Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/01/14. The contractual start date was in February 2009. The draft report began editorial review in May 2014 and was accepted for publication in May 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
The following coauthors have undertaken research or consultancy for companies that develop and manufacture smoking cessation medications: Robert West (Johnson & Johnson, GlaxoSmithKline, Pfizer), Tim Coleman (Pierre Fabre Laboratories) and Paul Aveyard (Pfizer).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Ussher et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
The problem of smoking in pregnancy
Maternal smoking in pregnancy is the main preventable cause of morbidity and death among women and infants. Smoking is associated with adverse pregnancy and birth outcomes, including miscarriage, stillbirth, prematurity, low birthweight, congenital abnormalities, and neonatal or sudden infant death. 1–3 Smoking also presents immediate risks for the mother, including placental abruption,4 as well as the longer-term risks reported for smokers in general. In addition, the children of mothers who smoke are twice as likely to become smokers. 5 Smoking in pregnancy is a global public health problem. In high-income countries the prevalence of smoking in pregnancy is typically between 10% and 25% and it appears to be reducing. 6–10 However, rates seem to be rapidly increasing in low- and middle-income countries. 11 In the UK it is estimated that 12% of women smoke during pregnancy7 and, as in other high-income countries, rates of smoking in pregnancy remain highest among younger women and those who are more socially disadvantaged. 7 Smoking cessation during pregnancy improves maternal and birth outcomes,12 yet only approximately 25% of pregnant smokers stop for at least part of their pregnancy and around two-thirds of these relapse after giving birth. 13
Treatments to aid smoking cessation in pregnancy
Face-to-face and ‘self-help’ behavioural support are the only two interventions that have been shown to help pregnant women to stop smoking. 12,14 Regular sessions of face-to-face behavioural support can increase smoking cessation rates in pregnancy by approximately 6%15 and there is a need to identify other interventions that are effective during pregnancy when combined with this support. The most effective therapy in non-pregnant smokers is a combination of behavioural support plus nicotine replacement therapy (NRT), bupropion or varenicline. 16–18 However, the efficacy of NRT during pregnancy is not known,19 and thus many pregnant women are reluctant to use it,20 and other smoking cessation medications are contraindicated during pregnancy. 19 There is a need to identify other non-pharmacological interventions that are effective for smoking cessation during pregnancy.
Evidence for physical activity aiding smoking cessation
Effective pharmaceutical aids for quitting are thought to work mainly through reducing cigarette cravings18 and there is good evidence from a meta-analysis21 to show that physical activity (PA) reduces these cravings, particularly at a moderate or vigorous intensity. Therefore, PA interventions could aid smoking cessation. For non-pregnant smokers, a Cochrane systematic review22 has considered the evidence for PA aiding cessation. The majority of the 15 randomised controlled trials (RCTs) reviewed had low statistical power to detect a meaningful difference between the treatment groups, with seven trials having < 25 participants in each treatment arm. Six adequately powered trials compared a group receiving a PA intervention combined with behavioural support with a group receiving behavioural support alone. Three of these studies showed significantly higher smoking abstinence rates in the PA group than in the control group at the end of treatment. 23–25 One of these studies also showed that a PA intervention increased abstinence compared with a control group at the 3-month follow-up and there was a benefit of exercise of borderline significance [relative risk (RR) 2.19, 95% CI 0.97 to 4.96; p = 0.05] at the 12-month follow-up. 23 A further study showed significantly higher abstinence rates for the exercise group than for the control group at the 3-month follow-up, but not at the end of treatment or at the 12-month follow-up. 26 The study with the most intensive PA intervention, entailing thrice-weekly sessions of supervised vigorous-intensity exercise, showed the strongest effect on abstinence. 23 The other studies involved PA interventions that were relatively less intense, particularly in terms of the extent of supervised exercise, and it is possible that supervised exercise is needed for efficacy. Adequately powered trials are needed that involve moderate-intensity exercise, which is likely to be more acceptable than vigorous exercise for most individuals. 27 Moderate-intensity PA (e.g. brisk walking) is recommended for pregnancy28 and has been shown to reduce cigarette cravings during pregnancy,29 and pilot work suggests that pregnant smokers are likely to be receptive to a PA intervention. 30
The effects of physical activity on maternal depression and weight gain
Important secondary outcomes included changes in maternal depression and weight. Antenatal and postnatal depression are important because they are common and are associated with harmful consequences for the mother and child. 31–38 Interventions are needed for preventing and treating these types of depression. Moreover, pregnant smokers are at a heightened risk of depression during and after pregnancy, and women who quit smoking during pregnancy are more likely to relapse if they experience depressive symptoms. 12,39,40 Thus, it is important that pregnant women who smoke or who are attempting to quit are offered effective interventions for depression. The London Exercise And Pregnant smokers (LEAP) trial was the first study to assess the effectiveness of a PA intervention for symptoms of antenatal and postnatal depression specifically among smokers.
Excessive gestational weight gain (GWG) is associated with adverse pregnancy outcomes, including large-for-gestational-age infants and caesarean section. 41,42 In addition, smoking cessation is associated with GWG. 43 Interventions for managing GWG are needed, especially among women attempting to quit smoking, and PA has potential in this regard. Observational studies have demonstrated an association between participation in PA and reduced risk of excessive GWG,44–46 whereas a recent meta-analysis using data from 10 RCTs showed an overall benefit of participation in PA compared with a control condition in terms of reducing GWG. 47 We are not aware of any studies that have examined the effect of a PA intervention on GWG in pregnant smokers. Among non-pregnant smokers, there is some evidence that PA interventions can limit post-smoking cessation weight gain. 48 The LEAP trial was the first large RCT to examine the effect of a PA intervention on preventing excessive GWG and postnatal weight retention.
Summary
In summary, smoking in pregnancy is extremely harmful for mother and baby and is an enduring global public health problem. Behavioural support is the only smoking cessation intervention shown to be effective in pregnancy. The evidence for PA programmes aiding smoking cessation is mixed and pregnancy provides a compelling rationale for their use because medication is contraindicated or ineffective. We conducted the LEAP RCT to assess the effectiveness of a PA intervention for smoking cessation during pregnancy.
Main objective
The main objective of the study was to investigate whether or not standard behavioural support for smoking cessation in pregnancy plus a PA intervention is more effective than behavioural support alone in achieving biochemically validated smoking cessation between a quit date and the end of pregnancy for women between 10 and 24 weeks’ gestation who currently smoke one or more cigarettes daily and who smoked at least five cigarettes daily before pregnancy. A further objective was to assess the cost-effectiveness of the intervention for achieving smoking cessation at the end of pregnancy.
Chapter 2 Methods
Trial design
The LEAP trial was a multicentre, pragmatic, randomised controlled, parallel-group trial of a PA intervention. Participants were monitored from their recruitment at between 10 and 24 weeks’ gestation until the end of pregnancy and were then followed up by telephone at 6 months after the birth. The trial protocol has been published. 49
Participants and recruitment
Eligibility criteria
Eligible participants were aged 16–50 years, were between 10 and 24 weeks’ gestation (subject to confirmation that they had a scan to show a viable pregnancy), were currently smoking at least one cigarette per day, were smoking at least five cigarettes per day before pregnancy, were prepared to quit smoking 1 week after enrolment and could confirm that they were able to walk continuously for at least 15 minutes. Women were excluded if they were unable to complete self-administered questionnaires in English (because of a lack of resources for translators) or if they reported any medical condition that might be exacerbated by exercise. There are no documented contraindications to moderate-intensity exercise but if a woman had been advised by her doctor or midwife not to take exercise during pregnancy, if she had any complications during her pregnancy or if she had been cautioned against taking exercise,28,50 a consultant obstetrician and gynaecologist at her hospital were consulted to check that it was safe for her to participate. Participants joining the trial were monitored at each treatment session for cautions to exercise and adverse events (AEs). Those with drug or alcohol dependence were excluded as the intervention described was not comprehensive enough to address these issues.
Although NRT is licensed for use in pregnancy, there is no evidence for its effectiveness at this time19 and many pregnant smokers prefer not to use it. 20 Allowing study participants to use NRT might create confounding; therefore, women who indicated that they wished to use NRT on commencing their quit attempt were excluded. Following guidelines,51 those women who were unable to stop smoking after their quit day and who expressed a wish to receive NRT were prescribed NRT by their general practitioner (GP). The participants’ GPs, midwives and obstetricians were informed of their patients’ participation in the trial.
Recruiting centres
Participants were recruited from 13 hospital antenatal clinics in England. Initially these were at hospitals in the Greater London area: St George’s Healthcare NHS Trust (St George’s Hospital), Chelsea and Westminster Hospital NHS Foundation Trust (Chelsea and Westminster Hospital), Guy’s and St Thomas’ NHS Foundation Trust (St Thomas’ Hospital), Croydon Health Services NHS Trust (Croydon University Hospital, previously known as Mayday Hospital), Imperial College Healthcare NHS Trust (Queen Charlotte’s and Chelsea Hospital and St Mary’s Hospital), Epsom and St Helier University Hospitals NHS Trust (Epsom Hospital) and Kingston Hospital NHS Foundation Trust (Kingston Hospital). Five further sites around England were later added to improve recruitment rates: Surrey and Sussex Healthcare NHS Trust (Crawley Hospital), West Middlesex University Hospital NHS Trust (West Middlesex University Hospital), Mid Cheshire Hospitals NHS Foundation Trust (Leighton Hospital), King’s College Hospital NHS Foundation Trust (King’s College Hospital) and Medway Foundation Trust (Medway Maritime Hospital).
Researchers
At each centre a dedicated research midwife, research nurse or research psychologist undertook all trial-related procedures, including delivering all of the interventions and administering all of the outcome measures. Researchers were trained by the chief investigator/trial manager (Professor Ussher) in research procedures, including screening, enrolment and consent procedures. They also attended certified Good Clinical Practice training. Researchers were trained to national standards to provide behavioural support for smoking cessation and PA. 52
Recruitment and consent
The smoking status of all pregnant women is routinely recorded in the hospital computerised patient administration system (PAS) at the first antenatal booking visit, which is typically at 9–14 weeks of gestation. At this time, the hospital midwife informs all women recorded as smokers that it is hospital policy to telephone them to offer smoking cessation support. This support would usually be offered by their local NHS Stop Smoking Service but during the period of recruitment to the study a trial researcher telephoned the women. The following methods of recruitment were used:
-
On recording women as smokers, hospital midwives routinely passed referral forms to the researcher. In some cases these referral forms would be available before the smoking status of the women was recorded in the PAS and in these cases the researcher extracted the women’s contact details from the referral forms rather than from the PAS.
-
In cases in which a woman’s smoking status appeared in the PAS before a midwife referral form was received, the researcher extracted the woman’s contact details from the PAS and telephoned her. Following our consultation with the Patient Information Advisory Group, the ethics committee gave us permission to contact all pregnant women recorded as smokers. This is because during the trial the researchers were considered as part of the clinical care team and it is routine practice to contact women in this way.
-
A flyer containing brief information about the trial was included in women’s packs for their first antenatal booking appointment and women were invited to call a researcher if they were interested in finding out more about the study.
-
Women who had seen posters advertising the study in hospitals or children’s centres could contact a researcher directly.
-
We had initially planned to distribute a questionnaire at the first ultrasound visit inviting women to take part. This approach was piloted at several sites in the first month of the study; however, it had a low response rate and was labour intensive and, therefore, was abandoned.
Those who were interested in receiving help with quitting were invited to join the trial or were offered referral to the primary care trust (PCT), as per usual practice. Those women expressing an interest in volunteering were screened for eligibility by the researcher by telephone (see Appendix 1) and eligible women were sent a participant information sheet.
After having the chance to consider the participant information sheet for at least 24 hours and to discuss the study with the researcher, women who volunteered were offered an appointment at a community-based children’s centre or at their local hospital. At the first appointment they gave their written informed consent before trial data were collected. In addition to trial participation, women were asked to give consent for researchers to have access to their and their child’s medical records, for information held by the NHS to be used to keep in touch with them and to follow their health status, and for the researcher to inform their GP, midwife and obstetrician about their participation in the study.
Interventions
The interventions followed Consolidated Standards of Reporting Trials (CONSORT) guidelines for non-pharmacological interventions. 53,54 Delivery of the interventions was standardised by training and by the therapists following manuals (see Appendices 2 and 3). The initial competence of the therapists was assessed by the trial manager/chief investigator by observing role-play scenarios during training. The fidelity of the interventions was monitored during the first 6 months by regular observations (at least five intervention sessions) by the trial manager/chief investigator, with the tasks listed in Appendices 2 and 3 used as a checklist. All sessions were face to face and one to one and were delivered in a private room at the hospital or in a community health centre/children’s centre. Social cognitive (learning) theory55 was the theoretical basis for the interventions. This theory recognises the interplay of individual factors (e.g. self-efficacy to quit smoking or increase PA) and social/environmental factors (e.g. social support) in health behaviour change. For each session that they attended, the women were paid £7 for their travel expenses.
Control group
Those in the control group received behavioural support for smoking cessation, which is generally provided by the NHS Stop Smoking Service to pregnant women as part of ‘usual care’. By extracting the elements of the intervention from written manuals and materials provided by the programme (see Appendix 2), the contents of the intervention were classified in accordance with the taxonomy of behaviour change techniques (BCTs) described by Michie and colleagues56 and used in individual behavioural support for smoking cessation (Table 1). Participants were offered six weekly sessions of 20 minutes of behavioural support for smoking cessation, commencing 1 week before the quit date and ending 4 weeks afterwards. The intervention (see Table 1) incorporated all 43 BCTs for smoking cessation defined by Michie and colleagues,56 except for the BCT ‘provide rewards contingent on successfully stopping smoking’, although financial rewards were offered to increase compliance (Table 2). Continued support was offered to women who failed to quit or who relapsed to smoking.
| Week | Session number | Session content | BCTs used (Michie categoriesa) |
|---|---|---|---|
| 1 | Session 1 (1 week before quit day) | Explain the treatment, including the timing of quit | RC4, BS4 |
| Measure expired CO level and explain purpose | RC3 | ||
| Assess and discuss current and past smoking behaviour | RI1 | ||
| Identify reasons for wanting and not wanting to quit | BM9 | ||
| Assess current motivation/confidence for quitting | RI2 | ||
| Discuss past attempts at quitting | RI3 | ||
| Prepare for the quit attempt | BM6, BS3 | ||
| Discuss use of social support | A2 | ||
| Advise on reducing smoking cues | BS8 | ||
| Advise subject to note the times when they are likely to relapse | BS6 | ||
| Facilitate relapse prevention planning and coping | BS2 | ||
| Identify barriers to quitting and address these barriers | BS1 | ||
| Emphasise choice (e.g. when they take their final smoke) | RD2 | ||
| Provide information about the consequences of smoking during pregnancy | BM1, RC5 | ||
| Explain about quitting abruptly, rather than cutting down | BM10 | ||
| For all sessions: | |||
| Allow time for questions | RC2 | ||
| Summarise | RC9 | ||
| Use reflective listening | RC7 | ||
| Elicit participant’s views | RC8 | ||
| Build a general rapport | RC1 | ||
| Give praise for progress | BM7 | ||
| Tailor the interactions | RD1 | ||
| 2 | Session 2 (quit day) | Look for reasons why the woman is a good prospect | BM2, BM3 |
| Explain about cigarette withdrawal symptoms and strategies for dealing with them | RC6 | ||
| Identify barriers to quitting and address these barriers | BS1 | ||
| Advise on avoiding social cues for smoking | BS11 | ||
| Advise on changing routine | BS7 | ||
| Advise on conserving mental resources | BS10 | ||
| Set graded tasks (e.g. take 1 hour/day at a time) | BS9 | ||
| 3 | Session 3 (1 week after quit day) | Check smoking status | BS5 |
| Assess withdrawal symptoms | RI4 | ||
| Reassure about the norms for these symptoms | RC10, BM5 | ||
| Advise subjects to monitor when they want to smoke | BS6 | ||
| Assess CO level and give feedback about whether or not reading has reduced | BM11, BM3 | ||
| Discuss planning and coping strategies to prevent relapse | BS2 | ||
| If they have relapsed ask them to commit to a new quit date | BM6 | ||
| Advise about use of NRT | A1 | ||
| Liaise with PCT about obtaining NRT | A3 | ||
| Encourage subject to see themselves as a non-smoker | BM8 | ||
| Remind them of lottery prize for attending all sessions | BM7 | ||
| 4 | Session 4 (2 weeks after quit day) onwards | Assess CO level | BM11 |
| Check smoking status | BS5 | ||
| If they are struggling offer further support from PCT | A5 | ||
| Discuss relapse prevention planning and coping strategies for after birth | BS2, BM8 | ||
| Emphasise importance of not having a single puff | BM6 | ||
| If subject has relapsed, set a new quit date and review use of NRT | A4 | ||
| Incentive occasion | Maximum financial incentive (£) | |
|---|---|---|
| PA group | Control group | |
| Annual lottery with three prizes of £100 for attending at least 80% of the treatment sessions | 100a | 100a |
| Travel expenses (£7) for each session attended | 98b (14 sessions) | 42b (six sessions) |
| Follow-up at end of pregnancy | 10a | 10a |
| Follow-up at 6 months after birth | 10a | 10a |
| ≥ 5 days of accelerometer data recorded | 25a | NA |
| Total | 243 | 162 |
Treatment group
In addition to behavioural support for smoking cessation, those in the PA group received a PA intervention, combining PA consultations and supervised exercise. By extracting the elements of the PA consultation from the written manuals and materials of the PA programme (Table 3), the contents of the PA consultation have been classified in accordance with the taxonomy of Michie and colleagues57 of BCTs used to help people change their PA behaviours. There were 14 sessions of supervised exercise, twice a week for 6 weeks (one session with behavioural support for smoking cessation) and then weekly for 2 weeks. Following a familiarisation session at the first visit, participants were advised to aim for 30 minutes of continuous treadmill walking during each session. Following guidelines,58 moderate-intensity exercise was prescribed according to age and current activity levels and was monitored using a polar heart-rate monitor. The intensity of exercise was also guided by a rating of perceived exertion59 (‘fairly light’ to ‘somewhat hard’) and by the ‘talk test’, which indicates that the intensity of activity is too high if it is not possible to hold a conversation.
| Week | Session number | Session content | BCTs used (Michie categoriesa) |
|---|---|---|---|
| 1 | Session 1 (1 week before quit day) | Review current PA and discuss PA benefits | 1, 2 |
| Explain and demonstrate use of treadmill and pedometer | 7, 21, 22, 26 | ||
| Check PA confidence levels using scaling questions | 16 | ||
| All sessions: | |||
| Agree PA goals | 10 | ||
| Provide weekly PA and step-count diaries | 16 | ||
| Allow time for questions, summarise, use reflective listening, elicit participant’s views, build a general rapport | NA | ||
| Give praise for effort and for achieving PA goals | 12, 13 | ||
| 1 | Session 2 | Review PA goals and effect of PA on cravings | 7, 9, 10 |
| Complete cost–benefit analysis for increasing PA | 2 | ||
| Identify PA barriers and problem solve | 8 | ||
| Explain and demonstrate exercises in booklet | 21, 22, 26 | ||
| Provide information on places to exercise | 20 | ||
| Discuss time management and exercise habits | 23, 38 | ||
| Plan social support | 29 | ||
| Provide weekly PA diary and step-count diary | 16 | ||
| 2 | Session 3 (quit day) | Review PA goals, set heart-rate targets on treadmill | 10 |
| Identify PA barriers and problem solve | 8 | ||
| Provide weekly PA diary and step-count diary | 16 | ||
| Check PA confidence levels with scaling questions | 8 | ||
| 3 | Session 4 (1 week after quit day) onwards | Review PA goals, set heart-rate targets on treadmill | 10 |
| Plan for relapse prevention/coping | 35 | ||
| Review exercises in booklet | 21, 22, 26 | ||
| Review social support | 29 | ||
| Use imagery to encourage identity as an ‘exerciser’ | 34 | ||
| Provide weekly PA diary and step-count diary | 16 | ||
| Reminder that sessions reduce to once per week for the last 2 weeks of the programme | 27 | ||
| Check PA confidence levels with scaling questions | 8 | ||
At the first two treadmill sessions and then on every other occasion women were offered a 20-minute PA consultation (total of nine sessions) on increasing their additional ‘home-based’ PA. The researcher worked through a booklet with each participant (see Appendix 4), which the participant retained. The intervention (see Table 3) incorporates 19 of 40 BCTs for increasing PA as defined by Michie and colleagues. 57 In general, the consultations aimed to identify opportunities to incorporate PA into women’s lives, to motivate them to use PA to aid smoking cessation and to help them use behavioural strategies to improve adherence to these plans. These consultations were tailored towards the women’s preferences for PA and their environment, including preference for type of PA, level of support from family or friends and availability of time and facilities for exercise. The participants were encouraged to view PA as a self-control strategy for reducing cigarette cravings and withdrawal60 and to maintain any increases in PA after their pregnancy. Following recommendations for pregnancy,28,61 the women were advised to be active for continuous periods of at least 10 minutes at a time, progressing towards accumulating 30 minutes of activity on at least 5 days of the week. The emphasis was on brisk walking, which is popular among pregnant smokers. 62 As a further option, a home-based antenatal exercise digital versatile disc (DVD) and booklet were provided. In addition, participants were given a pedometer for monitoring their daily steps (Digi-Walker SW-200; Great Performance Ltd, London, UK). Pedometers have been shown to increase activity levels in women63 and are acceptable during pregnancy64 and among pregnant smokers. 30 Participants were asked to log their daily steps, with the researcher calculating a 10% increment every 2 weeks, gradually progressing towards 10,000 steps a day. 65
Randomisation and blinding
An independent statistician generated a randomisation list using Stata version 11.2 (StataCorp LP, College Station, TX, USA), with random permuted blocks of random size stratified by recruitment centre. At enrolment the sequence was concealed from researchers, who had to confirm consent and eligibility on an online database before allocation was revealed. The online database was created by the Nottingham Clinical Trials Unit (NCTU) and held on a secure server in accordance with their standard operating procedures. Allocation was concealed from the participant until all baseline assessments had been completed. The sequence of treatment allocations was concealed until interventions had all been assigned and recruitment, data collection and laboratory analyses were complete. Thus, neither participants nor researchers were blinded to treatment allocation during intervention delivery or during outcome assessment.
