: 6.00 (5.20 to 6.40); activity limitation – visit 2: 6.25 (5.50 to 7.00), visit 6: 6.50 (5.75 to 7.00); emotional function: visit 2: 6.00 (4.67 to 6.67), visit 6: 6.00 (5.33 to 6.67); environmental stimuli: visit 2: 5.67 (5.00 to 6.67), visit 6: 6.33 (5.33 to 6.67) GQLI change (n = 78/78): 0 (–0.39 to 0.39) |
Analyses of median (IQR) change between visit 2 and visit 6: mAQLA overall: p = 0.197; mAQLQ symptoms: p = 0.041; activity limitation: p = 0.544; emotional function: p = 0.596; environmental stimuli: p = 0.193; GQLI: p = 0.666 |
FIGURE 18.
Meta-analysis of HRQoL outcomes. IV, inverse variable.
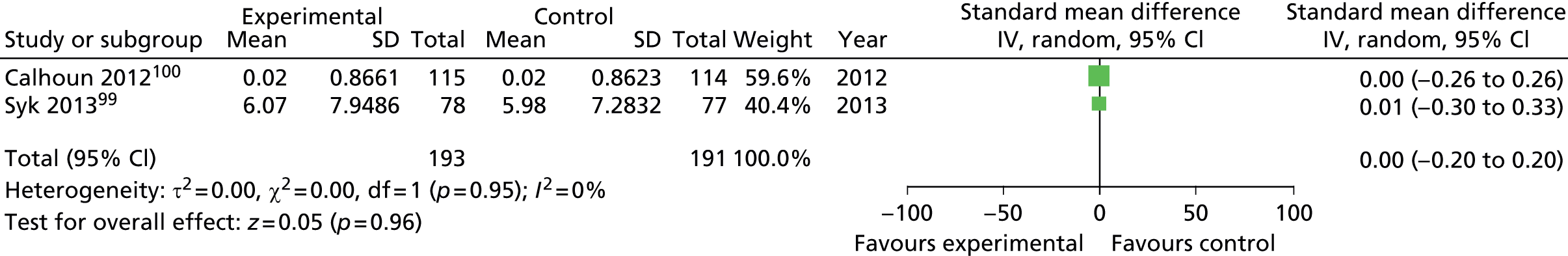
Four studies reported data for asthma control. 97–100 Smith et al. 97, Calhoun et al. 100 and Shaw et al. 98 reported no change in asthma control, whereas Syk et al. 99 reported a statistically significant difference in change in ACQ score from visit 2 to visit 6 between the two trial arms (Table 35). This matches the change seen in the AQLQ symptoms domain previously mentioned. Smith et al. ,97 Calhoun et al. 100 and Syk et al. 99 reported use of other medications; Smith et al. 97 and Calhoun et al. 100 reported no significant difference between groups for bronchodilator use, although in the study by Calhoun et al. 100 there was a trend towards less use in the intervention arm, and Syk et al. 99 reported non-significant trends towards greater numbers using LTRAs and higher mean use of LTRAs and SABAs (significance not reported) in the FeNO-controlled arm.
| Author, year | Outcome | Intervention | Control | Between-group difference |
|---|---|---|---|---|
| Asthma control | ||||
| Calhoun 2012100 | Night-time symptoms, difference from beginning to end of treatment period using model-based estimates (97.5% CI) | 0.01 (–0.00 to 0.02), p = 0.07 | 0.01 (–0.00 to 0.02), p = 0.11 | 0.00 (–0.02 to 0.02), p = 0.86 |
| Daytime symptoms, difference from beginning to end of treatment period using model-based estimates (97.5% CI) | –0.00 (–0.02 to 0.02), p = 0.86 | 0.01 (–0.00 to 0.03), p = 0.06 | –0.01 (–0.04 to 0.01), p = 0.17 | |
| ACQ score, difference from beginning to end of treatment period using model-based estimates (97.5% CI) | –0.01 (–0.15 to 0.12), p = 0.81 | 0.03 (–0.10 to 0.16), p = 0.64 | –0.04 (–0.23 to 0.15), p = 0.62 | |
| ASUI score, difference from beginning to end of treatment period using model-based estimates (97.5% CI) | 0.01 (–0.02 to 0.04) p = 0.40 | 0.01 (–0.02 to 0.03) p = 0.64 | 0.00 (–0.04 to 0.04) p = 0.79 | |
| Shaw 200798 | Asthma control | Data NR. No difference between groups in Juniper score throughout the study; however, in both groups the score decreased from baseline. Significance NR | ||
| Smith 200597 | Symptom score (daily score previous 7 days): final scores, mean (95% CI) | 0.4 (0.1 to 0.7) | 0.6 (0.4 to 0.9) | p = 0.23 |
| Nocturnal waking (nights/week, previous 7 days): final scores, mean (95% CI) | 0.2 (0.0 to 0.6) | 0.2 (0.0 to 0.4) | p = 0.89 | |
| Asthma score (% of days), mean (95% CI) | Score 0: 85.2 (78.4 to 92.0); score 1: 14.0 (7.4 to 20.6); score ≥ 2: 0.8 (0.3 to 1.3) | Score 0: 78.5 (70.4 to 86.6); score 1: 19.9 (12.3 to 27.5); score ≥ 2: 1.7 (0.3 to 3.1) | Between-group final scores (not change from baseline) p = 0.19 | |
| Syk 201399 | ACQ score change between visit 2 and 6, median (IQR) | –0.17 (–0.67 to 0.17) (n = 81/88) | 0 (–0.33 to 0.50) (n = 74/78) | p = 0.045 |
| Other medication use | ||||
| Calhoun 2012100 | Albuterol rescue use (puffs/day), difference from beginning to end of treatment period using model-based estimates (97.5% CI) | –0.04 (–0.10 to 0.02), p = 0.15 | 0.02 (–0.03 to 0.08), p = 0.30 | –0.06 (–0.14 to 0.02), p = 0.08 |
| Shaw 200798 | Medication use | NR | NR | NR |
| Smith 200597 | Bronchodilator use (occasions/day, previous 7 days), mean (95% CI) | 0.4 (0.1 to 0.7) | 0.4 (0.1 to 0.6) | p = 0.98 |
| Safety buffer criteria used | 16/436 assessments | NA | NA | |
| Syk 201399 | LTRA use, n/N (%) | 33/92 (35.9) | 19/85 (22.4) | p = 0.069 |
| Mean months on LTRA | 2.87 (4.42) | 1.81 (3.89) | p = 0.094 | |
| SABA use between visit 5 and visit 6 (8–12 months), median (IQR) | 1.56 (0.06 to 5.18) | 0.94 (0.03 to 2.81) | NR | |
No data were reported in the four studies for adverse events or mortality, although Calhoun et al. 100 reported one unrelated adverse event (hip surgery) in the control arm. Compliance was reported by Smith et al. 97 and Calhoun et al. 100 and was 85% and 89% in the intervention and control arms, respectively, in Smith et al. 97 and ≥ 95% (median) in both groups in Calhoun et al. 100 No test failure rates for NIOX MINO or NObreath were reported.
Children
Five studies103–107 that recruited children (plus adolescents and/or young adults) and compared FeNO- guided management to non-FeNO-guided management were identified from the initial search and a further two studies108,109 were identified during the update search. The study by Fritsch et al. 103 was based in Vienna, Austria; that by Szefler et al. 104 was based in the USA; that by Verini et al. 105 was based in Italy; that by Pijnenburg et al. 106 was based in Rotterdam, the Netherlands; that by Petsky et al. 107 was based in Australia; that by Peirsman et al. 109 was conducted in Belgium; and that by Pike et al. 108 was the first UK-based study identified on this topic in children. Fritsch et al. 103 received technical and analytical support from Aerocrine; one of the authors in the study by Szefler et al. 104 received speaker fees from Aerocrine; the study by Pijnenburg et al. 106 was supported by the Kroger Foundation/Sophia Children’s Hospital Foundation, although Aerocrine had provided a grant to the department; the study by Petsky et al. 107 was funded by the Royal Children’s Hospital Foundation, Asthma Foundation of Queensland; the study by Pike et al. 108 was funded by Sparks charity; the study by Peirsman et al. 109 was funded by Merck & Co., with equipment provided by Aerocrine; and Verini et al. 105 did not report their source of funding.
Quality assessment
Study quality varied, with no one study scoring well on every item and no item scoring well in every study (Figure 19). Of the studies included in the review, the study with the highest overall quality appeared to be that by Szefler et al. ,104 in which the only potential source of bias identified was study funding by a company with a commercial interest in FeNO measurement. The study with the lowest quality appeared to be that by Petsky et al. ,107 which was not scored as ‘low risk’ on any of the quality assessment items. As it was a conference abstract, Petsky et al. 107 was at especially high risk of selective reporting, whereas Verini et al. 105 was at risk of bias as the statistical comparison data were presented poorly (as discussed in the following sections). Potential sources of bias for the evidence base as a whole are discussed in the following sections.
FIGURE 19.
Methodological quality summary: review authors’ judgements about each methodological quality item for all included studies. Dark green circles with + sign, low risk of bias; light green circles with – sign, high risk of bias; medium green circles with ?, unclear risk of bias.
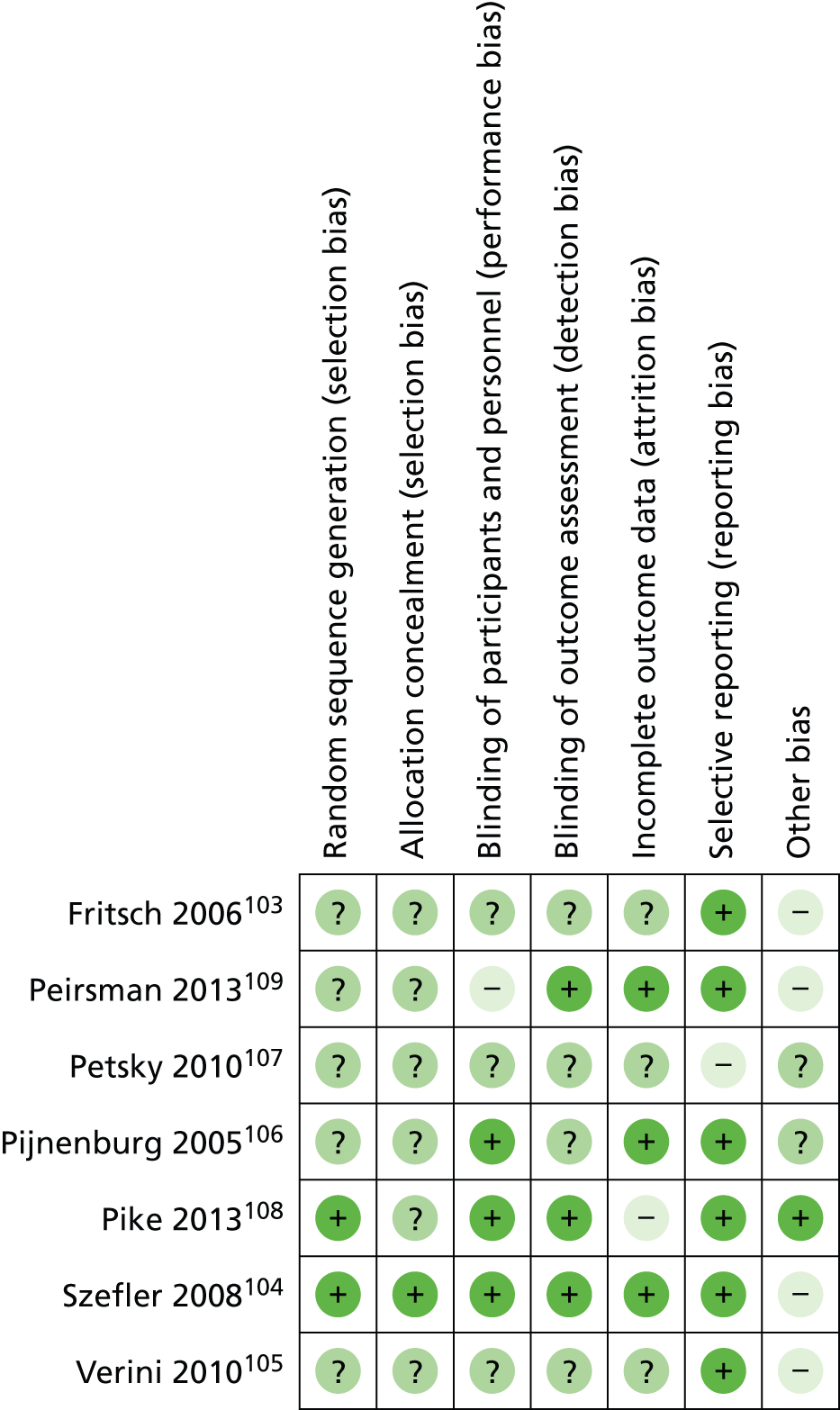
All of the included studies were described as randomised. However, only the study by Szefler et al. 104 provided sufficient information on sequence generation and allocation concealment. Pike et al. 108 did demonstrate random sequence generation but the method of allocation concealment was unclear. In all other studies the method of sequence generation and allocation concealment was not reported.
In terms of blinding, Szefler et al. ,104 Pijnenburg et al. 106 and Pike et al. 108 appeared to have performed adequate blinding for both participants and study personnel. Fritsch et al. 103 blinded participants but did not report whether this was the case for study personnel and so this study was rated as ‘unclear’ on this item. Peirsman et al. 109 blinded participants but not personnel. Neither Petsky et al. 107 nor Verini et al. 105 provided sufficient information to make a judgement on participant and personnel blinding and so these studies were rated as ‘unclear’. As many of the step-up/step-down protocols and criteria for exacerbations were based on participant symptom reporting and physician judgement, any potential lack of blinding in the studies could significantly affect the direction and size of the outcomes.
Szefler et al. ,104 Peirsman et al. 109 and Pike et al. 108 clearly stated that outcome assessment blinding had been performed. The poor reporting of outcome assessment blinding in the other studies means that unblinded outcome assessment may be a potentially important source of bias throughout this body of literature.
In terms of outcome data completeness, Pijnenburg et al. ,106 Szefler et al. 104 and Peirsman et al. 109 appeared to be at low risk of bias. There may have been some bias in terms of outcome data completeness in the remaining four studies. Fritsch et al. 103 did not report the reasons for participant withdrawal or correction for missing FeNO values and there may have been missing outcome data in the study by Petsky et al. ,107 but this is unknown as only a conference abstract of this study was identified. In the study by Verini et al. 105 it was reported that 64 patients were recruited; however, it was unclear whether this was the total number after dropout or whether no participants dropped out. Consequently, the study was rated as ‘unclear’ on outcome data completeness. Pike et al. 108 reported 23% of dropouts in the intervention arm but only 7% in the control arm and was scored as being at high risk of attrition bias.
Selective reporting risk may also have been present in some of the data. The study by Petsky et al. 107 was reported in a conference abstract and so this was rated as ‘high risk’ for this item. In the study by Fritsch et al. ,103 medication usage was reported as median (IQR) values rather than mean values and so these values could not be used in the planned meta-analysis; however, although planned, a meta-analysis was not possible because of study heterogeneity and so this bias did not affect the synthesis of data. The study by Verini et al. 105 was rated as having a low risk of bias for selective reporting; respiratory function and immunoallergological parameters were inadequately reported (i.e. no numerical data were provided; it was stated only that there were no significant between-group differences on these outcomes), but these outcomes were not of relevance to this review. Szefler et al. ,104 Pijnenburg et al. 106 Pike et al. 108 and Peirsman et al. 109 appear to have reported all of the outcomes that they set out to measure and so these studies were rated as ‘low risk’.
There were a number of further potential sources of bias in each of the studies. Fritsch et al. 103 and Szefler et al. 104 were both in receipt of sponsorship from the pharmaceutical industry and there was evidence of some such sponsorship in the studies by Peirsman et al. 109 and Pijnenburg et al. 106 The statistical comparison data reported in Verini et al. 105 was of poor quality in that most comparisons were presented as within-group longitudinal trends. Pike et al. ,108 Pijnenburg et al. ,106 Szefler et al. 104 and Fritsch et al. 103 all conducted run-in periods before randomisation, which have an unknown risk of bias attached to them. Finally, it was unclear whether there may have been additional sources of bias in the study by Petsky et al. ,107 as this research was presented as a conference abstract only.
The quality of the sampled literature was variable, with selective reporting being the most common potential source of bias. The studies by Fritsch et al. ,103 Petsky et al. 107 and Verini et al. 105 were rated as ‘unclear’ on the majority of quality items. Such ratings were given for those aspects of study design that were not clearly presented within the articles themselves, meaning that it was unclear whether there is likely to be bias in the conduct of the studies themselves or whether the lack of clarity was a result of inadequate reporting. Other common potential sources of bias were random sequence generation, allocation concealment and outcome assessor blinding. Pharmaceutical industry sponsorship was declared as the source of funding by Fritsch et al. 103 and Szefler et al. 104 and at least partially funded the activities of one author in the studies by Pijnenburg et al. 106 and Peirsman et al. 109 The studies at lowest risk of overall bias appeared to be those by Szefler et al. 104 and Pike et al. 108 (low risk on six and five of seven items respectively) and the studies by Szefler et al. ,104 Pike et al. 108 and Pijnenburg et al. 106 were the only studies in which it was possible to confirm blinding of both participants and study personnel. Possible lack of participant blinding may be a particularly important source of bias given that the study outcomes were largely based on subjective measurements (i.e. self-reporting of symptoms); blinding of study personnel may also be an important source of bias as they decide whether a patient’s medication step should be changed and there is some degree of interpretation in this decision. Finally, there was the possibility of selective reporting in Petsky et al. ,107 which may predispose the results to favour the intervention over the control.
Study details
All seven studies were RCTs, with varying degrees of blinding (see previous section). Study timelines are presented in Table 36. Study duration ranged from 6 months103 to 12 months. 105,107–109 Pijnenburg et al. ,106 Szefler et al. ,104 Fritsch et al. 103 and Pike et al. 108 reported run-in periods of 2, 3, 4 and 4–16 weeks respectively. In Pijnenburg et al. 106 and Fritsch et al. ,103 details of the run-in period were not provided. In Szefler et al. 104 patients were provided with a treatment programme based on previous treatment, adherence and control and in Pike et al. 108 patients were stabilised when necessary. Verini et al. 105 and Petsky et al. 107 reported no run-in. In addition, the frequency of visits varied from study to study. Fritsch et al. 103 and Szefler et al. 104 reported visits every 6–8 weeks, Pike et al. 108 every 2 months, Pijnenburg et al. 106 and Peirsman et al. 109 every 3 months and Verini et al. 105 every 6 months. Petsky et al. 107 did not report the frequency of visits and provided outcomes for 12 months only.
| Author, year | Timeline of study | Final assessment |
|---|---|---|
| Fritsch 2006103 | Visit 1: 4-week run-in. Visit 2: randomisation. Visit 3: visits at 6, 12, 18 and 24 weeks; symptoms, SABA use, anti-inflammatory treatment, FeNO and spirometry recorded. Bronchial challenge test (4.5% hypertonic saline) was carried out between the first and second visit | 24 weeks |
| Peirsman 2013109 | Visit 1: baseline. Visits 2–5: visit every 3 months; assessed symptom-free days (assessed daily), exacerbations, unscheduled asthma-related visits, hospital or emergency admissions, non-attendance at school and need for caregiver to take time off; FeNO recorded in all participants | 12 months |
| Petsky 2010107 | Spirometry, FeNO, QoL and asthma/cough diary every visit | 12 months |
| Pijnenburg 2005106 | Visit 0: 2-week run-in. Visit 1: randomisation, FeNO, FEV1, PD20, diary card. Visits 2–4: visit every 3 months; FeNO and symptoms (diary card previous 2 weeks) recorded at each visit. Visit 5: FeNO, FEV1, PD20, diary card | 9 months |
| Pike 2013108 | Unclear when baseline measurements performed. Visit 0: start of run-in period (4–16 weeks). Visit 1: randomisation. Visits 2–7: visit every 2 months for assessment – FeNO, exacerbations, symptoms and reliever use over previous 2 months recorded, treatment adherence assessed | 12 months |
| Szefler 2008104 | Visit 1: assessed asthma symptoms, pulmonary function, skin test, sensitivity, adherence and level of asthma control (NHLBI guidelines134). Run-in period of 3 weeks on a regimen based on standard treatment – physicians selected a treatment programme (one of six) based on previous treatment, adherence and asthma control. Included 10-minute session about adherence. Adherence measured during run-in period with Diskus inhaler and questionnaire. Centralised block randomisation and visit every 6–8 weeks for 46 weeks. Each visit: FeNO, days of symptoms, use of rescue drugs, pulmonary function, use of health care, adherence to treatment, missed days of school from asthma. Data were entered into computer and treatment option computed based on random allocation. In total, 546 were then randomly assigned to 46 weeks of either standard treatment or standard treatment modified on the basis of measurements of FeNO | 46 weeks |
| Verini 2010105 | Visit 1: baseline. Visit 2: 6 months. Visit 3: 12 months. ASS, asthma exacerbation frequency, asthma therapy score and immunoallergological and functional data recorded at each visit | 12 months |
The study and population characteristics are provided in Table 37. Eligibility criteria varied from study to study. With the exception of Verini et al. ,105 all studies included children with confirmed or persistent asthma. It is difficult to determine whether severity was comparable in the studies as scores have not been reported in a way that allowed comparison. Fritsch et al. 103 did not report severity; Szefler et al. 104 reported ACT scores; Verini et al. 105 and Peirsman et al. 109 classified participants on the basis of GINA scores; Pijnenburg et al. 106 reported mean daily symptom scores; and Pike reported exacerbations and OCS use in the previous year. Some insight into severity can be gained from considering the inclusion criteria and setting of each study. Three studies appeared to recruit patients who were uncontrolled. 104,105,107 Verini et al. 105 and Petsky et al. 107 recruited patients attending a specialist clinic, perhaps suggesting difficult to control patients, although in Verini et al. 105 patients had not yet started ICS therapy; an alternative explanation would be that all patients are sent to a specialist clinic before starting ICS therapy in this region and the patients were therefore not necessarily uncontrolled. Szefler et al. 104 recruited only patients with evidence of persistent or uncontrolled disease. Studies recruiting patients who are difficult to control are likely to capture the efficacy of FeNO for increasing control but not for decreasing ICS use in patients who are well controlled. Pike et al. 108 aimed to recruit patients with moderate to severe asthma and specified that children must be receiving therapy equivalent to stage 4 in the BTS/SIGN guidelines,8 as well as a requirement to demonstrate responsiveness to a bronchodilator (i.e. an increase in FEV1 of 15%). Fritsch et al. 103 recruited mild to moderate persistent asthma patients at an outpatients clinic. Pijnenburg et al. 106 recruited atopic asthma patients who were attending a children’s hospital, although it is not clear if this was a scheduled appointment, an emergency admission or just the location of the study follow-up and it is therefore unclear what level of severity of asthma these patients may have. As they had all had a stable dose of ICS for the previous 3 months, it may be reasonable to assume that these patients were reasonably well controlled. Peirsman et al. 109 recruited the broadest spectrum of patients, including mild to severe persistent asthmatics.
| Author, year | Study details | Inclusion/exclusion criteria | No. analysed/no. recruited | Age (years), sex | Spirometry, mean (SD) | Severity | FeNO (ppb) | Atopic, smokers | Medication use |
|---|---|---|---|---|---|---|---|---|---|
| Fritsch 2006103 | Setting: outpatient clinic, Vienna, Austria Funding: technical and analytical support from Aerocrine Study design: RCT, single blind, parallel groups |
Patients with mild to moderate persistent asthma with a positive skin prick test (SPT) or radioallergosorbent test (RAST > 1) to at least one of seven common aeroallergens (cat, dog, house dust mite, alternaria, birch pollen, hazelnut pollen and mixed grass pollen) in past medical history or at the time of recruitment | 47/52 I: 22 analysed, no. recruited unclear; C: 25 analysed, no. recruited unclear |
Children and adolescents (6–18 years) Mean (SD) age: I: 11.3 (3.4); C: 12.1 (2.8) Male 28/47 (59.6%) |
FEV1%, median (IQR): I: 101 (91.1 to 107.5); C: 93.7 (83.8 to 99.6) | NR | Median (IQR): I: 34.6 (17.5 to 58.6); C: 31 (20.8 to 54.8); difference NS | Atopic: assume 100% Smokers: NR |
ICS dose (µg/day), median (25%–75%): I: 230 (100 to 400); C: 140 (0 to 400) Beta-agonist puffs/day, median (IQR): I: 1 (0 to 7); C: 0 (0 to 2) |
| Peirsman 2013109 | Setting: Belgium (seven sites) Funding: Investigator Initiated Studies Program of Merck & Co. Study design: multicentre, single-blind, parallel-group RCT |
Mild to severe persistent asthma according to GINA guidelines125 for ≥ 6 months, allergic sensitisation (i.e. positive skin prick test and/or specific IgE antibodies against inhaled allergens), elevated FeNO values in the presence of allergic airway inflammation. Exclusion: significant comorbidity, acute exacerbation or administration of experimental medication 4 weeks before screening, hospitalisation and/or systemic corticosteroids in 12 weeks before screening or OCS dependence | 93/99 I: 47/49; C: 46/50 |
Children and young adolescent (5–14 years) Mean (SD) age: I: 10.6 (2.2); C: 10.7 (2.1) Male 66/99 (66.6%) |
FEV1% predicted: I: 92.9 (12.2); C: 89.0 (16.2); t-test: p = 0.18 | Mild/moderate/severe, n (%): I: 14 (28.6)/32 (65.3)/2 (4.1) (one missing value); C: 14 (28)/33 (66)/3 (6); chi-square: p = 0.92 | Median (IQR): I: 32.5 (13.8 to 72.0); C: 27.5 (16.3 to 47.3) | Atopic: assume 100% Smokers (household exposure): I: 5/48 (one missing value); C: 8/49 (one missing value) |
Daily ICS dose (µg), median (IQR): I: 320 (160 to 400); C: 320 (145 to 400) LABA use (n/N): I: 25/49; C: 23/50 |
| Petsky 2010107 | Setting: Australia (clinical setting NR) Funding: non-industry Study design: RCT |
Children with asthma and on ICS attending a paediatric specialist clinic. Excluded if other cardiorespiratory illness, if poorly compliant or if unable to take ICS or LABA | 63/63 I: 31/31; C: 32/32 |
Children (min. 4 years, max. NR) Mean age NR Male NR |
FEV1% predicted (95% CI): I baseline: 101.3 (90.9 to 111.7); I at 12 months: 106.1 (91.9 to 120.2); C baseline: 84.28 (63.9 to 104.7); C at 12 months: 91.16 (84.7 to 97.7) | NR | NR | Atopic: NR Smokers: NR |
NR |
| Pijnenburg 2005106 | Setting: children’s hospital, Rotterdam, the Netherlands Funding: some non-industry plus grant from Aerocrine to department Study design: parallel-group, double-blind RCT |
Children with atopic asthma who had been using ICS at a constant dose for previous 3 months | 85/89 I: 39/42; C: 46/47 |
Children and adolescents (6–18 years) Mean (SD) age: I: 11.9 (2.9); C: 12.6 (2.8) Male n = 55/85 (64.7%) |
FEV1% predicted: I 96 (14); C 99 (20) | Mean (SD) daily symptom score: I: 1.4 (2.0); C: 2.0 (2.4) | GM (range): I: 26.4 (5.6–134.9); C: 29.8 (3.1–117.5) | Atopic: I: 39/39 (100%); C: 46/46 (100%) Smokers: NR |
Mean (SD) daily beta2-agonist puffs: I: 0.4 (0.6); C: 0.4 (0.5) Initial ICS dose (µg), mean (SD): I: 762 (335); C: 746 (410) |
| Pike 2013108 | Setting: multiple outpatient clinics in the UK (n = 4) Funding: NR; authors state no conflict of interest Study design: RCT (assessor blinded) |
Age 6–17 years, treatment with 400 µg/day beclomethasone/budesonide or 200 µg/day fluticasone. Clinical asthma diagnosis based on symptoms including 15% increase in FEV1 with bronchodilator or diurnal PEF variability ≥ 15%. Exclusion criteria: inability to perform spirometry or FENO, cigarette smoking, poor treatment adherence, life-threatening exacerbation or need for maintenance oral prednisolone | 90/90 I: 34/44 completers (all analysed by ITT); C: 43/46 completers (all analysed via ITT) |
Children and adolescents (6–17 years) Mean (SD) age: I: 10.51 (2.6); C: 11.42 (2.69) Male 51/90 (56.7%) |
NR | Median (IQR) exacerbations in past year: I: 3.5 (2 to 8); C: 4.5 (2 to7) Median (IQR) OCSs in past year: I: 1 (0 to 3.5); C: 2 (0 to 3) |
Median (IQR): NR | Atopic: I: 81.1%; C: 88.4% Smokers (household exposure): I: 9.1%; C: 13.0% |
ICS dose (µg/day), median (25%–75%): I: 750 (400 to 1000); C: 800 (400 to 1000) Beta-agonist puffs/day, median (IQR): NR |
| Szefler 2008104 | Setting: 10 sites, USA Funding: non-industry, although one author received speaker fees from Aerocrine Study design: multicentre, double-blind, parallel-group RCT |
Eligibility restricted to residents of urban census tracts in which at least 20% of households had incomes below the federal poverty threshold. Eligible participants had been diagnosed with asthma by a physician. Those on long-term control included only if they had persistent asthma or evidence of uncontrolled disease; all others must have both symptoms of persistent asthma and evidence of uncontrolled disease. Excluded if adherence < 25% or a smoker | 534/546 I: 267/276; C: 267/270 |
Children and young adults (12–20 years) Mean (SD) age: 14.4 (2.1) Male 288/546 (53%) |
FEV1 (proportion of best FEV1): I: 95.9 (15.5); C: 95.7 (15.9) FEV1/FVC: I: 79.8 (9.0); C: 80.4 (8.3) |
ACT score in last month (range 5–25), mean (SD): I: 21.1 (3.6); C: 21.3 (3.2) | Median (25%–75%): at enrolment: 31.7 (14.1–65.4); at randomisation: I: 20.5 (11.5–45.3), C: 19.7 (10.9–38.0) | Atopic: NR Smokers: 0% |
NR |
| Verini 2010105 | Setting: secondary care hospital, Italy Funding: NR Study design: single-centre, parallel-group RCT |
Patients referred for allergic asthma – not all already on ICS | 64/64 I: 32/32; C: 32/32 |
Children and adolescents (6–17 years) Mean (SD) age: I: 10.7 (2.4); C: 11.3 (2.1) Male 36/64 (56.3) |
NR | GINA score 0: I: 7; C: 7 GINA score 1: I: 18; C: 19 GINA score 2 or 3: I: 7; C: 6 |
Baseline, mean (SD): I: 13.78 (12.31); C: NR | Atopic: 64/64 (100%) Smokers: NR |
NR |
Interestingly, atopy is a known confounder to FeNO measurements as atopic subjects have raised FeNO levels regardless of asthma status. Asthmatic atopic patients are thought to have the highest levels overall and so FeNO is theoretically able to distinguish between controlled and uncontrolled atopic asthmatics as well as between controlled and uncontrolled non-atopic asthmatics. 138 However, atopic asthma patients tend to have ICS-responsive asthma more often than non-atopic patients and so studies recruiting atopic patients will have limited generalisability. It is unclear whether studies in atopic patients will over- or underestimate efficacy or have no impact at all, although clinical input to the assessment suggested that it would be expected to increase estimates of efficacy as atopy is correlated with ICS responsiveness. Atopic patients were recruited by Peirsman et al. ,109 Fritsch et al. ,103 Verini et al. 105 and Pijnenburg et al. 106 Pike et al. 108 recruited only patients who demonstrated responsiveness to bronchodilators, but not all were atopic.
Petsky et al. 107 was the only study to include young children only, with all other studies including adolescents and/or young adults as well as young children, or adolescents only in the case of Szefler et al. 104 The studies also varied in terms of size (range 52103–546104) and baseline FeNO was inconsistently reported.
Table 38 describes the interventions used in each study. NIOX MINO was used in the two most recent studies;108,109 the NIOX device was used in three of the studies;103,104,106 Verini et al. 105 used the ECO MEDICS CLD 88 device; and it was unclear what device was used by Petsky et al. 107
| Author, year | Decisions based on flow rate, device and cut-off points | Step-up/step-down protocol | Doses |
|---|---|---|---|
| Fritsch 2006103 | Based on FeNO readings only Flow rate; device: 50 ml/second with the single-breath online method; NIOX device Cut-off: FeNO > 20 ppb |
In patients with stable asthma, an increased FeNO level was considered a sign of insufficient anti-inflammatory treatment (either because of insufficient dosing or because of low adherence to prescribed therapy); hence, aimed to improve adherence to therapy in patients on anti-inflammatory treatment with raised FeNO. These patients were provided with 2-week diary cards to record daily symptoms, beta-agonist use and controller medication requirements and telephone calls were made regularly to check adherence to therapy. Asymptomatic patients on therapy with beta-agonists on demand only, with normal lung function but increased FeNO levels, were prescribed low-dose steroids. Step up was performed irrespective of FeNO level if FEV1% predicted was < 80% and/or there were severe symptoms over the last 4 weeks and/or beta-agonist use involved six or more puffs over the last 14 days. If FeNO was raised in these patients they received 2-week diary cards as well. Step down was performed if FEV1% predicted was ≥ 80% and there were no or mild symptoms over the last 4 weeks and beta-agonist use involved fewer than six puffs over the last 14 days and FeNO was ≤ 20 ppb | Low-dose ICS: 2 × 100 µg fluticasone or 2 × 200 µg budesonide Low-dose ICS + LTRAs: 2 × 100 µg fluticasone or 2 × 200 µg budesonide + 5 mg montelukast once daily p.o. Low-dose ICS + LABAs: 2 × 100 µg fluticasone + 2 × 50 µg salmeterol or 2 × 200 µg budesonide + 2 × 12 µg formoterol High-dose ICS + LTRAs: 2 × 250 µg fluticasone or 2 × 400 µg budesonide + 5 mg montelukast once daily p.o. High-dose ICS + LABAs: 2 × 250 µg fluticasone + 2 × 50 µg salmeterol or 2 × 400 µg budesonide + 2 × 12 µg formoterol |
| Peirsman 2013109 | Based on FeNO readings and symptoms Flow rate; device: 50 ml/second; NIOX MINO Cut-off: 20 ppb |
Asthma classed as ‘controlled’ (≤ 20 ppb and symptoms controlled); ‘partly controlled’ (≤ 20 ppb and partly controlled symptoms); or uncontrolled (FeNO > 20 ppb regardless of symptoms). Medication changes were guided by participants’ baseline therapies | Controlled: if on ICS only: step down ICS 100 µg/day, if already < 100 µg stop and add LTRA; if on LTRA only, no change; if on ICS + LTRA, step down ICS 100 µg/day, if already < 100 µg, stop ICS; if on ICS + LABA, stop LABA Partly controlled: if on ICS only, consider adding LTRA; if on LTRA only, consider adding ICS 100 µg/day (max. 200 µg/day); if on ICS + LTRA, consider ICS step up by 100 µg/day (max. 400 µg/day, then add LABA); if on ICS + LABA, consider adding LTRA Uncontrolled: if on ICS only, add LTRA; if on LTRA only, add ICS 100 µg/day (max. 200 µg/day); if on ICS + LTRA, step up ICS by 100 µg/day (max. 400 µg, then add LABA); if on ICS + LABA, replace LABA with LTRA |
| Petsky 2010107 | Based on FeNO and atopy Flow rate; device: NR Cut-offs: NR |
NR (treatment adjusted according to exhaled nitric oxide result, monthly for 4 months, then every second month for 8 months) | NR |
| Pijnenburg 2005106 | Based on FeNO and symptoms Flow rate; device: presume 50 ml/second; NIOX analyser Cut-offs: > 30 ppb; ≤ 30 ppb + symptoms > 14 ≤ 30 ppb + symptoms ≤ 14 |
FeNO > 30 ppb = ICS increased; FeNO ≤ 30 ppb and symptoms > 14 = ICS stays the same; FeNO ≤ 30 ppb and symptoms ≤ 14 = ICS decreased | ICS doses: 100 µg: increase to 200 µg, decrease to 0 µg 200 µg: increase to 400 µg, decrease to 100 µg 400 µg: increase to 800 µg, decrease to 200 µg 500 µg: increase to 1000 µg, decrease to 250 µg 800 µg: increase to 1200 µg, decrease to 400 µg 1000 µg: increase to 1500 µg, decrease to 500 µg 1200 µg: increase to 1600 µg, decrease to 800 µg 1600 µg: increase to 2000 µg, decrease to 1200 µg 2000 µg: no further increase, decrease to 1000 µg |
| Pike 2013108 | Based on FeNO readings and symptoms Flow rate; device: flow rate NR; NIOX MINO Cut-offs: ≤ 15 ppb; > 15 ppb and ≤ 25 ppb; ≥ 25 or FeNO doubled from baseline |
FeNO ≥ 25 ppb or more than twice baseline:
|
Step 1: no ICS Step 2: beclomethasone 50 µg b.i.d. via spacer or budesonide 50 µg b.i.d. via spacer or turbohaler or fluticasone 50 µg once a day via spacer (or accuhaler) Step 3: beclomethasone 100 µg b.i.d. via spacer or budesamide 100 µg b.i.d. via spacer or turbohaler or fluticasone 100 µg once a day via spacer (or accuhaler) Step 4: beclomethasone 200 µg b.i.d. via spacer or budesamide 200 µg b.i.d. via spacer or turbohaler or fluticasone 100 µg once a day via spacer (or accuhaler) Step 5: trial of LABA. If ineffective, consider trial of LTRA Step 6: fluticasone 125 µg b.i.d. via spacer Step 7: fluticasone 250 µg b.i.d. via spacer Step 8: consider short course of prednisolone or other therapeutic options |
| Szefler 2008104 | Based on days/nights of symptoms (patient recall over past 2 weeks), FEV1 as percentage of personal best, FeNO Flow rate; device: 50 ml/second; NIOX device Cut-offs: 0–20, 20.1–30, 30.1–40, > 40 ppb |
Step up/down based on predefined levels of control: Level 1 – days of symptoms in past 2 weeks 0–3; nights of symptoms in past 2 weeks 0–1; % of personal best FEV1 ≥ 80%; FeNO 0–20 ppb. Medication would not change at this level or if at level 1 for two consecutive visits it may be stepped down Level 2 – days of symptoms in past 2 weeks 4–9; nights of symptoms in past 2 weeks 2; % of personal best FEV1 ≥ 80%; FeNO 20.1–30 ppb. Increase medication by one step Level 3 – days of symptoms in past 2 weeks 10–13; nights of symptoms in past 2 weeks 3–4; % of personal best FEV1 70–79%; FeNO 30.1–40 ppb. Increase medication by two steps Level 4 – days of symptoms in past 2 weeks 14; nights of symptoms in past 2 weeks 5–14; % of personal best FEV1 < 70%; FeNO > 40 ppb. Increase medication by three steps or two steps + prednisone |
Step 0 – no controller medication; rescue treatment with salbutamol as needed Step 1 – fluticasone by dry powder inhaler 100 µg/day Step 2 – fluticasone by dry powder inhaler 100 µg b.i.d. Step 3 – fluticasone by dry powder inhaler 100 µg/day and salmeterol 50 µg b.i.d. Step 4 – fluticasone by dry powder inhaler 250 µg/day and salmeterol 50 µg b.i.d. Step 5 – fluticasone by dry powder inhaler 500 µg/day and salmeterol 50 µg b.i.d. Step 6 – fluticasone by dry powder inhaler 500 µg/day and salmeterol 50 µg b.i.d. plus either low-dose theophylline or montelukast every day |
| Verini 2010105 | Based on GINA guidelines136 plus FeNO values Flow rate; device: flow rate NR; CLD 88 Cut-off: 12 ppb |
Values > 12 ppb lead to increased medication. Values < 12 ppb lead to a reduction in or maintenance of amount of drugs. Changes in drugs not reported. Unclear whether FeNO used at visit 2 only to guide therapy | NR |
None of the studies used the same protocol or cut-off points for the management of asthma with FeNO. The protocol of Fritsch et al. 103 was based on FeNO readings only. Other studies used a combination of FeNO levels and symptoms, with various cut-off points and numbers of cut-off points: Szefler et al. 104 specified three levels of cut-off, Pike et al. 108 specified two cut-off points, Petsky et al. 107 did not report cut-offs and all other studies used just one cut-off point. These cut-off points ranged from 12 ppb to 40 ppb. Treatments indicated at each step were also highly heterogeneous across studies, with Pike et al. ,108 Peirsman et al. ,109 Fritsch et al. 103 and Szefler et al. 104 indicating doses for ICSs, LTRAs and LABAs and Pijnenburg et al. 106 indicating doses only for ICSs. Verini et al. 105 and Petsky et al. 107 did not report doses. The treatment in the study by Pike et al. 108 was most similar to that in the BTS/SIGN guidelines,8 with some minor modifications. Importantly, in the studies by Szefler et al. ,104 Pike et al. 108 and Peirsman et al. ,109 step down could not occur on the basis of low FeNO levels if patients were still experiencing symptoms. This may have limited any potential reduction in ICS use for patients who were non-ICS responsive.
Table 39 provides details of the control group interventions. As with the interventions, none of the studies used the same criteria, protocols or treatment doses for the management of asthma in the control arm of the study, but, when reported, they all used the same treatment steps in both arms of the study. Management was typically guided by symptom severity and/or FEV1. Verini et al. 105 and Peirsman et al. 109 used GINA guidelines136 as a control and Pike et al. 108 used BTS/SIGN guidelines8 with some minor modifications.
| Author, year | Decisions based on | Step-up/step-down protocol | Doses |
|---|---|---|---|
| Fritsch 2006103 | Asthma control (symptoms, SABA use, lung function), as recommended in current (German) asthma guidelines139 | A step down in therapy was performed if FEV1% predicted was ≥ 80% and there were no or mild symptoms over the last 4 weeks and beta-agonist use involved fewer than six puffs over the last 14 days. A step up was performed in every other case | As intervention |
| Peirsman 2013109 | Symptoms, need for rescue treatment in past 2 weeks, spirometry (FEV1) based on GINA guidelines136 | GINA guidelines136 (specific step-up/step-down protocol not described) to determine if controlled, partly controlled or uncontrolled | As intervention |
| Petsky 2010107 | Symptoms/FEV1 | Unclear | NR |
| Pijnenburg 2005106 | Symptoms | Symptom score > 14 = ICS increase; symptom score ≤ 14 for first time = ICS stays the same; symptom score ≤ 14 for second time = ICS decrease Symptom score calculated as mean of daily scores for dyspnoea, wheezing and cough, during daytime and night-time, with each scored from 0 to 3, giving a max. score of 18, as well as use of beta2-agonists and percentage of symptom-free days. Calculated over previous 2 weeks for monitoring and over previous 4 weeks for end-point evaluation |
As intervention |
| Pike 2013108 | Symptom control | Therapy was stepped up if symptoms were poorly controlled and decreased if well controlled for ≥ 3 months, according to BTS/SIGN guidelines8 | As intervention |
| Szefler 2008104 | National Asthma Education and Prevention Program (symptoms, treatment use, lung function)140 | Control group received standard treatment based on the guidelines of the National Asthma Education and Prevention Program140 (i.e. as intervention but without FeNO measurements) | As intervention |
| Verini 2010105 | GINA guidelines136 (symptoms, SABA use, lung function) | GINA guidelines136 (specific step-up/step-down protocol not described) | NR |
Estimates of efficacy
All seven studies provided some data on asthma exacerbations, although it was unclear in some cases what the precise definition of an exacerbation was (Table 40).
| Author, year | Definition of outcomes | Intervention | Control | Between-group comparison |
|---|---|---|---|---|
| Hospital admission | ||||
| Peirsman 2013109 | One or more hospital admission | 1/43 (2.3%) | 1/43 (2.3%) | Chi-square test: p = 1.00 |
| Pike 2013108 | Requiring ≥ 8 hours of admission | 5 patients (11.4%) | 3 patients (6.5%) | p = 0.420 |
| Szefler 2008104 | One or more hospital admissions | 3.3% (SD 1.78%) | 4.1% (SD 1.98%) | Mean difference –0.8 (95% CI –4.0 to 2.3), p = 0.61 |
| Unscheduled use of health care | ||||
| Peirsman 2013109 | One or more unscheduled uses of health care | 6/44 (13.6%) | 15/43 (34.9%) | Chi-square test: p = 0.02 |
| Szefler 2008104 | One or more unscheduled uses of health care | 21.3% (SD 4.09%) | 22.7% (SD 4.19%) | Mean difference –1.4 (95% CI –9.3 to 6.7), p = 0.74 |
| OCS use | ||||
| Fritsch 2006103 | OCS use (no. of patients/group) | n = 2 | n = 2 | p = NS |
| Pijnenburg 2005106 | Prednisone courses | Eight events in 1 year = 8/39 = 0.21 per patient | 18 events in 1 year = 18/46 = 0.39 per patient | p = 0.60a |
| Szefler 2008104 | Prednisone courses | Mean 0.66 (SE 0.085) | Mean 0.84 (SE 0.085) | Mean difference 0.17 (95% CI –0.08 to 0.41), p = 0.14 |
| Any/wide definition of exacerbation | ||||
| Fritsch 2006103 | Exacerbation defined as oral steroid courses because of asthma symptoms over the last 4 weeks and/or unscheduled visit because of asthma symptoms over the last 4 weeks and/or increase in asthma symptoms from a symptom score of 0 or 1 to a score of 2 and/or decline in FEV1 > 10% compared with last visit (no. of patients/group) | 17/88 observations (18.2%) [sic]b | 22/99 observations (21.2%) [sic]b | p = NS |
| Peirsman 2013109 | Exacerbation as per GINA guidelines:136 an episode of progressive increased shortness of breath, coughing, wheezing or chest tightness or a combination of these symptoms | 18 per yearb | 35 per yearb | Mann–Whitney test: p = 0.02 |
| Petsky 2010107 | Asthma exacerbations (severity not described) | 6/31 (19.4%) | 15/32 (46.9%) | p = 0.021a |
| Pike 2013108 | Patients experiencing an exacerbation | 37 patients (84.1%)c | 38 patients (82.6%)c | p = 0.850 |
| Szefler 2008104 | Exacerbation defined as a composite outcome consisting of admissions to hospital, unscheduled visits and prednisone use | 37.0% (SD 4.83%) | 43.6% (SD 4.96%) | Mean difference –6.5% (95% CI –14.4 to 1.4), p = 0.11 |
| Verini 2010105 | ATS 2005 definition:35 number of episodes of coughing, dyspnoea and wheezing requiring SABAs | 0.83 (SD 0.98) per person-year | 1.85 (SD 1.34) per person-year | Between-group comparison NR |
| Other outcomes | ||||
| Peirsman 2013109 | Number of children with one or more exacerbation | 11/46 (23.9%) | 22/46 (47.8%) | Chi-square test: p = 0.02 |
| Peirsman 2013109 | One or more emergency room admission | 2/45 (4.4%) | 4/46 (8.7%) | Chi-square test: p = 0.41 |
| Szefler 2008104 | One or more prednisone course | 32.1% (SD 4.67%) | 42.0% (SD 4.94%) | Mean difference –10.3% (95% CI –18.5 to –2.2), p = 0.01 |
Three studies104,108,109 reported the number of patients (but not the rate per person-year) requiring hospitalisations. All three studies reported no difference between groups (see Table 40).
Data on exacerbations resulting in OCS use were reported in three studies. 103,104,106 Szefler et al. 104 reported rates per year and Pijnenburg et al. 106 reported the number of courses per group. In both cases rates were lower in the intervention arm. In the study by Szefler et al. 104 the rate per year was 0.66 (SE 0.085) in the FeNO group and 0.84 (SE 0.085) in the control group. It is not clear if this analysis calculated rates per person-year to account for missing data points. The mean difference was not statistically significant (0.17, 95% CI −0.08 to 0.41; p = 0.14). In the study by Pijnenburg et al. 106 the reviewer-calculated mean number of exacerbations per person was 0.21 (eight courses in 39 patients) in the FeNO group and 0.39 (18 courses in 46 patients) in the control group. The difference in the number of people experiencing an exacerbation in the study by Pijnenburg et al. 106 was non-significant. Fritsch et al. 103 reported no significant difference in the number of people requiring OCSs between groups.
Fritsch et al. ,103 Szefler et al. ,104 Verini et al. ,105 Petsky et al. ,107 Pike et al. 108 and Peirsman et al. 109 all reported this outcome using a broad definition of exacerbation (sometimes called treatment failure), but used different definitions to one another. Pijnenburg et al. 106 did not report this outcome. Lack of data allowing calculation of rates per person-year precluded meta analysis.
Five studies reported numerically fewer exacerbations or treatment failures in the intervention arm, but these differences were statistically significant in only two studies. Fritsch et al. 103 used a composite outcome that appeared to include moderate to severe exacerbations. There were 17 exacerbations out of 88 observations in the intervention group (reported as 18.2% in Fritsch et al. 103) compared with 22 exacerbations out of 99 observations in the control group (reported as 21.2% in Fritsch et al. 103). These data were not convertible to rates as some data points were missing in the study. The difference was not significant at the p < 0.05 level. Szefler et al. 104 also used a composite outcome, which appeared also to relate to moderate to severe exacerbations. This study reported the percentage of patients in each group with more than one exacerbation; these were 37.0% in the intervention group and 43.6% in the control group (risk ratio −6.5, 95% CI −14.4 to 1.4; p = 0.11). Verini et al. 105 reported events leading to the use of SABAs, which appeared to be likely to incorporate minor to major exacerbations. Rates per person-year were 0.83 (SD 0.98) and 1.85 (SD 1.34) in the intervention arm and control arm respectively. No between-group comparisons were presented for this outcome. Pike et al. 108 reported the number of patients who had ≥ 48 hours of increased asthma symptoms or therapy and showed no difference between the groups, with 37 (84.1%) patients in the intervention arm and 38 (82.6%) in the control arm experiencing an exacerbation (p = 0.850).
The two studies that reported a significant between-group difference were those by Petsky et al. 107 and Peirsman et al. 109 In the study by Petsky et al. ,107 exacerbations were not clearly defined but occurred in six out of 31 participants in the intervention group (19.4%) and 15 out of 32 participants in the control group (46.9%; p = 0.021). Peirsman et al. 109 reported statistically significantly fewer exacerbations of any severity (as defined using GINA guidelines136) in the intervention arm (18 events) than in the control arm (35 events; p = 0.02), although rates were not calculable because of missing information about the number of participants included in the analysis.
Table 41 provides details of ICS use in each study. Fritsch et al. 103 and Szefler et al. 104 reported statistically significantly higher ICS use in the intervention group, Pijnenburg et al. 106 reported very similar levels and the values in the remaining study105 (in terms of absolute numbers using ICSs) were difficult to interpret. Fritsch et al. 103 reported median (IQR) end-point values for ICS use in the intervention and control groups as 316 (200 to 500) µg and 241 (26 to 607) µg respectively (β = 0.20, p < 0.01). Pijnenburg et al. 106 reported the mean (standard error of the mean) cumulative ICS dose from visit 1 to visit 5 as 4407 (367) µg in the intervention group and 4332 (383) µg in the control group (p = 0.73). Szefler et al. 104 reported the between-group difference in use of fluticasone, which was 119 µg/day greater in the FeNO group by the final visit (95% CI 49 µg to 189 µg; p = 0.001). Finally, Verini et al. 105 reported the absolute number of participants using ICSs from each group at each time point (intervention group: T1 20, T2 19, T3 19; control group: T1 15, T2 22, T3 19). However, the baseline values for the groups in this study were not comparable and the absolute numbers of participants using ICSs, without concomitant data on dosages used, provide little understanding of between-group ICS use.
| Author, year | ICS type and measurement definition | Intervention | Control | Between-group difference |
|---|---|---|---|---|
| Fritsch 2006103 | Fluticasone and budesonide permitted. Data reported as median ICS dose (µg/day); unclear which ICS type the doses refer to | Baseline median (IQR) dose: 230 (100 to 400) µg; end point median (IQR) dose: 316 (200 to 500) µg | Baseline median (IQR) dose: 140 (0 to 400) µg; end point median (IQR) dose: 241 (26 to 607) µg | Repeated measures analysis: β = 10.20, p < 0.01 |
| Peirsman 2013109 | Budesonide or equivalent: | |||
| Median (IQR) cumulative ICS dose (calculated by summing daily ICS dose from visit 1 to visit 5) | Cumulative dose: 1280 (800 to 1800) µg | Cumulative dose: 1200 (675 to 1600) µg | p = NS | |
| Median (IQR) change in daily ICS dose from baseline | +100 (0 to + 400) µg | 0 (–200 to + 80) µg | p = 0.016 | |
| Petsky 2010107 | NR | NR | NR | NR |
| Pijnenburg 2005106 | Budesonide (2 mg max. daily dose permitted). Cumulative steroid dose (sum of mean daily steroid doses from visit 1 to visit 5) | Cumulative end point: 4407 (367) µg | Cumulative end point: 4332 (383) µg | p = 0.73 |
| Pike 2013108 | Beclomethasone, fluticasone and budesonide permitted. Data reported as median (IQR) ICS dose (µg/day) in terms of baseline, end point, change and total dose. Unclear which ICS type the doses refer to | Baseline dose: 750 (400 to 1000) µg/day; end point dose: 800 (400 to 1000) µg/day; dose change: 0 (–200 to 300) µg/day | Baseline dose: 800 (400 to 1000) µg/day; end point dose: 500 (400 to 1000) µg/day; dose change: 0 (–300 to 0) µg/day | Mann–Whitney rank-sum tests: baseline dose: p = 0.629; end point dose: p = 0.543; dose change: p = 0.297 |
| Szefler 2008104 | Fluticasone | NR | NR | Difference 119 µg/day (95% CI 49 to 189 µg; p = 0.001) (higher in intervention group) |
| Verini 2010105 | Unclear what ICS used. Measured in terms of absolute number of patients using per group at each time point | T1: 20, T2: 19, T3: 19 | T1: 15, T2: 22, T3: 19 | NR |
When studies recruiting patients who are or who are likely to be difficult to control103,104 were compared with the study that recruited patients who had been on a stable dose of ICS for 3 months,106 it was seen that the two studies recruiting the difficult to control groups saw an increase in ICS usage whereas the study that recruited stable patients saw similar levels of ICS use across both arms. This would be expected as the difficult to control group of patients is unlikely to need a dose reduction whereas patients who are stable may be eligible for such a reduction. In addition to this, the Szefler et al. 104 protocol did not allow step-down of ICSs on the basis of low FeNO levels if symptoms were still present, making step-down less likely to occur.
The two studies identified from the search update reported results that agree with previous findings. The study by Pike et al. ,108 which recruited more severe patients, saw a higher final median (IQR) ICS usage [800 (400 to 1000) µg vs. 500 (400 to 1000) µg] and higher upper and lower ends of the CIs [0 (–200 to 300) vs. 0 (–300 to 0) µg] for the median change from baseline in the intervention group, indicating that there was an increase in ICS use. However, the differences were not statistically significant. The study by Peirsman et al. ,109 which recruited a wider spectrum of patients but which also included severe asthmatics, also saw numerically higher doses in the intervention arm but, again, not statistically significantly so.
Table 42 provides HRQoL data. Only Petsky et al. 107 provided data on HRQoL and it was unclear which quality of life tool was used. In the intervention group, the baseline mean was 84.38 (95% CI 77.27 to 91.48), which rose to 86 (95% CI 74.84 to 97.1) at 12 months. Conversely, in the control group, the baseline mean of 86 (95% CI 81.49 to 90.51) dropped to 83.75 at 12 months (95% CI 78.6 to 88.9). If quality of life was measured with the European Quality of Life-5 Dimensions (EQ-5D), which seems likely, then higher values would indicate better quality of life and thus FeNO would be favoured. The end-point difference was statistically significant (p = 0.042), although it was unclear whether this comparison was for the change or for absolute end values.
| Author, year | Outcome | Intervention, mean (SD) | Control, mean (SD) | Comparison |
|---|---|---|---|---|
| Asthma control | ||||
| Fritsch 2006103 | Increase in symptoms to a score of 2 (severe), n/N (%) | 10/88 (11.4) | 11/99 (11.1) | NS at p < 0.05 |
| Unscheduled visits, n/N (%) | 5/88 (5.7) | 5/99 (5.1) | NS at p < 0.05 | |
| FEV1 decline > 10%, n/N (%) | 7/88 (8.0) | 13/99 (13.1) | NS at p < 0.05 | |
| Pijnenburg 2005106 | Change in mean symptom severity scores between visit 1 and visit 5 | 0.1 | 0.6 | Mean daily scores change p = 0.40 |
| Szefler 2008104 | Maximum days with symptoms | 1.93 (2.60) | 1.89 (2.69) | Mean difference 0.04 (95% CI −0.22 to 0.29), p = 0.78 |
| Days of wheeze | 1.71 (2.52) | 1.69 (2.64) | Mean difference 0.03 (95% CI −0.21 to 0.26), p = 0.83 | |
| Days of interference with activities | 0.87 (1.79) | 0.95 (1.98) | Mean difference −0.08 (95% CI −0.26 to 0.10), p = 0.38 | |
| Nights of sleep disruption | 0.52 (1.30) | 0.50 (1.25) | Mean difference 0.03 (95% CI −0.11 to 0.16), p = 0.71 | |
| Days of school missed | 0.19 (0.79) | 0.23 (0.84) | Mean difference −0.04 (95% CI −0.12 to 0.05), p = 0.38 | |
| ACT score in the last month | 21.89 (2.83) | 21.83 (2.88) | Mean difference 0.06 (95% CI −0.28 to 0.40), p = 0.72 | |
| Verini 2010105 | Symptom score (mean for ordinal data: intermittent asthma = 1; mild/moderate persistent asthma = 2; severe persistent asthma = 3) | T1: 1.09 (0.81); T2: 0.56 (0.75); T3: 0.75 (0.95) | T1: 1.09 (0.77); T2: 0.93 (0.61); T3: 0.92 (0.82) | NR |
| HRQoL | ||||
| Petsky 2010107 | HRQoL (metric not specified), mean (95% CI) | Baseline: 84.38 (77.27 to 91.48); 12 months: 86 (74.84 to 97.1) | Baseline: 86 (81.49 to 90.51); 12 months: 83.75 (78.6 to 88.9) | NR |
| Other medication use | ||||
| Fritsch 2006103 | Median (IQR) beta-agonist puffs/day | 1 (0 to 7) | 0 (0 to 2) | NR |
| Montelukast (LTRA) (unclear if median or mean) | 1.26 mg/day | 0 mg/day | p < 0.01 | |
| Peirsman 2013109 | Median (IQR) percentage of symptom-free days | 83.7 (27.1 to 91.9) | 79.6 (51.7 to 94) | Mann–Whitney test: p = 0.58 |
| Children who missed school, n/N, (%) | 10/46 (21.7) | 12/46 (26.1) | Chi-square test: p = 0.63 | |
| Children whose caregivers had to take time off, n/N, (%) | 6/45 (13.3) | 8/46 (17.4) | Chi-square test: p = 0.59 | |
| Nights of sleep disruption | 0.52 (1.30) | 0.50 (1.25) | 0.03 (95% CI −0.11 to 0.16), p = 0.71 | |
| Days of school missed | 0.19 (0.79) | 0.23 (0.84) | −0.04 (95% CI −0.12 to 0.05), p = 0.38 | |
| ACT score in the last month | 21.89 (2.83) | 21.83 (2.88) | 0.06 (95% CI −0.28 to 0.40), p = 0.72 | |
| Petsky 2010107 | – | – | – | – |
| Pijnenburg 2005106 | Beta-agonist use | NR | NR | p = 0.28 |
| Pike 2013108 | Change in FeNO (ppb), mean (95% CI) | +3.1 (–5.5 to 11.6) | + 3.3 (–8.5 to 15.1) | NS at p < 0.05 |
| Szefler 2008104 | Salmeterol (mean difference from baseline), µg/day | –6.5 | –12 | NR |
| Verini 2010105 | Mean of ordinal data, in which antihistamines, ketotifen and chromones = 1; specific immunotherapy, LABAs and LTRAs = 2; ICS = 3 | T1: 1.5 (0.7); T2: 1.43 (0.7); T3: 1.53 (0.6) | T1: 1.03 (0.9); T2: 1.62 (0.6); T3: 1.4 (0.7) | NR |
| Number of patients using LTRAs | T1: 8; T2: 8; T3: 11 | T1: 3; T2: 8; T3: 7 | NR | |
| Number of patients using no anti-inflammatory drugs | T1: 4; T2: 5; T3: 2 | T1: 14; T2: 2; T3: 6 | NR | |
Table 42 provides details of outcomes relating to asthma control and medication use. Four studies provided some data on asthma control, none of which demonstrated any significant effects favouring either the intervention or the control, although in the study by Verini et al. 105 significance was not reported. Furthermore, there was lack of uniformity in how asthma control was measured. Fritsch et al. 103 recorded the absolute number of participants per group whose symptom severity score increased to 2 (i.e. severe symptoms). Ten participants in the intervention group (11.4%), and 11 in the control group (11.1%) fulfilled this criterion (difference not significant). These researchers also reported the absolute number of participants per group who experienced a decline in FEV1 of > 10%: seven in the intervention group (8%) and 13 in the control group (13.1%; p = not significant). Szefler et al. 104 presented between-group differences for a number of symptomatic indicators of control, with higher numbers favouring the intervention. The measure comprised days of wheeze (risk ratio 0.04, 95% CI −0.22 to 0.29; p = 0.78), days of interference with activities (risk ratio 0.03, 95% CI −0.21 to 0.26; p = 0.83), nights of sleep disruption (risk ratio −0.08, 95% CI −0.26 to 0.10; p = 0.38), days of school missed (risk ratio 0.03, 95% CI −0.11 to 0.16; p = 0.71) and ACT score in the last month (risk ratio −0.04, 955 CI −0.12 to 0.05; p = 0.38). Pijnenburg et al. 106 calculated symptom scores based on diary card data for dyspnoea, wheezing and cough. Day and night were scored separately and each symptom was scored between 0 and 3, giving a maximum possible total score of 18. The change in mean daily scores between baseline and visit 5 was 0.1 in the intervention group and 0.6 in the control group (between-group difference p = 0.4). Finally, Verini et al. 105 measured symptom control using the GINA scale,136 which classified participants as having remission asthma (GINA score 0), intermittent asthma (GINA score 1) or persistent asthma (GINA score 2 or 3). The means (SDs) for these categorical data were presented for both groups at all three time points: at visit 1 the values were 1.09 (0.81) in the intervention group compared with 1.09 (0.77) in the control group; at visit 2 they were 0.56 (0.75) and 0.93 (0.61) respectively; and at visit 3 they were 0.75 (0.96) and 0.92 (0.82) respectively. No intergroup comparisons were conducted, although means in the intervention arm are numerically lower than those in the control arm.
With respect to additional medication use, three studies provided data using different metrics and mostly without formal comparison statistics. Szefler et al. 104 reported the mean difference from baseline in salmeterol usage; This was –6.5 µg/day in the intervention group and –12 µg/day in the control group (p-value not reported). Verini et al. 105 created a categorical measure of medication use in which antihistamines (e.g. ketotifen) and chromones = 1; specific immunotherapy, LABAs and LTRAs = 2; and ICSs = 3. The means (SDs) of these data were presented for both groups. In the intervention group the values were 1.5 (0.7) (visit 1), 1.43 (0.7) (visit 2) and 1.53 (0.6) (visit 3). For comparison, the values for the control group at the same time points were 1.03 (0.9), 1.62 (0.6) and 1.4 (0.7) respectively. Verini et al. 105 also provided absolute numbers using LTRA. For the intervention group these were 8, 8 and 11 at visits 1, 2 and 3 respectively and for the control group these were three, eight and seven respectively. Fritsch et al. 103 reported the median (IQR) number of beta-agonist puffs/day. In the intervention group this was one (0 to 7) whereas in the control group the number was zero (0 to 2). Overall, two studies reported numerically higher additional medication use in the intervention arm104,105
Studies found during the search update108,109 agreed with previous observations as neither reported a statistically significant difference in metrics of asthma control.
Data on adverse events, mortality and compliance were reported only in Szefler et al. 104 No statistically significant differences between the groups were reported for any adverse events or for mortality. For the intervention and control groups, respectively, adverse events were reported for eyes, ears, nose and throat (8.3% vs. 8.1%; p = 0.87), gastrointestinal disorders (13.4% vs. 14.1%; p = 0.78), haematology disorders (27.2% vs. 28.9%; p = 0.44), infections (55.8% vs. 52.2%; p = 0.46), musculoskeletal symptoms (15.9% vs. 18.5%; p = 0.44), nervous system disorders (34.4% vs. 33.7%; p = 0.20), respiratory signs and symptoms (33.7% vs. 34.1; p = 0.92) and skin symptoms (15.6% vs. 17.8%; p = 0.18). Medication compliance was shown to be 86% in the intervention group and 92% in the control group. No mortality was observed in either group.
Subgroups relevant to the review as defined in the scoping workshop
Pregnant women
Only one RCT of FeNO-guided management of asthmatic pregnant women was found. 102
The study by Powell et al. 102 was a double-blind, multicentre RCT conducted in Australia and funded from a number of industry and non-industry sources (including lecture fees from Aerocrine). In total, 203 participants were analysed out of 242 recruited. In the run-in period, patients not already using ICSs were started on budesonide at a dose of 200 µg twice a day. After randomisation, patients underwent monthly reviews and titration of the ICS dose. Patients were telephoned 2 weeks after each visit to assess symptoms and encourage adherence (Table 43). The study included no current smokers but did include 156/206 (75.7%) atopic patients (Table 44).
| Author, year | Study details | Timeline of study |
|---|---|---|
| Powell 2011102 | Setting: antenatal outpatients, Australia Funding: mixed; lecture fees from Aerocrine Study design: double-blind, parallel-group, multicentre RCT |
Visit 1: FeNO, spirometry, ACQ, atopy (specific IgE to aeroallergen); optimised self-management skills including inhaler technique, knowledge, action plan and adherence; 2-week run-in period after visit 1 – continued use of ICS budesonide turbuhaler or uncontrolled patients not using ICSs started on budesonide 200 µg b.i.d. Visit 2: randomisation; asthma symptoms, FeNO, spirometry, ACQ, quality of life questionnaires. Then, monthly review with research assistant: recorded clinical symptoms, ACQ, present treatment, FeNO, FEV1. FeNO, ACQ and treatment data sent to algorithm keeper who applied algorithm and sent treatment recommendation to research assistant who informed patient. If patient uncontrolled and at maximum dose, seen by investigator. Telephone assessments 2 weeks after visit to assess symptoms and encourage adherence |
| Author, year | Age (years), sex | No. analysed/no. recruited | Inclusion/exclusion criteria | Spirometry, mean (95% CI) | Severity, median (IQR) | FeNO (ppb), median (IQR) | Smokers; atopic, n/N (%) | Medication use |
|---|---|---|---|---|---|---|---|---|
| Powell 2011102 | Pregnant adults aged > 18 years Mean (SD) age 28 (5.4) Male 0/220 (0%) |
203/242 WBR: 22; I: 100/111; C: 103/109 |
Doctor’s diagnosis confirmed by respiratory physician’s diagnosis of asthma; non-smoking pregnant women between 12 and 20 weeks’ gestation with doctor’s diagnosis of asthma and who were using inhaled therapy in the last year | FEV1%: I: 95.1 (92.8 to 97. 4); C: 96.1 (93.5 to 98.7) FEV1/FVC: I: 79.7 (75.4 to 78.0); C: 80.63 (79.3 to 82.0) |
AQLQ-M: I: 0.8 (0.4 to 1.5); C: 1.0 (0.5 to 1.6) ACQ, mean (read off graph): I: 0.98; C: 1.01 |
I: 13.9 (6.6 to 32.0); C: 13.1 (7.5) | Current smokers: 0/203 (0); ex-smokers: 80/203 (39.4) Atopic: 156/206 (75.7) |
Days using beta-agonist in past week, median (IQR): I: 1.0 (0 to 5); C: 2.0 (0 to 6) ICS users, n/N (%): I: 46/111 (41.4); C: 47/109 (43.1) BDP-equivalent ICS dose (µg/day), median (IQR): I: 800 (400 to 800); C: 800 (400 to 1600) |
The study by Powell et al. 102 appeared to be at low risk of bias overall (see Figure 13). Randomisation and allocation concealment were performed well, meaning that the study was at low risk of selection bias. The study was double blind and made efforts to blind outcome assessors and is therefore at low risk of performance and detection bias. The study also seemed to be at low risk of attrition bias, with similar small numbers dropping out from each arm for reasons not related to treatment. Reporting bias did not seem to affect the study as far as could be ascertained from the journal publication. Commercial sponsorship puts the study at some risk of bias.
