Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/145/01. The contractual start date was in March 2013. The draft report began editorial review in February 2015 and was accepted for publication in August 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dr Noble has declared he reports grants from LEO Pharma during the conduct of the study; personal fees from LEO Pharma, grants from Pfizer Inc., personal fees from Bristol-Myers Squibb and personal fees from Boehringer Ingelheim, outside the submitted work. Professor Fitzmaurice has declared he is on the Health Technology Assessment Commissioning Board and Professor Hood is on the Clinical Trials Unit Standing Advisory Committee. Dr Cohen reports personal fees from Cardiff University during the conduct of the study; personal fees from Boehringer Ingelheim, grants and personal fees from Daiichi Sankyo, grants and personal fees from Bristol-Myers Squibb, personal fees from Johnson & Johnson, grants and personal fees from Pfizer Inc., personal fees from Portola Pharmaceuticals Inc., personal fees from Sanofi, personal fees from XO1 Ltd, and personal fees from Janssen Pharmaceutical, outside the submitted work. Dr Johnson reports grants from the National Institute of Health Research during the conduct of the study, and other grants from Mayne Pharma outside of the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Noble et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background, rationale and objectives
Venous thromboembolism (VTE) is the formation of a blood clot (thrombus) in a vein, which may displace from its original site and form an embolus. Most thrombi occur in the deep veins of the legs and are known as deep-vein thrombosis. Symptoms vary from leg pain and swelling to chest pain and breathlessness, and sudden collapse and death owing to embolism in the lungs. A deep-vein thrombus (DVT) also causes long-term swelling and ulceration of the legs, known as post-thrombotic syndrome, in one-third of people. The total cost to the NHS of managing VTE is estimated at £640M per year. 1 VTE occurs in 1 in 1000 patients and annually affects 6.5 million people worldwide. The rate is higher in the cancer population; in the UK over 250,000 people per year are diagnosed with cancer, up to 18% of whom will develop VTE. 2 The standard treatment of VTE is well established, consisting of 5 days’ anticoagulation with low-molecular-weight heparin (LMWH), followed by 3–6 months of warfarin. 3 However, the management of cancer-associated thrombosis (CAT) presents several challenges with a higher rate of both re-thrombosis and bleeding among cancer patients compared with those with non-malignant disease. 4
The impact of VTE on the cancer patient is substantial, conferring a worse prognosis compared with similar-stage cancer patients without VTE. 5,6 Furthermore, anticoagulation with warfarin is complicated by drug–drug interactions, variable drug absorption and changing nutritional status. 7 This inevitably has a practical impact on the delivery of anticancer therapies. Maintaining stable coagulation with warfarin is difficult and requires more frequent monitoring with blood tests, which adversely affects patients’ quality of life (QoL). 8 Current evidence-based guidelines recommend 6 months’ LMWH as a first-line therapy for CAT because of its greater efficacy. 4,9,10 However, patients with ongoing cancer remain at risk of VTE recurrence beyond 6 months and may therefore benefit from indefinite anticoagulation therapy. To date, there is no evidence that this is appropriate or cost-effective.
Summary of current evidence
Evidence for the use of LMWH is summarised in Table 1. Based on these data, guidelines from professional organisations (which include the American College of Clinical Pharmacy, the American Society of Clinical Oncology, the British Society for Haematology and the European Society for Medical Oncology) recommend LMWH for the treatment of CAT. However, there are important gaps in the current evidence base. In patients with CAT and ongoing cancer, anticoagulation brings even more challenges than the treated general cancer population. As the cancer progresses, so does the thrombotic tendency owing to increased tumour burden, releasing procoagulants, reduced mobility and, in pelvic cancers, additional stasis owing to local vessel occlusion. 14 Consensus recommends consideration of continuing anticoagulation indefinitely in this patient group, although the evidence supporting this is limited to case series and has not been economically evaluated. 15 As the thrombotic tendency increases with disease progression, so does the risk of bleeding associated with anticoagulation,16 therefore the choice of anticoagulant for indefinite anticoagulation in CAT will need to carefully balance efficacy and safety.
| Study | Design | Warfarin : LMWH | |
|---|---|---|---|
| Bleeding | Recurrent VTE | ||
| Meyer et al., 200211 | Patients with cancer and VTE randomised to 3 months of treatment with either the LMWH enoxaparin sodium (Clexane®, Sanofi, Paris, France) (1 mg/kg) or warfarin | Major
|
Warfarin: 3/75 (4%) Enoxaparin sodium: 2/71 (2.8%) (calculated from combined endpoint minus bleeding) |
| Lee et al., 200312 (CLOT) | Patients with active cancer presenting with acute VTE randomised to receive either the LMWH dalteparin sodium (Fragmin®, Pfizer Ltd, New York, NYC, USA) (200 IU/kg for 1 month followed by 150 IU/kg for 5 months) or oral anticoagulant therapy |
|
Oral anticoagulation therapy: 53/336 (15.8%) Dalteparin sodium: 27/336 (8%) |
Any bleeding (p = 0.09)
|
|||
| Hull et al., 200613 (LITE) | Patients with acute VTE and cancer randomised to receive either unfractioned heparin followed by warfarin for 84 days at a targeted INR of 2.5, or the LMWH tinzaparin sodium (Innohep®, LEO Pharma, Hurley, UK) (175 IU/kg) for 85 days | At 3 months
|
At 3 months
|
The past 5 years has seen the introduction of new oral anticoagulants including direct Xa inhibitors [rivaroxaban (Xarelto®, Bayer AG, Leverkusen, Germany), apixaban (Eliquis®, Bristol-Myers Squibb, New York City, NY, USA)] and direct thrombin inhibitors [dabigatran etexilate (Pradaxa®, Boehringer Ingelheim, Ingelheim am Rhein, Germany)]. Both dabigatran etexilate and rivaroxaban have been evaluated in the treatment of DVT and pulmonary embolism (PE) showing non-inferiority to warfarin with respect to recurrent VTE and bleeding profile. 17,18 Furthermore, the use of dabigatran etexilate and rivaroxaban has been cautioned in high-risk groups such as cancer patients until studies with representative numbers of cancer patients are available. 19–21 However, only 5% and 6.8% of patients, respectively, had cancer, and these agents are yet to demonstrate non-inferiority in this setting to the current gold standard (LMWH). As advanced cancer patients are at particular risk of recurrent thrombosis and bleeding, the use of the new oral anticoagulants in this study would be hard to justify ethically without convincing safety and efficacy data in this patient group. Therefore, to ensure the safety of this patient group, we proposed to test the feasibility of extending the use of the gold standard treatment, LMWH.
Research objectives
The purpose of this study was to address a specific gap in the evidence base for the management of cancer-associated VTE in patients with ongoing malignant disease. To address this evidence gap, a sufficiently powered randomised controlled trial (RCT) was needed to gain information relating to the sample group, which entails a vulnerable adult population of uncertain number and prognosis, and with an uncertainty around willingness for recruitment or likely attrition. Therefore, a trial was conducted specifically to look at the feasibility of progression to a phase III RCT, the primary outcome of which would be to determine the proportion of recurrent, symptomatic VTE in cancer patients receiving an additional 6 months’ LMWH.
The overarching aims of this study were to:
-
Identify practicalities of conducting a full RCT with regard to recruitment, retention and outcome measurement.
This would ensure all flagging and recruitment processes were running effectively, identify the number of eligible patients that can be recruited in a 1-year time frame, identify the dropout rate and assess the practical utility of measuring primary outcome measures, reporting processes and assessment tools within the context of a full RCT. A scoping exercise was also conducted to identify the likely spread of potential recruitment environments and thus gauge the degree of support a full RCT would attract.
-
To explore the barriers to progressing to a full RCT.
It was felt that in the event of sufficient numbers of patients being eligible for inclusion in the study, the barriers to recruitment were most likely to be logistical or attitudinal. The logistical challenges, such as how and where to identify patients for recruitment, were therefore evaluated through the pragmatic conduct of the feasibility study and scoping exercise. The attitudinal barriers that needed exploring lay with the attitudes of clinicians and those of the CAT patients being invited to participate. Without the support of clinicians to recruit to a full RCT, it would be impossible to conduct the study. Since consensus recommendations advocate the continuation of LMWH anticoagulation beyond 6 months in patients with CAT and ongoing cancer, it was thought possible that clinicians would be unwilling to enter such patients into a trial because of the belief that current practice (despite lack of evidence) is correct. The views of patients who either do not consent to the study or who withdraw from the study post randomisation need to be explored in order to understand any reasons why they may be unwilling to take part. Although a qualitative study has suggested LMWH is an acceptable intervention in the treatment of CAT,8 this study only interviewed patients receiving LMWH for 4 weeks and their views may not reflect those of a patient who has been self-injecting for 6 months. In addition, it did not offer insight into whether or not these patients would be willing to self-inject for a further 6 months. The corollary is that patients may be reluctant to stop a drug that has been used to treat a previously experienced condition and would not consent to a trial if there was a chance the LMWH would be stopped.
Primary outcomes
-
Number of eligible patients over 12 months.
A screening log was kept in each recruitment site to identify patients potentially meeting the inclusion criteria. Eligible patients who were approached about the trial and given the participant information sheet (PIS) were registered on a central database. This would help to inform the design of a main trial.
-
Number of recruited patients over 12 months (target recruitment rate of 30% of eligible patients).
Patients meeting the inclusion criteria were invited to participate in the study as outlined. The number of eligible participants consenting to randomisation was recorded.
-
Proportion of randomised participants with recurrent VTEs during follow-up.
The number of randomised patients experiencing recurrent symptomatic VTE was recorded to inform the sample size required for a full RCT. VTE was objectively confirmed through radiological investigation. Deep-vein thrombosis was confirmed through Doppler ultrasonography or venography. PE was confirmed through computerised tomography pulmonary angiography.
Secondary outcomes
-
Completion of trial protocol.
This was assessed 6 months after randomisation to ascertain the attrition rate because of death during the study period or patient choice. Participants choosing to withdraw from either arm of the study protocol were invited to participate in a qualitative interview to explore the reasons for withdrawal.
-
Costs.
The feasibility study also aimed to identify key cost drivers to inform the design of a future definitive trial.
-
Quality of life.
Participants’ QoL was measured using the EORTC QLQ-C30 (European Organisation for Research and Treatment of Cancer quality-of-life questionnaire for assessing health-related quality of life of cancer patients, Version 3.0) and the European Quality of Life-5 Dimensions – 5 levels (EQ-5D-5L) questionnaire at 3-monthly intervals for 6 months. The EORTC QLQ-C30 has become a benchmark measure of QoL in cancer patients. It contains five functional scales (physical, role, cognitive, emotional and social); three symptom scales (pain, nausea/vomiting and fatigue), global health and QoL, and several other single items. The EQ-5D-5L is a short QoL tool, designed to complement other QoL measures and is recommended by the National Institute for Health and Care Excellence (NICE) for use in economic analyses.
-
Symptom assessment.
Symptoms were assessed using the Edmonton Symptom Assessment System revised version (ESAS-r) at 3-monthly intervals for 6 months. The ESAS-r is used to capture participants’ perspective on their symptoms, providing an indication of symptom severity of nine symptoms: pain, tiredness, drowsiness, nausea, lack of appetite, depression, anxiety, shortness of breath and wellbeing. 22,23 In addition, we looked for symptoms likely to be specifically due to VTE: new or worse leg swelling/pain, new or worse breathlessness and pleuritic chest pain.
-
Attitudes of clinicians and patients.
The qualitative components of the trial were undertaken to explore the attitudes of clinicians recruiting to the study and of patients invited to participate in the trial, including perceived benefits and burdens, and reasons for withdrawal from the trial.
Study design
This was a mixed-methods study involving the following four components: (1) RCT, (2) embedded qualitative study, (3) patient pathway survey and (4) patient and public involvement.
Randomised controlled trial
The RCT compared ongoing LMWH treatment for CAT with cessation of LMWH at 6 months’ treatment (current licensed practice) in patients with locally advanced or metastatic cancer. The initial plan was to recruit patients in two stages. Stage 1 would be considered complete when 62 patients had been registered. If at least 15 out of these 62 participants accepted randomisation, then recruitment in stage 2 of the trial would occur, until 200 patients had been registered in total.
Embedded qualitative study
The embedded qualitative study was undertaken to explore attitudes towards participating in the study, potential barriers to and concerns about participation and factors influencing compliance with self-injecting (where appropriate). Interviews were planned with:
-
patients who did not wish to continue with LMWH treatment
-
trial participants in the intervention arm
-
trial participants in the control arm
-
carers of trial participants
-
participants who withdrew from the study.
Patient pathway survey
Individual telephone interviews with clinicians from oncology and haematology departments were conducted to aid the development of a national survey tool about the management of CAT.
A UK-wide survey exercise was also undertaken to identify current CAT models and pathways of care.
Patient and public involvement
The study design was developed in close association with Thrombosis UK (formerly Lifeblood). Furthermore, we have worked in partnership with our patient representatives Mr Harold Toone and Mr John Bell. Mr Bell, having received anticoagulation for CAT, gave the trial management group valuable insight and direction throughout the design, execution and completion of the trial. We have further plans to share the data with patient partners through the Thrombosis UK patient days.
Participating site selection
This study had been planned to be carried out at three types of participating sites within the UK, namely primary care and within the acute setting (haematology and oncology).
It was believed that the key clinician who managed the long-term anticoagulation of CAT varied across the UK; thus, it was felt that sites providing the best opportunity for recruitment might vary across the UK. In order to assess the feasibility of recruiting sufficient numbers to inform a full RCT, two recruitment settings in the acute sector and a primary care network were chosen for evaluation:
-
Oncology outpatients would be recruited at two hospital sites in Wales: Velindre Cancer Centre, Velindre NHS Trust (catchment population 1.5 million) and Aneurin Bevan CAT Clinic, Aneurin Bevan Health Board (catchment population 639,000).
-
Haematology outpatients would be recruited at three hospital sites in England: Warwick Hospital, South Warwickshire NHS Foundation Trust, and George Eliot Hospital, George Eliot Hospital NHS Trust, both of which fall under the remit of the Arden Cancer Research Network (catchment population 1 million); and Worcestershire Royal Hospital, Worcestershire Acute Hospitals NHS Trust.
-
Primary care research networks: Primary Care Research Network for Central England and The Midland Research Practices Consortium, using approximately 15 practices recruited from the following Clinical Commissioning Groups (CCGs) each in:
-
NHS Birmingham South Central CCG (catchment population 383,000).
-
NHS Coventry and Rugby CCG, NHS Warwickshire North CCG and NHS South Warwickshire CCG, all of which fall under the remit of the Arden Cluster (Coventry and Warwickshire) (catchment population 914,008).
-
Oxfordshire CCG (catchment population 689,500).
-
Chapter 2 The ALICAT randomised controlled trial
Introduction
This section of the report focuses on the RCT component of the ALICAT (Anticoagulation with Low-molecular-weight heparin In the treatment of Cancer-Associated Thrombosis) study. As recorded in Chapter 3, Background and rationale, only the oncology sites were opened for recruitment within the study time frame. As such, the methods will focus on the recruitment of patients within this setting alone. It will focus on the process whereby potential patients were identified for recruitment and record the number of patients who consented to participation.
Methods
Within the oncology sites the management of CAT was done exclusively through two dedicated CAT clinics. Potential patients were therefore identified at the point of referral to the clinic. Potential participants were screened by a National Institute of Health and Social Care Research (NISCHR) researcher and flagged up to the local principal investigator.
Patients meeting all the inclusion criteria and none of the exclusion criteria (listed below) were approached after 5 months of anticoagulation therapy and given information pertaining to the ALICAT trial.
Main inclusion criteria
-
Receiving LMWH for treatment of CAT for approximately 5 months.
-
Locally advanced or metastatic cancer.
-
Able to self-administer LMWH or have LMWH administered by a carer.
-
Able to give informed consent.
-
Aged ≥ 16 years.
Main exclusion criteria
-
Receiving a drug other than LMWH for CAT.
-
Contraindication to continuing anticoagulation therapy.
-
Confirmed recurrent VTE while receiving anticoagulation therapy.
-
Fitted with a prosthetic heart valve.
-
Pregnant and/or lactating female.
Eligible patients were approached by the investigator and invited to take part in the ALICAT trial when approaching the completion of their first 5 months’ treatment with LMWH for CAT.
Registration
Patients were posted the ALICAT trial PIS and consent form which explained the rationale behind randomisation and what would happen if they decided to take part in the trial, with their 5-month clinical appointment.
Prior to obtaining participant consent, the clinical trials nurse telephoned the trials unit to register the patient that had been approached and given the ALICAT trial PIS and consent form. A unique patient registration number was provided, but no patient information or identifiers were collected at this stage.
Randomisation
Randomisation was performed centrally at the trial centre once the participants had signed the consent form. Patients were randomised using block randomisation with varying block sizes. Randomisation used a 1 : 1 allocation ratio. At randomisation, the participants were given a unique participant trial number and the treatment allocation.
Participants randomised were given a participant diary card to record their trial medication (prescriptions and LMWH injections), concomitant medication, symptoms and use of health-care facilities. Participants randomised to the control arm of the trial were also given a participant diary card, but only needed to complete the symptom and use of health-care facilities sections.
Trial treatments
Currently, dalteparin is the only LMWH licensed specifically for the long-term treatment of CAT. However, enoxaparin and tinzaparin are still used in some practices for the treatment of CAT, and these three LMWHs were the trial investigational medicinal products in the ALICAT study.
As this was a pragmatic feasibility study, participating clinicians’ treatment practices did not alter. The type of LMWH used was recorded. Switching from one protocol investigational medicinal product to another protocol investigational medicinal product during the trial treatment period was allowed, if deemed necessary by the treating clinician.
Trial arm A: continue low-molecular-weight heparin
Participants randomised to trial arm A would have already received LMWH at treatment dose for 6 months and then continued the same drug at the same dose for a further 6 months.
Trial arm B: discontinue low-molecular-weight heparin
Participants randomised to arm B stopped LMWH treatment once a total of 6 months of the drug had been administered from the initial diagnosis of VTE.
Blinding
The ALICAT trial was open, i.e. unblinded.
Scheduling
The LMWH was given as a daily subcutaneous dose at the same time as the previously administered LMWH over the prior 6 months.
Measures of compliance/adherence
Compliance was monitored through review of participant diary cards during trial visits and as part of the qualitative interview study. Information from diary cards was transferred to the treatment case report form (CRF) by the research nurse.
Assessments at baseline
-
Confirmation of consent.
-
Disease history: diagnosis details of primary tumour.
-
Cancer treatment history: details of surgery, radiotherapy and/or chemotherapy.
-
History of index VTE: site of VTE and date diagnosed.
-
VTE treatment history: type of LMWH, dosage, details of whether LMWH is self-administered or carer-administered and details of other VTE treatments, for example a stent.
-
Physical assessment: Eastern Cooperative Oncology Group (ECOG) performance status and weight.
-
Current disease status: details of local recurrence, nodal and metastatic disease sites.
-
Haematology: white blood count, haemoglobin, absolute neutrophil count and platelets.
-
Urea and electrolytes: urea, sodium, potassium and creatinine.
-
Liver function test: albumin, alanine aminotransferase/aspartate aminotransferase, alkaline phosphatase and total bilirubin.
-
Bone profile: calcium and phosphate.
-
Comorbidities (baseline toxicity): any pre-existing conditions and current Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 grade.
-
Concomitant medication: all non-LMWH medication was recorded.
-
Use of NHS resources in previous 3 months [hospital admissions, general practitioner (GP) visits, nurse visits, etc.]. Details were taken from the patient diary booklet and patient notes.
-
Quality-of-life questionnaires:
-
EORTC QLQ-C30
-
EQ-5D-5L
-
ESAS-r.
-
Assessments during study
When arranging trial visits, the research nurse requested that the participant bring their diary booklet along to the clinic. Patients returned for two scheduled trial visits at week 12 and 26, but if the patient experienced a serious adverse event (SAE), a bleeding event or a recurrent VTE event, these were reported at the time until 30 days after the completion of the ALICAT trial.
Assessments undertaken at week 12
-
VTE treatment and compliance: current dose of LMWH, details of missed doses and dose modifications were taken from patient diary booklet.
-
Physical assessment: performance status ECOG, weight.
-
Haematology:* see Assessments at baseline.
-
Urea and electrolytes:* see Assessments at baseline.
-
Liver function test:* see Assessments at baseline.
-
Bone profile:* see Assessments at baseline.