Data collection
At baseline, researchers recorded demographic characteristics (including age, marital status, number of children, highest educational qualification, ethnicity, occupation, weeks of gestation and history of premature births) and smoking characteristics [including cigarettes smoked per day (now and before pregnancy), weekly urge to smoke (combining ratings of strength and frequency of urges),66,67 cigarette withdrawal symptoms,66,67 Fagerström Test for Cigarette Dependence (FTCD) score68,69 and partner’s smoking status]. Depression was assessed with the 10-item Edinburgh Postnatal Depression Scale (EPDS). 70 PA levels in the previous week (bouts of ≥ 10 minutes) were assessed for both groups using the 7-day PA interview (see Appendix 5). 71 Confidence levels with regard to taking up regular PA72 and stopping smoking73 were also recorded. Clothed weight (without shoes) was measured on a digital scale at the first antenatal booking visit by the midwife. The questionnaire showing assessments at baseline, including assessments repeated at further time points, is provided in Appendix 6. The timing of data collection is provided in Outcome measures.
Recording of adverse events
During all contacts, participants were asked about AEs. Medical records were examined monthly by research midwives for AEs and after delivery for maternal and infant outcome data. Researchers then summarised the descriptions in the case report forms and in the online study database. Descriptions were used to code the AEs according to standard terms in the Medical Dictionary for Regulatory Activities [see www.meddra.org (accessed 25 August 2015)]. Fetal deaths were recorded including miscarriage (non-live birth before 24 weeks’ gestation), stillbirth (non-live birth at ≥ 24 weeks’ gestation) and neonatal death (i.e. from live birth to 28 days).
Outcome measures
Timing of outcome measures
During the intervention period, the main assessment points were at 1 week, 4 weeks and 6 weeks after the quit day. The assessment at 1 week, when the vast majority of the sample was retained, was to assess the early impact of the intervention on cigarette withdrawal symptoms, urges to smoke, confidence for quitting and participating in PA and reports of PA. The 4-week assessment is a standard time for measuring short-term abstinence and is when the NHS Stop Smoking Service assesses abstinence. The 6-week assessment was timed to coincide with the end of the stop smoking programme. There were also follow-ups at the end of pregnancy and 6 months after the birth.
Primary outcome to end of pregnancy
The primary outcome was self-reported continuous abstinence from smoking between the quit date and the end of pregnancy, validated by exhaled carbon monoxide (CO) (Smokerlyzer; Bedfont Scientific Ltd, Maidstone, UK) or salivary cotinine (Salimetrics Europe Ltd, Newmarket, UK). Expired CO levels were assessed weekly up to 4 weeks after the quit day and at the end of pregnancy. Saliva cotinine levels were measured at 4 weeks after the quit day and at the end of pregnancy only among those who self-reported having smoked less than five cigarettes in total (on up to five occasions) since the quit day.
The primary outcome was operationalised as follows. Continuous abstinence was defined as having smoked less than five cigarettes in total (on up to five occasions) since the quit day. 74 Following an attempt to stop smoking, it is common for smokers to lapse on several occasions before finally succeeding in maintaining long-term abstinence; therefore, within the definition of continuous smoking abstinence it has become standard to allow five such lapses. With regard to exhaled CO, the criterion for confirming abstinence was a reading of < 8 parts per million (p.p.m.). 75 CO was assessed weekly up to 4 weeks after the quit day and at the end of pregnancy. With regard to salivary cotinine, the criterion for confirming abstinence was a value of < 10 ng/ml. 76 Cotinine was measured at 4 weeks post quit day and at the end of pregnancy.
The aim was to follow up women within 2 weeks of birth; however, it was acceptable for the primary outcome to be taken at any time between 36 weeks’ gestation and 10 weeks after the birth.
The primary outcome was dichotomous, that is, abstinent or non-abstinent. For a participant to be classed as abstinent from smoking at the end of pregnancy (i.e. positive primary outcome), the following criteria had to be satisfied:
-
At 4 weeks post quit (it was acceptable for this measure to be taken between 25 days and 6 weeks post quit):
-
‘Have you smoked at all since your quit day?’ = ‘no not even a puff’ or ‘yes just a few puffs’ or ‘yes, between one and five cigarettes’ or ‘missing’ (i.e. any response other than ‘yes, more than five cigarettes’) and CO is < 8 p.p.m. and/or cotinine is < 10 ng/ml or CO or cotinine is missing.
-
-
At the end of pregnancy:
-
‘Have you smoked at all since your quit day?’ = ‘no not even a puff’ or ‘yes just a few puffs’ or ‘yes, between one and five cigarettes’ (i.e. any response other than ‘yes, more than five cigarettes’) and CO is < 8 p.p.m. and/or cotinine is < 10 ng/ml.
-
The concentration of either exhaled CO or salivary cotinine was used to validate abstinence; if both measures were available both were required. Some women will not have data for self-report of smoking or biochemical validation at 4 weeks. If these women are confirmed as abstinent at the end of pregnancy it will be considered as a positive primary outcome.
For a participant to be considered as non-abstinent from smoking at the end of pregnancy (i.e. negative primary outcome), the following criteria had to be satisfied:
-
At 4 weeks or the end of pregnancy:
-
‘Have you smoked at all since your quit day?’ = ‘yes, more than five cigarettes’
-
CO or salivary cotinine values do not confirm abstinence
-
has withdrawn from the study (i.e. refuses follow-up)
-
fails to set a quit date that the follow-up assessment can be referenced against.
-
-
At the end of pregnancy:
-
refuses to allow biochemical validation
-
refuses to self-report number of cigarettes smoked
-
unable to contact to confirm smoking status (i.e. lost to follow-up).
-
Secondary outcomes
Biochemically validated continuous smoking abstinence was also assessed at 4 weeks after the quit day and 6 months after the birth. In addition, we assessed biochemically validated continuous smoking abstinence using a stricter criterion whereby no cigarettes were allowed after the quit day, at 4 weeks after the quit date, at the end of pregnancy and at 6 months postnatally. Self-reported smoking status at 6 months after the birth was reported by telephone and was not biochemically validated. Many women report that, rather than stopping smoking, they reduce their smoking during pregnancy77,78 and there is some evidence to suggest that a reduction in smoking of ≥ 50% is associated with an increased infant birthweight. 79 Therefore, levels of smoking reduction were assessed for those women who relapsed.
Other secondary outcome measures were changes in urge to smoke, tobacco withdrawal symptoms and confidence for stopping smoking and maintaining regular PA between baseline and 1 week after the quit day. We also assessed changes in depression between baseline, the end of pregnancy and 6 months after the birth, as well as changes in maternal weight between baseline, 4 weeks after the quit date and the end of pregnancy (the women were weighed again at the end of pregnancy by a researcher using the same method as used by the midwife at the first antenatal booking visit).
Further self-reports of PA levels were collected at weeks 1, 4 and 6 after the quit date and at both follow-ups (i.e. the end of pregnancy and 6 months postnatally). To validate self-reported PA levels, a 10% random subsample of participants had their PA levels objectively measured using an accelerometer (Model GT1M or GT3X; Actigraph, Pensacola, FL, USA). Only 90 women were asked to wear an accelerometer as our pilot work showed that most women would not tolerate waist-worn devices and at the start of the study validated wrist-worn accelerometers were not commercially available; therefore, as the only practicable alternative, we used self-reported PA levels in the primary analysis of PA. During the fourth week after the quit date, the accelerometer was worn over the right hip for 7 consecutive days, recording non-water-based activities during waking hours at 1-minute epochs. The Actigraph has been shown to be practicable and valid during pregnancy. 80–82
The duration of treadmill exercise and attendance rates were also recorded and use of NRT was monitored throughout the intervention period. At the end of pregnancy, follow-up participants were asked if they had received any face-to-face support to stop smoking during their pregnancy beyond that provided in the study.
Finally, the following birth and maternal outcomes were extracted from participants’ hospital records:
-
birthweight
-
gestational age at delivery
-
preterm birth (< 37 weeks’ gestation)
-
Apgar score
-
cord blood pH
-
neonatal intensive care unit admission
-
elective termination
-
maternal mortality
-
mode of delivery.
Statistical methods
The statistical analysis plan, which is presented in Appendix 7, was finalised before any analyses started. The analysis for the primary outcome was conducted by an independent statistician, with allocation to the two study groups concealed until the analysis was completed. Analyses were performed using Stata version 11.2 and IBM SPSS Statistics version 19 (IBM Corporation, Armonk, NY, USA). Throughout, a p-value of < 0.05 was taken to indicate statistical significance and 95% confidence intervals (CIs) were calculated.
Sample size
We anticipated a cessation rate of 15% in the control group on the basis that 9% of pregnant women who are smokers stop smoking with usual care after their first antenatal visit and that with behavioural support another 6–7% quit. 15 Based on pilot work,30 a cessation rate of 23% was anticipated in the treatment group. We calculated that 866 participants would provide 83% power at a 5% significance level (two-sided) to detect an absolute difference of 8 percentage points in the rate of the primary outcome between the two groups, corresponding to an odds ratio (OR) of 1.69 or a RR of 1.53.
The prime aim of assisting smoking cessation in pregnancy is to improve the outcome of the pregnancy. The latest version of the Cochrane review of psychosocial interventions for supporting women to stop smoking in pregnancy showed evidence that such interventions were effective in helping women stop smoking and improving perinatal outcome. 12 For example, the main subgroup in the review consisting of counselling compared with usual care showed a RR of 1.44 for achieving abstinence in late pregnancy, the same outcome that we used. No individual trial of smoking cessation in pregnancy detected differences in perinatal outcomes by intervention status, but the meta-analysis of all trials in the review showed evidence of this. When pooled together, these interventions produced the following RRs for the intervention compared with the control condition: low birthweight RR 0.82 (95% CI 0.71 to 0.94); preterm birth RR 0.82 (95% CI 0.70 to 0.96); increased mean birthweight 41 g (95% CI 18 g to 63 g). Thus, relative increases in the rate of cessation of a similar size to the one that we were aiming to detect have led to meaningful improvements in perinatal outcomes and would be expected to do so in this trial. A power of 80% is generally considered the minimum power for a trial. Based on available evidence, anticipated recruitment rates and budgeting constraints, we increased the power from the minimum of 80% to 83%. The trial as designed had adequate power for a plausible effect size.
Analysis for the primary outcome at the end of pregnancy
Analysis was on an intention-to-treat (ITT) basis; participants with missing outcome data were assumed to be smoking. 74 The proportion of women reporting continuous smoking abstinence at the end of pregnancy was compared between study groups using logistic regression, with adjustment for recruitment centre using fixed effects. Statistical significance was assessed with the likelihood ratio test, with the estimate of effect given as the OR and 95% CI. A secondary analysis adjusted for centre, nicotine dependence, age, depression, maternal educational level and partner’s smoking status, as potentially important prognostic baseline factors. 83 In addition, as it was observed that the vast majority of participants reported high levels of PA at baseline, we tested for an interaction between baseline PA [< 150 minutes per week of moderate- and vigorous-intensity PA (MVPA) vs. ≥ 150 minutes per week of MVPA] and the treatment effect for the primary outcome. For the primary outcome, to assess the influence of the assumption that missing data equals ‘smoking’ on the effect size, we used the Hedeker method to test various scenarios of the association between smoking and having missing data. 84 Other outcomes for smoking cessation were analysed in a similar way.
Analysis for secondary outcomes
For ratings of withdrawal symptoms, urge to smoke, confidence for quitting smoking and confidence for participating in PA we conducted a series of linear regressions with scores at 1 week post quit as the dependent variable and the two groups, recruitment centre and baseline scores as independent variables. We compared the use of NRT and behavioural support between the two groups using chi-squared tests.
Physical activity outcomes
Self-reported weekly minutes of MVPA were log-transformed (log base 10) to normality and the difference in self-reported PA between the groups over time was analysed using a mixed-effects model to account for within-person correlations over time. In this model, the difference between treatment groups at each time point was estimated with adjustment for visit time, baseline minutes of MVPA, the interaction of visit time and baseline minutes of MVPA, and recruitment centre. The accelerometer data were analysed using KineSoft software (version 3.3.76; Loughborough, UK). Files with at least 10 hours of valid wear time on ≥ 1 day were retained in the analyses. Standard cut-points were used to determine MVPA. 85 Consistent with the self-report data, only MVPA sustained for at least 10 minutes was included in the assessment of validity using correlational analysis. The validity of the self-reports was also assessed by examining the difference between the self-report data and the accelerometer data using a Bland–Altman plot. 86 The two study groups were compared (Mann–Whitney U-tests) for accelerometer reports of MVPA, both when restricting the analysis to bouts of > 10 minutes (to allow comparison with the self-report data) and when including all MVPA, irrespective of duration.
Fetal and maternal birth outcomes
For binary outcomes, fetal and maternal birth outcomes were compared using logistic regression adjusted for recruitment centre. For continuous outcomes we compared study group means using multiple linear regression, again with adjustment for recruitment centre. For fetal outcomes, the primary analysis was of singleton births. We also conducted a sensitivity analysis including multiple births, with clustering of outcomes accounted for using an approach previously published. 87 This adapts methodology previously created for use with cluster RCTs, assuming that each woman is regarded as the ‘cluster’ and her number of offspring as the cluster size. A chi-squared test was used to compare the total number of women or their infants who had at least one AE or serious adverse event (SAE).
Depression outcome
One participant was randomised but withdrew consent without reason before providing any data. Thus, the sample for the depression analysis consisted of 784 women. All 784 participants provided EPDS data at baseline, with 383 (48.9%) and 279 (35.6%) participants providing data at the end of pregnancy and 6 months postnatally, respectively. First, we checked whether or not those with EPDS data at the two follow-up points (end of pregnancy, 6 months postnatally) had similar baseline characteristics, quit rate at the end of pregnancy and amount of PA reported as those from the total trial sample. Then, we examined whether or not the baseline characteristics of the PA and control participants were similar in the subsamples with EPDS data at the two follow-up points.
To maximise statistical power, the EPDS data were treated as a continuous variable. We used a mixed-effect linear model, adjusted for visit time, baseline EPDS score, the interaction of visit time and baseline EPDS score, and recruitment centre, and presented the estimated difference in score at the end of pregnancy and at 6 months’ follow-up for the PA group compared with the control group. This model allows for correlation between the repeated measurements at the end of pregnancy and at 6 months. In a final linear mixed-effect model, analysis was further adjusted for the following potential predictors of postnatal depression: marital status, age at leaving full-time education (as a proxy for socioeconomic status), body mass index (BMI) and young age (i.e. age ≤ 20 years). At the end of pregnancy, as some of the women provided EPDS data before the birth and some after the birth, we used t-tests to explore whether or not EPDS scores were similar at these two times.
Maternal weight outcome
First, we compared the subsample providing maternal weight at the end of pregnancy (n = 271) with the main LEAP trial sample (n = 785) for baseline characteristics and key end-of-pregnancy outcomes that might be associated with weight gain (i.e. quit rates, PA levels and depression scores). As we are conducting separate analyses for those providing an end of pregnancy weight before the birth (GWG) and those providing an end of pregnancy weight after the birth (postnatal weight retention), we performed the latter comparison separately for the subsamples before and after birth. Second, in the combined sample at the end of pregnancy and in the subsamples providing an end of pregnancy weight before and after delivery we compared baseline characteristics between the two randomisation groups. Subsequent analysis adjusted for any baseline differences between groups that might affect weight.
For all analyses the main outcome was the mean change in maternal weight (i.e. weight in early pregnancy minus weight at the end of pregnancy), computed separately for the subsamples providing an end of pregnancy weight before and after birth. Weight change was first compared between the two randomisation groups using linear regression analyses, with adjustment for baseline weight and recruitment centre. All of the regression analyses were then further adjusted for the following potential prognostic factors for weight change during pregnancy: age and number of previous pregnancies. In a sensitivity analysis we further adjusted the results for baby’s weight, which is important because women who quit may have bigger babies than those who do not quit, and continuous rate of smoking abstinence at the end of pregnancy. For the subsamples providing an end of pregnancy weight before and after birth, the sensitivity analyses were limited to 140 and 131 participants, respectively, because of missing data for baby’s weight and because three sets of twins were excluded.
For the subsample with GWG (i.e. weight measured before birth) it is important to consider that women will have delivered at different weeks of pregnancy; therefore, besides using the change in the crude measure of weight, we computed the change in mean kilograms per gestational week. This was calculated by dividing the total weight gain by the number of weeks of pregnancy. The regression models used for crude weight change were then repeated using weight change adjusted for gestational weeks as the dependent variable. To assess whether or not weight change is modified by the presence of obesity at baseline we also added an interaction between the effect of PA and whether or not the individual was obese at baseline. Weight at early pregnancy was not added to this model because of its colinearity with the assessment of whether individuals were obese or non-obese.
Next, using Institute of Medicine guidance,88 we investigated what proportion of women gained excessive gestational weight (coded ‘yes’ or ‘no’) relative to their early pregnancy BMI. A woman was considered to have gained excessive gestational weight if she was underweight according to her early pregnancy BMI and her GWG was > 18 kg, her weight was healthy and her GWG was > 16 kg, she was overweight and her GWG was > 11.5 kg or she was obese and her GWG was > 9 kg. We used logistic regression analyses to compute ORs of excessive GWG for each BMI category and for the randomised groups. In the final logistic regression model, the results were adjusted for all prognostic factors that were used in the above main analyses, except weight at early pregnancy.
Ethics and governance
An independent Trial Steering Committee met once or twice per year to monitor the conduct and progress of the trial and to address any safety issues. London Wandsworth Research Ethics Committee granted national research ethical approval (reference number 08/H0803/177), with additional local approvals for each recruitment centre.
Trial management
The trial was co-ordinated from a central trial office located within St George’s, University of London (SGUL), with the day-to-day running supervised and organised by the trial manager and administrator. The trial was sponsored by SGUL and conducted in accordance with Good Clinical Practice guidelines. 89 The chief investigator/trial manager and administrator received Good Clinical Practice training. Monthly research staff meetings were held at SGUL. There were no stopping rules or plans for interim analysis.
The NCTU provided a web-based database and randomisation system and data management reports. The system was held on a secure server in the NCTU, had a full electronic audit trail and full back-ups of the database were made every 24 hours. All of the outcome data were entered directly into the online forms by the participants or researchers. The database included validation checks whereby responses not meeting expected criteria would be flagged so that data entry errors were minimised.
The National Institute for Health Research (NIHR) Primary Care Research Network adopted the study. For public and patient involvement in the study see Appendix 8.
The protocol49 has been published and includes a description of approved amendments made to the original protocol after the start of recruitment; details of these amendments are given in the following section.
Protocol amendments
-
For the follow-up at the end of pregnancy, the valid period for assessment was originally defined as from 38 weeks’ gestation to 2 weeks after the birth. As there were a number of women who could not be contacted during this time frame, the valid period was extended to 36 weeks’ gestation to 4 weeks after the birth (approved by the research ethics committee on 18 May 2010). However, because there were still some women being followed up later than 4 weeks after the birth, the valid period was further revised to 36 weeks’ gestation to 10 weeks after the birth (approved 31 January 2012). The aim remained to attempt to follow up as many women as possible within 2 weeks of the birth.
-
To provide the women with an incentive to complete the follow-ups at the end of pregnancy and 6 months postnatally, all women who completed these follow-ups were given a £10 shopping voucher for each of the follow-up sessions attended (approved 18 May 2010).
-
Originally, to be eligible women had to report smoking at least 10 cigarettes a day before their pregnancy. We found that a good number of women reported smoking five to nine cigarettes a day at this time. Therefore, we extended the eligibility criteria to include women smoking at least five cigarettes a day before pregnancy (approved 15 September 2010). These women are still likely to be dependent on smoking as there is evidence that women who say they were smoking five to nine cigarettes before pregnancy are back to smoking 14 cigarettes a day at 18 months postnatally. 90
-
Initially, women had to be between 12 and 24 weeks’ gestation to be eligible for the trial. However, after the trial started most of the hospital trusts began offering earlier antenatal booking appointments (before 12 weeks’ gestation) and, because we wished to recruit women as early as possible in pregnancy, we revised this eligibility criterion to 10–24 weeks’ gestation (approved 31 January 2012).
-
‘Partner’s smoking status’ was adjusted for in the final model for all of the outcomes related to smoking abstinence; this amendment was approved after publication of the protocol.
Trial extension
The NIHR agreed a 12-month time extension to the trial. This was necessary as the rate of recruitment had been slower than anticipated and several additional recruitment sites had been established. Through careful budgeting, mostly as a result of the researchers working fewer hours and the chief investigator taking the role of trial manager, the majority of this extension was funded within the original budget. An addition to the budget was awarded to extend the contract of the trial administrator.