An ECO MEDICS device was used to measure FeNO. Patients were categorised according to their FeNO values, with cut-offs being < 16 ppb, 16–29 ppb and > 29 ppb. Step-up and step-down criteria are described in Table 45; decisions were based on a combination of FeNO and asthma control levels, with asthma control levels directing the dose of LABA (formoterol) and FeNO levels controlling the dose of ICS (budesonide).
| Author, year | Decisions based on flow rate, device and cut-off points | Step-up/step-down protocol | Doses |
|---|---|---|---|
| Powell 2011102 | Based on FeNO and ACQ Flow rate; device: according to ATS guidelines 2005;35 ECOMEDICS (Duernten, Switzerland) Cut-offs: < 16 ppb, 16–29 ppb, > 29 ppb |
FeNO concentration used to adjust dose of ICS and ACQ used to adjust dose of LABA:
|
ICS – steps 1–5: budesonide 0, 100, 200, 400, 800 µg b.i.d. respectively LABA – step 1: salbutamol as required; steps 2–5: formoterol 6, 12, 24, 24 µg b.i.d. respectively |
Patients were managed according to asthma control as assessed by the ACQ (Table 46). Medication doses were somewhat different from those in the intervention arm, with different doses of budesonide and formoterol indicated.
| Author, year | Decisions based on | Step-up/step-down protocol | Doses |
|---|---|---|---|
| Powell 2011102 | ACQ guided | Well-controlled asthma (ACQ < 0.75) – reduce treatment one step; partially controlled asthma (ACQ 0.75–1.50) – no treatment change; uncontrolled asthma (ACQ > 1.5) – increase one step. Those at maximum dose referred to respiratory physician | Step 1: salbutamol as required; step 2: budesonide 200 µg b.i.d. + salbutamol as required; step 3: budesonide 400 µg b.i.d. + salbutamol as required; step 4: budesonide 400 µg + formoterol 12 µg b.i.d.; step 5: budesonide 800 µg b.i.d. + formoterol 24 µg b.i.d. |
Table 47 provides details of all outcomes of relevance to the review.
| Time of outcome | Definition of outcomes | Intervention | Control | Between-group comparison |
|---|---|---|---|---|
| Exacerbations | ||||
| Monthly until birth (max. approx. 30 weeks) | Exacerbations: an unscheduled visit to a doctor, presentation to the ER or admission to hospital or when OCSs used. Events separated by ≥ 7 days were counted as a second event | 0.288 per pregnancy [mean (SD) study time 17.8 (5.5) weeks] | 0.615 per pregnancy [mean (SD) study time 18.8 (3.8) weeks] | Incidence rate ratio 0.496 (95% CI 0.325 to 0.755), p = 0.001 |
| OCS use, mean (95% CI) | 0.08 (0.03 to 0.133) | 0.19 (0.08 to 0.31) | p-value OCS use: 0.042 | |
| Hospitalisations, mean (95% CI) | 0 (0 to 0) | 0.03 (–0.004 to 0.06) | p = 1.0 | |
| ER/labour ward visits, mean (95% CI) | 0.04 (0.001 to 0.07) | 0.02 (–0.01 to 0.04) | p = 0.399 | |
| Unplanned or unscheduled doctors’ visits, mean (95% CI) | 0.26 (0.16 to 0.36) | 0.56 (0.40 to 0.72) | p = 0.002 | |
| ICS use | ||||
| Difference in means (from baseline to last visit) (read off graph) (µg/day) | –210 | 50 | p = 0.043 | |
| BDP-equivalent ICS dose (µg/day), median (IQR) | 200 (0 to 400) | 0 (0 to 800) | p = 0.079 | |
| ICS users, n/N (%) | 76/111 (68.5) | 46/109 (42.2) | p < 0.0001 | |
| Other outcomes | ||||
| HRQoL, median (IQR) | ||||
| SF-12 physical component summary (low 0, high 100) | 47.7 (40.8 to 52.0) | 46.9 (38.2 to 51.8) | p = 0.89 | |
| SF-12 mental component summary (low 0, high 100) | 56.9 (50.2 to 59.3) | 54.2 (46.1 to 57.6) | p = 0.037 | |
| AQLQ-M: total score (good 0, poor 10) | 0.75 (0.38 to 1.25) | 0.81 (0.38 to 1.63) | p = 0.54 | |
| Asthma control (ACQ), mean (SD) | 0.56 (0.67) | 0.72 (0.80) | p = 0.046 | |
| Beta2-agonist use in past week, median (IQR) | 0 (0 to 3) | 1 (0 to 5) | p = 0.024 | |
| LABA users, n/N (%) | 45/111 (40.5) | 19/109 (17.4) | p < 0.0001 | |
| Adverse events, mortality, compliance and test failure rates | NR | NR | NR | |
The composite outcome of all exacerbations (in this case defined as an unscheduled visit to a doctor, presentation to the emergency room or admission to hospital or when an OCS was used) was reduced in the intervention arm, with a rate ratio of 0.496 (95% CI 0.325 to 0.755) (p = 0.001). It should be noted that, unlike the other studies in adults, the composite outcome in this study did not include moderate or minor exacerbations, but more closely matched the definitions of severe exacerbations given in other adult studies. Data for each element of the composite outcome were reported individually and from this it can be seen that this difference is mostly driven by the rate of OCS use and the rate of visits to the doctor. Mean OCS use was 0.08 (95% CI 0.03 to 0.133) in the intervention arm and 0.19 (95% CI 0.08 to 0.31) in the control arm and, unlike other studies in adults, this did reach statistical significance, with a p-value of 0.042. Similarly, the rate of visits to the doctor was 0.26 (95% CI 0.16 to 0.36) in the intervention arm and 0.56 (95% CI 0.40 to 0.72) in the control arm, with a p-value of 0.002. The other components of the exacerbation outcome (hospitalisations and emergency room/labour ward visits) did not differ between groups.
The change in mean values from baseline to the final visit for ICS use was a decrease of 210 µg/day in the intervention arm and an increase of 50 µg/day in the control arm. This difference was statistically significant at p = 0.043. Interestingly, more women in the intervention arm were taking ICSs (68.5% vs. 42.2%) and the median (IQR) ICS dose as beclomethasone dipropionate equivalent (µg/day) was higher in the intervention arm [200 (IQR 0 to 400)] than in the control arm [0 µg/day (IQR 0 to 800)], but not statistically significantly so (p = 0.079).
The median scores and p-value indicate a small but statistically significant difference in the Short Form questionnaire-12 items (SF-12) mental component summary score in favour of the intervention arm, with a median (IQR) score of 56.9 (50.2 to 59.3) compared with 54.2 (46.1 to 57.6) in the control arm (p = 0.037); however, there were no statistically significant differences in the SF-12 physical component summary score or the Asthma Quality of Life Questionnaire – Marks (AQLQ-M) total score.
The ACQ score at the end of the study indicated good control in both groups, with the mean (SD) indicating statistically significantly better control in the intervention group [0.56 (0.67)] than in the control group [0.72 (0.80); p = 0.046]. Beta2-agonist use in the past week was higher in the intervention arm (p = 0.024) and there were statistically significantly more LABA users in the intervention arm (p < 0.0001).
None of these outcomes was reported in the study.
An additional study by Mattes et al. ,124 a follow-up to the Powell et al. 102 study, was identified in the update search. This study was identified as a conference abstract although subsequent to the update search the full text was published. The study took the form of a prospective longitudinal birth cohort with the aim of determining the effect of better maternal asthma management on the number of episodes of wheezy illness in the first year of life. The abstract reports that children born to mothers who had been in the FeNO group were significantly less likely to suffer from recurrent bronchiolitis in the first year of life (OR 0.08, 95% CI 0.01 to 0.62; p = 0.016) than children born to mothers in the clinical treatment group. These results indicate that there may be wider benefits to asthma management with FeNO than have been captured in our economic model.
In pregnant women, the outcomes of exacerbation rate as well as OCS use on its own and doctors’ visits were all statistically significantly better in the intervention arm. ICS use and beta2-agonist use were also lower, although LABAs were used by more patients in the intervention arm than in the control arm. Asthma control was marginally better in the intervention arm and the mental component summary score of the SF-12 was also better, although the physical component summary score of the SF-12 and the total score for the AQLQ-M were not statistically significantly different between groups. In summary, the use of FeNO to guide asthma management in pregnant women appears to be as effective as if not more effective than the use of FeNO in other adults and appears to reduce exacerbations and ICS use. This may be because of increased efficacy in pregnant women or because of differences in step-up/step-down protocols. Notably, this protocol allowed for the step down of ICS use on the basis of FeNO levels alone, regardless of whether symptoms were still present or not. A follow-up study suggests that there may be more benefits for the children born to women who had asthma management with FeNO than have been captured in the economic model.
FeNO to assess compliance to treatment
One further study was identified for the management review. 141 This open-label RCT recruited patients aged 6–16 years with mild to moderate persistent asthma (n = 54) and excluded patients who had received oral or inhaled steroid treatment during the last 2 months, who had acute upper airway infection, who had a history of bad compliance (< 65% of prescribed medication) or who had any other serious illness. The trial consisted of a 4-week run-in period, a 4-week washout phase and a final randomised treatment phase in which only one group was treated with inhaled budesonide and FeNO was used to attempt to distinguish these groups, that is, the study explored FeNO as a tool to assess patient compliance. The study showed that FeNO was able to distinguish those who had been treated with ICSs more successfully than conventional lung function parameters. However, as the study did not examine the efficacy of FeNO for guiding management per se, the data could not be compared with that from other studies in the management review. It should also be noted that this potential benefit of using FeNO in the management of asthma will have been captured in the other RCT trials if the management protocol included investigations of compliance before stepping treatment up or down.
The elderly
In the absence of RCTs, other study designs were included to gather evidence for the use of FeNO in the management of asthma in the elderly. Five observational studies (three cohort,116–118 one nested case–control119 and one cross-sectional120 study), published between 2010 and 2012, were identified that evaluated the measurement of FeNO in elderly asthmatics. A further study125 was identified during the search update and a journal article of the abstract by Columbo et al. 117 was also identified. 126 A summary of the design, patient characteristics and outcomes of the included studies is provided in Table 48. The studies by Columbo et al. ,117 Ross and Baptist120 and Smith et al. 119 were based in the USA, that by Inoue et al. 116 was based in Japan, that by Roh118 was based in Korea and that by Bozek et al. 125 was based in Poland. Three studies reported where the patients were recruited from: Inoue et al. 116 recruited patients from an outpatient clinic whereas Smith et al. 119 and Bozek et al. 125 recruited from primary care. All patients in the intervention group had a diagnosis of asthma; however, how asthma was defined was not reported in any of the studies apart from that by Smith et al. ,119 which used the GINA guidelines. 129 The mean disease duration of asthma was reported in three studies116,117,120 and ranged from 14.4 years116 to 35 years. 117 The mean age in the intervention group ranged from 68119 to 73120 years, apart from in the study by Bozek et al. ,125 which recruited only patients aged > 80 years and which had a mean age of 84.1 (± 3.9) years. All studies included both sexes, with a slightly higher proportion of female participants, apart from the study by Roh,118 which had a higher proportion of male participants. Three studies had a comparative control group. 116,119,125 Bozek et al. 125 and Inoue et al. 116 compared FeNO values between elderly asthmatics and non-elderly asthmatics but with different age cut-offs and Smith et al. 119 compared asthmatics with non-asthmatics. The device for measuring FeNO was reported in four studies,117,119,120,125,126 with all four using NIOX MINO.
| Author, year | Study details | Population | FeNO (ppb) | Summary of outcomes |
|---|---|---|---|---|
| Bozek 2013125 (new paper from updated search) | To assess the differences in the clinical features of atopic bronchial asthma between patients aged > 80 years old and youth Setting: primary care Country: Poland Funding: NR Design: observational study |
Bronchial asthmatics aged at least 80 years (n = 207) EAs ≥ 80 years: n = 105; NEAs ≤ 32 years: n = 102 Mean age (years): EAs: 84.1 ± 3.9; NEAs: 22.1 ± 5.2 Sex male, n/N (%): EAs: 42/105 (40.0); NEAs: 67/102 (65.7) |
EA: 41.2 ± 15.04; EA smokers: 46.09 ± 25.33; EA non-smokers: 38.03 ± 13.2; NEA: 32.74 ± 12.62; NEA smokers: 39.09 ± 15.66; NEA non-smokers: 27.8 ± 11.09 Device: NIOX MINO Flow rate: 50 ml/second Measured in triplicate |
There was a statistical difference in FeNO levels (p < 0.05) between EAs and NEAs. The statistical difference between the two groups also extended to smokers and non-smokers. Authors’ conclusion: FeNO can be useful in the assessment of bronchial asthma in the elderly. The higher FeNO level observed in the elderly may be evidence of more advanced inflammation in the airways |
| Columbo 2012117 (abstract); Columbo 2013126 (journal article) | Role of FeNO measurements in elderly asthmatics. Follow-up for 1 year with evaluation at baseline and every 3 months Setting: NR Country: USA Funding: NR Design: observational study |
Stable elderly asthmatics (n = 30) (all asthmatics) Mean age (years): 71.6 ± 4.9 Sex male, n/N (%): 12/30 (40.0) Atopic, n/N (%): 21/30 (70.0) Rhinitis, n/N (%): 27/30 (90.0) GORD, n/N (%): 12/30 (40.0) Medication usage, n/N (%): ICS: 26/30 (86.7) (384 ± 378 µg/day); LABA: 20/30 (66.7); LTRAs: 19/30 (63.3); nasal steroids: 15/30 (50.0) |
Baseline: 18.2 ± 14.3 Device: NR Flow rate: NR Measured in triplicate |
FeNO was not elevated at baseline and did not significantly change throughout the follow-up period. No significant correlation was found between FeNO and FEV1/FVC (p > 0.55; 0.25, 0.10 and 0.26, respectively, at each time point), other spirometric values, ICS or ACT at any time point. Authors’ conclusion: in stable EAs, FeNO was not elevated and did not correlate with subjects’ demographics, comorbidities, treatment symptoms or spirometric values. Routine measurements of FeNO may not be clinically valuable in EAs |
| Inoue 2010116 (abstract) | Pathophysiological characteristics of asthma in the elderly Setting: outpatient clinic Country: Japan Funding: NR Design: observational retrospective study |
Clinically stable asthmatics, never or ex-smokers (< 5 pack-years) and receiving ICS therapy for at least 3 months (n = 136) EAs ≥ 65 years: n = 49; NEAs ≤ 60 years: n = 51 Mean age: NR Sex male, n/N (%): EAs: 12/49 (24.5); NEAs: 21/51 (41.2) |
NR | There was no difference in FeNO levels, percentage of induced sputum eosinophils and neutrophils or methacholine airway sensitivity or reactivity between EAs and NEAs |
| Roh 2012118 (abstract) (although described in text, data missing in table) | Patients performed spirometry and FeNO measurements and answered ACT questionnaire Setting: NR Country: Korea Funding: NR Design: observational study |
Patients with diagnosis of asthma aged > 65 years (n = 67) (all asthmatics) ACT ≤ 15 (very poorly controlled): n = 25 (37.3%); ACT 16–19 (not well controlled): n = 30 (44.8%); ACT ≥ 20 (well controlled): n = 12 (17.9%) Mean age (years): 72.3 (65–89a) Sex male, n/N (%): 57/67 (85.1) Medication usage: NR |
ACT ≤ 15:
|
No correlation was found between ACT/FeNO and FEV1/FeNO variables (p = 0.45 and 0.41 respectively). Author’s conclusion: in spite of these data, the clinical assessment of asthma should be based on a combined approach that involves clinical aspects, functional parameters and biomarkers of inflammation, because elderly patients may have reduced symptom perception and multiple comorbidities |
| Ross 2011120 (abstract) | Baseline data were collected on objective measures of asthma, including spirometry and FeNO, and were correlated with asthma QoL and asthma control Setting: NR Country: USA Funding: NR Design: cross-sectional study |
Subjects ≥ 65 years with a history of physician-diagnosed asthma (n = 77) (all asthmatics) Mean age (years): 73.2 Sex male, n/N (%): 16/70 (22.9) Medication usage: NR |
NR Device: NIOX MINO Flow rate: NR |
No correlation between spirometric/FeNO objective data and QoL or asthma control was noted |
| Smith 2012119 | Complete medical history taken and physical examination carried out. SPT of panel of common aeroallergens, spirometry and measurement of FeNO levels. Geriatric QoL and health status assessed through standardised questionnaire Setting: primary care (community-based family practice) Country: USA Funding: Department of Veterans Affairs Design: nested case–control study |
Asthmatics: older adults aged ≥ 60 years with symptoms consistent with asthma; control: ≥ 60 years, age matched, without asthma No. analysed/no. recruited: asthmatics: 32/36; control: 39/41; 6/77 were unable to perform FeNO measurements Mean age (whole cohort) (years): 68.7 ± 7.2 years Sex male, n/N (%): 18/77 (23.4) Passive smokers, n/N (%): 24/77 (31.2); smoking history: 19 ± 19 pack-years Asthma medication usage, n/N (%): ICS 6/36 (16.7%); combination inhaler: 12/36 (33.3%); LTMA 8/36 (22.2%); theophylline 4/36 (11.1%); albuterol 18/36 (50%) |
Asthmatics: 20.8 ± 17.3; control: 18.3 ± 9.8 (p = 0.5) Asthmatics treated with ICS: 21.4 ± 20.4; asthmatics not treated with ICS: 19.8 ± 14.3 (p = 0.8) Device: NIOX MINO Flow rate: 50 ml/second |
No statistically significant difference was seen in FeNO levels between asthmatics and control subjects (p = 0.5). No statistically significant difference was seen between ICS-treated and ICS-untreated asthmatics (p = 0.8) |
The results of the included studies are summarised in Table 48. Four of the six studies generally indicated a trend showing that FeNO measurements may not be clinically valuable in elderly asthmatics. Smith et al. 119 found no statistically significant difference in FeNO levels in elderly asthmatic subjects (20.8 ± 17.3 ppb) compared with elderly non-asthmatics (18.3 ± 9.8 ppb) (p = 0.5). Furthermore, no statistically significant difference was observed in FeNO levels between ICS-treated (21.4 ± 20.4 ppb) and -untreated asthmatics (19.8 ± 14.3 ppb) (p = 0.8). Columbo et al. 117 followed up stable elderly asthmatic patients for 1 year and evaluated FeNO measurements at baseline and every 3 months for 1 year. No significant correlation was found between FeNO levels and spirometric values, ICS usage or asthma control. Two further studies118,120 showed no correlation between FeNO levels and asthma control. Furthermore, one study,116 which evaluated the pathophysiological characteristics of asthma in elderly patients, found that there was no difference in FeNO levels, the percentage of induced sputum eosinophils and neutrophils or methacholine airway sensitivity or reactivity between elderly asthmatics and non-elderly asthmatics.
Bozek et al. 125 observed differences in FeNO levels between elderly and non-elderly asthmatics and concluded that FeNO can be useful in the assessment of bronchial asthma in the elderly. However, this study did not report data relating to correlations between measures of asthma presence/absence/severity/control and FeNO levels and concluded only that FeNO may be higher in older asthmatic patients than in non-elderly asthmatics.
Overall, these results should be interpreted with caution as data were derived from studies with lower-quality designs that have greater potential to produce biased results. In addition, three of these studies were reported in abstract form only and hence provide limited data. However, the majority of studies appear to indicate that FeNO is not useful in the elderly.
Adult smokers
Four studies5,121–123 were conducted in adult smokers. A summary of the design and patient characteristics of the four included studies is provided in Table 49. Two studies5,121 were conducted in Belgium, one123 in Serbia and one122 in New Zealand. With the exception of the study by Hromis et al. ,123 which did not provide details of the funding source, all studies received funding from one or more commercial sponsor. Kostikas et al. 122 received an unrestricted research grant from Aerocrine; Michils et al. 121 received technical funding from AstraZeneca; and Schleich et al. 5 received an unrestricted grant from GlaxoSmithKline, AstraZeneca and Novartis.
| Author | Study details | Population | FeNO measurements (ppb) | Outcomes |
|---|---|---|---|---|
| Hromis 2012123 (abstract) | Use of FeNO to assess asthma control in smoking patients; 4 weeks from the start of the study, a LABA (salmeterol, 30 non-smokers and 17 smokers) or an antileukotriene (montelukast 10 mg, 17 non-smokers and 14 smokers) was added. Changes in FeNO, FEV1 and ACT at baseline and after 4 weeks of treatment were measured Setting: NR Country: Serbia Funding: NR Design: randomised, open-label study |
Mild to moderate asthmatics with poor control (n = 83) Smokers: n = 31; non-smokers: n = 52 Age: NR Sex: NR Medication usage: low dose of ICS (400 µg beclomethasone dipropionate daily or equivalent) |
Smokers: baseline: 57; after 4 weeks’ treatment: 17 LABA added to ICS (reduction in FeNO): smokers: 22%, non-smokers: 32%; treated with antileukotriene (reduction in FeNO): smokers: 12%, non-smokers: 22% Device: NR Flow rate: NR |
The sequential changes in FeNO could be a useful marker of asthma control, regardless of smoking status. FeNO level also depends on the applied treatment |
| Kostikas 2011122 | Patients sequentially undertook FeNO measurements, EBC collection and spirometry with dry spirometer according to ATS 2005 guidelines.35 Subjects did not smoke for 2 hours before testing Setting: outpatient clinic Country: New Zealand Funding: Aerocrine Design: cohort study |
Asthmatic patients (n = 274) Group 1: ICS-untreated non-smokers: n = 48; group 2: ICS-untreated smokers: n = 32; group 3: ICS-treated non-smokers: n = 144; group 4: ICS-treated smokers: n = 50 Mean (SD) age (years): 50 (17) Sex male, n/N (%): 109/274 (40) Medication usage, n/N (%): ICS 194/274 (71) |
Median (IQR) Group 1: 30 (18–111) – well controlled 16 (14–21); partly controlled 40 (27–105),a uncontrolled 116 (63–145)a,b Group 2: 19 (14–22) – well controlled 16 (12–19); partly controlled 21 (15–38);a uncontrolled 22 (21–108)a Group 3: 23 (16–44) – well controlled 16 (12–20); partly controlled 28 (20–44);a uncontrolled 61 (35–78)a,b Group 4: 19 (14–25) – well controlled 17 (14–22); partly controlled 19 (13–25); uncontrolled 23 (17–74) Device: NIOX MINO Flow rate: 50 ml/second |
There was a statistically significant difference in FeNO values between controlled, partly controlled and uncontrolled asthma (p < 0.05) in non-smokers (groups 1 and 3). In group 4 (smokers treated with ICSs) there was no statistically significant difference in FeNO values between the three asthma control groups (p > 0.05). In group 2 (smokers untreated with ICSs) there was a significant difference in FeNO values between uncontrolled and well-controlled asthma but no difference from partly controlled asthma. The diagnostic performance of FeNO for the identification of not well-controlled (partly or uncontrolled) asthma was better in the non-smoker groups (group 1 FeNO cut-off > 22 ppb, sensitivity 87%, specificity 81%; group 3 FeNO cut-off > 27 ppb, sensitivity 64%, specificity 94%) than in the smoker groups (group 2 FeNO cut-off > 19 ppb, sensitivity 56%, specificity 75%; group 4 FeNO cut-off > 23 ppb, sensitivity 45%, specificity 87%) |
| Michils 2009121 | Evaluation of FeNO to predict asthma control in smoking patients. At each visit asthma treatment was adjusted according to GINA guidelines,112 regardless of ACQ score or FeNO values Setting: tertiary asthma clinic Country: Belgium, Funding: AstraZeneca Design: Retrospective, post hoc analysis study |
Adults with persistent asthma (n = 470) Current smokers: n = 59; non-smokers: n = 411 Mean (SD) age (years): smokers: 38 (11); non-smokers: 41 (16) Sex male, n/N (%): smokers: 34/59 (58); non-smokers: 195/411 (47) Medication usage: ICS dose (µg equivalents beclomethasone dipropionate per day), median (range): smokers: 500 (0–2000); non-smokers 250 (0–2000) |
GM (range): smokers: 18.1 (6.9 to 47.5); non-smokers: 33.7 (14.3 to 79.2) Device: LR2000 chemiluminescence analyser Flow rate: 50 ml/second |
Levels of FeNO were lower in smoking asthmatics. In smoking patients FeNO is unable to cross-sectionally discriminate for or against controlled vs. uncontrolled asthma (p = 0.39). FeNO cut-off was 50 ppb in non-smokers compared with 25 ppb in smokers. A decrease in FeNO of < 20% precludes asthma control improvement in non-smoking (NPV 78%) and smoking (NPV 72%) patients. An increase in FeNO of < 30% is unlikely to be associated with deterioration in asthma control in both groups. Authors’ conclusion: even in smokers, sequential changes in FeNO have a relationship with asthma control. This study indicates that cigarette smoking does not obviate the clinical value of measuring FeNO in asthma among smokers |
| Schleich 20105 | Use of FeNO to predict sputum eosinophil count of ≥ 3% Setting: secondary care Country: Belgium Funding: supported by the Interuniversity Project, GlaxoSmithKline, AstraZeneca and Novartis Design: retrospective, post hoc analysis |
Asthmatic patients (n = 295) Smokers: n = 58; non-smokers: n = 237 Median (IQR) age: 47.3 (14 to 83) Sex male, n/N (%): 131/295 (44.4%) Medication usage: NR |
Median (IQR): smokers: 17 (12 to 37); non-smokers: 35 (p = 0.003) Device: Niox (chemiluminescence) Flow rate: 50 ml/second |
FeNO levels in smokers were significantly lower than in non-smokers (p = 0.003) (the median FeNO level was two times higher in non-smokers than in current smokers). The FeNO level that identified a sputum eosinophil count of ≥ 3% was lower in smokers than in non-smokers (28 ppb vs. 46 ppb) (p = 0.066), with a sensitivity of 76% and specificity of 62% in smokers and a sensitivity of 58% and specificity of 82% in non-smokers. When combining all variables in the logistic model, FeNO and smoking were independent predictors of sputum eosinophilia. The optimum cut-off for FeNO sputum eosinophil count > 3% (the FeNO level that identified a sputum eosinophil count of ≥ 3%) ranged from 15 ppb for smoking non-atopic patients receiving a high dose of ICS to 58 ppb for non-smoking atopic patients not treated with a high dose of ICS. When FeNO values were compared with values expected according to the Dressel equation, the observed values were much higher. Authors conclusion: FeNO threshold needs to be adjusted for smokers compared with non-smokers when identifying the presence of sputum eosinophilia in an unselected asthma population |
Patients were recruited from a variety of settings including secondary care,5 tertiary care121 and outpatient clinics. 122 Eligibility criteria varied from study to study but all studies included smokers with confirmed or persistent asthma. The sample sizes of the included studies ranged from 83123 to 470,121 with the mean age of participants ranging from 38121 to 50122 years. Baseline FeNO levels were inconsistently reported: Schleich et al. 5 and Kostikas et al. 122 reported median values, Hromis et al. 123 reported mean values and Michils et al. 121 reported geometric means and ranges. All studies included patients who were being treated with ICSs except one study5 in which treatment was unclear. In all studies except that by Hromis et al. ,123 asthma control was evaluated according to the GINA guidelines129 and/or the ACT and Juniper’s ACQ;135 in the Hromis et al. 123 study it was unclear how asthma control was evaluated. The NIOX MINO device was used by Kostikas et al. ,122 Schleich et al. 5 used the Niox device, Michils et al. 121 used the LR2000 device and it was unclear which device was used by Hromis et al. 123 None of the studies used the same protocol or cut-off points for the management of asthma with FeNO.
The results are summarised in Table 49. In the study by Schleich et al. 5 the median FeNO level (17 ppb) in smokers was significantly lower than that in non-smokers (35 ppb) (p = 0.003). In addition, the FeNO threshold for identifying a sputum eosinophil count of ≥ 3% was significantly lower in smokers (28 ppb, sensitivity 76% and specificity 62%) than in non-smokers (46 ppb, sensitivity 58% and specificity 82%) (p = 0.066).
Michils et al. 121 reported that baseline FeNO levels were reduced in smoking asthmatics (18.1 ppb vs. 33.7 ppb in non-smoking asthmatics). Furthermore, when patients were treated with high to medium doses of ICSs, FeNO no longer had the ability to reflect an improvement in asthma control for smoking patients, whereas for non-smoking patients its ability was only slightly reduced. However, the authors suggested that FeNO can reflect asthma control in smoking patients provided that changes in FeNO values detected by repeated measurements are considered. A decrease in FeNO of < 20% precludes asthma control improvement in non-smoking (NPV 78%) and smoking (NPV 72%) patients. An increase in FeNO of < 30% is unlikely to be associated with a deterioration in asthma control in both groups (NPV 86% and 84% for non-smoking and smoking patients respectively).
In the study by Kostikas et al. ,122 non-smokers who were either treated or not treated with ICSs reported higher FeNO values in uncontrolled asthma than in partly or well-controlled asthma. In contrast, smokers not treated with ICSs showed significant differences in FeNO values between uncontrolled and well-controlled asthma but no difference from partly controlled asthma. Smokers treated with ICSs showed no statistically significant differences (p > 0.05) in FeNO values between the controlled, partly controlled or uncontrolled asthma groups. The diagnostic performance of FeNO for the identification of not well-controlled (partly or uncontrolled) asthma was better in the non-smoker groups (FeNO cut-off > 22 ppb, sensitivity 87%, specificity 81%; FeNO cut-off > 27 ppb, sensitivity 64%, specificity 94%) than in the smoker groups (FeNO cut-off > 19 ppb, sensitivity 56%, specificity 75%; FeNO cut-off > 23 ppb, sensitivity 45%, specificity 87%).
Hromis et al. 123 showed that FeNO levels are low in asthmatic smokers (17 ppb) compared with levels in asthmatic non-smokers (57 ppb); however, when treated with a LABA a reduction in FeNO values was observed in both non-smokers and smokers (32% vs. 22% respectively). A similar pattern was observed when both groups were treated with an antileukotriene (reduction of 22% in non-smokers and 12% in smokers). The authors concluded that the sequential changes in FeNO could be a useful marker of asthma control, regardless of smoking status. FeNO level also depends on the applied treatment.
In addition, a study by Lehtimaki et al. 142 reported that smoking can cause a small and transient but statistically significant increase in FeNO at 1 and 5 minutes after smoking, highlighting the importance that smokers abstain from smoking before FeNO measurements are undertaken.
Overall, the findings suggest that FeNO levels in adults tend to be lower in asthmatic smokers than in asthmatic non-smokers and there is some evidence to suggest that, when this group of patients is treated with ICSs, FeNO can no longer detect asthma control. However, the use of repeated measures and within-patient change from baseline cut-offs may be worthy of further investigation in higher-quality studies, with two studies121,123 providing promising data on this approach. However, as no high-quality RCT studies have been conducted in this group, the evidence on the effectiveness of using FeNO to guide the management of asthma in smokers is currently inconclusive.
Children exposed to tobacco smoke
A summary of the study design and patient characteristics of the three studies recruiting children exposed to tobacco smoke111–113 is provided in Table 50. Two of the studies were conducted in the USA112,113 and one in France. 111 None of the studies appeared to receive funding from commercial sponsors and all were observational studies. In the study by de la Riva-Velasco et al. ,113 which was a cohort study, the authors determined the relationship between FeNO levels and exposure to low levels of environmental tobacco smoke in children with asthma on ICS treatment. The study by Hanson et al. 112 was a retrospective chart review study that looked at the relationship between FeNO and multiple clinical variables in children aged 4–7 years. The study by Mahut et al. 111 was a cross-sectional study that evaluated whether exhaled FeNO was independently associated with underlying pathophysiological characteristics of asthma (e.g. airway tone and airway inflammation) and with clinical phenotypes of asthma. In two studies111,113 patients were recruited from outpatient clinics and in one study112 patients were recruited from an asthma allergy clinic. Eligibility criteria varied from study to study but all studies included children who had been exposed to tobacco smoke and who had a diagnosis of asthma. The sample sizes of the included studies ranged from 33113 to 169,111 with the mean age of participants ranging from 10113 to 10.5111 years. However, Hanson et al. 112 did not report the mean age but recruited children between the ages of 4 and 7 years. Baseline FeNO values were inconsistently reported (Mahut et al. 111 and de la Riva-Velasco et al. 113 reported median values and Hanson et al. 112 reported mean levels) and the studies also varied in terms of medication usage. De la Riva-Velasco et al. 113 included children who were being treated with ICSs113 whereas Mahut et al. 111 included patients who were being treated with ICSs, LABAs or beta-agonists on demand. Hanson et al. 112 failed to provide details on medication usage. A range of devices was used to measure FeNO levels. Hanson et al. 112 used the NIOX MINO device; Mahut et al. 111 used the Niox device; and de la Riva-Velasco et al. 113 used the NIOX Flex device. None of the studies used the same protocol or cut-off points for the management of asthma with FeNO.
| Author, year | Study details | Population | FeNO measurements (ppb) | Outcomes |
|---|---|---|---|---|
| de la Riva-Velasco 2012113 | Relationship between FeNO and exposure to low-level environmental tobacco smoke in children with asthma on ICSs Setting: outpatient clinic Country: USA Funding: supported by the Children’s Environmental Health Centre of the Hudson Valley and the Maria Fareri Children’s Hospital Foundation (authors declared no competing interest) Design: cohort study |
Children with clinically stable, mild or moderate persistent asthma taking a low or medium dose of ICS daily (n = 33) Subjects stratified based on urine cotinine levels: ≥ 1 ng/ml (ETS) n = 10; < 1 ng/ml (non-ETS) n = 23 Median (IQR) age (years): ETS exposed: 10.5 (9.5 to 10.9); non-ETS exposed: 10.0 (9 to 11) Sex male, n/N (%): ETS: 4/10 (40); non-ETS: 12/23 (52) Medication usage: n = 26 daily medium dose of ICS; n = 7 daily low dose of ICS |
Median (IQR) ETS: 9.6 (5.1 to 15.8); non-ETS: 23.9 (15.2 to 34.5) (p = 0.008) Device: NIOX Flex Flow rate: 50 ml/second |
Children on low to medium doses of ICSs with recent low-level tobacco smoke exposure have lower FeNO levels than subjects not exposed to tobacco smoke. Low-level ETS or third-hand smoke may be an important variable to consider when interpreting FeNO levels in school-aged children with asthma |
| Hanson 2012112 (abstract) | Characterisation of FeNO in children aged 4–7 years and analysis of its relationship with multiple clinical variables Setting: asthma/allergy clinic Country: USA Funding: NR Design: retrospective chart review |
Children aged 4–7 years who underwent FeNO testing (n = 80) Mean age: NR Sex: NR Medical usage: NR |
Mean (range): 18.7 (2.5–89) Device: NIOX MINO Flow rate: NR |
There was no statistically significant relationship between FeNO level and smoke exposure |
| Mahut 2011111 | To evaluate whether FeNO is associated with a phenotype of childhood asthma (exposure to tobacco) Setting: outpatient clinic Country: France Funding: NR (authors declared no competing interest) Design: cross-sectional study, single-centre cohort |
Asthmatic children (n = 169) Cluster 1 (asthmatic boys, well-controlled asthma, unexposed to tobacco): n = 79; cluster 2 (asthmatic girls, well-controlled asthma, unexposed to tobacco): n = 44; cluster 3 (asthmatic boys or girls, uncontrolled asthma associated with increase in airway tone, unexposed to tobacco): n = 11; cluster 4 (asthmatic boys or girls, uncontrolled asthma associated with small airway to lung size ratio, exposed to parental smoking): n = 35 Mean (SD) age (years): 10.5 (2.6) Medication usage, n/N (%): ICS: 87/169 (51); LABA: 73/169 (43); beta-agonist on demand: 82/169 (49) |
Median (25th–75th percentile) Cluster 1: 25 (14–45); cluster 2: 34 (19–51); cluster 3: 21 (9–49); cluster 4: 30 (14–52) (p = 0.58) Device: Niox Flow rate: 50 ml/second |
FeNO levels were not decreased by tobacco exposure in univariate analysis. FeNO levels were not different in the four clusters (p = 0.58) |
The results of the included studies were varied (see Table 50). Mahut et al. 111 reported that there was no statistically significant relationship between FeNO level and smoke exposure and concluded that FeNO is potentially helpful in asthma management. On the other hand, de la Riva-Velasco et al. 113 found that children on low to medium doses of ICSs with recent low-level environmental tobacco smoke exposure have lower median (IQR) FeNO levels [9.6 (5.1–15.8)] than subjects not exposed to environmental tobacco smoke [23.9 (15.2–34.5); p = 0.008]. The authors concluded that environmental tobacco smoke exposure or third-hand smoke (that which lingers after a cigarette is extinguished) may be an important variable to consider when interpreting FeNO levels in school-aged children with asthma.
Overall, the findings suggest that the potential efficacy of using FeNO to inform the management of asthma in children exposed to environmental tobacco smoke may be similar to that in children who have not been exposed, but that it may be necessary to consider a child’s exposure status when interpreting results as FeNO may be lower in these children. Whether this should involve the setting of lower cut-off points or whether a more qualitative interpretation should be made on a case-by-case basis or by comparing within-patient changes from baseline is unclear.
Discussion of the clinical evidence
Summary of key results
Equivalence of devices
The review of the equivalence of devices revealed that the level of agreement between devices is highly variable.
NIOX MINO compared with the Niox chemiluminescent device
There was most evidence available for a comparison between the Niox chemiluminescent device and NIOX MINO in adults. Devices gave similar mean FeNO values in five of eight studies, but higher values for NIOX MINO in three studies. Bland–Altman analysis (reported in four of eight studies) suggested that the limits of agreement were around 10 ppb in both directions when analysed on the absolute scale, with mean differences of between 0.5 and 1.5 ppb. Analysis on the log scale produced better limits of agreement in the study by Menzies et al. 39 but very wide limits in the study by Korn et al. 41 In a head-to-head comparison between two NIOX MINO devices,40 there was also evidence that not all NIOX MINO devices produce equivalent readings to one another, although another study comparing three devices found them to be equivalent to one another. 45 There was also evidence that agreement between NIOX MINO and Niox is worse at higher FeNO values, with all studies in which cohorts had mean values of < 30 ppb reporting better agreement and most Bland–Altman plots showing a multiplicative relationship. Agreement looked acceptable in children, with all limits of agreement falling between –4.4 and 8.9 ppb.
NIOX MINO compared with other chemiluminescent devices
Correlation co-efficients were generally good, with a correlation between 0.76 and 0.96. However, cohort means were far more variable, with some devices reading higher and some lower than NIOX MINO. The highest difference between cohort mean FeNO values was 47 ppb. Although individual devices may show good agreement with NIOX MINO, it is not possible to draw any solid conclusions as most devices were tested in only one or two studies and, as was seen in the comparison between NIOX MINO and Niox, results between studies appear to vary considerably.
NObreath compared with the Niox chemiluminescent device and other chemiluminescent devices
In the one study that compared NObreath with the Niox chemiluminescent device, a good level of agreement was seen in Bland–Altman analysis, but cut-off values derived by this study for the diagnosis of asthma differed by 10 ppb according to which device was used. 65 Other devices generally appeared to read higher than NObreath, but not by a consistent amount.
NObreath compared with NIOX MINO
Both studies reporting this comparison54,66 found that in most analyses NIOX MINO provided lower mean FeNO values than NObreath. This contradicts the available evidence for comparisons between NIOX MINO and Niox and between NObreath and Niox, in which NIOX MINO > Niox > NObreath.
NIOX VERO compared with NIOX MINO
Only one study provided data on this device. (Academic-in-confidence information has been removed.)
Test failure rates
The overall test failure rate for FeNO measurement in adults was generally low across all devices and most patients appear to be able to provide FeNO readings, provided that they are permitted sufficient measurement attempts. There may be a higher test failure rate in children using NIOX MINO.
Conclusion
Overall, it cannot be concluded that any two devices are equivalent in all situations. Although there may be situations in which they are similar, it appears to depend on the characteristics of the studies and cannot be generalised to all situations. Further research is required to identify what is driving the variability between studies and devices. However, as there is mostly a high degree of correlation between measurements across all devices, estimates of sensitivity and specificity are likely to be an accurate indication of the potential diagnostic accuracy of using FeNO to guide diagnosis, but the derived cut-off points are not likely to be interchangeable between devices.
Diagnostic accuracy of FeNO for the diagnosis of asthma
No end-to-end studies were identified and no cohort study provided a comparison between FeNO within a sequence of tests and a suitable reference standard of the same sequence of tests without FeNO. The review included 27 studies that estimated the diagnostic accuracy of either FeNO alone or FeNO in conjunction with another test compared with that of a variety of reference standards and in a variety of populations.
Adults presenting with symptoms of asthma (four studies)
It is difficult to draw any conclusion about the optimal cut-off for sensitivity and specificity because of the heterogeneity in the results, study designs and devices used.
-
The cut-off for the highest sum of sensitivity and specificity ranged from 20 ppb to 47 ppb amongst the studies and even results produced by the same authors in studies with high levels of homogeneity varied widely (25 ppb and 46 ppb in Schneider et al. 69 and Schneider et al. 71,72 respectively). Sensitivities ranged from 32% to 88% and specificities from 75% to 93%.
A range of cut-offs was not reported in all studies and it was not clear if the highest sensitivity or specificity value was available. From those that were reported:
-
when selecting the cut-off with the highest sensitivity, this ranged from 9 ppb to 15 ppb, with sensitivities ranging from 85% to 96% and specificities ranging from 13% to 48%
-
when selecting the cut-off with the highest specificity, this ranged from 47 ppb to 76 ppb, with sensitivities ranging from 55.6% to 13% and specificities ranging from 88.2% to 100%.
The consistently smaller range and higher values of specificities than sensitivities reported suggest that FeNO may be a more reliable and useful parameter to base diagnostic decisions on as a rule-in test than as a rule-out test. However, this balance will depend on the clinical and cost consequences of being TP, TN, FP and FN in each scenario.
Subset of patients at position A compared with airway reversibility or airway hyper-responsiveness (two studies)
These studies did not produce estimates of diagnostic accuracy that were noticeably different from those in the studies that recruited a potentially broader spectrum of patients:
-
The cut-off for the highest sum of sensitivity and specificity ranged from 27 to 36 ppb. Sensitivities from 77.8% to 87% and specificities from 60% to 92%.
A range of cut-offs was reported in only one study. 82 These reached 100% sensitivity and specificity at the lowest and highest cut-offs, respectively, in this cohort compared with this reference standard.
-
when selecting the cut-off with the highest sensitivity, this was 25 ppb, with a sensitivity of 100% and a specificity of 46.7%
-
when selecting the cut-off with the highest specificity, this was 100 ppb, with a sensitivity of 27.8% and a specificity of 100%.
Difficult-to-diagnose patients (four studies)
These studies all used some form of airway hyper-responsiveness as the reference standard. Surprisingly, estimates of sensitivity and specificity seemed largely comparable to those in the studies recruiting patients presenting to primary care with symptoms of asthma, with a reference standard of airway reversibility, ICS responsiveness and airway hyper-responsiveness.
Bobolea et al. 88 recruited a set of patients who were negative by MCT and compared FeNO with an adenosine challenge test:
-
This study produced 100% sensitivity (29.2% specificity) at a cut-off of 30 ppb, meaning that FeNO is likely to operate well as a rule-out test.
The other studies used MCT challenge in patients who were negative for asthma in previous tests:
-
Cut-offs for the highest sum of sensitivity and specificity ranged from 32 to 40 ppb compared with MCT, which is a slightly narrower range than those in the broader cohorts. Sensitivities ranged from 35.0% to 74.3% and specificities from 72.5% to 95%, which are similar ranges to those in the broader cohorts. This perhaps reflects the fact that airway reversibility is a highly accurate test for asthma and that the combined tests behave in a similar manner. If this is the case, it would also suggest that FeNO has similar diagnostic properties in difficult-to-diagnose patients as in the broader spectrum of patients.
A range of cut-offs was not reported in these studies.
Patients with chronic cough and difficult to diagnose (three studies)
These studies recruited patients with chronic cough who had tested negative for other causes. All three studies used a reference standard of ICS responsiveness. Cut-offs for the highest sum of sensitivity and specificity were also in the same range and sensitivity and specificity were somewhat better in two studies: 94.7% sensitivity and 76.3% specificity in Hsu et al. 73 and 90% sensitivity and 85% specificity in Hahn et al. 74 This is in accordance with the expectation that FeNO is a better marker of ICS responsiveness than of asthma itself. At this position in the pathway, FeNO may be a useful test to perform before ICS responsiveness to indicate which patients are likely to respond to a trial of treatment. Patients with a low FeNO level could go on for further asthma investigations (e.g. MCT) or be assumed to be non-asthmatic depending on whether a rule-in or a rule-out scenario is used.
Studies using FeNO in conjunction with another test as the index test (three studies)
These studies reported the diagnostic accuracy of FeNO in conjunction with another test. This evidence comes closest to testing FeNO in a diagnostic pathway but is still of very limited relevance to the decision problem. The improvements in diagnostic accuracy were modest (or negative when considering the sum of sensitivity and specificity) and necessitate the usual trade-off between sensitivity and specificity. As the two studies that reported accuracy data are derivation studies rather than validation studies, it is possible that the gains seen are an overestimate of increases in diagnostic accuracy. However, it would seem that using a combination of tests may have additional benefits to using FeNO on its own.
Children with symptoms of asthma (four studies)
In comparison to the adult cohorts, with a similar spectrum of patients and reference standards, the cut-offs derived are generally lower in studies in children but with similar ranges of estimates of sensitivity and specificity.
-
There was a high degree of agreement as to the cut-off that produces the highest sum of sensitivity and specificity, despite the heterogeneity in devices and reference standards, with values between 19 ppb and 21 ppb, which are consistently lower than those in adults. Estimates of sensitivity at these cut-off points were also wide-ranging, at 49–86%, which is similar to estimates in adult studies (32–88%). Again, as in adults (range 75–93%), specificity was more similar between studies, ranging from 76% to 89%.
-
When selecting the cut-off with the highest sensitivity, the results were similar to those in adult cohorts. Cut-offs ranged from 5 to 20 ppb (vs. 9–15 ppb in adults), sensitivity from 89% to 94% (vs. 85–96% in adults) and specificity from 14.1% to 70% (vs. 13–48% in adults).
-
When selecting the cut-off with the highest specificity, results were also similar to, but perhaps a little lower than, those in adult cohorts, ranging from 30 to 50 ppb (vs. 47–76 ppb in adults). Sensitivity ranged from 20% to 50% (vs. 13–55.6% in adults) and specificity ranged from 92% to 100% (vs. 88.2–100% in adults).
Adult smokers
Two studies were identified. Both recruited an unusual spectrum of patients who reported symptoms of asthma in response to a questionnaire, rather than a population presenting with symptoms.
Cut-off values were generally lower in smokers than in cohorts consisting of never, ex- and current smokers and it may be useful to consider a patient’s smoking status when interpreting results. It is difficult to determine how the fairly minor differences in cut-off points and diagnostic properties of FeNO across groups would affect cost-effectiveness and clinical utility in practice. However, it would appear that FeNO is able to distinguish between asthmatic and non-asthmatic smokers with similar accuracy as between non-smokers and ex-smokers, but different cut-off points may be required.
Children exposed to tobacco smoke
Evidence was limited with regard to children exposed to tobacco smoke and drew on studies reported in the section on the management of children exposed to tobacco smoke and from the above studies reported for adults. The overall conclusion was the same as for adult smokers: it may be necessary to consider a child’s exposure status when interpreting the results of FeNO testing for the diagnosis of asthma, as FeNO levels may be lower in children exposed to tobacco smoke.
Pregnant women
No diagnostic accuracy studies in pregnant women were identified. A cross-sectional study compared mean FeNO values in pregnant asthmatic women, non-pregnant asthmatic women, healthy pregnant women and healthy non-pregnant women. The study concluded that pregnancy does not alter FeNO levels in asthmatic or non-asthmatic women and that FeNO can distinguish between asthmatic and non-asthmatic pregnant and healthy women. However, it is unclear whether diagnostic accuracy would be equivalent to that reported in other studies with non-pregnant participants or a mix of pregnant and non-pregnant participants.
The elderly
No diagnostic accuracy studies in the elderly were identified. A case–control study115 investigated FeNO levels in elderly patients with eosinophilic airflow obstruction (sputum cell count > 3%) compared with elderly healthy controls. No significant difference was found in the mean FeNO values, suggesting that FeNO is not a good marker of eosinophilic airway inflammation in elderly patients. This indicates that FeNO is unlikely to act as a useful test for the diagnosis of asthma in the elderly.
FeNO-guided management in asthma
Adults (five studies)
There was a high degree of heterogeneity across studies in all aspects of study design, including levels of blinding, inclusion criteria, visit frequency, cut-off points selected, devices used, step-up/step-down protocols and medications controlled by the protocols. Only one study reported using UK guidelines in the comparator arm. 98
All studies reported a fall in exacerbation rate, although it appeared that this was mostly driven by mild and moderate exacerbations and was not always statistically significant.
Severe exacerbations:
-
An exploratory random-effects meta-analysis including all definitions of severe exacerbations (excluding the studies by Smith et al. 97 and Honkoop et al. 101) produced a pooled estimate of 0.94 (95% CI 0.66 to 1.34; p = 0.73). Reviewer-calculated rate ratios for major/severe exacerbations ranged from 0.79 (95% CI 0.66 to 0.94) to 1.29 (95% CI 0.83 to 2.03).
-
The addition of data from Honkoop et al. 101 with imputed SEs did not change the non-statistical significance of the estimate, with rate ratios ranging from 0.82 (95% CI 0.64 to 0.1.05; p = 0.11) to 0.89 (95% CI 0.67 to 1.17; p = 0.40).
-
The impact of the exclusion of the study by Smith et al. 97 from the analysis is unknown.
In a prespecified sensitivity analysis removing studies with wider definitions of severe exacerbations, only that by Syk et al. 99 remained in the analysis, with a rate ratio in favour of the control group at 1.29 (95% CI 0.83 to 2.03), although the difference was not statistically significant (p = 0.26). The inclusion of the study by Honkoop et al. 101 using imputed SE values resulted in a range of rate ratios from 0.91 (95% CI 0.47 to 1.77; p = 0.79) to 1.00 (95% CI 0.53 to 1.90; p = 1.00), indicating no significant difference between the intervention groups. Heterogeneity statistics were high, ranging from 80% to 53% and reflecting the opposite direction of effect reported in these two studies. Differences in study characteristics, step-up/step-down protocols and patients may account for differences in the direction of effect.
All definitions of exacerbations:
-
Three studies reported a composite outcome including all types of exacerbation. 97,99,100 Two reported fewer exacerbations in the FeNO arm, but the difference did not reach statistical significance. 97,100 Syk et al. ,99 however, did report a statistically significant difference in favour of the FeNO arm, with 0.22 exacerbations per person-year in the intervention arm and 0.41 in the control arm (p = 0.024).
-
An exploratory pooled analysis showed a statistically significant effect with a rate ratio of 0.53 (95% CI 0.46 to 0.61; p < 0.00001, I2 = 0%).
All studies reported some data on ICS use. Smith et al. 97 and Shaw et al. 98 reported mean ICS use at the end of the study, with mean differences of –270 µg/day (95% CI –112 to –430 µg/day; p = 0.003) and –338 µg/day (95% CI –640 to –37 µg/day; p = 0.028), respectively, in favour of FeNO-guided management. Syk et al. 99 showed a small increase in ICS use in the intervention arm compared with the control arm [586 µg (SE 454) vs. 540 µg (SE 317)]. Calhoun et al. 100 reported the mean value per month, although it is unclear if this was an average over the whole course of the study or the mean for the final month of the study. The means were very similar, at 1617 µg/month in the intervention arm and 1610 µg/month in the control arm.
-
An exploratory meta-analysis using standardised mean difference (as outcomes were not reported in a standardised way) showed an overall effect of –0.24 SDs in favour of the intervention, although this narrowly missed significance (95% CI –0.56 to 0.07; p = 0.13).
-
This may indicate that some step-up/step-down protocols were better at decreasing ICS use than others or it may be related to the characteristics of the study populations.
Inhaled corticosteroid use and exacerbations are likely to be inversely related, regardless of the use of FeNO. Whether the effects of FeNO-based management on these two related factors represents a cost-effective exchange is assessed through cost-effectiveness modelling in Chapter 4.
Three studies used versions of the AQLQ to measure quality of life. All showed no effect on the global score, but one study investigated domains and found a statistically significant difference in the symptoms score.
-
Asthma control either did not change97,98,100 or increased. 99
-
Smith et al. 97 and Calhoun et al. 100 reported no significant differences between groups in bronchodilator use and Syk et al. 99 reported greater numbers using and mean use of LTRAs (statistically non-significant) and SABAs (significance not reported) in the FeNO-controlled arm.
No asthma-related adverse events or deaths were reported.
Because of the high levels of heterogeneity with regard to multiple study characteristics and outcome definitions, it was not possible to draw any firm conclusions as to which step-up/step-down protocol or cut-off points offer the best efficacy. However, considering the evidence base as a whole, it would seem possible that FeNO-guided management of most descriptions may, during the first year of management, result in better management overall (fewer exacerbations), with the potential for no increase or a small reduction in ICS use.
Children (seven studies)
There was a high degree of heterogeneity across studies with regard to all aspects of study design, including levels of blinding, inclusion criteria, visit frequency, cut-off points selected, devices used, step-up/step-down protocols and medications controlled by the protocols. Only Pike et al. 108 reported using UK guidelines in the comparator arm.
-
Two studies recruited patients who appeared to be poorly controlled. 104,107 Pike et al. 108 recruited moderate to severe patients, some of whom will have been uncontrolled.
-
Peirsman et al. 109 recruited mild to severe asthmatics.
-
One study recruited patients who were mild to moderate persistent asthmatics. 103
-
One study recruited patients who had had a stable dose of ICS for the previous 3 months, suggesting that they were reasonably well controlled. 106
-
It was not possible to tell whether patients in the study by Verini et al. 105 were controlled or uncontrolled.
Three studies104,108,109 reported the number of patients (but not the rate per person-year) requiring hospitalisations. All three studies reported no difference between groups..
Three studies reported data on exacerbations resulting in OCS use. 103,104,106 Two reported fewer OCS courses in the FeNO arm, but the difference between groups was not statistically significant in the study by Szefler et al. ,104 whereas significance was unreported in the study by Pijnenburg et al. 106 Fritsch et al. 103 reported similar numbers of OCS courses in both arms.
Fritsch et al. ,103 Szefler et al. ,104 Verini et al. ,105 Petsky et al. ,107 Pike et al. 108 and Peirsman et al. 109 all reported exacerbations that were not defined as either major or minor and which had different definitions from one another. Five studies reported numerically fewer exacerbations or treatment failures in the intervention arm, but these differences were statistically significant in only two studies. 107,109 All studies showed at least a trend in favour of fewer exacerbations in the intervention arm. In the study by Petsky et al. ,107 exacerbations were not clearly defined but occurred in six out of 31 participants in the intervention group (19.4%) and 15 out of 32 in the control group (46.9%; p = 0.021). Peirsman et al. 109 reported statistically significantly fewer exacerbations of any severity (as defined using GINA guidelines136) in the intervention arm (18 events) than in the control arm (35 events; p = 0.02). Pijnenburg et al. 106 did not report this outcome.
With the exception of the study by Petsky et al. ,107 all of the studies provided some estimate of ICS use. Fritsch et al. 103 and Szefler et al. 104 reported statistically significantly higher ICS use in the intervention group; Pike et al. 108 and Peirsman et al. 109 reported higher levels of ICS use in the intervention group but not statistically significantly so; Pijnenburg et al. 106 reported very similar levels in the different groups; and the values in the remaining study (in terms of absolute numbers using ICSs) were difficult to interpret. The differences in the effects on ICS usage between studies may be the result of the specifics of the step-up/step-down protocols and/or the characteristics of the patients selected. In the case of the study by Pijnenburg et al. ,106 in which patients may have been generally better controlled at the outset, step down of ICS use may have been more likely than in the study by Szefler et al. ,105 in which patients were poorly controlled and ICS dose was perhaps more likely to be stepped up. Having said this, poorly controlled patients may be poorly controlled because they are non-responsive to corticosteroids, and use of FeNO testing may actually result in a decrease in ICS use if low FeNO levels always indicate a decrease in treatment. However, Szefler et al. ,104 Pijnenburg et al. ,106 Pike et al. 108 and Peirsman et al. 109 did not allow step down of ICS dose on the basis of low FeNO levels alone, meaning that uncontrolled ICS-unresponsive asthmatics for whom high doses of ICS were not appropriate could not have their doses lowered and mean ICS use may remain artificially high. It could be expected that this effect would be most pronounced in studies that recruited patients with severe asthma. As such, it may be that the increase in ICS usage in the study by Szefler et al. 104 is a function of the population selected as well as the management protocol.
Inhaled corticosteroid use and exacerbations are likely to be inversely related, regardless of the use of FeNO testing. Whether the effects of FeNO-based management on these two related factors represent a cost-effective exchange is assessed through cost-effectiveness modelling in Chapter 4.
This outcome was reported in only one study in abstract form107 and using an unknown tool. No conclusions can be confidently drawn from these data.
Four studies provided some data on asthma control, none of which demonstrated any statistically significant effects favouring either the intervention or the control. With respect to additional medication use, three studies provided data and there did not appear to be a clear direction of effect within the data.
Szefler et al. 104 reported no difference in adverse events between groups and no mortality was observed. The adverse events listed were disorders of the eyes, ears, nose and throat; gastrointestinal disorders; haematology disorders; infections; musculoskeletal symptoms; and skin symptoms.
Because of the high levels of heterogeneity with regard to multiple study characteristics and outcome definitions, it was not possible to draw any firm conclusion as to which step-up/step-down protocol or cut-off points offer the best efficacy. Results were generally not statistically significant, but this may be because of the small sample sizes in some cases; meta-analysis was precluded by the ability to calculate rate ratios. Because all but one study reported numerically smaller numbers or rates of exacerbations in the intervention arm, it would seem possible that FeNO-guided management of most descriptions could, during the first year of management, result in better management overall, despite the lack of statistically significant results in individual studies. Further larger studies are needed to clarify any treatment effect. It is unclear whether ICS use is likely to increase or decrease and this may depend on the details of the step-up/step-down protocols or the characteristics of the patients recruited to the trials in terms of control and severity.
Generalisability of the results to UK practice
Diagnostic review
Adults
Only studies with some relevance to UK practice were considered, of which not all used NIOX MINO or NObreath. The studies with the highest relevance to UK practice can be broken down into four types:
-
Studies of all patients presenting with symptoms of asthma and using a reference standard that includes the most common tests in the UK pathway. The most relevant studies in this category are those by Schneider et al. 69 and Schneider et al. ,71,72 who used the NIOX MINO device, and those by Smith et al. 83 and Smith et al. ,86 who used the Niox chemiluminescent device and an unknown device respectively.
-
Studies recruiting patients who are difficult to diagnose and using a reference test appropriate to UK practice:
-
Schleich et al. ,77 who used the Niox chemiluminescent device, and Pedrosa et al. 85 and Katsoulis et al. ,81 who used the NIOX MINO device.
-
Bobolea et al. ,88 who also used the NIOX MINO device and who selected a population of patients who were negative by MCT. The reference standard was the adenosine challenge test.
-
-
Studies recruiting patients with chronic cough who have already undergone other tests. This includes the studies by Hsu et al. ,73 Hahn et al. 74 and Prieto et al. 76 These are useful studies in terms of demonstrating that FeNO can predict ICS responsiveness in these patients, rather than a diagnosis of asthma. None of these studies used the NIOX MINO device.
-
Studies using FeNO in conjunction with another test compared with an appropriate reference standard:
-
Schleich et al. 77 recruited a difficult-to-diagnose group, combined FeNO with FEV1% < 101% and used a reference standard of MCT. It could be argued, however, that in the UK only patients negative by FEV1% would receive a MCT, in which case this combination may not be a useful one.
-
Cordeiro et al. 87 recruited patients presenting with symptoms of asthma and combined FeNO with airway reversibility to administration of a bronchodilator. This would be equivalent to FeNO being used to prevent a MCT, but some of the included patients would not have received a MCT under the UK pathway anyway. As such, it is again unclear how useful this combination of tests would be in UK practice.
-
Children
Only studies with some relevance to UK practice were considered. These studies, of all patients presenting with symptoms of asthma and using a reference standard that includes the most common tests in the UK pathway, can be broken down into the following groups:
-
Woo et al. ,96 who recruited patients in position A on the pathway and used the NIOX MINO device compared with a reference standard that roughly equates to UK practice.
-
Linkosalo et al. ,93 who used a Sievers NOA280i chemiluminescence device for patients in position A on the pathway with a reference standard of an exercise challenge test. Not all presenting patients would receive this test in UK practice. FeNO testing would be positioned before the exercise challenge test and could triage patients away from this.
-
Sivan et al. ,95 who used an ECO MEDICS device in patients at position A in the pathway compared with a reference standard similar to UK practice. In this study, FeNO replaces the whole pathway prior to ICS use.
Management review
Adults
Generalisability to UK practice is clear-cut in the study of adults by Shaw et al. ,98 which used UK guidelines as the comparator and was based in the UK. Patients were recruited from primary care and included mild to severe asthmatics (unless a severe exacerbation had been experienced in the previous 4 months) and atopic patients as well as non-atopic patients. Smokers were excluded and so the results may not be generalisable to this group. However, this study offers the best generalisability to UK practice in terms of setting, population and comparator.
However, if management protocols that are different from that used in Shaw et al. 98 were to be considered for recommendation, other studies may offer some useful data. Input from a clinician (Professor Ian Pavord, Nuffield Department of Medicine, University of Oxford, August 2013, personal communication) suggests that the management protocol described by Powell et al. ,102 in which symptoms control the LABA dose and FeNO levels control the ICS dose, is generally thought to be the best design. This study was conducted in pregnant women only and its generalisability to the whole asthma population is not assured. The protocol described by Shaw et al. 98 appears to be similar to this in that the FeNO level controls the ICS and LTRA doses whereas symptoms scored according to the Juniper scale control the SABA, LABA and theophylline doses. Importantly, this allows for low FeNO levels to result in a reduction in ICS dose regardless of symptoms. In practice, the extent of ICS dose reduction may be limited by current Commission on Human Medicine advice, which states that LABAs should not be prescribed without ICSs. 143
Children
Generalisability in studies recruiting children was less clear-cut. Only the study by Pike et al. 108 was set in the UK and used UK guidelines, but this study also recruited only moderate to severe asthmatics and may therefore not be generalisable to the whole population. This study was identified during the update search and it was not possible to incorporate it into the model because of time constraints. From the studies identified in the original search, and on the basis of reported quality, that by Szefler et al. 104 was at lowest risk of bias and, for patients who are uncontrolled, this may be the best study to base generalisations on. However, clinical input to the project (Professor Ian Pavord, August 2013, personal communication) indicated that this study has been criticised for not allowing a step down of ICS dose on the basis of low FeNO levels if symptoms are still present. In addition, the patient population in this study was patients who were uncontrolled, which may introduce bias in that patients will be less likely to be indicated for a step down of ICS dose.
For patients who are mild to moderate asthmatics, the study by Fritsch et al. 103 may be the best study to select for modelling as the study by Pijnenburg et al. 106 uses only symptoms to guide asthma management in the control arm, whereas that by Fritsch et al. 103 uses symptoms, SABA use and lung function tests, which is probably more comparable with UK practice. However, Fritsch et al. 103 used only FeNO to guide management and it would seem more likely that clinicians would use other measures such as symptom control and lung function to guide treatment. This would allow the stepping down of treatment based on FeNO values, but may also be less sensitive than using a combination of factors. Unfortunately, there is not a study in children that addresses this particular problem by combining FeNO testing with other indicators in a protocol that allows step down in the presence of low FeNO levels regardless of symptomatic control. Fritsch et al. 103 did not report data in a way that allowed calculation of rates of exacerbation per person-year and this study is of limited use for this reason. The study by Pijnenburg et al. 106 provided the necessary data and this study was selected for modelling.
The study by Peirsman et al. 109 was identified in the update search. A broad spectrum of patients was recruited but the study did not allow for step down of ICS use on the basis of FeNO levels alone and the treatment protocol was different from UK practice. This study was not used in the modelling because of time constraints.
Chapter 4 The cost-effectiveness of FeNO testing for the diagnosis and management of asthma
Introduction
This chapter presents an assessment of the cost-effectiveness of FeNO testing for the diagnosis and management of asthma. The chapter consists of two main sections: (1) a review of existing evidence relating to the cost-effectiveness of FeNO testing in the diagnosis and management of asthma and (2) an exposition of the methods and results of two de novo health economic models developed by the EAG to evaluate the cost-effectiveness of FeNO testing for the diagnosis and management of asthma.
The chapter is set out as follows. The following section describes the aims and objectives of the economic analysis. Review of existing evidence relating to the cost-effectiveness of FeNO testing for the diagnosis and management of asthma presents the methods and results of a critical review of existing economic evidence on FeNO testing for asthma; this includes published studies as well as other economic evidence submitted by the manufacturers of the FeNO devices considered in this assessment. This section also includes a summary of methodological problems and concerns associated with undertaking economic analyses of interventions for the diagnosis and management of asthma. Development of two de novo models to estimate the cost-effectiveness of FeNO testing for the diagnosis and management of asthma presents the methods of the de novo economic analyses undertaken by the EAG and De novo model results summarises the main findings of the analyses. The final discussion section highlights the key uncertainties surrounding the evidence base used to inform the de novo analysis.
Aims and objectives of the health economic assessment of FeNO testing
The purpose of this chapter is to assess the expected cost-effectiveness of NIOX MINO, NIOX VERO and NObreath compared with current standard care for the diagnosis and management of asthma. Importantly, there is uncertainty not only with respect to whether FeNO testing might represent a cost-effective use of health-care resources but also with respect to how FeNO testing should be used in the most cost-effective manner within existing asthma pathways. Thus, the economic analysis attempts to address the following questions:
-
What is the cost-effectiveness of FeNO testing using NIOX MINO, NIOX VERO and NObreath compared with current standard tests for the diagnosis of asthma in England and Wales?
-
Should FeNO testing be used alongside existing standard tests for the diagnosis of asthma?
-
Should FeNO testing be used in place of existing standard tests for the diagnosis of asthma?
-
-
What is the cost-effectiveness of FeNO testing using NIOX MINO, NIOX VERO and NObreath compared with standard guidelines for the management of asthma in England and Wales?
-
What are the key uncertainties relating to the cost-effectiveness of FeNO testing and how might these be resolved or reduced?
The next section presents the methods and results of a review of the existing evidence relating to the cost-effectiveness of FeNO testing.
Review of existing evidence relating to the cost-effectiveness of FeNO testing for the diagnosis and management of asthma
Purpose of the review
We undertook a systematic review of existing economic analyses of FeNO testing in the diagnosis of asthma and for the management of patients with diagnosed asthma. This also included a focused review of economic studies of other interventions for the diagnosis and/or management of asthma. The purpose of the review of existing health economic analyses was threefold:
-
to identify existing economic analyses of FeNO testing using NIOX MINO, NIOX VERO or NObreath for the diagnosis and/or management of asthma
-
to identify existing models that may be used to inform the structure of the de novo economic models developed by the EAG
-
to identify potentially relevant evidence sources to inform parameter values within the de novo economic models developed by the EAG.
Review methods
Methods used to identify existing economic studies
We undertook systematic searches across a range of electronic databases to identify published studies of FeNO testing for the diagnosis and/or management of asthma. We also searched for other economic studies of interventions for the diagnosis or management of asthma. All searches were undertaken by an information specialist (RW) during the period 30 May 2013 to 7 June 2013.
Four separate strands of searching were undertaken, which are detailed in the following sections.
Economic search 1: NIOX MINO/NObreath in either the diagnosis or the management of asthma (30 May 2013)
This search used free-text terms relating to NIOX MINO and NObreath (including manufacturer names), with the terms combined with a sensitive economic search filter.
Economic search 2: models of asthma and FeNO (30 May 2013)
This search used the search strategies developed for the management studies in the clinical effectiveness review (see Chapter 3, Clinical reviews search methodology) and combined these with a sensitive economic search filter. Studies that were found in the first search would also be retrieved in this search.
Economic search 3: asthma management models (3 June 2013)
This focused search used free-text terms for asthma combined with cost terms in the title and the economic model subject heading. A sensitive economic filter was not applied in this search.
Economic search 4: asthma diagnostic models (7 June 2013)
This focused search used free-text terms for asthma (as used in economic search 3) combined with a sensitive economic evaluations search filter and a diagnostic search filter.
These four searches are shown diagrammatically in Figure 20.
FIGURE 20.
Diagrammatic representation of the search approach.
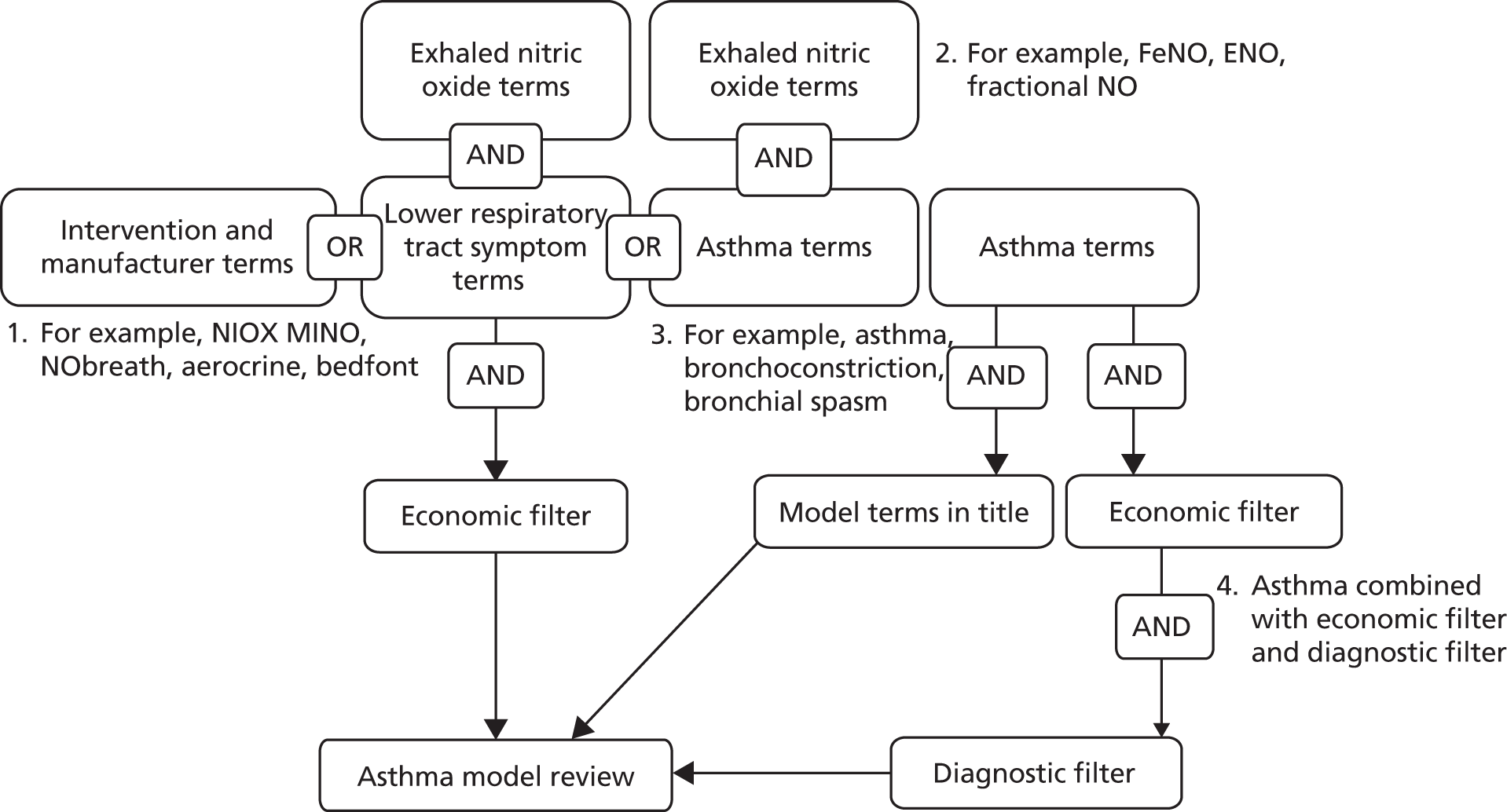
All of the above searches were performed within the following databases:
-
MEDLINE and MEDLINE-In-Process & Other Non-Indexed Citations (Ovid): 1948–present
-
EMBASE (Ovid): 1974–present
-
The Cochrane Library (Wiley Interscience):
-
CDSR: 1996–present
-
HTA database: 1995–present
-
NHS EED: 1995–present
-
-
SCIE (Web of Science): 1899–present
-
CPCI-S (Web of Science): 1990–present.