-
Toxicities: all side effects and adverse events were recorded on the CRF.
-
Concomitant medications: all non-LMWH medication was recorded.
-
Use of NHS resources: see Assessments at baseline.
-
Quality-of-life questionnaires:
-
EORTC QLQ-C30
-
EQ5D-5L
-
ESAS-r.
-
*No extra blood tests were required for the study; the latest routine blood test results were used.
End of treatment assessments at week 26
-
VTE treatment and compliance: see Assessments undertaken at week 12.
-
Physical assessment: see Assessments undertaken at week 12.
-
Haematology:* see Assessments at baseline.
-
Urea and electrolytes:* see Assessments at baseline.
-
Liver function test:* see Assessments at baseline.
-
Bone profile:* see Assessments at baseline.
-
Toxicities: see Assessments undertaken at week 12.
-
Concomitant medications: see Assessments undertaken at week 12.
-
Use of NHS resources: see Assessments at baseline.
-
Quality-of-life questionnaires:
-
EORTC QLQ-C30
-
EQ5D-5L
-
ESAS-r.
-
*No extra blood tests were required for the study; the latest routine blood test results were used.
Sample size calculation
Anticipating at least 200 eligible patients per year in total from all three recruitment settings, we assumed that not all patients who have been injecting LMWH for 5 months would agree to continue for a further 6 months. We considered that at least 30% would need to agree in order to make a phase III RCT worthwhile. Therefore, the ALICAT study aimed to assess the feasibility of randomisation by determining if at least 30% of potential patients would agree to randomisation. The precision of this proportion would then be calculated with a 95% confidence interval. The design of the phase III RCT would also be informed by estimating the proportion of patients who experienced recurrent VTEs during follow-up.
If less than 15% of eligible patients agreed to take part in the trial, a phase II RCT may not be suitable since it would not be possible to recruit suitable numbers, given that a larger phase III RCT could have more stringent eligibility criteria as a result of this feasibility study.
If at least 30% agreed to take part, then the larger RCT would be considered feasible. Using a Fleming’s single-stage design, setting p1 to 0.15 and p2 to 0.3, and with 5% significance and 90% power, then 62 eligible participants would need to be approached with details of the trial. This design required that at least 15 out of 62 participants consented to the trial. A two-stage sample size was therefore agreed if at least 15 out of the first 62 participants recruited to the trial accepted randomisation, then recruitment would continue into stage 2. If fewer than 15 patients agreed, randomisation within this population would be considered not feasible.
By expecting a maximum of 200 eligible patients, it would then be considered possible to produce a 95% confidence interval for the percentage willing to be randomised with a width of 13.7% or less. For example, if the percentage is 50%, then one could expect the 95% confidence interval to be 43.15% to 56.85%. If the percentage is 30%, a slightly smaller confidence interval of 23.7% to 36.3% would be expected. If only 150 patients were deemed eligible, then one would expect to calculate a 95% confidence interval for 50% randomised of 42.1% to 57.9%.
The target registration number for this feasibility trial was therefore 200 patients, with hope that at least 60 patients would be randomised into the two groups. Thirty patients per arm would provide enough power to create a 95% confidence interval around the risk of VTE recurrence, which would have a width of 34% or less. As an example, if it was found that the risk of VTE occurrence was 50% in the arm that stopped, one would be able to estimate a 95% confidence interval of approximately 33% to 67% or smaller. If the risk of VTE were 8%, the 95% confidence interval would be 0% to 19%.
Analysis
All analyses were to be performed on a full intention-to-treat basis (all patients randomised will be included) and all patients analysed according to their allocated group, whichever treatment they received. The percentage of registered patients who were randomised would be calculated, with 95% confidence intervals. Similarly, the percentage of patients experiencing VTE recurrence and bleeding events in both groups would be calculated. The percentage of patients who died within 6 months of randomisation would be calculated in both arms, along with the percentage of patients who successfully completed 6 months of trial treatment in the LMWH group. The median QLQ-C30 raw and linear transformed functional scales would be calculated and presented for both arms of the study. No formal subgroup analyses were planned. However, if any treatment effect was found one would investigate whether or not it is consistent across participant subgroups (defined by all pre-treatment factors collected), although this analysis would be exploratory in nature. Exploratory analyses may be conducted to aid hypothesis generation if a phase III RCT is subsequently developed.
Results
Set-up and site opening
Despite the completion to time of the protocol and associated consent forms/CRFs etc., the set-up of the study was impaired considerably by several procedural delays. The overarching ALICAT study was a mixed-methods study involving three different clinical environments spread across 10 trusts, health boards and CCGs, and involving 20 recruitment sites. As such, the contractual processes were more complex than anticipated and further complicated by these processes coinciding with the reconfiguration of primary care trusts to CCGs. Furthermore, it became apparent that having gained the necessary universal approvals within Wales under the auspices of the NISCHR, further approvals were required to meet the approvals within the National Institute for Health Research (NIHR).
Following discussion with the funders, it was agreed that recruitment efforts should focus on the oncology and haematology sites. At this point it was also agreed that a ‘drop dead’ target to recruit 15 out of 62 eligible patients by the end of July (a total of 6 months’ recruitment) should be met.
Recruitment to randomised controlled trial
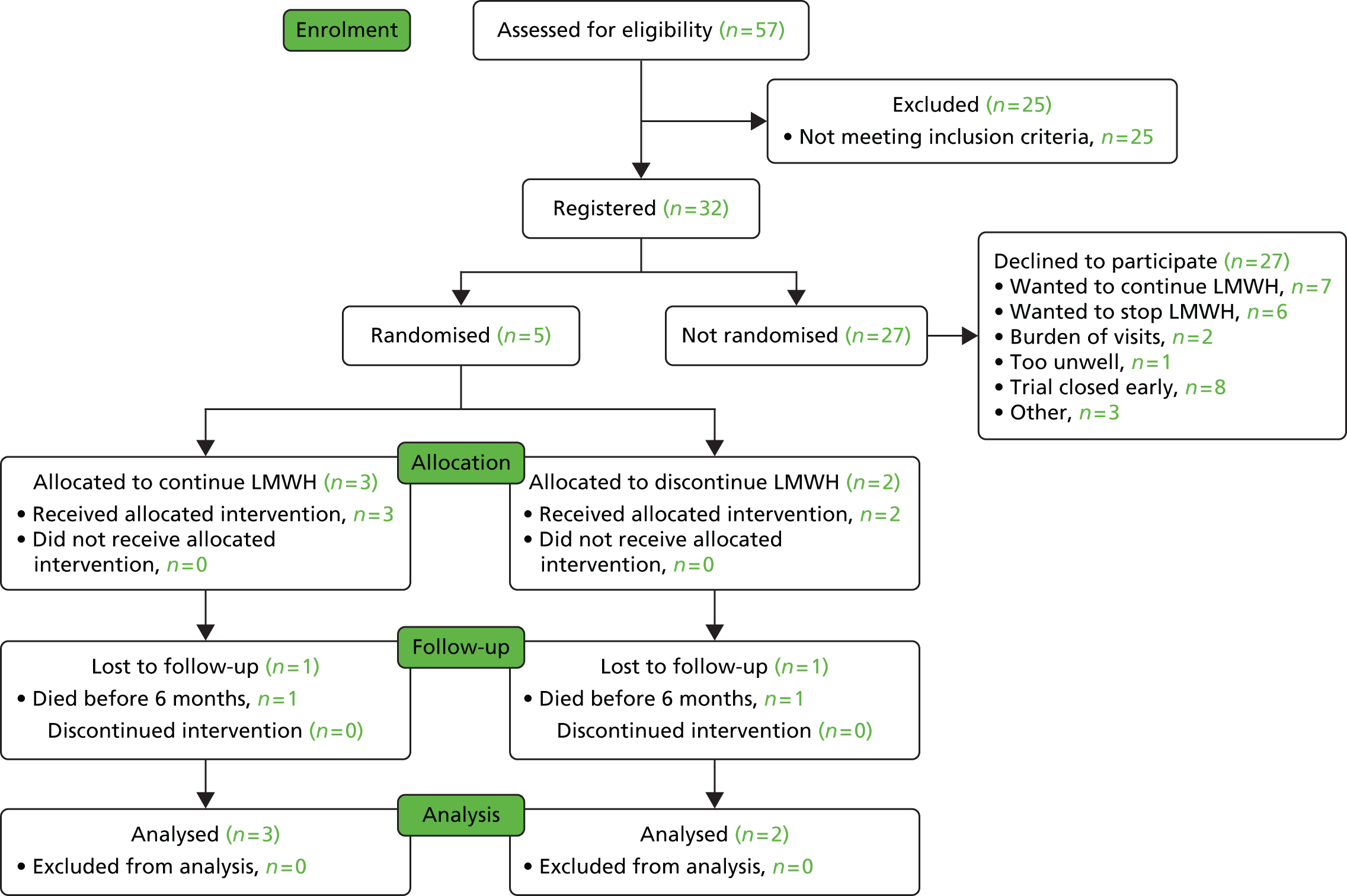
The delays in setting up the study led to the oncology sites opening 6 months late into the 12-month recruitment window and the first haematology site opening just before the recruitment window closed. The oncology sites were open for recruitment for 6 months between December 2013 and June 2014. During this time 57 patients were screened for eligibility, of which 26 did not meet the inclusion criteria. Of the remaining 32 patients, 27 declined participation in the RCT and five consented. These are detailed in Figures 1 and 2 and Table 2.
FIGURE 1.
Participant accrual.
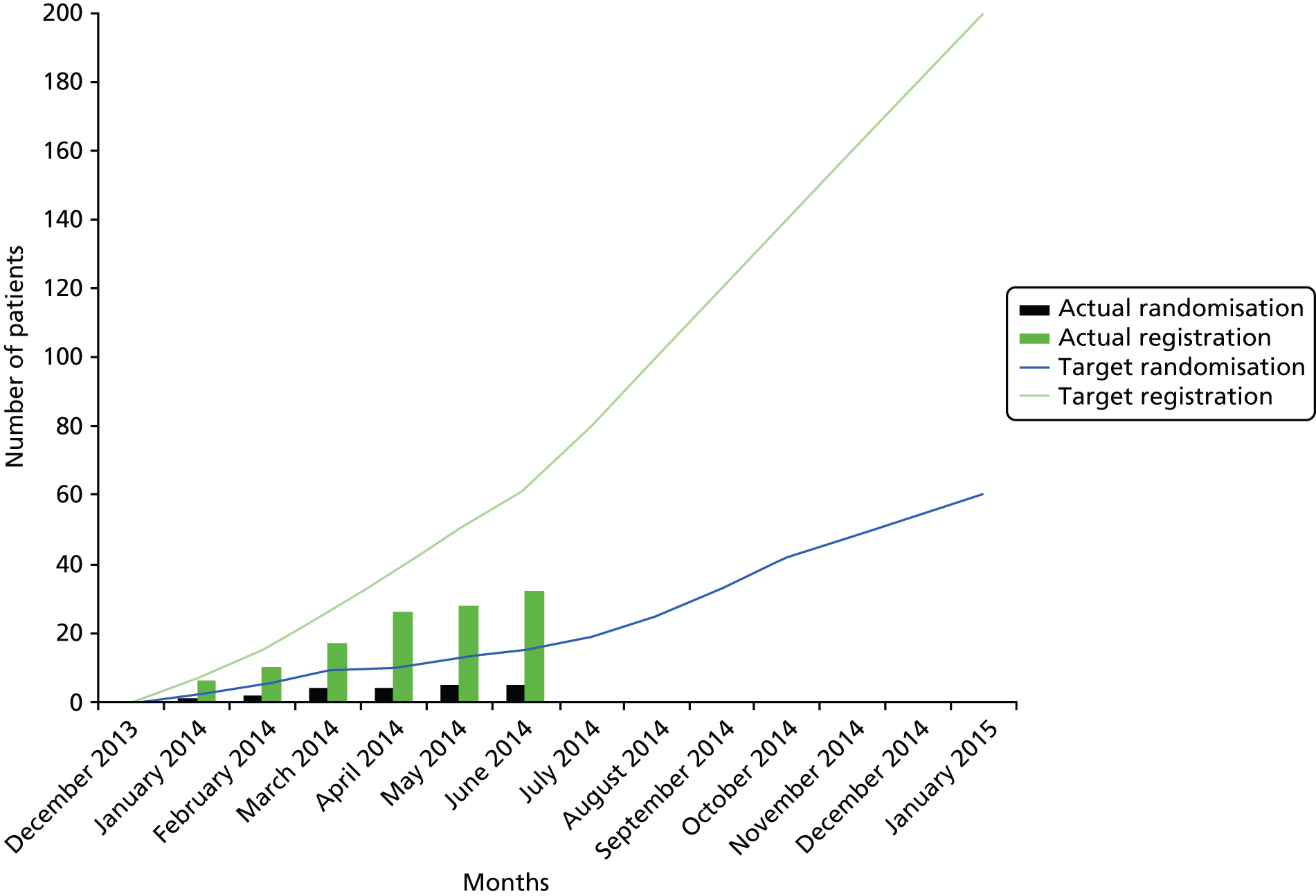
FIGURE 2.
The Consolidated Standards of Reporting Trials (CONSORT) diagram of recruitment.
| Recruitment centre | Registered | Randomised | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| December 2013 | January 2014 | February 2014 | March 2014 | April 2014 | May 2014 | June 2014 | Total | December 2013 | January 2014 | February 2014 | March 2014 | April 2014 | May 2014 | Total | |
| Velindre Cancer Centre – Velindre NHS Trust | 1 | 4 | 2 | 5 | 5 | 2 | 4 | 23 | 0 | 1 | 0 | 2 | 0 | 0 | 3 |
| Royal Gwent Hospital – Aneurin Bevan Health board, Wales, UK | 0 | 1 | 2 | 2 | 4 | 0 | 0 | 9 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| George Eliot Hospital – George Eliot Hospital NHS Trust | 0 | 0 | |||||||||||||
| Warwick Hospital – South Warwickshire NHS Foundation Trust | 0 | 0 | |||||||||||||
| Alexandra Hospital – Worcester Acute Hospitals NHS Trust | 0 | 0 | |||||||||||||
| 15 primary care GP practices | 0 | 0 | |||||||||||||
| Total | 32 | 5 | |||||||||||||
The initial aim had been to consent 15 of 62 (1 in 4.1) registered patients. Even taking into consideration slow opening of sites, the proportion of consenters was still too small (1 in 5.4) to project that keeping the study open longer would be possible to consent an adequate number of patients.
Overall feasibility
The results show that it is not feasible to conduct a phase III trial to investigate the optimum length of time required to treat VTE patients with anticoagulation therapy who have metastatic or locally advanced cancer.
Discussion
From this section of the study it is clear that a full RCT was not feasible. It could be argued that if it was possible to overcome the process-related delays, a higher rate of recruitment may be possible. Certainly, one cannot conclude that a study is impossible to recruit to by solely looking at recruitment figures; no patients were recruited or registered in the planned first 6 months of recruitment because no sites had opened by then. Arguably, the study could have been allowed to run for longer, especially since there was no opportunity to evaluate whether or not the haematology sites may prove a more fruitful recruitment environment. However, it is of relevance to note that recruitment to the oncology sites were led by the chief investigator for the study and, as such, the recruitment in these sites possibly over-represents the feasibility of recruitment. This is because the chief investigator will be more motivated to see the study succeed than the average site principal investigator. If it is not possible to recruit sufficient participants through the chief investigator’s sites, it is unlikely that other sites will do any better.
One lesson learned is to allow as much time as possible for set-up, particularly for complex mixed-methods studies.
Although the RCT component of the study demonstrated that it was not feasible to progress to a Phase III study, it did not conclusively indicate why we could not recruit the necessary numbers. This is explained, in part, in the next phase of the trial.
Chapter 3 Embedded qualitative study
This chapter of the report outlines an embedded qualitative study within a RCT. The methods will be presented, followed by a comprehensive overview of the findings. Finally, these findings will be placed in context and recommendations for practice and further research will be made.
Background and rationale
Venous thromboembolism is associated with reduced QoL in the general population,24,25 but this has not been formerly assessed through validated measures in patients with the additional burden of cancer. However, qualitative studies have explored patients’ experiences of the condition. A recent study in Wales highlighted the traumatic experience of CAT, including profound shock at diagnosis, distressing symptoms that impacted on everyday living and fear of prognosis. 26 Furthermore, a Canadian study identified that patients with CAT experienced frightening symptoms, which they recognised as potentially life-threatening and requiring urgent treatment. 27 Patients perceived CAT as an additional burden to their cancer and associated treatments, and while some felt the VTE was insignificant compared with their cancer diagnosis, others viewed it as a setback in their cancer journeys. 27
Treatment of VTE involves 5 days’ anticoagulation therapy with LMWH and subsequently 3–6 months’ warfarin. 2,3 However, patients with cancer experience higher rates of further thrombotic and haemorrhagic events. 4 Three RCTs have demonstrated the superiority of LMWH over warfarin for treating VTE in patients with cancer11–13 and clinical guidelines thus recommend 6 months’ weight-adjusted LMWH following CAT. 4,9,10,28 Although patients have discussed the discomfort of injecting LMWH, they also report this an acceptable trade-off to prevent a further thrombotic event. 26,27 Interestingly, patients have reported preference of LMWH over warfarin because of the regular blood tests required to manage the latter therapy. 8,24
Management of CAT beyond 6 months is more challenging. Patients with active cancer are at increased risk of further thrombotic events owing to disease progression, reduced performance status and chemotherapy,14,29,30 but conversely are also at increased risk of haemorrhage. There is thus the need to establish whether or not anticoagulation therapy should be continued beyond 6 months and, if so, for how long.
Methods
In order to explore clinicians’ attitudes towards the trial and patients’ and their relatives’ experiences of the trial, an embedded qualitative study was undertaken as part of the larger study. The embedded qualitative study included individual/group interviews, focus groups with clinicians and semistructured interviews with patients and their relatives.
Sampling strategy
Inclusion criteria
Patient interviews
-
A patient eligible to take part in the ALICAT RCT (RCT inclusion/exclusion criteria detailed in Appendix 1) who agreed for their details to be passed to the ALICAT study qualitative researcher.
-
Capacity to give informed consent.
-
Ability to communicate in English.
Relative interviews
-
A relative of a patient enrolled on the ALICAT trial, in either the intervention or control arm.
-
Capacity to give informed consent.
-
Ability to communicate in English.
Clinician focus groups
-
An oncologist, haematologist or clinician working in primary care (GP or practice nurse) attending one of the identified clinical conferences where we had planned to recruit.
-
Capacity to give informed consent.
-
Ability to communicate in English.
Recruitment and sampling
Patient and relative interviews
We had anticipated interviewing:
-
patients from the control arm (n = 10–15)
-
patients from the intervention arm (n = 10–15)
-
patients who withdrew from the RCT (n = 10–15)
-
patients who declined randomisation to the RCT (n = 10–15)
-
relatives of patients in the intervention or control arms (n = 10–15).
However, because of low recruitment and the sites closing early, fewer than anticipated patients and relatives were recruited.
A convenience sample of participants was planned from the RCT, a common sampling strategy for embedded qualitative studies within trials. 31 However, since there were only five eligible patients, all of them were approached. All eligible patients were approached by research nurses at the recruiting site. The research nurses provided the qualitative researcher with the contact details of patients who agreed to take part in an interview. The qualitative researcher contacted participants to discuss the interview study and, if they agreed, arranged a convenient time and location for the interview. LMWH is a home-based treatment and a proportion of relatives assumed a caring role in administering the medication. Relatives were therefore recruited by patients taking part in the RCT, enabling patients to have control about who they included. 32 The participants are displayed in Table 3.
| Recruitment site | Participants who declined randomisation, interviews completed (n) | Participants who declined to be interviewed or unable to contact (n) | Participants randomised, interviewed (n) | Participants randomised, agreed but unable to interview (n) | Relatives, interviewed (n) |
|---|---|---|---|---|---|
| Oncology site 1 | 5 | 2 | 2 (both intervention arm) | 1 (control arm, participant died) | 1 |
| Oncology site 2 | 3 | 2 | 0 | 0 | 0 |
| Total | 8 | 4 | 2 | 1 | 1 |
Focus groups
We had hoped to recruit 6–10 clinicians per focus group to enable a variety of perspectives while also ensuring that participants have the opportunity to take part in a dynamic discussion. 33 Furthermore, we had hoped to undertake two focus groups per clinical setting. However, recruitment to the focus groups was challenging and involved a considerable amount of organisation and resources.