Chapter 3 Results
Recruitment of participants
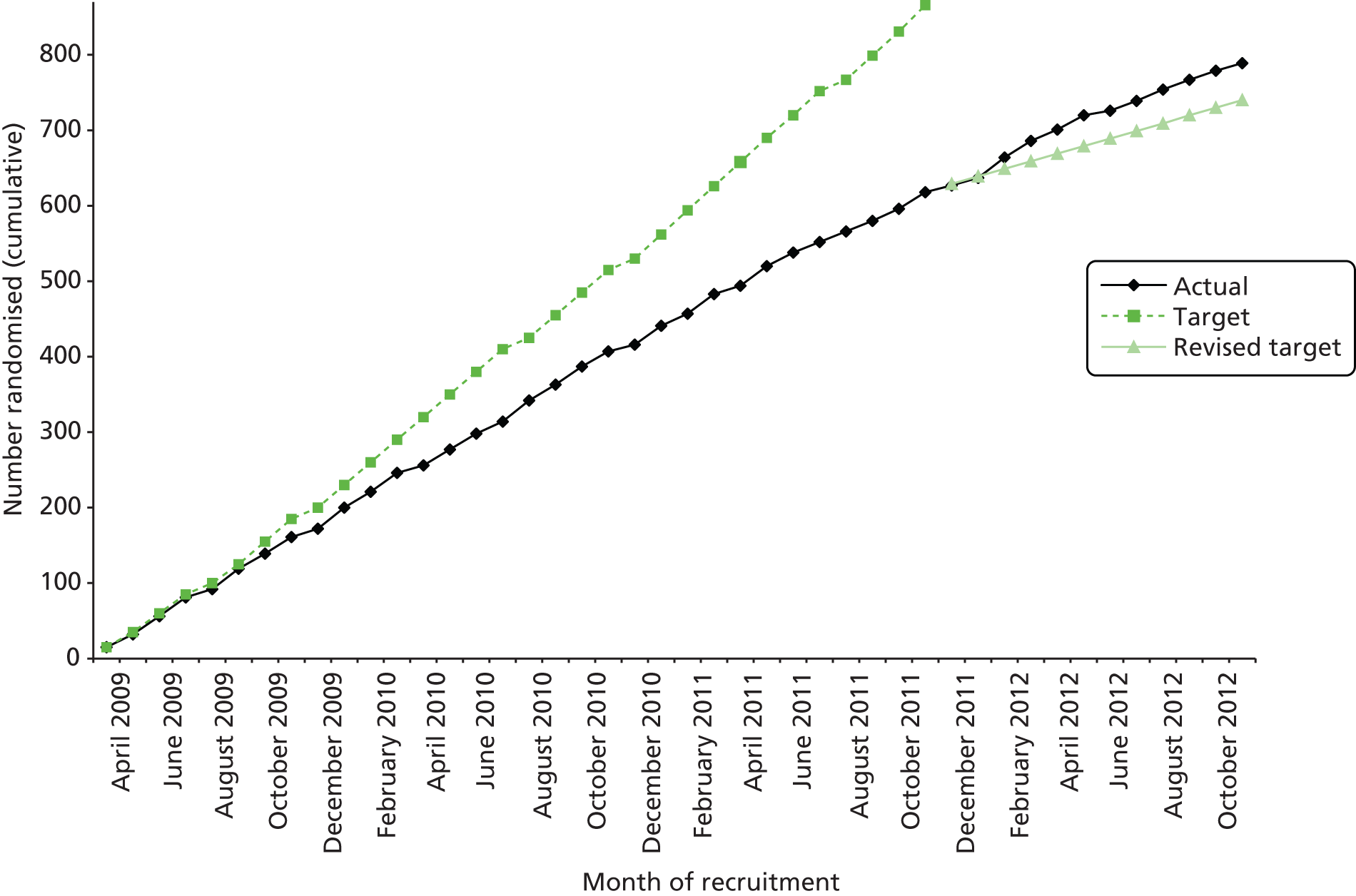
Recruitment took place between April 2009 and November 2012. Follow-up at 6 months after birth continued until January 2014. Figure 1 shows accrual for the original recruitment target of 866 participants, the revised target of 774 participants [agreed with the NIHR Health Technology Assessment (HTA) programme following approval of the trial extension] and the 789 participants who were actually randomised. Four women were excluded post randomisation. Two women (PA group) were enrolled twice in sequential pregnancies and their second enrolment was removed. The other two women (control group) were excluded because they were found to be ineligible at their baseline visit before any data were collected and had been randomised erroneously. When ineligible participants are mistakenly randomised into a trial it is acceptable, within an ITT approach, to exclude their data without risking bias. 91 Table 4 shows the recruitment numbers for each centre for the final sample size of 785 participants included in the analysis. As shown in Table 5, over two-thirds of participants were recruited through midwife referral and over one-quarter through direct calling, through extracting contact information from the PAS. Fewer than 4% of participants were recruited using the other methods combined.
FIGURE 1.
Cumulative trial recruitment.
| Centre | PA group (n = 392), n | Control group (n = 393), n | Total (N = 785), n (%) |
|---|---|---|---|
| St George’s Healthcare NHS Trust (St George’s Hospital) | 44 | 44 | 88 (11.2) |
| Chelsea and Westminster Hospital NHS Foundation Trust (Chelsea and Westminster Hospital) | 26 | 25 | 51 (6.5) |
| Imperial College Healthcare NHS Trust (Queen Charlotte’s and Chelsea Hospital) | 78 | 76 | 154 (19.6) |
| Imperial College Healthcare NHS Trust (St Mary’s Hospital) | 52 | 51 | 103 (13.1) |
| Guy’s and St Thomas’ NHS Foundation Trust (St Thomas’ Hospital) | 21 | 22 | 43 (5.5) |
| Croydon Health Services NHS Trust (Croydon University Hospital) | 38 | 37 | 75 (9.6) |
| Kingston Hospital NHS Foundation Trust (Kingston Hospital) | 40 | 41 | 81 (10.3) |
| Epsom and St Helier University Hospitals NHS Trust (Epsom Hospital) | 47 | 46 | 93 (11.8) |
| Surrey and Sussex Healthcare NHS Trust (Crawley Hospital) | 20 | 20 | 40 (5.1) |
| King’s College Hospital NHS Foundation Trust (King’s College Hospital) | 5 | 5 | 10 (1.3) |
| Medway Foundation Trust (Medway Maritime Hospital) | 6 | 7 | 13 (1.7) |
| West Middlesex University Hospital NHS Trust (West Middlesex Hospital) | 8 | 10 | 18 (2.3) |
| Mid Cheshire Hospitals NHS Foundation Trust (Leighton Hospital) | 7 | 9 | 16 (2.0) |
| Recruitment method | % | n |
|---|---|---|
| Midwife referral | 69.3 | 544 |
| Direct calling after consulting the PAS | 27.1 | 213 |
| Flyer/poster | 1.3 | 10 |
| Ultrasound questionnaire | 1.4 | 11 |
| Referral from PCT or other health professional | 0.9 | 7 |
| Total | 100 | 785 |
The CONSORT diagram (Figure 2) shows the flow of participants through the study to the primary end point at the end of pregnancy. Around 8100 women were recorded as smokers in the PAS. Of these, approximately one-quarter were uncontactable, just over one-quarter said that they were not interested in participating and just under one-third did not meet the inclusion criteria. A breakdown of the reasons for excluding participants is presented in Table 6. The main reason for exclusion was ‘reports smoking less than one cigarette a day’. Overall, of the 8096 women recorded as smokers at their first antenatal visit, 9.7% (785) were included in the ITT analysis. Of 785 pregnancies, 774 were singleton pregnancies, 10 were twin pregnancies and one was unknown as the woman withdrew consent.
FIGURE 2.
Numbers of participants who were enrolled in the study and included in the primary analysis. The participants lost to follow-up included some who had fetal or infant loss and were not assessed for smoking status.

| Reason for exclusion | % | n |
|---|---|---|
| Reports smoking less than one cigarette a day now | 27.5 | 710 |
| Gestation > 24 weeks | 26.1 | 673 |
| Gestation < 10 weeks | 8.0 | 207 |
| Unable to attend all visits | 13.9 | 359 |
| Wants to use NRT from commencing quit attempt | 11.2 | 290 |
| Poor English | 5.8 | 149 |
| Drug or alcohol problem | 3.1 | 80 |
| Reports smoking < 10 cigarettes a day before pregnancy | 2.1 | 53 |
| Medical contraindication to exercise | 1.2 | 31 |
| Age < 16 years | 0.7 | 17 |
| Unable to walk for 15 minutes | 0.5 | 13 |
| No permission to contact GP/obstetrician | 0.04 | 1 |
| Total | 100 | 2583 |
Baseline characteristics
Participants in the two groups had similar baseline characteristics (Table 7). Women were recruited to the trial at a mean gestational age of 16 weeks and on average they were 27 years of age. Over half (53.6%) were smoking at least 10 cigarettes a day. By self-report, 70% were achieving the recommendation of 150 minutes a week of MVPA. 27,28
| Characteristic | PA group (n = 391a), n (%) | Control group (n = 393), n (%) |
|---|---|---|
| Age (years), mean (SD) | 27.2 (6.1) | 27.8 (6.5) |
| Age at leaving full-time education (years), mean (SD)b | 17.8 (3.0) | 18.0 (3.3) |
| Maternal weight at first antenatal booking appointment (kg), mean (SD)c | 68.3 (14.4) | 70.4 (15.6) |
| BMI (kg/m2), mean (SD)c | 25.6 (5.0) | 26.6 (5.6) |
| Gestational age (weeks), mean (SD) | 15.6 (3.3) | 15.6 (3.3) |
| Number of cigarettes smoked daily before pregnancy, median (IQR) | 20 (12–20) | 20 (12–20) |
| Number of cigarettes smoked daily at randomisation, median (IQR) | 10 (5–12) | 10 (5–15) |
| FTCD score, median (IQR)d | 4 (2–5) | 4 (2–5) |
| Expired CO level (p.p.m.), median (IQR)e | 10 (7–14) | 10 (6–14) |
| Self-report of weekly MVPA (minutes), median (IQR) | 210 (125–350) | 225 (130–360) |
| Married or living with partner | 230 (58.8) | 221 (56.2) |
| Women with partner who smokesf | 261 (66.8) | 250 (63.6) |
| Caucasiang | 308 (78.8) | 298 (75.8) |
| Professional/managerial occupation | 46 (11.8) | 53 (13.5) |
| Smoked in a previous pregnancyh | 186 (78.2) | 193 (77.5) |
| EPDS score of ≥ 13 | 68 (17.4) | 75 (19.1) |
| Self-report of > 150 minutes per week of MVPA | 275 (70.3) | 273 (69.5) |
| Self-report walking as main type of PA | 301 (77.0) | 313 (79.6) |
| Parityi | ||
| 0–1 | 317 (81.1) | 309 (78.6) |
| 2–3 | 67 (17.1) | 75 (19.1) |
| ≥ 4 | 7 (1.8) | 9 (2.3) |
| Previous preterm birthj | 68 (17.4) | 61 (15.5) |
| Very or extremely high confidence for quitting smoking | 89 (22.8) | 98 (24.9) |
| Very or extremely confident of doing 30 minutes of PA on at least 5 days a week during pregnancy | 274 (70.1) | 277 (70.5) |
| Drinks alcohol more than twice a week | 6 (1.5) | 5 (1.3) |
| Consumes more than three alcoholic drinks on a drinking dayk | 14 (15.9) | 3 (3.8) |
Follow-up rates
At 4 weeks after the quit day, 316 (80.6%) women were successfully followed up in the PA group and 319 (81.2%) in the control group. At the end of pregnancy follow-up, 587 (74.8%) women were assessed before the birth and 198 (25.2%) were assessed after the birth. The overall follow-up rate for the primary outcome at the end of pregnancy (see Figure 2) was 88.8% (697 participants) and there was no evidence of a significant difference in the follow-up rate between study groups. Of the 88 participants (11.2%) who did not complete the assessments necessary for analysis of the primary outcome, 43 (48.9%) were known to have smoked from the follow-up assessments (PA group n = 19, control n = 24). In addition, of the remaining 45 participants (5.7% of total), 24 had a fetal or infant death and were not asked about smoking status, and one individual withdrew consent; all were assumed to be smoking at the end of pregnancy.
Rates of biochemical validation of smoking status
For the majority of participants who reported that they were not smoking, biochemical validation was obtained. At the end of pregnancy, validation rates for smoking status were 71.4% (30/42) in the PA group and 56.8% (25/44) in the control group (p = 0.158); at 4 weeks, the rates were 98.0% (50/51) and 100.0% (61/61) respectively (p = 0.272).
Attendance at treatment sessions and compliance with the physical activity intervention
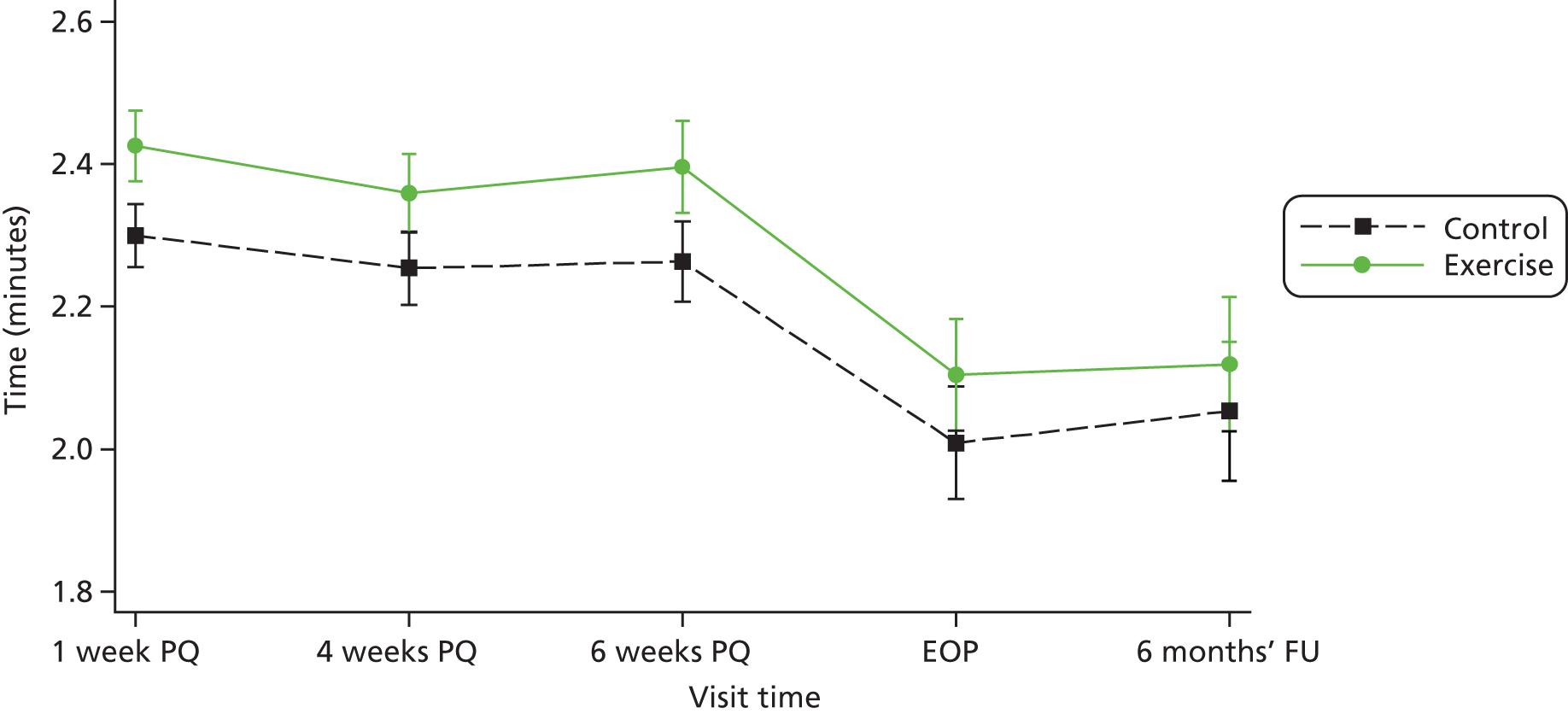
Participants attended a median of four of 14 treatment sessions in the PA group and three of six in the control group (Table 8). For the PA group compared with the control group, there was a 33% (95% CI 14% to 56%), 28% (95% CI 7% to 52%) and 36% (95% CI 12% to 65%) significantly greater increase in self-reported minutes of MVPA from baseline to 1 week, 4 weeks and 6 weeks respectively (see Table 8 and Figure 3). There was a decrease in self-reported minutes of PA at the end of pregnancy and at 6 months relative to baseline for both groups.
| Variable | PA group | Control group | Relative change in PA (95% CI): mixed-effect model for the log of PA | p-value |
|---|---|---|---|---|
| Self-reported weekly minutes of MVPA, n, median (IQR) | ||||
| Baseline | 391, 210 (125–350) | 393, 225 (130–360) | ||
| 1 week post quit | 162, 280 (190–425) | 206, 240 (140–420) | 1.33 (1.14 to 1.56) | < 0.001 |
| 4 weeks post quit | 135, 270 (180–420) | 157, 210 (120–340) | 1.28 (1.07 to 1.52) | 0.006 |
| 6 weeks post quit | 90, 277 (180–400) | 121, 220 (130–350) | 1.36 (1.12 to 1.65) | 0.002 |
| End of pregnancy | 188, 155 (100–240) | 187, 140 (60–240) | 1.25 (0.96 to 1.61) | 0.093 |
| 6-month follow-up | 147, 180 (80–330) | 136, 135 (60–285) | 1.16 (0.85 to 1.59) | 0.339 |
| Number of treatment sessions attended, n, median (IQR) | 391, 4 (2–8)a | 393, 3 (2–6) | NA | |
| Time walked on treadmill during supervised exercise (minutes), n, mean (SD) | ||||
| Baseline | 390, 12.2 (7.5) | NA | NA | |
| 1 week post quit | 163, 19.0 (8.5) | |||
| 4 weeks post quit | 134, 15.2 (10.8) | |||
| 6 weeks post quit | 90, 17.7 (10.9) | |||
FIGURE 3.
Comparisona of predicted self-reported levels of MVPA on a log-scale in the trial groups at different time points (bars represent CIs). EOP, end of pregnancy, FU, follow-up; PQ, post quit. a, Prediction on log scale from the mixed-effect model.
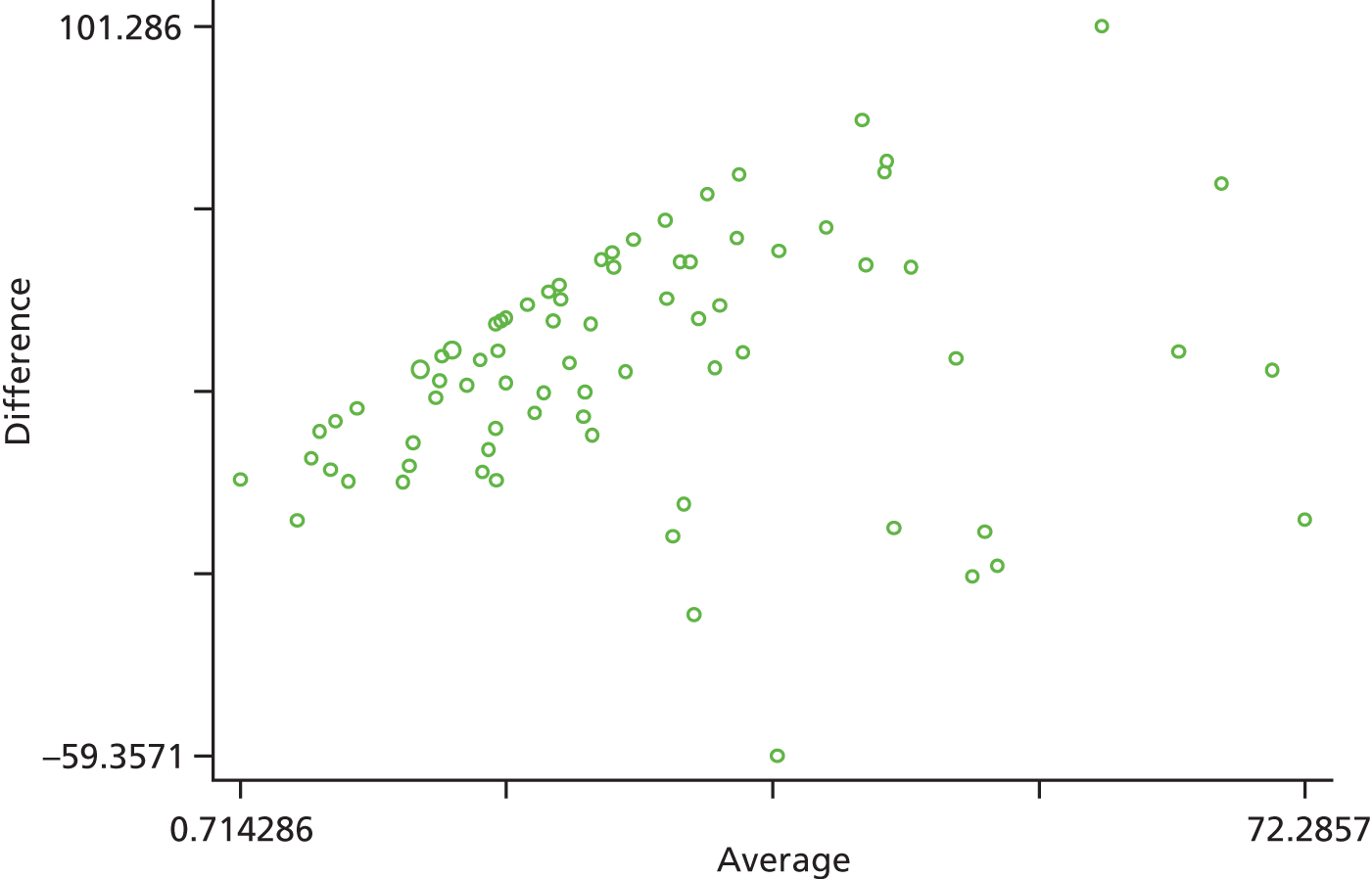
Of 90 participants asked to wear an accelerometer, 78 (86.7%) provided valid data (n = 37 PA group), 10 provided insufficient data and two were technical failures. Participants providing accelerometer data had similar baseline characteristics to those in the total sample. The majority (72%) had valid accelerometer data for at least 4 days. During the week of accelerometer wear, the median number of minutes of MVPA per day by self-report and according to accelerometer data was 38.2 [interquartile range (IQR) 25.4–54.5] and 7.8 (IQR 0–16.5) respectively. In total, 87% of participants self-reported higher levels of MVPA compared with the accelerometer data. Self-reports of minutes of MVPA per day were not significantly correlated with the accelerometer data (Spearman’s rho = 0.133, p = 0.247). Consistent with the correlation, using a Bland–Altman plot the mean difference between the self-report data and the accelerometer data for MVPA was 26.85 (95% CI 20.81 to 32.88) minutes (Figure 4). The median number of minutes of MVPA according to accelerometer data, when including only bouts of at least 10 minutes, was very similar for the PA group [7.5 (IQR 0–15.5)] and the control group [8.0 (IQR 0–16.2)] (p = 0.816). When including all MVPA, irrespective of duration, median activity levels per day tended to be higher for the PA group [38.00 (IQR 20.00–52.60)] than the control group [31.17 (IQR 19.00–44.10)], although this difference did not reach significance (p = 0.538).
FIGURE 4.
Bland–Altman plot of the difference between self-reported MVPA and accelerometer data for MVPA against the average of these measures (n = 78) showing the distribution of differences and how they relate to the average. Limits of agreement (reference range for difference) –26.698 to 80.390; range 0.714–72.286.
Only 28 women reported receiving face-to-face behavioural support for smoking cessation besides that offered in the trial and 60 women reported using NRT; the numbers reporting this support were similar in the two groups.