The economic MEDLINE search strategy is detailed in Appendix 13.
As noted in Chapter 3 (see Additional search for NIOX VERO), an additional separate search was also undertaken in August 2013 to identify evidence relating to NIOX VERO.
Inclusion and exclusion criteria for the review
Given the anticipated dearth of published economic analyses relating to FeNO, we adopted broad inclusion criteria for the review (Box 1).
-
Economic analyses of costs and consequences of interventions for the diagnosis and/or management of asthma in children and/or adults.
-
Studies reporting on the cost-effectiveness of NIOX MINO, NIOX VERO or NObreath for the diagnosis and/or management of asthma.
-
Letters, commentaries and editorials.
-
Economic studies that do not relate to diagnostic or management interventions.
-
Studies that do not relate to asthma.
-
Studies that do not involve (i) a model-based analysis, (ii) economic evaluations alongside trials or other forms of empirical clinical study or (iii) estimates of the costs and consequences of FeNO testing for the diagnosis of asthma.
Data sifting
The titles and abstracts of all records identified by the search were reviewed by one member of the research team (JM). The full texts of studies considered eligible for inclusion were then retrieved for a more detailed examination.
Critical appraisal methods
The identified studies of FeNO were critically appraised using the Drummond et al. 144 checklist for economic evaluations and the NICE reference case for diagnostic studies. 145 The identified studies were also informally assessed against current guidelines for the development and reporting of health economic models. 146 Studies of other interventions for the diagnosis and/or management of asthma were not subjected to a formal critical appraisal but were instead used to inform the design and development of the de novo health economic analyses (detailed in Development of two de novo models to estimate the cost-effectiveness of FeNO testing for the diagnosis and management of asthma).
Results of the review of FeNO testing for asthma diagnosis and/or management
Number and type of studies included in the review
The results of the four economic searches are presented in Table 51. A total of 1898 potentially relevant citations were identified from the four searches. The full texts of 27 studies were retrieved for further examination. The full text of one of these studies could not be retrieved and was excluded. Of the remainder, only two studies147,148 were identified that related to FeNO testing for the diagnosis and/or management of asthma. The focused searches did not identify any further cost–utility models of other interventions for the diagnosis of asthma. Sifting of the focused management model searches identified a further 13 studies149–161 that were used more generally to inform the model structure, although none of these related to FeNO testing. In addition, one additional management study13 that was detailed in the appendices of a UK HTA report was identified.
| Database | Search | |||
|---|---|---|---|---|
| 1. NIOX MINO/NObreath | 2. Asthma and FeNO models | 3. Asthma management models | 4. Asthma diagnostic models | |
| MEDLINE and MEDLINE-In-Process & Other Non-Indexed Citations | 2 | 29 | 311 | 338 |
| EMBASE | 7 | 144 | 420 | 590 |
| CDSR | 0 | 48 | 0 | 69 |
| HTA database | 4 | 8 | 4 | 0 |
| DARE | 0 | 2 | 3 | 14 |
| NHS EED | 1 | 2 | 119 | 12 |
| SCIE | 5 | 85 | 295 | 457 |
| CPI-S | 0 | 3 | 15 | 37 |
| Total unique citations | 14 | 269 | 567 | 1048 |
As part of the appraisal process, Aerocrine submitted evidence relating to the cost-effectiveness of NIOX MINO for the diagnosis and management of asthma (Aerocrine. Submission to NICE – Assessing the Impact of FeNO in the Management and Diagnosis of Asthma. Slideset and Microsoft Excel model, 2013). This submission included a Microsoft Excel 2010 spreadsheet model (Microsoft Corporation, Redmond, WA, USA) and a brief slideset. This submission is included as part of the economic review presented in this chapter. Aerocrine did not submit any economic evidence relating to the cost-effectiveness of the NIOX VERO device and Bedfont Scientific did not submit any evidence relating to either the effectiveness or the cost-effectiveness of the NObreath device.
Existing economic analyses of FeNO testing for the diagnosis of asthma
Methods and results of the included diagnostic studies
The searches included only one UK model-based published economic analysis relating to the diagnosis of asthma;147 this study assessed the cost-effectiveness of FeNO testing (specifically NIOX MINO) compared with standard diagnostic tests. This model has been published across two papers147,148 and also forms the basis of the Aerocrine submission to NICE for this appraisal. The general model structure and many of the evidence inputs are the same across these three analyses.
Price et al. 147 presents the methods and results of two economic analyses: (1) a model to assess the cost savings associated with using NIOX MINO for the diagnosis of asthma and (2) a model to assess the cost-effectiveness of NIOX MINO for the management of asthma. The model of asthma management is reviewed in detail in Existing economic analyses of FeNO testing for the management of asthma.
The conceptual form of the Price et al. 147 diagnostic model is presented in Figure 21. Within the model, the costs and outcomes of competing diagnostic strategies are modelled using a simple deterministic decision tree based on the true underlying probability of asthma and the operating characteristics of a variety of tests used for the diagnosis of asthma in the NHS. The population under evaluation within the model is reported to relate to ‘non-smoking adult patients with mild to severe asthma as seen in both primary and secondary care’ (p. 433). 147 The intervention is defined in the base-case analysis as FeNO testing using NIOX MINO alone, although a secondary analysis is also reported for a joint diagnostic modality consisting of NIOX MINO plus spirometry using FEV1 testing. The comparator within the base-case analysis is a blended comparison of standard diagnostic tests: (1) lung function testing, (2) reversibility test, (3) bronchial provocation and (4) sputum eosinophil count. The selection of tests included in the analysis was based on the BTS/SIGN asthma guidelines,8 although the source for the proportionate weighting of each of these is unclear within the Price et al. 147 paper. It should also be noted that current BTS/SIGN guidelines8 state that sputum induction is not in common usage and it currently remains a research tool. In contrast to the published Price et al. 147 model, the Aerocrine submission model does not adopt a blended comparison approach but instead evaluates each individual diagnostic test as a decision option in its own right.
FIGURE 21.
Model structure employed within the Price et al. 147 diagnostic model. Reproduced with permission from Price D, Berg J, Lindgren P. An economic evaluation of NIOX MINO airway inflammation monitor in the United Kingdom. Allergy 2009;64:431–8. 147 John Wiley & Sons. © 2009 The Authors. Journal compilation © 2009 Blackwell Munksgaard.
The model structure employs a single decision node whereby the model cohort is assumed to receive a single imperfect diagnostic intervention; those patients who receive an incorrect diagnosis are later assumed to achieve a correct diagnosis of either true asthma or not asthma. The published model estimates the costs associated with NIOX MINO compared with those of the blended comparison of standard diagnostic tests. The analysis takes the form of a comparative cost analysis and health outcomes are not explicitly considered in the published analysis (note that the number of misdiagnoses are not reported within the Price et al. 147 paper but could be easily calculated from the table of model input parameters). Diagnostic outcomes in terms of TPs, FPs, TNs and TPs are estimated explicitly within the Aerocrine model. Within the Price et al. 147 paper, costs are valued at 2005 prices. The model time horizon is undefined but relates to the time from presentation to correct diagnosis. No discounting is applied to costs.
The Price et al. 147 diagnostic model makes the following structural assumptions:
-
NIOX MINO will replace existing diagnostic tests rather than be used alongside them
-
time is not explicitly considered within the model with respect to the resolution of incorrect diagnoses (FPs or FNs)
-
negative health consequences [quality-adjusted life-year (QALY) losses] associated with incorrect diagnoses are not quantified within the model
-
all incorrect diagnoses are assumed to be corrected at the next outpatient visit.
The parameter values and evidence sources from which these are drawn are reported in Table 52.
| Parameter | Value | Source |
|---|---|---|
| Test operating characteristics | ||
| Sensitivity FeNO testing (flow rate 50 ml/second; > 20 ppb) | 0.88 | Smith et al.86 |
| Specificity FeNO testing (flow rate 50 ml/second; > 20 ppb) | 0.79 | |
| Sensitivity FeNO testing (flow rate 50 ml/second; > 33 ppb) + FEV1 < 80% predicted | 0.94 | aSmith and Taylor162 |
| Specificity FeNO testing (flow rate 50 ml/second; > 33 ppb) + FEV1 predicted < 80% | 0.93 | |
| Sensitivity PEF A%M > 21.6% | 0.43 | Hunter et al.163 |
| Specificity PEF A%M > 21.6% | 0.75 | |
| Sensitivity reversibility test: FEV1 > 2.9% improvement after salbutamol | 0.49 | |
| Specificity reversibility test: FEV1 > 2.9% improvement after salbutamol | 0.70 | |
| Sensitivity bronchial provocation: methacholine PC20 < 8 mg/ml | 0.91 | |
| Specificity bronchial provocation: methacholine PC20 < 8 mg/ml | 0.90 | |
| Sensitivity sputum eosinophil count > 1% | 0.72 | |
| Specificity sputum eosinophil count > 1% | 0.80 | |
| Disease characteristics | ||
| Asthma prevalence | 0.36 | Smith et al.86 |
| Comparator usage (blended comparison weightings) | ||
| Proportion using PEF charting | 0.485 | BTS/SIGN164 |
| Proportion using reversibility testing | 0.485 | |
| Proportion using bronchial provocation | 0.025 | |
| Proportion using sputum eosinophil count | 0.005 | |
| Cost parameters (£) | ||
| Cost NIOX MINO | 22.90 | Aerocrine |
| Cost peak flow charting (two visits) | 89.27 | NHS Reference Costs165 |
| Cost reversibility test | 29.27 | |
| Cost bronchial provocation | 48.50 | |
| Cost sputum eosinophil count | 48.50 | |
| Cost outpatient GP visit | 30.00 | Curtis and Netten166 |
| Cost outpatient lung practitioner | 44.00 | |
The headline results of the economic analysis are presented as a simple cost difference between NIOX MINO and the blended comparison of standard tests for asthma diagnosis. Uncertainty surrounding model input parameters was explored using simple one-way sensitivity analyses. These analyses include varying model parameters describing test sensitivity, true underlying asthma prevalence in the modelled population, the costs of NIOX MINO and other diagnostic tests, the number of additional visits required to resolve an initially incorrect diagnosis, a comparison of NIOX MINO with reversibility testing plus peak expiratory flow (PEF) charting and a comparison of NIOX MINO plus FEV1 and standard tests.
The diagnostic model results reported by Price et al. 147 are summarised in Table 53. In the base-case analysis, the authors report that the cost of an asthma diagnosis made using NIOX MINO was £29 per patient, or £43 less than when using standard diagnostic tests (£72 per patient).
| Scenario | NIOX MINO (£) | Standard tests (£) | Incremental cost (£) |
|---|---|---|---|
| Base case | 29 | 72 | –43 |
| Variation in test sensitivity –50% (all tests simultaneously) | 35 | 76 | –40 |
| Variation in test sensitivity +10% (all tests simultaneously) | 29 | 72 | –43 |
| Variation in test sensitivity –50% (bronchial provocation and sputum only) | 39 | 81 | –42 |
| Variation in test sensitivity +10% (bronchial provocation and sputum only) | 28 | 71 | –43 |
| Asthma prevalence set to 10% | 30 | 70 | –40 |
| Asthma prevalence set to 50% | 29 | 74 | –45 |
| Asthma prevalence set to 90% | 28 | 78 | –50 |
| NIOX MINO cost –50% | 18 | 72 | –54 |
| NIOX MINO cost +200% | 75 | 72 | 3 |
| Cost of standard diagnostic tests +50% | 29 | 72 | –43 |
| Cost of standard diagnostic tests +100% | 29 | 102 | –72 |
| Cost of standard diagnostic tests +150% | 29 | 131 | –102 |
| Cost of standard diagnostic tests +200% | 29 | 161 | –131 |
| Two visits for false diagnosis | 36 | 86 | –50 |
| Four visits for false diagnosis | 49 | 113 | –63 |
| NIOX MINO vs. reversibility + PEF charting | 29 | 131 | –102 |
| NIOX MINO + FEV1 testing vs. standard tests | 115 | 72 | 42 |
The results indicate that, within the base-case analysis, NIOX MINO is expected to produce cost savings (£43) compared with the blended comparison of standard diagnostic tests for asthma. These results do not account for potential health benefits associated with the improved accuracy of diagnosis. The sensitivity analysis indicates that NIOX MINO is expected to produce cost savings in all scenarios except (1) when the cost of NIOX MINO is increased by 200% and (2) within the comparison of NIOX MINO plus FEV1 testing compared with the blended comparison of current standard diagnostic tests.
The authors note that ‘is it is likely that, in practice, FeNO measurement will be used in conjunction with other tests rather than as their replacement. We examined this scenario and found that the combination of FeNO measurement plus lung function testing increased costs for diagnosing asthma by £42’ (p. 435). 147 Given the authors’ interpretation of the likely placement of NIOX MINO, it is unclear why the base-case analysis within the paper does not reflect this scenario and, given the proposed placement of FeNO within the existing pathway and the absence of quantified health outcomes within the Price et al. 147 diagnostic model, it is unclear whether the potential additional benefits associated with diagnosis using FeNO testing outweigh the opportunity costs associated with generating them.
The next section briefly outlines the economic analysis of NIOX MINO for asthma diagnosis as presented within the Aerocrine submission to NICE.
(ii) Additional analysis presented within the submitted Aerocrine diagnostic model
As noted earlier, Aerocrine also submitted a spreadsheet model to NICE as part of the appraisal process. The model was accompanied by a brief Microsoft PowerPoint slideset although this does not include a description of the intended base-case analysis results and little detail is provided supporting the structure, assumptions or choices regarding evidence used to inform the model parameters. The submitted Aerocrine model adopts a very similar structure and similar assumptions to those of the diagnostic model reported by Price et al. 147 It should be noted that, in the absence of a detailed written description of the Aerocrine submission model, it is difficult to provide a full critique of its methods and results. This task was further hindered as the worksheet tabs and many sets of calculations were structurally hidden within the Microsoft Excel worksheet, making formula auditing problematic.
The following differences should be noted between the Price et al. diagnostic model147 and the Aerocrine diagnostic model:
-
Differences in the specification of diagnostic options. The Aerocrine model assesses a different set of options compared with Price et al. :147
-
spirometry alone
-
spirometry and (if negative) MCT
-
spirometry and (if negative) FeNO testing
-
spirometry and FeNO testing
-
FeNO testing alone
-
spirometry and (if negative) sputum induction.
-
It should be noted that some of these options include sequences of diagnostic tests. These are implemented within the model by assuming that the probabilities of obtaining a positive or negative result from sequences of tests are uncorrelated with one another; in other words, the use of prior tests in a sequence will remove some candidates from the population, will alter the prevalence of true disease in the remaining population and may impact on the diagnostic accuracy of subsequent tests in that sequence. The validity of assuming no correlation between tests is questionable and no evidence is presented to support this. Within the Aerocrine submission model, all standard tests are evaluated as individual comparators in their own right rather than being combined and weighted within a blended comparison.
-
Different assumptions relating to the cost impact of misdiagnosis and resolution. The submitted model includes the costs of treating patients who are FP using ICSs over a 1-year time horizon; these treatment costs were not included in the Price et al. 147 diagnostic model. Conversely, the Aerocrine model does not include the assumptions made by Price et al. 147 regarding the costs of additional visits to resolve misdiagnosis.
-
Different parameter values and evidence sources. The Aerocrine diagnostic model includes some different parameter values from those in the Price et al. 147 diagnostic model. The parameter values and sources employed within the Aerocrine diagnostic model are detailed in Table 54.
| Parameter | Value | Source |
|---|---|---|
| Test operating characteristics | ||
| Sensitivity spirometry alone | 0.29 | Schneider et al.71 |
| Specificity PEF A%M > 21.6% | 0.90 | |
| Sensitivity FeNO testing + spirometry | 0.94 | aSmith and Taylor162 |
| Specificity FeNO testing + spirometry | 0.93 | |
| Sensitivity FeNO testing alone | 0.88 | Smith et al.86 |
| Specificity FeNO testing alone | 0.79 | |
| Sensitivity MCT | 0.91 | Hunter et al.163 |
| Specificity MCT | 0.90 | |
| Sensitivity sputum induction | 0.72 | |
| Specificity sputum induction | 0.80 | |
| Disease characteristics | ||
| Asthma prevalence | 0.36 | Smith et al.86 |
| Cost parameters (£) | ||
| Cost of spirometry | 1 | Source unclear |
| Cost of spirometry plus FeNO testing | 11 | Assumption |
| Cost of FeNO testing | 10 | Assumption |
| Cost of spirometry plus MCT | 63 | 2005 NHS Reference Costs (reported in Price et al.147) uplifted to 2012 values |
| Cost of spirometry and sputum induction | 63 | 2005 NHS Reference Costs (reported in Price et al.147) uplifted to 2012 values |
| Annual NHS cost for long-acting ICS (prescribing using standard guidelines) | 138 | BNF 51167 (reported in Price et al.147) uplifted to 2012 values |
It should be noted that the marginal per-test cost for NIOX MINO within the Aerocrine model is assumed to be £10.00; this is substantially lower than that assumed within the Price et al. 147 paper (£22.90). The manufacturer states that the model should mean this £10 cost can be amended depending on the aspects that local payers find relevant. The charge of £22.90 was noted by Price et al. 147 as is typical and includes all secondary care costs, but this cost also needs to be amended depending on the needs of to local payers. Most NHS costs are set; thus, the £22.90 charge may not be possible (Mr David Plotts, Director for Northern Europe & UK Managing Director, Aerocrine, 9 July 2013, personal communication).
Summary of the results of the Aerocrine diagnostic model
Table 55 provides the results presented within the Aerocrine diagnostic model. The results relate to a population of 840 patients; this population size is not justified within the model.
| Diagnostic option | No. of correct diagnoses (TPs and TNs) | No. of incorrect diagnoses (FPs an FNs) | Difference | Cost of incorrect diagnoses (diagnosis cost only) (£) | No. of FP diagnoses | Cost of FP diagnoses (£) | Cost of FP steroid use (£) |
|---|---|---|---|---|---|---|---|
| Spirometry alone | 572 | 268 | 303 | 268 | 54 | 54 | 7419 |
| Spirometry and (if spirometry negative) MCT | 719 | 121 | 597 | 4319 | 102 | 3102 | 14,096 |
| Spirometry and (if spirometry negative) FeNO testing | 659 | 181 | 478 | 1455 | 155 | 1171 | 21,441 |
| Spirometry and FeNO testing combined | 784 | 56 | 728 | 614 | 38 | 414 | 5193 |
| FeNO testing alone | 691 | 149 | 542 | 1492 | 113 | 1129 | 15,580 |
| Spirometry and (if spirometry negative) sputum induction | 629 | 211 | 419 | 9938 | 151 | 6150 | 20,773 |
The Aerocrine diagnostic model suggests that the combination of spirometry plus FeNO testing is expected to result in the greatest number of correct diagnoses and the fewest number of incorrect diagnoses. This is because of the assumed sensitivity and specificity of this combination (sourced from the expert review paper by Smith and Taylor162), both of which are higher than the values for all other tests included in the analysis.
The use of the Price et al. 147/Aerocrine diagnostic models to inform judgements about the cost-effectiveness of NIOX MINO compared with standard diagnostic tests for asthma is subject to a number of problems, which are detailed in the following sections.
Table 56 shows the extent to which the Price et al. 147 diagnostic model and the Aerocrine diagnostic model adhere to the NICE reference case for economic evaluations of diagnostic interventions. 145 Although the Price et al. 147 diagnostic model was not originally developed to inform this NICE appraisal, the model submitted by Aerocrine follows the same general approach and therefore should be interpreted in light of NICE’s reference case.
| Element of HTA | Reference case | EAG comments |
|---|---|---|
| Defining the decision problem | The scope developed by the NICE | The Price et al.147 diagnostic model was not developed specifically to inform the NICE diagnostic appraisal of FeNO, yet this same general model approach was employed within the Aerocrine submission. The intervention and comparators are generally in line with the NICE scope. However, the economic outcome does not include health consequences quantified in terms of health gains/losses. The population in both models is restricted to non-smoking adults with mild to severe asthma as seen in both primary and secondary care |
| Comparator | Therapies routinely used in the NHS, including technologies regarded as current best practice | Comparators include tests commonly used in the NHS for the diagnosis of asthma: bronchial provocation, lung function testing, reversibility testing and sputum eosinophil count. Sputum induction is not widely used in England and Wales. Importantly, the base-case analysis is presented as a blended comparison rather than as an incremental analysis between individual options. This is generally inappropriate as it may mask the most effective and/or the most cost-effective diagnostic option. Within the Aerocrine model, options are evaluated as individual diagnostic interventions. These include spirometry alone, spirometry and (if negative) MCT, spirometry and (if negative) FeNO, spirometry and FeNO, FeNO alone and spirometry and (if negative) sputum induction |
| Perspective on costs | NHS and Personal Social Services | A payer perspective was adopted by Price et al.;147 however, this is restricted to short-term costs only – treatment costs for diagnosed asthma are not included. The Aerocrine diagnostic model includes costs of diagnostic tests and treatment costs for FPs. The time horizon for costing is not explicit. Personal Social Services costs are not considered in either model |
| Perspective on outcomes | All health effects on individuals | Health gains and losses associated with correct/incorrect diagnoses are not reported by Price et al.147 The Aerocrine model reports numbers of TPs, FPs, TNs and FNs expected within a cohort of 840 patients |
| Type of economic evaluation | Cost–utility analysis | The Price et al.147 diagnostic analysis represents a cost comparison; although diagnostic outcomes are calculable, these are not reported. The Aerocrine model quantifies numbers of correct/incorrect diagnoses but does not value these in terms of health gains or losses |
| Synthesis of evidence on outcomes | Based on systematic review | Price et al.147 report that estimates of test sensitivity and specificity are based on three published papers identified by a systematic review of the literature.86,162,163 The Aerocrine submission does not present any detail regarding methods used to identify or select evidence used to inform its parameters. The full range of empirical evidence relating to the diagnostic accuracy of FeNO used in combination with other tests is not captured in either model |
| Measure of health effects | QALYs | Neither the Price et al.147 diagnostic model nor the Aerocrine diagnostic model measure or value health outcomes associated with correct/incorrect diagnoses |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | HRQoL is not captured in either model |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the public | HRQoL is not captured in either model |
| Discount rate | An annual rate of 3.5% on both costs and health effects | In both models, costs and outcomes are not discounted |
| Equity weighting | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | HRQoL is not captured in either model |
A key limitation of both the Price et al. 147 diagnostic model and the Aerocrine diagnostic model is that neither model attempts to value the health gains/losses resulting from correct/incorrect diagnoses of asthma. Although it may be reasonably inferred that a more sensitive and specific test will result in more correct diagnoses, and hence greater health gains from the use of that test, these factors are not captured within either model. Consequently, it is difficult to infer whether the health gains associated with a more sensitive and/or specific test outweigh the potential opportunity costs associated with displacing existing treatments and services.
The base-case analysis presented within the Price et al. 147 paper adopts a blended comparison approach. The results are not presented as an incremental comparison of the costs and consequences of NIOX MINO compared with individual comparator tests. This is misleading – although the base-case analysis suggests that NIOX MINO alone is more sensitive than the weighted mix of standard tests used to diagnose asthma, its sensitivity and specificity are both lower than those for bronchial provocation. It would be more appropriate to incrementally compare NIOX MINO against each individual diagnostic test; this is the approach adopted within the Aerocrine model submitted to NICE.
Both the Price et al. 147 diagnostic model and the Aerocrine diagnostic model reflect a situation in which NIOX MINO would replace existing standard tests for the diagnosis of asthma. The situation in which NIOX MINO is added to existing tests within the pathway, compared with those existing tests, as is suggested to be the more likely use of FeNO testing within the NHS by Price et al. ,147 is not adequately considered within the analysis. In addition, both models lack clarity with respect to the diagnostic setting in which the choice of diagnostic strategy is made (i.e. primary or secondary care).
The Price et al. 147 analysis crudely compares NIOX MINO with individual diagnostic tests. In reality, some patients may achieve a positive or negative diagnosis only following a sequence of tests. This is undoubtedly an issue relating to the available evidence base at the time of model development; however, this limitation should be borne in mind when interpreting the results reported within the Price et al. 147 paper. In contrast, the submitted Aerocrine model includes some test sequences; however, these do not reflect potential correlations between each test in the pathway (sensitivity and specificity are assumed to be random and uncorrelated between tests).
The Price et al. 147 model assumes that incorrect diagnoses are resolved at the next visit. Conversely, the Aerocrine model does not include the costs of additional visits required to resolve incorrect diagnoses, but instead attempts to capture the costs associated with ICS use in patients who are FP. Both of these factors reflect relevant costs to the NHS and should be included in any economic analysis of FeNO testing. In addition, the time horizon over which incorrect diagnoses prevail is unclear and no discounting is applied to cost estimates.
It is noteworthy that the estimates of test sensitivity and specificity for spirometry plus FeNO testing, the most favourable option within both diagnostic models included in this review, appear to have been derived from an expert review paper162 rather than from an empirical study. The expert review paper does make reference to the sensitivity and specificity estimates of 0.93 and 0.94 as used in the models and does provide an apparent (yet ambiguous) reference to two other empirical studies. 86,168 However, neither the Dupont et al. 168 study nor the Smith et al. 86 study referenced by Smith and Taylor162 report these estimates (or indeed any estimate of the joint sensitivity and specificity of FeNO testing plus FEV1). The credibility of these estimates cannot be verified by the EAG and hence the credibility of the Price et al. 147/Aerocrine model findings should be considered highly questionable.
Within the Aerocrine model, the sources of the costs of spirometry are unclear and the costs of NIOX MINO appear to be based solely on assumption (see earlier personal communication). The costs of NIOX MINO are substantially different between the two models (£22.90 vs. £10). It is unclear whether either estimate would reflect the true costs borne by the NHS. In addition, the methods used to identify and select evidence regarding test operating characteristics are particularly unclear within the Aerocrine model.
Both versions of the diagnostic model are evaluated deterministically using point estimates of parameters. Probabilistic sensitivity analysis (PSA) is not reported by Price et al. 147 and is not included in the submitted Aerocrine model.
It is reasonable to suggest that the existing evidence base relating to the cost-effectiveness of FeNO testing for the diagnosis of asthma is methodologically limited and should be interpreted with caution.
Existing economic analyses of FeNO testing for the management of asthma
Methods and results of included management studies
The Price et al. 147 study also included the methods and results of a separate model of the cost-effectiveness of FeNO testing using NIOX MINO for the management of asthma. The same model structure was also used in the German economic evaluation of FeNO testing for asthma reported by Berg and Lindgren. 148 In addition, the submission by Aerocrine also included an asthma management model based on the analysis published by Price et al. 147 No other published papers that evaluated FeNO testing for the management of asthma were identified.
The management model as described by Price et al. 147 uses a decision tree approach to evaluate the cost-effectiveness of NIOX MINO compared with standard guidelines for the management of asthma. The model adopts a UK health-care payer perspective and costs and outcomes are evaluated over a 1-year time horizon. The results are presented in terms of the incremental cost per QALY gained. Patients within the model were assumed to be non-smokers with mild to severe diagnosed asthma. Patients were assumed to be at step 3 and above as per GINA129 and BTS/SIGN164 guidelines, that is, receiving ICSs and LABAs for asthma management. Patients were assumed to visit their GP four times per year to determine the appropriate ICS dosage; it is unclear whether this applies to both groups or the FeNO management group only.
The two management strategies compared within the model were:
-
intervention: ICS dosage titration using NIOX MINO
-
comparator: ICS dosage titration based on standard guidelines.
The model uses different sources to inform parameters relating to the baseline risks and relative risks of exacerbation and ICS use. 2,97,169 Only one of these three studies involved asthma management according to BTS/SIGN guidelines. 2
The structure of the model is shown in Figure 22. The model assumes that patients are either well controlled or experience an exacerbation. Exacerbations are assumed to be either severe or mild to moderate. A proportion of the severe exacerbations are assumed to require hospitalisation whereas the remainder are assumed to be manageable on an outpatient basis. A mild to moderate exacerbation was defined as an exacerbation requiring a SABA in addition to usual medication; a severe exacerbation was defined as an exacerbation requiring corticosteroids (and, in some patients, hospitalisation). The successful control of exacerbations is assumed to be related to an improvement in HRQoL and a reduction in ICS use.
FIGURE 22.
Model structure employed within the Price et al. 147 management model. Reproduced with permission from Price D, Berg J, Lindgren P. An economic evaluation of NIOX MINO airway inflammation monitor in the United Kingdom. Allergy 2009;64:431–8. 147 John Wiley & Sons. © 2009 The Authors. Journal compilation © 2009 Blackwell Munksgaard.
The parameter values and evidence sources listed in the Price et al. 147 management model are shown in Table 57.
| Parameters | Value | Source |
|---|---|---|
| Baseline event probabilities | ||
| Exacerbation risk during 1 year | 0.71 | Jayaram et al.169 |
| Proportion of exacerbations that are severe | 0.23 | Jayaram et al.169 |
| Hospitalisation for severe exacerbations | 0.23 | Green et al.2 |
| Proportion of severe exacerbations requiring an outpatient visit | 0.75 | Andersson et al.170 |
| Mean number of severe exacerbations per year (overall population) | 2 | Jayaram et al.;169 Tattersfield et al.171 |
| Mean number of severe exacerbations per year (moderate to severe asthma) | 4 | Green et al.2 |
| Impact of FeNO management | ||
| Reduction in ICS dose | 0.42 | Smith et al.97 |
| Relative risk reduction of exacerbation | 0.29 | Jayaram et al.169 |
| Relative risk reduction of hospitalisation for severe exacerbation | 0.83 | Green et al.2 |
| Utility values | ||
| Well-controlled asthma | 0.93 | Szende et al.172 |
| Mild/moderate exacerbation | 0.65 | Szende et al.172 |
| Severe exacerbation | 0.52 | Szende et al.172 |
| Resource cost parameters (£) | ||
| Outpatient visit to GP | 30.00 | Curtis and Netten166 |
| Outpatient visit to lung specialist | 44.00 | Curtis and Netten166 |
| Hospitalisation for asthma | 2231.45 | BNF 51167 |
| Maintenance therapy (1 year) with LABA | 359.84 | BNF 51167 |
| Maintenance therapy (1 year) with ICS | 109.00 | BNF 51167 |
| Rescue therapy (1 week) with SABA | 7.38 | BNF 51167 |
| Rescue therapy (1 week) with oral prednisone | 5.13 | BNF 51167 |
In addition to the base-case analysis, the authors undertook 18 one-way sensitivity analyses. These include examining the impact of the baseline risk of exacerbations, health utilities, number of routine visits required per year, ICS dose reductions and costs of NIOX MINO on the cost-effectiveness of NIOX MINO compared with standard guidelines.
The model results reported by Price et al. 147 are presented in Table 58. For patients with moderate to severe asthma, FeNO monitoring was estimated to result in 0.004 additional QALYs compared with standard guidelines. FeNO monitoring was also estimated to result in cost savings of £554 per patient in this group. For patients with mild to moderate asthma, FeNO monitoring was estimated to result in 0.06 additional QALYs compared with standard guidelines. FeNO monitoring was also estimated to result in cost savings of £341 per patient in this group. Given its lower cost and increased QALY gain, FeNO monitoring was expected to dominate standard guidelines in both patient groups. It should be noted that the distinction between mild to moderate and moderate to severe in terms of input parameters is not entirely clear from the Price et al. 147 paper.
| Scenario | Cost (£) | QALYs | ICER | ||||
|---|---|---|---|---|---|---|---|
| NIOX MINO | Standard guidelines | Difference | NIOX MINO | Standard guidelines | Difference | ||
| Moderate to severe asthma | 628 | 1181 | –554 | 0.730 | 0.726 | 0.004 | Dominating |
| 1-year baseline risk of exacerbation of 0.35 (base case 0.71) | 589 | 915 | –326 | 0.857 | 0.83 | 0.027 | Dominating |
| Utility for moderate control of asthma of 0.76 (base case 0.65) | 666 | 1007 | –341 | 0.835 | 0.800 | 0.035 | Dominating |
| Different number of monitoring visits per year for mild to severe asthma (base case four visits) | |||||||
| Two visits per year | 620 | 828 | –208 | 0.785 | 0.726 | 0.059 | Dominating |
| Six visits per year | 712 | 1185 | –473 | 0.785 | 0.726 | 0.059 | Dominating |
| Different number of monitoring visits per year for moderate to severe asthma (base case, four visits) | |||||||
| Two visits per year | 582 | 1003 | –421 | 0.730 | 0.726 | 0.004 | Dominating |
| Six visits per year | 673 | 1360 | –687 | 0.730 | 0.726 | 0.004 | Dominating |
| Different NIOX MINO cost for mild to severe asthma | |||||||
| –50% | 620 | 1007 | –387 | 0.785 | 0.726 | 0.059 | Dominating |
| +50% | 712 | 1007 | –295 | 0.785 | 0.726 | 0.059 | Dominating |
| Different NIOX MINO cost for moderate to severe asthma | |||||||
| –50% | 582 | 1181 | –599 | 0.730 | 0.726 | 0.004 | Dominating |
| +50% | 673 | 1181 | –508 | 0.730 | 0.726 | 0.004 | Dominating |
| Different level of ICS dose reduction for mild to severe asthma (base case 42%) | |||||||
| 10% | 683 | 1007 | –324 | 0.785 | 0.726 | 0.059 | Dominating |
| 80% | 645 | 1007 | –362 | 0.785 | 0.726 | 0.059 | Dominating |
| Different level of ICS dose reduction for moderate to severe asthma (base case 42%) | |||||||
| 10% | 639 | 1181 | –543 | 0.730 | 0.726 | 0.004 | Dominating |
| 80% | 616 | 1181 | –565 | 0.730 | 0.726 | 0.004 | Dominating |
| Different relative risk reduction for exacerbation for mild to severe asthma (base case 29%) | |||||||
| 10% | 707 | 1007 | –300 | 0.747 | 0.726 | 0.021 | Dominating |
| 50% | 621 | 1007 | –386 | 0.828 | 0.726 | 0.102 | Dominating |
| Different relative risk reduction for hospitalisation for moderate to severe exacerbation (base case 0.83) | |||||||
| 10% | 869 | 1181 | –312 | 0.727 | 0.726 | 0.001 | Dominating |
| 100% | 571 | 1181 | –610 | 0.731 | 0.726 | 0.005 | Dominating |
| NIOX MINO in addition to rather than instead of standard lung function tests (added costs) | 1023 | 1007 | 17 | 0.785 | 0.726 | 0.059 | £279 per QALY gained |
The results of the simple sensitivity analyses indicate that, for all but one scenario (NIOX MINO in addition to rather than instead of standard lung function tests), NIOX MINO is expected to dominate standard guidelines. Within the last scenario, NIOX MINO in addition to standard lung function tests is expected to cost £279 per QALY gained compared with standard guidelines.
The schematic of the Aerocrine management model is presented in Figure 23.
FIGURE 23.
Management model submitted by Aerocrine. A&E, accident and emergency.

Table 59 presents the parameter values and evidence sources used in the Aerocrine management model; the column on the right hand side indicates whether the source and parameter value are the same as those in the published Price et al. 147 management model.
| Variable ID | Variable description | Value | Source/justification | Same as Price et al.147 model |
|---|---|---|---|---|
| P2 | Likelihood of exacerbation using FeNO monitoring for management | 0.369 | Jayaram et al.169 | No |
| P4 | Likelihood of exacerbation using standard care guidelines for asthma management | 0.520 | Akinbami et al.173 | No |
| P6 | Likelihood that exacerbations will be moderate to severe | 0.230 | Green et al.2 | Yes |
| P8 | Likelihood that mild to moderate asthma exacerbations will be treated at an emergency room or urgent care centre | 0.500 | Expert opiniona | Unclear |
| P10 | Likelihood that FeNO patient experiencing moderate to severe asthma exacerbations will be treated at an emergency room centre | 0.750 | Andersson et al.170 | Yes |
| P13 | Likelihood that standard care patient experiencing a moderate to severe exacerbation will require hospitalisation | 0.230 | Green et al.2 | Unclear |
| F1 | Reduction in ICS dose as a result of FeNO use | 0.42 | Smith et al.97 | Yes |
| F2 | Reduction in risk of hospitalisation for severe exacerbations as a result of FeNO use | 0.83 | Green et al.2 | Yes |
| F3 | Reduction in risk of exacerbations as a result of FeNO use | 0.29 | Jayaram et al.169 | Yes |
| C1 | Cost of FeNO monitoring | £10.00 | Assumption | Unclear |
| C2 | Cost of spirometry | £1.00 | Source unclear | Unclear |
| C3 | Annual cost of asthma medications for patients managed with FeNO | £536.04 | BNF 51167 (uplifted to 2012 prices) | Unclear |
| C4 | Annual cost of asthma medications for patients managed using standard guidelines | £594.00 | BNF 51167 (uplifted to 2012 prices) | Unclear |
| C5 | Cost per office visit to GP | £38.00 | Curtis and Netten166 (uplifted to 2012 prices) | Unclear |
| C6 | Cost per office visit (referral) to lung specialist | £144.00 | Curtis and Netten166 (uplifted to 2012 prices) | Unclear |
| C7 | Cost of A&E visit for asthma exacerbation | £81.00 | NHS Reference Costs174 | Unclear |
| C8A | Cost of rescue medications for moderate to severe exacerbations | £15.00 | BNF 51167 (uplifted to 2012 prices) | Unclear |
| C8B | Cost of rescue medications for mild to moderate exacerbations | £9.00 | BNF 51167 (uplifted to 2012 prices) | Unclear |
| C9 | Average hospital cost for asthma admission because of exacerbation | £867.00 | Weighted average of Healthcare Resource Group code DZ15A-F within NHS Reference Costs174 | Unclear |
| C10 | Annual number of check-ups for asthma management | 2 | Expert opinionb | Yes |
| C13 | Average annual number of exacerbations | 2 | Jayaram et al.169 | Yes |
| U1 | Utility value of asthma patients with good control | 0.93 | Szende et al.172 | Yes |
| U2 | Utility value of asthma patients with mildly reduced control | 0.76 | Szende et al.172 | Yes |
| U3 | Utility value of asthma patients with moderately reduced control | 0.65 | Szende et al.172 | Yes |
| U4 | Utility value of asthma patients with poor control | 0.52 | Szende et al.172 | Yes |
The management model submitted by Aerocrine is similar to the published Price et al. 147 management model in terms of its structure and both models share many common parameter values. However, the two models do not make identical assumptions and hence do not provide identical estimates of incremental costs and effects of FeNO monitoring compared with standard guidelines.
Although the Aerocrine management model does not present the incremental cost-effectiveness ratio (ICER) for FeNO monitoring compared with standard guidelines in the main results worksheet, elsewhere the model indicates that FeNO monitoring is expected to produce an additional 0.045 QALYs and reduces costs by £103.11 compared with standard care.
The use of the Price et al. 147/Aerocrine management models to inform judgements about the cost-effectiveness of NIOX MINO is subject to a number of methodological problems, as detailed in the following sections.
Table 60 shows the extent to which the Price et al. 147/Aerocrine management models adhere to the NICE reference case for economic evaluations of diagnostic interventions. 145
| Element of HTA | Reference case | EAG comments |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | The patient population is defined in both models as non-smoking adults diagnosed with mild to severe asthma. This population excludes children and smokers. The intervention and comparator are in line with the NICE scope |
| Comparator | Therapies routinely used in the NHS including technologies regarded as current best practice | The comparator is standard care without FeNO monitoring. This is appropriate although it should be noted that the studies used to inform the model parameters did not all use BTS/SIGN guidelines164 to guide treatment |
| Perspective on costs | NHS and Personal Social Services | The published Price et al.147 management model purports to have adopted a payer perspective. It appears that the submitted Aerocrine model adopts the same perspective although this is not explicitly stated in the model workbook |
| Perspective on outcomes | All health effects on individuals | Health outcomes reflect those accrued by NHS patients. Health gains are assumed to be influenced only by the level of control achieved, which is in turn assumed to be directly related to the incidence of exacerbations |
| Type of economic evaluation | Cost–utility analysis | The models take the form of a decision tree-based cost–utility analysis. This adopts a short time horizon (1 year). Longer-term costs and outcomes associated with FeNO monitoring are not considered within the Aerocrine management model or the published Price et al.147 management model |
| Synthesis of evidence on outcomes | Based on systematic review | Parameter values appear to have been selected in a non-systematic fashion. Estimates of relative reductions in exacerbations are drawn from different sources from estimates of reductions in medication use (the former relates to monitoring using sputum induction rather than FeNO testing but is assumed to be equivalent) |
| Measure of health effects | QALYs | The HRQoL impacts of different levels of control were estimated based on estimates from the literature |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | Health utilities were based on adequacy of asthma control rather than exacerbations per se, based on a study reported by Szende et al.172 Within this study, 228 consecutive adult outpatients and inpatients at four Hungarian sites completed a variety of HRQoL instruments including the EQ-5D. Utilities related to control were then qualitatively mapped to the incidence of different severities of exacerbation |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the public | Preference-based health utilities appear to have been generated using the UK EQ-5D tariff175 |
| Discount rate | An annual rate of 3.5% on both costs and health effects | Because of the short time horizon, costs and outcomes are not discounted |
| Equity weighting | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | No additional equity weighting was applied |
Price et al. 147 argue that the relative risk reduction associated with using FeNO monitoring may be overly conservative as the data used were drawn from a patient population including patients with mild asthma whereas the relative risk reductions may be greater in patients with more severe asthma. The validity of this statement is unclear and evidence to support this assertion is not presented in the paper.
Price et al. 147 also argue that some of the parameters, such as the effect of FeNO measurement on ICS usage, were based on patients in primary care, whereas other parameters, such as impact on exacerbations, were based on patients in secondary care.
The model adopts a very short time horizon (1 year). The impact of mortality and discounting over a longer horizon may alter the cost-effectiveness estimates presented.
The authors did not undertake PSA. Instead, the results are presented based on the point estimates of parameters and uncertainty analysis is restricted to one-way sensitivity analyses. It should be noted that the economic evaluation of NIOX MINO from the German perspective did include a full probabilistic analysis. 148 The reason for the exclusion of PSA in the UK models is unclear.
The methods used to identify and select evidence to inform the model parameters were not fully described in either the Price et al. 147 model or the Aerocrine model. It is unclear whether other evidence sources exist which indicate that different parameter values may be more appropriate. In particular, the model draws estimates of the relative reduction in exacerbations from FeNO monitoring from a study that used sputum induction monitoring rather than FeNO monitoring, hence assuming equivalence, despite the fact that exacerbation risk information was reported in the FeNO trial used to estimate reductions in ICS usage. 97
Several unit cost parameters within the Aerocrine model are based on those presented in the Price et al. 147 model, uplifted to 2012 values. For parameters such as drug costs and Healthcare Resource Groups, this is inappropriate as the British National Formulary and NHS Reference Costs are updated regularly to reflect current prices. Consequently, several of the cost estimates included in the submitted model may not reflect the prices paid by the NHS.
The Price et al. 147 management model and the Aerocrine management model assume a mean rate of two exacerbations per patient per year. It appears that this estimate was based on the results of a Phase II prospective trial of 117 adults reported by Jayaram et al. 169 This study reported that there was a total of 126 exacerbations in 63 patients, hence an average number of approximately two exacerbations per patient. However, the trial duration was > 1 year and the mean number of exacerbations per patient per year was reported by the study authors to be 0.75 in one arm of the trial and 1.02 in the other arm of the trial. 169 The Price et al. paper147 also mentions a second study171 used to inform this baseline exacerbation rate. In this latter study, the authors observed 425 severe exacerbations in 852 randomised patients over a 12-month period (approximate rate = 0.499 exacerbations per year). Both studies clearly indicate that the baseline exacerbation rate used in the Price et al. 147/Aerocrine models is substantially overestimated; hence, the expected benefits of FeNO testing are likely to be artificially inflated.
The Price et al. 147/Aerocrine management models make an assumption that the incidence of exacerbations is directly related to the level of asthma control and apply health utilities according to the incidence of exacerbations. The models apply these health utilities over the modelled time horizon (1 year) rather than to the duration over which the exacerbation occurs (hours to weeks). This is likely to substantially overestimate the health benefits associated with reducing exacerbations through improved dose titration.
The Aerocrine management model includes the use of expert opinion to inform a small number of parameters for which the authors could not identify relevant evidence. Although expert opinion is a valid source of evidence in such circumstances, no details are provided with respect to the sources of these judgements or the methods used to elicit them. In the absence of a written submission that presents these details, the credibility of such judgements remains unclear.
The existing models of FeNO monitoring for asthma indicate that NIOX MINO is expected to dominate standard guidelines. However, given the methodological concerns identified within the critical appraisal, these findings should be interpreted tentatively.
Other studies relating to the cost-effectiveness of asthma management strategies
Given the limited number of studies of FeNO testing for the management of asthma, we also reviewed other studies of interventions for asthma management to inform the key disease-specific factors that should, or could, be included in a cost-effectiveness model of FeNO testing for the management of asthma.
Thirteen studies149–154,157–161,176,177 (not related to FeNO testing) were included in the focused review of economic analyses of asthma management interventions; these studies are briefly summarised in Table 61.
| Author, year | Summary description |
|---|---|
| Studies reporting QALYs | |
| Briggs 2006150 | Cost–utility analysis undertaken alongside a clinical trial. The intervention was asthma treatment with salmeterol/fluticasone propionate in combination; the comparator was fluticasone propionate. Utility values for the model states were mapped from AQLQ scores. Within the GOAL study, patient treatment could be titrated upwards up to three times but not downwards. The amount of titration required was used to define three patient groups by asthma severity (stratum 1, stratum 2, stratum 3). The model states were ‘totally controlled’, ‘well controlled’, ‘not well controlled but without an exacerbation’ and ‘exacerbation’. The cycle length was 1 week. A multinomial regression approach using individual patient-level data from the trial was used to estimate the transition probabilities of moving between states over the course of each week |
| Doull 2007151 | Simple economic model comparing the cost-effectiveness of salmeterol xinafoate/fluticasone propionate combination inhalers with non-combination inhalers for adults and children with chronic asthma treated according to BTS/SIGN guidelines.178 Clinical effectiveness was estimated from meta-analyses comparing the percentage of symptom-free days for each treatment (%SFD). The definition of SFD was assumed to be consistent with that provided in the GOAL study. The %SFD was assumed to be time invariant; hence, differences in clinical effectiveness between treatment options were assumed to be entirely due to this parameter. A 1-year time horizon was used. QALY gains were estimated from AQLQ data recorded in the GOAL study using a mapping algorithm to the EQ-5D |
| Paggiaro 2011153 | A poster that discusses a patient-level Markov model. The decision problem concerns the cost-effectiveness of stepping down treatment according to GINA guidelines.129 Very limited detail on the methods was available |
| Peters 2002149 | UK technology assessment report evaluating submissions from several manufacturers of inhaler devices. Most (six out of eight) of the manufacturers submitted cost-minimisation analyses only. The assessment group did not develop a de novo model; instead, a QALY-based threshold analysis was performed |
| Wilson 2010152 | Economic evaluation comparing the addition of either a LTRA or a LABA for patients who were already receiving ICSs as part of asthma management and for whom a decision to add on additional treatment to improve their condition had been made. The analysis was based on a pragmatic trial involving 53 primary care practices. Patients judged to need add-on therapy were randomly assigned to receive either a LTRA or a LABA. The trial duration was 2 years. The patient age range included children and adults. The differences in EQ-5D and ACQ scores between the LTRA group and the LABA group were reported, together with differences in resource use |
| Studies not based on QALYs | |
| Andersson 2000177 | Poster abstract which argues that using 800 µg rather than 200 µg/day of budesonide is cost saving in patients with moderate asthma in the UK. Estimates were based on a survey of 20 physicians from the UK, Sweden and Spain |
| Barnes 1999155 | Poster abstract that summarises a meta-analysis comparing fluticasone propionate and budesonide for the treatment of asthma. The study appears mainly to be a cost–consequence analysis as it refers to differences in clinical parameters, such as morning PEF rate, successfully treated weeks and symptom-free days. The poster concludes that fluticasone propionate is both more clinically effective and cheaper than budesonide |
| Booth 1995154 | Cost comparison based on a RCT comparing fluticasone propionate (200 µg) via a diskhaler with budesonide (200 µg) via a reservoir dry powder device. The study provides estimates for the cost per successfully controlled week |
| Buxton 2004159 | Economic evaluation based on a 3-year international prospective RCT, the Steroid Treatment as Regular Therapy (START) trial. The trial compared budesonide against placebo combined with usual asthma therapy. The trial included patients from the UK although all costs were converted to US dollars for comparability. ICERs were calculated for the UK as well as for other countries, with the measure of health benefit being symptom-free days. Estimates for UK costs were based on only 39 patients |
| Everden 2002157 | Economic evaluation in children aged 6–17 years inclusive alongside a prospective multicentre open-label parallel-group study conducted in primary care in the UK and the Republic of Ireland (the FACT study). Most (> 95%) patients were at BTS step 1 or step 2 with a small proportion at step 3. The trial duration was 12 weeks. End points were change in SABA use (primary end point), PEF, number of poorly controlled days and quality of life evaluated using the Paediatric Asthma Quality of Life Questionnaire (PACLQ). When the clinical outcome was symptom-free days with no SABA use, use of eformoterol was estimated to rule out salmeterol by simple dominance, saving approximately 25 p per patient per day whilst resulting in approximately 10 additional symptom-free days over the 12-week period |
| Kemp 2010161 | Economic evaluation based on a retrospective analysis of patients recorded in the UK General Practice Research Database (GPRD) from 1997 to 2007. Patients were included in the analysis if they had been registered at the same practice, had a diagnosis of persistent asthma and had been receiving treatment with ICSs. Two patient populations were identified: an initiation population who had started ICSs and a step-up population who had been prescribed an increased ICS dose. Both populations had to have been followed up for at least 12 months on their current regimen. The clinical effectiveness and cost-effectiveness of three inhaler technologies were compared for these patient populations. The clinical outcome was ‘achieving asthma control within 1 year’. Asthma control was defined as a composite measure involving no hospital attendance for asthma, no OCS use and no consultation or hospital admission or attendance related to asthma |
| Price 2002158 | Markov model based on a 12-week RCT of patients diagnosed with asthma aged 12–70 years (FEV1 40–85% predicted). The main clinical outcome was the number of ‘successfully controlled weeks’. The intervention arm received fluticasone propionate whereas the comparator arm received salmeterol/fluticasone propionate combination. Health states included in the model were ‘successful control’, ‘hospital-managed exacerbation’, ‘primary care-managed exacerbation’, ‘suboptimal control’ and ‘treatment failure’ |
| Price 2007160 | Cost-minimisation analysis based on a 6-month, double-blind RCT. Resource use data were collected prospectively; these included medication costs and non-medication costs such as hospitalisations. The trial was international, with patients recruited from 16 countries. Costs were converted to 2004 UK costs. Patients were recruited if they were aged > 12 years at the time of recruitment and had been diagnosed with asthma at least 6 months previously and had been using ICSs continuously for at least 3 months. Compared with using ICSs alone, using budesonide/formoterol maintenance and reliever therapy was estimated to save the NHS around £90 per patient over the 6-month trial period |
Health outcomes and form of economic evaluation
Of the 13 studies included in the focused management review, five reported QALYs gained as the measure of health benefit. 149–153 One of these studies153 was published only in the form of a conference poster and provided very limited detail regarding the model structure. The study reported by Peters et al. 149 included only threshold analyses, indicating the necessary QALY impact to justify an incremental increase in cost. Of the eight studies that did not report QALYs, four were cost-effectiveness analyses. These studies reported health benefits in terms of:
Model structures
Several of the included economic evaluations were decision analyses conducted alongside clinical trials and did not explicitly involve the use of evidence synthesis or extrapolation. Three studies used Markov structures. 150,153,158 The Briggs et al. 150 and Price and Briggs158 studies both used similar methodologies. Each was based primarily on data from a single, although different, study. The Price and Briggs158 model categorised health states into five discrete categories: ‘successfully controlled’, ‘suboptimal control’, ‘primary care-managed exacerbation’, ‘hospital-managed exacerbation’ and ‘treatment failure’. Treatment failure was an absorbing state; patients could transition between any of the other states during a given Markov cycle. The cycle length was 1 week and so the assumption was made that an individual could not have more than one exacerbation within 1 week. The time horizon of the model was 12 weeks (equal to the duration of the RCT) and the analysis did not extrapolate anticipated lifetime effects of treatment. The model used the number of exacerbation-free weeks as the measure of health benefit; this disease-specific outcome measure is difficult to interpret from a policy context. The Briggs et al. 150 model was similar in that it was a model based on individual patient-level data from a single trial. This model adopted four discrete health states: ‘totally controlled’, ‘well controlled’, ‘not well controlled’ and ‘exacerbation’. These health states differ from those in the Price and Briggs model158 in that there were three non-exacerbation health states and only one exacerbation health state. This different categorisation implicitly reflects a different set of assumptions about the key factors that influence the clinical effectiveness and cost-effectiveness of different treatment options.
In addition to the studies identified by the search strategy described above, the Health Technology Assessment journal was searched from inception onwards for asthma management models. This search identified an additional asthma management model13 that was similar in structure to the Price and Briggs158 model. The report assessed the comparative effectiveness of different ICS treatments with or without LABAs for patients aged ≥ 12 years who had been diagnosed with chronic asthma. Unlike the Price and Briggs158 model, this model was a cost–utility analysis and therefore measured health benefits in terms of QALYs gained. The intention of this model was to represent clinical practice, as described in the BTS/SIGN guidelines,164 by including different separate health states to represent dosage levels corresponding to different BTS/SIGN treatment steps. For two steps, corresponding to step 2 and step 3 of the BTS/SIGN guidelines, the conceptual model is shown in Figure 24. The cycle length was 1 week and the time horizon was 5 years. The key disease-specific factors included in the model relate to whether the patient experiences an exacerbation within a model cycle and, if so, the severity of the exacerbation. By allowing transitions between different levels of treatment, however, changes in treatment in response to clinical events were also incorporated.
Discussion of the available economic evidence on the diagnosis and management of asthma using FeNO and other interventions
The review highlights a dearth of published studies reporting on the cost-effectiveness of FeNO testing for the diagnosis and/or management of asthma. Only one published UK cost-effectiveness model of asthma diagnosis was identified and included in the review;147 this model estimates the incremental costs of FeNO testing compared with existing standard tests for asthma. No other cost-effectiveness models of FeNO or other diagnostic tests were identified by the searches. Similarly, the review of economic analyses of asthma management interventions identified only one UK-published study of FeNO monitoring. 147 Modified versions of these FeNO management and diagnostic models were submitted to NICE by Aerocrine. No evidence was submitted by Aerocrine with respect to the expected cost-effectiveness of NIOX VERO. Bedfont Scientific did not submit any economic evidence relating to the cost-effectiveness of NObreath.
The wider review of economic analyses of asthma management interventions identified a number of other economic analyses, although few were undertaken within a formal modelling framework involving evidence synthesis and/or extrapolation. These models have the following features in common: (1) the use of a Markov modelling approach with generally short cycle lengths, typically 1 week in duration; (2) short time horizons; and (3) separate states for asthma exacerbations. Only two of the model-based studies reported QALYs as the measure of health outcome.
The available economic evidence for FeNO testing suggests that, in the diagnostic setting, monitoring using NIOX MINO may reduce the costs of diagnosis (depending on how it is used) compared with standard tests, whereas in the management setting monitoring using NIOX MINO may dominate standard guidelines. However, this evidence is subject to a number of methodological problems, questionable assumptions and weak evidence. The results of these existing analyses should be interpreted with caution.
Development of two de novo models to estimate the cost-effectiveness of FeNO testing for the diagnosis and management of asthma
Rationale for developing de novo models
This section describes the de novo economic models developed by the EAG to estimate the cost-effectiveness of FeNO testing (specifically using NIOX MINO, NIOX VERO or NObreath) compared with standard care for the diagnosis and management of asthma. The EAG analysis involves the development of two models: (1) a de novo model to assess the expected cost-effectiveness of FeNO testing in addition to or in place of standard tests for the diagnosis of asthma and (2) a de novo model to assess the expected cost-effectiveness of FeNO plus standard guidelines compared with standard guidelines for the management of patients with diagnosed asthma. Although these models are distinct, they form part of the same overall asthma service pathway, hence they share a number of parameter values and assumptions.
The EAG models were developed to attempt to resolve the problems identified with respect to the existing economic analyses of NIOX MINO (see Review of existing evidence relating to the cost-effectiveness of FeNO testing for the diagnosis and management of asthma) and to address gaps in the evidence relating to the cost-effectiveness of NObreath and NIOX VERO. It should be noted that, because of the limitations in the evidence base (see Chapter 3), the structures of the models are necessarily simple.
The decision to develop two models rather than a single model was made because the NICE scope reflects two distinct decision problems. Although the FeNO devices are the same in both the diagnostic setting and the management setting, the relevant populations and the way in which FeNO may influence decisions about appropriate clinical options for patients differ between settings; these potential effects are summarised in Table 62.
| Decision problem | Clinical population | Expected impact of FeNO testing |
|---|---|---|
| Diagnosis | Symptomatic patients with suspected asthma | FeNO testing, alone or in conjunction with other standard tests, may alter the proportion of correct and incorrect diagnoses amongst patients with suspected asthma. Changing the proportion of people with suspected asthma who are correctly/incorrectly diagnosed may then affect the expected downstream costs and health gains/losses |
| Management | Patients treated for diagnosed asthma | FeNO testing may influence the level of medication use and the rates of exacerbations experienced by patients diagnosed with asthma. This will influence the mean costs and health gains accrued by these patients |
At the outset, the EAG had intended to model a scenario in which FeNO testing is used both as a diagnostic option and as a management option. However, this analysis was not possible because of the necessary differences in the structures of the EAG diagnostic and management models.
Complexity and uncertainty surrounding the economic analysis of FeNO testing for the diagnosis and management of asthma
Given the limitations of the available evidence base (see Chapter 3), evaluating the expected cost-effectiveness of FeNO testing alone or in conjunction with other tests for the diagnosis of asthma is difficult. The BTS/SIGN guidelines8 for asthma diagnosis and management state that the absence of a gold standard definition of asthma means that it is impossible to make evidence-based recommendations on how to make a diagnosis of asthma. Further, differences in patient selection, methodological aspects of study design and the generalisability of studies to UK practice make the unbiased interpretation of the available diagnostic evidence extremely problematic. The current diagnostic pathway consists of a number of tests that may be used alone or in sequence; there is not a standard set of ways in which information from each of these tests should be evaluated and weighted when used together. The evidence base examined within this assessment, however, mostly relates to studies that estimate the operating characteristics of individual diagnostic tests used at particular points within this broader diagnostic pathway. In addition, the reference standards used within studies to estimate the sensitivities and specificities of other diagnostic tests are not always consistent or optimal, studies relate to different population groups and comparative (head-to-head) studies are few in number. As a consequence, there is considerable uncertainty surrounding the true diagnostic accuracy of FeNO testing and every other test used within the diagnostic pathway.
The uncertainty in the clinical evidence base is further compounded by the lack of available economic analyses. The review presented earlier (see Review of existing evidence relating to the cost-effectiveness of FeNO testing for the diagnosis and management of asthma) identified only one published economic model of options for asthma diagnosis147 (note that the same general model was used in the German economic evaluation reported by Berg and Lindgren148). Within this study, the authors highlight a key limitation in the scope of their analysis, that is, the analysis considers FeNO testing as a replacement for existing diagnostic tests; this limitation is masked somewhat by the inappropriate use of a blended comparison of multiple diagnostic tests, the absence of quantified health losses associated with misdiagnosis and the absence of a full incremental analysis. If all diagnostic tests can be substituted for one another for all patients with symptoms of asthma, as is implied by the design of the economic comparisons presented in the Price et al. 147 paper, then the most clinically effective option will be the diagnostic test with the greatest sensitivity and specificity (depending on the balance of health losses avoided by obtaining TP and TN diagnoses). Subject to the per-test costs and the costs and consequences of downstream tests used to correct misdiagnoses, this may or may not also represent the most cost-effective option. Downstream costs, sequences of diagnostic pathways and consequences of incorrect diagnoses are not fully addressed by the Price et al. 147 diagnostic model. The existing economic evidence base does not provide any information on the additional value of FeNO testing in conjunction with current standard tests for asthma diagnosis.
As noted by Price et al. ,147 in reality, FeNO testing is likely to play a role as an adjunct to existing tests currently used within the diagnostic pathway. Although Price et al. 147 attempt to consider the combination of FeNO testing plus FEV1, this is against the blended comparison of standard diagnostic tests and thus it still represents a replacement option. Current pathways for asthma diagnosis in adults and children are complex;8 within the Price et al. 147 diagnostic model, this complexity is avoided by the neat assumption that all misdiagnoses are resolved at some later point in time with one subsequent test (i.e. following misdiagnosis, the subsequent test is assumed to have perfect sensitivity and specificity, thereby correcting all previously incorrect diagnostic decisions). This is a substantial simplification. In reality, there may be a number of potential places in the existing pathway at which FeNO testing may provide additional diagnostic information to improve the diagnostic accuracy of current standard tests (see Figures 7 and 8), and misdiagnoses may prevail for months, years or, in some patients, indefinitely. These misdiagnoses may incur unnecessary treatment costs and health losses. The Price et al. 147/Aerocrine diagnostic models do not fully address these issues but instead ask the question, ‘What is the least expensive test for the diagnosis of asthma?’
An alternative and more sophisticated approach to evaluating the cost-effectiveness of FeNO in the diagnostic setting would involve assessing the diagnostic accuracy and cost-effectiveness of FeNO in addition to existing tests within the pathway. Such an analysis would address the question, ‘Where in the existing sequences of tests, if anywhere, should FeNO be added to provide the most cost-effective diagnostic pathway for patients with symptoms of asthma?’ This would require either (1) a similar model structure to that employed by Price et al. ,147 populated using studies that assess the accuracy of the whole diagnostic pathway for children and adults with and without FeNO testing, or (2) the development of a model that estimates the diagnostic outcomes of sequences of tests at each point in the pathway, which simulates the impact of changes in the true underlying prevalence of asthma conditional on the results of each test undertaken and which fully takes into account the impact of potential correlations between tests that may result in non-random test outcomes in particular patients (e.g. if test A is negative would test B also be negative in patient C?). For the former approach to be reliable, one would require studies that have assessed FeNO plus other tests against a reference standard as well as the standard tests (without FeNO) against the same reference standard, either by direct comparisons within the same study or by indirect comparisons across multiple studies with similar populations and study protocols. Price et al. 147 note that such data simply do not exist. The review presented in Chapter 3 did, however, identify several studies in which FeNO was used in conjunction with other tests within part of the diagnostic pathway. This evidence is, however, somewhat patchy. Interestingly, the Aerocrine diagnostic model does attempt to reflect sequential options – the latter modelling approach described above – despite the problems with the available evidence previously highlighted by Price et al. 147 The Aerocrine model thus assumes that sequential test outcomes are random and uncorrelated between tests. This represents a strong assumption that could lead to biased estimates of the cost-effectiveness of FeNO, the magnitude and direction of which are unclear.
These are important limitations relating to the evidence base that constrain what can be achieved through the development of any economic model of asthma diagnosis. It would be unfair to heavily criticise any model when the main limitations of that model are principally sourced from the weaknesses in the evidence used to inform it. Such weaknesses do, however, limit the confidence that can and should be placed in the results of the Price et al. 147/Aerocrine diagnostic models. In light of these issues, the de novo EAG diagnostic model attempts to resolve those weaknesses in the Price et al. 147 diagnostic model that can be resolved. Problems relating to the heterogeneity in the evidence base cannot be resolved by the EAG; hence, the results of the de novo model should also be interpreted tentatively. Insofar as the available evidence allows, the EAG de novo diagnostic model attempts to simultaneously address the following two questions:
-
As a replacement test – is FeNO expected to be more cost-effective than other existing tests used for the diagnosis of asthma?
-
As an adjunctive test – is the use of FeNO in conjunction with existing tests expected to be more cost-effective than using existing tests alone?
The economic analysis of asthma management is subject to fewer complexities because of the availability of more robust direct evidence sourced from RCTs. However, there remains a number of methodological and evidence issues. The most notable of these relate to differences in the frequency of FeNO monitoring between the trials, uncertainty regarding the longer-term benefits of FeNO monitoring over standard care, differences between studies in terms of the step-up/step-down treatment protocols used and associated issues relating to the generalisability of non-UK treatment guidelines and symptom management strategies to UK clinical practice. The economic analysis of FeNO monitoring addresses the following question:
-
What is the cost-effectiveness of FeNO monitoring compared with standard guidelines in the management of asthma?
The External Assessment Group asthma diagnostic model
Logic underpinning the diagnostic model structure
The EAG diagnostic model hinges on the expected costs and health losses associated with the misdiagnosis of asthma. If a patient has been misdiagnosed, their treatment will not be clinically optimal until their misdiagnosis has been corrected. Misdiagnosis has different implications for those patients who are FP and for those patients who are FN. For patients who are FP, suboptimal treatment means receiving treatment with asthma medication that will provide no health benefits to them (because they do not have the underlying disease). This means there is an additional cost to the NHS without additional health benefits for the patients. Furthermore, a proportion of patients with a FP diagnosis of asthma may have other more serious pathology that goes undetected (e.g. cancer or tuberculosis) because of an incorrect diagnosis of asthma. Conversely, for patients who are FN, suboptimal treatment means not receiving treatment with asthma medication when in reality they would have benefited from the treatment. Until this misdiagnosis is corrected, patients may suffer from poor asthma control and hence lower HRQoL because of asthma symptoms without adequate treatment. Poor asthma control can impact on a patient’s HRQoL during times without exacerbation and can also increase the duration of exacerbations. Clinically significant exacerbations are costly to the NHS and in the case of exacerbations requiring hospitalisation these costs may be substantial; hence, a patient with undiagnosed asthma may on balance be more costly to the NHS than a patient who is correctly treated for asthma. These patients may also go on to receive expensive and unnecessary tests such as imaging and referrals to specialists until their misdiagnosis is corrected.
An incorrect FN diagnosis may be corrected later following an asthma exacerbation, as a result of continued asthma-related symptoms that trigger subsequent appointments and investigations or even because of reconsideration of asthma after tests for other conditions produce negative findings. Similarly, an incorrect FP diagnosis may be corrected later as a result of the continued non-occurrence of exacerbations, a generally high level of HRQoL at very low treatment dosages, thus indicating that medications currently being taken by the patient may be unnecessary, or continued deterioration as a result of other more serious underlying pathology. Although it should be expected that the aggregate health consequences resulting from correct decisions should be better than those resulting from incorrect decisions, the implications for HRQoL and the costs of FP and FN diagnostic outcomes are not identical. Because of this, a diagnostic strategy that maximises the AUC on a receiver operating curve may not necessarily yield the most cost-effective strategy. The EAG diagnostic model is therefore intended to reflect the implications of test sensitivity and specificity for subsequent costs and health consequences for the full range of diagnostic options within the available evidence base.
Model structure and assumptions
Figure 25 presents the structure of the EAG diagnostic model. The model is implemented as a simple decision tree. The population under consideration may or may not have true underlying asthma (denoted θ in Figure 25). The model then uses estimates of sensitivity and specificity associated with each diagnostic test, or combination of tests, to estimate the expected probability that a patient will be diagnosed as having asthma or as not having asthma. Therefore, the model estimates the probability that a patient with asthma will be correctly or incorrectly diagnosed as TP or FN, respectively, and the probability that a patient without asthma will be correctly or incorrectly diagnosed as TN or FP respectively. The model makes the simplifying assumption that incorrect diagnoses (FNs and FPs) are resolved by subsequent tests after some period of time (see Evidence used to inform the External Assessment Group diagnostic and management model parameters). Unnecessary treatment costs and health losses resulting from misdiagnosis are explicitly captured in the model.
FIGURE 25.
Conceptual form of the EAG diagnostic model structure.
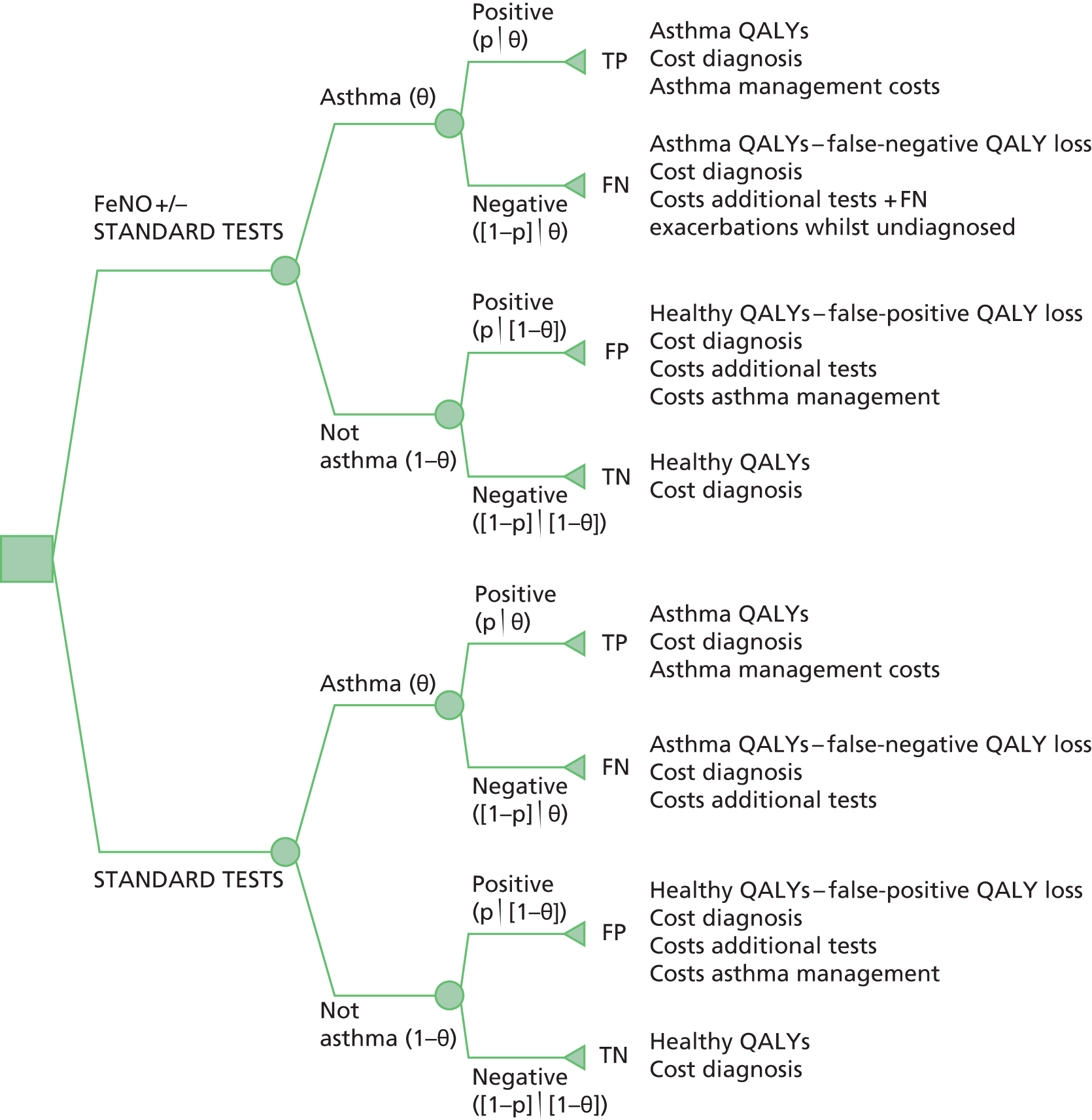
The diagnostic model estimates costs and health outcomes for each diagnostic option across four groups:
-
Patients who are TP (test sensitivity × prevalence) are assumed to require the initial diagnostic test(s) with no subsequent tests and are assumed to have their asthma controlled using ICSs plus LABAs.