A volunteer sample of clinicians from each clinical setting were recruited to the focus groups through advertising the group in the conference literature and at the venue, e-mails sent via the conference organisers and via social networking, with clinicians encouraged to contact the researchers or attend on the day. Overall, we conducted three focus groups (and one pilot interview), with between 3 and 11 clinicians per focus group. The participants are displayed in Table 4.
| Recruitment site | Number of participants | Number of focus groups |
|---|---|---|
| Primary care | 5 | 2 (one participant in first focus group) |
| GPs (n = 2) | ||
| Practice nurses (n = 3) | ||
| Oncology | 11 (all oncologists) | 1 |
| Haematology | 3 (all haematologists) | 1 |
| Total | 19 | 4 |
Semistructured interviews
This component of the study explored patients’ and their relatives’ attitudes towards participating in the RCT, and the most appropriate method for exploring these issues was semistructured interviews. 34
The interviews all took place in the person’s home for their convenience, and for the researcher to be able to speak to the person in their normal environment. 35 The qualitative researcher took written informed consent immediately prior to the interview, where appropriate. Interviews lasted up to 40 minutes. Participants who declined randomisation were interviewed as soon as possible after they had been approached to take part in the RCT. Participants who took part in the RCT, and the one relative, were interviewed following the study period.
The qualitative interviews followed a topic guide, but also allowed participants the flexibility to discuss issues important to them. 36 The topic guide was developed by three members of the research team (JB, AN and SN), incorporating the research objectives, previous clinical experience and previous CAT research experience. Patients who declined consent to the RCT were interviewed to explore their understanding of trial processes, their experiences of the first 5 months of LMWH treatment off-trial and reasons for non-consent. Patients who took part in the intervention or control arms of the trial were interviewed to explore their reasons for, and experiences of, participating in the trial; their views and attitudes towards equipoise; and the acceptability of LMWH treatment. The relative of a patient who took part in the RCT was interviewed to explore their experiences of caring for someone taking part in the ALICAT trial.
Focus groups
Focus groups were conducted with clinicians from oncology, haematology and primary care settings. For the ease of participants and to not limit the sample to clinicians working in one geographical area,37 focus groups were held at national meetings and educational events. This has previously been undertaken with clinicians who would otherwise be challenging to recruit to individual interviews of focus groups in other settings. 38 However, as stated in Focus groups, this approach proved challenging in reality.
The focus groups were facilitated by two experienced researchers, with one researcher taking the lead and the other ensuring that all participants had the opportunity to participate. It can be challenging to encourage participants to discuss issues relevant to them while ensuring that the research objectives are addressed33 and, therefore, a topic guide was developed addressing:
-
participants’ attitudes to recruiting to the study in terms of recruitment, equipoise, acceptability of intervention and outcome measures
-
participants’ experiences of, and attitudes to, prescribing LMWH, including whether or not they would extend treatment past 6 months.
The topic guide was developed by four members of the research team (JB, AN, SN and MJB), utilising the study objectives, clinical experience and previous CAT research experience. However, the researchers also encouraged participants to explore issues pertinent to them.
Data management and transcription
The focus groups and interviews were digitally audio-recorded and were transcribed in full and verbatim. Transcripts were anonymised and subsequently uploaded onto NVivo version 10 (QSR International, Warrington, UK) qualitative software for data storage and coding.
Data analysis
Data were analysed using the framework approach, an adaptable technique originally developed for use in applied policy research with clear objectives. 39 It is a methodical approach that follows a clear and documented process but, as with other qualitative approaches, it relies on the researcher to determine the quality of the analysis. 39 Framework analysis was carried out in line with Ritchie and Spencer’s39 five interconnected steps described in Table 5,39 using NVivo 10 to develop and manage the matrix.
| Title of step | Description |
|---|---|
| Familiarisation | The researcher immersed themselves in the data by relistening to interview recordings and rereading transcripts and field notes. The researcher documented central ideas and recurring themes |
| Identifying a thematic framework | An index of themes was created, informed by the original research aims around understanding recruitment and retention, but also by issues raised by the participants in the data |
| Indexing the data | The index was applied to each transcript by coding them with the themes from the thematic framework. During this process, the framework was adjusted, adding new themes and subthemes as they emerge. The adjusted framework was then applied to subsequent transcripts and reapplied to existing transcripts to ensure that all data was appropriately coded |
| Charting | A matrix was created of themes and participants. Data were lifted from the transcripts and arranged according to thematic references. The data were summarised by the researcher, rather than verbatim quotes included, and referenced back to the original data |
| Mapping and interpretation | Charts and research notes were reviewed to compare and contrast the perceptions and experiences of participants |
Trustworthiness
Trustworthiness in the research process and findings were promoted through interviewing patients, relatives and health-care professionals, enabling multiple perspectives of the ALICAT trial and CAT to be considered and compared. 40 The interviews and focus groups were reviewed by at least one other member of the research team, and the coding framework, developed by the qualitative researcher responsible for data generation, was tested by another researcher, promoting trustworthiness in the researcher’s interpretation of the data. The analysis process was also overseen by the qualitative research advisor.
Results: patients’ perspectives
Participants
Eight patients who declined randomisation to the ALICAT RCT were recruited to the embedded qualitative study. Four women and four men participated from the two oncology centres (OS1 and OS2), living varying distances from the centres in cities, towns and one village. Participants reported a number of cancer diagnoses including lung, bowel and breast, with two patients not disclosing this information. Four participants had experienced a PE, while two had DVTs in their legs, one had a DVT in her upper arm and one had a VTE in his peripherally inserted central catheter.
Overview of themes
In line with the framework approach, the coding framework was developed with reference to the protocol and interview schedules. Two overarching themes were identified from the data:
-
perceptions of the ALICAT study
-
perceptions of CAT and anticoagulation.
Each theme, with associated subthemes, is discussed in turn below.
The following abbreviations are used throughout the data extracts:
-
I: interviewer
-
NC: participant who did not consent to the RCT
-
P: participant.
Information about the ALICAT randomised controlled trial
Participants demonstrated various degrees of understanding of the RCT and what it entailed, but all were clear about why they wished not to participate.
Verbal information
Participants were all informed about the RCT when they attended the CAT clinic and the study was explained verbally to them by the consultant. The amount of information provided by the clinical team appeared to vary according to the participant, with some individuals reporting minimal information being delivered:
What were you told about it [the RCT]?
Um, not a lot really . . . I was really a bit confused.
Interview NC1
Did he tell you much about the, sort of, what would happen if you took part in the trial? What you would have to do?
No because I had already said I think that I was going to carry on as I was doing it, so there was no real need for him to go on.
Interview NC2
Other participants explained that the study was thoroughly explored, including trial processes and the relevance of the study:
What were you told about the actual trial? About ALICAT?
Um, he well he explained that all of the, you know, um that that it would be a randomised survey and that um fifty per cent of those who went into it would continue with the injections, fifty per cent wouldn’t, um and they would be monitored for a period of time see what, you know, whether there was any benefit in continuing . . . yeah, but pretty much that really um I mean he did explain a lot more.
Interview NC8
Written information
In addition to verbal information, seven of the eight patients interviewed explained that they had received a copy of the ALICAT study PIS. However, one participant reported that he did not:
Were you given sort of written information about it?
About the trial
The clinical trial
No no
OK
No all I had was the information that you gave me today.
Interview NC1
Other participants, who received the PIS for the RCT, discussed taking it home to read and consider the content:
Were you given any written information about the clinical trial?
Yes, there was something I read when I got home
OK
A few pages, I can’t remember what I did with it now but I read through it, it explained everything.
Interview NC2
One of his [CAT consultant] um assistants came and gave me a little chat and gave me a spreadsheet with information on just after that
OK
For me to take home and have a read and sort of digest it in my own time.
Interview NC4
Participants also reported using the PIS as an aide memoire to raise questions with the consultant and ultimately used the information to make their decision about taking part in the RCT:
I was given the written information about the um about the trial um and then he said, you know, ‘have a read of this and then when you come back next time we’ll have a chat as to whether you want to go on the trial or not’
OK
So that was when I went back last time and he said, you know, ‘do you mind’ you know ‘deciding what you want to do’ and I made my decision
Mm
I think based on what I read
Right, on the information sheet?
On the information sheet.
Interview NC6
Participants thus reported the usefulness of the study PIS to help them make their decision, in addition to verbal information about the study, reiterating the importance of ensuring all participants receive both methods of information communication.
Timing
Participants were eligible to take part in the clinical trial after they had injected LMWH for 6 months. However, the CAT clinic attended by all participants was a relatively new service. Therefore, most participants were told about the RCT before they reached the 6-month mark, often during their first or second appointment:
At what point did he [palliative care consultant] mention that there was this clinical trial that you might want to take part in?
Er I think it was the second time I saw him, he mentioned there was a there was a study going on . . . and I did I said then that er I’d consider er going on the study.
Interview NC3
At what point did he [palliative care consultant] tell you about the about ALICAT? The actual clinical trial?
. . . I can’t remember whether it was my first appointment or my second appointment, it might well have been the first appointment he mentioned it, but then he mention I think he mentioned it in more detail on the second appointment and said that when I got to 6 months I’d have to make a decision about whether I was going to continue with injections or not, and whether I was going to opt into the trial or no, so I said I’d talk to him in more detail when you get to the 6 months.
Interview NC6
Overall, participants reported having sufficient time to consider the ALICAT RCT, taking home written information about it to discuss with their families before making their decision:
One of his um assistants came and gave me a little chat and gave me a spreadsheet with information on
OK
Just after that for me to take home and have a read and sort of digest it in my own time
Yeah
There was no pressure or anything in fairness – it was up to me – I had a number of weeks to go away and have a look at it.
Interview NC4
Did you did you feel you had enough time to make that decision and have a good think about it?
Oh yes I wasn’t rushed at all I did it in in my own time, because even after I’d discussed it with the family, I think I left it another week where I was still mulling it over myself in case I thought I sort of thought differently after that time
Interview NC3
Having been prepared for the study and given written information about it, participants then returned to clinic to tell the consultant their decision:
He must have spoken to me about it before because I remember reading all the stuff about it and then telling him [my decision]
You went back to clinic
Yeah.
Interview NC8
However, despite taking home written information and returning to clinic at a later date, one participant described having already made her decision:
OK, and so when you were told about the trial and you obviously brought some information home, how long did you have to decide if you wanted to take part or not?
Well I’d already decided really.
Interview NC2
Finally, one participant (NC1) decided not to take part in the ALICAT RCT during his last appointment at the CAT clinic, when he was told about ALICAT for the first time and chose not to participate. This participant was, however, the exception and all other participants were given information and time to consider the study before they decided not to be randomised.
Participants’ reasons for declining randomisation to the ALICAT RCT are discussed next.
Reasons for declining randomisation
Participants all had clear reasons for deciding not to participate in the ALICAT RCT. The overwhelming reason was participants’ fear of experiencing a further VTE if they were to stop treatment, but other reasons included their dislike of injecting LMWH, not feeling well enough to participate in a RCT and wanting to try without treatment.
Risk and fear of further venous thromboembolism
The dominant reason, cited by five participants, for not wanting to participate in the RCT was their fear that if they ceased LMWH they would experience a further VTE. The emotional and physical pain caused by the event, and their realisation that a VTE could be fatal, motivated participants to carry on injecting LMWH beyond 6 months.
Participants perceived that their risk of a further VTE, or their VTE still being prevalent, was too great if they joined the RCT and ceased LMWH:
I didn’t want to take the chance
Take the chance, OK
That eh I thought there would be quite a good possibility that it would come back if I stopped.
Interview NC2
I said to him ‘look there’s no reason to suspect my blood clot has gone completely or that it won’t come back again because of the reason that it was there in the first place’ . . . the fact that my tumour’s still pressing on that same vein
Yeah
Suggests to me that there’s still that high risk that there’s a clot there.
Interview NC6
The post-operative thing was to have these needles, so I did for about 29 days . . . I stopped after the 29 days I stopped doing it and then a little bit later on, though I can’t quite remember the exact timing of it, er the um the blood clot occurred, so obviously the needles must have been doing me some good, you see, so then of course I thought ‘well if I stop taking them if I stop it it might come back again’ so you can see the logic behind what I’m thinking of.
Interview NC5
Some participants negated their decision to not join the trial, reasoning that if circumstances were different and their perceived risk was less, they would have taken part:
I think if if if I’d had the one episode, I’d have been happy to have taken part
Risk it yeah
And I could have had the risk either way
Yeah
But having had two episodes I I’m I’m afraid I wasn’t prepared to
No
Risk the third.
Interview NC3
Participants also discussed their understanding of their perceptions of risk, along with the serious repercussions of a further VTE.
Understanding the risk
Participants discussed their understanding of their perceived risk of experiencing further VTE if they were randomised to the control arm of the ALICAT RCT and ceased LMWH. Three participants reasoned that owing to their ongoing cancer, they remained at risk of experiencing another VTE:
I thought well the cancer is still there, there’s still a possibility that I would get the clot back.
Interview NC2
The reason as I said er because ah I don’t want it to happen again um although I’m not on er chemo at the moment
No
Um this it could still happen um through the actual cancer itself
Yes
So I’m a little bit wary of it you see um this is the reason um why I I can’t take part in the survey.
Interview NC5
Well the fact that my tumour’s still pressing on that same vein suggests to me that there’s still that high risk that there’s a clot there um so if I were to and I thought that was putting myself more at risk
At greater risk yeah
So the best thing to do was to say ‘well we’ll stick with the injections for the time being’.
Interview NC6
Other participants used the PIS provided about the ALICAT RCT or their discussions with the clinical team to support their decision-making, where they learned about the potential risks of taking part in the clinical trial:
As soon as we came to the paragraph where it said that if if you were in this 50% which you weren’t going to be having the injections there was the possibility of of um a clot reoccurring I think any chance that it could sort of block [indecipherable] from happening.
Interview NC4
We did discuss it and um ah I I’m not sure of the statistics as far as the blood clotting’s concerned, but he [CAT consultant] did say er there’s always that little chance, it’s not a big chance, there’ll be bleeding if if you stop taking the needles, but I he said that um he give me the figures it was um seven in a thousand but not seven per cent or something, which is very slight, but it is a slight risk
Mmm
But I don’t know what the statistics are for the for the blood clotting . . . you know, if I stop taking the needles . . . I don’t know what those statistics are, I would think it’s higher ah I did mention that I would have thought the chance of blood clotting will be greater than the chance of the bleeding, that’s what decided with my on that really to be honest with you.
Interview NC5
Repercussions of further venous thromboembolism
Participants discussed the potential repercussions of experiencing a further VTE, which varied from being required to have further investigations, to recognising VTE as a life-threatening event. NC2 described her preference of a daily injection compared with her experience of acute management of a VTE:
Um I thought no I think I’ll carry on rather than, you know, spending another day in hospital being prodded and probed like I was last time
OK
So that’s what decided me.
Interview NC2
NC3 repeatedly reported that he had experienced two episodes of VTE and, therefore, if he was randomised to cease LMWH, he was fearful that he would have another VTE that could be catastrophic. Like NC2, he also described his preference for a daily injection compared with taking this risk:
The thing that bothered me at that time was that I’d already had two episodes of of a blood clot and I thought if I was in the group that didn’t have the medication um there was a good chance that I’d I’d have another one and it could could have a lot more serious repercussions than if I just continued to take this medication.
Interview NC3
NC4 ultimately felt that the study was not suitable for him, as he had experienced a large VTE that he felt posed an ongoing threat if he were to stop LMWH, a risk he was unwilling to take:
When you’re told you’ve got a blood clot which is quite large, quite dangerous, which could kill you if you walk through the door and you’re possibly then going to stop having injections, which possibly stop you having um clots, to go down that avenue – it wasn’t the way I wanted to go
Interview NC4
Finally, two participants discussed the acuteness of a VTE and thus the risk it poses, hence their reluctance and ultimate refusal to join the ALICAT RCT:
Would er the main thing that did it was erm apprehension basically about er the clot and if a clot does occur it hits you like that bang
Yeah
And you haven’t got much choice.
Interview NC5
I know [CAT consultant] said that you know if that you know if you were unwell if you were in the the the non-medical group
Yes
Um you could go in and get it sorted, but it’s the time lag
Yes
Which which could be critical.
Interview NC3
Therefore, the decision to continue injecting LMWH and not take part in the RCT encompassed multiple factors, with information learned from different sources. Despite participants’ different perspectives about risk and their personal risk factors, ultimately they all perceived the risk of further VTE to be too great and they thus declined randomisation.
Dislike of low-molecular-weight heparin injections
Two participants sought to cease injecting LMWH past 6 months and, therefore, wished to not participate in the RCT. However, their reasons for wishing to discontinue LMWH varied. NC1 emotively expressed the pain and inconvenience caused by injecting, and his relief at being able to stop treatment:
I was just happy to get off of it to be honest with you, um it was more or less the same time every night, um and the pain as I said eh to me was terrible, horrific and a lot of bruising and things
Really
Um so really yeah, I was really glad to get off of those injections.
Interview NC1
However, NC8 had different reasons to NC1 for wishing to discontinue LMWH. While she strongly described her want to cease injecting, this was because of her desire to return to ‘normality’, rather than the side-effects of the actual procedure:
I was desperate to discontinue the injections
Of course
And I think that’s part of having had so many things pumped into you I wanted, you know, I suppose I just wanted to know how my body was really because you it it’s not you anymore you know
No
It’s having all this stuff affecting it and all these
Yeah
Side effects and you don’t actually know how you feel
No
And so I was very keen I have to say, I was predisposed I don’t want any further injections once the treatments finished I just want to try to get back to as much normality as I can
Interview NC8
While NC1 described feeling confused about the RCT and reported not receiving written information about it, NC8 felt confident in her decision to not enter the RCT and discontinue treatment. NC8 explained that although, like NC1, she was hoping to stop LMWH, she ensured the consultant was in agreement before making her final decision:
Although I mean [CAT consultant] said that you know there are, you know, there are risks with continuing, you know, so he he pointed out all of the risks of carrying on or not carrying on um so, you know, I I felt I felt well informed
Good
As in with my decision, even though I think I was predisposed
That you wanted to stop
Yeah
Yeah
If if if he was in agreement um that I could discontinue.
Interview NC8
Other
While the main two reasons for not opting in to the RCT were fear of VTE and disliking LMWH injections, two participants had other reasons for choosing not to be randomised.
While NC5 was fearful of experiencing a further VTE if he stopped LMWH treatment, which was his primary reason for declining randomisation to the trial, he also reported feeling generally unwell because of his ongoing cancer and thus not in good enough health to participate, despite feeling that the trial processes would not be too onerous:
I wouldn’t mind the blood tests, I don’t mind the questionnaires, but um apart from er the main reason, I didn’t feel I was quite well enough, well not very ill, well I’m ill, but I mean what I was saying was that I didn’t think I’d have the time to do it with what I’ve got.
Interview NC5
Furthermore, NC5 reported concern and anxiety about attending clinic appointments owing to faecal incontinence and urgency:
Cos um er I’ve got these problems I can explain with the bowel acting a certain way and I I got it’s difficult to make appointments and, you know, that sort of thing. So it will put a bit of a strain on me really, that’s the other reason you know . . . I can’t generally go out before two o’clock, because the way the bowels working at the moment, it’s working it’s working ok, but it’s working in such a way that um, er how can I put it er politely? I have to run sometimes, that’s the thing and it gets you in such a lightening way, it makes you a bit nervous
Interview NC5
Therefore, the multifaceted decisions made by patients when considering participation in this trial are clear.
One participant expressed unique reasons for declining randomisation to RCT, which differed to those of other participants. One of her reasons for deciding to cease LMWH and, therefore, not join the RCT was because of her perception that as the VTE had dispersed, and she was no longer experiencing symptoms, she no longer required treatment:
But then they found out there was a everything was alright and there was no clots so I thought I’d see how it went without without it. If I’m not getting any symptoms or anything there’s no point in having treatment is there? Just have regular check-ups.
Interview NC7
However, NC7 also conceived that she should cease LMWH and ‘see how she went’ without treatment, conceding that she could experience further VTE. She also alluded to the inconvenience of managing LMWH and hoping that by discontinuing treatment, and not taking part in the RCT, she could return to ‘normal’ life:
I just wanted to see if I how I went without it, you know, because it if I can cope with without all, you know, all that every day I can get on with my life then, cos I couldn’t imagine taking dozens of syringes on holidays, you know, I don’t mind half a dozen . . .