Smoking abstinence and reduction rates
There was no significant difference in smoking abstinence rates or smoking reduction rates between the two groups at follow-up (Table 9). The rate of validated continuous abstinence at the end of pregnancy (primary outcome) was 7.7% in the PA group and 6.4% in the control group (OR for PA group, adjusted for centre only, 1.21, 95% CI 0.70 to 2.10). At 4 weeks the validated abstinence rate was 12.8% in the PA group and 15.5% in the control group (OR, adjusted for centre only, 0.79, 95% CI 0.53 to 1.18). At 6 months postnatally the self-reported abstinence rate was 6.1% in the PA group and 4.1% in the control group (OR, adjusted for centre only, 1.55, 95% CI 0.81 to 2.97). Fully adjusted analyses yielded similar findings (at end of pregnancy: OR 1.37, 95% CI 0.78 to 2.41). The sensitivity analyses showed that the observed effect size and its statistical significance were independent of the influence of missing data for the primary outcome. There was no significant interaction between baseline self-reports of MVPA (< 150 vs. ≥ 150 minutes per week) and the treatment effect for the primary outcome [logistic regression model adjusted for site only: likelihood ratio test (LR chi-squared) = 2.31, p = 0.129; similar results were found for the fully adjusted model].
| Abstinence/reduction outcomesa | PA group (n = 392), n (%) | Control group (n = 393), n (%) | OR (95% CI)b | Adjusted OR (95% CI)c |
|---|---|---|---|---|
| Primary | ||||
| Self-reported continuous abstinence at end of pregnancy with biochemical validationd | 30 (7.7) | 25 (6.4) | 1.21 (0.70 to 2.10) | 1.37 (0.78 to 2.41) |
| Secondary | ||||
| Self-reported continuous abstinence at 4 weeks after quit day with biochemical validatione | 50 (12.8) | 61 (15.5) | 0.79 (0.53 to 1.18) | 0.87 (0.57 to 1.31) |
| Self-reported continuous abstinence at 6 months after birth | 24 (6.1) | 16 (4.1) | 1.55 (0.81 to 2.97) | 1.66 (0.82 to 3.37) |
| Self-reported lapse-free abstinence with biochemical validation | ||||
| At 4 weeks after quit day | 17 (4.3) | 16 (4.1) | 0.68 (0.38 to 1.22) | 0.74 (0.41 to 1.34) |
| At end of pregnancy | 20 (5.1) | 29 (7.4) | 1.07 (0.53 to 2.14) | 1.22 (0.60 to 2.48) |
| At 6 months after birth | 10 (2.6) | 10 (2.5) | 1.04 (0.43 to 2.56) | 1.12 (0.45 to 2.78) |
| PA group, n, mean (SD) | Control group, n, mean (SD) | Mean difference (95% CI) | Mean difference (95% CI) | |
| Self-reported reduction in no. of cigarettes smoked dailyf | ||||
| Between baseline and 4 weeks after quit day | 67, 4.3 (4.4) | 70, 4.0 (4.7) | 0.23 (–1.22 to 1.73) | 0.27 (–1.16 to 1.65) |
| Between baseline and end of pregnancy | 130, 4.0 (4.7) | 119, 2.9 (5.9) | 1.13 (–0.26 to 2.55) | 1.08 (–0.11 to 2.32) |
| Between baseline and 6 months after birth | 97, 1.4 (4.5) | 100, 1.0 (5.3) | 0.37 (–0.99 to 1.74) | 0.21 (–1.14 to 1.58) |
Withdrawal symptoms and urge to smoke
Tables 10 and 11 show the withdrawal symptom and urge to smoke scores, respectively, at baseline and 1 week post quit. When controlling for baseline score and treatment centre there was no significant group difference in total withdrawal score or urge to smoke score at 1 week post quit. When withdrawal symptoms were examined individually there were still no significant group differences.
| Follow-up | PA group, n, mean (SD) score | Control group, n, mean (SD) score | Linear regression: β (95% CI) for PA group vs. control group; p-valuea |
|---|---|---|---|
| Baseline | |||
| Withdrawal symptoms scale total score (range 1–35) | 391, 16.3 (4.9) | 393, 16.4 (4.7) | |
| Symptoms | |||
| Restless | 2.3 (1.2) | 2.3 (1.2) | |
| Irritable | 2.6 (1.2) | 2.7 (1.2) | |
| Depressed | 1.8 (1.1) | 1.7 (1.0) | |
| Hungry | 2.8 (1.3) | 3.0 (1.2) | |
| Poor concentration | 2.0 (1.1) | 2.0 (1.1) | |
| Poor sleep at night | 2.5 (1.3) | 2.5 (1.3) | |
| Anxious | 2.2 (1.2) | 2.2 (1.2) | |
| 1 week post quit | |||
| Withdrawal symptoms scale total score (range 1–35) | 163, 16.0 (4.6) | 206, 16.6 (4.6) | –0.56 (–1.45 to 0.33); 0.256 |
| Symptoms | |||
| Restless | 2.4 (1.1) | 2.4 (1.1) | |
| Irritable | 2.7 (1.1) | 2.9 (1.2) | |
| Depressed | 1.7 (1.0) | 1.8 (1.0) | |
| Hungry | 2.7 (1.2) | 2.9 (1.2) | |
| Poor concentration | 2.1 (1.0) | 2.1 (1.1) | |
| Poor sleep at night | 2.3 (1.2) | 2.3 (1.2) | |
| Anxious | 2.1 (1.0) | 2.2 (1.1) | |
| Follow-up | PA group, n, mean (SD) score | Control group, n, mean (SD) score | Linear regression: β (95% CI) for PA group vs. control group; p-valueb |
|---|---|---|---|
| Baseline | 391, 6.2 (2.0) | 393, 6.2 (1.9) | |
| 1 week post quit | 163, 5.3 (2.0) | 206, 5.5 (2.0) | –0.23 (–0.62 to 0.16); 0.242 |
Confidence for participating in physical activity and stopping smoking
Tables 12 and 13 present the scores for confidence for participating in PA and for stopping smoking, respectively, at baseline and 1 week post quit. When controlling for baseline score and treatment centre, ratings for confidence for participating in PA were significantly higher at 1 week post quit in the PA group than in the control group. When making the same adjustments, there was no significant group difference in confidence for quitting smoking at 1 week post quit.
| Follow-up | PA group, n, mean (SD) score | Control, n, mean (SD) score | Linear regression: β (95% CI) for PA group vs. control group; p-valuea |
|---|---|---|---|
| Baseline | 391, 3.9 (1.1) | 393, 3.9 (1.0) | |
| 1 week post quit | 163, 3.9 (1.0) | 157, 3.7 (1.0) | 0.30 (0.10 to 0.49); 0.002 |
| Follow-up | PA group, n, mean (SD) score | Control group, n, mean (SD) score | Linear regression: β (95% CI) for PA group vs. control group; p-valuea |
|---|---|---|---|
| Baseline | 391, 3.9 (0.9) | 393, 4.0 (1.0) | |
| 1 week post quit | 163, 4.4 (1.0) | 206, 4.3 (0.9) | 0.15 (–0.03 to 0.33); 0.097 |
Birth outcomes
Table 14 shows outcomes for singleton births. These outcomes were very similar between the two study groups except that there were significantly fewer deliveries by caesarean section in the PA group than in the control group (21.3% vs. 28.7%). Analyses that included twin births gave very similar findings.
| Fetal outcomes (singleton births only) | PA group (n = 384), n/N (%) | Control group (n = 391), n/N (%) | OR (95% CI)b |
|---|---|---|---|
| Miscarriagec | 6/383 (1.6) | 10/389 (2.6) | 0.60 (0.22 to 1.67) |
| Stillbirthc | 2/377 (0.5) | 2/379 (0.5) | 1.01 (0.14 to 7.24) |
| Neonatal deathc | 0 | 1/391 (0.3) | Not calculated |
| Preterm birth (< 37 weeks’ gestation) | 35/356 (9.8) | 26/348 (7.5) | 1.36 (0.80 to 2.31) |
| Low birthweight (< 2.5 kg) | 38/353 (10.8) | 44/359 (12.3) | 0.87 (0.55 to 1.38) |
| NICU admission | 27/352 (7.7) | 36/356 (10.1) | 0.74 (0.44 to 1.25) |
| Apgar score at 5 minutes < 7 | 8/344 (2.3) | 11/351 (3.1) | 0.74 (0.29 to 1.85) |
| Cord blood arterial pH < 7 | 2/130 (1.5) | 0/125 | Not calculated |
| Congenital abnormalitiesd | 9/346 (2.6) | 6/348 (1.7) | 1.43 (0.50 to 4.12) |
| Assisted vaginal delivery | 46/357 (12.9) | 32/359 (8.9) | 1.51 (0.94 to 2.43) |
| Caesarean delivery | 76/357 (21.3) | 103/359 (28.7) | 0.67 (0.48 to 0.95)e |
| PA group (n = 384), mean (SD) | Control group (n = 391), mean (SD) | Mean difference (95% CI)b | |
| Birthweight (kg) | (n = 354) 3.13 (0.58) | (n = 359) 3.15 (0.64) | –0.01 (−0.11 to 0.08) |
| Gestational age at delivery (weeks) | (n = 356) 39.24 (2.1) | (n = 348) 39.26 (2.1) | –0.02 (–0.36 to 0.31) |
Adverse events
There were similar numbers of AEs and SAEs in the two groups (Table 15). The total number of women or their infants who had at least one AE or SAE was 217 (55.4%) in the PA group and 219 (55.7%) in the control group (OR 0.99, 95% CI 0.75 to 1.32). The full breakdown of the data for less frequent AEs is provided in Appendix 9.
| Event | PA group (n = 392), n (%) | Control group (n = 393), n (%) |
|---|---|---|
| SAEs | ||
| Maternal death | 0 | 0 |
| Other eventsb | 12 (3.1) | 13 (3.3) |
| Maternal AEs potentially related to treatmentc | 2 (0.5) | 0 |
| Maternal AEs as probable complications of pregnancy | ||
| Vaginal bleeding or haemorrhage | 37 (9.4) | 35 (8.9) |
| Abdominal pain | 79 (20.2) | 83 (21.1) |
| Infection in pregnancy | 61 (15.6) | 55 (14.0) |
| Premature rupture of membranes at < 37 weeks’ gestation | 19 (4.8) | 16 (4.1) |
| Gestational diabetes | 7 (1.8) | 8 (2.0) |
| Gestational hypertension | 13 (3.3) | 13 (3.3) |
| Pre-eclampsia | 4 (1.0) | 11 (2.8) |
| Other, less frequent eventsd | 105 (26.8) | 114 (29.0) |
| Fetal AEs as probable complications of pregnancy | ||
| Decreased fetal movement | 41 (10.5) | 49 (12.5) |
| Intrauterine growth restriction | 15 (3.8) | 18 (4.6) |
| Other, less frequent eventsd | 7 (1.8) | 6 (1.5) |
| Neonatal AEs | 15 (3.8) | 14 (3.6) |
| Total AEs | 417 | 435 |
Maternal depression
All 784 participants provided EPDS data at baseline, with 383 (48.9%) and 279 (35.6%) participants providing these data at the end of pregnancy and at 6 months postnatally, respectively. The baseline characteristics of the subsamples used for the EPDS analysis at the two follow-up points were similar to those for the total trial sample. The baseline characteristics of the two trial groups were also similar in the subsamples at the end of pregnancy and at 6 months postnatally.
In both models the EPDS score was significantly higher in the PA group than in the control group at the end of pregnancy (Table 16). At this time there was a mean increase in EPDS score of 0.4 in the PA group and a mean reduction in EPDS score of 0.5 in the control group (mean difference between groups in fully adjusted model 0.95, 95% CI 0.08 to 1.83). When examining the data separately for end of pregnancy outcomes before and after the birth the findings were very similar. There was no significant difference in EPDS score between the groups at 6 months’ follow-up (fully adjusted mean difference 0.37, 95% CI –0.59 to 1.33).
| Follow-up | EPDS score, n, mean (SD) | β (difference between PA and control group, adjusted for baseline EPDS and centre onlyb) (95% CI); p-value | β (difference between PA and control group, fully adjustedc) (95% CI); p-value | |
|---|---|---|---|---|
| Control group | PA group | |||
| Baseline | 393, 7.7 (5.0) | 391, 7.6 (5.3) | 0 | 0 |
| End of pregnancy | 194, 7.2 (5.0) | 189, 8.0 (4.9) | 1.06 (0.19 to 1.94); 0.017 | 0.95 (0.08 to 1.83); 0.033 |
| 6 months | 133, 6.6 (4.7) | 146, 6.8 (4.8) | 0.52 (–0.45 to 1.50); 0.293 | 0.37 (–0.59 to 1.33); 0.450 |
Maternal weight
The combined sample for the analysis of maternal weight at the end of pregnancy was 271 participants, with 140 (51.7%) providing maternal weight before the birth and 131 (48.3%) providing maternal weight after the birth. The characteristics of these two subsamples (i.e. maternal weight provided before and after birth) were comparable with those of the main trial sample except that the rate of continuous smoking abstinence at the end of pregnancy was higher in both the subsample with maternal weight provided before delivery [n = 35 (25.0%)] and the subsample with maternal weight provided after delivery [n = 14 (10.7%)] than in the main sample [n = 55 (7.0%)]. In the combined sample at the end of pregnancy the characteristics were very similar between the two randomisation groups except that there was an approximately 1-kg difference in weight at early pregnancy between the two groups and therefore the results were adjusted for weight at early pregnancy. Rates of smoking abstinence were higher in the PA group than in the control group at the end of pregnancy [n = 29 (21.2%) vs. n = 20 (14.9%)] but this difference was not significant. When comparing the characteristics between the randomisation groups separately for the subsamples providing maternal weight before and after birth the findings were very similar.
At the end of pregnancy the mean GWG (subsample providing maternal weight before birth) in the PA group was 12.4 kg [standard deviation (SD) 6.1 kg] and in the control group was 11.2 kg (SD 7.0 kg) and the postnatal weight retention (subsample providing maternal weight after birth) was 4.7 kg (SD 7.2 kg) and 4.9 kg (SD 7.3 kg), respectively. With the basic adjustment the mean difference in GWG and postnatal weight retention for the PA group compared with the control group was 1.08 kg (95% CI –1.08 kg to 3.23 kg) and 0.11 kg (95% CI –2.27 kg to 2.49 kg), respectively (Table 17). In the fully adjusted model these mean differences were reduced and neither of them achieved statistical significance. The estimated mean weight change per gestation week was 0.31 kg (SD 0.15 kg) in the PA group and 0.27 kg (SD 0.17 kg) in the control group, and the difference was not significant. In contrast, the difference in estimated mean weight change per gestation week between the groups was significantly lower for those classed as obese at baseline than for those classed as non-obese at baseline (–0.12 kg, 95% CI –0.21 kg to –0.03 kg; p = 0.010). There was no significant interaction between the effect of PA compared with the control and whether or not the individual was obese at baseline (p = 0.599), such that, in those who were not obese, the difference between the PA group and the control group was 0.03 kg (95% CI –0.03 kg to 0.08 kg) and in those who were obese the difference between the PA group and the control group was 0.06 kg (95% CI –0.09 kg to 0.21 kg).
| Variable | PA group (n = 74), mean (SD) | Control group (n = 66), mean (SD) | Basic adjustment, mean difference PA vs. control (95% CI);a p-value | Fully adjusted, mean difference PA vs. control (95% CI);b p-value |
|---|---|---|---|---|
| Subsample at end of pregnancy with maternal weights measured before birth (n = 140) | ||||
| Early pregnancy weight | 68.3 (14.4) | 70.3 (15.6) | ||
| EOP weight | 80.7 (14.9) | 81.4 (14.7) | ||
| Weight change | 12.4 (6.1) | 11.2 (7.0) | 1.08 (–1.08 to 3.23); 0.325 | 0.92 (–1.15 to 2.99) |
| 0.99 (–0.98 to 2.95)c | ||||
| Weight change per gestational week | 0.31 (0.15) | 0.27 (0.17) | 0.04 (–0.01 to 0.10);d 0.113 | 0.02 (–0.03 to 0.08);e 0.449 |
| 0.02 (–0.04 to 0.08);c,e 0.555 | ||||
| PA group (n = 74), n (%) | Control group (n = 66), n (%) | OR adjusted for centre (95% CI);f p-value | Fully adjusted OR (95% CI);g p-value | |
| Excessive gestational weight relative to early pregnancy BMI | 30 (40.5) | 24 (36.4) | 1.26 (0.63 to 2.53); 0.520 | 1.35 (0.63 to 2.88); 0.440 |
| 1.36 (0.60 to 3.09);c 0.456 | ||||
| PA group (n = 63), mean (SD) | Control group (n = 68), mean (SD) | Basic adjustment, mean difference PA vs. control (95% CI);a p-value | Fully adjusted, mean difference PA vs. control (95% CI);b p-value | |
| Subsample at end of pregnancy with maternal weights measured after birth (n = 131) | ||||
| Early pregnancy weight | 66.1 (14.6) | 66.2 (13.8) | ||
| EOP weight | 70.7 (14.1) | 71.3 (13.4) | ||
| Weight change | 4.7 (7.2) | 4.9 (7.3) | –0.11 (–2.27 to 2.49); 0.928 | –0.25 (–2.59 to 2.09) |
| –0.19 (–2.53 to 2.15)c | ||||
According to Institute of Medicine guidelines88 the overall proportion of women with excessive GWG was 38.6%. The risk of excessive GWG was slightly higher in the PA group than in the control group (fully adjusted OR 1.35, 95% CI 0.63 to 2.88), but the difference was not significant (see Table 17). The risk was significantly higher for the overweight and obese categories than for the healthy weight category (overweight: fully adjusted OR 4.0, 95% CI 1.69 to 9.58; obese: fully adjusted OR 3.29, 95% CI 1.20 to 8.99). Those in the underweight category were less likely to gain excessive gestational weight than those with a healthy weight but this was not significant (fully adjusted OR 0.57, 95% CI 0.06 to 5.31). In sensitivity analyses, when adjusted for the effect of baby’s weight and rates of smoking abstinence, the mean difference in the weight change between the two groups was slightly changed and the effect of baby’s weight was significant in the regression models (see Table 17).
Chapter 4 Health economic analysis and results
Introduction
Several studies have investigated the potential cost saving of smoking cessation interventions in pregnancy92 but only one study could be identified that has used empirical data on costs to calculate the incremental cost-effectiveness of these interventions. 93 This chapter reports on an economic evaluation conducted alongside the LEAP trial to address the cost-effectiveness of a PA intervention plus behavioural support compared with behavioural support alone.
The objectives were:
-
to compare the costs associated with the control and intervention strategies
-
to estimate the consequences of these alternatives
-
to assess the cost-effectiveness of the PA intervention used in addition to behavioural support for smoking cessation at the end of pregnancy.
Methods
Overview
A cost-effectiveness analysis was undertaken to compare a PA intervention plus behavioural support with behavioural support only for women who were smoking in pregnancy. The main outcome for the economic evaluation was biochemically validated abstinence from smoking between a quit date and the end of pregnancy. As recommended by the National Institute for Health and Care Excellence (NICE),94 a cost–utility analysis with a fully incremental analysis was conducted from a NHS and personal social services viewpoint, including direct health effects (maternal smoking cessation) and costs (or cost savings) to the NHS. Women were eligible for inclusion in the trial if they were between 10 and 24 weeks’ gestation and outcomes were collected at the end of pregnancy (between 36 weeks’ gestation and 10 weeks post partum); therefore, the time horizon of the trial was up to 9 months. A clinical trial of NRT for smoking cessation (Smoking, Nicotine, and Pregnancy; SNAP)95,96 was approved by the NIHR HTA programme shortly before this study began and, as both studies used similar outcome measures, a similar approach has been taken to economic evaluation to permit comparison.
Cost estimation
Two main costings were included: first, the costs of the interventions and, second, the costs of caring for each woman and her infant during the period between randomisation and the immediate postnatal period (up to 6 weeks post partum).
Intervention costs
The cost of the interventions included training and time for staff to deliver the behavioural support and PA consultations (costed as Band 6 midwife, including overheads) as well as equipment and consumable costs [CO monitors (breath testing equipment), consumables associated with CO breath testing, equipment (treadmills and pedometers to count steps when walking), exercise DVDs, printing of PA manual for participants], and childcare costs. Participants in both arms were offered up to six sessions of behavioural support for smoking cessation. In addition, those in the PA arm were offered 14 sessions of supervised treadmill exercise and nine PA consultations, which were combined with the smoking cessation support. All of the support was provided face to face. The time spent providing support in the trial was multiplied by salary and overhead costs to calculate a cost per session.
The cost per use of CO monitoring in the trial was calculated by first summing the costs of equipment and consumables and then dividing the total cost by the total number of uses. The equipment and consumables included 12 CO monitors [two calibration kits, semi-disposable mouth pieces (assuming that these were changed every 60 uses), batteries (assuming that these were changed every 210 uses) and additional mouth pieces per each use (used in combination with semi-disposable mouth pieces)] and alcohol-free wipe per use. Assumptions concerning the need to change semi-disposable mouth pieces and batteries were taken from the SNAP trial. 96
It was assumed that a treadmill would last for 10 years and that three midwives would require training in that time; therefore, annual costs were calculated as one-tenth of the cost of a treadmill plus one-tenth of the cost of training three midwives, calculated as 1 day of PA training and 1 day of smoking cessation training received by each midwife. The costs of training were derived from the costs of training in the LEAP trial. In the control arm, only the cost of smoking cessation training was included. A professor of clinical psychology provided the smoking cessation training and a private PA consultant, specialising in exercise and pregnancy, delivered the PA training; both of these trainers regularly provide training in the NHS.
We wanted to present findings for an ‘average/typical’ hospital and so made the following additional assumptions: given a hospital with 5000 births per year with 600 (12%) pregnant smokers (based on national data)7 and using the recruitment rate in the LEAP trial of 9.7% (785/8096 eligible women randomised), 58 (9.7% of 600) women would be recruited annually per hospital. Therefore, per-participant costs for the treadmill and training were estimated by dividing the annual costs by 58. The robustness of the results to this assumption was tested in sensitivity analysis.
Health-care resource use costs
Information about antenatal and postnatal hospital admissions and mode of delivery was collected from maternal medical records and data on admissions to neonatal special care came from infant medical records.