-
Patients who are TN [test specificity × (1 – prevalence)] are assumed to incur the cost of the initial test(s) with no subsequent tests and are assumed to have a normal (general population) health status for the remainder of the model time horizon.
-
Patients who are FP [(1 – test sensitivity) × (1 – prevalence)] are assumed to incur the cost of the initial test(s) plus the costs of subsequent tests to correct their initial misdiagnosis. These patients are assumed to incur a reduction in health status and the costs of ICSs and LABAs until their misdiagnosis is corrected.
-
Patients who are FN [(1 – test sensitivity) × prevalence] are assumed to incur the cost of the initial test(s) plus the costs of subsequent tests to correct their initial misdiagnosis. These patients are assumed to lose health because of poor control until their asthma is correctly diagnosed. These patients are assumed to incur asthma management costs after their asthma is diagnosed for the remainder of the model time horizon. These patients also accrue costs associated with an increased rate of exacerbations until their misdiagnosis is corrected.
The diagnostic model makes the following key structural assumptions:
-
All misdiagnoses are eventually corrected within the patient’s lifetime. This assumption will bias against those options with greater diagnostic accuracy. The time to correct a FP diagnosis may be different from the time to correct a FN diagnosis.
-
The model time horizon for the analysis is set at 5 years. This exceeds the maximum time to correct a misdiagnosis in the base-case analysis (see Evidence used to inform the External Assessment Group diagnostic and management model parameters). In effect, this reflects a lifetime horizon because of the assumption that all misdiagnoses are corrected. Health benefits gained and costs accrued after the resolution of incorrect diagnoses will be the same between all competing diagnostic decision options.
-
FNs at initial diagnosis experience the same level of HRQoL after their misdiagnosis is corrected as patients who are initially correctly diagnosed as TPs.
-
FPs incur health losses until their misdiagnosis is corrected.
-
The health consequences of other serious conditions that may be mistaken for the symptoms of asthma (e.g. lung cancer, tuberculosis, COPD) are not reflected in the model.
-
Patients who are FN may experience an increased rate of exacerbations (compared with TPs) whilst their asthma remains uncontrolled.
-
Improved diagnostic accuracy has no impact on mortality.
-
All FeNO tests (NIOX MINO, NIOX VERO and NObreath) are assumed to have equivalent diagnostic accuracy.
-
FeNO, spirometry and reversibility testing can be undertaken in primary care. Airway hyper-responsiveness testing (MCT) and sputum induction are undertaken in secondary care.
-
Tests undertaken in primary care will involve two GP consultations and a nurse visit. Tests undertaken in secondary care will involve two attendances and a laboratory visit as well as a primary care visit for referral.
-
One additional primary care visit, one laboratory visit and two additional secondary care visits are required to achieve resolution of an incorrect diagnosis.
-
Because of a lack of evidence relating to the diagnostic accuracy of each test at each point in the pathway by patient age group, the model structure is ‘blunt’ in that differences between the diagnostic pathways for children and adults are not reflected.
Some of these assumptions are fairly strong and lack evidence to substantiate them. They are, however, relevant elements of the decision problem and thus require quantification. The impact of these assumptions is tested extensively in the sensitivity analysis (see De novo model results).
Table 63 summarises the calculations underpinning the expected costs and QALY gains associated with each terminal node within the model.
| Diagnostic test outcome | Expected cost | Expected QALY gain |
|---|---|---|
| TP | Diagnostic test costs + (time horizon × cost asthma management) | Time horizon × utility_asthma |
| FP | Diagnostic test costs + additional tests + (time to correct FP diagnosis × costs of asthma management) | [(Timehorizon – time to correct FP diagnosis) × utility_healthy] + [time to correct FP diagnosis × (utility_ healthy–disutility_asthma)] |
| TN | Diagnostic test costs | Time horizon × utility_healthy |
| FN | Diagnostic test costs + additional tests + (time to correct FN diagnosis × cost of increased severe exacerbations) + [(time horizon – time to correct FN diagnosis) × (costs of asthma management)] | [(Time horizon – time to correct FN diagnosis) × (utility_asthma)] + (time to correct FN diagnosis × disutility poor control) |
Scope of the External Assessment Group diagnostic model analysis
The model is intended to reflect a population of patients with symptoms of asthma as seen in primary and secondary care in England and Wales. Table 64 details the test options included in the EAG diagnostic model analysis and the setting in which these tests are assumed to be undertaken.
| Test(s) | Setting |
|---|---|
| FeNO > 25 ppb (using NIOX MINO, NIOX VERO or NObreath) | Primary care |
| FeNO 34 ppb (using NIOX MINO, NIOX VERO or NObreath) + FEV1 | Primary care |
| FeNO 19 ppb (using NIOX MINO, NIOX VERO or NObreath) + sputum induction | Secondary care |
| FeNO > 27 ppb (using NIOX MINO, NIOX VERO or NObreath) + bronchodilator reversibility | Primary care |
| FEV1/FVC | Primary care |
| PEF monitoring | Primary care |
| Bronchodilator reversibility | Primary care |
| Airway hyper-responsiveness (MCT) | Secondary care |
| Sputum induction | Secondary care |
All options are compared within a full incremental analysis. In line with the NICE reference case for diagnostic interventions,145 all costs and health outcomes are discounted at a rate of 3.5%. All costs are valued at 2012/13 prices. No subgroup analyses were conducted because of evidence limitations (a narrative review of subgroup analyses within the FeNO studies is presented in Chapter 3). The base-case analyses are drawn from the results of the probabilistic model and hence reflect the expectation of the mean. Further sensitivity analyses were undertaken deterministically using point estimates of parameters. PSA was used to generate information on the likelihood that each test is expected to produce the greatest net benefit over a range of willingness-to-pay thresholds.
It should be noted that originally the model included an additional combination of diagnostic options – FeNO plus FEV1 plus bronchodilator reversibility – based on the study reported by Fortuna et al. 70 However, as Fortuna et al. 70 reported that diagnostic accuracy was not improved compared with FeNO alone, incorporating this option into the model would result in a situation whereby it has the same modelled effectiveness and same modelled cost as FeNO testing alone. In reality, the use of spirometry, reversibility testing and FeNO testing would result in a small additional cost associated with consumables and/or minor drug costs compared with FeNO testing alone. Consequently, given the assumption of equivalence with FeNO testing alone and the expectation that test costs would be marginally higher than for FeNO testing alone, FeNO plus FEV1 plus bronchodilator reversibility would always be dominated. Hence, this option was excluded from the final economic analysis.
The External Assessment Group asthma management model
Logic underpinning the management model structure
The EAG asthma management model is principally concerned with the potential benefits associated with using FeNO monitoring to enable better disease control in patients who have been diagnosed with asthma. Patients with diagnosed asthma may receive ICSs, LABAs and other pharmacological treatments to maintain control of symptoms, minimise the impact of the disease on HRQoL and reduce the risk of serious complications of asthma. 8 Treatment in the UK follows a stepped approach, with escalation of medication until control is reached. The incidence of exacerbations generally indicates poor asthma control; these exacerbations also impact on patient’s HRQoL and may be expensive to manage. Monitoring of FeNO levels may provide information to allow for the better control of asthma, thereby resulting in a reduction in unnecessary medication use in patients who do not require such treatment, the maintenance of medication levels when appropriate and an increase in medication use in patients with poor disease control to avoid the health losses and costs associated with exacerbations.
Model structure
Figure 26 presents the structure of the de novo EAG management model. The model adopts a simple Markov framework with two states: (1) alive with diagnosed asthma and (2) dead. The model assumes that differences in HRQoL between treatment groups in the alive state are driven by the incidence of exacerbations whereas cost differences are influenced by the exacerbation rate and the mean level of medication use in each treatment group. Each exacerbation is associated with a reduction in HRQoL and a cost of management. Exacerbations that require hospitalisation are assumed to have a greater impact on HRQoL losses and are assumed to be more expensive to treat than other less severe exacerbations. Within each treatment group, the rate of exacerbations is modelled together with an estimate of required medication over time.
FIGURE 26.
Conceptual form of the EAG asthma management model.

The management model makes the following key structural assumptions:
-
Short-term impacts on exacerbations and medication use observed in the empirical studies associated with FeNO monitoring are assumed to be maintained in the longer term (indefinitely in the base case). Given the clinical evidence used to inform the analysis, this is a strong assumption that will favour FeNO.
-
Impacts of FeNO monitoring on costs and health outcomes occur only during the period in which FeNO monitoring is used (this applies only to the sensitivity analysis).
-
Exacerbations are associated with a short-term reduction in HRQoL.
-
The use of FeNO monitoring leads to impacts on exacerbations.
-
A proportion of severe exacerbations may require hospitalisation whereas the remainder may be managed in a primary care setting. Other less severe exacerbations may be managed at home.
-
Improved asthma management has no impact on mortality.
-
All FeNO devices (NIOX MINO, NIOX VERO and NObreath) are assumed to have an equivalent impact on dose titration decisions in the management setting.
Scope of the External Assessment Group management model analysis
The management model analysis compares the incremental costs of four options: FeNO monitoring using (1) NIOX MINO, (2) NIOX VERO and (3) NObreath against (4) standard guidelines in (a) children and (b) adults. It should be noted that each FeNO option also includes the use of guidelines, as determined by the clinical evidence used to inform the exacerbation rate and ICS use parameters. The starting age for the child subgroup is assumed to be 5 years whereas the starting age for the adult subgroup is assumed to be 18 years. The adult subgroup analysis also includes a separate subgroup analysis of FeNO monitoring in women who are pregnant. No further subgroup analyses were undertaken. The model adopts a lifetime horizon, all costs and health outcomes are discounted at 3.5% and all costs are valued at 2012/13 prices. The base-case analyses are drawn from the results of the probabilistic model and hence reflect the expectation of the mean. Further sensitivity analyses were undertaken deterministically using point estimates of parameters. PSA was used to generate information on the likelihood that each option is expected to produce the greatest net benefit over a range of willingness-to-pay thresholds.
Evidence used to inform the External Assessment Group diagnostic and management model parameters
Table 65 presents the parameter values, distributions and evidence sources used to inform the two models. These are described in more detail below.
| Parameter | Distributiona | Mean | Param1 | Param2 | Source |
|---|---|---|---|---|---|
| Diagnostic model parameters | |||||
| Diagnostic accuracy | |||||
| FeNO – sensitivity | Beta | 0.32 | 24.00 | 51.00 | Schneider et al.71,72 |
| FeNO – specificity | Beta | 0.93 | 79.00 | 6.00 | |
| FeNO + FEV1 – sensitivity | Beta | 0.24 | 20.00 | 62.00 | Schleich et al.77 |
| FeNO + FEV1 – specificity | Beta | 0.99 | 91.00 | 1.00 | |
| FeNO + sputum induction – sensitivity | Beta | 0.87 | 98.31 | 14.69 | Sivan et al.95 |
| FeNO + sputum induction – specificity | Beta | 0.86 | 24.03 | 4.07 | |
| FeNO + bronchodilator reversibility – sensitivity | Beta | 0.87 | 36.54 | 5.46 | Cordeiro et al.87 |
| FeNO + bronchodilator reversibility – specificity | Beta | 0.90 | 64.80 | 7.20 | |
| FEV1/FVC – sensitivity | Beta | 0.61 | 41.54 | 26.56 | Hunter et al.163 |
| FEV1/FVC – specificity | Beta | 0.60 | 11.37 | 7.58 | |
| PEF – sensitivity | Beta | 0.43 | 29.15 | 38.64 | |
| PEF – specificity | Beta | 0.75 | 14.21 | 4.74 | |
| Bronchodilator reversibility – sensitivity | Beta | 0.49 | 33.29 | 34.65 | |
| Bronchodilator reversibility – specificity | Beta | 0.70 | 13.28 | 5.69 | |
| Airway hyper-responsiveness (MCT) – sensitivity | Beta | 0.91 | 61.01 | 6.03 | |
| Airway hyper-responsiveness (MCT) – specificity | Beta | 0.90 | 22.43 | 2.49 | |
| Sputum induction – sensitivity | Beta | 0.72 | 48.91 | 19.02 | |
| Sputum induction – specificity | Beta | 0.80 | 15.26 | 3.81 | |
| Disease and population parameters | |||||
| Prevalence of true asthma | Beta | 0.47 | 412.00 | 469.00 | Schleich et al.,77 Sivan et al.,95 Fortuna et al.,70 Cordeiro et al.,87 Schneider et al.71,72 |
| Probability patient is male (children) | NA | 0.55 | – | – | Sivan et al.95 |
| Probability patient is male (adults) | NA | 0.40 | – | – | Schneider et al.69 |
| Patient age (years) at diagnosis (children) | NA | 5 | – | – | Assumption to reflect decision problem |
| Patient age (years) at diagnosis (adults) | NA | 18 | – | – | |
| Resource cost parameters | |||||
| NIOX MINO – marginal per-test cost | NA | £7.07 | – | – | Based on information provided by Bedfont Scientific and Aerocrine |
| NIOX VERO – marginal per-test cost | NA | £6.36 | – | – | |
| NObreath – marginal per-test cost | NA | £4.82 | – | – | |
| Primary care GP visit | Normal | £43.00 | £43.00 | £4.30b | Curtis179 |
| Primary care practice nurse visit | Normal | £13.69 | £13.69 | £1.39b | |
| Secondary care respiratory medicine outpatient visit | Normal | £204.29 | £204.29 | £30.64 | NHS Reference Costs174 |
| Secondary care laboratory visit | Normal | £203.29 | £203.29 | £30.49 | NHS Reference Costs174 |
| Number of additional primary care tests – FP | NA | 1.00 | – | – | Structural assumptions based on expert opinion |
| Number of additional secondary care tests – FP | NA | 2.00 | – | – | |
| Number of additional laboratory visits – FP | NA | 1.00 | – | – | |
| Number of additional primary care tests – FN | NA | 1.00 | – | – | |
| Number of additional secondary care tests – FN | NA | 2.00 | – | – | |
| Number of additional laboratory visits – FN | NA | 1.00 | – | – | |
| Annual rate of additional exacerbations in uncontrolled FNs | Normal | 1.02 | 1.02 | 0.10b | Assumption based on Jayaram et al.169 |
| Annual asthma drug management costs (children) | Normal | £201.00 | £10.00 | – | Main et al.180 |
| Annual asthma drug management costs (adults) | Normal | £231.00 | £10.00 | Shepherd et al.13 | |
| QALY gain/loss parameters | |||||
| Time (years) until correct diagnosis – FP | Normal | 1.50 | 1.50 | 0.26 | Expert opinion |
| Disutility FP | Assumed to be equal to asthma disutility (see below) | ||||
| Time (years) until correct diagnosis – FN | Normal | 0.67 | 0.67 | 0.17 | Expert opinion |
| Disutility poor asthma control | Beta | 0.04 | 1.39 | 33.35 | McTaggart-Cowan et al.181 |
| Disutility asthma | Beta | 0.05 | 49.92 | 1027.40 | Sullivan et al.182 |
| HRQoL non-asthma population | Multivariate normal | 0.96 | – | – | Ara and Brazier183 |
| Management model parameters | |||||
| Exacerbation rate parameters | |||||
| Duration (years) of FeNO monitoring benefit | NA | Lifetime | – | – | Assumption |
| FeNO annual exacerbation rate (children) | Log-normal | 0.36 | 0.36 | 0.00 | Szefler et al.104 |
| FeNO annual exacerbation rate (adults) | Log-normal | 0.33 | 0.33 | 0.09 | Shaw et al.98 |
| Guidelines annual exacerbation rate (children) | Log-normal | 0.47 | 0.47 | 0.00 | Szefler et al.104 |
| Guidelines annual exacerbation rate (adults) | Log-normal | 0.42 | 0.42 | 0.10 | Shaw et al.98 |
| HRQoL parameters | |||||
| Disutility severe hospitalised exacerbationc | Beta | 0.56 | 1.21 | 3.84 | Lloyd et al.184 |
| Disutility severe non-hospitalised exacerbation | Beta | 0.32 | 12.06 | 25.62 | |
| Duration (years) severe hospitalised exacerbation | Gamma | 0.08 | 15.62 | 0.00 | Expert opinion |
| Duration (years) severe non-hospitalised exacerbation | Gamma | 0.01 | 12.23 | 0.00 | |
| Resource cost parameters | |||||
| Additional FeNO monitoring visits year 1 | NA | 4 | – | – | Assumption based on BTS/SIGN8 recommendations |
| Additional FeNO monitoring visits subsequent years | NA | 4 | – | – | |
| RDI ICS use year 1 FeNO (children) | Normal | 0.98 | 0.98 | 0.05b | Szefler et al.104 |
| RDI ICS use years 2+ FeNO (children) | Normal | 0.97 | 0.97 | 0.05b | |
| RDI ICS use year 1 guidelines (children) | Normal | 0.87 | 0.87 | 0.05b | |
| RDI ICS use years 2+ guidelines (children) | Normal | 0.78 | 0.78 | 0.05b | |
| RDI ICS use year 1 FeNO (adults) | Normal | 1.20 | 1.20 | 0.05b | Shaw et al.98 |
| RDI ICS use years 2+ FeNO (adults) | Normal | 0.77 | 0.77 | 0.05b | |
| RDI ICS use year 1 guidelines (adults) | Normal | 1.06 | 1.06 | 0.05b | |
| RDI ICS use years 2+ guidelines (adults) | Normal | 1.27 | 1.27 | 0.05b | |
| Cost severe non-hospitalised exacerbation | Normal | £44.73 | £44.73 | – | Curtis,179 BNF185 |
| Cost severe hospitalised exacerbation | Normal | £1267 | £1267 | £253.34 | NHS Reference Costs174 |
Diagnostic test accuracy
Estimates of test accuracy for diagnostic tests were drawn from a number of separate studies70–72,77,87,95,163 based on the results of the systematic review (see Chapter 3). Tables 66 and 67 summarise the sources from which these estimates were derived and the actual values selected. As far as the evidence allows, the economic analysis included studies that presented estimates of the sensitivity and specificity of individual tests as well as combinations of FeNO plus other standard tests.
| Author, year | Study design | Population | Setting | Reference standard | Age range |
|---|---|---|---|---|---|
| Cordeiro 201187 | Retrospective (analysis of prospective database) | 114 patients referred to a general outpatient allergy clinic | Secondary care (the Netherlands) | History of typical respiratory symptoms and FEV1% improvement of > 12% and > 200 ml or a provocative concentration of histamine causing a > 20% decrease in FEV1% (PC20) of ≤ 8 mg/ml, according to GINA guidelines112 | Included those aged 7–83 years |
| Hunter 2002163 | Cross-sectional case–control study | 69 asthma patients, 20 pseudoasthma patients and 21 healthy subjects | Secondary care – single centre (UK) | Subjects with asthma had consistent clinical features, were symptomatic at the time of the investigations, had FEV1 predicted values of > 65% and had one or more of the following conditions: a provocative concentration of a substance (methacholine) causing a > 20% fall in FEV1 (PC20) of < 8 mg/ml; a > 15% increase in FEV1 10 minutes after receiving 200 µg of inhaled salbutamol; or a > 20% maximum within-day variability of PEF when measured twice daily for > 14 days | Mean age 44 years, range 15–70 years in asthma group |
| Schleich 201277 | Prospective cohort study | 174 steroid-naive patients with respiratory symptoms | Secondary care (Belgium) | Asthma was diagnosed based on airway hyper-responsiveness demonstrated by inhaled concentration of methacholine provoking a 20% fall in FEV1 (PC20) of < 16 mg/ml | Range 20–59 years |
| Sivan 200995 | Prospective, consecutive patients | 150 consecutive children referred for evaluation of possible asthma | Secondary care (Israel) | Patient’s history of two or more clinical exacerbations of wheezing documented by a physician, dyspnoea or cough relieved by bronchodilators, documented variability in FEV1 of ≥ 15% in response to bronchodilators at any time during the follow-up period (reversibility) or documented variability in FEV1 of ≥ 15% over time with or without controller medications (ICS or montelukast). The results of provocation tests were included when available. Children in whom asthma did not manifest within 18 months of follow-up were considered as not having asthma | Range 5–18 years |
| aSmith 200486 | Prospective cohort study | 47 consecutive patients referred by their GP to a pulmonary function laboratory for investigation of possible asthma | Secondary care (New Zealand) | Diagnosis of asthma made on the basis of the following: relevant symptom history (present in all patients) using the ATS criteria113 and a positive test for bronchial hyper-responsiveness and/or a positive response to a bronchodilator | Range 8–75 years |
| Schneider 200971,72 | Prospective, consecutive cohort study | 393 adults with symptoms suggestive of asthma | Private practice run by five pneumologists (Germany) | FEV1/FVC < 0.7% or FEV1% < 80% plus positive bronchodilator response = asthma. FEV1/FVC > 0.7% or FEV1% > 80% plus positive MCT = asthma | Unclear (adults) |
| Author, year | Test(s) | FeNO cut-off (ppb) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Cordeiro 201187 | FeNO + bronchodilator reversibility | > 27 | 87 | 90 |
| Hunter 2002163 | FEV1/FVC | NA | 61 | 60 |
| PEF | NA | 43 | 75 | |
| Bronchial reversibility | NA | 49 | 70 | |
| Airway hyper-responsiveness (MCT) | NA | 91 | 90 | |
| Sputum induction | NA | 72 | 80 | |
| Schleich 201277 | FeNO + FEV1 | > 34 | 24 | 99 |
| Schneider 200971,72 | FeNO | > 46 | 32 | 93 |
| Sivan 200995 | FeNO + sputum induction | > 19 | 87 | 89 |
The study reported by Schneider et al. 71,72 was used to inform estimates of the sensitivity and specificity of FeNO alone; this study was selected because of its broad population and high study quality and because the reference standard broadly reflects the BTS/SIGN guidelines. 8 This study used the NIOX MINO device. The study reported sensitivity and specificity across a range of cut-offs. The cut-off of > 46 ppb had the highest sum of sensitivity and specificity (32% and 93% respectively); hence, this estimate was used in the model. Additional diagnostic interventions involving FeNO plus other standard tests were included according to their availability;70,77,87,95 the FeNO cut-off values used from these studies were driven by the availability of reported estimates and were not based on choices made by the EAG. As noted earlier, the combination of FeNO + FEV1 bronchodilator reversibility was excluded from the final model because, based on data from Fortuna et al. 70 and the model costing assumptions, it will always be dominated by FeNO alone. Estimates of the operating characteristics of other standard tests for asthma diagnosis were drawn from Hunter et al. 163 This is consistent with the manufacturer’s economic analysis, although it should be noted that this study may introduce bias through the use of a case–control design. Sensitivity analyses were undertaken to examine whether the use of alternative estimates86 of the sensitivity and specificity of existing tests alters the cost-effectiveness of FeNO testing (see De novo model results).
Across all diagnostic options, test operating characteristics were derived directly from data reported in the study publications. Uncertainty surrounding sensitivity and specificity estimates was modelled using independent beta distributions based on patient numbers reported in the studies.
Because of the limitations in the evidence base, the model necessarily makes a number of unadjusted (naive) indirect comparisons between the included studies. As a consequence, the results of the health economic analysis may be subject to bias and confounding because of differences between studies in terms of study design, recruited populations and reference standards. This same limitation is evident in the Price et al. 147/Aerocrine diagnostic models and is unavoidable given the available evidence base. As the included studies did not provide sufficient information that would allow us to meaningfully discriminate between the sensitivity and specificity of all tests across population subgroups, we assumed that test operating characteristics were common to all patient populations. This assumption may not hold in reality.
Disease and population parameters
The true pre-test probability of asthma in undiagnosed patients was estimated as a weighted mean of the number of cases of asthma and non-asthma diagnosed in the studies used to inform the diagnostic test accuracy parameters. 69,70,77,86,87,95 We did not include the Hunter et al. 163 study in this calculation as it did not recruit consecutive patients because of its study design. Across these studies, 412 of 881 patients were diagnosed with asthma (probability = 0.47).
We estimated the probability that a patient is male using two studies. 69,95 This value is used only to estimate baseline HRQoL without asthma and thus does not impact on the model results.
Non-asthma utility
Preference-based HRQoL values for patients without asthma were estimated using a general population EQ-5D regression model reported by Ara and Brazier183 (modelled EQ-5D = 0.9508566 + 0.0212126 × male – 0.0002587 × age – 0.0000332 × age2). Uncertainty surrounding this regression equation was modelled using a multivariate normal distribution. As this parameter is common to all diagnostic comparator groups, it has no effect on the estimates of incremental health gain for the diagnostic tests included in the economic analysis.
Disutility associated with asthma
The disutility associated with asthma was taken from the catalogue of EQ-5D values reported by Sullivan et al. 182 Within this study, community-based UK preferences were applied to EQ-5D descriptive questionnaire responses in the US-based Medical Expenditure Panel Survey (MEPS). Sullivan et al. 182 used regression models to estimate the marginal disutility associated with a variety of diseases and conditions, assuming an additive model. Based on these models, the disutility for asthma was estimated to be –0.0463. Uncertainty surrounding this parameter was modelled using a beta distribution using bootstrapped confidence intervals provided in the supplementary appendices to the paper [available from http://mdm.sagepub.com/content/31/6/800/suppl/DC1 (accessed 1 August 2013)].
This disutility is applied indefinitely to all patients with asthma and to patients without asthma who test FP until their misdiagnosis is corrected. It should be noted that this disutility is unlikely to fully reflect health losses associated with the delayed diagnosis of more serious pathology such as cancer or tuberculosis.
Disutility associated with poor asthma control
The impact of poor asthma control on HRQoL was informed by a recent systematic review of studies that reported the use of the EQ-5D in patients with asthma. 186 Within this review, two studies were identified that reported the impact of loss of control on patients’ health status. 172,181 Within the study reported by Szende et al. ,172 228 consecutive adult outpatients and inpatients at four sites in Hungary completed the EQ-5D, the Short Form questionnaire-36 items (SF-36), the St George’s Respiratory Questionnaire and a direct time trade-off question. The patients’ level of asthma control was determined by physicians. EQ-5D estimates are reported for four health states: ‘good control’, ‘mildly reduced control, ‘moderately reduced control’ and ‘poor control’. EQ-5D estimates ranged from 0.93 for ‘good control’ to 0.52 for ‘poor control’. Within the study reported by McTaggart-Cowan et al. ,181 157 asthma patients completed the Health Utilities Index Mark 3 (HUI-3), the EQ-5D and the Short Form questionnaire-6 Dimensions (SF-6D). The degree of asthma control was self-reported by patients. The authors reported EQ-5D values for four health states: ‘very well controlled’, ‘well controlled’, ‘adequately controlled’ and ‘not controlled’. EQ-5D estimates ranged from 0.90 for ‘very well controlled’ to 0.80 for ‘not controlled’. The impact of loss of control is markedly different between these two studies. As Szende et al. 172 recruited inpatients and outpatients, it is very likely that a number of study subjects were identified because they were experiencing an exacerbation at the time at which they completed the questionnaire; this may overestimate their valuations of HRQoL. For this reason we derived disutilities from the study by McTaggart-Cowan et al. 181 We assumed that the health loss associated with poor control because of a FN diagnosis relates to the difference between the ‘well controlled’ state and the ‘not controlled’ state (mean disutility –0.04). Uncertainty surrounding this parameter was modelled using a beta distribution based on the mean difference between the two health states; this method ensures that the notionally better health state always has a monotonically better valuation than that for the notionally worse health state.
This disutility is applied to all patients with asthma who test FN until their misdiagnosis is corrected.
Time to resolution of incorrect diagnoses
There is a dearth of empirical evidence relating to the time required to resolve incorrect diagnoses (FPs and FNs); indeed, such studies would be difficult, if not impossible, to undertake prospectively. However, the time to resolve incorrect diagnoses is of direct relevance to the decision problem and must be quantified to evaluate the cost-effectiveness of alternative diagnostic options for asthma. Given the lack of empirical evidence relating to these parameters, we attempted to elicit these quantities from clinical experts. We asked six clinical experts (see Acknowledgements) the following questions:
-
For someone who has been incorrectly diagnosed as ‘not asthmatic’, how long on average do you think it will take for this incorrect diagnosis to be corrected? What is your 95% confidence interval around this average?
-
For someone who has been incorrectly diagnosed as ‘asthmatic’, how long on average do you think it will take for this incorrect diagnosis to be corrected? What is your 95% confidence interval around this average?
A total of four experts provided responses. One expert suggested, with considerable uncertainty, that the time to resolve a FN diagnosis may be in the region of 4–12 months whereas the time to resolve a FP diagnosis may be in the region of 12 months or longer. This expert indicated substantial uncertainty around these estimates.
The second expert stated that, for FNs, the time to correct a misdiagnosis will:
mainly depend on chronicity and persistence of asthma: (a) In those with chronic persistent asthma (BTS step 2 or higher); [the] mean will only be a few weeks with relatively tight c.i., as the patient will presumably not be given treatment, will become symptomatic and demands further investigations/treatment where the true diagnosis will be revealed by other methods i.e. lung function etc. (b) In those with mild intermittent/infection induced exacerbation, it may take much longer (mean [may be] months or even year or two with [a] wide CI) as they may not get regular symptoms so the diagnosis (no asthma) may seem correct until they are exposed to the trigger and become symptomatic or get an exacerbation.
With respect to FPs, the second expert stated that:
this is even more difficult to estimate but here the means and c.i. may be in years. With an incorrect diagnosis of asthma, patients are put on treatment and they may become asymptomatic (for other reasons e.g. placebo effect) and it is presumed that they are better because of treatment and hence continued on it. There is a reluctance to reduce treatment if patient[s] are doing well. This was one of the argument of using eNO (to monitor, not to diagnose), that by titrating asthma treatment with eNO you can manage airway inflammation better with lower doses of inhaled steroids.
The third expert stated that these questions were ‘impossible questions to answer’ but indicated that ‘misdiagnosis may never be corrected [for] both false-positive and false-negative’. In addition, the third expert stated that ‘patients may make the decision themselves and just stop going back to the doctor’ and that ‘asthmatic symptoms may come and go’. This expert also stated that a patient who has had asthmatic symptoms and who becomes asymptomatic might be considered an asymptomatic asthmatic or may be said to have had an incorrect diagnosis of asthma by someone who sees them when well. The expert also stated that these problems are the result of the absence of a reliable diagnostic test for asthma.
The fourth expert simply stated that these quantities are ‘unknowable’ but did suggest that the values quantified by the first expert were not unreasonable.
The fifth and sixth experts were not able to provide quantitative estimates.
Based on these responses, we assumed that the time to resolve a FN diagnosis has a mean of 8 months with a 95% CI of 4–12 months. We also assumed that the time to resolve a FP diagnosis has a mean of 18 months with a 95% CI of 12–24 months. Uncertainty surrounding these estimates was modelled using normal distributions. These estimates should be considered to be highly uncertain and are tested extensively in the sensitivity analysis.
Resource costs
Test costs
Calculating the likely marginal per-test cost for NIOX MINO, NIOX VERO and NObreath is somewhat complicated as the devices each have different lifetimes and test kits for each device are available at lower marginal costs if higher volumes of kits are purchased. The lifetimes of the NIOX MINO and NIOX VERO devices are determined either by time or by the number of tests undertaken (whichever limit is reached first).
The NIOX MINO device (Aerocrine) has a unit cost of £2100 and an effective unit lifetime of 3 years or 3000 tests (whichever comes first). The NIOX VERO device (Aerocrine) has a unit cost of £2310 and an effective unit lifetime of 5 years or 5000 tests (whichever comes first). The NObreath device (Bedfont Scientific) costs £1995 and, according to the manufacturer, has an unlimited unit lifetime.
Maintenance for NObreath is provided free of charge. No maintenance is required for NIOX MINO or NIOX VERO.
Test kits for NIOX MINO are available in packs of 300 at a price of £1350, in packs of 500 at a price of £2100 or in packs of 1000 at a price of £3950. Test kits for NIOX VERO are available in packs of 300 at a price of £1500, in packs of 500 at a price of £2200 or in packs of 1000 at a price of £4200. Mouthpieces for NObreath are available in packs of 50, 100, 300 or 1000 at prices of £195, £365, £995 and £2995 respectively.
The NObreath device requires replacement of the sensor unit every 2 years at a cost of £295. Besides test kits, NIOX MINO and NIOX VERO do not require any further consumables or incur any replacement costs.
Based on information provided by Bedfont Scientific and Aerocrine, Table 68 presents the estimated annuatised marginal per-test costs assuming a usage of 300 tests per device per year (this estimate is based on estimates of mean usage provided by Aerocrine). All calculations are based on the lifetime of the specific device and the lowest cost estimates for the required number of test kits at the assumed level of throughput and lifetime of the device. We assumed that, although the NObreath device has an unlimited life, advances in technology would lead to replacement of the device within 10 years. Annuatisation was undertaken assuming a rate of 3.5%.
| Item | NIOX MINO (£) | NIOX VERO (£) | NObreath (£) |
|---|---|---|---|
| Lifetime (years) | 3 | 5 | 10 |
| Total tests assumed per year | 300 | 300 | 300 |
| Equipment | 2100.00 | 2310.00 | 1995.00 |
| Test kits: 1000 mouthpieces | 3950.00 | 4200.00 | 2995.00 |
| Test kits: 500 mouthpieces | 2100.00 | 2200.00 | NA |
| Test kits: 300 mouthpieces | 1350.00 | 1500.00 | 995.00 |
| Test kits: 100 mouthpieces | NA | NA | 365.00 |
| Sensor replacements | NA | NA | 295.00 |
| Total cost over device lifetime | 6150.00 | 8910.00 | 12,455.00 |
| Annuatisation factor for specific device lifetime | 2.90 | 4.67 | 8.61 |
| Annuatised marginal per-test cost | 7.07 | 6.36 | 4.82 |
It should be noted that these marginal per-test costs do not include any costs associated with the education and training that may be required to teach NHS staff how to instruct patients to use the devices correctly to minimise test failure rates (see Chapter 7).
We assumed that spirometry, reversibility testing and FeNO testing can be carried out in primary care and would require two GP visits and one nurse visit. We assumed that sputum induction and airway hyper-responsiveness testing (MCT) would be undertaken in secondary care and would require two secondary care visits and one laboratory visit as well as an initial GP visit for referral (Dr John White, 17 July 2013, personal communication).
The unit cost of a GP visit was taken from Curtis et al. ;179 the economic analyses use an estimate of £43, which reflects the cost of an appointment lasting 11.7 minutes including direct staff costs and qualifications. Based on the same source179 the cost of a GP practice nurse visit was assumed to be £13.69, assuming a visit duration of 15.5 minutes. The secondary care attendance cost was based on the Healthcare Resource Group code for respiratory medicine attendances (cost £204.29). 174 The cost of a laboratory visit was based on the Healthcare Resource Group code for simple bronchodilator studies (cost £203.29). We assumed that SEs around these estimates were normally distributed, with a SE equal to 15% of the mean.
As HRGs are calculated using full economic costing, we assumed that all visit costs include the costs associated with capital, training, staff costs and procedure costs associated with all existing diagnostic tests for asthma. For the strategies that include FeNO testing, the marginal per-test cost of FeNO measurement was added to these visit costs (see Table 68).
Costs associated with resolving misdiagnoses
We assumed that incorrect diagnoses would be resolved at a later point in time. We crudely assumed that one additional primary care attendance, two additional secondary care attendances and one laboratory visit would be required to correctly diagnose FP and FN results. This is an assumption and should be interpreted with some caution.
Costs associated with loss of control for false negatives
The model assumes that patients with asthma who initially test negative experience an increased rate of exacerbations compared with that in TP patients who are correctly diagnosed and who receive treatment. It is likely that ethical implications associated with the design of an empirical research study to collect this information would be prohibitive. We assumed that FN patients would experience one exacerbation each year in which they remain undiagnosed; this was loosely based on the higher absolute exacerbation estimate for diagnosed patients reported by Jayaram et al. 169 The model assumes that a proportion of these exacerbations will require hospitalisation (see below).
Costs of asthma management
We assumed that, on average, patients would be at step 3 in the BTS/SIGN asthma guidelines. 8 Current technology appraisal guidance from NICE187,188 on the use of ICSs for children and adults recommends that the least expensive option is used and does not differentiate between drugs in terms of effectiveness. We derived estimates of the annual cost of combined inhalers from two previous HTA reports. 13,180 Main et al. 180 estimated the least expensive annual cost for combined inhalers to be £201 for children (Symbicort turbohaler). Shepherd et al. 13 estimated the least expensive annual cost of combined inhalers to be £231 for adults (Symbicort turbohaler). Scrutiny of the current version of the BNF185 indicates that the annual cost of these inhalers has not changed since the original HTA reports were published.
Additional management model parameters
General population mortality
The probability of dying from all causes was taken directly from current interim life tables189 and was applied according to the ratio of males to females with asthma.
Duration of benefit of FeNO monitoring
In the base-case analysis we assumed that the impact of FeNO monitoring on dose titration and exacerbations would be retained indefinitely over the patient’s lifetime. Although this is plausible, there is no long-term RCT evidence to support or refute this assumption. We examine the impact of this assumption within the sensitivity analysis.
Annual exacerbation rates with FeNO monitoring and standard care
Annual exacerbation rates with and without FeNO testing were derived for children from the RCT reported by Szefler et al. 104 and for adults from the RCT reported by Shaw et al. 98 Changes in ICS use with/without FeNO monitoring for the child and adult subgroups were also drawn from these trials.
The RCT reported by Shaw et al. 98 was selected for use in the adult subgroup as it was the only UK-based study included in the systematic review for adults (see Chapter 3), because it reflects BTS/SIGN guidelines8 and because it reported data on severe exacerbation rates and changes in ICS use (the relevant parameters for the model). The population within this RCT relates to adult non-smokers and never smokers who were deemed to be compliant with medication and who had not experienced a severe exacerbation within 4 weeks of study entry. This allowed for the inclusion of a broader range of severity compared with the other studies. Patients were aged between 20 and 81 years and were treated and followed up for 12 months.
Of the studies included in the systematic review for children (see Chapter 3), the study reported by Szefler et al. 104 appears to most closely reflect current UK practice; hence, this study was selected to inform the exacerbation rates and ICS use parameters for the child subgroup. Within this study, patients were either on long-term control treatment with symptoms of persistent asthma or evidence of uncontrolled disease or not on long-term control treatment with symptoms of persistent asthma and evidence of uncontrolled disease. Patients were treated and followed up for 46 weeks. This trial was undertaken in the US.
Szefler et al. 104 reported that 32.1% (SD 4.67%) of 276 patients in the FeNO group and 42.0% (SD 4.94%) of 270 patients in the control group received one or more courses of prednisone over the 46-week study period; this was taken as a proxy for severe exacerbations. The authors also reported that 3.3% (SD 1.78%) of patients in the FeNO group and 4.1% (SD 1.98%) of patients in the control group were hospitalised at least once. We used these data to estimate the annual rate of exacerbations for each arm (0.36 for the FeNO arm and 0.47 for the standard care arm). It should be noted that the data available in the paper relate to the number of patients experiencing exacerbation events rather than the number of exacerbation events. We calculated the probability that an exacerbation required hospitalisation by pooling the exacerbation and hospitalisation data for the two study arms (probability = 0.04).
Shaw et al. 98 reported 18 exacerbations in 12 patients from the FeNO group (n = 58) and 26 exacerbations in 19 patients from the control group (n = 60) over 42 weeks. This corresponds to an annual exacerbation rate per patient of 0.33 (SD 0.69) for the FeNO group and 0.42 (SD 0.79) for the control group. Shaw et al. 98 did not report the proportion of severe exacerbations requiring hospitalisation and so this probability was assumed to be the same as that observed in the Szefler et al. study.
Exacerbation rates were assumed to follow a log-normal distribution. The probability that an exacerbation requires hospitalisation was modelled using a beta distribution.
Impact of exacerbations on health-related quality of life
The impact of exacerbations on HRQoL was based on a valuation study reported by Lloyd et al. ;184 this study was identified from the systematic review reported by Davis. 186 Lloyd et al. 184 reported the impact of exacerbations on HRQoL in patients with moderate to severe asthma (BTS/SIGN levels 4 and 5) in the UK. Within this study, 112 patients completed a variety of health status questionnaires including the EQ-5D. Disutilities associated with severe non-hospitalised and severe hospitalised exacerbations (compared with ‘no exacerbation’) were calculated based on the differences between the valuations for the three states. Uncertainty surrounding these parameters was modelled using beta distributions based on the difference between each state and the next worst state; this method ensures that the notionally better health state always has a monotonically better valuation than that for the notionally worse health state. The disutility of a severe exacerbation resulting in hospitalisation (compared with no exacerbation) was estimated to be –0.56 whereas the impact of other exacerbations that do not result in hospitalisation (compared with no exacerbation) was estimated to be –0.32. Disutilities are assumed to be additive and are therefore not influenced by the baseline level of HRQoL.
Severe exacerbations not resulting in hospitalisation were assumed to last for 4 days whereas major exacerbations resulting in hospitalisation were assumed to last for 4 weeks. These quantities were based on subjective estimates provided by experts. These durations were assumed to follow gamma distributions with SEs fitted to capture the range of estimates elicited (2–6 weeks for exacerbations requiring hospitalisation and 3–7 days for other severe exacerbations).
Resource costs: additional costs of FeNO monitoring
We assumed that FeNO monitoring would be undertaken during routine GP visits and would require one additional nurse visit once every 3 months. 8 The marginal cost of FeNO monitoring was applied as the per-test cost plus the cost of a primary care nurse appointment.
Changes in medication (inhaled corticosteroid) use over time
We derived estimates of change in ICS use with and without FeNO monitoring in children from the RCT reported by Szefler et al. 104 and in adults from the RCT reported by Shaw et al. 98 We assumed that, during the period for which ICS use was observed in each study (12 months in the study by Shaw et al. 98 and 46 weeks in the study by Szefler et al. 104), ICS use would reflect the observed mean, with the relative dose intensity (RDI) calculated as the mean over the observed period divided by the baseline ICS dosage for each study arm. Beyond this point, we assumed that ICS use would remain constant at the level of the last observation for each study arm for the remainder of the duration over which FeNO monitoring impacts on exacerbations and titration decisions (Table 69).
Costs of managing exacerbations
We assumed that a proportion of exacerbations would require hospitalisation whereas the remainder could be managed in primary care. We assumed that severe exacerbations that do not require hospitalisation would require one GP attendance (cost £43.00) plus oral steroids for 5 days (cost £1.73), based on an earlier HTA report. 180 We derived the cost of asthma hospitalisation from current NHS Reference Costs174 (cost £1266.72).
Model evaluation
The model was evaluated probabilistically using standard Monte Carlo sampling techniques over 5000 random samples. Central estimates of cost-effectiveness are presented based on the expectation of the mean. Headline results are presented as ICERs, cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs). In addition, a large number of deterministic sensitivity analyses (DSAs) were undertaken; these analyses are detailed in the following sections. All incremental analyses were carried out using an automated tabular algorithm developed by one of the study authors (PT).
Deterministic sensitivity analyses undertaken using the diagnostic model
Scenario D1: point estimates of parameters
The model was evaluated using point estimates of parameters rather than the expectation of the mean.
Scenarios D2 and D3: alternative discount rates
The model was evaluated with discount rates of 0% for costs and QALYs (scenario D2) and 6% for costs and QALYs (scenario D3).
Scenario D4: all tests undertaken in secondary care
The model was run assuming that all tests are undertaken in a secondary care setting.
Scenarios D5 and D6: alternative asthma control disutilities for false negatives
The model was run assuming different disutilities for patients who are FN. In scenario D5, the most extreme disutility from the study by McTaggart-Cowan et al. 181 was assumed (‘very well controlled’ to ‘not controlled’ state disutility = –0.10). In scenario D6, the most extreme disutility from the study by Szende et al. 172 was assumed (‘good control’ to ‘poor control’ state disutility = –0.41).
Scenarios D7 and D8: alternative disutilities for false positives
The model was run assuming different disutilities for patients who are FP. In scenario D7, the base-case disutility applied to FPs was doubled whereas in scenario D8 this disutility was halved.
Scenarios D9 and D10: FeNO test costs
The model was evaluated assuming that the marginal per-test costs for all FeNO devices (NIOX MINO, NIOX VERO and NObreath) are double (scenario D9) or half (scenario D10) those assumed in the base-case analysis.
Scenarios D11–D13: alternative assumptions concerning the lifetime of the NObreath device
Within the base-case analysis, the NObreath device is assumed to have a fixed lifetime of 10 years (for costing purposes). In scenario D11 the analysis is repeated assuming a maximum lifetime for the NObreath device of 3 years (equal to the maximum lifetime of the NIOX MINO device). In scenario D12 the analysis is repeated assuming a maximum lifetime for the NObreath device of 5 years (equal to the maximum lifetime of the NIOX VERO device). In scenario D13 the analysis is repeated assuming a maximum lifetime for the NObreath device of 20 years (double that assumed in the base case). These alternative assumptions result in marginal per-test costs for the NObreath device of £14.32, £8.88 and £2.32 for scenarios D11, D12 and D13 respectively.
Scenarios D14 and D15: test visit costs
The model was evaluated assuming that all primary and secondary care visit costs are double (scenario D14) or half (scenario D15) those assumed in the base-case analysis. This includes the costs of initial visits and subsequent visits to resolve misdiagnosis.
Scenarios D16 and D17: false-negative exacerbation rate
The model was evaluated assuming that the base-case incremental exacerbation rate for FNs is double (scenario D16) or half (scenario D17) that assumed within the base-case analysis.
Scenarios D18 and D19: asthma treatment costs
The model was evaluated assuming that asthma treatment costs are double (scenario D18) or half (scenario D19) those assumed in the base-case analysis.
Scenarios D20–D25: time to resolve misdiagnosis
The model was evaluated assuming a range of different times to resolve initial misdiagnoses (both FNs and FPs) (2×, 3×, 4×, 5×, 10× and 0.5× the base-case time to correct diagnosis parameters in scenarios D20–D25 respectively). In these analyses the time horizon was set to be equal to the maximum time to resolve FP and FN results (note that this does not affect the incremental model results).
Scenarios D26 and D27: alternative sources for the diagnostic accuracy of FeNO monitoring alone
The base-case analysis used estimates of the diagnostic accuracy of FeNO monitoring from Schneider et al. 71,72 In scenario D26, the model was evaluated using alternative estimates from Schleich et al. ;77 at a cut-off of 34 ppb, the sensitivity and specificity of FeNO monitoring were 0.35 and 0.95 respectively. In DSA scenario D27, the model was evaluated using alternative estimates from Pedrosa et al. ;85 at a cut-off of 40 ppb, the sensitivity and specificity of FeNO monitoring were 0.74 and 0.73 respectively. Both of these studies include a difficult-to-diagnose population, although it should be noted that this is not the case for the other comparators in this scenario analysis.
Scenario D28: alternative source for the diagnostic accuracy of non-FeNO monitoring comparators
The base-case analysis draws estimates of sensitivity and specificity for individual comparators from the study reported by Hunter et al. 163 In scenario D28, the model was evaluated using estimates of diagnostic accuracy for FEV1/FVC, PEF and sputum induction from the comparative diagnostic study reported by Smith et al. 86
Scenarios D29–D31: ‘rule-out’ diagnostic decision approach
In scenarios D29–D31, the model was evaluated assuming a ‘rule-out’ diagnostic approach for all diagnostic tests. In these scenarios any patient who tests negative is ‘ruled out’ and treated as being not asthmatic (as per the base-case structure), whereas any patient testing positive is assumed to immediately undergo further tests to confirm the diagnosis. As a consequence, no patient loses health from initially testing FP. The ‘rule-out’ approach was evaluated over three scenarios: scenario D29 – base-case test characteristics for FeNO monitoring options; scenario 30 – best sensitivity for FeNO monitoring options; and scenario 31 – best specificity for FeNO monitoring options.
Scenarios D32–D34: ‘rule-in’ diagnostic decision approach
In scenarios D32–D34, the model was evaluated assuming a ‘rule-in’ diagnostic approach for all diagnostic tests. In this scenario any patient who tests positive is ‘ruled in’ and treated as being asthmatic (as per the base-case structure), whereas any patient testing negative is assumed to immediately undergo further tests to confirm the diagnosis. As a consequence, no patient loses health from initially testing FN. The ‘rule-in’ approach was evaluated over three scenarios: scenario D32 – base-case test characteristics for FeNO monitoring options; scenario D33 – best sensitivity for FeNO monitoring options; and scenario D34 – best specificity for FeNO monitoring options.
Deterministic sensitivity analyses undertaken using the management model
Scenario M1: point estimates of parameters
The model was evaluated using point estimates of parameters rather than the expectation of the mean.
Scenarios M2 and M3: alternative discount rates
The model was evaluated with discount rates of 0% for costs and QALYs (scenario M2) and 6% for costs and QALYs (scenario M3).
Scenario M4: pregnant women subgroup analysis
In this scenario the model was evaluated specifically for a subgroup consisting of women who are pregnant. This analysis was based on the RCT reported by Powell et al. 102 We estimated annual exacerbation rates of 0.58 and 1.26 for the FeNO monitoring and guidelines groups respectively. Mean ICS use over the study period was estimated to be approximately 77% of the baseline dose for the FeNO group and 102% of the baseline dose for the guidelines group. These estimates were assumed to apply for the first 5 months (the observed period in the trial). The final observations of 73% and 105% for the FeNO and guidelines groups, respectively, were assumed to be carried forward over the remainder of the time horizon.
Scenario M5: alternative source of exacerbation rates and ICS use for children
In scenario M5, the model was evaluated using alternative estimates of exacerbation rates and ICS use over time, based on the RCT reported by Pijnenburg et al. 106 We estimated exacerbation rates of 0.18 and 0.39 for the FeNO and guidelines groups respectively. ICS use over the 1-year follow-up period was similar in both groups: the RDI compared with the baseline dose was estimated to be 1.16 in both groups. Beyond the first year, the RDI was estimated to be 1.23 for the FeNO group and 1.22 for the guidelines group.
Scenarios M6 and M7: alternative sources of exacerbation rates and ICS use for adults
In scenarios M6 and M7, the model was evaluated using alternative estimates of exacerbation rates and ICS use over time, based on the RCTs reported by Smith et al. 119 (scenario M6) and Syk et al. 99 (scenario M7).
Using data from Smith et al. ,119 we estimated severe exacerbation rates of 0.16 and 0,17 for the FeNO and guidelines groups respectively. ICS use in the first year, relative to the first observation, was estimated to be 0.85 and 1.08 for the FeNO and guidelines groups respectively. ICS use based on the last observation was estimated to be 0.90 and 1.30 for the FeNO and guidelines groups respectively.
Using data from Syk et al. ,99 we estimated exacerbation rates of 0.09 and 0.07 for the FeNO and guidelines groups respectively. ICS use in the first year, relative to the first observation, was estimated to be 0.97 and 0.96 for the FeNO and guidelines groups respectively. ICS use based on the last observation was estimated to be 0.88 and 0.99 for the FeNO and guidelines groups respectively.
Scenarios M8–M17: alternative assumptions regarding the duration of impact of FeNO monitoring
A number of scenarios were undertaken to examine the impact of assuming alternative durations over which FeNO monitoring would impact on ICS use and exacerbations. The durations examined were 1 year, 2 years, 3 years, 4 years, 5 years, 10 years, 15 years, 20 years, 30 years and 40 years (scenarios M8–M17 respectively).
Scenarios M18 and M19: FeNO test costs
The model was evaluated assuming that the marginal per-test costs for all FeNO devices (NIOX MINO, NIOX VERO and NObreath) are double (scenario M18) or half (scenario M19) those assumed in the base-case analysis.
Scenarios M20–M22: alternative assumptions concerning the lifetime of the NObreath device
In scenario M20, the model was evaluated assuming a maximum lifetime for the NObreath device of 3 years (equal to the maximum lifetime of the NIOX MINO device). In scenario M21, the analysis was repeated assuming a maximum lifetime for the NObreath device of 5 years (equal to the maximum lifetime of the NIOX VERO device). In scenario M22, the analysis was repeated assuming a maximum lifetime for the NObreath device of 20 years (double that assumed in the base case). These result in marginal per-test costs for the NObreath device of £14.32, £8.88 and £2.32 for scenarios M20, M21 and M22 respectively.
Scenarios M23 and M24: nurse visits
The model was evaluated assuming that the number of nurse visits for the FeNO group was double (scenario M23) or half (scenario M24) the number applied in the base-case analysis.
Scenarios M25 and M26: alternative assumptions regarding exacerbation rates
The model was evaluated assuming that the exacerbation rates for the FeNO and guidelines groups are double (scenario M25) or half (scenario M26) those rates assumed in the base-case analysis.
Scenarios M27 and M28: alternative assumptions regarding exacerbation disutility
The model was evaluated assuming that the exacerbation disutilities for the FeNO and guidelines groups are double (scenario M27) or half (scenario M28) those disutilities assumed in the base-case analysis.
Scenario M29: mean observed inhaled corticosteroid use projected forward
The model was evaluated assuming that mean ICS use observed within the clinical trials is maintained over the remainder of the model time horizon.
Scenarios M30 and M31: alternative assumptions regarding inhaled corticosteroid dose change over time
The model was evaluated assuming that the mean RDI for ICSs in the FeNO and guidelines groups is double (scenario M30) or half (scenario M31) that assumed in the base-case analysis.
With the exception of scenarios M4–M7, all DSAs within the management model were undertaken in both the child subgroup and the adult subgroup.
Model validation methods
We took a number of measures to ensure the credibility of the models and their results. The conceptual models were discussed extensively amongst the EAG before implementation. The lead modeller (PT) checked the integrity of all model calculations and VBA (Visual Basic for Applications) programming whilst developing the model. The models were rechecked once they were complete. PT also rebuilt deterministic versions of both models in a more disaggregated form to ensure that all calculations were implemented as intended; these replicated models gave exactly the same results as the full models. All model input parameters and pre-model analyses were checked and inputted values were compared against the sources from which they were derived. The results of the models were compared against our a priori expectations, given the model structures and input parameters, and any discrepancies were investigated. A large number of sensitivity analyses and black-box tests were undertaken to ensure that the models were behaving as expected. Finally, the assessment report was peer reviewed by clinical experts, other researchers within the School of Health and Related Research (ScHARR), University of Sheffield, and NICE (see Acknowledgements).
De novo model results
Diagnostic model results (all patients)
Central estimates of cost-effectiveness: diagnosis (all patients)
Table 70 presents the central estimates of cost-effectiveness based on the probabilistic version of the diagnostic model. The results suggest that, across the 17 diagnostic options included in the economic analysis, the expected difference in QALY gains is likely to be very small. NIOX MINO and NIOX VERO, alone or in combination with other tests, are expected to be dominated as their marginal per-test cost is higher than that for NObreath. Airway hyper-responsiveness (MCT) is expected to produce the greatest QALY gain; this is because this option has both the highest sensitivity and the highest specificity of all of the tests included in the economic analysis. With the exception of FeNO (NObreath) plus bronchodilator reversibility, all other options are expected to be ruled out by simple dominance. The incremental cost-effectiveness of airway hyper-responsiveness compared with FeNO plus bronchodilator reversibility is expected to be approximately £1.125M per QALY gained. This information is presented on the absolute cost-effectiveness plane in Figure 27.
| Option | QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | Incremental cost per QALY gained (£) |
|---|---|---|---|---|---|
| Airway hyper-responsiveness (MCT) | 4.2834 | 1226.00 | 0.0005 | 539.92 | 1,125,074 |
| FeNO + bronchodilator reversibility (NObreath) | 4.2829 | 686.08 | – | – | – |
| FeNO + bronchodilator reversibility (NIOX VERO) | 4.2829 | 687.61 | – | – | Dominated |
| FeNO + bronchodilator reversibility (NIOX MINO) | 4.2829 | 688.33 | – | – | Dominated |
| FeNO + sputum induction (NObreath) | 4.2812 | 1265.78 | – | – | Dominated |
| FeNO + sputum induction (NIOX VERO) | 4.2812 | 1267.32 | – | – | Dominated |
| FeNO + sputum induction (NIOX MINO) | 4.2812 | 1268.03 | – | – | Dominated |
| FeNO + FEV1 (NObreath) | 4.2783 | 810.14 | – | – | Dominated |
| FeNO + FEV1 (NIOX VERO) | 4.2783 | 811.67 | – | – | Dominated |
| FeNO + FEV1 (NIOX MINO) | 4.2783 | 812.38 | – | – | Dominated |
| Sputum induction | 4.2774 | 1328.28 | – | – | Dominated |
| FeNO (NObreath) | 4.2771 | 819.94 | – | – | Dominated |
| FeNO (NIOX VERO) | 4.2771 | 821.47 | – | – | Dominated |
| FeNO (NIOX MINO) | 4.2771 | 822.18 | – | – | Dominated |
| PEF | 4.2719 | 877.91 | – | – | Dominated |
| Bronchodilator reversibility | 4.2710 | 886.27 | – | – | Dominated |
| FEV1/FVC | 4.2686 | 907.71 | – | – | Dominated |
FIGURE 27.
Cost-effectiveness plane: diagnosis (all patients).
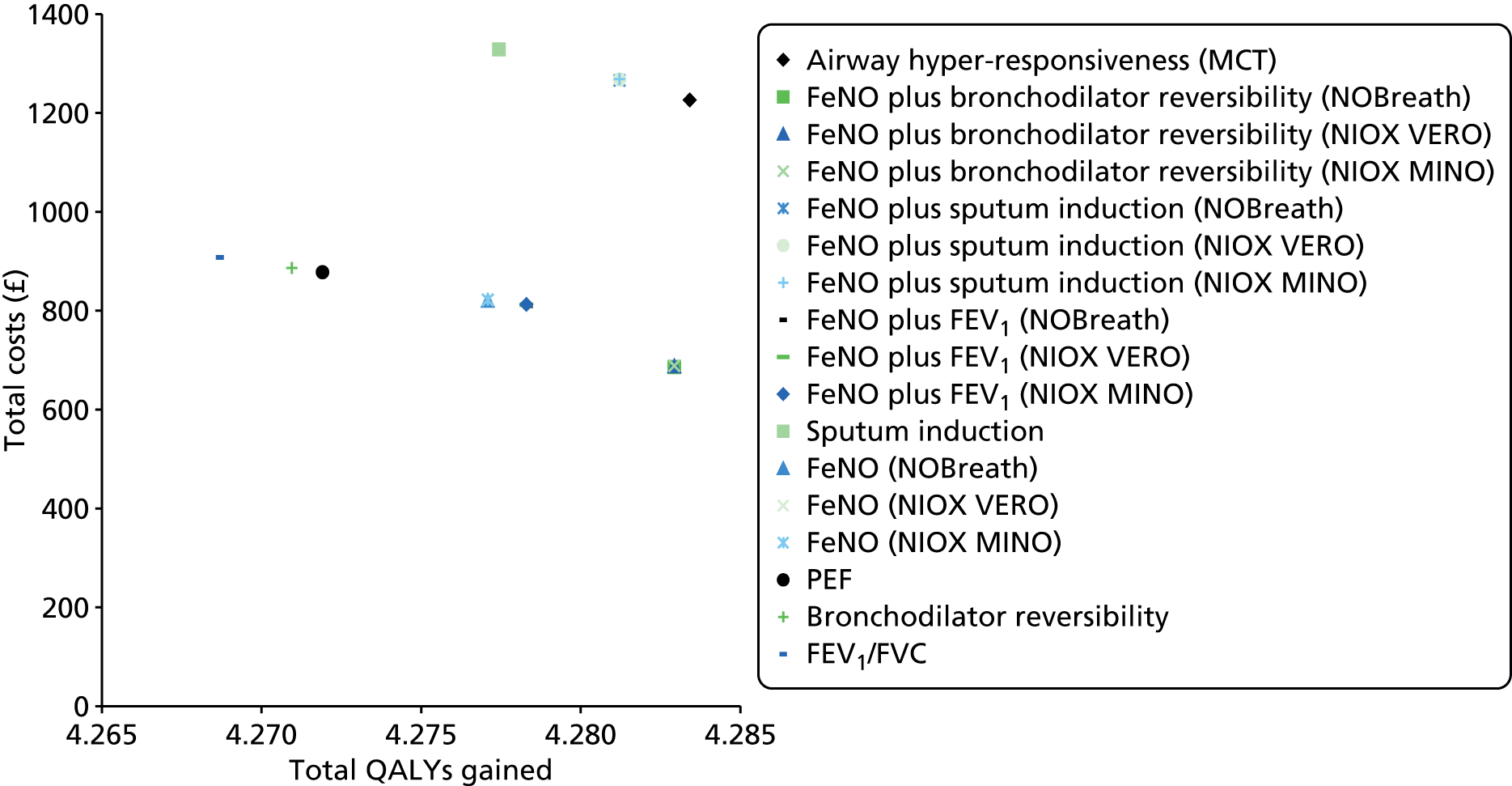
Uncertainty analysis: diagnosis (all patients)
Figure 28 presents CEACs for the diagnostic options. Assuming a willingness-to-pay threshold of £20,000 per QALY gained, FeNO plus bronchodilator reversibility (using NObreath) has the highest probability of producing the greatest amount of net benefit (probability = 0.98). Assuming a willingness-to-pay threshold of £30,000 per QALY gained, FeNO plus bronchodilator reversibility (using NObreath) also has the highest probability of producing the greatest amount of net benefit (probability = 0.95). These results are also summarised in Table 71.
FIGURE 28.
Cost-effectiveness acceptability curves: diagnosis (all patients).

| Option | Probability optimal: λ = £20,000 per QALY gained | Probability optimal: λ = £30,000 per QALY gained |
|---|---|---|
| FeNO (NIOX MINO) | 0.00 | 0.00 |
| FeNO (NIOX VERO) | 0.00 | 0.00 |
| FeNO (NObreath) | 0.00 | 0.00 |
| FeNO + FEV1 (NIOX MINO) | 0.00 | 0.00 |
| FeNO + FEV1 (NIOX VERO) | 0.00 | 0.00 |
| FeNO + FEV1 (NObreath) | 0.02 | 0.05 |
| FeNO + sputum induction (NIOX MINO) | 0.00 | 0.00 |
| FeNO + sputum induction (NIOX VERO) | 0.00 | 0.00 |
| FeNO + sputum induction (NObreath) | 0.00 | 0.00 |
| FeNO + bronchodilator reversibility (NIOX MINO) | 0.00 | 0.00 |
| FeNO + bronchodilator reversibility (NIOX VERO) | 0.00 | 0.00 |
| FeNO + bronchodilator reversibility (NObreath) | 0.98 | 0.95 |
| FEV1/FVC | 0.00 | 0.00 |
| PEF | 0.00 | 0.00 |
| Bronchial reversibility | 0.00 | 0.00 |
| Airway hyper-responsiveness (MCT) | 0.00 | 0.00 |
| Sputum induction | 0.00 | 0.00 |
Deterministic sensitivity analysis: diagnosis (all patients)
Tables 72–77 present the results of the DSAs. In all analyses the rank ordering of non-dominated options is maintained except when indicated by square brackets.
| Option | D1: point estimates of parameters (£) | D2: undiscounted costs and outcomes (£) | D3: discount rate = 6% (£) | D4: all tests in secondary care (£) | D5: disutility from McTaggart-Cowan et al.181 (£) | D6: disutility from Szende et al.172 (£) |
|---|---|---|---|---|---|---|
| Airway hyper-responsiveness (MCT) | 1,094,325 | 1,081,089 | 1,103,827 | Dominating | 437,730 | 106,763 |
| FeNO + bronchodilator reversibility (NObreath) | – | – | – | Dominated | – | – |
| FeNO + bronchodilator reversibility (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + bronchodilator reversibility (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Sputum induction | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| PEF | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Bronchodilator reversibility | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FEV1/FVC | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Option | D7: FP disutility doubled (£) | D8: FP disutility halved (£) | D9: FeNO marginal per-test cost doubled (£) | D10: FeNO marginal per-test cost halved (£) | D11: NObreath lifetime = 3 years (£) | D12: NObreath lifetime = 5 years (£) | D13: NObreath lifetime = 20 years (£) |
|---|---|---|---|---|---|---|---|
| Airway hyper-responsiveness (MCT) | 1,094,325 | 1,094,325 | 1,084,543 | 1,094,325 | 1,091,809 | 1,093,539 | 1,093,576 |
| FeNO + bronchodilator reversibility (NObreath) | – | – | – | – | – | – | – |
| FeNO + bronchodilator reversibility (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + bronchodilator reversibility (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Sputum induction | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| PEF | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Bronchodilator reversibility | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FEV1/FVC | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Option | D14: visit costs doubled (£) | D15: visit costs halved (£) | D16: FN exacerbation rate doubled (£) | D17: FN exacerbation rate halved (£) | D18: asthma treatment costs doubled (£) | D19: asthma treatment costs halved (£) |
|---|---|---|---|---|---|---|
| Airway hyper-responsiveness (MCT) | 2,196,057 | 543,459 | 1,090,925 | 1,096,025 | 1,100,100 | 1,091,438 |
| FeNO + bronchodilator reversibility (NObreath) | – | – | – | – | – | – |
| FeNO + bronchodilator reversibility (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + bronchodilator reversibility (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Sputum induction | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| PEF | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Bronchodilator reversibility | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FEV1/FVC | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Option | D20: misdiagnosis correction times ×2 (£) | D21: misdiagnosis correction times ×3 (£) | D22: misdiagnosis correction times ×4 (£) | D23: misdiagnosis correction times ×5 (£) | D24: misdiagnosis correction times ×10 (£) | D25: misdiagnosis correction times halved (£) |
|---|---|---|---|---|---|---|
| Airway hyper-responsiveness (MCT) | 556,717 | 377,547 | 287,986 | 234,270 | 126,982 | 2,169,614 |
| FeNO + bronchodilator reversibility (NObreath) | – | – | 3201 | 5111 | 8523 | – |
| FeNO + bronchodilator reversibility (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + bronchodilator reversibility (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NObreath) | Dominated | Dominated | – | – | – | Dominated |
| FeNO + FEV1 (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Sputum induction | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| PEF | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Bronchodilator reversibility | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FEV1/FVC | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Option | D26: FeNO operating characteristics from Schleich et al.77 (£) | D27: FeNO operating characteristics from Pedrosa et al.85 (£) | D28: other test operating characteristics from Smith et al.86 (£) |
|---|---|---|---|
| Airway hyper-responsiveness (MCT) | 1,094,325 | 1,094,325 | 1,094,325 |
| FeNO + bronchodilator reversibility (NObreath) | – | – | – |
| FeNO + bronchodilator reversibility (NIOX VERO) | Dominated | Dominated | Dominated |
| FeNO + bronchodilator reversibility (NIOX MINO) | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NObreath) | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX VERO) | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX MINO) | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NObreath) | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NIOX VERO) | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NIOX MINO) | Dominated | Dominated | Dominated |
| Sputum induction | Dominated | Dominated | Dominated |
| FeNO (NObreath) | Dominated | Dominated | Dominated |
| FeNO (NIOX VERO) | Dominated | Dominated | Dominated |
| FeNO (NIOX MINO) | Dominated | Dominated | Dominated |
| PEF | Dominated | Dominated | Dominated |
| Bronchodilator reversibility | Dominated | Dominated | Dominated |
| FEV1/FVC | Dominated | Dominated | Dominated |
| Option | D29: rule-out decision approach (base case) (£) | D30: rule-out decision approach (best sensitivity for FeNO) (£) | D31: rule-out decision approach (best specificity for FeNO) (£) | D32: rule-in decision approach (base case) (£) | D33: rule-out decision approach (best sensitivity for FeNO) (£) | D34: rule-in decision approach (best specificity for FeNO) (£) |
|---|---|---|---|---|---|---|
| Airway hyper-responsiveness (MCT) | 1,119,170 | 1,119,170 | 1,119,170 | 63,533 | 63,533 | Dominated |
| FeNO + bronchodilator reversibility (NObreath) | 6965 | 6965 | 6965 | – | – | Dominated |
| FeNO + bronchodilator reversibility (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + bronchodilator reversibility (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | – [rank 3] |
| FeNO + sputum induction (NObreath) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + sputum induction (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NObreath) | – | – | 5719 | Dominated | Dominated | 63,533 [rank 2] |
| FeNO + FEV1 (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO + FEV1 (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Sputum induction | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NObreath) | Dominated | Dominated | – | Extendedly dominated | Dominated | 94,020 [rank 1] |
| FeNO (NIOX VERO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FeNO (NIOX MINO) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| PEF | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| Bronchodilator reversibility | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| FEV1/FVC | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
The DSAs indicate the following:
-
Across the majority of scenarios, the cost-effectiveness frontier presented in the base-case analysis [which includes only airway hyper-responsiveness (MCT) and FeNO plus bronchodilator reversibility] is maintained. In most scenarios the majority of options are expected to be ruled out because of simple dominance.
-
The results based on the point estimates of parameters are similar to the results of the probabilistic analysis.
-
Discounting does not have a substantial effect on the cost-effectiveness of the non-dominated diagnostic options.
-
The disutility associated with loss of control in FNs has a substantial impact on the incremental cost-effectiveness of airway hyper-responsiveness (MCT) compared with FeNO plus bronchodilator reversibility.
-
The FP exacerbation rate has no impact on the results as both non-dominated options have the same specificity.
-
The cost of the various FeNO devices has only a negligible impact on the cost-effectiveness results for non-dominated options.
-
Longer misdiagnosis correction times substantially improve the cost-effectiveness of airway hyper-responsiveness (MCT) compared with FeNO plus bronchodilator reversibility.
-
The use of other sources for the operating characteristics of FeNO monitoring and standard tests does not impact on the cost-effectiveness of non-dominated options.
-
The use of a ‘rule-out’ decision approach may improve the comparative effectiveness and cost-effectiveness of FeNO monitoring in combination with either bronchodilator reversibility or FEV1.