. . . until I stopped the injections I didn’t know whether they were doing me any more good or whether there was, you know, once they said the clots were gone, but there was a risk of them coming back, well I they don’t know and I didn’t know wh whether until I tried for myself, you know
Yeah
So
OK
I think it’s possible they’ll come back.
Interview NC7
Finally, NC7 also discussed her altruistic reasons for declining randomisation to the RCT; however, these suggested that she did not fully understand the design of the study or what it meant to take part in a clinical trial. NC7 described not wanting to waste unnecessary medication that could be given to somebody else and also that owing to the uncertainty surrounding treatment length following VTE, the clinical team may be able to help other patients by monitoring her ceasing LMWH. Thus, NC7 seemed confused by the meaning and reality of participating in the RCT:
But as I said there’s um if I’d have carried on with it for months it might have been just . . . that that er medicine could be good for somebody who
Right
Who who
OK
Is on it, you know, but er he always said th they can’t tell they’re trying to find out one way or the other that’s why they’re asking all these questions’
But er I don’t think I’m going to be lucky enough to be able to go without these clots forever, I probably, you know, they might come back, but they don’t know without this research or they won’t know whether the effects were effects or which way. So if I can work out that I’m alright without them or if I I’m not then they can I, if somebody else is in the same situation they can put all the information together and work it out best for them as well. But until I try one way or the other they won’t know will they?
Interview NC7
Thus, despite some agreement between participants about their reasons for not consenting to the ALICAT RCT, it is clear that patients have individual factors that motivate them to decline randomisation to a trial.
Results: clinicians’ perspectives
Participants
Nineteen health-care professionals were recruited in total across four focus groups. The numbers per group and their specialties are shown in Table 6.
| Focus group 1 | Focus group 2 | Focus group 3 | Focus group 4 | Total |
|---|---|---|---|---|
| n = 1 (GP) | n = 11 (oncologists) | n = 3 (haematologists) | n = 4 (GPs, n = 1; practice nurses, n = 3) | 19 |
Overview of themes
In line with the framework approach, the coding framework was developed with reference to the protocol and focus group topic guide. Through the coding process of coding and analysis, the initial framework was refined twice. Two overarching themes were identified from the data:
-
management of VTE
-
perceptions of the ALICAT study.
Each theme, with associated subthemes as presented in the third and final framework, is discussed in turn below.
The following abbreviations are used throughout the data extracts:
-
I: interviewer
-
FG: focus group
-
P: participant.
Management of venous thromboembolism
Focus group participants were asked for their perceptions and experiences of managing VTE. This theme is split into three subthemes: (i) diagnosis of VTE; (ii) treatment of VTE; and (iii) responsibility for VTE management.
Diagnosis of venous thromboembolism
Focus group participants were asked about the processes and procedures they follow when identifying and diagnosing VTE, and the impact a diagnosis of VTE can have on patients.
There was some discussion among participants in FG1 and FG2 with regard to the procedures which would lead them to a diagnosis of VTE and the processes involved in the diagnosis of VTE.
One participant in FG1 described the procedure which he and his colleagues follow in Canada. The participant described how, on ‘suspicion at visit’ (FG1:P1) they would record either deep-vein thrombosis or VTE on the patient’s electronic medical record and refer to a radiologist from one of three hospitals in the area in order to arrange a Doppler scan. This participant stated that they did not follow set criteria or guidelines for diagnosing VTE, their ‘clinical suspicion’ was based on patients presenting with pain, usually in the leg or perhaps in the chest or arm. It is important to note that this participant is a GP and so approached the issue from a primary care perspective; in this regard a diagnosis of VTE in a patient presenting at general practice might indicate an underlying cancer:
Yep. Um um it usually begins with the suspicion at a visit the persons complaining of more often leg pain, but sometimes chest pain or arm pain. Um and by having a higher index of suspicion and knowing that most clinical manoeuvres are not terribly helpful or reliable um obtaining um err a err a venous Doppler study and we can usually organise that the same day or next day.
FG1
The participant recalled an incidence as an example as to why he and his colleagues go in their ‘suspicions’, rather than follow set guidance:
I mean the youngest person to my knowledge was only forty three and err it came as a big surprise. Then the big question is why
Yeah
Does he have this and about 6 months later we discovered pancreatic cancer
OK
but a year later he was dead
Right
so mostly err err index of clinical suspicion
OK
what else might it be?
FG1
With this example, the GP implied that solely relying on guidelines has the potential to miss those with VTE and hence treat them appropriately. This participant also commented that the system followed in his practice is one that both he and his colleagues have set up themselves as an improvement to the current process in place in Canada:
So in terms of this diagnostic process between you and um um the local hospitals
Uh hmm
Do you feel there could be any improvement in that process?
Umm err not so much in terms of requests generated by me or the practice . . . sometimes people in my practice end up in A&E [accident and emergency] or as we call it Emergency and err are diagnosed and may or may not be admitted to hospital and so because we don’t run with a list system in Canada the way you do here which this year you will probably see [Province name] my current province adopt a list system because the writing has been on the wall for only thirty 5 years or longer um the notification of admission and discharge from hospital is a nightmare in general practice in the different parts of Canada.
FG1
Also, this GP feels that there is a very good chance that a Doppler scan will confirm his suspicions, implying justification for the process he and his practice have adopted:
I don’t think I would change the process I err usually I mean I don’t call up my radiology colleagues that frequently and usually they have learned when I do call um it’s probably worth listening
OK
There is probably an eighty per cent chance that the err Doppler will show what we are worried about as opposed to err ohh [name] is just a worry wart.
FG1
This GP also noted that he would receive the results of the patient’s Doppler scan and anything of concern would be made known to him by the radiologist, emphasising the importance he places on the process.
The oncologists in FG3 made some reference to the processes and procedures for diagnosing a VTE, although this was more of a discussion and exchange of views than a clear indication of the processes they follow:
Once again playing devil’s advocate ‘cos I’m trying to keep the conversation lively not that I need to um the clot came from somewhere and so are you scanning your legs to see if that person who’s got a tiny clot’s got a massive DVT? I suppose that would be my question is
[All talk at once]
You clinically in the absence of a clinical
But 80% of PEs are asymptomatic for DVT
But it hasn’t changed the management of that patient because
Yeah
If I found the clot on the leg, that wouldn’t have been a driver
No
For me to go beyond the treatment
FG3
Processes and procedures were not discussed in the other two focus groups.
There was also some discussion, again among the primary care participants in FG1 and FG4, as to the impact a diagnosis of VTE can have on patients with cancer.
The GP in FG1 discussed the impact of a VTE diagnosis on patients presenting in primary care who may not be aware of an underlying cancer. While it is not simply a case of stating that because of a probable VTE a patient will have cancer, the participant emphasised that the possibility will need to be investigated and patients have a right to know what is happening and why:
But the fundamental question becomes why does this individual have this? and in the back of most people’s minds nurses, doctors, researchers is err is there an occult cancer that err it’s first and only manifestation is this . . . but in general most GPs in the UK and in Canada would start getting suspicious then well OK now what . . . Maybe we have sort of stabilised this but that is not the end of it (.) it’s like how far do you want to go
So you would look for other symptoms
Yeah
And act on those
And it may be that over a period of weeks before we put the whole picture together but just making that diagnosis of venous thromboembolism that’s err not the end of it
No
It’s like well now what and I guess having some sense of when do you reasonably exhaust investigations if not your patients and your patients’ families in terms of you don’t want to unnecessarily worry them but they need to know up front it might be something more.
FG1
Owing to the fact that these investigations will need to be undertaken may cause anxiety for patients and their families, the participant felt that it would be wrong not to keep them informed of the situation.
Similarly, the primary care professionals in FG4 commented that cancer alone can have considerable impact on patients’ QoL; to be diagnosed with VTE is another added complication:
Obviously when you’re being treated for cancer you having take a lot of drugs and have to make a lot of visits into hospital the last you need is another problem on top
Yeah
Affecting your quality of life anyway
Yeah
And needing hospital visits and treatment etc.
Yeah
Cos you’ve got enough going on (laughs).
FG4
While some discussion took place, predominantly among the primary care professionals, on the processes and procedures involved in diagnosing VTE and the potential impact such a diagnosis may have on patients, more discussion was held across all focus groups on the approaches to treating VTE.
Treatment of venous thromboembolism
Focus group participants spoke about the treatment options for VTE and the length of treatment, the extent to which patients are involved in decision making about treatment and monitoring.
The GP in FG1 said he would start patients on LMWH if there is a high suspicion of thrombosis, as the risk of waiting for a formal diagnosis would be greater than starting on LMWH unnecessarily:
I think to be honest err I am not alone but I think many of us if the err the index of suspicion is high enough might consider starting people on low-molecular-weight heparin until we confirm the diagnosis. The err risk of not doing that I think is much greater than the risk
OK
Than the risk of delaying diagnosis before starting someone on low-molecular-weight heparin.
FG1
This participant also said that he would prescribe LMWH rather than other agents because of his concern about the length of time they take to have any effect; this was of particular concern for him owing to the urgency of the situation these patients face:
What you would prescribe
Usually umm I err I guess it’s my sense of urgency
Right
Err you know I could start you on coumarin or warfarin but I am looking at three to 5 days before you are therapeutic . . . at least I have um some sense of reassurance that your low-molecular-weight heparin gets at it now
Yep exactly
As opposed to waiting.
FG1
Despite the fact that warfarin should not be prescribed for CAT, the same participant also said that he would, and had, prescribed warfarin for CAT before, commenting that there was some pressure to use some of the newer agents, which had become available:
So would you prescribe warfarin for a cancer-associated thrombosis?
Well I have and do um some of the newer products on the market there is a fair degree of pressure to start them . . . in our practice we haven’t started many people on the new products but um
Are you talking about the new oral agents or
Yes
Yeah OK um how about low-molecular-weight heparin versus warfarin for a cancer patient
Yes we have had people just stay on that um.
FG1
The haematologists in FG2 had some discussion about whether or not the site of the first thrombosis is important when considering treatment, although through this discussion it was acknowledged that regardless, the outcomes should be the same. The discussion then moved on to patients’ attitudes towards treatment and how previous experiences may influence how they feel:
Can I ask, would you change your mind or would you sort of consider the site of the first thrombosis as being important to you?
You
We know that if your first event’s in your lung
You probably would actually, you probably would, although saying that would you if it was an incidental finding on a scan as opposed to clinically relevant, I know that that’s important still, but that might influence you as well
But they’re supposed to have the same, data shows they have the same outcome
They do, but the patient themselves will have a different attitude
Yes
Because they’ll go ‘what’s all the fuss about’
Yeah
Whereas one who’s had a terrible PE will be a bit scared
Or a terribly swollen leg you know, ongoing pain.
FG2
Among the oncologists in FG3, there were some differences within the group as to how they would approach treatment of VTE and which agent they would use to treat it:
My view would be one is VTE is DVT and PE I wouldn’t routinely treat a DVT for three months even in a cancer patient. Secondly, my practice is
I would
For 6 months? Um it depends
For three months, I probably would actually
I mean it depends the second thing is what is active disease, because you know particular with ovarian cancer patients stage 3C you know they have microscopic disease give and take one or two patients but you know it’s so I wouldn’t call disease those patients I would definitely stop. Um fundamentally I think the question that we need to ask does it need to be indefinite or you know can there be a a longer time frame be longer and I also agree with you that it shouldn’t be inject, I mean it should be all forms of anticoagulation so if they’re not no longer on treatment a you know in the absence of good data for rivaroxaban or one of the newer agents, even warfarin should be allowed
Can I can I play devil’s advocate
Warfarin
No they hate the injections
[General chatter]
It must be a non-monitored one.
FG3
It is evident in the quote above that the oncologists would consider how patients may feel about the way in which the various treatment options are administered when deciding on which therapeutic agent to go with; for example, P1 (FG3) stated that patients ‘hate the injections’. Similarly, participants in FG4 also discussed patient preferences, with P4 commenting that they would imagine some patients would not like the injections and perhaps would prefer to be on warfarin with monitoring than go through daily injections:
As nurses you get a lot of comments already about people having to take extra tablets
[Group agreement and laughing]
So I think to take extra injections I can imagine would trouble some problems for some people
Some patients are quite really into if it’s going to benefit others partake in even if it is the inconvenience they they feel they’d like to participate and um but it’s their choice really um whether they want to be involved or not
OK
I know a lot of our patients would much rather have take warfarin every day and be monitored every so often and have that risk rather than have the injections they always seem so annoyed when we tell them we have to put them back on the injections
Right so you in your experience your patients prefer tablets to
Yeah I find it bizarre but yeah.
FG4
Patient involvement in decision-making is discussed further below.
On the subject of length of treatment, and more specifically whether or not to continue treatment after the initial 6 months, there were some mixed views although the overriding concern among participants across the different groups was the lack of evidence base for treatment beyond 6 months. The GP in FG1 stated that he and his colleagues would treat for a minimum of 6 months, but would feel more reassured if there were evidence to guide this. The decision to continue though would be taken on a case-by-case basis, taking into consideration whether or not the cancer has been cured, the advice of the surgeon and the patient’s lifestyle:
And so how how long do you prescribe um anticoagulation for these patients?
Well I wrote to be true to what I wrote you are probably looking at a minimum of 6 months but for us part of the reason I am interested in what you are attempting to do is I would feel more reassured if we had better clinical evidence to guide us and there isn’t
Yeah and there isn’t
So you know my some of my best consultants will say well probably a minimum of 6 months [name] maybe a year err and maybe of course they are being honest in terms of difficult err this is what I would do
Mmm
But if you ask me if it is based on evidence I have to admit no it’s pretty skimpy.
FG1
The haematologists in FG2 also commented that they would be reluctant to take patients off anticoagulation therapy at the 6-month mark if they still had an active cancer but other risk factors need to be taken into consideration as ‘. . . you shouldn’t be giving them injections they don’t need’ (FG2:P3).
But it’s only 6 months because
That was what the trial did
No it’s only 6 months because in all situations because we recognise that any sort of finite duration beyond 6 months makes no difference
Yup
I mean that happens is that your risk of recurrence when you discontinue, if your risk factors are prevalent for patients who have unprovoked thrombosis, are the same at 2 years as they were at 6 months and maybe even at three. So this 6-month period is just sort of a period that has been known as a sort of hyper-coagulable stage, but most people we know
Three months is probably enough
Well certainly in provoked events it certainly is, there’s been a meta-analysis looking at it for six versus three months for unprovoked events and just slightly in favour of 6 months, but that’s why it’s 6 months and that’s why if risk factors still prevail I think that’s what changes my mind about it and makes me think that the risk is still there.
FG2
Similarly, the oncologists in FG3 said they would continue with anticoagulation on a case-by-case basis:
There are already two counts doesn’t it . . . either we have patients that we pick up they’re in their first line treatment they’re disease free or they’re in remission then I would find it very hard to keep a [inaudible]
They’re not eligible
They’re ineligible OK
They’re ineligible
Then if they’ve got a lot of disease I would equally find it difficult to stop their anticoagulation because I think those are patients you have to have a def difficult discussion with to say you’re at ongoing risk, you’ve already had er venous thromboembolism or you’ve had you know DVT or a PE you’re already at risk. Do you want to continue? I mean I’d be encouraging those patients to carry on
But isn’t that exactly the point of doing research
A study to find out
You would encourage them to continue but you don’t actually know what the incidence is and then within the consequence I mean you know this is not something you should just make up as you ultimately you shouldn’t make it up as you go along
That’s what I was thinking because those are the ones can you can point
[General chatter]
I’m not so sure that’s the case because I mean if you’re accepting that malignancy as an increased risk, you’ve they’ve already proven that they’ve had a DVT or a PE so that they’ve already they’ve already succumbed to that and they’ve developed one so are you then saying well sorry you’ve got a big pelvic mass but I don’t actually believe you need longer term anticoagulation we can stop it
FG3
This quote demonstrates further evidence that the patient is considered, and to some degree involved in decisions concerning treatment, which is discussed further below.
As already indicated above, a common factor between groups though was that patient preferences would be taken into consideration and treatment options would be discussed with them, or ‘have a debate actually with the patient to be honest’ (FG3:P5):
Is there any reason you would prescribe one or the other
If people don’t mind their daily shot that’s OK
OK so it’s more down to patient preference
Yeah it’s down to patient preference.
FG1
The fact that this issue was discussed in conjunction with discussion about the treatment options clinicians would consider and the duration of treatment indicates the importance clinicians place on putting patients at the centre of these decisions.
Only the haematologists really discussed monitoring of patients and this was in relation to biomarkers:
It’s interesting that the situation where in terms of knowing what you want to do, something that’s a biomarker would be an involved biomarker would be incredible, now Svicar and his colleagues from the [incomprehensible] bunch did you read in the [incomprehensible] a couple of months ago? They presented some with patients with colorectal cancer and quantified tissue factor expression microparticles and they didn’t randomise the study, so they actually simply they took a normal value back, and I know microparticles are really difficult to do because it’s difficult to standardise, but what they did was effectively define the normal range er they sort of defined as high above the 75th percentile of that, and they then divided patients who presented into high micro particle levels or low. The low ones they just left them off treatment, the high ones they randomised them to low-molecular-weight heparin for prophylaxis or not, and it wasn’t the result
FG2
The GP in FG1 also commented that his practice has three international normalised ratio nurses who are responsible for checking levels and managing anticoagulation, although this was more part of a discussion about who has overall responsibility for VTE management, which is discussed below.
Responsibility for venous thromboembolism management
Participants across all focus groups discussed who is and/or should have responsibility for the management of VTE. The GP in FG1 commented that if oncology is involved in a patient’s care then they tend to manage the process, and as a result primary care can lose track of these patients:
And if you have patients who are eh who are perhaps seeing an oncologist
Mm Hmm
Do the eh do the oncologists tend to be involved in the prescription of anticoagulation or is it still to do with you as a GP
I eh well it depends on err er how we get there if someone has gone through the emergency room and admitted to a general hospital and if the diagnosis of a cancer is made and oncology is involved they will sort of start the whole process . . . In Canada at least some times if you go that route you may become lost to your GP because the oncology service tends to function in a more primary care mode . . . most of them most of them if I happen to be your family doctor you probably quickly will tell the oncology service to make sure that I get copies of reports and other such stuff . . . because remember we don’t function with a list um and they are usually very good er I will get copies of progress notes and so on um but sometimes there is a gap sometimes it can be a year or two while the person is going through cancer treatment and cancer management.
FG1
The haematologists in FG2 commented that these are not patients they would normally see, as oncology is not their specialty. That being said, if asked they would advise continuation of anticoagulation if the patient continues to have an active cancer, but ultimately they would leave the decision and responsibility with the oncologist:
I must say though that they’re not the patients that I tend to see or never sure about what to do with, they’re not typical patients
So are they the ones you would stop now?
Well I don’t think they’d even get to me in the first place, but then that’s my specialty, they’re not the complicated ones
Yeah I mean if I ever get asked I basically, because I don’t often see them, I’ll just get a phone call or a letter saying what do you think and my party line is if you’ve got active cancer then you need to carry on with treatment and then I leave it to the oncologist to decide you know how appropriate they feel that is for that particular patient.
FG2
In contrast to this the oncologists in FG3, while acknowledging that haematologists tend to defer to them, felt that it was not necessarily their place to be taking on the overall responsibility:
I’d like to say something – I’d like to say that I think that none of us in this room should be deciding whether these patients go onto long-term anticoagulation. We are all highly specialised in what we do and we would resent enormously if someone told us what we should be doing for their cancer treatment and we have specialists who deal with coagulant therapies and all those sort of things and these are the people who should be deciding and who would monitor and follow these patients and I have to say that is now what I do there specialists who are in hospital and I discuss these ca cases with them and I say ‘should this patient carry on what do you what’s your view?’
We’re actually when you do find a lot of them will say – a lot of the haematologists I work with will say you carry on
Or they defer to the oncologist
[A lot of talking]
You know well what’s what’s the diagnosis what do you think the risk you know
We’ve got to find the information let them [indecipherable]
They don’t.
FG3
The participants in FG4 felt that the responsibility for VTE management should be a combination of haematology and oncology ‘because we in primary care only follow their instructions . . . we don’t take the lead in starting any er antithrombotic treatment’ (FG4:P1). Therefore haematology and oncology should lead on the management plan:
OK . . . OK do you have a view then on whom you think should be leading the treatment on cancer-associated thrombosis? Any particular specialty?