Valuation of costs
All data were valued in monetary terms and unit costs were reported in UK pounds for the financial year 2012/13 (representing the end point of the trial). As follow-up did not continue beyond 9 months post randomisation, the question of discounting future costs did not arise. For standard NHS health care, UK unit costs were applied from national sources, increasing the generalisability of the results. Table 18 presents a summary of the resource use and unit costs.
| Resource item | Unit cost (£) | Unit | Source |
|---|---|---|---|
| PA group only | |||
| Treadmill | 775 | Per treadmill | LEAP trial estimation |
| Pedometer | 7.12 | Per pedometer | LEAP trial estimation |
| Printing | 0.58 | Nine-page PA manual | LEAP trial estimation |
| Exercise DVD | 0.80 | Per DVD | LEAP trial estimation |
| PA training costs | 120 | Per midwife trained | LEAP trial estimation |
| Both groups | |||
| Smoking cessation training | 250 | Per midwife trained | LEAP trial estimation |
| Band 6 (midpoint) midwife time (including overheads) | 31.95 | Per hour | Curtis97 |
| CO monitors and consumables | 1.37 | Per use | LEAP trial estimation |
| Childcare | 15 | Per crèche visit | LEAP trial estimation |
| Health-care use (both groups) | |||
| Maternal antenatal admission | 582.24 | Per day | Department of Health98 |
| Mode of delivery | |||
| Unassisted vaginal delivery | 2313.60 | Per obstetric delivery | Department of Health98 |
| Assisted vaginal delivery | 2788.86 | ||
| Caesarean section | 3848.83 | ||
| Miscarriage | 1376.76 | aPetrou et al.99 | |
| Baby admission to neonatal unit (assumes an average of 4 days in hospital) | 2967.18 | Per admission | Department of Health98 |
| Maternal postnatal admission | 782.68 | Per day | Department of Health98 |
Calculating costs
To calculate the cost of face-to-face support for each trial participant we multiplied the number of treatment sessions by the duration of support in minutes. For the PA group we assumed that the midwife engaged with the woman only when she was off the treadmill, except that during the first 5 minutes of each treadmill walking session the midwife provided some PA counselling; the estimate of 5 minutes is based on interviews with the researchers who provided the interventions. Besides these 5 minutes, for the rest of the time that the woman was on the treadmill the midwife was able to proceed with her normal work.
Example
Suppose that a patient has 11 treatment sessions lasting 471 minutes (including treadmill time) and uses the treadmill in 10 of these sessions for a total of 279 minutes. This patient will have spent 471 – 279 = 192 minutes in treatment sessions when not using the treadmill. It is assumed that the midwife contact time consists of these 192 minutes plus a further 5 minutes for each of the 10 sessions in which a treadmill was used. Total midwife time is therefore (471 – 279) + (10 × 5) = 242 minutes.
The cost of mode of delivery was established by calculating a weighted average of unit costs for different modes of delivery activities recorded in NHS reference costs. 98 A similar method was used to calculate an average cost of a maternal antenatal admission and postnatal admission, based on antenatal observations and investigations.
Outcome measure
The measure of health benefit for the economic evaluation was the same as the primary measure of clinical effectiveness in the LEAP trial: self-reported and biochemically validated maternal smoking cessation from the quit date to the end of pregnancy. Temporary smoking lapses of up to a total of five cigarettes (on up to five occasions) were permitted.
Analysis
An incremental cost-effectiveness analysis was undertaken, following the NICE guidance for health-care evaluations,94 comparing the additional costs of a PA intervention with those of behavioural support alone, as well as the additional benefits, to give a cost per additional quitter.
Results are first presented as the per-participant quit rate and costs including fixed costs apportioned as described earlier in Cost estimation. The same results have also been scaled up to the expected annual costs and outcomes for a typical hospital with 58 participants per year as used for the estimation of fixed costs.
When two interventions are compared in a cost-effectiveness analysis it may be the case that the more effective intervention is also the less costly. In this case, the strategy that is both more effective and less costly is said to dominate the other strategy. The dominating strategy is then unconditionally preferred regardless of any considerations of budget. When no dominance relationship exists, the results are summarised in the incremental cost-effectiveness ratio (ICER), which is the additional cost of achieving one extra quitter. It can be calculated as the expected per-participant cost of the intervention group over and above that of the control group divided by the expected difference in quit rate. The following is the formula for the ICER, where Δ represents change, C represents the costs, E represents the effects and subscripts I and C refer to the intervention and control respectively:
(Note that when a dominance relationship exists, the ICER as calculated above is negative, but the numerical value is not informative. This can be seen because doubling either ΔC or ΔE makes the dominance relationship stronger but these two possible changes have opposite effects on the numerical value of the ICER.)
A total of 785 women were included in the primary ITT analysis, 392 in the PA group and 393 in the control group. However, one individual in the PA group withdrew consent before providing any data or receiving any treatment and was therefore excluded from the economic evaluation. Thus, we analysed data for 391 in the PA group and 393 in the control group. Analyses were conducted in Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA).
There were no missing data for the effectiveness outcome (validated smoking cessation from quit date to the end of pregnancy) as any women without validated smoking cessation data were assumed to be smokers. The average resource use of each cost item was estimated from the available data for the relevant arm of the trial, effectively imputing an average for each woman with missing data. This was justified following preliminary analysis, which showed no significant association between any of the resource use items and key patient baseline characteristics associated with smoking cessation (age, FTCD score, EPDS score, partner’s smoking status, age leaving full-time education) plus quit status at the end of pregnancy. Age was significantly associated with mode of birth, such that those who were older were more likely to have a caesarean section. In addition, those with a higher EPDS score were significantly more likely to report at least one antenatal hospital admission and one postnatal admission. However, as neither age nor EPDS score was significantly associated with smoking status at the end of pregnancy, these associations with resource use were not considered a concern when imputing missing data.
Bootstrapping was used to assess the joint uncertainty in cost and effectiveness outcomes. Missing data were imputed individually for the relevant resource use items to produce a complete data set. Bootstrapped data sets of the same size as the original patient groups were then constructed by sampling at random with replacement. Incremental cost and effectiveness estimates were then calculated using the same methods as described earlier. This was repeated 2000 times to produce a joint distribution of incremental costs and effectiveness, which was plotted on a cost-effectiveness plane. 100 A cost-effectiveness acceptability curve101 was generated showing the proportion of bootstrapped replications that would be cost-effective at a range of threshold ICERs.
The baseline characteristics of age and nicotine dependence are reliable predictors of smoking cessation. Therefore, in a subgroup analysis, cost-effectiveness calculations were repeated using only data for the following subgroups: women aged < 28 years, women aged > 27 years, women with a FTCD score of < 4, women with a FTCD score of ≥ 4.
Results
In the PA group 30 of 391 women (7.7%) were abstinent from smoking with biochemical validation from the quit date to the end of pregnancy; the equivalent figure for the control group was 25 of 393 women (6.4%), a non-significant difference (see Chapter 3, Results).
Table 19 reports the health-care resources utilised in the two arms of the trial. Quantities of services were multiplied by the relevant unit costs in Table 18 to calculate the resource use for each woman. Maternal antenatal and postnatal hospital admissions and admissions to neonatal care were similar in the two groups. With regard to mode of birth, the PA group underwent more assisted and spontaneous vaginal births and the control group underwent more caesarean sections and miscarriages. A chi-squared test including all four modes of birth showed a significant difference between the groups (χ2 = 8.7, p < 0.035). On average, the PA group attended 5.3 of 14 treatment sessions offered and the control group attended 3.5 of six sessions offered. The total duration of treatment sessions was around twice that for the PA group compared with the control group.
| Resource item | PA group (n = 391) | Control group (n = 393) |
|---|---|---|
| Maternal antenatal hospital admissionsa | ||
| Missing data, n/N (%) | 46/391 (11.8) | 56/393 (14.2) |
| Admissions, n/N (%) | 85/345 (24.6) | 72/337 (21.4) |
| Average length of days per admission, mean (SD) | 2.9 (10.5) | 2.3 (2.5) |
| Baby admitted to neonatal unit, n/N (%)a | ||
| Missing data | 31/391 (7.9) | 35/393 (8.9) |
| Admissions | 29/360 (8.1) | 36/358 (10.1) |
| Maternal postnatal hospital admissionsa | ||
| Missing data, n/N (%) | 36/391 (9.2) | 42/393 (10.7) |
| Admissions, n/N (%) | 275/355 (77.5) | 271/351 (77.2) |
| Average length of days per admission, mean (SD) | 2.1 (1.6) | 2.2 (1.9) |
| Mode of birth, n/N (%) | ||
| Missing data | 20/391 (5.1) | 22/393 (5.6) |
| Assisted vaginal birthb | 49/391 (12.5) | 33/393 (8.4) |
| Caesarean section | 77/391 (19.7) | 104/393 (26.5) |
| Spontaneous vaginal birth | 239/391 (61.1) | 224/393 (57.0) |
| Miscarriage | 6/391 (1.5) | 10/393 (2.5) |
| Number of treatment sessions, mean (SD) | 5.3 (4.1) | 3.5 (1.9) |
| Total duration of treatment sessions (minutes), mean (SD) | 177.3 (146.0) | 86.8 (51.2) |
| Number of sessions using treadmill, mean (SD) | 4.9 (4.0) | NA |
| Total time on treadmill (minutes), mean (SD) | 93.3 (92.2) | NA |
| Number of crèche sessions,c mean | 0.46 | 0.22 |
The point estimate results shown in Table 20 suggest that the PA intervention is somewhat less costly than the control intervention, by a margin of £35 per participant, but produces a small increase in the expected number of quitters. Taken in isolation, this suggests that the PA intervention should be adopted, regardless of the decision-maker’s willingness to pay for an additional quitter. However, this result must be interpreted with great caution because there is considerable statistical uncertainty, as reflected in the scatterplot (Figure 5), which shows that neither the cost difference nor the effect difference is statistically significant as there are an appreciable number of points in each quadrant. This is confirmed by the cost-effectiveness acceptability curve (Figure 6), which shows that the proportion of bootstrapping replications deemed cost-effective does not exceed 80% for any willingness-to-pay threshold up to £50,000 per additional quitter.
| Group | Average per-participant intervention cost (£, 2012/13 prices) | Average per-participant resource use cost (£, 2012/13 prices) | Average per-participant cost (£, 2012/13 prices) | Quit rate (%) | Expected annual costa (£, 2012/13 prices) | Expected annual quittersa | ICER |
|---|---|---|---|---|---|---|---|
| PA | 83 | 4630 | 4713 | 7.7 | 273,343 | 4.45 | Not calculated: PA dominates the comparator in the point estimate result |
| Control | 56 | 4692 | 4748 | 6.4 | 275,373 | 3.69 | |
| Difference | 27 | –62 | −35 | 1.3 | −2029 | 0.76 |
FIGURE 5.
Cost-effectiveness scatterplot.
FIGURE 6.
Cost-effectiveness acceptability curve.

The ‘stripey’ effect in the cost-effectiveness scatterplot (see Figure 5) reflects the discrete nature of the outcome measure. One additional quitter selected in the bootstrapping moves the point to the next stripe. It appears that the estimated cost saving from the PA intervention is driven by the increased use of health-care resources in the control group, particularly with regard to the rate of caesarean section.
The results were calculated assuming that the costs for treadmill and midwife training would be based on a typical hospital size. The effect of these costs is negligible compared with other costs in the analysis and so varying this assumption makes no appreciable difference to the results. Table 21 presents illustrative results showing the effect on overall costs and the difference in costs of two possible scenarios. In the first scenario the per-participant fixed costs were halved, assuming that the treadmill and training would apply to twice as many participants (116 per year instead of 58). In the second scenario the per-participant fixed costs were doubled, assuming that the treadmill and training would apply only to half as many participants (29 per year).
| Scenario | Average cost per participant (£, 2012–13 prices) | Difference | |
|---|---|---|---|
| PA group | Control group | ||
| Base case | 4713 | 4748 | −35 |
| Halve per-participant fixed costs | 4711 | 4747 | −36 |
| Double per-participant fixed costs | 4716 | 4749 | −33 |
In a subgroup analysis, when repeating the calculations for the two categories of age and FTCD score, the point estimates no longer show PA dominating the control, but the bootstrapped results still occupy all four quadrants of the cost-effectiveness plane and, thus, it cannot be concluded that the intervention is cost-effective (or not) for any of these subgroups. Further details of the subgroup analysis are provided in Appendix 10.
Summary
Costs associated with the control and PA interventions were compared and the consequences of these alternatives were estimated. The main results of the cost-effectiveness analysis can be summarised as follows:
-
Mean costs were £35 per participant lower in the PA group than in the control group. This results from the fact that the mean intervention costs were £27 higher in the PA group than in the control group but health-care resource use costs were £62 higher in the control group. An important contributory factor to this result was the difference in rates of caesarean section (25% control group vs. 19.7% PA group).
-
Taken in isolation, the reduction in mean costs and increase in quit rate suggest that the PA intervention should be adopted, regardless of the decision-maker’s willingness to pay for an additional quitter. However, this result must be interpreted with great caution because there is considerable statistical uncertainty, as reflected in the scatterplot and accompanying cost-effectiveness acceptability curve.
-
Subgroup analysis was performed by age and dependency score. In each subgroup analysis there was still considerable uncertainty in both the incremental costs and the difference in quit rate.
Chapter 5 Discussion
Smoking outcomes
The trial showed that, among women who were recruited at 10–24 weeks of pregnancy, supplementing behavioural support with a PA intervention was no more effective than behavioural support alone in promoting smoking cessation at the end of pregnancy. At this time, the self-reported continuous smoking abstinence rate, with biochemical validation, was 1.3% higher among those in the PA group than among those in the control group, but this difference was not statistically significant.
Strengths and limitations
This is the first trial to assess the effect of a PA intervention on smoking cessation during pregnancy. Limitations of previous trials of PA interventions for smoking cessation were overcome22 through offering an intensive PA intervention (with support to increase PA as an aid for smoking cessation in addition to supervised exercise sessions), assessing and validating PA in both groups, using a robust outcome of continuous smoking abstinence and offering a pragmatic intervention that could be readily integrated into routine health care in the NHS.
This study was approximately twice as large as previous RCTs investigating PA interventions for smoking cessation delivered via face-to-face support. 22 As anticipated, around 10% of pregnant women recorded as smokers at their first antenatal booking visit were recruited. However, the overall number of women recorded as smokers was lower than anticipated; therefore, despite extending the recruitment period, we recruited only 91% of the target population of 866 (i.e. n = 789). Future studies may wish to aid recruitment by focusing on regions of the UK that have particularly high rates of smoking in pregnancy. Quit rates were lower than anticipated in our power calculation, which will also have reduced the power of the study. The CI around the OR for the effect of the intervention shows the level of precision of this estimate, given the achieved sample size, and implies that we cannot rule out up to a twofold increase in abstinence at the end of pregnancy in the intervention group compared with the control group at the upper end of the CI, although our point estimate suggests an effect of the intervention that is unlikely to be clinically meaningful. Additionally, at the 4-week follow-up point quit rates were higher and CIs narrower, and the difference in abstinence between the treatment group and the control group was minimal, even at the upper end of the CI. Thus, despite not reaching the target sample size, our results suggest that there is unlikely to be a clinically relevant difference between the groups. Quit rates were lower than in previous pregnancy trials with less rigorous outcome measures but were similar to those in studies using comparable outcome measures. 12 For example, two previous UK-based large trials of a smoking cessation intervention during pregnancy observed quit rates at the end of pregnancy of 7.6% and 7.8% in a group receiving only behavioural support for smoking;95,102 similarly, we observed a quit rate of 6.4% for this group.
Our finding of a lack of an effect of a PA intervention on smoking abstinence is consistent with most previous trials of PA for smoking cessation among non-pregnant smokers. However, these trials had low statistical power or used insufficiently intense interventions. 22 One adequately powered trial showed that regular, supervised, vigorous-intensity PA was effective for smoking cessation in women. 23 However, such an intensive intervention is not likely to be clinically suitable or appealing to most pregnant smokers and our finding remains that a pragmatic intervention based on moderate-intensity exercise was not effective for aiding smoking cessation during pregnancy.
Secondary outcomes
Cigarette withdrawal symptoms and urges to smoke
Reports of cigarette withdrawal symptoms and urges to smoke are important during smoking cessation as they predict relapse to smoking; pharmaceutical interventions are thought to largely work through reducing these symptoms. 18 There was no evidence of the PA intervention affecting changes in withdrawal symptoms or urges to smoke, rated for the last week, between baseline and 1 week of abstinence. The ratings for all of the withdrawal symptoms were fairly low at baseline (mean score 2.3, scale 1–5) and there may have been a floor effect for these items. The lack of a PA effect on cigarette withdrawal or urges to smoke is inconsistent with studies showing that brief bouts of PA have an acute effect on reducing urges to smoke among non-pregnant smokers21 and pregnant smokers. 29 However, these studies included temporarily abstinent smokers and findings from this study are likely to have more clinical relevance. It is possible that, among women attempting to stop smoking, bouts of PA have acute beneficial effects on reducing the urge to smoke but that these do not translate into benefits that extend across the week.
Confidence for participating in physical activity and stopping smoking
Increased confidence for participating in PA and stopping smoking (i.e. self-efficacy) tends to predict positive changes in these behaviours103,104 and, if the PA intervention has the potential to aid smoking cessation, we might expect it to increase self-efficacy in both of these domains. There was a significant difference in self-efficacy for PA between baseline and 1 week for the two groups; however, the difference was a modest 0.3 (scale 1–5) and was the result of scores remaining unchanged in the PA group and scores reducing (i.e. reduction in self-efficacy) in the control group. The difference in scores for self-efficacy for smoking cessation between baseline and 1 week was 0.2 (scale 1–6), which was not statistically significant. Thus, there was little evidence to show that the intervention positively influenced these processes.
Birth outcomes
Maternal and fetal birth outcomes were very similar between the two study groups, except that there were significantly fewer deliveries by caesarean section in the PA group than in the control group (difference of 7%). The finding of a lower incidence of caesarean sections in the PA group is consistent with the results of a recent meta-analysis of RCTs showing a significantly lower risk of caesarean delivery among women undergoing a PA intervention compared with a control group. 47 Ours is the first study to report this in pregnant smokers and it is a positive result as caesarean sections are more expensive to the NHS than other modes of delivery and there are complications associated with abdominal surgery. It has been hypothesised that this effect may be related to reduced birthweight or maternal weight in a more physically active group. 47 In the meta-analysis maternal weight, but not birthweight, was significantly reduced in the PA groups compared with the control groups, although for most of the individual studies either maternal weight was not measured or there was no significant reduction in weight. In the one study reporting a significantly lower risk of caesarean delivery among women undergoing a PA intervention compared with a control condition, maternal weight was not reported. Therefore, there is as yet no evidence to support either of these hypotheses. Moreover, in the current study neither maternal weight nor birthweight was significantly influenced by the intervention and so these are unlikely as explanations. There may be another mechanism operating, for example it is possible that more active women have more efficient uterine contractions and therefore are more likely to avoid caesarean section. Further studies are needed to replicate this finding and to explore underlying mechanisms.
Adverse events
There were similar numbers of AEs and SAEs in the two study groups and there were only two AEs potentially related to the PA intervention. This is reassuring as it suggests that a PA intervention is safe and is unlikely to increase these events in pregnant smokers.
Maternal weight
There was no evidence that randomisation led to reduced weight gain overall or reduced the tendency to gain excessive weight during gestation. Obesity did not modify the association between trial arm and weight gain.
The subsample of the LEAP participants included in the analysis of maternal weight study were much more likely to have stopped smoking than those who were not included. This was because abstinent participants had to return for biological confirmation of abstinence and they were obviously keen to return to show their success. Weighing participants was then possible. Although this is of some concern, it is a much less relevant concern here than it might appear. Post-cessation weight gain occurs only in those who sustain abstinence and therefore there is no potential for PA to ameliorate cessation-related weight gain in those not achieving abstinence. Consequently, the Cochrane review48 of interventions to prevent cessation-related weight gain confines the analysis to only participants who achieve abstinence in the intervention and control arms.
Pregnancy itself is a period when excessive weight gain occurs commonly, as shown in this trial, in which 38% of women gained an excessive amount of weight. PA might have been expected to ameliorate this, but there was no evidence it did so. The CIs were wide and encompassed modest effects that are clinically relevant. We can therefore conclude only that the PA programme had no large effects on GWG but may still have important, moderate-sized effects, although there is insufficient evidence to assess this.
Maternal depression
The PA intervention was no more effective than standard behavioural support for reducing depression scores at the end of pregnancy or 6 months after childbirth. Moreover, scores were significantly higher in the PA group than in the control group at the end of pregnancy, although the margin of this difference was very small and is unlikely to be clinically important. 105,106 AEs were very similar in the two groups and so this is unlikely to have affected this outcome. As for the smoking outcomes, there may have been limited potential to show a difference between the groups as both groups were already active at baseline and reported maintaining fairly high levels of PA through to the end of pregnancy. That said, the self-reported PA must be treated with caution as the accelerometer data suggest that these reports were overestimated and, among the sample with accelerometer data, levels of activity were similar for the two groups.
Another explanation for the findings relates to the population of interest and the requirements of the study/intervention. Those in the PA group were asked to change two health behaviours simultaneously (i.e. PA and smoking) while also coping with being pregnant, in addition to dealing with the demands of being asked to attend multiple treatment sessions. This might have demoralised these individuals and they may have found this difficult to achieve, resulting in marginally higher depression scores at the end of pregnancy.
In conclusion, although clinical guidelines recommend that pregnant women exercise for mental health benefits28,61,107 and there is a further recommendation that PA be used to treat and prevent depression among smokers,108 based on the current findings an intervention that offers supervised exercise combined with consultations to increase PA cannot be recommended for antenatal or postnatal depression in women attempting to quit smoking during pregnancy.
Economic evaluation
The total mean cost was £35 per participant lower in the PA group than in the control group. This results from the mean health-care resource use costs being £62 higher in the control group than in the PA group and the mean intervention costs being £27 higher in the PA group than in the control group. Control group health-care costs were higher mostly because of the higher rate of caesarean sections in this group (25% control group vs. 19.7% PA group). Considering the reduction in mean cost and increase in quit rate for the PA group compared with the control group, it could be recommended that the PA intervention be used as an aid for smoking cessation regardless of the decision-maker’s willingness to pay for an additional quitter. However, these results must be interpreted with extreme caution as there is considerably statistical uncertainty, as reflected in the scatterplot and accompanying cost-effectiveness acceptability curve.
Previous studies have investigated the cost-effectiveness of smoking cessation interventions during pregnancy. 92 However, these mainly US-based studies are limited by not including data for infant outcomes and therefore cannot easily be compared with this study. One previous study (the SNAP trial96), based in the UK, has considered infant outcomes and conducted a similar analysis as used in the present study. The SNAP trial assessed the cost-effectiveness of NRT for smoking cessation. The authors reported that the total mean cost of the intervention was £90.81 higher in the NRT group than in the usual-care group. While bearing in mind the high levels of statistical uncertainty present in both studies, this suggests that the PA intervention may be at least as cost-effective, if not more so, than the NRT intervention.