-
The use of a ‘rule-in’ decision approach may improve the effectiveness of FeNO monitoring plus FEV1; however, the ICER for this option (compared with FeNO monitoring plus bronchodilator reversibility) is in excess of £63,000 per QALY gained.
Management model results (children)
Central estimates of cost-effectiveness: management (children)
Table 78 presents the central estimates of cost-effectiveness based on the probabilistic version of the child management model. The results suggest that FeNO testing is expected to produce a small health benefit compared with guidelines alone (0.05 QALYs). FeNO testing is also expected to be more expensive than guidelines alone; this is because of the projected ICS use for the FeNO groups. The results also indicate, as expected, that NIOX MINO and NIOX VERO are expected to be dominated by NObreath because of their slightly higher marginal per-test cost. The incremental cost-effectiveness of NObreath compared with guidelines is expected to be approximately £45,213 per QALY gained. This information is presented on the absolute cost-effectiveness plane in Figure 29.
| Option | QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | Incremental cost per QALY gained (£) |
|---|---|---|---|---|---|
| Guidelines plus FeNO monitoring (NObreath) | 23.6767 | 8148.59 | 0.0506 | 2288.53 | 45,213 |
| Guidelines plus FeNO monitoring (NIOX VERO) | 23.6767 | 8314.30 | – | – | Dominated |
| Guidelines plus FeNO monitoring (NIOX MINO) | 23.6767 | 8391.53 | – | – | Dominated |
| Guidelines | 23.6261 | 5860.06 | – | – | – |
FIGURE 29.
Cost-effectiveness plane: management (children).
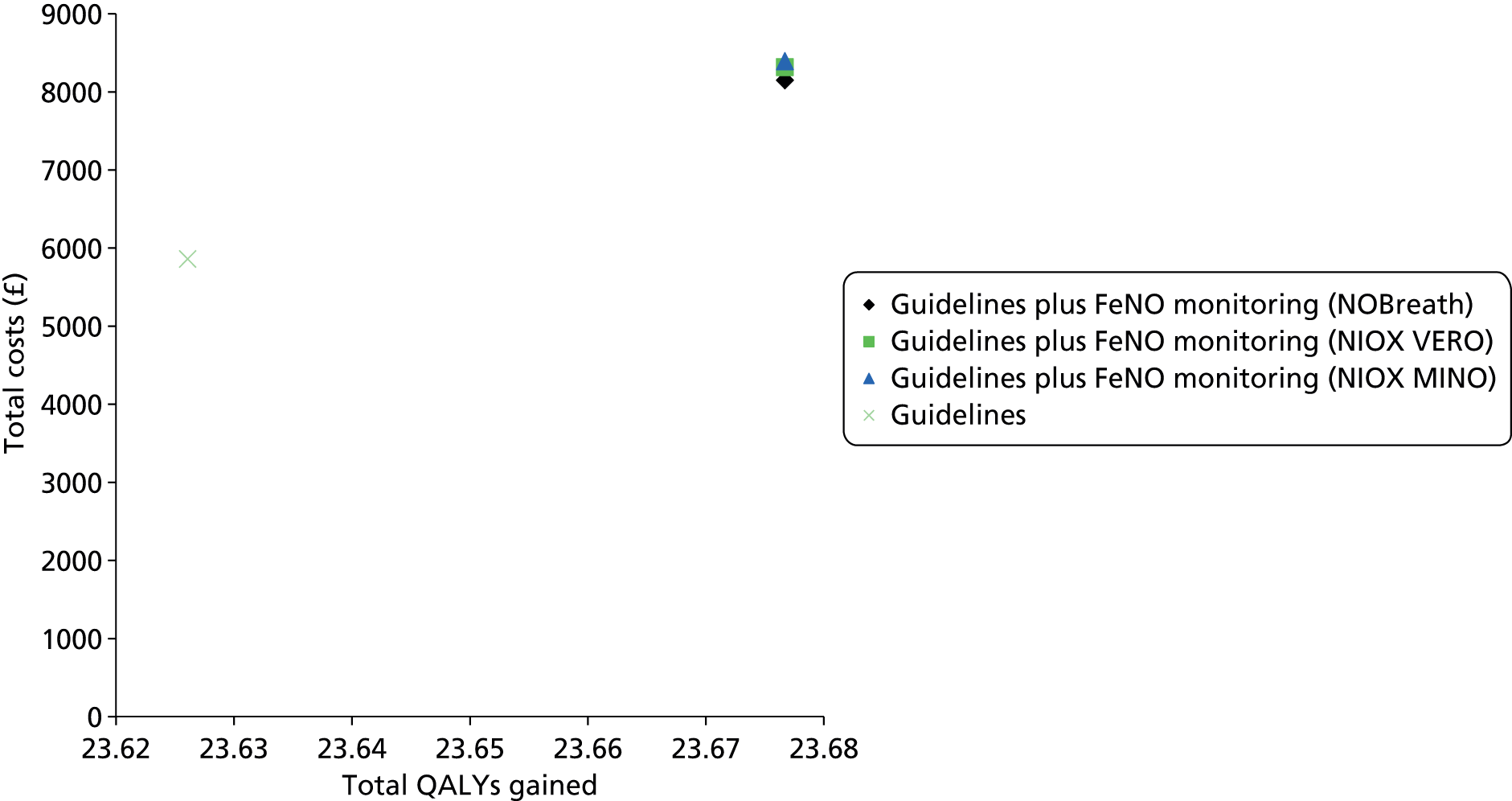
Uncertainty analysis: management (children)
Figure 30 presents CEACs for the management options in the child subgroup. These data are also summarised in Table 79. Assuming a willingness-to-pay threshold of £20,000 per QALY gained, use of guidelines alone has the highest probability of producing the greatest amount of net benefit (probability = 0.99). Assuming a willingness-to-pay threshold of £30,000 per QALY gained, use of guidelines alone also has the highest probability of producing the greatest amount of net benefit (probability = 0.91).
FIGURE 30.
Cost-effectiveness acceptability curves: management (children).

| Option | Probability optimal: λ = £20,000 per QALY gained | Probability optimal: λ = £30,000 per QALY gained |
|---|---|---|
| Guidelines | 0.99 | 0.91 |
| Guidelines plus FeNO monitoring (NIOX MINO) | 0.00 | 0.00 |
| Guidelines plus FeNO monitoring (NIOX VERO) | 0.00 | 0.00 |
| Guidelines plus FeNO monitoring (NObreath) | 0.01 | 0.09 |
Deterministic sensitivity analysis results
Table 80 presents the results of the DSAs.
| Scenario | Guidelines plus FeNO (NObreath) (£) | Guidelines plus FeNO (NIOX VERO) (£) | Guidelines plus FeNO (NIOX MINO) (£) | Guidelines (£) | |
|---|---|---|---|---|---|
| M1 | Point estimates of parameters | 45,138 | Dominated | Dominated | – |
| M2 | Undiscounted costs and outcomes | 46,894 | Dominated | Dominated | – |
| M3 | Discount rate = 6% | 44,555 | Dominated | Dominated | – |
| M5 | Analysis based on Pijnenburg et al.106 | 18,963 | Dominated | Dominated | – |
| M8 | FeNO impact = 1 year | Dominating | Dominated | Dominated | Dominated |
| M9 | FeNO impact = 2 years | Dominating | Dominated | Dominated | Dominated |
| M10 | FeNO impact = 3 years | Dominating | Dominated | Dominated | Dominated |
| M11 | FeNO impact = 4 years | Dominating | Dominated | Dominated | Dominated |
| M12 | FeNO impact = 5 years | 7598 | Dominated | Dominated | – |
| M13 | FeNO impact = 10 years | 27,660 | Dominated | Dominated | – |
| M14 | FeNO impact = 15 years | 34,337 | Dominated | Dominated | – |
| M15 | FeNO impact = 20 years | 37,674 | Dominated | Dominated | – |
| M16 | FeNO impact = 30 years | 41,025 | Dominated | Dominated | – |
| M17 | FeNO impact = 40 years | 42,721 | Dominated | Dominated | – |
| M18 | Marginal per-test FeNO cost doubled | 55,409 | Dominated | Dominated | – |
| M19 | Marginal per-test FeNO cost halved | 40,003 | Dominated | Dominated | – |
| M20 | NObreath lifetime = 3 years | 47,780 | Dominated | Dominated | – |
| M21 | NObreath lifetime = 5 years | 45,963 | Dominated | Dominated | – |
| M22 | NObreath lifetime = 20 years | 45,925 | Dominated | Dominated | – |
| M23 | FeNO nurse visits doubled | 84,564 | Dominated | Dominated | – |
| M24 | FeNO nurse visits halved | 25,425 | Dominated | Dominated | – |
| M25 | Exacerbation rates doubled | 19,891 | Dominated | Dominated | – |
| M26 | Exacerbation rates halved | 95,632 | Dominated | Dominated | – |
| M27 | Exacerbation disutility doubled | 31,479 | Dominated | Dominated | – |
| M28 | Exacerbation disutility halved | 52,844 | Dominated | Dominated | – |
| M29 | ICS observed mean carried forward | 37,452 | Dominated | Dominated | – |
| M30 | ICS change doubled | 56,206 | Dominated | Dominated | – |
| M31 | ICS change halved | 39,604 | Dominated | Dominated | – |
The DSAs indicate the following:
-
The results of the analysis using point estimates of parameters are similar to those produced using the probabilistic model.
-
NIOX MINO and NIOX VERO are expected to be consistently dominated by NObreath because of their higher marginal per-test costs.
-
Although the marginal per-test cost influences which device would be preferred, it does not have a substantial impact on the overall cost-effectiveness of FeNO monitoring compared with guidelines.
-
Discounting has little impact on the cost-effectiveness of FeNO monitoring.
-
The duration over which FeNO monitoring is assumed to impact on exacerbations and ICS use is a key parameter within the child subgroup. Shorter durations of impact improve the cost-effectiveness of FeNO monitoring.
-
The analysis based on data from Pijnenburg et al. 106 suggests a considerably more favourable ICER for FeNO monitoring compared with guidelines in children. This may be explained by the fact that the Szefler et al. 104 study was undertaken in uncontrolled patients and the study protocol did not allow therapy to be stepped down on the basis of low FeNO levels alone. This may in part explain why ICS use was higher for FeNO monitoring than for guidelines alone.
-
The model is sensitive to the rate of exacerbations (and associated health loss) and assumptions regarding the number of monitoring visits in which FeNO monitoring is used.
Management model results (adults)
Central estimates of cost-effectiveness: management (adults)
Table 81 presents the central estimates of cost-effectiveness based on the probabilistic version of the adult management model. FeNO testing is expected to produce a small incremental health gain compared with standard guidelines (0.04 QALYs). The results also suggest that NIOX MINO and NIOX VERO are expected to be dominated by NObreath (again, this is because of the slightly lower marginal per-test cost for this device). In this population subgroup, the NObreath device plus guidelines compared with guidelines alone is expected to cost approximately £2146 per QALY gained. This information is presented on the absolute cost-effectiveness plane in Figure 31.
| Option | QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | Incremental cost per QALY gained (£) |
|---|---|---|---|---|---|
| Guidelines plus FeNO monitoring (NObreath) | 21.9397 | 7377.61 | 0.0379 | 81.31 | 2146 |
| Guidelines plus FeNO monitoring (NIOX VERO) | 21.9397 | 7535.43 | – | – | Dominated |
| Guidelines plus FeNO monitoring (NIOX MINO) | 21.9397 | 7608.99 | – | – | Dominated |
| Guidelines | 21.9018 | 7296.30 | – | – | – |
FIGURE 31.
Cost-effectiveness plane: management (adults).

Uncertainty analysis: management (adults)
Figure 32 presents CEACs for the management options in the adult subgroup. Assuming a willingness-to-pay threshold of £20,000 per QALY gained, FeNO monitoring using NObreath plus guidelines has the highest probability of producing the greatest amount of net benefit (probability = 0.82). Assuming a willingness-to-pay threshold of £30,000 per QALY gained, FeNO monitoring using NObreath plus guidelines also has the highest probability of producing the greatest amount of net benefit (probability = 0.87). These results are summarised in Table 82.
FIGURE 32.
Cost-effectiveness acceptability curves: management (adults).
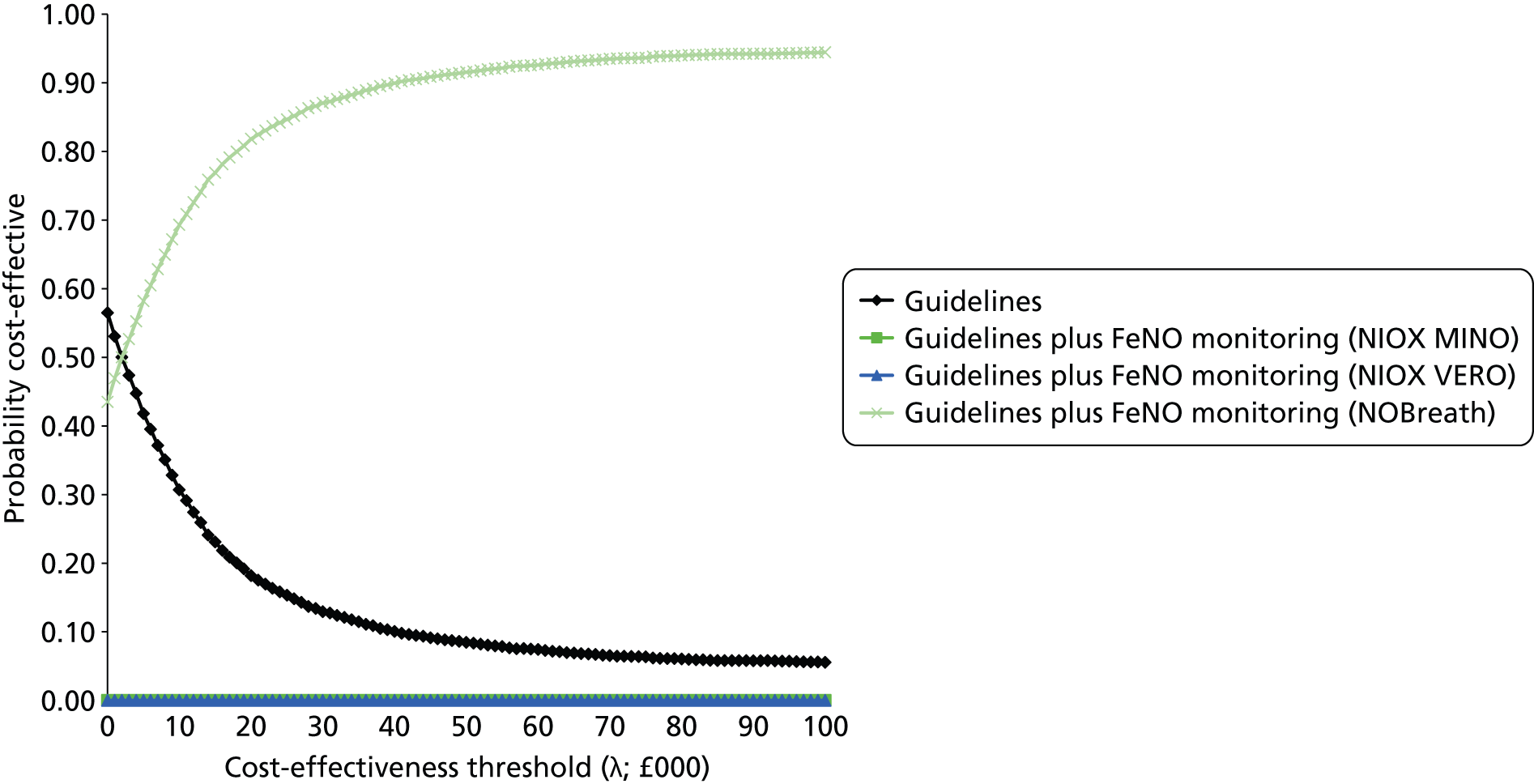
| Option | Probability optimal: λ = £20,000 per QALY gained | Probability optimal: λ = £30,000 per QALY gained |
|---|---|---|
| Guidelines | 0.18 | 0.13 |
| Guidelines plus FeNO monitoring (NIOX MINO) | 0.00 | 0.00 |
| Guidelines plus FeNO monitoring (NIOX VERO) | 0.00 | 0.00 |
| Guidelines plus FeNO monitoring (NObreath) | 0.82 | 0.87 |
Deterministic sensitivity analyses
Table 83 presents the results of the DSAs.
| Scenario | Guidelines plus FeNO (NObreath) (£) | Guidelines plus FeNO (NIOX VERO) (£) | Guidelines plus FeNO (NIOX MINO) (£) | Guidelines (£) | |
|---|---|---|---|---|---|
| M1 | Point estimates of parameters | 2248 | Dominated | Dominated | – |
| M2 | Undiscounted costs and outcomes | 740 | Dominated | Dominated | – |
| M3 | Discount rate = 6% | 3534 | Dominated | Dominated | – |
| M4 | Pregnant women subgroup | Dominating | Dominated | Dominated | – |
| M6 | Analysis based on Smith et al.119 | 184,095 | Dominated | Dominated | – |
| M7 | Analysis based on Syk et al.99 | Dominated | Dominated | Dominated | Dominating |
| M8 | FeNO impact = 1 year | 885,451 | Dominated | Dominated | – |
| M9 | FeNO impact = 2 years | 434,284 | Dominated | Dominated | – |
| M10 | FeNO impact = 3 years | 283,954 | Dominated | Dominated | – |
| M11 | FeNO impact = 4 years | 208,833 | Dominated | Dominated | – |
| M12 | FeNO impact = 5 years | 163,795 | Dominated | Dominated | – |
| M13 | FeNO impact = 10 years | 73,975 | Dominated | Dominated | – |
| M14 | FeNO impact = 15 years | 44,320 | Dominated | Dominated | – |
| M15 | FeNO impact = 20 years | 29,707 | Dominated | Dominated | – |
| M16 | FeNO impact = 30 years | 15,531 | Dominated | Dominated | – |
| M17 | FeNO impact = 40 years | 8898 | Dominated | Dominated | – |
| M18 | Marginal per-test FeNO cost doubled | 15,273 | Dominated | Dominated | – |
| M19 | Marginal per-test FeNO cost halved | Dominating | Dominated | Dominated | – |
| M20 | NObreath lifetime = 3 years | 5598 | Dominated | Dominated | – |
| M21 | NObreath lifetime = 5 years | 3294 | Dominated | Dominated | – |
| M22 | NObreath lifetime = 20 years | 3246 | Dominated | Dominated | – |
| M23 | FeNO nurse visits doubled | 52,246 | Dominated | Dominated | – |
| M24 | FeNO nurse visits halved | Dominating | Dominated | Dominated | – |
| M25 | Exacerbation rates doubled | Dominating | Dominated | Dominated | – |
| M26 | Exacerbation rates halved | 9958 | Dominated | Dominated | – |
| M27 | Exacerbation disutility doubled | 1563 | Dominated | Dominated | – |
| M28 | Exacerbation disutility halved | 2634 | Dominated | Dominated | – |
| M29 | ICS observed mean carried forward | 66,453 | Dominated | Dominated | – |
| M30 | ICS change doubled | Dominating | Dominated | Dominated | – |
| M31 | ICS change halved | 23,392 | Dominated | Dominated | – |
The DSAs indicate the following:
-
The results of the analysis using point estimates of parameters are very similar to those produced using the probabilistic version of the model.
-
NIOX MINO and NIOX VERO are expected to be consistently dominated by NObreath because of their higher marginal per-test costs.
-
FeNO monitoring using NObreath is expected to dominate standard guidelines in the subgroup of women who are pregnant.
-
Discounting has little impact on the cost-effectiveness of FeNO monitoring.
-
Although the marginal per-test cost influences which device would be preferred, it does not have a substantial impact on the overall cost-effectiveness of FeNO monitoring compared with guidelines.
-
The use of exacerbation rates from Syk et al. 99 and Smith et al. 119 has a substantial negative impact on the cost-effectiveness of FeNO monitoring.
-
The duration over which FeNO monitoring is assumed to impact on exacerbations and ICS use is a key driver of cost-effectiveness. It is noteworthy that, in the adult subgroup, cost-effectiveness improves over longer time horizons whereas the opposite is true in the child subgroup, in which cost-effectiveness worsens over longer time horizons. This is driven entirely by the observed differences in relative ICS use for FeNO monitoring and guidelines at the last observed time point in the trials.
-
The cost-effectiveness of FeNO monitoring is markedly less favourable when projected ICS use is modelled according to the mean ICS use observed in the trial reported by Shaw et al. 98
Discussion
Summary of cost-effectiveness evidence
There is very limited evidence available on the cost-effectiveness of FeNO testing for the diagnosis and/or management of asthma. The systematic review presented in this chapter identified one published UK model of FeNO testing in the diagnostic setting and one published model of FeNO testing in the management setting. These models were published within the same paper. 147 Aerocrine submitted a model of FeNO testing for diagnosis and a model of FeNO testing for management; these models were similar to, but not the same as, the published Price et al. 147 models.
The Price et al. 147 diagnostic model indicates that NIOX MINO is likely to be cost saving in comparison to other tests routinely used in the diagnosis of asthma. The model analysis presented by Price et al. 147 also suggests that NIOX MINO is expected to be more expensive than standard diagnostic tests when used in conjunction with other tests. The EAG critique of this model highlighted a number of problems including the use of a blended comparison, the questionable selection of evidence used to inform the model’s parameters and the absence of any quantified health consequences associated with diagnostic test outcomes. The Aerocrine diagnostic model is similar in structure to the published version but does not use a blended comparison approach and includes some updated parameter values. However, the Aerocrine model also fails to reflect the health consequences associated with correct or incorrect diagnostic outcomes. Because of their limited scope, these diagnostic models do not provide any information regarding the economic trade-off between potential additional health gains resulting from the more accurate diagnosis of asthma and the health loss associated with displacing existing services.
The Price et al. 147 management model compares FeNO monitoring using NIOX MINO with guidelines. This model was evaluated within a cost–utility framework and indicates that NIOX MINO produces more health gain at a lower cost than guidelines; in other words, NIOX MINO dominates management using guidelines alone. Aerocrine submitted a similar management model that included some different data and assumptions but ultimately produced the same conclusion as the published analysis reported by Price et al. 147 The EAG critique of these management models highlighted a number of problems including the use of a short time horizon, the selective use of efficacy evidence, the assumptions made regarding equivalence between sputum count monitoring and FeNO and invalid assumptions regarding the health losses associated with exacerbations. No economic evidence was submitted by the manufacturers for either NIOX VERO or NObreath. The EAG takes the view that neither the published Price et al. 147 models nor the submitted Aerocrine models represent a suitable basis for informing decision-making about the use of FeNO testing for the diagnosis or management of asthma.
In light of the problems with the available evidence, the EAG developed two de novo models:
-
a model to assess the cost-effectiveness of FeNO testing using NIOX MINO, NIOX VERO and NObreath in addition to, or in place of, existing tests compared with other diagnostic options commonly used in the diagnosis of asthma
-
a model to assess the cost-effectiveness of NIOX MINO, NIOX VERO and NObreath plus guidelines compared with guidelines alone for the management of asthma.
The EAG diagnostic model suggests that, across the diagnostic options included in the economic analysis, the expected difference in QALY gains is likely to be very small. Airway hyper-responsiveness (MCT) is expected to produce the greatest QALY gain; this is because this option has the highest sensitivity of all of the tests included in the economic analysis. All options that include NIOX MINO or NIOX VERO are expected to be dominated as their marginal per-test cost is higher than that for NObreath (assuming a device lifetime of 10 years). In the base-case analysis, all options except airway hyper-responsiveness (MCT) and FeNO monitoring (NObreath) plus bronchodilator reversibility testing are expected to be ruled out by simple dominance. The incremental cost-effectiveness of airway hyper-responsiveness (MCT) compared with FeNO monitoring (using NObreath) plus bronchodilator reversibility is expected to be £1.125M per QALY gained. The results of the analysis are particularly sensitive to assumptions about the duration of time required to resolve misdiagnoses, assumptions about health losses incurred by patients who are FNs, the costs of asthma management and the use of ‘rule-in’ and ‘rule-out’ diagnostic decision rules.
The EAG management model was evaluated across two subgroups: (1) children and (2) adults. Within both the child and the adult subgroup base-case analyses, FeNO testing is expected to produce a small incremental QALY gain compared with guidelines alone. In both subgroups, NIOX MINO and NIOX VERO are expected to be dominated as their marginal per-test costs are higher than that for NObreath. Within the child subgroup, the incremental cost-effectiveness of guidelines plus FeNO monitoring using NObreath compared with guidelines alone is expected to be approximately £45,200 per QALY gained. This ICER is influenced considerably by the assumed change in ICS use, which is applied over a lifetime horizon. Within the adult subgroup, FeNO monitoring using NObreath compared with guidelines alone is expected to cost approximately £2100 per QALY gained. A similarly favourable result was produced within a further analysis based on a subgroup of women who are pregnant. 102 Importantly, these positive results are not held when alternative trials are used to inform the analysis. 99,119 The results in the child and adult subgroups are particularly sensitive to assumptions regarding changes in ICS use over time, the number of nurse visits for FeNO monitoring and the duration over which FeNO monitoring is assumed to impact on exacerbations and ICS use.
Limitations of the External Assessment Group models
Although the EAG models presented here do resolve many of the problems identified within the Price et al. 147/Aerocrine models, the results drawn from these models remain subject to considerable uncertainty. These are briefly discussed in the following sections.
Limitations of the diagnostic model
The following represent the key limitations and uncertainties within the EAG diagnostic model:
-
Use of naive indirect comparisons. Limitations in the diagnostic evidence base meant that naive indirect comparisons across studies that assess the diagnostic accuracy of different tests were used in the model. The review presented in Chapter 3 highlighted considerable heterogeneity between these studies. As such, the results of the economic analysis of FeNO monitoring in the diagnostic setting should be interpreted with caution.
-
Non-systematic approach to including non-FeNO comparators. We did not undertake a systematic review of evidence concerning the diagnostic accuracy of existing tests used in the diagnosis of asthma but instead relied on studies picked up by our systematic review of FeNO studies. 86,163 Although a formal review of other tests (excluding FeNO monitoring) would be valuable, this was beyond the scope of the assessment and the time and resources available to the EAG precluded this work. It is likely that other studies exist and it is possible that these could be considered more relevant than the studies used in the EAG model.
-
Use of a ‘blunt’ model structure. We adopted a similar model structure to that of Price et al. ,147 which assesses options at a particular point in the diagnostic pathway rather than attempting to simulate the entire sequence of tests used throughout the pathway. This model development decision was taken because of limitations in the available evidence.
-
Uncertainty surrounding health losses associated with misdiagnosis. We crudely elicited estimates of the duration required to resolve a FN/FP diagnosis. Only one of our experts was able to tentatively quantify the likely values of these parameters. These estimates are very uncertain. There is also uncertainty surrounding the magnitude of the HRQoL loss as well as the duration over which this loss is incurred. It is possible that health losses associated with FP diagnoses in patients with more serious underlying pathology are underestimated. It is not clear how this uncertainty could be resolved empirically.
Limitations of the management model
The following represent the key limitations and uncertainties within the EAG management model:
-
Use of effectiveness evidence. The model uses individual studies within the child and adult subgroups. These studies were deemed by the EAG to most closely reflect asthma management in England and Wales. However, the Szefler et al. 104 study was undertaken in the USA and does not fully match BTS/SIGN guidelines8 on dose titration. Only the use of guidelines in the comparator group within the Shaw et al. 98 study can be considered as ‘standard’ within the UK.
-
Uncertainty surrounding the duration over which FeNO monitoring impacts on dose titration. In line with the NICE reference case, the EAG model reflects a lifetime horizon. There is, however, considerable uncertainty with respect to the duration over which FeNO monitoring would result in different exacerbation rates and ICS use compared with guidelines alone. Within the base-case analysis we assumed that this impact would be sustained indefinitely. The sensitivity analysis shows that this parameter is a key driver of cost-effectiveness.
Both the EAG diagnostic model and the EAG management model assume that all FeNO devices have the same diagnostic properties in terms of absolute FeNO measurements and how this translates into sensitivity and specificity. This necessary assumption may not hold in reality.
Areas for further research
Further research would be valuable to reduce some of the uncertainties detailed in the previous sections. In particular, comparative studies that include FeNO monitoring alongside the range of existing standard tests with a common population and a common reference standard of long-term follow-up of at least 1 year would be useful in assessing the comparative accuracies of these alternative diagnostic strategies. In addition, longer-term studies of FeNO monitoring in combination with standard UK management guidelines would be beneficial to better understand the long-term impacts on asthma medication use and exacerbations.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Beyond its likely clinical effectiveness and cost-effectiveness, a number of other factors relating to the implementation of FeNO testing in the NHS require consideration.
Training and education
The introduction of FeNO testing for the diagnosis and/or management of asthma has implications for training and education in terms of teaching NHS staff how to instruct patients to use the devices correctly to minimise test failure rates. Repeatability and accuracy of the devices are not dependent on patient performance as the devices will not produce a measurement if flow rate and length of exhalation limits are not met. The precise training and education requirements associated with introducing FeNO testing are dependent on whether it is routinely recommended and, if so, the setting that such recommendations relate to. Training may be required for primary care nurses and GPs or for secondary care staff or for both. It should be noted that these additional costs are not reflected in the marginal per-test costs used within the economic analysis presented in Chapter 4.
Purchasing of equipment and consumables
The diffusion of FeNO testing into routine NHS practice would involve the purchasing of additional equipment either for GP surgeries or for hospitals. Equipment costs include the costs of the devices, the replacement parts (NObreath only) and other consumables (test kit mouthpieces). The NIOX MINO and NIOX VERO devices both have a finite lifetime and would need to be replaced at a maximum of 3 years and 5 years respectively. The NObreath device does not have a finite lifetime but does require replacement sensor cells every 2 years. Each FeNO device requires the purchase of test kit mouthpieces; the volume purchased and the number of tests undertaken will influence the overall marginal per-test cost of each device for GP surgeries and trusts. Maintenance of the NObreath device is expected to be free of charge to the NHS. Aerocrine did not mention the cost of maintenance in its cost estimates.
Replacement of the NIOX MINO device with the newer NIOX VERO device
It is anticipated that the NIOX MINO device will soon be replaced with the newer NIOX VERO device. Both FeNO devices will be available for some time but, in the long term, the NIOX MINO device will eventually become redundant. It is likely that the NIOX VERO device will be less expensive (per test) than the NIOX MINO device and hence the justification for purchasing the NIOX MINO device is unclear.
Impact on the demand for current standard diagnostic tests
The introduction of FeNO testing in a diagnostic setting will likely have an impact on the demand for other existing standard tests currently used in the diagnosis of asthma. This change in the level of demand for existing standard tests will be dependent on how FeNO testing is incorporated into the existing asthma diagnosis pathway.
FeNO testing in children
The diagnostic and clinical evidence considered in this assessment is restricted to patients aged ≥ 5 years. The potential diagnostic/management benefit of FeNO use in younger children is unknown.
FeNO testing in older adults
Monitoring of FeNO levels does not seem to be a useful test in the diagnosis or management of older adults with asthma. In this population, other current standard tests and management approaches may be more applicable.
Patients with respiratory tract infections
Most studies included in this assessment (see Chapter 3) purposefully excluded patients with a recent respiratory tract infection. The diagnostic utility of FeNO testing in these patients is unclear. It may be more appropriate either to use standard diagnostic tests in these patients or to allow a period of recovery before using FeNO testing.
Chapter 6 Discussion
Statement of principal findings
Equivalence of devices
Although there was often a good correlation between FeNO measurement devices, equivalence of readings could not necessarily be assumed in all situations. Many studies concluded that the comparability of measurements between devices was within clinically acceptable limits; however, others went on to produce correction equations to correct for systematic bias in measurements. There was also no common justified definition of clinically acceptable differences and 95% limits of agreement were sometimes very wide (around 20 ppb). There seemed to be a generally consistent observation of poorer equivalence between FeNO devices at higher FeNO levels. The direction of disagreement varied between studies and between comparator devices.
However, as there is mostly a high degree of correlation between measurements across all devices, estimates of sensitivity and specificity are likely to be a reasonable indication of the potential diagnostic accuracy of using FeNO monitoring to guide diagnosis and management, but the derived cut-off points are not likely to be interchangeable between devices. As such, for the purpose of this assessment, sensitivities and specificities will be assumed to be interchangeable, but it cannot be assumed that the cut-off points that should be used to achieve them will be the same in each device, and there is still some doubt whether the same diagnostic accuracy would be achievable in all devices. This is an important issue that should be considered in the interpretation of the diagnostic accuracy review and the findings of the health economic analysis assessment presented within this report.
Test failure rates were generally low for all devices in adults, with the highest reported rate being 3.3%. With regard to children, there may be some problems with using the NIOX MINO device in younger children, with failure rates ranging from 5.5% to 27%. One study used the NObreath device with children and reported no test failures.
Diagnostic accuracy review
This review identified several groups of studies that were similar to one another in terms of the position of the patients in the UK pathway and the reference standards used. Groups were adults presenting with symptoms of asthma compared with most of or the entire UK pathway; a subset of adults presenting with symptoms of asthma compared with airway hyper-responsiveness; difficult-to-diagnose patients compared with airway hyper-responsiveness; patients with chronic cough who were difficult to diagnose compared with ICS responsiveness; and children with symptoms of asthma compared with various reference standards.
No meta-analysis was conducted in any group as clinical heterogeneity between studies was generally extremely high. Estimates of sensitivity and specificity were not consistent within groups and ranged widely in rule-in scenarios, rule-out scenarios and when the pair with the highest sum of sensitivity and specificity were selected. Table 84 summarises the results across studies and groups of studies. Given the wide-ranging estimates of sensitivity and specificity, together with heterogeneous cut-off points, it is difficult to draw any firm conclusions as to the diagnostic accuracy of FeNO monitoring in any situation and at any given cut-off point. Interestingly, there did not appear to be an obvious difference between the diagnostic accuracy of FeNO monitoring compared with the whole or parts of the UK pathway in patients who present with symptoms of asthma and the diagnostic accuracy of FeNO monitoring compared with airway hyper-responsiveness in patients who are difficult to diagnose. The large variation in estimates within groups may obscure any true underlying differences in the accuracy of FeNO monitoring between groups and compared with different reference standards.
| Patients | Reference standard | Number of studies | Highest sum of sensitivity and specificity | Rule-out scenario | Rule-in scenario | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range of cut-offs (ppb) | Range of sensitivity values (%) | Range of specificity values (%) | Range of cut-offs (ppb) | Range of sensitivity values (%) | Range of specificity values (%) | Range of cut-offs (ppb) | Range of sensitivity values (%) | Range of specificity values (%) | |||
| Adults with symptoms of asthma | Part or whole of the UK pathway | 4 | 20–47 | 32–88 | 75–93 | 9–15 | 85–96 | 13–48 | 47–76 | 13–55.6 | 88.2–100 |
| Subset of patients at position A | Airway reversibility or airway hyper-responsiveness | 2 | 27–36 | 77.8–87 | 60–92 | 25 | 100 | 46.7 | 100 | 27.8 | 100 |
| Difficult-to-diagnose patients | Airway hyper-responsiveness | 4 | 32–40 | 24.4–74.3 | 72.5–98.9 | NR | NR | NR | NR | NR | NR |
| Patients with chronic cough, difficult to diagnose | ICS responsiveness | 3 | 20–38 | 53–94.7 | 63–85 | NR | NR | NR | NR | NR | NR |
| Children with symptoms of asthma | Various | 4 | 19–21 | 49–86 | 76–89 | 5–20 | 89–94 | 14.1–70 | 30–50 | 20–50 | 92–100 |
However, some limited observations can be made. It would appear that FeNO monitoring was more often able to reach 100% specificity than 100% sensitivity and that ranges of specificity were generally tighter. This may indicate that it has the most potential for use as a rule-in test. It would also appear that FeNO cut-off points should probably be lower in children than in adults.
In addition to the above, two studies were found that reported results for FeNO monitoring in conjunction with another test in adults, one in those difficult to diagnose77 and one in patients of all ages with symptoms of asthma. 87 In both cases the addition of another test to the diagnostic protocol resulted in a change in diagnostic accuracy, but as this involved the usual trade-off between sensitivity and specificity it is difficult to tell if this represents an increase or decrease in clinical effectiveness and cost-effectiveness.
Evidence was limited in the subgroups defined a priori, namely pregnant women, the elderly and smokers/those subjected to environmental tobacco exposure.
-
Smokers. FeNO monitoring appeared to be able to distinguish between asthmatic and non-asthmatic adult smokers with similar accuracy as for non-smokers and ex-smokers. It would seem likely that the FeNO level is generally lower in smokers than in mixed cohorts and it may be useful to consider a patient’s smoking status when interpreting results or to select lower cut-off points for smokers. Limited data in children support the same conclusion as for adults.
-
The elderly. Available data were extremely limited and did not always provide appropriate comparisons between elderly asthmatics and elderly non-asthmatics. A case–control study indicated that FeNO monitoring is unlikely to be a useful test in the diagnosis of asthma in the elderly.
-
Pregnant women. A cross-sectional study indicated that pregnancy does not alter FeNO levels in asthmatics or non-asthmatics and that FeNO monitoring can distinguish between asthmatic and non-asthmatic pregnant and healthy women.
FeNO-guided management of asthma
Five studies on FeNO-guided management of asthma in adults were identified. There were high levels of heterogeneity in multiple study characteristics and outcome definitions and as such it was not possible to draw any firm conclusions as to which step-up/step-down protocol or cut-off points offer the best efficacy. All studies reported a fall in exacerbation rates per person-year, although it appeared that this was mostly driven by mild and moderate exacerbations and was statistically significant in only one study. 99 Exploratory pooled analyses showed no statistically significant difference for severe exacerbations and a statistically significant decrease in exacerbations in the intervention groups when considering the composite outcome of any severity of exacerbation. The effects on ICS use were heterogeneous, with two studies showing statistically significant decreases in ICS use in the FeNO-guided management groups, one study showing a very minor increase (significance not reported) and another showing very similar levels of use in each arm. This may indicate that some step-up/step-down protocols were better at decreasing ICS use than others or it may be because of the characteristics of the study populations. Pooled analysis showed decreased ICS use in the intervention arm, but the difference was not statistically significant. HRQoL was infrequently reported; two studies used versions of the AQLQ to measure quality of life and both showed no effect of FeNO monitoring on the global score, but one investigated domains and found a statistically significant difference in the symptoms score.
Despite the heterogeneity in the results, and the lack of statistically significant findings in individual studies, it would seem possible that, on the basis of an exploratory class-effects meta-analysis in adults, FeNO-guided management protocols of some or most designs may, during the first year of management, result in better management overall (considering all exacerbations), with either a small or a zero reduction in ICS use. Further larger studies are needed to clarify any treatment effects. There was no evidence relating to whether these effects would be maintained over a longer time period.
In pregnant women, the use of FeNO monitoring to guide asthma management appears to be as effective if not more effective than the use of FeNO monitoring to guide asthma management in other adults and appears to reduce exacerbations and ICS use. This may be because of increased efficacy in pregnant women or because of differences in step-up/step-down protocols. Notably, this protocol allowed for the step-down of ICS use on the basis of FeNO levels alone, regardless of whether symptoms were still present or not. A follow-up study suggests that there may be benefits to the children born to women who had FeNO-guided asthma management that have not been captured in the economic model.
Studies looking at FeNO-guided asthma management in the elderly were limited by study quality. The majority of studies suggest that FeNO monitoring is not useful in the elderly because of a lack of difference in FeNO levels between elderly asthmatics and elderly non-asthmatics and no correlation between FeNO levels, sputum eosinophils, asthma control, quality of life and so on in elderly asthmatics.
Levels of FeNO in smokers appeared to be generally lower than those in non-smokers but still appeared to be responsive to changes in control and eosinophils. Lower cut-off values for management might be necessary in smokers.
Seven studies in children were identified. One study appeared to recruit a group of patients who were well controlled whereas two others recruited patients who appeared to be poorly controlled. Both reported fewer severe exacerbations in the intervention arm, but not statistically significantly so. All studies reported a decrease in exacerbations (however defined) in the intervention arm, but only one reported a statistically significant reduction. The effects on ICS use were heterogeneous, with two studies showing a statistically significant increase in ICS use, one showing no difference, one being difficult to interpret and one further study not reporting this outcome. HRQoL was only reported within one study, although insufficient details were reported.
Because of the high levels of heterogeneity in multiple study characteristics and outcome definitions, it was not possible to draw any firm conclusions as to which step-up/step-down protocol or cut-off points offer the best efficacy for management. Results were generally not statistically significant but this may be because of small sample sizes in some cases; meta-analysis was precluded by the ability to calculate rate ratios. However, because all but one study reported numerically smaller numbers or rates of exacerbations in the intervention arm, it would seem possible that FeNO-guided management protocols of most descriptions could, during the first year of management, result in better management (fewer exacerbations) overall, despite the lack of statistically significant results in individual studies. Further larger studies are needed to clarify any treatment effect. It is unclear whether ICS use is likely to increase or decrease and this may depend on the details of the step-up/step-down protocols or the characteristics of the patients recruited to the trials in terms of control and severity.
Independent assessment of cost-effectiveness
The EAG developed two de novo models. The first model assesses the cost-effectiveness of FeNO testing using NIOX MINO, NIOX VERO and NObreath in addition to, or in place of, existing tests compared with other diagnostic options commonly used in the diagnosis of asthma. The second model assesses the cost-effectiveness of NIOX MINO, NIOX VERO and NObreath plus guidelines compared with guidelines alone for the management of asthma.
The EAG diagnostic model suggests that, across the 17 options included in the analysis, airway hyper-responsiveness (MCT) is expected to produce the greatest QALY gain. All options that include NIOX MINO or NIOX VERO are expected to be dominated as their marginal per-test costs are higher than that for NObreath. The incremental cost-effectiveness of airway hyper-responsiveness (MCT) compared with FeNO testing (using NObreath) plus bronchodilator reversibility is expected to be £1.125M per QALY gained. All other options are ruled out of the analysis because of simple dominance. The results of the analysis are particularly sensitive to assumptions about the duration of time required to resolve misdiagnoses, assumptions about health losses incurred by patients who are false-negative, the costs of asthma management and the use of ‘rule-in’ and ‘rule-out’ diagnostic decision rules.
The EAG management model was evaluated separately for the child and adult subgroups. Within both the child and adult subgroup analyses, FeNO monitoring plus guidelines is expected to produce a small incremental QALY gain compared with guidelines alone. NIOX MINO and NIOX VERO are expected to be dominated as their marginal per-test costs are higher than that for NObreath. Within the adult subgroup, FeNO monitoring using NObreath compared with guidelines alone is expected to cost approximately £2100 per QALY gained. A similarly favourable result was produced within a further analysis based on a subgroup of women who are pregnant. 102 Importantly, these positive results for the adult subgroup do not hold when alternative trials are used to inform the analysis. 97,99 Within the child subgroup, the incremental cost-effectiveness of guidelines plus FeNO monitoring using NObreath compared with guidelines alone is expected to be approximately £45,200 per QALY gained. A more favourable ICER was produced when the analysis was based on the trial reported by Pijnenburg et al. ;106 this may reflect differences in the characteristics of patients recruited to these trials, with the former trial being uncontrolled. The results in the child and adult subgroups are particularly sensitive to assumptions regarding changes in ICS use over time, the annual number of nurse visits for FeNO monitoring and the duration over which FeNO monitoring is assumed to impact on exacerbation rates and ICS use.
Generalisability of the results
Generalisability of the evidence relating to FeNO monitoring in the diagnosis of asthma
-
The clinical evidence was heterogeneous in terms of clinical characteristics and results and studies were selected for modelling based on their similarity to UK practice and similarity to the subgroups of interest as defined in the protocol (i.e. those who are difficult to diagnose or the wider population of those presenting with symptoms of asthma). As such, no single study can be generalised to the whole population and this should be noted when interpreting the results of this assessment.
-
Some of the subgroups of interest to the appraisal were not modelled. These groups were the elderly, pregnant women and smokers/those exposed to environmental tobacco smoke. This was because of limitations in the identified evidence. Only inferences as to the generalisability of results from other studies to these populations can be made.
-
The EAG model is ‘blunt’ in that it assumes that all misdiagnoses are assumed to be later corrected by subsequent tests. The model is not specific about what these tests are.
-
In addition, all but one95 of the studies used to inform the diagnostic accuracy parameters were undertaken in adults. As a consequence, the EAG model does not fully capture differences in the likely diagnostic pathways between child and adult subgroups.
Generalisability of the evidence relating to FeNO monitoring in the management of asthma
-
In adults, the studies used in the model were those by Shaw et al. ,98 Smith et al. 97 and Syk et al. 99 Each study has its own merits in terms of generalisability.
-
Shaw et al. 98 followed UK practice in terms of the comparator arm management strategy. They also recruited a population from primary care and included mild to severe asthmatics regardless of atopic status. Smokers were excluded and so it is not clear if the results can be generalised to the UK smoking population. It was also not clear which FeNO device was used.
-
Smith et al. 97 recruited what is likely to be a population with mild to moderately severe asthma and used a different step-up/step-down protocol in the control arm and the intervention arm. It is unclear to what extent this study could be generalisable to the UK population but it nevertheless provides some insight into the impact that different but plausible efficacy inputs have on the cost-effectiveness estimates.
-
The study by Syk et al. 99 is most notable for having recruited only atopic patients, only non-smokers and only mild to moderate asthmatics. This study is unlikely to have wide generalisability. However, again, it nevertheless provides some insight into the impact that different but plausible efficacy inputs have on the cost-effectiveness estimates.
-
-
In children, the two studies that were modelled were those by Szefler et al. 104 and Pijnenburg et al. ,106 largely because these two studies reported the most complete sets of data and recruited different populations. Again, each study has its own merits in terms of generalisability.
-
The study by Szefler et al. 104 had the lowest risk of bias amongst the studies available. It also recruited patients who were difficult to treat, one of the subgroups identified in the scope as being of special interest, and so generalisability may be limited to this group. However, the step-up/step down protocol within this trial did not allow for ICS use to be decreased on the basis of low FeNO levels alone, making it less likely that a decrease in ICS use will be seen in the intervention arm than in some other protocols. Therefore, the generalisability of this study largely depends on what type of step-up/step-down protocol is likely to be adopted in the UK.
-
Pijnenburg et al. 106 adopted inclusion criteria that were likely to result in a population of asthmatics who have more stable disease. The step-up/step-down protocol also does not allow for ICS use to be decreased on the basis of low FeNO levels alone, requiring that symptoms are also low. As such, the generalisability of this study also largely depends on what type of step-up/step-down protocol is likely to be adopted in the UK.
-
-
One study was found that recruited pregnant women. The management strategy allowed step down of ICS use on the basis of FeNO levels alone. This study can be generalised within the population of pregnant women.
Equivalence of devices
-
As the equivalence of devices is not assured, the generalisability of these results to all three devices is also not assured.
-
It is thought that estimates of diagnostic accuracy and efficacy in managing asthma are probably achievable by all devices, as correlation between measurements is good. However, the actual values that should be used as cut-offs in diagnosis and management are much more difficult to generalise and further research may be required to estimate the most appropriate values.
Strengths and limitations of the assessment
Strengths of the assessment
The assessment includes systematic reviews of the equivalence of devices, diagnostic accuracy, management efficacy and test failures, which have been undertaken according to robust and high-quality methods.
The scope of the assessment was agreed by NICE and the SCMs during an extensive scoping exercise.
The existing economic evidence base models have been formally critiqued using the Drummond et al. 144 checklist and assessed in terms of adherence of the individual studies to the NICE reference case. 145
The two economic models have been developed to a high standard and are based on the decision problem rather than being limited by the available empirical evidence. Both EAG models explicitly address the trade-off between expected additional health gains resulting from the more accurate diagnosis of asthma and the health losses associated with displacing existing services. Although many of the parameters included in these models are subject to considerable uncertainty, the use of a modelling framework helps elucidate which parameters are likely to be most important for decision-making.
The assessment report has been peer reviewed by NICE, other experienced HTA researchers and leading experts in the diagnosis and management of inflammatory airways diseases.
Limitations of the assessment
This assessment is subject to several limitations. It is important to note that these limitations are principally sourced in the evidence base rather than in the methods used to interrogate and evaluate it. Overall, the evidence base for this assessment was not of the highest quality. No end-to-end studies were found that estimated the clinical utility of FeNO testing in the diagnosis of asthma and no studies were found that used NIOX VERO or NObreath. As such, clinical validity studies were included and a review of the equivalence of devices was conducted. This leads to the following limitations:
-
The benefits and harms associated with the diagnosis of asthma using FeNO testing have been estimated based on modelling of the consequences of being TP, TN, FP and FN. This includes a large number of assumptions and extrapolations, many of which cannot be substantiated with empirical evidence.
-
The equivalence of devices is assumed and this may not hold true in practice. As such, FeNO cut-off values reported in the primary research may not be applicable to measurements using other devices.
-
The NObreath device will always dominate other devices as its efficacy has been assumed to be equivalent but its unit cost is less.
No study provided estimates relating to the additional diagnostic value of FeNO testing to the whole UK diagnostic pathway. This limits the scope of the economic analysis.
No short-term diagnosis of asthma is 100% accurate and as such all diagnostic studies included in the review had a flawed reference standard. However, in the absence of any alternative, these reference standards were considered to be 100% accurate. A better reference standard would have been long-term follow-up of patients; however, only one study95 used such a reference standard.
None of the management studies in children included a step-up/step-down protocol that allowed ICS use to be stepped down on the basis of FeNO levels alone. This will limit the degree to which ICS use can be reduced and means that one of the major putative benefits of FeNO management has not actually been assessed empirically: the identification of ICS non-responsive asthmatics who can be taken off ICS therapy with no loss of control.
The EAG diagnostic model is based on evidence identified through the systematic review of FeNO monitoring. The diagnostic accuracy of other non-FeNO comparators [spirometry, airway reversibility (MCT) and bronchodilator reversibility] was based on comparative studies identified through the review process. It is possible that other studies not identified within the review could be considered relevant to the model. The use of the Hunter et al. 163 case–control study does, however, mean that all non-FeNO diagnostic options are assessed consistently within the same study.
The EAG diagnostic model and the Price et al. 147/Aerocrine diagnostic models draw a number of naive indirect comparisons across studies; this is a limitation of the evidence base rather than of the assessment. It does, however, limit the confidence that can and should be placed in the findings of these diagnostic models.
The EAG management model is based on short-term evidence of the comparative efficacy of FeNO testing compared with guidelines. The extrapolation of these benefits to the longer term is subject to considerable uncertainty. Again, this limitation reflects the evidence base rather than the model itself.
Two previous systematic reviews of the effectiveness of FeNO monitoring to guide management were identified. Petsky et al. 31 performed a Cochrane review in 2008, which was updated with data from two new studies in 2009. A total of six studies were included in the update (two adult studies83,98 and four studies in children/adolescents103,104,106,190), all of which compared adjustments in asthma therapy based on FeNO monitoring with those based on conventional methods (typically clinical symptoms and spirometry). A meta-analysis was performed for seven outcomes: number of patients with more than one exacerbation, exacerbation rates, FEV1% predicted at the final study visit, FeNO level at the final visit, symptom score, ICS dose at the final visit and geometric mean change in FeNO level from baseline. There was some suggestion of benefits associated with FeNO monitoring for several outcomes, in particular the number of subjects with more than one exacerbation, exacerbation rates, FEV1% predicted at the final visit and geometric mean change in FeNO level from baseline; however, none of these results was statistically conclusive. There were also some results that suggested inconsistent effects between adult and child cohorts. FeNO monitoring appeared to have some beneficial effects on the symptom score in adults (mean difference –0.14, 95% CI –0.42 to 0.14) but not children (mean difference 0.04, 95% CI –0.11 to 0.20) and FeNO management lowered the ICS dose in adults (mean difference –450.03 µg, 95% CI –676.73 µg to –223.34 µg) but not children (mean difference 140.18 µg, 95% CI 28.94 µg to 251.43 µg). Furthermore, there were some limitations to the meta-analysis, particularly with respect to the studies in children. There was substantial clinical heterogeneity among the study cohorts, with no two studies using exactly the same step-up/step-down protocols. The study by de Jongste et al. ,190 which included a telemedical component, was not of relevance to our current assessment, making the results of this meta-analysis not directly applicable to this review.
It can be seen that there is a high degree of agreement between the Petsky et al. 31 review and our own review, especially with relation to the lack of statistically significant effects and some differences between adults and children. The strength of our review lies in the inclusion of subsequently published studies, the focus on exacerbation rates rather than the number of people with an exacerbation and the a priori separation of both children and pregnant women into different subgroups.
The second review was an academic-in-confidence manufacturer’s submission to NICE (Aerocrine. Meta Analysis of Asthma Exacerbation Rates with FeNO Guided Asthma Management. Aerocrine submission to NICE, 2013). This review has subsequently been published. 191 This review updated the meta-analyses of the number of patients with more than one exacerbation and exacerbation rates from the aforementioned Cochrane review31 with a study of FeNO-guided asthma management in pregnant women. 102 Inclusion of this study resulted in improvements on all measures of exacerbations, especially asthma exacerbation rates in adults (mean difference –0.27, 95% CI –0.42 to –0.12) and relative rate of asthma exacerbations in adults (relative rate 0.57, 95% CI 0.41 to 0.80). However, as it is known that pregnancy can substantially affect the course of asthma,192 it was arguably inappropriate to include the cohort of pregnant women in meta-analyses of adults with asthma. Indeed, in the current review of FeNO-guided management, we have interpreted the results of the Powell et al. 102 study of pregnant women separately from the main results for just this reason.
Research recommendations
This appraisal has been limited by several key evidence gaps that would benefit from further research. It could be argued that this technology is currently under-researched and that any conclusions drawn at this stage may be unduly affected by this lack of evidence. However, some of the problems with the evidence base seem intractable in terms of practicalities and it could also be argued that the available evidence does point towards some benefits of the technology, albeit benefits that are difficult to quantify with certainty.
Some key problems and suggested research priorities are listed here:
-
The equivalence of devices is not assured. There are several ways that this problem could be addressed, none of which offers a panacea:
-
Additional extensive equivalence testing of all devices in relation to one another to ascertain what is driving the heterogeneity in study results. This may be expensive and time-consuming and may still reveal high levels of disagreement between studies because of the evidence of variability between devices of the same design.
-
A network meta-analysis of the existing evidence. This was precluded in this project because of time and resource constraints. There is likely to be a high degree of uncertainty in any such analysis based on current evidence and its results may not be useful.
-
Derivation and validation studies conducted using the devices in question to develop unique cut-off points for each device for management and diagnosis. This may also be expensive and time-consuming.
-
Exploration of the option of using intrasubject relative change to assess control when managing asthma. There is already evidence relating to this approach but it appears to be in the comparatively early stages of development. This is not likely to be a useful option in diagnosis.
-
-
Cut-off values are highly variable and are largely based on derivation studies not validation studies. This is related to problems with the equivalence of devices. Possible research priorities relating to this include large validation studies (possibly preceded by derivation studies) to determine cut-off values in all populations of interest, using a number of available devices. Although expensive and time-consuming, these studies could be very valuable.
-
The clinical utility of the diagnosis of asthma using FeNO monitoring compared with the diagnosis of asthma using current practice is not informed by direct evidence. Possible research priorities relating to this include a study that charts the clinical utility of the diagnosis of asthma using FeNO monitoring compared with the diagnosis of asthma using current guidelines against a reference standard of long-term follow-up of diagnosis to correct for the misdiagnoses of both diagnostic approaches.
-
It is unclear which step-up/step-down protocol offers the best efficacy. Possible research priorities relating to this include:
-
Studies that compare different management protocols with one another. It may be that different protocols are necessary in different populations.
-
Studies that aim to derive the best cut-off points for management protocols. This may be influenced by the specifics of the step-up/step-down protocols.
-
-
It is unclear how treatment effects will progress over time. Long-term studies following patients for a number of years could address this evidence gap.
Larger RCTs of FeNO monitoring for asthma management are needed to clarify whether studies are failing to report significant effects because of underpowering.
Conclusions
There is considerable uncertainty associated with all analyses within this assessment. This is largely because of the limitations in the evidence base.
Studies using the devices that are the focus of this review were not available for all analyses and, in the absence of an alternative, equivalence has been assumed between devices. However, there is not a strong indication across the literature to support this assumption.
The clinical evidence relating to the use of FeNO monitoring for the diagnosis of asthma is highly heterogeneous and difficult to interpret in the context of the insertion of FeNO monitoring into a diagnostic pathway. This is compounded by a lack of certainty as to the equivalence of the devices used in the primary research studies to the devices that are the focus of this assessment.
The health economic analysis indicates that FeNO monitoring could have value in both the diagnostic setting and the management setting. In particular, the diagnostic model indicates that FeNO monitoring plus bronchodilator reversibility dominates many other diagnostic tests and may render airway hyper-responsiveness cost-ineffective. In the management setting, FeNO-guided management has the potential to appear cost-effective, although this is largely dependent on the expected duration over which it continues to impact on medication decisions. The conclusions drawn from both models require strong technical value judgements with respect to several aspects of the decision problem in which little or no empirical evidence exists.
Acknowledgements
The authors wish to thank Dr Clifford Godley, Dr Daryl Freeman, Professor Hasan Ashad and Dr John White who provided clinical advice and commented either on the draft report or on sections of the report. They also wish to thank Andrea Shippam (Programme Administrator, ScHARR) for providing administrative support and preparing and formatting the report. The authors also wish to thank Rachid Rafia for providing an internal peer review of the economic analysis and Eva Kaltenthaler for providing an internal peer review of the clinical review.
About the School of Health and Related Research (ScHARR)
The School of Health and Related Research is one of the nine departments that comprise the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research and the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the National Institute for Health Research (NIHR) Health Technology Assessment programme on behalf of a range of policy-makers, including NICE. ScHARR-TAG is part of a wider collaboration of a number of units from other regions including the Health Economics Research Unit and Health Services Research Unit, University of Aberdeen; the Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; the Liverpool Reviews & Implementation Group (LRiG), University of Liverpool; the Peninsula Technology Assessment Group (PenTAG), University of Exeter; the NHS Centre for Reviews and Dissemination, University of York; Warwick Evidence, University of Warwick; the BMJ Technology Assessment Group (BMJ-TAG), BMJ Evidence Centre; and Kleijnen Systematic Reviews Ltd, York.
Contribution of authors
Sue E Harnan (Research Fellow in Systematic Reviewing) acted as Principal Investigator for this assessment.
Sue E Harnan, Munira Essat (Research Associate in Systematic Reviewing) and Tim Gomersall (Research Associate in Systematic Reviewing) undertook the reviews of clinical effectiveness.
Paul Tappenden (Reader in Health Economic Modelling) and Jon Minton (Research Associate in Health Economic Modelling) undertook the review of existing health economic analyses.
Ruth Wong (Information Specialist) developed the electronic search strategies.
Paul Tappenden designed, developed and analysed the de novo EAG models.
Ian Pavord (Consultant Physician and Honorary Professor of Medicine), Mark Everard (Professor of Paediatric Respiratory Medicine) and Rod Lawson (Respiratory Physician) provided clinical guidance and commented on and edited the report.
Data sharing statement
Data relating to the clinical review chapter of this report can be obtained by request from the corresponding author, Sue Harnan. Data relating to the economic chapter can be obtained from Paul Tappenden (p.tappenden@sheffield.ac.uk). All other data can be obtained from the corresponding author, Sue Harnan.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- de Roberto M, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women. Am J Respir Crit Care Med 2000;162:68-74. http://dx.doi.org/10.1164/ajrccm.162.1.9907008.
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 2002;360:1715-21. http://dx.doi.org/10.1016/S0140-6736(02)11679-5.
- Wardlaw A, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull 2000;56:985-1003. http://dx.doi.org/10.1258/0007142001903490.
- Berry M, Morgan A, Shaw D, Parker D, Green R, Brightling C, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007;62:1043-9. http://dx.doi.org/10.1136/thx.2006.073429.
- Schleich FN, Seidel L, Sele J, Manise M, Quaedvlieg V, Michils A, et al. Exhaled nitric oxide thresholds associated with a sputum eosinophil count ≥ 3% in a cohort of unselected patients with asthma. Thorax 2010;65:1039-44. http://dx.doi.org/10.1136/thx.2009.124925.
- Pavord ID, Shaw DE, Gibson PG, Taylor DR. Inflammometry to assess airway diseases. Lancet 2008;372:1017-19. http://dx.doi.org/10.1016/S0140-6736(08)61421-X.
- Sur S, Crotty T, Kephart G, Hyma B, Colby T, Reed C, et al. Sudden-onset fatal asthma: a distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa?. Am Rev Respir Dis 1993;148:713-19. http://dx.doi.org/10.1164/ajrccm/148.3.713.
- British Guideline on the Management of Asthma: a National Clinical Guideline. Edinburgh and London: BTS/SIGN; 2012.
- Wenzel S. Asthma phenotypes: the evolution from clinical to molecular approaches. Nature Med 2012;18:716-25. http://dx.doi.org/10.1038/nm.2678.
- Gotshall R. Exercise-induced bronchoconstriction. Drugs 2002;62:1725-39. http://dx.doi.org/10.2165/00003495-200262120-00003.
- Anderson H, Gupta R, Strachan D, Limb E. 50 years of asthma: UK trends from 1955 to 2004. Thorax 2007;62:85-90. http://dx.doi.org/10.1136/thx.2006.066407.
- Hall J, Mindell J. Health Survey for England 2010: Volume 1. Chapter 2: Respiratory Symptoms and Disease in Adults. Leeds: Health & Social Care Information Centre; 2011.
- Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al. Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12190.
- Burr M, Davies B, Hoare A, Jones A, Williamson I, Holgate S, et al. A confidential inquiry into asthma deaths in Wales. Thorax 1999;54:985-9. http://dx.doi.org/10.1136/thx.54.11.985.
- Bucknall C, Slack R, Godley C, Mackay T, Wright S. Scottish Confidential Inquiry into Asthma Deaths (SCIAD), 1994–6. Thorax 1999;54. http://dx.doi.org/10.1136/thx.54.11.978.
- Sturdy P, Victor C, Anderson H, Bland J, Butland B, Harrison B, et al. Psychological, social and health behaviour risk factors for deaths certified as asthma: a national case–control study. Thorax 2002;57:1034-9. http://dx.doi.org/10.1136/thorax.57.12.1034.
- Sturdy P, Butland B, Anderson H, Ayres JG, Bland J, Harrison B, et al. Deaths certified as asthma and use of medical services: a national case–control study. Thorax 2005;60:909-15. http://dx.doi.org/10.1136/thx.2004.025593.
- Jones K, Berrill W, Bromly C, Hendrick D. A confidential enquiry into certified asthma deaths in the North of England, 1994–96: influence of co-morbidity and diagnostic inaccuracy. Respir Med 1999;93:923-7. http://dx.doi.org/10.1016/S0954-6111(99)90061-6.
- Brown P, Greville H, Finucane K. Asthma and irreversible airflow obstruction. Thorax 1984;39:131-6. http://dx.doi.org/10.1136/thx.39.2.131.
- Childhood Asthma Management Program Research Group . Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054-63. http://dx.doi.org/10.1056/NEJM200010123431501.
- Agertoft L, Pederson S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000;343:1064-9. http://dx.doi.org/10.1056/NEJM200010123431502.
- Craig R, Mindell J. Health Survey for England 2010: Volume 1 – Respiratory Health. Leeds: Health and Social Care Information Centre; 2011.
- Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-15. http://dx.doi.org/10.1164/rccm.9120-11ST.
- Kostikas K, Minas M, Papaioannou AI, Papiris S, Dweik RA. Exhaled nitric oxide in asthma in adults: the end is the beginning?. Curr Med Chem 2011;18:1423-31. http://dx.doi.org/10.2174/092986711795328436.
- Jatakanon A, Kharitonov S, Lim S, Barnes PJ. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax 1999;54:108-14. http://dx.doi.org/10.1136/thx.54.2.108.
- Jones SL, Herbison P, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, et al. Exhaled NO and assessment of anti-inflammatory effects of inhaled steroid: dose–response relationship. Eur Respir J 2002;20:601-8. http://dx.doi.org/10.1183/09031936.02.00285302.
- Silkoff PE, McClean P, Spino M, Erlich L, Slutsky AS, Zamel N, et al. Dose–response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest 2001;119:1322-8. http://dx.doi.org/10.1378/chest.119.5.1322.
- Aerocrine . Stakeholders Comments to NICE 2013. www.nice.org.UK/Nicemedia/Live/13864/65621/65621 (accessed 23 April 2014).
- Measurement of Exhaled Nitric Oxide Concentration in Asthma; NIOX MINO and NObreath. Final Scope. London: NICE; 2013.
- Minton J, Harnan S, Essat M, Wong R, Tappenden P. Measurement of Exhaled Nitric Oxide Concentration in Asthma: NIOX MINO and NObreath n.d. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013004149 (accessed 21 July 2015).
- Petsky HL, Cates CJ, Li A, Kynaston JA, Turner C, Chang AB. Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev 2009;4. http://dx.doi.org/10.1002/14651858.cd006340.pub3.
- Jartti T, Wendelin-Saarenhovi M, Heinonen I, Hartiala J, Vanto T. Childhood asthma management guided by repeated FeNO measurements: a meta-analysis. Paediatr Respir Rev 2012;13:178-83. http://dx.doi.org/10.1016/j.prrv.2011.11.002.
- Spahn AJD, Malka J, Mahr TA, Dorinsky PM. Meta analysis of asthma exacerbation rates in pediatric studies during asthma managed using fractional exhaled nitric oxide versus standard clinical parameters. J Allergy Clin Immunol 2013;131. http://dx.doi.org/10.1016/j.jaci.2012.12.1362.
- Clinical Guideline Development Methods. London: NICE; 2012.
- American Thoracic Society, European Respiratory Society . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med 2005;171:912-30. http://dx.doi.org/10.1164/rccm.200406-710ST.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration; 2011.
- Systematic Reviews – CRD’s Guidance for Undertaking Reviews in Healthcare. York: Centre for Reviews and Dissemination, University of York; 2009.
- Whiting P, Rutjes A, Westwood M, Mallett S, Deeks J, Reitsma J, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Menzies D, Nair A, Lipworth BJ. Portable exhaled nitric oxide measurement: comparison with the ‘gold standard’ technique. Chest 2007;131:410-14. http://dx.doi.org/10.1378/chest.06-1335.
- Grob NM, Laskowski D, Dweik RA. A technical report on exhaled nitric oxide measurement: asthma monitoring in athletes. J Breath Res 2008;2. http://dx.doi.org/10.1088/1752-7155/2/3/037027.
- Korn S, Telke I, Kornmann O, Buhl R. Measurement of exhaled nitric oxide: comparison of different analysers. Respirology 2010;15:1203-8. http://dx.doi.org/10.1111/j.1440-1843.2010.01847.x.
- Chen W, Purohit A, Barnig C, Casset A, de Blay F. Niox and Niox Mino: comparison of exhaled NO in grass pollen allergic adult volunteers. Allergy 2007;62:571-2. http://dx.doi.org/10.1111/j.1398-9995.2007.01334.x.
- Hemmingsson T, Linnarsson D, Gambert R. Novel hand-held device for exhaled nitric oxide-analysis in research and clinical applications. J Clin Monit Comput 2004;18:379-87. http://dx.doi.org/10.1007/s10877-005-1158-z.
- Pizzimenti S, Bugiani M, Piccioni P, Heffler E, Carosso A, Guida G, et al. Exhaled nitric oxide measurements: correction equation to compare hand-held device to stationary analyzer. Respir Med 2008;102:1272-5. http://dx.doi.org/10.1016/j.rmed.2008.04.006.
- Khalili B, Boggs PB, Bahna SL. Reliability of a new hand-held device for the measurement of exhaled nitric oxide. Allergy 2007;62:1171-4. http://dx.doi.org/10.1111/j.1398-9995.2007.01475.x.
- Alving K, Janson C, Nordvall L. Performance of a new hand-held device for exhaled nitric oxide measurement in adults and children. Respir Res 2006;7. http://dx.doi.org/10.1186/1465-9921-7-67.
- Vahlkvist S, Sinding M, Skamstrup K, Bisgaard H. Daily home measurements of exhaled nitric oxide in asthmatic children during natural birch pollen exposure. J Allergy Clin Immunol 2006;117:1272-6. http://dx.doi.org/10.1016/j.jaci.2006.03.018.
- McGill C, Malik G, Turner SW. Validation of a hand-held exhaled nitric oxide analyzer for use in children. Pediatr Pulmonol 2006;41:1053-7. http://dx.doi.org/10.1002/ppul.20491.
- Kalliola S, Malmberg P, Rito T, Pelkonen AS, Makela MJ. Can we use portable nitric oxide analyzer in young children?. Pediatr Pulmonol 2011;46:627-31. http://dx.doi.org/10.1002/ppul.21390.
- Ozier A, Girodet PO, Marthan R, Berger P. Reliability of the hand-held NIOX MINO device for monitoring asthma control. Fund Clin Pharmacol 2010;24.
- Ozier A, Girodet P-O, Bara I, Tunon de Lara JM, Marthan R, Berger P. Control maintenance can be predicted by exhaled NO monitoring in asthmatic patients. Respir Med 2011;105:989-96. http://dx.doi.org/10.1016/j.rmed.2011.01.006.
- Fortuna AM, Fexias T, Gonzalez M, Casan P. Determination of exhaled nitric oxide in healthy volunteers and asthmatic patients – comparison of two devices. Eur Respir J 2006;28.
- Fortuna AM, Feixas T, Casan P. Measurement of fraction of exhaled nitric oxide with the portable NIOX-MINO monitor in healthy adults. Arch Bronconeumol 2007;43:176-9. http://dx.doi.org/10.1016/S1579-2129(07)60044-5.
- Fukuhara A, Saito J, Sato S, Sato Y, Nikaido T, Saito K, et al. Validation study of asthma screening criteria based on subjective symptoms and fractional exhaled nitric oxide. Ann Allergy Asthma Immunol 2011;107:480-6. http://dx.doi.org/10.1016/j.anai.2011.09.002.
- Kim SH, Moon JY, Kwak HJ, Kim SI, Park DW, Kim JW, et al. Comparison of two exhaled nitric oxide analyzers: the NIOX MINO hand-held electrochemical analyzer and the NOA280i stationary chemiluminescence analyzer. Respirol 2012;17:830-4. http://dx.doi.org/10.1111/j.1440-1843.2012.02163.x.
- Yoon H, Kwak H, Kim T, Sohn J, Shin D, Park S, et al. Measurement of exhaled nitric oxide: comparison of two analyzers. Allergy 2011;66. http://dx.doi.org/10.1111/j.1440-1843.2012.02163.x.
- de Laurentiis G, Maniscalco M, Cianciulli F, Stanziola A, Marsico S, Lundberg JO, et al. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm Pharmacol Ther 2008;21:689-93. http://dx.doi.org/10.1016/j.pupt.2008.04.006.