Think it would be a combination of the haematologist and oncologist
Mm mm
They need to associate because we in primary care only follow their instructions
Right
We don’t take the lead in starting any er
OK
Antithrombotic treatment so I think it would be both of them together
OK
I agree that they would probably take the lead
Yeah
In actually managing making a plan management plan
And that’s haematologists and
Oncologists together yeah
Do you
And like it needs to be a joint decision.
FG4
How do you think that works in practically in in real life, do you think that joint decision working collaborative view works?
De sorry
I would sort of I would imagine it tends to be it depends who’s seeing them at what point in care but I would imagine the oncologist probably sees them more and we can get a haematology opinion, whether haematologists would actually come and visit a patient and get real in-depth vision of what what they are and and where they are in their treatment so far
Mm mm
I imagine it doesn’t occur so often
Right
But I would assume the discussion is probably not with the patient but by telephone usually
I think it would depend on the type of cancer as well because oncologist I think probably thinks like ovarian cancer, pancreatic cancers have got more of a risk of er of er coagulation than compared to other type of cancers so probably as you so rightly said oncologists probably is the lead person and together with the input from the haematologist they could make a plan
OK
Regarding individualised patients.
FG4
There was also some discussion in FG4 whether or not a joint decision between the two specialties should and could happen, although it was acknowledged that it would depend on who was seeing the patient at that particular point in time, the general opinion though was that it would be oncology. The participants then went on to suggest that, in an ideal world, a combined haematology/oncology clinic would benefit patients the most:
OK
Can they not have a combined clinic where you had a haematologist: and oncologist together?
OK
Rather than the patient having to come and see two different clinicians at different times
Like
Or on the same day its theoretical it’s not possible I know it isn’t but if it could be
But you’d think in an ideal world
Yeah
That might be a
In children’s services they they try to um have um family centred care where all your clinicians are together so in this case if that’s possible why not have something similar
FG4
However, regardless of this suggestion, the participants in FG4 still came back to the point that one health-care professional needs to be ultimately responsible and, while there was uncertainty, these participants continued to have a preference for that professional to be the oncologist:
I think somebody needs to have the ultimate decision because I think problems arise when you’ve got two clinicians with different opinions and it there needs to be somebody that says right this is what we’re going to do cos if it’s a joint decision who has the ultimate call?
Responsibility yeah
Right who do you think that should be then? Do you think that should be the haematologist or the oncologist?
I don’t know . . .
There is arguments for both isn’t there?
There is arguments for both but I don’t know I think the strength of it would lie probably with the the person who has the most contact with the patient, and knows the patient best so therefore possibly it would be the oncologist
Yeah I do agree with that
Yeah
Yes
Probably the oncologist.
FG4
Therefore, as with the haematologists, primary care professionals also feel that oncologists have more of a responsibility with regards to management of VTE.
Perceptions of the ALICAT study
Focus group participants were asked for their views on the ALICAT study. This theme is split into four subthemes: (i) study design; (ii) outcomes; (iii) recruitment; and (iv) ethics and risk.
Study design
Participants were asked for their perceptions on whether or not the trial had equipoise and how they would explain this to patients. They were also asked for their views on alternative study designs.
There were few comments made on the study design itself and issues concerning equipoise. Specific comments were made by participants in FG1 and FG4, the consensus being that this was an important topic and needed to be researched. The fact that participants across the group had already commented on the lack of evidence base would suggest a general underlying support for the ALICAT trial:
Cos it’s a question that needs an answer isn’t it?
FG4:P3
When it came to the issue of equipoise and how participants would approach this with patients were they to recruit to the trial, little discussion was offered. One of the haematologists in FG2 acknowledged the equipoise of the trial by stating:
And there’s something secure about a trial which means that, you know, you’re prepared to put people in because you know that that question is not answered you know
FG2
The participants in FG4, although noting that any research exposure they may have had in the past was minimal, commented on the potential to lead and bias participants:
. . . don’t think so the quality of data’s always difficult to get anyway isn’t it? Cos there’s always the possibility of subjectiveness creeping in and
Yeah
It’s difficult because you could lead people you know it’s got both arms so.
FG4
Therefore, the concern with potential bias is the implications for the quality of the data collected.
There were also some suggestions offered by participants as to possible alternative study designs for the ALICAT study. The haematologists in FG2 proposed the control arm could be a prophylactic dose:
You know what study would be more successful
Tell me
You know already probably that your control arm was to goes on to prophylactic doses
Mmm
Because it’s like a stepping stone down to nothing
Yup
And you would get round some of the ethics then um
And also what you are trying to do is secondary prevention
Yes
Yes
So prophylactic dose may be sufficient
Yeah
That’s very interesting, that could work. I’d certainly, from a clinician point of view, think that’s something to perhaps go for cause I’d just worry about, you know, basically if you’ve got a permanent malignancy the risk of recurrence is high
So what I’m hearing is that if ALICAT were to randomise people to continue beyond 12 months on full dose or to drop down to primary prophylaxis dose
That would make it easier to recruit
Yeah I think so yeah.
FG2
According to the oncologists in FG3, the ideal trial design would be no treatment versus warfarin versus heparin, after the initial 6-month period:
Yeah so that hasn’t there’s been no prospective randomised studies looking at what to do beyond 6 months
So an an ideal academic study – forget numbers for a second – would be randomising patients to nothing, warfarin or heparin
Yeah
After the 6-month period.
FG3
One participant in FG3 asked why warfarin could not be included in the ALICAT trial. This led to acknowledgement of the CLOT (LMWH vs. a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer) trial and the findings from this trial indicating warfarin to be inferior to LMWH:
Sorry I may have missed it, why was it that you couldn’t put warfarin – the extended period on warfarin – you said that you could in the newer agents
Well we could we could although the argument being that we we do have data to show that patients with um advanced cancer have a greater bleeding rate on warfarin, poorer control and also if a hypercoagulable state were arguably to progress with disease progression because you’ve got greater tumour bulk, the last thing you wanna do is give a drug which has already been shown not to be as good at as low weight molecular heparin at preventing it. And also if you’re gonna continue, arguably you’d continue with the er the drugs that is kind of indicated
And fundamentally I mean if you say active disease you’re effectively doing the CLOT trial and you know that warfarin isn’t as good.
FG3
The primary care participants in FG4 focused their discussions on alternative trial designs on the risk status of patients. They felt that perhaps the trial should focus more on higher risk patients, for example those with pancreatic or ovarian cancers, as they have more thrombogenic potential:
I don’t know about um I’m thinking like as I mentioned earlier a few of the cancers are associated with more thrombosis so would it be
Right
Sort of beneficial to isolate the higher risk um patients or take them on board more so um
OK
Getting a generalise and I’m not specialist in the area
No no no that’s ok so do you mean higher risk patients in terms of um cancer they have?
Yes because cancer like for example pancreatic cancer
Yeah
Ovarian cancers and things like that has got more slightly more thrombo thrombogenic potential so but there are not those patients need to be taken on board more um
Right
Rather than I mean something which is not so thrombogenic again
Yeah
As I said I am not a specialist but just a suggestion
OK so perhaps focus on the more higher risk
Yeah
Patients
Yeah.
FG3
Participants in FG4 also discussed other risk factors for VTEs and the implications they may have for the ALICAT trial:
Maybe sort of mobility um and if this patient has had major operation been in intensive care for months
Mmhmm
And then randomised and I mean obviously that would probably effect results
Right
But I don’t know
OK
To what extent because it’s randomised would it make a difference
Right
No I’m the same the other risk factors for VTEs it’s difficult to minimise the other risk factors apart from the cancers themselves
Mm mm
To make the trial as focused on the cancer and VTEs rather than other influences like mobility
Yeah
I see what you’re saying.
FG4
In summary, participants offered potential alternative study designs, although some may be less achievable than others.
Outcomes
Participants discussed which outcomes they thought were important and should be included in the trial design and how long they felt patients should be followed up for.
The priorities and perceptions for outcomes in the ALICAT trial were discussed in three of the focus groups (FG3 being the exception). The primary care participants in FG1 and FG4 felt that quality-of-life outcomes were of particular importance as well as gaining the perspectives of patients’ relatives as they are also involved:
Um as long as methodologically you know in addition to the usual quality-of-life assessments and so on there is a way to include the family and sometimes it is not the person with the illness or the disease who is doing the injections
Absolutely
And clearly it’s sort of family impact if you will or caregiver impact
Absolutel
Or burden
We’ll be interviewing relatives as well
OK.
FG1
What are your views on these um these sorts of measures for the trial element of the study? Do you have any opinions on them?
. . . it seems quite thorough actually testing actual results as well as how people feel about it as well and whether it makes a difference to them personally
Mmhmm
It’s good.
FG4
Think it’s really good that relatives’ attitudes are taken into account
Yeah
I think that’s really cos quite often they’ll say more than the patients they’re a bit more honest (laughs)
[Laughs] good
They tend to be the ones that tend to motivate, or dissuade, but more motivation if the patients agree then
OK, is there any any of these outcome measures that you personally would find the most interesting any of these stand out for you?
Should be the quality of life especially obviously if they’re cancer patients assume initially mention we don’t want extra problems for them so we try to maintain their quality of life so I think that would be a significant factor
OK
Which needs to be looked into
. . . I think the patients should be in the forefront, quality of life as well if it’s going to be benefit the patient that you’re not causing any harm because um but the diagnosis of cancer tend to think of the end result rather than going through the motions so yes
FG4
Participants in FG4 and FG1 thought it was also important to assess whether or not the continuation of anticoagulation was effective in preventing further VTEs:
obviously whether it’s effective otherwise
whether it’s
[Laugh]
The the additional anticoagulation
Yes
Yeah
How effective is it?
OK
In the end
When you say effective
Well does it prevent further
Further VTEs
Further VTEs yeah.
FG4
No no I think that the outcome measures are um because err I guess . . . events significant events is that included in here
In terms of
Do you mean like bleeding events or
Well err I think err I think if this were a strict drug trial we err would have a serious SAE
Oh yeah
Serious adverse event
Absolutely there would be
And significant adverse events and so on
Yes
And as long as there is a way to monitor
There would be safety monitoring
Events maybe specific to the injection
Mm hum
It may be specific to something else it may be unknown
Yeah
It may be a natural progresser
Yeah
Of the illness.
FG1
Participants in FG4 also gave their approval for the qualitative component to the trial; in particular they liked the fact that patients who withdrew from the trial would be provided opportunity to discuss their reasons for this:
I think it’s brilliant that you offer them an interview
Yeah
The withdrawn patients I think that’s really good
Yeah OK great
That’s useful information isn’t it for future
Yeah
Studies
Yeah
Basically what what’s what is the problems that make people drop out?
Most people will be quite happy to give you a reason
Yes.
FG4
Other suggestions for outcomes offered by the focus group participants included health economics (FG1:P1) and also clinical biomarkers such as microparticles as suggested by the haematologists in FG2:
So you’re talking D-dimers could be done, but would I mean tissue factor microparticles would be more challenging
Microparticles would be challenging
Microparticles are challenging
Yeah
You’d have to make sure you freeze an even amount of tissue
A single standardising methodology [incomprehensible]
[Incomprehensible]
An incredibly good [Incomprehensible]
But if one were [END OF SIDE A OF TAPE – TAPE TURNED OVER] Um feasibly one could actually um on could you know freeze blood and then analyse it all centrally somewhere
Yeah
Yeah yeah
Um so that would only be observational, it wouldn’t be able to be used in that situation to actually guide
No no you wouldn’t use it, you’d use it you’d just collect the sample to er um validate other people’s scores or develop a new biomarking style
To see if there’s correlation and obviously if there’s any correlation between outcome and that particular biomarker.
FG2
The GP in FG1 also commented on the importance of monitoring serious adverse events, including bleeding risk, and issues concerning the injections themselves:
No no I think that the outcome measures are um because err I guess events significant events is that included in here
In terms of
Do you mean like bleeding events or
Well err I think err I think if this were a strict drug trial we err would have a serious SAE
Oh yeah
Serious adverse event
Absolutely there would be
And significant adverse events and so on
Yes
And as long as there is a way to monitor
There would be safety monitoring
Events maybe specific to the injection
Mm hum
It may be specific to something else it may be unknown
Yeah
It may be a natural progresser
Yeah
Of the illness.
FG1
Participants in the other focus groups did not make any specific comments with regards to safety monitoring.
With regard to follow-up, the haematologists in FG2 commented that this was something the investigators would need to consider carefully, although offered little else in the way of opinion on the subject:
But how long are you going to follow them up for? 6 months?
They’re going to be in the trial for 6 months, they’ll be followed up for 12 months
Yeah you’re going to have to watch that quite carefully actually because you know you’ll probably have to have an ongoing significant statistical analysis to allow you to decide whether you should continue with the study or not
Absolutely
That’s a little bit tricky that because you can have quite high rate of events I think in the non-treatment arm
FG2
Similarly, the primary care participants in FG4, while discussing the issue, did not offer a full opinion on the duration of follow-up:
Do you think there are any others that have been missed that we could include that would be relevant here?
I just wonder would the the follow up of the VTE um occurrence within twelve months
Mmhmm
So usually its measured 5 years
Right
The recurrence rate in other literature but would there be an additional follow-up or would it would it be twelve months from when they initially started or would it be twelve months after the first 6 months of treatment?
FG4
In contrast to FG2 and FG4, the GP in FG1 felt that the target duration should be 2–3 years, although acknowledged that many patients are unlikely to still be alive:
Mm hum well the challenge with this is that every trial I have seen you know is keeping track of people now
Mm
Extra ordinary lengths are gone to sometimes
Do you mean follow up long term up
Yes
So we do have we are able to link into oh my gosh what’s it called we can we can link into the office of national statistics for very long term follow up not that some of these will have
No
Very long term but we can certainly track deaths at a later date um and we can build into the study I don’t know however long you felt was appropriate 12-month, 2-year, 3-year follow-up can be built into a future study
Yeah yeah I think two to 3 years is probably the target
Yep
OK
To go after but you are right um lots of people err won’t depending on
Mm
Depending on their condition will last that long but I think cracking that nut and it may very well be that it will push another study well you know if going to 2 years
Mm
Maybe we should be going longer term.
FG1
The oncologists in FG3 did not discuss the issue of follow-up.
Recruitment
Participants discussed in general terms whether or not they would, in theory, recruit to the ALICAT trial and offered their opinions on the number of participants which should be recruited to the trial as well as the challenges, motivators and facilitators to recruitment.
In terms of whether or not participants would recruit into the trial, no opinion was offered by the oncologists in FG3, and the GP in FG1 would not be eligible to recruit to the trial as he is based in Canada. Participants in FG4 said they would recruit to the trial, but only if patients were willing to take part, going back to the question of whether or not patients would be willing to continue with anticoagulation therapy:
Would you do you think you would refer your patients to the ALICAT trial?
Only if the patients are willing
Yeah
Then yes
You would be happy to
Yeah
OK
I’d be the same yes
Yeah
Cos it’s a question that needs an answer isn’t it?
Yeah
It’s just so if they were happy to
It’s just whether they were happy to have 6 months more injections
Yes
Do you think that might be an issue for some?
Yeah
Yes
Yeah some patients if they think that they don’t need it then they don’t want to have extra injections so it’s entirely up to the patient then we can advise them that it is obviously done for research purpose and things but it’s it is their concerns which is er most importance.
FG4
The haematologists in FG2 commented that, while currently they do not have access to eligible patients for the trial, there were situations where perhaps they could be involved in recruiting to the trial, demonstrating a degree of willingness:
Where do you think it’s going to be easiest to recruit these patients? Do you see these sorts of patients coming through your clinic?
I mainly get letters, I don’t very often see them, although that’s changing now as we have a nurse-led thrombosis clinic who’s going to see every single patient who gets a venous thrombosis, so whereas I only see the patients where a decision needs to be made about anticoagulation, um we will now actually see all these patients whether they’ve got cancer or not by a nurse, so we would actually be able to capture that data, um but before that it’s always been letter, rarely see them
No like you I don’t routinely see these patients but I get contacted continuously by colleagues to ask our opinion on them and we do have a fairly the actual unit’s a single entity, so I think I could probably recruit patients into that
[Names P1]?
Yeah I see the ones where it’s really uncertain, otherwise it’s advice like that, but we’re about to start seeing everyone at three months.
FG2
Although the GP in FG1 would not be able to recruit to the trial, he felt that if it were an option then a lot of his patients would be willing to take part in the trial and he, in turn, would be willing to recruit given the importance of the question the ALICAT trial is trying to answer:
And finally, what do you think your patients and their families would think about this trial? Would they be interested in taking part?
Yours can’t, but
Well obviously they can’t, but hypothetically
No but we were running this I think I think a lot of them would, particularly given that any reasonable clinician can say ‘we don’t the answer to this question’
Yeah
The guidelines don’t go that far, we think it’s a reasonable question to gain more information about, and you know we’d like you to consider being part of it, even though it might not
Mmm
Particularly benefit you, it may, but it likely won’t from a time point of view
Yeah
No I think, I think, when given the circumstance of this is what we don’t know, we don’t know how long to keep you on this medicine, these are some of the guidelines, but not much evidence after that
Mmm
Yeah
You can help clarify this.
FG1
There was some concern among the haematologists in FG2 that the numbers recruited to the trial would be particularly small; the target patient group would be those patients with active disease, who have completed their chemotherapy regime and who have a life expectancy of more than 6 months. The interviewer offered some examples of sample sizes to try and determine what would be acceptable to them, although the participants did not really enter the discussion a great deal, nor offer an opinion either way. The over-riding feeling was that this is something a statistician should decide:
If we’re saying 4% say 4% recurrence in the dalteparin arm or the low-molecular-weight heparin arm, would double that be, if you’ve got 4% recurrence
It depends on how many patients you’ve got, you need a power calculation
Yeah, cause 30 patients isn’t that many, so you probably do need from a statistical point of view, you probably will need to have a doubling of rate I should think
Yeah
But I don’t know, your statistician would need to decide that
Yeah
FG2
In terms of challenges to recruitment, both the haematologists (FG2) and oncologists (FG3) felt that some patients may be reluctant to join the trial if they wanted to continue with anticoagulation, running the risk of being randomised to the non-treatment arm:
I think the recruitment is would be quite difficult because I think what makes me decide is that if the risk factors that prevailed or were present at the time of the onset of the disease, of the venous thromboembolism, still there. That is has the patient still got cancer or other risk factors. I think it would be quite difficult to sort of think about randomising in that situation given the primary cause
I completely agree
Of the first event was still prevalent
I would say exactly the same actually; I would be reluctant to take those patients off anticoagulation, I mean certainly at the moment my advice is if they’ve got active cancer they need remain on it until the cancer’s in remission. And then big question then is, cause we then leave it to the oncologist to then decide when it should stop, because often these patients become palliative or get thrombocytopenia, and these sort of things, we leave them to decide when they feel the bleeding risks are getting so high that they should stop. Or the patient’s so ill that the injections aren’t appropriate anymore. So recruitment I think would be, from my experience and practice would be tricky.
FG2
The idea of low weight molecular heparin long-term I I think it I would find it very difficult to recruit patients in to it. That’s just
But turn it round [P1] are are there patients out there now who have active disease
Yeah
Who you actually stop your anticoagulation on
Yes
Or do you tend to carry it on?
Yes.
FG3
Participants in FG3 also felt that they key to getting this right was in making sure the patient group is clearly and correctly defined:
I still think there is something in then I think the question is defining what the patient group
[General agreement]
Is that you would want to put into that that’s what I’m grappling with at the moment because you know the
I I think P4’s absolutely right – those asymptomatic
You don’t want to be
They should fall out
And the 3C that have been optimally de-bulked and are on follow up
Yeah
‘Cos
Yeah
With no microscopic disease
Should not be there
Should be pulled out as well
I I think they’re in remission
Yes
[General agreement]
They’re not active
We could argue that they’re but they’re no they don’t have active disease in the context of what we’re talking about here I think.
FG3
Participants in FG2 also discussed this in relation to the problems other thrombosis trials have experienced in the past:
I mean all the original trials comparing warfarin with low-molecular-weight heparin stopped at 6 months, nobody is sure about what to do at 6 months so we have as a group all pretty much done the same thing
Mmm
Which is if their cancer’s in remission
With ongoing risk factor it’s like a permanent risk factor
If it’s not we, the thing is we don’t really know, cause often at the beginning at the diagnosis there are risk factors there, you know there’s surgical procedures, biopsies, that sort of thing that are not there at 6 months. So personally I would quite like to be able to randomise patients at that stage um but I do think you will struggle to recruit because all all studies involving randomisations in venous thrombosis treatments struggle to recruit, it’s just the nature of the disease.