The National Institute for Health and Care Excellence recommends that health outcomes should be measured in quality-adjusted life-years (QALYs) to facilitate comparisons between different health-care programmes. 94 Ideally, the value to society of each successful quitter in the LEAP trial would be estimated in QALYs but no method has been found for doing this that can be considered robust and reliable for use with pregnant smokers. QALYs are commonly calculated from generic health-related quality of life tools (e.g. the European Quality of Life-5 Dimensions or EQ-5D), which may be inappropriate for use in pregnant populations. Quality-of-life studies using generic measures have demonstrated that changes in quality of life, particularly declining physical functioning and vitality, occur over the course of pregnancy. 109 These substantial changes in quality of life may mask any potential short-term quality-of-life gains from smoking cessation. Moreover, existing models ignore QALY benefits to the fetus and do not take into account maternal morbidity during pregnancy. 92,93,110 It was anticipated that a model and systematic review being developed for another study at the University of Nottingham would be suitable for this purpose but this work has yet to be completed. However, once an appropriate economic model that values smoking in pregnancy is available we will use this to estimate cost-effectiveness in QALYs.
Interpretation and generalisability of the results for smoking outcomes
The trial had reasonably broad inclusion criteria and few exclusion criteria, and, therefore, the results are likely to be generalisable to most pregnant smokers, although the women were highly physically active in both groups at baseline. At baseline, compared with a survey of pregnant smokers similarly recruited in antenatal hospitals,111 participants were approximately twice as likely to report achieving the recommended 150 minutes per week of MVPA; this may be because more active women were attracted to a trial offering a PA intervention. Low attendance may have affected efficacy, with the PA group attending a median of only four (of 14) sessions. Low attendance was not explained by the two potentially treatment-related AEs in the PA group and there was no indication that the PA intervention increased the overall incidence of AEs. The vast majority of women who failed in their quit attempt stopped attending, suggesting that it was failure to quit that led to low attendance rather than low attendance leading to failure of the attempt. There was no evidence for the intervention influencing processes that might aid cessation, such as confidence for quitting, urges to smoke or withdrawal symptoms.
Bias in outcome ascertainment is unlikely to explain the findings as follow-up rates were equally high in the groups and the effect size was independent of the influence of missing outcome data. Fewer than 10% of participants reported using non-study behavioural support or NRT, with similar usage in the groups. Therefore, it is doubtful that this influenced the results.
Although the intervention group reported significantly higher PA levels throughout pregnancy relative to the control group, the self-reported PA scores in the control group were also relatively high at follow-up. For example, at the end of pregnancy the intervention group reported completing a median of 155 minutes of MVPA per week (22 minutes per day) and the control group reported completing a median of 140 minutes of MVPA per week (20 minutes per day). This suggests that some intervention contamination might have occurred in the control group and consequently there was an insufficient difference in PA levels between the groups to show an appreciable effect on depression outcomes. It is not clear why the control group participated in more PA than might be expected. One explanation could be that an atypical motivated sample of participants was recruited who were keen to be active regardless of their random group allocation; the high baseline PA scores would suggest this explanation is a possibility as participants were already achieving around 30 minutes per day of MVPA at baseline. The only previous trial of exercise for smoking cessation showing a long-term benefit for abstinence excluded more active women,23 and in less active women the LEAP intervention might have had more positive effects. However, there was no evidence to suggest that the treatment effect for the primary outcome was influenced by baseline levels of PA. Moreover, as participants were recruited from routine health-care settings, it seems likely that if the exercise intervention was offered as part of routine care women would be recruited who are active at a similar level to those taking part in the current trial.
It is also important to consider that, as previously observed,112–114 our accelerometer correlational analysis and Bland–Altman plot suggest that participants overestimated their self-reported PA levels. Moreover, participants could not be blinded to treatment allocation and the higher self-reported rates of activity in the PA group may be the result of a social desirability bias, such that there is a tendency to over-report PA levels with the belief that this will be positively viewed by the researchers. In addition, in the subsample using the accelerometer, accelerometer-derived PA levels were similar in the two trial groups, although when including all MVPA (rather than restricting it to bouts of > 10 minutes) the PA group tended to report more PA per week than the control group, which suggests that most increases in activity were likely to be in sporadic bouts lasting < 10 minutes. The accelerometer data were collected for only 10% of participants and therefore this finding must be treated with caution; however, it is possible that the non-significant effects for smoking abstinence emerged partly because the PA group failed to adhere fully to the behavioural goals of the intervention. If this is the case, it is unlikely that such an intervention offered in routine health care would raise PA levels sufficiently to have an impact on smoking cessation. On the other hand, although attendance at treatment sessions was low, the PA counselling may have increased PA even without attendance at all scheduled exercise sessions. A less pragmatic and more efficacy-oriented trial (e.g. with greater incentives to complete the exercise, such as financial incentives) with accelerometer measurement in the whole sample would be needed to establish whether or not PA per se aids smoking cessation during pregnancy.
Conclusion
During pregnancy, offering an intervention combining supervised exercise and PA counselling did not add to the effectiveness of behavioural support for smoking cessation. There was no evidence that the PA intervention increased AEs or had a harmful effect on birth outcomes, and there was some evidence that the PA intervention resulted in fewer caesarean sections. There was no evidence for the intervention reducing maternal depression or weight gain.
Recommendations for research (in priority order)
-
It is not recommended to fund further large-scale trials of PA for smoking cessation until much less expensive observational studies have been conducted to provide promising leads, for example related to the populations most suitable for such interventions and methods for increasing PA adherence.
-
Reasons for pregnant smokers’ low levels of attendance at supervised PA sessions should be investigated; findings could be used to increase attendance rates. For example, following on from recent work on barriers to PA,115–117 further research is needed to explore barriers to attendance and to PA adherence during pregnancy, and to assess whether or not these barriers vary during different stages of pregnancy and among women with different comorbidities, including gestational diabetes and obesity.
-
Further methods of increasing PA adherence among pregnant smokers need to be developed and tested. For example, financial incentives have shown some benefit for aiding smoking cessation in this population and they may be used in combination with PA to increase both attendance at exercise sessions and smoking cessation. 12 In addition, interventions are needed that provide regular prompts to remind women to exercise (e.g. text messages or brief telephone calls); such interventions have been successfully piloted with young women but not yet with pregnant women. 118
-
The reasons why few inactive pregnant smokers were attracted to a PA trial need to be identified and methods are needed to attract these less active pregnant smokers.
-
Studies are needed to establish whether or not the previously reported finding of a short bout of PA reducing cigarette cravings in pregnant smokers is a robust finding. So far, only one study has investigated this issue. If it is a robust finding, interventions need to be developed that can translate this benefit into prevention of smoking relapse.
-
There was no evidence of beneficial effects on maternal weight gain or depression. Studies are needed that focus on women who are at risk of higher maternal weight gain and on women who have high levels of depression at baseline.
-
Among pregnant smokers there was no evidence for a PA intervention having an added benefit for smoking cessation beyond that of usual care. However, it is possible that in some circumstances a PA programme alone may be more practical and may aid smoking cessation, and this needs to be assessed.
-
There were significantly fewer deliveries by caesarean section in the PA group than in the control group. Further studies are needed to replicate this finding and to explore the underlying mechanisms.
Implications for health care
There was no evidence that offering regular, supervised exercise and PA consultations, in addition to routine smoking cessation support, to women following their first antenatal visit is effective for aiding smoking cessation. Therefore, PA is not currently recommended for smoking cessation during pregnancy. One study showed that PA acutely reduces cigarette cravings in abstinent pregnant smokers29 and, among smokers in general, PA is recommended for reducing these cravings. 21,22 In the present study there was no evidence for the PA intervention moderating cravings/urges to smoke, but it is possible that there are some acute benefits of PA on reducing cravings during pregnancy and the recommendation to use PA to manage cravings acutely remains for all smokers, including those who are pregnant. The PA intervention did not show any benefit for reducing maternal depression; in fact, there was a slight increase in depression in the PA group and therefore the intervention cannot be recommended for antenatal or postnatal depression in women attempting to quit smoking during pregnancy. There was no evidence for an effect of the intervention on maternal weight gain and therefore the intervention cannot be currently recommended for moderating this weight gain. There was no evidence of an increased level of AEs in the PA group and there was some evidence for a reduced incidence of caesarean sections in the PA group; therefore, in line with current guidance, PA remains indicated for general health benefits in pregnancy, including among pregnant smokers.
Acknowledgements
We wish to thank all of the people listed here who contributed to the study, as well as all of the participants.
Trial team
Chief investigator: Michael Ussher.
Coapplicants/Trial Management Group: Paul Aveyard, Isaac Manyonda, Sarah Lewis, Robert West, Beth Lewis, Bess Marcus, Adrian H Taylor, Pelham Barton, Tim Coleman.
Trial managers: Michael Ussher, Noura Hamdi (year 1 only).
Trial administrator: Mary Apps.
Trial Steering Committee: Jim Thornton (Chair), Ann McNeil, Tim Coleman, Michael Ussher, Sue Cooper, Kim Watts, Serena Cox (patient and public involvement/lay member).
Statisticians: Sarah Lewis, Muhammad Riaz.
Data cleansing and database preparation: Sarah Kerry.
Health economists: Pelham Barton, Holly Essex.
Researchers: Julie Fuller, Maggie Hart, Bettina Wanninkhof, Ilia Papachristou, Gail Harding, Sarah Cleary, Ory Bolooki, Rachel Lex, Beth Steff, Zoe Magrath, Tracey Kilbane, Janet Brown, Caroline Dixon, Noura Hamdi.
Principal investigators (in recruiting centres): Isaac Manyonda (St George’s Healthcare NHS Trust), Mark Johnson (Chelsea and Westminster Hospital NHS Foundation Trust), Andrew Shennan (Guy’s and St Thomas’ NHS Foundation Trust), Raj Rai (Imperial College Healthcare NHS Trust), Ranee Thakar (Croydon Health Services NHS Trust), Hassan Shehata (Epsom and St Helier University Hospitals NHS Trust), Nick Anim-Nyame (Kingston Hospital NHS Foundation Trust), Katie Yiannouzis (King’s College Hospital NHS Foundation Trust), Maureen Royds-Jones (Surrey and Sussex Healthcare NHS Trust), Gill Perks (Medway Foundation Trust), Joanna Girling (West Middlesex University Hospital NHS Trust) and Simon Cunningham (Mid Cheshire Hospitals NHS Foundation Trust).
NCTU management: Dan Simpkins.
Cotinine analysis: Salimetrics Europe Ltd (Newmarket, UK), Dr Agnes Ernst.
We would also like to thank the following for their contributions to the study: Sherma Garcia and Tracey MacCormack for their help with data collection, and Dr Judith Ibison and Professor Andy Kent for their advice about the measurement of depression and interpretation of the findings for depression.
Contributions of authors
All authors made substantial contributions to the conception and design of the study and/or the acquisition of data and/or the analysis and interpretation of data, as listed below. All authors were involved in drafting the manuscript or revising it critically for important intellectual content. All authors approved the final version.
Michael Ussher (LEAP trial chief investigator/trial manager, Professor of Behavioural Medicine) was involved in the design, conduct, data acquisition, analysis and report-writing phases of the trial.
Sarah Lewis (Professor of Medical Statistics) was involved in the design, conduct, analysis and report-writing phases of the trial.
Paul Aveyard (Professor of Behavioural Medicine) was involved in the design, conduct, analysis and report-writing phases of the trial.
Isaac Manyonda (Professor and Consultant in Obstetrics and Gynaecology) was involved in the design, conduct and report-writing phases of the trial.
Robert West (Professor of Health Psychology) was involved in the design, conduct and report-writing phases of the trial.
Beth Lewis (Associate Professor, Behavioural Aspects of Physical Activity) was involved in the design, conduct and report-writing phases of the trial.
Bess Marcus (Professor of Psychiatry and Human Behaviour and Community Health) was involved in the design, conduct and report-writing phases of the trial.
Muhammad Riaz (Research Fellow in Medical Statistics) was involved in the data cleansing, analysis design, analysis and report-writing phases of the trial.
Adrian H Taylor (Professor of Exercise and Health Psychology) was involved in the design, conduct and report-writing phases of the trial.
Pelham Barton (Reader in Mathematical Modelling) was involved in the design, conduct, analysis and report-writing phases of the trial specifically for the economic analysis.
Amanda Daley (Senior Lecturer in Behavioural Medicine) was involved in the design, analysis and report-writing phases of the trial specifically for the outcomes related to depression and maternal weight.
Holly Essex (Research Fellow in Health Economics) was involved in the design, analysis and report-writing phases of the trial specifically for the economic analysis.
Dale Esliger (Senior Lecturer in the Measurement of Physical Activity) was involved in the design, conduct, analysis and report-writing phases of the trial specifically for accelerometer data outcomes.
Tim Coleman (Professor of Primary Care) was involved in the design, conduct, analysis and report-writing phases of the trial.
Publications
Ussher M, Aveyard P, Manyonda I, Lewis S, West R, Lewis B, et al. Physical activity as an aid to smoking cessation during pregnancy (LEAP) trial: study protocol for a randomized controlled trial. Trials 2012;13:186.
Ussher M, Lewis S, Aveyard P, Manyonda M, West R, Lewis B, et al. Physical activity for smoking cessation in pregnancy: randomised controlled trial. BMJ 2015;350:h2145.
Data sharing statement
The guarantor (MU) is willing to examine all requests for the full data set after a period of 3 years from the date of this publication. Participants did not give consent for data sharing but the data are anonymised and the risk of identification is low.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Kallen K. The impact of maternal smoking during pregnancy on delivery outcome. Eur J Public Health 2001;11:329-33. http://dx.doi.org/10.1093/eurpub/11.3.329.
- Rogers JM. Tobacco and pregnancy. Reprod Toxicol 2009;28:152-60. http://dx.doi.org/10.1016/j.reprotox.2009.03.012.
- Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev 2007;83:713-20. http://dx.doi.org/10.1016/j.earlhumdev.2007.08.002.
- Toivonen S, Heinonen S, Anttila M, Kosma VM, Saarikoski S. Reproductive risk factors, Doppler findings, and outcome of affected births in placental abruption: a population-based analysis. Am J Perinatol 2002;19:451-6. http://dx.doi.org/10.1055/s-2002-36868.
- Leonardi-Bee J, Jere ML, Britton J. Exposure to parental and sibling smoking and the risk of smoking uptake in childhood and adolescence: a systematic review and meta-analysis. Thorax 2011;66:847-55. http://dx.doi.org/10.1136/thx.2010.153379.
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res 2004;6:125-40. http://dx.doi.org/10.1080/14622200410001669187.
- Infant Feeding Survey 2010: Early Results. Leeds: NHS Information Centre for Health and Social Care; 2011.
- Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, et al. Trends in smoking before, during, and after pregnancy – Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ 2013;62:1-19.
- Al-Sahab B, Saqib M, Hauser G, Tamim H. Prevalence of smoking during pregnancy and associated risk factors among Canadian women: a national survey. BMC Pregn Childbirth 2010;10. http://dx.doi.org/10.1186/1471-2393-10-24.
- Tappin DM, MacAskill S, Bauld L, Eadie D, Shipton D, Galbraith L. Smoking prevalence and smoking cessation services for pregnant women in Scotland. Subst Abuse Treat Prev Policy 2010;5. http://dx.doi.org/10.1186/1747-597X-5-1.
- Richmond R. You’ve come a long way baby: women and the tobacco epidemic. Addiction 2003;98:553-7. http://dx.doi.org/10.1046/j.1360-0443.2003.00342.x.
- Chamberlain C, O’Mara-Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev 2013;10. http://dx.doi.org/10.1002/14651858.cd001055.pub4.
- Owen L, Penn G. Smoking and Pregnancy: A Survey of Knowledge Attitudes and Behaviour, 1992–1999. London: Health Development Agency; 1999.
- Naughton F, Prevost AT, Sutton S. Self-help smoking cessation interventions in pregnancy: a systematic review and meta-analysis. Addiction 2008;103:566-79. http://dx.doi.org/10.1111/j.1360-0443.2008.02140.x.
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.cd001055.pub3.
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2011;2. http://dx.doi.org/10.1002/14651858.cd006103.pub5.
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007;1. http://dx.doi.org/10.1002/14651858.cd000031.pub3.
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2012;11. http://dx.doi.org/10.1002/14651858.cd000146.pub4.
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2012;9. http://dx.doi.org/10.1002/14651858.cd010078.
- Ussher M, West R. Interest in nicotine replacement therapy among pregnant smokers. Tob Control 2003;12:108-9. http://dx.doi.org/10.1136/tc.12.1.108-a.
- Haasova M, Warren FC, Ussher M, Janse Van Rensburg K, Faulkner G, Cropley M, et al. The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data (IPD). Addiction 2012;108:26-37. http://dx.doi.org/10.1111/j.1360-0443.2012.04034.x.
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev 2014;1.
- Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomised controlled trial. Arch Intern Med 1999;159:1229-34. http://dx.doi.org/10.1001/archinte.159.11.1229.
- Marcus BH, Albrecht AE, Niaura RS, Abrams DB, Thompson PD. Usefulness of physical exercise for maintaining smoking cessation in women. Am J Cardiol 1991;68:406-7. http://dx.doi.org/10.1016/0002-9149(91)90843-A.
- Martin JE, Kalfas KJ, Patten CA, Polarek M, Hofstetter CR, Noto J, et al. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one-year results of project SCRAP-Tobacco. J Consult Clin Psychol 1997;65:190-4. http://dx.doi.org/10.1037/0022-006X.65.1.190.
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine Tob Res 2005;7:871-80. http://dx.doi.org/10.1080/14622200500266056.
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine . American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334-59. http://dx.doi.org/10.1249/MSS.0b013e318213fefb.
- American College of Obstetricians and Gynecologists . Exercise during pregnancy and the postpartum period. Obstet Gynecol 2003;46:496-9.
- Prapavessis H, De Jesus S, Harper T, Cramp A, Fitzgeorge L, Mottola MF, et al. The effects of acute exercise on cravings and withdrawal symptoms in temporary abstinent pregnant smokers. Addict Behav 2014;39:703-8. http://dx.doi.org/10.1016/j.addbeh.2013.10.034.
- Ussher M, Aveyard P, Coleman T, Straus L, West R, Marcus B, et al. Physical activity as an aid to smoking cessation during pregnancy: two feasibility studies. BMC Public Health 2008;8. http://dx.doi.org/10.1186/1471-2458-8-328.
- Gaynes BN, Gavin N, Melzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal Depression: Prevalence, Screening Accuracy and Screening Outcomes. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- Bolton HL, Hughes PM, Truton P, Sedgwick P. Incidence and demographic correlates of depressive symptoms during pregnancy in an inner London population. J Psychosom Obstet Gynaecol 1998;19:202-9. http://dx.doi.org/10.3109/01674829809025698.
- Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod 2009;24:146-53. http://dx.doi.org/10.1093/humrep/den342.
- Mancuso R, Schetter C, Rini C, Roesch S, Hobel C. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosom Med 2004;66:762-9. http://dx.doi.org/10.1097/01.psy.0000138284.70670.d5.
- Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry 2009;166:557-66. http://dx.doi.org/10.1176/appi.ajp.2008.08081170.
- Cooper P, Murray L, Wilson A, Romaniuk H. Controlled trial of the short- and long-term effect of psychological treatment of post-partum depression: I. Impact on maternal mood. Br J Psychiatry 2003;182:412-19. http://dx.doi.org/10.1192/bjp.182.5.412.
- Murray L. The impact of postnatal depression on infant development. J Child Psychol Psychiatry 1992;33:543-61. http://dx.doi.org/10.1111/j.1469-7610.1992.tb00890.x.
- Cooper PJ, Murray L. Course and recurrence of postnatal depression. Evidence for the specificity of the diagnostic concept. Br J Psychiatry 1995;166:191-5. http://dx.doi.org/10.1192/bjp.166.2.191.
- Allen AM, Prince CB, Dietz PM. Postpartum depressive symptoms and smoking relapse. Am J Prev Med 2009;36:9-12. http://dx.doi.org/10.1016/j.amepre.2008.09.020.
- Linares Scott TJ, Heil SH, Higgins ST, Badger GJ, Bernstein IM. Depressive symptoms predict smoking status among pregnant women. Addict Behav 2009;34:705-8. http://dx.doi.org/10.1016/j.addbeh.2009.04.003.
- Lan-Pidhainy X, Nohr EA, Rasmussen KM. Comparison of gestational weight gain-related pregnancy outcomes in American primiparous and multiparous women. Am J Clin Nutr 2013;97:1100-6. http://dx.doi.org/10.3945/ajcn.112.052258.
- Sunsaneevithayakul P, Titapant V, Ruangvutilert P, Sutantawibul A, Phatihattakorn C, Wataganara T, et al. Relation between gestational weight gain and pregnancy outcomes. J Obstet Gynaecol Res 2014;40:995-1001. http://dx.doi.org/10.1111/jog.12293.
- Rode L, Kjærgaard H, Damm P, Ottesen B, Hegaard H. Effect of smoking cessation on gestational and postpartum weight gain and neonatal birth weight. Obstet Gynecol 2013;122:618-25. http://dx.doi.org/10.1097/AOG.0b013e3182a10836.
- Haakstad LAH, Voldner N, Henriksen T, Bø K. Physical activity level and weight gain in a cohort of pregnant Norwegian women. Acta Obstet Gynecol Scand 2007;86:559-64. http://dx.doi.org/10.1080/00016340601185301.
- Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. J Am Diet Assoc 2003;103:48-54. http://dx.doi.org/10.1053/jada.2003.50001.
- Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol 2009;201:58e1-8. http://dx.doi.org/10.1016/j.ajog.2009.02.025.
- Domenjoz I, Kayser B, Boulvain M. Effect of physical activity during pregnancy on mode of delivery. Am J Obstet Gynecol 2014;211. http://dx.doi.org/10.1016/j.ajog.2014.03.030.
- Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev 2012;1. http://dx.doi.org/10.1002/14651858.cd006219.pub3.
- Ussher M, Aveyard P, Manyonda I, Lewis S, West R, Lewis B, et al. Physical activity as an aid to smoking cessation during pregnancy (LEAP) trial: study protocol for a randomized controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-186.
- Thomas S, Reading J, Shepard RJ. Revision of the physical activity readiness questionnaire. Can J Sport Sci 1992;17:338-45.
- Quitting Smoking in Pregnancy and Following Childbirth. London: NICE; 2010.
- Standard for Training in Smoking Cessation Treatments. London: Health Development Agency; 2003.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. http://dx.doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Davidson KW, Goldstein M, Kaplan RM, Kaufmann PG, Knatterund GL, Orleans CT, et al. Evidence-based behavioural medicine: what is it and how do we achieve it?. Ann Behav Med 2003;26:161-71. http://dx.doi.org/10.1207/S15324796ABM2603_01.
- Bandura A. Health promotion by social cognitive means. Health Educ Behav 2004;31. http://dx.doi.org/10.1177/1090198104263660.