- Peche R, Michils A, Baldassarre S, Mourid Z, Muylem A. Exhaled nitric oxide measured by using NIOX MINO and chemo-luminesence analyser: a comparative study. Eur Respir J 2007;30.
- Michils A, Peche R, Baldassarre S, Mourid Z, Van MA. Comparisons between portable and chemoluminescence exhaled nitric oxide measurements. Eur Respir J 2008;32:243-4. http://dx.doi.org/10.1183/09031936.00025308.
- Benchmark Testing Report. Comparison Testing Carried Out by Logan Fesearch Ltd for Bedfont Scientific Ltd – Reference the NObreath Nitric Oxide Monitoring Product. Rochester: Internal report supplied by Bedfont (manufacturers); 2009.
- Park YA, Lee JH, Lee YJ, Song TW, Kim KW, Sohn MH, et al. J Allergy Clin Immunol 2011;127. http://dx.doi.org/10.1016/j.jaci.2010.12.258.
- Schiller B, Hammer J, Barben J, Trachsel D. Comparability of a hand-held nitric oxide analyser with online and offline chemiluminescence-based nitric oxide measurement. Pediatr Allergy Immunol 2009;20:679-85. http://dx.doi.org/10.1111/j.1399-3038.2009.00853.x.
- Chladkova J, Havlinova Z, Chyba T, Krcmova I, Chladek J. Analysis of single-breath profiles of exhaled nitric oxide in children with allergy and asthma: guideline-derived plateau concentrations compared to results of automatic evaluation by two analyzers. J Asthma 2008;45:820-6. http://dx.doi.org/10.1080/02770900802312582.
- Boot J, de Ridder L, de Kam M, Calderon C, Mascelli M, Diamant Z. Comparison of exhaled nitric oxide measurements between Niox Mino electrochemical and Ecomedics chemiluminescence analyzer. Respir Med 2008;102:1667-71. http://dx.doi.org/10.1016/j.rmed.2008.06.021.
- Pisi R, Aiello M, Tzani P, Marangio E, Olivieri D, Chetta A, et al. Measurement of fractional exhaled nitric oxide by a new portable device: comparison with the standard technique. J Asthma 2010;47:805-9. http://dx.doi.org/10.3109/02770903.2010.485667.
- Antus B, Horvath I, Barta I. Assessment of exhaled nitric oxide by a new hand-held device. Respir Med 2010;104:1377-80. http://dx.doi.org/10.1016/j.rmed.2010.06.005.
- Kapande KM, McConaghy LA, Douglas I, McKenna S, Hughes JL, McCance DR, et al. Comparative repeatability of two handheld fractional exhaled nitric oxide monitors. Pediatr Pulmonol 2012;47:546-50. http://dx.doi.org/10.1002/ppul.21591.
- Kapande KM, Shields MD, McConaghy LA. Comparative repeatability of two handheld fractional exhaled nitric oxide monitors. Ir J Med Sci 2011;180. http://dx.doi.org/10.1002/ppul.21591.
- Schneider A, Schwarzbach J, Faderl B, Welker L, Karsch-Volk M, Jorres RA. FENO measurement and sputum analysis for diagnosing asthma in clinical practice. Respir Med 2013;107:209-16. http://dx.doi.org/10.1016/j.rmed.2012.10.003.
- Fortuna AM, Feixas T, Gonzalez M, Casan P. Diagnostic utility of inflammatory biomarkers in asthma: exhaled nitric oxide and induced sputum eosinophil count. Respir Med 2007;101:2416-21. http://dx.doi.org/10.1016/j.rmed.2007.05.019.
- Schneider A, Tilemann L, Schermer T, Gindner L, Laux G, Szecsenyi J, et al. Diagnosing asthma in general practice with portable exhaled nitric oxide measurement – results of a prospective diagnostic study. Respir Res 2009;10. http://dx.doi.org/10.1186/1465-9921-10-15.
- Schneider A, Tilemann L, Schermer T, Gindner L, Laux G, Szecsenyi J, et al. Diagnosing asthma in general practice with portable exhaled nitric oxide measurement – results of a prospective diagnostic study: FENO ≤ 16 ppb better than FENO ≤ 12 ppb to rule out mild and moderate to severe asthma. Respir Res 2009;10. http://dx.doi.org/10.1186/1465-9921-10-64.
- Hsu JY, Wang CY, Cheng YW, Chou MC. Optimal value of fractional exhaled nitric oxide in inhaled corticosteroid treatment for patients with chronic cough of unknown cause. J Chin Med Assoc 2013;76:15-9. http://dx.doi.org/10.1016/j.jcma.2012.08.010.
- Hahn PY, Morgenthaler TI, Lim KG. Use of exhaled nitric oxide in predicting response to inhaled corticosteroids for chronic cough. Mayo Clin Proc 2007;82:1350-5. http://dx.doi.org/10.4065/82.11.1350.
- Sato S, Saito J, Sato Y, Ishii T, Xintao W, Tanino Y, et al. Clinical usefulness of fractional exhaled nitric oxide for diagnosing prolonged cough. Respir Med 2008;102:1452-9. http://dx.doi.org/10.1016/j.rmed.2008.04.018.
- Prieto L, Ferrer A, Ponce S, Palop J, Marin J. Exhaled nitric oxide measurement is not useful for predicting the response to inhaled corticosteroids in subjects with chronic cough. Chest 2009;136:816-22. http://dx.doi.org/10.1378/chest.08-2942.
- Schleich FN, Asandei R, Manise M, Sele J, Seidel L, Louis R. Is FENO50 useful diagnostic tool in suspected asthma?. Int J Clin Pract 2012;66:158-65. http://dx.doi.org/10.1111/j.1742-1241.2011.02840.x.
- El Halawani SM, Ly NT, Mahon RT, Amundson DE. Exhaled nitric oxide as a predictor of exercise-induced bronchoconstriction. Chest 2003;124:639-43. http://dx.doi.org/10.1378/chest.124.2.639.
- Arora R, Thornblade CE, Dauby PA, Flanagan JW, Bush AC, Hagan LL. Exhaled nitric oxide levels in military recruits with new onset asthma. Allergy Asthma Proc 2006;27:493-8. http://dx.doi.org/10.2500/aap.2006.27.2904.
- Chancafe-Morgan J, Ramos-Quispe Y, Gomez-García R, Vargas-Espinal J, Puente-Maestú L. Validity of the fractional exhaled nitric oxide (FENO) for identification of bronchial hyperresponsiveness in a pulmonary function laboratory. Eur Respir J 2013;42.
- Katsoulis K, Ganavias L, Michailopoulos P, Bikas C, Dinapogias E, Kontakiotis T, et al. Exhaled nitric oxide as screening tool in subjects with suspected asthma without reversibility. Int Arch Allergy Immunol 2013;162:58-64. http://dx.doi.org/10.1159/000350221.
- Heffler E, Guida G, Marsico P, Bergia R, Bommarito L, Ferrero N, et al. Exhaled nitric oxide as a diagnostic test for asthma in rhinitic patients with asthmatic symptoms. Respir Med 2006;100:1981-7. http://dx.doi.org/10.1016/j.rmed.2006.02.019.
- Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med 2005;172:453-9. http://dx.doi.org/10.1164/rccm.200411-1498OC.
- de la Barra SL, Smith AD, Cowan JO, Herbison GP, Taylor DR. Predicted versus absolute values in the application of exhaled nitric oxide measurements. Respir Med 2011;105:1629-34. http://dx.doi.org/10.1016/j.rmed.2011.06.001.
- Pedrosa M, Cancelliere N, Barranco P, Lopez-Carrasco V, Quirce S. Usefulness of exhaled nitric oxide for diagnosing asthma. J Asthma 2010;47:817-21. http://dx.doi.org/10.3109/02770903.2010.491147.
- Smith AD, Cowan JO, Filsell S, McLachlan C, Monti-Sheehan G, Jackson P, et al. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med 2004;169:473-8. http://dx.doi.org/10.1164/rccm.200310-1376OC.
- Cordeiro D, Rudolphus A, Snoey E, Braunstahl G-J. Utility of nitric oxide for the diagnosis of asthma in an allergy clinic population. Allergy Asthma Proc 2011;32:119-26. http://dx.doi.org/10.2500/aap.2011.32.3419.
- Bobolea ID, Barranco P, Lopez-Carrasco V, Calderon O, Guillen D, Quirce S. Is methacholine challenge sufficient to rule out bronchial hyperresponsiveness in patients with suspected asthma?. J Allergy Clin Immunol 2012;129. http://dx.doi.org/10.1016/j.jaci.2011.12.870.
- Zhang YM, Lin JT, Su N, Chen X, Liu GL, Yu HX, et al. Values of fractional exhaled nitric oxide in the diagnosis of chronic cough. Chung-Hua I Hsueh Tsa Chih 2011;91:1254-8.
- Pizzimenti S, Heffler E, Piccioni P, Bugiani M, Migliore E, Guida G, et al. Usefulness of exhaled nitric oxide (FeNO) measured by a portable analyzer to diagnose cough variant asthma in a clinical setting of chronic cough. Allergy 2009;64.
- Mathew S, Cliff I, Agarwal S, Lim A, Allen M, Mustfa N. Relationship between exhaled nitric oxide and methacholine challenge test in suspected asthma. Am J Respir Crit Care Med 2011;183. http://ajrccm.atsjournals.org/cgi/reprint/183/1_MeetingAbstracts/A5554?sid = 0209d9f3-c74d-409d-8e6e-81ebe76d2328 (accessed 19 May 2015).
- Brannan JD, Adoni H, Daw L, Huang HC, Hurwitz M, Figurski D. Fraction exhaled NO in patients referred to pulmonary function laboratory (PFLAB) for mannitol challenge. Respirology 2013;18.
- Linkosalo L, Lehtimaki L, Holm K, Kaila M, Moilanen E. Relation of bronchial and alveolar nitric oxide to exercise-induced bronchoconstriction in atopic children and adolescents. Pediatr Allergy Immunol 2012;23:360-6. http://dx.doi.org/10.1111/j.1399-3038.2011.01223.x.
- Ramser M, Hammer J, Amacher A, Trachsel D. The value of exhaled nitric oxide in predicting bronchial hyperresponsiveness in children. J Asthma 2008;45:191-5. http://dx.doi.org/10.1080/02770900801890273.
- Sivan Y, Gadish T, Fireman E, Soferman R. The use of exhaled nitric oxide in the diagnosis of asthma in school children. J Pediatr 2009;155:211-16. http://dx.doi.org/10.1016/j.jpeds.2009.02.034.
- Woo SI, Lee JH, Kim H, Kang JW, Sun YH, Hahn YS. Utility of fractional exhaled nitric oxide (FENO) measurements in diagnosing asthma. Respir Med 2012;106:1103-9. http://dx.doi.org/10.1016/j.rmed.2012.03.022.
- Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 2005;352:2163-73. http://dx.doi.org/10.1056/NEJMoa043596.
- Shaw DE, Berry MA, Thomas M, Green RH, Brightling CE, Wardlaw AJ, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:231-7. http://dx.doi.org/10.1164/rccm.200610-1427OC.
- Syk J, Malinovschi A, Johansson G, Unden AL, Andreasson A, Lekander M, et al. Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: a randomized controlled trial. J Allergy Clin Immunol Pract 2013;1:639-48. http://dx.doi.org/10.1016/j.jaip.2013.07.013.
- Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA 2012;308:987-97. http://dx.doi.org/10.1001/2012.jama.10893.
- Honkoop P, Loijmans R, Termeer E, Snoeck-Stroband J, Assendelft P, Sterk P, et al. A cluster randomized trial comparing strict, partial, and FeNO-guided asthma control strategies in primary care. Eur Respir J 2013;42. http://dx.doi.org/10.1016/j.jaci.2014.07.016.
- Powell H, Murphy VE, Taylor DR, Hensley MJ, McCaffery K, Giles W, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011;378:983-90. http://dx.doi.org/10.1016/S0140-6736(11)60971-9.
- Fritsch M, Uxa S, Horak F, Putschoegl B, Dehlink E, Szepfalusi Z, et al. Exhaled nitric oxide in the management of childhood asthma: a prospective 6-months study. Pediatr Pulmonol 2006;41:855-62. http://dx.doi.org/10.1002/ppul.20455.
- Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet 2008;372:1065-72. http://dx.doi.org/10.1016/S0140-6736(08)61448-8.
- Verini M, Consilvio NP, Di Pillo S, Cingolani A, Spagnuolo C, Rapino D, et al. FeNO as a marker of airways inflammation: the possible implications in childhood asthma management. J Allergy 2010;2010. http://dx.doi.org/10.1155/2010/691425.
- Pijnenburg MW, Bakker EM, Hop WC, de Jongste JC. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med 2005;172:831-6. http://dx.doi.org/10.1164/rccm.200503-458OC.
- Petsky H, Li AM, Kynaston JA, Turner C, Chang AB. Dual-center randomised trial on tailored asthma therapy based on exhaled nitric oxide (FENO) versus routine clinical care. Am J Respir Crit Care Med 2010;181. http://dx.doi.org/10.1164/ajrccm-conference.2010.181.1_meetingabstracts.a3928.
- Pike K, Selby A, Price S, Warner J, Connett G, Legg J, et al. Exhaled nitric oxide monitoring does not reduce exacerbation frequency or inhaled corticosteroid dose in paediatric asthma: a randomised controlled trial. Clin Respir J 2013;7:204-13. http://dx.doi.org/10.1111/j.1752-699X.2012.00306.x.
- Peirsman EJ, Carvelli TJ, Hage PY, Hanssens LS, Pattyn L, Raes MM, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial [published online ahead of print September 4 2013]. Pediatr Pulmonol 2013. http://dx.doi.org/10.1002/ppul.22873.
- Malinovschi A, Backer V, Harving H, Porsbjerg C. The value of exhaled nitric oxide to identify asthma in smoking patients with asthma-like symptoms. Respir Med 2012;106:794-801. http://dx.doi.org/10.1016/j.rmed.2012.02.009.
- Mahut B, Peyrard S, Delclaux C. Exhaled nitric oxide and clinical phenotypes of childhood asthma. Respir Res 2011;12. http://dx.doi.org/10.1186/1465-9921-12-65.
- Hanson J, DeLurgio S, Williams D, Dinakar C. Ambulatory fractional exhaled nitric oxide (FENO) measurement in children 4–7 years of age. Ann Allergy Asthma Immunol 2012;109. http://dx.doi.org/10.1016/j.anai.2013.07.020.
- de la Riva-Velasco E, Krishnan S, Dozor AJ. Relationship between exhaled nitric oxide and exposure to low-level environmental tobacco smoke in children with asthma on inhaled corticosteroids. J Asthma 2012;49:673-8. http://dx.doi.org/10.3109/02770903.2012.701363.
- Tamasi L, Bohacs A, Bikov A, Andorka C, Rigo J, Losonczy G, et al. Exhaled nitric oxide in pregnant healthy and asthmatic women. J Asthma 2009;46:786-91. http://dx.doi.org/10.1080/02770900903090004.
- Simpson JL, McDonald VM, Gibson PG. Exhaled nitric oxide is not a marker of eosinophilic inflammation in older Australians. Respirology 2010;15. http://dx.doi.org/10.1111/crj.12017.
- Inoue H, Niimi A, Takeda T, Matsumoto H, Ito I, Otsuka K, et al. Pathophysiological characteristics of asthma in the elderly. Am J Respir Crit Care Med 2010;181. http://dx.doi.org/10.1164/ajrccm-conference.2010.181.1_meetingabstracts.a5096.
- Columbo M, Wong B, Panettieri RA, Rohr AS. Asthma in the elderly: the role of exhaled nitric oxide measurements. J Allergy Clin Immunol 2012;129. http://dx.doi.org/10.1016/j.jaci.2011.12.891.
- Roh YH. Usefulness of asthma control test questionnaire, FEV1 and exhaled nitric oxide level (FENO) for the clinical assessment of elderly asthma. World Allergy Organ J 2012;5. http://dx.doi.org/10.1097/01.WOX.0000412212.37057.12.
- Smith AM, Villareal M, Bernstein DI, Swikert DJ. Asthma in the elderly: risk factors and impact on physical function. Ann Allergy Asthma Immunol 2012;108:305-10. http://dx.doi.org/10.1016/j.anai.2012.02.022.
- Ross J, Baptist A. Factors associated with asthma quality of life and control among older adults. Ann Allergy Asthma Immunol 2011;107. http://dx.doi.org/10.1016/j.jaip.2012.12.003.
- Michils A, Louis R, Peche R, Baldassarre S, Van Muylem A. Exhaled nitric oxide as a marker of asthma control in smoking patients. Eur Respir J 2009;33:1295-301. http://dx.doi.org/10.1183/09031936.00154008.
- Kostikas K, Papaioannou AI, Tanou K, Giouleka P, Koutsokera A, Minas M, et al. Exhaled NO and exhaled breath condensate pH in the evaluation of asthma control. Respir Med 2011;105:526-32. http://dx.doi.org/10.1016/j.rmed.2010.10.015.
- Hromis S, Milutinov S, Zvezdin B, Kopitovic I, Maksimovic O. Exhaled nitric oxide in assessing of asthma control in smoking patients pilot study. Allergy 2012;67.
- Mattes J, Murphy V, Powell H, Gibson P. The effect of better asthma control in pregnancy on wheezy illnesses in infancy. Respirology 2013;18.
- Bozek A, Filipowski M, Fischer A, Jarzab J. Characteristics of atopic bronchial asthma in seniors over 80 years of age. Biomed Res Int 2013;2013. http://dx.doi.org/10.1155/2013/689782.
- Columbo M, Wong B, Panettieri RA, Rohr AS. Asthma in the elderly: the role of exhaled nitric oxide measurements. Respir Med 2013;107:785-7. http://dx.doi.org/10.1016/j.rmed.2013.01.018.
- Olaguibel JM, Parra A, Alvarez MJ, Quirce S, Lopez R. Measurements of fractional exhaled nitric oxide with 2 portable electrochemical sensors: a comparative study. J Investig Allergol Clin Immunol 2011;21:322-3.
- Bland M, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;327:307-10. http://dx.doi.org/10.1016/S0140-6736(86)90837-8.
- Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention 2007. www.ginasthma.org (accessed 19 May 2015).
- American Thoracic Society . Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis 1987;136:225-35. http://dx.doi.org/10.1164/ajrccm/136.1.225.
- Whiting P, Rutjes A, Reitsma J, Bossuyt P, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3. http://dx.doi.org/10.1186/1471-2288-3-25.
- Asher M, Keil U, Anderson H, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995;8.
- American Thoracic Society. Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children – 1999. Am J Respir Crit Care Med 1999;160:2104-17. http://dx.doi.org/10.1164/ajrccm.160.6.ats8-99.
- Report 3: Guidelines for the Diagnosis and Management of Asthma. Rockville, MD: National Heart, Lung, and Blood Institute; 2007.
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J n.d.;14:902-7. http://dx.doi.org/10.1034/j.1399-3003.1999.14d29.x.
- Global Strategy for Asthma Management and Prevention. Bethesda, MD: National Institutes of Health; 2002.
- Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-15. http://dx.doi.org/10.1164/rccm.9120-11ST.
- Scott M, Raza A, Karmaus W, Mitchell F, Grundy J, Kurukulaaratchy RJ, et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax 2010;65:258-62. http://dx.doi.org/10.1136/thx.2009.125443.
- Frischer T, Eber E, Eichler I, Horak E, Riedler J, Gotz M, et al. Consensus guidelines for drug therapy of bronchial asthma in children and adolescents. Wien Klin Wochenschr 1999;111:900-2.
- National Heart, Lung, and Blood Institute (NHLBI) . National Asthma Education and Prevention Program 2013. www.nhlbi.nih.gov/about/org/naepp (accessed 15 July 2015).
- Beck-Ripp J, Griese M, Arenz S, Koring C, Pasqualoni B, Bufler P, et al. Changes of exhaled nitric oxide during steroid treatment of childhood asthma. Eur Respir J 2002;19:1015-19. http://dx.doi.org/10.1183/09031936.02.01582001.
- Lehtimaki L, Turjanmaa V, Kankaanranta H, Saarelainen S, Hahtola P, Moilanen E. Increased bronchial nitric oxide production in patients with asthma measured with a novel method of different exhalation flow rates. Ann Med 2000;32:417-23. http://dx.doi.org/10.3109/07853890008995949.
- Medicines and Healthcare products Regulatory Agency . Asthma: Long-Acting Beta 2 Agonists: Reminder for Use in Children and Adults n.d. www.gov.uk/drug-safety-update/long-acting-2-agonists-reminder-for-use-in-children-and-adults (accessed 22 July 2015).
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Diagnostics Assessment Programme Manual. Manchester: NICE; 2011.
- Weinstein M, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health 2003;6:9-17. http://dx.doi.org/10.1046/j.1524-4733.2003.00234.x.
- Price D, Berg J, Lindgren P. An economic evaluation of NIOX MINO airway inflammation monitor in the United Kingdom. Allergy 2009;64:431-8. http://dx.doi.org/10.1111/j.1398-9995.2008.01855.x.
- Berg J, Lindgren P. Economic evaluation of FE(NO) measurement in diagnosis and 1-year management of asthma in Germany. Respir Med 2008;102:219-31. http://dx.doi.org/10.1016/j.rmed.2007.09.008.
- Peters J, Stevenson M, Beverley C, Lim JNW, Smith S. The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation. Health Technol Assess 2002;6. http://dx.doi.org/10.3310/hta6050.
- Briggs AH, Bousquet J, Wallace MV, Busse WW, Clark TJ, Pedersen SE, et al. Cost-effectiveness of asthma control: an economic appraisal of the GOAL study. Allergy 2006;61:531-6. http://dx.doi.org/10.1111/j.1398-9995.2006.01038.x.
- Doull I, Price D, Thomas M, Hawkins N, Stamuli E, Tabberer M, et al. Cost-effectiveness of salmeterol xinafoate/fluticasone propionate combination inhaler in chronic asthma. Curr Med Res Opin 2007;23:1147-59. http://dx.doi.org/10.1185/030079907X187982.
- Wilson EC, Price D, Musgrave SD, Sims EJ, Shepstone L, Murdoch J, et al. Cost-effectiveness of leukotriene receptor antagonists versus long-acting beta-2 agonists as add-on therapy to inhaled corticosteroids for asthma: a pragmatic trial. Pharmacoeconomics 2010;28:597-608. http://dx.doi.org/10.2165/11537550-000000000-00000.
- Paggiaro P, Buseghin G, Nicolini G, Patel S, Iannazzo S, Zaniolo O, et al. The cost-effectiveness of step down from high dose ICS/LABA combination therapy in asthma in the UK setting. Value Health 2011;14:A493-4. http://dx.doi.org/10.1016/j.jval.2011.08.1423.
- Booth PC, Capsey LJ, Langdon CG, Wells NEJ. A comparison of the cost-effectiveness of alternative prophylactic therapies in the treatment of adult asthma. Br J Med Econ 1995;8:65-72.
- Barnes NC, Thwaites RM, Price MJ. The cost-effectiveness of inhaled fluticasone propionate and budesonide in the treatment of asthma in adults and children. Respir Med 1999;93:402-7. http://dx.doi.org/10.1053/rmed.1999.0577.
- Payne DN, Qiu Y, Zhu J, Peachey L, Scallan M, Bush A, et al. Airway inflammation in children with difficult asthma: relationships with airflow limitation and persistent symptoms. Thorax 2004;59:862-9. http://dx.doi.org/10.1136/thx.2003.017244.
- Everden P, Lloyd A, Hutchinson J, Plumb J. Cost-effectiveness of eformoterol Turbohaler versus salmeterol Accuhaler in children with symptomatic asthma. Respir Med 2002;96:250-8. http://dx.doi.org/10.1053/rmed.2001.1258.
- Price MJ, Briggs AH. Development of an economic model to assess the cost-effectiveness of asthma management strategies. Pharmacoeconomics 2002;20:183-94. http://dx.doi.org/10.2165/00019053-200220030-00004.
- Buxton MJ, Sullivan SD, Andersson LF, Lamm CJ, Liljas B, Busse WW, et al. Country-specific cost-effectiveness of early intervention with budesonide in mild asthma. Eur Respir J 2004;24:568-74. http://dx.doi.org/10.1183/09031936.04.00108703.
- Price D, Wiren A, Kuna P. Cost-effectiveness of budesonide/formoterol for maintenance and reliever asthma therapy. Allergy 2007;62:1189-98. http://dx.doi.org/10.1111/j.1398-9995.2007.01466.x.
- Kemp L, Haughney J, Barnes N, Sims E, Von ZJ, Hillyer EV, et al. Cost-effectiveness analysis of corticosteroid inhaler devices in primary care asthma management: a real world observational study. Clinicoecon Outcomes Res 2010;2:75-8.
- Smith AD, Taylor DR. Is exhaled nitric oxide measurement a useful clinical test in asthma?. Curr Opin Allergy Clin Immunol 2005;5:49-56. http://dx.doi.org/10.1097/00130832-200502000-00010.
- Hunter C, Brightling C, Woltmann G, Wardlow A, Pavord I. A comparison of the validity of different diagnostic tests in adults with asthma. Chest 2002;121:1051-7. http://dx.doi.org/10.1378/chest.121.4.1051.
- British Guideline on the Management of Asthma, 2007 update. Edinburgh and London: BTS/SIGN; 2007.
- NHS Reference Costs 2005. London: Department of Health; 2005.
- Curtis L, Netten A. Unit Costs of Health and Social Care 2005. Canterbury: Personal Social Services Research Unit, University of Kent; 2005.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2006.
- Dupont LJ, Demedts MG, Verleden GM. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest 2003;123:751-6. http://dx.doi.org/10.1378/chest.123.3.751.
- Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, LemiÇùre C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J 2006;27:483-94. http://dx.doi.org/10.1183/09031936.06.00137704.
- Andersson F, Stahl E, Barnes PJ, LÇôfdahl CG, O’Byrne PM, Pauwels RA, et al. Adding formoterol to budesonide in moderate asthma – health economic results from the FACET study. Respir Med 2001;95:505-12. http://dx.doi.org/10.1053/rmed.2001.1078.
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O’Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. Am J Respir Crit Care Med 1999;160:594-9. http://dx.doi.org/10.1164/ajrccm.160.2.9811100.
- Szende A, Svensson K, Stahl E, Meszaros A, Berta GY. Psychometric and utility-based measures of health status of asthmatic patients with different disease control level. Pharmacoeconomics 2004;22:537-47. http://dx.doi.org/10.2165/00019053-200422080-00005.
- Akinbami L, Moorman J, Liu X. Asthma Prevalence, Health Care Use, and Mortality: United States, 2005–2009. National Health Statistics Reports 2011. www.cdc.gov/nchs/data/nhsr/nhsr032.pdf (accessed 19 May 2015).
- NHS Reference Costs 2011–12. London: Department of Health; 2012.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. http://dx.doi.org/10.1136/bmj.316.7133.736.
- Piacentini GL, Peroni DG, Bonafiglia E, Chinellato I, Bodini A, Boner AL. Childhood asthma control test (C-ACT) and nasal eosinophil inflammation in asthmatic children. Allergy 2010;65:796-7. http://dx.doi.org/10.1111/j.1398-9995.2009.02237.x.
- Andersson F, Borg S, Barnes PJ, Lofdahl CG, O’Byrne P, Postma D, et al. A high dose of inhaled budesonide is more cost-effective than a low dose in moderate asthma. J Allergy Clin Immunol 2000;105:S102-3. http://dx.doi.org/10.1016/S0091-6749(00)90734-6.
- British Guideline on the Management of Asthma. Edinburgh and London: BTS/SIGN; 2005.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Main C, Shepherd J, Anderson R, Rogers G, Thompson Coon J, Liu Z, et al. Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12200.
- McTaggart-Cowan H, Marra C, Yang Y, Brazier J, Kopec J, FitzGerald J, et al. The validity of generic and condition-specific preference-based instruments: the ability to discriminate asthma control status. Qual Life Res 2008;17:453-62. http://dx.doi.org/10.1007/s11136-008-9309-6.
- Sullivan P, Slejko J, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. http://dx.doi.org/10.1177/0272989X11401031.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007;16:22-7. http://dx.doi.org/10.3132/pcrj.2007.00002.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Davis S. A Review of the Psychometric Performance of the EQ-5D in Patients with Asthma. Sheffield: University of Sheffield; 2010.
- Inhaled Corticosteroids for the Treatment of Chronic Asthma in Children under the Age of 12 Years. London: NICE; 2007.
- Inhaled Corticosteroids for the Treatment of Chronic Asthma in Adults and in Children aged 12 Years and Over. London: NICE; 2008.
- Interim Life Tables, England and Wales, 2009–2011. London: ONS; 2011.
- de Jongste JC, Carraro S, Hop WC, Baraldi E. CHARISM Study Group . Daily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthma. Am J Respir Crit Care Med 2009;179:93-7. http://dx.doi.org/10.1164/rccm.200807-1010OC.
- Donohue JF, Jain N. Exhaled nitric oxide to predict corticosteroid responsiveness and reduce asthma exacerbation rates. Respir Med 2013;107:943-52. http://dx.doi.org/10.1016/j.rmed.2013.02.018.
- Tan KS, Thomson NC. Asthma in pregnancy. Am J Med 2000;109:727x-33. http://dx.doi.org/10.1016/S0002-9343(00)00615-X.
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000097.
Appendix 1 Search strategies for the clinical review
Shading indicates sets of related terms within the search strategy.
Management review: MEDLINE
-
NIOX MINO.mp.
-
aerocrine.mp.
-
(niox adj5 (monitor$ or chemiluminescence or analyser$ or sensor or device$ or desktop)).mp.
-
NObreath.mp.
-
bedfont.mp.
-
or/1-5
-
exp cough/
-
cough$.mp.
-
phlegm.mp.
-
sputum.mp.
-
mucus.mp.
-
wheez$.mp.
-
chest pain/
-
chest pain$.mp.
-
(chest adj5 tight$).tw.
-
((lower respiratory or lrt) adj5 symptom$).tw.
-
(lower airway adj5 symptom$).tw.
-
((trache$ or wind pipe or lung$ or bronch$) adj3 symptom$).tw.
-
exp lung/ or trachea/
-
symptom$.tw.
-
19 and 20
-
or/7-18,21
-
exp asthma/
-
asthma$.mp.
-
exp respiratory hypersensitivity/
-
exp bronchial hyperreactivity/
-
bronchial spasm/
-
bronchospas$.mp.
-
exp Bronchoconstriction/
-
bronchoconstric$.mp.
-
(bronch$ adj3 constrict$).mp.
-
(bronch$ adj5 spas$).mp.
-
(airway$ adj5 (obstruct$ or inflammation$)).mp.
-
((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
-
or/23-34
-
Nitric Oxide/
-
nitric oxide.mp.
-
36 or 37
-
(exhal$ or expir$ or alveolar or fractional).mp.
-
38 and 39 (5228)
-
exhaled NO.mp.
-
eno.mp.
-
fe?no$.mp.
-
(fractional adj2 NO).mp.
-
or/40-44
-
22 and 45
-
35 and 45
-
6 or 46 or 47
-
limit 48 to yr=“2009 -Current”
Systematic reviews search: MEDLINE
-
NIOX MINO.mp.
-
aerocrine.mp.
-
(niox adj5 (monitor$ or chemiluminescence or analyser$ or sensor or device$ or desktop)).mp.
-
NObreath.mp.
-
bedfont.mp.
-
or/1-5
-
exp cough/
-
cough$.mp.
-
phlegm.mp.
-
sputum.mp.
-
mucus.mp.
-
wheez$.mp.
-
chest pain/
-
chest pain$.mp.
-
(chest adj5 tight$).tw.
-
((lower respiratory or lrt) adj5 symptom$).tw.
-
(lower airway adj5 symptom$).tw.
-
((trache$ or wind pipe or lung$ or bronch$) adj3 symptom$).tw.
-
exp lung/ or trachea/
-
symptom$.tw.
-
19 and 20
-
or/7-18,21
-
exp asthma/
-
asthma$.mp.
-
exp respiratory hypersensitivity/
-
exp bronchial hyperreactivity/
-
bronchial spasm/
-
bronchospas$.mp.
-
exp Bronchoconstriction/
-
bronchoconstric$.mp.
-
(bronch$ adj3 constrict$).mp.
-
(bronch$ adj5 spas$).mp.
-
(airway$ adj5 (obstruct$ or inflammation$)).mp.
-
((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
-
or/23-34
-
Nitric Oxide/
-
nitric oxide.mp.
-
36 or 37
-
(exhal$ or expir$ or alveolar or fractional).mp.
-
38 and 39 (5228)
-
exhaled NO.mp.
-
eno.mp.
-
fe?no$.mp.
-
(fractional adj2 NO).mp.
-
or/40-44
-
22 and 45
-
35 and 45
-
6 or 46 or 47
-
meta-analysis as topic/
-
(meta analy$ or metaanaly$).tw.
-
Meta-Analysis/
-
(systematic adj (review$1 or overview$1)).tw.
-
“Review Literature as Topic”/
-
or/49-53 (96944)
-
(cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or cinhal or science citation index or bids or cancerlit).ab.
-
((reference adj list$) or bibliograph$ or hand-search$ or (relevant adj journals) or (manual adj search$)).ab.
-
((selection adj criteria) or (data adj extraction)).ab.
-
“review”/
-
57 and 58
-
comment/ or editorial/ or letter/
-
Animals/
-
Humans/
-
61 not (61 and 62)
-
60 or 63
-
54 or 55 or 56 or 59
-
65 not 64
-
48 and 66
Randomised controlled trials search: MEDLINE
-
NIOX MINO.mp.
-
aerocrine.mp.
-
(niox adj5 (monitor$ or chemiluminescence or analyser$ or sensor or device$ or desktop)).mp.
-
NObreath.mp.
-
bedfont.mp.
-
or/1-5
-
exp cough/
-
cough$.mp.
-
phlegm.mp.
-
sputum.mp.
-
mucus.mp.
-
wheez$.mp.
-
chest pain/
-
chest pain$.mp.
-
(chest adj5 tight$).tw.
-
((lower respiratory or lrt) adj5 symptom$).tw.
-
(lower airway adj5 symptom$).tw.
-
((trache$ or wind pipe or lung$ or bronch$) adj3 symptom$).tw.
-
exp lung/ or trachea/
-
symptom$.tw.
-
19 and 20
-
or/7-18,21
-
exp asthma/
-
asthma$.mp.
-
exp respiratory hypersensitivity/
-
exp bronchial hyperreactivity/
-
bronchial spasm/
-
bronchospas$.mp.
-
exp Bronchoconstriction/
-
bronchoconstric$.mp.
-
(bronch$ adj3 constrict$).mp.
-
(bronch$ adj5 spas$).mp.
-
(airway$ adj5 (obstruct$ or inflammation$)).mp.
-
((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
-
or/23-34
-
Nitric Oxide/
-
nitric oxide.mp.
-
36 or 37
-
(exhal$ or expir$ or alveolar or fractional).mp.
-
38 and 39 (5228)
-
exhaled NO.mp.
-
eno.mp.
-
fe?no$.mp.
-
(fractional adj2 NO).mp.
-
or/40-44
-
22 and 45
-
35 and 45
-
6 or 46 or 47
-
Randomized controlled trials as Topic/
-
Randomized controlled trial/
-
Random allocation/
-
randomized controlled trial.pt.
-
Double blind method/
-
Single blind method/
-
Clinical trial/
-
exp Clinical Trials as Topic/
-
controlled clinical trial.pt.
-
or/49-57
-
(clinic$ adj25 trial$).ti,ab.
-
((singl$ or doubl$ or treb$ or tripl$) adj (blind$ or mask$)).tw.
-
Placebos/
-
Placebo$.tw.
-
(allocated adj2 random).tw.
-
or/59-63
-
58 or 64
-
Case report.tw.
-
Letter/
-
Historical article/
-
66 or 67 or 68
-
exp Animals/
-
Humans/
-
70 not (70 and 71)
-
69 or 72
-
65 not 73
-
48 and 74
Diagnostic studies search: MEDLINE
-
NIOX MINO.mp.
-
aerocrine.mp.
-
(niox adj5 (monitor$ or chemiluminescence or analyser$ or sensor or device$ or desktop)).mp.
-
NObreath.mp.
-
bedfont.mp.
-
or/1-5
-
exp cough/
-
cough$.mp.
-
phlegm.mp.
-
sputum.mp.
-
mucus.mp.
-
wheez$.mp.
-
chest pain/
-
chest pain$.mp.
-
(chest adj5 tight$).tw.
-
((lower respiratory or lrt) adj5 symptom$).tw.
-
(lower airway adj5 symptom$).tw.
-
((trache$ or wind pipe or lung$ or bronch$) adj3 symptom$).tw.
-
exp lung/ or trachea/
-
symptom$.tw.
-
19 and 20
-
or/7-18,21
-
exp asthma/
-
asthma$.mp.
-
exp respiratory hypersensitivity/
-
exp bronchial hyperreactivity/
-
bronchial spasm/
-
bronchospas$.mp.
-
exp Bronchoconstriction/
-
bronchoconstric$.mp.
-
(bronch$ adj3 constrict$).mp.
-
(bronch$ adj5 spas$).mp.
-
(airway$ adj5 (obstruct$ or inflammation$)).mp.
-
((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
-
or/23-34
-
Nitric Oxide/
-
nitric oxide.mp.
-
36 or 37
-
(exhal$ or expir$ or alveolar or fractional).mp.
-
38 and 39 (5228)
-
exhaled NO.mp.
-
eno.mp.
-
fe?no$.mp.
-
(fractional adj2 NO).mp.
-
or/40-44
-
22 and 45
-
35 and 45
-
6 or 46 or 47
-
exp “Sensitivity and Specificity”/
-
sensitivity.tw.
-
specificity.tw.
-
((pre-test or pretest) adj probability).tw.
-
post-test probability.tw.
-
predictive value$.tw.
-
likelihood ratio$.tw.
-
or/49-55
-
48 and 56
Analytical validity studies search: MEDLINE
-
NIOX MINO.mp.
-
aerocrine.mp.
-
(niox adj5 (monitor$ or chemiluminescence or analyser$ or sensor or device$ or desktop)).mp.
-
NObreath.mp.
-
bedfont.mp.
-
or/1-5
Trial registers and websites search
ClinicalTrials.gov (www.clinicaltrials.gov/)
21 March 2013
16 studies found for niox
10 studies found for mino | asthma
12 studies found for aerocrine
No studies found for NObreath
No studies found for bedfont
31 studies found for fractional exhaled nitric oxide | asthma
metaRegister of Controlled Trials (www.controlled-trials.com/mrct/)
Three studies found for niox
Three studies found for mino
Two studies found for aerocrine
No studies found for NObreath
One study found for bedfont
Two studies found for fractional exhaled nitric oxide
Manufacturer and User Facility Device Experience (MAUDE) database (www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm)
Report date 1 January 2010–28 February 2013
No records were found with NIOX MINO
No records were found with aerocrine
No records were found with NObreath
No records were found with bedfont
EuroScan International Network (http://euroscan.org.uk/)
Two results for NIOX MINO
No records were found with aerocrine
No records were found with bedfont
No records were found with NObreath
13 results for fractional exhaled nitric oxide asthma
Appendix 2 Clarification of the scope: communication with specialist committee member clinicians
| Queries on scope and study selection | Study Reference Manager IDsa | Our thoughts | Clifford Godley | John White | Hasan Arshad | Our decision |
|---|---|---|---|---|---|---|
| Population | ||||||
| 1. Unselected populations (not selected on the basis of asthmatic symptoms but patients with symptoms may be selected from the wider cohort to establish diagnostic cut-off), e.g. birth cohorts, a school year, a college year, a group in a particular profession | 483, 525, 654, 661, 1109, 3011, 6204 | We would like to exclude these as they do not reflect how the device would work in UK clinical practice and the population selected would likely impact on the estimates of sensitivity and specificity | Agreed | Agree | Agree | Exclude unselected populations |
| 2. Very young children – what is the minimum age? a. Patients with infant wheeze – if we are including very young children, are these patients equivalent to patients with suspected asthma? |
6047, 5136 | We would like to exclude studies where patients are > 10% under 4 years of age, in line with Aerocrine’s CE documentation. Bedfont do not state a minimum age. Alternatively, we could consider a cut-off of 5 years, as the UK guidelines draw this distinction in their protocols, as children under this age cannot reliably perform spirometry, a key stage in the diagnostic process | Agreed | It would be good if there were an alternate measure but practical difficulties mean cut-off at 5 reasonable as you suggest | NO can be measured reliably in younger children. Potentially, it could be a very useful test (if it works) precisely because other diagnostic methods (spirometry, reversibility and bronchial hyper-responsiveness) are not practical. However, in the absence of ‘Gold standard’ it is difficult to conclude if NO is a useful diagnostic test. So agree to keep the minimum age 5 years | Exclude studies with > 10% of patients under 5 years of age |
| 3. All patients already have asthma and the reference standard is one of: a. responsiveness to ICS b. EIB c. severe asthma d. methacholine/mannitol responsiveness e. high levels of sputum eosinophilia |
350, 613, 6014, 6164, 7500 | a. Responsiveness to ICS studies could be seen to fall in-between diagnosis and management – see attached ‘Diagnostic pathway for asthma in children’ – is this useful? Is this diagnostic or management? Do any of the other reference standards have any potential use in patients already diagnosed with asthma? We would probably like to exclude if not | Responsiveness to ICS is effectively a diagnostic trial, a positive response to which lends support to the postulated diagnosis. I would therefore like to keep this group, particularly if symptoms relapse off steroids | It is both – initially diagnostic. Yes as they might suggest lack of ‘control’ like FeNO | ‘Response to steroid’ is primarily a management issue (although it can be argued that the test can be used to ‘diagnose’ steroid responsive asthma). I think this question is better considered with other management issues | No clear consensus – but keep these studies in, present separately. Latterly think only include if they are being used diagnostically for asthma |
| 4. Patients with chronic cough – are these patients equivalent to patients with asthma symptoms? These studies usually diagnose ICS responsiveness (but not whether they have asthma or not) or cough-variant asthma | 328, 5878, 6383, 6385 | Unsure what to do with these | Raised N > O > will be a useful diagnostic signpost in the investigation of chronic cough. Normal N > O > will not preclude cough variant asthma but will make it less likely | Include as area of clinical relevance | Keep this as it might be useful to know if NO helps to diagnose ‘cough variant asthma’ | Keep in if diagnose CVA [cough variant asthma], present separately |
| 5. Study includes only children with positive skin prick test (no asthma symptoms necessarily) | 6345 | We would like to exclude – includes only atopic children, not on basis of asthma symptoms, so does not match the population we are interested in in UK practice | Agreed | Others will have clearer view on this. I would include – sorry! | Agree | Exclude studies which select patients on the basis of positive skin prick test only |
| 6. Study includes only patients with severe refractory asthma or moderate asthma. Reference standard is one of: a. ICS responsiveness b. eosinophilic phenotype |
6321, 6257 | a. Responsiveness to ICS studies could be seen to fall in-between diagnosis and management – see attached ‘Diagnostic pathway for asthma in children’ – is this useful? Is this diagnostic or management?b. Is eosinophilic phenotype a useful outcome? Management or diagnostic? | I think it is used diagnostically in the first instance. If they have asthma I would include | As above. Yes as relates to accurate diagnosis and management strategy | a. As suggested above, responsiveness to steroid is more of a management issueb. NO is closely associated with eosinophilic asthma but in itself it is not a useful outcome in terms of management | Include studies which select patients with asthma and diagnose ICS responsiveness and/or eosinophilic phenotype. Present separately |
| 7. Study includes only patients with suspected occupational asthma; reference standard is positive specific inhalation challenge | 387 | Is this population too specific or is it useful? | Should be included – useful | Emphasis on this area, e.g. NICE QS [Quality Standards]. It could be useful | This population is distinct and it could be useful to see if NO can help to diagnose occupational asthma | Include studies in patients with occupational asthma |
| 8. Patients with suspected Western red cedar asthma or suspicion of any other very specific type of asthma (e.g. occupational asthma) | Is this population too specific or is it useful? | Useful | Think I might let you off here | Western red cedar asthma is too specific and targets a small population. However, occupational asthma in general is a significant problem | Include with occupational asthma studies | |
| 9. Only patients diagnosed with occupational asthma – reference standard/diagnostic target of ICS responsiveness | 617 | Is this population too specific or is it useful? | Useful | See 7 | See above | As 7 |
| 10. Study includes patients with rhinitis and asthma symptoms – useful group to include? | 106 | Is this population too specific or is it useful? | Useful | See 7 include | Asthma and rhinitis coexist in significant proportion of patients. This is therefore a large group and useful to include | Include studies in patients with rhinitis and asthma |
| 11. Population a little odd – see data in column 3 | 6184 | Inclusion criteria states ‘children 6 to 16 years of age referred to our pulmonary outpatient clinic for further diagnostic work-up of possible reactive airway disease’, but the results section then states ‘the major complaints leading to referral were: exercise-induced shortness of breath, physician diagnosed asthma, chronic cough or miscellaneous leading symptoms’, i.e. some patients were already diagnosed with asthma. Is this a relevant cohort? Is a physician diagnosis of asthma sufficiently unreliable to class these patients as ‘patients with symptoms suggestive of asthma’? Some other studies excluded such patients | My understanding is that more than 75% of patients with unexplained chronic cough referred to clinics are ultimately considered to have airways disease. Physicians diagnosed asthma patients should be included. This heterogeneous group of patients compromise the real world population. Raised NO is a most invaluable result | It’s mainly a clinical diagnosis – supported by response to Rx [medical prescription] etc. Recommendation is further tests if this doesn’t help. This is a further test! | I think this is an appropriate population of childhood asthma. I did not find the inclusion criteria unusual | Include this study |
| 12. Are patients with chronic cough and FEV1 > 80% predicted equivalent to the hard-to-diagnose group? | 6169 | If so, we can include this study | Yes | Yes | Yes | Include this study |
| Intervention | ||||||
| 13. Offline measurements – can we exclude studies that use this? | 6204, 7038, 525 | We would have to conduct an review of comparability between methods in order to include these studies and they would just add another source of heterogeneity to the results. We have enough data without including these studies. We would like to exclude | Agreed | I don’t know what offline means. Maybe that’s what I am, hence lack of understanding | Agree | Exclude offline measurement |
| 14. Tidal breathing measurements – can we exclude these (mostly in very young children) | If we are only including those aged 4 and up, this is irrelevant. We would like to exclude | Agreed | OK | Agree | Exclude tidal breathing methods | |
| 15. Studies that use a different flow rate but convert to FeNO 50 (FeNO measured at a flow rate of 50 ml/second) – can we exclude (this specifically relates to a RCT management study that was included in the Cochrane review – attached to e-mail) | 7704, Smith 2005 | We would like to exclude – not convinced that the ‘equivalence’ can be assured | Agreed | OK | As far as I can see the study is sound; so no reason to exclude the study | Include, but maybe do a subgroup analysis where the results are excluded |
| 16. Weird flow rate – please see attached study | 617 | Unsure what to do | Prob exclude | Could not find the study in the attachment | Send again to clinicians | |
| 17. Alveolar and bronchial NO measurements – can we exclude? | We would like to exclude – not the use intended by the manufacturer in this application | Agreed | OK. Not relevant or likely to be to routine practice | Agree | Exclude alveolar and bronchial NO measurements | |
| 18. Exhaled breath condensate – we have excluded these | We would like to exclude | Agreed | OK – as above | Agree | Exclude exhaled breath condensate studies | |
| 19. Laser spectroscopy – this is not in scope correct? | We would like to exclude | Agreed | OK | Agree | Exclude laser spectroscopy | |
| 20. Nasal NO measurements – can we exclude? | We would like to exclude | Agreed | Might tell you something different. Used in CF I think OK to exclude | Agree | Exclude nasal NO measurements | |
| 21. Portable FeNO devices other than NIOX MINO and NObreath: NO Vario and Medisoft devices – should we include these in the same way that we are including chemiluminescence evidence, as equivalent? We may not have any analytical validity study data to support the equivalence of devices | We would like to exclude, unless they provide data on a subgroup that we have no other data for. This is because we would have to review the equivalence evidence for these devices as well as NIOX MINO and NObreath, which widens the scope of that review | Agreed | Keen to see this as generic FeNO but would need equivalence data to allow that. If can’t be done then excluded by default | I am not sure of the validity or equivalency of NO Vario and Medisoft devices. I have not used them and have no experience. Unless the companies can provide these data, it might be better to exclude these studies | Include if we have equivalency studies that allow the comparison of NO Vario and Medisoft devices | |
| Reference standard/outcome/diagnostic target | ||||||
| 22. Is exercise-induced asthma/bronchoconstriction a useful diagnostic target? Are they the same thing even? Are they the same as the ‘airway hyper-responsiveness testing’ listed in UK guidelines? | 5171, 6014 (population all asthmatics), 6047 (population wheezy children) | Unsure what to do. If same as UK airway hyper-responsiveness testing, we can include | This is a form of hyperresponsivness testing along with methacholine and histamine challenge. It is diagnostic for asthma. Include | May not be the same. Suggest include | Exercise induced bronchial challenge provides a valid outcome. It estimates bronchial hyperresponsiveness just like methacholine or histamine bronchial challenge. For childhood asthma in general and exercise induced asthma in particular, it might be superior to other types of bronchial challenges | Include studies which use EIB as the reference standard |
| 23. Studies that diagnose asthma severity, usually in an already diagnosed asthma population | Unsure if useful to diagnose asthma severity – where population is all asthmatic, this may occupy an intermediate position between the diagnostic strategy and the management strategy that has not yet been captured in the review – as discussed above in ‘population’ item 3a | Useful if it facilitated the diagnosis of asthma or helps identify uncontrolled asthma | Not sure how this helps really | If NO can provide an objective indication of asthma severity in those already diagnosed with the condition, then this might be a valid use of the test | Include diagnosis of asthma severity in already diagnosed population. Exclude diagnosis of asthma severity in an undiagnosed population, unless this facilitates diagnosis of asthma or uncontrolled asthma | |
| 24. Studies that diagnose ICS responsiveness in a variety of populations, e.g.: a. chronic cough b. diagnosed asthmatics c. suspected asthma d. severe refractory asthma e. moderate asthma |
e.g. 6169, 613, 617, 5878, 6164, 6321 (sputum cell count), chronic cough papers 328, 6383, 6385 | For chronic cough, we would have to present this data separately, as the diagnosis may not always be one of asthma, just one of ICS responsiveness. However, it may be better to exclude for consistency. Would this data be useful to the committee? For asthmatic populations, this may occupy an intermediate position between the diagnostic strategy and the management strategy that has not yet been captured in the review – as discussed above in ‘population’ item 3a | Data would be very useful. I feel I am comfortable to consider that patients with chronic cough who demonstrate ICS responsiveness should be considered to have cough variant asthma | Data are pleural so ‘these data’. Sorry again! Anyway, ICS responsiveness will effectively be asthma though not all asthma is ICS responsive. Include if possible | Yes, it would be useful to know if NO can provide a reliable indication of steroid responsiveness in those with chronic cough. For other asthmatic populations, it would be useful to assess the value of NO in estimating steroid responsiveness, although, the answer is probably yes in most asthma phenotypes | Include studies which diagnose ICS responsiveness in those with or without asthma |
| 25. Asthma (as opposed to ICS responsiveness, i.e. diagnoses both eosinophilic and non-eosinophilic asthma) | This will influence the estimates of sensitivity and specificity we will get out of the review – if we are using ICS responsiveness as a reference standard, FeNO is likely to give higher diagnostic accuracy than if we are using a broader definition of asthma as the reference standard, if we believe that it is better at identifying eosinophilic asthma. It may be best to present both sets of data? Another thing to consider is what will be useful to the model – some of the RCT studies recruit atopic patients – so if we are matching data from the diagnostic review to the RCT data for the modelling, would we need to use the diagnostic studies which identify ICS responsive asthmatics to lead into the modelling of the management strategy? | Certainly it will be more important to highlight the differences between eosinophilic and neutrophilic asthma with regard to NO levels and likely steroid responsiveness | Agree probably | Agree, it would be better to present both sets of data. Studies show that NO is better in diagnosing atopic than non-atopic asthma. Indeed, some studies show that it is a marker for atopy, more than it is for asthma. Hence, it would be useful to assess the usefulness of NO (as a diagnostic test) separately in atopic and non-atopic asthma. I suspect the result will be very different | Include studies which use asthma as the diagnostic reference standard | |
| 26. Studies that use methacholine/mannitol or bronchial hyper-responsiveness challenge as the reference standard | 1109, 7500, 6184 | Include | Include | That is valid if the result of methacholine or mannitol bronchial challenge (bronchial hyperresponsiveness) is combined with symptoms and not used alone as a diagnostic marker of asthma | Include studies which use methacholine/mannitol or bronchial hyperresponsiveness challenge as the reference standard where this is combined with symptoms | |
| 27. Studies that use exposure to trigger (occupational asthma) as a specific inhalation challenge as the reference standard | 387 | Is the reference standard equivalent to a diagnosis of asthma? | Usually diagnosis of occupational asthma is more robust than the clinical diagnosis of non occupational asthma | Not always! | Fine, but need to consider this as a distinct asthma phenotype (occupational asthma) | Include studies in occupational asthma which use specific inhalation challenge as the reference standard, but present separately |
| 28. Eosinophilic and phenotype | 6257, 6164 | Similar to diagnosing ICS responsiveness and asthma – does this sit between diagnosis and management? Is it useful? | Sorry – couldn’t identify these papers | No, it is not useful | Send to clinicians again | |
| 29. Sputum eosinophilia | 350 | Is this a relevant reference standard? | Yes | Ian Pavord would say so | For eosinophilic asthma, yes. But that is not a useful phenotype to diagnose in terms of management in day to day practice | Include studies which use eosinophilia as the reference standard. Present separately |
| 30. Variety of possibly useless reference standards | 6345 (also recruited only children with positive skin prick test) | See Table 1 in attached document ID 6345 – are any of these a useful reference standard? | Sorry – couldn’t identify these papers | Can’t see it on any list | Could not find Table 1. As suggested before, studies recruiting those with positive skin test only i.e. atopy if combined with asthma should be considered separately as this asthma phenotype (atopic asthma) is likely to be sensitive to NO | Sent to clinicians |
Appendix 3 Data extraction forms
| Review type | Data extracted for | |||
|---|---|---|---|---|
| Study background | Methods and devices | Study sample characteristics | Results | |
| Analytical validity |
|
|
|
|
| Diagnostic |
|
|
Prevalence of asthma Prevalence of positive result by reference standard:
|
|
| Management |
|
|
|
|
Appendix 4 Quality assessment scoring criteria
Diagnostic studies
Risk of bias in diagnostic studies (for both child and adult populations) was assessed and described using the Bristol University QUADAS-2 tool. 38 The QUADAS-2 tool is structured around four domains of potential sources of bias in primary diagnostic studies: patient selection, index test, reference standard and flow and timing. There are signalling questions within each of these domains that allow researchers to overview the potential sources of bias therein and a summary domain score can be generated to provide an indication of the overall potential for bias in each aspect of a study’s methodology. These signalling questions, and our approach to scoring them, are detailed in the following sections. It should be noted that, in our risk of bias tables, we report only the domain summary scores, although we also narratively summarise our responses to signalling questions in the review text to support these judgements.
Domain 1: patient selection
Signalling question 1: Was a consecutive or a random sample of patients enrolled?
-
Score ‘yes’ if the report states that enrolment was consecutive or random.
-
Score ‘no’ if the report states that another method of sampling was used.
-
Score ‘unclear’ if insufficient information was provided to make a judgement.
Signalling question 2: Was a case–control design avoided?
-
Score ‘yes’ if the study was not a case–control study.
-
Score ‘no’ if the study was a case–control study.
-
Score ‘unclear’ if insufficient information was provided to make a judgement.
Signalling question 3: Did the study avoid inappropriate exclusions?
With respect to the current review, the population of interest was patients presenting with clinical characteristics of asthma or those who are ‘difficult to diagnose’, that is, patients who have already undergone some of the tests for asthma in the UK pathway and who have not yet been confirmed to have asthma. The review scope also sought data on subgroups, in particular women during pregnancy, older people and smokers/passive smokers. Hence, this question was answered with respect to these groups. When there were ambiguities, two reviewers would discuss whether the population was appropriate.
-
Score ‘yes’ if the study had appropriately selected patients conforming to the groups outline above.
-
Score ‘no’ if the study made inappropriate exclusions from the group it set out to select.
-
Score ‘unclear’ if insufficient information was provided to make a judgement.
Summary domain score: Could the selection of patients have introduced bias?
-
Score as ‘low risk’ if the study scored ‘yes’ on all of signalling questions above.
-
Score as ‘high risk’ if the study scored ‘no’ or ‘unclear’ on two or more of the individual items or on either of the case–control design or inappropriate exclusions questions.
-
Score as ‘unclear risk’ if the study scored ‘no’ or ‘unclear’ on signalling question 1.
Domain 2: index test
Signalling question 1: Were the index test results interpreted without knowledge of the results of the reference standard?
-
Score ‘yes’ if the index test results were interpreted blind to the reference standard results or the index test results were clearly interpreted before the reference standard results were known.
-
Score ‘no’ if the results of the reference standard (UK guidelines pathway) were already known or if parts of the reference standard results downstream of the position of the test in the UK pathway were already known. This will need to be scored with reference to the patient population.
-
Score ‘unclear’ if unclear.
Signalling question 2: If a threshold was used, was it prespecified?
-
Score ‘yes’ if prespecified cut-off values were used (validation study).
-
Score ‘no’ if cut-off values were fitted to the data (derivation study).
-
Score ‘unclear’ if unclear.
Summary domain score: Could the conduct or interpretation of the index test have introduced bias?
-
Score as ‘low risk’ if the study scored ‘yes’ on both signalling questions.
-
Score as ‘high risk’ if cut-off values were fitted to the data (as derivation studies are likely to overestimate the true diagnostic accuracy of a technology relative to clinical practice) or if the study scored ‘no’ or ‘unclear’ on both signalling questions.
-
Score as ‘unclear’ if the study scored ‘no’ or ‘unclear’ on signalling question 1 only.
Domain 3: reference standard
Signalling question 1: Is the reference standard likely to correctly classify the target condition?
No reference standard for asthma is 100% sensitive or specific. Possibly the only way that this could be achieved is with long-term follow-up, but even this might be confounded by the fact that asthma can remiss and develop (e.g. as a comorbidity) over time. Hence, this item was scored with respect to UK guidelines:
-
Score ‘yes’ if the reference standard conforms with all or part of the UK guidelines.
-
Score ‘no’ if the reference standard does not conform with UK guidelines, that is, it uses a test that is not within the UK guidelines.
-
Score ‘unclear’ if unclear.
Signalling question 2: Were the reference standard results interpreted without knowledge of the results of the index test?
-
Score ‘yes’ if the reference standard results were interpreted blind to the index test results or the reference standard results were clearly interpreted before the index test results were known.
-
Score ‘no’ if the results of the index test were known.
-
Score ‘unclear’ if unclear.
Summary domain score: Could the conduct or interpretation of the index test have introduced bias?
-
Score as ‘low risk’ if the study scored ‘yes’ on both signalling questions.
-
Score as ‘high risk’ if the study scored ‘no’ or ‘unclear’ on both signalling questions.
-
Score as ‘unclear’ if the study scored ‘no’ or ‘unclear’ on either of the two signalling questions.
Domain 4: flow and timing
Signalling question 1: Was there an appropriate interval between the index test(s) and the reference standard?
-
Score ‘yes’ if the tests were conducted consecutively.
-
Score ‘no’ if the index test and the reference test were conducted > 1 week apart.
-
Score ‘unclear’ if unclear.
Signalling question 2: Did all patients receive a reference standard?
-
Score ‘yes’ if yes.
-
Score ‘no’ if no.
-
Score ‘unclear’ if unclear.
Signalling question 3: Did patients receive the same reference standard?
-
Score ‘yes’ if yes.
-
Score ‘no’ if no.
-
Score ‘unclear’ if unclear.
Signalling question 4: Were all patients included in the analysis?
-
Score ‘yes’ if yes.
-
Score ‘no’ if no.
-
Score ‘unclear’ if unclear.
Summary domain score: Could the patient flow have introduced bias?
-
Score as ‘low risk’ if the study scored ‘yes’ on all signalling questions.
-
Score as ‘high risk’ if the study scored ‘no’ or ‘unclear’ on three or more items.
-
Score as ‘unclear risk’ if the study scored ‘no’ or ‘unclear’ on up to two items.