FG2
The primary care participants in FG4 felt that extra injections may be a barrier for some patients and this may make recruitment to the trial particularly challenging:
It may be a problem depending upon how many number of patients that they require because er I mean for instance once they are diagnosed they are going to different specialities and they’re spending quite a lot of time so they don’t they have to first digest information of cancer and that next thing would be whether or not to take part in the research I don’t know how much priority takes t that point in their life so
so for these patients they will have already um been receiving low-molecular-weight heparin for up to six that would be 6 months when they would enter this trial
Yeah
So for their cancer-associated thrombosis so they should have already received a cancer diagnosis and they’re receiving
Yes
Anticoagulation so this would be to see whether to extend it so at that point do you think that make would do you think that would make a difference?
It would depend if the patients are stable and they have come to the diagnosis and come to terms with the diagnosis so yes some patients may be still be struggling at that point they may be undergoing lots of treatments even at that time so for those patients it’s a bit difficult to predict what they have got in their minds.
FG4
Yeah I think it would depend on the individual patient obviously depends upon what stage of the treatment they are in if the pain that is their core
Everything’s [words incomprehensible]
With everything they will be happy to continue but if they think they’re still not come to terms with what is going on then yes it’s a difficult decision to predict at that point on what they want to do
Mm
But I mean as they rightly mentioned because they have been or already having it that gives us a chance to say yes that if are you happy to um continue it so there is a positive outlook for it anyway at that point.
FG4
Despite these challenges, the participants in FG4 also noted that some people who are already on injections may be fine to continue this. Furthermore, there was a sense that people having completed their 6-month course of treatment may be altruistic and be willing to take part in research to help others:
I think you might find it easier because they’re already on the injections and so therefore obviously not having any problems continuing and because there are I’m sure there will be a willingness as to want to help other cancer is such a big topic and people do do things that they wouldn’t normally do to try and help others in because it’s such a big problem for everybody you know
Right
It might give them cos they’ve been so they I mean they may have been quite helpless for the past 6 months and quite low giving them the sense of helping other people might [that would be quite nice for them [words incomprehensible]
Yeah it can be quite positive possible can imagine it might be quite positive for some people to think they can do something to possibly help themselves and others they might make a difference to themselves as well so.
FG4
OK um just as an aside then considering that this is obviously a trial so they will be randomly assigned to either continue with the injections or with their anticoagulation or to not um do you think that that the fact that they could be in one or the other group if they’re already having anticoagulation, do you think that might have an influence on whether or not they wish to take part?
I don’t think so because yes if it’s sort of willing to help other people that’s what their attitude in mind they wouldn’t have a problem with whatever group they are allocated to
OK
Because yes they have finished their 6 months of treatment anyway another one is sort of I think their willingness to go ahead and take part in the research so it’s I don’t think it should be a problem but
I suppose it depends on their motivation doesn’t it? For getting involved
Yeah.
FG4
In terms of facilitating recruitment into the trial, the primary care participants in FG4 offered a number of suggestions including education and support for participants throughout the trial, such as out-of-hours/clinic support and a helpline telephone number. Research nurses also would be a good source of support and help facilitate recruitment. Furthermore, recruiting a greater number of higher-risk patients might be beneficial for the trial itself:
OK do you think then there’s anything we could do to facilitate recruitment into the trial?
Education I think if they know and they understand um probably the more than we tell them then that would really help I think
And obviously good support while they’re going through the trial to ensure that they’re not feeling any extra problems or questions can be answered easily for them
Yeah
In case anything does crop up and obviously ease of having the injections so that may be to make the process of them having to have an extra 6 months as simple as possible if they’re in that type of
Yeah
Trial make it easier for them.
FG4
No I was going to say their support I think is most important because you’re taking part in a trial and you’re wondering are you just being seen just on a clinic you’d need like um out-of-hours support may be or out-of-clinic support just for somebody knowing that there is somebody there having the questions because normally when you attend clinics there’s so many things going on and you can’t take everything in so [words incomprehensible]
Yeah
Yeah I think probably something like that helpline number just in case they’ve got any queries about the research so that they can ring up and just to ask somebody obviously in hours time only it’s not out-of-hours because out-of-hours obviously we have got your er doctors and the A&E and things like that but I say in in in hour’s time there is some sort of helpline number in case they have queries about it
OK
They could ring up.
FG4
The GP in FG1 agreed with the interviewer’s suggestion that research support staff might help with recruitment by supporting the GP and minimising the impact on their workload:
Cost in staff into the project, so we know we’ve got them, so that’s another idea is to cost research support staff into the project so that when we open they’re ready to go out and support the GPs
I think that that would go a long way because any initiative, let me back up, any practice initiative that does not require the GP to work longer and harder.
FG1
The GP also discussed the infrastructure, which may or may not be in place in a particular GP practice. Larger practices are more likely to have the infrastructure and support in place to enable GPs to recruit into the study:
Well there may be but I suppose, you see I don’t think I’m that different from an average GP in the UK
You are because you’re here!
Particularly if if, I believe the clinical question is an important one for General Practice, maybe the size of the practice
OK
Maybe, I mean I was looking at maybe eight new
GPs
No eight new patients in a year
Oh OK
I’m working 5.5 days a week in an academic practice, cause the other half of my time I’m at the university
Mmhmm
Whereas if you have a GP in a practice where the average list is say 1800 people
Mmm
You know, a group of five or six GPs probably would have lots of experience with this
Yeah
Whereas if you’re a solo practitioner
And haven’t got time to do so much research activities
No if there’s a fairly robust infrastructure
So larger practices
Yeah, if you could take what was a highly functional PCT [primary care trust] if you will with six practices and so on
Mmm
So I don’t know how the Clinical Commissioning Groups are going, but they, it’s probably a rose by another name
Well yeah
But you need a minimum size I would think for it to be.
FG1
There was some discussion among the haematologists in FG2 and the primary care participants in FG4 about which cancers place patients at greater or lower risk of CAT and which patients, therefore, the trial should focus on or not. In FG2, breast cancer in particular was felt to be particularly challenging:
So you’re going to pick out your lower risks [incomprehensible]
Your aggressive pancreatic cancers you’re not going to stop
That’s right, I think aggressive might be different from dying
They’ll probably die before they’re 6 months
Aggressive adenocarcinomas, especially gastric pancreatic carcinoma someone who has metastatic metastatic breast cancer, so she’s got
Breast cancer with bone mets [metastases], she’s having bisphosphonate infusions and she had you know she’s had low-molecular-weight heparin for 6 months
Breast cancers are the lower-risk cancers, so I think for this trial I’d probably go I can probably do that
Prostate cancer with bone mets
Probably as well
Colorectal cancer with liver mets, not having any more treatment
[Laughs] I’m getting twitchy
No
[Laughs] lung cancer
[Incomprehensible]
With mets? Oh they’ve got to have mets
Well no they could have locally advanced lung cancer which was inoperable and they’ve had palliative radiotherapy or palliative chemo
Certainly brain tumours, pancreas, stomach, primary lung cancer you can almost certainly say no, I would not
I would probably be a bit more, I probably would maybe a bit more than these guys here
I guess what I’m hearing here is we’d be likely to recruit patients that were not representative of cancer-associated thrombosis
Yeah I agree with that
[Incomprehensible]
The ones you’re less worried about
You will
But breast cancer is an interesting one because it seems to be associated with lower-risk cancer-associated thrombosis, but people can have advanced disease and be treated palliatively for quite a long period of time I understand
You get quite –vein remissions as well I believe, it’s a tricky one.
FG2
The participants felt that breast cancer brings with it a perceived lower risk of CAT but patients could have the disease for a long time and be treated palliatively for a long time with extended remissions.
Ethics and risk
In terms of perceptions of whether or not the ALICAT trial is ethical, one area of potential concern raised by the GP in FG1 was the notion of an ‘enticement’ for clinicians (GPs) to recruit patients into research studies:
I think so I guess a sticking point for GPs in my community might be broaching perhaps an awkward circumstance in terms of is the GP being paid to enrol people
OK
In the study and it’s not just unique to GPs any any clinical researcher and of course. . .I err err so I guess what I am getting at is err what would the conversation be between me and someone in the practice
Yes there might be an enticement
Well that’s right and err I think an unusual err or a reasonable enticement . . . if it’s clear that I am being paid for the time and the energy of participating identifying people and so on
Yeah
But and so what it comes down to then is very much the matter of money and I think the degree to which GPs are willing we GPs are willing to for people to know
Yes
How much I am being paid by asking you to consider being in it
FG1
While the GP acknowledged that the payments really are to cover time taken to recruit and not really for profit, he raised a concern that such incentives may lead to a rush to recruit people by a certain date:
I’ve reviewed clinical trials where House Staff, Senior Registrar level, um may make [CAN]$1500 to $2000 for enrolling someone in the A&E department to do a trial, well yes and time out
Dodgy isn’t it
Dodgy yes! Quite dodgy, and um so that’s the sort of trial that
OK
That will get heavy
No there wouldn’t be any great enhancements for this, it would just cover time and maybe a little bonus
But no, but that’s the sort of trial where, you know, the red lights are on from the committee’s point of view, that’s not a reasonable inducement
Absolutely
And there’s no way a GP uh an oncologist, whomever the clinician is, is gonna be neutral on, you know, and I, particularly some trials you’ll see there’s a fine print that they have to be enrolled by this date
Mmm mmm
And and
Pressure yeah
This mad dash to enrol people.
FG1
The GP felt that it was vital to get the process for obtaining informed consent right given the importance of the question ALICAT is trying to answer:
You know is this worth doing on the surface it certainly seems so but then you know the sticking point often is how clear is the informed consent
Yeah
Is the IC [informed consent] as it relates to potential subjects and so that would be a critical issue in terms of you know.
FG1
The haematologists in FG2 were asked whether or not they thought there was a risk that clinicians would recruit into the trial for incentives. The haematologists felt that this was not really of concern as, despite instinctively feeling that anticoagulation should continue, ethical approval has been granted based on the fact the evidence base is lacking and there is a question that needs answering:
Is there a danger therefore that we could end up when do this feasibility to recruitment that we get a group of oncologists who go ‘no problem, happy to recruit, get my points in’ when your feeling as an oncologist [haematologist] is actually I’d be wary about recruiting into the study, and that’s a concern for me thinking patient
I think even though our gut feeling is you need to carry on with anticoagulation, there isn’t the evidence there to support that
That’s because we’ve had to decide that in the absence of evidence
Yeah yeah, so I don’t think you need to worry about that, I think if you’ve got ethical approval based on the data that’s out there, it doesn’t really matter what the knowledge base of the oncologists and the GPs are, because you’ve got the ethical approval based on the data that’s out there, so although we’ve got this gut feeling it’s not evidence based.
FG2
The haematologists also felt that one potential difficulty when recruiting patients to the ALICAT trial would be the fact that patients may wish to continue with anticoagulation. These participants felt that the very fact that ethical approval has been granted (and there was some surprise expressed among this group that ethical approval had been given) indicates that there is in fact no available evidence for patients to carry on with anticoagulation therapy. As a result there is the possibility that a greater number of lower-risk patients will be recruited into the trial:
No I’d read the details and see, I’d have to review all the data and see, you know, because as you said you’ve got ethical approval to stop treatment
Yep
So who am I to sit there and say no I want to carry on, cause you’ve got ethical approval, so that would indicate to me that the data is not there for me to say carry on
I think the situation you might find it a little patchy
Yeah
I think you’ll struggle to get to certain patients because in certain situations you’ll say this person can go into the study, but
Yeah
So you’re going to pick out your lower risks [incomprehensible]
Your aggressive pancreatic cancers you’re not going to stop
That’s right, I think aggressive might be different from dying
They’ll probably die before they’re 6 months
Aggressive adenocarcinomas, especially gastric pancreatic carcinoma.
FG2
Those patients with more aggressive cancers and who are at greater risk, may have died by the time their initial 6 months of treatment has passed, and so the study population again is more likely to comprise more lower-risk patients:
Well certainly you know in the context of unprovoked venous thromboembolism I think what most people think about would be that you’d be happy to keep patients off anticoagulation for a set period if the risk of annual risk recurrence is probably less than about 3%
Yeah
[Incomprehensible]
Yeah
[Incomprehensible]
Well I mean the risk of bleeding is 1% per year if you stay on anticoagulation, so your risk recurrence has got to be more than that
It’s got to be more than five in order to get more PE deaths than anticoagulant bleed deaths
Oh OK yes that’s right of course the [incomprehensible] rate yeah
But that’s just mortality rate
Yeah yeah
Yeah
Um so in a cancer patient I’m not sure.
FG2
Of course the other thing, as [names P1] said of course by the time you’ve looked at the patients after 6 months the most aggressive ones
Will have died yeah
Yeah, so it’s just a slightly altered group.
FG2
Similarly, the oncologists in FG3 also noted that it might be difficult to stop anticoagulation therapy with patients who have a lot of disease:
There are already two counts doesn’t it . . . Either we have patients that we pick up they’re in their first line treatment . . . They’re disease free or they’re in remission then I would find it very hard to keep a [inaudible]
They’re not eligible
They’re ineligible OK
They’re ineligible
Then if they’ve got a lot of disease I would equally find it difficult to stop their
Yeah
Anticoagulation because I think those are patients you have to have a def difficult discussion with to say you’re at ongoing risk, you’ve already had er venous thromboembolism or you’ve had you know DVT or a PE you’re already at risk. Do you want to continue? I mean I’d be encouraging those patients to carry on
But isn’t that exactly the point of doing research
A study to find out
You would encourage them to continue but you don’t actually know what the incidence is and then within the consequence I mean you know this is not something you should just make up as you ultimately you shouldn’t make it up as you go along.
FG3
However, it was also noted amongst the oncologists, that in terms of clinical trials in general, patients can, and are, randomised to receive ‘much more toxic treatments with probably as little benefit, [indecipherable] chemotherapy’ (FG3:P11).
Discussion
Overall, the health-care professionals across all focus groups felt positively towards the ALICAT trial. There was a consensus that the ALICAT trial was trying to address an important question which needs answering. While in their clinical practice the professionals are inclined to continue patients with anticoagulation therapy beyond 6 months, they recognised that this was without an evidence base to support their decisions. The professionals reported making these decisions on a case-by-case basis, assessing appropriate risk factors and taking patients’ preferences into consideration. There was also a sense among the professionals that oncology should take overall responsibility for the management of VTE, although this viewpoint was less strongly stated among the oncologists themselves.
While the professionals felt quite positive towards the ALICAT trial, there was some concern raised as to difficulties investigators may experience when trying to recruit to the trial. The professionals felt that while this was worthwhile and many patients may be willing to take part, some may feel they wish to continue with anticoagulation therapy; equally others may not and the chance that they may not be randomised to either option is a risk for attrition. There was also a concern that those patients at increased risk of VTE may not be referred to the trial as there would be a potential need to ensure they remained on anticoagulation therapy.
Chapter 4 Patient pathways
Introduction
Cancer-associated thrombosis can manifest at many different stages of a patient’s illness trajectory, but in each case there will be a process of diagnosis and treatment in which a variety of health professionals will be involved. In order to be able to target – and thereby optimise – recruitment for the proposed stage III ALICAT trial, we set out to map current points of contact between CAT patients and the health professionals in charge of their care.
We focused our investigation on oncology and haematology departments in UK hospitals, and in particular on pathways for CAT diagnosis with subsequent initiation, management and end-of-treatment decisions. We also tried to get insight into points and ways of communication between health professionals and patients as well as interprofessional ways of conveying information relevant to diagnosing and treating CAT.
This section of the ALICAT trial report will present the pathways mapping substudy, detailing both the interview and the online survey procedures and outcomes.
Methods
Between April and June 2014 we conducted structured telephone interviews with a convenience sample of seven oncologists and five haematologists, all working full-time and based in UK hospitals. In the first instance, outcomes from the telephone interviews were used to inform the design of an online survey for oncologists which was piloted in July and made available on Bristol Online Surveys from early August to the end of September 2014. As the time for keeping the survey live was limited we aimed to maximise response rates by targeting health professionals with a proven interest in research. Therefore we asked the National Cancer Research Institute to distribute the survey link to their oncology Clinical Studies Group members. The first request went out on 8 August 2014 with a reminder e-mail message exactly 1 month later. Participation in the survey was furthermore promoted using oncologist, GP and research colleagues’ Twitter accounts (Twitter, Inc., San Francisco, CA, USA).
To be able to contextualise survey outcomes, and to present, to some extent, a haematologist point of view, the telephone interviews were subjected to a more in-depth data analysis. For this, the interviews were transcribed verbatim and content coded using NVivo10 data analysis software. To test the coding framework, 20% of the interviews were double-coded by a second experienced qualitative researcher on the team. Overall agreement on coding categories and application of codes was high, but where agreement fell below 95% coding was discussed until agreement was reached. A small portion of the data was recoded as a result. Data excerpts presented use standard orthography and anonymised speaker identifiers [Health Professional (HP)1, HP2, HP3, etc.].
Results
Oncology survey
Curtailed by the more limited time available to complete the Pathway Mapping component of the ALICAT feasibility study, the study team focused on only one of the two professional groups initially targeted for online survey and set a maximum response time of just under 2 months. Survey results are presented in narrative summary, with tables presenting more detailed overviews of responses.
A total of 48 responses (43 complete, 5 incomplete to varying degrees) were received to the online survey, from across England, Wales and Scotland, representing a total of 23 different NHS organisations (Table 7). The majority of responders were consultants and worked in a specialist cancer clinic. Responders specialised in a broad range of cancer areas and saw more than 20 cancer patients a week.
| Characteristics | Number | Percentage |
|---|---|---|
| Sex | ||
| Male | 26 | 61 |
| Female | 17 | 40 |
| Age (years) | ||
| 30–39 | 3 | 7 |
| 40–49 | 26 | 61 |
| 50–59 | 13 | 30 |
| 60–70 | 1 | 2 |
| Geographical location | ||
| England | 26 (from 18 different NHS sites) | 61 |
| Scotland | 7 (from 2 different NHS sites) | 16 |
| Wales | 7 (from 3 different NHS sites) | 16 |
| Northern Ireland | 0 | 0 |
| Prefer not to say | 3 | 7 |
| Position | ||
| Consultant | 40 | 93 |
| Specialty registrar | 1 | 2 |
| Clinical nurse specialist | 1 | 2 |
| Other | 1 (nurse consultant) | 2 |
| Main place of worka | ||
| General hospital | 7 | 15 |
| Specialist cancer clinic | 35 | 73 |
| Thrombosis clinic | 3 | 6 |
| Other | 2 | 4 |
| Cancer specialtya | ||
| Gastrointestinal | 9 | 19 |
| Breast | 6 | 13 |
| Pancreatic | 5 | 10 |
| Lung | 5 | 10 |
| Gynaecological | 5 | 10 |
| Prostate | 6 | 13 |
| Melanoma | 2 | 4 |
| Haematological | 7 | 15 |
| Sarcoma | 5 | 10 |
| Other | 7 | 15 |
| Prefer not to say | 2 | 4 |
| Number of cancer patients seen every week | ||
| < 10 | 2 | 5 |
| 10–20 | 6 | 14 |
| 21–30 | 13 | 30 |
| 31–40 | 11 | 26 |
| > 40 | 11 | 26 |
Most clinicians saw 1 or 2 patients per month who had been newly diagnosed with CAT and 1 or 2 who they suspected of having CAT (Table 8). The most common place to refer patients suspected of CAT was radiology, with acute assessment units as the second most prominent category. For oncology patients, diagnoses were predominantly made by oncologists (both in- and outpatients). Almost two-thirds of clinicians reported having pathways in place for the diagnosis and management of CAT. The majority of these guidelines included the type of treatment, with just under half including who is responsible for treatment and treatment duration (Table 9).