- Michie S, Hyder N, Walia A, West R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict Behav 2011;36:315-19. http://dx.doi.org/10.1016/j.addbeh.2010.11.016.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;2:1479-98. http://dx.doi.org/10.1080/08870446.2010.540664.
- Mottola MF, Davenport MH, Brun CR, Inglis SD, Charlesworth S, Sopper MM. VO2 peak prediction and exercise prescription for pregnant women. Med Sci Sport Exer 2006;38:1389-95. http://dx.doi.org/10.1249/01.mss.0000228940.09411.9c.
- Borg GAV. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction 2007;102:534-43. http://dx.doi.org/10.1111/j.1360-0443.2006.01739.x.
- Exercise in Pregnancy: Statement 4. London: Royal College of Obstetricians and Gynaecologists; 2006.
- Rodriguez A, Bohlin G, Lindmark G. Psychosocial predictors of smoking and exercise during pregnancy. J Reprod Infant Psychol 2000;18:203-33. http://dx.doi.org/10.1080/713683039.
- Rooney B, Smalley K, Larson J, Havens S. Is knowing enough? Increasing physical activity by wearing a pedometer. Wisconsin Med J 2003;102:31-6.
- Downs DS, LeMasurier GC, DiNallo JM. Baby steps: pedometer-determined and self-reported leisure-time exercise behaviors of pregnant women. J Phys Act Health 2009;6:63-72.
- Prochaska JJ, Hall SM, Humfleet G, Munoz RF, Reus V, Gorecki J, et al. Physical activity as a strategy for maintaining tobacco abstinence: a randomized trial. Prev Med 2008;47:215-20. http://dx.doi.org/10.1016/j.ypmed.2008.05.006.
- West R, Russell M. Pre-abstinence smoke intake and smoking motivation as predictors of severity of cigarette withdrawal symptoms. Psychopharmacology 1985;87:334-6. http://dx.doi.org/10.1007/BF00432717.
- West R, Hajek P. Evaluation of the Mood and Physical Symptoms Scale (MPSS) to assess cigarette withdrawal. Psychopharmacology 2004;177:195-9. http://dx.doi.org/10.1007/s00213-004-1923-6.
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine Tob Res 2012;14:75-8. http://dx.doi.org/10.1093/ntr/ntr137.
- Heatherton T, Kozlowski L, Frecker T, Fagerström K. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86:1119-27. http://dx.doi.org/10.1111/j.1360-0443.1991.tb01879.x.
- Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EPDS). J Reprod Infant Psychol 1990;8:99-107. http://dx.doi.org/10.1080/02646839008403615.
- Blair SN, Haskell WL, Ho P, Paffenbarger P, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794-80.
- Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport 1992;63:60-6. http://dx.doi.org/10.1080/02701367.1992.10607557.
- West R, Willis N. Double-blind placebo controlled trial of dextrose tablets and nicotine patch in smoking cessation. Psychopharmacology 1998;136:201-4. http://dx.doi.org/10.1007/s002130050557.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. http://dx.doi.org/10.1111/j.1360-0443.2004.00995.x.
- SRNT Subcommittee on Biochemical Verification . Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4:149-59. http://dx.doi.org/10.1080/14622200210123581.
- Etzel RA. A review of the use of saliva cotinine as a marker of tobacco smoke exposure. Prev Med 1990;19:190-7. http://dx.doi.org/10.1016/0091-7435(90)90020-K.
- Windsor RA, Orleans CT. Guidelines and methodological standards for smoking cessation intervention research among pregnant women: improving the science and art. Health Educ Q 1986;13:131-61. http://dx.doi.org/10.1177/109019818601300203.
- Malchodi CS, Oncken C, Dornelas EA, Caramanica L, Gregonis E, Curry SL. The effects of peer counseling on smoking cessation and reduction. Obstet Gynecol 2003;101:504-10. http://dx.doi.org/10.1016/S0029-7844(02)03070-3.
- Windsor RA, Li CQ, Boyd NR, Hartmann KE. The use of significant reduction rates to evaluate health education methods for pregnant smokers: a new harm reduction behavioral indicator?. Health Educ Behav 1999;26:648-62. http://dx.doi.org/10.1177/109019819902600506.
- Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sport Exerc 2004;36:1750-60. http://dx.doi.org/10.1249/01.MSS.0000142303.49306.0D.
- DiNallo JM, Downs DS, Le Masurier G. Objectively assessing treadmill walking during the second and third pregnancy trimesters. J Phys Act Health 2012;9:21-8.
- Rousham EK, Clarke PE, Gross H. Significant changes in physical activity among pregnant women in the UK as assessed by accelerometry and self-reported activity. Eur J Clin Nutr 2006;60:393-400. http://dx.doi.org/10.1038/sj.ejcn.1602329.
- Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med 2002;21:2917-30. http://dx.doi.org/10.1002/sim.1296.
- Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction 2007;102:1564-73. http://dx.doi.org/10.1111/j.1360-0443.2007.01946.x.
- Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181-8. http://dx.doi.org/10.1249/mss.0b013e31815a51b3.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;327:307-10. http://dx.doi.org/10.1016/S0140-6736(86)90837-8.
- Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed?. BJOG 2004;111:213-19. http://dx.doi.org/10.1111/j.1471-0528.2004.00059.x.
- Rasmussen KM, Yaktine AL. Weight Gain During Pregnancy: Re-examining the Guidelines. Institute of Medicine (US) and National Research Council (US) Committee to Re-examine IOM Pregnancy Weight Guidelines. Washington, DC: National Academies Press; 2009.
- Medicines and Healthcare products Regulatory Agency . Good Clinical Practice for Clinical Trials 2014. www.gov.uk/guidance/good-clinical-practice-for-clinical-trials (accessed 8 September 2015).
- Lawrence T, Aveyard P, Croghan E. What happens to women’s self-reported cigarette consumption and urinary cotinine levels in pregnancy?. Addiction 2003;98:1315-20. http://dx.doi.org/10.1046/j.1360-0443.2003.00485.x.
- Fergusson D, Aaron SD, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652-4. http://dx.doi.org/10.1136/bmj.325.7365.652.
- Ruger JP, Emmons KM. Economic evaluations of smoking cessation and relapse prevention programs for pregnant women: a systematic review. Value Health 2008;11:180-90. http://dx.doi.org/10.1111/j.1524-4733.2007.00239.x.
- Ruger JP, Weinstein MC, Hammond SK, Kearney MH, Emmons KM. Cost-effectiveness of motivational interviewing for smoking cessation and relapse prevention among low-income pregnant women: a randomized controlled trial. Value Health 2008;11:191-8. http://dx.doi.org/10.1111/j.1524-4733.2007.00240.x.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, et al. Smoking, Nicotine, and Pregnancy (SNAP) trial team . A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med 2012;366:808-18. http://dx.doi.org/10.1056/NEJMoa1109582.
- Cooper S, Lewis S, Thornton JG, Marlow N, Watts K, Britton J, et al. Smoking, Nicotine And Pregnancy (SNAP) trial team . The SNAP trial: a randomised placebo-controlled trial of nicotine replacement therapy in pregnancy – clinical effectiveness and safety until 2 years after delivery, with economic evaluation. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18540.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal and Social Services Research Unit; 2013.
- NHS Reference Costs 2012–13: Appendix NSRC01: NHS Trust Reference Cost Schedules. London: Department of Health; 2013.
- Petrou S, Trinder J, Brocklehurst P, Smith L. Economic evaluation of alternative management methods of first-trimester miscarriage based on results from the MIST trial. BJOG 2006;113:879-89. http://dx.doi.org/10.1111/j.1471-0528.2006.00998.x.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-14. http://dx.doi.org/10.1177/0272989X9001000308.
- Van Hout BA, Al MJ, Gordon GS, Rutten FFH. Costs, effects and c/e-ratios alongside a clinical trial. Health Econ 1994;3:309-19. http://dx.doi.org/10.1002/hec.4730030505.
- Hajek P, West R, Lee A, Foulds J, Owen L, Eiser JR, et al. Randomized controlled trial of a midwife-delivered brief smoking cessation intervention in pregnancy. Addiction 2001;96:485-94. http://dx.doi.org/10.1046/j.1360-0443.2001.96348511.x.
- Gwaltney CJ, Metrik J, Kahler CW, Shiffman S. Self-efficacy and smoking cessation: a meta-analysis. Psychol Addict Behav 2009;23:56-6. http://dx.doi.org/10.1037/a0013529.
- Parschau L, Fleig L, Koring M, Lange D, Knoll N, Schwarzer R, et al. Positive experience, self-efficacy, and action control predict physical activity changes: a moderated mediation analysis. Br J Health Psychol 2013;18:395-406. http://dx.doi.org/10.1111/j.2044-8287.2012.02099.x.
- Matthey S. Calculating clinically significant change in postnatal depression studies using the Edinburgh Postnatal Depression Scale. J Affect Disord 2004;78:269-72. http://dx.doi.org/10.1016/S0165-0327(02)00313-0.
- Affonso DD, De AK, Horowitz JA, Mayberry LJ. An international study exploring levels of postpartum depressive symptomatology. J Psychosom Res 2000;49:207-16. http://dx.doi.org/10.1016/S0022-3999(00)00176-8.
- Antenatal and Postnatal Mental Health. London: NICE; 2007.
- Bernard P, Ninot G, Moullec G, Guillaume S, Courtet P, Quantin X. Smoking cessation, depression, and exercise: empirical evidence, clinical needs, and mechanisms. Nicotine Tob Res 2013;15:1635-50. http://dx.doi.org/10.1093/ntr/ntt042.
- Haas JS, Jackson RA, Fuentes-Afflick E, Stewart AL, Dean ML, Brawarsky P, et al. Changes in the health status of women during and after pregnancy. J Gen Intern Med 2005;20:45-51. http://dx.doi.org/10.1111/j.1525-1497.2004.40097.x.
- Flack S, Taylor M, Trueman P. Economic Analysis of Interventions for Smoking Cessation Aimed at Pregnant Women. York: York Health Economics Consortium; 2007.
- Ussher M, Ah-Yoon M, West R, Straus L. Factors associated with exercise participation and attitudes to exercise among pregnant smokers. J Smoking Cessation 2007;2:12-6. http://dx.doi.org/10.1375/jsc.2.1.12.
- Harrison CL, Thompson RG, Teede HJ, Lombard CB. Measuring physical activity during pregnancy. Int J Behav Nutr Phys Act 2011;8. http://dx.doi.org/10.1186/1479-5868-8-19.
- Bell R, Tennant PW, McParlin C, Pearce MS, Adamson AJ, Rankin J, et al. Measuring physical activity in pregnancy: a comparison of accelerometry and self-completion questionnaires in overweight and obese women. Eur J Obstet Gynecol Reprod Biol 2013;170:90-5. http://dx.doi.org/10.1016/j.ejogrb.2013.05.018.
- Goyder E, Hind D, Breckon J, Dimairo M, Minton J, Everson-Hock E, et al. A randomised controlled trial and cost-effectiveness evaluation of ‘booster’ interventions to sustain increases in physical activity in middle-aged adults in deprived urban neighbourhoods. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18130.
- Leppänen M, Aittasalo M, Raitanen J, Kinnunen TI, Kujala UM, Luoto R. Physical activity during pregnancy: predictors of change, perceived support and barriers among women at increased risk of gestational diabetes. Matern Child Health J 2014;18:2158-66. http://dx.doi.org/10.1007/s10995-014-1464-5.
- Redmond ML, Dong F, Frazier LM. Does the extended parallel process model fear appeal theory explain fears and barriers to prenatal physical activity?. Womens Health Issues 2015;25:149-54. http://dx.doi.org/10.1016/j.whi.2014.11.009.
- Santos PC, Abreu S, Moreira C, Lopes D, Santos R, Alves O, et al. Impact of compliance with different guidelines on physical activity during pregnancy and perceived barriers to leisure physical activity. J Sports Sci 2014;32:1398-408. http://dx.doi.org/10.1080/02640414.2014.893369.
- Fjeldsoe BS, Miller YD, Graves N, Barnett AG, Marshall AL. Randomised controlled trial of an improved version of MobileMums, an intervention for increasing physical activity in women with young children. Ann Behav Med 2015;49:487-99. http://dx.doi.org/10.1007/s12160-014-9675-y.
Appendix 1 Telephone screening sheet
Appendix 2 Therapist manual for delivering behavioural support for smoking cessation
Appendix 3 Therapist manual for delivering the physical activity intervention
Appendix 4 Participant physical activity booklet
Appendix 5 Physical activity questionnaire
Instructions for carrying out the 7-day physical activity recall interview
-
Label the days on the recall table (day 1 is yesterday).
-
Introduce interview: Explain that you will be asking about their physical activity, starting with yesterday and working backwards through the previous 7 days.
-
Define segments of the day: Say that you will be asking separately about activities for the morning, afternoon and evening.
-
Describe the type of activity that you are interested in: Explain that you are interested in any work, household or leisure activities lasting at least 10 minutes that are at least at a level of intensity that makes them breathe slightly harder than normal, makes them warmer and makes them aware that their heart is beating faster. Say that you are not interested in light activities such as desk work, strolling or light housework.
-
Ask about activities: For each segment of the day, ask the woman to recall activity episodes lasting at least 10 minutes and record the information in the table (round to the nearest 5 minutes). For each activity record the type of activity (using type codes) and duration of activity in minutes (e.g. ‘W 10’ denotes a 10-minute walk). For each day begin by asking ‘What did you do and where did you go on that morning’.
-
Record the number of minutes of activity for each day and the total minutes for the week. Enter the latter figure into the database.
| Day | Morning | Afternoon | Evening | Daily minutes of activity |
|---|---|---|---|---|
| 1 (yesterday) | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 7 | ||||
| Weekly total: |
Appendix 6 Questionnaires
All questionnaires were presented on an online server.
Baseline questionnaire
Additional questions asked post quit day only
Antenatal complications and birth out comes
Appendix 7 Statistical analysis plan
Appendix 8 Patient and public involvement
The LEAP trial was preceded by pilot work30 during which pregnant smokers and ex-smokers were interviewed concerning the recruitment methods, study design and interventions. The findings from these interviews were used in the design of the LEAP trial.
The Trial Steering Committe (TSC) included a lay member who approved the protocol and checked the patient information sheet and consent form, as well as being involved in monitoring all stages of the study. This individual was also involved in discussions concerning the dissemination of the findings.
The LEAP trial included qualitative interviews with women who were enrolled in the trial and with researchers delivering the interventions. This helped us to assess the acceptability of the intervention and women’s overall experience of being involved in the trial. These interviews also helped us to interpret the findings and to identify topics for future research.
Appendix 9 Supplementary data on adverse events
Serious adverse events
Physical activity group
-
Maternal AEs (105 events): early labour (n = 13), fainting/dizziness (n = 9), nausea or vomiting (n = 8), symphysis pubis dysfunction (n = 8), severe headaches (n = 7), chest pain (n = 6), obstetric cholestasis (n = 4), oligohydramnios (n = 6), generally unwell (n = 6), deep-vein thrombosis (n = 2), group B streptococcus (n = 3), pelvic pain (n = 3), polyhydramnios (n = 3), vaginal discharge (n = 3), dermatisis (n = 2), incompetent cervix (n = 2), uterine contractions during pregnancy (n = 2), abnormal antibodies, abnormal blood flow to placenta, anaemia, back pain, bicornate uterus, diarrhoea, Graves’ disease, hind waters rupture, palpitations, positive urine toxicology, post-partum haemorrhage, pregnancy-related tachycardia, right iliac fossa pain, sciatica, supraventricular tachycardia, thyroid problems, urinary incontinence, varicose veins (all n = 1).
-
Fetal AEs (14 events): congenital malformation (n = 10), abnormal fetal heart rate (n = 1), fetal kidney problem (n = 1), unstable lie (n = 2).
-
Neonatal AEs (15 events): premature birth at < 32 weeks (n = 5), congenital malformations (n = 9) [exomphalos (n = 2), talipes (n = 2), anomalous pulmonary venous drainage, bilateral cleft lip and palate, enlarged clitoris, perimembranous and muscular ventricular septal defect, small sacral skin tag (all n = 1)], shoulder dystocia (n = 1).
Control group
-
Maternal AEs (114 events): generally unwell (n = 11), symphysis pubis dysfunction (n = 11), fainting/dizziness (n = 10), oligohydramnios (n = 10), early labour (n = 8), nausea or vomiting (n = 8), obstetric cholestasis (n = 7), severe headaches (n = 6), vaginal discharge (n = 5), polyhydramnios (n = 4), thrombocytopenia (n = 2), back pain (n = 4), diarrhoea (n = 3), oedema (n = 3), epigastric pains (n = 2), placental insufficiency (n = 2), Bell’s palsy, chest pain, deep-vein thrombosis, epileptic fits, fever, group B streptococcus, irritable bowel syndrome, leaking pulmonary valve, pelvic pain, leg pain, persistent proteinuria, rectal bleeding, renal calculi, right iliac fossa pain, scoliosis, sickle cell anaemia, uterine contractions during pregnancy, vaginal pain (all n = 1).
-
Fetal AEs (14 events): congenital malformation (n = 9), unstable lie (n = 2), abnormal fetal heart rate, static growth on scan (< 10th centile), unprovoked decelerations of fetal heart rate (all n = 1).
-
Neonatal AEs (14 events): premature birth at < 32 weeks (n = 9), congenital malformations (n = 5) [chronic lung condition, extra toe on left foot, gastroschisis, renal dilatation of both kidneys, renal tract anomaly (all n = 1)].
Appendix 10 Results of subgroup cost-effectiveness analysis
The cost-effectiveness calculations were repeated using data from subgroups stratified by age and FTCD score. In each case, the population was divided approximately in half. The groups considered were age < 28 years, age > 27 years, FTCD score < 4, FTCD score ≥ 4. In this appendix the point estimate cost-effectiveness results are shown, followed by the cost-effectiveness scatterplot and cost-effectiveness acceptability curve derived from the same approach of imputation and bootstrapping as was used for the complete data set. Note that in each case there is no dominance relationship in the point estimate results but there is still considerable uncertainty shown on the cost-effectiveness plane and in the cost-effectiveness acceptability curves. A reasonable interpretation of these results is that there is no strong evidence of a subgroup effect.
| Group | Expected per-participant costs (£, 2012/13 prices) | Expected quit rate (%) | Expected annual costs (£, 2012/13 prices) | Expected annual quitters | ICER |
|---|---|---|---|---|---|
| PA | 4472 | 0.0542 | 259,348 | 3.14 | £84,000 per quitter. Note that this result taken in isolation would suggest that PA should be preferred at any willingness to pay below £84,000 per additional quitter. This is because the differences in costs and effectiveness are both negative. However, in context, a more reasonable interpretation is that there is no significant difference between the PA group results and the control group results for either cost or effectiveness |
| Control | 4757 | 0.0576 | 275,932 | 3.34 | |
| Difference | −286 | −0.0034 | −16,584 | −0.20 |
FIGURE 7.
Cost-effectiveness scatterplot for women aged < 28 years.
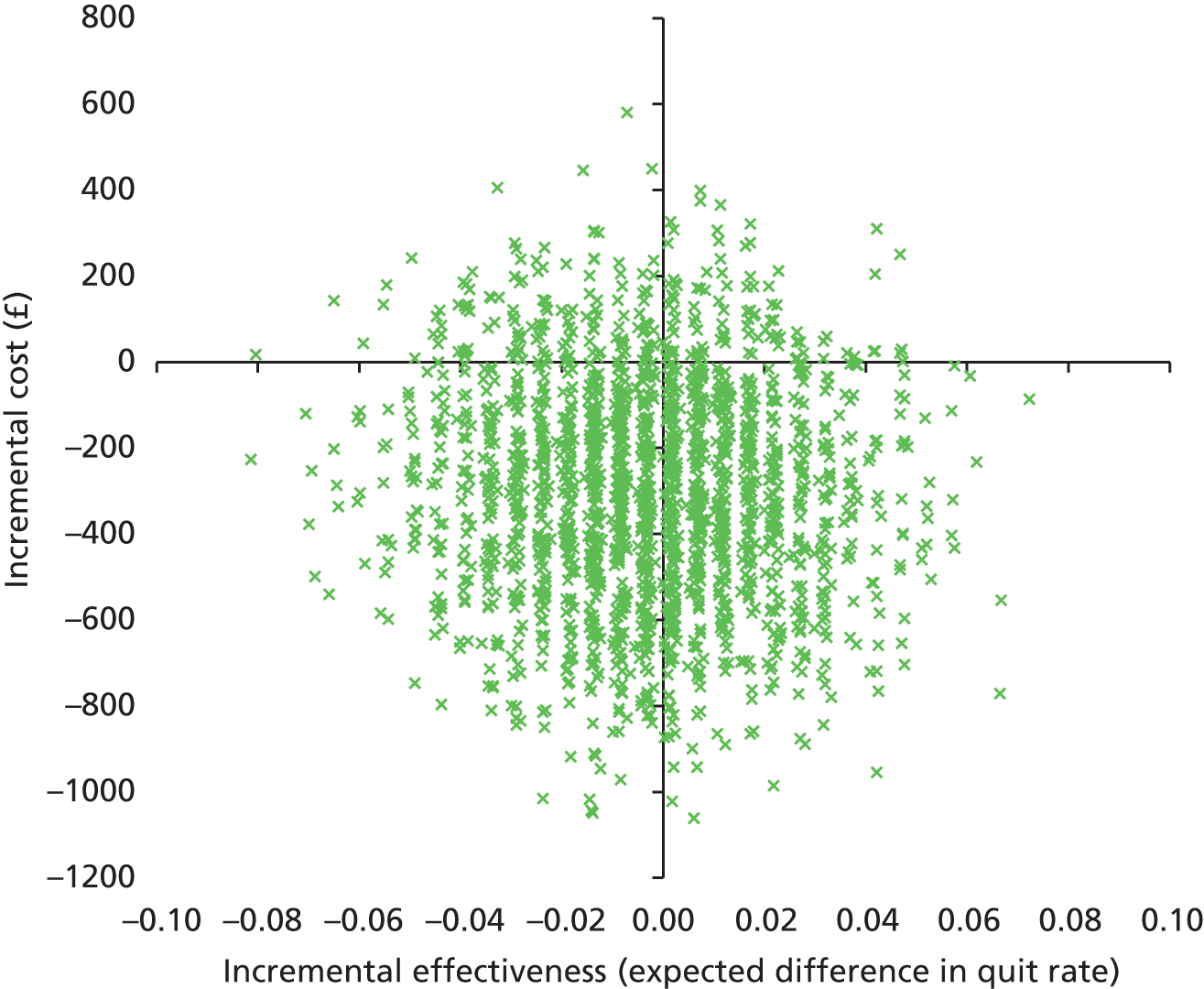
FIGURE 8.