Management studies
The quality of the FeNO-guided management studies in adults and children was assessed using the Cochrane Collaboration’s tool for assessing risk of bias in RCTs. 36 The tool is designed to address seven domains of bias: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and ‘other issues’. The tool provides a two-part assessment for risk of bias: the first part describes what was reported in the study for each domain and the second part consists of the review authors’ categorisation of the study as ‘low’, ‘high’ and ‘uncertain’ risk of bias (Table 85).
| Domain | Description | Review authors’ judgement |
|---|---|---|
| Sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups | Was the allocation sequence adequately generated? |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment | Was allocation adequately concealed? |
| Blinding of participants, personnel and outcome assessors [assessments should be made for each main outcome (or class of outcomes)] | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective | Was knowledge of the allocated intervention adequately prevented during the study? |
| Incomplete outcome data [assessments should be made for each main outcome (or class of outcomes)] | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with the total number of randomised participants), reasons for attrition/exclusions when reported and any re-inclusions in analyses performed by the review authors | Were incomplete outcome data adequately addressed? |
| Selective outcome reporting | State how the possibility of selective outcome reporting was examined by the review authors and what was found | Are reports of the study free of the suggestion of selective outcome reporting? |
| Other sources of bias | State any important concerns about bias not addressed in the other domains of the tool. If particular questions/entries were prespecified in the review’s protocol, responses should be provided for each question/entry | Was the study apparently free of other problems that could put it at a high risk of bias? |
The criteria for risk of bias judgements as outlined in the Cochrane Handbook36 (Table 86) were used to assign study ratings. As recommended by the Cochrane Handbook,36 we did not assign an overall numerical score for risk of bias in each study but discussed how potential sources of bias may be likely to affect the outcomes of the study.
| Sequence generation: Was the allocation sequence adequately generated? (Short form: Adequate sequence generation?) | |
| Criteria for a judgement of ‘yes’ (i.e. low risk of bias) | The investigators describe a random component in the sequence generation process such as:
|
| Criteria for the judgement of ‘no’ (i.e. high risk of bias) | The investigators describe a non-random component in the sequence generation process. Usually, the description would involve some systematic, non-random approach, e.g.:
|
| Criteria for the judgement of ‘unclear’ (uncertain risk of bias) | Insufficient information about the sequence generation process to permit judgement of ‘yes’ or ‘no’ |
| Allocation concealment: Was allocation adequately concealed? (Short form: Allocation concealment?) | |
| Criteria for a judgement of ‘yes’ (i.e. low risk of bias) | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgement of ‘no’ (i.e. high risk of bias) | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Criteria for the judgement of ‘unclear’ (uncertain risk of bias) | Insufficient information to permit judgement of ‘yes’ or ‘no’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, e.g. if the use of assignment envelopes is described but it remains unclear whether envelopes were sequentially numbered, opaque and sealed |
| Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated interventions adequately prevented during the study? (Short form: Blinding?) | |
| Criteria for a judgement of ‘yes’ (i.e. low risk of bias) | Any one of the following:
|
| Criteria for the judgement of ‘no’ (i.e. high risk of bias) | Any one of the following:
|
| Criteria for the judgement of ‘unclear’ (uncertain risk of bias) | Any one of the following:
|
| Incomplete outcome data: Were incomplete outcome data adequately addressed? (Short form: Incomplete outcome data addressed?) | |
| Criteria for a judgement of ‘yes’ (i.e. low risk of bias) | Any one of the following:
|
| Criteria for the judgement of ‘no’ (i.e. high risk of bias) | Any one of the following:
|
| Criteria for the judgement of ‘unclear’ (uncertain risk of bias) | Any one of the following:
|
| Selective outcome reporting: Are reports of the study free of the suggestion of selective outcome reporting? (Short form: Free of selective reporting?) | |
| Criteria for the judgement of ‘yes’ (i.e. high risk of bias) | Any of the following:
|
| Criteria for the judgement of ‘no’ (i.e. high risk of bias) | Any one of the following:
|
| Criteria for the judgement of ‘unclear’ (uncertain risk of bias) | Insufficient information to permit judgement of ‘yes’ or ‘no’. It is likely that the majority of studies will fall into this category |
| Other potential threats to validity: Was the study apparently free of other problems that could put it at a risk of bias? (Short form: Free of other bias?) | |
| Criteria for a judgement of ‘yes’ (i.e. low risk of bias) | The study appears to be free of other sources of bias |
| Criteria for the judgement of ‘no’ (i.e. high risk of bias) | There is at least one important risk of bias, e.g. the study:
|
| Criteria for the judgement of ‘unclear’ (uncertain risk of bias) | There may be a risk of bias but there is either:
|
Appendix 5 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)193 flow diagram (adapted) for the reviews of clinical evidence and for the update of clinical evidence conducted in September 2013. Additional search for NIOX Vero included in numbers for original search

| Study | Reason for exclusion | |
|---|---|---|
| 1 | National Institute of Allergy and Infectious Diseases. Evaluation of an asthma treatment strategy based on exhaled nitric oxide measurements in adolescents. ClinicalTrials.gov 2005 | Trial protocol |
| 2 | Abba AA, Habib SS, Beg MFS, Al Zoghaibi M. A comparative study of fraction of exhaled nitric oxide in stable chronic obstructive pulmonary disease and steroid naive asthma. In New Horizons Allergy Asthma Immunology 2009. pp. 199–204 | Not a RCT |
| 3 | Acembekiroglu S, Altintas D, Seydaoglu G, Ceter T, Yilmaz M, Bingol KG, et al. Seasonal variation of exhaled nitric oxide levels in children with allergic asthma that is sensitive to inhaled allergens. Allergy 2011;66:201 | Not a RCT |
| 4 | Agache I, Ciobanu C. Predictive value of lung function trend and FeNO for difficult asthma in children. J Invest Allergol Clin Immunol 2012;22:419–26 | Not a RCT |
| 5 | Alvarez-Gutierrez FJ, Medina-Gallardo JF, Perez-Navarro P, Martin-Villasclaras JJ, Etchegoren BM, Romero-Romero B, et al. Comparison of the Asthma Control Test (ACT) with lung function, levels of exhaled nitric oxide and control according to the Global Initiative for Asthma (GINA). Arch Bronconeumol 2010;46:370–7 | Foreign language |
| 6 | Anderson WJ, Lipworth BJ. Does body mass index influence responsiveness to inhaled corticosteroids in persistent asthma? Ann Allergy Asthma Immunol 2012;108:237–42 | Not a RCT |
| 7 | Andregnette-Roscigno V, Fernandez-Nieto M, Garcia Del Potro M, Aguado E, Sastre J. Correlation between tests to measure bronchial hyperreactivity and exhaled nitric oxide levels in asthmatic children. Allergy 2011;66:154 | No useable diagnostic data |
| 8 | Arnold DH, Gebretsadik T, Abramo TJ, Hartert TV, Arnold DH, Gebretsadik T, et al. Noninvasive testing of lung function and inflammation in pediatric patients with acute asthma exacerbations. J Asthma 2012;49:29–35 | Emergency care |
| 9 | Arochena L, Fernandez-Nieto M, Andregnette V, Garcia Del PM, Aguado E, Sastre J. Bronchial hyperresponsiveness in sportschildren; different methods to reach a diagnosis. J Allergy Clin Immunol 2012;129(2 Suppl. 1):AB2 | No useable diagnostic data |
| 10 | Artlich A, Hagenah JU, Jonas S, Ahrens P, Gortner L. Exhaled nitric oxide in childhood asthma. Eur J Pediatr 1996;155:698–701 | Wrong flow rate |
| 11 | Avital A, Uwyyed K, Berkman N, Godfrey S, Bar-Yishay E, Springer C. Exhaled nitric oxide and asthma in young children. Pediatr Pulmonol 2001;32:308–13 | Offline |
| 12 | Awabdy B, Balasubramanyam V, Parikh B, Peled N. Performance of the new insight ENO system to measure exhaled nitric oxide – comparison to chemiluminescence technologies. Am J Respir Crit Care Med 2010;181:A4283 | Wrong device |
| 13 | Ayars AG, Potter-Perigo S, Wight TN, Tilles SA, Altman LC. Comparative sensitivity of various indices in evaluating improvement in mild persistent asthma. J Allergy Clin Immunol 2012;129(2 Suppl. 1):AB75 | Not a RCT |
| 14 | Badzakova MG, Obocki K. Exhaled nitric oxide fraction in asthmatic children – well correlated with clinical control of asthma (in primary care). Allergy 2011;66:588 | Not a RCT |
| 15 | Baek HS, Kim HJ, Kim YD, Oh JW, Shin JH, Lee HB. Exhaled nitric oxide correlates with post-betaronchodilator improvement of FEV1 in chronic childhood asthma. J Allergy Clin Immunol 2010;125(2 Suppl. 1):AB50 | Not a RCT |
| 16 | Balinotti JE, Colom A, Kofman C, Teper AM. Association between nitric oxide and a clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2011;183:A5475 | Infants |
| 17 | Baptist AP, Sengupta R, Pranathiageswaran S, Wang Y, Ager J. Evaluation of exhaled nitric oxide measurements in the emergency department for patients with acute asthma. Ann Allergy Asthma Immunol 2008;100:415–19 | Emergency care |
| 18 | Baptist AP, Shah B, Wang Y, Ager J, Badr MS. Exhaled nitric oxide levels during treatment in patients hospitalized with asthma. Allergy Asthma Proc 2008;29:171–6 | Emergency care |
| 19 | Baptist AP, Khan FI, Wang Y, Ager J. Exhaled nitric oxide measurements in hospitalized children with asthma. J Asthma 2008;45:670–4 | Emergency care |
| 20 | Bar-Yishay E, Matyashchuk E, Mussaffi H, Prais D, Steuer G, Mei-Zahav M, et al. Fractional exhaled nitric oxide does not correlate with functional measures in pre-school wheezy children. Am J Respir Crit Care Med 2010;181:A3922 | No usable diagnostic data |
| 21 | Baraldi E, Scollo M, Zaramella C, Zanconato S, Zacchello F. A simple flow-driven method for online measurement of exhaled NO starting at the age of 4 to 5 years. Am J Respir Crit Care Med 2000;162:1828–32 | NO analytical data |
| 22 | Baraldi E, Azzolin NM, Zanconato S, Dario C, Zacchello F. Corticosteroids decrease exhaled nitric oxide in children with acute asthma. J Pediatr 1997;131:381–5 | Not a RCT |
| 23 | Baraldi E, Azzolin NM, Cracco A, Zacchello F. Reference values of exhaled nitric oxide for healthy children 6–15 years old. Pediatr Pulmonol 1999;27:54–8 | Tidal breathing |
| 24 | Barben J, Strippoli MP, Trachsel D, Schiller B, Hammer J, Kuehni CE, et al. Effect of mannitol dry powder challenge on exhaled nitric oxide in children. PLOS ONE 2013;8:e54521 | Not FeNO testing for diagnosis |
| 25 | Barreto M, Villa MP, Olita C, Martella S, Ciabattoni G, Montuschi P, et al. 8-Isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest 2009;135:66–73 | Not a RCT |
| 26 | Barreto M, La Penna F, Prete A, Bonafoni S, Negro V, Chialant D, et al. Exhaled breath temperature and other exhaled markers in children with asthma and rhinitis. Am J Respir Crit Care Med 2011;183:A1897 | No useable diagnostic data |
| 27 | Bastain TM, Islam T, Berhane KT, McConnell RS, Rappaport EB, Salam MT, et al. Exhaled nitric oxide, susceptibility and new-onset asthma in the Children's Health Study. Eur Respir J 2011;37:523–31 | Offline |
| 28 | Bautista AP, Eisenlohr CP, Lanz MJ. Nasal nitric oxide and nasal eosinophils decrease with levocetirizine in subjects with perennial allergic rhinitis. Am J Rhinol Allergy 2011;25:383–7 | Not asthma |
| 29 | Bayo AL, Tordera MP, Perez EM, Gisbert VM. Contribution of exhaled nitric oxide measurements to abbreviated bronchial challenge test protocols. Arch Bronconeumol 2008;44:402–7 | Population asthma and non-asthma |
| 30 | Becher G, Dietze S, Steinhaeusser W, Schmidtmann S, Beck E, Timm-Labsch B. Can we measure exhaled NO accurate and reproducible? Am J Respir Crit Care Med 2010;181:A4280 | No analytical data |
| 31 | Beigelman A, Mauger DT, Phillips BR, Zeiger RS, Taussig LM, Strunk RC, et al. Effect of elevated exhaled nitric oxide levels on the risk of respiratory tract illness in preschool-aged children with moderate-to-severe intermittent wheezing. Ann Allergy Asthma Immunol 2009;103:108–13 | Offline |
| 32 | Belda J, Parameswaran K, Lemiere C, Kamada D, O’Byrne PM, Hargreave FE, et al. Predictors of loss of asthma control induced by corticosteroid withdrawal. Can Respir J 2006;13:129–33 | Not a RCT |
| 33 | Bell MC, Evans MD, Tisler CJ, Gern J, Lemanske J, Jackson DJ. Early aeroallergen sensitization is associated with higher fractional exhaled nitric oxide levels in school age children independent of asthma diagnosis. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB46 | No useable diagnostic data |
| 34 | Berkman N, Avital A, Breuer R, Bardach E, Springer C, Godfrey S. Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax 2005;60:383–8 | Wrong flow rate |
| 35 | Berlyne GS, Parameswaran K, Kamada D, Efthimiadis A, Hargreave FE. A comparison of exhaled nitric oxide and induced sputum as markers of airway inflammation. J Allergy Clin Immunol 2000;106:638–44 | Wrong flow rate |
| 36 | Bernstein JA, Davis B, Alvarez-Puebla MJ, Nguyen D, Levin L, Olaguibel JM, et al. Is exhaled nitric oxide a useful adjunctive test for assessing asthma? J Asthma 2009;46:955–60 | No useable diagnostic data |
| 37 | Berry MA, Shaw DE, Green RH, Brightling CE, Wardlaw AJ, Pavord ID, et al. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy 2005;35:1175–9 | Wrong flow rate |
| 38 | Bisgaard H, Loland L, Oj JA. NO in exhaled air of asthmatic children is reduced by the leukotriene receptor antagonist montelukast. Am J Respir Crit Care Med 1999;160:1227–31 | Wrong flow rate |
| 39 | Bivins J, Ownby D, Waller J, Tingen M. Exhaled nitric oxide level and school absenteeism in rural high school students with current asthma. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB64 | No useable diagnostic data |
| 40 | Blain EA, Craig T, Weyant K. Exhaled nitric oxide (eNO) should not be used to exclude the diagnosis of asthma. Ann Allergy Asthma Immunol 2009;103(5 Suppl. 3):A60 | Case study |
| 41 | Bloemen K, Koppen G, Govarts E, Colles A, Van Den Heuvel R, Nelen V, et al. Application of non-invasive biomarkers in a birth cohort follow-up in relation to respiratory health outcome. Biomarkers 2010;15:583–93 | No useable diagnostic data |
| 42 | Bodini A, Peroni DG, Zardini F, Corradi M, Alinovi R, Boner AL, et al. Flunisolide decreases exhaled nitric oxide and nitrotyrosine levels in asthmatic children. Mediators Inflamm 2006;2006:31919 | Not a RCT |
| 43 | Bodini A, Peroni D, Loiacono A, Costella S, Pigozzi R, Baraldi E, et al. Exhaled nitric oxide daily evaluation is effective in monitoring exposure to relevant allergens in asthmatic children. Chest 2007;132:1520–5 | Not a RCT |
| 44 | Bohadana AB, Hannhart B, Ghezzo H, Teculescu D, Zmirou-Navier D. Exhaled nitric oxide and spirometry in respiratory health surveillance. Occup Med (Oxford) 2011;61:108–14 | Unselected population |
| 45 | Bommarito L, Migliore E, Bugiani M, Heffler E, Guida G, Bucca C, et al. Exhaled nitric oxide in a population sample of adults. Respiration 2008;75:386–92 | Offline |
| 46 | Boon M, Meyts I, Warnier G, Boeck KD. Exhaled nitric oxide: offline tidal breathing measurements are feasible in children and correlate with online single breath measurements. Pediatr Allergy Immunol Pulmonol 2010;23:201–6 | Online vs. offline |
| 47 | Boon M, Proesmans M, Meyts I, De Boeck K. Do composite scores of nNO and FENO improve diagnostic value? J Cyst Fibrosis 2012;11:S96 | Case–control study |
| 48 | Boot JD, de Kam ML, Mascelli MA, Miller B, van Wijk RG, de Groot H, et al. Nasal nitric oxide: longitudinal reproducibility and the effects of a nasal allergen challenge in patients with allergic rhinitis. Allergy 2007;62:378–84 | Nasal NO |
| 49 | Bora M, Alpaydin AO, Yorgancioglu A, Akkas G, Isisag A, Coskun AS, et al. Does asthma control as assessed by the asthma control test reflect airway inflammation? Multidiscip Resp Med 2011;6:291–8 | Not a RCT |
| 50 | Bosque-Garcia M, Asensio-De La Cruz O, Jaramillo-Hidalgo D, Valdesoiro-Navarrete L, Costa-Colomer J, Penas-Aguilera A, et al. Exhaled nitric oxide and asthma control measured by clinical score and exerciseinduced bronchoconstriction. Allergy Eur J Allergy Clin Immunol 2011;66:577–8 | Not a RCT |
| 51 | Bossley CJ, Saglani S, Kavanagh C, Payne DN, Wilson N, Tsartsali L, et al. Corticosteroid responsiveness and clinical characteristics in childhood difficult asthma. Eur Respir J 2009;34:1052–9 | Not a RCT |
| 52 | Bozek A, Jarzab J. Nasal nitric oxide measurements in patients with seasonal allergic rhinitis. Allergy Eur J Allergy Clin Immunol 2010;65:155 | No usable diagnostic data |
| 53 | Bozek A, Filipowska-Gronska A, Werynska-Kalemba M, Jarzab J. Nasal nitric oxide measurements in patients with seasonal allergic rhinitis of different age groups. Postepy Dermatol Alergol 2010;27:96–100 | Foreign language |
| 54 | Bozek A, Krajewska J, Jarzab J. Nasal nitric oxide and other diagnostic procedures in seasonal allergic rhinitis: elderly vs juvenile patients. Am J Otolaryngol 2011;32:105–8 | Nasal NO |
| 55 | Bratton DL, Lanz MJ, Miyazawa N, White CW, Silkoff PE. Exhaled nitric oxide before and after montelukast sodium therapy in school-age children with chronic asthma: a preliminary study. Pediatr Pulmonol 1999;28:402–7 | Wrong flow rate |
| 56 | Brightling CE, Green RH, Pavord ID. Biomarkers predicting response to corticosteroid therapy in asthma. Treat Respir Med 2005;4:309–16 | Review |
| 57 | Brindicci C, Ito K, Barnes PJ, Kharitonov SA. Differential flow analysis of exhaled nitric oxide in patients with asthma of differing severity. Chest 2007;131:1353–62 | FeNO testing did not guide step-up/step-down therapy |
| 58 | Brooks CR, Brogan SB, van Dalen CJ, Lampshire PK, Crane J, Douwes J, et al. Measurement of exhaled nitric oxide in a general population sample: a comparison of the Medisoft HypAir FE(NO) and Aerocrine NIOX analyzers. J Asthma 2011;48:324–8 | Wrong device |
| 59 | Bruce CT, Zhao D, Yates DH, Thomas PS. AMP challenge induces a decrease in FE(NO) in asthmatic subjects modulated by nedocromil. Eur J Clin Invest 2006;36:899–905 | FeNO testing did not guide step-up/step-down therapy |
| 60 | Bruce CT, Zhao D, Yates DH, Thomas PS. L-arginine reverses cigarette-induced reduction of fractional exhaled nitric oxide in asthmatic smokers. Inflammopharmacology 2010;18:9–16 | FeNO testing did not guide step-up/step-down therapy |
| 61 | Brusselle GG, Kardos P, Louis R, Schmoller T, Jorgensen L, Aubier M, et al. Budesonide/formoterol maintenance and reliever therapy at two different maintenance doses: effect on fractional excretion of nitric oxide (FENO). Am J Respir Crit Care Med 2010;181:A5407 | No useable diagnostic data |
| 62 | Buchvald F, Eiberg H, Bisgaard H. Heterogeneity of FeNO response to inhaled steroid in asthmatic children. Clin Exp Allergy 2003;33:1735–40 | FeNO testing did not guide step-up/step-down therapy |
| 63 | Buchvald F, Hermansen MN, Nielsen KG, Bisgaard H. Exhaled nitric oxide predicts exercise-induced bronchoconstriction in asthmatic school children. Chest 2005;128:1964–7 | All asthmatic not diagnostic |
| 64 | Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, et al. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol 2005;115:1130–6 | Measurement in healthy subjects |
| 65 | Bukstein D, Luskin AT, Brooks EA. Exhaled nitric oxide as a tool in managing and monitoring difficult-to-treat asthma. Allergy Asthma Proc 2011;32:185–92 | Not a RCT |
| 66 | Bukstein DA. Individualized dynamic phenotyping using fractional exhaled nitric oxide levels in children with asthma. Ann Allergy Asthma Immunol 2012;109:A52 | Not a RCT |
| 67 | Burnett M, Wegienka G, Havstad S, Ownby D, Cole JC, Zoratti E. The relationship of fractional exhaled nitric oxide levels to allergy and asthma biomarkers in young adults. J Allergy Clin Immunol 2011;127(2 Suppl. 1):AB58 | |
| 68 | Bush A. The use of inflammatory markers to guide therapy in children with severe asthma. ClinicalTrials.gov 2005 | Trial protocol |
| 69 | Byrnes CA, Dinarevic S, Busst CA, Shinebourne EA, Bush A. Effect of measurement conditions on measured levels of peak exhaled nitric oxide. Thorax 1997;52:697–701 | No analytical data |
| 70 | Cabral AL, Vollmer WM, Barbirotto RM, Martins MA. Exhaled nitric oxide as a predictor of exacerbation in children with moderate-to-severe asthma: a prospective, 5-month study. Ann Allergy Asthma Immunol 2009;103:206–11 | Not a RCT |
| 71 | Canady RG, Platts-Mills T, Murphy A, Johannesen R, Gaston B. Vital capacity reservoir and online measurement of childhood nitrosopnea are linearly related. Clinical implications. Am J Respir Crit Care Med 1999;159:311–14 | Online vs. offline |
| 72 | Cardinale F, De Benedictis FM, Muggeo V, Giordano P, Loffredo MS, Iacoviello G, et al. Exhaled nitric oxide, total serum IgE and allergic sensitization in childhood asthma and allergic rhinitis. Pediatr Allergy Immunol 2005;16:236–42 | Wrong flow rate |
| 73 | Carlstedt F, Lazowska D, Bornehag CG, Olin AC, Hasselgren M. Exhaled nitric oxide and urinary EPX levels in infants: a pilot study. Clin Mol Allergy 2011;9:8 | No useable diagnostic data |
| 74 | Carra S, Gagliardi L, Zanconato S, Scollo M, Azzolin N, Zacchello F, et al. Budesonide but not nedocromil sodium reduces exhaled nitric oxide levels in asthmatic children. Respir Med 2001;95:734–9 | Wrong flow rate |
| 75 | Carter R, Murphy A, Hargadon B, Agbetile J, Pavord ID, Wardlaw AJ, et al. Evaluating the role of triamcinolone in a difficult asthma service. Thorax 2010;65:A152 | Not a RCT |
| 76 | Carvalho-Pinto RM, Stelmach R, Angelini L, Santos DO, Dias-Junior S, Cukier A, et al. Is there a good tool to measure asthma control on severe asthma patients? Am J Respir Crit Care Med 2010;181:A2560 | Not a RCT |
| 77 | Castano R, Miedinger D, Malo JL, Desrosiers M. Nasal and exhaled nitric oxide monitoring during specific inhalation challenge using a portable analyser. J Allergy Clin Immunol 2011;127(2 Suppl. 1):AB53 | No analytical data |
| 78 | Castell B, Pike D, Masoli M. Difficult asthma: the Plymouth experience. Thorax 2011;66:A114 | Not a RCT |
| 79 | Castro-Rodriguez JA, Sardon O, Perez-Yarza EG, Korta J, Aldasoro A, Corcuera P, et al. Young infants with recurrent wheezing and positive asthma predictive index have higher levels of exhaled nitric oxide. J Asthma 2013;50:162–5 | Age < 5 years |
| 80 | Caudri D, Wijga AH, Hoekstra MO, Kerkhof M, Koppelman GH, Brunekreef B, et al. Prediction of asthma in symptomatic preschool children using exhaled nitric oxide, Rint and specific IgE. Thorax 2010;65:801–7 | Offline |
| 81 | Chai J-J, Cai B-Q. The normal value measurement of fractional concentration of exhaled nitric oxide in Chinese adults. Respirology 2011;16:196–7 | No analytical data |
| 82 | Chatkin JM, Ansarin K, Silkoff PE, McClean P, Gutierrez C, Zamel N, et al. Exhaled nitric oxide as a noninvasive assessment of chronic cough. Am J Respir Crit Care Med 1999;159:1810–13 | Wrong flow rate |
| 83 | Chawes BL, Buchvald F, Bischoff AL, Loland L, Hermansen M, Halkjaer LB, et al. Elevated exhaled nitric oxide in high-risk neonates precedes transient early but not persistent wheeze. Am J Respir Crit Care Med 2010;182:138–42 | Wrong flow rate |
| 84 | Chen E, Strunk RC, Bacharier LB, Chan M, Miller GE. Socioeconomic status associated with exhaled nitric oxide responses to acute stress in children with asthma. Brain Behav Immunity 2010;24:444–50 | Not a RCT |
| 85 | Cherot-Kornobis N, Hulo S, Edme JL, de Broucker V, Matran R, Sobaszek A. Analysis of nitrogen oxides (NOx) in the exhaled breath condensate (EBC) of subjects with asthma as a complement to exhaled nitric oxide (FeNO) measurements: a cross-sectional study. BMC Res Notes 2011;4:202 | No useable diagnostic data |
| 86 | Chinellato I, Piazza M, Peroni D, Sandri M, Chiorazzo F, Boner AL, et al. Bronchial and alveolar nitric oxide in exercise-induced bronchoconstriction in asthmatic children. Clin Exp Allergy 2012;42:1190–6 | Not a RCT |
| 87 | Chladkova J, Senkerik M, Havlinova Z, Krcmova I, Chladek J. Alveolar concentration and bronchial flux of nitric oxide: two linear modeling methods evaluated in children and adolescents with allergic rhinitis and atopic asthma. Pediatr Pulmonol 2012;47:1070–9 | Alveolar NO |
| 88 | Choi B, Jee H, Park Y, Kim C, Sohn M, Kim K. Relationship between exhaled nitric oxide and allergic inflammation or sensitization in children. J Allergy Clin Immunol 2009;123(2 Suppl. 1):S207 | No useable diagnostic data |
| 89 | Choi BS, Kim KW, Lee YJ, Baek J, Park HB, Kim YH, et al. Exhaled nitric oxide is associated with allergic inflammation in children. J Korean Med Sci 2011;26:1265–9 | Not FeNO testing for diagnosis |
| 90 | Chow JS, Leung AS, Li WW, Tse TP, Sy HY, Leung TF, et al. Airway inflammatory and spirometric measurements in obese children. Hong Kong Med J 2009;15:346–52 | No useable diagnostic data |
| 91 | Cibella F, Cuttitta G, La Grutta S, Melis MR, Bucchieri S, Viegi G. A cross-sectional study assessing the relationship between BMI, asthma, atopy, and eNO among schoolchildren. Ann Allergy Asthma Immunol 2011;107:330–6 | No useable diagnostic data |
| 92 | Ciprandi G, Tosca MA, Capasso M. Exhaled nitric oxide in children with allergic rhinitis and/or asthma: a relationship with bronchial hyperreactivity. J Asthma 2010;47:1142-7 | Data for both asthma and rhinitis |
| 93 | Ciprandi G, Tosca MA, Capasso M. High exhaled nitric oxide levels may predict bronchial reversibility in allergic children with asthma or rhinitis. J Asthma 2013;50:33–8 | Data for both asthma and rhinitis |
| 94 | Cirillo I, Ricciardolo FLM, Medusei G, Signori A, Ciprandi G. Exhaled nitric oxide may predict bronchial hyperreactivity in patients with allergic rhinitis. Int Arch Allergy Immunol 2013;160:322–8 | Not diagnosis of asthma |
| 95 | Clearie KL, Williamson PA, Vaidyanathan S, Short P, Goudie A, Burns P, et al. Disconnect between standardized field-based testing and mannitol challenge in Scottish elite swimmers. Clin Exp Allergy 2010;40:731–7 | Not FeNO for diagnosis |
| 96 | Clearie KL, Vaidyanathan S, Williamson PA, Goudie A, Short P, Schembri S, et al. Effects of chlorine and exercise on the unified airway in adolescent elite Scottish swimmers. Allergy 2010;65:269–73 | No useable diagnostic data |
| 97 | Clearie KL, Jackson CM, Fardon TC, Williamson PA, Vaidyanathan S, Burns P, et al. Supervised step-down of inhaled corticosteroids in the community – an observational study. Respir Med 2011;105:558–65 | Not a RCT |
| 98 | Cleveland C, Monforte SE, Spahn JD. Establishing normal exhaled nitric oxide (FeNO) values in young children. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB195 | No useable diagnostic data |
| 99 | Cohen J, Douma WR, Ten Hacken NH, Vonk JM, Oudkerk M, Postma DS, et al. Ciclesonide improves measures of small airway involvement in asthma. Eur Respir J 2008;31:1213–20 | Alveolar NO |
| 100 | Colon-Semidey AJ, Marshik P, Crowley M, Katz R, Kelly HW. Correlation between reversibility of airway obstruction and exhaled nitric oxide levels in children with stable bronchial asthma. Pediatr Pulmonol 2000;30:385–92 | Not a RCT |
| 101 | Columbo M, Wong B, Panettieri RA, Rohr AS. Asthma in the elderly: the role of exhaled nitric oxide measurements. J Allergy Clin Immunol 2012;129(2 Suppl. 1):AB8 | No useable diagnostic data |
| 102 | Consilvio NP, Di Pillo S, Verini M, de Giorgis T, Cingolani A, Chiavaroli V, et al. The reciprocal influences of asthma and obesity on lung function testing, AHR, and airway inflammation in prepubertal children. Pediatr Pulmonol 2010;45:1103–10 | Population obese |
| 103 | Consilvio NP, Di Pillo S, de Giorgis T, Cingolani A, Scaparrotta A, Rapino D, et al. The reciprocal influences of asthma and obesity on lung function, AHR and bronchial inflammation in prepubertal children. Paediatr Respir Rev 2010;11:S2–3 | No useable diagnostic data |
| 104 | Corradi M. What is new in the air? Monaldi Arch Chest Dis Pulm Ser 2002;57:227-8 | Editorial |
| 105 | Corradi M, Zinelli C, Caffarelli C. Exhaled breath biomarkers in asthmatic children. Inflamm Allergy Drug Targets 2007;6:150–9 | Review |
| 106 | Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild-to-moderate asthma. J Pediatr 2003;142:469-75 | Not a RCT |
| 107 | Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax 2010;65:384–90 | FeNO testing did not guide step-up/step-down therapy |
| 108 | Cowan DC, Hewitt RS, Cowan JO, Palmay R, Williamson A, Lucas SJ, et al. Exercise-induced wheeze: fraction of exhaled nitric oxide-directed management. Respirology 2010;15:683–90 | Not randomised to FeNO |
| 109 | Craig TJ, King TS, Lemanske RF Jr, Wechsler ME, Icitovic N, Zimmerman RR Jr, et al. Aeroallergen sensitization correlates with PC(20) and exhaled nitric oxide in subjects with mild-to-moderate asthma. J Allergy Clin Immunol 2008;121:671–7 | FeNO testing did not guide step-up/step-down therapy |
| 110 | Crane J, Wickens K, Beasley R, Fitzharris P. Asthma and allergy: a worldwide problem of meanings and management? Allergy Eur J Allergy Clin Immunol 2002;57:663–72 | Review |
| 111 | Crane J, Lampshire P, Wickens K, Epton M, Siebers R, Ingham T, et al. Asthma, atopy and exhaled nitric oxide in a cohort of 6-yr-old New Zealand children. Pediatr Allergy Immunol 2012;23:59–64 | No useable diagnostic data |
| 112 | Crater SE, Peters EJ, Martin ML, Murphy AW, Platts-Mills TAE. Expired nitric oxide and airway obstruction in asthma patients with an acute exacerbation. Am J Respir Crit Care Med 1999;159:806–11 | Wrong flow rate |
| 113 | Cristescu SM, Mandon J, Harren FJM, Merilainen P, Hogman M. Methods of NO detection in exhaled breath. J Breath Res 2013;7:017104 | Review |
| 114 | Crothall H, Custovic A, Simpson A, Kerry G, Belgrave D, Murray C. The relationship between exhaled nitric oxide, atopy and asthma in school aged children. Clin Exp Allergy 2012;42:1846 | No useable diagnostic data |
| 115 | Currie GP, Bates CE, Lee DKC, Jackson CM, Lipworth BJ. Effects of fluticasone plus salmeterol versus twice the dose of fluticasone in asthmatic patients. Eur J Clin Pharmacol 2003;59:11–15 | FeNO testing did not guide step-up/step-down therapy |
| 116 | Currie GP, Syme-Grant NJ, McFarlane LC, Carey FA, Lipworth BJ. Effects of low dose fluticasone/salmeterol combination on surrogate inflammatory markers in moderate persistent asthma. Allergy 2003;58:602–7 | Not a RCT |
| 117 | Currie GP, Lee DKC, Haggart K, Bates CE, Lipworth BJ. Effects of montelukast on surrogate inflammatory markers in corticosteroid-treated patients with asthma. Am J Respir Crit Care Med 2003;167:1232–8 | Wrong flow rate |
| 118 | Dahlen B, Lantz AS, Ihre E, Skedinger M, Henriksson E, Jorgensen L, et al. Effect of formoterol with or without budesonide in repeated low-dose allergen challenge. Eur Respir J 2009;33:747–53 | FeNO testing did not guide step-up/step-down therapy |
| 119 | Dal Negro R, Micheletto C, Tognella S, Turco P, Rossetti A, Cantini L. Assessment of inhaled BDP-dose dependency of exhaled nitric oxide and local and serum eosinophilic markers in steroids-naive nonatopic asthmatics. Allergy 2003;58:1018–22 | Wrong flow rate |
| 120 | Dallinga JW, Robroeks CMHH, van Berkel JJBN, Moonen EJC, Godschalk RWL, Jobsis Q, et al. Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin Exp Allergy 2010;40:68–76 | No useable diagnostic data |
| 121 | de Bot CM, Moed H, Bindels PJ, van Wijk RG, Berger MY, de Groot H, et al. Exhaled nitric oxide measures allergy not symptoms in children with allergic rhinitis in primary care: a prospective cross-sectional and longitudinal cohort study. Prim Care Respir J 2013;22:44–50 | No useable diagnostic data |
| 122 | de Gouw HW, Hendriks J, Woltman AM, Twiss IM, Sterk PJ. Exhaled nitric oxide (NO) is reduced shortly after bronchoconstriction to direct and indirect stimuli in asthma. Am J Respir Crit Care Med 1998;158:315–19 | FeNO testing did not guide step-up/step-down therapy |
| 123 | de Gouw HW, Grunberg K, Schot R, Kroes AC, Dick EC, Sterk PJ, et al. Relationship between exhaled nitric oxide and airway hyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur Respir J 1998;11:126–32 | Not RCT study |
| 124 | de Gouw HW, Marshall-Partridge SJ, Van der Veen H, Van Den Aardweg JG, Hiemstra PS, Sterk PJ. Role of nitric oxide in the airway response to exercise in healthy and asthmatic subjects. J Appl Physiol 2001;90:586–92 | Wrong flow rate |
| 125 | de Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax 2012;67:582–7 | Not a RCT |
| 126 | de Jongste JC, Carraro S, Hop WC, CHARISM Study Group, Baraldi E. Daily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthma. Am J Respir Crit Care Med 2009;179:93–7 | Daily monitoring |
| 127 | de Meer G, van Amsterdam JGC, Janssen NAH, Meijer E, Steerenberg PA, Brunekreef B. Exhaled nitric oxide predicts airway hyper-responsiveness to hypertonic saline in children that wheeze. Allergy 2005;60:1499–504 | Offline |
| 128 | de Winter-de Groot K, van der Ent CK. Measurement of nasal nitric oxide: evaluation of six different sampling methods. Eur J Clin Invest 2009;39:72–7 | Nasal NO |
| 129 | de Kluijver J, Evertse CE, Schrumpf JA, van der Veen H, Zwinderman AH, Hiemstra PS, et al. Asymptomatic worsening of airway inflammation during low-dose allergen exposure in asthma: protection by inhaled steroids. Am J Respir Crit Care Med 2002;166:294–300 | FeNO testing did not guide step-up/step-down therapy |
| 130 | Debley JS, Stamey DC, Cochrane ES, Gama KL, Redding GJ. Exhaled nitric oxide, lung function, and exacerbations in wheezy infants and toddlers. J Allergy Clin Immunol 2010;125:1228–34 | Infants aged < 2 years |
| 131 | Debley J, Stamey D, Cochrane E, Elliot M, Redding G. Exhaled nitric oxide predicts persistence of wheezing, exacerbations, and decline in lung function in wheezy infants and toddlers. Am J Respir Crit Care Med 2011;183:A1033 | Infants |
| 132 | Debley JS, Cochrane ES, Redding GJ, Carter ER. Lung function and biomarkers of airway inflammation during and after hospitalization for acute exacerbations of childhood asthma associated with viral respiratory symptoms. Ann Allergy Asthma Immunol 2012;109:114–20 | Not a RCT |
| 133 | Decimo F, Capristo C, Amelio R, Maiello N, Capristo AF, Miraglia Del GM, et al. Evaluation of bronchial hyperreactivity with mannitol dry powder challenge test in a paediatric population with intermittent allergic asthma or allergic rhinitis. Int J Immunopathol Pharmacol 2011;24:1069–74 | No useable diagnostic data |
| 134 | del Giudice MM, Brunese FP, Piacentini GL, Pedulla M, Capristo C, Decimo F, et al. Fractional exhaled nitric oxide (FENO), lung function and airway hyperresponsiveness in naive atopic asthmatic children. J Asthma 2004;41:759–65 | Unselected population |
| 135 | Delclaux C, Mahut B, Zerah-Lancner F, Delacourt C, Laoud S, Cherqui D, et al. Increased nitric oxide output from alveolar origin during liver cirrhosis versus bronchial source during asthma. Am J Respir Crit Care Med 2002;165:332–7 | Case–control study |
| 136 | Delclaux C, Sembach N, Claessens YE, Dolbeau G, Chevalier-Bidaud B, Renaud B, et al. Offline exhaled nitric oxide in emergency department and subsequent acute asthma control. J Asthma 2008;45:867–73 | Offline |
| 137 | Delgado-Corcoran C, Kissoon N, Murphy SP, Duckworth LJ. Exhaled nitric oxide reflects asthma severity and asthma control. Pediatr Crit Care Med 2004;5:48–52 | Not a RCT |
| 138 | Demange V, Bohadana A, Massin N, Wild P. Exhaled nitric oxide and airway hyperresponsiveness in workers: a preliminary study in lifeguards. BMC Pulm Med 2009;9:53 | Unselected population |
| 139 | Demange V, Wild P, Zmirou-Navier D, Tossa P, Bohadana A, Barbaud A, et al. Associations of airway inflammation and responsiveness markers in non asthmatic subjects at start of apprenticeship. BMC Pulm Med 2010;10:37 | Unselected population |
| 140 | Dente FL, Melosini L, Novelli F, Bacci E, Cianchetti S, Di Franco A, et al. Asthma control test (ACT) score is related to PEF variability and markers of airway inflammation in corticosteroids naives asthmatics. Am J Respir Crit Care Med 2010;181:A2738 | No useable diagnostic data |
| 141 | Deykin A, Massaro AF, Drazen JM, Israel E. Exhaled nitric oxide as a diagnostic test for asthma: online versus offline techniques and effect of flow rate. Am J Respir Crit Care Med 2002;165:1597–601 | Wrong flow rate |
| 142 | Deykin A, Lazarus SC, Fahy JV, Wechsler ME, Boushey HA, Chinchilli VM, et al. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol 2005;115:720–7 | Offline |
| 143 | Diaconu R, Diaconu C, Bica C, Bulucea D. Bronchial responsiveness and airway inflammation in various sports. Allergy 2010;65:195 | No useable diagnostic data |
| 144 | Diamant Z, Kuperus J, Baan R, Nietzmann K, Millet S, Mendes P, et al. Effect of a very late antigen-4 receptor antagonist on allergen-induced airway responses and inflammation in asthma. Clin Exp Allergy 2005;35:1080–7 | FeNO testing did not guide step-up/step-down therapy |
| 145 | Dichiaro CA, Baptist AP. Exhaled nitric oxide levels in African American children. Ann Allergy Asthma Immunol 2009;103(5 Suppl. 3):A71 | No useable diagnostic data |
| 146 | Dichiaro CA, Joiner TA, Hudson SA, Baptist AP. Factors influencing asthma control and quality of life in African American children. J Allergy Clin Immunol 2010;125(2 Suppl. 1):AB138 | No useable diagnostic data |
| 147 | Divjan A, Rosa M, Just AC, Sheares BJ, Perera FP, Miller RL, et al. IgE and symptoms by age 2 years predict FENO at age 5-7 years in a low-income urban New York City population. J Allergy Clin Immunol 2009;123(2 Suppl. 1):S19 | No useable diagnostic data |
| 148 | Divjan A, Rosa M, Reyes M, Hoepner L, Sheares BJ, Zhang H, et al. Exhaled NO at age 7–11 years is elevated with early life but not recent onset of allergic sensitization. Am J Respir Crit Care Med 2011;183:A4472 | No useable diagnostic data |
| 149 | Domingo C, Moreno A, Amengual MJ, Monton C, Suarez D, Pomares X. Omalizumab in the management of oral corticosteroid-dependent IGE-mediated asthma patients. Curr Med Res Opin 2011;27:45–53 | Not a RCT |
| 150 | Donohue KM, Miller RL, Perzanowski MS, Just AC, Hoepner LA, Arunajadai S, et al. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol 2013;131:736–42 | Not FeNO testing for diagnosis |
| 151 | Dressel H, Gross C, de la Motte D, Sultz J, Jorres RA, Nowak D. Educational intervention decreases exhaled nitric oxide in farmers with occupational asthma. Eur Respir J 2007;30:545–8 | Not a RCT |
| 152 | Dressel H, Gross C, de la Motte D, Sultz J, Jorres RA, Nowak D, et al. Educational intervention in farmers with occupational asthma: long-term effect on exhaled nitric oxide. J Invest Allergol Clin Immunol 2009;19:49–53 | Not a RCT |
| 153 | Dupont LJ, Demedts MG, Verleden GM. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest 2003;123:751–6 | Wrong flow rate |
| 154 | Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med 2010;181:1033–41 | Not a RCT |
| 155 | Ekerljung L, Bossios A, Lotvall J, Olin AC, Ronmark E, Wennergren G, et al. Multi-symptom asthma as an indication of disease severity in epidemiology. Eur Respir J 2011;38:825–32 | No useable diagnostic data |
| 156 | Fernandez-Nieto M, Sastre B, Sastre J, Lahoz C, Quirce S, Madero M, et al. Changes in sputum eicosanoids and inflammatory markers after inhalation challenges with occupational agents. Chest 2009;136:1308–15 | No data on FeNO testing for diagnosis |
| 157 | Fireman E, Toledano B, Soferman R, Moshe S, Sivan Y, Kivity S, et al. Airways eosiniphilic inflammation in the airways of astmatic children is correlated to particulate matter in induced sputum. Am J Respir Crit Care Med 2010;181:A1156 | No useable diagnostic data |
| 158 | Fortuna A, Feixas T, Gonzalez M, Casan P. Portable equipment (NIOX MINO, aerocrine) for determination of NO in respiratory air (FENO). Arch Bronconeumol 2006;42:420 | Foreign language |
| 159 | Franklin PJ, Turner SW, Le Souef PN, Stick SM. Exhaled nitric oxide and asthma: complex interactions between atopy, airway responsiveness, and symptoms in a community population of children. Thorax 2003;58:1048–52 | Wrong flow rate |
| 160 | Fuchs O, Latzin P, Singer F, Petrus N, Proietti E, Kieninger E, et al. Comparison of online single-breath vs. online multiple-breath exhaled nitric oxide in school-age children. Pediatr Res 2012;71:605–11 | Single breath vs. multiple breaths |
| 161 | Fujimura M, Ohkura N, Abo M, Furusho S, Waseda Y, Ichikawa Y, et al. Exhaled nitric oxide levels in patients with atopic cough and cough variant asthma. Respirology 2008;13:359–64 | Case–control study |
| 162 | Gill M, Graff GR, Adler AJ, Dweik RA. Validation study of fractional exhaled nitric oxide measurements using a handheld monitoring device. J Asthma 2006;43:731–4 | Inter-reliability |
| 163 | Grzelewski T, Grzelewska A, Majak P, Stelmach W, Kowalska A, Stelmach R, et al. Fractional exhaled nitric oxide (FeNO) may predict exercise-induced bronchoconstriction (EIB) in schoolchildren with atopic asthma. Nitric Oxide Biol Chem 2012;27:82–7 | Diagnosing EIB in asthmatics, i.e. not diagnostic of asthma |
| 164 | Hafkamp-de-Groen E, Mohangoo AD, de Jongste JC, van der Wouden JC, Moll HA, Jaddoe VW, et al. Early detection and counselling intervention of asthma symptoms in preschool children: study design of a cluster randomised controlled trial. BMC Public Health 2010;10:555 | Study design only |
| 165 | Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008;178:218–24 | Not a RCT |
| 166 | Hardaker K, Downie S, Kermode J, Farah C, Berend N, King G, et al. The predictors of airway hyperresponsiveness are different in younger and older asthmatics. Respirology 2010;15:A37 | No useable diagnostic data |
| 167 | Hardaker KM, Downie SR, Kermode JA, Farah CS, Brown NJ, Berend N, et al. Predictors of airway hyperresponsiveness differ between old and young patients with asthma. Chest 2011;139:1395–401 | No useable diagnostic data |
| 168 | Hemmingsson T, Horn A, Linnarsson D. Measuring exhaled nitric oxide at high altitude. Respir Physiol Neurobiol 2009;67:292–8 | Not in humans |
| 169 | Högman M, Malinovschi A, Norbäck D, Janson C. Added value with extended NO analysis in atopy and asthma. Clin Physiol Funct Imaging 2011;31:294–9 | No useable diagnostic data |
| 170 | Honkoop PJ, Loymans RJ, Termeer EH, Snoeck-Stroband JB, Bakker MJ, Assendelft WJ, et al. Asthma control cost–utility randomized trial evaluation (ACCURATE): the goals of asthma treatment. BMC Pulm Med 2011;11:53 | Ongoing study |
| 171 | Huang J, Yao T, Yeh K. Exhaled nitric oxide discriminates children with and without allergic sensitisation in a population-based study. Allergy 2011;66:198 | Unselected population |
| 172 | Hur G-Y, Oh JY, Choi J-H, Sim J-K, Min KH, Lee S-Y, et al. Mannitol challenge test, sputum eosinophils and exhaled nitric oxide (FENO) for diagnosis of asthma. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB64 | No useable diagnostic data |
| 173 | Imaoka M, Tanahashi T, Kishikawa R, Shimoda T, Iwanaga T. Gender-specific effect of overweight and obesity on airway inflammation in adults with asthma. J Allergy Clin Immunol 2011;127(2 Suppl. 1):AB98 | No useable diagnostic data |
| 174 | Imaoka M, Tanahashi T, Kishikawa R, Shimoda T, Iwanaga T. Overweight and obesity reduce exhaled nitric oxide levels in Japanese women with asthma. Allergy 2011;66:515 | No useable diagnostic data |
| 175 | Inoue H, Niimi A, Takeda T, Matsumoto H, Ito I, Otsuka K, et al. Pathophysiological characteristics of asthma in the elderly. Am J Respir Crit Care Med 2010;181:A5096 | No useable diagnostic data |
| 176 | Ishizuka T, Matsuzaki S, Aoki H, Yatomi M, Kamide Y, Hisada T, et al. Prevalence of asthma symptoms based on the European Community Respiratory Health Survey questionnaire and FENO in university students: gender differences in symptoms and FENO. Allergy Asthma Clin Immunol 2011;7:15 | No useable diagnostic data |
| 177 | Ito Y, Adachi Y, Itazawa T, Okabe Y, Adachi YS, Katsumuma T, et al. Comparison of exhalation time methods (6 sec vs. 10 sec) of a hand-held exhaled nitric oxide analyzer. Pediatr Pulmonol 2010;45:1005–8 | Measurement not in accordance with ATS 2005 guidelines35 |
| 178 | Jackson DJ, Virnig CM, Gangnon RE, Evans MD, Roberg KA, Anderson EL, et al. Fractional exhaled nitric oxide measurements are most closely associated with allergic sensitization in school-age children. J Allergy Clin Immunol 2009;124:949–53 | No useable diagnostic data |
| 179 | Jobsis Q, Raatgeep HC, Hop WC, Jongste JC. Controlled low flow off line sampling of exhaled nitric oxide in children. Thorax 2001;56:285–9 | Offline |
| 180 | Jobsis Q, Schellekens SL, Kroesbergen A, Hop WCJ, de Jongste JC. Off-line sampling of exhaled air for nitric oxide measurement in children: methodological aspects. Eur Respir J 2001;17:898–903 | Offline |
| 181 | Jung A, Summermatter S, Geidel C, Moller A, Menz G, Lauener R. Diagnostic value of nasal NO measurement using the NIOX MINO device. Atemwegs Lungenkr 2012;38:57–8 | Nasal NO |
| 182 | Jung M, Korn S, Taube C, Buhl R. Short-term reproducibility of non-invasive clinical and inflammatory parameters in asthma. Am J Respir Crit Care Med 2011;183:A4481 | No analytical data |
| 183 | Kelso JM. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized, controlled trial: commentary. Pediatrics 2006;118(Suppl. 1):S33 | Commentary |
| 184 | Kharitonov SA, Donnelly LE, Montuschi P, Corradi M, Collins JV, Barnes PJ, et al. Dose-dependent onset and cessation of action of inhaled budesonide on exhaled nitric oxide and symptoms in mild asthma. Thorax 2002;57:889–96 | FeNO testing did not guide step-up/step-down therapy |
| 185 | Khurana S, Larj M, Saatian B, Lerner LB, Smith S, Pietropaoli A, et al. Correlation of bronchodilator reversibility with exhaled nitric oxide levels and asthma severity. Am J Respir Crit Care Med 2011;183:A4474 | No useable diagnostic data |
| 186 | Kim S, Kim T, Sohn J, Yoon H, Shin D, Park S. Measurement of the exhaled nitric oxide in the assessment of chronic cough. J Allergy Clin Immunol 2009;123(2 Suppl. 1):S6 | No useable diagnostic data |
| 187 | Kim S-H, Kim TH, Sohn JW, Yoon HJ, Shin DH, Park SS. Measurement of exhaled and nasal nitric oxide in the diagnosis of chronic cough. Respirology 2009;14:A160 | No useable diagnostic data |
| 188 | Kim YH, Kim KW, Baek J, Park HB, Kim H, Song K-J, et al. Usefulness of impulse oscillometry and fractional exhaled nitric oxide in children with eosinophilic bronchitis. Pediatr Pulmonol 2013;48:221–8 | No useable diagnostic data |
| 189 | Klaassen EM, van de Kant KD, Jobsis Q, Hovig ST, van Schayck CP, Rijkers GT, et al. Symptoms, but not a biomarker response to inhaled corticosteroids, predict asthma in preschool children with recurrent wheeze. Mediators Inflamm 2012;2012:162571 | No useable diagnostic data |
| 190 | Konstantinou G, Xepapadaki P, Manousakis E, Makrinioti C, Kouloufakou-Gratsia K, Chatziioannou A, et al. Non-invasive evaluation of airway inflammation during virus-induced asthma exacerbations in atopic and non-atopic preschool-children. Allergy 2009;64:432–3 | FeNO testing in exacerbations |
| 191 | Konstantinou GN, Xepapadaki P, Manousakis E, Makrinioti H, Kouloufakou-Gratsia K, Saxoni-Papageorgiou P, et al. Assessment of airflow limitation, airway inflammation, and symptoms during virus-induced wheezing episodes in 4- to 6-year-old children. J Allergy Clin Immunol 2013;131:87–93 | No useable diagnostic data |
| 192 | Koopman M, Arets HG, Uiterwaal CS, van der Ent CK. Comparing 6 and 10 sec exhalation time in exhaled nitric oxide measurements in children. Pediatr Pulmonol 2009;44:340–4 | Measurement not in accordance with ATS 2005 guidelines35 |
| 193 | Kotaniemi-Syrjanen A, Malmberg LP, Malmstrom K, Pelkonen AS, Makela MJ. Factors associated with elevated exhaled nitric oxide fraction in infants with recurrent respiratory symptoms. Eur Respir J 2013;41:189–94 | Infants aged < 3 years |
| 194 | Krcmova I, Novosad J, Kralickova P, Kleiberova M, Chladkova J, Melicharova J. Asthma control test, FeNO, functional parameters, ECP and their correlation. Allergy 2009;64:301–2 | Not a RCT |
| 195 | Kumor M, Przybylowski T, Maskey-Warzechowska M, Hildebrand K, Fangrat A, Bielicki P, et al. [Reproducibility of exhaled nitric oxide (FENO) measurements in healthy subjects]. Pneumonol Alergol Pol 2004;72:395–9 | Foreign language |
| 196 | Larj MJ, Khurana S, Lerner LB, Smith SM, Aung T, Pietropaoli A, et al. Alveolar and airway nitric oxide correlations in asthma. Am J Respir Crit Care Med 2010;181:A4282 | Alveolar NO |
| 197 | Larson JL, Zeidler MR, Kleerup EC, Kim HJG, Tashkin DP. Correlation of alveolar nitric oxide with methacholine responsiveness in asthmatic subjects following a naturalistic cat challenge. Am J Respir Crit Care Med 2011;183:A4471 | Alveolar NO |
| 198 | Larson JL, Zeidler M, Kleerup E, Tashkin D. Evaluation of exhaled nitric oxide as a surrogate for airways hyperresponsiveness. J Invest Med 2011;59:214 | No useable diagnostic data |
| 199 | Latzin P, Kuehni CE, Baldwin DN, Roiha HL, Casaulta C, Frey U. Elevated exhaled nitric oxide in newborns of atopic mothers precedes respiratory symptoms. Am J Respir Crit Care Med 2006;174:1292–8 | No useable diagnostic data |
| 200 | Lee J, Lee BH, Lee S-H. Repeatability of successive measurements with a portable nitric oxide analyser in healthy Koreans. Chest 2011;140:213A | Repeatability |
| 201 | Lehtimaki L, Turjanmaa V, Kankaanranta H, Saarelainen S, Hahtola P, Moilanen E. Increased bronchial nitric oxide production in patients with asthma measured with a novel method of different exhalation flow rates. Ann Med 2000;32:417–23 | Device not in scope |
| 202 | Lemiere C, D’Alpaos V, Chaboillez S, Cesar M, Wattiez M, Chiry S, et al. Investigation of occupational asthma: sputum cell counts or exhaled nitric oxide? Chest 2010;137:617–22 | Offline |
| 203 | Lemiere C, Tremblay C, Bohadana A, Chaboillez S. Prognosis of the workers with non-eosinophilic occupational asthma. Am J Respir Crit Care Med 2011;183:A1172 | Not FeNO testing |
| 204 | Lex C, Dymek S, Heying R, Kovacevic A, Kramm CM, Schuster A. Value of surrogate tests to predict exercise-induced bronchoconstriction in atopic childhood asthma. Pediatr Pulmonol 2007;42:225–30 | Diagnosis of EIB not asthma |
| 205 | Li S, Lou XS, Ma Y, Han SL, Liu CH, Chen YZ, et al. [Exhaled nitric oxide levels in school children of Beijing]. Zhonghua Erke Zazhi 2010;48:148–52 | Foreign language |
| 206 | Linkosalo L, Lehtimaki L, Holm K, Kaila M, Moilanen E. Increased bronchial nitric oxide output is associated with exercise-induced bronchoconstriction in atopic children. Allergy 2009;64:72–3 | No useable diagnostic data |
| 207 | Linn WS, Rappaport EB, Berhane KT, Bastain TM, Avol EL, Gilliland FD. Exhaled nitric oxide in a population-based study of southern California schoolchildren. Respir Res 2009;10:28 | Wrong device |
| 208 | Linn WS, Rappaport EB, Berhane KT, Bastain TM, Salam MT, Gilliland FD, et al. Extended exhaled nitric oxide analysis in field surveys of schoolchildren: a pilot test. Pediatr Pulmonol 2009;44:1033–42 | No useable diagnostic data |
| 209 | Linn WS, Berhane KT, Rappaport EB, Bastain TM, Avol EL, Gilliland FD. Relationships of online exhaled, offline exhaled, and ambient nitric oxide in an epidemiologic survey of schoolchildren. J Expo Sci Environ Epidemiol 2009;19:674–81 | No useable diagnostic data |
| 210 | Little SA, Chalmers GW, MacLeod KJ, McSharry C, Thomson NC. Non-invasive markers of airway inflammation as predictors of oral steroid responsiveness in asthma. Thorax 2000;55:232–4 | Wrong flow rate |
| 211 | Lonnkvist K, Anderson M, Hedlin G, Svartengren M. Exhaled NO and eosinophil markers in blood, nasal lavage and sputum in children with asthma after withdrawal of budesonide. Pediatr Allergy Immunol 2004;15:351–8 | FeNO testing did not guide step-up/step-down therapy |
| 212 | Lund TK. Asthma in elite athletes: how do we manage asthma-like symptoms and asthma in elite athletes? Clin Respir J 2009;3:123 | Case–control study |
| 213 | Magori E, Hiltawsky K, Fleischer M, Simon E, Pohle R, von Sicard O, et al. Fractional exhaled nitric oxide measurement with a handheld device. J Breath Res 2011;5:027104 | Device not in scope |
| 214 | Mahut B, Peyrard S, Delclaux C. Exhaled nitric oxide and clinical phenotypes of childhood asthma. Respir Res 2011;12:65 | No useable diagnostic data |
| 215 | Malby Schoos AM, Chawes BL, Bonnelykke K, Bisgaard H. Fraction of exhaled nitric oxide and bronchial responsiveness are associated and continuous traits in young children independent of asthma. Chest 2012;142:1562–8 | No useable diagnostic data |
| 216 | Malik G, Turner SW. Deselecting the instant flow option on the NIOX© analyser increases the number of successful FENO measurements without altering the results. Eur Respir J 2005;26:Abstract 3907 | No comparison between devices |
| 217 | Malik G, Turner S. Is the ‘instant flow’ option on the NIOX analyser needed? Med Eng Physics 2007;29:72–5 | Wrong device |
| 218 | Malinovschi A, Janson C, Hogman M, Rolla G, Toren K, Norback D, et al. Both allergic and nonallergic asthma are associated with increased FE(NO) levels, but only in never-smokers. Allergy 2009;64:55–61 | No useable diagnostic data |
| 219 | Malinovschi A, Backer V, Harving H, Porsbjerg C. The value of exhaled nitric oxide to identify asthma in smoking patients with asthma-like symptoms. Respir Med 2012;106:794–801 | Incorrect reference standard |
| 220 | Malka-Rais J, Krawiec ME, Spahn JD. Are there differences in levels of impairment, risk, and biomarkers of inflammation in children vs. adults with severe persistent asthma? J Allergy Clin Immunol 2010;125(2 Suppl. 1):AB6 | No useable diagnostic data |
| 221 | Malka-Rais J, Davidson J, Krawiec ME, Spahn JD. Which parameters are useful in the determining asthma severity in childhood? Am J Respir Crit Care Med 2010;181:A2556 | No useable diagnostic data |
| 222 | Malmberg LP, Pelkonen AS, Mattila PS, Hammaren-Malmi S, Makela MJ. Exhaled nitric oxide and exercise-induced bronchoconstriction in young wheezy children – interactions with atopy. Pediatr Allergy Immunol 2009;20:673–8 | Age 3–7 years |
| 223 | Malmberg LP, Laatikainen T, von Hertzen L, Makela MJ, Vartiainen E, Haahtela T, et al. Exhaled nitric oxide in contrasting population samples of Finnish and Russian Karelia. Eur Respir J 2010;35:1416–18 | No useable diagnostic data |
| 224 | Maniscalco M, Lundberg JO. Hand-held nitric oxide sensor NIOX MINO for the monitoring of respiratory disorders. Expert Rev Respir Med 2010;4:715–21 | Review |
| 225 | Martin N, Lindley MR, Hargadon B, Monteiro W, Pavord ID. Airway dysfunction and inflammation in pool and non-pool based elite endurance athletes. Thorax 2010;65:A60–1 | No useable diagnostic data |
| 226 | Martin N, Lindley MR, Hargadon B, Monteiro W, Pavord ID. Airway dysfunction and inflammation in pool and non-pool based elite international athletes with symptoms suggesting exercise-induced asthma. Am J Respir Crit Care Med 2010;181:A3970 | No useable diagnostic data |
| 227 | Martin N, Lindley MR, Hargadon B, Monteiro W, Pavord ID. Airways dysfunction and eosinophilic inflammation in elite athletes with symptoms suggesting exercise-induced asthma. Thorax 2009;64:A72 | No useable diagnostic data |
| 228 | Martin N, Lindley MR, Hargadon B, Monteiro WR, Pavord ID. Airway dysfunction and inflammation in pool- and non-pool-based elite athletes. Med Sci Sports Exerc 2012;44:1433–9 | No useable diagnostic data |
| 229 | Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol 2007;119:73–80 | No useable diagnostic data |
| 230 | Martins P, Caires I, Pinto JR, da Mata PL, Torres S, Valente J, et al. The clinical use of exhaled nitric oxide in wheezing children. Rev Port Pneumol 2008;14:195–218 | Population not self-presenting for assessment |
| 231 | Matsunaga K, Hirano T, Akamatsu K, Koarai A, Sugiura H, Minakata Y, et al. Exhaled nitric oxide cutoff values for asthma diagnosis according to rhinitis and smoking status in Japanese subjects. Allergol Int 2011;60:331–7 | Case–control study |
| 232 | McCurdy MR, Bakhirkin YA, Tittel FK. Quantum cascade laser-based integrated cavity output spectroscopy of exhaled nitric oxide. Appl Physics B Lasers Optics 2006;85:445–52 | Laser spectroscopy |
| 233 | McCurdy MR, Bakhirkin Y, Wysocki G, Tittel FK. Performance of an exhaled nitric oxide and carbon dioxide sensor using quantum cascade laser-based integrated cavity output spectroscopy. J Biomed Optics 2007;12:034034 | Laser spectroscopy |
| 234 | McKinlay L, Williamson PA, Short PM, Fardon TC, Lipworth BJ. Proof of concept study to evaluate step-down therapy with inhaled corticosteroid alone or additive therapy on surrogate inflammatory markers in asthma. Br J Clin Pharmacol 2011;71:128–31 | FeNO testing did not guide step-up/step-down therapy |
| 235 | Menzies D, Jackson C, Mistry C, Houston R, Lipworth BJ. Symptoms, spirometry, exhaled nitric oxide, and asthma exacerbations in clinical practice. Ann Allergy Asthma Immunol 2008;101:248–55 | FeNO testing did not guide step-up/step-down therapy |
| 236 | Meyts I, Proesmans M, Van Gerven V, Hoppenbrouwers K, De Boeck K. Tidal off-line exhaled nitric oxide measurements in a pre-school population. Eur J Pediatr 2003;162:506–10 | No useable diagnostic data |
| 237 | Mgaloblishvili N, Gotua M, Rukhadze M, Dolidze N, Lomidze N, Abramidze T, et al. Exhaled nitric oxide and respiratory symptoms in the diagnosis of atopic asthma. Allergy 2009;64:179–80 | No useable diagnostic data |
| 238 | Mgaloblishvili N, Gotua M, Gamkrelidze A. Exhaled nitric oxide and asthma severity in georgian population. Allergy 2010;65:549 | No useable diagnostic data |
| 239 | Mi Q, Balzar S, Wenzel SE. Distinguishing the features of severe asthma: feature selection in the asthma dataset using linear support vector machines (SVM) approach. Am J Respir Crit Care Med 2011;183:A4301 | No useable diagnostic data |
| 240 | Miedinger D, Chhajed PN, Tamm M, Stolz D, Surber C, Leuppi JD. Diagnostic tests for asthma in firefighters. Chest 2007;131:1760–7 | Unselected population |
| 241 | Monforte S, Malka-Rais J, Spahn JD. The use of exhaled nitric oxide (FeNO) in the outpatient management of children with asthma. J Allergy Clin Immunol 2009;123:727 | No useable diagnostic data |
| 242 | Monforte S, Malka-Rais J, Spahn JD. The association of exhaled nitric oxide (FeNO) with asthma control and severity in children. J Allergy Clin Immunol 2010;125(2 Suppl. 1):AB186 | Not RCT study |
| 243 | Montella S, Alving K, Maniscalco M, Sofia M, De Stefano S, Raia V, et al. Measurement of nasal nitric oxide by hand-held and stationary devices. Eur J Clin Invest 2011;41:1063–70 | Nasal NO |
| 244 | Motomura C, Odajima H, Tezuka J, Murakami Y, Moriyasu Y, Kando N, et al. Effect of age on relationship between exhaled nitric oxide and airway hyperresponsiveness in asthmatic children. Chest 2009;136:519–25 | No useable diagnostic data |
| 245 | Motomura C, Odajima H, Tezuka J, Kodama T, Amimoto Y, Murakami Y, et al. Exhaled nitric oxide predicts bronchial responsiveness according to age in asthmatic children. Am J Respir Crit Care Med 2010;181:A3294 | No useable diagnostic data |
| 246 | Motomura C, Odajima H, Higashi N, Tezuka J, Honjo S, Okada K, et al. Exercise-induced bronchoconstriction in children with asthma: is there an association with urinary leukotriene E4 or exhaled nitric oxide. Pediatr Allergy Immunol Pulmonol 2012;25:208–12 | No useable diagnostic data |
| 247 | Muller KC, Jorres RA, Magnussen H, Holz O. Comparison of exhaled nitric oxide analysers. Respir Med 2005;99:631–7 | Wrong device |
| 248 | Munnik P, van der Lee I, Fijn J, van Eijsden LJ, Lammers JW, Zanen P. Comparison of eNO and histamine hyperresponsiveness in diagnosing asthma in new referrals. Respir Med 2010;104:801–7 | Not comparing relevant devices |
| 249 | Murata A, Kida K, Hasunuma H, Kanegae H, Ishimaru Y, Motegi T, et al. Environmental influence on the measurement of exhaled nitric oxide concentration in school children: special reference to methodology. J Nippon Med School 2007;74:30–6 | No useable diagnostic data |
| 250 | Musk AWB, Knuiman M, Hunter M, Hui J, Palmer L, Beilby J, et al. Patterns of airway disease and the clinical diagnosis of asthma in the Busselton population. Respirology 2010;15:A46 | No useable diagnostic data |
| 251 | Musk AW, Knuiman M, Hunter M, Hui J, Palmer LJ, Beilby J, et al. Patterns of airway disease and the clinical diagnosis of asthma in the Busselton population. Eur Respir J 2011;38:1053–9 | No useable diagnostic data |
| 252 | Nagase H, Toda T, Kamiyama A, Nakase Y, Sugimoto N, Yoshihara H, et al. Usefulness of measuring fractional exhaled nitric oxide (FeNO) in various respiratory diseases. J Allergy Clin Immunol 2011;127(2 Suppl. 1):AB7 | Unclear how patients recruited |
| 253 | Nakajima N, Mochizuki H, Muramatsu R, Hagiwara S, Mizuno T, Arakawa H, et al. Relationship between exhaled nitric oxide and small airway lung function in normal and asthmatic children. Allergol Int 2011;60:53–9 | No useable diagnostic data |
| 254 | Narang I, Ersu R, Wilson NM, Bush A. Nitric oxide in chronic airway inflammation in children: diagnostic use and pathophysiological significance. Thorax 2002;57:586–9 | Case–control study |
| 255 | Nelson BV, Sears S, Woods J, Ling CY, Hunt J, Clapper LM, et al. Expired nitric oxide as a marker for childhood asthma. J Pediatr 1997;130:423–7 | Case–control study |
| 256 | Nikasinovic L, Rouffai L, Dassonville C, Momas I, Just J. Nasal lavage fluid IL8 and fractional exhaled nitric oxide (FeNO) association in untreated asthmatic children. Allergy 2011;66:643–4 | No useable diagnostic data |
| 257 | Nishio K, Odajima H, Motomura C, Nakao F, Nishima S, Nishio K, et al. Exhaled nitric oxide and exercise-induced bronchospasm assessed by FEV1, FEF25–75% in childhood asthma. J Asthma 2007;44:475–8 | No useable diagnostic data |
| 258 | Obata H, Dittrick M, Chan H, Chan-Yeung. Sputum eosinophils and exhaled nitric oxide during late asthmatic reaction in patients with western red cedar asthma. Eur Respir J 1999;3:489–95 | Wrong flow rate |
| 259 | Olaguibel JM, Parra A, Alvarez MJ, Quirce S, Lopez R. Measurements of fractional exhaled nitric oxide with 2 portable electrochemical sensors: a comparative study. J Invest Allergol Clin Immunol 2011;21:322–3 | Healthy volunteers |
| 260 | Oros M, Codleanu C, Calapod L, Bogdan R, Bulacu E, Momarla C, et al. Is FENO of some help for pediatric outpatients with asthma? Am J Respir Crit Care Med 2010;181:A3301 | No useable diagnostic data |
| 261 | Oshikata C, Tsuburai T, Tsurikisawa N, Ono E, Higashi A, Fukutomi Y, et al. Cutoff point of the fraction of exhaled nitric oxide (FeNO) with the off-line method for diagnosing asthma and the effect of smoking on FeNO. Nihon Kokyuki Gakkai Zasshi 2008;46:356–62 | Foreign language |
| 262 | Perez-de-Llano LA, Carballada F, Castro AO, Pizarro M, Golpe R, Baloira A, et al. Exhaled nitric oxide predicts control in patients with difficult-to-treat asthma. Eur Respir J 2010;35:1221–7 | FeNO testing did not guide step-up/step-down therapy |
| 263 | Perzanowski MS, Divjan A, Mellins RB, Canfield SM, Rosa MJ, Chew GL, et al. Exhaled NO among 7-year-old children who attended Head Start in New York City. J Allergy Clin Immunol 2009;123(2 Suppl. 1):S171 | No useable diagnostic data |
| 264 | Perzanowski MS, Divjan A, Mellins RB, Canfield SM, Rosa MJ, Chew GL, et al. Exhaled NO among inner-city children in New York City. J Asthma 2010;47:1015–21 | No useable diagnostic data |
| 265 | Pijnenburg MW, Lissenberg ET, Hofhuis W, Ghiro L, Ho WC, Holland WP, et al. Exhaled nitric oxide measurements with dynamic flow restriction in children aged 4–8 yrs. Eur Respir J 2002;20:919–24 | Online vs. offline |
| 266 | Porsbjerg C, Brannan JD, Anderson SD, Backer V. Relationship between airway responsiveness to mannitol and to methacholine and markers of airway inflammation, peak flow variability and quality of life in asthma patients. Clin Exp Allergy 2008;38:43–50 | Diagnosis of airway hyper-responsiveness |
| 267 | Porsbjerg C, Lund TK, Pedersen L, Backer V. Inflammatory subtypes in asthma are related to airway hyperresponsiveness to mannitol and exhaled NO. J Asthma 2009;46:606–12 | Population all asthmatics |
| 268 | Porsbjerg C, Sverrild A, Thomsen SF, Backer V. The association between AHR to mannitol and to methacholine and exhaled NO in a random sample population. Respirology 2010;15:A47 | Unselected population |
| 269 | Prasad A, Langford B, Stradling JR, Ho LP. Exhaled nitric oxide as a screening tool for asthma in school children. Respir Med 2006;100:167–73 | No useable diagnostic data |
| 270 | Profita M, Montuschi P, Bonanno A, Riccobono L, Montalbano AM, Ciabattoni G, et al. Nasobronchial markers of oxidative stress and inflammation in atopic airway diseases. Pediatr Allergy Immunol 2009;20:58 | No useable diagnostic data |
| 271 | Puckett JL, Galant SP, Taylor RWE, Cirson LC, Warren JL, Guijon OL, et al. Bronchodilator response and airway nitric oxide flux correlate in a pediatric asthma population. J Allergy Clin Immunol 2009;123(2 Suppl. 1):S78 | No useable diagnostic data |
| 272 | Raulf-Heimsoth M, van Kampen V, Sucker K, Heinze E, Eliakopoulos C, Bruning T, et al. Application of non-invasive methods to assess current airway inflammation in health care workers 10 years after the latex ban in Germany. Allergy 2010;65:56 | No useable diagnostic data |
| 273 | Raulf-Heimsoth M, van Kampen V, Heinze E, Bernard S, Borowitzki G, Freundt S, et al. Comparison of different non-invasive methods for detection of allergic asthma. Adv Exp Med Biol 2013;755:55–63 | No useable diagnostic data |
| 274 | Rees PJ. Using exhaled NO concentrations to adjust inhaled corticosteroid dose maintained asthma control and reduced the dose. Evid Based Med 2006;11:20 | Commentary |
| 275 | Reyes RL, Tordera MP, Gisbert VM. Relationship between values for exhaled nitric oxide at a flow rate of 250 ml/s and levels obtained from a linear regression equation. Am J Respir Crit Care Med 2011;183:A4374 | Wrong flow rate |
| 276 | Robroeks CM, van de Kant KD, Jobsis Q, Hendriks HJ, van Gent R, Wouters EF, et al. Exhaled nitric oxide and biomarkers in exhaled breath condensate indicate the presence, severity and control of childhood asthma. Clin Exp Allergy 2007;37:1303–11 | Case–control study |