| Survey response | Number | Percentage |
|---|---|---|
| How many patients do you see who have been newly diagnosed with CAT? | ||
| None | 2 | 4 |
| 1 or 2 per month | 34 | 71 |
| 1 or 2 per week | 12 | 25 |
| 3 or more per week | 0 | 0 |
| In how many cases does this involve an incidental blood clot? | ||
| None | 4 | 8 |
| 1 or 2 per month | 39 | 81 |
| 1 or 2 per week | 5 | 10 |
| 3 or more per week | 0 | 0 |
| How many patients do you see who you suspect have a CAT? | ||
| None | 1 | 2 |
| 1 or 2 per month | 35 | 73 |
| 1 or 2 per week | 11 | 23 |
| 3 or more per week | 1 | 2 |
| Where do you refer patients you suspect may have a CAT?a | ||
| Do not refer | 6 | 13 |
| Radiology | 37 | 77 |
| Acute assessment unit | 13 | 27 |
| Community thrombosis clinic | 1 | 2 |
| Oncology colleague | 2 | 4 |
| Haematology colleague | 3 | 6 |
| Other | 4 | 8 |
| For how many patients do you make the actual diagnosis of CAT? | ||
| None | 3 | 6 |
| 1 or 2 per month | 36 | 81 |
| 1 or 2 per week | 6 | 13 |
| 3 or more per week | 0 | 0 |
| Who has the main responsibility for diagnosis of CAT in inpatients?a | ||
| Oncologist | 35 | 73 |
| Radiologist | 14 | 29 |
| General medical team | 15 | 31 |
| Thrombosis clinic | 3 | 6 |
| Other | 3 | 6 |
| Who has the main responsibility for diagnosis of CAT in outpatients?a | ||
| Oncologist | 37 | 77 |
| Radiologist | 14 | 29 |
| General medical team | 9 | 19 |
| Thrombosis clinic | 6 | 13 |
| Other | 5 | 10 |
| Are there established pathways for diagnosis and management of CAT? | ||
| Yes | 33 | 69 |
| No | 15 | 31 |
| Survey response | Number | Percentage |
|---|---|---|
| What do guidelines cover? | ||
| Who is responsible for treatment | 23 | 48 |
| Type of treatment | 30 | 63 |
| Duration of treatment | 20 | 42 |
| Other | 6 | 13 |
| Do you start patients with CAT on anticoagulation? | ||
| Yes | 44 | 98 |
| No | 1 | 2 |
| What is your treatment of choice for CAT?a | ||
| LMWH | 44 | 92 |
| Warfarin | 1 | 2 |
| Other | 2 | 4 |
| Who is usually involved in initial treatment decisions?a | ||
| My decision | 41 | 85 |
| Other oncologist | 8 | 17 |
| General medic | 5 | 10 |
| Haematologist | 5 | 10 |
| Clinical nurse specialist | 3 | 6 |
| Radiologist | 2 | 4 |
| GP | 3 | 6 |
| Patient | 8 | 17 |
| Other | 2 | 4 |
| Do patient preferences play a deciding role in whether or not to use LMWH or another anticoagulant? | ||
| Yes | 25 | 56 |
| No | 20 | 44 |
| Are you approached by others for advice on treatment of CAT? | ||
| Yes | 28 | 64 |
| No | 16 | 36 |
| If yes – who (n = 28)?a | ||
| Other oncologists | 9 | 32 |
| General medics | 15 | 54 |
| Haematologists | 4 | 14 |
| Clinical nurse specialists | 11 | 39 |
| GPs | 10 | 36 |
| Other | 2 | 7 |
| Where is the treatment of CAT managed?a | ||
| Own clinic consultations | 35 | 73 |
| Hospital ward | 16 | 33 |
| Specialist thrombosis clinic in hospital | 19 | 40 |
| Community thrombosis clinic | 7 | 15 |
| GP practice | 17 | 35 |
| Other | 8 | 17 |
| How often do you discuss anticoagulation management with your patients?a | ||
| Every consultation | 12 | 25 |
| Every other consultation | 6 | 13 |
| When changing dosage | 15 | 31 |
| When ending treatment | 14 | 29 |
| When relevant to changes in patient condition | 25 | 52 |
| Other | 2 | 4 |
| What other professionals are involved in prescribing and reviewing anticoagulation treatment in your patients?a | ||
| Nobody | 3 | 6 |
| Other oncologists | 16 | 33 |
| General medic | 8 | 17 |
| Haematologist | 11 | 23 |
| Clinical nurse specialist | 10 | 21 |
| Hospital thrombosis clinic | 22 | 46 |
| Community thrombosis clinic | 8 | 17 |
| GP | 25 | 52 |
| Other | 5 | 10 |
The overwhelming majority of responders made decisions about treatment themselves, although just under a half indicated that for type of treatment they would take patient preferences into account. The treatment of choice was LMWH. Most managed the CAT within their own clinic consultations and mainly discussed it with patients when their condition changed. GPs and hospital thrombosis clinics were also commonly involved in the management of patients with CAT.
Most LMWH was used for 4–6 months or for more than 6 months, although there were also a number of other options indicated which predominantly represented considerations of patient condition and other treatments in order to determine duration of LMWH (Table 10). The most common reasons for ending treatment were because of the guidelines or that the patient deteriorated. The clinicians responding were most likely to be the ones making the decision to end treatment.
| Survey response | Number | Percentage |
|---|---|---|
| If patients condition indicates use of LMWH, how long do you use it for?a | ||
| Do not use | 0 | 0 |
| 1–3 months | 4 | 8 |
| 4–6 months | 23 | 48 |
| Over 6 months | 19 | 40 |
| Other | 16 | 33 |
| What are your most common reasons for deciding to end treatment with LMWH?a | ||
| Guidelines | 29 | 60 |
| Patient deteriorated | 27 | 56 |
| Patient improved | 12 | 25 |
| Patient choice | 21 | 44 |
| Other | 7 | 15 |
| Who is usually involved in the decision to end treatment?a | ||
| My decision | 30 | 63 |
| Other oncologist | 12 | 25 |
| General medic | 5 | 10 |
| Haematologist | 9 | 19 |
| Clinical nurse specialist | 2 | 4 |
| Hospital thrombosis clinic | 10 | 21 |
| Community thrombosis clinic | 3 | 6 |
| GP | 11 | 23 |
| Other | 5 | 10 |
The majority of clinicians had previously put patients into research studies and would be happy to recommend patients to enter a trial at the end of 6 months of treatment with LMWH (Table 11). Most did not think that the current guidelines had enough evidence behind them. Slightly more clinicians agreed with a trial of full treatment against no treatment than full treatment against prophylactic treatment. Very few responders thought that the heterogeneity of the patients and their conditions meant that there was no place for a trial.
| Survey response | Number | Percentage |
|---|---|---|
| Have you ever recommended any of your patients participate in research? | ||
| Yes | 40 | 93 |
| No | 3 | 7 |
| Depending on their condition I would recommend my patients take part in a trial studying the continued use of LMWH after 6 months | ||
| Definitely | 13 | 30 |
| Probably | 17 | 40 |
| Possibly | 7 | 16 |
| Probably not | 5 | 12 |
| Definitely not | 1 | 2 |
| Current guidelines to stop LMWH after 6 months are based on sufficient evidence | ||
| Strongly agree | 0 | 0 |
| Agree | 2 | 5 |
| Neither agree nor disagree | 9 | 21 |
| Disagree | 26 | 61 |
| Strongly disagree | 6 | 14 |
| In a RCT on the use of LMWH after 6 months, patients should be randomised to full dose or stopping | ||
| Strongly agree | 4 | 9 |
| Agree | 21 | 49 |
| Neither agree nor disagree | 12 | 28 |
| Disagree | 5 | 12 |
| Strongly disagree | 1 | 2 |
| In a RCT on the use of LMWH after 6 months, patients should be randomised to full or prophylactic dose | ||
| Strongly agree | 3 | 7 |
| Agree | 21 | 49 |
| Neither agree nor disagree | 7 | 16 |
| Disagree | 11 | 26 |
| Strongly disagree | 1 | 2 |
| There are too many patient and disease variables to conduct a RCT on the continued use of LMWH | ||
| Strongly agree | 0 | 0 |
| Agree | 5 | 12 |
| Neither agree nor disagree | 8 | 19 |
| Disagree | 23 | 54 |
| Strongly disagree | 7 | 16 |
Interview data
Although the survey results provide a representative snapshot of current practice, they cannot capture individual enhancements to, or reservations about, accepted norms of professional engagement. Variations in practice, often described as being contingent on patients’ needs and circumstances, can potentially fall outside the categories a survey may provide. A choice was made, therefore, to investigate and incorporate more personal accounts of how some oncologists and haematologists experience the struggle of knowing what is best for their patients when independent evidence is thought to be lacking, or at the very least incomplete. This overview of interview results, therefore, is meant to contextualise the more categorical answers provided in the previous section. The aim is to show how in spite of systems operating well across a variety of settings, the decisions that need to be made can ultimately still be highly personal. For this component of the ALICAT study, these decisions are discussed in terms of how they may affect potential recruitment.
Pathways
Most interviewees report hospital- or trust-wide application of guidelines for dealing with occurrences of VTE and PE (Table 12). While current pathways are described as necessary and useful – ‘they move them through the system’ (HP9) – there is a sense that where CAT is concerned these guidelines have clear limitations. Existing guidelines stratify according to generic risk factors for VTE to generate a risk score/algorithm which enables medical staff to make treatment decisions. However, these guidelines tend to cover initial steps (up to diagnosis and treatment initiation), but not long-term management or end-of-treatment decisions. Prescribed pathways for management of any medical condition need to be highly specific to that condition. Oncological conditions are inherently complex and CAT forms only a part of that complexity. The treatment trajectories for CAT described by interviewees are inescapably tied in with the cancer treatments and necessarily reflect individual patients’ clinical needs and circumstances. In order to identify where recruitment to an ALICAT trial might be most prolific, the focus must be on these more elaborate treatment and management ‘pathways’ which go beyond the more narrow guidelines already officially in place and which may well differ between hospital sites and individual consultants.
| Patient presents with DVT or PE symptoms or asymptomatic find on staging scan | Diagnosis | LMWH treatment initiation | Treatment management | End of treatment decision |
|---|---|---|---|---|
| Radiologist ↓ Oncologist Oncologist ↓ Radiologist |
Oncologist | Oncologist (in conjunction with managing cancer treatment) | Oncologist (duration decision based on patient’s needs and circumstances) | |
| Acute medicine consultants/clinical assessment team | General medicine team starts patient on LMWH | Management passed on to relevant specialist teams (mainly oncology) | Oncologist (or other specialist) | |
| Ambulatory care team/specialist VTE service (often nurse led) | Thrombosis clinic team (nurse, pharmacist) | Managed in the community (thrombosis clinic, GP) | GP (in discussion with specialist) | |
| Haematologist (rarely involved in diagnosis) | Haematology deep-vein thrombosis clinic | Haematologist (advice on thrombosis or treatment complications and duration) | Haematologist | |
| GP (refers to acute medical team for diagnosis when suspecting blood clot) | Acute medical team or oncologist | LMWH prescribing (where allowed) | GP (often in discussion with specialist) |
Interviews focused on oncologists and haematologists as they were deemed most likely to see patients with CAT. However, in the interviewees’ descriptions of how they come to be involved in the treatment of such patients, a third group of health professionals emerged as playing a prominent role. Many patients ultimately diagnosed with CAT were described as entering the system through an acute medical assessment/medical decisions unit (either A&E or an ambulatory care team). From there, patients are then either admitted acutely and started on LMWH or initial anticoagulation is provided, the patient remains ambulatory and is referred on to an appropriate medical specialist. General medicine teams are reported as being involved in inpatient as well as outpatient treatments, for example, if a hospital has no outpatient ambulatory care team the patient can be admitted under the ambulatory care of an acute medical consultant. One interviewee stressed that a patient will only end up with an oncologist once the diagnosis of cancer has been made.
Between oncologists and haematologists there is a clear difference in how many CAT patients they actually diagnose. This ranges from one or two per week to one or two per month for oncologists, whereas the haematologists mention approximately one or two patients per year whose diagnosis they are actively involved in. Naturally, given the nature of the condition, oncologists have much closer involvement with CAT patients over a much longer period of time. Haematologists see themselves more as advisors to oncology colleagues when complications arise or when end-of-treatment decisions pose particular problems. This interview study, therefore, suggests that oncologists are the main/most significant point of contact for patients with CAT, with general medicine staff in clinical/acute assessment units offering a second opportunity for initially introducing potential trial participation.
The following sections will illustrate in some detail the current practice of managing patients with CAT as described by the interviewees. The aim is to highlight how variation in practice may need to be taken into account when devising proposed trial recruitment strategies. Especially relevant in this respect will be considerations about which part of the treatment trajectory should be the main focus of trial recruitment efforts.
Treatment initiation
As well as diagnosing CAT, treatment initiation often falls within the remit of emergency or general physicians. In hospitals where defined guidelines and pathways for thromboprophylaxis are in operation, treatment can also be initiated by clinical specialist nurses or pharmacists in dedicated ambulatory care or deep-vein thrombosis clinics. GPs are described as referring patients to be clinically assessed but not as initial prescribers. Where a patient is already in the care of an oncologist, this consultant will start the patient on anticoagulation or provide a recommendation to the expert team in the ambulatory care centre. LMWH therapy is considered the gold standard in treating CAT, needing little or no input from haematology.
Managing treatment
Oncologists are the main health professionals involved in the management of patients with cancer-associated thrombosis. As treatment with LMWH is deemed to need little active monitoring, patients who do not suffer complications can also remain in ambulatory care with ongoing prescribing deferred to the GP. For patients with active cancer, anticoagulation is described as ‘fitting around’ chemotherapy with anticancer treatment necessarily taking priority. Many of the oncologist interviewees say treatment of CAT is managed in their regular consultations with patients. Although monitoring of blood count and being alert to symptoms of thrombocytopenia tend to be part of these regular consultations there is, however, a danger that the focus may shift away from the VTE or PE, as described by one oncologist:
The fact these people have got VTE tends to drop to the bottom of the priority list. Well, not so much priority but what’s in your mind when you’re seeing them
Mhm
Because you’re focusing on their trea- on their chemotherapy and their cancer response so you know they’re diagnosed with VTE start their- doesn’t change what we do in terms of them their chemotherapy once they’re on Low Molecular Heparin. We just carry on and often we forget, I think, how long people have been on Heparin, for, you know, it’s something that drops down uhm you know when you see them in clinic your foc- your- all the consultation revolves around the chemotherapy and the cancer and the VTE you can forget.
Mhm
So when I say we’re sort of overseeing their trea- we are with the the giving the drugs out but we’re not uhm I don’t, you know, consultation rarely moved- would really focus on the VTE aspect
[three turns omitted]
Uhm but yeah so I think managing it is a little bit you know that obviously may mean different things to different people but even if (specifically) it means we give out the drug we’re seeing them regularly but not for their VTE but if someone is not on treatment or about to have a treatment break I would not see them but purely because of their VTE I would see them only if I need to from a cancer point of view.
HP10
If ‘managing CAT’ does indeed mean ‘different things to different people’, and the use of LMWH is seen as not requiring monitoring, the question of who exactly takes responsibility for a CAT patient’s care becomes a pertinent one, as indicated by the following interviewee:
If they go onto low-molecular-weight heparin it’s a bit sort of ad hoc as to who they end up with
Mhm
[May] have concerns that that they sometimes you know they get sent back to their GPs and you know you hope that their GPs pick it up but we don’t necessarily always you know have have a handle on this
Right can you explain a little bit more about why you would have concerns about this
Yeah because uhm it it because they started on the low-molecular-weight heparin there’s no monitoring required so theoretically they could say they well we don’t need an anticoagulant team you know they they I mean what are they going to do because there’s no need to initiate Warfarin or initiate a normal anticoagulant therefore you know we can just basically send this patient to the GP and ask the GP to carry on prescribing but it’s you know when they come to us we we we not necessarily we’re not geared towards on continuous follow-up so even if we do see them
Mhm mhm
You know my worry is we’ve seen them and we we you know we liaise with the GP about carry on prescribing. It’s like who’s who’s keeping an eye on that patient because there’s, we don’t uh, so does the oncologist keep an eye? Is it the GP that’s keeping an eye? You see, a plan might be put in place say 6 months of anticoagulation but is that- who who’s sort of keeping tabs on it? So we’ve had occasional patients who are still on low-molecular-weight heparin after a year or two because nobody’s actually made sure that they stopped their treatment
Right
So I think I think as a bunch of patients we are, you know, we we advise what should happen to them, but the lack of monitoring and the lack of ownership by the anticoagulant clinic means that, I think, you know, there there it’s like it’s like who’s keeping you know who’s keeping tabs who’s responsible.
HP11 (haematologist)
A workable trial recruitment strategy needs a focus on time points and/or specific health professionals as the best points of contact. However, if, as the interview data suggests, concepts like ‘treatment management’ and allocation of responsibility lack definition and certainty, the feasibility of recruiting the required number of patients may be thrown into doubt.
End of treatment: significant uncertainties
In the interviews, the one overarching theme in the discussion about end-of-treatment decisions is the uncertainty about what to do in cases where there is still evidence of active cancer after an initial 6 months. This is summed up in the following data excerpt:
When we come to the end of, you know, in in patients when they have come to the end of say their 6 months and they’re on chemotherapy then they’re asking ‘well, how long do I need to stay on this for?’ Then we have a discussion about significant uncertainties and say ‘you know, you’ve been on low-molecular-weight heparin and haven’t had another problem’ and we can’t really prove a negative, all we can do is when we stop if you didn’t have another problem we’d say that was the right thing to do and if you had another problem we’d say it was the wrong thing to do.
HP2 (oncologist)
One interviewee phrased current practice as ‘making it up as you go along’ (HP10), and another as ‘every time this happens we sit and scratch our heads and wonder are we doing the right thing for our patients or not’ (HP2). When dealing with patients with active cancer, practice is described as varying between continuing LMWH indefinitely at full dose to switching to prophylactic dose after 6 months, and stopping only when risks like significant bleeding become apparent and outweigh the perceived benefits.
Logic dictates that the end of recommended treatment (i.e. 6 months as per NICE guidelines) would be a good time point for a clinician to broach (again) potential participation in a trial investigating the effectiveness of using LMWH longer term. The question is whether the uncertainty permeating their own discourse about the topic would help or hinder in convincing patients of the need for a trial.
Communication
When asked about how they communicate with patients about the need to be on anticoagulation longer term, interviewees presented scenarios centring around enabling patients to make informed decisions. In some cases this entailed communicating the ‘significant uncertainty’ the health professional him or herself may feel. If this is done well, and right from the start of treatment as described in the data excerpt below, that could function as a useful introduction to potential trial participation as well:
What what what kind of discussions do you have with your patients about
Mhm well at the beginning I’ll say this is what I strongly suspect is the case and we need to manage that to prevent uhm you know a life-threatening event what we’re going to need to do is add in an injection everyday
Right
And then it’s always injection and uhm and I tell them why I prefer an injection but you are right there’s no evidence out there
Mhm
There’s a sense from some of the published articles in kind of early two thousands perhaps patients do better on low-molecular-weight heparin rather than Warfarin so (that has become our standard) practice
Mhm
Uhm and therefore I I’ll say to them this is what we’re going to do and then I give them the choice would you like to learn to inject yourself or not and then we have a time point and then I’ll say you’ll need to be on this for at least 6 months and it depends on how well your cancer is responding to treatment
Mhm
Actually I say to them there may be an argument of not coming off and we’ll we’ll discuss that when we get to 6 months
Right
I sort of put that in their brain at the beginning
HP9 (oncologist)
Discussion
It is evident from both survey and interview data that the standard pathways, which are currently in operation, are limited to diagnosis and treatment initiation for specific occurrences/types of symptoms/types of patients and are therefore only partially useful as a guide for recruitment optimisation. Moreover, as individual hospitals have different funding structures, location limitations and policy guidelines regarding CAT, it does not seem possible for a future ALICAT trial to implement a one-approach-fits-all recruitment scenario.
Although oncologists are by far the most prominent group of health professionals dealing with instances of CAT, general (emergency) physicians and/or staff in dedicated DVT clinics are also involved. They often make the initial diagnosis and start patients on anticoagulation treatment. Potentially, recruitment efforts could include these health professionals.
For CAT, treatment initiation and type of treatment preferred are fairly standard but there seems to be wide-ranging uncertainty about when to end treatment. There are marked differences of opinion about this across the professional groups (including GPs) so clearly more evidence is needed.
Chapter 5 Overall discussion and conclusions
Introduction
Cancer-associated thrombosis remains an important complication of the cancer journey and continues to increase in incidence. The current evidence only informs the management of CAT for the first 6 months of diagnosis, with no strong trial data to inform what should be done for patients with ongoing active cancer reaching 6 months’ anticoagulation treatment. Despite the need to address such a significant knowledge gap, this feasibility study strongly suggested that a Phase III RCT to answer this question was unlikely to recruit the necessary number of patients within a reasonable time frame that made funding such a study worthwhile. The lessons learned from the design, set-up and conduct of the ALICAT study have been highly informative and offer useful insights pertaining to the design of future similar studies. The challenges faced and lessons learned are discussed below. The challenges are categorised according to whether they are logistical or attitudinal.