Cost-effectiveness acceptability curve for women aged < 28 years.

| Group | Expected per-participant costs (£, 2012/13 prices) | Expected quit rate (%) | Expected annual costs (£, 2012/13 prices) | Expected annual quitters | ICER |
|---|---|---|---|---|---|
| PA | 4968 | 0.101 | 288,119 | 5.86 | £7200 per quitter. Note that this result taken in isolation would suggest that PA should be preferred at any willingness to pay above £7200 per additional quitter. However, in context, a more reasonable interpretation is that there is no significant difference between the PA group results and the control group results for either cost or effectiveness |
| Control | 4738 | 0.069 | 274,791 | 4.02 | |
| Difference | 230 | 0.032 | 13,328 | 1.84 |
FIGURE 9.
Cost-effectiveness scatterplot for women aged > 27 years.
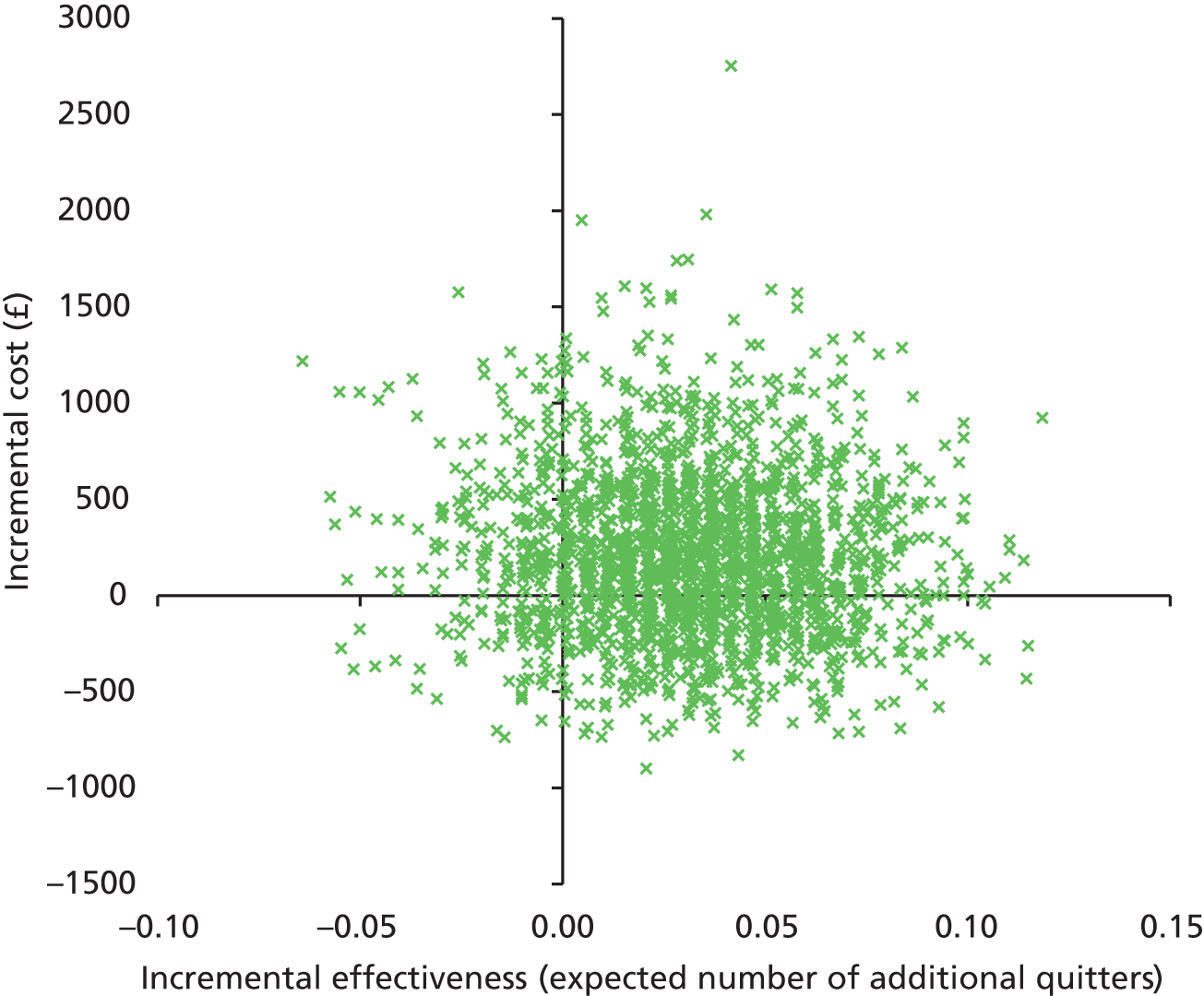
FIGURE 10.
Cost-effectiveness acceptability curve for women aged > 27 years.
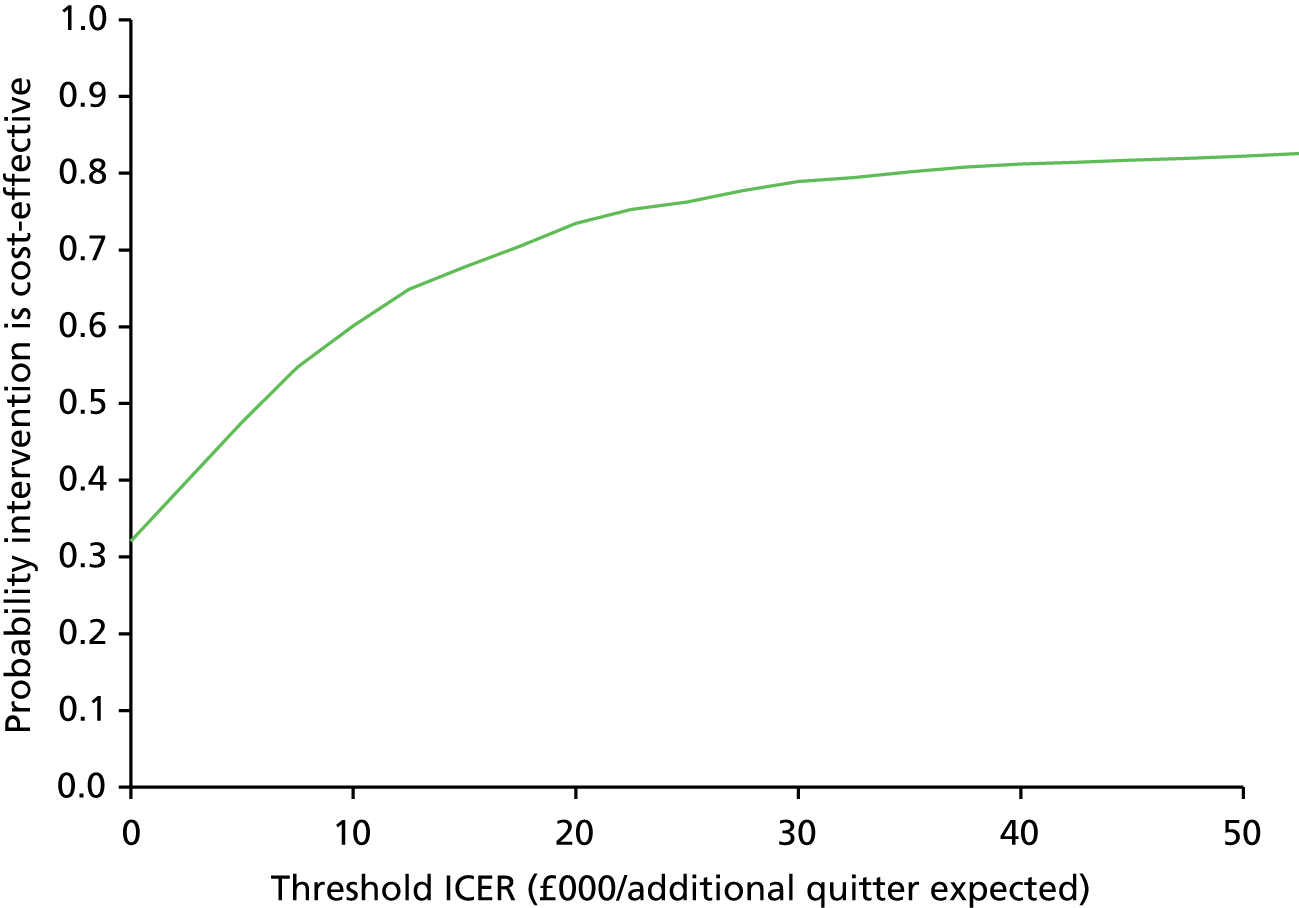
| Group | Expected per-participant costs (£, 2012/13 prices) | Expected quit rate (%) | Expected annual costs (£, 2012/13 prices) | Expected annual quitters | ICER |
|---|---|---|---|---|---|
| PA | 4620 | 0.122 | 267,945 | 7.06 | £4900 per quitter. Note that this result taken in isolation would suggest that PA should be preferred at any willingness to pay above £4900 per additional quitter. However, in context, a more reasonable interpretation is that there is no significant difference between the PA group results and the control group results for either cost or effectiveness |
| Control | 4435 | 0.084 | 257,242 | 4.89 | |
| Difference | 185 | 0.037 | 10,703 | 2.17 |
FIGURE 11.
Cost-effectiveness scatterplot for FTCD score of < 4.
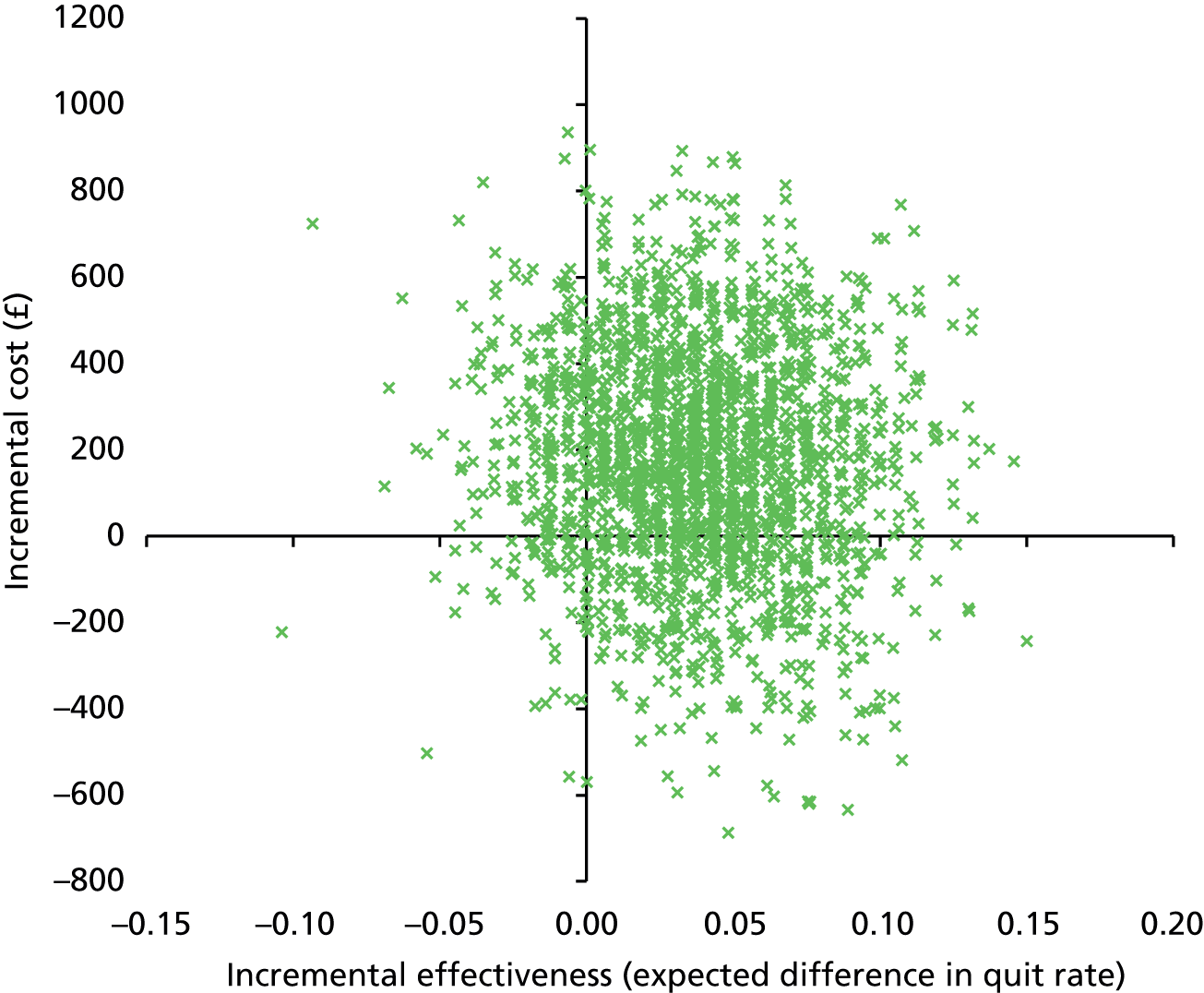
FIGURE 12.
Cost-effectiveness acceptability curve for FTCD score of < 4.
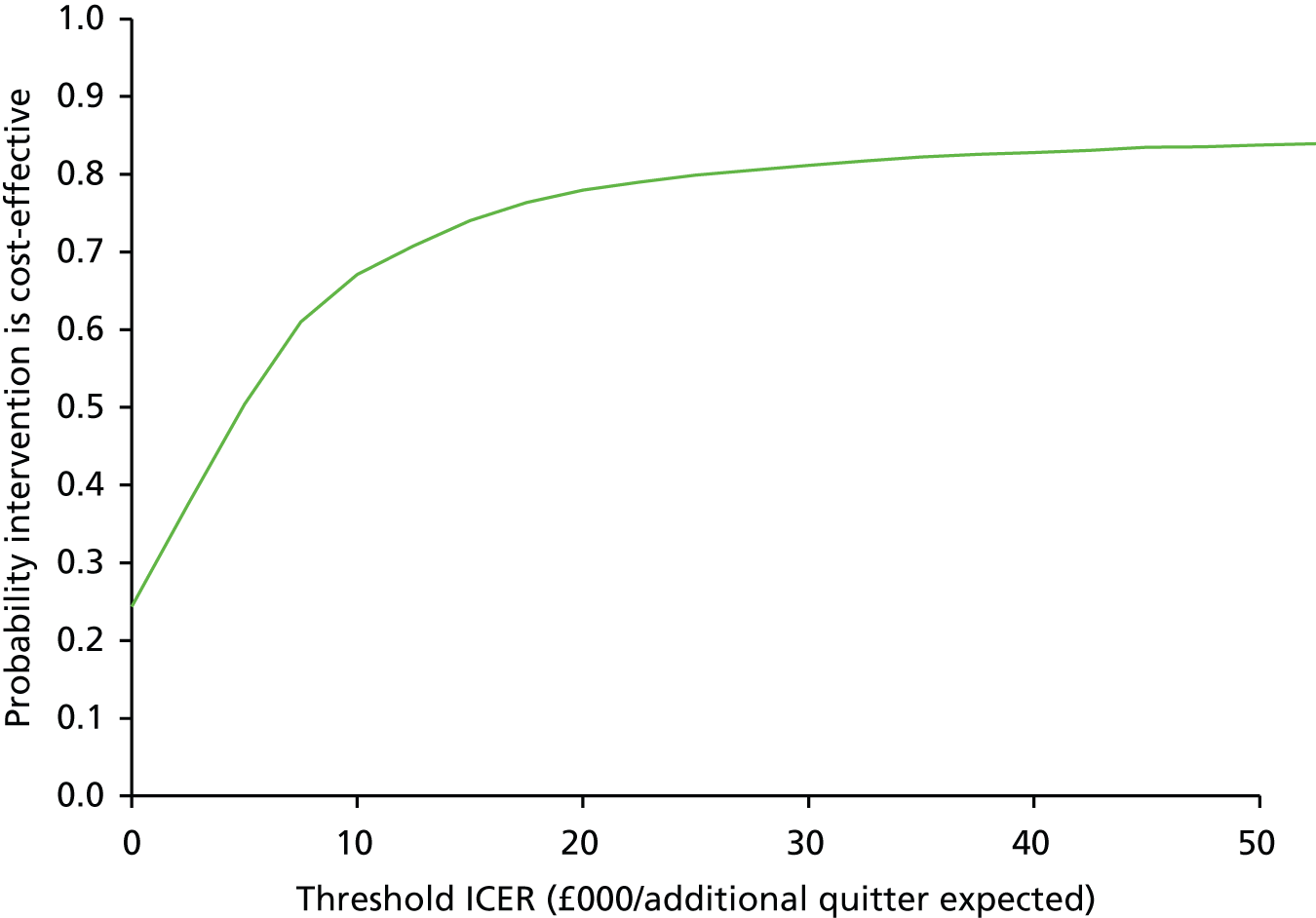
| Group | Expected per-participant costs (£, 2012/13 prices) | Expected quit rate (%) | Expected annual costs (£, 2012/13 prices) | Expected annual quitters | ICER |
|---|---|---|---|---|---|
| PA | 4772 | 0.0468 | 276,788 | 2.71 | £124,000 per quitter. Note that this result taken in isolation would suggest that PA should be preferred at any willingness to pay below £124,000 per additional quitter. This is because the differences in costs and effectiveness are both negative. However, in context, a more reasonable interpretation is that there is no significant difference between the PA group results and the control group results for either cost or effectiveness |
| Control | 4976 | 0.0485 | 288,630 | 2.81 | |
| Difference | −204 | −0.0016 | −11,842 | −0.10 |
FIGURE 13.
Cost-effectiveness scatterplot for FTCD score of ≥ 4.
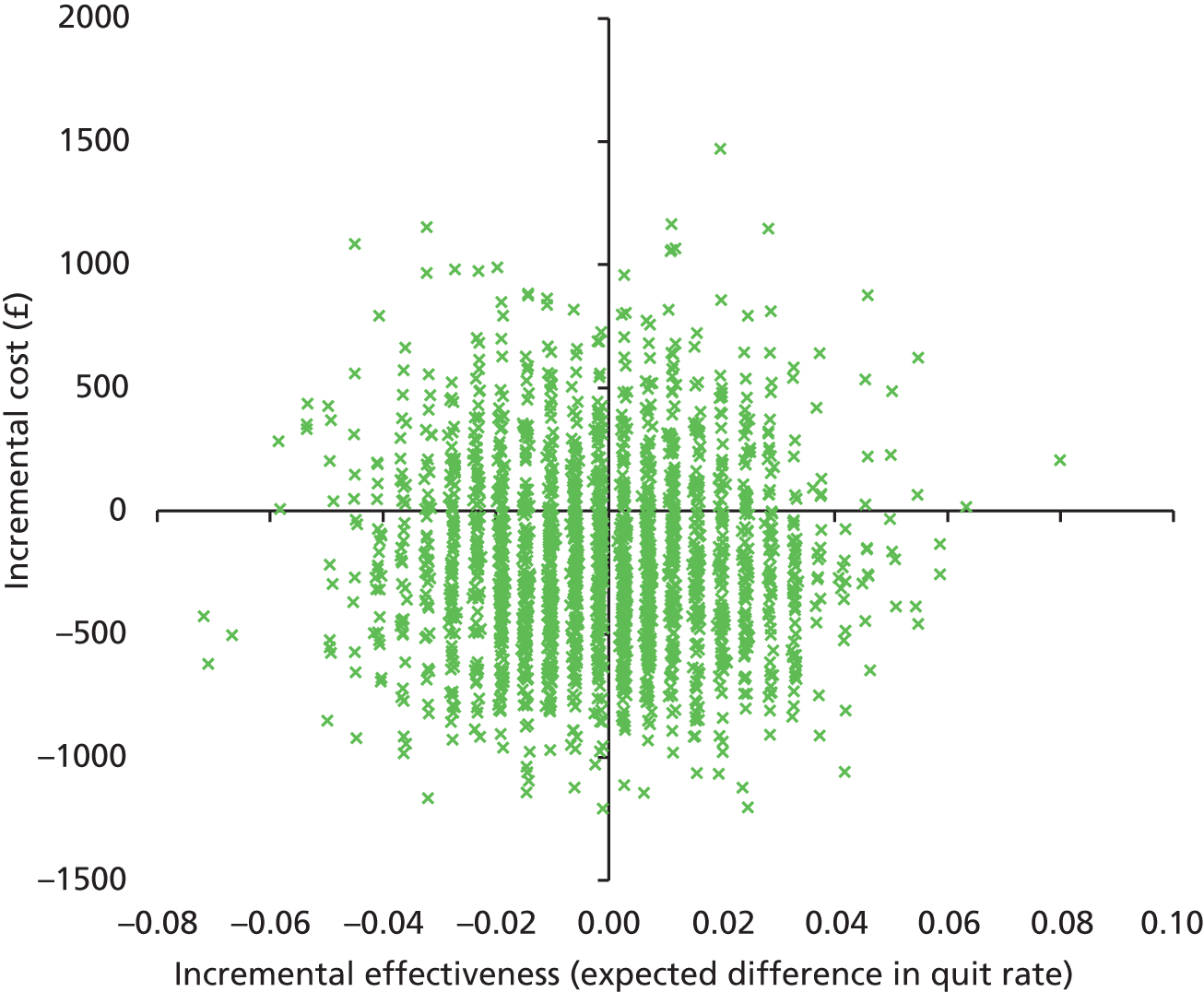
FIGURE 14.
Cost-effectiveness acceptability curve for FTCD score of ≥ 4.

List of abbreviations
- AE
- adverse event
- BCT
- behaviour change technique
- BMI
- body mass index
- CI
- confidence interval
- CO
- carbon monoxide
- CONSORT
- Consolidated Standards of Reporting Trials
- DVD
- digital versatile disc
- EPDS
- Edinburgh Postnatal Depression Scale
- FTCD
- Fagerström Test for Cigarette Dependence
- GP
- general practitioner
- GWG
- gestational weight gain
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- LEAP
- London Exercise And Pregnant smokers
- MVPA
- moderate- and vigorous-intensity physical activity
- NCTU
- Nottingham Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRT
- nicotine replacement therapy
- OR
- odds ratio
- PA
- physical activity
- PAS
- patient administration system
- PCT
- primary care trust
- p.p.m.
- parts per million
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- SGUL
- St George’s, University of London
- SNAP
- Smoking, Nicotine, and Pregnancy