| 277 | Rosa M, Divjan A, Johnson A, Hoepner L, Sheares B, Perera FP, et al. Flow-dependent and independent parameters of exhaled nitric oxide in 9 year-old children living in low-income NYC communities. J Allergy Clin Immunol 2009;123(2 Suppl. 1):S5 | No useable diagnostic data |
| 278 | Rosa MJ, Divjan A, Hoepner L, Sheares BJ, Diaz D, Gauvey-Kern K, et al. Fractional exhaled nitric oxide exchange parameters among 9-year-old inner-city children. Pediatr Pulmonol 2011;46:83–91 | Offline measurement |
| 279 | Rutgers SR, Meijer RJ, Kerstjens HA, van der Mark TW, Koeter GH, Postma DS, et al. Nitric oxide measured with single-breath and tidal-breathing methods in asthma and COPD. Eur Respir J 1998;12:816–19 | Case–control study |
| 280 | Ryan D, Thomas MD, Dorinsky PM, Burden A, Von Ziegenweidt J, Hutton C, et al. The role of exhaled nitric oxide in guiding asthma. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB205 | Not diagnostic study |
| 281 | Sachs-Olsen C, Lodrup Carlsen KC, Mowinckel P, Haland G, Devulapalli CS, Munthe-Kaas MC, et al. Diagnostic value of exhaled nitric oxide in childhood asthma and allergy. Pediatric Allergy Immunol 2010;21:e213–21 | Unselected population |
| 282 | Saito J, Fukuhara A, Sato Y, Sato S, Saito K, Nakagawa N, et al. [Differences of fractional exhaled nitric oxide (FeNO) levels performed using two different analyzers]. Nihon Kokyuki Gakkai Zasshi 2010;48:17–22 | Foreign language |
| 283 | Sakai T, Sugiyama N, Hirai K, Muramatsu R, Hagiwara S, Oh Y, et al. Consistently high levels of exhaled nitric oxide in children with asthma. Pediatr Int 2010;52:801–5 | No useable diagnostic data |
| 284 | Sanchez-Vidaurre S, Cruz MJ, Gomez-Olles S, Morell F, Munoz X. Diagnostic utility of exhaled breath condensate analysis in conjunction with specific inhalation challenge in individuals with suspected work-related asthma. Ann Allergy Asthma Immunol 2012;108:151–6 | No data on FeNO testing |
| 285 | Sardon PO, Aldasoro RA, Korta MJ, Mintegui AJ, Emparanza Knorr JI, Perez-Yarza EG, et al. [Agreement between two devices for measuring exhaled nitric oxide]. An Pediatr 2007;67:572–7 | Foreign language |
| 286 | Sardon PO, Perez-Yarza EG, Aldasoro RA, Korta MJ, Mintegui AJ, Emparanza Knorr JI, et al. [Fractional exhaled nitric oxide: validation of a 6 second exhalation time with two different analysers]. An Pediatr 2008;69:221–6 | Foreign language |
| 287 | Schleich FN, Seidel L, Sele J, Manise M, Quaedvlieg V, Michils A, et al. Exhaled nitric oxide thresholds associated with a sputum eosinophil count ≥ 3% in a cohort of unselected patients with asthma. Thorax 2010;65:1039–44 | Population all asthmatic |
| 288 | Scollo M, Zanconato S, Ongaro R, Zaramella C, Zacchello F, Baraldi E, et al. Exhaled nitric oxide and exercise-induced bronchoconstriction in asthmatic children. Am J Respir Crit Care Med 2000;161:1047–50 | Case–control study |
| 289 | Selby A, Clayton B, Grundy J, Pike K, Drew K, Raza A, et al. Are exhaled nitric oxide measurements using the portable NIOX MINO repeatable? Respir Res 2010;11:43 | Repeatability |
| 290 | Silkoff PE, Lent AM, Busacker AA, Katial RK, Balzar S, Strand M, et al. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol 2005;116:1249–55 | Diagnosis of Eosinophilic airway inflammation + phenotype |
| 291 | Simpson JL, McDonald VM, Gibson PG. Exhaled nitric oxide is not a marker of eosinophilic inflammation in older Australians. Respirology 2010;15:A53 | No useable diagnostic data |
| 292 | Smith AM, Villareal M, Bernstein DI, Swikert DJ. Asthma in the elderly: risk factors and impact on physical function. Ann Allergy Asthma Immunol 2012;108:305–10 | No useable diagnostic data |
| 293 | Sobrevia M, Segura N, Ferrer L, Lezaun A, Cubero J, Sierra J, et al. Influence of positive skin prick tests in FeNO measurement. Allergy 2010;65:675 | No useable diagnostic data |
| 294 | Sordillo J, Milton DK, Platts-Mills TA, Gold DR. Asthma symptoms, sensitization, and allergen exposure as predictors of exhaled NO. J Allergy Clin Immunol 2009;123(2 Suppl. 1):S22 | No useable diagnostic data |
| 295 | Sordillo JE, Webb T, Kwan D, Kamel J, Hoffman E, Milton DK, et al. Allergen exposure modifies the relation of sensitization to fraction of exhaled nitric oxide levels in children at risk for allergy and asthma. J Allergy Clin Immunol 2011;127:1165–72 | FeNO testing did not guide step-up/step-down therapy |
| 296 | Stahl MC, Arora R, Tucker M, Johnson T, Calabria C. A prospective evaluation of the fraction of exhaled nitric oxide and the subsequent diagnosis of asthma in military basic trainees. Ann Allergy Asthma Immunol 2009;103(5 Suppl. 3):A72 | Unselected population |
| 297 | Sverrild A, Porsbjerg C, Thomsen SF, Backer V. Airway hyperresponsiveness to mannitol and methacholine and exhaled nitric oxide: a random-sample population study. J Allergy Clin Immunol 2010;126:952–8 | Unselected population |
| 298 | Sverrild A, Malinovschi A, Porsbjerg C, Backer V, Alving K. Predicting airway hyperreactivity to mannitol using exhaled nitric oxide in an unselected sample of adolescents and young adults. Respir Med 2013;107:150–2 | Unselected population |
| 299 | Syed A, Rehman A, Akram M, Bukhari R. Role of FeNO in predicting asthma relapse and clinical relevance in children on inhaled corticosteroid. J Allergy Clin Immunol 2011;127(2 Suppl. 1):AB158 | No relevant outcomes |
| 300 | Tanaka H, Kitada J, Fujii M, Takahashi H. Diagnostic strategy for chorinic cough using FENO and impulse oscillometry. Am J Respir Crit Care Med 2011;183:A4386 | No useable diagnostic data |
| 301 | Tatyana S, Mitrova R, Ganeva M, Boyadjieva L, Markova R. Fractional exhaled nitric oxide and bronchodilator responsiveness in preschool children with asthma. Allergy 2011;66:197 | Age < 5 years |
| 302 | Taylor DR, Palmay R, Cowan JO, Herbison GP. Long term performance characteristics of an electrochemical nitric oxide analyser. Respir Med 2011;105:211–17 | Reproducibility and long-term performance in NIOX MINO only |
| 303 | Taylor DR, de la Barra SL, Herbison GP, Cowan JO, Smith AD. Predicted versus absolute values in the interpretation of exhaled nitric oxide measurements. Am J Respir Crit Care Med 2011;183:A4476 | No usable diagnostic data |
| 304 | Taylor ES, Smith AD, Cowan JO, Herbison GP, Taylor DR. Effect of caffeine ingestion on exhaled nitric oxide measurements in patients with asthma. Am J Respir Crit Care Med 2004;169:1019–21 | FeNO testing did not guide step-up/step-down therapy |
| 305 | Terada A, Fujisawa T, Iguchi K, Astuta J, Togari H. [Exhaled nitric oxide of childhood asthma]. Arerugi 1999;48:466–71 | Foreign language |
| 306 | Thijs W, Middeldorp S, Hiemstra PS, Rosendaal FR, Rabe KF. Reproducibility of exhaled NO measurements in overweight subjects. Am J Respir Crit Care Med 2010;181:A2530 | No comparison between devices |
| 307 | Thomas PS, Gibson PG, Wang H, Shah S, Henry RL. The relationship of exhaled nitric oxide to airway inflammation and responsiveness in children. J Asthma 2005;42:291–5 | Unselected population |
| 308 | Tossa P, Bohadana A, Demange V, Wild P, Michaely JP, Hannhart B, et al. Early markers of airways inflammation and occupational asthma: rationale, study design and follow-up rates among bakery, pastry and hairdressing apprentices. BMC Public Health 2009;9:113 | Study design only |
| 309 | Travers J, Marsh S, Aldington S, Williams M, Shirtcliffe P, Pritchard A, et al. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J Respir Crit Care Med 2007;176:238–42 | Unselected population |
| 310 | Tseliou E, Bessa V, Hillas G, Delimpoura V, Papadaki G, Roussos C, et al. Exhaled nitric oxide and exhaled breath condensate pH in severe refractory asthma. Chest 2010;138:107–13 | Population – severe refractory asthma |
| 311 | Tsuburai T, Tsurikisawa N, Higashi N, Tatsuno S, Fukutomi Y, Tanimoto H, et al. [Differences in fraction of exhaled nitric oxide values measured by two offline methods or NIOXmino in adult Japanese asthmatics]. Arerugi 2010;59:956–64 | Offline |
| 312 | Tsuburai T, Tsurikisawa N, Higashi N, Tatsuno S, Fukutomi Y, Tanimoto H, et al. The difference of the fraction of exhaled nitric oxide (FeNO) levels measured by off-line methods or NIOXmino in adult Japanese asthmatics. Nitric Oxide Biol Chem 2010;22:S93 | Foreign language |
| 313 | Turner SW, McSweeny C, Malik G. Comparisons of exhaled nitric oxide measurements using the NIOX and MINO analysers in children. Proc Am Thorac Soc 2006;A484 | Unable to obtain |
| 314 | Tworek D, Bochenska-Marciniak M, Kupczyk M, Kuprys-Lipinska I, Kuna P. [Lack of correlation between exhaled nitric oxide (eNO) and clinical indicators of the disease activity and quality of life in mild and moderate asthmatics]. Pneumonol Alergol Pol 2006;74:391–5 | Foreign language |
| 315 | van Amsterdam JGC, Zanen P, Somer S, van Loveren H, Opperhuizen A, Steerenberg PA. Flow dependency and off-line measurement of exhaled NO in children. Pediatr Allergy Immunol 2003;14:266–71 | Wrong flow rate |
| 316 | van de Kant KD, Koers K, Rijkers GT, Lima Passos V, Klaassen EMM, Mommers M, et al. Can exhaled inflammatory markers predict the response to inhaled corticosteroids in wheezing preschool children? Am J Respir Crit Care Med 2011;183:A4465 | Age < 5 years |
| 317 | van der Valk RJ, Caudri D, Savenije O, Koppelman GH, Smit HA, Wijga AH, et al. Childhood wheezing phenotypes and FeNO in atopic children at age 8. Clin Exp Allergy 2012;42:1329–36 | No useable diagnostic data |
| 318 | van Wonderen KE, van der Mark LB, Mohrs J, Geskus RB, van der Wal WM, van Aalderen WM, et al. Prediction and treatment of asthma in preschool children at risk: study design and baseline data of a prospective cohort study in general practice (ARCADE). BMC Pulm Med 2009;9:13 | Study design only |
| 319 | Vieira T, Fonseca J, Cruz L, Silva R, Ferreira A, Leblanc A, et al. Results of a school-based asthma assessment from the upKids questionnaire validation study. Allergy 2009;64:441–2 | No useable diagnostic data |
| 320 | Vieira T, Fonseca JA, Silva R, Cruz L, Ferreira AR, Leblanc A, et al. Validity of a questionnaire in a school-based allergic asthma screening- comparison with exhaled nitric oxide fraction and skin prick tests. Rev Port Imunoalergologia 2011;19:215–21 | Population – children with positive skin prick test |
| 321 | Vitruba J, Cap P. [Fractional exhaled nitric oxide and its correlation with bioptic results in chronic cough patients]. Cas Lek Cesk 2009;148:429–33 | Foreign language |
| 322 | Vitruba J, Cap P, Statsny B. Fractional exhaled nitric oxide and its correlation with biopsy results in chronic cough patients. Allergy 2011;66:575 | Not valid reference standard (biopsy results) |
| 323 | Wang C-C, Wang C-Y, Hsu J-Y. Evaluation the causes of chronic cough of unknown origin by a protocol based on result of fractional exhaled nitric oxide. Respirology 2009;14:A132 | No useable diagnostic data |
| 324 | Wanich NH, Kaplan MS. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Pediatrics 2009;124(Suppl. 2):S147 | Commentary |
| 325 | Warke TJ, Fitch PS, Brown V, Taylor R, Lyons JD, Ennis M, et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax 2002;57:383–7 | Population is mix of asthmatics and healthy people |
| 326 | Wedes SH, Khatri SB, Zhang R, Wu W, Comhair SA, Wenzel S, et al. Noninvasive markers of airway inflammation in asthma. Clin Transl Sci 2009;2:112–17 | Case–control study |
| 327 | Wildhaber JH, Hall GL, Stick SM. Measurements of exhaled nitric oxide with the single-breath technique and positive expiratory pressure in infants. Am J Respir Crit Care Med 1999;159:74–8 | No useable diagnostic data |
| 328 | Wildhaber JH, Moller A, Hall GL, Sennhauser FH, Stick S. Levels of exhaled nitric oxide in recurrently wheezy infants are decreased following inhaled steroid therapy. Schweiz Med Wochenschr 2000;130:529–34 | No useable diagnostic data |
| 329 | Yang CL, Simons E, Foty RG, To T, Dell SD. Parental report of asthma diagnosis superior to exhaled nitric oxide for measuring childhood asthma prevalence. Am J Respir Crit Care Med 2011;183:A6361 | Not FeNO |
| 330 | Yang CL, Simons E, Foty RG, Marshall L, Nelligan K, To T, et al. Questionnaire diagnosis of asthma leads to misclassification compared to guideline-based diagnosis. Am J Respir Crit Care Med 2011;183:A5466 | Case–control |
| 331 | Yao TC, Ou LS, Lee WI, Yeh KW, Chen LC, Huang JL, et al. Exhaled nitric oxide discriminates children with and without allergic sensitization in a population-based study. Clin Exp Allergy 2011;41:556–64 | Unselected population |
| 332 | Yawn B, Rickard K, Herje N, Dorinsky PM. Clinical outcomes of subjects with non-specific respiratory symptoms and high FENO who were not diagnosed with asthma: a retrospective review of outcomes 6 months following the initial evaluation. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB64 | Non-specific respiratory symptoms |
| 333 | Yoo Y, Bauer S, La KS, Seo HS, Seo SC, Song DJ, et al. Relationships between airway hyperresponsiveness to methacholine, blood eosinophil markers and FENO in asthmatic children. J Allergy Clin Immunol 2012;129(2 Suppl. 1):AB211 | No useable diagnostic data |
| 334 | Yoo Y, Bauer S, Harmin S, Seo S, Yoon W, Choung JT. Relationships between exhaled nitric oxide and atopy profiles (mono-sensitization/poly-sensitization) in children with asthma. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB62 | No useable diagnostic data |
| 335 | Zhang Y-M, Lin J-T, Su N, Chen X, Liu G-L, Yu H-X, et al. Values of fractional exhaled nitric oxide in the diagnosis of chronic cough. Nat Med J China 2011;91:1254–8 | Foreign language |
| 336 | Zhang YM, Lin JT. [The values of fractional exhaled nitric oxide in the diagnosis and treatment of chronic cough]. Chung-Hua Chieh Ho Ho Hu Hsi Tsa Chih 2011;34:504–8 | Foreign language |
| 337 | Zietkowski Z, Skiepko R, Tomasiak-Lozowska MM, Mroczko B, Szmitkowski M, Bodzenta-Lukaszyk A, et al. RANTES in exhaled breath condensate of allergic asthma patients with exercise-induced bronchoconstriction. Respiration 2010;80:463–71 | Case–control study |
| 338 | Zietkowski Z, Skiepko R, Tomasiak-Lozowska M, Bodzenta-Lukaszyk A. RANTES in exhaled breath condensate of allergic asthma patients with exercise-induced bronchoconstriction. Allergy 2010;65:148 | No useable diagnostic data |
| Study | Reason for exclusion | |
|---|---|---|
| 1 | Adachi Y. [Biomarkers in childhood asthma]. Arerugi 2013;62:124–30 | Not English language |
| 2 | Bozek A, Filipowski M, Fischer A, Jarzab J. Characteristics of atopic bronchial asthma in seniors over 80 years of age. Biomed Res Int 2013;2013:689782 | Not a diagnostic accuracy study – included in subgroup review of elderly |
| 3 | Columbo M, Wong B, Panettieri RA Jr, Rohr AS. Asthma in the elderly: the role of exhaled nitric oxide measurements. Respir Med 2013;107:785–7 | Not a diagnostic accuracy study – included in subgroup review of elderly |
| 4 | Gregoriano C, Abu HN, Maier S, Zogg S, Margelli HD, Miedinger D, et al. Predictive value of exhaled nitric oxide to predict exercise induced bronchoconstriction. Respiration 2013;85:605 | Unselected population |
| 5 | Grzelewski T, Witkowski K, Makandjou-Ola E, Grzelewska A, Majak P, Jerzynska J, et al. Diagnostic value of lung function parameters and FeNO for asthma in schoolchildren in large, real-life population. Pediatr Pulmonol 2014;49:632–40 | Unable to extract reliable data |
| 6 | Hsu JY, Wang CY, Cheng YW, Chou MC. Optimal value of fractional exhaled nitric oxide in inhaled corticosteroid treatment for patients with chronic cough of unknown cause. J Chin Med Assoc 2013;76:15–19 | Already included |
| 7 | Hur G-Y, Oh JY, Choi J-H, Sim J-K, Min KH, Lee S-Y, et al. Mannitol challenge test, sputum eosinophils and exhaled nitric oxide (FENO) for diagnosis of asthma. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB64 | Not enough information to data extract |
| 8 | Raulf-Heimsoth M, van Kampen V, Heinze E, Bernard S, Borowitzki G, Freundt S, et al. Comparison of different non-invasive methods for detection of allergic asthma. Adv Exp Med Biol 2013;755:55–63 | Wrong patient cohort – not symptoms of asthma |
| 9 | Ryan D, Thomas MD, Dorinsky PM, Burden A, Von Ziegenweidt J, Hutton C, et al. The role of exhaled nitric oxide in guiding asthma. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB205 | Not enough information to data extract |
| 10 | Schneider A, Schwarzbach J, Faderl B, Welker L, Karsch-Volk M, Jorres RA. FENO measurement and sputum analysis for diagnosing asthma in clinical practice. Respir Med 2013;107:209–16 | Already included |
| 11 | Voorend-van Bergen S, Vaessen-Verberne A, Landstra A, Brackel H, van den Berg N, de Jongste J, et al. FeNO and web-based monitoring in paediatric asthma managemant; the BATMAN study. Eur Respir J 2013;42(Suppl. 57):3014 | Not enough information to data extract |
Appendix 6 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)193 flow diagram (adapted) of lower levels of evidence identified during database interrogation for the subgroups relating to the elderly, smokers and pregnant women
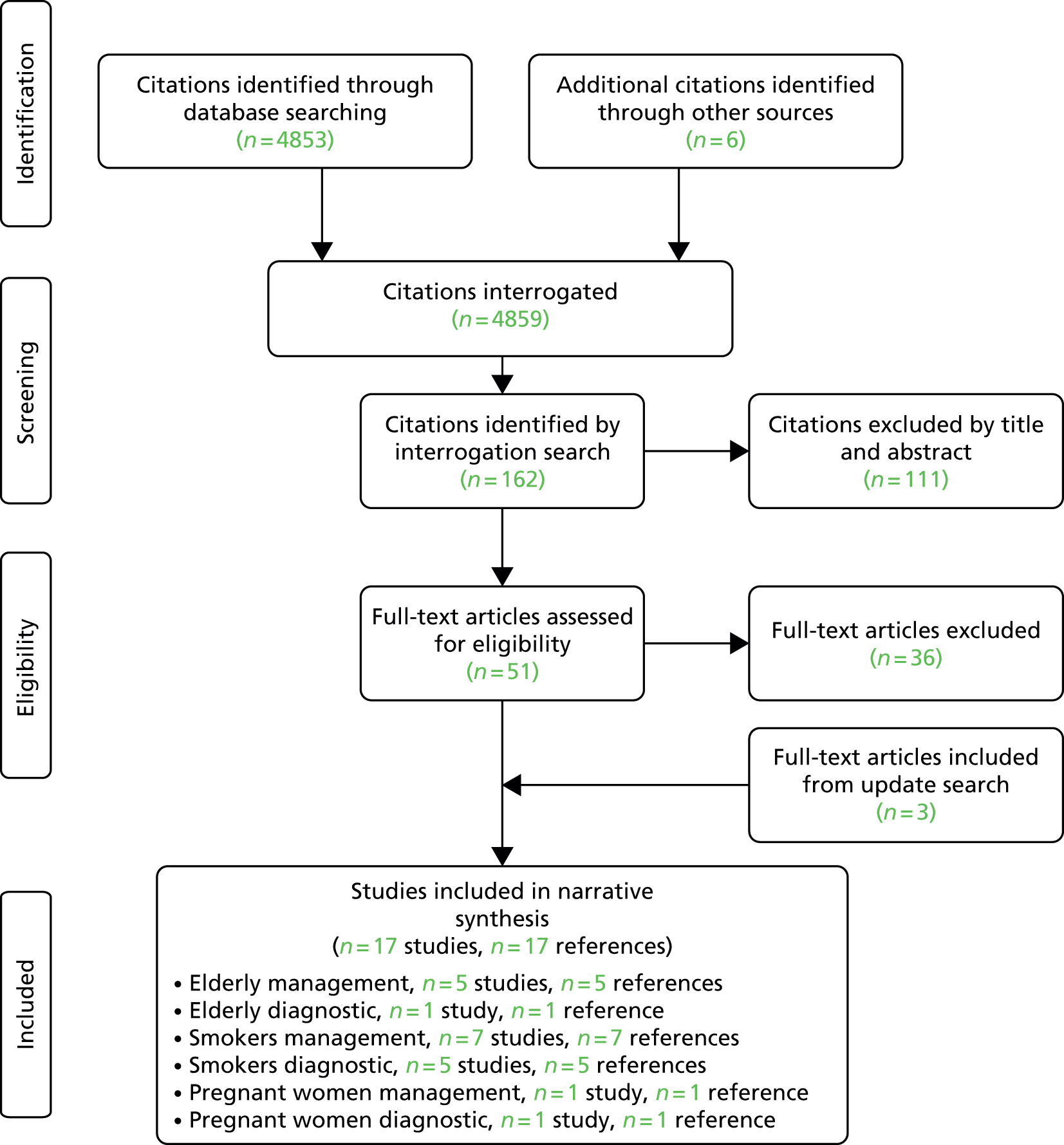
| Study | Reason for exclusion | |
|---|---|---|
| 1 | Baur X, Barbinova L. Latex allergen exposure increases exhaled nitric oxide in symptomatic healthcare workers. Eur Respir J 2005;25:309–316 | Non-asthmatics |
| 2 | Berry MA, Shaw DE, Green RH, Brightling CE, Wardlaw AJ, Pavord ID, et al. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy 2005;35:1175–9 | Wrong flow rate |
| 3 | Bivins J, Ownby D, Waller J, Tingen M. Exhaled nitric oxide level and school absenteeism in rural high school students with current asthma. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB64 | No data for smokers vs. non-smokers |
| 4 | Bohadana AB, Hannhart B, Ghezzo H, Teculescu D, Zmirou-Navier D. Exhaled nitric oxide and spirometry in respiratory health surveillance. Occup Med (Oxford) 2011;61:108–14 | Not all asthmatics |
| 5 | Bommarito L, Migliore E, Bugiani M, Heffler E, Guida G, Bucca C, et al. Exhaled nitric oxide in a population sample of adults. Respiration 2008;75:386–92 | Diagnostic – not all asthmatic |
| 6 | Bozek A, Krajewska J, Jarzab J. Nasal nitric oxide and other diagnostic procedures in seasonal allergic rhinitis: elderly vs juvenile patients. Am J Otolaryngol 2011;32:105–8 | Population not asthmatic |
| 7 | de la Barra SL, Smith AD, Cowan JO, Herbison GP, Taylor DR. Predicted versus absolute values in the application of exhaled nitric oxide measurements. Respir Med 2011;105:1629–34 | No data for smokers vs. non-smokers |
| 8 | Dinakar C, Lapuente M, Barnes C, Garg U. Real-life environmental tobacco exposure does not affect exhaled nitric oxide levels in asthmatic children. J Asthma 2005;42:113–18 | Offline |
| 9 | Gaku I, Risako S, Hiroyoshi W, Nene K, Mayuko T, Masanori W, et al. Smoking exacerbates airway inflammation in patients with asthma. Respirology 2010;15:61 | Mean FeNO levels only |
| 10 | Gemicioglu B, Guven K, Dogan I. FeNO in different asthma phenotypes. Allergy 2009;64:560 | Mean FeNO levels only |
| 11 | Gibson PG, Powell H, Giles W, Clifton V, Hensley M, Taylor DR, et al. Asthma exacerbations during pregnancy are reduced by inflammometry (FENO) guided asthma management: a randomised controlled trial. Am J Respir Crit Care Med 2011;183:A6414 | Management study |
| 12 | Gouvis-Echraghi R, Nikasinovic L, Bernard A, Herr-Breget M, Momas I, Just J. Passive smoke exposure lowers the fraction of exhaled nitric oxide in preschool children with recurrent wheeze. Allergy 2012;67:479 | Preschool |
| 13 | Hardaker K, Downie S, Kermode J, Farah C, Berend N, King G, et al. The predictors of airway hyperresponsiveness are different in younger and older asthmatics. Respirology 2010;15:A37 | Wrong flow rate |
| 14 | Hardaker KM, Downie SR, Kermode JA, Farah CS, Brown NJ, Berend N, et al. Predictors of airway hyperresponsiveness differ between old and young patients with asthma. Chest 2011;139:1395–1401 | Wrong flow rate |
| 15 | Hillas G, Kostikas K, Mantzouranis K, Bessa V, Kontogianni K, Papadaki G, et al. Exhaled nitric oxide and exhaled breath condensate pH as predictors of sputum cell counts in optimally treated asthmatic smokers. Respirology 2011;16:811–18 | Mean FeNO levels only |
| 16 | Jung A, Summermatter S, Geidel C, Moller A, Menz G, Lauener R. Diagnostic value of nasal NO measurement using the NIOX MINO device. Atemwegs Lungenkr 2012;38:57–8 | Nasal NO |
| 17 | Kostikas K, Papaioannou AI, Tanou K, Koutsokera A, Papala M, Gourgoulianis KI, et al. Portable exhaled nitric oxide as a screening tool for asthma in young adults during pollen season. Chest 2008;133:906–13 | Diagnostic AUCs reported for smokers vs. non-smokers |
| 18 | Leblanc A, Castro E, Castel-Branco M. Evolution and asthma control in pregnant women followed in an allergy Division. Allergy 2009;64:194–5 | Not diagnostic study |
| 19 | Lehtimaki L, Turjanmaa V, Kankaanranta H, Saarelainen S, Hahtola P, Moilanen E. Increased bronchial nitric oxide production in patients with asthma measured with a novel method of different exhalation flow rates. Ann Med 2000;32:417–23 | Wrong flow rate |
| 20 | Mahut B, Trinquart L, Le Bourgeois M, Becquemin MH, Beydon N, Aubourg F, et al. Multicentre trial evaluating alveolar NO fraction as a marker of asthma control and severity. Allergy 2010;65:636–44 | Adults mean FeNO levels only |
| 21 | Malinovschi A, Janson C, Hogman M, Rolla G, Toren K, Norback D, et al. Both allergic and nonallergic asthma are associated with increased FE(NO) levels, but only in never-smokers. Allergy 2009;64:55–61 | Diagnostic adults |
| 22 | Matsunaga K, Hirano T, Akamatsu K, Koarai A, Sugiura H, Minakata Y, et al. Differences in cutoff values of exhaled nitric oxide for asthma diagnosis according to rhinitis and smoking status. Am J Respir Crit Care Med 2011;183:A4480 | Diagnostic adults |
| 23 | Matsunaga K, Hirano T, Akamatsu K, Koarai A, Sugiura H, Minakata Y, et al. Exhaled nitric oxide cutoff values for asthma diagnosis according to rhinitis and smoking status in Japanese subjects. Allergol Int 2011;60:331–7 | Diagnostic adults |
| 24 | McCallister JW. Asthma in pregnancy: management strategies. Curr Opin Pulm Med 2013;19:13–17 | Review |
| 25 | Munnik P, van der Lee I, Fijn J, van Eijsden LJ, Lammers JW, Zanen P, et al. Comparison of eNO and histamine hyperresponsiveness in diagnosing asthma in new referrals. Respir Med 2010;104:801–7 | Diagnostic in adults, correction for smoking only |
| 26 | Nadif R, Matran R, Maccario J, Bechet M, Le Mouai N, Scheinmann P, et al. Passive and active smoking and exhaled nitric oxide levels according to asthma and atopy in adults. Ann Allergy Asthma Immunol 2010;104:385–93. [Erratum published in Ann Allergy Asthma Immunol 2010;105:97–8] | Mean FeNO levels only |
| 27 | Nadif R, Matran R, Maccario J, Bechet M, Le Moual N, Scheinmann P, et al. Passive and active smoking and exhaled nitric oxide levels according to asthma and atopy in adults. Ann Allergy Asthma Immunol 2010;105:97–8 | Mean FeNO levels only |
| 28 | Oshikata C, Tsuburai T, Tsurikisawa N, Ono E, Higashi A, Fukutomi Y, et al. Cutoff point of the fraction of exhaled nitric oxide (FeNO) with the off-line method for diagnosing asthma and the effect of smoking on FeNO. Nihon Kokyuki Gakkai Zasshi 2008;46:356–62 | Diagnostic adults |
| 29 | Persson MG, Zetterstrom O, Agrenius V, Ihre E, Gustafsson LE. Single-breath nitric oxide measurements in asthmatic patients and smokers. Lancet 1994;343:146–7 | Healthy only |
| 30 | Perzanowski MS, Divjan A, Mellins RB, Canfield SM, Rosa MJ, Chew GL, et al. Exhaled NO among inner-city children in New York City. J Asthma 2010;47:1015–21 | Wheeze not asthma |
| 31 | Powell H, Murphy VE, Taylor DR, Hensley MJ, McCaffery K, Giles W, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011;378:983–90 | Management study |
| 32 | Rouhos A, Ekroos H, Karjalainen J, Sarna S, Haahtela T, Sovijarvi AR, et al. Smoking attenuates increase in exhaled nitric oxide in atopic but not in nonatopic young adults with asthma. Int Arch Allergy Immunol 2010;152:226–32 | Mean FeNO levels only |
| 33 | Rutgers SR, Meijer RJ, Kerstjens HA, van der Mark TW, Koeter GH, Postma DS, et al. Nitric oxide measured with single-breath and tidal-breathing methods in asthma and COPD. Eur Respir J 1998;12:816–19 | Mean FeNO levels only |
| 34 | Shimoda T, Obase Y, Imaoka M, Kishikawa RT, Iwanaga T. Influence of cigarette smoking on airway inflammation and inhaled corticosteroid treatment in asthmatic patients. J Allergy Clin Immunol 2013;131(2 Suppl. 1):AB52 | Mean FeNO levels only |
| 35 | Spears M, Weir CJ, Smith AD, McSharry C, Chaudhuri R, Johnson M, et al. Bronchial nitric oxide flux (J'aw) is sensitive to oral corticosteroids in smokers with asthma. Respir Med 2011;105:1823–30 | Wrong flow rate |
| 36 | Taylor DR, de la Barra SL, Herbison GP, Cowan JO, Smith AD. Predicted versus absolute values in the interpretation of exhaled nitric oxide measurements. Am J Respir Crit Care Med 2011;183:A4476 | No data for smokers vs. non-smokers |
Appendix 7 Table of study characteristics for non-relevant adult diagnostics
| Author, year | Study design, funding | Country, setting, recruitment dates | Population | Device | Reference standard | n analysed/N recruited, reasons for withdrawals | Mean age (years) | Sex male , n/N (%) | Severity: FEV1% or FEV1/FVC | Mean FeNO (ppb) | Smokers | Atopic |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arora 200679 | Design: prospective Funding: US Air Force Surgeon General’s Office |
USA Specialist care Dates NR |
Adult (military recruits) with symptoms suggestive of asthma (position A) | Niox | Included both a consistent history with recurrent respiratory symptoms of non-productive cough, shortness of breath, chest tightness or wheezing with exertion or at rest and positive histamine bronchoprovocation [20% fall in FEV1 (PC20) of ≤ 8 mg/ml of histamine] | 172/172 | Asthmatic: 20 ± 2.7; non-asthmatic: 21 ± 2.7 | 85 (49.4) | FEV1% Asthmatic: mean 98 ± 13 (range 69–133); non-asthmatic: mean 107 ± 14 (range 89–135) |
Asthmatic: 30 ± 31; non-asthmatic: 19 ± 11 | 0/138 (0%) | NR |
| Fortuna 200770 | Design: prospective, consecutive Funding NR |
Spain Secondary care (outpatient clinic) Dates: October 2004–November 2005 |
Symptoms suggestive of asthma (position A) | N-6008 | Lung function tests (spirometry and bronchodilator response) and MCT following GINA guidelines112 | 50/57 n = 7 receiving OCS treatment at the time of study |
Asthmatic: 37 (range 18–68); non-asthmatic: 38 (range 18–64) | 21/50 (42) | FEV1% Asthmatic: mean 94 ± 19; non-asthmatic: mean 99 ± 10 |
Asthmatic: 40 ± 31; non-asthmatic: 18 ± 23 | Asthmatic: n = 3 smokers, n = 4 ex-smokers; non-asthmatic: n = 4 smokers, n = 3 ex-smokers | NR |
| Fukuhara 201154 | Design: prospective Funding NR; authors reported no conflict of interests |
Japan Secondary care Dates: May 2007–June 2007 |
Symptoms suggestive of asthma (position A) | NA623 | (1) At least one of the subjective symptoms of recurrent cough, wheezing or dyspnoea; (2) at least two of the three criteria of induced sputum eosinophilia, airway hyper-responsiveness and reversible airway obstruction; (3) exclusion of other lung diseases | 61/97 n = 36 unable to complete all tests |
55.6 (range 17–81) | 31/61 (50.8) | 96.1% (95% Cl 90.1% to 102.0%) | 74.5 (95% Cl 56.2 to 92.8) | n = 6 current smokers; n = 13 ex-smokers; n = 42 non-smokers | 14/61 (23%) |
| Mathew 2011,91 Sato 200875 | Design: prospective Funding NR |
UK Secondary care Dates NR |
Difficult to diagnose (position B) | NR | MCT | 84/84 | NR | 36/84 (42.9) | NR | NR | NR | NR |
| Pizzimenti 200990 | Design: prospective, consecutive Funding NR |
Italy Secondary care (outpatient clinic) Dates NR |
Patients with chronic cough (position A) | NIOX MINO | MCT (PD20 FEV1 < 800 µ) | 156/156 | NR | 64/156 (41.0) | NR | 34.1 (95% Cl 28.5 to 39.5) | 14/156 (9%) | 74/156 (47.4%) |
| Zhang 201189 | Design: prospective Funding NR |
China Secondary care October 2009–September 2010 |
Chronic cough with normal chest radiographs (position A) | NIOX MINO | Diagnosis of cough variant asthma, eosinophilic bronchitis and other based on sputum cell counts, pulmonary function test, bronchial hyper-responsiveness, 24-hour oesophageal pH monitoring, skin prick test and serum IgE | 106/106 | NR | NR | NR | NR | NR | NR |
Appendix 8 Table of the highest sum of sensitivity and specificity, highest sensitivity and highest specificity for non-relevant studies
| Author, year | Population | Device | Reference standard | Highest sum of sensitivity and specificity | Rule-out sensitivity | Rule-in specificity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cut-off (ppb) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||||
| Position A vs. whole pathway | ||||||||||||||||||
| Arora 200679 | Adults Position A |
Niox | Included both a consistent history with recurrent respiratory symptoms of non-productive cough, shortness of breath, chest tightness or wheezing with exertion or at rest and positive histamine bronchoprovocation [20% fall in FEV1 (PC20) of ≤ 8 mg/ml of histamine] | > 17 | 63 | 58.8 | 86.14 | 28.17 | > 6 | 96.4 | 0 | 79.64 | 0.00 | > 46 | 16.7 | 100 | 100 | 22.81 |
| Fortuna 200770 | Adults Position A |
N-6008 | Lung function tests (spirometry and bronchodilator response) and MCT following GINA guidelines112 | ≥ 20 | 77 | 64 | 62.96 | 78.26 | – | – | – | – | – | – | – | – | – | – |
| Fukuhara 201154 | Adults Position A |
NA623 | (1) At least one of the subjective symptoms of recurrent cough, wheezing or dyspnoea; (2) at least two of the three criteria of induced sputum eosinophilia, airway hyper-responsiveness and reversible airway obstruction; (3) exclusion of other lung diseases | 40 | 78.6 | 89.5 | 94.28 | 65.38 | – | – | – | – | – | – | – | – | – | – |
| Pizzimenti 200990 | Unspecified age group Position A |
NIOX MINO | MCT (PD20 FEV1 < 800 µ) | 55 | 10 | 67.2 | 39.28 | 97.66 | – | – | – | – | – | – | – | – | – | – |
| Zhang 201189 | Unspecified age group Position A |
NIOX MINO | Diagnosis of cough variant asthma, eosinophilic bronchitis and other based on sputum cell counts, pulmonary function test, bronchial hyper-responsiveness, 24-hour oesophageal pH monitoring, skin prick test and serum IgE | 40 | 75 | 86 | 76.31 | 85.29 | – | – | – | – | – | – | – | – | – | – |
| Position B | ||||||||||||||||||
| Mathew 201191 Sato 200875 |
Unspecified age group Position B |
NR | Airway reversibility, positive response to ICS, airway hyper-responsiveness (MCT) | NR | NR | NR | 8.7 | 70.49 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
Appendix 9 Table detailing the reference standards used in relevant adult diagnostic studies
| Author, year | Details of reference standard | Summarised as |
|---|---|---|
| Position A vs. whole pathway | ||
| Schneider 200971,72 |
|
FEV1, FEV1/FVC, airway reversibility, airway hyper-responsiveness (MCT) |
| Schneider 201369 | If FEV1 < 80% predicted, patient received salbutamol plus whole-body plethysmography 20 minutes later. Obstructive airway disease diagnosed if FEV1/FVC was ≤ 0.70. Classified as asthma according to clinical symptoms and history plus change in FEV1 ≥ 12% compared with baseline and ≥ 200 ml and if lung function increased to predicted normal range. Classified as incomplete bronchodilator response if response was ≤ 12% compared with baseline and ≥ 200 ml and lung volumes remained below predicted. Classified as COPD according to clinical symptoms and history plus FEV1 after salbutamol < 12% compared with baseline and < 200 ml. If FEV1 ≥ 80% predicted, bronchial provocation performed to determine bronchial hyper-responsiveness to methacholine according to the 1-concentration–4-step dosimeter protocol. Asthma diagnosed if 20% fall in FEV1 from baseline after inhaling methacholine stepwise until the maximum concentration (16 mg/ml) or doubling of airway resistance (Raw) and its increase to ≥ 2.0 kPa/second. The pneumologist was blinded to the FeNO results and made diagnostic decisions on basis of medical history, physical examination, spirometry, whole-body plethysmography and bronchial provocation results | FEV1, FEV1/FVC, airway reversibility, hyper-responsiveness (MCT) |
| Smith 200486 | Relevant symptom history (ATS 1987 guidelines113) and a positive test for bronchial hyper-responsiveness (provocative dose of hypertonic saline resulting in a 15% fall in FEV1 of < 20 ml) and/or a positive response to bronchodilator (increase in FEV1 of ≥ 12% from baseline 15 minutes after inhaled albuterol) | Airway reversibility, positive response to ICS, airway hyper-responsiveness (MCT) |
| Smith 200583 | ATS 1987113 diagnostic criteria plus one or more of:
|
Airway reversibility, positive response to ICS, airway hyper-responsiveness (MCT) |
| Position A vs. airway reversibility | ||
| de la Barra 201184 | Positive response to bronchodilator (increase in FEV1 of ≥ 12% from baseline 15 minutes after inhaled albuterol) | Airway reversibility |
| Subset of Position A vs. airway reversibility or airway hyper-responsiveness | ||
| Cordeiro 201187 | History of typical respiratory symptoms and FEV1% improvement of > 12% and > 200 ml or PC20 histamine of ≤ 8 mg/ml, according to GINA guidelines129 | Airway reversibility, airway hyper-responsiveness (histamine) |
| Heffler 200682 | Asthma confirmed based on typical symptoms and > 12% improvement in FEV1 in response to salbutamol or a dose of methacholine needed to cause a 20% fall from baseline in FEV1 (PD20) of < 800 µg | Airway hyper-responsiveness (MCT) or airway reversibility |
| Difficult to diagnose vs. airway hyper-responsiveness | ||
| Bobolea 201288 | Adenosine challenge test (PC20 < 400 mg/ml) | Adenosine challenge test |
| Katsoulis 201381 | Dose of methacholine needed to cause a 20% fall from baseline in FEV1 (PD20) according to ATS guidelines133 | Airway hyper-responsiveness (MCT) |
| Pedrosa 201085 | Consistent symptoms and a positive methacholine bronchial challenge. Patients stopped asthma medication before the test. The test was performed according to ATS 1999 guidelines133 and was considered positive when a decrease in FEV1 from baseline of ≥ 20% or higher was obtained after methacholine inhalation | Airway hyper-responsiveness (MCT) |
| Schleich 201277 | Asthma diagnosed based on airway hyper-responsiveness (MCT) provoking a 20% fall in FEV1 of < 16 mg/ml. Subjects were characterised as atopic if they had at least one positive skin prick test (wheal > 3 mm compared with negative control) or specific IgE (> 0.35 kU/l) for at least one common aeroallergen (cat, dog, house dust mites, grass pollen, tree pollen and a mixture of moulds) | Airway hyper-responsiveness (MCT) |
| Suspected EIB vs. exercise challenge test | ||
| El Halawani 200378 | Exercise challenge test was performed on a treadmill with an incremental work rate (up to 14 minutes of symptom-limited exercise). Treadmill speed began at 2 miles/hour and increased by 1 mile/hour every 2 minutes. Treadmill grade began at 10%, increasing to 15% after 8 minutes. The targeted heart rate was 85% predicted maximum and maintained for 2 minutes. Spirometry was performed every 5 minutes after exercise for a total of 30 minutes. Pulmonary functioning discontinued when a fall in FEV1 of 15% from baseline was demonstrated | Exercise challenge |
| Position F with chronic cough vs. ICS responsiveness | ||
| Hahn 200774 | ICS responsiveness assessed 1–16 months after diagnostic tests | ICS responsiveness |
| Hsu 201373 | Complete improvement of cough on ICS treatment with 250 µg twice per day for at least 2 weeks | ICS responsiveness |
| Prieto 200976 | Responsiveness to FP was identified by a reduction of > 50% in the mean daily cough symptom scores during the 4 weeks of the fluticasone propionate trial compared with the baseline period | ICS responsiveness |
Appendix 10 Table detailing the inclusion and exclusion criteria of the studies considered of most relevance to the review
| Author, year | Details of inclusion and exclusion criteria | Categorised as |
|---|---|---|
| Position A vs. whole pathway | ||
| Schneider 200971,72 | Patients presenting to their GP for the first time with complaints suggestive of obstructive airway disease; presentation of symptoms such as dyspnoea, coughing or expectoration for > 2 months, thus leading to clinical suspicion of obstructive or restrictive airway disease as most important differential diagnoses (‘indicated population’). GPs were advised to exclude patients with respiratory tract infections preceding the evaluation by 6 weeks. Patients with a previously established diagnosis of obstructive airway disease were excluded. Other exclusion criteria related to well-known contraindications for bronchodilator reversibility testing or bronchial provocation, namely untreated hyperthyreosis, unstable coronary artery disease and cardiac arrhythmia. Pregnancy also led to exclusion | Position A |
| Schneider 201369 | Patients presenting for the first time with symptoms such as dyspnoea, cough or phlegm for > 2 months, leading to the clinical suspicion of obstructive or restrictive airway disease (‘indicated population’). Patients were advised not to smoke on the day of investigation and not to use inhaler medication for 12 hours before lung function testing. Exclusions: patients with respiratory tract infections within the last 6 weeks; previously established diagnosis of chronic obstructive airway disease; known contraindications for bronchodilator reversibility testing or bronchial provocation, namely untreated hyperthyreosis, unstable coronary artery disease and cardiac arrhythmia; pregnancy | Position A |
| Smith 200486 | Patients referred by their GP for investigation of possible bronchial asthma with symptoms for a minimum of 6 weeks. No patient had been referred for specialist consultation. Exclusions: those who used oral or inhaled corticosteroids in the previous 4 weeks and those with a respiratory tract infection in the previous 6 weeks | Position A |
| Smith 200583 | Patients referred by their GP for investigation of persistent, undiagnosed respiratory symptoms lasting for at least 6 weeks. Exclusions: use of ICSs or OCSs in the previous 4 weeks, respiratory tract infection in the previous 6 weeks, other established respiratory diagnosis or significant comorbidity; smokers were not excluded | Position A |
| Position A vs. airway reversibility | ||
| de la Barra 201184 | New undiagnosed symptoms of cough, wheeze or dyspnoea of ≥ 6 weeks in duration | Position A |
| Subset of Position A vs. airway reversibility or airway hyper-responsiveness | ||
| Cordeiro 201187 | All new patients who were referred to a general outpatient allergy clinic from January 2007 to September 2007. Patients using ICSs or oral corticosteroids within 6 weeks of the first visit were excluded from data analysis | Position A |
| Heffler 200682 | Nasal symptoms for > 4 days per week over > 8 weeks; asthma-like symptoms during the past 2 months. Exclusions: use of steroids or any other anti-inflammatory drugs in the last 2 months, current smoker (within the past 12 months), previous diagnosis of asthma, respiratory infection within the past 6 weeks | Position A |
| Difficult to diagnose vs. airway hyper-responsiveness | ||
| Bobolea 201288 | Patients with suspected asthma who had normal spirometry, a negative bronchodilator test, a negative methacholine challenge [provocative concentration inducing a 20% fall in FEV1 (PC20) > 16 mg/ml)] | Difficult to diagnose |
| Katsoulis 201381 | Patients with one positive answer for respiratory symptoms. Exclusions: pre-existing asthma diagnosis, treatment with asthma-related medication, 12% reversibility after bronchodilation and 200 ml FEV1, respiratory infection within the last 8 weeks, recent ex-smokers | Difficult to diagnose |
| Pedrosa 201085 | Those reporting persistent symptoms consistent with asthma (shortness of breath, wheezing and/or cough) regardless of atopic status who showed normal spirometry and who had a negative bronchodilator test. Exclusions: as per ATS 1999 guidelines133 for bronchial challenge test | Difficult to diagnose |
| Schleich 201277 | Patients were referred to a respiratory physician for a methacholine challenge to detect asthma. Subjects referred to methacholine challenge were those in whom the bronchodilating test failed to demonstrate reversible airways obstruction or those in whom baseline spirometric values were normal giving a low probability for a bronchodilating test to be significant. Patients had either (1) baseline FEV1 ≥ 80% predicted and a FEV1/FVC ratio ≥ 70% or (2) baseline FEV1 < 80% predicted and a FEV1/FVC ratio < 70% plus bronchodilation < 12% from baseline and 200 ml after 400 µg inhaled salbutamol Exclusions: patients already receiving ICSs | Difficult to diagnose |
| Suspected EIB vs. exercise challenge test | ||
| El Halawani 200378 | Patients with suspected asthma who had normal spirometry, a negative bronchodilator test, negative methacholine challenge [provocative concentration inducing a 20% fall in FEV1 (PC20) > 16 mg/ml] | EIB |
| Position F with chronic cough vs. ICS responsiveness | ||
| Hahn 200774 | Age > 18 years, uncontrolled chronic cough (> 8 weeks), normal/non-localising chest radiograph, documented MCT results and measurement of NO levels within 1 day of each other. Only patients who had started ICS therapy or who had their current ICS doses altered were included. Exclusions: current smokers and users of angiotensin-converting enzyme inhibitors | Difficult to diagnose with chronic cough |
| Hsu 201373 | Patients with a history of chronic cough of > 8 weeks’ duration and who did not stop coughing after treatment for upper airway cough syndrome or gastro-oesophageal reflux disease. Exclusions: obvious chest radiograph abnormalities, current smokers/smoking history of > 10 pack-years | Difficult to diagnose with chronic cough |
| Prieto 200976 | Chronic cough of at least 8 weeks’ duration with no evidence of any other lung disease, non-smokers, not currently being treated with angiotensin-converting enzyme inhibitors or beta-blockers, not previously received treatment with ICSs or OCSs or not experienced a respiratory tract infection in the previous 4 weeks. Each subject required to have a FEV1 of at least 80% predicted | Difficult to diagnose with chronic cough |
Appendix 11 Table of results for all diagnostic studies in adults
| Author, year | Prevalence of positive result by reference standard, n/N (%) | FeNO cut-off (ppb) | TP (n) | FP (n) | FN (n) | TN (n) | Sensitivity asthma (95% CI) (%) | Specificity asthma (95% CI) (%) |
|---|---|---|---|---|---|---|---|---|
| Position A | ||||||||
| de la Barra 201184 | NR | 25 | 10 | 17 | 2 | 23 | 83.3 | 57.5 |
| 40 | 9 | 12 | 3 | 28 | 75 | 70 | ||
| 50 | 7 | 8 | 5 | 32 | 58.3 | 80 | ||
| 70 | 5 | 5 | 7 | 35 | 41.7 | 87.5 | ||
| 90 | 5 | 3 | 7 | 37 | 41.7 | 92.5 | ||
| 110 | 3 | 2 | 9 | 38 | 25 | 95 | ||
| 130 | 2 | 2 | 10 | 38 | 16.7 | 95 | ||
| 150 | 2 | 2 | 10 | 38 | 16.7 | 95 | ||
| Fortuna 200770 | Induced sputum (Eos%) 16/50 (32.0); bronchodilator test 13/50 (26.0); FEV1 < 80% 5/50 (10.0) | ≥ 20 ppb | 17 | 10 | 5 | 18 | 77 | 64 |
| Fukuhara 201154 | 42/61 (68.9) | 40 | 33 | 2 | 9 | 17 | 78.6 | 89.5 |
| Schneider 200971,72 | 75/160 (46.9) | > 20 | 48 | 36 | 27 | 49 | 64 (53 to 74) | 58 (47 to 77) |
| > 12 | 64 | 65 | 11 | 20 | 85 (76 to 92) | 24 (16 to 34) | ||
| > 16 | 52 | 40 | 23 | 45 | 69 (58 to 79) | 53 (42 to 63) | ||
| > 35 | 24 | 14 | 51 | 71 | 32 (25 to 42) | 84 (74 to 90) | ||
| > 46 | 24 | 6 | 51 | 79 | 32 (23 to 43) | 93 (85 to 97) | ||
| > 76 | 10 | 0 | 65 | 85 | 13 (7 to 23) | 100 (96 to 100) | ||
| Schneider 201369 | 154/393 (39.2) | > 9 | 146 | 209 | 7 | 31 | 96 (91 to 98) | 13 (9 to 18) |
| > 12 | 135 | 167 | 23 | 68 | 85 (79 to 90) | 29 (23 to 35) | ||
| > 16 | 105 | 128 | 46 | 114 | 70 (62 to 76) | 47 (41 to 54) | ||
| > 20 | 91 | 89 | 62 | 151 | 60 (52 to 67) | 63 (57 to 69) | ||
| > 25 | 75 | 59 | 79 | 180 | 49 (41 to 57) | 75 (69 to 80) | ||
| > 35 | 50 | 29 | 104 | 210 | 33 (26 to 40) | 88 (83 to 91) | ||
| > 41 | 42 | 20 | 112 | 219 | 27 (21 to 35) | 92 (87 to 94) | ||
| > 42 | 40 | 20 | 114 | 219 | 26 (20 to 33) | 92 (87 to 94) | ||
| > 43 | 39 | 19 | 115 | 220 | 25 (19 to 32) | 92 (88 to 95) | ||
| > 44 | 39 | 19 | 115 | 220 | 25 (19 to 32) | 92 (88 to 95) | ||
| > 45 | 38 | 19 | 116 | 220 | 23 (17 to 31) | 92 (88 to 95) | ||
| > 46 | 38 | 17 | 116 | 222 | 27 (21 to 35) | 92 (87 to 94) | ||
| > 71 | 27 | 7 | 127 | 232 | 18 (12 to 24) | 97 (94 to 99) | ||
| Smith 200486 | 17/47 (36.2) | > 20 | 14 | 6 | 2 | 22 | 88 | 79 |
| Smith 200583 | 27/52 (51.9) | ≥ 15 | 22 | 13 | 5 | 12 | 81.5 | 48 |
| > 47 | 15 | 2 | 12 | 23 | 55.6 | 92 | ||
| < 15 | 5 | 12 | 22 | 13 | 18.5 | 52 | ||
| Subset of Position A | ||||||||
| Cordeiro 201187 | 42/114 (36.8) | 27 | 33 | 6 | 9 | 66 | 78 | 92 |
| Heffler 200682 | 18/48 (37.5) | > 10 | 18 | 29 | 0 | 1 | 100 | 3.3 |
| > 15 | 18 | 26 | 0 | 4 | 100 | 13.3 | ||
| > 20 | 18 | 20 | 0 | 10 | 100 | 33.3 | ||
| > 25 | 18 | 16 | 0 | 14 | 100 | 46.7 | ||
| > 30 | 14 | 15 | 4 | 15 | 77.8 | 50 | ||
| > 34 | 14 | 14 | 4 | 16 | 77.8 | 53.3 | ||
| > 36 | 14 | 12 | 4 | 18 | 77.8 | 60 | ||
| > 40 | 11 | 11 | 7 | 19 | 61.1 | 63.3 | ||
| > 45 | 11 | 8 | 7 | 22 | 61.1 | 73.3 | ||
| > 50 | 10 | 7 | 8 | 23 | 55.6 | 76.7 | ||
| > 55 | 9 | 6 | 9 | 24 | 50 | 80 | ||
| > 60 | 9 | 4 | 9 | 26 | 50 | 86.7 | ||
| > 65 | 8 | 4 | 10 | 26 | 44.4 | 86.7 | ||
| > 75 | 8 | 3 | 10 | 27 | 44.4 | 90 | ||
| > 80 | 7 | 1 | 11 | 29 | 38.9 | 96.7 | ||
| > 85 | 5 | 1 | 13 | 29 | 27.8 | 96.7 | ||
| > 100 | 5 | 0 | 13 | 30 | 27.8 | 100 | ||
| Pizzimenti 200990 | 14/156 (9.0) | 55 | 11 | 17 | 3 | 125 | 78 | 88 |
| Difficult to diagnose | ||||||||
| Bobolea 201288 | 6/30 (20.0) | > 30 | 6 | 17 | 0 | 7 | 100 | 29.2 |
| Katsoulis 201381 | 48/112 (42.9) | > 30 | 24 | 12 | 24 | 52 | 49 (34 to 64) | 82 (71 to 90) |
| > 25 | 24 | 16 | 24 | 48 | 51 (36 to 66) | 75 (63 to 85) | ||
| > 20 | 31 | 26 | 17 | 38 | 64 (49 to 78) | 60 (47 to 72) | ||
| > 15 | 35 | 33 | 13 | 31 | 73 (58 to 85) | 49 (37 to 62) | ||
| > 10 | 39 | 39 | 9 | 25 | 81 (64 to 91) | 39 (29 to 50) | ||
| Mathew 201191 | 20/84 (23.8) | NR | 2 | 21 | 18 | 43 | 10 | 67.2 |
| Pedrosa 201085 | 35/114 (30.7) | 40 | 26 | 22 | 9 | 57 | 74.3 | 72.5 |
| Schleich 201277 | 82/174 (47.1) | 34 | 29 | 4 | 53 | 88 | 35.4 | 95.4 |
| Schneider 200971,72 | Subjects with unsuspicious spirometric results: 49/101 (48.5) | > 46 | 17 | 5 | 32 | 47 | 35 (23 to 49) | 90 (79 to 96) |
| > 15 | 38 | 29 | 11 | 23 | 78 (63 to 89) | 45 (34 to 57) | ||
| Difficult to diagnose with chronic cough | ||||||||
| aHahn 200774 | 38/64 (59.4) | 35 | 36 | 5 | 2 | 21 | 95 (83 to 99) | 80 (62 to 92) |
| 38 | 34 | 4 | 4 | 22 | 90 (76 to 96) | 85 (76 to 96) | ||
| aHsu 201373 | 38/81 (46.9) | 33.9 | 36 | 9 | 2 | 34 | 94.7 | 76.3 |
| 30 | 37 | 14 | 1 | 29 | 97.4 | 65.8 | ||
| Prieto 200976 | 19/43 (44.2) | 20 | 10 | 9 | 9 | 15 | 53 | 63 |
| Sato 200875 | 48/71 (67.6) | 38.8 | 38 | 2 | 10 | 21 | 79.2 | 91.3 |
| Zhang 201189 | 39/106 (36.8) | 40 | 29 | 9 | 10 | 58 | 75 | 86 |
| 36a | 32 | 5 | 7 | 62 | 82 | 93 | ||
| EIB | ||||||||
| El Halawani 200378 | 7/49 (14.3)b | < 12 | 7 | 29 | 0 | 13 | 100 | 31 |
| Other | ||||||||
| Arora 200679 | 138/172 (80.2) | > 6 | 133 | 34 | 5 | 0 | 96.4 | 0 |
| > 7 | 131 | 33 | 7 | 1 | 94.6 | 2.9 | ||
| > 8 | 130 | 31 | 8 | 3 | 94.2 | 8.8 | ||
| > 9 | 127 | 30 | 11 | 4 | 92 | 11.8 | ||
| > 10 | 119 | 28 | 19 | 6 | 86.2 | 17.6 | ||
| > 11 | 115 | 26 | 23 | 8 | 83.3 | 23.5 | ||
| > 12 | 113 | 25 | 25 | 9 | 81.9 | 26.5 | ||
| > 13 | 110 | 21 | 28 | 13 | 79.7 | 38.2 | ||
| > 14 | 102 | 19 | 36 | 15 | 73.9 | 44.1 | ||
| > 15 | 98 | 19 | 40 | 15 | 71 | 44.1 | ||
| > 16 | 92 | 17 | 46 | 17 | 66.7 | 50 | ||
| > 17 | 87 | 14 | 51 | 20 | 63 | 58.8 | ||
| > 18 | 83 | 14 | 55 | 20 | 60.1 | 58.8 | ||
| > 19 | 78 | 13 | 60 | 21 | 56.5 | 61.8 | ||
| > 20 | 73 | 11 | 65 | 23 | 52.9 | 67.6 | ||
| > 25 | 56 | 7 | 82 | 27 | 40.6 | 79.4 | ||
| > 30 | 45 | 7 | 93 | 27 | 32.6 | 79.4 | ||
| > 40 | 32 | 3 | 106 | 31 | 23.2 | 91.2 | ||
| > 46 | 23 | 0 | 115 | 34 | 16.7 | 100 | ||
| Brannan 201392 | 76/401 (19.0) | 47 | 23 | 12 | 44 | 322 | 30.2 | 96.3 |
| Chancafe-Morgan 201380 | 12/30 (40.0) | 35 | 9 | 3 | 3 | 15 | 75 | 83.3 |
Appendix 12 Table of results for all diagnostic studies in children
| Author, year | Prevalence of positive result by reference standard, n/N (%) | FeNO cut-off (ppb) | TP (n) | FP (n) | FN (n) | TN (n) | Sensitivity asthma (%) | Specificity asthma (%) |
|---|---|---|---|---|---|---|---|---|
| Linkosalo 201293 | 18/30 (60.0) | 10 | 16 | 8 | 2 | 4 | 89 | 33 |
| 20 | 13 | 2 | 5 | 10 | 72 | 83 | ||
| 30 | 9 | 1 | 9 | 11 | 50 | 92 | ||
| 40 | 7 | 1 | 11 | 11 | 39 | 92 | ||
| 50 | 2 | 1 | 16 | 11 | 11 | 92 | ||
| Ramser 200894 | 105/169 (62.1) | 10 | 75 | 24 | 45 | 25 | 76 | 36 |
| 20 | 49 | 50 | 17 | 53 | 49 | 76 | ||
| 30 | 33 | 66 | 12 | 58 | 33 | 83 | ||
| 40 | 23 | 76 | 7 | 63 | 23 | 90 | ||
| 50 | 20 | 79 | 5 | 65 | 20 | 93 | ||
| Sivan 200995 | 106/150 (70.7) | 15 | 62 | 13 | 7 | 31 | 90 | 70 |
| 18 | 87 | 7 | 19 | 37 | 82 | 84 | ||
| 19 | 59 | 5 | 10 | 39 | 86 | 89 | ||
| 25 | 52 | 5 | 17 | 39 | 75 | 89 | ||
| > 20 or < 15 | 58 | 4 | 7 | 32 | 89 | 88 | ||
| Woo 201296 | 167/245 (68.2) | > 50 | 24 | 0 | 143 | 78 | 14.4 | 100 |
| > 45 | 29 | 0 | 138 | 78 | 17.4 | 100 | ||
| > 41 | 39 | 0 | 128 | 78 | 23.4 | 100 | ||
| > 40 | 41 | 1 | 126 | 77 | 24.6 | 98.7 | ||
| > 35 | 54 | 1 | 113 | 77 | 32.3 | 98.7 | ||
| > 30 | 71 | 4 | 96 | 74 | 42.5 | 94.9 | ||
| > 25 | 83 | 6 | 84 | 72 | 49.7 | 92.3 | ||
| > 24 | 84 | 7 | 83 | 71 | 50.3 | 91 | ||
| > 23 | 86 | 7 | 81 | 71 | 51.5 | 91 | ||
| > 22 | 90 | 10 | 68 | 68 | 53.9 | 87.2 | ||
| > 21 | 95 | 10 | 68 | 68 | 56.9 | 87.2 | ||
| > 20 | 101 | 15 | 63 | 63 | 60.5 | 80.8 | ||
| > 15 | 120 | 26 | 52 | 52 | 71.9 | 66.7 | ||
| > 10 | 134 | 43 | 35 | 35 | 80.2 | 44.9 | ||
| > 5 | 157 | 67 | 11 | 11 | 94 | 14.1 | ||
| 22 | 93 | 9 | 51 | 51 | 72.1 | 85 |
Appendix 13 MEDLINE search strategies for the economic review
Use of NIOX MINO/NObreath for either the diagnosis or the management of asthma (30 May 2013)
-
niox mino.mp.
-
aerocrine.mp.
-
(niox adj5 (monitor$ or chemiluminescence or analyser$ or sensor or device$ or desktop)).mp.
-
nobreath.mp.
-
bedfont.mp.
-
or/1-5
-
exp “Costs and Cost Analysis”/
-
Economics/
-
exp Economics, Hospital/
-
exp Economics, Medical/
-
Economics, Nursing/
-
exp models, economic/
-
Economics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp Budgets/
-
budget$.tw.
-
ec.fs.
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimi$)).ab.
-
(economic$ or pharmacoeconomic$ or pharmaco-economic$).ti.
-
(price$ or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(fee or fees).tw.
-
(value adj2 (money or monetary)).tw.
-
quality-adjusted life years/
-
(qaly or qalys).af.
-
(quality adjusted life year or quality adjusted life years).af.
-
or/7-28
-
6 and 28
Models of asthma and FENO testing (30 May 2013)
-
niox mino.mp.
-
aerocrine.mp.
-
(niox adj5 (monitor$ or chemiluminescence or analyser$ or sensor or device$ or desktop)).mp.
-
nobreath.mp.
-
bedfont.mp.
-
or/1-5
-
exp cough/
-
cough$.mp.
-
phlegm.mp.
-
sputum.mp.
-
mucus.mp.
-
wheez$.mp.
-
chest pain/
-
chest pain$.mp.
-
(chest adj5 tight$).tw.
-
((lower respiratory or lrt) adj5 symptom$).tw.
-
(lower airway adj5 symptom$).tw.
-
((trache$ or wind pipe or lung$ or bronch$) adj3 symptom$).tw.
-
exp lung/ or trachea/
-
symptom$.tw.
-
19 and 20
-
or/7-18,21
-
exp asthma/
-
asthma$.mp.
-
exp respiratory hypersensitivity/
-
exp bronchial hyperreactivity/
-
bronchial spasm/
-
bronchospas$.mp.
-
exp Bronchoconstriction/
-
bronchoconstric$.mp.
-
(bronch$ adj3 constrict$).mp.
-
(bronch$ adj5 spas$).mp.
-
(airway$ adj5 (obstruct$ or inflammation$)).mp.
-
((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
-
or/23-34
-
Nitric Oxide/
-
nitric oxide.mp.
-
36 or 37
-
(exhal$ or expir$ or alveolar or fractional).mp.
-
38 and 39
-
exhaled NO.mp.
-
eno.mp.
-
fe?no$.mp.
-
(fractional adj2 NO).mp.
-
or/40-44
-
22 and 45
-
35 and 45
-
6 or 46 or 47
-
exp “Costs and Cost Analysis”/
-
Economics/
-
exp Economics, Hospital/
-
exp Economics, Medical/
-
Economics, Nursing/
-
exp models, economic/
-
Economics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp Budgets/
-
budget$.tw.
-
ec.fs.
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimi$)).ab.
-
(economic$ or pharmacoeconomic$ or pharmaco-economic$).ti.
-
(price$ or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(fee or fees).tw.
-
(value adj2 (money or monetary)).tw.
-
quality-adjusted life years/
-
(qaly or qalys).af.
-
(quality adjusted life year or quality adjusted life years).af.
-
or/49-69
-
48 and 70
Asthma management models (3 June 2013)
-
exp asthma/
-
asthma$.mp.
-
exp respiratory hypersensitivity/
-
exp bronchial hyperreactivity/
-
bronchial spasm/
-
bronchospas$.mp.
-
exp Bronchoconstriction/
-
bronchoconstric$.mp.
-
(bronch$ adj5 spas$).mp.
-
(airway$ adj5 (obstruct$ or inflammation$)).mp.
-
((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
-
exp models, economic/
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimi$)).ti.
-
((cost$ or economic) adj5 model$).ti.
-
or/1-11
-
or/12-14
-
15 and 16
Asthma diagnostic models (7 June 2013)
-
exp asthma/
-
asthma$.mp.
-
exp respiratory hypersensitivity/
-
exp bronchial hyperreactivity/
-
bronchial spasm/
-
bronchospas$.mp.
-
exp Bronchoconstriction/
-
bronchoconstric$.mp.
-
(bronch$ adj3 constrict$).mp.
-
(bronch$ adj5 spas$).mp.
-
(airway$ adj5 (obstruct$ or inflammation$)).mp.
-
((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
-
or/1-12
-
exp “Costs and Cost Analysis”/
-
Economics/
-
exp Economics, Hospital/
-
exp Economics, Medical/
-
Economics, Nursing/
-
exp models, economic/
-
Economics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp Budgets/
-
budget$.tw.
-
ec.fs.
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimi$)).ab.
-
(economic$ or pharmacoeconomic$ or pharmaco-economic$).ti.
-
(price$ or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(fee or fees).tw.
-
(value adj2 (money or monetary)).tw.
-
quality-adjusted life years/
-
(qaly or qalys).af.
-
(quality adjusted life year or quality adjusted life years).af.
-
or/14-34
-
exp “Sensitivity and Specificity”/
-
sensitivity.tw.
-
specificity.tw.
-
((pre-test or pretest) adj probability).tw.
-
post-test probability.tw.
-
predictive value$.tw.
-
likelihood ratio$.tw.
-
diagnostic$.ti,ab.
-
or/36-43
-
13 and 35 and 44
Glossary
- Airway hyper-responsiveness
- Synonymous with bronchial hyper-responsiveness and an indicator of asthma. Usually assessed using a bronchial challenge test. In a bronchial challenge test an agent such as histamine or methacholine is inhaled. If these agents trigger bronchospasm at a significantly lower threshold than normal an individual is considered to have airway hyper-responsiveness.
- Airway reversibility
- Airway obstruction that improves when a bronchodilator or corticosteroids are taken.
- Antihistamine
- A drug that inhibits the action of histamine in the body and which may be effective in treating allergic asthma.
- Area under the curve
- A measure of the diagnostic accuracy of a technology based on the geometric inspection of a receiver operating characteristic plot, which plots true-positive rate against false-positive rate. A technology with perfect diagnostic accuracy will have an area under the curve of 1, a technology that is no better than chance will have an area under the curve of 0.5 and a technology that miscategorises on every occasion will have an area under the curve of zero.
- Atopy/atopic disorder
- A predisposition towards the development of some forms of allergic hypersensitivity. Atopy is considered to be a risk factor for asthma.
- Attrition bias
- A statistical bias caused by systematic differences in rates of attrition in the control and intervention arms of a study. For example, the intervention may make some patients receiving it better but may cause others to experience severe side effects and be more likely to leave the study.
- Bland–Altman plot
- Also known as a difference plot and used to estimate the level of agreement between two devices or assays used for measuring the same thing. Observations are paired and the mean of the paired observations is plotted against the difference in estimates between the two devices for the same observation.
- Bronchoconstriction
- Constriction of the airways in the lungs as a result of the action of surrounding smooth muscle, airway inflammation or excessive production of mucus because of allergy or irritation from air friction, overcooling or drying of the airways. It is characterised by coughing, wheezing and shortness of breath.
- Chemiluminescence
- A broad range of methods in which light is emitted as a result of a chemical reaction. Used to detect the presence and level of nitric oxide in exhaled breath.
- Chronic obstructive pulmonary disease
- A lung disease in which airflow is persistently poor because of lung tissue damage and dysfunction of the small airways. Some treatment for chronic obstructive pulmonary disease is similar to that for asthma but, unlike asthma, chronic obstructive pulmonary disease is usually acquired rather than inherited and the prognosis and health-related quality of life are poorer.
- Cut-off
- In a binary categorisation exercise, a value within a range of values used to categorise observations into one of two mutually exclusive groups. With respect to the fraction of exhaled nitric oxide devices considered in this assessment, the cut-off threshold is expressed as parts per billion of nitric oxide in exhaled breath; those with values above the threshold are considered ‘positive’ and those with values below the threshold are considered ‘negative’.
- Detection bias
- Detection bias refers to systematic differences between groups in how outcomes are determined. This usually occurs as a result of preconceptions about treatment efficacy. As such, blinding (or masking) of outcome assessors may reduce the risk that knowledge of which intervention was received, rather than the intervention itself, affects outcome measurement. Blinding of outcome assessors can be especially important for assessment of subjective outcomes such as degree of postoperative pain. The outcome assessor can be the patient when outcomes are self-assessed.
- Diagnostic accuracy
- The effectiveness of a diagnostic test in correctly categorising patients as either ‘positive’ or ‘negative’. There are several ways that this can be expressed, for example the area under the curve or the sum of sensitivity and specificity.
- Exacerbation
- A worsening of symptoms that may be acute or subacute. In the case of asthma, this can also be termed an ‘asthma attack’. Symptoms include shortness of breath, wheezing, cough and chest tightness. Exacerbations also lead to decreases from baseline in lung function, such as forced expiratory volume in the first second.
- Extended dominance
- The state when a strategy under study is both less effective and more costly than a linear combination of two other strategies with which it is mutually exclusive.
- False negative
- An individual who has been incorrectly categorised as a member of the category ‘negative’ in a binary categorisation exercise when the only other possible classification is ‘positive’, for example someone who has asthma but who has been categorised as not having asthma.
- False positive
- An individual who has been incorrectly categorised as a member of the category ‘positive’ in a binary categorisation exercise when the only other possible classification is ‘negative’, for example a patient incorrectly diagnosed with asthma.
- Forced expiratory volume in the first second
- The volume of air expelled by a patient within the first second.
- Fraction of exhaled nitric oxide
- The concentration of nitric oxide in exhaled breath, also known as fractional exhaled nitric oxide.
- Index test
- A diagnostic test whose sensitivity and specificity are assessed by comparing its categorisations (positive, negative) with another diagnostic test, known as a reference standard, which is assumed to have perfect sensitivity and specificity. In this assessment the index test is the fraction of exhaled nitric oxide.
- Inhaled corticosteroid responsiveness
- The degree to which the asthma condition improves in response to treatment with inhaled corticosteroids.
- Juniper score
- A quality of life measure for patients with asthma.
- Negative predictive value
- The probability that a patient who has been categorised as ‘negative’ really is negative.
- Peak expiratory flow rate
- The maximum rate of expiration of breath, as measured by a peak flow metre. Considered a measure of lung function.
- Pearson correlation
- A measure ranging between –1 and 1 indicating the degree and direction of linear dependence between two variables. Values close to zero indicate no/very low correlation and values close to 1 indicate very high correlation.
- Performance bias
- A statistical bias caused by the control and treatment groups receiving different standards of care or being exposed to factors other than the interventions of interest.
- Positive predictive value
- The probability that a patient who has been categorised as ‘positive’ really is positive.
- Receiver operating characteristic plot
- A graph that plots the joint sensitivity and specificity of a diagnostic test at a range of cut-off thresholds.
- Reference standard
- A diagnostic test used to estimate the sensitivity and specificity of another diagnostic test, known as an index test. The reference standard is assumed to have perfect sensitivity and specificity and so, when both tests categorise something differently, the index test categorisation is assumed to be incorrect (either a false negative or a false positive).
- Reporting bias
- Reporting bias refers to systematic differences between reported and unreported findings. In any given study, analyses with statistically significant differences between intervention groups are more likely to be reported than analyses with non-significant differences. This is also known as outcome reporting bias or selective reporting bias. Reporting bias can also occur when results are reported in such a way that they cannot be included in a meta-analysis.
- Selection bias
- Systematic differences in the baseline characteristics of the intervention and control groups. Randomisation should result in study groups with similar baseline characteristics but this can be subverted if there is a lack of allocation concealment (preventing foreknowledge of forthcoming allocations).
- Sensitivity
- The proportion of ‘positives’ within a population undergoing diagnostic testing who are identified as such.
- Simple dominance
- When a given treatment alternative is less effective and more expensive than its comparator.
- Specificity
- The proportion of ‘negatives’ within a population undergoing diagnostic testing who are identified as such.
- Spirometry
- Lung function tests based on the measurement of exhaled air under controlled conditions using a device called a spirometer.
- Standardised mean difference
- A summary statistic showing the difference between two groups, calculated as the difference in mean outcomes between two groups divided by the standard deviation of scores for all study participants. This can be used to meta-analyse data for an outcome that has been measured using different metrics.
- True negative
- An individual who has been correctly categorised as a member of the category ‘negative’ in a binary categorisation exercise when the only other possible classification is ‘positive’, for example someone who has been correctly identified as not asthmatic.
- True positive
- An individual who has been correctly categorised as a member of the category ‘positive’ in a binary categorisation exercise when the only other possible classification is ‘negative’, for example someone who has been correctly diagnosed with asthma.
List of abbreviations
- ACQ
- Asthma Control Questionnaire
- ACT
- Asthma Control Test
- AQLQ
- Asthma Quality of Life Questionnaire
- AQLQ-M
- Asthma Quality of Life Questionnaire – Marks
- ATS
- American Thoracic Society
- AUC
- area under the curve
- BTS
- British Thoracic Society
- CCRCT
- Cochrane Central Register of Controlled Trials
- CDSR
- Cochrane Database of Systematic Reviews
- CE
- Conformité Européenne
- CEAC
- cost-effectiveness acceptability curve
- COPD
- chronic obstructive pulmonary disease
- CPCI-S
- Conference Proceedings Citation Index – Science
- CRD
- Centre for Reviews and Dissemination
- DARE
- Database of Abstracts of Reviews of Effects
- DSA
- deterministic sensitivity analysis
- EAG
- External Assessment Group
- EIB
- exercise-induced bronchoconstriction
- EQ-5D
- European Quality of Life-5 Dimensions
- FeNO
- fractional exhaled nitric oxide [also known as fraction of exhaled nitric oxide and exhaled nitric oxide (ENO)]
- FEV1
- forced expiratory volume in the first second
- FEV1%
- percentage of predicted forced expiratory volume in the first second
- FEV1/FVC
- forced expiry volume in the first second divided by forced vital capacity (Tiffeneau–Pinelli index)
- FN
- false negative
- FP
- false positive
- FVC
- forced vital capacity
- GINA
- Global Initiative for Asthma
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICS
- inhaled corticosteroid
- IQR
- interquartile range
- LABA
- long-acting beta2-agonist
- LTRA
- leukotriene receptor antagonist
- mAQLQ
- mini Asthma Quality of Life Questionnaire
- MAUDE
- Manufacturer and User Facility Device Experience
- MCT
- methacholine challenge test
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NPV
- negative predictive value
- OCS
- oral corticosteroid
- ONS
- Office for National Statistics
- PEF
- peak expiratory flow
- PEFR
- peak expiratory flow rate
- ppb
- parts per billion
- PPV
- positive predictive value
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies – second revision
- RCT
- randomised controlled trial
- RDI
- relative dose intensity
- RR
- relative risk
- SABA
- short-acting beta2-agonist
- SCIE
- Science Citation Index Expanded
- SCM
- specialist committee member
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SIGN
- Scottish Intercollegiate Guidelines Network
- TN
- true negative
- TP
- true positive
Note
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed academic-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of academic-in-confidence data removed and replaced by the statement ‘academic-in-confidence information removed’ is available on the NICE website: www.nice.org.uk. The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.