Challenges: procedural
On reflection, the design of the ALICAT study was an ambitious one. Although trials units have considerable experience co-ordinating multicentre studies, undertaking a RCT in three clinical environments and across two nations brought additional challenges, some of which could be avoided if all stakeholders involved in clinical research streamlined their processes, minimised repetition of processes and addressed bottlenecks arising from arguably unnecessary bureaucracy. Although the best research questions are the simple ones and simple trial designs are more likely to complete recruitment, one should not always pick the low-hanging fruit. By only focusing on the simple common questions, one will neglect delivering the answers to more complex or rare problems.
One site’s opening was delayed by 2 months while awaiting approval from the trust research and development (R&D) radiation lead to sign off the study, to say there were no additional radiation risks posed by the trial. What took 1 day for approval in one organisation took 56 days in another, with no excuse offered save consultant workload. Such examples reinforce the responsibility that all those who commit to involvement in research have. One cancelled risk review committee here, one R&D lead who is off sick and not backfilled there, can lead to a 6-month delay in a trial set-up before any legitimate concerns are raised.
The slightly different approvals processes between the NISCHR in Wales and NIHR in England led to a significant delay in setting up sites outside of Wales. This suggests a degree of disconnect between nations, which should be remediable.
Another challenge identified through the pathways mapping section of the study was the fact that CAT is managed differently across health organisations with no clear consensus nor consistency for ownership. Although the management of thrombosis is usually a haematology issue, which is then taken on by primary care, this is not the case for CAT. Haematology services seem best suited to manage CAT; it is frequently taken on by the patient’s oncologist who often does not have the time or equivalent expertise to manage the more complex cases that may arise. From the perspective of the feasibility study, the mapping exercise highlighted the breadth of environments and clinical pathways across which CAT is managed, thereby outlining the challenge of recruiting to such a RCT.
Challenges: attitudinal
Even if it were possible to establish a robust recruitment strategy and environment, it appears that we would still be unlikely to recruit sufficient patients to the study. From the RCT component of the ALICAT trial, only 5 of the 32 eligible patients identified consented to randomisation, suggesting that patients do not wish to participate in such trials. Furthermore, the scoping exercises suggest that clinicians likewise may be reticent to recruit to such a study since they already hold fixed views regarding the management of VTE beyond 6 months. These issues are discussed further in Influence of prior experience and Belief in equipoise.
Influence of prior experience
From the embedded qualitative study, it was possible to ascertain reasons for patients declining randomisation into the RCT and these seemed equally divided between a fear of randomisation to continue LMWH and a fear of randomisation for stopping it. It is clear that the patient experience of the index VTE event had a strong influence on their attitudes and beliefs regarding anticoagulation; in particular, there appears to be a divide between those diagnosed with symptomatic and incidental VTE.
For those patients with symptomatic VTE, there remains a strong association with a distressing symptomatic event and a subsequent improvement of symptoms with commencing LMWH treatment. Such patients lived with a concern that by stopping the LMWH, the VTE would come back and were therefore reticent to risk participation into a study, which may discontinue the drug. Conversely, those with incidental and, hence, largely asymptomatic VTE had few associations with distressing symptoms. Rather, they perceived the requirement to self-inject for 6 months an inconvenience, which they could not wait to desist from. Participation in a study, which may risk further anticoagulation, did not appeal.
Belief in equipoise
The responses from patients declining randomisation, suggest they were influenced largely by prior experience of VTE and injecting LMWH. While they acknowledged an evidence deficit in the management of VTE they made their decisions based on their fixed views on whether or not they wished to continue LMWH, and not whether or not they needed to. To some extent, they used the evidence deficit to justify their decision and continuing or not continuing their LMWH in the absence of strong data did not appear to cause distress.
From the clinician interviews and focus groups there was acknowledgement of the lack of evidence around the management of VTE beyond 6 months. However, anticoagulation beyond 6 months off-license had become such custom and practice that most clinicians held fixed beliefs that this was the best form of treatment. These beliefs were further reinforced by satisfactory, if not formally evaluated, clinical outcomes. Interestingly, the haematology views were based far more on knowledge of the existing data and understanding the complexities of thrombosis and haemostasis, and as a group gave the haematologist a cogent case for why they feared the study lacked equipoise. Of those clinicians willing to randomise patients to the study, there was a clear sense that they would ‘cherry pick’ the cases in which they perceived there was less certainty about VTE recurrence risk. As such, those perceived to be more thrombogenic would not be approached, thereby leading to a highly biased sampling strategy.
Although there were established views that patients should continue with LMWH, there was acknowledgement that the required ongoing dose needed clarification.
It was interesting to observe that clinicians would be more willing to recruit to a study which randomised patients to weight-adjusted (full-dose) LMWH versus a primary thromboprophylaxis dose. This appears to be a more acceptable study design for clinicians and is likely to overcome any challenges of clinician recruitment. It is not clear whether or not this would lead to increased consent from patients; it is unlikely to change the views of patients with incidental VTE, as their reason for declining randomisation was to ensure they stopped the LMWH. However, the remaining majority of potential patients may find this study design more acceptable.
Priorities for future research
The management of CAT will remain a clinical challenge as it is a complication of cancer, which is increasing in parallel with the use of chemotherapy in patients with metastatic and curable disease.
Currently research strategies have focused on CAT as a single entity, which behaves consistently across the different tumour types. In reality, is seems counterintuitive to research the properties of CAT in breast cancer patients alongside those with advanced pancreatic cancer since the diseases have different levels of tumour-related thrombogenicity, extrinsic thrombotic risk factors and overall prognoses. An urgent consideration for the CAT research world is to focus research strategies on specific tumour types. One such option would be to focus on a tumour which is known to be less thrombogenic overall but still carries uncertainty of best management at the 6-month anticoagulation stage. Such a cancer would be breast cancer, which is not only common, but also common as a metastatic disease with a favourable overall prognosis.
Another area of research that is much needed is an adequately powered non-inferiority trial between one of the new oral anticoagulants and LMWH. To date, the data support the use of new oral anticoagulants in the non-cancer population, with non-inferiority to warfarin. However, it is yet to be evaluated head to head in the cancer population against LMWH. If an oral agent were to demonstrate non-inferiority to the current injectable gold standard, this would negate the reasons for non-recruitment based on disliking injections.
Conclusion
The ALICAT study has established that at the current time it is not feasible to recruit sufficient patients with advanced cancer and VTE to a RCT comparing 6 months’ anticoagulation with indefinite anticoagulation. The reasons for this are multifactorial, and include practical and procedural challenges, patients’ attitudes and those of the recruiting clinicians. Current practice to continue long-term anticoagulation, although not evidence based, is supported strongly by consensus and clinician experience. A study to confirm or refute that current practice is the most appropriate treatment regime is unlikely to be supported by clinicians in particular, until current practice is demonstrated to be financially unsustainable or clinically unsafe.
Acknowledgements
The ALICAT trial is a NIHR Health Technology Assessment programme-funded trial. Participating hospital sites are providing LMWH from local clinical supplies.
Study sponsor: Cardiff University, Cardiff, UK.
The other members of the ALICAT Trial Management Group include: Richard Adams [Acting Director, Wales Cancer Trials Unit (WCTU)], Gareth Griffiths (Former Director, WCTU), Terri Kitson (Data Manager, WCTU), Peter Rose (Consultant Haematologist, Warwick Hospital), Trevor Baglin (Consultant Haematologist, Addenbrooke’s Hospital), Loys Richards (Safety Officer, WCTU), Helen Falconer (Research Governance Officer, Cardiff University), Usman Malik (Principal Pharmacist, Velindre NHS Trust), Sue Kearney (Research Nurse, Velindre NHS Trust), Harold Toone (Research Partner) and John Bell (Research Partner).
Contribution of authors
Simon I Noble: study design, data analysis and manuscript write-up.
Annmarie Nelson: study design, data analysis and manuscript write-up.
David Fitzmaurice: study design and data analysis.
Marie-Jet Bekkers: study design, data analysis and manuscript write-up.
Jessica Baillie: data analysis and manuscript write-up.
Stephanie Sivell: data analysis and manuscript write-up.
Joanna Canham: data analysis.
Joanna D Smith: data analysis.
Angela Casbard: study design, data analysis and manuscript write-up.
Ander Cohen: study design and data analysis.
David Cohen: study design and data analysis.
Jessica Evans: data analysis.
Kate Fletcher: data analysis.
Miriam Johnson: study design and data analysis.
Anthony Maraveyas: study design and data analysis.
Hayley Prout: data analysis and manuscript write-up.
Kerenza Hood: study design, data analysis and manuscript write-up.
All the authors approved the final manuscript.
Data sharing statement
Available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Smith JD, Baillie J, Baglin T, Griffiths G, Casbard A, Cohen D, et al. A feasibility study to inform the design of a randomized controlled trial to identify the most clinically and cost effective anticoagulation length with low molecular weight heparin in the treatment of cancer associated thrombosis (ALICAT): study protocol for a mixed-methods study. Trials 2014;15:122. http://dx.doi.org/10.1186/1745-6215-15-122
References
- Report of the Independent Expert Working Group on the Prevention of Venous Thromboembolism in Hospitalised Patients. London: Department of Health; 2007.
- Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer 2010;102:S2-9. http://dx.doi.org/10.1038/sj.bjc.6605599.
- Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, et al. Guidelines on oral anticoagulation with warfarin – fourth edition. Br J Haematol 2011;154:311-24. http://dx.doi.org/10.1111/j.1365-2141.2011.08753.x.
- Noble SI, Shelley MD, Coles B, Williams SM, Wilcock A, Johnson MJ. Management of venous thromboembolism in patients with advanced cancer: a systematic review and meta-analysis. Lancet Oncol 2008;9:577-84. http://dx.doi.org/10.1016/S1470-2045(08)70149-9.
- Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine 1999;78:285-91. http://dx.doi.org/10.1097/00005792-199909000-00001.
- Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846-50. http://dx.doi.org/10.1056/NEJM200012213432504.
- Noble S. The challenges of managing cancer related venous thromboembolism in the palliative care setting. Postgrad Med 2007;83:671-4. http://dx.doi.org/10.1136/pgmj.2007.061622.
- Noble SI, Finlay IG. Is long-term low-molecular-weight heparin acceptable to palliative care patients in the treatment of cancer related venous thromboembolism? A qualitative study. Palliat Med 2005;19:197-201. http://dx.doi.org/10.1191/0269216305pm1008oa.
- Khorana AA, Streiff MB, Farge D, Mandala M, Debourdeau P, Cajfinger F, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol 2009;27:4919-26. http://dx.doi.org/10.1200/JCO.2009.22.3214.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:454S-545S. http://dx.doi.org/10.1378/chest.08-0658.
- Meyer G, Marjanovic Z, Valcke J, Lorcerie B, Gruel Y, Solal-Celigny P, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med 2002;162:1729-35. http://dx.doi.org/10.1001/archinte.162.15.1729.
- Lee A, Levine M, Baker R, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53. http://dx.doi.org/10.1056/NEJMoa025313.
- Hull R, Pineo G, Brant R, Mah AF, Burke N, Dear R, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006;119:1062-72. http://dx.doi.org/10.1016/j.amjmed.2006.02.022.
- Johnson MJ, Sproule MW, Paul J. The prevalence and associated variables of deep venous thrombosis in patients with advanced cancer. Clin Oncol 1999;11:105-10. http://dx.doi.org/10.1053/clon.1999.9023.
- Noble SI, Hood K, Finlay IG. The use of long-term low-molecular weight heparin for the treatment of venous thromboembolism in palliative care patients with advanced cancer: a case series of sixty two patients. Palliat Med 2007;21:473-6. http://dx.doi.org/10.1177/0269216307080816.
- Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100:3484-8. http://dx.doi.org/10.1182/blood-2002-01-0108.
- Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52. http://dx.doi.org/10.1056/NEJMoa0906598.
- Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510. http://dx.doi.org/10.1056/NEJMoa1007903.
- Peetz D, Lackner KJ. Dabigatran versus warfarin for venous thromboembolism. N Engl J Med 2010;362. http://dx.doi.org/10.1056/NEJMc1000696.
- Teachey DT. Dabigatran versus warfarin for venous thromboembolism. N Engl J Med 2010;362. http://dx.doi.org/10.1056/NEJMc1000696.
- Romualdi E, Donadini MP, Ageno W. Oral rivaroxaban after symptomatic venous thromboembolism: the continued treatment study (EINSTEIN-Extension study). Expert Rev Cardiovasc Ther 2011;9:841-4. http://dx.doi.org/10.1586/erc.11.62.
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9.
- Watanabe SM, Nekolaichuk C, Beaumont C, Johnson L, Myers J, Strasser F. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage 2011;41:456-68. http://dx.doi.org/10.1016/j.jpainsymman.2010.04.020.
- Elman E, Kahn S. The post-thrombotic syndrome after upper extremity deep venous thrombosis in adults: a systematic review. Thromb Res 2006;117:609-14. http://dx.doi.org/10.1016/j.thromres.2005.05.029.
- van Korlaar I, Vossen C, Rosendaal F, Bovill EG, Cushman M, Naud S, et al. The impact of venous thrombosis on quality of life. Thromb Res 2004;114:11-8. http://dx.doi.org/10.1016/j.thromres.2004.04.007.
- Seaman S, Nelson A, Noble S. Cancer-associated thrombosis, low-molecular weight heparin, and the patient experience: a qualitative study. Patient Pref Adherence 2014;8:453-61. http://dx.doi.org/10.2147/PPA.S58595.
- Mockler A, O’Brien B, Emed J, Ciccotosto G. The experience of patients with cancer who develop venous thromboembolism: an exploratory study. Oncol Nurs Forum 2012;3:E233-40. http://dx.doi.org/10.1188/12.ONF.E233-E240.
- Farge D, Debourdeau P, Beckers M, Baglin C, Bauersachs RM, Brenner B, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost 2013;11:56-70. http://dx.doi.org/10.1111/jth.12070.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715-22. http://dx.doi.org/10.1001/jama.293.6.715.
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007;110:2339-46. http://dx.doi.org/10.1002/cncr.23062.
- Lewin S, Glenton C, Oxman A. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3496.
- Beanlands H, Horsburgh M, Fox S, Howe A, Locking-Cusolito H, Pare K, et al. Caregiving by family and friends of adults receiving dialysis. Nephrol Nurs J 2005;32:621-31.
- Barbour R. Doing Focus Groups. Los Angeles, CA: Sage Publications; 2007.
- Patton M. Qualitative Research and Evaluation Methods. Thousand Oaks, CA: Sage Publications; 2002.
- Creswell J. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Thousand Oaks, CA: Sage Publications; 2003.
- Gillham B. The Research Interview. New York, NY: Continuum; 2000.
- Krueger R, Casey M. Focus Groups: A Practical Guide for Applied Research. Thousand Oaks, CA: Sage Publications; 2000.
- Edwards A, Sivell S, Dundon J, Elwyn G, Evans R, Gaff C, et al. Effective Risk Communication in Clinical Genetics: A Systematic Review. Cardiff: Cardiff Centre for Health Sciences Research: Cardiff University; 2006.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analyzing Qualitative Data. London: Routledge; 1994.
- Guba E, Lincoln Y. Fourth Generation Evaluation. Newbury Park, CA: Sage Publications; 1989.
Appendix 1 Tables for randomised controlled trial results
| Participant characteristics | Arm A (LMWH) | Arm B |
|---|---|---|
| Participants (n) | 3 | 2 |
| Age (years), mean | 67 | 51 |
| ECOG score (n) | ||
| 0–1 | 2 | 2 |
| 2–4 | 1 | 0 |
| Type of LMWH (n) | ||
| Dalteparin | 2 | 2 |
| Tinzaparin | 0 | 0 |
| Enoxaparin | 1 | 0 |
| Concomitant aspirin (n) | 0 | 0 |
| Site of historical VTE event (n) | ||
| PE lung | 2a | 2 |
| DVT leg | 2a | 0 |
| Bilateral DVT | 0 | 0 |
| Elsewhere | 0 | 0 |
| Patients with any bleeding events during initial LMWH treatment (n) | 0 | 0 |
| Site of bleeding event | N/A | N/A |
| Patients with any major bleeding event (n) | N/A | N/A |
| Participant characteristics | Arm A (LMWH) | Arm B |
|---|---|---|
| Participants (n) | 3 | 2 |
| Type of LMWH allocated (n) | ||
| Dalteparin | 2 | 0 |
| Tinzaparin | 0 | 0 |
| Enoxaparin | 1 | 0 |
| Participants who did not follow their allocated treatment arm during the trial (n) | 0 | 0 |
| Participant characteristics | Arm A (LMWH) | Arm B |
|---|---|---|
| Participants (n) | 3 | 2 |
| Patients completing 6 months’ follow-up according to allocated trial arm (n) | 2 | 1 |
| Arm B patients restarting LMWH | N/A | 0 |
| Weeks on LMWH treatment | ||
| Participant 1 | 28 | N/A |
| Participant 2 | 26 | N/A |
| Participant 3 (died week 19) | 12 | N/A |
| Withdrawn from LMWH treatment pre-6 months (n) | 0 | 0 |
| Change to non-LMWH anticoagulant | N/A | N/A |
| Toxicity | N/A | N/A |
| Participant choice | N/A | N/A |
| Clinician choice | N/A | N/A |
| Non-concordance | N/A | N/A |
| Disease progression | N/A | N/A |
| Other | N/A | N/A |
| Died before 6-month follow-up (n) | 1 | 1 |
| Participant characteristics | Arm A (LMWH) | Arm B |
|---|---|---|
| Participants (n) | 3 | 2 |
| Number of participants with any recurrent VTE during follow up (%) | 0 | 0 |
| Total number of events reported | ||
| PE lung | N/A | N/A |
| DVT leg | N/A | N/A |
| Bilateral DVT | N/A | N/A |
| Elsewhere | N/A | N/A |
| Number of events per patient, n (%) | ||
| 1 | N/A | N/A |
| 2 | N/A | N/A |
| 3 | N/A | N/A |
| 4 | N/A | N/A |
| Mean VTE events per patient randomised | N/A | N/A |
| Confirmed by Doppler scan or venography, n (%) | N/A | N/A |
| Outcome, n (%) | N/A | N/A |
| LMWH treatment modified or started | N/A | N/A |
| Caval filter inserted | N/A | N/A |
| Death | N/A | N/A |
| Questionnaire administered | Baseline | 3 months | 6 months | |||
|---|---|---|---|---|---|---|
| Arm A (LMWH) | Arm B | Arm A (LMWH) | Arm B | Arm A (LMWH) | Arm B | |
| EORTC-C30: patients completing questionnaire (n) | 3 | 2 | 3 | 1 | 2 | 0 |
| Questions completed (%) | 100 | 98 | 99 | 100 | 100 | N/A |
| EQ-5D-5L: patients completing questionnaire (n) | 3 | 2 | 3 | 1 | 2 | 0 |
| Questions completed (%) | 100 | 100 | 100 | 100 | 100 | N/A |
| ESAS-r: patients completing questionnaire (n) | 3 | 2 | 3 | 1 | 2 | 0 |
| Questions completed (%) | 93 | 95 | 100 | 90 | 95 | N/A |
Glossary
- Participant
- An individual who has given written, informed consent and is participating in trial-related activities.
- Patient
- A patient under care who may be eligible for the trial but has not yet consented to participate in any trial-related activities.
- Sponsor
- The primary organisation that oversees, and is responsible for, the clinical trial.
List of abbreviations
- A&E
- accident and emergency
- ALICAT
- Anticoagulation with Low-molecular-weight heparin In the treatment of Cancer-Associated Thrombosis trial
- CAT
- cancer-associated thrombosis
- CCG
- Clinical Commissioning Group
- CLOT
- low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer trial
- CRF
- case report form
- DVT
- deep-vein thrombus
- ECOG
- Eastern Cooperative Oncology Group
- EORTC QLQ-C30
- European Organisation for Research and Treatment of Cancer quality-of-life questionnaire for assessing health-related quality of life of cancer patients (Version 3.0)
- EQ-5D-5L
- European Quality of Life-5 Dimensions – 5 levels
- ESAS-r
- Edmonton Symptom Assessment System (revised version)
- GP
- general practitioner
- LMWH
- low-molecular-weight heparin
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NISCHR
- National Institute of Health and Social Care Research
- PE
- pulmonary embolism
- PIS
- participant information sheet
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- VTE
- venous thromboembolism